Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/67. The contractual start date was in April 2016. The draft report began editorial review in November 2016 and was accepted for publication in July 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Hashim U Ahmed reports grants and personal fees from SonaCare Medical, Sophiris Bio Inc. and Galil Medical and grants from TROD Medical outside the submitted work. He conducts private practice in London for the diagnosis and treatment of patients with potential prostate cancer through insurance schemes and self-paying patients in addition to his NHS practice. Ahmed El-Shater Bosaily reports grants and non-financial support from University College London Hospitals/University College London Biomedical Research Centre, grants and non-financial support from The Royal Marsden NHS Foundation Trust and the Institute for Cancer Research Biomedical Research Centre and non-financial support from the Medical Research Council’s Clinical Trials Unit during the conduct of the study. Mark Emberton reports grants from Sophiris Bio Inc., grants from TROD Medical, other support from STEBA Biotech, grants and other support from SonaCare Medical and other support from Nuada Medical and London Urology Associates, outside the submitted work. Rita Faria reports grants from the National Institute for Health Research during the conduct of the study. Richard Graham Hindley reports payment as a Clinical Director from Nuada Medical during the period of recruitment. Alexander Kirkham reports other support from Nuada Medical outside the submitted work. Derek J Rosario reports personal fees from Ferring Pharmaceuticals Ltd and grants from Bayer Pharmaceuticals Division, outside the submitted work. Iqbal Shergill reports grants from Ipsen and Astellas Pharma Inc. and non-financial support from Boston Scientific and Olympus, outside the submitted work. Eldon Spackman reports personal fees from Astellas Pharma Canada, Inc., outside the submitted work. Mathias Winkler reports grants and personal fees from Zicom-Biobot (Singapore), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Rationale
There are approximately 40,000 new cases of prostate cancer annually in the UK. The reported incidence has doubled in the last 15 years, mainly as a result of the increased use of serum prostate-specific antigen (PSA) testing in healthy men. As a result, prostate cancer has become the most commonly diagnosed cancer in men. 1 Many, if not most, prostate cancers that are currently detected are clinically non-significant (CNS) (i.e. would have no clinical impact during the man’s remaining life expectancy). Although the UK has no formal population-based PSA screening programme, the rising numbers of cases in the UK are primarily the result of increased awareness and incidental PSA testing. The existing diagnostic pathway will, if left unchecked, result in a further rise in the number of CNS cases identified and in the associated costs and harms of treatment, without necessarily reducing the risk of dying from the disease.
This position is exemplified in two large randomised controlled trials (RCTs) of population-based prostate cancer screening using serum PSA testing compared with standard practice. First, a large RCT of prostate cancer screening in the USA2 showed no evidence of a survival benefit when comparing an annual screening strategy (with an approximately 85% rate of screening) with usual care, which included quite considerable rates of PSA testing (approximately 46–52%). Second, the European Randomized Study of Screening for Prostate Cancer3 showed a modest reduction in the risk of death from prostate cancer in men who were screened every 4 years, from 8.2% to 4.8% [risk ratio 0.8, 95% confidence interval (CI) 0.65 to 0.98] at 9 years’ follow-up. The number of participants needed to screen was 1410 and the number needed to treat was 48 to prevent one death from prostate cancer over a 10-year period. 4 This benefit was maintained up to 13 years’ follow-up. 5
Once diagnosed, the lack of benefit for radical treatment of CNS cancer compared with a strategy of monitoring has been exemplified in three RCTs. First, the Scandinavian Prostate Cancer Group – 4 (SPCG-4) trial6 randomised men who were clinically diagnosed (not from screening). Most of these men had clinically significant (CS) medium- to high-risk cancers and radical prostatectomy (RP) showed a survival benefit at long-term follow-up compared with watchful waiting (a less active form of surveillance than that used in the modern era). 6 Treatment conferred significant harms for patients. 7 Second, the Prostate Cancer Intervention versus Observation Trial (PIVOT)8 randomised men diagnosed early through PSA screening in the USA between watchful waiting and RP and found no cancer-specific survival benefit, although subgroup analyses showed that men with high-risk disease did survive longer with treatment and there was a possible benefit, albeit marginal, for those with intermediate-risk cancers. Most recently, the Prostate Testing for Cancer and Treatment (ProtecT) trial in the UK9 found that, in 1643 men with localised prostate cancer detected as a result of PSA-based screening, there was no statistically significant difference in prostate-specific or all-cause mortality at 10 years in men assigned to active surveillance, surgery or radiotherapy; however, rates of disease progression and metastasis were higher with active surveillance. Commentators were quick to voice their concern that overdiagnosis, and hence overtreatment and associated morbidity, would increase further if PSA screening was adopted more widely. 10,11 However, if a diagnostic method was available that was more specific to clinically significant prostate cancer, the beneficial effect of screening and subsequent treatment on mortality could be retained, but overdiagnosis and overtreatment would be minimised.
Current clinical pathway
At present, a man is judged to be at risk of harbouring prostate cancer if he has any of the following: a raised serum PSA level (present in the majority of cases), an abnormal digital rectal examination (DRE), a positive family history of prostate cancer or a specific ethnic risk profile. 12 The diagnostic pathway of interest to this work relates to men with signs and symptoms that are suspicious of prostate cancer who have been referred for confirmatory diagnostic testing. In these men, prostate cancer, if present, is typically localised. Men with signs and symptoms of locally advanced cancer or metastatic cancer are often managed in a different manner with staging whole-body scans and the institution of systemic therapy with or without confirmatory prostate biopsy.
Men in whom there is diagnostic uncertainty are currently advised to have a transrectal ultrasound (TRUS)-guided prostate biopsy. 13 Men with a negative TRUS-guided biopsy result are advised to consider multiparametric magnetic resonance imaging (mpMRI) (using T2- and diffusion-weighted imaging) to determine whether or not another biopsy is needed. Between 59,000 and 80,000 men have a TRUS-guided biopsy in the UK each year,14 although this is likely to be an underestimate considering that three to four TRUS-guided biopsies are usually carried out to diagnose one man with cancer.
Men diagnosed with localised prostate cancer in England are managed according to their risk of progression (Table 1). The risk of progression is assessed on the basis of their serum PSA concentration, Gleason score and clinical stage. PSA is a protein produced by the prostate gland; an elevated PSA level is a sign that cancer may be present. The Gleason pattern classifies prostate cells obtained in the TRUS-guided biopsy by level of differentiation, which is related to the degree of aggressiveness of prostate cancer. Cancer cells can be classified from Gleason grade 1 (well differentiated and lower risk) to Gleason grade 5 (poorly differentiated and higher risk). 13 The Gleason score is the sum of the grades of the two most common types of cells. For example, if the man’s most common cells are Gleason grade 3 and Gleason grade 4, the Gleason pattern is 3 + 4 = 7. The clinical stage is assessed by the urologist by DRE with or without imaging scans, although the majority of men diagnosed with prostate cancer also undergo pelvic and prostate magnetic resonance imaging (MRI) after the biopsy.
| Risk | PSA level (ng/ml) | Gleason score | Stage | ||
|---|---|---|---|---|---|
| Low | < 10 | AND | ≤ 6 | AND | T1–T2a |
| Intermediate | 10–20 | OR | 7 | OR | T2b |
| High | > 20 | OR | 8–10 | OR | ≥ T2c |
The 2014 National Institute for Health and Care Excellence (NICE) clinical guideline13 recommends that men with low-risk prostate cancer are offered active surveillance, as described in Table 2. Men with intermediate- or high-risk cancer should be offered active treatment. In men with intermediate-risk cancer, the 2014 NICE clinical guideline recommends RP or radical radiotherapy but considers active surveillance or high-dose-rate brachytherapy with external-beam radiotherapy as possible options. Men with high-risk cancer should be offered RP, radical radiotherapy or high-dose-rate brachytherapy with external-beam radiotherapy. Interestingly, the NICE guidance also recommends prostate MRI as a baseline test at the commencement of active surveillance. Under the current NICE guidance and clinical practice, therefore, almost all men who have a TRUS-guided biopsy are recommended to undergo MRI after biopsy.
| Timing | Testsa | Frequency |
|---|---|---|
| Enrolment in active surveillance | mpMRI if not previously carried out | Not applicable |
| Year 1 | PSA level | Every 3–4 monthsb |
| PSA kinetics | Throughout active surveillancec | |
| DRE | Every 6–12 monthsd | |
| Prostate rebiopsy | At 12 months | |
| Year 2 and beyond | PSA level | Every 3–6 monthsb |
| PSA kinetics | Throughout active surveillancec | |
| DRE | Every 6–12 monthsd |
The current clinical pathway and potential for imaging
Men undergo prostate biopsy in the absence of accurate imaging that can visualise a suspicious lesion, as ultrasonography is used to identify the prostate, not the suspect lesion. The result is that biopsies are taken ‘blindly’ from areas of the gland. Although protocols stipulate that the biopsies should sample certain regions in a fanlike manner, studies have shown that this is often not the case and that the biopsies are clustered. 15 This results in a random, rather than systematic, deployment of the needle. This approach (Figure 1) contrasts markedly with that used for other cancers, in which the physician either visualises (e.g. using endoscopy) or images (e.g. using mammography) a suspect lesion in order to guide a biopsy needle to it.
FIGURE 1.
Current and proposed diagnostic pathway for prostate cancer. Note that the proposed future pathway is not the pathway taken by patients in the Prostate Magnetic Resonance Imaging Study. Reproduced from El-Shater Bosaily et al. 16 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
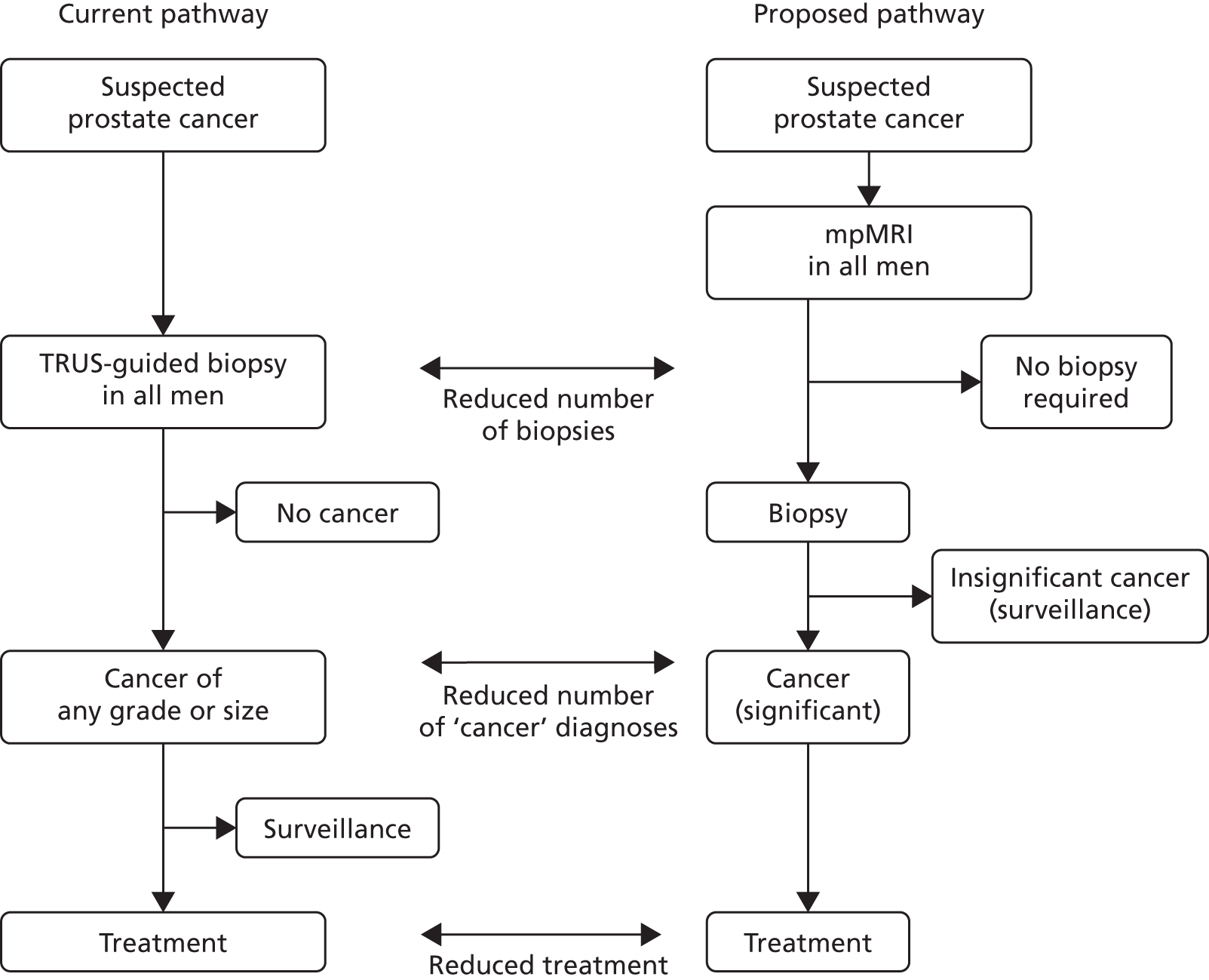
The use of mpMRI prior to TRUS-guided biopsy could offer several important advantages:
-
less overdiagnosis – in that fewer CNS prostate cancers are detected by avoiding unnecessary biopsy of men who do not have CS cancer
-
less overtreatment – as fewer CNS prostate cancers are detected
-
increased detection of CS prostate cancers – by directing biopsies to areas of the prostate that appear abnormal on mpMRI
-
improved characterisation of individual cancers – as a result of more representative biopsy sampling
-
reduced complications (including sepsis and bleeding) – as fewer men are biopsied and fewer biopsies are taken in men who are biopsied.
In addition, a revised diagnostic pathway based on the findings of the PROstate Magnetic resonance Imaging Study (PROMIS) also has the potential to offer a more cost-effective use of NHS resources. This is explored in the accompanying economic evaluation.
Limitations of transrectal ultrasound-guided biopsy
-
Overdetection of CNS prostate cancer: men who undergo TRUS-guided biopsy have a 25% chance of being diagnosed with prostate cancer. 17 This compares with a lifetime risk of 6–8% for symptomatic prostate cancer, illustrating the overdiagnosis of harmless cancers in men who undergo TRUS-guided biopsies (Figure 2). 18
-
Underdetection of CS prostate cancer: TRUS-guided biopsies have an estimated false-negative rate of 30–45%, although the true false-negative rate is unknown as few studies have applied a detailed reference test to those men who have a negative TRUS-guided biopsy. 19,20 Although the clinician takes 10–12 biopsies in a manner that attempts to obtain representative tissue within the peripheral zone (Figure 3, left-hand image), several parts of the gland are not well sampled (a systematic error). The anterior part of the gland may be missed as a result of its greater distance from the rectum (Figure 3, middle and right-hand image). Tissue in the midline is missed as a result of efforts to avoid the urethra, whereas the apex of the prostate is often difficult to access by the transrectal route.
-
Inaccurate risk stratification: TRUS-guided biopsies can be unrepresentative of the true burden of cancer as a result of random sampling error (Figure 4). Either the size or the grade of cancer may be underestimated if the cancer tissue obtained in a TRUS-guided biopsy is not representative. 21 Figure 4 illustrates how accurate estimation of tumour size will depend on hitting the centre of a lesion. At present, because these lesions are not visualised, this relies purely on chance. However, improved risk stratification is likely if MRI results can be used to guide deployment of needles in biopsies.
Equally, the pathological status derived from TRUS-guided biopsies can be unreliable if the test is reapplied, for instance in active surveillance, not only in discriminating clinically important cancer from clinically unimportant prostate cancer but also in attributing a non-cancer status from a cancer status, in approximately one-quarter of men subject to serial testing. 22
-
Side effects: TRUS-guided biopsy is associated with a number of complications, the most important being urinary tract infection (in 1–8% of cases), which can result in life-threatening sepsis (in 1–4% of cases). Haematuria (in 50% of cases), haematospermia (in 30% of cases), pain/discomfort (in most cases), dysuria (in most cases) and urinary retention (in 1% of cases) can also be expected. 23–27
FIGURE 2.
Overdetection of CNS prostate cancer.

FIGURE 3.
Underdetection of CS prostate cancer.

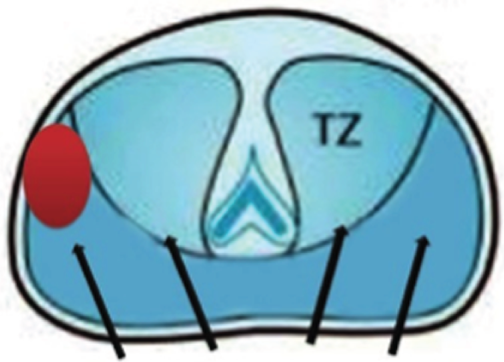

FIGURE 4.
Inaccurate risk stratification.

Multiparametric magnetic resonance imaging in diagnosing prostate cancer
The available evidence suggests that MRI can achieve both sensitivity and specificity of between 70% and 90% for the detection of CS prostate cancer. 28 However, a systematic review of the literature29 found the quality of the initial studies evaluating MRI to be disappointing. 30 They repeatedly showed low sensitivity and specificity as well as high interobserver variability, even when using high-resolution endorectal MRI. 31–37 Much has changed since these early reports, including an appreciation of the impact of post-biopsy changes on MRI; technological improvements such as increasing magnetic field strength (from 0.5 T to 1.5 T and 3.0 T); shorter pulse sequences enabling faster image acquisition; and the introduction of functional imaging in the form of diffusion weighting (DW) and dynamic contrast enhancement (DCE).
The main types of magnetic resonance images available are those produced by T2 weighting, DW and DCE. Multiparametric approaches (mpMRI, combining these three sequences together) have also been investigated. 38–48 Although their samples are small, single-centre case series have found an advantage of using two or three magnetic resonance sequences rather than just one. A recent systematic review49 incorporating studies that reported after PROMIS started has demonstrated the following ranges in accuracy metrics in studies using mapping biopsies and radical whole-mount prostatectomy specimens (often in retrospective case series): sensitivity, 73–95%; specificity, 28–87%; positive predictive value (PPV), 34–93%; and negative predictive value (NPV), 3–98%. However, no studies prior to PROMIS have prospectively evaluated the clinical validity of mpMRI in the population of interest (men at risk of harbouring prostate cancer who have not had a prior prostate biopsy) against an accurate and appropriate reference standard within a multicentre study in a double-blinded fashion.
Limitations of the magnetic resonance imaging literature
There are important limitations of previous studies investigating the diagnostic accuracy of MRI for prostate cancer:
-
Biopsy artefact: studies mostly evaluate MRI after biopsy; however, the biopsy procedure can affect what is seen on MRI, which can result in an increase in false-positive or false-negative results.
-
Limited application: studies mostly evaluate only the peripheral zone of the prostate, ignoring up to one-third of prostate cancers.
-
Segmentation: when each region of interest is segmented to achieve a sufficient number of data sets, increasing the power of the analysis and accuracy by incorrectly treating each region of interest as independent.
-
Poor reference standard: most studies use RP, leading to selection bias as those undergoing surgery tend to have burdens of cancer that are distinct from men with an abnormal PSA level, and patients choosing other treatments can never be evaluated. 50 Co-registration of a magnetic resonance image to a RP specimen is challenging because of shrinkage (10–20%), distortion, tissue loss as a result of ‘trimming’ (10%), orientation and absent perfusion.
These deficiencies, which mean that there is a discernible lack of level 1 evidence on the accuracy of mpMRI, probably account for the limited acceptance of MRI in contemporary prostate cancer diagnostic pathways. 51
The optimal reference standard: template prostate mapping
Template prostate mapping (TPM) biopsy was selected as the reference test for this study as it meets the required specification for our defined population when using 5-mm sampling52 (Figure 5). TPM-biopsy:
-
Produces a histological map of the entire prostate in three dimensions. 52–55
-
Has an estimated sensitivity and a NPV in the order of 95% for CS cancers when assessed against RP. 56–58
-
Avoids selection bias because all men exposed to the index test can be exposed to the reference standard.
-
Has a similar side-effect profile to that of TRUS-guided biopsy, with three important differences. TPM-biopsy:
-
carries a significantly lower risk of urosepsis (< 0.5%), as the needles do not traverse the rectal mucosa23 (urosepsis is the most serious complication of TRUS-guided biopsy)
-
confers a higher risk of self-limiting failure to void urine (5%) as a result of greater gland swelling; the risk is 5% compared with 1–2% associated with TRUS-guided biopsy55,59,60
-
requires general or spinal anaesthesia.
-
FIGURE 5.
Illustration of a TPM-biopsy procedure. Reproduced from El-Shater Bosaily et al. 16 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Although the accuracy of TPM-biopsy is high in the diagnosis of prostate cancer, it is not currently recommended as standard practice as it requires general anaesthesia and theatre time and so cannot be used routinely in the tens of thousands of men undergoing TRUS-guided biopsy. 61
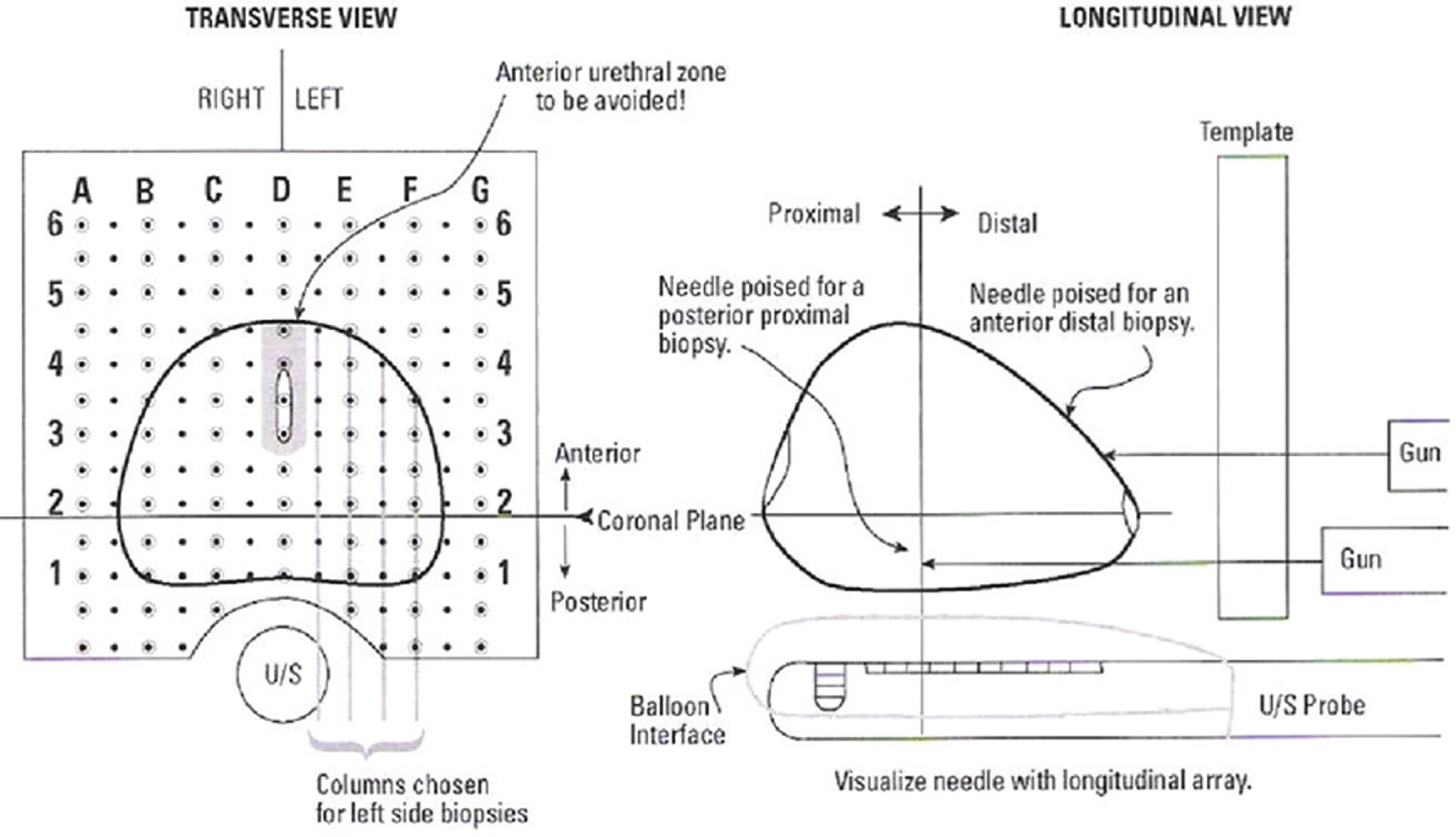
Template prostate mapping biopsy is carried out in accordance with a set protocol and so can be conducted blind to any clinical or imaging data. Mapping using 5-mm sampling is obtained using core needles inserted via a brachytherapy grid fixed on a stepper placed against the perineum with the patient under general anaesthesia in the lithotomy position. In most prostates, two biopsies at each grid point are required to sample the full craniocaudal gland length.
Aims and objectives
The purpose of PROMIS was to assess the clinical validity (sensitivity, specificity, PPV and NPV) of mpMRI for the detection of CS prostate cancer and compare these accuracy metrics with those of TRUS-guided biopsy.
In particular, PROMIS aimed to evaluate whether or not mpMRI improves the ability to detect, as well as rule out, CS prostate cancer in a group of men at risk of harbouring prostate cancer, who have not had a previous biopsy and who would normally be advised to undergo a prostate biopsy as part of standard care. PROMIS was designed to determine whether or not it is appropriate for men with a risk factor for harbouring CS cancer to first undergo mpMRI to select who should have a prostate biopsy and who might safely forgo a first prostate biopsy. In other words, we sought to determine whether or not mpMRI could be used as a triage test prior to a first biopsy (see Figure 1). 62
The main objectives of the trial were to:
-
Assess the ability of mpMRI to identify men who can safely avoid unnecessary biopsy.
-
Assess the ability of the mpMRI-based pathway to improve the rate of detection of CS cancers compared with TRUS-guided biopsy, by evaluating the diagnostic accuracy of both mpMRI (the index test) and TRUS-guided biopsy (standard test) against an accurate reference standard, TPM-biopsy.
-
Estimate the cost-effectiveness of a mpMRI-based diagnostic pathway. Using data from the main study and the wider literature, the study considered the implications of alternative diagnostic strategies for NHS costs and men’s quality-adjusted survival duration.
Chapter 2 Methods
Study design
This chapter is partially reproduced from El-Shater Bosaily et al. 16 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The protocol is also available at www.ctu.mrc.ac.uk/research/documents/cancer_protocols/promis_protocol_v4 (accessed 12 December 2017).
The PROMIS study was a prospective validating paired-cohort study representing level 1b evidence for diagnostic studies. 63 The population of interest was men at risk of prostate cancer who are usually recommended to undergo a first prostate biopsy within standard care. The study was conducted at 11 NHS hospitals in England. To compare the diagnostic accuracy of mpMRI (the index test) and TRUS-guided biopsy (the current standard), both must be individually compared with a reference standard, TPM-biopsy. Therefore, all participants in the study underwent all three tests (mpMRI, TPM-biopsy and TRUS-guided biopsy), with TPM-biopsy followed by TRUS-guided biopsy carried out as a combined biopsy procedure (CBP). The trial schema is shown in Figure 6. Each test was conducted blind to all of the other test results and reported independently of the other tests.
FIGURE 6.
Trial schema. Reproduced from El-Shater Bosaily et al. 16 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

A patient and public representative (Robert Oldroyd) was involved in the study, in all aspects relating to the design, the writing of the patient information and consent process, the conduct throughout the study as a member of the Trial Management Group, the critical review of the results and the dissemination of the results to both clinician and patient audiences. He contributed to the Trial Management Group deliberations on the study concept and design for the duration of the trial, at both face-to-face meetings and during teleconferences. As a lay member of the Nottingham 1 Research Ethics Committee (REC) from 2006 until 2015, he contributed expertise on ethics issues, including elements of the project proposal presented to the London–Hampstead REC, which he attended with Mark Emberton in March 2011. As a former prostate cancer patient, he voiced initial concerns about the patient volunteer burden in the study. He rewrote the patient information sheet (reducing the initial 28 pages to 14 pages) to make it more acceptable to the REC and revised the consent form. He commented fully on aspects of the study report, clarifying some sections to make them more accessible to lay readers. He spoke about PROMIS at the Nottingham Prostate Cancer UK conference in March 2017.
PROMIS was designed to overcome several shortcomings that are associated with other studies reported in the literature. These shortcomings prompted the following decisions to be made around the design of PROMIS:
-
All patients were biopsy naive and underwent mpMRI prior to any biopsy, so there was no biopsy artefact.
-
mpMRI was evaluated for all anatomical zones of the prostate including peripheral and transition zones.
-
The study was powered so that the primary outcome was derived using the whole prostate as the sector of analysis rather than segmented sectors of the prostate.
-
The study used an accurate reference test that can be applied to all men at risk.
The study was also designed to avoid or minimise a number of biases that are inherent in the current literature:
-
Spectrum and selection biases were avoided by recruiting men at risk of prostate cancer and applying all tests to all of the men.
-
Work-up bias was eliminated by ensuring that patients and clinicians remained blinded to all imaging test results until the biopsies were carried out and reported.
-
Reviewer/reporter bias was avoided by ensuring that the radiologist was blinded to the reference test and the pathologist was blinded to the imaging. The radiology report was submitted prior to the biopsies.
-
Incorporation bias was minimised by ensuring that TPM-biopsies and TRUS-guided biopsies followed a standard accepted protocol.
Patient eligibility
Patients were eligible for registration into the study if they fulfilled all of the inclusion criteria and none of the exclusion criteria (Box 1). Men with a PSA level of > 15 ng/ml were excluded as the higher prevalence of prostate cancer in this subgroup means that mpMRI is unlikely to be used as a triage test in these men. Men whose prostate volume on mpMRI was found to be > 100 ml were withdrawn from the study. Men were required to give written informed consent before participating.
-
Men aged ≥ 18 years who are at risk of prostate cancer and have been advised to have a prostate biopsy.
-
Serum PSA level of ≤ 15 ng/ml within the previous 3 months.
-
Suspected stage ≤ T2 on rectal examination (organ confined).
-
Fit for general/spinal anaesthesia.
-
Fit to undergo all protocol procedures including a TRUS-guided biopsy.
-
Signed informed consent.
-
Treated using 5-alpha-reductase inhibitors at time of registration or during the prior 6 months.
-
Previous history of prostate biopsy, prostate surgery or treatment for prostate cancer (interventions for benign prostatic hyperplasia/bladder outflow obstruction are acceptable).
-
Evidence of a urinary tract infection or history of acute prostatitis within the last 3 months.
-
Contraindication to MRI (e.g. claustrophobia, a pacemaker, an estimated glomerular filtration rate of ≤ 50 ml/minute).
-
Any other medical condition precluding procedures described in the protocol.
-
Contraindications for MRI (history of hip replacement surgery, metallic hip replacement or extensive pelvic orthopaedic metal work).
Reproduced from El-Shater Bosaily et al. 16 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Ethics considerations
The study was carried out in accordance with the principles of the Declaration of Helsinki64 and the UK Research Governance Framework version 265 and received UK REC approval on 16 March 2011 from the London–Hampstead REC. PROMIS has been registered on ClinicalTrials.gov (NCT01292291).
Test procedures
The index test: multiparametric magnetic resonance imaging
In PROMIS, mpMRI was standardised to the minimal requirements advised by a European consensus meeting,66 the European Society of Urogenital Radiology67 and the British Society of Urogenital Radiology guidelines. 68 T1-weighted, T2-weighted, diffusion-weighted [apparent diffusion coefficient (ADC) maps and long-b scan] and dynamic gadolinium contrast-enhanced imaging was acquired using a 1.5-T scanner and a pelvic phased array (Table 3).
| TR | TE | Flip angle, ° | Plane | Slice thickness [mm (gap)] | Matrix size | Field of view (mm) | Time from scan | |
|---|---|---|---|---|---|---|---|---|
| T2 TSE | 5170 | 92 | 180 | Axial, coronal, sagittal | 3 (10%) | 256 × 256 | 180 × 180 | 3 minutes 54 seconds (ax) |
| VIBE at multiple flip angles for T1 calculation (optional) | ||||||||
| VIBE fat sat | 5.61 | 2.52 | 15 | Axial | 3 | 192 × 192 | 260 × 260 | Continue for ≥ 5 minutes 30 seconds after contrast |
| Diffusion (b = 0, 150, 500, 1000) | 2200 | Minimum (< 98) | Axial | 5 | 172 × 172 | 260 × 260 | 5 minutes 44 seconds (16 averages) | |
| Diffusion (b = 1400) | 2200 | Minimum (< 98) | Axial | 5 | 172 × 172 | 320 × 320 | 3 minutes 39 seconds (32 averages) |
Endorectal coils were not used as there is no consensus on their role in minimal scanning requirements. 66 Magnetic resonance spectroscopy was not included because evidence from a large prospective multicentre study at the time showed no benefit of spectroscopy for localisation of prostate cancer compared with T2-weighted imaging alone. 69 We decided to use only 1.5-T scanners as these were more widely available in the UK health-care setting and most studies in the literature had reported the accuracy of mpMRI results based on 1.5-T scanners alone.
Quality assurance and reporting
A robust quality control process was used to maintain the quality of scans and ensure uniformity across all centres. All MRI scanners and individual mpMRI scans underwent quality control checks by an independent commercial imaging clinical research organisation appointed through open tender (IXICO plc, London, UK). Prior to site initiation, the lead radiologist (Alexander Kirkham) reviewed a number of prostate MRI scans from each centre and gave iterative feedback on improving scan quality.
During the study, scans deemed to be of insufficient quality were repeated prior to biopsy. In addition, a standardised operating procedure for mpMRI reporting was adopted in line with the recommendations of the European consensus meeting66 and the European Society of Urogenital-Radiology prostate MRI guidelines. 67 This was convened before publication of the more recent Prostate Imaging Reporting and Data System (PI-RADS) mpMRI reporting consensus. 67 Subsequent comparisons of the Likert and PI-RADS reporting schemes have yielded similar results. 70,71
At each centre, mpMRI scans were reported by dedicated urological radiologists who had undergone centralised training provided by the lead centre [University College London Hospital (UCLH)]. Radiologists were provided with clinical details including PSA levels, DRE findings and any other risk factors such as a family history of prostate cancer.
Images were reported in sequence, with T2-weighted images reported first, T2-weighted and diffusion-weighted images reported together and then a third report issued for T2-weighted with diffusion-weighted and dynamic contrast-enhanced scans together. The reporting form is shown in Figure 7. Future analyses will investigate whether or not both diffusion-weighted and dynamic contrast-enhanced images are required. As DCE requires a contrast agent (with its need for intravenous access, medical supervision and contrast-related risks) and an additional 10–15 minutes of scan time, it will be useful to determine whether or not this additional resource use and cost is necessary.
FIGURE 7.
Multiparametric magnetic resonance imaging reporting form.
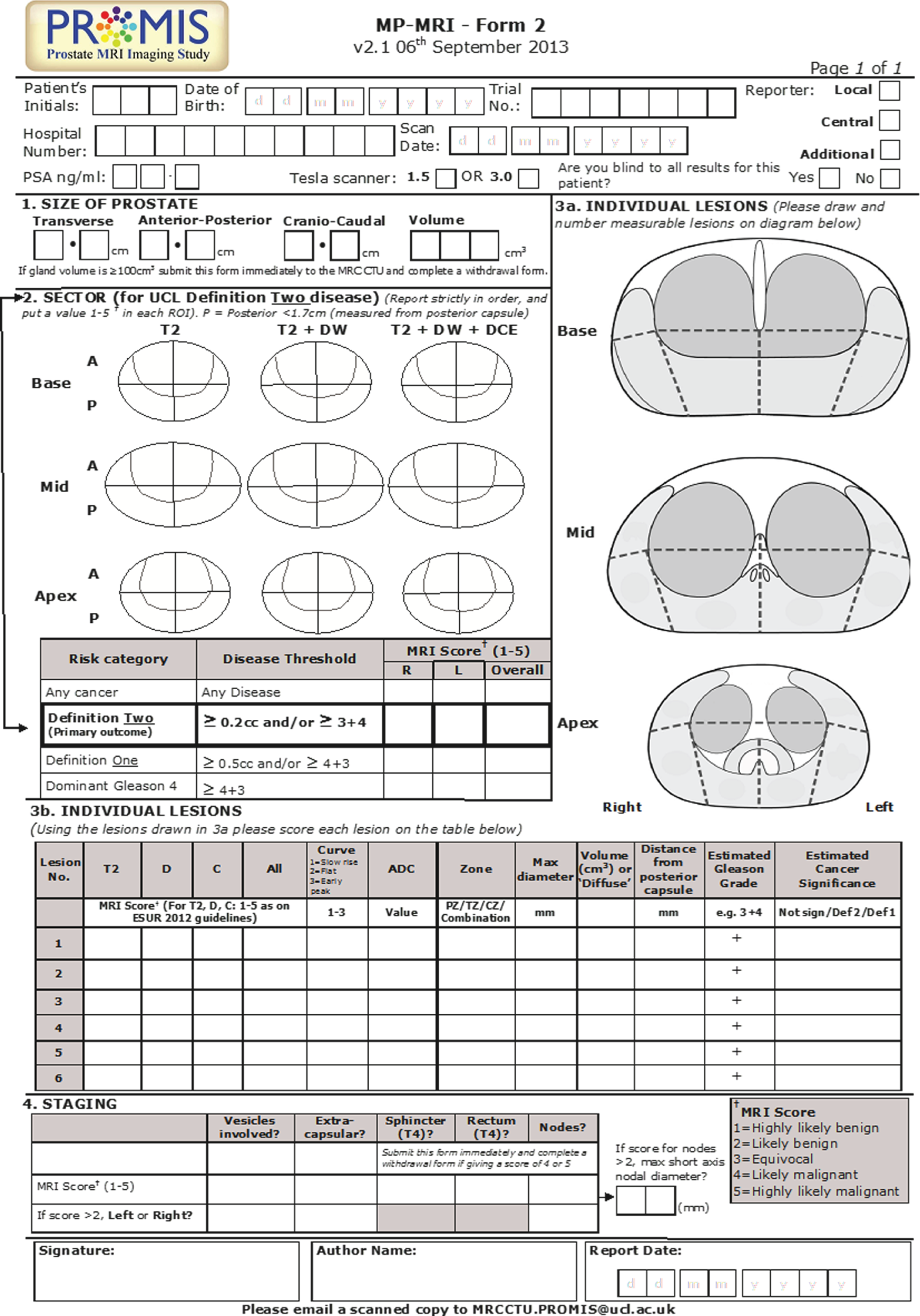
A five-point Likert scoring system66,67,72 was used to indicate the probability of cancer (1, highly likely to be benign; 2, likely to be benign; 3, equivocal; 4, likely to be malignant; 5, highly likely to be malignant). The prostate was divided into 12 regions of interest and each region was scored from 1 to 5. In addition, each lesion was identified and scored separately and the longest axial diameter, lesion volume, ADC value and contrast enhancement curve type were recorded. 73–76 From these observations, an overall score of 1–5 (using the same definitions) was assigned to the whole prostate. This was carried out for ‘any cancer’ and for definitions 1 and 2 of CS cancer (see Definitions of clinically significant prostate cancer).
For the primary outcome, an overall score of ≥ 3 was used to indicate a suspicious scan in relation to CS cancer (i.e. a positive mpMRI score). This reflects the level at which further tests (e.g. biopsy) would be considered if mpMRI were to be introduced into the diagnostic pathway in the future. To assess interobserver agreement, 132 scans from the lead site were rereported by a blinded second radiologist based at that site. The values used in the analyses were those from the original reporter.
The results of the mpMRI could be unblinded by the radiologist if the mpMRI revealed an enlarged prostate volume of > 100 ml, which would be impossible to sample every 5 mm because of the interference of the bony pelvic arch. This might have reduced the number of ‘negative’ prostate results in the final analyses. The mpMRI could also be unblinded if there was evidence of stage T4 prostate cancer or involved lymph nodes or colorectal/bladder invasion. This might have had a detrimental impact on the performance characteristic of mpMRI, as such tumours were more likely to be detected. The presence of other cancers such as bladder or colorectal cancers was also a criterion for withdrawal. Withdrawal was deemed appropriate in these men as expedited referral for biopsy and treatment was required. These withdrawals were unlikely to have an impact on the primary or secondary outcomes.
The standard test: transrectal ultrasound-guided biopsy
Transrectal ultrasound-guided biopsy of the prostate was carried out after the TPM-biopsy (see The reference test: transperineal template prostate mapping biopsy), under the same general/spinal anaesthesia. This was to ensure that the results for the reference test (i.e. TPM-biopsy) were obtained in an optimal fashion in a biopsy-naive gland that had not undergone swelling and distortion. It also theoretically minimised the risk of infection as the potential for faecal contamination was restricted to the end of the procedure. The surgeon carrying out the biopsy procedure was blind to the mpMRI results so that suspicious areas would not be targeted during the TRUS-guided biopsy and the fidelity of the TPM-biopsy reference test was maintained. TRUS-guided biopsies were taken as per international guidelines77 and incorporated 10–12 core biopsies. Each core was identified and potted separately. The TPM-biopsies and TRUS-guided biopsy sets from individual patients were sent to different pathologists to minimise review bias and work-up bias.
The reference test: transperineal template prostate mapping biopsy
Centres were selected for their prior experience in carrying out TPM-biopsies and training was provided to all centres to enable them to conduct TPM-biopsies in accordance with the PROMIS protocol.
Definitions of clinically significant prostate cancer
Disease significance was defined using criteria that have been previously developed and validated for use with TPM-biopsy for the detection of primary Gleason grades of ≥ 478 and of a cancer core length that is predictive of the presence of lesions of ≥ 0.5 ml. 79–82 Gleason scoring was based on the most frequent pattern and not on the highest grade detected on histological analysis.
The primary definition of CS prostate cancer used a histological target condition on TPM-biopsy that incorporated the presence of a Gleason score of ≥ 4 + 3 and/or a cancer core length of ≥ 6 mm of any Gleason score, in any location (definition 1). This was chosen as the primary outcome on the basis that few physicians would disagree that any man with this burden of cancer would require treatment. A secondary definition of CS disease was also used (a cancer core length of ≥ 4 mm and/or a Gleason score of ≥ 3 + 4; definition 2). A further definition of clinical significance (any Gleason pattern of ≥ 7) was added at the request of reviewers at The Lancet and this was treated as an exploratory definition (definition 3).
Monitoring of adverse events
The expected side effects of combining the TPM-biopsy and TRUS-guided biopsy procedures were discussed with patients before registration. The rate of serious adverse events (SAEs) was monitored weekly by an independent Trial Steering Committee.
Outcome measures
The primary outcome measures were the diagnostic accuracy of mpMRI, TRUS-guided biopsy and TPM-biopsy using the primary mpMRI and histology definitions, as measured by sensitivity, specificity, PPV and NPV. The same parameters using combinations of alternative definitions of disease for each test were investigated as secondary outcomes. The other outcomes reported were inter-rater agreement between tests and adverse events (AEs).
Magnetic resonance imaging outcome measures
For the primary outcomes, a MRI score of ≥ 3 to indicate a positive MRI result (primary analysis) was used. MRI scores of 1 or 2 were used to identify a group of men who might be able to avoid biopsy. Other reporting thresholds and radiological criteria were recorded and will form exploratory tertiary analyses in subsequent reports.
Translational research objectives
PROMIS was an ideal setting for assessing the utility of biomarkers (from urine and blood) to identify men with CS prostate cancer. It was, to our knowledge, the first time that a broad spectrum of men at risk have been evaluated using an optimal biopsy technique that accurately characterises the presence, size and grade of prostate cancer. A comprehensive bank of tissue samples (serum, plasma, germline DNA, urine) was collected from men prior to biopsy, to enable urinary and serum biomarkers to be analysed with respect to the detection of CS prostate cancer on TPM-biopsy. The results of these analyses will be reported at a later date.
Sample size
Power calculations were performed in relation to (1) the precision of the estimates for the accuracy of mpMRI relative to TPM-biopsy in terms of the primary definition of CS cancer, (2) a head-to-head comparison of mpMRI with TRUS-guided biopsy and (3) an assumed underlying prevalence of CS cancer of 15% according to the primary definition. 19,20,83,84 For the less stringent definition (definition 2), it was assumed that 25% of participants would have CS prostate cancer as detected by the reference standard.
The largest sample size obtained from the power calculations around (1) and (2) in the previous paragraph was 714 (see protocol16); this was considered to be the maximum number of men required to have all three tests (mpMRI, TPM-biopsy and TRUS-guided biopsy), based on the assumption that mpMRI and TRUS-guided biopsy are uncorrelated.
Assuming a specificity of 77%, in order to demonstrate that the lower 95% CI is ≥ 70%, we would require 407 cases of CNS prostate cancer. This is equivalent to a total of 479 men for definition 1 and 543 men for definition 2. Assuming a sensitivity of 75%, in order to demonstrate that the lower 95% CI for sensitivity is ≥ 60%, we would require 97 cases of CS prostate cancer. This is equivalent to a total of 647 men for definition 1 and 388 for definition 2. These estimates of sensitivity and specificity were considered realistic based on current unpublished and published literature. 85,86
It was assumed that TRUS-guided biopsy detects 48% of CS prostate cancers83,87 and that mpMRI would detect ≥ 70%; these were conservative estimates. Using McNemar’s test for paired binary observations,88 in order to show an absolute increase in the proportion of CS cancers detected of ≥ 22% (from 48% to 70%) with a power of 90% and a two-sided alpha of 5%, a total of 107 cases are required. This is equivalent to a total study population of 714 men for definition 1 and 428 men for definition 2. Table 4 shows the required sample size for McNemar’s test for different levels of agreement between mpMRI and TRUS-guided biopsy. The shaded regions reflect the scenario in which virtually all cancers are detected by either mpMRI or TRUS-guided biopsy, in which there is extremely low agreement between mpMRI and TRUS-guided biopsy. This is very unlikely but is included for completeness.
| mpMRI results | TRUS-guided biopsy result (for true cases)a | Required number of casesb | Required sample size | ||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | ||||||
| –ve | +ve | –ve | +ve | Prevalence of 15% (definition 1) | Prevalence of 25% (definition 2) | ||
| Sensitivity = 70% | 0.29 | 0.23 | 0.01 | 0.47 | 48 | 321 | 192 |
| 0.25 | 0.27 | 0.05 | 0.43 | 66 | 441 | 264 | |
| Independence assumptionc | 0.156 | 0.364 | 0.144 | 0.336 | 107 | 714 | 428 |
| 0.05 | 0.47 | 0.25 | 0.23 | 153 | 1021 | 612 | |
| 0.01 | 0.51 | 0.29 | 0.19 | 170 | 1134 | 680 | |
The independent Trial Steering Committee carried out an a priori interim review after 50 participants had received all three tests. Although a higher than anticipated prevalence of any cancer was observed, no changes to the target sample size were recommended.
Statistical analysis
All statistical analyses were carried out in accordance with a statistical analysis plan agreed before the data were inspected. Stata® version 13.0 (StataCorp LP, College Station, TX, USA) was used to carry out the analyses. The primary analysis was based on all evaluable data, excluding men without all three test results and any data that were rejected as part of the external mpMRI quality control/quality assurance process.
For each comparison, 2 × 2 contingency tables were used to present the results and calculate the diagnostic accuracy estimates with 95% CIs. Given the paired nature of the test results, McNemar’s tests were used for the head-to-head comparisons of sensitivity and specificity between mpMRI and TRUS-guided biopsy. Because the PPV and NPV are dependent on disease prevalence, a generalised estimating equation (GEE) logistic regression model was used to compare the PPV and NPV for mpMRI and TRUS-guided biopsy against those for TPM-biopsy. 89,90
The sensitivities, specificities and predictive values were calculated for mpMRI based on the overall radiological score for mpMRI [assigned to definition 2 on the mpMRI case report form (CRF)] and definition 1 for CS cancer on TPM-guided biopsy.
The format of the 2 × 2 table is shown in Table 5. Specificity = d/(c + d), where d = the number of men testing negative on mpMRI and negative for CS cancer on TPM-biopsy and c = the number of men testing positive on mpMRI and negative for CS cancer on TPM-biopsy. The NPV = d/(b + d), where d = the number of men testing negative on mpMRI and negative for CS cancer on TPM-biopsy and b = the number of men testing negative on mpMRI who test positive for CS cancer on TPM-biopsy. Sensitivity = a/(a + b), where a = the number of men testing positive on mpMRI and positive for CS on TPM-biopsy and b = the number of men testing negative on mpMRI who have CS cancer on TPM-biopsy. The PPV = a/(a + c), where a = the number of men testing positive on mpMRI and positive for CS on TPM-biopsy and c = the number of men testing positive on mpMRI who do not have CS cancer on TPM-biopsy.
| TPM-biopsy | mpMRI | ||
|---|---|---|---|
| +ve | –ve | Total | |
| +ve | a | b | a + b |
| –ve | c | d | c + d |
| Total | a + c | b + d | |
For the comparison of TRUS-guided biopsy and mpMRI, McNemar’s test was used to compare the agreement between mpMRI (radiological score of ≥ 3 assigned to definition 2 on the mpMRI CRF) and TRUS-guided biopsy (definition 1) in the subset of men found to have CS prostate cancer on TPM-biopsy according to definition 1. For the secondary analysis, all analyses that were carried out for definition 1 were repeated for definition 2 and, at the request of The Lancet reviewers, for any Gleason score of ≥ 7.
Exploratory analyses will be performed in the future to evaluate the impact on the sensitivity, specificity and predictive values of mpMRI when each of the 12 regions of interest are correlated between mpMRI and TPM-biopsy. Agreement between mpMRI and TPM-biopsy in identifying CS cancer in the same region will be based on a nearest neighbourhood approach. 91 Sensitivity to this approach will be tested by also presenting results according to complete match (the most stringent rule) and using a left/right rule (the less stringent rule). The sensitivity, specificity and predictive values of each of the individual MRI reporting sequence combinations, namely T2, T2 + DW and T2 + DW + DCE, will inform additional important tertiary analyses in subsequent publications.
Odds ratios (ORs) represent the odds of each test correctly detecting the presence or absence of disease. For specificity and NPV, the coding logic was reversed as the correct test result is a negative test result. Ratios are presented for TRUS-guided biopsy relative to mpMRI, so ratios of > 1 favour TRUS-guided biopsy and ratios of < 1 favour mpMRI.
Chapter 3 Results
The main results of PROMIS have been published by Ahmed et al. 92 Sections of this chapter have been reproduced from Ahmed et al. 92 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Recruitment and screening
A total of 740 participants were registered to the trial from 11 NHS hospitals in England. Recruitment took place between May 2012 and November 2015. Details of the screening and participating centres are given in Appendix 1.
Flow of participants through the study
Figure 8 shows the flow of participants through the study. Of the 740 men registered, 164 subsequently withdrew from the study before completing all three tests. Reasons for withdrawal are presented in Table 6. Most withdrawals took place before the combined biopsy was carried out; the most common reason for withdrawal was the discovery of a large prostate volume (> 100 ml), which was a mandatory withdrawal criterion. A total of 576 men were included in the final analysis. For the analysis set, the median (range) time between mpMRI and the combined biopsy was 38 days (1–190 days). The median (range) time between registration and the end-of-study visit was 111 days (31–421 days).
FIGURE 8.
Participant flow through the study. Reproduced from Ahmed et al. 92 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

| Reason | Timing of withdrawal | ||||
|---|---|---|---|---|---|
| Before MRI | Before CBP | During CBP | After CBP | Total | |
| Ineligible | 1 | 2 | 0 | 1 | 4 |
| Unblinded | 0 | 2 | 0 | 0 | 2 |
| Large prostate | 1 | 46 | 21 | 1 | 69 |
| Stage T4 or nodal disease | 0 | 5 | 0 | 0 | 5 |
| Clinical reasonsa | 5 | 15 | 0 | 1 | 21 |
| Did not want biopsy | 4 | 17 | 0 | 0 | 21 |
| Did not want to wait: sought private medical treatment | 1 | 4 | 0 | 0 | 5 |
| No longer wished to participate | 5 | 21 | 0 | 0 | 26 |
| Other | 0 | 10 | 0 | 1 | 11 |
| Total | 17 | 122 | 21 | 4 | 164 |
Baseline characteristics
Table 7 summarises the baseline characteristics for the 576 men who were included in the final analysis and the 164 men who withdrew after registration. The group of participants who withdrew had a similar age and PSA level to the included group, but were slightly less likely to report a family history of prostate cancer.
| Characteristic [missing data, n] | Men included in the final analysis (N = 576) | Withdrawals (N = 164) |
|---|---|---|
| Mean (SD) age, years [0] | 63.4 (7.6) | 64.5 (7.5) |
| Ethnicity, n (%) [1] | ||
| White | 502 (87) | 136 (83) |
| Mixed | 6 (1) | 2 (1) |
| Asian or Asian British | 16 (3) | 5 (3) |
| Black or black British | 39 (7) | 16 (10) |
| Other | 12 (2) | 4 (3) |
| Family history of prostate cancer, n (%) [7] | ||
| Yes | 127 (22) | 27 (17) |
| No | 442 (78) | 130 (83) |
| BMI (kg/m2), mean (SD) [62] | 27.8 (4.4) | 28.6 (5.2) |
| PSA level (ng/ml), mean (SD) [range] [0] | 7.1 (2.9) [0.5–15.0] | 7.1 (2.7) [1.0–14.7] |
Cancer prevalence by template prostate mapping biopsy
The prevalence of any cancer as assessed by the reference test, TPM-biopsy, was 71% (95% CI 67% to 75%). The prevalence of CS cancer according to the primary definition (a Gleason score of ≥ 4 + 3 and/or a cancer core length of ≥ 6 mm) was 40% (95% CI 36% to 44%). The prevalence of CS cancer according to the secondary definition (a Gleason score of ≥ 3 + 4 and/or a cancer core length of ≥ 4 mm) was 57% (95% CI 53% to 62%). Using the exploratory definition of any Gleason pattern of ≥ 7, the prevalence of CS cancer was 53% (308/576).
Cancer prevalence according to each definition as detected by TPM-biopsy is summarised in Table 8, together with the number of cancers that were identified to have perineural or lymphovascular involvement. A summary of the cancer grades according to TPM-biopsy pathology results is given in Appendix 1.
| Prevalence | TPM-biopsy (N = 576), n (%) [95% CI] |
|---|---|
| Any cancer | 408 (71) [67 to 75] |
| PNI | 156 |
| LVI | 3 |
| CS cancer, primary definition (Gleason score of ≥ 4 + 3 and/or cancer core length of ≥ 6 mm) | 230 (40) [36 to 44] |
| PNI | 133 |
| LVI | 3 |
| CS cancer, secondary definition (Gleason score of ≥ 3 + 4 and/or cancer core length of ≥ 4 mm) | 331 (57) [53 to 62] |
| PNI | 155 |
| LVI | 3 |
| CS cancer, exploratory definition (Gleason score of ≥ 7) | 308 (53) [49 to 58] |
| PNI | 152 |
| LVI | 3 |
Diagnostic accuracy of transrectal ultrasound-guided biopsy compared with template prostate mapping biopsy (primary outcome)
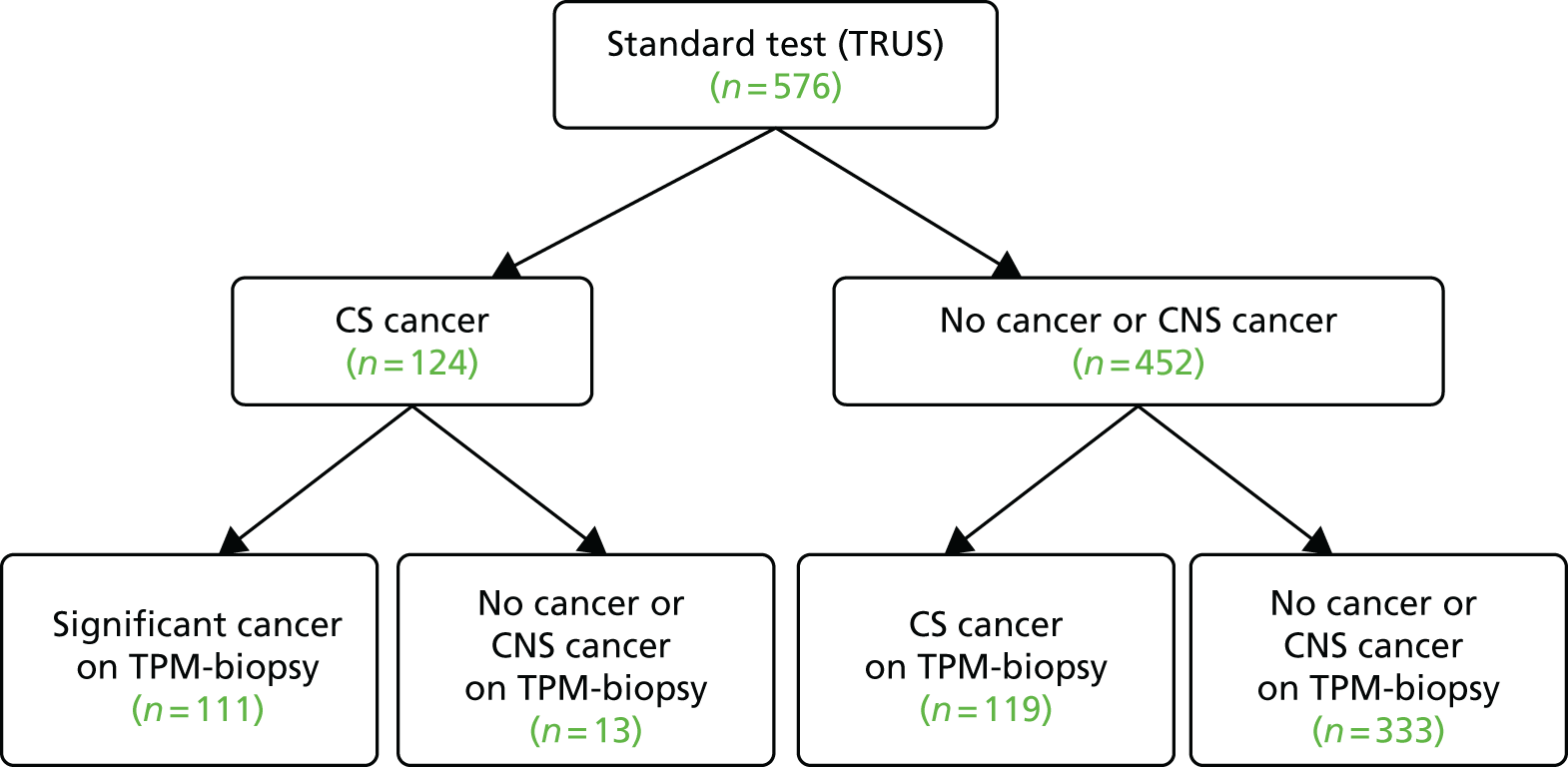
The diagnostic accuracy results for TRUS-guided biopsy compared with TPM-biopsy are shown in Table 9 and Figure 9. Cancer was detected in 286 out of 576 men (50%, 95% CI 45% to 54%), of whom 65 had perineural invasion and one had lymphovascular invasion. CS cancer according to the primary definition was detected in 124 out of 576 men (22%, 95% CI 18% to 25%), of whom 48 had perineural invasion and one had lymphovascular invasion.
| TRUS-guided biopsy | TPM-biopsy, n | ||
|---|---|---|---|
| CS cancer (+) | NC or CNS cancer (–) | Total | |
| CS cancer (+) | 111 | 13 | 124 |
| NC or CNS cancer (–) | 119 | 333 | 452 |
| Total | 230 | 346 | 576 |
FIGURE 9.
Diagnostic accuracy of the detection of CS cancer for TRUS-guided biopsy compared with TPM-biopsy.
A summary of the cancer grades according to TRUS-guided biopsy pathology results is given in Appendix 1. Appendix 1 also provides two case illustrations showing the correlation of TRUS-guided biopsy and mpMRI with TPM-biopsy, one for a man harbouring CS cancer and one for a man with no cancer.
Diagnostic accuracy of multiparametric magnetic resonance imaging compared with template prostate mapping biopsy (primary outcome)
Multiparametric magnetic resonance imaging results
In the group of 576 men included in the final analysis, the mean volume of the prostate was 48 ml [standard deviation (SD) 20 ml]. Six men had a prostate volume of > 100 ml as they had been entered into the trial before this threshold was adopted as an exclusion criterion mid-way through the study. The Trial Management Group decided that these six men should remain in the study as they had been fully sampled using TPM-biopsy.
The distribution of mpMRI scores is presented in Table 10. Agreement between mpMRI and TRUS-guided biopsy for the detection of CS cancer is shown in Appendix 1.
| Primary MRI score | Participants, n (%) |
|---|---|
| 1: highly likely benign | 23 (4) |
| 2: likely benign | 135 (23) |
| 3: equivocal | 163 (29) |
| 4: likely malignant | 120 (21) |
| 5: highly likely malignant | 135 (23) |
| Total | 576 (100) |
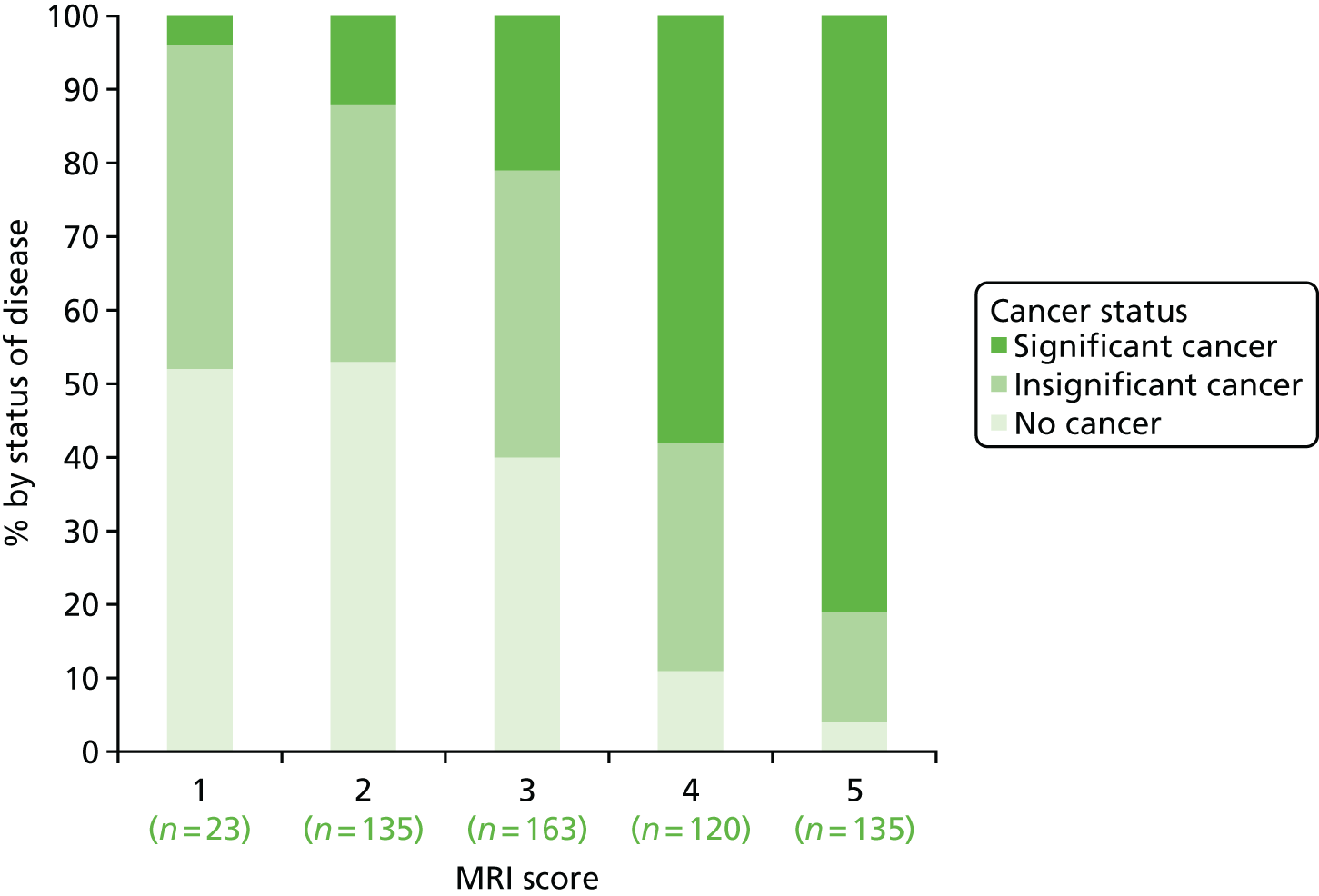
The diagnostic results for mpMRI compared with TPM-biopsy are shown in Table 11 and Figure 10. The sensitivity of mpMRI for CS cancer was 93% (95% CI 88% to 96%) and the NPV was 89% (95% CI 83% to 94%). The specificity of mpMRI was 41% (95% CI 36% to 46%), with PPV being 51% (95% CI 46% to 56%). The proportion of CS disease by mpMRI score is shown in Figure 11.
| mpMRI | TPM-biopsy, n | ||
|---|---|---|---|
| CS cancer (+) | NC or CNS cancer (–) | Total | |
| CS cancer (+) | 213 | 205 | 418 |
| NC or CNS cancer (–) | 17 | 141 | 158 |
| Total | 230 | 346 | 576 |
FIGURE 10.
Diagnostic accuracy of the detection of CS cancer for mpMRI compared with TPM-biopsy. Pie charts represent actual mpMRI scores from 1 to 5. Reproduced from Ahmed et al. 92 © The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

FIGURE 11.
Proportion of men with no cancer, insignificant cancer and significant cancer based on TPM-biopsy by mpMRI score. Reproduced from Ahmed et al. 92 © 2015 The Authors. Published by Elsevier Inc. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
A negative mpMRI result was recorded for 158 men (27%). Of these, 17 were found to have CS cancer on TPM-biopsy (see Table 15 and Figure 10). All 17 had Gleason grade 3 + ≤ 4, and core lengths of between 6 mm and 12 mm (Table 12). Of the 119 significant cancers missed by TRUS-guided biopsy, 13 had a Gleason score of 4 + 3, 99 had a Gleason score of 3 + 4 and 7 had a Gleason score of 3 + 3 (see Table 12).
| Gleason grade | Number of cases missed (range of maximum cancer core length, mm), by trial arm | |
|---|---|---|
| mpMRI (N = 17) | TRUS-guided biopsy (N = 119) | |
| 3 + 3 | 1 (8) | 7 (6–11) |
| 3 + 4 | 16 (6–12) | 99 (6–14) |
| 4 + 3 | 0 | 13 (3–16) |
Head-to-head comparison of the diagnostic accuracy of multiparametric magnetic resonance imaging and transrectal ultrasound-guided biopsy compared with template prostate mapping biopsy (primary outcome)
Multiparametric magnetic resonance imaging was more accurate than TRUS-guided biopsy in terms of both sensitivity (93% compared with 48%, respectively; McNemar’s test ratio 0.52, 95% CI 0.45 to 0.60) and NPV (89% compared with 74%, respectively; GEE model estimate for OR 0.34, 95% CI 0.21 to 0.5; p < 0.0001). A total of 119 significant cancers were missed by TRUS-guided biopsy, of which 13 had a Gleason score of 4 + 3, 99 had a Gleason score of 3 + 4 and 7 had a Gleason score of 3 + 3 (see Table 12). However, compared with mpMRI, TRUS-guided biopsy showed better specificity (41% compared with 96%, respectively; McNemar’s test ratio 2.34, 95% CI 2.08 to 2.68; p < 0.0001) and a better PPV (51% compared with 90%; GEE model estimate for OR 8.2, 95% CI 4.7 to 14.3; p < 0.0001].
Clinical implications of introducing multiparametric magnetic resonance imaging into the diagnostic pathway
We considered the implications of using mpMRI in clinical practice by comparing a strategy of an initial TRUS-guided biopsy for all men (standard care) with two alternative strategies. In both alternative strategies, mpMRI would be used as a triage test and only men with a suspicious mpMRI result (i.e. a Likert score of ≥ 3) would go on to have a biopsy (Table 13), with the remainder receiving active surveillance or being discharged. In the first alternative strategy – the worst-case scenario – a standard TRUS-guided biopsy would be carried out but mpMRI would not be used to direct needle deployment. The TRUS-guided biopsy results for each patient have been used to calculate overdiagnosis. In the second alternative strategy – the best-case scenario – the TRUS-guided biopsy needle deployment would be guided by the mpMRI findings and the results presented assume that such targeted biopsies would achieve similar diagnostic accuracy to that of TPM-biopsy. 93,94 We expect the actual scenario to lie somewhere between these best- and worst-case scenarios.
| Outcome | Strategy | ||
|---|---|---|---|
| TRUS-guided biopsy-only pathway | mpMRI triage followed by non-directed TRUS-guided biopsy (worst-case scenario)a | mpMRI triage followed by MRI-directed TRUS-guided biopsy (best-case scenario)b | |
| Primary biopsy carried out, n (%) [95% CI] | 576 (100) [99 to 100] | 418 (73) [69 to 76] | 418 (73) [69 to 76] |
| Overdiagnosis (CNS cancer detected), n (%) [95% CI] | 90 (16) [13 to 19] | 62 (11) [8 to 14] | 121 (21) [18 to 25] |
| Significant cancer correctly detected, n (%) [95% CI] | 111 (19) [16 to 23] | 105 (18) [15 to 22] | 213 (37) [33 to 41] |
For both of these scenarios, 158 out of 576 men (27%) might avoid a primary biopsy because they would have a non-suspicious mpMRI result with a low (1 in 10) probability of harbouring significant cancer. For the worst-case scenario, an absolute reduction in the overdiagnosis of CNS cancers might be seen, with 28 out of 576 (5%) fewer cases (relative reduction of 31%, 95% CI 22% to 42%) than with the current standard. However, in this worst-case scenario, important information on tumour location would not be used, resulting in the number of CS cancers that are detected being 1% lower than in standard care.
Under the best-case scenario, overdiagnosis might increase to 21% [i.e. there would be 31/576 (5%) more cases]. However, this figure is based on the probability of detecting CNS cancers on TPM-biopsy and, therefore, is an overestimation. Nonetheless, if the mpMRI information was used for biopsy deployment in this scenario, it might also lead to 102 out of 576 (18%) more cases of CS cancer being detected than in the standard pathway of TRUS-guided biopsy for all (see Table 13). We envisage that, in practice, the actual impact of including mpMRI in the pathway would lie somewhere between the best- and worst-case scenarios.
Diagnostic accuracy of transrectal ultrasound-guided biopsy and multiparametric magnetic resonance imaging for other definitions of clinically significant cancer
In total, 203 out of 576 cases (35%, 95% CI 31% to 39%) had CS cancer on TRUS-guided biopsy according to the secondary definition [University College London (UCL) definition 2: Gleason grade of ≥ 3 + 4 and/or any grade of cancer length of ≥ 4 mm]. Table 14 presents the definitions and prevalence according to TPM-biopsy. Of these 203 cases with UCL definition 2 disease, 63 had perineural invasion and one had lymphovascular invasion. For the exploratory definition (any Gleason pattern of ≥ 7), 151 out of 576 cases (26%, 95% CI 23% to 30%) had CS disease. Of these 151 cases with disease with a Gleason grade of ≥ 7, 54 had perineural invasion and one had lymphovascular invasion.
| TPM-biopsy definition of clinical significance | Prevalence of disease on TPM-biopsy, n (%) [95% CI] | Test attribute | mpMRI, % (95% CI) | TRUS-guided biopsy, % (95% CI) | Test ratioa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Secondary (Gleason score of ≥ 3 + 4 and/or cancer score lenght of ≥ 4 mm) | 331 (57) [53 to 62] | Sensitivity | 87 (83 to 90) | 60 (55 to 65) | 0.69 (0.64 to 0.76) | < 0.0001 |
| Specificity | 47 (40 to 53) | 98 (96 to 100) | 2.11 (1.85 to 2.41) | < 0.0001 | ||
| PPV | 69 (64 to 73) | 98 (95 to 100) | 22.7 (8.6 to 59.9) | < 0.0001 | ||
| NPV | 72 (65 to 79) | 65 (60 to 70) | 0.70 (0.52 to 0.96) | 0.025 | ||
| Exploratory [any Gleason pattern of ≥ 7 (≥ 3 + 4)] | 308 (53) [49 to 58] | Sensitivity | 88 (84 to 91) | 48 (43 to 54) | 0.55 (0.49 to 0.62) | < 0.0001 |
| Specificity | 45 (39 to 51) | 99 (97 to 100) | 2.22 (1.94 to 2.53) | < 0.0001 | ||
| PPV | 65 (60 to 69) | 99 (95 to 100) | 40.8 (10.2 to 162.8) | < 0.0001 | ||
| NPV | 76 (69 to 82) | 63 (58 to 67) | 0.53 (0.38 to 0.73) | < 0.0001 |
Diagnostic accuracy results for the secondary and exploratory definitions of CS cancer are shown in Table 14. Table 15 shows the histological characteristics of cancers missed by TRUS-guided biopsy and mpMRI for all histological definitions.
| Definition of clinical significance | Missed cases, n | |
|---|---|---|
| mpMRI | TRUS-guided biopsy | |
| Primary (N = 230) | 17
|
119
|
| Secondary (N = 331) | 44
|
132
|
| Exploratory (Gleason score of ≥ 7) (N = 308) | 38
|
159
|
Interobserver and intraobserver variability in the reporting of multiparametric magnetic resonance imaging scores
Blinded, double reporting was available for the mpMRI scans of 132 men. For this group, agreement between radiologists in the detection of CS cancer according to the primary definition was 80% (Table 16). This corresponded to a Cohen’s kappa statistic of 0.5 [moderate agreement according to the Landis and Koch classification,95 in which agreement is graded as excellent (κ ≥ 0.80), good (κ = 0.60–0.79), moderate (κ = 0.40–0.59), poor (κ = 0.20–0.39) or very poor (κ < 0.20)]. Cohen’s kappa statistics indicate how much better the agreement is over the agreement that would have occurred by chance (the expected agreement). We propose to carry out further analyses on interobserver and intraobserver variability and all of the mpMRI scans have been archived for future training and quality assurance purposes.
| Radiologist 1 | Number of cases by mpMRI score | |||||
|---|---|---|---|---|---|---|
| Radiologist 2 | ||||||
| 1 | 2 | 3 | 4 | 5 | Total | |
| 1 | 0 | 4 | 1 | 0 | 0 | 5 |
| 2 | 0 | 19 | 15 | 0 | 0 | 34 |
| 3 | 0 | 9 | 33 | 5 | 0 | 47 |
| 4 | 0 | 1 | 10 | 7 | 5 | 23 |
| 5 | 0 | 0 | 0 | 1 | 22 | 23 |
| Total | 0 | 33 | 59 | 13 | 27 | 132 |
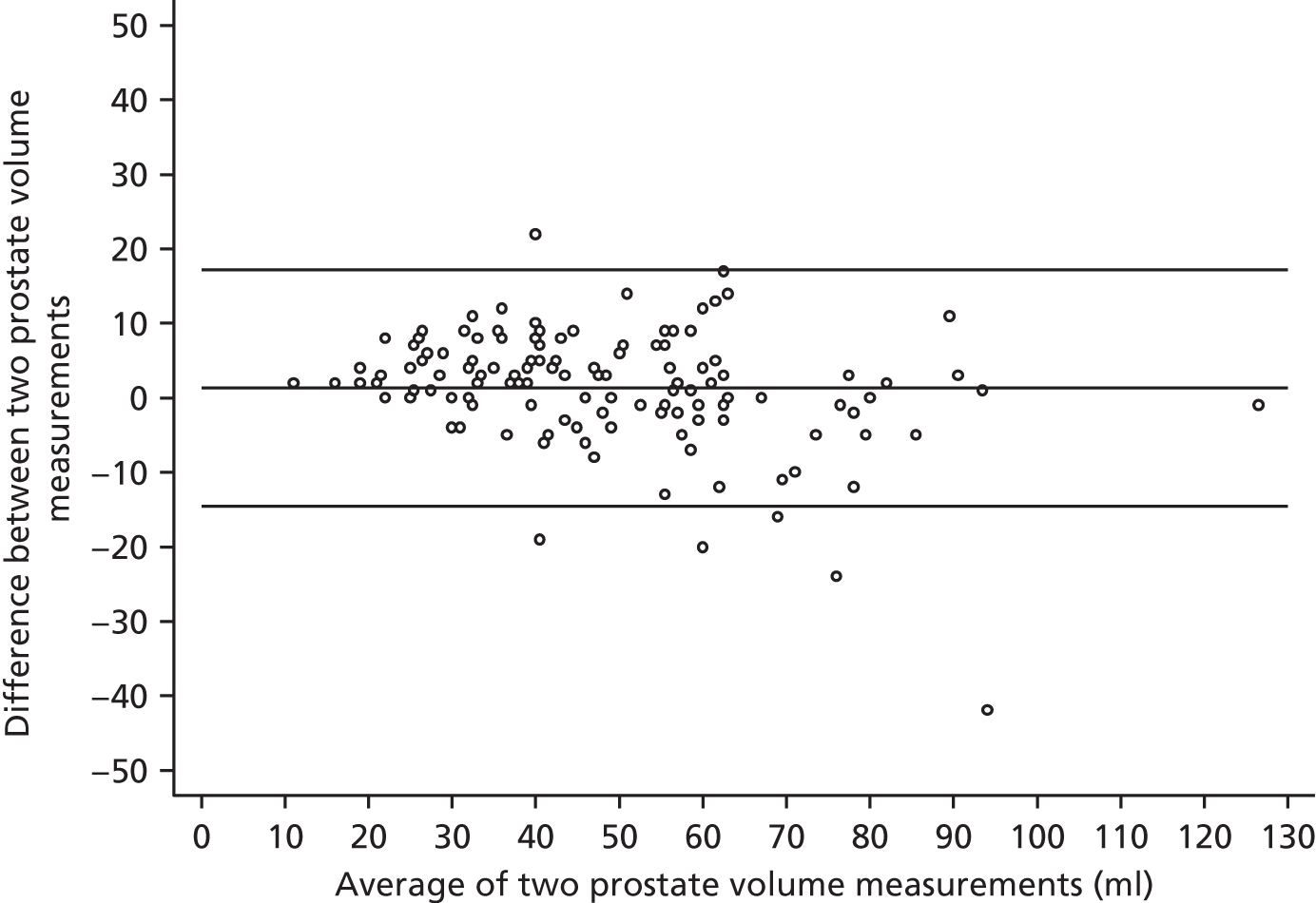
Agreement between radiologists for prostate volume measurements by mpMRI is shown in Figure 12, using the Bland–Altman method, which plots the difference between measurements against the average of the measurements. The average prostate volume was 48 ml. Overall, the mean difference between volumes was +1.3 ml (95% CI –0.04 ml to +2.69 ml]. The plot indicates that the interobserver reproducibility between radiologists for prostate volume measurement was approximately ±15 ml. There was a tendency for poorer agreement with larger volumes and this association was significant according to Pitman’s test, as well as simple linear regression.
FIGURE 12.
Bland–Altman plot for volume measurements between two radiologists.
There was no difference in diagnostic accuracy between the lead radiology site (UCLH, responsible for training all other sites) and the other sites (see Appendix 1 for comparison).
Health-related quality of life
Participants filled in EuroQol-5 Dimensions, three-level version (EQ-5D-3L) questionnaires at enrolment, after undergoing mpMRI, after undergoing the CBP and at the end of the study, which was, on average, 42 days after the CBP. As part of the economic evaluation, the EQ-5D-3L profiles were converted into preference-based index scores using the UK tariff. 96 The index scores after each test were compared with the values at enrolment. There was no evidence of a change in index score between post mpMRI and baseline (change = 0.008, 95% CI –0.002 to 0.018). However, there was a large and statistically significant negative impact after the CBP, with a change of –0.176 (95% CI –0.203 to –0.149). The health-related quality of life (HRQoL) implications of the various diagnostic strategies are explored further in the economic evaluation (see Chapters 4–8).
Safety
This section summarises the safety information for the CBP, which involved TPM-biopsy followed by TRUS-guided biopsy under the same anaesthesia. The CBP was carried out for the purposes of the study only; it is important to note that the CBP would not be required in routine clinical practice under any protocols developed as a result of PROMIS.
Risk of sepsis
There were nine cases of sepsis after the CBP during the study. There was one case of sepsis prior to the CBP. This equates to a post-CBP risk of sepsis of 9 out of 601 (1.5%, 95% CI 0.7% to 2.8%).
Risk of serious adverse events and side effects
A SAE is defined as any event that leads to death, a life-threatening situation, inpatient hospitalisation, persistent or significant disability, a congenital anomaly/birth defect or another important medical condition. 97 There were 44 reports of SAEs during the study. This equates to a risk of 44 out of 740 (5.9%, 95% CI 4.4% to 7.9%). Twenty-eight of the events (64%) involved the urogenital system, with the most common events being urinary retention and urinary tract infections or urosepsis. As reported in Risk of sepsis, there were 10 cases of sepsis. There were no deaths up to the time limit for reporting SAEs (30 days after the last study visit). All SAEs, including sepsis cases, were independently reviewed by the independent Data Monitoring Committee to ensure that the rate of sepsis was no higher than that reported for TRUS-guided biopsy on its own. No safety concerns were raised during these reviews.
Most men (88%) experienced at least one side effect. Table 17 summarises the numbers of any side effects documented at the patients’ last study visit.
| Side effect (n with missing data) | n (%) |
|---|---|
| mpMRI | |
| Pain/discomfort (15) | 11 (2) |
| Allergic reaction to contrast medium (16) | 1 (< 1) |
| Other | 3 (< 1) |
| CBP | |
| Pain/discomfort (13) | 362 (64) |
| Dysuria (17) | 256 (46) |
| Haematuria (11) | 380 (67) |
| Haematospermia (51) | 291 (55) |
| Erectile dysfunction (requiring medication, injection therapy or devices) (48) | 76 (14) |
| Urinary tract infection (only if confirmed by a laboratory test) (11) | 32 (6) |
| Systemic urosepsis (9) | 8 (1) |
| Acute urinary retention (12) | 58 (10) |
| Symptoms associated with general/spinal anaesthesia (43) | 19 (4) |
| Other | 65 (11) |
| Total patients with any side effect (8) | 501 (88) |
Chapter 4 Economic evaluation approach
Overview
The economic evaluation conducted for PROMIS is presented in four chapters. Chapter 4 states the aims and objectives of the economic evaluation and describes the approach used. Chapter 5 describes the evaluation of the short-term outcomes associated with using the different diagnostic tests to detect CS cancer. Chapter 6 describes the evaluation of the long-term outcomes associated with treatment compared with monitoring of men with prostate cancer. Chapter 7 combines the results of Chapters 5 and 6 and presents conclusions on the cost-effectiveness of the diagnostic strategies.
Introduction
Prostate cancer is the most common cancer in men, with approximately 47,000 new cases diagnosed in the UK in 2013. It is the second most common cause of death for men in the UK, with approximately 11,000 deaths per year. 1 As discussed in Chapter 1, prostate cancer is divided into risk groups based on a combination of PSA level, Gleason score (i.e. cancer grade) and stage (see Table 1). Low-risk cancer is considered to be CNS because it is unlikely to have a clinical impact during the man’s remaining lifetime. The term ‘clinically insignificant’ is also commonly used. Intermediate-risk cancer and high-risk cancer have a greater risk of progression and are considered to be CS.
Currently in the UK, all men considered to be at risk of having prostate cancer are invited to undergo a biopsy, usually of the TRUS-guided type, to identify and classify the cancer. The biopsy procedure has adverse health consequences, albeit mostly temporary, and misses a considerable proportion of CS cancers.
There are two possible ways of improving the current practice of TRUS-guided biopsy for all men considered to be at risk: (1) choose another test that is better at detecting CS cancer (better sensitivity) or (2) incorporate additional tests prior to biopsy that might inform the decision to proceed to biopsy (triage test) and/or might improve the sensitivity of biopsy. The mpMRI is in the latter category, because it may enable the selection of patients who should have a biopsy and also provide information to enable targeted biopsies of suspicious areas within the prostate. The mpMRI can be used in combination with TRUS-guided biopsy or other biopsy types to better detect CS cancer and avoid unnecessary biopsies.
The different ways of combining mpMRI and biopsy form a range of diagnostic strategies for prostate cancer. These diagnostic strategies have different costs and health outcomes, in both the short term and the long term. The short-term costs and health outcomes depend on the prices of the tests and their direct health effects and detection rates in men with and without CS cancer. The long-term costs and health outcomes relate to the downstream consequences of treatment decisions, which in turn depend on the detection rates of each test. The short- and long-term costs and health outcomes together determine the most cost-effective way in which to use mpMRI and biopsy (i.e. the cost-effective diagnostic strategy).
To inform decisions on how best to use the tests in combination, decision modelling is required to combine the data generated in PROMIS with external information. The clinical data generated in PROMIS provide information on how accurate mpMRI and biopsy are at detecting CS and CNS prostate cancer. However, the data do not provide information on the effect of using mpMRI and biopsy in combination (e.g. the proportion of CS cancers detected when using mpMRI to direct the TRUS-guided biopsy to suspicious lesions). External information is also required to predict the long-term costs and health outcomes of the alternative strategies, based on the proportions who are correctly diagnosed with CS prostate cancer, that is, the quality-adjusted survival and lifetime costs of men according to their cancer status and diagnostic classification. Decision modelling structures the available evidence using a mathematical model, combining the different pieces of information to produce an estimate of the costs and health outcomes of the different diagnostic strategies. Health outcomes are typically expressed as quality-adjusted life-years (QALYs), a measure that weights life expectancy with its HRQoL.
An evaluation of the effectiveness of the alternative strategies can rely on the health outcomes associated with each, the most effective strategy being the one conferring the most health to the potential population of users. To ascertain cost-effectiveness, however, the consequences of the additional costs that some of the strategies impose on the NHS also need to be considered. This requires information on the health opportunity cost of diverting resources to more costly interventions. The health opportunity cost is the health benefit forgone by other patients if their interventions are no longer funded in order to release resources for other more costly diagnostic strategies. The cost-effective diagnostic strategy is the strategy that achieves the greatest health gain net of the health opportunity cost.
Previous research98 has estimated that £13,000 in additional costs displaces 1 QALY. This is the health opportunity cost for the NHS of offering a more costly intervention and is often referred to as the cost-effectiveness threshold. Therefore, the most cost-effective diagnostic strategy is the one that achieves the most QALYs net of its costs, converted into QALYs forgone using the health opportunity cost of £13,000. NICE uses a higher value, of between £20,000 and £30,000 per QALY, to make recommendations to the NHS on the cost-effectiveness of new interventions. 99 This means that an additional £20,000–30,000 is assumed to impose on the NHS a loss of 1 QALY.
Aims and objectives of the economic evaluation
The aim of the economic evaluation was to identify the most cost-effective diagnostic strategy in men with suspected localised prostate cancer from the perspective of the UK NHS, using information on TRUS-guided biopsy, mpMRI and TPM-biopsy. The evaluation makes use of information generated in PROMIS, supplemented by external evidence as appropriate, and compares the various ways that these diagnostic tests can be used to diagnose prostate cancer.
The specific objectives of the economic evaluation were to:
-
define potential diagnostic strategies involving combinations of TRUS-guided biopsy, mpMRI and TPM-biopsy that could reasonably be used in clinical practice
-
estimate the proportions of men correctly identified with prostate cancer, both CNS and CS, and men with cancer who have been missed, for each diagnostic strategy
-
estimate the direct HRQoL and NHS costs associated with each diagnostic strategy
-
evaluate the expected quality-adjusted survival and NHS costs of men depending on their diagnostic classifications, treatment decisions and true disease status
-
compare the quality-adjusted survival and NHS costs of all diagnostic strategies incrementally to identify the most cost-effective strategy for a given cost-effectiveness threshold range
-
identify the main drivers of cost-effectiveness and explore how they affect the results
-
characterise the uncertainty around the results and identify the key areas of uncertainty, which should be prioritised for future research.
Framework of analyses
The cost-effectiveness of the different diagnostic strategies involving mpMRI, TRUS-guided biopsy and TPM-biopsy depends on two aspects. First, it depends on the diagnostic performance, direct impact on HRQoL (mainly through AEs) and costs (mainly depending on the price and level of use of each diagnostic technology) of the combination of tests forming each diagnostic strategy in correctly classifying men with their true disease status. These form the short-term outcomes of testing. Second, it depends on the long-term health benefits and costs of managing the disease, which in turn depend on the men’s true disease status and clinical pathway. These form the long-term outcomes of testing. The short-term and long-term outcomes of testing, once combined, allow the cost-effectiveness of the diagnostic strategies to be compared. For this reason, the decision model is divided into two components: (1) to evaluate short-term outcomes and (2) to evaluate long-term outcomes. The decision models were developed in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA), with some auxiliary calculations on diagnostic accuracy carried out in Stata version 13.0. The results of the short-term and long-term models are combined to evaluate the cost-effectiveness of the different diagnostic strategies over the patients’ lifetime.
Chapter 5 Evaluation of short-term outcomes
Methods
A decision tree (see Figure 13) is used to represent the diagnostic process and to evaluate the probability that men are classified as having no cancer, CNS cancer and CS cancer, conditional on their true disease status, for every given diagnostic strategy. The diagnostic strategies consist of clinically feasible combinations of mpMRI, TRUS-guided biopsy and TPM-biopsy; each are evaluated using the different cancer definitions and cut-off points.
For each diagnostic strategy, the decision tree evaluates:
-
the proportion of men classified as having no cancer, CNS cancer and CS cancer, conditional on their true disease status
-
the proportion of men undergoing each diagnostic test
-
the direct costs of diagnosis, which consist of the costs of testing and the cost of managing the adverse effects of the tests
-
the direct health outcomes of diagnosis, in terms of the HRQoL impact of the tests
-
diagnostic efficiency, that is, the trade-off between the accuracy of the diagnostic strategies in detecting CS cancer and their direct costs.
Population
The target population was men with suspected prostate cancer who had been referred to secondary care for further investigation. Their true disease status was defined according to the cancer risk categories recommended by NICE13 and used in the randomised controlled study comparing expectant management with active treatment. 6 The groups are:
-
men with no cancer
-
men with low-risk cancer – a PSA level of ≤ 10 ng/ml and a Gleason score of ≤ 6
-
men with intermediate-risk cancer – a PSA level of > 10 ng/ml and ≤ 20 and a Gleason score of 7
-
men with high-risk cancer – a Gleason score of ≥ 8.
In order to inform the model inputs, the true disease status of men was ascertained using the results of TPM-biopsy and complemented by TRUS-guided biopsy if cancer had not been detected on TPM-biopsy but had been detected on TRUS-guided biopsy.
Diagnostic tests
PROMIS classifies men with cancer as having either CS cancer or CNS cancer. The definitions of CS cancer are discussed in Chapter 2 and are restated in the following section. These definitions correspond to the classification by cancer risk recommended by NICE. 13 Low-risk cancer is assumed to be equivalent to CNS cancer. Intermediate-risk and high-risk cancer form the group with CS cancer.
Multiparametric magnetic resonance imaging
Multiparametric magnetic resonance imaging is an imaging technique that can be used to report the physiological properties of the prostate tissue and its morphology. The type of MRI used in PROMIS is mpMRI with T2 weighting, DW and DCE. Each technique helps to distinguish between normal tissue and cancer tissue. Detection rates in mpMRI (with T2 weighting, DW and DCE) vary widely in the literature. A recent systematic review49 reported that the correct classification of men with CS cancer ranged from 58% to 96% (sensitivity), whereas the correct classification of men without CS cancer ranged from 23% to 87% (specificity).
In PROMIS, the lesions observed by mpMRI are classified according to suspicion of cancer and, if cancer is suspected, suspicion of CS cancer. There are two CS cancer definitions for mpMRI (Table 18). The primary mpMRI outcome is definition 2. Definition 2 defines CS cancers as those with a volume of ≥ 0.2 ml and/or a Gleason score of ≥ 3 + 4. As a secondary outcome, CS cancers are defined as those with a volume of ≥ 0.5 ml and/or a Gleason score of ≥ 4 + 3 (definition 1). The radiologist uses the information from the scan to score lesions according to suspicion of cancer and CS cancer for each definition using a five-point Likert scale. This scale ranges from 1 (very low suspicion) to 5 (very high suspicion). The cut-off point for considering a lesion suspicious enough to warrant further investigation can vary from 2, in which all men with a score of ≥ 2 are referred, to 5, in which only men with a score of 5 are referred. In PROMIS, a cut-off point of 3 was adopted. Note that mpMRI is unable to diagnose cancer with certainty; instead, it classifies lesions according to the degree of suspicion of cancer and CS cancer.
| Outcome | mpMRI definition |
|---|---|
| Primary | 2: a lesion volume of ≥ 0.2 ml and/or Gleason score of ≥ 3 + 4 |
| Secondary | 1: a lesion volume of ≥ 0.5 ml and/or Gleason score of ≥ 4 + 3 |
Transrectal ultrasound-guided biopsy
In TRUS-guided biopsy, a probe is inserted through the anus to obtain 10–12 small samples of tissue from the prostate, guided by an ultrasound scan. The TRUS-guided biopsy has perfect specificity in that if cancer is detected it undoubtedly means that cancer is present. However, TRUS-guided biopsies miss 30–45% of CS cancers. 2,3 TRUS-guided biopsy has some adverse health effects, which are generally transient. 100 Up to 20% of men would refuse a repeat biopsy, even if it was clinically indicated. 101
In PROMIS, two definitions are used to classify men on the basis of their TRUS-guided biopsy results (Table 19). The primary TRUS-guided biopsy outcome defines CS cancer as cancer that exhibits a dominant Gleason pattern of ≥ 4 and/or any Gleason pattern of ≥ 5 and/or a cancer core length of ≥ 6 mm. As a secondary outcome, CS cancer is defined as lesions with any Gleason pattern of ≥ 4 and/or a cancer core length of ≥ 4 mm.
| Outcome | TRUS-guided biopsy definition |
|---|---|
| Primary | 1: dominant Gleason pattern of ≥ 4 and/or any Gleason pattern of ≥ 5 and/or cancer core length of ≥ 6 mm |
| Secondary | 2: any Gleason pattern of ≥ 4 and/or cancer core length of ≥ 4 mm |
A TRUS-guided biopsy may be used as a first test for all men or it may be used a second time following an initial TRUS-guided biopsy or following mpMRI (once or twice if the first biopsy did not detect cancer). The sensitivity of TRUS-guided biopsy is likely to change depending on whether it is used for a first or a second biopsy, even if only because the patients undergoing a second biopsy differ from those undergoing a first biopsy. The sensitivity of TRUS-guided biopsy is also likely to differ if it is undertaken before or after mpMRI, as mpMRI can be used to guide the second biopsy. For these reasons, this study considers six types of TRUS-guided biopsy, listed in Table 20.
| Code | Test |
|---|---|
| T | TRUS-guided biopsy before mpMRI |
| Q | TRUS-guided biopsy after a TRUS-guided biopsy that did not detect cancer |
| R | TRUS-guided biopsy after a TRUS-guided biopsy that detected CNS cancer |
| W | TRUS-guided biopsy after a suspicious mpMRI result |
| Z | TRUS-guided biopsy after a TRUS-guided biopsy that did not detect cancer and a suspicious mpMRI result |
| S | TRUS-guided biopsy after a TRUS-guided biopsy that detected CNS cancer and a suspicious mpMRI result |
Template prostate mapping biopsy
A TPM-biopsy involves taking a large number of tissue samples from the prostate using a grid sampling system. TPM-biopsy is widely regarded as the gold standard as it correctly identifies nearly all men with CS cancer, who can then receive treatment. 53–58,102 However, it is costly and causes adverse effects. On balance, TPM-biopsy identifies the greatest proportion of men with CS cancer, but may have a higher cost and greater HRQoL implications. For this analysis, the reference standard is the combination of TPM-biopsy and TRUS-guided biopsy.
Diagnostic strategies
Each of the three diagnostic tests (mpMRI, TRUS-guided biopsy and TPM-biopsy) can be used in a different sequence to diagnose prostate cancer. The sequence of tests is determined by whether or not, and when, to use mpMRI; the type of biopsy (TRUS-guided or TPM); and whether or not men can be rebiopsied. Although TPM-biopsy can correctly diagnose all men, it involves the use of more health-care resources and hence has greater costs and may affect HRQoL. Therefore, although it may not be cost-effective to use TPM-biopsy in all men, it may be worthwhile using it in men selected according to their mpMRI results, TRUS-guided biopsy results or a combination of the two.
Four key assumptions are made in the design of the diagnostic strategies under evaluation:
-
The only tests considered are mpMRI, TRUS-guided biopsy and TPM-biopsy. This follows from PROMIS, which compared mpMRI and TRUS-guided biopsy with TPM-biopsy.
-
There can be up to three tests in one diagnostic strategy. Diagnostic episodes may be repeated over time but this is not explicitly modelled in this analysis.
-
A diagnostic strategy can include up to two biopsies.
-
If included in the strategy, mpMRI can be used only once.
Tables 21–24 identify the 32 diagnostic strategies compared in this work. Table 21 shows the strategies starting with mpMRI prior to TRUS-guided biopsy (M1–M7) and Table 23 shows the strategies starting with TRUS-guided biopsy prior to TRUS-guided biopsy (T1–T9). Strategies M1–M7 and T1–T9 do not include TPM-biopsy. Tables 22 and 24 show the equivalent strategies but including TPM-biopsy as the last biopsy, replacing TRUS-guided biopsy. Therefore, strategies N1–N7 start with mpMRI and include TPM-biopsy as the last biopsy in the test sequence and strategies P1–P7 start with a biopsy and include TPM-biopsy as the last biopsy in the test sequence. This means that in strategy P1, which includes only one biopsy for all men, all men receive TPM-biopsy. In strategy P2, all men receive TRUS-guided biopsy and men who were classified as having no cancer receive TPM-biopsy. Men are classified as having ‘de facto’ no cancer in strategies in which men with suspected CNS cancer on mpMRI are not retested with TRUS-guided biopsy. In other words, men with suspected CNS cancer on mpMRI are not referred for active surveillance because they have not been diagnosed with CNS cancer. Instead, they are discharged to primary care where their PSA level is monitored annually. In contrast, men in whom TRUS-guided biopsy detected CNS cancer are referred for active surveillance.
| Strategy | First test | Result | Second test | Result | Third test | Result | Final result |
|---|---|---|---|---|---|---|---|
| M1 | mpMRI | NC | NC | ||||
| CNS cancer | NC | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M2 | mpMRI | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M3 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M4 | mpMRI | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M5 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M6 | mpMRI | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| M7 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer |
| Strategy | First test | Result | Second test | Result | Third test | Result | Final result |
|---|---|---|---|---|---|---|---|
| N1 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N2 | mpMRI | NC | NC | ||||
| CNS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N3 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N4 | mpMRI | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N5 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N6 | mpMRI | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| N7 | mpMRI | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer |
| Strategy | First test | Result | Second test | Result | Third test | Result | Final result |
|---|---|---|---|---|---|---|---|
| T1 | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T2 | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T3 | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T4 | TRUS-guided biopsy | NC | TRUS-guided biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T5 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | NC | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T6 | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | mpMRI | NC | CNS cancer | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T7 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | NC | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | mpMRI | NC | NC | ||||
| CNS cancer | NC | ||||||
| CS cancer | TRUS-guided biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T8 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| T9 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | mpMRI | NC | CNS cancer | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TRUS-guided biopsy (S) | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer |
| Strategy | First test | Result | Second test | Result | Third test | Result | Final result |
|---|---|---|---|---|---|---|---|
| P1 | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P2 | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P3 | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P4 | TRUS-guided biopsy | NC | TPM-biopsy | NC | NC | ||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P5 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | NC | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P6 | TRUS-guided biopsy | NC | NC | ||||
| CNS cancer | mpMRI | NC | CNS cancer | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P7 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | NC | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | mpMRI | NC | NC | ||||
| CNS cancer | NC | ||||||
| CS cancer | TPM-biopsy | CNS cancer | CNS cancer | ||||
| CS cancer | CS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P8 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| P9 | TRUS-guided biopsy | NC | mpMRI | NC | NC | ||
| CNS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CS cancer | TPM-biopsy | NC | NC | ||||
| CNS cancer | CNS cancer | ||||||
| CS cancer | CS cancer | ||||||
| CNS cancer | mpMRI | NC | CNS cancer | ||||
| CS cancer | CS cancer |
Figure 13 shows how these strategies can be translated into decision trees, using strategies M1 and T1 as examples. The black circles represent chance nodes, in which the outcome depends on a probability. In strategy M1, all men receive mpMRI. Men identified as having suspected CS cancer on mpMRI receive TRUS-guided biopsy. The TRUS-guided biopsy can classify these men as not having cancer if no cancer is detected or as having CNS or CS cancer, depending on the characteristics of the lesions. In strategy T1, men receive only one single test, TRUS-guided biopsy. The biopsy can classify men as having no cancer, CNS cancer or CS cancer.
FIGURE 13.
Decision trees. (a) Strategy M1: mpMRI in all men and TRUS-guided biopsy in men with suspected CS cancer; and (b) strategy T1: all men have TRUS-guided biopsy.
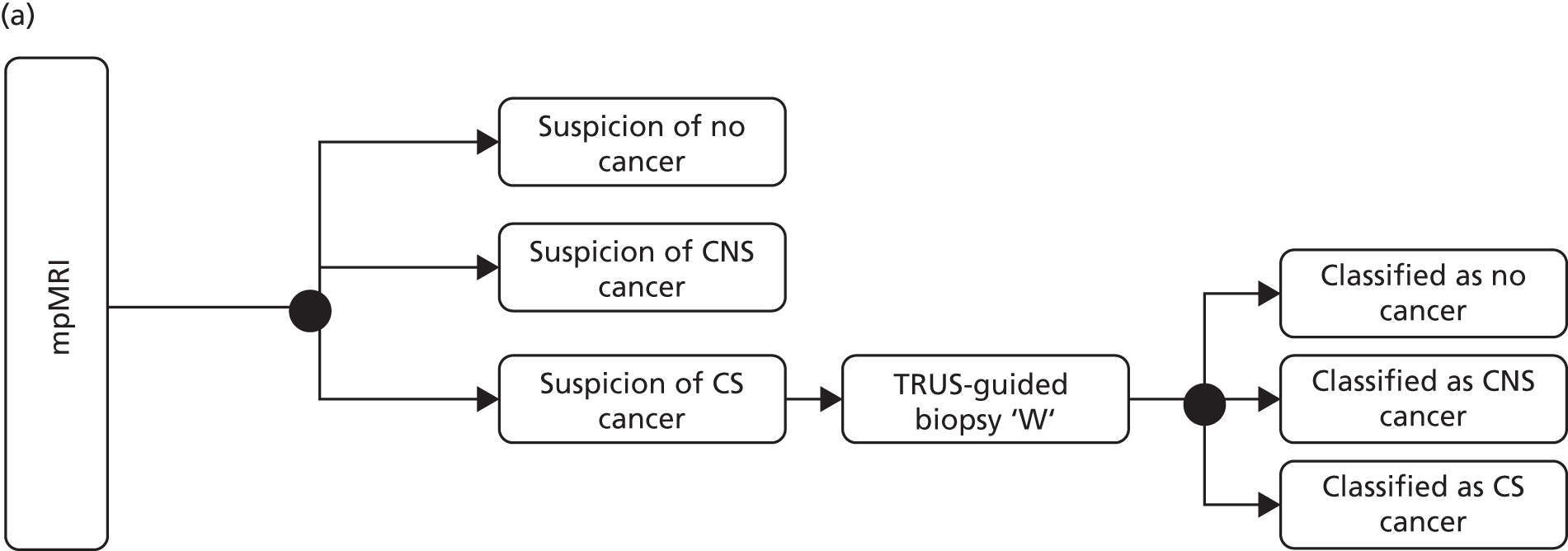
This study evaluates each of the 32 diagnostic strategies using each of the two diagnostic definitions for mpMRI, TRUS-guided biopsy and TPM-biopsy (see Tables 18 and 19) and between two and five cut-off points on the mpMRI Likert scale for suspicion of cancer. In total, the 32 different diagnostic strategies form 383 substrategies:
-
In strategies M1–M7, all men undergo mpMRI, which will then indicate whether one or two TRUS-guided biopsies are required. Strategies M1–M7 are tested over two mpMRI definitions, four cut-off points and two TRUS-guided biopsy definitions, forming 112 possible substrategies.
-
In strategies N1–N7, all men undergo mpMRI to inform the need for a TRUS-guided biopsy or TPM-biopsy. In strategies N1 and N2, all men receive mpMRI and, depending on the result, some men receive TPM-biopsy. Therefore, in strategies N1 and N2, two mpMRI definitions can be used, each over four cut-off points. This forms 16 substrategies. In N3–N7, the two mpMRI definitions, four cut-off points and two TRUS-guided biopsy definitions apply, forming 80 substrategies. Therefore, in total, there are 96 substrategies.
-
In strategies T1–T9, all men undergo a TRUS-guided biopsy, which can be subsequently followed by mpMRI and potentially a second TRUS-guided biopsy. Strategies T1–T4 include only TRUS-guided biopsy and, therefore, are tested over two definitions of CS cancer, forming eight possible combinations. Strategies T5–T9 are tested over two mpMRI definitions, four cut-off points and two TRUS-guided definitions, forming 80 possible combinations. Therefore strategies T1–T9 form 88 possible substrategies.
-
In strategies P1–P9, all men undergo either a TRUS-guided biopsy or a TPM-biopsy and may undergo mpMRI to inform whether or not they should have a second biopsy (TRUS-guided or TPM). Strategy P1 consists of a TPM-biopsy for all men, which constitutes the reference test and uses the primary outcome as the definition for CS cancer against which all other tests are compared. Strategies P2–P4 are tested over the two TRUS-guided biopsy definitions. Strategies P5–P9 are tested over the two mpMRI definitions, four cut-off points and two TRUS-guided definitions. As a result, there are 87 possible substrategies.
The diagnostic strategies were labelled according to their test combination first (M1–M7, N1–N7, T1–T9 or P1–P9) and then according to their TRUS-guided biopsy definition (1 or 2), MP-MRI definition (1 or 2) and cut-off point (2–5). For example, substrategy M1 125 refers to test combination M1, in which all men were first assessed using MP-MRI definition 2 and cut-off point 5 and then those with suspected CS cancer were followed up with TRUS-guided biopsy definition 1.
Strategy P1 (testing all men with TPM-biopsy) represents the value of a perfect immediate test. TPM-biopsy is taken here as the reference test, which correctly identifies the true disease status of all men. TPM-biopsy is unlikely to be feasible in clinical practice, given its HRQoL and resource implications and the large number of men referred to secondary care with suspected prostate cancer. The inclusion of strategy P1 aims to show the difference in benefits between (1) a perfect immediate test that is costly (P1) and (2) less costly and less accurate strategies composed of sequences of tests, in which TPM-biopsy can be used in a small proportion of men.
Substrategy M7 123 corresponds to the PROMIS-proposed diagnostic pathway. This involves using mpMRI in all men followed by TRUS-guided biopsy in men whose mpMRI result indicates suspected CS cancer according to definition 2 and cut-off point 3. TRUS-guided biopsy is evaluated according to definition 1. Men in whom TRUS-guided biopsy does not detect CS cancer are rebiopsed and classified according to definition 1.
Substrategy T7 123 corresponds most closely to the strategy recommended in the 2014 NICE clinical guidelines. 13 It involves TRUS-guided biopsy for all men. Men in whom CS cancer according to definition 1 was not detected receive mpMRI. Men with suspected CS cancer on mpMRI using definition 2 and cut-off point 3 receive a second TRUS-guided biopsy, evaluated according to definition 1. However, in some hospitals, mpMRI is not available for the diagnosis of prostate cancer and the usual diagnostic strategy is T4 1––. In T4 1––, all men receive TRUS-guided biopsy assessed according to definition 1. Men in whom CS cancer was not detected are rebiopsied.
Model inputs
Diagnostic performance
The diagnostic performance of the tests is based as far as possible on the data collected in the diagnostic study. This provides the diagnostic performance of mpMRI and TRUS-guided biopsy, each used alone, compared with the reference standard of TPM-biopsy. The diagnostic performance is likely to be different if the tests are used in combination. This is for two reasons. First, additional information is known, which can improve the performance of the test. For example, a TRUS-guided biopsy conducted after mpMRI is likely to be more accurate given that mpMRI can provide information on where the lesions are located. Second, the characteristics of the men who have been referred for the second test are likely to be different. For example, the group of men with cancer in whom an initial TRUS-guided biopsy did not find cancer are likely to have lesions with different characteristics from those in the group of biopsy-naive men. Consequently, external data are used to inform the diagnostic performance of repeat biopsy, MRI-targeted biopsy and MRI-targeted repeat biopsy.
The diagnostic performance of the tests is represented as a series of conditional probabilities. The probabilities of the tests’ classifications given the true disease status are identified in Table 25. The true disease status is defined in terms of cancer risk because this is the classification commonly used, including in the NICE clinical guidelines. 13 The true cancer status is the most severe result of TPM-biopsy and TRUS-guided biopsy. Both TPM-biopsy and TRUS-guided biopsy have perfect specificity in that they can classify men as having cancer only if cancer is present.
| Disease status | Test classification | ||
|---|---|---|---|
| NC detected | CNS cancer detected | CS cancer detected | |
| NC | p(NC|NC): probability of detecting NC given that NC exists | p(CNS|NC): probability of detecting CNS cancer given that NC exists | p(CS|NC): probability of detecting CS cancer given that NC exists |
| Low-risk cancer | p(NC|low): probability of detecting NC given that low-risk cancer exists | p(CNS|low): probability of detecting CNS cancer given that low-risk cancer exists | p(CS|low): probability of detecting CS cancer given that low-risk cancer exists |
| Intermediate-risk cancer | p(NC|int): probability of detecting NC given that intermediate-risk cancer exists | p(CNS|int): probability of detecting CNS cancer given that intermediate-risk cancer exists | p(CS|int): probability of detecting CS cancer given that intermediate-risk cancer exists |
| High-risk cancer | p(NC|high): probability of detecting NC given that high-risk cancer exists | p(CNS|high): probability of detecting CNS cancer given that high-risk cancer exists | p(CS|high): probability of detecting CS cancer given that high-risk cancer exists |
Uncertainty is considered by including the diagnostic performance parameters in the model as probability distributions. In order to capture the uncertainty and the correlation in diagnostic performance, the individual patient data obtained in PROMIS were bootstrapped 1000 times. Bootstrapping was used to account for the correlation between tests. Diagnostic performance obtained from the literature is incorporated as a beta distribution for counts or a log-normal distribution for relative probabilities. Details are presented in Appendix 2.
The analysis of the individual patient data for the purposes of the economic evaluation has some different features from that conducted for PROMIS. Therefore, the diagnostic inputs used in the decision model are not exactly the same as those reported in Chapter 3. This is for three reasons. First, the disease categories used in the economic model, with which the test classifications are compared, are different. The clinical study uses CS compared with CNS, which includes no cancer. The economic evaluation uses four categories: (1) no cancer, (2) low-risk cancer (equivalent to CNS cancer), (3) intermediate-risk cancer and (4) high-risk cancer. Intermediate- and high-risk cancers are CS cancers. Second, in PROMIS, compared with TRUS-guided biopsy, TPM-biopsy found either no cancer or a lower-grade cancer in nine men. Hence, the results of the TPM-biopsy in these nine men were changed to match the results of the TRUS-guided biopsy. Third, the definition of CS cancer in PROMIS takes into consideration the cancer core length, whereas the NICE risk definitions13 do not. This caused an inconsistency between the risk subgroups assigned to seven men because of their cancer core length. These seven men were excluded from our analysis.
Table 26 summarises the diagnostic performance evidence for each type of TRUS-guided biopsy and its source. The model requires information on the diagnostic performance of the six types of TRUS-guided biopsy (tests ‘T’, ‘Q’, ‘R’, ‘W’, ‘Z’ and ‘S’).
| Test | Data source | Definition | Probability of having each test result given the true disease status, mean (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p(NC|low)a | p(CNS|low)b | p(NC|int)c | p(CNS|int)d | p(CS|int)e | p(NC|high)f | p(CNS|high)g | p(CS|high)h | |||
| Ti | PROMIS | Primary | 0.65 (0.55 to 0.75) | 0.35 (0.25 to 0.45) | 0.24 (0.20 to 0.29) | 0.42 (0.36 to 0.47) | 0.34 (0.29 to 0.39) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Secondary | 0.65 (0.55 to 0.75) | 0.35 (0.25 to 0.45) | 0.24 (0.20 to 0.29) | 0.17 (0.13 to 0.21) | 0.59 (0.54 to 0.64) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) | ||
| Qj | Roehl et al.103 | Primary | 0.55 (0.53 to 0.57) | 0.45 (0.43 to 0.47) | 0.55 (0.53 to 0.57) | 0.25 (0.22 to 0.28) | 0.20 (0.17 to 0.23) | 0.55 (0.53 to 0.57) | 0.00 (0.00 to 0.00) | 0.45 (0.43 to 0.47) |
| Secondary | 0.55 (0.53 to 0.57) | 0.45 (0.43 to 0.47) | 0.55 (0.53 to 0.57) | 0.10 (0.07 to 0.13) | 0.35 (0.32 to 0.38) | 0.55 (0.53 to 0.57) | 0.00 (0.00 to 0.00) | 0.45 (0.43 to 0.47) | ||
| Rk | Barzell et al.104 | 1 | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) | 0.00 (0.00 to 0.00) | 0.75 (0.55 to 0.88) | 0.25 (0.12 to 0.45) | 0.00 (0.00 to 0.00) | 0.75 (0.55 to 0.88) | 0.25 (0.12 to 0.45) |
| 2 | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) | 0.00 (0.00 to 0.00) | 0.75 (0.63 to 0.85) | 0.25 (0.15 to 0.37) | 0.00 (0.00 to 0.00) | 0.75 (0.63 to 0.85) | 0.25 (0.15 to 0.37) | ||
| Wl | PROMIS and Schoots et al.105 | Primary | 0.80 (0.67 to 0.89) | 0.20 (0.11 to 0.33) | 0.20 (0.15 to 0.24) | 0.37 (0.31 to 0.44) | 0.43 (0.36 to 0.52) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Secondary | 0.79 (0.66 to 0.89) | 0.21 (0.11 to 0.34) | 0.15 (0.09 to 0.21) | 0.11 (0.06 to 0.16) | 0.74 (0.65 to 0.84) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) | ||
| Zm | Schoots et al.105 | Same for both definitions | 0.67 (0.02 to 1.00) | 0.33 (0.00 to 0.98) | 0.05 (0.02 to 0.09) | 0.08 (0.03 to 0.17) | 0.87 (0.74 to 0.96) | 0.05 (0.02 to 0.09) | 0.08 (0.03 to 0.17) | 0.87 (0.74 to 0.96) |
| Sn | Schoots et al.105 (assumption) | Same for both definitions | 0.67 (0.02 to 1.00) | 0.33 (0.00 to 0.98) | 0.05 (0.02 to 0.09) | 0.08 (0.03 to 0.17) | 0.87 (0.74 to 0.96) | 0.05 (0.02 to 0.09) | 0.08 (0.03 to 0.17) | 0.87 (0.74 to 0.96) |
Information on the diagnostic performance of test ‘T’ (first TRUS-guided biopsy without prior mpMRI) is obtained directly from the individual patient data collected in PROMIS.
The diagnostic performance of test ‘Q’ (TRUS-guided biopsy after a TRUS-guided biopsy that did not detect cancer) is obtained from Roehl et al. 103 Roehl et al. 103 report the number of cancers detected in men who underwent a series of TRUS-guided biopsies. The total number of cancers detected is used to calculate the prevalence of prostate cancer in the study population of 0.38 (n = 963 men with cancer; N = 2526 men in the initial population). After the second TRUS-guided biopsy, 143 men were classified as having cancer. Therefore, the probability of detecting cancer given that cancer exists is 0.45. These cancers consist of CNS and CS cancers; however, no information is provided on the proportion of CNS cancers compared with CS cancers or how the probability of detection varies by cancer significance. In the absence of this information, it is assumed that the proportion misclassified is the same as for the initial TRUS-guided biopsy (test ‘T’) for intermediate- and high-risk cancers. In mathematical terms:
where p(T = CNS|int) is the probability of test ‘T’ classifying an individual as having CNS cancer given that intermediate-risk cancer exists, p(T = CS|int) is the probability of test ‘T’ classifying an individual as having CS cancer given that intermediate-risk cancer exists, p(Q = CNS|int) is the probability of test ‘Q’ classifying an individual as having CNS cancer given that intermediate-risk cancer exists and p(Q = CS|int) is the probability of test ‘Q’ classifying an individual as having CS cancer given that intermediate-risk cancer exists.
Test ‘R’ (TRUS-guided biopsy in men who were classified as having CNS cancer on the first TRUS-guided biopsy) can classify men as having CNS or CS cancer, because the first TRUS-guided biopsy has already detected CNS cancer. The diagnostic performance of test ‘R’ is obtained from Barzell et al. 104 Barzell et al. 104 compared repeat TRUS-guided biopsy with TPM-biopsy in men in whom CNS cancer had been detected. There are five definitions for clinical significance. 82,106,107 The UCL definitions82 are the ones that most closely resemble the definitions used in PROMIS and, therefore, ensure comparability. UCL definition 1 defines CS cancer as localised cancer with a maximum total cancer core length of 10 mm, a maximum cancer core length of 6 mm and a Gleason score of at least 4 + 3 or 3 + 5. UCL definition 2 defines CS cancer as a maximum total cancer core length of 6 mm, a maximum cancer core length of 4 mm and a Gleason score of at least 3 + 4. Within PROMIS, the total cancer core lengths were not used because the additive value of this factor was marginal in the original description. For definition 1, the probability of test ‘R’ (second TRUS-guided biopsy) detecting CS cancer given that CS cancer is present is 0.24 (10 men tested positive out of 41 men with cancer), whereas for definition 2 the equivalent value is 0.25 (17 men tested positive out of 69 men with cancer). The model uses these data for the sensitivity of test ‘R’ in detecting CS cancer: UCL definition 1 for definition 1 TRUS-guided biopsy and UCL definition 2 for definition 2 TRUS-guided biopsy.
Test ‘W’ (TRUS-guided biopsy after a suspicious mpMRI result) is likely to be more sensitive than test ‘T’ (TRUS-guided biopsy without a prior mpMRI). The mpMRI provides information on the area most likely to have cancer lesions, which can subsequently be sampled by the TRUS-guided biopsy. Schoots et al. 105 report the relative sensitivity of TRUS-guided biopsy targeted by mpMRI and TRUS-guided biopsy in men with suspicious mpMRI results (cut-off point of ≥ 3) for any cancer and by clinical significance. They report a relative sensitivity of MRI-targeted, TRUS-guided biopsy compared with TRUS-guided biopsy of 1.20 (95% CI 1.09 to 1.32) for CS cancer and 0.56 (95% CI 0.37 to 0.85) for ‘insignificant cancer’, assumed to be equivalent to CNS cancer. This represents the ratio between the probability of detecting CS and CNS cancer, respectively, in men whose mpMRI result is suspicious of cancer, from using the mpMRI scan to guide the TRUS-guided biopsy compared with conducting a TRUS-guided biopsy blind. Therefore, this relative sensitivity is applied to the probability of TRUS-guided biopsy detecting CS and CNS cancer in men with a mpMRI result that was suspicious of cancer (score of ≥ 3) observed in PROMIS.
The performance of test ‘Z’ (TRUS-guided biopsy after a suspicious mpMRI result and no cancer on prior TRUS-guided biopsy) is also likely to be different from the sensitivity of test ‘T’ (first TRUS-guided biopsy). PROMIS does not provide information on this because the men received only one TRUS-guided biopsy. Schoots et al. 105 report the probability of mpMRI-guided TRUS-guided biopsy after a previous negative biopsy detecting CNS and CS cancer. Because the probability of classifying a CS cancer as CNS or as no cancer is not reported, it is assumed that the distribution of intermediate- or high-risk cancers detected by the TRUS-guided biopsy targeted by mpMRI, and after a previous negative biopsy, is the same as in the initial TRUS-guided biopsy. In mathematical terms:
where p(T = NC|int) is the probability of test ‘T’ classifying an individual as having no cancer given that intermediate-risk cancer exists, p(T = CNS|int) is the probability of test ‘T’ classifying an individual as having CNS cancer given that intermediate-risk cancer exists, p(Z = NC|int) is the probability of test ‘Z’ classifying an individual as having no cancer given that intermediate-risk cancer exists and p(Z = CNS|int) is the probability of test ‘Z’ classifying an individual as having CNS cancer given that intermediate-risk cancer exists. In the absence of further evidence, the diagnostic performance of test ‘Z’ in men with intermediate-risk cancer is assumed to be representative of the performance of the test in men with high-risk cancer.
Test ‘S’ (second TRUS-guided biopsy following a first TRUS-guided biopsy that detected CNS cancer and a mpMRI scan suspicious for cancer), similarly to test ‘R’, can classify men only into CNS or CS cancer because the previous TRUS-guided biopsy has already detected CNS cancer. No evidence was found on test ‘S’. Its performance is assumed to be equivalent to that of test ‘Z’, as reported in Schoots et al. ,105 because they both represent MRI-guided TRUS-guided second biopsy. The probability of test ‘S’ detecting CS cancer given that CS cancer exists is the sensitivity of MRI-guided TRUS-guided biopsy post previous negative TRUS-guided biopsy for CS cancer detection as reported in Schoots et al. 105 (95% CI 0.71 to 0.95; p = 0.87). The probability of test ‘S’ detecting CNS cancer given that CS cancer exists is its complement (95% CI 0.05 to 0.29; p = 0.13).
The diagnostic performance of mpMRI was also obtained from PROMIS and is summarised in Table 27 for different definitions and cut-off points. In PROMIS, lesions were classified by degree of suspicion for any cancer and CS cancer using a five-point Likert scale. The score is converted into test classifications (suspicion of no cancer, suspicion of CNS cancer and suspicion of CS cancer) according to cut-off points 2–5.
| Disease status | mpMRI classification, mean (95% CI) | ||
|---|---|---|---|
| No suspicion of cancer | Suspicion of CNS cancer | Suspicion of CS cancer | |
| Primary definition (definition 2) cut-off point 2 | |||
| NC | 0.00 (0.00 to 0.00) | 0.07 (0.03 to 0.11) | 0.93 (0.89 to 0.97) |
| Low-risk cancer | 0.00 (0.00 to 0.00) | 0.08 (0.02 to 0.14) | 0.92 (0.86 to 0.98) |
| Intermediate-risk cancer | 0.01 (0.00 to 0.02) | 0.01 (0.00 to 0.02) | 0.98 (0.97 to 1.00) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Primary definition (definition 2) cut-off point 3 | |||
| NC | 0.33 (0.26 to 0.40) | 0.17 (0.11 to 0.23) | 0.50 (0.43 to 0.58) |
| Low-risk cancer | 0.28 (0.19 to 0.38) | 0.16 (0.08 to 0.24) | 0.56 (0.46 to 0.67) |
| Intermediate-risk cancer | 0.08 (0.05 to 0.11) | 0.05 (0.02 to 0.07) | 0.87 (0.83 to 0.91) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Primary definition (definition 2) cut-off point 4 | |||
| NC | 0.86 (0.80 to 0.91) | 0.03 (0.01 to 0.05) | 0.11 (0.06 to 0.17) |
| Low-risk cancer | 0.75 (0.66 to 0.84) | 0.04 (0.01 to 0.09) | 0.21 (0.13 to 0.29) |
| Intermediate-risk cancer | 0.30 (0.25 to 0.35) | 0.04 (0.02 to 0.07) | 0.65 (0.60 to 0.71) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Primary definition (definition 2) cut-off point 5 | |||
| NC | 0.96 (0.93 to 0.99) | 0.01 (0.00 to 0.02) | 0.03 (0.01 to 0.06) |
| Low-risk cancer | 0.98 (0.94 to 1.00) | 0.00 (0.00 to 0.00) | 0.02 (0.00 to 0.06) |
| Intermediate-risk cancer | 0.60 (0.54 to 0.65) | 0.03 (0.01 to 0.05) | 0.38 (0.32 to 0.44) |
| High-risk cancer | 0.23 (0.04 to 0.45) | 0.00 (0.00 to 0.00) | 0.77 (0.55 to 0.96) |
| Secondary definition (definition 1) cut-off point 2 | |||
| NC | 0.00 (0.00 to 0.00) | 0.23 (0.17 to 0.29) | 0.77 (0.71 to 0.83) |
| Low-risk cancer | 0.00 (0.00 to 0.00) | 0.20 (0.12 to 0.29) | 0.80 (0.71 to 0.88) |
| Intermediate-risk cancer | 0.01 (0.00 to 0.02) | 0.06 (0.03 to 0.09) | 0.93 (0.90 to 0.96) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Secondary definition (definition 1) cut-off point 3 | |||
| NC | 0.33 (0.26 to 0.40) | 0.41 (0.33 to 0.49) | 0.26 (0.19 to 0.33) |
| Low-risk cancer | 0.28 (0.19 to 0.38) | 0.40 (0.30 to 0.50) | 0.32 (0.22 to 0.41) |
| Intermediate-risk cancer | 0.08 (0.05 to 0.11) | 0.18 (0.13 to 0.22) | 0.74 (0.69 to 0.79) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | 1.00 (1.00 to 1.00) |
| Secondary definition (definition 1) cut-off point 4 | |||
| NC | 0.86 (0.80 to 0.91) | 0.08 (0.04 to 0.13) | 0.06 (0.02 to 0.10) |
| Low-risk cancer | 0.75 (0.66 to 0.84) | 0.14 (0.08 to 0.22) | 0.11 (0.05 to 0.18) |
| Intermediate-risk cancer | 0.30 (0.25 to 0.35) | 0.24 (0.19 to 0.28) | 0.46 (0.41 to 0.52) |
| High-risk cancer | 0.00 (0.00 to 0.00) | 0.06 (0.00 to 0.18) | 0.94 (0.82 to 1.00) |
| Secondary definition (definition 1) cut-off point 5 | |||
| NC | 0.96 (0.93 to 0.99) | 0.02 (0.00 to 0.04) | 0.02 (0.00 to 0.05) |
| Low-risk cancer | 0.98 (0.94 to 1.00) | 0.01 (0.00 to 0.04) | 0.01 (0.00 to 0.04) |
| Intermediate-risk cancer | 0.60 (0.54 to 0.65) | 0.17 (0.13 to 0.22) | 0.23 (0.18 to 0.28) |
| High-risk cancer | 0.23 (0.04 to 0.45) | 0.16 (0.00 to 0.35) | 0.61 (0.38 to 0.84) |
It was assumed that no loss of precision would result from conducting mpMRI after a TRUS-guided biopsy. Biopsy can cause bleeding and inflammation that can take a few months to subside. It was assumed that mpMRI would take place after this period to maintain the diagnostic performance observed in PROMIS.
Health-related quality of life
Health-related quality of life is measured using a preference-based scale on which a value of 0 represents death and a value of 1 represents full health.
Table 28 shows the HRQoL estimates used for the different tests. The underpinning assumptions and values were drawn from findings in PROMIS and the wider literature.
| Parameter | Value, mean (95% CI) | Source or rationale |
|---|---|---|
| Change post mpMRI | Assumed zero | The change post mpMRI in PROMIS was negligible |
| Change post TRUS-guided biopsy | Assumed zero | Essink-Bot et al.108 found no impact of TRUS-guided biopsy on HRQoL |
| Change post TPM-biopsy | –0.176 (–0.15 to –0.20) | Obtained from the PROMIS individual patient data. Duration of decrement assumed to be 2 weeks based on Merrick et al.55 and Tsivian et al.109 |
The men taking part in PROMIS filled in EQ-5D-3L questionnaires at enrolment, after mpMRI, after the CBP (TRUS-guided biopsy and TPM-biopsy) and at the end of the study (on average 42 days after the CBP). The EQ-5D-3L profiles were converted into preference-based index scores using the ‘UK tariff’, which is based on the preferences of a sample of the UK public. 96 The index scores after each test were compared with the values at enrolment. The results show no evidence of a change in index score when comparing post-mpMRI scores with baseline scores (mean difference 0.008, 95% CI –0.002 to 0.018). The data also show a large and statistically significant mean impact of the CBP of –0.176 (95% CI –0.203 to –0.149). Because the two procedures were undertaken concomitantly, these data cannot inform the effects of each individual biopsy. The literature reports that TRUS-guided biopsy (carried out under local anaesthesia) has only minor and transient adverse effects23 and no discernible effect on HRQoL. 108
In contrast, there is some evidence that TPM-biopsy affects urinary, bowel and erectile function,55,109 which could plausibly have an impact on HRQoL. The available evidence suggests that these effects are also transient and resolve within 30 days to 6 weeks. 21,55,57,109 Given this, the model base case assumes that mpMRI and TRUS-guided biopsy have no effect on HRQoL. In the model, TPM-biopsy is assumed to decrease HRQoL by 0.176, as observed in PROMIS, over a time period of 2 weeks. This is incorporated in the model as a normal distribution (mean –0.176, standard error 0.014).
Resource use and costs
The decision tree considers the direct cost of the diagnostic tests and the costs associated with the resources used to manage their adverse effects.
Resource use resulting from adverse effects
In PROMIS, data on health-care resource use were collected for each man at their end-of-study visit, but it was not possible to ascribe the resource use to a specific diagnostic test. Therefore, the resource use associated with the adverse effects of each test was obtained from the literature. For TRUS-guided biopsy, resource use is informed by Rosario et al. 27 Rosario et al. 27 prospectively observed 1147 men in the UK 35 days after TRUS-guided biopsy. During this period, 15 men (1.3%) were admitted to hospital, 92 men (8.0%) consulted a general practitioner, 14 men (1.2%) consulted the urology department nurse and 13 men (1.1%) consulted another source of medical advice. For TPM-biopsy, resource use is informed by Pepe and Aragona. 26 Pepe and Aragona26 report the prevalence of complications, including hospitalisations and accident and emergency (A&E) admissions, in men with suspected prostate cancer who underwent fortransperineal prostate biopsy with 12, 18 or > 24 samples (saturation biopsy), at 15–20 days after the biopsy. Of the 630 men included, 10 men (1.6%) and 91 men (14.4%) experienced a hospital admission and an A&E admission, respectively. Consultations with general practitioners or other health-care contacts were not reported. The mpMRI is assumed to involve no health-care resource use caused by adverse effects. Counts of resource use are incorporated in the model as beta distributions. These are summarised in Table 29.
| Parameter | Value, % (n/N) | Source |
|---|---|---|
| Associated with TRUS-guided biopsy | ||
| Hospital admission | 1.3 (15/1147) | Rosario et al.27 |
| Consultation with general practitioner | 8.0 (92/1147) | |
| Consultation with urology department nurse | 1.2 (14/1147) | |
| Sought other medical advice | 1.1 (13/1147) | |
| Associated with TPM-biopsy | ||
| Hospital admission | 1.6 (10/630) | Pepe and Aragona26 |
| A&E admission | 14.4 (91/630) | |
Unit costs
The model evaluates the cost of testing, which includes the cost of each of the diagnostic tests that form the diagnostic strategies and the cost of managing their adverse effects. The costs refer to the 2014–15 price year. For the base case, the unit costs are based on hospital care costs in the Reference Costs 2014–15110 and the Unit Costs of Health and Social Care 2015. 111 In the scenarios, the unit costs are based on the payment-by-results (PbR) tariff for 2014/15112 and the Unit Costs of Health and Social Care 2015. 111 All of these sources provide the cost of a procedure or appointment to the NHS taking into account capital, overheads and staffing costs. These costs are included in the model deterministically because their variation is a result of the heterogeneity in the patient population rather than parameter uncertainty that can be resolved with additional research. Table 30 shows the unit costs used in the base case.
| Parameter | Value (£) | Source |
|---|---|---|
| Diagnostic tests | ||
| TRUS-guided biopsy | 403 | ‘LB76Z Transrectal ultrasound guided biopsy of the prostate’ |
| TPM-biopsy | 1370 | ‘LB77Z Transperineal template biopsy of prostate’ |
| mpMRI, T2 weighting, DW and DCE | 182 | ‘RD03Z MRI scan of one area with pre- and post-contrast’ |
| Management of AEs from testing | ||
| A&E admission | 132 | Average cost of emergency medicine |
| Hospital admission | 587 | Average cost of non-elective admission, short stay |
| Urology department nurse | 94 | Unit cost of ‘WF01A: non-admitted face-to-face attendance, follow-up, urology’ |
| Other costs that are common to both scenarios | ||
| General practitioner | 44 | Curtis111 |
| Other health-care advice | 7.90 | Curtis111 |
Analytical methods
The decision tree compares the diagnostic outcomes, short-term costs and health outcomes (measured as HRQoL) of the different diagnostic strategies. The results are probabilistic in that they represent the average over 1000 Monte Carlo simulations sampled from the distributions of the parameter inputs. 113 The 95% CIs are calculated as the 97.5 and 2.5 percentiles of the Monte Carlo simulations.
Results are presented for the 32 strategies over the two definitions of TRUS-guided biopsy for CS cancer, the two definitions of mpMRI for CS cancer and the four cut-off points. These form 16 substrategies, as detailed in Table 31. The substrategies are labelled as follows:
-
The first number indicates the definition of CS cancer for TRUS-guided biopsy: the primary definition is definition 1 and the secondary definition is definition 2. Substrategies 112–5 and 122–5 use TRUS-guided biopsy definition 1, the primary CS definition.
-
The second number indicates the definition of CS cancer for mpMRI: the primary definition is definition 2 and the secondary definition is definition 1. Substrategies 122–5 and 222–5 use definition 2, the primary CS cancer definition.
-
The third number indicates the cut-off point: ≥ 2, ≥ 3, ≥ 4 and 5. For example, type 112 uses the TRUS-guided biopsy definition 1, the mpMRI definition 2 and the cut-off point of ≥ 2.
-
There are substrategies for which one or more of the definitions or cut-off points are not applicable; for example, in T1, only the definition of TRUS-guided biopsy is applicable (1 or 2) because mpMRI is not performed. These substrategies have a dash (–) to represent where the definition or cut-off point is not applicable. Therefore, T1 under the TRUS-guided biopsy definition 1 is represented as T1 1––.
| Substrategy | CS definition | mpMRI cut-off point | |
|---|---|---|---|
| TRUS-guided biopsy | mpMRI | ||
| 112 | 1: primary | 1: secondary | 2 |
| 113 | 1: primary | 1: secondary | 3 |
| 114 | 1: primary | 1: secondary | 4 |
| 115 | 1: primary | 1: secondary | 5 |
| 122 | 1: primary | 2: primary | 2 |
| 123a | 1: primarya | 2: primarya | 3a |
| 124 | 1: primary | 2: primary | 4 |
| 125 | 1: primary | 2: primary | 5 |
| 212 | 2: secondary | 1: secondary | 2 |
| 213 | 2: secondary | 1: secondary | 3 |
| 214 | 2: secondary | 1: secondary | 4 |
| 215 | 2: secondary | 1: secondary | 5 |
| 222 | 2: secondary | 2: primary | 2 |
| 223 | 2: secondary | 2: primary | 3 |
| 224 | 2: secondary | 2: primary | 4 |
| 225 | 2: secondary | 2: primary | 5 |
Results
The results are presented in terms of the men referred for testing:
-
the proportion of men with CS cancer detected; this corresponds to the sensitivity of the diagnostic strategies in detecting CS cancer
-
the number of TRUS-guided biopsies; this can vary from zero to two
-
the proportion of men who receive TPM-biopsy
-
the HRQoL loss attributable to the adverse effects of testing; this applies only to TPM-biopsy
-
diagnostic efficiency as the proportion of CS cancers detected per pound spent in testing.
The diagnostic frontier is evaluated by plotting the costs and outcomes of the alternative diagnostic substrategies. The line formed by the strategies with the most CS cancers detected per pound spent is the efficiency frontier and identifies the strategies that represent an efficient use of resources for a certain level of funding. Strategies above the line will detect the same proportion of CS cancers but at a higher cost.
Detection of clinically significant cancer
Figures 14–17 show the probability of detecting CS cancer (i.e. the strategies’ true-positive rate or sensitivity) for each diagnostic substrategy. The diagnostic strategies are represented on the horizontal axis and the probability of detecting CS cancer is represented on the vertical axis. The different substrategies across the different definitions and mpMRI cut-off points are represented as points. The vertical bars represent the 95% CI for the probability of detecting CS cancer for each substrategy. The figures are organised by mpMRI cut-off point, from ≥ 2 to 5, to aid interpretation. Appendix 3 (see Tables 49 and 50) presents the same data in tabular form.
FIGURE 14.
Sensitivity of strategies with a mpMRI cut-off point of ≥ 2.
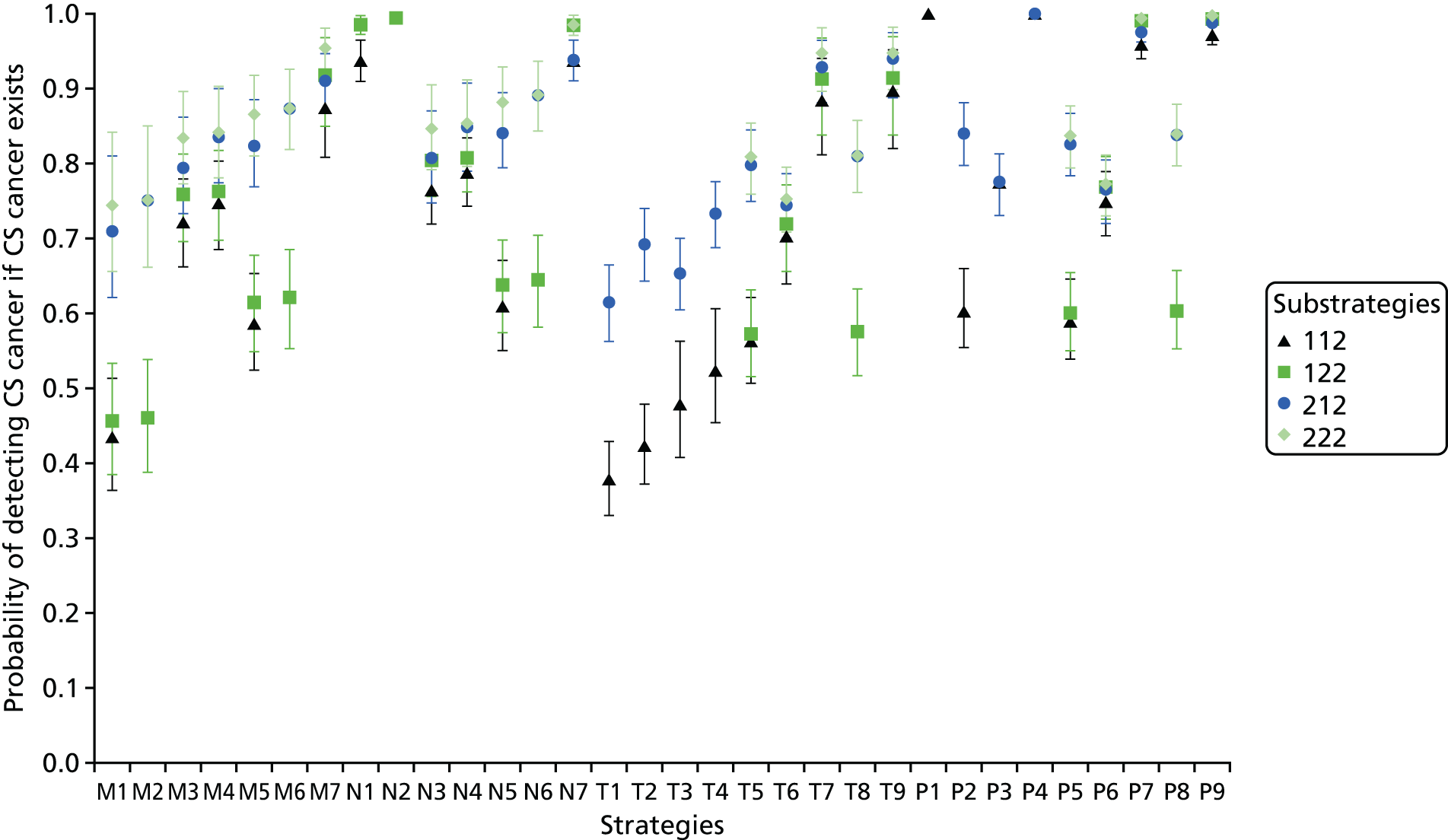
FIGURE 15.
Sensitivity of strategies with a mpMRI cut-off point of ≥ 3.

FIGURE 16.
Sensitivity of strategies with a mpMRI cut-off point of ≥ 4.

FIGURE 17.
Sensitivity of strategies with a mpMRI cut-off point of 5.

The probability of detecting CS cancer varies between strategies and within the same strategy, depending on the CS cancer definitions and cut-off points used. The primary TRUS-guided definition for CS cancer (definition 1) detects up to 0.29 fewer CS cancers than the secondary definition (definition 2). This reflects the stringent primary definition for CS cancers. The implication is that the substrategies using definition 2 always detect more CS cancers than those using definition 1, for a given strategy, mpMRI definition and mpMRI cut-off point.
The difference in the detection of CS cancer between the two mpMRI definitions for CS cancer is smaller than that between the TRUS-guided definitions and depends on the mpMRI cut-off point. On average, the primary CS cancer definition for mpMRI (definition 2: lesions with a volume of ≥ 0.2 ml and/or a Gleason score of ≥ 3 + 4; substrategies 122–5 and 222–5) detects 0.01–0.18 more CS cancers than the secondary definition (definition 1: lesions with a volume of ≥ 0.5 ml and/or a Gleason score of ≥ 4 + 3; substrategies 112–5 and 212–5). This is because definition 2 (primary) is less stringent and hence more sensitive in detecting CS cancers.
Lower mpMRI cut-off points detect a large proportion of CS cancers. For example, substrategies using a mpMRI cut-off point of ≥ 2 (substrategies 112, 122, 212 and 222; see Figure 14) detect nearly all CS cancers. In contrast, in substrategies using a high mpMRI cut-off point, such as those using a cut-off point of 5 (substrategies 115, 125, 215 and 225; see Figure 17), a smaller proportion of cancers is detected.
The most sensitive combination of definitions and mpMRI cut-off point to detect CS cancer includes the secondary TRUS-guided biopsy definition (definition 2), the primary mpMRI definition (definition 2) and a PI-RADS cut-off point of ≥ 2 (i.e. substrategy 222). This will be referred to hereafter as the ‘most sensitive definition’. The least sensitive combination of definitions and mpMRI cut-off point uses the primary TRUS-guided biopsy definition (definition 1), the secondary mpMRI definition (definition 1) and a mpMRI cut-off point of 5 (i.e. substrategy 115). This will be referred to hereafter as the ‘least sensitive definition’.
As evident in Figures 14–17, strategies including TPM-biopsy detect the most CS cancers. Strategies P1 (all men are tested with TPM-biopsy) and P4 (all men in whom TRUS-guided biopsy did not detect cancer or detected CNS cancer are retested with TPM-biopsy) detect all CS cancers.
Some strategies at specific cut-off points and definitions detect most CS cancers:
-
P9 consists of TRUS-guided biopsy in all men, mpMRI in men in whom no CS cancer was detected and rebiopsy with TPM-biopsy in men whose mpMRI result was suspicious of CS cancer (in men for whom TRUS-guided biopsy detected CNS cancer) or suspicious of any cancer (in men for whom TRUS-guided biopsy did not detect cancer). P9 using the most sensitive definition (substrategy P9 222) detects all CS cancers. The proportion of CS cancers detected decreases to a minimum of 56% when using the least sensitive definition for mpMRI and TRUS-guided biopsy, definition 1 (substrategy P9 115).
-
P7 consists of testing all men with TRUS-guided biopsy; men in whom no cancer or CNS cancer is detected are retested with mpMRI and men with suspected CS cancer are rebiopsied with TPM-biopsy. This detects 99% of CS cancers under the most sensitive definition for mpMRI and TRUS-guided biopsy, definition 2 (substrategy P7 222), and 52% under the least sensitive definition (substrategy P7 115).
-
N1 consists of testing all men with mpMRI and conducting TPM-biopsy in all men with suspected CS cancer. It detects 98% of CS cancers under the most sensitive definition of mpMRI, definition 2 (substrategy N1 222), and 28% under the least sensitive definition (substrategy N1 115).
-
N2 consists of testing all men with mpMRI and conducting TPM-biopsy in all men with suspected cancer. It detects 99% of CS cancers under the most sensitive definition of mpMRI, definition 2 (substrategy N2 222), and 44% under the least sensitive definition (substrategy N2 115).
-
T7 and T9 detect 95% of all CS cancers under the most sensitive definitions. In T7 and T9, all men receive TRUS-guided biopsy. In T7, men in whom CS cancer was not detected undergo mpMRI and those with suspected CS cancer are rebiopsied. In T9, men in whom CS cancer was not detected undergo mpMRI. Men whose first biopsy indicated no cancer receive a second biopsy if the mpMRI result is suspicious for any cancer. Men whose first biopsy indicated CNS cancer receive a second biopsy if the mpMRI result is suspicious for CS cancer.
-
M7 consists of testing all men with mpMRI, conducting TRUS-guided biopsy in men with suspected CS cancer and rebiopsying those in whom CS cancer was not detected. This strategy detects 95% of CS cancers under the most sensitive definition (substrategy M7 222). Under the least sensitive definition, M7 detects 26% of CS cancers (substrategy M7 115).
Given that the accuracy data for each diagnostic strategy are drawn from sampled data, there is some uncertainty regarding the sensitivity of each substrategy in detecting CS cancer. This is greater in strategies with smaller test sequences that do not include TPM-biopsy, such as T1 [which detects 38% of CS cancers (95% CI 33% to 43%)] and M1 [which detects 44% of CS cancers (95% CI 36% to 51%)].
The PROMIS-proposed substrategy, M7 123, detects 82% of CS cancers (95% CI 75% to 87%). M7 consists of testing all men with mpMRI, conducting TRUS-guided biopsy in men with suspected CS cancer and rebiopsying those in whom CS cancer was not detected. This compares favourably with T4 1––, the standard care substrategy, which does not include mpMRI and which detects 52% of CS cancers (95% CI 45% to 61%). T4 consists of testing all men with TRUS-guided biopsy and repeating this biopsy in those men in whom CS cancer was not detected. The model calculates that the substrategy most similar to the NICE recommendations,13 T7 123, detects 85% of CS cancers (95% CI 78% to 91%). In T7, men in whom CS cancer was not detected undergo mpMRI and those men with suspected CS cancer are rebiopsied.
Biopsies
Number of transrectal ultrasound-guided biopsies
One of the aims of introducing mpMRI in the diagnostic pathway for prostate cancer is to avoid unnecessary biopsies. Figure 18 shows the number of TRUS-guided biopsies carried out per man referred for diagnosis for each substrategy. Note that this number can vary only from zero to two because the diagnostic strategies were constrained to include up to two biopsies. Strategies P1, N1 and N2 do not include TRUS-guided biopsy and hence the average number of TRUS-guided biopsies per referred man is zero.
FIGURE 18.
Number of TRUS-guided biopsies carried out per man referred for testing for each substrategy.
The strategies starting with TRUS-guided biopsy involve at least one TRUS-guided biopsy per patient; however, some involve more. Strategy T4, under TRUS-guided biopsy definition 1, has the highest mean number of TRUS-guided biopsies per man referred, at 1.79. In this strategy, all men have TRUS-guided biopsy and all men in whom CS cancer is not detected undergo a second TRUS-guided biopsy. Because, under CS cancer definition 1, fewer CS cancers are detected, 79% of men receive a second TRUS-guided biopsy. Other strategies starting with TRUS-guided biopsy with a high number of TRUS-guided biopsies per referred man are strategies T7 and T9, with a maximum of 1.75 and 1.78, respectively. In strategy T7, all men receive TRUS-guided biopsy and men in whom CS cancer was not detected receive mpMRI. Men with suspected CS cancer on mpMRI receive a second TRUS-guided biopsy. Strategy T9 is similar, but also includes rebiopsy in men in whom the mpMRI result showed suspected CNS cancer after an initial TRUS-guided biopsy that did not detect cancer. Strategies with TRUS-guided biopsy definition 1 for CS cancer and low cut-off points for the mpMRI score have a greater proportion of men referred for a second biopsy. Therefore, the average number of TRUS-guided biopsies per referred man increases.
Strategies starting with mpMRI (M1–M7 and N1–N7) have a generally lower mean number of TRUS-guided biopsies per referred man. However, in two strategies, > 50% of men receive two TRUS-guided biopsies; these are strategies M6 and M7, under the TRUS-guided biopsy definition 1 and cut-off point of ≥ 2, in which the average number of TRUS-guided biopsies is 1.51 and 1.66, respectively. In strategy M6, men with suspected cancer on mpMRI receive a TRUS-guided biopsy and are rebiopsied if no cancer is detected. In strategy M7, men with suspected CS cancer on mpMRI receive a TRUS-guided biopsy and are rebiopsied if CS cancer is not detected.
Number of template prostate mapping biopsies
Figure 19 shows the proportion of men who undergo TPM-biopsy. As expected, given the structure of the strategies, strategies M1–M7 and T1–T9 do not involve TPM-biopsy. Strategies N1–N7 and P1–P9 include TPM-biopsy as the last biopsy in the series. Some strategies involve TPM-biopsy in all men referred for diagnosis: P1, which consists of offering TPM-biopsy to all men with no prior testing, and N2, in which men are first tested with mpMRI and men with suspected cancer receive TPM-biopsy, under the cut-off point of ≥ 2. Under this cut-off point, all men referred for diagnosis have suspected cancer and, therefore, receive TPM-biopsy.
FIGURE 19.
Proportion of men who receive a TPM-biopsy.
Cost of testing
Figure 20 shows the costs of testing (see Appendix 3, Tables 51 and 52, for the results in tabular form). The least costly test (at £244, 95% CI £232 to £257) is strategy M1 (mpMRI for all men and TRUS-guided biopsy for men in whom there is suspicion of CS cancer) using the least sensitive definition. This strategy is the least costly because it includes only one biopsy and, using this definition and cut-off point, mpMRI results in only 15% of men being referred for TRUS-guided biopsy. Using more sensitive definitions, a greater proportion of men are referred for TRUS-guided biopsy.
FIGURE 20.
Costs of testing.
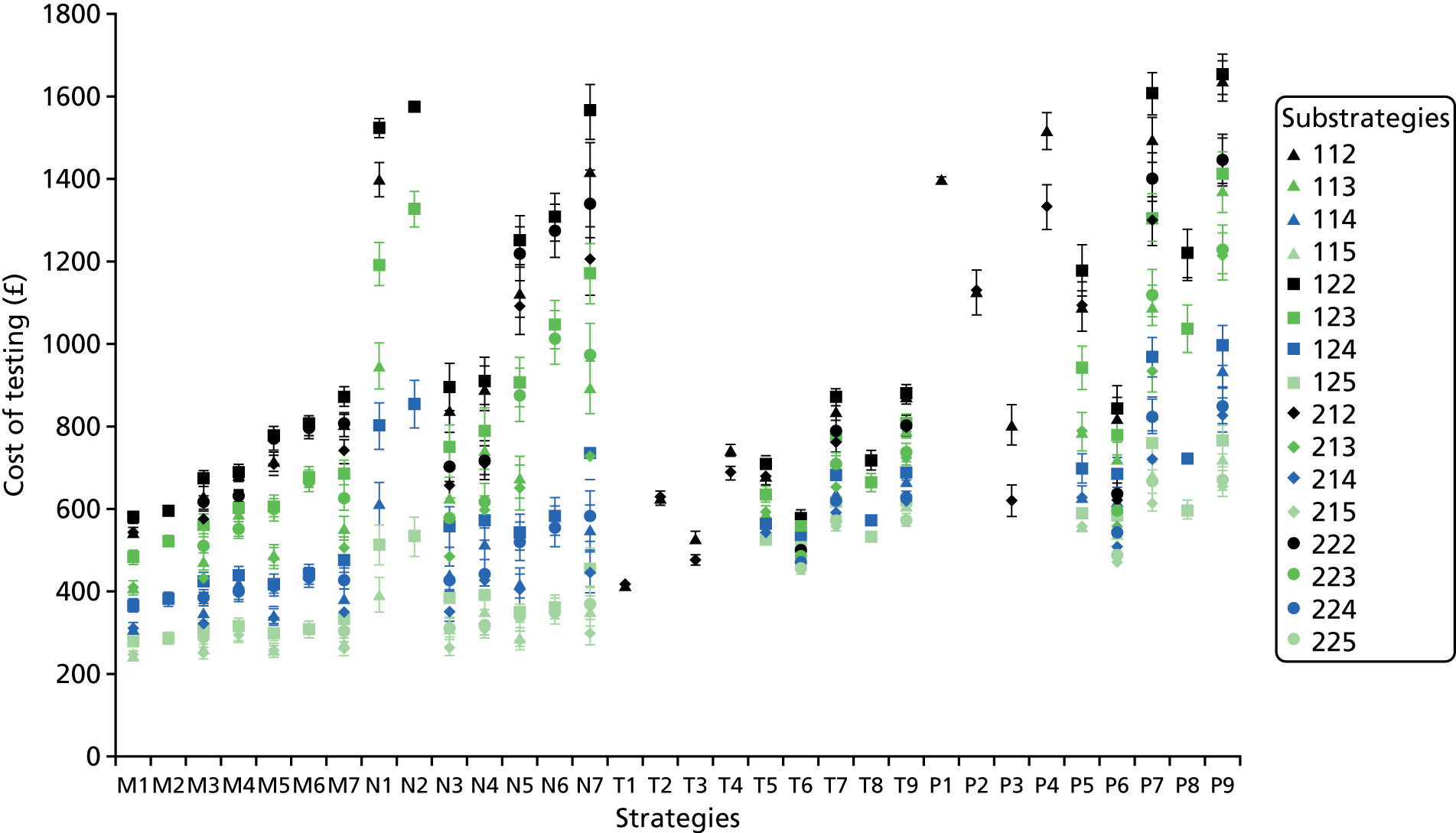
The most costly substrategy is P9 112, at £1654 (95% CI £1604 to £1702) per man referred. This substrategy consists of TRUS-guided biopsy in all men, mpMRI in men in whom no CS cancer was detected and rebiopsy with TPM-biopsy in men whose mpMRI result was suspicious of CS cancer (in men in whom TRUS-guided biopsy detected CNS cancer) or suspicious of any cancer (in men in whom TRUS-guided biopsy did not detect cancer) using the TRUS-guided biopsy definition 1, the secondary mpMRI definition 2 and the cut-off point of ≥ 2.
The 95% CIs are relatively narrow, at up to 9% greater or lower than the average cost. Uncertainty regarding the cost of testing arises from uncertainty regarding the probability that each test correctly classifies men, which determines whether or not men receive a subsequent test, and the risk of AEs for strategies involving biopsy.
The PROMIS-proposed substrategy, M7 123, costs £687 (95% CI £657 to £719). This is less than the cost of T4 1––, the standard care substrategy without mpMRI, at £742 (95% CI £727 to £757), and the cost of the NICE-proposed substrategy T7 123, at £780 (95% CI £760 to £801).
Health-related quality of life
Only strategies involving TPM-biopsy are associated with a HRQoL loss, because mpMRI and TRUS-guided biopsy are assumed not to have HRQoL implications. The HRQoL loss depends on the proportion of men receiving TPM-biopsy and thus, as expected, strategy P1 (in which all men receive TPM-biopsy) presents the biggest loss at –0.007 QALYs. The strategy with the next greatest HRQoL loss is N2 (mpMRI for all men and TPM-biopsy for men in whom there is suspicion of cancer) at the cut-off point of ≥ 2, irrespective of the mpMRI definition.
Diagnostic efficiency
Figure 21 shows the relationship between the proportion of CS cancers detected and the expected cost of testing. The strategies forming the line (called a frontier) are efficient because they offer the greatest detection of CS cancers for different costs of testing. Strategies above the efficiency frontier are inefficient because they are expected to detect fewer CS cancers for the same cost. Table 32 details the strategies forming the efficiency frontier. None of these strategies, except P4, has HRQoL implications because they do not include TPM-biopsy. Neither the PROMIS-proposed substrategy (M7 123) nor any of the standard care substrategies (T4 1–– and T7 123) form the diagnostic efficiency frontier.
FIGURE 21.
Diagnostic efficiency.

| Strategy | TRUS-guided biopsy definition | mpMRI definition | mpMRI cut-off point | Detection of CS cancer, mean (95% CI) (%) | Cost (£) of testing, mean (95% CI) |
|---|---|---|---|---|---|
| M1: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer | 2 | 1 | 5 | 22 (17 to 27) | 244 (232 to 257) |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer at the first biopsy receive a second TRUS-guided biopsy | 2 | 1 | 5 | 24 (20 to 29) | 250 (237 to 264) |
| 2 | 2 | 5 | 35 (30 to 41) | 290 (273 to 306) | |
| M4: mpMRI for all men and TRUS-guided biopsy in men with suspicion of any cancer; men with suspected CS cancer on mpMRI and in whom CNS cancer was detected at the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 5 | 37 (32 to 43) | 296 (280 to 312) |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 5 | 40 (35 to 46) | 307 (287 to 328) |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer at the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 4 | 57 (51 to 64) | 385 (366 to 404) |
| M4: mpMRI for all men and TRUS-guided biopsy in men with suspicion of any cancer; men with suspected CS cancer on mpMRI and in whom CNS cancer was detected at the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 4 | 60 (54 to 67) | 400 (382 to 420) |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 4 | 65 (60 to 71) | 430 (404 to 458) |
| T6: TRUS-guided biopsy for all men; men classified with CNS cancer receive mpMRI and men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 74 (69 to 78) | 488 (473 to 503) |
| 2 | 2 | 2 | 75 (71 to 79) | 500 (484 to 517) | |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 3 | 85 (81 to 89) | 628 (597 to 660) |
| T7: TRUS-guided biopsy for all men; men classified as having NC or CNS cancer receive mpMRI and men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 91 (86 to 94) | 709 (688 to 730) |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 2 | 95 (92 to 98) | 807 (777 to 833) |
| P4: TRUS-guided biopsy in all men and TPM-biopsy for men in whom the biopsy did not detect CS cancer | 2 | Not applicable | Not applicable | 100 (100 to 100) | 1332 (1278 to 1385) |
Summary of findings
-
Multiparametric magnetic resonance imaging, TRUS-guided biopsy and TPM-biopsy can be used in 32 different combinations to diagnose prostate cancer. Multiparametric magnetic resonance imaging can be used as a first test for all men referred to secondary care or following a negative TRUS-guided biopsy result. Likewise, TRUS-guided biopsy can be used as a first test or as a targeted test following mpMRI. The different combinations have been evaluated for different diagnostic definitions and cut-off points, forming 383 diagnostic substrategies. Our analysis was informed by the evidence obtained in PROMIS, together with published studies on repeated TRUS-guided biopsy and TRUS-guided biopsy in combination with mpMRI. 103–105
-
The short-term outcomes relate to the immediate consequences of using the different diagnostic strategies in men with suspected CS cancer. These are the proportion of CS cancers detected (sensitivity), the number of TRUS-guided biopsies, the proportion of men receiving TPM-biopsy and the cost of testing (which includes the cost of managing any adverse effects from testing). The proportion of CS cancers detected and the cost of testing form the cost-efficiency plane and frontier.
-
The strategies considered represent a range of levels of use of TRUS-guided biopsy, from 0 to 1.79 biopsies per man. The strategies considered also represent a range of levels of use of TPM-biopsy, from strategies in which no men receive TPM-biopsy to those in which all men receive this test.
-
The proportion of CS cancers detected depends on the diagnostic definition and mpMRI cut-off point. The most sensitive strategies use the TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off point 2. The least sensitive strategies use the TRUS-guided biopsy definition 1, mpMRI definition 1 and cut-off point 5.
-
P1 and P4 detect all CS cancers. Some strategies under the most sensitive definitions and cut-off point detect almost all CS cancers: N1, N2, P7 and P9, involving TPM-biopsy; T7 and T9, involving TRUS-guided biopsy as the first test; and M7, involving mpMRI as the first test.
-
The diagnostic strategies cost between £244 and £1654 per man to the UK NHS.
-
A limited number of strategies form the cost-efficiency frontier. These strategies detect the greatest proportion of CS cancer by level of spending (on testing and adverse effects management). Of these, only four substrategies detect ≥ 80% of CS cancers: M7 (substrategies 223 and 222), T7 (substrategy 223) and P4 (substrategy 2––).
-
In M7, men whose mpMRI result is suspicious of CS cancer receive TRUS-guided biopsy and men in whom no CS cancer was detected receive a second biopsy.
-
In T7, all men receive TRUS-guided biopsy and men in whom TRUS-guided biopsy does not detect CS cancer undergo mpMRI. Men in whom the mpMRI result is suspicious of CS cancer receive a second biopsy.
-
In P4, all men receive TRUS-guided biopsy and men in whom TRUS-guided biopsy does not detect CS cancer undergo TPM-biopsy.
-
Chapter 6 Evaluation of long-term outcomes
The cost-effectiveness of the diagnostic strategies depends not only on their short-term outcomes, described in Chapter 5, but also on the long-term outcomes of the subsequent management decisions. To consider long-term outcomes, information is required on the lifetime costs, survival and quality-adjusted survival of men conditional on their true disease status and the treatment decision, with these last two aspects being defined by the diagnostic classification outlined in Table 33.
| Disease status | Diagnostic classification | ||
|---|---|---|---|
| NC detected | CNS cancer detected | CS cancer detected | |
| NC | Primary care follow-up | Not applicablea | Not applicablea |
| Low-risk cancer | Primary care follow-up | Active surveillance | Not applicablea |
| Intermediate-risk cancer | Primary care follow-up | Active surveillance | Radical treatment |
| High-risk cancer | Primary care follow-up | Active surveillance | Radical treatment |
The clinical effectiveness and cost-effectiveness of treatments will determine the value of the upstream diagnostic strategies. For this reason, ideally, alternative ways of delivering primary care management (for patients identified as having no cancer), alternative active surveillance protocols (for those identified with CNS cancer) and alternative treatment choices for CS cancer should be evaluated to determine the best diagnostic strategy/test combination. If it is not possible to do so, then the post-testing health-care choices modelled need to be the most effective/cost-effective, so that the value of testing is appropriately considered. Otherwise, the cost-effectiveness evaluation of the diagnostic strategies may be biased. For example, more-sensitive diagnostic strategies are favoured if the net health of radical treatment for intermediate- and high-risk (i.e. CS) cancers is overestimated. Conversely, less sensitive diagnostic strategies are favoured if the net health of primary care follow-up and active surveillance in CS cancers is overestimated.
No study was identified that provided the lifetime costs and quality-adjusted survival of men with low-, intermediate- and high-risk cancer managed with primary care, active surveillance and radical treatment. Therefore, the 2014 NICE clinical guideline,13 taken to represent the best available evidence in terms of management and treatment recommendations for prostate cancer in the UK setting, was reviewed. Clinical guidelines are informed by reviews of the clinical effectiveness and cost-effectiveness evidence, and bespoke cost-effectiveness modelling, deliberated by an expert committee. They make recommendations on how patients should be managed in light of the best available evidence and to make the best use of the available resources. The aim is to replicate in the long-term model the recommended management options.
Evidence on long-term outcomes
National Institute for Health and Care Excellence recommendations and supporting evidence
As discussed in Chapter 4, the 2014 NICE clinical guideline13 recommends that men diagnosed with localised prostate cancer are managed depending on the risk of cancer progression. Men with low-risk cancer should be offered active surveillance. Men with an intermediate risk should be offered RP or radical radiotherapy (as radical external-beam radiotherapy or as radiotherapy with hormones) but can also consider active surveillance or high-dose rate brachytherapy with external-beam radiotherapy. Men with high-risk cancer should be offered RP or radical radiotherapy (as radical external-beam radiotherapy or as radiotherapy with hormones). These recommendations are based on the findings of systematic reviews and cost-effectiveness analyses conducted for the 201413 and 2008114 NICE clinical guidelines on prostate cancer.
The 2014 NICE clinical guideline13 developed a protocol for the active surveillance of men with low-risk cancer (see Table 2) based on clinical consensus, informed by a systematic review of the protocols for active surveillance in 16 cohorts and a survey of cancer networks in England and Wales. 114 No health economic evidence was identified on the most cost-effective way of monitoring men diagnosed with low-risk cancer.
The recommendation of radical treatment for intermediate- and high-risk prostate cancer is based on the systematic review and cost-effectiveness analysis conducted for the 2008 NICE clinical guideline. 114 The systematic review identified one RCT comparing RP with watchful waiting, the SPCG-4 trial,115 that was considered to be applicable to the UK context. At the time of this review, the SPCG-4 trial had reported up to 10 years of follow-up. 115,116
Another systematic review of the economic evidence found five studies but considered only two studies117,118 to be relevant to the UK context. Hummel et al. 117 evaluated the cost-effectiveness of different management options, including active surveillance, from the NHS perspective. The results were driven by the AEs from the different treatments because it was assumed that the rate of cancer progression and death were not affected by the treatments. Calvert et al. 118 compared watchful waiting with RP in men with low- or intermediate-risk cancer (Gleason grades 5–7) from a NHS perspective. Although RP increased survival rates, it also caused a reduction in HRQoL because of the associated AEs. As a result, RP resulted in fewer health benefits and higher costs than watchful waiting.
Given the evidence from the 10-year follow-up of the SPCG-4 trial, and based on these data, a new cost-effectiveness analysis was conducted to compare the costs and health outcomes of RP with those of alternative management options for men with clinically localised prostate cancer. 13 The alternative management options included brachytherapy, standard external-beam radiotherapy, intensity-modulated radiation therapy, high-intensity focused ultrasonography, cryotherapy and watchful waiting. Health outcomes were expressed as QALYs. Costs were expressed in Great British pounds (GBP) from a 2006 price base from the perspective of the UK NHS. The effect of treatment was based on the 10-year follow-up of the SPCG-4 trial. 119 Because the SPCG-4 trial evaluated RP compared with watchful waiting, the cost-effectiveness of different therapies was explored in a threshold analysis. The time horizon was 20 years.
Radical prostatectomy was found to be more costly than watchful waiting (£10,619 compared with £6185, respectively). It was effective in extending survival (10.19 life-years compared with 9.69 life-years, respectively) and improving quality-adjusted survival (7.52 vs. 6.96 QALYs) if AEs were not considered. However, if AEs were considered, RP was associated with lower quality-adjusted survival (6.36 QALYs vs. 6.63 QALYs) and, therefore, it was dominated by watchful waiting. Probabilistic sensitivity analysis was not conducted. The univariate sensitivity analysis suggested that the results were sensitive to the rate of AEs, but robust to plausible changes in the cost of RP, the cost of watchful waiting and the survival benefit from RP. Subgroup analysis by cancer risk was not carried out. The threshold analysis suggested that modest increases in quality-adjusted survival are required for other treatment options to be cost-effective compared with watchful waiting. However, these increases in quality-adjusted survival depend not only on the clinical effectiveness of treatments but also on their AE profiles.
Given the cost-effectiveness evidence, the 2008 NICE clinical guideline114 recommended, and the 2014 NICE clinical guideline13 maintained, that men with intermediate-risk localised prostate cancer should be offered RP or radical radiotherapy. This recommendation includes men with high-risk localised prostate cancer when there is a realistic prospect of long-term disease control.
Clinical effectiveness of radical treatment in the recent literature
The 2014 NICE clinical guideline13 recommends RP or radical radiotherapy for men with intermediate- or high-risk prostate cancer, who are classified in PROMIS as having CS cancer. However, and as discussed in the previous section, the 2014 NICE guideline found no clear evidence on the clinical effectiveness and cost-effectiveness of RP compared with radical radiotherapy.
For the current study, the literature was examined for more recent systematic reviews, namely Xiong et al. 120 and Wallis et al. 101 These have also been inconclusive. Xiong et al. 120 conducted a systematic review and network meta-analysis of RCTs comparing RP, external-beam radiotherapy, watchful waiting and cryotherapy for localised prostate cancer. No treatment reached significance at the 95% credible interval superior although hypofractionated conformal low-dose radiotherapy was the treatment most likely to be clinically effective (OR vs. watchful waiting 0.53, 95% credible interval 0.10 to 2.88). Wallis et al. 101 conducted a systematic review and meta-analysis of RCTs and cohort and case–control studies comparing RP and radiotherapy for localised prostate cancer. They found that RP statistically significantly reduces all-cause death [hazard ratio (HR) 1.63, 95% CI 1.54 to 1.73] and death attributable to prostate cancer (HR 2.08, 95% CI 1.76 to 2.47). However, this study has been criticised for not appropriately accounting for the risk of bias in observational studies, because men who receive RP are typically younger and healthier than men who receive radiotherapy.
Given these issues, the reviews were examined for RCTs comparing RP or radiotherapy with expectant management strategies such as watchful waiting. These were the studies by Graversen et al. ,121 Wilt122 Bill-Axelson et al. (the SPCG-4 study)6,115,119,123 and Widmark. 124 The trial by Graversenet al. 121 was not included in this review because it recruited participants 4–5 decades in the past and, therefore, is unlikely to reflect the current standard of care. The trial by Widmark124 which compared radiotherapy with watchful waiting, was excluded because it has been published only in abstract format. Therefore, two studies were included: Wilt,8 who reported on PIVOT, and Bill-Axelson et al. ,6,115,119,123 who reported on the SPCG-4 RCT (Table 34). Both studies compare RP with observation. RP reduces the risk of all-cause death, death attributable to prostate cancer and progression, although the magnitude of the effect may depend on the cancer risk of progression. In the absence of RCT evidence on radiotherapy compared with watchful waiting (or other types of deferred treatment such as active monitoring) in men with intermediate- and high-risk cancer, RP was selected in the base case as the treatment of choice for men with intermediate- and high-risk prostate cancer.
| Item | Study | |
|---|---|---|
| Wilt8 | Bill-Axelson et al.6,115,119,123 | |
| Country | USA | Sweden, Finland and Iceland |
| Enrolment period | 1994–2002 | 1989–99 |
| Stage (see Table 1) | T1–T2 | T1b–T2 |
| Interventions and sample size per group | Observation, n = 367; prostatectomy, n = 364 | Observation, n = 348; prostatectomy, n = 347 |
| Outcomes | Overall survival, cancer survival, bone metastases | Overall survival, cancer mortality, distant metastasis, local progression |
| Follow-up (years) | 10 | 18 |
| Subgroups | Age, ethnicity, Charlson Comorbidity Index, performance status, PSA level and cancer risk | Age and cancer risk |
| Entire study population | Non-SS: RP reduces risk of all-cause death and cancer death; SS: RP reduces risk of bone metastases | SS: RP reduces risk of all-cause death, cancer death, distant metastases and local progression |
| Intermediate risk | Non-SS: RP reduces risk of cancer death and bone metastases; SS: RP reduces risk of all-cause death | SS: RP reduces risk of all-cause death, cancer death, distant metastases and local progression |
| High risk | Non-SS: RP reduces the risk of all-cause death and cancer death; SS: RP reduces the risk of bone metastases | Non-SS: RP reduces risk of all-cause death, cancer death and distant metastases; SS: RP reduces risk of local progression |
The clinical effectiveness of radical prostatectomy for clinically significant cancer
Following the 2014 NICE clinical guideline,13 the decision model assumes that men classified as having CS cancer receive RP. The results from the SPCG-4 trial and PIVOT suggest that the treatment effect depends on the risk of cancer progression. Therefore, the model should include the treatment effect specific to each cancer risk subgroup. This treatment effect can be obtained from one of the two RCTs identified or from a meta-analysis of these studies if they are sufficiently homogeneous to allow pooling.
Both the SPCG-4 trial and PIVOT include subgroup analysis by cancer risk. In the SPCG-4 trial, low risk was defined as a PSA level of < 10 ng/ml and either a Gleason score of < 7 or a World Health Organization grade of 1; high risk was defined as a PSA level of ≥ 20 ng/ml or a Gleason score of > 7; and the intermediate-risk group consisted of men who did not fulfil the low-risk or high-risk criteria. 6 The risk stratification was similar in PIVOT. Low risk was defined as a PSA level of ≤ 10 ng/ml, a Gleason score of ≤ 6 and tumour stage T1–T2a; intermediate risk was defined as a PSA level of > 10 ng/ml and ≤ 20 ng/ml, a Gleason score of 7 and tumour stage T2b; and high risk was defined as men who were not low risk or intermediate risk but whose cancer was still clinically localised. 8 These two definitions are similar to the risk stratification in the 2014 NICE clinical guidelines. 13
To choose the approach for the base case (meta-analysis or single study), the participant characteristics of the SPCG-4 trial and PIVOT were compared with the characteristics of participants enrolled in PROMIS. The inclusion criteria for PROMIS were men aged ≥ 18 years at risk of prostate cancer who had been referred for biopsy, a serum PSA level of ≤ 15 ng/ml within the previous 3 months, suspected stage of ≤ T2 on rectal examination and fit to undergo all study procedures. In PROMIS, men with intermediate-risk CS cancer had a mean age of 64.9 years (SD 7.3 years) and their mean PSA level was 8.0 ng/ml (SD 3.0 ng/ml) and men with high-risk CS cancer had a mean age of 66.8 years (SD 6.5 years) and their mean PSA level was 8.3 ng/ml (SD 3.4 ng/ml). Their mean age is similar to that of men in the SPCG-4 trial but, within the risk subgroups, their mean PSA level is lower. In the SPCG-4 trial, men with intermediate-risk cancer had a mean PSA level of approximately 11.5 ng/ml (SD not reported) and men with high-risk cancer had a mean PSA level of approximately 25–29 ng/ml (SD not reported). It is not possible to compare the PROMIS intermediate- and high-risk subgroup characteristics with those of PIVOT because PIVOT does not report baseline characteristics by subgroup. When comparing the entire PIVOT population with the SPCG-4 trial population, the mean age in PIVOT is slightly older, the mean PSA level is lower and the mean clinical stage is less severe, because approximately half of the men in PIVOT had stage T2 cancer compared with three-quarters of the men in the SPCG-4 trial. Because the SPCG-4 trial population appears to be at a higher risk of cancer progression than the PROMIS population, PIVOT was selected as the source of the treatment effect for RP in men with intermediate- and high-risk CS cancer. Recently, the ProtecT study,9 comparing active surveillance, RP and radiotherapy in men with localised prostate cancer, has reported results. This study was not considered because it does not report results by cancer risk subgroup. Access to individual-level data from these studies was not sought.
Methods
Overview
The long-term model aims to predict the lifetime QALYs and costs of men with no cancer (who are followed in primary care), low-risk cancer (who can be followed in primary care or managed with active surveillance) and intermediate- and high-risk cancer (who can be followed in primary care, be managed with active surveillance or receive radical treatment). As discussed earlier, the NICE cancer risk classifications13 are broadly equivalent to the PROMIS cancer significance definitions: low risk is equivalent to CNS cancer and intermediate risk and high risk are equivalent to CS cancer.
Ideally, the long-term model would be informed by a RCT in which men with CNS (low-risk) cancer were randomised to receive primary care management or active surveillance and men with CS (intermediate- and high-risk) cancer were randomised to receive primary care management, active surveillance or radical treatment. However, only two RCTs were identified that provide relevant evidence: the SPCG-4 trial6,115,119,123 and PIVOT. 124 As discussed earlier, because men in the SPCG-4 trial appear to have more severe cancer than men in PROMIS, even within their risk subgroups, PIVOT is used as the source of evidence for the base case. This assumes that observation in PIVOT is equivalent to the NICE-recommended active surveillance protocol13 in its consequences for health outcomes.
Note that PIVOT stratified patients into subgroups of risk using an imperfect test (TRUS-guided biopsy) and thus a proportion of patients with a higher risk of cancer were misclassified and included in lower-risk groups. Given the absence of alternative evidence, the analysis takes the evidence as it is. In addition, the long-term outcomes of men with cancer who were misclassified as having no cancer and who are followed in primary care are assumed to be equivalent to those of men who are managed with observation in PIVOT. The implications of this assumption are explored in the sensitivity analysis. In addition, the PIVOT observation protocol8 is assumed to be equivalent to the NICE active surveillance protocol13 in its detection of progression and referral to subsequent treatment.
To predict the lifetime QALYs and costs of men with cancer, information is required on the length of time that men spend with both localised and metastatic disease. A decision model can predict the lifetime QALYs and costs of men with cancer, using information on their probability of developing metastases, their probability of death without metastases and their probability of death once metastases have developed. There are a number of challenges raised by the data on PIVOT reported by Wilt et al. 8 to inform the cost and QALYs:
-
Some men in PIVOT are alive at the time of reporting. Therefore, the model needs to extrapolate the survival of these men based on the available information.
-
Wilt et al. 8 report the cumulative prevalence of metastases at 4, 8 and 12 years, which does not allow for the direct estimation of the probability of developing metastasis.
-
Wilt et al. 8 do not report the prevalence of death in men who have progressed to metastases. In other words, this does not allow for the direct estimation of the probability of death from metastasis.
For these reasons, the modelling of long-term outcomes takes a three-step approach:
-
The life-years from diagnosis for each subgroup and management option are predicted using a partitioned model based on all-cause mortality reported in PIVOT.
-
A plausible set of transition probabilities between progression-free survival, metastases and death are selected using the life-years predicted from data on the prevalence of metastasis from PIVOT8 and the prevalence of death following metastasis from another study [Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE)]. 125
-
The lifetime QALYs and costs are predicted using the plausible set of transition probabilities obtained from step 2.
In summary, for the prediction of long-term outcomes, the model assumes that the health outcomes of men in PIVOT are representative of the health outcomes of men with prostate cancer. Hence, men with CS cancer who are classified as such are assumed to be equivalent to the men with intermediate- and high-risk cancer who are allocated to RP. Men with CS cancer who are classified as having no cancer or as having CNS cancer are assumed to be equivalent to the men with intermediate- and high-risk cancer who are allocated to observation. Men with CNS cancer who are classified as having no cancer or as having CNS cancer are assumed to be equivalent to men with low-risk cancer who are allocated to observation. For the calculation of costs, the modelling of long-term outcomes assumes that men classified as having CS cancer receive active treatment in the form of RP. Men classified as having CNS cancer receive active surveillance. Men classified as having no cancer are discharged to primary care.
Modelling approach
Step 1: life-years
Appendix 4 reports the modelling approach in detail. In brief, the survival curves reported in the supplementary material of Wilt et al. 8 for each subgroup allocated to observation were digitised using WebPlotDigitizer version 3.12 (Ankit Rohatgi, Austin, TX, USA), a free online tool. Alternative parametric distributions were assessed for goodness of fit with the available data. The Weibull distribution was selected across the three cancer risk subgroups. In the extrapolation period, the Weibull distribution predicted lower hazards of death than those of the general population. Therefore, at relevant times, the hazard of death of the general population was used to predict survival.
Step 2: transition probabilities
Step 1 obtains the life-years for each subgroup and each treatment option. However, the prediction of quality-adjusted survival (i.e. QALYs) and costs requires information about the health state in which these life-years are lived, namely whether with progression-free or metastatic disease. This requires information on the transition probabilities from progression-free disease to metastasis or death and from metastasis to death. This is obtained with a Markov model with three health states (progression-free disease, metastatic disease and death), evaluated by calibration (Figure 22). The model is calibrated to the life-years predicted in step 1, the proportion of patients with metastases in Wilt et al. 8 and a plausible range of values for the probability of dying after metastasis from the standard care arm of the STAMPEDE trial. 125
FIGURE 22.
Structure of the long-term calibration model.
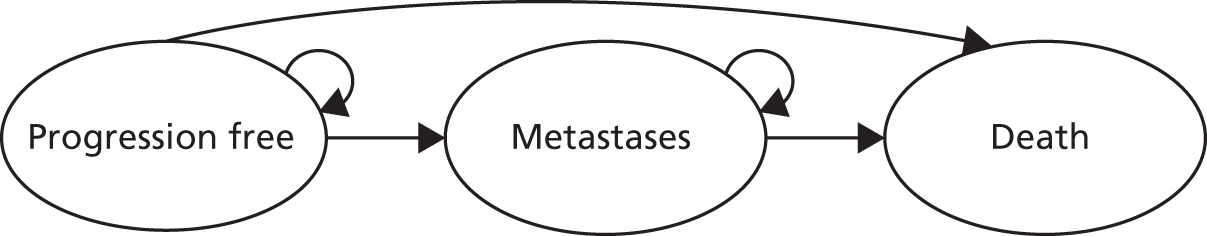
Specifically, the calibration model aimed to find a plausible set of transition probabilities that fit the above criteria. It randomly draws numbers between 0 and 1 from the transitions from progression-free disease to metastatic disease and death. For the transition from metastatic disease to death, the model draws a random number from within the 95% CI of the cumulative incidence of all-cause death from the metastatic disease subgroup allocated to the standard care arm of the STAMPEDE trial. 125 A 10% leeway is given to allow for differences in the disease severity of patients and their management between the STAMPEDE trial and PIVOT. STAMPEDE is a RCT set in the UK that compares treatments for men with locally advanced and metastatic prostate cancer. James et al. 125 report the survival outcomes of men with newly diagnosed metastatic prostate cancer who were allocated to the current standard of care, androgen deprivation therapy.
The calibration model records the transition probabilities that meet three conditions of plausibility: (1) the life-years are within the 95% CI predicted in step 1, (2) the cumulative incidence of metastases at 12 years is within the 95% CI of PIVOT and (3) the transition probability from progression-free disease to death is smaller than the transition probability from metastatic disease to death. The calibration model is run until 1000 plausible sets of transition probabilities for each subgroup are found.
Step 3: lifetime quality-adjusted life-years and costs
Lifetime QALYs and costs are predicted by the same three-state Markov model and the plausible set of transition probabilities from step 2. A 3.5% discount rate is used to calculate the present value of future benefits (QALYs) and costs, in line with current NICE guidance. 99
Model inputs
The inputs for HRQoL and costs are reported in Table 35. The model assumes that the active treatment of choice is RP. Men diagnosed with CNS cancer receive active surveillance, as recommended by NICE. 13 HRQoL in men with no cancer is calculated as a function of the men’s age using the Ara and Brazier127 regression model. Men with localised cancer are assumed to have the same HRQoL as similarly aged men with no cancer. In other words, localised cancer is assumed to have no effect on men’s HRQoL. The treatment and management of localised cancer is also assumed to have no HRQoL implications. This is based on the findings of Korfage et al. ,129 who found no effect of treatment on HRQoL post RP. The decrement from metastatic disease is calculated from Torvinen et al. 126 Torvinen et al. 126 assessed HRQoL in different stages of prostate cancer using the EQ-5D, 15D and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC-30) in Finland between 2009 and 2010. Men with prostate cancer were divided into five mutually exclusive groups: (1) local disease in the first 6 months after diagnosis (n = 47), (2) local disease 0.5–1.5 years after diagnosis or recurrence (n = 158), (3) local disease > 1.5 years after diagnosis (n = 317), (4) metastatic disease (n = 89) and (5) palliative care (n = 19). The decrement for metastatic disease is estimated for the model as the difference between the weighted average of the local disease EQ-5D score and the weighted average of the metastatic disease EQ-5D score.
| Parameter | Value | Source |
|---|---|---|
| HRQoL | ||
| Decrement from metastatic disease | –0.137 | Torvinen et al.:126 calculated as the difference between the average EQ-5D score reported for localised cancer and the average EQ-5D score reported for metastatic cancer. The probabilistic value is sampled from the difference between the distributions (parameterised as beta distributions) |
| Age-related decrement | Ara and Brazier127 | |
| Constant | 0.9508566 | |
| Coefficient on male | 0.0212126 | |
| Coefficient on age | –0.0002587 | |
| Coefficient on age squared | –0.0000332 | |
| Unit costs (all deterministic) | ||
| Radical treatment total (one-off cost) | £4667 | 2014/15 National Tariff Payment System:112 average of the PbR tariff for ‘LB21Z Bladder neck open procedures – male’ and ‘LB22Z Laparoscopic bladder neck procedures – male’ |
| RP surgery | £4275 | |
| Urology follow-up | £91 | 2014/15 National Tariff Payment System:112 PbR tariff for ‘WF01A: Follow-up attendance single professional’ |
| First surgical consultation | £210 | 2014/15 National Tariff Payment System:112 PbR tariff for ‘WD01B: First attendance single professional’ |
| Follow-up surgical consultation | £91 | 2014/15 National Tariff Payment System:112 PbR tariff for ‘WF01A: Follow-up attendance single professional’ |
| Active surveillance total (per year) | £138 | 2014/15 National Tariff Payment System:112 PbR tariff for ‘WF01A: Follow-up attendance single professional’ and cost of PSA test in primary care, conducted three times per year |
| Follow-up surgical consultation | £91 | |
| PSA testing in primary care | £16 × 3 | |
| AEs (per year) | £205 | Weighted average of the cost of managing AEs by the incidence of each AE |
| Sexual dysfunction (per year) | £282 | 2014/15 National Tariff Payment System:112 PbR tariff for ‘LB43Z: Treatment of erectile dysfunction’ |
| Urinary incontinence (per year) | £279 | NICE13 and Unit Costs of Health and Social Care 2015:111 management with containment pads, from NICE clinical guideline 2014, inflated from 2008–9 to 2014–15 prices |
| Bowel dysfunction (per year) | £1707 | NICE13 and Unit Costs of Health and Social Care 2015:111 mean weighted cost of sigmoidoscopy, laser therapy, enemas and blood transfusion, from the 2014 NICE clinical guideline, inflated from 2008–9 to 2014–15 prices |
| Metastatic cancer (per year) | £1876 | Calculated from Lord et al.128 (605.45 years of life predicted from the end of progression-free disease to death + 39.92 years from fourth-line palliative care treatment to chemotherapy; the lifetime cost of treatment is £1,072,554 (price year 2010–11), which inflated to 2014–15 prices is £1,136,124111 |
| Incidence of AEs | ||
| Following radical treatment | Wilt et al.:8 RP group, incidence over 2 years converted to 1-year probability | |
| Sexual dysfunction | 231/285 | |
| Urinary incontinence | 49/287 | |
| Bowel dysfunction | 35/286 | |
| Under active surveillance | Wilt et al.:8 observation group, incidence over 2 years converted to 1-year probability | |
| Sexual dysfunction | 18/284 | |
| Urinary incontinence | 124/281 | |
| Bowel dysfunction | 32/282 | |
Radical treatment is costed as the cost of RP. The cost of RP includes the cost of surgery and follows the approach taken for the 2014 NICE clinical guideline. 13 It includes the procedure cost, taken as the average PbR tariff for ‘LB21Z Bladder neck open procedures – male’ and ‘LB22Z Laparoscopic bladder neck procedures – male’ (both combined day case/ordinary elective spell tariff), the urology follow-up appointment (‘WF01A: Follow-up attendance single-professional’), a first surgical consultation (‘WD01B: First attendance single-professional’) and a follow-up surgical consultation (‘WF01A: Follow-up attendance single-professional’). Active surveillance is costed as an annual cost corresponding to a once-yearly outpatient appointment (‘WF01A: Follow-up attendance single-professional’) and three PSA tests in primary care.
Every year, men incur an annual cost for the management of AEs. This cost is calculated as the weighted average of the incidence of AEs multiplied by the unit cost of managing those AEs. The incidence of AEs is obtained from PIVOT. Wilt122 reports patient-reported urinary, erectile and bowel dysfunction at 2 years. The cost of managing AEs is obtained from the PbR tariff for the management of erectile dysfunction (PbR tariff for LB43Z) and from the 2014 NICE clinical guideline13 for the management of urinary incontinence and bowel dysfunction (inflated to 2014–15 prices).
Men with no cancer are modelled to have the life expectancy and HRQoL of the UK general population127,130 and incur the costs of a once-yearly PSA test in primary care. 111
Results
Step 1: life-years
Table 36 shows the input parameters and life-years predicted from the PIVOT survival curves reported in Wilt et al. 8 In PIVOT, men were aged, on average, 67 years. Their life expectancy using 2012–14 UK life tables130 is 18.38 years. The model predicts a life expectancy of 16.26 years (95% CI 14.77 to 17.26 years) for men with low-risk (CNS) cancer managed with active surveillance, 13.38 years (95% CI 11.59 to 15.09 years) for men with intermediate-risk (CS) cancer managed with active surveillance, 15.65 years (95% CI 13.95 to 16.93 years) for men with intermediate-risk (CS) cancer managed with radical treatment, 11.15 years (95% CI 9.16 to 13.47 years) for men with high-risk (CS) cancer managed with active surveillance and 13.23 years (95% CI 10.91 to 15.42 years) for men with high-risk (CS) cancer managed with radical treatment.
| Subgroup | Management | Mean (95% CI) | ||
|---|---|---|---|---|
| Scale | Shape | Life-years | ||
| Low-risk cancer | Active surveillancea | 0.019 (0.015 to 0.023) | 1.278 (1.248 to 1.306) | 16.26 (14.77 to 17.26) |
| Intermediate-risk cancer | Active surveillancea | 0.025 (0.022 to 0.029) | 1.358 (1.312 to 1.403) | 13.38 (11.59 to 15.09) |
| Radical treatmentb | 0.018 (0.015 to 0.021) | 15.65 (13.95 to 16.93) | ||
| High-risk cancer | Active surveillancea | 0.027 (0.021 to 0.034) | 1.463 (1.436 to 1.489) | 11.15 (9.16 to 13.47) |
| Radical treatmentb | 0.020 (0.015 to 0.025) | 13.23 (10.91 to 15.42) | ||
The life expectancy of men with low-risk cancer is lower than that of men with no cancer, but this is explained by two factors. First, PIVOT was set in the USA and recruited men between 1994 and 2002, predominantly in the Veterans Affairs Healthcare system; their life expectancy is likely to be lower than the current life expectancy in the UK for men of a similar age. Second, and to address the heavy tail predicted by the Weibull parametric model, the hazard rate applied is the maximum between the hazard rate for death of men with no cancer (from UK life tables) and that of men with low-risk cancer. Nevertheless, the differences in life expectancy are as expected: men with low-risk (CNS) cancer have a longer life expectancy; men with intermediate- and high-risk (CS) cancer have a lower life expectancy, but it is higher if they are treated with RP.
Step 2: transition probabilities
Table 37 shows the transition probabilities predicted by the calibration model. The transition probabilities from progression-free disease to metastases vary by subgroup and management option. The model predicts that men with low-risk (CNS) cancer have a yearly probability of 0.008 of progressing to metastases; this is similar to the probability in men with intermediate- and high-risk (CS) cancer who have received radical treatment. The model predicts that men with intermediate- and high-risk (CS) cancer who received watchful waiting have more than double the probability of progressing to metastases. The predicted probabilities from progression-free disease, and from metastases, to death are similar across the subgroups.
| Subgroup | Management | Transition probabilities, mean (95% CI) | ||
|---|---|---|---|---|
| Progression-free disease to metastases | Progression-free disease to death | Metastases to death | ||
| Low-risk cancer | Active surveillancea | 0.008 (0.004 to 0.013) | 0.050 (0.043 to 0.058) | 0.139 (0.058 to 0.226) |
| Intermediate-risk cancer | Active surveillancea | 0.018 (0.010 to 0.026) | 0.064 (0.049 to 0.078) | 0.145 (0.071 to 0.223) |
| Radical treatmentb | 0.007 (0.003 to 0.011) | 0.054 (0.045 to 0.063) | 0.142 (0.062 to 0.226) | |
| High-risk cancer | Active surveillancea | 0.022 (0.011 to 0.034) | 0.080 (0.058 to 0.101) | 0.157 (0.087 to 0.226) |
| Radical treatmentb | 0.008 (0.002 to 0.014) | 0.070 (0.053 to 0.085) | 0.148 (0.071 to 0.225) | |
Step 3: lifetime quality-adjusted life-years and costs
Table 38 shows the lifetime QALYs and costs, discounted at 3.5% and predicted using the plausible set of transition probabilities. The model predicts that men with low-risk (CNS) cancer who are managed with active surveillance have 8.45 QALYs (95% CI 7.99 to 8.94 QALYs) and accrue health-care costs of £3994 (95% CI £3301 to £4894) over their lifetime. Men with intermediate-risk (CS) cancer managed with active surveillance have 7.29 QALYs (95% CI 6.65 to 8.03 QALYs) and accrue health-care costs of £4130 (95% CI £3215 to £5351) over their lifetime. Men with high-risk (CS) cancer who receive radical treatment have 8.23 QALYs (95% CI 7.69 to 8.79 QALYs) and accrue health-care costs of £7041 (95% CI £6353 to £7959) over their lifetime.
| Subgroup | Management | Mean (95% CI) | |
|---|---|---|---|
| Lifetime QALYsa | Lifetime costs (£)a | ||
| Low-risk cancer | Active surveillanceb | 8.45 (7.99 to 8.94) | 3994 (3301 to 4894) |
| Intermediate-risk cancer | Active surveillanceb | 7.29 (6.65 to 8.03) | 4130 (3215 to 5351) |
| Radical treatmentc | 8.23 (7.69 to 8.79) | 7041 (6353 to 7959) | |
| High-risk cancer | Active surveillanceb | 6.38 (5.59 to 7.36) | 3764 (2804 to 5001) |
| Radical treatmentc | 7.21 (6.42 to 8.18) | 6796 (6112 to 7746) | |
The incremental cost-effectiveness ratio (ICER) for radical treatment compared with active surveillance in men with intermediate-risk (CS) cancer is £3067 per QALY gained. The probability that radical treatment is cost-effective is 0.91 assuming that the health opportunity cost (i.e. cost-effectiveness threshold) is £13,000 per QALY gained; it is 0.93 for a health opportunity cost of £20,000 per QALY gained and 0.94 for a health opportunity cost of £30,000 per QALY gained.
Men with high-risk (CS) cancer managed with active surveillance have 6.38 QALYs (95% CI 5.59 to 7.36 QALYs) and accrue health-care costs of £3764 (95% CI £2804 to £5001) over their lifetime. Men with high-risk (CS) cancer who receive radical treatment have 7.21 QALYs (95% CI 6.42 to 8.18 QALYs) and accrue health-care costs of £6796 (95% CI £6112 to £7746) over their lifetime.
The ICER for active treatment compared with active surveillance in men with high-risk (CS) cancer is £3602 per QALY gained. The probability that radical treatment is cost-effective is 0.78 for a health opportunity cost of £13,000 per QALY gained, 0.81 for a health opportunity cost of £20,000 per QALY gained and 0.82 for a health opportunity cost of £30,000 per QALY gained. The implication is that radical treatment is highly likely to be cost-effective compared with active surveillance for men with CS cancer.
Summary
-
The 2014 NICE clinical guideline13 recommends that men with low-risk cancer, equivalent to CNS cancer, should receive active surveillance and be offered radical treatment if their cancer progresses. It recommends that men with intermediate- and high-risk cancer, equivalent to CS cancer, should receive radical treatment if there are reasonable chances of a cure. These recommendations are based on limited quantitative evidence on the cost-effectiveness of active surveillance and active treatment for these patients.
-
Two good-quality RCTs were identified on the clinical effectiveness of RP compared with watchful waiting for the management of prostate cancer by risk subgroup: the SPCG-4 trial6,115,119,123 and PIVOT. 8,122 The patients taking part in PIVOT were found to be more comparable to the patients in PROMIS; therefore, PIVOT was selected to inform the prediction of long-term outcomes. No evidence was found on the clinical effectiveness of other treatments by risk subgroup. Consequently, RP was taken as the treatment of choice in patients referred for radical treatment.
-
Men with low-risk cancer managed with active surveillance have a life expectancy of 16.26 years on average. Men with intermediate-risk cancer who receive active surveillance have a life expectancy of 13.38 years on average; this increases to 15.65 years if they receive radical treatment. Men with high-risk cancer who receive active surveillance have a life expectancy of 11.15 years on average; this increases to 13.23 years if they receive radical treatment.
-
Radical treatment was found to be cost-effective compared with active surveillance in men with intermediate-risk and high-risk cancer, with an ICER of £3067 per QALY gained and £3602 per QALY gained, respectively. The probability that radical treatment is cost-effective ranges between 0.74 and 0.93.
Chapter 7 Cost-effectiveness of diagnostic strategies
The most cost-effective strategy is the strategy that offers the greatest net health, evaluated at three values of health opportunity cost (£13,000, £20,000 and £30,000 per QALY gained). Net health is calculated by converting the costs associated with each diagnostic strategy into health forgone using the aforementioned values of health opportunity cost and subtracting this from the direct health outcomes of each strategy. The health benefits and costs of the diagnostic strategies are evaluated by combining the results of the short-term model, described in Chapter 5, with those of the long-term model, described in Chapter 6.
Following TRUS-guided biopsy, men classified as having no cancer are assumed to be discharged, men classified as having CNS cancer are assumed to receive active surveillance as advised by the NICE clinical guideline13 and men classified as having CS cancer are assumed to receive immediate radical treatment.
In the model:
-
The costs and health outcomes of men with intermediate- and high-risk cancer who receive radical treatment are assumed to represent the costs and health outcomes of men with CS cancer who are correctly classified.
-
The costs and health outcomes of men with intermediate- and high-risk cancer who receive active surveillance are assumed to represent the costs and health outcomes of men with CS cancer who are incorrectly classified as being of low risk or having no cancer.
-
The costs and health outcomes of men with low-risk cancer who receive active surveillance are assumed to represent the costs and health outcomes of men with CNS cancer.
The costs and health outcomes of each strategy are the weighted average of the costs and health benefits of each group of men, defined by their true cancer status and diagnostic classification, weighted by the sensitivity and specificity of the diagnostic strategies for classifying them.
Analytical methods
In order to consider uncertainty, analyses were run probabilistically. The 1000 simulations from the model predicting short-term outcomes were considered together with the 1000 simulations of the model predicting long-term outcomes to calculate the distribution of overall QALYs and costs associated with each diagnostic strategy. Results are presented in terms of their averages and 95% CIs, calculated using the percentile method. The expected overall costs and QALYs are shown in the cost-effectiveness plane and a cost-effectiveness frontier is drawn. The cost-effectiveness frontier is formed by the substrategies that offer the most health outcomes per unit of cost. Substrategies to the left of the frontier are not cost-effective, because those in the frontier achieve more health benefits for the same cost. The probability that a strategy is cost-effective is shown in the cost-effectiveness acceptability frontier. The probability that a strategy is cost-effective was calculated as the proportion of simulations in which this strategy offers the most health benefits net of its costs, for a given value of health opportunity cost. 113 The cost-effectiveness acceptability frontier indicates the probability that the cost-effective alternative, on average (i.e. the strategy with the highest average net health value), will be cost-effective. 131 Remaining sensitivity analyses were conducted deterministically unless significant changes were observed that warranted in-depth exploration with probabilistic sensitivity analysis.
Sensitivity analysis
Bivariate sensitivity analysis on the unit cost of tests
A sensitivity analysis varied the unit costs of TRUS-guided biopsy and mpMRI by ± 50% from their base-case values (£182 and £403, respectively) and used four unit costs of TPM-biopsy: (1) base case (£1370), (2) base case + 25% (£1713), (3) base case – 25% (£1028) and (4) microcosting conducted for PROMIS (£1872). The results are presented in area graphs showing the cost-effective diagnostic strategy for each combination.
Scenario using payment-by-results tariff unit costs
The unit costs of the tests can depend on the source of information. The NHS reference costs110 are used in the base case. These reflect the average cost across all health-care providers. The cost charged to commissioners is, however, indicated in the PbR tariff. 112 The unit costs of TRUS-guided biopsy and mpMRI are similar between the NHS reference costs and PbR tariff, at £403 compared with £339, respectively, and £182 compared with £188, respectively. In contrast, the unit cost of TPM-biopsy is different between the two sources; £931 (NHS reference costs)110 compared with £1370 (PbR tariff). 112 Because the unit costs of the tests is anticipated to have an impact on the cost-effectiveness results, the model was run probabilistically using the PbR tariff for the unit costs.
Threshold sensitivity analysis
Additional threshold sensitivity analysis was carried out, aiming to identify the parameters that can have an impact on the results and indicate the changes in values that change the cost-effective strategy. This analysis is run deterministically; this means that the point estimates are used rather than the full distribution to reduce the running time of the decision model. Table 39 summarises the threshold sensitivity analysis and its rationale.
| Analysis | Rationale |
|---|---|
| SA1: relative sensitivity of MRI-targeted TRUS-guided biopsy (‘W’) in detecting CS cancer | The inputs used in the base case are obtained from the systematic review and meta-analysis by Schoots et al.105 TRUS-guided and mpMRI definitions and cut-off points used in the studies included in the meta-analysis may not directly map to those used in PROMIS. Increased sensitivity of ‘W’ biopsy will improve the cost-effectiveness profile of strategies starting with mpMRI. Increased sensitivity of ‘Z’ biopsy will improve the cost-effectiveness of strategies including a second biopsy post negative biopsy and suspicious mpMRI |
| SA2: relative sensitivity of MRI-targeted second TRUS-guided biopsy (‘Z’) in detecting CS cancer | |
| SA3: prevalence of intermediate-risk cancer vs. low-risk cancer | Men in PROMIS had a higher than expected prevalence of intermediate-risk (CS) cancer (0.53) and a lower than expected probability of not having cancer (0.28); men referred for diagnosis may have a lower probability of having intermediate-risk cancer and a higher probability of not having cancer. This can affect the cost-effectiveness results because the increased probability of CS cancer favours more sensitive strategies |
| SA4: probability of NC | |
| SA5: risk of death from TRUS-guided biopsy | There is some evidence that TRUS-guided biopsy may increase the risk of death, mainly because of sepsis, although this is uncertain (see Appendix 5). The base case assumes that biopsy has no risk of death. Including risk of death from TRUS-guided biopsy will improve the health outcomes of strategies with fewer TRUS-guided biopsies |
| SA6: reduced quality-adjusted survival from incorrect classification as NC | In the base case, men incorrectly classified as having NC have the same outcomes as men incorrectly classified as having low-risk cancer. However, their quality-adjusted survival may be lower because they may not be tested in the future as frequently as men classified as having low-risk cancer |
| SA7: reduced clinical effectiveness of radical treatment | The base case assumes that radical treatment in men with intermediate-risk (CS) cancer is cost-effective, as indicated by the results of the prediction of long-term outcomes, which is based on PIVOT. However, radical treatment may be less effective in improving survival. Therefore, the cost-effectiveness of radical treatment may have been overestimated. This scenario tests how reductions in the effect of radical treatment affect the cost-effectiveness of the diagnostic strategies |
| SA8: HRQoL impact of TRUS-guided biopsy | The base case assumes that TRUS-guided biopsy has no HRQoL consequences. However, this may be incorrect because this is a procedure that requires anaesthesia and may have complications. This sensitivity analysis tests the impact of assuming that TRUS-guided biopsy has a fraction of the impact of TPM-biopsy, from 10% of its impact to its full impact (–0.176), over the same period of 2 weeks |
Impact of repeated testing over time
In the base case, men with intermediate-risk cancer who are misclassified as having low-risk cancer are assumed to receive active surveillance. Their long-term outcomes are predicted from the intermediate-risk subgroup of the observation arm of PIVOT. This assumes that men misclassified will not receive radical treatment in the future. In practice, however, there is the possibility that men are correctly classified in one of the monitoring episodes of the active surveillance protocol and receive radical treatment at that point. The evidence on repeated testing and monitoring is, however, sparse. The population of individuals for retesting (i.e. those who initially tested negative) will differ from the overall PROMIS population and is likely to differ by the diagnostic strategy initially implemented. This means that the accuracy of retesting cannot be drawn from the general literature but needs to be drawn from studies that specifically focus on retesting. In addition, between the initial test and retesting, the disease may progress in risk category, the rate of which is unknown. For these reasons, the analysis cannot explicitly consider retesting. However, exploratory analyses were conducted by considering that a proportion of men misclassified in year 0 (i.e. in the first diagnostic episode), and who have not progressed to metastatic disease in subsequent years, can be correctly classified through retesting. These patients are assumed to receive radical treatment at that time point. Given the absence of information, the outcomes after radical treatment are unchanged.
A threshold sensitivity analysis finds the smallest change in parameter values that change the cost-effective strategy from the base case. The lifetime discounted QALYs and costs for treatment delays are calculated in the long-term model. In order to do this, the model assumes that treatment is received 1–5 years post referral from primary care. The model calculates the lifetime discounted QALYs and costs as the weighted average of the QALYs and costs for delays each year weighted by the proportion of men detected each year.
Value of future research
In addition to the uncertainty from the structural assumptions explored above, the cost-effectiveness results are also uncertain because of uncertainty in the parameter inputs, as represented by their CIs (or other measures of uncertainty such as standard errors). The cost-effectiveness results are presented in terms of their expected values, CIs and probability that a strategy is cost-effective. This means that there is a probability that the strategy expected to be cost-effective is actually not cost-effective. This has consequences in terms of the net health achieved from choosing this strategy. The difference between the net health achieved from choosing this strategy and the net health achieved by choosing the strategy that is always cost-effective represents the health losses attributable to uncertainty. These health losses can also be thought of as the health gain from solving this uncertainty through future research (i.e. the value of future research). The value of future research in health terms can be converted into monetary units to indicate the maximum investment to solve this uncertainty. 113
Base-case results
Health outcomes
Figure 23 shows the health outcomes achieved by each diagnostic substrategy; 95% CIs are not shown because they greatly overlap (see Appendix 3, Tables 53 and 54, for full results). The health outcomes vary between 8.30 and 8.75 QALYs.
FIGURE 23.
Base-case results: health benefits of each diagnostic strategy.

The substrategy that achieves the least favourable health outcomes is M1 115, at 8.29 QALYs (95% CI 7.94 to 8.68 QALYs). In M1 115, all men receive mpMRI and men with suspected CS cancer according to the mpMRI definition 1 and cut-off point 5 receive TRUS-guided biopsy. Men who have CS cancer using the TRUS-guided biopsy definition 1 receive radical treatment; otherwise, men receive active surveillance. This is the strategy that detects the fewest CS cancers (sensitivity 0.15, 95% CI 0.12 to 0.19; see Appendix 3, Tables 49 and 50). Therefore, using this strategy, the majority of men with CS cancer are missed and do not receive immediate treatment.
The substrategy that achieves the most favourable health outcomes is P4 2––, at 8.74 QALYs (95% CI 8.41 to 9.06 QALYs). All men receive TRUS-guided biopsy at definition 2 of clinical significance, which is the most sensitive in detecting CS cancers. Men in whom CS cancer was not detected receive TPM-biopsy. P4 2–– detects all CS cancers and minimises the proportion of men who receive TPM-biopsy and who experience the associated reduction in HRQoL.
The PROMIS-proposed substrategy (M7 123) achieves 8.65 QALYs (95% CI 8.35 to 8.95 QALYs). The standard care substrategy without mpMRI (T4 1––) achieves 8.49 QALYs (95% CI 8.19 to 8.80 QALYs). The strategy deemed to be the most similar to the NICE-recommended strategy13 achieves 8.66 QALYs (95% CI 8.36 to 8.97 QALYs).
Costs
Figure 24 shows the lifetime costs of using each diagnostic substrategy; 95% CIs are not shown to improve readability (see Appendix 3, Tables 55 and 56, for full results). The costs vary between £3500 and nearly £6300 per man referred for testing. Similar to the results for health outcomes, the substrategies using the lowest mpMRI cut-off point (≥ 2, represented in black), mpMRI definition 2 and TRUS-guided definition 2 have the highest costs. These substrategies have the highest costs of testing (see Figure 20 and Appendix 3, Tables 51 and 52) and the highest treatment costs, because more men with CS cancer are referred for treatment.
FIGURE 24.
Base-case results: costs of each diagnostic strategy.
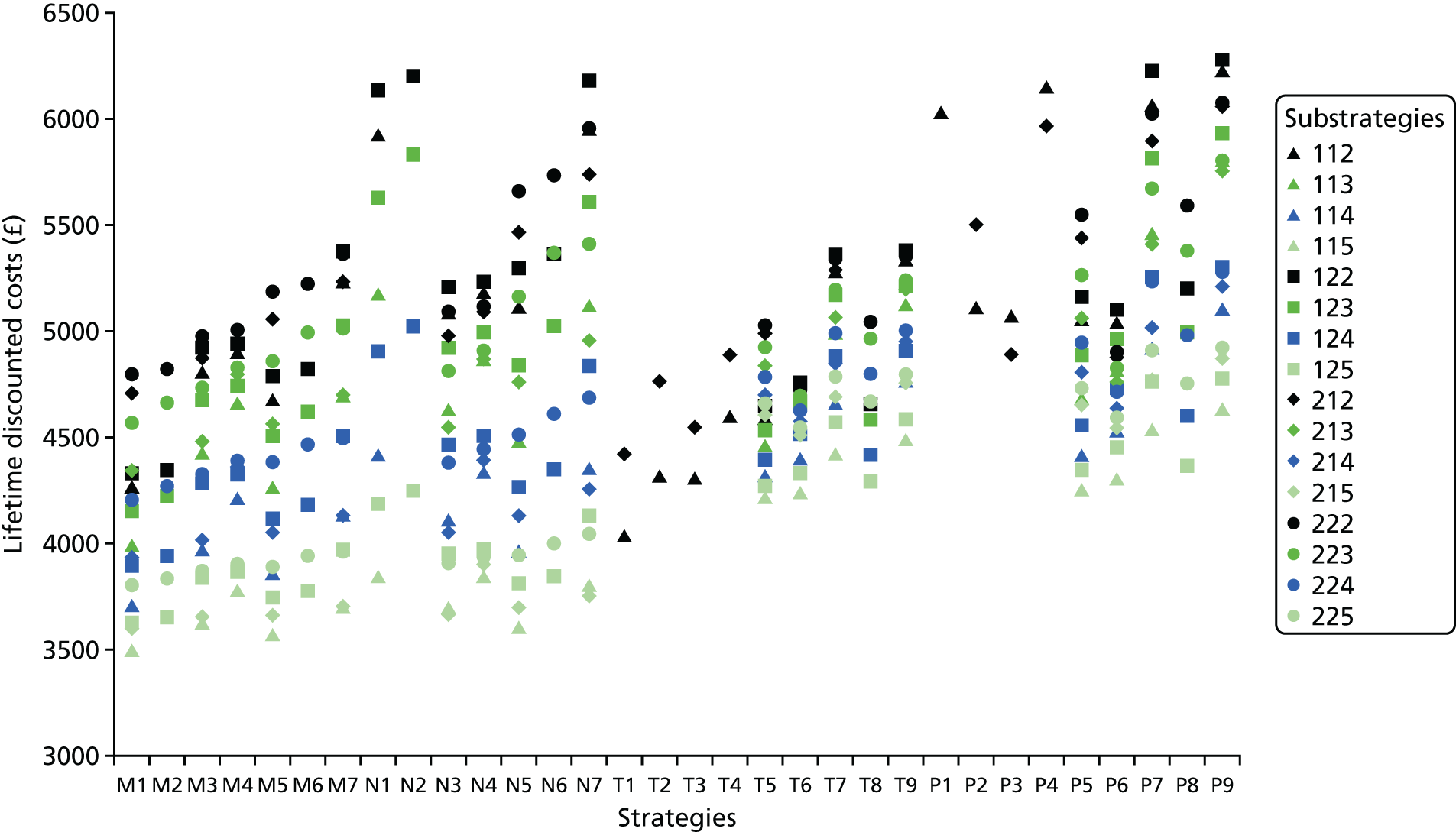
The substrategy with the lowest costs is M1 115, at £3497 (95% CI £3007 to £4115), which is also the substrategy that achieves the least favourable health outcomes, as discussed in Health outcomes. The smallest proportion of men are referred for TRUS-guided biopsy using the mpMRI definition 1 and cut-off point 5. The smallest proportion of men are referred for radical treatment, which is more costly than active surveillance, using the TRUS-guided biopsy definition 1.
The substrategy with the greatest costs is P9 122, at £6277 (95% CI £5858 to £6808). In P9 122, all men receive TRUS-guided biopsy and men in whom TRUS-guided biopsy at definition 1 did not detect CS cancer receive mpMRI. Men in whom mpMRI at definition 2 and cut-off point of ≥ 2 was suspicious of CS cancer are referred to TPM-biopsy. P9 122 detects almost all CS cancers (0.99, 95% CI 0.98 to 1.00) (see Appendix 3, Table 49) and these men then receive radical treatment, which is more costly than active surveillance. P9 122 is also more costly in the short term as a result of the large number of tests involved.
The PROMIS-proposed substrategy (M7 123) costs £5027 (95% CI £4609 to £5512). The standard care substrategy without mpMRI (T4 1––) costs £4603 (95% CI £4174 to £5044). The strategy deemed to be the most similar to the NICE-recommended strategy13 costs £5173 (95% CI £4755 to £5664).
Cost-effectiveness results
Figure 25 shows each diagnostic substrategy in terms of its average costs and QALYs and defines the strategies that achieve the most QALYs per pound spent (i.e. the cost-effectiveness frontier, represented as a black line). Substrategies above the cost-effectiveness frontier cannot be considered to be cost-effective. This figure does not include the uncertainty around the results to improve readability. Table 40 presents the same information, including uncertainty, in a tabular format.
FIGURE 25.
Cost-effectiveness plane.
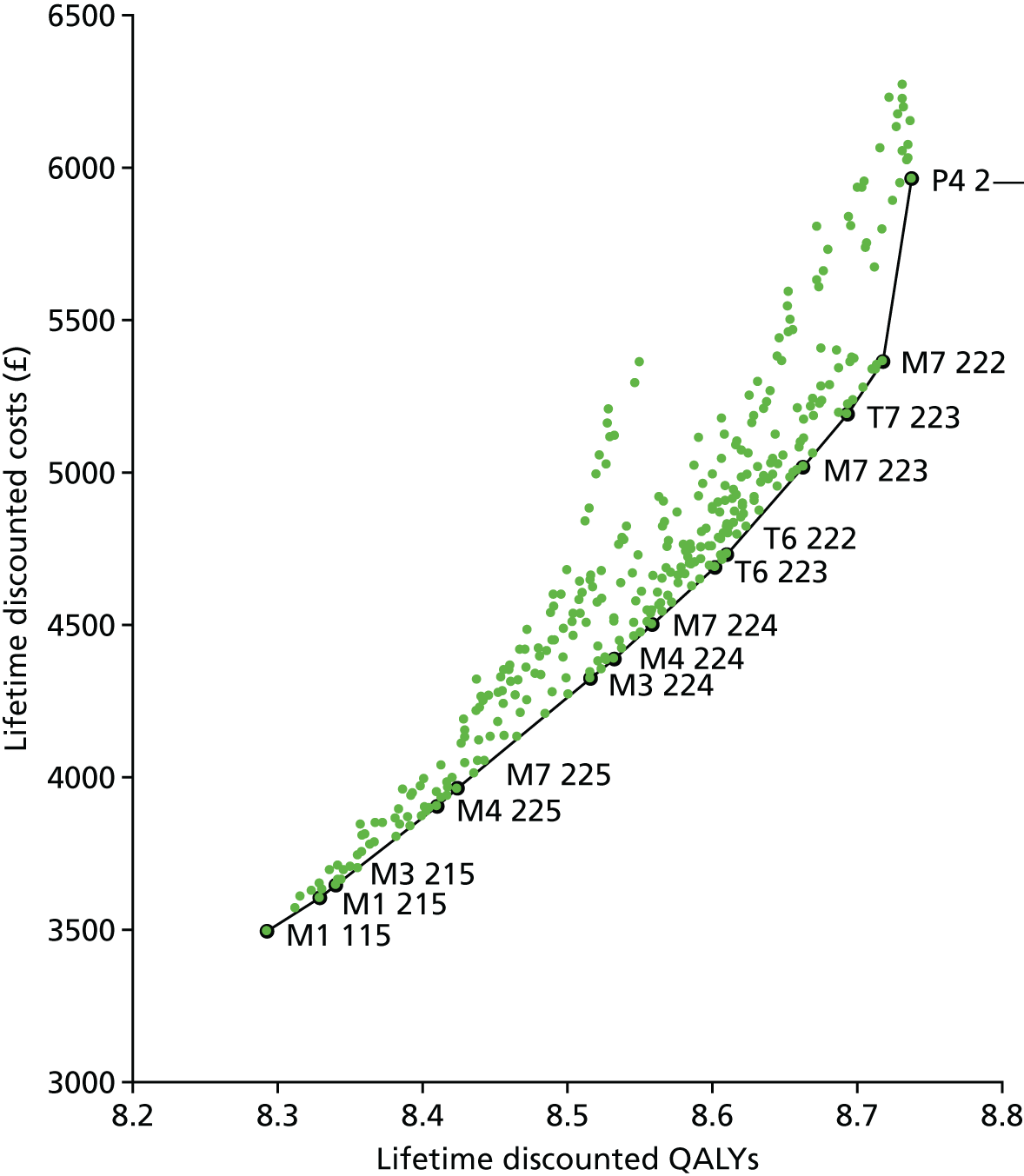
| Strategy | Definition | mpMRI cut-off point | QALYs, mean (95% CI) | Costs, mean (95% CI) (£) | ICER (£) | Net health at | |||
|---|---|---|---|---|---|---|---|---|---|
| TRUS-guided biopsy | mpMRI | £13,000 per QALY | £20,000 per QALY | £30,000 per QALY | |||||
| M1: mpMRI for all men and TRUS-guided biopsy in men suspicious for CS cancer | 1 | 1 | 5 | 8.29 (7.94 to 8.68) | 3497 (3007 to 4115) | 8.024 | 8.118 | 8.176 | |
| 2 | 1 | 5 | 8.33 (7.99 to 8.69) | 3608 (3137 to 4191) | 3081 | 8.051 | 8.148 | 8.208 | |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer on the first biopsy receive a second TRUS-guided biopsy | 2 | 1 | 5 | 8.34 (8.01 to 8.70) | 3648 (3180 to 4218) | 3630 | 8.059 | 8.157 | 8.218 |
| M4: mpMRI for all men and TRUS-guided biopsy in men with suspicion of any cancer; men with suspected CS cancer at mpMRI and in whom CNS cancer was detected on the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 5 | 8.41 (8.11 to 8.74) | 3909 (3477 to 4415) | 3738 | 8.109 | 8.214 | 8.279 |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 5 | 8.42 (8.13 to 8.74) | 3965 (3539 to 4455) | 3867 | 8.119 | 8.226 | 8.292 |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer on the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 4 | 8.52 (8.23 to 8.82) | 4325 (3915 to 4774) | 3921 | 8.183 | 8.300 | 8.372 |
| M4: mpMRI for all men and TRUS-guided biopsy in men with suspicion of any cancer; men with suspected CS cancer at mpMRI and in whom CNS cancer was detected on the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 4 | 8.53 (8.24 to 8.83) | 4390 (3979 to 4833) | 4031 | 8.194 | 8.312 | 8.386 |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 4 | 8.56 (8.27 to 8.86) | 4502 (4094 to 4937) | 4250 | 8.212 | 8.333 | 8.408 |
| T6: TRUS-guided biopsy for all men; men classified as having CNS cancer receive mpMRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 8.60 (8.31 to 8.90) | 4693 (4289 to 5127) | 4393 | 8.241 | 8.367 | 8.445 |
| 2 | 2 | 2 | 8.61 (8.32 to 8.91) | 4732 (4327 to 5166) | 4633 | 8.246 | 8.373 | 8.452 | |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 3 | 8.66 (8.37 to 8.97) | 5021 (4612 to 5496) | 5501 | 8.276 | 8.412 | 8.495 |
| T7: TRUS-guided biopsy for all men; men classified as having NC or CNS cancer receive mpMRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 8.69 (8.38 to 9.00) | 5194 (4780 to 5682) | 5778 | 8.293 | 8.433 | 8.519 |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 2 | 8.72 (8.40 to 9.04) | 5367 (4947 to 5876) | 7076 | 8.304 | 8.449 | 8.538 |
| P4: TRUS-guided biopsy in all men and TPM-biopsy in men in whom CS cancer was not detected | 2 | Not applicable | 8.74 (8.41 to 9.06) | 5968 (5550 to 6490) | 30,084 | 8.278 | 8.439 | 8.538 | |
Across the three values of health opportunity cost used (£13,000, £20,000 and £30,000 per QALY gained), the most cost-effective substrategy is M7 222. P4 2–– is on the margin of cost-effectiveness using the health opportunity value of £30,000 per QALY; the difference in net health between P4 2–– and M7 222 at this value is only 0.0001 QALYs.
In the restricted comparison between the PROMIS-proposed substrategy of M7 123, the standard care no-mpMRI strategy of T4 1–– and the substrategy deemed to be the most similar to the NICE-recommended substrategy13 (T7 123), T7 123 is most cost-effective (ICER £8520 per QALY vs. M7 123). The ICER of M7 123 compared with T4 1–– is £2730 per QALY. In the complete comparison of the 383 strategies, these three strategies have zero probability of being cost-effective.
Figure 26 shows the cost-effectiveness acceptability frontier. The cost-effectiveness acceptability curve plots the options that are cost-effective in terms of their probability of cost-effectiveness. The most cost-effective option is the option that offers the most benefits net of its cost, for a given value of health opportunity cost. For example, at the health opportunity cost of £20,000 per QALY, the cost-effective option is M7 222. M7 222 is the option that offers the most benefits on average. In some of the simulations, other strategies are cost-effective. Therefore, the probability that M7 222 is cost-effective corresponds to the proportion of simulations in which M7 222 offers the most benefits, net of its costs. The cost-effectiveness acceptability curve is useful in evaluations with a large number of options, which is the case here.
FIGURE 26.
Cost-effectiveness acceptability frontier.

At health opportunity costs lower than £3800 per QALY, M1 115 is the cost-effective substrategy (the substrategy that detects the fewest CS cancers but is also the least costly). Between the health opportunity costs of £3000 and £7500 per QALY, a number of substrategies appear to be cost-effective (M1 215 to T6 222 in Figure 25), but these are associated with a low probability of being cost-effective. At health opportunity costs of > £7500 up to £30,084 per QALY, M7 222 is the most cost-effective substrategy; the probability of this strategy being cost-effective ranges from 0.31 to 0.48 depending on the value of the health opportunity cost. At the health opportunity cost of ≥ £30,084, P4 2–– is the substrategy that is most likely to be cost-effective. The probability ranges between 0.44 and 0.63 for values of health opportunity cost up to £50,000 per QALY.
Threshold sensitivity analysis
Table 41 shows the results of the threshold sensitivity analysis. Sensitivity analysis 1 tests changes in the relative sensitivity of MRI-targeted TRUS-guided biopsy (‘W’ biopsy). In the base case, relative sensitivity is obtained from Schoots et al. ,105 at 1.2 (95% CI 1.09 to 1.32). This translates into a sensitivity of 0.74 (95% CI 0.65 to 0.84) using the TRUS-guided biopsy definition 2. Reductions in the sensitivity of MRI-targeted TRUS-guided biopsy (biopsy ‘W’) in detecting CS cancer change the cost-effective substrategy from M7 222 to T7 222 at the health opportunity cost of £13,000 per QALY gained, to T9 222 at the health opportunity cost of £20,000 per QALY gained and to P4 2–– at the health opportunity cost of £30,000 per QALY gained. The cost-effective substrategy is robust to increases in sensitivity.
| Analysis | Cost-effective substrategy at the health opportunity cost of | ||
|---|---|---|---|
| £13,000 per QALY gained | £20,000 per QALY gained | £30,000 per QALY gained | |
| Base case | M7 222 | M7 222 | M7 222 |
| SA1: changes in relative sensitivity of MRI-targeted TRUS-guided biopsy (‘W’) in detecting CS cancer; sensitivity of TRUS-guided biopsy at base case = 1.2 | |||
| 1.00–1.10 | T7 222 | T9 222 | P4 2–– |
| 1.15–1.19 | M7 222 | M7 222 | P4 2–– |
| 1.20–1.50 | M7 222 | M7 222 | M7 222 |
| SA2: changes in sensitivity of MRI-targeted second TRUS-guided biopsy (‘Z’) in detecting CS cancer; sensitivity of TRUS-guided biopsy at base case = 0.87 | |||
| 0.92–1.00 | T7 222 | T9 222 | T9 222 |
| SA3: prevalence of intermediate-risk cancer vs. low-risk cancer; base case = 0.53 | |||
| 0.35–0.53 | No changes from base case | ||
| SA4: probability of NC; base case = 0.28 | |||
| 0.28–0.53 | No changes from base case | ||
| SA5: risk of death from biopsy that changes cost-effective substrategy; no risk at base case | |||
| 0.5–1.0% | M7 222 | P1 | P1 |
| 1.5% | N1 123 | P1 | P1 |
| 2.0% | N2 114 | N2 123 | P1 |
| SA6: reduced quality-adjusted survival from incorrect classification as NC (QALY reduction) | |||
| 0.01 | M7 222 | M7 222 | P4 2–– |
| 0.09 | M7 222 | P4 2–– | P4 2–– |
| ≥ 0.10 | T9 222 | P4 2–– | P4 2–– |
| SA7: reduced clinical effectiveness of radical treatment | |||
| –10% | T7 223 | M7 222 | M7 222 |
| –15% | M1 215 | T7 223 | M7 222 |
| –20% | M1 115 | M1 115 | T6 222 |
| SA8: HRQoL impact of TRUS-guided biopsy | |||
| 10% of TPM-biopsy impact | M7 222 | M7 222 | P4 2–– |
| 60% of TPM-biopsy impact | M7 222 | M7 222 | P1 ––– |
| Same impact as TPM-biopsy | M7 222 | M7 222 | M7 222 |
Sensitivity analysis 2 tests changes in the sensitivity of MRI-targeted second TRUS-guided biopsy in men in whom the first TRUS-guided biopsy is negative for cancer. The base case uses a sensitivity of 0.87 (95% CI 0.74 to 0.96), which is obtained from Schoots et al. 105 Increases in the sensitivity of MRI-targeted second TRUS-guided biopsy in men with a prior negative biopsy result (biopsy ‘Z’) change the cost-effective substrategy to T7 222 at £13,000 per QALY and T9 222 at £20,000 and £30,000 per QALY.
Sensitivity analysis 3 tests changes in the proportion of intermediate-risk (CS) cancer compared with low-risk (CNS) cancer. A lower proportion of intermediate-risk (CS) cancer favours the cost-effectiveness of less sensitive, less resource-intensive strategies. The value used in the base case is 0.53, obtained from the individual patient data collected in PROMIS. The results are robust to changes in the proportion of intermediate-risk (CS) cancer compared with low-risk (CNS) cancer.
Sensitivity analysis 4 tests changes in the probability of no cancer. As for sensitivity analysis 3, a higher probability of no cancer (and, therefore, a lower probability of cancer and CS cancer) favours less-sensitive and less-resource-intensive strategies. The value used in the base case is 0.28, which was obtained from PROMIS. Changes in the proportion of men with cancer do not affect which substrategy is the most cost-effective.
Sensitivity analysis 5 tests the impact of including the risk of death from TRUS-guided biopsy. Some studies suggest that the risk of death from biopsy can be up to 0.01 (see Appendix 5 for a review). Including a risk of death of 0.5–1.5% (i.e. 5000–15,000 men die per 1,000,000 men receiving a biopsy) from complications of biopsy changes the cost-effective strategy to P1 at £20,000 and £30,000 per QALY gained; including a risk of death of 2.0% changes the cost-effective strategy to P1 at £30,000 per QALY.
Sensitivity analysis 6 tests the impact of reduced quality-adjusted survival from incorrect classification as no cancer. A reduction in health gains of 0.01 QALYs from incorrect classification as no cancer changes the cost-effective substrategy from M7 222 to P4 2–– at £30,000 per QALY gained. Reductions of ≥ 0.10 QALYs change the cost-effective substrategy from M7 222 to T9 222 at £13,000 per QALY gained and from M7 222 to P4 2–– at £20,000 and £30,000 per QALY gained.
Sensitivity analysis 7 tests the impact of reducing the clinical effectiveness of radical treatment. The base case is informed by PIVOT,122 which shows that radical treatment in men with intermediate-risk (CS) cancer is cost-effective. However, radical treatment may be less effective in improving survival than suggested by PIVOT. 8 Therefore, the cost-effectiveness of radical treatment may have been overestimated. Reducing the effectiveness of radical treatment will favour diagnostic substrategies that are less costly and detect fewer CS cancers, which will subsequently be directed for treatment.
The results are sensitive to the clinical effectiveness of radical treatment. Reducing the effectiveness by 10% changes the cost-effective substrategy at the health opportunity cost of £13,000 per QALY from M7 222 to T7 223. Subsequent reductions change the cost-effective substrategy to M1 215 and then to M1 115. At the health opportunity cost of £20,000 per QALY, the cost-effective substrategy changes to T7 223 if the effectiveness of radical treatment is 15% lower; larger reductions change the cost-effective substrategy to M1 115. At the health opportunity cost of £30,000 per QALY, reductions beyond 20% change the cost-effective substrategy from M7 222 to T6 222. These results suggest that, if radical treatment is less effective than suggested by PIVOT, less sensitive and less costly diagnostic strategies are more likely to be cost-effective. These results also shed light on the impact of alternative treatment options. Some men receive radiotherapy instead of radical treatment. If radiotherapy is as cost-effective as radical treatment, the cost-effective diagnostic strategy is the same. If radiotherapy is less beneficial, its ICER compared with active surveillance is greater. The sensitivity analysis (sensitivity analysis 7) suggests that reductions in the benefits of radical treatment of < 10% do not change the cost-effective strategy. Larger reductions change the cost-effective strategy to less costly but also less sensitive strategies such as T7 223.
Sensitivity analysis 8 tests the impact of a reduction in HRQoL attributable to TRUS-guided biopsy. The results are robust in that M7 222 remains the cost-effective substrategy at the lower bounds of the health opportunity cost. At the £30,000 per QALY threshold, the cost-effective substrategy changes to P4 2— for small HRQoL reductions. As the reduction is more pronounced, the cost-effective strategy changes to P1—.
Bivariate sensitivity analysis on the cost of diagnostic tests
Figure 27–38 show the results of the bivariate sensitivity analysis on the unit costs of TRUS-guided biopsy and mpMRI, by the cost of TPM-biopsy. The unit costs are varied by ± 50%; the unit cost of TRUS-guided biopsy is represented on the vertical axis, whereas the unit cost of mpMRI is represented on the horizontal axis. Each figure refers to a unit cost of TPM-biopsy (base case at £1370, +25% at £1713, –25% at £1028 and the cost estimated for PROMIS at £1872) and a health opportunity cost (£13,000, £20,000 and £30,000 per QALY). The square delineated in black represents the base-case unit cost of TRUS-guided biopsy and mpMRI. The figures show the cost-effective substrategy for a combination of unit costs, distinguished by colours.
FIGURE 27.
Bivariate sensitivity analysis: TPM-biopsy cost = £1370, health opportunity cost = £13,000 per QALY.
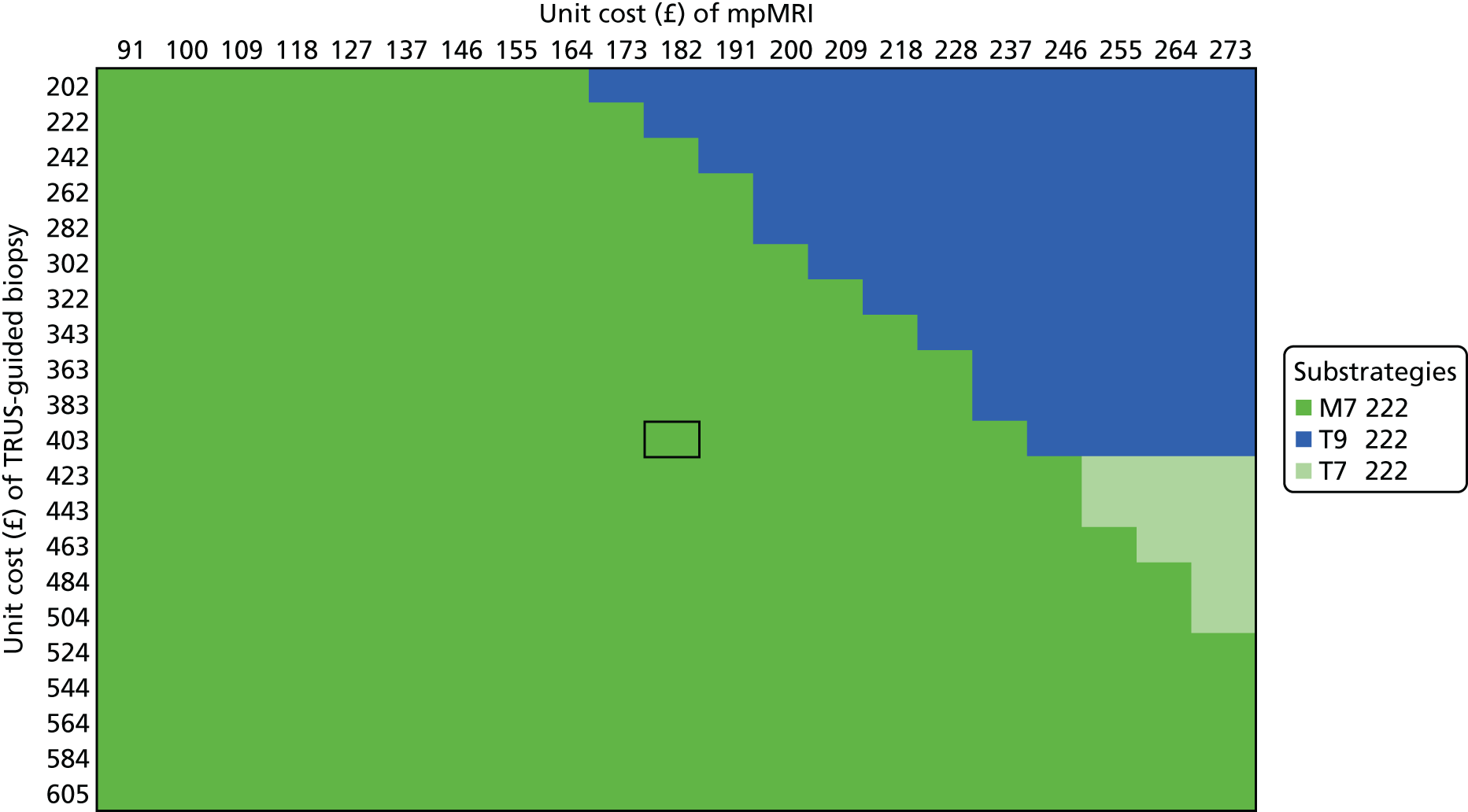
FIGURE 28.
Bivariate sensitivity analysis: TPM-biopsy cost = £1370, health opportunity cost = £20,000 per QALY.

FIGURE 29.
Bivariate sensitivity analysis: TPM-biopsy cost = £1370, health opportunity cost = £30,000 per QALY.
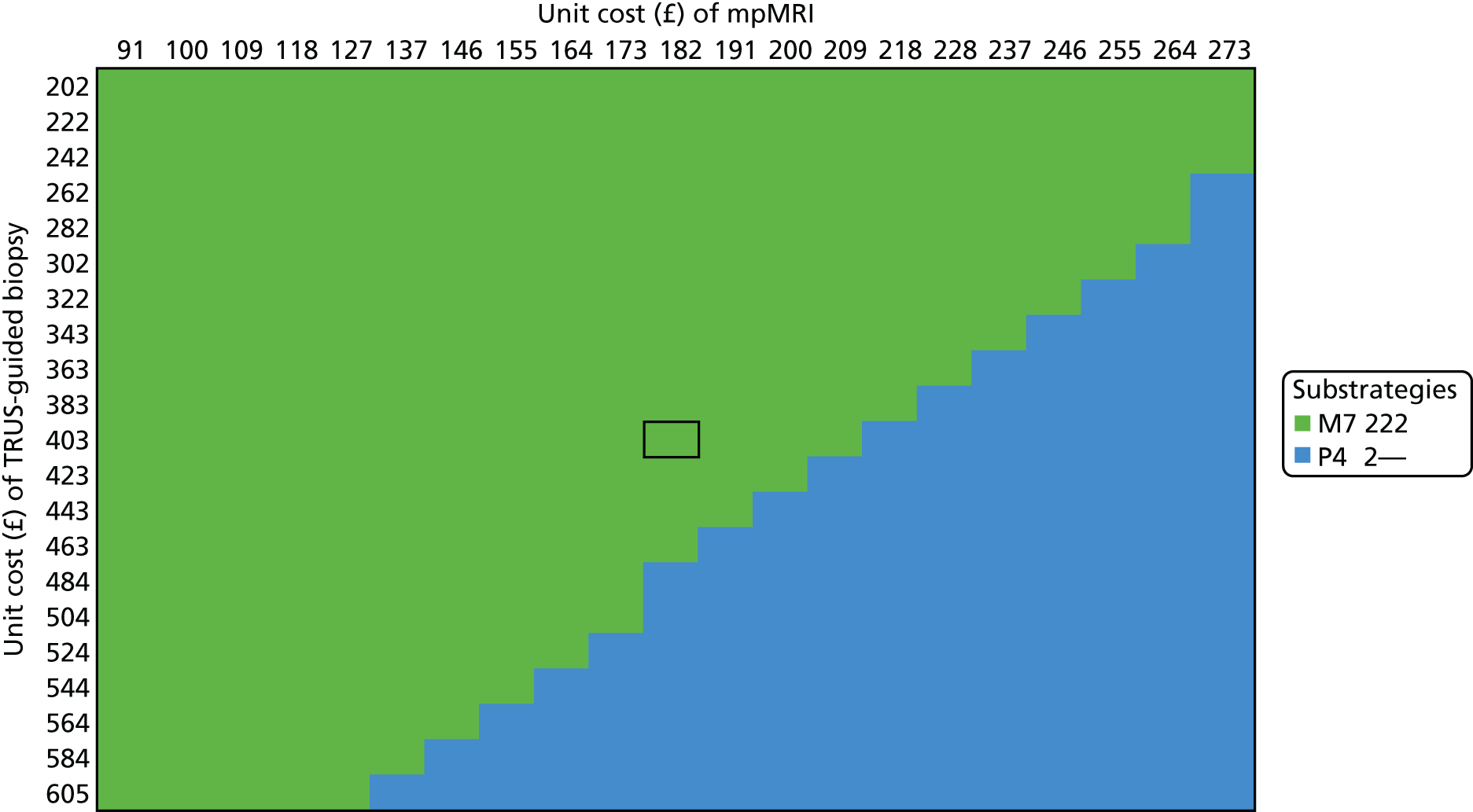
FIGURE 30.
Bivariate sensitivity analysis: TPM-biopsy cost = £1713, health opportunity cost = £13,000 per QALY.
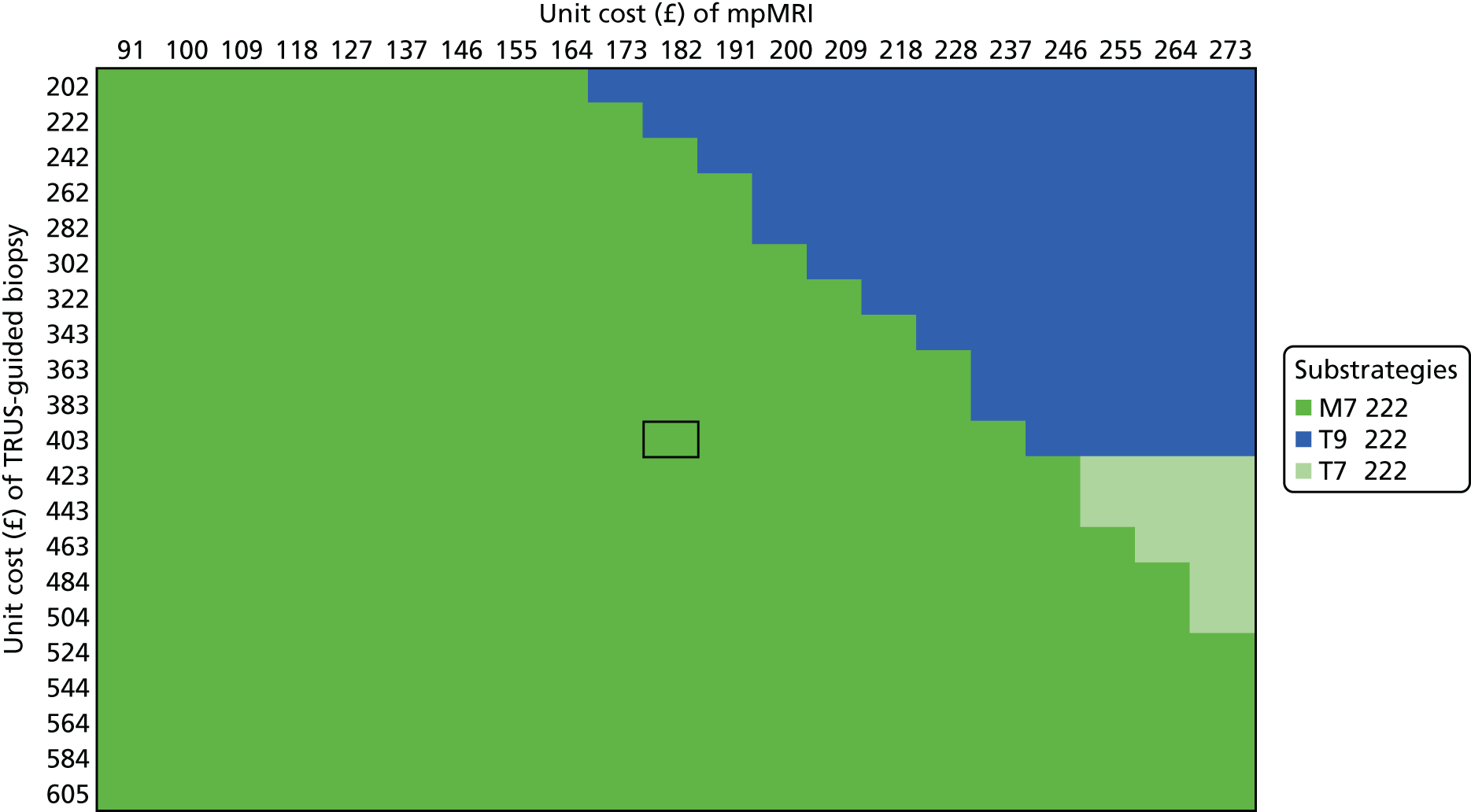
FIGURE 31.
Bivariate sensitivity analysis: TPM-biopsy cost = £1713, health opportunity cost = £20,000 per QALY.

FIGURE 32.
Bivariate sensitivity analysis: TPM-biopsy cost = £1713, health opportunity cost = £30,000 per QALY.

FIGURE 33.
Bivariate sensitivity analysis: TPM-biopsy cost = £1028, health opportunity cost = £13,000 per QALY.
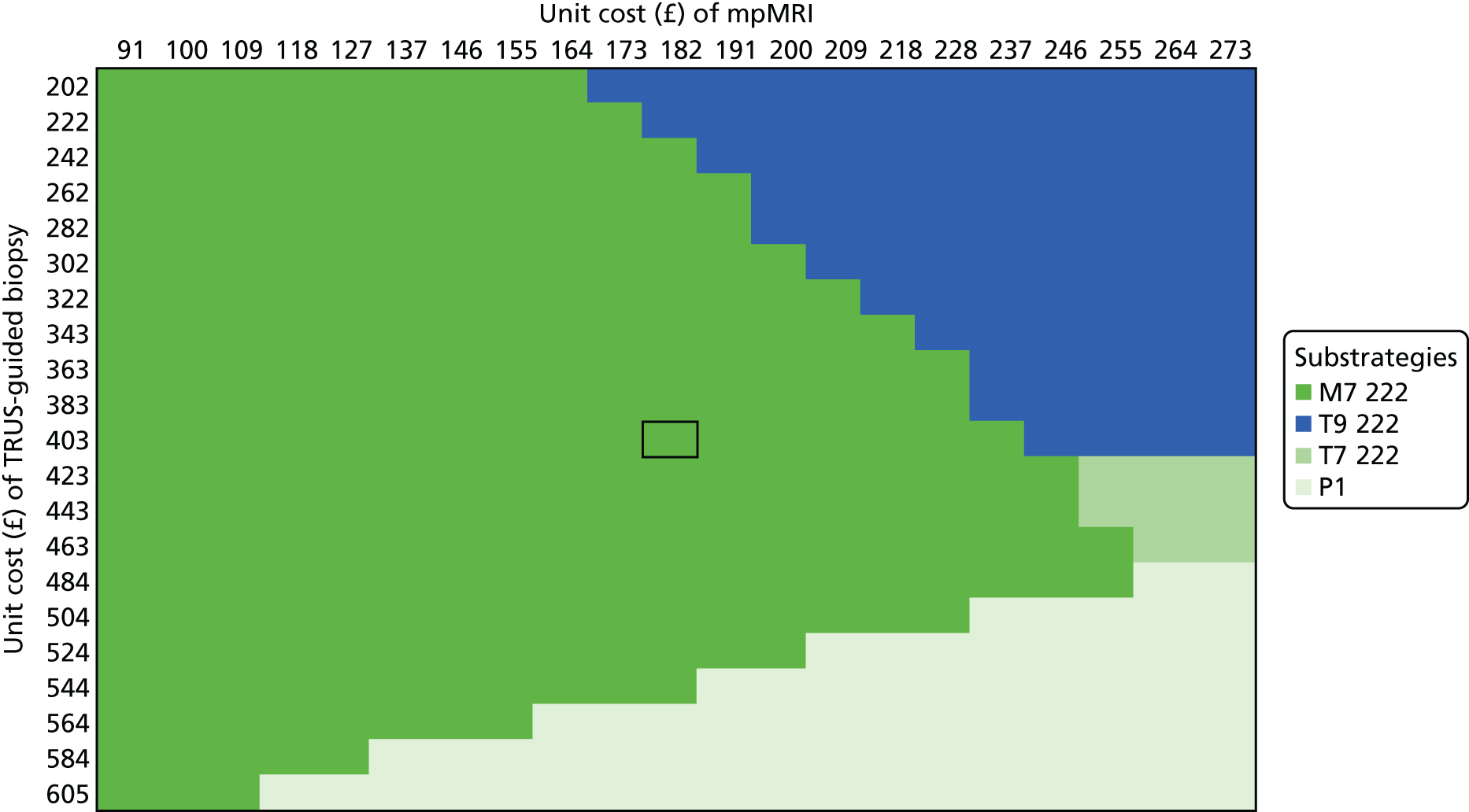
FIGURE 34.
Bivariate sensitivity analysis: TPM-biopsy cost = £1028, health opportunity cost = £20,000 per QALY.
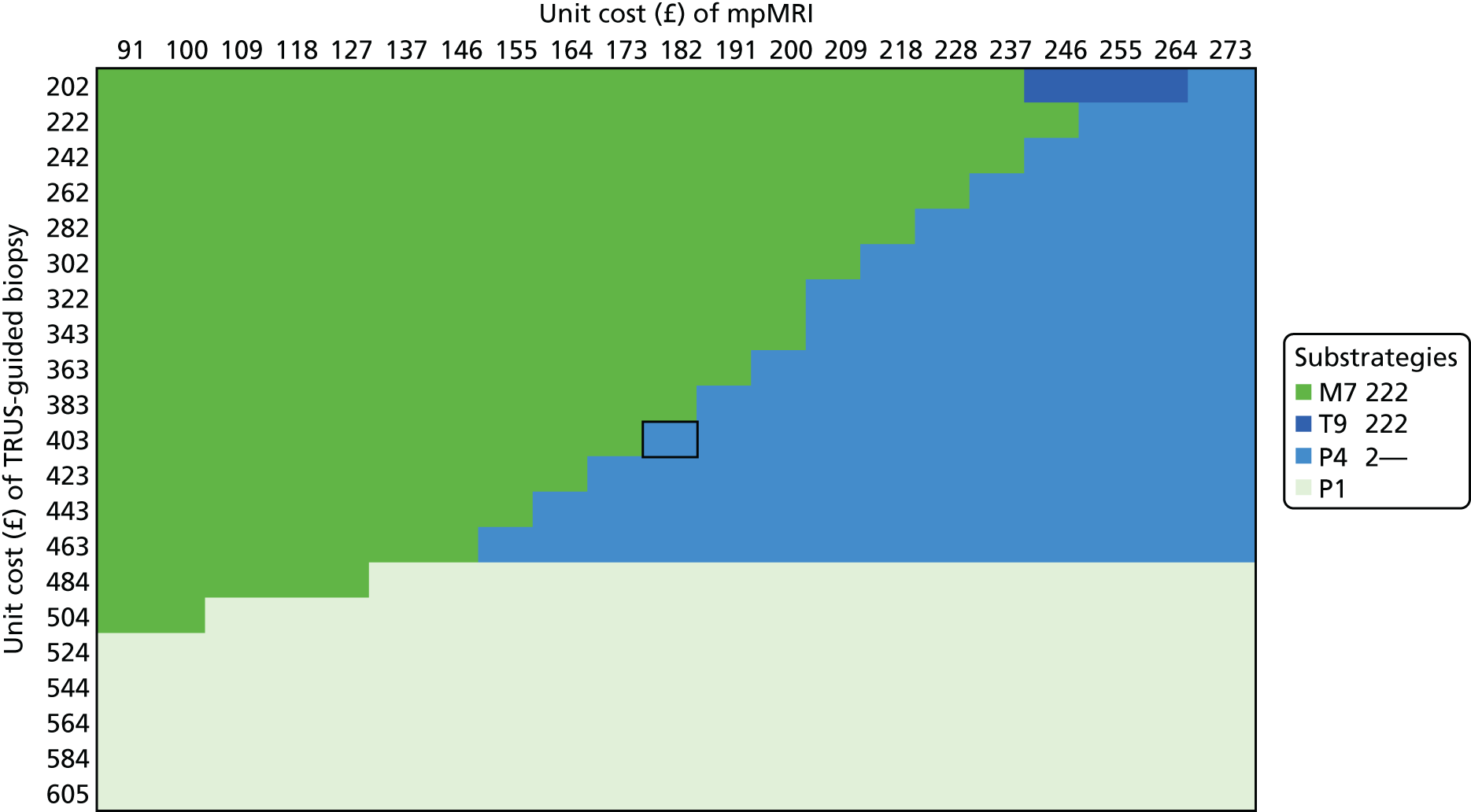
FIGURE 35.
Bivariate sensitivity analysis: TPM-biopsy cost = £1028, health opportunity cost = £30,000 per QALY.
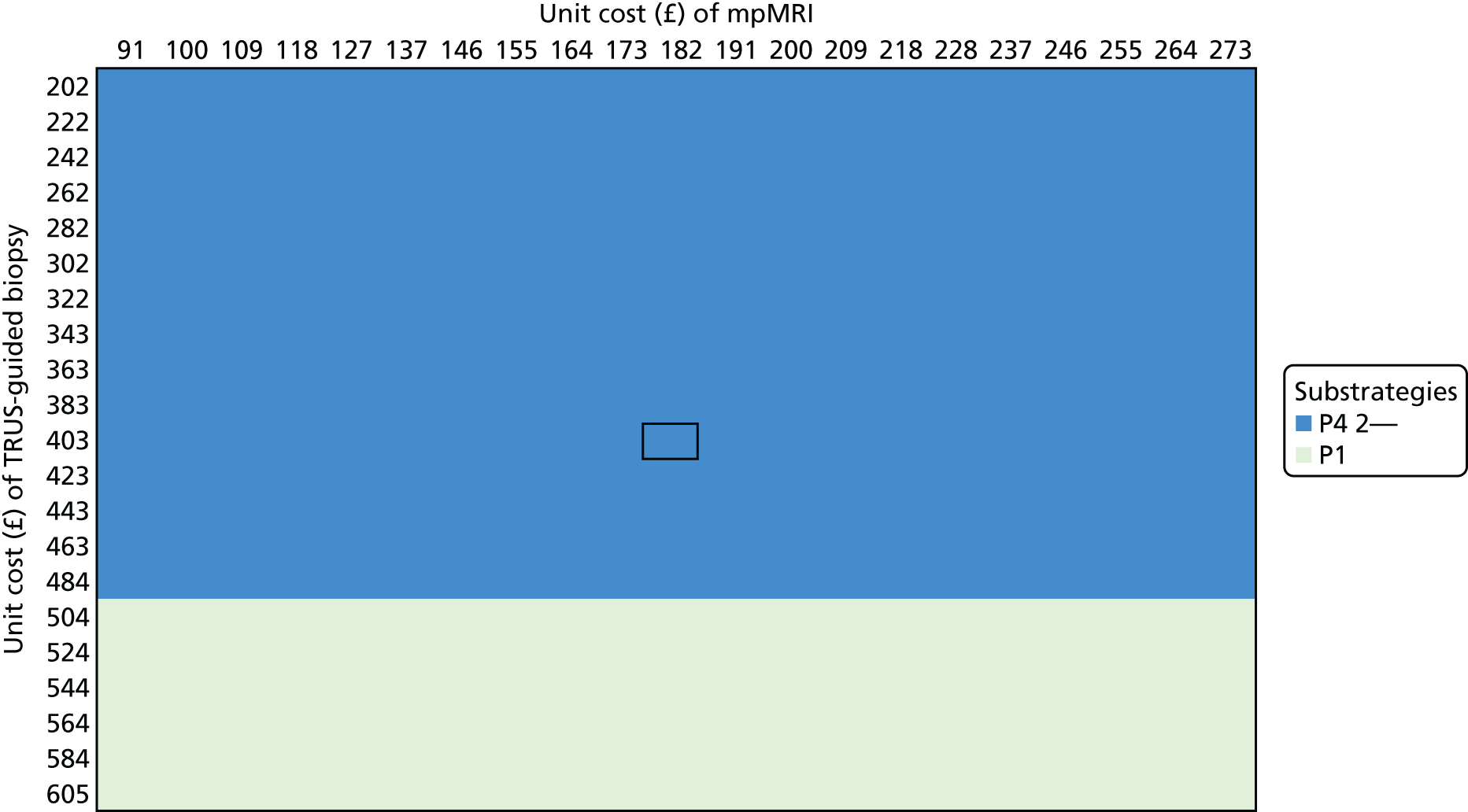
FIGURE 36.
Bivariate sensitivity analysis: TPM-biopsy cost = £1872, health opportunity cost = £13,000 per QALY.
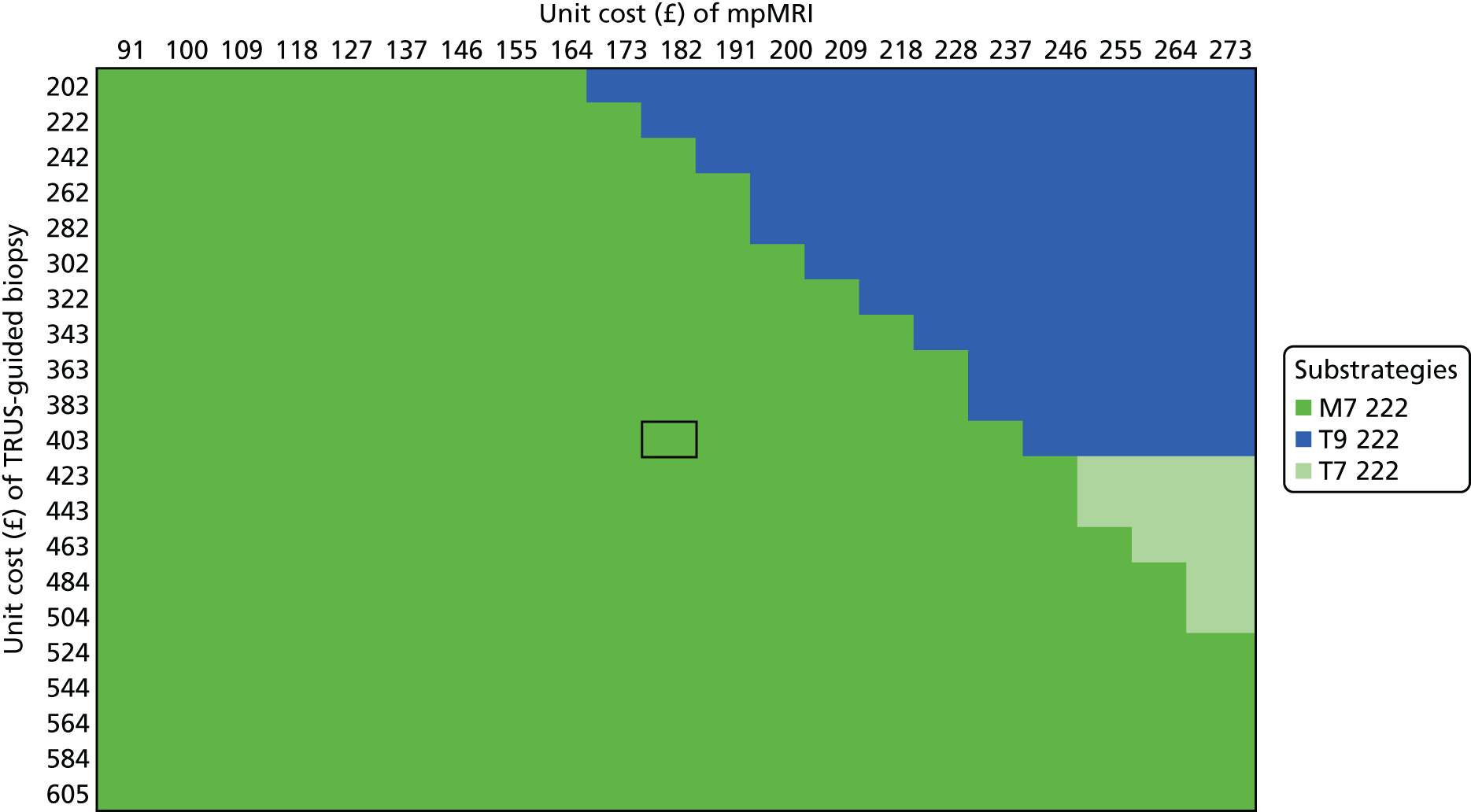
FIGURE 37.
Bivariate sensitivity analysis: TPM-biopsy cost = £1872, health opportunity cost = £20,000 per QALY.
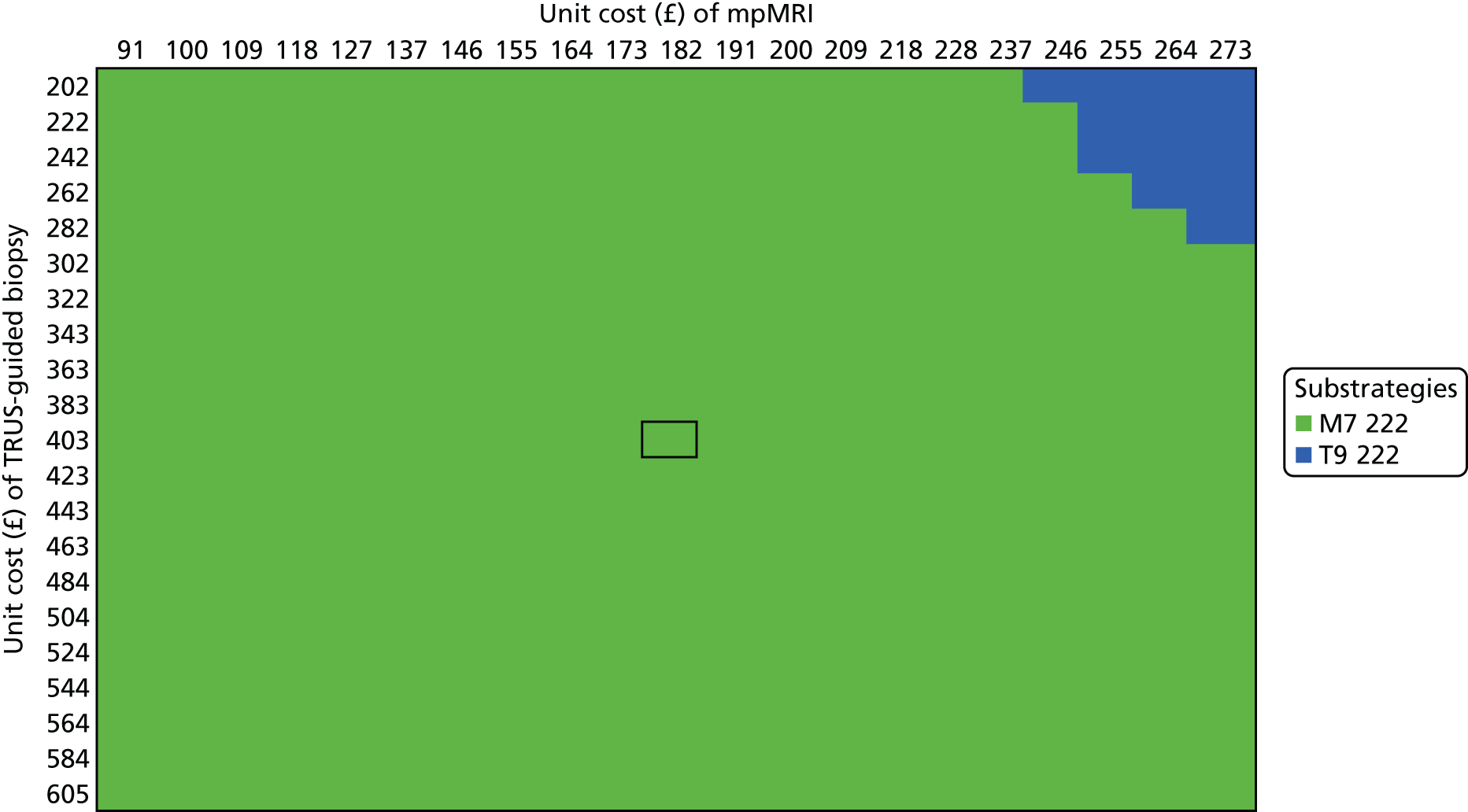
FIGURE 38.
Bivariate sensitivity analysis: TPM-biopsy cost = £1872, health opportunity cost = £30,000 per QALY.

Figure 27 shows the cost-effective substrategy using the base-case cost for TPM-biopsy at the health opportunity cost of £13,000. M7 222 is the cost-effective substrategy unless the cost of mpMRI is increased. The cost-effective substrategy is T9 222 if the unit cost of mpMRI is increased and the unit cost of TRUS-guided biopsy is reduced. If both the unit cost of mpMRI and the unit cost of TRUS-guided biopsy are increased, the cost-effective substrategy is T7 222. At the higher opportunity cost of £20,000 per QALY (see Figure 28), the cost-effective substrategy is robust as M7 222, unless very high unit costs of mpMRI are coupled with very low unit costs of TRUS-guided biopsy. At £30,000 per QALY (see Figure 29), the cost-effective substrategy is also M7 222, unless the costs of mpMRI and TRUS-guided biopsy increase. Figures 30–32 and Figures 36–38, using higher unit costs for TPM-biopsy, show a similar pattern. Figures 33–35 show the results for a lower cost of TPM-biopsy (£1028). The cost-effective strategy is more sensitive to changes in the unit costs of TRUS-guided biopsy and mpMRI using this unit cost. At the health opportunity cost of £13,000 per QALY, M7 222 is cost-effective. However, this can change to T9 222, T7 222 or P1 depending on the unit costs of mpMRI and TRUS-guided biopsy. At the health opportunity cost of £20,000 per QALY, M7 222 is cost-effective only at lower costs of mpMRI. The cost-effective substrategy for a wider range of unit costs is P4 2–– or P1.
There is some variation in the unit costs of the tests. These figures can be used to indicate the cost-effective substrategy for a given combination of unit costs. For example, the average unit cost of TRUS-guided biopsy using the code LB76Z is £403. However, 20,502 men received it as a day case procedure, at a cost of £533, whereas 17,846 men received it as an outpatient procedure, at a cost of £223. At the TPM-biopsy cost of £1397, similar to the base-case average NHS reference cost,110 M7 222 is cost-effective unless the cost of mpMRI increases to > £197 per scan.
The results suggest that the unit costs of the tests, particularly TPM-biopsy, are a driver of the cost-effectiveness results. Higher TPM-biopsy costs favour the cost-effectiveness of highly sensitive strategies, such as M7 222, which do not include TPM-biopsy. Very high mpMRI costs favour strategies that start with TRUS-guided biopsy rather than mpMRI. However, at lower TPM-biopsy costs, and depending on the health opportunity cost adopted, the cost-effective strategy may involve TPM-biopsy for a large number of men, such as in P1 and P4 2––.
Scenario: using the payment-by-results tariff for the unit costs of the tests
Figure 39 shows the cost-effectiveness plane for the scenario using the PbR tariff for the unit costs of the tests. The substrategies in the cost-effectiveness frontier are similar to those in in the cost-effectiveness frontier in the base case. Four strategies forming the frontier start with mpMRI (M1, M3, M4 and M7) and five strategies start with biopsy (T1, T6, T7, P4 and P1). The cost-effective strategy depends on the value of the health opportunity cost. At £13,000 per QALY gained, the cost-effective substrategy is M7 222. On average, M7 222 achieves the highest net health, at 8.307 QALYs. At £20,000 and £30,000 per QALY gained, the cost-effective substrategy is P4 2––. However, the difference between P4 2–– and P1, the next best substrategy, is small, at 0.001 QALYs.
FIGURE 39.
Cost-effectiveness plane for the scenario using the PbR tariff.
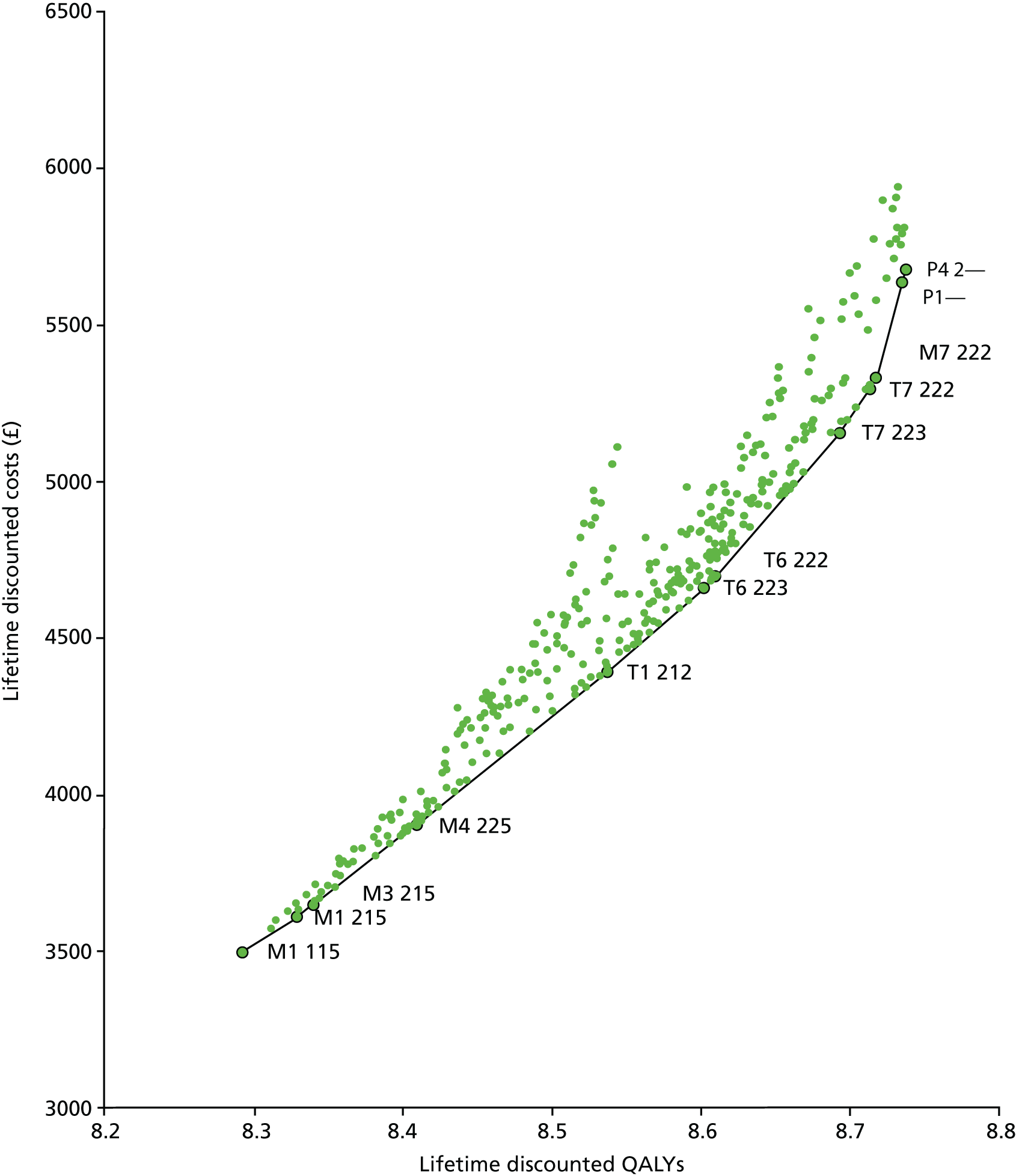
Figure 40 shows the cost-effectiveness acceptability frontier. As with the base-case results, the substrategy most likely to be cost-effective at values of health opportunity cost of < £3000 per QALY gained is M1 115. There is a region of uncertainty around £3000–6000 per QALY gained, where a number of substrategies appear in the frontier. M7 222 is most likely to be cost-effective up to a value of health opportunity cost of £17,500 per QALY. P4 2–– is the substrategy most likely to be cost-effective at values of > £17,500 per QALY gained.
FIGURE 40.
Cost-effectiveness acceptability frontier for the scenario using the PbR tariff.
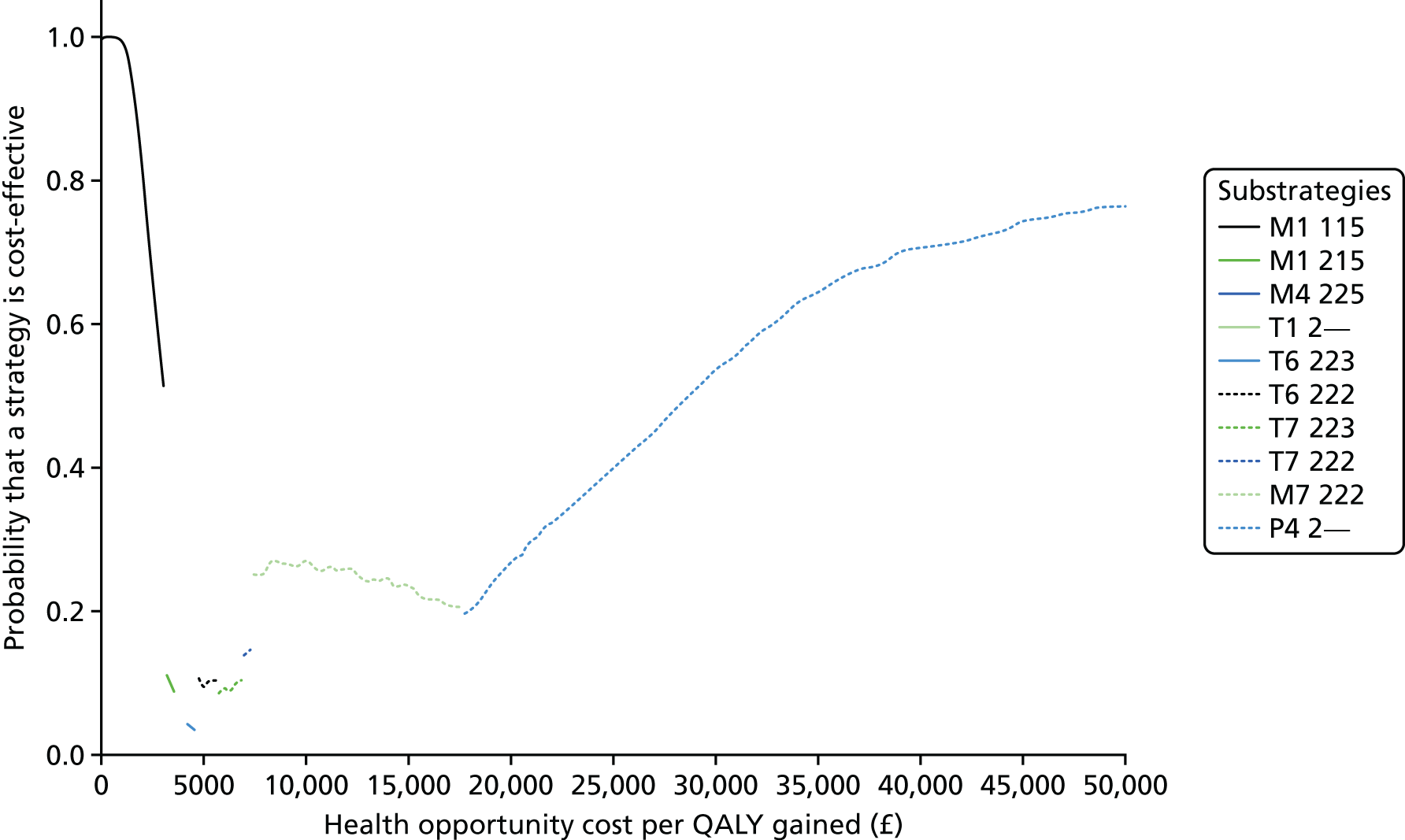
The threshold sensitivity analyses described earlier were also conducted using the PbR tariff for the unit costs (see Appendix 6). In general, the results are similar to those of the base case. In addition to M7 222 and P4 2––, the substrategies that emerge as potentially cost-effective are typically T7 222, T9 222 and P1.
Scenario: the impact of repeated testing over time
Table 42 shows the results of the sensitivity analysis of repeat testing. In the base case, M7 222 is the cost-effective substrategy. It correctly classifies 95.0% of men with intermediate-risk cancer; 2.0% of these men are misclassified as having low-risk cancer and 3.0% are misclassified as having no cancer. This analysis shows that only when ≥ 45.0% of the men misclassified, that is, 2.5% of the men with intermediate-risk cancer who are incorrectly classified, are correctly classified at subsequent testing rounds does the cost-effective strategy change. The cost-effective substrategy M7 222 changes to T7 222 at the health opportunity cost of £13,000 per QALY and to T9 222 at the health opportunity costs of £20,000 and £30,000 per QALY. This means that, if men with intermediate-risk cancer who are incorrectly classified can be correctly classified and treated in the future, less-sensitive and less-costly diagnostic strategies are favoured.
| Proportion of men reclassified in the future (%) | Cost-effective substrategy at the health opportunity cost of | ||
|---|---|---|---|
| £13,000 per QALY gained | £20,000 per QALY gained | £30,000 per QALY gained | |
| 0–40 | M7 222 | M7 222 | M7 222 |
| 45 | M7 222 | T9 222 | T9 222 |
| 50–100 | T7 222 | T9 222 | T9 222 |
Value of future research
Table 43 shows the value of future research in solving the uncertainty regarding which substrategy is cost-effective. The cost-effective substrategy is M7 222. The net health achieved is the average of the net health achieved in each of the 1000 simulations, which represent possible realisations of the parameter values given the current evidence. This is between 8.304 and 8.538 QALYs per man referred for testing. Hypothetically, under perfect information, between 8.320 and 8.551 QALYs per man referred could be achieved. This is the average of the maximum net health in each of the possible realisations of the parameter values. The difference between the maximum of the net health achievable and the net health achieved on average by the cost-effective strategy corresponds to the health losses because of parameter uncertainty. 113 These health losses correspond to the upper bound of the value of future research to resolve this uncertainty. Depending on the unit costs used and the value of the health opportunity cost, the health loss because of parameter uncertainty is between 0.012 and 0.015 QALYs per man referred for testing.
| Results | Health opportunity cost of | ||
|---|---|---|---|
| £13,000 per QALY gained | £20,000 per QALY gained | £30,000 per QALY gained | |
| Cost-effective substrategy | M7 222 | M7 222 | M7 222 |
| Net health of cost-effective substrategy | 8.304 | 8.449 | 8.538 |
| Maximum net health achievable | 8.320 | 8.461 | 8.551 |
| Health loss because of parameter uncertainty per man referred for testing | 0.015 | 0.012 | 0.013 |
| Health loss because of parameter uncertainty per 39,000 men referred per year | 585 | 468 | 507 |
| Health loss because of parameter uncertainty over 5 years | 2734 | 2187 | 2369 |
| Value of additional research per year (£) | 7,605,000 | 9,360,000 | 10,140,000 |
| Value of additional research over 5 years (£) | 35,538,767 | 43,740,021 | 47,385,023 |
According to NHS Reference Costs 2014–15,110 there are approximately 39,000 TRUS-guided prostate biopsies performed each year in the NHS. If each of these biopsies corresponds to a new patient referred for testing, the health losses attributable to additional research are between 468 and 585 QALYs. Over 5 years, this amounts to 2187–2734 QALYs lost. This means that, if this uncertainty was resolved, there would be 2187–2734 QALYs gained. The investment warranted by this uncertainty can be calculated by converting the health gains from perfect information into monetary units using the health opportunity cost. The maximum investment warranted by this uncertainty is between £7.6M and £10.1M over a 1-year time horizon and between £35.5M and £47.4M over a 5-year time horizon.
Chapter 8 Discussion of the diagnostic study
PROMIS is the first study to present blinded data on the diagnostic accuracy of both mpMRI and TRUS-guided biopsy compared with an accurate reference test in biopsy-naive men with suspected prostate cancer. It is also the largest registered trial to date in this at-risk population. PROMIS represents level 1b evidence for the assessment of diagnostic accuracy. The study design allowed highly reliable and precise accuracy estimates for both TRUS-guided biopsy and mpMRI to be obtained across a number of centres, and the conduct and reporting of each test was standardised and performed blind to the other test results.
Generalisability and cancer prevalence
Despite the number of withdrawals following consent, there were no significant differences between those who withdrew after registration and those who completed all of the tests. We would expect this to be the case as the mpMRI findings were blinded to patients and physicians. Withdrawal after consent of the men with large prostates may have led to a reduction in the number of men with no cancer or CNS cancers, because larger prostates tend to lead to an elevated PSA level as a result of benign prostatic hyperplasia. Although a significant number of men were screened in order to recruit our cohort, the screening log data indicated that there were no differences in age or PSA level between patients who were screened as eligible for the study but who did not enter the study and those who did enter the study.
The prevalence of any cancer or CS cancer was much higher than we anticipated. This was a function of the combined biopsy strategy – a diagnostic approach that has rarely been tried before. The effect was the identification of a large, and almost certainly representative, sample of men with prostate cancer from the cohort referred for biopsy. This figure might have also been influenced by our withdrawal of men with large prostates (> 100 ml), which could not be biopsied using the reference test.
Nonetheless, our data are not outliers with respect to the literature. Previous TPM-biopsy studies have found a higher prevalence of CS prostate cancer than studies using TRUS-guided biopsy. 82,132 No single sampling system identifies all disease. The combination of TRUS-guided biopsy and TPM-biopsy probably represents the best detection strategy that is available.
The degree to which the prevalence of CS prostate cancer is contingent on the sampling method used does raise questions about where the threshold of clinical significance should be placed. This highlights the uncertainty around what constitutes clinical significance in prostate cancer. 133 PROMIS was conducted within a health-care system that does not have a formal prostate cancer screening programme in place. In jurisdictions where screening has been in place for some time, it is likely that the prevalence of CS cancer among men undergoing biopsy will be lower than in the UK, where a formal population-based PSA screening programme is not recommended.
Diagnostic accuracy: findings and implications
On TPM-biopsy, 408 men (71%) who were tested had cancer (CNS and CS cancer), of which 230 cases (40%) were CS according to the primary definition. The primary definition of clinical significance used in our study was a Gleason score of ≥ 4 + 3 of any length and/or a maximum cancer core length of ≥ 6 mm of any grade in any location on TPM-biopsy. Using our primary reporting schema for mpMRI (as described in Chapter 3), with a score of ≥ 3 considered a suspicious scan, mpMRI was more sensitive than TRUS-guided biopsy (93%, 95% CI 88% to 96% vs. 48%, 95% CI 42% to 55%; p < 0.0001) and less specific (41%, 95% CI 36% to 46% vs. 96%, 95% CI 94% to 98%; p < 0.0001). Furthermore, using these primary definitions and thresholds, the NPV of mpMRI was 89% and the PPV was 51%.
The relatively low specificity and PPV seen with mpMRI mean that 73% of patients would test positive on mpMRI and would still require a biopsy. Because of the low specificity of mpMRI, 50% of this 73% (or 36% of men in absolute terms) would undergo an unnecessary biopsy after mpMRI triage. In the current diagnostic pathway, 100% of men have biopsies and about 60% (346/576) of these biopsies might be considered unnecessary as they were carried out in men without CS cancer.
Our results show that, of the 27% of men who would not receive a biopsy on the basis of their mpMRI result, 10% (17 men in our sample) would have CS cancer missed.
Using mpMRI to triage men might allow 158 out of 576 (27%) men to avoid a primary biopsy, resulting in 28 out of 576 (5%) fewer CNS cancers being diagnosed. If subsequent TRUS-guided biopsy needles were directed by mpMRI findings, an estimate of up to 102 out of 576 more cases (18%) of CS cancer might be detected compared with the standard pathway of TRUS-guided biopsy for all.
PROMIS was unable to test the strategy of targeting biopsies to suspicious areas because of the blinded nature of the study design. In the above paragraph, we assumed that deployment of the needles should be guided by the mpMRI findings and might be equivalent to the high accuracy of a TPM-biopsy strategy. The PRECISION trial has recently tested this assumption by randomising men to either systematic TRUS-guided biopsy or a MRI-targeted approach. It found that fewer men were biopsied, fewer cancers of a Gleason score of 6 were found and greater numbers of men with cancer of a Gleason score of ≥ 7 were detected by using mpMRI prior to biopsy. 134
Intraobserver and interobserver variability in the reporting of multiparametric magnetic resonance imaging
PROMIS was intentionally designed to be conducted at multiple sites. When the analysis was stratified between the central training site (UCLH) and non-UCLH sites, the results were almost identical between the two groups, which was reassuring.
Agreement between radiologists has implications in terms of quality assurance if mpMRI were to be rolled out nationally. Note that the interobserver agreement results in PROMIS indicate that there was moderate agreement in mpMRI scores between two independent radiologists. Our results show that variation on the Likert scale was usually by 1 point. Nonetheless, this is an important consideration and highlights the need for a robust training programme for radiologists.
Furthermore, there was no difference in diagnostic accuracy between the UCLH site and the non-UCLH sites. For both groups, the percentage of CS cases missed is about 3% and the percentage of men who may be able to avoid a biopsy is about 27%.
Context within the literature
Our results support the findings of recent systematic reviews assessing the diagnostic accuracy of mpMRI. 49,135 The reviews found sensitivities of 58–96%, NPVs of 63–98% and specificities of 23–87%. The broad ranges are attributable to the studies being conducted at single centres, each of which used different target conditions on different reference standards and different thresholds for designating a positive scan. Other limitations common to most of the studies were retrospective analysis, non-blinding of imaging findings (making them vulnerable to incorporation and reporting biases) and comparison of mpMRI with reference tests that were inaccurate (i.e. TRUS-guided biopsy) or inappropriate (i.e. radical treatment).
One other prospective study compared mpMRI with TPM-biopsy. 136,137 This study reported a sensitivity of 96%, specificity of 36%, NPV of 92% and PPV of 52% for the detection of CS cancer, which was defined as a Gleason score of 7–10 with > 5% at Gleason grade 4, ≥ 20% positive cores or ≥ 7 mm cancer. This was a single-centre, non-blinded study that permitted scanners with two magnetic field strengths (1.5 T or 3.0 T), used fewer sampling cores in the TPM-biopsy protocol and did not include TRUS-guided biopsy, which is the current standard test. 138
Service provision and health-care system issues
We have shown that high-quality mpMRI can be delivered in a multicentre setting in the UK NHS. However, we have not considered the impact that this could have on health-care settings from a capacity perspective in terms of access to high-quality mpMRI and have not formally evaluated aspects of radiology training to ensure high-quality reporting nor the need or otherwise of high-quality MRI-targeted biopsies.
In health-care systems where rates of prior PSA testing are higher, the proportion of men with CS prostate cancer according to our definition is likely to be lower. If mpMRI were used as a triage test in these settings, the proportion of men who could potentially avoid a primary biopsy might be larger. Furthermore, the recent ProtecT trial9 demonstrates no cancer-specific survival benefit over active monitoring when men are treated with RP or radical radiotherapy, although there was a beneficial effect in reducing time to metastases. 139 With the majority of patients having low-risk disease in ProtecT, weight is added to the need for strategies, such as mpMRI, that reduce the diagnosis of CNS cancer and are better able to identify higher-risk disease. We acknowledge that, in the longer term, the number and type of patients being referred to secondary care may change depending on the future validation of potential screening strategies. 140
We note that, although a positive MRI result is associated with a very high OR of harbouring CS prostate cancer and, as a result, has dominated prediction models that incorporate MRI and blood or urine biomarkers,141 there is still much work to do in this area to explore the incremental value of using an inexpensive and easily applied biomarker as a triage test prior to MRI or as a companion test, particularly in relation to an ‘indeterminate’ MRI scan. Many of the MRI studies being planned will incorporate urine and blood-based biomarkers as well as existing validated risk calculators. 140
Limitations of the diagnostic study
PROMIS had some limitations. First, TPM-biopsy is limited by the size of the template grid equipment and interference with needle insertion from the bony pubic arch. This meant that some men with very large prostates (> 100 ml) had to be excluded from the study, which may result in a slight decrease in the proportion of true negatives in the study population. However, although it is too invasive for routine use in the clinic, TPM-biopsy offered the precision required for a highly accurate reference test by virtue of its uniform 5-mm sampling density over the entire prostate. 142
Second, we acknowledge that PROMIS represents a selected group, although it is encouraging that men who were subsequently withdrawn from the study did not differ from those who completed it in terms of the baseline data we collected. We also acknowledge that these men may differ in terms of other characteristics that we did not measure.
Third, some systematic error may have been introduced into the standard test by the sequence in which TPM-biopsy and TRUS-guided biopsy were performed, and we were unable to control for this. Carrying out TPM-biopsy before TRUS-guided biopsy may have contributed to the poor accuracy of the standard test because of swelling, distortion and tissue disruption. The sequencing was based on patient safety considerations and on the need to preserve the integrity of the reference test. Infection is a major risk associated with TRUS-guided biopsy because of breaching of the rectal mucosa. Participants might have been exposed to excess risk if bacteria had been introduced into the prostate prior to multiple needle deployments of an inherently sterile TPM-biopsy.
Fourth, because of the need for blinding, the targeting of suspicious lesions identified in MRI was not possible, and we cannot accurately assess the clinical utility of a MRI-targeted biopsy approach. Fifth, although we included some measurements of interobserver variability, these were between two expert readers. Further work is required to measure the interobserver variability of expert and non-expert reporters. Last, we acknowledge that likelihood ratios and area under the receiver operating characteristic curves were not part of the prespecified analysis plan. These metrics provide an overall measure of test performance and clarify the relative strengths and weaknesses of each test, particularly as likelihood ratios are independent of disease prevalence.
The mpMRI scans were performed using a magnetic field strength of 1.5 T. This was chosen because it is widely available and because we assumed that any benefit shown was likely to be at least maintained at higher magnetic field strengths, where it is possible to achieve greater signal-to-noise ratios.
Finally, we acknowledge the benefits of communicating risk using the 1–5 ordinal scale conferred by the Gleason grade group. 143 Our end points cannot, unfortunately, be reclassified using Gleason grade group as they incorporated histopathology for our primary end point (Gleason grade 4 + 3 or Gleason grade group 3) and secondary end point (Gleason grade 3 + 4 or Gleason grade group 2), and the cancer core length thresholds, which are not part of the Gleason grade group definitions. One of the principal outcomes of PROMIS is that a normal MRI result was not associated with any Gleason grade group of ≥ 3.
Recommendations for further research
PROMIS is a unique data set that will continue to be an important resource for further research. Future work is likely to include the following.
-
modelling of prognostic factors for CS cancer and development and validation of a risk score calculator for the presence of CS cancer
-
radiological issues:
-
optimisation of sequencing (T2, DW, DCE)
-
reporting methodology
-
training and quality assurance for scanners and radiologists
-
-
pathology issues:
-
characterisation of disease
-
correlation between TPM-biopsy and MRI lesion location
-
-
biomarker/translational research analyses using the subset of blood and urine samples that have been collected.
Conclusions
Transrectal ultrasound-guided biopsy performs poorly as a diagnostic test for CS prostate cancer. Multiparametric magnetic resonance imaging identifies a large proportion of men with suspected CS cancer who will then have TRUS-guided biopsy for diagnosis, in which the biopsy strategy should be guided by the mpMRI findings. Using mpMRI as a triage test avoids biopsy in up to 27% of men with non-CS cancer or no cancer who were referred for testing. However, mpMRI as a triage test missed 17 men with CS cancer (3% of the whole PROMIS cohort) as a result of a biopsy not being performed.
Chapter 9 Discussion of the economic evaluation
Main findings
Multiparametric magnetic resonance imaging, TRUS-guided biopsy and TPM-biopsy can be used in a range of combinations and with different definitions and cut-off points to detect CS cancer in men referred to secondary care for further investigation. The combinations, definitions and cut-off points form 383 diagnostic strategies, which detect 15–100% of CS cancers, depending on the way that the tests re used in combination, their definitions for CS cancer and the cut-off point used. Strategies using the TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off point 2 detect the largest proportion of CS cancers compared with the same strategies using other definitions and cut-off points. The HRQoL consequences of testing are small and relate only to TPM-biopsy. The diagnostic strategies cost between £244 and £1653 for the UK NHS.
The quality-adjusted life expectancy and health-care costs of men diagnosed with prostate cancer at an average age of 67 years depend on the severity of their cancer and on their treatment. Men with low-risk cancer who are managed with active surveillance have an average life expectancy of 16.26 years. Men with intermediate-risk cancer who receive active surveillance have an average life expectancy of 13.38 years; this increases to 15.65 years if they are treated with radical treatment. Men with high-risk cancer who receive active surveillance have an average life expectancy of 11.15 years; this increases to 13.23 years if they are treated with radical treatment. These estimates translate to discounted lifetime health benefits and costs, which informed the outcomes of men undergoing each diagnostic strategy. In order to assess the cost-effective strategy, the results of the short-term model on the costs and outcomes of testing were combined with the results of the long-term model on the costs and outcomes of radical treatment and observation.
In the base case, using NHS reference costs,110 the cost-effective strategy at the health opportunity costs of £13,000, £20,000 and £30,000 per QALY gained involves testing all men with mpMRI at definition 2, cut-off point 2 for CS cancer, using MRI-targeted TRUS-guided biopsy at definition 2 to detect CS cancer and rebiopsying those men in whom CS cancer was not detected (substrategy M7 222). M7 222 achieves 8.72 discounted QALYs (95% CI 8.40 to 9.04 discounted QALYs) and costs £5367 (95% CI £4947 to £5876). M7 222 detects 95% of CS cancers (95% CI 92% to 98%).
A main driver of cost-effectiveness is the unit cost of each test, particularly TPM-biopsy. At a higher TPM-biopsy unit cost (e.g. £1872 per test), the cost-effective substrategy is M7 222 unless the unit cost of mpMRI is almost doubled and the unit cost of TRUS-guided biopsy is maintained or reduced. At a lower TPM-biopsy unit cost (e.g. £931), M7 222 is cost-effective if the unit cost of mpMRI is reduced, unless the £13,000 per QALY health opportunity cost is used. A reduced unit cost of TPM-biopsy favours the substrategies that include TPM-biopsy and that detect more CS cancers, such as P4 2––.
Under base-case assumptions, the results are generally robust to uncertain assumptions. Apart from M7 222 and P4 2––, the substrategies that emerge as potentially cost-effective in the sensitivity analysis are generally T7 222, T9 222 and P1. With T7 222 and T9 222, all men receive TRUS-guided biopsy as the first test and men classified as having no cancer or CNS cancer receive mpMRI. In T7, men with suspected CS cancer receive a second TRUS-guided biopsy (MRI guided). In T9, men with suspected CS cancer, as well as men with suspected CNS cancer on mpMRI but a prior no cancer result on the first biopsy, receive a second TRUS-guided biopsy (MRI guided). These strategies appear to be potentially cost-effective according to the TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off point of 2. In P1–––, all men receive TPM-biopsy and no further tests.
The value-of-information analysis indicates the magnitude of health losses because of uncertainty in the parameter estimates. It suggests that up to 0.015 QALYs per man referred for testing could be gained if better information was available. Given the large number of men referred for testing each year in the UK, this relatively small health loss translates into a large loss at the population level. Future research that resolves this uncertainty is worth up to £9.3M. More research is required to be conducted on which specific parameters most contribute to the health losses attributable to uncertainty, in order to better target investments in future research.
Comparison with other studies
The cost-effectiveness of mpMRI in combination with TRUS-guided biopsy has been subject to increasing research. A recent systematic review on the cost-effectiveness of mpMRI for the diagnosis of prostate cancer144 found five studies, although only three compared mpMRI as a first screening test in combination with TRUS-guided biopsy in an incremental analysis. 13,145,146 In addition, another study has been published more recently. 147 These studies compared up to two ways of using mpMRI, either as a first screening test to determine which men should receive MRI-targeted TRUS-guided biopsy13,146,147 or as MRI-targeted TRUS-guided biopsy for all men13 or for men with a previous negative biopsy result. 145 None of the studies had access to individual patient data; therefore, all inputs were obtained from the literature or expert opinion. As in the current evaluation, mpMRI was found to be cost-effective as a first test before TRUS-guided biopsy.
Two of these studies were conducted in a UK setting. 13,145 Mowatt et al. 145 compared targeted TRUS-guided biopsy with systematic extended core TRUS-guided biopsy. The population was men who had suspected prostate cancer with a prior negative or inconclusive biopsy and with an indication for repeat biopsy. This study found T2-weighted MRI-targeted TRUS-guided biopsy to be cost-effective, although the health gains and additional costs compared with the least costly strategy (systematic extended-core TRUS-guided biopsy) were very small.
The economic evaluation conducted for the 2014 NICE clinical guideline13 compared a strategy of mpMRI followed by MRI-targeted TRUS-guided biopsy in men with a MRI scan suspicious of cancer with TRUS-guided biopsy. The population was newly referred men with symptoms of prostate cancer. The mpMRI + TRUS-guided biopsy strategy achieved lower health benefits, but was also less costly, than the TRUS-guided biopsy alone. Although the ICER for MP-MRI+TRUS-guided biopsy compared with TRUS-guided biopsy alone was approximately £16,000 per QALY, which is generally considered to be cost-effective, the cost savings were found to be insufficient to justify the lower health benefits.
Strengths
To our knowledge, this is the first study to examine the cost-effectiveness of all of the possible ways of using mpMRI, TRUS-guided biopsy and TPM-biopsy to diagnose prostate cancer and distinguish between CNS and CS cancer. Having access to the individual patient data from the PROMIS clinical study facilitated the comparison of not only the different combinations of tests, but also different definitions and diagnostic cut-off points to define the disease. This makes this study the most comprehensive cost-effectiveness analysis of alternative diagnostic strategies for prostate cancer to date.
This evaluation was informed primarily by the diagnostic information collected in PROMIS. PROMIS is the first multicentre study to evaluate the diagnostic performance of TRUS-guided biopsy and mpMRI in a blinded fashion compared with a reference test of TPM-biopsy. Therefore, it forms the best available evidence on TRUS-guided biopsy and mpMRI. Additional information on the diagnostic performance of repeat tests and tests performed in combination was sought from the literature, and recent systematic reviews were used whenever possible. 23,105
This study explicitly distinguishes between the short-term outcomes of the diagnostic strategies and the long-term outcomes from the subsequent management decisions informed by diagnosis. Furthermore, it overcomes the limitations in the available evidence on long-term outcomes with a novel calibration method to obtain the quality-adjusted survival and lifetime costs. Extensive sensitivity analysis explores the sensitivity of the results to parameter uncertainty and to assumptions regarding the management of men in clinical practice. Probabilistic sensitivity analysis indicates the impact that parameter uncertainty had on the results. Value-of-information analysis shows the magnitude of health losses attributable to parameter uncertainty and the potential for investment in future research.
Limitations
This study has some limitations, mostly related to the fact that evidence on the long-term outcomes of men with prostate cancer and on the diagnostic performance of tests in repeated testing episodes is itself limited. Data on the long-term outcomes of men with prostate cancer, by risk subgroup and management, were obtained from a recently published RCT, PIVOT. 8 Given that only summary data were available, we developed a method to predict quality-adjusted survival and costs from information on overall survival, cumulative incidence of metastases and mortality following metastases. It assumes that the probabilities of disease progression and death are constant over time, which is unrealistic. However, this is unlikely to have a major impact on the results because the aim is to obtain long-term outcomes on average for radical treatment or active surveillance, rather than selecting between treatments.
Importantly, the long-term outcomes of men with prostate cancer who are incorrectly classified as having no cancer were assumed to be equivalent to those of men allocated to active surveillance. These men would not be recruited for studies on prostate cancer; therefore, the consequences of misdiagnosis are, by definition, unknown. The cost-effectiveness of less-sensitive diagnostic strategies is favoured if the long-term outcomes of men who are incorrectly classified as having no cancer were overestimated. The implication is that the base case may be biased towards M7 222 and against P4 2–– and P1–––, which detect all cancers. For this reason, the sensitivity analysis establishes the minimum reduction in health outcomes that misclassified men would need to experience to change the cost-effective strategy. A reduction as small as 0.01 QALYs, equivalent to 3.7 days in full health, changes the cost-effective strategy at the health opportunity cost of £30,000 per QALY. Reductions of ≥ 0.1 QALYs, equivalent to 37 days in full health, change the strategies at £13,000 and £20,000 per QALY. As a benchmark, 1–2% increases in the hazard of all-cause death (HR 1.01–1.02) reduce life expectancy by 0.07–0.13 years in men with intermediate-risk cancer. The implication is that small changes in survival may have a large impact on the results. Therefore, the follow-up of men with suspected prostate cancer but in whom cancer was not detected requires further investigation and may have an impact on the most cost-effective diagnostic strategy.
It was not possible to account for the impact of repeated tests over time on the outcomes and costs of men who are managed with active surveillance. As discussed earlier, these men are likely to be different in their cancer characteristics from the men first referred for diagnosis. Furthermore, the cancer may progress in size and differentiation at an unknown rate. To some extent, the impact of repeated testing is included in the long-term outcomes, having been based on the active surveillance group of PIVOT,8 which included repeated testing and the possibility of treatment over time. However, if the sensitivity of the watchful waiting protocol in PIVOT is lower than that of the active surveillance protocol recommended by NICE,13 the long-term outcomes of men misclassified as having low-risk cancer may have been underestimated. This was the rationale of the threshold sensitivity analysis to find the smallest proportion of reclassified men that would change the cost-effective strategy. However, it is difficult to relate this minimum proportion to the sensitivity of the active surveillance protocol recommended by NICE. Consequently, more research is required on the sensitivity of repeated testing and its implications upstream to the cost-effectiveness of diagnostic strategies.
Remaining areas of uncertainty and areas for further research
The cost-effective diagnostic strategy depends on the cost-effectiveness of active treatment; however, the long-term outcomes of men with prostate cancer remain uncertain and the evidence base, perhaps, remains insufficient. The 2014 NICE clinical guideline13 recommends that men with low-risk cancer should be offered active surveillance, whereas men with intermediate- and high-risk cancer should be offered radical treatment. These recommendations are mostly based on expert consensus rather than formal quantitative evaluations to identify for whom treatment should be recommended, how to monitor men with lower-risk cancer and which treatments should be prioritised. The lack of evidence on management decisions has upstream implications for the cost-effectiveness of diagnostic strategies because their costs and benefits are partly determined by the costs and benefits of active surveillance and treatment. If treatment is more beneficial than suggested by the evidence from PIVOT,8 the benefits of more-sensitive strategies have been underestimated. Conversely, if treatment is less beneficial, the benefits of less-sensitive strategies have been underestimated. This is explored in the threshold sensitivity analysis, which showed that the less-sensitive and less-costly diagnostic strategies become cost-effective for a 15–20% reduction in the clinical effectiveness of radical treatment.
The ProtecT study9, which compared RP, radiotherapy and active monitoring in men with prostate cancer, may help resolve this uncertainty. At the time of conducting this analysis, the 10-year follow-up results are available, but only for the entire trial population. Active treatment showed no effect on mortality, although few deaths occurred, but a significant reduction in cancer progression (HR 0.39, 95% CI 0.27 to 0.54). This is similar to the results of PIVOT8 (relative risk for bone metastasis 0.44, 95% CI 0.25 to 0.76). However, this refers to the entire trial population, almost 80% of whom had low-risk cancer and, therefore, would not be offered active treatment according to NICE’s recommendations. 13 Evidence is required on the effect of active treatment specifically in men with intermediate- and high-risk (CS) cancer, for whom the NICE guidelines13 recommend treatment.
Moreover, the predicted long-term outcomes are affected by the misclassification of men by TRUS-guided biopsy in PIVOT. 8 In PIVOT, patients were diagnosed, enrolled and classified by risk on the basis of a biopsy result and not on the basis of their true disease status. The biopsy is an imperfect test in that it produces a considerable proportion of false-negative results, with evidence from PROMIS suggesting that only 34% of intermediate-risk patients are identified as having CS cancer. The implications are twofold. First, the men enrolled in PIVOT may not be representative of the men with cancer in the general population. This is only an issue if the patients who were missed by the biopsy have a different prognosis from the prognoses of the patients enrolled in PIVOT. Second, the PIVOT risk groups may have been misclassified and this may introduce bias in the prediction of long-term outcomes and the relative clinical effectiveness of radical treatment, and, therefore, may introduce bias to the benefits of correctly detecting CS cancer. Quality-adjusted survival may have been underestimated in the high-risk subgroup if the sensitivity of TRUS-guided biopsy is higher for higher cancer severity; hence, the high-risk subgroup may consist of men with the most severe cancer, who have worse prognoses. Because only 18 out of 576 men in PROMIS had high-risk cancer, any bias arising is unlikely to affect the results.
Quality-adjusted survival may also have been underestimated in the intermediate- and low-risk subgroups in PIVOT if these were contaminated by more severe patients. This may be an issue particularly for long-term outcomes post radical treatment if its effects are more pronounced in the intermediate-risk subgroup. If the quality-adjusted survival of men treated with radical treatment was more underestimated than that of men managed with observation, the benefits of more-sensitive strategies in detecting CS cancer may have been underestimated. This issue can be resolved only with better-quality evidence on the outcomes of men with prostate cancer, based on a perfect test such as TPM-biopsy for their diagnosis and classification.
There is some uncertainty regarding the costs of the tests, particularly TPM-biopsy, which affects the results. The NHS reference cost for TPM-biopsy is approximately 25% higher than the PbR tariff, which makes strategies with TPM-biopsy less likely to be cost-effective.
In principle, the unit costs of resource use should reflect the cost to the budget holder under whose perspective the analysis is conducted (i.e. the UK NHS). 148 This would suggest that appropriate unit costs should reflect what the NHS actually pays external individuals or organisations – in this case, what hospitals pay staff and the suppliers of equipment, services and consumables. Hence, unit costs would be based on the mean reference costs reported by relevant hospital trusts. However, the UK NHS is devolved into a range of organisations: NHS England, clinical commissioning groups, hospital trusts, etc. When a patient is managed in secondary care, the hospital is generally reimbursed by the relevant clinical commissioning group that has referred them, according to the PbR tariff. The PbR tariff is informed by NHS reference costs, adjusted for inflation and changes in efficiency. If the specific perspective of the analysis is that of clinical commissioning groups, for example, the PbR tariff may be the more relevant basis for estimating unit costs. The impact of changes in the unit costs is provided in the multivariate sensitivity analysis (see Chapter 7, Threshold sensitivity analysis).
The value-of-information analysis explored the expected costs to population health associated with parameter uncertainty and the maximum expected benefit associated with investment in further research. It shows that, although the expected health loss attributable to parameter uncertainty is small at the per-patient level, it sums to a considerable amount at the population level over 5 years, given the large number of men referred for investigation. In order to inform which research to commission in the future, the value-of-information analysis should be developed further. This could take a number of avenues. The expected value of perfect parameter information can indicate which parameters are driving the uncertainty and have greater consequences, and hence should be prioritised for investment. The expected value of sample information can be explored in order to help inform the most cost-effective study design and sample size to obtain information on a specific parameter. There is also an important question regarding the structural uncertainties in the model. Specifically, the model assumes that men receiving expectant management have the outcomes of men in the observation arm of PIVOT. However, in practice, these men may receive active treatment over time. This was explored in the scenario analysis. However, future research could extend the model to account for this assumption as a parameter and explore its impact in the value-of-information analysis. There is little guidance on how to develop this work in practice and future methodological research may address this gap.
The results suggest that the most cost-effective strategy is using mpMRI as the first test, rather than TRUS-guided biopsy. It may be difficult to implement this change, because it implies a shift in resources from histopathology to imaging departments. Although financial resources are more straightforward to transfer, there may be issues around capacity, namely related to skilled staff and capital investment in equipment. Consequently, more consideration and research may be required on how best to implement this policy and manage change.
This study suggests that mpMRI is cost-effective as the first test for the diagnosis of prostate cancer when followed by MRI-targeted TRUS-guided biopsy in men in whom mpMRI suggests suspicion for CS cancer and a second biopsy if no CS cancer is found. The cost-effective strategy uses the most sensitive definitions and cut-off point for CS cancer: definition 2 for TRUS-guided biopsy (any Gleason pattern of ≥ 4 and/or cancer core length of ≥ 4 mm) and definition 2 for mpMRI (lesion volume of ≥ 0.2 ml and/or a Gleason score of ≥ 3 + 4) at the cut-off point of ≥ 2 on the Likert scale for suspicion of CS cancer.
This study makes it clear that the value of the diagnostic test depends on the value of the treatment options that follow. Here, the most cost-effective strategy is one that detects almost all men with CS cancer because active treatment was found to be highly cost-effective in this population. The findings are sensitive to the costs of the tests, hence the motivation for the bivariate sensitivity analysis indicating the most cost-effective strategy for a wide range of unit costs of each test. This can inform decisions in contexts facing different sets of prices. The findings are also sensitive to the sensitivity of MRI-targeted TRUS-guided biopsy compared with TRUS-guided biopsy alone, which was tested in the threshold sensitivity analysis. As with any research, the findings are grounded on the evidence used. The cost-effectiveness of diagnostic tests depends on the cost-effectiveness of subsequent treatment decisions. If radical treatment is less beneficial than PIVOT suggests, the scope for investment in diagnosis is smaller, and less-sensitive and less-costly strategies are favoured. This study makes clear how the value of treatment can have upstream implications for the value of the diagnostic tests.
Chapter 10 Conclusions
The results from PROMIS suggest that a diagnostic strategy that incorporates mpMRI as an initial test in unscreened men referred for prostate biopsy may be useful in three ways. First, it is likely to reduce the proportion of men having unnecessary biopsies. Second, fewer men with clinically important prostate cancer will be missed. Third, the incorporation of mpMRI may enhance the cost-effectiveness of the prostate cancer diagnostic and therapeutic pathway.
Acknowledgements
Editorial assistance with the manuscript was provided by Jo Whelan of Textpharm Ltd, funded by UCL.
We would like to thank Sarah Willis, Research Fellow in Health Economics at the London School of Hygiene & Tropical Medicine, for useful discussions on the cost-effectiveness modelling of prostate cancer.
We would like to thank every man who agreed to take part in the study. Most were motivated by a strong desire to improve the prostate cancer diagnostic pathway. Some were motivated by the greater diagnostic precision that was conferred through participation. All agreed to a CBP under general anaesthesia that has rarely been applied before. We are all hugely indebted to them.
PROMIS has been fortunate to receive input and advice from a wide range of experts in their respective fields. The full PROMIS group is detailed in the following sections.
Trial Management Group
-
Professor Mark Emberton (Chief Investigator; Urologist), UCLH.
-
Mr Hashim Ahmed (Co-chief Investigator; Urologist), UCLH.
-
Dr Ahmed El-Shater Bosaily (Clinical Research Fellow), UCLH.
-
Dr Alex Kirkham (Radiologist), UCLH.
-
Dr Alex Freeman (Pathologist), UCLH.
-
Dr Charles Jameson (Pathologist), UCLH.
-
Mr Richard Graham Hindley (Urologist), Basingstoke and North Hampshire Hospital.
-
Dr Christopher Parker (Translational Research), Royal Marsden Hospital.
-
Professor Colin Cooper (Translational Research), Royal Marsden Hospital.
-
Robert Oldroyd (Patient Representative).
-
Professor Richard Kaplan (Programme Lead; Oncologist), Medical Research Council Clinical Trials Unit (MRC CTU at UCL).
-
Dr Louise Brown (Project Lead; Statistician), MRC CTU at UCL.
-
Dr Rhian Gabe (Statistician), University of York.
-
Dr Yolanda Collaco-Moraes (Clinical Operations Manager), MRC CTU at UCL.
-
Cybil Adusei and Katie Ward (Trial Managers), MRC CTU at UCL.
-
Sophie Stewart, Katie Thompson, Claire Mulrenan, Hannah Gardner and Alex Maytum (Data Managers), MRC CTU at UCL.
-
Carlos Diaz-Montana (Data Programmer), MRC CTU at UCL.
-
Dr Chris Coyle (Clinical Fellow), MRC CTU at UCL.
-
Professor Mark Sculpher (Health Economics), University of York.
-
Ms Rita Faria (Health Economics), University of York.
-
Dr Eldon Spackman (Health Economics), University of York.
Trial Steering Committee (also acted as Data Monitoring Committee)
-
Dr David Guthrie (Chairperson; Oncologist), Derbyshire Royal Infirmary.
-
Professor John Chester (Oncologist), University of Cardiff.
-
Professor Richard Cowan (Oncologist), Christie Hospital Manchester.
-
Professor Michael Jewitt (Urologist), University of Toronto.
Participating centres (number of men registered and team members)
-
University College London Hospital (304): H Ahmed [Principal Investigator (PI)], A El-Shater Bosaily, M Emberton, A Kirkham, S Punwani, A Freeman, C Jameson, M Hung, J Coe, V Abu, R Scott and M Ahmed.
-
Basingstoke and North Hampshire Hospital (130): R Hindley (PI), P Aslet, A Emara, A Chetwood, D Peppercorn, A Thrower, H El-Mahallawi, A Mustajab, H Alkhazaraji, A Edwards and J Smith.
-
Southampton General Hospital (55): T Dudderidge (PI), J Dyer, J Smart, K Tung, H Markham, B Birch, R Lockyer, A Lodge, L Dando and J Gwilt.
-
Southmead Hospital, Bristol (44): R Persad (PI), M Elmahdy, S Pandian, D Gillatt, J Ash-Miles, M Sohail, C Shiridzinomwa and A Treasure.
-
Charing Cross Hospital, Imperial College London (41): M Winkler (PI), T Barwick, V Stewart, L Honeyfield, N Qazi, B Statton, N Ngo, K Ansu, S Edwards and E Temple.
-
Wrexham Maelor Hospital (39): I Shergill (PI), S Agarwal, K Pradeep, M Atkinson and S Ackerley.
-
Royal Hallamshire Hospital, Sheffield (36): D Rosario (PI), J Catto, F Salim, S Morgan and J Howson.
-
Musgrove Park Hospital, Taunton (36): N Burns-Cox (PI), K Gordon, A Birring, A Maccormick, P Burn, D Paterson and H Routley.
-
Frimley Park Hospital (25): S Bott (PI), H Evans, G Kousparos, AM Silvanto, A Mann, J Amero, A Pilcher and N Pereira.
-
The Whittington Hospital (18): M Ghei (PI), TT Shah, J Kumaradevan, A Trinidade, R Katz, D Arul, L Harbin, V Conteh and A Verjee.
-
Maidstone Hospital (12): A Henderson (PI), S Ghosh, P McMillan, J James, G Russell, E Bernsten, R Casey, T Nolan and D Day.
Contributions of authors
Louise Clare Brown contributed to the diagnostic study design, the statistical analysis, the first draft of the manuscript (diagnostic study section) and the interpretation and critical review of the manuscript (diagnostic study section).
Hashim U Ahmed contributed to the diagnostic study design, the statistical analysis, the first draft of the manuscript (diagnostic study section) and the interpretation and critical review of the manuscript (diagnostic study section).
Rita Faria developed the economic evaluation design, undertook the economic modelling and analysis, wrote the first and subsequent drafts of the economic chapters, and critically revised the economic chapters.
Ahmed El-Shater Bosaily contributed to the acquisition of data, diagnostic test reporting and the first draft of the manuscript (diagnostic study section).
Rhian Gabe contributed to the diagnostic study design and statistical analysis.
Richard S Kaplan contributed to the diagnostic study design, the statistical analysis and the interpretation and critical review of the manuscript (diagnostic study section).
Mahesh Parmar contributed to the interpretation and critical review of the manuscript (diagnostic study section).
Yolanda Collaco-Moraes contributed to the acquisition of data and diagnostic test reporting.
Katie Ward contributed to the acquisition of data and diagnostic test reporting.
Richard Graham Hindley contributed to the acquisition of data (as a PI in a participating site).
Alex Freeman contributed to the acquisition of data and diagnostic test reporting.
Alexander Kirkham contributed to the acquisition of data and diagnostic test reporting.
Robert Oldroyd contributed to the diagnostic study concept and initial design and the interpretation and critical review of the manuscript (diagnostic study section).
Chris Parker contributed to the diagnostic study concept and initial design and the interpretation and critical review of the manuscript (diagnostic study section).
Simon Bott contributed to the acquisition of data (as a PI in a participating site).
Nick Burns-Cox contributed to the acquisition of data (as a PI in a participating site).
Tim Dudderidge contributed to the acquisition of data (as a PI in a participating site).
Maneesh Ghei contributed to the acquisition of data (as a PI in a participating site).
Alastair Henderson contributed to the acquisition of data (as a PI in a participating site).
Rajendra Persad contributed to the acquisition of data (as a PI in a participating site).
Derek J Rosario contributed to the acquisition of data (as a PI in a participating site).
Iqbal Shergill contributed to the acquisition of data (as a PI in a participating site).
Mathias Winkler contributed to the acquisition of data (as a PI in a participating site).
Marta Soares supervised the economic evaluation design, the economic modelling and analysis, and critically reviewed the economic chapters.
Eldon Spackman commented on the economic evaluation design, modelling and analysis, conducted the analysis of the impact of the tests on health-related quality of life, and critically reviewed the economic chapters.
Mark Sculpher oversaw the economic evaluation design, modelling and analysis, and critically reviewed the economic chapters.
Mark Emberton contributed to the diagnostic study concept and initial design, the first draft of the manuscript (diagnostic study section) and the interpretation and critical review of the manuscript (diagnostic study section).
All authors reviewed and approved the final manuscript.
Publications
El-Shater Bosaily A, Parker C, Brown LC, Gabe R, Hindley RG, Kaplan R, et al. PROMIS – prostate MR imaging study: a paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp Clin Trials 2015;42:26–40.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22.
Faria R, Soares MFO, Spackman DE, Hashim A, Brown L, Kaplan R, et al. Optimising the diagnosis of prostate cancer in the era of multi-parametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging study (PROMIS). Eur Urol 2018;73:23–30.
Wilt TJ, Ahmed HU. Prostate cancer screening and the management of clinically localized disease. BMJ 2013;346:f325.
El-Shater Bosaily A, Valerio M, Hu Y, Freeman A, Jameson C, Brown L, et al. The concordance between the volume hotspot and the grade hotspot: a 3-D reconstructive model using the pathology outputs from the PROMIS trial. Prostate Cancer Prostatic Dis 2016;19:258–63.
Data sharing statement
University College London encourages data sharing and we propose to make PROMIS an openly accessible research study for future investigators. All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Prostate Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Five (accessed 28 August 2016).
- Andriole GL, Crawford ED, Grubb RL III, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-19. https://doi.org/10.1056/NEJMoa0810696.
- Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-32. https://doi.org/10.1016/S1470-2045(10)70146-7.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. https://doi.org/10.1056/NEJMoa0810084.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. https://doi.org/10.1016/S0140-6736(14)60525-0.
- Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370:932-42. https://doi.org/10.1056/NEJMoa1311593.
- Bill-Axelson A, Garmo H, Holmberg L, Johansson JE, Adami HO, Steineck G, et al. Long-term distress after radical prostatectomy versus watchful waiting in prostate cancer: a longitudinal study from the Scandinavian Prostate Cancer Group-4 randomized clinical trial. Eur Urol 2013;64:920-8. https://doi.org/10.1016/j.eururo.2013.02.025.
- Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. https://doi.org/10.1056/NEJMoa1113162.
- Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415-24. https://doi.org/10.1056/NEJMoa1606220.
- Parker C, Emberton M. Screening for prostate cancer appears to work, but at what cost?. BJU Int 2009;104:290-2. https://doi.org/10.1111/j.1464-410X.2009.08689.x.
- Barry MJ. Screening for prostate cancer – the controversy that refuses to die. N Engl J Med 2009;360:1351-4. https://doi.org/10.1056/NEJMe0901166.
- Wilt TJ, Ahmed HU. Prostate cancer screening and the management of clinically localized disease. BMJ 2013;346. https://doi.org/10.1136/bmj.f325.
- Prostate Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence; 2014.
- Cross T, McPhail S. Clinical Guideline Prostate Cancer: Diagnosis and Treatment. An Assessment of Need. A Report to the National Collaborating Centre for Cancer. Cardiff: National Collaborating Centre for Cancer; 2008.
- Han M, Chang D, Kim C, Lee BJ, Zuo Y, Kim HJ, et al. Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. J Urol 2012;188:2404-9. https://doi.org/10.1016/j.juro.2012.07.107.
- El-Shater Bosaily A, Parker C, Brown LC, Gabe R, Hindley RG, Kaplan R, et al. PROMIS – PROstate MR Imaging Study: a paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp Clin Trials 2015;42:26-40. https://doi.org/10.1016/j.cct.2015.02.008.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215-24. https://doi.org/10.1056/NEJMoa030660.
- Bangma CH, Roemeling S, Schröder FH. Overdiagnosis and overtreatment of early detected prostate cancer. World J Urol 2007;25:3-9. https://doi.org/10.1007/s00345-007-0145-z.
- Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop?. J Urol 2001;166:1679-83. https://doi.org/10.1016/S0022-5347(05)65652-2.
- Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol 2007;52:1309-22. https://doi.org/10.1016/j.eururo.2007.08.006.
- Kulkarni GS, Al-Azab R, Lockwood G, Toi A, Evans A, Trachtenberg J, et al. Evidence for a biopsy derived grade artifact among larger prostate glands. J Urol 2006;175:505-9. https://doi.org/10.1016/S0022-5347(05)00236-3.
- Porten SP, Whitson JM, Cowan JE, Cooperberg MR, Shinohara K, Perez N, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 2011;29:2795-800. https://doi.org/10.1200/JCO.2010.33.0134.
- Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. https://doi.org/10.1016/j.eururo.2013.05.049.
- Batura D, Gopal Rao G. The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention. J Antimicrob Chemother 2013;68:247-9. https://doi.org/10.1093/jac/dks401.
- Abdelkhalek MA, Abdelshafy M, Elhelaly HA, El Nasr MK. Hemospermia after transrectal ultrasound (TRUS)-guided prostatic biopsy: a prospective study. J Egypt Soc Parasitol 2012;42:63-70. https://doi.org/10.12816/0006295.
- Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology 2013;81:1142-6. https://doi.org/10.1016/j.urology.2013.02.019.
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344. https://doi.org/10.1136/bmj.d7894.
- Kurhanewicz J, Vigneron D, Carroll P, Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol 2008;18:71-7. https://doi.org/10.1097/MOU.0b013e3282f19d01.
- Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate?. Eur Urol 2006;50:1163-74. https://doi.org/10.1016/j.eururo.2006.06.025.
- Ahmed HU, Kirkham A, Arya M, Illing R, Freeman A, Allen C, et al. Is it time to consider a role for MRI before prostate biopsy?. Nat Rev Clin Oncol 2009;6:197-206. https://doi.org/10.1038/nrclinonc.2009.18.
- Bernstein MR, Cangiano T, D’Amico A, Chittams J, Hardy C, Whittington RD, et al. Endorectal coil magnetic resonance imaging and clinicopathologic findings in T1c adenocarcinoma of the prostate. Urol Oncol 2000;5:104-7. https://doi.org/10.1016/S1078-1439(99)00049-6.
- Cornud F, Flam T, Chauveinc L, Hamida K, Chretien Y, Vieillefond A, et al. Extraprostatic spread of clinically localized prostate cancer: factors predictive of pT3 tumor and of positive endorectal MR imaging examination results. Radiology 2002;224:203-10. https://doi.org/10.1148/radiol.2241011001.
- May F, Treumann T, Dettmar P, Hartung R, Breul J. Limited value of endorectal magnetic resonance imaging and transrectal ultrasonography in the staging of clinically localized prostate cancer. BJU Int 2001;87:66-9. https://doi.org/10.1046/j.1464-410x.2001.00018.x.
- Outwater EK, Petersen RO, Siegelman ES, Gomella LG, Chernesky CE, Mitchell DG. Prostate carcinoma: assessment of diagnostic criteria for capsular penetration on endorectal coil MR images. Radiology 1994;193:333-9. https://doi.org/10.1148/radiology.193.2.7972739.
- Rifkin MD, Zerhouni EA, Gatsonis CA, Quint LE, Paushter DM, Epstein JI, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med 1990;323:621-6. https://doi.org/10.1056/NEJM199009063231001.
- Schiebler ML, Yankaskas BC, Tempany C, Spritzer CE, Rifkin MD, Pollack HM, et al. MR imaging in adenocarcinoma of the prostate: interobserver variation and efficacy for determining stage C disease. AJR Am J Roentgenol 1992;158:559-62. https://doi.org/10.2214/ajr.158.3.1738994.
- Schnall MD, Pollack HM. Magnetic resonance imaging of the prostate gland. Urol Radiol 1990;12:109-14. https://doi.org/10.1007/BF02923982.
- Chen M, Dang HD, Wang JY, Zhou C, Li SY, Wang WC, et al. Prostate cancer detection: comparison of T2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol 2008;49:602-10. https://doi.org/10.1080/02841850802004983.
- Futterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006;241:449-58. https://doi.org/10.1148/radiol.2412051866.
- Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis – correlation with biopsy and histopathology. J Magn Reson Imaging 2006;24:108-13. https://doi.org/10.1002/jmri.20626.
- Kumar V, Jagannathan NR, Kumar R, Das SC, Jindal L, Thulkar S, et al. Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging 2006;24:541-8. https://doi.org/10.1016/j.mri.2006.01.001.
- Macura KJ. Multiparametric magnetic resonance imaging of the prostate: current status in prostate cancer detection, localization, and staging. Semin Roentgenol 2008;43:303-13. https://doi.org/10.1053/j.ro.2008.06.002.
- Mazaheri Y, Shukla-Dave A, Hricak H, Fine SW, Zhang J, Inurrigarro G, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging – correlation with pathologic findings. Radiology 2008;246:480-8. https://doi.org/10.1148/radiol.2462070368.
- Noworolski SM, Henry RG, Vigneron DB, Kurhanewicz J. Dynamic contrast-enhanced MRI in normal and abnormal prostate tissues as defined by biopsy, MRI, and 3D MRSI. Magn Reson Med 2005;53:249-55. https://doi.org/10.1002/mrm.20374.
- Reinsberg SA, Payne GS, Riches SF, Ashley S, Brewster JM, Morgan VA, et al. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol 2007;188:91-8. https://doi.org/10.2214/AJR.05.2198.
- Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging 2007;25:146-52. https://doi.org/10.1002/jmri.20793.
- van Dorsten FA, van der Graaf M, Engelbrecht MR, van Leenders GJ, Verhofstad A, Rijpkema M, et al. Combined quantitative dynamic contrast-enhanced MR imaging and (1)H MR spectroscopic imaging of human prostate cancer. J Magn Reson Imaging 2004;20:279-87. https://doi.org/10.1002/jmri.20113.
- Yoshimitsu K, Kiyoshima K, Irie H, Tajima T, Asayama Y, Hirakawa M, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging 2008;27:132-9. https://doi.org/10.1002/jmri.21181.
- Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015;68:1045-53. https://doi.org/10.1016/j.eururo.2015.01.013.
- Harlan SR, Cooperberg MR, Elkin E, Lubeck DP, Meng M, Mehta SS, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol 2003;170:1804-7. https://doi.org/10.1097/01.ju.0000091641.34674.11.
- Ahmed HU, Calleary J, Arya M, Emberton M, Illing RO, Allen C. Re: dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2007;177.
- Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol 2008;26:506-10. https://doi.org/10.1016/j.urolonc.2008.03.005.
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate – a 4-year experience. Urology 2007;70:S27-35. https://doi.org/10.1016/j.urology.2007.06.1126.
- Li H, Yan W, Zhou Y, Ji Z, Chen J. Transperineal ultrasound-guided saturation biopsies using 11-region template of prostate: report of 303 cases. Urology 2007;70:1157-61. https://doi.org/10.1016/j.urology.2007.07.072.
- Merrick GS, Taubenslag W, Andreini H, Brammer S, Butler WM, Adamovich E, et al. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int 2008;101:1524-9. https://doi.org/10.1111/j.1464-410X.2008.07542.x.
- Bott SR, Henderson A, Halls JE, Montgomery BS, Laing R, Langley SE. Extensive transperineal template biopsies of prostate: modified technique and results. Urology 2006;68:1037-41. https://doi.org/10.1016/j.urology.2006.05.033.
- Crawford ED, Wilson SS, Torkko KC, Hirano D, Stewart JS, Brammell C, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int 2005;96:999-1004. https://doi.org/10.1111/j.1464-410X.2005.05801.x.
- Furuno T, Demura T, Kaneta T, Gotoda H, Muraoka S, Sato T, et al. Difference of cancer core distribution between first and repeat biopsy: in patients diagnosed by extensive transperineal ultrasound guided template prostate biopsy. The Prostate 2004;58:76-81. https://doi.org/10.1002/pros.10298.
- Buskirk SJ, Pinkstaff DM, Petrou SP, Wehle MJ, Broderick GA, Young PR, et al. Acute urinary retention after transperineal template-guided prostate biopsy. Int J Radiat Oncol Biol Phys 2004;59:1360-6. https://doi.org/10.1016/j.ijrobp.2004.01.045.
- Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg 2005;75:48-50. https://doi.org/10.1111/j.1445-2197.2005.03284.x.
- Transperineal Template Biopsy and Mapping of the Prostate. London: National Institute for Health and Care Excellence; 2010.
- Bossuyt PM, Irwig L, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ 2006;332:1089-92. https://doi.org/10.1136/bmj.332.7549.1089.
- Levels of Evidence 1. Oxford: University of Oxford; 2013.
- World Medical Association . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects 2018. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 15 June 2018).
- Research Governance Framework for Health and Social Care. London: Department of Health and Social Care; 2005.
- Dickinson L, Hashim UA, Clare A, Jelle OB, Brendan C, Jurgen JF, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2010;59:477-94. https://doi.org/10.1016/j.eururo.2010.12.009.
- Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. https://doi.org/10.1007/s00330-011-2377-y.
- Kirkham AP, Haslam P, Keanie JY, McCafferty I, Padhani AR, Punwani S, et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol 2013;68:1016-23. https://doi.org/10.1016/j.crad.2013.03.030.
- Weinreb JC, Blume JD, Coakley FV, Wheeler TM, Cormack JB, Sotto CK, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy – results of ACRIN Prospective Multi-institutional Clinicopathologic Study 1. Radiology 2009;251:122-33. https://doi.org/10.1148/radiol.2511080409.
- Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and Likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol 2013;201:W612-18. https://doi.org/10.2214/AJR.12.10173.
- Rastinehad AR, Waingankar N, Turkbey B, Yaskiv O, Sonstegard AM, Fakhoury M, et al. Comparison of multiparametric MRI scoring systems and the impact on cancer detection in patients undergoing MR US fusion guided prostate biopsies. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0143404.
- Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway?. J Magn Reson Imaging 2013;37:48-5. https://doi.org/10.1002/jmri.23689.
- Li B, Du Y, Yang H, Huang Y, Meng J, Xiao D. Magnetic resonance imaging for prostate cancer clinical application. Chin J Cancer Res 2013;25:240-9.
- Franiel T, Hamm B, Hricak H. Dynamic contrast-enhanced magnetic resonance imaging and pharmacokinetic models in prostate cancer. Eur Radiol 2011;21:616-26. https://doi.org/10.1007/s00330-010-2037-7.
- Ocak I, Bernardo M, Metzger G, Barrett T, Pinto P, Albert PS, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol 2007;189. https://doi.org/10.2214/AJR.06.1329.
- Ito H, Kamoi K, Yokoyama K, Yamada K, Nishimura T. Visualization of prostate cancer using dynamic contrast-enhanced MRI: comparison with transrectal power Doppler ultrasound. Br J Radiol 2003;76:617-24. https://doi.org/10.1259/bjr/52526261.
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011;59:61-7. https://doi.org/10.1016/j.eururo.2010.10.039.
- Stark JR, Perner S, Stampfer MJ, Sinnott JA, Finn S, Eisenstein AS, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3?. J Clin Oncol 2009;27:3459-64. https://doi.org/10.1200/JCO.2008.20.4669.
- Kepner GR, Kepner JV. Transperineal prostate biopsy: analysis of a uniform core sampling pattern that yields data on tumor volume limits in negative biopsies. Theor Biol Med Model 2010;7:1-13. https://doi.org/10.1186/1742-4682-7-23.
- Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RCN, Hoedemaeker RF, van Leenders GJLH, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011;185:121-5. https://doi.org/10.1016/j.juro.2010.08.082.
- Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. https://doi.org/10.1002/1097-0142(19930201)71:3+<933::AID-CNCR2820711408>3.0.CO;2-L.
- Ahmed HU, Hu Y, Carter T, Arumainayagam N, Lecornet E, Freeman A, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J Urol 2011;186:458-64. https://doi.org/10.1016/j.juro.2011.03.147.
- Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, Curtis R, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis 2010;13:71-7. https://doi.org/10.1038/pcan.2009.42.
- Dall’Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, Klotz LH, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer 2008;112:1650-9. https://doi.org/10.1002/cncr.23373.
- Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection – a multireader study. Radiology 2009;250:145-51. https://doi.org/10.1148/radiol.2501080207.
- Türkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL. The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol 2010;16:186-92. https://doi.org/10.4261/1305-3825.DIR.2537-08.1.
- Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 2006;175:1605-12. https://doi.org/10.1016/S0022-5347(05)00957-2.
- Machin D, Campbell MJ, Tan SB, Tan SH, Machin D, Campbell MJ, et al. Sample Size Tables for Clinical Studies. Hoboken, NJ: Wiley-Blackwell; 2009.
- Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2000;56:345-51. https://doi.org/10.1111/j.0006-341X.2000.00345.x.
- Wang W, Davis CS, Soong SJ. Comparison of predictive values of two diagnostic tests from the same sample of subjects using weighted least squares. Stat Med 2006;25:2215-29. https://doi.org/10.1002/sim.2332.
- Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection – histopathologic correlation. Radiology 2010;255:89-9. https://doi.org/10.1148/radiol.09090475.
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. https://doi.org/10.1016/S0140-6736(16)32401-1.
- Radtke JP, Kuru TH, Boxler S, Alt CD, Popeneciu IV, Huettenbrink C, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 2015;193:87-94. https://doi.org/10.1016/j.juro.2014.07.098.
- Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 2013;189:860-6. https://doi.org/10.1016/j.juro.2012.10.009.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- US Food and Drug Administration (FDA) . What Is a Serious Adverse Event? n.d. www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm (accessed 7 September 2016).
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Access 2015;19.
- Guide to the Processes of Technology Appraisal. London: NICE; 2014.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-90. https://doi.org/10.1056/NEJMoa1113135.
- Wallis CJ, Saskin R, Choo R, Herschorn S, Kodama RT, Satkunasivam R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol 2016;70:21-30. https://doi.org/10.1016/j.eururo.2015.11.010.
- Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol 2009;27:4321-6. https://doi.org/10.1200/JCO.2008.20.3497.
- Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol 2002;167:2435-9. https://doi.org/10.1016/S0022-5347(05)64999-3.
- Barzell WE, Melamed MR, Cathcart P, Moore CM, Ahmed HU, Emberton M. Identifying candidates for active surveillance: an evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J Urol 2012;188:762-7. https://doi.org/10.1016/j.juro.2012.04.107.
- Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MM. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. https://doi.org/10.1016/j.eururo.2014.11.037.
- Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer 1993;71:3582-93. https://doi.org/10.1002/1097-0142(19930601)71:11<3582::AID-CNCR2820711120>3.0.CO;2-Y.
- Goto Y, Ohori M, Arakawa A, Kattan MW, Wheeler TM, Scardino PT. Distinguishing clinically important from unimportant prostate cancers before treatment: value of systematic biopsies. J Urol 1996;156:1059-63. https://doi.org/10.1016/S0022-5347(01)65702-1.
- Essink-Bot ML, de Koning HJ, Nijs HG, Kirkels WJ, van der Maas PJ, Schröder FH. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst 1998;90:925-31. https://doi.org/10.1093/jnci/90.12.925.
- Tsivian M, Abern MR, Qi P, Polascik TJ. Short-term functional outcomes and complications associated with transperineal template prostate mapping biopsy. Urology 2013;82:166-70. https://doi.org/10.1016/j.urology.2013.01.071.
- Department of Health and Social Care . Reference Costs 2014–15 2016. www.gov.uk/government/uploads/system/uploads/attachment_data/file/477919/2014-15_Reference_costs_publication.pdf (accessed 18 August 2016).
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS England Monitor . 2014/15/National/Tariff/Payment/System 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/477919/2014-15_Reference_costs_publication.pdf (accessed 28 August 2016).
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Prostate Cancer: Diagnosis and Treatment. London: NICE; 2008.
- Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84. https://doi.org/10.1056/NEJMoa043739.
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlén BJ, et al. Scandinavian Prostatic Cancer Group Study Number 4 . Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002;347:790-6. https://doi.org/10.1056/NEJMoa021483.
- Hummel S, Paisley S, Morgan A, Currie E, Brewer E. Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7330.
- Calvert NW, Morgan AB, Catto JW, Hamdy FC, Akehurst RL, Mouncey P, et al. Effectiveness and cost-effectiveness of prognostic markers in prostate cancer. Br J Cancer 2003;88:31-5. https://doi.org/10.1038/sj.bjc.6600630.
- Bill-Axelson A, Holmberg L, Filén F, Ruutu M, Garmo H, Busch C, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst 2008;100:1144-54. https://doi.org/10.1093/jnci/djn255.
- Xiong T, Turner RM, Wei Y, Neal DE, Lyratzopoulos G, Higgins JP. Comparative efficacy and safety of treatments for localised prostate cancer: an application of network meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004285.
- Graversen PH, Nielsen KT, Gasser TC, Corle DK, Madsen PO. Radical prostatectomy versus expectant primary treatment in stages I and II prostatic cancer. A fifteen-year follow-up. Urology 1990;36:493-8. https://doi.org/10.1016/0090-4295(90)80184-O.
- Wilt TJ. The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy with watchful waiting for men with clinically localized prostate cancer. JNCI Monographs 2012;2012:184-90. https://doi.org/10.1093/jncimonographs/lgs041.
- Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. https://doi.org/10.1056/NEJMoa1011967.
- Widmark A. Prospective Randomized Trial Comparing External Beam Radiotherapy Versus Watchful Waiting in Early Prostate Cancer (T1b-T2, pN0, Grade 1–2, M0) n.d.
- James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the ‘Docetaxel Era’: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 2015;67:1028-38. https://doi.org/10.1016/j.eururo.2014.09.032.
- Torvinen S, Färkkilä N, Sintonen H, Saarto T, Roine RP, Taari K. Health-related quality of life in prostate cancer. Acta Oncol 2013;52:1094-101. https://doi.org/10.3109/0284186X.2012.760848.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Lord J, Willis S, Eatock J, Tappenden P, Trapero-Bertran M, Miners A, et al. Economic modelling of diagnostic and treatment pathways in National Institute for Health and Care Excellence clinical guidelines: the Modelling Algorithm Pathways in Guidelines (MAPGuide) project. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17580.
- Korfage IJ, Essink-Bot ML, Borsboom GJ, Madalinska JB, Kirkels WJ, Habbema JDF, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. Int J Cancer 2005;116:291-6. https://doi.org/10.1002/ijc.21043.
- National Life Tables, United Kingdom Statistical Bulletins. London: Office for National Statistics; 2015.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- de Groot JA, Bossuyt PM, Reitsma JB, Rutjes AW, Dendukuri N, Janssen KJ, et al. Verification problems in diagnostic accuracy studies: consequences and solutions. BMJ 2011;343. https://doi.org/10.1136/bmj.d4770.
- Valerio M, Anele C, Bott SR, Charman SC, van der Meulen J, El-Mahallawi H, et al. The prevalence of clinically significant prostate cancer according to commonly used histological thresholds in men undergoing template prostate mapping biopsies. J Urol 2016;195:1403-8. https://doi.org/10.1016/j.juro.2015.11.047.
- Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. PRECISION Study Group Collaborators . MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767-7. https://doi.org/10.1056/NEJMoa1801993.
- de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014;202:343-51. https://doi.org/10.2214/AJR.13.11046.
- Thompson JE, Moses D, Shnier R, Brenner P, Delprado W, Ponsky L, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol 2014;192:67-74. https://doi.org/10.1016/j.juro.2014.01.014.
- Thompson JE, van Leeuwen PJ, Moses D, Shnier R, Brenner P, Delprado W, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol 2016;195:1428-35. https://doi.org/10.1016/j.juro.2015.10.140.
- El-Shater Bosaily A, Arya M, Punwani S, Emberton M, Kirkham A, Freeman A, et al. Re: multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study: J. E. Thompson, D. Moses, R. Shnier, P. Brenner, W. Delprado, L. Ponsky, M. Pulbrook, M. Bohm, A.-M. Haynes, A. Hayen and P. D. Stricker J Urol 2014;192:67–74. J Urol 2015;193:735-6.
- Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109-18. https://doi.org/10.1016/S1470-2045(14)70361-4.
- Grönberg H, Adolfsson J, Aly M, Nordström T, Wiklund P, Brandberg Y, et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol 2015;16:1667-76. https://doi.org/10.1016/S1470-2045(15)00361-7.
- National Institute for Health and Care Excellence (NICE) . Diagnosing Prostate Cancer: PROGENSA PCA3 Assay and Prostate Health Index. 2015. www.nice.org.uk/guidance/dg17 (accessed 8 May 2018).
- Crawford ED, Rove KO, Barqawi AB, Maroni PD, Werahera PN, Baer CA, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate 2013;73:778-87. https://doi.org/10.1002/pros.22622.
- Berney DM, Beltran L, Fisher G, North BV, Greenberg D, Møller H, et al. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer 2016;114:1078-83. https://doi.org/10.1038/bjc.2016.86.
- Willis SR, van der Meulen J, Valerio M, Miners A, Ahmed HU, Emberton M. A review of economic evaluations of diagnostic strategies using imaging in men at risk of prostate cancer. Curr Opin Urol 2015;25:483-9. https://doi.org/10.1097/MOU.0000000000000220.
- Mowatt G, Scotland G, Boachie C, Cruickshank M, Ford JA, Fraser C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17200.
- de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modelling study from a health care perspective. Eur Urol 2014;66:430-6. https://doi.org/10.1016/j.eururo.2013.12.012.
- Cerantola Y, Dragomir A, Tanguay S, Bladou F, Aprikian A, Kassouf W. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy in diagnosing prostate cancer. Urol Oncol 2016;34. https://doi.org/10.1016/j.urolonc.2015.09.010.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol 2011;186:1830-4. https://doi.org/10.1016/j.juro.2011.06.057.
- Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 2010;183:963-8. https://doi.org/10.1016/j.juro.2009.11.043.
- Carmignani L, Picozzi S, Spinelli M, Di Pierro S, Mombelli G, Negri E, et al. Bacterial sepsis following prostatic biopsy. Int Urol Nephrol 2012;44:1055-63. https://doi.org/10.1007/s11255-012-0145-9.
- Simsir A, Kismali E, Mammadov R, Gunaydin G, Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy?. Urol Int 2010;84:395-9. https://doi.org/10.1159/000296290.
- Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schroder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol 2012;61:1110-4. https://doi.org/10.1016/j.eururo.2011.12.058.
- Hasegawa T, Shimomura T, Yamada H, Ito H, Kato N, Hasegawa H, et al. Fatal septic shock caused by transrectal needle biopsy of the prostate; a case report. Kansenshogaku Zasshi 2002;76:893-7. https://doi.org/10.11150/kansenshogakuzasshi1970.76.893.
- Kumagi A, Ogawa D, Koyama T, Takeuchi I, Oyama I. A case report of Fourneir’s gangrene in a diabetic patient induced by transrectal prostate biopsy (TRPB). Nihon Hinyokika Gakkai Zasshi 2002;93:648-51. https://doi.org/10.5980/jpnjurol1989.93.648.
- Gallina A, Suardi N, Montorsi F, Capitanio U, Jeldres C, Saad F, et al. Mortality at 120 days after prostatic biopsy: a population-based study of 22,175 men. Int J Cancer 2008;123:647-52. https://doi.org/10.1002/ijc.23559.
- Carlsson SV, Holmberg E, Moss SM, Roobol MJ, Schröder FH, Tammels TL, et al. No excess mortality after prostate biopsy: results from the European Randomized Study of Screening for Prostate Cancer. BJU Int 2011;107:1912-7. https://doi.org/10.1111/j.1464-410X.2010.09712.x.
- Wu MW, Sevilla EM, Raman L, Consigliere D, Siow WY, Tiong HY. Incidence of complications after transrectal ultrasonography-guided biopsy of the prostate in a local tertiary institution. Singapore Med J 2011;52:752-7.
Appendix 1 Supplementary clinical results
Centres
Participants were recruited from the following NHS hospitals in England: UCLH (lead centre), Basingstoke and North Hampshire Hospital, Imperial College/Charing Cross Hospital, Musgrove Park Hospital, Maidstone Hospital, Southmead Hospital, Whittington Hospital, Wrexham Maelor Hospital, Royal Hallamshire Hospital, Frimley Park Hospital and Southampton General Hospital.
Screening
Screening log data were of adequate quality in 6 of the 11 centres. These centres provided 608 of the 740 registrations. In summary, 608 out of 1618 men (38%) who were screened at these centres were registered into the study. However, about half of the 1010 non-registered men were ineligible according to the entry criteria (n = 450) and in 68 men there was a valid clinical reason for non-entry. For 378 men (37%), the reason for not registering was patient or clinician refusal; in addition, 11 men (1%) experienced delays but would have been eligible. According to the baseline characteristics we were able to collect on these non-registered men, there were no strong differences in age or PSA level between the 389 men who were either delayed or refused entry and those who consented to be registered into PROMIS.
Results of diagnostic tests (supplementary)
| Gleason gradesa | Any cancer (N = 408) | CS according to definition | ||
|---|---|---|---|---|
| 1a (N = 230) | 2a (N = 331) | 3a (N = 308) | ||
| Cancer core length (mm), mean (SD) | 6.3 (3.8) | 9.1 (2.7) | 7.4 (3.4) | 7.5 (3.4) |
| 3 + 3, n | 100 | 10b | 23b | 0 |
| 3 + 4, n | 252 | 164b | 252 | 252 |
| 3 + 5, n | 1 | 1 | 1 | 1 |
| 4 + 3, n | 44 | 44 | 44 | 44 |
| 4 + 5, n | 7 | 7 | 7 | 7 |
| 5 + 4, n | 4 | 4 | 4 | 4 |
| Gleason gradesa | Any cancer (N = 286) | CS according to definition | ||
|---|---|---|---|---|
| 1a (N = 124) | 2a (N = 203) | 3a (N = 151) | ||
| Cancer core length (mm), mean (SD) | 5.2 (4.1) | 8.4 (4.3) | 6.6 (4.1) | 6.7 (4.4) |
| 3 + 3, n | 135 | 26b | 52b | 0 |
| 3 + 4, n | 103 | 50b | 103 | 103 |
| 3 + 5, n | 1 | 1 | 1 | 1 |
| 4 + 3, n | 34 | 34 | 34 | 34 |
| 4 + 4, n | 6 | 6 | 6 | 6 |
| 4 + 5, n | 4 | 4 | 4 | 4 |
| 5 + 3, n | 1 | 1 | 1 | 1 |
| 5 + 4, n | 2 | 2 | 2 | 2 |
| mpMRI | TRUS-guided biopsy, n | Total | |
|---|---|---|---|
| + CS cancer | – NC or CNS cancer | ||
| + CS cancer | 115 | 303 | 418 |
| – NC or CNS cancer | 9 | 149 | 158 |
| Total | 124 | 452 | 576 |
Case illustrations
Figure 41 illustrates a case showing correlation of TRUS-guided biopsy and mpMRI with TPM-biopsy for a man harbouring CS cancer. Axial mpMRI scans are shown in a man with a PSA level of 12.4 ng/ml and normal DRE. The mpMRI shows a 39-ml prostate volume and a right-sided lesion (Likert score of 5/5) on (clockwise from top left) T2-weighted, diffusion-weighted high b-value and ADC map and dynamic contrast enhanced images (all other areas had a Likert score of 1–2/5). TRUS-guided biopsy detected no cancer and TPM-biopsy found a Gleason score of 4 + 3 and a cancer core length of 4 mm in a total of 8 out of 70 scores taken (primary definition of CS prostate cancer) that was concordant with the mpMRI. On the TPM-biopsy diagram, each white or coloured box represents an area biopsied with one core. TRUS-guided biopsy showed no cancer.
FIGURE 41.
A case showing correlation of TRUS-guided biopsy and mpMRI with TPM-biopsy for a man harbouring CS cancer. (a) mpMRI; (b) TPM-biopsy; and (c) TRUS-guided biopsy. ASAP, atypical small acinar proliferation; G3, Gleason 3; HGPIN, high-grade prostate intraepithelial neoplasia; Inflamm, inflammation; ISUP, International Society of Urological Pathology; Lymphovas, Lymphovascular.
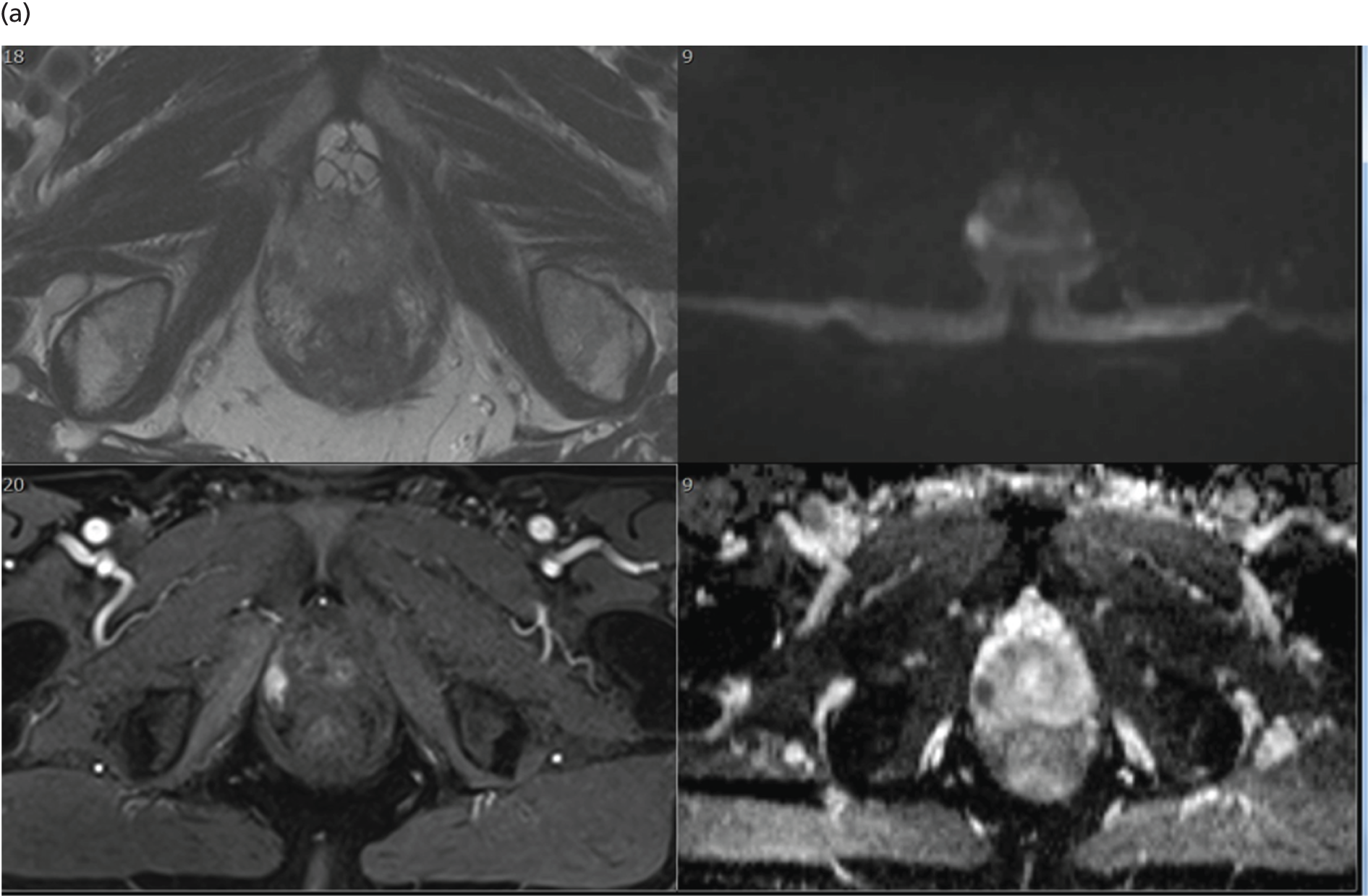
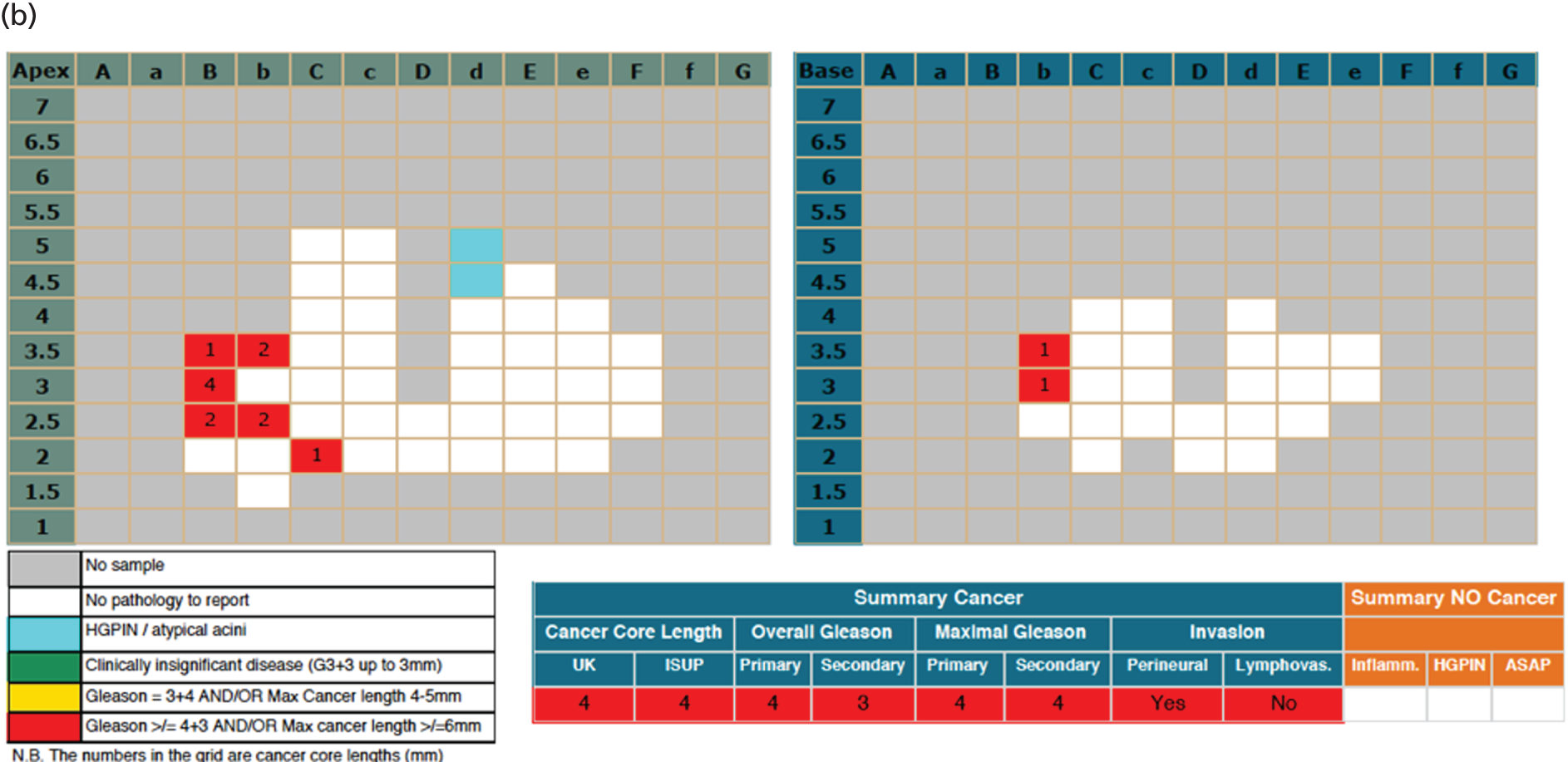
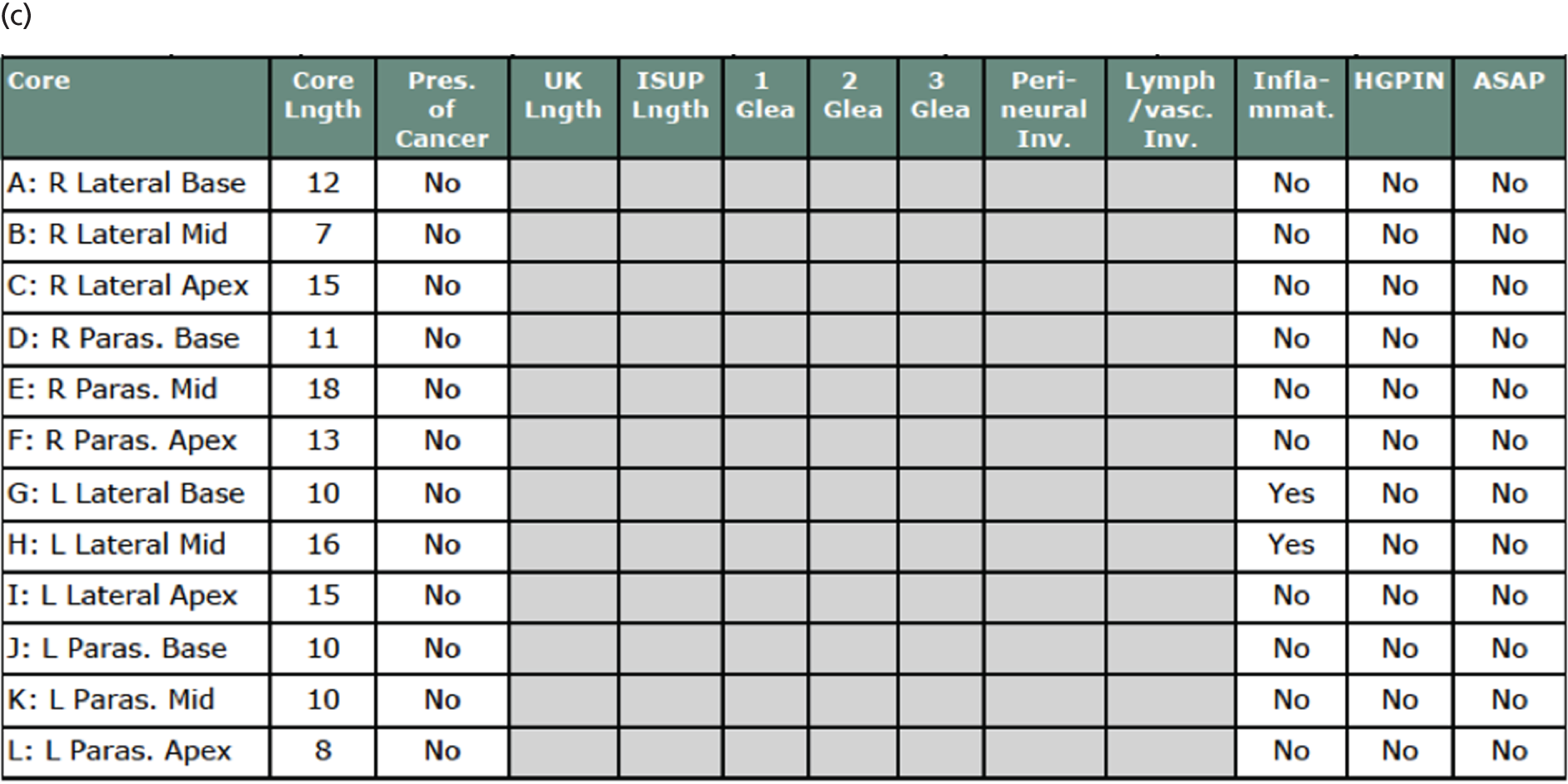
Figure 42 illustrates a case showing correlation of TRUS-guided biopsy and mpMRI with TPM-biopsy for a man harbouring no cancer. It shows a series of images demonstrating mpMRI in a man with a PSA level of 8.9 ng/ml and a normal DRE, showing a 45-ml prostate volume and no suspicious area lesion on mpMRI (T2-weighted image only shown). This was reported as a Likert score of 2. TPM-biopsy and TRUS-guided biopsy detected no cancer.
FIGURE 42.
A case showing correlation of TRUS-guided biopsy and mpMRI with TPM-biopsy for a man harbouring no cancer. (a) mpMRI; (b) TPM-biopsy; and (c) TRUS-guided biopsy. ASAP, atypical small acinar proliferation; G3, Gleason 3; HGPIN, high-grade prostate intraepithelial neoplasia; Inflamm, inflammation; ISUP, International Society of Urological Pathology; Lymphovas, Lymphovascular.

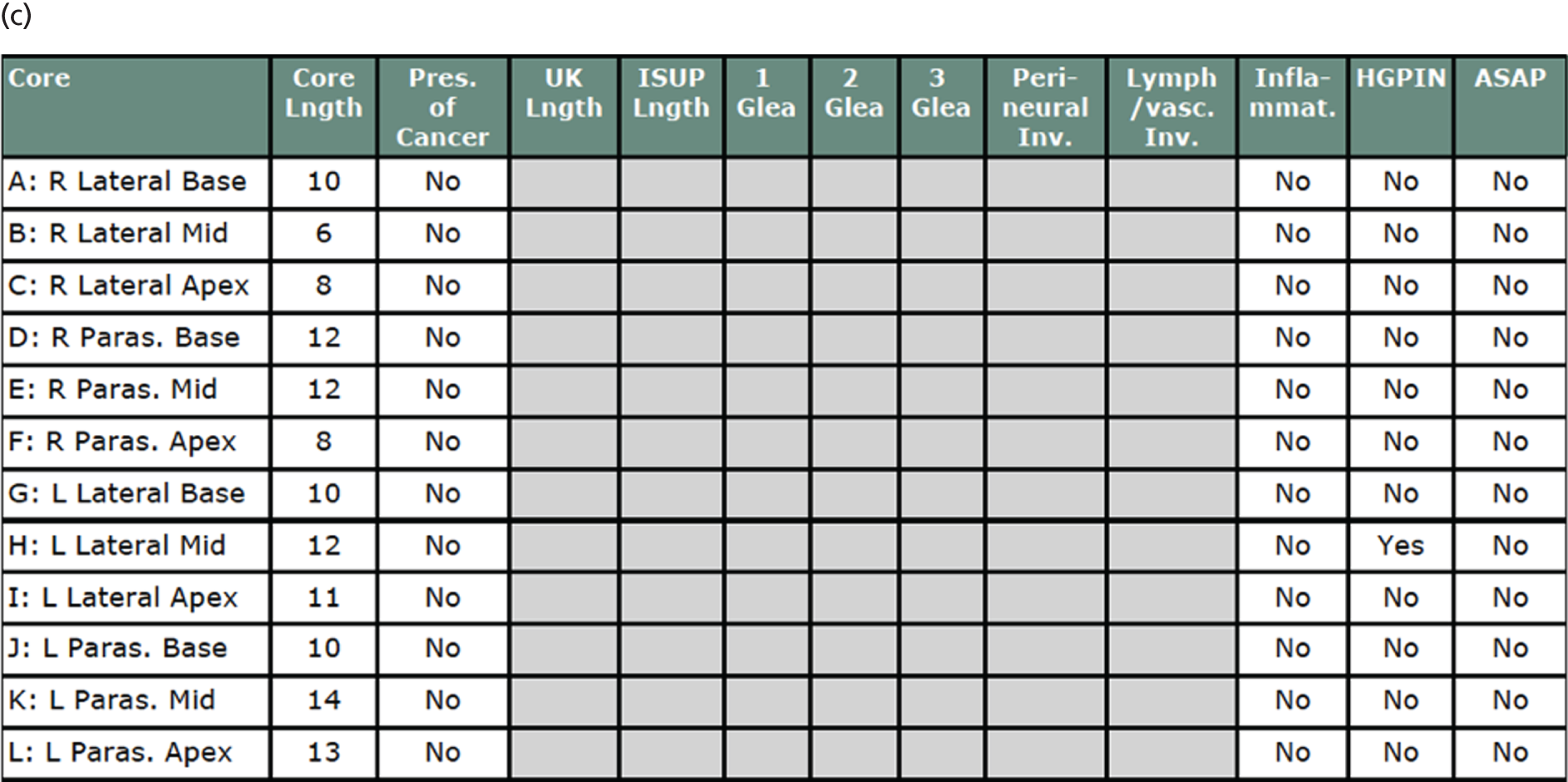
Clinical implications of introducing multiparametric magnetic resonance imaging
Two scenarios were explored. In the best-case scenario (Figure 43), the results of mpMRI would be used to direct TRUS-guided biopsy. In the worst-case scenario (Figure 44), TRUS-guided biopsy after mpMRI would be conducted without direction from mpMRI.
FIGURE 43.
Flow chart showing best-case scenario (TRUS-guided biopsy directed by mpMRI).

FIGURE 44.
Flow chart showing worst-case scenario (TRUS-guided biopsy not directed by mpMRI).

Best-case scenario
-
Biopsies, n = 418 (73% of total).
-
Significant cancer detected, n = 213 (37% of total; 93% of 230 with TPM sig disease).
-
Significant cancer missed, n = 17 (3% of total; 7% of 230 with TPM sig disease).
-
Overdiagnosis if insignificant cancers offered active surveillance, n = 0.
-
Overdiagnosis if insignificant cancers are treated, n = 121 (21% of total; 35% of 346 without TPM sig disease).
Worst-case scenario
-
Biopsies, n = 418 (73% of total).
-
Significant cancer detected, n = 77 + 105 = 182 (32% of total; 79% of 230 with TPM sig disease).
-
Significant cancer missed, n = 17 + 31 = 48 (8% of total; 21% of 230 with TPM sig disease).
-
Overdiagnosis, n = 244 – 182 = 62 (11% of total; 18% of 346 without TPM sig disease).
Agreement between sites for multiparametric magnetic resonance imaging measurements
In order to determine whether or not the lead radiology site (UCLH, responsible for training all other sites) demonstrated different diagnostic accuracy from the non-UCLH sites, the results were compared for the primary outcome analysis between mpMRI definition 2, cut-off point 3 and TPM-biopsy definition 1 variables for the detection of CS cancer. The results were almost identical (Tables 47 and 48 and Figures 45 and 46). For both groups, the proportion of CS cases that were missed was about 3% and the proportion of men who may be able to avoid a biopsy was about 27%.
| mpMRI | TPM-biopsy, n | ||
|---|---|---|---|
| + CS | – CNS | Total | |
| + CS | 85 | 75 | 160 |
| – CNS | 6 | 56 | 62 |
| Total | 91 | 131 | 222 |
| mpMRI | TPM-biopsy, n | ||
|---|---|---|---|
| + CS | – CNS | Total | |
| + CS | 128 | 130 | 258 |
| – CNS | 11 | 85 | 96 |
| Total | 139 | 215 | 354 |
FIGURE 45.
Results for detection of CS cancer for mpMRI (definition 2, cut-off point 3) relative to TPM-biopsy (definition 1) for the UCLH site alone.
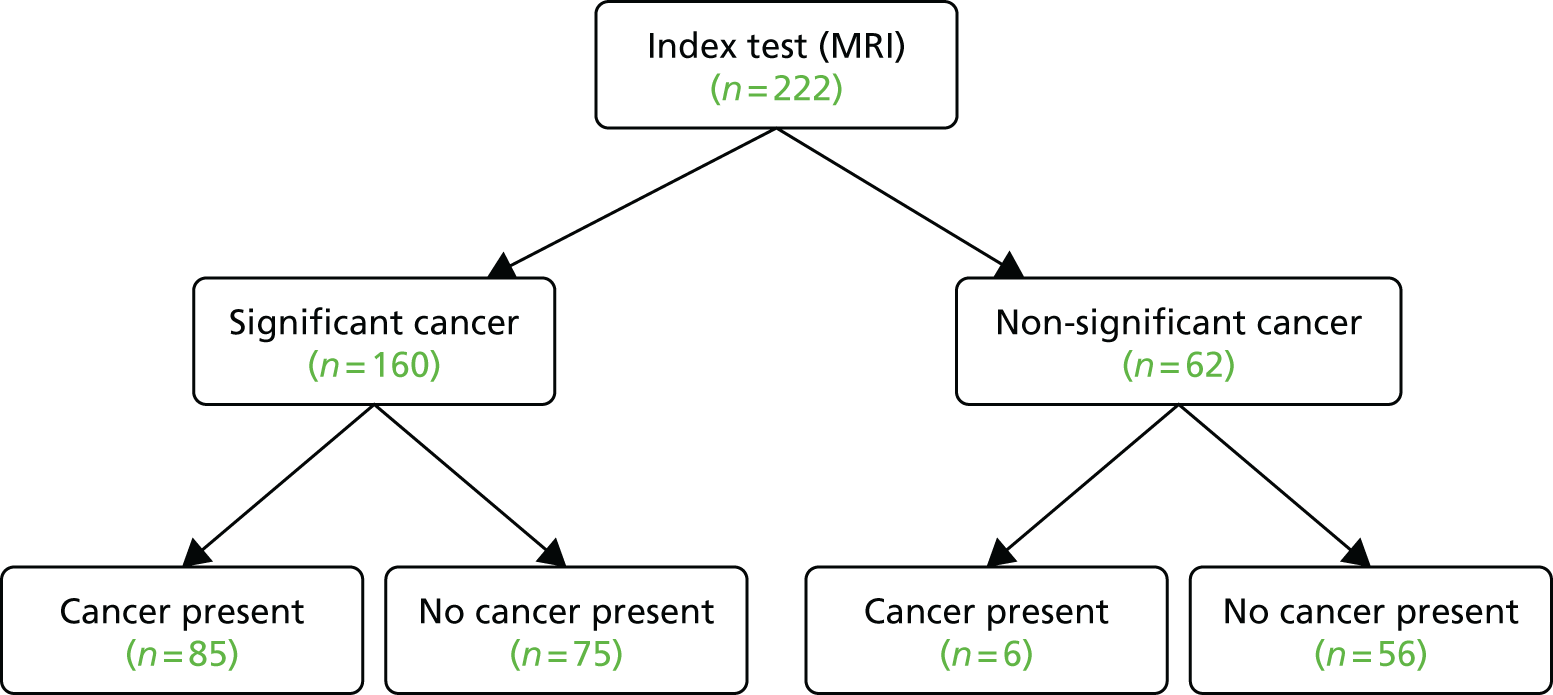
FIGURE 46.
Results for detection of CS cancer for mpMRI (definition 2, cut-off point 3) relative to TPM-biopsy (definition 1) for non-UCLH sites.

Appendix 2 Analysis of individual patient data on the diagnostic performance of multiparametric magnetic resonance imaging and transrectal ultrasound-guided biopsy
This analysis was carried out for the purposes of the economic evaluation. Figure 47 depicts the analysis of the individual patient data collected in PROMIS to obtain simulations of the diagnostic performance of mpMRI and TRUS-guided biopsy.
FIGURE 47.
Analysis of individual patient data.
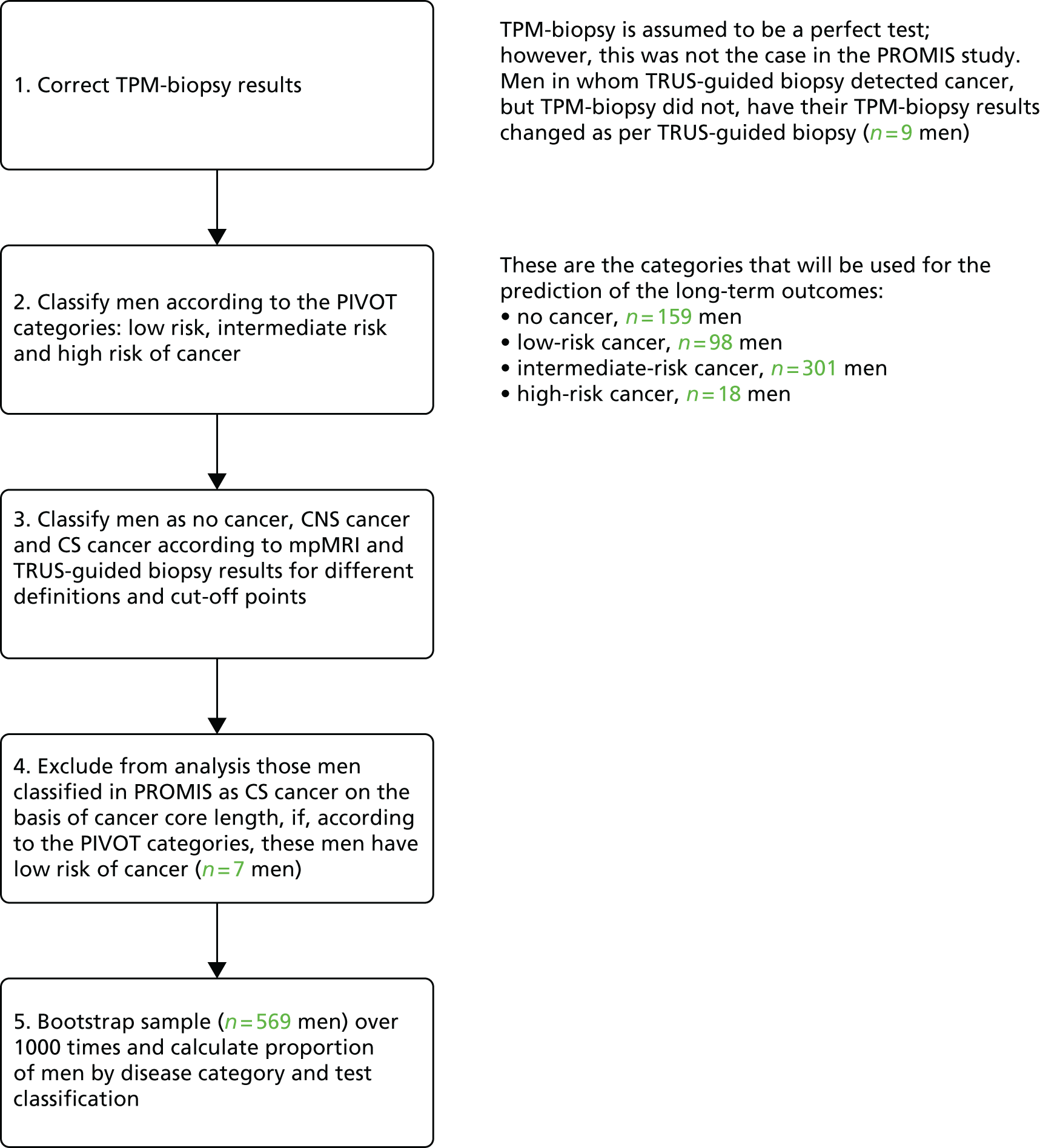
Appendix 3 Full results of the economic analyses
Tables 49–56 show the full results of the economic analyses.
| Strategy | Sensitivity by substrategy, average (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 0.44 (0.36 to 0.51) | 0.36 (0.30 to 0.42) | 0.24 (0.20 to 0.29) | 0.15 (0.12 to 0.19) | 0.46 (0.38 to 0.53) | 0.41 (0.35 to 0.48) | 0.32 (0.27 to 0.38) | 0.21 (0.17 to 0.25) |
| M2 | 0.46 (0.39 to 0.54) | 0.43 (0.36 to 0.50) | 0.34 (0.29 to 0.40) | 0.22 (0.18 to 0.27) | 0.46 (0.39 to 0.54) | 0.43 (0.36 to 0.50) | 0.34 (0.29 to 0.40) | 0.22 (0.18 to 0.27) |
| M3 | 0.72 (0.66 to 0.78) | 0.58 (0.52 to 0.64) | 0.39 (0.34 to 0.44) | 0.22 (0.18 to 0.27) | 0.76 (0.70 to 0.81) | 0.68 (0.62 to 0.73) | 0.52 (0.47 to 0.58) | 0.33 (0.28 to 0.38) |
| M4 | 0.75 (0.68 to 0.80) | 0.66 (0.59 to 0.71) | 0.48 (0.43 to 0.54) | 0.29 (0.25 to 0.34) | 0.76 (0.70 to 0.82) | 0.70 (0.64 to 0.75) | 0.54 (0.48 to 0.60) | 0.34 (0.29 to 0.39) |
| M5 | 0.59 (0.52 to 0.65) | 0.48 (0.42 to 0.54) | 0.32 (0.27 to 0.37) | 0.19 (0.15 to 0.23) | 0.61 (0.55 to 0.68) | 0.55 (0.49 to 0.61) | 0.43 (0.37 to 0.48) | 0.27 (0.23 to 0.31) |
| M6 | 0.62 (0.55 to 0.68) | 0.58 (0.52 to 0.64) | 0.45 (0.40 to 0.51) | 0.29 (0.24 to 0.33) | 0.62 (0.55 to 0.68) | 0.58 (0.52 to 0.64) | 0.45 (0.40 to 0.51) | 0.29 (0.24 to 0.33) |
| M7 | 0.87 (0.81 to 0.93) | 0.70 (0.64 to 0.76) | 0.46 (0.40 to 0.52) | 0.26 (0.21 to 0.31) | 0.92 (0.85 to 0.97) | 0.82 (0.75 to 0.87) | 0.63 (0.57 to 0.69) | 0.39 (0.33 to 0.44) |
| N1 | 0.94 (0.91 to 0.96) | 0.75 (0.70 to 0.80) | 0.49 (0.44 to 0.55) | 0.28 (0.23 to 0.33) | 0.98 (0.97 to 1.00) | 0.88 (0.84 to 0.91) | 0.67 (0.62 to 0.73) | 0.41 (0.36 to 0.47) |
| N2 | 0.99 (0.98 to 1.00) | 0.92 (0.89 to 0.95) | 0.71 (0.66 to 0.77) | 0.44 (0.38 to 0.49) | 0.99 (0.98 to 1.00) | 0.92 (0.89 to 0.95) | 0.71 (0.66 to 0.77) | 0.44 (0.38 to 0.49) |
| N3 | 0.77 (0.72 to 0.81) | 0.62 (0.56 to 0.67) | 0.41 (0.36 to 0.46) | 0.23 (0.19 to 0.28) | 0.80 (0.76 to 0.85) | 0.72 (0.67 to 0.76) | 0.55 (0.50 to 0.61) | 0.34 (0.29 to 0.39) |
| N4 | 0.79 (0.74 to 0.83) | 0.69 (0.64 to 0.74) | 0.50 (0.45 to 0.56) | 0.30 (0.26 to 0.35) | 0.81 (0.76 to 0.85) | 0.74 (0.69 to 0.78) | 0.57 (0.52 to 0.63) | 0.35 (0.30 to 0.41) |
| N5 | 0.61 (0.55 to 0.67) | 0.49 (0.44 to 0.55) | 0.33 (0.29 to 0.38) | 0.19 (0.16 to 0.23) | 0.64 (0.57 to 0.70) | 0.57 (0.51 to 0.63) | 0.44 (0.39 to 0.50) | 0.28 (0.24 to 0.32) |
| N6 | 0.64 (0.58 to 0.70) | 0.60 (0.54 to 0.66) | 0.47 (0.42 to 0.53) | 0.29 (0.25 to 0.34) | 0.64 (0.58 to 0.70) | 0.60 (0.54 to 0.66) | 0.47 (0.42 to 0.53) | 0.29 (0.25 to 0.34) |
| N7 | 0.94 (0.91 to 0.96) | 0.75 (0.70 to 0.80) | 0.49 (0.44 to 0.55) | 0.28 (0.23 to 0.33) | 0.98 (0.97 to 1.00) | 0.88 (0.84 to 0.91) | 0.67 (0.62 to 0.73) | 0.41 (0.36 to 0.47) |
| T1 | 0.38 (0.33 to 0.43) | a | a | a | a | a | a | a |
| T2 | 0.42 (0.37 to 0.48) | a | a | a | a | a | a | a |
| T3 | 0.48 (0.41 to 0.56) | a | a | a | a | a | a | a |
| T4 | 0.52 (0.45 to 0.61) | a | a | a | a | a | a | a |
| T5 | 0.56 (0.51 to 0.62) | 0.52 (0.47 to 0.58) | 0.47 (0.42 to 0.52) | 0.42 (0.38 to 0.48) | 0.57 (0.52 to 0.63) | 0.55 (0.50 to 0.61) | 0.51 (0.46 to 0.56) | 0.45 (0.40 to 0.50) |
| T6 | 0.70 (0.64 to 0.75) | 0.63 (0.58 to 0.69) | 0.54 (0.49 to 0.59) | 0.46 (0.41 to 0.51) | 0.72 (0.66 to 0.77) | 0.68 (0.62 to 0.73) | 0.60 (0.55 to 0.66) | 0.51 (0.46 to 0.56) |
| T7 | 0.89 (0.81 to 0.94) | 0.78 (0.71 to 0.84) | 0.63 (0.57 to 0.68) | 0.51 (0.45 to 0.56) | 0.91 (0.84 to 0.97) | 0.85 (0.78 to 0.91) | 0.73 (0.67 to 0.79) | 0.58 (0.53 to 0.64) |
| T8 | 0.57 (0.52 to 0.63) | 0.56 (0.51 to 0.62) | 0.52 (0.46 to 0.57) | 0.46 (0.41 to 0.51) | 0.57 (0.52 to 0.63) | 0.56 (0.51 to 0.62) | 0.52 (0.46 to 0.57) | 0.46 (0.41 to 0.51) |
| T9 | 0.90 (0.82 to 0.95) | 0.81 (0.75 to 0.87) | 0.68 (0.62 to 0.73) | 0.54 (0.49 to 0.60) | 0.91 (0.84 to 0.97) | 0.86 (0.79 to 0.91) | 0.74 (0.68 to 0.80) | 0.59 (0.53 to 0.64) |
| P1 | 1.00 (1.00 to 1.00) | a | a | a | a | a | a | a |
| P2 | 0.60 (0.55 to 0.66) | a | a | a | a | a | a | a |
| P3 | 0.77 (0.73 to 0.81) | a | a | a | a | a | a | a |
| P4 | 1.00 (1.00 to 1.00) | a | a | a | a | a | a | a |
| P5 | 0.59 (0.54 to 0.64) | 0.54 (0.49 to 0.60) | 0.48 (0.43 to 0.53) | 0.43 (0.38 to 0.48) | 0.60 (0.55 to 0.65) | 0.57 (0.53 to 0.63) | 0.53 (0.48 to 0.58) | 0.46 (0.42 to 0.51) |
| P6 | 0.75 (0.70 to 0.79) | 0.67 (0.62 to 0.72) | 0.56 (0.51 to 0.61) | 0.47 (0.42 to 0.52) | 0.77 (0.72 to 0.81) | 0.72 (0.68 to 0.77) | 0.64 (0.59 to 0.68) | 0.53 (0.48 to 0.58) |
| P7 | 0.96 (0.94 to 0.98) | 0.84 (0.80 to 0.87) | 0.67 (0.62 to 0.71) | 0.52 (0.47 to 0.58) | 0.99 (0.98 to 1.00) | 0.92 (0.90 to 0.94) | 0.79 (0.74 to 0.82) | 0.61 (0.56 to 0.66) |
| P8 | 0.60 (0.55 to 0.66) | 0.59 (0.54 to 0.64) | 0.54 (0.49 to 0.59) | 0.47 (0.42 to 0.52) | 0.60 (0.55 to 0.66) | 0.59 (0.54 to 0.64) | 0.54 (0.49 to 0.59) | 0.47 (0.42 to 0.52) |
| P9 | 0.97 (0.96 to 0.98) | 0.88 (0.85 to 0.90) | 0.72 (0.67 to 0.76) | 0.56 (0.51 to 0.61) | 0.99 (0.98 to 1.00) | 0.93 (0.91 to 0.95) | 0.80 (0.75 to 0.83) | 0.62 (0.57 to 0.67) |
| Strategy | Sensitivity by substrategy, average (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 0.71 (0.62 to 0.81) | 0.57 (0.50 to 0.66) | 0.38 (0.32 to 0.45) | 0.22 (0.17 to 0.27) | 0.74 (0.66 to 0.84) | 0.66 (0.58 to 0.76) | 0.51 (0.44 to 0.59) | 0.32 (0.27 to 0.38) |
| M2 | 0.75 (0.66 to 0.85) | 0.70 (0.61 to 0.79) | 0.54 (0.47 to 0.63) | 0.34 (0.28 to 0.40) | 0.75 (0.66 to 0.85) | 0.70 (0.61 to 0.79) | 0.54 (0.47 to 0.63) | 0.34 (0.28 to 0.40) |
| M3 | 0.79 (0.73 to 0.86) | 0.64 (0.58 to 0.71) | 0.42 (0.37 to 0.48) | 0.24 (0.20 to 0.29) | 0.83 (0.77 to 0.89) | 0.74 (0.68 to 0.81) | 0.57 (0.51 to 0.64) | 0.35 (0.30 to 0.41) |
| M4 | 0.84 (0.77 to 0.90) | 0.76 (0.70 to 0.83) | 0.59 (0.52 to 0.66) | 0.36 (0.31 to 0.42) | 0.84 (0.78 to 0.90) | 0.78 (0.71 to 0.84) | 0.60 (0.54 to 0.67) | 0.37 (0.32 to 0.43) |
| M5 | 0.82 (0.77 to 0.88) | 0.66 (0.60 to 0.73) | 0.44 (0.38 to 0.49) | 0.25 (0.20 to 0.29) | 0.86 (0.81 to 0.92) | 0.77 (0.72 to 0.83) | 0.59 (0.53 to 0.65) | 0.37 (0.32 to 0.42) |
| M6 | 0.87 (0.82 to 0.93) | 0.81 (0.76 to 0.86) | 0.63 (0.57 to 0.69) | 0.39 (0.33 to 0.45) | 0.87 (0.82 to 0.93) | 0.81 (0.76 to 0.86) | 0.63 (0.57 to 0.69) | 0.39 (0.33 to 0.45) |
| M7 | 0.91 (0.87 to 0.95) | 0.73 (0.68 to 0.78) | 0.48 (0.43 to 0.54) | 0.27 (0.22 to 0.32) | 0.95 (0.92 to 0.98) | 0.85 (0.81 to 0.89) | 0.65 (0.60 to 0.71) | 0.40 (0.35 to 0.46) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 0.81 (0.75 to 0.87) | 0.65 (0.59 to 0.71) | 0.43 (0.37 to 0.49) | 0.24 (0.20 to 0.29) | 0.85 (0.79 to 0.90) | 0.76 (0.70 to 0.81) | 0.58 (0.52 to 0.64) | 0.36 (0.31 to 0.42) |
| N4 | 0.85 (0.79 to 0.91) | 0.77 (0.71 to 0.84) | 0.59 (0.52 to 0.66) | 0.36 (0.31 to 0.42) | 0.85 (0.80 to 0.91) | 0.79 (0.73 to 0.85) | 0.61 (0.55 to 0.68) | 0.38 (0.32 to 0.44) |
| N5 | 0.84 (0.79 to 0.89) | 0.68 (0.62 to 0.74) | 0.44 (0.39 to 0.50) | 0.25 (0.20 to 0.30) | 0.88 (0.84 to 0.93) | 0.79 (0.74 to 0.84) | 0.61 (0.55 to 0.66) | 0.37 (0.32 to 0.43) |
| N6 | 0.89 (0.84 to 0.94) | 0.83 (0.78 to 0.87) | 0.64 (0.58 to 0.70) | 0.39 (0.34 to 0.45) | 0.89 (0.84 to 0.94) | 0.83 (0.78 to 0.87) | 0.64 (0.58 to 0.70) | 0.39 (0.34 to 0.45) |
| N7 | 0.94 (0.91 to 0.96) | 0.75 (0.70 to 0.80) | 0.49 (0.44 to 0.55) | 0.28 (0.23 to 0.33) | 0.98 (0.97 to 1.00) | 0.88 (0.84 to 0.91) | 0.67 (0.62 to 0.73) | 0.41 (0.36 to 0.47) |
| T1 | 0.61 (0.56 to 0.66) | b | b | b | b | b | b | b |
| T2 | 0.69 (0.64 to 0.74) | b | b | b | b | b | b | b |
| T3 | 0.65 (0.60 to 0.70) | b | b | b | b | b | b | b |
| T4 | 0.73 (0.69 to 0.78) | b | b | b | b | b | b | b |
| T5 | 0.80 (0.75 to 0.84) | 0.76 (0.71 to 0.81) | 0.71 (0.66 to 0.75) | 0.66 (0.61 to 0.71) | 0.81 (0.76 to 0.85) | 0.79 (0.74 to 0.83) | 0.74 (0.70 to 0.79) | 0.69 (0.64 to 0.74) |
| T6 | 0.74 (0.70 to 0.79) | 0.72 (0.67 to 0.76) | 0.68 (0.63 to 0.72) | 0.65 (0.60 to 0.69) | 0.75 (0.71 to 0.79) | 0.74 (0.69 to 0.78) | 0.71 (0.66 to 0.75) | 0.67 (0.62 to 0.71) |
| T7 | 0.93 (0.88 to 0.96) | 0.86 (0.82 to 0.90) | 0.77 (0.72 to 0.81) | 0.69 (0.65 to 0.74) | 0.95 (0.90 to 0.98) | 0.91 (0.86 to 0.94) | 0.83 (0.79 to 0.87) | 0.74 (0.69 to 0.78) |
| T8 | 0.81 (0.76 to 0.86) | 0.79 (0.75 to 0.84) | 0.75 (0.70 to 0.80) | 0.69 (0.64 to 0.74) | 0.81 (0.76 to 0.86) | 0.79 (0.75 to 0.84) | 0.75 (0.70 to 0.80) | 0.69 (0.64 to 0.74) |
| T9 | 0.94 (0.89 to 0.97) | 0.90 (0.85 to 0.93) | 0.82 (0.77 to 0.86) | 0.73 (0.68 to 0.77) | 0.95 (0.90 to 0.98) | 0.92 (0.87 to 0.95) | 0.84 (0.80 to 0.88) | 0.75 (0.70 to 0.79) |
| P1 | a | b | b | b | b | b | b | b |
| P2 | 0.84 (0.80 to 0.88) | b | b | b | b | b | b | b |
| P3 | 0.77 (0.73 to 0.81) | b | b | b | b | b | b | b |
| P4 | 1.00 (1.00 to 1.00) | b | b | b | b | b | b | b |
| P5 | 0.83 (0.78 to 0.87) | 0.78 (0.74 to 0.82) | 0.72 (0.67 to 0.76) | 0.67 (0.62 to 0.71) | 0.84 (0.79 to 0.88) | 0.81 (0.77 to 0.85) | 0.76 (0.72 to 0.80) | 0.70 (0.65 to 0.75) |
| P6 | 0.76 (0.72 to 0.80) | 0.73 (0.69 to 0.77) | 0.69 (0.64 to 0.73) | 0.65 (0.60 to 0.70) | 0.77 (0.73 to 0.81) | 0.75 (0.71 to 0.79) | 0.72 (0.68 to 0.76) | 0.67 (0.63 to 0.72) |
| P7 | 0.97 (0.96 to 0.99) | 0.90 (0.87 to 0.92) | 0.79 (0.75 to 0.83) | 0.70 (0.65 to 0.75) | 0.99 (0.99 to 1.00) | 0.95 (0.93 to 0.97) | 0.87 (0.83 to 0.90) | 0.76 (0.72 to 0.80) |
| P8 | 0.84 (0.80 to 0.88) | 0.82 (0.78 to 0.86) | 0.77 (0.73 to 0.81) | 0.71 (0.66 to 0.75) | 0.84 (0.80 to 0.88) | 0.82 (0.78 to 0.86) | 0.77 (0.73 to 0.81) | 0.71 (0.66 to 0.75) |
| P9 | 0.99 (0.98 to 0.99) | 0.94 (0.92 to 0.96) | 0.85 (0.81 to 0.88) | 0.74 (0.70 to 0.78) | 1.00 (0.99 to 1.00) | 0.96 (0.95 to 0.97) | 0.88 (0.85 to 0.90) | 0.77 (0.72 to 0.81) |
| Strategy | Cost of testing by substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 544 (531 to 556) | 409 (392 to 427) | 310 (294 to 325) | 244 (232 to 257) | 581 (573 to 588) | 482 (467 to 499) | 366 (349 to 383) | 280 (265 to 295) |
| M2 | 596 (592 to 600) | 522 (509 to 536) | 381 (364 to 399) | 287 (272 to 301) | 596 (592 to 600) | 522 (509 to 536) | 381 (364 to 399) | 287 (272 to 301) |
| M3 | 631 (611 to 654) | 474 (451 to 499) | 349 (327 to 371) | 264 (248 to 280) | 674 (656 to 693) | 562 (539 to 587) | 423 (398 to 447) | 311 (291 to 332) |
| M4 | 684 (667 to 701) | 587 (568 to 607) | 421 (399 to 442) | 306 (287 to 324) | 689 (672 to 707) | 602 (581 to 624) | 438 (415 to 461) | 318 (298 to 338) |
| M5 | 716 (692 to 739) | 488 (464 to 514) | 342 (322 to 363) | 257 (242 to 273) | 780 (759 to 800) | 608 (583 to 635) | 418 (397 to 442) | 301 (284 to 320) |
| M6 | 807 (790 to 826) | 678 (653 to 702) | 441 (418 to 465) | 310 (292 to 328) | 807 (790 to 826) | 678 (653 to 702) | 441 (418 to 465) | 310 (292 to 328) |
| M7 | 804 (776 to 831) | 553 (524 to 583) | 382 (354 to 408) | 277 (258 to 296) | 873 (850 to 896) | 687 (657 to 719) | 475 (447 to 504) | 333 (310 to 357) |
| N1 | 1399 (1357 to 1438) | 946 (890 to 1004) | 613 (561 to 664) | 392 (349 to 435) | 1524 (1500 to 1547) | 1193 (1142 to 1245) | 801 (745 to 857) | 512 (464 to 560) |
| N2 | 1575 (1565 to 1585) | 1328 (1284 to 1371) | 853 (797 to 912) | 534 (485 to 581) | 1575 (1565 to 1585) | 1328 (1284 to 1371) | 853 (797 to 912) | 534 (485 to 581) |
| N3 | 839 (784 to 897) | 628 (581 to 677) | 443 (404 to 483) | 309 (284 to 339) | 895 (836 to 953) | 749 (696 to 805) | 557 (508 to 605) | 386 (350 to 422) |
| N4 | 891 (838 to 946) | 741 (698 to 788) | 514 (476 to 554) | 352 (323 to 380) | 911 (853 to 967) | 789 (737 to 844) | 572 (525 to 621) | 392 (356 to 428) |
| N5 | 1124 (1065 to 1186) | 676 (625 to 728) | 419 (386 to 457) | 288 (266 to 313) | 1252 (1192 to 1311) | 906 (848 to 967) | 542 (500 to 588) | 351 (324 to 381) |
| N6 | 1308 (1249 to 1366) | 1046 (987 to 1107) | 582 (536 to 629) | 364 (336 to 393) | 1308 (1249 to 1366) | 1046 (987 to 1107) | 582 (536 to 629) | 364 (336 to 393) |
| N7 | 1420 (1348 to 1487) | 894 (831 to 961) | 552 (497 to 609) | 353 (318 to 392) | 1566 (1495 to 1629) | 1173 (1098 to 1245) | 733 (672 to 795) | 457 (413 to 505) |
| T1 | 415 (412 to 420) | a | a | a | a | a | a | a |
| T2 | 627 (610 to 644) | a | a | a | a | a | a | a |
| T3 | 531 (516 to 547) | a | a | a | a | a | a | a |
| T4 | 742 (727 to 757) | a | a | a | a | a | a | a |
| T5 | 681 (658 to 705) | 591 (573 to 608) | 544 (531 to 556) | 523 (513 to 533) | 707 (683 to 730) | 636 (616 to 657) | 564 (550 to 578) | 532 (522 to 543) |
| T6 | 571 (550 to 592) | 541 (524 to 558) | 511 (497 to 525) | 488 (477 to 500) | 578 (557 to 599) | 559 (541 to 579) | 531 (515 to 547) | 501 (488 to 515) |
| T7 | 837 (816 to 858) | 717 (700 to 736) | 639 (625 to 655) | 595 (585 to 607) | 870 (849 to 890) | 780 (760 to 801) | 680 (663 to 697) | 618 (606 to 633) |
| T8 | 719 (695 to 743) | 665 (642 to 687) | 571 (557 to 586) | 534 (523 to 545) | 719 (695 to 743) | 665 (642 to 687) | 571 (557 to 586) | 534 (523 to 545) |
| T9 | 875 (855 to 894) | 791 (772 to 810) | 667 (652 to 683) | 607 (596 to 619) | 882 (861 to 901) | 809 (790 to 829) | 687 (671 to 705) | 620 (608 to 635) |
| P1 | 1398 (1392 to 1405) | a | a | a | a | a | a | a |
| P2 | 1127 (1070 to 1179) | a | a | a | a | a | a | a |
| P3 | 805 (754 to 854) | a | a | a | a | a | a | a |
| P4 | 1517 (1472 to 1561) | a | a | a | a | a | a | a |
| P5 | 1091 (1031 to 1150) | 787 (741 to 833) | 628 (601 to 655) | 558 (542 to 577) | 1179 (1117 to 1240) | 940 (890 to 995) | 698 (664 to 733) | 590 (569 to 614) |
| P6 | 819 (767 to 871) | 720 (677 to 762) | 618 (585 to 653) | 539 (518 to 563) | 844 (789 to 899) | 781 (732 to 829) | 686 (647 to 727) | 585 (556 to 614) |
| P7 | 1496 (1440 to 1550) | 1092 (1044 to 1143) | 831 (789 to 872) | 682 (655 to 713) | 1608 (1554 to 1658) | 1305 (1248 to 1363) | 968 (922 to 1017) | 760 (726 to 797) |
| P8 | 1219 (1154 to 1278) | 1037 (980 to 1093) | 722 (686 to 759) | 597 (575 to 622) | 1219 (1154 to 1278) | 1037 (980 to 1093) | 722 (686 to 759) | 597 (575 to 622) |
| P9 | 1638 (1589 to 1686) | 1373 (1319 to 1424) | 936 (893 to 982) | 722 (694 to 754) | 1654 (1604 to 1702) | 1414 (1361 to 1466) | 996 (949 to 1045) | 767 (733 to 803) |
| Strategy | Cost of testing by substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 544 (531 to 556) | 409 (392 to 427) | 310 (294 to 325) | 244 (232 to 257) | 581 (573 to 588) | 482 (467 to 499) | 366 (349 to 383) | 280 (265 to 295) |
| M2 | 596 (592 to 600) | 522 (509 to 536) | 381 (364 to 399) | 287 (272 to 301) | 596 (592 to 600) | 522 (509 to 536) | 381 (364 to 399) | 287 (272 to 301) |
| M3 | 577 (560 to 595) | 431 (412 to 451) | 323 (305 to 341) | 250 (237 to 264) | 617 (603 to 633) | 511 (492 to 532) | 385 (366 to 404) | 290 (273 to 306) |
| M4 | 630 (618 to 643) | 545 (528 to 561) | 394 (377 to 413) | 292 (276 to 308) | 632 (620 to 646) | 551 (534 to 570) | 400 (382 to 420) | 296 (280 to 312) |
| M5 | 707 (682 to 731) | 481 (456 to 507) | 338 (317 to 358) | 255 (240 to 271) | 770 (746 to 791) | 599 (572 to 625) | 412 (390 to 435) | 298 (279 to 316) |
| M6 | 797 (777 to 818) | 669 (642 to 694) | 434 (411 to 458) | 306 (287 to 323) | 797 (777 to 818) | 669 (642 to 694) | 434 (411 to 458) | 306 (287 to 323) |
| M7 | 740 (710 to 769) | 503 (475 to 532) | 350 (328 to 374) | 261 (244 to 278) | 807 (777 to 833) | 628 (597 to 660) | 430 (404 to 458) | 307 (287 to 328) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 657 (620 to 703) | 484 (450 to 522) | 353 (328 to 380) | 264 (246 to 283) | 704 (663 to 750) | 580 (543 to 625) | 429 (399 to 463) | 312 (289 to 336) |
| N4 | 709 (672 to 753) | 598 (565 to 633) | 424 (400 to 452) | 306 (286 to 326) | 719 (678 to 766) | 620 (583 to 663) | 445 (414 to 478) | 319 (296 to 342) |
| N5 | 1092 (1023 to 1157) | 651 (597 to 706) | 403 (369 to 442) | 280 (258 to 306) | 1218 (1147 to 1284) | 876 (812 to 941) | 520 (474 to 568) | 339 (310 to 370) |
| N6 | 1275 (1210 to 1339) | 1015 (950 to 1080) | 558 (509 to 608) | 350 (320 to 383) | 1275 (1210 to 1339) | 1015 (950 to 1080) | 558 (509 to 608) | 350 (320 to 383) |
| N7 | 1206 (1118 to 1286) | 726 (653 to 796) | 446 (398 to 500) | 300 (272 to 333) | 1341 (1251 to 1423) | 973 (891 to 1051) | 583 (522 to 645) | 371 (333 to 411) |
| T1 | 415 (412 to 420) | b | b | b | b | b | b | b |
| T2 | 627 (610 to 644) | b | b | b | b | b | b | b |
| T3 | 476 (463 to 489) | b | b | b | b | b | b | b |
| T4 | 687 (671 to 705) | b | b | b | b | b | b | b |
| T5 | 681 (658 to 705) | 591 (573 to 608) | 544 (531 to 556) | 523 (513 to 533) | 707 (683 to 730) | 636 (616 to 657) | 564 (550 to 578) | 532 (522 to 543) |
| T6 | 496 (480 to 511) | 477 (464 to 490) | 462 (452 to 472) | 451 (443 to 459) | 500 (484 to 517) | 488 (473 to 503) | 471 (460 to 484) | 457 (448 to 466) |
| T7 | 762 (738 to 785) | 653 (635 to 671) | 590 (577 to 604) | 559 (549 to 569) | 792 (769 to 816) | 709 (688 to 730) | 620 (605 to 637) | 574 (562 to 586) |
| T8 | 719 (695 to 743) | 665 (642 to 687) | 571 (557 to 586) | 534 (523 to 545) | 719 (695 to 743) | 665 (642 to 687) | 571 (557 to 586) | 534 (523 to 545) |
| T9 | 799 (776 to 822) | 727 (705 to 747) | 618 (603 to 634) | 570 (559 to 582) | 804 (781 to 828) | 737 (715 to 759) | 627 (611 to 644) | 576 (564 to 588) |
| P1 | a,b | b | b | b | b | b | b | b |
| P2 | 1127 (1070 to 1179) | b | b | b | b | b | b | b |
| P3 | 620 (580 to 660) | b | b | b | b | b | b | b |
| P4 | 1332 (1278 to 1385) | b | b | b | b | b | b | b |
| P5 | 1091 (1031 to 1150) | 787 (741 to 833) | 628 (601 to 655) | 558 (542 to 577) | 1179 (1117 to 1240) | 940 (890 to 995) | 698 (664 to 733) | 590 (569 to 614) |
| P6 | 623 (582 to 663) | 560 (530 to 591) | 509 (489 to 531) | 472 (459 to 486) | 639 (595 to 683) | 596 (560 to 633) | 541 (515 to 569) | 491 (474 to 509) |
| P7 | 1299 (1238 to 1358) | 932 (884 to 979) | 721 (689 to 757) | 615 (595 to 637) | 1402 (1341 to 1462) | 1120 (1066 to 1181) | 823 (782 to 867) | 666 (639 to 695) |
| P8 | 1219 (1154 to 1278) | 1037 (980 to 1093) | 722 (686 to 759) | 597 (575 to 622) | 1219 (1154 to 1278) | 1037 (980 to 1093) | 722 (686 to 759) | 597 (575 to 622) |
| P9 | 1442 (1383 to 1500) | 1213 (1157 to 1269) | 826 (786 to 869) | 655 (631 to 682) | 1448 (1387 to 1507) | 1229 (1171 to 1288) | 851 (808 to 896) | 673 (645 to 702) |
| Strategy | Overall QALYs by substrategy, average (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 8.44 (8.14 to 8.76) | 8.40 (8.09 to 8.73) | 8.34 (8.01 to 8.70) | 8.29 (7.94 to 8.68) | 8.45 (8.15 to 8.78) | 8.43 (8.13 to 8.75) | 8.38 (8.07 to 8.72) | 8.32 (7.99 to 8.69) |
| M2 | 8.46 (8.16 to 8.78) | 8.44 (8.14 to 8.76) | 8.39 (8.08 to 8.73) | 8.33 (7.99 to 8.69) | 8.46 (8.16 to 8.78) | 8.44 (8.14 to 8.76) | 8.39 (8.08 to 8.73) | 8.33 (7.99 to 8.69) |
| M3 | 8.60 (8.30 to 8.90) | 8.52 (8.23 to 8.82) | 8.42 (8.12 to 8.74) | 8.33 (8.00 to 8.69) | 8.61 (8.32 to 8.91) | 8.57 (8.28 to 8.87) | 8.49 (8.20 to 8.80) | 8.38 (8.07 to 8.72) |
| M4 | 8.61 (8.32 to 8.91) | 8.56 (8.27 to 8.86) | 8.47 (8.18 to 8.78) | 8.37 (8.06 to 8.71) | 8.62 (8.32 to 8.92) | 8.58 (8.29 to 8.88) | 8.50 (8.21 to 8.81) | 8.39 (8.09 to 8.73) |
| M5 | 8.52 (8.24 to 8.82) | 8.46 (8.17 to 8.77) | 8.38 (8.06 to 8.72) | 8.31 (7.97 to 8.68) | 8.54 (8.26 to 8.83) | 8.50 (8.22 to 8.81) | 8.44 (8.14 to 8.75) | 8.36 (8.03 to 8.71) |
| M6 | 8.54 (8.26 to 8.84) | 8.52 (8.23 to 8.82) | 8.45 (8.16 to 8.77) | 8.36 (8.05 to 8.71) | 8.54 (8.26 to 8.84) | 8.52 (8.23 to 8.82) | 8.45 (8.16 to 8.77) | 8.36 (8.05 to 8.71) |
| M7 | 8.68 (8.38 to 8.99) | 8.58 (8.30 to 8.89) | 8.46 (8.17 to 8.77) | 8.35 (8.03 to 8.70) | 8.70 (8.39 to 9.02) | 8.65 (8.35 to 8.95) | 8.55 (8.26 to 8.84) | 8.42 (8.12 to 8.74) |
| N1 | 8.70 (8.39 to 9.02) | 8.61 (8.31 to 8.90) | 8.47 (8.18 to 8.78) | 8.36 (8.04 to 8.70) | 8.73 (8.40 to 9.05) | 8.67 (8.37 to 8.98) | 8.57 (8.28 to 8.86) | 8.43 (8.13 to 8.75) |
| N2 | 8.73 (8.40 to 9.06) | 8.69 (8.38 to 9.01) | 8.59 (8.30 to 8.89) | 8.44 (8.14 to 8.76) | 8.73 (8.40 to 9.06) | 8.69 (8.38 to 9.01) | 8.59 (8.30 to 8.89) | 8.44 (8.14 to 8.76) |
| N3 | 8.62 (8.32 to 8.91) | 8.54 (8.25 to 8.84) | 8.43 (8.13 to 8.75) | 8.34 (8.00 to 8.70) | 8.64 (8.34 to 8.94) | 8.59 (8.30 to 8.88) | 8.50 (8.22 to 8.81) | 8.39 (8.09 to 8.73) |
| N4 | 8.63 (8.34 to 8.93) | 8.58 (8.28 to 8.87) | 8.48 (8.19 to 8.79) | 8.37 (8.06 to 8.71) | 8.64 (8.34 to 8.94) | 8.60 (8.31 to 8.89) | 8.51 (8.22 to 8.82) | 8.40 (8.09 to 8.73) |
| N5 | 8.53 (8.25 to 8.83) | 8.47 (8.18 to 8.78) | 8.39 (8.07 to 8.72) | 8.32 (7.97 to 8.69) | 8.55 (8.26 to 8.84) | 8.51 (8.23 to 8.81) | 8.45 (8.15 to 8.76) | 8.36 (8.04 to 8.71) |
| N6 | 8.55 (8.27 to 8.84) | 8.53 (8.24 to 8.82) | 8.46 (8.17 to 8.77) | 8.37 (8.05 to 8.71) | 8.55 (8.27 to 8.84) | 8.53 (8.24 to 8.82) | 8.46 (8.17 to 8.77) | 8.37 (8.05 to 8.71) |
| N7 | 8.70 (8.39 to 9.02) | 8.61 (8.31 to 8.90) | 8.47 (8.19 to 8.78) | 8.36 (8.04 to 8.70) | 8.73 (8.40 to 9.06) | 8.67 (8.37 to 8.98) | 8.57 (8.28 to 8.86) | 8.43 (8.13 to 8.75) |
| T1 | 8.41 (8.11 to 8.74) | a | a | a | a | a | a | a |
| T2 | 8.44 (8.14 to 8.75) | a | a | a | a | a | a | a |
| T3 | 8.47 (8.16 to 8.78) | a | a | a | a | a | a | a |
| T4 | 8.49 (8.19 to 8.80) | a | a | a | a | a | a | a |
| T5 | 8.51 (8.22 to 8.81) | 8.49 (8.20 to 8.80) | 8.46 (8.17 to 8.78) | 8.44 (8.14 to 8.75) | 8.52 (8.23 to 8.82) | 8.50 (8.22 to 8.81) | 8.48 (8.19 to 8.79) | 8.45 (8.15 to 8.77) |
| T6 | 8.58 (8.29 to 8.88) | 8.55 (8.26 to 8.84) | 8.50 (8.21 to 8.81) | 8.46 (8.16 to 8.77) | 8.59 (8.30 to 8.89) | 8.57 (8.28 to 8.87) | 8.53 (8.25 to 8.83) | 8.48 (8.19 to 8.80) |
| T7 | 8.68 (8.38 to 9.00) | 8.62 (8.33 to 8.93) | 8.55 (8.26 to 8.84) | 8.48 (8.19 to 8.79) | 8.69 (8.38 to 9.02) | 8.66 (8.36 to 8.97) | 8.60 (8.31 to 8.91) | 8.52 (8.24 to 8.82) |
| T8 | 8.52 (8.23 to 8.82) | 8.51 (8.22 to 8.81) | 8.49 (8.19 to 8.80) | 8.45 (8.16 to 8.77) | 8.52 (8.23 to 8.82) | 8.51 (8.22 to 8.81) | 8.49 (8.19 to 8.80) | 8.45 (8.16 to 8.77) |
| T9 | 8.69 (8.38 to 9.01) | 8.64 (8.35 to 8.95) | 8.57 (8.29 to 8.87) | 8.50 (8.21 to 8.81) | 8.70 (8.39 to 9.02) | 8.67 (8.37 to 8.98) | 8.61 (8.31 to 8.91) | 8.52 (8.24 to 8.82) |
| P1 | 8.73 (8.40 to 9.06) | a | a | a | a | a | a | a |
| P2 | 8.53 (8.25 to 8.83) | a | a | a | a | a | a | a |
| P3 | 8.62 (8.33 to 8.92) | a | a | a | a | a | a | a |
| P4 | 8.74 (8.41 to 9.06) | a | a | a | a | a | a | a |
| P5 | 8.52 (8.24 to 8.82) | 8.50 (8.21 to 8.80) | 8.47 (8.17 to 8.78) | 8.44 (8.14 to 8.75) | 8.53 (8.25 to 8.83) | 8.51 (8.23 to 8.81) | 8.49 (8.20 to 8.80) | 8.46 (8.16 to 8.77) |
| P6 | 8.61 (8.31 to 8.90) | 8.57 (8.27 to 8.86) | 8.51 (8.23 to 8.81) | 8.46 (8.16 to 8.77) | 8.62 (8.33 to 8.91) | 8.59 (8.30 to 8.89) | 8.55 (8.26 to 8.84) | 8.49 (8.21 to 8.80) |
| P7 | 8.72 (8.40 to 9.04) | 8.65 (8.36 to 8.95) | 8.56 (8.29 to 8.86) | 8.49 (8.20 to 8.80) | 8.73 (8.40 to 9.06) | 8.70 (8.38 to 9.00) | 8.63 (8.33 to 8.93) | 8.54 (8.25 to 8.83) |
| P8 | 8.53 (8.25 to 8.83) | 8.52 (8.24 to 8.82) | 8.50 (8.21 to 8.80) | 8.46 (8.17 to 8.77) | 8.53 (8.25 to 8.83) | 8.52 (8.24 to 8.82) | 8.50 (8.21 to 8.80) | 8.46 (8.17 to 8.77) |
| P9 | 8.72 (8.40 to 9.05) | 8.67 (8.38 to 8.98) | 8.59 (8.30 to 8.89) | 8.51 (8.22 to 8.81) | 8.73 (8.40 to 9.06) | 8.70 (8.39 to 9.01) | 8.63 (8.34 to 8.94) | 8.54 (8.26 to 8.83) |
| Strategy | Overall QALYs by substrategy, average (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 8.59 (8.29 to 8.89) | 8.52 (8.22 to 8.82) | 8.41 (8.11 to 8.74) | 8.33 (7.99 to 8.69) | 8.61 (8.31 to 8.90) | 8.56 (8.27 to 8.86) | 8.48 (8.19 to 8.79) | 8.38 (8.06 to 8.72) |
| M2 | 8.61 (8.31 to 8.91) | 8.58 (8.29 to 8.88) | 8.50 (8.21 to 8.80) | 8.39 (8.08 to 8.73) | 8.61 (8.31 to 8.91) | 8.58 (8.29 to 8.88) | 8.50 (8.21 to 8.80) | 8.39 (8.08 to 8.73) |
| M3 | 8.63 (8.33 to 8.93) | 8.55 (8.26 to 8.85) | 8.44 (8.14 to 8.76) | 8.34 (8.01 to 8.70) | 8.65 (8.35 to 8.95) | 8.61 (8.31 to 8.90) | 8.52 (8.23 to 8.82) | 8.40 (8.09 to 8.73) |
| M4 | 8.65 (8.35 to 8.96) | 8.62 (8.32 to 8.92) | 8.52 (8.24 to 8.82) | 8.40 (8.10 to 8.73) | 8.66 (8.35 to 8.96) | 8.62 (8.33 to 8.92) | 8.53 (8.24 to 8.83) | 8.41 (8.11 to 8.74) |
| M5 | 8.65 (8.35 to 8.95) | 8.56 (8.27 to 8.86) | 8.44 (8.15 to 8.76) | 8.34 (8.01 to 8.70) | 8.67 (8.37 to 8.98) | 8.62 (8.33 to 8.92) | 8.53 (8.24 to 8.82) | 8.41 (8.11 to 8.73) |
| M6 | 8.67 (8.38 to 8.98) | 8.64 (8.35 to 8.94) | 8.55 (8.26 to 8.84) | 8.42 (8.12 to 8.74) | 8.67 (8.38 to 8.98) | 8.64 (8.35 to 8.94) | 8.55 (8.26 to 8.84) | 8.42 (8.12 to 8.74) |
| M7 | 8.69 (8.38 to 9.01) | 8.60 (8.31 to 8.90) | 8.47 (8.18 to 8.77) | 8.35 (8.03 to 8.70) | 8.72 (8.40 to 9.04) | 8.66 (8.37 to 8.97) | 8.56 (8.27 to 8.86) | 8.42 (8.13 to 8.74) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 8.64 (8.34 to 8.93) | 8.56 (8.26 to 8.85) | 8.44 (8.14 to 8.76) | 8.34 (8.01 to 8.70) | 8.66 (8.36 to 8.96) | 8.61 (8.32 to 8.91) | 8.52 (8.23 to 8.82) | 8.40 (8.10 to 8.73) |
| N4 | 8.66 (8.36 to 8.96) | 8.62 (8.32 to 8.92) | 8.53 (8.24 to 8.82) | 8.40 (8.10 to 8.73) | 8.66 (8.36 to 8.96) | 8.63 (8.33 to 8.93) | 8.54 (8.25 to 8.83) | 8.41 (8.11 to 8.74) |
| N5 | 8.65 (8.35 to 8.95) | 8.57 (8.28 to 8.87) | 8.45 (8.15 to 8.76) | 8.35 (8.02 to 8.70) | 8.68 (8.38 to 8.98) | 8.63 (8.34 to 8.93) | 8.53 (8.25 to 8.83) | 8.41 (8.11 to 8.74) |
| N6 | 8.68 (8.38 to 8.99) | 8.65 (8.35 to 8.95) | 8.55 (8.26 to 8.85) | 8.42 (8.12 to 8.74) | 8.68 (8.38 to 8.99) | 8.65 (8.35 to 8.95) | 8.55 (8.26 to 8.85) | 8.42 (8.12 to 8.74) |
| N7 | 8.71 (8.39 to 9.02) | 8.61 (8.31 to 8.91) | 8.47 (8.19 to 8.78) | 8.36 (8.04 to 8.70) | 8.73 (8.40 to 9.06) | 8.67 (8.38 to 8.98) | 8.57 (8.28 to 8.86) | 8.43 (8.13 to 8.75) |
| T1 | 8.54 (8.25 to 8.83) | b | b | b | b | b | b | b |
| T2 | 8.58 (8.29 to 8.88) | b | b | b | b | b | b | b |
| T3 | 8.56 (8.27 to 8.85) | b | b | b | b | b | b | b |
| T4 | 8.60 (8.31 to 8.90) | b | b | b | b | b | b | b |
| T5 | 8.64 (8.34 to 8.94) | 8.61 (8.33 to 8.91) | 8.59 (8.30 to 8.88) | 8.56 (8.27 to 8.86) | 8.64 (8.35 to 8.94) | 8.63 (8.34 to 8.93) | 8.61 (8.32 to 8.91) | 8.58 (8.29 to 8.88) |
| T6 | 8.61 (8.31 to 8.90) | 8.59 (8.30 to 8.89) | 8.57 (8.28 to 8.87) | 8.55 (8.27 to 8.85) | 8.61 (8.32 to 8.91) | 8.60 (8.31 to 8.90) | 8.59 (8.30 to 8.88) | 8.57 (8.27 to 8.86) |
| T7 | 8.70 (8.39 to 9.02) | 8.67 (8.37 to 8.97) | 8.62 (8.33 to 8.92) | 8.58 (8.29 to 8.88) | 8.71 (8.40 to 9.03) | 8.69 (8.38 to 9.00) | 8.65 (8.36 to 8.96) | 8.60 (8.31 to 8.91) |
| T8 | 8.64 (8.35 to 8.95) | 8.63 (8.34 to 8.94) | 8.61 (8.32 to 8.91) | 8.58 (8.29 to 8.88) | 8.64 (8.35 to 8.95) | 8.63 (8.34 to 8.94) | 8.61 (8.32 to 8.91) | 8.58 (8.29 to 8.88) |
| T9 | 8.71 (8.39 to 9.03) | 8.69 (8.38 to 9.00) | 8.64 (8.35 to 8.95) | 8.60 (8.30 to 8.90) | 8.71 (8.40 to 9.04) | 8.70 (8.39 to 9.01) | 8.66 (8.36 to 8.96) | 8.61 (8.31 to 8.91) |
| P1 | a,b | b | b | b | b | b | b | b |
| P2 | 8.65 (8.36 to 8.96) | b | b | b | b | b | b | b |
| P3 | 8.62 (8.33 to 8.92) | b | b | b | b | b | b | b |
| P4 | 8.74 (8.41 to 9.06) | b | b | b | b | b | b | b |
| P5 | 8.65 (8.35 to 8.95) | 8.62 (8.34 to 8.92) | 8.59 (8.30 to 8.89) | 8.57 (8.28 to 8.86) | 8.65 (8.36 to 8.96) | 8.64 (8.35 to 8.94) | 8.61 (8.33 to 8.91) | 8.58 (8.29 to 8.88) |
| P6 | 8.62 (8.32 to 8.91) | 8.60 (8.31 to 8.90) | 8.58 (8.29 to 8.87) | 8.56 (8.27 to 8.85) | 8.62 (8.33 to 8.91) | 8.61 (8.32 to 8.90) | 8.59 (8.30 to 8.89) | 8.57 (8.28 to 8.86) |
| P7 | 8.72 (8.40 to 9.05) | 8.69 (8.38 to 8.99) | 8.63 (8.34 to 8.93) | 8.58 (8.30 to 8.88) | 8.73 (8.41 to 9.06) | 8.71 (8.40 to 9.02) | 8.67 (8.37 to 8.98) | 8.61 (8.32 to 8.91) |
| P8 | 8.65 (8.36 to 8.96) | 8.64 (8.35 to 8.95) | 8.62 (8.33 to 8.92) | 8.59 (8.29 to 8.89) | 8.65 (8.36 to 8.96) | 8.64 (8.35 to 8.95) | 8.62 (8.33 to 8.92) | 8.59 (8.29 to 8.89) |
| P9 | 8.73 (8.40 to 9.05) | 8.71 (8.39 to 9.02) | 8.66 (8.36 to 8.96) | 8.60 (8.31 to 8.90) | 8.73 (8.41 to 9.06) | 8.72 (8.40 to 9.03) | 8.67 (8.38 to 8.98) | 8.62 (8.33 to 8.91) |
| Strategy | Costs by substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 4260 (3828 to 4755) | 3995 (3538 to 4502) | 3714 (3235 to 4291) | 3497 (3007 to 4115) | 4329 (3900 to 4814) | 4156 (3718 to 4659) | 3897 (3444 to 4439) | 3627 (3156 to 4217) |
| M2 | 4351 (3926 to 4834) | 4227 (3787 to 4724) | 3941 (3484 to 4466) | 3651 (3184 to 4239) | 4351 (3926 to 4834) | 4227 (3787 to 4724) | 3941 (3484 to 4466) | 3651 (3184 to 4239) |
| M3 | 4817 (4401 to 5258) | 4430 (4015 to 4863) | 3985 (3541 to 4491) | 3633 (3158 to 4211) | 4917 (4507 to 5359) | 4672 (4261 to 5104) | 4282 (3862 to 4744) | 3848 (3409 to 4368) |
| M4 | 4908 (4499 to 5351) | 4662 (4250 to 5088) | 4212 (3786 to 4702) | 3788 (3340 to 4328) | 4938 (4530 to 5384) | 4743 (4332 to 5171) | 4326 (3909 to 4779) | 3872 (3431 to 4383) |
| M5 | 4678 (4263 to 5118) | 4268 (3836 to 4743) | 3867 (3407 to 4403) | 3571 (3090 to 4172) | 4787 (4372 to 5227) | 4511 (4091 to 4953) | 4121 (3681 to 4619) | 3747 (3286 to 4299) |
| M6 | 4824 (4411 to 5267) | 4624 (4204 to 5060) | 4184 (3755 to 4667) | 3780 (3330 to 4332) | 4824 (4411 to 5267) | 4624 (4204 to 5060) | 4184 (3755 to 4667) | 3780 (3330 to 4332) |
| M7 | 5235 (4809 to 5728) | 4703 (4292 to 5146) | 4139 (3704 to 4633) | 3707 (3239 to 4269) | 5374 (4949 to 5879) | 5027 (4609 to 5512) | 4507 (4083 to 4941) | 3968 (3534 to 4454) |
| N1 | 5935 (5514 to 6457) | 5178 (4761 to 5640) | 4421 (3976 to 4920) | 3849 (3376 to 4412) | 6135 (5696 to 6659) | 5630 (5208 to 6129) | 4906 (4479 to 5357) | 4189 (3756 to 4689) |
| N2 | 6201 (5764 to 6740) | 5837 (5403 to 6349) | 5024 (4608 to 5489) | 4252 (3813 to 4749) | 6201 (5764 to 6740) | 5837 (5403 to 6349) | 5024 (4608 to 5489) | 4252 (3813 to 4749) |
| N3 | 5093 (4684 to 5542) | 4638 (4227 to 5071) | 4112 (3668 to 4629) | 3696 (3220 to 4264) | 5210 (4785 to 5670) | 4923 (4522 to 5357) | 4464 (4043 to 4925) | 3949 (3510 to 4475) |
| N4 | 5184 (4772 to 5639) | 4870 (4462 to 5293) | 4340 (3919 to 4820) | 3850 (3403 to 4389) | 5231 (4811 to 5694) | 4995 (4591 to 5434) | 4508 (4088 to 4956) | 3973 (3532 to 4490) |
| N5 | 5122 (4720 to 5565) | 4484 (4052 to 4949) | 3962 (3502 to 4488) | 3611 (3128 to 4208) | 5297 (4895 to 5736) | 4842 (4422 to 5277) | 4270 (3826 to 4759) | 3812 (3346 to 4361) |
| N6 | 5363 (4960 to 5807) | 5027 (4618 to 5469) | 4352 (3921 to 4842) | 3850 (3405 to 4393) | 5363 (4960 to 5807) | 5027 (4618 to 5469) | 4352 (3921 to 4842) | 3850 (3405 to 4393) |
| N7 | 5955 (5536 to 6477) | 5126 (4711 to 5583) | 4360 (3915 to 4850) | 3809 (3342 to 4360) | 6177 (5755 to 6692) | 5609 (5192 to 6108) | 4837 (4410 to 5281) | 4134 (3703 to 4630) |
| T1 | 4038 (3602 to 4537) | a | a | a | a | a | a | a |
| T2 | 4324 (3900 to 4798) | a | a | a | a | a | a | a |
| T3 | 4317 (3884 to 4772) | a | a | a | a | a | a | a |
| T4 | 4603 (4174 to 5044) | a | a | a | a | a | a | a |
| T5 | 4604 (4192 to 5036) | 4451 (4040 to 4901) | 4315 (3898 to 4783) | 4221 (3801 to 4702) | 4646 (4235 to 5077) | 4539 (4122 to 4977) | 4397 (3983 to 4851) | 4276 (3864 to 4750) |
| T6 | 4721 (4303 to 5150) | 4580 (4180 to 5007) | 4394 (3995 to 4838) | 4242 (3833 to 4711) | 4755 (4331 to 5190) | 4673 (4266 to 5099) | 4523 (4127 to 4954) | 4336 (3934 to 4795) |
| T7 | 5286 (4856 to 5778) | 4992 (4574 to 5454) | 4671 (4268 to 5108) | 4424 (4027 to 4883) | 5363 (4939 to 5868) | 5173 (4755 to 5664) | 4882 (4480 to 5327) | 4574 (4176 to 5006) |
| T8 | 4661 (4254 to 5089) | 4582 (4169 to 5011) | 4418 (4006 to 4869) | 4287 (3874 to 4754) | 4661 (4254 to 5089) | 4582 (4169 to 5011) | 4418 (4006 to 4869) | 4287 (3874 to 4754) |
| T9 | 5343 (4916 to 5844) | 5124 (4709 to 5594) | 4774 (4365 to 5211) | 4490 (4096 to 4933) | 5378 (4952 to 5879) | 5217 (4799 to 5707) | 4903 (4497 to 5351) | 4585 (4187 to 5014) |
| P1 | 6035 (5596 to 6572) | a | a | a | a | a | a | a |
| P2 | 5118 (4715 to 5547) | a | a | a | a | a | a | a |
| P3 | 5073 (4668 to 5531) | a | a | a | a | a | a | a |
| P4 | 6153 (5728 to 6688) | a | a | a | a | a | a | a |
| P5 | 5058 (4650 to 5489) | 4681 (4257 to 5117) | 4421 (4006 to 4896) | 4267 (3846 to 4748) | 5164 (4761 to 5598) | 4883 (4472 to 5308) | 4561 (4148 to 5017) | 4352 (3936 to 4822) |
| P6 | 5046 (4643 to 5491) | 4819 (4418 to 5246) | 4539 (4133 to 4985) | 4313 (3902 to 4786) | 5102 (4693 to 5545) | 4965 (4567 to 5395) | 4731 (4329 to 5163) | 4451 (4056 to 4904) |
| P7 | 6065 (5647 to 6581) | 5462 (5049 to 5930) | 4921 (4515 to 5358) | 4541 (4139 to 4988) | 6227 (5804 to 6751) | 5810 (5398 to 6325) | 5254 (4854 to 5713) | 4764 (4364 to 5185) |
| P8 | 5207 (4807 to 5636) | 4997 (4585 to 5428) | 4601 (4185 to 5046) | 4368 (3951 to 4835) | 5207 (4807 to 5636) | 4997 (4585 to 5428) | 4601 (4185 to 5046) | 4368 (3951 to 4835) |
| P9 | 6230 (5814 to 6749) | 5809 (5404 to 6299) | 5113 (4719 to 5570) | 4643 (4245 to 5084) | 6277 (5858 to 6808) | 5936 (5528 to 6448) | 5297 (4902 to 5763) | 4780 (4380 to 5205) |
| Strategy | Costs by substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 4706 (4282 to 5165) | 4347 (3918 to 4807) | 3934 (3482 to 4458) | 3608 (3137 to 4191) | 4798 (4370 to 5265) | 4571 (4144 to 5042) | 4209 (3776 to 4690) | 3807 (3367 to 4337) |
| M2 | 4825 (4395 to 5300) | 4665 (4249 to 5119) | 4274 (3836 to 4744) | 3844 (3400 to 4370) | 4825 (4395 to 5300) | 4665 (4249 to 5119) | 4274 (3836 to 4744) | 3844 (3400 to 4370) |
| M3 | 4878 (4469 to 5324) | 4478 (4071 to 4914) | 4015 (3572 to 4519) | 3648 (3180 to 4218) | 4981 (4562 to 5451) | 4729 (4321 to 5173) | 4325 (3915 to 4774) | 3872 (3438 to 4380) |
| M4 | 4997 (4582 to 5460) | 4796 (4382 to 5248) | 4355 (3939 to 4804) | 3884 (3448 to 4395) | 5008 (4590 to 5473) | 4823 (4404 to 5275) | 4390 (3979 to 4833) | 3909 (3477 to 4415) |
| M5 | 5056 (4649 to 5515) | 4566 (4148 to 5003) | 4054 (3622 to 4554) | 3665 (3202 to 4238) | 5185 (4769 to 5658) | 4863 (4449 to 5319) | 4386 (3966 to 4834) | 3900 (3465 to 4403) |
| M6 | 5226 (4811 to 5698) | 4995 (4589 to 5451) | 4466 (4051 to 4906) | 3943 (3511 to 4439) | 5226 (4811 to 5698) | 4995 (4589 to 5451) | 4466 (4051 to 4906) | 3943 (3511 to 4439) |
| M7 | 5228 (4823 to 5732) | 4698 (4291 to 5139) | 4135 (3713 to 4623) | 3705 (3242 to 4261) | 5367 (4947 to 5876) | 5021 (4612 to 5496) | 4502 (4094 to 4937) | 3965 (3539 to 4455) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 4978 (4568 to 5438) | 4547 (4142 to 4983) | 4055 (3615 to 4554) | 3667 (3196 to 4232) | 5089 (4664 to 5565) | 4817 (4409 to 5266) | 4384 (3975 to 4829) | 3903 (3466 to 4410) |
| N4 | 5097 (4681 to 5572) | 4865 (4454 to 5317) | 4395 (3981 to 4840) | 3903 (3463 to 4411) | 5115 (4697 to 5596) | 4910 (4487 to 5365) | 4449 (4036 to 4886) | 3939 (3508 to 4442) |
| N5 | 5469 (5062 to 5944) | 4757 (4339 to 5202) | 4133 (3697 to 4637) | 3697 (3238 to 4267) | 5661 (5251 to 6132) | 5165 (4745 to 5626) | 4513 (4104 to 4960) | 3952 (3514 to 4457) |
| N6 | 5732 (5326 to 6204) | 5368 (4960 to 5826) | 4611 (4197 to 5055) | 3999 (3567 to 4497) | 5732 (5326 to 6204) | 5368 (4960 to 5826) | 4611 (4197 to 5055) | 3999 (3567 to 4497) |
| N7 | 5742 (5326 to 6247) | 4958 (4552 to 5408) | 4255 (3821 to 4730) | 3756 (3291 to 4304) | 5952 (5522 to 6464) | 5410 (5002 to 5889) | 4688 (4276 to 5114) | 4047 (3615 to 4534) |
| T1 | 4423 (4016 to 4852) | b | b | b | b | b | b | b |
| T2 | 4762 (4351 to 5196) | b | b | b | b | b | b | b |
| T3 | 4548 (4142 to 4967) | b | b | b | b | b | b | b |
| T4 | 4888 (4485 to 5306) | b | b | b | b | b | b | b |
| T5 | 4989 (4585 to 5435) | 4835 (4428 to 5275) | 4699 (4289 to 5132) | 4605 (4197 to 5045) | 5030 (4625 to 5479) | 4923 (4519 to 5370) | 4782 (4374 to 5220) | 4661 (4259 to 5097) |
| T6 | 4716 (4310 to 5149) | 4652 (4249 to 5081) | 4574 (4180 to 5003) | 4511 (4117 to 4939) | 4732 (4327 to 5166) | 4693 (4289 to 5127) | 4628 (4226 to 5057) | 4550 (4156 to 4982) |
| T7 | 5282 (4866 to 5787) | 5064 (4656 to 5544) | 4851 (4446 to 5296) | 4694 (4290 to 5131) | 5339 (4918 to 5846) | 5194 (4780 to 5682) | 4987 (4578 to 5455) | 4787 (4387 to 5214) |
| T8 | 5045 (4638 to 5495) | 4967 (4565 to 5413) | 4803 (4394 to 5240) | 4671 (4268 to 5108) | 5045 (4638 to 5495) | 4967 (4565 to 5413) | 4803 (4394 to 5240) | 4671 (4268 to 5108) |
| T9 | 5339 (4920 to 5843) | 5196 (4788 to 5691) | 4954 (4550 to 5417) | 4760 (4358 to 5192) | 5354 (4936 to 5866) | 5237 (4827 to 5734) | 5008 (4601 to 5478) | 4798 (4400 to 5227) |
| P1 | a,b | b | b | b | b | b | b | b |
| P2 | 5502 (5095 to 5962) | b | b | b | b | b | b | b |
| P3 | 4888 (4489 to 5334) | b | b | b | b | b | b | b |
| P4 | 5968 (5550 to 6490) | b | b | b | b | b | b | b |
| P5 | 5442 (5039 to 5900) | 5066 (4649 to 5507) | 4805 (4382 to 5244) | 4651 (4247 to 5092) | 5548 (5151 to 6011) | 5268 (4853 to 5718) | 4946 (4531 to 5388) | 4736 (4331 to 5181) |
| P6 | 4874 (4475 to 5316) | 4759 (4362 to 5193) | 4637 (4241 to 5059) | 4540 (4145 to 4967) | 4903 (4504 to 5345) | 4830 (4429 to 5273) | 4719 (4322 to 5150) | 4597 (4200 to 5024) |
| P7 | 5894 (5479 to 6409) | 5402 (4982 to 5899) | 5019 (4614 to 5470) | 4769 (4363 to 5203) | 6028 (5612 to 6555) | 5675 (5266 to 6207) | 5242 (4837 to 5708) | 4910 (4512 to 5343) |
| P8 | 5591 (5187 to 6053) | 5381 (4975 to 5832) | 4986 (4566 to 5428) | 4753 (4345 to 5192) | 5591 (5187 to 6053) | 5381 (4975 to 5832) | 4986 (4566 to 5428) | 4753 (4345 to 5192) |
| P9 | 6058 (5651 to 6575) | 5750 (5338 to 6261) | 5211 (4807 to 5671) | 4871 (4469 to 5306) | 6077 (5666 to 6599) | 5801 (5391 to 6320) | 5285 (4877 to 5758) | 4926 (4529 to 5358) |
Appendix 4 Modelling approach for long-term outcomes
The following figures show the digitised curves for all-cause death (Figure 48) and death attributable to prostate cancer (Figure 49) from PIVOT8 for the men allocated to watchful waiting.
FIGURE 48.
Digitised curves for all-cause death for men allocated to watchful waiting in PIVOT (Wilt et al. , 2012). 8
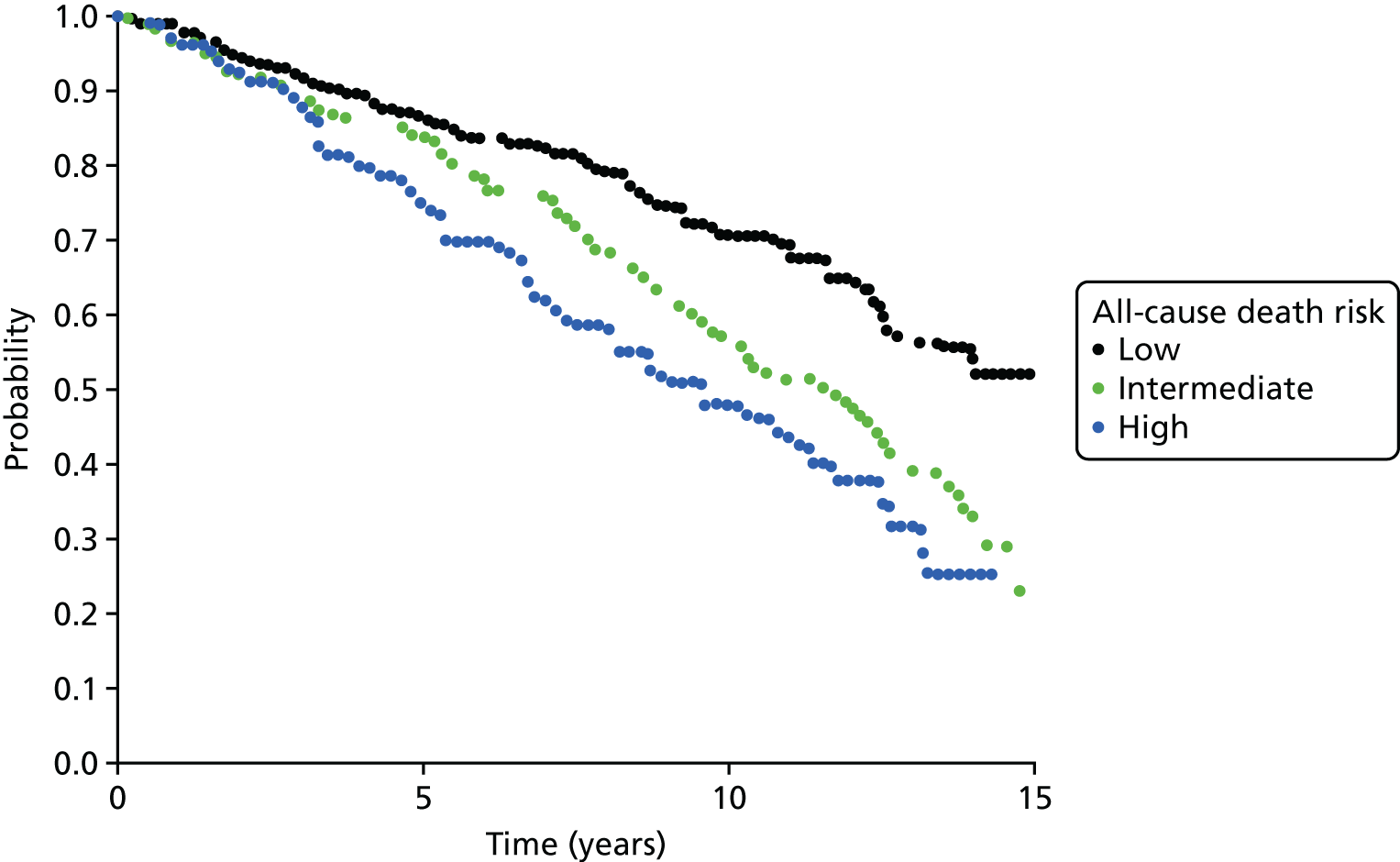
FIGURE 49.
Digitised curves for death attributable to prostate cancer for men allocated to watchful waiting in PIVOT (Wilt et al. , 2012). 8
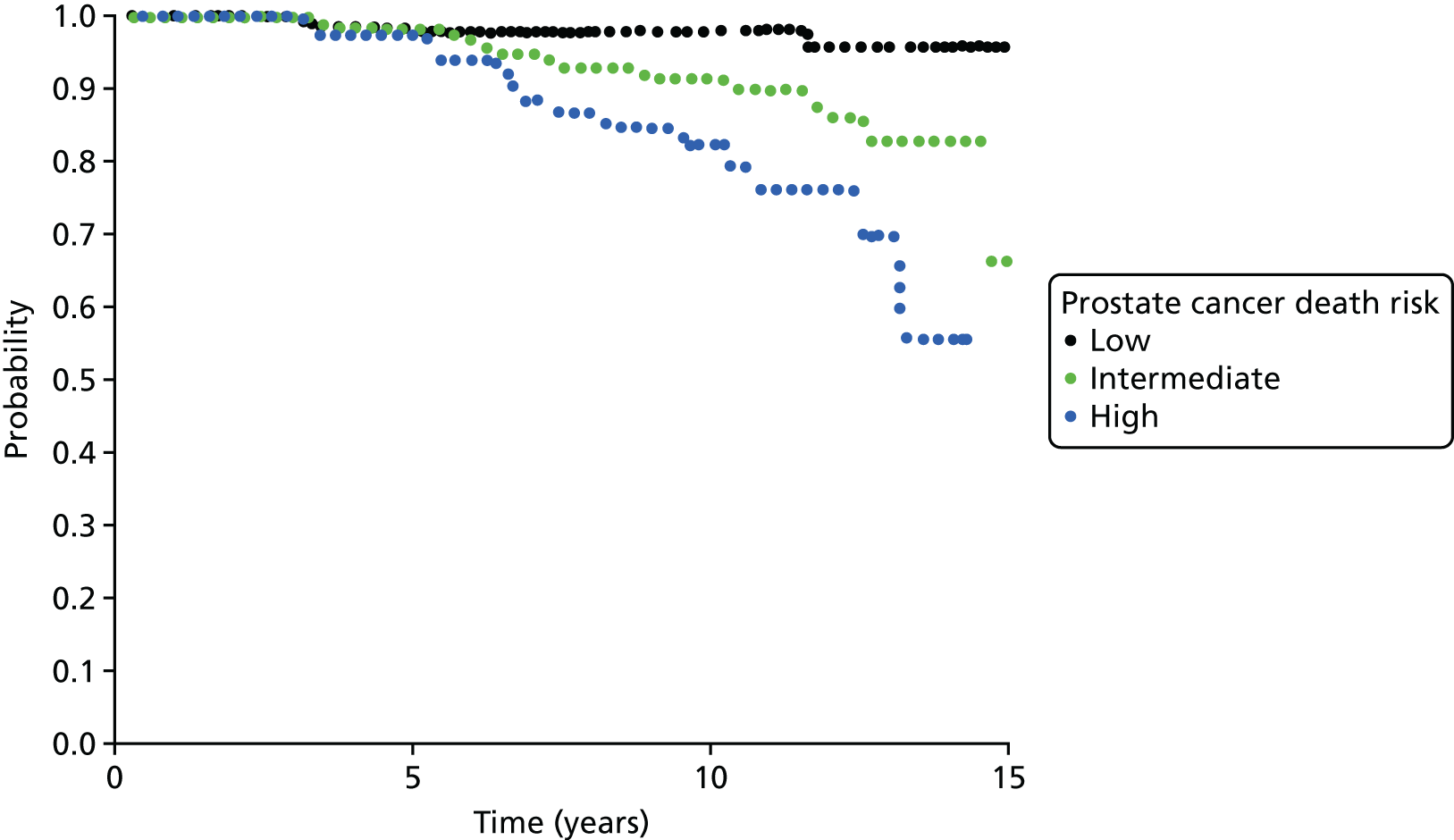
Parametric models were fitted to these data in order to extrapolate the probability of survival being the follow-up of PIVOT. Broadly, two options were examined:
-
parametric model for all-cause death using either Weibull or exponential regression
-
parametric model for death attributable to prostate cancer using Weibull, exponential or linear regression, in addition to all-cause death from the UK general population.
Figures 50–52 show the performance of the two approaches. Using a parametric model for all-cause death has better face validity than using death attributable to prostate cancer added to all-cause death from the general population. The decrease in probability close to year 35 is because the models assume a probability of death of 1 after the age of 100 years. The Weibull curve has a better fit and statistical goodness of fit (not shown) over the three subgroups and, therefore, it was selected.
FIGURE 50.
Curve fitting for men with low-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012). 8
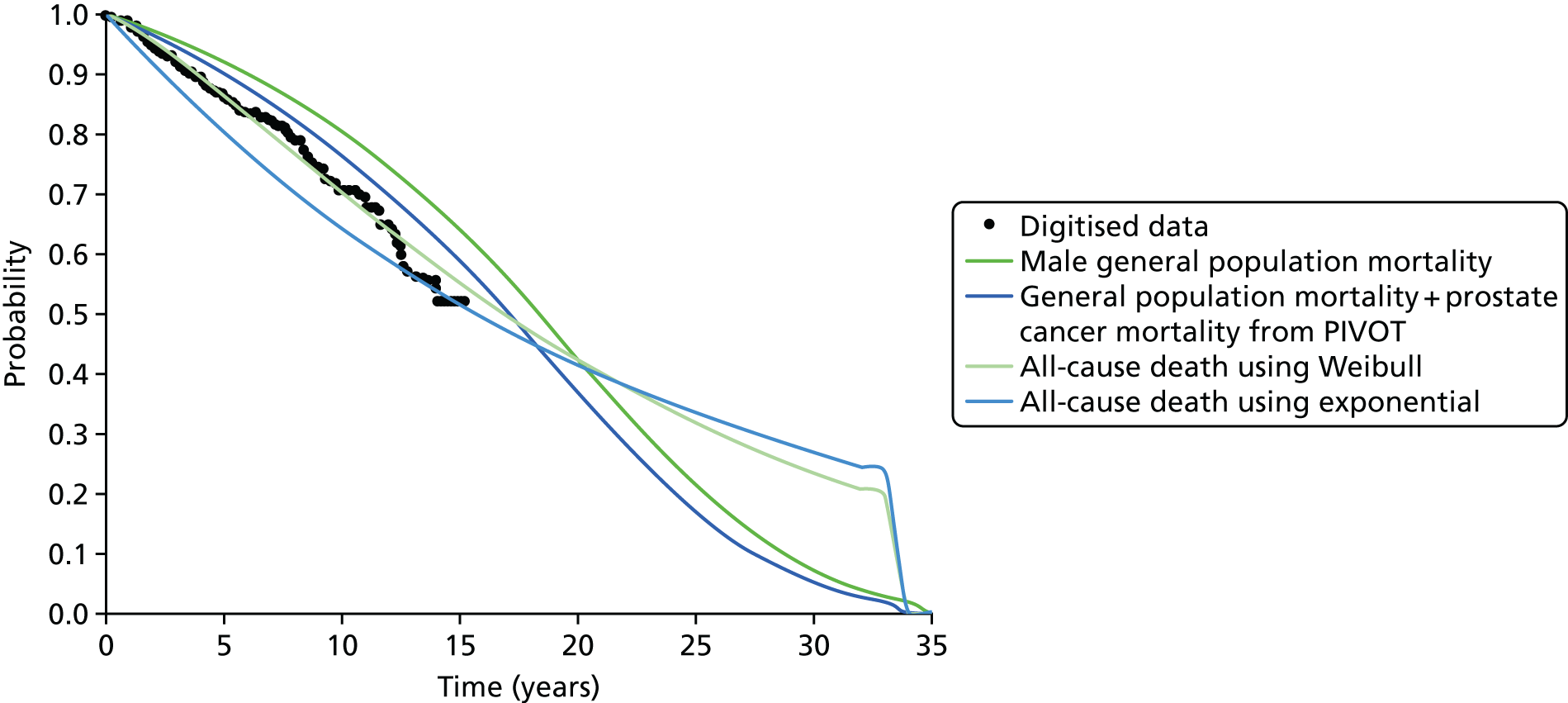
FIGURE 51.
Curve fitting for men with intermediate-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012). 8

FIGURE 52.
Curve fitting for men with high-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012). 8
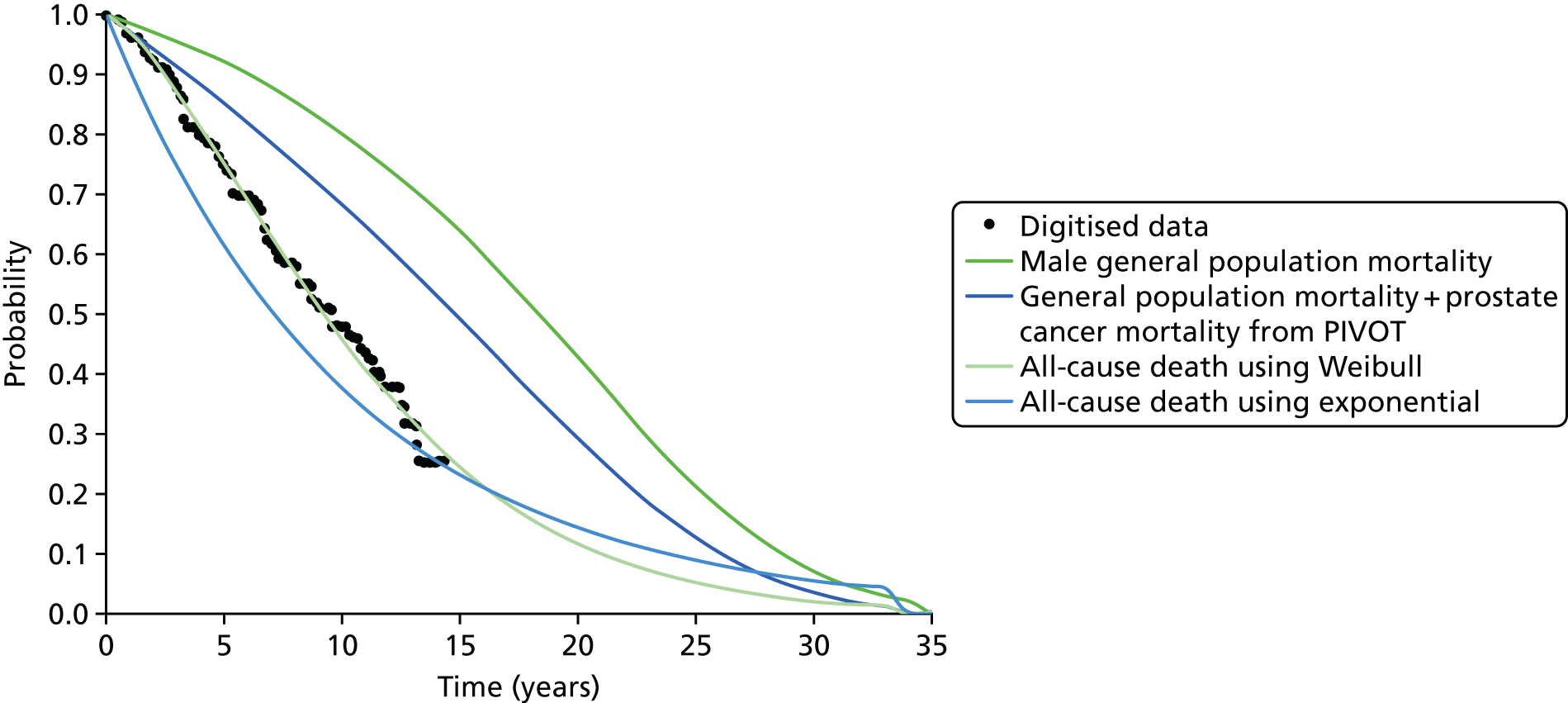
Figures 53–55 show the modelled life expectancy obtained by combining the hazard rate for death predicted by the Weibull model and the hazard rate for death of the general population. These estimates are used in the calibration model in step 2 (above) to obtain transition probabilities.
FIGURE 53.
Life expectancy for men with low-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012)8 predicted with step 1 (above).
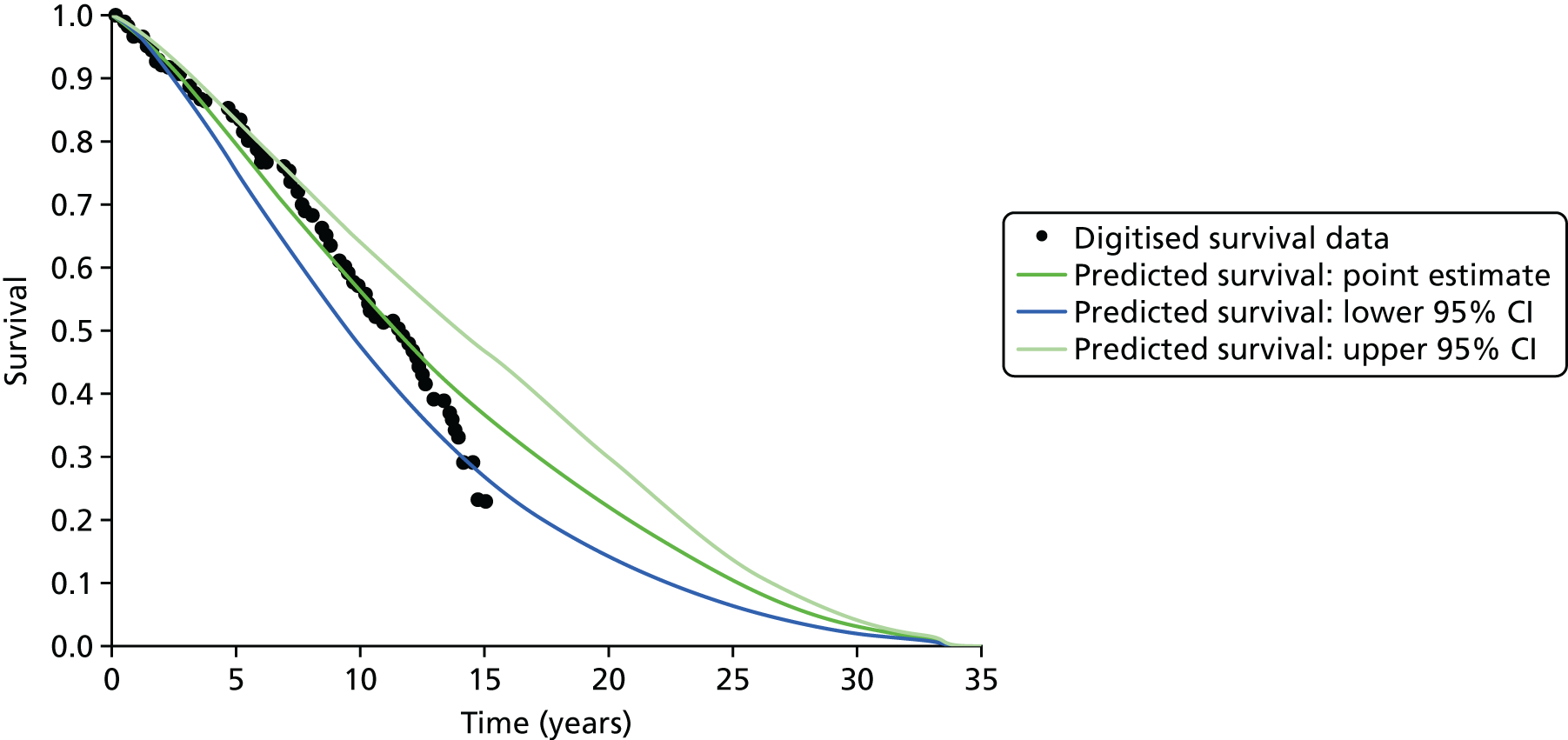
FIGURE 54.
Life expectancy for men with intermediate-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012)8 predicted with step 1 (above).
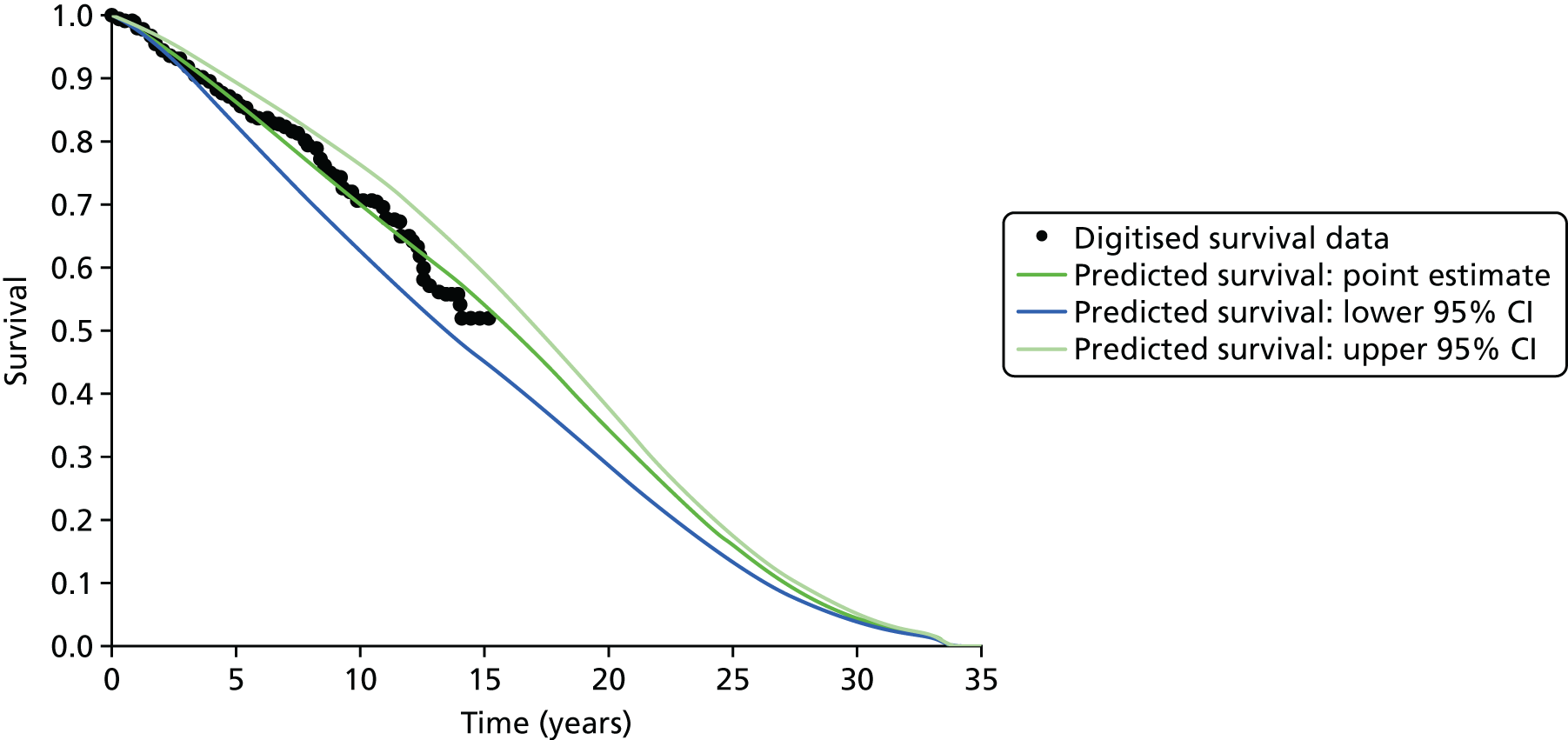
FIGURE 55.
Life expectancy for men with high-risk cancer allocated to watchful waiting in PIVOT (Wilt et al. , 2012)8 predicted with step 1 (above).
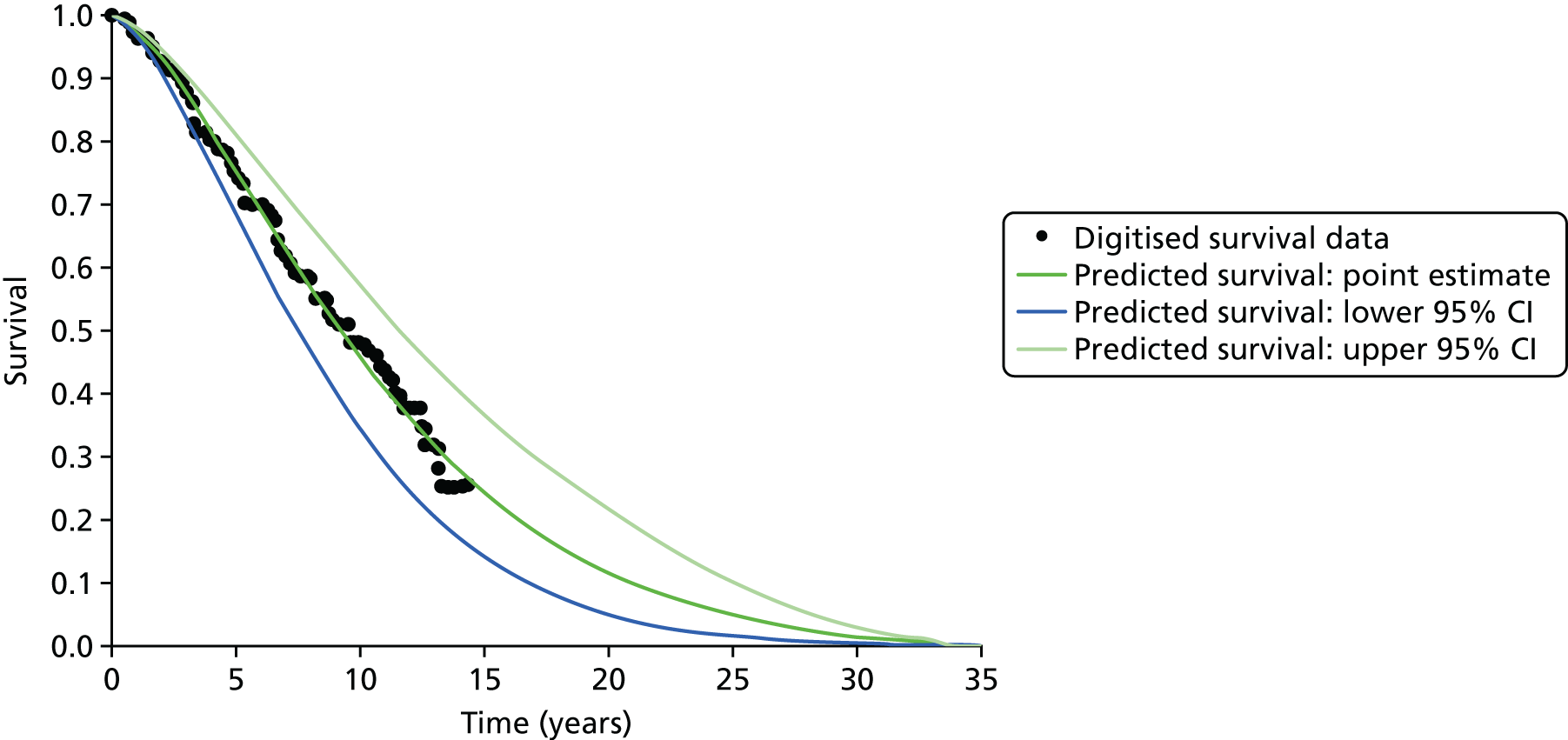
Appendix 5 Transectal ultrasound-guided biopsy
There were concerns that TRUS-guided biopsy may be associated with a small risk of death as a result of sepsis, which is not included in the base-case model. Therefore, the Loeb et al. 23 systematic review on the complications of prostate biopsy was examined for studies on the risk of death from TRUS-guided biopsy.
Loeb et al. 23 identified 10 studies on the risk of death following TRUS-guided biopsy: Loeb et al. 149 (2011), Nam et al. ,150 Carmignani et al. ,151 Simsir et al. ,152 Loeb et al. 153 (2012), Hasegawa et al. ,154 Kumagai et al. ,155 Gallina et al. ,156 Carlsson et al. 157 and Wu et al. 158 Of these, Hasegawa et al. 154 and Kumagai et al. 155 are individual case reports of a death following transrectal biopsy in Japan and are reported in Japanese; hence, they were not examined further. It was not possible to obtain the full text of Wu et al. ,158 although Loeb et al. 23 reported that no deaths had occurred in this study. It was also not possible to obtain the full text of Simsir et al. ,152 a retrospective cohort study set in Turkey. The remaining six studies have been reviewed in detail and are summarised in Table 57. One study found that biopsied men had an increased risk of death compared with non-biopsied men. 156 However, this study has limitations in that a limited range of variables were available to make the biopsied group and control group more comparable; specifically, a measure of comorbidities was not available for the control group.
| Study | Methods | Results | Comments |
|---|---|---|---|
| Carlsson et al.157 (BJU Int 2011;107:1912–7) (various northern European countries) | Death associated with prostate biopsy among participants of the European Randomized Study of Screening for Prostate Cancer. Compared cohort of screen-positive men (biopsied) with cohort of screen-negative men (no biopsy) |
|
No evidence that prostate biopsy is associated with increased risk of death |
| Carmignani et al.151 (Int Urol Nephrol 2012;44:1055–63) (Italy) | Monitoring of major septic complications after biopsy at 3 days, 7 days and 1 month after transrectal biopsy |
|
No deaths occurred |
| Gallina et al.156 (Int J Cancer 2008;123:647–52) (Canada) | 120-day death rate after prostate biopsy and in a convenience sample of participants who did not undergo biopsy |
|
|
| Loeb et al.149 (2011) (J Urol 2011;186:1830–4) (USA) | 30-day hospitalisation rates and primary diagnosis for hospital admissions between men who underwent biopsy and random sample of controls. Adjusted for predictors of serious infections and non-infectious complications |
|
|
| Loeb et al.153 (2012) (Eur Urol 2012;61:1110–4) (the Netherlands) | Complications following prostate biopsy reported in the European Randomized Study of Screening for Prostate Cancer (Rotterdam section) |
|
No deaths occurred |
| Nam et al.150 J Urol 2013;189(Suppl 1):S12–17 (Canada) | 30-day hospital admission and mortality attributable to urological complications |
|
|
Appendix 6 Scenario analysis using the payment-by-results tariff
Whereas the NHS reference costs reflect the average current cost across all health-care providers, the PbR tariff reflects the charge to primary care commissioners, who refer men to secondary care for testing. The reference costs are used to set prices, such as the PbR tariff, for services. Given that the perspective of the analysis is the NHS, the unit costs should be obtained from the NHS reference costs. However, these may not reflect the costs to commissioners, who make the referral decision. Therefore, sensitivity analysis is presented using PbR tariff unit costs.
Unit costs of tests
The costs used are shown in Table 58. The PbR tariff does not provide a cost for ‘LB76Z Transrectal ultrasound guided biopsy of the prostate’, used in the base case for TRUS-guided biopsy; hence, the Healthcare Resource Group code ‘LB27Z prostate or bladder neck minor endoscopic procedure male’ for an outpatient appointment is used instead, with a cost of £339. This is similar to the approach taken for the 2014 NICE clinical guideline,13 although it used NHS reference costs and added the cost of histopathology. Using this approach, the NHS reference cost for TRUS-guided biopsy would be £342 (£223 for the LB27Z code for an outpatient appointment + £119 for histopathology). Similarly, the PbR tariff does not provide the cost for ‘LB77Z Transperineal template biopsy of prostate’. The 2014 NICE clinical guideline used the LB27Z code for a day case. Using NHS reference costs, the TPM-biopsy cost would be £830 (£533 for the LB27Z code for a day case + £297 for histopathology). The PbR tariff does include the cost for LB27Z as an ordinary elective spell at £931; therefore, this is used to represent the tariff for TPM-biopsy. The unit cost for mpMRI is obtained from the code ‘RD03Z MRI scan of one area with pre- and post-contrast’, at £188 including reporting. The cost of a hospitalisation is the average tariff for a non-elective admission of £3173. The cost of an A&E visit is the average tariff for type 1 and type 2 departments of £134. An outpatient appointment is the cost of the code ‘WF02A first attendance single professional medical oncology’, at £91.
| Parameter | Value (£) | Source |
|---|---|---|
| Diagnostic tests | ||
| TRUS-guided biopsy | 339.00 | ‘LB27Z prostate or bladder neck minor endoscopic procedure male as outpatient’110 |
| TPM-biopsy | 931.00 | ‘LB27Z prostate or bladder neck minor endoscopic procedure male as elective admission’110 |
| mpMRI T2 DW DCE | 188.00 | ‘RD03Z MRI scan of one area with pre- and post-contrast’110 |
| Management of AEs from testing | ||
| A&E admission | 134.00 | Average cost of admission in type 1 and type 2 departments110 |
| Hospital admission | 3173.00 | Average cost of non-elective admission |
| Urology department nurse | 91.00 | ‘WF02A First attendance single professional Medical oncology’110 |
| Other costs, common to both scenarios | ||
| GP | 44.00 | Equivalent to a GP appointment, with an average duration of 11.7 minutes112 |
| Other health-care advice | 7.90 | Cost of nurse-led telephone triage112 |
Results
Costs of testing using the payment-by-results tariff
Tables 59 and 60 show the costs of testing using the PbR tariff, presenting the average and 95% CI for each substrategy. The costs of testing using the PbR tariff are similar to those using NHS reference costs for the strategies involving mpMRI and TRUS-guided biopsy. The unit costs are similar, at £182 (NHS reference cost) compared with £188 (PbR tariff) for mpMRI and £403 (NHS reference costs) compared with £339 (PbR tariff) for TRUS-guided biopsy. There is a bigger difference, of up to £398, in the cost of strategies involving TPM-biopsy. This is mainly because the NHS reference cost for TPM-biopsy is £1370, £439 more than the PbR tariff. The costs of adverse effects from testing are smaller using NHS reference costs than using the PbR tariff; however, the differences are small.
| Strategy | Cost of testing per substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 524 (505 to 545) | 399 (381 to 418) | 307 (291 to 323) | 246 (235 to 258) | 558 (539 to 581) | 467 (446 to 489) | 359 (342 to 377) | 279 (265 to 294) |
| M2 | 572 (553 to 596) | 504 (484 to 526) | 373 (356 to 393) | 285 (271 to 300) | 572 (553 to 596) | 504 (484 to 526) | 373 (356 to 393) | 285 (271 to 300) |
| M3 | 605 (576 to 636) | 459 (433 to 486) | 343 (322 to 366) | 264 (248 to 280) | 645 (617 to 676) | 540 (513 to 571) | 411 (387 to 436) | 308 (289 to 328) |
| M4 | 654 (627 to 684) | 564 (539 to 592) | 410 (387 to 435) | 303 (286 to 320) | 659 (631 to 690) | 578 (550 to 608) | 426 (402 to 452) | 314 (295 to 334) |
| M5 | 684 (650 to 719) | 472 (446 to 502) | 337 (317 to 357) | 258 (244 to 273) | 743 (708 to 781) | 584 (551 to 618) | 407 (385 to 433) | 299 (282 to 316) |
| M6 | 769 (734 to 807) | 649 (616 to 685) | 428 (404 to 455) | 307 (290 to 325) | 769 (734 to 807) | 649 (616 to 685) | 428 (404 to 455) | 307 (290 to 325) |
| M7 | 765 (726 to 807) | 532 (501 to 566) | 373 (348 to 399) | 276 (258 to 294) | 830 (789 to 872) | 657 (619 to 697) | 460 (431 to 491) | 328 (306 to 350) |
| N1 | 1059 (1021 to 1100) | 735 (693 to 782) | 496 (458 to 536) | 338 (309 to 369) | 1149 (1118 to 1184) | 912 (873 to 960) | 631 (589 to 678) | 425 (390 to 461) |
| N2 | 1185 (1159 to 1221) | 1008 (970 to 1051) | 668 (626 to 717) | 440 (405 to 477) | 1185 (1159 to 1221) | 1008 (970 to 1051) | 668 (626 to 717) | 440 (405 to 477) |
| N3 | 735 (690 to 780) | 555 (517 to 595) | 402 (371 to 434) | 292 (271 to 316) | 783 (737 to 829) | 657 (615 to 701) | 495 (457 to 534) | 354 (326 to 384) |
| N4 | 783 (741 to 826) | 660 (625 to 699) | 468 (437 to 502) | 332 (308 to 355) | 797 (753 to 844) | 695 (654 to 738) | 509 (473 to 548) | 360 (333 to 389) |
| N5 | 939 (888 to 991) | 590 (550 to 633) | 385 (357 to 416) | 277 (259 to 298) | 1038 (987 to 1089) | 770 (722 to 823) | 485 (452 to 521) | 330 (308 to 353) |
| N6 | 1082 (1030 to 1132) | 879 (827 to 929) | 516 (479 to 557) | 341 (318 to 365) | 1082 (1030 to 1132) | 879 (827 to 929) | 516 (479 to 557) | 341 (318 to 365) |
| N7 | 1150 (1089 to 1210) | 746 (694 to 800) | 480 (439 to 523) | 324 (297 to 353) | 1263 (1201 to 1319) | 960 (900 to 1022) | 621 (574 to 670) | 405 (370 to 441) |
| T1 | 386 (366 to 409) | a | a | a | a | a | a | a |
| T2 | 582 (549 to 620) | a | a | a | a | a | a | a |
| T3 | 493 (467 to 525) | a | a | a | a | a | a | a |
| T4 | 689 (653 to 733) | a | a | a | a | a | a | a |
| T5 | 642 (605 to 682) | 558 (529 to 590) | 514 (490 to 543) | 495 (474 to 521) | 666 (628 to 707) | 601 (567 to 637) | 534 (507 to 562) | 504 (481 to 531) |
| T6 | 536 (505 to 568) | 508 (481 to 539) | 480 (455 to 507) | 458 (436 to 484) | 542 (512 to 577) | 525 (496 to 557) | 499 (472 to 529) | 471 (448 to 497) |
| T7 | 792 (753 to 834) | 681 (649 to 715) | 609 (582 to 638) | 568 (544 to 594) | 823 (782 to 868) | 740 (701 to 779) | 647 (617 to 680) | 589 (564 to 617) |
| T8 | 677 (638 to 720) | 627 (593 to 664) | 540 (513 to 570) | 506 (483 to 534) | 677 (638 to 720) | 627 (593 to 664) | 540 (513 to 570) | 506 (483 to 534) |
| T9 | 827 (788 to 872) | 750 (713 to 789) | 635 (605 to 666) | 579 (555 to 606) | 834 (794 to 879) | 766 (729 to 807) | 653 (622 to 687) | 591 (566 to 619) |
| P1 | 1001 (974 to 1036) | a | a | a | a | a | a | a |
| P2 | 895 (845 to 943) | a | a | a | a | a | a | a |
| P3 | 664 (621 to 705) | a | a | a | a | a | a | a |
| P4 | 1174 (1130 to 1220) | a | a | a | a | a | a | a |
| P5 | 899 (845 to 947) | 681 (642 to 722) | 567 (539 to 600) | 518 (494 to 545) | 962 (906 to 1012) | 791 (743 to 837) | 617 (583 to 651) | 540 (515 to 569) |
| P6 | 691 (645 to 736) | 620 (582 to 659) | 547 (514 to 580) | 490 (465 to 517) | 709 (661 to 755) | 663 (619 to 706) | 595 (559 to 632) | 523 (494 to 554) |
| P7 | 1204 (1154 to 1255) | 915 (871 to 962) | 728 (693 to 767) | 622 (595 to 651) | 1285 (1235 to 1335) | 1068 (1018 to 1120) | 827 (788 to 870) | 678 (646 to 713) |
| P8 | 990 (932 to 1043) | 860 (811 to 908) | 634 (598 to 669) | 545 (519 to 574) | 990 (932 to 1043) | 860 (811 to 908) | 634 (598 to 669) | 545 (519 to 574) |
| P9 | 1306 (1257 to 1355) | 1117 (1071 to 1166) | 804 (766 to 844) | 651 (622 to 684) | 1317 (1269 to 1366) | 1146 (1098 to 1197) | 847 (807 to 891) | 683 (650 to 719) |
| Strategy | Cost of testing per substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 524 (505 to 545) | 399 (381 to 418) | 307 (291 to 323) | 246 (235 to 258) | 558 (539 to 581) | 467 (446 to 489) | 359 (342 to 377) | 279 (265 to 294) |
| M2 | 572 (553 to 596) | 504 (484 to 526) | 373 (356 to 393) | 285 (271 to 300) | 572 (553 to 596) | 504 (484 to 526) | 373 (356 to 393) | 285 (271 to 300) |
| M3 | 555 (531 to 581) | 419 (399 to 442) | 319 (301 to 337) | 251 (239 to 265) | 592 (567 to 619) | 494 (471 to 519) | 376 (356 to 397) | 288 (273 to 304) |
| M4 | 603 (580 to 630) | 525 (503 to 549) | 385 (366 to 406) | 291 (276 to 306) | 606 (581 to 634) | 531 (507 to 556) | 390 (371 to 413) | 294 (279 to 310) |
| M5 | 675 (640 to 711) | 465 (439 to 495) | 332 (313 to 353) | 256 (242 to 270) | 734 (697 to 772) | 575 (542 to 610) | 401 (378 to 426) | 295 (279 to 313) |
| M6 | 759 (723 to 798) | 640 (607 to 677) | 422 (398 to 449) | 303 (286 to 321) | 759 (723 to 798) | 640 (607 to 677) | 422 (398 to 449) | 303 (286 to 321) |
| M7 | 706 (664 to 747) | 486 (455 to 518) | 344 (323 to 368) | 261 (246 to 278) | 768 (725 to 808) | 602 (565 to 641) | 418 (392 to 446) | 304 (285 to 324) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 605 (570 to 642) | 452 (424 to 482) | 337 (316 to 360) | 260 (245 to 276) | 646 (609 to 683) | 536 (503 to 571) | 404 (378 to 432) | 302 (282 to 322) |
| N4 | 653 (618 to 689) | 558 (528 to 589) | 404 (381 to 429) | 299 (282 to 317) | 660 (623 to 698) | 573 (539 to 608) | 418 (392 to 447) | 308 (289 to 328) |
| N5 | 916 (860 to 974) | 571 (528 to 617) | 373 (345 to 405) | 271 (253 to 291) | 1014 (958 to 1067) | 748 (694 to 803) | 469 (432 to 507) | 321 (298 to 345) |
| N6 | 1058 (1003 to 1112) | 856 (801 to 913) | 499 (462 to 542) | 331 (308 to 356) | 1058 (1003 to 1112) | 856 (801 to 913) | 499 (462 to 542) | 331 (308 to 356) |
| N7 | 997 (929 to 1062) | 625 (569 to 682) | 404 (369 to 443) | 285 (263 to 308) | 1102 (1031 to 1166) | 817 (752 to 883) | 514 (468 to 561) | 344 (314 to 374) |
| T1 | 386 (366 to 409) | b | b | b | b | b | b | b |
| T2 | 582 (549 to 620) | b | b | b | b | b | b | b |
| T3 | 442 (418 to 469) | b | b | b | b | b | b | b |
| T4 | 638 (603 to 680) | b | b | b | b | b | b | b |
| T5 | 642 (605 to 682) | 558 (529 to 590) | 514 (490 to 543) | 495 (474 to 521) | 666 (628 to 707) | 601 (567 to 637) | 534 (507 to 562) | 504 (481 to 531) |
| T6 | 463 (437 to 491) | 446 (422 to 472) | 432 (410 to 456) | 421 (401 to 445) | 467 (441 to 497) | 456 (431 to 483) | 440 (418 to 466) | 427 (406 to 451) |
| T7 | 720 (681 to 761) | 618 (588 to 653) | 560 (534 to 589) | 531 (508 to 558) | 748 (708 to 791) | 670 (634 to 710) | 588 (560 to 619) | 545 (521 to 573) |
| T8 | 677 (638 to 720) | 627 (593 to 664) | 540 (513 to 570) | 506 (483 to 534) | 677 (638 to 720) | 627 (593 to 664) | 540 (513 to 570) | 506 (483 to 534) |
| T9 | 755 (714 to 797) | 687 (651 to 727) | 586 (558 to 616) | 542 (518 to 570) | 759 (718 to 801) | 697 (659 to 738) | 595 (566 to 627) | 547 (523 to 576) |
| P1 | a,b | b | b | b | b | b | b | b |
| P2 | 895 (845 to 943) | b | b | b | b | b | b | b |
| P3 | 532 (496 to 569) | b | b | b | b | b | b | b |
| P4 | 1042 (991 to 1089) | b | b | b | b | b | b | b |
| P5 | 899 (845 to 947) | 681 (642 to 722) | 567 (539 to 600) | 518 (494 to 545) | 962 (906 to 1012) | 791 (743 to 837) | 617 (583 to 651) | 540 (515 to 569) |
| P6 | 542 (505 to 581) | 497 (467 to 529) | 461 (435 to 488) | 435 (412 to 459) | 554 (514 to 595) | 523 (489 to 558) | 484 (455 to 513) | 448 (424 to 474) |
| P7 | 1056 (1001 to 1106) | 793 (750 to 837) | 642 (609 to 681) | 567 (541 to 595) | 1130 (1075 to 1183) | 928 (876 to 980) | 715 (676 to 756) | 603 (574 to 638) |
| P8 | 990 (932 to 1043) | 860 (811 to 908) | 634 (598 to 669) | 545 (519 to 574) | 990 (932 to 1043) | 860 (811 to 908) | 634 (598 to 669) | 545 (519 to 574) |
| P9 | 1158 (1103 to 1210) | 994 (943 to 1043) | 718 (680 to 757) | 595 (568 to 628) | 1162 (1106 to 1213) | 1006 (955 to 1056) | 735 (695 to 777) | 608 (579 to 642) |
Diagnostic efficiency
Figure 56 shows the relationship between the proportion of CS cancers detected and the average cost of testing using the PbR tariff. Table 61 shows the strategies forming the frontier in tabulated format. There are small differences compared with using NHS reference costs in that some other strategies now appear at the frontier. T1 2 —, in which all men receive TRUS-guided biopsy and no further testing at the TRUS-guided biopsy definition 2; T7 222, and P1 ---, in which all men receive TPM-biopsy and no further testing.
FIGURE 56.
Efficiency plane using the PbR tariff for unit costs.

| Strategy | Definition | mpMRI cut-off point | Detection of CS cancers, average (95% CI) | Cost of testing, average (95% CI) (£) | |
|---|---|---|---|---|---|
| TRUS-guided biopsy | mpMRI | ||||
| M1: mpMRI for all men and TRUS-guided biopsy in men suspicious of CS cancer | 2 | 1 | 5 | 0.22 (0.17 to 0.27) | 246 (235 to 258) |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer on the first biopsy receive a second TRUS-guided biopsy | 2 | 1 | 5 | 0.24 (0.20 to 0.29) | 251 (239 to 265) |
| 2 | 2 | 5 | 0.35 (0.30 to 0.41) | 288 (273 to 304) | |
| M4: mpMRI for all men and TRUS-guided in men with suspicion of any cancer; men with suspected CS cancer at mpMRI and in whom CNS cancer was detected on the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 5 | 0.37 (0.32 to 0.43) | 294 (279 to 310) |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 5 | 0.40 (0.35 to 0.46) | 304 (285 to 324) |
| T1: TRUS-guided biopsy in all men and no further testing | 2 | N/A | N/A | 0.61 (0.56 to 0.66) | 386 (366 to 409) |
| T6: TRUS-guided biopsy for all men; men classified as CNS cancer receive MRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 0.74 (0.69 to 0.78) | 456 (431 to 483) |
| 2 | 2 | 2 | 0.75 (0.71 to 0.79) | 467 (441 to 497) | |
| T7: TRUS-guided biopsy for all men; men classified as NC or CNS cancer receive mpMRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 0.91 (0.86 to 0.94) | 670 (634 to 710) |
| 2 | 2 | 2 | 0.95 (0.90 to 0.98) | 748 (708 to 791) | |
| M7: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 2 | 0.95 (0.92 to 0.98) | 768 (725 to 808) |
| P1: TPM-biopsy in all men | N/A | N/A | N/A | 1.00 (1.00 to 1.00) | 1001 (974 to 1036) |
The frontier is defined by 12 strategies at different definitions and cut-off points. The strategies in the frontier in which all men receive mpMRI as the first test are:
-
M1 – men whose mpMRI scan is suspicious of CS cancer receive a TRUS-guided biopsy. This strategy is in the frontier at the TRUS-guided biopsy definition 2, mpMRI definition 1 and cut-off point 5.
-
M3 – men whose mpMRI scan is suspicious of CS cancer receive a TRUS-guided biopsy; men in whom CNS cancer was detected receive a second TRUS-guided biopsy to rule out CS cancer. This strategy is in the frontier at the TRUS-guided biopsy definition 2, mpMRI definition 1 and 2 and cut-off point 5.
-
M4 – men whose mpMRI scan is suspicious of any cancer receive TRUS-guided biopsy; men in whom the mpMRI showed suspected CS cancer but the TRUS-guided biopsy detected CNS cancer receive a second TRUS-guided biopsy. This strategy is in the frontier at the TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off point 5.
-
M7 – men whose mpMRI scan is suspicious of CS cancer receive TRUS-guided biopsy; men in whom no CS cancer was detected receive a second biopsy. This strategy is in the frontier at the TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off points 2 and 5.
The strategies in the frontier in which all men receive a TRUS-guided biopsy as the first test are:
-
T1 – all men receive TRUS-guided biopsy and no other tests. This strategy is in the frontier under TRUS-guided biopsy definition 2.
-
T6 – all men receive TRUS-guided biopsy; men in whom TRUS-guided biopsy detects CNS cancer have mpMRI; men in whom the mpMRI scan is suspicious of CS cancer receive a second biopsy. This strategy is in the frontier under TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off points 2 and 3.
-
T7 – all men receive TRUS-guided biopsy; men in whom TRUS-guided biopsy does not detect CS cancer have mpMRI; men in whom the mpMRI scan is suspicious of CS cancer receive a second biopsy. This strategy is in the frontier under TRUS-guided biopsy definition 2, mpMRI definition 2 and cut-off points 2 and 3.
-
P1 – all men receive TPM-biopsy and no other tests.
Lifetime costs
Figure 57 shows the lifetime costs using a diagnostic strategy that uses the PbR tariff for the unit costs; 95% CIs are not shown to improve readability. Tables 62 and 63 show the results in tabulated format, including 95% CIs. The costs vary between £3500 and close to £6000 per man referred for testing. The substrategy with the lowest cost is M1 115, at £3499 (95% CI £3008 to £4113), which is also the substrategy that achieves the fewest health benefits. The substrategy with the greatest costs is P9 122, at £5950 (95% CI £5521 to £6478).
FIGURE 57.
Scenario results: costs of each diagnostic strategy using the PbR tariff.
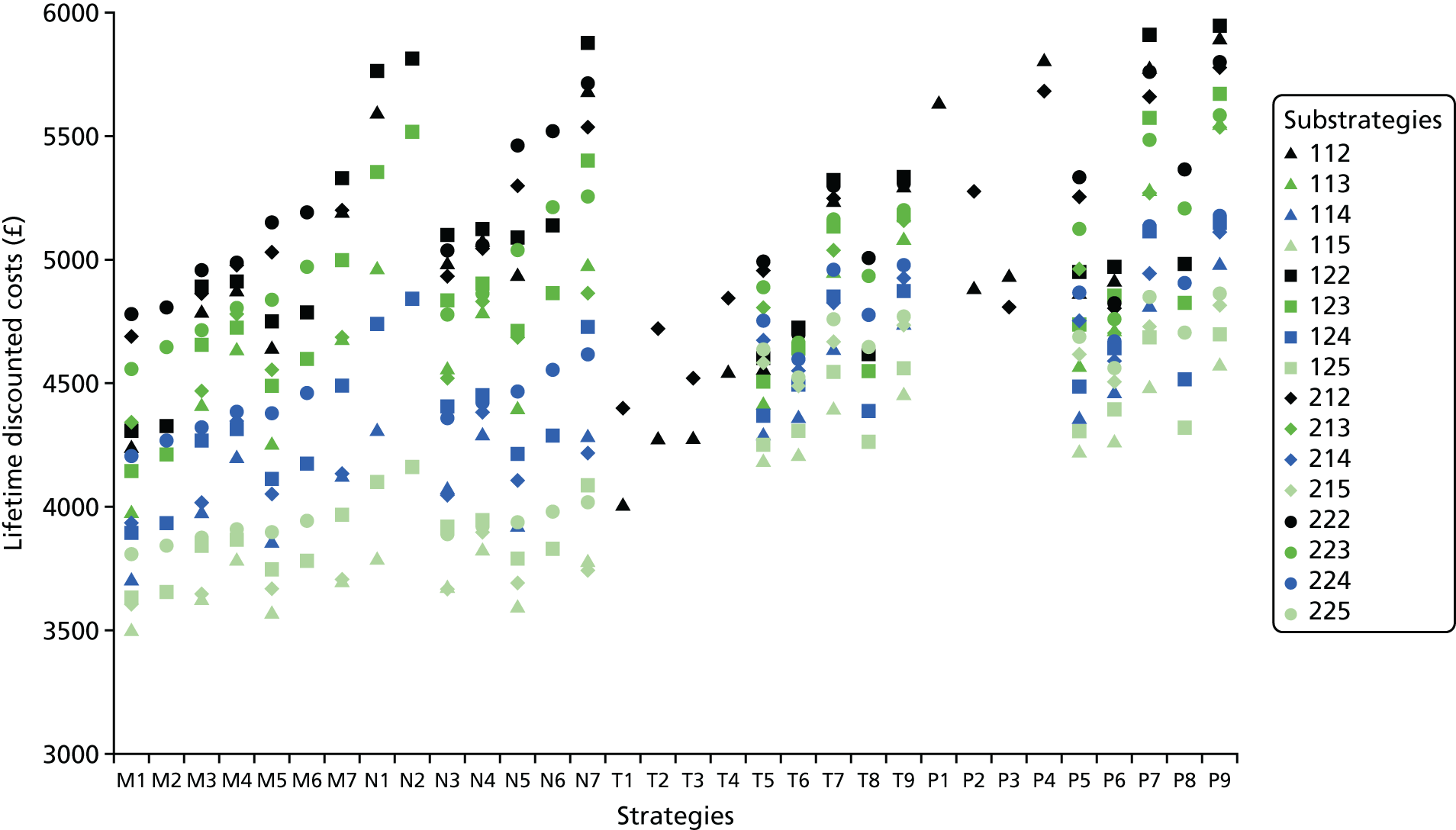
| Strategy | Lifetime costs per substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 112 | 113 | 114 | 115 | 122 | 123 | 124 | 125 | |
| M1 | 4240 (3800 to 4743) | 3985 (3535 to 4502) | 3711 (3234 to 4285) | 3499 (3008 to 4113) | 4307 (3877 to 4794) | 4141 (3698 to 4648) | 3890 (3436 to 4433) | 3626 (3156 to 4218) |
| M2 | 4328 (3897 to 4811) | 4209 (3771 to 4713) | 3933 (3478 to 4464) | 3650 (3183 to 4240) | 4328 (3897 to 4811) | 4209 (3771 to 4713) | 3933 (3478 to 4464) | 3650 (3183 to 4240) |
| M3 | 4791 (4383 to 5229) | 4415 (4003 to 4845) | 3979 (3536 to 4489) | 3633 (3157 to 4210) | 4887 (4482 to 5341) | 4651 (4240 to 5085) | 4271 (3851 to 4735) | 3844 (3404 to 4366) |
| M4 | 4878 (4473 to 5323) | 4639 (4231 to 5072) | 4201 (3778 to 4690) | 3785 (3333 to 4327) | 4908 (4502 to 5360) | 4719 (4304 to 5152) | 4314 (3886 to 4765) | 3868 (3429 to 4383) |
| M5 | 4646 (4237 to 5082) | 4252 (3822 to 4723) | 3862 (3400 to 4393) | 3572 (3089 to 4173) | 4751 (4340 to 5184) | 4486 (4062 to 4931) | 4111 (3677 to 4607) | 3745 (3284 to 4300) |
| M6 | 4785 (4373 to 5223) | 4595 (4173 to 5029) | 4172 (3738 to 4652) | 3777 (3331 to 4331) | 4785 (4373 to 5223) | 4595 (4173 to 5029) | 4172 (3738 to 4652) | 3777 (3331 to 4331) |
| M7 | 5197 (4768 to 5692) | 4682 (4275 to 5125) | 4130 (3695 to 4632) | 3706 (3240 to 4264) | 5331 (4908 to 5834) | 4997 (4578 to 5474) | 4492 (4073 to 4928) | 3963 (3530 to 4451) |
| N1 | 5595 (5173 to 6117) | 4967 (4551 to 5431) | 4305 (3864 to 4800) | 3795 (3322 to 4350) | 5759 (5325 to 6289) | 5348 (4927 to 5845) | 4736 (4317 to 5175) | 4101 (3671 to 4593) |
| N2 | 5811 (5373 to 6350) | 5517 (5085 to 6017) | 4840 (4425 to 5292) | 4158 (3723 to 4656) | 5811 (5373 to 6350) | 5517 (5085 to 6017) | 4840 (4425 to 5292) | 4158 (3723 to 4656) |
| N3 | 4989 (4579 to 5440) | 4565 (4159 to 4989) | 4071 (3629 to 4587) | 3679 (3204 to 4250) | 5097 (4688 to 5566) | 4832 (4434 to 5267) | 4403 (3981 to 4859) | 3918 (3483 to 4437) |
| N4 | 5076 (4668 to 5537) | 4789 (4390 to 5218) | 4293 (3872 to 4773) | 3830 (3385 to 4370) | 5118 (4711 to 5585) | 4900 (4495 to 5342) | 4446 (4021 to 4889) | 3942 (3505 to 4458) |
| N5 | 4937 (4532 to 5374) | 4398 (3966 to 4864) | 3928 (3467 to 4450) | 3600 (3119 to 4199) | 5083 (4686 to 5510) | 4706 (4288 to 5136) | 4213 (3771 to 4705) | 3791 (3330 to 4341) |
| N6 | 5137 (4739 to 5568) | 4860 (4445 to 5297) | 4286 (3852 to 4774) | 3826 (3385 to 4371) | 5137 (4739 to 5568) | 4860 (4445 to 5297) | 4286 (3852 to 4774) | 3826 (3385 to 4371) |
| N7 | 5686 (5271 to 6200) | 4978 (4571 to 5436) | 4288 (3850 to 4770) | 3780 (3313 to 4331) | 5874 (5455 to 6401) | 5397 (4981 to 5895) | 4726 (4318 to 5170) | 4082 (3658 to 4574) |
| T1 | 4009 (3566 to 4509) | a | a | a | a | a | a | a |
| T2 | 4279 (3851 to 4752) | a | a | a | a | a | a | a |
| T3 | 4279 (3837 to 4742) | a | a | a | a | a | a | a |
| T4 | 4550 (4123 to 5002) | a | a | a | a | a | a | a |
| T5 | 4565 (4157 to 4997) | 4418 (4002 to 4863) | 4286 (3867 to 4747) | 4193 (3777 to 4672) | 4605 (4195 to 5035) | 4503 (4095 to 4940) | 4367 (3950 to 4824) | 4248 (3830 to 4714) |
| T6 | 4685 (4277 to 5115) | 4547 (4146 to 4972) | 4363 (3955 to 4815) | 4213 (3803 to 4693) | 4719 (4309 to 5156) | 4638 (4237 to 5067) | 4490 (4096 to 4922) | 4306 (3902 to 4767) |
| T7 | 5242 (4818 to 5735) | 4956 (4552 to 5414) | 4640 (4233 to 5081) | 4397 (3995 to 4855) | 5316 (4886 to 5819) | 5132 (4719 to 5619) | 4848 (4447 to 5294) | 4545 (4144 to 4978) |
| T8 | 4619 (4209 to 5048) | 4545 (4133 to 4973) | 4387 (3967 to 4838) | 4258 (3837 to 4720) | 4619 (4209 to 5048) | 4545 (4133 to 4973) | 4387 (3967 to 4838) | 4258 (3837 to 4720) |
| T9 | 5296 (4865 to 5793) | 5082 (4671 to 5543) | 4742 (4331 to 5186) | 4462 (4061 to 4907) | 5330 (4899 to 5833) | 5174 (4755 to 5664) | 4869 (4464 to 5318) | 4555 (4155 to 4988) |
| P1 | 5637 (5197 to 6175) | a | a | a | a | a | a | a |
| P2 | 4886 (4485 to 5306) | a | a | a | a | a | a | a |
| P3 | 4933 (4527 to 5380) | a | a | a | a | a | a | a |
| P4 | 5810 (5382 to 6352) | a | a | a | a | a | a | a |
| P5 | 4866 (4457 to 5301) | 4575 (4166 to 5014) | 4360 (3938 to 4826) | 4226 (3805 to 4703) | 4946 (4546 to 5372) | 4734 (4320 to 5157) | 4481 (4068 to 4925) | 4302 (3887 to 4770) |
| P6 | 4917 (4511 to 5362) | 4719 (4317 to 5147) | 4468 (4058 to 4910) | 4264 (3854 to 4740) | 4967 (4549 to 5409) | 4848 (4446 to 5285) | 4641 (4246 to 5075) | 4389 (3991 to 4841) |
| P7 | 5774 (5360 to 6290) | 5285 (4879 to 5759) | 4819 (4418 to 5260) | 4481 (4076 to 4940) | 5904 (5485 to 6442) | 5573 (5159 to 6082) | 5113 (4710 to 5570) | 4682 (4290 to 5107) |
| P8 | 4978 (4578 to 5400) | 4820 (4423 to 5246) | 4514 (4102 to 4951) | 4316 (3900 to 4777) | 4978 (4578 to 5400) | 4820 (4423 to 5246) | 4514 (4102 to 4951) | 4316 (3900 to 4777) |
| P9 | 5898 (5480 to 6427) | 5553 (5140 to 6038) | 4981 (4587 to 5432) | 4572 (4170 to 5015) | 5940 (5521 to 6478) | 5668 (5253 to 6169) | 5148 (4749 to 5609) | 4696 (4304 to 5123) |
| Strategy | Lifetime costs per substrategy, average (95% CI) (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| 212 | 213 | 214 | 215 | 222 | 223 | 224 | 225 | |
| M1 | 4686 (4259 to 5148) | 4337 (3902 to 4799) | 3931 (3477 to 4461) | 3610 (3140 to 4192) | 4776 (4346 to 5242) | 4556 (4124 to 5028) | 4202 (3770 to 4685) | 3806 (3367 to 4335) |
| M2 | 4802 (4366 to 5274) | 4646 (4228 to 5113) | 4266 (3829 to 4734) | 3842 (3399 to 4367) | 4802 (4366 to 5274) | 4646 (4228 to 5113) | 4266 (3829 to 4734) | 3842 (3399 to 4367) |
| M3 | 4856 (4450 to 5297) | 4466 (4057 to 4901) | 4011 (3569 to 4517) | 3649 (3183 to 4219) | 4956 (4545 to 5420) | 4712 (4304 to 5155) | 4317 (3907 to 4766) | 3870 (3436 to 4377) |
| M4 | 4971 (4558 to 5439) | 4776 (4366 to 5230) | 4346 (3929 to 4791) | 3882 (3443 to 4396) | 4981 (4571 to 5449) | 4802 (4394 to 5258) | 4380 (3967 to 4820) | 3907 (3472 to 4415) |
| M5 | 5024 (4620 to 5483) | 4551 (4133 to 4991) | 4049 (3612 to 4545) | 3666 (3203 to 4238) | 5149 (4726 to 5622) | 4839 (4421 to 5295) | 4375 (3957 to 4820) | 3897 (3462 to 4398) |
| M6 | 5188 (4763 to 5665) | 4966 (4563 to 5423) | 4454 (4042 to 4894) | 3940 (3508 to 4438) | 5188 (4763 to 5665) | 4966 (4563 to 5423) | 4454 (4042 to 4894) | 3940 (3508 to 4438) |
| M7 | 5194 (4793 to 5695) | 4680 (4278 to 5124) | 4129 (3710 to 4620) | 3706 (3240 to 4260) | 5328 (4904 to 5848) | 4994 (4583 to 5475) | 4490 (4075 to 4920) | 3962 (3537 to 4455) |
| N1 | a | a | a | a | a | a | a | a |
| N2 | a | a | a | a | a | a | a | a |
| N3 | 4926 (4516 to 5385) | 4515 (4112 to 4950) | 4040 (3602 to 4542) | 3663 (3191 to 4230) | 5031 (4618 to 5504) | 4773 (4369 to 5223) | 4358 (3948 to 4806) | 3893 (3456 to 4400) |
| N4 | 5041 (4632 to 5522) | 4825 (4425 to 5280) | 4375 (3961 to 4817) | 3896 (3459 to 4408) | 5056 (4647 to 5537) | 4863 (4459 to 5318) | 4422 (4010 to 4859) | 3929 (3500 to 4433) |
| N5 | 5293 (4884 to 5768) | 4678 (4262 to 5118) | 4103 (3671 to 4597) | 3688 (3226 to 4260) | 5457 (5043 to 5935) | 5037 (4623 to 5499) | 4462 (4046 to 4900) | 3934 (3502 to 4432) |
| N6 | 5515 (5096 to 5983) | 5209 (4802 to 5660) | 4552 (4139 to 4990) | 3980 (3550 to 4470) | 5515 (5096 to 5983) | 5209 (4802 to 5660) | 4552 (4139 to 4990) | 3980 (3550 to 4470) |
| N7 | 5532 (5127 to 6041) | 4857 (4458 to 5310) | 4212 (3784 to 4686) | 3742 (3279 to 4296) | 5712 (5304 to 6237) | 5254 (4849 to 5740) | 4618 (4209 to 5046) | 4020 (3601 to 4518) |
| T1 | 4393 (3983 to 4826) | b | b | b | b | b | b | b |
| T2 | 4718 (4311 to 5159) | b | b | b | b | b | b | b |
| T3 | 4514 (4108 to 4942) | b | b | b | b | b | b | b |
| T4 | 4839 (4439 to 5273) | b | b | b | b | b | b | b |
| T5 | 4950 (4549 to 5398) | 4802 (4392 to 5253) | 4670 (4262 to 5103) | 4577 (4170 to 5019) | 4990 (4581 to 5440) | 4887 (4482 to 5334) | 4751 (4340 to 5192) | 4632 (4225 to 5073) |
| T6 | 4683 (4278 to 5118) | 4621 (4227 to 5057) | 4544 (4140 to 4981) | 4482 (4076 to 4916) | 4699 (4298 to 5134) | 4661 (4262 to 5099) | 4597 (4203 to 5032) | 4520 (4117 to 4952) |
| T7 | 5240 (4821 to 5748) | 5030 (4619 to 5509) | 4821 (4409 to 5275) | 4666 (4261 to 5105) | 5295 (4873 to 5806) | 5155 (4740 to 5655) | 4955 (4547 to 5429) | 4759 (4357 to 5200) |
| T8 | 5004 (4595 to 5457) | 4929 (4524 to 5373) | 4772 (4358 to 5212) | 4643 (4233 to 5080) | 5004 (4595 to 5457) | 4929 (4524 to 5373) | 4772 (4358 to 5212) | 4643 (4233 to 5080) |
| T9 | 5294 (4876 to 5811) | 5157 (4747 to 5650) | 4923 (4519 to 5382) | 4731 (4325 to 5169) | 5309 (4888 to 5827) | 5197 (4782 to 5696) | 4975 (4569 to 5450) | 4769 (4369 to 5212) |
| P1 | a,b | b | b | b | b | b | b | b |
| P2 | 5270 (4854 to 5730) | b | b | b | b | b | b | b |
| P3 | 4801 (4398 to 5246) | b | b | b | b | b | b | b |
| P4 | 5678 (5260 to 6208) | b | b | b | b | b | b | b |
| P5 | 5250 (4842 to 5698) | 4960 (4540 to 5407) | 4744 (4325 to 5190) | 4611 (4200 to 5052) | 5331 (4920 to 5790) | 5118 (4712 to 5581) | 4865 (4454 to 5315) | 4686 (4279 to 5130) |
| P6 | 4794 (4390 to 5235) | 4697 (4302 to 5132) | 4589 (4184 to 5022) | 4503 (4095 to 4942) | 4818 (4414 to 5263) | 4758 (4360 to 5199) | 4662 (4269 to 5098) | 4554 (4156 to 4977) |
| P7 | 5651 (5238 to 6181) | 5264 (4856 to 5764) | 4940 (4542 to 5388) | 4720 (4311 to 5152) | 5756 (5340 to 6293) | 5483 (5067 to 5999) | 5134 (4727 to 5601) | 4847 (4446 to 5287) |
| P8 | 5363 (4943 to 5821) | 5204 (4800 to 5655) | 4898 (4483 to 5350) | 4701 (4293 to 5142) | 5363 (4943 to 5821) | 5204 (4800 to 5655) | 4898 (4483 to 5350) | 4701 (4293 to 5142) |
| P9 | 5775 (5359 to 6300) | 5531 (5116 to 6047) | 5102 (4690 to 5559) | 4811 (4410 to 5253) | 5792 (5378 to 6319) | 5578 (5161 to 6098) | 5170 (4761 to 5643) | 4861 (4462 to 5301) |
Cost-effectiveness using payment-by-results tariff
Figure 58 shows the cost-effectiveness plane for the scenario using the PbR tariff as the source of unit costs for the tests and related complications. Table 64 shows the results in tabulated format for the strategies in the cost-effectiveness frontier. The strategies in the cost-effectiveness frontier are similar to those in the cost-effectiveness frontier in the base case. Four strategies forming the frontier start with mpMRI: M1, M3, M4 and M7; five strategies start with biopsy: T1, T6, T7, P4 and P1. The cost-effective strategy depends on the value of health opportunity cost. At £13,000 per QALY, the cost-effective substrategy is M7 222. On average, M7 222 achieves the highest net health at 8.307 QALYs. At £20,000 and £30,000 per QALY gained, the cost-effective strategy is P4 2––. However, the difference between P4 2–– and P1, the next best strategy, is small at 0.001 QALYs.
FIGURE 58.
Cost-effectiveness plane for the scenario using the PbR tariff.
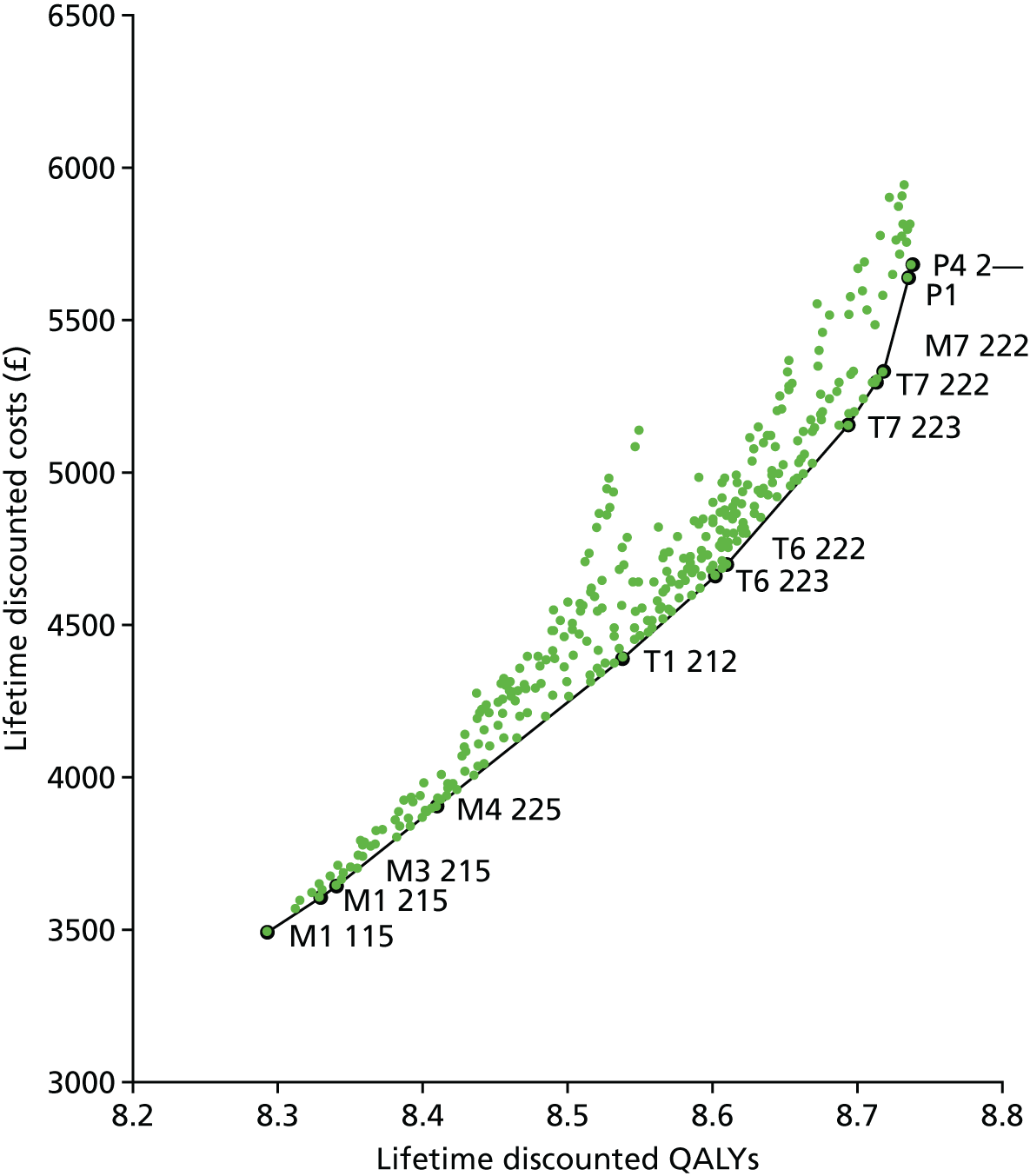
| Strategy | Definition | mpMRI cut-off point | QALYs, average (95% CI) | Costs, average (95% CI) (£) | ICER (£) | Net health at | |||
|---|---|---|---|---|---|---|---|---|---|
| TRUS-guided biopsy | mpMRI | £13,000 per QALY | £20,000 per QALY | £30,000 per QALY | |||||
| M1: mpMRI for all men and TRUS-guided biopsy in men suspicious of CS cancer | 1 | 1 | 5 | 8.29 (7.94 to 8.68) | 3499 (3008 to 4113) | 8.023 | 8.118 | 8.176 | |
| 2 | 1 | 5 | 8.33 (7.99 to 8.69) | 3610 (3140 to 4192) | 3081 | 8.051 | 8.148 | 8.208 | |
| M3: mpMRI for all men and TRUS-guided biopsy in men with suspected CS cancer; men with CNS cancer on the first biopsy receive a second TRUS-guided biopsy | 2 | 1 | 5 | 8.34 (8.01 to 8.70) | 3649 (3183 to 4219) | 3588 | 8.059 | 8.157 | 8.218 |
| M4: mpMRI for all men and TRUS-guided in men with suspicion of any cancer; men with suspected CS cancer at mpMRI and in whom CNS cancer was detected on the first biopsy receive a second TRUS-guided biopsy | 2 | 2 | 5 | 8.41 (8.11 to 8.74) | 3907 (3472 to 4415) | 3691 | 8.109 | 8.214 | 8.279 |
| T1: TRUS-guided biopsy in all men and no further testing | 2 | N/A | N/A | 8.54 (8.25 to 8.83) | 4393 (3983 to 4826) | 3793 | 8.200 | 8.318 | 8.391 |
| T6: TRUS-guided biopsy for all men; men classified as CNS cancer receive MRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 8.60 (8.31 to 8.90) | 4661 (4262 to 5099) | 4184 | 8.243 | 8.369 | 8.446 |
| 2 | 2 | 2 | 8.61 (8.32 to 8.91) | 4699 (4298 to 5134) | 4524 | 8.249 | 8.375 | 8.453 | |
| T7: TRUS-guided biopsy for all men; men classified as NC or CNS cancer receive mpMRI; men with suspected CS cancer receive a second TRUS-guided biopsy | 2 | 2 | 3 | 8.69 (8.38 to 9.00) | 5155 (4740 to 5655) | 5539 | 8.296 | 8.435 | 8.521 |
| 2 | 2 | 2 | 8.71 (8.40 to 9.03) | 5295 (4873 to 5806) | 6954 | 8.305 | 8.448 | 8.536 | |
| M7: mpMRI for all men; TRUS-guided biopsy in men with suspected CS cancer; rebiopsy with TRUS-guided biopsy for those in whom CS cancer was not detected | 2 | 2 | 2 | 8.72 (8.40 to 9.04) | 5328 (4904 to 5848) | 7442 | 8.307 | 8.451 | 8.539 |
| P1: TPM-biopsy for all men | N/A | N/A | N/A | 8.73 (8.40 to 9.06) | 5637 (5197 to 6175) | 17,503 | 8.301 | 8.453 | 8.547 |
| P4: TRUS-guided biopsy in all men and TPM-biopsy in men in whom CS cancer was not detected | 2 | N/A | N/A | 8.74 (8.41 to 9.06) | 5678 (5260 to 6208) | 17,511 | 8.300 | 8.453 | 8.548 |
Threshold sensitivity analysis
Table 65 shows the results of the sensitivity analysis for the scenario using PbR tariff costs for the unit cost of tests and resource use associated with testing. In summary:
-
A relative sensitivity of < 1.15 for MRI-targeted TRUS-guided biopsy (‘W’biopsy) changes the cost-effective strategy at the health opportunity cost of £13,000 per QALY gained from M7 222 to T7 222. A relative sensitivity of > 1.30 (sensitivity > 0.80) changes the cost-effective strategy at £20,000 per QALY gained from P4 2–– to M7 222. Changes of > 1.43 (sensitivity > 0.88) change the cost-effective strategy at £30,000 from P4 2–– to M7 222. The implication is that the cost-effectiveness of M7 222 is improved if the sensitivity of MRI-targeted TRUS-guided biopsy is improved.
-
Increasing the sensitivity of MRI-targeted second TRUS-guided biopsy in men with a prior negative biopsy (‘Z’ biopsy) to 0.90 changes the cost-effective substrategy at £20,000 per QALY gained from P4 2— to M7 222. Increasing it further to 0.91 changes the cost-effective substrategy at £13,000 per QALY from M7 222 to T7 222. Further increases change the cost-effective substrategy to T9 222. In T9, all men receive TRUS-guided biopsy; men in whom the biopsy did not detect CS cancer receive mpMRI. Men in whom the first TRUS-guided biopsy had not detected cancer but the mpMRI indicates that cancer is present receive a second TRUS-guided biopsy. Men in whom the first TRUS-guided biopsy had detected CNS cancer but the mpMRI indicates CS cancer receive a second TRUS-guided biopsy.
-
A lower proportion of intermediate-risk cancer changes the cost-effective strategy to P1—, depending on the cost-effectiveness thresholds. A proportion between 0.35 and 0.43 changes the cost-effective strategy to M7 222 at £20,000 per QALY gained.
-
Increasing the proportion of men with no cancer to 0.41 and 0.53 changes the cost-effective strategy at £20,000 per QALY gained to P1 and M7 222, respectively. At £30,000 per QALY gained, the cost-effective strategy changes to P1.
-
There are no changes in the cost-effective strategy for risk of death between 0.0001% and 0.005%, that is, between 1 and 50 in 1,000,000 men who have a biopsy die from biopsy-related complications. A risk of death from biopsy of 0.01% (i.e. 100 in 1,000,000 men who have a biopsy die from biopsy-related complications) changes the cost-effective strategy at £20,000 from P4 2–– to P1. Increasing the risk further to 0.05% (i.e. 500 in 1,000,000 men die) changes the cost-effective strategy at £30,000 from P4 2–– to P1. From a risk of 0.05% to 0.5% (i.e. 500 to 5000 in 1,000,000 men die), the cost-effective strategy across the range of values of health opportunity cost is P1. A risk of 2% (i.e. 20,000 in 1,000,000 men die) changes the cost-effective strategy at £13,000 per QALY to N1 123; in this strategy, all men receive mpMRI and men in whom the mpMRI scan is suspicious of CS cancer receive TPM-biopsy.
-
Changes in the cost-effective strategy at £13,000 per QALY gained occur when the health loss from the incorrect classification is ≥ 0.02 QALYs. Reductions of < 0.2 QALYs do not change the cost-effective strategy at £20,000 and £30,000 per QALY gained.
-
The cost-effective strategy changes to less-costly and less-sensitive strategies if the clinical effectiveness of RP is reduced.
| Analysis | Cost-effective substrategy at the health opportunity cost of | ||
|---|---|---|---|
| £13,000 per QALY | £20,000 per QALY | £30,000 per QALY | |
| Base case | M7 222 | P4 2–– | P4 2–– |
| SA1: changes in relative sensitivity of MRI-targeted TRUS-guided biopsy (‘W’) in detecting CS cancer; sensitivity in base case = 1.2 | |||
| 1–1.15 | T7 222 | P4 2–– | P4 2–– |
| 1.16–1.25 | M7 222 | P4 2–– | P4 2–– |
| 1.30–1.42 | M7 222 | M7 222 | P4 2–– |
| 1.43–1.50 | M7 222 | M7 222 | M7 222 |
| SA2: changes in the sensitivity of MRI-targeted second TRUS-guided biopsy (‘Z’) in detecting CS cancer; sensitivity in base case = 0.87 | |||
| 0.90 | M7 222 | M7 222 | P4 2–– |
| 0.91 | T7 222 | M7 222 | P4 2–– |
| 0.92–0.93 | T7 222 | T9 222 | P4 2–– |
| 0.94–1.00 | T9 222 | T9 222 | T9 222 |
| SA3: prevalence of intermediate-risk vs. low-risk cancer; base case = 0.53 | |||
| 0.49–0.52 | M7 222 | P1 --- | P4 2–– |
| 0.44–0.48 | M7 222 | P1 --- | P1 --- |
| 0.35–0.43 | M7 222 | M7 222 | P1 --- |
| SA4: probability of NC; base case = 0.28 | |||
| 0.30–0.33 | M7 222 | P1 --- | P4 2–– |
| 0.34–0.41 | M7 222 | P1 --- | P1 --- |
| 0.42–0.53 | M7 222 | M7 222 | P1 --- |
| SA5: risk of death from biopsy | |||
| 0.01% | M7 222 | P1 --- | P4 2–– |
| 0.05% | M7 222 | P1 --- | P1 --- |
| 0.5% | P1 --- | P1 --- | P1 --- |
| 2% | N1 123 | P1 --- | P1 --- |
| SA6: reduced quality-adjusted survival from incorrect classification as NC | |||
| QALY reduction 0.02–0.06 | P1 --- | P4 2–– | P4 2–– |
| SA7: reduced effectiveness of RP | |||
| 5% | M7 222 | M7 222 | P4 2–– |
| 10% | T7 222 | M7 222 | M7 222 |
| 15% | M1 215 | T7 223 | M7 222 |
| 20–30% | M1 115 | M1 115 | T6 222 |
List of abbreviations
- A&E
- accident and emergency
- ADC
- apparent diffusion coefficient
- AE
- adverse event
- CBP
- combined biopsy procedure
- CI
- confidence interval
- CNS
- clinically non-significant
- CRF
- case report form
- CS
- clinically significant
- DCE
- dynamic contrast enhancement
- DRE
- digital rectal examination
- DW
- diffusion weighting
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GEE
- generalised estimating equation
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- mpMRI
- multiparametric magnetic resonance imaging
- MRC CTU
- Medical Research Council Clinical Trials Unit
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- OR
- odds ratio
- PbR
- payment by results
- PI
- principal investigator
- PI-RADS
- Prostate Imaging Reporting and Data System
- PIVOT
- Prostate Cancer Intervention versus Observation Trial
- PPV
- positive predictive value
- PROMIS
- PROstate Magnetic resonance Imaging Study
- ProtecT
- Prostate Testing for Cancer and Treatment
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RP
- radical prostatectomy
- SAE
- serious adverse event
- SD
- standard deviation
- SPCG-4
- Scandinavian Prostate Cancer Group – 4
- STAMPEDE
- Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy
- TPM
- template prostate mapping
- TRUS
- transrectal ultrasound
- UCL
- University College London
- UCLH
- University College London Hospital
