Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/39. The contractual start date was in August 2010. The draft report began editorial review in May 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Joanne Blair undertakes paid advisory work for, and has received funding for research, to attend academic meetings and to support a nursing salary from, Novo Nordisk Ltd (Gatwick, UK), a pharmaceutical company that manufactures some of the insulins used in the SubCutaneous Insulin: Pumps or Injections? (SCIPI) study. The work undertaken by Joanne Blair for Novo Nordisk Ltd relates to growth hormone therapy and not diabetes. Mohammed Didi has received payment for advisory work and funding to attend academic meetings and to support a nursing salary from Novo Nordisk Ltd. The work undertaken by Mohammed Didi for Novo Nordisk Ltd relates to growth hormone therapy and not diabetes. Mohammed Didi has received expenses from Merck Serono Ltd (Feltham, UK) to attend educational meetings. Carrol Gamble is a member of the Efficacy and Mechanism Evaluation Programme Board of the National Institute for Health Research. Dyfrig Hughes is a member of the Health Technology Assessment (HTA) Clinical Trials Board (2010–16), the HTA Funding Teleconference (2015–16) and the Pharmaceuticals Panel (2008–12). John W Gregory reports grants from Cardiff University during the conduct of the study, and membership of the HTA Commissioning Board (2010–14). John W Gregory received speaker fees from Pfizer Inc. and Eli Lilly and Company (Basingstoke, UK), fees for attending an advisory board for Eli Lilly and Company and financial assistance to attend annual meetings of the European Society for Paediatric Endocrinology from Sanofi Genzyme (Guildford, UK), Merck Serono Ltd, Ipsen and Novo Nordisk Ltd.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Blair et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Type 1 diabetes (T1D) is one of the most common chronic diseases of childhood, affecting > 28,000 children and young people in the UK. 1 In Europe, the incidence of childhood T1D is rising, and it is estimated that between 2005 and 2020 the number of children diagnosed per year will increase from 15,000 to 24,400 and will double in children aged < 5 years. 2
The treatment of T1D and its complications presents a considerable burden to patients, the NHS and society, and, as the number of affected patients increases, so do the projected costs. Currently, there is no cure for T1D, and so it is essential that insulin therapies are optimised to enable the best possible quality of life (QoL) for patients and effective use of NHS resources, while minimising the risk of acute and long-term complications.
The use of intensive insulin treatment regimens, in the form of multiple daily injections (MDI) (≥ 4) and continuous subcutaneous insulin infusion (CSII; or ‘insulin pumps’), is associated with a reduction in the risk of developing long-term vascular complications of T1D3 and both treatments are used widely in the NHS.
Treatment with MDI utilises insulin analogues with different pharmacokinetic properties. Once or twice a day, a long-acting insulin is administered using a pen injection device, which delivers insulin just beneath the skin into the subcutaneous tissues. This gives a relatively stable concentration of insulin throughout a 24-hour period. Additional injections of a short-acting insulin analogue are given every time ≥ 10 g of carbohydrates is consumed or when blood glucose concentrations rise above an acceptable level.
The term ‘CSII’ refers to the use of a small pump that infuses insulin, via a fine catheter and needle, to the subcutaneous tissues just under the skin. Using this technology, there is the potential to deliver insulin with a more physiological profile than can be achieved by treatment with MDI. Background insulin infusion rates can be increased and decreased to mimic the normal diurnal patterns of insulin secretion and physiological responses to exercise, illness, fasting, etc. Insulin boluses are infused by patients when > 5 g of carbohydrates is consumed or when blood glucose concentrations increase above an acceptable level. It seems logical to assume that the more physiological profile of insulin that can be achieved from CSII, together with a markedly reduced need to self-inject, would result in improved glycaemic control, reduced frequency and severity of hypoglycaemic events and improved QoL.
In 2008, a National Institute for Health and Care Excellence (NICE) guideline4 recommended that children aged ≥ 12 years who cannot achieve glycosylated haemoglobin (HbA1c) concentrations of < 8.5% without disabling low blood glucose concentrations (hypoglycaemia), despite a high level of care, should be offered a trial of CSII. Children aged < 12 years should be offered CSII therapy from diagnosis of T1D if MDI is considered impractical or inappropriate. Data from 28,400 paediatric patients were submitted to the 2015/16 National Paediatric Diabetes Audit (NPDA),1 of whom 30% were treated with CSII, compared with 23% in the previous year. Of the 18,500 patients aged < 14 years, most would fulfil the age criteria for CSII therapy, and data reporting HbA1c concentrations suggest that many of the older children would also qualify for treatment on the basis of poor glycaemic control.
Glycaemic control, intensive insulin therapy and complications of diabetes
In the short term, failure to manage T1D adequately may result in episodes of hypoglycaemia that may result in seizures in the most extreme circumstances or high blood glucose concentrations (hyperglycaemia), which may result in life-threatening episodes of diabetic ketoacidosis (DKA) if left untreated. However, it is the long-term vascular complications of T1D that have the greatest impact on morbidity, mortality and well-being. These can be broadly classified as macrovascular complications (coronary artery disease, peripheral arterial disease and stroke) and microvascular complications (diabetic nephropathy, neuropathy and retinopathy).
The risk of acquiring these complications of T1D is directly related to glycaemic control, duration of T1D, insulin sensitivity and weight. By the time patients enter adult health-care services, many will have lived with T1D for ≥ 10 years, including a period of pubertal growth and development when changes in lifestyle, relationships within families and with peers, normal risk-taking behaviour and physiological insulin resistance make glycaemic control particularly challenging.
In 1993, The Diabetes Control and Complications Research Group3 published conclusive evidence that both the degree and duration of hyperglycaemia are critical determinants of the risk of both microvascular complications and macrovascular complications. The median HbA1c concentration was lower in patients treated intensively with either MDI or CSII (7.3%) than in patients treated using conventional insulin regimes (9.1%). In patients with established T1D who were observed over a period of 6.5 years, the risk of acquiring retinopathy or nephropathy was reduced by 76% and 39%, respectively, and of having a cardiovascular event was reduced by 41% in those treated with CSII or MDI. Glycaemic control exerted greatest influence on acquisition or progression of complications; however, following correction for the HbA1c concentration, those patients who were treated with intensive regimes demonstrated a persistent benefit.
Data from historical paediatric cohorts have confirmed that the link between glycaemic control and microvascular complications is established in childhood. 5 Furthermore, there is a strong relationship between improved glycaemic control in children and young people and a fall over time in the prevalence of childhood-onset retinopathy and nephropathy. 6
Glycaemic control in the first year after diagnosis has a long-lasting effect on glycaemic control in later life and, independently of glycaemic control, on the prevalence of complications. Two British, single-centre, retrospective studies, together reporting data from > 300 patients, reported that HbA1c concentration at 6 months following diagnosis of T1D was an independent predictor of long-term glycaemic control up to 10 years later. 7,8 In a larger Swedish cohort of > 1550 children, HbA1c concentrations at 3–15 months following diagnosis was related to poorer glycaemic control and increased risk of microalbuminaemia and retinopathy in young adult life. 9 These data highlight the importance of researching interventions that may influence glycaemic control during the critical first year of diagnosis.
Data relating to glycaemic control in childhood and macrovascular complications in adult life are sparse because of the long natural history of the disease. However, markers of an increased risk of cardiovascular complications have been validated and used to identify modifiable risk factors in childhood and adolescence. The SEARCH for Diabetes in Youth (SEARCH) study10 has produced evidence that T1D affects (increases) arterial stiffness, a validated measure of risk for cardiovascular events and mortality in adult life, by late adolescence and early adult life. When data from a mixed cohort of patients with T1D and healthy control participants were analysed using multivariate analyses, other correlates of increased arterial stiffness included adiposity, blood pressure, lipid profile, ethnicity (black and hispanic ethnicities), blood pressure and microalbuminuria. In addition to the long-term vascular complications of poor glycaemic control, patients are subject to a number of health and social disadvantages during childhood. Hospital admission rates are nearly five times higher for children with T1D than for the background population, with the poorest children and those in the youngest age group being most likely to require admission. 11 Frequent episodes of severe hypoglycaemia and sustained hyperglycaemia have been associated with changes on functional brain magnetic resonance imaging, impaired memory and poorer cognitive outcomes. 12,13 Patients with poor glycaemic control may experience poor growth, particularly during puberty,14 and poor glycaemic control has also been associated with an increased risk of childhood depression and use of antidepressants. 15
Prevalence of vascular complications of type 1 diabetes
In the UK, data describing patient demographics, insulin treatment regimes, compliance with NICE guidelines and clinical outcomes are collected in the NPDA. In the 2015/16 NPDA report,1 9.7% of patients had microalbuminuria, an early marker of evolving diabetic nephropathy, and 13.8% had an abnormality on screening for retinopathy. Nearly one-third of patients were hypertensive, 20% had elevated blood cholesterol and 21% of those aged ≥ 12 years were obese. In a quantitative epidemiological systematic review of data from young adults (aged 18–30 years),16 there was some evidence of diabetic retinopathy in 50% of patients, with more severe features presenting in 10% of patients. In the same population, one in six patients had evidence of evolving diabetic nephropathy and half were hypertensive. 16
The data for adult patients for 2014/15 have yet to be reported in the National Diabetes Audit. However, the 2012/13 audit17 reported that, compared with the background population, adults with T1D are 139% more likely to be admitted to hospital with angina, 94% more likely to be admitted to hospital with myocardial infarction, 126% more likely to be admitted to hospital with heart failure, 63% more likely to be admitted to hospital with a stroke, 400% more likely to be admitted to hospital for a major amputation, 817% more likely to be admitted to hospital for a minor amputation and 272% more likely to be admitted to hospital for renal replacement therapy.
There can be little doubt that the burden of T1D complications has a profound impact on the QoL and career prospects of patients. In a study of 91 young adults in their mid-thirties who had lived with T1D for nearly 30 years,18 the mortality rate was 10 times higher than in a control population, owing to diabetic nephropathy and trauma, including suicide. People with T1D were less likely to be employed and more likely to need social welfare. Long-term complications of T1D were the primary predictor of an adverse outcome, with patients with T1D and no complications showing no significant differences from the control population. 18
Treatment of childhood type 1 diabetes and glycaemic control in the NHS
In 2015/16, the NPDA reported data from approximately 28,400 patients aged < 19 years. 1 Intensive insulin regimes were used by 82% of patients: 54% were treated with MDI, having four or more insulin injections a day, and 28% were treated with CSII, an increase from 14% in 2011.
The NPDA also reports trends of improving glycaemic control over time, with the number of patients achieving the target HbA1c concentration of < 58 mmol/mol, rising from 15.8% in 2012/13 to 27.0% in 2015/16. In the current NICE guideline for the treatment of childhood T1D, the target HbA1c concentration has been reduced to 6.5% (48 mmol/mol). 19
The Paediatric Diabetes Best Practice Tariff Criteria20 were introduced in the UK in 2012. These allow for a payment of £3189 per patient per year, linked to the provision of core clinical services for paediatric T1D care. For most children’s diabetes services, the additional funding made available by the Paediatric Diabetes Best Practice Tariff Criteria20 enabled the recruitment of more specialist staff and the relative impact of this increase in resources compared with the introduction of CSII therapy on the improvement in glycaemic control reported in the NPDA is unknown.
National databases of children and young people with T1D are maintained in a number of other countries worldwide, enabling clinical outcomes to be compared between countries. In general, data from the UK compare poorly with those from other countries: a publication reported data from 201221 that were held in the NPDA data set, the Prospective Diabetes Follow-up Registry (DPV) from Germany and Austria, and the T1D Exchange database (T1D Exchange) from the USA. The data showed that glycaemic control was the poorest in the NPDA, which also had the lowest rate of CSII usage. In Britain, the disadvantage of poor glycaemic control during childhood appears to persist into adult life. When HbA1c concentrations were compared between national registries, reporting data from all age groups, from the UK, Germany, Denmark, Latvia and Austria, only Latvian patients had poorer glycaemic control. 22
Evidence for the use of continuous subcutaneous insulin infusion in childhood
Evidence for CSII therapy comes from three areas: single-centre observational studies, national database reports and randomised controlled trials (RCTs).
Single-centre observational studies
A number of observational studies examining the effect of CSII therapy on glycaemic control have been published. 23–33 In these studies, authors report longitudinal data examining within-patient change in HbA1c concentrations following the introduction of CSII or cross-sectional data comparing glycaemic control in patients treated with MDI with those treated with CSII. In general, the introduction of CSII therapy is associated with an improvement in glycaemic control in longitudinal observational studies,23–27,34 coupled with a reduction in the number of severe hypoglycaemic episodes23–25 and, in some studies, weight loss,25,26 a reduction in insulin requirements26,27 and an improvement in QoL. 23,24 Other authors have reported a transient improvement in glycaemic control, with a return to baseline values 6 months after the start of CSII therapy. 28 Transient improvements in markers of vascular function, blood pressure, HbA1c concentration and glycaemic variability have also been reported 3 weeks after the introduction of CSII; however, at 12 months these measurements returned to baseline values and HbA1c concentrations deteriorated. 29
Cross-sectional studies report a less favourable effect of CSII on glycaemic control and QoL. 30,31,35 This difference may be explained by the fact that longitudinal studies followed patients who were changing from MDI to CSII, presumably because treatment outcomes with MDI were unsatisfactory, whereas those who were treated with MDI in cross-sectional studies are more likely to be satisfied with their treatment and to have acceptable glycaemic control.
Reduced glycaemic variability, but not HbA1c concentration was reported in a cross-sectional study of 22 children treated with CSII compared with 26 children treated with MDI. 32 Insulin requirements and high-density lipoprotein cholesterol levels were also lower in those treated with CSII. Adolescents with established T1D treated with CSII for > 12 months have been reported to have reduced glycaemic variability and a reduction in the prevalence of microvascular disease compared with those treated with MDI. 33
National database reports
An association between superior glycaemic control and CSII treatment has been reported in the DPV, T1D Exchange and NPDA data sets. 36 Higher rates of CSII use are reported in girls, whereas those from ethnic minorities and the most deprived patients are least likely to use CSII, even in health-care settings with universal funding. 37–39 In these studies, lower socioeconomic status and ethnicity were also predictors of poorer glycaemic control, even when the effect of CSII use was accounted for. This demonstrated that the characteristics of those who use CSII are the same as the characteristics that facilitate good glycaemic control, raising important issues of bias.
Randomised controlled trials
A number of small RCTs have been undertaken in children and young people,2,7,35,40–48 and these are summarised in Table 1. A meta-analysis49 of RCTs investigating the outcomes of children with T1D treated with CSII compared with those treated with MDI assessed six small studies. 34,35,44–47 The largest number of children included in a single study was 32. In total, 165 children and young people were included in these six studies, of which three included pre-school children only, and 78 children in total were aged > 5 years. In general, the period of observation was brief: ≤ 7 months in five studies. A statistically significant difference in HbA1c concentrations between children treated with CSII and MDI was demonstrated at 3 months, but this was modest [–0.24%, 95% confidence interval (CI) –0.41% to –0.07%; p < 0.001]. The insulin dose, reported in three out of the six studies, was significantly lower in children treated with CSII [0.22 international units (IU)/kg/day, 95% CI 0.31 to 0.14 IU/kg/day; p < 0.001], whereas the incidences of ketoacidosis and severe hypoglycaemic events did not differ.
| Study design | Observation period | Baseline characteristics | Between-treatment findings at study completion | |||
|---|---|---|---|---|---|---|
| n | Age (years)a | HbA1c (%)a | Duration of T1D (years)a | |||
| Crossover RCT40 (2003) | 6 months for each arm | 16 | Median: 14.2 (range 14.1–17.5) | CSII: 8.6 ± 0.8 | Not reported |
No difference in HbA1c concentrations or severe hypoglycaemia CSII: greater treatment satisfaction |
| MDI: 8.5 ± 1.4 | ||||||
| Parallel RCT35 (2004) | 16 weeks | 32 | CSII: 12.5 ± 3.2 | CSII: 8.2 ± 1.1 | CSII: 6.8 ± 3.8 |
HbA1c: 7.2% (CSII) vs. 8.1% (MDI); p < 0.05 Insulin doses: 0.9 unit/kg/day (CSII) vs. 1.2 unit/kg/day (MDI); p < 0.003 No difference in QoL |
| MDI: 13.0 ± 2.9 | MDI: 8.1 ± 1.2 | MDI: 5.6 ± 4.0 | ||||
| Parallel RCT41 (1982) | 6 days | 16 | CSII: 13.3 ± 4.0 | CSII: 10.5 ± 2.9 | Not reported |
No significant difference in blood glucose (mean of seven samples/day) or 24-hour glycosuria MDI arm: more frequent morning hypoglycaemia |
| MDI: 12.9 ± 2.4 | MDI: 10.7 ± 1.1 | |||||
| Parallel RCT42 (2008) | CSII: 10.5 months | 38 | CSII: 10.0 ± 3.0 | CSII: 8.3 ± 0.8 | CSII: 5.6 ± 3.3 | No significant difference in QoL or glycaemic control |
| MDI: 3.5 months, then 7 months for CSII | MDI: 10.0 ± 3.7 | MDI: 8.4 ± 1.1 | MDI: 4.7 ± 2.9 | |||
| Parallel RCT43 (2008) | 2 years | 72 | CSII: 11.8 ± 4.9 | CSII: 8.2 ± 0.4 | CSII: 12.2 ± 2.0 (number of days between diagnosis and entering the trial) |
No difference in HbA1c concentrations or severe hypoglycaemia Insulin doses: 0.7 unit/kg/day (CSII) vs. 1.1 unit/kg/day (MDI); p = 0.001 CSII: greater treatment satisfaction |
| MDI: 12.3 ± 4.5 | MDI: 8.4 ± 0.5 | MDI: 10.4 ± 1.7 (between diagnosis and entering the trial) | ||||
| Crossover RCT43 (2004) | 3.5 months for each arm | 23 | Arm A: 11.9 ± 1.4 | Arm A: 8.0 ± 1.1 | Arm A: 5.3 ± 1.9 |
No significant difference in HbA1c concentrations severe hypoglycaemia, DKA or QoL CSII: greater treatment satisfaction |
| Arm B: 11.9 ± 1.5 | Arm B: 8.3 ± 0.7 | Arm B: 6.3 ± 2.6 | ||||
| Parallel RCT44 (2004) | 6 months | 42 | CSII: 3.8 ± 0.8 | CSII: 9.0 ± 0.6 | CSII: 1.8 ± 0.6 | No difference in HbA1c concentrations or severe hypoglycaemia |
| MDI: 3.7 ± 0.7 | MDI: 9.0 ± 0.6 | MDI: 1.8 ± 0.6 | ||||
| Parallel RCT45 (2005) | 24 weeks | 26 | CSII: 3.9 ± 0.4 | CSII: 7.4 ± 0.5 | CSII: 1.2 ± 0.3 | No difference in HbA1c concentrations or severe hypoglycaemia |
| MDI: 3.8 ± 0.4 | MDI: 7.6 ± 0.3 | MDI: 1.6 ± 0.3 | CSII: fathers reported greater improvement in QoL | |||
| Crossover RCT46 (2003) | 3.5 months for each arm | 23 | Median: 11.9 (range 9.3–13.3) | Median: 8.9 (range 6.1–10.1) | Median: 6.0 (range 2.5–11.0) |
No difference in HbA1c concentrations Continuous glucose monitoring during CSII: greater duration target range, less time in hypoglycaemic range, more frequent hyperglycaemic readings |
| Parallel RCT47 (2005) | 12 months | 22 | 3.6 ± 1.0 | 8.0 ± 0.8 | 1.4 ± 0.6 | No difference in HbA1c concentrations QoL, episodes of severe hypoglycaemia or DKA |
| Parallel RCT48 (2009) |
CSII: 12 months MDI: 6 months for then 6 months for CSII |
35 | 3.7 ± 0.8b | 8.9 ± 0.6a | 1.6 ± 0.6a | No difference in HbA1c concentrations neurocognitive or parenting stress parameters |
In summary, there is no conclusive evidence that treatment with CSII is or is not superior to MDI for glycaemic control or other clinical outcomes. This was in part attributable to the small number of patients studied, short observation periods and issues of bias.
Acceptability of continuous subcutaneous insulin infusion and multiple daily injections
The success of treatment with either MDI or CSII is likely to be heavily influenced by patients’ and parents’ views on the acceptability of each treatment modality. Studies examining this aspect of treatment are very limited. Data reporting parental stress and QoL in studies comparing clinical outcomes during treatment with MDI and CSII are summarised in Table 1.
Discontinuation rates for CSII are reported to be < 20%, implying a high level of satisfaction with CSII therapy. 50–52 Older children, girls and those with poor glycaemic control are more likely to revert to MDI. Reasons for discontinuing CSII therapy include greater sense of disease, difficulties in doing sports, poorer sense of well-being during CSII therapy, wearing an insulin pump, embarrassment, pain at the site of needle insertion, poor glycaemic control, fear and dislike of frequent blood glucose monitoring. 53–55
Qualitative work undertaken in a study of 19 parents with children aged < 12 years who had changed from MDI to CSII reported the benefits of CSII therapy to include no longer having to administer painful injections, fewer dietary restrictions, improved quality of family life and improved glycaemic control. 56 When patients and parents were interviewed in another study, they also reported improved glycaemic control, in addition to increased lifestyle flexibility and participation in social activities, during CSII therapy. 57 However, parents also reported increased demands on them during CSII therapy, primarily relating to increased blood glucose monitoring. These data give only a limited insight into the acceptability of either treatment. It is likely that patients who participated in these studies changed from MDI to CSII treatment because MDI treatment was unsatisfactory, and studies of unselected cohorts of patients are required to examine acceptability of both treatments.
Costs of treatment of childhood type 1 diabetes and its complications
The cost of T1D to the NHS in the UK is significant: estimates of expenditure range from £1B to £1.8B per year,58,59 and this is expected to rise further, and is projected to account for 2% of total NHS expenditure over the next two decades. 58 The proportion of this cost that is attributable to paediatric T1D services and the budgetary implications of switching patients to CSII is unknown. The number of children and young people aged < 19 years old in the UK with a diagnosis of diabetes is 31,500;60 however, this may be a conservative estimate as not all children aged > 15 years old are managed in paediatric care settings and the true prevalence could be as high as 42,000. The majority of paediatric patients (95.1%) have T1D60 requiring insulin therapy, which is currently administered by CSII in 24% of 10- to 14-year-olds and by MDI in 67%, with the remainder on mixed-methods treatment (three injections per day or fewer). 61 Based on published estimates of MDI and CSII costs62 and the modelled costs of mixed insulins,19 the current annual treatment costs for children and young people with T1D range from £52M to £70M. This would rise to between £79M and £106M if all were converted to using CSII. Additional costs relating to the management of T1D and its complications may be more than three times higher than treatment costs alone,58 suggesting total current annual costs of between £179M and £241M, rising to between £272M and £366M if all patients used CSII.
In 2011, the year that the SubCutaneous Insulin: Pumps or Injections? (SCIPI) study opened to recruitment, it was also estimated that patients with T1D took 830,000 sickness days from work at a cost of £94M, and additional costs associated with T1D during work were > £91M. Premature death accounted for a further 37,000 lost working years, at an estimated cost of £0.6B. 58
It is clear from these observations that T1D represents a significant threat to the well-being and life expectancy of affected patients, a significant cost to society in loss of productivity and economic output and an increasing threat to the NHS as its prevalence continues to rise, particularly in the youngest patients, who will require NHS treatment for the longest period of time. It is critical, therefore, to identify and manage factors that may influence the natural history of the disease and the risk of complications.
Rationale for research
The role of intensive insulin therapy in optimising glycaemic control and thereby reducing the risk of vascular complications of T1D is unquestioned. The optimal way in which to achieve this and the cost-effectiveness of the tools currently available are unknown. A number of observational studies and small RCTs have compared the outcomes of children and young people treated with CSII with those treated with MDI. However, in studies reported to date, there are concerns relating to bias, small patient numbers and short observation periods, and no RCT is directly applicable to the health-care environment in the UK.
Aims and objectives
The SCIPI study is a pragmatic RCT that compares the outcomes of infants, children and young people treated with CSII with those treated with MDI from the time of diagnosis of T1D. The study protocol was developed during a period in which children’s diabetes care in the UK was changing rapidly with the widespread introduction of CSII.
The aim of the SCIPI study was to provide robust clinical data, together with a careful health economics appraisal, to inform the place of CSII therapy in the treatment of individual patients from diagnosis of T1D, and within national diabetes treatment strategies. This study compared CSII with MDI during infancy, childhood and adolescence to identify which treatment facilitates superior glycaemic control and to examine the impact that treatment modalities have on other predictors of vascular complications of T1D, adverse events (AEs) and QoL.
The study was designed with an internal pilot prior to progression to the full study. The objectives of the pilot and full study are detailed in the following sections.
Internal pilot study
Primary objective
-
To acquire an understanding of the acceptability of randomisation to MDI or CSII at diagnosis of T1D in children and young people.
Secondary objectives
-
To define the characteristics of patients who consent and those who do not consent to randomisation.
-
To generate data to confirm the size of the standard deviation (SD) used in the sample size calculation of the full study.
Full study
Primary objective
-
To compare glycaemic control, assessed by HbA1c concentrations 12 months after diagnosis of T1D in infants, children and adolescents receiving CSII with those receiving MDI.
Secondary objectives
To compare the following outcomes in children and adolescents receiving CSII with those receiving MDI:
-
clinical effectiveness
-
safety
-
growth
-
quality of life
-
cost-effectiveness.
Summary
Type 1 diabetes is a common disease of childhood, associated with complex treatment regimens and lifelong complications that pose a significant burden to individual patients. The cost to society and the NHS is also significant, as patients may not fulfil their potential in the workplace, may be more dependent on state assistance and may need intensive and expensive therapies throughout their lifetime.
There is the potential to modify the disease trajectory of patients by optimising treatment, particularly in children who will live with T1D for many years. Data from national registries suggest that children in the UK have less satisfactory glycaemic control than those in other health economies in which access to health care is also unrestricted. Some researchers have linked improved glycaemic control to higher rates of CSII use; however, the characteristics of those who are treated with CSII are the same as those that favour good glycaemic control, and the relative contribution of these factors is unclear.
Chapter 2 Trial design and methods
In developing the SCIPI study protocol, we aimed to address areas of potential bias identified in previous studies, to recruit a population of patients that was sufficiently large to enable us to be able to report, with confidence, differences in glycaemic control between treatment groups and to study secondary outcome measures that should give some insight into the evolving risk profile for macrovascular complications. A key part of the SCIPI study is the health economics assessment that examines how the additional investment in CSII may be offset against long-term health costs and QoL; this is reported separately in Chapter 4. To ensure acceptability of the study design and protocol across clinics in the UK, the study protocol was written in close collaboration with the Endocrinology and Diabetes Clinical Study Group of the Medicines for Children Research Network (MCRN). While developing the study protocol, we aimed to address three critical issues, discussed in the following sections.
Measures taken to address bias in patient populations
Previous RCTs have recruited patients with established T1D who are treated with MDI and randomised them to continue with treatment with MDI or to change to CSII. This recruitment strategy may introduce bias in favour of CSII, as patients who are satisfied with treatment with MDI and who have good glycaemic control are less likely to be approached or to participate in a study than those in whom treatment is less satisfactory. Furthermore, previous experience of treatment success or failure is likely to have an impact on the use of a treatment in the future. In order to address this potential bias, we recruited patients from the time of diagnosis of T1D.
We continued to address the internal pilot study objectives throughout the full study, by maintaining screening logs documenting patients’ demographic data (age, sex, ethnicity and deprivation score derived from their postcode) and the reasons why patients were ineligible, why eligible patients were not approached to participate and why they declined. This enabled us to identify differences in the characteristics of those who consented to participate and those who declined and also to identify major barriers to recruitment.
Measures taken to address potential bias in treatment arms
There are two key areas of concern that were addressed within the SCIPI study design that could otherwise lead to bias between treatment arms.
Education
At the introduction of CSII therapy, there is a period of intensive education in which patients and their families consolidate their understanding of the relationship between insulin, carbohydrate consumption, exercise and periods of ill health. Intensive blood glucose monitoring is undertaken to determine the distribution of insulin infused across 24 hours, together with planned increases and decreases in infusion rates to account for periods of illness, exercise, etc.
In previous studies, it was not possible to determine whether the beneficial effect of CSII on glycaemic control was because of the method of insulin delivery or because of the period of intensive education and insulin dose titration. To address this educational imbalance, the SCIPI study protocol was designed such that both treatment arms were required to receive core diabetes education, as defined by the International Society for Pediatric and Adolescent Diabetes. 63 We then defined a minimum set of contacts between members of the diabetes team, patients and families in the period following diagnosis, and documented how long additional episodes of education or advice lasted. This information was included in the health economics assessment and used to detect imbalance in education and support across treatment arms.
Glucometers
The glucometers provided with insulin pumps advise patients of the dose of insulin required for a given quantity of carbohydrate, accounting for the patient’s blood glucose concentration measured at that time. If blood glucose is measured when carbohydrates are not going to be consumed, and the blood glucose reading is high, the glucometer prompts the patient to take an additional dose of insulin, calculated to return the blood glucose to the target range.
At the time that the SCIPI study opened to recruitment, glucometers issued routinely to patients treated with MDI did not advise on insulin doses, placing them at risk of miscalculating doses or failing to recognise when additional doses may be required. In the SCIPI study protocol, we addressed this potential source of bias by ensuring that both treatment arms used the same F. Hoffman-La Roche AG’s Expert glucometer (Basel, Switzerland).
Measures taken to ensure generalisability of study findings
It was important to generate data that could be used to inform local and national treatment strategies. To achieve this, the SCIPI study needed to recruit and retain participants who were representative of children and young people treated in the NHS. This required that the protocol be deliverable in a wide range of clinics and patient populations, including large clinics in university hospitals and smaller clinics in district general hospitals. To ensure a high retention rate, clinic, participant and parent acceptability needed to be high. The trial design aimed to achieve minimal disruption to normal clinic routines and appointments by drawing on clinical information collected routinely, ensuring that study visits coincided with the times of routine clinic appointments and including minimal additional tasks for patients and their carers.
Study design
The SCIPI study was designed as a pragmatic, open-label two-arm multicentre RCT comparing use of CSII with use of MDI in children and young people aged 7 months to 15 years who were newly diagnosed with T1D. The study included an internal pilot with a sample size of 30 participants to estimate the rate of consent to randomisation and to define the characteristics of recruited participants.
Progression from the internal pilot to the full study was based on the following criteria:
-
At least 50% of patients who are eligible and are invited to participate in the pilot study are successfully recruited.
-
Demographic characteristics that are significantly associated with glycaemic control (age, ethnicity, sex, deprivation score) are not significantly different in the group of patients who are recruited compared with those who decline.
The trial protocol has been published previously. 64 A schematic representation of the study design is given in Figure 1.
FIGURE 1.
Study design. HUI2, Health Utilities Index Mark 2; PedsQL, Pediatric Quality of Life Inventory.

Ethics approval and research governance
This trial compared alternative methods of insulin delivery via Conformité Européenne (CE)-marked medical devices employed for their intended purpose; therefore, this trial was not considered to be a clinical investigation under The Medical Devices Regulations 2002. 65 The trial did fall within the remit of the European Union Directive 2001/20/EC,66 transposed into UK law as Medicines for Human Use (Clinical Trials) Regulations 200467 as amended.
The protocol was approved by the Liverpool East Research Ethics Committee (reference number 10/H1002/80) and the Medicines and Healthcare products Regulatory Agency (clinical trial authorisation number 21362/0002/001-0001). The trial was funded by the National Institute for Health Research Health Technology Assessment programme (08/14/39) and included on the International Standard Randomised Controlled Trial Number registry (ISRCTN29255275) and the European Union Drug Regulating Authorities Clinical Trials database (EudraCT 2010-023792-25). Site-specific approval was obtained at all recruiting sites.
The study opened on protocol version 1.0 and the final approved version of the protocol was version 7.0. Protocol amendments are summarised in Table 2, and full details are provided in the study protocol (www.journalslibrary.nihr.ac.uk/programmes/hta/081439; accessed 15 February 2017).
| Protocol version (date) | Key amendments |
|---|---|
| 2.0 (30 March 2011) |
Inclusion criteria amendment to include patients and parents able to complete study material HbA1c samples will be collected, analysed and destroyed according to local clinical practice rather than analysis at a central laboratory Pharmacovigilance: only related SAEs and related AEs will be reported for this trial. RUSAEs related to medical devices will be reported as per user vigilance reporting |
| 3.0 (1 July 2011) |
The time period to start the randomised treatment was changed from within 3–5 days to within 10 days. The study information will be provided and the consent should occur as close to the time of diagnosis as possible, ideally between the time of diagnosis (day 0) and day 5 Timelines for providing information and approaching the patient for consent will be recorded on the screening log Internet randomisation system to be used instead of telephone, with randomisation envelopes as a backup. Up to this point all randomisations were completed using backup envelopes PedsQL (QoL) questionnaire booklets were removed from baseline and will only be administered at 6- and 12-month study visit |
| 4.0 (17 August 2012) |
Revision to the inclusion criteria, change to patients and parents able to comply with the treatment regimen and study visits Additions to exclusion criteria list: g. Known thyroid condition in a non-euthyroid state h. Known coeliac disease and unable to maintain a gluten-free diet Exclusions previously specified within the protocol but not numbered within the exclusion criteria Revision to the exclusion criteria: change to a. have a sibling with existing T1D rather than first degree relative Additional guidance and change to recruitment window period to 14 days and further guidance on patients being approached and consented as soon after diagnosis as possible HbA1c samples to be collected, analysed and destroyed at a central laboratory |
| 5.0 (21 January 2015) |
Removal of the following from the eligibility criteria: patients aged ≥ 8 years are able to comply with the treatment regimen and study visits Addition of Omnipod® (Ypsomed Ltd, Escrick, UK) as a pump that can be supplied in line with normal clinical practice Addition of text permitting use of the insulin detemir (Levemir®, Novo Nordisk Ltd, Gatwick, UK) |
| 6.0 (16 February 2016) | Update of contact members and their details |
| 7.0 (1 August 2016) |
Updated the HbA1c concentration recommendations in line with the recent NICE guidance: change to the secondary objective. HbA1c concentrations reduced from 7.5% to 6.5% and HbA1c concentrations also provided in mmol Change to secondary end point – percentage of participants in each group with HbA1c concentrations reduced from < 7.5% to < 6.5%. Added partial remission and height as end points Outcomes may be presented as mmol/mol or equivalent % |
Selection of study sites
Participants were recruited from 15 centres in England and Wales, which serve demographically diverse populations. To be eligible to participate in the study, centres had to have ≥ 10 participants treated with CSII within their clinic population and have sufficient resources to deliver the clinical aspects of the study protocol.
To inform our selection of recruiting centres, we drew on the experience of the investigators of the Delivering Early Care In Diabetes Evaluation (DECIDE) study,68 another RCT that recruited paediatric patients at diagnosis of T1D. This study was delivered across eight children’s diabetes centres. We invited the principal investigators who had recruited well to the DECIDE study to recruit patients to the SCIPI study.
Participants
The study recruited infants, children and young people who were newly diagnosed with T1D.
Inclusion criteria
Participants were considered for inclusion in the trial if they met the following criteria:
-
they have newly diagnosed T1D using standard diagnostic criteria69
-
they are aged 7 months to 15 years (inclusive)
-
parent/legal representative of the patient is willing to give consent for the study
-
parent/legal representative of the patient is able to comply with the treatment regimen and study visits.
Exclusion criteria
Participants with the following characteristics were excluded from the trial:
-
previous treatment for diabetes
-
haemoglobinopathy
-
co-existing pathology conditions likely to affect glycaemic control (e.g. cystic fibrosis)
-
psychological or psychiatric disorders (e.g. eating disorder)
-
receipt of medication likely to affect glycaemic control (e.g. systemic or high-dose topical corticosteroid) or growth hormone therapy
-
allergy to a component of insulin aspart (Novorapid®, Novo Nordisk Ltd, Gatnick, UK) or insulin glargine (Lantus®, Sanofi, Guildford, UK)
-
sibling with existing T1D
-
known thyroid condition in a non-euthyroid state
-
known coeliac disease and inability to maintain a gluten-free diet.
Recruitment procedure
At the time that the SCIPI study opened, children’s diabetes teams were notified within 72 hours of presentation of all infants, children and young people who were newly diagnosed with T1D. This possible delay in the identification of potential participants was addressed when the Paediatric Diabetes Best Practice Tariff Criteria20 were introduced in 2011/12, shortly after the study opened. It is a requirement for centres receiving this additional payment that newly diagnosed patients are discussed with a senior member of the children’s diabetes team within 24 hours of presentation, and that all new patients are seen by a member of the children’s diabetes team on the next working day. All potentially eligible participants were therefore identified promptly.
Screening
Sites maintained detailed screening logs of all patients with newly diagnosed T1D. The screening logs collected data on the number of patients who:
-
were assessed for eligibility at diagnosis of T1D
-
met the study inclusion criteria
-
were eligible at screening, were invited to consent and, subsequently, gave consent to participate
-
were eligible at screening, were invited to consent but did not consent to participate in the study (with details of the reasons why consent was not given)
-
were eligible at screening but consent was not sought (with details of the reasons for not seeking consent).
Dates and times of consent process milestones were also recorded along with the deprivation score calculated from patients’ postcodes and ethnicity. These data were used to monitor and inform the recruitment process and to fulfil the internal pilot objectives.
Informed consent
Participants and families were given verbal and written information about the study at the time of diagnosis of T1D. Patient information leaflets were developed in collaboration with the MCRN Young Person’s Advisory Group, ‘Stand Up, Speak UP!’ Three age-appropriate information leaflets for children aged < 8 years, 8–12 years and > 12 years were prepared to ensure that study information was accessible to children of all ages. A separate information sheet was prepared for parents or legal guardians.
To support recruitment, and to address issues of patient preference, the SCIPI study team also produced a video. This was aimed at patients and families interested in taking part in the study, and gave a balanced patient’s perspective of treatment with both methods of insulin delivery from some of the children, young people and their parents who had participated in the study. Four children and young people took part in the video; two were treated with MDI and two with CSII. The video was intended to be viewed after the SCIPI study had been introduced to the family by their diabetes team and the SCIPI information leaflet had been read.
The video was approved by the main research ethics committee in December 2013. The video went live on the SCIPI study website on 14 February 2014 (www.scipitrial.org.uk/families.html; accessed 15 February 2017) and was available for use at SCIPI study sites from 17 February 2014.
Consent was obtained by an appropriately trained and experienced member of the research or diabetes staff. The timing of consent was dependent on the needs and wishes of the patient and family. However, consent had to be obtained within a time frame that allowed for the randomised treatment to start within 14 days of diagnosis of T1D.
Randomisation, concealment and blinding
Once eligibility criteria were confirmed and informed consent, and assent when appropriate, had been obtained, participants were randomised to treatment with MDI or CSII, using a secure (24-hour) web-based randomisation programme controlled centrally by the MCRN division of the Clinical Trials Research Centre Clinical Trials Unit (CTU). A personal login (username and password), provided by the MCRN CTU, was required to access the randomisation system.
The randomisation code list was generated by a statistician within the MCRN CTU who was otherwise independent of the study, using random variable block sizes of 2 and 4. Participants were randomised to either MDI or CSII treatment in a ratio of 1 : 1, stratified by recruitment site and age using three bands (7 months to < 5 years, 5 years to < 12 years and ≥ 12 years).
The allocated treatment arm was communicated immediately to the patient and family and the treating health-care professionals, with the randomised treatment to start within 14 days of diagnosis.
Owing to the nature of the interventions, it was not possible to blind either the participants or clinical teams.
Treatment group allocation
Multiple daily injections
Participants randomised to treatment with MDI were treated with two insulin analogues: insulin aspart, a short-acting insulin, and insulin glargine or insulin detemir, long-acting insulin analogues. Insulin was delivered using ‘pen’ injection devices, which contain cartridges of insulin that are administered via a fine needle at the tip of the pen and injected using a plunger at the top of the pen.
Insulin aspart is a short-acting insulin analogue licensed for the treatment of T1D in adults, adolescents and children aged 2–17 years. It should be used in children aged < 2 years only under careful supervision; however, the rapid onset and offset of action of this insulin make it particularly attractive in the management of young children. For this reason, it is widely used in young children with T1D. Insulin aspart was administered every time ≥ 10 g of carbohydrates was consumed.
Insulin glargine is a long-acting insulin analogue, administered once or twice daily. It is not currently licensed for use in children aged < 6 years; however, the use of insulin glargine in this age group has been associated with a reduction in hypoglycaemia and improved metabolic control. 70 For these reasons, it is widely used in MDI treatment regimens in UK paediatric practice.
In protocol version 5.0, the use of detemir was added to the long-acting insulin analogues permitted at the initiation of MDI therapy. This amendment was made in recognition that a number of recruiting sites started treatment with MDI, using insulin detemir at the time of diagnosis, and were reluctant to change to insulin glargine following randomisation to MDI. This insulin has been studied in the age group recruited to the SCIPI study and found to be safe and effective. 71
Continuous subcutaneous insulin infusion
Insulin aspart was administered using CSII insulin pumps. The F. Hoffman-La Roche AG insulin pump (Basel, Switzerland) was selected at the start of the study, following discussion with the participating centres. This pump was used widely at the time the study opened, and participating centres had experience in its use. The use of the F. Hoffman-La Roche AG insulin pump also enabled us to use the same glucometer across both treatment arms. This glucometer includes a ‘bolus wizard’, which calculates insulin doses according to blood glucose readings and carbohydrate consumption. The use of this glucometer ensured consistency in the insulin bolus doses across treatment arms.
The F. Hoffman-La Roche AG insulin pumps and consumables were provided at a 25% discounted price for the study participants. However, this is a pragmatic study and treating clinicians were able to use other insulin pumps when this was considered to be in the patient’s best interest. A small number of Medtronic (Medtronic Ltd, Minneapolis, MI, USA) and Omnipod® pumps (Ypsomed Ltd, Escrick, UK) were also used in some study centres. In these instances, patients used the appropriate glucometer for their pump.
Participants were given insulin aspart using basal insulin infusion with bolus doses of insulin aspart when ≥ 5 g of carbohydrate was consumed.
Starting dose calculations: multiple daily injections and continuous subcutaneous insulin infusion
The initial total daily dose of insulin was calculated from body weight. In prepubertal participants, a dose of 0.5 units/kg body weight/day was used, and in pubertal participants 0.7 units/kg body weight/day was used.
For the MDI arm, 50% of the calculated dose was given as a single injection either as insulin glargine or detemir, injected into the anterior-lateral aspect of the thigh, arm, abdomen or the upper outer quadrant of the buttocks.
For the CSII arm, 50% of the calculated dose was given as a continuous 24-hour infusion [0.5 × body weight (kg) ÷ 2 ÷ 24 = hourly rate].
The remaining 50% of the daily dose was given as three divided preprandial doses at mealtimes in both arms. If the doses were not equal, more insulin was given before breakfast and the evening meal than at lunchtime to account for diurnal variation in insulin sensitivity.
It was recommended that blood glucose readings should be undertaken at least four times a day: before breakfast, before the midday meal, before the evening meal and before supper/bed.
Insulin dose modifications
Correction doses were calculated according to the ‘100’ rule. 72 Insulin doses were titrated according to home blood glucose readings, as per local routine clinical advice. The diabetes clinical team supported insulin dose titration according to participant needs. Telephone contact was also used as an opportunity to provide support and education regarding the management of T1D. The frequency and duration of all contact between participants and their local clinical service were logged. All participants and their parents had 24-hour telephone access for support and advice throughout the study, as is standard practice. Participants on CSII treatment also had 24/7 access to the pump manufacturer’s helpline for assistance for technical problems relating to the pump.
Education
At entry to the study, all participants completed a structured educational programme delivered to participants and their families in accordance with the standards of the International Society for Pediatric and Adolescent Diabetes. 63
Participants and their families were educated in:
-
type 1 diabetes
-
the use and administration of insulin
-
hyperglycaemia and correction doses
-
hypoglycaemia symptoms and treatment
-
exercise
-
sick day rules
-
carbohydrate counting
-
the benefits of maintaining optimal glycaemic control for long-term health
-
blood glucose monitoring.
All the participants were trained in the use of the MDI regimen and the F. Hoffman-La Roche AG Expert glucometer; participants undergoing CSII treatment were also trained in the use of CSII pumps.
Additional diabetic education was organised to suit individual needs of the participant and family. The dietitian met the participant and their family to assess their diet and educate them in carbohydrate counting. All contact was recorded within the participant follow-up.
To support recruiting sites in the initiation of CSII so soon after diagnosis of T1D, treatment guidelines and written patient information were available from the lead site. These resources were used at the discretion of the recruiting sites.
Baseline assessment
At entry to the study, we collected the following routine biochemical data recorded in medical case notes:
-
blood pH at diagnosis
-
blood glucose at diagnosis
-
presentation with DKA
-
glycosylated haemoglobin
-
thyroid function tests
-
tissue transglutaminase antibody titres, or other screening test for coeliac disease
-
titres of anti-glutamic acid decarboxylase and anti-islet cell antibodies.
These baseline tests were not required as part of the SCIPI study protocol, but results were collected if available.
Baseline measurements of height and weight were recorded on the day that the participants commenced randomised treatment to allow for rehydration in participants who were dehydrated at diagnosis of T1D. The Health Utilities Index (HUI) questionnaire was completed and information was collected relating to prescribed concomitant medications. The Health Utilities Index Mark 2 (HUI2) was used for the base-case analysis and the Health Utilities Index Mark 3 (HUI3) (based on a Canadian tariff) was used in the sensitivity analysis.
Follow-up
Study visits were timed to coincide with the times of routine clinic appointments at 3, 6, 9 and 12 months from the date of randomisation. A window of 15 days either side of the appointment time was permitted to allow for holidays, clinic availability and other commitments. A schematic representation of follow-up assessments is given in Table 3. At each visit, permanent and temporary changes in the delivery of insulin were recorded under the heading ‘Review of insulin use’.
| Procedures | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline (randomisation) | Follow-up (months after randomisation) | |||||
| Diagnosis | Prior to start of treatment | 3 | 6 | 9 | 12 | |
| Assessment of eligibility criteria | ✗ | |||||
| Signed consent form | ✗a | |||||
| Randomisation | ✗ | |||||
| Review of concomitant medications | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Review of insulin use (insulin requirements) | ||||||
| Treatment diaries | ✗ | ✗ | ✗ | ✗ | ||
| Prescriptions | ✗ | ✗ | ✗ | ✗ | ||
| CSII pumps download | ✗ | ✗ | ✗ | ✗ | ||
| Blood glucose measurement | ✗b | ✗ | ✗ | ✗ | ✗ | |
| Blood pH measurement | ✗ | |||||
| Demographics | ✗ | |||||
| Study intervention | ✗c | |||||
| Physical examination | ||||||
| Height | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Weight | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Injection sites | ✗ | ✗ | ✗ | ✗ | ||
| Symptom-directed | (✗) | (✗) | (✗) | (✗) | ||
| Assessment of related AEs | ||||||
| Incidence of severe hypoglycaemia | (✗) | (✗) | (✗) | (✗) | ||
| Incidence of DKA | (✗) | (✗) | (✗) | (✗) | ||
| Other AEs | (✗) | (✗) | (✗) | (✗) | ||
| Related to diabetes | (✗) | (✗) | (✗) | (✗) | ||
| Clinical laboratory | ||||||
| HbA1c analysisd | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Other routine testse (e.g. chemistry, haematology, urinalysis) | (✗) | (✗) | (✗) | (✗) | (✗) | |
| Questionnaires | ||||||
| Participant-completed PedsQL | (✗) | (✗) | ||||
| Parent-completed PedsQL | ✗ | ✗ | ||||
| Participant-completed HUI Questionnairef | (✗) | (✗) | (✗) | (✗) | (✗) | |
| Parent-completed HUI Questionnairef | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Resource use | ||||||
| RN-completed CRF | ✗ | ✗ | ✗ | ✗ | ✗ | |
Measures
Primary end point
The primary outcome measure was glycaemic control (HbA1c) concentrations 12 months after diagnosis.
Capillary blood samples were collected from finger-pricks into small capillary tubes and were analysed in two separate locations. At study sites, most samples were analysed using portable instrumentation at outpatient clinics. Samples were also analysed at the clinical pathology laboratory in Alder Hey Children’s NHS Foundation Trust. Samples were transported to the central laboratory through the post or via bespoke courier systems that ensure that the samples are received the next day.
Quality assurance of the measurement of glycosylated haemoglobin levels
Portable instrumentation is calibrated regularly with local laboratories. All laboratories involved in the measurement of HbA1c concentrations are obliged to participate in external quality assurance schemes, ensuring that laboratories with equivalent equipment are able to produce results that are comparable to each other. For almost all HbA1c measurements (local and central), the biochemical methodology employed was immunoassay.
Since June 2009, HbA1c assays have been calibrated against the International Federation of Clinical Chemistry and Laboratory Medicine standardised values,73 and centralised analysis should no longer be necessary; therefore, costs to support central analysis were not provided within the study budget. However, the preference in the trial design was for central analysis and this was included when funds were identified to support this (changed from local to central analysis in protocol version 4.0, August 2012).
To determine whether or not central analysis was required, a limits of agreement analysis was performed when 590 paired samples were available. Based on the results of this analysis,74 samples continued to be analysed both locally and centrally.
Data from local samples were usually recorded in two units of measurement: mmol/mol and % (percentage of total haemoglobin). The HbA1c concentration measured at the central laboratory was recorded in mmol/mol only. The following formula was used for conversion between the two units of measurement:
Secondary end points
Percentage of participants in each group with a glycosylated haemoglobin level of < 48 mmol/mol at 12 months after diagnosis
At the time the SCIPI study protocol was written, the target HbA1c concentration was < 58 mmol/mol. However, in the most recent NICE guideline, the target has been reduced to 48 mmol/mol. 19 This lower figure was used in our analysis to ensure that the findings are relevant to current clinical practice; however, as sites would have previously worked to the 58-mmol/mol threshold, the results for this are also presented.
Incidence of severe hypoglycaemia
Severe hypoglycaemia was defined according to the criteria of the American Diabetes Association: a hypoglycaemic episode that required the assistance of another person to administer carbohydrate, glucagon or other treatments. 75
Incidence of diabetic ketoacidosis
Diabetic ketoacidosis was defined according to the criteria of the European Society of Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society:76 blood glucose level of > 11 mmol/l, with a venous pH of < 7.3 and/or bicarbonate concentration of < 15 mmol/l.
Change in body mass index standard deviation score
Height was measured using a fixed stadiometer and weight was measured using electronic scales. Body mass index (BMI) was calculated according to the formula:
Standard deviation scores (SDSs) were derived from 2007 World Health Organization growth data. 77,78
Insulin requirements (unit/kg/day)
Insulin usage was downloaded from F. Hoffman-La Roche AG’s Expert glucometers for participants in both treatment arms. For participants who were treated with CSII, insulin usage was also downloaded from insulin pumps, and for participants who were treated with MDI, data were retrieved from patient-held records. Finally, to guard against significant over-reporting and for the health economics assessment, the general practitioner (GP) of the participant was contacted for details of issued prescriptions.
Percentage of patients in partial remission at 12 months
Rates of partial remission were calculated at each time point, according to insulin dose-adjusted HbA1c (IDAA1c) level. This measure is calculated according to the formula:
Health-related quality of life
Health-related QoL was assessed using version 3 of the diabetes module of the Pediatric Quality of Life Inventory (PedsQL) questionnaire,79,80 completed at routine clinic appointments at 6 and 12 months. The questionnaire was not completed at baseline because patients and parents are asked to rate their experience of living with T1D in the previous month. Given that patients were recruited at diagnosis of T1D, these questions were not relevant.
The PedsQL has age-specific questionnaires to be completed by children (aged 5–7 years old, 8–12 years old and 13–16 years old). The parent-reported PedsQL includes an additional age band for children aged 2–4 years.
Sample size
A difference in HbA1c concentration of 0.5% is widely recognised as the threshold used by the US Food and Drug Administration and the pharmaceutical industry to determine effectiveness of any new oral hypoglycaemic agents. A meta-analysis of 20 studies comparing CSII with MDI detected an improvement of 0.61% in adults, suggesting that, in addition to this estimate being the minimum clinically important, it is also a realistic difference to detect. 81–100 To achieve 80% power, a sample size of 143 in each group was required to detect a difference in means of 0.50, assuming that the common SD is 1.50 using a two-group t-test with a 0.05 two-sided significance level. Allowing for 10% loss to follow-up increased the sample size to a total of 316 participants (158 per group). The estimate used for the SD in the sample size calculation was taken from an unpublished audit at Alder Hey Children’s NHS Foundation Trust (Joanne Blair, Mohammed Didi, Princy P, Atrayee Ghatak, Alder Hey Children’s NHS Foundation Trust, 2009, personal communication) based on children matching the inclusion criteria for this proposed study.
Statistical methods
The analysis and reporting of the SCIPI study was undertaken in accordance with the Consolidated Standards of Reporting Trials (CONSORT)101 and the International Conference on Harmonisation E9 guidelines. 102 The main features of the statistical analysis plan are included here with a full and detailed statistical analysis plan provided as a separate document [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/081439/# (accessed 26 July 2018)]. All statistical analyses were undertaken using SAS® software (version 9.2; SAS Institute Inc., Cary, NC, USA). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates US registration.
A two-sided p-value of ≤ 0.05 was used to declare statistical significance for all analyses. Similarly, all CIs were calculated at the 95% level. No adjustment for multiplicity was made to adjust the type 1 error rate for secondary outcomes. Relevant results from other studies already reported in the literature were taken into account in the interpretation of results.
The primary analysis followed the intention-to-treat (ITT) principle as far as practically possible; a secondary analysis using the per-protocol approach was conducted for the primary outcome. The purpose of the per-protocol approach is to consider the robustness of the conclusions reached from the analysis using the ITT principle, which includes protocol deviations.
Glycosylated haemoglobin, a continuous outcome, was compared between the trial groups using mixed-model regression with 12-month HbA1c as the dependent variable, treatment group as an explanatory factor and the randomisation stratification variables (age group and centre) as covariates; centre was fitted as a random effect. The mean and SD of HbA1c concentrations were reported for each age group and treatment group. The mean difference in HbA1c concentrations and 95% CI between treatment groups was given as the estimated age group- and centre-adjusted treatment effect calculated by the fitted mixed-model regression. Central measures of HbA1c concentration were used in preference to local measurements; local measurements were used if central ones were not available.
Secondary continuous outcomes were analysed as per the primary outcome methods. For binary outcomes, the number and percentage of participants with the outcome are reported overall and for each treatment group. The difference between groups is tested using the chi-squared test. Relative risks with 95% CI are presented.
The safety analysis data set contains all participants who were randomised and received at least one dose of insulin via the randomised treatment. Participants’ AEs/serious adverse events (SAEs) were included in the treatment group the participant was actually receiving at the time of AE/SAE onset to take into account any participants who permanently changed their mode of insulin delivery at any point during the trial. The number of related AEs occurring and the number and percentage of participants involved were reported by treatment arm. The incidence rates of total numbers of AEs/SAEs were calculated for each treatment group in person-days using the incidence density ratio (IDR). The IDR is the number of patients with at least one new AE per population at risk in a given time period. The denominator is the sum of the person-time in years for each treatment group (accounting for treatment switches) of the at-risk population.
The statistical analysis of the data follows the standard operating procedures of the Clinical Trials Research Centre, which requires independent programming of the primary outcome and safety analyses by an independent statistician.
Study oversight and role of funders
The Trial Management Group (TMG), comprising the chief investigator, other lead investigators (clinical and non-clinical), members of the MCRN CTU and three parent contributors, was responsible for the day-to-day running and management of the trial. The membership of the oversight committees was suggested by members of the TMG to the trial funders and appointed by the funders with their constitution following funder requirements.
The Trial Steering Committee (TSC) consisted of an independent chairperson, Dr Peter E Clayton, two independent experts in the fields of diabetes and endocrinology, Dr Christine P Burren and Dr Ian Craigie, an expert in medical statistics, Professor Gordon D Murray, and a parent contributor, Mrs Christina McRoe. The role of the TSC was as the executive decision-making committee considering the recommendations of the Independent Data and Safety Monitoring Committee (IDSMC). Monitoring reports viewed by the TSC were not split by treatment group.
The IDSMC consisted of an independent chairperson, Professor Stephen Greene, plus two independent members, Professor John Wilding, an expert in the field of endocrinology, and Dr Arne Ring, an expert in medical statistics. The IDSMC was responsible for reviewing and assessing recruitment, interim monitoring of safety and effectiveness, trial conduct and external data. The IDSMC provided recommendations to the TSC concerning the continuation of the trial and viewed accumulating data split by treatment group.
Chapter 3 Results
Recruitment
The study opened to recruitment on 16 May 2011 and was closed on 30 January 2017.
At the outset of the study, we planned to recruit 316 patients within 30 months across eight study sites. The recruitment rate had been informed by the DECIDE trial68 and the study sites were selected to build on the experience and expertise acquired by local researchers during the DECIDE trial.
All study sites had to secure funds to meet the excess treatment costs of the study protocol. This process incurred some considerable delays, and in some centres was not possible. Recruitment rates were also slower than expected and, consequently, the recruitment curve was revised in month 18 to allow for an additional 12 months of recruitment and again in month 40 to allow a further 6 months. The number of study sites was increased from 8 to 15. The recruitment graph showing the initial, and revised, predictions and the observed recruitment numbers is displayed in Appendix 1.
To increase recruitment, key protocol amendments were made (protocol version 3.0 to 4.0) that increased the permitted time window for recruitment from diagnosis to 14 days and softened eligibility criteria to place emphasis on ability to comply with treatment regimens rather than complete study questionnaires and to support inclusion of children with parents with T1D but to maintain exclusion of siblings with T1D.
To optimise consent rates, screening log data were used to inform the recruitment strategy. Screening data indicated that patients who consented to participate were approached sooner after diagnosis than those who declined. In the light of these data, clinical teams and research nurses (RNs) were encouraged to share information about the study as soon as possible following diagnosis.
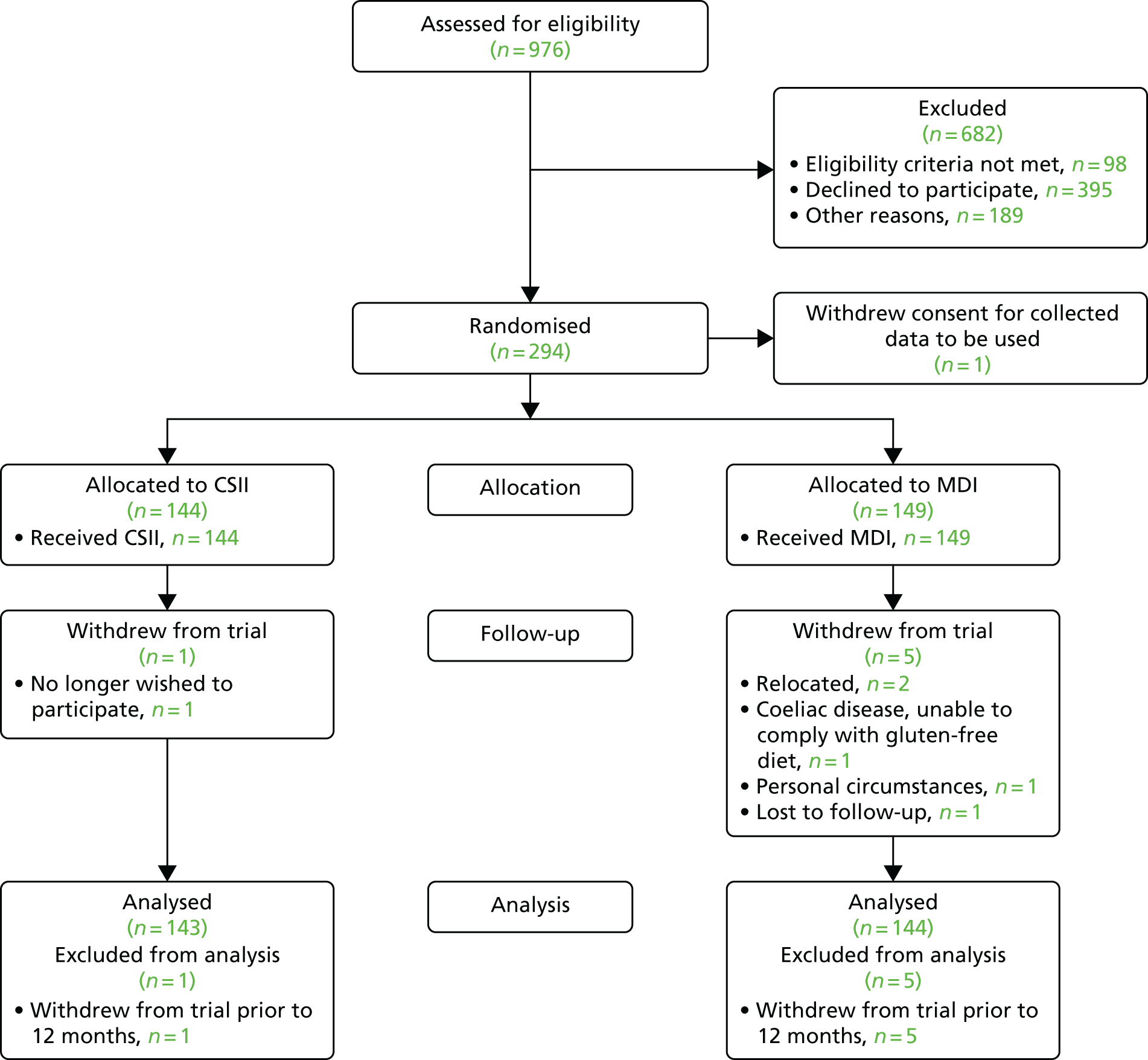
In total, 976 patients were diagnosed with T1D in the 15 study centres in England and Wales during the 48-month recruitment period, of whom 98 (10%) did not meet the study eligibility criteria. One hundred and eighty-nine patients (22%) were not approached about participation. Of these, 115 (61%) were considered to be unsuitable by treating clinicians for reasons other than the study exclusion criteria, 52 patients (28%) were not approached because staffing levels at the site were too low at the time of diagnosis to deliver the study protocol and no reason was recorded for 22 (12%). Of the remaining 689 patients, 294 (42.7%) consented to participate in the study; however, one randomised participant withdrew consent for their data to be used immediately following randomisation, leaving 293 randomised participants.
Patients and families were invited to share their reasons for declining to participate in the study. The main reason given was patient preference for one of the trial arms. Strong patient preference had been expected at the design stage, and of the 395 eligible participants who declined consent, 36 (9%) cited a preference for CSII therapy and 259 (66%) cited a preference for MDI.
In response to this strong patient preference and to support an informed decision, we made a short film in which four SCIPI study participants (two randomised to CSII and two randomised to MDI) and their parents shared their experiences of diagnosis and living with T1D and the treatment in each of the study arms. Although the film was generally well received, we did not observe any increase in recruitment rates following its introduction and the issue of patient preference persisted throughout the trial.
A CONSORT flow diagram illustrating the pathway of patients from diagnosis to consent and randomisation and through the study protocol is given in Figure 2.
FIGURE 2.
The CONSORT flow chart.
The purpose of the SCIPI study was to generate evidence that could be applied to the population of patients treated in children’s diabetes services in the NHS. For this reason, it was important to demonstrate that patient characteristics, in particular those known to be associated with glycaemic control, did not differ between those who consented and those who declined to participate. The baseline characteristics of patients who were invited to participate in the study are given in Appendix 1, Table 28. There was no difference in age, sex or ethnicity between the group of patients who consented and the group of patients who declined. The median deprivation score for those who declined because they had a strong preference for CSII was 27.7 (range 16.6–63.9), compared with 17.0 (range 1.62–77.23) for those who consented and 17.96 (range 1.18–74.35) for those who stated a strong preference for MDI.
Internal pilot
The internal pilot was planned after 30 patients had been recruited. Of the 89 eligible patients approached for consent, 30 provided consent and 59 declined (33.7% consent rate, 95% CI 23.9% to 43.5% consent rate). The first patient was randomised on 31 May 2011, and 30 patients were randomised by 3 July 2012. A review of the consent rates by recruiting centre identified lower consent rates in one centre that started patients on three daily injections at diagnosis, rather than MDI. Excluding this site, the consent rate increased (26/60 patients; 43.3%, 95% CI 30.8% to 55.9%). Screening data comparing characteristics of eligible consenting participants did not differ from those declining for age, sex, ethnicity or deprivation score. A review of the SD used in the sample size calculation to the accrued data indicated that the study should continue to the planned sample size. The recommendation of the IDSMC was that the SCIPI study should progress to the full study.
Baseline comparability
There was no difference in age, sex, ethnicity or deprivation score between treatment arms (Table 4). Baseline data also showed that auxological and biochemical characteristics did not differ between treatment groups at baseline (Table 5).
| Demographic variable | Treatment arm | Total | |
|---|---|---|---|
| CSII | MDI | ||
| Age at randomisation (years) | |||
| n (missing) | 144 (0) | 149 (0) | 293 (0) |
| Median (IQR) | 9.9 (5.7–12.2) | 9.4 (5.8–12.5) | 9.8 (5.7–12.3) |
| Minimum, maximum | 0.8, 16 | 0.7, 15.4 | 0.7, 16 |
| Age category, n (%) | |||
| n (missing) | 144 (0) | 149 (0) | 293 (0) |
| 7 months to < 5 years | 33 (22.9) | 32 (21.5) | 65 (22.2) |
| 5 years to < 12 years | 71 (49.3) | 76 (51) | 147 (50.2) |
| 12 years to 15 years | 40 (27.8) | 41 (27.5) | 81 (27.6) |
| Sex, n (%) | |||
| n (missing) | 144 (0) | 149 (0) | 293 (0) |
| Female | 71 (49.3) | 69 (46.3) | 140 (47.8) |
| Male | 73 (50.7) | 80 (53.7) | 153 (52.2) |
| Ethnicity, n (%) | |||
| n (missing) | 143 (1) | 146 (3) | 289 (4) |
| Asian or Asian British | 4 (4.2) | 7 (6.1) | 10 (5.6) |
| Black or British black | 0 (0) | 3 (2.1) | 3 (1) |
| British white | 124 (86.7) | 118 (80.8) | 242 (83.7) |
| Mixed | 4 (2.8) | 6 (4.1) | 10 (3.5) |
| Other white | 9 (6.3) | 10 (6.9) | 19 (6.0) |
| Deprivation scorea | |||
| n (missing) | 137 (7) | 143 (6) | 280 (13) |
| Median (IQR) | 19.4 (8.9–37.9) | 14.7 (7.8–31.8) | 17 (8.4–35.8) |
| Minimum, maximum | 1.8, 77.1 | 1.6, 77.2 | 1.6, 77.2 |
| Demographic variable | Treatment arm | Total | |
|---|---|---|---|
| CSII | MDI | ||
| BMI SDS | |||
| n (missing) | 124 (20) | 132 (17) | 256 (37) |
| Mean (SD) | 0.2 (1.3) | 0.1 (1.4) | 0.1 (1.3) |
| Median (IQR) | 0.2 (–0.7 to 0.9) | 0.1 (–0.8 to 1) | 0.2 (–0.8 to 1) |
| Minimum, maximum | –2.9, 4.2 | –4.6, 3.5 | –4.6, 4.2 |
| Height SDS | |||
| n (missing) | 124 (20) | 132 (17) | 256 (37) |
| Mean (SD) | 0.3 (1.1) | 0.3 (1.1) | 0.3 (1.1) |
| Median (IQR) | 0.1 (–0.4 to 1.1) | 0.4 (–0.3 to 1.1) | 0.3 (–0.4 to 1.1) |
| Minimum, maximum | –2.3, 3.3 | –4.8, 2.4 | –4.8, 3.3 |
| Local HbA1c measurement (mmol/mol) | |||
| n (missing) | 122 (22) | 122 (27) | 244 (49) |
| Mean (SD) | 105.9 (24.2) | 103.6 (26.3) | 104.7 (25.2) |
| Median (IQR) | 105 (87 to 122) | 103 (83 to 126) | 104.7 (85.5 to 125) |
| Minimum, maximum | 58, 184 | 55, 172.1 | 55, 184 |
| Central HbA1c measurement (mmol/mol) | |||
| n (missing) | 64 (80) | 71 (78) | 135 (158) |
| Mean (SD) | 101.2 (24.9) | 96.4 (24) | 98.7 (24.4) |
| Median (IQR) | 102.5 (81.5 to 127) | 93 (79 to 119) | 100 (80 to 126) |
| Minimum, maximum | 38, 130 | 50, 130 | 38, 130 |
| HbA1c concentrationa (mmol/mol) | |||
| n (missing) | 132 (12) | 131 (18) | 263 (30) |
| Mean (SD) | 104.6 (24.4) | 102.6 (26.7) | 103.6 (25.5) |
| Median (IQR) | 105 (87.5 to 127) | 103 (81 to 127) | 105 (84 to 127) |
| Minimum, maximum | 38, 184 | 50, 172.1 | 38, 184 |
| Blood glucose (mmol/l) | |||
| n (missing) | 141 (3) | 146 (3) | 287 (6) |
| Mean (SD) | 26.8 (9.2) | 26.9 (10) | 26.9 (9.6) |
| Median (IQR) | 26.2 (20.2 to 32.5) | 25.6 (19.5 to 32.6) | 25.7 (19.7 to 32.5) |
| Minimum, maximum | 5.1, 56.0 | 5.7, 69.5 | 5.1, 69.5 |
| Blood pH | |||
| n (missing) | 127 (17) | 133 (16) | 260 (33) |
| Mean (SD) | 7.3 (0.2) | 7.3 (0.1) | 7.3 (0.2) |
| Median (IQR) | 7.4 (7.3 to 7.4) | 7.4 (7.2 to 7.4) | 7.4 (7.3 to 7.4) |
| Minimum, maximum | 6, 7.6 | 6.8, 7.5 | 6, 7.6 |
The measurement of autoantibodies was determined by local protocols, with some centres testing only patients in whom the diagnosis of T1D was felt to be uncertain. Eleven patients randomised to the MDI arm tested positive for coeliac disease on antibody testing, compared with four randomised to the CSII arm.
Retention and adherence
Figure 3 summarises the time point and number of patients who made a permanent change to their randomly allocated insulin delivery method, the time point and number of patients who decided to end their follow-up in the SCIPI study and the number of patients who attended each scheduled follow-up. In total, only six participants withdrew from the SCIPI study prior to the 12-month follow-up, and all remaining 287 patients (97.0%) attended the 12-month follow-up visit. See Figure 2 for a summary of the reasons for withdrawals.
FIGURE 3.
Retention and adherence. a, Permanent change of insulin delivery from randomised treatment but continuing follow-up; b, patient changed from MDI to injections ter die sumendus (TDS) regime.

Permanent changes to insulin delivery
All participants initially received their insulin via their randomly allocated device.
Twenty-two patients (15%) randomised to CSII changed to MDI, and 31 patients (21%) randomised to MDI changed to CSII. Table 6 summarises the time point and age group of participants who switched between treatment arms.
| Age (years) | CSII to MDI | MDI to CSII | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Time point (months) | Total (%) | n | Time point (months) | Total (%) | ||||||||
| 0 | 0–3 | 3–6 | 6–9 | 9–12 | 0–3 | 3–6 | 6–9 | 9–12 | |||||
| < 5 | 33 | 1 | 0 | 0 | 0 | 0 | 1 (3.0) | 32 | 3 | 4 | 2 | 3 | 12 (37.5) |
| 5–11 | 71 | 2 | 5 | 0 | 2 | 1 | 10 (14.1) | 76 | 1 | 5 | 4 | 4 | 14 (18.4) |
| ≥ 12 | 40 | 0 | 6 | 2 | 2 | 1 | 11 (27.5) | 41 | 1 | 2 | 1 | 0 | 4 (9.8) |
| Total | 144 | 3 | 11 | 2 | 4 | 2 | 22 (15.3) | 149 | 5 | 11 | 7 | 7 | 30 (20.1) |
In the youngest age group, more than one-third of patients randomised to treatment with MDI switched to CSII, whereas only one patient (3%) switched from CSII to MDI. In the oldest age group, the converse was true, with more than twice as many participants switching from CSII to MDI; however, in the middle age group, switching between treatment arms was more balanced.
When movement between treatment arms was examined by study centre, we found two centres in which there was dominance for switch from MDI to CSII, with one centre switching four out of five patients from MDI to CSII and none from CSII to MDI. In another centre, 9 out of 16 patients randomised to MDI switched to CSII and 3 out of 14 patients randomised to CSII switched to MDI. In other centres, movement between treatment arms was more balanced (see Appendix 2, Figure 12).
Data were collected relating to the decision to switch treatment arms and a summary is displayed in Table 7, with more detailed data in Appendix 2, Table 29. The most frequently cited reasons for switching treatment arm were parent or patient preference. The majority of the participants moving from CSII to MDI had a last HbA1c measurement above the NICE recommendations applicable at the time of the switch; however, a larger proportion of those moving from MDI to CSII had HbA1c measurements within the NICE recommendations. 19
| Age (years) | N | HbA1c concentration higher than recommended guideline (n) | Decision-makers | Reasons (frequency) | |||
|---|---|---|---|---|---|---|---|
| Parent | Patient | Clinician | Frequency | ||||
| Permanent change from CSII to MDI | |||||||
| < 5 | 1 | 1 | ✗ | 1 | Parent preference (n = 1) | ||
| 5–11 | 10 | 9 | ✗ | 4 |
Patient/parent preference (n = 10) Poor concordance with treatment (n = 1) Poor control, frequent hyperglycaemia (n = 1) Pain at cannula site (n = 1) Did not want pump (n = 1) |
||
| ✗ | 1 | ||||||
| ✗ | ✗ | 3 | |||||
| ✗ | ✗ | ✗ | 1 | ||||
| ✗ | ✗ | 1 | |||||
| ≥ 12 | 10 | 9 | ✗ | 2 |
Patient/parent preference (n = 10) Poor concordance with treatment (n = 1) Poor control, frequent hyperglycaemia (n = 1) |
||
| ✗ | ✗ | 4 | |||||
| ✗ | ✗ | ✗ | 4 | ||||
| ✗ | ✗ | 1 | |||||
| Permanent change from MDI to CSII | |||||||
| < 5 | 12 | 10 | ✗ | 2 |
Age related (n = 2) Patient/parent preference (n = 7) Poor control, frequent hyperglycaemia (n = 5) Bruising at injection site (n = 1) |
||
| ✗ | 2 | ||||||
| ✗ | ✗ | 8 | |||||
| 5–11 | 14 | 5 | ✗ | 2 |
Patient/parent preference (n = 13) Poor concordance with treatment (n = 2) Poor control, frequent hyperglycaemia (n = 1) |
||
| ✗ | ✗ | 2 | |||||
| ✗ | ✗ | ✗ | 9 | ||||
| ✗ | ✗ | 1 | |||||
| ≥ 12 | 4 | 1 | ✗ | 1 | Patient/parent preference (n = 4) | ||
| ✗ | ✗ | 1 | |||||
| ✗ | ✗ | ✗ | 1 | ||||
| ✗ | ✗ | 1 | |||||
| Permanent change from MDI to injections TDS regime | |||||||
| 5–11 | 1 | 1 | ✗ | ✗ | 1 | Patient/parent preference (n = 4) | |
Protocol deviations
Protocol deviations are summarised in Table 8. The most common major protocol deviation was the 12-month visit taking place outside the preferred time window. The median number of days outside the visit window was 2.0 [interquartile range (IQR) –5 to 10 days] and 2.5 (IQR –7 to 9.5 days) for the CSII and MDI arms, respectively.
| Protocol deviations | Treatment arm, n (%) | Overall (N = 293), n (%) | |
|---|---|---|---|
| CSII (N = 144) | MDI (N = 149) | ||
| Relating to inclusion and exclusion criteria | 0 (0) | 0 (0) | 0 (0) |
| Relating to treatment and follow-up visits | |||
| At least one major | 57 (39.6) | 77 (51.7) | 134 (45.7) |
| Start of study treatment from diagnosis being > 10 days (protocol version 3.0) | 1 (0.7) | 1 (0.7) | 2 (0.7) |
| Start of study treatment from diagnosis being > 14 days (protocol version 4.0) | 1 (0.7) | 3 (2) | 4 (1.4) |
| Scheduled 12-month follow-up visit falling outside the ± 15-day window | 36 (25) | 49 (32.9) | 85 (29) |
| Permanent change of insulin delivery | 22 (15.3) | 31a (20.8) | 53a (18.1) |
| Usage of non-protocol-specified insulinb | 10 (6.9) | 13 (8.7) | 23 (7.8) |
| At least one minor | 70 (48.6) | 87 (58.4) | 157 (53.6) |
| At least three minor | 0 (0) | 0 (0) | 0 (0) |
| Scheduled 3-month follow-up visit falling outside the ± 15-day window | 34 (23.6) | 44 (29.5) | 78 (26.6) |
| Scheduled 6-month follow-up visit falling outside the ± 15-day window | 43 (29.9) | 48 (32.2) | 91 (31.1) |
| Scheduled 9-month follow-up visit falling outside the ± 15-day window | 44 (30.6) | 39 (26.2) | 83 (28.3) |
| At least one major and/or at least three minor | 57 (39.6) | 77 (51.7) | 134 (45.7) |
Primary outcome: glycosylated haemoglobin measured at 12 months
Data on HbA1c concentrations were available for 97% of participants (CSII, n = 143; MDI, n = 142).
The results for the primary outcome for the primary ITT analysis and the per-protocol analysis are provided in Table 9. The per-protocol analysis population excludes participants who had a major protocol deviation (see Table 8) or three or more minor deviations.
| HbA1c concentration (mmol/mol) at 12 months | Treatment arm | Total | Meana,b (95% CI) | Meana difference between treatment groups (CSII – MDI) (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| CSII | MDI | CSII | MDI | ||||
| Primary analysis: ITT analysis (age group) | |||||||
| 7 months to < 5 years | |||||||
| n (missing)c | 33 (0) | 31 (1) | 64 (1) | 60.9 (58.5 to 63.3) | 58.5 (56.1 to 60.9) | 2.4 (–0.4 to 5.3) | 0.09 |
| Mean (SD) | 63.9 (12.1) | 58.4 (9.9) | 61.2 (11.3) | ||||
| 5 years to < 12 years | |||||||
| n (missing)c | 70 (1) | 72 (4) | 142 (5) | ||||
| Mean (SD) | 58 (11.4) | 59.3 (11.4) | 58.7 (11.4) | ||||
| 12 years to < 16 years | |||||||
| n (missing)c | 40 (0) | 39 (2) | 79 (2) | ||||
| Mean (SD) | 61.3 (13.3) | 54.7 (14.7) | 58.1 (14.3) | ||||
| Per-protocol analysis (age group) | |||||||
| 7 months to < 5 years | |||||||
| n (missing)d | 23 (10) | 11 (21) | 34 (31) | 60.2 (56.4 to 63.9) | 59.3 (55.3 to 63.3) | 0.9 (–3.2 to 5) | 0.67 |
| Mean (SD) | 62.6 (13.1) | 56.2 (11) | 60.5 (12.6) | ||||
| 5 years to < 12 years | |||||||
| n (missing)d | 41 (30) | 32 (44) | 73 (74) | ||||
| Mean (SD) | 57.9 (11.8) | 59.7 (10.8) | 58.7 (11.3) | ||||
| 12 years to < 16 years | |||||||
| n (missing)d | 23 (17) | 23 (18) | 46 (35) | ||||
| Mean (SD) | 57.6 (14.2) | 57.8 (16.8) | 57.7 (15.4) | ||||
The HbA1c measurement was marginally higher in the CSII group than in the MDI group (mean difference 2.4 mmol/mol; 95% CI –0.4 to 5.3 mmol/mol), although the observed difference was not statistically significant. This result was consistent when compared with the per-protocol analysis (see Tables 4 and 5 for comparison of baseline characteristics of all randomised participants and Appendix 2, Table 30, for baseline characteristics of the per-protocol analysis population). A summary of the data sources (local or central laboratory) used in the primary ITT analysis and the per-protocol analysis is provided in Table 10.
| Laboratory value used for primary outcome analysis | Analysis, n (%) | |||
|---|---|---|---|---|
| ITT | Per-protocol | |||
| CSII | MDI | CSII | MDI | |
| Aged < 5 years | ||||
| Central | 22 (66.7) | 26 (83.9) | 15 (65.2) | 11 (100) |
| Local | 11 (33.3) | 5 (16.1) | 8 (34.8) | – |
| Aged 5–11 years | ||||
| Central | 58 (82.9) | 58 (80.6) | 34 (82.9) | 27 (84.4) |
| Local | 12 (17.1) | 14 (19.4) | 7 (17.1) | 5 (15.6) |
| Aged ≥ 12 years | ||||
| Central | 32 (80) | 33 (84.6) | 18 (78.3) | 20 (87) |
| Local | 8 (20) | 6 (15.4) | 5 (21.7) | 3 (13) |
| Total | ||||
| Central | 112 (77.8) | 117 (78.5) | 67 (77.0) | 58 (87.9) |
| Local | 31 (21.5) | 25 (16.8) | 20 (23.0) | 8 (12.1) |
Exploratory analyses including HbA1c concentrations measured at baseline as a continuous explanatory variable in the age-adjusted model did not alter the SCIPI study conclusions for CSII compared with MDI at 12 months [least square mean difference between treatment groups (CSII – MDI) 2.9; 95% CI –0.02 to 5.9], but did suggest the importance of early baseline values for 12-month measurements (HbA1c concentration baseline coefficient estimate 0.07; standard error 0.03; 95% CI 0.01 to 0.13).
An additional exploratory analysis considered the impact of the deprivation score by including it in the age strata-adjusted model and found that it did not alter conclusions [least square mean difference (CSII – MDI) 2.2; 95% CI –0.7 to 5.0]. The model suggested that higher deprivation score was associated with higher HbA1c concentrations at 12 months (0.03, standard error 0.04, 95% CI –0.05 to 0.12).
Mean profile plots are provided for each age stratum in Figure 4. The results from including an age stratum by treatment group interaction with age as a main effect in the model are provided in Table 11. The least mean square estimates are 61.5 (95% CI 59.1 to 63.9) for CSII, 57.9 (95% CI 55.5 to 60.3) for MDI and 3.6 (95% CI 0.6 to 6.6) for the difference (CSII – MDI).
FIGURE 4.
Glycosylated haemoglobin (mmol/mol) mean profile plots by age stratum. (a) Participants aged < 5 years; (b) participants aged 5–11 years; and (c) participants aged ≥ 12 years. Not a prespecified analysis in the statistical analysis plan.
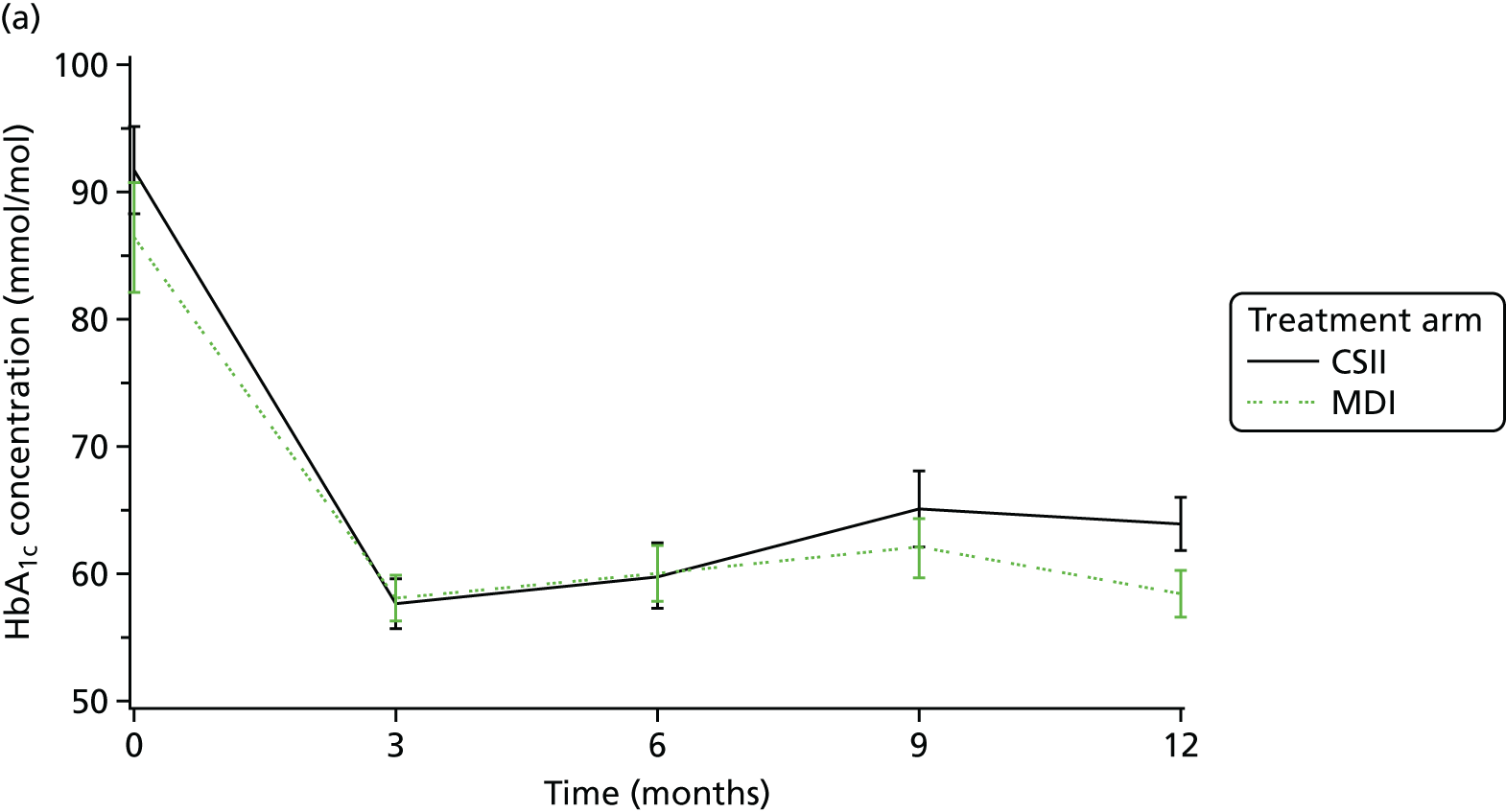

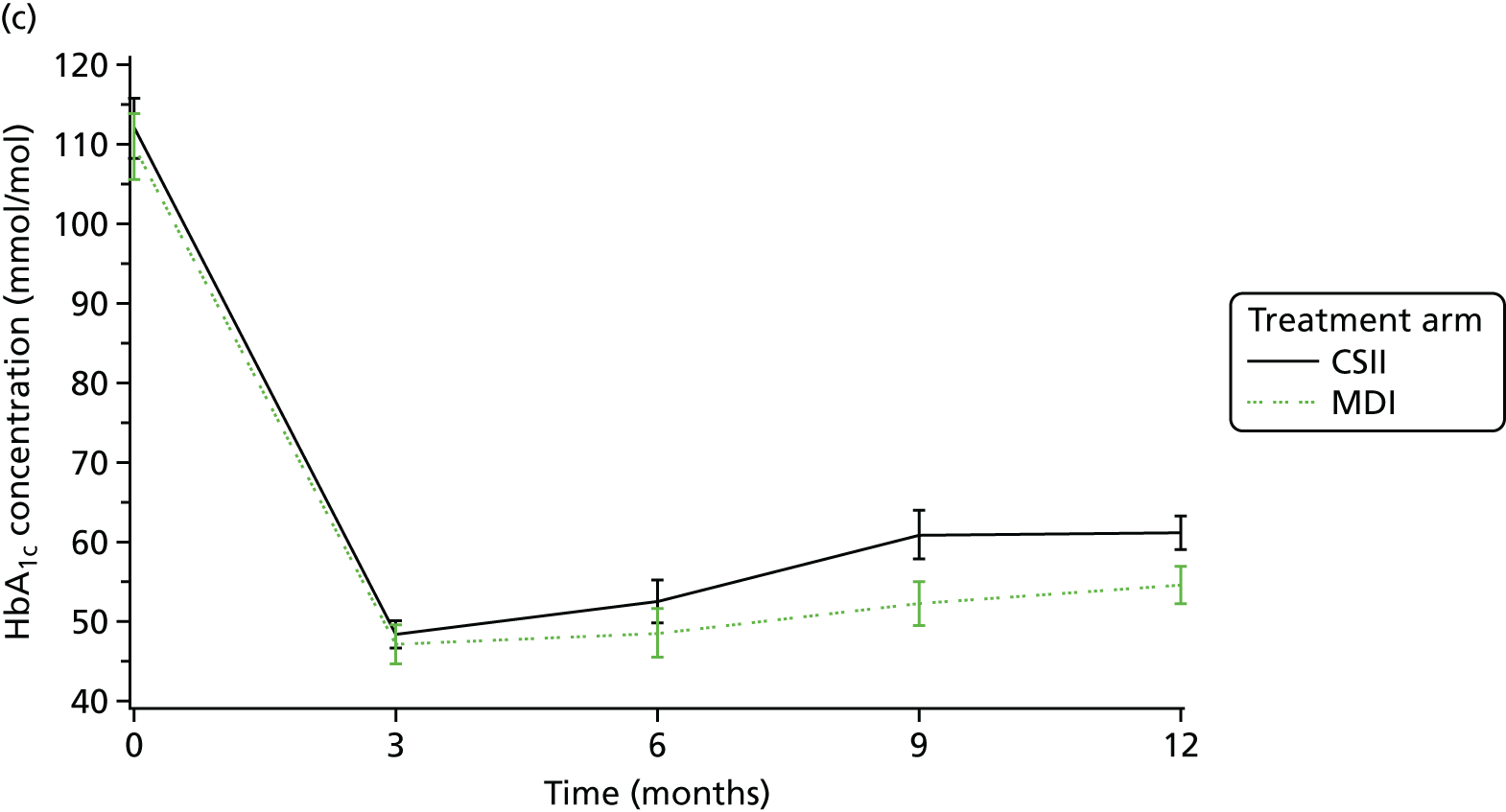
| Effect (CSII – MDI) | Estimatea | Standard error | 95% CI |
|---|---|---|---|
| Treatment × ≥ 12 years | 6.5001 | 2.7026 | 1.1788 to 11.8213 |
| Treatment × 5–11 years | –1.2540 | 2.0148 | –5.2210 to 2.7130 |
| Treatment × < 5 years | 5.5902 | 3.0029 | –0.3223 to 11.5027 |
Figure 5 displays the consistency of treatment effect across the SCIPI study centres. The consistency of treatment effect across sites is evident by the overlapping CIs. There is no statistical evidence for differences between sites from the chi-squared test for heterogeneity (p = 0.51) and I2 (0%). Figure 6 displays the treatment effect in 6-month periods to consider potential presence of a learning curve from the first randomisations. The period of 0–6 months includes the first 6 months of randomisations within each centre. The graph does not suggest a pattern of reducing treatment effect by time.
FIGURE 5.
Forest plot of HbA1c concentrations at 12 months by site. IV, inverse variance.
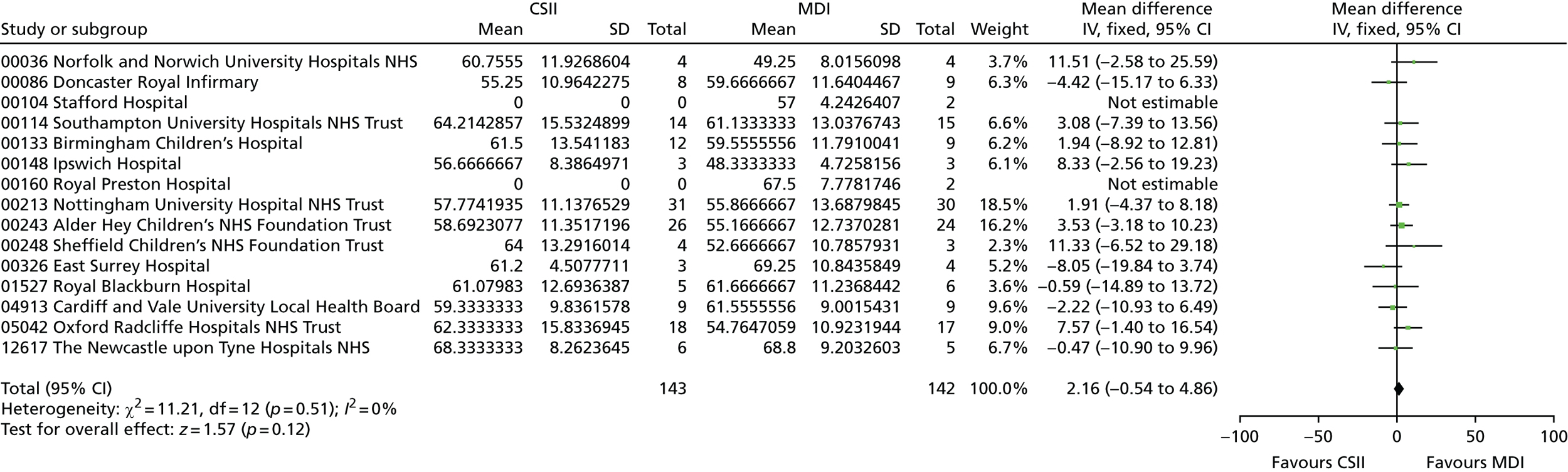
FIGURE 6.
Forest plot to consider treatment effect learning curve. Not prespecified analysis in the statistical analysis plan. IV, inverse variance.

Secondary outcomes
The target HbA1c value that was recommended by NICE19 at the start of the SCIPI study was < 58 mmol/mol. In August 2015, this was revised to be < 48 mmol/mol. To reflect current practice, the secondary outcome that had previously been < 58 mmol/mol was changed in a protocol amendment (version 7.0). However, as the majority of the SCIPI study participants would have been randomised and followed up with diabetes teams aiming to attain HbA1c values of < 58 mmol/mol, the results for both are presented in Table 12. Greater proportions of participants on the MDI arm attained values below the target thresholds; however, results are not statistically significant.
| Target HbA1c concentration | Treatment arm, n (%) | Overall (N = 293), n (%) | Relative risk (95% CI) | p-valuea | |
|---|---|---|---|---|---|
| CSII (N = 144) | MDI (N = 149) | ||||
| < 48 mmol/mol | 22 (15.4) | 29 (20.4) | 51 (17.9) | 0.75 (0.46 to 1.25) | 0.28 |
| Missing | 1 | 5 | |||
| < 58 mmol/mol | 66 (46.2) | 78 (54.9) | 144 (50.5) | 0.84 (0.67 to 1.06) | 0.16 |
| Missing | 1 | 5 | |||
| Severe hypoglycaemiab | 6 (4.2) | 2 (1.3) | 8 (2.7) | 3.1 (0.6 to 15.1) | 0.17 |
| Missing | 10 | 0 | |||
| DKA | 2 (1.4) | 0 | 2 (0.7) | 5.2 (0.3 to 106.8) | 0.24 |
| Missing | 10 | 0 | |||
| Partial remission | 21 (24.4) | 21 (32.8) | 42 (28.0) | 0.74 (0.45 to 1.24) | 0.28 |
| Missing | 58 | 85 | |||
The incidences of severe hypoglycaemia and DKA were low, with only eight and two participants experiencing these events, respectively. Although participants on CSII had a greater event rate, for both events, the wide CIs reflect the low number of event rates and, hence, a high level of uncertainty.
A higher proportion of participants randomised to MDI were in partial remission at 12 months. However, levels of missing data were high for this outcome because of the lack of availability of the daily insulin dose data required to calculate its occurrence. The formula used to calculate partial remission is available in the statistical analysis plan, which is available on the National Institute for Health Research website (www.journalslibrary.nihr.ac.uk/programmes/hta/081439/#/; accessed 13 February 2018).
There was no significant difference in change in BMI SDS or height SDS between those randomised to CSII and those randomised to MDI (Table 13). Insulin requirements were significantly lower for those randomised to MDI than for those randomised to CSII (see Table 13). Similarly, with partial remission, the low availability of dose data increased levels of missing data. Owing to the availability of CSII data, levels of missing data are higher in the MDI arm.
| Summary | Treatment arm | Total | CSII – MDIa (95% CI) | p-value | |
|---|---|---|---|---|---|
| CSII | MDI | ||||
| Change in BMI SDS | |||||
| 7 months to < 5 years | |||||
| n (missing) | 124 (20) | 132 (17) | 256 (37) | 0.1 (0 to 0.3) | 0.13 |
| Mean (SD) | 0.2 (1.3) | 0.1 (1.3) | 0.1 (1.3) | ||
| Median (IQR) | 0.2 (–0.7 to 0.9) | 0.1 (–0.8 to 1) | 0.2 (–0.8 to 1) | ||
| Minimum, maximum | –2.9, 4.2 | –4, 3.5 | –4, 4.2 | ||
| 5 years to < 12 years | |||||
| na (missing) | 142 (1) | 138 (6) | 280 (7) | ||
| Mean (SD) | 0.8 (1.1) | 0.7 (1) | 0.7 (1.1) | ||
| Median (IQR) | 0.8 (0.1 to 1.5) | 0.6 (0 to 1.4) | 0.6 (0 to 1.4) | ||
| Minimum, maximum | –1.8, 4.1 | –2.7, 3 | –2.7, 4.1 | ||
| 12 years to < 16 years | |||||
| na (missing) | 122 (21) | 122 (22) | 244 (43) | ||
| Mean (SD) | 0.6 (0.8) | 0.5 (0.8) | 0.5 (0.8) | ||
| Median (IQR) | 0.5 (0.1 to 1) | 0.4 (–0.1 to 0.9) | 0.5 (0 to 0.9) | ||
| Minimum, maximum | –1.4, 3.4 | –1.1, 5.3 | –1.4, 5.3 | ||
| Change in height SDS | |||||
| 7 months to < 5 years | |||||
| n (missing) | 124 (20) | 132 (17) | 256 (37) | –0.1 (–0.2 to 0) | 0.10 |
| Mean (SD) | 0.3 (1.1) | 0.3 (1) | 0.3 (1) | ||
| Median (IQR) | 0.1 (–0.4 to 1.1) | 0.4 (–0.3 to 1.1) | 0.3 (–0.3 to 1.1) | ||
| Minimum, maximum | –2.3, 3.3 | –2.9, 2.4 | –2.9, 3.3 | ||
| 5 years to < 12 years | |||||
| na (missing) | 142 (2) | 138 (11) | 280 (13) | ||
| Mean (SD) | 0.2 (1.1) | 0.3 (1) | 0.3 (1) | ||
| Median (IQR) | 0.1 (–0.6 to 1) | 0.3 (–0.3 to 1) | 0.2 (–0.5 to 1) | ||
| Minimum, maximum | –2.1, 3.5 | –2.7, 2.3 | –2.7, 3.5 | ||
| 12 years to < 16 years | |||||
| na (missing) | 122 (22) | 122 (27) | 244 (49) | ||
| Mean (SD) | –0.1 (0.5) | 0 (0.4) | 0 (0.4) | ||
| Median (IQR) | 0 (–0.2 to 0.2) | 0 (–0.2 to 0.2) | 0 (–0.2 to 0.2) | ||
| Minimum, maximum | –2.8, 0.7 | –1.7, 1.6 | –2.8, 1.6 | ||
| Insulin requirements (unit/kg/day) | |||||
| 7 months to < 5 years | |||||
| n (missing) | 24 (9) | 13 (19) | 37 (28) | 0.1 (0.0 to 0.2) | 0.01 |
| Mean (SD) | 0.7 (0.2) | 0.7 (0.1) | 0.7 (0.2) | ||
| Median (IQR) | 0.8 (0.6 to 0.8) | 0.6 (0.6 to 0.7) | 0.7 (0.6 to 0.8) | ||
| Minimum, maximum | 0.3, 1.2 | 0.4, 0.9 | 0.3, 1.2 | ||
| 5 years to < 12 years | |||||
| n (missing) | 45 (26) | 38 (38) | 83 (64) | ||
| Mean (SD) | 0.6 (0.2) | 0.6 (0.3) | 0.6 (0.3) | ||
| Median (IQR) | 0.6 (0.5 to 0.8) | 0.6 (0.4 to 0.7) | 0.6 (0.4 to 0.8) | ||
| Minimum, maximum | 0.2, 1.2 | 0, 1.2 | 0, 1.2 | ||
| 12 years to < 16 years | |||||
| n (missing) | 18 (22) | 13 (28) | 31 (50) | ||
| Mean (SD) | 0.8 (0.2) | 0.5 (0.4) | 0.7 (0.3) | ||
| Median (IQR) | 0.8 (0.6 to 0.9) | 0.7 (0 to 0.8) | 0.7 (0.5 to 0.9) | ||
| Minimum, maximum | 0.4, 1.2 | 0, 0.9 | 0, 1.2 | ||
Quality of life
Tables 14 and 15 present results for QoL as measured by the PedsQL instruments for parents and children, respectively, stratified by instrument age category. Results for each domain are available in Appendix 2, Tables 31 and 32.
| Summary | Time point | |||||
|---|---|---|---|---|---|---|
| 6 monthsa | 12 months | |||||
| CSII | MDI | Total | CSII | MDI | Total | |
| Parents/carers of 2- to 4-year-old children | ||||||
| n | 24 | 18 | 42 | 20 | 17 | 37 |
| Median (IQR) | 72.8 (62.9–77.2) | 61.8 (55.5–74.1) | 68.8 (57–76.8) | 75 (62.1–82.1) | 70.5 (54.7–79.5) | 73.2 (60.7–80.4) |
| Minimum, maximum | 54.5, 98.6 | 46.7, 81.6 | 46.7, 98.6 | 52.4, 99.1 | 40.2, 90.2 | 40.2, 99.1 |
| Parents/carers of 5- to 7-year-old children | ||||||
| n | 13 | 23 | 36 | 16 | 17 | 33 |
| Median (IQR) | 70.5 (63.5–85.7) | 69.6 (65.2–80.4) | 69.8 (65.1–80.8) | 75.6 (60.4–86.6) | 72.3 (64.7–76) | 73.2 (63.4–81.3) |
| Minimum, maximum | 51.8, 90.2 | 54.7, 84.4 | 51.8, 90.2 | 45.5, 94.6 | 49.1, 86.6 | 45.5, 94.6 |
| Parents/carers of 8- to 12-year-old children | ||||||
| n | 58 | 56 | 114 | 54 | 55 | 109 |
| Median (IQR) | 73.2 (65.2–84.2) | 68.8 (63.4–78.8) | 70.5 (64.3–81.5) | 74.5 (65.9–81.3) | 67.9 (57.1–77.7) | 70.5 (61.6–80.4) |
| Minimum, maximum | 31.3, 94.6 | 34.8, 95.8 | 31.3, 95.8 | 39.5, 100 | 34.2, 99.1 | 34.2, 100 |
| Parents/carers of 13- to 16-year-old children | ||||||
| n | 29 | 28 | 57 | 38 | 34 | 72 |
| Median (IQR) | 73.2 (59.8–84.3) | 67.6 (55.6–74.9) | 69.6 (58.9–80.4) | 67.3 (58–80.7) | 67.4 (59.8–79.5) | 67.3 (58.9–80.1) |
| Minimum, maximum | 42, 99.1 | 35.1, 98.2 | 35.1, 99.1 | 41.1, 96.4 | 19.6, 91.1 | 19.6, 96.4 |
| Summary | Time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| CSII | MDI | Total | CSII | MDI | Total | |
| 5- to 7-year-old children | ||||||
| n | 11 | 21 | 32 | 13 | 18 | 31 |
| Median (IQR) | 75 (51.5–80.4) | 70.9 (67.9–80.4) | 71.4 (67–80.4) | 78.6 (60.7–85.7) | 76.4 (60.7–81.6) | 76.8 (60.7–82.1) |
| Minimum, maximum | 41.1, 83.4 | 55.4, 87.5 | 41.1, 87.5 | 39.3, 89.3 | 30.4, 85.7 | 30.4, 89.3 |
| 8- to 12-year-old children | ||||||
| n | 58 | 54 | 112 | 54 | 53 | 107 |
| Median (IQR) | 79.1 (66.6–84.8) | 71.5 (64.3–80.4) | 75.9 (66.3–83.6) | 81.7 (70.7–88.4) | 76.8 (64.3–85.7) | 77.7 (70.5–87.8) |
| Minimum, maximum | 45.5, 94.1 | 40.2, 99.1 | 40.2, 99.1 | 44.6, 100 | 39.8, 96.4 | 39.8, 100 |
| 13- to 16-year-old children | ||||||
| n | 29 | 29 | 58 | 37 | 33 | 70 |
| Median (IQR) | 79.5 (71.4–87.6) | 75.9 (67–82.1) | 76.9 (69.6–85.7) | 75 (63.4–84.8) | 73.2 (60.7–79.3) | 74.7 (60.7–84.8) |
| Minimum, maximum | 43.8, 99.1 | 50, 98.2 | 43.8, 99.1 | 47.3, 95.5 | 35.7, 94.6 | 35.7, 95.5 |
In parent-reported QoL, there was a tendency for parents with children randomised to CSII to report higher QoL scores than those on MDI. This was consistent across instrument age strata, the 6- and 12-month follow-up time points and the child-reported scores. Estimates of the least mean squares difference between treatment groups (CSII – MDI) were 4.1 (95% CI 0.6 to 7.6; p = 0.02) for parent-reported and 3.1 (95% CI –0.6 to 6.8; p = 0.1) for child-reported QoL. It should be noted, however, that child-reported QoL starts from age 5 years but the parent-reported QoL starts from age 2 years and that, in general, the size of the differences and CI widths are similar.
Safety analysis
Table 12 reports on incidences of hypoglycaemia and DKA in the ITT analysis population. In this section, the data set contains all participants who were randomised and received at least one dose of trial medication. For AEs, the method of insulin delivery that the participants were receiving at the time of AE onset was used, rather that the allocated treatment, to take into account that participants could temporarily or permanently switch treatment. The total number of events experienced and the number of participants experiencing at least one event are provided along with the IDR (the number of patients with at least one new AE per population at risk in a given time period):
-
The incidence of related AEs by treatment group was 54 in 36 patients who were on CSII at the time of the AE. IDR was 25.0 patients with at least one event per 100 person-years and 17 AEs in 16 patients who were on MDI at the time of the AE. IDR was 10.5 patients with at least one event per 100 person-years.
-
The incidence of related SAEs by treatment group was 14 SAEs in nine patients who were on CSII at the time of the SAE. IDR was 6.2 patients with at least one event per 100 person-years and eight SAEs in eight patients who were on MDI at the time of the SAE. IDR was 5.3 patients with at least one event per 100 person-years.
Tables 16 and 17 provide the summary of related AEs and SAEs, respectively.
| Description | Treatment arm | Total (296.1 total person-years, N = 293 patients) | ||||
|---|---|---|---|---|---|---|
| CSII (144.1 total person-years, N = 144 patients) | MDI (151.9 total person-years, N = 149 patients) | |||||
| Events (n) | Patients, n (IDR) | Events (n) | Patients, n (IDR) | Events (n) | Patients, n (IDR) | |
| All | ||||||
| DKA | 2 | 2 (1.4) | 0 | 0 (0) | 2 | 2 (0.7) |
| Insulin administration error | 2 | 2 (1.4) | 5 | 5 (3.3) | 7 | 6 (2) |
| Pump failure | 4 | 3 (2.1) | 0 | 0 (0) | 4 | 3 (1) |
| Severe hypoglycaemia | 6 | 6 (4.2) | 2 | 2 (1.3) | 8 | 8 (2.7) |
| Site infections | 8 | 7 (4.9) | 0 | 0 (0) | 8 | 7 (2.4) |
| Other – specify | 32 | 22 (15.3) | 10 | 10 (6.6) | 42 | 32 (10.8) |
| Device | ||||||
| DKA | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Pump failure | 4 | 3 (2.1) | 0 | 0 (0) | 4 | 3 (1) |
| Severe hypoglycaemia | 2 | 2 (1.4) | 0 | 0 (0) | 2 | 2 (0.7) |
| Site infections | 8 | 7 (4.9) | 0 | 0 (0) | 8 | 7 (2.4) |
| Other – specify | 14 | 11 (7.6) | 3 | 3 (2) | 17 | 14 (4.7) |
| Carer error | ||||||
| Insulin administration error | 1 | 1 (0.7) | 4 | 4 (2.6) | 5 | 5 (1.7) |
| Other – specify | 5 | 2 (1.4) | 0 | 0 (0) | 5 | 2 (0.7) |
| Meter error | ||||||
| Other – specify | 3 | 3 (2.1) | 1 | 1 (0.7) | 4 | 4 (1.4) |
| Incidental illness | ||||||
| Insulin administration error | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Other – specify | 5 | 5 (3.5) | 3 | 3 (2) | 8 | 8 (2.7) |
| Other | ||||||
| DKA | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Insulin administration error | 0 | 0 (0) | 1 | 1 (0.7) | 1 | 1 (0.3) |
| Severe hypoglycaemia | 4 | 4 (2.8) | 2 | 2 (1.3) | 6 | 6 (2) |
| Other – specify | 5 | 4 (2.8) | 3 | 3 (2) | 8 | 7 (2.4) |
| Description | Treatment arm | Total (296.1 total person-years, N = 293 patients) | ||||
|---|---|---|---|---|---|---|
| CSII (144.1 total person-years, N = 144 patients) | MDI (151.9 total person-years, N = 149 patients) | |||||
| Events (n) | Patients, n (IDR) | Events (n) | Patients, n (IDR) | Events (n) | Patients, n (IDR) | |
| All | ||||||
| DKA | 2 | 2 (1.4) | 0 | 0 (0) | 2 | 2 (0.7) |
| Insulin administration error | 2 | 2 (1.4) | 3 | 3 (2) | 5 | 4 (1.4) |
| Pump failure | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Severe hypoglycaemia | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Site infections | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Other – specify | 8 | 6 (4.2) | 5 | 5 (3.3) | 13 | 11 (3.7) |
| Device | ||||||
| DKA | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Pump failure | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Severe hypoglycaemia | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Site infections | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Other – specify | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Carer error | ||||||
| Insulin administration error | 1 | 1 (0.7) | 2 | 2 (1.3) | 3 | 3 (1) |
| Other – specify | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Meter error | ||||||
| Other – specify | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Incidental illness | ||||||
| Insulin administration error | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Other – specify | 5 | 4 (2.8) | 2 | 2 (1.3) | 7 | 6 (2) |
| Other | ||||||
| DKA | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Insulin administration error | 0 | 0 (0) | 1 | 1 (0.7) | 1 | 1 (0.3) |
| Severe hypoglycaemia | 1 | 1 (0.7) | 0 | 0 (0) | 1 | 1 (0.3) |
| Other – specify | 3 | 2 (1.4) | 3 | 3 (2) | 6 | 5 (1.7) |
Chapter 4 Economic evaluation
Introduction
Economic evaluations
Economic evaluations of CSII versus MDI have been reviewed by Cummins et al. 103 and, more recently, by Roze et al. ,104 Pozzilli et al. 105 and ourselves using targeted searches of the PubMed, Web of Science, Scopus, Cumulative Index to Nursing and Allied Health Literature, ProQuest and Cochrane databases. In total, 10 fully reported economic evaluations were identified. 62,81,103,106–112 Most are limited in their transferability to the paediatric setting, as they relate to adult populations, and only two evaluations consider children and adolescent populations. 106,107
Cohen et al. 106 examined the cost-effectiveness of CSII with MDI in adults and in children/young adults with T1D from the perspective of the Australian single-payer health-care system. They applied the Center for Outcomes Research (CORE) diabetes model (CDM) to simulate the lifetime progression of diabetes in adolescent patients with the following baseline characteristics: a mean age of 17.1 years, a mean duration of diabetes of 6.3 years and a mean HbA1c concentration of 8.9%. 113,114
The effectiveness of CSII in the base-case analysis was based on the results of a meta-analysis of 52 studies that included 1500 patients of all ages. 115 Compared with MDI, the meta-analysis suggested that treatment with CSII for ≥ 1 year was associated with a mean decrease in baseline HbA1c concentration of 1.2%. The base-case analysis assumed no differences between interventions in the frequency of hypoglycaemic events. Annual costs of CSII and MDI were based on pump costs (and assumed an 8-year pump life), insulin costs, consumables, self-monitoring of blood glucose and medical professional assistance with pump initiation, maintenance and operation. The costs of managing complications and health state utilities were taken from published sources. All costs and outcomes were discounted at an annual rate of 5%.
The results indicated gains in discounted quality-adjusted life-years (QALYs) of 0.560 and a cost increase of AU$41,779 in CSII compared with MDI. The incremental cost per QALY gained was AU$74,661, which is marginally cost-effective at the Australian threshold for cost-effectiveness. This was sensitive to change in HbA1c concentration: a reduction to 0.51% for CSII versus MDI increased the incremental cost-effectiveness ratio (ICER) to AU$114,818. Reducing the effective lifespan of the insulin pump from 8 to 6 years increased the ICER by 11%.
St Charles et al. 107 modelled the cost-effectiveness of CSII in T1D populations, including children and young adults, based on a third-party US payer perspective. Patients’ baseline demographics, risk factors and pre-existing complications were taken from Doyle et al. 35 and the Diabetes Control Complications Trial Research Group secondary intervention cohort,3 with a mean age of 13.0 years (range 8–21 years), a mean duration of diabetes of 5.6 years and a mean HbA1c concentration of 8.2%.
For their base-case analysis, the authors assigned a greater reduction in HbA1c concentration to CSII (0.9%) than to MDI (0.1%), based on a 16-week randomised trial of 32 children and young adults with T1D. 35 They further assumed an insulin pump lifetime of 7 years and 50% lower hypoglycaemia event rates in CSII based on an observational study116 and reviews of outpatient insulin therapy in T1D patients. 117–119 Costs and outcomes were extrapolated over a lifetime using the CDM and discounted at a rate of 3% per annum.
The base-case ICER, of US$27,195 per QALY gained, was sensitive to (1) the lifespan of the pump, rising to US$40,652 per QALY gained for a 4-year lifetime, (2) the HbA1c concentration improvement with CSII, rising to US$37,326 per QALY gained with a reduction in HbA1c concentration of 0.675%, and (3) the hypoglycaemia event rate, with the ICER increasing to US$45,595 per QALY gained and no improvement in CSII over MDI. The model was less sensitive to changes in the discount rate. The higher treatment costs associated with CSII in comparison with MDI (US$37,636 over a lifetime) were more than twice the expected savings from fewer complications (US$16,172).
An important limitation of both studies106,107 was that they modelled the costs and consequences of CSII and MDI using data from disparate sources. They relied on small studies for the estimated improvement in HbA1c concentration and made assumptions relating to the frequency of hypoglycaemic events and lifetime rates of T1D-related complications. The modelled outputs were very sensitive to these assumptions. Other long-term issues, including adherence and patient preference, could also potentially influence the cost-effectiveness of CSII, and although the CDM is a widely validated120 and widely accepted103 model in adult diabetes, there is no supporting evidence of validity in paediatric populations. 19
Aim
The aim of the economic evaluation of the SCIPI study was to estimate the cost–utility, based on an assessment of the incremental costs per QALY gained, of treating paediatric T1D patients with CSII versus MDI. The costing perspective was that of the NHS in England. Within-trial QALYs were derived from patients’, parents’ or guardians’ responses to the HUI Quetionnaire using the HU12 algorithm. Costs were derived by measuring health-care resource use associated with both CSII and MDI, including (1) purchase of pumps and pen devices, (2) consumables, (3) management of AEs, (4) procedures, (5) insulin, (6) hospitalisations and (7) contact with health-care professionals including GPs, school nurses, as an outpatient and at the accident and emergency (A&E) department. Differences between intervention groups in the primary outcome would indicate the need for a lifetime extrapolation using the CDM, otherwise a 12-month time horizon would be adopted.
Methods
Resource use
The perspective of the analysis was that of the NHS in England, with the expectation the major cost drivers would be devices and consumables, such as cartridge sets, batteries and infusion sets for CSII, insulin use, glucose self-monitoring and outpatient visits. 104,106 Other potential high-cost resource usages were considered, including A&E department attendance, hospital inpatient stays (including intensive care units) and all contacts with health-care professionals, including GPs, hospital doctors, school nurses and other specialists in person, and by telephone, fax, text or e-mail.
The measurement of resource use required complementary approaches using data collected as part of the trial and as part of routine care. Patients’ use of health-care services was obtained from:
-
Electronic patient-linked information costing system (PLICS) data and/or patient administration system (PAS) data of participating hospitals – PLICS and PAS data were used as a primary source for identifying inpatient stays and use of a paediatric intensive care unit (PICU) or high-dependency unit (HDU).
-
Baseline forms – RNs completed the relevant sections of the baseline forms to identify the devices that were assigned to SCIPI study patients [Medtronic pump, F. Hoffman-La Roche AG pump, MDI, F. Hoffman-La Roche AG Expert glucometer, Contour® Glucometer (Ascensia Diabetes Care, Newbury, UK)]. The baseline forms also recorded patients’ self-reported contact with hospital services and health-care professionals in the 3 months prior to randomisation. When the patient was unsure of this, RNs contacted the patient’s GP or referred to the patient’s hospital notes. These forms were the primary source of baseline resource used.
-
Three-monthly patient questionnaires – RNs completed the relevant sections of the patient questionnaires in face-to-face interviews with trial participants, their parents or guardians to identify overnight hospital stays, number of nights, reason for admission and type of ward. Another section of the patient questionnaire was used to identify contacts with health-care professionals including GPs, consultants, non-consultant grade medical doctors, nurses, dietitians, psychologists, infusion specialists, social workers as well as the places or means of contact: A&E department, outpatient, general practice, school visits, home visits telephone, fax, text and e-mail. When the patient was unsure, RNs contacted the patient’s GP or referred to the patient’s hospital notes. Patient questionnaires were used as a primary source of data for treatment as an outpatient, in an A&E department, by a GP or by other health-care professional contacts. They were used as a secondary source of data for inpatient, PICU or HDU stays, which was especially relevant if the patients had attended a different hospital for treatment.
-
Adverse event or SAE forms – RNs completed AE and SAE forms when trial participants were admitted to hospital with severe hypoglycaemia, DKA, site infections, pump failures, insulin administration errors or other diabetes and device-related causes. This information was used as a secondary source of data for inpatient stays and use of PICU or HDU stays, especially relevant if the patients had attended a different hospital for treatment.
-
Insulin prescription forms – insulin prescription forms were used as a primary source of insulin usage, as these are more likely to reflect the quantity of insulin dispensed and, therefore, the true cost of insulin. RNs contacted GPs and hospital personnel at 3-monthly intervals to record the number of boxes or vials of insulin prescribed to patients, along with the dates and insulin type. Each box contained five × 300-unit cartridges or five × 300-unit pens and each vial contained 1000 units. For young children, GPs occasionally prescribe two or three cartridges or pens; however, no part-boxes were reported in the case report form (CRF) and no assumptions were made based on patient age.
-
Three-monthly insulin usage forms – RNs documented patient usage of insulin in the 4 weeks prior to each 3-monthly visit through face-to-face interviews with trial participants, their parents and/or guardians. These data were used if their projected 12-month insulin usage exceeded the usage implied in the insulin prescription form.
-
Electronic pump download data – RNs downloaded insulin usage data from the patient insulin pumps and these were entered into an electronic database. These data were used if the 12-month insulin usage exceeded the usage implied in the insulin prescription and/or insulin usage forms.
Unit costs
All resource use was valued in monetary terms using appropriate unit costs estimated at the time of analysis (cost year was 2016). Healthcare Resource Group (HRG) codes were used as the main currency of the economic analysis for inpatient stays and were obtained directly from electronic PLICS/PAS data or assigned based on the description of the condition and complications when recorded in the patient questionnaire, AE or SAE forms. HRG codes have the advantage of most closely reflecting actual payments, with cost codes being allocated based on the latest available national tariff121 (these being bundled care packages, reimbursed at a national level according to the NHS Payment by Results scheme; Table 18). The scheduled 3-monthly visits were also costed using the national tariff because these formed part of the ongoing multidisciplinary team integrated package of care approach recommended by NICE19 and were not costed elsewhere. Unbundled care packages reimbursed at a local level, such as PICU and HDU stays, were costed using the latest available National Schedule costs. 122 Standard sources were used for the unit costs of all other primary health-care, A&E and outpatient contacts,123 with consultation time estimated by discussing with 15 RNs representing all recruiting sites (Table 19). The costs of needles, test strips and insulin were based on those detailed in the British National Formulary124 and the costs of pumps and estimated annual consumables were obtained from the suppliers (F. Hoffman-La Roche AG and Medtronic) (Tables 20 and 21). For the base-case analysis, insulin pump costs were divided by four to represent a 4-year lifetime and, for MDI patients, two refillable pens (1 × basal and 1 × bolus) were factored in at an estimate of £40 each per year.
| HRG code | HRG name | Elective | Non-elective | Costs for excess days (£ per day) | ||
|---|---|---|---|---|---|---|
| Cost (£) | Trim-point (days) | Cost (£) | Trim-point (days) | |||
| BZ01Z | Enhanced cataract surgery | 982 | 5 | 1944 | 12 | 242 |
| CZ05T | Tonsillectomy, 18 years and under without CC | 1083 | 5 | 1013 | 5 | 330 |
| FZ20E | Appendicectomy procedures, 18 years and under without major CC | 2403 | 5 | 2379 | 5 | 268 |
| FZ24E | Major therapeutic endoscopic upper or lower GI tract procedures, between 2 and 18 years | 1366 | 5 | 1177 | 5 | 268 |
| FZ62Za | Endoscopic or intermediate upper GI tract procedures, between 2 and 18 years | 864 | 5 | 893 | 5 | 268 |
| FZ63Z | Combined upper and lower GI tract diagnostic endoscopic procedures | 513 | 5 | 694 | 5 | 209 |
| HA73B | Minor elbow and lower arm procedures for trauma, 18 years and under | 1192 | 5 | 1192 | 5 | 317 |
| PA03B | Febrile convulsions, 1 year and over | 659 | 5 | 584 | 5 | 288 |
| PA04A | Headaches and migraines, with CC | 883 | 5 | 697 | 5 | 288 |
| PA04B | Headaches and migraines, without CC | 673 | 5 | 550 | 5 | 288 |
| PA11Z | Acute upper respiratory tract infection and common cold | 566 | 5 | 484 | 5 | 288 |
| PA12Z | Asthma or wheezing | 599 | 5 | 702 | 5 | 288 |
| PA17A | Intermediate infections with CC | 1121 | 5 | 1261 | 8 | 288 |
| PA19A | Viral infections with length of stay 1 day or less | 435 | 5 | 455 | 5 | 288 |
| PA21A | Infectious or non-infectious gastroenteritis, with CC | 1728 | 8 | 913 | 5 | 288 |
| PA25B | Major gastrointestinal disorders without CC | 1352 | 5 | 1103 | 5 | 288 |
| PA26B | Other gastrointestinal disorders without CC | 835 | 5 | 589 | 5 | 288 |
| PA28B | Feeding difficulties and vomiting, without CC | 828 | 5 | 551 | 5 | 288 |
| PA29Z | Abdominal pain | 691 | 5 | 580 | 5 | 288 |
| PA50Z | Ingestion poisoning or allergies | 453 | 5 | 503 | 5 | 288 |
| PA58Z | Examination, follow-up, special screening or other admissions, with length of stay 0 days | 427 | 5 | 346 | 5 | 288 |
| PA63B | Head, neck and ear disorders with length of stay 1 day or more, with CC | 1524 | 5 | 994 | 5 | 288 |
| PA67Z | Diabetes, with ketoacidosis or coma | 1459 | 10 | 1055 | 6 | 288 |
| PA68Z | Diabetes, without ketoacidosis or coma | 1207 | 5 | 954 | 5 | 288 |
| PA69Z | Nephritic and nephrotic renal diseases | 662 | 5 | 1350 | 9 | 288 |
| WA21Y | Other procedures or health-care problems, without CC | 467 | 5 | 579 | 5 | 198 |
| XB07Zb | Paediatric critical care high dependency | 1004 | N/A | N/A | N/A | N/A |
| XB05Zb | Paediatric critical care intensive care basic | 1670 | N/A | N/A | N/A | N/A |
| Profession | Cost (£) | |||||
|---|---|---|---|---|---|---|
| Surgery | Home | Telephone, e-mail and texts | Hospital outpatient | A&E | School visit | |
| Advanced nurse including infusion specialist and diabetes nurse | 22.75 | 37.92 | 9.10 | 45.50 | 30.33 | 37.92 |
| GP | 64.50 | 87.75 | 26.63 | – | – | – |
| Social worker (children’s services) | 39.50 | 39.50 | 39.50 | – | – | 39.50 |
| Hospital consultant | – | – | 22.83 | 68.50 | 45.67 | – |
| Specialist registrar | – | 13.68 | 12.00 | 36.00 | 24.00 | – |
| Community nurse | 38.00 | 38.00 | 38.00 | 38.00 | – | 38.00 |
| General practice nurse | 14.75 | – | 5.60 | – | – | – |
| Dietician | 19.00 | 19.00 | 6.33 | 19.00 | 12.67 | 19.00 |
| Psychologist | 69.50 | 69.50 | 23.17 | 69.50 | 46.33 | 69.50 |
| Consumable or device | Annual cost (£) | Rationale | Source |
|---|---|---|---|
| F. Hoffman-La Roche AG pump | 594 | Unit cost of £2375 with a 4-year lifespan | Supplier |
| F. Hoffman-La Roche AG pump consumables (e.g. reservoirs, cannula, batteries) | 1818 | Supplier | |
| Medtronic pump | 749 | Unit cost of £2995 with a 4-year lifespan | Supplier |
| Medtronic pump consumables (e.g. reservoirs, cannula, batteries) | 2377 | Supplier | |
| Pen injectors (various) | 80 | 1 × basal and 1 × bolus at £40 each | Based on children’s pen device suppliers in the UK |
| Needles for pen devices | 87 | Four injections a day at a cost of £5.95 per 100 needles | British National Formulary 124 |
| Blood glucose monitoring | 577 | NICE recommendation of five tests a day, Accu-Chek Aviva test strips (F. Hoffman-La Roche AG, Basel, Switzerland), at £15.79 per 50 tests | British National Formulary 124 |
| Insulin | Type | Cost (£ per unit) |
|---|---|---|
| Insulin aspart (Novorapid®, Novo Nordisk Ltd, Gatwick, UK) | Short acting | 0.0141 |
| Insulin detemir (Levemir®, Novo Nordisk Ltd, Gatwick, UK) | Intermediate/long acting | 0.0290 |
| Insulin aspart/protamine-crystallised insulin aspart in the ratio 30 : 70 (NovoMix 30, Novo Nordisk Ltd, Gatwick, UK) | Short/intermediate acting | 0.0199 |
| Insulin lispro (Humalog®, Eli Lilly and Company Ltd, Basingstoke, UK) | Short acting | 0.0166 |
| Insulin glargine (Lantus®, Sanofi, Guildford, UK) | Intermediate/long acting | 0.0307 |
| Biphasic isophane insulin (Humulin® M3, Eli Lilly and Company Ltd, Basingstoke, UK) | Short/intermediate acting | 0.0166 |
Cost analysis
All hospital stays recoded in patient questionnaires and the baseline form were costed irrespective of whether or not they were related to diabetes. Bundled national tariff costs were based on the hospital spell and incorporated excess ward days and whether the case was elective or emergency. 125 Tariff codes were obtained primarily from PLICS and PAS data supplied by the participating hospitals but, if unavailable, were referenced to the associated form (AE, SAE, baseline or patient questionnaire) and an appropriate HRG code was assigned based on reason for admission, condition and any complications. Locally negotiated unbundled costs were similarly identified and costs were assigned directly from the National Schedule.
If a hospitalisation spanned a period preceding and following randomisation, an adjustment was made to apportion costs between baseline and post-baseline values. Patients who were admitted to hospital n days before randomisation and spending N days in hospital after randomisation had their total costs calculated as:
Training comprised multiple sessions covering single visits. Although full details of how many patients and their family members attended each training session were not recorded, the majority were believed to be one-to-one sessions. However, because the majority of training was conducted in outpatient clinics, the costs of training were excluded from the total to avoid the potential for double-counting.
Blood glucose monitoring was costed on the minimum NICE recommendation of five tests per day. 19 Identical blood glucose monitoring costs were added to the consumables costs supplied by the pump manufacturers for the CSII intervention group. The costs of consumables in the MDI intervention group comprised blood glucose monitoring and disposable needles, based on an estimate of four insulin injections a day. 19 Total costs for each patient were calculated from the sum of the costs of ward, outpatient care, A&E, primary care, device, consumables and prescribed insulin.
Patients’ use of health-care resources and total costs were calculated for the ITT population, with summary statistics generated by intervention group.
Outcomes
Within-trial QALYs were calculated as the area under the utility–time curve over 12 months, with utilities calculated from patients’ (aged ≥ 12 years) or their parents’ or guardians’ responses to the HUI questionnaire taken at baseline and then at 3-, 6-, 9- and 12-month visits. The HUI questionnaire has been validated in paediatric populations126,127 and was previously suggested by NICE for use in children,128 and considers the six attributes of sensation, mobility, emotion, cognition, self-care and pain. These further expand into vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain using the Sheffield algorithm, which was used to generate a single UK-derived preference-based utility score. 129
The parents of patients < 3 years of age at randomisation were not required to answer the HUI questionnaire. Proxy assessment is recommended for the 3- to 12-year-old category, and self-assessment and/or proxy is recommended for patients > 12 years of age.
The minimum requirements for the base-case calculation of QALYs were utility measurements at baseline and at 12 months, with linear interpolation for any missing data points in between. When both parents and patients completed a HUI questionnaire in the same visit, base-case preference was given to the patient if they were aged 12 years and over at randomisation, and to the parent or guardian if the patient was under 12 years old.
Incremental analysis
In the base-case analysis, the cost-effectiveness of CSII was evaluated by its ICER calculated according to the formula:
in which, ΔCosts is the difference in mean total costs between intervention groups and ΔQALY is the difference in mean QALYs between intervention groups.
Uncertainty analysis
Mean costs and QALYs and differences between intervention groups in costs and QALYs were based on a bootstrapped analysis (bias-corrected and accelerated) using 10,000 replicates. The 95% central range was based on the 2.5 and 97.5 percentiles of the bootstrap values.
Uncertainty in the ICER was represented in cost-effectiveness acceptability curves, which presented the probability of CSII being cost-effective for given ceiling thresholds of costs per QALY. 130. Estimates of ICERs were compared with the £20,000 to £30,000 per QALY threshold of cost-effectiveness set by NICE. 131
Sensitivity analysis
Robustness of the base-case analysis was tested with respect to costs by (1) applying 2- and 6-year life cycles to the pumps, whereby the purchase cost of the pump was divided by 2 and 6 and (2) considering insulin costs based on quantity administered.
Sensitivity analyses concerning outcomes used (1) utilities and QALYs based on HUI3-derived Canadian tariff scores132 and (2) mean baseline utility imputation and multiple imputation with chained equations (MICE)133 to assess the impact of missing data on HUI2-derived scores. Explanatory variables considered included (1) utility at baseline and at 3 and 6 months; (2) QALYs calculated up to 9 months, centre, baseline cost, age, ethnicity, sex, weight, blood pH, blood glucose and HbA1c concentration; (3) mean baseline utility imputation and the last observation carried forward (LOCF) approach, as recommended in the HUI manual;132 and (4) HUI2-derived full parental or full patient responses to the HUI questionnaires.
All analyses adhered to the health economics analysis plan (see Appendix 3), and were performed using Stata® version 13 (StataCorp LP, College Station, TX, USA), and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 134
Exploratory analysis
The contribution of the baseline variables, of age group, centre, BMI, sex, baseline cost and ethnicity to total costs and of age group, centre, BMI, sex, baseline utility and ethnicity to QALYs, were tested separately in regression models. This allowed for identification of any particular high-cost subgroup and adjustment for any imbalanced covariates to account for their confounding effect. 135 Given the expected non-normality of both costs and QALYs, generalised linear models were implemented using a range of families and links and goodness of fit established using the Modified Park test.
A further planned exploratory analysis was to extrapolate the trial results to estimate lifetime costs and benefits and to capture long-term microvascular and macrovascular complications using the CDM model. 136 This extrapolation was to use a 3.5% annual discount rate for costs and outcomes accruing after the first year. 131 Clinical and physiological progression parameters were to be as presented in the NICE guideline19 and updated with relevant data from the SCIPI study.
Results
Resource use and costs
Complete resource use data were available for all patients.
Patients’ use of health-care resources and corresponding NHS costs were comparable in both intervention groups for the 3 months preceding randomisation (Table 22). The mean cost in the CSII group was £1530 (95% CI £1408 to £1779), compared with £1392 (95% CI £1267 to £1530) in the MDI intervention group. Although there were more hospital outpatient visits in the CSII group, there was no difference in the mean total costs of these visits (£189, 95% CI –£29 to £417). The main cost drivers for the 3 months preceding randomisation were inpatient stays (contributing to 69% of the total) and training sessions (contributing to 14%), which were carried out primarily as outpatient appointments.
| Item of resource use (units) | Treatment arm, mean (95% CI) | Difference (95% CI) | |
|---|---|---|---|
| CSII (n = 144) | MDI (n = 149) | ||
| Health-care professional contacts (number of telephone calls, faxes, texts or e-mails) | 3.32 (2.7 to 3.97) | 2.58 (1.96 to 3.3) | 0.74 (–0.18 to 1.65) |
| Scheduled outpatient (number of visits) | 1 (1 to 1) | 1 (1 to 1) | 0 (0 to 0) |
| Unscheduled outpatient visits (number of visits) | 2.19 (1.77 to 2.63) | 1.12 (0.89 to 1.37) | 1.07 (0.6 to 1.59) |
| A&E department (number of attendances) | 0.77 (0.56 to 0.99) | 0.62 (0.42 to 0.88) | 0.15 (–0.18 to 0.46) |
| Other hospital (e.g. ward visits) (number) | 0.49 (0.2 to 0.82) | 0.44 (0.18 to 0.79) | 0.04 (–0.38 to 0.47) |
| GP visits (number of contacts) | 0.25 (0.17 to 0.34) | 0.21 (0.14 to 0.28) | 0.04 (–0.07 to 0.16) |
| Home visits (number of contacts) | 1 (0.78 to 1.24) | 0.99 (0.75 to 1.25) | 0.01 (–0.33 to 0.34) |
| School visits (number of contacts) | 0.3 (0.2 to 0.41) | 0.24 (0.17 to 0.32) | 0.06 (–0.07 to 0.19) |
| Training sessions (recorded sessions) | 14.6 (12.99 to 16.22) | 13.83 (12.7 to 14.99) | 0.77 (–1.17 to 2.74) |
| HRG code | |||
| PA21A Infectious or non-infectious gastroenteritis, with CC | 0.02 (0 to 0.05) | 0 (0 to 0) | 0.02 (0 to 0.04) |
| PA67Z Diabetes, with ketoacidosis or coma | 0.13 (0.07 to 0.2) | 0.11 (0.06 to 0.17) | 0.02 (–0.07 to 0.11) |
| PA68Z Diabetes, without ketoacidosis or coma | 0.74 (0.65 to 0.83) | 0.72 (0.64 to 0.81) | 0.02 (–0.1 to 0.14) |
| PA69Z Nephritic and nephrotic renal diseases | 0 (0 to 0) | 0.01 (0 to 0.02) | –0.01 (–0.02 to 0) |
| XB05Z Paediatric critical care intensive care basic | 0.01 (0 to 0.02) | 0.02 (0 to 0.06) | –0.01 (–0.06 to 0.03) |
| XB07Z Paediatric critical care high dependency | 0.15 (0.03 to 0.32) | 0.05 (0.01 to 0.11) | 0.09 (–0.04 to 0.27) |
Resource use for the 12 months following randomisation is reported in Table 23. Inpatient stays and A&E department visits decreased considerably relative to the 3 months preceding randomisations, although health-care professional contacts, especially outpatient and GP clinic visits, and contacts via texts, e-mails and telephone calls increased.
| Item of resource use (units) | Treatment arm, mean (95% CI) | Difference (95% CI) | |
|---|---|---|---|
| CSII (n = 144) | MDI (n = 149) | ||
| Health-care professional contacts (number of telephone calls, faxes, texts or e-mails) | 21.15 (18.31 to 23.99) | 16.87 (14.62 to 19.25) | 4.29 (0.64 to 7.98) |
| Scheduled outpatient (number of visits) | 4 (4 to 4) | 4 (4 to 4) | 0 (0 to 0) |
| Unscheduled outpatient visits (number of visits) | 10.85 (9.6 to 12.08) | 10.81 (9.65 to 11.98) | 0.05 (–1.63 to 1.73) |
| A&E department (number of attendances) | 0.85 (0.51 to 1.24) | 0.4 (0.24 to 0.58) | 0.44 (0.07 to 0.88) |
| Other hospital (e.g. ward visits) (number) | 0.33 (0.1 to 0.67) | 0.26 (0.11 to 0.44) | 0.06 (–0.23 to 0.43) |
| GP visits (number of contacts) | 1.46 (1.16 to 1.78) | 1.2 (0.99 to 1.42) | 0.26 (–0.11 to 0.64) |
| Home visits (number of contacts) | 2.45 (1.91 to 3.1) | 1.99 (1.6 to 2.42) | 0.46 (–0.23 to 1.22) |
| School visits (number of contacts) | 1.4 (1.14 to 1.69) | 1.5 (1.19 to 1.85) | –0.1 (–0.53 to 0.32) |
| Training sessions (recorded sessions)a | 24.79 (21.87 to 27.92) | 22.45 (19.38 to 25.54) | 2.34 (–1.89 to 6.61) |
| Insulin (mean prescribed daily units) | 71.61 (62.76 to 81.78) | 66.32 (58.81 to 74.2) | 5.05 (–6.6 to 17.53) |
| HRG code | |||
| FZ62Z Diagnostic and intermediate procedures on the upper GI tract | 0.01 (0 to 0.02) | 0.02 (0 to 0.05) | –0.01 (–0.04 to 0.02) |
| PA21A Infectious or non-infectious gastroenteritis, with CC | 0.01 (0 to 0.03) | 0.01 (0 to 0.02) | 0.01 (–0.01 to .b) |
| PA25B Major gastrointestinal disorders without CC | 0.02 (0 to 0.05) | 0.01 (0 to 0.02) | 0.01 (–0.01 to 0.05) |
| PA67Z Diabetes, with ketoacidosis or coma | 0 (0 to 0) | 0.01 (0 to 0.02) | –0.01 (–0.02 to .b) |
| PA68Z Diabetes, without ketoacidosis or coma | 0.17 (0.08 to 0.27) | 0.07 (0.03 to 0.12) | 0.09 (0 to 0.21) |
| Other HRGs | 0.09 (0.04 to 0.15) | 0.07 (0.03 to 0.12) | 0.02 (–0.06 to 0.09) |
Patients who were randomised to CSII had more than twice as many A&E department visits and inpatient stays relating to the management of diabetes (HRG code PA68Z) as those in the MDI group, although the absolute numbers of attendances were low (Table 24). Over the 12-month period, health-care professionals made an average of 4.3 (95% CI 0.6 to 8.0) more contacts by text, e-mail or telephone with patients in the CSII group than with patients in the MDI group.
| Item of resource use | Treatment arm, mean cost (£) (95% CI) | Difference in mean cost (£) (95% CI) | |
|---|---|---|---|
| CSII (n = 144) | MDI (n = 149) | ||
| Consumables (e.g. needles, infusion sets, reservoirs) | 1841 (1826 to 1861) | 664 (664 to 664) | 1177 (1162 to 1197) |
| Device (pump 4-year lifespan or two pen devices) | 600 (596 to 606) | 80 (80 to 80) | 520 (516 to 526) |
| Insulin (prescribed) | 422 (364 to 486) | 482 (426 to 541) | –60 (–142 to 24) |
| Health-care professional contacts (telephone calls, faxes, texts or e-mails) | 138 (117 to 162) | 108 (92 to 124) | 30 (3 to 59) |
| Scheduled outpatients visits | 434 (434 to 434) | 434 (434 to 434) | 0 (0 to 0) |
| Unscheduled outpatient visits | 309 (272 to 346) | 328 (292 to 366) | –19 (–71 to 33) |
| Inpatient stays costed from HRG codes | 387 (245 to 553) | 219 (142 to 306) | 168 (5 to 352) |
| A&E | 26 (16 to 39) | 13 (8 to 19) | 13 (2 to 27) |
| Other hospital (e.g. ward visits) | 3 (1 to 7) | 3 (1 to 5) | 1 (–3 to 5) |
| GP visits | 71 (56 to 88) | 57 (45 to 69) | 15 (–5 to 35) |
| Home visits | 106 (80 to 138) | 83 (66 to 100) | 23 (–9 to 59) |
| School visits | 53 (43 to 64) | 56 (44 to 69) | –3 (–19 to 13) |
| Concomitant medications | 12 (8 to 17) | 15 (8 to 23) | –2 (–12 to 6) |
| Total cost | 4404 (4197 to 4642) | 2541 (2412 to 2672) | 1863 (1620 to 2137) |
Data on prescribed insulin were available for all but seven patients; for these patients, data on dose administered were used in the analysis. There were no significant differences in either the quantity or the cost of prescribed insulin between the two intervention groups.
Mean total and disaggregated costs, with their 95% bootstrapped CIs, are reported in Table 25. Consumables, devices (for insulin pumps) and inpatient stays were the main drivers of the differences in costs between the groups. These contributed 63%, 28% and 9%, respectively. Each of these was higher in the CSII group. The annualised cost of the devices differed by £520 between groups; consumables, which included needles, infusion sets and reservoirs, differed by £1177 (£1841 CSII vs. £664 MDI).
| Treatment arm, mean (95% CI) | Difference, mean (95% CI) | ICER | ||
|---|---|---|---|---|
| CSII (n = 109) | MDI (n = 105) | |||
| Costs (£) | 4404 (4197 to 4642) | 2541 (2412 to 2672) | 1863 (1620 to 2137) | CSII dominated |
| QALYs | 0.910 (0.892 to 0.927) | 0.916 (0.899 to 0.933) | –0.006 (–0.031 to 0.018) | |
The mean total 12-month costs for CSII were £4404 (median £4010, range £2995–13,175, 95% CI £4197 to £4642, n = 144) and for MDI were £2541 (median £2351, range £1295–5930, 95% CI £2412 to £2672, n = 149).
Disaggregated costs for prescribed insulin, outpatient visits and GP visits were not significantly different between the intervention groups and the extra costs associated with A&E department visits and electronic communications made only a modest (2.3%) contribution to the overall difference in mean total costs (£1863, 95% CI £1620 to £2137). The costs of training, covering the period from 15 days prior to randomisation to the end of follow-up, were £1230 (95% CI £1125 to £1337) in the CSII group and £1001 (95% CI £905 to £1100) in the MDI group.
Health utilities and quality-adjusted life-years
The HUI questionnaires were not completed for all patients. Thirteen of the 14 patients for whom there were no HUI data were aged < 3 years at randomisation and so were excluded from the analysis. Baseline utilities were missing for 23 patients in the CSII group and 14 patients in the MDI group.
For patients with complete baseline utility assessment, mean values were 0.870 (95% CI 0.847 to 0.892, n = 115) for the CSII group, compared with 0.888 (95% CI 0.866 to 0.909, n = 127) for the MDI group. Two hundred and fourteen patients (CSII group, n = 109; MDI group, n = 105) were included in the base-case analysis. Changes in utilities and each of the HUI domains (vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain) over the 12-month period are presented in Figures 7 and 8, respectively. Both intervention groups provided improvements in patients’ happiness (emotion) and how they perceived pain perception. However, there were no significant differences in QALYs between intervention groups. For the CSII group, these were 0.910 QALYs (95% CI 0.892 to 0.927 QALYs), and for the MDI group these were 0.916 QALYs (95% CI 0.899 to 0.933 QALYs), which was a difference of –0.006 QALYs (95% CI –0.031 to 0.018 QALYs).
FIGURE 7.
Mean utility values (95% CI) over 12 months for the base-case analysis.
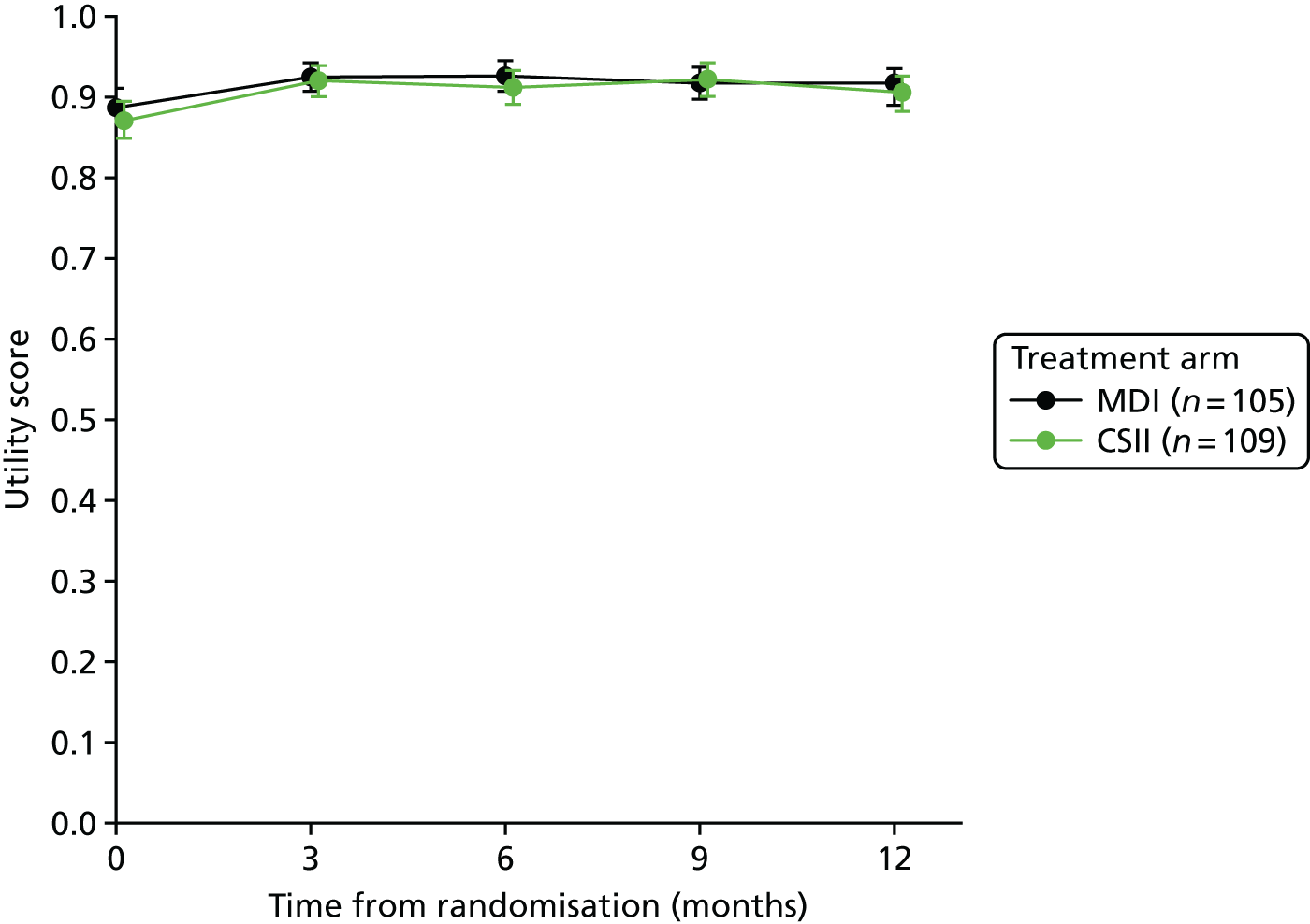
FIGURE 8.
Distribution of responses to HUI domain, by intervention group, over time. (a) Vision; (b) hearing; (c) speech; (d) ambulation; (e) dexterity; (f) emotion; (g) cognition; and (h) pain.

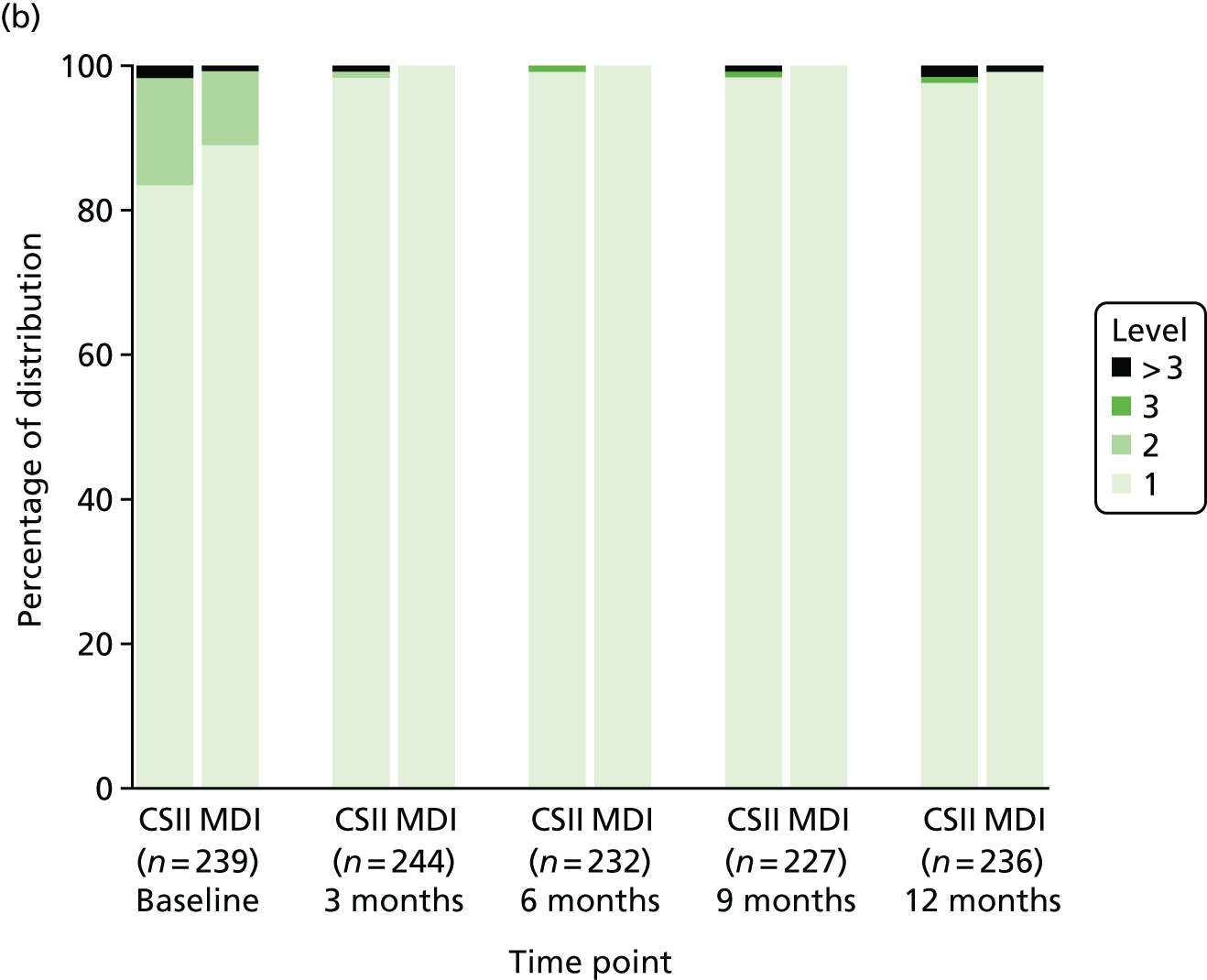

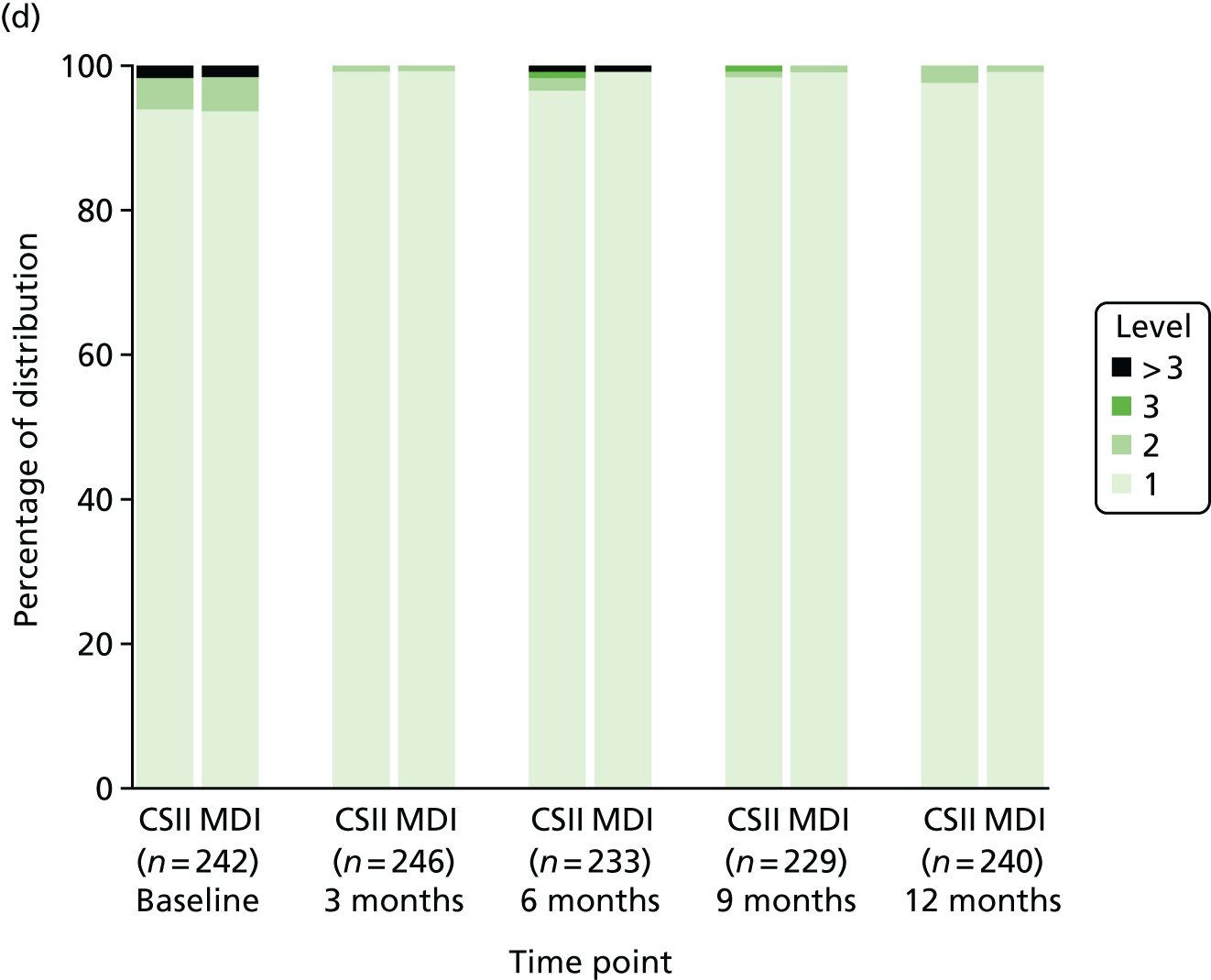
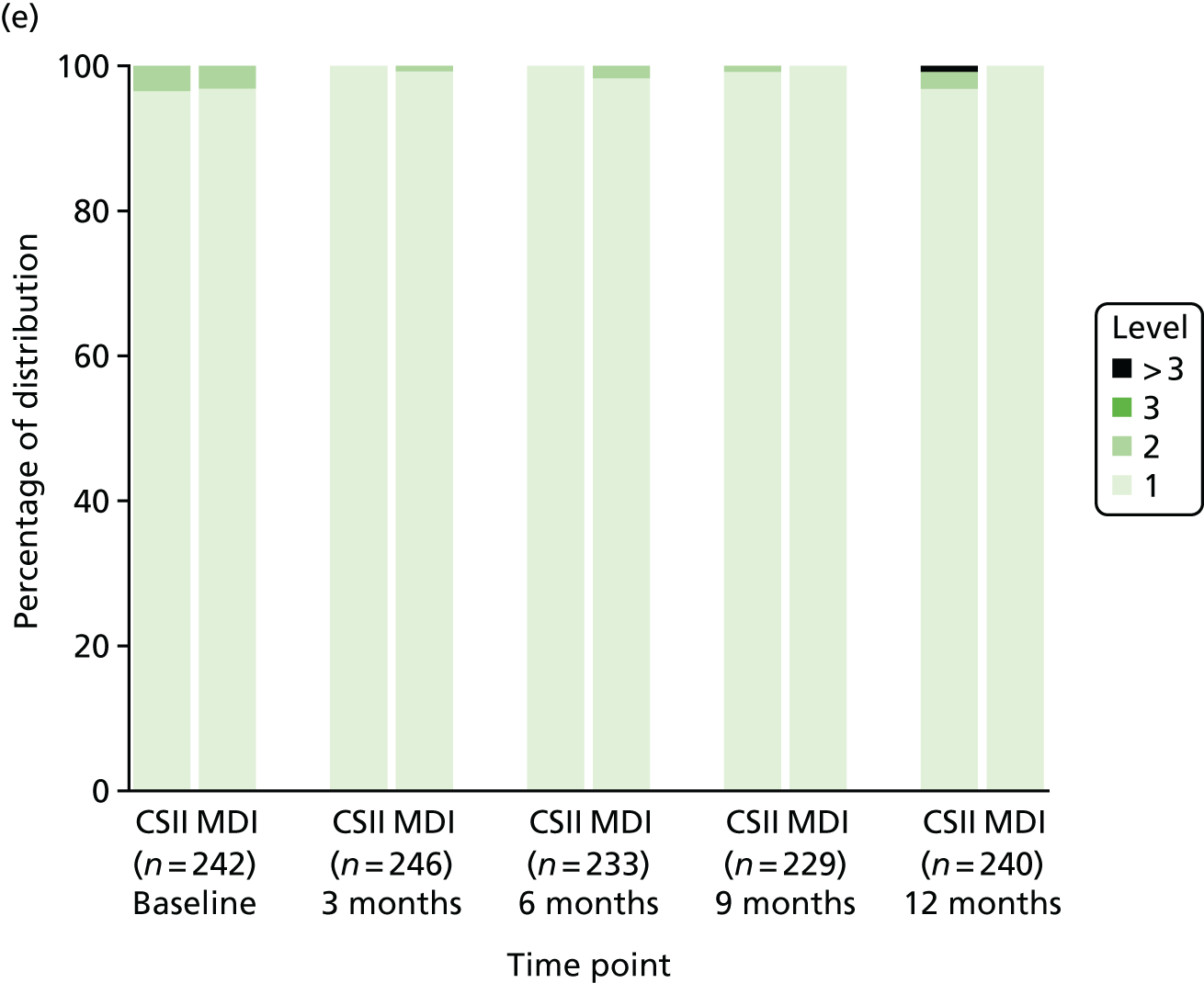


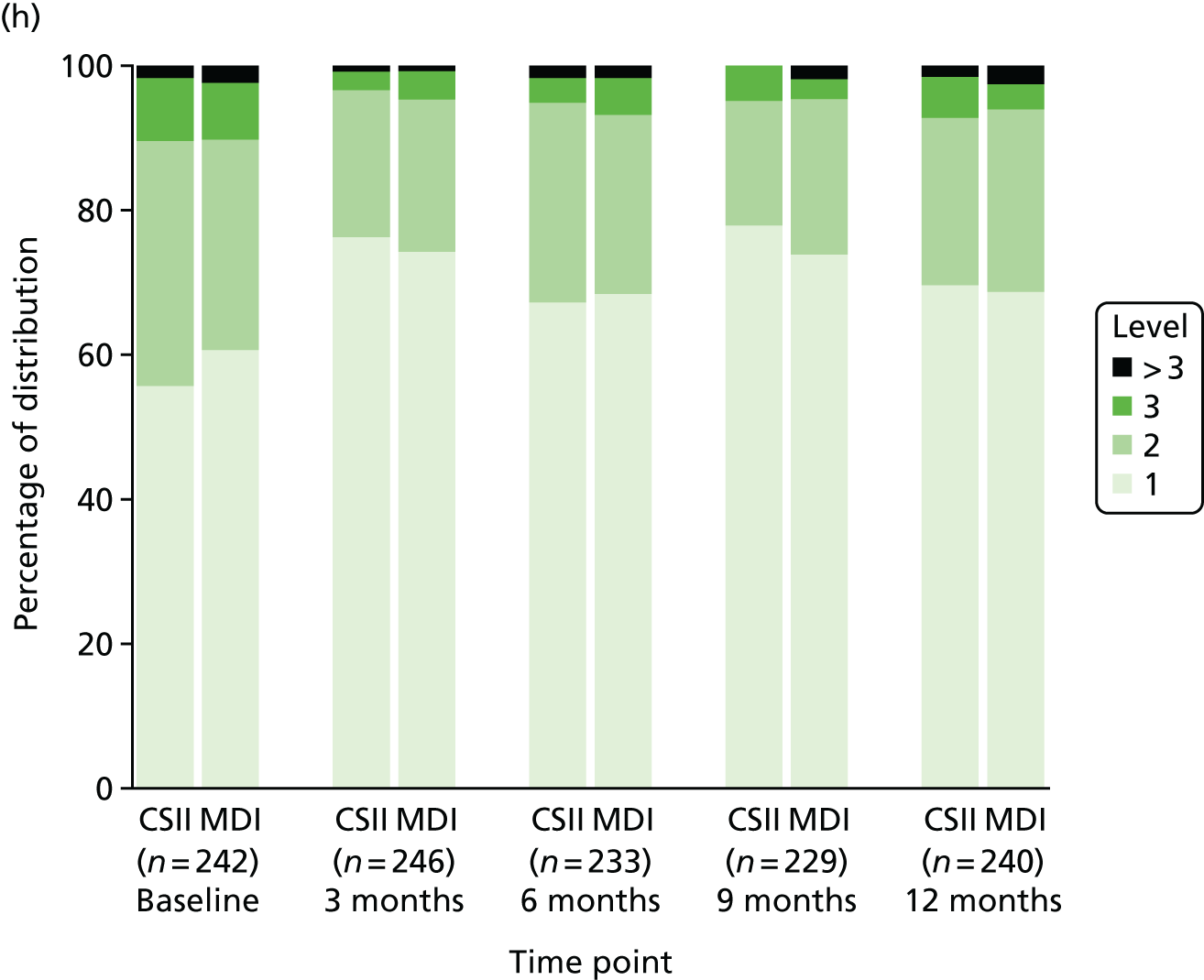
Incremental analysis
The ICER is indeterminate, as CSII was dominated by MDI over the 12-month time horizon of analysis (see Table 25). As the differences in costs favoured MDI (£1863 less expensive) and as there was no difference in benefits (–0.006 QALYs), through cost minimisation, MDI is more cost-effective.
Uncertainty analysis
The cost-effectiveness plane, which presents the difference in costs on the vertical axis and difference in effects on the horizontal axis, is shown in Figure 9. Sixty-nine per cent of iterations were in the north-west quadrant, reflecting the probability of CSII being dominated by MDI. The corresponding cost-effectiveness acceptability curve (Figure 10) indicates that CSII is not cost-effective, even at very high thresholds, consistent with the certainty of there being a large cost difference with no improvement in QALYs.
FIGURE 9.
Cost-effectiveness plane presenting the joint uncertainty in incremental costs and QALYs.
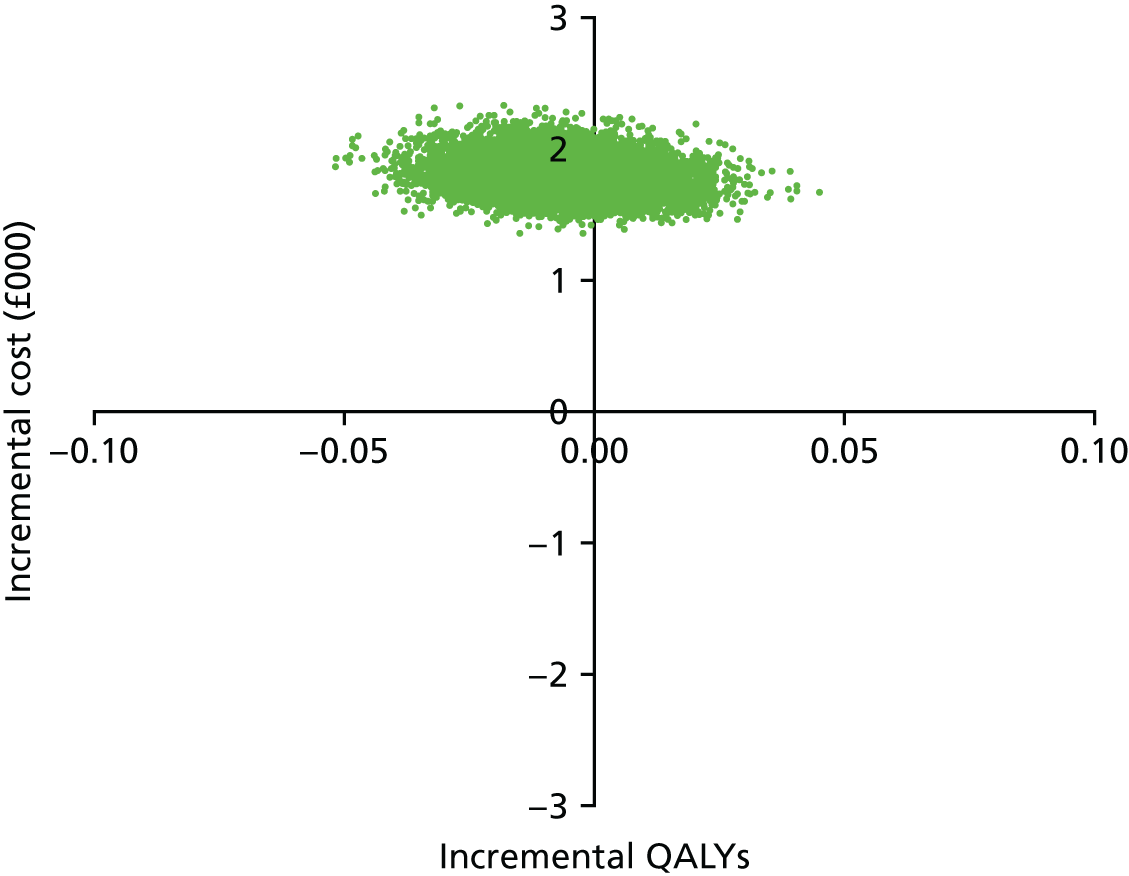
FIGURE 10.
Cost-effectiveness acceptability curve indicating the probability of CSII being cost-effective for different thresholds of cost-effectiveness. (a) Proportion 0–1; and (b) proportion 0–0.01.
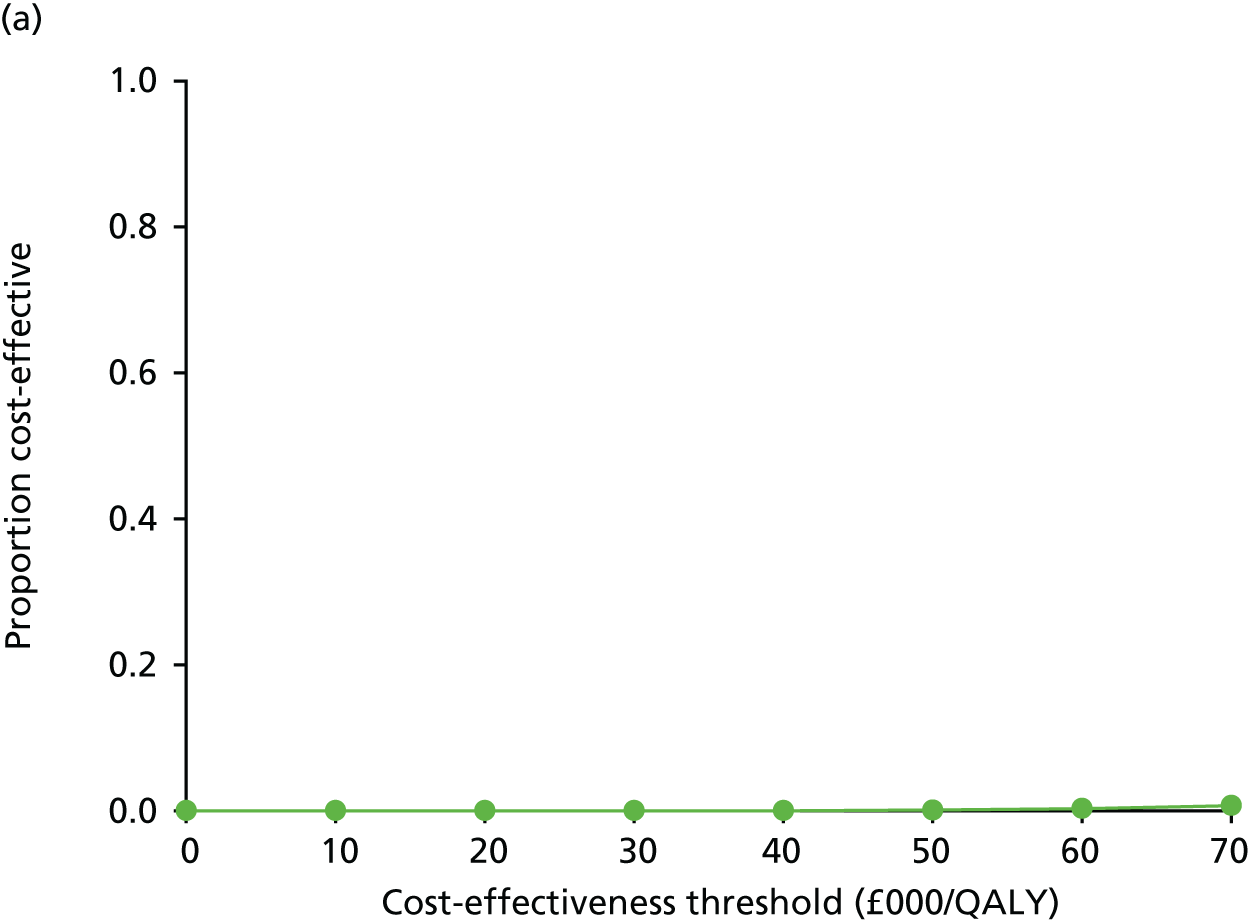
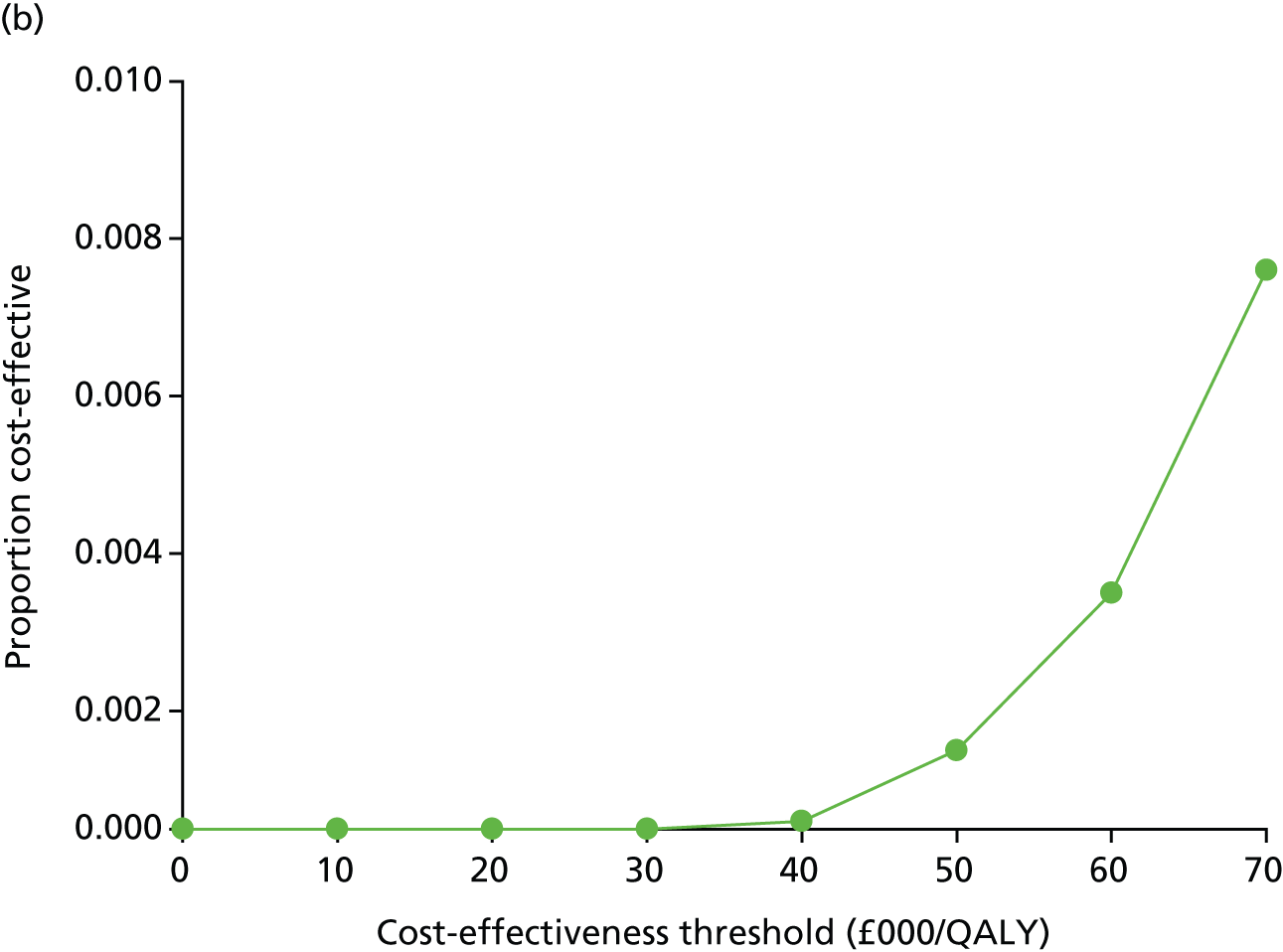
Sensitivity analysis
An assumption of insulin pumps being replaced every 2 years increased the cost difference between CSII and MDI to £2463 (95% CI £2218 to £2738), but although less frequent replacement (every 6 years) lowered the cost difference to £1663 (95% CI £1420 to £1937), CSII remained dominated.
An analysis based on the quantity of insulin administered, as opposed to prescribed, resulted in a mean cost of £4102 (95% CI £3915 to £4318) in the CSII group and £2192 (95% CI £2080 to £2310) in the MDI group, which was a difference of £1910 (95% CI £1690 to £2151).
All sensitivity analyses concerning the calculation of QALYs consistently indicated that there was no difference over 12 months (Table 26). Based on the Canadian tariff HUI3 scores, the difference between group means (CSII – MDI) was –0.004 QALYs (95% CI –0.036 to 0.029 QALYs). Alternative approaches for dealing with missing data, in which 29 additional patients in the CSII group and 36 additional patients in the MDI group were included, yielded similar QALY differences. Using MICE, this was –0.004 QALYs (95% CI –0.022 to 0.014 QALYs) and, based on LOCF, the difference was –0.001 QALYs (95% CI –0.022 to 0.020 QALYs). HUI2-derived QALY differences based on full parental and full patient responses to the HUI questionnaires were, respectively, –0.001 QALYs (95% CI –0.024 to 0.023 QALYs) and –0.006 QALYs (95% CI –0.031 to 0.018 QALYs).
| Scenario | Treatment arm | Difference, mean (95% CI) | |||
|---|---|---|---|---|---|
| CSII | MDI | ||||
| n | Mean (95% CI) | n | Mean (95% CI) | ||
| QALYs (base case) | 105 | 0.910 (0.892 to 0.927) | 109 | 0.916 (0.899 to 0.933) | –0.006 (–0.031 to 0.018) |
| QALYs (Canadian tariffs)a | 105 | 0.908 (0.884 to 0.929) | 109 | 0.912 (0.887 to 0.933) | –0.004 (–0.036 to 0.029) |
| MICE-imputed QALYs | 138 | 0.910 (0.896 to 0.923) | 141 | 0.915 (0.903 to 0.926) | –0.004 (–0.022 to 0.014) |
| LOCF-imputed QALYs | 138 | 0.911 (0.896 to 0.925) | 141 | 0.912 (0.896 to 0.926) | –0.001 (–0.022 to 0.020) |
| Parental QALYs | 105 | 0.914 (0.897 to 0.930) | 109 | 0.915 (0.899 to 0.931) | –0.001 (–0.024 to 0.023) |
| Child QALYs | 105 | 0.910 (0.892 to 0.927) | 109 | 0.916 (0.899 to 0.932) | –0.006 (–0.031 to 0.018) |
None of the sensitivity analyses affected the base-case result of CSII being dominated by MDI.
Exploratory analysis
Cost distributions for both intervention groups deviated from normality, with p-values for both skewness and kurtosis normality testing being < 0.01. However, based on the Akaike information criterion for different model specifications, an ordinary least squares regression was optimal for both costs and QALYs.
In the cost regression, 46% of the variability in total costs could be explained, with intervention group, recruiting centre and pre-randomisation costs being significant. The adjusted cost difference between the intervention groups was comparable to the base-case analysis at £1850 (95% CI £1600 to £2100).
In the QALY regression, 41% of the variability in total costs could be explained, with only baseline utility being significant. The adjusted difference in QALYs between intervention groups was consistent with the base-case analysis at 0.000 QALYs (95% CI –0.019 to 0.020 QALYs).
As there were no differences between intervention groups in HbA1c concentrations at 12 months, and no indication of CSII being cost-effective given the large difference in cost, longer-term extrapolation was considered to be futile, especially given the lack of validation of diabetes models in children.
Chapter 5 Discussion
Summary of findings
The results of the SCIPI study clearly demonstrate that, across the range of ages from 7 months to 15 years, treatment with CSII is not superior to treatment with MDI during the first year of treatment for T1D and is not a cost-effective treatment. At 12 months following diagnosis, HbA1c concentrations differed by only 2.4 mmol/mol (p = 0.1), being lower in participants treated with MDI and with our study having been powered to detect a difference of > 0.5%, approximately 5 mmol/mol. The percentage of participants whose HbA1c concentration was within the target range (< 48 mmol/mol) at 12 months differed between the treatment arms by 5% (CSII, 15.4%; MDI, 20.4%) and was also not significantly different (p = 0.28).
The proportion of patients in the CSII group who experienced AEs (28%) was nearly three times that in the MDI group (10%). During the course of the study, there were 11 severe AEs (nine episodes of severe hypoglycaemia and two of DKA), of which nine occurred in patients treated with CSII.
Patient-reported disease-related QoL did not differ between treatment arms, although parent-reported measures of QoL showed higher scores for patients who were treated with CSII, indicating a superior QoL. However, it should be noted that child-reported QoL starts from age 5 years, whereas the parent-reported QoL starts from age 2 years, and that, in general, the size of the differences and CI widths are similar between child- and parent-reported measures.
Patients randomised to CSII had more than twice as many A&E department visits and inpatient stays relating to the management of T1D as those in the MDI group. Over the 12-month period, health-care professionals had, on average, 4.3 (95% CI 0.6 to 8.0) more contacts (texts, e-mails and telephone calls) with participants in the CSII group than with those in the MDI group. The mean total costs were £1863 (95% CI £1620 to £2137) higher in the CSII group than in the MDI group, with the majority of this difference (£1177) attributable to the additional cost of consumables and device (annualised cost of £600 for CSII vs. £80 for pen devices). There were no significant differences in QALYs between the CSII and MDI groups (–0.006 QALYs, 95% CI –0.031 to 0.018 QALYs).
In summary, the SCIPI study achieved excellent completeness of primary outcome data for assessment of efficacy of CSII compared with MDI. There were no differences in key baseline characteristics between groups at randomisation. There was no difference in efficacy between CSII and MDI in any of the primary ITT analyses. In addition, there was no difference in efficacy between CSII and MDI in the per-protocol ITT analyses. The economic evaluation indicated that CSII does not represent a cost-effective option for the management of T1D in patients representative of the SCIPI study population. There was also no evidence of a difference in QALY gain over the 12-month period of observation in participants treated with CSII compared with those receiving MDI, and the cost differential was such that, even for willingness-to-pay values far in excess of the NICE £30,000 per QALY threshold for cost-effectiveness, CSII remained dominated by MDI.
Patient recruitment and retention
The large and diverse population of patients recruited to the SCIPI study, coupled with a high retention rate (97%), enabled us to overcome elements of bias inherent in previous RCTs and observational studies. The characteristics of study participants show that the treatment arms were well balanced for factors known to influence glycaemic control, including deprivation score, sex and ethnicity. Screening log data show that these characteristics were also well balanced between the groups of patients who consented to participate in the study and those who declined. The distribution of ages in the study population reflects the known demographic of childhood T1D, with approximately 50% of participants being in the age range of 5–12 years.
Of those who declined to participate, most (66%) cited a strong preference to continue treatment with MDI, although a smaller number (9%) cited a strong preference for CSII. We noted that the small number of patients and parents who declined to participate in the study, stating a strong preference for CSII (n = 36), had a higher deprivation index (were more deprived) than those who declined stating a strong preference for MDI (n = 259) and those who participated in the study. National database studies37–39 report an association with CSII use and deprivation, with the most deprived patients being least likely to be treated with CSII. Our data suggest that this association is established sometime later after the diagnosis of T1D.
Recruitment rates showed marked variability between study sites, ranging from 16% to 82% of eligible patients. Both the highest- and lowest-recruiting centres had participated in the DECIDE trial68 and so were experienced in the recruitment of paediatric patients to research studies at the time of diagnosis of T1D. In the lowest-recruiting centre, intensive insulin therapy was introduced later in the first year of treatment and patients were treated with four or fewer injections per day at the time of diagnosis. A requirement to either increase the number of injections or to learn a new method of insulin delivery is likely to have been least attractive to this population.
We recognised that patients who were given information about the study closest to the time of diagnosis were more likely to consent than those who were approached later. This may be because a preference for MDI had yet to be established or may reflect qualities of the research staff that increased the likelihood of patients consenting to participate in the study.
We speculate that the strong preference that patients stated for MDI at the time they were invited to participate in the study is likely to be temporary. It would have been ideal to undertake qualitative work to understand more about the reasons for this preference and how it may change over time. A higher recruitment rate may have been achieved had the window for recruitment been increased to 1 month from diagnosis.
These observations underscore the difficulty in predicting recruitment rates to clinical trials. The target recruitment rate of 50% was set, in part, to ensure that a highly selected cohort of patients was not recruited. Despite our lower recruitment rate, we have been able to demonstrate that the characteristics of those who consented to participate did not differ significantly from those who declined, and we are confident that we have studied a population of patients representative of the background population of paediatric patients treated for T1D in the NHS setting.
The non-participation of patients with a strong preference for one treatment arm may limit the generalisability of our data. It would be interesting to learn more about the reasons behind this strong preference early in the diagnosis of T1D and how this may change over time. A qualitative component within the SCIPI study would have been beneficial.
By randomly allocating treatment to either MDI or CSII, we have overcome the bias in observational studies, in which treatment selection by patients, carers and health-care professionals results in over-representation of favourable patient characteristics in those treated with CSII and under-representation of these characteristics in those treated with MDI.
Previous RCTs of CSII and MDI have recruited patients with established T1D already being treated with MDI, and have randomised them to continue with MDI treatment or to change to CSII treatment. This recruitment strategy may introduce bias in favour of CSII, as patients who are satisfied with treatment with MDI and who have good glycaemic control are less likely to be approached or to participate in the study than those in whom treatment is less satisfactory. Furthermore, previous experience of treatment success or failure with MDI may influence treatment outcomes: if a patient dissatisfied with MDI treatment consents to participate in a RCT, it is possible that randomisation to CSII will result in renewed commitment to the management of T1D; however, randomisation to continue unsatisfactory treatment with MDI may result in further disengagement. Another factor to consider is the extent to which any studies reporting benefits of CSII over MDI in established patients reflect the effects of the intensive education and increased contact with health-care professionals required before starting CSII rather than inherent benefits arising directly from CSII. These possible effects on outcomes were balanced in our study design by the selection of newly diagnosed patients, all of whom would have required a similar intensity of education. We elected to recruit patients at diagnosis of T1D to overcome this element of bias. However, it may be that the generalisability of our findings beyond the first year of T1D is therefore limited.
Fifty-three patients moved between treatment arms: 30 changed from MDI to CSII, 22 from CSII to MDI and one from MDI to ‘injections TDS regime’. In one study site, four out of five patients who were randomised to MDI were changed to CSII and none from CSII to MDI, and, in another site, 9 out of 16 patients who were randomised to MDI were changed to CSII and 3 out of 14 changed from CSII to MDI. It is likely that there is a degree of bias towards CSII in these centres and this may account, in part, for the minor imbalance in movement between treatment arms. We also observed an imbalance in movement between treatment arms in the youngest age group, favouring a switch from MDI to CSII. This may also reflect a bias towards treatment with CSII in this age group, perhaps because of the difficulties of administering frequent injections in children in an age group who eat frequently and unpredictably throughout the day. The administration of very small insulin doses in the youngest children can also be difficult using MDI and this can be accommodated more easily using CSII.
When we examined the reasons for changing between treatment arms, we found that HbA1c concentration was above the target range in 20 out of 22 patients (90.9%) at the time of changing from CSII to MDI and in 16 out of 30 patients (53%) at the time of changing from MDI to CSII. Severe or frequent hypoglycaemia was not reported as a reason for changing treatment arm. Our data suggest that, following randomisation, patient and parent preference was the strongest determinant of insulin delivery device.
Effectiveness of continuous subcutaneous insulin infusion and multiple daily injections therapy
Glycaemic control
We selected glycaemic control, measured as HbA1c concentration 1 year after the diagnosis of T1D, as the primary outcome measure of treatment efficacy and the percentage of patients in whom HbA1c concentrations were within the target range as a secondary outcome measure. Since the publication of the findings of the Diabetes Control Complications Trial Research Group,3 HbA1c concentration has been recognised as the most robust predictor of the risk of long-term T1D complications, and there is some evidence that this is particularly true during the first year after diagnosis. 7–9 Using this measurement of glycaemic control, we found no difference between treatment arms, at either 6 or 12 months following diagnosis. Previous RCTs comparing MDI and CSII have reported similar findings. 43,45,47,48 The significance of our results for the management of patients beyond 1 year is uncertain. However, there is good evidence that glycaemic control in the first year following diagnosis is a predictor of long-term glycaemic control and the risk of vascular complications in young adult life. 7–9
A number of alternative outcome measures could have been used to compare the effectiveness of MDI and CSII, including glycaemic variability and preservation of pancreatic function. In vitro and animal studies have demonstrated that fluctuating glucose concentrations cause more cell damage and oxidative stress generation and induce monocyte–endothelial adhesion and atherogenesis to a greater degree than sustained hyperglycaemia. 137,138 It has also been reported that decreased, as well as increased, glycaemic variability may influence the expression of genes that protect cells from the toxic effects of hypoglycaemia, when compared with normal blood glucose profiles. 139
Glycaemic variability has been seen to be lower in children treated with CSII than in those treated with MDI, despite there being no difference in HbA1c concentrations. 32,140 In one cross-sectional cohort study, surrogate markers of oxidative stress and cyclo-oxygenase activity were also favourable and glycaemic control was superior in patients treated with CSII than in those treated with MDI. However, in studies in which treatment arms are not randomly assigned it may be difficult to determine whether the observed differences in glycaemic control and variability are a result of the mode of insulin delivery or patient characteristics that cluster with each treatment arm.
Although there is good evidence linking glycaemic variability and microvascular and macrovascular complications in type 2 diabetes, the evidence is less convincing for T1D. 141,142 This may be, in part, because the best measure of glycaemic variability (e.g. coefficient of variation of fasting plasma glucose, mean amplitude of glycaemic excursions, SD of blood glucose) has yet to be determined and because previous studies have used self-monitored blood glucose readings rather than continuous blood glucose monitoring. For now, it seems that HbA1c concentrations remains the most appropriate primary outcome measure in studies of treatment effectiveness but measures of glycaemic variability should be considered as important secondary outcomes measures in future studies.
Partial remission and insulin dose
During the partial remission phase (PRP), there is some recovery of pancreatic islet cell function, insulin requirements fall and incidents of hypoglycaemia and hyperglycaemia occur less frequently and are less severe. Glycaemic control may be near perfect on minimal doses of insulin. In a study of over 3000 children with newly diagnosed T1D, approximately 70% experienced a PRP, which lasted 9 months (range 0–21 months). Younger age at diagnosis and female sex were associated with a lower prevalence of PRP. 143 Those who present with DKA or who have a lower pH or higher antibody titres at diagnosis are also less likely to experience PRP.
The longer-term implications of the PRP are unclear, although a recent study related PRP with the risk of microvascular complications; 7 years following diagnosis, young adults who did not experience a PRP were more likely to have one or more microvascular complication. 144
A number of measures of beta cell function have been proposed for the definition of PRP, including measurements of basal and stimulated C-peptide. 145,146 However, these measurements require an early-morning attendance at hospital, which requires a delay in eating breakfast and the administration of the first morning dose of insulin. It may be especially difficult and inconvenient for patients treated with MDI to ensure an adequate ‘washout’ period following a dose of a long-acting insulin analogue for measurements of C-peptide to be meaningful.
In our original study protocol, we had not intended to undertake studies of residual pancreatic function. However, the publication of a new, validated method of assessing pancreatic reserve, based on insulin dose and HbA1c concentration gave us the opportunity to explore this phenomenon in our population using data collected to inform other study outcomes. 147 This measure of residual pancreatic function was defined from a group of 275 patients aged < 16 years at diagnosis of T1D. Residual β-cell function was assessed by stimulated C-peptide measured 1, 6 and 12 months following diagnosis. To develop the definition, a multivariate analysis was performed, which demonstrated a negative correlation between stimulated C-peptide, HbA1c concentration and insulin dose. From these observations, the authors developed a formula for IDAA1c, in which a C-peptide response of > 300 pmol/l correlated well with IDAA1c ≤ 9 (R2 = 31%).
Using this method, IDAA1c, we found no difference in the percentage of patients in PRP at either 6 or 12 months. When considering these data, it is important to note that the determination of IDAA1c relies on accurate recording of insulin doses.
For both treatment arms, insulin doses were downloaded from the F. Hoffman-La Roche AG Expert glucometer. For those randomised to CSII, insulin doses were also downloaded from insulin pumps and for those randomised to MDI data were obtained from patient-held records. The amount of insulin prescribed by the patients’ family practitioners was also recorded. Data from these multiple sources showed that patients treated with MDI received significantly lower doses than those treated with CSII; this is in contrast to previous studies in which patients treated with MDI received higher doses of insulin. 35,46,82
Patients randomised to the MDI arm of the study were advised that an insulin injection should be given every time ≥ 10 g of carbohydrates was consumed. It is likely that this will deter some patients from consuming carbohydrates between mealtimes. Patients randomised to the CSII arm of the study were given a lower threshold for the administration of an insulin bolus, 5 g of carbohydrate, in part because the administration of an insulin bolus is less intrusive than the administration of an injection. By giving this advice, we may have encouraged higher insulin usage in children treated with CSII.
Insulin usage differed between treatment arms only in patients aged ≥ 12 years, when those treated with CSII reported greater insulin use than those treated with MDI. This may reflect poorer reporting during adolescence and/or a reduction in the intensity of insulin injections as patients become increasingly independent.
Our data are subject to these methodological limitations, but, although recognising that these exist, we found no evidence that patients treated with CSII have lower insulin requirements than those treated with MDI or that the prevalence of the PRP was different between treatment arms at any time point.
Growth and weight gain
In common with previous studies, children recruited to the SCIPI study had a height SDS that was just above the mean (0.3, SD 1.0) of our reference population at diagnosis of T1D. 148 Growth during the first year of T1D was normal in both treatment arms and there was no difference in either baseline height SDS or change in height SDS between treatment arms.
Gain in weight has been reported to be greater in children treated with MDI than in those treated with CSII,46 and this may be important, as obesity increases the risk of long-term macrovascular complications. In the SCIPI study protocol, baseline weight measurements were taken on the day that the randomised treatment was started, to allow time for adequate rehydration in children who were dehydrated at diagnosis. The baseline BMI SDS was normal and, similar to height SDS, it was just above the mean for the reference population (0.1, SD 1.3). In both treatment arms, BMI SDS increased: to 0.8 (SD 1.1) in the CSII arm and 0.7 (SD 1.0) in the MDI arm. This trend for weight gain may be of clinical concern if it continues in subsequent years.
Adverse events
The incidence of severe AEs was too low for us to draw any meaningful conclusions regarding the relative risk or benefits of either treatment. However, it is noteworthy that, of the 11 severe AEs that were reported (nine episodes of severe hypoglycaemia and two of DKA), nine occurred in patients treated with CSII. Previous RCTs have also reported a low prevalence of severe hypoglycaemia and DKA and concluded that there is no significant difference between treatment arms. 43–47
It is possible that AEs were under-reported. This is unlikely for DKA but may be more likely for episodes of hypoglycaemia, which may be considered to be an inevitable part of T1D treatment. We recorded only severe hypoglycaemia, and frequent and less severe episodes of hypoglycaemia may also be debilitating.
A perceived advantage of CSII therapy is a reduction in the number of episodes of severe hypoglycaemia compared with treatment with MDI. 42,149 In 2010, Health Technology Assessment published a report that compared the clinical effectiveness and cost-effectiveness of CSII and MDI, which concluded that there were fewer problems with hypoglycaemic episodes in patients treated with CSII than those treated with MDI. 81 However, this systematic review included studies of MDI using Neutral Protamine Hagedorn insulin, which is associated with a higher incidence of hypoglycaemia than the insulin analogues used in modern MDI regimens. 71
More than twice as many patients randomised to treatment with CSII as those randomised to treatment with MDI experienced AEs (28% vs. 10%, respectively), with the IDR being 24 for patients treated with CSII and 9 for patients treated with MDI.
A number of studies describing AEs during CSII therapy in childhood have been reported. In a large clinic population of 235 children, 45% of patients had experienced at least one insulin pump-related AE in the preceding 12 months, 8% required hospital treatment and 25% required a replacement insulin pump. 150 A very similar prevalence of CSII-related AEs (46% of treated patients) has been reported independently by another group,151 and these data were supported further by a systematic review152,153 of AEs in adult and childhood CSII therapy, which found that > 40% of patients per year had an insulin pump-related AE, with hyperglycaemia and DKA occurring most commonly. The risk of AEs is reported to be lower in those who have used CSII for longer, higher in children than in adults and unrelated to socioeconomic status or glycaemic control. 151
We found no evidence of a reduced risk of severe hypoglycaemia, DKA or other AEs in patients treated with CSII.
Quality of life
It was our intention to minimise disruption to the routines of patients and the clinics in order to achieve a high level of recruitment and retention. For this reason, the number of additional tasks patients and parents were asked to perform was kept to a minimum. We decided to restrict assessments of QoL to a simple questionnaire-delivered tool, the diabetes module of the PedsQL instrument. 79 Patients who were aged ≥ 5 years completed questionnaires according to age (5–8, 9–12 and ≥ 13 years) and parents completed questionnaires for children aged 2–4, 5–8, 9–12 and ≥ 13 years.
We observed some discordance in the reports from parents and patients, as parents reported higher scores and, therefore, better QoL in those treated with CSII than in those treated with MDI but there was no difference in the scores reported by patients. The differences in parental scores were most notable in the youngest children, and particularly in the treatment domains for the youngest children.
Discordance in parental and patient reporting in measures of QoL is well recognised. In a large study154 of 3402 children with T1D who were aged 5–18 years, which also used the PedsQL questionnaire, children reported a better QoL than that reported by their parent/proxy, except children aged 5–7 years in whom parent/proxy QoL reports were higher. The authors of this study concluded that both sources of reporting are valuable but that when discrepancies exist between patients and parent/proxy reports, preference should be given to the patient’s report. 154
Economic evaluation
The economic evaluation indicates that CSII does not represent a cost-effective option for the management of T1D in patients who were representative of the SCIPI study population. There was no evidence of a difference in QALY gain over the 12-month period of observation compared with MDI, and the cost differential was such that, even for willingness-to-pay values far in excess of the NICE £30,000 per QALY threshold for cost-effectiveness, CSII remained dominated by MDI.
The principal cost drivers, accounting for 91% of the difference between intervention groups, were the consumables and insulin pumps. These costs were not offset by any reduction in the prescribing of insulin or lower use of health-care services. In fact, patients who were randomised to CSII had more than twice as many A&E department visits and inpatient stays relating to the management of T1D as those in the MDI group. This may be attributable to the higher number of AEs associated with the use of CSII.
Sensitivity analyses confirmed the robustness of the results to different assumptions regarding the lifetime of the CSII insulin pump, the use of alternative methods for calculating utilities and of approaches to estimate the overall costs of insulin.
The analysis benefited from having complete cost data, achieved by obtaining resource use data from multiple sources, including routinely collected data and questionnaires. There were some missing responses to the HUI questionnaire, but different approaches of imputation and use of different tariff scores resulted in QALY estimates that were consistent with the base-case analysis. It should be acknowledged, however, that the trial was not powered to detect a statistically significant difference in QALYs between intervention groups.
The main limitation of the analysis concerns the short period of follow-up. The impacts of T1D and treatment modalities on QALYs are unlikely to be evident in the first year of treatment. Rather, QALY decrements are most likely to result from the long-term microvascular and macrovascular complications of T1D, and their associated impacts on health-related QoL and survival. In the absence of data from extended follow-up, the conventional approach of modelling life-long costs and consequences is usually taken. However, models that are not validated in paediatric populations, especially in the context of interventions that are not more clinically effective or likely to be cost-effective, would have limited credibility and offer little contribution to the understanding of longer-term economic outcomes.
Strengths and limitations
It is a major strength of the SCIPI study that a large and diverse population of patients has been recruited from a wide range of clinical settings across the UK, with near-complete retention at the conclusion of the study protocol. Our patient population is representative of the background population of patients diagnosed with T1D in study centres during the recruitment period of the study. We are confident that our data are generalisable to children treated for T1D in the NHS setting. Our recruitment strategy has also overcome the main sources of bias inherent in previous studies.
Using well-established and widely accepted measures of treatment effectiveness, we found no evidence that treatment of diabetes in the first year after diagnosis with CSII therapy is superior to treatment with MDI other than higher QoL on parent/proxy reporting.
We recruited patients at diagnosis of T1D to address important issues of bias inherent in some previous studies. However, our findings may not be applicable beyond the first year of diagnosis. The presence and duration of the PRP may mask subtle differences in therapeutic benefits and glycaemic control between treatment groups. Patient and parent education and knowledge may enable more sophisticated use of CSII over time and this may also confer an advantage compared with MDI in established patients, as suggested by some other studies.
We also recognise that the use of CSII at diagnosis of T1D was not routine practice at the time that the SCIPI study opened to recruitment, and outcomes may have improved over time as diabetes teams have gained experience in the use of this treatment modality. However, we also note that the primary outcome measurement was obtained 1 year after diagnosis, when all participants and professionals would have a minimum of 12 months’ experience of both CSII and MDI, and was not a composite of measurements taken over the first year.
It is important to recognise that there is a strongly held view among health-care professionals, patients and families that CSII therapy is a valuable tool in the treatment of T1D, and is superior to treatment with MDI. It may be that we have not used the correct tools to identify the reasons for this or that the tools we have used are not sensitive enough to identify the benefits of treatment with CSII. This may be especially true for measures of QoL, and the absence of a measure of treatment satisfaction. Anecdotal reports from patients and parents often cite QoL as the main advantage of CSII therapy.
Qualitative work, examining the beliefs of physicians prescribing CSII, found there was acceptance that CSII therapy was not necessarily associated with superior glycaemic control compared with MDI. 155 This was attributed to the complexity of CSII therapy and the failure of patients and families to use it to its full potential, in part, owing to a lack of support in the community and at school. The same study found a strong attraction to CSII because of its status as a novel technology, as a necessary step towards more sophisticated and successful therapies that should be embraced and encouraged. However, the authors also recognised that parents may feel pressurised to allow use of CSII in their children, given the strong advocacy for CSII therapy from support groups and some health-care providers. Perhaps most importantly, physicians reported social benefits of the use of CSII: allowing adolescent patients to become more independent and reducing parental stress around the unpredictable eating habits of young children and the need for frequent insulin injections. The physicians also felt that CSII therapy facilitated the physician–patient relationship. In order to fully understand the drivers behind the widespread adoption of CSII, tools will need to be developed that accurately harness this information.
Chapter 6 Conclusions
Implications for diabetes treatment strategies
The purpose of the SCIPI study was to generate objective data to inform national paediatric treatment strategies and individual patient care. We speculated that, in the absence of benefit of CSII therapy, resources may be better invested in services that are likely to benefit a wider range of patients.
In the NPDA of 2015/16,1 2800 children aged < 15 years were diagnosed with T1D. Given that 30% of patients are currently treated with CSII, our data suggest that the additional cost to the NHS of treating newly diagnosed patients with CSII in preference to MDI would reach approximately £1.5M per year, with no objective evidence of benefit, at least in the year following diagnosis.
The data from the SCIPI study should reassure families that there is no evidence that MDI therapy is inferior to CSII during the first year of T1D treatment. We have provided new evidence to clinicians that the driver for the adoption of CSII therapy at diagnosis of T1D should not be improved glycaemic control or AEs; indeed, we would encourage consideration of the increased prevalence of AEs in those treated with CSII.
The social benefits of CSII have not been quantified in this study, and perhaps the balance between cost and benefits in this domain is an issue for wider discussion between funders, health-care professionals, patients and families.
Future research
The findings of the SCIPI study may be applicable only during the first year following diagnosis of T1D. On completion of the study protocol, participants and their families were invited to consent for the collection of routine clinical data for a further 9 years. We therefore have the opportunity to learn more about the longer-term trajectories of this valuable and unique cohort of patients and whether or not outcomes diverge between treatment groups over time.
It is important to recognise that many patient advocacy groups and health-care professionals have a strong belief in the benefit of CSII. The measures of QoL used in the SCIPI study may not have been sensitive enough to detect the QoL benefits reported by the advocates of CSII, and we have not measured generic QoL or the impact of either treatment on social function across childhood and into adolescence. More detailed investigation in these domains may contribute to the critical analysis of the place of CSII in national diabetes strategies.
The need to examine how resources are distributed has become increasingly evident as relationships between socioeconomic status, CSII therapy and glycaemic control are reported. Observational cohort studies from across the world report superior glycaemic control and increased CSII use in children from more affluent families, and their parents have lower unemployment rates and higher educational levels than those children treated with MDI, whereas socioeconomic deprivation and ethnic minority status are associated with poor glycaemic control, independent of CSII use. 38,39,156,157 Unless efforts are made to correct this disparity, health-care resources will continue to be drawn away from the most vulnerable patients to support CSII therapy, with little evidence of benefit. We propose that research should now focus on interventions that may help to address the disparity in outcomes between the most affluent and disadvantaged children.
There is currently no validated, structured education programme for paediatric patients who are newly diagnosed with T1D, and previous studies of structured education have not demonstrated a beneficial effect on glycaemic control. 158 It may be that education to establish good management habits at the time of diagnosis will be more effective than in established patients, in whom poor management habits are likely to be entrained and instigating change may be difficult. The benefits of structured, validated education at diagnosis of T1D deserve further evaluation.
In this study, we did not record whether or not patients used CSII to its full potential. It is possible that enhanced CSII use would have resulted in improved glycaemic control and that this may be achievable through further education, training and support in the community. There may be a place for researching these elements of CSII use. However, such a package of care is likely to require increased investment, and this additional cost needs to be considered in the light of the health economic data reported in the SCIPI study. Very significant improvements in glycaemic control would be necessary for CSII to be seen as cost-effective. Should such a project be undertaken, it would be essential to also examine the effect of enhanced education and support in the community on glycaemic control in children treated with MDI.
Acknowledgements
We would like to thank the external members of the TSC for their advice and support for the project: Professor Peter Clayton (University of Manchester), Dr Christine Burren (Bristol Children’s Hospital), Dr Ian Craigie (Royal Hospital for Sick Children, Glasgow), Professor Gordon Murray (University of Edinburgh), Mrs Christine Manning and Mrs Christina McRoe (patient and parent representatives).
Our thanks also go to the IDSMC: Professor Stephen Green (University of Dundee), Professor John Wilding (University of Liverpool) and Dr Arne Ring (University of Leicester).
We would like to thank the SCIPI study co-ordinators: Rachel Breen (Senior Trials Manager), Amit Hansrahani, Rachel McMahon, Lola Awoyale, Emma Cwiklinski, Abigail Bennett and Amy Tao.
We are grateful to all the patients and their families for their generosity and commitment to the study. We would like to thank the principal investigators and RNs who recruited patients and supported them during the study: Birmingham Children’s Hospital – Professor Tim Barrett, Kimberly Dale, Catherine Lambden, Kate Penny-Thomas, Lucy Cooper, Lydiah Makusha and Charlotte Lilley; Royal Blackburn Hospital – Dr Chris Gardener, Chloe Rishton and Heather Collier; University Hospital of Wales, Cardiff – Linda Phillips; Doncaster Royal Infirmary – Dr Anuja Natarajan, Lynda Viles, Janet Shackleton and Sarah Besley; Ipswich Hospital – Dr Jackie Buck and John Hassler Hurst; Alder Hey Children’s Hospital, Liverpool – Dr Atrayee Ghatak, Ceri Evans and Dawn Case; Mid Staffordshire NHS Foundation Trust – Dr Olumuyiwa Oso, Marie Phipps, Carol Parton, Julie Hollins and Kelly Armor; Great North Children’s Hospital, Newcastle – Dr Tim Cheetham and Evelyn Thomson; Norfolk and Norwich University NHS Foundation Trust – Dr Nandu Thalange, Louisa Fear, Faye Stubbs, Joanna Watts and Louise Pointer; Nottingham Children’s Hospital – Dr Tabitha Randell and Caroline Saddington; Oxford Children’s Hospital – Dr Julie Edge, Isabel Reffell and Jane Bowen-Morris; Royal Preston Hospital – Dr Omolola Ayoola, Angela Waktare and Elaine McDonald; Sheffield Children’s Hospital – Dr Neil Wright and Linda Viles; Southampton Children’s Hospital – Dr Nicola Trevelyan, Ellen-May Anderson, Rebecca Cutts, Sarah Coulbeck, Gillian Crouch, Lisa-Marie Lueddeke and Louise Summerton; and East Surrey Hospital – Dr Neemisha Jain, Linda Bailey and Fiona Riley.
We would also like to thank Paul Newland, who oversaw the analysis of blood samples at Alder Hey Children’s Hospital.
Contributions of authors
Joanne Blair (Chief Investigator and Consultant Paediatric Endocrinologist) developed the study protocol in collaboration with co-investigators. She oversaw the delivery of the study, prepared study update reports, oversaw clinical aspects of the statistical analysis plan and clinical interpretation of study data and led the preparation of the final report. She was Chairperson of the Trial Management Committee.
Carrol Gamble (Senior Statistician) contributed to the study design, protocol development, and data capture methods and led the CTU involvement including all monitoring activities, the development of the statistical analysis plan and the study statistical analyses. She contributed to the preparation of the final report (drafting, reviewing and editing).
Andrew McKay (Study Statistician) undertook the final statistical analyses under the supervision of Carrol Gamble, prepared data for reports throughout the study, prepared data tables and figures for the final report. He was a member of the Trial Management Committee.
Dyfrig Hughes (Lead Health Economist) contributed to protocol development, oversaw the health economic aspects of the study and the final analyses, contributed to the final report (drafting, reviewing and editing). He was a member of the Trial Management Committee.
Colin Ridyard (Health Economist) undertook health economics analysis, managed all aspects of health economics elements of the study, contributed to the final report (drafting, reviewing and editing). He was a member of Trial Management Committee.
Matthew Peak (Co-Investigator and Director of Research, Alder Hey Children’s Hospital) contributed to protocol development, provided guidance on study delivery, supported interpretation of clinical data and contributed to the final report (drafting and reviewing). He was a member of the Trial Management Committee.
John W Gregory (Co-Investigator, Professor in Paediatric Endocrinology and Honorary Consultant) contributed to protocol development, provided guidance on study delivery, supported interpretation of clinical data and contributed to the final report (reviewing and editing). He was a member of the Trial Management Committee.
Mohammed Didi (Co-Investigator and Consultant Paediatric Endocrinologist) contributed to protocol development, provided guidance on study delivery, supported interpretation of clinical data and contributed to the final report (reviewer). He was a member of the Trial Management Committee.
Francesca Annan (Co-Investigator and Paediatric Diabetes Dietician) contributed to study design, prepared educational materials for study sites and contributed to the final report (reviewer).
Emma Bedson (Senior Trials Manager) gave guidance and support on all aspects of governance and study delivery and supported the preparation of progress reports. She contributed to the final report (reviewer) and was a member of the Trial Management Committee.
Keith Thornborough (RN and Paediatric Diabetes Nurse Specialist) recruited patients, provided guidance and expertise to RNs in study sites, led RN teleconferences and face-to-face meetings and supported interpretation of clinical study data. He contributed to the final report (reviewer) and was a member of the Trial Management Committee.
Publications
Blair JC, Peak M, Gregory JW. What is the best way to deliver subcutaneous insulin to infants, children, and young people with type 1 diabetes mellitus? BMJ 2011;343:d5221.
Blair J, Gregory JW, Peak M. Insulin delivery by multiple daily injections or continuous subcutaneous insulin infusion in childhood: addressing the evidence gap. Pract Diabetes 2012;29:47–8.
Blair J, Gregory JW, Hughes D, Ridyard CH, Gamble C, McKay A, et al. Study protocol for a randomised controlled trial of insulin delivery by continuous subcutaneous infusion compared to multiple daily injections. Trials 2015;16:163.
Arch BN, Blair J, McKay A, Gregory JW, Newland P, Gamble C. Measurement of HbA1c in multicentre diabetes trials – should blood samples be tested locally or sent to a central laboratory: an agreement analysis. Trials 2016;17:517.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Paediatric Diabetes Audit 2015–16 Report 1: Care Processes and Outcomes. London: Royal College of Paediatrics and Child Health; n.d.
- Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. EURODIAB study group . Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009;373:2027-33. https://doi.org/10.1016/S0140-6736(09)60568-7.
- Diabetes Control Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86. https://doi.org/10.1056/NEJM199309303291401.
- Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus. London: NICE; 2008.
- Olsen B, Johannesen J, Sjølie A, Borch-Johnsen K, Hougarrdss P, Thorsteinsson B, et al. Metabolic control and prevalence of microvascular complications in young Danish patients with type 1 diabetes mellitus. Diabet Med 1999;16:79-85. https://doi.org/10.1046/j.1464-5491.1999.00024.x.
- Downie E, Craig ME, Hing S, Cusumano J, Chan AK, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care 2011;34:2368-73. https://doi.org/10.2337/dc11-0102.
- Lawes T, Franklin V, Farmer G. HbA1c tracking and bio-psychosocial determinants of glycaemic control in children and adolescents with type 1 diabetes: retrospective cohort study and multilevel analysis. Pediatr Diabetes 2014;15:372-83. https://doi.org/10.1111/pedi.12100.
- Jackson C, Wernham E, Elder C, Wright N. Early glycaemic control is predictive of long-term control: a retrospective observational study. Pract Diabetes 2013;30:16-8. https://doi.org/10.1002/pdi.1734.
- Samuelsson U, Steineck I, Gubbjornsdottir S. A high mean-HbA1c value 3–15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood – a pilot study using two nation-wide population based quality registries. Pediatr Diabetes 2014;15:229-35. https://doi.org/10.1111/pedi.12085.
- Shah AS, Wadwa RP, Dabelea D, Hamman RF, D’Agostino R, Marcovina S, et al. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes 2015;16:367-74. https://doi.org/10.1111/pedi.12279.
- Sayers A, Thayer D, Harvey JN, Luzio S, Atkinson MD, French R, et al. Evidence for a persistent, major excess in all cause admissions to hospital in children with type-1 diabetes: results from a large Welsh national matched community cohort study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-005644.
- Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: the structural and functional integrity of the developing brain. Pediatr Diabetes 2013;14:541-53. https://doi.org/10.1111/pedi.12088.
- Tonoli C, Heyman E, Roelands B, Pattyn N, Buyse L, Piacentini MF, et al. Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J Diabetes 2014;6:499-513. https://doi.org/10.1111/1753-0407.12193.
- Marcovecchio ML, Heywood JJ, Dalton RN, Dunger DB. The contribution of glycemic control to impaired growth during puberty in young people with type 1 diabetes and microalbuminuria. Pediatr Diabetes 2014;15:303-8. https://doi.org/10.1111/pedi.12090.
- Plener PL, Molz E, Berger G, Schober E, Mönkemöller K, Denzer C, et al. Depression, metabolic control, and antidepressant medication in young patients with type 1 diabetes. Pediatr Diabetes 2015;16:58-66. https://doi.org/10.1111/pedi.12130.
- James S, Gallagher R, Dunbabin J, Perry L. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: systematic literature review. BMC Res Notes 2014;7. https://doi.org/10.1186/1756-0500-7-593.
- National Diabetes Audit 2012–2013. Report 1: Care Processes and Treatment Targets. London: Royal College of Paediatrics and Child Health; 2014.
- Ingberg CM, Palmér M, Åman J, Larsson S. Social consequences of insulin-dependent diabetes mellitus are limited: a population-based comparison of young adult patients vs healthy controls. Diabet Med 1996;13:729-33. https://doi.org/10.1002/(SICI)1096-9136(199608)13:8<729::AID-DIA147>3.0.CO;2-A.
- Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management. London: NICE; 2015.
- Randell T. Paediatric Diabetes Best Practice Tariff Criteria 2012. www.cypdiabetesnetwork.nhs.uk/national-network/best-practice-tariff (accessed July 2018).
- Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the US, Austria, and Germany. Diabetes Care 2015;38:1876-82. https://doi.org/10.2337/dc15-0780.
- McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 2015;32:1036-50. https://doi.org/10.1111/dme.12676.
- Júlíusson PB, Graue M, Wentzel-Larsen T, Søvik O. The impact of continuous subcutaneous insulin infusion on health-related quality of life in children and adolescents with type 1 diabetes. Acta Paediatr 2006;95:1481-7. https://doi.org/10.1080/08035250600774114.
- McMahon S, Airey F, Marangou DA, McElwee KJ, Carne CL, Clarey AJ, et al. Insulin pump therapy in children and adolescents: improvements in key parameters of diabetes management including quality of life. Diabet Med 2005;22:92-6. https://doi.org/10.1111/j.1464-5491.2004.01359.x.
- Nimri R, Weintrob N, Benzaquen H, Ofan R, Fayman G, Phillip M. Insulin pump therapy in youth with type 1 diabetes: a retrospective paired study. Pediatrics 2006;117:2126-31. https://doi.org/10.1542/peds.2005-2621.
- Sulli N, Shashaj B. Long-term benefits of continuous subcutaneous insulin infusion in children with type 1 diabetes: a 4-year follow-up. Diabet Med 2006;23:900-6. https://doi.org/10.1111/j.1464-5491.2006.01935.x.
- Sulli N, Shashaj B. Continuous subcutaneous insulin infusion in children and adolescents with diabetes mellitus: decreased HbA1c with low risk of hypoglycemia. J Pediatr Endocrinol Metab 2003;16:393-400. https://doi.org/10.1515/JPEM.2003.16.3.393.
- Raile K, Noelle V, Landgraf R, Schwarz H. Weight in adolescents with type 1 diabetes mellitus during continuous subcutaneous insulin infusion (CSII) therapy. J Pediatr Endocrinol Metab 2002;15:607-12. https://doi.org/10.1515/JPEM.2002.15.5.607.
- Harrington J, Peña AS, Wilson L, Gent R, Dowling K, Baghurst P, et al. Vascular function and glucose variability improve transiently following initiation of continuous subcutaneous insulin infusion in children with type 1 diabetes. Pediatr Diabetes 2013;14:504-11. https://doi.org/10.1111/pedi.12050.
- Hathout EH, McClintock T, Sharkey J, Kytsenko D, Hartwick N, Hadley-Scofield M, et al. Glycemic, auxologic, and seasonal aspects of continuous subcutaneous insulin infusion therapy in children and young adults with type 1 diabetes. Diabetes Technol Ther 2003;5:175-81. https://doi.org/10.1089/152091503321827830.
- Alemzadeh R, Palma-Sisto P, Parton EA, Holzum MK. Continuous subcutaneous insulin infusion and multiple dose of insulin regimen display similar patterns of blood glucose excursions in pediatric type 1 diabetes. Diabetes Technol Ther 2005;7:587-96. https://doi.org/10.1089/dia.2005.7.587.
- Schreiver C, Jacoby U, Watzer B, Thomas A, Haffner D, Fischer DC. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): a cross-sectional cohort study. Clin Endocrinol 2013;79:641-7. https://doi.org/10.1111/cen.12093.
- Zabeen B, Craig ME, Virk SA, Pryke A, Chan AK, Cho YH, et al. Insulin pump therapy is associated with lower rates of retinopathy and peripheral nerve abnormality. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0153033.
- Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med 2004;158:677-84. https://doi.org/10.1001/archpedi.158.7.677.
- Doyle EA, Weinzimer SA, Steffen AT, Ahern JAH, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care 2004;27:1554-8. https://doi.org/10.2337/diacare.27.7.1554.
- Sherr JL, Hermann JM, Campbell F, Foster NC, Hofer SE, Allgrove J, et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87-91. https://doi.org/10.1007/s00125-015-3790-6.
- Thompson RJ, Agostini K, Potts L, Luscombe J, Christie D, Viner R, et al. Deprivation and ethnicity impact on diabetes control and use of treatment regimen. Diabet Med 2013;30:491-4. https://doi.org/10.1111/dme.12023.
- McKergow E, Parkin L, Barson DJ, Sharples KJ, Wheeler BJ. Demographic and regional disparities in insulin pump utilization in a setting of universal funding: a New Zealand nationwide study. Acta Diabetol 2017;54:63-71. https://doi.org/10.1007/s00592-016-0912-7.
- Khanolkar AR, Amin R, Taylor-Robinson D, Viner RM, Warner JT, Stephenson T. Young people with type 1 diabetes of non-white ethnicity and lower socio-economic status have poorer glycaemic control in England and Wales. Diabet Med 2016;33:1508-15. https://doi.org/10.1111/dme.13079.
- Cohen D, Weintrob N, Benzaquen H, Galatzer A, Fayman G, Phillip M. Continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with type I diabetes mellitus: a randomized open crossover trial. J Pediatr Endocrinol Metab 2003;16:1047-50. https://doi.org/10.1515/JPEM.2003.16.7.1047.
- Meschi F, Beccaria L, Vanini R, Szulc M, Chiumello G. Short-term subcutaneous insulin infusion in diabetic children. Comparison with three daily insulin injections. Acta Diabetol Lat 1982;19:371-5. https://doi.org/10.1007/BF02629260.
- Nuboer R, Borsboom GJ, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes 2008;9:291-6. https://doi.org/10.1111/j.1399-5448.2008.00396.x.
- Skogsberg L, Fors H, Hanas R, Chaplin J, Lindman E, Skogsberg J. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes 2008;9:472-9. https://doi.org/10.1111/j.1399-5448.2008.00390.x.
- DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. J Pediatr 2004;145:380-4. https://doi.org/10.1016/j.jpeds.2004.06.022.
- Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care 2005;28:1277-81. https://doi.org/10.2337/diacare.28.6.1277.
- Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics 2003;112:559-64. https://doi.org/10.1542/peds.112.3.559.
- Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care 2005;28:15-9. https://doi.org/10.2337/diacare.28.1.15.
- Nabhan ZM, Kreher NC, Greene DM, Eugster EA, Kronenberger W, DiMeglio LA. A randomized prospective study of insulin pump vs. insulin injection therapy in very young children with type 1 diabetes: 12-month glycemic, BMI, and neurocognitive outcomes. Pediatr Diabetes 2009;10:202-8. https://doi.org/10.1111/j.1399-5448.2008.00494.x.
- Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 2009;10:52-8. https://doi.org/10.1111/j.1399-5448.2008.00440.x.
- Hofer S, Heidtmann B, Raile K, Fröhlich-Reiterer E, Lilienthal E, Berghaeuser MA, et al. Discontinuation of insulin pump treatment in children, adolescents, and young adults. A multicenter analysis based on the DPV database in Germany and Austria. Pediatr Diabetes 2010;11:116-21. https://doi.org/10.1111/j.1399-5448.2009.00546.x.
- de Vries L, Grushka Y, Lebenthal Y, Shalitin S, Phillip M. Factors associated with increased risk of insulin pump discontinuation in pediatric patients with type 1 diabetes. Pediatr Diabetes 2011;12:506-12. https://doi.org/10.1111/j.1399-5448.2010.00701.x.
- Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 2006;29:2355-60. https://doi.org/10.2337/dc06-1141.
- Lombardo F, Scaramuzza AE, Iafusco D. Failure of glycated hemoglobin drop after continuous subcutaneous insulin infusion initiation may indicate patients who discontinue: a 4-year follow-up study in children and adolescents with type 1 diabetes. Acta Diabetologica 2012;49:99-105. https://doi.org/10.1007/s00592-011-0344-3.
- Babar GS, Ali O, Parton EA, Hoffmann RG, Alemzadeh R. Factors associated with adherence to continuous subcutaneous insulin infusion in pediatric diabetes. Diabetes Technol Ther 2009;11:131-7. https://doi.org/10.1089/dia.2008.0042.
- Binek A, Rembierz-Knoll A, Polańska J, Jarosz-Chobot P. Reasons for the discontinuation of therapy of personal insulin pump in children with type 1 diabetes. Pediatr Endocrinol Diabetes Metab 2016;21:65-9. https://doi.org/10.18544/PEDM-21.02.0026.
- Rankin D, Harden J, Noyes K, Waugh N, Barnard K, Lawton J. Parents’ experiences of managing their child’s diabetes using an insulin pump: a qualitative study. Diabet Med 2015;32:627-34. https://doi.org/10.1111/dme.12683.
- Alsaleh FM, Smith FJ, Thompson R, Al-Saleh MA, Taylor KM. Insulin pump therapy: impact on the lives of children/young people with diabetes mellitus and their parents. Int J Clin Pharm 2014;36:1023-30. https://doi.org/10.1007/s11096-014-9990-1.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- Kanavos P, van den Aardweg S, Schurer W. Diabetes Expenditure, Burden of Disease and Management in 5 EU Countries. London: London School of Economics Health and Social Care; 2012.
- Diabetes: Facts and Stats. Version 3. London: Diabetes UK; 2014.
- National Paediatric Diabetes Audit Reports. London: Royal College of Paediatrics and Child Health; 2015.
- Roze S, Valentine W, Zakrzewska K, Palmer A. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Diabet Med 2005;22:1239-45. https://doi.org/10.1111/j.1464-5491.2005.01576.x.
- Swift P. ISPAD clinical practice consensus guidelines 2006–2007. Diabetes education. Pediatr Diabetes 2007;8. https://doi.org/10.1111/j.1399-5448.2007.00232.x.
- Blair J, Gregory JW, Hughes D, Ridyard CH, Gamble C, McKay A, et al. Study protocol for a randomised controlled trial of insulin delivery by continuous subcutaneous infusion compared to multiple daily injections. Trials 2015;16. https://doi.org/10.1186/s13063-015-0658-5.
- The Medical Devices Regulations 2002. London: The Stationery Office; 2002.
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001. Brussels: European Commission; 2001.
- Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery Office; 2004.
- Townson JK, Gregory JW, Cohen D, Channon S, Harman N, Davies JH, et al. Delivering Early Care In Diabetes Evaluation (DECIDE): a protocol for a randomised controlled trial to assess hospital versus home management at diagnosis in childhood diabetes. BMC Pediatrics 2011;11. https://doi.org/10.1186/1471-2431-11-7.
- Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation. Geneva: WHO; 2006.
- Colino E, López-Capapé M, Golmayo L, Alvarez MA, Alonso M, Barrio R. Therapy with insulin glargine (Lantus) in toddlers, children and adolescents with type 1 diabetes. Diabetes Res Clin Pract 2005;70:1-7. https://doi.org/10.1016/j.diabres.2005.02.004.
- Thalange N, Bereket A, Larsen J, Hiort LC, Peterkova V. Insulin analogues in children with type 1 diabetes: a 52-week randomized clinical trial. Diabet Med 2013;30:216-25. https://doi.org/10.1111/dme.12041.
- Walsh J, Roberts R. Pumping Insulin. Everything You Need for Success on a Smart Insulin Pump. San Diego, CA: Torrey Pines; 2006.
- Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med 2002;40:78-89. https://doi.org/10.1515/CCLM.2002.016.
- Arch BN, Blair J, McKay A, Gregory JW, Newland P, Gamble C. Measurement of HbA1c in multicentre diabetes trials – should blood samples be tested locally or sent to a central laboratory: an agreement analysis. Trials 2016;17. https://doi.org/10.1186/s13063-016-1640-6.
- Workgroup on Hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245-9. https://doi.org/10.2337/diacare.28.5.1245.
- Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics 2004;113:e133-e40. https://doi.org/10.1542/peds.113.2.e133.
- WHO Child Growth Standards: LengthHeight-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-For-Age: Methods and Development. Geneva: World Health Organization; 2006.
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660-7. https://doi.org/10.2471/BLT.07.043497.
- Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care 2003;26:631-7. https://doi.org/10.2337/diacare.26.3.631.
- Varni JW, Curtis BH, Abetz LN, Lasch KE, Piault EC, Zeytoonjian AA. Content validity of the PedsQL™ 3.2 Diabetes Module in newly diagnosed patients with type 1 diabetes mellitus ages 8–45. Qual Life Res 2013;22:2169-81. https://doi.org/10.1007/s11136-012-0339-8.
- Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8430.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000;23:1232-5. https://doi.org/10.2337/diacare.23.9.1232.
- Bak JF, Nielsen OH, Pedersen O, Beck-Nielsen H. Multiple insulin injections using a pen injector versus insulin pump treatment in young diabetic patients. Diabetes Res 1987;6:155-8.
- Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care 1996;19:324-7. https://doi.org/10.2337/diacare.19.4.324.
- Chiasson JL, Ducros F, Poliquin-Hamet M, Lopez D, Lecavalier L, Hamet P. Continuous subcutaneous insulin infusion (Mill-Hill Infuser) versus multiple injections (Medi-Jector) in the treatment of insulin-dependent diabetes mellitus and the effect of metabolic control on microangiopathy. Diabetes Care 1984;7:331-7. https://doi.org/10.2337/diacare.7.4.331.
- Haakens K1, Hanssen KF, Dahl-Jørgensen K, Vaaler S, Aagenaes O, Mosand R. Continuous subcutaneous insulin infusion (CSII), multiple injections (MI) and conventional insulin therapy (CT) in self-selecting insulin-dependent diabetic patients. A comparison of metabolic control, acute complications and patient preferences. J Intern Med 1990;228:457-64. https://doi.org/10.1111/j.1365-2796.1990.tb00263.x.
- Home PD, Capaldo B, Burrin JM, Worth R, Alberti KG. A crossover comparison of continuous subcutaneous insulin infusion (CSII) against multiple insulin injections in insulin-dependent diabetic subjects: improved control with CSII. Diabetes Care 1982;5:466-71. https://doi.org/10.2337/diacare.5.5.466.
- Nathan DM, Lou P, Avruch J. Intensive conventional and insulin pump therapies in adult type I diabetes. A crossover study. Ann Intern Med 1982;97:31-6. https://doi.org/10.7326/0003-4819-97-1-31.
- Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic–ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes Nutr Metab 1988;1:289-96.
- Saurbrey N1, Arnold-Larsen S, Møller-Jensen B, Kühl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabet Med 1988;5:150-3. https://doi.org/10.1111/j.1464-5491.1988.tb00962.x.
- Schiffrin A, Belmonte MM. Comparison between continuous subcutaneous insulin infusion and multiple injections of insulin. A one-year prospective study. Diabetes 1982;31:255-64. https://doi.org/10.2337/diab.31.3.255.
- Schmitz A, Christiansen JS, Christensen CK, Hermansen K, Mogensen CE. Effect of pump versus pen treatment on glycaemic control and kidney function in long-term uncomplicated insulin-dependent diabetes mellitus (IDDM). Dan Med Bull 1989;36:176-8.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001;24:1722-7. https://doi.org/10.2337/diacare.24.10.1722.
- Ziegler D, Dannehl K, Koschinsky T, Toeller M, Gries FA. Comparison of continuous subcutaneous insulin infusion and intensified conventional therapy in the treatment of type I diabetes: a two year randomized study. Diabetes Nutr Metab Clin Exp 1990;3:203-13.
- Burkart W, Hanker JP, Schneider HP. Complications and fetal outcome in diabetic pregnancy. Intensified conventional versus insulin pump therapy. Gynecol Obstet Invest 1988;26:104-12. https://doi.org/10.1159/000293680.
- Carta Q, Meriggi E, Trossarelli GF, Catella G, Dal Molin V, Menato G, et al. Continuous subcutaneous insulin infusion versus intensive conventional insulin therapy in type I and type II diabetic pregnancy. Diabetes Metab 1986;12:121-9.
- Coustan DR, Reece EA, Sherwin RS, Rudolf MC, Bates SE, Sockin SM, et al. A randomized clinical trial of the insulin pump vs intensive conventional therapy in diabetic pregnancies. JAMA 1986;255:631-6. https://doi.org/10.1001/jama.1986.03370050073024.
- Nosari I, Maglio ML, Lepore G. Is continuous subcutaneous insulin infusion more effective than intensive conventional insulin therapy in the treatment of pregnant diabetic women?. Diabetes Nutr Metab 1993;6:33-7.
- Schiffrin A, Parikh S, Marliss EB, Desrosiers MM. Metabolic response to fasting exercise in adolescent insulin-dependent diabetic subjects treated with continuous subcutaneous insulin infusion and intensive conventional therapy. Diabetes Care 1984;7:255-60. https://doi.org/10.2337/diacare.7.3.255.
- Tamborlane WV, Batas SE, Rudolf MC. Comparison of continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with insulin-dependent diabetes. Adv Diabetol 1989;2:24-7.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Topic E9 Statistical Principles for Clinical Trials. (CPMP/ICH/363/96). London: European Medicines Agency; 1998.
- Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14110.
- Roze S, Smith-Palmer J, Valentine W, Portu S, Nørgaard K, Pickup J. Cost-effectiveness of continuous subcutaneous insulin infusion versus multiple daily injections of insulin in type 1 diabetes: a systematic review. Diabet Med 2015;32:1415-24. https://doi.org/10.1111/dme.12792.
- Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev 2016;32:21-39. https://doi.org/10.1002/dmrr.2653.
- Cohen N, Minshall ME, Sharon-Nash L, Zakrzewska K, Valentine WJ, Palmer AJ. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia. PharmacoEconomics 2007;25:881-97. https://doi.org/10.2165/00019053-200725100-00006.
- St Charles M, Lynch P, Graham C, Minshall ME. A cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: a third-party US payer perspective. Value Health 2009;12:674-86. https://doi.org/10.1111/j.1524-4733.2008.00478.x.
- Conget Donlo I, Serrano Contreras D, Rodríguez Barrios JM, Levy Mizrahi I, Castell Abat C, Roze S. Cost-utility analysis of insulin pumps compared to multiple daily doses of insulin in patients with type 1 diabetes mellitus in Spain. Rev Esp Salud Publica 2006;80:679-95.
- Nørgaard K, Sohlberg A, Goodall G. Cost-effectiveness of continuous subcutaneous insulin infusion therapy for type 1 diabetes. Ugeskr Laeger 2010;172:2020-5.
- Petkova E, Petkova V, Konstantinova M, Petrova G. Economic evaluation of continuous subcutaneous insulin infusion for children with diabetes - a pilot study: CSII application for children – economic evaluation. BMC Pediatr 2013;13. https://doi.org/10.1186/1471-2431-13-155.
- Scuffham P, Carr L. The cost-effectiveness of continuous subcutaneous insulin infusion compared with multiple daily injections for the management of diabetes. Diabet Med 2003;20:586-93.
- St Charles ME, Sadri H, Minshall ME, Tunis SL. Health economic comparison between continuous subcutaneous insulin infusion and multiple daily injections of insulin for the treatment of adult type 1 diabetes in Canada. Clin Ther 2009;31:657-67. https://doi.org/10.1016/j.clinthera.2009.03.013.
- Mohsin F, Craig ME, Cusumano J, Chan AK, Hing S, Lee JW, et al. Discordant trends in microvascular complications in adolescents with type 1 diabetes from 1990 to 2002. Diabetes Care 2005;28:1974-80. https://doi.org/10.2337/diacare.28.8.1974.
- Australian National Diabetes Information Audit and Benchmarking. Sydney, NSW: National Association of Diabetes Centres: Weston, ACT; 2004.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079-87. https://doi.org/10.2337/diacare.26.4.1079.
- Linkeschova R, Raoul M, Bott U, Berger M, Spraul M. Less severe hypoglycaemia, better metabolic control, and improved quality of life in type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med 2002;19:746-51. https://doi.org/10.1046/j.1464-5491.2002.00713.x.
- DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003;289:2254-64. https://doi.org/10.1001/jama.289.17.2254.
- Bode BW, Sabbah HT, Gross TM, Fredrickson LP, Davidson PC. Diabetes management in the new millennium using insulin pump therapy. Diabetes Metab Res Rev 2002;18:14-20. https://doi.org/10.1002/dmrr.205.
- Bruttomesso D, Pianta A, Crazzolara D, Scaldaferri E, Lora L, Guarneri G, et al. Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med 2002;19:628-34. https://doi.org/10.1046/j.1464-5491.2002.00750.x.
- McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health 2014;17:714-24. https://doi.org/10.1016/j.jval.2014.07.007.
- National Tariff 2016–2017 Annex A. London: NHS England; 2016.
- National Schedule 2014–2015. London: Department of Health and Social Care; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. https://doi.org/10.1002/hec.1785.
- Thorrington D, Eames K. Measuring health utilities in children and adolescents: a systematic review of the literature. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0135672.
- Tarride JE, Burke N, Bischof M, Hopkins RB, Goeree L, Campbell K, et al. A review of health utilities across conditions common in paediatric and adult populations. Health Qual Life Outcomes 2010;8. https://doi.org/10.1186/1477-7525-8-12.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- McCabe CJ, Stevens KJ, Brazier JE. Utility scores for the Health Utilities Index Mark 2: an empirical assessment of alternative mapping functions. Med Care 2005;43:627-35. https://doi.org/10.1097/01.mlr.0000163666.00471.8e.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Guide to the Methods of Technology Appraisal 2013. Process and Methods [PMG9]. London: NICE; 2013.
- Furlong W, Feeny D, Torrance G. Health Utilities Index (HUI) Procedures Manual: Algorithm for Determining HUI Mark 2 (HUI2)/Mark 3 (HUI3) Health Status Classification Levels, Health States Single-attribute Level Utility Scores and Overall Health-related Quality of Life Utility Scores from 15-item Self-complete Health Status Questionnaires. Dundas, ON: Health Utilities Inc.; 2002.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Nixon RM, Thompson SG. Methods for incorporating covariate adjustment, subgroup analysis and between-centre differences into cost-effectiveness evaluations. Health Econ 2005;14:217-29. https://doi.org/10.1002/hec.1008.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20:S5-S26. https://doi.org/10.1185/030079904X1980.
- Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 2001;281:E924-E30. https://doi.org/10.1152/ajpendo.2001.281.5.E924.
- Del Guerra S, Grupillo M, Masini M, Lupi R, Bugliani M, Torri S, et al. Gliclazide protects human islet beta-cells from apoptosis induced by intermittent high glucose. Diabetes Metab Res Rev 2007;23:234-8. https://doi.org/10.1002/dmrr.680.
- Kuricová K, Pácal L, Šoupal J, Prázný M, Kaňková K. Effect of glucose variability on pathways associated with glucotoxicity in diabetes: Evaluation of a novel in vitro experimental approach. Diabetes Res Clin Pract 2016;114:1-8. https://doi.org/10.1016/j.diabres.2016.02.006.
- Bruttomesso D, Crazzolara D, Maran A, Costa S, Dal Pos M, Girelli A, et al. In type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med 2008;25:326-32. https://doi.org/10.1111/j.1464-5491.2007.02365.x.
- Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab 2010;12:288-98. https://doi.org/10.1111/j.1463-1326.2009.01160.x.
- Lin K, Huang K, Yang C. Glycemic variability: clinical and prognostic significance. Diabetes Res Open J 2015;1:48-53. https://doi.org/10.17140/DROJ-1-108.
- Nagl K, Hermann JM, Plamper M, Schröder C, Dost A, Kordonouri O, et al. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes 2016;18:428-34. https://doi.org/10.1111/pedi.12413.
- Niedzwiecki P, Pilacinski S, Uruska A, Adamska A, Naskret D, Zozulinska-Ziolkiewicz D. Influence of remission and its duration on development of early microvascular complications in young adults with type 1 diabetes. J Diabetes Complications 2015;29:1105-11. https://doi.org/10.1016/j.jdiacomp.2015.09.002.
- Komulainen J, Lounamaa R, Knip M, Kaprio EA, Akerblom HK. Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Childhood Diabetes in Finland Study Group. Arch Dis Child 1996;75:410-15. https://doi.org/10.1136/adc.75.5.410.
- Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250-64. https://doi.org/10.2337/diabetes.53.1.250.
- Mortensen HB, Hougaard P, Swift P, Hansen L, Holl RW, Hoey H, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1384-90. https://doi.org/10.2337/dc08-1987.
- DiLiberti JH, Carver K, Parton E, Totka J, Mick G, McCormick K. Stature at time of diagnosis of type 1 diabetes mellitus. Pediatrics 2002;109:479-83. https://doi.org/10.1542/peds.109.3.479.
- Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care 1999;22:1779-84. https://doi.org/10.2337/diacare.22.11.1779.
- Wheeler BJ, Donaghue KC, Heels K, Ambler GR. Family perceptions of insulin pump adverse events in children and adolescents. Diabetes Technol Ther 2014;16:204-7. https://doi.org/10.1089/dia.2013.0315.
- Scaramuzza AE, Dell’Acqua M, Macedoni M, Zuccotti GV. Insulin pump therapy in children with type 1 diabetes: the dark side of the moon. J Diabetes Sci Technol 2013;7:1095-7. https://doi.org/10.1177/193229681300700435.
- Ross PL, Milburn J, Reith DM, Wiltshire E, Wheeler BJ. Clinical review: insulin pump-associated adverse events in adults and children. Acta Diabetol 2015;52:1017-24. https://doi.org/10.1007/s00592-015-0784-2.
- Ross P, Gray AR, Milburn J, Kumarasamy IM, Wu F, Farrand S, et al. Insulin pump-associated adverse events are common, but not associated with glycemic control, socio-economic status, or pump/infusion set type. Acta Diabetol 2016;53:991-8. https://doi.org/10.1007/s00592-016-0897-2.
- Yi-Frazier JP, Hilliard ME, Fino NF, Naughton MJ, Liese AD, Hockett CW, et al. Whose quality of life is it anyway? Discrepancies between youth and parent health-related quality of life ratings in type 1 and type 2 diabetes. Qual Life Res 2016;25:1113-21. https://doi.org/10.1007/s11136-015-1158-5.
- Shulman R, Miller FA, Daneman D, Guttmann A. Valuing technology: a qualitative interview study with physicians about insulin pump therapy for children with type 1 diabetes. Health Policy 2016;120:64-71. https://doi.org/10.1016/j.healthpol.2015.10.006.
- Lin MH, Connor CG, Ruedy KJ, Beck RW, Kollman C, Buckingham B, et al. Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther 2013;15:929-34. https://doi.org/10.1089/dia.2013.0132.
- Schiel R, Burgard D, Perenthaler T, Stein G, Kramer G, Steveling A. Use and effectiveness of Continuous Subcutaneous Insulin Infusion (CSII) and Multiple Daily Insulin Injection Therapy (MIT) in children, adolescents and young adults with type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes 2016;124:99-104. https://doi.org/10.1055/s-0042-101155.
- Price K, Knowles J, Fox M, Wales JK, Heller S, . Eiser C4 . Effectiveness of the Kids in Control of Food (KICk–OFF) structured education course for 11–16 year olds with type 1 diabetes. Diabet Med 2016;33:192-203. https://doi.org/10.1111/dme.12881.
Appendix 1 Recruitment
FIGURE 11.
Predicted and actual recruitment.
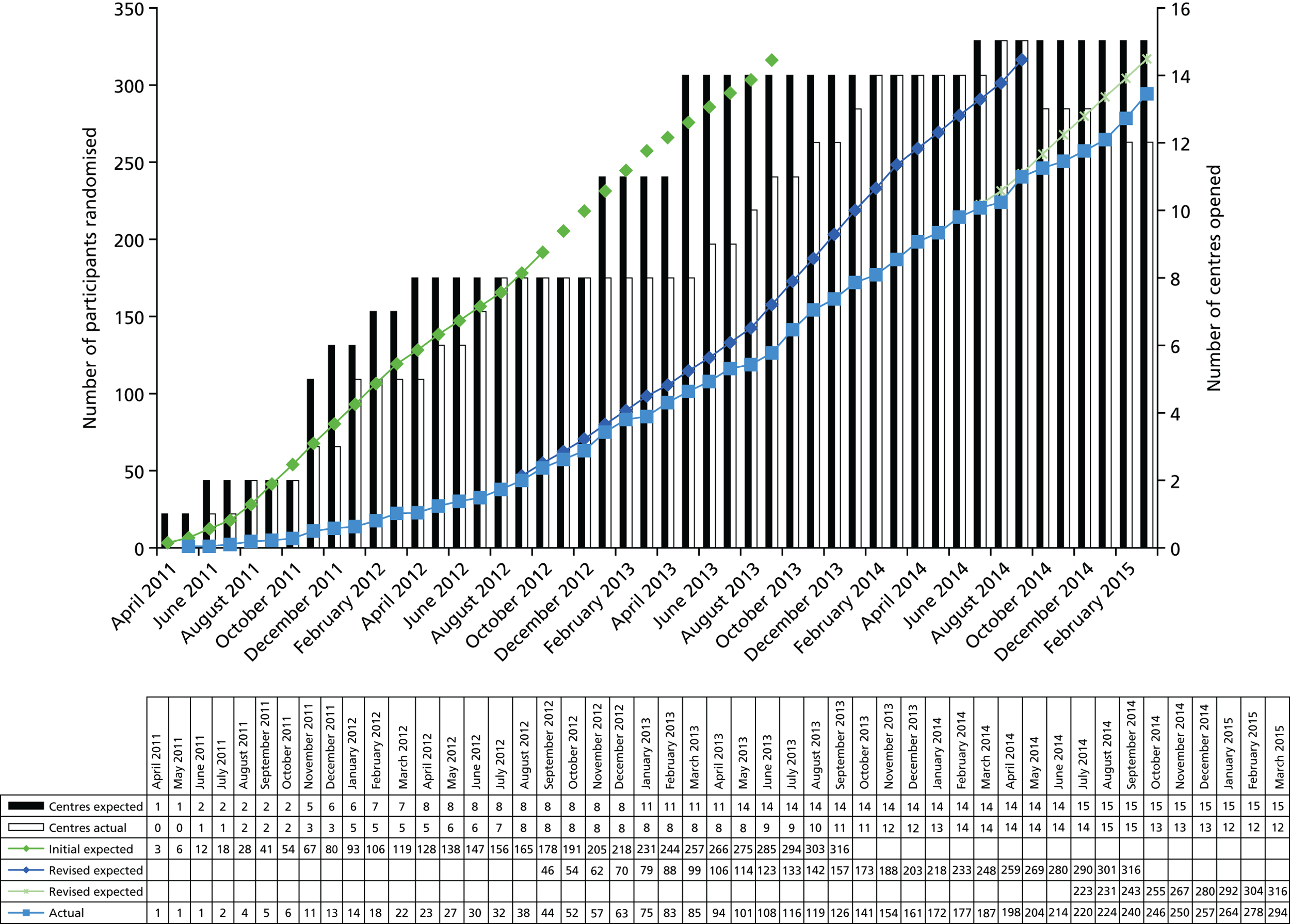
Screening and consent by site with the affect of protocol amendments that were aimed to improve recruitment are provided in Table 27.
| Site (initiation date) | [A] | [B] | [C] | [D] | [E] | [F] | Randomised | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total screens | Total not eligible (%) [dA] | Total eligible (%) [dA] | Eligible but consent not sought | Eligible and consent sought but refused | Total consented (%) [dE + F] | |||||||||||
| Lack of trained staff (%) [dD] | Reason not recorded (%) [dD] | Consultant decision (%) [dD] | Total (%) [dC] | MDI preference (%) [dE] | CSII preference (%) [dE] | Other (%) [dE] | Total (%) [E] | |||||||||
| ≤ version 3 | ≥ version 4 | ≤ version 3 | ≥ version 4 | ≤ version 3 | ≥ version 4 | |||||||||||
| Total | 976 | 98 (10) | 878 (90) | 18 (38.3) | 34 (23.9) | 0 (0) | 22 (15.5) | 29 (61.7) | 86 (60.6) | 189 (21.5) | 259 (65.6) | 36 (9.1) | 100 (25.3) | 395 (57.3) | 294 (42.7) | 294 |
| Alder Hey (16 May 2011) | 163 | 19 (11.7) | 144 (88.3) | 16 (94.1) | 19 (55.9) | 0 (0) | 0 (0) | 1 (5.9) | 15 (44.1) | 51 (35.4) | 33 (80.5) | 3 (7.3) | 5 (12.2) | 41 (44.1) | 52 (55.9) | 52 |
| Newcastle (18 July 2011) | 90 | 7 (7.8) | 83 (92.2) | 0 (0) | 2 (18.2) | 0 (0) | 1 (9.1) | 2 (100) | 8 (72.7) | 13 (15.7) | 41 (69.5) | 4 (6.8) | 14 (23.7) | 59 (84.3) | 11 (15.7) | 11 |
| Birmingham (27 October 2011) | 97 | 9 (9.3) | 88 (90.7) | 1 (5.9) | 3 (15) | 0 (0) | 4 (20) | 16 (94.1) | 13 (65) | 37 (42) | 12 (40) | 12 (40) | 6 (20) | 30 (58.8) | 21 (41.2) | 21 |
| Cardiff (19 December 2011) | 68 | 7 (10.3) | 61 (89.7) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 2 (3.3) | 34 (85) | 1 (2.5) | 5 (12.5) | 40 (67.8) | 19 (32.2) | 19 |
| Oxford (23 December 2011) | 135 | 16 (11.9) | 119 (88.1) | 0 (0) | 6 (40) | 0 (0) | 4 (26.7) | 6 (100) | 5 (33.3) | 21 (17.6) | 35 (56.5) | 2 (3.2) | 25 (40.3) | 62 (63.3) | 36 (36.7) | 36 |
| Doncaster (26 April 2012) | 39 | 3 (7.7) | 36 (92.3) | 1 (50) | 0 (0) | 0 (0) | 1 (33.3) | 1 (50) | 2 (66.7) | 5 (13.9) | 9 (75) | 2 (16.7) | 1 (8.3) | 12 (38.7) | 19 (61.3) | 19 |
| Southampton (12 June 2012) | 77 | 11 (14.3) | 66 (85.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (100) | 12 (100) | 15 (22.7) | 14 (66.7) | 0 (0) | 7 (33.3) | 21 (41.2) | 30 (58.8) | 30 |
| Nottingham (20 July 2012) | 86 | 7 (8.1) | 79 (91.9) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 4 (80) | 5 (6.3) | 11 (84.6) | 0 (0) | 2 (15.4) | 13 (17.6) | 61 (82.4) | 61 |
| Blackburn (24 May 2013) | 48 | 4 (8.3) | 44 (91.7) | 0 (0) | 0 (0) | 0 (0) | 6 (60) | 0 (0) | 4 (40) | 10 (22.7) | 18 (78.3) | 3 (13) | 2 (8.7) | 23 (67.6) | 11 (32.4) | 11 |
| Mid Staffordshire (16 July 2013) | 22 | 2 (9.1) | 20 (90.9) | 0 (0) | 2 (33.3) | 0 (0) | 1 (16.7) | 0 (0) | 3 (50) | 6 (30) | 6 (50) | 2 (16.7) | 4 (33.3) | 12 (85.7) | 2 (14.3) | 2 |
| East Surrey (23 August 2013) | 45 | 3 (6.7) | 42 (93.3) | 0 (0) | 0 (0) | 0 (0) | 3 (50) | 0 (0) | 3 (50) | 6 (14.3) | 21 (75) | 3 (10.7) | 4 (14.3) | 28 (77.8) | 8 (22.2) | 8 |
| Ipswich (23 October 2013) | 26 | 2 (7.7) | 24 (92.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (100) | 6 (25) | 6 (50) | 1 (8.3) | 5 (41.7) | 12 (66.7) | 6 (33.3) | 6 |
| Sheffield (4 December 2013) | 32 | 5 (15.6) | 27 (84.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (25) | 2 (10) | 13 (65) | 20 (74.1) | 7 (25.9) | 7 |
| Norfolk and Norwich (17 January 2014) | 39 | 3 (7.7) | 36 (92.3) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 7 (87.5) | 8 (22.2) | 11 (57.9) | 1 (5.3) | 7 (36.8) | 19 (67.9) | 9 (32.1) | 9 |
| Preston (10 July 2014) | 9 | 0 (0) | 9 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 3 (75) | 4 (44.4) | 3 (100) | 0 (0) | 0 (0) | 3 (60) | 2 (40) | 2 |
| Demographic variable | Consent obtained | Patient declined | ||
|---|---|---|---|---|
| MDI preference | CSII preference | Other reason | ||
| Age (years) | ||||
| N | 293 | 259 | 36 | 100 |
| Mean | 8.98 | 10 | 8.1 | 8.85 |
| SD | 4.13 | 3.76 | 4.27 | 4.09 |
| Median | 9.69 | 10.48 | 8.075 | 9.08 |
| Minimum | 0.7 | 0.41 | 1.28 | 0.98 |
| Maximum | 16 | 16 | 15.15 | 15.7 |
| Missing (n) | 1 | 0 | 0 | 0 |
| Age, n (%) | ||||
| N | 293 | 259 | 36 | 100 |
| Birth to 6 months | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) |
| 7 months to 4 years | 67 (22.9) | 32 (12.4) | 9 (25) | 23 (23) |
| 5–11 years | 146 (49.8) | 135 (52.1) | 18 (50) | 52 (52) |
| 12–15 years | 79 (27) | 90 (34.7) | 9 (25) | 25 (25) |
| ≥ 16 years | 1 (0.3) | 1 (0.4) | 0 (0) | 0 (0) |
| Missing | 1 | 0 | 0 | 0 |
| Sex, n (%) | ||||
| N | 293 | 259 | 36 | 100 |
| Female | 140 (47.8) | 121 (46.7) | 17 (47.2) | 46 (46) |
| Male | 153 (52.2) | 138 (53.3) | 19 (52.8) | 54 (54) |
| Missing | 1 | 0 | 0 | 0 |
| Ethnicity, n (%) | ||||
| N | 292 | 259 | 36 | 100 |
| Asian or Asian British | 6 (2.1) | 4 (1.5) | 1 (2.8) | 4 (4) |
| Black or British black | 3 (1) | 2 (0.8) | 2 (5.6) | 1 (1) |
| British white | 242 (82.9) | 228 (88) | 25 (69.4) | 81 (81) |
| Chinese | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Indian | 4 (1.4) | 1 (0.4) | 0 (0) | 2 (2) |
| Mixed | 10 (3.4) | 6 (2.3) | 2 (5.6) | 1 (1) |
| Not stated | 3 (1) | 12 (4.6) | 1 (2.8) | 4 (4) |
| Other | 5 (1.7) | 1 (0.4) | 0 (0) | 1 (1) |
| Other white | 14 (4.8) | 3 (1.2) | 1 (2.8) | 5 (5) |
| Pakistani | 5 (1.7) | 2 (0.8) | 4 (11.1) | 1 (1) |
| Missing | 2 | 0 | 0 | 0 |
| Deprivation scorea | ||||
| N | 280 | 241 | 33 | 94 |
| Mean | 23.26 | 24.01 | 28.51 | 21.65 |
| SD | 18.53 | 18.19 | 16.57 | 16.17 |
| Median | 17.045 | 17.96 | 27.65 | 17.06 |
| Minimum | 1.62 | 1.18 | 3.9 | 2.95 |
| Maximum | 77.23 | 74.35 | 63.86 | 71.91 |
| Missing (n) | 14 | 18 | 3 | 6 |
Appendix 2 Supplementary results
FIGURE 12.
Permanent change to insulin delivery method split by site: forest plot (ITT). There were 53 permanent changes in total (22 were randomised to CSII and 31 were randomised to MDI). However, one permanent switch of insulin delivery method was from MDI to ‘Injections TDS regime’. This has not been included in the meta-analysis as an event. M–H, Mantel–Haenszel.

| Randomisation number | Timing of change (months) | Age at permanent change (years) | Last HbA1c concentration measurement prior to switch (mmol/mol) | Person(s) who made decision | Reason(s) |
|---|---|---|---|---|---|
| Permanent change from CSII to MDI | |||||
| < 5 years | |||||
| 00213102 | 0 | 1.84 | 110 | Parent | Parent preference |
| 5–11 years | |||||
| 00243224 | 0–3 | 6.55 | 72 | Patient | Participant preference |
| 05042218 | 0–3 | 10.2 | 83 | Patient | Participant preference |
| 00213218 | 6–9 | 10.35 | 68 | Clinician; parent; patient |
Poor concordance with treatment Participant preference Parent preference |
| 05042212 | 0–3 | 10.55 | 117 | Parent; patient |
Participant preference Parent preference |
| 00114207 | 0–3 | 10.58 | 105 | Parent; patient |
Participant preference Parent preference Other: pain at cannula site |
| 05042214 | 0 | 10.72 | 114 | Clinician; parent |
Poor glycaemic control – b. frequent hyperglycaemia Parent preference |
| 00133207 | 6–9 | 10.8 | N/A | Patient | Participant preference |
| 00133209 | 9–12 | 11.24 | 58b | Patient | Participant preference |
| 05042207 | 0 | 11.71 | 38 | Parent; patient | Other: participant was not sure he wanted a pump to deliver insulin |
| 00243219 | 0–3 | 11.95 | 98 | Parent | Participant preference |
| ≥ 12 years | |||||
| 00133302 | 6–9 | 12.8 | 65 | Parent; patient |
Participant preference Parent preference |
| 00114302 | 0–3 | 13.15 | N/A | Parent; patient |
Participant preference Parent preference |
| 00213313 | 3 | 13.28 | 59 | Clinician; parent; patient |
Participant preference Parent preference |
| 00213307 | 0–3 | 13.32 | 105 | Parent; patient |
Participant preference Parent preference |
| 01527305 | 0–3 | 13.46 | 103 | Parent; patient | Participant preference |
| 05042301 | 0–3 | 14.05 | 129 | Patient | Participant preference |
| 00213304 | 6–9 | 14.7 | 89 | Clinician; patient | Poor concordance with treatment |
| 00248302 | 0–3 | 14.95 | 126 | Clinician; parent; patient | Participant preference |
| 00213318 | 3 | 15.04 | 50 | Patient | Participant preference |
| 00114306 | 0–3 | 15.13 | 130 | Clinician; parent; patient | Participant preference |
| 00243313 | 9–12 | 15.38 | 63 | Clinician; parent; patient |
Poor glycaemic control – b. frequent hyperglycaemia Participant preference Parent preference |
| Permanent change from MDI to CSII | |||||
| < 5 years | |||||
| 00213109 | 0–3 | 0.96 | 68 | Clinician; parent | Other: age related |
| 00036102 | 0–3 | 2.2 | 90 | Parent | Parent preference |
| 00243104 | 9–12 | 2.38 | 73 | Clinician; parent |
Poor glycaemic control – b. frequent hyperglycaemia Parent preference |
| 00213101 | 3–6 | 2.66 | 66 | Clinician; parent |
Poor glycaemic control – a. debilitating hypoglycaemia Poor glycaemic control – b. frequent hyperglycaemia |
| 00133104 | 9–12 | 3.42 | 72 | Clinician | Poor glycaemic control – b. frequent hyperglycaemia |
| 00213107 | 0–3 | 3.54 | 89 | Clinician; parent |
Poor glycaemic control – a. debilitating hypoglycaemia Parent preference |
| 00213103 | 3–6 | 3.72 | 39 | Clinician; parent | Other: young age needs smaller insulin quantities |
| 00114105 | 9–12 | 3.98 | 79 | Parent | Other: bruising from injections |
| 00114101 | 3–6 | 4.15 | 51.9 | Clinician; parent | Parent preference |
| 05042104 | 6–9 | 4.47 | 60 | Clinician; parent |
Parent preference Other: frequent hypoglycaemia |
| 00114103 | 6–9 | 4.78 | 78 | Clinician; parent |
Participant preference Parent preference |
| 00036103 | 3–6 | 5.17 | 54 | Clinician | Parent preference |
| 5–11 years | |||||
| 00114202 | 0–3 | 5.36 | 58.5 | Clinician; parent; patient |
Participant preference Parent preference |
| 00086210 | 9–12 | 6.39 | 59 | Clinician; parent; patient |
Poor glycaemic control – b. frequent hyperglycaemia Participant preference Parent preference |
| 00213229 | 6–9 | 7.64 | 51b | Clinician; parent | Poor concordance with treatment |
| 00114206 | 3–6 | 7.67 | 46 | Clinician; parent; patient |
Participant preference Parent preference |
| 00114213 | 6–9 | 8.01 | 53b | Parent; patient |
Participant preference Parent preference |
| 00326201 | 3–6 | 8.59 | 53 | Patient | Participant preference |
| 00114208 | 3–6 | 8.7 | 47 | Clinician; parent; patient |
Participant preference Parent preference |
| 00213223 | 9–12 | 8.96 | 61 | Clinician; parent; patient |
Participant preference Parent preference |
| 00326204 | 3–6 | 9.87 | 54b | Clinician; parent; patient | Participant preference |
| 00213226 | 6–9 | 11.02 | 43b | Clinician; parent; patient |
Poor concordance with treatment Participant preference Parent preference |
| 05042217 | 9–12 | 11.42 | 58 | Patient | Participant preference |
| 00213211 | 3–6 | 12.02 | 43 | Clinician; parent; patient |
Participant preference Parent preference |
| 00114209 | 6–9 | 12.31 | 64 | Clinician; parent; patient |
Participant preference Parent preference |
| 05042216 | 9–12 | 12.42 | 44 | Parent; patient |
Participant preference Parent preference |
| ≥ 12 years | |||||
| 00213306 | 3–6 | 12.61 | 38 | Clinician; parent; patient |
Participant preference Parent preference Other: fear of hypoglycaemia |
| 00036303 | 0–3 | 12.69 | 133 | Parent; patient |
Participant preference Parent preference |
| 00114301 | 3–6 | 14.44 | 38.8 | Clinician; patient | Parent preference |
| 00036301 | 6–9 | 15.69 | 32 | Patient | Participant preference |
| Permanent change from MDI to injections TDS regime | |||||
| 5–11 years | |||||
| 12617501 | 9 | 10.06 | 66 | Clinician; parent |
Participant preference Parent preference |
| Baseline characteristic | Intervention arm | Total | |
|---|---|---|---|
| CSII | MDI | ||
| Age at randomisation (years) | |||
| n | 87 | 66 | 153 |
| Mean (SD) | 8.6 (4.2) | 9.7 (4.2) | 9.1 (4.2) |
| Median (IQR) | 9.1 (4.7–12.2) | 10.1 (7.1–13.7) | 9.3 (5.7–12.5) |
| Minimum, maximum | 0.8, 16 | 0.9, 15.3 | 0.8, 16 |
| Age (strata) category, n (%) | |||
| n | 87 | 66 | 153 |
| 7 months to < 5 years | 23 (26.4) | 11 (16.7) | 34 (22.2) |
| 5 years to < 12 years | 41 (47.1) | 32 (48.5) | 73 (47.7) |
| 12–15 years | 23 (26.4) | 23 (34.8) | 46 (30.1) |
| Age category (EudraCT), n (%) | |||
| n | 87 | 66 | 153 |
| Infants and toddlers | 6 (6.9) | 3 (4.5) | 9 (5.9) |
| Children | 58 (66.7) | 40 (60.6) | 98 (64.1) |
| Adolescents | 23 (26.4) | 23 (34.8) | 46 (30.1) |
| Sex, n (%) | |||
| n | 87 | 66 | 153 |
| Female | 44 (50.6) | 29 (43.9) | 73 (47.7) |
| Male | 43 (49.4) | 37 (56.1) | 80 (52.3) |
| Ethnicity, n (%) | |||
| n | 86 | 65 | 151 |
| Asian or Asian British | 0 (0) | 2 (3.1) | 2 (1.3) |
| Black or British black | 0 (0) | 3 (4.6) | 3 (2) |
| British white | 78 (90.7) | 51 (78.5) | 129 (85.4) |
| Indian | 2 (2.3) | 1 (1.5) | 3 (2) |
| Mixed | 3 (3.5) | 5 (7.7) | 8 (5.3) |
| Not stated | 0 (0) | 0 (0) | 1 (0.7) |
| Other | 2 (2.3) | 3 (4.6) | 5 (3.3) |
| Pakistani | 1 (1.2) | 0 (0) | 1 (0.7) |
| Deprivation score | |||
| n | 82 | 62 | 144 |
| Mean (SD) | 21.7 (18.9) | 21 (16.8) | 21.4 (18) |
| Median (IQR) | 13.7 (8.4–31.8) | 15.1 (8.4–30.9) | 14.4 (8.4–31.4) |
| Minimum, maximum | 2.1, 67.9 | 1.6, 73.9 | 1.6, 73.9 |
| Summary | Time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| CSII | MDI | Total | CSII | MDI | Total | |
| Child (diabetes) | ||||||
| Children 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 12 | 22 | 34 | 14 | 19 | 33 |
| Mean (SD) | 56.8 (13.4) | 61 (10.4) | 59.5 (11.5) | 66.7 (16.9) | 59.6 (16) | 62.6 (16.5) |
| Median (IQR) | 54.5 (50–63.6) | 59.1 (50–72.7) | 56.8 (50–68.2) | 68.2 (63.6–79.3) | 63.6 (50–72.7) | 63.6 (54.5–72.7) |
| Minimum, maximum | 31.8, 81.8 | 40.9, 77.3 | 31.8, 81.8 | 27.3, 90.9 | 22.7, 77.3 | 22.7, 90.9 |
| Children 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 59 | 55 | 114 | 57 | 55 | 112 |
| Mean (SD) | 63 (16.9) | 62.3 (15) | 62.7 (15.9) | 68.7 (15.8) | 63.9 (16.1) | 66.4 (16.1) |
| Median (IQR) | 65.9 (49.6–77.3) | 63.6 (50–74.4) | 63.6 (50–75) | 70.5 (54.5–79.5) | 61.4 (50–77.3) | 65.9 (52.3–79.5) |
| Minimum, maximum | 25, 100 | 29.5, 100 | 25, 100 | 36.4, 100 | 37.2, 93.2 | 36.4, 100 |
| Children 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 31 | 60 | 37 | 33 | 70 |
| Mean (SD) | 68.2 (16.4) | 64.3 (15.3) | 66.2 (15.8) | 62.9 (16.8) | 61.6 (17.3) | 62.3 (16.9) |
| Median (IQR) | 65.9 (56.8–75) | 61.4 (56.8–72.7) | 63.6 (56.8–73.9) | 61.4 (54.5–71.9) | 59.1 (52.3–72.7) | 61.1 (52.3–72.7) |
| Minimum, maximum | 40.9, 100 | 40.9, 100 | 40.9, 100 | 27.3, 100 | 29.5, 90.9 | 27.3, 100 |
| Child (treatment I) | ||||||
| Children 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 12 | 22 | 34 | 14 | 19 | 33 |
| Mean (SD) | 77.3 (18.3) | 79.8 (16.1) | 79 (16.7) | 81.3 (16.8) | 75.7 (22.2) | 78 (20) |
| Median (IQR) | 76.6 (62.5–93.8) | 82.8 (75–87.5) | 78.1 (75–87.5) | 75 (75–100) | 75 (62.5–87.5) | 75 (62.5–100) |
| Minimum, maximum | 50, 100 | 37.5, 100 | 37.5, 100 | 50, 100 | 12.5, 100 | 12.5, 100 |
| Children 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 59 | 54 | 113 | 57 | 55 | 112 |
| Mean (SD) | 79.3 (17.9) | 74.7 (19.6) | 77.1 (18.8) | 83.7 (15.5) | 77.2 (20.5) | 80.5 (18.3) |
| Median (IQR) | 87.5 (68.8–93.8) | 75 (68.8–87.5) | 81.3 (68.8–93.8) | 87.5 (75–93.8) | 81.3 (62.5–93.8) | 81.3 (75–93.8) |
| Minimum, maximum | 31.3, 100 | 12.5, 100 | 12.5, 100 | 25, 100 | 18.8, 100 | 18.8, 100 |
| Children 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 31 | 60 | 38 | 33 | 71 |
| Mean (SD) | 79.7 (21.2) | 79.5 (14.2) | 79.6 (17.8) | 76.6 (21.2) | 74.8 (15.3) | 75.8 (18.6) |
| Median (IQR) | 87.5 (75–93.8) | 81.3 (75–87.5) | 81.3 (75–93.8) | 81.3 (68.8–93.8) | 75 (62.5–87.5) | 75 (68.8–87.5) |
| Minimum, maximum | 6.3, 100 | 43.8, 100 | 6.3, 100 | 25, 100 | 31.3, 100 | 25, 100 |
| Child (treatment II) | ||||||
| Children 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 12 | 22 | 34 | 13 | 19 | 32 |
| Mean (SD) | 80.1 (19.3) | 83.2 (12.9) | 82.1 (15.3) | 82.3 (18.1) | 79.9 (15.3) | 80.9 (16.3) |
| Median (IQR) | 85.7 (67.9–95.4) | 83.7 (73.5–92.9) | 85.7 (71.4–92.9) | 85.7 (73.5–100) | 78.6 (71.4–92.9) | 80.1 (72.4–95.4) |
| Minimum, maximum | 42.9, 100 | 57.1, 100 | 42.9, 100 | 35.7, 100 | 55.1, 100 | 35.7, 100 |
| Children 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 59 | 54 | 113 | 57 | 55 | 112 |
| Mean (SD) | 87.9 (11.9) | 83.7 (14.5) | 85.9 (13.3) | 89.6 (11.6) | 84.8 (17.6) | 87.2 (15) |
| Median (IQR) | 92.9 (82.1–96.4) | 85.7 (77.6–96.4) | 89.3 (78.6–96.4) | 92.9 (82.1–100) | 92.9 (75–100) | 92.9 (82.1–100) |
| Minimum, maximum | 45.9, 100 | 39.3, 100 | 39.3, 100 | 53.1, 100 | 16.3, 100 | 16.3, 100 |
| Children 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 31 | 60 | 38 | 33 | 71 |
| Mean (SD) | 90.1 (13.8) | 87.2 (10.7) | 88.6 (12.3) | 84 (16.7) | 85.3 (12.4) | 84.6 (14.8) |
| Median (IQR) | 96.4 (87.2–100) | 85.7 (82.1–96.4) | 92.9 (82.1–98) | 91.3 (75–96.4) | 85.7 (78.6–96.4) | 89.3 (75–96.4) |
| Minimum, maximum | 50, 100 | 60.7, 100 | 50, 100 | 42.9, 100 | 50, 100 | 42.9, 100 |
| Child (worry) | ||||||
| Children 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 11 | 21 | 32 | 14 | 19 | 33 |
| Mean (SD) | 74.2 (26.2) | 77.8 (24.9) | 76.6 (25) | 77.4 (31.1) | 68.4 (28.8) | 72.2 (29.7) |
| Median (IQR) | 66.7 (66.7–100) | 83.3 (66.7–100) | 83.3 (66.7–100) | 100 (50–100) | 66.7 (50–100) | 83.3 (50–100) |
| Minimum, maximum | 16.7, 100 | 16.7, 100 | 16.7, 100 | 16.7, 100 | 0, 100 | 0, 100 |
| Children 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 59 | 54 | 113 | 57 | 53 | 110 |
| Mean (SD) | 81.2 (16.8) | 75.8 (20.7) | 78.6 (18.9) | 82.5 (17.7) | 78 (23.4) | 80.3 (20.6) |
| Median (IQR) | 83.3 (75–91.7) | 79.2 (58.3–91.7) | 83.3 (66.7–91.7) | 83.3 (75–100) | 83.3 (66.7–100) | 83.3 (66.7–100) |
| Minimum, maximum | 33.3, 100 | 25, 100 | 25, 100 | 16.7, 100 | 8.3, 100 | 8.3, 100 |
| Children 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 31 | 60 | 38 | 33 | 71 |
| Mean (SD) | 80.5 (22.1) | 77.2 (17.9) | 78.8 (19.9) | 73 (23.2) | 74.5 (23.3) | 73.7 (23.1) |
| Median (IQR) | 83.3 (66.7–100) | 83.3 (66.7–91.7) | 83.3 (66.7–91.7) | 75 (50–100) | 75 (58.3–100) | 75 (50–100) |
| Minimum, maximum | 25, 100 | 33.3, 100 | 25, 100 | 33.3, 100 | 25, 100 | 25, 100 |
| Child (communication) | ||||||
| Children 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 11 | 22 | 33 | 14 | 18 | 32 |
| Mean (SD) | 56.1 (38.2) | 77.3 (23.9) | 70.2 (30.5) | 57.1 (33.1) | 80.6 (25.7) | 70.3 (31) |
| Median (IQR) | 50 (16.7–100) | 83.3 (66.7–100) | 66.7 (50–100) | 58.3 (33.3–83.3) | 83.3 (66.7–100) | 75 (50–100) |
| Minimum, maximum | 0, 100 | 33.3, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| Children 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 55 | 113 | 54 | 54 | 108 |
| Mean (SD) | 82.2 (18.3) | 72 (25.3) | 77.2 (22.5) | 79.7 (23.7) | 75.6 (26.6) | 77.6 (25.2) |
| Median (IQR) | 83.3 (75–100) | 75 (58.3–91.7) | 83.3 (66.7–100) | 91.7 (66.7–100) | 83.3 (58.3–100) | 83.3 (58.3–100) |
| Minimum, maximum | 16.7, 100 | 0, 100 | 0, 100 | 8.3, 100 | 0, 100 | 0, 100 |
| Children 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 29 | 58 | 38 | 33 | 71 |
| Mean (SD) | 86.2 (15.5) | 81.6 (19.7) | 83.9 (17.7) | 81.6 (18.1) | 76.8 (26.4) | 79.3 (22.3) |
| Median (IQR) | 91.7 (75–100) | 83.3 (75–100) | 87.5 (75–100) | 83.3 (66.7–100) | 83.3 (66.7–100) | 83.3 (66.7–100) |
| Minimum, maximum | 50, 100 | 16.7, 100 | 16.7, 100 | 33.3, 100 | 0, 100 | 0, 100 |
| Questionnaire age group | Time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| CSII | MDI | Total | CSII | MDI | Total | |
| Parent (diabetes) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 25 | 18 | 43 | 22 | 18 | 40 |
| Mean (SD) | 62 (16) | 58.1 (11.1) | 60.4 (14.1) | 62.7 (17.1) | 56.2 (16.2) | 59.8 (16.8) |
| Median (IQR) | 62 (54.5–65.9) | 58.1 (47.7–65.9) | 61.4 (47.7–65.9) | 59.1 (47.7–75) | 56.8 (43.2–68.2) | 56.8 (46.6–72.7) |
| Minimum, maximum | 27.3, 97.7 | 38.6, 75 | 27.3, 97.7 | 33.1, 97.7 | 27.3, 81.8 | 27.3, 97.7 |
| Parents/carers: children aged 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 14 | 23 | 37 | 17 | 18 | 35 |
| Mean (SD) | 66.2 (12) | 61.4 (13.9) | 63.2 (13.2) | 68.3 (15.4) | 60.8 (15.7) | 64.5 (15.8) |
| Median (IQR) | 65.9 (59.1–77.3) | 59.1 (50–72.7) | 61.4 (54.5–72.7) | 65.9 (56.8–77.3) | 61.4 (53.7–72.7) | 65.9 (53.7–75) |
| Minimum, maximum | 43.2, 84.1 | 38.6, 90.9 | 38.6, 90.9 | 45.5, 95.5 | 13.6, 81.8 | 13.6, 95.5 |
| Parents/carers: children aged 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 58 | 116 | 55 | 55 | 110 |
| Mean (SD) | 62.9 (14.9) | 62.7 (15.3) | 62.8 (15) | 66.2 (14.6) | 60.8 (16.2) | 63.5 (15.6) |
| Median (IQR) | 63.6 (52.3–75) | 61.4 (52.3–72.7) | 61.4 (52.3–72.7) | 65.9 (52.3–75.2) | 61.4 (47.7–74.4) | 63.6 (52.3–75) |
| Minimum, maximum | 25, 93.2 | 36.4, 95.5 | 25, 95.5 | 43.2, 100 | 29.5, 97.7 | 29.5, 100 |
| Parents/carers: children aged 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 30 | 29 | 59 | 38 | 34 | 72 |
| Mean (SD) | 63.9 (17.4) | 60.3 (18.1) | 62.1 (17.7) | 60.3 (17.7) | 61.5 (14) | 60.9 (15.9) |
| Median (IQR) | 58 (52.3–81.8) | 61.4 (50–72.7) | 59.1 (52.1–75) | 59.1 (47.7–68.2) | 61.4 (54.5–70.5) | 61.4 (50–70.5) |
| Minimum, maximum | 36.4, 97.7 | 24.8, 100 | 24.8, 100 | 27.3, 100 | 22.7, 90.9 | 22.7, 100 |
| Parent (treatment I) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 25 | 18 | 43 | 21 | 18 | 39 |
| Mean (SD) | 79.8 (20.5) | 69.8 (20.7) | 75.6 (20.9) | 80.7 (18.9) | 73.6 (20.8) | 77.4 (19.9) |
| Median (IQR) | 87.5 (62.5–100) | 68.8 (56.3–93.8) | 75 (56.3–100) | 87.5 (68.8–93.8) | 81.3 (68.8–87.5) | 81.3 (68.8–87.5) |
| Minimum, maximum | 37.5, 100 | 31.3, 100 | 31.3, 100 | 31.3, 100 | 18.8, 100 | 18.8, 100 |
| Parents/carers: children aged 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 14 | 23 | 37 | 17 | 18 | 35 |
| Mean (SD) | 63.8 (28.4) | 69.3 (19.2) | 67.2 (22.9) | 72.8 (21.5) | 69.8 (21.1) | 71.3 (21.1) |
| Median (IQR) | 62.5 (50–87.5) | 68.8 (56.3–81.3) | 68.8 (56.3–81.3) | 81.3 (56.3–87.5) | 71.9 (56.3–87.5) | 75 (56.3–87.5) |
| Minimum, maximum | 0, 100 | 31.3, 100 | 0, 100 | 37.5, 100 | 25, 100 | 25, 100 |
| Parents/carers: children aged 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 57 | 115 | 55 | 55 | 110 |
| Mean (SD) | 71.3 (20.3) | 67.9 (20.8) | 69.6 (20.5) | 69.1 (18.7) | 66.9 (19.6) | 68 (19.1) |
| Median (IQR) | 75 (56.3–87.5) | 68.8 (56.3–87.5) | 68.8 (56.3–87.5) | 68.8 (56.3–81.3) | 62.5 (56.3–81.3) | 68.8 (56.3–81.3) |
| Minimum, maximum | 25, 100 | 12.5, 100 | 12.5, 100 | 25, 100 | 25, 100 | 25, 100 |
| Parents/carers: children aged 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 30 | 29 | 59 | 38 | 34 | 72 |
| Mean (SD) | 65.2 (22.3) | 61.3 (18.1) | 63.3 (20.3) | 67.3 (22.1) | 62.3 (21.9) | 64.9 (22) |
| Median (IQR) | 68.8 (43.8–81.3) | 62.5 (50–75) | 62.5 (50–81.3) | 68.8 (50–81.3) | 62.5 (43.8–81.3) | 68.8 (50–81.3) |
| Minimum, maximum | 25, 100 | 31.3, 100 | 25, 100 | 25, 100 | 12.5, 100 | 12.5, 100 |
| Parent (treatment II) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 25 | 18 | 43 | 22 | 18 | 40 |
| Mean (SD) | 85.4 (13.6) | 74.2 (16.6) | 80.8 (15.7) | 88.6 (10.9) | 82.7 (14.8) | 85.9 (13) |
| Median (IQR) | 91.8 (82.1–96.4) | 73.2 (61.2–85.7) | 82.1 (69.4–96.4) | 92.3 (85.7–92.9) | 87.8 (75–93.9) | 90.8 (80.4–93.4) |
| Minimum, maximum | 49, 100 | 40.8, 100 | 40.8, 100 | 59.7, 100 | 45.9, 100 | 45.9, 100 |
| Parents/carers: children aged 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 14 | 23 | 37 | 16 | 18 | 34 |
| Mean (SD) | 84.2 (14.4) | 82.8 (9.8) | 83.3 (11.6) | 84.8 (14.3) | 77.6 (15.3) | 81 (15.1) |
| Median (IQR) | 91.3 (75–96.4) | 82.1 (75–92.9) | 85.7 (75–93.9) | 85.7 (70.2–100) | 76.8 (64.3–92.9) | 82.1 (65.3–96.4) |
| Minimum, maximum | 57.1, 98 | 64.3, 100 | 57.1, 100 | 64.3, 100 | 50, 98 | 50, 100 |
| Parents/carers: children aged 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 58 | 116 | 55 | 55 | 110 |
| Mean (SD) | 86.4 (12.7) | 80.9 (15.3) | 83.7 (14.3) | 86.8 (13.5) | 81.1 (16.6) | 83.9 (15.3) |
| Median (IQR) | 87.2 (77.6–98) | 82.1 (71.4–92.9) | 85.7 (73.5–96.4) | 89.3 (81.6–100) | 85.7 (67.9–92.9) | 89.3 (75–96.4) |
| Minimum, maximum | 50, 100 | 39.3, 100 | 39.3, 100 | 44.9, 100 | 16.3, 100 | 16.3, 100 |
| Parents/carers: children aged 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 29 | 58 | 38 | 34 | 72 |
| Mean (SD) | 84.6 (15.1) | 79.4 (15) | 82 (15.1) | 80.7 (16.4) | 77.7 (19.6) | 79.3 (17.9) |
| Median (IQR) | 85.7 (77.6–98) | 82.1 (64.3–92.9) | 85.7 (73.5–93.9) | 81.9 (64.3–96.4) | 78.6 (67.9–92.9) | 81.6 (67.9–96.4) |
| Minimum, maximum | 42.9, 100 | 55.1, 100 | 42.9, 100 | 39.3, 100 | 32.1, 100 | 32.1, 100 |
| Parent (worry) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 25 | 18 | 43 | 21 | 18 | 39 |
| Mean (SD) | 63 (29.5) | 45.4 (28.6) | 55.6 (30.1) | 47.9 (28.1) | 48.6 (31.9) | 48.2 (29.5) |
| Median (IQR) | 58.3 (33.3–91.7) | 41.7 (25–58.3) | 50 (33.3–83.3) | 41.7 (33.3–75) | 54.2 (25–66.7) | 50 (25–75) |
| Minimum, maximum | 8.3, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| Parents/carers: children aged 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 14 | 23 | 37 | 17 | 18 | 35 |
| Mean (SD) | 61.3 (26.7) | 75.4 (22) | 70 (24.5) | 61.3 (29.3) | 67.1 (25.2) | 64.3 (27) |
| Median (IQR) | 58.3 (33.3–83.3) | 83.3 (58.3–91.7) | 75 (50–91.7) | 66.7 (33.3–83.3) | 75 (50–83.3) | 75 (41.7–83.3) |
| Minimum, maximum | 25, 100 | 25, 100 | 25, 100 | 16.7, 100 | 0, 100 | 0, 100 |
| Parents/carers: children aged 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 58 | 116 | 55 | 55 | 110 |
| Mean (SD) | 72.1 (20.1) | 64.4 (21.3) | 68.2 (21) | 68.2 (24.3) | 67.7 (25.3) | 68 (24.7) |
| Median (IQR) | 75 (58.3–83.3) | 66.7 (50–83.3) | 66.7 (50–83.3) | 75 (50–91.7) | 66.7 (50–91.7) | 70.8 (50–91.7) |
| Minimum, maximum | 25, 100 | 25, 100 | 25, 100 | 8.3, 100 | 0, 100 | 0, 100 |
| Parents/carers: children aged 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 30 | 29 | 59 | 38 | 34 | 72 |
| Mean (SD) | 69.2 (24.4) | 65.2 (23.5) | 67.2 (23.8) | 71.9 (21) | 66.9 (23.6) | 69.6 (22.3) |
| Median (IQR) | 75 (50–91.7) | 66.7 (50–83.3) | 66.7 (50–83.3) | 75 (50–83.3) | 75 (50–83.3) | 75 (50–83.3) |
| Minimum, maximum | 8.3, 100 | 16.7, 100 | 8.3, 100 | 33.3, 100 | 8.3, 100 | 8.3, 100 |
| Parent (communication) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 24 | 19 | 43 | 21 | 17 | 38 |
| Mean (SD) | 83.7 (25.2) | 74.1 (28.7) | 79.5 (26.9) | 81.3 (25.7) | 70.6 (34) | 76.5 (29.8) |
| Median (IQR) | 100 (75–100) | 75 (58.3–100) | 91.7 (66.7–100) | 91.7 (75–100) | 83.3 (50–100) | 91.7 (50–100) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 | 25, 100 | 0, 100 | 0, 100 |
| Parents/carers: children aged 5–7 years old (6- and 12-month follow-up) | ||||||
| n | 13 | 23 | 36 | 17 | 17 | 34 |
| Mean (SD) | 82.7 (22.4) | 84.1 (22.5) | 83.6 (22.1) | 72.5 (32.1) | 80.9 (18.8) | 76.7 (26.3) |
| Median (IQR) | 100 (66.7–100) | 91.7 (75–100) | 95.8 (75–100) | 83.3 (50–100) | 83.3 (66.7–100) | 83.3 (66.7–100) |
| Minimum, maximum | 41.7, 100 | 16.7, 100 | 16.7, 100 | 0, 100 | 41.7, 100 | 0, 100 |
| Parents/carers: children aged 8–12 years old (6- and 12-month follow-up) | ||||||
| n | 58 | 57 | 115 | 54 | 55 | 109 |
| Mean (SD) | 78.7 (22.4) | 75.7 (26.2) | 77.2 (24.3) | 80.1 (22.8) | 67.2 (26.3) | 73.6 (25.4) |
| Median (IQR) | 83.3 (66.7–100) | 83.3 (58.3–100) | 83.3 (58.3–100) | 87.5 (75–100) | 75 (50–91.7) | 75 (58.3–100) |
| Minimum, maximum | 16.7, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| Parents/carers: children aged 13–16 years old (6- and 12-month follow-up) | ||||||
| n | 29 | 28 | 57 | 38 | 34 | 72 |
| Mean (SD) | 77 (28.9) | 67.3 (24.1) | 72.2 (26.9) | 73.2 (26.1) | 72.8 (30.7) | 73 (28.2) |
| Median (IQR) | 83.3 (75–100) | 66.7 (50–91.7) | 83.3 (50–100) | 75 (50–100) | 79.2 (58.3–100) | 75 (58.3–100) |
| Minimum, maximum | 0, 100 | 25, 100 | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| Parent (overall) | ||||||
| Parents/carers: children aged 2–4 years old (6- and 12-month follow-up) | ||||||
| n | 24 | 18 | 42 | 20 | 17 | 37 |
| Mean (SD) | 72.5 (13) | 64 (11.5) | 68.9 (12.9) | 73 (12.7) | 67 (15.7) | 70.2 (14.3) |
| Median (IQR) | 72.8 (62.9–77.2) | 61.8 (55.5–74.1) | 68.8 (57–76.8) | 75 (62.1–82.1) | 70.5 (54.7–79.5) | 73.2 (60.7–80.4) |
| Minimum, maximum | 54.5, 98.6 | 46.7, 81.6 | 46.7, 98.6 | 52.4, 99.1 | 40.2, 90.2 | 40.2, 99.1 |
Appendix 3 Health economics analysis plan
This health economic analysis plan provides a detailed and comprehensive description of the pre-planned economic analyses for the SCIPI study. The planned economic analysis described within this document is compliant with those specified in brief within the SCIPI study protocol version 6.0 (16 February 2016). This health economic analysis plan comprehensively describes the planned final analysis.
These planned analyses will be performed by health economists at the Centre for Health Economics and Medicines Evaluation, Bangor University, Bangor, UK.
All analyses will be performed using Stata version 13 and reported according to CHEERS.
Overview
Within the SCIPI study, the cost-effectiveness of CSII compared with insulin administered subcutaneously by MDI will be assessed from a UK NHS perspective. The cost-effectiveness will be based on the incremental cost per QALY gained. The economic analysis will have two parts: (1) a trial-based evaluation of the first 12 months from diagnosis and, if one intervention is clinically superior, (2) a lifetime extrapolation of costs and outcomes, based on an economic model of diabetes.
Data sources
Resource use
Data on health-care resource use are based on:
-
PLICS data and/or PAS data of participating hospitals.
-
The trial CRFs, which will record health-care resource items (outpatient visits, primary care and hospital stays) for the 3 months prior to randomisation at baseline and then at 3, 6, 9 and 12 months following randomisation. All hospital stays will be measured irrespective of whether or not they are related to diabetes.
-
Prescription records, patient diaries and electronic downloads from pumps, to estimate the number of units of insulin used by patients.
Unit costs
All resource use will be valued in monetary terms using UK unit costs current at the time of analysis. HRGs will be used as the main currency of the economic analysis for inpatient stays with cost codes allocated based on the latest available national tariff. Obsolete national tariff and schedule codes will be uplifted using the hospital price index according to the current version of the compendium of Unit Costs of Health and Social Care. 123 This resource will also be the source of unit costs that will be applied to primary health-care and outpatient contacts.
Bundled national tariff costs will be based on the hospital spell and incorporated excess ward days, market forces factor and whether the case was elective or emergency. Tariff codes will be obtained primarily from PLICS and PAS data but if unavailable they will be assigned by reference to CRFs and an appropriate HRG code will be assigned based on condition and comorbidities. Similarly, appropriate HRGs will be applied to unassignable national tariff HRG codes appearing in the PLICS and PAS data.
Unbundled costs such as intensive care unit stays are not expected to be common; however, if they do arise they will be identified from reference to (1) lists of AEs, (2) CRF code 4 critical care, (3) PLICS data or (4) PAS data. Appropriate HRG codes for unbundled costs will be assigned from the National Schedule of reference costs. 122
Insulin and concomitant medication costs will be based on those detailed in the British National Formulary. 124
Utilities
Within-trial health utilities will be estimated from patients’ (aged ≥ 12 years) and their parents’ or guardians’ responses to the HUI questionnaire and the application of UK tariff scores.
Data analysis
Costs
Within-trial total costs for each patient will be calculated from the sum of ward, outpatient, A&E, primary care, disposable devices, insulin and concomitant medication usage. An annualised cost will be assigned for the insulin pump and accoutrements.
Costs at baseline, relating to the 3 months preceding randomisation, will be calculated from CRF data with reference to PAS and PLICS data when available. This will relate to all primary and secondary care activity.
If a hospitalisation is observed for the period subsequent to randomisation, an adjustment may be necessary to apportion costs, given that ward costs relate to episodes of care that could start prior to randomisation. Patients admitted to hospital n days before randomisation and spending N days in hospital after randomisation will have their total costs calculated as:
Patients’ use of health-care resources and total costs will be calculated for the ITT population, with summary statistics generated by intervention group. Differences between intervention groups in mean lengths of stay and costs will be compared with reference to bias-corrected and accelerated bootstrapped 95% CIs, based on 10,000 replicates.
Imbalances in baseline covariates, should any be present in spite of randomisation, may result in biased estimates of cost-effectiveness. If there are imbalances in important clinical or demographic variables, we will implement suitable regression techniques.
Outcomes
A QALY profile over the 12-month trial period will be estimated from HUI2/HUI3 derived utilities based on the area under the curve, assuming the trapezoidal rule.
Incremental analysis
The trial-based cost-effectiveness analysis of CSII will be evaluated by its ICER, which will be calculated according to:
where ΔCosts is the difference in total costs between interventions (cost CSII – cost MDI), and ΔQALY is the difference in utility between interventions (QALY CSII – QALY MDI).
Uncertainty analysis
The joint uncertainty in costs and outcomes at 12 months will be considered by non-parametric bootstrapping resampling (10,000) of patient costs and QALYs. Uncertainty will be represented using a cost-effectiveness acceptability curve to present the probability of CSII being cost-effective for given ceiling thresholds for costs per QALY gained. Estimates of ICERs will be compared with the £20,000–30,000-per-QALY threshold of cost-effectiveness set by NICE.
Sensitivity analyses
A range of sensitivity analyses will be considered, including different calculations for estimating the costs of pumps, use of alternative (Canadian) tariff scores for the HUI3 algorithm and application of MICE for imputing missing cost and utility data, when necessary.
Exploratory analyses
The contribution of baseline variables to total costs and QALYs will be examined using regression models. Given the expected skewed nature of outcomes, we will specify generalised linear models using (1) total cost per patient and (2) total QALYs per patient, as the dependent variable, and explanatory variables for accounting for baseline cost, baseline HbA1c concentration, age, centre and other covariates, as specified in the statistical analysis plan, as well as the intervention group. The Modified Park test will be used to identify the preferred distributional family based on the lowest chi-squared test value. For each prediction model, the link function will be identified using the Pearson correlation test, the Pregibon link test and the modified Hosmer–Lemeshow test. When all three tests yield non-significant p-values, the link function is said to fit well.
If there are significant differences in HbA1c concentrations between intervention groups at 12 months, we will conduct an exploratory analysis in which trial results will be extrapolated to estimate lifetime costs and benefits and to capture long-term microvascular and macrovascular complications, using the CDM. We acknowledge that the CDM has not been validated for populations comparable with those in the SCIPI study; however, this exploratory analysis will aim to assess potential lifetime benefits and incremental cost per QALY of tighter glycaemic control in early diagnosed paediatric patients with T1D.
The CDM will be initialised with a cohort profile consistent with each intervention group of the SCIPI study, in terms of demographic and clinical characteristics. A conservative estimate of treatment effect having been reached at 12 months from randomisation will be assumed to calculate the risk factor trajectories over time for blood pressure, lipids and HbA1c concentration using the default setting of the UK Prospective Diabetes Study (UKPDS) panel equations. Costs inbuilt to the CDM will be used to estimate lifetime total cost per patient. Costs and QALYs in the extrapolated phase will be discounted at 3.5% per annum.
Appendix 4 Patient and public involvement
We have aimed to describe patient and public involvement throughout this report. A summary is provided below.
We have worked with young people and parent contributors from study design to delivery.
The MCRN group of children and young people, ‘Stand Up, Speak UP!’, advised us on the contents and presentation of patient information leaflets and consent forms.
Three parent contributors were recruited to the TMG, which met by teleconference every month, and one parent contributor joined the TSC. Our parent contributors were particularly valuable during the recruitment period of the study. They advised us of the impact of the diagnosis of T1D and how and when to approach patients and their families in a sensitive manner, and helped us to anticipate barriers to recruitment.
When it became evident that recruitment rates were significantly influenced by patient preference for MDI, we approached completed participants from both arms of the study to participate in the study video. Participants and their parents shared their experiences of the diagnosis of T1D and of living with their randomised treatment and their reasons for participating in clinical research. This video was very well received by health-care professionals, families and patients.
Once recruitment rates had been optimised, we entered a phase of the study in which the TMG discussions focused on issues relating to governance and internal study management. During this period, it was more difficult to engage our parent contributors and to ensure that the discussions were relevant and interesting to them.
As results became available, we invited our parent contributors to help us to interpret them and set them in the context of living with childhood T1D. These discussions have been very helpful and have motivated us to learn more about our study cohort, in particular how the experiences of those treated with MDI and CSII may have differed in ways that were not identified through the study questionnaires. The importance of extending the observation period was also discussed. We will apply for further funding to undertake this research in the near future, and we hope to continue to work with our parent contributors to develop and deliver new study protocols.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- BMI
- body mass index
- CDM
- Center for Outcomes Research (CORE) diabetes model
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSII
- continuous subcutaneous insulin infusion
- CTU
- Clinical Trials Unit
- DECIDE
- Delivering Early Care In Diabetes Evaluation
- DKA
- diabetic ketoacidosis
- DPV
- Prospective Diabetes Follow-up Registry
- GP
- general practitioner
- HbA1c
- glycosylated haemoglobin
- HDU
- high-dependency unit
- HRG
- Healthcare Resource Group
- HUI
- Health Utilities Index
- HUI2
- Health Utilities Index Mark 2
- HUI3
- Health Utilities Index Mark 3
- ICER
- incremental cost-effectiveness ratio
- IDAA1c
- insulin dose-adjusted HbA1c
- IDR
- incidence density ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- IQR
- interquartile range
- ITT
- intention to treat
- IU
- international unit
- LOCF
- last observation carried forward
- MCRN
- Medicines for Children Research Network
- MDI
- multiple daily injections
- MICE
- multiple imputation with chained equations
- NICE
- National Institute for Health and Care Excellence
- NPDA
- National Paediatric Diabetes Audit
- PAS
- patient administration system
- PedsQL
- Pediatric Quality of Life Inventory
- PICU
- paediatric intensive care unit
- PLICS
- patient-linked information costing system
- PRP
- partial remission phase
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RN
- research nurse
- SAE
- serious adverse event
- SCIPI
- SubCutaneous Insulin: Pumps or Injections?
- SD
- standard deviation
- SDS
- standard deviation score
- T1D
- type 1 diabetes
- TDS
- ter die sumendus
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
