Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/45. The contractual start date was in April 2011. The draft report began editorial review in February 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jan E Clarkson reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study. Alison M McDonald reports grants from the NIHR HTA programme during the conduct of the study. John DT Norrie reports grants from the University of Aberdeen and grants from the University of Glasgow outside the submitted work. He was a member of the NIHR HTA Commissioning Board (2010–16), is currently a member of the NIHR Editorial Board (2015–present) and is currently the deputy chairperson of the NIHR HTA General Board (2016–present). Marjon van der Pol reports grants from the NIHR HTA programme during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Ramsay et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The subsequent chapters of this monograph describe Improving the Quality of Dentistry (IQuaD), a National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme-funded trial testing the relative effectiveness of different types of oral hygiene advice (OHA) and the optimal frequency of periodontal instrumentation (PI). The trial protocol has been published. 1
The reason for the trial
Background
Epidemiology
Periodontal disease is an inflammatory disease that affects the soft and hard tissues supporting teeth. This disease is largely preventable, yet it remains the major cause of poor oral health worldwide and is the primary cause of tooth loss in older adults. 2,3 Severe periodontitis is the sixth most prevalent human disease, with a standardised prevalence of 11.2%. 4
The categorisation of periodontal disease is based on which of the tissues surrounding and supporting the teeth are affected and is classified into two broad categories: (1) gingivitis and (2) periodontitis. Gingivitis is a reversible condition characterised by inflammation and bleeding at the gingival margin. The gum becomes swollen and red because of the inflammation and will bleed easily on probing. It is a prerequisite for periodontitis and is also a risk indicator for caries progression. Periodontitis is the irreversible destruction and loss of the supporting periodontal structures (periodontal ligament, cementum and alveolar bone). 5 The result can be unsightly gingival recession, sensitivity of the exposed root surface, root caries (decay), mobility and drifting of teeth and, ultimately, tooth loss.
Gingivitis and periodontitis are a continuum of the same inflammatory disease6 but it is currently not possible to predict if and/or when an individual will progress from the reversible gingivitis to irreversible periodontitis. Accumulation of microbial dental plaque is the primary aetiological factor for gingivitis and periodontitis, as well as dental caries. However, progression of the disease is known to be affected by other factors including genetics (host’s defence mechanisms to bacterial infection), calculus, smoking and systemic comorbidities including type 2 diabetes mellitus. 7–10 Although several risk factors have been identified, the lack of certainty as to whether or not, and when, they may cause progression to irreversible periodontitis in an individual causes a challenge for the profession.
The 2009 UK Adult Dental Health Survey (ADHS)11 provides evidence that the majority of UK adults might be at risk of developing periodontal disease: 66% of dentate adults had visible plaque, indicating that tooth brushing was ineffective, and 68% had calculus in at least one sextant of the mouth. Gingival bleeding was demonstrated in 54% of dentate adults and 45% of dentate adults had periodontitis (defined by at least one site with a clinical probing depth of ≥ 4 mm), increasing with age from 19% in those aged 16–24 years to 61% in those aged 75–84 years. Indicators of severe disease (at least one site with a clinical probing depth of ≥ 6 mm) also increased with age, affecting 14% of those aged ≥ 65 years. Only 17% of dentate adults had excellent periodontal health, which was defined by the ADHS 200911 as ‘no bleeding, no calculus, no periodontal probing depths of 4 mm or more and in the case of adults aged 55 or above, no loss of periodontal attachment over 4 mm or more anywhere in their mouth’.
As microbial dental plaque accumulation is considered the most important risk factor for periodontitis, its disruption or removal is a key component of prevention, combined with the control/management of other risk factors (e.g. smoking). 12,13
Prevention strategies aim to prevent the inflammatory process from destroying the periodontal attachment as well as prevent the recurrence of inflammation in successfully treated patients. 14
Individuals and dental care professionals have different roles to play in prevention. Effective individual self-care (tooth brushing and interdental aids) for plaque control is considered the foundation stone of successful periodontal prevention and therapy of disease. 13 The current annual public spend on oral care products in the UK alone is £950M. 15 The dental care professionals’ role in prevention and periodontal treatment involves providing patients with OHA (self-care) and PI. There is no agreed published content of OHA, but the overall aim of this intervention is to encourage effective self-care. PI (or ‘scale and polish’) comprises removal of plaque and plaque retentive factors [e.g. calculus (tartar) deposits] which, together with the removal of overhanging restorations (poorly adapted dental fillings), facilitate adequate patient-performed oral self-care. In the UK, almost all of this treatment is provided by general dental practitioners and dental hygienists/therapists in the primary care setting. The British Society of Periodontology16 advises that consideration be given to referral to a specialist periodontist of patients exhibiting severe or aggressive forms of periodontal diseases.
Periodontal instrumentation is one of the most frequently provided treatments in general dental practices. In the year 2014/15, there were 2.2 million claims for this simple periodontal treatment in Scotland, costing £31.5M. 17 This represents 8% of the total NHS budget spend of general and public dental services and 25% of the monetary value of all fee-per-item treatments provided in these settings. There were also 2910 claims for intensive scaling, at a cost of £200,000. During this time frame in England, it was estimated that 12.9 million courses of treatment involved PI for adults and approximately 0.9 million courses for children. 18
Dental reimbursement in Scotland is a retrospective ‘fee-per-item’ service for which dentists are primarily reimbursed for the number of individual treatments provided following completion of a course of treatment. In 2006, England’s dental health service moved from a similar model to a prospective ‘Unit of Dental Activity’ (UDA) system. Dentists agree contracts in advance with health boards to provide a prespecified number of UDAs. In both health-care systems, non-exempt patients face a patient copayment. In Scotland, the copayment equals 80% of the fee-per-item payment up to a prespecified upper limit. In England, there is a fixed copayment according to the band of treatment provided.
Evidence base
Despite evidence of an association between sustained good oral hygiene and a low incidence of periodontal disease and caries in adults,19 there is a lack of strong and reliable evidence to inform clinicians of the relative clinical effectiveness (if any) of different types of OHA that can be delivered in a dental setting.
Prior to the start of the IQuaD trial, a number of relevant systematic reviews20–22 evaluating OHA had been conducted, with some inconsistency in their findings. A recent systematic review23 of psychological approaches to behaviour change for improved plaque control in periodontal management reported benefits of using goal-setting, self-monitoring and planning for improving oral hygiene. Patient understanding of the benefits of behaviour change and the seriousness of periodontal disease were also considered important by the authors of the review. 23 A number of the included trials were of short duration, had non-experimental designs and were rated as being at high risk of bias. A meta-analysis of the available trials was not possible and the results and recommendations should be interpreted with caution.
The evidence to inform clinicians of the clinical effectiveness and optimal frequency of PI is mixed. A Cochrane systematic review24 of routine scale and polish (PI) for periodontal health in adults found insufficient evidence to determine the effects of routine PI treatments, providing little guidance for policy-makers, dental professionals or patients. Only three trials25–27 were eligible for inclusion and all were rated as being at an unclear risk of bias. Given that PI is routinely provided in general dental practice, it is noteworthy that only one of the eligible trials25 was conducted in primary care. Following baseline PI, Jones et al. 25 compared no PI delivery with 6-monthly PI or 12-monthly PI, with a 24-month clinical follow-up. The trial25 recruited only periodontally healthy participants. The Cochrane systematic review24 assessed the body of evidence for PI as being of low quality. The need for further well-conducted trials of sufficient duration, including research investigating patients’ willingness to pay (WTP), was highlighted. 28
The relative effectiveness of OHA and PI was assessed in the IQuaD trial, a robust, adequately powered randomised controlled trial (RCT) in primary dental care.
The questions the IQuaD trial addressed
Aim
The aim of this study was to compare the clinical effectiveness and cost-effectiveness of theory-based, personalised OHA or PI at different time intervals (no PI, 12-monthly PI or 6-monthly PI), or their combination, with routine care in improving periodontal health in dentate adults attending general dental practice.
Objectives
The primary objectives were to test the clinical effectiveness and cost-effectiveness of the following dental management strategies:
-
personalised OHA versus routine OHA
-
6-monthly PI versus 12-monthly PI
-
6-monthly PI versus no PI.
The secondary objectives were to:
-
test the clinical effectiveness and cost-effectiveness of a combination of personalised OHA and PI at different time intervals
-
measure dentist/hygienist beliefs relating to giving OHA, PI and maintenance of periodontal health.
Chapter 2 Methods of the study
Study design
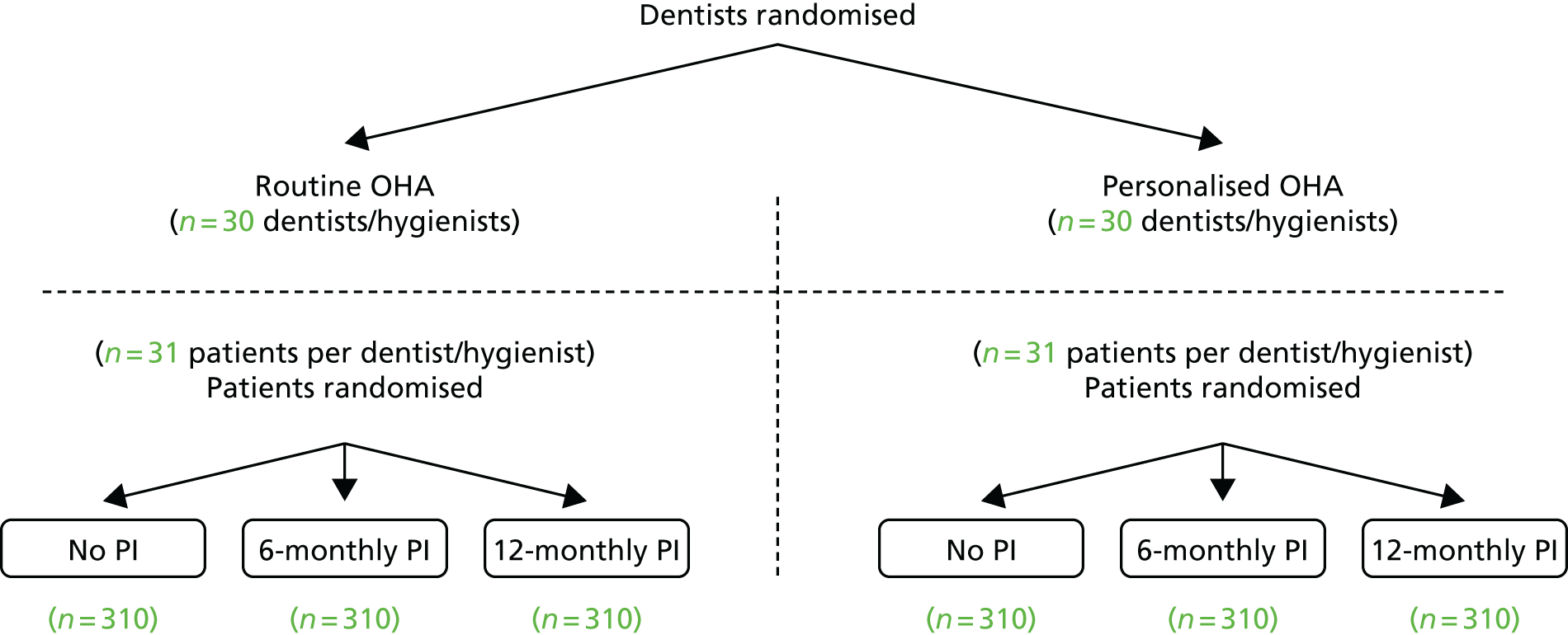
This study was a multicentre, cluster randomised controlled, open trial with blinded outcome evaluations and 3-year follow-ups. The comparisons were made within a factorial design using a combination of cluster and individual participant randomisation. Dental practices were randomised to either a routine or personalised OHA group. All participants were seen in the same dental practice by a dentist or hygienist (a ‘cluster’) and received either routine (current practice) or personalised OHA depending on their dental practice’s allocation. This was the optimal design to address the concern that contamination could happen if each dentist delivered routine OHA to some participants and personalised OHA to others. To test the effects of PI, each individual participant was randomised to one of three groups: (1) no PI, (2) 6-monthly PI (current practice) or (3) 12-monthly PI (Figure 1).
FIGURE 1.
Study design.
Ethics approval and consent
Favourable ethics opinion for the IQuaD trial was confirmed by the East of Scotland Research Ethics Service on 24 March 2011 [Research Ethics Committee (REC) reference number 10/S0501/65].
Participants and procedure
Recruitment and consent of dental practices
Recruitment of dental practices was achieved by a variety of methods. The IQuaD trial was presented at a number of national conferences and promoted within dental professional publications to raise awareness of the trial within the dental profession. In addition, the Scottish Dental Practice Based Research Network and the Faculty of General Dental Practitioners provided trial information to their members. A series of trial information and recruitment evenings were organised across Scotland and north-east England. Potential dentist participants were sent personalised invitation letters to these events in which the reasons for the trial, design of the trial and practice involvement were described, and dental professionals were given the opportunity to discuss participation with the trial team. Information packs about the trial were posted to dentists who were unable to attend a meeting.
Trial team members telephoned dental practices to follow up the trial information letters and noted interest of involvement. Principal dentists (usually the owner of the practice) who were interested in the trial were asked to provide written consent for the dental practice (cluster) to participate. All participating dentists and hygienists within a dental practice were individually invited to complete a consent form and the clinician belief questionnaire (CBQ) prior to cluster randomisation. Participating dental practices were asked to identify dates in advance for training in trial processes and at least three dates for the screening and recruitment of potentially eligible participants.
Recruitment and consent of participants
The identification of potential participants in each dental practice was supported by the Scottish Primary Care Research Network in Scotland and the UK Clinical Research Network in England. A variety of patient appointment management strategies are utilised within dental practices across the UK. Therefore, the IQuaD trial developed a flexible and pragmatic participant recruitment strategy that aimed to adapt to each dental practice’s usual appointment management system. Some dental practices arrange routine check-up appointments for their patients up to 6 months or 1 year in advance, while other practices send letters or mobile phone text message reminders to their patients when their routine dental examinations are due, asking them to contact the dental practice to make an appointment. Dental practices were asked to send an invitation letter to attend a trial screening session, along with a patient information sheet and baseline questionnaire to potentially eligible participants who were due to attend for a routine dental examination and who were regular attenders and did not have severe periodontitis [Basic Periodontal Examination (BPE) score of 4]. This was sent, at most, 6 weeks in advance of the patient’s routine dental examination appointment. Patients who were not interested in taking part were asked to contact their practice for an alternative appointment.
Trial outcome assessor (OA) teams, consisting of qualified dental hygienists/dental therapists and dental nurses employed by the trial, attended the screening and recruitment sessions in participating dental practices to obtain consent from potentially eligible participants and collect the baseline clinical measurements and questionnaires of consented participants.
At the screening appointment, the dentist was available to discuss the trial with potential participants and answer any questions. The OA was present at this appointment and answered any questions specifically related to the trial. Patients who did not wish to take part were seen by their dentist/hygienist, who provided an examination, OHA and/or PI as normal. Potentially eligible participants provided written informed consent to the trial before the baseline clinical outcomes were measured by the OA to confirm a patient’s eligibility for the trial. Reasons for ineligibility or declining to participate were recorded at this session. Participants excluded from the trial solely because of a BPE score of 4 or * (furcation involvement) were not randomised to a PI allocation and were given the opportunity to consent to follow-up by annual postal questionnaires and clinical follow-up in a separate cohort group (see study documentation, available at www.journalslibrary.nihr.ac.uk/programmes/hta/090145/#/; accessed October 2017). All participants received a baseline PI and OHA from their own dentist or hygienist after the baseline outcome measures and questionnaires were collected by the OA team prior to participants being informed of their randomised PI allocation.
Inclusion/exclusion criteria
Dental practices
Inclusion criteria
-
NHS provider for adult patients.
-
Primary care provider.
-
Willing to follow protocol.
Exclusion criteria
-
Providing only private dental care to adults.
-
Unwilling to follow protocol.
Participants
Inclusion criteria
Adult patients (aged ≥ 18 years) with periodontal health, gingivitis or moderate periodontitis (BPE score of 0, 1, 2 or 3) who:
-
were dentate
-
had attended for a check-up at least twice in the previous 2 years
-
received their dental care in part or in full as a NHS patient.
Exclusion criteria
-
Patients with periodontal disease with a BPE score of 4 (clinical probing depth of > 6 mm and/or furcation involvements or attachment loss of ≥ 7 mm) in any sextant on the basis that more extensive periodontal care was indicated.
-
Patients with an uncontrolled chronic medical condition (e.g. diabetes mellitus, immunocompromised).
Trial outcome assessor training
Before participant recruitment and 3 years’ clinical follow-up, the OA teams were trained in the recording of the trial clinical outcomes. The training was delivered by trial collaborators who have extensive experience of clinical periodontal research (PH, GM). The emphasis of the training was on the consistency of the scoring, both intra- and interassessor, to achieve assessor alignment.
The practical steps of clinical outcome assessment were agreed on in advance, including sequence of outcome assessment; time allocation; sequence around the mouth; isolation as well as the angulation, positioning and pressure of the University of North Carolina (UNC)-15 probe to ensure a standardised approach across the OA teams.
It is widely accepted that methods of assessing gingival inflammation that provoke gingival bleeding do not allow for repeated assessment. 29 The training for the primary outcome of gingival inflammation as bleeding therefore involved OA group discussion of the technique of assessment, scoring definition, as well as demonstrated photograph and clinical examples. This was repeated for the outcome of calculus.
Periodontal probing depths were recorded at six sites on all erupted teeth using a probing force of approximately 25 g on an independent, but similar, cohort of patients to those recruited to the study at the Newcastle upon Tyne and Dundee dental schools. Assessments by an experienced clinical periodontal researcher (LH) were used the reference standard. Intra- and interoutcome assessor scores were recorded and kappa scores of ≥ 0.60 achieved, with over 95% of scores within 1 mm.
Trial interventions
Both cluster-level intervention (OHA) and individual participant-level intervention (PI) were delivered by the dental practice dentist or dental hygienist in line with each individual dental practice’s usual practice.
Oral hygiene advice
Routine oral hygiene advice
Routine OHA was defined as the OHA currently being provided by the dental practices. There is no published information describing ‘routine’ OHA, but anecdotal evidence suggests that it is often minimal (e.g. ‘you need to brush your teeth more frequently’) or not provided at all.
Personalised oral hygiene advice
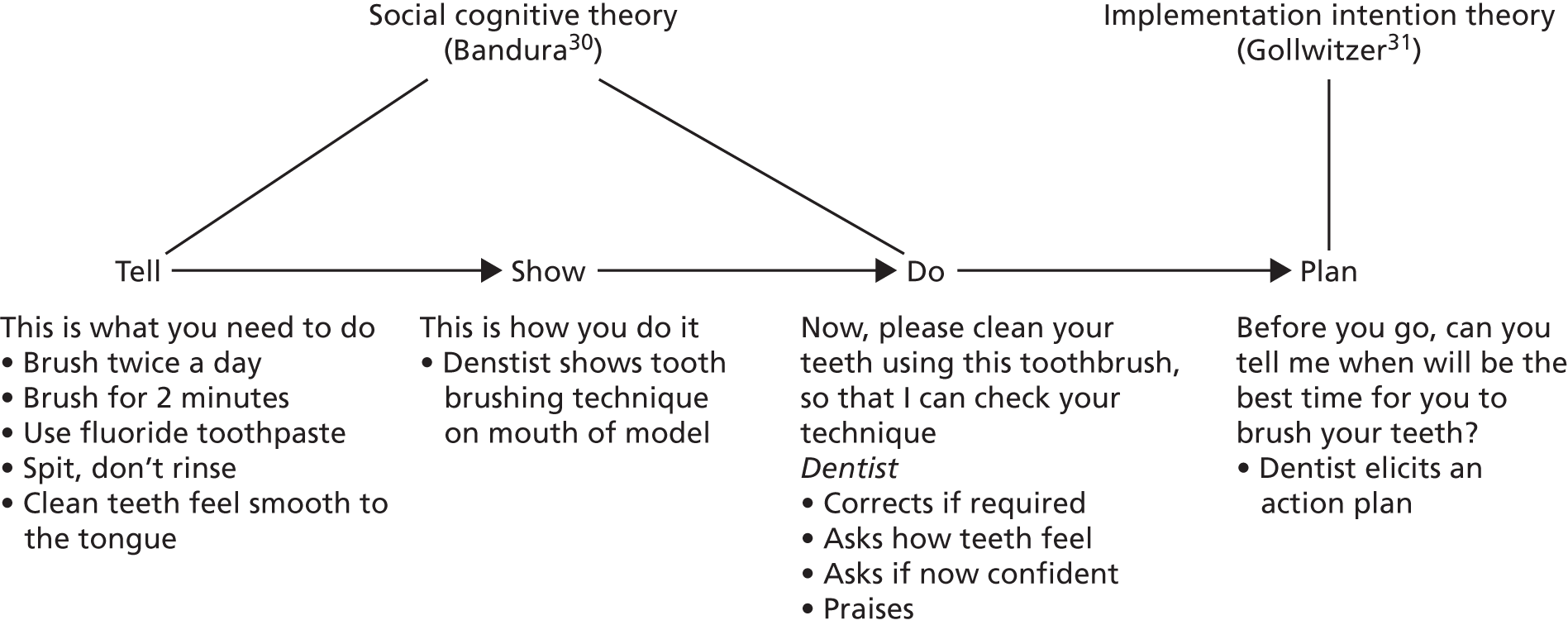
The personalised OHA intervention used two well-established psychological models for explaining and influencing behaviour (Figure 2). The first was a pre-motivational model, social cognitive theory. 30 This theory proposes that a key variable influencing motivation and behaviour is self-efficacy, assessed as a person’s confidence in their ability to perform behaviour. According to this model, the sources of self-efficacy are doing the behaviour (performance: cleaning their teeth), seeing someone else do it (vicarious example/modelling: the dentist demonstrated to patients how to use the oral health-care tools to clean their teeth on a model of the mouth), being encouraged to perform it (verbal persuasion: the dentist helped the patient make oral hygiene a habit with the right plan) and how we feel afterwards (physical state: the dentist discussed biofeedback, highlighting what clean teeth would feel like). Based on this model, an intervention to influence oral hygiene behaviour should target oral hygiene self-efficacy via these sources. The second model was implementation intention theory,31 a post-motivational model designed to help people put their intentions into practice, bridging the gap between motivation and behaviour. This theory proposes that making an explicit action plan about where and when a behaviour will be performed increases the likelihood of performing it. Action plans work by setting up a cue to remind the individual to perform the behaviour. Therefore, oral hygiene behaviours are likely to be very sensitive to action planning, as they can easily be linked to other behaviours most people do every day, for example tooth brushing after the cue of eating a meal or before going to bed.
FIGURE 2.
The OHA intervention behavioural framework.
The intervention was tested in a study32 consisting of 84 dental practices and 799 patients. The results of this pilot study32 supported the theoretically framed intervention as an effective method of influencing oral hygiene beliefs and behaviour. Participants who received the intervention had significantly better behavioural (timing, duration, method), cognitive (confidence, planning) and clinical (plaque, gingival bleeding) outcomes than the participants in the control group receiving routine care.
Training in the delivery of the personalised oral hygiene advice
Training in the delivery of the personalised OHA intervention was provided by a clinical member of the trial team to all dentists/hygienists within a dental practice randomised to this allocation. The content and the delivery of the intervention were standardised as a series of steps (see study documentation, available at www.journalslibrary.nihr.ac.uk/programmes/hta/090145/#/; accessed October 2017) designed to take place within an average primary care consultation, taking approximately 5 minutes in total.
A training DVD demonstrating a consultation using the steps was also developed and provided on a memory stick to all practices assigned to personalised OHA intervention (available at http://dentistry.dundee.ac.uk/nihr-hta-iquad-trial; accessed October 2017). The practices were also provided with laminated instruction sheets for use in the dental surgery. Dentists/hygienists retained these training resources in order to be able to undertake self-directed training as required throughout the trial. New clinicians appointed to any of the IQuaD trial dental practices (clusters) throughout the trial were invited to take part and provide consent to IQuaD if they had taken over the care of any IQuaD trial participants. Full training in trial methodology and intervention delivery was provided, as detailed above.
Frequency of oral hygiene advice
At baseline, all participants received OHA in accordance with the cluster-level randomisation allocation. Reinforcement of OHA was provided at the discretion of the dentist/hygienist during the trial.
Periodontal instrumentation
The definition of PI was as used in standard practice and would include the removal of plaque and calculus from the crown and root surfaces using manual or ultrasonic scalers, with no adjunctive subgingival therapy (e.g. antibiotics)33 and the appropriate management of plaque retention factors.
Baseline periodontal instrumentation
A full mouth supra- and subgingival PI was carried out by the practice dentist/hygienist on all participants prior to the participant or dental clinician being aware of the trial allocation. No time limit was set on this treatment and dentists/hygienists were instructed to scale the teeth and root surfaces until they were free of all deposits and were smooth to probing.
Experimental periodontal instrumentation
Experimental groups received a PI at 6- or 12-monthly intervals in accordance with the individual participant-level randomisation. Participants allocated to the no-PI groups attended their dentist at time intervals determined by current practice. However, participants and dental practices were advised that every trial participant should be invited to attend their dentist for a routine examination appointment at least every 12 months.
Randomisation
Practice allocation to oral hygiene advice group
Recruited dental practices were allocated to routine or personalised OHA by minimisation on two factors: (1) practice employed a dental hygienist (yes/no) and (2) practice size (one or two dentists in practice/three or more dentists). This cluster-level randomisation was conducted using the automated, central randomisation service at the Centre for Healthcare Randomised Trials (CHaRT), University of Aberdeen, after the dental practice consent form was received at the Trial Coordinating Office in Dundee (TCOD) and before any potential participant had been approached.
Participant allocation to periodontal instrumentation group
Participants were allocated to the PI trial arms using the automated, central randomisation service at the CHaRT, University of Aberdeen, with access by both telephone and web. Allocation took place once the OA had completed the baseline outcome assessment and was minimised on (1) absence of gingival bleeding on probing (yes/no), (2) highest sextant BPE score (BPE score of < 3/BPE score of 3) and (3) currently smoking (yes/no).
The OAs were informed that allocation had taken place but were blinded to allocation, with the actual allocation transmitted to the TCOD. A letter was sent to all participants to inform them of their PI allocation and all participants received a £25 gift voucher in recognition of their contribution to the study. The TCOD provided each individual recruited dental practice with a list of projected dates for PI and review (no-PI group), according to the individual participant-level allocation, to be delivered throughout the trial for all their trial participants. The list was updated and re-sent by TCOD on an annual basis to each practice.
Descriptive measures
Participant descriptive measures were collected at baseline and annually by self-administered postal questionnaire. They included the time since the last visit at the dental practice, the type of attendee (regular vs. non-regular), the self-reported number of PIs and OHA received in the last 12 months and by whom, smoking status and the type of toothbrush (manual vs. electric). Descriptive measures are presented in Table 1 by year and randomised group, using either mean, standard deviation (SD) or n (%), as appropriate.
| Participant dental characteristics | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| Age (years), mean (SD), n | 47.8 (15.8), 623 | 47.9 (15.7), 625 | 47.8 (15.8), 626 | 47.4 (16.1), 1008 | 48.3 (15.3), 866 |
| Male | 223 (36) | 229 (37) | 225 (36) | 387 (38) | 290 (33) |
| Date of last visit to the dental practice | |||||
| < 1 year ago | 569 (91) | 547 (88) | 553 (88) | 893 (89) | 776 (90) |
| 1–2 years ago | 38 (6) | 57 (9) | 49 (8) | 79 (8) | 65 (8) |
| > 2 years ago | 5 (1) | 4 (1) | 6 (1) | 9 (1) | 6 (1) |
| Missing | 11 (2) | 17 (3) | 18 (3) | 27 (3) | 19 (2) |
| Last course of treatment was | |||||
| NHS | 557 (89) | 573 (92) | 575 (92) | 918 (91) | 787 (91) |
| Private | 10 (2) | 9 (1) | 8 (1) | 15 (1) | 12 (1) |
| Combination | 40 (6) | 23 (4) | 24 (4) | 41 (4) | 46 (5) |
| Missing | 16 (3) | 20 (3) | 19 (3) | 34 (3) | 21 (2) |
| Do you think of yourself as | |||||
| A regular attendee | 565 (91) | 561 (90) | 574 (92) | 903 (90) | 797 (92) |
| Someone who sees a dentist when in pain or trouble | 43 (7) | 47 (8) | 31 (5) | 76 (8) | 45 (5) |
| Missing | 15 (2) | 17 (3) | 21 (3) | 29 (3) | 24 (3) |
| Last time you went to the dental practice were you given OHA? | |||||
| Yes | 410 (66) | 411 (66) | 420 (67) | 688 (68) | 553 (64) |
| No | 192 (31) | 187 (30) | 179 (29) | 275 (27) | 283 (33) |
| Missing | 21 (3) | 27 (4) | 27 (4) | 45 (4) | 30 (3) |
| By whom? | |||||
| Dentist | 298 (48) | 293 (47) | 291 (46) | 484 (48) | 398 (46) |
| Hygienist | 81 (13) | 85 (14) | 97 (15) | 141 (14) | 122 (14) |
| Both | 29 (5) | 27 (4) | 27 (4) | 53 (5) | 30 (3) |
| Missing | 215 (35) | 220 (35) | 211 (34) | 330 (33) | 316 (36) |
| Last time you went to the dental practice were you given a scale and polish? | |||||
| Yes | 371 (60) | 364 (58) | 381 (61) | 619 (61) | 497 (57) |
| No | 230 (37) | 236 (38) | 217 (35) | 341 (34) | 342 (39) |
| Missing | 22 (4) | 25 (4) | 28 (4) | 48 (5) | 27 (3) |
| By whom? | |||||
| Hygienist | 106 (17) | 108 (17) | 114 (18) | 182 (18) | 146 (17) |
| Dentist | 247 (40) | 236 (38) | 244 (39) | 394 (39) | 333 (38) |
| Missing | 270 (43) | 281 (45) | 268 (43) | 432 (43) | 387 (45) |
| What type of toothbrush do you normally use? | |||||
| Manual | 413 (66) | 397 (64) | 382 (61) | 647 (64) | 545 (63) |
| Electric | 180 (29) | 192 (31) | 205 (33) | 300 (30) | 277 (32) |
| Do not use toothbrush | 14 (2) | 18 (3) | 16 (3) | 28 (3) | 20 (2) |
| Missing | 16 (3) | 18 (3) | 23 (4) | 33 (3) | 24 (3) |
| Do you normally pay for dental treatments? | |||||
| Yes | 453 (73) | 436 (70) | 452 (72) | 716 (71) | 625 (72) |
| No | 155 (25) | 170 (27) | 150 (24) | 260 (26) | 215 (25) |
| Missing | 15 (2) | 19 (3) | 24 (4) | 32 (3) | 26 (3) |
| Do you have dental insurance? | |||||
| Yes | 19 (3) | 20 (3) | 24 (4) | 32 (3) | 31 (4) |
| No | 581 (93) | 583 (93) | 573 (92) | 932 (92) | 805 (93) |
| Missing | 23 (4) | 22 (4) | 29 (5) | 44 (4) | 30 (3) |
| How often do you prefer to have a scale and polish? | |||||
| Never | 13 (2) | 13 (2) | 8 (1) | 21 (2) | 13 (2) |
| Once every 2 years | 25 (4) | 20 (3) | 13 (2) | 38 (4) | 20 (2) |
| Once a year | 117 (19) | 122 (20) | 121 (19) | 193 (19) | 167 (19) |
| Twice a year | 269 (43) | 268 (43) | 289 (46) | 451 (45) | 375 (43) |
| Three times a year | 65 (10) | 65 (10) | 59 (9) | 89 (9) | 100 (12) |
| Four times a year | 67 (11) | 71 (11) | 56 (9) | 103 (10) | 91 (11) |
| More often | 26 (4) | 19 (3) | 25 (4) | 32 (3) | 38 (4) |
| Missing | 41 (7) | 47 (8) | 55 (9) | 81 (8) | 62 (7) |
Outcome measures
Primary outcomes
-
Clinical: gingival inflammation/bleeding on probing at the gingival margin at the 3-year follow-up.
-
Patient centred: oral hygiene self-efficacy at the 3-year follow-up.
-
Economic: net benefits (mean WTP minus mean costs).
Secondary outcomes
-
Clinical: (1) calculus, (2) clinical probing depth, (3) additional PI and (4) referral. (All of which were collected at 3-year follow-up.)
-
Patient-centred: (1) dental quality of life (QoL), (2) oral health behaviour and (3) knowledge. (All of which were collected during 3 years’ annual follow-up.)
-
Economic: costs to the NHS and patients; WTP.
-
Providers: beliefs relating to giving OHA and maintenance of periodontal health.
Note: the Periodontal Advisory Group considered that clinical attachment loss and plaque cannot be measured reliably; therefore, neither was included as outcomes.
Collection of clinical outcome measures
Gingival inflammation was measured according to the Gingival Index of Löe34 by running the UNC probe circumferentially around each tooth just within the gingival sulcus or pocket. After 30 seconds, bleeding was recorded as being present or absent on the buccal and lingual surfaces. The primary outcome (gingival inflammation/bleeding) was calculated by adding all the sites at which bleeding was observed and dividing these sites by the number of sites (twice the number of teeth) and presented as a percentage.
The colour-coded UNC periodontal probe was used to measure clinical probing depth and presence of calculus. Clinical probing depths were measured for all teeth (excluding third molars) at six sites per tooth: (1) mesiobuccal, (2) midbuccal, (3) distobuccal, (4) mesiolingual/palatal, (5) mid-lingual/palatal and (6) distolingual/palatal. Clinical probing depth was calculated as the mean of the six different sites measured per tooth and it is presented in mm.
Calculus was calculated by adding all the sites where calculus was observed and dividing it by the number of teeth and presented as a percentage.
The sequence of scoring was gingival inflammation/bleeding, clinical probing depths and calculus.
Collection of patient-centred outcome measures
Patient-centred outcomes were measured at baseline and annually by self-administered postal questionnaire.
The full details of the calculations used to generate each patient-reported outcome are available in Appendix 1, Section 1: methods for computing patient-reported outcomes.
Oral health belief outcomes
The questions for measuring patient-centred oral health belief outcomes were derived from social cognitive theory30 and the theory of planned behaviour. 31
The primary patient-centred outcome was self-efficacy, assessed as a person’s confidence in their ability to perform several different oral health behaviours. Each behaviour was measured using a 7-point scale scored from 1 (not at all confident) to 7 (extremely confident), with 7 being the best outcome.
Perceived behaviour control (PBC) outcome, assessed in terms of perceived ease or difficulty of performing several different oral health behaviours, was measured using a 7-point scale varying from 1 (strongly agree) to 7 (strongly disagree), with 7 being the best outcome.
Attitude outcome (perceived consequences of the behaviour) was measured using a 7-point scale varying from 1 to 7 (strongly agree to strongly disagree), with 7 being the best outcome.
Subjective norm outcome (perceptions of social pressure to perform the behaviour) was measured using three questions with points varying from 1 (strongly agree) to 7 (strongly disagree), with 7 being the best outcome.
Oral health behaviour outcome
Patient-reported oral health behaviour outcome was measured using four questions (about duration and frequency of brushing, frequency of flossing and frequency of interdental brushes use). Each response varied from 0 to 3, with a score of 3 being the best possible behaviour. The best value between flossing and interdental brushes was used as a measure of interdental cleaning behaviour. The responses for each question were summed to produce a summary score ranging from 0 to 9, with 9 being the best outcome.
Intention outcome
Intention (motivation to perform a behaviour) was measured using three questions (about duration and frequency of brushing and frequency of flossing). Each response varied from 0 to 3, with a score of 3 being the best possible intention. The responses were summed to produce a summary score ranging from 0 to 9, with 9 being the best outcome.
Quality of life
Quality of life was measured using the Oral Health Impact Profile-14 (OHIP-14). 35 The OHIP-14 is a 14-question, oral health-specific, patient-centred measure of symptoms experienced in the previous 12 months. The questions are scored from 0 (never) to 4 (very often) and summed to produce a summary score ranging from 0 to 56, with 56 being the worst outcome.
Additional periodontal instrumentation outcome
The additional PI outcome assessed at the 3-year follow-up was the number of participants self-reporting in their annual questionnaire that they had received any private PI at any time during the trial. It was based on the question, ‘In the last 12 months have you received a private scale and polish?’.
Having a plan to floss or brush
Participants were asked in all questionnaires if they had a plan to brush better and if they had a plan to floss better. If they answered ‘yes’ to either question, they were considered to have a plan. This outcome was also used as a measure of fidelity of the personalised OHA intervention.
Dentist beliefs
Clinician belief questionnaires were collected at baseline and at the 3-year follow-up. At baseline, the following variables were measured: self-efficacy, attitude, PBC, intention, subjective norm and action planning. All of the variables are measured on a scale of 1 to 7, except subjective norm (measured from 1 to 49) and intention (measured from 0 to 100). At follow-up, the variables measured were the same. Again, all variables varied from 1 to 7, except for subjective norm (varies from 1 to 49). The full details of the calculations used to generate each dentist belief outcome are available in Appendix 1, Section 1: methods for computing patient-reported outcomes.
Post hoc outcomes
Self-reported bleeding was assessed using one question with the scale varying from 1 (never) to 5 (very often) at the 3-year follow-up in the annual questionnaire.
Tertiary measures
Dental appearance was measured using four different questions (e.g. ’How clean and pleasant do your teeth look and feel after you brush and after a scale and polish’?). Each scale varied from 1 (not at all clean/not at all pleasant) to 7 (could not get any cleaner/extremely pleasant) and were summarised separately using the mean responses, with 7 being the best outcome.
Sensitivity was measured using four different questions. First, it was measured through a binary (yes/no) question: ‘Do you experience sensitivity in your teeth?’. Second, it was measured through three different scales, the first varying from rarely sensitive to always sensitive and the other two scales varying from 1 to 7 (never to all the time, and no pain to worst imaginable pain). These tertiary outcomes were self-reported at the 3-year follow-up.
Fidelity measures
Fidelity measures were collected at baseline and annually by self-administered postal questionnaire. These included whether or not participants had a plan to start brushing and/or flossing better and whether or not, after brushing, the patients did, or intended to, ‘spit, but not rinse’.
Dental practice compliance with the protocol was monitored in two ways: (1) through annual face-to-face visits by a member of the trial office team and (2) through an annual audit of six participants. All practices received at least one face-to-face visit between baseline and follow-up. This was to ensure practice compliance with the protocol and an understanding of their role, to answer any queries the practice staff had and to build and maintain a rapport with the practices to ensure a smooth transition into the follow-up phase of the trial. The annual audit of six participants (two from each PI allocation) was conducted with each practice to check if participants had been contacted to attend an appointment according to their allocated treatment group. If ≥ 50% of these six random participants had not been contacted or invited to attend, this triggered a telephone call to the practice to check the trial processes and, if required, a visit to review protocol was arranged.
Number of periodontal instrumentations received (routine data and self-report)
The self-reported number of PIs received in the previous 12 months was collected annually by postal questionnaire. If participants replied that they had not received any PI in the previous 12 months, this response was recorded as a zero for those 12 months. If participants replied that they received PI in the previous 12 months but did not indicate how many times, these responses were set as missing. The total number of received PIs over the 3 years was calculated by summing the number of times the participant self-reported receiving PI over the course of the trial.
Routine treatment data were obtained from Information Services Division (ISD) Scotland and the NHS Business Services Authority (BSA) in England for the time period of 2010 to 2016. The routine data informed on the number of PI received throughout the trial, by counting the number of claims for PIs made by dentists for each participant. Further details regarding how these variables were calculated can be found in Chapter 3.
Data collection
Assessment for eligibility and informed consent was achieved at the screening stage. Clinical outcome assessment was carried out at baseline and the 3-year follow-up. Participants were sent a trial questionnaire at baseline, 12 months, 24 months and 36 months following recruitment. Clinicians were asked to complete a belief questionnaire at baseline and at the 3-year follow-up.
Baseline
Dentists and/or hygienists were asked to complete the CBQ at baseline, after each individual dental practice had consented to take part in the IQuaD trial.
Patient-centred outcomes were collected at baseline by self-administered questionnaire.
Arrangement of clinical assessment appointments has been outlined in the recruitment section above.
Clinical outcomes were measured at baseline by trained OAs who were blinded to allocation. Gingival inflammation/bleeding scores, calculus, clinical probing depth and BPE scores were measured by the OA and recorded on the baseline clinical chart by the dental research nurse who was a member of the trial team.
At the baseline appointment, the OAs also collected personal details from participants, including preferred contact method (telephone, e-mail) and contact details.
Annual follow-up
Patient-centred outcomes were collected annually by self-administered postal questionnaires.
Like the baseline questionnaire, the annual follow-up questionnaire contained questions on self-efficacy, PBC, attitude, subjective norm, behaviour, OHIP-14, dental appearance and sensitivity. Questions designed to collect the descriptive measures were also included in this questionnaire, as were questions on dental costs for the health economic outcomes. Annual follow-up questionnaires at year 1 and year 2 post randomisation were posted from the CHaRT trial office to participants’ home addresses, along with a covering letter and reply-paid envelope for return of the completed questionnaire. Those participants who failed to return their questionnaire within 3 weeks were sent a reminder letter, a further copy of the questionnaire and a reply-paid envelope. If questionnaires were returned to the trial office marked ‘return to sender’, every effort was made to obtain updated contact details from the participant’s dental practice.
Three-year follow-up
As at baseline, dentists and hygienists were asked to complete the clinician CBQ. These were collected by the OAs when they visited the dental practices to collect participant clinical outcomes.
Patient-centred outcomes were collected at the 3-year follow-up using the annual follow-up questionnaire, with an additional question on self-reported bleeding. The questionnaire was sent to participants at least 3 weeks before the date of the first 3-year follow-up appointment made at the dental practice where they were recruited. Participants who had not returned a questionnaire by the time of their follow-up appointment were asked to complete a shortened one-page version of the follow-up questionnaire containing questions on the primary patient-centred outcome (self-efficacy) at that appointment. Participants who did not attend the follow-up appointment and who had not returned a questionnaire were sent a reminder letter, a further copy of the questionnaire and a reply-paid envelope to return the completed questionnaire to the CHaRT trial office.
All trial participants were invited to attend a trial follow-up assessment appointment by their dental practice either at the time of their routine check-up or at a separate trial assessment appointment at which the clinical outcomes were measured by trained OAs who were blinded to allocation. As at baseline, gingival inflammation/bleeding scores, clinical probing depths and calculus were measured by the OAs and recorded on the follow-up clinical chart by the dental research nurse who was a member of the trial team.
Participants who could not attend were contacted and given the option of attending on at least one other day or time.
All participants who attended the follow-up assessment received a certificate of appreciation for their participation in the trial, details of when the trial results would be published and a £25 gift voucher in recognition of their contribution.
Sample size
The study was powered to detect a difference of 7.5% in the number of gingival sites showing bleeding on probing at 3 years’ follow-up between routine and personalised OHA and for each pairwise comparison across both routine and personalised OHA groups. An OHA exploratory trial32 in the same population demonstrated that, at baseline, 35% of gingival sites were bleeding on probing with a SD of 25%.The PI Cochrane review24 suggested that a reduction of 15% of sites with bleeding was a plausible reduction for 6-monthly PI. If the effect is assumed to be linear, halving the number of PIs should half the expected difference of 15% of sites. If the effect is non-linear and the difference is > 7.5%, the trial would be adequately powered. A smaller effect would be of questionable clinical significance. There is some evidence that personalised OHA can reduce the number of gingival sites that bleed on probing by approximately 7.5%. 32
Oral hygiene advice
Assuming a conservative estimate of the intracluster correlation coefficient (ICC) of 0.05, a cluster RCT of 50 dentists collecting information from 25 patient participants each (25 × 25 = 625 patient participants per arm) was expected to have 90% power to detect a difference of 7.5%. Should the ICC be 0.1, the trial would still have approximately 80% power to detect a difference of 7.5%.
Periodontal instrumentation
Given that the comparison of routine versus personalised OHA required 625 participants in each arm, equal randomisation 1 : 1 : 1 (no PI, 12-monthly PI, 6-monthly PI) of participants implied 208 in each of the six groups. Assuming no interaction effect, the corresponding PI groups could be combined across both routine and personalised OHA groups, requiring 416 patients allocated to each PI group. Based on a sample size of 416 in each group, the trial has in excess of 95% power for each pairwise comparison to detect a difference of 7.5% in the percentage of gingival sites that bleed on probing.
Interaction
A substantive interaction effect between the PI interventions and the personalised OHA was not expected. Assuming an ICC of 0.05, the trial had 80% power to detect an interaction effect of 7.5%. Should the ICC be 0.1, the trial had approximately 80% power to detect an interaction effect of 10%.
The total number of dentists required was 50, and the total number of participants was 1248 (6 × 208). A previous trial36 in general dental practice suggests that we should expect to lose a small number of dental practices from the trial for reasons such as practices amalgamating with other practices or restricting NHS patients; a conservative assumption of 17% attrition for dentists and 20% for participants was anticipated, requiring a minimum of 60 dentists and 1860 participants. Each dentist was required to recruit, on average, 31 participants to ensure 25 at follow-up.
Collection of providers outcomes
Differences in the frequency of PI visits would have an impact on clinicians’ costs and benefits. The effect on incomes, job satisfaction and changes to the level of fees on the provision of PI were assessed using self-reported questionnaires administered to clinicians over the duration of the trial.
Statistical analyses of outcomes
Statistical analyses were based on all participants randomised. Three principal comparisons and their interactions were tested in the factorial design. The principal comparisons were between (1) all those allocated to routine OHA and all those allocated to personalised OHA, (2) all those allocated to no PI and those allocated to 6-monthly PI and (3) all those allocated to 12-monthly PI and those allocated to 6-monthly PI.
Reflecting the clustered nature of the data, the clinical outcomes were compared using mixed models with dental practice as a random effect, and with adjustment for minimisation variables [practice employs dental hygienist (yes/no) and practice size (one or two dentists in practice/three or more dentists) for the cluster-level randomisation and absence of gingival bleeding on probing (yes/no), highest sextant BPE score (BPE score of < 3/BPE score of 3) and currently smoking (yes/no) for the patient-level randomisation] and participant baseline values (when available) as fixed effects. Patient-centred outcomes were compared using a mixed model with a random effect for the participant (to take into account the three-level nested and correlated data structure: a variable number of observations nested within participants and participants grouped in dental practices) and another for the centre and adjusting for the same variables as before. Statistical significance was at the 5% level, with corresponding confidence intervals (CIs) derived.
Pre-planned subgroup analyses on the primary outcome included exploration of participant age (< 45 years, 45–64 years or ≥ 65 years), smoking status (non-smoker or smoker), periodontal disease severity (no clinical signs or presence of gingival bleeding on probing) and intervention provider (dentist or practice hygienist). Post hoc subgroup analyses were undertaken by region (Scotland or England) and clinical pocket depth (four or more sites with clinical pocket depth of ≥ 4 mm, or fewer than four sites). These analyses were conducted by including a subgroup by treatment interaction term in the primary outcome model described above. Conservative levels of statistical significance (p < 0.01) were sought, reflecting the exploratory nature of these pre-planned and post hoc subgroup analyses.
Non-response analysis
Descriptive data are presented, comparing the baseline characteristics of participants who did and who did not attend the 3-year follow-up clinical appointment. The t-test (continuous outcomes) and chi-squared test (dichotomous outcomes) were used to estimate the statistical significance of the differences between responders and non-responders.
Missing items
Missing items were dealt with according to the relevant published recommendations for the specific instruments. 35 For missing items with no published recommendation, self-efficacy, PBC, attitude and subjective norm were calculated as a mean of the items available. Behaviour and intention were calculated using complete cases.
Sensitivity analysis
To assess the possible effect of outliers, treatment effects by individual dental practices were explored to assess the influence of between-centre differences in the primary clinical outcome: bleeding. Any practices where the treatment effect excluded zero from the 99% CI and had at least twice the target difference estimate (either –15% or 15%) were excluded from the analysis comparing no PI with 6-monthly PI, as well as from the subgroup analysis.
Trial oversight
The University of Dundee acted as sponsor for the study. The trial was co-ordinated from the TCOD in the Dental Health Services Research Unit, University of Dundee, which provided day-to-day support for the dental practices and OAs/research nurses. The TCOD was responsible for transacting the randomisation of dental practices, collecting trial data (including baseline questionnaires) and co-ordination of participant follow-up. CHaRT, in the Health Services Research Unit, University of Aberdeen, provided the database applications and information technology (IT) programming for the trial, hosted the randomisation system, co-ordinated the participant follow-up questionnaires, provided experienced trial management guidance and took responsibility for all statistical aspects of the trial [including interim reports to the Trial Steering Committee (TSC) and the Data Monitoring Committee (DMC)]. A Project Management Committee (PMG) met monthly, chaired by the chief investigator, comprising co-investigators in Newcastle upon Tyne and key members of the TCOD and CHaRT. A Trial Management Committee (TMC) met biannually, chaired by the chief investigator, comprising co-investigators and key members of the TCOD and CHaRT. A Periodontal Advisory Group was convened to provide expert clinical advice to the TMC.
Independent TSCs and DMCs were also established. The TSC comprised an independent chairperson (a professor and honorary consultant of dental public health) and two further independent members (both members of the public acting as patient representatives). The TSC met approximately annually over the course of the trial. The DMC comprised an independent chairperson (a professor of restorative dentistry) and two further independent members (a professor of dental public health and a statistician). The DMC met approximately annually.
Patient and public involvement
Prior to the start of the IQuaD trial, patients were involved with the trial design and provided invaluable feedback on trial recruitment and communication strategies. Patients also contributed to the content and layout of the trial invitation, trial newsletters and the design of patient participant questionnaires. This ensured that trial participants could understand and easily complete these materials. Members of the public also contributed to trial oversight through membership of the TSC, including helping to interpret the trial findings and the preparation of the monograph.
Protocol amendments after trial initiation
A number of protocol revisions were made after trial initiation. These included:
-
an amended start date, list of grant holders, TSC members and DMC members
-
clarification of the measurement of gingival bleeding and the list of grant holders was updated
-
an amended timescale for sending patient information to ‘at most 6 weeks’, in line with routine practice for scheduling check-up appointments
-
an amended end date, in line with approved contract variation.
Adaptations of study administrative processes (e.g. the use of additional letters, revisions to letters) were also implemented.
Chapter 3 Health economic methods
The health economics analysis assessed the cost–benefit of theory-based, personalised OHA or PI at different time intervals (no PI, 12-monthly PI or 6-monthly PI), or their combination for improving periodontal health in dentate adults attending general dental practice. A cost–benefit analysis (CBA) reporting incremental net benefit, relative to standard care (routine OHA with 6-monthly PI), was conducted alongside the cluster RCT. Costs were assessed from a NHS perspective and a wider societal perspective including both NHS and participant costs. Benefits were assessed using a general population’s WTP for personalised OHA and PI, estimated from a discrete choice experiment (DCE). All analyses were based on the intention-to-treat (ITT) principle.
Cost–benefit analysis is based on the concept of maximising societal welfare including a wider, more holistic measure of value, going beyond narrow measures, such as quality-adjusted life-years, which are not appropriate in the context of dentistry. In CBA, both costs and benefits are measured in monetary terms and net benefits (benefits – costs) can be directly interpreted as either welfare increasing or welfare decreasing. The CBA conducted here is an incremental analysis of alternative policies of OHA with/without PI in terms of costs and benefits (WTP). The analysis focuses on whether moving from standard care to an alternative policy increases or decreases welfare.
Resource use and costs
Health service costs and Participant costs describe the methods for generating NHS and participant perspective costs, respectively.
Health services costs
Use of NHS dental services
Resource utilisation data for NHS treatments at dental practices over the trial follow-up period were collected using routine sources held by the ISD of the Scottish Government and the NHS BSA in England. Dentists are paid on the basis of claims and, therefore, relatively rich and reliable routine data on NHS dental care provided are available. Dental claims data were linked to the trial data set on an individual level to each trial participant.
Different remuneration systems are in place across the UK, which has implications for the level of detail that is available on resource use.
In Scotland, NHS payments are based on fee-for-service contracts. There is a high level of detail, as fees are attached to each individual item of service. This includes a separate fee for PI. Although there is a separate fee for intensive hygiene instruction, this cannot be claimed if a PI was claimed within the same course of treatment or within the previous 5 months.
In England, dentists are reimbursed according to contracts negotiated at (1) national level [General Dental Service (GDS) contracts negotiated through the British Dental Association] or (2) local level (personal dental contracts). The former accounts for 85% of dental contracts in England and forms the basis for the trial costing analysis. 37 Under the GDS contracts, each dental practice is contracted to provide an agreed amount of dental treatment, described in terms of UDAs. Treatments provided to participants are grouped into four bands based on the complexity of the treatment. Each band carries a set number of UDAs,38 as follows:
-
Band 1 (check-up, radiography, advice, PI) = 1 UDA.
-
Band 2 (band 1 treatments plus fillings, extractions, root canal treatments) = 3 UDAs.
-
Band 3 (band 1 and band 2 work plus crowns, dentures, bridges) = 12 UDAs.
-
Urgent band = 1.2 UDAs.
A number of services do not fall into any of the patient charge bands in their own right, but are awarded UDAs when provided outside other banded treatments. The number of UDAs for such treatments is as follows:
-
Arrest of bleeding = 1.2 UDAs.
-
Bridge repair = 1.2 UDAs.
-
Denture repair = 1.2 UDAs.
-
Issue of prescriptions = 0 UDAs.
-
Removal of sutures = 1 UDA.
As dentists in England are reimbursed on the basis of UDAs, the level of detail on resource use is clearly much lower than in Scotland. The claim form (FP17) in England collects some further information on treatments provided, including PI, but these are not associated with additional payments. Given the structure of the contract, it is less likely that there will be a difference in costs across the different policies in England as PIs and personalised OHA are not associated with any additional UDAs if they are provided alongside other treatments such as a dental check-up.
Costs were attached directly to items of service (Scotland) and treatment course bands (England) using the appropriate NHS unit cost. Unlike other NHS services, patients contribute to the cost of their dental care. The cost to the NHS is therefore equal to the unit cost minus the appropriate patient charge. The Scottish Statement of Dental Remuneration – Amendment No 130 Letter39 was used to attach unit costs to the treatment items in Scotland. In England, the cost of a UDA delivered under the GDS contracts can vary widely by practice. Determining an average cost per UDA across England is not straightforward. The 2009 Steele report40 suggested that the value of a UDA was approximately £25, but that actual practice-specific values ranged widely from £17 to £40. Work informing National Institute for Health and Care Excellence (NICE) public health guidance on oral health promotion41 assumed a mean cost of £25 (95% CI £15 to £35). We conducted a descriptive analysis of published data for contract payments in England in 2014/1537 and found that the median gross value (prior to deduction of participant charges) of a UDA (after removal of payments for units of orthodontic activity) is still approximately £25, ranging from £20 to £33 (5th to 95th percentile). Therefore, we use a unit value of £25 per UDA for the analysis. The NHS cost per UDA is varied in the sensitivity analysis to explore the impact of practice-level variation on results.
Some patients do not pay a co-charge (e.g. those in receipt of income support, pension credit, universal credit or tax credit, as well as those aged < 18 years, in full-time education or who are pregnant or who have given birth in the last 12 months). Table 2 summarises the most common breakdown of costs in England and Scotland. When participants were exempt from treatment charges, the full cost was attributed to the NHS budget. We tested for any cross-group differences in the proportion of participants exempt from charges, as any differences could bias predictions of incremental NHS costs. We also repeated the cost analysis assuming that all participants were exempt, that is, the NHS bears the full cost.
| Region | ||||||
|---|---|---|---|---|---|---|
| England | Scotland | |||||
| Treatment category | Total UDA cost (£) | aPatient charge (£)38 | NHS cost (if charge is paid) (£) | Treatment category | Patient chargea | NHS cost |
| Band 1 | 25.00 | 18.50 | 6.50 | Check-ups and case assessments | 0% of full cost | 100% of full cost |
| Band 2 | 75.00 | 50.50 | 24.50 | Other treatments | 80% of full cost | 20% of full cost |
| Band 3 | 300.00 | 219.00 | 81.00 | Maximum charge per course of treatment | £384.00 | Unlimited |
| Urgent | 30.00 | 18.50 | 11.50 | |||
Data are in the form of courses of treatment that have a commencement date and a claim payment date (Scotland) or a completion date (England). The base-case analysis excludes the baseline and final study visits, as treatments provided at these visits are not part of routine care nor the trial intervention. The exception is the provision of personalised OHA at the baseline visit. However, as explained above, these are typically not associated with additional payments in either region. A sensitivity analysis was conducted to explore the impact of including an additional fee for personalised OHA.
The baseline visit is excluded from the analysis by removing all treatment claims with an acceptance date < 90 days following the baseline examination date. Owing to practice-specific approaches to calling participants for their final study visit, some had their final study visit prior to randomisation and others after 3 years post randomisation. To remove all final visits from the claims data, we excluded all treatment acceptance dates at > 2.5 years following the baseline study visit. This implies that, after exclusion of all baseline and final study visits, the follow-up period for the CBA is 2.25 years. Sensitivity analysis explores the impact on net benefits of including all the baseline and final clinic visits in the analysis.
Routine data downloads were taken in August 2016 (ISD, Scotland) and November 2016 (NHS BSA, England). For Scotland, data are a complete and accurate reflection of resource use up to 3 months prior to data download (i.e. May 2016). For England, the data are likely to be an accurate reflection of resource use up to April 2016, reflecting the end of the financial year, by which time dental practices are required to have submitted all relevant information. The final clinic examinations took place in July 2016. Given that the base-case analysis excludes claims with treatment acceptance dates beyond 2.5 years post randomisation, the latest treatment claims in the data set have acceptance dates in February 2016 and, therefore, all data are assumed to provide an accurate and complete reflection of resource use for both regions.
All costs are presented in 2014/15 GBP values and discounted at a rate of 3.5% per annum. 42 A sensitivity analysis varying the discount rate was conducted.
Costs of other NHS services during follow-up
Annual questionnaires collected data on use of other NHS services for problems related to participants’ teeth. These included secondary care resource use [hospital inpatient data, outpatient consultations, day-case procedures and accident and emergency (A&E) attendances] together with general practitioner (GP) appointments and contact with NHS 24 (Scotland) or NHS Direct (England). As is common with participant-reported resource use, a number of conservative assumptions were made to address issues of discrepancy in the reported data:
-
When respondents reported that they did not attend a service but entered a number of visits, it was assumed that no resource use was incurred.
-
When respondents stated that they attended hospital as an inpatient for problems related to their teeth but provided no further information, it was conservatively assumed that such responses should be treated as day-case admissions. National data43,44 show that the majority of hospital admissions in the UK for dental problems are day-case procedures.
Rates of secondary care visits, including outpatient, day-case and hospital admissions, were checked against practice records and average rates in the general population to assess their validity. The outcomes of the validation exercise are reported in Chapter 5.
Participant costs
Three sources of participant cost were considered for the analysis: (1) charges for NHS dental care, (2) privately purchased dental care products and treatments and (3) time and travel costs (for participants and companions) associated with visits to the dental practice.
Charges for NHS dental care
Participant charges were sourced directly from the routine data sets described in Health services costs. Participant charges were based on the co-charge that should have been collected at the dental practice, as opposed to the actual value paid by the participant. This reflects the way in which dental practices are paid.
Self-purchased dental care products and private dental care
Participants reported how often they used interdental brushes, electric toothbrushes and manual toothbrushes in the annual questionnaires, as well as how often they replaced electric toothbrush heads. These resource use data were combined with an assumed average market price for four commonly available products, including a range of high- and low-cost items. Details of unit costs applied are provided in Appendix 2, Section 1: additional detailed methods for costing, the discrete choice experiment and mapping discrete choice experiment valuations to the trial outcomes. Resource use was multiplied by the average unit cost to generate a cost per participant.
Furthermore, in each annual questionnaire, participants were asked to recall and report the total cost of private PI and any other privately purchased dental care that they incurred over the previous year. It should be noted that, although participants were asked to report how often they received private PI, it may be difficult in some scenarios to determine whether treatment was private or via the NHS because, for example, many NHS dentists may also offer private PI. For this reason, there may be a risk of double-counting from the participant perspective, but only if NHS co-charges were interpreted as private treatment. All reported data were summed across the questionnaires to generate a cost to each participant of self-purchased dental care products and stated private care costs.
Costs of time and travel
Participants incur costs travelling to, and attending, NHS dental appointments. The number of visits to the dentist was collected from the routine data sets, conservatively assuming that each unique treatment acceptance date (rather than each claimed item) relates to a single dental visit. The true costs may be higher if a course of treatment involved more than one visit to the dentist. The opportunity cost to participants and companions of making a return journey to visit the dental practice was estimated using responses to the participant time and travel cost questions in the baseline questionnaire. It is assumed that the data collected on opportunity cost at baseline are applicable to each subsequent visit to the dental practice. National unit cost sources (Table 3) were used to value time commitment of attendance (including travelling to and from) at a dental appointment, as well as the opportunity cost based on the reported activity forgone through attendance. The calculated opportunity cost for a single visit was multiplied by the number of treatment courses from the routine data to generate a total participant level estimate of the opportunity cost of time and travel.
| Activity | Assumptions made | Unit cost (£) | Reference |
|---|---|---|---|
| Paid work | Median weekly wage: £528; 39.1 hours per week | 13.50 | Annual Survey of Hours and Earnings: 2015 Provisional Results, ONS45 |
| Self-employed | Average of two reports, 52 weeks, 40 hours per weeka | 15.13 |
The Boox Report 2014, Boox46 Cost Converter, EPPI Centre47 Self-employed Workers in the UK – 2014, ONS48 |
| Transport | Cost per mileb | 0.45 | Expenses and Benefits: Business Travel Mileage for Employees’ Own Vehicles, HMRC (approved millage rate)49 |
| Caring for a relative or friend | Median gross weekly pay: £341; 39.2 hours per weekc | 8.70 | NHS Pay Review Body, p. 17 (2015 values), DHSC50 |
| Leisure activities | Value of non-working time | 6.81 | WebTAG: TAG data book, July 2016, TAG51 |
| Childcare | Assumed as paid work | 13.50 | Annual Survey of Hours and Earnings: 2015 Provisional Results, ONS45 |
| Voluntary work | Assumed as paid work | 13.50 | Annual Survey of Hours and Earnings: 2015 Provisional Results, ONS45 |
| Unemployed | Value of non-working time | 6.81 | WebTAG: TAG data book, July 2016, TAG51 |
| Retired | Value of non-working time | 6.81 | WebTAG: TAG data book, July 2016, TAG51 |
| Parking | Participant’s individual costs | Various | Participant questionnaires |
| Housework | Cost of housework in the NHS, assumed annual salary £21,000 gross, 2012 values inflated to 2015 | 10.56 | NHS Pay Review Body, p. 17 (2015 values), DHSC50 |
Combined participant and NHS perspective costs
Data from Health services costs and Participant costs were combined to generate a wider combined NHS and participant perspective of the costing analysis.
Statistical analysis of cost data
Cost data were analysed according to best practice methodology for cluster randomised trials. 52–54 We used multilevel mixed-effects models that account for clustering of the data through a cluster-level random effect. 52 Models for cost data were estimated using the meglm command in Stata® 14 (StataCorp LP, College Station, TX, USA) specifying a gamma family and log-link function for cost data to account for skewness of the distribution. The decision to use this model for costs was based on a combination of the results of a modified Park’s test, which identified both an inverse Gaussian and gamma distribution to be acceptable, and software restriction (only gamma family allowed within Stata’s meglm command). The log-link function was decided on as it allowed easier model convergence and had a preferred Akaike information criterion score than an identity link. The models were estimated by maximum likelihood. Incremental costs were estimated as the mean cost difference between randomised policies (relative to the policy most representative of standard care: routine OHA with 6-monthly PI), together with 95% CIs. All models were adjusted for baseline characteristics (age, age-squared and sex) as well as minimisation variables (cluster level: hygienist employed at practice and practice size; individual level: smoker, gingival bleeding at baseline and BPE score band).
Owing to the high level of completeness, descriptive analyses of NHS dental costs and participant co-charges were based on complete cases. Participant costs were presented and analysed using multiple imputation of missing cost data from participant annual questionnaires. For the analysis of total costs within the CBA, the small proportion of missing routine data were also imputed to complete the data set. The imputation process followed best practice guidelines. 55,56 All imputation was completed using Stata’s multiple imputation procedure. Missing cost data were imputed at the level of cost component (e.g. missing data on private PI imputed for each annual questionnaire). Costs were imputed using predictive mean matching accounting for the repeated measures nature of the costs from the annual participant questionnaires. The average of the five closest values was used for each imputed data point. Data were imputed separately for each randomised group to preserve the allocation of participants. Imputation models were adjusted for cluster, age and sex as well as minimisation covariates. Sixty imputed data sets were used to ensure stable results and were combined using Rubin’s rules to generate estimates of costs. 57
Benefits (willingness to pay)
The main outcome measure in the economic evaluation is WTP, estimated using a DCE administered via online survey panels to a representative sample of the UK general population. The DCE was administered to a separate sample from the general population while the trial was ongoing and before the results of the trial were known. A DCE is a survey method for which participants are asked to make hypothetical choices between different goods or, in this case, different packages of dental care provided over 3 years (the follow-up period of the trial). An underlying assumption of all DCEs is that a treatment’s or service’s value depends on its attributes and the levels of those attributes. By including the price proxy within the DCE (i.e. the annual cost of each dental package), we can obtain a monetary valuation for any given dental treatment package. These WTP estimates are used as a measure of benefit within the CBA. Ethics approval for the conduct of the DCE substudy was obtained from the College Ethics Review Board at the University of Aberdeen (REF 2015/12/1278).
Selection of attributes and levels
The relevant attributes were, to a large extent, determined by the trial. Preferences needed to be elicited for the specific interventions (PI and personalised OHA) and for the outcomes that may vary across the arms of the trial (i.e. self-reported bleeding gums and perception of appearance and feel of cleanliness). We followed recommended practice to assess whether or not these attributes were important to individuals and whether or not there were any other important attributes that should be included in the DCE. We conducted a literature review and focus groups (FGs) with the general population to develop the final list of attributes and levels to present in the DCE (see Appendix 2, Section 2: research conducted for questionnaire development). We also engaged with, and sought advice from, a range of stakeholders, including clinical dental experts, practising dentists and hygienists, and patient representatives on our trial advisory group about the DCE content. Table 4 shows the final list of attributes and levels included in the DCE.
| Attributes | Levels |
|---|---|
| Dental advice | No detailed or personalised advice |
| Detailed and personalised advice from the dentist | |
| Detailed and personalised advice from the hygienist | |
| Scale and polish | None |
| One per year from the dentist | |
| One per year from the hygienist | |
| Two per year from the dentist | |
| Two per year from the hygienist | |
| Your teeth will look and feel | Very unclean |
| Unclean | |
| Moderately clean | |
| Clean | |
| Very clean | |
| In 3 years’ time, your gums will bleed | Never |
| Hardly ever | |
| Occasionally | |
| Fairly often | |
| Very often | |
| The cost to you | £10 per year (total cost: £30 over 3 years) |
| £20 per year (total cost: £60 over 3 years) | |
| £50 per year (total cost: £150 over 3 years) | |
| £100 per year (total cost: £300 over 3 years) | |
| £200 per year (total cost: £600 over 3 years) |
Additional levels were added for provider of service (dentist/hygienist) as this was found to be an important consideration in the FGs. The FGs showed that respondents would have difficulty understanding and attaching value to the primary clinical trial outcome (gingival inflammation/bleeding). Therefore, we included the self-reported frequency of bleeding measure from the participant questionnaire instead. Respondents were informed that having bleeding gums increases the risk of tooth loss in the future. The look and feel attribute was also based on the measures used within the same questionnaire. The cost attribute was framed as an annual commitment over a 3-year time period (trial follow-up). The levels of the cost attribute were determined from a number of sources, including the FGs, baseline trial data on WTP for PI and a supplementary questionnaire asked to FG respondents. Figure 3 provides an example choice set included in the DCE.
FIGURE 3.
Example choice task. Note: the example choice set shown here is for a respondent categorised as having poor dental health. See Appendix 2, Section 1: additional detailed methods for costing, the discrete choice experiment and mapping discrete choice experiment valuations to the trial outcomes, for more information on segmentation of choice sets based on dental health category. This hypothetical respondent has chosen Dental Care Package B and is willing to pay £100 per year for the package.

Experimental design
Choices presented to respondents in the DCE were drawn from the set of all possible attribute and level combinations. With a single three-level attribute and four five-level attributes, the total number of combinations in the full factorial is given as 31 × 54 = 1875, resulting in [(1875 × 1874) ÷ 2] = 1.76 million unique choice sets. Following standard practice in DCE literature, a D-efficient design was used. 58 Ngene version 1.1.2 software (Ngene, ChoiceMetrics Pty Ltd, Sydney, NSW, Australia) was used to create a main effects design consisting of 30 choice set questions. To ensure a manageable number of choice tasks, the tasks were split into three blocks, each with 10 questions. Two further choice questions were added to each block to check the validity of responses as follows: (1) choice 11 repeated choice 5 to test for consistency of preferences and (2) choice 12 was a non-satiation (dominance) test in which one option should be preferred over its alternatives if individuals value PI polish and personalised OHA. Therefore, each respondent completed a total of 12 choice tasks. The responses to the internal validity choice tasks were not included in the analysis; therefore, a total of 10 choice sets were analysed for each respondent.
As respondents were likely to vary considerably in terms of how many PIs they usually receive and the extent to which they experience bleeding gums, a segmented pivoted design was used to increase realism. For example, it is unrealistic that a respondent who has no bleeding gums and never has a PI would suddenly experience bleeding very often if they opt out. Respondents were assigned to one of three segments [(1) good, (2) moderate or (3) poor dental health] based on self-reported frequency of bleeding gums and the look and feel of their teeth (both attributes in the DCE). The pivoted design tailored the presented levels based on the segment to which the respondents were assigned. For example, respondents categorised as having ‘good’ dental health would never be presented with the worst level of bleeding gums and unclean teeth, as it would be highly unlikely for dental health to deteriorate so quickly because of forgoing PI or detailed OHA. The list of attribute levels included in the experimental design for the good, moderate and poor dental health segments can be found in Appendix 2.
Questionnaire development
Think-aloud interviews were used to test the wording and understanding of the questionnaire. Extensive piloting was used to assess the best way of reducing hypothetical bias and to ensure that attributes and levels were sensible, realistic and tradable to the majority of respondents. Full details of all methods used to select attributes and levels (literature searches and FGs) and test their feasibility in the survey (think aloud and piloting) are outlined in Appendix 2, Section 2: research conducted for questionnaire development.
The survey was divided into three sections. Section 1 asked about respondents’ experiences of dental care, how often they attend their dental practice and who they are treated by. Section 2 included questions about bleeding gums and aesthetic appearance to determine the appropriate version of the DCE to present. Section 3 concluded the survey with demographic questions and a number of debriefing questions about respondents’ views and attitudes to dental topics. The questionnaire was designed using Qualtrics (Provo, UT, USA).
Data collection
Data were collected using Qualtrics online panels. The survey was nationally representative, with population census quotas for age (among adult population), sex and region sought. We oversampled in Scotland (n = 125) to enable subgroup analysis across the main participating regions in the trial (England/Scotland). We further sought to achieve a mix of respondents with recent experience of dental services and without. During the pilot phase, we attempted to achieve 30% of responses from non-regular attenders, defined as not having seen the dentist in the previous 2 years. 11 However, we were unable to achieve this sample in the pilot and relaxed the target to a more achievable 10% for the main phase of data collection. Responses were anonymised and respondents were free to leave the survey at any point without having to give a reason for doing so. Respondents who completed the survey were reimbursed in a manner determined by the survey panel from which they were partaking.
Data analysis
The DCE data were analysed using best practice methods59 and followed random utility maximisation theory. 60 The utility of the option (Vj) is a linear function of the attributes and levels presented to the respondents, where:
Alternative-specific constant (ASC) is accounting for latent or unobserved utility associated with choosing any package, regardless of the attribute levels, β represents the marginal utilities associated with the attributes and levels. All categorical variables were included in the model using effects coding. The advantage of effects coding is that the reference level of an attribute is uncorrelated with the ASC. The reference categories for the effects coded attributes are no OHA, no PI, never bleeding and having teeth that look and feel very clean. For example, the value for ‘no advice’, βno advice, is retrieved as the negative sum of β1 Advice D + β2 Advice H.
The marginal WTP of an attribute level is equal to the marginal rate of substitution between that level and the cost attribute. For ease of interpretation, we also estimate the difference in WTP between the reference level and the attribute level. For example, the annual marginal WTP for OHA from the hygienist compared with the reference level (no OHA) is equal to:
Mixed logistic regression models in preference space were used to analyse the data to determine the impact of the attributes and levels on preferences for dental care services and outcomes. The ASC was assumed random and normally distributed for all models, reporting mean and SD. All other attribute levels were fixed effects, reporting mean coefficients only. The final model was estimated using the maximum simulated likelihood method, with 2000 Halton draws. All models were estimated using Stata 14 software.
The model was re-estimated separately using interaction terms for several pre-determined subgroups including (1) sex; (2) region, to determine if system of reimbursement has an impact on preferences; (3) smoking status, to determine if current or previous smokers’ preferences differ from the overall group; and (4) household income, to determine if greater ability to pay (annual income of ≥ £20,800) had an impact on preferences. Further subgroup analyses explored if the preferences of those with experience of PI or of treatment from a hygienist differed from the overall sample. We planned to conduct subgroup analysis by dental attendance (regular/non-regular attenders) but were unable to achieve sufficient numbers of respondents attending the dentist less often than every 2 years to power such an analysis. The impact that subgroups had on the main attribute level effects was assessed by the significance of interaction terms between subgroup identifier and attribute level coefficient. Likelihood ratio tests were used to test the joint significance of the interaction terms.
Estimating benefits (willingness to pay) for each trial participant
Benefits measured as WTP were estimated for each trial participant by attaching WTP values from the DCE to the relevant treatments provided (PI and personalised OHA) and to the relevant trial outcomes (self-reported bleeding when brushing and aesthetic outcome). The DCE provided estimates of WTP for zero, one and two PIs provided per year or a total of zero, three or six PIs over the trial period. A step-wise linear utility function was used to estimate WTP for the number of PIs other than those presented in the DCE. It was assumed that the provider of the baseline PI (dentist or hygienist) also delivered the intervention over follow-up. The impact that the results of this assumption have is also explored in a sensitivity analysis.
Willingness to pay for personalised OHA was attached based on randomised group, as all participating practices complied with their allocation to provide either personalised or routine OHA at the baseline visit. For the remaining attributes, including aesthetic outcome and frequency of bleeding gums when brushing, WTP values were directly attached at an individual level in the trial. The full mapping process for each attribute to the trial interventions and outcomes can be found in Appendix 2.
Discounting was applied at a rate of 3.5% per annum. 42 Discounted marginal WTPs were summed to generate a total WTP for each trial participant. Total WTP data were analysed using multilevel mixed-effects models with a random effect capturing cluster effects to account for the hierarchical nature of the trial. A normal distribution was assumed for benefits data; hence, the analyses were implemented using Stata’s mixed command. All models were adjusted for individual- and cluster-level minimisation variables, age and sex. The base-case analysis undertakes statistical imputation of missing data on benefits (i.e. WTP mapped to trial outcomes). Data were imputed for annual questionnaires at the level of WTP for each outcome (e.g. bleeding gums), following a similar procedure to that outlined for costs.
Cost–benefit analysis
The CBA compares the costs and benefits of alternative policies of OHA with/without PI with standard care (routine OHA with 6-monthly PI). Both costs and benefits are measured in monetary terms and net benefits can therefore be directly estimated (benefits – cost). The incremental net benefits indicate whether moving from standard care to an alternative policy increases or decreases overall societal welfare.
Statistical analysis of (incremental) net benefit
The imputed data sets were used to estimate net benefits and incremental net benefits. Net benefit was calculated for each participant in the trial according to Equation 3:
Multilevel mixed-effects models were used to analyse the data, implemented using Stata’s ‘mi estimate: mixed’ command following the approach of Gomes et al. 52,53 These models can explicitly recognise clustering in parameter estimates though the incorporation of cluster-level random effects (ujc, uje), while also adjusting for cluster- and individual-level covariates (minimisation variables: age and sex). The model addresses both individual- and cluster-level correlation between costs and benefits, using a bivariate normal distribution of the error terms. The model assumes linear additive effects for treatments and covariates. Restricted maximum likelihood was used to estimate the model for the base-case analysis. Interaction terms were included to retrieve the true impact of randomisation on costs/benefits. 54 Stata’s mimargins command was used to retrieve the values of the reference category of cost/benefit from the imputed data sets and the nlcom command was used to calculate incremental costs, incremental benefits and incremental net benefits across groups, together with 95% CIs.
Presentation of cost–benefit analysis results
The base-case perspective is that of the UK NHS. A wider perspective, including both NHS and participant costs, was considered as a secondary analysis. All results are presented as incremental costs, incremental benefits and incremental net benefits, relative to standard care. Note that exclusion of dominated policy options is not required in the absence of a threshold value of a ratio between costs and benefits (as would be the case in a cost–utility analysis).
Confidence ellipses, based on parametric calculations using the model variance–covariance matrix, were used to illustrate the uncertainty in each comparison of costs and benefits graphically on the cost–benefit plane. 61 The confidence ellipses illustrate the probability that each intervention is less/more costly and less/more beneficial than standard care. All policies with positive incremental net benefits are associated with higher levels of welfare than standard care. Note that this can be generated by a higher level of benefit and/or a lower level of cost of the policy. The strategy with the greatest incremental net benefit would be considered the preferred policy from a welfare perspective. The analysis is solely based on incremental changes in net benefits between policies and, as is common for economic evaluations alongside trials, makes no statement about the magnitude of the underlying net benefits of specific policies.
Analysis of uncertainty
The confidence ellipses of the cost–benefit plane illustrate the joint uncertainty surrounding estimates of incremental costs and incremental benefits on results. Further deterministic sensitivity analyses, outlined in Table 5, investigated the impact of assumptions around cost data, benefit estimation and model structure on the results.
| Base-case analysis | Sensitivity analysis | Justification for approach |
|---|---|---|
| Cost of personalised OHA incorporated according to routine claims database | Requirement of an additional consultation to provide personalised OHA | There was no additional cost of providing personalised OHA for most participants. An additional fee is included in sensitivity analysis according to the SDR for intensive hygiene instruction |
| Baseline and final visits excluded from analysis | Baseline and final treatment visits included | Base-case analysis pragmatically excludes treatment initiated at trial-driven visits. Sensitivity analysis incorporates all visits, including treatment requirements identified at baseline (treatment claim acceptance dates on or after randomisation date) or at follow-up (treatment acceptance dates included up to 3 years post randomisation) trial visits. Changes applied to both costs and WTP |
| Mapping aesthetic DCE outcome to a Right now response to the trial data | Mapping aesthetic DCE outcome to a After brushing teeth response to the trial data | Analysis conducted to address uncertainty about the most appropriate trial outcome to map marginal WTP onto |
| Unit cost per UDA assigned at the national average level (£25) | Vary the cost per UDA ± 20% | Payment per UDA substantially varies across practices. The base-case analysis gives the best estimate for the average cost per UDA in England, giving the most generalisable results. The sensitivity analysis explores the impact of practice level variation |
| Costs and benefits discounted at a rate of 3.5% per annum | Varying the discount rate between 0% and 6% | Standard exploration of methodological uncertainty |
| Professional delivering baseline intervention also delivered follow-up appointments (i.e. dentist or hygienist) | Explore assumptions in which all OHA and PI delivered by dentist; all OHA and PI delivered by hygienist | Anecdotal evidence from the trial suggests that the professional delivering baseline intervention may not have delivered all treatments for a participant over the follow-up period. The sensitivity analysis explores the likely impact of this assumption on results |
Cost–consequences analysis
In addition to the results of the CBA conducted for the primary economic analysis, we further considered all of the available information across all components of the study, using a narrative cost–consequences analysis (CCA) framework. The CCA is a useful approach to summarise all the study information succinctly for decision-makers. We use a balance sheet approach summarising the advantages and disadvantages of each policy option under consideration. The CCA includes the following information:
-
the results of clinical effectiveness, using both primary and secondary outcomes defined in the protocol
-
the results of all economic analyses, including costs to the NHS and participants, general population preferences for PI/OHA (results from the DCE) and overall incremental net benefits
-
a summary of information sourced from the CBQ investigating the practitioner’s perception of the advantages and disadvantages of different policy strategies.
No attempt was made to quantitatively synthesise the information across different categories. The results of the CCA are presented qualitatively as a means to aid decision-makers’ understanding of the overall outcomes of the trial as well as the trade-offs between clinical and economic outcomes.
Chapter 4 Trial results and clinical effectiveness
This chapter describes the clinical effectiveness results of the IQuaD trial. The chapter starts with an explanation of how the trial groups were derived. It then describes the study groups at trial entry. The results at the annual follow-up points are then reported, followed by a formal statistical analysis of the data for the principal measures of outcome.
Recruitment to the study
Recruitment of dental practices began in October 2011 and continued until May 2013. Participant recruitment to the trial began in February 2012 and continued until July 2013. Data were closed to follow-up on 2 September 2016. The derivation of the main study groups and their progress through the stages of follow-up in the trial is shown in Figure 4, in the form of a Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
FIGURE 4.
The Consolidated Standards of Reporting Trials (CONSORT) diagram. PAQ, patient annual questionnaire; PRX, post-randomisation exclusion. a, one was a post-randomisation exclution; b, four were post-randomisation exclusions.

Dental practices
Sixty-eight dental practices were initially recruited in total, with 49 in Scotland and 19 in England: 34 practices were allocated to give routine OHA and 34 to provide personalised OHA. From the 68 practices, four were excluded post randomisation in the routine OHA arm and one in the personalised OHA arm. The exclusions were due to a trial procedural error in the early recruitment phase of the study whereby practices were randomised when a dentist in a practice had verbally given consent to take part but before the practice principal dentist (usually the owner of the practice) had signed a consent form for the practice to take part in the trial. The principal dentist did not give permission to use their practice. The practice study intervention allocation was not known to any potential dentist, practice principal dentist (or patient) participants at the time of non-consent. Selection bias due to the post-randomisation exclusions was not, therefore, possible. Hence, there were 63 recruiting practices in total.
Participants
A total of 2341 patients were screened for trial entry and 183 (8%) of the screened patients were found to be ineligible (Table 6). The primary reason for ineligibility was due to a BPE score of 4 or * (160 patients). From those ineligible as a result of a BPE score of 4 or *, 144 (90%) patients agreed to join a separate cohort group. Further details about the cohort participants are given in Chapter 6. There were 281 patients eligible for the study who were not recruited. The main reason for non-recruitment was that the patient did not attend the baseline appointment (see Table 6).
| Total ineligible/total declined | OHA, n (%) | |
|---|---|---|
| Personalised (N = 1239) | Routine (N = 1102) | |
| Total declined | 123 (10) | 158 (14) |
| Unable to complete the study | 4 (< 1) | 24 (2) |
| Did not attend baseline appointment | 64 (5) | 72 (7) |
| No reason stated | 53 (4) | 61 (6) |
| Observing Ramadan | – | 1 (< 1) |
| Probes locked in safe and patient could not wait | 1 (< 1) | – |
| Did not have time to consider | 1 (< 1) | – |
| Total ineligible | 106 (9) | 77 (7) |
| BPE score of 4 or * | 92 (7) | 68 (6) |
| Uncontrolled medical condition | 13 (1) | 5 (1) |
| Too nervous | 1 (< 1) | 2 (< 1) |
| Not a regular attendee | – | 2 (< 1) |
Participants were recruited in 63 dental practices (see Appendix 3). A total of 1877 participants were recruited to the study, with 867 in the routine OHA arm (n = 289 allocated to receive no PI, n = 290 allocated to receive 6-monthly PI and n = 288 allocated to receive 12-monthly PI) and 1010 in the personalised OHA arm (n = 334 allocated to receive no PI, n = 337 allocated to receive 6-monthly PI and n = 339 allocated to receive 12-monthly PI). Of the total participants, 1348 (72%) were recruited across Scotland, including 121 (6%) recruited from the Scottish islands. No dental practice recruited > 2.5% of the participants. The average cluster size was 30.6 and 28.9 participants for personalised OHA and routine OHA practices, respectively. Three participants were subsequently identified to be non-NHS patients at consent (and therefore ineligible) and were thus excluded post randomisation.
Description of the groups at trial entry
Practice characteristics
Table 7 provides the dental practice characteristics and beliefs. The majority of practices employed hygienists and had three or more dentists. Participating dentists and hygienists completed a questionnaire at baseline (the CBQ) on their beliefs about providing OHA. Scores for belief variables varied from 1 to 7, where 7 represented the most positive outcome (e.g. the highest level of confidence or the most positive attitude). Intention and subjective norm were the exceptions: intention varied from 0 to 100, where 100 represented the highest level of intention, and subjective norm varied from 1 to 49, where 49 represented the highest level of subjective norm. The results suggested that dental professionals had positive attitudes towards, and high levels of intention of, providing OHA. There was little perceived influence of peers in the ability of the respondents to provide OHA (subjective norm) (see Table 7). Overall, there were no important differences between groups on any of the practice characteristics or beliefs at baseline.
| Dental practices | OHA, n (%) | |
|---|---|---|
| Personalised (N = 33)a | Routine (N = 30)a | |
| Employs hygienists | 24 (73) | 24 (80) |
| Has three or more dentists | 25 (76) | 24 (80) |
| Region | ||
| Scotland | 24 (72.7) | 20 (66.7) |
| England | 9 (27.3) | 10 (33.3) |
| Health professional beliefs | OHA, n (%) | |
| Personalised (N = 87)b | Routine (N = 75)b | |
| Dentist | 70 (81) | 63 (84) |
| Hygienist | 17 (20) | 12 (16) |
| Beliefs, mean (SD), n | ||
| Self-efficacy | 5.9 (0.7), 81 | 5.9 (0.7), 67 |
| Attitude | 5.4 (0.7), 81 | 5.4 (0.7), 67 |
| PBC | 3.7 (1.0), 81 | 3.7 (0.8), 67 |
| Intention | 90.0 (14.2), 77 | 85.7 (17.2), 64 |
| Has a plan to give OHA | 2.2 (1.4), 79 | 2.4 (1.4), 67 |
| Has a plan to give PI | 2.2 (1.3), 78 | 2.3 (1.3), 66 |
| Subjective norm | 13.5 (9.2), 78 | 11.3 (7.3), 67 |
Participant characteristics
Sociodemographic factors and dental characteristics
Participant and sociodemographic characteristics are shown in Table 1. Tables 1 and 8–16 follow a consistent format to aid interpretation of the main cluster and participant comparisons. Columns 2–4 of data represent the participant-level comparison and show the no PI, 12-monthly PI and 6-monthly PI groups, respectively. Columns 5 and 6 of data represent the cluster-level comparison and show personalised OHA and routine OHA, respectively.
The average age of participants was 48 years; the majority (around 65%) were women and regular attendees of the dental practice (> 90% reported that their last visit to the dentist was within 2 years prior to baseline). Around 60% reported having received OHA and a PI at their last dental appointment. For those who reported receiving one treatment or the other, the dentist was most often the provider of the OHA or PI. Overall, 64% of patients used a manual toothbrush and > 90% had no dental insurance. There were no substantive differences between groups on any of the sociodemographic factors or dental characteristics.
Clinical characteristics
Table 8 displays the results of the clinical assessment at baseline. The mean number of teeth per participant was 24. The highest sextant BPE score was ≤ 2 in two-thirds of the participants. The mean per cent of sites bleeding was 33%, and 35% of teeth had calculus present. The mean clinical probing depth was 1.8 mm and between 10% and 12% of participants in each group had four or more sites with a clinical probing depth of ≥ 4 mm.
| Clinical characteristics | Randomised group, mean (SD), n | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| Present teeth | 23.7 (4.5), 623 | 23.6 (4.5), 625 | 23.7 (4.7), 626 | 23.6 (4.6), 1008 | 23.7 (4.5), 866 |
| Highest sextant BPE score, n (%) | |||||
| 0 | – | 4 (1) | 1 (0) | 2 (0) | 3 (0) |
| 1 | 40 (6) | 42 (7) | 38 (6) | 73 (7) | 47 (5) |
| 2 | 376 (60) | 374 (60) | 377 (60) | 590 (59) | 537 (62) |
| 3 | 207 (33) | 205 (33) | 210 (34) | 343 (34) | 279 (32) |
| Gingival bleeding, n (%) | 608 (98) | 610 (98) | 612 (98) | 991 (98) | 839 (97) |
| Current smoker, n (%) | 135 (22) | 143 (23) | 147 (23) | 213 (21) | 212 (24) |
| % of sites bleeding | 33.5 (23.8), 623 | 32.5 (23.9), 625 | 32.4 (22.9), 626 | 34.3 (23.2), 1008 | 31.0 (23.7), 866 |
| % of teeth with calculus | 35.5 (26.3), 623 | 35.9 (27.4), 625 | 34.9 (26.8), 626 | 33.7 (25.8), 1008 | 37.4 (27.8), 866 |
| Mean clinical probing depth (mm) | 1.8 (0.3), 623 | 1.8 (0.3), 625 | 1.8 (0.3), 626 | 1.8 (0.3), 1008 | 1.8 (0.3), 866 |
| Four or more sites with a clinical probing depth of ≥ 4 mm, n (%) | 64 (10) | 75 (12) | 70 (11) | 117 (12) | 92 (11) |
There were no important imbalances across the randomised groups.
Patient-reported outcomes
The baseline patient-reported outcomes summary is shown in Table 9. More details about individual questions that produced the scales are given in Appendix 1.
| Patient-reported outcomes | Randomised group, mean (SD), n | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12 monthly (N = 625) | 6 monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| Self-efficacy | 5.2 (1.1), 612 | 5.3 (1.2), 607 | 5.2 (1.2), 607 | 5.2 (1.2), 976 | 5.2 (1.2), 850 |
| PBC | 4.5 (1.2), 607 | 4.5 (1.2), 603 | 4.5 (1.2), 606 | 4.5 (1.2), 972 | 4.4 (1.2), 844 |
| Attitude | 5.8 (1.2), 611 | 5.9 (1.1), 605 | 5.7 (1.3), 607 | 5.8 (1.2), 977 | 5.8 (1.2), 846 |
| Subjective norm | 5.3 (1.1), 601 | 5.3 (1.2), 598 | 5.2 (1.1), 597 | 5.3 (1.1), 958 | 5.2 (1.1), 838 |
| Behaviour | 4.7 (1.7), 608 | 4.7 (1.8), 604 | 4.8 (1.7), 605 | 4.6 (1.7), 971 | 4.9 (1.7), 846 |
| Intention | 5.5 (1.7), 560 | 5.5 (1.8), 568 | 5.6 (1.8), 556 | 5.4 (1.8), 903 | 5.6 (1.7), 781 |
| OHIP-14 | 6.0 (7.4), 595 | 6.4 (8.1), 591 | 6.5 (8.3), 591 | 5.9 (7.6), 952 | 6.7 (8.3), 825 |
Self-efficacy, the patient-reported primary outcome, was, on average, 5.2 points (on a scale of 1 to 7 points with 7 points meaning very confident).
Cognition variables
Perceived behaviour control was, on average, 4.5 points; attitude towards oral health behaviour was very positive and was, on average, 5.8 points and subjective norm was 5.3 points (scales of 1 to 7, with 7 meaning very confident).
Behaviour
The mean self-reported oral hygiene behaviour score was 4.7 points. The scale varies from zero (poorest hygiene behaviour) to 9 (perfect hygiene behaviour). The behaviour scale had space for improvement across the three behaviours measured: (1) frequency of brushing, (2) duration of brushing and (3) frequency of interdental cleaning (either flossing or the use of interdental brushes). In both brushing frequency and duration, around 10% of participants scored the maximum of 3 points (brushed more than twice a day, for longer than 2 minutes). Regarding interdental cleaning, around 20% of participants scored the maximum of 3 points (floss or use interdental brushes every day).
Intention
The mean intention to perform good OHA practice was 5.5 points (on a scale from 0 to 9, with 9 being the best outcome).
Quality of life
The mean score on the oral health QoL scale, OHIP-14, was 6.3 points on a scale form 0 to 56 (with 0 indicating perfect oral health QoL). The OHIP-14 75th percentile was 9 and the 99th percentile was 39, illustrating a generally good oral health-related QoL in this population.
The baseline participant self-reported data suggested that participants were confident that they could perform good toothbrushing practice, thought oral hygiene practice was a positive thing to do, were influenced by their peers to do it and intended to do it. However, the participants did not currently actually perform good oral hygiene practice and reported that there were external factors, PBC, that limited their ability to do so. Overall, across the randomised groups, there were no apparent imbalances in the patient-reported outcomes.
Trial follow-up
Attendance at 3-year clinical examination
The primary clinical outcome was collected at the 3-year clinical follow-up. Overall, 71% of the participants attended the appointment (see Appendix 1). Twelve participants were known to have died by the end of the 3-year follow-up. The main reasons for non-attendance were that the practice was unable to contact the participant (41%), the participant was unable to attend (30%) and the participant did not want to attend (13%). There were no important differences between groups for the reasons of non-attendance.
A comparison of baseline characteristics between those who did and those who did not attend the final appointment was undertaken. The results in Table 10 show that only a few factors differed between attenders and non-attenders. Attenders were, on average, older (50 vs. 43 years). Although there was a statistically significant difference in the OHIP-14 score, with the mean score reported by non-attenders higher than that reported by attenders, the difference of 1.3 points (0.15 of the SD of the score) was not thought to be clinically important. There was no evidence that non-attenders had different levels of disease, with BPE scores, bleeding, calculus and probing depths all very similar to those seen in attenders.
| Baseline characteristics | Attendance, mean (SD), n | p-value | |
|---|---|---|---|
| Attender (N = 1327) | Non-attender (N = 547) | ||
| Age (years) | 49.9 (15.1), 1327 | 42.7 (16.1), 547 | < 0.001 |
| Male, n (%) | 490 (37) | 187 (34) | 0.27 |
| BPE score, n (%) | 0.28 | ||
| 0 | 5 (0) | ||
| 1 | 82 (6) | 38 (7) | |
| 2 | 788 (59) | 339 (62) | |
| 3 | 452 (34) | 170 (31) | |
| Bleeding n (%) | 0.78 | ||
| Yes | 1295 (98) | 535 (98) | |
| No | 32 (2) | 12 (2) | |
| Self-efficacy | 5.2 (1.1), 1306 | 5.2 (1.2), 520 | 0.50 |
| OHIP-14 | 5.9 (7.3), 1276 | 7.2 (9.2), 501 | 0.003 |
| % of sites bleeding | 32.4 (23.1), 1327 | 33.9 (24.4), 547 | 0.20 |
| % of calculus | 34.7 (26.3), 1327 | 37.2 (28.0), 547 | 0.08 |
| Mean clinical probing depth (mm) | 1.8 (0.3), 1327 | 1.8 (0.3), 547 | 0.64 |
Annual questionnaire returns at years 1, 2 and 3
Approximately 77% of participants completed a follow-up questionnaire at 3 years. There were no substantive differences in response rates between the randomised groups. The overall rates of return of follow-up questionnaires at 1, 2 and 3 years are shown in Appendix 1.
Participant dental characteristics at 3 years
The participant dental characteristics at 3 years are shown in Table 11. The profile of characteristics is similar across the groups and similar to baseline responses (e.g. smoking status, type of toothbrush used, preferences for two PIs per year; see Tables 1 and 8). Characteristics for years 1 and 2 are given in Appendix 1, Section 2: participant dental characteristics (years 1 and 2).
| Patient and dental characteristics | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| Date of last visit to the dental practice (years ago) | |||||
| < 1 | 393 (63) | 386 (62) | 383 (61) | 630 (63) | 532 (61) |
| 1–2 | 17 (3) | 21 (3) | 12 (2) | 24 (2) | 26 (3) |
| > 2 | 3 (0) | 2 (0) | – | 4 (0) | 1 (0) |
| Missing | 210 (34) | 216 (35) | 231 (37) | 350 (35) | 307 (35) |
| Do you think of yourself as | |||||
| A regular attendee | 399 (64) | 394 (63) | 388 (62) | 632 (63) | 549 (63) |
| Someone who sees a dentist when in pain or trouble | 13 (2) | 16 (3) | 6 (1) | 24 (2) | 11 (1) |
| Missing | 211 (34) | 215 (34) | 232 (37) | 352 (35) | 306 (35) |
| Last time you went to the dental practice were you given OHA? | |||||
| Yes | 281 (45) | 339 (54) | 327 (52) | 534 (53) | 413 (48) |
| No | 131 (21) | 70 (11) | 67 (11) | 121 (12) | 147 (17) |
| Missing | 211 (34) | 216 (35) | 232 (37) | 353 (35) | 306 (35) |
| By whom? | |||||
| Dentist | 210 (34) | 217 (35) | 178 (28) | 357 (35) | 248 (29) |
| Hygienist | 39 (6) | 65 (10) | 83 (13) | 90 (9) | 97 (11) |
| Both | 30 (5) | 56 (9) | 66 (11) | 86 (9) | 66 (8) |
| Missing | 344 (55) | 287 (46) | 299 (48) | 475 (47) | 455 (53) |
| In the last 12 months, did you receive a scale and polish? | |||||
| Yes | 210 (34) | 334 (53) | 354 (57) | 491 (49) | 407 (47) |
| No | 199 (32) | 72 (12) | 40 (6) | 161 (16) | 150 (17) |
| Missing | 214 (34) | 219 (35) | 232 (37) | 356 (35) | 309 (36) |
| By whom? | |||||
| Dentist | 145 (23) | 212 (34) | 198 (32) | 317 (31) | 238 (27) |
| Hygienist | 54 (9) | 111 (18) | 138 (22) | 151 (15) | 152 (18) |
| Both | 8 (1) | 8 (1) | 13 (2) | 16 (2) | 13 (2) |
| Missing | 416 (67) | 294 (47) | 277 (44) | 524 (52) | 463 (53) |
| Smoked in the last 12 months? | |||||
| Yes | 50 (8) | 55 (9) | 45 (7) | 76 (8) | 74 (9) |
| No | 362 (58) | 355 (57) | 349 (56) | 580 (58) | 486 (56) |
| Missing | 211 (34) | 215 (34) | 232 (37) | 352 (35) | 306 (35) |
| What type of toothbrush do you normally use? | |||||
| Manual | 233 (37) | 223 (36) | 199 (32) | 345 (34) | 310 (36) |
| Electric | 158 (25) | 154 (25) | 167 (27) | 261 (26) | 218 (25) |
| Do not use toothbrush | 21 (3) | 31 (5) | 27 (4) | 48 (5) | 31 (4) |
| Missing | 211 (34) | 217 (35) | 233 (37) | 354 (35) | 307 (35) |
| How often do you prefer to have a scale and polish? | |||||
| Never | 19 (3) | 12 (2) | 11 (2) | 24 (2) | 18 (2) |
| Once every 2 years | 20 (3) | 9 (1) | 15 (2) | 20 (2) | 24 (3) |
| Once a year | 108 (17) | 113 (18) | 50 (8) | 144 (14) | 127 (15) |
| Twice a year | 184 (30) | 196 (31) | 212 (34) | 322 (32) | 270 (31) |
| Three times a year | 27 (4) | 40 (6) | 49 (8) | 71 (7) | 45 (5) |
| Four times a year | 41 (7) | 25 (4) | 46 (7) | 47 (5) | 65 (8) |
| More often | 4 (1) | 10 (2) | 10 (2) | 19 (2) | 5 (1) |
| Missing | 220 (35) | 220 (35) | 233 (37) | 361 (36) | 312 (36) |
Statistical analyses
Primary outcomes
Gingival inflammation/bleeding
The pre-chosen clinical primary outcome was mean gingival bleeding at the 3-year follow-up. The means and 95% CI for baseline and follow-up for each randomised group are displayed in Figure 5. All randomised groups followed a similar pattern: bleeding increased from baseline (mean 32% of sites) to 3-year follow-up (mean 39% of sites). The differences between groups and corresponding 95% CIs are shown in Table 12.
FIGURE 5.
Gingival inflammation/bleeding (mean and 95% CI), by randomised allocation. (a) OHA delivery and (b) PI interval.


| Primary outcomes | Estimate, p-value (95% CI) | ||
|---|---|---|---|
| No PI vs. 6-monthly PI | 12-monthly PI vs. 6-monthly PI | Personalised OHA vs. routine OHA | |
| Gingival inflammation/bleeding | |||
| Unadjusted | 0.71 (–2.0 to 3.7), 0.579 | –0.53 (–3.4 to 2.3), 0.715 | 0.02 (–6.3 to 6.4), 0.985 |
| Adjusted for baseline bleeding | 0.8 (–1.7 to 3.2), 0.534 | 0.1 (–2.4 to 2.5), 0.947 | –1.9 (–8.1 to 4.3), 0.549 |
| Adjusted for baseline bleeding and minimisation variables | 0.87 (–1.6 to 3.3), 0.481 | 0.11 (–2.3 to 2.5), 0.929 | –2.5 (–8.3 to 3.3), 0.393 |
| Self-efficacy | |||
| Unadjusted | –0.017 (–0.133 to 0.098), 0.767 | –0.082 (–0.198 to 0.033), 0.162 | 0.002 (–0.144 to 0.147), 0.984 |
| Adjusted for baseline self-efficacy | –0.098 (–0.189 to –0.007), 0.035 | –0.027 (–0.118 to 0.064), 0.564 | 0.003 (–0.110 to 0.115), 0.962 |
| Adjusted for baseline self-efficacy and minimisation variables | –0.028 (–0.119 to 0.063), 0.543 | –0.097 (–0.188 to –0.006), 0.037 | 0.017 (–0.089 to 0.123), 0.750 |
Three types of analyses are reported: (1) an unadjusted analysis, (2) an adjusted-for-baseline bleeding analysis and (3) a fully adjusted model accounting for baseline bleeding and minimisation variables (this is the prespecified primary model). Under ITT analysis, there was no evidence of a statistical or clinical difference between those randomised to receive 6-monthly PI and those randomised to receive no PI (difference 0.87%, 95% CI –1.6% to 3.3%; p = 0.481). Similarly, there was no evidence of a difference between 6-monthly PI and 12-monthly PI (difference 0.11%, 95% CI –2.3% to 2.5%; p = 0.929). The 95% CIs were small enough to exclude the prespecified clinically important difference of 7.5% in bleeding. There was also little evidence of a difference between participants randomised to personalised OHA and those randomised to routine OHA (difference –2.5%, 95% CI –8.3% to 3.3%; p = 0.393), although the 95% CI did not entirely rule out a 7.5% reduction. The results were robust to other adjusted/unadjusted models. The interaction between personalised OHA and 6-monthly PI was 1.7% (95% CI –3.8% to 7.3%). The interaction demonstrated that a participant receiving both personalised OHA and 6-monthly PI would have a further 1.7% bleeding reduction compared with just adding the individual effects of 6-monthly PI with personalised OHA. The interaction term ruled out an additional 7.5% reduction. The ICC at follow-up for bleeding for all participants was 0.23 (95% CI 0.16 to 0.31). A full description of the means (SD) for gingival inflammation at each time point is given in Appendix 1, Section 3: clinical and patient-reported outcomes by year of follow-up.
Self-efficacy
The pre-chosen patient-centred primary outcome was self-efficacy at the 3-year follow-up. The means and 95% CIs for baseline and follow-up for each randomised group are displayed in Figure 6, by randomised allocation. All groups followed a similar pattern. The score was similar from baseline (mean 5.2 points) up to the year 3 questionnaire (mean 5.3 points) in both the PI and the OHA groups. The differences between groups and corresponding 95% CIs are shown in Table 12. Under ITT analysis, there was no evidence of a difference between those randomised to receive 6-monthly PI and those randomised to receive no PI (difference –0.028, 95% CI –0.119 to 0.063; p = 0.543). Between those randomised to receive 6-monthly and those randomised to receive 12-monthly PI, there was a statistically significant difference, favouring the 6-monthly PI (difference –0.097, 95% CI –0.188 to –0.006; p = 0.037). However, this result is not clinically significant. There was also no evidence of a difference between participants randomised to personalised OHA and those randomised to routine OHA (difference 0.017, 95% CI –0.089 to 0.123; p = 0.750). The interaction coefficient between personalised OHA and 6-monthly PI was –0.007 (95% –0.22 to 0.20), suggesting no evidence of important interaction between interventions. The self-efficacy ICC for all participants was 0.06 (95% CI 0.04 to 0.09). A full description of the means (SD) for self-efficacy at each time point is given in Appendix 1, Section 3: clinical and patient-reported outcomes by year of follow-up.
FIGURE 6.
Self-efficacy score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.


Secondary outcomes
A full description of the means (SD) for the secondary outcomes at each time point is given in Appendix 1, Section 3: clinical and patient-reported outcomes by year of follow-up.
The clinical secondary outcomes were calculus and mean clinical probing depth collected at the 3-year follow-up. The patient-reported secondary outcomes were PBC; attitude; subjective norm; behaviour and intention collected at 1, 2 and 3 years; and private PI received throughout the trial collected at year 3. Self-reported bleeding was a post hoc secondary outcome collected at 3 years. The differences between groups, and corresponding 95% CIs, are shown in Table 13.
| Secondary outcomes | Estimate, p-value (95% CI) | ||
|---|---|---|---|
| No PI vs. 6-monthly PI | 12-monthly PI vs. 6-monthly PI | Personalised OHA vs. routine OHA | |
| Clinical | |||
| % of calculus | 8.0 (5.4 to 10.7), < 0.001 | 1.6 (–1.0 to 4.2), 0.231 | 2.3 (–5.8 to 10.3), 0.577 |
| Mean clinical probing depth (mm) | 0.003 (–0.024 to 0.030), 0.808 | 0.022 (–0.004 to 0.049), 0.102 | –0.024 (–0.084 to 0.036), 0.433 |
| Patient-centred outcomes | |||
| PBC | 0.02 (–0.09 to 0.13), 0.704 | –0.01 (–0.12 to 0.10), 0.838 | –0.02 (–0.12 to 0.07), 0.620 |
| Attitude | –0.137 (–0.273 to –0.001), 0.048 | –0.093 (–0.229 to 0.044), 0.183 | –0.019 (–0.134 to 0.097), 0.754 |
| Subjective norm | –0.07 (–0.18 to 0.04), 0.198 | –0.06 (–0.17 to 0.05), 0.272 | –0.04 (–0.14 to 0.06), 0.473 |
| Behaviour | –0.125 (–0.256 to 0.007), 0.064 | –0.164 (–0.296 to –0.032), 0.015 | 0.072 (–0.063 to 0.207), 0.297 |
| Intention | –0.10 (–0.25 to 0.05), 0.186 | –0.14 (–0.29 to 0.00), 0.054 | 0.09 (–0.06 to 0.25), 0.253 |
| OHIP-14 | 0.35 (–0.23 to 0.93), 0.239 | 0.41 (–0.17 to 0.99), 0.169 | –0.33 (–0.86 to 0.20), 0.229 |
| Private PIs, odds ratio (95% CI), p-value | 0.5 (0.3 to 0.7), 0.001 | 0.8 (0.6 to 1.2), 0.302 | 1.0 (0.7 to 1.6), 0.908 |
| Has a plan to better brush or floss, odds ratio (95% CI), p-value | 1.1 (0.9 to 1.3), 0.563 | 0.94 (0.77 to 1.16), 0.635 | 1.1 (0.9 to 1.4), 0.579 |
| Self-reported bleeding | –0.01 (–0.14 to 0.12), 0.886 | –0.03 (–0.16 to 0.10), 0.666 | –0.06 (–0.17 to 0.06), 0.333 |
Calculus
The calculus means and 95% CIs for baseline and follow-up for each randomised group are displayed in Figure 7. The mean level of calculus decreased across the no PI, 12-monthly and 6-monthly PI groups. The calculus level was statistically significantly higher in the no-PI group than in the 6-monthly PI group (mean 8.0%, 95% CI 5.4% to 10.7%, p < 0.001). There was no evidence of a difference in mean calculus between 12-monthly PI and 6-monthly PI or between personalised and routine OHA (Table 13).
FIGURE 7.
Calculus percentage (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.
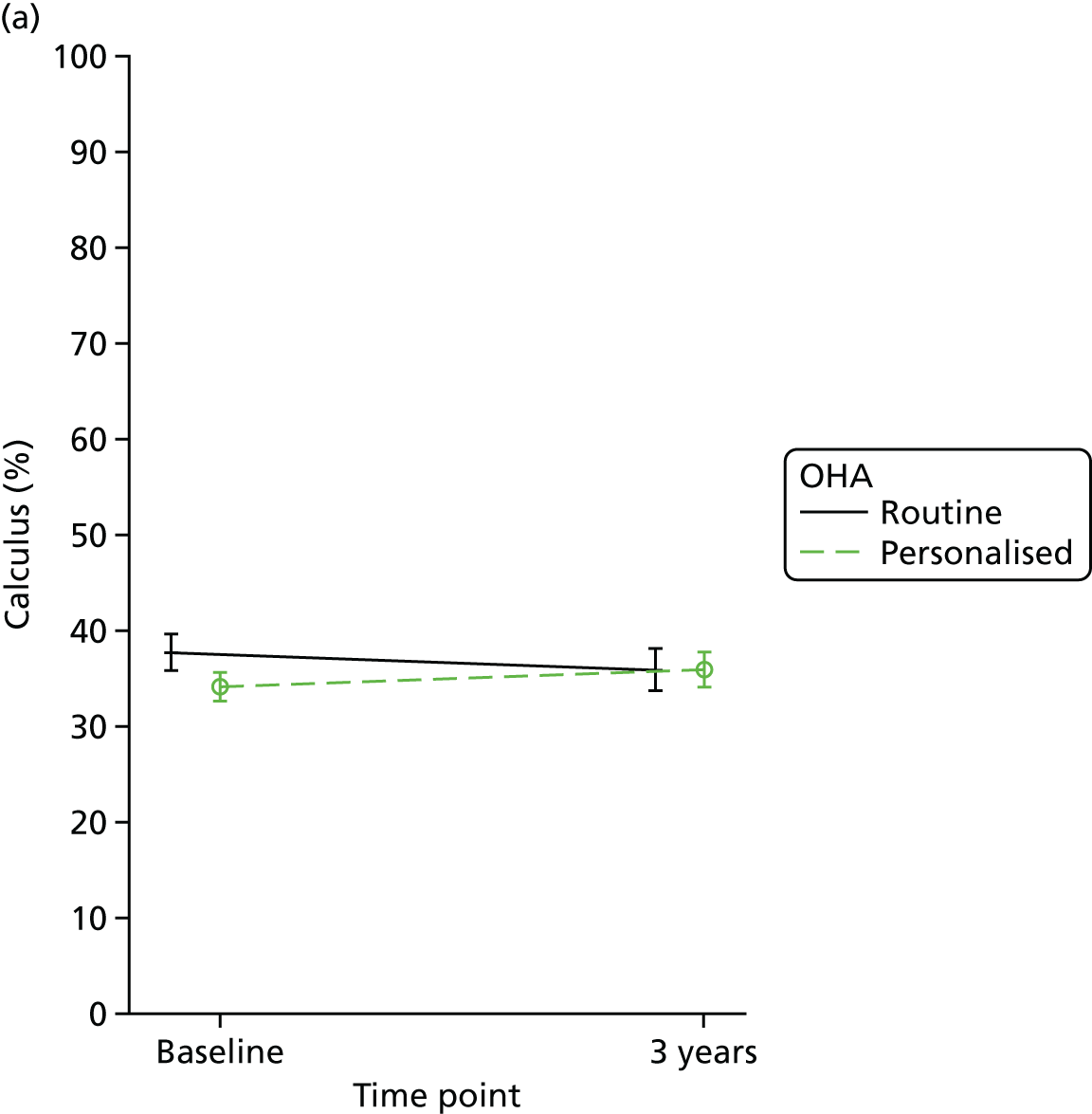
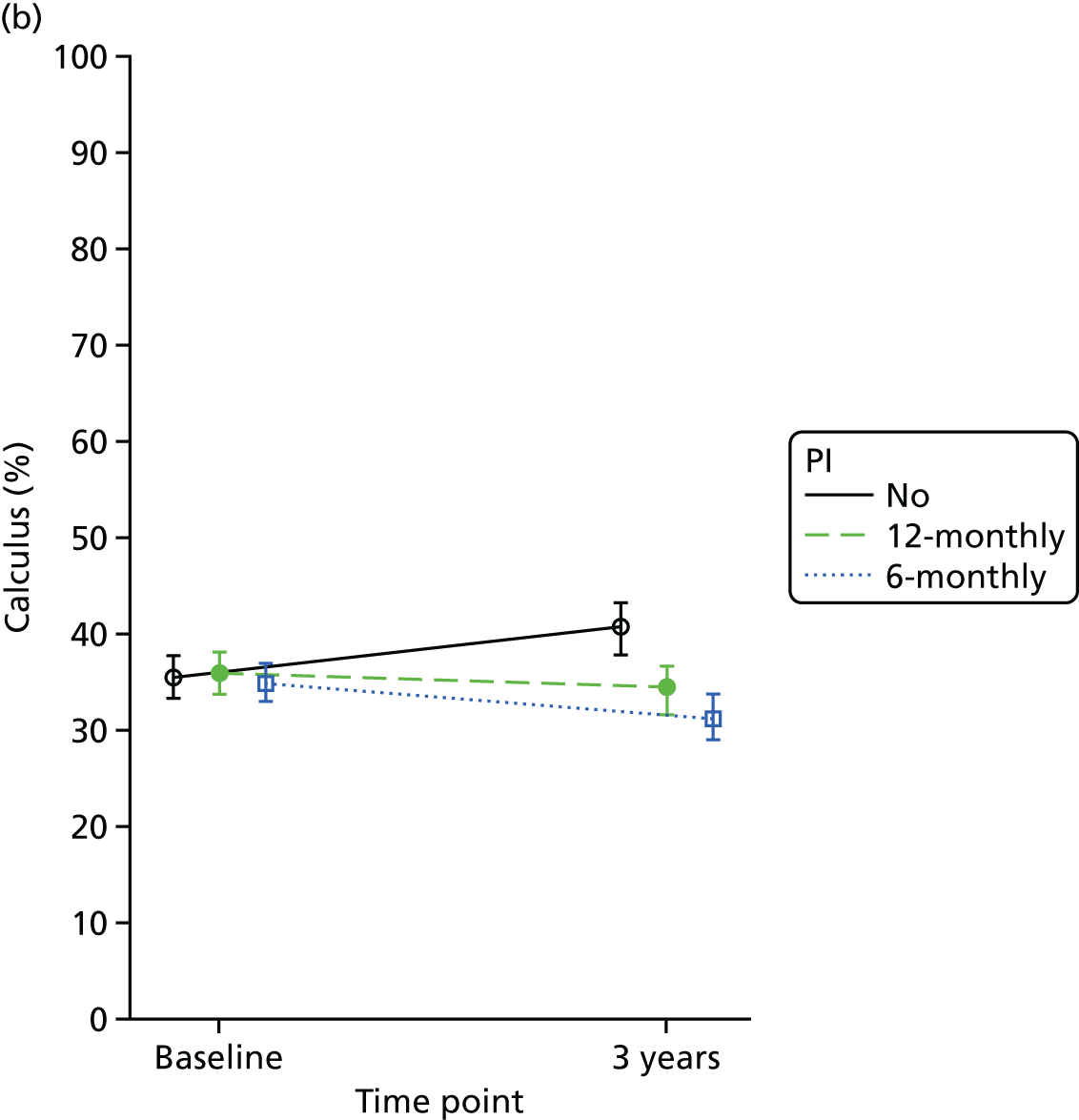
Clinical probing depth
The mean clinical probing depth and 95% CI for baseline and follow-up for each randomised group are displayed in Figure 8. There was no evidence of changes from the baseline clinical pocket depths and no evidence of differences between any of the randomised comparisons (see Table 13).
FIGURE 8.
Mean clinical probing depth (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.
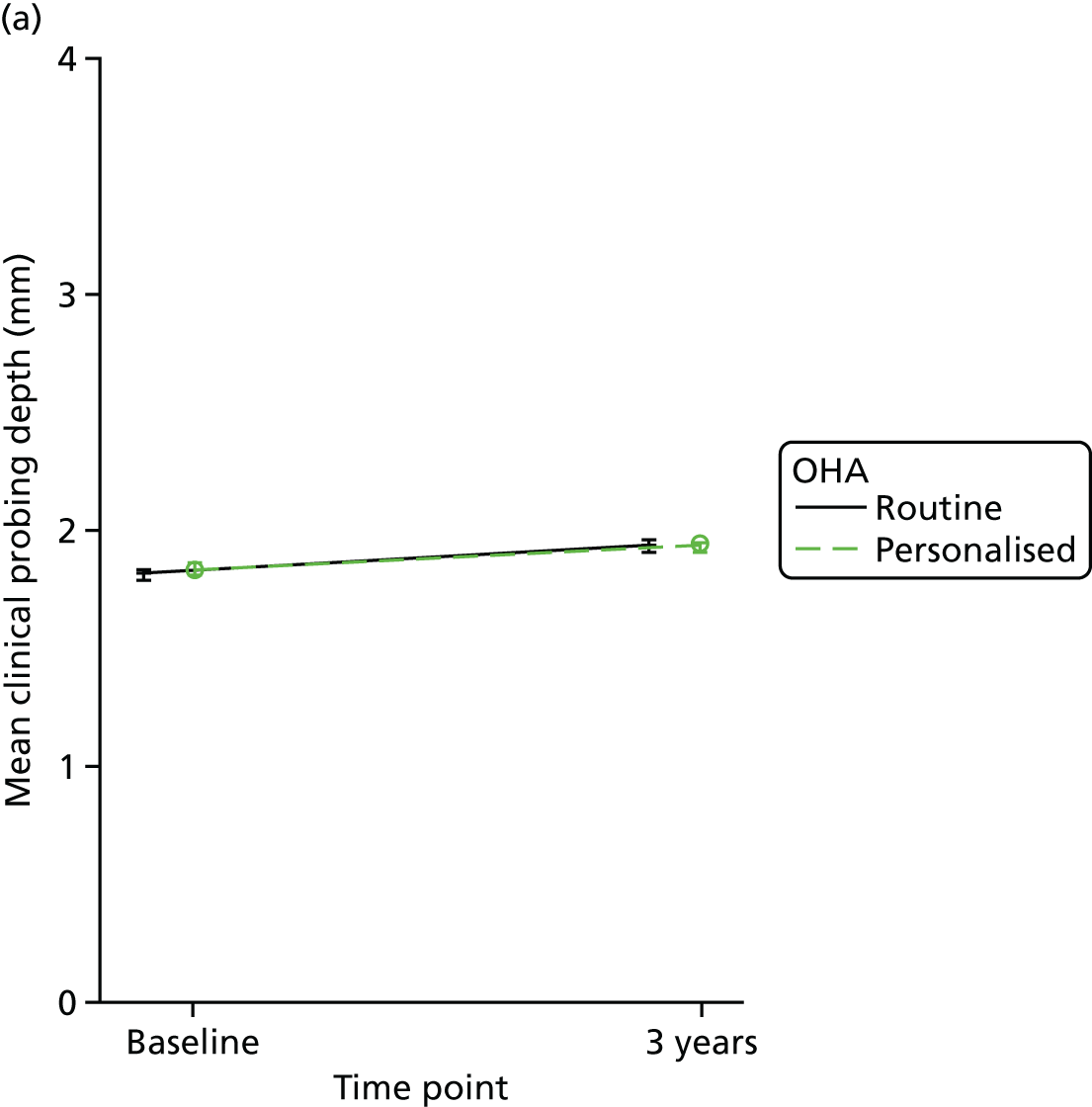

Patient-reported outcomes
The patient-reported outcomes (PBC, attitude and subjective norm) are shown in Figures 9–11. There was no evidence of any clinically important differences between the randomised groups in any of the cognitive variables at any follow-up time (see Table 13). Attitude was statistically significantly different between participants randomised to 6-monthly PI and those randomised to no PI, favouring a better attitude to oral hygiene behaviour in the no-PI group (difference –0.137, 95% CI –0.273 to –0.001; p = 0.048). However, the 95% CI excludes the possibility of a clinically important difference.
FIGURE 9.
Perceived behaviour control score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.

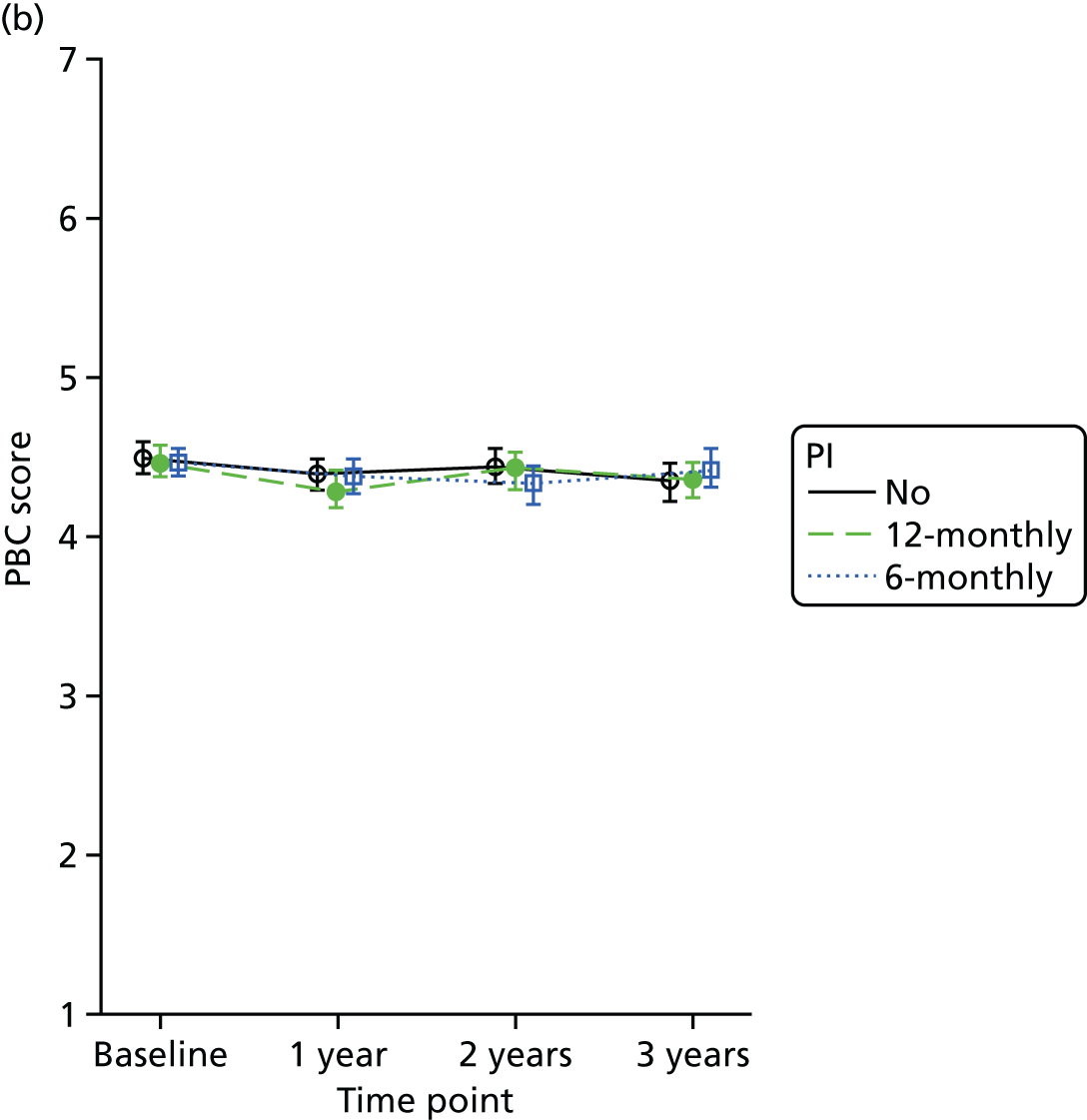
FIGURE 10.
Attitude score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.
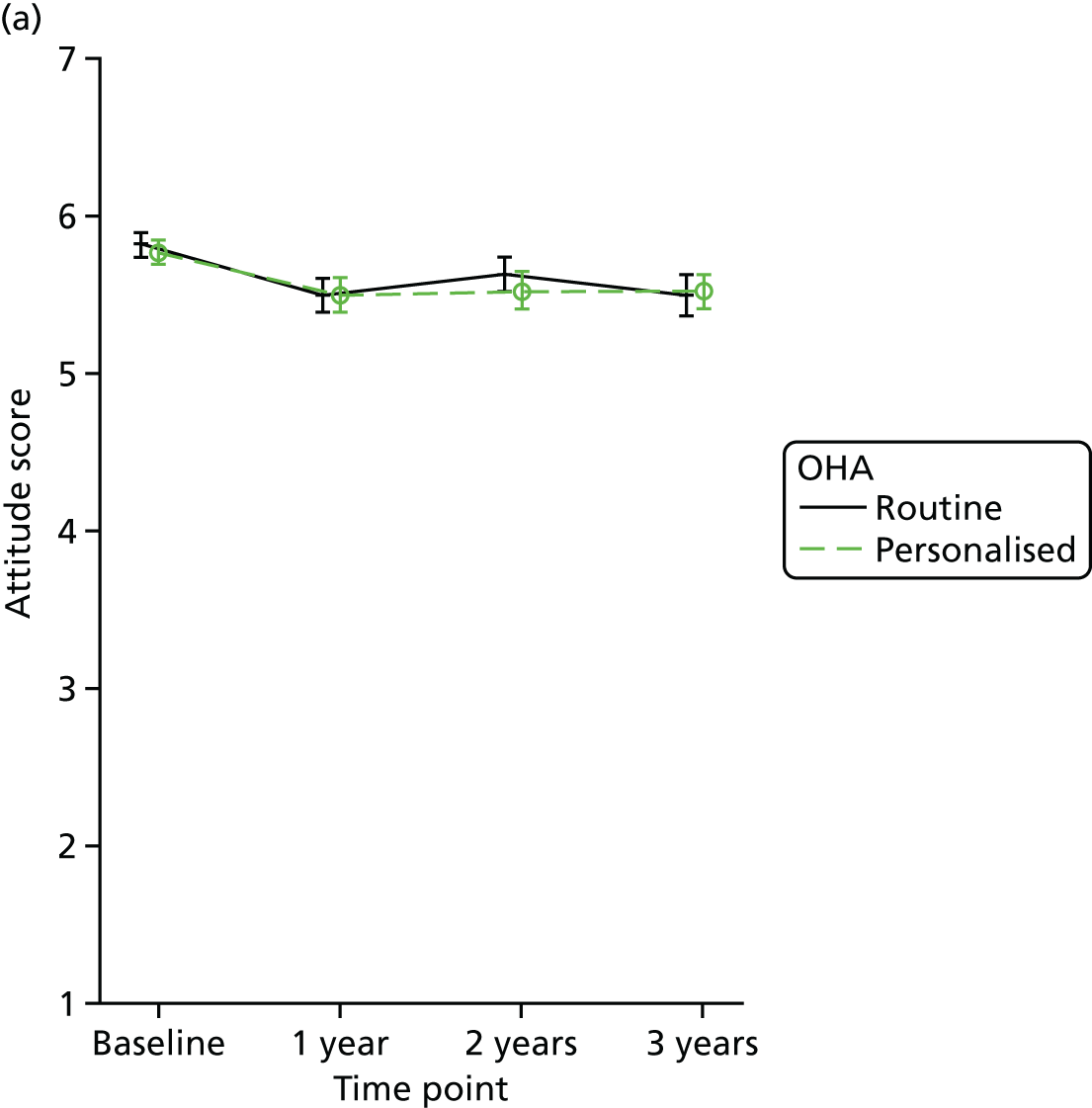

FIGURE 11.
Subjective norm score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.
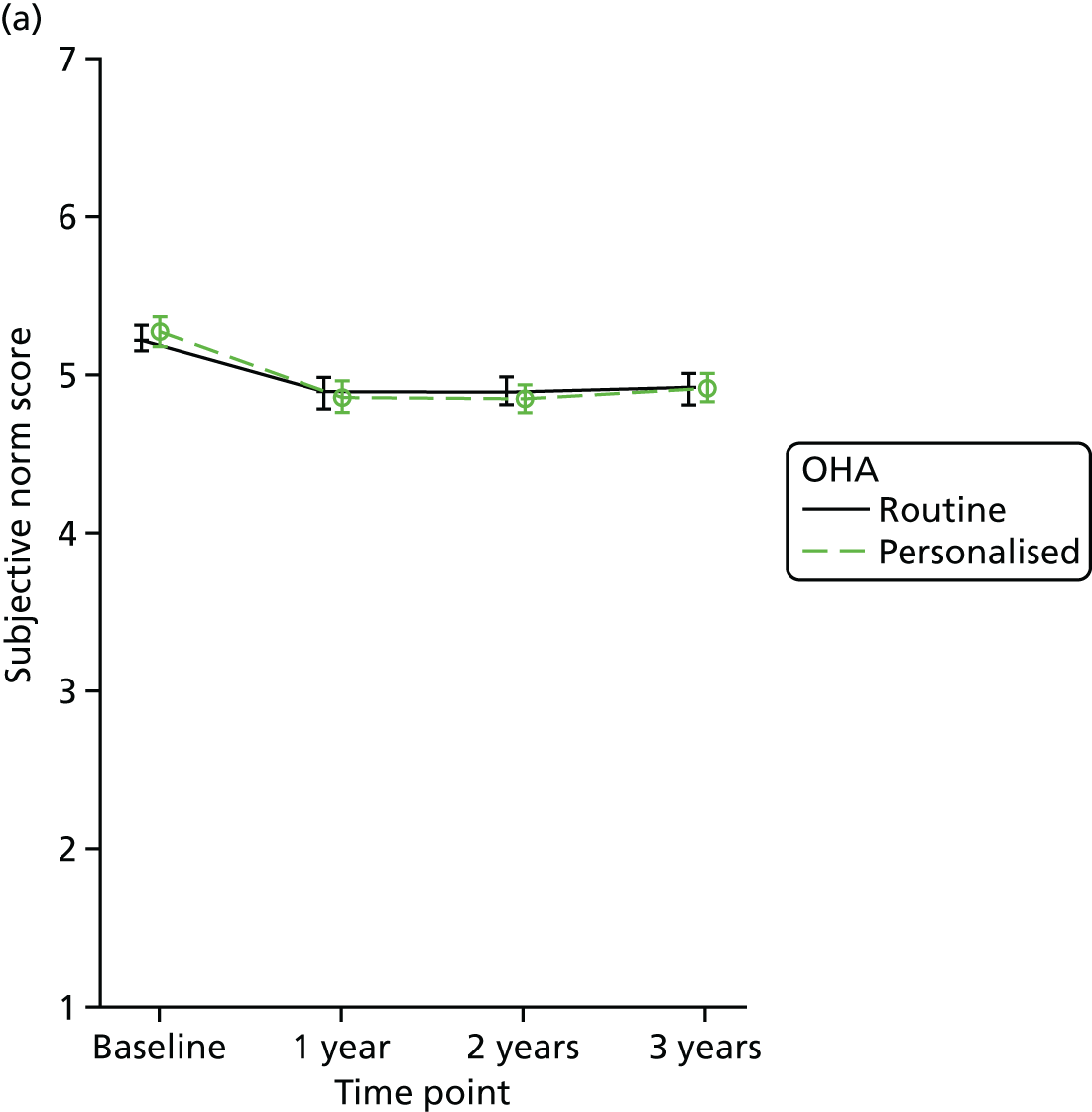

Behaviour score
The mean behaviour score and 95% CI for baseline and follow-up for each randomised group are displayed in Figure 12. The behaviour score was significantly different between participants randomised to the 6-monthly PI and those randomised to 12-monthly PI, favouring those in the 6-monthly group (difference –0.164, 95% CI –0.296 to –0.032; p = 0.015) (see Table 13). However, the 95% CI excludes the possibility of a clinically important difference. Therefore, there was no evidence of any clinically important differences between the randomised groups.
FIGURE 12.
Behaviour score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.


Intention score
The mean intention score and 95% CI for baseline and follow-up for each randomised group are displayed in Figure 13. There was no evidence of any clinically or statistically significant differences between the randomised groups for the intention score at any of the follow-up times (see Table 13).
FIGURE 13.
Intention score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.


Oral Health Impact Profile-14
The OHIP-14 score was overall very low (representing a good oral QoL) across the randomised groups at all follow-up time points (Figure 14), varying from mean 6.0 at baseline to mean 5.0 at 3 years. There was no evidence of any clinically important differences between the randomised groups for OHIP-14 scores at any of the follow-up times (see Table 13).
FIGURE 14.
The OHIP-14 score (mean and 95% CI), by randomised allocation. (a) OHA and (b) frequency of PI.

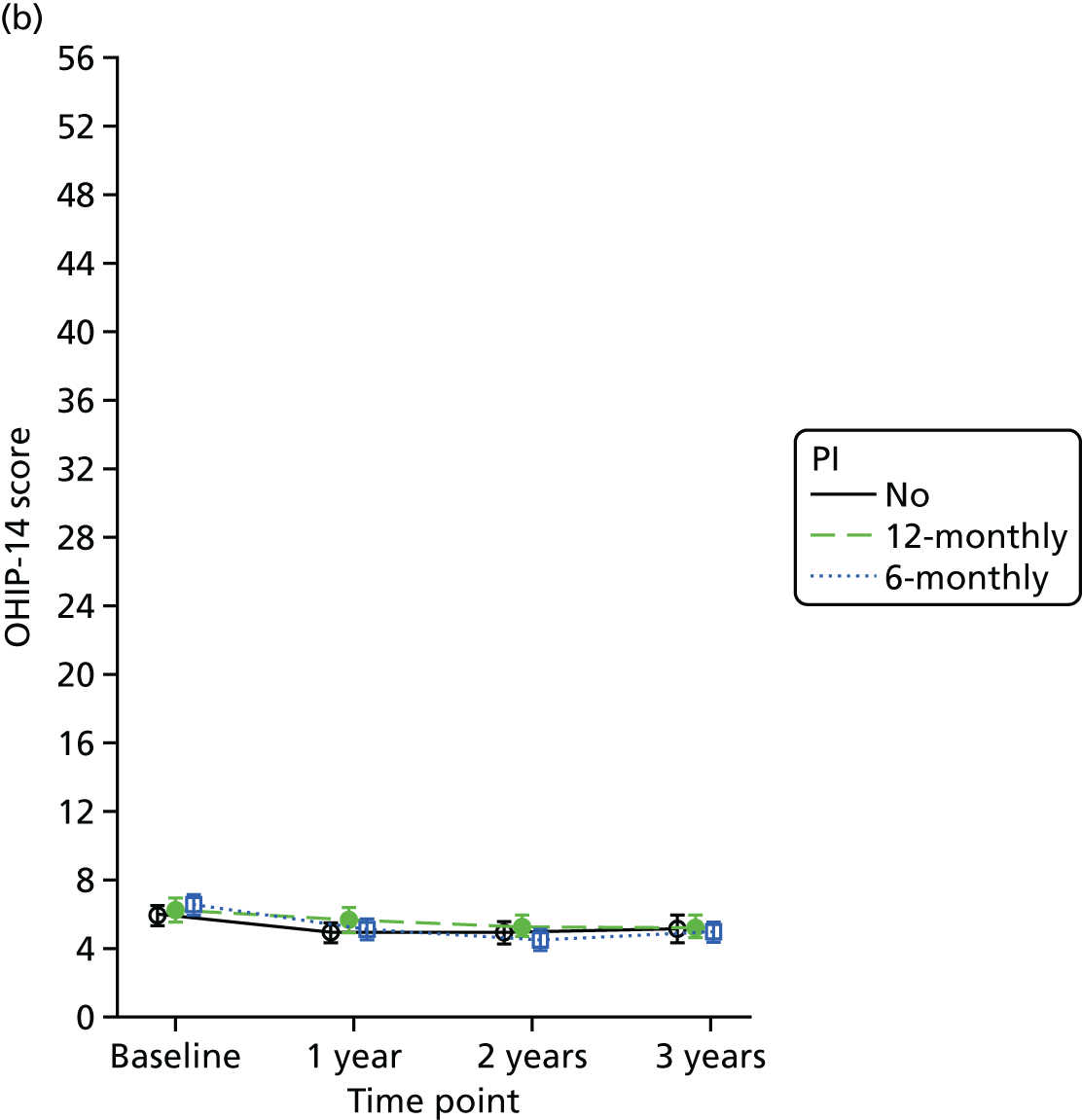
Private periodontal instrumentation received during the trial
There was a significant difference in the self-report of having received at least one private PI during the trial between the participants randomised to 6-monthly PI and the participants randomised to no PI. Participants in the 6-monthly PI had 50% greater odds of reporting a private PI than those in the no-PI group (odds ratio 0.5, 95% CI 0.3 to 0.7; p = 0.001). There was no evidence of a difference between the other randomised groups in private PI received during the trial (see Table 13). This is likely to reflect the number of PIs received in each group and a misunderstanding of the difference between a private and NHS PI rather than a real effect.
Having a plan to brush or floss better
There was no evidence of a difference between PI frequencies or OHA randomised groups in having a plan to brush or floss better over the course of the trial (see Table 13). This variable was also used as a measure of fidelity (see Fidelity of interventions).
Self-reported bleeding (post hoc outcome)
There was no evidence of a difference between the randomised groups for self-reported bleeding at 3 years (see Table 13).
Clinician beliefs at follow-up
The majority (around 78%) of respondents to the CBQ at follow-up were dentists. Every scale regarding the provider’s beliefs varied from 1 to 7, except for subjective norm, which varied from 1 to 49 (Table 14). Self-efficacy was high in both randomised groups (around 6 out of 7) and PBC was low. The overall attitude towards OHA and PI was around 3.5 out of 7, which was worse than at baseline, when the value was around 5 out of 7. The comparability between the follow-up and baseline CBQ is limited since the responders to both questionnaires were not always the same clinicians. Professionals did not report strong feelings of change in self-efficacy regarding both OHA and PI delivery over the course of the trial (mean of around 3 and similar between randomised groups). There was no evidence of a difference between randomised groups for any of the provider’s beliefs.
| Clinician beliefs | OHA | Personalised OHA vs. routine OHA, estimate (95% CI; p-valuea) | |
|---|---|---|---|
| Personalised (N = 44) | Routine (N = 40) | ||
| Profession, n (%) | |||
| Hygienist | 11 (25) | 7 (18) | – |
| Dentist | 33 (75) | 33 (83) | – |
| Clinician beliefs, mean (SD), n | |||
| Self-efficacy | 6.2 (0.6), 44 | 6.0 (0.6), 40 | 0.16 (–0.12 to 0.44; 0.254) |
| PBC | 3.0 (1.0), 44 | 2.8 (1.0), 40 | 0.15 (–0.26 to 0.57; 0.472) |
| Attitude | 3.7 (0.5), 44 | 3.5 (0.7), 40 | 0.15 (–0.11 to 0.41; 0.270) |
| Subjective norm | 13.2 (7.8), 43 | 11.3 (8.5), 40 | 1.87 (–1.49 to 5.22; 0.275) |
| Intention | 5.5 (1.2), 43 | 5.4 (1.0), 39 | 0.12 (–0.39 to 0.62; 0.648) |
| Has the plan about advice changed throughout the trial? | 3.7 (1.8), 43 | 3.3 (2.1), 39 | 0.36 (–0.39 to 1.11; 0.350) |
| Has the plan about providing PI changed throughout the trial? | 3.1 (1.8), 43 | 3.1 (1.8), 39 | –0.02 (–0.77 to 0.73; 0.949) |
Tertiary outcomes
Appendix 1, Section 4: tertiary outcomes (at baseline and 3 years) provides the self-reported data on dental sensitivity and appearance for baseline and 3 years. Possible answers to all the questions varied from 1 point (not clean at all/not at all pleasant) to 7 points (could not get any cleaner/extremely pleasant). At baseline, patients reported feeling that their teeth were clean after brushing (on average, 5.8 points), but cleaner after PI (on average, 6.6 points). The same was reported for how clean their teeth look (after brushing 5.3 points vs. after a PI 6.2 points), how pleasant they feel (5.8 points after brushing vs. 6.3 points after PI) and how pleasant they look (5.1 points after brushing vs. 5.7 points after PI). Around half of the patients reported feeling sensitivity in their teeth. Dental sensitivity and appearance characteristics were balanced across randomised groups.
At 3 years, participants reported that their teeth felt and looked clean and pleasant after brushing (on average, around 5.5 points out of 7 points) and even more clean and pleasant after PI (on average, around 6.4 points out of 7 points). The average scores were similar across randomised groups, indicating that having fewer PIs throughout the trial did not influence the participants’ perceptions.
Fidelity of interventions
Number of periodontal instrumentations received throughout the trial
Table 15 presents descriptive information from routine data and participant self-reported questionnaire data regarding the number of PIs claimed during the course of the trial. It includes participants with a clinical follow-up assessment and routine data available. The number of PIs reported in Table 15 excluded the baseline PI given to all participants. There was a separation between the number of PIs (claimed or self-reported) in each randomised PI group: the no-PI group claimed on average one PI throughout the trial, the 12-monthly PI group claimed around two PIs and the 6-monthly PI group claimed three PIs. Personalised and routine OHA had a similar number of PIs throughout the trial. The self-reported number of PIs was similar to the routinely collected data; however, the similarity was due to participants over- or underestimating PIs compared with the routine data (25% of the participants self-reported the same number of PIs as those claimed in the routine data; 47% self-reported having more PIs than those claimed; and 28% self-reported having fewer PIs than those claimed). There was clear evidence of a separation in the number of PIs given as randomised.
| Number of PIs received | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 427) | 12-monthly (N = 433) | 6-monthly (N = 426) | Personalised (N = 699) | Routine (N = 587) | |
| Routine data | |||||
| 0 | 197 (46) | 61 (14) | 41 (10) | 168 (24) | 131 (22) |
| 1 | 108 (25) | 89 (21) | 58 (14) | 133 (19) | 122 (21) |
| 2 | 60 (14) | 192 (44) | 56 (13) | 176 (25) | 132 (22) |
| 3 | 42 (10) | 52 (12) | 103 (24) | 118 (17) | 79 (13) |
| 4 | 13 (3) | 33 (8) | 127 (30) | 85 (12) | 88 (15) |
| ≥ 5 | 7 (2) | 6 (1) | 41 (10) | 19 (3) | 35 (6) |
| Mean (SD), n | 1.0 (1.2), 427 | 1.8 (1.1), 433 | 2.8 (1.5), 426 | 1.8 (1.4), 699 | 2.0 (1.5), 587 |
| Self-report | |||||
| 0 | 154 (36) | 62 (14) | 19 (4) | 122 (17) | 113 (19) |
| 1 | 98 (23) | 88 (20) | 59 (14) | 142 (20) | 103 (18) |
| 2 | 66 (15) | 94 (22) | 81 (19) | 128 (18) | 113 (19) |
| 3 | 29 (7) | 77 (18) | 43 (10) | 74 (11) | 75 (13) |
| 4 | 18 (4) | 42 (10) | 71 (17) | 74 (11) | 57 (10) |
| ≥ 5 | 14 (3) | 21 (5) | 105 (25) | 74 (11) | 66 (11) |
| Missing | 48 (11) | 49 (11) | 48 (11) | 85 (12) | 60 (10) |
| Mean (SD), n | 1.2 (1.4), 379 | 2.0 (1.4), 384 | 3.1 (1.6), 378 | 2.1 (1.6), 614 | 2.1 (1.7), 527 |
In addition to using the routine and self-reported data, the dental practice data monitoring showed that all practices were compliant. A telephone call was made to each practice on two occasions; however, no practices required further follow-up by the trial manager.
Routine check-ups were similar across the randomised groups, with an overall mean of 3.8 visits and a SD of 1.6 (i.e. participants attended the dental practice with the same frequency across groups).
Personalised and routine oral hygiene advice
Personalised OHA was given as intended to all participants at the baseline visit. Four questions in the participant annual questionnaire were used to further measure the continued fidelity of the OHA: (1) ‘Usually, when you finish brushing your teeth do you . . .?’, (2) ‘What do you intend to do when you finish brushing your teeth in the future?’ (related to the use of mouth wash after brushing), (3) ‘Do you have a plan about when you will start brushing your teeth better?’ and (4) ‘Do you have a plan about when you will start flossing your teeth better?’. Correct answers to the first two questions (i.e. ‘I do spit but not rinse after brushing’ or ‘I should spit but not rinse after brushing’), as well as having a plan about when to start brushing or flossing better, were considered as proxy indicators that the personalised OHA was delivered. At baseline, the routine and personalised groups were balanced for each question (around 28% of participants answered that they spit but do not rinse after brushing, 28% that they intended to do that and 25% that they had a plan to brush or floss better). At year 1, 29% of participants in the routine group said that they spit but do not rinse, or intended to do so, compared with around 40% in the personalised group. At the end of the trial, in the 3-year follow-up questionnaire, around 29% of the participants in the routine arm reported that they spit but do not rinse or intended to do so, compared with 39% in the personalised arm. Around 20% of the participants in either arm had a plan to brush or floss better. This was similar across the years. Participants did not necessarily need to have a plan to brush or floss better if they did not need one (one of the options in the questionnaire). There was evidence, from the answer ‘I do spit but not rinse after brushing’, that the personalised OHA group retained/received the OHA as intended.
Subgroup analyses
Prespecified subgroup analyses for gingival bleeding included currently a smoker (yes/no), BPE score (of < 3 or of 3), age (< 45/45–64/> 64 years) and whether or not the practice employed a hygienist (yes/no). Post hoc subgroup analyses were undertaken for participants with four or more sites with a clinical probing depth of ≥ 4 mm (yes/no) and region (Scotland/England). Figures 15 and 16 show the means and 99% CIs for the differences in bleeding at 3 years in the subgroups for PI frequency and OHA, respectively. There was no evidence that any of the subgroups were statistically significantly different at the 1% level (Table 16).
FIGURE 15.
Subgroup results for PI allocation: difference between arms, by subgroup.

FIGURE 16.
Subgroup results for OHA allocation: difference between arms, by subgroup.

| Subgroups | Mean (99% CI; p-value) | |
|---|---|---|
| Other PI vs. 6-monthly PI | Personalised OHA vs. routine OHA | |
| Scotland vs. England | –4.8 (–11.0 to 1.4; 0.048) | –2.9 (–19.5 to 13.6; 0.649) |
| BPE score of 3 vs. < 3 | 4.7 (–1.1 to 10.5; 0.038) | –2.5 (–8.1 to 3.1; 0.254) |
| Smoker vs. non-smoker | 0.0 (–7.3 to 7.4; 0.992) | –0.3 (–7.2 to 6.7; 0.922) |
| Employs hygienist vs. does not | 2.1 (–4.3 to 8.5; 0.396) | –11.3 (–29.0 to 6.5; 0.102) |
| Clinical pocket depth (mm) ≥ 4 vs. < 4 | 2.8 (–6.1 to 11.6; 0.417) | 0.8 (–7.7 to 9.2; 0.811) |
| Age (years) 45–64 vs. < 45 | 2.5 (–3.7 to 8.8; 0.291) | 0.4 (–5.5 to 6.3; 0.855) |
| Age (years) > 65 vs. < 45 | 2.2 (–5.9 to 10.2; 0.487) | 3.9 (–3.6 to 11.4; 0.177) |
Sensitivity analyses
Missing data
Possible mechanisms of missingness were investigated by modelling baseline predictors of participants missing the final examination (see Table 10). Only age was identified as a predictor. Clinical severity of disease indicators (bleeding, calculus and clinical pocket depths) were not predictors. There were no differences between randomised groups in the proportion of missing data. In addition, the main reason for missing the final examination was that the participant was no longer contactable by the dental practice and, therefore, unlikely to be related to the study interventions. Given these findings, no further missing data sensitivity analyses were undertaken.
Outliers
To test the sensitivity of the primary outcome to outliers, we explored potential between-practice differences influencing the primary analyses. Practices where the treatment difference was twice the target difference (either –15% or +15%) and excluded zero from the 99% CI were excluded from the analysis comparing no PI with 6-monthly PI. A positive treatment difference was found in favour of the 6-monthly PI versus other PI. Three practices in England met the criteria and were excluded as part of the sensitivity analysis. They had the following treatment difference estimates +19.4; +24.2; and +24.7. After excluding the three outlier practices from the main analysis, the treatment effect of 6-monthly PI versus no PI was –0.38 (95% CI –2.9 to 2.1), of 6-monthly PI versus 12-monthly PI was –0.18 (95% CI –2.7 to 2.3) and of personalised OHA versus routine OHA was –2.1 (95% CI –8.1 to 4.0). The overall I2 for the practice effects in the trial was 27%. When the outliers were removed, the I2 was 0% and the interaction mean in the subgroup analysis for region changed from –4.8 (95% CI –11 to 1.4; p = 0.048) to –2.3 (95% CI –9.0 to 4.5; p = 0.387).
Chapter 5 Results of economic analysis
Resource use and costs
NHS dental care resource use
Data were linked for 1337 out of 1346 (99%) trial participants randomised in Scotland (after post-randomisation exclusion) and 477 out of 525 (91%) trial participants randomised in England. It was not possible to link data for 11 participants in Scotland, but data were available for two participants randomised in England having some dental treatment in Scotland. Linkage was not possible for 48 participants in England. A further participant had claims in both Scotland and England. For this individual, claims were summed across both data sets, with the participant remaining allocated to their region of randomisation for the purposes of reporting results.
Table 17 describes the total number of treatment claims provided to all randomised participants with linked data over the 2.25-year follow-up period (excluding baseline and final study visits) by randomised group. The majority of claims in England fell into band 1 treatments. There were relatively few emergency claims and free treatments across all groups. The highest number of claims in Scotland was for diagnostic assessments, including clinical examinations and radiographs. There were 2516 periodontal treatments provided, accounting for 23% of claims. Among the remaining categories, the greatest number of claims were for conservative treatments [2506 claims (23%)] such as fillings, with relatively few surgical extractions or more intensive treatments. There were clear differences across groups in terms of numbers of periodontal treatments reflecting the different number of PIs delivered in each randomised trial arm. Overall, owing to the restrictions of the reimbursement system, there were only 11 claims for ‘preventative care’ (including intensive hygiene instruction), all in the personalised OHA cluster. Detailed information on items claimed in Scotland can be found in Appendix 2, Section 3: detailed claims for Scottish routine data.
| Region | OHA, n | Overall total, n | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | ||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | ||
| Scotland | (N = 192) | (N = 193) | (N = 195) | (N = 580) | (N = 250) | (N = 255) | (N = 252) | (N = 757) | (N = 1337) |
| Category of claima | |||||||||
| Diagnosis | 679 | 666 | 698 | 2043 | 873 | 894 | 912 | 2679 | 4722 |
| Preventative care | 0 | 0 | 0 | 0 | 7 | 4 | 0 | 11 | 11 |
| Periodontal | 268 | 343 | 530 | 1141 | 251 | 466 | 658 | 1375 | 2516 |
| Conservative treatments | 370 | 351 | 361 | 1082 | 462 | 495 | 467 | 1424 | 2506 |
| Surgical treatments | 59 | 86 | 62 | 207 | 83 | 98 | 69 | 250 | 457 |
| Prostheses | 28 | 36 | 19 | 83 | 39 | 63 | 54 | 156 | 239 |
| Orthodontic | 1 | 4 | 0 | 5 | 0 | 0 | 0 | 0 | 5 |
| Other | 53 | 55 | 72 | 180 | 67 | 110 | 85 | 262 | 442 |
| Total | 1458 | 1541 | 1742 | 4741 | 1782 | 2130 | 2245 | 6157 | 10,898 |
| England | (N = 79) | (N = 80) | (N = 88) | (N = 247) | (N = 77) | (N = 77) | (N = 76) | (N = 230) | (N = 477) |
| Band 1b | 156 | 173 | 204 | 533 | 118 | 161 | 156 | 435 | 968 |
| Band 2 | 74 | 75 | 62 | 211 | 52 | 66 | 63 | 181 | 392 |
| Band 3 | 15 | 18 | 12 | 45 | 8 | 17 | 13 | 38 | 83 |
| Band urgent | 24 | 20 | 27 | 71 | 24 | 24 | 20 | 68 | 139 |
| Free | 1 | 1 | 0 | 2 | 0 | 6 | 0 | 6 | 8 |
| Total | 270 | 287 | 305 | 862 | 202 | 274 | 252 | 728 | 1590 |
Table 18 shows that the numbers of PIs delivered to all randomised participants with linked data were evenly balanced across clusters. The mean number of reported PIs was lower in England than in Scotland. It should be noted that the number of PIs in England may be under-reported as only one qualifying procedure for a band 1 treatment needs to be reported when filling in the clinical data set (e.g. a clinical examination).
| Region | OHA, n | Overall total, n | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | ||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | ||
| Scotland | (N = 192) | (N = 193) | (N = 195) | (N = 580) | (N = 250) | (N = 255) | (N = 252) | (N = 757) | (N = 1337) |
| 0 | 68 | 29 | 28 | 125 | 109 | 35 | 21 | 165 | 290 |
| 1 | 50 | 49 | 24 | 123 | 70 | 54 | 45 | 169 | 292 |
| 2 | 28 | 74 | 31 | 133 | 40 | 111 | 40 | 191 | 324 |
| 3 | 30 | 23 | 32 | 85 | 24 | 37 | 66 | 127 | 212 |
| 4 | 11 | 14 | 63 | 88 | 6 | 14 | 70 | 90 | 178 |
| ≥ 5c | 5 | 4 | 17 | 26 | 1 | 4 | 10 | 15 | 41 |
| Mean (SD) | 1.4 (1.4) | 1.8 (1.2) | 2.7 (1.7) | 2.0 (1.6) | 1.0 (1.1) | 1.8 (1.2) | 2.6 (1.4) | 1.8 (1.4) | 1.9 (1.5) |
| England | (N = 79) | (N = 80) | (N = 88) | (N = 247) | (N = 77) | (N = 77) | (N = 76) | (N = 230) | (N = 477) |
| 0 | 40 | 20 | 25 | 85 | 44 | 27 | 20 | 91 | 176 |
| 1 | 28 | 16 | 18 | 62 | 16 | 15 | 17 | 48 | 110 |
| 2 | 7 | 35 | 14 | 56 | 9 | 22 | 12 | 43 | 99 |
| 3 | 1 | 7 | 10 | 18 | 7 | 4 | 13 | 24 | 42 |
| 4 | 1 | 2 | 9 | 12 | 0 | 8 | 8 | 16 | 28 |
| ≥ 5c | 2 | 0 | 12 | 14 | 1 | 1 | 6 | 8 | 22 |
| Mean (SD) | 0.8 (1.1) | 1.4 (1.0) | 2.0 (1.9) | 1.4 (1.5) | 0.8 (1.2) | 1.4 (1.4) | 1.9 (1.6) | 1.4 (1.5) | 1.4 (1.5) |
NHS dental care costs
Table 19 presents complete-case NHS dental costs for the UK and at the regional level based on the appropriate region-level unit costs applied to resource use data. Incremental differences are presented for each randomised policy, compared with standard care.
| Region | OHA, n | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | |||||||||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| UK | (N = 271) | (N = 273) | (N = 283) | (N = 827) | (N = 327) | (N = 332) | (N = 328) | (N = 987) | ||||||||
| Total | 73.23 | 118.04 | 81.37 | 141.64 | 73.75 | 104.78 | 76.10 | 122.16 | 62.42 | 116.13 | 85.43 | 131.62 | 82.84 | 134.19 | 76.94 | 127.87 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PI | –3.12 (–18.18 to 11.93) | 0.14 (–15.42 to 15.69) | – | N/A | –14.91 (–34.18 to –4.36) | 11.67 (–11.00 to 34.33) | 6.23 (–15.70 to 28.16) | N/A | ||||||||
| Scotland | (N = 192) | (N = 193) | (N = 195) | (N = 580) | (N = 250) | (N = 255) | (N = 252) | (N = 757) | ||||||||
| Diagnosis | 24.27 | 10.43 | 24.30 | 11.08 | 24.88 | 12.36 | 24.49 | 11.31 | 23.24 | 10.83 | 23.93 | 11.90 | 25.46 | 11.10 | 24.21 | 11.31 |
| Prevention | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 1.54 | 0.17 | 2.35 | 0.00 | 0.00 | 0.12 | 1.63 |
| Periodontal | 7.72 | 12.70 | 9.90 | 12.76 | 14.20 | 16.73 | 10.63 | 14.43 | 5.65 | 11.03 | 10.27 | 13.45 | 11.53 | 12.06 | 9.16 | 12.47 |
| Conservative | 23.40 | 55.90 | 22.66 | 46.77 | 25.15 | 61.25 | 23.74 | 54.90 | 25.54 | 77.32 | 33.53 | 86.20 | 32.02 | 97.44 | 30.39 | 87.35 |
| Surgical | 1.06 | 3.19 | 2.01 | 6.36 | 1.99 | 11.64 | 1.69 | 7.90 | 1.53 | 6.34 | 1.73 | 7.58 | 1.33 | 4.90 | 1.53 | 6.37 |
| Prosthesis | 5.04 | 34.19 | 7.34 | 37.11 | 4.87 | 32.32 | 5.75 | 34.55 | 3.49 | 23.70 | 7.65 | 33.28 | 7.09 | 32.13 | 6.09 | 30.05 |
| Orthodontic | 1.22 | 16.88 | 0.44 | 6.07 | 0.00 | 0.00 | 0.55 | 10.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other | 0.95 | 3.30 | 0.68 | 2.24 | 1.02 | 3.33 | 0.88 | 3.00 | 1.58 | 14.61 | 1.49 | 6.32 | 1.38 | 5.95 | 1.49 | 9.77 |
| Total | 63.68 | 86.38 | 67.32 | 82.51 | 72.12 | 93.08 | 67.73 | 87.37 | 61.24 | 102.71 | 78.76 | 120.40 | 78.82 | 119.90 | 72.99 | 114.83 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PI | –9.11 (–23.62 to 5.41) | –6.60 (–21.39 to 8.20) | – | N/A | –13.54 (–33.83 to 6.76) | 5.45 (–17.37 to 28.28) | 4.16 (–18.46 to 26.75) | N/A | ||||||||
| England | (N = 79) | (N = 80) | (N = 88) | (N = 247) | (N = 77) | (N = 77) | (N = 76) | (N = 230) | ||||||||
| Band 1 | 19.65 | 24.90 | 21.65 | 22.88 | 20.40 | 26.63 | 20.56 | 24.82 | 13.73 | £17.37 | 22.41 | 24.49 | 20.11 | 19.73 | 18.74 | 20.98 |
| Band 2 | 32.31 | 59.37 | 42.38 | 113.02 | 26.49 | 46.21 | 33.50 | 77.58 | 23.77 | £41.97 | 36.37 | 78.65 | 35.51 | 57.62 | 31.87 | 61.31 |
| Band 3 | 38.63 | 130.37 | 46.57 | 133.00 | 25.08 | 97.55 | 36.38 | 120.48 | 23.39 | £111.83 | 39.70 | 114.59 | 35.96 | 123.45 | 33.00 | 116.40 |
| Urgent | 5.49 | 21.48 | 4.31 | 11.24 | 5.42 | 13.31 | 5.08 | 15.81 | 5.38 | £15.13 | 6.10 | 15.23 | 4.58 | 10.82 | 5.35 | 13.84 |
| Free | 0.35 | 3.15 | 0.36 | 3.24 | 0.00 | 0.00 | 0.23 | 2.56 | 0.00 | £0.00 | 1.84 | 9.61 | 0.00 | 0.00 | 0.62 | 5.61 |
| Total | 96.44 | 170.85 | 115.27 | 225.57 | 77.39 | 127.47 | 95.75 | 177.72 | 66.27 | £152.54 | 106.41 | 162.10 | 96.16 | 173.67 | 89.59 | 163.14 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PI | 23.24 (–22.22 to 68.70) | 22.09 (–23.63 to 67.80) | – | N/A | –17.67 (–62.30 to 26.95) | 33.73 (–27.62 to 95.08) | 13.87 (–40.83 to 68.60) | N/A | ||||||||
The mean cost per trial participant over the 2.25-year follow-up period (excluding baseline and final study visits) ranged from £62.42 to £85.43 across the randomised groups. The SDs are generally large, suggesting relatively large variation in costs across participants. Data were somewhat skewed to the left, with some outliers incurring very high costs; however, these were spread evenly across groups.
The average cost is lowest for the no-PI groups. However, none of the differences in mean cost between standard care (routine OHA and 6-monthly PI) and the other policies considered was statistically significant at a 5% level. The largest difference in costs is between standard care and a policy of no PI with personalised OHA (mean difference –£14.91; 95% CI –£34.18 to £4.36).
Table 19 also reports the NHS costs by region. This is important given the differences in reimbursement systems and the level of detail on resource use. Average per-participant costs are higher in England than in Scotland. However, the overall results are the same across the two regions: there are no statistically significant differences in costs between standard care and the other five groups. The CIs for incremental costs are generally wider for England, which is not surprising given the smaller sample sizes.
The Scottish data provide much more detail on resource use than the English data and can, therefore, provide additional insights into differences in different cost categories. The costs of periodontal treatments were expected to be lower for no PI and 12-monthly PI than for standard care, and this was found to be the case. Mean cost differences of periodontal treatments in Scotland for each randomised group, compared with routine OHA and 6-monthly PI were as follows: routine OHA, no PI, –£7.47 (95% CI –£11.22 to –£3.72); routine OHA, 12-monthly PI, –£5.11 (95% CI –£8.96 to –£1.26); personalised OHA, no PI, –£9.31 (95% CI –£13.30 to –£5.32); personalised OHA, 6-monthly PI –£2.00 (95% CI –£6.76 to £2.75); and personalised OHA, 12-monthly PI, –£3.78 (95% CI –£8.29 to £0.73). There were no significant differences across groups in any of the other categories of cost. Given the relatively low average NHS costs of PI, the differences in PI costs did not lead to differences in the total costs.
Participant exemptions from NHS co-charges can have an impact on the split of dental charges between NHS and participant. Therefore, it is important to explore whether or not there are differences in the proportion of participants exempt across the randomised groups. Table 20 shows that around 24% of participants were exempt from charges. There were no significant differences across randomised groups in terms of the proportion of participants exempt from payment (χ2 6.82; p-value of 0.234). As a robustness check, we also analysed the data assuming that all participants are exempt, that is, the NHS pays the full cost of dental care provided. Relative to standard care, for this exploratory analysis, incremental costs were as follows: routine OHA, no PI, –£4.76 (95% CI –£30.53 to £21.01); routine OHA, 12-monthly PI, –£1.89 (95% CI –£28.04 to £24.27); personalised OHA, no PI, –£25.65 (95% CI –£53.15 to £1.85); personalised OHA, 12-monthly PI, £10.95 (95 CI –£20.17 to £42.06); and personalised OHA, 6-monthly PI, £11.21 (95% CI –£19.97 to £42.40). Furthermore, using similar regression models as for the main results, there was no evidence that exemption status had an impact on the number of PIs delivered (p = 0.518). The main conclusion was the same: there was no statistically significant difference in costs across the randomised groups.
| Region | Proportion charge to participant (%) | OHA, n (%) | Overall total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | |||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | |||
| UK | (N = 271) | (N = 273) | (N = 283) | (N = 827) | (N = 327) | (N = 332) | (N = 328) | (N = 987) | (N = 1814) | |
| Exempt | Region-specific | 62 (23) | 68 (25) | 81 (29) | 211 (26) | 71 (22) | 88 (27) | 70 (21) | 229 (23) | 440 (24) |
| Non-exempt | 209 (77) | 205 (75) | 202 (71) | 616 (74) | 256 (78) | 244 (73) | 258 (79) | 758 (77) | 1374 (76) | |
| Scotlanda | (N = 192) | (N = 193) | (N = 195) | (N = 580) | (N = 250) | (N = 255) | (N = 252) | (N = 757) | (N = 1337) | |
| Employment support allowance | 0 | 4 (2) | 1 (1) | 4 (2) | 9 (2) | 4 (2) | 3 (1) | 5 (2) | 12 (2) | 21 (2) |
| Pay full patient charge | 100 | 152 (79) | 141 (73) | 134 (69) | 427 (74) | 193 (77) | 186 (73) | 201 (80) | 580 (77) | 1007 (75) |
| Help with health care | Various | 0 (0) | 2 (1) | 3 (2) | 5 (1) | 2 (1) | 3 (1) | 2 (1) | 7 (1) | 12 (1) |
| Income support | 0 | 3 (2) | 11 (6) | 7 (4) | 21 (4) | 4 (2) | 7 (3) | 10 (4) | 21 (3) | 42 (3) |
| Jobseeker’s allowance | 0 | 2 (1) | 8 (4) | 8 (4) | 18 (3) | 8 (3) | 8 (3) | 6 (2) | 22 (3) | 40 (3) |
| Maternity/newborn | 0 | 7 (4) | 3 (2) | 3 (2) | 13 (2) | 7 (3) | 9 (4) | 5 (2) | 21 (3) | 34 (3) |
| Pension/tax credits | 0 | 24 (13) | 27 (14) | 36 (18) | 87 (15) | 32 (13) | 39 (15) | 23 (9) | 94 (12) | 181 (14) |
| England | (N = 79) | (N = 80) | (N = 88) | (N = 247) | (N = 77) | (N = 77) | (N = 76) | (N = 230) | (N = 477) | |
| Child | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 2 (1) | 2 (0) |
| Exempt | 0 | 22 (28) | 16 (20) | 20 (23) | 58 (23) | 14 (18) | 18 (23) | 18 (24) | 50 (22) | 108 (23) |
| Not exempt | According to band | 57 (72) | 64 (80) | 68 (77) | 189 (77) | 63 (82) | 58 (75) | 57 (75) | 178 (77) | 367 (77) |
Other NHS-incurred costs
The analysis protocol set out to measure costs to the NHS of primary and secondary care, based on annual questionnaire data, for problems related to participants’ teeth. There were 193 out of 1630 (12%) participants who reported at least one contact with secondary care services over the 3 years of follow-up. This relatively high number of participants reporting secondary care use was unexpected given the relatively good dental health of the trial population. A validation exercise was conducted by comparing secondary care contacts from the trial with the general population using routine data and by checking self-report data against practice records. In terms of the routine data, the average annual rate of outpatient consultations in the trial (1 : 19) was six times greater than the corresponding number of consultations in the general population in Scotland (1 : 121) and three times greater than the corresponding rate in England (1 : 70). A similar result was found when inpatient admissions were compared, with an average annual rate among trial participants (1 : 70) 10 times higher than the general population in Scotland (1 : 722) and over three times higher than the general population rate in England (1 : 249). It should be noted that the general population rates for England and Scotland should not be directly compared as the data are based on different assumptions. For 80 out of the 193 trial participants reporting resource use (23 in England and 57 in Scotland), data were checked against dental practice records for evidence of referral for secondary care treatment (across all hospitals, dental or otherwise). Fewer than one in four reports were validated against practice records. Table 21 summarises the results of the validation exercise.
| Validation against routine ISD data | Trial data (UK)a | Trial data (England)a | General population rate (England)b,c | Trial data (Scotland)a | General population rate (Scotland)c |
|---|---|---|---|---|---|
| Outpatients | |||||
| Year 1 | 1 : 18 | 1 : 19 | 1 : 70b | 1 : 15 | 1 : 121d |
| Year 2 | 1 : 18 | 1 : 18 | 1 : 17 | ||
| Year 3 | 1 : 23 | 1 : 22 | 1 : 26 | ||
| Average annual rate | 1 : 19 | 1 : 20 | 1 : 19 | ||
| Inpatients | |||||
| Year 1 | 1 : 40 | 1 : 41 | 1 : 249b | 1 : 39 | 1 : 722e |
| Year 2 | 1 : 42 | 1 : 46 | 1 : 35 | ||
| Year 3 | 1 : 129 | 1 : 164 | 1 : 85 | ||
| Average annual rate | 1 : 70 | 1 : 84 | 1 : 53 | ||
| Validation against practice records | % with a referral in practice notes | % with a referral in practice notes | |||
| One or more contacts with secondary care over 3 yearsf | 1 : 10 | 1 : 11 | 3 : 23 (13) | 1 : 10 | 16 : 57 (28) |
Given the poor level of validation of other NHS resource use against routine data, the lack of evidence of dental care referrals in practice records and the fact that the general population may be expected to have, on average, poorer rather than better dental health, the data on use of NHS resources have been excluded from the base-case analysis. Appendix 2, Section 4: participant-reported contact with non-dental health services presents descriptive data for primary and secondary care resource use falling outside the dental budget for information only. The data are excluded from all subsequently reported results.
Participant-incurred costs
Participant charges for NHS dental care
Table 22 reports complete-case data on participant-incurred charges for NHS dental treatments, provided to all randomised participants with linked routine data. The average charge to participants over the 2.25-year follow-up period ranged from £48.95 to £61.33.
| Region | OHA, co-charges (£) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | |||||||||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| UK | (N = 271) | (N = 273) | (N = 283) | (N = 827) | (N = 327) | (N = 332) | (N = 328) | (N = 987) | ||||||||
| Total | 59.96 | 80.16 | 53.94 | 68.50 | 60.97 | 70.65 | 58.32 | 73.21 | 48.95 | 67.12 | 60.64 | 99.87 | 61.33 | 77.92 | 56.99 | 82.95 |
| Scotlandb,c | (N = 192) | (N = 193) | (N = 195) | (N = 580) | (N = 250) | (N = 255) | (N = 252) | (N = 757) | ||||||||
| Diagnosis | 3.15 | 4.69 | 2.35 | 3.25 | 2.73 | 3.54 | 2.74 | 3.88 | 3.47 | 4.31 | 3.34 | 6.09 | 2.95 | 4.07 | 3.25 | 4.91 |
| Prevention | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.66 | 0.09 | 0.79 | 0.00 | 0.00 | 0.05 | 0.59 |
| Periodontal | 11.78 | 15.34 | 13.95 | 13.58 | 22.08 | 20.37 | 15.97 | 17.25 | 8.27 | 11.42 | 14.54 | 13.38 | 23.38 | 18.71 | 15.41 | 16.05 |
| Conservative | 30.41 | 55.62 | 23.07 | 38.94 | 26.23 | 50.89 | 26.56 | 48.99 | 30.27 | 59.32 | 31.92 | 76.69 | 25.65 | 55.99 | 29.29 | 64.67 |
| Surgical | 1.65 | 5.51 | 1.76 | 6.31 | 1.42 | 4.48 | 1.61 | 5.48 | 1.85 | 7.72 | 1.48 | 4.47 | 1.14 | 4.68 | 1.49 | 5.80 |
| Prosthesis | 4.33 | 22.77 | 1.19 | 9.70 | 1.06 | 8.47 | 2.19 | 15.12 | 3.32 | 18.41 | 5.55 | 26.21 | 5.14 | 27.99 | 4.68 | 24.57 |
| Orthodontic | 0.00 | 0.00 | 1.75 | 24.28 | 0.00 | 0.00 | 0.58 | 14.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Other | 0.57 | 2.34 | 1.00 | 2.78 | 0.76 | 2.51 | 0.77 | 2.55 | 0.70 | 2.00 | 0.98 | 2.79 | 1.19 | 4.53 | 0.96 | 3.29 |
| Total | 51.88 | 76.16 | 45.07 | 55.86 | 54.27 | 64.49 | 50.42 | 66.01 | 47.94 | 71.22 | 57.89 | 102.78 | 59.46 | 77.36 | 55.13 | 85.05 |
| England | (N = 79) | (N = 80) | (N = 88) | (N = 247) | (N = 77) | (N = 77) | (N = 76) | (N = 230) | ||||||||
| Treatment band 1 | 27.44 | 28.89 | 30.02 | 29.07 | 33.93 | 30.79 | 30.59 | 29.64 | 22.32 | 23.63 | 26.82 | 24.76 | 27.82 | 28.00 | 25.64 | 25.52 |
| Treatment band 2 | 33.06 | 48.67 | 24.30 | 42.46 | 24.10 | 37.27 | 27.03 | 42.88 | 23.85 | 42.42 | 23.24 | 39.64 | 23.03 | 42.33 | 23.37 | 41.30 |
| Treatment band 3 | 16.17 | 56.77 | 18.16 | 67.66 | 14.35 | 53.36 | 16.16 | 59.17 | 2.84 | 24.96 | 16.40 | 66.36 | 13.64 | 61.89 | 10.95 | 54.38 |
| Urgent | 2.93 | 9.67 | 2.87 | 8.15 | 3.44 | 9.34 | 3.09 | 9.05 | 3.20 | 6.82 | 2.52 | 7.96 | 3.05 | 9.35 | 2.92 | 8.08 |
| Free | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 79.61 | 86.53 | 75.34 | 88.97 | 75.82 | 81.12 | 76.88 | 85.13 | 52.20 | 51.85 | 68.97 | 89.20 | 67.54 | 79.97 | 62.88 | 75.40 |
Opportunity costs of time and travel to dental appointments
Appendix 2, Section 5: unit opportunity cost of time and travel to dental appointments reports the descriptive data used to calculate the unit opportunity costs of time and travel for trial participants attending NHS dental appointments. Overall, 1308 out of 1873 (70%) participants completed the appropriate baseline questions and had linked routine data on the number of visits to the dentist to enable a full calculation of per-participant unit opportunity costs of attending dental appointments (for both participants and any companions they took with them to the dentist). The average opportunity cost of time and travel for a single dental visit ranged from £9.85 to £11.07 across groups. The average companion cost was much lower, ranging from £0.85 to £1.35, because only a small proportion (≈15%) of participants were accompanied to their dental appointment. The total number of visits to a dental practice for NHS care was between three and four across the groups.
Other private dental care costs
Participant-reported costs for dental care items include the cost of toothbrushes, replacement heads, interdental brushes and private dental care costs, including PI and other private care. Respondents in the 6-monthly PI group were more likely to self-report having private PI. However, as noted in Chapter 4, this potentially reflects a misunderstanding of what constitutes private care. Furthermore, an assessment of the validity of self-reported PI indicated a mismatch between self-reported data and routine information on PIs. Therefore, there is the potential for double-counting of participant-incurred costs as NHS-provided treatments may have been interpreted as private PIs in some cases. For this reason, the reader should exercise caution when interpreting any differences in participant-reported private PI costs.
Twenty respondents reported exceptionally high cost values for ‘other private treatment’, > £1000 (maximum = £11,100) for private dental care in at least one annual questionnaire (some reported high-cost items in more than one questionnaire). These high-cost items were unevenly spread across the randomised groups (n = 4, 1, 5, 3, 5 and 2) and led to skewed distributions and potentially misleading mean estimates. Such treatments included crowns, dentures and orthodontics. These high-cost items were verified with the practice in 14 out of 22 (64%) cases, with the remainder having no record of private treatments at their NHS practice. These high-cost items (> £1000 per year) were removed from the analysis given that there is no feasible clinical link between these treatments and the provision of PI or OHA.
Total participant costs
Table 23 details all participant-incurred costs. Overall, charges for NHS care represented a small proportion of total participant costs (< 20%), with the largest cost attributed to the purchase of interdental brushes. Total participant costs ranged from £265 to £345 across the groups. No PI with personalised OHA was less costly from the participant’s perspective (mean difference, compared with routine OHA and 6-monthly PI, –£64.11, 95% CI –£112.33 to –£15.88). The between-group difference is driven primarily by differences in the use of interdental brushes in England. There were no other significant differences between groups from the participant perspective. Owing to missing data across specific questions in each of the three annual participant questionnaires, participant costs are reported on the basis of multiple imputation of missing cost component data (at the item and questionnaire level). Missing opportunity costs of time and travel are imputed at the total cost level (number of visits multiplied by the calculated unit cost).
| Region | OHA, cost (£) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | |||||||||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| UK | (N = 289) | (N = 287) | (N = 290) | (N = 866) | (N = 334) | (N = 338) | (N = 335) | (N = 1007) | ||||||||
| Private PI | 5.75 | 20.59 | 5.67 | 23.10 | 12.04 | 34.56 | 7.83 | 26.94 | 4.23 | 20.88 | 7.71 | 26.23 | 9.18 | 24.51 | 7.04 | 24.06 |
| Other private dental care | 33.26 | 142.49 | 31.58 | 94.19 | 30.22 | 109.12 | 31.68 | 116.98 | 20.54 | 90.63 | 18.54 | 80.00 | 23.78 | 87.21 | 20.94 | 86.01 |
| Electric toothbrushes | 19.24 | 38.94 | 18.00 | 34.67 | 25.04 | 44.36 | 20.77 | 39.63 | 17.91 | 31.86 | 23.20 | 52.44 | 15.91 | 31.47 | 19.02 | 39.95 |
| Manual toothbrushes | 20.59 | 12.46 | 19.65 | 12.51 | 18.83 | 12.61 | 19.69 | 12.54 | 18.46 | 12.47 | 19.14 | 12.95 | 19.10 | 12.78 | 18.90 | 12.73 |
| Interdental brushes | 101.28 | 191.70 | 120.06 | 202.15 | 129.81 | 208.14 | 117.06 | 200.95 | 101.17 | 194.15 | 104.98 | 189.31 | 120.49 | 197.30 | 108.88 | 193.62 |
| Electric toothbrush (heads) | 19.40 | 21.77 | 19.95 | 21.94 | 21.27 | 20.92 | 20.21 | 21.55 | 18.74 | 20.72 | 21.05 | 20.98 | 19.00 | 22.86 | 19.60 | 21.55 |
| Participant NHS charges | 60.30 | 80.75 | 54.72 | 69.17 | 61.44 | 71.10 | 58.83 | 73.84 | 49.00 | 67.19 | 60.49 | 99.50 | 61.20 | 77.76 | 56.92 | 82.76 |
| Time and travel costs | 43.55 | 51.33 | 47.31 | 57.12 | 46.44 | 48.35 | 45.77 | 52.36 | 34.57 | 36.17 | 42.75 | 56.46 | 44.36 | 50.95 | 40.57 | 48.81 |
| Total participant costs | 303.38 | 281.03 | 316.95 | 267.39 | 345.10 | 301.61 | 321.85 | 284.02 | 264.62 | 249.57 | 297.85 | 271.66 | 313.02 | 263.00 | 291.87 | 262.16 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PIa | –39.80 (–83.94 to 4.33) | –23.48 (–70.00 to 23.04) | – | N/A | –64.11 (–112.33 to –15.88) | –16.64 (–68.28 to 35.00) | –30.28 (–81.59 to 21.03) | N/A | ||||||||
| Scotland | (N = 196) | (N = 195) | (N = 195) | (N = 586) | (N = 252) | (N = 255) | (N = 252) | (N = 759) | ||||||||
| Private PI | 4.62 | 13.89 | 4.92 | 22.18 | 5.89 | 18.76 | 5.14 | 18.57 | 3.01 | 14.92 | 6.11 | 21.41 | 7.24 | 18.71 | 5.45 | 18.63 |
| Other private dental care | 30.41 | 135.64 | 30.89 | 95.63 | 22.06 | 78.27 | 27.79 | 105.95 | 16.03 | 60.77 | 15.17 | 69.50 | 22.72 | 87.20 | 17.96 | 73.32 |
| Electric toothbrushes | 15.96 | 30.60 | 16.96 | 34.88 | 25.30 | 42.44 | 19.40 | 36.49 | 18.01 | 32.95 | 19.03 | 35.54 | 15.40 | 29.86 | 17.49 | 32.88 |
| Manual toothbrushes | 20.23 | 12.04 | 20.18 | 12.15 | 19.02 | 12.55 | 19.81 | 12.24 | 18.87 | 12.24 | 18.77 | 12.74 | 19.62 | 12.76 | 19.08 | 12.58 |
| Interdental brushes | 79.08 | 170.38 | 97.77 | 182.56 | 107.58 | 192.33 | 94.78 | 182.11 | 99.52 | 193.14 | 93.75 | 177.23 | 107.55 | 185.92 | 100.25 | 185.41 |
| Electric toothbrush (heads) | 18.01 | 21.53 | 17.26 | 20.62 | 20.99 | 20.70 | 18.75 | 21.00 | 17.81 | 20.24 | 20.44 | 20.85 | 17.98 | 21.98 | 18.75 | 21.05 |
| Participant NHS charges | 51.75 | 75.85 | 45.07 | 55.80 | 54.51 | 64.53 | 50.45 | 65.93 | 47.91 | 71.13 | 58.12 | 102.86 | 59.46 | 77.36 | 55.17 | 85.02 |
| Time and travel costs | 39.86 | 44.44 | 46.53 | 56.87 | 43.28 | 45.98 | 43.22 | 49.41 | 38.16 | 37.63 | 45.27 | 60.37 | 48.16 | 54.86 | 43.87 | 52.02 |
| Total participant cost | 259.92 | 249.03 | 279.58 | 246.81 | 298.64 | 245.20 | 279.34 | 247.21 | 259.32 | 240.28 | 276.66 | 259.72 | 298.11 | 252.42 | 278.03 | 251.19 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PIa | –44.10 (–93.67 to 5.47) | –24.78 (–77.38 to 27.81) | – | N/A | –41.13 (–93.09 to 10.82) | –27.65 (–80.89 to 25.60) | –3.78 (–59.28 to 51.72) | N/A | ||||||||
| England | (N = 93) | (N = 92) | (N = 95) | (N = 280) | (N = 82) | (N = 83) | (N = 83) | (N = 248) | ||||||||
| Private PI | 8.14 | 30.15 | 7.28 | 24.96 | 24.68 | 51.98 | 13.47 | 38.47 | 8.00 | 32.90 | 12.62 | 37.05 | 15.06 | 36.41 | 11.91 | 35.51 |
| Other private care | 39.27 | 156.39 | 33.04 | 91.42 | 46.98 | 153.28 | 39.84 | 137.02 | 34.41 | 148.49 | 28.88 | 105.68 | 26.99 | 87.56 | 30.08 | 116.25 |
| Electric toothbrushes | 26.17 | 51.84 | 20.19 | 34.28 | 24.48 | 48.22 | 23.63 | 45.41 | 17.57 | 28.38 | 36.00 | 84.64 | 17.45 | 36.04 | 23.70 | 56.15 |
| Manual toothbrushes | 21.35 | 13.32 | 18.54 | 13.22 | 18.44 | 12.76 | 19.44 | 13.14 | 17.20 | 13.13 | 20.25 | 13.58 | 17.53 | 12.77 | 18.33 | 13.20 |
| Interdental brushes | 148.08 | 224.00 | 167.30 | 232.34 | 175.44 | 231.56 | 163.68 | 228.94 | 106.23 | 198.18 | 139.46 | 219.77 | 159.80 | 224.89 | 135.28 | 215.02 |
| Electric toothbrush (heads) | 22.32 | 22.05 | 25.65 | 23.59 | 21.85 | 21.41 | 23.25 | 22.37 | 21.59 | 21.98 | 22.92 | 21.36 | 22.12 | 25.20 | 22.21 | 22.85 |
| Participant NHS charges | 78.32 | 87.89 | 75.17 | 88.16 | 75.68 | 81.50 | 76.39 | 85.61 | 52.37 | 53.53 | 67.79 | 88.56 | 66.51 | 79.17 | 62.26 | 75.34 |
| Time and travel costs | 51.34 | 62.90 | 48.98 | 57.86 | 52.93 | 52.46 | 51.10 | 57.75 | 23.53 | 28.69 | 35.01 | 41.47 | 32.83 | 34.26 | 30.49 | 35.48 |
| Total participant costs | 394.98 | 321.23 | 396.15 | 292.16 | 440.48 | 376.48 | 410.80 | 331.94 | 280.89 | 277.00 | 362.93 | 297.55 | 358.29 | 289.51 | 334.25 | 289.59 |
| Mean (95% CI) cost difference vs. routine OHA with 6-monthly PIb | –25.41 (–113.77 to 62.95) | –14.18 (–109.65 to 81.29) | – | N/A | –125.59 (–219.85 to –31.33) | –20.18 (–132.27 to 91.91) | –43.81 (–148.79 to 61.16) | N/A | ||||||||
Benefits
Discrete choice experiment
Participant characteristics
The DCE was administered online and 667 respondents completed the full questionnaire. Each respondent completed 10 choice tasks (after exclusion of dominance and consistency checks), leading to a total of 20,010 observations in the data set. The average median survey completion time was 17 minutes (interquartile range 13–24 minutes). Table 24 shows the characteristics of the DCE respondent sample.
| Characteristic | Mean | SD |
|---|---|---|
| Age (years) | 51 | 16 |
| Number of participants (n = 667) | % of total | |
| Sex | ||
| Male | 308 | 46.18 |
| Female | 359 | 53.82 |
| Currently living in | ||
| England | 482 | 72.26 |
| Scotland | 125 | 18.74 |
| Wales | 44 | 6.60 |
| Northern Ireland | 14 | 2.10 |
| Isle of Man | 2 | 0.30 |
| Currently a smoker | ||
| Yes | 98 | 14.69 |
| No | 447 | 67.02 |
| Previously a smoker | 122 | 18.29 |
| Annual gross income (£) | ||
| < 20,800 | 247 | 37.03 |
| 20,800–41,600 | 211 | 31.63 |
| ≥ 41,600 | 119 | 17.84 |
| Prefer not to answer/blank | 90 | 13.49 |
| Educational attainment | ||
| O levels/SVQ (level 1 or 2)/1 A level | 183 | 27.44 |
| ≥ 2 A levels/SVQ (level 3) | 101 | 15.14 |
| Degree | 164 | 24.59 |
| Professional qualifications | 81 | 12.14 |
| Apprentice qualification | 13 | 1.95 |
| Vocational/foreign/other/none | 125 | 18.74 |
| Employment | ||
| Any paid employment | 326 | 48.88 |
| Unemployed or seeking work | 24 | 3.60 |
| Retired | 185 | 27.74 |
| Student | 20 | 3.00 |
| Other | 112 | 16.79 |
| Self-reported dental health | ||
| Very poor | 6 | 0.90 |
| Poor | 28 | 4.20 |
| Fair | 200 | 29.99 |
| Good | 315 | 47.23 |
| Very good | 118 | 17.69 |
| Self-reported general health | ||
| Very poor | 10 | 1.50 |
| Poor | 47 | 7.05 |
| Fair | 169 | 25.34 |
| Good | 343 | 51.42 |
| Very good | 98 | 14.69 |
Most respondents were from England, but there was over-representation of the general population in Scotland to enable region-level subgroup analysis. In total, 67% of the sample never smoked. There was an even spread across the sample regarding educational attainment and 77% of the sample were either in paid work or retired. Overall, the majority of respondents reported that they were in fair or better general health and dental health. A total of 37 (6%), 232 (35%) and 398 (60%) respondents completed the worst, moderate and best segmented versions of the DCE, respectively.
Table 25 presents descriptive statistics detailing the DCE participants’ experience of dental care services. Most respondents were registered with a dental practice. A range of methods of payment for dental care were reported. A total of 62% of the sample had seen a dental hygienist in the past and 87% of the sample had had a PI in the past, with 77% having one at least annually. Respondents in the sample had more experience of contact with dental hygienists and having a PI than the general population: 47% had experienced treatment from a hygienist and 80% had had a PI. 11 Overall, the majority in the sample were regular dental attenders (86%), defined as seeing the dentist every 2 years or more often. By comparison, our sample had fewer regular attenders than the general population (the ADHS 200911 found that 71% of respondents attend the dentist at least every 2 years). This may be due to an element of self-selection into the DCE survey.
| Characteristic | Number of participants | % of total |
|---|---|---|
| Registered with a dental practice | ||
| Yes | 636 | 95.35 |
| No | 26 | 3.90 |
| Do not know | 5 | 0.75 |
| Normally pay for dental care | ||
| Co-charge | 307 | 46.03 |
| NHS pays all cost | 127 | 19.04 |
| Private: pay full cost | 143 | 21.44 |
| Dental treatment plan | 65 | 9.75 |
| Dental insurance | 12 | 1.80 |
| Never had dental care | 6 | 0.90 |
| Do not know | 7 | 1.05 |
| Ever been to visit a dental hygienist | ||
| Yes | 414 | 62.07 |
| No | 213 | 31.93 |
| Do not know | 40 | 6.00 |
| Normally have a PI | ||
| > every 3 months | 10 | 1.52 |
| Every 3 months | 65 | 9.89 |
| Every 6 months | 309 | 47.03 |
| Every year | 121 | 18.42 |
| Every 2 years | 22 | 3.35 |
| < every 2 years | 46 | 7.00 |
| Never | 84 | 12.79 |
| Regular attendancea | ||
| Attends at least every 2 years | 570 | 85.84 |
| Attends less often | 94 | 14.16 |
Results of analysis models
The results of the mixed logit model are presented in Table 26.
| Attribute/level | Model estimates | WTP (£) | ||
|---|---|---|---|---|
| Mean WTP compared with mean attribute level | 95% CI | Mean WTP compared with reference level | ||
| No OHAa | –0.143*** | –13.50 | –18.56 to –8.45 | – |
| Personalised OHA from dentist | 0.122*** | 11.50 | 6.61 to 16.39 | 25.00 |
| Personalised OHA from hygienist | 0.021 | 2.00 | –2.89 to 6.90 | 15.50 |
| No PIa | –0.721*** | –68.24b | –76.52 to –59.96 | – |
| 12-month PI from dentist | 0.194*** | 18.31b | 10.68 to 25.93 | 86.55 |
| 12-month PI from hygienist | –0.067* | –6.32b | –13.63 to 0.98 | 61.92 |
| 6-month PI from dentist | 0.315*** | 29.83b | 22.23 to 37.43 | 98.07 |
| 6-month PI from hygienist | 0.279*** | 26.43b | 19.21 to 33.65 | 94.67 |
| Bleeding gums | ||||
| Nevera | 0.385*** | 36.40 | 26.82 to 45.98 | – |
| Hardly ever | 0.307*** | 29.03 | 20.55 to 37.52 | –7.37 |
| Occasionally | –0.046 | –4.40 | –11.40 to 2.61 | –40.80 |
| Fairly often | –0.073 | –6.90 | –16.61 to 2.80 | –43.30 |
| Very often | –0.572*** | –54.13 | –73.37 to –34.90 | –90.53 |
| Teeth look and feel | ||||
| Very unclean | –0.899*** | –85.03 | –104.55 to –65.52 | – |
| Unclean | –0.393*** | –37.12 | –47.24 to –27.01 | 47.91 |
| Moderately clean | 0.139*** | 13.10 | 5.97 to 20.23 | 98.13 |
| Clean | 0.514*** | 48.59 | 39.91 to 57.26 | 133.62 |
| Very cleana | 0.639*** | 60.47 | 50.76 to 70.18 | 145.50 |
| Annual cost | –0.011*** | – | – | – |
| ASC | ||||
| Mean | 0.465*** | 43.95 | 29.49 to 58.41 | –86.42c |
| SD | 1.470*** | |||
| Log likelihood | –5174.58 | |||
| Number of observations | 20,010 | |||
| Number of respondents | 667 | |||
| AIC | 10,383 | |||
| BIC | 10,518 | |||
The positive value for the ASC indicates a preference among the general population to have any package of dental care as opposed to none. The significance of the SD around the constant term indicates the presence of significant preference heterogeneity for opting into a dental care package, with some respondents more likely to opt out (choosing no dental care package). Across all choice sets, 288 (43%) respondents always opted in to the choice task and 45 (7%) always opted out, choosing no dental care package.
Respondents valued having both personalised OHA and PI regardless of provider. They preferred to receive personalised OHA and 12-monthly PI from the dentist. However, who provided the care was less important for the 6-monthly PI. As expected, respondents prefer to have gums that do not bleed and teeth that look and feel clean. The negative coefficient on the annual cost attribute indicates that respondents prefer dental packages that cost less.
Willingness-to-pay estimates, together with their CIs, should be interpreted as the general population’s valuation of each attribute and level. The differences between WTP values indicate how much the general population values moving from one health-care package to another. For example, the general population would be willing to pay an additional £90.53 per year over 3 years to move from having bleeding gums very often (very often: –£54.13) to never having bleeding gums (never: £36.40). They would be willing to pay £145.50 per year to move from having teeth that look and feel very unclean to teeth that look and feel very clean. The difference between these valuations indicates that the general population attaches a greater value to shifts in average aesthetic outcome than to changes in frequency of bleeding 3 years after signing up to the dental package.
The WTP estimates can thus be used to calculate the general population’s WTP for packages of dental care. Assuming that a participant in the trial received no OHA/6-monthly PI from the dentist, hardly ever had bleeding gums at the final annual questionnaire and had teeth that, on average over the period, looked and felt moderately clean, the general population would be willing to pay £93.93 per year for this package (i.e. £43.95 – £13.50 + £29.83 + £20.55 + £13.10). Following a similar approach, the results of the DCE are mapped to each individual to enable a CBA to be completed alongside the trial.
Validity of responses
The estimates of annual WTP for PI (relative to none) fall within a feasible range of prices charged in the private market for PI (i.e. between £49 and £86 per PI provided by the dentist). In terms of the internal validity checks, 535 (80%) respondents passed the consistency of preferences check (i.e. answering the same way for exactly the same choice twice). A total of 498 (75%) respondents passed the non-satiation (dominance) test. Very few respondents failed both tests (≈5%). The high percentage passing such tests is in line with other DCE studies. 65
Analysis of subgroups
Subgroup analyses were conducted for participant characteristics by sex, region and income, as well as experience of PI and dental hygienists. Table 27 describes the impact of subgroup membership on preferences, through interaction terms between effects coded categorical variables and attribute-level main effects. Subgroup effects were considered significant at a p-value of < 0.01.
| Attribute levels | Base case | Hygienist experiencea | Region (Scotland)b | Sex (f)c | Income (> £20,800)d | Smoker (ever)e | Experience of PIf |
|---|---|---|---|---|---|---|---|
| Main effects | |||||||
| Personalised OHA from dentist | 0.122*** | 0.133*** | 0.143*** | 0.124*** | 0.121*** | 0.140*** | 0.144*** |
| Personalised OHA from hygienist | 0.021 | 0.016 | 0.027 | 0.019 | 0.022 | 0.011 | –0.027 |
| 12-monthly PI from dentist | 0.194*** | 0.199*** | 0.232*** | 0.194*** | 0.193*** | 0.208*** | 0.170*** |
| 12-monthly PI from hygienist | –0.067* | –0.065 | –0.089* | –0.071* | –0.065* | –0.100** | –0.171*** |
| 6-monthly PI from dentist | 0.315*** | 0.309*** | 0.316*** | 0.323*** | 0.315*** | 0.319*** | 0.280*** |
| 6-monthly PI from hygienist | 0.279*** | 0.274*** | 0.281*** | 0.283*** | 0.277*** | 0.294*** | 0.261*** |
| Bleeding gums | |||||||
| Hardly ever | 0.307*** | 0.313*** | 0.337*** | 0.303*** | 0.312*** | 0.301*** | 0.280*** |
| Occasionally | –0.046 | –0.032 | –0.094** | –0.055 | –0.043 | –0.053 | –0.143** |
| Fairly often | –0.073 | –0.058 | –0.012 | –0.073 | –0.084 | –0.055 | –0.057 |
| Very often | –0.572*** | –0.599*** | –0.591*** | –0.556*** | –0.573*** | –0.608*** | –0.429** |
| Teeth look and feel | |||||||
| Very unclean | –0.899*** | –0.989*** | –0.887*** | –0.845*** | –0.868*** | –0.901*** | –0.719*** |
| Unclean | –0.393*** | –0.368*** | –0.308*** | –0.392*** | –0.400*** | –0.398*** | –0.311*** |
| Moderately clean | 0.139*** | 0.183*** | 0.109** | 0.120*** | 0.134*** | 0.138*** | 0.049 |
| Clean | 0.514*** | 0.497*** | 0.448*** | 0.494*** | 0.508*** | 0.506*** | 0.402*** |
| Annual cost | –0.011*** | –0.011*** | –0.011*** | –0.011*** | –0.011*** | –0.011*** | –0.012*** |
| ASC | |||||||
| Mean | 0.465*** | 0.402*** | 0.448*** | 0.459*** | 0.472*** | 0.417*** | 0.239** |
| SD | 1.470*** | 1.435*** | 1.476*** | 1.473*** | 1.459*** | 1.464*** | 1.420*** |
| Interaction terms for subgroup with main attribute level effects | |||||||
| Personalised OHA from dentist | –0.034 | 0.032 | 0.011 | –0.018 | 0.044 | –0.023 | |
| Personalised OHA from hygienist | 0.017 | 0.010 | 0.022 | 0.021 | –0.028 | 0.058 | |
| 12-monthly PI from dentist | –0.007 | 0.062 | –0.021 | –0.009 | 0.036 | 0.030 | |
| 12-monthly PI from hygienist | 0.013 | –0.032 | 0.027 | –0.025 | –0.082* | 0.130** | |
| 6-monthly PI from dentist | 0.020 | –0.001 | 0.103** | 0.053 | 0.012 | 0.055 | |
| 6-monthly PI from hygienist | 0.001 | –0.004 | 0.071* | 0.069* | 0.033 | 0.025 | |
| Bleeding gums | |||||||
| Hardly ever | –0.022 | 0.045 | 0.069 | 0.098** | –0.018 | 0.033 | |
| Occasionally | –0.046 | –0.075* | 0.004 | 0.076** | –0.018 | 0.110 | |
| Fairly often | –0.036 | 0.094 | –0.080 | –0.059 | 0.057 | –0.026 | |
| Very often | 0.057 | –0.024 | –0.075 | –0.204** | –0.083 | –0.160 | |
| Teeth look and feel | |||||||
| Very unclean | 0.187 | 0.028 | –0.334*** | 0.117 | –0.004 | –0.213 | |
| Unclean | –0.077 | 0.124* | –0.216*** | –0.067 | –0.003 | –0.106 | |
| Moderately clean | –0.111*** | –0.047 | 0.160*** | –0.047 | –0.004 | 0.104 | |
| Clean | 0.070 | –0.102* | 0.177*** | –0.014 | –0.020 | 0.140* | |
| Annual cost | 0.002*** | 0.000 | –0.002*** | 0.001*** | –0.001** | 0.002*** | |
| ASC (mean) | 0.307*** | –0.024 | –0.064 | 0.081 | –0.134* | 0.320*** | |
| Likelihood ratio test | |||||||
| Likelihood ratio | 2176 | 2068 | 2177 | 2163 | 2132 | 2148 | 2025 |
| χ2 (df = 16) | 86.44 | 16.82 | 93.27 | 42.23 | 20.02 | 81.19 | |
| p-value | 0.0000 | 0.3976 | 0.0000 | 0.0004 | 0.2195 | 0.0000 | |
The likelihood ratio tests show no overall effect on preferences (jointly across all model parameters) for UK region or smoking status. For subgroups for which the tests indicated an impact of subgroup on preferences, the following findings were observed:
-
Respondents with experience of the hygienist valued the dental care packages more highly (disutility associated with cost attribute was lower). They also gained less utility from teeth that look and feel only moderately clean.
-
The significant interaction effect on the ASC with experience of PI indicates that those with experience of PI are more likely to commit to a dental care package. They also valued the dental care packages more highly.
-
Neither experience of seeing the dental hygienist or of having PI affected the preferences of respondents for having these services, or for whether it is the dentist or hygienist who provides the service.
-
Females in the sample gained greater utility from dental care packages in which teeth look and feel clean or very clean. Similarly, they experienced greater disutility from teeth that look and feel moderately clean or unclean.
-
As expected, the cost attribute had less of an impact on the preferences of higher-income respondents.
The subgroup analyses indicate that, although there were some differences across subgroups, in general, the direction of attribute-level effect remained similar with respondents preferring PI and personalised OHA, less bleeding and teeth that look and feel clean and healthy, as well as preferring lower-cost dental care packages.
Benefits: willingness to pay
Table 28 presents the mean WTP values across randomised groups, including the component parts of total WTP based on the mapping of DCE results to treatment received and trial outcomes. The average total benefits in terms of WTP ranged from £79.09 to £207.65 across the groups. The policy of 6-monthly PI and personalised OHA is associated with the highest average benefit. The difference in benefits between this policy and standard care (routine OHA and 6-monthly PI) is statistically significant. Compared with standard care, having no PI or a 12-monthly PI and routine OHA is associated with significantly lower benefits. There was no significant difference between the benefits for personalised OHA with either 12-monthly PI or no PI and standard care, implying that a reduction in PI can be compensated for by the provision of personalised OHA. The findings were consistent across regions. The average benefit was generally lower in England, as PIs were more likely to be provided by hygienists in England than in Scotland (following the assumption that the provider of baseline OHA/PI would consistently provide the trial intervention over follow-up).
| Region | OHA, WTP (£) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Routine OHA | Personalised OHA | |||||||||||||||
| No PI | 12-monthly PI | 6-monthly PI | Total | No PI | 12-monthly PI | 6-monthly PI | Total | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| UK | (N = 289) | (N = 287) | (N = 290) | (N = 866) | (N = 334) | (N = 338) | (N = 335) | (N = 1007) | ||||||||
| Constant | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 |
| OHA | –30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | 19.80 | 9.66 | 19.99 | 9.56 | 19.88 | 9.62 | 19.89 | 9.60 |
| PI | –82.92 | 70.59 | –56.55 | 60.80 | –27.43 | 76.87 | –55.60 | 73.29 | –93.73 | 63.83 | –51.77 | 61.96 | –21.60 | 70.98 | –55.65 | 71.97 |
| Bleeding | 30.02 | 45.86 | 35.28 | 41.60 | 29.98 | 47.63 | 31.74 | 45.15 | 33.86 | 45.78 | 33.48 | 42.39 | 35.25 | 44.17 | 34.19 | 44.10 |
| Look and feel | 63.12 | 61.06 | 67.05 | 59.84 | 75.61 | 58.32 | 68.60 | 59.96 | 69.52 | 61.83 | 66.69 | 62.00 | 75.24 | 58.34 | 70.47 | 60.84 |
| Total WTP | 79.09 | 97.68 | 114.25 | 95.04 | 146.46 | 104.91 | 113.30 | 103.01 | 128.46 | 104.80 | 167.28 | 95.92 | 207.65 | 116.31 | 167.87 | 110.72 |
| Mean (95% CI) benefit difference vs. routine OHA with 6-monthly PI | –67.65 (–86.50 to –48.81) | –30.75 (–48.65 to –12.85) | – | N/A | –17.72 (–39.37 to 3.93) | 19.70 (–1.64 to 41.04) | 61.67 (40.19 to 83.14) | N/A | ||||||||
| Scotland | (N = 196) | (N = 195) | (N = 195) | (N = 586) | (N = 252) | (N = 255) | (N = 252) | (N = 759) | ||||||||
| Constant | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 |
| OHA | –£30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | 23.16 | 7.13 | 23.03 | 7.28 | 23.25 | 7.03 | 23.14 | 7.14 |
| PI | –69.16 | 74.79 | –45.71 | 61.52 | –9.42 | 72.91 | –41.47 | 74.08 | –89.21 | 64.70 | –42.46 | 58.52 | –8.77 | 66.05 | –46.80 | 71.16 |
| Bleeding | 24.97 | 45.56 | 31.52 | 43.20 | 26.13 | 49.98 | 27.52 | 46.37 | 34.40 | 45.06 | 31.86 | 41.91 | 35.07 | 45.42 | 33.77 | 44.13 |
| Look and feel | 62.85 | 60.67 | 65.45 | 61.41 | 73.63 | £59.80 | 67.31 | 60.76 | 69.88 | 63.25 | 66.36 | 61.97 | 75.74 | 56.53 | 70.64 | 60.76 |
| Total WTP | 87.75 | 101.92 | 119.81 | 99.26 | 158.86 | 106.56 | 122.15 | 106.57 | 137.12 | 105.57 | 177.67 | 93.15 | 224.17 | 112.50 | 179.65 | 109.82 |
| Mean (95% CI) benefit difference vs. routine OHA with 6-monthly PI | –71.63 (–94.39 to –48.86) | –39.09 (–61.03 to –17.14) | – | N/A | –22.58 (–45.90 to 0.74) | 14.31 (–8.62 to 37.24) | 64.09 (40.95 to 87.22) | N/A | ||||||||
| England | (N = 93) | (N = 92) | (N = 95) | (N = 280) | (N = 82) | (N = 83) | (N = 83) | (N = 248) | ||||||||
| Constant | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 | 98.89 | 0.00 |
| OHA | –30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | –30.38 | 0.00 | 9.45 | 9.07 | 10.68 | 9.75 | 9.65 | 9.20 | 9.93 | 9.32 |
| PI | –111.93 | 49.83 | –79.52 | 52.57 | –64.42 | 71.67 | –85.16 | 62.04 | –107.61 | 59.31 | –80.38 | 63.77 | –60.55 | 71.58 | –82.75 | 67.67 |
| Bleeding | 40.66 | 44.84 | 43.15 | 36.97 | 37.89 | 41.45 | 40.54 | 41.17 | 32.17 | 48.04 | 38.46 | 43.62 | 35.79 | 40.34 | 35.49 | 44.05 |
| Look and feel | 63.67 | 62.04 | 70.44 | 56.33 | 79.71 | 55.07 | 71.31 | 58.18 | 68.40 | 57.29 | 67.71 | 62.17 | 73.72 | 63.75 | 69.96 | 61.13 |
| Total WTP | 60.92 | 85.69 | 102.59 | 84.67 | 120.74 | 96.88 | 94.81 | 92.56 | 101.50 | 97.95 | 135.36 | 97.61 | 157.50 | 113.78 | 131.70 | 105.67 |
| Mean (95% CI) benefit difference vs. routine OHA with 6-monthly PI | –59.82 (–89.86 to –29.78) | –15.06 (–44.61 to 14.48) | N/A | –12.45 (–51.68 to 26.79) | 25.39 (–13.44 to 64.21) | 45.46 (6.08 to 84.86) | N/A | |||||||||
The differences in benefits observed between groups were driven primarily by the differences in WTP for PI and personalised OHA. For example, the 6-monthly PI with routine OHA had a higher WTP (–£27.43) associated with PIs than no PI with routine OHA (–£82.92), a difference of £55.49. Note that the negative mean WTPs for PI across all groups are due to the relatively few PIs provided across all groups, and the use of effects coding in which WTP values are estimated relative to the mean of the attribute. In terms of interpretation, the focus should therefore be on differences in WTP for PI across groups. Similarly, for example, 6-monthly PI with personalised OHA had a higher WTP for the OHA component (£19.88) than did 6-monthly PI with routine OHA (–£30.38), an additional WTP of £50.26 for the OHA provided in the personalised group. There were some differences in benefits across regions, driven by the provider of care (more treatments assumed to be provided by hygienists in England than in Scotland). The differences across groups appear to be driven mainly by the services provided rather than the bleeding or aesthetic outcomes. This observation matches the findings reported in the clinical effectiveness chapters.
Cost–benefit analysis
Base-case analysis: NHS perspective
The CBA reports incremental net benefits (incremental benefits – incremental costs) for each policy, compared with standard care (routine OHA, with 6-monthly PI). Table 29 shows results from the base-case NHS dental health-care cost perspective. The average incremental net benefit ranged from –£69 to £48 across the groups. For the base-case analysis, personalised OHA with 6-monthly PI is associated with the highest positive incremental net benefit compared with standard care and the difference is statistically significant. Therefore, this is the preferred option from a welfare-maximising perspective. The only other policy that is associated with positive incremental net benefits is personalised OHA with 12-monthly PI, although it should be noted that the difference in net benefits between this policy and routine care is not statistically significant.
| Region | Mean (SD) costs | Mean difference in costsb (95% CI) vs. routine OHA (6-monthly PI) | Mean (SD) benefits | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI) | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI) |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 74 (106) | – | 146 (105) | – | – |
| No PI (n = 288) | 75 (122) | 2 (–19 to 22) | 79 (98) | –67 (–86 to –48) | –69 (–97 to –41) |
| 12-monthly PI (n = 285) | 81 (140) | 8 (–12 to 29) | 114 (95) | –30 (–48 to –12) | –38 (–65 to –12) |
| Personalised OHA | |||||
| No PI (n = 333) | 62 (116) | –6 (–28 to 16) | 128 (105) | –17 (–38 to 4) | –11 (–37 to 15) |
| 12-monthly PI (n = 338) | 86 (133) | 18 (–4 to 40) | 167 (96) | 21 (0 to 42) | 3 (–23 to 29) |
| 6-monthly PI (n = 335) | 83 (134) | 15 (–8 to 37) | 208 (116) | 63 (42 to 84) | 48 (22 to 74) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 159 (107) | – | – |
| No PI (n = 195) | 64 (87) | –9 (–28 to 11) | 88 (102) | –71 (–94 to –48) | –63 (–92 to –34) |
| 12-monthly PI (n = 193) | 67 (83) | –4 (–23 to 16) | 120 (99) | –39 (–61 to –17) | –35 (–64 to –6) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–28 to 18) | 137 (106) | –23 (–49 to 3) | –18 (–46 to 10) |
| 12-monthly PI (n = 255) | 80 (121) | 12 (–11 to 35) | 178 (93) | 14 (–11 to 40) | 2 (–25 to 29) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–11 to 35) | 224 (112) | 64 (38 to 89) | 52 (24 to 79) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 79 (129) | – | 121 (97) | – | – |
| No PI (n = 93) | 99 (172) | 25 (–25 to 74) | 61 (86) | –59 (–90 to –29) | –84 (–143 to –25) |
| 12-monthly PI (n = 92) | 111 (214) | 32 (–17 to 81) | 103 (85) | –15 (–44 to 15) | –47 (–104 to 11) |
| Personalised OHA | |||||
| No PI (n = 81) | 65 (149) | –13 (–68 to 43) | 102 (98) | –10 (–49 to 29) | 3 (–57 to 62) |
| 12-monthly PI (n = 83) | 107 (161) | 31 (–25 to 87) | 135 (98) | 28 (–11 to 66) | –3 (–62 to 56) |
| 6-monthly PI (n = 83) | 95 (169) | 19 (–37 to 74) | 157 (114) | 48 (9 to 87) | 29 (–30 to 89) |
Confidence ellipses on the cost-effectiveness plane for each comparison are reported in Appendix 2 and show that personalised OHA with 6-monthly PI has a high probability of generating a positive incremental net benefit (i.e. with plots to the right of the diagonal line on the cost–benefit plane). Routine OHA with either no PI or 12-monthly PI is associated with negative incremental net benefits compared with standard care, and the differences in net benefits are statistically significant. Figure 19, Appendix 2 shows that there is more uncertainty surrounding the incremental net benefits associated with personalised OHA (no PI or 12-monthly PI) relative to routine OHA (6-monthly PI), with confidence ellipses crossing the diagonal.
The main conclusion is consistent across regions, although the difference in positive incremental net benefits for personalised OHA and 6-monthly PI is not statistically significant for England. This may be caused by the relatively smaller sample in England.
Wider perspective cost–benefit analysis
Table 30 presents the CBA results using the NHS and participant perspectives for costs. The preferred policy option is the same as in the base-case analysis, namely personalised OHA with 6-monthly PI. The incremental net benefit is higher than in the base-case analysis (£68 vs. £48). Note that the CI is much wider, reflecting the relatively large variation in participant costs. The preferred policy varies across regions when using the wider perspective. For England, personalised OHA with no PI generates the largest incremental net benefit. This result is driven by the lower cost of this policy compared with standard care. As noted in Total participant costs, this appears to be driven mainly by the lower costs of interdental brushes. The extent to which this can be attributable directly to the intervention is questionable. Furthermore, conclusions about participant-reported costs should be interpreted in the light of concerns about double-counting of private/NHS PI.
| Region | Mean (SD) costs | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI) | Mean (SD) benefits | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI) | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI) |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 420 (323) | – | 146 (105) | – | – |
| No PI (n = 288) | 378 (312) | –41 (–93 to 11) | 79 (98) | –68 (–86 to –49) | –26 (–81 to 28) |
| 12-monthly PI (n = 285) | 398 (302) | –17 (–69 to 36) | 114 (95) | –31 (–49 to –13) | –14 (–70 to 42) |
| Personalised OHA | |||||
| No PI (n = 333) | 327 (279) | –75 (–126 to –25) | 128 (105) | –17 (–39 to £4) | 58 (7 to 110) |
| 12-monthly PI (n = 338) | 384 (302) | –21 (–73 to 30) | 167 (96) | 20 (–1 to 41) | 41 (–11 to 94) |
| 6-monthly PI (n = 335) | 396 (295) | –6 (–57 to 46) | 208 (116) | 62 (41 to 83) | 68 (15 to 120) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 371 (257) | – | 159 (107) | – | – |
| No PI (n = 195) | 323 (264) | –49 (–106 to 8) | 88 (102) | –72 (–94 to –49) | –23 (–83 to 37) |
| 12-monthly PI (n = 195) | 347 (255) | –25 (–83 to 33) | 120 (99) | –39 (–61 to –17) | –14 (–75 to 47) |
| Personalised OHA | |||||
| No PI (n = 252) | 320 (266) | –41 (–96 to 14) | 137 (106) | –22 (–47 to 2) | 18 (–38 to 74) |
| 12-monthly PI (n = 255) | 356 (291) | –13 (–68 to 42) | 178 (93) | 14 (–10 to 38) | 27 (–29 to 84) |
| 6-monthly PI (n = 252) | 377 (284) | 15 (–40 to 70) | 224 (112) | 64 (40 to 88) | 49 (–7 to 105) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 520 (411) | – | 121 (97) | – | – |
| No PI (n = 93) | 494 (371) | –20 (–125 to 86) | 61 (86) | –60 (–90 to –29) | –40 (–148 to 68) |
| 12-monthly PI (n = 92) | 507 (360) | 6 (–102 to 115) | 103 (85) | –15 (–45 to 15) | –21 (–131 to 89) |
| Personalised OHA | |||||
| No PI (n = 81) | 346 (319) | –154 (–265 to –43) | 102 (98) | –12 (–52 to 28) | 142 (31 to 253) |
| 12-monthly PI (n = 83) | 469 (320) | –14 (–129 to 100) | 135 (98) | 26 (–14 to 66) | 40 (–73 to 153) |
| 6-monthly PI (n = 83) | 453 (322) | –40 (–153 to 72) | 157 (114) | 46 (5 to 86) | 86 (–27 to 200) |
This result may have also caused the second preferred policy for the UK as a whole to be different from the base-case analysis. The second preferred policy for the base-case analysis was personalised OHA with 12-monthly PI (incremental net benefit of £3) while the second preferred policy when taking the wider perspective was personalised OHA with no PI (incremental net benefit of £58).
Sensitivity analysis results
Table 31 reports the incremental net benefit results for a range of sensitivity analyses. All sensitivity analyses were conducted using NHS-perspective dental care costs. Further details of mean and incremental costs and benefits are presented for each analysis in Appendix 2, Section 6: detailed results of further health economics analyses, at a regional and UK level. Overall, the conclusions of our analyses remain robust to the range of sensitivity analyses undertaken. In all cases, a policy of personalised OHA with 6-monthly PI was associated with the greatest incremental net benefit relative to standard care. This was statistically significant for all scenarios except those for which an additional charge was included for personalised OHA. This analysis was conducted to reflect the likely opportunity cost of time spent delivering personalised OHA if it were to be rolled out as a policy. However, under current reimbursement structures, such a charge is unlikely to be directly incurred by the NHS. In all cases, routine OHA with no PI was associated with the lowest incremental net benefit relative to standard care. It should be noted that, across all sensitivity analyses, the results were driven by high estimates of WTP for PI in particular. Therefore, our results and conclusions are most applicable to decision-makers wishing to maximise societal welfare.
| Policy | Incremental net benefits (95% CI) (£) vs. routine OHA (6-monthly PI) | |||||
|---|---|---|---|---|---|---|
| Base-case analysis | Including baseline and final visits | Discounting at 0% | Discounting at 6% | Assuming all PI and OHA delivered by dentist | Assuming all PI and OHA delivered by hygienist | |
| Routine OHA | ||||||
| 6-monthly PI (n = 289) | – | – | – | – | – | – |
| No PI (n = 288) | –69 (–97 to –41) | –68 (–103 to –33) | –70 (–99 to –42) | –67 (–93 to –40) | –70 (–98 to –43) | –61 (–88 to –34) |
| 12-monthly PI (n = 285) | –38 (–65 to –12) | –33 (–67 to 1) | –40 (–68 to –12) | –38 (–65 to –11) | –37 (–65 to –10) | –37 (–64 to –10) |
| Personalised OHA | ||||||
| No PI (n = 333) | –11 (–37 to 15) | 9 (–24 to 42) | –13 (–40 to 15) | –10 (–36 to 15) | –8 (–34 to 19) | –18 (–44 to 8) |
| 12-monthly PI (n = 338) | 3 (–23 to 29) | 25 (–8 to 57) | 1 (–26 to 28) | 4 (–22 to 29) | 8 (–18 to 35) | –13 (–39 to 13) |
| 6-monthly PI (n = 335) | 48 (22 to 74) | 69 (36 to 102) | 49 (21 to 76) | 47 (22 to 73) | 55 (28 to 82) | 29 (2 to 55) |
| Policy | Incremental net benefits (95% CI) (£) vs. routine OHA (6-monthly PI) | |||||
| Base-case analysis | Additional cost for personalised OHA | Decreasing the unit price of a UDA (–20%) | Increasing the unit price of a UDA (+20%) | Alternative mapping of aesthetic outcome from the DCE | ||
| Routine OHA | ||||||
| 6-monthly PI (n = 289) | – | – | – | – | – | |
| No PI (n = 288) | –69 (–97 to –41) | –68 (–101 to –36) | –66 (–91 to –40) | –68 (–97 to –39) | –64 (–89 to –39) | |
| 12-monthly PI (n = 285) | –38 (–65 to –12) | –39 (–70 to –7) | –36 (–61 to –10) | –40 (–69 to –10) | –37 (–61 to –12) | |
| Personalised OHA | ||||||
| No PI (n = 333) | –11 (–37 to 15) | –54 (–104 to –4) | –12 (–37 to 13) | –9 (–38 to 19) | –5 (–29 to 18) | |
| 12-monthly PI (n = 338) | 3 (–23 to 29) | –48 (–100 to 5) | 3 (–21 to 28) | 2 (–27 to 30) | 7 (–16 to 31) | |
| 6-monthly PI (n = 335) | 48 (22 to 74) | 28 (–15 to 70) | 49 (23 to 74) | 48 (20 to 77) | 44 (20 to 68) | |
Cost–consequences analysis
The balance sheet of costs and outcomes outlined in Table 32 narratively summarises the advantages and disadvantages of the different policy considerations within a cost–consequences framework. The analysis makes no attempt to conduct any further quantitative synthesis of economic findings.
| Policy | Analysis | Advantages/positives | Disadvantages/negatives |
|---|---|---|---|
| Routine OHA (6-monthly PI): SC | DCE | 6-monthly PI is preferred by the general population | Personalised is preferable to routine OHA |
| Clinical effectiveness | – | No evidence of clinical benefit | |
| Incremental costs (£) (vs. SC) | N/A | N/A | |
| Incremental benefits (£) (vs. SC) | N/A | N/A | |
| INB (£) (vs. SC) | N/A | N/A | |
| Routine OHA (no PI) | DCE | – | Least preferred policy by the general population |
| Clinical effectiveness | A policy of full disinvestment in PI would not adversely affect bleeding gums | – | |
| Incremental costs (£) (vs. SC) | NHS –3 (95% CI –18 to 12) | N/A | |
| Participant –40 (95% CI –84 to 4) | |||
| Incremental benefits (£) (vs. SC) | N/A | –68 (95% CI –87 to –49) | |
| INB (£) (vs. SC) | N/A | –69 (95% CI –97 to –41) | |
| Routine OHA (12-monthly PI) | DCE | – | 12-monthly PI preferred to none but 6-monthly OHA would be more highly valued |
| Clinical effectiveness | – | No evidence of clinical benefit of PI | |
| Incremental costs (£) (vs. SC) | Participant –23 (95% CI –70 to 23) | NHS 0 (95% CI –15 to 16) | |
| Incremental benefits (£) (vs. SC) | N/A | –31 (95% CI –49 to –13) | |
| INB (£) (vs. SC) | N/A | –38 (95% CI –65 to –12) | |
| Personalised OHA (no PI) | DCE | Personalised OHA preferred to routine | Policies with delivery of 12-monthly or 6-monthly PI would be preferred |
| Clinical effectiveness | – | No evidence of clinical benefit | |
| Incremental costs (£) (vs. SC) | NHS –15 (95% CI –34 to 4) | N/A | |
| Participant –64 (95% CI –112 to –16) | |||
| Incremental benefits (£) (vs. SC) | N/A | –18 (95% CI –39 to 4) | |
| INB (£) (vs. SC) | N/A | –11 (95% CI –37 to 15) | |
| Personalised OHA (12-monthly PI) | DCE | Personalised OHA preferred to routine; reduction in PI could, in part, be compensated for by personalised OHA | – |
| Clinical effectiveness | – | No evidence of clinical benefit | |
| Incremental costs (£) (vs. SC) | Participant –17 (95% CI –68 to 35) | NHS 6 (95% CI –16 to 28) | |
| Incremental benefits (£) (vs. SC) | 20 (95% CI –2 to 41) | N/A | |
| INB (£) (vs. SC) | 3 (95% CI –23 to 29) | N/A | |
| Personalised OHA (6-monthly PI) | DCE | Most preferred policy by the general population | – |
| Clinical effectiveness | – | No evidence of clinical benefit | |
| Incremental costs (£) (vs. SC) | Participant –30 (95% CI –82 to 21) | NHS 12 (95% CI –11 to 34) | |
| Incremental benefits (£) (vs. SC) | 62 (95% CI 40 to 83) | N/A | |
| INB (£) (vs. SC) | 48 (95% CI 22 to 74) | N/A |
The data presented in the balance sheet indicate the trade-offs decision-makers need to consider when determining the most efficient policy of PI and OHA to implement. It shows that, although personalised OHA with 6-monthly PI is the most cost-beneficial policy, owing to the high value placed on the service by the general population, there is no clear evidence of clinical benefit, or changes to provider beliefs. The preferred policy depends on the viewpoint taken. If the aim is to maximise welfare from a fixed NHS budget, then the CBA analysis shows that personalised OHA with 6-monthly PI is the preferred policy. If the aim is to maximise health from a fixed NHS budget, then it could be argued that no PI is the preferred policy given the lack of evidence of clinical benefit and given that it was associated with the lowest average costs (although it should be noted that differences were not significant).
Chapter 6 Cohort
Introduction
The pragmatic design of the IQuaD trial (reported in Chapter 2) outlined recruitment of healthy individuals or those with mild to moderate forms of periodontal disease. As one of the intervention groups received no PI, the IQuaD Periodontal Advisory Group recommended that potential participants exhibiting more severe forms of disease, as defined by BPE scores of 4 or *, should not be recruited into the experimental trial. The most advanced form of periodontal disease affects only a relatively small percentage of the population11 and there is a paucity of long-term data for these individuals in the primary care setting. A prospective longitudinal cohort study was therefore conducted in conjunction with the IQuaD trial, involving those individuals who were found to be ineligible for the IQuaD trial but who consented to be followed up for 3 years.
Methods
The IQuaD cohort study was conducted in conjunction with the IQuaD trial and the recruitment protocol of dental practices and participants is summarised in Chapter 2.
Ethics approval
Favourable ethics approval for the IQuaD trial was confirmed by the East of Scotland Research Ethics Service on 24 March 2011 (REC reference number 10/S0501/65).
Recruitment and consent
The IQuaD trial and IQuaD cohort recruitment were conducted in 63 IQuaD trial dental practices in Scotland and north-east England from February 2012 to July 2013. All participants in the cohort study had previously consented to participate in the IQuaD trial. Following baseline clinical screening and outcome measurement, potential participants were advised that they were not eligible to be included in the trial if they were found to have a BPE score of 4 or * or had an uncontrolled medical condition. They were then invited to participate in the IQuaD cohort study and signed consent to take part in this prospective longitudinal study. The IQuaD cohort study participants subsequently received a routine dental examination from their general dental practitioners, who were advised that the patient was ineligible for the IQuaD trial. The IQuaD cohort study participants received usual care and were not part of the experimental design.
Outcomes
The clinical and patient-centred outcomes were the same as the IQuaD trial outcomes (outlined in methods). The IQuaD cohort participants completed the same baseline questionnaire as the IQuaD trial participants and were also sent an annual questionnaire for 3 years.
Statistical analyses
No statistical analyses were planned and only descriptive data of the longitudinal cohort were reported.
Results
Baseline characteristics
Of the 2341 potential participants who attended for a baseline clinical outcome assessment, 160 were found to have a BPE score of 4 and/or *. These potential participants were invited to participate in the IQuaD cohort study, with 16 (10%) declining to participate (reasons outlined in Table 6), resulting in 144 (90%) participants being recruited to the longitudinal study.
Clinical outcomes
Clinical outcome assessment, measurement and outcome mean calculations followed the same protocol as the IQuaD trial (outlined in Chapter 2, Collection of clinical outcome measures). At baseline, the 144 cohort participants had a mean number of 22 (SD 4.7) teeth present, with gingival inflammation/bleeding recorded in 35.1% (mean) (SD 23.4%) of sites. Supragingival calculus was recorded on 40.6% (mean) (SD 30.2%) of teeth and the mean clinical probing depth of the cohort patients was 2.2 mm (SD 0.4 mm).
Similar to the IQuaD trial, a number of recruited participants were not available for follow-up clinical assessment; 85 (59%) IQuaD cohort participants attended for follow-up clinical assessment at approximately 3 years following recruitment. When considering only the 85 participants who attended for both clinical assessment appointments, the baseline clinical characteristics were as follows: a mean number of 21.6 (SD 5) teeth present, 37.0% (mean) (SD 24%) of sites with recorded gingival inflammation, mean supragingival calculus of 36.0% (SD 27%) and mean clinical probing depth of 2.2 mm (SD 0 mm). A total of 97% (n = 82) of these participants had a BPE score of 4 or * at baseline; the remaining three participants, who were ineligible due to medical history reasons, had a BPE score of 3 (1%, n = 1) or 2 (2%, n = 2).
Patient-centred outcomes
The collection, assessment and mean score calculations of the participant-centred outcomes are the same as in the IQuaD trial outlined in Chapter 2.
At baseline, the cohort participants’ self-efficacy, assessed as confidence in one’s ability to perform a behaviour and measured using a 7-point scale scored from 1 (not at all confident) to 7 (extremely confident), was, on average, 5.0 points (SD 1.2 points; completed the questionnaire, n = 131).
Cognitions
Perceived behaviour control score, assessed in terms of perceived ease or difficulty of performing the behaviour and measured using a 7-point scale varying from 1 (strongly agree) to 7 (strongly disagree), was, on average, 4.1 points (SD 1.4 points; completed the questionnaire, n = 131).
Attitude towards oral health behaviour, measured using a scale varying from 1 (strongly agree) to 7 (strongly disagree), was very positive, averaging 5.6 points (SD 1.2 points; completed the questionnaire, n = 131).
Subjective norm, assessed as attitude towards the behaviour and perceptions of social pressure to perform the behaviour and measured using a scale of 1 (strongly agree) to 7 (strongly disagree) scored, on average, 4.9 points (SD 1.3 points; completed the questionnaire, n = 131).
The score of mean intention to perform good oral hygiene practice was 5.5 points (SD 1.8 points; completed the questionnaire, n = 114), which was measured using questions to which responses were scored from 0 to 3 (the best possible intention), with 9 being the best possible score.
Behaviour
On the self-reported oral hygiene behaviours scale, which ranges from 0 to 9, with 9 being the best possible behaviour, the cohort group had an average score of 4.9 points (SD 1.7 points; completed the questionnaire, n = 130). Like the IQuaD trial participants, the cohort group showed room for improvement on this scale.
Quality of life
The self-reported QoL was measured using the OHIP-14, a 14-item oral health-specific patient-centred outcome referring to symptoms in the past 12 months. Each item is scored from to 0 to 4 (very often) and the scores are summed to produce a summary score ranging from 0 to 56, with 56 being the worst outcome. The cohort participants’ mean score at baseline was 8.5 points (SD 9.5 points; completed the questionnaire, n = 126).
Follow-up characteristics
Clinical
Follow-up examinations were conducted from May 2015 to July 2016. At the 3-year clinical follow-up, the 85 participants had an average of 21.2 (SD 5.4) teeth with mean gingival inflammation/bleeding recorded at 41% (SD 25.5%) of sites. More teeth (mean 43.5%, SD 33.1%) exhibited supragingival calculus and the mean clinical probing depths had increased minimally by 0.1 mm to 2.3 mm (SD 0.5 mm).
Patient-centred outcomes
At the 3-year follow-up, the cohort participant patient-centred outcomes were similar to the baseline scores. The average self-efficacy score was 5.1 points (SD 1.1 points; completed the questionnaire, n = 101) on a scale of 1–7.
Cognitions
The average PBC, attitude and subjective norm scores were 4.4 points (SD 1.3 points; completed the questionnaire, n = 87), 5.2 points (SD 1.6 points; completed the questionnaire, n = 87) and 4.5 points (SD 1.4 points; completed the questionnaire, n = 86), respectively, with each measured on a scale of 1–7. The 3-year follow-up average intention score was 5.4 points (SD 1.8 points; completed the questionnaire, n = 83) on a scale from 0 to 9, with 9 being the best possible score.
Behaviour
On the self-reported oral hygiene behaviours scale, which ranges from 0 to 9, with 9 being the best possible behaviour, the cohort group had a mean score of 5.4 points (SD 1.8 points; completed the questionnaire, n = 87).
Quality of life
The mean score on the OHIP-14, which measures self-reported QoL, was 7.8 points (SD 8.0 points; completed the questionnaire, n = 83).
Discussion
The IQuaD cohort study provided a unique opportunity to monitor NHS primary care patients with severe periodontal disease over a 3-year time period. The end-point outcome of periodontal disease is tooth loss, which is thought to be concentrated in a relatively small number of individuals with severe periodontal disease. There is a general paucity of long-term data on individuals with severe periodontal disease in primary care receiving routine care (which may include specialist periodontist referral). Among the 85 participants with baseline and follow-up data, there were only minimal changes in the mean scores of present teeth (–0.4 teeth), gingival inflammation (+4%), supragingival calculus (+7.5%) and mean clinical probing depths (+0.1 mm). Similarly, there were only minimal changes in participant-reported outcomes: self-efficacy (+0.1 points; 1–7 scale), PBC (+0.2 points; 1–7 scale), attitude (–0.3 points; 1–7 scale), subjective norm (–0.4 points; one to seven scale), behaviour (+0.3 points, 0–9 scale) and intention (no change). Interestingly, the patient-centred outcome scores (including QoL) were largely similar to those of the IQuaD trial participants.
The IQuaD cohort study has a number of limitations, including the high attrition rate, with only 59% of participants being available for 3-year clinical follow-up. The recruitment strategy of the IQuaD trial meant that only those individuals who had a BPE score of ≤ 3 at their most recent examination appointment were invited to the trial recruitment sessions. Therefore, it is unclear how representative this relatively self-selecting group of participants was, considering that the participants were all regular attenders at their primary care dental practice who had previously shown no signs of severe periodontal disease (as defined by a BPE score of 4).
It would be inappropriate to draw definitive conclusions from the outcomes, but it appears that periodontal condition and participant-centred outcomes did not markedly deteriorate or improve in the 85 IQuaD cohort participants over the course of this 3-year study.
Chapter 7 Discussion/conclusions
The IQuaD trial involving regular adult NHS dental attenders (with no or early signs of periodontitis) has shown that, over a 3-year period, scheduling 6-monthly or 12-monthly PIs has no additional beneficial effect (over not providing this treatment unless desired or recommended) on primary clinical (gingival inflammation/bleeding) and patient-centred (self-reported) outcomes and that there is no difference between personalised OHA and routine OHA (current practice). However, patients value, and are willing to pay for, both interventions, with greater financial value placed on PI than on OHA.
The IQuaD trial is the first pragmatic cluster RCT to evaluate PI and OHA in NHS dental practices. These interventions are the most frequently performed treatments in dentistry, with considerable cost to the NHS and society. The aim of this RCT in primary care dental practice was to provide evidence for the benefit or not of these routine treatments on the periodontal health of adults with no or early signs of periodontal disease.
The primary clinical outcome, gingival inflammation/bleeding, is a measure of gingivitis, a recognised precursor of periodontitis, caused by plaque retention, and is reversible with effective plaque removal. The PI in the IQuaD trial involved professional mechanical removal of plaque and calculus. Effective oral hygiene (self-care) involves tooth brushing with appropriate interdental cleaning, which, if undertaken effectively, will be sufficient to prevent or resolve gingivitis.
At baseline, most participants (98%) experienced gingival inflammation/bleeding, with, on average, 33% of sites affected. The prevalence of gingival inflammation/bleeding measured at baseline was similar to that reported in other studies25 and confirmed that, if effective, the trial interventions had the opportunity/potential to improve periodontal health.
At the 3-year follow-up, there was no evidence of a difference in gingival inflammation/bleeding between those randomised to PI on a 6-monthly basis and those who were randomised to receive no PI (difference 0.87%, 95% CI –1.6% to 3.3%; p = 0.481). Similarly, there was no evidence of a difference between scheduled 6-monthly PI and scheduled 12-monthly PI (difference 0.11%, 95% CI –2.3% to 2.5%; p = 0.929). We are confident in the finding of no clinical benefit for 6-monthly PI over other frequencies of PI because the 95% CIs were small enough to exclude the prespecified clinically important difference in bleeding of 7.5%. Of the secondary clinical outcomes, a difference between groups was found only for calculus, with those participants in the no-PI group having 8% (95% CI 5.4% to 10.7%; p = 0.001) more sites with calculus than the 6-monthly PI group after 3 years. Although there was a statistically significant difference, the clinical significance or relevance of this result has to be questioned because there was no associated improvement in the primary clinical outcome of gingival inflammation/bleeding.
All participants received PI at baseline and were then randomised to be offered this treatment 6-monthly, 12-monthly or not at all for the 3-year duration of the trial. Participants’ dental practices were asked to recall their participants for PI in keeping with their randomised allocation; however, a PI could be provided if either the participant requested it or the clinician thought it necessary. The pragmatic nature of the trial meant that scheduling of dental appointments and the intervals between PIs varied, as they do in routine practice. Prior to and since the publication of NICE dental recall guidelines,66 common practice is to provide a PI at the same time as a dental recall visit and for this to be planned every 6 months. The intention was that participants randomised to the 6-monthly PI group would receive this frequency of care.
The claims recorded in the routine data collected for the IQuaD trial confirmed that the number and interval between PIs varied within groups and demonstrated clear separation in the mean number of PIs between groups. At the 3-year follow-up, there was a threefold difference in the mean number of PIs (1.1, 2.0 and 3.0 for the no-PI, 12-monthly and 6-monthly groups, respectively). National routine data67 suggest that, in the NHS, the most frequent interval between visits with a PI is 9 months and this reflects the experience of participants in the 6-monthly PI group. The finding that 46% of participants randomised to the no-PI group did not request, and were not thought to need, this treatment (PI) indicates that almost half of participants were not clinically compromised for the duration of the trial and would not have experienced any benefit had this treatment been provided.
Although participants with moderate to severe periodontal disease (BPE score of 4 or *) were excluded from the trial, adults with a spectrum of periodontal disease and a wide age range were included. Using the baseline data, these groups were included in subgroup analyses along with dental practice/cluster employment of a hygienist, smoking habit and a post hoc criterion for moderate periodontitis evidenced by four or more pockets with a clinical probing depth of ≥ 4 mm. The IQuaD trial found no evidence of statistically significant differences at the 1% level for the prespecified and post hoc subgroup analyses and the primary clinical outcome of gingival inflammation/bleeding. However, the results suggest that there may be some clinical benefit of 6-monthly PI compared with other frequencies for participants with BPE scores of 3 at baseline (difference –4.7% 99% CI –10.5% to 1.1%; p = 0.038). Current clinical guidance from the British Society of Periodontology16,68 recommends providing PI for early signs of periodontitis (BPE score of 3 with bleeding or other risk factors) and these results suggest a possible benefit to such a targeted approach.
The IQuaD trial found no evidence of a difference between participants randomised to personalised OHA and those randomised to routine advice (current practice) for any outcome. Although the 95% CI for the difference in gingival inflammation/bleeding (–2.5%, 95% CI –8.3% to 3.3%; p = 0.393) does not exclude the prespecified clinically important difference of 7.5%, it is unlikely that the personalised OHA has any impact > 8%. The results were robust to other adjusted/unadjusted models. Similarly to PI frequency, there was no evidence that any of the subgroup analyses were statistically significant at the 1% level. Although we found no evidence of a difference between the two OHA interventions, we have evidence of intervention fidelity from participant-reported responses in their follow-up questionnaires about desired oral health behaviours such as spit but not rinse, which leads us to believe that the personalised intervention was indeed delivered as intended. Participants having a plan for brushing or flossing better was another variable assessed. The randomised groups (personalised vs. routine) had similar levels of participants reporting to have a plan. This might reflect the fact that the question was asked too late (it would have been around 1 year between the participants making a plan with their clinicians and receiving their first participant annual questionnaire). Regardless, there is no good evidence that clinicians in the personalised arm delivered the intervention in a way that promoted planning of oral hygiene behaviour.
The personalised OHA was designed as a brief intervention (under 10 minutes) appropriate to be delivered in primary care dental practice. OHA is considered integral within a NHS recall/check-up appointment and only rarely attracts an additional fee. The trial intervention was designed 10 years ago using psychological behaviour change models; since that time national clinical guidance documents that provide similar advice have been disseminated in both England16 and Scotland. 69 Dentists randomised to deliver routine OHA were asked to conform to their current practice. Therefore, one possible explanation of the outcome is that the personalised OHA was similar to current practice and therefore did not result in any additional benefit compared with current practice.
The confidence (self-efficacy) of participants to perform effective oral hygiene (i.e. to clean their teeth and gums regularly and well) was chosen to be the primary patient-centred outcome because gingivitis can be prevented with effective oral hygiene. 34 The IQuaD trial found participants to be moderately confident in performing good oral hygiene and the frequency of PI or type of OHA did not make a difference. The statistically significant difference found between those randomised to receive 6-monthly and 12-monthly PI, favouring 6-monthly PI (difference –0.097, 95% CI –0.188 to 0.006; p = 0.037), is most likely due to the very precise CI. The difference considered clinically significant was one-quarter of a SD around 0.275 in the self-efficacy scale and the CI excludes that value.
For all other clinical and patient-centred outcomes, the IQuaD trial found no evidence to suggest that the frequency of PI or whether or not OHA was personalised made a difference.
The health economics analysis assessed the costs and benefits of alternative policies of OHA with or without PI in terms of costs and benefits (WTP). The analysis focused on whether moving from standard care to an alternative policy increases or decreases welfare. The results showed that there were no statistically significant differences in total NHS costs for any of the policies compared with standard care (6-monthly PI and routine OHA). In terms of point estimates of total mean NHS costs, the policy of no PI and personalised OHA was associated with the lowest NHS costs (£62.42), followed by the policy of no PI and routine OHA (£72.23).
In Scotland, the fee-for-service structure allowed an assessment solely of the costs of periodontal treatments. Relative to standard care, and given the structure of current contracts, a policy of no PI (with routine OHA) would significantly reduce NHS spending on periodontal treatments by £7.47 per person over 2.25 years. A policy of offering routine OHA with 12-monthly PI would save £5.11 per person on periodontal treatments over the same time frame. However, we cannot definitively conclude cost savings to the overall NHS dental budget. To make this statement would require the assumption that the lack of statistical significance in total NHS costs is due to a lack of statistical power and the relatively large SDs around high-cost treatments, rather than any causal effects of disinvesting from PI. Given the lack of evidence of any clinical differences across policies, it could be argued that such an assumption may be valid, and that cost savings could be achieved, at least in the Scottish system, without having an adverse impact on the primary clinical outcome (gingival inflammation/bleeding).
When taking a wider perspective (NHS and participant), the policy of personalised OHA and no PI was less costly than standard care. However, this difference was mainly driven by the lower costs of interdental brushes in England and it is questionable whether or not it is attributable to the policy. The total cost differences between all other policies and standard care were not statistically significant.
The results of the DCE show strong evidence that the general population valued both PI and personalised OHA even when controlling for frequency of bleeding gums and aesthetics (the look and feel of teeth). Owing to the relatively high value placed by the general population on PI and personalised OHA, the policy of 6-monthly PI and personalised OHA was associated with the highest incremental benefit compared with standard care (£61.67). The policy of 12-monthly PI and personalised OHA was also associated with a positive but lower incremental benefit (£19.70) but the difference was not statistically significant.
The results of the CBA showed that the policy of 6-monthly PI with personalised OHA was associated with the largest incremental net benefit compared with standard care [£48 when taking a NHS cost perspective and £68 when taking a wider perspective (NHS and participant)]. The most efficient approach depends on the perspective taken. From a welfare maximisation perspective, the analysis shows that personalised OHA with 6-monthly PI is cost-beneficial, with the greatest incremental net benefit relative to standard care. It should be noted that this finding was driven primarily by differences in benefits rather than NHS-incurred costs.
From a health maximisation perspective, the no-PI approach generates the lowest average costs (though differences are not statistically significant), without detrimentally affecting clinical outcomes. The overall health economic conclusions were robust to a range of sensitivity analyses undertaken to explore the impact of uncertainty in key assumptions on results.
Comparisons with other randomised clinical trials
The Cochrane systematic review24 of routine scale and polish (PI) for periodontal health in adults (updated in 2013) reported that there was insufficient evidence to determine the effects of routine PI treatment. Three studies25–27 were identified, one of which was conducted in a similar setting to the IQuaD trial (three NHS general dental practices in England, involving 369 participants). The trial inclusion criteria were less pragmatic than those in the IQuaD trial, with individuals with BPE scores of > 2, diabetes mellitus and those aged > 60 years excluded. Jones et al. 25 compared PI intervals of 6-monthly PI, 12-monthly PI or 24-monthly PI (no PI throughout the trial). There was no evidence of any clinical differences between these different PI intervals provided for gingival health (gingival inflammation/bleeding). The Cochrane review is currently being updated by two authors of the monograph and will include the results of the IQuaD trial. The updated search of 5 December 2016 identified the IQuaD trial as the only new eligible study to be included in the updated review. When synthesising the data of both trials, the treatment effect is mean difference 0.00 (95% CI –0.03 to 0.03; p = 0.87) in gingival health, as shown in Figure 17.
FIGURE 17.
Routine PI for periodontal health in adults. (a) Forest plot of comparison 1: PI vs. no PI (control), outcome 1.1 gingivitis at 24 months; and (b) forest plot of comparison 1: PI vs. no PI (control), outcome 1.2 calculus at 24 months. df, degrees of freedom; IV, inverse variance.
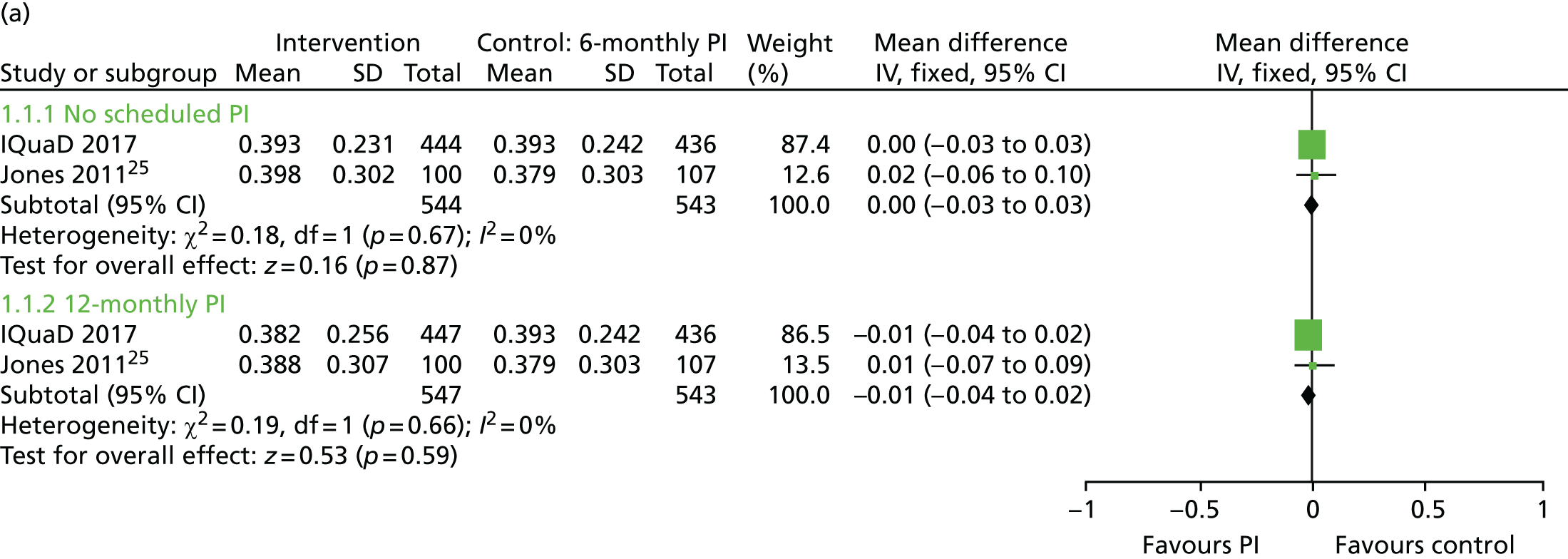
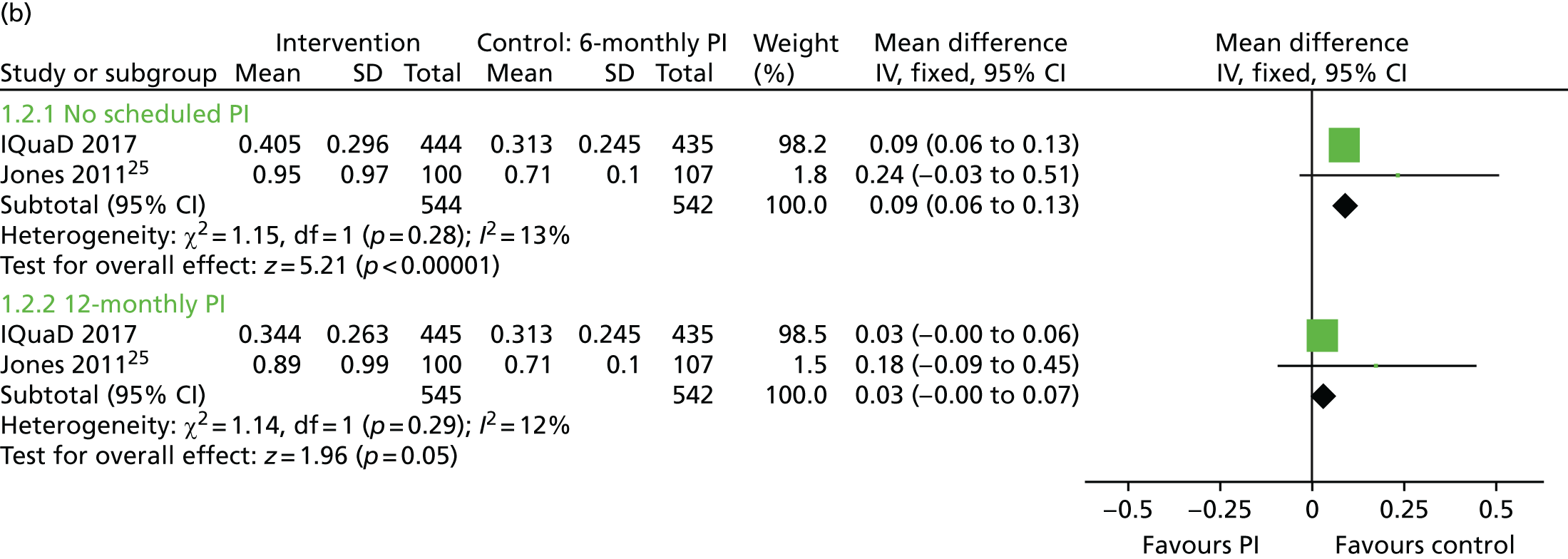
A recent systematic review23 of psychological approaches to behaviour change for improved plaque control in periodontal management reported benefits of using goal-setting, self-monitoring and planning for improving oral hygiene. A number of the included trials were of short duration, of a non-experimental design and were rated as having a high risk of bias. A meta-analysis was not possible and the results and recommendations should be interpreted with caution. An ongoing Cochrane systematic review of delivering one-to-one oral hygiene in a variety of dental settings has identified a number of trials investigating enhanced oral hygiene techniques (Soldani FA, Young L, Jones K, Walsh T, Clarkson JE. Bradford District Care NHS Foundation Trust. 2017). The intensity of the interventions varied greatly and for a number of trials the risk of bias is rated as unclear or high. We have presented a diagrammatic display of the studies’ effects to demonstrate the spread of effect size, direction and lack of CI overlap, highlighting the uncertainty of treatment clinical effectiveness (Figure 18).
FIGURE 18.
One-to-one OHA provided in a dental setting for oral health. Forest plot of comparison 1: personalised OHA vs. routine OHA, outcome 1.1 gingivitis. IV, inverse variance.
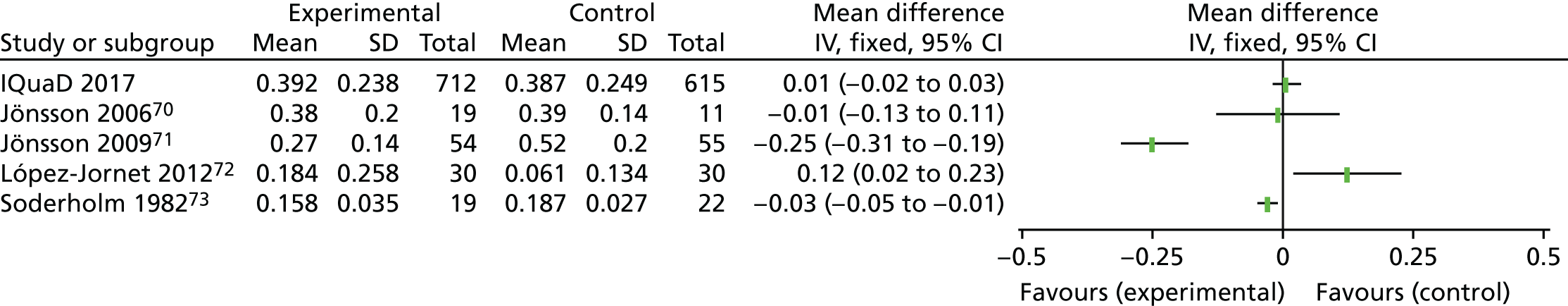
Strengths
The IQuaD trial was a pragmatic trial in primary care dental practice designed to provide evidence for the clinical effectiveness and cost-effectiveness of the most frequently provided treatment in dentistry, PI, and the impact of personalised OHA. Despite the length of time that has elapsed since the trial began, uncertainty still exists for providers of dental care worldwide regarding the benefit of these common components of routine dental care.
Surprisingly, there have been few studies comparing different frequencies of PI, and none that have combined it with different approaches to providing OHA in a setting such as the NHS. Reviews evaluating PI frequency have included studies that involve few providers and recruit only participants with no or very early signs of periodontal disease (BPE scores of 0–2). One review concluded that there was little value in providing PI without OHA; this was largely influenced by the results of a study with an intensive advice programme involving several 45-minute appointments, which does not reflect routine NHS practice. 74
The strengths of the IQuaD trial include the recruitment and retention of a large number of centres (n = 63), with wide representation of geographical, clinical and dental practice characteristics and operating in two different contractual systems in the UK. Recruitment and retention in trials is a frequent challenge, but an achievement for the IQuaD trial was the recruitment of 87% of the potentially eligible patients and the retention at 3 years of 1450 participants with 1327 (71%) providing clinical data. The randomised groups were balanced/similar at baseline and the reasons for loss to follow-up were related to the inability of practices to contact their patients; therefore, we were confident in the robustness of the results regardless of the missing data. The dental behaviour and clinical characteristics of the participants mean that the findings of the IQuaD trial are generalisable to regular dental attenders in the NHS and, therefore, similar third-party funding systems. The cohort study provided long-term data on NHS primary care patients with moderate to severe periodontal disease, which has previously been lacking.
The pragmatic design of the trial did not prevent fidelity to the interventions with evidence of separation in the frequency of PIs between groups and of the delivery of personalised OHA. Conducting the trial in a primary care NHS setting with a design that involved minimal requirements for the dental practices, blind outcome assessment and access to routine data are strengths. The interventions delivered were not independently observed; however, we believe that they were delivered as in current dental practice and the fidelity information supports this.
The health economic evaluation has a number of strengths. To our knowledge, this trial provides the only evidence worldwide regarding the efficient allocation of NHS resources to the delivery of PI and personalised OHA. Uniquely among clinical trials of dental care interventions, we implement a CBA, using a DCE to capture the value placed on both the processes and outcomes of care by the general population. The values of the general population are most relevant as they provide the funding for health care in a tax-based system such as the NHS. The approach is novel within dentistry and has a number of distinct advantages over traditional cost-effectiveness and cost–utility analyses as it incorporates the simultaneous valuation of multiple processes and outcomes which are relevant to dental care recipients.
In terms of the within-trial analysis, we use best practice methodology, incorporating the most advanced recommendations for analysis of cluster randomised trials together with the appropriate use of missing data models to minimise the potential for bias.
Limitations
As with all evaluations, it is important to acknowledge the limitations of the trial.
A weakness was not collecting detailed information about the reasons for additional PIs. A possible weakness is that gingival inflammation, measured as bleeding on probing, is a measure for which calibration is not possible. The difference in bleeding measures between baseline and follow-up probably reflects assessor’s measurement error, but they were equally distributed across practice and participant randomised allocations, avoiding bias between randomised groups. The ICC for the primary clinical measure, bleeding on probing, was higher (0.24) than originally planned for (0.10), and most likely the observed difference was influenced by the ‘assessor effect’. The trial was still powered to detect the clinically important difference due to statistical adjustment for other factors such as the baseline measures of bleeding and the minimisation variables.
The different contracts across Scotland and England provided several challenges to the health economic evaluation. First, the differences in costs across the policies were likely to vary across the regions as PI attracts a separate payment in Scotland while it is included in band 1 treatments in England (which also include clinical check-ups). Second, the different contracts may provide different incentives for providing care. Some evidence suggests that health-care providers have a tendency to supply more services under fee-for-service than under other reimbursement contracts, but, in the IQuaD trial, we found no evidence of this, as average costs were actually higher in England than in Scotland. One could also make an argument that different contracts may have an impact on the private–public mix of services, for example the referral rate for private PI. These issues potentially limit the direct comparability of Scottish and English cost data. However, this difference did not bias average UK results, as the ratio of English to Scottish practices was similar across the different arms of the trial. Furthermore, although subgroup analyses exploring regional-level resource use, cost and incremental net benefits show substantial variation across regions, the overall recommendations from the CBA remain unchanged.
Although the DCE was a useful method for obtaining WTP estimates for PI, personalised OHA and trial outcomes, it did not provide insights into why individuals value these attributes. Although source of value is not relevant from a CBA perspective, policy-makers may want some insights into this given the current climate of severe financial constraints. The results showed that the public value PI and personalised OHA even when controlling for self-reported bleeding and aesthetics. There are a number of potential explanations for this finding:
-
Respondents may have assumed that PI and personalised OHA were associated with clinical benefits other than bleeding and aesthetics.
-
PI and personalised OHA represent another interaction with a dentist or hygienist, which provides an opportunity to identify any dental health issues and to provide reassurance.
-
Respondents may simply value PI because that is what they always have (habitual behaviours) and/or their dentist has always recommended that they have a PI.
-
Respondents might value having the option of PI and personalised OHA in a dental care package (option value).
-
Respondents might want to minimise the regret that they may feel if their dental health gets worse and they did not have PIs or personalised OHA.
-
It is possible that the general population value the additional PI service received as part of attending for a band 1 treatment in England. It is possible that withdrawal of PI may have an impact on the attendance for routine check-ups; however, that consequence was not observed in the trial participants when all groups continued to attend for dental check-ups at a similar rate.
Further research is required to explore the reasons behind the positive value attached to PI.
The economic analysis is based on the price paid by the NHS for dental treatments under the current contracts and structures in place in England and Scotland. Thus, our analysis is the most accurate reflection of the current impact on NHS budgets of delivering alternative policies. However, there are limitations associated with this. First, the price may not be an accurate reflection of the cost of time and materials to the dentists of delivering the respective interventions. Owing to contract structures, it is likely that there are substantial differences between price and cost. Second, if the way in which dental practices are paid were to change substantially under new contracts, the results of our costing analyses would need to be considered in the light of such changes. Third, we have not explicitly estimated the opportunity costs of alternative policies (i.e. the benefits forgone from not having those resources available to expand or implement other policies). This is an important consideration, given a fixed NHS budget; however, the issue is rarely explicitly incorporated within economic evaluation frameworks. It should be noted that, for this study, incremental net benefit results were driven by differences in benefits rather than costs, so the notion of opportunity costs is less relevant in this scenario, as NHS costs were similar across policies.
The majority of economic evaluations of health-care interventions tend to extrapolate trial outcomes over a longer time horizon using decision-modelling studies. For this study, we have conducted a CBA alongside the RCT. We have not attempted to extrapolate trial results as there is a paucity of evidence to directly predict the probability of final clinical end points (such as tooth loss or long-term caries), or to determine when such events may happen. This is a limitation of our analysis. However, to attempt such a modelling exercise, given the paucity of available data, could generate misleading conclusions for policy-makers. Furthermore, given that there was no evidence of short-term clinical benefit, one would not expect any differences in long-term tooth loss or caries across different policy options in this population with relatively healthy dentitions. Compared with other areas of health economic evaluation, there is a lack of evidence generally to inform the population of economic decision models in dentistry and further research is required to bridge this gap.
Despite attempting to collect information on the wider NHS perspective, it was found that rates of secondary care consultations exceeded what might be expected in the general population. After a validation exercise, the data were excluded. Reasons for the poor validity may include a participant’s difficulty in determining what was meant by the questionnaire text asking to report ‘admissions for problems related to your teeth’ or issues of recall bias over a full year of recall, or the question may have been misinterpreted, with participants providing details of all contacts with secondary care, rather than dental care-specific attendances. Future studies should explore direct linkage to Scottish Morbidity Reports data in Scotland and Hospital Episode Statistics data in England to obtain more accurate estimates at a participant level. The impact of omitting these data on the results is likely to be minimal, given that resource use was evenly distributed across groups.
Patient and public involvement and engagement
Prior to the start of the IQuaD trial, patients were involved with the trial design and provided invaluable feedback on trial recruitment and communication strategies. Patients also contributed to the content and layout of the trial invitation, trial newsletters and the design of patient participant questionnaires. This ensured that trial participants could understand and easily complete these materials. Members of the public also contributed to trial oversight through membership of the TSC, including helping to interpret the trial findings and preparation of the monograph. We found that the most effective feedback was provided in face-to-face meetings with trial staff and patient and public involvement (PPI) personnel. This provided an opportunity for clarification and more comprehensive and constructive feedback. In preparation for oversight committee meetings and the final PMG meeting, the PPI representative found that the opportunity for a pre-meeting session to clarify issues made their participation of these meetings easier. The results were discussed with the Periodontal Advisory Group and Chief Dental Officers of the UK.
Generalisability
The IQuaD trial was designed pragmatically to investigate OHA and PI delivery to NHS patients in dental primary care that did not exhibit moderate or severe periodontitis.
The 63 recruiting dental practices were situated across Scotland and the north-east of England, with 72% of practices employing dental hygienists and 75% having three or more dentists working in the practice.
In the year to 31 March 2014, 84.4% of the population of Scotland was registered with a NHS dentist, 76.4% of whom had attended the dentist in the past 2 years. The ADHS 200911 reported that over half of the UK population had attended a dental practice within the last 3 years. The IQuaD trial recruited a total of 1877 participants at their routine dental examination, with 1348 (72%) of these participants recruited across Scotland, including 121 (6%) recruited from the Scottish islands. Over 90% of participants reported having attended a dental practice at least once over the previous 2 years.
At baseline, around 60% of participants reported having received OHA and a PI during their last dental appointment. The mean percentage of sites with gingival inflammation/bleeding was 33%, and 35% of teeth had calculus present. The mean clinical probing depth was 1.8 mm. Two-thirds of participants had BPE scores of ≤ 2, and between 10% and 12% of participants in each group had four or more pockets with a clinical probing depth of ≥ 4 mm.
We are confident that the practices and participants recruited to the IQuaD trials are a true representation of those adults who are periodontally healthy or have very early periodontal disease and attend NHS dental practices across Scotland and England.
Recommendations for research
-
Research is needed to assess the clinical effectiveness and cost-effectiveness of providing multifaceted periodontal care packages (e.g. OHA, oral care products, PI) in primary dental care for those with periodontitis.
-
Research is required to better understand the source of WTP values and the extent to which this is influenced by perceptions and current practice.
-
Further research is required to explore the relative value of different data sources for estimating resource use in dentistry including routine data, patient-reported data and practice records.
Acknowledgements
The authors wish to thank Mark Forrest and the programming team at CHaRT; Cynthia Fraser, our information specialist, for assistance with referencing; Moira Swan, who was the dental research nurse and part of the OA team in Newcastle upon Tyne; Louise Campbell for secretarial support and data management; our original statistician in the group, Andy Elders; senior IT manager Gladys Macpherson; senior trial administrator at the TCOD Marilyn Laird; Luke Vale for his involvement with the design of the health economic analysis at the inception of the trial; Maria Dimitrova, who assisted the health economists in the collection of unit costs; staff of the Scottish Primary Care Research Network, who assisted with screening eligible patients at dental practices; staff of the North East Commissioning Support Unit who assisted with research payments to dental practices in the north-east; members of the TMC and Periodontal Advisory Group for their ongoing advice and support of the trial; the independent members of the TSC and DMC; and the staff at recruitment sites who facilitated recruitment, treatment and follow-up of trial participants.
The Health Services Research Unit and the Health Economics Research Unit is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate.
Trial Management Committee
Paul Averley, Graham Chadwick, Andy Hall, Penny Hodge, Isobel Madden, Wendy McCombes, Nigel Pitts, David Ricketts, Margaret Ross, Martin Tickle and Helen Worthington.
Independent members of the Trial Steering Committee
Tina Halford-McGuff, Dione Macmorran, the late Eleanor Grey, James McCaul and Elizabeth Treasure (chairperson).
Independent members of the Data Monitoring Committee
Pollyanna Hardy, Peter Robinson and Damien Walmsley (chairperson).
Members of the Periodontal Advisory Committee
Mary Cullinan, Mark Ide, Ian Needleman, Tim Newton and Greg Seymour.
Recruitment sites
We would like to thank the staff and participants of the following dental practices:
Ardmillan Dental Practice, Baillieston Dental Care, Bank Street Dental Surgery (formally known as Peacock Dental Surgery), Birch Valley Dental Clinic, Bridge of Don Dental Practice, Bute Dental Surgery, Care Dental – Crieff, Chong Kwan Dental Centre, City Health Clinic, Clark and Watson Family Dental Practice, Clyde Dental Practice, David Gilchrist Dental Surgery, Dental Care Perth Ltd, Dental Plus, Discovery Dental Care, Drumbrae Dental Surgery, Dunbar Dental Practice, Duns Dental Practice, East Neuk Dental Practice, Framwellgate Dental Surgery, Consett, Grange Dental Centre (formally known as Gosforth Dental), Hetton Dental Practice, Invergowrie Dental Practice, JL Barrack Dental Practice, Kenton Lane Dental Practice, King Street Dental Practice (Orkney), Laurencekirk Dental Practice, L C Milton Dental Practice (Barrhead Dental practice), Lochboisdale Dental Clinic (South Uist), Long and Gilmour Dental Care, Lux Dental (formally known as K H Stirling), Mastrick Dental Centre, Montgomery Street Dental Care, Mr S B Pabary and Associates, Mullins Dental Practice, Mydentist – Dean Road, Mydentist – Front Street (formally known as Front Street Dental Practice), Nepali Dental Practice, Number One Dental Practice, Osborne Dental Practice, Park View Family Dental (formally known as Mr A I Robson and Associates Dental Practice), P A Penney Dental Practice, Perfect Smile Blaydon (formally known as Blaydon Dental Practice), Pickering Dental Care Ltd, Princes Street Dental Practice, Riverview Dental Practice, S. Rankin, Selkirk Dental Practice, Shotley Bridge Dental Care, Silver Dental Practice, Smith and Smith Dental Practice, St Leonards Dental Practice, Stirling Dental Care, Stobswell Dental Practice (formally known as Karolak and Iwanowicz), Stuart Eaborn Dental Practice, Sunderland Road Dental Practice, The Hollies Dental Practice, West End Dental Practice, Whickham Village Dental Practice, Whitburn Cosmetic Dental Clinic (formally known as Whitburn Dental Practice), Wishaw Cross Dental Care and Woodside Dental Practice.
Contributions of authors
Craig R Ramsay (Professor and Triallist) contributed to the conception and design of the trial, the conduct of the trial, the statistical analysis, the interpretation of the results and the writing/editing of the report.
Jan E Clarkson (Professor and Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Anne Duncan (Trials Manager) was responsible for the day-to-day management of the trial, contributed to the interpretation of data and made significant contributions to drafting the monograph.
Thomas J Lamont (Clinical Research Fellow) was responsible for the conduct of the trial in Dundee, contributed to recruitment of participants and clinical data collection, contributed to the interpretation of data and made significant contributions to drafting the monograph.
Peter A Heasman (Professor and Triallist) contributed to the design of the trial, was responsible for the conduct of the trial in Newcastle upon Tyne, contributed to recruitment of dentists, contributed to the interpretation of data and made significant contributions to writing and editing the report.
Dwayne Boyers (Research Fellow) undertook the trial-based health economics analysis and drafted the chapter on health economic methods, Chapter 3, and results of the economic analysis, Chapter 5, under the supervision of Marjon van der Pol.
Beatriz Goulão (Research Fellow and Statistician) conducted the statistical analyses, contributed to drafting the statistical methods section in Chapter 2 and drafted the clinical effectiveness results in Chapter 4 under the supervision of Craig R Ramsay.
Debbie Bonetti (Health Psychologist) led on the conception and design of the patient-reported self-efficacy questionnaire, the CBQ and the development of the personalised OHA intervention, contributed to analysis and interpretation of behavioural data and CBQ, and contributed to reviewing and approving the final report.
Rebecca Bruce (Trials Data Co-ordinator) was responsible for the day-to-day data management of the trial, contributed to interpreting the data and reviewing and approving the final report.
Jill Gouick (Research Dental Nurse and OA) was responsible for recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
Lynne Heasman (Research Hygienist and OA) was responsible for recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
Laura A Lovelock-Hempleman (Research Hygienist and OA) was responsible for recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
Lorna E Macpherson (Trials Administrator) was responsible for the day-to-day management of the trial, and contributed to the interpretation of data and to drafting and approving the monograph.
Giles I McCracken (Clinical Senior Lecturer and Triallist) contributed to the design of the trial, was responsible for the conduct of the trial in Newcastle upon Tyne, contributed to recruitment of dentists, contributed to the interpretation of data and reviewed and approved the final report.
Alison M McDonald (Senior Trials Manager) was responsible for overseeing management of the trial, and contributed to the interpretation of data and to reviewing and approving the monograph.
Fiona McLaren-Neil (Trials Administrator) was responsible for overseeing management of the trial, and contributed to the interpretation of data and to reviewing and approving the monograph.
Fiona E Mitchell (Research Dental Nurse and OA) was responsible for recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
John DT Norrie (Professor and Triallist) contributed to the design of the trial, the interpretation of the data and reviewing and approving the monograph.
Marjon van der Pol (Professor and Health Economist) contributed to the design of the trial, the interpretation of the data and contributed to the writing and editing of the final report.
Kirsty Sim (Research Hygienist and OA) was responsible for the recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
James G Steele (Professor and Triallist) contributed to the design of the trial, the interpretation of the data and reviewing and approving the monograph.
Alex Sharp (Research Dental Nurse and OA) was responsible for the recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
Graeme Watt (Research Hygienist and OA) was responsible for the recruitment of participants and collection of clinical data outcomes, and contributed to interpreting the data and reviewing and approving the final report.
Helen V Worthington (Professor and Triallist) contributed to the design of the trial, the interpretation of the data and reviewing and approving the monograph.
Linda Young (Research Manager and Economist) contributed to the design of the trial, the interpretation of the data and reviewing and approving the monograph.
Publications
Heasman PA, Macpherson LE, Haining SA, Breckons M. Clinical research in primary dental care. BDJ 2015;219:159–163.
Data sharing statement
All available data can be obtained by contacting the corresponding author. See the HTA programme website for further project information.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Clarkson JE, Ramsay CR, Averley P, Bonetti D, Boyers D, Campbell L, et al. IQuaD dental trial; improving the quality of dentistry: a multicentre randomised controlled trial comparing oral hygiene advice and periodontal instrumentation for the prevention and management of periodontal disease in dentate adults attending dental primary care. BMC Oral Health 2013;13. https://doi.org/10.1186/1472-6831-13-58.
- Papapanou PN. Epidemiology of periodontal diseases: an update. J Int Acad Periodontol 1999;1:110-16.
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000 2012;60:15-39. https://doi.org/10.1111/j.1600-0757.2011.00425.x.
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 2014;93:1045-53. https://doi.org/10.1177/0022034514552491.
- Neely AL, Holford TR, Löe H, Anerud A, Boysen H. The natural history of periodontal disease in man. Risk factors for progression of attachment loss in individuals receiving no oral health care. J Periodontol 2001;72:1006-15. https://doi.org/10.1902/jop.2001.72.8.1006.
- Kinane DF, Attström R. European Workshop in Periodontology group B . Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J Clin Periodontol 2005;32:130-1. https://doi.org/10.1111/j.1600-051X.2005.00823.x.
- Chapple IL, Genco R. working group 2 of the joint EFP/AAP workshop . Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;40:S106-S12. https://doi.org/10.1111/jcpe.12077.
- Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol 2013;40:S8-S19. https://doi.org/10.1111/jcpe.12064.
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol 2013;40:S51-S69. https://doi.org/10.1111/jcpe.12060.
- Tonetti MS, Van Dyke TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol 2013;40:S24-S9. https://doi.org/10.1111/jcpe.12089.
- Steele J, O’Sullivan I. Executive Summary: Adult Dental Health Survey 2009 2011. http://content.digital.nhs.uk/catalogue/PUB01086/adul-dent-heal-surv-summ-them-exec-2009-rep2.pdf (accessed 1 February 2017).
- Chapple IL, Van der Weijden F, Doerfer C, Herrera D, Shapira L, Polak D, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol 2015;42:S71-6. https://doi.org/10.1111/jcpe.12366.
- Tonetti MS, Chapple IL, Jepsen S, Sanz M. Primary and secondary prevention of periodontal and peri-implant diseases: introduction to, and objectives of the 11th European Workshop on Periodontology consensus conference. J Clin Periodontol 2015;42:S1-S4. https://doi.org/10.1111/jcpe.12382.
- Tonetti MS, Eickholz P, Loos BG, Papapanou P, van der Velden U, Armitage G, et al. Principles in prevention of periodontal diseases: consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol 2015;42:S5-11. https://doi.org/10.1111/jcpe.12368.
- Key Note . Market Update – Toiletries 2014. www.keynote.co.uk/market-update/retail/toiletries (accessed 1 February 2017).
- The Good Practitioner’s Guide to Periodontology. Selby: BSP; 2016.
- Primary Care in Dentistry in Scotland, Annual Report 2014/15. Edinburgh: NHS National Services Scotland; 2016.
- NHS . NHS Dental Statistics for England 2015–16, Annual Report 2016. www.content.digital.nhs.uk/catalogue/PUB21701 (accessed 1 February 2017).
- Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 2004;31:749-57. https://doi.org/10.1111/j.1600-051X.2004.00563.x.
- Kay E, Locker D. A systematic review of the effectiveness of health promotion aimed at improving oral health. Community Dent Health 1998;15:132-44.
- Renz A, Ide M, Newton T, Robinson PG, Smith D. Psychological interventions to improve adherence to oral hygiene instructions in adults with periodontal diseases. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD005097.pub2.
- Sprod AJ, Anderson R, Treasure ET. Effective Oral Health Promotion: Literature Review. Technical Report 20. Cardiff: Health Promotion Wales; 1996.
- Newton JT, Asimakopoulou K. Managing oral hygiene as a risk factor for periodontal disease: a systematic review of psychological approaches to behaviour change for improved plaque control in periodontal management. J Clin Periodontol 2015;42:S36-S46. https://doi.org/10.1111/jcpe.12356.
- Worthington HV, Clarkson JE, Bryan G, Beirne PV. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD004625.pub4.
- Jones CL, Milsom KM, Ratcliffe P, Wyllie A, Macfarlane TV, Tickle M. Clinical outcomes of single-visit oral prophylaxis: a practice-based randomised controlled trial. BMC Oral Health 2011;11. https://doi.org/10.1186/1472-6831-11-35.
- Lightner LM, O’Leary JT, Drake RB, Crump PP, Allen MF. Preventive periodontic treatment procedures: results over 46 months. J Periodontol 1971;42:555-61. https://doi.org/10.1902/jop.1971.42.9.555.
- Listgarten MA, Schifter CC, Laster L. 3-year longitudinal study of the periodontal status of an adult population with gingivitis. J Clin Periodontol 1985;12:225-38. https://doi.org/10.1111/j.1600-051X.1985.tb00920.x.
- Gaunt F, Devine M, Pennington M, Vernazza C, Gwynnett E, Steen N, et al. The cost-effectiveness of supportive periodontal care for patients with chronic periodontitis. J Clin Periodontol 2008;35:67-82. https://doi.org/10.1111/j.1600–051X.2008.01261.x.
- Hefti AF, Preshaw PM. Examiner alignment and assessment in clinical periodontal research. Periodontol 2000 2012;59:41-60. https://doi.org/10.1111/j.1600-0757.2011.00436.x.
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health 1998;13:623-49. https://doi.org/10.1080/08870449808407422.
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. Am Psychol 1999;54:493-50. https://doi.org/10.1037/0003-066X.54.7.493.
- Clarkson JE, Young L, Ramsay CR, Bonner BC, Bonetti D. How to influence patient oral hygiene behavior effectively. J Dent Res 2009;88:933-7. https://doi.org/10.1177/0022034509345627.
- Chapple IL, Hill K. Getting the message across to periodontitis patients: the role of personalised feedback. Int Dent J 2008;58:294-306. https://doi.org/10.1111/j.1875-595X.2008.tb00207.x.
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 1967;38:610-16. https://doi.org/10.1902/jop.1967.38.6.610.
- Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol 1997;25:284-90. https://doi.org/10.1111/j.1600-0528.1997.tb00941.x.
- Clarkson JE, Turner S, Grimshaw JM, Ramsay CR, Johnston M, Scott A, et al. Changing clinicians’ behavior: a randomized controlled trial of fees and education. J Dent Res 2008;87:640-4. https://doi.org/10.1177/154405910808700701.
- NHS Payments to General Practice, England, 2014–15. Leeds: NHS Digital; 2015.
- A Guide to NHS Dental Publications. Leeds: NHS Digital; 2015.
- The Scottish Government . Statement of Dental Remuneration – Amendment No 130 Letter 2015. www.scottishdental.org/library/statement-of-dental-remuneration-amendment-no-130-letter/ (accessed 1 February 2017).
- Steele J. NHS Dental Services in England: An Independent Review 2009. www.sigwales.org/wp-content/uploads/dh_101180.pdf (accessed 1 February 2017).
- Lord J, Longworth L, Singh J, Onyimadu O, Fricke J, Bayliss S, et al. Oral Health Guidance – Economic Analysis of Oral Health Promotion Approaches for Dental Teams. Birmingham & Brunel Consortium External Assessment Centre; 2015.
- Guide to the Methods of Technology Appraisal. PMG9. London: NICE; 2013.
- NHS Reference Costs 2014 to 2015. London: Department of Health and Social Care; 2015.
- Costs Book Tables 2015. Edinburgh: ISD Scotland; 2015.
- Annual Survey of Hours and Earnings: 2015 Provisional Results. London: ONS; 2015.
- Boox . The Boox Report: Analysis of the UK’s Self-Employed Workforce 2014. www.boox.co.uk/wp-content/uploads/2014/01/The-Boox-Report-2014-Download.pdf (accessed 1 February 2017).
- Cost Converter. London: EPPI Centre, Social Science Research Unit, University College London; 2016.
- Self-employed Workers in the UK – 2014. London: ONS; 2014.
- Expenses and Benefits: Business Travel Mileage for Employees’ Own Vehicles. London: HMRC; 2016.
- NHS Pay Review Body. Review for 2012. London: Department of Health and Social Care; 2012.
- Transport Analysis Guidance (TAG). WebTAG: TAG Data Book, July 2016 2016. www.gov.uk/government/publications/webtag-tag-data-book-july-2016 (accessed 1 February 2017).
- Gomes M, Ng ES, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomised trials. Med Decis Making 2012;32:350-61. https://doi.org/10.1177/0272989X11418372.
- Gomes M, Grieve R, Nixon R, Edmunds WJ. Statistical methods for cost-effectiveness analyses that use data from cluster randomized trials: a systematic review and checklist for critical appraisal. Med Decis Making 2012;32:209-20. https://doi.org/10.1177/0272989X11407341.
- Gomes M, Grieve R, Nixon R, Ng ES, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ 2012;21:1101-18. https://doi.org/10.1002/hec.2812.
- Gomes M, Diaz-Ordaz K, Grieve R, Kenward MG. Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies: an application to cluster randomized trials. Med Decis Making 2013;33:1051-63. https://doi.org/10.1177/0272989X13492203.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004.
- Johnson FR, Lancsar E, Marshall D, Kilambi V, Muhlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health 2013;16:3-13. https://doi.org/10.1016/j.jval.2012.08.2223.
- Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403-13. https://doi.org/10.1016/j.jval.2010.11.013.
- McFadden D, Zarembka P. Frontiers in Econometrics. New York, NY: Academic Press; 1974.
- McIntosh E. Using discrete choice experiments within a cost-benefit analysis framework: some considerations. PharmacoEconomics 2006;24:855-68. https://doi.org/10.2165/00019053-200624090-00004.
- Hospital Outpatient Activity - 2014-15. London: NHS Digital; 2015.
- Hospital Episode Statistics, Admitted Patient Care - England, 2014–15. London: NHS Digital; 2015.
- Office for National Statistics, National Records of Scotland, Northern Ireland Statistics & Research Agency . 2011 Census Aggregate Data 2016. https://discover.ukdataservice.ac.uk/doi/2011-census-aggregate (accessed 1 February 2017).
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. https://doi.org/10.1007/s40273-014-0170-x.
- Dental Checks: Intervals Between Oral Health Reviews. Clinical Guideline. London: NICE; 2014.
- Tilley CJ, Chalkley MJ. Measuring access to health services: General Dental Services in Scotland. Br Dent J 2005;199:599-601. https://doi.org/10.1038/sj.bdj.4812905.
- Basic Periodontal Examination (BPE). Selby: BSP; 2016.
- Prevention and Treatment of Periodontal Diseases in Primary Care: Dental Clinical Guidance. Dundee: SDCEP; 2014.
- Jönsson B, Lindberg P, Oscarson N, Ohrn K. Improved compliance and self-care in patients with periodontitis–a randomized control trial. Int J Dent Hyg 2006;4:77-83. https://doi.org/10.1111/j.1601-5037.2006.00175.x.
- Jönsson B, Ohrn K, Oscarson N, Lindberg P. The effectiveness of an individually tailored oral health educational programme on oral hygiene behaviour in patients with periodontal disease: a blinded randomized-controlled clinical trial (one-year follow-up). J Clin Periodontol 2009;36:1025-34. https://doi.org/10.1111/j.1600-051X.2009.01453.x.
- López-Jornet P, Fabio CA, Consuelo RA, Paz AM. Effectiveness of a motivational–behavioural skills protocol for oral hygiene among patients with hyposalivation. Gerodontology 2012;31:288-95. https://doi.org/10.1111/ger.12037.
- Soderholm G, Nobreus N, Attstrom R, Egelberg J. Teaching plaque control. I. A five-visit versus a two-visit program. J Clin Periodontol 1982;9:203-13. https://doi.org/10.1111/j.1600-051X.1982.tb02060.x.
- Needleman I, Nibali L, Di Iorio A. Professional mechanical plaque removal for prevention of periodontal diseases in adults – systematic review update. J Clin Periodontol 2015;42:S12-35. https://doi.org/10.1111/jcpe.12341.
- NHS Business Services Authority . FP17 Processing and Payments n.d. www.nhsbsa.nhs.uk/fp17-processing-and-payments (accessed 1 February 2017).
- Dyer TA, Owens J, Robinson PG. What matters to patients when their care is delegated to dental therapists?. Br Dent J 2013;214. https://doi.org/10.1038/sj.bdj.2013.275.
- Sonneveld RE, Brands WG, Bronkhorst EM, Welie JV, Truin GJ. Patients’ priorities in assessing organisational aspects of a general dental practice. Int Dent J 2013;63:30-8. https://doi.org/10.1111/idj.12001.
- Sonneveld RE, Wensing M, Bronkhorst EM, Truin GJ, Brands WG. The estimation of patients’ views on organisational aspects of a general dental practice by general dental practitioners: a survey study. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-263.
- Gussy M, Dickson-Swift V, Adams J. A scoping review of qualitative research in peer-reviewed dental publications. Int J Dent Hyg 2013;11:174-9. https://doi.org/10.1111/idh.12008.
- Guzeldemir E, Toygar HU, Cilasun U. Pain perception and anxiety during scaling in periodontally healthy subjects. J Periodontol 2008;79:2247-55. https://doi.org/10.1902/jop.2008.080152.
- Stenman J, Hallberg U, Wennstrom JL, Abrahamsson KH. Patients’ attitudes towards oral health and experiences of periodontal treatment: a qualitative interview study. Oral Health Prev Dent 2009;7:393-401.
- Mussard J, Ashley FA, Newton JT, Kendall N, Crayford TJ. What do you think of your dentist? A dental practice assessment questionnaire. J Eval Clin Pract 2008;14:181-4. https://doi.org/10.1111/j.1365-2753.2006.00826.x.
- Ohrn K, Hakeberg M, Abrahamsson KH. Dental beliefs, patients’ specific attitudes towards dentists and dental hygienists: a comparative study. Int J Dent Hyg 2008;6:205-13. https://doi.org/10.1111/j.1601-5037.2008.00300.x.
Appendix 1 Clinical effectiveness outcomes
Section 1: methods for computing patient-reported outcomes
Table 33 describes the calculations used to produce the outcomes in the CBQ at baseline.
| Outcome | Questionnaire item(s) | Scoring |
|---|---|---|
| Self-efficacy | Q1 (a) to (g) | Mean of all Q1 |
| Attitude | Q2 (a) to (d) | Mean of: 2(a) + 2(b) + 2(c) + 2(d) + 4(a) + 4(b) + 4(c)R* + 3(a) + 3(b) + 3(c) + 3(d) + 5(a) + 5(b) + 5(c)R* |
| Q3 (a) to (d) | ||
| Q4 (a) to (c) | ||
| Q5 (a) to (c) | ||
| PBC | Q4 (d), Q5 (d) | Mean of: 4(d) + 9(a) + 9(b) + 9(c) + 9(d) + 9(e) + 9(f) + 9 (g) + 5(d) |
| Q9 (a) to (f) | ||
| Intention | Q7 (a) to (c) | Mean of: 7(a) + 7(b) |
| Subjective norm | Q11 (a) to (c) |
Mean of: subjective norm advice + subjective norm treat Subjective norm advice = mean of 11(a) × 13(a) + 11(b) × 13(b) + 11(c) × 13(c) Subjective norm treat = mean of 12(a) × 13(a) + 12(b) × 13(b) + 12(c) × 13(c) |
| Q12 (a) to (c) | ||
| Q13 (a) to (c) | ||
| Has a plan to give OHA | Q8 | 8(a) |
| Has a plan to give PI | 8(b) |
Table 34 describes the calculations used to produce the outcomes in the CBQ at follow-up.
| Outcome | Questionnaire item(s) | Scoring |
|---|---|---|
| Self-efficacy | Follow-up CBQ | Mean of all Q1 items |
| Q1 (a) to (g) | ||
| Attitude | Follow-up CBQ | Mean of: 2(a) + 2(b) + 2(c) + 2(d) + 4(a) + 4(b) + 4(c)R* + 3(a) + 3(b) + 3(c) + 3(d) + 5(a) + 5(b) + 5(c)R* |
| Q2 (a) to (d) | ||
| Q3 (a) to (d) | ||
| Q4 (a) to (c) | ||
| Q5 (a) to (c) | ||
| PBC | Follow-up CBQ | Mean of: 4(d) + 9(a) + 9(b) + 9(c) + 9(d) + 9(e) + 9(f) + 9(g) + 5(d) + 10(a) + 10(b) + 10(c) + 10(d) + 10(e) + 10(f) + 10(g) |
| Q4 (d) | ||
| Q5 (d) | ||
| Q6 (d) | ||
| Q9 (a) to (g) | ||
| Q10 (a) to (g) | ||
| Intention | Follow-up CBQ | Mean of: 7(a) + 7(b) |
| Q7 (a) to (b) | ||
| Subjective norm | Follow-up CBQ |
Mean of: subjective norm advice + subjective norm treat Subjective norm advice = mean of 11(a) × 14(a) + 11(b) × 14(b) + 11(c) × 14(c) Subjective norm treat = mean of 12(a) × 14(a) + 12(b) × 14(b) + 12(c) × 14(c) |
| Q11 (a) to (c) | ||
| Q12 (a) to (c) | ||
| Q14 (a) to (c) | ||
| Has a plan to give OHA |
Follow-up CBQ Q8 |
8(a) |
| Has a plan to give PI | 8(b) |
Table 35 describes the calculations used to produce the patient-reported outcomes. All outcomes were calculated for each year of the patient annual questionnaire (baseline and years 1, 2 and 3).
| Outcome | Questionnaire item(s) | Scoring |
|---|---|---|
| Self-efficacy | Section 3 | Mean of all Q1 items |
| Q1 (a) to (g) | ||
| PBC | Section 3 | Mean of: 2(a)R* + 2(b) + 2(c) + 2(d) + 2(e) + 3(a) + 3(b) + 3(c) |
| Q2 (a) to (e) | ||
| Q5 (a) to (c) | ||
| Subjective norm | Section 3 | Mean of: 4(a)R* + 4(b) + 4(f) |
| Q4 (a), (b) and (f) | ||
| Attitude | Section 3 | Mean of: 3(a)R* + 3(b) + 3(c)R* + 3(d)R* + 3(e)R* + 3(f)R* + 3(g)R* + 5(d)R* + 5(e)R* |
| Q3 (a) to (f) | ||
| Q5 (d) and (e) | ||
| Behaviour | Section 2 | Q1 + Q2 + Q8 or Q12 (the highest of the two) |
| Q1, Q2, Q8 and Q12 | ||
| Intention | Section 2 | Q4 + Q5 + Q10 |
| Q4, Q5 and Q10 | ||
| OHIP | Section 4 | According to instrument instructions |
| Q1–14 |
Section 2: participant dental characteristics (years 1 and 2)
Section 2 shows the participant dental characteristics at follow-up at year 1 (Table 36) and at year 2 (Table 37).
| Participant dental characteristics | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 860) | |
| Date of last visit to the dental practice (years ago) | |||||
| < 1 | 449 (72) | 445 (71) | 458 (73) | 715 (71) | 637 (74) |
| 1–2 | 23 (4) | 23 (4) | 4 (1) | 31 (3) | 19 (2) |
| > 2 | – | 1 (0) | 1 (0) | – | 2 (0) |
| Missing | 151 (24) | 156 (25) | 163 (26) | 262 (26) | 208 (24) |
| Do you think of yourself as | |||||
| A regular attendee | 452 (73) | 446 (71) | 457 (73) | 718 (71) | 637 (74) |
| Someone who sees a dentist when in pain or trouble | 20 (3) | 22 (4) | 6 (1) | 28 (3) | 20 (2) |
| Missing | 151 (24) | 157 (25) | 163 (26) | 262 (26) | 209 (24) |
| In the last 12 months, did you receive OHA? | |||||
| Yes | 343 (55) | 378 (60) | 406 (65) | 635 (63) | 492 (57) |
| No | 124 (20) | 88 (14) | 51 (8) | 103 (10) | 160 (18) |
| Missing | 156 (25) | 159 (25) | 169 (27) | 270 (27) | 214 (25) |
| By whom? | |||||
| Dentist | 241 (39) | 233 (37) | 209 (33) | 395 (39) | 288 (33) |
| Hygienist | 61 (10) | 89 (14) | 110 (18) | 132 (13) | 128 (15) |
| Both | 43 (7) | 59 (9) | 89 (14) | 116 (12) | 75 (9) |
| Missing | 278 (45) | 244 (39) | 218 (35) | 365 (36) | 375 (43) |
| In the last 12 months, did you receive a scale and polish? | |||||
| Yes | 269 (43) | 353 (56) | 434 (69) | 575 (57) | 481 (56) |
| No | 201 (32) | 115 (18) | 27 (4) | 172 (17) | 171 (20) |
| Missing | 153 (25) | 157 (25) | 165 (26) | 261 (26) | 214 (25) |
| By whom? | |||||
| Dentist | 180 (29) | 219 (35) | 253 (40) | 354 (35) | 298 (34) |
| Hygienist | 79 (13) | 120 (19) | 154 (25) | 189 (19) | 164 (19) |
| Both | 6 (1) | 12 (2) | 22 (4) | 24 (2) | 16 (2) |
| Missing | 358 (57) | 274 (44) | 197 (31) | 441 (44) | 388 (45) |
| Smoked in the last 12 months? | |||||
| Yes | 66 (11) | 77 (12) | 70 (11) | 109 (11) | 104 (12) |
| No | 407 (65) | 394 (63) | 392 (63) | 642 (64) | 551 (64) |
| Missing | 150 (24) | 154 (25) | 164 (26) | 257 (25) | 211 (24) |
| What type of toothbrush do you normally use? | |||||
| Manual | 283 (45) | 280 (45) | 266 (42) | 443 (44) | 386 (45) |
| Electric | 168 (27) | 166 (27) | 167 (27) | 271 (27) | 230 (27) |
| Do not use brush | 21 (3) | 26 (4) | 28 (4) | 38 (4) | 37 (4) |
| Missing | 151 (24) | 153 (24) | 165 (26) | 256 (25) | 213 (25) |
| How often do you prefer to have a scale and polish? | |||||
| Never | 17 (3) | 14 (2) | 7 (1) | 22 (2) | 16 (2) |
| Once every 2 years | 19 (3) | 15 (2) | 7 (1) | 23 (2) | 18 (2) |
| Once a year | 109 (17) | 127 (20) | 70 (11) | 149 (15) | 157 (18) |
| Twice a year | 233 (37) | 213 (34) | 266 (42) | 398 (39) | 314 (36) |
| Three times a year | 39 (6) | 55 (9) | 46 (7) | 74 (7) | 66 (8) |
| Four times a year | 42 (7) | 37 (6) | 50 (8) | 68 (7) | 61 (7) |
| More often | 10 (2) | 8 (1) | 11 (2) | 13 (1) | 16 (2) |
| Missing | 154 (25) | 156 (25) | 169 (27) | 261 (26) | 218 (25) |
| Participant dental characteristics | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 860) | |
| Date of last visit to the dental practice (years ago) | |||||
| < 1 | 400 (64) | 408 (65) | 400 (64) | 645 (64) | 563 (65) |
| 1–2 | 22 (4) | 20 (3) | 3 (0) | 23 (2) | 22 (3) |
| > 2 | 5 (1) | – | 2 (0) | 4 (0) | 3 (0) |
| Missing | 196 (31) | 197 (32) | 221 (35) | 336 (33) | 278 (32) |
| Do you think of yourself as | |||||
| A regular attendee | 411 (66) | 403 (64) | 399 (64) | 642 (64) | 571 (66) |
| Someone who sees a dentist when in pain or trouble | 17 (3) | 25 (4) | 5 (1) | 28 (3) | 19 (2) |
| Missing | 195 (31) | 197 (32) | 222 (35) | 338 (34) | 276 (32) |
| In the last 12 months, did you receive OHA? | |||||
| Yes | 302 (48) | 346 (55) | 343 (55) | 550 (55) | 441 (51) |
| No | 123 (20) | 79 (13) | 64 (10) | 121 (12) | 145 (17) |
| Missing | 198 (32) | 200 (32) | 219 (35) | 337 (33) | 280 (32) |
| By whom? | |||||
| Dentist | 226 (36) | 221 (35) | 189 (30) | 368 (37) | 268 (31) |
| Hygienist | 37 (6) | 73 (12) | 90 (14) | 92 (9) | 108 (12) |
| Both | 32 (5) | 48 (8) | 61 (10) | 87 (9) | 54 (6) |
| Missing | 328 (53) | 283 (45) | 286 (46) | 461 (46) | 436 (50) |
| In the last 12 months, did you receive a scale and polish? | |||||
| Yes | 219 (35) | 342 (55) | 370 (59) | 494 (49) | 437 (50) |
| No | 204 (33) | 83 (13) | 37 (6) | 172 (17) | 152 (18) |
| Missing | 200 (32) | 200 (32) | 219 (35) | 342 (34) | 277 (32) |
| By whom? | |||||
| Dentist | 153 (25) | 213 (34) | 216 (35) | 317 (31) | 265 (31) |
| Hygienist | 51 (8) | 119 (19) | 139 (22) | 155 (15) | 154 (18) |
| Both | 6 (1) | 8 (1) | 12 (2) | 16 (2) | 10 (1) |
| Missing | 413 (66) | 285 (46) | 259 (41) | 520 (52) | 437 (50) |
| Smoked in the last 12 months? | |||||
| Yes | 56 (9) | 47 (8) | 50 (8) | 78 (8) | 75 (9) |
| No | 370 (59) | 380 (61) | 355 (57) | 592 (59) | 513 (59) |
| Missing | 197 (32) | 198 (32) | 221 (35) | 338 (34) | 278 (32) |
| What type of toothbrush do you normally use? | |||||
| Manual | 255 (41) | 233 (37) | 218 (35) | 369 (37) | 337 (39) |
| Electric | 148 (24) | 165 (26) | 166 (27) | 265 (26) | 214 (25) |
| Do not use brush | 24 (4) | 29 (5) | 22 (4) | 36 (4) | 39 (5) |
| Missing | 196 (31) | 198 (32) | 220 (35) | 338 (34) | 276 (32) |
| How often do you prefer to have a scale and polish? | |||||
| Never | 14 (2) | 12 (2) | 10 (2) | 20 (2) | 16 (2) |
| Once every 2 years | 24 (4) | 7 (1) | 7 (1) | 22 (2) | 16 (2) |
| Once a year | 98 (16) | 120 (19) | 59 (9) | 137 (14) | 140 (16) |
| Twice a year | 210 (34) | 204 (33) | 225 (36) | 351 (35) | 288 (33) |
| Three times a year | 30 (5) | 40 (6) | 48 (8) | 68 (7) | 50 (6) |
| Four times a year | 39 (6) | 34 (5) | 38 (6) | 50 (5) | 61 (7) |
| More often | 6 (1) | 5 (1) | 13 (2) | 14 (1) | 10 (1) |
| Missing | 202 (32) | 203 (32) | 226 (36) | 346 (34) | 285 (33) |
Section 3: clinical and patient-reported outcomes by year of follow-up
Section 3 shows descriptive data for the clinical (Tables 38 and 39) and patient-reported (Tables 40 and 41) outcomes.
| Attended 3-year examination | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| 444 (71) | 447 (72) | 436 (70) | 712 (71) | 615 (71) | |
| Clinical outcomes | Time point | Randomised group, mean (SD), n | ||||
|---|---|---|---|---|---|---|
| PI | OHA | |||||
| No | 12-monthly | 6-monthly | Personalised | Routine | ||
| Present teeth | Baseline | 23.4 (4.7), 444 | 23.5 (4.5), 447 | 23.8 (4.4), 436 | 23.4 (4.7), 712 | 23.7 (4.3), 615 |
| Year 3 | 23.6 (4.8), 444 | 23.6 (4.6), 447 | 23.9 (4.5), 436 | 23.5 (4.8), 712 | 23.8 (4.3), 615 | |
| % of sites bleeding | Baseline | 32.8 (22.8), 444 | 31.5 (23.7), 447 | 32.8 (22.9), 436 | 33.7 (23.1), 712 | 30.8 (23.1), 615 |
| Year 3 | 39.3 (23.1), 444 | 38.2 (25.6), 447 | 39.3 (24.2), 436 | 39.2 (23.8), 712 | 38.7 (24.9), 615 | |
| % of teeth with calculus present | Baseline | 35.0 (26.0), 444 | 35.9 (27.4), 447 | 33.3 (25.3), 436 | 33.3 (25.1), 712 | 36.4 (27.4), 615 |
| Year 3 | 40.5 (29.6), 444 | 34.4 (26.3), 445 | 31.3 (24.5), 435 | 35.7 (27.1), 711 | 35.1 (27.2), 613 | |
| Mean clinical pocket depth (mm) | Baseline | 1.8 (0.3), 444 | 1.8 (0.3), 447 | 1.8 (0.3), 436 | 1.8 (0.3), 712 | 1.8 (0.3), 615 |
| Year 3 | 1.9 (0.3), 444 | 1.9 (0.3), 446 | 1.9 (0.3), 436 | 1.9 (0.3), 712 | 1.9 (0.3), 614 | |
| Mean clinical pocket depth (four sites with clinical pocket depth of ≥ 4 mm), n (%) | Baseline | 47 (11) | 58 (13) | 47 (11) | 89 (13) | 63 (10) |
| Year 3 | 63 (14) | 62 (14) | 59 (14) | 98 (14) | 86 (14) | |
| Year | Randomised group, n (%) | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| 1 | 476 (76) | 472 (76) | 463 (74) | 753 (75) | 658 (76) |
| 2 | 430 (69) | 428 (68) | 408 (65) | 674 (67) | 592 (68) |
| 3 | 494 (79) | 486 (78) | 472 (75) | 780 (77) | 672 (78) |
| Outcomes | Time point | Randomised group, mean (SD), n | ||||
|---|---|---|---|---|---|---|
| PI | OHA | |||||
| No | 12-monthly | 6-monthly | Personalised | Routine | ||
| Self-efficacy | Baseline | 5.2 (1.1), 490 | 5.2 (1.2), 477 | 5.2 (1.2), 463 | 5.2 (1.2), 766 | 5.2 (1.2), 664 |
| Year 1 | 5.2 (1.1), 418 | 5.1 (1.1), 417 | 5.2 (1.1), 407 | 5.2 (1.1), 665 | 5.2 (1.1), 577 | |
| Year 2 | 5.3 (1.1), 398 | 5.2 (1.2), 394 | 5.3 (1.1), 386 | 5.2 (1.1), 632 | 5.3 (1.2), 546 | |
| Year 3 | 5.3 (1.1), 493 | 5.2 (1.1), 485 | 5.3 (1.1), 472 | 5.3 (1.1), 780 | 5.2 (1.1), 670 | |
| PBC | Baseline | 4.4 (1.2), 486 | 4.4 (1.2), 474 | 4.5 (1.2), 462 | 4.5 (1.2), 764 | 4.4 (1.2), 658 |
| Year 1 | 4.4 (1.2), 418 | 4.3 (1.2), 417 | 4.4 (1.2), 409 | 4.4 (1.2), 666 | 4.3 (1.2), 578 | |
| Year 2 | 4.4 (1.2), 395 | 4.5 (1.3), 394 | 4.3 (1.3), 386 | 4.4 (1.3), 630 | 4.4 (1.2), 545 | |
| Year 3 | 4.4 (1.3), 412 | 4.4 (1.2), 411 | 4.4 (1.2), 397 | 4.4 (1.3), 660 | 4.4 (1.2), 560 | |
| Attitude | Baseline | 5.8 (1.2), 489 | 5.9 (1.1), 475 | 5.7 (1.3), 463 | 5.8 (1.2), 767 | 5.8 (1.2), 660 |
| Year 1 | 5.4 (1.6), 418 | 5.5 (1.5), 417 | 5.7 (1.4), 409 | 5.5 (1.5), 666 | 5.5 (1.5), 578 | |
| Year 2 | 5.6 (1.4), 397 | 5.6 (1.5), 394 | 5.6 (1.4), 386 | 5.5 (1.5), 632 | 5.6 (1.4), 545 | |
| Year 3 | 5.5 (1.6), 414 | 5.6 (1.4), 410 | 5.6 (1.5), 397 | 5.5 (1.5), 659 | 5.5 (1.5), 562 | |
| Subjective norm | Baseline | 5.3 (1.1), 479 | 5.3 (1.2), 469 | 5.3 (1.1), 453 | 5.3 (1.1), 749 | 5.2 (1.1), 652 |
| Year 1 | 4.9 (1.2), 412 | 4.9 (1.2), 416 | 5.0 (1.2), 408 | 4.9 (1.2), 663 | 4.9 (1.2), 573 | |
| Year 2 | 4.9 (1.1), 392 | 4.9 (1.1), 390 | 4.9 (1.1), 384 | 4.9 (1.1), 624 | 4.9 (1.1), 542 | |
| Year 3 | 4.9 (1.2), 409 | 4.9 (1.2), 409 | 4.9 (1.1), 396 | 4.9 (1.2), 654 | 4.9 (1.1), 560 | |
| Behaviour score | Baseline | 4.7 (1.7), 485 | 4.7 (1.8), 476 | 4.8 (1.7), 463 | 4.6 (1.7), 762 | 4.9 (1.8), 662 |
| Year 1 | 5.0 (1.7), 418 | 5.1 (1.7), 416 | 5.2 (1.7), 406 | 5.0 (1.7), 665 | 5.2 (1.7), 575 | |
| Year 2 | 5.1 (1.6), 398 | 5.1 (1.7), 396 | 5.3 (1.6), 383 | 5.1 (1.6), 632 | 5.2 (1.6), 545 | |
| Year 3 | 5.1 (1.7), 412 | 5.1 (1.7), 411 | 5.4 (1.6), 395 | 5.1 (1.7), 658 | 5.2 (1.6), 560 | |
| Intention score | Baseline | 5.5 (1.8), 445 | 5.4 (1.8), 451 | 5.5 (1.8), 418 | 5.3 (1.8), 707 | 5.6 (1.7), 607 |
| Year 1 | 5.7 (1.7), 400 | 5.6 (1.7), 401 | 5.7 (1.7), 393 | 5.7 (1.7), 642 | 5.6 (1.7), 552 | |
| Year 2 | 5.6 (1.7), 387 | 5.6 (1.7), 377 | 5.7 (1.7), 373 | 5.6 (1.7), 614 | 5.7 (1.7), 523 | |
| Year 3 | 5.6 (1.7), 403 | 5.6 (1.7), 390 | 5.8 (1.7), 384 | 5.7 (1.7), 636 | 5.7 (1.7), 541 | |
| OHIP | Baseline | 5.6 (7.0), 476 | 6.0 (7.3), 466 | 6.1 (7.5), 456 | 5.8 (7.4), 752 | 6.1 (7.2), 646 |
| Year 1 | 4.9 (6.5), 415 | 5.6 (7.0), 407 | 5.0 (6.6), 395 | 4.9 (6.5), 652 | 5.4 (7.0), 565 | |
| Year 2 | 4.7 (6.5), 389 | 5.4 (7.0), 387 | 4.4 (6.0), 381 | 4.8 (6.5), 619 | 5.0 (6.6), 538 | |
| Year 3 | 5.2 (6.8), 408 | 5.3 (6.7), 399 | 4.9 (6.7), 387 | 4.8 (6.5), 641 | 5.5 (6.9), 553 | |
| Has a plan to either brush or floss better | Baseline | 0.3 (0.4), 494 | 0.2 (0.4), 486 | 0.3 (0.4), 472 | 0.3 (0.4), 780 | 0.2 (0.4), 672 |
| Year 1 | 0.2 (0.4), 494 | 0.2 (0.4), 486 | 0.2 (0.4), 472 | 0.2 (0.4), 780 | 0.2 (0.4), 672 | |
| Year 2 | 0.2 (0.4), 494 | 0.2 (0.4), 486 | 0.2 (0.4), 472 | 0.2 (0.4), 780 | 0.2 (0.4), 672 | |
| Year 3 | 0.2 (0.4), 494 | 0.2 (0.4), 486 | 0.2 (0.4), 472 | 0.2 (0.4), 780 | 0.2 (0.4), 672 | |
| Have you had bleeding from your gums when brushing your teeth? | Year 3 | 2.2 (1.0), 484 | 2.2 (1.0), 473 | 2.2 (1.0), 459 | 2.2 (1.0), 777 | 2.2 (1.0), 639 |
| Received private PI, n (%) | Year 3 | 75 (15) | 103 (21) | 106 (22) | 149 (19) | 135 (20) |
| Missing, n (%) | 228 (46) | 221 (45) | 227 (48) | 372 (48) | 304 (45) | |
Section 4: tertiary outcomes (at baseline and 3 years)
Section 4 describes the tertiary measures at baseline (Table 42) and 3-year follow-up (Table 43).
| Tertiary outcomes at baseline | Randomised group | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| How clean do your teeth feel, mean (SD), n | |||||
| After you brush | 5.8 (0.9), 605 | 5.8 (0.9), 608 | 5.9 (0.9), 605 | 5.8 (0.9), 975 | 5.8 (0.9), 843 |
| After a scale and polish | 6.6 (0.8), 574 | 6.5 (0.9), 589 | 6.6 (0.8), 574 | 6.6 (0.9), 927 | 6.6 (0.8), 810 |
| How clean do your teeth look, mean (SD), n | |||||
| After you brush | 5.3 (1.2), 608 | 5.3 (1.3), 603 | 5.3 (1.2), 601 | 5.3 (1.2), 970 | 5.2 (1.2), 842 |
| After a scale and polish | 6.2 (1.1), 575 | 6.1 (1.2), 585 | 6.1 (1.1), 567 | 6.2 (1.1), 928 | 6.1 (1.1), 799 |
| How pleasant do your teeth feel, mean (SD), n | |||||
| After you brush | 5.8 (1.1), 607 | 5.7 (1.1), 604 | 5.8 (1.1), 600 | 5.8 (1.1), 971 | 5.7 (1.1), 840 |
| After a scale and polish | 6.3 (1.0), 574 | 6.3 (1.0), 581 | 6.3 (1.0), 565 | 6.3 (1.0), 924 | 6.3 (1.1), 796 |
| How pleasant do your teeth look, mean (SD), n | |||||
| After you brush | 5.0 (1.4), 608 | 5.0 (1.4), 603 | 5.1 (1.4), 598 | 5.1 (1.4), 968 | 5.0 (1.4), 841 |
| After a scale and polish | 5.8 (1.4), 579 | 5.7 (1.3), 580 | 5.7 (1.4), 566 | 5.7 (1.4), 923 | 5.7 (1.4), 802 |
| Do you experience sensitivity in your teeth? n (%) | |||||
| Yes | 304 (49) | 293 (47) | 282 (45) | 445 (44) | 434 (50) |
| No | 304 (49) | 309 (49) | 323 (52) | 527 (52) | 409 (47) |
| Missing | 15 (2) | 23 (4) | 21 (3) | 36 (4) | 23 (3) |
| How sensitive are your teeth? n (%) | |||||
| Rarely sensitive | 59 (9) | 44 (7) | 41 (7) | 82 (8) | 62 (7) |
| Sometimes sensitive, rarely interfering with what I eat or drink | 166 (27) | 152 (24) | 135 (22) | 221 (22) | 232 (27) |
| Sometimes sensitive, occasionally interfering with what I eat or drink | 79 (13) | 90 (14) | 98 (16) | 135 (13) | 132 (15) |
| Always sensitive | 13 (2) | 17 (3) | 10 (2) | 21 (2) | 19 (2) |
| Missing | 306 (49) | 322 (52) | 342 (55) | 549 (54) | 421 (49) |
| How sensitive are your teeth? | 2.1 (0.8), 317 | 2.3 (0.8), 303 | 2.3 (0.7), 284 | 2.2 (0.8), 459 | 2.2 (0.7), 445 |
| On contact with hot and cold, how often do you get pain? | 3.1 (1.4), 321 | 3.1 (1.4), 321 | 3.0 (1.4), 295 | 3.1 (1.4), 480 | 3.1 (1.3), 457 |
| On contact with hot and cold, how severe is the pain? | 2.9 (1.3), 319 | 2.8 (1.3), 321 | 2.9 (1.2), 292 | 2.8 (1.3), 477 | 2.9 (1.2), 455 |
| Tertiary outcomes at 3 years | Randomised group | ||||
|---|---|---|---|---|---|
| PI | OHA | ||||
| No (N = 623) | 12-monthly (N = 625) | 6-monthly (N = 626) | Personalised (N = 1008) | Routine (N = 866) | |
| How clean do your teeth feel, mean (SD), n | |||||
| After you brush | 6.0 (0.8), 408 | 6.0 (0.8), 408 | 6.1 (0.8), 395 | 6.0 (0.8), 656 | 6.0 (0.8), 555 |
| After a scale and polish | 6.6 (0.6), 397 | 6.6 (0.8), 407 | 6.7 (0.7), 386 | 6.7 (0.7), 643 | 6.6 (0.7), 547 |
| How clean do your teeth look, mean (SD), n | |||||
| After you brush | 5.5 (1.2), 406 | 5.4 (1.2), 405 | 5.6 (1.2), 394 | 5.5 (1.2), 652 | 5.5 (1.2), 553 |
| After a scale and polish | 6.3 (1.0), 391 | 6.1 (1.1), 404 | 6.2 (1.1), 383 | 6.2 (1.1), 639 | 6.2 (1.1), 539 |
| How pleasant do your teeth feel, mean (SD), n | |||||
| After you brush | 5.9 (1.0), 408 | 5.9 (1.0), 408 | 6.0 (1.0), 392 | 6.0 (1.0), 652 | 5.9 (1.0), 556 |
| After a scale and polish | 6.5 (0.9), 393 | 6.4 (1.0), 404 | 6.5 (0.9), 385 | 6.5 (0.9), 640 | 6.4 (1.0), 542 |
| How pleasant do your teeth look, mean (SD), n | |||||
| After you brush | 5.3 (1.3), 406 | 5.2 (1.3), 407 | 5.4 (1.3), 391 | 5.3 (1.3), 650 | 5.2 (1.4), 554 |
| After a scale and polish | 5.9 (1.3), 396 | 5.8 (1.4), 404 | 5.9 (1.3), 382 | 5.9 (1.3), 638 | 5.9 (1.4), 544 |
| Do you experience sensitivity in your teeth?, n (%) | |||||
| Yes | 161 (26) | 165 (26) | 157 (25) | 241 (24) | 242 (28) |
| No | 235 (38) | 224 (36) | 224 (36) | 385 (38) | 298 (34) |
| Missing | 227 (36) | 236 (38) | 245 (39) | 382 (38) | 326 (38) |
| How sensitive are your teeth? n (%) | |||||
| Rarely sensitive | 47 (8) | 46 (7) | 38 (6) | 69 (7) | 62 (7) |
| Sometimes sensitive, rarely interfering with what I eat or drink | 73 (12) | 87 (14) | 85 (14) | 131 (13) | 114 (13) |
| Sometimes sensitive, occasionally interfere | 50 (8) | 51 (8) | 48 (8) | 73 (7) | 76 (9) |
| Always sensitive | 9 (1) | 3 (0) | 1 (0) | 4 (0) | 9 (1) |
| Missing | 444 (71) | 438 (70) | 454 (73) | 731 (73) | 605 (70) |
| How sensitive are your teeth?, mean (SD), n | 2.1 (0.9), 179 | 2.1 (0.8), 187 | 2.1 (0.7), 172 | 2.0 (0.8), 277 | 2.1 (0.8), 261 |
| On contact with hot and cold, how often do you get pain?, mean (SD), n | 2.8 (1.5), 199 | 2.8 (1.2), 196 | 2.8 (1.3), 183 | 2.7 (1.2), 299 | 3.0 (1.4), 279 |
| On contact with hot and cold, how severe is the pain?, mean (SD), n | 2.5 (1.2), 199 | 2.7 (1.2), 195 | 2.7 (1.2), 183 | 2.6 (1.2), 298 | 2.6 (1.2), 279 |
Appendix 2 Health economics
Section 1: additional detailed methods for costing, the discrete choice experiment and mapping discrete choice experiment valuations to the trial outcomes
Calculations of unit costs
The cost of consumer products was based on a sample of commonly available products in the marketplace. Given that data on the expense of replacing products (e.g. toothbrushes, interdental brushes) were not collected, we have taken a sample of products to represent the variability and the range available. This is not intended to be an exhaustive list of products.
| Item | Type | Unit cost (£) | Reference |
|---|---|---|---|
| Interdental brushes | Superdrug Totalcare interdental brushes (6 pack) | 2.49 | Superdrug (www.superdrug.com; accessed 1 February 2017) |
| TePe interdental brushes 0.4 mm (6 pack) | 3.05 | Superdrug (www.superdrug.com; accessed 1 February 2017) | |
| Boots Expert TePe interdental brushes 0.4 mm (6 pack) | 3.25 | Boots (www.boots.com; accessed 1 February 2017) | |
| TePe interdental brush blue 0.6 mm (6 pack) | 5.70 | British Corner Shop (www.britishcornershop.co.uk; accessed 1 February 2017) | |
| Average | 3.62 (0.60 each) | ||
| Electric toothbrush | Oral-B® (Proctor & Gamble Co., Cincinatti, OH, USA) Genius 9000 Black | 140.00 | Boots (www.boots.com; accessed 1 February 2017) |
| Oral-B® Pro 600 | 49.99 | Superdrug (www.superdrug.com; accessed 1 February 2017) | |
| Philips (Amsterdam, the Netherlands) Sonicare DiamondClean | 249.99 | Boots (www.boots.com; accessed 1 February 2017) | |
| Colgate® (Colgate-Palmolive, New York, NY, USA) ProClinical® C350 | 59.99 | Boots (www.boots.com; accessed 1 February 2017) | |
| Oral-B® Vitality Precision | 18.99 | Tesco (www.tesco.com; accessed 1 February 2017) | |
| Average | 103.79 | ||
| Manual toothbrush | Sensodyne® (GlaxoSmithKline plc, GSK House, Middlesex, UK) Precision Toothbrush | 3.00 | Tesco (www.tesco.com; accessed 1 February 2017) |
| Aquafresh® (GSK) Complete Care Medium | 2.50 | Tesco (www.tesco.com; accessed 1 February 2017) | |
| Colgate® 360 Max White | 4.00 | Asda (www.asda.com; accessed 1 February 2017) | |
| Colgate® Slim Soft | 1.22 | Sainsbury’s (www.sainsburys.co.uk; accessed 1 February 2017) | |
| Average | 2.68 | ||
| Heads for electric toothbrush | Oral-B® Precision Clean brush heads (4 pack) | 18.75 (4.69 each) | Superdrug (www.superdrug.com; accessed 1 February 2017) |
| Philips Sonicare Pro Results Standard Sonic toothbrush heads (4 pack) | 25.00 (6.25 each) | Philips (www.philips.co.uk; accessed 1 February 2017) | |
| Colgate® ProClinical Sensitive refill brush heads (4 pack) | 18.99 (4.75 each) | Boots (www.boots.com; accessed 1 February 2017) | |
| Oral-B® Sensitive Clean brush heads (4 pack) | 16.00 (4.00 each) | Sainsbury’s (www.sainsburys.co.uk; accessed 1 February 2017) | |
| Average | 19.69 (4.92 each) |
Discrete choice experiment attributes and levels by segment of design
| Attribute | Dental Health | |||||
|---|---|---|---|---|---|---|
| Good | Moderate | Poor | ||||
| Levels considered for choice sets | Opt-out | Levels considered for choice sets | Opt-out | Levels considered for choice sets | Opt-out | |
| Detailed and personalised OHA | All | None | All | None | All | None |
| Frequency of PI | All | None | All | None | All | None |
| Bleeding gums | Never | Occasional | Hardly ever | Fairly often | Occasional | Very often |
| Hardly ever | Occasional | Fairly often | ||||
| Occasional | Fairly often | Very often | ||||
| Look and feel | Very clean | Moderately clean | Clean | Unclean | Moderately clean | Very unclean |
| Clean | Moderately clean | Unclean | ||||
| Moderately clean | Unclean | Very unclean | ||||
| Cost (£) | All | 0 | All | 0 | All | 0 |
Mapping discrete choice experiment valuations to trial outcomes
| DCE outcome | Trial outcome/variable | Mapping method/notes/assumptions |
|---|---|---|
| WTP value for routine or personalised OHA provided by dentist/hygienist | Type of OHA by randomisation |
|
| Provider of initial intervention | ||
| WTP value for different annual frequency of PI, provided by hygienist/dentist | Frequency of PI (routine data) |
|
| Provider (participant questionnaire) | ||
| WTP value for frequency of bleeding gums | Frequency of bleeding gums at the 3-year follow-up time point |
|
| WTP value for aesthetic outcome (teeth look and feel clean and healthy) | How clean do your teeth look and feel ‘right now’? |
|
| The annual cost to you | N/A |
|
Section 2: research conducted for questionnaire development
Literature review
The initial set of draft attributes and levels presented to our advisory group for feedback was informed by a structured review of the literature regarding patient preferences for the processes and outcomes of dental care. The review searched for qualitative studies and surveys from 2000 to 2014 of preferences, experiences, attitudes, beliefs or opinions of adult patients or the general population. Studies were included if they related to routine or preventative dental care. The literature review focused on studies which could inform the selection of attributes for the DCEs. In brief, 15 articles were included and assessed. There was clear qualitative evidence on processes and organisational aspects of care that patients value. Continuity of care, and being treated ‘as a person, not as a patient’ were viewed positively. 76–78 Dental anxiety and fear were significant barriers to attending the dentist. 79,80 The review reaffirmed the importance of costs,76,81,82 and provider of care. 76,83
There was less information on preferences for dental health outcomes, such as bleeding gums (the primary clinical end point for the trial). Therefore, it was crucial to include this as an attribute and to ensure that it was framed correctly within the DCE. There was no information from the literature about the trade-offs between aesthetic and clinical outcomes, particularly for people having PI. The lack of relevant literature motivated the decision to undertake primary FG research.
Focus groups
After development of an initial set of attributes and levels important for the trial, and based on the literature available, we ran FG discussions to see if these issues were important to the general population (potential preventative dental care patients) and to determine if we omitted anything relevant and important for our research questions.
Eighteen respondents from the general population in Aberdeen were recruited into four FG sessions. Each group consisted of four or five respondents. FG discussions were recorded, transcribed and analysed following a qualitative thematic framework. The FG protocol was approved by the University of Aberdeen College Ethics Review Board. The FGs provided a rich data set with varied demographic characteristics. Saturation was achieved after the second group.
Focus group results are discussed in the context of developing attributes and levels for the DCEs. The goods to be valued within the DCE were all found to be important to participants, namely frequency of visits, PI and OHA. Participants preferred to avoid dental pain, tooth loss and bad breath. Bleeding gums were important, but only after the facilitator encouraged discussion of this issue. Bleeding was associated with brushing. A description of how bleeding from a clinical point of view might translate into brushing is presented in the DCE to ground the attribute in reality. Dental pain was not directly included as an attribute as it would dominate all of the other attributes in the choice sets, limiting the ability to value any potential differences between groups in trial outcomes. As aesthetic improvement was a prominent reason in the FGs for having a PI, we included an attribute asking respondents to value how clean their teeth look and feel. This aesthetic attribute was intended to value wider, non-health, benefits of PI or OHA.
Focus group data were further used to frame the questions and explain the attributes in the DCEs. For example, many respondents were unaware of who a dental hygienist was, and their knowledge was clearly dependent on experience. A comprehensive description of the roles and responsibilities of a dental hygienist was thus included. There was a preference for personalised care; however, the meaning of ‘personalised’ is specific to each individual. The survey therefore included a detailed description of what we meant by ‘personalised’ OHA, in line with the intervention delivered in the trial. Furthermore, many FG participants tended to merge their preferences for PI with a routine visit to the dentist as the two often happen at the same visit. In order to obtain preferences for PI, as opposed to a regular check-up, we tailored the explanation of the PI attribute, emphasising the difference between PI and routine check-ups.
Focus group data identified important contextual information. For example, respondents who had frequent bleeding tended to be less bothered by it. Respondents with experience of a dental hygienist had a more positive view of their role. Fear, anxiety, continuity of care and trust were important drivers of preferences. These findings shaped the selection of a number of contextual questions for inclusion at the end of the DCE surveys.
Think-aloud interviews
Once the final design was decided, we recruited five members of the general population and five colleagues to work through the survey, providing comments on the content and framing of the questions. We asked respondents to focus on areas of the questionnaire that were difficult to understand or any problems that they experienced as they went along. Particular attention was paid to how the choice tasks were interpreted and if there was any evidence of decision heuristics or attribute non-attendance. In general, respondents reacted well to the questionnaire. A number of minor bugs in the process were identified and corrected and some minor changes were made to clarify the instructions for the choice tasks, in particular explaining what should and should not be considered when making the choices. No changes were made to the DCE design at this stage as all attributes and levels were found to be acceptable to respondents.
Pilot survey
We conducted a comprehensive pilot study with an online representative sample of the general population. A major concern with the external validity of DCE responses relates to the issue of hypothetical bias, in which the respondent’s stated preferences are not as they would act in reality. A particularly problematic issue is that, in the presence of hypothetical bias, respondents often overstate the value they place on a good or service. The implication is biased estimates of WTP and, hence, an overvaluation of services in a CBA. Hypothetical bias may therefore contribute to incorrect policy recommendations. There are many potential causes of hypothetical bias and much literature on how best to address it.
We compared a number of suggested approaches, including a cheap talk script, a consequentiality script and asking respondents to sign an honesty oath. All approaches were compared with a standard practice approach of reminding respondents about the availability of close substitutes and asking them to consider their budget constraints when answering the choice tasks. The pilot questionnaire was administered to a nationally representative sample of the general population, with oversampling (n = 100) in Scotland. The Qualtrics survey platform was used to deliver the survey online.
To determine the presence or otherwise of hypothetical bias, we estimated marginal WTP and predicted uptake from the DCE. We compared results across groups. We found no clear evidence of differences across groups. While our sample was small, we were reasonably confident that the base-case approach was sufficient for the main study.
The pilot study was also used to assess the direction of effect of the included attributes and levels. The pilot data were analysed using both simple conditional logit model and mixed logit models, finding that all attributes and levels had good face validity, with the expected coefficient signs and we found that WTP estimates were within the extremes of the levels of the cost attribute presented (i.e. no attribute level was valued at a WTP value of > £200 per year). Therefore, we concluded that the pilot version without correction for hypothetical bias was appropriate for the main phase of data collection.
Section 3: detailed claims for Scottish routine data
| Routine data claim | OHA, number of claims | Overall total, n (n = 1337) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | ||||||||
| No PI (n = 192) | 12-monthly PI (n = 193) | 6-monthly PI (n = 195) | Total (n = 580) | No PI (n = 250) | 12-monthly PI (n = 255) | 6-monthly PI (n = 252) | Total (n = 757) | ||
| Clinical | 498 | 495 | 522 | 1515 | 608 | 629 | 673 | 1910 | 3425 |
| Simple scaling and polishing | 253 | 333 | 519 | 1105 | 238 | 447 | 644 | 1329 | 2434 |
| Small film | 160 | 149 | 162 | 471 | 232 | 229 | 208 | 669 | 1140 |
| Composite or synthetic resin | 95 | 112 | 94 | 301 | 117 | 134 | 122 | 373 | 674 |
| Amalgam | 103 | 102 | 106 | 311 | 129 | 109 | 122 | 360 | 671 |
| Additional fee for each visit | 28 | 39 | 27 | 94 | 35 | 43 | 31 | 109 | 203 |
| Fee per course of treatment | 28 | 39 | 27 | 94 | 35 | 43 | 31 | 109 | 203 |
| Incisal acid etch | 26 | 33 | 30 | 89 | 44 | 42 | 27 | 113 | 202 |
| Glass ionomer, silicate or silico-phosphate | 28 | 27 | 33 | 88 | 23 | 39 | 30 | 92 | 180 |
| Additional fee for first inlay or crown in the same arch | 17 | 11 | 18 | 46 | 29 | 33 | 31 | 93 | 139 |
| Stoning and smoothing surface of a tooth | 5 | 9 | 8 | 22 | 21 | 38 | 31 | 90 | 112 |
| Treatment of sensitive cementum or dentine | 6 | 13 | 19 | 38 | 14 | 25 | 15 | 54 | 92 |
| Extensive clinical | 13 | 11 | 11 | 35 | 23 | 14 | 18 | 55 | 90 |
| Refixing or recementing a crown | 15 | 13 | 12 | 40 | 15 | 18 | 14 | 47 | 87 |
| Bonded full or jacket crown – non-precious metal | 11 | 8 | 10 | 29 | 17 | 18 | 20 | 55 | 84 |
| Treatment urgently required for acute conditions | 14 | 16 | 11 | 41 | 10 | 16 | 12 | 38 | 79 |
| Other treatment claims | 158 | 131 | 133 | 422 | 192 | 253 | 216 | 661 | 1083 |
| Total claims | 1458 | 1541 | 1742 | 4741 | 1782 | 2130 | 2245 | 6157 | 10,898 |
Section 4: participant-reported contact with non-dental health services
| NHS resource use item | OHA, n/N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | ||||||||
| No PI (n = 289) | 12-monthly PI (n = 287) | 6-monthly PI (n = 290) | Total (n = 866) | No PI (n = 334) | 12-monthly PI (n = 338) | 6-monthly PI (n = 335) | Total (n = 1007) | ||
| NHS 24 | Year 1 | 9/204 (4) | 7/203 (3) | 4/200 (2) | 20/607 (3) | 4/242 (2) | 6/233 (3) | 6/240 (3) | 16/715 (2) |
| Year 2 | 5/196 (3) | 5/186 (3) | 6/179 (3) | 16/561 (3) | 6/207 (3) | 3/214 (1) | 2/209 (1) | 11/630 (2) | |
| Year 3 | 1/186 (1) | 2/177 (1) | 3/170 (2) | 6/533 (1) | 4/207 (2) | 2/215 (1) | 1/209 (0) | 7/631 (1) | |
| GP | Year 1 | 26/204 (13) | 24/205 (12) | 20/200 (10) | 70/609 (11) | 25/244 (10) | 28/237 (12) | 17/240 (7) | 70/721 (10) |
| Year 2 | 21/196 (11) | 20/188 (11) | 18/179 (10) | 59/563 (10) | 24/208 (12) | 19/213 (9) | 19/209 (9) | 62/630 (10) | |
| Year 3 | 23/187 (12) | 9/179 (5) | 7/168 (4) | 39/534 (7) | 20/207 (10) | 17/217 (8) | 13/209 (6) | 50/633 (8) | |
| A&E | Year 1 | 10/202 (5) | 9/204 (4) | 4/198 (2) | 23/604 (4) | 3/242 (1) | 10/236 (4) | 7/239 (3) | 20/717 (3) |
| Year 2 | 9/196 (5) | 6/186 (3) | 9/178 (5) | 24/560 (4) | 7/207 (3) | 8/214 (4) | 4/210 (2) | 19/631 (3) | |
| Year 3 | 1/188 (1) | 1/176 (1) | 3/169 (2) | 5/533 (1) | 3/207 (1) | 4/215 (2) | 3/208 (1) | 10/630 (2) | |
| Outpatients | Year 1 | 13/204 (6) | 12/203 (6) | 18/202 (9) | 43/609 (7) | 14/243 (6) | 12/237 (5) | 10/239 (4) | 36/719 (5) |
| Year 2 | 13/196 (7) | 12/188 (6) | 12/178 (7) | 37/562 (7) | 16/209 (8) | 8/214 (4) | 10/210 (5) | 34/633 (5) | |
| Year 3 | 13/188 (7) | 7/179 (4) | 8/171 (5) | 28/538 (5) | 9/207 (4) | 9/216 (4) | 8/209 (4) | 26/632 (4) | |
| Inpatients | Year 1 | 10/203 (5) | 5/202 (2) | 5/199 (3) | 20/604 (3) | 3/243 (1) | 7/236 (3) | 3/239 (1) | 13/718 (2) |
| Year 2 | 7/194 (4) | 5/185 (3) | 6/176 (3) | 18/555 (3) | 5/208 (2) | 4/212 (2) | 1/208 (0) | 10/628 (2) | |
| Year 3 | 3/188 (2) | 1/177 (1) | 1/167 (1) | 5/532 (1) | 1/207 (0) | 1/212 (0) | 2/209 (1) | 4/628 (1) | |
Section 5: unit opportunity cost of time and travel to dental appointments
| Time and travel costs | OHA, n, mean cost (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Routine | Personalised | |||||||
| No PI (N = 289) | 12-monthly PI (N = 287) | 6-monthly PI (N = 290) | Total (N = 866) | No PI (N = 334) | 12-monthly PI (N = 338) | 6-monthly PI (N = 335) | Total (N = 1007) | |
| Participant time and travel costs | ||||||||
| Participant mode of transport, n (%) | ||||||||
| Data completeness | 284 (98) | 281 (98) | 283 (98) | 848 (98) | 326 (98) | 329 (97) | 324 (97) | 979 (97) |
| Walked | 57 (20) | 64 (22) | 66 (23) | 187 (22) | 77 (23) | 76 (22) | 72 (21) | 225 (22) |
| Cycled | 7 (2) | 5 (2) | 5 (2) | 17 (2) | 8 (2) | 8 (2) | 4 (1) | 20 (2) |
| Private car | 186 (64) | 173 (60) | 177 (61) | 536 (62) | 209 (63) | 213 (63) | 212 (63) | 634 (63) |
| Bus | 27 (9) | 35 (12) | 32 (11) | 94 (11) | 25 (7) | 24 (7) | 31 (9) | 80 (8) |
| Taxi | 1 (0) | 3 (1) | 0 (0) | 4 (0) | 5 (1) | 2 (1) | 2 (1) | 9 (1) |
| Other, non-specified | 3 (1) | 0 (0) | 1 (0) | 4 (0) | 1 (0) | 2 (1) | 1 (0) | 4 (0) |
| Metro | 1 (0) | 0 (0) | 1 (0) | 2 (0) | 0 (0) | 2 (1) | 1 (0) | 3 (0) |
| Boat | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 2 (1) | 0 (0) | 3 (0) |
| Train | 2 (1) | 1 (0) | 1 (0) | 4 (0) | 0 (0) | 0 (0) | 1 (0) | 1 (0) |
| Missing | 5 (2) | 6 (2) | 7 (2) | 18 (2) | 8 (2) | 9 (3) | 11 (3) | 28 (3) |
| Transportation costs, n, mean (£) (SD, £) | 227, 3.46 (4.70) | 238, 3.62 (6.32) | 238, 3.47 (5.54) | 267, 3.46 (6.35) | 274, 3.60 (5.11) | 703, 3.52 (5.56) | 274, 3.63 (8.40) | 815, 3.56 (6.75) |
| Minutes travelling/waiting, n, mean (SD) | 281, 31 (25.56) | 276, 31.2 (27.81) | 279, 31.36 (25.29) | 318, 28.72 (23.66) | 322, 29.88 (27.4) | 836, 31.19 (26.21) | 326, 29.91 (28.11) | 966, 29.51 (26.46) |
| Participant alternative activity, n (%) | ||||||||
| Data completeness | 281 (97) | 276 (96) | 276 (95) | 833 (96) | 324 (97) | 328 (97) | 323 (96) | 975 (97) |
| Housework | 59 (20) | 57 (20) | 55 (19) | 171 (20) | 58 (17) | 60 (18) | 75 (22) | 193 (19) |
| Child care | 24 (8) | 17 (6) | 17 (6) | 58 (7) | 19 (6) | 31 (9) | 20 (6) | 70 (7) |
| Caring for a friend/relative | 6 (2) | 6 (2) | 3 (1) | 15 (2) | 7 (2) | 6 (2) | 5 (1) | 18 (2) |
| Unemployed | 14 (5) | 11 (4) | 15 (5) | 40 (5) | 9 (3) | 18 (5) | 14 (4) | 41 (4) |
| Paid work | 114 (39) | 118 (41) | 114 (39) | 346 (40) | 138 (41) | 140 (41) | 138 (41) | 416 (41) |
| Voluntary work | 3 (1) | 5 (2) | 5 (2) | 13 (2) | 6 (2) | 2 (1) | 4 (1) | 12 (1) |
| Leisure activities | 35 (12) | 47 (16) | 46 (16) | 128 (15) | 66 (20) | 47 (14) | 48 (14) | 161 (16) |
| Other non-specified | 5 (2) | 8 (3) | 8 (3) | 21 (2) | 8 (2) | 13 (4) | 10 (3) | 31 (3) |
| Studying | 12 (4) | 3 (1) | 5 (2) | 20 (2) | 5 (1) | 6 (2) | 4 (1) | 15 (1) |
| Retired | 5 (2) | 3 (1) | 5 (2) | 13 (2) | 7 (2) | 3 (1) | 4 (1) | 14 (1) |
| Self-employed | 2 (1) | 1 (0) | 1 (0) | 4 (0) | 1 (0) | 2 (1) | 1 (0) | 4 (0) |
| Long-term sick/disabled | 2 (1) | 0 (0) | 2 (1) | 4 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Missing | 8 (3) | 11 (4) | 14 (5) | 33 (4) | 10 (3) | 10 (3) | 12 (4) | 32 (3) |
| Opportunity cost, alternative activities, n, mean (£) (SD, £) | 280, 7.16 (5.10) | 274, 7.20 (5.80) | 274, 7.23 (5.36) | 320, 6.42 (4.54) | 323, 6.99 (5.77) | 828, 7.20 (5.42) | 325, 6.90 (6.12) | 968, 6.77 (5.52) |
| Participant unit cost for time and travel, n, mean (£) (SD, £) | 222, 10.53 (8.42) | 234, 11.07 (10.70) | 230, 10.83 (9.71) | 261, 9.85 (9.59) | 272, 10.74 (9.87) | 686, 10.81 (9.66) | 270, 10.61 (13.26) | 803, 10.41 (11.04) |
| Companion time and travel costs | ||||||||
| Participant accompanied, n (%) | ||||||||
| Yes | 52 (18) | 36 (13) | 38 (13) | 126 (15) | 55 (16) | 50 (15) | 41 (12) | 146 (14) |
| No | 220 (76) | 231 (80) | 233 (80) | 684 (79) | 265 (79) | 268 (79) | 274 (82) | 807 (80) |
| Missing | 17 (6) | 20 (7) | 19 (7) | 56 (6) | 14 (4) | 20 (6) | 20 (6) | 54 (5) |
| Relationship of companiona | ||||||||
| Partner | 21 (36) | 16 (41) | 15 (35) | 52 (37) | 29 (51) | 25 (45) | 14 (33) | 68 (44) |
| Other relative | 16 (28) | 14 (36) | 19 (44) | 49 (35) | 15 (26) | 16 (29) | 13 (30) | 44 (28) |
| Paid companion | 0 (0) | 0 (0) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Friend | 2 (3) | 1 (3) | 0 (0) | 3 (2) | 0 (0) | 1 (2) | 0 (0) | 1 (1) |
| Other | 19 (33) | 8 (21) | 8 (19) | 35 (25) | 13 (23) | 13 (24) | 16 (37) | 42 (27) |
| Companion travel costs | ||||||||
| Bus fare, n, mean (£) (SD, £) | 34, 0.16 (0.75) | 28, 0.08 (0.30) | 30, 0.34 (0.84) | 92, 0.19 (0.68) | 29, 0.21 (0.53) | 27, 0.26 (0.77) | 24, 0.12 (0.42) | 80, 0.20 (0.59) |
| Total costs, n, mean (£) (SD, £)b | 263, 0.38 (2.55) | 263, 0.09 (0.59) | 265, 0.22 (1.60) | 791, 0.23 (1.77) | 309, 0.15 (0.85) | 311, 0.20 (1.00) | 308, 0.23 (1.34) | 928, 0.19 (1.08) |
| Companion alternative activity, n (%) | ||||||||
| Housework | 5 (9) | 3 (8) | 6 (15) | 14 (11) | 12 (23) | 11 (21) | 3 (8) | 26 (18) |
| Child care | 3 (5) | 1 (3) | 3 (8) | 7 (5) | 1 (2) | 4 (8) | 4 (11) | 9 (6) |
| Caring for a relative/friend | 3 (5) | 1 (3) | 3 (8) | 7 (5) | 1 (2) | 3 (6) | 1 (3) | 5 (4) |
| Unemployed | 0 (0) | 1 (3) | 3 (8) | 4 (3) | 1 (2) | 0 (0) | 0 (0) | 1 (1) |
| Paid work | 12 (21) | 7 (19) | 6 (15) | 25 (19) | 11 (21) | 11 (21) | 9 (24) | 31 (22) |
| Voluntary work | 0 (0) | 1 (3) | 1 (3) | 2 (2) | 2 (4) | 0 (0) | 0 (0) | 2 (1) |
| Leisure activities | 18 (32) | 15 (42) | 10 (25) | 43 (33) | 14 (27) | 13 (25) | 14 (37) | 41 (29) |
| Other non-specified | 4 (7) | 3 (8) | 7 (18) | 14 (11) | 7 (13) | 5 (10) | 1 (3) | 13 (9) |
| Studying | 9 (16) | 3 (8) | 1 (3) | 13 (10) | 1 (2) | 3 (6) | 4 (11) | 8 (6) |
| Retired | 2 (4) | 1 (3) | 0 (0) | 3 (2) | 1 (2) | 2 (4) | 2 (5) | 5 (4) |
| Disabled | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (1) |
| Companion unit cost (£) for time and travel | 260, 1.35 (4.57) | 260, 0.85 (2.94) | 260, 1.03 (4.07) | 780, 1.08 (3.92) | 301, 0.89 (3.28) | 306, 0.98 (3.16) | 304; 0.96 (3.72) | 911, 0.94 (3.40) |
| Average number of visits to dentist | 271, 3.57 (1.99) | 273, 3.75 (2.17) | 283, 3.9 (2.37) | 827, 3.74 (2.19) | 327, 3.21 (1.87) | 331, 3.61 (1.96) | 328, 3.74 (1.87) | 986, 3.52 (1.91) |
| Total cost (£) of time and travel:complete case | 186, 44.08 (51.69) | 201, 47.53 (57.18) | 195, 47.16 (48.61) | 582, 46.30 (52.61) | 234, 35.16 (36.66) | 249, 43.67 (58.72) | 243, 44.38 (50.50) | 726, 41.17 (49.72) |
| Total cost (£) of time and travel: imputation of missing data | 289, 43.55 (51.33) | 287, 47.31 (57.12) | 290, 46.44 (48.35) | 866, 45.77 (52.36) | 334, 34.57 (36.17) | 338, 42.75 (56.46) | 335, 44.36 (50.95) | 1007, 0.57 (48.81) |
Section 6: detailed results of further health economic analyses
FIGURE 19.
Confidence ellipses for base-case health economic results. NHS dental costs: (a) routine OHA, no PI vs. routine OHA, 6-monthly PI; (b) routine OHA, 12-monthly PI vs. routine OHA, 6-monthly PI; (c) personalised OHA, no PI vs. routine OHA, 6-monthly PI; (d) personalised OHA, 6-monthly PI vs. routine OHA, 6-monthly PI; and (e) personalised OHA, 12-monthly PI vs. routine OHA, 6-monthly PI.



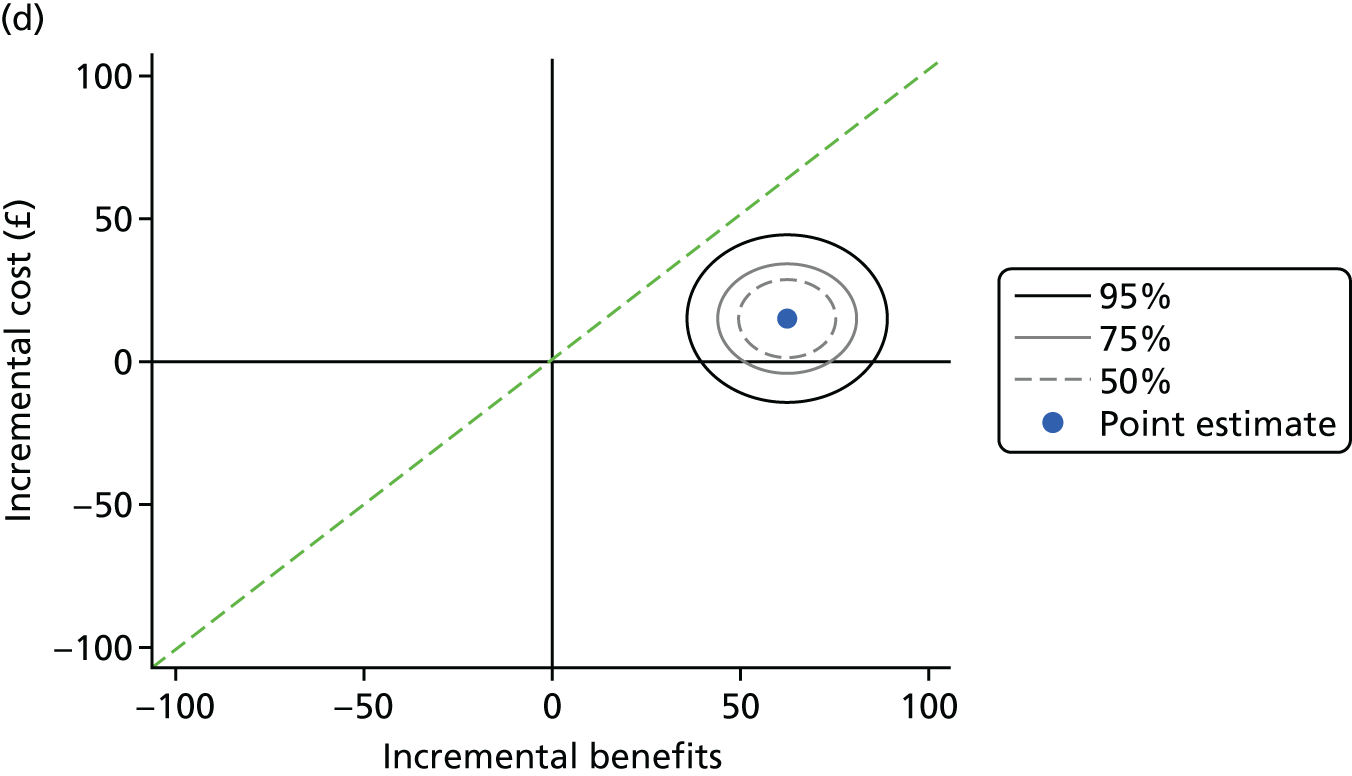
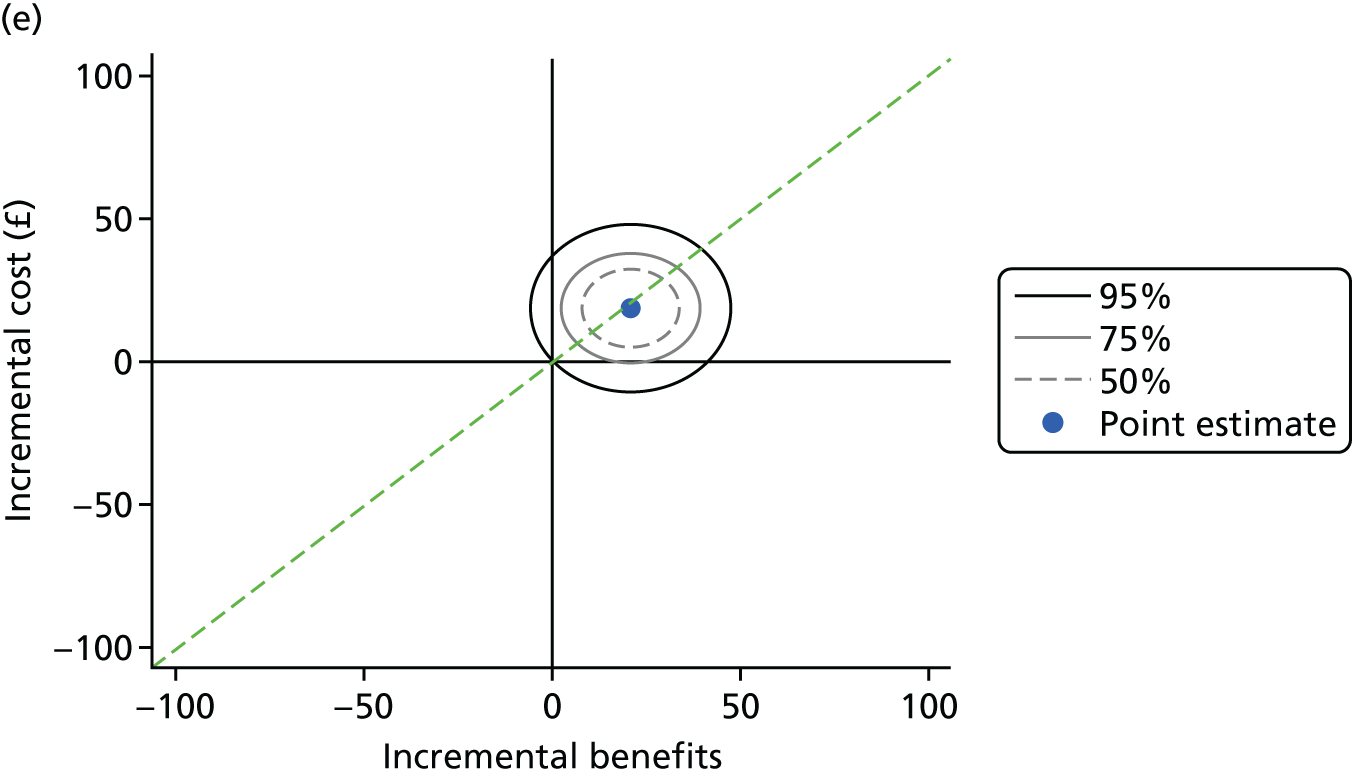
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 97 (123) | – | 231 (139) | – | – |
| No PI (n = 288) | 97 (134) | 2 (–22 to 25) | 164 (134) | –67 (–92 to –42) | –68 (–103 to –33) |
| 12-monthly PI (n = 285) | 105 (160) | 10 (–13 to 33) | 205 (133) | –23 (–48 to 1) | –33 (–67 to 1) |
| Personalised OHA | |||||
| No PI (n = 333) | 87 (132) | –3 (–31 to 25) | 236 (145) | 6 (–23 to 35) | 9 (–24 to 42) |
| 12-monthly PI (n = 338) | 110 (150) | 21 (–8 to 49) | 277 (134) | 45 (17 to 74) | 25 (–8 to 57) |
| 6-monthly PI (n = 335) | 109 (164) | 19 (–9 to 47) | 317 (150) | 88 (59 to 117) | 69 (36 to 102) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 101 (118) | – | 251 (136) | – | – |
| No PI (n = 195) | 88 (102) | –12 (–36 to 12) | 192 (130) | –59 (–89 to –29) | –47 (–85 to –9) |
| 12-monthly PI (n = 195) | 97 (116) | –2 (–26 to 22) | 226 (133) | –24 (–54 to 5) | –22 (–60 to 15) |
| Personalised OHA | |||||
| No PI (n = 252) | 87 (114) | –5 (–34 to 23) | 260 (141) | 7 (–26 to 41) | 13 (–23 to 49) |
| 12-monthly PI (n = 255) | 107 (142) | 13 (–16 to 41) | 302 (123) | 45 (12 to 77) | 32 (–3 to 67) |
| 6-monthly PI (n = 252) | 108 (152) | 14 (–14 to 43) | 345 (138) | 92 (58 to 125) | 77 (42 to 113) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 89 (133) | – | 189 (137) | – | – |
| No PI (n = 93) | 115 (183) | 32 (–23 to 86) | 105 (122) | –83 (–124 to –43) | –115 (–185 to –45) |
| 12-monthly PI (n = 92) | 122 (227) | 33 (–20 to 87) | 161 (123) | –24 (–65 to 17) | –58 (–126 to 11) |
| Personalised OHA | |||||
| No PI (n = 81) | 85 (175) | –2 (–66 to 63) | 161 (133) | –15 (–69 to 38) | –14 (–84 to 56) |
| 12-monthly PI (n = 83) | 119 (173) | 35 (–30 to 101) | 199 (138) | 30 (–24 to 84) | –5 (–76 to 65) |
| 6-monthly PI (n = 83) | 111 (197) | 27 (–38 to 92) | 233 (157) | 60 (5 to 116) | 34 (–38 to 105) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 77 (109) | – | 150 (110) | – | – |
| No PI (n = 288) | 78 (126) | 2 (–20 to 23) | 81 (102) | –69 (–88 to –49) | –70 (–99 to –42) |
| 12-monthly PI (n = 285) | 85 (147) | 9 (–12 to 30) | 117 (99) | –31 (–50 to –12) | –40 (–68 to –12) |
| Personalised OHA | |||||
| No PI (n = 333) | 65 (120) | –6 (–29 to 17) | 130 (110) | –18 (–41 to 4) | –13 (–40 to 15) |
| 12-monthly PI (n = 338) | 90 (139) | 19 (–4 to 43) | 170 (100) | 20 (0 to 41) | 1 (–26 to 28) |
| 6-monthly PI (n = 335) | 86 (139) | 15 (–8 to 38) | 212 (120) | 64 (42 to 86) | 49 (21 to 76) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 75 (96) | – | 163 (112) | – | – |
| No PI (n = 195) | 66 (90) | –9 (–29 to 12) | 90 (106) | –73 (–97 to –49) | –64 (–95 to –34) |
| 12-monthly PI (n = 195) | 70 (86) | –4 (–24 to 17) | 122 (103) | –40 (–63 to –17) | –36 (–66 to –6) |
| Personalised OHA | |||||
| No PI (n = 252) | 63 (106) | –5 (–29 to 19) | 139 (112) | –25 (–53 to 2) | –20 (–50 to 9) |
| 12-monthly PI (n = 255) | 83 (127) | 13 (–11 to 37) | 181 (97) | 14 (–12 to 39) | 1 (–27 to 29) |
| 6-monthly PI (n = 252) | 82 (124) | 13 (–11 to 36) | 229 (117) | 65 (38 to 92) | 52 (23 to 81) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 82 (132) | -- | 123 (102) | -- | -- |
| No PI (n = 93) | 102 (177) | 25 (–27 to 76) | 62 (89) | –60 (–92 to –29) | –85 (–146 to –24) |
| 12-monthly PI (n = 92) | 116 (224) | 35 (–16 to 86) | 105 (89) | –14 (–47 to 18) | –50 (–111 to 12) |
| Personalised OHA | |||||
| No PI (n = 81) | 68 (156) | –12 (–69 to 45) | 103 (100) | –11 (–52 to 30) | 1 (–62 to 64) |
| 12-monthly PI (n = 83) | 113 (171) | 35 (–24 to 93) | 136 (102) | 26 (–14 to 67) | –9 (–71 to 54) |
| 6-monthly PI (n = 83) | 98 (175) | 20 (–38 to 78) | 162 (116) | 50 (9 to 90) | 30 (–32 to 91) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 72 (103) | – | 144 (102) | – | – |
| No PI (n = 288) | 72 (117) | 1 (–19 to 21) | 79 (95) | –65 (–84 to –47) | –67 (–93 to –40) |
| 12-monthly PI (n = 285) | 79 (139) | 8 (–11 to 28) | 113 (91) | –29 (–48 to –11) | –38 (–65 to –11) |
| Personalised OHA | |||||
| No PI (n = 333) | 60 (112) | –6 (–27 to 16) | 127 (102) | –16 (–36 to 5) | –10 (–36 to 15) |
| 12-monthly PI (n = 338) | 84 (129) | 18 (–4 to 39) | 166 (93) | 21 (1 to 41) | 4 (–22 to 29) |
| 6-monthly PI (n = 335) | 81 (131) | 15 (–7 to 36) | 205 (112) | 62 (41 to 83) | 47 (22 to 73) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 71 (91) | – | 157 (103) | – | – |
| No PI (n = 195) | 62 (84) | –9 (–28 to 10) | 87 (99) | –70 (–93 to –47) | –62 (–90 to –33) |
| 12-monthly PI (n = 195) | 66 (80) | –4 (–23 to 15) | 119 (95) | –38 (–62 to –15) | –34 (–64 to –5) |
| Personalised OHA | |||||
| No PI (n = 252) | 60 (100) | –5 (–27 to 17) | 136 (103) | –22 (–48 to 3) | –18 (–45 to 10) |
| 12-monthly PI (n = 255) | 78 (118) | 12 (–11 to 34) | 176 (91) | 15 (–10 to 40) | 3 (–24 to 30) |
| 6-monthly PI (n = 252) | 77 (117) | 12 (–11 to 34) | 221 (108) | 63 (37 to 88) | 51 (24 to 78) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 76 (125) | – | 118 (94) | – | – |
| No PI (n = 93) | 95 (165) | 23 (–26 to 72) | 61 (84) | –56 (–85 to –27) | –79 (–136 to –22) |
| 12-monthly PI (n = 92) | 109 (213) | 33 (–15 to 82) | 101 (82) | –13 (–42 to 15) | –47 (–103 to 10) |
| Personalised OHA | |||||
| No PI (n = 81) | 63 (144) | –12 (–65 to 42) | 101 (94) | –8 (–46 to 30) | 3 (–54 to 61) |
| 12-monthly PI (n = 83) | 104 (157) | 31 (–23 to 86) | 133 (94) | 27 (–10 to 65) | –4 (–62 to 54) |
| 6-monthly PI (n = 83) | 93 (166) | 20 (–34 to 73) | 155 (109) | 48 (9 to 88) | 28 (–30 to 87) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 74 (106) | – | 153 (107) | – | – |
| No PI (n = 288) | 74 (119) | 1 (–20 to 21) | 83 (99) | –70 (–89 to –51) | –70 (–98 to –43) |
| 12-monthly PI (n = 285) | 81 (141) | 9 (–12 to 29) | 122 (96) | –29 (–48 to –10) | –37 (–65 to –10) |
| Personalised OHA | |||||
| No PI (n = 333) | 62 (115) | –6 (–27 to 16) | 137 (104) | –13 (–35 to 8) | –8 (–34 to 19) |
| 12-monthly PI (n = 338) | 87 (134) | 18 (–3 to 40) | 178 (97) | 27 (6 to 47) | 8 (–18 to 35) |
| 6-monthly PI (n = 335) | 83 (134) | 15 (–7 to 37) | 220 (113) | 70 (48 to 91) | 55 (28 to 82) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 161 (107) | – | – |
| No PI (n = 195) | 64 (86) | –8 (–28 to 11) | 89 (104) | –72 (–95 to –49) | –64 (–93 to –34) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–23 to 16) | 122 (99) | –39 (–62 to –17) | –35 (–64 to –6) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–27 to 18) | 141 (106) | –20 (–46 to 6) | –15 (–44 to 13) |
| 12-monthly PI (n = 255) | 80 (121) | 12 (–11 to 35) | 184 (95) | 19 (–6 to 44) | 7 (–20 to 35) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–10 to 35) | 231 (110) | 69 (43 to 95) | 57 (29 to 85) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 78 (128) | – | 135 (106) | – | – |
| No PI (n = 93) | 95 (167) | 21 (–28 to 71) | 69 (87) | –65 (–97 to –34) | –86 (–145 to –28) |
| 12-monthly PI (n = 92) | 112 (216) | 33 (–16 to 83) | 122 (90) | –9 (–42 to 24) | –43 (–103 to 17) |
| Personalised OHA | |||||
| No PI (n = 81) | 65 (148) | –13 (–68 to 42) | 123 (97) | –3 (–43 to 36) | 9 (–50 to 69) |
| 12-monthly PI (n = 83) | 108 (165) | 33 (–23 to 88) | 160 (101) | 38 (–3 to 79) | 6 (–56 to 67) |
| 6-monthly PI (n = 83) | 95 (170) | 19 (–36 to 74) | 185 (116) | 62 (20 to 103) | 43 (–19 to 104) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 74 (106) | – | 126 (100) | – | – |
| No PI (n = 288) | 74 (119) | 1 (–20 to 21) | 65 (89) | –60 (–78 to –43) | –61 (–88 to –34) |
| 12-monthly PI (n = 285) | 81 (141) | 9 (–12 to 29) | 95 (89) | –29 (–46 to –11) | –37 (–64 to –10) |
| Personalised OHA | |||||
| No PI (n = 333) | 62 (115) | –6 (–27 to 15) | 100 (94) | –24 (–43 to –4) | –18 (–44 to 8) |
| 12-monthly PI (n = 338) | 87 (134) | 18 (–3 to 39) | 130 (88) | 5 (–14 to 24) | –13 (–39 to 13) |
| 6-monthly PI (n = 335) | 83 (134) | 15 (–7 to 36) | 167 (105) | 43 (23 to 63) | 29 (2 to 55) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 131 (102) | – | – |
| No PI (n = 195) | 64 (86) | –8 (–28 to 11) | 68 (92) | –63 (–84 to –41) | –54 (–83 to –25) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–23 to 16) | 94 (92) | –37 (–58 to –16) | –33 (–61 to –4) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–27 to 17) | 103 (96) | –28 (–53 to –4) | –24 (–51 to 4) |
| 12-monthly PI (n = 255) | 80 (121) | 12 (–10 to 34) | 133 (87) | –1 (–24 to 22) | –13 (–40 to 14) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–10 to 34) | 175 (103) | 44 (19 to 68) | 31 (4 to 59) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 78 (128) | – | 115 (97) | – | – |
| No PI (n = 93) | 95 (167) | 21 (–28 to 71) | 58 (82) | –56 (–86 to –27) | –78 (–135 to –20) |
| 12-monthly PI (n = 92) | 112 (216) | 33 (–16 to 83) | 98 (81) | –13 (–45 to 18) | –47 (–106 to 13) |
| Personalised OHA | |||||
| No PI (n = 81) | 65 (148) | –14 (–67 to 39) | 89 (88) | –19 (–54 to 17) | –5 (–64 to 54) |
| 12-monthly PI (n = 83) | 108 (165) | 32 (–22 to 85) | 118 (91) | 15 (–22 to 51) | –17 (–78 to 43) |
| 6-monthly PI (n = 83) | 95 (170) | 18 (–35 to 71) | 141 (106) | 35 (–2 to 72) | 17 (–43 to 77) |
| Region | Mean (SD) costs, £a | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 74 (106) | – | 147 (104) | – | – |
| No PI (n = 288) | 75 (121) | 1 (–26 to 28) | 79 (98) | –67 (–85 to –49) | –68 (–101 to –36) |
| 12-monthly PI (n = 285) | 81 (140) | 8 (–18 to 35) | 115 (95) | –30 (–48 to –12) | –39 (–70 to –7) |
| Personalised OHA | |||||
| No PI (n = 333) | 109 (215) | 37 (–13 to 87) | 129 (104) | –17 (–37 to 3) | –54 (–104 to –4) |
| 12-monthly PI (n = 338) | 135 (190) | 66 (16 to 116) | 166 (96) | 19 (–2 to 39) | –48 (–100 to 5) |
| 6-monthly PI (n = 335) | 105 (177) | 34 (–5 to 72) | 207 (117) | 61 (40 to 82) | 28 (–15 to 70) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 159 (105) | – | – |
| No PI (n = 195) | 64 (86) | –9 (–40 to 23) | 88 (102) | –72 (–94 to –50) | –64 (–102 to –26) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–36 to 27) | 120 (98) | –39 (–62 to –17) | –35 (–74 to 3) |
| Personalised OHA | |||||
| No PI (n = 252) | 122 (231) | 52 (–12 to 116) | 138 (105) | –23 (–46 to 0) | –75 (–141 to –10) |
| 12-monthly PI (n = 255) | 143 (196) | 75 (11 to 140) | 177 (93) | 12 (–11 to 35) | –63 (–130 to 4) |
| 6-monthly PI (n = 252) | 106 (177) | 36 (–12 to 84) | 223 (113) | 62 (39 to 86) | 26 (–27 to 80) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 79 (130) | – | 120 (96) | – | – |
| No PI (n = 93) | 98 (170) | 23 (–28 to 73) | 62 (86) | –57 (–87 to –27) | –80 (–140 to –21) |
| 12-monthly PI (n = 92) | 112 (214) | 33 (–17 to 83) | 103 (85) | –13 (–43 to 17) | –46 (–104 to 12) |
| Personalised OHA | |||||
| No PI (n = 81) | 69 (152) | –9 (–65 to 47) | 103 (97) | –8 (–47 to 31) | 1 (–59 to 61) |
| 12-monthly PI (n = 83) | 111 (164) | 35 (–22 to 91) | 132 (98) | 26 (–13 to 65) | –9 (–70 to 52) |
| 6-monthly PI (n = 83) | 102 (177) | 25 (–32 to 82) | 158 (113) | 49 (11 to 87) | 24 (–37 to 85) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 69 (96) | – | 146 (105) | – | – |
| No PI (n = 288) | 68 (104) | 0 (–19 to 18) | 79 (98) | –66 (–85 to –48) | –66 (–91 to –40) |
| 12-monthly PI (n = 285) | 74 (119) | 7 (–12 to 25) | 115 (95) | –29 (–48 to –11) | –36 (–61 to –10) |
| Personalised OHA | |||||
| No PI (n = 333) | 59 (107) | –5 (–25 to 16) | 128 (105) | –17 (–38 to 5) | –12 (–37 to 13) |
| 12-monthly PI (n = 338) | 81 (124) | 18 (–3 to 38) | 166 (97) | 21 (0 to 42) | 3 (–21 to 28) |
| 6-monthly PI (n = 335) | 78 (124) | 15 (–6 to 35) | 207 (115) | 63 (41 to 85) | 49 (23 to 74) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 159 (107) | – | – |
| No PI (n = 195) | 64 (86) | –8 (–28 to 11) | 88 (102) | –71 (–94 to –49) | –63 (–92 to –34) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–23 to 16) | 120 (99) | –39 (–61 to –17) | –35 (–64 to –6) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–28 to 18) | 137 (106) | –23 (–49 to 3) | –18 (–47 to 10) |
| 12-monthly PI (n = 255) | 80 (121) | 12 (–11 to 35) | 178 (94) | 14 (–11 to 39) | 2 (–25 to 29) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–11 to 35) | 225 (110) | 64 (38 to 90) | 52 (24 to 80) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 63 (103) | – | 118 (97) | – | – |
| No PI (n = 93) | 76 (133) | 17 (–22 to 57) | 61 (85) | –56 (–87 to –26) | –73 (–123 to –24) |
| 12-monthly PI (n = 92) | 89 (173) | 27 (–13 to 67) | 104 (85) | –10 (–42 to 22) | –37 (–89 to 14) |
| Personalised OHA | |||||
| No PI (n = 81) | 52 (119) | –9 (–55 to 36) | 101 (97) | –8 (–47 to 30) | 1 (–50 to 52) |
| 12-monthly PI (n = 83) | 86 (132) | 27 (–19 to 73) | 132 (98) | 28 (–12 to 67) | 0 (–53 to 53) |
| 6-monthly PI (n = 83) | 76 (136) | 16 (–30 to 62) | 154 (113) | 48 (8 to 88) | 32 (–21 to 84) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 79 (117) | – | 146 (105) | – | – |
| No PI (n = 288) | 80 (136) | 2 (–21 to 25) | 79 (98) | –66 (–85 to –48) | –68 (–97 to –39) |
| 12-monthly PI (n = 285) | 89 (164) | 11 (–12 to 34) | 115 (95) | –29 (–48 to –11) | –40 (–69 to –10) |
| Personalised OHA | |||||
| No PI (n = 333) | 65 (125) | –7 (–31 to 17) | 128 (105) | –16 (–38 to 5) | –9 (–38 to 19) |
| 12-monthly PI (n = 338) | 92 (145) | 19 (–5 to 43) | 166 (97) | 21 (0 to 41) | 2 (–27 to 30) |
| 6-monthly PI (n = 335) | 87 (146) | 15 (–9 to 39) | 207 (115) | 63 (42 to 85) | 48 (20 to 77) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 159 (107) | – | – |
| No PI (n = 195) | 64 (86) | –8 (–28 to 11) | 88 (102) | –71 (–94 to –49) | –63 (–92 to –34) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–23 to 16) | 120 (99) | –39 (–61 to –17) | –35 (–64 to –6) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–28 to 18) | 137 (106) | –23 (–49 to 3) | –18 (–47 to 10) |
| 12-monthly PI (n = 255) | 80 (121) | 12 (–11 to 35) | 178 (94) | 14 (–11 to 39) | 2 (–25 to 29) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–11 to 35) | 225 (110) | 64 (38 to 90) | 52 (24 to 80) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 94 (154) | – | 118 (97) | – | – |
| No PI (n = 93) | 114 (200) | 26 (–34 to 85) | 61 (85) | –57 (–87 to –26) | –82 (–148 to –16) |
| 12-monthly PI (n = 92) | 134 (259) | 40 (–19 to 100) | 104 (85) | –10 (–42 to 22) | –51 (–119 to 17) |
| Personalised OHA | |||||
| No PI (n = 81) | 78 (178) | –16 (–80 to 49) | 101 (97) | –9 (–49 to 31) | 7 (–61 to 74) |
| 12-monthly PI (n = 83) | 130 (198) | 39 (–27 to 105) | 132 (98) | 27 (–14 to 69) | –12 (–81 to 58) |
| 6-monthly PI (n = 83) | 114 (204) | 22 (–43 to 88) | 154 (113) | 47 (6 to 89) | 25 (–44 to 94) |
| Region | Mean (SD) costs, £ | Mean difference in costs (95% CI) vs. routine OHA (6-monthly PI), £ | Mean (SD) benefits, £ | Mean difference in benefits (95% CI) vs. routine OHA (6-monthly PI), £ | Incremental net benefits (95% CI) vs. routine OHA (6-monthly PI), £ |
|---|---|---|---|---|---|
| UK | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 289) | 74 (105) | – | 177 (92) | – | – |
| No PI (n = 288) | 74 (118) | 1 (–20 to 21) | 113 (85) | –64 (–79 to –49) | –64 (–89 to –39) |
| 12-monthly PI (n = 285) | 81 (141) | 9 (–12 to 29) | 148 (78) | –28 (–43 to –13) | –37 (–61 to –12) |
| Personalised OHA | |||||
| No PI (n = 333) | 62 (116) | –6 (–28 to 17) | 166 (84) | –11 (–29 to 7) | –5 (–29 to 18) |
| 12-monthly PI (n = 338) | 86 (133) | 18 (–4 to 40) | 203 (78) | 26 (8 to 44) | 7 (–16 to 31) |
| 6-monthly PI (n = 335) | 83 (134) | 15 (–8 to 37) | 235 (100) | 59 (40 to 77) | 44 (20 to 68) |
| Scotland | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 195) | 72 (93) | – | 190 (92) | – | – |
| No PI (n = 195) | 63 (86) | –9 (–28 to 11) | 123 (89) | –67 (–86 to –49) | –59 (–85 to –33) |
| 12-monthly PI (n = 195) | 67 (82) | –4 (–23 to 16) | 153 (82) | –38 (–56 to –20) | –34 (–59 to –8) |
| Personalised OHA | |||||
| No PI (n = 252) | 61 (103) | –5 (–27 to 17) | 173 (86) | –18 (–39 to 3) | –13 (–37 to 11) |
| 12-monthly PI (n = 255) | 79 (121) | 12 (–11 to 34) | 212 (75) | 18 (–3 to 40) | 7 (–18 to 31) |
| 6-monthly PI (n = 252) | 79 (120) | 12 (–10 to 34) | 253 (95) | 62 (41 to 84) | 50 (26 to 75) |
| England | |||||
| Routine OHA | |||||
| 6-monthly PI (n = 94) | 78 (127) | – | 150 (88) | – | – |
| No PI (n = 93) | 95 (165) | 21 (–28 to 70) | 93 (72) | –57 (–81 to –33) | –79 (–134 to –23) |
| 12-monthly PI (n = 92) | 112 (215) | 34 (–15 to 83) | 137 (68) | –10 (–34 to 13) | –45 (–99 to 10) |
| Personalised OHA | |||||
| No PI (n = 81) | 66 (150) | –12 (–68 to 44) | 142 (73) | –1 (–36 to 35) | 11 (–45 to 68) |
| 12-monthly PI (n = 83) | 108 (162) | 32 (–24 to 89) | 176 (81) | 36 (0 to 71) | 3 (–53 to 60) |
| 6-monthly PI (n = 83) | 95 (170) | 19 (–37 to 75) | 180 (94) | 38 (3 to 74) | 19 (–37 to 76) |
Appendix 3 Recruitment
FIGURE 20.
Target vs. actual practice accrual.

FIGURE 21.
Target vs. actual participant accrual. Note: a delay to staff appointments (research hygienists and dental research nurses) resulted in a delay to the start of recruitment of dental practices and participants against the initial projected target accrual.
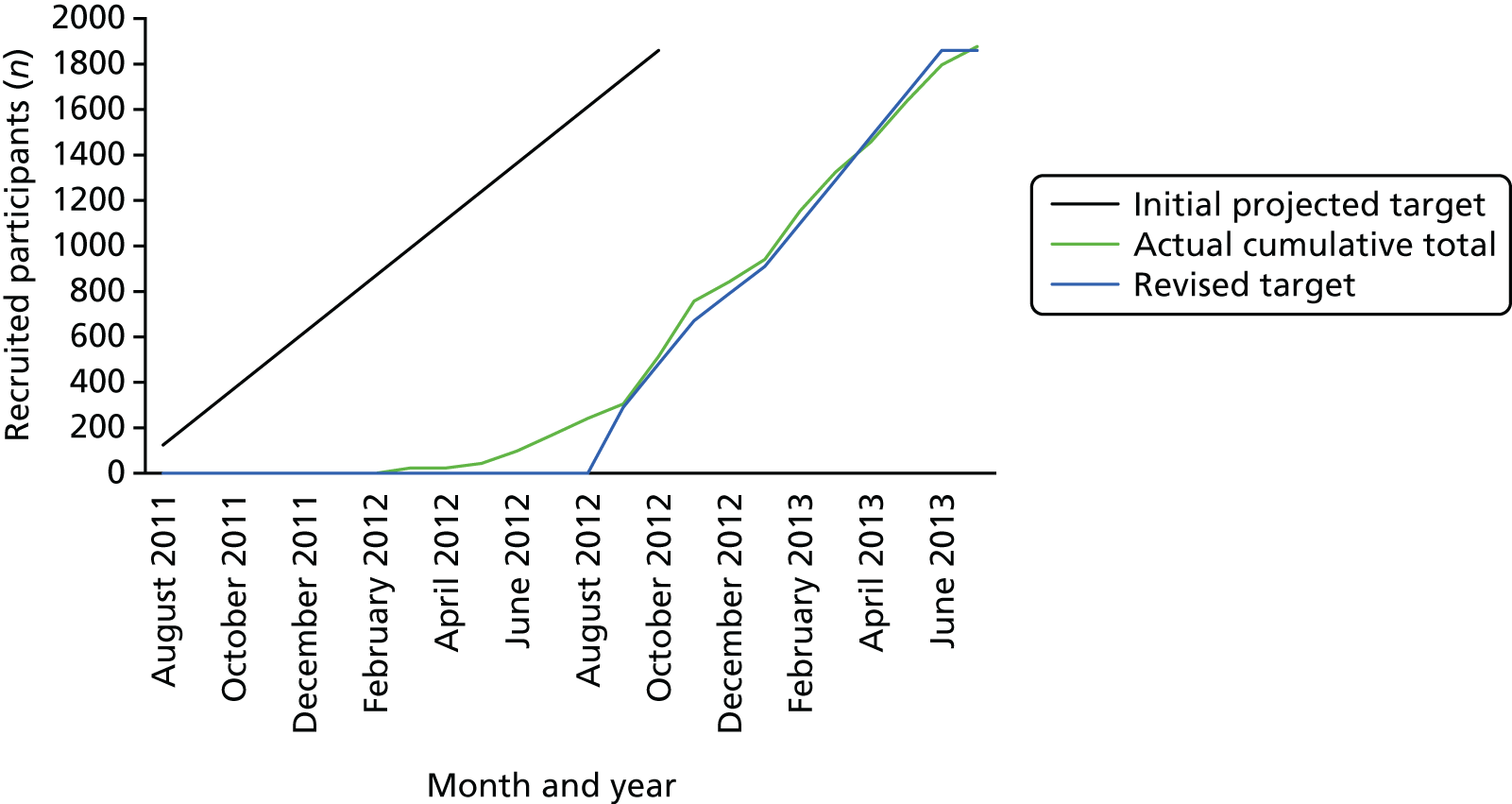
| Centre | OHA | Randomisation date | ||
|---|---|---|---|---|
| Personalised | Routine | First participant | Last participant | |
| Scotland | ||||
| Dental Care Glenrothes | – | 31 | 2 March 2012 | 18 May 2012 |
| Discovery Dental Care | 33 | – | 17 July 2012 | 28 September 2012 |
| Mullins Dental Practice | 35 | – | 11 June 2012 | 15 June 2012 |
| Laurencekirk Dental Practice | – | 13 | 17 May 2012 | 30 May 2012 |
| Woodside Dental Practice | – | 32 | 19 July 2012 | 16 August 2012 |
| Baillieston Dental Care | 31 | – | 19 June 2012 | 24 October 2012 |
| P A Penney Dental Practice | – | 14 | 26 July 2012 | 20 September 2012 |
| Wishaw Cross Dental Care | 31 | – | 6 August 2012 | 18 October 2012 |
| Clyde Dental | – | 23 | 18 July 2012 | 23 October 2012 |
| S. Rankin | 31 | – | 1 October 2012 | 5 December 2012 |
| Barrhead Dental practice | 29 | – | 13 August 2012 | 10 September 2012 |
| The Hollies Dental Practice | – | 39 | 29 October 2012 | 1 November 2012 |
| Chong Kwan and Associates | 32 | – | 5 November 2012 | 9 November 2012 |
| Duns Dental Practice | – | 35 | 9 October 2012 | 2 November 2012 |
| Clark and Watson | – | 33 | 26 September 2012 | 14 November 2012 |
| Dental Care Perth | – | 18 | 23 October 2012 | 25 February 2013 |
| Dental Plus | 31 | – | 3 October 2012 | 20 December 2012 |
| Stirling Dental Care | 31 | – | 5 November 2012 | 25 March 2013 |
| West End Dental Practice | 32 | – | 15 November 2012 | 13 December 2012 |
| JL Barrack Dental Practice | – | 31 | 31 October 2012 | 24 January 2013 |
| Selkirk | 34 | – | 22 October 2012 | 9 November 2012 |
| Bridge of Don Dental Practice | – | 38 | 19 November 2012 | 10 December 2012 |
| Ardmillan Dental Practice | – | 34 | 11 December 2012 | 22 January 2013 |
| Drumbrae Dental Surgery | 16 | – | 13 November 2012 | 11 January 2013 |
| Pickering Dental Care Ltd | – | 25 | 5 December 2012 | 6 May 2013 |
| Karolak and Iwanowicz | – | 22 | 14 January 2013 | 15 April 2013 |
| City Health Clinic | – | 34 | 25 January 2013 | 23 May 2013 |
| Care Dental (Crieff) | 31 | – | 7 January 2013 | 3 June 2013 |
| Dunbar Dental Practice | 43 | – | 4 February 2013 | 8 February 2013 |
| Long and Gilmour Dental Care | 22 | – | 11 March 2013 | 25 June 2013 |
| Riverview Dental Practice | – | 30 | 10 April 2013 | 25 June 2013 |
| Montgomery Street Dental | 32 | – | 29 March 2013 | 22 May 2013 |
| Invergowrie Dental | 32 | – | 17 January 2013 | 15 March 2013 |
| East Neuk Dental | – | 30 | 25 January 2013 | 12 April 2013 |
| Mastrick Dental | 32 | – | 12 February 2013 | 3 April 2013 |
| Birch Valley Dental Clinic | 34 | – | 19 February 2013 | 21 February 2013 |
| Whitburn Dental Practice | – | 34 | 27 March 2013 | 19 June 2013 |
| K H Stirling | 29 | – | 21 March 2013 | 16 May 2013 |
| Bute Dental Practice | – | 34 | 27 May 2013 | 31 May 2013 |
| Peacocks Dental Surgery | 22 | – | 20 May 2013 | 24 May 2013 |
| St Leonards | 32 | – | 1 May 2013 | 3 May 2013 |
| Lochboisdale, South Uist | 41 | – | 1 July 2013 | 5 July 2013 |
| King Street, Orkney | 46 | – | 10 June 2013 | 14 June 2013 |
| D A Gilchrist | – | 36 | 26 June 2013 | 3 July 2013 |
| England | ||||
| Mr A I Robson and Associates | 31 | – | 6 September 2012 | 18 October 2012 |
| Mr S B Pabary and Associates | 31 | – | 13 November 2012 | 30 April 2013 |
| Sunderland Road Dental Practice | – | 31 | 12 September 2012 | 6 November 2012 |
| Framwellgate Dental Practice | 24 | – | 18 September 2012 | 5 February 2013 |
| Osborne Dental Practice | 26 | – | 12 June 2012 | 3 December 2012 |
| Hetton Dental | – | 24 | 15 November 2012 | 24 April 2013 |
| Shotley Bridge | 31 | – | 29 November 2012 | 11 December 2012 |
| Number One | – | 14 | 18 February 2013 | 4 July 2013 |
| Stuart Eaborn Dentist | 31 | – | 5 February 2013 | 23 April 2013 |
| Dean Road Dental Surgery | 33 | – | 10 January 2013 | 11 March 2013 |
| Smith and Smith | – | 33 | 28 February 2013 | 14 March 2013 |
| Kenton Lane | – | 31 | 15 April 2013 | 28 May 2013 |
| Whickham Village | 34 | – | 13 February 2013 | 28 March 2013 |
| Blaydon Dental | – | 32 | 5 March 2013 | 27 March 2013 |
| Gosforth Dental | – | 31 | 15 May 2013 | 5 June 2013 |
| Nepali Dental | – | 30 | 15 May 2013 | 4 July 2013 |
| Princes Street | – | 32 | 28 May 2013 | 27 June 2013 |
| Front Street, Consett | 7 | – | 5 June 2013 | 5 June 2013 |
| Silver Dental Practice | – | 23 | 20 June 2013 | 4 July 2013 |
List of abbreviations
- *
- furcation involvement
- ADHS
- Adult Dental Health Survey
- ASC
- alternative-specific constant
- BPE
- Basic Periodontal Examination
- BSA
- Business Services Authority
- CBA
- cost–benefit analysis
- CBQ
- clinician belief questionnaire
- CCA
- cost–consequences analysis
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- DCE
- discrete choice experiment
- DMC
- Data Monitoring Committee
- FG
- focus group
- GDS
- General Dental Service
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- IQuaD
- Improving the Quality of Dentistry
- ISD
- Information Services Division
- IT
- information technology
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- OA
- outcome assessor
- OHA
- oral hygiene advice
- OHIP-14
- Oral Health Impact Profile-14
- PBC
- perceived behaviour control
- PI
- periodontal instrumentation
- PMG
- Project Management Committee
- PPI
- patient and public involvement
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- TCOD
- Trial Coordinating Office in Dundee
- TMC
- Trial Management Committee
- TSC
- Trial Steering Committee
- UDA
- Unit of Dental Activity
- UNC
- University of North Carolina
- WTP
- willingness to pay
