Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/66/02. The contractual start date was in March 2013. The draft report began editorial review in February 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jain Holmes received consultancy fees as an employee of Obair Associates to train and mentor FRESH therapists and was subsequently granted PhD Studentship funding from the University of Nottingham and UK Occupational Therapy Research Foundation to work on this study. She was a paid employee of Obair Associates Ltd during the conduct of the study. Tracey Sach was a member of the Health Technology Assessment Efficient Study Designs Board during the conduct of the trial.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Radford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In 2011, the UK’s National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme identified the need to evaluate the feasibility of conducting a definitive randomised controlled trial (RCT) into the clinical effectiveness and cost-effectiveness of vocational rehabilitation (VR) interventions to support a return to work (RTW) for people with traumatic brain injury (TBI). The RCT should use interventions likely to be feasible for routine NHS delivery and compare these with usual NHS rehabilitation [i.e. usual care (UC)], which does not typically include interventions to support a RTW. This report describes the work commissioned to address these issues and a mixed-methods process evaluation which sought to understand factors likely to affect the conduct and outcomes of the definitive RCT.
Traumatic brain injury
Traumatic brain injury is defined as an injury to the brain caused by a trauma to the head. 1 It is a leading cause of death and a major cause of long-term disability in working-age adults. 2–4 More than 1 million people attend UK emergency departments for head injuries annually, with over 160,000 admitted to hospital. 5,6 Approximately 1.3 million people in the UK live with the consequences of TBI. 6 The economic and societal impact is considerable,7–9 costing the economy an estimated £15B per year. 10 This includes premature death, health and social care costs and lost productivity. TBI is also a known cause of personal bankruptcy. 11 However, the human cost far outweighs the economic impact. People who survive TBI are more likely to suffer from depression12 and have poor quality of life (QoL). 13,14 Even those without prior history of mental illness are twice as likely to suffer from mental health problems later in life. 10
Traumatic brain injury resulting from a blow to the head, blast waves from an explosion, swift acceleration or deceleration, or a foreign object penetrating the brain2,15 causes damage to brain tissues. In closed head injuries, it is the shearing movement and swelling of the brain within the skull following impact that causes damage. Open head injuries are caused by an object penetrating the skull and entering the brain16 or by skull fractures resulting from road traffic accidents (RTAs), falls and sports injuries. Although closed head injuries are more common than open head injuries, they are often more difficult to treat17 as they tend to result in diffuse damage affecting different areas of the brain.
The severity of TBI ranges from mild to severe and is usually determined by measures such as the duration of coma or post-traumatic amnesia (PTA), Glasgow Coma Scale (GCS) scores and the nature and extent of functional impairments following the injury. 18 A minor or mild TBI is usually defined by a GCS score of 13–15. Moderate TBI is usually defined as resulting in PTA lasting < 24 hours, a GCS score of 9–12 or a loss of consciousness lasting between 15 minutes and 6 hours. Severe TBI is usually diagnosed if the patient has a GCS score of 3–8, has been unconscious for ≥ 6 hours or has been in PTA ≥ 24 hours. 19,20 However, definitions of TBI severity vary widely across studies.
Traumatic brain injury symptoms may be physical, cognitive, perceptual, behavioural or emotional21,22 and include movement problems, seizures, impaired speech, fatigue, memory loss, decision-making difficulties, inattention, difficulty in planning or initiating tasks, inability to express thoughts, and visual and sensory problems. 22 It is often the hidden cognitive, psychological and emotional sequelae that present some of the greatest problems for people with TBI. They include reduced awareness, anxiety, anger, depression and apathy,21 and may affect social participation, independence and self-confidence, which, ultimately, have an impact on QoL.
The long-term outcomes of TBI depend on a number of factors, such as the location and severity of the injury,22 and are difficult to predict as they do not necessarily correlate with the amount of observable damage to the brain. 18,23,24 Restricted activity and social participation are common consequences. Although 70% of people who incur moderate or severe TBI are likely to sustain permanent neurological damage, resulting in long-term physical, cognitive, emotional and behavioural problems that interfere with work,25–27 many people who incur milder injuries also experience long-term impairments that limit participation in daily life activities, including work. 28,29 Doctor et al. 30 studied all consecutive patients with TBI at a level I trauma centre in the USA. Participants had to have positive evidence of TBI [e.g. any period of loss of consciousness, PTA of at least 1 hour and computerised tomography (CT) evidence of a brain lesion]. They stratified mild TBI from other severity levels using the GCS and found that workers with mild TBI were almost 3.5 times more likely to be unemployed at 1 year post injury than the general population, after controlling for age, sex and education [relative risk 3.46; confidence interval (CI) 2.87 to 4.28]. 30 Whatever the extent of the damage, TBI may be further complicated by the distressing nature of the injury and the survivor’s and family’s psychological response to it.
Return to work following traumatic brain injury
Returning to work is a primary rehabilitation goal following a TBI. Data on the reported employment rates of people with TBI vary widely between studies. Methodological differences such as sampling methods, definitions of work, length of follow-up and loss to follow-up make drawing comparison between studies difficult. 3 A systematic review31 identified that only 41% (range 30–65%) of people who were in work prior to their TBI were working at 1 and 2 years post injury. Those who do not return to work within 2 years post injury are unlikely to ever return. 31,32 For those who do, retaining work is also problematic. Many TBI survivors return prematurely but drop out once the impact of the brain injury on their job is realised. 33 Longer-term follow-up studies suggest a downwards trend in job type and earning potential as TBI survivors return to, but fail to retain, work, spiralling into more menial and poorly paid employment. 34 Although some people with mild TBI may successfully return to work without external support,35 a significant proportion (24%, range 5–56%) encounter difficulties,28,36,37 and approximately 35% are able to find only part-time work. 38 The challenge is in identifying who needs help. 35
Traumatic brain injury sequelae, including physical, sensory, communication, cognitive, behavioural, emotional, financial and social difficulties, can all have an impact on a person’s ability to work. 2,39,40 Cognitive problems especially may present considerable problems in the workplace40 and, unless the TBI survivor and employer are aware of them, they can be misinterpreted. For example, reduced ability to initiate activities may be misconstrued as laziness, yawning when losing attention as a sign of boredom and increased irritability due to fatigue as rudeness. In addition, reduced insight, which affects the ability to accurately self-monitor and adjust performance, is regarded as a poor indicator for RTW success even in people who are otherwise independent in activities of daily living (ADL). 41–44
Emotional difficulties such as anxiety and depression, which are common following TBI,44,45 have also been associated with lower levels of post-injury employment. 41,46–48 Even in those with mild TBI, post-concussive symptoms, fatigue and mild balance problems may be predictive of employment outcomes. 44,49–52 Moreover, the increased risk of seizures can preclude people from driving and doing certain jobs. 44,53–55 Ensuring that timely mechanisms are in place to identify and address these problems, irrespective of TBI severity, would seem important.
Therefore, although study heterogeneity and the known difficulty in following up people with TBI over time56 may explain some of the difference in reported outcomes, inadequate VR is also a plausible explanation for poor rates of return and job retention difficulties.
Vocational rehabilitation
Vocational rehabilitation is defined as whatever helps someone with a health problem to return to, or remain in, work. 57 It involves helping people find work, helping those who are in work but having difficulty, and supporting career progression in spite of illness or disability. The primary aim is to optimise work participation. 58
Early Specialist Traumatic brain injury Vocational Rehabilitation (ESTVR) is an adaptive form of rehabilitation that depends on identifying problems that the person with TBI has. ESTVR therapists then advise that person and their employer on how to adapt their behaviour and activities so that the problems can be managed by the patient and accommodated in the workplace. The person with TBI can then continue to be involved in work. The need for such an intervention was based on the reported prevalence of employment difficulties for people with TBI38 and findings from our previous pilot study,59 which suggested that for people with more moderate and severe injuries, continued involvement in work was unlikely to happen without support beyond 3 months, and that 10% fewer people with mild injuries would be in work at 12 months post injury without VR.
Vocational rehabilitation targets not only the brain-injured person but also their family, the work environment and the employer; crossing boundaries between health, employment, social care and welfare services. Therefore, it is a complex intervention,60 and one that needs to be individually tailored to the person and the local context, which makes it difficult to standardise.
Enabling people who have capacity to work to do so is a UK Government priority. 61 Clinical guidelines and professional recommendations62–64 state that VR should be provided for people with TBI. Ensuring people who develop long-term health conditions can return to, and remain in, work is both a government health outcome priority65 and a recognised role for health-care professionals (HCPs). 66 Despite this, NHS provision does not typically extend to VR for TBI survivors. 67 Health-based services supporting people with TBI in returning to work are rare in the UK and estimated to meet < 10% of the need. 68
Evidence for vocational rehabilitation for people with traumatic brain injury
There is a lack of evidence to support the clinical effectiveness or cost-effectiveness of VR for people with TBI. Two systematic reviews3,69 of RTW following TBI have concluded that there is a need for well-conducted experimental and observational studies. Saltychev et al. ,3 in a systematic review of pre- and post-injury predictors of vocational outcome, identified 80 studies, comprising 12 controlled (eight RCTs, two controlled clinical trials and two observational reports) and 68 uncontrolled observational reports. They found no strong evidence that vocational outcomes after TBI could be predicted or improved. However, most of the studies included in the review were generic studies of neurorehabilitation rather than trials evaluating the effectiveness of a VR intervention.
In a systematic review examining the effectiveness of VR in TBI, Graham et al. 69 identified three small RCTs: two of military populations in the USA37,70 and one of civilians in Hong Kong. 71
Salazar et al. 70 compared an intensive in-hospital cognitive rehabilitation intervention and integrated work programmes delivered by a neuropsychologist, occupational therapist (OT) and speech pathologist (n = 67) with an in-home rehabilitation programme including TBI education and individual counselling from a psychiatric nurse (n = 53). Participants were active US military personnel with moderate to severe TBI within 90 days of injury. At 1 year, there were no significant differences between groups in fitness for military duty, RTW rate, cognition, physical or verbal aggression, or QoL. However, among those who were unconscious for > 1 hour (n = 75), the proportion who returned to work was 7% higher in the cognitive rehabilitation and VR group, although the difference was not significant (95% CI –10% to 24%; p = 0.43).
Twamley et al. 37 compared a 1-year supported employment programme with the same programme plus CogSMART (for the first 3 months). Participants were US military veterans with mild to moderate TBI (n = 50). The authors hypothesised that augmenting work rehabilitation with compensatory cognitive training may improve functional outcomes because compensatory interventions can be individually tailored to each person’s job search process and job duties. CogSMART sessions were delivered by an employment specialist and included strategies to improve sleep, fatigue, headaches and tension, and compensatory cognitive strategies for prospective memory, attention, learning and memory, and executive functioning. At 12 months, there was no difference in employment between the groups. However, those also receiving CogSMART had significant reductions in post-concussive symptoms, improvements in prospective memory functioning, less severe post-traumatic stress disorder and less depression, which suggested that adding CogSMART to supported employment might improve post-concussive symptoms and prospective memory.
Man et al. 71 compared 12 sessions of three-dimensional artificial intelligence (AI), virtual reality-based VR with 12 sessions of psychoeducational VR with a vocational trainer. People with mild to moderate TBI were recruited from rehabilitation facilities in Hong Kong (n = 50). The programmes included similar problem-solving tasks, instructions and provided time to practise skills. Of the 40 participants self-reporting employment outcomes at 6 months, 8 out of 20 in the AI-VR group were employed compared with only 4 out of 20 in the psychoeducational VR group [odds ratio (OR) 2.20, 95% CI 0.46 to 10.57].
Graham et al. 69 concluded that VR for people with TBI may improve employment status but no programme was more effective than its comparator. No studies compared VR with UC or a non-VR attention control group, or reported secondary employment outcomes such as hours worked, wages earned, absenteeism, presenteeism or self-efficacy.
Since this review,69 two further VR in TBI trials72,73 have been published. O’Connor et al. 72 compared VR enhanced by cognitive rehabilitation and a computer-based homework programme with supportive client-centred therapy in a small pilot trial in US veterans with mild TBI and mental illness (n = 18). At 12 months, they identified small to moderate effects in the VR group on employment outcomes (more people competitively employed and more days worked). The 12-session VR programme, led by therapists and psychologists, included strategies to manage cognitive difficulties in the workplace, enhance skills to recognise and control unhelpful behaviours at work, deal with negative emotions, and foster positive relationships among coworkers and employers. There was also a computer-based homework programme, in addition to support, from a VR specialist. This feasibility study was small and hampered by poor adherence with therapeutic strategies in both groups, although programme attendance was similar, and high withdrawal rates. Of the 25 participants randomised, six withdrew or were withdrawn in the first two study sessions owing to mental health destabilisation (n = 3), an unexpected move out of the area (n = 2) and dissatisfaction with the control group assignment (n = 1).
Trexler et al. 73 examined the effectiveness of resource facilitation (RF) (i.e. a partnership that supports people to make informed choices and achieve rehabilitation goals, involving active engagement with a previous employer) in 44 people with mild to moderate acquired brain injury (ABI) who were working pre injury (n = 22, 13 with TBI) and a UC control group (n = 22, 10 with TBI). At 15 months, 17 out of 22 participants in the RF group with a goal of returning to work, volunteering or returning to education were successful (11 had returned to paid employment, three became volunteers and three returned to education), compared with 12 out of 22 participants in the UC control group meeting return-to-work/volunteering/education goals (10 remained employed and two returned to education). RF participants with a work-related goal had seven times higher odds of returning to productive activity than the control participants (95% CI 1.25 to 39.15).
However, although this case-co-ordinated approach in ABI seems positive, the trial73 took place in a single centre, numbers were small and there were only 23 participants with TBI (mostly mild). The intervention was staff resource intensive, requiring three more staff than UC, and lasting for 15 months. Both intervention and UC participants had access to acute and outpatient rehabilitation services, neuropsychological services and specialised day treatment programmes. However, the total amount of RF and outpatient therapies provided to the VR and UC groups was not reported, meaning that it is difficult to identify if both groups received similar levels of outpatient therapy and if differences are attributable to RF VR. Data specific to people with TBI were not reported. Interestingly, more participants in the RF group than in the UC group were employed in professional and executive positions to which they returned, which may also have contributed to the favourable outcome. Therefore, this study requires replication on a larger scale elsewhere.
Overall, current evidence for effectiveness of VR in TBI is limited and inconclusive. Of the five VR trials37,70–73 published to date, three37,70,72 have been with US military personnel or veterans. Military studies differ from those in civilian populations as participants present with different challenges (e.g. post-traumatic stress disorder) but also opportunities (e.g. better motivation, adherence and greater opportunities for redeployment within the military).
The effectiveness of neurological rehabilitation in traumatic brain injury on work outcomes
Other trials have compared various neurological rehabilitation interventions (e.g. cognitive rehabilitation,74,75 multidisciplinary rehabilitation programmes,76,77 individually tailored intervention78 and telephone-based motivational interviewing79) for people with TBI and reported work outcomes. These interventions did not include VR and no significant differences in work outcomes were identified.
In the only two large trials35,36 of early intervention following head injury, Wade et al. 36 offered intervention following routine review of all patients (n = 314) presenting to accident and emergency (A&E) with TBI. Eligible patients were prospectively randomised to a TBI specialist rehabilitation team (n = 184) or a group receiving existing standard services (n = 130). Intervention group participants were assessed 7–10 days post TBI to identify their rehabilitation needs and offered interventions as appropriate, for example information, signposting, telephone support or further outpatient intervention (46%) from different TBI specialist HCPs, including, but not specific to, advice and support with aspects of work. 80 At 6 months post TBI, 132 trial and 86 control participants were followed up (attrition: 32% intervention, 34% control) and assessed on a social disability measure. Severity of TBI was estimated from duration of PTA as mild (n = 79, 40%), moderate (n = 62, 32%) or severe (n = 55, 28%). Intervention group participants had significantly less social disability (p = 0.01) than the control group, suggesting that early intervention by a TBI specialist service can significantly reduce social morbidity post TBI. The amount of intervention increased with length of PTA. However, most participants receiving extensive services had mild to moderate TBI, suggesting that persistent TBI problems may not be severity related. In a similar trial35 (n = 1156), 71% of the 478 participants followed up at 6 months (41% response rate) had returned to some form of work or education, with no differences in work outcomes between groups (i.e. both 71%). However, in a subgroup analysis of participants with PTA lasting > 1 hour and admitted to hospital (n = 71), those in the intervention group were significantly less likely to report problems in work, relationships and social and leisure activities as measured by the Rivermead Head Injury Follow-up questionnaire (p = 0.04), suggesting that this could be a group that might benefit most from this support.
Limitations of previous studies
There are very few RCTs of VR in TBI and no cost-effectiveness evaluations of VR.
Previous trials of VR in TBI are typically small, military-based and of low methodological quality, with limitations in blinding, incomplete data, high attrition and selective outcome reporting. The definitions used for employment are wide-ranging (e.g. from homemaker to competitive employment) and time points at which employment outcomes are measured (3, 6 or 12 months) vary between studies, making trial comparisons difficult. Studies included mixed samples including TBI, stroke and other cerebral pathologies, or a range of different TBI severities.
Randomised controlled trials of general neurological rehabilitation in TBI including work outcomes and VR as part of a neurorehabilitation programme have not described the VR provided in sufficient detail for replication or implementation into clinical services. None of the trials included economic evaluation. Observational studies contribute to the evidence base but cannot provide robust, reliable evidence of clinical effectiveness that generalises to the target clinical population.
Justification for the current study
A single-centre cohort comparison study of ESTVR developed in the NHS and delivered by an OT supported by a TBI case manager (CM) was compared with usual NHS rehabilitation in people with TBI of all severities (n = 94). 59 The primary focus of the ESTVR was to prevent job loss by identifying people early after injury and optimising the opportunity to return to work with an existing employer. The findings suggested that better work outcomes (job retention at 12 months post TBI) may be achieved through early occupational therapy targeted at job retention,59 particularly for those with moderate or severe injury, although a clinically meaningful 10% difference was also observed in people with mild TBI at 12 months post injury.
However, as ESTVR was already part of a specialist TBI service and it was delivered by one therapist in a single centre, it is unclear whether or not the successful outcomes were attributable to ESTVR and whether or not it is deliverable by OTs elsewhere.
The lack of RCTs of VR and of rehabilitation in people with TBI and the known difficulty in conducting RCTs of complex health interventions,81 mean that a feasibility study is needed prior to a definitive RCT. The aims are to test the feasibility of conducting a multicentre RCT comparing ESTVR delivered by NHS OTs in addition to usual NHS rehabilitation (i.e. UC) with UC alone on work outcomes at 12 months post TBI. The feasibility study should also explore factors related to the trial process and outcomes, intervention delivery, training provided and factors likely to affect its clinical implementation in the NHS.
Structure of the Health Technology Assessment monograph
The FRESH (Facilitating Return to work through Early Specialist Health-based interventions) study consisted of a feasibility RCT, a feasibility health economic evaluation and an embedded mixed-methods process evaluation. Chapter 2 describes the design of ESTVR, the development and delivery of ESTVR training package for OTs (training, manual and mentoring) and its evaluation. Chapter 3 reports the aims, methods and findings of the feasibility RCT. Chapter 4 describes the feasibility economic evaluation and Chapter 5 summarises the aims, methods and findings of the process evaluation. The study’s key findings are discussed in Chapter 6 and the study conclusion is in Chapter 7. The appendix and supplementary material files contain details of the study components.
Chapter 2 Intervention and training package development
Aims and objectives
The aim of this chapter is to describe the ESTVR intervention and the development of the ESTVR training package.
The development of a programme theory and logic model
The starting point for the trial and subsequent process evaluation was a clear description of the intervention and its underlying theory (i.e. assumptions about how it would work in context). These assumptions included theories based on evidence from the literature and those based on experience or ‘common sense’ (see Appendix 1 for a summary of ESTVR).
The ‘theory of change’ underpinning ESTVR
For people who survive TBI and who are employed or studying at the time of injury, advice not to return to work too soon or make a premature decision about the ability to return to work is an important pre-discharge need. However, hospitals do not routinely identify and record whether or not people who have a TBI are employed pre injury. Information and advice about work is not routinely provided by HCPs for people who survive TBI, and work ability is rarely assessed early after injury. When advice is given, it can sometimes be unhelpful. For example, HCPs sometimes advise a person that they will be unable to return to work, based on an assumption that this involves returning to the same job with the same roles and responsibilities and the same working hours. However, a RTW can involve returning to the same employer but doing a modified or entirely different job. Or it might mean returning to work for a new employer, or doing the same, doing a modified or doing a different job than pre injury. Moreover, disability discrimination law requires employers to make reasonable adjustments to accommodate the injured person in the workplace. Adjustments can include more breaks, reductions in working hours, reduced responsibilities, increased supervision, flexitime working, working from home and receiving help from other people or agencies, including rehabilitation. They can also include modifications to the work environment.
Therefore, health-based support for returning to work after TBI has typically been deficient in meeting TBI survivors’ work needs. ESTVR was designed to bridge the gap between existing TBI rehabilitation services and employers and the voluntary sector in supporting survivors in a RTW. Tested in a single-centre cohort comparison, we found that the intervention may be effective in supporting job retention at 12 months post TBI.
The implicit theory of change on which ESTVR is based can be expressed as follows:
Traumatic brain injury results in physical, psychological, behavioural and emotional changes that are likely to have an impact on the capacity to return to, and remain in, work. The ability to early identify work needs after TBI is missing from TBI rehabilitation services and there is a VR knowledge and skills gap.
Implementing mechanisms for identifying TBI survivors who are employed at the time of injury, educating TBI care teams about ‘return to work’, and teaching OTs with TBI knowledge, basic skills in VR, disability discrimination, how to evaluate jobs and assess work capability, and how to match TBI survivor’s abilities to job demand, engage with employers and other employment sector stakeholders, go into the workplace and how to negotiate reasonable adjustments and a phased RTW, will enable TBI rehabilitation services to support stroke survivors in a RTW. This should result in a higher proportion of TBI survivors returning to, and remaining in, work in the first 12 months post TBI.
Logic model
A logic model was generated based on the programme theory and the intervention manual to visually depict how the intervention works and describe the resources needed, the intended activities, the hypothesised mechanisms underpinning the intervention and intended outcomes82 (Table 1).
| Resources | Core process inputs from TBI/VR OT | Short-term impacts | Impacts | Health outcomes |
|---|---|---|---|---|
|
Facilitating legal framework and policies Skilled, knowledgeable TBI/VR therapist and patient with a job Colocation: crossing boundaries between health, employment, charities Multistakeholder engagement |
Intervenes within 4 weeks of injury, advises on impact of injury and RTW | Patient does not make rapid RTW decisions | Patient and employer satisfaction | Prevent job loss |
| Provides ongoing education, advice and emotional support to patient and family | Patient aware of available support and how to access | Patient confident and able to self-manage | Improved physical and mental health | |
| Co-ordinates patient’s VR across all sectors | Vocational case co-ordinator has early and regular contact with the trauma survivor, family and other stakeholders (e.g. employer, GP, DWP, OH) |
Stakeholders report to one key contact, all aware of stakeholders involved and work towards RTW Conflicting RTW advice prevented |
Personal and financial well-being | |
| Communicates openly in writing with stakeholders regarding work performance | Patient and stakeholders aware of residual problems and coping strategies | Everyone is heard, no confusing communications | ||
| Assesses impact of TBI on person and job, analyses impact on work ability | Patient and employer aware of impact of TBI on work |
Considered decisions made re RTW Patient and employer satisfaction |
||
|
Delivers individually tailored VR in the community Explores alternatives to pre-injury employment when existing employment not feasible or sustainable |
Patient and stakeholders aware of residual TBI problems |
Employment environment optimised Workplace accommodations in place |
||
| Adapts workplace, negotiates phased RTW, provides feedback on performance | Coping strategies in place |
Phased RTW facilitated Patient able to cope with work |
Reduced sickness absence Reduced health resource use |
|
| Monitors RTW to ensure work stability | Referrals made for relevant support | Contributes to economy | Patient in sustainable employment |
Logic models provide stakeholders with a road map describing a sequence of events that connect the health intervention with its desired results and impact (improvement in patient outcomes and retaining work). 83 The process of mapping the intervention helped identify the resources and activities (core inputs) required to achieve the goals (outputs and outcomes) and overall sustained improvements (impact).
The logic model was informed by the meaningfulness (personal opinions, values, thoughts, beliefs, positive or negative experiences and perceptions of TBI service users) and the appropriateness of the intervention (i.e. how ESTVR meets expectations and the extent to which ESTVR fits with what is considered state of the art) from the perspective of researchers and TBI service users. 82
To develop the model, three of the authors (KR, JP, JH) experienced in delivering VR for people with an ABI analysed the component parts of the ESTVR intervention described in the training manual to identify:
-
core activities/processes leading to the desired outcomes
-
necessary resources (considered essential to intervention delivery and outcome success, e.g. the knowledge and expertise of the therapist, supportive policies and legal frameworks that facilitate workplace accommodations and prevent disability discrimination)
-
immediate impacts (influence on patient and stakeholder decisions and awareness that facilitate longer-term outcomes, e.g. patient’s increased awareness of problems, employer awareness of injury impact)
-
longer-term impacts (resulting from immediate outcomes, e.g. RTW, sustained work)
-
health outcomes (benefits of the intervention at a person, employer, health organisation and societal level, e.g. prevention of long-term work disability, cost savings).
The logic model was then used to design and develop an intervention fidelity checklist for use during monitoring visits (see Report Supplementary Material 1), an intervention content pro forma for recording the intervention delivered (see Report Supplementary Material 2) and to inform the interview topic guides (see Report Supplementary Material 3).
ESTVR
ESTVR was based on an intervention initially delivered by the Nottingham Traumatic Brain Injury Service (NTBIS), and described and evaluated by Radford et al.,59 Phillips84 and Phillips et al. 85 It is an early, specialist, health-based, case co-ordination model. People with TBI who are employed or studying full-time are identified early (within 8 weeks) following injury and the intervention is aimed at preventing job loss and optimising employment and education outcomes by ensuring the person can return to work/study. It is delivered by HCPs with specialist knowledge of TBI and VR-specific knowledge working in the NHS. The VR is based on best practice recommendations64 and is either delivered by an OT, supported by a health-based TBI specialist CM, or by an OT adopting a case-co-ordination role. 86 Its aim is to prevent job loss in people who are employed or studying at injury onset.
The intervention is based on the premise that a RTW need not involve a return to the same job with the same roles and responsibilities but could include a return to some form of work or an adapted or modified job with different roles and responsibilities with an existing employer, and that these adaptations could be negotiated as part of the employer’s requirement to make reasonable adjustments to support their employee in a RTW.
The ESTVR intervention seeks to lessen the impact of TBI by assessing the person’s role as a worker and finding acceptable strategies to overcome problems (e.g. assessing and addressing new physical, cognitive or psychological disabilities which might have a direct impact on work activities in relation to job demands). In the ESTVR model, it is intended that the OT provides pre-work training to prepare the person for work by establishing structured routines with gradually increasing activity levels, provides opportunities to practise work skills (e.g. computer use to increase concentration, cooking to practise multitasking), liaises with employers/tutors and disability employment advisors (DEAs) to advise about the effects of the brain injury and to plan and monitor graded work return, conducts worksite visits and job evaluations, identifies the need for workplace or job adaptations and serves as the link between health and Department for Work and Pensions (DWP) services to access additional support.
The TBI CM co-ordinates the overall TBI care package, provides support, education and advice to the person with TBI, family and others (e.g. NHS staff, social services, Headway and solicitors) and remaining in contact while there are achievable rehabilitation goals.
The intervention involves eight core processes as depicted in the logic model:
-
early intervention from a TBI team [i.e. within 8 weeks (ideally 4 weeks) of injury to provide information and advice about the impact of TBI and RTW (e.g. maintaining contact with employer/tutor)] to prevent job loss
-
assessment of the impact of TBI on the individual and the job, and analysis of the impact of TBI on the person’s work ability
-
provision of individually tailored education and rehabilitation to support a RTW
or, when return to the pre-existing employer is not feasible or sustainable, exploration of alternatives to pre-injury employment/learning:
-
optimisation of the employment/learning environment (the OT negotiates a graded RTW and any necessary accommodations to the job role, responsibilities, coworker or supervisory support and environmental adaptations needed for reintegration, and provides feedback on performance)
-
monitoring of the RTW to ensure that the person can maintain employment
The CM/VR therapist:
-
provides ongoing education, advice and emotional support to the person with TBI and their family throughout the VR process
-
co-ordinates the return to work/education rehabilitation across all sectors
-
communicates openly in writing with stakeholders about work performance at all stages of the return to work/education process.
Early intervention
The purpose of intervening early is to ensure advice (1) not to make premature decisions about relinquishing work and (2) to maintain contact with the employer is given to patients, family members and other key NHS service providers involved in the person’s care.
The assessment involves:
-
asking questions about occupational status and vocational aspirations and needs
-
responding to the person with TBI’s questions and concerns about return to work, education or training. It sometimes involves input from other relevant stakeholders who form part of a wider multidisciplinary team co-ordinated by the VR therapist or CM including NHS HCPs such as medical consultant, clinical psychologists and physiotherapists and external agencies (e.g. Jobcentre Plus, occupational health).
The rehabilitation involves:
-
providing interventions (directly related to the person’s work or study) that promote optimal recovery and management of difficulties, including one or more of the following –
-
developing skills or behaviours necessary for work or study
-
restoring work-related routines
-
activities to improve attention, work/study tolerance and stamina
-
extending coping strategies (e.g. fatigue management) for use in the workplace or for study.
-
-
planning a return to work/study with relevant agreed accommodations, such as equipment, graded return, voluntary trial, restricted hours/duties, advice/support in the workplace, job coaching, support from work colleagues, off-site support (e.g. from the OT or other relevant agencies). In the case of study return, these may include adjustments to course, learning-support equipment, individual learning support, examination support and personal support (e.g. personal tutor). These plans take account of family and personal circumstances and the required motor sensory and cognitive–behavioural and emotional skills necessary to a return to work/education.
Education involves:
-
advising the person with TBI not to return to work too soon (i.e. until the impact of the TBI is understood and coping strategies formulated)
-
educating the person, family and employer about difficulties likely to affect work/study (e.g. functional ability, insight and ability to work or return to work/study)
-
developing strategies for the person to explain the effects of their TBI to others
-
providing written information for participants and employers (e.g. about managing fatigue, job accommodations and informing the Driver and Vehicle Licensing Agency about driving) (see Report Supplementary Material 4).
Communication and co-ordination includes:
-
establishing which other community services [e.g. ABI team, speech and language therapy (SALT)] are involved and communicating with them
-
supporting the person prior to, and during, meetings with employers and other stakeholders (including clarifying what was said and agreed in meetings)
-
seeking the person’s consent to contact the employer, education provider and/or training provider to discuss needs
-
establishing and maintaining communication with employer and informing them of rehabilitation goals
-
consulting with occupational health, DEA, Jobcentre Plus occupational psychologist or other VR service providers to discuss relevant action if there is doubt about a client’s ability to cope with a supervised and graded RTW
-
providing clear written and verbal advice about appropriate timing, and gradual increases in hours and responsibilities
-
liaison with the relevant occupational health department for advice or, when unavailable, consulting the NHSPlus website
-
agreeing and discussing the disclosure of information to an employer or occupational health provider (providing clients with draft submissions for comment prior to disclosure)
-
agreeing and discussing the RTW plans with the client and a family member (when possible)
-
negotiating and agreeing workplace accommodations, such as equipment, graded return, voluntary trial, restricted hours/duties, advice/support in the workplace, job coaching, support from work colleagues, off-site support (e.g. from the OT or other relevant agencies) with the employer. In the case of study return, these may include adjustments to course, learning-support equipment, individual learning support, examination support and personal support (e.g. personal tutor).
Monitoring involves:
-
reviewing progress with ongoing advice, support and feedback for person and employer (supervisor and work colleagues, as appropriate) and obtaining feedback from family members about the impact of work on personal life, family life and relationships
-
liaising with DEAs when long-term adjustment and support are needed (e.g. major adjustments to work duties, specialist equipment or help with travel to work).
The intervention is individually tailored and delivered in the community, clinic or workplace over weeks or months based on participants’ individual needs. In our earlier cohort comparison,85 participants had a mean of 6.5 sessions of occupational therapy and 4.7 1-hour-long sessions of case management over the 12-month intervention period. Half of the sessions were delivered in person and half by telephone or e-mail. This ‘direct’ contact with the person accounts for about two-thirds of the therapist’s overall time. The rest is spent in administration and travel.
In the single-centre cohort study, ESTVR was delivered by a single experienced OT trained in VR. ESTVR was developed based on best practice recommendations64 over a number of years and evaluated pragmatically in the context in which it was developed. What remained unclear was whether or not OTs in other contexts could be trained to deliver it so that its effectiveness could be tested in a RCT. The intervention needed to be described such that the key intervention components were clear before an intervention training manual could be developed. Therefore, the starting point for this study was to manualise the intervention and train OTs in the three sites to deliver it.
Development, delivery and evaluation of the ESTVR training package
The OTs employed on FRESH needed to learn how to deliver the ESTVR intervention over the 12-month duration.
Development of the manual
A group of experts in VR TBI and training was formed [Julie Phillips (JP), Jain Holmes (JH), Yash Bedekar (YB) and Ruth Tyerman (RT)], including one service user (TJ). Three development meetings were held, supplemented by e-mail and telephone communication. The manual was based on the existing NTBIS manual and a description of the intervention delivered by Phillips et al. 85 in an earlier study. Phillips was interviewed to elicit information about the core activities, process, outcomes, impacts and resources essential to ESTVR delivery. These are illustrated in the logic model (see Report Supplementary Material 5). Manuals developed for other rehabilitation trials were used to inform the content and design. 87–90
The manual was presented in hard copy, accompanied by an electronic copy on a memory stick and was issued to OTs during training.
Manual content
The first five chapters followed a typical patient journey from hospital admission to RTW and covered the aims of ESTVR, examples of core intervention activities, the role of the OT and the CM, information on the frequency of visits and a section on common problems related to RTW following TBI (see Report Supplementary Material 4). To optimise implementation, the OTs were encouraged to adapt the manual to their local context (e.g. by adding local contact details for Jobcentre Plus, Headway groups, etc.).
Development, delivery and evaluation of the training
Training involved didactic teaching, case vignette discussions and role play. This multimodal approach was considered the best way to achieve learning. 91 Training was scheduled to take place within 1 month of trial recruitment to ensure that learning was current. However, trial recruitment was delayed by 3 months in two centres and by 6 months in the third. Refresher training was scheduled 6 months after the initial training to provide a top-up and an opportunity for shared learning once OTs had case experience. Training was delivered over 2 days by training group members. The OTs were taught together to provide peer support.
At the end of day 1, homework (case study problem-solving) was given to embed learning. A video of the training was made available to OTs as an additional resource.
It was assumed that the OTs would already be knowledgeable about TBI but have little understanding of VR. Therefore, pre-training reading materials were VR specific.
A learning needs analysis was used to assess the OTs’ knowledge and confidence on TBI and VR 1 month before, and immediately following, the training on a scale of 1 (not very confident or knowledgeable) to 5 (very confident and knowledgeable) (see Report Supplementary Material 6).
The evaluation of the training is reported in Chapter 5.
Mentoring
Mentoring was used to supplement training and ensure ESTVR was delivered with fidelity, a method found successful in implementing other complex therapy interventions. 92 The FRESH trial OTs received mentoring from one of four mentors assigned to them. Mentors were the same people who delivered the VR training. Mentors were intended to provide approximately 1 hour of support per month consistent with typical clinical supervision practice. Mentoring was delivered flexibly and included meetings, telephone calls, e-mails or text messages as agreed between the OT and mentor.
Mentoring was evaluated in interviews with OTs and the content and quantity was measured. The findings are reported in Chapter 5.
Chapter 3 Feasibility randomised controlled trial
This chapter describes the design, conduct and findings of the feasibility trial according to CONSORT 2010 statement: extension to randomised pilot and feasibility trials. 93,94 This chapter is used to address the study’s primary objective and the first 10 of the 12 secondary objectives listed below, although the process evaluation, presented in Chapter 5, also contributes to the evaluation of the protocol detailed in objective one. The remaining objectives (11 and 12), relating to the acceptability of the intervention to TBI patients, staff and employers, and the views of TBI patients and staff on recruitment and the acceptability of randomisation, are listed here to give an overall picture of the FRESH study but are not addressed until Chapter 5.
Methods
Study design
The FRESH study was a pragmatic, multicentre, individually randomised parallel-group controlled feasibility trial comparing ESTVR plus UC with UC alone, with both a feasibility economic evaluation and an embedded process evaluation.
Aim
To assess the feasibility of conducting a multicentre, two-arm randomised (1 : 1) controlled trial comparing ESTVR delivered by NHS therapists in addition to usual NHS rehabilitation with usual NHS rehabilitation alone (UC), and of measuring its effects and cost-effectiveness on work (work return and job retention) and health outcomes at 12 months post TBI.
Objectives
To:
-
assess the integrity of the study protocol (e.g. inclusion/exclusion criteria, staff training, adherence to intervention, and identify reasons for non-adherence)
-
estimate the proportion of potentially eligible TBI patients recruited and identify reasons for non-recruitment (missed, medical, logistic, other)
-
estimate the recruitment rate (by centre)
-
determine the spectrum of TBI severity among recruits
-
estimate the proportion of participants lost to follow-up and the reasons for loss to follow-up
-
describe the completeness of data collection for potential primary outcome(s) for a definitive trial
-
explore potential gains in using face-to-face data collection rather than postal data collection
-
determine the most appropriate method(s) of measuring key outcomes (RTW, job retention)
-
investigate how RTW is related to mood, well-being, function, work capacity, social participation, QoL and carer strain
-
estimate the parameters necessary to calculate the sample size for a larger trial (e.g. rate of RTW at 12 months in the control group)
-
explore the views of TBI patients and staff on recruitment and the acceptability of randomisation
-
determine whether or not ESTVR can be delivered in a way that is acceptable to TBI patients, staff and employers [views of TBI patients, staff and employers on the interventions (ESTVR vs. UC)].
Summary of design and methods of randomised controlled trial
Figure 1 summarises the study method in line with the recommendations93 for individually randomised controlled trials of non-pharmacological interventions.
FIGURE 1.
Study method CONSORT flow chart. CONSORT, Consolidated Standards of Reporting Trials.
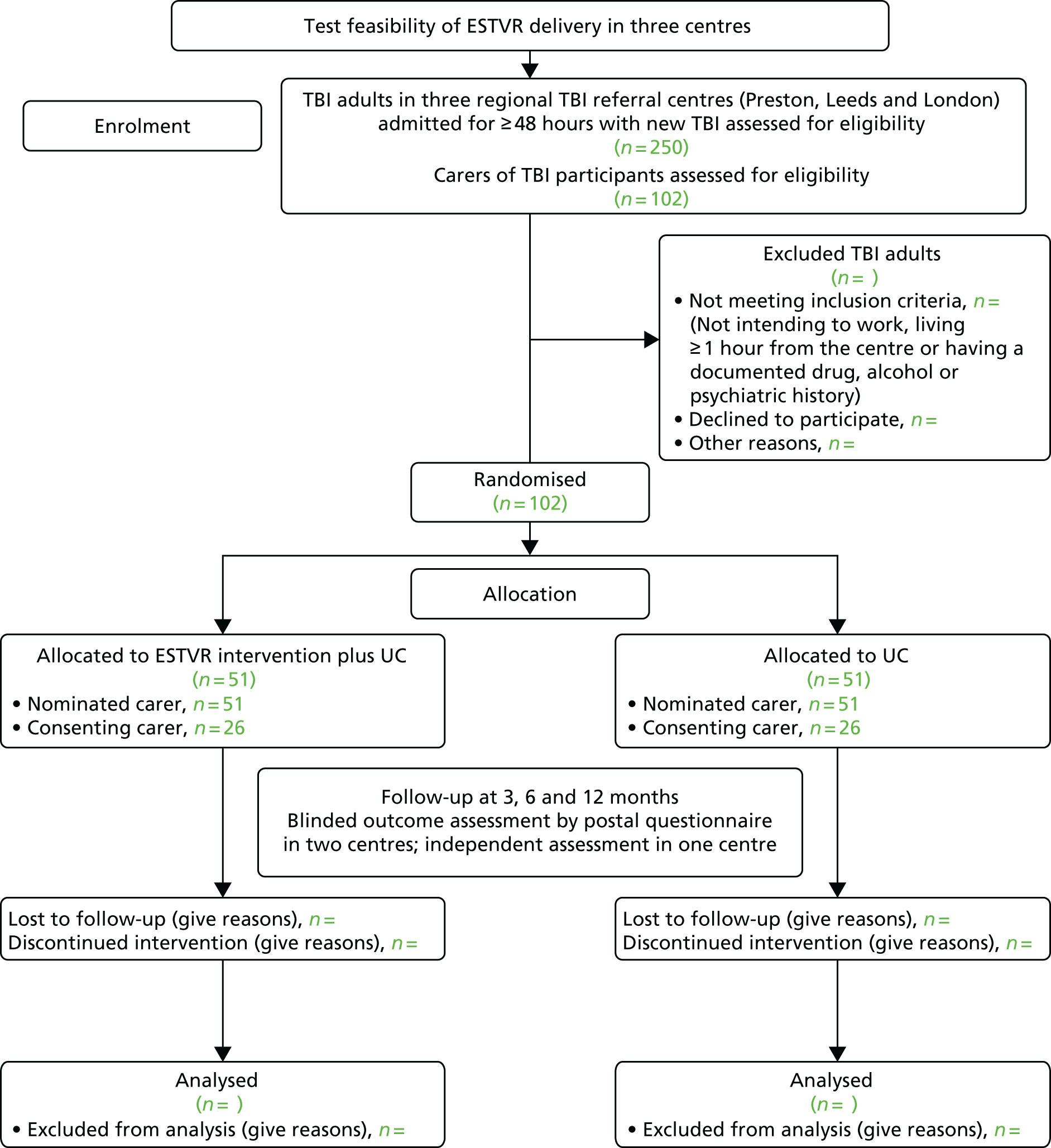
Setting and locations
The study was sponsored and co-ordinated by the University of Nottingham and the feasibility trial was co-ordinated and managed by the University of Central Lancashire Clinical Trials Unit (CTU). It involved three sites; each a designated major trauma centre (MTC).
Participants were identified initially during an inpatient stay but the intervention was delivered in other settings, for example outpatients, domiciliary visits, the workplace and other community settings.
Three sites were involved in the study.
-
Site 1 was in the North West.
-
Site 2 was in London.
-
Site 3 was in Yorkshire.
These sites were involved in FRESH from the start of the study in March 2013 until the end (last patients identified January 2015 and completed outcome assessments in January 2016). We planned to open all sites to recruitment for 12 months in September 2013.
Eligibility criteria
Inclusion
Patients were eligible for the trial if they were:
-
adults (aged ≥ 16 years)
-
living in the health communities served by the three trial sites
-
admitted for ≥ 48 hours with a new TBI
-
in work (paid or unpaid) or in full-time education prior to their injury
-
within 8 weeks of TBI.
Exclusion
Patients who met the inclusion criteria were excluded from the trial if they:
-
did not intend to return to work/study
-
were unable to provide consent for themselves
-
lived > 1 hour (or reasonable) travelling distance from the recruiting centre.
People with a language barrier resulting from TBI (e.g. aphasia) or for whom English was not their first language were not excluded. We planned to seek help from family members and interpreters to include people who met the inclusion criteria wherever possible.
Changes to eligibility criteria during the trial
No changes to eligibility criteria were made during the trial, although we considered widening the recruitment window from 8 weeks to 12 weeks post TBI. However, following discussion, it was decided not to amend the recruitment window but to collect data about repatriation in two centres (sites 2 and 3) to assess the potential impact on recruitment.
Carers
Carers of TBI participants were eligible if they were nominated during the baseline assessment visit by a consenting TBI participant as the person (spouse, partner, parent or other) with whom they had most contact.
Inclusion criteria
Carers of adults (aged ≥ 16 years) living in health communities surrounding sites 1, 2 and 3 and admitted for ≥ 48 hours with a new TBI who were in work (paid or unpaid) or in full-time education prior to their injury.
Screening, recruitment and baseline assessment
Screening process
Potential TBI participants were identified by members of the usual clinical care team using existing TBI registers. A screening log was used by the research assistant (RA) or research therapist to monitor and identify recruitment against eligibility criteria. Every person with TBI admitted to hospital and who fitted the inclusion criteria during the trial recruitment period was entered onto the screening log by the RA or Clinical Local Research Network (CLRN) research nurse. The minimum data recorded were age, gender, meeting eligibility criteria (yes/no) and consented (date) or reason for non-consent. The status of eligible patients (refused, consented) and reasons for refusal (when given) were recorded on the screening log. In addition, information about employment status was gathered to ascertain fit with inclusion criteria.
Completeness of screening was verified by cross-checking with existing trauma and local TBI registers. This was done by staff deployed to support recruitment by the participating trust and administrative staff from the clinical care team who were familiar with local mechanisms and trust policies and procedures. In two sites, this included RAs (a study-specific RA in one site), and in another this was the local CLRN research nurse, working within the trust.
Recruitment process
The initial approach was made by a member of the patient’s UC team, who provided information sheets and notified the research team. The research team (RA or CLRN research nurse) in each centre informed potential participants of all aspects pertaining to participation in the study. Patients fitting the inclusion criteria but who were discharged before being seen by a member of the research team were sent a participant information sheet with a covering letter from the consultant informing them about the project. This letter also stated that a researcher would contact them to ask if they were interested in taking part. If the patient expressed an interest, then an appointment was made for the researcher to visit, answer any questions and, if applicable, take informed written consent. Patients were given a minimum of 24 hours to consider the information prior to consent being taken. The status of eligible patients (refused, consented) and reasons for refusal (when given) were recorded on the screening log. In addition, the screening log included additional information about the employment status and type of injury (e.g. fall, assault, RTA).
Consenting participants were asked to nominate a carer (spouse, partner, parent or person with whom they had most contact) during the baseline assessment. Carers were sent a carer’s information sheet and covering letter from the consultant informing them about the project, and stating that a researcher would contact them to ascertain their interest in taking part. Interested carers were visited by a member of the research team who took written consent. Only carers nominated by a participant with TBI were approached.
The process for obtaining participant (patient and carer)-informed consent was in accordance with research ethics committee guidance and good clinical practice. The investigator, or their nominee, and the participant both signed and dated the informed consent form before the person could participate.
Baseline assessments
Baseline assessments were completed as soon as possible following consent but prior to randomisation. All baseline measures were collected face to face by the RA or research nurse either in hospital or at the participant’s home if they had been discharged at the time of recruitment.
Traumatic brain injury participants
A minimal amount of basic demographic information required for randomisation was collected from each participant by the RA or research therapist at the baseline visit (see schedule of outcome measures in Table 2).
Carers
Consenting carers in the three sites were sent a brief questionnaire including a measure of carer strain95 and asking about the impact of the TBI participant’s injury on their working hours and income.
Sample size
The sample size was based on an expectation to recruit approximately one-third of patients fitting the eligibility criteria (e.g. 100 participants from 300 patients approached over 12 months). This was intended to enable us to estimate the recruitment rate to within ± 6% (with 95% confidence) and the attrition rate to within ± 7% (with 95% confidence) (assuming an attrition rate of ≤ 15%). We anticipated that not all TBI participants would have, or be willing to pass on, carer details; however, we believed that at least 30% of carers identified by TBI participants would be recruited.
Randomisation and allocation concealment
Once TBI patients had consented to join the trial and completed their baseline assessment, participants were randomised to UC or ESTVR plus UC. The allocation sequence was computer generated via an algorithm which implemented stratified (by site) randomisation using random permuted blocks of randomly varying size. It was created by Nottingham CTU in accordance with its standard operating procedure and held on a secure server.
The randomisation system was accessed via the web by the RA or the CLRN research nurse performing the recruitment in each site and an automatically generated e-mail was sent to the nominated site staff informing them of the allocation; a similar e-mail, but without details of the intervention allocation, was sent to the trial management and data management staff at Lancashire CTU. These e-mails also contained the unique participant identification code, which was generated by the randomisation system and then used on patient questionnaires, on other trial documents and in the electronic database. The documents and database also used participant initials and date of birth for verification purposes.
Carers were not randomised.
Intervention and control conditions
ESTVR
Participants randomised to the intervention group received all usual NHS rehabilitation interventions but, in addition, they received the ESTVR (as required) targeted at job retention.
ESTVR is an early, TBI-specialist, VR, job retention intervention. It was developed in Nottingham by an OT and is routinely delivered as part of usual NHS rehabilitation by the NTBIS. It was evaluated in a single-centre cohort comparison study59 and the results suggested a positive influence on 12-month work outcomes in those who received it. ESTVR is a case co-ordination model85 based on best practice guidelines for VR following ABI. 63 It is delivered by an OT and supported by a health-based CM, both of whom have knowledge and skills in working with people with a TBI and in VR. Most interventions are delivered in the community.
People with TBI are identified early (at point of injury) and the intervention aims to prevent job loss. The VR intervention seeks to lessen the impact of TBI by assessing the patient’s role as a worker and finding acceptable strategies to overcome problems (e.g. assessing and addressing new disabilities which might have a direct impact on work activities). The intervention process follows three stages: (1) assessment, (2) intervention and (3) monitoring and review. Detailed assessment of the person’s occupational status and vocational aspirations and functional capacity for work is followed by intervention to prepare the person with TBI for work by providing pre-work training and establishing structured routines with gradually increasing activity levels and the opportunity to practise work skills (e.g. computer use to increase concentration, cooking to practise multitasking). The OT liaises with employers/tutors and employment services to advise about the effects of TB and to plan and monitor graded work return, conduct worksite visits and job evaluations, identifies the need for workplace or job adaptations, and serves as the link between health and employment services to access additional support. During ‘monitoring and review’, progress is reviewed and ongoing advice, support and feedback is provided for the TBI patient, their family and employer (supervisor and work colleagues, as appropriate) with ongoing liaison with employment services, if needed. TBI CMs co-ordinate the overall TBI care package, provide support, education and advice to patients, family and others (e.g. NHS staff, social services, Headway and solicitors), remaining in contact with patients and families while there are achievable rehabilitation goals.
The intervention was tailored to individual needs according to the following menu of components:
-
assessing people’s functional capacity for work
-
detailed job evaluation and safety assessment
-
liaison with employers regarding necessary accommodations (equipment and adaptations) and graduated RTW programmes
-
individual work-related goal setting and problem-solving sessions
-
partnership working with statutory and voluntary service providers such as disability employment and benefits advisors and Headway
-
negotiating voluntary work placements
-
providing information and advice to TBI patients, their families and employers, and counselling.
A more detailed description of the intervention is provided in Chapter 2 and Appendix 1.
Control: usual NHS rehabilitation
Participants allocated to the control group availed themselves of whatever usual health and social care services were available to them in their area. Usual NHS rehabilitation differed in each area but none of the sites had existing specialist VR services.
Usual care was measured by including resource use questions in the follow-up questionnaires. Additional information about efforts to support people with TBI in a RTW in the control group was gathered in qualitative interviews with UC participants.
Concomitant therapy
Continued use of NHS/social services department/third-sector services alongside the ESTVR intervention was anticipated. There were no known issues with the intervention and concomitant treatments; therefore, no concomitant treatments were excluded. We attempted to capture and describe concomitant therapy by including questions intended to capture the nature and quantity of concomitant therapy and any intervention received by the control group. This included information on participants’ use of other community rehabilitation, social care and third-sector services.
Training programme
A manualised training programme developed in advance of the trial and based on the original Nottingham Pilot study59,86 was delivered centrally by the chief investigator (KR) and VR expert OTs (JP, JH, YB and RT) to the OTs from each of the three centres. Therapists were trained to adopt a vocational case co-ordination/case management role in addition to delivering VR (see Chapter 2).
Therapists were asked to maintain their usual clinical notes and, in addition, to record the intervention content delivered in each intervention session in 10-minute units using an intervention fidelity pro forma (see Report Supplementary Material 2) based on the training manual. Pro formas captured face-to-face activity, travel, non-face-to-face activity [participant-related activity that did not involve direct face-to-face contact (e.g. telephone calls, letter writing)] and administration (e.g. writing clinical notes).
Monitoring and mentoring
Training and intervention delivery was supported by monthly telephone and e-mail mentoring to ensure that the intervention was delivered as intended and that the therapists felt confident in its delivery.
One hour per month of mentoring time was allocated per therapist in accordance with usual clinical practice for NHS supervision. Mentoring was delivered by therapists with expertise in VR for people with TBI (JP, JH, YB and RT). Pairing of mentors with therapists was done geographically.
In addition to the formally agreed 60-minute monthly mentoring sessions, therapists were supported by telephone and via e-mail, ensuring that they always had access to someone who could help with issues/queries as they arose. Mentoring was recorded using a mentoring record form, which also served as a mentoring checklist (see Report Supplementary Material 7). The content of the mentoring sessions is reported in the process evaluation in Chapter 5.
Intervention delivery was also quality monitored and fidelity checks were carried out to assess adherence to the ESTVR therapy process described in the manual. Further detail on the assessment of fidelity can be found in Chapter 5.
Regular, 3-monthly fidelity monitoring visits were conducted in each site by the therapy co-ordinator (JP). Therapists were asked to bring clinical case notes and to supply completed intervention fidelity pro formas for their current caseload of participants in advance of each visit. The intervention fidelity pro formas were collected and returned to the University of Nottingham for data entry and analysis.
During fidelity monitoring visits, review of case notes was used to check that the process of assessment had been completed and goals for therapy were clearly stated, as described in the therapy manual. This meant that impairments and functional limitations had been identified and RTW goals had been articulated by the participant. A discussion with the therapist about the rationale for their treatment plans provided an opportunity to ask questions or seek advice. In addition, checks were made to ensure that information had been provided to participants.
An intervention fidelity checklist (see Report Supplementary Material 1) was used to monitor whether or not the intervention delivered by trial therapists was consistent with the ESTVR core process components described in the training manual and whether or not the content was consistent with that delivered in the Nottingham pilot study. 59
The OT therapy co-ordinator (JP) carried out a minimum of four direct monitoring sessions with each of the four therapists during the intervention delivery period. In total, 16 sessions of direct monitoring were conducted.
Once completed, the fidelity pro formas and fidelity checklist were collected by the therapy co-ordinator and used to ensure intervention fidelity. In addition to these fidelity monitoring processes, therapists were interviewed about factors affecting intervention fidelity as part of the process evaluation reported in Chapter 5.
In addition to the direct monitoring and mentoring, the OTs were invited to present, to their peers, details of two participants with whom they were working at a workshop 3 months after recruitment commenced.
Outcome assessments
All outcomes for TBI participants and carers were assessed at 3, 6 and 12 months post randomisation. Outcome measures from both TBI participants and carers were collected by post in two centres and assessments were collected face to face by a RA masked to treatment allocation in one centre. In the two centres using postal data collection, participants requesting help to complete the measures were offered a home visit by a RA. Similar steps to minimise missing data were taken irrespective of the mode of data collection (postal or face to face) using personal contact by telephone and text messaging to prompt returns when 2 weeks had elapsed since the due date, using an agreed protocol (see Report Supplementary Material 8). Key data, including RTW information, were collected by telephone normally when 60 days had elapsed since the questionnaire due date.
As the likely primary measure of effectiveness for the main trial was work status at 12 months, defined as competitive employment (full- or part-time paid work in an ordinary work setting, paid at the market rate96) at 12 months post randomisation, data were collected on participants who reported having returned to:
-
work in the same role with an existing employer
-
a different role with an existing employer
-
work with a different employer (i.e. new work in the same or a different role)
-
self-employed work.
The proposed secondary outcome measures of effectiveness for a potential effectiveness trial were also collected from TBI participants and were as follows:
-
Perception of mood, using the Hospital Anxiety and Depression Scale (HADS). 97
-
Perception of functional ability, using the Nottingham Extended Activities of Daily Living (NEADL) scale. 98
-
Perception of participation ability using the Community Integration Questionnaire (CIQ). 99
-
Perception of health-related QoL using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 100
-
Perception of productivity at work using the Work Productivity and Activity Impairment (WPAI v2) questionnaire. 101
-
Use of health, social care, and broader resources, using bespoke resource use questions.
-
Perception of work self-efficacy, measured using a single question from the Work Ability Index (WAI) (Ilmarinen et al. ). 102
-
TBI recovery (at 12 months only) for comparison with other TBI studies, measured using the Glasgow Outcome Scale (GOS) score. 103 Responses were recorded into one of five categories: (1) death, (2) persistent vegetative state, (3) severe disability, (4) moderate disability and (5) low disability.
Detail of the secondary outcome measures and time points for administration are summarised in the schedule in Table 2. For each TBI participant, the measures described above were collected at recruitment to the study (baseline) and at 3, 6 and 12 months post randomisation.
| Measure | Baseline | Follow-up time points | ||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||
| For TBI participants | ||||
| Demographic information | ✓ | – | – | – |
| Duration PTA | ✓ | – | – | – |
| GCS score | ✓ | – | – | – |
| Duration unconsciousness | ✓ | – | – | – |
| Specific VR-focused questions | ✓ | ✓ | ✓ | ✓ |
| EQ-5D-3L | ✓ | ✓ | ✓ | ✓ |
| HADS | ✓ | ✓ | ✓ | ✓ |
| NEADL | ✓ | ✓ | ✓ | ✓ |
| CIQ | ✓ | ✓ | ✓ | ✓ |
| Resource use of health, social care and broader | ✓ | ✓ | ✓ | ✓ |
| Self-efficacy (single question from the WAI) | ✓ | ✓ | ✓ | ✓ |
| WPAI v2 | ✓ | ✓ | ✓ | ✓ |
| GOS score | ✓ | |||
| For carers | ||||
| CSI | ✓ | ✓ | ✓ | ✓ |
| Questions about specific impact on carer’s work | ✓ | ✓ | ✓ | ✓ |
At each time point, consenting carers were asked:
-
to complete the self-reported Caregiver Strain Index (CSI)95
-
to answer questions about the financial impact on the carer of the TBI participant’s injury at 3, 6 and 12 months post randomisation (see Table 2).
The outcome measures were compiled into booklets (see Report Supplementary Material 9 and 10) for ease of completion.
Blinding
Participants were not blinded to the intervention group allocation. The RAs and research nurses conducting recruitment and baseline assessments were not blinded to the intervention group allocation, with the exception of the RA in site 3, who was responsible for collecting postal and face-to-face follow-up outcome measures. Participants were asked not to mention group allocation to the RAs on arrival. However, it was anticipated that the RA could become unblinded (e.g. by reference to the name of the therapist).
Other members of the research team, including the chief investigator, health economist and all Lancashire CTU staff, including the trial statistician, were blinded to group allocation. They remained blinded until all interventions were assigned, recruitment and data collection were completed and the Statistical and Health Economics Analysis Plan (SHEAP) was approved by Lancashire CTU and the chief investigator. When the databases were locked, the intervention arm codes were released by Nottingham CTU; the data analysis was performed using codes for the intervention arms, with the exception of the health economic analysis. The health economic analysis was blinded as much as possible [i.e. the majority of resource use items were valued and utility values scored with estimation of quality-adjusted life-years (QALYs), without knowledge of the intervention group], but unblinded once intervention costs were assigned to participants and the final analysis conducted.
Participant withdrawal criteria
Participants could be withdrawn from the study either at their own request or at the discretion of the principal investigator (PI). The participants were made aware that this would not affect their future care. Participants were also made aware (via the information sheet and consent form) that, should they withdraw, the data already collected could not be erased and might still be used in the final analysis. Participants could also withdraw from the intervention without ceasing participation in the trial.
Safety evaluation
Given the nature of the trial intervention, no serious adverse reactions were anticipated. Therefore, no specific safety investigations were proposed and no additional safeguards were put in place over and above those adhered to by any OT in the delivery of therapy. It was not anticipated that any special conditions needed to be imposed for monitoring safety over and above those for eliciting and recording adverse events. However, as this was feasibility work, it was envisaged that safety factors which may need to be accounted for in a larger trial might be revealed and described.
Adverse events were classified as follows (some were included in more than one of the categories below):
-
deterioration in a participant’s physical or psychological health resulting in inpatient acute admissions, use of emergency, health or social care services.
Safety was assessed by collecting all adverse outcomes considered to be related to the ESTVR intervention. As the side effects of the intervention were unknown, we hoped to identify them in order to inform the design of future trials. Therefore, we collected outcome data potentially related to the intervention, including:
-
accidental injury resulting from non-compliance with equipment or workplace adaptations recommended by the FRESH VR OTs
-
work accidents resulting in injury requiring hospital treatment (hospitalisation due to a work-related injury was classified as a serious adverse outcome)
-
incidents of aggression (defined as excessive verbal aggression, physical aggression against objects, physical aggression against self and physical aggression against others) of the participant towards the researcher, staff or others (e.g. work colleagues)
-
attempted suicide (hospitalisation due to attempted suicide was classified as a serious adverse outcome).
In order to provide formal reassurance that the intervention was of extremely low risk, the Study Steering Committee (SSC) was provided with a report detailing adverse events and safety outcomes. These have also been analysed and details are included in this report.
Identification of adverse events
Questions that helped to identify adverse events were included in the questionnaire booklets at 3, 6 and 12 months. The information was extracted by the RA collecting outcome data in site 3 and the Lancashire CTU trial staff from responses to questions regarding hospital and general practitioner (GP) visits reported in service use questions. These were enhanced by records of any deaths (obtained from hospital records, GP contact and/or reports from carers and VR therapists during the trial).
Other adverse events were identified ad hoc by the FRESH VR OT and research staff in study sites (e.g. incidents of aggression, defined as excessive verbal aggression, physical aggression against objects, physical aggression against self or physical aggression against others towards researcher collecting outcome data in site 3). However, events identified only in this way were analysed separately as these data were not available in the UC arm and were fully attributed to individual participants only when the intervention arms were identified to avoid unblinding the Lancashire CTU team.
Progression criteria
Feasibility criteria for progression to the main trial were approved by the Trial Management Committee and SSC in May 2015. Feasibility will be demonstrated:
-
if at least two TBI patients per month are recruited on average and, when less than two, if strategies to achieve this target are identified
-
no fewer than 5% of TBI patients screened are recruited
-
at least 25% of eligible TBI patients are recruited and, when < 25%, if strategies are identified to achieve this
-
at least 30% of eligible carers are recruited
-
fewer than 40% withdraw or are lost to follow-up or, when > 40%, if strategies to overcome identified barriers are identified
-
fewer than 30% withdraw from the intervention or if strategies to reduce this are identified when > 30%
-
bespoke work questions are completed in 90% of completed returned questionnaires and when strategies to improve completion are identified if < 90%
-
fewer than 30% of participants are lost to follow-up and strategies to reduce to 20% are identified
-
loss to follow-up of the primary end point is < 30% at 12 months, with strategies identified to reduce this to 20%
-
most participants interviewed state that the intervention is acceptable
-
most participants interviewed indicate that randomisation is acceptable
-
most of the therapists interviewed consider the training to be useful and acceptable and, when this was not considered the case, if strategies for improvement are identified.
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, version 23 (IBM Corporation, Armark, NY, USA) and Stata® version 14 (StataCorp LP, College Station, TX, USA). A detailed statistical analysis plan (the SHEAP) was written by the trial statistician, in consultation with the SSC and Trial Management Group, prior to unblinding of the data.
Demographics and baseline characteristics
Demographic and baseline characteristics were summarised using:
-
mean [standard deviation (SD)] or median [interquartile range (IQR)], as appropriate, if quantitative (continuous or count)
-
median (IQR) or frequency (%), as appropriate, if ordinal
-
frequency (%) if nominal (dichotomous or polychotomous)
-
both overall and within intervention groups.
Recruitment characteristics
Monthly trends in recruitment across sites were graphed to assess patterns in recruitment rate over time. This was intended to help evaluate potential barriers to recruitment, inform strategies to maximise recruitment and determine the potential duration of recruitment in any subsequent trial.
Site-specific and overall eligibility and recruitment rates were estimated as point estimate with 95% CI. CIs were calculated using exact (binomial) methods.
Participant attrition
Traumatic brain injury and carer participant attrition rates at each follow-up time point were estimated overall, by site and by follow-up mode; each rate estimate has been provided as point estimate with 95% CI. CIs were calculated using exact (binomial) methods. Numbers of TBI and carer participants dropping out for various reasons have also been presented.
In addition, baseline characteristics (including intervention arm allocation) affecting TBI participant attrition were investigated using logistic regression. Baseline characteristics [site, age, sex, severity of TBI, education level, marital status, ethnic group, living alone, occupational group, length of inpatient stay, cause of injury, PTA (as either a continuous or categorical variable), post-traumatic unconsciousness (as either a continuous or categorical variable), pre-injury work status, baseline ADL, baseline anxiety, baseline depression, baseline work self-efficacy, baseline percentage work time missed owing to health, baseline percentage activity impairment owing to health and intervention arm] were added individually. Any term significant at the 20% significance level was considered for inclusion in a multiple logistic regression model. However, selection of terms for this model was restricted by the small sample size. To investigate the joint effect of terms significant (p < 0.2) at the first stage of the modelling, terms were included in the model based on a forward stepwise selection procedure using a 5% significance level (for inclusion and exclusion); categories were merged as necessary to limit sparseness of categories and, when non-linear effects were deemed possible (e.g. when scale scores are skewed), scales were categorised. Statistical significance of variables was assessed using the likelihood ratio test.
Outcome measures
All outcome measures were summarised at each time point overall, by intervention group and by site using:
-
mean (SD), or median (IQR), as appropriate, if quantitative (continuous)
-
median (IQR) or frequency (%), as appropriate, if ordinal
-
frequency (%) if nominal (dichotomous or polychotomous).
Summaries have been within intervention groups, within sites and within site by intervention groups. Descriptive analysis of work status data over time was performed to investigate within-individual trends in work status to aid selection of the primary work status-related outcome for a subsequent effectiveness trial, should this trial demonstrate feasibility.
Generalised linear models for continuous, ordinal and dichotomous outcomes were used to compare the two intervention groups (defined according to the intention-to-treat population, but with no imputation) on outcome data. Baseline measures of the outcomes variables were included as covariates (or factors) in the models for outcome data. Measures of effectiveness and relative effectiveness (differences between means or ORs, presented as ‘ESTVR relative to UC’ as appropriate) were estimated and 95% CIs presented; no p-values have been presented.
Exploratory logistic modelling was used to investigate factors previously found to be related to work return and estimates of intervention effectiveness will be adjusted for baseline factors which were found (i.e. significant at the 5% level using a likelihood ratio test) or deemed likely to affect the work status at 12 months post randomisation. A decision for inclusion of terms was also dictated by model convergence, which was not achievable for some potential models owing to the small sample and sparseness of the data.
Investigation of the distribution of responses for health outcome measures and of patterns in work status over time was performed to inrefers to imputationform the design (primary outcome, follow-up duration, analysis, sample size, etc.) of a future trial. Key parameters (e.g. percentage in work at 12 months in control arm) were also estimated (with CIs) to inform the design of the potential future trial.
Statistics (frequency, percentage) describing the nature and extent of missing data were produced for each outcome, summarised by (1) planned mode of data collection method (face to face and postal) and (2) treatment arm. No imputation of missing data was performed.
Adverse outcomes
Adverse events were presented as frequencies (%), overall and by intervention group.
Ethics arrangements and research governance
Integrated Research Application System (IRAS) approval was granted (13/EM/0353), together with site-specific approvals for each individual centre. Research and development (R&D) approval was obtained from each participating trust. The trial was conducted in accordance with the International Council for Harmonisation – Good Clinical Practice104 and the Research Governance Framework for Health and Social Care. 105
The SSC comprised an independent chairperson (AB); six independent members (all of whom had expertise in rehabilitation research, clinical trials, TBI, statistics or all of the above); two non-independent members of the study team including the chief investigator (KR) and trial statistician (CS); and four non-independent observers: the trial health economics lead (TS), the director of the Lancashire CTU (CW), a patient and public involvement (PPI) representative (TJ) and the senior trial manager (DE).
As this was a feasibility study and no serious adverse reactions were anticipated, an independent Data Monitoring Ethics Committee was not required, but a subgroup of the SSC was established to consider adverse events. This was chaired by an independent statistician (MD) and included a consultant neurologist (DW) (both with experience in research trials) and a rehabilitation medic (NB).
Amendments to the study following commencement
All amendments were carried out following consultation with IRAS.
A request was made in November 2013 for approval following revisions to the protocol: (1) to remove reference to named researchers and (2) regarding the definitions and methods of reporting adverse events, identifying some as expected outcomes of the study intervention and defining others more clearly in terms of severity. Data about these outcomes were then routinely gathered at the timed follow-up points, using additional related questions in the 3-, 6- and 12-month follow-up questionnaires. We also revised the participant information sheets and carer information sheets to reflect that, in the event of a TBI participant’s formal withdrawal or death, no further attempts would be made to follow up their nominated carer.
No changes were made to the trial outcomes after the trial commenced.
Further amendments were requested in July 2014, which included the following.
-
Corrections to the inclusion and exclusion criteria, namely removal of ‘intention to work’, limiting inclusion to those who were in work (paid or unpaid) or in full-time education prior to their injury.
-
Clarification of the procedure for carer follow-up, making the distinction between face-to-face follow-up in site 3 and postal follow-up in sites 1 and 2.
-
Production of a short version of the information sheet to accompany the more detailed information sheet. This was because our PPI representative told us that the original approved version was too long and detailed for someone who had recently had a head injury. We wanted to help people early after injury understand what the study was about and what taking part would involve for them.
-
A variation to the contract with the fund-giver (HTA) asking for permission to extend recruitment by 4 months until 31 December 2014 and extend the end date of the study by 6 months to 31 August 2016 to allow for 12-month follow-up data collection and analysis. This was due to the 4-month delay in obtaining R&D approvals and difficulties in securing the Excess Treatment Costs to pay for the therapy input.
These amendments were approved on 23 July 2014.
A further substantial amendment to patient information sheets and consent forms to state that, in the event of a participant’s death, no further attempts would be made to contact carers, was given chairperson’s approval by the Northampton Research Ethics Committee (13/EM/03530) on 23 July 2014 but rejected by sites. A request was made to revert to the original forms on 10 October 2014 and approved.
Recruitment rate issues
The recruitment start date was delayed. After lower than anticipated recruitment, a 3-month extension to enable recruitment to continue for the planned 12 months was granted by the funder following agreement from all participating sites to continue in the trial.
Following initial recruitment difficulties, a recruitment workshop was held and written materials produced to assist with recruitment, including a list of ‘frequently asked questions’ and a crib sheet to help the non-therapists describe the VR intervention (see Report Supplementary Material 11). These were sent to each centre. More information about the utility of these materials and suggestions for optimising recruitment in any future trial are provided in the process evaluation in Chapter 5.
Results
This section reports on the process of recruiting and conducting the 3-, 6- and 12-month outcome assessments with participants (patients and carers) in the feasibility RCT, before presenting the outcome data relating to clinical effectiveness. Particular emphasis is given to 12-month outcomes as these reflect the time point at which effectiveness would be evaluated in a RCT.
The results are organised around the first 10 objectives listed at the start of this chapter (see Methods, Objectives), although objective 1 will be addressed primarily in the summary of the results.
Randomised controlled trial process
Following delays, two sites opened for recruitment in December 2013 and one site (site 3) opened in February 2014. After lower than anticipated recruitment during the early stages, a 3-month extension was granted by the funder following agreement from all participating sites to continue in the trial. Recruitment took place over the full 12 months planned in the trial protocol in two sites and 11 months in the third. However, the RA did not commence until 1 March 2013 in site 2, with the site relying on the OT to attempt recruitment during the first month following its opening. The trial closed to recruitment after 14 January 2015, having allowed a 2-week window for consent for those with injuries sustained up until 31 December 2014. A total of 78 participants were recruited and randomised in equal numbers to the ESTVR and UC arms. The overall screening, recruitment, randomisation, treatment and follow-up process data are presented in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 2).
FIGURE 2.
Study CONSORT diagram.

Screening and recruitment
Objective 2: to estimate the proportion of potentially eligible traumatic brain injury patients recruited and identify reasons for non-recruitment (missed, medical, logistic, other)
In total, 1446 patients were screened, with 200 (14%) eligible for recruitment, 1167 (81%) ineligible and 79 (5%) with unknown eligibility, mainly owing to being ‘discharged prior to screening’. Once patients were identified as eligible for the study, they were approached by a RA or research nurse for consent. Of the 200 who were eligible, 78 (39%, 95% CI 32% to 46%) (5% of those screened) were recruited to the trial. The majority of those ineligible (779, 67%) were either unemployed (216, 19%) or retired (563, 48%). Other common reasons were remaining in hospital < 48 hours (95, 8%), living approximately > 1 hour away (105, 9%) and not competent to provide consent (79, 7%). Fifty-seven (5%) of those screened were recorded as not having had a TBI, 39 (3%) had died and small numbers were not planning to return to work (n = 7), were aged < 16 years (n = 5) or were enrolled in a competing trial (n = 1).
Objective 3: to estimate the recruitment rate (by centre)
The estimated recruitment rate (overall 39%, 95% CI 32% to 46%) varied somewhat by site, from 32% (95% CI 21% to 44%) in site 1 to 35% (95% CI 24% to 49%) in site 2 and 49% (95% CI 37% to 62%) in site 3. Site 3 met its recruitment target of 34 in 11 months. Sites 1 and 2 each recruited 22 participants in 12 months, albeit with differing patterns of recruitment across the trial, with site 2 recruiting to target for the first 6 months of the trial (n = 17) and recruitment at site 1 improving towards the end of the recruitment period (Figure 3).
FIGURE 3.
Recruitment by site against target (two sites technically opened in late December 2013 but did not recruit anyone until January 2014).
A total of 76 (38% of those eligible) patients declined to participate, 29 (15%) did not respond to a letter inviting them to take part following discharge (lost to follow-up) and 17 (9%) did not participate for other reasons [seven (4%) were not approached owing to behavioural safety issues highlighted by the clinical team, six (3%) gave incorrect personal information and four (2%) were erroneously excluded].
Some of the 76 people who declined volunteered a reason. The most common set of reasons was that they believed that they did not require help or that the intervention would be of no benefit, in some cases owing to them being back at work, although 16 patients reported that it was ‘too much’ for them to participate in the trial (see Report Supplementary Material 12, Table 4).
Carers of TBI patients were nominated by recruited patients. In total, 52 carers were nominated by 78 patients and 32 (62%, 95% CI 47% to 75% of those nominated) were recruited.
The ESTVR intervention was intended to be delivered early (within 8 weeks) after TBI. The mean time from injury to first face-to-face contact with a FRESH therapist was 35.3 working days (range 7–97 working days) indicating that most participants’ intervention commenced within 8 weeks (56 days) of TBI. Further statistics relating to times between injury onset and recruitment and between injury and intervention commencing are provided in Report Supplementary Material 12, Table 5. The success of randomising participants and delivering the intervention within the early recovery/post-acute phase is discussed further in Chapter 5.
Those who consented were similar to those who declined in the characteristics collected (mean age 38.9 years and 39.1 years respectively; percentage male 85% and 80% respectively).
Objective 4: to determine the spectrum of traumatic brain injury severity among recruits
Overall, 41 (56%) of those recruited had a minor TBI (GCS score of 13–15). Of those who responded to specific questions about amnesia or unconsciousness, 61 (79%) reported having experienced PTA and 59 (79%) reported having been unconscious.
The most common causes of injury were falls (n = 30, 39%) and RTAs (n = 31, 40%), although 19 of those who had fallen were randomised to the intervention arm and 19 of those who had suffered a RTA were randomised to the control arm (Table 3).
| Characteristic | Intervention arm | Overall (n = 78) | |
|---|---|---|---|
| UC (n1 = 39) | VR (n2 = 39) | ||
| Sex | |||
| Male | 32 (82) | 34 (87) | 66 (85) |
| Female | 7 (18) | 5 (13) | 12 (15) |
| Age (years), mean (SD) | 38.0 (13.8) | 40.6 (13) | 39.3 (13.4) |
| Marital status | |||
| Married or living with a partner | 19 (49) | 22 (56) | 41 (53) |
| In a long-term relationship but not cohabiting | 6 (15) | 5 (13) | 11 (14) |
| Single | 12 (31) | 10 (26) | 22 (28) |
| Divorced or separated | 2 (5) | 2 (5) | 4 (5) |
| Lives alone or with others | |||
| Lives alone | 5 (13) | 8 (21) | 13 (17) |
| Lives with family/spouse or significant other | 34 (87) | 31 (80) | 65 (83) |
| Highest education level | |||
| Degree, higher degree or equivalent qualification | 13 (33) | 14 (36) | 27 (35) |
| Higher education qualification | 8 (21) | 5 (13) | 13 (17) |
| GCE, A level or equivalent qualification | 3 (8) | 4 (10) | 7 (9) |
| GCSE grades A*–C or equivalent | 8 (21) | 9 (23) | 17 (22) |
| Other qualifications | 1 (3) | 3 (8) | 4 (5) |
| No qualifications | 6 (15) | 4 (10) | 10 (13) |
| Ethnic group | |||
| White | 35 (90) | 34 (87) | 69 (88) |
| Black Caribbean | 2 (5) | 1 (3) | 3 (4) |
| Asian | 2 (5) | 2 (5) | 4 (5) |
| Other | 0 (0) | 2 (5) | 2 (3) |
| Occupational grouping (n1 = 32, n2 = 36, n = 68) | |||
| Managers, directors and senior officials | 4 (13) | 4 (11) | 8 (12) |
| Professional occupations | 5 (16) | 6 (17) | 11 (16) |
| Associate professional and technical occupations | 6 (19) | 2 (6) | 8 (12) |
| Administrative and secretarial occupations | 2 (6) | 4 (11) | 6 (9) |
| Skilled trades occupations | 8 (25) | 8 (22) | 16 (24) |
| Caring, leisure and other service occupations | 0 (0) | 1 (3) | 1 (2) |
| Sales and customer service occupations | 1 (3) | 1 (3) | 2 (3) |
| Process, plant and machine operatives | 2 (6) | 4 (11) | 6 (9) |
| Elementary occupations | 4 (13) | 6 (17) | 10 (15) |
| Number of days in hospital, median (IQR) | 7 (4–11) | 10 (5–18) | 9 (4–14.3) |
| Cause of injury | |||
| Fall | 11 (28) | 19 (49) | 30 (39) |
| RTA | 19 (49) | 12 (31) | 31 (40) |
| Assault | 7 (18) | 6 (15) | 13 (17) |
| Othera | 2 (5) | 2 (5) | 4 (5) |
| Categorised lowest GCS (n1 = 36, n2 = 37, n = 73) | |||
| Severe (≤ 8) | 8 (22) | 11 (30) | 19 (26) |
| Moderate (9–12) | 7 (19) | 6 (16) | 13 (18) |
| Minor (13–15) | 21 (58) | 20 (54) | 41 (56) |
| Experienced PTA (n1 = 39, n2 = 38, n = 77) | |||
| Yes | 31 (80) | 30 (79) | 61 (79) |
| Duration of PTA (hours), median (IQR) [range] (n1 = 27, n2 = 28, n = 55)b | 24 (3–96) [0.1–240] | 60 (24–168) [0.3–336] | 48 (5–120) [0.1–336] |
| Unconscious after injury (n1 = 37, n2 = 38, n = 75) | |||
| Yes | 27 (73) | 32 (84) | 59 (79) |
| Time unconscious after injury (hours), median (IQR) [range] (n1 = 17, n2 = 24, n = 41)b | 1 (0.2–48) [0.02–840] | 1 (0.5–21) [0.02–336] | 1 (0.4–36) [0.02–840] |
| Injury date to baseline questionnaire completion (days), median (IQR) | 21 (8–34) | 22 (10–39) | 22 (8–36.3) |
| Site | |||
| 1 | 11 (28.2) | 11 (28.2) | 22 (28.2) |
| 2 | 11 (28.2) | 11 (28.2) | 22 (28.2) |
| 3 | 17 (43.6) | 17 (43.6) | 34 (43.6) |
The mean age of participants was 39.3 years and most (n = 66, 85%) were male. A total of 88% described themselves as white. Forty-one (53%) participants were married and 40 (51%) had a higher education qualification (degree or other). Most (n = 63, 81%) participants reported that their health was worse than 12 months previously, with a mean NEADL score of 9.5 points. Other demographic and health characteristics are summarised in Table 7.
Stratification achieved balance by site (see Table 3) and differences between arms on other demographic measures were generally small (see Table 3). Differences between arms on self-reported measures of health were generally small, although, on average, the UC group was slightly less severely impaired (in ADL, community integration and anxiety levels) and reported being more able to work effectively (higher work self-efficacy, lower percentage work time missed due to health impairment while working and overall work impairment) (Table 4).
| Measure | Intervention arm | Overall (n = 78) | |
|---|---|---|---|
| UC (n1 = 39) | VR (n2 = 39) | ||
| EQ-5D-3L VAS, mean (SD) | 56.2 (25.7) | 53.4 (22.1) | 54.8 (23.9) |
| NEADL, mean (SD), higher scores indicate greater independence | |||
| Mobility (valid range 0–6 points) | 2.7 (2.3) | 2.3 (2.4) | 2.5 (2.4) |
| Kitchen (valid range 0–5 points) | 2.9 (1.8) | 2.7 (1.7) | 2.8 (1.8) |
| Domestic (valid range 0–5 points) | 1.5 (2.0) | 1.5 (2.2) | 1.5 (2.1) |
| Leisure (valid range 0–6 points) | 2.7 (1.3) | 2.5 (1.3) | 2.6 (1.3) |
| Total score (valid range 0–22 points) | 9.9 (6.4) | 9 (6.7) | 9.5 (6.5) |
| CIQ, mean (SD) | |||
| Home integration (valid range 0–10) | 4.4 (2.9) | 4.5 (2.9) | 4.5 (2.9) |
| Social integration (valid range 0–12) | 7.5 (2.5) | 6.3 (2.7) | 6.9 (2.7) |
| Social productivity (valid range 0–7) (n1 = 39, n2 = 37, n = 76) | 4.8 (1.6) | 5 (1.7) | 4.9 (1.6) |
| Total score (valid range 0–29) (n1 = 39, n2 = 37, n = 76) | 16.6 (4.8) | 16.0 (5.0) | 16.3 (4.9) |
| HADS | |||
| Anxiety | |||
| Normal (0–7) | 26 (67%) | 24 (62%) | 50 (64%) |
| Borderline abnormal (8–10) | 7 (18%) | 8 (21%) | 15 (19%) |
| Abnormal (11–21) | 6 (15%) | 7 (18%) | 13 (17%) |
| Depression (n1 = 38, n2 = 39, n = 77) | |||
| Normal (0–7) | 23 (61%) | 25 (64%) | 48 (62%) |
| Borderline abnormal (8–10) | 9 (24%) | 9 (23%) | 18 (23%) |
| Abnormal (11–21) | 6 (16%) | 5 (13%) | 11 (14%) |
| Self-efficacy single question from WAI, mean (SD) (valid range 0–10) | 5.2 (3.1) | 4.6 (3.2) | 4.9 (3.2) |
| WPAI v2, mean (SD) | |||
| Per cent work time missed due to health (n1 = 8, n2 = 7, n = 15) | 52.2 (44.0) | 65.0 (47.0) | 58.2 (44.2) |
| Per cent impairment while working due to health (n1 = 6, n2 = 6, n = 12) | 36.7 (34.4) | 81.7 (24.0) | 59.2 (36.8) |
| Per cent overall work impairment due to health (n1 = 8, n2 = 7, n = 15) | 63.6 (34.9) | 87.1 (23.6) | 74.6 (31.5) |
| Per cent activity impairment due to health | 52.3 (33.9) | 60.5 (29.5) | 56.4 (31.8) |
| EQ-5D-3L | |||
| Mobility | |||
| No problems | 16 (41) | 17 (44) | 33 (42) |
| Some problems | 18 (46) | 19 (49) | 37 (47) |
| Confined to bed | 5 (13) | 3 (8) | 8 (10) |
| Self-care | |||
| No problems | 27 (69) | 26 (67) | 53 (68) |
| Some problems | 9 (23) | 12 (31) | 21 (27) |
| Unable to wash or dress | 3 (8) | 1 (3) | 4 (5) |
| Usual activities | |||
| No problems | 7 (18) | 2 (5) | 9 (12) |
| Some problems | 15 (39) | 18 (46) | 33 (42) |
| Unable to do usual activities | 17 (44) | 19 (49) | 36 (46) |
| Pain/discomfort | |||
| No pain or discomfort | 12 (31) | 9 (23) | 21 (27) |
| Moderate pain or discomfort | 25 (64) | 26 (67) | 51 (65) |
| Extreme pain or discomfort | 2 (5) | 4 (10) | 6 (8) |
| Anxiety/depression | |||
| Not anxious or depressed | 25 (64) | 23 (59) | 48 (62) |
| Moderately anxious or depressed | 10 (26) | 14 (36) | 24 (31) |
| Extremely anxious or depressed | 4 (10) | 2 (5) | 6 (8) |
| Health compared to previous 12 months | |||
| Better | 1 (3) | 0 (0) | 1 (1) |
| Much the same | 10 (26) | 4 (10) | 14 (18) |
| Worse | 28 (72) | 35 (90) | 63 (81) |
Two consenting carers did not return the baseline questionnaire; one of those returned the 3-month questionnaire. Most carers were female and were the spouse or partner of the TBI participant. More control group carer participants were a parent of the TBI participant (47% vs. 7%) (Table 5).
| TBI carer demographic and carer strain measures | Intervention arm | Overall (n = 30) | |
|---|---|---|---|
| UC (n1 = 16) | VC (n2 = 14) | ||
| Sexa | |||
| Male | 2 (12) | 2 (13) | 4 (13) |
| Female | 15 (88) | 13 (87) | 28 (88) |
| Age (years), mean (SD) | 48.6 (11.4) | 44.9 (17.2) | 46.9 (14.3) |
| Relationship to participanta | |||
| Spouse | 7 (41) | 8 (53) | 15 (47) |
| Partner | 2 (12) | 6 (40) | 8 (25) |
| Parent | 8 (47) | 1 (7) | 9 (28) |
| Sibling | 0 (0) | 0 (0) | 0 (0) |
| Friend | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| CSI, mean (SD) | 6.0 (4.1) | 5.4 (3.1) | 5.7 (3.6) |
| CSI – categorised | |||
| All item responses are negative | 3 (19) | 1 (7) | 4 (13) |
| 1–6 item responses are positive | 5 (31) | 7 (50) | 12 (40) |
| ≥ 7 item responses are positive | 8 (50) | 6 (43) | 14 (47) |
Treatment fidelity
Delivery of the ESTVR intervention is described fully in Chapter 5 and detailed data on the amount and content of ESTVR or UC delivered in each arm are presented in Chapters 4 and 5. In summary, 39 participants were randomised to receive the ESTVR intervention. One participant did not respond to attempts to contact them. The remaining 38 participants were deemed to have received ESTVR as described in the treatment manual. However, there were some issues identified with its implementation in the context of a RCT, which would need to be addressed in a definitive trial. These are described in Chapter 5.
Adverse events
Only one (1%) participant had a single reportable adverse event, which was an incident of (verbal) aggression of an ESTVR participant towards their OT (see Report Supplementary Material 12, Table 52).
Participant follow-up
Objective 5: to estimate the proportion of participants lost to follow-up and the reasons for loss to follow-up
Return rates and reasons for non-return of questionnaires for the 78 TBI participants and 32 carers recruited for the trial are reported below. Return rates for each follow-up time point are based on the actual number of questionnaires returned divided by the total numbers of TBI participants or carers. Table 6 shows the return rate, both overall and by site, for each follow-up time point. In total, 52 out of 78 (66.7%, 95% CI 55.1% to 76.9%) TBI participants responded at 12 months. None had died by 12 months, but four had withdrawn consent (one in the intervention group and three in the control group) and 22 further TBI participants did not complete a questionnaire at 12 months (nine in the intervention group and 13 in the control group) (see Figure 2). In a few cases, data on primary outcomes were available in the absence of a fully completed questionnaire (shown in Table 6 as ‘overall + key primary data’).
| Return rate | Follow-up time point | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| TBI participants | ||||
| Overall (n = 78) | 78 (100, 95% CI 95.3 to 100) | 65 (83.3, 95% CI 73.2 to 90.8) | 62 (79.5, 95% CI 68.8 to 87.8) | 52 (66.7, 95% CI 55.1 to 76.9) |
| Overall + key primary dataa (n = 78) | N/A | 68 (87.2, 95% CI 77.7 to 93.7) | 65 (83.3, 95% CI 73.2 to 90.8) | 52 (66.7, 95% CI 55.1 to 76.9) |
| Carers | ||||
| Overall (n = 32) | 30 (93.8, 95% CI 79.2 to 99.2) | 26 (81.3, 95% CI 63.6 to 92.8) | 23 (71.9, 95% CI 53.3 to 86.3) | 22 (68.8, 95% CI 50.5 to 83.9) |
Response rates for carers were marginally lower. At baseline, 30 out of 32 (93.8%, 95% CI 79.2% to 99.2%) questionnaires were returned; at 3 months, 26 out of 32 (81.3%, 95% CI 63.6% to 92.8%) were returned; at 6 months, 23 out of 32 (71.9%, 95% CI 53.3% to 86.3%) were returned; and at 12 months, there were 22 out of 32 (68.8%, 95% CI 50.5% to 83.9%) returned.
The characteristics affecting completion of TBI participant questionnaires at 12 months are shown in Table 7. Non-responders were significantly more likely, at baseline, to have been younger (p = 0.005), not living alone (p = 0.033), more anxious (p = 0.018) and to have lower mean percentage activity impairment due to health scores (p = 0.004).
| Characteristic | Overall (n = 78) | Non-responder (n1 = 26) | Responder (n2 = 52) | p-value |
|---|---|---|---|---|
| Age (years) at recruitment, mean (SD) | 39.3 (13.4) | 33.4 (13.4) | 42.3 (12.6) | 0.005 |
| Site | 0.26 | |||
| 1 | 22 (28) | 4 (15) | 18 (35) | |
| 2 | 22 (28) | 9 (35) | 13 (25) | |
| 3 | 34 (44) | 13 (50) | 21 (40) | |
| Sex | 0.51a | |||
| Male | 66 (85) | 21 (81) | 45 (87) | |
| Female | 12 (15) | 5 (19) | 7 (14) | |
| Categorised lowest GCS score (n1 = 25, n2 = 48, n = 73) | 0.89a | |||
| Severe (≤ 8) | 19 (26) | 6 (24) | 13 (27) | |
| Moderate (9–12) | 13 (18) | 4 (16) | 9 (19) | |
| Minor (13–15) | 41 (56) | 15 (60) | 26 (54) | |
| Education | 0.87a | |||
| Degree, higher degree or equivalent qualification | 27 (35) | 7 (27) | 20 (39) | |
| Higher education | 13 (17) | 6 (23) | 7 (14) | |
| A level or equivalent | 7 (9) | 4 (15) | 3 (6) | |
| GCSE grades A*–C or equivalent | 17 (22) | 3 (12) | 14 (27) | |
| Other qualifications | 4 (5) | 3 (12) | 1 (2) | |
| No qualifications | 10 (13) | 3 (12) | 7 (14) | |
| Marital status | 0.54a | |||
| Married or living with a partner | 41 (53) | 11 (42) | 30 (58) | |
| In a long-term relationship but not cohabiting | 11 (14) | 5 (19) | 6 (12) | |
| Single | 22 (28) | 9 (35) | 13 (25) | |
| Divorced or separated | 4 (5) | 1 (4) | 3 (6) | |
| Widowed | 0 (0) | 0 (0) | 0 (0) | |
| Ethnic group | 0.64a | |||
| White | 69 (88) | 22 (85) | 47 (90) | |
| Black | 3 (4) | 1 (4) | 2 (4) | |
| Asian | 4 (5) | 1 (4) | 3 (6) | |
| Other | 2 (3) | 2 (8) | 0 (0) | |
| Living alone or with others | 0.033 | |||
| Lives alone | 13 (17) | 2 (8) | 11 (21) | |
| Lives with family/spouse or significant other | 65 (83) | 24 (92) | 41 (79) | |
| Occupational groupb (n1 = 20, n2 = 48, n = 68) | 0.88a | |||
| Managers, directors and senior officials | 8 (12) | 2 (10) | 6 (13) | |
| Professional occupations | 11 (16) | 2 (10) | 9 (19) | |
| Associate professional and technical occupations | 8 (12) | 3 (15) | 5 (10) | |
| Administration and secretarial occupations | 6 (9) | 2 (10) | 4 (8) | |
| Skilled trades occupations | 16 (24) | 5 (25) | 11 (23) | |
| Caring, leisure and other service occupations | 1 (2) | 0 (0) | 1 (2) | |
| Sales and customer service occupations | 2 (3) | 1 (5) | 1 (2) | |
| Process, plan and machine operatives | 6 (9) | 1 (5) | 5 (10) | |
| Elementary occupations | 10 (15) | 4 (20) | 6 (13) | |
| Length of inpatient stay (days), mean (SD) | 13.7 (16.8) | 14.1 (15.6) | 13.5 (17.5) | 0.94a |
| Cause of injury | 0.67 | |||
| Fall | 30 (39) | 6 (23) | 24 (46) | |
| RTA | 31 (40) | 15 (58) | 16 (31) | |
| Assault | 13 (17) | 5 (19) | 8 (15) | |
| Other | 4 (5) | 0 (0) | 4 (8) | |
| Duration of PTA (hours) (n1 = 24, n2 = 47, n = 71) | 0.36a | |||
| Below or equal overall median | 39 (55) | 15 (63) | 24 (51) | |
| Above overall median | 32 (45) | 9 (38) | 23 (49) | |
| Duration of post-traumatic unconsciousness (hours) (n1 = 22, n2 = 35, n = 57) | 0.95a | |||
| Below or equal overall median | 34 (60) | 13 (59) | 21 (60) | |
| Above overall median | 23 (40) | 9 (41) | 14 (40) | |
| Pre-injury work status | 0.18 | |||
| Work | 66 (85) | 19 (73) | 47 (90) | |
| Work and study | 3 (4) | 1 (4) | 2 (4) | |
| On sick leave | 2 (3) | 1 (4) | 1 (2) | |
| Study | 7 (9) | 5 (19) | 2 (4) | |
| Baseline NEADL, mean (SD) | 9.5 (6.5) | 8.5 (6.1) | 9.9 (6.8) | 0.36a |
| Baseline HADS anxiety, mean (SD) | 6.6 (4.1) | 8.0 (5.0) | 5.9 (3.3) | 0.018 |
| Baseline HADS depression, mean (SD) | 6.6 (4.2) | 7.6 (4.6) | 6.1 (3.9) | 0.29a |
| Baseline self-efficacy, mean (SD) | 4.9 (3.2) | 5.8 (3.4) | 4.5 (3) | 0.21 |
| Baseline percentage work time missed due to health, mean (SD) | 58.2 (44.2) | 34.6 (42.9) | 69.9 (42) | 0.18 |
| Baseline activity impairment due to health, mean (SD) | 56.4 (31.8) | 45.4 (34.4) | 61.9 (29.2) | 0.004 |
| Intervention arm | ||||
| UC | 39 (50) | 16 (62) | 23 (44) | 0.44 |
| ESTVR | 39 (50) | 10 (39) | 29 (56) | |
Objective 6: to explore potential gains in using face-to-face rather than postal data collection
Completeness of follow-up
There was no evidence of gains in using face-to-face rather than postal data collection for TBI participants. At each time point, the response rate was slightly lower at the site which collected data primarily by face-to-face interview, although this site suffered all four TBI participant withdrawals (see Report Supplementary Material 12, Table 7). Analysis has been by ‘intention-to-collect’ as it may be that the mode of data collection had an impact on the risk of withdrawal.
Effectiveness of follow-up telephone calls and other contacts
The number of attempted contacts to collect outcome data at 3, 6 and 12 months, by site, planned mode of completion and overall are given in Table 8. When data were collected by face-to-face data collection, participant contact was necessary to make an appointment, whereas with postal data collection, the follow-up protocol required a telephone call or other personal contact only if the questionnaire was not returned within 2 weeks of the due date. Despite this, differences in the distribution of the number of contact attempts between data collection modes were small and evident only towards the upper tail of the distribution (see Table 8).
| Participants and contact attempts | Time point | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||||
| Postal (n1 + n2 = 44) | Face to face (n3 = 34) | Postal (n1 + n2 = 44) | Face to face (n3 = 31) | Postal (n1 + n2 = 44) | Face to face (n3 = 31) | |
| Number (%) of participants to be contacted | 33 (75) | 34 (100) | 33 (75) | 31 (100) | 34 (77) | 27 (87) |
| Total contact attempts, median (IQR) [range] | 4 (3–6) [1–10] | 3 (1–8) [1–33] | 5 (2–6) [1–16] | 5 (2–10) [1–22] | 5 (2–6) [1–8] | 5 (2–10) [1–16] |
Table 9 summarises the distribution of the number of telephone contacts for those for whom RTW status was or was not collected. At each time point, for those for whom RTW information was eventually collected, the upper quartile was 6, indicating that substantial effort was made to collect outcomes and that this was frequently successful.
| Participants and contact attempts | Time point | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||||
| Collection of RTW status (nc = 68) | Non-collection of RTW status (nn = 10) | Collection of RTW status (nc = 65) | Non-collection of RTW status (nn = 10) | Collection of RTW status (nc = 52) | Non-collection of RTW status (nn = 23) | |
| Number (%) of participants to be contacted | 54 (83) | 13 (100) | 51 (82) | 13 (100) | 42 (81) | 19 (83) |
| Total contact attempts, median (IQR) [range] | 3 (1–6) [1–18] | 7.5 (5–10) [1–33] | 3.5 (2–6) [1–22] | 7.5 (5–13) [4–20] | 4 (2–6) [1–14] | 6 (5–8) [1–16] |
Timing of responses
Median (IQR) time to outcome assessment for those responding to the follow-up request was around the intended time points at 3 months (+ 23 days, IQR 11–48 days), 6 months (+ 23.5 days, 7–61.3 days) and 12 months (+ 18.5 days, 8.3–43.8 days). The distribution of response times was similar across time points for the postal data collection mode at around 20 days after the due date. At both 3 and 6 months, the median time difference was slightly greater for face-to-face than for postal follow-up, but at 12 months this difference was rather less. Differences between time due and time completed for TBI participant questionnaires at follow-up are summarised overall and by planned mode of completion in Report Supplementary Material 12, Table 9.
Objective 7 [to determine the most appropriate method(s) of measuring key outcomes (return to work, work retention)] and objective 8 [to describe the completeness of data collection for potential primary outcome(s) for a definitive trial]
Objectives 7 and 8 are considered jointly as the completeness of data collection is one of the factors affecting the choice of the most appropriate method of measuring key outcomes. Individual RTW questions were well completed, with few missing data.
A total of 57 (84%) participants reported that they were in work or education at 3 months [28 (85%) in the control group and 29 (83%) in the intervention group]. Of the 11 (16%) participants reporting not being in work/study, nine were planning to return to work and two were not. However, of the 57 participants reporting that they were working or in full-time education, 18 (26% of the total) were technically employed but ‘off sick’, so only 39 (57% of the total) were classified as working [three participants were studying and eight participants reported neither working nor being ’off sick’ (see Report Supplementary Material 12, Table 41)].
The responses to these questions highlight the complexity of determining work outcomes by self-report, although some of the difficulty in interpreting the responses may be attributed to poor question wording. In particular, for question 4a, ‘At present I am not working due to my brain injury’, which, with possible responses ‘Yes’ and ‘No’, appeared to cause confusion in some participants (questions 4b, ‘I feel I cannot work at present as a result of my brain injury’, and 4c, ‘I feel I will never be able work again as a result of my brain injury’, had possible responses ‘Agree’ and ‘Disagree’) as it inferred a double negative. There are also concerns that participants may report worse outcomes as these may influence benefits and insurance claims.
At 3, 6 and 12 months, there were few problems (other than the non-return of questionnaires) with incomplete (or invalid) responses to the further questions which contributed to the proposed primary health outcomes (see Report Supplementary Material 12, Table 14).
Using an algorithm to determine the employment outcomes from responses to questions about work and education, 41 (60%) participants were classified as competitively employed or in full-time study at 3 months, with an additional person in voluntary employment in the control group, making a total of 42 (62%) participants [24 (73%) in the control group and 18 (51%) in the intervention group] classified as in purposeful occupation or studying (Table 10).
| Follow-up time point, work/educational outcome | Intervention arm | Overall | ||
|---|---|---|---|---|
| UC | VR | |||
| 3 months | n = 33a | n = 35b | n = 68 | |
| Competitively employed or in full-time study | ||||
| Yes | 23 (70) | 18 (51) | 41 (60) | |
| No | 10 (30) | 17 (49) | 27 (40) | |
| In purposeful occupation or studying | ||||
| Yes | 24 (73) | 18 (51) | 42 (62) | |
| No | 9 (2) | 17 (49) | 26 (38) | |
| 6 months | n = 31a | n = 34b | n = 65 | |
| Competitively employed or in full-time study | ||||
| Yes | 21 (68) | 24 (71) | 45 (69) | |
| No | 10 (32) | 10 (29) | 20 (31) | |
| In purposeful occupation or studying | ||||
| Yes | 21 (68) | 25 (74) | 46 (71) | |
| No | 10 (32) | 9 (27) | 19 (29) | |
| 12 months | n = 23a | n = 29b | n = 52 | ORc (95% CI) |
| Competitively employed or in full-time studya | 0.23 (0.04 to 1.18) | |||
| Yes | 21 (91) | 19 (66) | 40 (77) | |
| No | 2 (9) | 10 (35) | 12 (23) | |
| In purposeful occupation or studyingb | 0.26 (0.05 to 1.40) | |||
| Yes | 21 (91) | 20 (69) | 41 (79) | |
| No | 2 (9) | 9 (31) | 11 (21) | |
The self-reported employment/educational outcomes of the 65 participants for whom RTW data were available at 6 months post randomisation indicated that 46 (71%) participants were in work (paid or unpaid) or education [21 (68%) in the control group and 25 (74%) in the intervention group] (see Table 10). A total of 20 participants (32%) reported that they were not working owing to their injury and 12 (19%) felt that they could not work owing to their injury (eight in the intervention group). At 6 months, three (5%) participants felt that they would never be able to work because of their injury. All were in the intervention group. One person had retired but not because of their injury. Two-thirds (n = 37) of respondents were earning < £1600 per month, while 18 (30%) were earning < £800 per month.
The employment/educational outcomes of the 52 participants for whom primary outcome data were available at 12 months are also presented in Table 10. At 12 months post randomisation, 41 (79%) participants were in paid or voluntary work of > 1 hour per week or in education (of at least 5 hours per week), 20 (69%) in the intervention group and 21 (91%) in the control group. A total of 40 (77%) participants were in competitive employment or full-time study (an average of at least 12 hours per week). However, only 23 out of 39 (59%) control group participants responded compared with 29 out of 39 (74%) in the intervention group.
Most (n = 28, 54%) of the respondents returned to the same job with an existing (pre-injury) employer or a different job with an existing employer. Three people (6%) reported having changed jobs by 12 months, and four were self-employed. By 12 months, two people had retired (one due to injury), seven (14%) felt unable to work owing to their injury and three (6%) felt that they would never be able to work as a result of the injury (two in the intervention group). The majority (n = 40) of participants earned < £2500 per month, with 13 (27%) earning < £800 per month.
Of those returning to study, three (6%) participants returned to the same course at the same college and one (2%) started a new course at a different college.
The only factors potentially related to 12-month in-work outcomes were education level, with those educated to degree level or above (p = 0.004) and randomised to UC (p = 0.036) being more likely to be in competitive employment or study. Findings were similar for being in purposeful occupation or studying, although the effect of intervention arm did not achieve statistical significance (p = 0.094) (Table 11).
| Factor | Competitive employment or full-time study | Purposeful occupation or studying | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.97 (0.91 to 1.04) | 0.38 | 0.98 (0.92 to 1.05) | 0.57 |
| Sex | 0.35 | 0.38 | ||
| Male | 1 (–) | 1 (–) | ||
| Female | 0.34 (0.04 to 3.25) | 0.38 (0.04 to 3.20) | ||
| Marital status | 0.43 | 0.16 | ||
| Married or living with a partner | 1 (–) | 1 (–) | ||
| In a long-term relationship but not cohabiting | 0.25 (0.01 to 4.24) | 0.29 (0.02 to 4.92) | ||
| Single | 0.29 (0.04 to 2.13) | 0.15 (0.02 to 0.93) | ||
| Divorced or separated | 0.16 (0.01 to 3.01) | 0.16 (0.01 to 3.60) | ||
| Educationa,b | 0.004 | 0.013 | ||
| A-level or equivalent or GCSE grades A*–C or equivalent/other qualifications/no qualifications | 1 (–) | 1 (–) | ||
| Degree, higher degree or equivalent qualification/higher education | 9.77 (1.70 to 56.31) | 6.75 (1.26 to 36.03) | ||
| TBI severity | 0.93 | 0.93 | ||
| Severe | 1 (–) | 1 (–) | ||
| Moderate | 0.99 (0.08 to 11.73) | 0.68 (0.07 to 6.40) | ||
| Minor | 1.35 (0.21 to 8.57) | 1.29 (0.22 to 7.72) | ||
| Occupational group | 0.82 | 0.90 | ||
| Managers, directors and senior officials/professional occupations | 1 (–) | 1 (–) | ||
| Associate professional and technical occupations/skilled trades occupations/caring, leisure and other service occupations/sales and customer service occupations | 0.54 (0.06 to 4.53) | 0.60 (0.07 to 6.13) | ||
| Process, plan and machine operatives/ elementary occupations | 0.84 (0.09 to 8.05) | 0.66 (0.07 to 6.13) | ||
| Length of inpatient stay | 1.01 (0.97 to 1.05) | 0.71 | 1.00 (0.96 to 1.04) | 0.97 |
| Percentage work time missed owing to healthc | – | – | – | – |
| Percentage activity impairment | 1.00 (0.97 to 1.02) | 0.86 | 1.00 (0.98 to 1.03) | 0.96 |
| HADS anxiety categoryc | – | – | ||
| Normal (0–7) | – | – | ||
| Borderline abnormal (8–10) | – | – | ||
| Abnormal (11–21) | – | – | ||
| HADS depression category | 0.44 | 0.45 | ||
| Normal (0–7) | 1 (–) | 1 (–) | ||
| Borderline abnormal (8–10) | 0.72 (0.10 to 4.91) | 0.99 (0.15 to 6.71) | ||
| Abnormal (11–21) | 0.25 (0.03 to 2.07) | 0.29 (0.04 to 2.09) | ||
| NEADL score | 1.03 (0.90 to 1.17) | 0.69 | 1.02 (0.90 to 1.16) | 0.73 |
| Self-efficacy score | 1.02 (0.80 to 1.29) | 0.90 | 0.98 (0.78 to 1.24) | 0.87 |
| Intervention arma | 0.036 | 0.094 | ||
| Control | 1 (–) | 1 (–) | ||
| Intervention | 0.17 (0.03 to 1.04) | 0.22 (0.04 to 1.30) | ||
Completeness of baseline demographic and clinical data
In terms of the collection of baseline demographic and health data, general questions about sex, marital status, live alone or with others, highest education level, ethnic group, occupation and cause of injury were answered by all participants. Other calculated information, such as age and number of days in hospital, was also complete. However, there were minor problems in classifying injury severity as data pertaining to GCS scores [completion in 73 out of 78 (94%) participants] and duration of PTA [completion in 55 out of 61 (90%) of those with PTA, with one further participant failing to indicate whether or not they had suffered PTA] and considerable amounts of missing data for duration of unconsciousness (completion in 41 out of 59 who reported unconsciousness with three further participants failing to indicate whether or not they had been unconscious following their injury). Further details of response rates for individual questions can be found in Report Supplementary Material 12, Tables 14–32, (patients) and Report Supplementary Material 12, Tables 33–36 (carers).
Completeness of secondary outcome data at baseline and follow-up
Health profile data from the EuroQol-5 Dimensions, three-level version were almost 100% complete for participants who returned questionnaires, except in site 3, where data for one person were missing (when data collected face to face were incomplete). EuroQol-5 Dimensions Visual Analogue Scale (EQ-5D VAS) data were 100% complete for participants who returned questionnaires (see Report Supplementary Material 12, Tables 18 and 20).
For the NEADL, all subscales (and hence the total) were almost complete (at least 98% complete) for participants who returned their questionnaires (see Report Supplementary Material 12, Table 22).
For returned CIQs, subscales were mostly complete, with some missing data for two people at baseline for the productivity subscale, for one person in the leisure subscale at 3 months and one or two participants with missing data in all subscales at 6 and 12 months (see Report Supplementary Material 12, Table 24).
Completeness of HADS data for participants who returned questionnaires was high; there were missing data for one person at 6 and 12 months, and for one person on the depression subscale at baseline (see Report Supplementary Material 12, Table 26).
Completion of the Work Productivity and Activity Impairment Questionnaire Specific Health Problem v2.0 (WPAI: SHP) subscales for participants who reported that they were in work was generally lower than for other questionnaires. At baseline, only 12 (75%) of those reporting that they were in work completed the percentage impairment measures relating to work, although completion rates were higher, at approximately 90% or greater, at follow-up (Table 12).
| Subscale | Time point | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| Per cent work time missed owing to health | 15 (94) | 32 (91) | 34 (89) | 36 (95) |
| Per cent impairment while working owing to health | 12 (75) | 34 (97) | 35 (92) | 37 (97) |
| Per cent overall work impairment owing to health | 12 (75) | 32 (91) | 34 (89) | 36 (95) |
| Per cent activity impairment due to healtha (n1 = 22, n2 = 22, n3 = 34, n = 78) | 78 (100) (n = 78) | 65 (100) (n = 65) | 59 (95) (n = 62) | 50 (96) (n = 52) |
Among those who returned questionnaires, data for the single question on work self-efficacy were missing for only one person at 3 months, one at 6 months and one at 12 months (see Report Supplementary Material 12, Table 29).
This was coded successfully by the research team from the responses to other questions for all participants who returned questionnaires at 12 months post randomisation (see Report Supplementary Material 12, Table 30).
Among carers who returned questionnaires, the CSI was completed by all but one carer at 12 months (see Report Supplementary Material 12, Table 34).
Objective 9: to investigate how return to work is related to mood, function, work capacity, social participation, quality of life and carer strain
Relationships between the secondary outcome measures relating to mood (HADS anxiety, HADS depression), function (NEADL total score), work capacity (self-efficacy and per cent activity impairment due to health), social participation (CIQ total score), QoL (EQ-5D-3L VAS) and carer strain (CSI), suggested two primary outcome measures (‘In competitive employment or full-time study at 12 months’ and ‘In purposeful occupation or studying at 12 months’) and the other outcome measures were investigated using logistic regression.
Both competitive employment (or full-time study) and purposeful employment (or study) were significantly related to impairment owing to each of health, function, self-efficacy and community integration, with the former also significantly related to depression. The strongest relationships were with the CIQ total score (pseudo-R2 0.55 and 0.42, respectively), with that with self-efficacy the next strongest (pseudo-R2 0.27 and 0.26, respectively) (Table 13). There was no significant relationship with anxiety, QoL or carer strain (see Table 13).
| Secondary outcome variable | In competitive employment or full-time study | In purposeful occupation or studying | ||
|---|---|---|---|---|
| p-value | bPseudo-R2 | p-value | bPseudo-R2 | |
| HADS anxiety score | 0.89 | 0.00 | 0.86 | 0.00 |
| HADS depression score | 0.034 | 0.09 | 0.076 | 0.06 |
| Per cent activity impairment owing to health | 0.013 | 0.13 | 0.014 | 0.13 |
| NEADL total score | 0.006 | 0.17 | 0.033 | 0.09 |
| Self-efficacy | 0.003 | 0.27 | 0.003 | 0.26 |
| CIQ total score | 0.001 | 0.55 | 0.001 | 0.42 |
| EQ-5D-3L VAS | 0.091 | 0.05 | 0.063 | 0.067 |
| CSI | 0.55 | 0.02 | 0.42 | 0.05 |
Objective 10: to estimate parameters necessary to calculate sample size for a larger trial (e.g. rate of return to work at 12 months in control group)
Whether the primary outcome for an effectiveness trial is chosen to be ‘In competitive employment or full-time study’ or ‘In purposeful occupation or studying’, the crude estimate of the control group ‘in-work’ rate is 21 out of 23 (91%, 95% CI 72% to 99%). However, the control group response rate was only 59%, with those not in competitive employment or full-time study at 6 months appearing more likely to drop out by 12 months (5 out of 10, 50%) than those in competitive employment or full-time study at 6 months (3 out of 21, 14%), thus raising two feasibility questions:
-
What is the best (least biased) estimate of the control group ‘in-work’ rate at 12 months?
As the control group ‘in-work’ rate is around 70% at both 3 and 6 months, it is unlikely that the 12-month rate would be substantially greater than this, although three of those not working at 6 months were working at 12 months. Therefore, it would seem plausible that an estimate of the control group ‘in-work (or study)’ rate for the population recruited to this trial would be of the order of 80%. However, if a population with more severe TBI were recruited, the ‘in-work’ rate would be expected to be lower.
Under this assumption (i.e. a control group ‘in-work’ rate of 80%), a total sample of 2424 participants providing 12-month outcome data would be required to detect a 5% difference between the intervention and control groups in the ‘in-work (or study)’ rate, with 90% power using a 5% significance level (using a chi-squared test); 1014 responders would be required to detect a 7.5% between-groups difference. Therefore, assuming an attrition rate of no greater than 25%, randomising 3232 participants would provide 90% power to detect a 7.5% difference, and 1352 participants would provide the same power to detect a 5% difference.
-
Can the trial be designed to reduce the attrition rate and, in particular, reduce the differential effect on response of:
-
working status (those in work may be more likely to respond)?
-
intervention arm (those in the intervention arm may be more likely to respond)?
-
Unless the recruitment and attrition issues (particularly the latter) can be addressed, it would seem unlikely that a trial would be able to provide convincing evidence of effectiveness on a RTW outcome.
Sample size calculation based on the Community Integration Questionnaire
If the CIQ, rather than the ‘in-work’ rate, is chosen as the primary outcome measure for a full trial, then the parameters required for the sample size calculation would be the SD and the minimally important difference in means. The minimal important difference is not established in the literature, but the research team believes it to be in the range of 1–2 points, possibly towards the middle or lower end of this range: a difference of 1.5 points is almost 10% of the mean baseline CIQ score (16.3 points). The point estimate of the CIQ residual SD from the 12-month inferential analysis (linear model) was 5.02. Based on this, and using a linear model (adjusted for baseline CIQ) to achieve 90% power using a 5% significance level, to detect a minimal important difference on the CIQ of:
-
1 point – 1062 participants (531 per arm) would need to provide outcome data at 12 months, equating to randomising 1416 participants if 12-month attrition is 25%
-
1.5 points – 474 participants (237 per arm) would need to provide outcome data at 12 months, equating to randomising 632 participants if 12-month attrition is 25%
-
2 points – 268 participants (134 per arm) would need to provide outcome data at 12 months, equating to randomising 358 participants if 12-month attrition is 25%.
Specific feasibility criteria for progression to the main trial
Feasibility will be shown if at least two TBI patients per month are recruited on average and, if the average number of patients recruited monthly is less than two, strategies to achieve this target are identified:
-
Met in one site (site 3) and on average overall (2.2 per month); however, two sites recruited at slightly under two patients per month (22 each in 12 months, 1.8 per month). A number of different intrinsic and extrinsic factors influenced recruitment in each site. These included the deployment of network support, patient repatriation, the lack of a designated trauma unit in one centre, lack of access to A&E records, construction of a helipad near one centre, which affected admissions (trauma admissions triaged to another local major trauma centre), and the characteristics of the local population in one centre (most at, or near, retirement age). Recruitment was most successful (3.1 per month) in the site with a RA dedicated to the project, supervised by the local PI.
Feasibility will be demonstrated if no fewer than 5% of TBI patients screened are recruited:
-
A total of 78 out of 1446 screened (5.4%, 95% CI 4.3% to 6.7%) were recruited.
Feasibility will be demonstrated if ≥ 25% of eligible TBI patients are recruited and, when < 25%, strategies are identified to achieve this:
-
A total of 78 out of 200 (39.0%, 95% CI 32.2% to 46.1%) of eligible participants were recruited.
Feasibility will be demonstrated if the proportions of recruited participants with mild TBI and moderate or severe TBI are equal or if recruitment strategies to address any observed imbalance are identified:
-
The proportion of participants recruited who had incurred a mild TBI (56%) was higher than the proportion with moderate or severe TBI (44%), as categorised by the GCS score. The imbalance is thought to have been due to repatriation of a higher proportion of more severely injured patients requiring ongoing rehabilitation as part of the newly configured major trauma pathways.
-
As part of this study, estimates based on the consent rate and the number of patients fitting the inclusion criteria who were repatriated in a given time period in one site suggested that the recruitment targets and injury severity balance would have been achieved by extending the recruitment window to 12 weeks and with the necessary resource for recruiting repatriated patients.
Feasibility will be shown if at least 30% of eligible carers are recruited:
-
The 78 TBI participants nominated 45 carers and 32 (61.5%, 95% CI 47.0% to 74.7%) were recruited. Carers were typically female and either a spouse/partner (72%) or parent (28%) of the TBI participant.
Feasibility will be demonstrated if < 40% withdraw or are lost to follow-up or, when > 40%, if strategies to overcome identified barriers are identified:
-
In total, 52 out of 78 (67%) questionnaires were available for analysis at 12 months, from 23 and 29 participants in the UC and ESTVR intervention groups, respectively. Follow-up of the primary end point at 12 months was also 67%. Therefore, at 12 months, 33.3% (95% CI 23.1% to 44.9%) of TBI participants did not provide RTW outcome data (22 non-response and four withdrawals). Additional strategies to reduce loss to follow-up in a future trial were identified. These included text messages and telephone calls from an identified number (having asked participants at recruitment to accept the trial telephone number as an identified number) and text messages with a link to an online questionnaire to facilitate response in smartphone users and people who return to work.
Feasibility will be shown if < 30% withdraw from the intervention or if > 30% withdraw but strategies to reduce this rate are identified:
-
Two participants (5%) disengaged from the intervention. One declined further support having being advised that he was not legally permitted to drive, one lost his job, transferred to long-term benefits and declined further help, and a further two could not be contacted (one moved to a new care home) after minimal intervention.
Feasibility will be demonstrated if bespoke work questions are answered in >90% of returned questionnaires or, if answered in < 90% of questionnaires, strategies to improve completion are identified:
-
Overall, all bespoke RTW questions were answered at all time points by ≥ 90% of participants, except for ‘Reason left work/education or changed job/course (Q23)’, which just failed to meet this criterion at the 6-month follow-up time point. However, the completion rate was 100% at the other time points, and this apparently lower rate was due to a single non-respondent out of nine (as this question was often not applicable). Hence, we concluded that this feasibility criterion had been met.
Feasibility will be demonstrated by < 30% loss to follow-up of the primary end point at 12 months with strategies identified to reduce this rate to 20%:
-
Attrition was estimated to be 33.3%. Despite the follow-up protocol implemented by the CTU and efforts by the RA at site 3, no RTW information was collected for those who failed to complete the full questionnaire. It will be important to consider the use of technology to increase both the ongoing contact with trial participants and to collect RTW information. This may also help reduce the differential attrition between the control and intervention arms.
Feasibility will be demonstrated if most participants interviewed state that the intervention is acceptable:
-
Almost all of the participants interviewed stated that the intervention was acceptable and useful. Participants liked knowing that the FRESH OT was there if needed. However, two reported feeling fraudulent because they did not feel that the TBI had left them in need of rehabilitation. They valued particular elements, including letters to the participant and employer, specific support to prepare for work, negotiating a RTW and support once back at work. Participants thought that FRESH should be available in the future to them and their employer and that it should be driven by the health professional.
Feasibility will be demonstrated if most participants interviewed indicate that randomisation is acceptable:
-
None of the participants interviewed indicated that randomisation was unacceptable or declined participation because they wanted to ensure randomisation to the intervention group.
Feasibility will be demonstrated if most of the therapists interviewed consider the training to be useful and acceptable and, if this was not the case, if strategies for improvement are identified:
-
All of the therapists interviewed considered the training to be useful and considered that all three elements of the training package (training, manual and mentoring) were essential. Training increased therapists’ confidence to deliver ESTVR. However, they wanted training to converge with the start of recruitment and to include more detail about the study and delivering interventions within a trial context. They requested more opportunities for knowledge sharing around cases. The most valued aspects of the training package were case discussions and mentoring to support individual tailoring of the intervention and its local implementation.
The findings indicate that training packages should include detailed intervention descriptions in order to facilitate implementation.
Summary
We set out to assess the integrity of the study protocol (e.g. inclusion/exclusion criteria, staff training, adherence to the intervention, and to identify reasons for non-adherence). This study found the protocol to be robust. The feasibility objectives were achieved or, when not achieved, strategies for achieving targets were identified. Therefore, overall feasibility has been demonstrated so this key feasibility objective was met.
The trial achieved 76% of its target recruitment. The recruitment rate overall was 6.5 per month. However, this varied by centre. Site 1 recruited 22 patients (32% of those eligible), site 2 recruited 22 patients (35% of those eligible) and site 3 recruited 34 patients (49% of those eligible), with site 3 achieving the 12-month target of 34 patients in 11 months (a rate of 3.1 per month) and the other centres fluctuating and achieving rates of 1.8 per month. However, site 3 had a dedicated RA for face-to-face follow-up and who could deploy time flexibly for recruitment. The reasons for the fluctuations and mechanisms to improve recruitment are well understood.
More people with a mild TBI (56% of participants) were recruited than those with moderate or severe TBI. The imbalance is thought to have occurred owing to repatriation following the establishment of MTCs with new care pathways.
As part of this study, estimates based on the consent rate and the number of patients fitting the inclusion criteria who were repatriated in a given time period in one site suggested that the recruitment targets and injury severity balance would have been achieved by extending the recruitment window to 12 weeks and with the necessary resource for recruiting repatriated patients.
The impact of patients displaced or repatriated, and the option of extending the recruitment window from 8 to 12 weeks, was explored using data from a single centre (site 3). This suggested that an extension from 8 to 12 weeks would have meant that we would have achieved the recruitment target. However, as more severe cases are often transferred to other sites, additional resources and approvals would need to be in place to support recruitment across a wider geographical area.
Although the overall recruitment target of 102 was not met, the reasons for this are well understood and this knowledge will inform the design of the definitive trial. The ideal model for recruitment in a future trial is to have a RA embedded in the research team and supervised by the local PI, with support and training from the CTU.
Identifying people who had sustained a TBI was, in some cases, problematic. Not everyone who sustains a head injury is scanned for the presence of brain injury and not all injuries to the brain are present on a scan. Because of this, it was not a requirement of FRESH that participants’ TBI was confirmed by CT. Instead, we relied on local clinical procedures for the identification of patients with TBI. This lack of a clear definition of TBI may have affected eligibility and inflated the proportion of patients screened. Moreover, the establishment of MTCs contributed to difficulties in identifying people who sustain mild TBI, as this was sometimes missed or poorly recorded in patients admitted with other severe injuries.
In one site, there was no designated trauma ward and, therefore, no simple mechanism for identifying potential participants [Trauma Audit and Research Network (TARN) registers are often retrospectively completed and capture patients admitted for only ≥ 72 hours]. All patients admitted to A&E with a suspected ‘head injury’ were screened in an attempt to identify those who had sustained a TBI. As all of these patients were entered onto the screening log, the proportion of participants recruited in relation to the number screened was low.
A total of 39% of potentially eligible patients and 5.5% of TBI patients screened were recruited. The most common reasons for non-recruitment were that participants were discharged before being seen and did not respond to recruitment materials by post or that participants had already returned to work or did not feel that they needed support to return to work. In total, 76 (38% of eligible) refused consent.
The findings suggest that TBI patients are willing to be randomised to either receive support with RTW or not.
A total of 56% of participants had incurred a mild TBI, 18% a moderate TBI and 26% a severe TBI. When compared with the pilot study, more people with mild TBI were included in FRESH. This can be explained, in part, by the institution of MTCs operating a ‘hub and spoke’ model, with organised pathways of care, which resulted in the repatriation of potential participants with moderate or severe TBI to referring hospitals or rehabilitation units, which were geographically dispersed and for which additional approvals and resources were required for recruitment.
The higher proportion of people with mild TBI in this study will have had an impact on the 12-month rate of RTW; if this is high in the control arm then it may be deemed that an effectiveness trial is not required given the limited gains in this population. Therefore, it is important that a more balanced sample including more people with moderate or severe injuries can be recruited to demonstrate gains within the 12-month follow-up period.
Carers of TBI patients were nominated by recruited patients. In total, 45 carers were nominated by 78 patients and 31 (69%) were recruited. Proportionately fewer eligible carers were recruited in site 3 than in other centres.
At baseline, across all three centres, the return rate was 78 out of 78 (100%). At 3 months, the return rate was 65 out of 78 (83%); at 6 months, 62 out of 78 (79%); and at 12 months, 52 out of 78 (67%). Only four of the 26 ‘lost to follow-up’ at 12 months formally withdrew, so reasons for non-response are generally not known. However, those who did not respond at 12 months were significantly more likely to be younger, not live alone, be more anxious and have lower mean percentage health-related activity impairment at baseline. Furthermore, at least in the control group, they appeared more likely to have been out of work at 6 months.
Carers’ response rates were similar to, or slightly lower than, those of the TBI participants. At baseline, 30 out of 32 (94%) questionnaires were returned; at 3 months, 26 out of 32 (81%) were returned; at 6 months, 23 out of 32 (72%) were returned; and at 12 months, there were 22 out of 32 (69%) returns. Four carers formally withdrew because the nominated carer was a partner and the relationship ended.
Follow-up in TBI participants is known to be difficult and we have shown that it can be achieved using postal follow-up with text message prompts and telephone assistance. The Lancashire CTU’s success in securing excellent return rates resulted from a team of well-trained staff, who followed a well-considered and robust protocol to enhance response. The team also developed rapport with trial participants. However, the different response rates observed between groups suggests that those in the control group, in particular, may require an incentive to remain in the trial and contact should be made between the 6- and 12-month follow-up points. The possibility of the 12-month work status affecting non-response (a ‘not missing at random’ missing data mechanism) should be considered when designing and analysing a full trial.
Postal follow-up appears to be comparable to face-to-face follow-up and is less resource intensive.
Other than non-return of questionnaires, there were few problems with incomplete (or invalid) responses to primary and secondary health outcome related questions.
Measuring RTW was possible in people with TBI but complicated by several factors:
-
The interchangeable use of the terms ‘work’ and ‘employment’ by participants. A person can be technically employed but ‘off sick’, but may still refer to their work status as being ‘in work’.
-
The interplay between benefit eligibility, insurance claims and work status. Some participants may intend to stay ‘off sick’ to remain eligible for sickness benefits or insurance payouts.
Future studies should give clear definitions within the questionnaire and offer reassurance that there is no communication between the researchers and the DWP or insurance companies. It proved difficult to obtain an unbiased estimate of the RTW rate in the control arm, which is the key parameter required for a sample size calculation for an effectiveness trial. Attrition appeared to be dependent on work status and also intervention group. So, although > 90% of the control arm respondents were working or studying at 12 months, it is likely that those out of work were less likely to respond than those in work, particularly control group participants, who may have felt dissatisfied with the trial as a result of having been allocated to that arm. However, unless this can be overcome, it would appear that an effectiveness trial of ESTVR using a work status variable at 12 months would not provide convincing evidence of effectiveness, due to a combination of the high control group attrition rate and the inherent bias in the intervention effect estimate.
Return to work was found to be most strongly related to social participation (community integration) and work self-efficacy, with a weaker relationship with impairment and, for competitive work or full-time study, with depression.
Chapter 4 The feasibility of conducting an economic evaluation of vocational rehabilitation compared with usual care alone following traumatic brain injury
Introduction
Traumatic brain injury is a major event in a person’s life that can have long-term consequences in terms of reduced health, independence and employability. Together this equates to reduced QoL and increased costs for the individual, their family and society. A recent review7 of research on the economic burden of TBI included 10 studies, of which only two were from the UK. The authors concluded that:
Further research is needed to estimate the economic burden of these participants on healthcare providers and social services and how this can impact current health policies and practices.
Humphreys et al. 7
One paper10 estimates, based on data from the USA, Australia and Europe, that the annual cost of TBI in developed countries is 0.8% of yearly GDP, with the annual cost of TBI in the UK being £15B. If VR is found to be effective at helping people retain, or return to, work while at the same time being cost-effective, this could help to substantially reduce this economic burden.
Economic evaluations are an essential component of the health technology assessment process to ensure that the interventions evaluated offer value for money as well as clinical benefit. Definitive trials are expensive to conduct; therefore, it is important to assess the feasibility of running a definitive trial before it is funded to ensure that funds are not wasted on trials that are unlikely to be feasible to run. The FRESH trial assesses the feasibility of designing a definitive trial of VR after TBI and this chapter contributes to this endeavour by assessing the feasibility of undertaking a concurrent economic evaluation of VR following TBI.
Aims and perspective
A definitive study of clinical effectiveness and cost-effectiveness can be successful only if appropriate methods of measurement are employed. This component of the study aimed to assess the feasibility of conducting a definitive economic evaluation of the cost-effectiveness of VR after TBI and undertake an early-stage cost-effectiveness analysis of VR after TBI in order to inform a definitive study.
The objectives of the economic component of this study were to:
-
identify relevant resources and outcomes likely to change as a result of VR
-
assess the feasibility of collecting resource use and outcome data for a cost-effectiveness analysis within a fully powered trial by evaluating completion rates
-
consider how data collection instruments should be designed for a definitive study
-
demonstrate the feasibility of conducting a within-trial cost-effectiveness analysis, reporting early-stage estimates of incremental costs and effects, in a complete-case analysis.
Although the National Institute for Health and Care Excellence (NICE)106 recommends a NHS and Personal Social Services (PSS) perspective in the reference case, it is recognised that, for certain conditions, a broader perspective may be important when interventions are likely to have an impact on the costs and benefits felt by other government sectors. These resources and outcomes are recommended as being presented separately from the reference case. It should be noted that the NICE guidance106 does not permit the inclusion of productivity costs in either the reference case or non-reference case. This reflects the methodological challenge of measuring such costs. In this study, the aim of the intervention is to help people back into work or to retain work after TBI; therefore, changes in productivity are central to any economic analysis. Given the methodological challenges of measuring productivity costs,107,108 this report presents the resource use (in units of time) and costs separately.
Methods
Feasibility of identifying, measuring and valuing resource use
Identification of potentially relevant resource use
The resource use items were identified for inclusion on the basis of experience from a cohort study59 undertaken on VR after TBI. The aim was to be as broad as possible in order to identify which resource use items are potentially important to capture in a definitive trial.
Feasibility of measuring resource use
Participants were asked to complete the bespoke resource use questions (see Report Supplementary Material 9 and 10 for copies of the questionnaires used) at baseline and at 3, 6, and 12 months via face-to-face interview or postal questionnaire, depending on their centre.
Delivery of the intervention (including staff training)
The OTs delivering the intervention recorded how much time (in minutes) they spent on face-to-face contacts, non-face-to-face contacts, travel, administration, notes, mentoring and training. These were costed assuming that a band 7 OT would be delivering the intervention. Mentoring was designed to be delivered remotely via e-mail and telephone calls.
The training provided to intervention OTs was costed as incurred, but it should be noted that, in a definitive study, training would probably be more efficient to provide as more OTs would attend fewer events. For this reason, the training element was included only in sensitivity analyses (SAs) and not the base case. A venue for the training was assumed to cost £200 to hire for the day, with £15 per person for refreshments. Mileage was assumed to be £0.56109 per mile and mileage travel was estimated using the government statistics110 on average free-flow speeds for an assumed average 120 minute return journey per OT or trainer attending.
It was recognised during the design phase that it would be hard for participants to distinguish between contacts that were part of the VR intervention and those that would be considered UC. For instance, participants might receive occupational therapy from the intervention OT or through their usual service. As a result, it was felt that it would be impracticable to ask participants to record only contacts that were not part of the intervention; thus, participants were free to record all of their health and social care contacts. Separately, intervention therapists recorded and collated information on all staff training (including who led the training; who attended; and where, when, and for how long it occurred) and VR intervention therapists’ timings (for contact and non-contact time related to participant activity). Thus, it was possible to measure, and subsequently cost, the intervention. However, in order to avoid double counting the therapists, recorded face-to-face visits were not included in the base-case economic analysis. Instead, this information is reported separately and included within a SA to provide a range on estimates. In addition, the concordance between number of visits reported by the intervention therapists and the number of OT visits reported by participants in the intervention group was investigated.
NHS and Personal Social Services utilisation
NHS resource use in primary, secondary and community care along with PSSs (including community care assistant time, Meals on Wheels, etc.) were recorded via the bespoke questionnaires administered at baseline and at 3, 6 and 12 months via postal questionnaires (two centres) or face-to-face interviews (one centre).
Productivity of the person with traumatic brain injury and their main carer (if applicable)
Productivity costs (time off work and presenteeism) were recorded in two ways in order to assess which approach was the most practicable for participants to complete and for researchers to value. The first approach used the published instrument WPAI v2101 and the second approach measured productivity using bespoke questions (see questions 9 and 29 for the participant and questions 33 and 34 for the carer in the participant questionnaire in Report Supplementary Material 9 or questions 4 and 5 in the carer questionnaire in Report Supplementary Material 10). Both were collected at baseline and at 3, 6 and 12 months via postal questionnaires or face-to-face interviews.
Other services (including government employment agencies, private service, etc.)
Participants were also asked to self-report any other services that either they or their carer used or paid for (such as alternative therapies, legal fees), or that were incurred by other public sector agencies (such as government employment services) as a result of their TBI. These were collected at baseline and at 3, 6 and 12 months via postal questionnaires or face-to-face interviews.
Feasibility of valuing resource use
The point of valuing resource use was to check that all unit costs could be sourced and that the way data were captured was amenable to valuation, to again inform the definitive trial.
NHS and PSS resource items were valued using published unit costs for a common price year (2015). 109,111,112
Medications were costed using the published 2015 Prescription Cost Analysis, England,112 a data set that details the number of items, as well as the net ingredient costs of all prescriptions dispensed in the community in England. As participants were asked to report the name, duration, dosage and number of times taken a day of any medications taken, it was possible to calculate the quantity of each medication taken. Using this value multiplied by the net ingredient cost per quantity reported in the Prescription Cost Analysis (PCA),112 a total cost for each medication could be calculated. When data were not complete, several assumptions were made. The first assumed that when the size/dosage of a medication was not reported, the most commonly prescribed strength/size of that medication, as reported in the PCA,112 was used. For instance, if paracetamol was stated but not the strength, the net ingredient cost per quantity was used for paracetamol 500 mg because that strength is recorded in the PCA112 as having been prescribed most often. Second, if the number of times a prescription was taken and/or duration was missing, a per-item cost was used. Finally, some participants reported taking medications only when needed, pro re nata. When this occurred, as it was not possible to quantify the actual amount of medication taken, the net ingredient cost per item was used and taken to be the cost of that medication.
Productivity costs were valued using the human capital approach, which states that lost productivity is equivalent to the wage rate. 107 Two approaches to value this time were used to provide a range around estimates: (1) participants’ self-reported income (taking the mid-point of the reported income band) versus (2) using the published mean hourly wage rate from the Annual Survey of Hours and Earnings Statistical bulletins. 113
Participant and carer out-of-pocket expenses were valued using the amount self-reported by participants in the questionnaire. Other public sector agency costs were sought from published sources.
Feasibility of measuring outcomes using the EuroQol-5 Dimensions, three-level version
Measurement of outcomes
Effectiveness, for use in preliminary cost-effectiveness analysis, was expressed as the difference in percentage who returned/maintained in work.
Utility was measured using the EQ-5D-3L, as recommended by NICE in their reference case,106 at baseline and at 3, 6 and 12 months in order to check the level of completeness achievable with this particular study population. Utility values were attached to the EQ-5D-3L using the York A1 tariff. 114 An early-stage cost–utility analysis was undertaken to inform the value of undertaking a definitive trial. QALYs for the study time frame were estimated using linear interpolation and area-under-the-curve analysis with and without baseline adjustment. 115 Where total area under the curve was estimated as:
for each intervention group, where U0 was utility at baseline, U3 utility at 3 months, U6 utility at 6 months and U12 utility at 12 months.
The primary purpose of valuing outcomes was, first, to check data completeness and, second, to assess if the expected effects of VR on health-related QoL were being picked up, to inform the definitive trial.
Statistical analysis
Feasibility of collecting resource use and outcome data
The completeness of the resource use data collected was analysed first using descriptive statistics to estimate the number and percentage of participants with data available for each resource item by sector. This was repeated for each time point (baseline, 3, 6 and 12 months), first by centre and then overall for VR intervention compared with UC. The completeness of EQ-5D-3L responses is also given as number and percentage of participants with a value recorded overall and for each of the five dimensions for all study time points (baseline, 3, 6 and 12 months). Overall completeness, in terms of number (percentage) of participants with complete cost and utility data, that could be entered into the complete-case early stage economic evaluation is also reported.
Feasibility of conducting a within-trial economic evaluation
Given the 12-month timeframe for the study, costs and outcomes were not discounted. 116 All analysis was conducted in Stata version 14.2 or Microsoft Excel® 2016 (Microsoft Corporation, Redmond, WA, USA) and, when necessary, 0.05 was taken as the level of significance.
Having looked at the completeness of the data, mean (SD) resource use and costs (Great British pounds, 2015 price year) were estimated for each resource item by intervention group and overall as mean difference (95% CI) for all study time points (baseline, 3, 6 and 12 months) independently and in total (i.e. summed for the total 12-month period). This analysis was designed to help identify any resource items that were likely to change significantly as a result of the VR intervention and, thus, that ought to be collected carefully in any future definitive trial.
The mean (SD) utility values estimated using the EQ-5D-3L and EQ-5D VAS at each study time point for each intervention group were also presented, along with the mean (SD) unadjusted QALYs based on both EQ-5D-3L and EQ-5D VAS. The mean difference (95% CI) in utility and QALYs between groups were also presented.
In order to undertake a value-of-information analysis to estimate the value of undertaking further research on VR intervention for TBI, an early-stage cost–utility analysis was conducted using a complete-case analysis approach, including only participants for whom complete cost and effectiveness data at each time point were available. In line with the statistical analyses, missing data were not imputed.
The early-stage economic evaluation is a within-feasibility study analysis. This means that costs and benefits were evaluated only for the study follow-up period (12 months). Costs and outcomes in both groups of the study were estimated using the methods described above. The information on costs and benefits was used to conduct an early-stage complete-case incremental economic analysis comparing VR to UC alone after TBI. Incremental cost-effectiveness ratios (ICERs) were calculated using accepted methodology. 116,117 When the ICER value may be difficult to interpret, for example when the intervention is shown to be both less costly and less effective than the comparator, the net monetary benefit (NMB) (λΔE – ΔC, where lambda equals the willingness-to pay-threshold, ΔE the change in outcomes and ΔC the change in costs between the VR intervention group and the UC group) was calculated instead.
The statistical analysis estimated both the unadjusted and adjusted estimates, and the latter controlled for any differences in baseline characteristics (e.g. the cost regression adjusted for recruiting centre and baseline cost, while the QALY regression adjusted for baseline utility and recruiting centre). The adjusted analyses used a regression-based approach (seemingly unrelated regression equations118) to estimate incremental costs and QALYs.
As cost data were skewed, we used non-parametric bootstrapping to estimate adjusted mean (95% CI) incremental cost and mean (95% CI) incremental QALY gain estimates. Bootstrapping was also used to estimate cost-effectiveness acceptability curves,119–121 which show the probability of each intervention group being the most cost-effective option at different monetary valuations of the outcome variable (QALYs). A range of ceiling ratio [or willingness to pay (WTP) per QALY] values were tested, including the £20,000 and £30,000 per QALY thresholds used by NICE in cost–utility calculations. 106 The purpose of undertaking the early-stage cost–utility analysis, using the data generated in the feasibility study, was to estimate the expected value of perfect information (EVPI), which provides an upper estimate of the value of undertaking future research to reduce the level of uncertainty associated with the decision about whether or not to allocate scarce resources to the provision of VR after TBI. The EVPI is estimated by the probability of making the wrong decision being multiplied by the consequences of that wrong decision. 122 The EVPI can be estimated on a per-participant basis, or scaled up to reflect the total population potentially eligible for VR following a TBI. We report both per-participant EVPI as well as population EVPI. Population EVPI is estimated for 1-year, 5-year, and 10-year periods, where potential population likely to benefit was estimated to be 22,431 (13.8% of 162,544, where 13.8% is the overall eligibility rate of all those screened to enter the FRESH feasibility trial and 162,544 is an estimate of the number of admissions for TBI per annum in the UK). 6 When the time horizon exceeded 1 year in the value-of-information analysis, costs were discounted at a rate of 3.5%. Therefore, this analysis can inform whether or not the cost and benefits of future research are likely to be less than the value to be gained in terms of reduced uncertainty from undertaking the research and, as such, indicate whether or not it might be worth proceeding to a definitive trial.
Sensitivity analyses
Five SAs were undertaken, details and results of these can be found in Report Supplementary Material 13.
Results
In line with the statistical analysis plan, missing data were not imputed; rather, a complete-case analysis was undertaken. As a feasibility study, the focus was on observing the practicality of collecting different types of data in different ways and of the practicality of valuing the data given the format in which they were collected.
Data completeness
The main findings in terms of completeness were that most participants who returned a questionnaire answered all questions, so that incompleteness can be largely attributed to the failure of some participants to return a questionnaire at all, either because they droppped out of the study completely or because they did not return a questionnaire at one follow-up point. Overall levels of data completion were high, suggesting that the questionnaires and approach to administering them were appropriate for this participant population.
Table 14 shows the reasons for exclusion from the complete-case analysis. It can be seen that complete resource use and utility data at 3, 6 and 12 months were available for 37 (47%) (VR intervention, n = 19; UC, n = 18) participants, enabling them to be entered into the complete-case analysis that is presented in the sections that follow. Of the 14 participants from whom resource use data were missing, two were also missing utility data; in only one case were utility data but not cost data missing. It can be seen that dropout was higher in the control group, although the number missing resource use data for those still in the study at 12 months was higher in the VR intervention group.
| Reason for exclusion (n) | Intervention arm | Overall (N = 78) | |
|---|---|---|---|
| VR (N = 39) | UC (N = 39) | ||
| Dropped out at 3 months | 4 | 6 | 10 |
| Dropped out at 6 months | 1 | 2 | 3 |
| Dropped out at 12 months | 5 | 8 | 13 |
| Total participants at 12 months | 29 | 23 | 52 |
| Missing resource use dataa | 10 | 4 | 14 |
| Missing utility dataa | 0 | 1 | 1 |
| Number in complete-case analysis | 19 | 18 | 37 |
The detailed tables reporting completion rates can be found in Report Supplementary Material 14. Report Supplementary Material 14, Tables 11–15, show the number and percentage of participants by centre with data available for each resource item for each of the four time points and over the entire 12-month study period. For some resource items, completeness was recorded for two variables. First, one variable (Y/N variable) in the data set was used to record ‘yes’ or ‘no’ for whether or not the participant had reported any use of the item and a second variable (number) was used to record the number of contacts. This was repeated by intervention group and is detailed in Report Supplementary Material 14, Tables 16–20. Notably, at baseline, there was 100% completeness for all NHS and social service resource items. Although resource items under a broader perspective were largely 100% complete, there were a few items which were not, including at Preston (WPAI v2 and lost wages) and at Leeds (DEA). Job Centre, other DWP services, benefits advisor and solicitor data were less complete but still largely > 95% complete. Data completeness declined at 3 and 6 months but improved at 12 months. The one centre using face-to-face administration of questionnaires (Leeds) did tend to have slightly more complete data than the two postal centres at 3, 6 and 12 months but only by a few percentage points, which is unlikely to justify the additional cost involved in administering face-to-face questionnaires for all participants. Data completeness was usually above 90% per resource item and the least complete item was OT visits recorded at the London centre at 6 months, which had 72% data completeness. A comparison of data completeness by intervention group showed that levels were fairly similar at baseline and 3 months but at 6 months (typically 88% completeness in the VR group compared with 100% in the UC group) and 12 months (typically 97% completeness in the VR group compared with 100% in the UC group) the VR intervention group had lower levels of completeness. Overall, 38 (49% of the participants entered into the study at the start) participants had complete resource use data across the 12-month study period.
Tables 11–15 in Report Supplementary Material 14 show the number and percentage of participants by centre with data available for the EQ-5D-3L overall, for each dimension and for the EQ-5D VAS for each of the four time points and across the entire 12-month study period. This was repeated by intervention group and is detailed in Tables 16–20 in Report Supplementary Material 14. The same general overall conclusion found for resource use applies to the utility data: if participants answered one question, they were most likely to answer all questions. The only exception to this was one participant in the Leeds centre at 12 months who did not complete the anxiety and depression dimension of the EQ-5D-3L. Data were 100% complete at baseline for all centres. Data completion was always ≥ 95% for overall EQ-5D-3L score in the by-centre analysis except at the London centre at 3 months. Data completeness was always above 91% for the overall EQ-5D-3L score in the by-intervention group analysis. Data completeness was lower in the UC group at 3 months (94% vs. 97% in VR group) and 12 months (96% vs. 100% in the VR group), but lower in the VR group at 6 months (91% vs. 100% in the UC group). Overall, 49 (63% of those who entered into the study at the start) participants had complete utility data across the 12-month study period such that they could be included in the early-stage cost–utility analysis, conditional on having complete resource use data.
Although it has been shown that it is feasible to collect health economic data to assess the cost-effectiveness of VR following TBI in a subsequent definitive study, this is not without potential challenges that would need to be mitigated in order to minimise dropout and missing data in a definitive study on a larger scale.
Analysis of resource use and costs (NHS and Personal Social Services perspective)
The unit costs and their source for each resource item are presented in Table 15 (NHS and PSS perspective). When deriving these unit costs, several assumptions were made. For inpatient visits, a weighted average was calculated based on the number and cost of procedures reported in the Department of Health and Social Care’s reference costs,111 considering only non-elective stays. A similar approach was taken when calculating the unit cost for being admitted, whereby a weighted average of all emergency medicine admitted categories (excluding dead on arrival) was taken. When it was not possible to find a unit cost for the 2015 price year, costs were inflated using a web-based tool. 123 For resource items for which participants could provide a variety of responses, such as medications and outpatient visits, these were individually costed, and, thus, a single unit cost is not provided within Table 15.
| Resource item | Unit cost (£) | Source |
|---|---|---|
| Secondary health-care resource use | ||
| Inpatient visits (per night) | 538.00 | Department of Health and Social Care 2015111 |
| A&E | 107.00 | Department of Health and Social Care 2015111 |
| Admitted to hospital | 295.97 | Department of Health and Social Care 2015111 |
| Outpatient visits | Various | Department of Health and Social Care 2015111 |
| Primary and community health-care resource use | ||
| GP (per 11.7-minute consultation) | 37.00 | PSSRU 2015109 |
| Practice nurse (per 15.5-minute consultation) (duration sourced from PSSRU 2014124) | 12.14 | PSSRU 2015109 |
| NHS walk-in centre (assumed to have the same unit cost as a GP visit) | 37.00 | PSSRU 2015109 |
| OT (per 30-minute consultation) (duration sourced from PSSRU 2010125) | 20.50 | PSSRU 2015109 |
| Physiotherapist (per 23.3-minute consultation) (duration sourced from PSSRU 2013126) | 13.20 | PSSRU 2015109 |
| SALT (per 30-minute consultation) (duration sourced from PSSRU 2010125) | 34.00 | PSSRU 2015109 |
| Psychologist (per hour consultation) | 138.44a | PSSRU 2014124 |
| Medication | Various | Health and Social Care Information Centre112 |
| Social care resource use | ||
| Social worker (per 30-minute consultation) (duration assumed) | 27.50 | PSSRU 2015109 |
| Community care assistant (per hour) | 37.12a | PSSRU 2014124 |
| Meals on Wheels (per meal) | 6.62a | PSSRU 2014124 |
| Other | Various | Department of Health and Social Care 2015111 |
Baseline resource use and costs (NHS and Personal Social Services perspective)
The mean baseline costs for the VR intervention group was £250.34 (SD £786.59) and £266.32 (SD £805.15) for the UC group. The mean difference between the two groups was –£15.98 (95% CI –£374.97 to £343.00). Mean (SD) resource use and cost per participant at baseline from a NHS and PSS perspective is presented in Tables 16 and 17, by intervention group.
| Health and social care appointmentsa | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 39) | UC (n = 39) | ||||
| Mean | SD | Mean | SD | ||
| Secondary health-care resource use | |||||
| Inpatient visits | 0.051 | 0.223 | 0.026 | 0.160 | 0.025 (–0.062 to 0.113) |
| A&E | 0.103 | 0.307 | 0.051 | 0.223 | 0.051 (–0.070 to 0.172) |
| Admitted | 0.077 | 0.270 | 0.026 | 0.160 | 0.051 (–0.049 to 0.151) |
| Outpatient visits | 0.462 | 1.166 | 0.615 | 0.963 | –0.154 (–0.636 to 0.329) |
| Primary and community health-care resource use | |||||
| GP | 0.359 | 0.584 | 0.436 | 0.680 | –0.077 (–0.363 to 0.209) |
| Practice nurse | 0.103 | 0.384 | 0.077 | 0.270 | 0.026 (–0.124 to 0.175) |
| NHS walk-in centre | 0.000 | 0.000 | 0.026 | 0.160 | –0.026 (–0.077 to 0.025) |
| OT | 0.282 | 1.025 | 0.333 | 1.924 | –0.051 (–0.746 to 0.644) |
| Physiotherapist | 0.077 | 0.480 | 0.128 | 0.656 | –0.051 (–0.311 to 0.208) |
| SALT | 0.00 | 0.000 | 0.103 | 0.641 | –0.103 (–0.307 to 0.102) |
| Medication | 2.256 | 2.061 | 1.949 | 1.761 | 0.308 (–0.557 to 1.172) |
| Total health visits | 1.436 | 1.997 | 1.821 | 3.691 | –0.385 (–1.723 to 0.954) |
| Social care resource use | |||||
| Social worker | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Community care assistant | 0.128 | 0.801 | 0.000 | 0.000 | 0.128 (–0.127 to 0.384) |
| Meals on Wheels | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Other | 0.103 | 0.307 | 0.051 | 0.223 | 0.051 (–0.070 to 0.172) |
| Total social care visits | 0.231 | 0.842 | 0.051 | 0.223 | 0.179 (–0.098 to 0.457) |
| Total visits | 1.667 | 2.355 | 1.872 | 3.708 | –0.205 (–1.609 to 1.196) |
| Health and social care costs | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 39) | UC (n = 39) | ||||
| Mean | SD | Mean | SD | ||
| Secondary health-care costs | |||||
| Inpatient visits | 137.95 | 705.99 | 124.15 | 775.34 | 13.79 (–320.63 to 348.22) |
| A&E | 10.97 | 32.89 | 5.49 | 23.91 | 5.49 (–7.48 to 18.45) |
| Admitted | 22.77 | 79.90 | 7.59 | 47.39 | 15.18 (–14.45 to 44.80) |
| Outpatient visits | 41.30 | 94.79 | 86.53 | 140.72 | –45.23 (–99.34 to 8.88) |
| Primary and community health-care costs | |||||
| GP | 13.28 | 21.62 | 16.13 | 25.17 | –2.85 (–13.43 to 7.74) |
| Practice nurse | 1.25 | 4.66 | 0.93 | 3.28 | 0.31 (–1.50 to 2.13) |
| NHS walk-in centre | 0.00 | 0.00 | 0.95 | 5.92 | –0.95 (–2.84 to 0.95) |
| OT | 5.78 | 21.01 | 6.83 | 39.44 | –1.05 (–15.30 to 13.20) |
| Physiotherapist | 1.02 | 6.34 | 1.69 | 8.66 | –0.68 (–4.10 to 2.75) |
| SALT | 0.00 | 0.00 | 1.74 | 10.89 | –1.74 (–5.22 to 1.73) |
| Medication | 6.84 | 14.06 | 13.64 | 60.32 | –6.79 (–26.55 to 12.96) |
| Total health costs | 241.16 | 787.80 | 265.68 | 805.23 | –24.52 (–383.79 to 334.75) |
| Social care costs | |||||
| Social worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Community care assistant | 4.76 | 21.02 | 0.00 | 0.00 | 4.76 (–4.72 to 14.24) |
| Meals on Wheels | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Other | 4.42 | 20.15 | 0.64 | 4.03 | 3.77 (–2.78 to 10.33) |
| Total social care costs | 9.18 | 35.30 | 0.64 | 4.03 | 8.53 (–2.80 to 19.86) |
| Total costs | 250.34 | 786.59 | 266.32 | 805.15 | –15.98 (–374.97 to 343.00) |
Intervention resource use and costs
The mean cost of providing the VR intervention was £591.81 (SD £339.92) per participant based on the data recorded by the VR therapists themselves. This cost included OT time undertaking face-to-face contacts, non-face-to-face work, travel time and costs, mentoring, administration and notes.
As reported in Methods, in the base-case economic evaluation we excluded the cost of OT time for face-to-face contacts to avoid potential double counting with OT appointments recorded by participants themselves. The mean cost of the VR intervention, without face-to-face contacts included, was £177.28 (SD £68.08) and it is this cost that is included in the base-case economic evaluation.
To explore whether or not OT-reported face-to-face contacts in the VR group were in line with the number of OT appointments reported by participants in their questionnaires, we compared mean (SD) estimates of the number of OT appointments reported by therapist and participant as well as estimating the level of agreement between the number of OT appointments reported by therapist and participant using Lin’s concordance correlation coefficient. Therapists recorded a mean of 6.16 (SD 6.47) visits, compared with 10.21 (SD 12.47) visits recorded by participants [mean difference –4.05 visits (95% CI –8.20 to 0.10 visits)]. In most instances, the number reported by the OT was lower than that reported by the participant, with only five participants (26%) of the complete cases recording fewer visits than those recorded by the OT. This may suggest that participants are recording both the intervention visits as well as any additional occupational therapy appointments received as part of UC. Lin’s concordance correlation coefficient was estimated to be 0.574 using only those included in the complete-case analysis or 0.587 for all cases with OT data available. This indicates that there was moderate agreement127 between OT and participant reports, suggesting that the majority of participants are likely to be including their intervention OT visits in their questionnaire responses.
Wider NHS and Personal Social Services resource use and costs over the 12-month study period
The mean (SD) and mean difference (95% CI) for resource use and costs can be seen in Tables 18 and 19, respectively. The mean cost per participant across the entire study period (12 months) in the VR intervention group was £2659.55 (SD £4402.83) without including the estimated intervention costs and £2845.43 (SD £4406.69) when these were included, compared with £3834.81 (SD £4666.32) for the UC group. Without adjustment, the early-stage incremental mean cost per participant was –£989.38 (95% CI –£4017.34 to £2038.59) and, when adjusting for centre and baseline costs, was –£1030.64 (95% CI –£3840.45 to £1779.17).
| Health and social care visits | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 19) | UC (n = 18) | ||||
| Mean | SD | Mean | SD | ||
| Secondary health care | |||||
| Inpatient visits | 0.105 | 0.315 | 0.500 | 0.707 | –0.395 (–0.757 to 0.033) |
| A&E | 0.368 | 0.684 | 0.556 | 0.784 | –0.187 (–0. 677 to 0.303) |
| Admitted | 0.105 | 0.459 | 0.278 | 0.575 | –0.173 (–0.519 to 0.174) |
| Outpatient visits | 6.737 | 12.662 | 7.333 | 6.808 | –0.596 (–7.438 to 6.245) |
| Primary and community health care | |||||
| GP | 9.789 | 18.359 | 3.667 | 3.029 | 6.122 (–2.781 to 15.027) |
| Practice nurse | 2.312 | 5.508 | 0.444 | 0.856 | 1.871 (–0.796 to 4.539) |
| NHS walk-in centre | 0.000 | 0.000 | 3.778 | 15.536 | –3.778 (–11.008 to 3.452) |
| OT | 10.211 | 12.470 | 5.333 | 8.684 | 4.877 (–2.333 to 12.088) |
| Physiotherapist | 3.579 | 8.362 | 1.500 | 3.698 | 2.079 (–2.279 to 6.437) |
| SALT | 0.368 | 1.383 | 0.222 | 0.732 | 0.146 (–0.598 to 0.891) |
| Medication | 3.211 | 5.339 | 1.722 | 1.994 | 1.488 (–1.232 to 4.208) |
| Social care | |||||
| Social worker | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Community care assistant | 0.105 | 0.459 | 0.333 | 1.414 | –0.228 (0.922 to 0.466) |
| Meals on Wheels | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Other | 0.105 | 0.315 | 0.111 | 0.323 | –0.006 (–0.219 to 0.207) |
| Health and social care costs | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 19) | UC (n = 18) | ||||
| Mean | SD | Mean | SD | ||
| Partial intervention costs | |||||
| Non-face-to-face OT time | 185.88 | 77.10 | 0.00 | 0.00 | 185.88 (–148.96 to 222.80) |
| Secondary health-care costs | |||||
| Inpatient visits | 1076.00 | 4315.19 | 1823.22 | 4539.42 | –747.22 (–3702.32 to 2207.87) |
| A&E | 39.42 | 73.19 | 59.44 | 83.87 | –20.02 (–72.48 to 32.43) |
| Admitted | 31.15 | 135.80 | 82.21 | 170.04 | –51.06 (–153.48 to 51.36) |
| Outpatient visits | 826.85 | 1146.83 | 1320.99 | 1596.13 | –494.13 (–1417.89 to 429.62) |
| Primary and community health-care costs | |||||
| GP | 362.21 | 679.29 | 135.67 | 112.08 | 226.54 (–102.90 to 555.99) |
| Practice nurse | 28.11 | 66.87 | 5.40 | 10.39 | 22.72 (–9.67 to 55.10) |
| NHS walk-in centre | 0.00 | 0.00 | 139.78 | 574.82 | –139.78 (–407.28 to 127.73) |
| OT | 209.32 | 255.64 | 109.33 | 178.02 | 99.98 (–47.83 to 247.80) |
| Physiotherapist | 3.58 | 8.36 | 1.50 | 3.70 | 2.08 (–2.28 to 6.44) |
| SALT | 6.26 | 23.51 | 3.78 | 12.45 | 2.49 (–10.17 to 15.15) |
| Medication | 72.74 | 157.89 | 21.94 | 36.03 | 50.79 (–26.65 to 128.24) |
| Total health costs | 2655.65 | 4401.75 | 3703.26 | 4667.61 | –1047.61 (–4074.36 to 1979.14) |
| Social care costs | |||||
| Social worker | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Community care assistant | 3.91 | 17.03 | 12.37 | 52.50 | –8.47 (–34.22 to 17.29) |
| Meals on Wheels | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Other | 0.00 | 0.00 | 119.18 | 358.77 | –119.18 (286.14 to 47.79) |
| Total social care costs | 3.91 | 17.03 | 131.55 | 358.26 | –127.64 (–294.56 to 39.28) |
| Total costs (excluding intervention) | 2659.55 | 4402.83 | 3834.81 | 4666.32 | –1175.25 (–4201.93 to 1851.42) |
| Total costs (including intervention) | 2845.43 | 4406.69 | 3834.81 | 4666.32 | –989.38 (–4017.34 to 2038.59) |
The percentage of total cost accounted for by each individual resource item in the base-case analysis is presented in Report Supplementary Material 14, Table 21. From this it can be seen that inpatient visits, outpatient visits and GP visits were the biggest contributors to total costs from a health and social care perspective, where the percentage of total costs accounted for by these categories of costs were inpatient visits (40.46%), outpatient visits (31.09%) and GP visits (13.62%) for the VR intervention and inpatient visits (47.54%), outpatient visits (34.45%) and visits to NHS walk-in centres (3.64%) in the UC group.
Outcomes
In terms of the clinical outcome in the feasibility study, 15 (79%) participants returned to work or education in the VR group and 16 (89%) in the UC group. It should be noted that these figures are different from those reported in Chapter 3, because only the complete cases were considered.
The mean (SD) utility per participant at each time point (baseline, 3, 6 and 12 months) by intervention group is presented in Table 20 along with estimates of QALYs for the study duration. It can be seen that, considering only complete cases, the mean number of QALYs per participant was 0.6512 (SD 0.2781) in the VR intervention group, compared with 0.7292 (SD 0.1609) in the UC group. The early-stage incremental mean QALYs per participant was –0.0780 (95% CI –0.2308 to 0.0748) without adjustment and was –0.0506 (95% CI –0.1715 to 0.0702) with adjustment for baseline and centre.
| Estimate and measurement time point (n VR, n UC) | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR | UC | ||||
| Mean | SD | Mean | SD | ||
| EQ-5D-3L: baseline (39, 39) | 0.4435 | 0.3070 | 0.4878 | 0.3349 | –0.0443 (–0.1892 to 0.1006) |
| EQ-5D-3L: 3 months (34, 31) | 0.6873 | 0.2946 | 0.6375 | 0.3075 | 0.0497 (–0.0995 to 0.1990) |
| EQ-5D-3L: 6 months (31, 31) | 0.6907 | 0. 3493 | 0.7498 | 0.1989 | –0.0591 (–0.2035 to 0.0853) |
| EQ-5D-3L: 12 months (29, 22) | 0.7477 | 0.2854 | 0.8305 | 0.1970 | –0.0828 (–0.2256 to 0.0600) |
| QALYs (EQ-5D-3L): area under the curve (27, 22) | 0.6679 | 0.2527 | 0.7309 | 0.1697 | –0.0630 (–0.1898 to 0.0638) |
| QALYs (EQ-5D-3L): area under the curve (complete case, n = 19; UC, n = 18) | 0.6512 | 0.2781 | 0.7292 | 0.1609 | –0.0780 (–0.2308 to 0.0748) |
| EQ-5D VAS: baseline (39, 39) | 0.5336 | 0.2210 | 0.5623 | 0.2571 | –0.0287 (–0.1369 to 0.0794) |
| EQ-5D VAS: 3 months (32, 31) | 0.7388 | 0.1733 | 0.6935 | 0.2048 | 0.0452 (–0.0503 to 0.1407) |
| EQ-5D VAS: 6 months (31, 30) | 0.7006 | 0.2554 | 0.7380 | 0.1606 | –0.0374 (–0.1471 to 0.0724) |
| EQ-5D VAS: 12 months (29, 23) | 0.7714 | 0.1778 | 0.7978 | 0.1617 | –0.0264 (–0.1223 to 0.0694) |
| QALYs (EQ-5D VAS): area under the curve (27, 22) | 0.7009 | 0.1714 | 0.7470 | 0.1307 | –0.0460 (–0.1353 to 0.0432) |
| QALYs (EQ-5D VAS) area under the curve (complete case, n = 19; UC, n = 18) | 0.6722 | 0.1786 | 0.7565 | 0.1307 | –0.0842 (–0.1914 to 0.0229) |
Report Supplementary Material 14, Table 22, shows the estimated utility at different time points and QALYs for the study period by intervention group and data collection method. It can be seen that both utility and QALYs were higher in the postal group than the face-to-face questionnaire group for both the intervention and UC groups; however, as a result of the small sample sizes, it could be that one or two individuals in the face-to-face questionnaire group influenced the mean values.
Value-of-information analysis
The purpose of undertaking the early stage cost–utility analysis, using the data generated in the feasibility study, was to demonstrate that a within-trial economic evaluation is feasible and to estimate the EVPI to provide an upper estimate of the value of undertaking future research to reduce the level of uncertainty associated with the decision about whether or not to allocate scarce resources to the provision of VR after TBI.
Early-stage cost-effectiveness analysis
The difference in mean cost per participant was –£989.38 (95% CI –£4017.34 to £2038.59) unadjusted or –£1030.64 (95% CI –£3840.45 to £1779.17) when adjusting for centre and baseline costs, meaning that the VR intervention group was, on average, cheaper than the UC group. However, in the VR group, fewer people returned to, or retained, work: 15 (79%) participants returned to work or education, compared with 16 (89%) in the UC group. As it is not clear what cost saving a decision-maker might be willing to make at the expense of fewer people returning to work, we do not estimate an ICER or net benefit estimate using the clinical outcome but note that, although this could be done, the result would be difficult to interpret.
Early-stage cost–utility analysis
As above, the difference in mean cost per participant was –£1030.64 (95% CI –£3840.45 to £1779.17) when adjusting for centre and baseline costs, meaning that the VR intervention was, on average, cheaper than UC. The incremental mean QALYs per participant was –0.0506 (95% CI –0.1715 to 0.0702) with adjustment for baseline and centre. Figure 4 illustrates the cost-effectiveness plane comparing incremental costs and QALYs for the VR intervention with UC in the base-case analysis.
FIGURE 4.
Cost-effectiveness plane for the comparison of the VR intervention and UC, based on 10,000 bootstrapped cost–effect pairs, using QALYs for the base-case analysis.

The NMB of the intervention was calculated, assuming a WTP of £20,000 and £30,000 per QALY generated, in line with NICE guidance. 106 The NMB for the VR intervention group, when considering the intervention costs in the analysis, was £18.64 (95% CI –£3355.00 to £3032.98) when using a £20,000 WTP per QALY threshold and –£487.36 (95% CI –£4655.34 to £3159.67) at the WTP threshold of £30,000 per QALY. At a WTP threshold of £20,000, the NMB is above zero, which indicates that TBI may be cost-effective at currently accepted thresholds. It should be noted this is based on only the limited data within this feasibility study, although it does demonstrate the uncertainty that currently exists.
The probability of VR being cost-effective at a WTP threshold of £20,000 (£30,000) per QALY was 47.00% (39.03%) (Figure 5).
FIGURE 5.
Cost-effectiveness acceptability curve showing the probability that VR intervention is cost-effective compared with UC for different values of WTP per QALY.
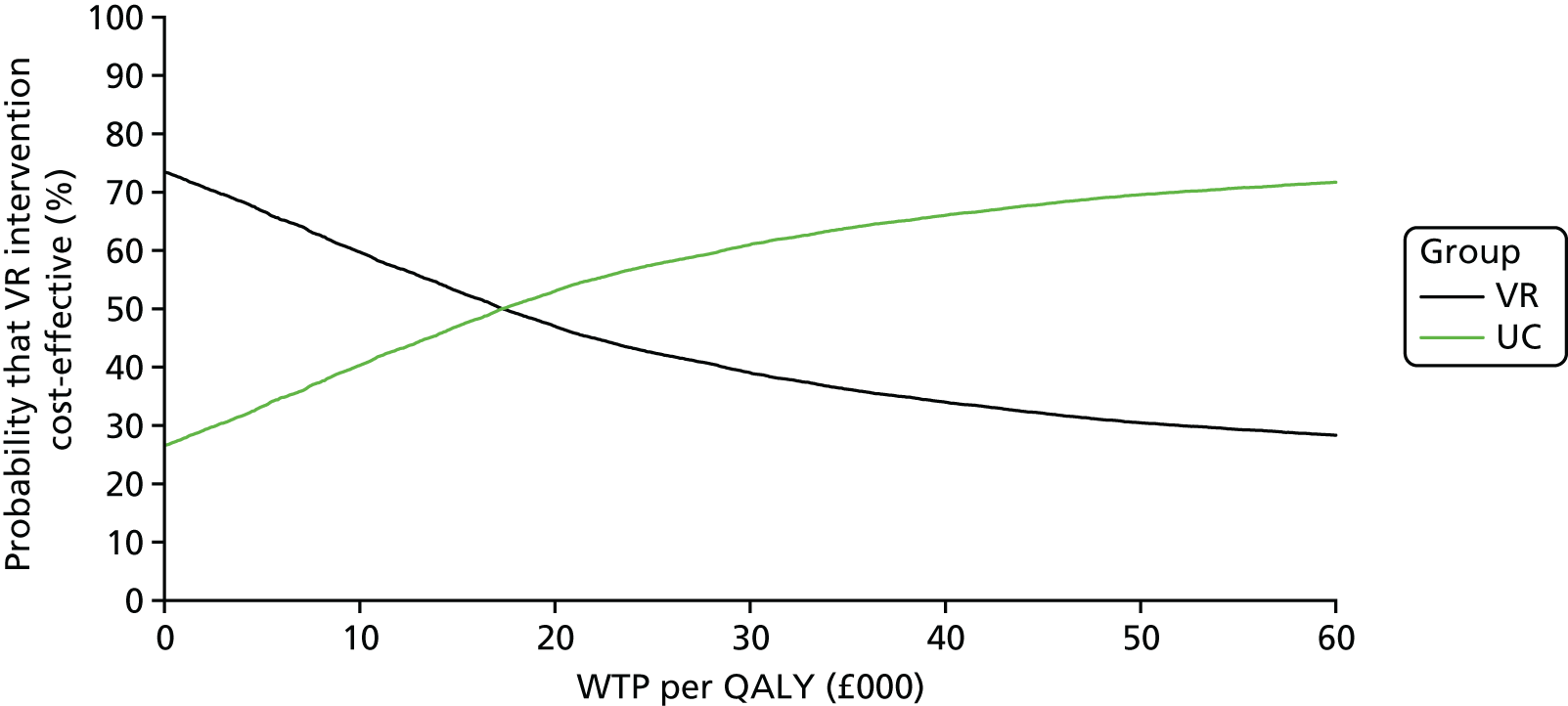
The EVPI was £3077.17 (£3741.49) per participant at a threshold ICER of £20,000 (£30,000). This can be seen in Figure 6.
FIGURE 6.
Expected value of perfect information in the base-case analysis.

The population EVPI was estimated as £69,024,213.37 (£83,925,662.63) over a 1-year time horizon with a WTP per QALY of £20,000 (£30,000) (Figure 7). These figures indicate that the cost of future research is likely to be less than the value to be gained in terms of reducing the uncertainty surrounding the decision about whether or not to adopt VR for TBI, and, thus, proceeding to a definitive trial would be of value.
FIGURE 7.
Population EVPI in the base-case analysis.
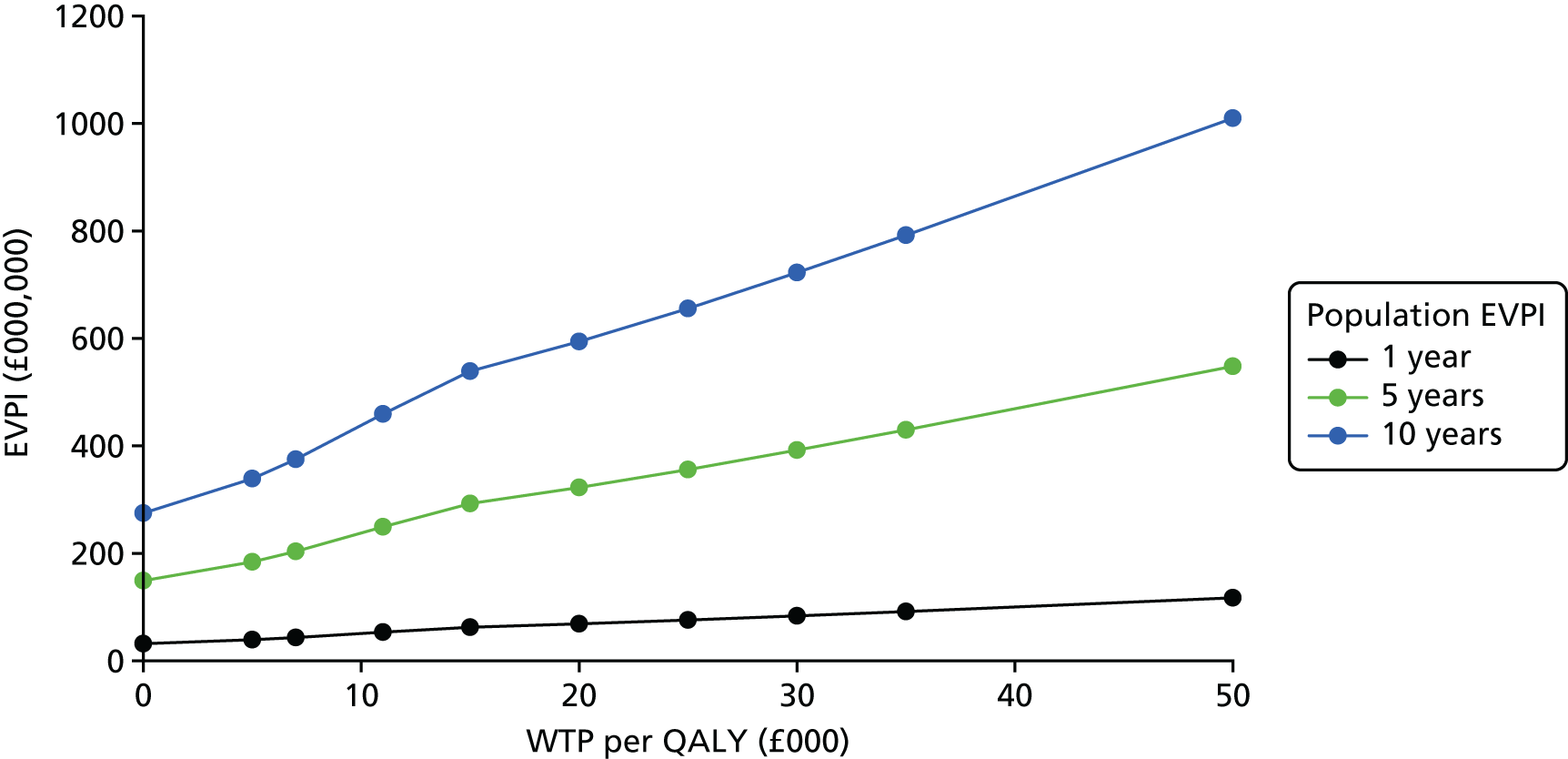
Sensitivity analysis
A number of SAs were undertaken and the summary results of these can be seen in Table 21, with more detail provided in Report Supplementary Material 13. Detailed results for SA2 incorporating the broader perspective are discussed here to reflect the potential importance of these in the context of VR and TBI.
| Analysis | n | Mean difference (95% CI) [unadjusted (adjusted)] | EVPI per participant (£) at £20,000 (£30,000) WTP threshold | |
|---|---|---|---|---|
| Cost (£) | QALYs | |||
| Base case | 37 |
–989.38 (–4017.34 to 2038.59) [–1030.64 (–3840.45 to 1779.17)] |
–0.0780 (–0.2308 to 0.0748) [–0.0506 (–0.1715 to 0.0702)] |
3077.17 (3741.49) |
| SA1: f2f + training + BC | 37 |
–605.79 (–3630.42 to 2418.85) [–647.67 (–3449.90 to 2154.57)] |
–0.0780 (–0.2308 to 0.0748) [–0.0507 (–0.1715 to 0.0702)] |
2857.20 (3538.93) |
| SA2: BC + broader | 14 |
739.29 (–14,534.70 to 16,013.27) [2421.53 (–11,927.18 to 16,770.25)] |
–0.0766 (–0.3175 to 0.01643) [–0.0428 (–0.2219 to 0.1362)] |
5755.15 (5782.21) |
| SA3: SA1 + broader | 14 |
1102.45 (–14,184.82 to 16,389.73) [2751.04 (–11,615.21 to 17,117.28)] |
–0.0766 (–0.3175 to 0.01643) [–0.0429 (–0.2219 to 0.1361)] |
5823.00 (5862.28) |
| SA4: postal base case | 19 |
–1845.59 (–7656.83 to 3965.67) [–1836.62 (–7170.40 to 3497.16)] |
–0.0304 (–0.2311 to 0.1704) [0.0187 (–0.1627 to 0.2001)] |
3154.50 (4122.66) |
| SA5: face-to-face base case | 18 |
189.90 (–2008.08 to 2387.87) [454.01 (–1687.07 to 2595.10)] (adjusted for baseline) |
–0.0977 (–0.3202 to 0.1247) [–0.0833 (–0.2323 to 0.0657)] (adjusted for baseline) |
1495.26 (2018.66) |
Sensitivity analysis 2: base case including wider societal costs
Given the nature of the impact of TBI on participants, the costs of TBI and the benefits of any intervention aimed at supporting people with TBI are likely to be incurred beyond the NHS and PSS sectors. To establish the feasibility of measuring and valuing resource use beyond health and social care, and the importance of these costs for TBI, we incorporated wider resource use questions.
The unit costs and their source for each resource item are presented in Table 22 (broader perspective). When it was not possible to find a unit cost for the 2015 price year, costs were inflated using a web-based tool. 123
| Cost item | Unit cost | Source |
|---|---|---|
| Participant and carer lost wages | – | Participant questionnaire ASHE, 2015113 (mean hourly rate), used for wage when using average method |
| Participant and carers’ out-of-pocket costs | – | Individual extra costs as reported on study questionnaire |
| DEA (per visit) | 43.29a | Jobcentre Plus, Radford et al.59 |
| Job Centre | 140.40a | Jobcentre Plus, Radford et al.59 |
| Work-focused interviews: existing claimant | 29.25a | Jobcentre Plus, Radford et al.59 |
| Other services arranged by the DWP (i.e. Access to Work) | 43.29a | Assume same costs as DEA, Radford et al.59 |
| Benefits advisor | 43.29a | Assume same costs as DEA, Radford et al.59 |
| Employers costs | – | Individual extra costs as reported on study questionnaire |
| Solicitor (per hour) | 195.42 | Based on the average hourly prices reported by gov.uk [URL: www.gov.uk/guidance/solicitors-guideline-hourly-rates (accessed 21 December 2017)] |
| Average hourly rate of pay | 15.27 | ASHE, 2015.113 Based on mean earnings of both men and women |
Baseline costs (broader perspective)
The mean baseline costs were £1557.46 (SD £2194.71) for the VR intervention group and £1561.27 (SD £2393.50) for the UC group. The mean difference between the two groups was –£3.81. Mean (SD) resource use and cost per participant at baseline are presented in Tables 23 and 24, respectively, by intervention group.
| Broader perspective units | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 39) | UC (n = 39) | ||||
| Mean | SD | Mean | SD | ||
| Employer resource use | |||||
| Equipment items | 0.026 | 0.160 | 0.00 | 0.00 | 0.026 (–0.025 to 0.077) |
| WPAI v2 productivity (%) | 0.729 | 3.489 | 2.371 | 8.750 | –1.642 (–4.646 to 1.362) |
| Public sector resource use | |||||
| DEA (per visit) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Job Centre | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Other services arranged by the DWP (i.e. Access to Work) | 0.000 | 0.000 | 0.051 | 0.223 | –0.051 (–0.124 to 0.021) |
| Benefits advisor | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 (0.000 to 0.000) |
| Solicitor | 0.053 | 0.324 | 0.103 | 0.502 | –0.050 (–0.242 to 0.143) |
| Total public sector visits | 0.053 | 0.324 | 0.154 | 0.709 | –0.101 (–0.353 to 0.150) |
| Participant and carer resource use | |||||
| Participant and carers’ out-of-pocket purchases | 0.436 | 0.502 | 0.256 | 0.4424 | 0.179 (–0.034 to 0.393) |
| Participant time off work (number of hours) | 145.614 | 160.702 | 116.654 | 133.071 | 28.960 (–37.582 to 95.501) |
| Carers’ time off work (number of hours) | 76.438 | 116.479 | 63.742 | 80.726 | 12.697 (–58.949 to 84.342) |
| Broader perspective units | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 39) | UC (n = 39) | ||||
| Mean | SD | Mean | SD | ||
| Employer costs | |||||
| Equipment items | 0.03 | 0.16 | 0.00 | 0.00 | 0.03 (–0.03 to 0.08) |
| WPAI v2 productivity | 16.00 | 79.75 | 32.76 | 126.27 | –16.76 (–64.39 to 30.87) |
| Public sector costs | |||||
| DEA (per visit) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Job Centre | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Other services arranged by the DWP (i.e. Access to Work) | 0.00 | 0.00 | 2.22 | 1.55 | –2.22 (–5.31 to 0.87) |
| Benefits advisor | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 (0.00 to 0.00) |
| Solicitor | 10.00 | 62.45 | 20.00 | 97.96 | –10.00 (–47.05 to 27.05) |
| Participant and carer costs | |||||
| Participant and carers’ out-of-pocket purchases | 67.00 | 176.54 | 18.74 | 59.56 | 48.26 (–11.16 to 107.68) |
| Participant lost wages | 1480.43 | 2175.93 | 1520.31 | 2353.06 | –39.88 (–1062.00 to 982.25) |
| Carers’ lost wages (VR 15, UC 17) | 852.26 | 1605.87 | 868.19 | 1475.02 | –15.93 (–1128.24 to 1096.38) |
| Total broader costs excluding carer wages/WPAI v2 | 1557.46 | 2194.71 | 1561.27 | 2393.50 | –3.81 (–1039.48 to 1031.86) |
| Total broader costs including carer lost wagesa | 1885.25 | 2639.26 | 1939.71 | 2557.84 | –54.46 (–1226.62 to 1117.69) |
The mean cost per participant across the entire study period (12 months) in the VR intervention group was £16,306.94 (SD £10,255.40), compared with £15,567.66 (SD £13,577.72) for the UC group. Without adjustment, the early-stage incremental mean cost per participant was £739.29 (95% CI –£14,534.70 to £16,013.27) and, when adjusting for centre and baseline costs, it was £2421.53 (95% CI –£11,927.18 to £16,770.25). Note that the number of participants included in the complete-case analysis of the broader perspective was less than in the NHS and PSS perspective analysis, with just five in the VR group and nine in the UC group.
The percentage of total cost accounted for by each individual resource item according to the broader perspective is presented in Report Supplementary Material 14, Table 21. From a broader perspective, it was carers’ lost wages (33.11%), participant lost wages (26.66%) and inpatient visits (25.35%) that were the biggest contributors to total cost for the VR group, compared with participant lost wages (59.32%), carers’ lost wages (16.27%) and outpatient visits (11.65%) for the UC group.
It is clear from Tables 23 and 24 that productivity changes are significant following TBI; therefore, it is important to explore how best to capture and cost this information in a definitive trial.
Table 25 reports the mean (95% CI) productivity costs according to the different methods used, across the whole time period of the study for the complete cases using the broader perspective. In the VR intervention group, the mean cost of lost earnings was calculated as £5746.25 using the bespoke valuation method or £11,076.72 using average wage. In comparison, it was estimated that those receiving UC lost £7379.94 in wages based on their reported earnings and £7229.38 when using the Annual Survey of Hours and Earnings (ASHE). 113 However, when using the WPAI v2 for the VR intervention group, the mean loss of earnings was £11,165.05, and £16,447.90 when calculated using a mean wage. Using the reported earnings, it was estimated that those receiving UC lost £8516.30 in wages compared with £8583.65 when using a mean wage from the ASHE. 113
| Method of measuring productivity cost | Intervention arm | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| VR (n = 5) | UC (n = 9) | ||||
| Mean | SD | Mean | SD | ||
| Estimated using reported earnings | |||||
| Participants’ lost productivity: bespoke | 4301.24 | 6678.20 | 9234.32 | 13,600.38 | –4933.07 (–19218.71 to 9352.57) |
| Participants’ lost productivity: WPAI v2 (presenteeism) | 546.20 | 971.12 | 899.18 | 866.70 | –352.98 (–1450.20 to 744.24) |
| Participants’ lost productivity: WPAI v2 (working and not working) | 13,965.16 | 9305.70 | 12,060.35 | 16,240.82 | 1904.82 (–15,483.01 to 19,292.64) |
| Carers’ lost productivity: bespoke (VR 5, UC 9) | 5341.01 | 10,781.95 | 2532.33 | 4678.22 | 2808.68 (–6067.11 to 11,684.47) |
| Estimated using average earningsa (using ASHE)113 | |||||
| Participants’ lost productivity: bespoke | 7942.15 | 13,982.86 | 10,952.23 | 16,168.51 | –3010.08 (–21,815.75 to 15,795.59) |
| Participants’ lost productivity: WPAI v2 (presenteeism) | 1067.26 | 2079.05 | 2021.80 | 2544.57 | –954.54 (–3870.55 to 1961.48) |
| Participants’ lost productivity: WPAI v2 (working and not working) | 16,269.20 | 10,775.09 | 14,074.19 | 18,182.86 | 2195.01 (–17,367.35 to 21,757.37) |
| Carers’ lost productivity: bespoke (VR 5, UC 9) | 5083.17 | 9498.24 | 3227.79 | 5873.16 | 1855.38 (–6997.71 to 10,708.46) |
In the analysis carried out, it was the bespoke method using participant-reported wage costs that was included (as part of the broader perspective). Although the bespoke approach was used, each of the methods has distinct advantages and disadvantages that need to be carefully considered ahead of a definitive trial, considering whether to use the bespoke method or the WPAI v2, as well as whether to use each individual’s reported salary or to use an average. For example, when using individual salaries, participants were asked to report which band their salary was within (see question 29 in Report Supplementary Material 9), as opposed to their exact salary, because it was felt that participants may be less willing to report their exact wage. In doing this, it was necessary for the purposes of calculations to use the mid-point of each band, reducing the advantage of accuracy in using the reported salary method.
Importantly, when using the WPAI v2, it was possible to consider the cost of presenteeism, which was not something captured when using the bespoke method. As can be seen in Table 25, from presenteeism alone, there were substantial costs. However, this method was more complex to cost than the bespoke method, owing to the way the WPAI v2 questions were structured. When looking at all cases with data available on productivity at 12 months (see Report Supplementary Material 14, Table 23), broadly similar results can be seen.
In SA2, a broader perspective including wider societal costs was taken. This included the cost of participants’ and carers’ lost wages, calculated using the bespoke method. Note that the number of participants with complete cases was reduced when considering the broader perspective costs, and, thus, the sample was reduced to five in the VR group and nine in the UC group.
Within this analysis, it was found that the mean incremental cost per participant in the VR intervention group was £739.29 (95% CI –£14,534.70 to £16,013.27) without adjustment and £2421.53 (95% CI –£11927.18 to £16,770.25) with adjustment for centre and baseline costs. The mean incremental QALY gain per participant in the VR intervention group was –0.0766 (95% CI –0.3174 to 0.1643) without adjustment and –0.0428 (95% CI –0.2219 to 0.1362) with adjustment for baseline and centre. It can be seen that UC dominated, as the VR intervention was both costlier and less effective than UC in this broader perspective.
The EVPI was £5755.15 (£5782.21) per participant at a threshold ICER of £20,000 (£30,000).
Summary
This chapter has explored the feasibility of identifying and collecting resource use and outcome data for use in a health economic evaluation. It has also demonstrated the feasibility of conducting a within-trial cost–utility analysis and estimated the potential value of undertaking future research on VR following TBI.
Although data completeness was largely good for those participants actually completing the questionnaires, dropout (higher in the control group) and missing resource use data for those still in the study at 12 months (higher in the VR intervention group) meant that only 37 (47.4%) out of 78 participants (47.4%) could be included in the complete-case analysis in the base case. Overall, complete resource use data were available for 38 out of 78 (48.7%) participants, compared with 51 out of 78 (65.4%) participants for QoL. Therefore, while it has been shown that it is feasible to collect health economic data to assess the cost-effectiveness of VR following TBI in a subsequent definitive study, this is not without potential challenges that would need to be overcome.
A relatively small number of participants (n = 32, 41.0%) had a carer complete the carer questionnaires and, thus, it would seem important to try and capture information on carer time and cost inputs via the participant questionnaires in a definitive study.
Given the percentage of total cost accounted for by broader costs, taking such a perspective would be important in a definitive study. The bespoke method of collecting information on productivity changes seemed the easiest to value. The WPAI v2, while collecting information on presenteeism, considers only those in work and so involved an additional step to cost changes to productivity for those not in work. WPAI v2 was difficult to use in terms of estimating and valuing the costs of changes to productivity. Using the mean hourly wage as reported in ASHE113 was more straightforward than asking participants to report which income band they fell within. Using published wage rates would mean that a future study could ask one less question (i.e. drop the income question from participant questionnaires).
A definitive trial will need to re-evaluate how best to capture the intervention costs distinct from other wider NHS resource use. It is unclear if participants are able to differentiate between VR intervention OT visits and community OT visits that were not part of the intervention. The moderate agreement found between therapist- and participant-recorded OT visits seems to suggest that participants probably included intervention visits in their response to questionnaires. As they were not asked to exclude them, we cannot be sure if they would be able to do this in practice. We dealt with this in the analysis by undertaking SAs including and excluding the sessions recorded by the intervention OTs themselves in order to provide a range on the possible estimates of costs and early stage cost-effectiveness.
From the value-of-information analysis conducted, it is likely that the cost of future research is lower than the potential value of undertaking the research (in terms of level of uncertainty around the decision to adopt or not VR for TBI) and, thus, proceeding to a definitive trial might be of value.
Chapter 5 Process evaluation
Aims and objectives of the process evaluation
Process evaluations are recommended alongside trials of complex interventions to help understand and interpret outcomes and inform future implementation. 128 We conducted a parallel process evaluation, nested within the feasibility trial to (1) identify VR outcomes important to TBI survivors, (2) assess fidelity of implementation (e.g. enablers of, and barriers to, intervention deployment), (3) understand UC and (4) explore factors affecting the running of a definitive trial.
The process evaluation was to address the following questions:
-
Was the training seen as acceptable and useful by therapists who received it?
-
What changes are needed for a future trial?
-
Did therapists deliver the ESTVR intervention as intended?
-
What, and how much, was delivered (when, where and how often)?
-
What factors affected intervention deployment in each group?
-
Was the ESTVR intervention perceived as useful and acceptable by those (patients and employers) who received it and was UC considered acceptable by control participants?
-
To what extent was ESTVR already being delivered in UC?
-
What additional resources (NHS/social care/other) were used by intervention and control group participants?
-
What factors will influence the use of ESTVR in the context of usual NHS rehabilitation?
-
What factors will affect running a definitive trial?
-
What were the practical clinical issues for the screening, recruitment and consent of participants?
-
What were the practical difficulties, comprehensibility and emotional load required to complete outcome measures?
To inform the choice of primary outcome for a definitive trial an embedded study set out to identify:
-
What are the most important primary outcomes of VR for people with TBI from the perspective of newly injured and long-term survivors of TBI, service providers and employers?
Methodology
Approaches to obtaining process information
The process evaluation used quantitative process indicator data (content of treatment records, fidelity checklists, mentoring pro formas and clinical OT records) collected as part of the trial and qualitative data collection methods (interviews and focus groups with participants, employers and NHS staff).
Quantitative data collection methods
To tell us which ESTVR intervention components were delivered and how much time was spent in its deployment, ESTVR ‘content’ was recorded by the treating OTs following each intervention session using a pro forma developed by the authors85 and modified for use in this study (see Report Supplementary Material 2). Time spent delivering individual intervention components in 10-minute units was measured.
Therapists were also asked to maintain their usual clinical notes, which were photocopied and anonymised for analysis. At study end, an independent researcher extracted information from clinical notes that was not recorded on pro formas (e.g. referrals to other services).
To capture data on the frequency, content and impact of mentoring, mentors completed a mentoring record form (see Report Supplementary Material 7) following each mentoring session, which was signed off by both parties. Supplementary mentoring data were gathered from e-mails, text messages and written summaries of telephone calls. The frequency and total time spent in mentoring was calculated and qualitative data used to evaluate intervention fidelity.
The extent to which ESTVR occurred in usual care (the routine rehabilitation of people with traumatic brain injury)
To describe the extent to which ESTVR occurred in UC, we mapped local service provision before and after the trial intervention period using a self-administered postal questionnaire (see Report Supplementary Material 15) previously developed for an England-wide survey67 of VR for people with long-term neurological conditions (LTNCs) and adapted for TBI. The questionnaire was based on the best practice guidelines for VR for people with LTNCs. 63 The questionnaire included 44 items in two parts (A and B):
-
Part A explored funding sources, referral numbers and waiting times, black and minority ethic groups served, time established, vocational needs addressed and interventions provided, cross-partnership working, training, professionals involved in delivery, timing and nature of intervention delivered, audit, evaluation and factors influencing service development. Both general and specialist VR services for people with TBI completed this section.
-
Part B explored the components of the specialist VR in more depth, including assessment and intervention, relationships with other agencies and people involved in service delivery.
Most questions were closed or semi-closed. However, questions probing perceptions about resources and barriers to, and enablers of, service development required free-text responses and were analysed separately. Details of known VR services for people with TBI in the FRESH catchment (within 1 hour’s drive from each recruitment centre) were requested.
All respondents completed part A and only those identifying themselves as a ‘specialist VR TBI service’ in accordance to fit with a working definition completed part B:
A specialist VR service for people following a traumatic brain injury is characterised by, a multi-disciplinary team with expertise in TBI and expertise in VR who through shared education and learning and by working with employees and employers in the work-place can meet the needs of the majority of their patients/clients.
Reproduced with permission from Playford et al. 67
We used data from an earlier mapping study67 to identify VR providers in health services in each recruitment centre. We also used the knowledge of PIs and local therapists to identify UC providers. Questionnaires were sent in three tranches to services most likely to be involved in health-based TBI VR service delivery within 1 hour’s drive of each FRESH recruitment site. The three tranches were:
Tranche 1 – FRESH-trained therapists and PIs in FRESH sites.
Tranche 2 – services identified by FRESH therapists and PIs.
Tranche 3 – additional services in FRESH catchment identified by respondents to tranche 2.
To optimise response, services with no named recipient were contacted by telephone to identify a recipient. In services for which the respondents’ contact details were already known, questionnaires were sent by e-mail. A description of the mailing strategy is given below and illustrated in Figure 8:
-
questionnaire sent by e-mail and hard copy posted with personalised covering letter and stamped-addressed envelope (see Report Supplementary Material 15)
-
for questionnaires not returned within 2 weeks, reminder e-mail and questionnaire sent
-
subsequent reminder sent 2 weeks after the first
-
personalised e-mail thanking respondents on receipt of completed questionnaire
-
respondents asked to identify other local service(s) delivering VR for people with TBI. Questionnaires sent to services not previously identified, followed by steps a–d.
FIGURE 8.
Vocational rehabilitation service mapping. A4E, Action for Employment; DP, Diane Playford.
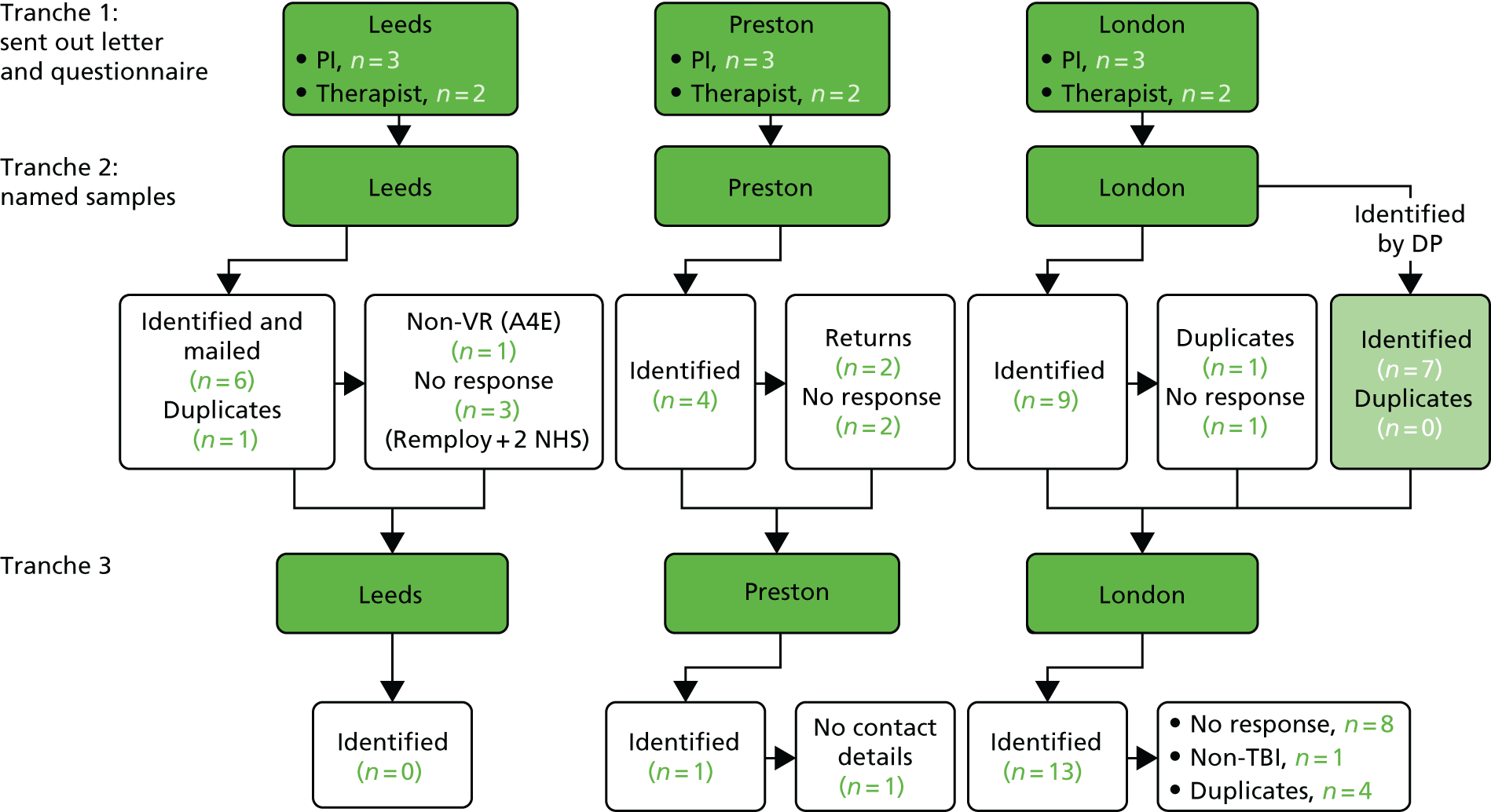
The e-mail address, covering letters and reminders were drafted in accordance with Dillman. 129 In London, additional services were identified in partnership with co-applicant Dr Diane Playford, who was conducting an online survey of VR services for people with all LTNCs in London. Questionnaires from respondents to that survey within the FRESH site 2 study catchment and who had identified themselves as providing VR services for people with sudden-onset neurological conditions (including TBI and stroke) were forwarded to the project team and data were extracted on those provided TBI services.
The project team identified services providing VR for people with TBI and, when doubt existed, contacted respondents for clarification. Only those providing VR services for people with TBI were included in this mapping survey. Identified services and respondents were asked to complete the questionnaire at study outset and at the end.
Data from questionnaires were entered into Microsoft Excel and descriptive analysis undertaken to identify service delivery patterns, service features and types of interventions offered.
The questionnaire enabled us to identify and describe components of VR service delivery in UC, differences between UC and ESTVR in each centre at study outset, and to describe changes in UC during the study’s course.
Content of usual care and ESTVR
To describe the content of UC and ESTVR, we used a combination of methods.
For patients randomised to the intervention, data from the content of treatment pro formas and OT records were coded to describe content, and resource use data from follow-up questionnaires were used to describe the additional support accessed by participants, as part of their ‘usual care’.
Patients randomised to UC completed resource use questions at 3, 6 and 12 months to identify the content of UC. Resource use is reported in Chapter 4.
Practical issues relating to the deployment of the intervention
To identify practical issues in deployment of the intervention during the trial, a clinical academic research fellow OT (JP) conducted 3-monthly ‘fidelity monitoring’ visits to NHS staff in recruiting centres to monitor:
-
intervention fidelity
-
practical clinical issues for participants’ screening, recruitment and consent.
Intervention fidelity data were gathered from FRESH therapists using an implementation fidelity checklist (see Report Supplementary Material 1) based on the training manual and ESTVR logic model (see Chapter 2). Each component of the ESTVR process was rated on a five-point scale (delivered ‘always’, ‘often’, ‘sometimes’, ‘seldom’ or ‘never’, where ‘always’ scored 1 point and ‘never’ scored 5 points). Fidelity monitoring aimed to ensure that the intervention was delivered as intended and to identify barriers to, and facilitators of, ESTVR delivery in each site.
In addition, qualitative interviews with treating therapists and participants were used to identify factors affecting intervention delivery.
Screening, recruitment and consent data were collected from NHS staff in recruiting sites and site screening logs, baseline data case report forms and consent forms, and by informal discussion during monitoring visits by Lancashire CTU trial managers.
Information from content pro formas and clinical notes was inputted into Microsoft Excel. To estimate the overall time spent in delivering ESTVR, activities were coded as face-to-face activity, travel, non-face-to-face activity (participant-related activity not involving direct face-to-face contact, e.g. telephone calls, letter writing) and administration (clinical note keeping). Pro formas were collected every 3 months by a RA (JP) during site fidelity monitoring visits. The number of face-to-face contacts per therapist was calculated from completed pro formas. Additional face-to-face and non-face-to-face contacts recorded in therapists’ clinical notes but not on a pro forma were calculated and combined to form total face-to-face and non-face-to-face contacts. Unrecorded travel time for visits documented in clinical notes was imputed based on a mean travel time per participant. If travel time was missing, the mean per visit for the individual therapist was used.
Qualitative data collection methods
Qualitative data collection methods included:
-
Focus groups and individual semistructured interviews with TBI service users’ and NHS staff with a role in managing, commissioning or delivering TBI rehabilitation (five in each site) to explore their views of:
-
the usefulness and acceptability of the ESTVR training package
-
the utility of materials/measures and recruitment methods
-
barriers to, and facilitators of, ESTVR implementation and contextual factors influencing sustainability and outcome success
-
factors affecting intervention deployment in each group.
-
Semistructured interviews with FRESH trial therapists were used to explore their views on the acceptability and usefulness of training and supporting materials and mentoring systems, perceived changes in practice resulting from training, and the anticipated and actual effects (including costs) of implementation on supporting services. A topic guide (see Chapter 2) informed by the logic model was used. Interviews addressed the OTs’ professional experience, effectiveness of the training and experiences of implementing it in practice and suggested improvements for future training.
Interviews were conducted by telephone by a researcher not involved in training delivery. Data collection was iterative, allowing for refinement of the topic guide between interviews.
A description of the methods used in developing the training package (manual, teaching and mentoring) and its content is given in Chapter 2.
-
Individual interviews with 30 service users (15 ESTVR and 15 UC) explored:
-
acceptability and usefulness of the ESTVR or UC intervention
-
practical difficulties, comprehensibility and emotional load required to complete outcome measures.
-
A matrix was created to identify and select service users from different sites with different demographics, injury severity levels and employer type.
Employers of trial participants randomised to ESTVR and who consented to their employer being approached by the research team, were invited to participate in individual telephone interviews lasting around 45 minutes. Employers were asked about the ESTVR intervention they had received to identify the most and least useful components. We anticipated interviewing between 10 and 20 employers as not all TBI participants have an employer or agree to employer contact.
Face-to-face or telephone interviews were conducted by one of three RAs (JH, JP or RM). Participants were invited by letter and followed up within 1 week by telephone. During the call, verbal consent was sought, participants were asked to sign and return a consent form and a date for the interview was arranged. Topic guides (see Chapter 2 and Report Supplementary Material 3) for all interviews were informed by the logic model.
-
End of study meetings were held in each recruiting centre. Participants, therapists and NHS staff involved in trial delivery were invited to discuss the study conduct and barriers to, and enablers of, future ESTVR implementation. These groups took place towards the end of the study and were facilitated at each centre by the chief investigator and a research fellow using a small working groups approach.
-
Primary outcomes of VR considered important to people with TBI, service providers and employers.
To identify primary outcomes of importance, we held focus groups and interviews with service users, service providers and employers. We interviewed trial participants prior to randomisation to explore what outcomes they wanted from VR. We held two focus groups: one with survivors of TBI (n = 10) of mixed severity and at different times since injury, identified in partnership with Headway; and one with TBI service providers in health (n = 10), including therapists and other rehabilitation providers recruited via specialist conferences and special interest groups. We interviewed employers with experience of employing a person with TBI (n = 10). They were recruited via service providers, people with a TBI, a regional occupational health group and by writing to local businesses. Interviews and groups explored the notion of important outcomes of a health-based VR intervention for people with TBI in response to the following question:
Currently not everyone who suffers a TBI receives NHS support to help them return to work. If you were to receive a NHS service designed to help you return to work after brain injury, what would be the most important outcomes for you?
Group participants were asked to write down what they considered the most important outcomes, without conferring. Nominal group technique130 was used to prioritise identified outcomes. As with the Delphi process, it aims to achieve group consensus and facilitate equal participation.
Trial participants were asked the same question in face-to-face interviews with a RA prior to randomisation. Responses were recorded as free text, entered into Microsoft Excel and thematically analysed. Two researchers discussed the themes to identify and agree priorities.
Owing to availability and travel constraints, employers were questioned by e-mail or telephone. Identified outcomes were typed and returned to respondents by e-mail to confirm accuracy. Duplicate responses were combined into a single outcome statement with a definition. All outcomes were then collated and returned to employers to check accuracy. Employers then ranked their top six outcomes and the amalgamated outcomes for all employers were returned to the group by e-mail for verification and amendments until consensus was reached.
Data from the four groups were combined with data from qualitative interviews, held later with trial participants, and actual outcome data from the feasibility trial to identify possible primary outcomes for a definitive trial.
All interview and focus group participants were afforded a minimum of 24 hours to read participant information sheets and the opportunity to ask questions before written consent was sought.
Qualitative data analysis
The ESTVR logic model was used to inform the design of data collection tools (interview topic guides and fidelity checklists) and to guide qualitative data analysis and interpretation.
Interviews were digitally recorded and field notes made to capture inaudible or other contextual information. All interviews and groups were fully transcribed and data uploaded to NVivo version 10 (QSR International, Warrington, UK) software for management. Analysis was conducted by at least two research team members. It was an iterative process that involved reflection on data collected, refinement of the topic guide for subsequent interviews and iterative testing of interpretation through research team discussions. Analysis used the Framework approach131 and combined both inductive (new insights emerging from the data) and deductive (informed by the logic model) approaches.
Results
Was the training seen as acceptable and useful by the therapists who received it?
Interviews with FRESH therapists took place approximately 4 months after they had seen their first patient (range 2–6 months) and 7 months after the initial training. All therapists considered the training useful and considered that all three elements of the package (training, manual and mentoring) were essential. Training increased therapists’ confidence to deliver ESTVR, but they requested more opportunities for discussing cases. They liked the training manual but needed support to contextualise the contents and relied on it less over time. The most valued aspects of the package were case discussions and mentoring to support individual tailoring and local implementation.
The training was intended to upskill therapists already experienced in working with people with a brain injury, with VR knowledge. However, four out of the five OTs already had VR experience. Three qualified in countries where it is widely implemented.
For contingency reasons, we made video recordings and electronic copies of training materials to enable therapists who could not attend or wanted to revisit the training to do so. However, NHS computer systems prevented downloads and access to links. Subsequently material was provided on USB. It is not known whether or not the therapists made use of these resources. Although videos are useful, the therapists preferred face-to-face learning.
The manual was seen as integral to the training package. It was designed to be adapted for local use by adding details of relevant NHS rehabilitation, TBI and employer support services, referral routes and assessment tools. The therapists valued the detailed description of the intervention in the manual describing it as a ‘VR bible’ (OT3), but some forgot about some resources between initial and refresher training.
Although therapists identified learning needs relating to the clinical intervention, they were more concerned about delivering it as part of a trial and their role in the trial. Information about the trial design and issues that might affect outcomes (e.g. contamination) was included in the training, but more time should be spent on this in future as NHS therapists have limited research experience. Mentors reinforced information provided in the training and helped problem-solve trial-related issues.
Qualitative evaluation of the training package suggests that the OTs absorbed the knowledge necessary to implement ESTVR. Therapists found the training package acceptable and useful, and highlighted factors important to consider when developing and delivering training packages for future rehabilitation trials. These included spending more time discussing research processes and individual cases, including more details about the study in the manual, and reducing the delay between training and intervention delivery.
Interviews also identified releasing NHS staff as a barrier to running a future trial:
They’ve got no leeway to release anyone . . . They just are running on such low staffing levels that they can’t afford, even if they’re being funded for it, they can’t afford to release someone for a day a week.
OT4
Did the therapists deliver the ESTVR intervention as intended?
What and how much was delivered (when, where and how often)?
This section describes the early specialist TBI VR delivered using Template for Intervention Description and Replication (TIDieR) reporting guidelines. 132
Participants
Thirty-nine participants were randomised to receive the intervention. One did not respond to attempts to contact; therefore, 38 were seen by the four FRESH OTs (Table 26) and their intervention records (content pro formas and therapy notes) were included in the analysis.
| Therapist | Severity of TBI | No GCS score recorded | ||
|---|---|---|---|---|
| Severe (GCS score of 3–8) | Moderate (GCS score of 9–12) | Mild (GCS score of 13–15) | ||
| A (n = 11) | 2 | 1 | 7 | 1 |
| B (n = 6) | 2 | 1 | 3 | 0 |
| C (n = 4) | 1 | 1 | 2 | 0 |
| D (n = 17) | 5 | 4 | 7 | 1 |
| Total | 10 | 7 | 19 | 2 |
The mean age of participants was 40.4 years (SD 6.3 years, range 16–62 years); 87% (n = 33) were male, 50% (n = 19) were classified as having a mild TBI and 27 (71%) were working full-time prior to injury. The demographic characteristics of intervention group participants are given in Table 27.
| Demographic characteristic | Severity of TBI | No GCS score recorded | Total | ||
|---|---|---|---|---|---|
| Severe (GCS score of 3–8) | Moderate (GCS score of 9–12) | Mild (GCS score of 13–15) | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| N | 10 (26) | 7 (18) | 19 (50) | 2 (5) | 38 (100) |
| Age (years) mean (SD), range | 42.2 (16.3), 26–62 | 38.0 (15.7), 17–61 | 38.4 (10.9), 16–54 | 58.0 (4.2), 55–61 | 40.4 (16.3), 16–62 |
| Gender | |||||
| Male | 9 (90) | 6 (86) | 16 (84) | 2 (100) | 33 (87) |
| Female | 1 (10) | 1 (14) | 3 (16) | 0 (0) | 5 (13) |
| Marital status | |||||
| Married/living with a partner | 6 (27) | 3 (14) | 12 (54) | 1 (5) | 22 (58) |
| Single | 4 (40) | 3 (43) | 6 (32) | 1 (50) | 14 (37) |
| Divorced | 0 (0) | 1 (14) | 1 (5) | 0 (0) | 2 (5) |
| Cause of accident | |||||
| RTA | 4 (40) | 3 (43) | 5 (26) | 1 (50) | 13 (34) |
| Fall | 5 (50) | 2 (29) | 10 (53) | 0 (0) | 17 (45) |
| Assault | 1 (10) | 2 (29) | 3 (16) | 0 (0) | 6 (16) |
| Othera | 0 (0) | 0 (0) | 1 (5) | 1 (50) | 2 (5) |
| Work status | |||||
| Full-time | 10 (100) | 4 (57) | 12 (63) | 1 (50) | 27 (71) |
| Part-timeb | 0 (0) | 0 (0) | 5 (26) | 0 (0) | 5 (11) |
| Flexible hours | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 1 (3) |
| Not specified | 0 (0) | 2 (29) | 0 (0) | 1 (50) | 3 (8) |
| Unemployed | 0 (0) | 1 (14) | 1 (5) | 0 (0) | 2 (5) |
Data analysed
In total, 38 sets of OT records (notes) and 699 (42–248 per therapist) content pro formas were included in the analysis.
When and how much intervention was delivered?
The mean time from injury to first face-to-face contact with a FRESH therapist was 35.3 working days (range 7–97 working days). The mean variation between therapists was 17.9 working days (range 26.8–44.7 working days).
Duration, intensity and dose
The intervention lasted approximately 8 months (mean duration 173.4 working days, range 8–327 working days). This varied between therapists from 164.9 to 197.2 working days (Table 28).
| Therapist | Time period, mean (SD), range | |||
|---|---|---|---|---|
| From randomisation to first contact | From injury to first contact | From injury to discharge | Duration of interventiona | |
| A | 4.6 (4.1), 1–16 | 25.4 (14.7), 5–54 | 203 (88.4), 34–304 | 182 (88.8), 8–267 |
| B | 8.5 (7.4), 3–22 | 41.3 (37.9), 11–92 | 202.5 (135.9), 73–417 | 166 (108.0), 59–327 |
| C | 8.5 (3.5), 5–12 | 19.0 (16.6), 5–43 | 210.5 (66.4), 151–287 | 197.2 (72.6), 125–280 |
| D | 9.4 (6.0), 2–24 | 24.4 (12.2), 4–41 | 186.5 (92.8), 44–294 | 164.9 (92.4), 17–275 |
| Total | 7.8 (5.7), 2–24 | 26.8 (19.6), 4–92 | 196.3 (93.7), 34–417 | 173.4 (89.2), 8–327 |
There were no significant differences in intervention duration according to TBI severity, although participants with a mild TBI received the longest duration of intervention [190.2 working days (SD 91.17 working days), range 8–327 working days] and participants with a severe TBI received the shortest duration of intervention [154.2 working days (SD 84.4 working days, range 22–267 working days)]. For participants with a moderate TBI, intervention lasted a mean of 164.7 working days [SD 100.6 working days (range 17–262 working days)] (see Report Supplementary Material 16, Table 1).
Face-to-face visits
The mean number of face-to-face visits was 6.3 (range 5–9.5 visits) (see Report Supplementary Material 16, Table 2). Seven participants had > 10 visits. The number of visits was unrelated to TBI severity. Twenty (8%) face-to-face visits were recorded in therapists’ clinical notes but not on the content pro formas (range 1–9 visits per therapist).
How much intervention was delivered? (dose)
The number of visits per participant was highest in the first month then reduced in frequency. In month 1, the mean number of face-to-face visits per participant was 1.5 (range 1.27–1.72), and in month 12 it was 0.21 (range 0–0.31).
How often did the interventions occur? (frequency)
Intervention was most intense at the outset of the intervention period but frequency reduced over time. People with a more severe TBI appeared to receive less support than those with milder injuries. In month 1, participants with a severe TBI received, on average, 0.50 visits (range 0–2 visits), people with a moderate TBI received a mean of 2.4 visits (range 2–9 visits) and those with mild TBI received a mean of 1.68 visits (range 2–13 visits). In month 12, participants classified as having a severe TBI received, on average, no visits and those with a mild or moderate TBI received, on average, between 0 and 2 visits (mean 0.31 visits and 0.28 visits, respectively).
How did FRESH therapists spend their time?
Overall, 60% per cent of therapists’ time was spent in patient-facing and patient-related activity, 16% on administration and 24% on travel (Figure 9).
FIGURE 9.
Overall time spent by all the therapists per participant.

The proportion of time spent in patient-facing and patient-related activity was similar for each therapist (therapist A = 65%, therapist B = 58%, therapist C = 54% and therapist D = 59%). There was a difference in the amount of time therapists spent travelling. This ranged from 14% to 34%. London-based therapists spent almost 15% more time in travel. Injury severity did not appear to influence intervention time. The combined amount of face-to-face and non-face-to-face time was similar, ranging from 60% to 64%.
What intervention was delivered? (content)
Therapists’ activity was measured against the core components ESTVR identified in the logic model. One ’core component’ involved working with employers in supporting the TBI participants’ RTW. However, FRESH therapists had direct contact with only 14 (37%) of participants’ employers, non-direct contact (when the participant acts as a conduit of information between the therapist and employer) with 20 (53%) employers and gave ‘advice only’ (when the patient is advised on how to broker their own RTW with an employer) to four (10%) participants. The extent to which the therapists engaged directly with employers differed between therapists.
The main focus of the intervention received by trial participants was help in preparing for a RTW and assisting in the RTW (Figure 10). Very little therapy time was spent on personal or domestic ADL or non-work-related physical activities. Time spent dealing with current issues, goal setting and providing psychological support varied between therapists. However, this may be related to differences in the way therapists classified their activity.
FIGURE 10.
Components of occupational therapy face-to-face sessions.
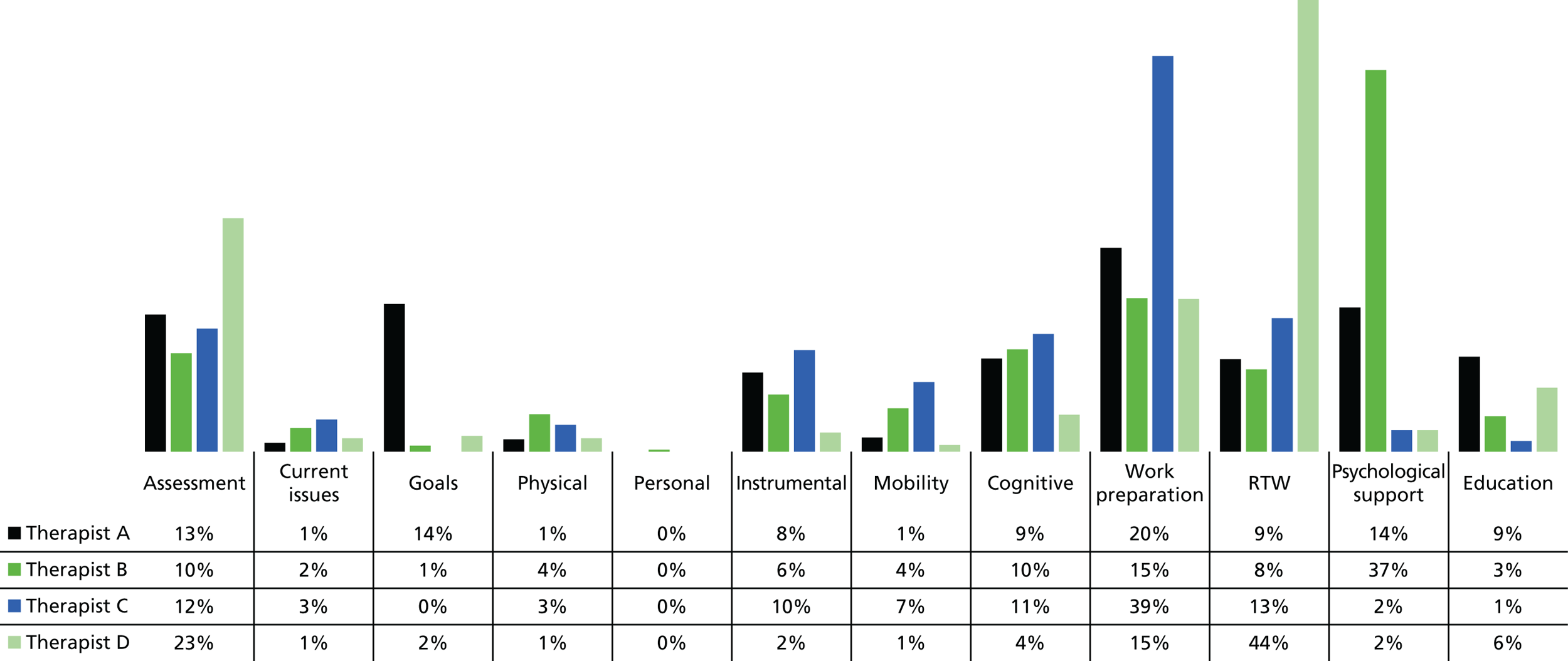
Overall, therapists spent most of their non-direct contact time dealing with participants, families and employers, followed by occupational health and human resources (Figure 11). They also liaised with other health professionals, specifically other OTs and psychologists. Nobody reported having contact with DEAs or other DWP services. There was little liaison with solicitors or other private providers. Other liaison included alcohol services and the school of a parent with a TBI.
FIGURE 11.
Non face-to-face time. Other health professionals refers to CM, physiotherapist, psychologist and cognitive–behavioural therapist. HR, human resources; OH, occupational health.

Other services involved
For 25 (71%) participants, the FRESH therapist was the first point of rehabilitation contact. However, five (13%) participants were visited by a neurorehabilitation or ABI team member before the FRESH OT. FRESH OTs referred 20 participants (53%) on to other services including Headway, brain injury or community neurorehabilitation teams, DEAs or Access to Work. At 12 months, therapists made onward referrals for four participants who they felt needed ongoing support. Referrals were for community brain injury or neurorehabilitation teams, musculoskeletal physiotherapy and neuropsychology.
Where did the intervention take place?
Most (56%) intervention took place in participants’ homes; 11% took place in the workplace and 13% took place elsewhere. The venue was unrecorded in 20% of therapy records.
Tailoring the intervention
Intervention was individually tailored in dose, frequency and intensity to suit participants’ needs and the local therapy context. Some participants were self-employed, which meant that there was no employer to liaise with. Tailoring was also evidenced in the pro formas and the therapists’ clinical notes as variations in the way ESTVR was delivered. For example, some participants who had returned to work early were too busy to meet the therapist in person at the intended frequency and the therapist had to find alternative ways of working to deliver the necessary support (e.g. by telephone). In addition to the expected individual tailoring, three participants had much more intervention in terms of the dose, frequency and duration than the others. Two participants had returned to their former employment but were experiencing difficulties associated with their TBI, workplace relationships and specific work tasks. A third, who incurred a severe TBI and for whom local rehabilitation services were lacking or non-existent, required extra help in working towards the vocational goals and the participant and family members needed education and emotional support to adjust to and manage the TBI impact. This person was unable to sustain work.
Intervention fidelity
Fidelity checklists (see Report Supplementary Material 17) for each therapist suggest that the intervention was delivered as intended and that the core ESTVR process was almost ‘always’ or ‘often’ followed by all therapists. However, there were differences in the ways therapists worked, which were influenced by caseload and participants’ individual needs and circumstances. For example, one therapist’s caseload involved few people with an employer or who permitted employer engagement and one participant with long-standing alcohol problems who was previously self-employed and for whom a RTW was not possible. This meant that some components (e.g. ‘advice not to return to work too soon’ and ‘establishing and maintaining contact with the employer’) could not easily be assessed. The two private-practice therapists seldom made contact with participants while they were still in hospital as early discharge limited opportunity. The parts of the process that were least often followed were completing the case management assessment form and ‘progress monitoring’. However, as the FRESH therapists were fulfilling both the CM and OT role, they felt no need to duplicate effort by completing both the OT and the CM assessments.
The OT mentor (JP) who conducted the fidelity monitoring visits confirmed that standards were met during the trial. However, therapy delivery at one site raised concern as risk assessments of the home were not routinely conducted and the checklist highlighted concerns about whether or not interventions were explicitly work focused. This was closely monitored and extra support provided through mentoring until the monitor was satisfied that the intervention was being delivered as intended.
Mentoring
Most (74%) mentoring was delivered by e-mail or by telephone (22%). Total mentoring time per therapist ranged from 365 to 2214 minutes over the trial duration. Mentoring frequency was flexible and driven by therapist need. Therapists could approach mentors for additional advice outside agreed mentoring sessions. Such ad hoc sessions tended to be shorter (≈10 minutes) than pre-planned sessions lasting 1 hour.
The FRESH therapists required between 5 and 28 minutes (mean 14 minutes) of mentoring per 7.5 hours of intervention delivered and received between 68 and 201 minutes per participant. Additional support was needed for a therapist with no previous community rehabilitation experience.
In total, 136 e-mails, 40 telephone calls and seven face-to-face meetings were recorded as mentoring activity. A mentoring record form was completed for each telephone call and face-to-face meeting. E-mails were treated as a record. Therefore, 183 mentoring records were available for analysis.
Mentoring supported intervention fidelity, problem-solving in individual cases and local implementation in the context of the trial.
The mentoring records offered insight into the interaction between the FRESH OTs and mentors, and the quality of the intervention. The FRESH OTs sought mentors’ approval on decisions, activities and reports as a means of quality assurance. In particular, they wanted feedback on reports to employers or occupational health providers to ensure that the language and structure were appropriate. Mentors offered solutions to problems and made suggestions for enhancing intervention delivery, for example suggesting that the FRESH OT ask the rehabilitation consultant to provide a report for an employer.
Mentoring also acted as a portal on the trial, highlighting local issues with screening and recruitment and communication, and early warning of participants considering withdrawal.
In this study, four mentors who had worked together to develop and deliver the training package met to discuss and agree the handling of issues arising during mentoring. In a future trial, mentors may require training to ensure consistency in approach, opportunity to discuss handling of sensitive issues (e.g. trial therapists competency), guidance on the mentoring role and purpose (i.e. performance mentoring rather than other types of mentoring), and use of time. The mentoring record form could be modified to record fidelity issues routinely and highlight potential clinical implementation issues.
Did the intervention meet patients’ and employers’ expectations?
Acceptability was measured by interviewing 30 trial participants, six employers and the four FRESH therapists to seek their views on the interventions (ESTVR and UC) and, in the case of patients and staff only, their views on recruitment and the acceptability of randomisation. We sought to understand what service interventions were most valued in practice by an employee with TBI and which by an employer.
Patients
One-third of participants interviewed had incurred a mild TBI and two-thirds had either a moderate or a severe TBI.
Most participants interviewed described significant problems as a result of their TBI. While work was clearly the most important and valued outcome among trial participants, social and leisure activities and work–life balance were also considered important:
Well I was studying at the time, when I fell, and I. I mean, I was doing night school, so I was working and then going to night school. I think although it’s been hard to come to terms with not being able to do everything that I did, I think work is ultimately the most important. And then doing one or two things at the weekend is fine but yeah, ultimately work
2017
I took, I still do take great delight in little improvements, such as a little victory little challenges. For me, it was a big thing when I could go shopping by myself, when I could read a book, watch a film, meet friends, not in my front room with no noise, no distractions, just meet friends down the pub or in a café, for instance, to begin to return to normal life.
2019
She helped me with writing out a timetable for me. I mean, one of the problems that I get is just motivation. I have a tendency for my mind to go blank or to lose the thread easily. I can lose track of what I’m supposed to be doing.
2020
Almost all participants interviewed valued the intervention and found it useful. They particularly valued the practical nature of the support including the therapists’ letters to the participant and employer, support in preparing for work return, negotiating a RTW and support once back at work. They valued clear, simple explanations about TBI as a follow-up to information provided in the aftermath of the accident:
She came to two meetings with me at [workplace]. . . . we’d meet beforehand and we’d talk about it and then she’d go away and e-mail me a letter that she’d written and then I’d change or just tell her what wasn’t quite right, and then she’d change it and then put it together in a very compact way and then print it off and bring it.
1006
She was very good at giving practical suggestions. I mean, the guy in East XXX was also excellent, but maybe because of my condition at the time, he was more philosophical. FRESH OT was also happy to have a philosophical conversation but would also say, ‘You need to write yourself a short to-do list. You need to work out a way of filtering out all unnecessary distractions around you so that you’re not distracted.
2019
One person was unhappy that he was unable to return to work after disclosing his brain injury and seizures to his employer. Three people found the intervention intense or felt, at times, overwhelmed with information. One patient could not be contacted following randomisation, two patients withdrew and a further two disengaged from ESTVR. This is about the same as might be expected in usual NHS rehabilitation and is consistent with TBI other studies:
She helped me with writing out a timetable for me. I mean, one of the problems that I get is just motivation. I have a tendency for my mind to go blank or to lose the thread easily. I can lose track of what I’m supposed to be doing, FRESH OT helped me get some structure to my day and it certainly is an aspiration, yeah.
2020
What were the practical difficulties, comprehensibility and emotional load required to complete outcome measures?
None of the participants interviewed indicated that randomisation was unacceptable or declined participation because they wanted to ensure randomisation to the intervention group:
Yes, she has, yeah. Before we got into the HR element of coming back to work and seeing the [work] doctor – she’s been present at all those meetings – we met before and we discussed about what’s actually possible and how to approach the meetings and what to put on the table, basically, and say this is what I can do. Yeah, she’s been there and she’s supported me and, like I said, just coming along to the meetings and things.
3002
The main problems reported by participants regarding the practical difficulties, comprehensibility and emotional load required to complete outcome measures were concerned with participants’ memory problems. Many of the patients asked could not recall receiving or completing questionnaires, or said that they forgot to complete it because they were too busy. Two reported needing help to interpret questions or complete questionnaires.
In several cases, responses were complicated by the fact that the brain injury was only one of a number of serious injuries incurred at the same time and participants were not sure how to respond to the questions when factors other than the brain injury limited their abilities.
Research assistants who completed baseline measures with participants found questions to be repetitive and reported that participants provided different responses to similar questions.
Employers
Employers believed that the intervention was extremely valuable for both the employer and employee. They had little, if any, experience of supporting a person with TBI to return to work. They valued timely communication and the FRESH OT visiting the workplace to gain an understanding of the organisation and context, and to understand how the employee would cope. They appreciated the therapist acting as an advocate for the employee and arranging meetings between the parties. They valued learning about TBI and its impact on the employee’s work ability. Employers appreciated advice about adaptations. They welcomed practical advice in planning a phased RTW (e.g. a RTW timetable), guidance about which work tasks to begin with and how to upgrade tasks, and advice on legal requirements regarding driving. They valued the opportunity for progress monitoring following a RTW to allow setbacks to be overcome and reassessment of the employee’s status over time. Employers recognised the cost attached to supporting a person with TBI to return to work but they were not able to quantify this and reflected that, on the whole, the benefits outweighed the costs:
. . . I talked to [recruiter] about that when she’s been and also sent her an e-mail afterwards. Two things, one is they’re really difficult to fill in on paper because you can’t distinguish between the brain injury and the other injuries, there were lots of questions – I can’t remember all the questions – for example, there’s a question about driving. I can’t drive but it’s got nothing to do with my brain injury, and you can’t distinguish that on the questionnaire.
2019
What factors will affect the running of the definitive trial?
What were the practical clinical issues with regard to the screening, recruitment and consent of participants?
There were a number of difficulties associated with the running of the trial, particularly during setup, and issues with screening, recruitment and consent.
During setup, delays in appointing research staff, staff sickness and difficulties in securing Excess Treatment Costs from the newly reconfigured Clinical Commissioning Groups (CCGs) delayed the start of recruitment. Despite considerable effort in negotiation, the deployment of network support failed to materialise in one site, despite adoption.
Patients fitting the inclusion criteria for the study were sometimes not admitted to a designated trauma unit and were therefore, geographically dispersed across the recruitment site.
The identification and screening of potential participants was complicated as recruiting sites kept incomplete clinical registers. In two sites, recruiting staff had no access to A&E lists to identify patients admitted with head injury/TBI or the TARN register to cross-reference participants on the screening log.
Identifying people who had sustained a TBI was problematic. Not all those who sustain a head injury are scanned for brain injury and not all injuries to the brain are present on a scan. For this reason, it was not a requirement of the study that participants’ TBI was confirmed by CT. Instead, we relied on local clinical procedures for diagnosis. This lack of a clear TBI definition may have affected eligibility and inflated the proportion of patients screened. In one site, there was no designated trauma ward and, therefore, no simple mechanism for identifying potential participants (TARN registers are often retrospectively completed and capture only patients admitted for ≥ 72 hours).
The establishment of ‘hub and spoke’ MTCs at the same time as the trial compromised recruitment. The ‘hub and spoke’ nature and new care pathways meant that people admitted to the units with moderate and severe injuries were rapidly repatriated elsewhere and could not be recruited. This affected the proportions of people recruited with either moderate or severe TBI and who are, arguably, most likely to benefit from the intervention.
Mild TBI was sometimes missed or poorly recorded in patients admitted with other severe injuries.
The characteristics of the local trauma populations differed between sites. In one site, the majority of potential participants were already at, or near, retirement age and local unemployment was high. In another, the workforce was transient and affected by migration, and the potential participants less likely to be in long-term relationships, which affected carer recruitment.
We also found that staff needed fairly detailed training in how to recruit a person with a TBI and in how to describe the ESTVR intervention. An additional training workshop was run with knowledge sharing around best practice and ‘top tips’ for recruitment. Additional resources were generated to aid recruiting staff.
Research staff at the end-of-study meetings reported that assessing participants’ capacity to consent relied on clinical NHS staff providing guidance but, often, information was conflicting. They reported concerns that some people were incapable of providing informed consent because of the behaviours being displayed (e.g. incoherent, falling asleep) and, therefore, waited until behaviours changed. PTA needs to be measured to avoid complications.
In one site, the amount of study paperwork was considered overwhelming and to have interfered with actual time available to approach participants.
Staff in each site were highly motivated to achieve recruitment targets. However, the flexibility and skills of recruiting staff may have differentially affected recruitment. Childline training helped one RA talk to, and recruit, some otherwise ‘hard to reach’ people. Flexible working enabled one RA to recruit people during visiting hours (with family present) in the evenings and at weekends.
Recruitment was further complicated because many TBI patients often feel well while in hospital early after injury/at the time of recruitment and may not anticipate having difficulties with resuming work or perceive the need for rehabilitation and, therefore, considered the study not relevant to them.
End-of-site visits and interviews with therapists and therapy managers revealed several factors that may affect the running of a future trial.
-
Local PIs and site-specific research staff needed additional training in screening and in how to administer baseline measures.
-
The internal transfer of participant information (demographic data, cause of TBI and relevant safety information) between recruiting staff and FRESH OTs sometimes took up to 10 days. E-mails facilitated rapid sharing of safety information and enabled therapists to monitor new participants and gauge workload. Creating a NHS e-mail address for the private therapists in one site facilitated the passage of information and communication with other service providers.
-
Recruiting or deploying experienced OTs to the trial was problematic (despite securing Excess Treatment Costs). Senior therapy managers were extremely busy and lacked research knowledge, resulting in delays in appointing and training therapy staff. Service restructuring resulted in fewer experienced therapists in one site and limited options to backfill therapists’ existing clinical caseloads when resource was deployed to the trial. This led to clinical team frustration. Line managers suggested that increasing the number of hours allocated to the FRESH OT role would be both more attractive to potential FRESH OTs and force senior managers to ensure adequate cover for existing service demands. One line manager believed that existing services were expected to absorb the commitment of the trial therapists’ allocation of time to the study, within existing resource.
What factors will affect the use of ESTVR in the context of usual NHS rehabilitation?
Interviews were held with 15 NHS staff with a role in delivering, managing or commissioning TBI rehabilitation (five in each site).
Macro-level societal gaps were identified in rehabilitation pathways for people with TBI and this was echoed by the UC participants interviewed.
Community neurological rehabilitation experience was seen as essential for therapists delivering ESTVR; even FRESH OTs with community experience initially found it challenging to work outside their normal geographical boundaries.
A communication gap between occupational therapy services and commissioners poses a threat to the future delivery of VR for people with TBI.
Occupational therapists and their managers felt that the intervention was worthwhile but believed that commissioners needed to be convinced. They felt that they had little influence over commissioning. Commissioners agreed that VR was likely to be beneficial, but were unlikely to commission services that were both specialised and highly specific. Some commissioners believed VR was already being provided under general rehabilitation service level agreements. However, NHS therapists were unclear whether or not they were permitted to deliver VR.
To what extent was ESTVR already being delivered in usual care?
Mapping
Pre trial (round 1)
In tranche 1, three services were identified by PIs and therapists in site 3, two in site 1 and two in site 2. All were sent questionnaires.
A further six services were identified by respondents from tranche 1 and mailed but one was a duplicate and one not a VR service. All were mailed. Three did not respond to multiple attempts to follow up (one was a Remploy service and two were NHS services). In site 1, four additional services were identified by respondents and mailed a questionnaire; two responded and two did not. In site 2, nine services were identified by respondents in tranche 1, one was a duplicate, eight were sent questionnaires, seven responded and one did not.
In total, 36 services were identified and sent questionnaires, and 24 responses were received. Eight (33%) services identified themselves as a specialist VR service provider and completed part B, which included more detailed questions about the components of the VR service delivered. Six of the respondents were based near site 2 and two near site 3.
None of the site 1-based services identified themselves as a specialist VR provider.
Some respondents to part A, who indicated that they were not a VR specialist service, said that they were working towards it or that they offered help in RTW as part of a multidisciplinary neurological, community or trauma rehabilitation team. Some indicated that while they were not advertised as VR specialist, they were TBI specialist and OTs addressed work needs routinely under ‘key life roles and occupational performance’ areas.
Four part B respondents also said that they were ‘not VR specialist’ but indicated fit with the definition given. Most indicated that they provided VR as part of a multidisciplinary rehabilitation or occupational therapy service and VR was only a small part of their role. This was either because (1) the patients were significantly disabled both physically and cognitively and many would never be able to access vocational roles, (2) staff and financial resources for providing VR were limited or unfunded (in the case of one charity), (3) they were a unidisciplinary service and did not have close links with local job coaches or other multidisciplinary team members (e.g. SALT) and (4) VR was not seen as a priority for the service and could not be provided unless the client had needs for multidisciplinary rehabilitation, which the service was funded to provide. Therefore, people who only needed VR could not access it.
We cross-referenced the identified services with the sites, which indicated that one of the FRESH participants in site 2 might have been able to access three out of six services identified (the others were not from the area). However, all of the services identified had their own referral acceptance criteria and access was dependent on postcodes and local funding agreements with the CCGs.
In site 3, one of the identified services could only be accessed by clients with complex needs. A need for help with RTW alone was not sufficient to access the support; therefore, people with mild or moderate TBI were likely to have been excluded. A neighbouring TBI-specific service in site 3 offered a very comprehensive range of VR services on the basis of partnership working with DWP services. It indicated that it had access to placement officers, job brokers, job coaches, employment advisors and occupational health and careers advisors in addition to the core rehabilitation team, which included clinical psychologists, psychology assistants and access to a doctor of rehabilitation medicine. However, the VR aspect of the service was relatively newly established and the actual funded resource limited to OT and psychology. Three of the FRESH trial participants would have met the eligibility criteria for this service.
What did they do?
Only one of the identified services was a VR-specific service, which was run by a charity in site 2. It offered a comprehensive VR service, including help to access work and work placements, job brokerage and financial advice. However, this fell outside the FRESH participants’ catchment.
The other VR specialist services identified provided VR as part of a community rehabilitation or occupational therapy service, specialising in TBI or neurological conditions and VR was only a small part of the service remit.
Professions working within a service
All of the services had occupational therapy and most had physiotherapy and clinical psychology input. Two also had access to a cognitive–behavioural therapy and one had a nurse team member. Only two had access to, or employed, employment professionals such as careers advisors, placement officers and job coaches or brokers. Only one service (site 3) had access to social workers, VR CMs or ergonomists. Most had access to a doctor by referral. One, a site 3-based charity and the only VR-specific service, had a careers advisor, placement officer, medical and psychological input, job coach and job broker.
Components of assessment
All of the services indicated that they offered a comprehensive assessment of a person’s vocational needs, which took into account education and personal and employment history, past and current rehabilitation, the impact of the TBI on cognition, sensation, physical function, behaviour, psychological and emotional well-being, vocational interest and work goals. They also assessed the demands of the current work role and work skills, reviewed employer feedback, carried out observational assessments on a person’s work performance, behaviour and attitude, assessed physical and environmental barriers to work including access and equipment, and carried out an assessment of the job role and person specification.
None of the services offered residential assessment, only one offered medical examination, two did not conduct functional capacity for work and only three offered simulated work assessment opportunities or assessed finances.
Relationships with other agencies
The services all liaised and referred clients to and from other agencies including GPs, NHS rehabilitation services, occupational health, DWP, social and educational services, and employers. One service stated that it accepted referrals from NHS service professionals only.
Vocational interventions
All eight services provided most aspects of a comprehensive specialist VR service. The majority offered VR in addition to TBI rehabilitation to people who had jobs to return to and to those who did not. All except one went into or liaised with the workplace. The service that did not offer work site visits, work with coworkers or give advice about physical adaptations, was a charitable sector service that was VR-rather than TBI-specific and specialised in finding new work, including training in transferable skills for work and help with job seeking. It offered no rehabilitation off-site for a specific job or role. One NHS provider offered no advice about assistive technology and two were unable to help with job progression once a person had returned to work and wanted to change jobs. Only half of the services offered follow-up to monitor progress. Few of the services offered careers advice or supported access to training schemes and work placements for people who were out of work. All said that they helped people withdraw from work.
Post trial (round 2)
At the end of the trial, 38 services were identified from existing responses and by following the same procedure as in round 1. All were sent a questionnaire and 26 responded. In total, 14 services were identified as a VR-specialist service for people with TBI from having completed part B in round 1 or 2. Of the 26 respondents in round 2, only six services identified themselves as a specialist provider. One additional service identified as specialist in round 1, indicated that it no longer had the resources to provide VR.
Four of the respondents were the same specialist VR providers as identified in rounds 1 and 2 were newly identified specialist services (one was one of the private-practice therapists who delivered the trial intervention site 2). The second was a service that did not previously identify itself as a specialist provider.
Five of the services were based geographically close to site 2 and one was near site 3. Of the specialist service providers identified but who did not respond, three were NHS services located near site 1, two near site 2 (one NHS, one charitable sector) and two near site 3 (one NHS, one employment sector).
The descriptions of the VR provided by these six services were as described in round 1. All were specialist providers of VR for people with TBI. Most were NHS services of which the VR was only one component of a TBI, neurological rehabilitation or occupational therapy service. They all adopted a multidisciplinary team approach to meeting the work needs of TBI survivors. However, the VR was usually delivered by one or two key professionals, which in the NHS were mostly OTs and psychologists, with some physiotherapy and in the charitable or independent sector, OTs working with other employment specialists, including job coaches and CMs. Not all of these services could be accessed by FRESH participants.
Summary
Our findings suggest that some services providing VR for people with TBI are located geographically close to each site. Some were NHS-based; others were in the charitable sector. Most were NHS community neurological rehabilitation services, some were brain injury specific. The majority provide rehabilitation to a select few people with complex needs resulting from TBI as part of usual NHS rehabilitation. Access was determined by postcode and tightly defined referral criteria, geographical boundaries and complex commissioning arrangements. This meant that the VR, which was resource limited and not the primary purpose of the service, was restricted to selected people fitting the criteria. In some cases the primary purpose of a service was multidisciplinary rehabilitation and a need for help with RTW alone was insufficient to access support, with the result that people with mild or moderate TBI were likely to have been excluded. Cross-referencing of the mapping data with trial participants in the two sites where specialist services were identified suggests that only four trial participants may have met the criteria for referral.
Therefore, specialist VR services existing within geographical proximity to sites could not routinely be accessed by trial participants as part of UC. These findings might explain why so many of the UC patients we spoke to said that they had not received any help with returning to work. As we found in an earlier mapping study, people with visible disabilities were more likely to access support and, once in the system, were referred between services; however, those who did not get in, by contrast, received little if any help. 134 These findings resonate with those of participants interviewed in this study. Some reported having received no help, whereas a small number reported receiving help in returning to work from services like those described here (i.e. a comprehensive specialist VR intervention delivered as part of a TBI specialist or neurorehabilitation team). However, our interview matrix is likely to have shaped the perspective on support available in UC by selecting two-thirds of people with moderate or severe injuries in a trial sample in which 56% had incurred mild TBI.
Although some NHS services may have indicated that participants had access to employment expertise and resources to support patients with accessing new work, careers training, career planning, work placements, financial advice and overcoming access issues to work through existing relationships with employment sector (DWP) services, in reality the changing landscape of DWP provision means that this is likely to have been extremely limited. The resource use data (see Chapter 4) supports this and suggests that only one or two trial participants received help from DWP services.
We were aware of services in site 1 that provided similar levels of support to those described here; however, these services did not indicate that they were a specialist provider of VR and did not complete part B to provide the detail on the VR service provided. As the definition of specialist relied on the respondents’ judgement, it is likely that some services that provide this type of support may not have been reliably identified using these methods and that the actual number of services may be greater. However, the pattern of provision and access issues are likely to remain unchanged.
The only new services identified at the end of the trial were both in site 2. One was a private-practice therapist who was trained to deliver the ESTVR intervention in site 2, and the other was a service that completed the survey in round 1, but identified itself only as a specialist provider in round 2. This was a NHS multidisciplinary neurological rehabilitation team with VR offered as part of the service. It is thought unlikely that the trial influenced the service change in this service.
Interviews with trial participants
The mapping identified only one VR-specific service local to site 2. However, a small amount of VR was being provided as part of usual NHS rehabilitation. Interviews with 15 ESTVR participants revealed that three had received support with RTW from local ABI teams. In five cases, ESTVR participants saw a neurological rehabilitation or ABI team before FRESH but it was not known whether or not this included support with RTW. One participant was confused with having several different OTs involved in their care:
I was in hospital in [site XX] but when I got discharged home I came to [home town]. And then the OTs from [home town] Hospital came to see me. I’m slightly confused about it actually because I saw several different OTs. FRESH OT saw me but a female OT came to see me and a male OT came to see me, and I’m not quite sure what their roles were. One was definitely a community OT and the other was definitely a neuro OT, and then FRESH OT saw me as well, but that wasn’t part of the NHS care, that was part of the study.
1005
Participants in the UC group indicated a lack of information and no routinely available support for RTW. In one site, rehabilitation was restricted to people who had limitations in basic ADL:
I mean, to be perfectly honest, if I’d never come into contact with FRESH OT, I don’t know what would have happened because I would have been just handed to, I can’t remember, this team, the one at XXX, who I don’t really think have dealt with it well at all. I mean, they basically wrote me off. And actually, the woman who came to see me, the OT that came to see me, I remember her saying, ‘The thing is, some of the clients I go to see, they can’t even fill the kettle up,’ and she said, ‘But you can make yourself a cup of tea. I don’t need to be here.’ [Laughter.]
2020
Participants also reported a lot of support from family members including parents and partners:
Most support has come from my wife basically. She’s a doctor as well, she was working but she basically stopped working for quite a few months in fact ended up not working longer than me – effectively to look after me in fact. And so lots of support came from her but then that caused other issues [indistinguishable].
3002
Only four UC participants reported having received support from a brain injury service and, of those, three (one in each site) received an intervention similar to ESTVR, which they valued. Intervention ranged from a one-off visit by a health professional to weekly (or more frequent) visits over several months. This was mainly provided by community ABI services:
Just sort of getting me to do various exercises and sort of let me know where I have problems with returning to work because of my brain injury. And also they attended a couple of meetings with my bosses to just start a process there.
2018
So she sent it [the letter] to my doctor, my doctor then topped and tailed it and then he sent it on to my HR Team. She didn’t liaise directly, no. She was quite happy to write her recommendations and then my employers just accepted that. But [large IT firm] are such a forward-thinking employer, it’s not as if you’re talking about some back-street garage who’s going to try and screw me for just £10 an hour. I would have been very surprised if my employers had kicked back on the recommendations that a skilled professional in a Brain Trauma Injury Team made, backed up by a letter from my doctor. They're just not healthcare professionals; they would have no basis on which to challenge that diagnosis.
1005
NHS community ABI OT, they called her from XXX [hospital]. She was brilliant, she was. You know, she sorted them out, work, and she told them how it could affect me and how it will affect me and she were brilliant, she were.
1006
Patient-reported resource use data (see Chapter 4) indicated that people receiving ESTVR had twice as much OT and more GP visits than those receiving UC, but UC participants consumed more social care resources (care assistants), paid more visits to NHS walk-in centres, were more frequent attenders at A&E and had more hospital admissions. For all other health services utilised, it appeared that those receiving UC were comparable with those in ESTVR.
What are the most important primary outcomes of vocational rehabilitation?
Trial participants with a new TBI (n = 78) were interviewed at approximately 3 weeks post injury. Responses were available for 72 people. In total, 48 long-term survivors of TBI volunteered, 23 were excluded because they did not have a TBI (n = 16), lived too far away (n = 4) or said that they felt unable to cope in group situations (n = 3). A total of 25 people with TBI were invited and 14 responded, but one person did not turn up. Therefore, 13 people with long-term TBI took part. The severity of their injuries was unknown. Two people were accompanied to the group as they could not travel independently (Table 29). Eighteen employers were identified and invited, four did not respond and two were excluded as they had no experience of TBI. Therefore, 12 employers participated. Participants in each group came from a wide geographical area and represented a variety of post-TBI experience.
| Demographic characteristic | Participants | |||
|---|---|---|---|---|
| Newly injured people with TBI (n = 72) | Long-term TBI survivors (n = 13) | Service providers (n = 13) | Employers (n = 12) | |
| Participants | FRESH study participants. All in work or full-time education prior to injury and admitted to hospital for ≥ 48 hours |
Eight males, five female All in work or education at time of injury At time of focus group: four in full-time work, five in part-time work, two in voluntary work, one retired and one no set activity |
OTs (n = 11), social worker/CM (n = 1), counsellor (n = 1) | OH doctor (n = 1), OH nurse (n = 1), human resource manager (n = 1), line managers (n = 7), DEA (n = 1), personal-injury solicitor(n = 1) |
| Age (years) | Median 43 (range 16–62) | Median 40 (range 25–61) | Not requested | Not requested |
| Time post injury | Mean 24 days post injury (SD 22 days) | Median 10 years post injury (range 2–39 years) | N/A | N/A |
| Experience | N/A | N/A | Median 19 years’ experience as a service provider (range 1–40 years) | The solicitor, DEA and one manager knew > 5 people with TBI, one OH doctor had seen two people with TBI, eight people had experience of only one person with TBI |
| Work | All employed or in full-time education prior injury |
All employed or in full-time education prior injury 9 out of 13 employed full- or part-time at time of group |
10 provided VR | All had experience of people with TBI in a work capacity |
People with a new diagnosis of TBI prioritised RTW and symptom management. People with long-term TBI rated self-confidence and ‘assessment of brain function’ as their top two most important outcomes. People with long-term TBI differentiated between self-confidence and self-esteem, defining self-confidence as having task-specific confidence and self-esteem as how they felt about themselves more generally (Table 30).
| Outcome rating | New TBI (n = 78) | Long-term TBI (n = 13) |
|---|---|---|
| 1 | Resume work | Maintain/improve self-confidence |
| 2 | Adequate management of symptoms | Assessment of brain function |
| 3 | Resume previous activities | Fatigue management at home and work |
| 4 | Manage symptoms at work | Education on impact of TBI |
| 5 | Adequate finances | Maintain/improve self-esteem |
| 6 | Knowing what to expect | Positive encouragement to meet others with TBI |
Service providers prioritised quality of work–life balance and insight into the impact of the injury on the person with TBI. For employers, the most important outcomes were communication between the employer, employee and the therapist, and understanding the impact of the injury on the person with TBI (Table 31).
| Outcome rating | Service providers (n = 13) | Employers (n = 12) |
|---|---|---|
| 1 | Quality of work–life balance | Effective communication between the employer, employee and the therapist |
| 2 | Insight into how the brain injury has affected the person with TBI overall and how they use strategies to deal with their problems | Employers to understand the effect of brain injury on the employee and their job |
| 3 | Enabling people with TBI to explore own goals, including (work) alternatives | The person with brain injury having a good QoL whether or not this involves work |
| 4 | Return to paid work | Employer informed about the employee’s physical, cognitive and functional ability to work |
| 5 | Measurement of work readiness | Early prediction of employee’s potential capacity so that realistic options can be explored |
| 6 = | Return to unpaid work | Phased or graded RTW |
| 6 = | People with TBI being aware of how the TBI has affected them in relation to their work role |
Summary
In people with TBI, outcome priorities differed according to time since injury. Newly injured people said that a RTW and symptom management were the most important outcomes. Employers prioritised communication between employer, employee and NHS VR services, and understanding the TBI impact on workability; service providers said QoL and insight were more important than work outcomes. People late after injury, nine of whom were employed, prioritised self-confidence and having a comprehensive assessment of their brain function to understand the impact of TBI on all daily life activities, not just work.
The long-term survivors did not prioritise returning to work. This may be because early after injury, and particularly while still in hospital, participants have limited knowledge of the consequences of their injury or may have reduced insight into its effects due to either impaired awareness or lack of opportunity to test out their abilities. They may not initially recognise the full impact of their injury on their ability to work or acknowledge any potential long-term consequences. 135 It is only over time that people realise the full impact. 136 Moreover, for participants late after injury who are employed, a RTW may no longer be a goal. Finding ways of coping with the effects of their TBI across a broad range of daily life activities and having work–life balance was seen as more important than a RTW itself. This could be because they are no longer faced with financial concerns and fears for the future that motivate the newly injured person to return ‘to normal’ and retain financial independence.
For all TBI survivors, the second most important outcome was the need for all relevant parties to understand the effects of TBI on the individual. Newly injured participants called this ‘knowing what to expect’ and those with long-term TBI referred to ‘education about TBI’ and understanding their ‘brain function’. Therapists referred to the need for ‘self-awareness’ and ‘insight’ into a person’s ability, while employers said that they wanted to understand the TBI impact on the employees’ work ability. These findings are similar to others that have highlighted that understanding TBI and its impact on the individual is an important outcome of rehabilitation. 135,137,138 All stakeholders felt that, without this understanding, it would be difficult to facilitate a successful RTW or any other activity. Therefore, some means of measuring a person’s understanding of the TBI impact on their ability to work may be an important measure of TBI VR.
All except newly injured participants differentiated between understanding their brain injury and coping with it. Long-term TBI survivors made specific suggestions for coping outcomes including measures of confidence, self-esteem and the ability to manage fatigue.
The employers we interviewed ranked communication as their primary outcome. Those with some experience of employing a person with TBI highlighted understanding the effects of the brain injury and doing the right thing for their employee. Although they felt that RTW was important, they also highlighted the need for good QoL, irrespective of whether or not this involved work.
Service providers rated the opportunity to explore a person’s own goals and achieve a good work–life balance. Interestingly, service providers struggled to identify discrete ‘outcomes’ of VR. They emphasised the importance of the rehabilitation process and the need for the person with TBI to understand how their brain injury had affected them. Inherent in their suggestions were that measuring aspects of process was more important than the outcome, and that having better measures of the intervention process was the only way to link the intervention with its outcomes.
Both service providers’ and employers’ responses were more aligned with people living long term with TBI than with the more recently injured. This may reflect their experiences of working with people with more severe TBI problems later after injury and over time. Both service providers and employers acknowledged that returning to employment following TBI was difficult and not always possible.
Overall summary of findings
This process evaluation adopted a broad approach and gathered data from across the trial, at all time points and from multiple stakeholders, using mixed methods. This enabled triangulation of findings and a thorough understanding of factors likely to affect the running of a definitive trial.
Was the training seen as acceptable and useful?
The qualitative evaluation of the training package and mentoring records suggests that the OTs absorbed the knowledge necessary to implement ESTVR within the trial. Therapists valued the whole training package (training, manual and mentoring) but especially the case discussions and mentoring, which increased their confidence to deliver ESTVR, tailor it to the person and overcome implementation barriers. Therapists wanted the training to include more detailed descriptions of the trial and the trial processes, and more opportunities to meet with the other therapists to discuss cases.
In future, pre-training materials and the training manual should include more detailed descriptions of the trial so that more training time can be dedicated to discussing concerns about delivering the intervention in the context of a trial and minimising contamination, how to complete the study paper work and case discussions. Training could be delivered early and implemented by trained therapists in UC before recruitment commences. Case vignettes could be used to assess therapists’ competency and confidence to deliver the intervention before recruitment begins. Therapists with previous community experience may be easier to train and mentor.
Did the therapists deliver the ESTVR intervention as intended?
Analysis of 38 sets of occupational therapy records and 699 content pro formas indicated that therapy was delivered as intended and was flexibly tailored in dose, duration and intensity to individual need. It started approximately 5 weeks after TBI and involved a mean of 6.3 face-to-face contacts (18 hours) delivered over 8 months, in community (home or work) settings, often with family members present. Intervention duration varied according to injury severity. Participants with a mild TBI received the most intervention. Most (60%) intervention was directly patient facing or patient related.
The FRESH therapists had direct contact with 14 (37%) employers, non-direct contact (participant acted as a conduit of information) with 20 (53%) and advised participants on how to broker their own RTW with an employer in four (10%) cases, as per the participants’ wishes. These findings are consistent with those from the earlier pilot study59 and a similar feasibility trial in stroke survivors. 139
Measuring the intervention enabled us to understand what was delivered, how closely it resembled that intended (e.g. by training), whether or not the core ESTVR process was followed (according to the logic model) and which components were being implemented, omitted or altered. These data triangulated with data from interviews with the therapists, participants and NHS staff, highlighting factors affecting intervention fidelity and considerations for training and mentoring for a future study.
Similar fidelity issues, relating to ‘resources’ necessary to deliver ESTVR were noted across sites and included differences attributable to individual participants’ needs, employer access, local rehabilitation service provision and therapist expertise. Although these resulted in individual variation, the core ESTVR process, as depicted by the logic model, was followed and the minimum standards as set out in the therapy manual were met in each case. Other local contextual factors such as the deployment of OT time, access to administrative support and access to NHS e-mail and postal systems and clinical registers posed a threat to ESTVR delivery.
Mentoring was essential to supporting intervention fidelity and delivery in the context of a trial and should be considered an essential resource. The therapists required about 15 minutes of mentoring per day of intervention delivered or between 1 and 3 hours per participant. One therapist with no previous community rehabilitation experience needed more support. The mentoring also offered a novel window on the research process, highlighting local issues with screening and recruitment, thus enabling timely intervention. This warrants further investigation.
Some difficulties were observed in therapists’ intervention records, highlighting the need for training in completing trial documentation and recording withdrawal and start and end dates for the intervention. Triallists need to be conscious of not adding to the administrative burden of the trial therapists.
Did the intervention meet patients’ and employers’ expectations?
Almost all participants interviewed found the intervention useful. They particularly valued the practical nature of the support including therapists’ letters to the participant and employer, and support in preparing for, and negotiating, a RTW. Only two patients withdrew from the intervention. This is about the same as might be expected in usual NHS rehabilitation. Employers valued timely communication and appreciated the therapist acting as an advocate for the employee. They valued learning about TBI impact on the employees work ability, advice about adaptations and practical support in planning a phased RTW and monitoring progress.
To what extent was ESTVR already being delivered in usual care?
The mapping identified only one VR-specific service local to one of the sites. However, other services were identified that provided VR to people with TBI as part of usual NHS rehabilitation. Strict referral and access criteria, tight geographical boundaries and limited resources meant that these were largely restricted to people with a need for multidisciplinary rehabilitation. Only four trial participants would have been eligible to access services identified in the mapping that were identified as providing an ESTVR-like intervention.
Interviews with 15 ESTVR participants revealed that three ESTVR and three UC participants received support with RTW from local neurorehabilitation teams.
Patient-reported resource use data indicated that people receiving ESTVR had twice as much OT and more GP and practice nurse visits than UC participants, but UC participants paid more visits to NHS walk-in centres. The intervention group had fewer A&E attendances and admissions, and used fewer social care resources. For all other health services utilised, the two groups appeared comparable. These findings suggest that no additional resource to support RTW was available in UC.
What were the practical difficulties, comprehensibility and emotional load required to complete outcome measures?
Only two patients said that they needed help to complete questionnaires. Some said responses were complicated because the TBI was only one of a number of serious injuries incurred at the same time and they were not sure how to respond.
What factors will affect the use of ESTVR in the context of usual NHS rehabilitation?
A communication gap between occupational therapy services and commissioners poses a threat to the future delivery of VR for people with TBI. A future trial would benefit from having a commissioner and senior therapy service manager in an advisory role to facilitate setup and translation of findings into services.
What factors will affect the running of the definitive trial?
Securing Excess Treatment Costs from the newly reconfigured CCGs delayed recruitment. Despite negotiation, network support failed to materialise in one site.
Deploying experienced OTs to the trial was problematic. Options to backfill therapists’ existing clinical caseloads were limited. Senior therapy managers were extremely busy, resulting in delays in appointing and training therapists. The intervention costs were based on an average amount of therapy time delivered to participants in the pilot study59 and intended to be delivered flexibly according to participant recruitment and need. However, this was interpreted as ‘one day per week’ by service and line managers and the therapists attempted to contain trial activities into a single day, which was inconsistent with fluctuating trial caseloads. Time spent discussing the need for flexible working with trial therapists and their managers is needed during setup.
Line managers told us that existing services were expected to deploy therapy time to deliver the trial intervention from existing therapy resources and that there was no backfill. They felt than allocating more than 1 day might have positively influenced action to backfill and should be considered in future trial design.
A future trial should engage commissioners, therapy services, research networks and R&D officers early to ensure ‘buy-in’ and explore optimal methods for recruiting and deploying OTs and for recruiting potential participants in each site.
Identifying and screening potential participants was complicated. Not all patients fitting the inclusion criteria were admitted to a designated trauma unit and they were geographically dispersed across sites. In two sites, recruiting staff had no access to clinical registers to identify patients admitted with head injury. Mild TBI was sometimes missed or poorly recorded in patients admitted with other severe injuries. The lack of a clear definition of TBI may have affected eligibility and inflated the proportion of patients screened. A future trial should include a working definition of TBI rather than rely on local clinical diagnostic procedures, which differed between centres.
New care pathways in the newly established ‘hub and spoke’ MTCs resulted in people with moderate and severe injuries being rapidly repatriated, affecting recruitment of those most likely to benefit from the intervention. A future study should identify local referral ‘spokes’ and include additional resources for recruitment there.
The characteristics of the local trauma populations differed between sites. In one site, most potential participants were at, or near, retirement age and local unemployment was high. In another, the workforce was transient and potential participants less likely to be in long-term relationships, affecting carer recruitment.
What are the most important outcomes of ESTVR?
Our findings suggest that what constitutes a successful outcome of VR differs according to the stakeholder. Across all groups, most of the identified outcomes focused on broader rehabilitation outcomes, which is unsurprising given that, in usual NHS rehabilitation, VR is delivered as a component of a more generic rehabilitation process following TBI. These broader outcomes may be important in terms of adjustment and adapting to life with a brain injury, particularly when returning to work or education cannot be achieved and alternatives need to be explored.
Return to work was only one of several important rehabilitation outcomes identified by people with TBI, service providers and employers. Measuring ‘return to work’ alone may be insufficient and does not reflect the complex nature of TBI or the VR process. In addition to work outcomes, future VR trials should measure outcomes including QoL, self-confidence, employer communication, and constructs such as understanding the effects of TBI on the person and their work ability, and the process of rehabilitation, for example phased work re-entry.
Chapter 6 Discussion
Key findings
The FRESH study demonstrated feasibility across the majority of its objectives and, when these were not met, strategies for addressing these in a definitive trial were identified. However, two main challenges remain, namely how to:
-
recruit those people with TBI most likely to benefit from the intervention
-
reduce attrition, particularly in the UC group.
Recruitment
At 12 months post randomisation, 91% of UC respondents reported that they had returned to some form of work, suggesting that their problems did not affect their ability to return to work within the first year. However, in contrast, qualitative data indicated that most of those treated in the intervention arm, and many of the UC participants interviewed, had considerable problems that had an impact on their ability to work. Those receiving ESTVR greatly valued this intervention.
This dichotomy may have been influenced by two factors, which will impact any definitive trial:
-
problems with recruitment, resulting in a sample with a disproportionately large number of participants with mild TBI
-
non-response at 12 months observed to be higher in the UC group than the intervention group, impacting on the potential validity of RTW data between the ESTVR and UC arms.
Recruitment of people with moderate and/or severe TBIs was affected by the establishment of ‘hub and spoke’ MTCs when the study was funded. New care pathways for ‘major trauma’ meant that people with moderate or severe injuries requiring ongoing rehabilitation were rapidly repatriated to designated rehabilitation units or local referring hospitals before they could be recruited, or lived further away and, therefore, no longer met the inclusion criteria. Any future trial would need to include resources necessary to identify, recruit and treat repatriated patients.
Recruitment was also affected by the study design, which assumed that patients could be recruited for VR early after TBI. However, persuading people with TBI, still in hospital, that they might later encounter difficulties in the workplace was problematic. Of those who declined and offered a reason, 34 (45%) said that they could not think about work at this time, did not think they needed help or felt that ESTVR would not be beneficial. Perhaps offering a more generic form of rehabilitation might have had greater appeal. Alternative recruitment mechanisms including timing of recruitment need to be explored in future studies. For example, offering all potential participants a set follow-up appointment post discharge (e.g. 2–4 weeks) to assess progress may have been more appropriate and have facilitated recruitment.
Recruitment was further compromised by the lack of a clear definition of TBI. Not everyone who sustains a head injury is scanned for brain injury and not all brain injuries are detectable on a scan. For this reason, it was not a requirement of FRESH that TBI was confirmed by CT and, instead, diagnosis relied on local clinical procedures. This lack of a clear definition may have affected eligibility and inflated the proportion screened. In one site, there was no designated trauma ward and, therefore, no simple mechanism for identifying potential participants (TARN registers are often retrospectively completed and capture only patients admitted for ≥ 72 hours). All patients admitted to A&E with a suspected ‘head injury’ were screened to identify those with TBI. As all these patients were entered onto the screening log, the proportion recruited in relation to the number screened was low. Screening was further complicated by head injuries missed when patients were being treated for other serious injuries simultaneously.
Problems in defining and recording TBI in medical records are not new36 and difficulties identifying injury severity are well documented in studies of TBI rehabilitation. 2,140 Presence and duration of PTA are often poorly recorded in medical notes and are difficult to assess retrospectively. While this is possible using standardised interview methods, such as the Rivermead PTA protocol, it requires skilled clinicians experienced in TBI assessment. 19 These difficulties are further exacerbated by the non-linear relation between injury severity (measured by the GCS) and functional and vocational outcome. 24,141 Future studies could combine data from case notes with self-report or follow standardised protocols to obtain more reliable estimates of PTA.
Although the recruitment rate was satisfactory, actual recruitment from the total of eligible patients was low; 61% of eligible patients were not recruited, and some are likely to have had significant problems. Even if the target recruitment rate of 34 participants per year (slightly exceeded at one site) could be achieved across a larger number of sites in an effectiveness trial, recruitment overall would be difficult. For example, if RTW were retained as the primary outcome measure and attrition at 12 months reduced to 25%, a total of 1352 participants would need to be recruited to give 90% power to detect a 7.5% increase in the 12-month RTW rate; this would require recruitment from 20 sites for 24 months.
However, as reported by potential participants during recruitment and TBI participants during the study, there may be more important outcomes of VR than RTW itself. This was the perspective of TBI survivors late after injury, who prioritised improved self-efficacy and social interaction among their top six outcomes (along with assessment of brain function, education on TBI impact, improved self-esteem and fatigue management). Therapists also prioritised work–life balance, gaining insight into TBI impact and enabling individuals to explore their own goals above return to paid work. These findings suggest that adjusting to TBI is an important rehabilitation outcome even if the focus of the intervention is work. This is further supported by the finding that, at 12 months, a measure of social participation (the CIQ) and a measure of work self-efficacy (a single question from the WAI) were quite strongly related to who was working or studying at 12 months. Therefore, it is possible that a broader outcome measure, relating to ability to work, confidence in working or a broader ability to participate in work or other activities might be a better and potentially more sensitive outcome. This suggestion is supported by other TBI rehabilitation studies indicating positive effects of TBI rehabilitation on participation outcomes. 36,73,74 However, future studies would need to be designed to isolate the effects of the VR over and above a comparator. 69,142
On this basis, if the CIQ was the primary outcome for a definitive trial then, based on a linear model (adjusted for baseline CIQ), to achieve 90% power with 5% significance to achieve a minimal important difference of 1.5 points (i.e. the difference between the means at 12 months), would require 474 participants (237 per arm). This would equate to randomising 632 participants if 12-month attrition was 25%. This could be achieved by recruiting from 12 sites for a little over 18 months at the rate of 34 participants per year.
Attrition
Poor response to follow-up at 12 months in the UC group and the injury severity imbalance is likely to have inflated the estimate of people with TBI who return to, and remain in, work without support at 12 months post injury. People with milder TBI in the UC group who were not in work at 6 months were also less likely to respond at 12 months than those who were in work at 6 months. The observed figure of 91% of UC respondents in work or study at 12 months is higher than published elsewhere. Systematic reviews and other prospective follow-up studies have indicated this to be in the region of 71–76% in people with milder injuries,28,35 which is closer to the rate we observed at 6 months. As people with more severe injuries are less likely to drop out, providing they can be recruited, there remains potential for research on a group who are more likely to benefit from the intervention and in whom the potential for attrition is reduced.
High attrition rates in studies of TBI populations is a well documented problem. 35,56,143–145 Incentivisation might be key; a recent trial73 (n = 44) of RF, a vocational intervention in the USA, followed and retained 100% of participants (23 with TBI) to 15 months by incentivising them with a US$100 fee on completion.
Given the high RTW rates in control participants, it could be argued that interventions should be offered only to people with TBI who report work problems or require help accessing work because they are unemployed. However, our inclusion criteria limited recruitment to people employed at the time of injury because we tested the feasibility of delivering and measuring a job retention intervention. This was because little, if any, advice or information about work is routinely provided prior to discharge following TBI. In addition, the disparate and limited VR services for people with ABIs in England67,146 means that problems often go undetected or inappropriate advice about work is given by HCPs, resulting in job loss.
The ESTVR model differs from those used for people who have no job to return to or who cannot return to an existing role and who, therefore, require retraining and job brokerage to find suitable work, both of which services are more resource intensive. ESTVR aimed to prevent job loss by offering early advice to participants, family, employers and other HCPs. It maintains that a RTW does not necessarily involve returning to the same job with the same roles and responsibilities or working hours as previously, rather it can involve an adapted form of work with an existing employer. The danger is that without early intervention to prevent job loss, therapists’ time will be consumed in delivering resource-intensive job finding and retraining interventions later to those who have unfortunately lost their job. However, it remains to be understood how best to identify those that will benefit most from this support.
Process evaluation
Participants interviewed included people with TBI of all severities who reported significant problems following TBI and valued the intervention received. About 20% interviewed from each arm, and in each site, reported having received an intervention similar to ESTVR. Therefore, it is possible that such rehabilitation is already being delivered as part of UC. In five cases, another ABI service intervened before ESTVR and so, although it is not known whether or not a work-related intervention was offered, this does suggest that early intervention is happening, albeit to a limited extent, in UC.
Work return following TBI needs to be both safe and sustainable in the longer term. It is plausible that attrition in UC was biased towards those who were no longer working and that long-term work sustainability in people with mild TBI needs to be better understood. 28
We have demonstrated that ESTVR delivery was feasible. OTs familiar with working in the community may be more amenable to training and mentoring. Therefore, upskilling community OTs with specialist VR experience was considered the best way of delivering the intervention. Mentoring was an essential mechanism for supporting intervention fidelity. Similar findings have been reported elsewhere. 147,148 Mentoring also acted as a window on the trial, highlighting local issues with screening and recruitment that enabled timely intervention.
Economic evaluation
The feasibility of identifying and collecting resource use data for use in a health economic evaluation was explored. Although data completeness was good (> 80%) for participants completing the questionnaires, dropout and missing data for those still in the study at 12 months meant that only 37 out of the original 78 participants (47.4%) could be included in the early-stage complete-case, base-case analysis. Therefore, although it is feasible to measure and value health economic data to assess cost-effectiveness of VR following TBI, a subsequent definitive study would not be without potential challenges, particularly around retention.
The feasibility findings also suggest that, in a definitive trial, it would be important to take a broader perspective when measuring and valuing costs, and re-evaluate how best to capture intervention costs distinct from other wider NHS resource use. Despite the challenges in undertaking a definitive study of VR following TBI in those in work or education at the time of injury, the estimates in the value-of-information analysis suggest that the cost of future research is likely to be lower than the potential value of undertaking the research.
Organisational issues
The study was compromised by a number of organisational problems including failure of CLRN nurses to recruit any patients; the variable way in which head injury is managed in different centres, which affected the identification of people with TBI; and difficulties in recruiting or deploying experienced OTs on the trial (despite securing Excess Treatment Costs). There were limited options for backfilling clinical caseloads of staff deployed to the trial, which led to team frustration. Line managers suggested that this could be solved by increasing the number of hours allocated to the FRESH OT role, which would not only be more attractive to OTs but might force senior managers to ensure adequate cover for existing demands. As the total amount of time allocated to FRESH approximated to 1 day per week, some believed that existing OT services were expected to absorb this commitment within existing resources.
Research in the context of other studies
There have been four small TBI-specific randomised controlled pilot or feasibility trials of vocational interventions in TBI. Although all showed positive gains in employment status following TBI rehabilitation, none was more effective than its comparator intervention. 6 Yet, as three37,70,72,149 were US military based and one71 was a civilian study in China comparing a virtual reality training programme with standard VR, they are not directly relevant to this study.
In a recent systematic review of rehabilitation for people with ABIs, Turner-Stokes et al. 150 identified 19 studies involving 3480 participants, comparing multidisciplinary rehabilitation with routinely available local services or lower levels of intervention. Within the subgroup of five studies for mild TBI (1180 patients), they found ‘strong evidence’ that most individuals make a good recovery when appropriate information is provided, without the need for additional specific interventions, whereas those with moderate to severe injury require intervention. They also found some evidence that rehabilitation early after injury results in better outcomes and ‘strong evidence’ that more intensive programmes are associated with earlier functional gains in moderate to severe injury, and ‘moderate evidence’ to suggest that continuing outpatient therapy might sustain this. 150
Five trials35,36,70,78,151 (1180 patients) that were targeted primarily at increasing participation (social integration, RTW, etc.) and reducing post-concussion symptoms included patients in the milder ambulatory category. Four35,36,78,151 compared a programme of treatment as needed (which consisted largely of community-based rehabilitation) with a lesser intervention (Paniak et al. 151: information only; Wade et al. 35,36: standard follow-up, which usually meant that no further input). All were rated as high-quality RCTs. The authors summated that an intervention provided to a totally unselected group of patients with mild TBI was not effective (both treatment and control intervention groups made substantial gains in terms of enhanced participation, including RTW, and it was concluded that ‘strong evidence’ suggests that most patients with mild TBI make a good recovery and those with PTA of < 1 hour, usually not admitted to hospital, need no specific intervention; patients with PTA of > 1 hour do benefit from routine follow-up contact to receive information and advice; and a subgroup of patients with moderate to severe injury benefit from a higher level of intervention but may not present themselves unless routine follow-up is provided).
These findings are supported by a systematic review of mild TBI involving four studies (1309 participants), two from Canada,152,153 one from the USA30 and one from the Netherlands154 that found that most workers with mild TBI returned to work within 3–6 months after injury, 76% (152 out of 201) of the patients reported full RTW at 6 months post injury, suggesting that mild TBI is not a significant risk factor for long-term work disability. 28 However, one study153 reported that only 44% of people with mild TBI had returned to work within 6–9 months of injury.
In a trial36 of early intervention following head injury, in which TBI specialist team rehabilitation was offered following routine review of all patients presenting to A&E with head injury, at 7–10 days, participants (n = 184) who received the specialist input, which was decided on the basis of presenting problems and ranged from signposting to further rehabilitation, had significantly less social disability (p = 0.01) 6 months after injury than controls (n = 130). The findings suggest that early intervention by a TBI specialist service significantly reduced social morbidity at 6 months after head injury. Although the amount of service provided increased with length of PTA, most people who received extensive rehabilitation services had incurred mild to moderate injuries, suggesting that persistent problems may not be severity related. In a similar trial35 of 1156 patients in the preceding year, 71% of 478 participants who were followed up at 6 months (41%) had returned to some form of work or education at 6 months. Although there were no differences in work outcomes between the intervention and control groups (both 71%), a subgroup analysis of 71 patients with PTA lasting > 1 hour who were admitted to hospital found that those who received the specialist rehabilitation were significantly less likely to report problems in work, relationships, and social and leisure activities at 6 months as measured by the Rivermead head injury follow-up questionnaire (Mann–Whitney U-test: z = –2.07; p = 0 04), suggesting a group who might benefit most from the support.
The findings from these two trials35,36 might imply that, in an unselected group of people with TBI, it may be possible to triage people to receive greater or lesser amounts of specialist intervention on the basis of symptom severity and need. Such a triage-based hierarchical model is not dissimilar to that proposed by Eva et al. 155 for supporting cancer survivors in a RTW and to that proposed by Frank and Thurgood. 156
The only VR-specific study with which we can directly compare the results is the single-centre cohort comparison study59,85 on which this multicentre feasibility trial was based. The current intervention provided was similar in dose and intensity but slightly shorter in duration than that in the single-centre study,85 which was pragmatic. However, the two studies did differ, first, in that the single-centre study was not a randomised study and, second, in the fact that the initial study took place before the establishment of MTCs and recruited more participants with moderate or severe TBI (17% moderate, 40% severe).
Strengths and limitations
Strengths
-
The protocol was robust. The majority of feasibility objectives were met or, when not achieved, strategies for achieving targets in a definitive trial were identified. The trial achieved 76% of its target recruitment. One site achieved target, and further mechanisms to increase recruitment, including recruiting more people with moderate or severe TBI, were identified.
-
Patients with TBI were willing to be randomised.
-
Carers were nominated by 45 TBI participants and 69% were successfully recruited.
-
Data completeness was good (> 80%) for participants completing questionnaires; 90% of work questions were completed. It was feasible to measure and value health economic data to assess the cost-effectiveness of VR following TBI, although this was not without potential challenges, particularly around retention and missing resource use data, which would need to be further mitigated against in a definitive trial.
-
NHS therapists in three geographical locations were successfully trained to deliver ESTVR alongside usual NHS rehabilitation. The intervention was found to be safe, well received and highly valued by TBI participants and employers.
Limitations
-
Target recruitment was not achieved in two centres; however, mechanisms to ensure that this could be met in a future study have been identified.
-
Participant retention at 12 months was low; however, those with more severe injury were more likely to remain in the study. Therefore, recruitment of more people with moderate and severe TBI may improve retention. We have suggested how this might be achieved.
-
It proved difficult to obtain an unbiased estimate of the RTW rate in the control arm – the key parameter required for a sample size calculation for an effectiveness trial; 91% of control participants were in work or studying at 12 months, suggesting no need for a definitive trial. However, it appeared that people with milder injuries who were out of work were less likely to respond, particularly UC participants, who may have felt dissatisfied with the trial. Unless this can be overcome, it would appear that an effectiveness trial of ESTVR using a work status variable at 12 months would not provide convincing evidence of effectiveness, owing to a combination of high control group attrition and the inherent bias in the intervention effect estimate. However, adopting the suggested strategies for recruitment and follow-up, maintaining contact with participants between 6 and 12 months and incentivising participants to return questionnaires may provide a way forward.
-
Some control participants were provided with an intervention to support them in RTW.
Chapter 7 Conclusions
FRESH demonstrated feasibility across most objectives and, when feasibility criteria were not met, strategies to achieve them in a definitive trial were identified.
We recruited at a satisfactory rate but actual recruitment from the total number of eligible patients was low: 61% of eligible patients were not recruited and some are likely to have had significant problems. We also recruited a disproportionate number of people with mild TBI, who, as was demonstrated in UC, may be a low-risk population for long-term problems with employment or whose employment problems may be sufficiently well managed by their employer to enable them to remain in work, if not always performing as they did prior to their injury.
Strategies for improved recruitment (and for recruiting more people with moderate and severe injuries) in a larger study have been identified. However, some issues remain unclear in terms of the design of a definitive trial, in particular, how to reduce attrition of the primary end point at 12 months, especially among UC participants. However, successfully recruiting more people with moderate and severe TBI may have a positive impact on attrition rates and ensure that rehabilitation is directed to those who most need it.
It was feasible to collect health economic data to assess the cost-effectiveness of VR following TBI. Although challenges remain in terms of minimising dropout and missing resource use data in a definitive study, the estimates found in the value-of-information analysis suggest that the cost of a future trial is likely to be lower than the potential value of undertaking the research.
We have demonstrated that the intervention was feasible to deliver. However, OTs already familiar with working in the community may be easier to train and mentor. Therefore, upskilling community OTs with specialist VR experience may be the best way of delivering the intervention.
Research implications
-
People with TBI can be recruited and randomised to a feasibility trial of a VR intervention but the timing of recruitment needs further testing. A two-stage recruitment process that enables potential participants to return home and live with their injury before recruitment is recommended.
-
The intervention was no more expensive than UC and the estimates found in the value-of-information analysis suggest that the cost of future research is likely to be lower than the potential value of undertaking the research.
-
People with TBI can successfully complete outcome assessments but further strategies are needed to improve retention to 12 months.
-
Return to work was most strongly related to social participation (community integration) and work self-efficacy. These findings support those of our primary outcome interview study and suggest that an outcome measure relating to a broader ability to participate in work or other activities, such as the Community Integration Questionnaire, might potentially be more sensitive.
-
NHS OTs in different geographical locations can be trained to deliver early VR for people with TBI, with mentoring support to ensure intervention fidelity. Training should cover research processes and allow more time for discussing cases and addressing therapists’ concerns about delivering the intervention as part of a trial.
-
A future study should include a working definition of TBI and explore optimal methods for identifying and screening potential participants in each site, including access to NHS registers and systems.
-
Early engagement with NHS commissioners, therapy services, research networks and local R&D to explore options for deploying NHS therapists to research and network support for recruitment may reduce delays and recruitment problems. Additional training may be needed for research and network staff in recruiting people with TBI to a complex rehabilitation intervention.
Therefore, a multicentre trial of VR is feasible and warranted, but further work is needed to test the suggested strategies for optimising recruitment of people with moderate and severe TBI (recruiting from referring ‘spokes’ for up to 12 weeks post injury), and identifying people with mild TBI with problems that might threaten work stability or affect RTW success (by seeking initial consent to follow-up and screening for problems after discharge from hospital) to ensure that intervention is delivered to those that most need it. In any definitive trial, additional stratification factors for randomisation, including TBI severity and age, should be considered, and subgroup analysis conducted to determine whether intervention effectiveness differs between those with mild and moderate/severe TBI. Although optimising recruitment may have a positive impact on retention and follow-up rates, implementation and evaluation of additional strategies (e.g. incentivisation, mini text surveys, cross-referencing with DWP data) to reduce the amount of missing primary outcome data, particularly in UC, remains important. Given the likelihood that outcome data are not missing at random, SAs should also be performed.
Implications for clinical practice
The FRESH study was a feasibility trial; therefore, no conclusions about the effectiveness of early VR for people with TBI can be drawn.
A mismatch in understanding between NHS therapists and local commissioners about VR delivery may determine whether or not the vocational needs of people with TBI are met.
Acknowledgements
The Excess Treatment Costs were funded by CCGs in Leeds and London and by the participating NHS trust in Preston.
The north-west CLRN provided staff to assist with the identification of eligible participants, organised most efficiently by Gemma Whitely and Gillian Martin.
We are grateful to our programme managers Nick Eaton and Hazel Church at the NIHR HTA for their open and supportive communications throughout the study and to Angela Shone for her input to the SSC and research governance.
The FRESH grant holders were Kate Radford, Caroline Watkins, Chris Sutton, Bipin Bhakta, Julie Phillips, Avril Drummond, Marion Walker, David Shakespeare, Diane Playford, Tracey Sach, Trevor Jones, Richard Greenwood, Lelia Duley, Andy Tyerman, Gemma Whiteley, Jain Holmes and Alison Hammond.
The authors would like to express their thanks to the following:
-
people with TBI, their employers and their carers who participated in the study
-
the OTs who delivered the ESTVR intervention – Susan Hughes, Suzanne Henshall, Paul Morris and Elaine Thornton
-
our PIs – Rory O’Connor, Karen Hoffman and David Shakespeare
-
the supportive staff in the major trauma units, occupational therapy departments and neurological rehabilitation wards in the three participating NHS sites and, in particular, the therapy service managers who facilitated the identification and deployment of local NHS therapists, namely Jacqueline Benstead and Jane Savage
-
our VR specialists, expert trainers and mentors – Ruth Tyerman, Andy Tyerman, Julie Phillips, Jain Holmes and Yash Bedekar, Kate Kerry and John Parker
-
our service users – Trevor Jones, Jonathan Franchi and Steph Parvin
-
research staff over the 3 years, especially Julie Phillips, Jain Holmes, Lyndsay Howard, Richard Morris, Jose Antonio Merchán-Baeza, Ali Gibson and Emma McManus
-
RAs and research nurses, those who assisted in identifying and recruiting participants, namely Emma Jane Mallas, Eleanor Bruce, Alex Williams and Sean McMullan
-
trial administrator Patricia Dziunka for her invaluable support in co-ordinating activities across sites, organising meetings and producing the trial newsletter
-
the SSC external members – Audrey Bowen, Derick Wade, Anne Chamberlain, Michael Dewey, Nicola Brain and Mark Chesman
-
members of the Lancashire CTU who had substantial involvement in running the trial including Chris Sutton, Denise Forshaw, Caroline Watkins, Jane Burnell, Alison Hadley, Mal Auton and Ali Gibson
-
members of the Nottingham CTU for randomisation and input to the Trial Management Group.
Particular thanks go to Professor Bipin Bhakta, an inspirational leader and rehabilitation friend who assisted in the development of this study and sadly passed away before its completion.
Research partnerships: service users
Trevor Jones suffered a TBI in 2004. He was a co-applicant who had worked with us on a previous bid and assisted in the development of this proposal. Trevor was a member of the Trial Management Group and an observer on the SSC. He assisted in focus groups with other TBI survivors, contributed to the development and delivery of the VR training, assisted in a recruitment workshop for research staff and commented on drafts of all patient-facing documents. Trevor also helped in analysing anonymised interviews. Trevor talks about his role in a podcast, which can be accessed via www.nottingham.ac.uk/go/fresh (accessed 19 December 2017).
Richard Morris, representing Headway, was a member of the SSC until becoming RA to the study in 2015. Richard supported us in publicising a call for focus group participants via Headway’s Facebook (Facebook, Inc., Menlo Park, CA, USA) page and assisted in qualitative interview data analysis. Both Trevor and Richard have helped prepare papers for publication, outputs for the lay public and this report.
Two additional service users, Johnathan Franchi and Stephanie Parvin assisted in training delivery, and Jonathan, an IT specialist, assisted in recording the training.
Contributions of authors
Kate Radford (Associate Professor in Rehabilitation Research) was chief investigator, responsible for the design and conduct of the study and drafting the report, except Chapter 4.
Chris Sutton (Trial Statistician) managed the input from the Lancashire CTU, led the data collection and analysis and assisted in writing Chapter 3.
Tracey Sach (Professor of Health Economics) led the economic evaluation, wrote Chapter 4 and was a member of the TSC.
Jain Holmes (RA) assisted in developing and delivering the training package, mentoring the therapists and analysing the process evaluation data. She contributed to drafting Chapter 5.
Caroline Watkins (Lancashire CTU Lead) led the input from the Lancashire CTU, chaired the operational group and was a member of the TMG. She commented on drafts of the report.
Denise Forshaw (Senior Trial Manager) was responsible for trial management and contributed to drafts of Chapter 3.
Trevor Jones (PPI Lead) assisted in bid development, supported data analysis and wrote the Plain English summary.
Karen Hoffman (PI) assisted in identifying and recruiting the trial therapists, assisted in study setup and recruitment training and was a member of the TMG.
Rory O’Connor (PI) assisted in the study setup, the choice of outcome measures, supervised the RA in face-to-face follow-up in site 3, was a member of the TMG and wrote the Abstract.
Ruth Tyerman (VR expert trainer and mentor) assisted in developing the training manual, trained and mentored the fresh OTs and contributed to drafts of Chapter 5.
Jose Antonio Merchán-Baeza (RA) analysed the content and fidelity data and contributed to drafts of Chapter 5.
Richard Morris (RA) analysed the qualitative data, assisted in conducting interviews, and proofread, compiled and formatted the report.
Emma McManus (Health Economics RA) conducted the economic analysis evaluation and drafted Chapter 4.
Avril Drummond contributed to the TMG and assisted in proofreading the report.
Marion Walker was a member of the SSC and assisted in writing the executive summary.
Lelia Duley advised on study setup, trial management and contributed to the TMG.
David Shakespeare (PI) assisted in the study setup and recruitment.
Alison Hammond contributed to the project design, advised on the report structure and assisted in revisions.
Julie Phillips (Research OT) assisted in developing and delivering the training package, mentoring therapists, interviewing participants and analysing the content of intervention and process evaluation data. She contributed to writing the report.
Data sharing statement
All available data from this report can be obtained by contacting the corresponding author. Please e-mail Kate.Radford@nottingham.ac.uk.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone's privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Headway – The Brain Injury Association . Types of Brain Injury 2017. www.headway.org.uk/about-brain-injury/individuals/types-of-brain-injury/ (accessed 3 January 2017).
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008;7:728-41. https://doi.org/10.1016/S1474-4422(08)70164-9.
- Saltychev M, Eskola M, Tenovuo O, Laimi K. Return to work after traumatic brain injury: systematic review. Brain Inj 2013;27:1516-27. https://doi.org/10.3109/02699052.2013.831131.
- Walsh RS, Fortune DG, Gallagher S, Muldoon OT. Acquired brain injury: combining social psychological and neuropsychological perspectives. Health Psychol Rev 2014;8:458-72. https://doi.org/10.1080/17437199.2012.733914.
- Hassan Z, Smith M, Littlewood S, Bouamra O, Hughes D, Biggin C, et al. Head injuries: a study evaluating the impact of the NICE head injury guidelines. Emerg Med J 2005;22:845-9. https://doi.org/10.1136/emj.2004.021717.
- Headway – The Brain Injury Association . Statistics 2015. www.headway.org.uk/about-brain-injury/further-information/statistics/ (accessed 2 December 2016).
- Humphreys I, Wood RL, Phillips CJ, Macey S. The costs of traumatic brain injury: a literature review. Clinicoecon Outcomes Res 2013;5:281-7. https://doi.org/10.2147/CEOR.S44625.
- Tuominen R, Joelsson P, Tenovuo O. Treatment costs and productivity losses caused by traumatic brain injuries. Brain Inj 2012;26:1697-701. https://doi.org/10.3109/02699052.2012.722256.
- Rickels E, von Wild K, Wenzlaff P. Head injury in Germany: a population-based prospective study on epidemiology, causes, treatment and outcome of all degrees of head-injury severity in two distinct areas. Brain Inj 2010;24:1491-504. https://doi.org/10.3109/02699052.2010.498006.
- Centre for Mental Health . Traumatic Brain Injury and Offending: An Economic Analysis 2016. www.centreformentalhealth.org.uk/traumatic-brain-injury (accessed 24 July 2016).
- Relyea-Chew A, Hollingworth W, Chan L, Comstock BA, Overstreet KA, Jarvik JG. Personal bankruptcy after traumatic brain or spinal cord injury: the role of medical debt. Arch Phys Med Rehabil 2009;90:413-19. https://doi.org/10.1016/j.apmr.2008.07.031.
- Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: prevalence, risk factors and implications for clinical management. Brain Inj 2007;21:1335-51. https://doi.org/10.1080/02699050701785542.
- Berger E, Leven F, Pirente N, Bouillon B, Neugebauer E. Quality of life after traumatic brain injury: a systematic review of the literature. Restor Neurol Neurosci 1999;14:93-102.
- Polinder S, Haagsma JA, van Klaveren D, Steyerberg EW, van Beeck EF. Health-related quality of life after TBI: a systematic review of study design, instruments, measurement properties, and outcome. Popul Health Metr 2015;13. https://doi.org/10.1186/s12963-015-0037-1.
- Menon DK, Schwab K, Wright DW, Maas AI. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health . Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010;91:1637-40. https://doi.org/10.1016/j.apmr.2010.05.017.
- BrainLine.org . Brain Trauma, Concussion, and Coma 2017. www.brainline.org/content/2009/06/brain-trauma-concussion-and-coma_pageall.html (accessed 12 January 2017).
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2010.
- BrainandSpinalCord.org . Prognosis of a TBI 2017. www.brainandspinalcord.org/prognosis-of-a-tbi/ (accessed 10 January 2017).
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT, Caldwell FE. Measurement of post-traumatic amnesia: how reliable is it?. J Neurol Neurosurg Psychiatr 1997;62:38-42. https://doi.org/10.1136/jnnp.62.1.38.
- Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 2014;13:844-54. https://doi.org/10.1016/S1474-4422(14)70120-6.
- Daisley A, Tams R, Kischka U. Head Injury: The Facts. Oxford: Oxford University Press; 2009.
- National Institute of Neurological Disorders and Stroke . Traumatic Brain Injury Information Page 2017. www.ninds.nih.gov/Disorders/All-Disorders/Traumatic-Brain-Injury-Information-Page (accessed 5 January 2017).
- Headway – The Brain Injury Association . How Severe Is the Brain Injury? 2017. www.headway.org.uk/about-brain-injury/individuals/types-of-brain-injury/traumatic-brain-injury/how-severe-is-the-brain-injury/ (accessed 11 January 2017).
- Ponsford J. Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation 2013;32:803-15. https://doi.org/10.3233/NRE-130904.
- Andelic N, Hammergren N, Bautz-Holter E, Sveen U, Brunborg C, Røe C. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurol Scand 2009;120:16-23. https://doi.org/10.1111/j.1600-0404.2008.01116.x.
- Mansfield E, Stergiou-Kita M, Cassidy JD, Bayley M, Mantis S, Kristman V, et al. Return-to-work challenges following a work-related mild TBI: the injured worker perspective. Brain Inj 2015;29:1362-9. https://doi.org/10.3109/02699052.2015.1053524.
- Sendroy-Terrill M, Whiteneck GG, Brooks CA. Aging with traumatic brain injury: cross-sectional follow-up of people receiving inpatient rehabilitation over more than 3 decades. Arch Phys Med Rehabil 2010;91:489-97. https://doi.org/10.1016/j.apmr.2009.11.011.
- Cancelliere C, Kristman VL, Cassidy JD, Hincapié CA, Côté P, Boyle E, et al. Systematic review of return to work after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil 2014;95:S201-9. https://doi.org/10.1016/j.apmr.2013.10.010.
- Theadom A, Parag V, Dowell T, McPherson K, Starkey N, Barker-Collo S, et al. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract 2016;66:e16-23. https://doi.org/10.3399/bjgp16X683161.
- Doctor JN, Castro J, Temkin NR, Fraser RT, Machamer JE, Dikmen SS. Workers’ risk of unemployment after traumatic brain injury: a normed comparison. J Int Neuropsychol Soc 2005;11:747-52. https://doi.org/10.1017/S1355617705050836.
- van Velzen J, van Bennekom C, Edelaar M, Sluiter JK, Frings-Dresen M. How many people return to work after acquired brain injury?: a systematic review. Brain Inj 2009;23:473-88. https://doi.org/10.1080/02699050902970737.
- Kendall E, Muenchberger H, Gee T. Vocational rehabilitation following traumatic brain injury: a quantitative synthesis of outcome studies. J Vocat Rehabil 2006;25:149-60.
- Pössl J, Jürgensmeyer S, Karlbauer F, Wenz C, Goldenberg G. Stability of employment after brain injury: a 7-year follow-up study. Brain Inj 2001;15:15-27. https://doi.org/10.1080/02699050150209093.
- Johnson R. How do people get back to work after severe head injury? A 10 year follow-up study. Neuropsychol Rehabil 1998;8:61-79. https://doi.org/10.1080/713755552.
- Wade DT, Crawford S, Wenden FJ, King NS, Moss NE. Does routine follow up after head injury help? A randomised controlled trial. J Neurol Neurosurg Psychiatr 1997;62:478-84. https://doi.org/10.1136/jnnp.62.5.478.
- Wade DT, King NS, Wenden FJ, Crawford S, Caldwell FE. Routine follow up after head injury: a second randomised controlled trial. J Neurol Neurosurg Psychiatr 1998;65:177-83. https://doi.org/10.1136/jnnp.65.2.177.
- Twamley E, Jak A, Delis D, Bondi M, Lohr J. Cognitive Symptom Management and Rehabilitation Therapy (CogSMART) for Veterans with traumatic brain injury: pilot randomized controlled trial. J Rehabil Res Dev 2014;51. https://doi.org/10.1682/JRRD.2013.01.0020.
- Cuthbert JP, Harrison-Felix C, Corrigan JD, Bell JM, Haarbauer-Krupa JK, Miller AC. Unemployment in the United States after traumatic brain injury for working-age individuals: prevalence and associated factors 2 years postinjury. J Head Trauma Rehabil 2015;30:160-74. https://doi.org/10.1097/HTR.0000000000000090.
- Vocational Rehabilitation: The Way Forward: A Working Party Report. London: British Society of Rehabilitation Medicine; 2003.
- Bootes K, Chapparo CJ. Cognitive and behavioural assessment of people with traumatic brain injury in the work place: occupational therapists’ perceptions. Work 2002;19:255-68.
- Franulic A, Carbonell CG, Pinto P, Sepulveda I. Psychosocial adjustment and employment outcome 2, 5 and 10 years after TBI. Brain Inj 2004;18:119-29. https://doi.org/10.1080/0269905031000149515.
- Shames J, Treger I, Ring H, Giaquinto S. Return to work following traumatic brain injury: trends and challenges. Disabil Rehabil 2007;29:1387-95. https://doi.org/10.1080/09638280701315011.
- Björkdahl A. The return to work after a neuropsychological programme and prognostic factors for success. Brain Inj 2010;24:1061-9. https://doi.org/10.3109/02699052.2010.494588.
- McNamee S, Walker W, Cifu DX, Wehman PH. Minimizing the effect of TBI-related physical sequelae on vocational return. J Rehabil Res Dev 2009;46:893-908. https://doi.org/10.1682/JRRD.2008.08.0106.
- Kersel DA, Marsh NV, Havill JH, Sleigh JW. Psychosocial functioning during the year following severe traumatic brain injury. Brain Inj 2001;15:683-96. https://doi.org/10.1080/02699050010013662.
- Corrigan JD, Bogner JA, Mysiw WJ, Clinchot D, Fugate L. Life satisfaction after traumatic brain injury. J Head Trauma Rehabil 2001;16:543-55. https://doi.org/10.1097/00001199-200112000-00003.
- Catalano D, Pereira AP, Wu MY, Ho H, Chan F. Service patterns related to successful employment outcomes of persons with traumatic brain injury in vocational rehabilitation. NeuroRehabilitation 2006;21:279-93.
- Ponsford J, Draper K, Schönberger M. Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc 2008;14:233-42. https://doi.org/10.1017/S1355617708080272.
- Johnson V, Marek M, Ranalli N, Smith D. Assessment of Traumatic Brain Injury, Acute 2009. http://bestpractice.bmj.com/best-practice/monograph/515.html (accessed 1 March 2009).
- McCrimmon S, Oddy M. Return to work following moderate-to-severe traumatic brain injury. Brain Inj 2006;20:1037-46. https://doi.org/10.1080/02699050600909656.
- Sherer M, Davis LC, Sander AM, Caroselli JS, Clark AN, Pastorek NJ. Prognostic importance of self-reported traits/problems/strengths and environmental barriers/facilitators for predicting participation outcomes in persons with traumatic brain injury: a systematic review. Arch Phys Med Rehabil 2014;95:1162-73. https://doi.org/10.1016/j.apmr.2014.02.006.
- Walker WC, Marwitz JH, Kreutzer JS, Hart T, Novack TA. Occupational categories and return to work after traumatic brain injury: a multicenter study. Arch Phys Med Rehabil 2006;87:1576-82. https://doi.org/10.1016/j.apmr.2006.08.335.
- Rapport LJ, Bryer RC, Hanks RA. Driving and community integration after traumatic brain injury. Arch Phys Med Rehabil 2008;89:922-30. https://doi.org/10.1016/j.apmr.2008.01.009.
- Radford KA, Lincoln NB, Murray-Leslie C. Validation of the stroke drivers screening assessment for people with traumatic brain injury. Brain Inj 2004;18:775-86. https://doi.org/10.1080/02699050310001657394.
- Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure 2000;9:453-7. https://doi.org/10.1053/seiz.2000.0458.
- Langley J, Johnson S, Slatyer M, Skilbeck CE, Thomas M. Issues of loss to follow-up in a population study of traumatic brain injury (TBI) followed to 3 years post-trauma. Brain Inj 2010;24:939-47. https://doi.org/10.3109/02699052.2010.491494.
- Waddell G, Burton AK, Kendall NAS. Vocational Rehabilitation. What Works, for Whom, and When? Report for the Vocational Rehabilitation Task Group 2008.
- Escorpizo R, Finger ME, Glässel A, Cieza A. An international expert survey on functioning in vocational rehabilitation using the international classification of functioning, disability and health. J Occup Rehabil 2011;21:147-55. https://doi.org/10.1007/s10926-010-9276-y.
- Radford K, Phillips J, Drummond A, Sach T, Walker M, Tyerman A, et al. Return to work after traumatic brain injury: cohort comparison and economic evaluation. Brain Inj 2013;27:507-20. https://doi.org/10.3109/02699052.2013.766929.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- 2010 to 2015 Government Policy: Employment. London: The Stationery Office; 2013.
- Turner-Strokes L. Rehabilitation Following Acquired Brain Injury: National Clinical Guidelines. London: Royal College of Physicians; 2003.
- Vocational Assessment and Rehabilitation for People with Long-term Neurological Conditions: Recommendations for Best Practice. London: BSRM; 2010.
- Tyerman A, Meehan M. Vocational Assessment and Rehabilitation After Acquired Brain Injury: Inter-agency Guidelines. London: Royal College of Physicians; 2004.
- NHS Outcomes Framework 2015 to 2016. Edinburgh: Department of Health and Social Care; 2014.
- Black CD, Frost D. Health at Work – An Independent Review of Sickness Absence. London: The Stationery Office; 2011.
- Playford E, Radford K, Burton C, Gibson A, Jellie B, Sweetland J, et al. Mapping Vocational Rehabilitation Services for People with Long Term Neurological Conditions: Summary Report. London: Department of Health and Social Care; 2011.
- Deshpande P, Turner-Stokes L. Survey of Vocational Rehabilitation Services Available to People with Acquired Brain Injury in the UK. Vocational Assessment and Rehabilitation After Acquired Brain Injury. Inter-agency Guidelines. London: Royal College of Physicians; 2004.
- Graham CW, West MD, Bourdon JL, Inge KJ, Seward HE, Graham CW. Employment interventions for return to work in working-aged adults following traumatic brain injury: a systematic review. Camp Syst Rev 2016;6.
- Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: a randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. JAMA 2000;283:3075-81. https://doi.org/10.1001/jama.283.23.3075.
- Man DWK, Poon WS, Lam C. The effectiveness of artificial intelligent 3-D virtual reality vocational problem-solving training in enhancing employment opportunities for people with traumatic brain injury. Brain Inj 2013;27:1016-25. https://doi.org/10.3109/02699052.2013.794969.
- O’Connor MK, Mueller L, Kwon E, Drebing CE, O’Connor AA, Semiatin A, et al. Enhanced vocational rehabilitation for Veterans with mild traumatic brain injury and mental illness: pilot study. J Rehabil Res Dev 2016;53:307-20. https://doi.org/10.1682/JRRD.2014.10.0231.
- Trexler LE, Parrott DR, Malec JF. Replication of a prospective randomized controlled trial of resource facilitation to improve return to work and school after brain injury. Arch Phys Med Rehabil 2016;97:204-10. https://doi.org/10.1016/j.apmr.2015.09.016.
- Cicerone KD, Mott T, Azulay J, Sharlow-Galella MA, Ellmo WJ, Paradise S, et al. A randomized controlled trial of holistic neuropsychologic rehabilitation after traumatic brain injury. Arch Phys Med Rehabil 2008;89:2239-49. https://doi.org/10.1016/j.apmr.2008.06.017.
- Vanderploeg RD, Schwab K, Walker WC, Fraser JA, Sigford BJ, Date ES, et al. Rehabilitation of traumatic brain injury in active duty military personnel and veterans: Defense and Veterans Brain Injury Center randomized controlled trial of two rehabilitation approaches. Arch Phys Med Rehabil 2008;89:2227-38. https://doi.org/10.1016/j.apmr.2008.06.015.
- Powell J, Heslin J, Greenwood R. Community based rehabilitation after severe traumatic brain injury: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2002;72:193-202. https://doi.org/10.1136/jnnp.72.2.193.
- Zhu X, Poon W, Chan CC, Chan SS. Does intensive rehabilitation improve the functional outcome of patients with traumatic brain injury (TBI)? A randomized controlled trial. Brain Inj 2007;21:681-90. https://doi.org/10.1080/02699050701468941.
- Elgmark Andersson E, Emanuelson I, Björklund R, Stålhammar DA. Mild traumatic brain injuries: the impact of early intervention on late sequelae. A randomized controlled trial. Acta Neurochir (Wien) 2007;149:151-9. https://doi.org/10.1007/s00701-006-1082-0.
- Bell KR, Brockway JA, Hart T, Whyte J, Sherer M, Fraser RT, et al. Scheduled telephone intervention for traumatic brain injury: a multicenter randomized controlled trial. Arch Phys Med Rehabil 2011;92:1552-60. https://doi.org/10.1016/j.apmr.2011.05.018.
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. Interventions and service need following mild and moderate head injury: the Oxford Head Injury Service. Clin Rehabil 1997;11:13-27. https://doi.org/10.1177/026921559701100104.
- Borglin G. Mixed methods in health service research – where do we go from here?. BMC Health Serv Res 2016;16.
- Richards DA, Hallberg IR. Complex Interventions in Health: An Overview of Research Methods. Abingdon: Routledge; 2015.
- Logic Model Development Guide. Battle Creek, MI: WK Kellogg Foundation; 2004.
- Phillips J. Return to Work after Traumatic Brain Injury: A Cohort Comparison Study and Feasibility Economic Analysis. Nottingham: University of Nottingham; 2013.
- Phillips J, Drummond A, Radford K, Tyerman A. Return to work after traumatic brain injury: recording, measuring and describing occupational therapy intervention. Br J Occup Ther 2010;73:422-30. https://doi.org/10.4276/030802210X12839367526138.
- Fadyl JK, McPherson KM. Approaches to vocational rehabilitation after traumatic brain injury: a review of the evidence. J Head Trauma Rehabil 2009;24:195-212. https://doi.org/10.1097/HTR.0b013e3181a0d458.
- das Nair R, Lincoln N, Ftizsimmons D, Brain N, Montgomery A, Bradshaw L, et al. Rehabilitation of memory following brain injury: a phase III randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-6.
- Logan PA, Armstrong S, Avery TJ, Barer D, Barton GR, Darby J, et al. Rehabilitation aimed at improving outdoor mobility for people after stroke: a multicentre randomised controlled study (the Getting out of the House Study). Health Technol Assess 2014;18. https://doi.org/10.3310/hta18290.
- Garfield A, Holmes J, Ford E. Low Mood and MS – A Group Adjustment Course. Nottingham: University of Nottingham; 2013.
- Walker MF, Sunderland A, Fletcher-Smith J, Drummond A, Logan P, Edmans JA, et al. The DRESS trial: a feasibility randomized controlled trial of a neuropsychological approach to dressing therapy for stroke inpatients. Clin Rehabil 2012;26:675-85. https://doi.org/10.1177/0269215511431089.
- Petzold A, Korner-Bitensky N, Menon A. Using the knowledge to action process model to incite clinical change. J Contin Educ Health Prof 2010;30:167-71. https://doi.org/10.1002/chp.20077.
- McCormack B, Rycroft-Malone J, DeCorby K, Hutchinson AM, Bucknall T, Kent B, et al. A realist review of interventions and strategies to promote evidence-informed healthcare: a focus on change agency. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-107.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feas Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-18.
- Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Crowther R, Marshall M, Bond GR, Huxley P. Vocational rehabilitation for people with severe mental illness. Cochrane Database Syst Rev 2001;2. https://doi.org/10.1002/14651858.CD003080.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Nouri F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- Willer B, Rosenthal M, Kreutzer JS, Gordon WA, Rempel R. Assessment of community integration following rehabilitation for traumatic brain injury. J Head Trauma Rehabil 1993;8:75-87. https://doi.org/10.1097/00001199-199308020-00009.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65. https://doi.org/10.2165/00019053-199304050-00006.
- Tuomi K, Ilmarinen J, Jahkola A, Katajarinne L, Tulkki A, Ilmarinen J. Ageing and Work. Helsinki: Institute of Occupational Health; 1993.
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480-4. https://doi.org/10.1016/S0140-6736(75)92830-5.
- International Council for Harmonisation . Efficacy Guidelines E6 R1: Good Clinical Practice 1996. www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed 1 August 2016).
- Research Governance Framework for Health and Social Care. London: Department of Health and Social Care; 2005.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/article/PMG9/chapter/Foreword (accessed 24 July 2016).
- Pritchard C, Sculpher M. Productivity Costs: Principles and Practice in Economic Evaluation. London: Office of Health Economics; 2000.
- Sach TH, Whynes DK. Measuring indirect costs: is there a problem?. Appl Health Econ Health Policy 2003;2:135-9.
- Curtis L, Burns H. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Department for Transport . Free Flow Vehicle Speed Statistics: Great Britain 2015 2016. www.gov.uk/government/uploads/system/uploads/attachment_data/file/533244/free-flow-vehicle-speeds-great-britain-2015.pdf (accessed 19 December 2017).
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 22 April 2016).
- Health and Social Care Information Centre . Prescription Cost Analysis, England 2015. https://data.gov.uk/dataset/prescription-cost-analysis-england (accessed 17 May 2016).
- Office for National Statistics . Annual Survey of Hours and Earnings Statistical Bulletins. Annual Survey of Hours and Earnings: 2015 Provisional Results n.d. www.ons.gov.uk/ons/rel/ashe/annual-survey-of-hours-and-earnings/index.html (accessed 7 July 2016).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. https://doi.org/10.1111/j.1524-4733.2008.00358.x.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-40. https://doi.org/10.1002/(SICI)1099-1050(199707)6:4<327::AID-HEC282>3.0.CO;2-W.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6:51-9. https://doi.org/10.1332/174426410X482999.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Dillman DA. Mail And Internet Surveys: The Tailored Design Method. New York, NY: John Wiley & Sons; 2000.
- Delbecq AL, Van de Ven AH, Gustafson DH. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Processes. Glenview, IL: Scott Foresman; 1975.
- Ritchie J, Lewis J, Nicholls CM, Ormston R. Qualitative Research Practice: A Guide For Social Science Students and Researchers. London: Sage; 2013.
- Hoffmann TC, Erueti C, Glasziou PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f3755.
- Malec JF, Buffington AL, Moessner AM, Degiorgio L. A medical/vocational case coordination system for persons with brain injury: an evaluation of employment outcomes. Arch Phys Med Rehabil 2000;81:1007-15. https://doi.org/10.1053/apmr.2000.6980.
- Sinclair E, Radford K, Grant M, Terry J. Developing stroke-specific vocational rehabilitation: a soft systems analysis of current service provision. Disabil Rehabil 2014;36:409-17. https://doi.org/10.3109/09638288.2013.793410.
- Svendsen HA, Teasdale TW. The influence of neuropsychological rehabilitation on symptomatology and quality of life following brain injury: a controlled long-term follow-up. Brain Inj 2006;20:1295-306. https://doi.org/10.1080/02699050601082123.
- Gilworth G, Eyres S, Carey A, Bhakta BB, Tennant A. Working with a brain injury: personal experiences of returning to work following a mild or moderate brain injury. J Rehabil Med 2008;40:334-9. https://doi.org/10.2340/16501977-0169.
- Tsaousides T, Warshowsky A, Ashman TA, Cantor JB, Spielman L, Gordon WA. The relationship between employment-related self-efficacy and quality of life following traumatic brain injury. Rehabil Psychol 2009;54. https://doi.org/10.1037/a0016807.
- O’Callaghan A, McAllister L, Wilson L. Insight vs readiness: factors affecting engagement in therapy from the perspectives of adults with TBI and their significant others. Brain Inj 2012;26:1599-610. https://doi.org/10.3109/02699052.2012.698788.
- Grant M. Developing, Delivering and Evaluating Stroke Specific Vocational Rehabilitation: A Feasibility Randomised Controlled Trial. Nottingham: University of Nottingham; 2014.
- Marshman LA, Jakabek D, Hennessy M, Quirk F, Guazzo EP. Post-traumatic amnesia. J Clin Neurosci 2013;20:1475-81. https://doi.org/10.1016/j.jocn.2012.11.022.
- Laureys S, Piret S, Ledoux D. Quantifying consciousness. Lancet Neurol 2005;4:789-90. https://doi.org/10.1016/S1474-4422(05)70230-1.
- Brasure M, Lamberty GJ, Sayer NA, Nelson NW, MacDonald R, Ouellette J, et al. Participation after multidisciplinary rehabilitation for moderate to severe traumatic brain injury in adults: a systematic review. Arch Phys Med Rehabil 2013;94:1398-420. https://doi.org/10.1016/j.apmr.2012.12.019.
- Vikane E, Hellstrøm T, Røe C, Bautz-Holter E, Assmus J, Skouen JS. Missing a follow-up after mild traumatic brain injury – does it matter?. Brain Inj 2014;28:1374-80. https://doi.org/10.3109/02699052.2014.919532.
- Jourdan C, Bayen E, Bahrami S, Ghout I, Darnoux E, Azerad S, et al. Loss to follow-up and social background in an inception cohort of patients with severe traumatic brain injury: results from the PariS-TBI study. J Head Trauma Rehabil 2016;31:E42-8. https://doi.org/10.1097/HTR.0000000000000147.
- Krellman JW, Kolakowsky-Hayner SA, Spielman L, Dijkers M, Hammond FM, Bogner J, et al. Predictors of follow-up completeness in longitudinal research on traumatic brain injury: findings from the National Institute on Disability and Rehabilitation Research traumatic brain injury model systems program. Arch Phys Med Rehabil 2014;95:633-41. https://doi.org/10.1016/j.apmr.2013.10.016.
- Tyerman A. Vocational rehabilitation after traumatic brain injury: models and services. NeuroRehabilitation 2012;31:51-62. https://doi.org/10.3233/NRE-2012-0774.
- Sambunjak D, Straus SE, Marusić A. Mentoring in academic medicine: a systematic review. JAMA 2006;296:1103-15. https://doi.org/10.1001/jama.296.9.1103.
- Abdullah G, Rossy D, Ploeg J, Davies B, Higuchi K, Sikora L, et al. Measuring the effectiveness of mentoring as a knowledge translation intervention for implementing empirical evidence: a systematic review. Worldviews Evid Based Nurs 2014;11:284-300. https://doi.org/10.1111/wvn.12060.
- Twamley EW, Thomas KR, Gregory AM, Jak AJ, Bondi MW, Delis DC, et al. CogSMART compensatory cognitive training for traumatic brain injury: effects over 1 year. J Head Trauma Rehabil 2015;30:391-40. https://doi.org/10.1097/HTR.0000000000000076.
- Turner-Stokes L, Pick A, Nair A, Disler PB, Wade DT. Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD004170.pub3.
- Paniak C, Toller-Lobe G, Durand A, Nagy J. A randomized trial of two treatments for mild traumatic brain injury. Brain Inj 1998;12:1011-23. https://doi.org/10.1080/026990598121927.
- Kristman VL, Côté P, Hogg-Johnson S, Cassidy JD, Van Eerd D, Vidmar M, et al. The burden of work disability associated with mild traumatic brain injury in Ontario compensated workers: a prospective cohort study. Open Occup Health Saf J 2010;2:1-8. https://doi.org/10.2174/1876216601002010001.
- Friedland JF, Dawson DR. Function after motor vehicle accidents: a prospective study of mild head injury and posttraumatic stress. J Nerv Ment Dis 2001;189:426-34. https://doi.org/10.1097/00005053-200107000-00003.
- Stulemeijer M, van der Werf S, Borm GF, Vos PE. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J Neurol Neurosurg Psychiatry 2008;79:936-42. https://doi.org/10.1136/jnnp.2007.131250.
- Eva G, Playford D, Sach T, Barton G, Risebro H, Radford K, et al. Thinking Positively About Work. Delivering Work Support and Vocational Rehabilitation for People with Cancer. London: University College London Institute of Neurology; 2012.
- Frank AO, Thurgood J. Vocational rehabilitation in the UK: opportunities for health-care professionals. Int J Ther Rehabil 2006;13:126-34. https://doi.org/10.12968/ijtr.2006.13.3.21364.
Appendix 1 Summary of ESTVR
Purpose of this document
This document aims to provide a non-specialist summary of the intervention to be evaluated, providing core information on when, where, how often and by whom the intervention is delivered to those participants randomised to this arm of the trial. It also summarises the ESTVR process and core activities of the intervention, emphasising ‘minimum standards’.
Aims of the intervention
ESTVR aims to prevent job loss in people who were employed prior to their TBI by adopting a case co-ordination model that involves identifying employed people within 8 weeks of injury, assessing the TBI on the person and its impact on work activities, roles and responsibilities and finding acceptable ways of overcoming problems. This includes the use of strategies to cope with TBI-related problems (e.g. fatigue management); liaison with employers and other statutory and voluntary service providers involved in the person’s care including health and social care, employment-related and voluntary services (e.g. Headway) planning; preparing for, and negotiating, a phased RTW and supporting adjustment to disability over time; and by adapting the work environment by making recommendations to modify or to overcome problems (e.g. physical adaptations, additional breaks, changes to working hours, roles and responsibilities and supernumerary support). Family members and employers receive support to promote their understanding of the TBI impact on the person and their work ability.
When, where and how often
-
Intervention starts about 5 weeks after admission to hospital.
-
It lasts a maximum of 52 weeks with one or two contacts per month, but a variable number of contacts dependent on individual participant need.
-
Early sessions take place in inpatient facilities, often a specialist trauma or rehabilitation unit when possible and include family and carers when possible.
-
Many people will be discharged from inpatient care after the first few days or weeks when the intervention will continue in the community, including the patient’s home or workplace or another mutually agreed venue.
By whom
The intervention is designed, implemented and monitored by qualified OTs registered with the Health Professionals Council and (ideally) employed by NHS trusts. Participating OTs will be trained in the intervention. OTs deliver all of the face-to-face contacts with patients.
Content of the intervention
The intervention is multifaceted and must be tailored to individual needs and abilities.
However, the ESTVR process involves the following core activities.
1. Assessment
As a minimum, participants receive initial and ongoing assessment from a qualified OT using a range of rehabilitation and vocational needs assessment tools to:
-
assess the impact of the TBI on the participant’s function, family and work roles
-
assess the work/study role, work duties/functions and work/job demands
-
identify specific work goals and a VR plan
-
risk assess the home, community and work environment to enable safe home/community visits.
2. Early education and advice
As a minimum, an OT/CM with TBI and VR expertise makes contact with the participant in hospital or at home (within 10 days) to establish rapport and provide early education, advice and support to the participant and family. This involves:
-
advice to not make premature decisions about RTW (i.e. until the impact of the TBI is understood and coping strategies formulated)
-
advice to keep employers informed of their situation
-
information about the role of the OT/CM.
Education and advice continues throughout the VR process (OT every 1–2 weeks, CM every 6–8 weeks), individually tailored to the participant, family and employer.
The OT/CM provides emotional support to the participant and family.
3. Co-ordination and communication
As a minimum, participants receive a co-ordinated programme where the OT/CM establishes which other health, DWP, private provider and voluntary services involved and:
-
collects relevant information about other organisations and services involved in the participant’s care and co-ordinates VR activity
-
actively liaises, and communicates in writing, with everyone involved in the participants care, to agree work goals and ensure that consistent advice is given about returning to work
-
works with the participant to ensure that non-work-focused activities and rehabilitation continues
-
establishes and maintains communication with the employer as required and informs them of rehabilitation goals
-
supports the participant prior to, and after, meetings with the employer and other stakeholders to clarify what was said and agreed
-
plans rehabilitation and RTW in consultation with the participant
-
agrees discharge from the ESTVR intervention mutually with the participant
-
informs all other parties of the end of the intervention.
4. Flexible, individually tailored preparation to return to work
As a minimum, participants receive a flexible, individually tailored VR programme where:
-
RTW options are explored with all parties
-
learning opportunities are provided to support RTW
-
work skills/functions are practised or retraining is organised
-
strategies are developed with the participant (and family) to communicate the impact of their TBI to colleagues and others
-
coping strategies are developed with the participant (and family) to manage the effects of their TBI in everyday life and at work/study
-
alternatives to pre-injury employment are explored in cases for which return to pre-existing employer is not feasible or is unsustainable.
5. Optimisation of the employment environment
As a minimum, participants receive support to negotiate a graded RTW for reintegration which involves the OT:
-
monitoring factors that may limit the participant’s capacity to carry out job tasks safely or that may limit job stability or threaten longer-term sustainability over the RTW period (every 2–5 days in the early days after work return, reducing to every 2 months later)
-
supporting the participant to seek and accept feedback about their (work) function
-
reinforcing coping skills to deal with potential risks to job retention.
Employer liaison ends at a mutually agreed time when the participant, employer and OT feel that the work situation is safe and sustainable or when the trial ends (whichever is sooner).
List of abbreviations
- A&E
- accident and emergency
- ABI
- acquired brain injury
- ADL
- activities of daily living
- AI
- artificial intelligence
- ASHE
- Annual Survey of Hours and Earnings
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- CIQ
- Community Integration Questionnaire
- CLRN
- Clinical Local Research Network
- CM
- case manager
- CONSORT
- Consolidated Standards of Reporting Trials
- CSI
- Caregiver Strain Index
- CT
- computerised tomography
- CTU
- Clinical Trials Unit
- DEA
- disability employment advisor
- DWP
- Department for Work and Pensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D VAS
- EuroQol-5 Dimensions Visual Analogue Scale
- ESTVR
- Early Specialist Traumatic brain injury Vocational Rehabilitation
- EVPI
- expected value of perfect information
- FRESH
- Facilitating Return to work through Early Specialist Health-based interventions
- GCS
- Glasgow Coma Scale
- GOS
- Glasgow Outcome Scale
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HCP
- health-care professional
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRAS
- Integrated Research Application System
- LTNC
- long-term neurological condition
- MTC
- major trauma centre
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NTBIS
- Nottingham Traumatic Brain Injury Service
- OR
- odds ratio
- OT
- occupational therapist
- PCA
- Prescription Cost Analysis
- PI
- principal investigator
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PTA
- post-traumatic amnesia
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RA
- research assistant
- RCT
- randomised controlled trial
- RF
- resource facilitation
- RTA
- road traffic accident
- RTW
- return to work
- SA
- sensitivity analysis
- SALT
- speech and language therapy
- SD
- standard deviation
- SHEAP
- Statistical and Health Economics Analysis Plan
- SSC
- Study Steering Committee
- TARN
- Trauma Audit and Research Network
- TBI
- traumatic brain injury
- UC
- usual care
- VR
- vocational rehabilitation
- WAI
- Work Ability Index
- WPAI: SHP
- Work Productivity and Activity Impairment Questionnaire Specific Health Problem v2.0
- WPAI v2
- Work Productivity and Activity Impairment v2
- WTP
- willingness to pay
