Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/75. The contractual start date was in April 2011. The draft report began editorial review in September 2016 and was accepted for publication in February 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Maya H Buch reports grants from Pfizer and Chugai Pharmaceutical Co. Ltd (Roche), and personal fees from AstraZeneca, Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd (Roche), Sandoz and R-Pharm, during the conduct of the study. Claire Hulme reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, during the conduct of the study, and was a member of the NIHR HTA Commissioning Board during the conduct of the study. Paul Emery reports grants and personal fees from Pfizer, Merck Sharp & Dohme Corp., AbbVie, Bristol-Myers Squibb, UCB Pharma Ltd, Roche, Novartis, Samsung, Sandoz, and Eli Lilly and Company, during the conduct of the study. Sue Pavitt is a member of the Efficacy and Mechanism Evaluation Board and NIHR Clinical Trials Unit Board and has been a recipient of NIHR Clinical Trials Unit Support funding. Linda Sharples, Sarah Brown and Claire Davies report grants from NIHR HTA programme during the conduct of the study. Christopher McCabe reports that historically he worked as a paid consultant for a number of pharmaceutical companies. He has also done paid extensive work for the NHS.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Rheumatoid arthritis (RA), the most common autoimmune disease in the Western world,1 is a chronic and systemic inflammatory arthritis that affects 0.8% of the UK population. 2 RA is the largest cause of treatable disability in the Western world. 3,4 Patients with RA suffer considerable pain, stiffness and swelling and, if not adequately controlled, sustain various degrees of joint destruction, deformity and significant functional decline. RA has a considerable health and socioeconomic impact, as a result of both hospitalisation and loss of employment, with over 50% of patients work-disabled within 10 years of diagnosis. 5–7
Rheumatoid arthritis is associated with significant comorbidity and increased mortality compared with the general population,8 largely because the prevalence of premature cardiovascular disease9 is as high as that seen in patients with other major cardiovascular disease risk factors, such as type 2 diabetes,10 and, in fact, is the cause of death of 48% of patients with RA. RA-related inflammation and disease activity over time are associated with increased cardiovascular disease risk in patients with RA,11–14 which further emphasises the importance of ensuring optimal and effective disease control.
As compared with other chronic diseases, such as hypertension and type 2 diabetes, treatment of RA previously employed a gradual ‘step-up’ strategy with the use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). 15 The concept of the ability to modify disease course started to be realised in the 1990s,16 with key studies demonstrating the importance of early diagnosis and expedient implementation of csDMARD therapy,17–19 which remain the cornerstones of management of RA. These paradigms have been consolidated by strategy trials and meta-analysis on the radiographic benefit. 20–24
The National Institute for Health and Care Excellence’s rheumatoid arthritis management guidance
The National Institute for Health and Care Excellence (NICE)’s clinical guidance for the management of RA25 recommends, in people with newly diagnosed active RA, a combination of csDMARDs [including methotrexate (MTX) and at least one other csDMARD, plus short-term glucocorticoids] as first-line treatment as soon as possible, ideally within 3 months of the onset of persistent symptoms (and within 6 weeks of diagnosis by a rheumatologist).
Methotrexate is thus recommended as the optimal first-line treatment strategy25,26 either as monotherapy or in combination. Nevertheless, it had become clear that poor response (even if initially effective) remained a feature with most csDMARDs over time, with progression of joint damage and functional decline. In addition, a high incidence of toxicity has been observed with these drugs. 27 Such obstacles to therapy, combined with data suggesting limited alteration in long-term outcome, even in those patients showing a response, are an argument for more effective therapy. 28
Biological therapies
This unmet clinical need fuelled continued research into our understanding of RA, which led to significant advances by the 1990s. Inflammation was recognised to be a result of imbalance between pro-inflammatory cytokines such as tumour necrosis factor (TNF) alpha [as well as interleukin 1(IL-1), IL-6 and others] and anti-inflammatory cytokines such as IL-4 and IL-10. In RA, an excess of pro-inflammatory cytokines, in particular TNF and IL-1, has been shown to be responsible for disrupting this balance towards continued inflammation and cartilage and bone damage, and thus critical in driving RA pathogenesis. 29 This understanding was complemented with significant advances in biotechnology. Following in vitro and in vivo work, the most compelling evidence for a key role for TNF stemmed from studies in which marked clinical benefit was observed in patients with RA treated with a chimeric anti-TNF monoclonal antibody. 30 The subsequent introduction of several costly, but highly effective, tumour necrosis factor inhibitor (TNFi) therapies marked the start of a new era in biologic disease-modifying antirheumatic drug (bDMARD) development for RA. 31–33
Tumour necrosis factor inhibitors
Tumour necrosis factor inhibitor drugs [etanercept (Enbrel®; Pfizer, New York City, NT, USA), infliximab (REMICADE®, Janssen Pharmaceutical, Beerse, Belgium), adalimumab (HUMIRA®; Abbott, now AbbVie, North Chicago, IL, USA), certolizumab pegol (CZP) (CIMZIA®; UCB, Brussels, Belgium) and golimumab (Simponi®; Janssen Pharmaceutical)] in combination with MTX produce better outcomes in RA than in placebo or treatment with MTX alone. 31–37 TNFi drugs, however, differ in several respects:
-
molecule type [chimeric (mouse–human) monoclonal antibody (infliximab), fully human monoclonal antibody (adalimumab, golimumab), pegylated Fab fragment of a humanised monoclonal antibody (CZP) and a TNF receptor fusion protein (etanercept)]38
-
target (etanercept binds both TNF and another cytokine, lymphotoxin alpha)39
-
binding affinity to TNF
-
clinical administration (intravenous vs. subcutaneous).
Non-tumour necrosis factor inhibitors
Following the development of TNFis, recognition of other key cytokines and immune cells in RA pathogenesis43 led to the development of additional bDMARDs: rituximab (MabThera; Roche, Basel, Switzerland), a chimeric anti-CD20-depleting monoclonal antibody,44 tocilizumab (Actemra®; Roche), an IL-6 receptor monoclonal antibody45 and abatacept (Orencia®; Bristol-Myers Squibb, New York City, NY, USA), a recombinant fusion protein T-cell co-stimulation blocking agent. 46 All of these bDMARDs demonstrated significant benefits compared with placebo and MTX in MTX-inadequate response44,47,48 and TNFi-inadequate response49–51 groups, respectively.
The clinical unmet need
Tumour necrosis factor inhibitor is the most frequently used first-line bDMARD. Despite the extensive benefits of TNF-directed bDMARDs, a significant proportion, 20–40%, of patients with RA who have MTX-inadequate response and treated with TNFi52 fail to achieve sufficient response (primary non-response) or lose responsiveness over time (secondary non-response). 36,52
Thus, following initial TNFi-inadequate response, two broad approaches could theoretically be employed to manage patients: switching to alternative TNFi therapy or switching to a bDMARD with another mode of action. 26
The National Institute for Health and Care Excellence’s technology appraisal for biologic disease-modifying antirheumatic drugs
At the time of the SWITCH study, a NICE technology appraisal recommended TNFi use if disease is severe, that is, a Disease Activity Score of 28 joints (DAS28) of > 5.1 units and disease has not responded to at least two conventional disease-modifying antirheumatic drugs (DMARDs), including MTX. Initially, adalimumab, etanercept and infliximab53 (and later on CZP54 and golimumab55) were recommended by NICE as first-line bDMARD therapy for the treatment of patients with RA who had failed to respond to, or had been intolerant of, at least two csDMARDs including MTX. 56
The current NICE technology appraisal 37556 has updated possible first-line bDMARD options and now recommends use not only of one of the five TNFi, but also of tocilizumab57 and abatacept,58 which are also approved by NICE for first-line bDMARD therapy following MTX-inadequate response. 58 Nevertheless, TNFi remains the most frequently used first-line bDMARD (both in the UK and worldwide).
Non-response to tumour necrosis factor inhibitor
In the context of first-line bDMARD TNFi failure, NICE guidance recommends using rituximab as second-line bDMARD. 59 Switching to alternative TNFi, abatacept or tocilizumab, is permitted only if patients have had an inadequate response to rituximab57 or are intolerant of rituximab57,59 or if rituximab is contraindicated. 57,59 This is in the absence of any trial data demonstrating that rituximab is more appropriate than the alternative bDMARDs. Of note is the fact that this technology appraisal guidance applies to bDMARD use with background MTX. For individuals who are unable to take MTX, TNFi switching is permitted. This guidance has not been comprehensively updated following approval of tocilizumab and abatacept as first-line bDMARDs.
It is the absence of robust trial data to support NICE’s guidance regarding the process to follow in the event of failure of initial TNFi treatment (discussed below), effectively limiting the treatment choice to rituximab, which we recognise is not effective for all individuals, that is the basis for the SWITCH study.
Switching between tumour necrosis factor inhibitors
Observational studies
Several early-phase uncontrolled studies and an initial small randomised study suggested benefit in switching between TNFi agents. 60–70 A report of extremely high responses on alternative TNFi agent in specific subgroups of patients62 also indicated the potential value and the need to explore this approach further. A literature review71 documented 29 reports on switching from one TNFi to another in RA, with the data largely indicating benefit of switching from a first TNFi to a second, with switching for secondary non-response likely to be more effective. A systematic review reported similar findings. 72
Randomised controlled trials
No randomised controlled trials (RCTs) of switching between adalimumab, etanercept and infliximab have been conducted in patients in whom these three established TNFi have failed. The rationale and argument for switching between different TNFi drugs, however, were further strengthened by a large, international, multicentre, randomised, Phase II study73 that investigated 461 patients who had previously received and either failed or were intolerant of one or more TNFis. Patients were randomised to either golimumab (50 mg or 100 mg every 4 weeks) or placebo. American College of Rheumatology 20 (ACR20) response rates at week 14 were significantly higher in the golimumab groups than in the placebo group (35% and 38% vs. 18%, respectively). More recently, two RCT studies,74,75 one with open-label evaluation,74 have demonstrated significant efficacy of CZP in prior TNFi treatment failures. In a small but first prospective RCT,74 patients in whom an initial TNFi was stopped because of secondary non-response (i.e. the initial response to the TNFi was lost) were randomised to 12 weeks of either CZP (n = 27) or placebo (n = 10), followed by an open-label CZP 12-week period. The primary end point was the proportion of patients reaching an ACR20 response by week 12, observed in 61.5% of patients in the CZP group, compared with 0% of patients in the placebo group. Placebo patients who were switched blindly to CZP attained similar results seen with CZP in weeks 0–12. As this result was highly significant, study inclusion was terminated after entry of 33.6% of the originally planned 102 patients. The REALISTIC study, a 28-week Phase IIIb study, assessed safety and maintenance of response to CZP in a diverse population of RA patients, stratified by prior TNFi exposure, concomitant MTX use and disease duration.
Switching to non-tumour necrosis factor inhibitor biologic disease-modifying antirheumatic drug therapies
Randomised controlled trials
Randomised controlled trials49–51 and their long-term extension studies (LTEs)76–78 have demonstrated the benefits of non-TNFi bDMARDs over placebo/MTX following TNFi-inadequate response.
The randomised evaluation of long-term efficacy of rituximab in RA (REFLEX) study evaluated the efficacy of rituximab versus placebo in patients receiving MTX who had failed at least one TNFi. 49 Significantly more rituximab-treated patients than placebo-treated patients achieved ACR20, American College of Rheumatology 50 (ACR50), American College of Rheumatology 70 (ACR70) and moderate to good European League Against Rheumatism (EULAR) responses at week 24. Of note, despite a significant reduction in Disease Activity Score in the rituximab group, the mean DAS28 at week 24 was still high, at 5.1 units (a reduction of 1.83 from 6.9 at baseline). 49 In the LTE study (and thus a selected subgroup), rituximab showed sustained effects on joint damage progression. 76 The ATTAIN (A Therapeutic Trial of Afatinib In the Neoadjuvant Setting) study51 compared the efficacy of abatacept and placebo/MTX in patients with TNFi-inadequate response and found that significantly more patients in the abatacept group achieved ACR20, ACR50 and ACR70 responses, impressive quality-of-life results and improvement in DAS28 (a reduction in Disease Activity Score of > 1.2 in 70% in the abatacept group vs. 18.2% in the placebo group)51 and patients continued to maintain these improvements throughout the 2-year LTE study. 77 The RA study in anti-TNF failures (RADIATE) study compared the efficacy of tocilizumab (8 mg/kg or 4 mg/kg) plus MTX to placebo plus MTX in patients with one or more TNFi-inadequate responses. 50 At week 24, more patients in the tocilizumab groups than in the placebo group achieved ACR20, ACR50 and ACR70 responses and good or moderate EULAR responses. 50 The efficacy of tocilizumab was maintained for up to 4.2 years during the LTE study. 78
Switching to a second tumour necrosis factor inhibitor or alternative class biologic disease-modifying antirheumatic drug
Observational studies
A number of observational studies have compared clinical response after switching to either rituximab or alternative TNFi in patients who failed initial TNFi treatment. 79–82 As summarised below, most have suggested better efficacy on switching to rituximab, although there are also reports of equivalent clinical responses in patients who switched to either alternative TNFi or rituximab following failure of one or more TNFi therapies. 83,84 These observational studies, however, had several design limitations, such as small sample sizes,79 selection bias,79–82 pooling all causes of TNFi failure81 and missing data,79–82 although they tried to address these issues by calculating propensity scores and using multivariable analysis techniques. 79–82
Analysis of patients with RA in the Swiss Clinical Quality Management in Rheumatic Diseases RA registry (SCQM-RA),79,80 who had treatment failure with at least one TNFi and were switched either to alternative TNFi or to one cycle of rituximab, showed that switching to rituximab may be more effective than switching to another TNFi. Furthermore, when the motive for switching was ineffectiveness of the TNFi, patients who received rituximab achieved a significantly better improvement in DAS28 at 6 months than patients who received alternative TNFi. 80 However, when the reason for switching was other causes, the improvement in DAS28 was similar in the two groups. 80 The same registry also reported that rituximab was as effective as alternative TNFi in preventing joint erosions in patients who had previous treatment failure with a TNFi. 83 A study of 1300 patients with RA on the British Society of Rheumatology (BSR)’s Biologics Register who had a failed response to their first TNFi treatment and were switched to a single cycle of either rituximab or alternative TNFi found that patients who switched to rituximab had better EULAR responses and were more likely to achieve improvement in their Health Assessment Questionnaire (HAQ) scores. 81 More recently, a global observational real-life study (SWITCH-RA)82 also showed that among patients with RA who failed to respond to, or were intolerant of, a single previous TNFi, those who were switched to rituximab achieved significantly better clinical responses at 6 months than those patients who were switched to a second TNFi. However, further subgroup analysis showed that these differences were observed only in seropositive [to either or both of rheumatoid factor (RF) and anti-citrullinated peptide antibody (ACPA)] patients who switched because of lack of efficacy of the first TNFi. 82
An observational study from the US Consortium of Rheumatology Researchers of North America (CORRONA) cohort85 reported clinical effectiveness of abatacept versus subsequent TNFi in patients with RA following one or more TNFi drug failures, using propensity scoring to reduce bias attributable to systematic prescribing practices. Six- and/or 12-month response outcomes with change in disease activity, remission rates based on the Clinical Disease Activity Index (CDAI) and modified DAS28 (mDAS28) and American College of Rheumatology (ACR) response rates were evaluated. The main analysis included all patients regardless of the reason for switching in the main analysis, with inadequate response to prior TNFi addressed in a sensitivity analysis. For the primary outcome (minimum clinically important difference in the change in CDAI score of 4.3) and the secondary outcomes, no differences between the two treatments were recorded.
Head-to-head comparisons
Gottenberg et al. 86 reported a 52-week pragmatic open-label RCT that randomised 300 patients who did not respond to a first TNFi to receive either alternative TNFi or an alternative-mechanism bDMARD (abatacept, rituximab or tocilizumab). This was a superiority trial, with the primary outcome of a good or moderate EULAR response at 6 months. Primary outcome was achieved in a significantly greater proportion of patients in the non-TNFi group, with 70% achieving a good or moderate EULAR response, compared with 52% in the second TNFi group. Similar differences were observed from week 12 and persisted at week 52, with, in addition, significantly better low disease activity and remission rates. Although an instructive and randomised study, the multiple treatment options included within the non-TNFi group limit the extent to which these data can inform on which specific targeted agent should be considered.
In contrast, a recent preliminary report from a Dutch randomised trial of 144 patients with RA who had failed a first TNFi and were randomised (1 : 1 : 1) to receive alternative TNFi, abatacept or rituximab, showed that there was comparable improvement in the DAS28, HAQ scores and Short Form questionnaire-36 items (SF-36) outcome measures over a 12-month period. 87 Rituximab therapy was the most cost-effective of the three (although this finding may not be true in other countries with different health-care provision and pricing structures). 87 Further studies with larger sample sizes and inclusion of tocilizumab in the treatment options are needed to confirm these results.
Serology and response
Compared with rituximab, and potentially the other two non-TNFi bDMARDs, a key benefit of the TNFi appears to be its suitability in both seropositive (either or both of RF and ACPA positive) and particularly seronegative disease. 82 Seronegative antibody status (seen in up to 25–30% of patients with RA) is associated with a poorer response to rituximab84,88,89 and better response rates have been demonstrated in antibody-positive patients treated with rituximab, which was most evident in the TNFi failure group;84 perhaps intuitive in the light of its target and rationale for use. Recent studies have also demonstrated that abatacept may be more effective in seropositive (ACPA-positive) patients. 90–93 Furthermore, a Japanese study of 58 patients with RA treated with tocilizumab (including 22 patients who previously received a TNFi) reported that a high titre of immunoglobulin M RFs at baseline was the only variable to be associated with CDAI remission at 24 weeks. 94 However, a larger French cohort study95 of 208 patients with RA did not find an association between seropositivity at baseline and a EULAR response after 24 weeks of tocilizumab therapy.
Additional clinical factors for consideration
Apart from antibody status, certain patients will not be appropriate for rituximab therapy, and coexisting pathologies, such as inflammatory bowel disease and psoriasis, that are also treated with TNFi may make other agents less appropriate. Rituximab, for example, has been associated with the development of psoriasis in patients with no previous history of the disease,96 although it is recognised that bDMARDs, including TNFi therapy, can induce paradoxical clinical manifestations such as pustular psoriasis. 97,98
Summary comments
Despite the benefits of recent advances in the management of RA, no universally effective treatment exists. It remains unclear how best to utilise the alternative bDMARDs following an initial TNFi-inadequate response. Although large observational studies have been performed, the need for more direct comparisons to provide sufficiently robust evidence to inform clinicians is necessary. Results from recent trials are emerging. Nevertheless, data suggesting that subgroups are more responsive to a particular targeted therapy (seronegativity and TNFi99) highlight the importance of including such factors in study design to avoid prematurely discounting alternative TNFi drug as an effective therapeutic option, particularly in the context of resistant and aggressive disease cohorts. In addition, optimal bDMARD choice based on the nature of prior inefficacy (primary or secondary) has not been addressed to date. Despite several treatment options now available, no large-scale head-to-head comparisons investigating the efficacy of sequential biologic treatments have been conducted to date.
The SWITCH trial100 was a well-designed randomised trial in this therapeutic area, which also aimed to explore the more refined clinical questions that would thus provide clear guidance to clinicians. This study aimed to evaluate whether or not alternative class bDMARDs compared with rituximab (the NICE-preferred second-line option) were comparable in efficacy and safety outcomes. The results of this study were expected to contribute to the development of a rational treatment algorithm and more judicious and cost-effective management, in particular to allow individualised treatment regimens rather than switching all patients to a single (and potentially unsuccessful and toxic) therapy. The trial was stopped early by the funding committee because of unforeseen interruptions and lengthy site set-up, which, thus, impacted on the inability to recruit to target on time. Although the results presented in this report are not sufficiently powerful to address these aims, we expect that the controlled data will inform the emerging evidence base and can be included in meta-analyses.
Chapter 2 Clinical trial methods
Objectives
In patients with RA who had failed treatment to an initial TNFi (according to NICE guidance), the objectives of this study were as follows.
Primary objective
The primary objective was to determine whether or not an alternative-mechanism TNFi or abatacept is non-inferior to rituximab in disease response at 24 weeks post randomisation.
Secondary objectives
The secondary objectives were:
-
to compare alternative TNFis and abatacept with rituximab for disease response, quality of life, toxicity and safety over 48 weeks
-
to undertake an evaluation of the cost-effectiveness of switching patients to alternative TNFi, abatacept or rituximab
-
to compare structural and bone density outcomes for abatacept and alternative TNFis with those for rituximab over 48 weeks using plain radiography and bone densitometry score.
Exploratory objectives
The exploratory objectives were:
-
to determine the optimal sequence of treatments by assessing whether or not the response to the second treatment in patients with RA is affected by which initial TNFi the patients failed treatment on (TNFi monoclonal or TNFi receptor fusion protein)
-
to evaluate whether or not the response to the second treatment (alternative TNFi, abatacept or rituximab) is affected by whether or not the patient was a primary (no initial response) or secondary (loss of an initial response) response failure to their initial TNFi therapy
-
to ascertain whether or not seropositive (to either or both of RF and ACPA) and seronegative patients with RA behave differently in their response and disease outcome measures across the three treatment arms, particularly with respect to rituximab.
Design
The study was a multicentre, Phase III, open-label, non-inferiority, parallel-group, three-arm RCT comparing the clinical effectiveness and cost-effectiveness of alternative TNFi and abatacept (separately) with that of rituximab in patients with RA who have failed an initial TNFi treatment.
Patients were randomised on a 1 : 1 : 1 basis to receive one of the following:
-
alternative TNFi:
-
etanercept if patient had initial failure of a monoclonal antibody: infliximab, adalimumab, certolizumab or golimumab
-
OR
-
monoclonal antibody: infliximab, adalimumab, certolizumab or golimumab if patient had initial failure of etanercept (choice of monoclonal TNFi at investigator’s discretion)
-
abatacept
-
rituximab.
Patients received randomised treatment during the interventional phase to a maximum of 48 weeks and were subsequently followed up to a maximum of 96 weeks in the observational phase.
The study was reviewed and approved by the National Research Ethics Service, Research Ethics Committee Leeds (West) (reference number 11/H1307/6) and was registered as an International Standard Randomised Controlled Trial number 89222125 and with ClinicalTrials.gov identifier NCT01295151. The trial protocol100 can be accessed at http://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/1471-2474-15-452.
Patient and public involvement
Ailsa Bosworth, Chief Executive and Founder of the National Rheumatoid Arthritis Society, was the patient and public involvement (PPI) member on the Trial Management Group and provided valuable PPI input to the development of the SWITCH trial proposal and on key decisions throughout the trial.
There was also involvement from a PPI representative on the Trial Steering Committee, Sandra Purdy, who provided input into the patient information sheet and other trial documentation intended for use by patients. Through membership of the Trial Steering Committee, the PPI representative also provided input into the design and conduct of the trial through annual meetings.
Participants
Patients attending hospital-based rheumatology outpatient departments throughout the UK, who had been diagnosed with RA, were receiving MTX, had not responded to (at least two) csDMARD therapy (including MTX) and had experienced an inadequate response to treatment with one TNFi were invited to be screened for eligibility in the trial if they:
-
were male or female and aged ≥ 18 years
-
had a diagnosis of RA as per the ACR/EULAR 2010 classification criteria confirmed at least 24 weeks prior to the screening visit
-
failed csDMARD therapy according to NICE/BSR guidelines,101 that is failure of at least two csDMARDs including MTX
-
had persistent RA disease activity despite having been treated with a current initial TNFi agent for at least 12 weeks. Active RA was defined as:
-
primary non-response defined as failing to improve DAS28 by > 1.2 units or failing to achieve a DAS28 of ≤ 3.2 units within the first 12–24 weeks of starting the initial TNFi treatment (this may include patients who have shown a reduction in DAS28 of > 1.2 units but still demonstrate an unacceptably high disease activity in the physician’s judgement with evidence of an overall DAS28 of ≥ 3.2 units)
-
OR
-
secondary non-response defined as lack of efficacy of the first TNFi treatment (having demonstrated prior satisfactory response) as per clinician judgement, with the reason for cessation of the first TNFi treatment other than intolerance
-
were MTX dose stable for 4 weeks prior to the screening visit and to be continued for the duration of the study
-
were on non-steroidal anti-inflammatory drugs (NSAIDs) and/or corticosteroids (oral prednisolone not exceeding 10 mg daily), on an unchanged regimen for at least 4 weeks prior to the screening visit and were expected to remain on a stable dose until the baseline assessments have been completed
-
provided written informed consent prior to any trial-specific procedures.
Patients were excluded if they met any one of the following criteria.
-
They had had major surgery (including joint surgery) within 8 weeks prior to the screening visit or planned major surgery within 52 weeks following randomisation.
-
They had inflammatory joint disease of different origin, mixed connective tissue disease, Reiter’s syndrome, psoriatic arthritis, systemic lupus erythematosus, or any arthritis with onset prior to 16 years of age.
-
They had received doses of prednisolone of > 10 mg/day within the 4 weeks prior to the screening visit.
-
They had received intra-articular or intramuscular steroid injections within 4 weeks prior to the screening visit.
-
They had previously received more than one TNFi drug OR any other bDMARD for the treatment of RA.
-
They were unable or unwilling to stop treatment with a prohibited DMARD (i.e. synthetic DMARD aside from MTX, e.g. oral or injectable gold, chloroquine, hydroxychloroquine, ciclosporin, azathioprine, leflunomide, sulfasalazine) prior to the start of protocol treatment.
-
They had been treated with any investigational drug in the last 12 weeks prior to the start of protocol treatment.
-
They had other comorbidities including acute, severe infections, uncontrolled diabetes, uncontrolled hypertension, unstable ischaemic heart disease, moderate/severe heart failure (class III/IV of the New York Heart Association functional classification system102), active bowel disease, active peptic ulcer disease, recent stroke (within 12 weeks before the screening visit), or any other condition which, in the opinion of the investigator, would put them at risk if they participated in the study or would make implementation of the protocol difficult.
-
They had experienced any major episode of infection requiring hospitalisation or treatment with intravenous antibiotics within 12 weeks of start of the treatment protocol or oral antibiotics within 4 weeks of start of the protocol treatment.
-
They were at significant risk of infection that, in the opinion of the investigator, would put them at risk if they participated in the study [e.g. leg ulceration, indwelling urinary catheter, septic joint within 52 weeks (or ever if a prosthetic joint is still in situ)].
-
They had known active current or a history of recurrent bacterial, viral, fungal, mycobacterial or other infections including herpes zoster [for tuberculosis (TB) and hepatitis B and C, see below], but excluding fungal infections of nail beds as per clinical judgement.
-
They had untreated active current or latent TB. Patients should have been screened for latent TB (as per BSR’s guidelines) within 24 weeks prior to the screening visit and, if positive, treated following local practice guidelines prior to the start of protocol treatment.
-
They had active current hepatitis B and/or C infection. Patients should have been screened for hepatitis B and C within 24 weeks prior to the screening visit and, if positive, excluded from the study.
-
The had primary or secondary immunodeficiency (history of or currently active) unless related to primary disease under investigation.
-
In the case of women, they were pregnant or lactating or were women of child-bearing potential (WCBP) who were unwilling to use an effective birth control measure while receiving treatment and after the last dose of protocol treatment, as indicated in the relevant summary of product characteristics (SmPC) or investigator’s brochure (IB).
-
In the case of men, their partners were WCPB who were unwilling to use an effective birth control measure while receiving treatment and after the last dose of protocol treatment as indicated in the relevant SmPC/IB.
-
They were known to have significantly impaired bone marrow function as a result of, for example, significant anaemia, leucopenia, neutropenia or thrombocytopenia, defined by the following laboratory values at the time of the screening visit:
-
haemoglobin level of < 8.5 g/dl
-
platelet count of < 100 × 109/l
-
white blood cell count of < 2.0 × 109/l
-
neutrophil count of < 1 × 109/l
-
-
They were known to have severe hypoproteinaemia at the time of the screening visit as a result of, for example, nephrotic syndrome or impaired renal function, defined by:
-
a serum creatinine concentration of > 150 µmol/l.
-
The eligibility criteria were based on BSR’s guidelines on the use of TNFi. 101 Important exclusion criteria that are adhered to in clinical practice were applied in this study.
Recruitment
Patients were approached during standard clinic visits for the management of their RA, or were identified by waiting lists, registries or reviews of case records, and sent a personalised letter inviting them to participate. Patients were provided with verbal and written details about the trial and had as long as they required to consider participation. Assenting patients provided written consent before being registered into the trial and formally assessed for eligibility. Patients at Chapel Allerton Hospital also had the option of giving informed consent for blood and tissue samples to be taken for the SWITCH trial biobank for future scientific research. The participant information sheet and consent forms are provided in Appendix 1.
Interventions
Abatacept
Abatacept is a selective T-cell co-stimulation blocking agent that is a fusion protein composed of the Fc region of the immunoglobulin G1 (IgG1) fused to the extracellular domain of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).
Alternative tumour necrosis factor inhibitors
Etanercept
Etanercept is a human TNF receptor p75Fc fusion protein produced by recombinant deoxyribonucleic acid (rDNA) technology. Patients randomised to receive alternative TNFis whose initial TNFi was a monoclonal antibody received etanercept as their intervention.
Monoclonal antibodies
For patients randomised to receive alternative TNFi whose initial TNFi was etanercept, the allocation was to one of four anti-TNF monoclonal antibodies. Within this group of interventions, allocation was at the discretion of the treating clinician.
-
Adalimumab: a recombinant fully human IgG1 monoclonal antibody specific for TNF produced in a mammalian cell expression system.
-
CZP: a recombinant (Fc-free) humanised antibody Fab fragment against TNF and conjugated to polyethylene glycol.
-
Infliximab: a chimeric (human–murine) IgG1 monoclonal antibody produced by rDNA technology.
-
Golimumab: a fully human IgG1 monoclonal antibody to TNF.
Rituximab (control)
A genetically engineered chimeric (human–murine) monoclonal antibody against the B-cell protein marker CD20 (clusters of differentiation 20).
Efficacy of rituximab to placebo was established in a similar patient population in the REFLEX study. 49
Table 1 illustrates the treatment regimen including mode of administration and dose, for each of the three treatment arms. The intervention period was 48 weeks, achieved via treatment regimens administered for a minimum of 24 weeks.
| Treatment arm | Treatment description |
|---|---|
| Rituximab | A single dose of 1 g as an intravenous infusion administered at days 0 (week 0) and 15 (week 2). In line with standard practice, a patient who lost an initial 6-month (week 24) response, as per NICE’s guidance, could receive a further cycle of rituximab after a minimum of 6 months following the first dose. The second cycle of rituximab was, again, given at a dose of 1 g; two intravenous infusions administered at a 2-week interval, for example week 24 and 26. Prior to receiving rituximab, 100 mg of intravenous methylprednisolone was given as a premedication |
| Abatacept | Solution for subcutaneous injection: 125 mg per syringe (125 mg/ml). Administered at a dose of 125 mg at week 0 and once weekly thereafter for a minimum of 24 weeks |
| Alternative TNFi | |
| Etanercept | A single dose of 50 mg by subcutaneous injection weekly for a minimum of 24 weeks (unless not tolerated) |
| Adalimumab | A single dose of 40 mg by subcutaneous injection every 2 weeks for a minimum of 24 weeks (unless not tolerated) |
| Infliximab | A dose of 3 mg/kg per intravenous infusion, administered on a day-case unit or equivalent at weeks 0, 2 and 6 and then every 8 weeks thereafter for a minimum of 24 weeks |
| CZP | Single dose of 400 mg by subcutaneous injection at weeks 0, 2 and 4 and then at a dose of 200 mg every 2 weeks thereafter for a minimum of 24 weeks |
| Golimumab | Dose of 50 mg by subcutaneous injection every 4 weeks for a minimum of 24 weeks. Available as IMP within the SWITCH trial following approval in November 2013 |
Study procedures
Screening and baseline assessments
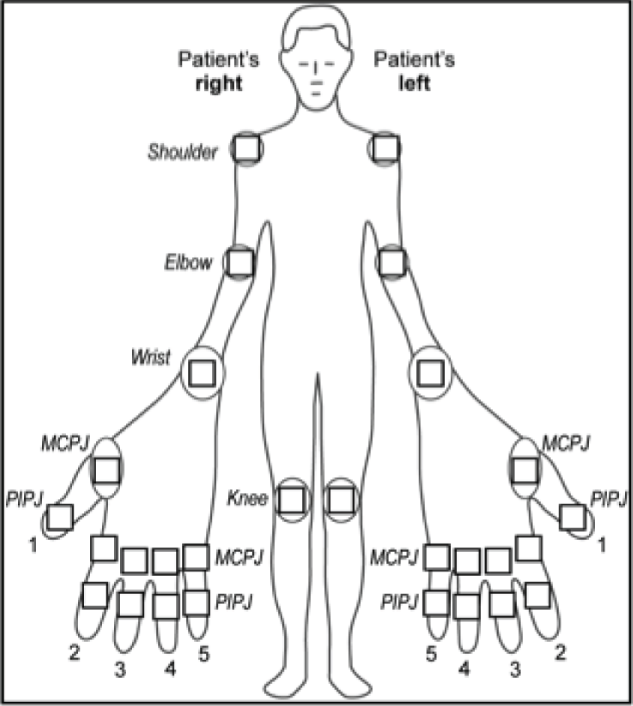
Following written informed consent and prior to any trial-related procedures, patients were registered into the study. All patients had a screening assessment within 4 weeks prior to the baseline assessment (and, when applicable, the assessment was repeated at the baseline assessment) to establish eligibility. The clinical assessment included a medical history, a physical examination, which included measurements of height, weight and vital signs, electrocardiography (ECG), chest radiography and a screen for TB (if not performed within specified time window prior to the screening visit). In addition, a 28 swollen joint count (SJC) and tender joint count (TJC) were performed (Figure 1), and blood and urine tests [haematology, blood chemistry, C-reactive protein (CRP) level test, erythrocyte sedimentation rate (ESR), serological tests, hepatitis B and C screen, a pregnancy test and urinalysis] were undertaken. At the baseline assessment, a further blood test was undertaken to assess glucose levels and lipid profiles.
FIGURE 1.
Manikin showing joints to be included in the SJC and TJC. MCPJ, metacarpophalangeal joint; PIPJ, proximal interphalangeal joint.
At screening and baseline assessments, patients completed a Global Assessment of Arthritis using a visual analogue scale (VAS) and reported the extent of their early-morning stiffness. The clinician assessed the Global Disease Activity using a VAS. At the baseline assessment, patients completed a Global Assessment of Pain VAS and an assessment of their general health using a VAS.
Intervention and observational phase assessments
Randomised patients attended clinic assessment visits at weeks 12, 24, 36 and 48 in the interventional phase and at weeks 60, 72, 84 and 96 in the observational phase. Patients allocated to the subcutaneous TNFi or abatacept therapies had additional standard assessment for safety purposes (usually week 4) in line with local practice. At the Leeds Chapel Allerton Hospital site, biological samples from patients consenting to the SWITCH trial biobank substudy were collected prior to commencement of the trial treatment and at weeks 2, 4, 12, 24 and 48 or at the time of early discontinuation, and stored for future research. See Appendix 5, Tables 33–35, for the schedule of events for rituximab, infliximab and subcutaneous bDMARDs.
Outcome measures
Primary outcome measure
The primary outcome measure was the absolute reduction from baseline in DAS28 at 24 weeks post randomisation. DAS28 is a measure of disease activity in RA. 103,104 The composite score is calculated as a function of the number of tender and swollen joints (total 28 joints), the ESR and the patient’s global assessment of their arthritis measured using a VAS (see Appendix 6, Box 1).
Secondary outcome measures
The following outcomes were measured over 48 weeks (at each of the visit time points).
Clinical measures
-
DAS28.
-
Reduction in DAS28 of ≥ 1.2 units.
-
Low disease activity rate and remission rate: low disease activity is defined as 2.6 < DAS28 ≤ 3.2 units and remission as DAS28 of ≤ 2.6 units (see Appendix 6, Table 36).
-
EULAR response scores: EULAR response criteria are applied to the DAS28 and classify patients as good, moderate or non-responders using the DAS28 and the absolute reduction in the DAS28 from baseline (see Appendix 6, Table 37).
-
ACR20, ACR50 and ACR70:105 composite measures developed for RA. These are defined as a relative improvement (reduction) from baseline of at least 20% (or 50% or 70% for ACR50 and ACR70, respectively) in TJCs and SJCs and a relative 20% (or 50% or 70% for ACR50 and ACR70, respectively) improvement in three out of the five following criteria:
-
Patient Global Assessment of Arthritis (VAS)
-
Physician Global Assessment of Disease Activity (VAS)
-
Patient Global Assessment of Pain (VAS)
-
Patient assessment of physical function as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI) questionnaire
-
Results of laboratory test for inflammatory markers (either ESR or CRP level).
-
-
CDAI:104,106 a composite outcome measure consisting of the number of tender joints (i.e. 28-joint count), the number of swollen joints (i.e. 28-joint count), a patient global assessment of disease activity (measured via a 100-mm VAS) and Physician Global Assessment of Disease Activity (measured via a 100-mm VAS). Appendix 6,Table 38, provides the response categories for CDAI.
-
Simplified Disease Activity Index (SDAI):104,107 a composite outcome measure consisting of the number of tender joints (28-joint count), the number of swollen joints (28-joint count), the Patient Global Assessment of Disease Activity (measured via a 100-mm VAS), the Physician Global Assessment of Disease Activity (measured via a 100-mm VAS) and CRP level (mg/dl). Appendix 6,Table 39 provides the response categories for SDAI.
-
In-remission rates according to the ACR/EULAR Boolean criteria: this is defined as SJC, TJC, patient global assessment and CRP level scores all ≤ 1. 108
The use of 28-joint count-based outcome measures is well accepted and established in RA trials. This is based on prior evaluation of the performance between 28- and 66-joint count assessments. 109–111 However, we acknowledge on an individual patient level, RA activity outside the 28 joints may be missed and thus influence individual disease activity and response assessments.
Quality of life
-
The Health Assessment Questionnaire Disability Index112 includes 20 questions across eight domains relating to physical function and the need for any help or aids to undertake daily activities. The extent of disability is scored on a scale from 0 (no disability) to 3 (severe disability) for each item relating to rising, dressing, walking and other activities; patients are then asked to list any aids or devices required to undertake such activities. The use of help or aids increases the category score from 0 or 1 to 2 if it has been indicated that aids/help are required in that category. If the category score is already a 2 or 3, no adjustment is made. The total score is derived by taking the maximum score across all domains (0–24) and dividing by 8 to provide an average score (0–3), with higher scores representing greater disability.
-
The Hospital Anxiety and Depression Scale (HADS)113 describes the degree to which patients feel anxious and/or depressed. It comprises 14 questions for each symptom, and each question has four possible responses, ranging from 0, representing no anxiety/depression, to 3, representing high anxiety/depression. Responses are totalled to provide two scales, one for each domain, with a measurement range from 0 to 21.
-
The Rheumatoid Arthritis Quality of Life (RAQoL)114 questionnaire is a specific disease activity measure for RA. It is a 30-item questionnaire, the response to each item being yes (score as 1) or no (score as 0), that ascertains the extent of RA symptoms experienced. The maximum RAQoL score is 30.
Safety
Toxicity is defined as any symptom or event requiring permanent cessation of treatment.
Imaging
Plain radiographs of hands and feet were requested (for later scoring to calculate the modified Genant score) and bone densitometry scans for T-scores of unilateral neck of femur and lumbar spine were obtained at baseline and week 48 in a subgroup of patients recruited at centres with the facilities to do the imaging. Note that the plain radiographs were not scored because of the inability to secure additional resource centrally to conduct the analysis (compounded by the early termination/underpowered study).
Safety monitoring
Adverse events (AEs) and adverse reactions (ARs) were monitored throughout the trial and recorded at each treatment visit. An AE was defined as any untoward medical occurrence in a trial patient that does not necessarily have a causal relationship with the treatment. An AR was defined as any untoward and unexpected responses to an investigational medicinal product (IMP) related to any dose administered.
A serious adverse event (SAE) or a suspected serious adverse reaction (SSAR) was defined as any untoward medical occurrence or effect that resulted in death, persistent or significant disability or incapacity or a congenital anomaly or birth defect, or was life-threatening, or required inpatient hospitalisation or prolongation of existing hospitalisation or may have jeopardised the patient necessitating medical or surgical intervention to prevent one of the outcomes stated in Outcome measures.
Expected common SAEs related to RA were the development of major extra-articular manifestations of disease, for example vasculitis, and blood dyscrasia associated with disease activity. Expected serious ARs common to all treatments were allergic reactions, injection site/infusion reaction, blood dyscrasias, serious infections, diarrhoea, new infections, toxic epidermal necrolysis, Stevens–Johnson syndrome or severe rash, pulmonary fibrosis, renal failure, neurological impairment, new autoimmunity and cardiovascular abnormalities.
All SAEs, regardless of the suspected relationship to the trial treatment, were reported to the Clinical Trials Research Unit within 24 hours of the research staff becoming aware of the event. SAEs were followed up until the event had resolved or a trial outcome had been reached. All AEs/ARs and SAEs were monitored from randomisation until a maximum of 30 days (later revised to 32 days) after the last dose of randomised treatment during the interventional phase (week 48 maximum). Beyond this, only SAEs considered to be related to the randomised treatment administered during the interventional phase were reported.
Patient withdrawal
Patients could withdraw from the trial at any time without explanation, and continue to receive treatment as per standard clinical practice. Patient withdrawal was categorised as withdrawal of consent for: further trial treatment only, further trial treatment and visits but willing to have follow-up data collected or further trial treatment and follow-up information.
Sample size and power calculation
A total of 429 evaluable patients were required to have 80% power to demonstrate non-inferiority of either abatacept or alternative TNFi to rituximab at the 5% significance level. A total of 143 evaluable patients in each treatment group provided 80% power for the lower limit of the two-sided 95% confidence interval (CI) for the true difference in the reduction in the DAS28 (abatacept/alternative TNFi – rituximab) to lay above –0.6 units, assuming no difference between treatment groups and a standard deviation (SD) between patients of 1.8 units (the REFLEX study49). Allowing for a loss to follow-up of 10%, a total of 477 patients were to be recruited. No adjustment for multiplicity of the comparisons of each treatment group to rituximab was made. 115,116
The proposed non-inferiority margin of –0.6 units in the reduction in the DAS28 at 24 weeks post randomisation corresponds to the maximum difference in a reduction in the DAS28 that is considered to be of no clinical relevance and is the threshold for the clinical distinction of ‘inferiority’ (corresponds to the maximum change in the DAS28 within patients with a low or moderate disease activity that is classified as ‘no response’ by the EULAR criteria). A DAS28 of 0.6 units is also the reported measurement error. 117
For the analysis of the secondary outcome measures to compare quality of life, toxicity and safety at 24 weeks between treatment arms the sample size of 143 evaluable patients per group would detect a standardised effect size of 0.33 (small to medium by the definition of Cohen118), with 80% power and two-sided 5% significance level.
After opening, the trial underwent a major redesign. The original target sample size was 870, based on 80% power to determine whether or not abatacept or alternative TNFi are non-inferior to rituximab at 24 weeks post randomisation in the proportion of patients achieving a DAS28 reduction of ≥ 1.2 units without toxicity. The corresponding non-inferiority margin was set at 12% (as an absolute difference) and assumed a response rate of 65% in the rituximab arm. The original trial design was also powered for a definitive subgroup analysis to determine if there was a differential treatment response between seropositive and seronegative patients. Owing to the challenges in trial setup and associated poor initial patient recruitment, and reassessment of important end points, the primary outcome measure was changed from a binary to a continuous outcome that (following consensus discussion with the principal investigators) was still considered clinically relevant. This allowed a reduction in sample size to 477 patients while still ensuring a trial of clinical relevance. The previous planned definitive subgroup analysis was relegated to an exploratory analysis. The trial redesign was unanimously supported by the Data Monitoring and Ethics Committee and the Trial Steering Committee, was approved by the funder and received ethics approval.
Randomisation
Randomisation took place once eligibility was confirmed and baseline assessments and questionnaires were complete. Patients were randomised in a 1 : 1 : 1 allocation ratio to receive alternative TNFi, abatacept or rituximab. Treatment group allocation used a computer-generated minimisation program incorporating a random element of 0.8, to ensure that treatment groups were well balanced for the following minimisation factors: centre, disease duration (< 5 years or ≥ 5 years), non-response (primary or secondary) and RF/ACPA status (either of RF or ACPA positive, or both RF and ACPA negative).
Both registration and randomisation were performed centrally using an automated 24-hour telephone system based at the Clinical Trials Research Unit. Centres completed a log of all patients aged > 18 years with RA whose treatment had failed for an initial TNFi agent and were considered for the trial but who were not registered for screening or randomised, either because of ineligibility or because of refusal to participate.
Blinding
Blinding of patients and the treating clinicians to treatment allocation was not possible in the trial because of the nature and mode of administration of the different treatment regimens.
Analysis
Formal analyses were conducted using a two-sided 5% level of significance, with exception of the primary analysis, which used a one-sided 2.5% level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The statistical analysis plan is provided in Appendix 7.
The trial planned for one interim analysis to allow for early stopping of either abatacept or alternative TNFi if inferiority compared with rituximab was demonstrated. This analysis would have taken place once 50% of patients (i.e. 239) had reached 24 weeks of follow-up. Following the early termination of the trial, there was no basis for an interim analysis.
Patient populations
All patients recruited into the trial were included in the analysis using ‘intention to treat’ (ITT) and analysed according to the randomised allocation.
A per protocol (PP) population analysis was also undertaken; patients who deviated from the protocol or failed to comply with the required treatment regimen were excluded (see Appendix 7 for further details of exclusions). For the analysis of the primary outcome measure, non-inferiority needed to be demonstrated in both the ITT and PP populations in order to infer non-inferiority.
The complete-case population included all patients with complete data for the relevant outcome measure summarised.
The safety population included all patients who received at least one dose of treatment and is summarised by treatment received.
Missing data
Multiple imputation by chained equations was used to impute missing values at the component level for the DAS28 and ACR20,119 under the assumption that the data were ‘missing at random’. The minimisation factors of disease duration, RF/ACPA status and non-response category and the values of the component end points at all visits from baseline to week 48 (for DAS28 components) and to week 24 (for ACR components) were included in the imputation models. Centre was not included because of the small number of patients recruited in each centre.
Imputations were performed separately for each of the three treatment groups; 27 imputed data sets were created for each treatment group for the primary end point DAS28 and 22 for the secondary end-point ACR20, corresponding to the maximum percentage of missing data across all end points and time points. Predictive mean matching was used to select a value to impute from the three observed values closest to the fitted value in each imputation. 120 The composite end points were then derived in each of the fully imputed data sets (see Appendix 7 for further detail).
Primary outcome measure
A mixed-effects linear regression model was fitted to the primary end point, absolute reduction in the DAS28 at 24 weeks, with covariates corresponding to the minimisation factors and treatment group; centre was fitted as a random effect.
Algebraic representation for the mixed-effects linear regression model is:
Parameter estimates from the mixed-effects models across each of the fully imputed data sets were combined using Rubin’s rules. 119,121 Estimates of each treatment effect and corresponding 95% CIs and p-values were reported in relation to the predefined non-inferiority margin of –0.6 units on the reduction in the DAS28 at 24 weeks post randomisation.
The primary analysis model was fitted to the ITT, PP and complete-case populations. Non-inferiority in both the ITT and PP patient populations was required in order to conclude non-inferiority. A sensitivity analysis was completed on the ITT population with an additional covariate, baseline DAS28, fitted to the primary analysis model.
Key secondary outcome measures
Disease Activity Score of 28-joint scores over 48 weeks
Initially a random coefficient linear regression model was fitted to the DAS28 over time, with covariates entered for the minimisation factors, baseline DAS28, treatment group, time and time-by-treatment interaction; centre, patient and patient-by-time interaction random effects were fitted. However, as there was evidence of non-constant residual error variance, a multivariable covariance pattern model was fitted to the scores over time (at weeks 12, 24, 36 and 48), with the same covariates entered for the minimisation factors (excluding centre), baseline DAS28, treatment group, time and time-by-treatment interaction, and an unstructured covariance pattern was specified. Centre was not fitted as a random effect, as there was no centre component of variation in 24 of the 27 imputed data sets.
Algebraic representation for the random coefficient linear regression model is:
Algebraic representation for the covariance pattern model is:
where m and n denote two (arbitrary) different visit time points.
Disease Activity Score of 28 joints response over 48 weeks (reduction in Disease Activity Score of 28 joints of ≥ 1.2)
A multivariable covariance pattern logistic model was fitted to the response variable, achieving a reduction in the DAS28 of ≥ 1.2 units over time (at weeks 12, 24, 36 and 48), with covariates entered for the minimisation factors (excluding centre), baseline DAS28, treatment group, time and time-by-treatment interaction. An unstructured covariance pattern was specified. Centre was not fitted as a random effect because the model failed to converge.
where m and n denote two (arbitrary) different visit time points.
American College of Rheumatology 20 at week 24
A multivariable logistic regression model was fitted to ACR20 at 24 weeks post randomisation, with covariates entered for the minimisation factors (excluding centre) and treatment group. Centre was not fitted as a random effect, as there was no centre component of variation.
Algebraic representation of the analysis model:
In all analyses estimation of the treatment effects was of primary interest, but hypothesis testing was also performed. Treatment group effects were tested and the significance level is presented based on the Wald test (because of limitations of the likelihood ratio test for imputed data sets119). Model fit was assessed informally by examination of standardised residuals.
Additional secondary outcome measures
All additional secondary outcome measures, including further measures of disease activity and quality of life were summarised by treatment group and compared informally using descriptive statistics. The predefined subgroup analyses to evaluate the treatment modification effect of RF/ACPA status, initial TNFi group failed on and non-response category on the DAS28 were summarised by treatment group. In addition, treatment compliance, toxicity and safety were summarised.
Summary of protocol amendments
Appendix 8 provides a summary of the key protocol amendments throughout the trial.
Chapter 3 Clinical trial results
Patient recruitment
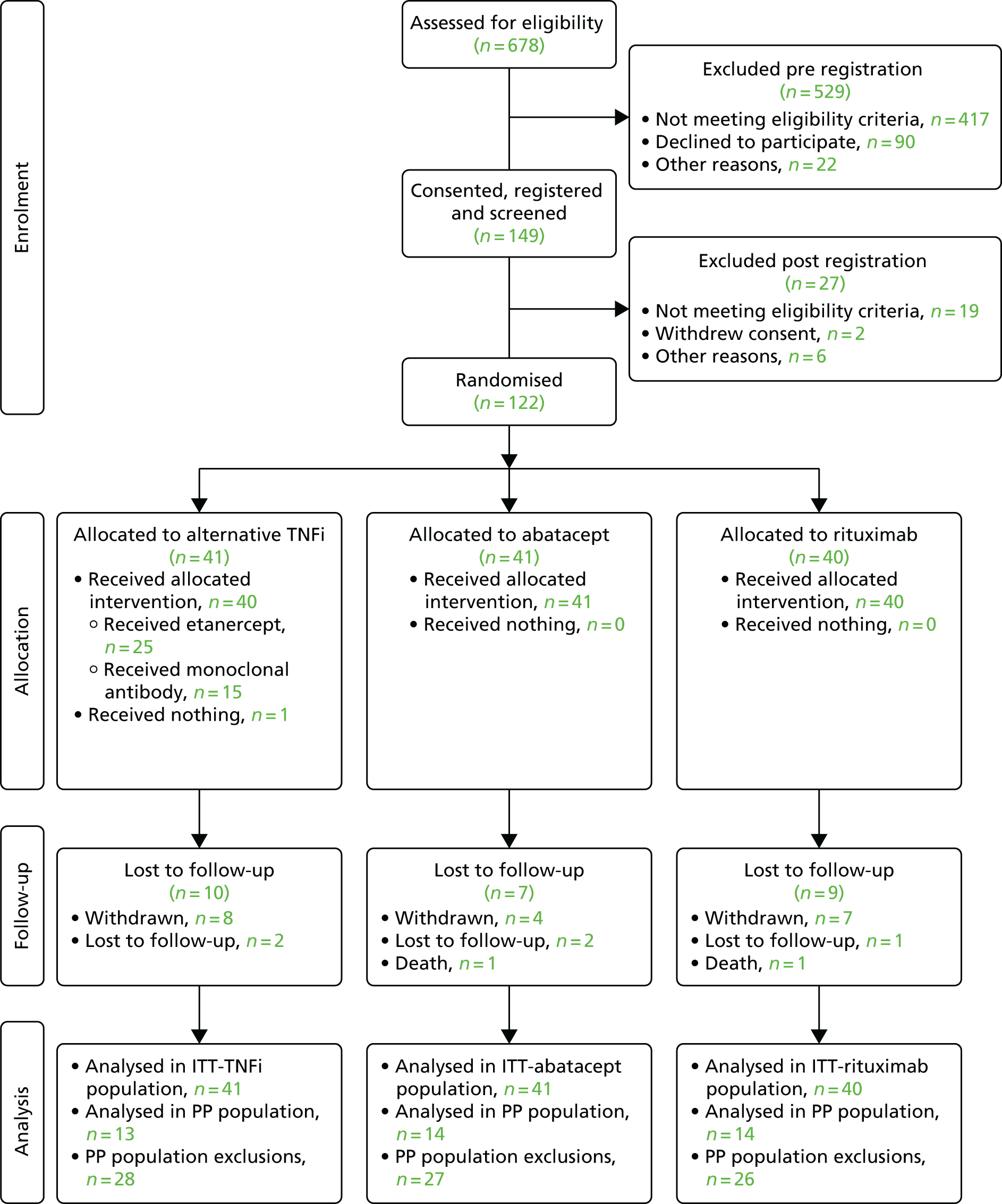
Between July 2012 and December 2014, 678 patients were screened for the trial across 35 centres. A total of 529 patients were excluded at screening (pre-registration), 417 of whom failed to meet the eligibility criteria. The main reasons for failing to meet the eligibility criteria were that they had not failed an initial TNFi agent (n = 95), were not on a stable dose of MTX over the previous 28 days (n = 92) and had received more than one TNFi drug or other biological agent (n = 72). A total of 149 patients gave written informed consent and were registered onto the trial.
Twenty-seven patients were excluded post registration, of whom 19 did not meet the eligibility criteria, two withdrew consent and six were excluded for other reasons (rescreening required, previous hepatitis infection, raised alkaline phosphatase levels, awaiting cancer diagnosis/treatment, patient not contactable, unknown reason). The remaining 122 patients were randomised to treatment. Following early trial termination because of the withdrawal of funding, the last patient was randomised on 18 December 2014.
A summary of the number of patients considered, registered and randomised by centre is provided in Appendix 9,Table 40. The flow of patients from initial assessment through to the end of follow-up is shown in Figure 2 (Consolidated Standards of Reporting Trials diagram). Full reasons for ineligibility and non-consent are provided in Appendix 9, Tables 41–43.
FIGURE 2.
The flow of patients through the SWITCH trial (Consolidated Standards of Reporting Trials diagram).
Recruitment target
Figure 3 displays the projected recruitment against the actual recruitment across all centres during the trial recruitment period. Although the target for recruitment of 16 centres was reached by September 2013, the number of eligible patients identified by centres was much lower than expected (see Appendix 9, Table 40). Barriers to reaching the target included a 9-month halt to recruitment because of unforeseen contractual issues with a home health-care company, longer times for centre set-up, primarily because of the commissioning environment with significant geographical variability in receptiveness of Clinical Commissioning Groups (CCGs) to approve RCTs that included non-NICE-approved therapies and delays to approval.
FIGURE 3.
Graph showing actual recruitment and projected recruitment when the trial was terminated. Projection 1 assumes 35 centres opened. Projection 2 assumes 31 centres opened. (XX), number of centres open.
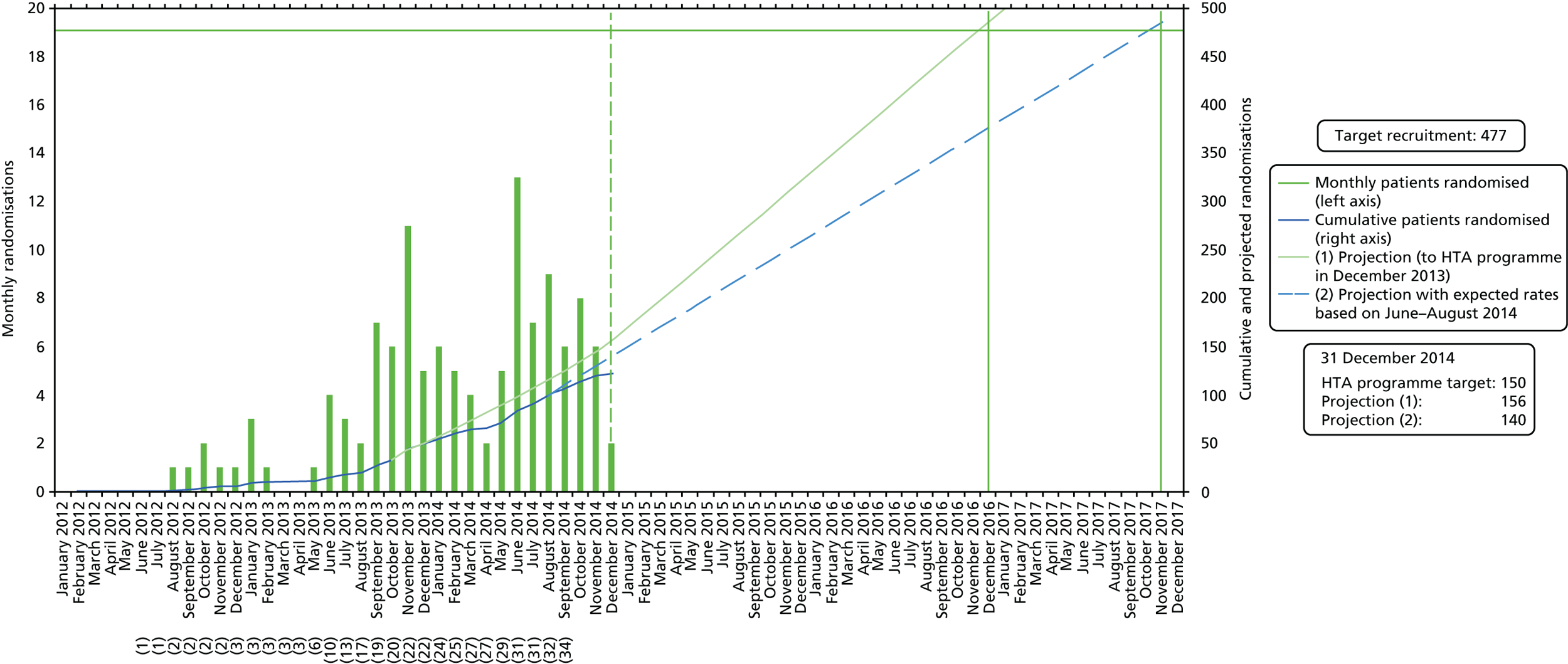
Although the patient recruitment rate improved as new centres were initiated, overturning the deficit accrued from the above delays was not feasible within the planned recruitment period. Based on the number of centres opened and observed recruitment rates, recruitment to November 2017 would have been required to reach the target of 477 patients, at an additional cost of £450,000. Consequently, the Health Technology Assessment (HTA) programme monitoring panel withdrew funding in November 2014; the trial closed to recruitment in December 2014.
The study closure patient information sheet and article for the National Rheumatoid Arthritis Society web page (and other relevant electronic forums) are in Appendices 3 and 4, respectively.
Randomisation
The overall mean randomisation rate was 0.26 patients per month, that is, 3.12 patients per centre per year, across 35 centres. Twenty-eight centres randomised at least one patient and only seven centres randomised more than five patients, with the co-ordinating hospital (Chapel Allerton) providing 32 (26%) of all the randomised patients.
Of the 122 patients randomised to the treatment group, 41 were allocated to alternative TNFi, 41 were allocated to abatacept and 40 were allocated to rituximab.
The median time from the centre opening to randomisation of the first patient was 3.8 months (95% CI 2.5 to 7 months). Two centres did not randomise their first patient until more than 12 months after opening, and two further centres recruited no patients despite being open for more than 12 months.
Generalisability of the patient population randomised
Summaries of the clinical and demographic variables collected on the non-registration logs for patients considered for enrolment, registered but not randomised, and for patients randomised are provided in Appendix 9, Table 44. Age distribution and sex of registered, consented and non-randomised patients were similar. The proportion of patients who were RF seropositive or ACPA positive was also similar between patients registered but not randomised and those patients who were randomised; however, there was a large proportion of patients for whom RF and ACPA status was unknown among non-registered patients, making an assessment of generalisability on RF/ACPA status difficult.
Withdrawals
A summary of follow-up attendance up to the end of the 48-week intervention phase is provided in Figure 4.
FIGURE 4.
Summary of follow-up attendance up to the end of the 48-week intervention phase. DNA, did not attend.

Six patients withdrew consent to continue with randomised treatment and follow-up, two patients on alternative TNFi, one on abatacept and three on rituximab. Two further patients, one on alternative TNFi and one on abatacept, withdrew from follow-up during the observational period after week 48. An additional patient (2.4%) on abatacept withdrew from treatment but continued to have follow-up assessments and a further patient, also on abatacept (2.4%), was withdrawn from the study because of an AE (chest infection).
Appendix 9, Table 45 provides a full list of the reasons for withdrawal.
Two patients on alternative TNFi were lost to follow-up by 48 weeks: one patient (2.4%) on abatacept and one on rituximab.
Protocol deviations
Two patients were eligibility violations, one randomised to alternative TNFi and one to rituximab, both of whom continued to receive their allocated treatment and were followed up. One patient had juvenile-onset RA and one received sulfasalazine and hydroxychloroquine prior to the start of protocol treatment, had not received MTX and had started taking NSAIDs (naproxen) within 4 weeks prior to the screening visit.
A further patient randomised to alternative TNFi had a susceptibility to myeloma if given a monoclonal antibody and a clinical judgement was made to withdraw this patient before the allocated treatment and follow-up.
Eighty-one patients (66.4%) deviated from the protocol in some way, which resulted in exclusion from the PP population, corresponding to 28 (68.3%) patients on alternative TNFi, 27 (65.9%) on abatacept and 26 (65.0%) on rituximab. The most common protocol deviation was receiving steroid treatment within 6 weeks of an end-point assessment (35 patients; 28.7%), followed by not being compliant with treatment up to week 24 (based on information obtained via direct questioning) (27 patients; 22.1%), and receiving additional contraindicated treatment (23 patients; 18.9%). Appendix 10, Table 46, provides a further summary of reasons for protocol deviations resulting in exclusion from the PP population.
Treatment compliance
All except one patient received their allocated treatment. One patient was randomised to receive alternative TNFi (monoclonal antibody) but before treatment commenced the treating clinician withdrew the patient because of the presence of a comorbidity that precluded treatment with a monoclonal antibody TNFi.
Rituximab
All 40 patients randomised to rituximab received at least one infusion of rituximab. By week 12, all infusions had been given in line with the protocol, although infusions for four patients had been delayed (because of patient choice, clinician examination delaying first dose, AE and out-of-range pre-treatment tests). A total of 35 (87.5%) patients were known to be at least 80% compliant with treatment up to week 24.
Abatacept
All 41 patients randomised to receive abatacept received at least one injection of abatacept. A total of 29 patients (70.7%) were known to be at least 80% compliant with treatment up to week 24.
Alternative tumour necrosis factor inhibitor
Forty out of 41 patients (97.6%) randomised to the alternative TNFi treatment arm received their allocated treatment. A total of 31 patients (75.6%) were known to be at least 80% compliant with their randomised treatment up to week 24.
Etanercept
Twenty-five patients were assigned to receive etanercept as a result of being randomised to alternative TNFi. All 25 received at least one injection of etanercept.
Monoclonal antibody
The remaining 16 patients randomised to an TNFi were assigned to receive a monoclonal antibody, the choice of which was at the clinician’s discretion.
Adalimumab
Ten patients received adalimumab as a result of allocation to a monoclonal antibody and all 10 received at least one injection.
Certolizumab pegol
Only one patient received CZP as a result of allocation to a monoclonal antibody. With the exception of one missed injection between baseline and week 12, this patient received all injections in line with the protocol up to week 36.
Golimumab
Three patients received golimumab as a result of allocation to a monoclonal antibody. All three patients received all injections to at least week 24.
Infliximab
One patient received infliximab as a result of allocation to alternative TNFi. Up to week 24, infusions were delivered in line with the protocol.
Baseline characteristics
Baseline characteristics are presented in Tables 2–6. The mean age was 56.7 years (SD 12.2 years; range 24–81 years). A total of 102 patients (83.6%) were female.
| Minimisation factor | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Disease duration category, n (%) | ||||
| < 5 years | 16 (39.0) | 15 (36.6) | 14 (35.0) | 45 (36.9) |
| ≥ 5 years | 25 (61.0) | 26 (63.4) | 26 (65.0) | 77 (63.1) |
| Disease duration (years) | ||||
| Median (IQR) | 5.9 (3.9–12.3) | 6.9 (4.0–15.4) | 7.0 (3.9–15.6) | 6.7 (3.9–14.2) |
| Range | 0.4–35.2 | 0.6–43.5 | 1.3–33.7 | 0.4–43.5 |
| Missing | 0 | 1 | 0 | 1 |
| RA/ACPA seropositivity, n (%) | ||||
| RF seropositive or ACPA positive | 36 (87.8) | 31 (75.6) | 33 (82.5) | 100 (82.0) |
| Both RF seronegative and ACPA negative | 5 (12.2) | 10 (24.4) | 7 (17.5) | 22 (18.0) |
| Non-response category, n (%) | ||||
| Primary | 15 (36.6) | 15 (36.6) | 15 (37.5) | 45 (36.9) |
| Secondary | 26 (63.4) | 26 (63.4) | 25 (62.5) | 77 (63.1) |
| Patient characteristic | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Sex, n (%) | ||||
| Male | 8 (19.5) | 2 (4.9) | 10 (25.0) | 20 (16.4) |
| Female | 33 (80.5) | 39 (95.1) | 30 (75.0) | 102 (83.6) |
| Patient age (years) | ||||
| Mean (SD) | 54.2 (9.98) | 58.1 (13.89) | 57.8 (12.37) | 56.7 (12.21) |
| Median (IQR) | 56.9 (45.5–59.8) | 60.5 (45.2–66.9) | 57.0 (52.4–67.4) | 57.3 (46.7–65.4) |
| Range | 34.2–73.6 | 28.8–81.7 | 24.5–81.1 | 24.5–81.7 |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 30.1 (7.25) | 29.2 (5.74) | 30.4 (6.80) | 29.9 (6.60) |
| Median (IQR) | 28.7 (25.0–34.0) | 28.4 (24.3–34.5) | 29.0 (25.4–33.5) | 29.0 (24.9–34.1) |
| Missing | 1 | 2 | 2 | 5 |
| Smoking status, n (%) | ||||
| Non-smoking (never smoked) | 12 (29.3) | 17 (41.5) | 21 (52.5) | 50 (41.0) |
| Past smoker | 18 (43.9) | 13 (31.7) | 11 (27.5) | 42 (34.4) |
| Current smoker | 11 (26.8) | 11 (26.8) | 8 (20.0) | 30 (24.6) |
| Prior comorbidities, n (%) | ||||
| Hypertension | 15 (36.6) | 13 (31.7) | 14 (35.0) | 42 (34.4) |
| Osteoarthritis | 11 (26.8) | 14 (34.1) | 8 (20.0) | 33 (27.0) |
| Hyperchloesterolaemia | 7 (17.1) | 8 (19.5) | 10 (25.0) | 25 (20.5) |
| Depression | 7 (17.1) | 7 (17.1) | 4 (10.0) | 18 (14.8) |
| Thyroid dysfunction | 8 (19.5) | 5 (12.2) | 2 (5.0) | 15 (12.3) |
| Asthma | 6 (14.6) | 3 (7.3) | 4 (10.0) | 13 (10.7) |
| Diabetes | 4 (9.8) | 1 (2.4) | 5 (12.5) | 10 (8.2) |
| Cancer | 3 (7.3) | 1 (2.4) | 1 (2.5) | 5 (4.1) |
| Bowel disease | – | 2 (4.9) | 1 (2.5) | 3 (2.5) |
| Ischaemic heart disease | 1 (2.4) | – | 2 (5.0) | 3 (2.5) |
| Emphysema/chronic bronchitis | 1 (2.4) | 1 (2.4) | – | 2 (1.6) |
| Myocardial infarction | – | – | 2 (5.0) | 2 (1.6) |
| Peptic ulcer disease | – | 1 (2.4) | 1 (2.5) | 2 (1.6) |
| Stroke | – | 1 (2.4) | 1 (2.5) | 2 (1.6) |
| Chronic liver disease | 1 (2.4) | – | – | 1 (0.8) |
| Epilepsy | 1 (2.4) | – | – | 1 (0.8) |
| Peripheral vascular disease | – | – | 1 (2.5) | 1 (0.8) |
| Renal disease | – | 1 (2.4) | – | 1 (0.8) |
| Treatment history | Treatment arm, n (%) | Total (n = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Type of initial TNFi that failed | ||||
| Monoclonal antibody | 25 (61.0) | 23 (56.1) | 22 (55.0) | 70 (57.4) |
| Etanercept | 16 (39.0) | 18 (43.9) | 18 (45.0) | 52 (42.6) |
| Previous TNFi agent | ||||
| Adalimumab | 10 (24.4) | 10 (24.4) | 8 (20.0) | 28 (23.0) |
| CZP | 11 (26.8) | 9 (22.0) | 5 (12.5) | 25 (20.5) |
| Etanercept | 16 (39.0) | 18 (43.9) | 18 (45.0) | 52 (42.6) |
| Golimumab | 2 (4.9) | – | 4 (10.0) | 6 (4.9) |
| Infliximab | 2 (4.9) | 4 (9.8) | 5 (12.5) | 11 (9.0) |
| Measure of disease activity | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Experience early-morning stiffness?, n (%) | ||||
| Yes | 39 (95.1) | 39 (95.1) | 40 (100.0) | 118 (96.7) |
| No | 2 (4.9) | 2 (4.9) | – | 4 (3.3) |
| TJC | ||||
| Mean (SD) | 15.3 (6.40) | 15.1 (7.39) | 17.4 (8.13) | 15.9 (7.33) |
| Missing | 0 | 0 | 1 | 1 |
| SJC | ||||
| Mean (SD) | 9.9 (6.43) | 8.8 (5.54) | 10.0 (6.64) | 9.5 (6.19) |
| Missing | 0 | 0 | 1 | 1 |
| ESR (mm/hour) | ||||
| Median (IQR) | 19.0 (8.0–27.0) | 34.0 (17.0–54.0) | 27.0 (9.0–44.0) | 26.0 (11.0–43.0) |
| Missing | 0 | 2 | 2 | 4 |
| CRP level (mg/l) | ||||
| Median (IQR) | 5.0 (4.0–15.5) | 9.0 (5.0–27.0) | 6.0 (5.0–15.0) | 6.0 (5.0–18.0) |
| Missing | 1 | 2 | 1 | 4 |
| DAS28 | ||||
| Mean (SD) | 5.9 (1.05) | 6.2 (1.08) | 6.2 (1.28) | 6.1 (1.13) |
| Missing | 1 | 3 | 5 | 9 |
| CDAI score | ||||
| Mean (SD) | 38.6 (13.12) | 36.6 (13.34) | 39.6 (13.68) | 38.3 (13.31) |
| Missing | 1 | 3 | 4 | 8 |
| SDAI score | ||||
| Mean (SD) | 39.8 (13.98) | 38.8 (13.87) | 41.9 (14.49) | 40.2 (14.04) |
| Missing | 2 | 5 | 5 | 12 |
| Physician Global Assessment of Disease Activity VAS (mm) | ||||
| Median (IQR) | 67.0 (56.0–75.0) | 66.0 (58.0–84.0) | 65.0 (53.0–84.2) | 66.0 (57.0–79.0) |
| Missing | 0 | 2 | 1 | 3 |
| Patient-reported outcome measure | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Patient Global Assessment of Arthritis VAS (mm) | ||||
| Median (IQR) | 70.5 (62.0–83.0) | 67.5 (52.0–79.5) | 74.0 (53.0–85.0) | 71.0 (56.0–83.0) |
| Missing | 1 | 1 | 3 | 5 |
| Patient Assessment of General Health VAS (mm) | ||||
| Median (IQR) | 56.5 (45.5–72.0) | 62.0 (47.8–68.5) | 61.0 (46.0–74.0) | 59.0 (47.0–70.0) |
| Missing | 1 | 1 | 3 | 5 |
| Patient Global Assessment of Pain VAS (mm) | ||||
| Median (IQR) | 70.5 (59.0–82.5) | 69.5 (57.5–79.0) | 77.0 (55.0–85.0) | 71.0 (58.0–81.0) |
| Missing | 1 | 1 | 3 | 5 |
| HAQ-DI score | ||||
| Median (IQR) | 1.9 (1.4–2.1) | 1.9 (1.6–2.3) | 1.9 (1.4–2.3) | 1.9 (1.5–2.1) |
| Missing | 1 | 1 | 1 | 3 |
| RAQoL score | ||||
| Median (IQR) | 21.6 (15.0–24.5) | 22.0 (14.0–25.5) | 22.0 (15.0–25.0) | 22.0 (15.0–25.0) |
| Missing | 1 | 1 | 2 | 4 |
| HADS score | ||||
| Median (IQR) | 13.5 (8.0–20.0) | 17.0 (10.0–22.0) | 14.0 (11.0–19.0) | 15.0 (10.0–21.0) |
| Missing | 1 | 1 | 3 | 5 |
Minimisation factors
The median disease duration was 6.7 years (range 0.4–43.5 years) (see Table 2). Seventy-seven patients (63.1%) were secondary non-responders and 100 patients (82.0%) were RF seropositive or ACPA positive.
Demographics
Ninety patients (73.8%) had a current comorbidity, with the most frequently reported being hypertension (42 patients; 34.4%), osteoarthritis (33 patients; 27.0%) and hypercholesterolaemia (25 patients; 20.5%) (see Table 3).
Treatment history
Seventy patients (57.4%) had previously failed to respond to a monoclonal antibody TNFi agent. The most common first TNFi agent used was etanercept (52 patients; 42.6%), followed by adalimumab (28 patients; 23.0%) and then CZP (25 patients; 20.5%) (see Table 4).
A total of 24 patients (19.7%) had received some form of steroid or corticosteroid within 4 weeks of screening, with oral prednisolone the most frequently reported (22 patients;18.0%). Sixty patients (49.2%) reported receiving NSAIDs within 4 weeks of screening, with naproxen, diclofenac and ibuprofen most frequently reported. The most common non-bDMARDs used prior to participating were sulfasalazine (91 patients; 74.6%), hydroxychloroquine (84 patients; 68.9%) and leflunomide (26 patient;, 21.3%) (see Appendix 11).
Baseline disease activity and individual component measures
The mean number of TJCs and SJCs at baseline was 15.9 (SD 7.3) and 9.5 (SD 6.2), respectively. The median ESR was 26.0 mm/hour (quartiles 11.0 mm/hour and 43.0 mm/hour) and the median CRP level was 6.0 mg/l (quartiles 5.0 mg/l and 18.0 mg/l) (see Table 5).
The mean DAS28 at baseline was 6.1 units (SD 1.1 units), with 76.2% patients having high disease activity (see Table 5 and Appendix 12, Table 59). The mean CDAI score was 38.3 (SD 13.3), with 82.0% patients categorised as having high disease activity (see Table 5 and Appendix 12, Table 63). Furthermore, the mean SDAI score was 40.2 (SD 14.0) and 77.9% patients were categorised as having high disease activity (see Table 5 and Appendix 12, Table 65).
The median Physician Global Assessment Disease Activity score was 66.0 (quartiles 57.0 and 79.0).
Baseline patient-reported outcomes
The median global assessment of arthritis, general health and pain scores, as rated by the patient, were 71.0 (quartiles 56.0 and 83.0), 59.0 (quartiles 47.0 and 70.0) and 71.0 (quartiles 58.0 and 81.0), respectively (see Table 6). The median HAQ-DI score was 1.9 (quartiles 1.5 and 2.1), the median RAQoL score was 22.0 (quartiles 15.0 and 25.0) and the median HADS score was 15.0 (quartiles 10.0 and 21.0) (see Table 6).
Comparability of baseline characteristics between groups
The baseline characteristics were balanced across the three treatment groups, with the following notable exceptions. A higher percentage of females were randomised to abatacept (95.1%, compared with 80.5% and 75.0% randomised to alternative TNFi and rituximab, respectively). A greater proportion of patients in the alternative TNFi or abatacept arms were current or past smokers (70.7% and 68.5%, respectively) than in the rituximab arm (47.5%). A higher proportion of patients on abatacept had osteoarthritis, whereas more patients on rituximab had a history of hypercholesterolaemia. A greater proportion of patients on alternative TNFi and abatacept had a history of depression and also a history of thyroid dysfunction. Furthermore, a slightly greater proportion of patients on the alternative TNFi or rituximab had a history of diabetes (see Table 3). A slight imbalance in the RF/ACPA status was apparent, whereby a greater proportion of patients on alternative TNFi were seropositive than those taking abatacept and rituximab (see Table 2). The slight imbalances observed are consistent with the random allocation to the treatment group and result from the small sample size.
Tables 47–51 in Appendix 10 summarise the baseline characteristics for the PP population.
Primary and secondary outcomes
Although the results are presented in accordance with the planned analysis, the trial is underpowered for our planned objectives relating to the primary and secondary outcome measures.
Primary outcome
Intention-to-treat patient population
Tables 7 and 8 and Figure 5 present the results of the primary end-point analysis for the ITT patient population.
| Effect | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | 1.50 | 0.72 to 2.28 | < 0.001 |
| Randomised treatment: alternative TNFi vs. rituximab | 0.30 | –0.45 to 1.05 | 0.436 |
| Randomised treatment: abatacept vs. rituximab | 0.04 | –0.72 to 0.79 | 0.927 |
| RF/anti-CCP seropositivity: both seronegative vs. either seropositive | –0.17 | –1.00 to 0.66 | 0.690 |
| Disease duration: ≥ 5 years vs. < 5 years | –0.05 | –0.70 to 0.60 | 0.883 |
| Non-responder type: secondary vs. primary | –0.45 | –1.11 to 0.21 | 0.181 |
| Treatment arm | Adjusted mean DAS28 reduction at week 24 (95% CI) | Treatment comparison | Difference in mean DAS28 reductions (experimental – rituximab 95% CI) | Probabilitya (δ > –0.6) |
|---|---|---|---|---|
| Rituximab (n = 40) | 1.17 (0.56 to 1.77) | – | – | – |
| Alternative TNFi (n = 41) | 1.47 (0.85 to 2.08) | Alternative TNFi vs. rituximab | 0.30 (–0.45 to 1.05) | 0.0094 |
| Abatacept (n = 41) | 1.20 (0.62 to 1.78) | Abatacept vs. rituximab | 0.04 (–0.72 to 0.79) | 0.0493 |
FIGURE 5.
Primary end-point analysis: difference in mean reduction in the DAS28 at 24 weeks post randomisation between alternative TNFi, abatacept and rituximab – ITT population analysis. The dotted line at zero is the null difference value and the thick dashed line denotes the non-inferiority margin.

For the comparison between alternative TNFi and rituximab, the lower limit of the 95% CI for the difference in the mean reduction in the DAS28 lies above the predefined, non-inferiority limit of –0.6 units; the difference in the mean reduction in DAS28 (alternative TNFi – rituximab) at 24 weeks post randomisation was 0.3 units (95% CI –0.45 to 1.05 units). Therefore, alternative TNFi was non-inferior to rituximab in the ITT patient population.
For the comparison between abatacept and rituximab, the lower limit of the 95% CI lay just below the predefined non-inferiority limit; the difference in mean reduction in the DAS28 at 24 weeks (abatacept – rituximab) was 0.04 units (95% CI –0.72 to 0.79 units). Therefore, abatacept was not shown to be non-inferior to rituximab in the ITT patient population.
Summaries of the missing values for the component end points of the DAS28 by treatment group are presented in Appendix 13, Tables 69 and 70.
Sensitivity analysis
Table 9 and Figure 6 present results of the sensitivity analysis on the primary end point for the ITT patient population, adjusting for the baseline DAS28.
| Treatment arm | Adjusted mean DAS28 reduction at week 24 (95% CI) | Treatment comparison | Baseline-adjusted difference in mean DAS28 reductions (experimental – rituximab 95% CI) | Probabilitya (δ > 0.6) |
|---|---|---|---|---|
| Rituximab (n = 40) | 1.07 (0.53 to 1.60) | – | – | – |
| Alternative TNFi (n = 41) | 1.60 (1.05 to 2.15) | Alternative TNFi vs. rituximab | 0.54 (–0.12 to 1.20) | < 0.001 |
| Abatacept (n = 41) | 1.11 (0.60 to 1.62) | Abatacept vs. rituximab | 0.05 (–0.61 to 0.70) | 0.026 |
FIGURE 6.
Sensitivity analysis on the primary end point: difference in the mean reduction in the DAS28 at 24 weeks post randomisation between alternative TNFi, abatacept and rituximab.
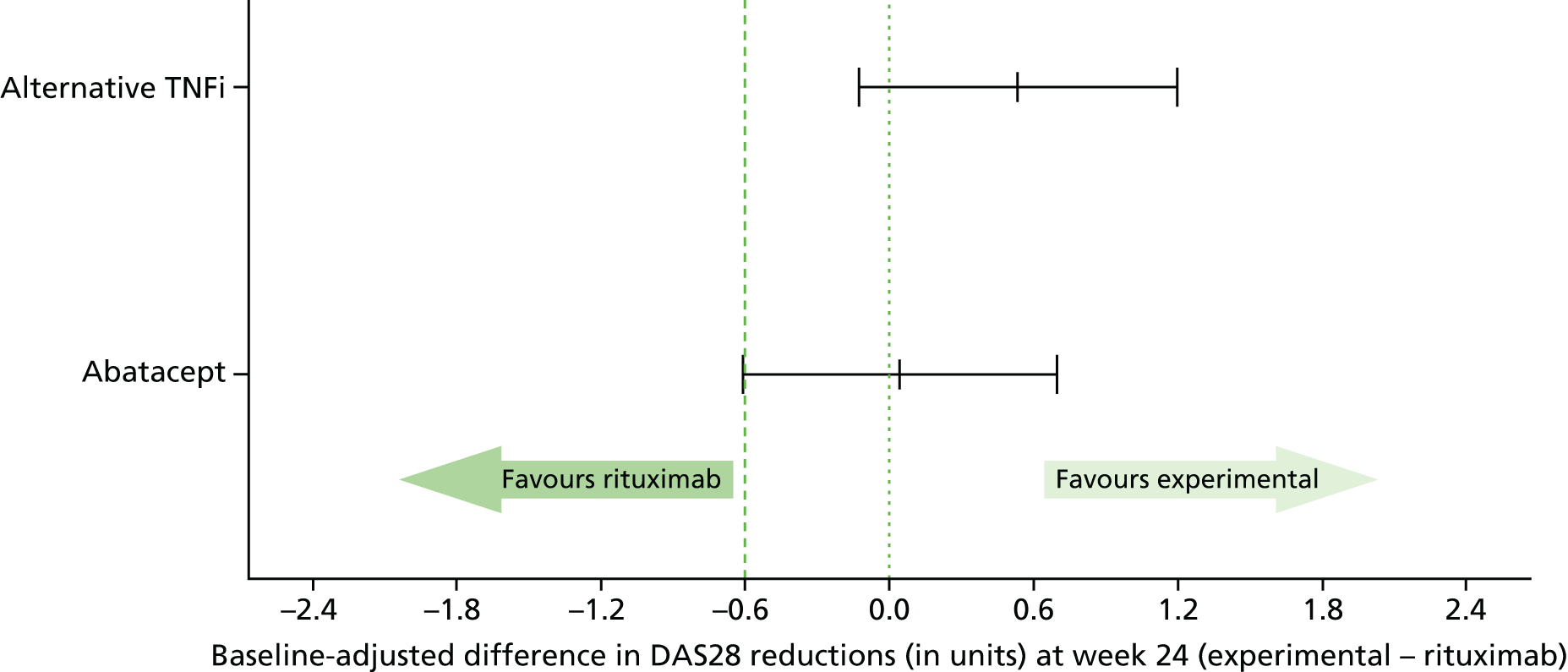
For the comparison between alternative TNFi and rituximab, the lower limit of the 95% CI for the difference in the mean reduction in the DAS28 lies above the predefined non-inferiority limit of –0.6 units; the difference in mean reduction in the DAS28 (alternative TNFi – rituximab) at 24 weeks post randomisation was 0.54 units (95% CI –0.12 to 1.20 units). Therefore, again alternative TNFi was non-inferior to rituximab in the ITT patient population.
For the comparison between abatacept and rituximab, the lower limit of the 95% CI lay just below the predefined non-inferiority limit; the difference in the mean reduction in the DAS28 at 24 weeks (abatacept – rituximab) was 0.05 units (95% CI –0.61 to 0.70 units). Therefore, there was only marginal evidence that abatacept was non-inferior to rituximab in the ITT patient population.
Per protocol population
A total of 41 patients were included in the PP population with 13 (31.7%), 14 (34.1%) and 14 (35.0%) in alternative TNFi, abatacept and rituximab treatment groups, respectively. Owing to the small number of patients in the PP population, only the primary outcome has been analysed.
Tables 10 and 11 and Figure 7 provide the results of the primary end-point analysis for the PP population.
| Effect | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept | 2.07 | 0.91 to 3.23 | 0.001 |
| Randomised treatment: alternative TNFi vs. rituximab | –0.58 | –1.72 to 0.55 | 0.312 |
| Randomised treatment: abatacept vs. rituximab | –0.15 | –1.27 to 0.98 | 0.796 |
| RF/anti-CCP seropositivity: both seronegative vs. either seropositive | –0.73 | –1.96 to 0.50 | 0.245 |
| Disease duration: ≤ 5 years vs. > 5 years | –0.05 | –1.10 to 1.01 | 0.930 |
| Non-responder type: secondary vs. primary | –0.05 | –0.99 to 0.88 | 0.908 |
| Treatment group | Adjusted mean DAS28 reduction at week 24 (95% CI) | Treatment comparison | Difference in mean DAS28 reductions (experimental – rituximab 95% CI) | Probabilitya (δ > –0.6) |
|---|---|---|---|---|
| Rituximab (n = 14) | 1.66 (0.77 to 2.55) | – | – | – |
| Alternative TNFi (n = 13) | 1.07 (0.11 to 2.03) | Alternative TNFi vs. rituximab | –0.58 (–1.72 to 0.55) | 0.489 |
| Abatacept (n = 14) | 1.51 (0.70 to 2.31) | Abatacept vs. rituximab | –0.15 (–1.27 to 0.98) | 0.216 |
FIGURE 7.
Primary end-point analysis: difference in the mean reduction in the DAS28 at 24 weeks post randomisation between alternative TNFi, abatacept and rituximab – PP population.

For the comparison of alternative TNFi versus rituximab, the lower limit of the 95% CI for the difference in the mean reduction in the DAS28 was below the predefined non-inferiority limit of –0.6 units; the difference in the mean reduction in the DAS28 at 24 weeks post randomisation (alternative TNFi – rituximab) was –0.58 units (95% CI –1.72 to 0.55 units). Therefore, non-inferiority of alternative TNFi to rituximab was not demonstrated in the PP population and so we cannot conclude that alternative TNFi is non-inferior to rituximab.
Similarly, for the comparison of abatacept versus rituximab, the lower limit of the 95% CI for the difference in the mean reduction in the DAS28 was below –0.6 units; the difference in the mean reduction in the DAS28 at 24 weeks post randomisation (abatacept – rituximab) was –0.15 units (95% CI –1.27 to 0.98 units). Therefore, non-inferiority of abatacept to rituximab was not demonstrated in the PP population. Hence, a conclusion of non-inferiority of abatacept to rituximab was not reached in both analyses (although in an underpowered cohort).
Complete-case analysis population
The primary end-point analysis was also conducted on the population of all patients with complete data at both baseline and week 24. Table 12 and Figure 8 provide the results of the primary end-point analysis in the complete-case analysis population.
| Treatment group | Adjusted mean DAS28 reduction at week 24 (95% CI) | Treatment comparison | Difference in mean DAS28 reductions (experimental – rituximab 95% CI) | Probabilitya (δ > –0.6) |
|---|---|---|---|---|
| Rituximab (n = 32) | 1.14 (0.44 to 1.85) | – | – | – |
| Alternative TNFi (n = 36) | 1.24 (0.57 to 1.91) | Alternative TNFi vs. rituximab | 0.10 (–0.71 to 0.91) | 0.044 |
| Abatacept (n = 34) | 1.10 (0.47 to 1.74) | Abatacept vs. rituximab | –0.04 (–0.86 to 0.79) | 0.090 |
FIGURE 8.
Primary end-point analysis: difference in the mean reduction in the DAS28 at 24 weeks post randomisation between alternative TNFi, abatacept and rituximab – complete-case population.
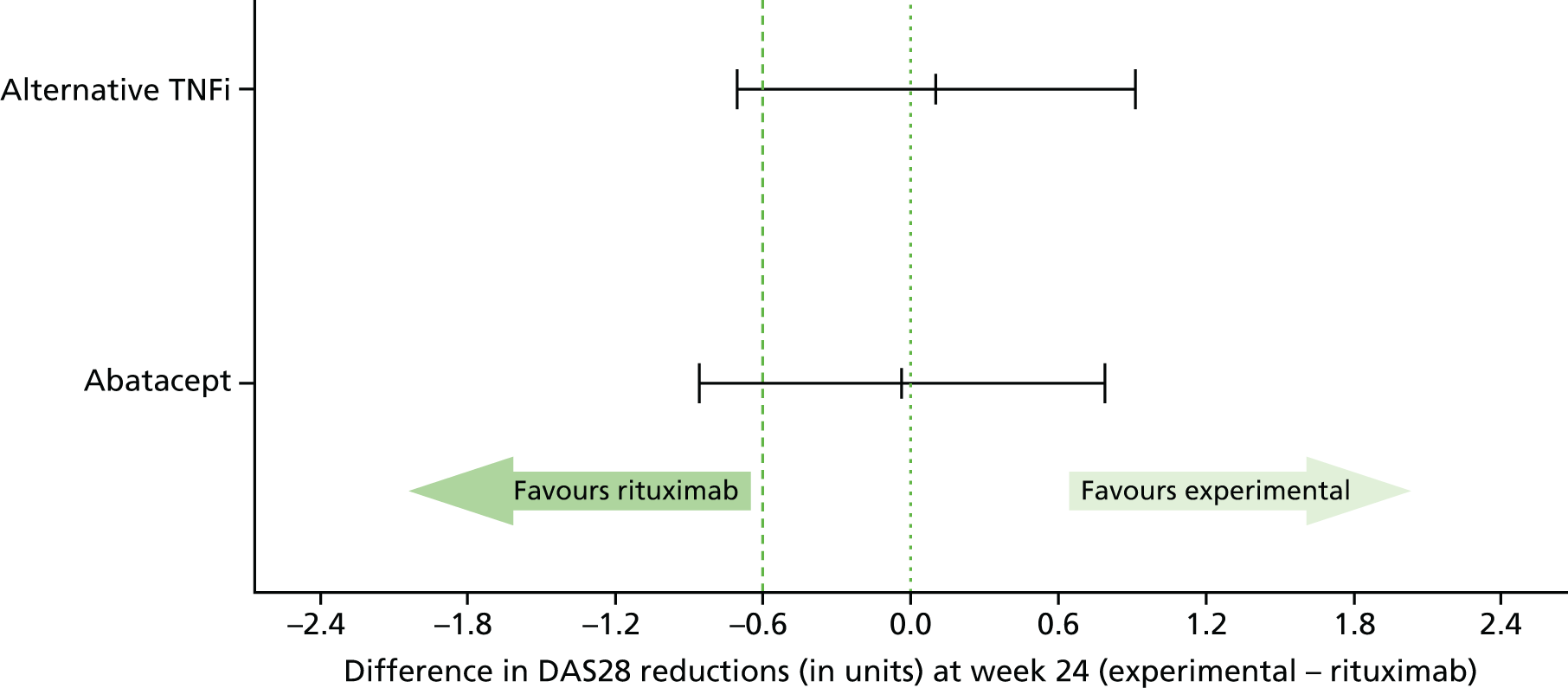
For both alternative TNFi and abatacept, the lower limit of the 95% CI for the true difference in the mean reduction in the DAS28 lay just below the predefined non-inferiority limit of –0.6 units. The difference in the mean reduction in the DAS28 at 24 weeks post randomisation for alternative TNFi compared with rituximab was 0.10 units (95% CI –0.71 to 0.91 units) and for abatacept relative to rituximab was –0.04 units (95% CI –0.86 to 0.79 units). Therefore, non-inferiority of alternative TNFi and abatacept to rituximab was not demonstrated in the complete-case analysis population.
Summary statistics for the DAS28 at baseline, week 24 and the corresponding reduction for the complete-case population is summarised in Appendix 12, Table 58.
Exploratory subgroup analysis
Table 13 presents the least squares means and corresponding 95% CIs of the reduction in the DAS28 at week 24 by RF/ACPA seropositivity status, initial TNFi type and non-responder status to an initial bDMARD for the ITT population; corresponding summary statistics for the complete-case population are provided in Appendix 12, Table 53. The results of the subgroup analysis are not sufficiently precise to draw definitive conclusions and, therefore, only informal comparisons between treatment groups were made. All results should be interpreted cautiously.
| Subgroup | Treatment arm, mean (95% CI) | ||
|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | |
| RF/ACPA seropositivity status | |||
| Both RF seronegative and ACPA seronegative | 1.76 (0.23 to 3.29) | 1.64 (0.57 to 2.70) | 0.09 (–1.20 to 1.39) |
| RF seropositive and/or ACPA seropositive | 1.50 (0.93 to 2.07) | 1.13 (0.50 to 1.75) | 1.47 (0.87 to 2.06) |
| Non-response category | |||
| Primary | 1.15 (0.25 to 2.05) | 1.84 (0.97 to 2.71) | 1.45 (0.56 to 2.35) |
| Secondary | 1.48 (0.72 to 2.24) | 0.68 (–0.07 to 1.44) | 0.84 (0.09 to 1.58) |
| Type of TNFi failed | |||
| Etanercept | 1.64 (0.71 to 2.56) | 1.50 (0.64 to 2.36) | 1.38 (0.50 to 2.26) |
| Monoclonal antibody | 1.38 (0.64 to 2.12) | 0.99 (0.25 to 1.73) | 1.02 (0.26 to 1.78) |
Rheumatoid factor/anti-citrullinated peptide antibody seropositivity effect on treatment response
It was hypothesised that patients who were RF/ACPA seronegative would have a greater response to alternative TNFi or abatacept than to rituximab.
The DAS28 improvements at week 24 among patients who were RF or ACPA seropositive appeared to be similar in the alternative TNFi and rituximab groups, although a small improvement was observed in the abatacept group. However, among patients who were seronegative, no improvement in the rituximab group was apparent, with a greater improvement observed in the alternative TNFi and abatacept groups. However, as only approximately 18% of patients were seronegative, the conclusions that could be drawn are limited.
Primary or secondary non-responder status (on an initial tumour necrosis factor inhibitor) on treatment response
Primary non-responders appeared to show greater improvement, on average, on abatacept and rituximab than on alternative TNFi, although the reverse was observed for secondary non-response patients.
Initial alternative tumour necrosis factor inhibitor failed on treatment response
In the case of patients who previously did not respond to etanercept, similar improvements in the DAS28 at week 24 were observed across all treatment groups, although, among those who previously failed to respond to a monoclonal antibody, alternative TNFi (fusion protein, etanercept) appeared to confer a greater improvement than abatacept or rituximab.
Secondary outcomes
Disease Activity Score of 28 joints over time
Table 14 provides the parameter estimates for the model for the DAS28 up to week 48. Table 15 presents the adjusted DAS28 and corresponding difference from rituximab at each time point up to week 48. Figure 9 provides a graphical representation of the adjusted means and corresponding 95% CIs from the model. The covariance matrix is provided in Appendix 12, Table 55.
| Effect | Parameter estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept (rituximab at week 12) | 2.72 | 1.48 to 3.96 | < 0.0001 |
| Baseline DAS28 | 0.38 | 0.21 to 0.55 | < 0.0001 |
| Randomised treatment | |||
| Alternative TNFi vs. rituximab | –0.18 | –0.72 to 0.35 | 0.503 |
| Abatacept vs. rituximab | –0.04 | –0.57 to 0.48 | 0.870 |
| RF/ACPA status: both seronegative vs. either RF/ACPA seropositive | 0.19 | –0.32 to 0.69 | 0.475 |
| Years since diagnosis: ≥ 5 years vs. 0–4 years | –0.16 | –0.57 to 0.25 | 0.440 |
| Non-response type: secondary vs. primary | 0.13 | –0.27 to 0.53 | 0.524 |
| Visit | |||
| 24 weeks | –0.09 | –0.54 to 0.36 | 0.701 |
| 36 weeks | –0.14 | –0.52 to 0.24 | 0.477 |
| 48 weeks | –0.29 | –0.75 to 0.17 | 0.218 |
| Interaction effect for | |||
| Alternative TNFi | |||
| At 24 weeks | –0.32 | –0.96 to 0.33 | 0.338 |
| At 36 weeks | –0.53 | –1.11 to 0.05 | 0.071 |
| At 48 weeks | –0.20 | –0.88 to 0.47 | 0.552 |
| Abatacept | |||
| At 24 weeks | –0.01 | –0.65 to 0.63 | 0.976 |
| At 36 weeks | 0.05 | –0.51 to 0.60 | 0.871 |
| At 48 weeks | 0.10 | –0.55 to 0.75 | 0.755 |
| Treatment arm | Adjusted mean (95% CI) | Difference in adjusted means (95% CI) | p-value |
|---|---|---|---|
| 12 weeks | |||
| Rituximab | 5.09 (4.69 to 5.48) | – | – |
| Abatacept | 5.03 (4.63 to 5.43) | –0.04 (–0.57 to 0.48) | 0.870 |
| Alternative TNFi | 4.89 (4.47 to 5.32) | –0.18 (–0.72 to 0.35) | 0.503 |
| 24 weeks | |||
| Rituximab | 4.99 (4.50 to 5.47) | – | – |
| Abatacept | 4.93 (4.46 to 5.41) | –0.05 (–0.71 to 0.60) | 0.872 |
| Alternative TNFi | 4.49 (3.99 to 4.99) | –0.50 (–1.16 to 0.16) | 0.137 |
| 36 weeks | |||
| Rituximab | 4.94 (4.49 to 5.39) | – | – |
| Abatacept | 4.94 (4.49 to 5.39) | 0.00 (–0.60 to 0.61) | 0.995 |
| Alternative TNFi | 4.22 (3.75 to 4.70) | –0.71 (–1.32 to 0.11) | 0.022 |
| 48 weeks | |||
| Rituximab | 4.79 (4.28 to 5.29) | – | – |
| Abatacept | 4.84 (4.38 to 5.31) | 0.06 (–0.59 to 0.71) | 0.859 |
| Alternative TNFi | 4.40 (3.88 to 4.92) | –0.39 (–1.04 to 0.27) | 0.249 |
FIGURE 9.
Adjusted mean DAS28 over the 48-week intervention period and the comparisons of alternative TNFi and abatacept with rituximab over 48 weeks. Adjusted mean DAS28 over the 48-week intervention period (top, left axis). Estimated differences between each intervention arm and rituximab at each time point (bottom, right axis).
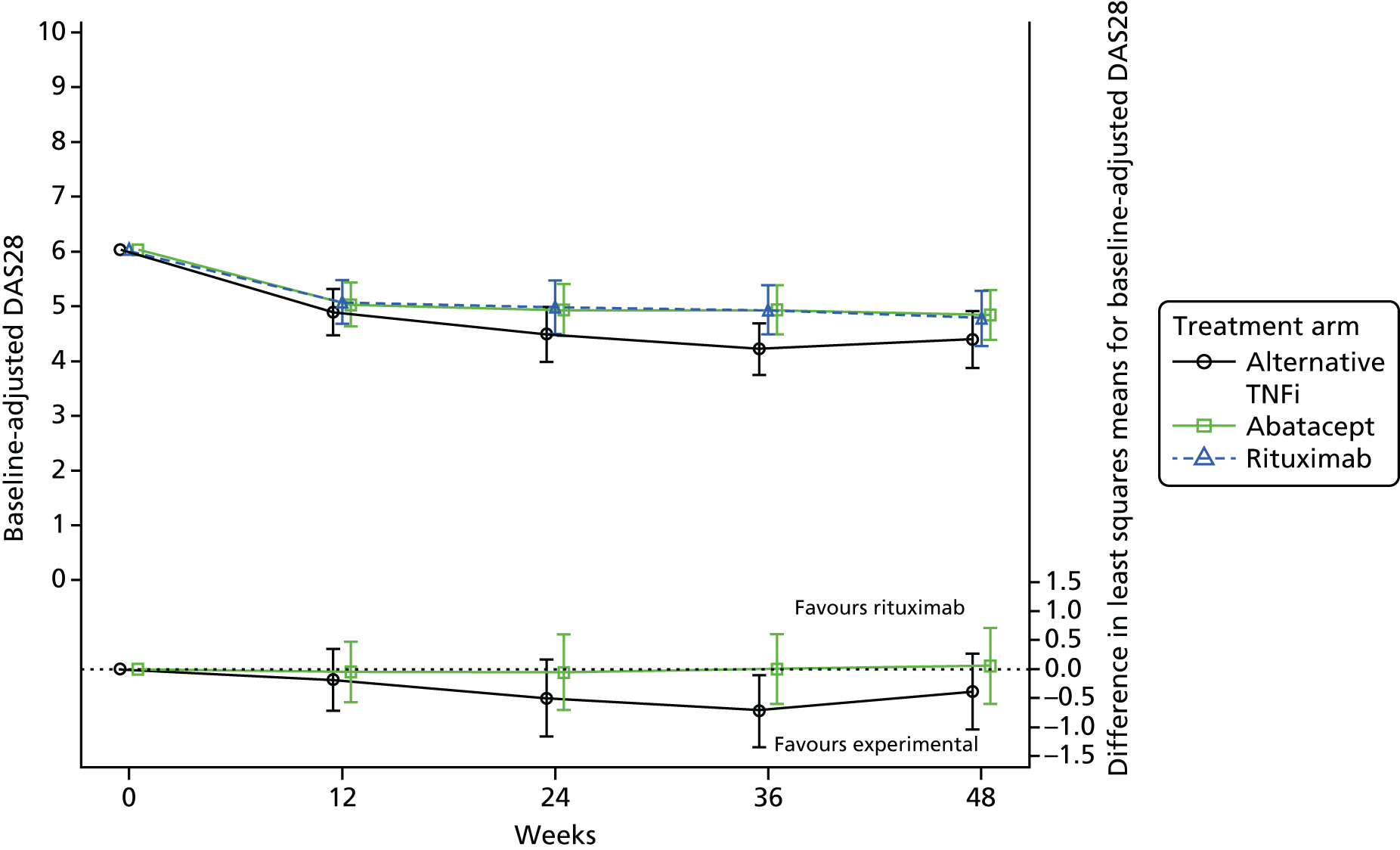
There was no evidence of a treatment effect for abatacept compared with rituximab at any of the time points (see Table 15). This analysis showed significant evidence of a difference between alternative TNFi and rituximab at week 36 (–0.71 units, 95% CI –1.32 to –0.11 units; p = 0.022), although this difference was not maintained at week 48.
From 24 weeks post randomisation, the 95% CIs for the mean DAS28 in the alternative TNFi group exclude values greater than 4.99, suggesting that the mean DAS28 for this group is lower than the DAS28 threshold for high disease activity (i.e. DAS28 > 5.1 units). For abatacept and rituximab the estimated 95% CIs for the mean DAS28 include values corresponding to high disease activity at all time points.
Reduction in the Disease Activity Score of 28 joints of ≥ 1.2 units over time
The frequency of patients achieving a DAS28 response over 48 weeks for the complete-case population is provided in Appendix 12, Table 54.
Parameter estimates for the model are given in Table 16. Table 17 shows the odds of achieving DAS28 ≥ 1.2 units in each group and the odds ratios relative to rituximab at each time point up to week 48. Figure 10 provides a graphical representation of the fitted values from the model. The covariance matrix is provided in Appendix 12, Table 56.
| Effect | Odds ratio (95% CI) | p-value |
|---|---|---|
| Intercept | < 0.001 | |
| Baseline DAS28 | 2.23 (1.65 to 3.02) | < 0.001 |
| Randomised treatment | ||
| Alternative TNFi vs. rituximab | 1.07 (0.38 to 3.02) | 0.904 |
| Abatacept vs. rituximab | 1.22 (0.44 to 3.39) | 0.701 |
| Seropositivity: RF/ACPA both seronegative vs. either RF/ACPA seropositive | 0.74 (0.33 to 1.65) | 0.464 |
| Years since diagnosis: ≥ 5 years vs. 0–4 years | 1.56 (0.82 to 2.97) | 0.178 |
| Non-response type: secondary vs. primary | 0.96 (0.51 to 1.81) | 0.888 |
| Visit | ||
| 24 weeks | 0.98 (0.45 to 2.13) | 0.963 |
| 36 weeks | 1.17 (0.49 to 2.77) | 0.726 |
| 48 weeks | 1.77 (0.64 to 4.90) | 0.269 |
| Interaction effect for | ||
| Alternative TNFi | ||
| At 24 weeks | 2.37 (0.81 to 6.94) | 0.116 |
| At 36 weeks | 1.79 (0.56 to 5.72) | 0.327 |
| At 48 weeks | 1.32 (0.33 to 5.33) | 0.699 |
| Abatacept | ||
| At 24 weeks | 0.95 (0.33 to 2.76) | 0.930 |
| At 36 weeks | 0.92 (0.28 to 2.98) | 0.888 |
| At 48 weeks | 0.75 (0.19 to 2.93) | 0.675 |
| Treatment arm | Adjusted odds ratio of response (95% CI) | Adjusted odds ratio of response vs. rituximab (95% CI) | p-value |
|---|---|---|---|
| 12 weeks | |||
| Rituximab | 0.65 (0.29 to 1.42) | – | – |
| Abatacept | 0.79 (0.38 to 1.65) | 1.22 (0.44 to 3.39) | 0.701 |
| Alternative TNFi | 0.69 (0.32 to 1.50) | 1.07 (0.38 to 3.02) | 0.905 |
| 24 weeks | |||
| Rituximab | 0.64 (0.29 to 1.39) | – | – |
| Abatacept | 0.74 (0.35 to 1.55) | 1.16 (0.42 to 3.24) | 0.771 |
| Alternative TNFi | 1.61 (0.75 to 3.46) | 2.53 (0.90 to 7.07) | 0.077 |
| 36 weeks | |||
| Rituximab | 0.76 (0.33 to 1.74) | – | – |
| Abatacept | 0.85 (0.40 to 1.81) | 1.12 (0.37 to 3.42) | 0.839 |
| Alternative TNFi | 1.44 (0.66 to 3.12) | 1.91 (0.65 to 5.60) | 0.240 |
| 48 weeks | |||
| Rituximab | 1.15 (0.49 to 2.71) | – | – |
| Abatacept | 1.05 (0.50 to 2.19) | 0.91 (0.30 to 2.73) | 0.869 |
| Alternative TNFi | 1.61 (0.72 to 3.62) | 1.40 (0.47 to 4.19) | 0.543 |
FIGURE 10.
Adjusted odds of achieving a DAS28 response and odds ratios of a response over 48 weeks. Covariance pattern model of a DAS28 response over time. Unstructured covariance pattern. Random centre effect excluded, baseline score adjusted. Estimated odds of achieving a DAS28 response at follow-up (top, left axis). Odds ratios of response of alternative TNFi and abatacept to rituximab at each time point (bottom, right axis).
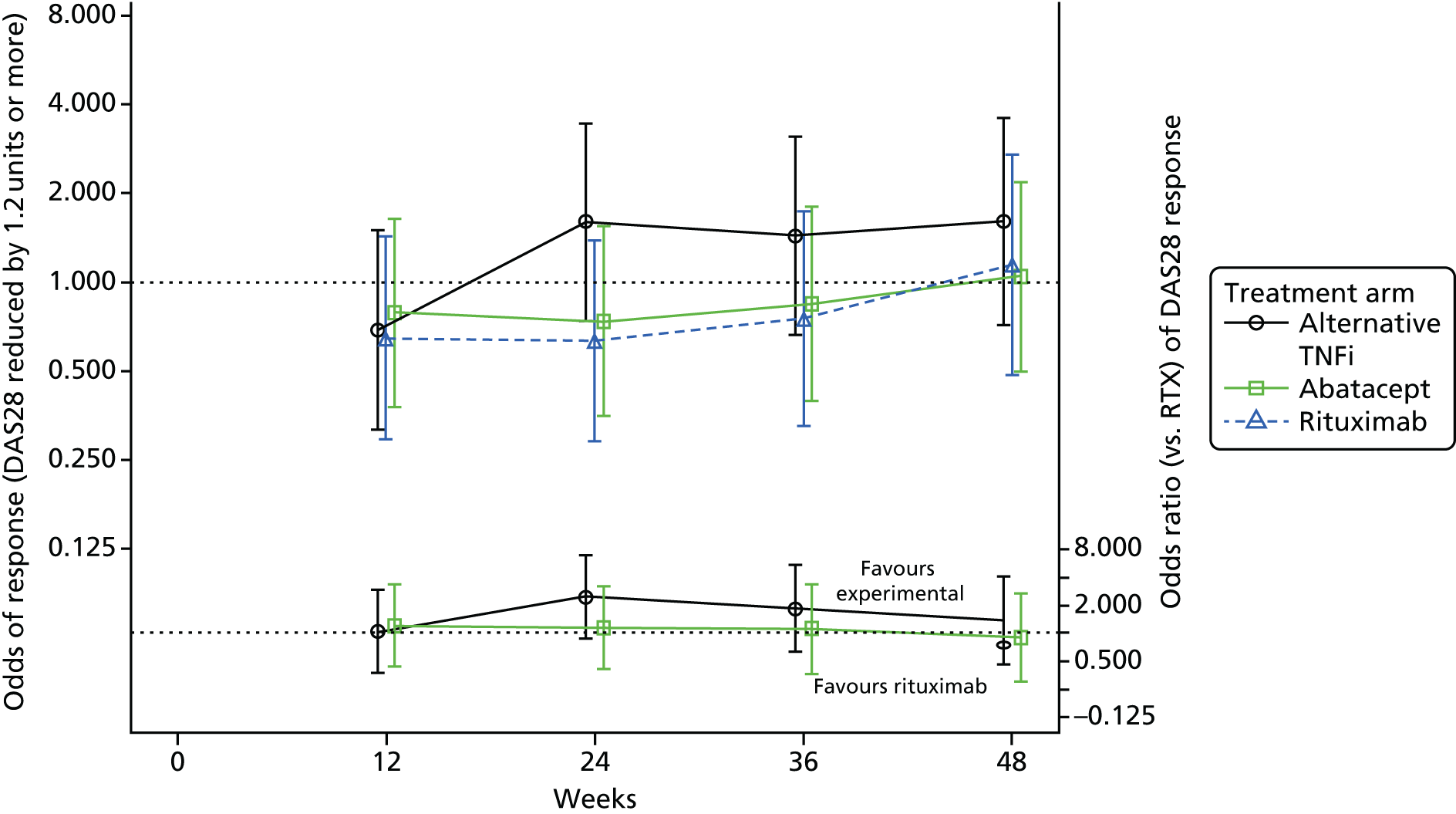
There was no evidence of a treatment effect for either alternative TNFi or abatacept compared with rituximab after adjusting for the minimisation factors and baseline covariates, at any of the time points (see Table 17).
American College of Rheumatology 20 response at week 24
Table 18 provides the parameter estimates for the model and Table 19 provides the adjusted odds ratios for achieving ACR20 response at 24 weeks post randomisation. Figure 11 provides a graphical representation of the fitted values from the model.
| Parameter | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Intercept | 0.062 | ||
| Randomised treatment | |||
| Alternative TNFi vs. rituximab | 2.06 | 0.77 to 5.53 | 0.150 |
| Abatacept vs. rituximab | 1.19 | 0.44 to 3.21 | 0.736 |
| RF/ACPA status: both seronegative vs. either seropositive | 1.39 | 0.48 to 3.99 | 0.539 |
| Disease duration: ≥ 5 years vs. < 5 years | 1.73 | 0.73 to 4.12 | 0.216 |
| Non-responder type: secondary vs. primary | 0.55 | 0.24 to 1.26 | 0.161 |
| Treatment group | Adjusted odds ratio of response (95% CI) | Adjusted odds ratio of response vs. rituximab (95% CI) | p-value |
|---|---|---|---|
| Rituximab | 0.43 (0.20 to 0.94) | ||
| Abatacept | 0.51 (0.24 to 1.08) | 1.19 (0.44 to 3.21) | 0.736 |
| Alternative TNFi | 0.89 (0.41 to 1.96) | 2.06 (0.77 to 5.53) | 0.150 |
FIGURE 11.
Odds ratios of an ACR20 response relative to rituximab at 24 weeks post randomisation: ITT patient population.
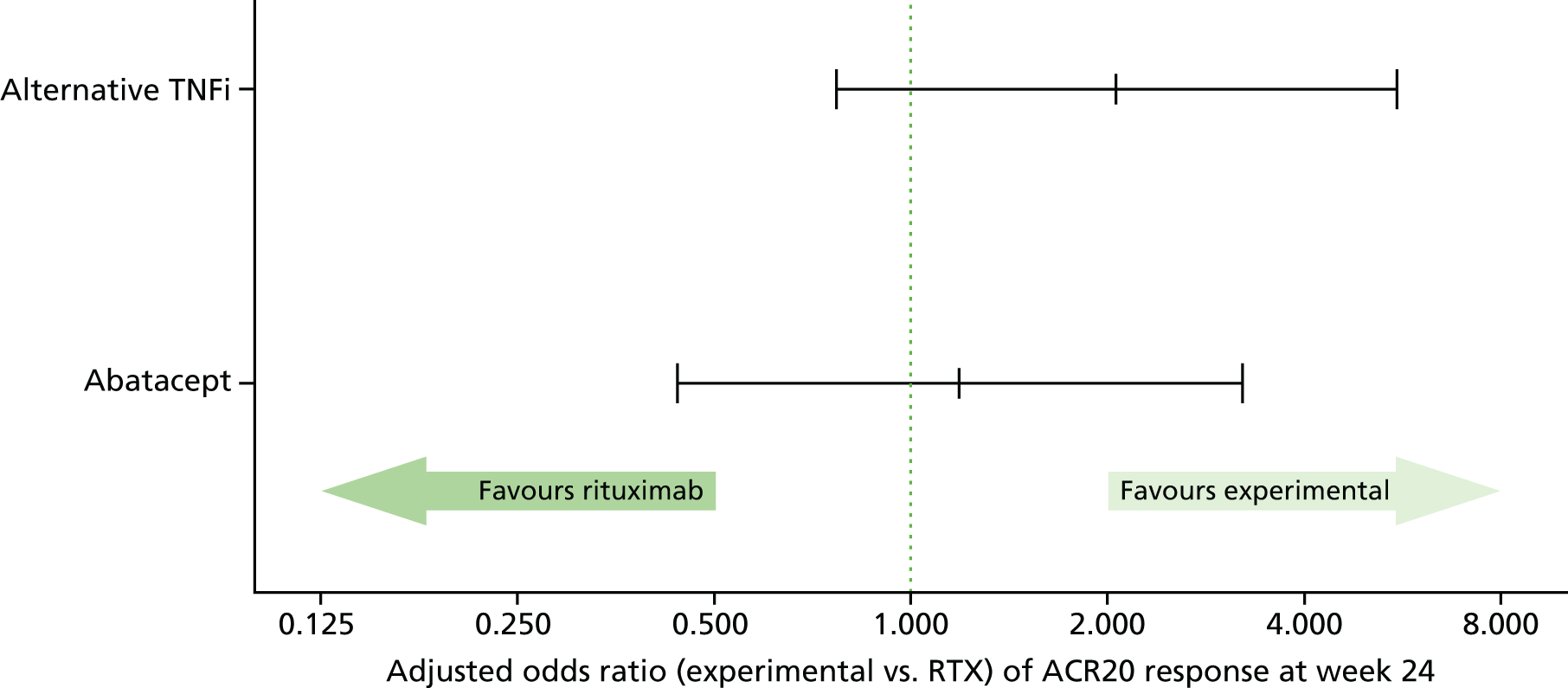
There was no evidence of a difference in the odds of achieving an ACR20 response at 24 weeks post randomisation in either intervention compared with rituximab (OR 2.06, 95% CI 0.77 to 5.53 and OR 1.19, 95% CI 0.44 to 3.21 for alternative TNFi and abatacept, respectively).
Summaries of the missing values for the component end points of the ACR20 by treatment group are presented in Appendix 13, Tables 69 and 70.
Additional secondary outcomes
Clinical assessment of disease activity over 48 weeks
Appendix 12, Tables 54 and 57–65, summarise measures of disease activity over 48 weeks by randomised treatment group. Summary statistics for each of the secondary outcomes over the observational period (weeks 60–96) are provided in Appendix 12, Tables 58–65. A brief description of the findings is given below. There were too few patients to make firm inferences from any of these analyses.
American College of Rheumatology 20, American College of Rheumatology 50 and American College of Rheumatology 70 response
The proportion of patients on alternative TNFi who achieved an ACR20 response appeared to increase over time, reaching 54.8% by week 48, although, among patients on abatacept, the proportion decreased to 35.5% by week 48 and, among those on rituximab, it had increased by week 48 to 42.9% after an initial reduction at week 24 (27.0%), potentially reflecting the need for a repeat cycle of rituximab in the second 6-month period. The proportion of patients achieving an ACR50 response generally increased over time on alternative TNFi and rituximab and plateaued after week 24 for abatacept. The proportion of patients achieving an ACR70 fluctuated over time across all three treatment groups; overall, only 11.8% achieved an ACR70 by week 48 (see Appendix 12, Table 57).
Disease Activity Score of 28 joints and Disease Activity Score of 28 joints response category
The mean reduction in the DAS28 over 48 weeks was greater among patients on alternative TNFi than among those on rituximab, whereas a similar mean reduction in the DAS28 was apparent in the abatacept and rituximab groups [alternative TNFi mean at 48 weeks, 1.6 units (SD 1.64 units); abatacept mean at 48 weeks, 1.4 units (SD 1.38 units); and rituximab mean at 48 weeks, 1.2 units (SD 1.49 units)] (see Appendix 12, Table 58).
The proportion of patients who achieved DAS28 low disease activity or remission at 24 weeks was similar in the alternative TNFi and rituximab groups, but lower in the abatacept group (19.5%, 20.0% and 14.6%, respectively). The proportion of patients achieving remission at week 24 showed a similar pattern (alternative TNFi, 9.8%; rituximab, 10.0%; abatacept, 7.3%). Furthermore, in the alternative TNFi group, this figure continued to increase, reaching 26.8% by week 48; in contrast, in the abatacept and rituximab groups, it fell, to 7.3% and 7.5%, respectively (see Appendix 12, Table 59). The proportion of patients reaching remission at week 48 showed a corresponding increase in the alternative TNFi group (12.2%), but in the abatacept and rituximab groups, this fell to 4.9% and 5.0%, respectively.
European League Against Rheumatism response
The proportion of patients with a good EULAR response at 24 weeks was similar in the alternative TNFi and rituximab groups, at 19.5% and 17.5%, respectively, but was lower in the abatacept group, at 12.2%. Furthermore, in the alternative TNFi group, the proportion of patients achieving a good response generally increased over time, reaching 26.8% at week 48, whereas in the abatacept and rituximab groups, a reduction to 4.9% and 5.0%, respectively, was observed (see Appendix 12, Table 60).
American College of Rheumatology/Boolean remission
A minority of patients achieved ACR/Boolean remission status over the 48 weeks. At 24 weeks, two patients (4.9%) on alternative TNFi and one patient (2.4%) on abatacept reached ACR/Boolean remission, compared with none on rituximab. By week 48 the frequency remained very low, with only one patient (2.4%) on alternative TNFi and a further one patient (2.4%) on abatacept reaching this status (see Appendix 12, Table 61).
Clinical Disease Activity Index
Similar improvements over 48 weeks were observed in CDAI in the alternative TNFi and rituximab groups,, with a lower improvement observed in the abatacept group [median improvement of 19.3 (quartiles 5.7 and 28.8), 20.3 (quartiles 5.3 and 32.3) and 14.1 (quartiles 5.9 and 29.2) respectively] (see Appendix 12, Table 62).
Simplified Disease Activity Index
Like the CDAI, improvement in the SDAI over 48 weeks was similar in the alternative TNFi and rituximab groups, but lower in the abatacept group [median improvement of 20.1 (quartiles 7.9 and 27.2), 20.1 (quartiles 5.3 and 34.0) and 13.7 (quartiles 6.3 and 31.2) respectively] (see Appendix 12, Table 64).
Patient-reported outcomes over 48 weeks
Figures 12–15 summarise the patient-reported outcomes of the HAQ-DI, RAQoL and HADS and Figures 16–18 summarise the Patient Global Assessment of Pain, Patient Global Assessment of Arthritis and Patient Assessment of General Health VAS scores over 48 weeks by treatment group. Further summaries over the 96 weeks are provided in Appendix 12, Tables 66 and 67.
FIGURE 12.
Box and whisker plot: HAQ-DI score over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.
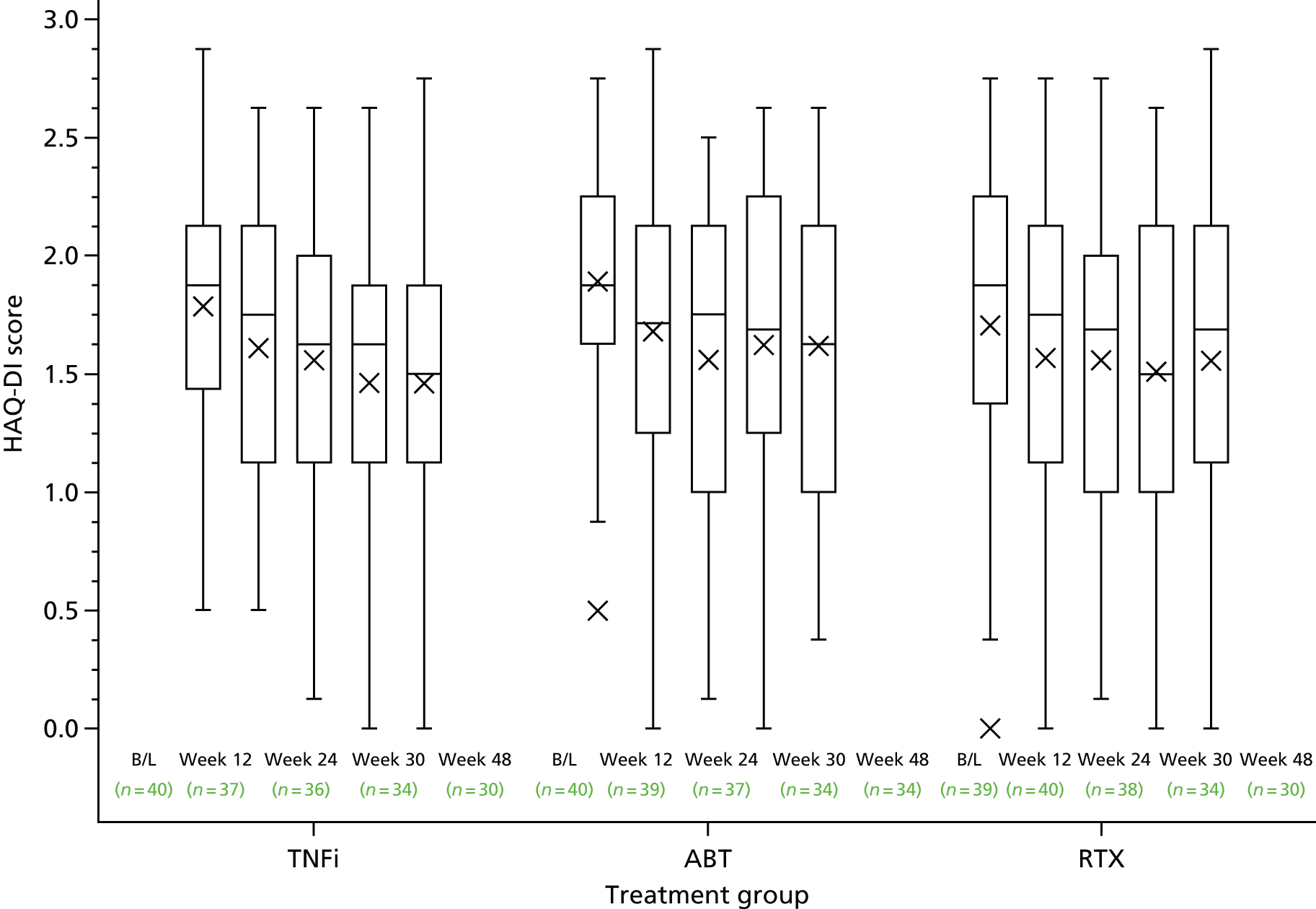
FIGURE 13.
Box and whisker plot: RAQoL over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.

FIGURE 14.
Box and whisker plot: HADS anxiety scale over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.

FIGURE 15.
Box and whisker plot: HADS depression scale over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.

FIGURE 16.
Box and whisker plot: Patient Global Assessment of Pain over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.
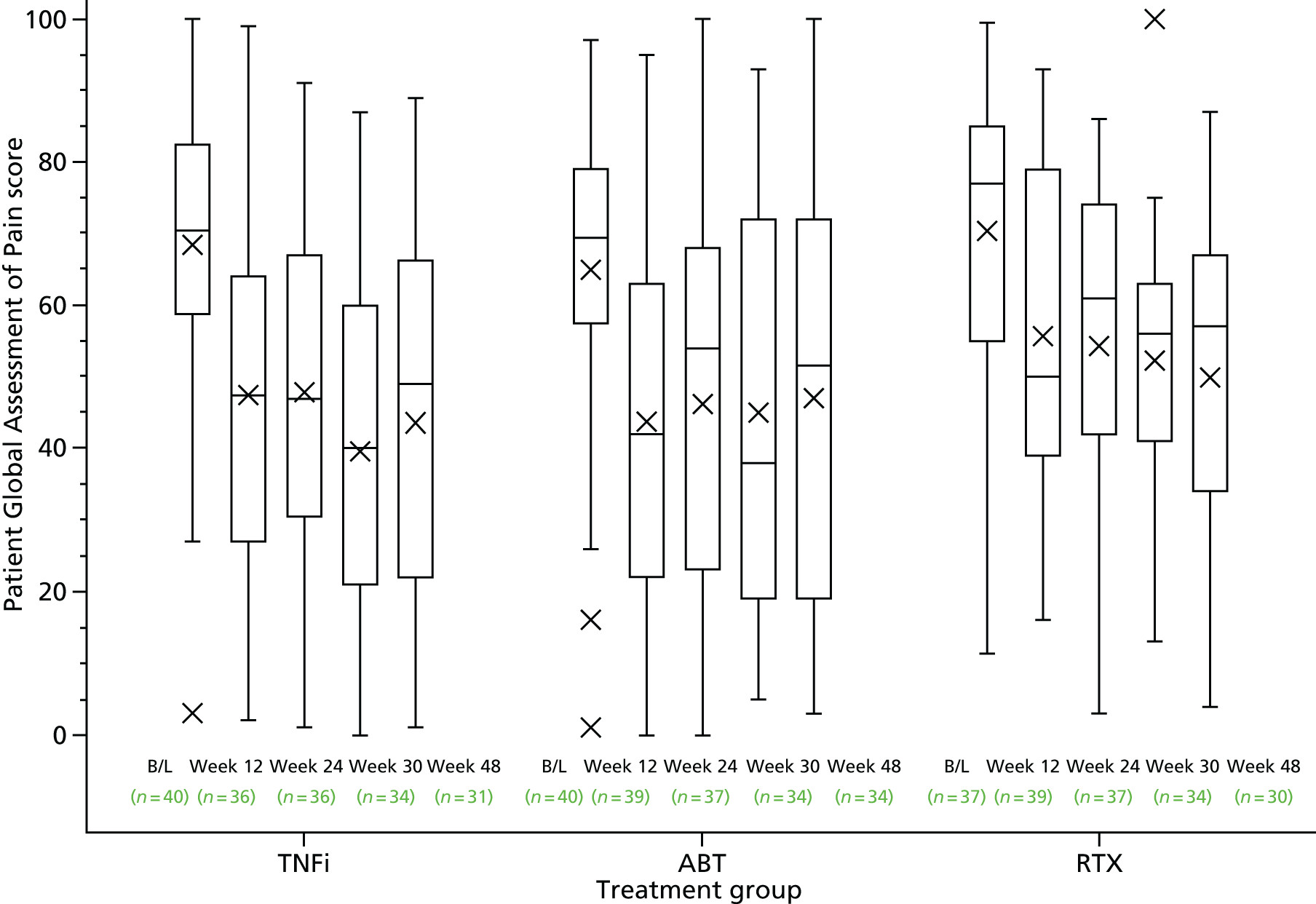
FIGURE 17.
Box and whisker plot: Patient Global Assessment of Arthritis over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.
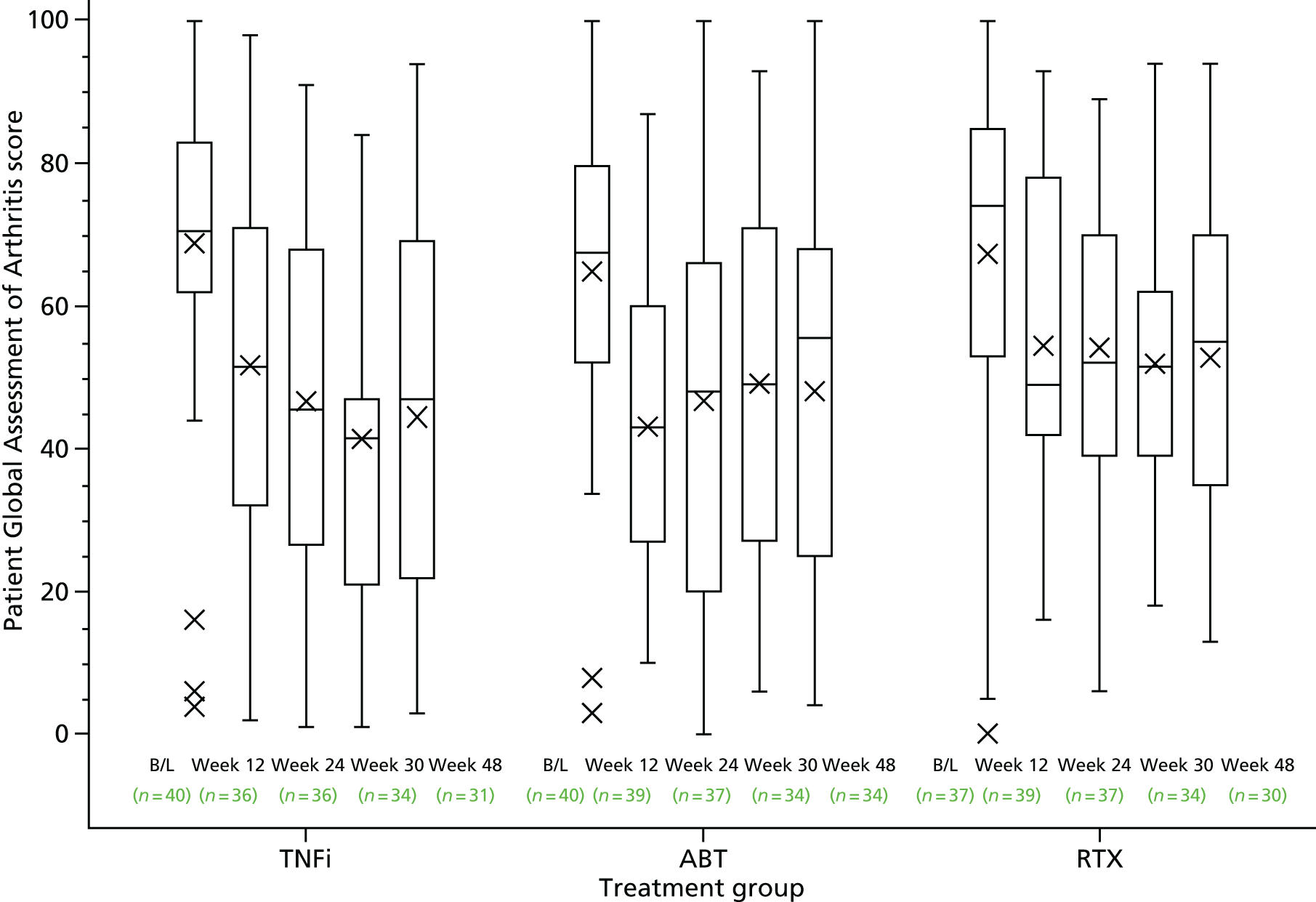
FIGURE 18.
Box and whisker plot: Patient Assessment of General Health over 48 weeks by treatment group. X inside the bars corresponds to the mean value; X outside the bars corresponds to outlier values. B/L, baseline.

Health Assessment Questionnaire Disability Index, Rheumatoid Arthritis Quality of Life and Hospital Anxiety and Depression Scale
Overall, there was an improvement in the HAQ-DI over the 48-week period across all three treatment groups. The median HAQ-DI score decreased to 1.5 (quartiles 1.1 and 1.9) in the alternative TNFi group, to 1.6 (quartiles 1.0 and 2.1) in abatacept group and to 1.7 (quartiles 1.1 and 2.1) the rituximab group (see Figure 12). Similarly, a general improvement in the RAQoL was observed over time, with no notable differences between treatment groups [median score of 19.0 (quartiles 9.0 and 23.0) was reported at 48 weeks in the alternative TNFi group, 17.5 (quartiles 11.4 and 24.0) in the abatacept group and 19.5 (quartiles 12.0 and 25.0) in the rituximab group] (see Figure 13). Small improvements in the HADS scores over the 48-week period were observed in the alternative TNFi and abatacept groups [median scores for anxiety and depression of 6 (quartiles 3.0 and 11.0) and 4.5 (quartiles 2.0, 9.0), respectively, in the alternative TNFi group and of 7.0 (quartiles 4.0 and 10.0) and 5.0 (quartiles 3.0 and 7.0), respectively, in the abatacept group], whereas no notable improvement was apparent in the rituximab group [median scores of 8.0 (quartiles 5.0 and 12.0) and 5.5 (quartiles 4.0 and 9.0), respectively] (see Figures 14 and 15).
Patients’ global assessment of pain, arthritis and general health
A marked improvement in the patients’ global assessments of pain was apparent in all three treatment groups by 12 weeks post randomisation; thereafter small fluctuations in median pain scores over time were observed (see Figure 16). The median pain VAS scores at 48 weeks were 49.0 (quartiles 22.0 and 66.0), 51.5 (quartiles 19.0 and 72.0) and 57.0 (quartiles 34.0 and 67.0) in the alternative TNFi, abatacept and rituximab groups, respectively; the observed higher median score for rituximab may in part be explained by the higher median score observed at baseline.
Similarly, an initial marked improvement in the patients’ global assessment of their arthritis by 12 weeks post randomisation was apparent across all three treatment groups (see Figure 17). In the alternative TNFi arm, this improvement continued to 36 weeks, followed by a slight deterioration by 48 weeks [median 47.0 (quartiles 22.0 and 69.0)]. In comparison, following the initial 12-week improvement, in the abatacept and rituximab groups there was a slight deterioration in patients’ global assessment of their arthritis by 48 weeks [median 55.5 (quartiles 25.0 and 68.0) and 55.0 (quartiles 35.0 and 70.0), respectively].
Overall, an improvement in patients’ general health was observed over time, with no notable difference between treatment groups; the median score at 48 weeks was 48.0 (quartiles 26.0 and 63.0), 49.0 (quartiles 27.0 and 67.0) and 52.0 (quartiles 31.0 and, 64.0) in the alternative TNFi, abatacept and rituximab groups, respectively (see Figure 18).
Bone densitometry
A total of 33 (60.0%) patients in the centres that had the facilities underwent a bone densitometry scan at their baseline assessment, with 14 (25.5%) patients undergoing scans at the week 48 assessment. Appendix 12, Table 68, presents the densitometry scores at baseline and 48 weeks for the subgroup of patients.
Safety
Serious adverse events
A full listing of the SAEs and SSARs is included in Appendix 14, Tables 71–74. Ten SAEs were reported in nine patients. Of these, three events in three patients were considered to be related to trial medications and classed as SSARs. There were no suspected unexpected serious adverse reactions (SUSARs).
One patient in the TNFi arm, receiving infliximab, had a SSAR of autoimmune hepatitis but recovered with sequelae. Two patients who received abatacept experienced SSARs (one experienced angiooedema and recovered and one contracted pneumonia and died) and a further two patients who received abatacept experienced SAEs (one experienced chest pain/epigastric pain, which remained unresolved, and one contracted a chest infection but recovered). Finally, three patients who received rituximab each experienced a SAE: one patient developed malignant melanoma and subbsequently died; one suffered a broken coccyx bone, attributable to collapsing, but recovered with sequelae, and a third experienced abdominal pain, but later recovered. A further patient who was randomised to receive rituximab experienced two SAEs occurring prior to first infusion: a flare of RA and left basal pneumonia, from both of which the patient recovered.
As summarised above, two patients died following the development of a SAE. One patient receiving rituximab had developed malignant melanoma, while a second patient developed pneumonia, considered by the treating physicians to be related to abatacept treatment, as well as another illness.
No pregnancies were reported in any of the trial patients or in any partners of the trial patients.
Adverse events
Overall, 243 non-SAEs were reported in 90 patients (Table 20). Twelve events resulted in a permanent cessation of treatment. Table 20 summarises the number of non-SAEs reported by treatment received. A listing of all AEs is provided in Appendix 14, Table 75.
| AEs | Treatment arm | Total | ||
|---|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | ||
| Number of patients with one or more AE | 28 | 31 | 31 | 90 |
| Number of AEs reported | 83 | 73 | 87 | 243 |
| Number of AEs per patient | ||||
| Mean (SD) | 2.1 (2.27) | 1.8 (1.68) | 2.2 (2.17) | 2.0 (2.04) |
| Median (IQR) | 1.0 (0–3) | 1.0 (1–3) | 2.0 (1–3) | 1.0 (0–3) |
| Range | 0–8 | 0–6 | 0–8 | 0–8 |
A total of 10 patients experienced one or more AE or SAE that resulted in a permanent cessation of treatment: four patients on alternative TNFi (9.8%), two on abatacept (4.9%) and four on rituximab (10.0%).
Chapter 4 Health economic analysis
Introduction
As described in Chapter 1, DMARDs are used in the early stages of management of RA. However, even when there is an initial positive response, treatment efficacy often reduces over time. bDMARDs are usually given to patients experiencing insufficient response to conventional DMARDs, but at a markedly higher cost of around £9500 per patient per year, compared with around £450 per year for conventional therapy. 122 During 2007–8, expenditure on bDMARDs for the treatment of RA alone ranged between £0.8M and £3.5M per acute trust, with expenditure on bDMARDs accounting for the highest pharmaceutical spend within some trusts. 122
Tumour necrosis factor inhibitor drugs are a type of bDMARD that have been found to be costly but highly effective. 31–33 However, NICE currently approves only rituximab following TNFi non-response, with the use of alternative TNFi being permitted only when rituximab (and/or MTX that is co-prescribed) is contraindicated.
An economic evaluation was conducted to estimate the cost-effectiveness of alternative TNFi or abatacept compared with the current practice of rituximab in patients with RA who have failed treatment with an initial TNFi. The economic evaluation was conducted alongside the SWITCH clinical trial so that only the data collected within the (reduced) trial were analysed. Originally, the health economic analysis included a within-trial analysis and a decision analytical model. Given the early termination of the trial and the consequent reduced period of follow-up, the health economic analysis was adapted to include a within-trial cost-effectiveness analysis over 48 weeks and a value of information analysis to inform future research.
Methods
Aim and end points
The primary aim of this analysis was to assess the cost-effectiveness of the use of abatacept or alternative TNFi compared with the current practice of rituximab in patients with RA who have failed treatment with an initial TNFi. The primary end point was the cost per quality-adjusted life-year (QALY) gained. The methods used for this within-trial analysis were guided by the recommendations from the NICE methods guide. 123
Perspective and time frame
The study adopted a NHS and Public Social Services perspective for cost evaluation, but a broader societal perspective was adopted for secondary analysis to incorporate costs to patients and productivity costs. Costs and benefits for the base-case analysis were calculated for the study period of 48 weeks. As the time frame of the trial was < 1 year, discounting of the costs and benefits was not required.
Measurement of effectiveness
This analysis used the QALY as the main outcome measure. QALYs are a generic measure of health state that take account of both quality and length of life such that 1 QALY is equal to 1 year in full health. 124
Health-related quality of life was measured using the EuroQol 5 Dimensions, 3 levels (EQ-5D-3L). The EuroQol 5 Dimensions (EQ-5D) is a commonly used generic measure of health-related quality of life and is NICE’s preferred outcome measure for cost-effectiveness analyses. 123 The questionnaire comprises five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain consists of three levels: no problems, some problems and severe problems. 125
The EQ-5D-3L was administered at baseline and at follow-up visits in weeks 12, 24, 36 and 48. Responses were converted to health state utility values using the UK general population time trade-off tariff values. 126
Measurement of costs
Health-care resource utilisation data were collected using patient self-reported questionnaires covering primary care [e.g. general practitioner (GP) and nurse visits] and secondary care (e.g. hospital stays/visits) resource use over the trial period (see Appendix 2). The questionnaires also captured personal costs to patients related to RA (e.g. travel to/from hospital and cost of aids) and any impact the disease had on their income over the trial period. The resource-use questionnaires were completed at weeks 12, 24, 36 and 48 by the research teams at the participating centres and were supplemented by case report forms (CRFs) capturing data on hospital inpatient or outpatient visits. When there were discrepancies between the CRFs and the patient-completed forms, the CRFs were given precedence. Unit costs for health service staff and resources were obtained from the Personal Social Services Research Unit (PSSRU) report in 2015, entitled Unit Costs of Health and Social Care 2015,127 and NHS Reference Costs 2014 to 2015. 128 For medications, a unit cost per treatment received was assigned. The Commercial Medicines Unit’s electronic market Information Tool (eMit) was used to cost the drugs when possible. 129 However, when drugs were not listed on eMit, costs were taken from the British National Formulary (BNF). 130 When unit costs were not available, targeted literature searches were used to provide the relevant costs which were inflated to 2015 prices (pounds sterling) using an online inflator. 131 Unit costs are presented in Appendix 15, Table 76.
Assumptions related to medication use
A number of assumptions were required in order to measure the costs related to the drugs used within the trial:
-
When the patient’s weight was needed to calculate the dose of trial medication, the baseline weight was used. This applied to the one patient using infliximab and, in this case, the associated cost was not affected if the patient’s weight at each clinical assessment had been used instead.
As no stop date was recorded for the trial drugs, the number of doses was deduced from the CRFs making the following assumptions.
-
If the records showed that all infusions were received as per the protocol, the full protocol-defined allocation for the relevant time period was allocated to that patient.
-
In patients who were reported to have received some randomised treatment but the infusions received did not follow protocol because they were delayed, it was assumed that the patient received the full allocation of trial medication.
-
In patients who were reported to have received some randomised treatment but for whom infusions received did not follow protocol because treatment was stopped, it was assumed that the patient received half the allocation of trial medication for that time period.
-
If it was indicated that some of the randomised treatment had been received but it was not reported whether or not all the treatment had been received or whether or not there had been any modifications to the treatment protocol, it was assumed that the full allocation of treatment for that time period had been received.
-
For treatments administered by injection, if the number of missing injections was recorded, the number of missing injections was taken from the full protocol-defined allocation for the relevant time period.
-
For treatments administered by injection, if some treatments were missed but the number of treatments missed was not recorded, then it was assumed that half of the allocation for the relevant time period was received.
The following assumptions were applied in order to cost the concomitant drugs used within the trial period.
-
When dose was not recorded, it was assumed that the standard dose was received.
-
When it was indicated that it was an ongoing drug with no start or end date recorded, it was assumed that it was taken for the full 48 weeks.
-
For those patients who took MTX, it was assumed that it was taken orally at an average dose of 15 mg per week based on the relevant literature and expert opinion. 132
Missing data
The base-case analysis was conducted using only complete cases. That is, patients were included in the analysis if they had no missing resource use data as well as no missing quality-of-life data. In the case of resource use, no missing data were defined as a resource use form having been completed at all time points. For the resource use questionnaires, if a patient recorded that they had used a form of health care (e.g. GP visit) but did not record the number of visits, the mean number of visits was imputed. For quality of life, complete data were defined as a completed quality-of-life questionnaire returned at each time point.
Sensitivity analyses were conducted using imputed data so that all patients were included in the analysis. Two imputation methods were explored: mean imputation and multiple imputation. For the mean imputation, when a QALY value for a given time point was missing, the mean of the non-missing QALYs for the trial arm at that time point was imputed. The same approach was taken to impute missing cost data for resource use. For the multiple imputation, costs for each follow-up and total QALYs were imputed by chained equations using predictive mean matching. 133 Forty-five data sets were imputed (reflecting the percentage of incomplete cases), which were then combined using Rubin’s rules. 119,134
Analysis
The primary analysis was a cost-effectiveness analysis of the three relevant treatment arms of the trial. A complete-case analysis was the primary method for analysing the trial data and an ITT analysis was undertaken as a sensitivity analysis.
Resource use and costs were quantified and analysed using analysis of variance and independent sample t-tests. Owing to the small sample size and, subsequently, the potential violation of the underlying normality assumption when using t-tests, the robustness of the results was checked using a non-parametric bootstrap.
Health-state utilities were used to calculate QALYs using an area under the curve approach:
where EQ-5DBaseline, EQ-5D12, EQ-5D24, EQ-5D36 and EQ-5D48 are the EQ-5D scores at baseline, week 12, week 24, week 36 and week 48, respectively; 0.230769 represents 12 weeks out of 52 for each time period:
Total costs and QALYs for each arm of the trial were calculated. For the secondary analysis, a wider cost perspective was adopted to include the total costs incurred by the patients.
Incremental cost-effectiveness ratios (ICERs) were calculated. 135 An ICER represents the additional cost per QALY gained for each intervention compared with the next best alternative and is calculated as follows for treatment A relative to treatment B:
where CostA and CostB are the mean costs and QALYA and QALYB are the mean QALYs for groups A and B. An intervention was judged to be cost-effective using the lower limit of the NICE acceptance threshold of £20,000 per incremental QALY (λ = £20,000) as the decision rule for the analysis. 123
The level of sampling uncertainty around the ICER was determined using non-parametric bootstrapping (with replacement) to generate 10,000 estimates of incremental costs and benefits. These were then plotted on the cost-effectiveness plane to visualise the uncertainty around the mean incremental costs and effects. The expected ICERs for the primary analysis were estimated from the means of bootstrapped cost and outcome distributions.
Net monetary benefit (NMB) values were also calculated. Net benefit (NB) combines cost-effectiveness and willingness to pay to give an explicit monetary valuation of the health outcome. It is calculated by rearranging the ICER calculation and incorporating a proposed willingness-to-pay threshold value per QALY. 135 The expected value of the NMB was calculated for each treatment. Treatments with positive NMBs provide more health benefit than is displaced by the associated opportunity costs and should be adopted. The treatment with the highest positive NMB is the most cost-effective. 135 The probability that the treatments were cost-effective was evaluated by generating estimates of NMB for a range of cost-effectiveness thresholds (λ). This analysis was presented as a cost-effectiveness acceptability curve. 136,137 The cost-effectiveness acceptability curve provides decision-makers with useful information regarding the risk of making a wrong decision; however, the decision to fund or not fund a treatment should be made on the expected value of the NMB.
Net monetary benefit is derived for each patient as:
Sensitivity analyses
The following scenario sensitivity analyses were conducted to test the robustness of the conclusions drawn from the results.
-
Mean imputed data: an analysis was conducted using singly imputed data for missing QALYs and costs to enable an assessment of cost-effectiveness using data from all patients.
-
Multiple imputation: an analysis was conducted using multiply imputed data for missing QALYs and costs.
-
Adjust baseline: an analysis was conducted to evaluate the effect of adjusting for baseline differences in EQ-5D score (using an ordinary least squares regression and adjustment: total QALYs over 48 weeks were regressed on trial arm, EQ-5D score at baseline, age at baseline and sex).
-
Subcutaneous MTX: patients could have taken MTX orally or by subcutaneous injection but, as the method was not recorded, at baseline an assumption was made that MTX was taken orally by all patients. Therefore, sensitivity analysis was conducted that explored the alternative scenario that MTX was instead taken via subcutaneous injection by all patients.
Secondary analysis assessed the effect of taking a broader, societal cost perspective. The analysis uses health and social sector costs together with the addition of patient out-of-pocket costs plus values from the EQ-5D to estimate QALYs (replicating the primary analysis). As in the primary analysis the base case used complete cases. Sensitivity analyses using mean imputation and multiple imputation were undertaken.
Value of information analysis
Value of information analysis was conducted to estimate the potential gains from the elimination of uncertainty as a result of conducting additional research. As decisions based on current information are uncertain (because of imperfect information) there is a chance that the wrong decision will be made, resulting in costs being incurred in the form of health benefit and cost of resources forgone. Given the very small sample size, the decision uncertainty is large and, therefore, the value of information analysis is especially important. The expected value of perfect information (EVPI) is derived from the expected costs associated with the uncertainty in decisions. The EVPI provides the maximum value that a health-care system should be willing to pay for additional evidence to eliminate uncertainty in parameter estimates to inform future decisions, and gives an upper bound for the value of additional research. As information is valuable to all patients with a disease (not just one patient) EVPI can be expressed for the population of patients who could benefit. 138,139 The EVPI was calculated for the population of the UK who have RA as follows:
The bootstrap simulation provides estimates of costs and benefits and, therefore, NB. Eθmaxj NB(j, θ) is the expected NB with perfect information, which is the mean value of NMB in the set when the intervention with the higher NMB is chosen for each simulation, and maxjEθNB(j, θ) is the expected NB with current information, which is obtained when the intervention with the higher expected NB is chosen across all simulations. 140
All of the analyses were conducted in Stata® (version 14, StataCorp LP, College Station, TX, USA) and Microsoft Excel® (2013, Microsoft Corporation, Redmond, WA, USA).
Results
Sample
Of the 122 patients recruited to the trial, 70 patients with complete resource use data and EQ-5D results (25 rituximab, 24 abatacept and 21 alternative TNFi) were included in the base-case analysis.
Baseline characteristics of the 70 patients analysed in the complete case are presented in Table 21 (see Table 3 for baseline characteristics of the ITT population). In all treatment arms more than two-thirds of the patients were female. The average weight was slightly lower in the abatacept group than in the other treatment groups. Fewer patients in the alternative TNFi arm were non-smokers and a higher percentage were past smokers than in the other treatment arms. There was some variation between arms in baseline EQ-5D scores, with the alternative TNFi group having the highest scores. However, the difference between the scores was not statistically significant.
| Patient characteristic | Treatment arm | ||
|---|---|---|---|
| Rituximab (n = 25) | Abatacept (n = 24) | Alternative TNFi (n = 21) | |
| Age (years) | |||
| Mean (SD) | 58.51 (11.55) | 57.17 (13.90) | 56.53 (9.31) |
| Sex, n (%) | |||
| Male | 7 (28) | 1 (4) | 5 (24) |
| Female | 18 (72) | 23 (96) | 16 (76) |
| Weight (kg) | |||
| Mean (SD) | 85.26 (21.05) | 75.86 (15.79) | 81.91 (20.24) |
| Smoking status, n (%) | |||
| Non-smoking | 10 (40) | 9 (37.5) | 5 (23.8) |
| Past smoker | 10 (40) | 9 (37.5) | 10 (47.6) |
| Current smoker | 5 (20) | 6 (25) | 6 (28.6) |
| EQ-5D score | |||
| Mean (SD) | 0.31 (0.34) | 0.39 (0.32) | 0.46 (0.27) |
| DAS28 | |||
| Mean (SD) | 6.14 (1.34) | 6.23 (0.97) | 5.77 (0.78) |
| Missing | 3 | 3 | 0 |
| HAQ score | |||
| Mean (SD) | 1.74 (0.84) | 1.8 (0.59) | 1.75 (0.5) |
| Missing | 0 | 1 | 0 |
Resource use and costs
Table 22 shows the average resource use of patients with complete resource use data over the trial period in each trial arm (rituximab, n = 29; abatacept, n = 26; and alternative TNFi, n = 25). Further breakdown of resource use is provided in Appendix 15, Table 79. Average health-care costs over the trial period are presented in Table 23. These are broken down further in Appendix 15, Table 80. The mean (SD) total costs of community health and social services were £927.17 (£1238.60) for the rituximab group, £601.20 (£553.70) for the abatacept group and £557.80 (£513.42) for the alternative TNFi group. The mean (SD) total costs for hospital and residential care services were £1112.71 (£1137.51) for the rituximab group, £862.61 (£788.43) for the abatacept group and £957.78 (£678.42) for the alternative TNFi group. All groups have large SDs for these costs, particularly rituximab, which are driven by the skewed distribution of costs, floor effects (zero costs for each cost component were observed for a proportion of patients) and by a number of high-cost outliers. This is shown in the box and whisker plots in Figure 19. It would be wrong to overinterpret differences between the groups given the outliers, skewness and small sample. Despite having the highest resource use cost, the rituximab treatment group incurred the lowest mean total NHS cost along the whole treatment pathway. The trial medication costs reported in Table 23 include the cost of administering the drugs as well as the cost of the medication. The unit costs of the trial medication are reported in Appendix 15, Table 77, and a breakdown of the cost of administering each of the trial drugs is presented in Appendix 15, Table 78, for other relevant unit costs. As costs are assigned per treatment received, we note that there may be cases in which retreatment occurs within the trial period. In these cases, the costs for retreatment are included in the analysis but some of the benefits that may extend beyond the end of the trial could be excluded. This may be particularly relevant for rituximab, as 20 patients received re-infusion within the trial period.
FIGURE 19.
Box and whisker plots of the distributions of the resource use costs by treatment group. (a) Total community health and social services cost and (b) total hospital and residential care services cost. X inside the bars corresponds to the mean value; and X outside the bars corresponds to outlier values.
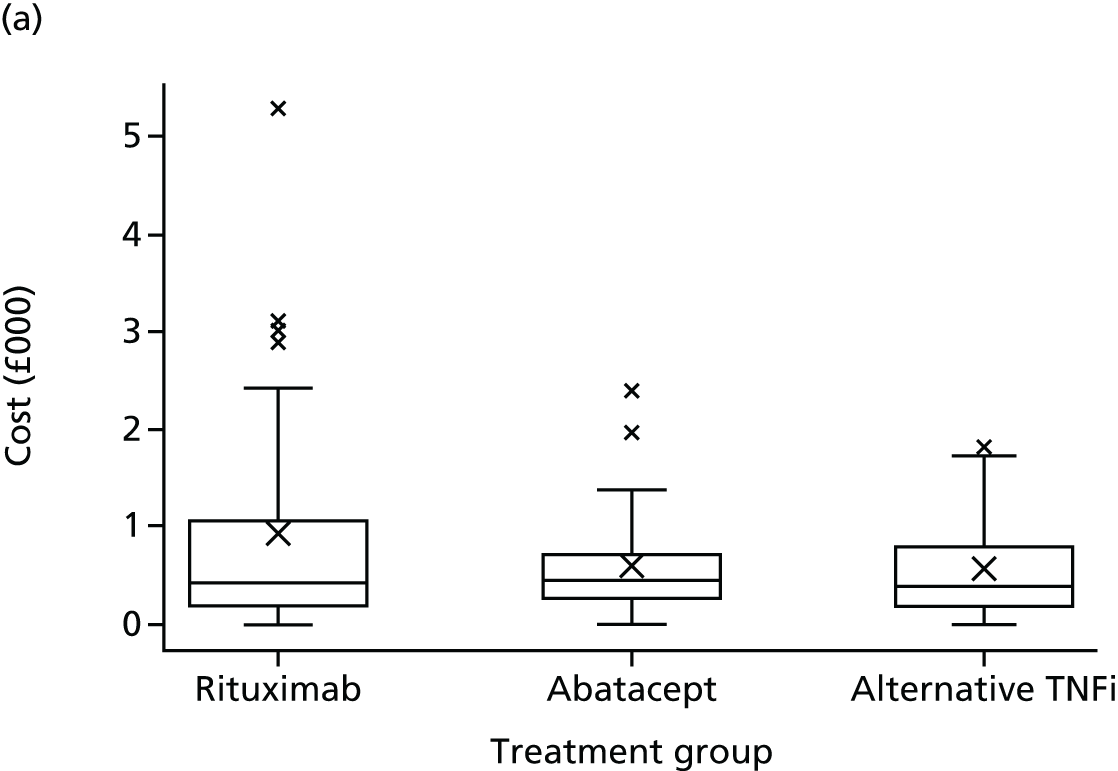
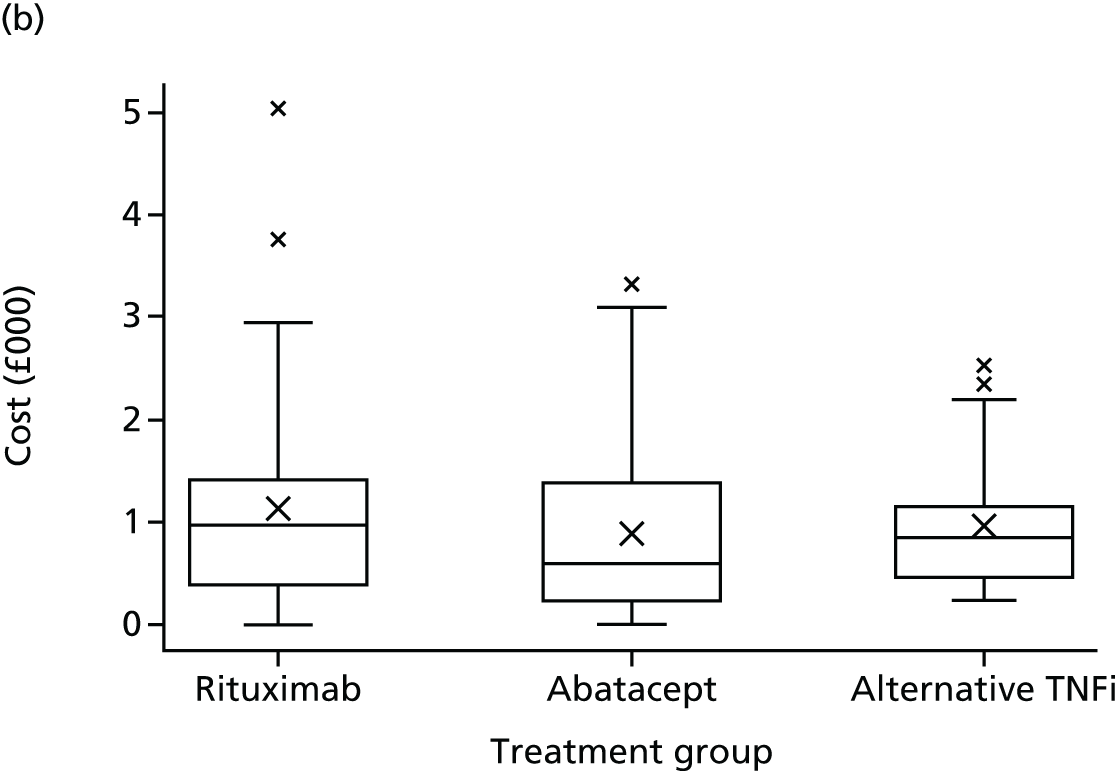
| Resource use | Treatment arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | |||||||
| Mean (SD) | Minimum | Maximum | Mean (SD) | Minimum | Maximum | Mean (SD) | Minimum | Maximum | |
| Community health and social services | |||||||||
| GP surgery visit | |||||||||
| Face to face | 9.10 (10.88) | 0 | 43 | 6.19 (4.84) | 0 | 23 | 6.32 (4.91) | 0 | 15 |
| Telephone/e-mail | 2.34 (4.15) | 0 | 19 | 0.57 (1.79) | 0 | 9 | 1.44 (2.48) | 0 | 10 |
| GP home visit | |||||||||
| Face to face | 0.24 (0.99) | 0 | 5 | 0.07 (0.39) | 0 | 2 | 0.04 (0.2) | 0 | 1 |
| District nurse | |||||||||
| Face to face | 0.72 (1.87) | 0 | 9 | 0.81 (1.55) | 0 | 6 | 1.28 (2.30) | 0 | 7 |
| Telephone/e-mail | 0.34 (0.19) | 0 | 1 | 0.27 (0.96) | 0 | 4 | 0.08 (0.4) | 0 | 2 |
| Social worker | |||||||||
| Face to face | 0.10 (0.41) | 0 | 2 | 0 | 0 | 0 | 0 (0) | 0 | 0 |
| Telephone/e-mail | 0.07 (0.37) | 0 | 1 | 0 | 0 | 0 | 0 (0) | 0 | 0 |
| Physiotherapist | |||||||||
| Face to face | 3.14 (7.00) | 0 | 30 | 2.88 (8.12) | 0 | 40 | 1.36 (3.38) | 0 | 14 |
| Telephone/e-mail | 0.41 (1.88) | 0 | 10 | 0.04 (0.20) | 0 | 1 | 0 (0) | 0 | 0 |
| Occupational therapist | |||||||||
| Face to face | 0.41 (1.43) | 0 | 6 | 0.54 (1.07) | 0 | 4 | 0.32 (1.6) | 0 | 8 |
| Telephone/e-mail | 0.07 (0.26) | 0 | 2 | 0.04 (0.20) | 0 | 1 | 0 (0) | 0 | 0 |
| Podiatrist | |||||||||
| Face to face | 2.24 (4.28) | 0 | 18 | 2.12 (4.10) | 0 | 14 | 1.96 (4.21) | 0 | 14 |
| Telephone/e-mail | 0.28 (1.16) | 0 | 6 | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 |
| Counsellor | |||||||||
| Face to face | 0.31 (1.67) | 0 | 9 | 0 (0) | 0 | 0 | 0.24 (0.88) | 0 | 4 |
| Telephone/e-mail | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 | 0.16 (0.8) | 0 | 4 |
| Psychologistb | |||||||||
| Face to face | 0.14 (0.74) | 0 | 4 | 0 (0) | 0 | 0 | 0 (0) | 0 | 0 |
| Home helpb | |||||||||
| Face to face | 3.72 (12.46) | 0 | 48 | 0.46 (2.59) | 0 | 12 | 0.48 (2.4) | 0 | 12 |
| Hospital or residential care services | |||||||||
| Hospital inpatient stay | 0.79 (2.27) | 0 | 10 | 0 (0) | 0 | 0 | 0.08 (0.28) | 0 | 1 |
| Hospital outpatient clinic | 3.48 (2.94) | 0 | 12 | 4.15 (2.60) | 0 | 8 | 4.76 (3.38) | 0 | 16 |
| Hospital day centre | 1.76 (2.41) | 0 | 8 | 1.08 (2.59) | 0 | 10 | 1.2 (2.40) | 0 | 9 |
| Hospital accident and emergency department | 0.34 (0.94) | 0 | 4 | 0.19 (0.98) | 0 | 5 | 0.4 (0.28) | 0 | 7 |
| Health-care cost (£) | Treatment arm | ||
|---|---|---|---|
| Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | |
| Community health and social services | |||
| Mean (SD) | 927.17 (1238.6) | 601.2 (553.7) | 557.8 (513.42) |
| Minimum | 0 | 0 | 0 |
| Maximum | 5291.57 | 2388 | 1818.23 |
| Hospital and residential care services | |||
| Mean (SD) | 1112.71 (1137.51) | 862.61 (788.43) | 957.78 (678.42) |
| Minimum | 0 | 0 | 224 |
| Maximum | 5039.21 | 3322 | 2523 |
| Trial medicationa | |||
| Mean (SD) | 6633.49 (3197.01) | 11,584.52 (4733.65) | 6939.19 (2679.69) |
| Minimum | 2024.96 | 1407.6 | 902.28 |
| Maximum | 16,199.68 | 15,015.6 | 9353.64 |
| MTX | |||
| Mean (SD) | 32.36 (5.27) | 30.92 (3.42) | 35.14 (3.09) |
| Minimum | 14.4 | 21.6 | 31.2 |
| Maximum | 38.4 | 33.6 | 45.6 |
| Other concomitant medication | |||
| Mean (SD) | 396.78 (1830.36) | 519.51 (1168.58) | 807.93 (1909.68) |
| Minimum | 0 | 0 | 0 |
| Maximum | 9907.84 | 3590.59 | 7014.68 |
| Total NHS | |||
| Mean (SD) | 9102.51 (3375.77) | 13,598.77 (4092.09) | 9297.84 (2007.36) |
| Minimum | 4716.78 | 2690.88 | 1462.19 |
| Maximum | 18,064.16 | 17,661.2 | 12,573.96 |
| Patient costs | |||
| Mean (SD) | 1081.66 (2020.47) | 387.79 (655.19) | 947.07 (2521.82) |
| Minimum | 0 | 0 | 0 |
| Maximum | 9028.5 | 2552 | 12676 |
| Total all | |||
| Mean (SD) | 10,184.17 (3509.55) | 13,986.55 (4382.28) | 10,244.91 (3298.72) |
| Minimum | 4874.25 | 2692.23 | 1462.19 |
| Maximum | 18,292.16 | 20,213.2 | 21,882.63 |
Independent-sample t-tests were undertaken to explore differences in the mean total NHS costs associated with treatment groups. However, the t-test is based on the assumption that the data are normally distributed, which is violated, and asymptotic results surrounding normality of the mean costs are not robust because of the small sample size. Consequently, a non-parametric bootstrap of the difference in costs was conducted as a check on the robustness of the standard Student t-tests. 124 Independent-sample t-tests indicated that there was a significant difference in the mean total NHS costs associated with the rituximab treatment group and the abatacept treatment group (p = 0.0003). There was also a significant difference between the mean costs for the alternative TNFi group and the abatacept group (p = 0.0002), but there was no statistically significant difference between the mean costs associated with the rituximab group and the alternative TNFi group (p = 0.6772). The bootstrap confirmed the results of the t-test.
Quality-of-life data
The mean (SD) EQ-5D scores for each trial arm, at each time point, are presented in Table 24. There was an apparent increase in mean EQ-5D score from baseline to week 12 in all treatment groups. Although there were small fluctuations in scores, this initial improvement was more or less maintained up to week 48 in all arms of the trial. All three treatment groups show an increase in EQ-5D score from baseline to week 48. Despite the apparent slightly higher baseline EQ-5D score for the alternative TNFi group, statistical tests indicated that there was no statistically significant difference in EQ-5D scores at baseline (p = 0.2592). This was explored further in a sensitivity analysis adjusting for baseline differences in EQ-5D score.
| Time point | Treatment arm | ||
|---|---|---|---|
| Rituximab | Abatacept | Alternative TNFi | |
| Baseline | |||
| Mean (SD) | 0.36 (0.33) | 0.34 (0.33) | 0.42 (0.29) |
| n valid (missing) | 37 (3) | 36 (5) | 40 (1) |
| Week 12 | |||
| Mean (SD) | 0.51 (0.25) | 0.52 (0.29) | 0.59 (0.23) |
| n valid (missing) | 38 (2) | 39 (2) | 37 (4) |
| Week 24 | |||
| Mean (SD) | 0.48 (0.29) | 0.55 (0.29) | 0.57 (0.24) |
| n valid (missing) | 36 (4) | 37 (4) | 33 (8) |
| Week 36 | |||
| Mean (SD) | 0.52 (0.23) | 0.54 (0.32) | 0.59 (0.29) |
| n valid (missing) | 34 (6) | 33 (8) | 33 (8) |
| Week 48 | |||
| Mean (SD) | 0.51 (0.29) | 0.50 (0.29) | 0.55 (0.28) |
| n valid (missing) | 30 (10) | 33 (8) | 29 (12) |
Table 25 shows the mean EQ-5D change scores between baseline and each of the follow-up time points. Statistical analysis using analysis of variance indicated that the variation among groups in the changes in EQ-5D scores was not statistically significant (p = 0.7071).
| Time point | Treatment arm | ||
|---|---|---|---|
| Rituximab | Abatacept | Alternative TNFi | |
| Baseline to week 12 | |||
| Mean (SD) | 0.19 (0.33) | 0.17 (0.32) | 0.14 (0.25) |
| n | 35 | 34 | 36 |
| Baseline to week 24 | |||
| Mean (SD) | 0.13 (0.37) | 0.22 (0.32) | 0.11 (0.29) |
| n | 34 | 33 | 32 |
| Baseline to week 36 | |||
| Mean (SD) | 0.15 (0.35) | 0.20 (0.39) | 0.13 (0.37) |
| n | 32 | 29 | 32 |
| Baseline to week 48 | |||
| Mean (SD) | 0.18 (0.38) | 0.12 (0.37) | 0.10 (0.34) |
| n | 28 | 29 | 28 |
Missing data
Seventy-four patients had complete EQ-5D scores across all time points. The remaining 48 had EQ-5D scores missing for at least one of the time periods. All 122 patients completed resource use questionnaires in the first two time periods (12 and 24 weeks), but the response rate dropped in the following two time periods to 98 forms returned (79%) at 36 weeks and 89 forms returned (71%) at 48 weeks.
Cost-effectiveness results
The costs and outcomes from the observed data are presented in Table 26. As the sample size of the observed data is small, the average costs and effects from the non-parametric bootstrap provide a more accurate and robust estimate of the distribution of the population costs and effects. Table 27 shows the costs and QALYs gained for each treatment arm, the incremental costs and QALYs of the relevant comparisons and the resulting ICERs calculated from the bootstrap sample. The treatments are arranged in order of increasing cost so that the incremental costs and QALYs refer to the comparison between the neighbouring treatments in the table. The abatacept treatment group had the highest QALYs gained over the trial period, although the rituximab group had the lowest. The mean total cost was highest for the abatacept treatment group and lowest for the rituximab treatment group.
| Treatment group | Total cost (£), mean (SD) | QALYs, mean (SD) |
|---|---|---|
| Rituximab (n = 25) | 9367.27 (3215.13) | 0.46 (0.18) |
| Alternative TNFi (n = 21) | 9680.23 (1263.71) | 0.52 (0.14) |
| Abatacept (n = 24) | 13,475.09 (4173.22) | 0.53 (0.17) |
| Treatment group | Total cost (£), mean (SD) | Incremental cost (£) | QALYs, mean (SD) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Rituximab | 9367.80 (624.70) | 0.46 (0.04) | |||
| Alternative TNFi compared with rituximab | 9673.77 (268.03) | 305.96 | 0.52 (0.03) | 0.06 | 5332.02 |
| Abatacept compared with alternative TNFi | 13,441.77 (833.12) | 3768.00 | 0.53 (0.03) | 0.02 | 253,967.96 |
The results suggest that switching to alternative TNFi would be cost-effective compared with rituximab, as QALY gains are higher and costs are only slightly higher, leading to an ICER value of £5332.02 per QALY gained. This is well below the NICE acceptance threshold (λ = £20,000), which indicates that switching to alternative TNFi would be a cost-effective treatment option. Conversely, the abatacept group has much higher costs and only marginal gains in QALYs compared with the alternative TNFi treatment group. This results in an ICER value of £253,967.96 per QALY gained, indicating that switching to abatacept compared with switching to alternative TNFi drug would not be cost-effective, as this ICER value is well above the NICE cost/QALY threshold. However, these results should be interpreted very cautiously given the small number of cases and the substantial probability of a very small QALY difference, resulting in a divisor close to zero. Moreover, the SD is likely to be underestimated.
Figure 20 shows the joint distribution of incremental costs and incremental effects in the cost-effectiveness plane for alternative TNFi compared with rituximab and alternative TNFi compared with abatacept. The wide spread of the ‘clouds’ shows the high degree of uncertainty around the central results. This is to be expected given the small sample sizes.
FIGURE 20.
Scatterplot on the cost-effectiveness plane: alternative TNFi vs. rituximab and abatacept vs. TNFi.
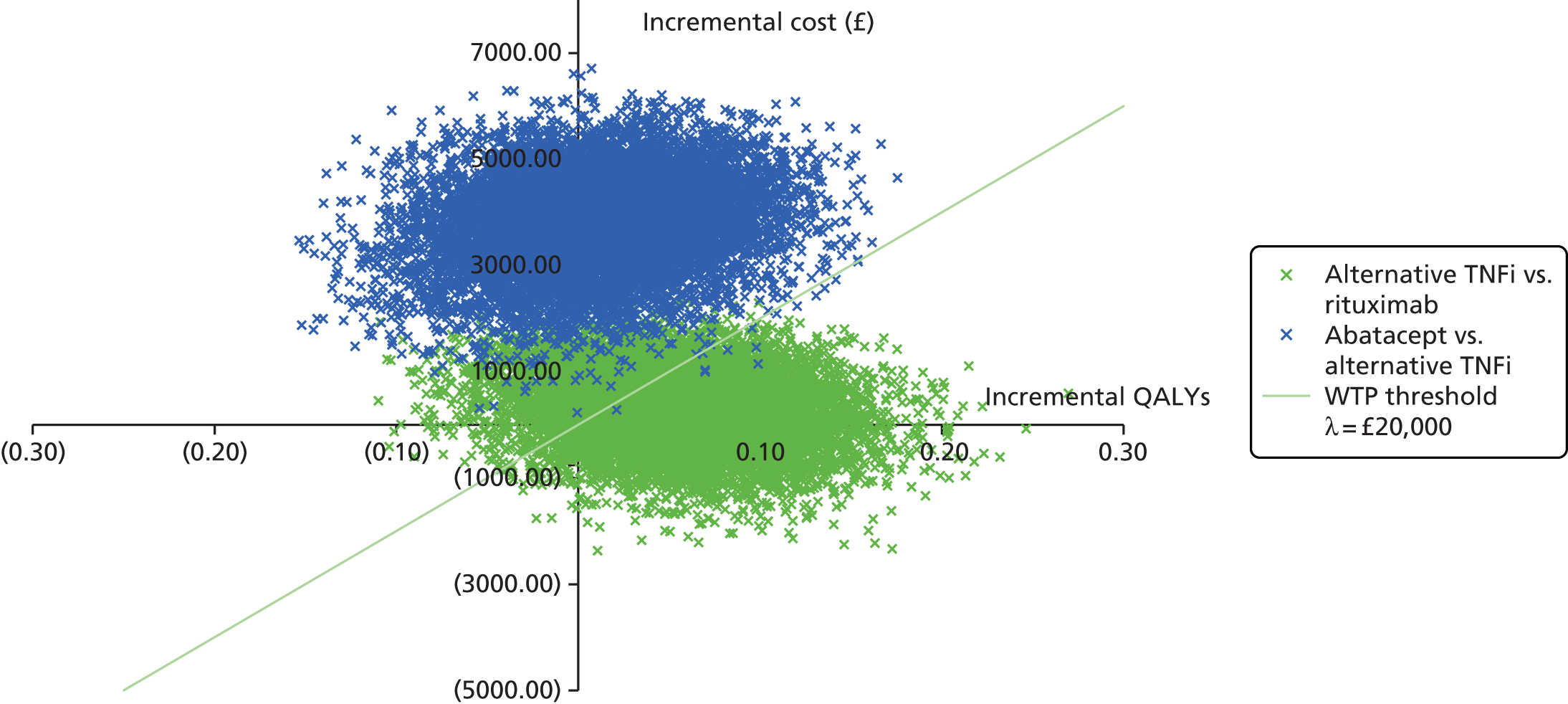
Net benefit
The NMB for each treatment group is presented in Table 28. Given the decision rule, the NMB results indicate that rituximab and abatacept are not cost-effective treatments, although alternative TNFi has a positive NMB, indicating that it is cost-effective. These results suggest that switching to rituximab following an initial TNFi failure, as recommended in the current NICE guidance, is not the most efficient use of NHS resources. The probability that the treatments are cost-effective is presented in the cost-effectiveness acceptability curve shown in Figure 21. This shows that rituximab has the highest probability of being cost-effective for very low threshold values but that at threshold values > £6000 alternative TNFi has the highest probability of being cost-effective. At a £20,000 threshold, there is a 74.5% probability that alternative TNFi is cost-effective. Abatacept is never the most likely to be cost-effective and has a low probability of cost-effectiveness even at high threshold values.
| Treatment group | Expected value NMB | SD of the estimates | 95% CI |
|---|---|---|---|
| Rituximab | –160 | 1076 | –2336 to 1877 |
| Abatacept | –2790 | 947 | –4594 to –921 |
| Alternative TNFi | 681 | 659 | –643 to 1943 |
FIGURE 21.
Cost-effectiveness acceptability curve of an TNFi, rituximab and abatacept.

Sensitivity analysis
The costs and benefits from the observed data for each scenario explored in the sensitivity analyses are presented in Table 29. For each scenario the observed data were used to conduct a non-parametric bootstrap to provide the cost-effectiveness results, which are presented in Table 30.
| Treatment group | Cost (£), mean (SD) | QALYs, mean (SD) |
|---|---|---|
| Mean imputed data | ||
| Alternative TNFi (n = 41) | 9011.85 (1874.19) | 0.51 (0.14) |
| Rituximab (n = 40) | 11,611.90 (15,601.15) | 0.45 (0.15) |
| Abatacept (n = 41) | 12,484.63 (3792.04) | 0.48 (0.18) |
| Multiple imputation | ||
| Alternative TNFi (n = 41) | 7313.11 (2641.83) | 0.54 (0.11) |
| Rituximab (n = 40) | 7563.66 (3466.92) | 0.49 (0.15) |
| Abatacept (n = 41) | 9757.76 (5469.32) | 0.53 (0.13) |
| Adjust baseline | ||
| Rituximab (n = 25) | 9367.27 (3215.13) | 0.48 (0.03) |
| Alternative TNFi (n = 21) | 9680.23 (1263.71) | 0.51 (0.02) |
| Abatacept (n = 24) | 13,475.09 (4173.22) | 0.51 (0.01) |
| Subcutaneous MTX | ||
| Rituximab (n = 25) | 10,126.88 (3168.76) | 0.46 (0.18) |
| Alternative TNFi (n = 21) | 10,519.07 (1281.09) | 0.52 (0.14) |
| Abatacept (n = 24) | 14,217.46 (4219.53) | 0.53 (0.17) |
| Sensitivity analysis | Total cost (£), mean (SD) | Incremental cost (£) | QALY, mean (SD) | Incremental QALY | ICER (£) |
|---|---|---|---|---|---|
| Mean imputed data | |||||
| Alternative TNFi | 9016.65 (283.76) | 0.52 (0.02) | |||
| Rituximab compared with alternative TNFi | 11,675.67 (2428.85) | 2659.01 | 0.45 (0.02) | –0.07 | Dominated |
| Abatacept compared with alternative TNFi | 12,489.87 (583.13) | 3473.21 | 0.48 (0.03) | –0.04 | Dominated |
| Multiple imputation | |||||
| Alternative TNFi | 7311.22 (407.52) | 0.54 (0.02) | |||
| Rituximab compared with alternative TNFi | 7577.46 (545.39) | 266.24 | 0.49 (0.02) | –0.05 | Dominated |
| Abatacept compared with alternative TNFi | 9747.11 (847.59) | 2435.89 | 0.53 (0.02) | –0.01 | Dominated |
| Adjust baseline | |||||
| Rituximab | 9358.71 (632.32) | 0.48 (0.01) | |||
| Alternative TNFi compared with rituximab | 9681.06 (267.89) | 322.35 | 0.51 (0.00) | 0.03 | 10,948.76 |
| Abatacept compared with alternative TNFi | 13,470.63 (830.90) | 3789.57 | 0.51 (0.00) | 0.00 | Dominated |
| Subcutaneous MTX | |||||
| Rituximab | 10,123.97 (616.01) | 0.46 (0.04) | |||
| Alternative TNFi compared with rituximab | 10,519.20 (273.74) | 395.24 | 0.52 (0.03) | 0.06 | 6863.26 |
| Abatacept compared with alternative TNFi | 14,214.03 (840.88) | 3694.83 | 0.53 (0.03) | 0.02 | 237,955.53 |
Given that only complete cases were used for the base-case analysis, sensitivity analyses using mean imputation and multiple imputation were conducted, so that all randomised patients could be included. In each case, this does not alter the conclusion drawn from the base-case analysis, as alternative TNFi dominates all other treatment arms and is the optimal treatment option. We note that in the case of the mean imputation the increase in precision of these estimates is spurious, as the uncertainty attributable to imputation of a single value is ignored. Moreover, the imputed value did not take the characteristics of the missing cases into account; however, these are accounted for in the multiple imputation. Changing the administration of MTX to subcutaneous injection and adjusting for baseline differences also supports the results of the base-case analysis, with alternative TNFi remaining the most cost-effective treatment. Variances of population distributions are known to be underestimated in small samples, which is exacerbated by the high level of skewness in cost data, and we note that these sensitivity analyses have a large effect on the SD of the estimates (i.e. standard error). Consequently, we should be cautious when interpreting these results.
Secondary analyses
Secondary analyses incorporating costs using a wider social perspective were also conducted. As such, the costs to the patient as well as health-care provider costs were included. Initially, this perspective was explored using complete cases: those patients with complete health-care cost and quality-of-life data (rituximab, n = 25; abatacept, n = 24; and alternative TNFi, n = 21). In the complete-case analysis, those observations without complete cost and quality-of-life data were excluded. In addition, the wider social cost perspective was explored using imputed health-care cost and quality-of-life data in a similar way to the primary analysis. This enabled the inclusion of the whole sample in the analysis. The total costs and QALYs for each analysis, obtained from the observed data, are presented in Table 31. The observed data were used to conduct a non-parametric bootstrap to provide the cost-effectiveness results which are presented in Table 32. Including societal costs does not alter the conclusions drawn from the primary analyses, with alternative TNFi continuing to be the most cost-effective treatment.
| Treatment group | Cost (£), mean (SD) | QALYs, mean (SD) |
|---|---|---|
| Complete case | ||
| Rituximab (n = 25) | 10,234.14 (3398.08) | 0.46 (0.18) |
| Alternative TNFi (n = 21) | 10,801.87 (2952.71) | 0.52 (0.14) |
| Abatacept (n = 24) | 13,786.91 (4365.49) | 0.53 (0.17) |
| Mean imputed data | ||
| Alternative TNFi (n = 41) | 10,543.76 (6729.35) | 0.51 (0.14) |
| Rituximab (n = 40) | 12,582.66 (15,489.78) | 0.45 (0.15) |
| Abatacept (n = 41) | 13,177.04 (4319.81) | 0.48 (0.18) |
| Multiple imputation | ||
| Rituximab (n = 40) | 8482.35 (3918.54) | 0.49 (0.15) |
| Alternative TNFi (n = 41) | 8855.76 (6511.18) | 0.54 (0.11) |
| Abatacept (n = 41) | 10,461.48 (5720.53) | 0.53 (0.13) |
| Treatment group | Cost (£), mean (SD) | Incremental cost (£) | QALYs, mean (SD) | Incremental QALYs | ICER (£/QALY) |
|---|---|---|---|---|---|
| Complete case | |||||
| Rituximab | 10,229.65 (660.23) | 0.46 (0.04) | |||
| Alternative TNFi compared with rituximab | 10,811.13 (626.02) | 581.48 | 0.52 (0.03) | 0.06 | 10,145.42 |
| Abatacept compared with alternative TNFi | 13,785.52 (872.09) | 2974.39 | 0.53 (0.04) | 0.02 | 188,842.00 |
| Mean imputed data | |||||
| Alternative TNFi | 10,538.72 (1040.50) | 0.51 (0.02) | |||
| Rituximab compared with alternative TNFi | 12,606.74 (2423.23) | 2068.02 | 0.45 (0.02) | –0.06 | Dominated |
| Abatacept compared with alternative TNFi | 13,173.90 (673.21) | 2635.17 | 0.48 (0.03) | –0.04 | Dominated |
| Multiple imputation | |||||
| Alternative TNFi | 8490.97 (599.60) | 0.49 (0.02) | |||
| Rituximab compared with alternative TNFi | 8853.82 (997.76) | 362.85 | 0.54 (0.02) | 0.05 | 7647 |
| Abatacept compared with alternative TNFi | 10,449.61 (883.59) | 1595.79 | 0.53 (0.02) | –0.01 | Dominated |
Value of information analysis
There are 690,000 people with RA in the UK. 3,141 The population EVPI, at the NICE cost-effectiveness threshold value of lambda (λ = £20,000), is £129,227,589. The population EVPI for other values of lambda is plotted in Figure 22. This indicates that it would be highly valuable to the NHS to reduce the current uncertainty regarding the effectiveness of alternative TNFi compared with rituximab in the management of RA.
FIGURE 22.
Expected value of perfect information: population.

Conclusions
Owing to the sample size, the conclusions drawn from the cost-effectiveness analyses should be treated with caution. The analysis shows that switching to alternative TNFi following an initial TNFi failure may be a cost-effective option compared with rituximab, although switching to abatacept is unlikely to be cost-effective. Although the conclusion was robust to several alternative sensitivity analyses and was also corroborated when taking a broader societal perspective, which includes the costs incurred by patients there are caveats given that the full sample size was not achieved within the study. The value of information analysis indicates that it would be highly valuable to the NHS to reduce the current uncertainty regarding the effectiveness of alternative TNFi compared with rituximab in the management of RA.
Chapter 5 Discussion
Principal findings
Primary outcome
Owing to the early termination of the trial by the funders, the ‘SWITCH’ study was substantially underpowered in its objective to demonstrate non-inferiority of either alternative TNFi or abatacept to rituximab in terms of a reduction in the DAS28 at 24 weeks post randomisation. In the context of the low number of patients, alternative TNFi was non-inferior to rituximab in the ITT patient population, in that the estimate of the treatment effect excluded the non-inferiority margin of –0.6 units, but non-inferiority was not demonstrated in the PP population, which was our prespecified requirement for demonstrating non-inferiority overall. Non-inferiority of abatacept to rituximab was not demonstrated in either patient population; the 95% CI did not exclude the non-inferiority margin of –0.6 units and is therefore a plausible value.
As the trial was not permitted to recruit the target number of patients, the main interpretation of the results is based on the estimated treatment effect and corresponding precision. In the ITT patient population, the estimated mean difference in the reduction in the DAS28 after 24 weeks between alternative TNFi and rituximab was 0.30 units (95% CI –0.45 to 1.05 units); the corresponding estimate for the difference between abatacept and rituximab was 0.04 units (95% CI –0.72 to 0.79 units). Hence, the treatment effect in the ITT patient population is estimated with a precision of ± 0.75 units (corresponding to the half-width of the 95% CI).
Following exclusion of patients according to the prespecified criteria, the number of patients included in the PP population resulted in the treatment effect for both interventions compared with rituximab being estimated with very low precision. The estimated treatment effect for alternative TNFi compared with rituximab was –0.58 units (95% CI –1.72 to 0.55 units) and for abatacept was –0.15 units (95% CI –1.27 to 0.98 units) for the DAS28 at 24 weeks. Therefore, the treatment effect was estimated with a precision of ± 1.13 units, so there is large uncertainty in the estimate of the treatment effect in the PP population.
Exploratory subgroup analysis
Subgroup effect estimates in this underpowered study should be interpreted cautiously; however, this component of the study was particularly novel and important clinically.
The suggestion of an association between negative serological status and poorer response to rituximab was consistent with meta-analyses,3–84 a recent RCT of the first-line use of a bDMARD (as opposed to following first TNFi failure as with the ‘SWITCH’ study) in which non-inferiority of rituximab compared with a TNFi in seropositive patients142 was observed and observational registry data. 82 The dependency of individual CCG receptiveness to secure such agreements, however, increases the potential for regional inconsistency in prescribing options and approach. Complete SWITCH data could have pushed for inclusion in future technology appraisals.
Primary and secondary non-response to an initial TNFi is well recognised and an important marker of the mechanisms for treatment failure, with secondary non-response suggesting a pharmacokinetic basis for failure and primary non-response suggesting the wrong target. 143 The results suggest that primary non-response may benefit from a change in class of bDMARD, whereas secondary non-response can be circumvented by use of alternative TNFi; these are consistent with other published literature, albeit from uncontrolled cohort studies.
Secondary outcomes
Rheumatoid arthritis is a chronic disease that in the main requires long-term DMARD therapy. RCTs usually comprise a primary end point at week 24 (or earlier) that provides guidance only on short-term outcome whereas, in practice, reassurance on the maintenance (durability) of response is equally relevant. The secondary outcomes at weeks 36 and 48 go some way to providing this additional context. In the ‘SWITCH’ study, there was evidence of a greater improvement in disease activity (via the DAS28) at week 36 for alternative TNFi than for rituximab, although this difference was not maintained at week 48. There was no evidence of a difference in the DAS28 between abatacept and rituximab at any time point. However, when assessing disease activity in terms of the odds of achieving a DAS28 response (≥ 1.2 units), there was no evidence of a difference for either intervention compared with rituximab at any of the time points. Moreover, there was no evidence of a difference in the odds of achieving an ACR20 response at 24 weeks post randomisation for either intervention relative to rituximab.
In addition to demonstrating a reduction in the DAS28, the overall disease activity status and setting of a target of at least low disease activity (if not remission) is now established as an important goal, which is coined as part of a ‘treat to target’ management approach of RA. 144 In the ‘SWITCH’ study, the proportions of patients on alternative TNFi and rituximab who achieved DAS28 low disease activity or remission at 24 weeks were similar, whereas the proportion of patients who achieved low disease activity or remission on abatacept was lower. Furthermore, among patients on alternative TNFi, the proportion achieving low disease activity or remission continued to increase to week 48, whereas among those on abatacept and rituximab this proportion fell between 24 and 48 weeks.
Functional and quality-of-life patient-reported outcome measures remain important indicators of patient well-being. Overall, a general improvement in HAQ-DI, RAQoL and the Patient Global Assessment of General Health was apparent over time, with no notable differences between treatment groups. There was a marked initial improvement in the Patient Global Assessment of Pain and Patient Global Assessment of Arthritis at 12 weeks across all three treatment groups. Small improvements in the HADS anxiety and depression scores over the 48-week period were observed in patients treated with alternative TNFi or abatacept, whereas no notable improvement was apparent in those receiving rituximab.
The safety profile was similar for all three treatments. Ten SAEs were reported in nine patients, of which three events in three patients were considered to be related to trial medications. There were no SUSARs reported. Two patients died, in both cases following the development of a SAE (one each in the rituximab and abatacept groups). Ten patients experienced toxicity resulting in a permanent cessation of treatment (four patients on alternative TNFi, two on abatacept and four on rituximab).
The most common protocol deviations related to receiving steroid treatment within 6 weeks of an end-point assessment, not being compliant with treatment to 24 weeks and receiving additional contraindicated treatment, all of which were likely to have contributed to a less conservative estimate of the mean treatment effect relative to the ITT patient population.
Strengths and weaknesses
The principal strength of the ‘SWITCH’ study design was its emphasis on evaluation of a more defined and refined patient population, in keeping with the overall ambitions of the medical community for a more precise, tailored approach to medicine. This in itself, however, posed its own challenges, such as in recruitment. To date, almost all TNFi failure studies (RCTs and observationa studiesl) have included any cause of failure (inefficacy and toxicity/intolerance), limiting the strength in application of the data on an individual patient level and also the potential mechanistic insights that can be drawn from clinical studies (‘reverse translation’). The SWITCH study permitted the enrolment of only patients in whom TNFi had been found to be ineffective; although this is the predominant reason for failure, it will have limited the eligible patient pool. In addition, evaluating only patients on concomitant MTX (to recognise MTX synergy with bDMARD) will have limited the available recruitment pool further. In hindsight, accepting a less precise approach by including all patients (any cause of TNFi failure and TNFi with/without MTX combination) with sensitivity analysis adjusting for MTX combination would have been more pragmatic and reduced some of the challenges in recruitment.
Nevertheless, the scientific design and rigorous conduct of this, albeit small, trial means that the SWITCH study will contribute to the evidence base for future research in RA, including in meta-analyses, and, hopefully, encourage future study design to address the factors such as those included in our exploratory subgroup analysis.
The obvious major weakness was the early termination, which resulted in a small number of patients recruited into the study. This naturally led to uncertainty in providing definitive conclusions. Despite this, the study identifies some indicators of response, which, although not definitive, provide support for, in particular, alternative TNFi rather than rituximab as a second-line bDMARD therapy in the management of RA. Furthermore, although an exploratory outcome, further diminished with the small sample size, the absence of a response to rituximab in the seronegative population is consistent with the published meta-analyses in efficacy trials. 84 These results, thus, further support that the hypothesis that an alternative bDMARD to rituximab may be preferable if a patient is seronegative. Moreover, if secondary non-response has resulted from a first TNFi, then it may also be more appropriate to consider a second TNFi.
It is important to emphasise, however, that no single treatment (or sequence) is appropriate for all patients and no single study will be able to address this completely. Although these and other complementary data may not provide definitive evidence on tailoring therapy, they do provide initial evidence to support a clinical judgement and increase the chance of treatment success on which subsequent studies can build. This approach to stratification of treatment would represent an advance to the current status of generally prescribing rituximab to all patients, thereby dismissing the potential benefits of switching to alternative therapies in patients who fail TNFi.
A further limitation of the design was the open-label nature of the study. Although blinding patients and treating clinicians to the allocated intervention would have reduced the risk and impact of any assessment bias, it would have been impractical to implement: each of the seven distinct bDMARDs in the SWITCH study involved a different route of delivery and dosing regimen, thus requiring multiple dummy infusions (necessitating additional inpatient attendance) and injections to maintain the blind. Such a treatment schedule was given careful consideration by our PPI advisor, and it was concluded that it would have imposed considerable burden on patients and was unethical, potentially either reducing recruitment further or increasing the rate of attrition throughout the study. However, the fact that all patients received an active therapy may have attenuated any bias introduced by the lack of blinding.
When the SWITCH study was designed, it included all three classes of bDMARD available for evaluation as second-line therapies when taken in combination with MTX, thereby reflecting the full range of therapies available. After the trial design and initiation, tocilizumab was approved as a first-line bDMARD and, hence, represented an additional option for the TNFi-inadequate response RA patient cohort. In addition, NICE has also approved the use of tocilizumab as a monotherapy, broadening the available therapies when given as monotherapy (without concomitant MTX).
The trial design allowed a pragmatic approach with clinician choice of the monoclonal antibody if the patient previously received etanercept. Moreover, a fourth monoclonal antibody, golimumab, was introduced into the alternative TNFi arm during the recruitment phase, thereby allowing further clinician choice across all available TNFis and ensuring further generalisability of the trial results to clinical practice. Although this pragmatic design may have introduced some heterogeneity into the results, it reflected current practice in the NHS and ensured that the EULAR guidance, recommending discussion between patient and treating clinician in the choice of bDMARD, was adhered to; this was considered important in order to maximise recruitment.
Recruitment
It is important to provide information on recruitment strategies in the SWITCH study in order to inform future trials. We would refer the reader to the more exhaustive case study (Maya H Buch, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds and National Institute for Health Research (NIHR) Leeds Musculoskeletal Biomedical Research Unit, Leeds Teaching Hospitals NHS Trust, Leeds, UK, 2015) that systematically dissected each stage of the SWITCH study, from time of grant submission to active recruitment period. Some of the points and strategies to overcome the challenges faced in the SWITCH study are summarised below.
Despite attempts by the investigators to elicit reliable pre-trial recruitment estimates from centres, the numbers of eligible patients proposed were optimistic. In addition, the prolonged grant application and contracting processes resulted in a loss of momentum and the presence of competing studies that usurped the SWITCH study in some centres. Furthermore, centres reported research staff shortages and had to rely on clinical nurse specialist services to identify patients. Despite this, we recruited significantly more study sites than originally planned to address recruitment challenges. A clinical research fellow was appointed to support centres and to advise on co-ordination of research and clinical teams, but the heterogeneity in service provision meant that this had limited success. They were, however, effective in navigating the clinical challenges of patient recruitment and provided specific clinical guidance that aided recruitment. Other strategies for recruitment included clinic posters, leaflets, patient websites, e-bulletins and social media via the National Rheumatoid Arthritis Society. Fortnightly news flashes, monthly teleconferences and training days were also initiated to support centres.
As rituximab was the only NICE-approved treatment option in this context, a number of CCGs would not fund the experimental arms, so that centres were expected to underwrite the cost of these drugs (which was not a realistic option), although NICE provided clarification that its guidance applied to routine practice only and these treatments should be funded if part of a well-designed RCT. Such guidance was not mandated, meaning that CCGs could deviate from this and restrict participation by some centres, and many attempts to establish a dialogue with CCGs proved lengthy and unsuccessful. Discussions with pharmaceutical companies resulted in supplies of abatacept but not before additional delays attributable to negotiations and contract preparation.
The NHS routinely uses home health-care companies to deliver medication to patients’ homes. These companies raised concerns regarding their regulatory authority to deliver trial IMPs only following activation of the initial three centres, which resulted in extended discussions, risk assessments and revisions to the protocol, during which recruitment was halted for 9 months at the beginning of the recruitment period.
Ultimately, as a result of discussions initiated with the NIHR Clinical Research Network (CRN) Coordinating Centre to overcome these obstacles, the CRN used the SWITCH trial as a case study to highlight the challenges in trial set-up, including areas for improvement in the CRN, feasibility and centre-specific approvals process and the insufficient resources (Maya H Buch, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds and NIHR Leeds Musculoskeletal Biomedical Research Unit, Leeds Teaching Hospitals NHS Trust, Leeds, UK, 2015).
The recruitment estimates may have been moderated had we repeated the recruitment survey once the study had been set up, and this is something that we would recommend as routine prior to study activation. Earlier engagement with the Medicines and Healthcare products Regulatory Agency, NIHR CRN and NICE may also have improved the trial status, although their influence on the NHS landscape and commissioning groups remains debatable. Clinician experience suggests that CCG approach to NICE guidance and TAs can be variable. To be able to deliver a fully ‘independent’ study to the diverse portfolio of studies, involvement of pharmaceutical partners was deliberately not considered at the start. Following the experience with the SWITCH study, however, this remains a naive aspiration, and, certainly, approaching industry to support IMP provision from the outset in future clinical trials of an IMP is vital to militate against CCG lack of support and to prevent delays. Finally, given the complexity of the governance and practical requirements of a clinical trial of an IMP and lengthy approvals process, it is important that the set-up period for such trials is not underestimated and that any estimates of patient recruitment are based on robust local audits rather than clinician judgement.
Health economic evaluation
Principal findings
The trial-based cost-effectiveness analysis indicated that the current NICE guidance to switch to rituximab after initial TNFi failure was not the most cost-effective use of NHS resources. Instead, switching to alternative TNFi drug following an initial failure was the most cost-effective treatment option. Switching to alternative TNFi was more costly, but provided more QALYs than rituximab over a 48-month period. Moreover, switching to abatacept was not cost-effective compared with switching to alternative TNFi, as the associated costs were much higher and the QALY gain was small.
All three trial arms showed an increase in mean EQ-5D score over the 12-month trial period, with an initial increase from baseline to 12 weeks that was more or less maintained to 48 weeks. The small (non-significant) differences in QALYs resulting from these treatments could relate to the benefits being measured only over the trial period of 48 weeks, meaning that longer-term benefits could not be considered. In the primary analysis costs from a health and social services perspective and a wider social perspective were highest for the abatacept group and lowest for the rituximab group, but only slightly lower than the costs for the alternative TNFi group. These costs-effectiveness results were driven by the (statistically significant) difference in costs between abatacept and the other treatment groups. These results may change in longer follow-up if the side-effect profiles of the treatments are different when the drugs are used for prolonged periods, or effectiveness persists for different durations.
Base-case cost-effectiveness results were not sensitive to assumptions in a small number of deterministic sensitivity analyses. In line with the NICE reference case that indicates that costs to patients may be included in cost-effectiveness evaluations, results from a secondary analysis including the costs incurred by patients remained consistent, indicating that switching to alternative TNFi is cost-effective but switching to abatacept is not. However, these results should be treated with caution because of the small sample size, for which estimation of asymmetrical cost distributions is particularly difficult.
An estimate of the value of perfect information suggested that further research, in order that robust economic decisions are made, is worth in the order of £129M. This suggests that the early termination of the study by the funders may have imposed extremely large costs (either financial or in terms of health forgone) on the NHS, through allowing a high degree of decision uncertainty to persist. It is acknowledged, however, that, even had the trial achieved its expected sample size, it is unlikely to have addressed all uncertainty.
Strengths and weaknesses of the economic analysis
The main strength of this analysis lies in the randomised controlled design of the study, which enabled the collection of high-quality data over the 48-week time horizon of the trial that were subsequently used in this analysis.
The small sample size achieved led to greater uncertainty in the conclusions drawn from the analyses and limited the scope of the analyses that could be performed. Only complete cases were used for the primary analysis. This meant that an even smaller sample was used for the primary analysis and the results must be treated with caution because of the potential for bias and the high likelihood of overestimation of the level of precision in the estimates of cost-effectiveness. The value of perfect information estimate indicates that further research is likely to be of value. Given the relatively short duration of follow-up, consideration of longer-term outcomes would be beneficial as part of any future research.
Meaning of the study
The costs associated with TNFi drugs and abatacept are relatively high because of the larger number of treatments that are given weekly or fortnightly (see Table 1), compared with the costs of rituximab, which is given in weeks 1 and 2 with a potential repeat at 6 months. However, when all health economic data were taken into account, to give the full costs incurred of the treatments over 48 weeks from a health and social services perspective, the difference in overall costs between the TNFis used in this trial and rituximab is quite small. The overall costs associated with abatacept are much higher. With only a small, non-significant, difference in the change in EQ-5D scores between trial arms, it is these differences in costs that drive the results and show that the use of alternative TNFi following an initial TNFi failure could be viable as a cost-effective treatment option alongside the currently approved treatment, rituximab.
Unanswered questions and further research
Although the analysis conducted here provides useful insights into the costs and effects of the treatment options in the trial period of 48 weeks, further research is required to provide evidence on the cost-effectiveness for alternative TNFi over a longer time horizon beyond 48 weeks. This could involve the development of an economic model that would also allow an extension to the value of information analysis conducted here to include the expected value of partial perfect information. This would provide a clearer idea of the areas around which further research should be conducted. Additional research would also be beneficial using a larger sample size to reduce the uncertainty in the conclusions drawn from the analysis and to ensure that a representative sample of the patient group is captured.
The existing evidence base has been limited to numerous small trials, whereas the ambition of the SWITCH randomised trial was to deliver a large-scale definitive trial that would have represented a paradigm shift in the RA community, delivering the largest RA pragmatic trial undertaken in the UK. Early termination of the SWITCH trial limits the conclusions that may be drawn and is therefore considered a lost opportunity to obtain definitive evidence on cost-effectiveness of either treatment option. However, the data presented may be used in meta-analysis in future research.
Comparison with other studies
The only studies comparable with the SWITCH trial are the preliminary reports of the French study [Rotation of anti-TNF Or Change of class of biologic (ROC)] by Gottenberg et al. 145 and the Dutch study by Manders et al. 87 Although the ROC study is an instructive randomised trial that reached its primary outcome (non-TNFi bDMARD significantly superior to alternative TNFi), the multiple treatment options included within the non-TNFi randomised group limit the extent to which these data can inform the optimal targeted agent. The study by Manders et al. 87 found no significant difference between alternative TNFi, abatacept and rituximab, but further details on whether or not these treatments are equivalent are needed. Moreover, the study reported that, of the three treatments, rituximab was the most cost-effective and that treating patients with alternative TNFi was more cost-effective than treating patients with abatacept. However, similar to the SWITCH trial, this study is limited in what conclusions can be made because of the low number of patients recruited.
A requirement for non-inferiority trials is the assumption of assay sensitivity (constancy assumption), that is, establishing that the active control arm, rituximab, would be superior to placebo in the setting of the SWITCH trial. 146 One previous study, the REFLEX trial,49 established the efficacy of rituximab compared with placebo in patients receiving MTX who had failed more than one treatment with a TNFi. The SWITCH trial was similar in a number of respects. The intervention was substantially the same over the 24-week period during which the primary end point was to be assessed; the primary and key secondary end points of the DAS28 reduction and ACR20 response were assessed at week 24, which was the key assessment time point in the REFLEX study. Moreover, the enrolled population had a similar age range and disease duration. Notable differences include the REFLEX study requiring a minimum level of disease activity in order for a patient to be enrolled, the difference in blinding (which had a much simpler requirement in the REFLEX study’s two-arm placebo-controlled study) and that patients were eligible if they had failed treatment with more than one TNFi and had demonstrated intolerance to prior TNFi therapy. The reduction in disease activity for rituximab in the SWITCH trial was sufficiently similar to that reported in the REFLEX study, supporting the conclusion that the SWITCH trial fulfilled the assay sensitivity requirement for a non-inferiority trial.
Implications for health care and future research
The clinical question of whether or not alternative bDMARDs to rituximab are comparable in efficacy and safety outcomes in patients with RA who had not responded adequately to an initial TNFi bDMARD and MTX treatment remains unresolved. The lack of evidence, which is based on a single treatment (rituximab) being appropriate for all patients, limits guidance options.
In addition, NICE acknowledges this and has explicitly stated the need for comparative studies to inform future guidance. 25 In response to this unmet need, the scale and ambition of the SWITCH study was impressive, planned as one of the largest RA trials. It was, thus, particularly unfortunate that the trial was prematurely stopped. This has again made contribution to a meta-analysis the best outcome of this trial, although this also means the loss of a more definitive UK NHS-relevant answer; this is particularly disheartening when substantial investment into the study had already been made. Had the SWITCH trial been permitted to recruit to target, definitive evidence on whether or not either of the interventions were non-inferior to rituximab may have been provided, which may have opened up further treatment options for patients.
The ‘SWITCH’ study design also aimed to serve as a driver to the RA community to develop novel trial designs into our clinical trial repertoire, such that we can begin to address some of the challenges that currently impede delivery of the promise of personalised medicine in RA. 147 Incorporating stratification based on RF/ACPA seropositivity status and primary/secondary non-response to an initial TNFi would have allowed an exploratory analysis to determine if there was any evidence of a differential treatment response in these subgroups of patients, an aspect which no other RCT to date has attempted to do.
Finally, more seamless and integrated data capture is vital to support successful delivery of, in particular, large-scale definitive studies. Health informatics and electronic health records linking the needs of NHS with the NIHR and clinical research environment represents a core area for development to effectively embed research in the NHS.
The ‘SWITCH’ study provided several learning points for the academic community that will inform future initiatives. Many of the challenges illustrated deficiencies in the NHS organisational approach and infrastructure that are needed if it is to successfully deliver NIHR research, detailed by way of a case study initiated by the NIHR CRN (Maya H Buch, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds and NIHR Leeds Musculoskeletal Biomedical Research Unit, Leeds Teaching Hospitals NHS Trust, Leeds, UK, 2015).
Trial activity in the clinical area of RA is in its relative infancy compared with other high-prevalence clinical areas, such as cancer and cardiovascular disease. The SWITCH trial began to harness the academic and clinical community’s commitment to trial activity and established a RCT research network, setting up a total of 35 centres: a massive achievement in itself that was truly representative of the wider rheumatology community. Continuing and ultimately delivering on the SWITCH trial, therefore, would have not just answered the trial question but also would have represented a substantial achievement for the RA community. The SWITCH study was therefore considered as an early investment for future (independent) RA trials that would allow the rheumatology community to build up its experience incrementally and, with this progression of trial design and better integration of research in the NHS, trial efficiency and landscape would have evolved. We hope future considerations take account of the SWITCH trial experience using the case study to support the rheumatology community in delivering on its ambition to improve the lives of people with RA (and other musculoskeletal conditions).
Acknowledgements
Clinical Trials Research Unit staff, University of Leeds
Dr Janine Gray (Principal Statistician and Co-applicant).
Mr Howard Collier [Data Manager (Acting)].
Mrs Catherine Reynolds (Data Manager).
Dr Catherine Fernandez (Head of Trial Management).
Miss Suzanne Hartley (former Head of Trial Management).
Cris Bunker (Senior Programmer).
Trial Management Group
Mrs Nuria Navarro Coy (former Senior Trial Manager).
Professor Anne-Maree Keenan (Co-applicant).
Professor Anthony Redmond (Co-applicant).
Mrs Ailsa Bosworth (Patient Representative and Co-applicant).
Data Monitoring Committee
Dr Robin Butler (Independent Clinician and Chairperson), Robert Jones and Agnes Hunt, Orthopaedic Hospital, Oswestry.
Dr Duncan Porter (Independent Clinician), University of Glasgow.
Professor Hazel Inskip (Independent Methodologist), University of Southampton.
Trial Steering Committee
Professor Ernest Choy (Independent Clinician and Chairperson), University of Cardiff.
Dr Andrew Ostor (Independent Clinician), Addenbrookes Hospital, Cambridge.
Dr Martyn Lewis (Independent Statistician), University of Keele.
Dr Laura Bojke (Independent Health Economist), University of York.
Ms Sandra Purdy (Independent Member and Patient Representative).
National Institute for Health Research Clinical Research Network
Nancy Lester (Business Development Director), CRN Executive Team.
Tomasz Kurdziel (Research Support and Management Lead), CRN: Yorkshire and the Humber.
Richard Wylde (Continuous Improvement Manager) Leeds and York Partnership NHS Foundation Trust.
Participating centres
With thanks to all research staff at participating centres who arranged set-up of the study at their centres, provided and cared for trial patients and collected trial data. Special thanks to the principal investigators:
John Isaacs, Royal Victoria Infirmary, Newcastle.
Ian Bruce, Manchester Royal Infirmary, Manchester.
Euthalia Roussou, King George Hospital, Essex.
Srinivasan Venkat Chalam, Cannock Chase Hospital, Mid Staffordshire.
Christopher Buckley, Birmingham City Hospital.
Alison Kinder, Leicester Royal Infirmary, Leicester.
Lindsay Robertson, Derriford Hospital, Plymouth.
Sarah Westlake, Poole Hospital, Poole.
Paul Thompson, Poole Hospital, Poole.
Raashid Luqmani, Oxford Radcliffe Hospitals NHS Trust, Oxford.
Chris Deighton, Royal Derby Hospital, Derby.
Shabina Sultan, Airedale General Hospital, Steeton.
Mohamed Nisar, Queen’s Hospital, Burton upon Trent.
Luke Gompels, Musgrove Park Hospital, Taunton.
John Pauling, Royal National Hospital for Rheumatic Diseases, Bath.
Rod Hughes, Ashford and St Peters NHS Trust, Lyne.
Vadivelu Saravanan, Queen Elizabeth Hospital, Gateshead.
Yusuf Patel, Hull Royal Infirmary, Hull.
Devesh Mewar, Broadgreen Hospital, Liverpool.
Bhaskar Dasgupta, Southend Hospital, Southend-on-Sea.
Costantino Pitzalis, Barts and The London NHS Trust (Mile End and Whipps Cross), London.
Hector Chinoy, Salford Royal NHS Foundation Trust.
Cathy Lawson, Harrogate District Hospital, Harrogate.
Bruce Kirkham, Guy’s Hospital, London.
Dayavathi Ashok, County Durham & Darlington NHS Foundation Trust.
James Maxwell, Royal Hallamshire Hospital, Sheffield.
Shaheena Haque, University Hospital of South Manchester, Wythenshawe.
Pippa Watson, University Hospital of South Manchester, Wythenshawe.
Wahab Al-Allaf, New Cross Hospital (Royal Wolverhampton NHS Trust).
John Harvie, Raigmore Hospital, Inverness.
James Taylor, Northampton General Hospital, Northampton.
Ernest Wong, Queen Alexandra Hospital, Portsmouth.
Matthew Roy, Bristol Royal Infirmary.
Claire Gorman, Homerton University Hospital.
Sam Panthakalam Eastbourne District General Hospital, Eastbourne.
Participants
Thank you to all trial patients for their essential contribution to the trial.
Contributions of authors
Sarah Brown (Principal Statistician, Statistics) provided statistical supervision on the trial, led the drafting of the monograph and interpreted the results.
Colin C Everett (Senior Statistician, Statistics) conducted the statistical analysis and interpreted the results.
Kamran Naraghi (Clinical Research Fellow, Rheumatology) contributed to the conduct and monitoring of the trial and clinical queries.
Claire Davies (Senior Trial Manager, Trial Management) oversaw and co-ordinated the running of the trial, trial monitoring and negotiations on centre closures.
Bryony Dawkins (Health Economist, Health Economics) conducted the heath economic analysis.
Claire Hulme (Professor of Health Economics, Health Economics) had overall responsibility for the reporting of the health economics analysis.
Christopher McCabe (Professor of Health Economics, Health Economics) reviewed the planned analyses and development of the cost-effectiveness chapter and contributed to the writing up of the final set of results.
Sue Pavitt (Professor in Translational and Applied Health Research, Dentistry) provided specialist musculoskeletal expertise in delivery of the trial.
The SWITCH trial was conceived by Paul Emery (Professor of Rheumatology, Rheumatology) and Maya H Buch (Professor of Rheumatology, Rheumatology). Maya H Buch also had overall responsibility for the trial.
Linda Sharples (Professor of Statistics, Statistics) had overall responsibility for the statistics and research methodology.
All authors contributed to the writing of the report and had the opportunity to critically revise it.
Publication
Navarro Coy NC, Brown S, Bosworth A, Davies CT, Emery P, Everett CC, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord 2014;15:452.
Data sharing statement
The data sets during and/or analysed during the SWITCH trial will be available from the corresponding author on reasonable request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903-11. https://doi.org/10.1016/S0140-6736(01)06075-5.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Markenson JA. Worldwide trends in the socioeconomic impact and long-term prognosis of rheumatoid arthritis. Semin Arthritis Rheum 1991;21:4-12. https://doi.org/10.1016/0049-0172(91)90046-3.
- Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med 1990;322:1277-89. http://dx.doi.org/10.1056/NEJM199005033221805.
- Weinblatt ME. Rheumatoid arthritis: treat now, not later!. Ann Intern Med 1996;124:773-4. https://doi.org/10.7326/0003-4819-124-8-199604150-00012.
- Barrett EM, Scott DG, Wiles NJ, Symmons DP. The impact of rheumatoid arthritis on employment status in the early years of disease: a UK community-based study. Rheumatology 2000;39:1403-9. https://doi.org/10.1093/rheumatology/39.12.1403.
- Young A, Dixey J, Kulinskaya E, Cox N, Davies P, Devlin J, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the Early RA Study (ERAS). Ann Rheum Dis 2002;61:335-40. https://doi.org/10.1136/ard.61.4.335.
- Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690-7. http://dx.doi.org/10.1002/art.24092.
- Kaplan MJ. Cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol 2006;18:289-97. http://dx.doi.org/10.1097/01.bor.0000218951.65601.bf.
- Peters MJ, Van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Care Res 2009;61:1571-9. https://doi.org/10.1002/art.24836.
- Mantel Ä, Holmqvist M, Jernberg T, Wållberg-Jonsson S, Askling J. Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur Heart J 2015;36:3413-22. https://doi.org/10.1093/eurheartj/ehv461.
- Douglas KM, Pace AV, Treharne GJ, Saratzis A, Nightingale P, Erb N, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis 2006;65:348-53. https://doi.org/10.1136/ard.2005.037978.
- Mantel Ä, Holmqvist M, Nyberg F, Tornling G, Frisell T, Alfredsson L, et al. Risk Factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: a nested case–control study. Arthritis Rheum 2015;67:2845-54. https://doi.org/10.1002/art.39267.
- Arts EE, Fransen J, den Broeder AA, Popa CD, van Riel PL. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis 2015;74:998-1003. https://doi.org/10.1136/annrheumdis-2013-204531.
- Wilske KR, Healey LA. Challenging the therapeutic pyramid: a new look at treatment strategies for rheumatoid arthritis. J Rheumatol Suppl 1990;25:4-7.
- Wright V, Amos R. Do drugs change the course of rheumatoid arthritis?. Br Med J 1980;280:964-6. https://doi.org/10.1136/bmj.280.6219.964-a.
- Nell VP, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology 2004;43:906-14. http://dx.doi.org/10.1093/rheumatology/keh199.
- Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med 2001;111:446-51. https://doi.org/10.1016/S0002-9343(01)00872-5.
- van Aken J, Lard LR, le Cessie S, Hazes JM, Breedveld FC, Huizinga TW. Radiological outcome after four years of early versus delayed treatment strategy in patients with recent onset rheumatoid arthritis. Ann Rheum Dis 2004;63:274-9. https://doi.org/10.1136/ard.2003.010298.
- Korpela M, Laasonen L, Hannonen P, Kautiainen H, Leirisalo-Repo M, Hakala M, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum 2004;50:2072-81. http://dx.doi.org/10.1002/art.20351.
- Verstappen SM, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, Borg EJt, et al. Five-year follow-up of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum 2003;48:1797-807. https://doi.org/10.1002/art.11170.
- Landewé RB, Boers M, Verhoeven AC, Westhovens R, van de Laar MA, Markusse HM, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum 2002;46:347-56. https://doi.org/10.1002/art.10083.
- Maillefert JF, Combe B, Goupille P, Cantagrel A, Dougados M. Long term structural effects of combination therapy in patients with early rheumatoid arthritis: five year follow up of a prospective double blind controlled study. Ann Rheum Dis 2003;62:764-6. https://doi.org/10.1136/ard.62.8.764.
- Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum 2006;55:864-72. http://dx.doi.org/10.1002/art.22353.
- Rheumatoid Arthritis in Adults: Management. London: NICE; 2015.
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492-509. http://dx.doi.org/10.1136/annrheumdis-2013-204573.
- Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Baillieres Clin Rheumatol 1995;9:619-32. https://doi.org/10.1016/S0950-3579(05)80305-X.
- Brooks PM. Clinical management of rheumatoid arthritis. Lancet 1993;341:286-90. https://doi.org/10.1016/0140-6736(93)92628-7.
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996;14:397-440. http://dx.doi.org/10.1146/annurev.immunol.14.1.397.
- Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum 1993;36:1681-90. https://doi.org/10.1002/art.1780361206.
- Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med 1997;337:141-7. http://dx.doi.org/10.1056/NEJM199707173370301.
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932-9. https://doi.org/10.1016/S0140-6736(99)05246-0.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35-4. http://dx.doi.org/10.1002/art.10697.
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594-602. http://dx.doi.org/10.1056/NEJM200011303432202.
- Keystone E, Heijde DVD, Mason D, Landewé R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319-29. https://doi.org/10.1002/art.23964.
- Emery P, Fleischmann RM, Moreland LW, Hsia EC, Strusberg I, Durez P, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272-83. http://dx.doi.org/10.1002/art.24638.
- Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10420.
- Mewar D, Wilson AG. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol 2011;162:785-91. http://dx.doi.org/10.1111/j.1476-5381.2010.01099.x.
- Buch MH, Conaghan PG, Quinn MA, Bingham SJ, Veale D, Emery P. True infliximab resistance in rheumatoid arthritis: a role for lymphotoxin alpha?. Ann Rheum Dis 2004;63:1344-6. http://dx.doi.org/10.1136/ard.2003.014878.
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002;301:418-26. https://doi.org/10.1124/jpet.301.2.418.
- Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-α monoclonal antibody cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine 1995;7:251-9. https://doi.org/10.1006/cyto.1995.0029.
- Scallon BJ, Trinh H, Nedelman M, Brennan FM, Feldmann M, Ghrayeb J. Functional comparisons of different tumour necrosis factor receptor/IgG fusion proteins. Cytokine 1995;7:759-70. https://doi.org/10.1006/cyto.1995.0091.
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344:907-16. http://dx.doi.org/10.1056/NEJM200103223441207.
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572-81. http://dx.doi.org/10.1056/NEJMoa032534.
- Smolen JS, Schoels MM, Nishimoto N, Breedveld FC, Burmester GR, Dougados M, et al. Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis 2013;72:482-92. https://doi.org/10.1136/annrheumdis-2012-202469.
- Buch MH, Boyle DL, Rosengren S, Saleem B, Reece RJ, Rhodes LA, et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann Rheum Dis 2009;68:1220-7. https://doi.org/10.1136/ard.2008.091876.
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144:865-76. https://doi.org/10.7326/0003-4819-144-12-200606200-00003.
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987-97. https://doi.org/10.1016/S0140-6736(08)60453-5.
- Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793-806. http://dx.doi.org/10.1002/art.22025.
- Emery P, Keystone E, Tony H, Cantagrel A, Van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008;67:1516-23. https://doi.org/10.1136/ard.2008.092932.
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114-23. https://doi.org/10.1056/NEJMoa050524.
- Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009;11. https://doi.org/10.1186/ar2666.
- Adalimumab, Etanercept and Infliximab for the Treatment of Rheumatoid Arthritis. London: NICE; 2007.
- Certolizumab Pegol for the Treatment of Rheumatoid Arthritis. London: NICE; 2010.
- Golimumab for the Treatment of Rheumatoid Arthritis after the Failure of Previous Disease-modifying Anti-rheumatic Drugs. London: NICE; 2011.
- Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis not Previously Treated with DMARDs or After Conventional DMARDs only have Failed. London: NICE; 2016.
- Tocilizumab for the Treatment of Rheumatoid Arthritis. London: NICE; 2012.
- Abatacept for Treating Rheumatoid Arthritis after the Failure of Conventional Disease-modifying Anti-rheumatic Drugs. London: NICE; 2013.
- Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis after the Failure of a TNF Inhibitor. London: NICE; 2010.
- Buch MH, Bingham SJ, Bejarano V, Bryer D, White J, Reece R, et al. Therapy of patients with rheumatoid arthritis: outcome of infliximab failures switched to etanercept. Arthritis Rheum 2007;57:448-53. http://dx.doi.org/10.1002/art.22617.
- Buch MH, Emery P. Nonresponse to tumor necrosis factor antagonists – is there any point in re-treatment?. Nat Clin Pract Rheumatol 2006;2:288-9. https://doi.org/10.1038/ncprheum0210.
- Buch MH, Seto Y, Bingham SJ, Bejarano V, Bryer D, White J, et al. C-reactive protein as a predictor of infliximab treatment outcome in patients with rheumatoid arthritis: defining subtypes of nonresponse and subsequent response to etanercept. Arthritis Rheum 2005;52:42-8. http://dx.doi.org/10.1002/art.20711.
- Gomez-Reino JJ, Carmona L. BIOBADASER Group . Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 2006;8. https://doi.org/10.1186/ar1881.
- Wick M, Ernestam S, Lindblad S, Bratt J, Klareskog L, Van Vollenhoven R. Adalimumab (Humira®) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade®) or etanercept (Enbrel®): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol 2005;34:353-8. https://doi.org/10.1080/03009740510026887.
- van Vollenhoven RF. Switching between biological agents. Clin Exp Rheumatol 2004;22:115-21.
- Hansen KE, Hildebrand JP, Genovese MC, Cush JJ, Patel S, Cooley DA, et al. The efficacy of switching from etanercept to infliximab in patients with rheumatoid arthritis. J Rheumatol 2004;31:1098-102.
- van Vollenhoven R, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis 2003;62:1195-8. https://doi.org/10.1136/ard.2003.009589.
- Hyrich KL, Lunt M, Watson KD, Symmons DP, Silman AJ. British Society for Rheumatology Biologics Register . Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13-20. https://doi.org/10.1002/art.22331.
- Hjardem E, Østergaard M, Pødenphant J, Tarp U, Andersen LS, Bing J, et al. Do rheumatoid arthritis patients in clinical practice benefit from switching from infliximab to a second tumor necrosis factor alpha inhibitor?. Ann Rheum Dis 2007;66:1184-9. https://doi.org/10.1136/ard.2006.054742.
- Nikas SN, Voulgari PV, Alamanos Y, Papadopoulos CG, Venetsanopoulou AI, Georgiadis AN, et al. Efficacy and safety of switching from infliximab to adalimumab: a comparative controlled study. Ann Rheum Dis 2006;65:257-60. https://doi.org/10.1136/ard.2005.039099.
- Carmona L, Ortiz A, Abad MA. How good is to switch between biologics? A systematic review of the literature. Acta Reumatol Port 2007;32:113-28.
- Lopez-Olivo M, Roundtree A, Ortiz Z, Skidmore B, Suarez-Almazor M. Switching between anti-TNF agents treatments for rheumatoid arthritis: a systematic review. Ann Rheum Dis n.d.
- Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009;374:210-21. https://doi.org/10.1016/S0140-6736(09)60506-7.
- Schiff MH, von Kempis J, Goldblum R, Tesser JR, Mueller RB. Rheumatoid arthritis secondary non-responders to TNF can attain an efficacious and safe response by switching to certolizumab pegol: a phase IV, randomised, multicentre, double-blind, 12-week study, followed by a 12-week open-label phase. Ann Rheum Dis 2014;73:2174-7. http://dx.doi.org/10.1136/annrheumdis-2014-205325.
- Weinblatt ME, Fleischmann R, van Vollenhoven RF, Emery P, Huizinga TWJ, Cutolo M, et al. Twenty-eight-week results from the REALISTIC phase IIIb randomized trial: efficacy, safety and predictability of response to certolizumab pegol in a diverse rheumatoid arthritis population. Arthritis Res Ther 2015;17. http://dx.doi.org/10.1186/s13075-015-0841-9.
- Cohen SB, Keystone E, Genovese MC, Emery P, Peterfy C, Tak PP, et al. Continued inhibition of structural damage over 2 years in patients with rheumatoid arthritis treated with rituximab in combination with methotrexate. Ann Rheum Dis 2010;69:1158-61. http://dx.doi.org/10.1136/ard.2009.119222.
- Genovese M, Schiff M, Luggen M, Becker J, Aranda R, Teng J, et al. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis 2008;67:547-54. https://doi.org/10.1136/ard.2007.074773.
- Emery P, Tony H, Kremer J. Long-term efficacy of tocilizumab (TCZ) in RA patients (PTS) who have inadequate response to anti-TNF therapy (TNF-IR). Ann Rheum Dis 2011;70.
- Finckh A, Ciurea A, Brulhart L, Kyburz D, Möller B, Dehler S, et al. B cell depletion may be more effective than switching to an alternative anti–tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti–tumor necrosis factor agents. Arthritis Rheum 2007;56:1417-23. https://doi.org/10.1002/art.22520.
- Finckh A, Ciurea A, Brulhart L, Möller B, Walker UA, Courvoisier D, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent?. Ann Rheum Dis 2010;69:387-93. http://dx.doi.org/10.1136/ard.2008.105064.
- Soliman MM, Hyrich KL, Lunt M, Watson KD, Symmons DP, Ashcroft DM. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res 2012;64:1108-15.
- Emery P, Gottenberg J, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Taboada VM, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis 2015:979-84. https://doi.org/10.1136/annrheumdis-2013-203993.
- Finckh A, Möller B, Dudler J, Walker UA, Kyburz D, Gabay C. physicians of the Swiss Clinical Quality Management for Rheumatoid Arthritis . Evolution of radiographic joint damage in rituximab-treated versus TNF-treated rheumatoid arthritis cases with inadequate response to TNF antagonists. Ann Rheum Dis 2012;71:1680-5. https://doi.org/10.1136/annrheumdis-2011-201016.
- Isaacs JD, Cohen SB, Emery P, Tak PP, Wang J, Lei G, et al. Effect of baseline rheumatoid factor and anti-citrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis 2013;72:329-36. https://doi.org/10.1136/annrheumdis-2011-201117.
- Harrold LR, Reed GW, Kremer JM, Curtis JR, Solomon DH, Hochberg MC, et al. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis 2013:430-6. https://doi.org/10.1136/annrheumdis-2013-203936.
- Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, et al. non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA 2016;316:1172-80. http://dx.doi.org/10.1001/jama.2016.13512.
- Manders SH, Kievit W, Adang E, Brus HL, Moens HJ, Hartkamp A, et al. Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial. Arthritis Res Ther 2015;17. http://dx.doi.org/10.1186/s13075-015-0630-5.
- Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis 2011;70:1575-80. https://doi.org/10.1136/ard.2010.148759.
- Sellam J, Hendel-Chavez H, Rouanet S, Abbed K, Combe B, Le Loët X, et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum 2011;63:933-8. http://dx.doi.org/10.1002/art.30233.
- Gottenberg JE, Ravaud P, Cantagrel A, Combe B, Flipo RM, Schaeverbeke T, et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis 2012;71:1815-19. http://dx.doi.org/10.1136/annrheumdis-2011-201109.
- Gottenberg J, Courvoisier D, Hernandez M, Iannone F, Lie E, Canhão H, et al. Brief report: association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: results from the Pan-European Registry Analysis. Arthritis Rheum 2016;68:1346-52. https://doi.org/10.1002/art.39595.
- Pieper J, Herrath J, Raghavan S, Muhammad K, van Vollenhoven R, Malmström V. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol 2013;14. https://doi.org/10.1186/1471-2172-14-34.
- Nüßlein HG, Alten R, Galeazzi M, Lorenz H-M, Nurmohamed MT, Bensen WG, et al. Prognostic factors for abatacept retention in patients who received at least one prior biologic agent: an interim analysis from the observational, prospective ACTION study. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0636-9.
- Kawashiri S-y, Kawakami A, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, et al. In rheumatoid arthritis patients treated with tocilizumab, the rate of clinical disease activity index (CDAI) remission at 24 weeks is superior in those with higher titers of IgM-rheumatoid factor at baseline. Mod Rheumatol 2011;21:370-4. https://doi.org/10.3109/s10165-010-0409-0.
- Pers YM, Fortunet C, Constant E, Lambert J, Godfrin-Valnet M, De Jong A, et al. Predictors of response and remission in a large cohort of rheumatoid arthritis patients treated with tocilizumab in clinical practice. Rheumatology 2014;53:76-84. http://dx.doi.org/10.1093/rheumatology/ket301.
- Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum 2007;56:2715-18. http://dx.doi.org/10.1002/art.22811.
- Cohen JD, Bournerias I, Buffard V, Paufler A, Chevalier X, Bagot M, et al. Psoriasis induced by tumor necrosis factor-alpha antagonist therapy: a case series. J Rheumatol 2007;34:380-5.
- Roux CH, Brocq O, Leccia N, Giacchero D, Breuil V, Albert C, et al. New-onset psoriatic palmoplantaris pustulosis following infliximab therapy: a class effect?. J Rheumatol 2007;34:434-7.
- Potter C, Hyrich KL, Tracey A, Lunt M, Plant D, Symmons DP, et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis 2009;68:69-74. http://dx.doi.org/10.1136/ard.2007.084715.
- Navarro Coy NC, Brown S, Bosworth A, Davies CT, Emery P, Everett CC, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-452.
- Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFalpha blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology 2005;44:157-63. https://doi.org/10.1093/rheumatology/keh464.
- Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. Boston, MA: Little, Brown & Co; 1994.
- Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44-8. https://doi.org/10.1002/art.1780380107.
- Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res (Hoboken) 2011;63:14-36. http://dx.doi.org/10.1002/acr.20621.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993;36:729-40. https://doi.org/10.1002/art.1780360601.
- Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796-806. http://dx.doi.org/10.1186/ar1740.
- Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003;42:244-57. https://doi.org/10.1093/rheumatology/keg072.
- Bykerk VP, Massarotti EM. The new ACR/EULAR remission criteria: rationale for developing new criteria for remission. Rheumatology 2012;51:vi16-20. http://dx.doi.org/10.1093/rheumatology/kes281.
- Fuchs HA, Pincus T. Reduced joint counts in controlled clinical trials in rheumatoid arthritis. Arthritis Rheum 1994;37:470-5. https://doi.org/10.1002/art.1780370406.
- Prevoo ML, van Riel PL, van ‘t Hof MA, van Rijswijk MH, van Leeuwen MA, Kuper HH, et al. Validity and reliability of joint indices. A longitudinal study in patients with recent onset rheumatoid arthritis. Br J Rheumatol 1993;32:589-94. https://doi.org/10.1093/rheumatology/32.7.589.
- Smolen JS, Breedveld FC, Eberl G, Jones I, Leeming M, Wylie GL, et al. Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum 1995;38:38-43. https://doi.org/10.1002/art.1780380106.
- Pincus T, Summey JA, Soraci SA, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford health assessment questionnaire. Arthritis Rheum 1983;26:1346-53. http://dx.doi.org/10.1002/art.1780261107.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- de Jong Z, van der Heijde D, McKenna SP, Whalley D. The reliability and construct validity of the RAQoL: a rheumatoid arthritis-specific quality of life instrument. Br J Rheumatol 1997;36:878-83. https://doi.org/10.1093/rheumatology/36.8.878.
- O’Keefe DJ. Colloquy: Should familywise alpha be adjusted?. Hum Comm Res 2003;29:431-47. https://doi.org/10.1111/j.1468-2958.2003.tb00846.x.
- Proschan MA, Follmann DA. Multiple comparisons with control in a single experiment versus separate experiments: why do we feel differently?. Am Stat 1995;49:144-9. https://doi.org/10.1080/00031305.1995.10476132.
- van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800-5. https://doi.org/10.1136/annrheumdis-2012-202281.
- Cohen JD. Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 1988.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Morris TP, White IR, Royston P. Tuning multiple imputation by predictive mean matching and local residual draws. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-75.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Services for People with Rheumatoid Arthritis. London: The Stationery Office; 2009.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- EuroQol Research Foundation . How to Use EQ-5D 2016. www.euroqol.org/about-eq-5d/how-to-use-eq-5d.html (accessed February 2016).
- Dolan P. Modeling valuations for EuroQol health states. Medical Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS Reference Costs 2014 to 2015. London: DH; 2015.
- Drugs and Pharmaceutical Electronic Market Information (eMit). London: Commercial Medicines Unit, Department of Health; 2015.
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; n.d.
- Campbell and Cochrane Economics Methods Group and the Evidence for Policy and Practice Information and Co-ordinating Centre . CCEMG – EPPI-Centre Cost Converter 2014. http://eppi.ioe.ac.uk/costconversion/ (accessed January 2016).
- Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8110.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Economics 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8310.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Health Technology Assessment. London: Springer; 2015.
- What is RA?. 2014.
- Porter D, van Melckebeke J, Dale J, Messow CM, McConnachie A, Walker A, et al. Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet 2016. https://doi.org/10.1016/S0140-6736(16)00380-9.
- Tak PP. A personalized medicine approach to biologic treatment of rheumatoid arthritis: a preliminary treatment algorithm. Rheumatology 2012;51:600-9. https://doi.org/10.1093/rheumatology/ker300.
- Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. http://dx.doi.org/10.1136/ard.2009.123919.
- Gottenberg J, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D. Therapeutic Strategy in patients with rheumatoid arthritis and insufficient response to a 1st anti-TNF: results of the multicenter randomized controlled ‘ROC’ Trial. [Abstract]. Arthritis Rheum 2013;65.
- D’Agostino RB, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues–the encounters of academic consultants in statistics. Stat Med 2003;22:169-86. https://doi.org/10.1002/sim.1425.
- Buch MH, Pavitt S, Parmar M, Emery P. Creative trial design in RA: optimizing patient outcomes. Nat Rev Rheumatol 2013;9:183-94. http://dx.doi.org/10.1038/nrrheum.2013.5.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU; 2014.
- Marshall JK, Blackhouse G, Goeree R, Brazier N, Irvine E. Infliximab for the Treatment of Crohn’s Disease: A Systematic Review and Cost–Utility Analysis. Ottawa, ON: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 2002.
Appendix 1 Participant information sheet and consent forms
Appendix 2 Participant health social care expenditure
Appendix 3 Study closure patient information sheet
Appendix 4 SWITCH study closure patient information Article for National Rheumatoid Arthritis Society web page and other relevant electronic forums
You may remember that last year we published details of a new clinical trial, called SWITCH, on our website and Facebook page, amongst other areas. This trial was designed to compare three types of drugs in the treatment of RA (abatacept, rituximab and TNF inhibitors).
We are sorry to say this trial will not be able to accept any new participants after all. The study recruited 122 of the 477 participants it was looking to recruit, but unfortunately the study’s funders (the NIHR HTA programme) have had to withdraw their future funding for the study. The reason they made this difficult decision was simply because they could not justify the additional time frame and, therefore, the resource, that would be needed to recruit all the participants to complete the study.
The study organisers are very keen to stress to all the trial participants and anyone else taking the drugs being used in the study that the decision was in no way due to any concerns about the safety of the study; the study is still safe for all current participants to continue with. The decision was purely due to the difficulties in justifying additional finances needed to support finding enough participants including within an acceptable time frame. Unfortunately, without the full numbers of participants entered into the trial, the study team will be unable to reach the significant conclusions they have intended. However, they still hope to be able to look for potential patterns of how well the treatments work which will inform further research in this area, and possibly combine the results with those from other studies, in order to strengthen any findings.
All participants who were still being seen by the research teams will have been contacted to discuss their future treatment. However, if you were previously involved in the trial and have any further questions please contact the research team who were looking after you whilst you were on the study and they will be happy to answer any queries you might have.
Appendix 5 Schedule of events for each treatment
| Event | Study phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Baseline | Interventional | Observational | |||||||||
| Study week 0 (≤ – 4 weeks) | Study week 0 | Study week 2a (+5 days) | Study week 12a | Study week 24a | Study week 26a (+5 days) | Study week 36a | Study week 48a | Study week 60a | Study week 72a | Study week 84a | Study week 96a | |
| Assessment/procedure | ||||||||||||
| Study treatment: rituximab | ✗ | ✗ | ✗ | ✗ | ||||||||
| Informed consent and registration | ✗ | |||||||||||
| Inclusion/exclusion | ✗ | |||||||||||
| Randomisation | ✗ | |||||||||||
| Demographic data | ✗ | |||||||||||
| Medical and recent surgical history | ✗ | |||||||||||
| Pregnancy test (urine) | ✗ | |||||||||||
| Chest radiographyb and 12-lead ECG | ✗ | |||||||||||
| Hepatitis B and C screening | ✗ | |||||||||||
| TB screeningb | ✗ | |||||||||||
| Urinalysis | ✗ | |||||||||||
| Immunoglobulins | ✗ | |||||||||||
| Serological test (RF, ACPA, ANA test and anti-dsDNA antibodies) | ✗ | ✗ | ||||||||||
| Haematology test (FBC); blood chemistry (U&E, LFT); and CRP and ESR | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Glucose and lipid profile | ✗ | ✗ | ✗ | |||||||||
| Unplanned surgery details | ✗ | ✗ | ✗ | ✗ | ||||||||
| Concomitant medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Physical examination and vital signs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 28-joint count (TJC and SJC) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Patient Assessment of General Health VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Patient Global Assessment of Arthritis VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Patient Global Assessment of Pain VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Physician Global Assessment of Disease Activity VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Morning stiffness (minutes) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| HAQ-DI | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| RAQoL | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| HADS | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| EQ-5D | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Health Utilities Index | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Health, social care use and expenditure | ✗ | ✗ | ✗ | ✗ | ||||||||
| Inpatient/outpatient hospital form | ✗ | ✗ | ✗ | ✗ | ||||||||
| Dorsal–posterior radiography of hands and feetc | ✗ | ✗ | ||||||||||
| Bone densitometry scanc | ✗ | ✗ | ||||||||||
| Optional biobank samples | ✗ | ✗d | ✗ | ✗ | ✗ | ✗ | ||||||
| AEs | Monitor during trial treatment | |||||||||||
| Event | Study phase | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Baseline | Interventional | Observational | ||||||||||||||
| Study week 0 (≤ – 4 weeks) | Study week 0 | Study week 2a (±2 days) | Study week 6a (±2 days) | Study week 12a | Study week 14a (±1 week) | Study week 22a (±1 week) | Study week 24a | Study week 30a (±1 week) | Study week 36a | Study week 38a (±1 week) | Study week 46a (±1 week) | Study week 48a | Study week 60a | Study week 72a | Study week 84a | Study week 96a | |
| Assessment/procedure | |||||||||||||||||
| Study treatment: infliximab | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| Informed consent and registration | ✗ | ||||||||||||||||
| Inclusion/exclusion | ✗ | ||||||||||||||||
| Randomisation | ✗ | ||||||||||||||||
| Demographic data | ✗ | ||||||||||||||||
| Medical and recent surgical history | ✗ | ||||||||||||||||
| Pregnancy test (urine) | ✗ | ||||||||||||||||
| Chest radiographyb and 12-lead ECG | ✗ | ||||||||||||||||
| Hepatitis B and C screening | ✗ | ||||||||||||||||
| TB screeningb | ✗ | ||||||||||||||||
| Urinalysis | ✗ | ||||||||||||||||
| Immunoglobulins | ✗ | ||||||||||||||||
| Serological test (RF, ACPA, ANA test and anti-dsDNA antibodies) | ✗ | ✗ | |||||||||||||||
| Haematology test (FBC); blood chemistry (U&E, LFT); and CRP and ESR | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Glucose and lipid profile | ✗ | ✗ | ✗ | ||||||||||||||
| Unplanned surgery details | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Concomitant medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Physical examination and vital signs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 28-joint count (TJC and SJC) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Patient Assessment of General Health VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Patient Global Assessment of Arthritis VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Patient Global Assessment of Pain VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Physician Global Assessment of Disease Activity VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Morning stiffness (minutes) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| HAQ-DI | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| RAQoL | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||
| HADS | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||
| EQ-5D | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Health Utilities Index | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Health, social care use and expenditure | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Inpatient/outpatient hospital form | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Dorsal–posterior radiography of hands and feetc | ✗ | ✗ | |||||||||||||||
| Bone densitometry scanc | ✗ | ✗ | |||||||||||||||
| Optional biobank samples | ✗ | ✗d | ✗ | ✗ | ✗ | ✗ | |||||||||||
| AEs | Monitor during trial treatment | ||||||||||||||||
| Event | Study phase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Baseline | Interventional | Observational | |||||||||
| Study week 0 (≤ – 4 weeks) | Study week 0 | Study week 2a | Study week 4 safety visita | Study week 12a | Study week 24a | Study week 36a | Study week 48a | Study week 60a | Study week 72a | Study week 84a | Study week 96a | |
| Assessment/procedure | ||||||||||||
| Study treatment: subcutaneous IMP | ✗4 | |||||||||||
| Informed consent and registration | ✗ | |||||||||||
| Inclusion/exclusion | ✗ | |||||||||||
| Randomisation | ✗ | |||||||||||
| Demographic data | ✗ | |||||||||||
| Medical and recent surgical history | ✗ | |||||||||||
| Pregnancy test (urine) | ✗ | |||||||||||
| Chest radiographyb and 12-lead ECG | ✗ | |||||||||||
| Hepatitis B and C screening | ✗ | |||||||||||
| TB screeningb | ✗ | |||||||||||
| Urinalysis | ✗ | |||||||||||
| Immunoglobulins | ✗ | |||||||||||
| Serological test (RF, ACPA, ANA test and anti-dsDNA antibodies) | ✗ | ✗ | ||||||||||
| Haematology test (FBC); blood chemistry (U&E, LFT); and CRP and ESR | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Glucose and lipid profile | ✗ | ✗ | ✗ | |||||||||
| Unplanned surgery details | ✗ | ✗ | ✗ | ✗ | ||||||||
| Concomitant medication | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Physical examination and vital signs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| 28-joint count (TJC and SJC) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Patient Assessment of General Health VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Patient Global Assessment of Arthritis VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Patient Global Assessment of Pain VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Physician Global Assessment of Disease Activity VAS | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Morning stiffness (minutes) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| HAQ-DI | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| RAQoL | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| HADS | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| EQ-5D | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Health Utilities Index | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Health, social care use and expenditure | ✗ | ✗ | ✗ | ✗ | ||||||||
| Inpatient/outpatient hospital form | ✗ | ✗ | ✗ | ✗ | ||||||||
| Dorsal–posterior radiography of hands and feetc | ✗ | ✗ | ||||||||||
| Bone densitometry scanc | ✗ | ✗ | ||||||||||
| Optional biobank samples | ✗ | ✗d | ✗ | ✗ | ✗ | ✗ | ||||||
| AEs | Monitor during trial treatment | |||||||||||
Appendix 6 Disease activity response categories
The DAS28 used for the primary end-point analysis is a composite measure of four items:
-
TJC: (range 0–28)
-
SJC: (range 0–28)
-
ESR: (range 0–99)
-
Patient-completed VAS of Global Assessment of Arthritis, to answer the question ‘Considering all of the ways your arthritis has affected you, mark on the line below how you feel your arthritis is today’ (VAS: range ‘very well’ = 0 mm – ‘very poor’ = 100 mm)
With these four items, the DAS28 is calculated in the following manner:
where loge is the natural logarithm function, and x is the square root function.
| Response category | DAS28 values |
|---|---|
| High | > 5.1 |
| Moderate | > 3.2 but ≤ 5.1 |
| Low | > 2.6 but ≤ 3.2 |
| Remission | ≤ 2.6 |
| DAS28 values | DAS28 improvement since baseline | ||
|---|---|---|---|
| > 1.2 | ≤ 1.2 and ≥ 0.6 | < 0.6 | |
| ≤ 3.2 | Good response | ||
| > 3.2 but ≤ 5.1 | Moderate response | Moderate response | |
| > 5.1 | No response | ||
| Disease activity | CDAI values |
|---|---|
| High disease activity | < 22 |
| Moderate disease activity | > 10 but ≤ 22 |
| Low disease activity | > 2.8 but ≤ 10 |
| Remission | ≥ 0 but ≤ 2.8 |
| Disease activity | SDAI values |
|---|---|
| High disease activity | < 26 |
| Moderate disease activity | > 11 but ≤ 26 |
| Low disease activity | > 3.3 but ≤ 11 |
| Remission | ≥ 0 but ≤ 3.3 |
Appendix 7 Statistical analysis plan
Appendix 8 Key protocol amendments
| Version of patient information sheet containing amendment | Additional document created | Description of the protocol amendment |
|---|---|---|
| 4.0 | N/A | Amendment from intravenous formulation of abatacept to subcutaneous formulation following agreement from manufacturers of abatacept to provide trial supplies. This also required changes to other documentation to:
|
| N/A | Abatacept participant letter | Approval was obtained for a letter to provide to participants that explained a discrepancy between the expiry date given on the internal and external packaging of abatacept |
| 4.0 | N/A | Inclusion of the option for subcutaneous IMPs to be sourced and delivered to participants’ homes by third-party home health-care providers as per local hospital practice |
| 5.0 | N/A | Clarification included that when local practice indicates the use of a home health-care provider for the delivery of subcutaneous IMPs, the trial procedures will map onto the established standard care practices in place at each individual site in terms of services and record-keeping and retention requirements |
| 6.0 | N/A | Addition of golimumab to the alternative-mechanism TNFi arm following feedback from sites that the use of golimumab was becoming more commonplace and, therefore, the ability to use this may expand the field of potential patients/increase pragmatism of study would reflect standard practice more closely |
| 6.0 | N/A | Modification of the primary end point from a dichotomous end point (whether or not the patient achieved a reduction of > 1.2 units in the DAS28 with no toxicity) to a continuous end point (change in the DAS28) |
| N/A | Patient advert, version 1.0 | Designed to advertise the trial directly to patients, with the intention that sites display the patient advert in patient waiting rooms, etc. In addition, information contained within the advert was intended to be used via various means, for example patient websites, e-bulletins and social media for the purpose of advertising the trial to the wider RA community |
| N/A | Patient information summary sheet, version 1.0 | A participant information summary sheet was created to summarise and complement the main PIS/ICD before the patient reads the main PIS/ICD following feedback from patient and public involvement representatives that the current PIS/ICD was lengthy and a supplementary summary sheet would be beneficial |
| 7.0 | N/A | Corrections of errors noted in the research ethics committee form and the patient information sheet relating to the amount of radiation that patients would be exposed to as part of the imaging aspects of the study |
| N/A | N/A | Research and development form amended to enable the use of participant identification centres, as a number of investigators suggested that they had clinics at other sites where eligible patients may be seen and who they would be able to refer to their main site in order to screen for participation |
Appendix 9 Screening and withdrawals
| Centre name and number | Patient was ineligible (n = 417) | Patient was eligible but did not consent (n = 90) | Clinician preferred particular treatment (n = 12) | Clinician wishes to continue current treatment, despite non-response (n = 5) | Did not attend (n = 2) | No reason given for non-registration (n = 3) | Considered, registered, but not randomised (n = 27) | Randomised (n = 122) | Total (n = 678) |
|---|---|---|---|---|---|---|---|---|---|
| Chapel Allerton Hospital, Leeds; N00482 | 7 | 5 | – | – | – | – | 8 | 32 | 52 |
| Cannock Chase Hospital; N00473 | 29 | 5 | – | – | – | – | 2 | 8 | 44 |
| Manchester Royal Infirmary; N00080 | 28 | 6 | – | – | – | – | 3 | 6 | 43 |
| Airedale General Hospital; N00074 | 2 | 4 | – | – | 1 | – | 2 | 6 | 15 |
| Derriford Hospital, Plymouth; N00118 | 5 | – | – | – | – | – | 2 | 6 | 13 |
| King George Hospital, Ilford; N00165 | 6 | – | – | – | – | – | 1 | 6 | 13 |
| Queen Elizabeth Hospital, Gateshead; N00071 | 2 | 4 | – | – | – | – | – | 6 | 12 |
| Royal National Hospital for Rheumatic Diseases, Bath; N02220 | 15 | 7 | 1 | – | – | – | 1 | 5 | 29 |
| Birmingham City Hospital; N00346 | 24 | 6 | – | – | – | – | – | 5 | 35 |
| Hull Royal Infirmary; N00078 | 13 | – | – | – | – | – | 1 | 4 | 18 |
| Royal Hallamshire Hospital, Sheffield; N00093 | 20 | 9 | – | – | – | – | – | 4 | 33 |
| Leicester Royal Infirmary; N00031 | 17 | 5 | – | – | – | 2 | – | 4 | 28 |
| Queen’s Hospital, Burton upon Trent; N00178 | 16 | 6 | 1 | – | – | – | – | 4 | 27 |
| University Hospital, North Durham; N00170 | 5 | 1 | 1 | – | – | – | 1 | 3 | 11 |
| Poole Hospital; N00108 | 53 | 2 | – | – | – | 1 | – | 3 | 59 |
| Northampton General Hospital; N00038 | 2 | – | – | – | – | – | – | 3 | 5 |
| New Cross Hospital, Wolverhampton; N00034 | – | – | – | – | – | – | – | 3 | 3 |
| Salford Royal Infirmary; N00400 | – | – | – | – | – | – | – | 3 | 3 |
| Broadgreen Hospital, Liverpool; N00589 | 3 | – | – | – | – | – | – | 2 | 5 |
| Royal Victoria Infirmary, Newcastle; N00072 | 31 | 8 | 4 | – | – | – | 2 | 1 | 46 |
| Nuffield Orthopaedic Centre, Oxford; N00282 | 71 | 1 | 3 | 5 | – | – | 1 | 1 | 82 |
| Royal Derby Hospital; N00168 | 34 | 5 | 1 | – | – | – | – | 1 | 41 |
| Queen Alexandra Hospital, Portsmouth; N00110 | 11 | 3 | – | – | – | – | – | 1 | 15 |
| Guy’s Hospital, London; N00241 | 4 | 2 | – | – | – | – | – | 1 | 7 |
| Darlington Memorial Hospital; N00068 | – | 3 | – | – | – | – | – | 1 | 4 |
| Harrogate District Hospital; N00076 | – | – | – | – | – | – | – | 1 | 1 |
| Bristol Royal Infirmary; N00117 | – | – | – | – | – | – | – | 1 | 1 |
| Musgrove Park Hospital, Taunton; N00306 | – | – | – | – | – | – | – | 1 | 1 |
| Royal London Hospital (Ex Mile End); N01705 | 2 | 2 | – | – | – | – | 1 | – | 5 |
| St Peter’s Hospital, Ashford; N00052 | 2 | – | 1 | – | – | – | 1 | – | 4 |
| Raigmore Hospital, Inverness; N00355 | – | 2 | – | – | – | – | 1 | – | 3 |
| Southend Hospital; N00049 | 14 | 2 | – | – | 1 | – | – | – | 17 |
| Wythenshawe Hospital, Manchester; N00172 | 1 | 2 | – | – | – | – | – | – | 3 |
| Reason for ineligibility | Total (n = 417), n (%) |
|---|---|
| Has not experienced RS disease activity on an initial TNFi agent | 95 (22.8) |
| Has not been on a stable dose of MTX for 28 days prior to screening | 92 (22.1) |
| Has received more than one TNFi drug OR any other biological therapy | 72 (17.3) |
| Has not failed conventional DMARD therapy (including MTX) | 32 (7.7) |
| Has another comorbidity | 23 (5.5) |
| Has not had a diagnosis of RA | 14 (3.4) |
| Has inflammatory joint disease of different origin | 9 (2.2) |
| Is pregnant, lactating or a woman of child-bearing potential | 8 (1.9) |
| Has scheduled or anticipated surgery | 7 (1.7) |
| Has received intra-articular or intramuscular steroid injections | 7 (1.7) |
| No reason provided | 7 (1.7) |
| Is under 18 years of age | 5 (1.2) |
| Is unable to provide written informed consent prior to trial | 4 (1.0) |
| Is unable or unwilling to stop treatment with a prohibited DMARD | 4 (1.0) |
| Has untreated active current or latent TB | 4 (1.0) |
| Is known to have active current or history of recurrent infections. | 3 (0.7) |
| Has received doses of prednisolone > 10 mg/day | 2 (0.5) |
| Has had treatment with any investigational drug in the last 90 days before study drug admin. | 2 (0.5) |
| Has significantly impaired bone marrow function. | 2 (0.5) |
| Is currently on NSAIDs and/or corticosteroids but not an unchanged regimen | 1 (0.2) |
| Unable/unwilling to stop etanercept ≥ 4 weeks or infliximab/adalimumab/certolizumab ≥ 8 weeks prior to study drug administration | 1 (0.2) |
| Has had a major episode of infection | 1 (0.2) |
| Is at significant risk of infection | 1 (0.2) |
| Has severe hypoproteinaemia | 1 (0.2) |
| Other | 20 (4.8) |
| Reason for non-consent | Total (n = 90), n (%) |
|---|---|
| Does not want to be involved in the research | 32 (35.6) |
| Refused without any reason | 19 (21.1) |
| Patient preference for or against one or more treatments | 10 (11.1) |
| Took/taken part in competing study | 9 (10.0) |
| Language difficulties | 4 (4.4) |
| Feels poorly or unwell | 3 (3.3) |
| Patient refused to be randomised | 3 (3.3) |
| Considered study schedule compliance to be burdensome | 2 (2.2) |
| Patient did not respond | 2 (2.2) |
| Other reason | 6 (6.7) |
| Reason for ineligibility | Total, n |
|---|---|
| Persistent RA disease activity | 6 |
| Stable dose of MTX | 4 |
| Stable regimen of NSAIDs | 2 |
| Had or anticipated major surgery | 2 |
| Untreated active current TB | 2 |
| Steroid injections within 28 days before screening | 2 |
| Prior regimens | 2 |
| Participant failed conventional DMARD therapy | 1 |
| Major episode of infection | 1 |
| Significant risk of infection | 1 |
| Recurrent bacterial, viral, fungal or mycobacterial infections | 1 |
| Significantly impaired bone marrow function | 1 |
| Other comorbidities | 1 |
| Patient characteristic | Considered, but not registered (N = 529) | Consented and registered, but not randomised (N = 27) | Randomised (N = 122) | Total (N = 678) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 56.9 (13.67) | 56.9 (14.15) | 56.6 (12.21) | 56.9 (13.41) |
| Range | 2.0–86.0 | 30.4–81.1 | 24.4–81.6 | 2.0–86.0 |
| Missing | 36 | 0 | 0 | 36 |
| Sex, n (%) | ||||
| Male | 103 (19.5) | 5 (18.5) | 21 (17.2) | 129 (19.0) |
| Female | 418 (79.0) | 22 (81.5) | 101 (82.8) | 541 (79.8) |
| Not known | 8 (1.5) | – | – | 8 (1.2) |
| RF status, n (%) | ||||
| RF seropositive | 250 (47.3) | 17 (63.0) | 82 (67.2) | 349 (51.5) |
| RF seronegative | 69 (13.0) | 5 (18.5) | 38 (31.1) | 112 (16.5) |
| Not known | 210 (39.7) | 5 (18.5) | 2 (1.6) | 217 (32.0) |
| ACPA status, n (%) | ||||
| Positive | 154 (29.1) | 16 (59.3) | 76 (62.3) | 246 (36.3) |
| Negative | 58 (11.0) | 4 (14.8) | 35 (28.7) | 97 (14.3) |
| Not known | 317 (59.9) | 7 (25.9) | 11 (9.0) | 335 (49.4) |
| Centre | Patient number | Randomised treatment | Date of randomisation | Date of withdrawal request | Withdrawn from further trial treatment | Follow-up as per trial schedule | Collection of further data from notes | Withdrawal reason |
|---|---|---|---|---|---|---|---|---|
| 00080 | 00023 | Monoclonal antibody | 14 August 2013 | 11 November 2013 | Yes | No | No | Lack of efficacy |
| 00118 | 00060 | Etanercept | 2 January 2014 | 12 May 2015 | Yes | No | Yes | Flares of arthritis and poor response from trial drugs. Patient agrees to switch to a new biologic |
| 00038 | 00126 | Etanercept | 10 September 2014 | 21 May 2015 | N/A | No | Yes | Because of ill health she has missed a lot of time at work and cannot take any more time off to attend research appointments |
| 00118 | 00068 | Abatacept | 12 February 2014 | 12 May 2015 | Yes | No | Yes | Poor response from the randomised medication and worsening arthritis and frequent flares |
| 00118 | 00109 | Abatacept | 18 July 2014 | 12 May 2015 | Yes | No | Yes | Poor response from trial drugs. Flare of arthritis and unwilling to continue in trial. Participant switching to rituximab |
| 00118 | 00109 | Abatacept | 18 July 2014 | 14 April 2015 | Yes | No | No | Participant was requesting to switch biologic because of reduced efficacy and because of a house move. No longer wanted appointments |
| 00178 | 00134 | Abatacept | 9 October 2014 | 12 December 2014 | No | – | – | Owing to chest infection principal investigator wishes to withdraw patient from the study |
| 00117 | 00145 | Abatacept | 17 November 2014 | 14 August 2015 | Yes | Yes | – | Would like to get pregnant |
| 00482 | 00007 | Rituximab | 11 December 2012 | 23 August 2013 | Yes | No | No | Patient does not want further treatment or any follow-up appointments, declined at every level. The patient declined any further treatment from the rheumatology department in general |
| 00080 | 00017 | Rituximab | 13 June 2013 | 17 June 2014 | Yes | No | No | No reason given |
| 00093 | 00135 | Rituximab | 7 October 2014 | 19 March 2015 | Yes | No | Yes | Participant’s choice |
Appendix 10 Per protocol population summary tables
| Reason for exclusion | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Excluded from the PP population | 28 (68.3) | 27 (65.9) | 26 (65.0) | 81 (66.4) |
| Unacceptable eligibility violation | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Week 24 assessment did not occur within 30 weeks of baseline | ||||
| Failed because week 24 visit did not occur | 4 (9.8) | 3 (7.3) | – | 7 (5.7) |
| Failed because week 24 was > 30 weeks from baseline | 5 (12.2) | 2 (4.9) | 7 (17.5) | 14 (11.5) |
| Received additional contraindicated treatment | 10 (24.4) | 9 (22.0) | 4 (10.0) | 23 (18.9) |
| Protocol treatment interrupted for > 28 days | – | 3 (7.3) | – | 3 (2.5) |
| Participant was under- or overdosed | – | – | – | – |
| Received steroid treatment within 6 weeks of an end-point assessment | 10 (24.4) | 13 (31.7) | 12 (30.0) | 35 (28.7) |
| Not compliant with MTX up to week 24 | 5 (12.2) | 3 (7.3) | 4 (10.0) | 12 (9.8) |
| Not compliant with treatment up to week 24 | 10 (24.4) | 12 (29.3) | 5 (12.5) | 27 (22.1) |
| Minimisation factor | Treatment arm, n (%) | Total (N = 41), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 13) | Abatacept (N = 14) | Rituximab (N = 14) | ||
| Centre name and number | ||||
| Chapel Allerton Hospital, Leeds; N00482 | 3 (23.1) | 2 (14.3) | 5 (35.7) | 10 (24.4) |
| King George Hospital, Ilford; N00165 | 2 (15.4) | 2 (14.3) | 1 (7.1) | 5 (12.2) |
| Royal Hallamshire Hospital, Sheffield; N00093 | 2 (15.4) | 1 (7.1) | – | 3 (7.3) |
| Derriford Hospital, Plymouth; N00118 | 1 (7.7) | 1 (7.1) | 1 (7.1) | 3 (7.3) |
| Airedale General Hospital; N00074 | 1 (7.7) | 1 (7.1) | – | 2 (4.9) |
| Hull Royal Infirmary; N00078 | – | 1 (7.1) | 1 (7.1) | 2 (4.9) |
| Poole Hospital; N00108 | 1 (7.7) | – | 1 (7.1) | 2 (4.9) |
| University Hospital, North Durham; N00170 | – | – | 2 (14.3) | 2 (4.9) |
| Broadgreen Hospital, Liverpool; N00589 | 1 (7.7) | 1 (7.1) | – | 2 (4.9) |
| New Cross Hospital, Wolverhampton; N00034 | – | 1 (7.1) | – | 1 (2.4) |
| Darlington Memorial Hospital; N00068 | – | 1 (7.1) | – | 1 (2.4) |
| Queen Elizabeth Hospital, Gateshead; N00071 | 1 (7.7) | – | – | 1 (2.4) |
| Manchester Royal Infirmary; N00080 | – | – | 1 (7.1) | 1 (2.4) |
| Queen Alexandra Hospital, Portsmouth; N00110 | – | – | 1 (7.1) | 1 (2.4) |
| Bristol Royal Infirmary; N00117 | – | 1 (7.1) | – | 1 (2.4) |
| Queen’s Hospital, Burton upon Trent; N00178 | 1 (7.7) | – | – | 1 (2.4) |
| Musgrove Park Hospital, Taunton; N00306 | – | 1 (7.1) | – | 1 (2.4) |
| Salford Royal Infirmary; N00400 | – | – | 1 (7.1) | 1 (2.4) |
| Cannock Chase Hospital; N00473 | – | 1 (7.1) | – | 1 (2.4) |
| Disease duration | ||||
| < 5 years | 3 (23.1) | 4 (28.6) | 4 (28.6) | 11 (26.8) |
| ≥ 5 years | 10 (76.9) | 10 (71.4) | 10 (71.4) | 30 (73.2) |
| RA/ACPA seropositivity | ||||
| RF seropositive and/or anti-CCP seropositive | 12 (92.3) | 9 (64.3) | 12 (85.7) | 33 (80.5) |
| Both RF seronegative and anti-CCP seronegative | 1 (7.7) | 5 (35.7) | 2 (14.3) | 8 (19.5) |
| Non-response category | ||||
| Primary | 4 (30.8) | 6 (42.9) | 8 (57.1) | 18 (43.9) |
| Secondary | 9 (69.2) | 8 (57.1) | 6 (42.9) | 23 (56.1) |
| Patient characteristic | Treatment arm | Total (N = 41) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 13) | Abatacept (N = 14) | Rituximab (N = 14) | ||
| Participant sex, n (%) | ||||
| Male | 4 (30.8) | 1 (7.1) | 6 (42.9) | 11 (26.8) |
| Female | 9 (69.2) | 13 (92.9) | 8 (57.1) | 30 (73.2) |
| Derived patient age (years) | ||||
| Mean (SD) | 55.3 (9.40) | 53.4 (15.00) | 58.1 (11.82) | 55.6 (12.21) |
| Range | 40.8–67.0 | 28.8–76.2 | 41.0–81.1 | 28.8–81.1 |
| Missing | 0 | 0 | 0 | 0 |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 29.8 (5.45) | 29.9 (6.23) | 30.9 (5.34) | 30.2 (5.54) |
| Median (IQR) | 28.3 (27.4–34.1) | 27.9 (24.6–36.8) | 29.0 (26.4–33.5) | 29.0 (25.3–34.9) |
| Missing | 0 | 2 | 1 | 3 |
| Smoking status, n (%) | ||||
| Non-smoking (never smoked) | 3 (23.1) | 6 (42.9) | 7 (50.0) | 16 (39.0) |
| Past smoker | 5 (38.5) | 4 (28.6) | 6 (42.9) | 15 (36.6) |
| Current smoker | 5 (38.5) | 4 (28.6) | 1 (7.1) | 10 (24.4) |
| Prior comorbidities, n (%) | ||||
| Asthma | 3 (23.1) | – | 1 (7.1) | 4 (9.8) |
| Bowel disease | – | – | 1 (7.1) | 1 (2.4) |
| Cancer | – | – | 1 (7.1) | 1 (2.4) |
| Depression | 3 (23.1) | 2 (14.3) | – | 5 (12.2) |
| Diabetes | – | 1 (7.1) | 3 (21.4) | 4 (9.8) |
| Hypercholesterolaemia | – | 4 (28.6) | 4 (28.6) | 8 (19.5) |
| Hypertension | 4 (30.8) | 7 (50.0) | 5 (35.7) | 16 (39.0) |
| Ischaemic heart disease | 1 (7.7) | – | 2 (14.3) | 3 (7.3) |
| Myocardial infarction | – | – | 2 (14.3) | 2 (4.9) |
| Osteoarthritis | 4 (30.8) | 3 (21.4) | 5 (35.7) | 12 (29.3) |
| Peptic ulcer disease | – | 1 (7.1) | 1 (7.1) | 2 (4.9) |
| Peripheral vascular disease | – | – | 1 (7.1) | 1 (2.4) |
| Stroke | – | – | 1 (7.1) | 1 (2.4) |
| Thyroid dysfunction | 3 (23.1) | 3 (21.4) | – | 6 (14.6) |
| Disease activity/treatment history | Treatment arm | Total (N = 41) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 13) | Abatacept (N = 14) | Rituximab (N = 14) | ||
| Disease duration (years) | ||||
| Median (IQR) | 9.3 (5.7–17.5) | 6.5 (4.4–10.6) | 8.1 (4.0–15.3) | 8.0 (4.4–14.3) |
| Range | 2.2–35.2 | 1.1–20.4 | 1.3–33.7 | 1.1–35.2 |
| Missing | 0 | 0 | 0 | 0 |
| TJC (/28) | ||||
| Mean (SD) | 13.2 (5.55) | 17.5 (7.98) | 21.1 (6.96) | 17.4 (7.51) |
| Missing | 0 | 0 | 0 | 0 |
| SJC (/28) | ||||
| Mean (SD) | 8.3 (5.38) | 9.4 (6.16) | 12.3 (8.13) | 10.0 (6.73) |
| Missing | 0 | 0 | 0 | 0 |
| Does the participant experience early-morning stiffness?, n (%) | ||||
| Yes | 12 (92.3) | 14 (100.0) | 14 (100.0) | 40 (97.6) |
| No | 1 (7.7) | – | – | 1 (2.4) |
| ESR (mm/hour) | ||||
| Median (IQR) | 14.0 (8.0–22.0) | 23.5 (12.0–34.0) | 26.5 (15.0–42.0) | 21.0 (11.0–32.0) |
| Missing | 0 | 0 | 0 | 0 |
| CRP level (mg/l) | ||||
| Median (IQR) | 5.0 (3.8–10.1) | 8.5 (5.0–19.0) | 6.5 (6.0–21.0) | 6.0 (4.5–16.5) |
| Range | 1.0–66.0 | 1.0–58.2 | 2.1–78.0 | 1.0–78.0 |
| Type of TNFi failed (derived), n (%) | ||||
| Monoclonal antibody | 10 (76.9) | 7 (50.0) | 8 (57.1) | 25 (61.0) |
| Etanercept | 3 (23.1) | 7 (50.0) | 6 (42.9) | 16 (39.0) |
| Previous TNFi agent, n (%) | ||||
| Adalimumab | 2 (15.4) | 3 (21.4) | 4 (28.6) | 9 (22.0) |
| CZP | 6 (46.2) | 3 (21.4) | – | 9 (22.0) |
| Etanercept | 3 (23.1) | 7 (50.0) | 6 (42.9) | 16 (39.0) |
| Golimumab | 1 (7.7) | – | 1 (7.1) | 2 (4.9) |
| Infliximab | 1 (7.7) | 1 (7.1) | 3 (21.4) | 5 (12.2) |
| Disease activity | Treatment arm | Total (n = 41) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 13) | Abatacept (n = 14) | Rituximab (n = 14) | ||
| DAS28 (baseline) | ||||
| Mean (SD) | 5.3 (0.73) | 6.2 (0.85) | 6.6 (1.22) | 6.0 (1.09) |
| Missing | 0 | 0 | 1 | 1 |
| CDAI | ||||
| Mean (SD) | 32.8 (12.80) | 41.0 (12.97) | 47.0 (13.43) | 40.3 (14.02) |
| Missing | 0 | 1 | 1 | 2 |
| SDAI | ||||
| Mean (SD) | 32.8 (13.22) | 42.6 (13.17) | 48.8 (14.27) | 41.6 (14.76) |
| Missing | 1 | 1 | 1 | 3 |
| Physician Global Assessment of Disease Activity (mm) | ||||
| Median (IQR) | 60.0 (45.0–70.0) | 66.0 (57.3–81.0) | 65.0 (62.0–84.3) | 63.5 (53.5–79.0) |
| Missing | 0 | 1 | 0 | 1 |
| Patient-reported outcome | Treatment arm | Total (n = 41) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 13) | Abatacept (n = 14) | Rituximab (n = 14) | ||
| Patient Global Assessment of Arthritis VAS (mm) | ||||
| Median (IQR) | 68.0 (49.0–76.0) | 69.5 (64.0–81.0) | 74.0 (48.0–88.0) | 72.0 (56.5–81.5) |
| Missing | 0 | 0 | 1 | 1 |
| Patient Assessment of General Health VAS (mm) | ||||
| Median (IQR) | 58.0 (47.0–74.0) | 61.5 (51.0–67.0) | 67.0 (29.0–72.0) | 61.0 (47.5–71.0) |
| Missing | 0 | 0 | 1 | 1 |
| Patient Global Assessment of Pain VAS (mm) | ||||
| Median (IQR) | 66.0 (51.0–78.0) | 73.0 (64.0–79.0) | 78.0 (61.0–95.0) | 73.0 (58.5–86.0) |
| Missing | 0 | 0 | 1 | 1 |
| HAQ-DI score | ||||
| Median (IQR) | 1.9 (1.8–2.1) | 1.8 (1.6–2.0) | 2.0 (1.4–2.5) | 1.9 (1.6–2.1) |
| Missing | 0 | 0 | 1 | 1 |
| RAQoL score | ||||
| Median (IQR) | 22.0 (20.0–24.0) | 21.5 (14.0–28.0) | 21.0 (15.0–24.0) | 21.6 (15.5–25.5) |
| Missing | 0 | 0 | 1 | 1 |
| HADS score | ||||
| Median (IQR) | 13.0 (8.0–19.0) | 16.0 (9.0–22.0) | 11.0 (8.0–16.0) | 13.0 (8.0–20.0) |
| Missing | 0 | 0 | 1 | 1 |
| HADS anxiety score | ||||
| Median (IQR) | 6.0 (4.0–11.0) | 8.5 (6.0–12.0) | 5.0 (4.0–10.0) | 6.5 (4.0–11.5) |
| Missing | 0 | 0 | 1 | 1 |
| HADS depression score | ||||
| Median (IQR) | 6.0 (4.0–8.0) | 7.5 (4.0–9.0) | 6.0 (3.0–10.0) | 6.0 (3.5–9.0) |
| Missing | 0 | 0 | 1 | 1 |
| Visit | Treatment arm | Total (n = 41) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 13) | Abatacept (n = 14) | Rituximab (n = 14) | ||
| DAS28 | ||||
| Baseline | ||||
| Mean (SD) | 5.3 (0.73) | 6.2 (0.85) | 6.6 (1.22) | 6.0 (1.09) |
| Missing | 0 | 0 | 1 | 1 |
| 12 weeks | ||||
| Mean (SD) | 4.2 (0.97) | 4.7 (1.20) | 4.8 (1.27) | 4.6 (1.16) |
| Missing | 1 | 1 | 1 | 3 |
| 24 weeks | ||||
| Mean (SD) | 3.9 (0.99) | 4.6 (1.51) | 4.4 (1.78) | 4.3 (1.46) |
| Missing | 0 | 1 | 1 | 2 |
| 36 weeks | ||||
| Mean (SD) | 3.7 (0.99) | 4.8 (1.44) | 5.1 (1.36) | 4.5 (1.40) |
| Missing | 0 | 1 | 1 | 2 |
| 48 weeks | ||||
| Mean (SD) | 3.8 (1.16) | 4.5 (0.84) | 5.0 (1.44) | 4.5 (1.27) |
| Missing | 3 | 3 | 1 | 7 |
| DAS28 improvement | ||||
| 12 weeks | ||||
| Mean (SD) | 1.0 (1.18) | 1.5 (0.91) | 1.5 (0.97) | 1.4 (1.02) |
| Missing | 1 | 1 | 2 | 4 |
| 24 weeks | ||||
| Mean (SD) | 1.4 (1.08) | 1.6 (1.37) | 2.2 (1.75) | 1.7 (1.42) |
| Missing | 0 | 1 | 2 | 3 |
| 36 weeks | ||||
| Mean (SD) | 1.6 (1.26) | 1.5 (1.33) | 1.5 (1.17) | 1.5 (1.22) |
| Missing | 0 | 1 | 1 | 2 |
| 48 weeks | ||||
| Mean (SD) | 1.6 (1.58) | 1.8 (0.83) | 1.4 (1.40) | 1.6 (1.27) |
| Missing | 3 | 3 | 2 | 8 |
Appendix 11 Supplementary baseline data
| Baseline characteristic | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Centre name and number | ||||
| Chapel Allerton Hospital, Leeds; N00482 | 10 (24.4) | 11 (26.8) | 11 (27.5) | 32 (26.2) |
| Cannock Chase Hospital; N00473 | 2 (4.9) | 2 (4.9) | 4 (10.0) | 8 (6.6) |
| Queen Elizabeth Hospital, Gateshead; N00071 | 2 (4.9) | 1 (2.4) | 3 (7.5) | 6 (4.9) |
| Airedale General Hospital; N00074 | 2 (4.9) | 3 (7.3) | 1 (2.5) | 6 (4.9) |
| Manchester Royal Infirmary; N00080 | 2 (4.9) | – | 4 (10.0) | 6 (4.9) |
| Derriford Hospital, Plymouth; N00118 | 2 (4.9) | 3 (7.3) | 1 (2.5) | 6 (4.9) |
| King George Hospital, Ilford; N00165 | 2 (4.9) | 2 (4.9) | 2 (5.0) | 6 (4.9) |
| Birmingham City Hospital; N00346 | 2 (4.9) | 2 (4.9) | 1 (2.5) | 5 (4.1) |
| Royal National Hospital for Rheumatic Diseases, Bath; N02220 | 1 (2.4) | 3 (7.3) | 1 (2.5) | 5 (4.1) |
| Leicester Royal Infirmary; N00031 | 1 (2.4) | 2 (4.9) | 1 (2.5) | 4 (3.3) |
| Hull Royal Infirmary; N00078 | 1 (2.4) | 1 (2.4) | 2 (5.0) | 4 (3.3) |
| Royal Hallamshire Hospital, Sheffield; N00093 | 2 (4.9) | 1 (2.4) | 1 (2.5) | 4 (3.3) |
| Queen’s Hospital, Burton upon Trent; N00178 | 2 (4.9) | 2 (4.9) | – | 4 (3.3) |
| New Cross Hospital, Wolverhampton; N00034 | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Northampton General Hospital; N00038 | 3 (7.3) | – | – | 3 (2.5) |
| Poole Hospital; N00108 | 2 (4.9) | – | 1 (2.5) | 3 (2.5) |
| University Hospital, North Durham; N00170 | – | 1 (2.4) | 2 (5.0) | 3 (2.5) |
| Salford Royal Infirmary; N00400 | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Broadgreen Hospital, Liverpool; N00589 | 1 (2.4) | 1 (2.4) | – | 2 (1.6) |
| Darlington Memorial Hospital; N00068 | – | 1 (2.4) | – | 1 (0.8) |
| Royal Victoria Infirmary, Newcastle upon Tyne; N00072 | 1 (2.4) | – | – | 1 (0.8) |
| Harrogate District Hospital; N00076 | – | – | 1 (2.5) | 1 (0.8) |
| Queen Alexandra Hospital, Portsmouth; N00110 | – | – | 1 (2.5) | 1 (0.8) |
| Bristol Royal Infirmary; N00117 | – | 1 (2.4) | – | 1 (0.8) |
| Royal Derby Hospital; N00168 | – | 1 (2.4) | – | 1 (0.8) |
| Guy’s Hospital, London; N00241 | 1 (2.4) | – | – | 1 (0.8) |
| Nuffield Orthopaedic Centre, Oxford; N00282 | – | – | 1 (2.5) | 1 (0.8) |
| Musgrove Park Hospital, Taunton; N00306 | – | 1 (2.4) | – | 1 (0.8) |
| Previous DMARDs | ||||
| Azathioprine | ||||
| Previously used, and stopped | 4 (9.8) | 3 (7.3) | – | 7 (5.7) |
| Not used | 36 (87.8) | 38 (92.7) | 39 (97.5) | 113 (92.6) |
| Not known | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Chloroquine | ||||
| Previously used, cessation unknown | 1 (2.4) | – | – | 1 (0.8) |
| Not used | 39 (95.1) | 41 (100.0) | 39 (97.5) | 119 (97.5) |
| Not known | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Ciclosporin | ||||
| Previously used, and stopped | 1 (2.4) | 2 (4.9) | 2 (5.0) | 5 (4.1) |
| Not used | 39 (95.1) | 39 (95.1) | 37 (92.5) | 115 (94.3) |
| Not known | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Hydroxychloroquine | ||||
| Previously used, and stopped | 33 (80.5) | 23 (56.1) | 24 (60.0) | 80 (65.6) |
| Previously used, but unwilling to stop | 1 (2.4) | – | – | 1 (0.8) |
| Previously used, cessation unknown | – | 1 (2.4) | 2 (5.0) | 3 (2.5) |
| Not used | 7 (17.1) | 17 (41.5) | 13 (32.5) | 37 (30.3) |
| Not known | – | – | 1 (2.5) | 1 (0.8) |
| Leflunomide | ||||
| Previously used, and stopped | 11 (26.8) | 4 (9.8) | 11 (27.5) | 26 (21.3) |
| Not used | 29 (70.7) | 37 (90.2) | 28 (70.0) | 94 (77.0) |
| Not known | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Oral/injectable gold | ||||
| Previously used, and stopped | 2 (4.9) | 2 (4.9) | 2 (5.0) | 6 (4.9) |
| Not used | 38 (92.7) | 39 (95.1) | 37 (92.5) | 114 (93.4) |
| Not known | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Sulfasalazine | ||||
| Previously used, and stopped | 23 (56.1) | 32 (78.0) | 34 (85.0) | 89 (73.0) |
| Previously used, but unwilling to stop | 1 (2.4) | – | – | 1 (0.8) |
| Previously used, cessation unknown | – | 1 (2.4) | – | 1 (0.8) |
| Not used | 16 (39.0) | 8 (19.5) | 6 (15.0) | 30 (24.6) |
| Not known | 1 (2.4) | – | – | 1 (0.8) |
| Penicillamine | ||||
| Previously used, and stopped | – | 2 (4.9) | 1 (2.5) | 3 (2.5) |
| Not used | 41 (100.0) | 39 (95.1) | 39 (97.5) | 119 (97.5) |
| Unknown DMARD | ||||
| Previously used, and stopped | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Not used | 40 (97.6) | 41 (100.0) | 39 (97.5) | 120 (98.4) |
| Received steroids within 4 weeks of screening visit | ||||
| None | 32 (78.0) | 33 (80.5) | 33 (82.5) | 98 (80.3) |
| Oral prednisolone | 8 (19.5) | 8 (19.5) | 6 (15.0) | 22 (18.0) |
| Intramuscular methylprednisolone | – | – | 1 (2.5) | 1 (0.8) |
| Intramuscular triamcinolone | 1 (2.4) | – | – | 1 (0.8) |
| NSAIDs 4 weeks prior to screen | ||||
| Yes | 24 (58.5) | 14 (34.1) | 22 (55.0) | 60 (49.2) |
| No | 16 (39.0) | 27 (65.9) | 18 (45.0) | 61 (50.0) |
| Missing | 1 (2.4) | – | – | 1 (0.8) |
Appendix 12 Secondary outcomes
| Exploratory subgroup | Treatment arm | Total | ||
|---|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | ||
| RF/ACPA status | ||||
| RF and ACPA seronegative | n = 5 | n = 10 | n = 7 | n = 22 |
| Mean (SD) | 1.8 (1.63) | 1.2 (2.05) | –0.2 (1.76) | 1.0 (1.91) |
| Median (IQR) | 1.0 (0.9 to 2.7) | 1.3 (–0.1 to 2.5) | 0.3 (–1.2 to 0.9) | 1.0 (0.2 to 1.5) |
| Missing, n | 1 | 2 | 3 | 6 |
| RF or ACPA seropositive | n = 36 | n = 31 | n = 33 | n = 100 |
| Mean (SD) | 1.4 (1.25) | 1.2 (1.65) | 1.5 (1.90) | 1.4 (1.59) |
| Median (IQR) | 1.6 (0.5 to 2.1) | 1.2 (0.1 to 2.2) | 1.4 (0.5 to 2.5) | 1.4 (0.4 to 2.2) |
| Missing, n | 4 | 5 | 5 | 14 |
| Initial alternative TNFi failed | ||||
| Monoclonal antibody | n = 25 | n = 23 | n = 22 | n = 70 |
| Mean (SD) | 1.3 (1.25) | 0.9 (1.58) | 1.1 (1.96) | 1.1 (1.57) |
| Median (IQR) | 1.4 (0.4 to 1.9) | 0.7 (–0.4 to 1.7) | 1.3 (0.4 to 1.9) | 1.2 (0.3 to 1.9) |
| Missing, n | 2 | 4 | 5 | 11 |
| Etanercept | n = 16 | n = 18 | n = 18 | n = 52 |
| Mean (SD) | 1.6 (1.36) | 1.6 (1.85) | 1.5 (1.97) | 1.6 (1.72) |
| Median (IQR) | 1.7 (0.9 to 2.2) | 1.6 (0.4 to 2.6) | 1.5 (–0.2 to 2.4) | 1.6 (0.4 to 2.4) |
| Missing, n | 3 | 3 | 3 | 9 |
| Initial non-responder status | ||||
| Primary non-response | n = 15 | n = 15 | n = 15 | n = 45 |
| Mean (SD) | 1.2 (1.51) | 1.7 (2.14) | 1.7 (1.78) | 1.5 (1.76) |
| Median (IQR) | 1.1 (0.3 to 2.2) | 1.4 (0.0 to 3.3) | 1.5 (0.1 to 3.2) | 1.4 (0.3 to 2.6) |
| Missing, n | 1 | 4 | 3 | 8 |
| Secondary non-response | n = 26 | n = 26 | n = 25 | n = 77 |
| Mean (SD) | 1.5 (1.13) | 1.0 (1.48) | 1.1 (2.05) | 1.2 (1.57) |
| Median (IQR) | 1.6 (0.9 to 2.1) | 1.2 (–0.2 to 1.8) | 0.9 (0.3 to 1.8) | 1.3 (0.3 to 2.0) |
| Missing, n | 4 | 3 | 5 | 12 |
| Visit | Treatment arm, n/N (%) | Total, n/N (%) | ||
|---|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | ||
| Week 12 | 14/34 (41.2) | 19/35 (54.3) | 16/34 (47.1) | 49/103 (47.6) |
| Week 24 | 21/36 (58.3) | 17/34 (50.0) | 17/32 (53.1) | 55/102 (53.9) |
| Week 36 | 18/34 (52.9) | 16/31 (51.6) | 15/29 (51.7) | 49/94 (52.1) |
| Week 48 | 19/30 (63.3) | 18/30 (60.0) | 13/23 (56.5) | 50/83 (60.2) |
| Visit | Time point | |||
|---|---|---|---|---|
| 12 weeks | 24 weeks | 36 weeks | 48 weeks | |
| 12 weeks | 2.11 (1.56 to 2.66) | |||
| 24 weeks | 1.04 (0.63 to 1.44) | 1.69 (1.23 to 2.15) | ||
| 36 weeks | 0.82 (0.38 to 1.25) | 1.05 (0.64 to 1.47) | 1.90 (1.36 to 2.44) | |
| 48 weeks | 0.72 (0.38 to 1.07) | 0.83 (0.49 to 1.16) | 0.74 (0.39 to 1.08) | 1.38 (1.00 to 1.75) |
| Visit | Time point | |||
|---|---|---|---|---|
| 12 weeks | 24 weeks | 36 weeks | 48 weeks | |
| 12 weeks | 1.03 (0.75 to 1.30) | |||
| 24 weeks | 0.40 (0.18 to 0.63) | 1.08 (0.79 to 1.37) | ||
| 36 weeks | 0.28 (0.07 to 0.50) | 0.27 (0.06 to 0.47) | 0.95 (0.70 to 1.21) | |
| 48 weeks | 0.48 (0.26 to 0.70) | 0.44 (0.21 to 0.68) | 0.20 (0.00 to 0.40) | 1.09 (0.80 to 1.38) |
| Visit | Treatment arm, n/N (%) | Total, n/N (%) | ||
|---|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | ||
| ACR20 | ||||
| Week 12 | 12/37 (32.4) | 16/37 (43.2) | 14/37 (37.8) | 42/111 (37.8) |
| Week 24 | 16/36 (44.4) | 11/35 (31.4) | 10/37 (27.0) | 37/108 (34.3) |
| Week 36 | 16/34 (47.1) | 12/32 (37.5) | 11/32 (34.4) | 39/98 (39.8) |
| Week 48 | 17/31 (54.8) | 11/31 (35.5) | 12/28 (42.9) | 40/90 (44.4) |
| ACR50 | ||||
| Week 12 | 6/37 (16.2) | 5/37 (13.5) | 3/39 (7.7) | 14/113 (12.4) |
| Week 24 | 8/37 (21.6) | 7/36 (19.4) | 3/38 (7.9) | 18/111 (16.2) |
| Week 36 | 7/34 (20.6) | 6/32 (18.8) | 3/33 (9.1) | 16/99 (16.2) |
| Week 48 | 9/31 (29.0) | 6/32 (18.8) | 6/29 (20.7) | 21/92 (22.8) |
| ACR70 | ||||
| Week 12 | 1/37 (2.7) | 1/39 (2.6) | 0/40 (0.0) | 2/116 (1.7) |
| Week 24 | 3/37 (8.1) | 3/36 (8.3) | 2/38 (5.3) | 8/111 (7.2) |
| Week 36 | 6/34 (17.6) | 4/32 (12.5) | 0/34 (0.0) | 10/100 (10.0) |
| Week 48 | 5/31 (16.1) | 3/32 (9.4) | 3/30 (10.0) | 11/93 (11.8) |
| Visit | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| DAS28 | ||||
| Baseline | ||||
| Mean score (SD) | 5.9 (1.05) | 6.2 (1.08) | 6.2 (1.28) | 6.1 (1.13) |
| Missing, n | 1 | 3 | 5 | 9 |
| 12 weeks | ||||
| Mean score (SD) | 4.7 (1.33) | 5.0 (1.34) | 5.0 (1.22) | 4.9 (1.29) |
| Missing, n | 7 | 4 | 2 | 13 |
| 24 weeks | ||||
| Mean score (SD) | 4.3 (1.32) | 4.9 (1.60) | 4.9 (1.55) | 4.7 (1.51) |
| Missing, n | 5 | 4 | 3 | 12 |
| 36 weeks | ||||
| Mean score (SD) | 4.0 (1.35) | 4.9 (1.47) | 4.9 (1.25) | 4.6 (1.41) |
| Missing, n | 7 | 8 | 9 | 24 |
| 48 weeks | ||||
| Mean score (SD) | 4.1 (1.58) | 4.8 (1.24) | 4.8 (1.42) | 4.6 (1.44) |
| Missing, n | 11 | 8 | 13 | 32 |
| 60 weeks | ||||
| Mean score (SD) | 3.5 (1.40) | 4.1 (1.44) | 4.8 (1.19) | 4.1 (1.42) |
| Missing, n | 27 | 26 | 29 | 82 |
| 72 weeks | ||||
| Mean score (SD) | 3.4 (1.71) | 4.9 (1.47) | 4.9 (0.93) | 4.5 (1.53) |
| Missing, n | 33 | 28 | 32 | 93 |
| 84 weeks | ||||
| Mean score (SD) | 3.3 (1.46) | 3.8 (1.48) | 5.1 (1.28) | 4.1 (1.54) |
| Missing, n | 35 | 34 | 34 | 103 |
| 96 weeks | ||||
| Mean score (SD) | 2.6 (1.52) | 3.2 (1.34) | 4.8 (2.07) | 3.3 (1.73) |
| Missing, n | 36 | 37 | 37 | 110 |
| Absolute reduction in the DAS28 | ||||
| 12 weeks | ||||
| Mean reduction in score (SD) | 1.1 (1.30) | 1.3 (1.27) | 1.1 (1.16) | 1.1 (1.24) |
| Missing, n | 7 | 6 | 6 | 19 |
| 24 weeks | ||||
| Mean reduction in score (SD) | 1.4 (1.28) | 1.2 (1.72) | 1.3 (1.94) | 1.3 (1.64) |
| Missing, n | 5 | 7 | 8 | 20 |
| 36 weeks | ||||
| Mean reduction in score (SD) | 1.6 (1.36) | 1.2 (1.68) | 1.2 (1.18) | 1.3 (1.42) |
| Missing, n | 7 | 10 | 11 | 28 |
| 48 weeks | ||||
| Mean reduction in score (SD) | 1.6 (1.64) | 1.4 (1.38) | 1.2 (1.49) | 1.4 (1.50) |
| Missing, n | 11 | 11 | 17 | 39 |
| 60 weeks | ||||
| Mean reduction in score (SD) | 2.1 (1.37) | 2.3 (1.31) | 1.3 (1.89) | 2.0 (1.52) |
| Missing, n | 27 | 28 | 30 | 85 |
| 72 weeks | ||||
| Mean reduction in score (SD) | 2.4 (1.53) | 1.4 (1.28) | 1.1 (2.18) | 1.7 (1.63) |
| Missing, n | 33 | 31 | 34 | 98 |
| 84 weeks | ||||
| Mean reduction in score (SD) | 2.6 (1.10) | 1.3 (1.57) | 2.1 (1.04) | 2.1 (1.25) |
| Missing, n | 35 | 37 | 35 | 107 |
| 96 weeks | ||||
| Mean reduction in score (SD) | 3.3 (1.18) | 3.0 (–) | 2.3 (1.58) | 3.0 (1.16) |
| Missing, n | 36 | 40 | 38 | 114 |
| DAS28 category | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Baseline | ||||
| High disease activity (DAS28) | 33 (80.5) | 32 (78.0) | 28 (70.0) | 93 (76.2) |
| Moderate disease activity (DAS28) | 7 (17.1) | 6 (14.6) | 7 (17.5) | 20 (16.4) |
| Missing | 1 (2.4) | 3 (7.3) | 5 (12.5) | 9 (7.4) |
| 12 weeks | ||||
| High disease activity (DAS28) | 11 (26.8) | 16 (39.0) | 18 (45.0) | 45 (36.9) |
| Moderate disease activity (DAS28) | 19 (46.3) | 18 (43.9) | 17 (42.5) | 54 (44.3) |
| Low disease activity (DAS28) | 3 (7.3) | 2 (4.9) | 2 (5.0) | 7 (5.7) |
| Remission (DAS28) | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Missing | 7 (17.1) | 4 (9.8) | 2 (5.0) | 13 (10.7) |
| 24 weeks | ||||
| High disease activity (DAS28) | 9 (22.0) | 15 (36.6) | 20 (50.0) | 44 (36.1) |
| Moderate disease activity (DAS28) | 19 (46.3) | 16 (39.0) | 9 (22.5) | 44 (36.1) |
| Low disease activity (DAS28) | 4 (9.8) | 3 (7.3) | 4 (10.0) | 11 (9.0) |
| Remission (DAS28) | 4 (9.8) | 3 (7.3) | 4 (10.0) | 11 (9.0) |
| Missing | 5 (12.2) | 4 (9.8) | 3 (7.5) | 12 (9.8) |
| 36 weeks | ||||
| High disease activity (DAS28) | 7 (17.1) | 17 (41.5) | 15 (37.5) | 39 (32.0) |
| Moderate disease activity (DAS28) | 17 (41.5) | 12 (29.3) | 13 (32.5) | 42 (34.4) |
| Low disease activity (DAS28) | 5 (12.2) | 2 (4.9) | 1 (2.5) | 8 (6.6) |
| Remission (DAS28) | 5 (12.2) | 2 (4.9) | 2 (5.0) | 9 (7.4) |
| Missing | 7 (17.1) | 8 (19.5) | 9 (22.5) | 24 (19.7) |
| 48 weeks | ||||
| High disease activity (DAS28) | 8 (19.5) | 14 (34.1) | 14 (35.0) | 36 (29.5) |
| Moderate disease activity (DAS28) | 11 (26.8) | 16 (39.0) | 10 (25.0) | 37 (30.3) |
| Low disease activity (DAS28) | 6 (14.6) | 1 (2.4) | 1 (2.5) | 8 (6.6) |
| Remission (DAS28) | 5 (12.2) | 2 (4.9) | 2 (5.0) | 9 (7.4) |
| Missing | 11 (26.8) | 8 (19.5) | 13 (32.5) | 32 (26.2) |
| 60 weeks | ||||
| High disease activity (DAS28) | 1 (2.4) | 5 (12.2) | 4 (10.0) | 10 (8.2) |
| Moderate disease activity (DAS28) | 7 (17.1) | 5 (12.2) | 6 (15.0) | 18 (14.8) |
| Low disease activity (DAS28) | 2 (4.9) | 1 (2.4) | 1 (2.5) | 4 (3.3) |
| Remission (DAS28) | 4 (9.8) | 4 (9.8) | – | 8 (6.6) |
| Missing | 27 (65.9) | 26 (63.4) | 29 (72.5) | 82 (67.2) |
| 72 weeks | ||||
| High disease activity (DAS28) | 1 (2.4) | 5 (12.2) | 3 (7.5) | 9 (7.4) |
| Moderate disease activity (DAS28) | 2 (4.9) | 6 (14.6) | 5 (12.5) | 13 (10.7) |
| Low disease activity (DAS28) | 2 (4.9) | 1 (2.4) | – | 3 (2.5) |
| Remission (DAS28) | 3 (7.3) | 1 (2.4) | – | 4 (3.3) |
| Missing | 33 (80.5) | 28 (68.3) | 32 (80.0) | 93 (76.2) |
| 84 weeks | ||||
| High disease activity (DAS28) | – | 2 (4.9) | 4 (10.0) | 6 (4.9) |
| Moderate disease activity (DAS28) | 3 (7.3) | 3 (7.3) | 1 (2.5) | 7 (5.7) |
| Low disease activity (DAS28) | 1 (2.4) | – | 1 (2.5) | 2 (1.6) |
| Remission (DAS28) | 2 (4.9) | 2 (4.9) | – | 4 (3.3) |
| Missing | 35 (85.4) | 34 (82.9) | 34 (85.0) | 103 (84.4) |
| 96 weeks | ||||
| High disease activity (DAS28) | – | – | 2 (5.0) | 2 (1.6) |
| Moderate disease activity (DAS28) | 1 (2.4) | 3 (7.3) | – | 4 (3.3) |
| Low disease activity (DAS28) | 1 (2.4) | – | – | 1 (0.8) |
| Remission (DAS28) | 3 (7.3) | 1 (2.4) | 1 (2.5) | 5 (4.1) |
| Missing | 36 (87.8) | 37 (90.2) | 37 (92.5) | 110 (90.2) |
| EULAR response category | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| 12 weeks | ||||
| Good response | 4 (9.8) | 3 (7.3) | 1 (2.5) | 8 (6.6) |
| Moderate response | 13 (31.7) | 18 (43.9) | 20 (50.0) | 51 (41.8) |
| No response | 17 (41.5) | 14 (34.1) | 13 (32.5) | 44 (36.1) |
| Missing | 7 (17.1) | 6 (14.6) | 6 (15.0) | 19 (15.6) |
| 24 weeks | ||||
| Good response | 8 (19.5) | 5 (12.2) | 7 (17.5) | 20 (16.4) |
| Moderate response | 18 (43.9) | 15 (36.6) | 13 (32.5) | 46 (37.7) |
| No response | 10 (24.4) | 14 (34.1) | 12 (30.0) | 36 (29.5) |
| Missing | 5 (12.2) | 7 (17.1) | 8 (20.0) | 20 (16.4) |
| 36 weeks | ||||
| Good response | 9 (22.0) | 3 (7.3) | 3 (7.5) | 15 (12.3) |
| Moderate response | 15 (36.6) | 14 (34.1) | 13 (32.5) | 42 (34.4) |
| No response | 10 (24.4) | 14 (34.1) | 13 (32.5) | 37 (30.3) |
| Missing | 7 (17.1) | 10 (24.4) | 11 (27.5) | 28 (23.0) |
| 48 weeks | ||||
| Good response | 11 (26.8) | 2 (4.9) | 2 (5.0) | 15 (12.3) |
| Moderate response | 8 (19.5) | 18 (43.9) | 12 (30.0) | 38 (31.1) |
| No response | 11 (26.8) | 10 (24.4) | 9 (22.5) | 30 (24.6) |
| Missing | 11 (26.8) | 11 (26.8) | 17 (42.5) | 39 (32.0) |
| 60 weeks | ||||
| Good response | 6 (14.6) | 4 (9.8) | – | 10 (8.2) |
| Moderate response | 5 (12.2) | 7 (17.1) | 6 (15.0) | 18 (14.8) |
| No response | 3 (7.3) | 2 (4.9) | 4 (10.0) | 9 (7.4) |
| Missing | 27 (65.9) | 28 (68.3) | 30 (75.0) | 85 (69.7) |
| 72 weeks | ||||
| Good response | 5 (12.2) | 1 (2.4) | – | 6 (4.9) |
| Moderate response | 2 (4.9) | 5 (12.2) | 3 (7.5) | 10 (8.2) |
| No response | 1 (2.4) | 4 (9.8) | 3 (7.5) | 8 (6.6) |
| Missing | 33 (80.5) | 31 (75.6) | 34 (85.0) | 98 (80.3) |
| 84 weeks | ||||
| Good response | 3 (7.3) | – | 1 (2.5) | 4 (3.3) |
| Moderate response | 3 (7.3) | 2 (4.9) | 3 (7.5) | 8 (6.6) |
| No response | – | 2 (4.9) | 1 (2.5) | 3 (2.5) |
| Missing | 35 (85.4) | 37 (90.2) | 35 (87.5) | 107 (87.7) |
| 96 weeks | ||||
| Good response | 4 (9.8) | – | 1 (2.5) | 5 (4.1) |
| Moderate response | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Missing | 36 (87.8) | 40 (97.6) | 38 (95.0) | 114 (93.4) |
| ACR/EULAR Boolean remission | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Baseline | ||||
| No | 39 (95.1) | 38 (92.7) | 35 (87.5) | 112 (91.8) |
| Missing | 2 (4.9) | 3 (7.3) | 5 (12.5) | 10 (8.2) |
| 12 weeks | ||||
| No | 31 (75.6) | 38 (92.7) | 38 (95.0) | 107 (87.7) |
| Missing | 10 (24.4) | 3 (7.3) | 2 (5.0) | 15 (12.3) |
| 24 weeks | ||||
| Yes | 2 (4.9) | 1 (2.4) | – | 3 (2.5) |
| No | 32 (78.0) | 36 (87.8) | 36 (90.0) | 104 (85.2) |
| Missing | 7 (17.1) | 4 (9.8) | 4 (10.0) | 15 (12.3) |
| 36 weeks | ||||
| Yes | 2 (4.9) | – | – | 2 (1.6) |
| No | 30 (73.2) | 34 (82.9) | 34 (85.0) | 98 (80.3) |
| Missing | 9 (22.0) | 7 (17.1) | 6 (15.0) | 22 (18.0) |
| 48 weeks | ||||
| Yes | 1 (2.4) | 1 (2.4) | – | 2 (1.6) |
| No | 30 (73.2) | 31 (75.6) | 28 (70.0) | 89 (73.0) |
| Missing | 10 (24.4) | 9 (22.0) | 12 (30.0) | 31 (25.4) |
| 60 weeks | ||||
| Yes | 1 (2.4) | 2 (4.9) | – | 3 (2.5) |
| No | 12 (29.3) | 13 (31.7) | 11 (27.5) | 36 (29.5) |
| Missing | 28 (68.3) | 26 (63.4) | 29 (72.5) | 83 (68.0) |
| 72 weeks | ||||
| Yes | 1 (2.4) | – | – | 1 (0.8) |
| No | 6 (14.6) | 13 (31.7) | 7 (17.5) | 26 (21.3) |
| Missing | 34 (82.9) | 28 (68.3) | 33 (82.5) | 95 (77.9) |
| 84 weeks | ||||
| Yes | 1 (2.4) | – | – | 1 (0.8) |
| No | 5 (12.2) | 7 (17.1) | 4 (10.0) | 16 (13.1) |
| Missing | 35 (85.4) | 34 (82.9) | 36 (90.0) | 105 (86.1) |
| 96 weeks | ||||
| Yes | 1 (2.4) | 1 (2.4) | – | 2 (1.6) |
| No | 4 (9.8) | 3 (7.3) | 3 (7.5) | 10 (8.2) |
| Missing | 36 (87.8) | 37 (90.2) | 37 (92.5) | 110 (90.2) |
| Visit | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| CDAI score | ||||
| Baseline | ||||
| Median score (IQR) | 38.7 (27.8 to 46.5) | 37.4 (28.3 to 47.9) | 39.3 (29.3 to 51.0) | 38.2 (28.8 to 48.0) |
| Missing, n | 1 | 3 | 4 | 8 |
| 12 weeks | ||||
| Median score (IQR) | 18.5 (11.8 to 37.2) | 17.8 (10.2 to 36.7) | 22.0 (13.6 to 30.2) | 21.2 (12.5 to 35.7) |
| Missing, n | 6 | 2 | 2 | 10 |
| 24 weeks | ||||
| Median score (IQR) | 15.4 (9.1 to 28.5) | 22.1 (8.9 to 36.4) | 23.5 (12.2 to 36.1) | 20.8 (9.8 to 32.4) |
| Missing, n | 6 | 5 | 5 | 16 |
| 36 weeks | ||||
| Median score (IQR) | 14.6 (9.2 to 28.8) | 24.1 (8.5 to 32.3) | 20.6 (13.9 to 30.6) | 18.8 (9.6 to 30.4) |
| Missing, n | 8 | 8 | 6 | 22 |
| 48 weeks | ||||
| Median score (IQR) | 19.3 (5.4 to 25.3) | 17.2 (13.5 to 25.6) | 19.7 (10.8 to 30.9) | 17.7 (9.9 to 28.1) |
| Missing, n | 10 | 9 | 12 | 31 |
| 60 weeks | ||||
| Median score (IQR) | 16.9 (3.4 to 29.8) | 13.1 (6.1 to 26.7) | 16.1 (10.5 to 30.9) | 16.1 (6.1 to 28.5) |
| Missing, n | 27 | 27 | 29 | 83 |
| 72 weeks | ||||
| Median score (IQR) | 7.7 (4.3 to 25.7) | 18.4 (14.6 to 33.5) | 15.1 (12.8 to 31.2) | 15.5 (9.9 to 33.5) |
| Missing, n | 33 | 29 | 32 | 94 |
| 84 weeks | ||||
| Median score (IQR) | 7.6 (3.9 to 17.1) | 11.8 (5.0 to 31.8) | 28.5 (11.4 to 30.9) | 12.6 (4.1 to 29.7) |
| Missing, n | 34 | 33 | 35 | 102 |
| 96 weeks | ||||
| Median score (IQR) | 2.6 (1.2 to 14.0) | 8.3 (5.4 to 15.1) | 28.7 (11.4 to 33.6) | 10.4 (3.0 to 24.8) |
| Missing, n | 36 | 37 | 37 | 110 |
| Change in CDAI score | ||||
| 12 weeks | ||||
| Median change in score (IQR) | –12.4 (–21.4 to –1.6) | –15.6 (–22.6 to –6.8) | –16.2 (–26.5 to –5.8) | –14.6 (–23.1 to –5.7) |
| Missing, n | 6 | 5 | 4 | 15 |
| 24 weeks | ||||
| Median change in score (IQR) | –17.8 (–25.7 to –10.6) | –14.7 (–22.3 to 0.4) | –17.0 (–25.1 to –4.9) | –16.1 (–24.8 to –4.5) |
| Missing, n | 6 | 8 | 8 | 22 |
| 36 weeks | ||||
| Median change in score (IQR) | –17.5 (–23.3 to –11.7) | –15.5 (–24.1 to 0.6) | –19.0 (–26.7 to –8.0) | –17.7 (–24.8 to –6.6) |
| Missing | 8 | 11 | 8 | 27 |
| 48 weeks | ||||
| Median change in score (IQR) | –19.3 (–28.8 to –5.7) | –14.1 (–29.2 to –5.9) | –20.3 (–32.3 to –5.3) | –18.5 (–29.2 to –5.7) |
| Missing, n | 10 | 12 | 15 | 37 |
| 60 weeks | ||||
| Median change in score (IQR) | –19.5 (–27.7 to –10.0) | –20.5 (–31.6 to –7.7) | –12.8 (–38.8 to –9.9) | –19.5 (–31.0 to –9.9) |
| Missing, n | 27 | 27 | 29 | 83 |
| 72 weeks | ||||
| Median change in score (IQR) | –20.0 (–28.4 to –11.1) | –9.3 (–17.3 to –2.1) | –13.3 (–27.0 to –0.7) | –14.5 (–26.3 to –4.5) |
| Missing, n | 33 | 30 | 33 | 96 |
| 84 weeks | ||||
| Median change in score (IQR) | –20.6 (–27.7 to –15.5) | –9.9 (–25.5 to 5.8) | –23.5 (–30.0 to –7.6) | –19.1 (–27.7 to –2.7) |
| Missing, n | 34 | 34 | 35 | 103 |
| 96 weeks | ||||
| Median change in score (IQR) | –25.2 (–32.7 to –19.1) | 1.2 (–16.3 to 4.1) | –12.9 (–18.5 to 1.0) | –16.3 (–25.2 to 1.0) |
| Missing, n | 36 | 38 | 37 | 111 |
| CDAI category | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Baseline | ||||
| High disease activity | 36 (87.8) | 32 (78.0) | 32 (80.0) | 100 (82.0) |
| Moderate disease activity | 4 (9.8) | 5 (12.2) | 4 (10.0) | 13 (10.7) |
| Low disease activity | – | 1 (2.4) | – | 1 (0.8) |
| Missing | 1 (2.4) | 3 (7.3) | 4 (10.0) | 8 (6.6) |
| 12 weeks | ||||
| High disease activity | 15 (36.6) | 18 (43.9) | 18 (45.0) | 51 (41.8) |
| Moderate disease activity | 16 (39.0) | 12 (29.3) | 14 (35.0) | 42 (34.4) |
| Low disease activity | 4 (9.8) | 8 (19.5) | 6 (15.0) | 18 (14.8) |
| Remission | – | 1 (2.4) | – | 1 (0.8) |
| Missing | 6 (14.6) | 2 (4.9) | 2 (5.0) | 10 (8.2) |
| 24 weeks | ||||
| High disease activity | 13 (31.7) | 18 (43.9) | 19 (47.5) | 50 (41.0) |
| Moderate disease activity | 13 (31.7) | 6 (14.6) | 10 (25.0) | 29 (23.8) |
| Low disease activity | 7 (17.1) | 11 (26.8) | 4 (10.0) | 22 (18.0) |
| Remission | 2 (4.9) | 1 (2.4) | 2 (5.0) | 5 (4.1) |
| Missing | 6 (14.6) | 5 (12.2) | 5 (12.5) | 16 (13.1) |
| 36 weeks | ||||
| High disease activity | 13 (31.7) | 18 (43.9) | 15 (37.5) | 46 (37.7) |
| Moderate disease activity | 6 (14.6) | 6 (14.6) | 14 (35.0) | 26 (21.3) |
| Low disease activity | 11 (26.8) | 6 (14.6) | 5 (12.5) | 22 (18.0) |
| Remission | 3 (7.3) | 3 (7.3) | – | 6 (4.9) |
| Missing | 8 (19.5) | 8 (19.5) | 6 (15.0) | 22 (18.0) |
| 48 weeks | ||||
| High disease activity | 11 (26.8) | 11 (26.8) | 13 (32.5) | 35 (28.7) |
| Moderate disease activity | 10 (24.4) | 14 (34.1) | 9 (22.5) | 33 (27.0) |
| Low disease activity | 8 (19.5) | 6 (14.6) | 5 (12.5) | 19 (15.6) |
| Remission | 2 (4.9) | 1 (2.4) | 1 (2.5) | 4 (3.3) |
| Missing | 10 (24.4) | 9 (22.0) | 12 (30.0) | 31 (25.4) |
| 60 weeks | ||||
| High disease activity | 6 (14.6) | 5 (12.2) | 4 (10.0) | 15 (12.3) |
| Moderate disease activity | 2 (4.9) | 2 (4.9) | 5 (12.5) | 9 (7.4) |
| Low disease activity | 3 (7.3) | 5 (12.2) | 2 (5.0) | 10 (8.2) |
| Remission | 3 (7.3) | 2 (4.9) | – | 5 (4.1) |
| Missing | 27 (65.9) | 27 (65.9) | 29 (72.5) | 83 (68.0) |
| 72 weeks | ||||
| High disease activity | 2 (4.9) | 5 (12.2) | 3 (7.5) | 10 (8.2) |
| Moderate disease activity | 2 (4.9) | 5 (12.2) | 4 (10.0) | 11 (9.0) |
| Low disease activity | 3 (7.3) | 2 (4.9) | 1 (2.5) | 6 (4.9) |
| Remission | 1 (2.4) | – | – | 1 (0.8) |
| Missing | 33 (80.5) | 29 (70.7) | 32 (80.0) | 94 (77.0) |
| 84 weeks | ||||
| High disease activity | 1 (2.4) | 3 (7.3) | 3 (7.5) | 7 (5.7) |
| Moderate disease activity | 2 (4.9) | 1 (2.4) | 1 (2.5) | 4 (3.3) |
| Low disease activity | 3 (7.3) | 3 (7.3) | – | 6 (4.9) |
| Remission | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Missing | 34 (82.9) | 33 (80.5) | 35 (87.5) | 102 (83.6) |
| 96 weeks | ||||
| High disease activity | 1 (2.4) | – | 2 (5.0) | 3 (2.5) |
| Moderate disease activity | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Low disease activity | – | 3 (7.3) | – | 3 (2.5) |
| Remission | 3 (7.3) | – | – | 3 (2.5) |
| Missing | 36 (87.8) | 37 (90.2) | 37 (92.5) | 110 (90.2) |
| Visit | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| SDAI score | ||||
| Baseline | ||||
| Median score (IQR) | 39.8 (27.9 to 47.5) | 38.0 (29.7 to 50.4) | 40.6 (29.9 to 52.4) | 39.8 (29.5 to 51.4) |
| Missing, n | 2 | 5 | 5 | 12 |
| 12 weeks | ||||
| Median score (IQR) | 19.4 (12.3 to 38.6) | 19.1 (12.1 to 37.5) | 23.3 (15.8 to 36.1) | 21.0 (13.0 to 37.5) |
| Missing, n | 11 | 3 | 3 | 17 |
| 24 weeks | ||||
| Median score (IQR) | 15.9 (9.6 to 30.7) | 23.1 (9.9 to 38.2) | 23.9 (12.7 to 39.8) | 21.6 (9.9 to 35.1) |
| Missing, n | 8 | 5 | 6 | 19 |
| 36 weeks | ||||
| Median score (IQR) | 14.7 (9.5 to 29.1) | 24.6 (9.5 to 35.4) | 21.4 (14.4 to 31.6) | 19.9 (10.2 to 31.6) |
| Missing, n | 10 | 8 | 6 | 24 |
| 48 weeks | ||||
| Median score (IQR) | 19.8 (6.0 to 25.7) | 19.2 (14.1 to 31.3) | 20.3 (12.0 to 32.1) | 19.4 (11.1 to 29.8) |
| Missing, n | 10 | 10 | 12 | 32 |
| 60 weeks | ||||
| Median score (IQR) | 23.0 (4.8 to 30.3) | 17.2 (6.8 to 28.7) | 16.6 (11.0 to 31.8) | 17.2 (8.0 to 30.3) |
| Missing, n | 28 | 28 | 29 | 85 |
| 72 weeks | ||||
| Median score (IQR) | 11.0 (5.1 to 41.1) | 21.6 (16.1 to 39.0) | 19.3 (11.7 to 38.0) | 18.7 (11.0 to 38.0) |
| Missing, n | 34 | 29 | 33 | 96 |
| 84 weeks | ||||
| Median score (IQR) | 12.7 (4.4 to 19.6) | 10.3 (4.1 to 37.1) | 30.5 (20.5 to 36.6) | 17.2 (6.3 to 31.9) |
| Missing, n | 35 | 34 | 36 | 105 |
| 96 weeks | ||||
| Median score (IQR) | 3.1 (1.7 to 14.5) | 8.8 (5.7 to 15.6) | 29.2 (11.5 to 35.9) | 10.6 (3.3 to 25.3) |
| Missing, n | 36 | 37 | 37 | 110 |
| Change in SDAI score | ||||
| 12 weeks | ||||
| Median change in score (IQR) | –13.6 (–21.4 to 2.7) | –14.9 (–23.1 to –8.5) | –17.2 (–27.4 to –6.0) | –15.1 (–24.3 to –5.3) |
| Missing, n | 12 | 8 | 6 | 26 |
| 24 weeks | ||||
| Median change in score (IQR) | –18.2 (–26.7 to –9.3) | –14.9 (–22.5 to –0.5) | –18.2 (–27.3 to –5.2) | –16.6 (–25.7 to –5.0) |
| Missing, n | 9 | 10 | 9 | 28 |
| 36 weeks | ||||
| Median change in score (IQR) | –19.9 (–24.7 to –11.8) | –15.0 (–23.5 to 0.6) | –19.7 (–28.0 to –8.7) | –18.2 (–25.5 to –6.7) |
| Missing, n | 11 | 12 | 9 | 32 |
| 48 weeks | ||||
| Median change in score (IQR) | –20.1 (–27.2 to –7.9) | –13.7 (–31.2 to –6.3) | –20.1 (–34.0 to –5.3) | –19.7 (–30.5 to –5.6) |
| Missing, n | 11 | 15 | 15 | 41 |
| 60 weeks | ||||
| Median change in score (IQR) | –21.6 (–29.5 to –8.6) | –23.2 (–38.7 to –14.2) | –12.9 (–38.3 to –9.9) | –21.9 (–32.1 to –10.4) |
| Missing, n | 29 | 30 | 29 | 88 |
| 72 weeks | ||||
| Median change in score (IQR) | –20.8 (–31.6 to –9.1) | –10.7 (–19.5 to –2.2) | –16.7 (–26.8 to –2.5) | –13.3 (–26.8 to –2.5) |
| Missing, n | 34 | 31 | 34 | 99 |
| 84 weeks | ||||
| Median change in score (IQR) | –21.7 (–28.8 to –15.0) | –1.3 (–25.4 to 5.8) | –16.9 (–32.9 to –3.9) | –20.7 (–28.8 to –1.3) |
| Missing, n | 35 | 36 | 36 | 107 |
| 96 weeks | ||||
| Median change in score (IQR) | –25.3 (–33.8 to –20.3) | –8.1 (–19.8 to 3.6) | –13.8 (–25.5 to 1.0) | –20.1 (–25.5 to –11.0) |
| Missing, n | 36 | 39 | 37 | 112 |
| SDAI category | Treatment arm, n (%) | Total (N = 122), n (%) | ||
|---|---|---|---|---|
| Alternative TNFi (N = 41) | Abatacept (N = 41) | Rituximab (N = 40) | ||
| Baseline | ||||
| High disease activity | 34 (82.9) | 30 (73.2) | 31 (77.5) | 95 (77.9) |
| Moderate disease activity | 5 (12.2) | 5 (12.2) | 4 (10.0) | 14 (11.5) |
| Low disease activity | – | 1 (2.4) | – | 1 (0.8) |
| Missing | 2 (4.9) | 5 (12.2) | 5 (12.5) | 12 (9.8) |
| 12 weeks | ||||
| High disease activity | 12 (29.3) | 16 (39.0) | 14 (35.0) | 42 (34.4) |
| Moderate disease activity | 14 (34.1) | 16 (39.0) | 17 (42.5) | 47 (38.5) |
| Low disease activity | 4 (9.8) | 5 (12.2) | 6 (15.0) | 15 (12.3) |
| Remission | – | 1 (2.4) | – | 1 (0.8) |
| Missing | 11 (26.8) | 3 (7.3) | 3 (7.5) | 17 (13.9) |
| 24 weeks | ||||
| High disease activity | 11 (26.8) | 17 (41.5) | 13 (32.5) | 41 (33.6) |
| Moderate disease activity | 13 (31.7) | 7 (17.1) | 15 (37.5) | 35 (28.7) |
| Low disease activity | 7 (17.1) | 11 (26.8) | 4 (10.0) | 22 (18.0) |
| Remission | 2 (4.9) | 1 (2.4) | 2 (5.0) | 5 (4.1) |
| Missing | 8 (19.5) | 5 (12.2) | 6 (15.0) | 19 (15.6) |
| 36 weeks | ||||
| High disease activity | 11 (26.8) | 15 (36.6) | 13 (32.5) | 39 (32.0) |
| Moderate disease activity | 8 (19.5) | 9 (22.0) | 16 (40.0) | 33 (27.0) |
| Low disease activity | 9 (22.0) | 7 (17.1) | 5 (12.5) | 21 (17.2) |
| Remission | 3 (7.3) | 2 (4.9) | – | 5 (4.1) |
| Missing | 10 (24.4) | 8 (19.5) | 6 (15.0) | 24 (19.7) |
| 48 weeks | ||||
| High disease activity | 7 (17.1) | 8 (19.5) | 10 (25.0) | 25 (20.5) |
| Moderate disease activity | 13 (31.7) | 18 (43.9) | 12 (30.0) | 43 (35.2) |
| Low disease activity | 9 (22.0) | 4 (9.8) | 5 (12.5) | 18 (14.8) |
| Remission | 2 (4.9) | 1 (2.4) | 1 (2.5) | 4 (3.3) |
| Missing | 10 (24.4) | 10 (24.4) | 12 (30.0) | 32 (26.2) |
| 60 weeks | ||||
| High disease activity | 5 (12.2) | 5 (12.2) | 3 (7.5) | 13 (10.7) |
| Moderate disease activity | 3 (7.3) | 3 (7.3) | 5 (12.5) | 11 (9.0) |
| Low disease activity | 3 (7.3) | 3 (7.3) | 3 (7.5) | 9 (7.4) |
| Remission | 2 (4.9) | 2 (4.9) | – | 4 (3.3) |
| Missing | 28 (68.3) | 28 (68.3) | 29 (72.5) | 85 (69.7) |
| 72 weeks | ||||
| High disease activity | 2 (4.9) | 5 (12.2) | 2 (5.0) | 9 (7.4) |
| Moderate disease activity | 1 (2.4) | 5 (12.2) | 4 (10.0) | 10 (8.2) |
| Low disease activity | 3 (7.3) | 2 (4.9) | 1 (2.5) | 6 (4.9) |
| Remission | 1 (2.4) | – | – | 1 (0.8) |
| Missing | 34 (82.9) | 29 (70.7) | 33 (82.5) | 96 (78.7) |
| 84 weeks | ||||
| High disease activity | 1 (2.4) | 3 (7.3) | 3 (7.5) | 7 (5.7) |
| Moderate disease activity | 2 (4.9) | – | 1 (2.5) | 3 (2.5) |
| Low disease activity | 2 (4.9) | 3 (7.3) | – | 5 (4.1) |
| Remission | 1 (2.4) | 1 (2.4) | – | 2 (1.6) |
| Missing | 35 (85.4) | 34 (82.9) | 36 (90.0) | 105 (86.1) |
| 96 weeks | ||||
| High disease activity | 1 (2.4) | – | 2 (5.0) | 3 (2.5) |
| Moderate disease activity | 1 (2.4) | 1 (2.4) | 1 (2.5) | 3 (2.5) |
| Low disease activity | – | 3 (7.3) | – | 3 (2.5) |
| Remission | 3 (7.3) | – | – | 3 (2.5) |
| Missing | 36 (87.8) | 37 (90.2) | 37 (92.5) | 110 (90.2) |
| Visit | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| HAQ-DI score | ||||
| Baseline | ||||
| Median score (IQR) | 1.9 (1.4–2.1) | 1.9 (1.6–2.3) | 1.9 (1.4–2.3) | 1.9 (1.5–2.1) |
| Missing, n | 1 | 1 | 1 | 3 |
| 12 weeks | ||||
| Median score (IQR) | 1.8 (1.1–2.1) | 1.7 (1.3–2.1) | 1.8 (1.1–2.1) | 1.8 (1.1–2.1) |
| Missing, n | 4 | 2 | 0 | 6 |
| 24 weeks | ||||
| Median score (IQR) | 1.6 (1.1–2.0) | 1.8 (1.0–2.1) | 1.7 (1.0–2.0) | 1.6 (1.0–2.1) |
| Missing, n | 5 | 4 | 2 | 11 |
| 36 weeks | ||||
| Median score (IQR) | 1.6 (1.1–1.9) | 1.7 (1.3–2.3) | 1.5 (1.0–2.1) | 1.6 (1.1–2.0) |
| Missing, n | 7 | 7 | 6 | 20 |
| 48 weeks | ||||
| Median score (IQR) | 1.5 (1.1–1.9) | 1.6 (1.0–2.1) | 1.7 (1.1–2.1) | 1.6 (1.1–2.0) |
| Missing, n | 11 | 7 | 10 | 28 |
| 60 weeks | ||||
| Median score (IQR) | 1.6 (1.0–2.0) | 1.8 (1.3–2.4) | 1.6 (1.0–2.0) | 1.6 (1.1–2.1) |
| Missing, n | 27 | 24 | 30 | 81 |
| 72 weeks | ||||
| Median score (IQR) | 1.3 (0.5–1.9) | 1.6 (1.5–2.4) | 1.4 (0.8–1.7) | 1.6 (0.9–1.9) |
| Missing, n | 33 | 28 | 32 | 93 |
| 84 weeks | ||||
| Median score (IQR) | 1.8 (0.8–2.0) | 1.4 (1.1–1.6) | 1.5 (0.8–2.4) | 1.4 (0.9–1.9) |
| Missing, n | 34 | 32 | 34 | 100 |
| 96 weeks | ||||
| Median score (IQR) | 1.0 (0.4–2.0) | 1.1 (0.8–1.1) | 1.4 (0.4–2.5) | 1.1 (0.4–1.7) |
| Missing, n | 36 | 37 | 37 | 110 |
| RAQoL score | ||||
| Baseline | ||||
| Median score (IQR) | 21.6 (15.0–24.5) | 22.0 (14.0–25.5) | 22.0 (15.0–25.0) | 22.0 (15.0–25.0) |
| Missing, n | 1 | 1 | 2 | 4 |
| 12 weeks | ||||
| Median score (IQR) | 19.0 (11.5–24.0) | 17.0 (11.8–23.0) | 19.0 (12.0–26.0) | 18.5 (12.0–24.0) |
| Missing, n | 5 | 2 | 1 | 8 |
| 24 weeks | ||||
| Median score (IQR) | 18.0 (9.0–24.0) | 20.5 (8.9–26.0) | 20.0 (13.0–25.0) | 19.0 (9.0–25.0) |
| Missing, n | 6 | 7 | 2 | 15 |
| 36 weeks | ||||
| Median score (IQR) | 17.3 (8.0–22.0) | 16.8 (10.3–25.0) | 18.0 (13.0–22.0) | 17.5 (10.0–23.0) |
| Missing, n | 7 | 7 | 7 | 21 |
| 48 weeks | ||||
| Median score (IQR) | 19.0 (9.0–23.0) | 17.5 (11.4–24.0) | 19.5 (12.0–25.0) | 18.4 (11.0–23.0) |
| Missing, n | 11 | 7 | 10 | 28 |
| HADS total score | ||||
| Baseline | ||||
| Median total score (IQR) | 13.5 (8.0–20.0) | 17.0 (10.0–22.0) | 14.0 (11.0–19.0) | 15.0 (10.0–21.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median total score (IQR) | 12.0 (9.0–18.0) | 13.0 (9.0–22.0) | 13.5 (11.0–20.5) | 13.0 (9.0–20.0) |
| Missing, n | 4 | 3 | 0 | 7 |
| 24 weeks | ||||
| Median total score (IQR) | 12.5 (6.0–18.5) | 13.0 (8.0–17.0) | 15.0 (10.0–20.0) | 14.0 (8.0–19.0) |
| Missing, n | 5 | 5 | 3 | 13 |
| 36 weeks | ||||
| Median total score (IQR) | 9.5 (5.0–17.0) | 13.0 (7.0–18.0) | 13.0 (11.0–18.0) | 13.0 (7.0–18.0) |
| Missing | 7 | 8 | 6 | 21 |
| 48 weeks | ||||
| Median total score (IQR) | 12.5 (4.0–19.0) | 12.0 (7.0–16.0) | 13.0 (10.0–20.0) | 13.0 (7.0–19.0) |
| Missing, n | 11 | 8 | 10 | 29 |
| HADS anxiety score | ||||
| Baseline | ||||
| Median anxiety score (IQR) | 7.0 (4.0–10.5) | 9.0 (6.0–12.0) | 8.0 (6.0–11.0) | 8.0 (5.0–11.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median anxiety score (IQR) | 6.0 (4.0–9.0) | 7.0 (5.0–10.0) | 8.0 (6.0–12.0) | 7.0 (5.0–11.0) |
| Missing, n | 4 | 3 | 0 | 7 |
| 24 weeks | ||||
| Median anxiety score (IQR) | 6.0 (3.0–9.5) | 6.0 (4.0–9.5) | 8.0 (6.0–11.0) | 7.0 (4.0–10.0) |
| Missing, n | 5 | 5 | 3 | 13 |
| 36 weeks | ||||
| Median anxiety score (IQR) | 6.0 (3.0–10.0) | 6.0 (4.0–11.0) | 8.0 (6.0–10.0) | 7.0 (3.0–10.0) |
| Missing, n | 7 | 8 | 6 | 21 |
| 48 weeks | ||||
| Median anxiety score (IQR) | 6.0 (3.0–11.0) | 7.0 (4.0–10.0) | 8.0 (5.0–12.0) | 7.0 (4.0–10.0) |
| Missing, n | 11 | 8 | 10 | 29 |
| HADS depression score | ||||
| Baseline | ||||
| Median depression score (IQR) | 6.5 (4.0–9.0) | 7.0 (4.0–10.0) | 6.0 (4.0–9.0) | 6.0 (4.0–9.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median depression score (IQR) | 6.0 (3.0–8.0) | 7.0 (4.0–9.0) | 6.0 (4.0–9.0) | 6.0 (4.0–9.0) |
| Missing, n | 4 | 3 | 0 | 7 |
| 24 weeks | ||||
| Median depression score (IQR) | 6.0 (2.0–9.5) | 6.0 (2.5–9.0) | 6.0 (3.0–9.0) | 6.0 (3.0–9.0) |
| Missing, n | 5 | 5 | 3 | 13 |
| 36 weeks | ||||
| Median depression score (IQR) | 4.0 (2.0–8.0) | 5.0 (4.0–8.0) | 5.5 (4.0–8.0) | 5.0 (3.0–8.0) |
| Missing, n | 7 | 8 | 6 | 21 |
| 48 weeks | ||||
| Median depression score (IQR) | 4.5 (2.0–9.0) | 5.0 (3.0–7.0) | 5.5 (4.0–9.0) | 5.0 (3.0–9.0) |
| Missing, n | 11 | 8 | 10 | 29 |
| Visit | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Patient Global Assessment of Pain VAS | ||||
| Baseline | ||||
| Median, mm (IQR) | 70.5 (59.0–82.5) | 69.5 (57.5–79.0) | 77.0 (55.0–85.0) | 71.0 (58.0–81.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median, mm (IQR) | 47.5 (27.0–64.0) | 42.0 (22.0–63.0) | 50.0 (39.0–79.0) | 47.5 (30.0–70.0) |
| Missing, n | 5 | 2 | 1 | 8 |
| 24 weeks | ||||
| Median, mm (IQR) | 47.0 (30.5–67.0) | 54.0 (23.0–68.0) | 61.0 (42.0–74.0) | 51.0 (28.0–70.0) |
| Missing, n | 5 | 4 | 3 | 12 |
| 36 weeks | ||||
| Median, mm (IQR) | 40.0 (21.0–60.0) | 38.0 (19.0–72.0) | 56.0 (41.0–63.0) | 48.5 (24.0–63.0) |
| Missing, n | 7 | 7 | 6 | 20 |
| 48 weeks | ||||
| Median, mm (IQR) | 49.0 (22.0–66.0) | 51.5 (19.0–72.0) | 57.0 (34.0–67.0) | 51.0 (22.0–68.0) |
| Missing, n | 10 | 7 | 10 | 27 |
| 60 weeks | ||||
| Median, mm (IQR) | 64.0 (32.0–71.0) | 48.5 (15.5–63.5) | 47.0 (27.0–83.0) | 58.0 (27.0–71.0) |
| Missing, n | 27 | 25 | 29 | 81 |
| 72 weeks | ||||
| Median, mm (IQR) | 42.0 (12.5–53.0) | 56.0 (31.0–87.0) | 52.0 (24.0–85.0) | 45.0 (26.0–62.5) |
| Missing, n | 33 | 28 | 33 | 94 |
| 84 weeks | ||||
| Median, mm (IQR) | 57.0 (16.0–71.0) | 41.0 (14.0–66.0) | 67.0 (29.0–85.0) | 57.0 (16.0–71.0) |
| Missing, n | 34 | 32 | 34 | 100 |
| 96 weeks | ||||
| Median, mm (IQR) | 30.0 (7.0–36.0) | 30.5 (3.0–60.0) | 36.0 (31.0–89.0) | 33.5 (7.0–53.0) |
| Missing, n | 36 | 37 | 37 | 110 |
| Patient Global Assessment of Arthritis VAS | ||||
| Baseline | ||||
| Median, mm (IQR) | 70.5 (62.0–83.0) | 67.5 (52.0–79.5) | 74.0 (53.0–85.0) | 71.0 (56.0–83.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median, mm (IQR) | 51.5 (32.0–71.0) | 43.0 (27.0–60.0) | 49.0 (42.0–78.0) | 48.0 (30.0–69.0) |
| Missing, n | 5 | 2 | 1 | 8 |
| 24 weeks | ||||
| Median, mm (IQR) | 45.5 (26.5–68.0) | 48.0 (20.0–66.0) | 52.0 (39.0–70.0) | 48.0 (31.0–69.0) |
| Missing, n | 5 | 4 | 3 | 12 |
| 36 weeks | ||||
| Median, mm (IQR) | 41.5 (21.0–60.0) | 49.0 (27.1–71.0) | 51.5 (39.0–62.0) | 49.0 (28.0–66.0) |
| Missing, n | 7 | 7 | 6 | 20 |
| 48 weeks | ||||
| Median, mm (IQR) | 47.0 (22.0–69.0) | 55.5 (25.0–68.0) | 55.0 (35.0–70.0) | 53.0 (26.0–69.0) |
| Missing, n | 10 | 7 | 10 | 27 |
| 60 weeks | ||||
| Median, mm (IQR) | 60.0 (21.0–69.0) | 44.0 (21.0–71.5) | 66.0 (27.0–77.0) | 54.0 (24.0–70.0) |
| Missing, n | 27 | 25 | 29 | 81 |
| 72 weeks | ||||
| Median, mm (IQR) | 40.0 (18.0–54.5) | 48.0 (38.0–77.0) | 56.5 (19.5–64.5) | 47.0 (29.0–65.0) |
| Missing, n | 33 | 28 | 32 | 93 |
| 84 weeks | ||||
| Median, mm (IQR) | 51.0 (18.0–64.0) | 56.0 (24.0–63.0) | 53.0 (24.0–85.0) | 53.5 (24.0–72.0) |
| Missing, n | 34 | 32 | 34 | 100 |
| 96 weeks | ||||
| Median, mm (IQR) | 25.0 (11.0–35.0) | 32.5 (6.0–57.5) | 43.0 (24.0–81.0) | 30.0 (10.5–54.5) |
| Missing, n | 36 | 37 | 37 | 110 |
| Patient Global Health Assessment of General Health | ||||
| Baseline | ||||
| Median, mm (IQR) | 56.5 (45.5–72.0) | 62.0 (47.8–68.5) | 61.0 (46.0–74.0) | 59.0 (47.0–70.0) |
| Missing, n | 1 | 1 | 3 | 5 |
| 12 weeks | ||||
| Median, mm (IQR) | 46.5 (25.5–64.5) | 46.0 (23.0–60.0) | 53.0 (34.0–70.0) | 50.0 (28.0–64.0) |
| Missing, n | 5 | 2 | 1 | 8 |
| 24 weeks | ||||
| Median, mm (IQR) | 46.0 (32.5–63.0) | 48.0 (24.0–68.0) | 42.0 (27.0–65.0) | 47.0 (27.0–64.0) |
| Missing, n | 5 | 4 | 3 | 12 |
| 36 weeks | ||||
| Median, mm (IQR) | 38.5 (19.0–55.0) | 55.0 (30.0–70.0) | 49.0 (40.0–58.0) | 48.0 (29.0–60.0) |
| Missing | 7 | 7 | 7 | 21 |
| 48 weeks | ||||
| Median, mm (IQR) | 48.0 (26.0–63.0) | 49.0 (27.0–67.0) | 52.0 (31.0–64.0) | 50.0 (28.0–63.0) |
| Missing, n | 11 | 7 | 10 | 28 |
| 60 weeks | ||||
| Median, mm (IQR) | 54.5 (24.0–61.0) | 43.0 (22.0–55.0) | 46.0 (24.0–65.0) | 47.5 (24.0–61.0) |
| Missing, n | 27 | 24 | 29 | 80 |
| 72 weeks | ||||
| Median, mm (IQR) | 43.5 (21.5–56.0) | 50.0 (33.0–75.0) | 50.0 (25.0–72.0) | 48.5 (27.5–69.5) |
| Missing, n | 33 | 28 | 33 | 94 |
| 84 weeks | ||||
| Median, mm (IQR) | 46.0 (18.0–60.0) | 46.0 (31.0–55.0) | 74.5 (66.0–82.0) | 49.5 (31.0–71.0) |
| Missing, n | 34 | 32 | 34 | 100 |
| 96 weeks | ||||
| Median, mm (IQR) | 24.0 (20.0–37.0) | 20.5 (10.0–31.0) | 43.0 (20.0–89.0) | 22.5 (20.0–42.0) |
| Missing, n | 36 | 37 | 37 | 110 |
| Bone densitometry parameter | Treatment arm | Total (n = 55) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 17) | Abatacept (n = 20) | Rituximab (n = 18) | ||
| Baseline | ||||
| Bone densitometry scan been performed, n (%) | ||||
| Yes | 11 (64.7) | 12 (60.0) | 10 (55.6) | 33 (60.0) |
| No | 6 (35.3) | 8 (40.0) | 8 (44.4) | 22 (40.0) |
| Neck of femur densitometry | ||||
| Median t-score (IQR) | –0.8 (–1.6 to –0.2) | –0.5 (–0.8 to 0.2) | –1.0 (–1.3 to 0.3) | –0.7 (–1.3 to 0.1) |
| Missing, n | 6 | 9 | 8 | 23 |
| Neck of femur densitometry | ||||
| Median z-score (IQR) | –0.6 (–0.8 to 0.4) | 0.4 (0.0 to 1.1) | 0.4 (–0.7 to 0.7) | 0.4 (–0.7 to 0.8) |
| Missing, n | 7 | 9 | 8 | 24 |
| Spine densitometry | ||||
| Median t-score (IQR) | –0.2 (–1.9 to 1.2) | 0.4 (–0.8 to 1.1) | –1.3 (–1.8 to 0.5) | –0.3 (–1.3 to 1.1) |
| Missing, n | 6 | 9 | 8 | 23 |
| Spine densitometry | ||||
| Median z-score (IQR) | 0.2 (–0.4 to 1.2) | 1.6 (0.6 to 2.7) | 0.3 (–0.2 to 1.5) | 0.6 (–0.1 to 2.1) |
| Missing, n | 7 | 9 | 8 | 24 |
| Week 48 | ||||
| Bone densitometry scan been performed, n (%) | ||||
| Yes | 6 (35.3) | 5 (25.0) | 3 (16.7) | 14 (25.5) |
| No | 11 (64.7) | 15 (75.0) | 15 (83.3) | 41 (74.5) |
| Neck of femur densitometry | ||||
| Median t-score (IQR) | –0.8 (–2.1 to 0.4) | –0.7 (–0.8 to –0.7) | –0.3 (–2.0 to 1.5) | –0.7 (–1.1 to –0.3) |
| Missing, n | 11 | 15 | 15 | 41 |
| Neck of femur densitometry | ||||
| Median z-score (IQR) | –0.7 (–1.6 to –0.7) | 0.7 (0.0 to 0.9) | 0.1 (–1.2 to 0.6) | –0.1 (–0.7 to 0.6) |
| Missing, n | 12 | 15 | 15 | 42 |
| Spine densitometry | ||||
| Median t-score (IQR) | –1.2 (–1.9 to –0.1) | 0.5 (–2.2 to 2.4) | –0.9 (–2.2 to –0.3) | –0.9 (–1.9 to –0.1) |
| Missing, n | 11 | 16 | 15 | 42 |
| Spine densitometry | ||||
| Median z-score (IQR) | –0.7 (–1.2 to 0.0) | 1.8 (0.5 to 4.1) | 0.0 (–1.3 to 0.7) | 0.0 (–1.0 to 0.7) |
| Missing, n | 11 | 16 | 15 | 42 |
Appendix 13 Data missingness
| Component (visit) | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Alternative TNFi | Abatacept | Rituximab | ||||
| n | % | n | % | n | % | |
| CRP level | ||||||
| Baseline | 1 | 2.4 | 2 | 4.9 | 1 | 2.5 |
| 12 weeks | 9 | 22.0 | 3 | 7.3 | 1 | 2.5 |
| 24 weeks | 6 | 14.6 | 3 | 7.3 | 1 | 2.5 |
| 36 weeks | 9 | 22.0 | 7 | 17.1 | 5 | 12.5 |
| 48 weeks | 10 | 24.4 | 8 | 19.5 | 9 | 22.5 |
| ESR | ||||||
| Baseline | 0 | 0.0 | 2 | 4.9 | 2 | 5.0 |
| 12 weeks | 5 | 12.2 | 4 | 9.8 | 1 | 2.5 |
| 24 weeks | 4 | 9.8 | 3 | 7.3 | 1 | 2.5 |
| 36 weeks | 7 | 17.1 | 8 | 19.5 | 8 | 20.0 |
| 48 weeks | 11 | 26.8 | 7 | 17.1 | 10 | 25.0 |
| HAQ-DI | ||||||
| Baseline | 1 | 2.4 | 1 | 2.4 | 1 | 2.5 |
| 12 weeks | 4 | 9.8 | 2 | 4.9 | 0 | 0.0 |
| 24 weeks | 5 | 12.2 | 4 | 9.8 | 2 | 5.0 |
| 36 weeks | 7 | 17.1 | 7 | 17.1 | 6 | 15.0 |
| 48 weeks | 11 | 26.8 | 7 | 17.1 | 10 | 25.0 |
| Patient Global Assessment of Arthritis VAS | ||||||
| Baseline | 1 | 2.4 | 1 | 2.4 | 3 | 7.5 |
| 12 weeks | 5 | 12.2 | 2 | 4.9 | 1 | 2.5 |
| 24 weeks | 5 | 12.2 | 4 | 9.8 | 3 | 7.5 |
| 36 weeks | 7 | 17.1 | 7 | 17.1 | 6 | 15.0 |
| 48 weeks | 10 | 24.4 | 7 | 17.1 | 10 | 25.0 |
| Patient Global Assessment of Pain VAS | ||||||
| Baseline | 1 | 2.4 | 1 | 2.4 | 3 | 7.5 |
| 12 weeks | 5 | 12.2 | 2 | 4.9 | 1 | 2.5 |
| 24 weeks | 5 | 12.2 | 4 | 9.8 | 3 | 7.5 |
| 36 weeks | 7 | 17.1 | 7 | 17.1 | 6 | 15.0 |
| 48 weeks | 10 | 24.4 | 7 | 17.1 | 10 | 25.0 |
| Physician Global Assessment of Disease Activity VAS | ||||||
| Baseline | 0 | 0.0 | 2 | 4.9 | 1 | 2.5 |
| 12 weeks | 5 | 12.2 | 2 | 4.9 | 1 | 2.5 |
| 24 weeks | 5 | 12.2 | 4 | 9.8 | 3 | 7.5 |
| 36 weeks | 8 | 19.5 | 8 | 19.5 | 5 | 12.5 |
| 48 weeks | 10 | 24.4 | 8 | 19.5 | 10 | 25.0 |
| SJC | ||||||
| Baseline | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 |
| 12 weeks | 4 | 9.8 | 2 | 4.9 | 0 | 0.0 |
| 24 weeks | 4 | 9.8 | 3 | 7.3 | 1 | 2.5 |
| 36 weeks | 7 | 17.1 | 7 | 17.1 | 5 | 12.5 |
| 48 weeks | 10 | 24.4 | 8 | 19.5 | 10 | 25.0 |
| TJC | ||||||
| Baseline | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 |
| 12 weeks | 4 | 9.8 | 2 | 4.9 | 0 | 0.0 |
| 24 weeks | 4 | 9.8 | 3 | 7.3 | 1 | 2.5 |
| 36 weeks | 7 | 17.1 | 7 | 17.1 | 5 | 12.5 |
| 48 weeks | 10 | 24.4 | 8 | 19.5 | 10 | 25.0 |
| Patient-level missingness | Treatment arm | Total (n = 122) | ||
|---|---|---|---|---|
| Alternative TNFi (n = 41) | Abatacept (n = 41) | Rituximab (n = 40) | ||
| Missing DAS28 components (up to week 48 per patient) | ||||
| Mean, n (SD) | 2.6 (4.94) | 2.1 (3.78) | 2.0 (2.98) | 2.2 (3.97) |
| Median, n (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–4.0) | 0.0 (0.0–3.5) | 0.0 (0.0–4.0) |
| Range | 0.0–17.0 | 0.0–16.0 | 0.0–12.0 | 0.0–17.0 |
| Missing, n | 0 | 0 | 0 | 0 |
| All DAS28 components completed over 48 weeks, n (%) | ||||
| All completed | 26 (63.4) | 24 (58.5) | 21 (52.5) | 71 (58.2) |
| One or more incomplete | 15 (36.6) | 17 (41.5) | 19 (47.5) | 51 (41.8) |
| Missing ACR response components (up to week 48 per patient) | ||||
| Mean, n (SD) | 5.5 (9.85) | 4.2 (7.53) | 4.0 (6.00) | 4.6 (7.93) |
| Median, n (IQR) | 1.0 (0.0–8.0) | 1.0 (0.0–8.0) | 1.0 (0.0–8.0) | 1.0 (0.0–8.0) |
| Range | 0.0–35.0 | 0.0–32.0 | 0.0–23.0 | 0.0–35.0 |
| Missing, n | 0 | 0 | 0 | 0 |
| All ACR response components completed over 48 weeks, n (%) | ||||
| All completed | 19 (46.3) | 19 (46.3) | 19 (47.5) | 57 (46.7) |
| One or more incomplete | 22 (53.7) | 22 (53.7) | 21 (52.5) | 65 (53.3) |
| Missing ACR response components (up to week 24 per patient) | ||||
| Mean, n (SD) | 2.0 (4.85) | 1.4 (3.66) | 0.8 (1.74) | 1.4 (3.66) |
| Median, n (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) |
| Range | 0.0–19.0 | 0.0–16.0 | 0.0–7.0 | 0.0–19.0 |
| Missing, n | 0 | 0 | 0 | 0 |
| All ACR response components completed over 24 weeks, n (%) | ||||
| All completed | 27 (65.9) | 29 (70.7) | 29 (72.5) | 85 (69.7) |
| One or more incomplete | 14 (34.1) | 12 (29.3) | 11 (27.5) | 37 (30.3) |
Appendix 14 Safety line listings
| Treatment Randomised | Medical Dictionary for Regulatory Activities’ System Organ Class | SAE code | SAE in medical terms | SAE description | Age (years) | Sex | Seriousness criteria | Causality | Suspected to be related to | Expectedness | Date of registration | Randomisation date | Date event became serious |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative TNFi | Hepatobiliary disorders | N00072/00024/001 | Autoimmune Hepatitis | Persistently raised/rising liver enzyme levels despite cessation of MTX co-therapy | 34 | Female | 3 | Trial medications | Infliximab | Expected | 7 August 2013 | 4 September 2013 | 27 April 2014 |
| Abatacept | Skin and subcutaneous tissue disorders | N00400/00074/001 | Angiooedema | Participant woke up feeling unwell after first injection of abatacept, husband confirmed she had signs of angiooedema (swollen tongue, swollen face) and swollen hands. No rash, pruritus, no other symptoms/signs reported. It is unclear if breathing was somehow compromised but if affirmative likely not to be severe, as patient took co-codamol and went to bed again. The principal investigator has informed patient of the potential seriousness of symptoms and strongly advised to seek medical attention if happens again | 43 | Female | 6 | Trial medications | Abatacept | Expected | 4 March 2014 | 4 March 2014 | 10 April 2014 |
| Abatacept | Infections and infestations | N02220/00077/001 | Pneumonia | Pneumonia leading to sepsis. Stroke – left middle cerebral artery | 81 | Female | 1–3 | Trial medications, 998 to 999 (COPD, smoking history) | Abatacept | Expected | 12 March 2014 | 31 March 2014 | 10 October 2014 |
| Treatment Randomised | Medical Dictionary for Regulatory Activities’ System Organ Class | SAE code | SAE in medical terms | Recovery date | Duration (days) | Outcome | First ever trial medication | Product form | First trial dose | Date most recent dose | Most recent dosing schedule | Most recent route |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative TNFi | Hepatobiliary disorders | N00072/00024/001 | Autoimmune hepatitis | 30 September 2014 | 157 | Recovered with sequelae | Infliximab | Intravenous | 10 September 2013 | 10 February 2014 | 261 mg | Intravenous |
| Abatacept | Skin and subcutaneous tissue disorders | N00400/00074/001 | Angiooedema | 11 April 2014 | 2 | Recovered | Abatacept | Subcutaneous injection | 9 April 2014 | 8 July 2014 | 125 mg, weekly | Subcutaneous |
| Abatacept | Infections and infestations | N02220/00077/001 | Pneumonia | Death | Abatacept | Subcutaneous injection | 31 March 2014 |
| Treatment Randomised | Medical Dictionary for Regulatory Activities’ System Organ Class | SAE code | SAE in medical terms | SAE description | Age | Sex | Seriousness criteria | Causality | Date of registration | Randomisation date | Date event became serious | Recovery date |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abatacept | N00168/00146/001 | Chest pain/epigastric pain | Constant epigastric/chest pain for past 2 weeks. Increasingly worse. Prior admission to King’s Mill Hospital and discharged with no diagnosis. Today (5 February 2015) seen in the accident and emergency department at Royal Derby Hospital and admitted for surgical assessment. Patient was admitted to hospital for a third occasion on 22 February 2015 for the same medical condition. 24 hours stay. Nothing abnormal detected. Investigations continue | 36 | Female | 3 | 10 November 2014 | 11 November 2014 | 25 January 2015 | |||
| Abatacept | Infections and infestations | N00178/00134/001 | Chest Infection | Persistent cough, chest Infection | 81 | Female | 3 | 999 (contact with family with viral chest infection) | 24 September 2014 | 9 October 2014 | 11 December 2014 | 29 December 2014 |
| Rituximab | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N00071/00037/001 | Malignant melanoma | Presented to the accident and emergency department with fungating and partially necrotic mass posteromedial knee. Magnetic resonance imaging highly suggestive of sarcoma. Referred to Freeman Hospital. Biopsies confirm melanoma with positive groin nodes. Referred for surgery | 66 | Male | 1–3 | 999 [Humira (previous medication)] | 4 October 2013 | 14 October 13 | 27 February 14 | |
| Rituximab | Musculoskeletal and connective tissue disorders | N00473/00045/001 | Flare of RA | Admitted via the accident and emergency department with ‘flare of RA’ suspected to have underlying chest infection. CRP level > 100. Secondary to left basal pneumonia. Signs/symptoms = generalised joint pain. Admitted 24 January 2014. Discharged 29 January 2014 | 58 | Male | 3 | 998 (pneumonia) | 5 November 2013 | 13 November 2013 | 24 January 2014 | 29 January 14 |
| Rituximab | Respiratory, thoracic and mediastinal disorders | N00473/00045/002 | Left basal pneumonia | Flu-like symptoms, pyrexia, pleuritic chest pain and productive cough. Admitted 24 January 2014. Discharged 29 January 2014 | 58 | Male | 3 | 999 (community acquired) | 5 November 2013 | 13 November 2013 | 24 January 2014 | 10 February 2014 |
| Rituximab | Musculoskeletal and connective tissue disorders | N00473/00114/001 | Collapse and broken coccyx | As a result of collapse in the bathroom the patient sustained a broken coccyx | 75 | Female | 3 | 998 (suspected neurological condition) | 25 July 2014 | 7 August 2014 | 22 January 2015 | 27 January 2015 |
| Rituximab | Gastrointestinal disorders | N00482/00008/001 | Abdominal pain | Vomiting and two episodes of diarrhoea | 53 | Female | 3 | 999 (scarring from hysterectomy) | 8 January 2013 | 22 January 2013 | 7 May 2013 | 8 May 2013 |
| Treatment Randomised | Medical Dictionary for Regulatory Activities’ System Organ Class | SAE code | SAE in medical terms | Duration (days) | Outcome | First ever trial medication | Product form | First trial dose | Date most recent dose | Most recent dosing schedule | Most recent route |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abatacept | N00168/00146/001 | Chest pain/epigastric pain | Condition still present and unchanged | Abatacept | Subcutaneous injection | 14 November 2014 | 4 February 2015 | 125 mg weekly | Subcutaneous | ||
| Abatacept | Infections and infestations | N00178/00134/001 | Chest infection | 19 | Recovered | Abatacept | Subcutaneous injection | 9 October 2014 | 27 November 2014 | 125 mg weekly | Subcutaneous |
| Rituximab | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N00071/00037/001 | Malignant melanoma | Death | Rituximab | Intravenous | 23 October 2013 | 6 November 2013 | 1 g | Intravenous | |
| Rituximab | Musculoskeletal and connective tissue disorders | N00473/00045/001 | Flare of RA | 6 | Recovered | a | . . . | ||||
| Rituximab | Respiratory, thoracic and mediastinal disorders | N00473/00045/002 | Left basal pneumonia | 18 | Recovered | a | . . . | ||||
| Rituximab | Musculoskeletal and connective tissue disorders | N00473/00114/001 | Collapse and broken coccyx | 6 | Recovered with sequelae | Rituximab | Intravenous | 4 September 2014 | 24 September 2014 | 1 g | Intravenous |
| Rituximab | Gastrointestinal disorders | N00482/00008/001 | Abdominal pain | 2 | Recovered | Rituximab | Intravenous | 24 January 2013 | 7 February 2013 | 1 g | Intravenous |
| Treatment randomised | Centre name and number | Patient number | First reported | AE description | New or pre-existing event? | Intensity | Causality | Expectedness | (For infections) Requested treatment with antibiotics? | SAE or SUSAR? | Stopping of treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | Northampton General Hospital; N00038 | 00119 | Week 12 | URTI (sore throat, runny nose) | New | Moderate | Unrelated | Unexpected | No | No | No |
| Adalimumab | Northampton General Hospital; N00038 | 00119 | Week 12 | Non-cardiac chest pain | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Adalimumab | Manchester Royal Infirmary; N00080 | 00023 | Week 12 | Worsening RA | New | Moderate | Probably | Unexpected | N/A | No | Permanent |
| Adalimumab | Cannock Chase Hospital; N00473 | 00028 | Week 48 | Nausea | Pre-existing | Mild | Unrelated | Unexpected | No | No | No |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 12 | LRTI, lower respirating chest infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 12 | Exacerbation of COPD | New | Moderate | Possibly | Expected | No | No | Temporary |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 24 | Benign cyst on right breast | New | Mild | Unlikely | Unexpected | No | No | No |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 24 | Cold symptoms | New | Mild | Probably | Expected | Yes | No | Temporary |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 36 | Infective exacerbation of COPD | Pre-existing | Moderate | Unrelated | Unexpected | Yes | No | Temporary |
| Adalimumab | Chapel Allerton Hospital, Leeds; N00482 | 00032 | Week 48 | Exacerbation of COPD | Pre-existing | Severe | Possibly | Expected | Yes | No | Temporary |
| Adalimumab | Broadgreen Hospital, Liverpool; N00589 | 00110 | Week 12 | Rash – maculopapular grade 1 | New | Moderate | Possibly | Unexpected | N/A | No | No |
| CZP | Birmingham City Hospital; N00346 | 00086 | Week 12 | Vomiting | New | Moderate | Unlikely | Unexpected | No | No | Temporary |
| Golimumab | Airedale General Hospital; N00074 | 00088 | Week 36 | Chest infection | New | Moderate | Probably | Expected | Yes | No | Temporary |
| Golimumab | Airedale General Hospital; N00074 | 00088 | Week 48 | Fatigue | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Golimumab | Airedale General Hospital; N00074 | 00088 | Week 48 | Mouth ulcers | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Golimumab | Airedale General Hospital; N00074 | 00088 | Week 48 | Poor sleep | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 24 | Abnormal liver blood test | New | Moderate | Possibly | Unexpected | No | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 24 | One episode of heart rate rise to 146 b.p.m + feeling faint | New | Mild | Possibly | Unexpected | No | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 36 | Nasal sores crusts | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 36 | Reduced liver function blood results stopped MTX temporarily | New | Moderate | Almost certainly | Unexpected | N/A | No | Temporary |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 48 | Chest infection | New | Mild | Possibly | Unexpected | No | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 48 | Sore throat and earache | New | Mild | Unlikely | Unexpected | No | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 48 | Nasal crusts/ulcers | New | Mild | Probably | Unexpected | No | No | No |
| Golimumab | Derriford Hospital, Plymouth; N00118 | 00133 | Week 48 | Styes both eyes | New | Mild | Unlikely | Unexpected | No | No | No |
| Infliximab | Royal Victoria Infirmary, Newcastle; N00072 | 00024 | Week 12 | Sore throat | New | Mild | Possibly | Expected | No | No | No |
| Infliximab | Royal Victoria Infirmary, Newcastle; N00072 | 00024 | Week 24 | Elevated ALT levels in blood samples taken on 10 February 2014 | New | Moderate | Possibly | Expected | N/A | No | Permanent |
| Etanercept | Leicester Royal Infirmary; N00031 | 00094 | Week 24 | Viral infection | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Etanercept | Leicester Royal Infirmary; N00031 | 00094 | Week 24 | Haemoglobin decreased | New | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Leicester Royal Infirmary; N00031 | 00094 | Week 24 | Abdominal pain | New | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Leicester Royal Infirmary; N00031 | 00094 | Week 24 | ↑left wrist pain | Pre-existing | Mild | Unlikely | Expected | No | No | No |
| Etanercept | Northampton General Hospital; N00038 | 00112 | Week 24 | Chest infection | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Etanercept | Queen Elizabeth Hospital, Gateshead; N00071 | 00096 | Week 12 | Reaction around injection site | New | Moderate | Almost certainly | Expected | No | No | Permanent |
| Etanercept | Airedale General Hospital; N00074 | 00147 | Week 12 | Nausea | New | Moderate | Unrelated | Expected | N/A | No | Temporary |
| Etanercept | Derriford Hospital, Plymouth; N00118 | 00060 | Week 12 | Nausea | New | Moderate | Probably | Expected | N/A | No | No |
| Etanercept | Derriford Hospital, Plymouth; N00118 | 00060 | Week 36 | Pain in both feet | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Etanercept | Derriford Hospital, Plymouth; N00118 | 00060 | Week 36 | Pimples on neck | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Etanercept | King George Hospital, Ilford; N00165 | 00046 | Week 12 | UTI requiring oral antibiotics | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Etanercept | King George Hospital, Ilford; N00165 | 00046 | Week 12 | Erythematous lesion at Enbrel injection site | New | Moderate | Almost certainly | Expected | N/A | No | Temporary |
| Etanercept | King George Hospital, Ilford; N00165 | 00046 | Week 24 | Pain with swelling in the tummy area where injection was given | New | Mild | Almost certainly | Expected | N/A | No | No |
| Etanercept | King George Hospital, Ilford; N00165 | 00057 | Week 12 | UTI | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Etanercept | King George Hospital, Ilford; N00165 | 00057 | Week 12 | Twisted (right) knee | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Etanercept | King George Hospital, Ilford; N00165 | 00057 | Week 24 | Upper molar extraction abscess | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Etanercept | Queen’s Hospital, Burton upon Trent; N00178 | 00073 | Week 12 | Itching erythema | New | Mild | Almost certainly | Expected | No | No | No |
| Etanercept | Queen’s Hospital, Burton upon Trent; N00178 | 00073 | Week 48 | Right haemorrhagic branch retinal vein occlusion (retinal vascular disorder CTCAE description) | New | Moderate | Unrelated | Unexpected | No | No | No |
| Etanercept | Queen’s Hospital, Burton upon Trent; N00178 | 00121 | Week 12 | Tonsillitis | New | Moderate | Unrelated | Expected | Yes | No | Temporary |
| Etanercept | Queen’s Hospital, Burton upon Trent; N00178 | 00121 | Week 12 | Injection site reaction | New | Moderate | Almost certainly | Expected | No | No | No |
| Etanercept | Queen’s Hospital, Burton upon Trent; N00178 | 00121 | Week 12 | Subconjunctival haemorrhage | New | Mild | Unrelated | Expected | No | No | No |
| Etanercept | Guy’s Hospital, London; N00241 | 00064 | Week 12 | Migraine | New | Moderate | Unrelated | Expected | N/A | No | No |
| Etanercept | Guy’s Hospital, London; N00241 | 00064 | Week 24 | Migraine | Pre-existing | Mild | Unrelated | Expected | No | No | No |
| Etanercept | Guy’s Hospital, London; N00241 | 00064 | Week 36 | Migraine | Pre-existing | Moderate | Unrelated | Expected | N/A | No | No |
| Etanercept | Guy’s Hospital, London; N00241 | 00064 | Week 48 | Right flank abdominal pain | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Etanercept | Salford Royal Infirmary; N00400 | 00084 | Week 12 | URTI | New | Mild | Unrelated | Unexpected | No | No | No |
| Etanercept | Salford Royal Infirmary; N00400 | 00084 | Week 24 | Mouth ulcers | New | Moderate | Unlikely | Unexpected | No | No | No |
| Etanercept | Salford Royal Infirmary; N00400 | 00084 | Week 24 | Toothache | New | Moderate | Unlikely | Unexpected | No | No | No |
| Etanercept | Salford Royal Infirmary; N00400 | 00084 | Week 36 | Rash left hand | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Etanercept | Salford Royal Infirmary; N00400 | 00084 | Week 48 | Hay fever | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 12 | Injection site reaction | New | Moderate | Almost certainly | Unexpected | N/A | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 24 | Sickness | New | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 24 | Diarrhoea | New | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 24 | Throat infection | New | Mild | Unlikely | Unexpected | Yes | No | Temporary |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 24 | Eye infection | New | Mild | Unlikely | Unexpected | Yes | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 36 | Vaginal thrush | New | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 36 | Sinusitis | New | Mild | Unlikely | Unexpected | Yes | No | No |
| Etanercept | Cannock Chase Hospital; N00473 | 00041 | Week 48 | Sinusitis | Pre-existing | Mild | Unlikely | Unexpected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00001 | Week 12 | (Left) lower backache | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00004 | Week 12 | Injection site reaction | New | Mild | Almost certainly | Expected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00004 | Week 48 | Facial rash and chest rash, non-blanching | New | Moderate | Possibly | Expected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 12 | Bruising | New | Mild | Possibly | Unexpected | N/A | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 12 | Chest infection | New | Moderate | Unlikely | Unexpected | Yes | No | Temporary |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 12 | Nausea | New | Mild | Possibly | Unexpected | N/A | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 24 | Sickness with (nausea) etanercept | New | Moderate | Almost certainly | Expected | N/A | No | Permanent |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 24 | Bruising | New | Mild | Possibly | Unexpected | N/A | No | Permanent |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 24 | Chest infection | New | Moderate | Probably | Expected | Yes | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00018 | Week 48 | Cut (right) shin | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00059 | Week 48 | Cold | New | Mild | Probably | Expected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00065 | Week 48 | Cellulitis | New | Moderate | Probably | Unexpected | Yes | No | Temporary |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00070 | Week 48 | Diarrhoea | New | Mild | Missing | Unexpected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00070 | Week 48 | Cold | New | Mild | Missing | Expected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00070 | Week 48 | Fall | New | Moderate | Missing | Unexpected | No | No | No |
| Etanercept | Chapel Allerton Hospital, Leeds; N00482 | 00070 | Week 48 | Tooth infection | New | Moderate | Missing | Expected | Yes | No | No |
| Etanercept | Royal National Hospital for Rheumatic Diseases, Bath; N02220 | 00044 | Week 12 | Swelling of ankles in the evening | New | Mild | Possibly | Unexpected | N/A | No | No |
| Etanercept | Royal National Hospital for Rheumatic Diseases, Bath; N02220 | 00044 | Week 12 | Blocked nose (on SmPC) | New | Mild | Probably | Unexpected | N/A | No | No |
| Etanercept | Royal National Hospital for Rheumatic Diseases, Bath; N02220 | 00044 | Week 12 | Right shoulder/neck pain | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 24 | Mouth ulcer | Pre-existing | Moderate | Possibly | Unexpected | No | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 36 | Nose bleeds | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 36 | Dizziness | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 36 | Swollen ankles | Pre-existing | Moderate | Unrelated | Expected | N/A | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 36 | Slurring | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Leicester Royal Infirmary; N00031 | 00087 | Week 36 | Sore mouth | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Darlington Memorial Hospital; N00068 | 00097 | Week 12 | Feeling generally a bit low and lethargic | New | Mild | Unlikely | Expected | No | No | No |
| Abatacept | Darlington Memorial Hospital; N00068 | 00097 | Week 12 | Cool hands and feet | New | Mild | Possibly | Expected | No | No | No |
| Abatacept | Darlington Memorial Hospital; N00068 | 00097 | Week 12 | ↓ haemoglobin levels | New | Moderate | Unlikely | Unexpected | No | No | No |
| Abatacept | Darlington Memorial Hospital; N00068 | 00097 | Week 24 | Flare of RA | New | Moderate | Possibly | Unexpected | N/A | No | No |
| Abatacept | Queen Elizabeth Hospital, Gateshead; N00071 | 00034 | Week 12 | Infected leg ulcer | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | Queen Elizabeth Hospital, Gateshead; N00071 | 00034 | Week 12 | Ear infection | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | Queen Elizabeth Hospital, Gateshead; N00071 | 00034 | Week 12 | Flu-like symptoms | New | Mild | Unlikely | Unexpected | No | No | Temporary |
| Abatacept | Queen Elizabeth Hospital, Gateshead; N00071 | 00034 | Week 48 | Short of breath? Attributable to MTX | New | Moderate | Possibly | Unexpected | – | No | Temporary |
| Abatacept | Airedale General Hospital; N00074 | 00033 | Week 12 | URTI | New | Moderate | Probably | Expected | No | No | No |
| Abatacept | Airedale General Hospital; N00074 | 00078 | Week 12 | UTI | New | Moderate | Unlikely | Expected | Yes | No | No |
| Abatacept | Airedale General Hospital; N00074 | 00078 | Week 24 | Middle ear infection | New | Moderate | Unlikely | Expected | Yes | No | Temporary |
| Abatacept | Airedale General Hospital; N00074 | 00078 | Week 36 | Back pain | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Airedale General Hospital; N00074 | 00078 | Week 36 | (Ingrowing toe nail) inflamed big toe | New | Mild | Unrelated | Unexpected | No | No | No |
| Abatacept | Airedale General Hospital; N00074 | 00078 | Week 48 | UTI | Pre-existing | Moderate | Unlikely | Expected | Yes | No | Temporary |
| Abatacept | Bristol Royal Infirmary; N00117 | 00145 | Week 24 | Campylobacter gastroenteritis | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00040 | Week 12 | Chest infection | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00040 | Week 12 | Urinary infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00040 | Week 24 | Tiredness | New | Mild | Possibly | Expected | No | No | No |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00040 | Week 48 | Urinary infection | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00068 | Week 12 | Infected left big toe | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Derriford Hospital, Plymouth; N00118 | 00068 | Week 48 | Patient has a cold and feeling unwell. Treated with amoxicillin 21 January 2015 to 1 week out of 52 and oxytetracycline 4 February 2015 to 1/52 | New | Moderate | Unlikely | Unexpected | Yes | Yes | Temporary |
| Abatacept | King George Hospital, Ilford; N00165 | 00051 | Week 12 | Elevated ALP (145 IU/l – range 30 to 130 IU/l) | New | Mild | Possibly | Unexpected | N/A | No | No |
| Abatacept | King George Hospital, Ilford; N00165 | 00052 | Week 12 | (Right) axilla infection of hair follicles | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | King George Hospital, Ilford; N00165 | 00052 | Week 24 | Gastroenteritis | New | Mild | Unlikely | Unexpected | No | No | Temporary |
| Abatacept | Royal Derby Hospital; N00168 | 00146 | Week 12 | Chest pain | New | Severe | Unrelated | Unexpected | N/A | Yes | Temporary |
| Abatacept | Royal Derby Hospital; N00168 | 00146 | Week 24 | Upper abdominal pain | Pre-existing | Moderate | Unrelated | Unexpected | N/A | Yes | Temporary |
| Abatacept | University Hospital, North Durham; N00170 | 00071 | Week 12 | UTI | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | University Hospital, North Durham; N00170 | 00071 | Week 12 | Mouth ulcers | New | Mild | Possibly | Unexpected | N/A | No | No |
| Abatacept | University Hospital, North Durham; N00170 | 00071 | Week 12 | Sore throat | New | Mild | Possibly | Unexpected | N/A | No | No |
| Abatacept | University Hospital, North Durham; N00170 | 00071 | Week 12 | Abscess tooth, tooth out | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Abatacept | Musgrove Park Hospital, Taunton; N00306 | 00089 | Week 36 | Right wrist swollen and painful 120-mg Depo injection given | New | Moderate | Missing | Expected | No | No | No |
| Abatacept | Birmingham City Hospital; N00346 | 00061 | Week 12 | Upper respiratory indigestion | New | Moderate | Possibly | Unexpected | No | No | Temporary |
| Abatacept | Birmingham City Hospital; N00346 | 00061 | Week 24 | Upper respiratory injection | Pre-existing | Moderate | Possibly | Unexpected | No | No | No |
| Abatacept | Birmingham City Hospital; N00346 | 00061 | Week 24 | Occasional wheeze | New | Moderate | Possibly | Unexpected | No | No | No |
| Abatacept | Birmingham City Hospital; N00346 | 00079 | Week 24 | UTI | New | Moderate | Unlikely | Unexpected | Yes | No | No |
| Abatacept | Birmingham City Hospital; N00346 | 00079 | Week 48 | Nausea | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 12 | Bruising | New | Mild | Possibly | Unexpected | N/A | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 12 | Angiooedema face, tongue, hands | New | Moderate | Possibly | Unexpected | N/A | Yes | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 12 | Angiooedema right cheek | New | Moderate | Possibly | Unexpected | N/A | Yes | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 24 | Mouth ulcers | New | Mild | Possibly | Unexpected | No | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 24 | Sickness/nausea | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 24 | Hypertension | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 24 | Neck pain | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 36 | Flare of RA (left wrist and left hip) | – | Missing | Missing | Missing | – | Missing | Missing |
| Abatacept | Salford Royal Infirmary; N00400 | 00074 | Week 48 | Pain in hip | Pre-existing | Moderate | Unrelated | Expected | No | No | No |
| Abatacept | Cannock Chase Hospital; N00473 | 00106 | Week 36 | Mouth ulcers | New | Mild | Possibly | Unexpected | No | No | No |
| Abatacept | Cannock Chase Hospital; N00473 | 00106 | Week 36 | Chest (URTI) infection | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Cannock Chase Hospital; N00473 | 00106 | Week 36 | Cellulitis | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00002 | Week 12 | (Right) tooth abscess | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00002 | Week 12 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00006 | Week 36 | UTI | New | Moderate | Unlikely | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00009 | Week 48 | Flu illness with URTI | New | Moderate | Unrelated | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00014 | Week 24 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00016 | Week 12 | Back pain after getting up from chair | New | Severe | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00016 | Week 12 | Nausea and change in smell | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00021 | Week 24 | Foot ulcer right MTP 1 | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00026 | Week 12 | Mild hair loss | New | Mild | Possibly | Expected | N/A | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00026 | Week 24 | Swelling and lumps on both sides of the neck | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00026 | Week 48 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00038 | Week 36 | Sinusitis | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00038 | Week 36 | Laryngitis | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00102 | Week 36 | Cold | New | Mild | Probably | Expected | No | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00103 | Week 36 | Cold | New | Mild | Probably | Expected | No | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00137 | Week 12 | URTI | New | Moderate | Probably | Expected | Yes | No | No |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00137 | Week 36 | Tooth abscess | New | Moderate | Probably | Expected | Yes | No | Temporary |
| Abatacept | Chapel Allerton Hospital, Leeds; N00482 | 00137 | Week 48 | Hair loss | Pre-existing | Moderate | Probably | Expected | N/A | No | Permanent |
| Abatacept | Broadgreen Hospital, Liverpool; N00589 | 00130 | Week 12 | Laryngeal inflammation | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00077 | Week 12 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00077 | Week 12 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00077 | Week 12 | Infected dog scratch | New | Moderate | Unrelated | Unexpected | Yes | No | Temporary |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00123 | Week 36 | UTI low backache. No fever, dysuria, frequency | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00123 | Week 36 | UTI low backache no fever, dysuria, frequency | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Abatacept | Royal National Hospital for Rheumatic Diseases, Bath N02220 | 00125 | Week 12 | Severe flare – widespread pain and stiffness, bed/chairbound, assessed by out-of-hours GP | New | Severe | Unrelated | Unexpected | N/A | No | No |
| Rituximab | New Cross Hospital, Wolverhampton; N00034 | 00142 | Week 36 | Rash – forearms and trunk | New | Mild | Possibly | Unexpected | No | No | No |
| Rituximab | Queen Elizabeth Hospital, Gateshead; N00071 | 00031 | Week 24 | Vomited (once) | New | Mild | Possibly | Unexpected | N/A | No | No |
| Rituximab | Queen Elizabeth Hospital, Gateshead; N00071 | 00037 | Week 24 | Metastatic melanoma | New | Life-threatening | Unrelated | Unexpected | N/A | Yes | Permanent |
| Rituximab | Queen Elizabeth Hospital, Gateshead; N00071 | 00066 | Week 12 | Rash | New | Moderate | Possibly | Unexpected | No | No | No |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 12 | White cells 3.4 × 109/l (below normal limit) | New | Mild | Unrelated | Expected | No | No | Temporary |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 12 | Neutrophils 1.74 × 109/l (below normal limit) | New | Mild | Unrelated | Expected | No | No | Temporary |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 24 | White cell count (3.1 × 109/l, 3.3 × 109/l, 3.3 × 109/l) | New | Mild | Unrelated | Expected | No | No | Temporary |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 24 | Low neutrophils (1.45 × 109/l, 1.45 × 109/l, 1.62 × 109/l) | New | Mild | Unrelated | Expected | No | No | Temporary |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 36 | Cystitis | New | Moderate | Unrelated | Expected | Yes | No | No |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 36 | Raised ALT (5S g/l) | Pre-existing | Mild | Unrelated | Expected | N/A | No | Temporary |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 48 | Around 15 November 2014 croaky voice | New | Moderate | Possibly | Expected | No | No | No |
| Rituximab | Airedale General Hospital; N00074 | 00058 | Week 48 | Around 15 November 2014 (ongoing) blocked nose (took paracetamol on the weekend when she felt worse) | New | Moderate | Possibly | Expected | No | No | No |
| Rituximab | Hull Royal Infirmary; N00078 | 00149 | Week 12 | Ruptured sebaceous cyst | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Hull Royal Infirmary; N00078 | 00149 | Week 24 | Urine infection | New | Mild | Unrelated | Unexpected | Yes | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00013 | Week 12 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00013 | Week 48 | Neutropenia | New | Moderate | Unrelated | Unexpected | N/A | No | Temporary |
| Rituximab | Manchester Royal Infirmary; N00080 | 00017 | Week 12 | Widespread soft tissue tenderness | New | Mild | Possibly | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00017 | Week 12 | No sleep for past 2 days | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00017 | Week 36 | Longstanding wind in stomach | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00056 | Week 24 | Low white cell count | New | Moderate | Almost certainly | Unexpected | N/A | No | Permanent |
| Rituximab | Manchester Royal Infirmary; N00080 | 00056 | Week 24 | Mouth ulcer | New | Moderate | Almost certainly | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00092 | Week 12 | Increased fatigue | New | Mild | Possibly | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00092 | Week 12 | Slight breathlessness at night | New | Mild | Possibly | Unexpected | N/A | No | No |
| Rituximab | Manchester Royal Infirmary; N00080 | 00092 | Week 36 | Left hand injury | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Poole Hospital; N00108 | 00075 | Week 12 | Rash to upper arms | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Poole Hospital; N00108 | 00075 | Week 36 | Submitted Yellow Card for severe skin reaction | New | Moderate | Probably | Unexpected | No | No | Permanent |
| Rituximab | Queen Alexandra Hospital, Portsmouth; N00110 | 00148 | Week 12 | Infusion reaction on first rituximab infusion 27 November 2014. Treated with intravenous hydrocortisone infusion stopped then restarted: no further infusion reactions | New | Moderate | Unlikely | Unexpected | No | No | No |
| Rituximab | Queen Alexandra Hospital, Portsmouth; N00110 | 00148 | Week 48 | Patient reports hair loss – MTX dose reduced to 10 mg weekly | New | Mild | Unrelated | Expected | N/A | No | No |
| Rituximab | Derriford Hospital, Plymouth; N00118 | 00025 | Week 24 | Abscess on mouth mucosa | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Rituximab | Derriford Hospital, Plymouth; N00118 | 00025 | Week 48 | Lower back pain | Pre-existing | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 12 | Suspected UTI | New | Moderate | Unlikely | Unexpected | Yes | No | No |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 24 | Chest infection | Pre-existing | Moderate | Probably | Expected | Yes | No | Temporary |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 24 | Chest infection | Pre-existing | Moderate | Probably | Expected | Yes | No | Temporary |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 24 | Exacerbation of asthma | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 24 | Shingles | New | Moderate | Probably | Expected | Yes | No | No |
| Rituximab | King George Hospital, Ilford; N00165 | 00030 | Week 36 | (Right) sided suspected pleurisy | New | Severe | Unlikely | Unexpected | Yes | No | Temporary |
| Rituximab | University Hospital, North Durham; N00170 | 00083 | Week 12 | ALT raised | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00083 | Week 24 | Eye infection treated with antibiotic eye ointment by GP | New | Mild | Possibly | Unexpected | Yes | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00083 | Week 36 | Flare | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00083 | Week 48 | (Light) knee swollen (inner) painful | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00107 | Week 12 | 22 October 2014 shortness of breath | New | Moderate | Unlikely | Unexpected | Yes | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00107 | Week 24 | Chest infection | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Rituximab | University Hospital, North Durham; N00170 | 00107 | Week 24 | Vertigo | New | Moderate | Possibly | Unexpected | N/A | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 12 | Neutropenia, 0.69 × 109/l | New | Moderate | Possibly | Expected | No | No | Temporary |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 24 | URTI | New | Moderate | Possibly | Expected | No | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 24 | Burn on hand | New | Mild | Unrelated | Unexpected | No | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 24 | Headaches | New | Mild | Probably | Expected | No | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 24 | Temporomandibular pain | New | Mild | Unrelated | Expected | No | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 36 | Carditis, 9 February 2015, co-amoxiclav | New | Moderate | Unrelated | Expected | Yes | No | No |
| Rituximab | Nuffield Orthopaedic Centre, Oxford; N00282 | 00104 | Week 36 | Viral infection, 2–3 weeks | New | Mild | Possibly | Expected | No | No | No |
| Rituximab | Birmingham City Hospital; N00346 | 00132 | Week 12 | Rash skin | New | Moderate | Probably | Expected | N/A | No | No |
| Rituximab | Salford Royal Infirmary; N00400 | 00113 | Week 36 | Gastritis | New | Moderate | Unrelated | Expected | No | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00036 | Week 12 | Worsening depression (following bereavement) | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00036 | Week 24 | Leg cramps at night | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00036 | Week 36 | Upper back pain | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00036 | Week 36 | Unstable hypertension | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00036 | Week 36 | Elevated lipids | Pre-existing | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00045 | Week 48 | Mouth ulcers | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00045 | Week 48 | Chest infection | New | Mild | Unlikely | Expected | No | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00098 | Week 12 | Diabetic peripheral neuropathy (longstanding) | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00098 | Week 24 | Left trochanteric bursitis | New | Moderate | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00098 | Week 36 | Hair thinning | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00098 | Week 36 | Intermittent numbness of upper limbs | New | Mild | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 12 | UTI | New | Mild | Possibly | Expected | Yes | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 12 | Tremor, right hand | New | Mild | Unrelated | Unexpected | N/A | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 24 | Cough | New | Moderate | Unlikely | Unexpected | No | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 24 | UTI | New | Moderate | Unlikely | Unexpected | Yes | No | No |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 36 | Cough | New | Moderate | Unlikely | Unexpected | No | No | Permanent |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 36 | Sterile pyuria | New | Moderate | Unlikely | Unexpected | No | No | Permanent |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 36 | Oral thrush | New | Moderate | Unlikely | Unexpected | N/A | No | Permanent |
| Rituximab | Cannock Chase Hospital; N00473 | 00114 | Week 36 | Flare of RA | New | Moderate | Unlikely | Unexpected | N/A | No | Permanent |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00003 | Week 24 | Skin rash (left wrist) | New | Mild | Possibly | Unexpected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00003 | Week 24 | Lower respiratory chest infection | New | Mild | Possibly | Unexpected | Yes | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00003 | Week 48 | (Left) ear deafness? Eustachian tube dysfunction | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00003 | Week 48 | Cough and dry 1 month out of 12 | New | Moderate | Possibly | Unexpected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00007 | Week 12 | Lower respiratory chest infection (January 2013) | New | Moderate | Possibly | Unexpected | Yes | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00007 | Week 12 | Lower respiratory chest infection (7 March 2013) | New | Moderate | Possibly | Unexpected | Yes | No | Temporary |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00007 | Week 24 | Chest infection | New | Mild | Possibly | Unexpected | Yes | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00008 | Week 24 | Acute abdominal pain over previous hysterectomy scar | New | Severe | Unlikely | Unexpected | N/A | Yes | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00015 | Week 24 | (Right) groin pain | New | Mild | Unrelated | Expected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00019 | Week 12 | Urticarial rash with faster infusion rituximab | New | Moderate | Almost certainly | Expected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00019 | Week 24 | Headaches twice with MTX | Pre-existing | Mild | Possibly | Expected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00019 | Week 36 | Tooth infection | New | Moderate | Probably | Expected | Yes | No | Temporary |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00047 | Week 48 | Sinusitis | New | Moderate | Probably | Expected | Yes | No | Temporary |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00055 | Week 48 | Chest infection | New | Moderate | Probably | Expected | Yes | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00080 | Week 36 | Chest infection | New | Moderate | Possibly | Expected | Yes | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00082 | Week 36 | Blepharitis (left eye) | New | Moderate | Unlikely | Unexpected | N/A | No | No |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00101 | Week 36 | UTI (cystitis) | New | Moderate | Probably | Expected | Yes | No | Temporary |
| Rituximab | Chapel Allerton Hospital, Leeds; N00482 | 00101 | Week 48 | Wound infection | New | Moderate | Possibly | Expected | Yes | No | Temporary |
Appendix 15 Supplementary health economics tables
| Type of service | Cost (£) | Unit of measure | Notes | Source |
|---|---|---|---|---|
| Community-based health and social services | ||||
| GP, surgery visit | 44.00 | Per visit | GP, per patient contact lasting 11.7 minutes including direct care staff costs | PSSRU Unit Costs of Health and Social Care 2015, p. 177127 |
| GP, surgery telephone | 27.00 | Per telephone call | GP, per telephone consultation lasting 7.1 minutes | PSSRU Unit Costs of Health and Social Care 2015, p. 177127 |
| GP, home visit | 90.00 | Per home visit | (Per patient contact lasting 11.7 minutes + average 12-minute travel time) × £3.80/minute cost of patient | PSSRU Unit Costs of Health and Social Care 2015, p. 177127 |
| District nurse face to face | 37.26 | Per visit | District nurse, adult, face to face | NHS Reference Costs 2014 to 2015 128 |
| District nurse telephone/e-mail | 16.53 | District nurse, adult non-face to face | NHS Reference Costs 2014 to 2015 128 | |
| Social worker face to face | 79.00 | Per visit | Assuming 1-hour appointment | PSSRU Unit Costs of Health and Social Care 2015, p. 188127 |
| Social worker telephone/e-mail | 9.34 | Assuming telephone consultation lasting 7.1 minutes, based on cost per hour | PSSRU Unit Costs of Health and Social Care 2015, p. 188127 | |
| Physiotherapist face to face | 52.00 | Per appointment | Community physiotherapist mean cost for one-to-one contact | NHS Reference Costs 2014 to 2015 128 |
| Physiotherapist telephone/e-mail | 35.00 | Physiotherapy non-admitted non-face-to-face follow-up, consultant led | NHS Reference Costs 2014 to 2015 128 | |
| Occupational therapist face to face | 44.00 | Per appointment | NHS community occupational therapist | PSSRU Unit Costs of Health and Social Care 2015, p. 191127 |
| Occupational therapist telephone/e-mail | 9.00 | Occupational therapy consultant-led non-admitted non-face-to-face follow-up (first cost = £17.00) | NHS Reference Costs 2014 to 2015 128 | |
| Podiatrist face to face | 39.63 | Per appointment | NHS Reference Costs 2014 to 2015 128 | |
| Podiatrist telephone/e-mail | 18.00 | Podiatry non-admitted non-face-to-face follow-up (first cost = £30.00) | NHS Reference Costs 2014 to 2015 128 | |
| Counsellor face to face | 50.79 | Per appointment | Assuming 1-hour appointment | PSSRU Unit Costs of Health and Social Care 2014, p. 51148 and EPPI-Centre Cost Converter to 2015 price, URL: http://eppi.ioe.ac.uk/costconversion/Default.aspx |
| Counsellor telephone/e-mail | 6.01 | Assuming telephone consultation lasting 7.1 minutes, based on cost per hour | PSSRU Unit Costs of Health and Social Care 2014, p. 51148 and EPPI-Centre Cost Converter to 2015 price, URL: http://eppi.ioe.ac.uk/costconversion/Default.aspx | |
| Psychiatrist or psychologist face to face | 61.96 | Per visit | Clinical psychologist per hour | PSSRU Unit Costs of Health and Social Care 2014, p. 183148 and EPPI-Centre Cost Converter to 2015 price, URL: http://eppi.ioe.ac.uk/costconversion/Default.aspx |
| Psychiatrist or psychologist telephone/e-mail | 34.00 | Clinical psychology non-admitted non-face-to-face follow-up (first cost = £31.00) | NHS Reference Costs 2014 to 2015 128 | |
| Home help or care workers face to face | 24.00 | Per session | Face-to-face 1-hour weekday session | PSSRU Unit Costs of Health and Social Care 2015, p. 192127 |
| Home help or care workers telephone/e-mail | 2.84 | Assuming telephone consultation lasting 7.1 minutes, based on cost per hour | PSSRU Unit Costs of Health and Social Care 2015, p. 192127 | |
| Practice nurse | 12.14 | Per 15.5-minute consultation | Based on £47 per hour | PSSRU Unit Costs of Health and Social Care 2015, p. 174127 |
| Specialist nurse telephone | 7.69 | Assuming telephone consultation lasting 7.1 minutes, based on £65 per hour | PSSRU Unit Costs of Health and Social Care 2015, p. 172127 | |
| Hydrotherapy pool | 27.00 | Physiotherapy non-admitted, non-face to face, non-consultant led | NHS Reference Costs 2014 to 2015 128 | |
| Hospital-based or residential care services | ||||
| Hospital inpatient stay | 303.00 | Per day | General ward, non-elective inpatients – excess bed-days | NHS Reference Costs 2014 to 2015 128 |
| Hospital day centre | 160.00 | Per visit | Inpatient specialist palliative care, same day | NHS Reference Costs 2014 to 2015 128 |
| Hospital outpatient clinic | 112.00 | Per visit | Weighted average of all outpatient attendances | PSSRU Unit Costs of Health and Social Care 2015, p. 107127 |
| Hospital accident and emergency department | 132.00 | Per visit | Emergency medicine | NHS Reference Costs 2014 to 2015 128 |
| Nursing home | 88.71 | Per day | Assume cost for 1 day and night equals the reported private sector nursing home cost per week/7 | PSSRU Unit Costs of Health and Social Care 2015, p. 37127 |
| Residential home | 72.00 | Per day | Assume cost for 1 day and night equals the reported private sector residential home cost per week/7 | PSSRU Unit Costs of Health and Social Care 2015, p. 38127 |
| Inpatient procedures | ||||
| Oral surgery (dental clearance) | 154.00 | Oral surgery, extraction of multiple teeth aged ≥ 19 years | NHS Reference Costs 2014 to 2015 128 | |
| Excision of cystic swelling | 2054.30 | Minor foot procedures for non-trauma, inpatient elective | NHS Reference Costs 2014 to 2015 128 | |
| Rheumatology visits | ||||
| Day-case rheumatology | 421.00 | Day case inflammatory, spine, joint or connective tissue disorders, with CC score 0–2 | DH’s NHS Reference Costs 2014 to 2015 (HD23J)128 | |
| Outpatient rheumatology, first attendance | 162.00 | Non-admitted face to face, first | DH’s NHS Reference Costs 2014 to 2015 (WF01B)128 | |
| Outpatient rheumatology, follow-up attendance | 91.00 | Non-admitted face to face follow-up | DH’s NHS Reference Costs 2014 to 2015 (WF01A)128 | |
| Staff nurse | 36.00 | Cost per hour | PSSRU’s Unit Costs of Health and Social Care 2015127 | |
| Nurse specialist | 45.00 | Cost per hour | PSSRU’s Unit Costs of Health and Social Care 2015127 | |
| Medication | Dose | Cost per dose | Unit cost (£) | Description | Source |
|---|---|---|---|---|---|
| Rituximab |
First cycle: 1 g as an intravenous infusion at days 0 (week 0) and 15 (week 2; + 5 days) Second cycle: 1 g as an intravenous infusion at 2-week interval |
£1746.30 | 873.15 | 10 mg/ml of a 50-ml vial concentrate for intravenous infusion | BNF130 |
| Abatacept | 125 mg by subcutaneous injection at week 0 and once weekly thereafter for a minimum of 24 weeks | £302.40 | 302.40 | 125-mg prefilled pen or prefilled syringe | BNF130 |
| Infliximab | 3 mg/kg per intravenous infusion to be administered at weeks 0, 2 and 6 then 8-weekly thereafter for minimum 24 weeks | 419.62 | 100-mg vial | BNF130 | |
| Etanercept | 50 mg by subcutaneous injection weekly for minimum 24 weeks | £178.75 | 178.75 | 50-mg prefilled pen or prefilled syringe | BNF130 |
| Adalimumab | 40 mg by subcutaneous injection every 2 weeks for a minimum of 24 weeks | £352.14 | 352.14 | 40-mg prefilled pen or prefilled syringe | BNF130 |
| CZP | 400 mg by subcutaneous injection at weeks 0, 2 and 4, then 200 mg every 2 weeks thereafter for a minimum of 24 weeks | 400-mg dose, £715.00; 200-mg dose, £357.50 | 357.50 | 200-mg prefilled syringe | BNF130 |
| Golimumab | 50 mg of self-administered subcutaneous injection monthly, same date each month | £762.97 | 762.97 | 50-mg prefilled pen or prefilled syringe | BNF130 |
| Treatment | Hours of nurse supervision for intravenous drug administration per treatment | Intravenous equipment required per treatmenta | Educational visit, outpatient, rheumatology (first attendance) | Safety check: visit staff nurse (week 4) | Administration cost (£) |
|---|---|---|---|---|---|
| Etanercept | – | – | 1 | 1 | 198 |
| Adalimumab | – | – | 1 | 1 | 198 |
| CZP | – | – | 1 | 1 | 198 |
| Abatacept | – | – | 1 | 1 | 198 |
| Golimumab | – | – | 1 | 1 | 198 |
| Infliximab | 2 | 1 | – | – | 98.66 |
| Rituximab | 7 | 1 | – | – | 278.66 |
| Resource | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 weeks | 24 weeks | 36 weeks | 48 weeks | |||||||||
| Treatment arm | ||||||||||||
| Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | |
| Community Health and Social Services | ||||||||||||
| GP surgery visit | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 1.48 (1.70) | 1.23 (2.1) | 1 (1.26) | 2.1 (2.78) | 1.46 (1.56) | 1.32 (1.44) | 2.52 (4.47) | 1.81 (1.39) | 2.2 (2.87) | 3 (3.74) | 1.69 (1.83) | 1.8 (1.76) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 6 | 10 | 3 | 10 | 6 | 4 | 20 | 5 | 12 | 12 | 6 | 6 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0.86 (1.73) | 0.12 (0.33) | 0.44 (1.26) | 0.31 (0.93) | 0.15 (0.46) | 0.48 (1.05) | 0.45 (1.15) | 0.77 (0.27) | 0.32 (0.9) | 0.72 (2.37) | 0.23 (1.18) | 0.2 (0.58) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 6 | 1 | 5 | 4 | 2 | 4 | 5 | 1 | 3 | 12 | 6 | 2 |
| GP home visit | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0.14 (0.52) | 0 | 0 | 0.1 (0.56) | 0.08 (0.39) | 0 | 0 | 0 | 0.04 (0.2) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 1 |
| District nurse | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0.14 (0.58) | 0.27 (0.83) | 0.44 (1.04) | 0.14 (0.58) | 0.23 (0.82) | 0.32 (0.9) | 0.21 (0.77) | 0.19 (0.69) | 0.04 (0.2) | 0.24 (1.12) | 0.12 (0.59) | 0.48 (1.23) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 6 | 3 | 5 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0 | 0.15 (0.78) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 (0.19) | 0.12 (0.59) | 0.08 (0.4) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 2 |
| Social worker | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0.03 (0.19) | 0 | 0 | 0 | 0 | 0 | 0.07 (0.26) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0.03 (0.19) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 (0.19) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Physiotherapist | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0.38 (0.19) | 0.54 (1.9) | 0 | 0.52 (2.05) | 0 | 0.6 (1.94) | 0.76 (2.85) | 1.81 (7.91) | 0.64 (1.87) | 1.48 (3.3) | 0.54 (1.82) | 0.12 (0.44) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 8 | 7 | 0 | 10 | 0 | 7 | 12 | 40 | 7 | 12 | 8 | 2 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 (0.37) | 0 | 0 | 0.34 (1.86) | 0.04 (0.2) | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 10 | 1 | 0 |
| Occupational therapist | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0 | 0.15 (0.54) | 0 | 0.1 (0.41) | 0.08 (0.39) | 0.04 (0.2) | 0.1 (0.41) | 0 | 0.2 (1) | 0.21 (0.68) | 0.31 (0.62) | 0.08 (0.4) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 5 | 3 | 2 | 2 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 (0.37) | 0.04 (0.2) | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 |
| Podiatrist | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0.31 (0.76) | 0.27 (0.83) | 0.24 (0.72) | 0.31 (0.76) | 0.35 (1.02) | 0.48 (0.96) | 0.69 (1.83) | 0.62 (1.3) | 0.92 (2.56) | 0.93 (1.98) | 0.88 (1.86) | 0.32 (1.22) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 3 | 3 | 3 | 3 | 4 | 3 | 9 | 4 | 12 | 8 | 6 | 6 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0.07 (0.37) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.21 (1.11) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| Counsellor | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0.1 (0.56) | 0 | 0.16 (0.8) | 0.1 (0.56) | 0 | 0.08 (0.4) | 0.1 (0.56) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 3 | 0 | 4 | 3 | 0 | 2 | 3 | 0 | 0 |
| Telephone/e-mail | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.16 (0.8) | 0 | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Psychologistc | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 (0.19) | 0 | 0 | 0.1 (0.56) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 |
| Home helpc | ||||||||||||
| Face to face | ||||||||||||
| Mean (SD) | 0.83 (3.09) | 0.46 (2.35) | 0 | 0.83 (3.09) | 0 | 0.48 (2.4) | 0.83 (3.09) | 0 | 0 | 1.24 (3.72) | 0 | 0 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 12 | 12 | 0 | 12 | 0 | 12 | 12 | 0 | 0 | 12 | 0 | 0 |
| Hospital or residential care service | ||||||||||||
| Hospital inpatient stay | ||||||||||||
| Mean (SD) | 0 | 0 | 0 | 0.21 (0.94) | 0 | 0 | 0.59 (1.66) | 0 | 0 | 0 | 0 | 0.08 (0.28) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 0 | 0 | 5 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 1 |
| Hospital outpatient clinic | ||||||||||||
| Mean (SD) | 0.59 (1.02) | 1.12 (1.24) | 1.44 (2.75) | 0.86 (1.03) | 0.88 (1.03) | 1.36 (0.95) | 1.1 (1.11) | 1.27 (1.04) | 0.88 (0.97) | 0.93 (1) | 0.88 (0.95) | 1.08 (1.04) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 4 | 5 | 14 | 3 | 3 | 3 | 4 | 3 | 2 | 2 | 2 | 3 |
| Hospital day centre | ||||||||||||
| Mean (SD) | 0.45 (1.02) | 0 | 0.12 (0.6) | 0.17 (0.66) | 0.23 (0.82) | 0.12 (0.6) | 0.48 (1.12) | 0.31 (0.97) | 0.4 (1.32) | 0.66 (1.37) | 0.54 (1.73) | 0.56 (1.32) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 3 | 0 | 3 | 3 | 3 | 3 | 4 | 4 | 6 | 5 | 8 | 4 |
| Hospital accident and emergency department | ||||||||||||
| Mean (SD) | 0 | 0.08 (0.39) | 0.12 (0.6) | 0.17 (0.54) | 0.08 (0.39) | 0.08 (0.4) | 0.14 (0.58) | 0.04 (0.2) | 0 | 0.03 (0.19) | 0 | 0.2 (0.58) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 0 | 2 | 3 | 2 | 2 | 2 | 3 | 1 | 0 | 1 | 0 | 2 |
| Health-care cost | Cost | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Total costs | ||||||||||||||
| 12 weeks | 24 weeks | 36 weeks | 48 weeks | ||||||||||||
| Treatment arm | Treatment arm | Treatment arm | Treatment arm | Treatment arm | |||||||||||
| Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | Rituximab (n = 29) | Abatacept (n = 26) | TNFi (n = 25) | |
| Total community health and social services costs | |||||||||||||||
| Mean (SD) | 148.36 (189.81) | 126.36 (163.97) | 81.79 (97.53) | 190.07 (248.13) | 95.56 (92.58) | 154.59 (213.56) | 240.88 (351.98) | 214.09 (415.49) | 191.57 (258.95) | 347.86 (567.01) | 165.19 (209.73) | 129.85 (155.14) | 927.17 (1238.6) | 601.2 (553.7) | 557.8 (513.42) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 818.89 | 651.78 | 267 | 916 | 358.3 | 890.20 | 1297.63 | 2168 | 1003.56 | 2649.79 | 829.78 | 570.15 | 5291.57 | 2388 | 1818.23 |
| Total hospital and residential care service costs | |||||||||||||||
| Mean (SD) | 175.48 (242.04) | 188.12 (241.07) | 267.04 (399.20) | 254.35 (524.83) | 222.49 (332.29) | 199.04 (166.25) | 454.93 (555.61) | 230.42 (225.73) | 192.92 (312.42) | 227.93 (521.64) | 221.58 (290.24) | 298.78 (273.42) | 1112.71 (1137.51) | 862.61 (788.43) | 957.78 (678.42) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 224 |
| Maximum | 999 | 972 | 1846 | 2802.21 | 1230 | 704 | 1901 | 743 | 1209 | 1024 | 1280 | 864 | 5039.21 | 3322 | 2523 |
| Trial medication costs (inclusive of drug administration cost) | |||||||||||||||
| Mean (SD) | 3980.09 (376.03) | 3966.37 (542.51) | 2302.18 (377.75) | 418.96 (1255.21) | 2721.6 (1443.94) | 1662.59 (864.53) | 1675.83 (2029.94) | 2535.51 (1611.71) | 1625.14 (865.73) | 578.56 (1443.18) | 2361.05 (1710.67) | 1349.28 (1003.26) | 6633.49 (3197.01) | 11584.52 (4733.65) | 6939.19 (2679.69) |
| Minimum | 2024.96 | 1407.6 | 902.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2024.96 | 1407.6 | 902.28 |
| Maximum | 4049.92 | 4129.2 | 3415.5 | 4049.92 | 3628.8 | 2288.91 | 4049.92 | 3628.8 | 2288.91 | 4049.92 | 3628.8 | 2288.91 | 16199.68 | 15015.6 | 9353.64 |
| Other medication costs | |||||||||||||||
| MTX | |||||||||||||||
| Mean (SD) | 9.93 (2.1) | 8.03 (1.65) | 10.18 (2.52) | 7.7 (1.49) | 7.66 (1.8) | 8.64 (1.39) | 7.53 (2.38) | 7.66 (1.36) | 8.16 (1.83) | 8.35 (1.57) | 7.87 (2.02) | 8.16 (1.55) | 32.36 (5.27) | 30.92 (3.42) | 35.14 (3.09) |
| Minimum | 2.4 | 2.4 | 7.2 | 4.8 | 2.4 | 4.8 | 2.4 | 4.8 | 4.8 | 7.2 | 2.4 | 4.8 | 14.4 | 21.6 | 31.2 |
| Maximum | 14.4 | 9.6 | 19.2 | 12 | 12 | 9.6 | 12 | 12 | 12 | 12 | 9.6 | 9.6 | 38.4 | 33.6 | 45.6 |
| Other concomitant medicationb | |||||||||||||||
| Mean (SD) | – | – | – | – | – | – | – | – | – | – | – | – | 396.78 (1830.36) | 519.51 (1168.58) | 807.93 (1909.68) |
| Minimum | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 0 |
| Maximum | – | – | – | – | – | – | – | – | – | – | – | – | 9907.84 | 3590.59 | 7014.68 |
| Total NHS costsc | |||||||||||||||
| Mean (SD) | 4313.87 (378.37) | 4288.88 (604.15) | 2661.18 (565.53) | 871.08 (1339.2) | 3047.32 (1310.12 | 2024.87 (939.16) | 2379.18 (1897.29) | 2987.68 (1628.37) | 2017.8 (823.93) | 1141.6 (1532.23) | 2755.38 (1649.07) | 1786.07 (909.02) | 9102.51 (3375.77) | 13598.77 (4092.09) | 9297.84 (2007.36) |
| Minimum | 2967.85 | 1766 | 999.88 | 7.2 | 2.4 | 137.23 | 7.2 | 248.8 | 7.2 | 7.2 | 231.2 | 9.6 | 4716.78 | 2690.88 | 1462.19 |
| Maximum | 5122.32 | 5198.8 | 4201 | 4466.12 | 4840 | 3268.8 | 4994.52 | 6028 | 3268 | 5410.78 | 4611.99 | 2857.06 | 18064.16 | 17661.2 | 12573.96 |
| Total societal costs | |||||||||||||||
| Mean (SD) | 183.74 (361.21) | 118.88 (265.37) | 356.33 (1276.82) | 246.76 (479.87) | 65.63 (103.15) | 310.14 (824.62) | 211.71 (817.26) | 136.05 (342.1) | 176.93 (430.94) | 447.95 (1676.83) | 72.27 (105.59) | 103.68 (171.63) | 1081.66 (2020.47) | 387.79 (655.19) | 947.07 (2521.82) |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum | 1553.85 | 960 | 6428.5 | 1936.5 | 337.5 | 4102.5 | 4415 | 1592 | 2120 | 9015 | 440 | 600 | 9028.5 | 2552 | 12676 |
| Total all c | |||||||||||||||
| Mean (SD) | 4497.61 (565.02) | 4407.76 (693.58) | 3017.51 (1421.18) | 1148.69 (1479.19) | 3215.29 (1209.9) | 2335 (1051.97) | 2590.89 (2225.42) | 3123.73 (1806.39) | 2194.72 (873.79) | 1589.55 (2149.22) | 2827.65 (1674.5) | 18889.75 (915.7) | 10184.17 (3509.55) | 13986.55 (4382.28) | 10244.91 (3298.72) |
| Minimum | 3058.45 | 1766 | 999.88 | 23.4 | 574.36 | 167.23 | 7.2 | 251 | 7.2 | 23.7 | 253.38 | 9.6 | 4874.25 | 2692.23 | 1462.19 |
| Maximum | 5963.07 | 5748.8 | 9211.1 | 5160.12 | 5147.48 | 4522.1 | 9073.41 | 7620 | 3659 | 9208.6 | 4794.68 | 3114.81 | 18292.16 | 20213.2 | 21882.63 |
List of abbreviations
- ACPA
- anti-citrullinated peptide antibody
- ACR
- American College of Rheumatology
- ACR20
- American College of Rheumatology 20
- ACR50
- American College of Rheumatology 50
- ACR70
- American College of Rheumatology 70
- AE
- adverse event
- AR
- adverse reaction
- bDMARD
- biologic disease-modifying antirheumatic drug
- BNF
- British National Formulary
- BSR
- British Society of Rheumatology
- CCG
- Clinical Commissioning Group
- CDAI
- Clinical Disease Activity Index
- CI
- confidence interval
- CRF
- case report form
- CRN
- Clinical Research Network
- CRP
- C-reactive protein
- csDMARD
- conventional synthetic disease-modifying antirheumatic drug
- CZP
- certolizumab pegol
- DAS28
- Disease Activity Score of 28 joints
- DMARD
- disease-modifying antirheumatic drug
- ECG
- electrocardiography
- eMit
- electronic market information tool
- EQ-5D
- EuroQol 5 Dimensions
- EQ-5D-3L
- EuroQol 5 Dimensions, 3 levels
- ESR
- erythrocyte sedimentation rate
- EULAR
- European League Against Rheumatism
- EVPI
- expected value of perfect information
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HAQ
- Health Assessment Questionnaire
- HAQ-DI
- Health Assessment Questionnaire Disability Index
- HTA
- Health Technology Assessment
- IB
- investigator’s brochure
- ICER
- incremental cost-effectiveness ratio
- IgG1
- immunoglobulin G1
- IL
- interleukin
- IMP
- investigational medicinal product
- IR
- inadequate response
- ITT
- intention to treat
- LTE
- long-term extension study
- MTX
- methotrexate
- NB
- net benefit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NSAID
- non-steroidal anti-inflammatory drug
- PP
- per protocol
- PPI
- patient and public involvement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RAQoL
- Rheumatoid Arthritis Quality of Life
- RCT
- randomised controlled trial
- rDNA
- recombinant deoxyribonucleic acid
- REFLEX
- randomised valuation of long-term efficacy of rituximab in rheumatoid arthritis study
- RF
- rheumatoid factor
- SAE
- serious adverse event
- SD
- standard deviation
- SDAI
- Simplified Disease Activity Index
- SJC
- swollen joint count
- SmPC
- summary of product characteristics
- SSAR
- suspected serious adverse reaction
- SUSAR
- suspected unexpected serious adverse reaction
- TB
- tuberculosis
- TJC
- tender joint count
- TNF
- tumour necrosis factor
- TNFi
- tumour necrosis factor inhibitor
- VAS
- visual analogue scale