Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/179/01. The contractual start date was in April 2015. The draft report began editorial review in April 2017 and was accepted for publication in December 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan A Michaels reports grants outside the submitted work from the National Institute for Health Research (NIHR) Programme Grants for Applied Research programme (grant number RP-PG-1210-12009). Janet T Powell report grants from NIHR (HTA 07/37/64) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Thompson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background and aims
In the UK, ultrasonographic screening for abdominal aortic aneurysms (AAAs) is currently offered to men aged 65 years but not to women. Until recently, the prevalence of AAAs in women was substantially lower than that for men. 1 However, women now account for 34% of all deaths due to ruptured AAA. 2 AAA ruptures are fatal in about 80% of cases and women with a small AAA have been found to have a fourfold higher risk of rupture than men. 3 Moreover, the prevalence of smoking has been rising in younger women, and so, in the future, AAAs may become even more common in women.
Thus, research is needed to assess the cost-effectiveness of screening women for AAAs. Only one study,4 published in 2006, has attempted to address this question; this modelling study was based on a prevalence of AAA in women of 1.1% and suggested that screening may be cost-effective at US$6000 per life-year gained. However, more data are now available to inform such modelling, and a more sophisticated and realistic model can be used to provide more reliable results. One of the conclusions of the literature review undertaken by LeFevre and the US Preventive Services Task Force5 was that high-quality modelling studies need to be conducted to determine whether or not AAA screening is beneficial in women. An international consensus group6 also identified that targeted AAA screening of women is an area for future development to reduce deaths from AAAs. Furthermore, there is international debate regarding the optimal clinical management strategy for women who have been diagnosed with an AAA. 7
Population-based screening for abdominal aortic aneurysms
Screening men for abdominal aortic aneurysms
The NHS Abdominal Aortic Aneurysm Screening Programme (NAAASP)8 for men aged 65 years was launched in England in 2009, and similar programmes have subsequently been introduced to other parts of the UK. In NAAASP, an aortic diameter of ≥ 3.0 cm, as measured by ultrasound, is used as a diagnosis of an AAA. Men with smaller aortic diameters are reassured and not followed up further. Men with AAAs of 3.0- to 4.4-cm diameter enter a surveillance programme with annual follow-up scans, while those with AAAs of diameter 4.5–5.4 cm have follow-up scans every 3 months. Men with AAAs whose diameter is initially ≥ 5.5 cm, or which expand to that diameter during surveillance, are referred for consideration of elective surgery.
The scientific evidence supporting the implementation of NAAASP came from the results of four randomised trials9 of AAA screening that almost exclusively recruited men. These trials showed that AAA-related mortality in men could be halved by offering AAA screening along with appropriate clinical follow-up that included elective surgery when an AAA reached a threshold size. Long-term modelling based on the largest of these trials, the Multicentre Aneurysm Screening Study (MASS),10 showed that AAA screening in men aged 65 years was extremely cost-effective, with an estimated cost of £3000 per quality-adjusted life-year (QALY) gained. 11
This cost-effectiveness estimate came under scrutiny later, because initial data from NAAASP8 showed an AAA prevalence of 1.5% in men aged 65 years, rather than 4.9% as observed in the MASS trial. 10 Revision of the long-term model to reflect this lower prevalence as well as the attendance rates observed in NAAASP and updated cost estimates increased the cost per QALY. Nevertheless, NAAASP was still estimated to be highly cost-effective, at £7400 per QALY gained. 12 Indeed, provided the AAA prevalence was above 0.35%, it was estimated that screening would be cost-effective at a willingness to pay (WTP) of £20,000 per QALY. This could imply that screening women for AAAs might also be cost-effective.
Screening women for abdominal aortic aneurysms
The prevalence of AAAs in women aged 65 or 70 years may be around 0.5%. 13–15 Moreover, it is known that women have an AAA rupture rate about fourfold of that in men for a given AAA diameter,3 although their AAA growth rates are similar. 16 Women may also have worse outcomes after AAA surgery than men,17,18 for example, because of their typically shorter aneurysm necks. 19 A higher proportion of women are turned down for both elective and emergency surgery. 20 Some of these differences between women and men would probably favour systematic AAA screening in women, whereas others would not.
There are a number of reasons why the design of an optimal AAA screening programme for women might differ from that currently adopted for men. The prevalence of AAA increases with age, and women have a greater life expectancy than men, so screening women at age 70 years might be more beneficial than screening them at age 65 years. The diameter of the aorta ss typically smaller aortic in women than in men,21 and the aortic diameter that define an aneurysm could be lowered from the conventional 3.0 cm. Because AAA rupture rates are higher in women, it may be advisable to reduce the diameter threshold for considering elective surgery below the usual 5.5 cm.
There is no prospect of being able to undertake a randomised trial to answer these questions in women. First, such a trial would have to be an order of magnitude bigger than the MASS trial10 of 68,000 men and with a similar length of follow-up (13 years). 22 Second, a single trial could not address the relative merits of different designs of a screening and intervention programme. The best practical way in which these questions can be addressed is by undertaking a detailed modelling exercise, which is described here.
Aims and objectives
The overall aim is to estimate the cost-effectiveness of systematic population-based AAA screening for women. Offering ultrasound screening for AAAs to women is compared with a policy of no systematic screening. Outcomes are in terms of AAA-related mortality, life expectancy, elective AAA operations, emergency AAA operations, costs and cost-effectiveness. Cost-effectiveness is expressed as cost per life-year gained and, using age-dependent quality-of-life (QoL) norms, cost per QALY gained.
Some input parameters are very uncertain, or even unknown, for women. Therefore, a key component of the research is to evaluate the uncertainty in conclusions by both probabilistic and deterministic sensitivity analyses. In addition, some possible departures from the design of the AAA screening programme in men are evaluated.
Although this project has a UK focus, its results have implications for the development of AAA screening programmes internationally, as is evident, for example, from the recent recommendations on AAA screening from the US Preventive Services Task Force. 5
Scientific objectives
Objective 1
To adapt a previously validated multistate model of AAA screening in men as a more flexible individual simulation model (see Chapter 2).
The work is based on adapting the previously developed long-term Markov model based on the MASS trial. 10 However, as the design of an optimal AAA screening programme for women may require some quite substantial modifications compared with that adopted for men, it is necessary first to translate the existing model into the more flexible format of an individual simulation model. This enables relevant potential modifications (e.g. regarding age at screening, surgical threshold or surveillance intervals) to be more easily and efficiently assessed.
Objective 2
To obtain information from the published literature, where possible, on input parameters for this model, relevant to women rather than men (see Chapter 3).
Information on parameters for women, often from outside the UK, is available in published papers. These include the prevalence of AAAs in women in Sweden14 and operative mortality rates after rupture. 23,24 The most recent systematic review of mortality following elective surgery was published in 2010;25 this needs to be updated to provide further evidence for endovascular aneurysm repair (EVAR). New systematic reviews to assess the proportion of women suitable for EVAR, with currently available endografts, and the rates of non-intervention for elective AAA repair are also necessary.
Objective 3
To seek other information or data sources on input parameters for women that are not available in the published literature (see Chapters 4–6).
In the case of some parameters, little or no published information relates specifically to women. This applies to the proportion of elective and emergency AAA operations that are carried out by EVAR rather than by open repair (a key issue for costs and maybe effects), as well as long-term AAA mortality after repair. Hence, we search out data sources that might provide relevant estimates, including the UK National Vascular Registry (NVR),26 the international Vascunet database27 and the English Hospital Episode Statistics (HES). 28 We also obtain additional information on women specifically from particular studies, for example, about reintervention rates after surgery and resource use for costing purposes, from the endovascular aneurysm repair trial 1 (EVAR-1)29 and Immediate Management of Patients with Ruptured aneurysm: Open versus Endovascular Repair (IMPROVE)30 trial. Dropout rates from surveillance were obtained from local audit data in Leicester (Professor Matthew J Bown, University of Leicester, 2016, personal communication) and London (Professor Janet T Powell, Imperial College London, 2016, personal communication).
Objective 4
To run the adapted model for women to estimate cost-effectiveness and to assess the impact of parameter uncertainty on the conclusions using probabilistic and deterministic sensitivity analyses (see Chapter 7).
We first provide cost-effectiveness estimates for women based on the same screening programme design as used in NAAASP. 8 Given new values of the input parameters for women, we run the model to obtain estimates of AAA-related mortality, all-cause mortality, numbers of elective and emergency operations, life expectancy and costs. In addition, by using age-related population norms for QoL,31 we also estimate quality-adjusted life expectancy. The principal results are reported as incremental cost-effectiveness ratios (ICERs) in terms of Great British pounds (£) per QALY gained or incremental net monetary benefit (INMB). Many of the input parameters have uncertainty intervals that are used in a probabilistic sensitivity analysis (PSA), thus, providing an uncertainty interval for the estimated cost-effectiveness. Deterministic sensitivity analyses (DSAs) are used to explore the impact of different choices of parameter values on the incremental costs, effects and ICERs.
Objective 5
To assess modifications of the AAA screening programme used for men that may be more appropriate and cost-effective for women (see Chapter 8).
Some of the design characteristics might be altered to provide a screening programme that is more appropriate for women, with potentially greater cost-effectiveness. A number of aspects are considered: (1) increasing the age at which screening is offered, (2) lowering the threshold AAA diameter at which elective surgery is considered, (3) lowering the aortic diameter that defines an AAA, (4) lengthening the surveillance intervals for the smallest AAAs and (5) evaluating rescreening of all women at a later age.
Patient and public involvement group
At the outset of the project, there were no aneurysm-related projects in the INVOLVE database. 32 Therefore, we established a female patient and public involvement (PPI) group to provide project-specific input and help direct the dissemination of the outputs from this research project (see Appendix 1). The PPI group was used to monitor the progress of the project, assist in interpretation of results from a lay perspective and help prepare the Plain English summary. The PPI group now forms an ongoing resource for future work in this area.
Modified objectives
Two objectives set out in the original grant application have not been pursued. One is related to the evaluation of targeted AAA screening of at-risk groups of women, for example, female smokers or those with a family history of AAAs. This objective was not pursued for three reasons. First, the PPI group expressed a strong view against the idea of selective screening of women (see Appendix 1) and very much favoured a population-based approach. Second, it became apparent that it was already difficult to find information on key model parameters for women in general. It would be even harder to find evidence relevant to specific high-risk groups. For example, it might be anticipated that smokers would differ from the general population in terms of AAA prevalence, rates of attendance at screening, AAA growth and rupture rates, incidental detection and dropout rates, operative mortalities, costs of surgery and reinterventions, and non-AAA (competing) mortality; estimates of all these parameters would be necessary to evaluate the cost-effectiveness of AAA screening for female smokers. Third, AAA mortality in other women (e.g. non-smokers) would be unaltered, raising issues of both overall effectiveness at the population level and of societal equity.
The second of the original objectives was to estimate the expected value of obtaining more information on influential parameters, for which estimated values are very imprecise in women. This objective was also not pursued for two reasons. First, it became clear that there was a more fundamental problem of whether or not certain parameter estimates obtained were fully relevant for current women in the UK; it was not just an issue of their imprecision. Second, and as agreed with the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme at an interim progress report, the computational demands of undertaking such expected value of information analyses within a complex individual simulation model were too great, and the research should be focused on the more crucial objectives.
Input parameters required
A clinically realistic model for AAA screening is complex (see Chapter 2), and has many input parameters that require estimation. The parameters include those related to screening, AAA growth and rupture, and surveillance (Table 1); elective and emergency operations (Table 2); and parameters reflecting costs, QoL and competing mortality from non-AAA causes (Table 3). These tables indicate the sections in Chapters 3–6 of this report that describe the way in which these parameters are estimated.
| Parameter | Assumptions | Sources of data | Section of report |
|---|---|---|---|
| Screening | |||
| Reinvitation proportion | Applies to all ages | NAAASP8 | Chapter 4 , Screening |
| Attendance proportion | Varies with age | Literature review, Uppsala14 and Chichester33 | Chapter 4 , Screening |
| Non-visualisation proportion | Applies to all ages | NAAASP8 | Chapter 4 , Screening |
| AAA size distribution at screening | Depends on prevalence | NAAASP,8 Uppsala14 and Viborga | Chapter 4 , Screening |
| Prevalence proportion | Varies according to age at screening | Systematic review34 | Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysm in women and Chapter 4, Screening |
| AAA growth and rupture | |||
| AAA growth | Based on underlying AAA diameter plus measurement error | Women in 11 RESCAN surveillance studies35 | Chapter 4 , Growth and rupture rates of abdominal aortic aneurysm in women |
| AAA rupture | Based on underlying AAA diameter | Women in six RESCAN surveillance studies35 | Chapter 4 , Growth and rupture rates of abdominal aortic aneurysm in women |
| Surveillance | |||
| Surveillance intervals | Varies with measured AAA diameter | MASS10 and NAAASP8 | Chapter 1 , Population-based screening for abdominal aortic aneurysm |
| Dropout rate from surveillance | Assumed constant | NAAASP,8 Leicesterb and Imperialc | Chapter 4 , Surveillance |
| Incidental detection rate | Assumed constant | New Zealand,36 Manchesterd and MASS10 | Chapter 4 , Surveillance |
| Delay from ≥ 5.5-cm scan to consultation | Assumed constant | NAAASP8 | Chapter 4 , Surveillance |
| Consultation scan | CT scan not ultrasound scan | RESCAN35 | Chapter 4 , Surveillance |
| Decision at consultation | Those not undergoing surgery never receive surgery | Systematic review37 | Chapter 3, Proportion of women versus men not offered an intervention, and Chapter 4,Surveillance |
| Delay from consultation scan to elective surgery | Assumed constant | NAAASP8 | Chapter 4 , Surveillance |
| Parameter | Assumptions | Sources of data | Section of report |
|---|---|---|---|
| Elective operations | Parameters may vary with age, AAA diameter | ||
| Proportion receiving EVAR vs. open repair | NVR,26 HES28 and systematic review37 | Chapter 3, Suitability of women versus men for standard endovascular repair, and Chapter 5, Elective operations | |
| EVAR 30-day operative mortality | Assumed immediate (not 30 days) | NVR,26 HES28 and systematic review37 | Chapter 3, 30-day operative mortality in women versus men and Chapter 5, Elective operations |
| Open repair 30-day operative mortality | Assumed immediate (not 30 days) | NVR,26 HES28 and systematic review37 | Chapter 3, 30-day operative mortality in women versus men, and Chapter 5, Elective operations |
| Reintervention rate after successful EVAR | Constant rate within two time periods | EVAR-138 | Chapter 5 , Elective operations |
| Reintervention rate after successful open repair | Constant rate | EVAR-138 | Chapter 5 , Elective operations |
| Long-term AAA mortality rate after successful EVAR | Constant rate | EVAR-138 | Chapter 5 , Elective operations |
| Long-term AAA mortality rate after successful open repair | Constant rate | EVAR-138 | Chapter 5 , Elective operations |
| Emergency operations | Symptomatic AAAs excluded from modelling; parameters may vary with age | ||
| % operated after rupture | Assumed constant | Literature review and IMPROVE24 | Chapter 3 , Mortality following ruptured abdominal aortic aneurysm in women |
| Proportion receiving EVAR vs. open repair | NVR26 and HES28 | Chapter 5 , Emergency operations for ruptured abdominal aortic aneurysm | |
| EVAR 30-day operative mortality | Assumed immediate (not 30 days) | NVR,26 HES28 and literature review | Chapter 3, Mortality following ruptured abdominal aortic aneurysm in women and Chapter 5, Emergency operations for ruptured abdominal aortic aneurysm |
| Open repair 30-day operative mortality | Assumed immediate (not 30 days) | NVR,26 HES28 and literature review | Chapter 3, Mortality following ruptured abdominal aortic aneurysm in women, and Chapter 5, Emergency operations for ruptured abdominal aortic aneurysm |
| Reintervention rate after successful EVAR | Constant rate | IMPROVE30 | Chapter 5 , Emergency operations for ruptured abdominal aortic aneurysm |
| Reintervention rate after successful open repair | Constant rate | IMPROVE30 | Chapter 5 , Emergency operations for ruptured abdominal aortic aneurysm |
| Long-term AAA mortality rate after successful EVAR | Constant rate | IMPROVE30 | Chapter 5 , Emergency operations for ruptured abdominal aortic aneurysm |
| Long-term AAA mortality rate after successful open repair | Constant rate | IMPROVE30 | Chapter 5 , Emergency operations for ruptured abdominal aortic aneurysm |
| Parameter | Assumptions | Sources of data | Sections of report |
|---|---|---|---|
| Costs | 2014/15 prices | ||
| Invitation, reinvitation | NAAASP8 | Chapter 6 , Unit costs | |
| Screening scan | NAAASP8 | Chapter 6 , Unit costs | |
| Surveillance scan | NAAASP8 | Chapter 6 , Unit costs | |
| Consultation for elective surgery | Average of 1.6 consultations per woman | MASS10 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Elective EVAR repair | Includes all costs for primary admission | EVAR-1,38 HES28 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Elective open repair | Includes all costs for primary admission | EVAR-1,38 HES28 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Emergency EVAR repair | Includes all costs for primary admission | IMPROVE,24 HES28 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Emergency open repair | Includes all costs for primary admission | IMPROVE,24 HES28 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Surveillance after operations | Current practice, on average | Chapter 6 , Unit costs | |
| Reintervention after EVAR | Average across types of reintervention | EVAR-138 and NHS Reference Costs 2014 to 201539 | Chapter 6 , Unit costs |
| Miscellaneous | |||
| Non-AAA mortality rate | Depends only on age | ONS 2012–14 data40 | Chapter 6 , Quality of life and competing mortality |
| QoL utilities | Depend only on age | Population norms | Chapter 6 , Quality of life and competing mortality |
| QoL harms of screening | None | MASS10 | Chapter 6 , Quality of life and competing mortality |
| QoL harms of surgery | None | MASS10 | Chapter 6 , Quality of life and competing mortality |
Chapter 2 A discrete event simulation model for evaluating the cost-effectiveness of an abdominal aortic aneurysm screening programme
Previous economic evaluations11,12 of AAA screening for men have been implemented using a multistate Markov model. The original model was based on data from 4 years of follow-up of the MASS trial10 and gave an estimated mean cost per QALY gained of £2970 (95% uncertainty interval £2030 to £5430) at 2000–1 prices, over a 30-year time frame. The model was later updated to use data from 10 years of follow-up in the MASS trial10, data on prevalence and baseline aortic sizes from NAAASP, estimates of growth and rupture rates from the RESCAN collaboration,12,35 and 2013–14 prices. With these updated parameters, the mean cost per QALY gained was estimated to be £7370 (95% uncertainty interval £5470 to £9440).
The Markov model, as implemented, calculated the mean numbers of events and mean costs every 3 months over a certain time frame (e.g. 30 years), based on the expected number of persons occupying each of the model states. This allowed the model to be simple [e.g. it could be easily implemented in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA)], but makes it inflexible. First, it is difficult to change the state structure (i.e. adding or removing states from the model). This problem arose when the model was adapted to include more small AAA sizes for a recent evaluation of different surveillance policies. 35 Second, events are constrained to occur within each cycle of 3 months, and the amount of time spent in each state is always a multiple of the cycle length. Third, it is difficult to make modifications to the screening programme, such as changing the size threshold for diagnosis of an aneurysm or the size threshold for consideration for surgery.
In this project, a discrete event simulation (DES) was used instead of a Markov model, in which each individual has their own sequence of events that occur in continuous time. The DES was created using the freely available statistical programming language R (The R Foundation for Statistical Computing, Vienna, Austria). 41 This chapter describes the development of the DES, its model structure and the main associated assumptions. Specifically, it addresses objective 1 in Chapter 1, Scientific objectives. A validation exercise is then presented in which the DES is compared with 4-year results from the MASS trial,10 in which input parameters relevant to a population of 65-year-old men, similar to those from the MASS trial,10 are used. This exercise was conducted to verify that the computer program worked properly and that the DES was a reasonably accurate model. The DES was then run over a 30-year time horizon and the cost-effectiveness estimates were compared with those previously published.
Model structure
Figure 1 shows the pathways that an individual can take through the DES, starting from the time when they are invited or not invited to screening and continuing to the time when they die. Events that can occur during an individual’s lifetime are represented by rectangles and the arrows show the order in which events can occur. Some events incur costs that are relevant to the assessment of the screening programme; these are indicated in Figure 1 with circled ‘£’ signs. The DES simulates a number of individuals and summarises the events that they experience over time and the costs incurred.
FIGURE 1.
The sequences of events that are possible in the DES. Circled ‘£’ sign: event incurring costs. Note: although omitted from the figure, post-surgery surveillance and reinterventions can also occur. Adapted with permission from Sweeting et al. 42 © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 licence.

For each simulated individual, a set of patient characteristics is first generated. These include their age, initial aortic diameter and the rate at which their aortic diameter changes over time (see Modelling aortic growth and abdominal aortic aneurysm rupture). The DES then adopts an event-scheduling approach by generating a sequence of events for each individual and the times at which they may occur, using a list of events that are ‘scheduled’ for the future [future events list (FEL)]. The DES has an explicit simulation clock and chooses the event that has the earliest sampled time, and records it in the individual’s sequence of events. It then schedules, reschedules or cancels other events as necessary, updating the FEL. The process is repeated until death or censoring.
For each individual, scheduled events may or may not actually happen because of competing risks. For example, if a person’s scheduled time of death from non-AAA causes occurs prior to their scheduled time of AAA rupture, then the rupture will not occur.
As implemented, the DES in fact creates two copies of each person that are identical in terms of their baseline characteristics and their scheduled times of rupture and non-AAA death. These can be regarded as twins or a pair of clones. One twin/clone is invited to screening and the other is not. The purpose of this is to reduce the variation in the final health-economic outputs of the model. 43,44
Event types
Table 4 lists the possible events that can occur, when they are first scheduled, whether or not repeat events can occur and when they are ‘cancelled’ from the FEL.
| Event | Initial scheduled time | Can event be rescheduled (occur multiple times)? | Can event be cancelled? |
|---|---|---|---|
| Invitation to screening | Immediately (invited group only) | No | No |
| Require reinvitation | Immediately for a proportion of individuals (invited group only) | No | No |
| Attend screening | Immediately for a proportion of individuals (invited group only) | No | No |
| Non-visualisation of the aorta | Immediately for a proportion of those who attend screening (invited group only) | No | No |
| Incidental detection | After individual’s aortic diameter reaches diagnosis threshold (e.g. 3.0 cm) for those not currently in surveillance | Yes, following dropout from the screening programme | Yes, if rupture or non-AAA death occur first or the individual drops below the diagnosis threshold |
| Surveillance scan | Following screen-detected AAA below intervention threshold (e.g. 5.5 cm) or after incidental detection | Yes, after previous surveillance scan, after contraindication or after incidental detection | Yes, if dropout, rupture or non-AAA death occur first |
| Dropout (from surveillance) | Following screen-detected AAA or incidental detection | Yes, following incidental detection | Yes, if consultation, rupture or non-AAA death occur first |
| Consultation | Following a measured AAA diameter above the intervention threshold | Yes, following any repeat surveillance scan that measures AAA diameter above the intervention threshold | Yes, if dropout, rupture or non-AAA death occur first |
| Contraindicated | Immediately for a proportion of those who receive a consultation | No | No |
| Decide to elective surgery | Immediately for a proportion of those who receive a consultation | No | No |
| Decide on return to surveillance | Immediately for a proportion of those who receive a consultation | Yes, following a repeat consultation | No |
| Elective surgery (open and EVAR separately) | Following decision for elective surgery | No | Yes, if rupture or non-AAA death occur first |
| Rupture | Any time from start | No | Yes, if elective surgery or non-AAA death occur first |
| Emergency surgery (open and EVAR separately) | Immediately for a proportion of those who rupture | No | No |
| Surveillance following surgery (elective or emergency) | Following elective or emergency operation | Yes, after previous post-surgery surveillance scan | Yes, if non-AAA or AAA death occur first |
| Reintervention following surgery (elective or emergency) | Following elective or emergency operation | Yes, after a previous reintervention | Yes, if non-AAA or AAA death occur first |
| AAA death | Following rupture, emergency surgery or elective surgery | No | Yes, if non-AAA death occurs first |
| Non-AAA death | Any time from start | No | Yes, if AAA death occurs first |
Screening and monitoring
We refer to an initial ultrasound scan as a ‘screening’ scan and a subsequent check-up scan as a ‘surveillance’ scan. An individual who does not respond to the initial invitation to screening is reinvited, and may either attend and be screened or fail to attend screening. Invitation, reinvitation and screening all incur costs. In a small proportion of individuals who attend screening, visualisation of the aorta will be unsuccessful; these individuals will be discharged from the screening programme. For a person who is successfully screened, there are three possibilities: (1) if the aortic size, measured using an ultrasound scan, is less than the diagnosis threshold (currently 3.0 cm), then repeat surveillance is not needed and the individual is discharged; (2) if the aortic size is greater than or equal to the diagnosis threshold and less than the intervention threshold (currently 5.5 cm), then the individual is entered into surveillance and a surveillance scan is scheduled depending on the measured AAA size (in NAAASP, this is 1 year for AAAs of diameter 3.0–4.4 cm and 3 months for AAAs of diameter 4.5–5.4 cm); and (3) if the aortic size is greater than or equal to the intervention threshold (currently 5.5 cm), then a consultation with a vascular surgeon is scheduled. The model is flexible enough to allow any of the diameter thresholds and/or surveillance times to be modified. For example, a consultation for elective surgery could be scheduled earlier by changing the intervention diameter threshold from 5.5 to 5.0 cm.
Individuals whose AAA measures less than the diagnosis threshold at any of the surveillance scans (i.e. excluding the initial screening scan) are kept in the surveillance programme and have another surveillance scan scheduled as usual. Ultrasound scans are assumed to give imprecise measurements of the underlying aortic diameter (a latent parameter that changes over time and is defined for each individual by an aortic growth model; see Modelling aortic growth and abdominal aortic aneurysm rupture).
Dropout and incidental detection
Following the screening scan, if an individual remains in the screening programme, then a future ‘dropout from surveillance’ time is scheduled to account for a certain proportion of individuals who will drop out of the screening programme over time. If individuals are not under active follow-up in the screening programme (e.g. those in the non-invited group and those in the invited group whose screening scan was normal), then an incidental detection time is scheduled. Individuals become at risk of incidental detection only once their underlying aortic diameter reaches the diagnosis threshold, and their incidental detection time is, therefore, scheduled at some time after this occurrence. However, if the diameter is decreasing over time (a rare, but possible, occurrence when simulating many individuals), then incidental detection is allowed to occur only up until the time at which the diameter of that individual’s AAA drops below the diagnosis threshold. Incidental detection and dropout times are both generated from an exponential distribution with a fixed rate.
In the case of individuals who drop out of the screening programme, a further incidental detection time is scheduled, and, for those entering the screening programme via incidental detection, a further dropout time is scheduled. Hence, individuals can repeatedly drop out and come back into surveillance, although this will be a rare phenomenon in practice.
Consultation with a vascular surgeon
If an individual has a consultation with a vascular surgeon then their aortic diameter is remeasured using a computerised tomography (CT) scan, which may give a different reading to an ultrasound scan (see Consultation scan: computerised tomography scan versus ultrasound scan). For example, CT may give a systematically higher reading of the aortic diameter than ultrasound, and may also have a different measurement error. If the measured size on the CT scan is less than the intervention threshold, then the individual is returned to surveillance – that is, a new surveillance scan is scheduled. Otherwise, either an elective operation is scheduled or surgery is deemed to be contraindicated for that individual. If elective surgery is contraindicated, no surgery is scheduled and the individual remains under a defined surveillance protocol until their aneurysm ruptures or they die from a non-AAA cause. The DES allows the non-AAA death rate to change among individuals in whom surgery is contraindicated (e.g. owing to comorbidities, the death rate in this group is generally much higher than that of the general population).
Emergency and elective surgery
If an individual’s AAA ruptures, then they will either receive emergency surgery or die before they reach the operating table (in which case their death is recorded as AAA related). Both emergency and elective surgery carry an initial operative (30-day) mortality risk and a longer-term AAA-related mortality risk to account for future complications and secondary ruptures. Surgery can be via either EVAR or open repair; the probability that an individual will undergo EVAR is a parameter of the DES. The DES is flexible enough to allow specification of operative and longer-term risks separately for EVAR and open repair, emergency and elective, together with associating different costs to each type of repair. The initial 30-day postoperative mortality is implemented in the DES as an immediate event.
The model also allows the user to specify whether or not longer-term (> 30 days) postoperative AAA-related events can occur, such as reinterventions or postoperative surveillance, which incur costs. Such events can be scheduled at the time of operation or after the occurrence of a postoperative event (to allow for recurrent events), and the rate and cost of postoperative events can depend on the type of operation (EVAR or open) and whether the operation was in the elective or emergency setting. These model extensions are not applied in the validation model described in this chapter, but are considered in the inputs to the DES for women (see Chapter 5).
Finally, the DES is also flexible enough to allow operative events (i.e. the proportion receiving EVAR, or the proportion who do not survive 30 days postoperatively) to depend on the age and AAA diameter of the individual at the time of surgery. These risk factors are incorporated using logistic regression models, with the user specifying log-odds ratios for covariates associated with each event. As above, these DES model extensions are considered in Chapter 5 in the model for women.
Death from non-abdominal aortic aneurysm causes
Rates of non-AAA deaths can be input into the DES using age-specific rates (e.g. from population mortality statistics). The model then calculates the conditional survival curve from age at screening and simulates for each pair of individuals a time of non-AAA death by sampling from a Uniform(0,1) distribution and evaluating the inverse function of the survival distribution.
Example sequences of events
Table 5 shows example sequences of events from the DES for four pairs of twins (i.e. individuals with identical baseline characteristics). Like most individuals, pairs 1 and 2 have rather short sequences of events and die of non-AAA causes – each twin dies at the same time, so the only difference between them is that more money was spent on the twin who was invited to screening. Pairs 3 and 4 were chosen because they have longer sequences of events.
| Time (years) | Invited to screening | Not invited to screening |
|---|---|---|
| Pair 1 | ||
| 0.00 | Invited to screening | – |
| 0.00 | Require a reinvitation | – |
| 0.00 | Screened (ultrasound measurement of 2.82 cm) | – |
| 11.46 | Died of non-AAA causes | Died of non-AAA causes |
| Pair 2 | ||
| 0.00 | Invited to screening | – |
| 0.00 | Fail to attend screening | – |
| 11.44 | Died of non-AAA causes | Died of non-AAA causes |
| Pair 3 | ||
| 0.00 | Invited to screening | – |
| 0.00 | Screened (ultrasound measurement of 4.35 cm) | – |
| 1.00 | Surveillance scan (ultrasound measurement of 4.64 cm) | – |
| 1.25 | Surveillance scan (ultrasound measurement of 5.25 cm) | – |
| 1.50 | Surveillance scan (ultrasound measurement of 5.43 cm) | – |
| 1.75 | Surveillance scan (ultrasound measurement of 6.08 cm) | – |
| 1.95 | Consultation (CT measurement of 5.04 cm) | – |
| Returned to surveillance | ||
| 2.20 | Surveillance scan (ultrasound measurement of 4.85 cm) | – |
| 2.45 | Surveillance scan (ultrasound measurement of 5.18 cm) | – |
| 2.70 | Surveillance scan (ultrasound measurement of 6.12 cm) | – |
| 2.89 | Consultation (ultrasound measurement of 5.71 cm) | – |
| Decide on elective surgery | ||
| 3.05 | Elective surgery (open repair) | – |
| 5.11 | – | Ruptured AAA (diameter of 7.13 cm) |
| Emergency surgery (open repair) | ||
| 6.24 | Died of non-AAA causes | Died of non-AAA causes |
| Pair 4 | ||
| 0.00 | Invited to screening | – |
| 0.00 | Screened (ultrasound measurement of 7.34 cm) | – |
| 0.19 | Consultation (CT measurement of 6.91 cm) | – |
| Decide on elective surgery | ||
| 0.36 | Elective surgery (open repair) | |
| 0.54 | – | Ruptured AAA |
| AAA death | ||
| 1.81 | Died of non-AAA causes | – |
For pair 3, the twin who is invited to screening attends and is found to have a 4.35-cm AAA. He has a surveillance scan 1 year later and then at 3-month intervals once his aneurysm is observed to be ≥ 4.5 cm. At 1.75 years, the ultrasound scan finds his aortic size to be 6.08 cm. This high reading is largely due to measurement error and his true aortic size is only 5.16 cm. Nevertheless, he is referred for a consultation, in which the more accurate CT scan, 71 days later, measures the aneurysm at 5.04 cm and he is, therefore, returned to surveillance. This person continues to attend his surveillance scans. About 1 year later his aneurysm is again measured to be > 5.5 cm and he has another consultation. This time the large aneurysm is confirmed by the CT scan and it is decided that he should have elective surgery. The surgery is successful and he lives for another 3 years.
His twin, who is not invited to screening, experiences a rupture, with a large aneurysm of 7.13 cm, but receives emergency surgery and survives. In the end, the two twins die at the exact same time due to non-AAA causes, so there are no gains in life-years in this example. However, the screening programme has saved overall costs by avoiding a costly emergency operation – the first twin instead has the safer and cheaper elective surgery.
Pair 4 is another example in which the screening programme works well. The twin who is invited to screening lives longer as a result of the screening. His aneurysm is detected straight away, he is referred for a consultation and he has elective surgery, which is successful. The other twin’s AAA ruptures and he dies without getting to the hospital in time to have emergency surgery.
Modelling aortic growth and abdominal aortic aneurysm rupture
The model for aortic growth
In the DES, the evolution of an individual’s aortic diameter over time must be taken into account as it affects many aspects of the health economic model, namely (1) when an individual can be diagnosed, (2) planned surveillance intervals, (3) when an intervention can be considered, (4) the risk of rupture, (5) the probability of receiving EVAR rather than open repair and (6) the operative mortality risk. Therefore, the evolution of the aortic diameter over time is modelled using a continuous-time linear mixed model, which allows the underlying diameter and a measured diameter (using ultrasound or CT) to be determined at any time point. Let yij be the aortic diameter, as measured using ultrasound, of person i at time tij, j = 1,. . .,ni; so yi0 is the baseline diameter as measured at screening. The linear mixed model is as follows:
where
Each person has two random effects: (1) their own intercept (true baseline log-diameter), b0i, and (2) their own slope (rate of growth), b1i, measured on the log-diameter scale. Correlation between an individual’s underlying baseline log-diameter and slope is allowed as b0i and b1i have a bivariate normal distribution with correlation parameter ρ. The parameters σ20 and σ21 determine the between-person variability of the intercepts and slopes, respectively, while σ2w determines the amount of variability due to measurement error.
The linear mixed model is fitted using data from repeated ultrasound measurements of the aortic diameter from cohorts of AAA patients such as from the MASS trial10 or RESCAN studies. 35 These cohorts are restricted to the diameter range 3.0–5.5 cm. As a result, model extrapolation is used to infer true baseline diameters and growth rates for individuals outside this range.
Baseline diameter distribution and derived random effects
The baseline diameter distribution is a particularly important aspect of the DES, because it determines how many persons have aneurysms at the time at which screening would be implemented and has a great effect on how many develop aneurysms in subsequent years. The full specification of the model is that yi0 follows a fixed baseline distribution, which we specify using external data sources (e.g. data on measured diameters from the first 700,000 men screened in NAAASP), and an individual’s random effects b0i and b1i are then generated conditional on their observed baseline diameter. Following evaluation of the performance of the aortic growth model, it was decided to use the following rules to generate an individual’s random-effects (full details of the reasons for these choices are given in Appendix 2).
If yi0 ≥ 3.0, generate random effects from the linear mixed model posterior distribution
As estimated parameters from the linear mixed model are strictly relevant only to baseline diameters of ≥ 3.0 cm, for individuals in this range, b0i and b1i are generated from their bivariate normal distribution conditional on the observed diameter, yi0:
where
If yi0 < 3.0, set an individual’s true baseline diameter to their observed diameter
If the observed baseline diameter, yi0, measures < 3.0 cm, then we set b0i = log(yi0). This avoids shrinkage of the true baseline diameter upwards towards the mean in the AAA cohort used to fit the linear mixed model [as estimated by exp(β0)].
If 2.0 < yi0 ≤ 3.0, generate an individual’s rate of growth from their posterior distribution conditional on b0i
If 2.0 ≤ yi0 < 3.0, then b1i is generated from a univariate normal distribution conditional on b0i:
where
If yi0 < 2.0, set rate of growth to zero
This rule means that, if the aortic diameter is < 2.0 cm at baseline, no aneurysm will develop during the individual’s lifetime. It was felt that, in this range, the model-extrapolated estimates of growth could not be relied on, and instead it was assumed that in these individuals the aorta would never grow to be aneurysmal within their lifetime.
The rules set out here ensure that extrapolated growth rates < 3.0 cm are sensible and approximately follow empirical data from a group of men with aortic diameters of 2.6–2.9 cm followed over time in the Gloucestershire study. 45 Further technical details are given in Appendix 2.
Prevalence
The user of the DES specifies the baseline diameter distribution as an input as well as, optionally, the prevalence of AAAs, which is the probability that a baseline diameter is greater or equal to the diagnosis threshold (e.g. 3.0 cm). If provided, this optional input then reweights the baseline diameter distribution accordingly (further details of this reweighting procedure are given in Chapter 4).
Calculation of a person’s aortic diameter at any time
The aortic diameter measured at an individual’s initial screening scan is taken to be yi0, because this ensures the correct prevalence of AAA at screening.
When an individual’s aortic diameter at time t > 0 is needed, one of the following formulas is used.
The measurement error is expressed differently in the formulas for ultrasound and CT scans. This has been done as a matter of convenience since estimates of σw come from the mixed-effects model that uses ultrasound measurements and is fitted on the log-diameter scale. Meanwhile, estimates of µCT and σCT will generally originate from the literature and are assumed to be additive on the diameter scale.
The model for abdominal aortic aneurysm rupture
The model for AAA rupture is the survival component of a joint longitudinal and survival model. According to this model, the hazard of rupture is:
where γ is the log-baseline hazard and α is the log-hazard ratio associated with a 1-unit increase in log-aortic diameter (the expression in the inner brackets; see The model for aortic growth). In reality, the hazard of rupture will increase with the aortic diameter, and this is the case if α is positive.
The hazard function corresponds to a Gompertz distribution with shape parameter αb1i and rate parameter exp(γ + αb0i). Therefore, rupture times are generated from this distribution for each pair of individuals from the time of screening.
Improving the efficiency of the discrete event simulation and conducting probabilistic sensitivity analyses
Once a sequence of events has been created for a person, this can be used to calculate health-economic quantities for them, namely their life-years, the total cost of the events that they experience, and their discounted life-years, costs and QALYs. Discounting is applied at 3.5% per year for both costs and life-years, whereas QALYs are calculated based on UK population norms for QoL. 31
A single run of the DES consists of simulating a large number of individuals, calculating their health-economic quantities and finding the means of these quantities over all the individuals. Differences in mean life-years and costs between the individuals in the invited and non-invited groups can then be calculated and the ICER and INMB for a given WTP can be obtained.
Convergence
Differences in life-years and costs between the invited to screening and not invited to screening groups are generally small, as the prevalence of AAAs is relatively low and, therefore, a screening programme will benefit only a small proportion of the population. Thus, the model needs to be run on millions of individuals in order to obtain accurate estimates of incremental life-years. However, if interest is primarily in incremental costs and life-years (rather than absolute estimates for each group), then this approach is computationally inefficient. A pair of twins who have an aortic diameter less than the diagnosis threshold at screening should follow exactly the same life-course, as the twin who is invited to screening will be found to be normal on screening and will no longer be followed up. The only difference between the twins is that the twin invited for screening will incur extra screening costs. For this reason, if accurate estimates are required of incremental life-years, incremental costs, the ICER and INMB, then the DES can be run by selectively sampling only individuals above the diagnosis threshold. The mean incremental costs and life-years are then calculated in this subgroup and are weighted by the prevalence (proportion of individuals in the population who are above the diagnosis threshold at screening). The population below the diagnosis threshold is never sampled but has zero mean incremental life-years and mean incremental costs determined by the mean screening costs in the invited to screening group. These are weighted by (1 – prevalence) and are added to the incremental mean costs in those sampled above the threshold. An example of the convergence of the ICER using the selective sampling approach versus not using the approach is shown in Appendix 2.
Probabilistic sensitivity analysis
A PSA is conducted by running the main analysis a large number of times using a different set of parameter inputs each time to account for uncertainty in the parameters. Each of the main analyses that are performed in the PSA produces an estimate of the incremental cost and an estimate of the incremental effectiveness, and these quantities can then be viewed in a scatterplot and a cost-effectiveness acceptability curve (CEAC) can be generated.
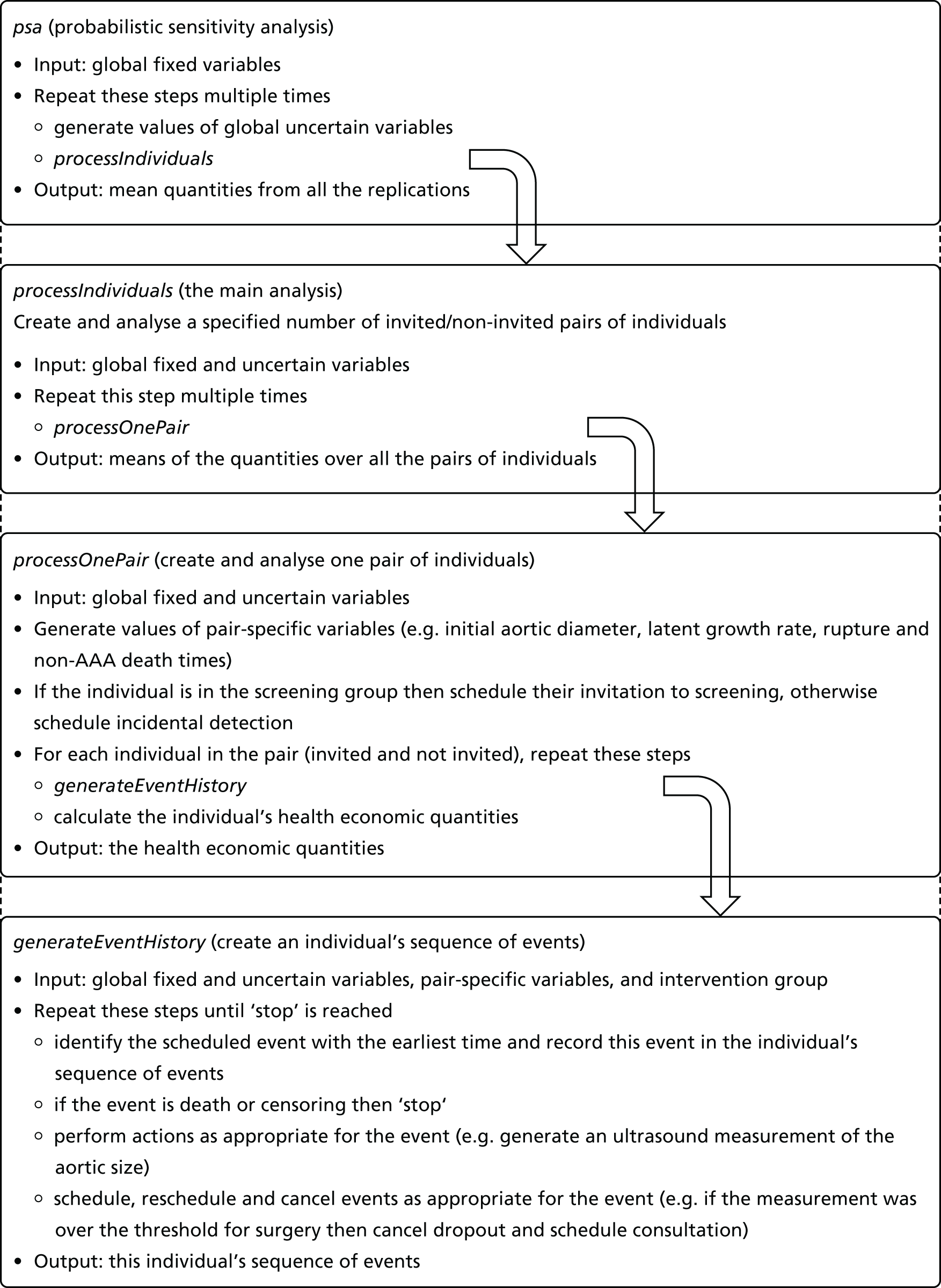
Figure 2 shows how the functions in the DES form a hierarchy in which each function runs the function below it multiple times. At the top is a function named ‘psa’, which performs a PSA. Next is ‘processIndividuals’, which conducts the main analysis. Within this function is ‘processOnePair’, which generates a pair of twins and calculates their health-economic quantities. Embedded into this function is ‘generateEventHistory’, which generates a single sequence of events for an individual. This is run twice by ‘processOnePair’, once for the invited twin and once for the non-invited twin.
FIGURE 2.
Abdominal aortic aneurysm screening DES: hierarchy of functions.
Distributions for parameters
Model parameters that feature as uncertain parameters in a PSA are one of three types, and are dealt with using different distributions within the PSA:
-
A probability (e.g. probability of attendance) is generated from a Beta distribution in the PSA.
-
A rate (e.g. rate of incidental detection) is generated from a Gamma distribution in the PSA.
-
Coefficients from a regression model (e.g. a logistic model for a probability, the linear mixed-effects regression model for aortic growth or the survival analysis regression model for AAA rupture) or transformations of the coefficients are generated for each regression in combination using a multivariate normal distribution in the PSA. For example, the regression coefficients in the linear mixed model for aortic growth are generated in a PSA from a multivariate normal distribution of the transformed parameter vector (β0,β1,logσ0,logσ1,tanh–1ρ,logσW), while the regression coefficients in the model for AAA rupture (γ and α) are generated from a bivariate normal distribution in the PSA.
Validating the discrete event simulation in men
Validating against 4-year data from the Multicentre Aneurysm Screening Study in men
To validate the DES, a model was developed based on inputs used in the original Markov model developed for men. 11 The values of the parameters used are shown in Table 27, Appendix 2, alongside distributions placed on the parameters for PSA. The baseline distribution was taken from the first 700,000 screened men in NAAASP and was then reweighted to give an AAA prevalence of 4.97%, as observed in the MASS trial. 10 A further reweighting was then undertaken to ensure that 70.8% of the individuals’ aneurysms were small (3.0–4.4 cm), 16.7% were medium (4.5–5.4 cm) and 12.5% were large (≥ 5.5 cm) at screening, as seen in the MASS trial. 10 Non-AAA death rates were taken directly from those observed in the MASS trial. 10 Costs were the same as those used originally:11 invitation (£1.31), reinvitation (£1.28), screening ultrasound scan (£19.08), surveillance ultrasound scan (£46.04), consultation for elective surgery (£309.88), elective open repair (£6908.75) and emergency open repair (£11,175.63).
The screening programme evaluated was as specified in the MASS trial10 (1-year monitoring for AAAs of 3.0- to 4.4-cm diameter, 3-month monitoring for AAAs of 4.5- to 5.4-cm diameter and consideration for elective surgery for AAAs ≥ 5.5-cm diameter). The DES was run for a population of men aged 69 years (mean age of the MASS trial10), for a mean follow-up of 4 years (with random censoring between 3 and 5 years to mimic censoring in the 4-year MASS trial10 results) and the total number of events were compared with those observed in the 4-year MASS trial10 data.
Table 6 shows the numbers of key events in the two groups over a 4-year period as estimated by the DES. The DES was run using 107 pairs of individuals, and then the estimated numbers of events that occurred was scaled to be relevant to the size of the invited and control (non-invited) groups in the MASS trial. 10 For comparison, the observed numbers in the MASS trial10 are also given, together with the expected-to-observed (E/O) ratio expressed as a percentage. The E/O ratio is within ± 20% for all events except for contraindications resulting from screen-detected AAAs. The DES was deemed accurate enough in estimating the numbers of key events in both arms, and the timing of these events also adequately followed the occurrence of the events in the trial. Further results from this validation exercise are given elsewhere, including cumulative numbers of key events over time. 46
| Event | MASS10 observed (n) | DESa (n) | DES (% of MASS10) |
|---|---|---|---|
| No invitation group | |||
| Elective operation | 100 | 98 | 98 |
| Emergency operation | 62 | 69 | 111 |
| Rupture | 138 | 157 | 114 |
| Contraindicated for elective surgery | N/A | 17 | N/A |
| AAA death | 113 | 122 | 108 |
| Non-AAA death | 3750 | 3708 | 99 |
| Invited group | |||
| Elective operation | |||
| Resulting from screen detection | 295 | 332 | 113 |
| Resulting from incidental detection | 31 | 27 | 87 |
| Emergency operation | 28 | 31 | 109 |
| Rupture | 66 | 70 | 105 |
| Contraindicated for elective surgery | |||
| Resulting from screen detection | 41 | 54 | 131 |
| Resulting from incidental detection | N/A | 4 | N/A |
| AAA death | 65 | 65 | 100 |
| Non-AAA death | 3694 | 3712 | 100 |
| Loss to recall follow-up | 290 | 281 | 97 |
Validating against 30-year contemporary Markov model in men
The DES was then extended to run for 30 years, with inputs updated to reflect more contemporaneous estimates for men. 12 In particular, national mortality statistics were used for non-AAA death rates, the baseline diameter distribution from NAAASP was used directly (hence giving an AAA prevalence of 1.34%) and both EVAR and open repair for elective mortality were considered along with long-term postoperative mortality. Costs were the same as previously specified:12 invitation (£1.70), reinvitation (£1.70), screening ultrasound scan (£32.20), surveillance ultrasound scan (£68.00), consultation for elective surgery (£435.25), elective open repair (£11,532.69), elective EVAR (£13,345.66) and emergency open repair (£19,984.75).
The estimated life-years and costs were compared with the previously published estimates from a Markov model. 12 Table 7 shows that the results, although not identical, provide a similar conclusion regarding the cost-effectiveness of the AAA screening programme over a 30-year period. The DES estimates a higher gain in life-years but with similar incremental costs to the 30-year Markov model and as such the estimated ICER is about £1000 less. Nevertheless, both models suggest that the programme is highly cost-effective.
| Outcome | 30-years | |
|---|---|---|
| Markov model12 | DES | |
| No invitation group | ||
| Life-years | 12.719 | 12.556 |
| QALYs | 9.921 | 9.647 |
| Cost (£) | 269 | 364 |
| Invited group | ||
| Life-years | 12.727 | 12.567 |
| QALYs | 9.928 | 9.655 |
| Cost (£) | 316 | 414 |
| Difference | ||
| Life-years | 0.0084 | 0.01026 |
| QALYs | 0.0067 | 0.00777 |
| Cost (£) | 47 | 50 |
| ICER (£) | ||
| Discounted | 5758 (95% CI 4285 to 7410) | 4876 (95% CI 3727 to 6839) |
| Discounted, quality adjusted | 7370 (95% CI 5467 to 9443) | 6440 (95% CI 4920 to 9063) |
Summary
The DES developed and described in this chapter provides a flexible and comprehensive way to assess the cost-effectiveness of AAA screening under a number of possible screening scenarios. The DES is applied to contemporary data for women in Chapter 7, while different screening programme options are evaluated in Chapter 8. The model allows the assessment of parameter uncertainty through PSAs. The DES has been validated against 4-year outcomes as observed in the MASS trial10 and was found to perform adequately. The model also gave comparable results with respect to previously published 30-year cost-effectiveness results, and further internal validation of the model has been undertaken (e.g. see Appendix 2 regarding long-term growth and rupture rates).
Chapter 3 Systematic reviews of the current prevalence of screen-detected abdominal aortic aneurysms and management of abdominal aortic aneurysms in women
The prevalence of AAAs and the efficacy of interventions to prevent ruptures are pivotal to assessing the likely benefit of AAA screening in women. Therefore, we undertook a series of four systematic reviews of contemporary (year 2000 or later) AAA prevalence and management in women:
-
the prevalence of screen-detected AAAs
-
the proportion of AAAs suitable for endovascular repair
-
the proportion of patients with AAAs not offered repair
-
the 30-day operative mortality following either endovascular or open repair.
We also undertook a narrative review of the outcome in women following rupture, the fifth piece of work in this chapter.
Specifically, this chapter addresses objective 2 in Chapter 1, Scientific objectives, providing evidence on the prevalence of AAAs (see Table 1) and of parameters related to elective surgery (see Table 2), as well as a literature review of parameters related to emergency surgery for ruptured AAAs (see Table 2). The chapter is a summary of two published papers,34,37 in which more discussion of the results is provided. Some of the tables and figures are adapted from these papers in the British Journal of Surgery and Lancet with permission.
The systematic reviews were conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines47 and registered in the international prospective register of systematic reviews (PROSPERO) database [www.crd.york.ac.uk/PROSPERO/ (accessed 1 March 2017); registration numbers CRD42015020444 and CRD42016043227]. For the reviews, MEDLINE and EMBASE databases were searched, using a combination of controlled vocabulary [medical subject heading (MeSH) or Emtree®] terms and free-text terms in ProQuest Dialog™. Clinicaltrials.gov (http://clinicaltrials.gov), Current Controlled Trials [www.controlled-trials.com/ (accessed 1 March 2017)] and the National Research Register (UK) were also searched for details of ongoing or unpublished studies, complemented by hand-searching the abstracts of the 2015 and 2016 annual meetings of the Society for Vascular Surgery (North America) and the European Society for Vascular Surgery. The quality of studies was assessed using the relevant Newcastle–Ottawa scores. 48 Searches were restricted to the major European languages. The MeSH headings, search dates for each review and the inclusion and exclusion criteria are shown in Table 8, and the PRISMA search strategies and flow charts are given in Appendix 3 (Boxes 1–4 and Figures 19–22).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Prevalence reviewa | |
| Screening date year 2000 onwards; final search date 13 January 2016 | Review articles |
| Women ≥ 60 years of age | Editorials |
| All ethnic groups | Letters |
| Population described clearly | Case reports |
| Screening of ≥ 1000 women | Studies of people with known cardiovascular disease |
| Ultrasound or CT for aortic diameter measurement | |
| EVAR suitability reviewb | |
| Published 1 January 2005 to 2 September 2016 | Review articles |
| Sex-specific data | Editorials |
| Population of all or nearly all of the patients considered for AAA repair | Letters |
| Morphological criteria for suitability clearly defined with measurements or device IFU | Case reports |
| ≥ 20 women | Studies including only patients with EVAR |
| CT with 1-mm slices and 3D reconstruction | |
| Non-intervention reviewc | |
| Published 1 January 2005 to 2 September 2016 | Review articles |
| Sex-specific data | Editorials |
| Population of all or nearly all of the patients considered for AAA repair | Letters |
| Team decision whether or not repair offered | Case reports |
| ≥ 20 women | Studies including only patients with EVAR |
| 30-day operative mortality reviewd | |
| Published 1 January 2009e to 26 August 2016 | Review articles |
| Study period after year 2000 | Editorials |
| Sex-specific 30-day mortality data | Letters |
| ≥ 50 women | Case reports |
| Studies that only provide hazard ratios | |
| Studies that only report in-hospital mortality | |
Random-effects meta-analyses of proportions across studies were undertaken on a logit scale and transformed back to the probability scale for presentation.
Current prevalence of screen-detected abdominal aortic aneurysms in women
The literature search identified seven studies,14,49–54 all based on ultrasound screening. Of these, only three14,49,50 were based on screening using population registers: two51,52 were screening studies of people in the USA offered free screening by advertisement and two53,54 were screening studies of those paying a fee to the Lifeline screening programme, also recruited by advertisement. For the Norwegian study,49 data for women with an aortic diameter of ≥ 3.0 cm were obtained from the corresponding author. Women with a known AAA were excluded from screening in all studies. Only one study14 reported the exact method of ultrasound diameter measurement (anterior–posterior or transverse, based on inner to inner wall, outer to outer wall or leading edge to leading edge). Variation in the method of measurement could produce considerable heterogeneity, as there is up to a 6 mm difference between inner to inner and outer to outer wall diameters.
Correspondence with authors provided further details of several studies,51–53 and one author52 provided an eighth unpublished study, a follow-on to their earlier study. Data were extracted, wherever possible, by age band and smoking status. The main US Lifeline screening study did not report on smoking status; however, smoking status was available for a subgroup of women, with sponsored screening, and this was included only for assessment of the effect of smoking on prevalence. 13 One excluded study55 reported on physician-initiated screening (with both ultrasound and CT) in a socioeconomically deprived population in the USA and did not define the specific criteria for screening; however, it provided additional useful information about the effects of smoking on prevalence.
An estimate of the prevalence was made from each study (number of women with an AAA divided by the number of women who were screened successfully). Three studies49,53,54 included women < 60 years of age in their screening. As the present review excludes younger women, only those aged ≥ 60 years from these studies were included.
The characteristics of the included studies are summarised in Table 9. Two studies53,54 of very large cohorts were identified (about 1.4 and 0.9 million women, respectively, aged ≥ 60 years), mainly self-referred for self-purchased Lifeline screening, from the USA and the UK and Ireland. Smaller studies offering free screening based on population registers were from Sweden,14 Norway49 and Italy,50 but only two14,49 of these were of very high quality, and, in total, this type of study contributed only 11,003 women. With the three further studies offering, by advertisement, sponsored free screening in the USA, this gave an overall total of 1,537,633 women screened in eight separate studies, with a pooled prevalence of AAAs of 0.74% [95% confidence interval (CI) 0.53% to 1.03%] in women aged ≥ 60 years, but with considerable heterogeneity (see Figure 23, Appendix 3).
| Study | Selection for screening | Screening dates | Country | No. of women screened (% attendance) | Age range (years) | Never smoked, n (current smokers) (%) | N–O score* | No. of AAAs (% prevalence) |
|---|---|---|---|---|---|---|---|---|
| Forsdahl et al.49 | Population based, free | 2001 | Norway | 1956 (85†) | 61 to ≥ 80 | 35 (25) | 9 | 30 (1.53) |
| Ogata et al.51 | Self-referred, free | 2001–4 | USA | 1298 (n.a.) | 60–89 | n.a. (9.2) | 5 | 19 (1.46) |
| Hupp et al.52 | Self-referred, free | 2000–6 | USA | 4982 (n.a.) | 60–89 | n.a. | 7 | 47 (0.94) |
| Savji et al.54‡ | Mainly self-referred, self-purchased | 2003–8 | USA | 1,428,316 (n.a.) | 61–100 | n.a. | 6 | 6229 (0.44) |
| Hupp (unpublished) | Self-referred, free | 2006–8 | USA | 3060 (n.a.) | 66–105 | 22 (n.a.) | 7 | 28 (0.92) |
| Svensjö et al.14 | Population based, free | 2007–9 | Sweden | 5140 (74) | 70 | 56 (10) | 9 | 19 (0.37) |
| Palombo et al.50 | Population based, free | 2007–9 | Italy | 3907 (48) | ≥ 65 | n.a. | 7 | 43 (1.10) |
| Bulbulia et al.53 | Self-referred, self-purchased | 2008–12 | UK, Ireland | 88 974 (n.a.) | 60 to ≥ 80 | n.a. | 6 | 278 (0.31) |
The overall prevalence of AAAs increased rapidly with age: 0.43% at 61–70 years, 1.15% at 71–80 years and 1.68% in those aged ≥ 81 years (Figure 3). However, there was considerable heterogeneity even for these pooled estimates (I2 = 74–94%), and in every age band the prevalence was lowest in the self-referred cohorts and highest in the Norwegian population register-based cohort. However, when relative risks were assessed, there was more consistency between studies (I2 = 0–49%) than seen with the absolute risks. Compared with the 60- to 69-year age group, the prevalence was 2.7 (95% CI 1.8 to 4.2) times higher in the 70- to 79-year age group and 4.3 (95% CI 4.0 to 4.7) times higher among women aged ≥ 80 years.
FIGURE 3.
Prevalence of AAAs in women aged ≥ 60 years, by 10-year age groups. References for studies are in Table 9. Reproduced from Ulug et al. 34 with permission. © 2016 The Authors. BJS published by John Wiley & Sons Ltd on behalf of BJS Society Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.

Only four studies reported on prevalence by smoking status (see Table 9), although the recording of smoking status was not uniform. Hupp (Dr Jon A Hupp, Anne Arundel Medical Center, Annapolis, MD, USA, 2016, unpublished) recorded those who remembered having smoked > 100 cigarettes in their lifetime, which is the definition used by the US Preventive Services Task Force. 5 The overall prevalence was lower for never smokers (0.28%) than for ever smokers (1.34%) (see Figure 24, Appendix 3). Three studies reported the prevalence in current smokers 2.08%,14 4.63%49 and 2.82%. 51 The study by Jahangir et al. 55 provides support for this effect as the association between AAAs and former smoking had a hazard ratio of 3.4, rising to 9.2 in current smokers.
Summary
This review provided an overall AAA prevalence of 0.74% for women aged ≥ 60 years, with the prevalence increasing sharply with age and current smoking. The overall prevalences of 0.43% for the 61- to 70-year age group and of 1.15% for the 71- to 80-year age group are used in the modelling (see Chapters 7 and 8) as the prevalences for women aged 65 and 75 years, respectively.
Suitability of women versus men for standard endovascular repair
After searching and evaluation, only five papers based on five studies56–60 were eligible for inclusion in the meta-analysis. One study also included suitability for endovascular sealing technology but used a selected population. 61 All the studies focused on standard endovascular repair and did not consider the use of fenestrated grafts. The characteristics of the included studies are summarised in Table 29, Appendix 3. Only one58 of these studies included > 100 women; most were small, and the quality of these studies was not good. The criteria of morphological suitability for EVAR were different in each study: three studies56,58,59 included all patients with an aneurysm (including those not offered intervention), one study60 did not specify which patients were being considered for EVAR and one study57 considered only patients who had undergone elective repair. The largest study58 has published two further updates62,63 but neither provided sufficient information to merit inclusion in the review. The threshold AAA diameter for inclusion ranged from 4 cm to 5 cm. In total, there was information for 1507 men, but only 400 women, with the proportion considered suitable for EVAR ranging from 25% to 47%. The overall estimate of suitability for EVAR in women was 34.0% (95% CI 25.4% to 43.8%) compared with 53.6% (95% CI 46.4 to 60.6%) in men, both overall estimates having significant heterogeneity (Figure 4a).
FIGURE 4a.
Proportion of patients morphologically suitable for EVAR in women and men separately. References for studies are in Table 29, Appendix 3. Reproduced from Ulug et al. 37 © 2017 The Authors. Published by Elsevier Ltd. This is an open access article under CC BY-NC-ND licence.

FIGURE 4b.
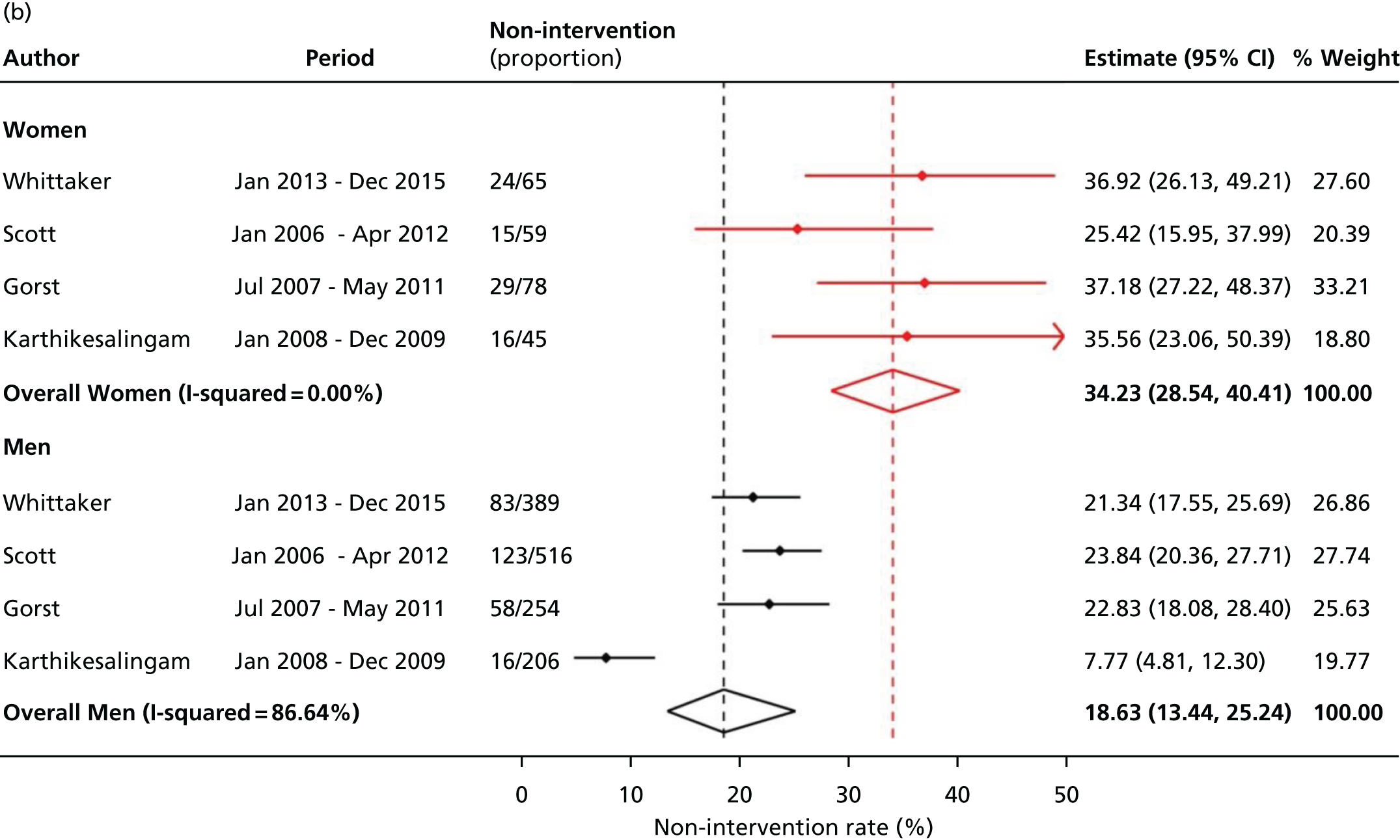
Non-intervention rates in women and men separately. References for studies are in Table 30, Appendix 3. Reproduced from Ulug et al. 37 © 2017 The Authors. Published by Elsevier Ltd. This is an open access article under CC BY-NC-ND licence.
Some studies considered relaxing the morphological criteria for EVAR, which increased the proportion of women suitable for EVAR. For instance, in the largest study,58 reducing the eligible neck length to > 7.5 mm (from > 15 mm) increased the proportion of women suitable for EVAR from 63 out of 251 (25%) to 113 out of 251 (45%). The 2014 Swedish study56 also considered relaxation of the minimum iliac diameter from 7.5 mm (Cook Zenith Flex®) or 8 mm (Gore Excluder or Medtronic Endurant) to 6 mm, which would have increased the proportion of women eligible for EVAR from 27% to 39%. The type of endograft considered also affects the proportion of women suitable for EVAR. For instance, in one excluded study61 that considered both conventional endografts and endovascular sealing, just 41% (32/78) of women were suitable for the Gore Excluder graft but 78% (61/78) would have been eligible for the Nellix endovascular sealing technology. 61 In the largest study,58 evidence was provided showing how suitability for EVAR declined with increasing aneurysm diameter, with almost no women being suitable for EVAR if their AAA diameter exceeded 6.5 cm. However, the other four studies56,57,59,60 provided few demographic or clinical details, so it was not possible to investigate how the suitability for EVAR in women might depend on age or other characteristics.
Summary
Overall, only 34% of women are suitable for standard endovascular repair (compared with 54% for men). Even with devices newer than those considered in this systematic review, the proportion of women suitable for endovascular repair according to the manufacturer’s instructions for use is likely to reach only 40%.
Proportion of women versus men not offered an intervention
Searching and evaluation yielded four publications, all from the UK: two papers20,64 and two abstracts (both with additional information from the authors). 65,66 All four studies were retrospective, with a total of just 1365 men and 247 women; the studies were only of fair quality (see Table 30, Appendix 3). The overall results showed greater heterogeneity for men and suggested that one-third (34.2%, 95% CI 28.5% to 40.4%) of potentially eligible women were not offered or were refused AAA repair, this proportion being about double the non-intervention rate in men, 18.6% (95% CI 13.4% to 25.2%) (see Figure 4b). The difference in non-intervention rates between men and women was highest for the earliest study at a specialist tertiary referral centre. 20
Surgical registries and national databases do not record or report the numbers of patients with an AAA who either are morphologically suitable for EVAR or are denied elective repair. In the case of the latter, the only data we identified came from four single-centre series in the UK, where the decisions about repair are made at a multidisciplinary team meeting: presumably the women not offered repair had extensive comorbidities and had a high risk of early postoperative death. Only one of these series has provided detailed follow-up data for those initially assigned to a non-intervention policy. 64 The authors found that after 3 years only about one-third of these patients remained alive and that 37% had died of rupture.
Summary
Overall, 34% of women with clinically relevant an AAA (usually ≥ 5.5 cm in diameter) were not offered an elective repair of their intact aneurysm, after consideration at a multidisciplinary team meeting. The non-intervention proportion in women is twice as high as in men.
Thirty-day operative mortality in women versus men
After searching and evaluation, seven papers18,67–72 based on seven studies met the inclusion criteria. Among these, one study72 reported on perioperative mortality in a combined cohort from 1992 to 2012, but the 30-day operative mortality data for the late era (2003–2012) were obtained from the corresponding author. Similarly, the corresponding author of a study investigating the outcomes of primary infrarenal AAA repairs in the Swedish Vascular Registry (Swedvasc) between 1994 and 2010 provided data on 30-day mortality for the time period 2006–10. 69 All studies included consecutive patients undergoing EVAR and/or open repair for infrarenal AAAs between 1 January 2000 and 31 December 2013. One further study73 was identified and the few patients with repairs before 2000 were excluded. Therefore, eight studies18,67–73 were included in the meta-analysis. All eight studies provided data for intact infrarenal aneurysms only; there were two studies72,73 that excluded symptomatic AAAs. One very large study74 of an English administrative database (2002–13) was excluded because much of the 30-day mortality was not aneurysm related. A rather similar study,75 but for endovascular repair only, based on the same database for the years 2006–15, was not identified in searches carried out by 26 August 2016.
The characteristics of the included studies are summarised in Table 31, Appendix 3. There were two population-based studies, one with 765 from Sweden69 and one including 5421 women from the USA. 71 Other, mostly smaller, studies, based on either single centre or voluntary registries, were all from the USA;18,67,68,70,72 in total, this type of study contributed 2438 women. Individual patient data meta-analysis of four prospective randomised controlled trials (RCTs) [EVAR-1,38 Dutch Randomised Endovascular Aneurysm Management (DREAM),76 US Open Versus Endovascular Repair (OVER)77 and French Anevrysme de l'aorte abdominale: Chirurgie versus Endoprothese (ACE)78 trials] contributed data for 148 women. This gave a total of 8772 women operated on in eight separate studies, with an overall 30-day mortality of 2.23% after EVAR (95% CI 1.86% to 2.68%) with no heterogeneity (Figure 5a), and of 5.37% after open repair (95% CI 4.18% to 6.88%) with some heterogeneity (Figure 5b). These data contrast with the results for a much larger cohort of 33,803 men operated on in these same studies with an overall 30-day mortality of 1.29% (95% CI 0.96% to 1.72%) after EVAR and 2.82% (95% CI 1.88% to 4.22%) after open repair; both overall estimates were subject to considerable heterogeneity (see Figure 5a and b). The Medicare study71 provided more than half the numbers of both men and women. When this study was removed from the meta-analysis, the 30-day mortality for women changed little: overall mortality 2.55% (95% CI 1.83% to 3.55%) and 4.72% (95% CI 3.83% to 5.82%) for EVAR and open repair, respectively.
FIGURE 5a.
The 30-day mortality after EVAR for intact AAAs, in women and men separately. References for studies are in Table 31, Appendix 3. Reproduced from Ulug et al. 37 © 2017 The Authors. Published by Elsevier Ltd. This is an open access article under CC BY-NC-ND licence.

FIGURE 5b.
The 30-day mortality after open repair for intact AAAs, in women and men separately. References for studies are in Table 31, Appendix 3. Reproduced from Ulug et al. 37 © 2017 The Authors. Published by Elsevier Ltd. This is an open access article under CC BY-NC-ND licence.

Data on confounding factors such as age, AAA diameter, number of symptomatic AAAs included and comorbidities were sparse, so that the influence of such variables could not be evaluated.
A comprehensive systematic review and meta-analysis25 of sex differences in mortality after either EVAR or open repair of AAAs was published in 2010. This review included the English-language literature data from 1995 to July 2009 and, for operative mortality, used either 30-day or in-hospital mortality (the latter is usually lower than 30-day mortality). The review concluded that operative mortality was higher in women than men: overall odds ratio 2.51 (95% CI 1.72 to 3.69) for EVAR and 1.50 (95% CI 1.33 to 1.69) for open repair. The results, particularly for open repair, were dominated by a 20-year review (1980–2000) from the USA with 81,384 women. 79 All but two of the 21 papers that offered data for EVAR included < 60 women. We focused on 30-day mortality only in more recent material, published since January 2009; this time the included studies had more data for EVAR and the lowest number of women included in any study was 121. Again, we observed that mortality for both EVAR and open repair was higher in women than in men, but that mortality rates for EVAR were lower than for open repair. Although the overall mortality rates have decreased since the earlier systematic review, the odds ratio for women versus men has changed little. The mortality rate following open repair in women would appear to be unacceptably high.
Summary
Overall, the 30-day operative mortality for intact AAAs in women is almost twice as high as in men. For EVAR, the pooled operative mortality in women was 2.2% and 5.4% for open repair.
Mortality following ruptured abdominal aortic aneurysms in women
The mortality of women following AAA rupture depends on how many reach hospital alive, how many are turned down for, or refuse, AAA repair, and mortality following emergency repair, either endovascular (EVAR) or open repair. There is no recent evidence concerning the proportion of women who reach hospital alive versus those who do not. Moreover, given the sometimes unreliable reporting of causes of death, the number of women dying from a ruptured AAA outside hospital may be an underestimate. There is a suspicion that women may not fare as well as men following rupture of their AAA. 17,80 This narrative review also considers the late mortality (after 3–5 years) of women after successful emergency AAA repair.
Non-intervention rates for emergency repair of ruptured abdominal aortic aneurysms in women
Earlier work by Anjum and Powell81 reported that up to 75% of women with a ruptured AAA did not receive an emergency repair, but the source data from English Hospital Episode Statistics (HES) did not permit full differentiation between patients who did and did not reach hospital alive. Later work used stratified matching by age and sex to compare non-corrective in-hospital treatment rates in the USA and England for the years 2005–2010. 82 The English data showed that non-corrective treatment was selected for about 40% women aged < 75 years, rising to over 80% for women aged ≥ 85 years; in the USA, the comparable rates were about 20% and 60%, respectively. In England, the rate of non-corrective treatment for men was far lower, about 15% for those aged < 75 years and about 40% for those aged ≥ 85 years. Data from northern Norway83 show that, for the period 2010–14, 42% of women underwent non-corrective treatment in hospital, compared with 17% of men.
These data can be supplemented by further data from the IMPROVE trial centres, where approximately 25% patients assessed by the vascular team were not considered to be candidates for emergency repair. The relative proportions of women and men considered not to be candidates for (or refusing) emergency repair were very different: 161 out of 255 (63%) and 107 out of 548 (20%) respectively. Those not considered for repair were older than those who underwent repair.
Operative mortality after emergency repair for rupture in women
There are few sources of information for cohorts including more than 100 women. A summary of the main sources (published and unpublished since 2000, often with < 100 patients) is given in Table 10. The rates in all sources are likely to depend on the proportion of patients turned down for repair, information that is rarely provided (see Non-intervention rates for emergency repair of ruptured abdominal aortic aneurysm in women). Lower mortality is likely to be reported when the proportion of patients receiving an intervention is lower (selection of the best surgical candidates).
| Trial or study (recruitment period) | Number of women | 30-day mortality | 1-year mortality | 2-year mortality | 3-year mortality | ||||
|---|---|---|---|---|---|---|---|---|---|
| EVAR | Open | EVAR | Open | EVAR | Open | EVAR | Open | ||
| AJAX,84 2004–11,a the Netherlands | 17 | 2/8 | 3/9 | 3/8 | 3/9 | N/A | N/A | ||
| ECAR,85 2008–13,a France | 10 | 0/5 | 0/5 | 1/3 | 2/4 | N/A | N/A | ||
| IMPROVE,24 2009–13,a mainly the UK | 133b | 26/70 | 36/63 | 28/70 | 38/63 | N/A | 35/69 | 44/63 | |
| VSGNE18 2003–11, USA | 84 | 6/22 | 30/62 | 10/22 | 36/62 | N/A | N/A | ||
| Norway,83 2007–12 | 21 | – | 12/21a | – | 13/21 | N/A | N/A | ||
| Total | 245 | 34/105 (32%) | 81/160 (51%) | 42/103 (41%) | 92/159 (58%) | N/A | N/A | ||
| HES 2010–14 for those aged > 65 years, England | 995 | 49/215 (22.8%) | 300/780 (38.5%) | 33.7% | 47.0% | 37.4% | 50.7% | 45.4% | 54.1% |
These data all suggest that 30-day operative mortality is higher after open repair than EVAR. This also was identified in an earlier large cohort Medicare study17 (1995–2006), in which 30-day mortality in women after EVAR was 41% versus 53% after open repair. Similarly Vascunet (international registry collaboration) shows lower in-hospital mortality in women after EVAR versus open repair, 36% and 44%, respectively (Professor Maarit Venermo, University of Helsinki, 2016, personal communication).
Late mortality after emergency repair of ruptured abdominal aortic aneurysms in women
There is a paucity of information regarding survival beyond 30 days for women who have undergone repair of a ruptured AAA. Several publications in the endovascular era since 2000 have assessed mid-term survival between 1 and 5 years following the repair of a ruptured AAA. Although the results are not separated by sex, in the multivariate analyses the odds or hazard ratios reported for women range from 1.1 to 1.4, indicating a higher mortality overall in women. Further details, with a breakdown of results by sex, were requested for two of these cohorts with a strong population base, the Amsterdam region, the Netherlands87 and New England, USA (VSGNE),88 but were not available. This leaves the only data to 1 year and beyond, as shown in Table 10, with a total of only 245 women. Table 10 also shows unpublished 2- and 3-year survival data from HES and the data for the IMPROVE trial. 30
The only published longer-term follow-up comes from the earlier large Medicare data set from 1995 to 2006 with 48,865 participants (23.5% women). Among women, the 5-year survival following EVAR was 32% (95% CI 25% to 39%), compared with 19% (95% CI 18% to 21%) following open repair.
Summary
The turn-down rate or non-corrective treatment rate of women with ruptured AAAs is not usually reported but is likely to be at least 40% and rises with age. Recent data indicate that, overall, the 30-day mortality in women is about 40% but probably is lower after EVAR compared with open repair. Although longer-term data for women are scant, overall about half the patients who undergo repair are alive at 3 years. The differential mortality between EVAR and open repair observed at 30 days appears to be preserved at 1 year and data from HES suggest that this difference is maintained at 2 years, with possibly some attenuation by 3 years.
Chapter 4 Screening, abdominal aortic aneurysm growth and rupture, and surveillance parameters for women
This chapter describes the sources of data and modelling approaches used to obtain important parameters relevant to screening, AAA growth, rupture and surveillance for women invited to an AAA screening programme. At the end of each section, the base-case estimate that is used for the economic modelling in Chapter 7 is presented, together with other estimates used in sensitivity analyses. This chapter addresses part of objective 3 in Chapter 1, Scientific objectives, and the parameters listed in Table 1. A summary of all these parameter estimates is provided in Table 32, Appendix 4.
Screening
Reinvitation
No information on the proportion of women who would be reinvited to AAA screening following non-attendance could be found. Therefore, the reinvitation rate is based on data in men from NAAASP. Table 33, Appendix 4, shows the numbers invited and attending screening in the 2013/14 and 2014/15 cohorts in NAAASP. The total attendance rate was 242,674/300,667 (80.7%) in 2013/14 and 236,936/293,709 (80.7%) in 2014/15. The proportion reinvited, which affects the overall costs of the screening programme, is calculated as the number who did not attend the first appointment minus the number who declined screening, all divided by the total number offered an appointment. The proportion who are reinvited stays at a constant 23.9% across the 2 years. This is higher than the 13.6% reinvited in the MASS trial,10 which was used as the basis for the health economic evaluation in men. Uncertainty in this figure for the PSA is very low if the number of reinvitations and total number are used directly as parameters of a Beta distribution (see Table 32, Appendix 4).
In the base-case analysis, a reinvitation rate of 23.9% is used based on NAAASP data for men from 2013/14 and 2014/15.
Attendance rate
The attendance rate is an important consideration and will influence the cost-effectiveness of any AAA screening programme. Evidence regarding the potential attendance rate for screening programmes involving both men and women is summarised here.
Participation of women in colorectal cancer screening
Screening for colorectal cancer is based on testing for faecal occult blood, with test kits posted to individuals’ homes and the completed test kits returned to the screening centre by post. The results of the first 2.6 million invitations to colorectal screening (October 2006 to January 2009) have been analysed in some detail. 89 Overall, the uptake (returned kits) was 51% in men and 56% in women. In women, the uptake rate was the same in those aged 60–64 years as in those aged 65–69 years (although uptake in men increased from 49% to 53% over these age bands). The overall uptake was 54%, which compares favourably with similar screening programmes in Australia90 and the Netherlands91 (uptake rates of 46%90 and 49%,91 respectively).
However, because the uptake is relatively low, with scope for improvements, the barriers to participation have been analysed in some detail. 89,92 In common with many other screening programmes, including NAAASP, socioeconomic status is of great importance, with deprived areas reporting lower uptake rates; non-white ethnic groups also appear to have a lower screening uptake, although the difference may be lower in women than in men. Furthermore, among women, there appears to be dislike of the actual screening test, contributing towards much lower participation in colorectal screening than in breast screening. 92 The lower uptake rates in men, and change with age, have been attributed to the difficulty of completing the test kit while out at work.
In summary, for UK colorectal cancer screening, uptake is higher in women than in men, but there is no change in uptake in the age range 60–69 years.
Population screening for abdominal aortic aneurysms in women
There is limited experience, but the main results come from the Chichester screening RCT33 in the early 1990s. This showed that the uptake of AAA ultrasound screening was lower in women than in men and declined with age, particularly in those aged > 75 years. Table 11 shows the attendance rate at screening by age and sex from the 7887 men and women in the group invited to screening.
| Age (years) | Total screened (n) | Total invited (n) | Accepted (%) |
|---|---|---|---|
| 65 | |||
| Men | 169 | 210 | 80.5 |
| Women | 218 | 300 | 72.7 |
| 66–70 | |||
| Men | 922 | 1208 | 76.3 |
| Women | 1123 | 1635 | 68.7 |
| 71–75 | |||
| Men | 676 | 919 | 73.6 |
| Women | 905 | 1364 | 66.3 |
| 76–80 | |||
| Men | 575 | 868 | 66.2 |
| Women | 806 | 1383 | 58.3 |
Although this is a relatively small, now dated, sample (4682 women), the results from a more recent study (2007–9) of 6925 women from two Swedish counties showed only a slight increase in uptake: 74% of 70-year-old women accepted the invitation to screening. 14 This contrasts with an acceptance rate for 65-year-old Swedish men of 85% for national AAA screening, 2006–10. 93 Furthermore, the MASS trial94 showed that the uptake in men aged 65–69 years was similar to the uptake at 70–74 years (81% and 79%, respectively). The uptake rate in NAAASP is very similar at around 80% (see Reinvitation).
In summary, uptake rates in AAA screening may be lower (by up to 10%) in women than in men. Increasing the age of screening attenuates the participation in screening.
Summary
Data in Table 11 from the Chichester study33 are used in the modelling: for 65-year-old women in the base-case analysis (see Chapter 7) and for other age groups when assessing different screening strategies (see Chapter 8). A Beta(218,82) distribution is used to account for parameter uncertainty in the base-case PSA.
Non-visualisation
There is no information on the proportion of women whose aortic diameter would be non-visualised at a screening session, and so this information has also been obtained from NAAASP (see Table 34, Appendix 4). Based on NAAASP data for men from 2013/14 and 2014/15 combined, the non-visualisation proportion (after attempts on two separate occasions) is very low, at 0.35%, considerably lower than the 1.21% reported in the MASS trial. 10
A non-visualisation rate of 0.35%, based on NAAASP data for men, is used in the base-case analysis.
Aortic diameter distribution
A crucial consideration when screening a population of women is the distribution of aortic diameters and, related to this, the prevalence of AAAs detected at screening (i.e. the proportion of diameters detected above the diagnosis threshold, e.g. diameter of ≥ 3.0 cm). We obtained data on the full aortic diameter distribution in women from two sources: (1) 5140 women from Uppsala and Dalarna, Sweden, aged 70 years who were screened using leading edge to leading edge diameter measurements between 2007 and 2009,14 and (2) 570 women from Viborg, Denmark, aged 67 years who were screened using outer-to-outer wall diameter measurements in 2015 (Professor Jes Lindholt, personal communication).
A third source of information comes from the first 700,000 men screened in NAAASP, using inner to inner wall diameter measurements. Although not directly relevant, this large source of information may still be useful if it is suitably reweighted (see Prevalence of abdominal aortic aneurysms) so that it has the same prevalence of AAAs as seen in women. Table 12a compares each data source in terms of the proportion of screened individuals by 0.5-cm categories of aortic diameter. These results show that the prevalence of AAAs ≥ 3.0 cm is higher in the population of men in NAAASP than in the population of women screened in Sweden and Denmark, as is the prevalence of aortic diameters ≥ 2.0 cm (18.3% in NAAASP vs. 8.4% and 14.0% in Sweden and Denmark, respectively). This latter size range is important as it is aortic diameters of this size that are allowed to grow and potentially rupture within the DES model (see Chapter 2, Modelling aortic growth and abdominal aortic aneurysm rupture). The mean aortic diameters are 1.79 cm and 1.66 cm in NAAASP men and Swedish women, respectively. The mean diameter could not be accurately calculated in the Danish women as aortic diameter was available only in 0.5 cm categories. In the Swedish study, the standard deviation (SD) of the distribution is 0.26 cm, resulting in an aortic diameter of 2.5 cm being 3.2 SDs above the mean (or 51% higher) and one of 3.0 cm being 5.2 SDs above the mean (or 81% higher). In comparison, in men (NAAASP), 2.5 cm is 2.0 SDs above the mean (40% higher) and 3.0 cm is 3.4 SDs above the mean (68% higher). Outer–outer diameter measurements are expected to be larger than leading edge–leading edge or inner–inner measurements, which makes it even more surprising that the prevalence of AAAs was 0% in the Viborg study.
| Aortic diameter (cm) | Uppsala and Dalarna, Sweden (N = 5140 women), n (%) | Viborg, Denmark (N = 570 women), n (%) | NAAASP (N = 700,000 men), n (%) |
|---|---|---|---|
| < 1.0 | 1 (0.02) | 0 (0.0) | 37 (0.01) |
| 1.0–1.4 | 909 (17.7) | 131 (23.0) | 49,147 (7.0) |
| 1.5–1.9 | 3796 (73.9) | 359 (63.0) | 522,513 (74.6) |
| 2.0–2.4 | 385 (7.5) | 77 (13.5) | 108,988 (15.6) |
| 2.5–2.9 | 30 (0.6) | 3 (0.5) | 9927 (1.4) |
| 3.0–4.4 (small AAA) | 16 (0.31) | 0 (0.00) | 7605 (1.09) |
| 4.5–5.4 (medium AAA) | 3 (0.06) | 0 (0.00) | 1028 (0.15) |
| ≥ 5.5 (large AAA) | 0 (0.00) | 0 (0.00) | 755 (0.11) |
| Prevalence (≥ 3.0) | 0.37% | 0.00% | 1.34% |
| Aortic diameter (cm) | Uppsala and Dalarna, Sweden (reweighted) (%) | NAAASP (reweighted) (%) |
|---|---|---|
| < 1.0 | 0.02 | 0.01 |
| 1.0–1.4 | 17.24 | 8.05 |
| 1.5–1.9 | 73.93 | 76.49 |
| 2.0–2.4 | 7.75 | 14.01 |
| 2.5–2.9 | 0.63 | 1.01 |
| 3.0–4.4 (small AAA) | 0.356 | 0.426 |
| 4.4–5.4 (medium AAA) | 0.074 | 0.004 |
| ≥ 5.5 (large AAA) | 0.000 | 0.001 |
| Prevalence (≥ 3.0) | 0.43% | 0.43% |
Prevalence of abdominal aortic aneurysms
The estimates obtained from the systematic review of AAA prevalence in women (see Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysms in women) are used to inform the proportion of the aortic diameter distribution, that is, ≥ 3.0 cm. The pooled prevalence estimate from this systematic review was found to be 0.74% (95% CI 0.53% to 1.03%) overall and 0.43% (95% CI 0.23% to 0.80%) in 60- to 69-year-old women. This is higher than the estimate found in either the Swedish or Danish studies, but lower than that seen in NAAASP. To use this information from the systematic review, each of the aortic distributions described is reweighted. This has the effect of shifting the distribution in order for the desired prevalence to be achieved. A linear reweighting approach is taken using the following algorithm.
Let pold be the prevalence of AAAs calculated in the aortic diameter distribution being considered and pnew be the prevalence that we wish to recalibrate the distribution to (e.g. 0.43% for 60- to 69-year-old women). Each aortic diameter size x (accurate to 1 mm) has an associated probability weight w(x) indicating the proportion of individuals in the distribution who were screened with that diameter. The weights sum up to 1. It follows that:
Given the desired prevalence, pnew, calculate new weights, w*(x), as follows:
where f(x) = a + bx is a linear function of x. The conditions that must be satisfied are:
and
A pair of simultaneous equations can, therefore, be obtained to give the solutions:
and
After reweighting, some of the new weights may be negative. If this occurs, these are set to zero and then a further reweighting step is performed to ensure the weights above the diagnosis threshold (e.g. 3.0 cm) sum to the desired prevalence. Applying this algorithm to the aortic diameter distributions shown in Table 12a, using the estimated prevalence of 0.43% found from the systematic review for 60- to 69-year-old women (see Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysms in women), gives the distributions shown in Table 12b. Note that this approach could not be used with the Viborg data as there were no cases of AAAs reported in this screening study (and, hence, the slope parameter b is infinite). It can be seen that the reweighted Swedish and NAAASP distributions are different, with a higher proportion of ≥ 2.0-cm aortic diameters in NAAASP. Furthermore, no AAAs of diameters of ≥ 5.5 cm were found in the Swedish study. This would have an important impact in the modelling, giving rise to no AAAs that are immediately referred for elective surgery.
Summary
The reweighted NAAASP distribution is used in the base-case analysis. The distribution is reweighted to have 0.43% prevalence, as found in the 60- to 69-year age group in the systematic review. In a one-way sensitivity analysis, the reweighted NAAASP distribution is replaced with the reweighted Swedish aortic diameter distribution. Two other DSAs are conducted to assess the robustness of results to a doubling or a halving of the prevalence. Within the PSA, uncertainty in the estimated prevalence of 0.43% is incorporated to assess how this affects uncertainty in the health economic outputs. To do this, in repeated PSA iterations, the prevalence is drawn from a normal distribution on the logit scale since this was the scale used to perform the meta-analysis (see Table 32, Appendix 4). For each draw from this distribution, a reweighted NAAASP distribution is calculated. When assessing ages other than 65-year-old women, the prevalence is changed accordingly to the age-specific estimates from the systematic review.
Growth and rupture rates of abdominal aortic aneurysms in women
Data from observational surveillance studies of AAAs in the diameter range 3.0–5.4 cm in the RESCAN collaborative project16 were used to estimate growth and rupture rates in women. Eleven studies from RESCAN35 recruited women (see Table 35, Appendix 4, for a descriptive summary of these studies).
Growth modelling
A mixed-effects model was used to model the longitudinal AAA diameter trajectories for each woman in each of the 11 studies. A model was fitted separately within each study assuming a linear relationship between log-AAA diameter and time since entry into the study (see Chapter 2, Modelling aortic growth and abdominal aortic aneurysm rupture, for the rationale to using this model). The model allowed a separate intercept and slope parameter for each individual through the use of random effects. For individual i with measurement j at time tij years after study entry, the AAA diameter yij (cm) is modelled as:
where (21)ϵij∼N(0,σw2), (22)β=(β1β0) and (23)G=(σ02ρσ0σ1ρσ0σ1σ12).
β0 and β1 represent the mean intercept and slope of the AAA diameter trajectories on the log-scale, respectively, while b0i and b1i allow for individual variation about the intercept and slope (random effects). The random effects for each patient are correlated and come from a bivariate normal distribution. Parameter estimates obtained from this model for each study are shown in Table 36, Appendix 4, and a forest plot for the average factor increase in AAA diameter per year (exp β1) is shown in Figure 6. In a second stage, study-specific estimates are pooled via multivariate random-effects meta-analysis (overall estimates shown in Table 36, Appendix 4). On average, AAA diameter increases by 5% per year, but with considerable heterogeneity between both studies and people. The average and distribution of AAA growth rates in women and men are in fact similar. 35
As described in Chapter 2, Modelling aortic growth and abdominal aortic aneurysm rupture, the approach taken in the DES is first to sample baseline diameters from our chosen distribution (see Aortic diameter distribution) and then generate random effects for each individual conditional on their baseline diameter, using the parameter estimates obtained from the overall linear mixed-effects model.
Table 37, Appendix 4, shows the estimated proportion of individuals predicted to cross the intervention threshold (5.5 cm) within 5 and 10 years, in the absence of any deaths. For comparison, the empirical rates estimated in the 11 RESCAN35 studies for women in the absence of any competing risks (e.g. deaths) are also shown. The data from the 11 RESCAN35 studies are naively pooled to estimate the empirical rates. The predicted rates lie within the 95% CIs for the observed rates in all size/threshold categories.
Summary
In the base-case analysis, growth rate parameters are taken from the overall parameters estimated by two-stage meta-analysis, as given in Table 36, Appendix 4. The PSA uses the variance–covariance matrix of these parameters to propagate uncertainty through to health economic outputs (see footnote to Table 32, Appendix 4).
Rupture rates
Rupture data were available in only 6 out of the 11 RESCAN studies35 that provided growth data (Edinburgh and Leeds did not record rupture information and the Propranolol, PIVOTAL and Swedish studies did not have rupture events in both men and women; see Table 35, Appendix 4). Characteristics of these six studies are shown in Table 38, Appendix 4.
A joint growth and rupture model was fitted to the data separately within each study before pooling estimates using multivariate random-effects meta-analysis. As ruptures were rare, we used data from both men and women and allowed for sex differences in the baseline AAA diameter and rate of rupture by including sex as a covariate in both the longitudinal (growth) and survival (rupture) submodels. As described in Growth modelling, a linear relationship between log (diameter) and time was assumed to model the growth of an aneurysm. The hazard of rupture was related to an individual’s current predicted (log) AAA diameter, mi(t), and their sex as follows:
Pooled estimates obtained from the rupture submodel are shown in Table 39, Appendix 4, together with an estimate of between-study heterogeneity as given by the I2 statistic. The association between the risk of rupture and AAA diameter is depicted in Figure 7 for women, predicted from each study-specific model and from the pooled estimates. For comparison purposes, the empirical observed rates of rupture by 0.5 cm categories are also shown. The pooled model trajectory gives a reasonable fit to the overall data. Study-specific estimates can be seen to vary considerably. The pooled rate of rupture reaches 1 per 100 person-years at a predicted diameter of 4.2 cm.
FIGURE 7.
Comparison of observed rates of rupture (with 95% CIs) and those predicted from study-specific models and a pooled model, from RESCAN studies. 35

Summary
In the base-case analysis, estimates for parameters relating to the risk of rupture are obtained from the pooled multivariate meta-analysis. Parameter uncertainty in the PSA is accounted for using the estimated variance–covariance matrix. Estimates are shown in Tables 32 and 39, Appendix 4.
Surveillance
The clinical effectiveness and cost-effectiveness of a screening programme will be reliant on the operation of a surveillance programme for detected AAAs, and will be affected by (1) the rate of dropout from the screening programme, (2) the rate of incidental detection for individuals found to have an AAA not through the screening programme and (3) the efficiency of the programme in ensuring consultations and surgical operations are performed in a timely manner. Sources of data for each of these three parameters are addressed in this section.
Dropout
Data on the rate at which women drop out from regular surveillance were kindly made available from two AAA surveillance programmes that have recruited both men and women with incidentally detected AAAs: (1) the Leicester AAA surveillance programme (81 women and 353 men between September 2004 and September 2015; Professor Matthew J Bown, personal communication) and (2) the Imperial College AAA surveillance programme (28 women and 97 men recruited in 2010; Professor Janet T Powell, personal communication). Dropout information from Leicester was available from January 2014 onwards and, hence, only individuals still in the surveillance programme from 2014 onwards were considered (n = 389). Dropout was defined as any of the following reasons for leaving surveillance: (1) discharged, (2) patient cancelled, (3) moved location, (4) referral for other surgery and (5) other. For the Imperial College AAA surveillance programme, information on dropouts was collected from 2010 to 2015 and included the following reasons: (1) did not attend and (2) moved away. Only year of scan was recorded and, hence, follow-up time was an integer defining year of last scan minus year of first scan. In this study, 30 individuals had only one scan recorded and were excluded from the analysis. Table 13 shows the rate of dropout for women and men from the two screening programmes along with the estimated hazard ratio between men and women from a Cox regression model. There was no evidence from either of the screening programmes of a differential dropout rate between women and men.
| Item | Leicester surveillance programme | Imperial College surveillance programme | NAAASP |
|---|---|---|---|
| Number in surveillance | |||
| Women | 72 | 23 | – |
| Men | 317 | 72 | 10,734 |
| Dropout from surveillance (n) | |||
| Women | 7 | 7 | – |
| Men | 28 | 8 | 1072 |
| Rate of dropout per person-year | |||
| Women | 7/74 = 0.0945 | 7/80 = 0.0875 | – |
| Men | 28/338 = 0.0827 | 8/209 = 0.0383 | 1072/19,650 = 0.0546 |
| Hazard ratio (men vs. women) | 0.887 (95% CI 0.385 to 2.045); p = 0.78 | 0.516 (95% CI 0.186 to 1.427); p = 0.20 | – |
A further source of data on dropout rates in men is NAAASP. NAAASP includes 13,271 men who were under surveillance (11,136 screen detected, 2135 self-referrals) up to 4 April 2016. Follow-up was defined as the date of first scan to the date of last scan or status update date, whichever came later. Of these individuals, 2537 in whom only one scan was recorded were excluded from these analyses. Dropout was defined as any of the following reasons for leaving the programme: (1) appointment missed, (2) declined, (3) non-visualised, (4) out of cohort, (5) surveillance ceased or (6) temporarily ineligible. These additional data are also summarised in Table 13. The dropout rate was estimated to be 5.5 per 100 person-years. A sensitivity analysis including the 2537 individuals with only one scan (giving them a very small follow-up time) gave a very similar estimated rate (5.6 per 100 person-years). There was also little evidence of a difference in dropout rates between self-referred and screen-detected individuals (p = 0.081).
Summary
From these analyses, there is little evidence of a difference in dropout rates between men and women. Therefore, owing to the large sample size of NAAASP and the fact that any screening programme for women is likely to be incorporated within NAAASP’s screening processes, the dropout rate estimated in NAAASP (5.46 per 100 person-years) is used in the base-case analysis. This is lower than the dropout rate seen in the MASS trial10 (8.20 per 100 person-years), which was originally used to model the cost-effectiveness in men, although an updated model for men used a dropout rate of 5.57 per 100 person-years,12 which closely reflects the figure seen in NAAASP. A Gamma(1072,19650) distribution is used in PSA to account for uncertainty in the dropout rate. In DSAs, the dropout rate is doubled and halved to investigate the effect on key health economic quantities.
Incidental detection
Data from electronic hospital records of women aged ≥ 65 years undergoing CT scanning were obtained from the University Hospital of South Manchester in 2014; 2494 women underwent an abdominal CT during this period and 65 AAAs were identified. Of these, 53 were newly identified AAAs, but only seven were referred on to vascular surgeons to be followed up with surveillance or elective surgery. The population (women aged ≥ 65 years) of the referral catchment area for the university hospital is approximately 24,500. Assuming that 181 (0.74%) of these women have an aneurysm (see Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysms in women), this would indicate an incidental detection rate to a surveillance programme of approximately 7/181 = 3.9 per 100 person-years for women aged ≥ 65 years with an AAA. This is similar to the rate of 4.6 per 100 person-years used in the most recent health economic model for men. 12
Further data come from a study conducted in Canterbury, New Zealand,36 in which 167 new incidental AAAs were detected in men and women from CT scans over a period of 4.25 years. About one-quarter of all detected AAAs (incidental and known) were in women. Assuming this proportion also applies to the incidental AAAs and that 97% of AAAs were in individuals aged ≥ 65 years, there would be approximately 40 AAAs detected in women aged ≥ 65 years. From census data, the 2006 population of women aged ≥ 65 years for the catchment area (Canterbury, West Coast and Timaru regions of South Island, New Zealand) was approximately 43,500. Assuming that 321 (0.74%) of these women had an aneurysm (see Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysm in women), this would indicate an incidental detection rate of approximately 40/(321 × 4.25) = 2.93 per 100 person-years for women aged ≥ 65 years with an AAA. This is also quite similar to the rate of 4.6 per 100 person-years used in the most recent health economic model for men. 12
Summary
An incidental detection rate of 2.93 per 100 person-years, as estimated from the New Zealand study,36 is used in the base-case analysis. A Gamma(40,1364.25) distribution is used in the PSA to account for uncertainty in the incidental detection rate. In DSAs, the incidental detection rate is doubled and halved to investigate the effect on key health economic quantities.
Delay from ≥ 5.5-cm scan to consultation
Data from NAAASP for the years 2013/14 and 2014/15 indicate that 981 men in total were referred to vascular services, of whom 947 (97%) received a consultation. The mean time from referral scan to consultation was 10.6 days, much lower than the mean delay of 71 days observed in the MASS trial. 10
A time delay from referral screen to consultation of 10.6 days based on NAAASP is used in the modelling.
Consultation scan: computerised tomography scan versus ultrasound scan
At consultation, an AAA is confirmed (or otherwise) using a CT scan. The measurement of the AAA diameter made with this CT scan may be systematically higher than that seen on an ultrasound scan. Evidence for this comes from the RESCAN collaboration35 in which four studies measured diameters using both ultrasound and CT scans. CT measurements were, on average, significantly larger than ultrasound measurements {Leeds 3.91 mm [standard error (SE) 0.33 mm], PIVOTAL 1.75 mm (SE 0.21 mm), Galdakao 1.77 mm (SE 0.10 mm), Stirling 2.46 mm (SE 0.27 mm)}. A pooled estimate from these studies suggests an average increase of 2.44 mm.
There is also evidence that the measurement error for a CT scan may be different from that based on an ultrasound scan. A paper by Singh et al. 95 suggests that a CT measurement of AAA diameters has interobserver ‘variability’ of 5.2 mm (defined as 1.96 multiplied by the SD of interobserver differences). This equates to a CT measurement error SD of 1.9 mm. This is lower than the estimated ultrasound measurement error SD from the RESCAN35 model for a large AAA, approximately 55exp(–2.96) = 2.9 mm for a 5.5-cm aneurysm (see Table 36, Appendix 4).
In the modelling, the mean observed diameter from a CT scan is assumed to be 2.44 mm higher than that obtained from an ultrasound scan, with a measurement error SE of 1.9 mm.
Decision at consultation: proportion returned to surveillance
The DES programmed for men (see Chapter 2) used the observed CT scan diameter at consultation to decide whether or not the individual should be returned to surveillance, with those with AAAs of measured diameter < 5.5 cm returned to surveillance. Based on the CT measurements, 13.7% of consultations resulted in an individual being returned to surveillance. This is a much higher rate than the 36 out of 947 men (3.8%) who were ‘inappropriate referrals’ (AAA diameter of < 5.5 cm, other or not stated) in NAAASP data. Nevertheless, in our modelling, the proportion of women who are returned to surveillance after a consultation is derived from the proportion of CT measurements that are < 5.5 cm.
Decision at consultation: non-intervention rate in women not returned to surveillance
Women may refuse surgical intervention, or may be turned down because of contraindications. Information on the non-intervention rate in women not returned to surveillance (i.e. the proportion turned down for elective surgery or refusing an operation) comes from four hospitals in the UK (see Chapter 3, Proportion of women versus men not offered an intervention). The overall non-intervention rate is 34% (95% CI 29% to 40%), with no between-study heterogeneity (I2 = 0%).
The proportion of individuals elective surgery in whom surgery is deemed to be contraindicated is based on the pooled estimate from four UK hospitals of 0.3423. The PSA is based on a normal(–0.653, 0.1352) distribution for the logit pooled probability.
Decision at consultation: proportion who will receive elective surgery
The proportion of women who receive elective surgery is defined in the model based on the remaining population who are not turned down, refuse surgery or are returned to surveillance.
Delay from consultation to elective surgery
Among 827 individuals in whom surgery took place in NAAASP, the mean time from referral to surgery was 81.4 days. Assuming that the mean time from consultation to referral was 10.6 days, this would imply a mean time from consultation to surgery of 70.8 days, slightly higher than the mean delay of 59 days observed in the MASS trial. 10 In our modelling, a time delay from consultation to surgery of 70.8 days is used for everyone for whom surgery is planned, based on NAAASP data.
Chapter 5 Surgery-related parameters for women
Crucial parameters in any AAA screening model are those that relate to surgical AAA repair. These include operative mortality rates for both EVAR and open repairs, for both elective and emergency operations. For example, high postoperative mortality rates following elective AAA repair would reduce any benefits of a screening programme. Also important are the rates of reinterventions and the long-term AAA-related mortality rates after these operations. Of particular relevance to the assessment of the clinical effectiveness of AAA screening in women is the evidence that both postoperative morbidity and mortality are higher in women than in men. 74 This may negatively affect the clinical effectiveness of AAA screening in women.
This chapter provides estimates of the parameters for women listed in Table 2, addressing part of objective 3 in Chapter 1, Scientific objectives.
Sources of data
Data on operations and patient outcomes were available for the UK from the NVR,26 for England and Wales from HES,28 and internationally from the voluntary Vascunet register. 27 Postoperative data on reinterventions can, in principle, be extracted from HES by linking records, as can long-term mortality from HES–Office for National Statistics (ONS) linkage. We also used published data and other particular data sets to provide information on these parameters; these are described later in this chapter.
National Vascular Registry
The submission of data to the NVR by vascular units is voluntary, but it is generally thought to be about 90% complete. 26 Data are entered into the NVR by surgeons at the time of surgery and/or at the time of discharge from hospital. The registry covers all types of vascular surgery, including elective and emergency AAA repairs. Under a data sharing agreement with the Healthcare Quality Improvement Partnership, individual-level data were obtained for all AAA repairs reported to the NVR from 1 January 2010 to 31 December 2014. The initial year was set at 2010 to focus on recent practice and because this is when EVAR became reliably recorded in the NVR. NVR provides data on AAA size and in-hospital mortality (rather than 30-day mortality). For men, incidentally detected and screen-detected AAAs are sometimes (but not always) distinguished; for women, it is assumed that all AAAs have been incidentally detected as no systematic screening was in place in the UK during the period covered by the data extract.
Hospital Episode Statistics
Summary tabular data were made available from HES28 for the same time period as the NVR data extract (1 January 2010 to 31 December 2014). To comply with confidentiality requirements, cells in the tables with values of 5 or below were either merged with neighbouring categories or supressed. Identifying operations as AAA repairs is more difficult in HES than the NVR, as one admission may generate multiple hospital episodes each recorded separately in HES. Data on both 30-day and in-hospital mortality can be extracted from HES.
Vascunet
Vascunet is an international register of vascular surgical procedures, and includes data principally from mainland Europe but also some from the UK and Australasia. 27 Submission of data to this register is performed much less routinely than for the NVR, and Vascunet should be regarded as far from complete. Nevertheless, it provides an interesting comparator as it includes data from outside the UK. Summarised tabular data from Vascunet were obtained for the years 2010–13.
Use of National Vascular Registry data as a source of parameter estimates in women
As the NVR provides the most detailed data, it is the principal source of evidence we use for surgical parameters for women. This section describes the principles employed.
The NVR allows modelling of individual data with respect to sex (men vs. women), age and AAA diameter. Most of the information is in the form of proportions (p), for example, the proportion of patients receiving EVAR, or the proportion of patients undergoing EVAR who die in hospital. We use logistic regression models, including all possible sex interactions, to provide estimates for women in two ways, as described here. The SEs from these regressions are used to represent parameter uncertainty in the PSA via correlated normal distributions. The data in the NVR on whether the AAA was detected by screening or incidentally are available only for men (as there was no screening for women during this time period) and, even for men, this information is only about 35% complete. As we are primarily concerned with estimates for women, we do not include this variable in the logistic regression models.
First, we consider just the overall proportion for women derived from the simple logistic regression:
where sex is coded as ‘0’ for women and ‘1’ for men. The parameter aF is the log-odds for women and aM is the log-odds ratio comparing men with women. We use expit(aF) = exp(aF)/[1 + exp(aF)] as the estimated probability for women.
In a second analysis, we use the more detailed logistic regression:
Here, b1F is the change in the log-odds per year of age and b1M is the difference in this log-odds between men and women. Similarly, b2F is the change in log-odds per cm increase in AAA diameter and b2M is the difference in this log-odds between men and women. Subtracting the values of age (80 years) and AAA diameter (6.0 cm) reduces the correlations between parameter estimates; the intercept aF now refers to a woman aged 80 years with an AAA diameter of 6.0 cm. For example, we use Equation 27 to estimate the relevant proportion for a woman aged 71.2 years with an AAA of diameter 6.2 cm, as expit (aF – 8.8 × b1F + 0.2 × b2F).
In this second analysis, we include all the terms in the logistic regression (Equation 27), whether or not they are statistically significant. We use linear terms for age and AAA diameter. We do not model trends according to calendar time, as the purpose here is to use relevant recent evidence; any extrapolation to the future would likely be very unreliable. One slight disadvantage of the second model is that the few patients with missing values of age or AAA diameter in the NVR have to be omitted.
Elective operations
We separate AAA operations into those that were planned (i.e. electively for large AAAs) and those that were performed either urgently (e.g. for symptomatic AAAs) or as an emergency (i.e. for AAA rupture). This section focuses on elective operations.
Proportion receiving endovascular aneurysm repair for elective abdominal aortic aneurysm surgery
Open AAA repair and EVAR have different immediate mortality rates and different subsequent rates of reinterventions and AAA-related mortality,96 so the proportion of women receiving each type of operation needs to be estimated.
Data for women from the NVR and HES are shown in Table 14a. The NVR reports fewer operations than HES for the same period (around 80% of the HES total), reflecting under-reporting in the NVR. The overall proportion of EVAR operations is 58.6% in the NVR and very similar to the HES at 60.6%. These overall rates conceal strong trends: the use of EVAR increases with age and decreases with AAA diameter (Figure 8).
| Source | Open repair (n) | EVAR (n) | % EVAR |
|---|---|---|---|
| NVR | 922 | 1306 | 58.6 |
| HES | 1066 | 1642 | 60.6 |
| Vascunet | 2137 | 2726 | 56.1 |
| Source | EVAR repairs (n) | Deaths (n) | % deaths |
|---|---|---|---|
| NVR in-hospital | 1306 | 23 | 1.8 |
| HES in-hospital | 1642 | 27 | 1.6 |
| HES 30-day | 1642 | 37 | 2.3 |
| Vascunet 30-day | 2726 | 54 | 2.0 |
| Source | Open repairs (n) | Deaths (n) | % deaths |
|---|---|---|---|
| NVR in-hospital | 922 | 64 | 6.9 |
| HES in-hospital | 1066 | 64 | 6.0 |
| HES 30-day | 1066 | 75 | 7.0 |
| Vascunet 30-day | 2137 | 142 | 6.6 |
FIGURE 8.
Proportion of women receiving elective EVAR in the NVR (with 95% CIs). (a) By age, and a superimposed logistic regression fit (for women with an AAA of diameter 6.2 cm, the mean in the NVR data); and (b) by AAA diameter, and a superimposed logistic regression fit (for women aged 76.8 years, the mean in the NVR data).


For comparison, the overall use of EVAR in men in the NVR was 63.5% and there were similar trends according to age and aneurysm diameter as in women. 97 Data from Vascunet give the overall proportion of women receiving EVAR as 56.1%, similar to the figures from the NVR and HES (see Table 14a).
We use the data from the NVR as described in Use of NVR data as a source of parameter estimates in women to provide estimates of the proportion of women receiving EVAR in the model (see Table 40, Appendix 5). In the base-case analysis, the overall proportion is simply that observed in the NVR (i.e. 58.6%).
Proportion who are morphologically suitable to receive endovascular aneurysm repair for elective abdominal aortic aneurysm surgery
In a sensitivity analysis, we instead use the proportion of women whose AAA was considered morphologically suitable for elective EVAR. This may be different from the proportion of women who receive EVAR in practice (i.e. in the NVR or HES). An estimate of this is provided by the systematic review of the literature described in Chapter 3, Suitability of women versus men for standard endovascular repair.
Across the five studies included, the pooled estimate of suitability for EVAR according to the manufacturers’ instructions for use (IFU) was 34% (95% CI 25% to 44%). This is substantially less than the NVR estimate of 59% for the proportion of women receiving EVAR. Assuming that only those within IFU receive EVAR, the proportion of 34% could be used in place of 59%, although the consequent effects on postoperative mortality for both EVAR and open repair are unknown. More recent data, which could not be included in the systematic review (see Chapter 3, Suitability of women versus men for standard endovascular repair) but assess the use of newer endografts, suggest that 40% of women are eligible for EVAR within the IFU.
Elective endovascular aneurysm repair operative mortality
Overall in-hospital or 30-day postoperative mortality rates from the NVR and HES in women undergoing elective EVAR are shown in Table 14b. There were too few deaths to show any convincing trends according to age or AAA diameter in women. The overall figures for in-hospital mortality from the NVR and HES are very similar, 1.8% and 1.6%, respectively. From the HES data, 30-day mortality is somewhat greater than in-hospital mortality (2.3% vs. 1.6%).
For comparison, the overall in-hospital mortality in the NVR for men was 0.7%, lower than in women, with evidence of increasing mortality with age. 97 Data from Vascunet give an overall value of 2.0% for 30-day mortality in women, slightly lower than the figure of 2.3% from HES (see Table 14b). In the systematic literature review (see Chapter 3, Thirty-day operative mortality in women versus men), the overall estimate of 30-day mortality for women after elective EVAR was 2.2% (95% CI 1.9% to 2.7%), similar to the figure from HES.
For the modelling, we adjust the NVR in-hospital mortality to reflect the (greater) 30-day mortality. Thus, we use the NVR data to estimate the log-odds of in-hospital mortality (see Table 40, Appendix 5) according to Equation 26 or 27 in Use of National Vascular Registry data as a source of parameter estimates in women, but then add the log-odds ratio corresponding to the 30-day mortality compared with the in-hospital mortality in HES (namely log-odds of 2.3% vs. 1.6% = 0.370) before transforming back to the probability scale. Working on the log-odds scale ensures that probabilities cannot exceed 1. For the base-case analysis, this gives an overall 30-day mortality estimate of 2.4%. In the PSA, we ignore the fact that the difference between 30-day and in-hospital mortality from HES is estimated with error.
Elective open abdominal aortic aneurysm repair operative mortality
Overall, postoperative mortality rates for women following elective open AAA repair are shown in Table 14c. In-hospital mortality rates are much higher than those after elective EVAR: 6.9% and 6.0% in the NVR and HES, respectively. In HES, as for elective EVAR, the 30-day mortality rate is slightly higher than the in-hospital mortality rate, 7.0% vs. 6.0%. The in-hospital mortality rate in the NVR increased with age, but not convincingly with AAA diameter (Figure 9).
FIGURE 9.
In-hospital mortality rate in women receiving elective open AAA repair in the NVR (with 95% CIs). (a) By age, and a superimposed logistic regression fit (for women with an AAA of diameter 6.2 cm, the mean in the NVR data); and (b) by AAA diameter, and a superimposed logistic regression fit (for women with AAA at age 76.8 years, the mean in the NVR data).


Compared with women, the overall in-hospital mortality was lower in men: 4.0% and 3.8% in the NVR and HES, respectively. 97 The Vascunet data show an overall 30-day mortality rate of 6.6% for women, again quite similar to HES (see Table 14c). In the systematic literature review (see Chapter 3, Thirty-day operative mortality in women versus men), the overall estimate of 30-day mortality for women after elective open repair was 5.4% (95% CI 4.2% to 6.9%), somewhat lower than the figure from HES.
To estimate the 30-day mortality rate for women (see Table 40, Appendix 5), we follow the procedures in Use of National Vascular Registry data as a source of parameter estimates in women along with a similar conversion from in-hospital to 30-day mortality (as described at the end of Elective endovascular aneurysm repair operative mortality). For the base-case analysis, this gives an overall 30-day mortality estimate of 8.1%.
Reintervention rate after successful elective surgery
A ‘successful operation’ is taken to mean that the patient is alive 30 days after the operation. The NVR does not provide information on reintervention rates after the initial hospitalisation for AAA repair. In principle, such reinterventions can be extracted from HES data, but the correct linking of subsequent hospitalisations for individuals that are related to the initial AAA repair (as opposed to other related or unrelated conditions) is fraught with difficulty. Moreover, the length of follow-up available in the 2010–14 HES data is limited to a maximum of 5 years. Thus, we base our estimates on the long-term follow-up (up to 15 years) of the EVAR-1 trial38 of 1252 patients with a large AAA (diameter of ≥ 5.5 cm) randomised to either open AAA repair or EVAR.
There are some drawbacks of the EVAR-1 trial38 data for our purpose. The first is that about 90% of the patients in the trial were men. The second is that the trial patients were restricted to those deemed both fit for open repair and anatomically suitable for EVAR, whereas the groups receiving open repair or EVAR in practice include additional patients. Furthermore, rather than analysing the trial by randomised group from the date of randomisation, we present the data by operation received from the date of operation, omitting patients who did not receive an operation; this makes only a slight difference for the EVAR-1 trial38 since 93% of patients received their randomly allocated surgical intervention, and the median delay between randomisation and surgery was only 40 days. Patients in whom EVAR was converted to open AAA repair in the initial admission are classified as open repairs. We include all reinterventions, excluding the first 30 days following the operation, whether they are first or subsequent ones, and express them as a rate per 100 person-years. We also note that there was strict adherence to the IFU in the EVAR-1 trial,38 and for both EVAR and open repairs reintervention rates rise where morphology is outside the IFU.
Reinterventions are taken to include the following AAA-related conditions: added stent, staple or ligation, type I–III endoleaks, embolisation of endoleak, sclerosis, conversion to open repair, aneurysmal extension above or below original graft, thrombosis of graft limb, graft infection, incisional hernia, false femoral aneurysm, fem-fem graft, FEVAR, axillo bi-fem, distal limb procedure/revascularisation, reoperation of open repair, replacement stent graft and amputation. Reinterventions for laparotomy-related complications were not initially included in the EVAR-1 trial. 38
Based on these definitions, the analysis is based on 1172 patients (1065 men and 107 women) who survived 30 days after their operation, rather than the 1252 originally randomised in EVAR-1. 38 The number of reinterventions occurring in these patients was 262, over a period of up to 15 years, constituting 9321 person-years of observation. The rate of these reinterventions over time is depicted in Figure 25, Appendix 5. The rate of reinterventions is much higher after EVAR than after open AAA repair. The rates can be adequately represented by exponential distributions (constant hazard over time) within periods of 31–120 days and > 120 days after the operation; Weibull distributions did not provide a better fit to the data.
There was substantial evidence that the rates of reintervention differed between women and men, and that this sex effect differed for EVAR and open AAA repair (see Figure 26, Appendix 5); the p-value for including the main effect of sex and its interaction with operation type was 0.006. Reintervention rates were higher in women than in men after EVAR, but lower in women than in men after open AAA repair. Thus, we use the reintervention rates for women alone as parameters in our modelling (see Table 15). For example, the rate of reinterventions for days 31–120 after successful EVAR is estimated as 3 per 15 woman-years or 20.3 per 100 woman-years. For the PSA, we use a Gamma(3,15) distribution to reflect the number of reinterventions and woman-years in the EVAR-1 trial data. 38 There were no reinterventions after 30 days after open AAA repair in 388 woman-years of observation (Table 15); we combine these two periods after 30 days, and apply a zero rate in the base-case analysis. In a sensitivity analysis, we use data from the DREAM76 and OVER77 trials in men to estimate an alternative reintervention rate.
| Item | Men | Women | ||
|---|---|---|---|---|
| Number/person-years | Rate per 100 person-years (SE) | Number/person-years | Rate per 100 person-years (SE) | |
| Reinterventions after EVAR | ||||
| 31–120 days | 20/135 | 14.8 (3.3) | 3/15 | 20.3 (11.7) |
| > 120 days | 153/4221 | 3.6 (0.3) | 27/421 | 6.4 (1.2) |
| Reinterventions after open repair | ||||
| 31–120 days | 5/125 | 4.0 (1.8) | 0/11 | 0.0 |
| > 120 days | 53/4017 | 1.3 (0.2) | 0/377 | |
| AAA-related mortality | ||||
| > 30 days after EVAR | 34/4436.3 | 0.766 (0.131) | 8/444.7 | 1.799 (0.636) |
| > 30 days after open repair | 3/4291.1 | 0.070 (0.040) | 2/400.8 | 0.499 (0.353) |
Long-term abdominal aortic aneurysm-related mortality rate after successful elective repair
For similar reasons as for reinterventions, we use the long-term EVAR-1 trial data38 in preference to HES–ONS data. The latter has limited follow-up available and it is doubtful that AAA-related mortality can be reliably defined based on death certification. For example, many of the deaths occurring within 30 days of an AAA operation are not categorised as AAA related in the HES–ONS data set. We also note that data to 14 years for all-cause mortality from the DREAM trial76 are similar to those from the EVAR-1 trial. 38
Following the same principles as for reinterventions, the rates of AAA-related deaths in the EVAR-1 trial38 after successful AAA repair are shown in Figure 27, Appendix 5; AAA-related deaths include all those within 30 days of any AAA surgery. For AAA-related deaths occurring > 30 days after operation, an exponential model fit to the data was reasonable. There was strong evidence of an increased hazard for females (hazard ratio 2.72, 95% CI 1.35 to 5.46; p = 0.005). Therefore, we use AAA-related mortality rates for women alone in our modelling (see Table 15). For example, the AAA-related mortality after successful EVAR is estimated as 8 per 444.7 woman-years, or 1.8 per 100 woman-years. For the PSA, we use a Gamma(8,444.7) distribution to reflect the number of deaths and woman-years in the EVAR-1 trial data. 38
Emergency operations for ruptured abdominal aortic aneurysms
A similar set of parameters as described for elective operations are required for emergency operations. We define emergency surgery as that done for an acute rupture, whereas urgent surgery is undertaken for a symptomatic AAA. In the NVR data, this distinction is recorded. In the HES data, these cannot be directly separated, but have been approximated by classifying those operations done on the same day as admissions as emergencies. We disregard the consideration of symptomatic AAAs in our modelling: operations are either emergency or elective. For emergency operations, we do not include AAA diameter in the logistic regression models. This is because the post-rupture assessment of AAA diameter (as recorded in the NVR) is not a reliable assessment of the pre-rupture AAA diameter (as used in the individual simulation modelling).
Proportion operated on after an abdominal aortic aneurysm rupture
Many patients with an AAA rupture die before getting to hospital or the operating theatre, are turned down or refuse AAA repair. So, the proportion of patients with an AAA rupture receiving an operation is an important parameter that crucially influences the survival rate after an AAA rupture. However, estimates of this parameter for women are not easy to obtain.
We have taken data from the literature and from recruitment to the randomised trials of EVAR versus open repair for ruptured AAAs (such as the IMPROVE trial30) to provide relevant estimates (see Chapter 3, Mortality following ruptured abdominal aortic aneurysm in women). The conclusion is the overall proportion of women with a ruptured AAA who receive an emergency repair is low, at around 25%. We use this figure in our modelling, but allow considerable uncertainty (95% uncertainty interval 15–35%) in the PSA.
Proportion receiving endovascular aneurysm repair for an abdominal aortic aneurysm rupture
The numbers of women in the NVR and HES receiving open repair or EVAR for ruptured AAAs are shown in Table 16a. The proportion of operations identified in HES that are also reported in the NVR is lower than for elective operations (around 70%). This may underlie the more substantial difference in the reported proportions receiving EVAR: 16.8% in the NVR compared with 22.4% in HES. There is an increasing rate of EVAR use with age (Figure 10a).
| Source | Open repair (n) | EVAR (n) | % EVAR |
|---|---|---|---|
| NVR | 653 | 132 | 16.8 |
| HES | 845 | 244 | 22.4 |
| Vascunet (urgent + emergency) | 1069 | 328 | 23.5 |
| Source | EVAR repairs (n) | Deaths (n) | % deaths |
|---|---|---|---|
| NVR in-hospital | 132 | 33 | 25.0 |
| HES in-hospital | 244 | 31 | 12.7 |
| HES 30-day | 244 | 48 | 19.7 |
| Vascunet in-hospital (urgent + emergency) | 254 | 53 | 20.9 |
| Source | Open repairs (n) | Deaths (n) | % deaths |
|---|---|---|---|
| NVR in-hospital | 653 | 260 | 39.8 |
| HES in-hospital | 845 | 284 | 33.6 |
| HES 30-day | 845 | 319 | 37.8 |
| Vascunet in-hospital (urgent + emergency) | 927 | 318 | 34.3 |
FIGURE 10.
Emergency surgery data in the NVR. (a) Proportion of women receiving EVAR for an AAA rupture by age in the NVR, and a superimposed logistic regression fit; and (b) in-hospital mortality rate in women receiving open repair for an AAA rupture by age in the NVR, and a superimposed logistic regression fit.


For comparison, the overall proportion of men receiving EVAR for emergency operations in the NVR was 19.4%;97 an increasing trend with age was again evident. Data from Vascunet give the overall proportion of women receiving EVAR as 23.5% (see Table 16a), but do not distinguish urgent and emergency cases.
In our analysis, we use the data from the NVR, first, because of the potential coding problems with HES and, second, because individual-level data were available for incorporating the influence of age. We use the same methods as before, described in Use of National Vascular Registry data as a source of parameter estimates in women, but ignore any effect of AAA diameter. Parameter estimates from the logistic regressions are given in Table 41, Appendix 5; in the base-case analysis, the overall proportion for women is simply that observed in the NVR (i.e. 16.8%).
Emergency endovascular aneurysm repair operative mortality
The in-hospital and 30-day mortality rates from the NVR and HES are shown in Table 16b. The overall in-hospital mortality rate from the NVR is 25.0%, substantially greater than the 12.7% reported in HES. This may reflect mortality events being missed in HES when patients are transferred to another hospital (e.g. for rehabilitation or long-term nursing care) and subsequently dying. The transfer of care results in the end of a HES episode. There were increasing mortality rates with increasing age. As for elective operations, the 30-day mortality rate determined by HES–ONS linked data is greater than the in-hospital mortality rate (19.7% vs. 12.7%). Vascunet provided very incomplete data for 30-day mortality after urgent and emergency operations (which are combined in Vascunet), in contrast to the data for 30-day mortality after elective operations shown earlier. So we report the Vascunet in-hospital mortality data, which are more complete, giving a figure of 20.9% after EVAR (see Table 16b). The literature review (see Chapter 3, Mortality following ruptured abdominal aortic aneurysms in women) suggested a 30-day mortality rate of 32%. Some of the differences may relate to how EVAR converted to open repair (which has very high mortality) and is categorised in the different studies and to the differential use of anaesthesia types in different countries.
For comparison, the in-hospital mortality rate for men in the NVR is 20.7%,97 slightly lower than the 25.0% for women.
In our modelling for women, again we use the NVR data for in-hospital mortality and make an adjustment to reflect 30-day mortality based on HES (as in Elective endovascular aneurysm repair operative mortality). For the base-case analysis, this yields an overall 30-day mortality rate of 35.9%. Parameter estimates from the logistic regressions are given in Table 41, Appendix 5.
Emergency open abdominal aortic aneurysm repair operative mortality
The corresponding mortality rates for women after open AAA repair are shown in Table 16c. The overall in-hospital mortality rate is 39.8% in the NVR, compared with 33.6% in HES. An increasing mortality rate with age was again evident (see Figure 10b). Again, in HES–ONS, the 30-day mortality was greater than the in-hospital mortality (37.8% vs. 33.6%, respectively). Vascunet data give the in-hospital mortality rate as 34.3%, which is similar to HES, but does not distinguish emergency and urgent cases. The literature review (see Chapter 3, Thirty-day operative mortality in women versus men) suggested a 30-day mortality rate of 51%.
For comparison, the in-hospital mortality rate for men in the NVR is 36.9%,97 slightly lower than the 39.8% for women.
We use the same methods as before to provide estimates from the logistic regressions in Table 41, Appendix 5. For the base-case analysis, this yields an overall 30-day mortality rate of 44.2%.
Reintervention rates after successful emergency surgery
Again, a ‘successful operation’ is taken to mean that the patient is alive 30 days after the operation. Obtaining information on reintervention rates after emergency AAA operations, especially for women, is difficult. For similar reasons as for elective operations, we based estimates on the IMPROVE trial,30 the largest and longest trial of EVAR versus open repair for ruptured AAAs. The trial randomised patients with a ruptured AAA either to a policy of EVAR if possible, compared with open repair. We have access to the provisional unpublished 3-year follow-up data from the IMPROVE trial,30 and are able to report reinterventions by operation received, for the group of patients with confirmed ruptured AAAs. The time between randomisation and operation (if received) in IMPROVE30 is very short (median 0.7 hours), so we use time since randomisation as the time scale.
The IMPROVE trial data30 were limited by the fact that only about 20% of the patients were women, and that the available follow-up extends only to 3 years. In the trial, about 50% of the ruptured AAA patients had died within 3 years. Because of the available follow-up, we consider the period after 30 days after the operation as one period, include all reinterventions, whether they are first or subsequent ones, and express this as a rate per 100 person-years. The reinterventions included are as listed in Reintervention rate in successful elective surgery.
The reinterventions data from the IMPROVE trial30 are summarised in the upper two rows of Table 17. Because there are possible differences in rates between men and women, we use the data for women alone despite the small numbers. Thus, the rate of reinterventions after 30 days after EVAR is estimated as 15.8 per 100 woman-years and correspondingly after open repair as 2.3 per 100 woman-years.
| Item | Men | Women | ||
|---|---|---|---|---|
| Number/person-years | Rate per 100 person-years (SE) | Number/person-years | Rate per 100 person-years (SE) | |
| Reinterventions > 30 days after EVAR | 29/267 | 10.9 (2.0) | 9/57 | 15.8 (5.3) |
| Reinterventions > 30 days after open repair | 25/410 | 6.1 (1.2) | 2/85 | 2.3 (1.7) |
| AAA-related mortality > 30 days after EVAR | 4/406 | 0.985 (0.493) | 0/87 | 0.0 |
| AAA-related mortality > 30 days after open repair | 9/626 | 1.437 (0.479) | 2/124 | 1.163 (1.140) |
Long-term abdominal aortic aneurysm-related mortality rate after successful emergency surgery
For similar reasons as before (see Long-term abdominal aortic aneurysm-related mortality rate after successful elective repair), we use the IMPROVE trial30 data to estimate the long-term AAA-related mortality rates after emergency surgery in women (lower two rows of Table 17). For the period after 30 days after emergency EVAR, the rate is estimated as 0; correspondingly, after emergency open repair, the rate is estimated as 1.2 per 100 woman-years.
Comparability of National Vascular Registry and Hospital Episode Statistics data
Both the NVR and HES are large data sets that can provide information about patients with an aortic aneurysm. Each suffers from some drawbacks. The NVR is voluntary and may be incomplete and, although the submission rates are high, selective censoring may have an impact on estimates of less frequent events and mortality. It is also a procedure-based registry, which does not contain information about longer-term follow-up or include patients with aneurysms who do not undergo procedures. However, it does include rich clinical data regarding risk factors, and anatomical and procedural information that is not included in HES.
Hospital Episode Statistics is primarily an administrative data set in which patients with an aneurysm can be identified based on procedural and diagnostic codes. Although there are concerns about accuracy, in recent years both data quality and coverage have improved and HES data have been found to be useful in studying mortality rates. The exact information will differ between the data sources as, apart from missing data and true coding errors, there are differences in definition of cases owing to the need to interpret multiple diagnostic and procedural codes in HES for categorising procedures. However, HES does provide information about longer-term readmission rates and repeat procedures and can be linked to ONS data to provide long-term mortality estimates.
It is notable that, for elective procedures, operative mortality rates were very similar in both the NVR and HES (see Table 14). The main discrepancy between the NVR and HES data related to emergency procedures (see Table 16). This is likely to be due to the aforementioned limitations of both data sets. NVR mortality rates were consistently higher than those in HES, but especially so for emergency EVAR. One factor may be the method by which these data are recorded. NVR data entry is completed by the surgeon performing the procedure whereas HES is based on hospital coding data. In the NVR data set, patients undergoing urgent repair of a non-ruptured AAA is specifically captured. These patients were excluded from our analysis of NVR data. Such patients are not coded specifically in HES and, therefore, may be inadvertently coded as a ruptured AAA because they underwent an unplanned operation and were admitted as an emergency. In the analysis of the HES data used here, patients with coding records inconsistent with a ruptured AAA were excluded, but it remains possible that some ‘urgent’ patients with a non-ruptured AAA remained in the HES dataset. These patients have better outcomes than true ruptured AAAs and may account for the lower mortality seen in HES.
Chapter 6 Costs and miscellaneous parameters for women
The original AAA Markov model11 assessing the cost-effectiveness of a one-off invitation to screening for men aged 65 years used cost estimates from the MASS trial98 presented in 2000/1 prices. These costs were subsequently uprated to 2010/11 prices, incorporating changes in surgical repair resource use and unit costs. Contemporary screening costs were acquired from NAAASP, and cost estimates were updated to reflect the increased use of EVAR. 35
In this previous study35 a bottom-up costing was not feasible, but more recent randomised trial surgical resource use data were available. Data from the EVAR-1 trial99 were used to estimate the costs of elective open repair and EVAR. Contemporary registry data from the National Vascular Registry (NVR)26 were utilised to update significant components of resource use [operation length, hospital length of stay (LOS)], and general NHS inflation was accounted for. The MASS trial98 was used to estimate the cost of emergency repairs, with emergency procedures assumed to be limited to open repair on the basis of appropriate National Institute for Health and Care Excellence (NICE) guidance. 100 Similar to open repair, major components of resource use were updated using registry data from the NVR.
For the current modelling, new estimates of costs were necessary for three reasons: to reflect (1) changes in unit costs since 2010/11, (2) possible trends in procedure resource use and (3) potential differences in resource use between men and women. This chapter provides estimates of these costs and a few remaining parameters (see Table 3), representing the final aspect of objective 3 in Chapter 1, Scientific objectives.
Unit costs
All costs are considered from a NHS perspective, rather than from a societal or personal perspective, and are presented in 2014/15 prices.
Screening costs
Screening costs were taken from NAAASP (Professor Jonothan Earnshaw, Gloucestershire Hospitals NHS Foundation Trust, 2012, personal communication) and updated to reflect general health service inflation to 2014/15 prices. 101 The cost of screening women was assumed to be the same as in the programme for men (Table 18).
| Resource use item | Cost 2010/11 (£) | Updated cost 2014/15 (£) |
|---|---|---|
| Invitation to screen | 1.70 | 1.80 |
| First scan | 32.20 | 34.11 |
| Surveillance scan | 68.00 | 72.03 |
Pre-surgical consultation costs
In previous modelling, the cost of a pre-surgical consultation was based on data from the MASS trial98 and subsequently uprated for general NHS inflation. 35 This estimate was from data collected from a subsample of the full trial population. On average, 1.6 consultations were conducted before elective surgery. Unit costs came from the finance departments of centres involved in the trial. In the current modelling, the number of consultations was assumed to be the same as observed in the MASS trial,98 but the unit cost was updated using contemporary estimates from the Department of Health and Social Care’s NHS Reference Costs 2014 to 2015. 39 The unit cost comprised a weighted mean of face-to-face consultant-led outpatient visits for vascular surgery, cardiothoracic surgery and cardiac surgery specialties. The new estimate of the cost of pre-surgical consultations was £328.64, rather than £435.25 as used before. 35
Costs of elective and emergency abdominal aortic aneurysm repair
A similar approach was adopted to estimate contemporary costs for women undergoing surgical AAA repair, given the infeasibility of conducting detailed microcosting. Cost estimates were taken from UK-based randomised trials (EVAR-138 and IMPROVE24) focusing on women-specific data. These were updated using registries to provide robust data on the general AAA repair population and reflect potential trends in hospital LOS. LOS data were available from HES28 and the NVR26,97 between 2010 and 2014. Hospital stay constitutes the largest component of resource use, with significant differences between men and women, and includes that incurred by renal dialysis in the primary admission. Unlike previous modelling, which had limited emergency surgery to only open repair, an estimate of the cost of emergency EVAR was required, given evidence of its increased use in this setting from both the NVR and HES.
For elective repair costs, the EVAR-1 trial38 was again utilised. EVAR-1 recruited patients between 1999 and 2004 in 38 UK centres. 38 Women-specific elective AAA repair costs were obtained from the EVAR-1 trial38 investigators in 2014/15 prices and updated using LOS data from HES. These costs related to the primary admission. HES LOS data were preferred to the NVR as HES is a more complete database of AAA repairs, and additional analysis using more accurate coding was possible.
The components of total cost comprising mean vascular ward and critical care stay were removed and replaced with women-specific mean LOS data from HES, multiplied by unit costs obtained from NHS Reference Costs 2014 to 2015. 39 Elective AAA repair LOS observed in HES was significantly lower than in the EVAR-1 trial38 for EVAR repair, particularly the general vascular ward stay; however, open repair hospital LOS was similar. Updated costs are shown in Tables 19a and b.
| Elective EVAR | EVAR-138 LOS (n = 60) (days) | EVAR-138 cost (n = 60) (£) | HES LOS (n = 1491) (days) | Updated cost 2014/15 prices (£) |
|---|---|---|---|---|
| Vascular ward | 13.1 | 4463 | 5.8 | 1984 |
| Critical care | 2.7 | 3084 | 1.0 | 1142 |
| Othera | N/A | 10,758 | N/A | 10,758 |
| Total cost | 18,306 | 13,884 |
| Elective open repair | EVAR-138 LOS (n = 54) (days) | EVAR-138 cost (n = 54) (£) | HES LOS (n = 1009) (days) | Updated cost 2014/15 prices (£) |
|---|---|---|---|---|
| Vascular ward | 10.07 | 3444 | 10.0 | 3420 |
| Critical care | 4.17 | 4764 | 3.7 | 4227 |
| Othera | N/A | 5413 | N/A | 5413 |
| Total cost | 13,621 | 13,060 |
| Emergency EVAR | IMPROVE30 LOS (n = 29) (days) | IMPROVE30 cost (n = 29) (£) | HES LOS (n = 380) (days) | Updated cost 2014/15 prices (£) |
|---|---|---|---|---|
| Vascular ward | 8.1 | 2308 | 10.2 | 3488 |
| Critical care | 3.1 | 3627 | 2.2 | 2513 |
| Otherb | N/A | 10,152 | N/A | 10,152 |
| Total cost | 16,088 | 16,154 |
| Emergency open repair | IMPROVE30 LOS (n = 69) (days) | IMPROVE30 cost (n = 69) | HES LOS (n = 1044) (days) | Updated cost 2014/15 prices (£) |
|---|---|---|---|---|
| Vascular ward | 6.2 | 1961 | 21.0 | 7182 |
| Critical care | 6.3 | 7617 | 3.7 | 4227 |
| Otherb | N/A | 6204 | N/A | 6204 |
| Total cost | 15,783 | 17,613 |
Emergency AAA repair cost data for women were obtained from the IMPROVE trial30 investigators in 2011/12 prices for the primary admission. The trial recruited patients between 2009 and 2013 in 29 UK centres and one Canadian centre. Data provided were restricted to those patients with a confirmed AAA rupture and according to treatment received rather than randomised group. In the trial, an ‘EVAR where possible’ strategy was adopted and analysed by intention to treat, so that patients in that group did not always receive EVAR.
Components of total cost comprising general vascular ward and critical care stay were removed and the remaining costs were inflated for general NHS inflation to 2014/15 prices using published indices. 101 Women-specific HES data were used to update LOS and were multiplied by unit costs from NHS Reference Costs 2014 to 2015. 39 Critical care LOS observed in HES was lower for both EVAR and open repair, although mean stay on vascular ward was higher. Updated costs are shown in Table 19c and d.
For comparison, HES data on AAA repair for men were utilised to update costs from the EVAR-138 and IMPROVE24 trials using the same approach. Elective AAA repair, both EVAR and open repair, was estimated to be less costly for men than for women (EVAR £12,993, open repair £11,712), largely due to a lower observed LOS. For emergency repair, costs for men were higher than for women (EVAR £18,045, open repair £17,995) because of longer critical care stays. This could be related to the higher mortality rate among women undergoing AAA repair, reducing LOS, although the pattern of general ward stay between men and women was dissimilar.
Surveillance costs
Post-surgery surveillance resource use was based on expert opinion [one vascular surgeon (MJB) and one vascular biologist (JTP) on the study team] of additional imaging performed in UK clinical practice. All unit costs were obtained from NHS Reference Costs 2014 to 2015. 39 For open repair, it was assumed that patients received one 6-week follow-up consultation. The cost of this consultation was assumed to be the same unit cost as pre-surgical consultation (£196.79). For EVAR, patients were assumed to have annual surveillance for their lifetime, consisting of one consultation (£196.79) and one ultrasound scan. A weighted mean of unit costs of an ultrasound scan with duration of ≥ 20 minutes (RD42Z-RD43Z) was obtained (£61.37).
Reintervention costs
Reintervention costs were incorporated into the model explicitly using data from the EVAR-1 trial. 38 The cost, reflecting mean resource use, of a reintervention during the 10-year follow-up of the EVAR-1 trial38 was estimated for women only for EVAR and open repair. The costs were £7546 and £8986, respectively. These costs were assumed to be the same for reinterventions occurring after elective and emergency repairs.
Sensitivity analyses for costs
Because of the nature of the costing exercise, which produced surgical cost estimates with components combined from different sources (both randomised trial data and observational data), a formal estimate of the associated stochastic precision could not be computed. Therefore, imprecision in unit costs was included in the PSA conducted by representing a 95% uncertainty interval from 20% lower to 25% higher costs as a symmetrical normal distribution for log-costs. The impact of changes in costs was also explored in DSAs by varying the costs of screening, surveillance and surgical operation by –20% or + 25% (see Costs).
Quality of life and competing mortality
Quality of life in the population
There is limited evidence that an AAA-screened population has a lower health-related QoL than the general population. 10 For the purpose of calculating QALYs, the life-years accrued of all women, screened and unscreened, in the model were adjusted using UK population EuroQol-5 Dimensions (EQ-5D) utility survey data, specific to women. 102 The QoL weights used were as follows: 0.81 for ages 55–64 years, 0.78 for ages 65–74 years and 0.71 for ages of ≥ 75 years.
Quality of life after surgery
An additional consideration relates to the QoL of those who undergo surgery. Trials of elective AAA repair indicate that there is a reduction in QoL following repair, but that this is transient. 103 In the EVAR-1 trial,99 mean EQ-5D utility (range 0–1) score in the EVAR arm was 0.74, 0.73, 0.71 and 0.74 at baseline and 1-month, 3-month and 12-month follow-up, respectively. Using a visual analogue scale [(VAS); scale 0–100] the corresponding figures were 70.82, 70.20, 69.69 and 71.29, respectively. In the open repair arm, mean EQ-5D utility score was 0.74, 0.67, 0.73 and 0.75 at baseline and 1-month, 3-month and 12-month follow-up, respectively (corresponding figures for the VAS were 70.78, 64.09, 71.36 and 72.53, respectively). Given the focus on a whole-screened population, these small differences in QoL are not likely to have a material bearing on results, and it is more important to reflect age-related differences in QoL.
Given the nature of emergency repair and the patient’s condition at randomisation and baseline, comparative utility scores are harder to acquire. However, at the 12-month compared with the 3-month follow-up in the IMPROVE trial,30 mean EQ-5D utility score was 0.01 higher for EVAR and 0.04 higher for open repair. Again, it was considered that these differences were not material in terms of long-term modelling for a whole population.
Non-abdominal aortic aneurysm mortality
Mortality not related to an AAA is a competing risk, in that AAA screening will be less effective when such competing mortality is higher. Age-specific non-AAA mortality rates for women were estimated using two data sets: (1) overall life tables and (2) rates of death by age and cause. Life tables for 2012–14 were available from the ONS for the UK population. 40 Overall annual mortality risks were adjusted by subtracting the AAA-specific death rates for women among the UK population, using ONS cause-of-death data. 104
Previous modelling exercises of AAA screening based on the MASS trial98 have noted that the non-AAA mortality after being turned down for elective surgery was higher than that in the general population. 11,12 This is because comorbidities are a major reason for surgery being contraindicated. In previous modelling for men, the corresponding non-AAA mortality rate was taken directly from data in the MASS trial. 98 However, such comorbidities occur at the same rate in both the invited and non-invited groups, although in the latter they may be largely unobserved. Hence, including an increased non-AAA mortality rate after being turned down for elective surgery, which occurs mainly in the group invited to screening, unfairly biases the results against screening. With this understanding, the current modelling for women does not include a similar increase in the rate of non-AAA mortality. This provides fair estimates of incremental costs, life-years and QALYs (i.e. the differences between the invited and non-invited groups). However, it will very slightly overestimate absolute life-years and QALYs, and maybe absolute costs as well, because the women following contraindication to elective surgery are on average being assumed to survive for longer than they may do in reality.
Chapter 7 Cost-effectiveness analyses for women based on current NHS Abdominal Aortic Aneurysm Screening Programme policy
Using the women-specific parameter estimates generated in Chapters 3–6, this chapter addresses objective 4 in Chapter 1, Scientific objectives. It presents the cost-effectiveness of a screening programme for women, based on an identical protocol that is currently implemented by NAAASP for men. Specifically, estimates are presented for the cost-effectiveness of a one-off invitation to screening for an AAA for women aged 65 years, in which women whose aortic diameter measures ≥ 3.0 cm at the first screening are entered into the surveillance programme, with annual ultrasound scans for AAAs that measure 3.0–4.4 cm and 3-monthly scans for AAAs that measure 4.5–5.4 cm. Women are considered for elective surgery once their AAA diameter reaches ≥ 5.5 cm. A PSA is conducted, along with a range of DSAs, to investigate the impact of changing parameter values on the cost-effectiveness results.
Base-case analysis
The base-case analysis uses the best available evidence for the input parameters, based on systematic reviews, registry data, cohort studies, other hospital data and contemporary costs, as described in Chapters 3–6. These parameters are listed in full in Table 20. The prevalence for women aged 65 years is assumed to be 0.43%, with average growth and rupture rates of 1.5 mm per year and 0.2 per 100 woman-years for a 3.0-cm AAA, respectively, increasing to 2.5 mm per year and 2.7 per 100 woman-years for a 5.0-cm AAA, respectively. The DES model, based on a 30-year time horizon, is run on 10 million pairs of individuals to obtain reliable estimates of the health economic quantities and counts key events occurring within the non-invited and invited to screening groups. Parameter uncertainty is accounted for through a PSA in which the DES model is run 1000 times on 500,000 pairs of individuals, using a different set of input parameters in each run. Input parameters are drawn from suitable distributions, as detailed in Table 20.
| Parameter | Source (base case) | Base case | PSA | DSA |
|---|---|---|---|---|
| Screening | ||||
| Reinvitation proportion | NAAASP8 | 142,127/594,376 ≈ 0.239 | None | None |
| Attendance proportion | Chichester33 | 218/300 ≈ 0.727 | Beta(218,82) | None |
| Non-visualisation proportion | NAAASP8 | 1652/470,531 ≈ 0.0035 | None | None |
| AAA size distribution at screening | NAAASP8 | NAAASP distribution, reweighted to give 0.0043 prevalence | NAAASP distribution based on uncertain prevalence | Uppsala distribution, reweighted to give 0.0043 prevalence |
| Prevalence proportion | Systematic review34 | 0.0042756 | Based on normal (–5.45054, 0.323212) distribution for logit(p) | (a) 0.0021378 (b) 0.0085512 |
| AAA growth and rupture | ||||
| AAA growth | RESCAN35 | Mixed linear model for log-AAA diameter (see Chapter 4) | Using variance–covariance matrix for the six parameters (see Chapter 4) | None |
| AAA rupture | RESCAN35 | Joint model for log-rupture rates and log-underlying AAA diameter (see Chapter 4) | Using variance–covariance matrix for the two parameters (see Chapter 4) | None |
| Surveillance | ||||
| Dropout from surveillance | NAAASP8 | 1072/19,650 ≈ 0.0546 per year | Gamma(1072,19650) | (a) 0.0273 per year (b) 0.1092 per year |
| Incidental detection rate | New Zealand36 | 40/1364.25 ≈ 0.0293 per year | Gamma(40,1364.25) | (a) 0.0147 per year (b) 0.0586 per year |
| Delay from ≥ 5.5-cm scan to consultation | NAAASP8 | 10.6 days | None | None |
| Consultation scan | RESCAN,35 Singh et al.95 | CT is on average 0.244 cm greater than ultrasound; measurement error SD of 0.19 cm for CT | None | None |
| Decision at consultation: proportion returned to surveillance | N/A | Modelled directly from AAA measurements by CT | N/A | None |
| Decision at consultation: non-intervention proportion | Meta-analysis from four hospitals37 | 0.34226 of those not returned to surveillance | Based on normal (–0.65324, 0.135022) distribution for logit(p) | None |
| Decision at consultation: proportion elective surgery | N/A | 1 – 0.34226 = 0.65774 of those not returned to surveillance | Obtained by subtraction | None |
| Delay from consultation scan to elective surgery | NAAASP8 | 70.8 days | None | None |
| Elective operations | ||||
| Proportion receiving EVAR vs. open repair | NVR26 | 0.586 | Based on normal(0.348, 0.0432) for logit(p) | (a) Dependence of logit(p) on (age-80) and (AAA diameter-6.0) (b) 0.3396 based on systematic review of EVAR suitability |
| EVAR 30-day operative mortality | NVR,26 HES28 |
Expit[logit(23/1306) + F1] ≈ 0.024 F1 = log{[37/(1642–37)]/[27/(1642–27)]} |
Based on normal(–4.022, 0.2102) + F1 for logit(p) | (a) Dependence of logit(p) on (age-80) and (AAA diameter-6.0) (b) 0.0223 based on systematic review |
| Open repair 30-day operative mortality | NVR,26 HES28 |
Expit[logit(64/922) + F2] ≈ 0.081 F2 = log{[75/(1066–75)]/[64/(1066–64)]} |
Based on normal(–2.596, 0.1302) + F2 for logit(p) | (a) Dependence of logit(p) on (age-80) and (AAA diameter-6.0) (b) 0.0537 based on systematic review (c) 0.05 |
| Reintervention rate after successful EVAR | EVAR-1 RCT38 | 20.3 and 6.4 per 100 woman-years during 31–120 and > 120 days, respectively | Based on Gamma(3,15) and Gamma(27,421), respectively | None |
| Reintervention rate after successful open repair | EVAR-1 RCT38 | 0.0 | None | DSA based on DREAM76/OVER77 RCT rates in men, as these trials include incisional hernias. Overall rate across the two trials combined, 4.4 and 2.9 per 100 woman-years during 31–120 and > 120 days, respectively |
| Long-term AAA mortality rate after successful EVAR | EVAR-1 RCT38 | 1.799 per 100 woman-years | Based on Gamma(8,444.7) | None |
| Long-term AAA mortality rate after successful open repair | EVAR-1 RCT38 | 0.499 per 100 woman-years | Based on Gamma(2,400.8) | None |
| Emergency operations | ||||
| % operated after rupture | Literature review and the IMPROVE RCT24 | 0.25 | Based on normal(0.25, 0.052), with truncation to within [0,1] | None |
| Proportion receiving EVAR vs. open repair | NVR26 | 0.168 | Based on normal(–1.599, 0.0952) for logit(p) | Dependence of logit(p) on (age-80) |
| EVAR 30-day operative mortality | NVR26 and HES28 |
Expit[logit(33/132) + F3] ≈ 0.359 F3 = log{[48/(244–48)]/[31/(244–31)]} |
Based on normal(–1.099, 0.2102) + F3 for logit(p) | (a) Dependence of logit(p) on (age-80) (b) 0.32 based on systematic review |
| Open repair 30-day operative mortality | NVR26 and HES28 |
Expit[logit(260/653) + F4] ≈ 0.442 F4 = log{[319/(845–319)]/[284/(845–284)]} |
Based on normal(–0.413, 0.0802) + F4 for logit(p) | (a) Dependence of logit(p) on (age-80) (b) 0.51 based on systematic review |
| Reintervention rate after successful EVAR | IMPROVE RCT24 | 15.8 per 100 woman-years | Based on Gamma(9,57) | None |
| Reintervention rate after successful open repair | IMPROVE RCT24 | 2.3 per 100 woman-years | Based on Gamma(2,85) | None |
| Long-term AAA mortality rate after successful EVAR | IMPROVE RCT24 | 0.0 | None | 0.985 per 100 woman-years based on men |
| Long-term AAA mortality rate after successful open repair | IMPROVE RCT24 | 1.613 per 100 woman-years | Based on Gamma(2,124) | 1.437 per 100 woman-years based on men |
| Costs | ||||
| Invitation, reinvitation | NAAASP8 | £1.80 | In all cases: based on normal[log(base-case estimate), 0.1142] for log-costs |
In all cases: (a) base-case estimate × 0.80 (b) base-case estimate ×1.25 |
| Screening scan | NAAASP8 | £34.11 | ||
| Surveillance scan | NAAASP8 | £72.03 | ||
| Consultation for elective surgery | MASS10 and NHS Reference Costs 2014 to 201539 | £328.64 | ||
| Elective EVAR repair | EVAR-1,38 HES28 and NHS Reference Costs 2014 to 201539 | £13,844 | ||
| Elective open repair | EVAR-1,38 HES28 and NHS Reference Costs 2014 to 201539 | £13,060 | ||
| Emergency EVAR repair | IMPROVE,24 HES28 and NHS Reference Costs 2014 to 201539 | £16,154 | ||
| Emergency open repair | IMPROVE,24 HES28 and NHS Reference Costs 2014 to 201539 | £17,613 | ||
| Surveillance after operations | Expert opinion and NHS Reference Costs 2014 to 201539 | £258.16 annually after EVAR, £196.79 at 6 weeks after open repair | ||
| Reintervention after EVAR | EVAR-138 | £7546 | ||
| Reintervention after open repair | EVAR-138 | £8986 | ||
| Miscellaneous | ||||
| Non-AAA mortality rate | ONS40 | ONS 2012–14 data by single year of age, ages 65–94 years | None | None |
| Overall QoL/utilities | EQ-5D102 | 0.81 for age 55–64 years, 0.78 for age 65–74 years and 0.71 for age ≥ 75 years | None | None |
| QoL harms of screening | MASS10 | No effect | None | None |
| QoL harms of surgery | EVAR-138 and IMPROVE24 | No effect | None | None |
| Discounting rates | N/A |
(a) Undiscounted (b) 3.5% per year for costs, 3.5% per year for life-years |
None | None |
Numbers of key events
Table 21 shows the numbers of women in the non-invited and invited to screening groups that experience key events from the base-case run of the DES model over 30 years. About three-quarters of all elective operations in the invited to screening group occur through incidental detection, indicating that many AAAs are not initially detected at age 65 years, owing to non-attendance or an aneurysm that has yet to develop. Elective operations following screen detection of an AAA occur predominantly in the 68- to 78-year age group (Figure 11). In total, 86% of the population die by the age of 95 years in the non-invited group and 0.83% die of AAA-related causes. Screening prevents approximately 2500 AAA deaths in this population of 10 million women, with the percentage who die of AAA-related causes in the invited to screening group being reduced to 0.80%. The relative risk reduction is 3.0%; 4100 women need to be invited to screening to save one death from an AAA.
| Event | Number of events | |
|---|---|---|
| Not invited to screening | Invited to screening | |
| Emergency open surgery | 18,957 | 18,108 |
| Emergency EVAR surgery | 3915 | 3728 |
| Elective open surgery | ||
| Incidentally detected | 9039 | 8221 |
| Screen detected | 0 | 2718 |
| Total | 9039 | 10,939 |
| Elective EVAR surgery | ||
| Incidentally detected | 12,743 | 11,551 |
| Screen detected | 0 | 4010 |
| Total | 12,743 | 15,561 |
| AAA ruptures | 91,759 | 87,855 |
| AAA deaths | 82,932 | 80,476 |
| Non-AAA deaths | 8,552,257 | 8,554,234 |
| Reinterventions | ||
| After elective open | 0 | 0 |
| After elective EVAR | 4367 | 5776 |
| After emergency open | 1582 | 1480 |
| After emergency EVAR | 1522 | 1447 |
| Total | 7471 | 8703 |
| Surveillance measurements | ||
| Entered surveillance | 94,371 | 115,699 |
| After open surgery | 18,688 | 19,951 |
| After EVAR surgery | 13,606 | 16,087 |
| After contraindication | 10,638 | 13,133 |
| Total | 137,303 | 164,870 |
| Contraindications | ||
| Incidentally detected | 11,469 | 10,581 |
| Screen detected | 0 | 3402 |
| Total | 11,469 | 13,983 |
| Dropout from surveillance | 23,563 | 35,101 |
FIGURE 11.
Cumulative elective operations, emergency operations and AAA deaths in the base-case analysis ages 65–95 years among 10 million women. (a) Elective operations; (b) emergency operations; and (c) AAA deaths.


The difference in numbers of emergency operations and AAA deaths between the invited and non-invited groups accrues gradually over the 30-year period after initial screening (see Figure 11). No effect is evident within the first 5 years as only a small proportion of women are initially over the diameter threshold for elective intervention. This is in contrast to the MASS trial10 of screening in men, in which the benefit of screening was apparent at an earlier stage. In the non-invited group, 0.92% of women have a ruptured AAA over the course of 30 years, compared with 0.88% of the invited group (see Table 21).
Health economic outputs
Estimates of mean life-years, QALYs and costs per woman invited to screening for the base-case are given in Table 22. The group not invited to screening has an average life expectancy from age 65 years of 20.5429 years, which increases by 0.00285 years for the group invited to screening. The gain of 0.00285 life-years equals 1.04 days of life; this average figure reflects the very many who have no change in life expectancy through screening, the few who gain (some substantially) because rupture of the AAA is prevented and the very few who lose by dying in elective surgery.
| Sensitivity analysis change | Base-case | Baseline aorta distribution and prevalence | Dropout and incidental detection rates | |||||
|---|---|---|---|---|---|---|---|---|
| Uppsala distribution (0.43% prevalence) | Halve prevalence (0.21%) | Double prevalence (0.86%) | Halve both ratesa | Double both ratesb | ||||
| No screening | Screeningc | Difference | Difference | Difference | Difference | Difference | Difference | |
| Life-years | ||||||||
| Undiscounted | 20.5429 | 20.5458 | 0.00285 | 0.00504 | 0.00123 | 0.00840 | 0.00399 | 0.00154 |
| Discounted | 13.9338 | 13.9353 | 0.00153 | 0.00286 | 0.00065 | 0.00472 | 0.00212 | 0.00084 |
| Discounted, QA | 10.4474 | 10.4485 | 0.00110 | 0.00207 | 0.00047 | 0.00342 | 0.00152 | 0.00061 |
| Costs (£) | ||||||||
| Undiscounted | 88.40 | 124.57 | 36.170 | 39.569 | 31.238 | 49.330 | 40.381 | 31.761 |
| Discounted | 49.56 | 83.55 | 33.990 | 37.179 | 30.187 | 44.770 | 36.905 | 30.803 |
| ICER (per life-year or QALY) (£) | ||||||||
| Undiscounted | 12,685 | 7849 | 25,405 | 5871 | 10,115 | 20,647 | ||
| Discounted | 22,180 | 12,987 | 46,346 | 9477 | 17,393 | 36,543 | ||
| Discounted, QA | 30,955 | 17,931 | 64,841 | 13,107 | 24,302 | 50,888 | ||
| INMB, discounted, QA (£) | ||||||||
| Lambda of £20,000 | –12.03 | 4.29 | –20.88 | 23.55 | –6.53 | –18.70 | ||
| Lambda of £30,000 | –1.05 | 25.02 | –16.22 | 57.71 | 8.65 | –12.64 | ||
For health economic analyses, life-years and costs are both discounted at 3.5% per annum. The increase in QALYs associated with screening is 0.00110 years per woman invited to screening. Mean discounted costs associated with screening and AAA-related events increase by £33.99 from £49.56 to £83.55. Overall, this gives an ICER of £22,000 per life-year gained and £31,000 per QALY gained for the base-case screening strategy versus no screening. Therefore, the INMB [calculated as (net discounted QALYs × WTP) – net discounted costs] is negative for both £20,000 and £30,000 per QALY thresholds (see Table 22). This suggests that a screening programme for women based on the current NAAASP implementation is unlikely to be cost-effective.
Uncertainty from the PSA in the estimated incremental QALYs and incremental costs is shown in Figure 12a. The scatterplot lies in the north-east quadrant, where invitation to screening is both more effective and more expensive. However, the majority of the points lie above the threshold of £20,000 per QALY gained and about half lie above a £30,000 threshold (shown in Figure 12a by the diagonal lines that pass through the origin). There is considerable uncertainty in the incremental discounted QALYs. In particular, the prevalence of AAAs is highly correlated with the estimated incremental QALYs and, as this quantity is not precisely known, it is a key driver behind the large amount of uncertainty. We express uncertainty on the INMB scale: an INMB of –£12.03 (95% uncertainty interval –£27.88 to £22.12) per woman invited is estimated if a QALY is valued at £20,000, and an INMB of –£1.05 (–£23.76 to £54.79) per woman invited if a QALY is valued at £30,000. A CEAC (Figure 12b) indicates that the screening programme, as implemented, is unlikely to be considered cost-effective: there is a < 20% probability that the programme would be cost-effective at a threshold of £20,000 per QALY.
FIGURE 12.
Cost-effectiveness from the PSA of the base-case model. (a) Results on the cost-effectiveness plane; the green and blue lines indicate WTP thresholds of £20,000 and £30,000 per QALY, respectively; and (b) CEAC.
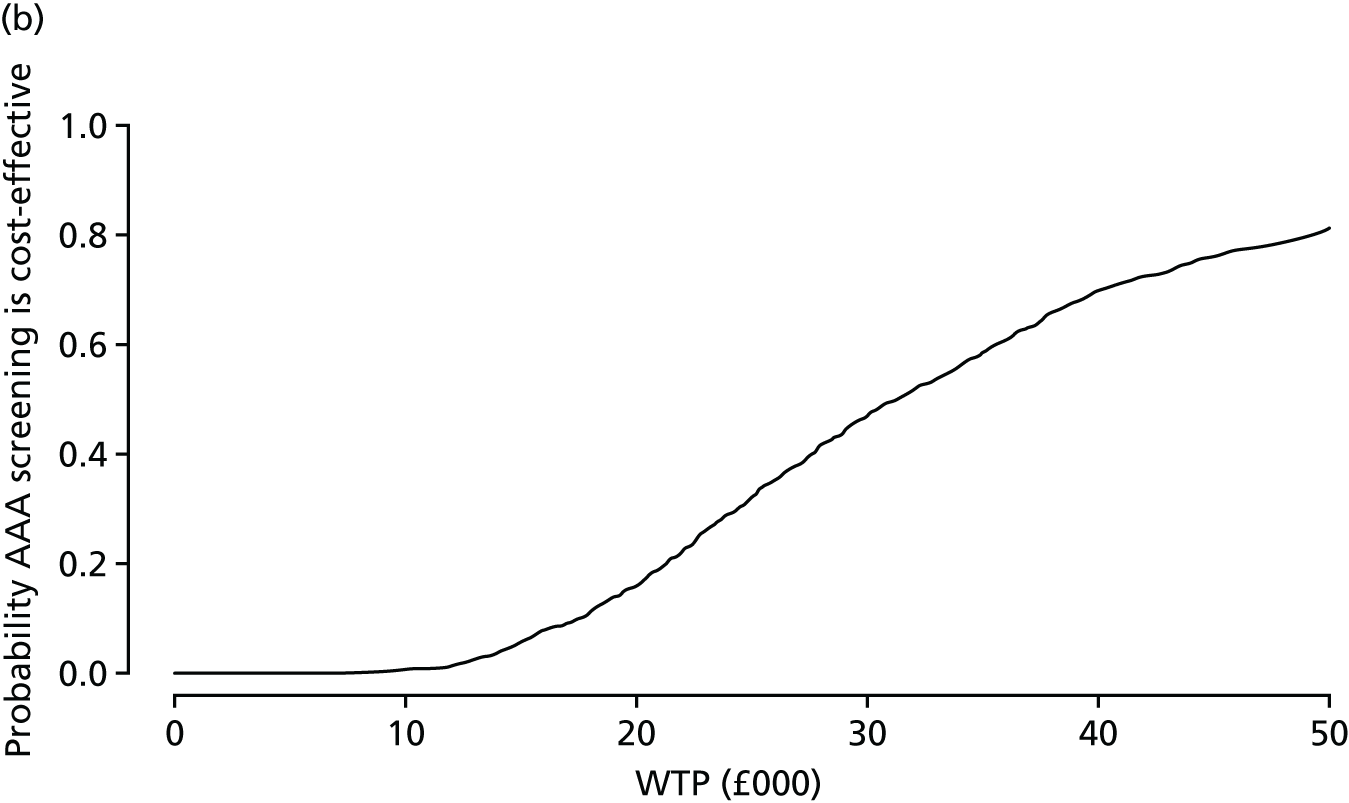
Deterministic sensitivity analyses
A total of 15 one-way DSAs are undertaken to investigate the robustness of the cost-effectiveness estimates to changes in parameter inputs. The sensitivity analyses investigated are detailed here, and Table 23 (and see Table 22) shows the results for incremental life-years, QALYs and costs, and cost-effectiveness estimates.
| Sensitivity analysis change | Elective surgery parametersa | Emergency surgery parametersa | Parameters affecting postoperative complications | Costs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Difference | ||||||||||
| Dependent on age and AAA diameter | Based on systematic literature reviews | Open repair operative mortality of 5%b | Dependent on age | Based on systematic literature reviews | Increasing the reintervention rate after elective open repair and increasing AAA mortality rate after emergency repairc | 20% lower costs of screening, surveillance and consultation | 25% higher costs of screening, surveillance and consultation | 20% lower costs of elective surgery, and 25% higher costs of emergency surgery | 25% higher costs of elective surgery, and 20% lower costs of emergency surgery | |
| Life-years | ||||||||||
| Undiscounted | 0.00296 | 0.00304 | 0.00293 | 0.00284 | 0.00291 | 0.00286 | 0.00285 | 0.00285 | 0.00285 | 0.00285 |
| Discounted | 0.00159 | 0.00163 | 0.00158 | 0.00152 | 0.00156 | 0.00154 | 0.00153 | 0.00153 | 0.00153 | 0.00153 |
| Discounted, QA | 0.00114 | 0.00117 | 0.00113 | 0.00109 | 0.00112 | 0.00110 | 0.00110 | 0.00110 | 0.00110 | 0.00110 |
| Costs (£) | ||||||||||
| Undiscounted | 35.923 | 35.097 | 36.173 | 36.165 | 36.153 | 36.807 | 30.106 | 43.751 | 34.532 | 38.031 |
| Discounted | 33.824 | 33.329 | 33.991 | 33.989 | 33.982 | 34.373 | 28.095 | 41.359 | 32.758 | 35.405 |
| ICER per life-year or QALY) (£) | ||||||||||
| Undiscounted | 12,153 | 11,534 | 12,331 | 12,744 | 12,416 | 12,864 | 10,558 | 15,344 | 12,111 | 13,338 |
| Discounted | 21,257 | 20,409 | 21,506 | 22,306 | 21,716 | 22,353 | 18,333 | 26,988 | 21,376 | 23,103 |
| Discounted, QA | 29,656 | 28,481 | 29,998 | 31,135 | 30,308 | 31,196 | 25,586 | 37,666 | 29,833 | 32,244 |
| INMB, discounted, QA (£) | ||||||||||
| Lambda of £20,000 | –11.01 | –9.92 | –11.33 | –12.16 | –11.56 | –12.34 | –6.13 | –19.40 | –10.80 | –13.44 |
| Lambda of £30,000 | 0.39 | 1.78 | 0.00 | –1.24 | –0.35 | –1.32 | 4.85 | –8.42 | 0.18 | –2.46 |
Baseline aortic diameter distribution and prevalence
We first consider how changes in the aortic diameter distribution and prevalence affect the cost-effectiveness of the screening programme. Although the base-case model is derived from the aortic size distribution from 700,000 men in NAAASP, a sensitivity analysis is based on the Uppsala study14 aortic size distribution, undertaken in 5140 women (see Chapter 4, Screening). After reweighting both distributions to give the desired prevalence (0.43% ≥ 3.0 cm), the Uppsala study14 had a much higher proportion of medium and large AAAs (0.074% vs. 0.005% in the reweighted NAAASP distribution; see Table 12b). In addition, we investigate the effect of halving the prevalence, from 0.43% to 0.21%, and doubling the prevalence, from 0.43% to 0.86%, from the base-case model.
A notable change in the ICER is seen when changing the baseline aortic diameter distribution from the (weighted) NAAASP distribution to the (weighted) Uppsala distribution (see Table 22). There is a small increase in costs, due to an increase in elective operations, but the mean difference in life-years almost doubles owing to timely elective operations taking place and the subsequent reduction in ruptures. The effect is a screening programme that is more cost-effective, with the ICER reduced to £18,000 per QALY gained and an INMB gain of £4.29 per woman invited if a QALY is valued at £20,000. This effect becomes even clearer if the prevalence of AAAs is doubled to 0.86%, with the ICER decreasing to £13,000 per QALY gained, and an INMB gain of £23.55 per woman invited if a QALY is valued at £20,000. As expected, the ICER increases substantially if the prevalence is halved, to £65,000 per QALY gained.
Dropout and incidental detection rates
We next consider the effect of a change in the dropout and incidental detections rates. First, the dropout and the incidental detection rates are halved, from 5.5 to 2.7 per 100 person-years and from 2.9 to 1.5 per 100 person-years, respectively. Following this, the dropout and the incidental detection rates are doubled, from 5.5 to 10.9 per 100 person-years and from 2.9 to 5.9 per 100 person-years, respectively.
Halving the dropout rate ensures that a larger number of individuals stay in the surveillance programme, giving a greater chance of preventing a rupture via an elective operation should their AAA grow large enough. This increases the cost of the programme, but a greater number of life-years are gained owing to the increase in AAA treatment. In addition, life-years are lost in the non-invited group owing to the halving of the incidental detection rate. This results in a more cost-effective programme than in the base-case, with an ICER of £24,000 per QALY gained (see Table 22). Conversely, an increase in the dropout and incidental detection rates results in a greater ICER of £51,000 per QALY gained, attributable to a greater relative reduction in the incremental life-years than in the reduction in the incremental costs.
Parameters affecting elective operations
We now consider the effects age and AAA size have on an individual’s suitability for elective EVAR surgery and their operative mortality rates, and whether or not changes in these parameters affect the cost-effectiveness of the screening programme. First, the percentage receiving elective EVAR and the elective operative mortality rates for EVAR and open repair are allowed to depend on age and AAA diameter (see Chapter 5). Next, the percentage receiving elective EVAR and the elective operative mortality rates are based on the systematic reviews (see Chapter 3). Finally, the elective open AAA repair operative mortality, estimated from the NVR26 and HES,28 is decreased from 8.1% to 5%; this might be regarded as a potentially attainable target after a performance improvement programme.
These changes all have a similar, but small, effect on the cost-effectiveness estimates, with ICERs in the range £28,000–30,000 (see Table 23). Although the incremental costs are similar or slightly lower than in the base case, the incremental QALYs are slightly higher. A reduction in the elective open repair operative mortality to 5% has little effect on the cost-effectiveness estimates when compared with the base case, which suggests that cost-effectiveness is largely unaffected by even quite substantial changes in elective open AAA repair mortality.
Parameters affecting emergency operations
As earlier, we first allow the percentage receiving emergency EVAR, and emergency operative mortality rates for EVAR and open repair, to depend on age. Second, the percentage receiving emergency EVAR, and emergency operative mortality rates are based on the literature reviews (see Chapter 3, Mortality following ruptured abdominal aortic aneurysms in women).
Allowing emergency operations to depend on age has limited overall effect on the cost-effectiveness, with an ICER of £31,000 per QALY gained. The effect of the systematic review point estimates also has little effect on the cost-effectiveness estimates, resulting in an ICER of £30,000 per QALY gained (see Table 23).
Reintervention rates following successful abdominal aortic aneurysm repair
Uncertainties in the reintervention rates estimated as zero in the base-case analysis were not included in the PSA. We consider complications following both elective and emergency operations, by increasing the reintervention rate after successful elective open repair from 0.0 to 4.4 (31–120 days) and 2.9 (> 120 days) per 100 person-years (based on men in the DREAM76 and OVER77 trials), and increasing the long-term AAA mortality rate after successful emergency EVAR repair from 0.0 to 0.985 per 100 person-years (based on men in the IMPROVE trial30). This has almost no effect on the cost-effectiveness of the screening programme for women (see Table 23). This highlights the fact that overall cost-effectiveness is relatively insensitive to changes in rates of events that affect only a small proportion of the population (e.g. those with an AAA who have undergone and survived an elective or emergency operation). Therefore, although there were no long-term data for women about reinterventions after successful emergency surgery, it is reassuring that this parameter is unlikely to have any substantial effect on overall cost-effectiveness estimates.
Costs
Finally, we consider the effect of costs, by means of combinations of alterations to the unit costs in different stages of the screening programme. First, we lower the costs of screening, surveillance and consultation by 20%. This is followed by the increase in screening, surveillance and consultation costs of 25%, a symmetrical increase on a log-scale. Next, we consider opposing decreases and increases in costs of elective and emergency surgeries: a 20% lower cost of elective surgery and 25% higher cost of emergency surgery, then a 25% increase in the cost of elective surgery and 20% decrease in the cost of emergency surgery.
The decrease and increase in costs associated with screening and surveillance result in an expected increase and decrease in the incremental costs with no change in the incremental life-years. This has the effect of decreasing and increasing the ICER by approximately 20% to £26,000 and £38,000 per QALY gained, respectively (see Table 23). A smaller effect on the ICER was seen when the elective and emergency surgery costs were varied. Decreasing elective surgery costs and increasing emergency surgery costs makes the screening programme more cost-effective, but only slightly, with the ICER decreasing to £30,000 per QALY gained. Similarly, increasing elective surgery costs and decreasing emergency surgery costs increases the ICER to only £32,000 per QALY gained.
Conclusions
Based on our best estimates, an AAA screening programme for women, as currently implemented by NAAASP, is unlikely to be considered cost-effective for the NHS, with an estimated ICER of £31,000 per QALY gained compared with the NICE valuation of a QALY (£20,000–30,000). However, this conclusion is sensitive to the prevalence of AAAs in 65-year-old women and the distribution of aortic sizes among AAAs in women. We have shown that, if the prevalence is as high as 0.86%, then the ICER would be lowered to £13,000 per QALY gained and screening could be considered cost-effective. This prevalence is below that estimated from two out of the six studies included in our systematic review for women aged < 70 years (see Figure 3). Similarly, if more women who are detected with an AAA at screening have a medium or large AAA (as indicated in the Uppsala distribution of aorta sizes), then the programme could also be considered cost-effective. This highlights the urgent need to obtain robust evidence about both the prevalence and aortic diameter distribution in the UK population of women at ages that could be considered for screening.
Other key parameters that could change conclusions regarding cost-effectiveness are the rate at which women drop out from a screening programme and the rate at which AAAs are incidentally detected. The latter parameter in particular is very difficult to estimate, and good-quality data on this are lacking. Halving the incidental detection and dropout rates would decrease the ICER to £24,000 per QALY gained.
Finally, we have shown in this chapter that varying the rates and costs associated with elective and emergency operations does not change the cost-effectiveness results very much since they affect a relatively small proportion of the population. This provides some confidence that the results are robust to moderate deviations in estimates for these parameters.
Chapter 8 Screening options for women
A more cost-effective screening programme for women may depart from the options used for men in a number of ways. Given their lower AAA prevalence and longer life expectancy, inviting women to screening at a higher age may be more cost-effective than doing so at age 65 years. In addition, given the higher AAA rupture risk in women than men, the threshold for considering elective surgery might be lowered from 5.5 cm. The ‘prevalence’ may also be increased by considering an aortic diameter < 3.0 cm as defining an AAA in women, and including this group in the surveillance programme. Surveillance intervals might be lengthened for this group and, indeed, others with the smallest AAAs, in order to reduce costs. Finally, women might be offered rescreening after some years, even if they were screened as normal initially.
In this chapter, each of these options is first investigated separately. Then the options are combined to find the most cost-effective joint option. Therefore, this chapter addresses objective 5 in Chapter 1, Scientific objectives.
Single screening options
The options considered include changing the age at screening or the AAA diameter for considering elective surgery. These changes affect the age and AAA diameter at which women receive elective operations, and the age when AAAs rupture. Thus, it is important that all these analyses include the effects of age and AAA diameter on the parameters for elective operations and the effect of age on those for emergency surgery. Thus, we start with a ‘reference case’ for this chapter, which combines the two relevant sensitivity analyses in Chapter 7, Parameters affecting elective operations and Chapter 7, Parameters affecting emergency operations, so that these age and AAA diameter effects are included.
Reference case
This includes the effects of age and AAA diameter on parameters for elective operations, and the effect of age on parameters for emergency operations, but does not make any other changes. Neither of these sensitivity analyses in Chapter 7 changed the cost-effectiveness estimates very much compared with the base case. Therefore, their combination is also similar to the base case (Table 24 Reference case columns), with an ICER of £30,000 per QALY gained. The numbers of key events are provided for this reference case in Table 25 (Reference case columns), which are, again, similar to the base case (see Table 21).
| Option, surveillance interval changes | Reference case | Screening age (years) | Intervention threshold | Diagnosis threshold | Surveillance intervals | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age and AAA diameter effects on operation parameters included | 70 | 75 | 5.0 cm, 3 months for 4.0–4.9 cm | 4.5 cm, 3 months for 3.5–4.4 cm | 2.5 cm, 1 year for 2.5–2.9 cm | 2.5 cm, 5 years for 2.5–2.9 cm | 2 years for 3.0–3.9 cm | Rescreen < 3.0 cm every5 years | |||
| No screening | Screeninga | Difference | Difference | Difference | Difference | Difference | Difference | Difference | Difference | Difference | |
| Life-years | |||||||||||
| Undiscounted | 20.5451 | 20.5480 | 0.00291 | 0.00366 | 0.00520 | 0.00337 | 0.00374 | 0.00403 | 0.00405 | 0.00285 | 0.00591 |
| Discounted | 13.9351 | 13.9367 | 0.00156 | 0.00216 | 0.00346 | 0.00181 | 0.00200 | 0.00211 | 0.00212 | 0.00153 | 0.00296 |
| Discounted, QA | 10.4484 | 10.4495 | 0.00112 | 0.00154 | 0.00246 | 0.00130 | 0.00143 | 0.00151 | 0.00152 | 0.00110 | 0.00211 |
| Costs (£) | |||||||||||
| Undiscounted | 90.33 | 126.23 | 35.899 | 39.759 | 47.719 | 38.201 | 41.255 | 45.215 | 41.818 | 34.686 | 154.928 |
| Discounted | 50.55 | 84.36 | 33.806 | 36.849 | 44.084 | 35.892 | 38.925 | 40.378 | 37.636 | 32.802 | 114.687 |
| ICER (per life-year or QALY) (£) | |||||||||||
| Undiscounted | 12,335 | 10871 | 9180 | 11,333 | 11,022 | 11,231 | 10,317 | 12,151 | 26,233 | ||
| Discounted | 21,620 | 17,034 | 12,741 | 19,802 | 19,443 | 19,174 | 17,732 | 21,386 | 38,737 | ||
| Discounted, QA | 30,170 | 23,966 | 17,946 | 27,628 | 27,151 | 26,817 | 24,798 | 29,844 | 54,294 | ||
| INMB, discounted, QA (£) | |||||||||||
| Lambda of £20,000 | –11.40 | –6.10 | 5.05 | –9.91 | –10.25 | –10.26 | –7.28 | –10.82 | –72.44 | ||
| Lambda of £30,000 | –0.19 | 9.28 | 29.61 | 3.08 | 4.08 | 4.79 | 7.90 | 0.17 | –51.32 | ||
| Event | Reference case (see Reference case) | Combined option 1 (see Detailed results for option 1) | ||
|---|---|---|---|---|
| Not invited to screening (n) | Invited to screening (n) | Not invited to screening (n) | Invited to screening (n) | |
| Emergency open surgery | 18,824 | 18,126 | 14,911 | 13,037 |
| Emergency EVAR surgery | 4533 | 4260 | 3780 | 3325 |
| Elective open surgery | ||||
| Incidentally detected | 6097 | 5267 | 5487 | 3802 |
| Screen detected | 0 | 2960 | 0 | 5966 |
| Total | 6097 | 8227 | 5487 | 9768 |
| Elective EVAR surgery | ||||
| Incidentally detected | 15,555 | 14,351 | 18,262 | 14,652 |
| Screen detected | 0 | 3597 | 0 | 12,337 |
| Total | 15,555 | 17,948 | 18,262 | 26,989 |
| AAA ruptures | 92,346 | 88,389 | 74,653 | 65,545 |
| AAA deaths | 83,877 | 81,311 | 68,864 | 63,205 |
| Non-AAA deaths | 8,550,791 | 8,552,846 | 8,497,888 | 8,502,202 |
| Reinterventions | ||||
| After elective open | 0 | 0 | 0 | 0 |
| After elective EVAR | 5054 | 6192 | 5428 | 9134 |
| After emergency open | 1606 | 1466 | 1086 | 908 |
| After emergency EVAR | 1612 | 1549 | 1249 | 1023 |
| Total | 8272 | 9207 | 7763 | 11,065 |
| Surveillance measurements | ||||
| Entered surveillance | 95,290 | 115,463 | 138,346 | 207,259 |
| After open surgery | 15,446 | 16,984 | 12,391 | 15,239 |
| After EVAR surgery | 16,090 | 18,168 | 17,530 | 25,460 |
| After contraindication | 10,905 | 13,056 | 11,681 | 18,522 |
| Total | 137,731 | 163,671 | 179,948 | 226,480 |
| Contraindications | ||||
| Incidentally detected | 11,725 | 10,565 | 12,606 | 9889 |
| Screen detected | 0 | 3410 | 0 | 9671 |
| Total | 11,725 | 13,975 | 12,606 | 19,560 |
| Dropout from surveillance | 24,050 | 35,226 | 38,340 | 84,809 |
Age at screening
We consider raising the screening age from 65 years to 70 or 75 years. The AAA prevalence at age 65 years was 0.43% (95% CI 0.23% to 0.80%), based on the systematic review for women aged < 70 years (see Chapter 3, Current prevalence of screen-detected abdominal aortic aneurysms in women), and increased to 1.15% (95% CI 0.59% to 2.24%) for women aged 75 years, based on the 70- to 79-year age group in the systematic review. Interpolating linearly on a logit scale between these two estimates gives an AAA prevalence of 0.70% (95% CI 0.37% to 1.34%) at age 70 years. The attendance rate at screening was 72.7% at age 65 years, and this is estimated to decrease to 67.6% at age 70 years and to 62.3% at age 75 years (see Chapter 4, Screening). Both of these factors are taken into account when adjusting the screening age.
As shown in Table 24, offering screening at age 70 or 75 years increased the gain in QALYs per woman invited to screening by factors of about 1.4 and 2.2, respectively, compared with the reference case. The costs per woman invited decreased in both groups compared with the reference case, with the incremental cost being larger, especially for age 75 years screening. The estimated ICERs were £24,000 per QALY gained for age 70 years screening and £18,000 for age 75 years screening. The more favourable ICER for age 75 years screening gives rise to a positive INMB at a threshold of £20,000 per QALY.
The improved cost-effectiveness at higher ages illustrates the importance of AAA prevalence. This offsets the disadvantages incurred through a lower life expectancy (16.4 years at age 70 years and 12.6 years at age 75 years, compared with 20.5 years at age 65 years) resulting from the increased mortality rates from non-AAA causes, as well as the increased mortality from elective operations at higher ages (see Chapter 5). However, two points should be noted. First, the non-intervention rate for elective operations used in the model is not age dependent, as there was insufficient evidence to quantify this in the systematic review (see Proportion of women versus men not offered an intervention). This is not clinically realistic, and it is appreciated that non-intervention rates probably increase markedly with age, especially for women in whom EVAR is not suitable (as open repair operations in elderly women have the greatest risk). This implies that the results for screening, at age 75 years in particular, may be overly optimistic. Second, by screening at a higher age, AAA deaths at younger ages are not prevented. From the unscreened group in the base-case analysis (see Figure 11c), one can see that about 5% of all AAA deaths occur between the ages of 65 and 70 years, and about 15% between the ages of 65 and 75 years. These earlier deaths are also associated with the greatest number of life-years lost. Although this does not affect the cost-effectiveness of screening at higher ages, it reduces the benefit of screening at the population level.
Abdominal aortic aneurysm diameter for considering surgery
We consider reducing the threshold of the AAA diameter for considering elective surgery to 5.0 or 4.5 cm. The surveillance intervals were also altered in these scenarios, so that a 3-month interval applied for the 1.0 cm range below the threshold (i.e. 4.0–4.9 cm when the threshold is 5.0 cm, and 3.5–4.4 cm when the threshold is 4.5 cm). This is in keeping with the use of 3-month intervals for 4.5- to 5.4-cm AAAs in both the base case and reference case in which the threshold is 5.5 cm.
Using lower thresholds increased the incremental life-years and QALYs compared with the reference case (see Table 24). Both options slightly increased the incremental costs because of a greater number of elective operations, especially in the invited group. The consequence is that for a 5.0-cm threshold the ICER was £28,000 per QALY gained, while, for a 4.5-cm threshold, the ICER was £27,000, both slightly lower than the reference case.
Aortic diameter defining an abdominal aortic aneurysm
We consider reducing the aortic diameter for defining an AAA in women to 2.5 cm. In both the Swedish and Danish data, (see Chapter 4, Screening), 2.5 cm is roughly 1.5 times the average aortic diameter in women (which is sometimes suggested as an appropriate definition of an AAA105) and roughly 3 SDs above the mean. Based on an AAA prevalence of 0.43% for an aortic diameter of ≥ 3.0 cm at age 65 years, the AAA prevalence becomes 1.44% for an aortic diameter of ≥ 2.5 cm. For the 2.5- to 2.9-cm group, which are now entered into the surveillance programme, we consider two choices of surveillance intervals: (1) 1 year (as in the 3.0–4.4 cm group) or (2) 5 years (as has been suggested for men with subaneurysmal aortic diameters106).
Both options similarly increased the life-years and QALYs gained (see Table 24), but only by about one-third compared with the reference case. However, they also increased the incremental costs, through more surveillance scans in the invited group, especially in the first option, which employed 1-year surveillance intervals, and more elective operations. As a result, the cost-effectiveness estimates were more favourable than in the reference case, with ICERs of £27,000 and £25,000 in the two cases, respectively. However, the programme would still not be considered cost-effective at a threshold of £20,000 per QALY.
Surveillance intervals and rescreening
Finally, we consider changing the surveillance intervals. First, we increase the surveillance intervals for the smallest AAAs (3.0–3.9 cm) to 2 years, an option that improved cost-effectiveness slightly for men. 35 Second, we consider a programme in which all women are rescreened every 5 years from age 65 years upwards. In essence, this includes everyone with an aortic diameter of < 3.0 cm into a monitoring programme with a surveillance interval of 5 years.
In this first option, unsurprisingly, the number of life-years and QALYs gained is very slightly lower, but the incremental cost is also slightly lower than the reference case through fewer surveillance scans (see Table 24, penultimate column). This yields an ICER of £30,000 per QALY gained, hardly changed compared with the reference case. The second option has dramatic effects on both the life-years and QALYs gained, which are approximately doubled compared with the reference case, and the incremental cost, which is increased more than three-fold (see Table 24). The result is not favourable in terms of cost-effectiveness, with an estimated ICER of £54,000 per QALY gained.
Combined screening options
We take the more favourable options considered, and combine them in an attempt to identify options for an AAA screening programme in women that would be better than the ones considered so far. Of particular interest is whether or not any combined options yield an ICER < £20,000 per QALY. We avoid the possibility of screening at age 75 years, for the reasons given earlier (see Age at screening). We consider 12 options in total, for screening age (either 65 or 70 years), intervention threshold (AAA diameter of 4.5, 5.0 or 5.5 cm) and diagnosis threshold (aortic diameter of either 2.5 cm or 3.0 cm). Surveillance intervals are set as 3 months for the 1 cm interval below the intervention threshold, 5 years for 2.5–2.9 cm, if applicable, and 1 year otherwise. Five of the 12 possible options have already been considered in Table 24; results from the additional seven options are shown in Table 26.
| Outcome | Option 1 (70 years screening age, 5.0-cm intervention threshold,a 2.5-cm diagnosis threshold)a | Option 2 (70 years screening age, 5.0-cm intervention threshold,a 3.0-cm diagnosis threshold)a | Option 3 (65 years screening age, 5.0-cm intervention threshold,a 2.5-cm diagnosis threshold)a | Option 4 (70 years screening age, 5.5-cm intervention threshold,a 2.5-cm diagnosis threshold)a | Option 5 (70 years screening age, 5.0-cm intervention threshold,a 2.5-cm diagnosis threshold)a | Option 6 (70 years screening age, 4.5-cm intervention threshold,a 3.0-cm diagnosis threshold)a | Option 7 (65 years screening age, 4.5-cm intervention threshold,a 2.5-cm diagnosis threshold)a | ||
|---|---|---|---|---|---|---|---|---|---|
| No screening | Screeningb | Difference | Difference | Difference | Difference | Difference | Difference | Difference | |
| Life-years | |||||||||
| Undiscounted | 16.4305 | 16.4353 | 0.00484 | 0.00416 | 0.00465 | 0.00430 | 0.00524 | 0.00450 | 0.00515 |
| Discounted | 11.8599 | 11.8627 | 0.00281 | 0.00246 | 0.00244 | 0.00251 | 0.00303 | 0.00264 | 0.00268 |
| Discounted, QA | 8.7257 | 8.7277 | 0.00200 | 0.00175 | 0.00174 | 0.00178 | 0.00215 | 0.00187 | 0.00192 |
| Costs (£) | |||||||||
| Undiscounted | 84.53 | 134.93 | 50.395 | 43.584 | 45.812 | 44.901 | 58.111 | 48.694 | 51.407 |
| Discounted | 52.76 | 97.83 | 45.066 | 40.418 | 40.954 | 40.264 | 52.134 | 45.491 | 45.939 |
| ICER (per life-year or QALY) (£) | |||||||||
| Undiscounted | 10,420 | 10,480 | 9842 | 10,436 | 11,094 | 10,830 | 9977 | ||
| Discounted | 16,016 | 16,449 | 16,800 | 16,072 | 17,229 | 17,235 | 17,126 | ||
| Discounted, QA | 22,540 | 23,149 | 23,492 | 22,615 | 24,271 | 24,281 | 23,972 | ||
| INMB, discounted, QA (£) | |||||||||
| Lambda of £20,000 | –5.08 | –5.50 | –6.09 | –4.66 | –9.17 | –8.02 | –7.61 | ||
| Lambda of £30,000 | 14.91 | 11.96 | 11.34 | 13.15 | 12.30 | 10.72 | 11.55 | ||
Combined options considered
The results in Table 24 suggest that a favourable combined option might be obtained by screening at age 70 years, employing an intervention threshold of 5.0 cm and a diagnosis threshold of 2.5 cm. These options considered singly gave ICERs of £24,000, £28,000 and £25,000, respectively. Combining them is presented as option 1 in Table 26, and shows increases in both QALYs gained and in incremental costs compared with both the reference case and the options considered singly (see Table 24). However, the ICER is estimated as £23,000 per QALY, which represents only a slight improvement in cost-effectiveness.
Given that option 1 did not reduce the ICER to below £20,000 per QALY, all the other additional combinations were also investigated, being presented as options 2–7 in Table 26. A number of conclusions can be drawn from the total 12 options considered: (1) many of the options yield very similar ICERs, with, for example, 9 out of the 12 between £22,500 and £25,000 per QALY; (2) the combinations employing screening at age 65 years and a diagnosis threshold of 3.0 cm are clearly not the best options; (3) screening at age 70 years is almost uniformly more cost-effective than screening at age 65 years; (4) a diagnosis threshold of 2.5 cm is uniformly more cost-effective than one of 3.0 cm; and (5) no option gave an ICER below £20,000 per QALY.
The best choice of intervention threshold is moot. Except when screening at age 65 years using a 3.0-cm diagnosis threshold, all three intervention thresholds (4.5 cm, 5.0 cm and 5.5 cm) yield very similar ICERs. The lower thresholds give rise to greater QALYs gained but also to greater incremental costs from an increased number of elective operations. However, since the increases in QALYs and costs are proportionally similar, the ICERs remain similar.
Given the uncertainty in the ICERs, it is not possible to be definitive about which combined option is ‘best’ in terms of cost-effectiveness. However, option 1 gave the lowest ICER estimate and the next to largest gain in life-years and QALYs. We now examine this option in more detail.
Detailed results for option 1
The numbers of key events for this option, which is the best in terms of overall cost-effectiveness, are shown in Table 25 (right-hand side). Compared with the reference case, there are fewer AAA ruptures, emergency operations and AAA deaths due to screening at the higher age of 70 years. The relative reductions in each of these outcomes in the screening group are 12%, 12% and 8%, compared with 4%, 4% and 3% in the reference case, respectively. However, there are more elective operations in this screening option than in the reference case, owing to the lower intervention threshold, with a relative increase of 55% compared with 21%.
The cumulative numbers of elective operations, emergency operations and AAA deaths for option 1 are shown in Figure 13. Compared with the corresponding figures for the base-case (see Figure 11), the separation of the lines for the invited and non-invited groups is greater. For emergency operations and AAA deaths, the separation gradually increases over the whole of the 25-year time course from age 70 to 95 years, with no suggestion, for example, of a marked benefit in the early years after screening. A marked early benefit might be anticipated only if there were a substantial number of women discovered at screening who were near or over the AAA diameter threshold for considering surgery.
FIGURE 13.
Cumulative elective operations, emergency operations, and AAA deaths among 10 million women in combined screening option 1. (a) Elective operations; (b) emergency operations; and (c) AAA deaths.

A PSA was carried out for this case, using 1000 runs for different parameter values representing their uncertainty distributions, each run including 500,000 pairs of women. This involved using the variance–covariance matrix from the logistic regressions that quantified the relations between age and AAA diameter on parameters for elective and emergency operations (see Chapter 5). Random draws of parameter values were taken from the relevant multivariate normal distributions. A normal distribution for the uncertainty of the altered AAA prevalence estimate (on a logit scale) was used, together with a Beta distribution for the altered attendance rate. The uncertainty in other parameters was as in the base-case analysis (see Table 11).
The distribution of incremental costs and QALYs is shown on the cost-effectiveness plane in Figure 14a. As for the base-case (see Figure 12a), there is a wide spread for incremental QALYs and a lesser spread for incremental costs. The centre of the distribution lies between the lines representing the thresholds of £20,000 and £30,000 per QALY. The INMB at a threshold of £20,000 per QALY is –£5.08 (95% uncertainty interval –£31.53 to £69.98), whereas at a threshold of £30,000 it is £14.91 (95% uncertainty interval –£25.18 to £135.16). The cost-effectiveness estimate is more favourable than that for the base-case analysis, there being about a 40% chance that this screening programme would be cost-effective at a threshold of £20,000 per QALY, as indicated in the CEAC shown in Figure 14b.
FIGURE 14.
Cost-effectiveness from the PSA of combined screening option 1. (a) Results on the cost-effectiveness plane (the green and blue lines indicate WTP thresholds of £20,000 and £30,000 per QALY, respectively) and (b) CEAC.
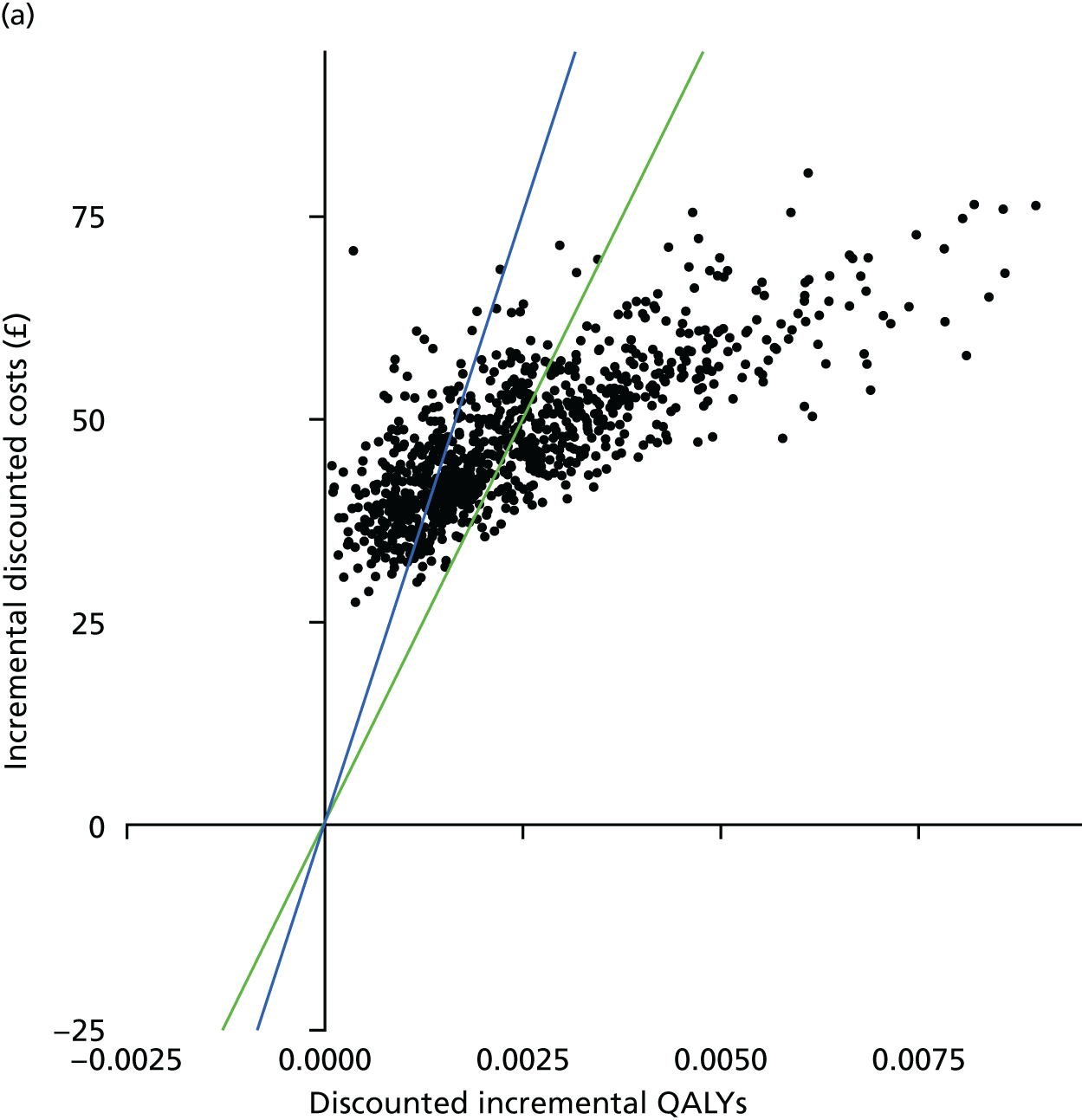
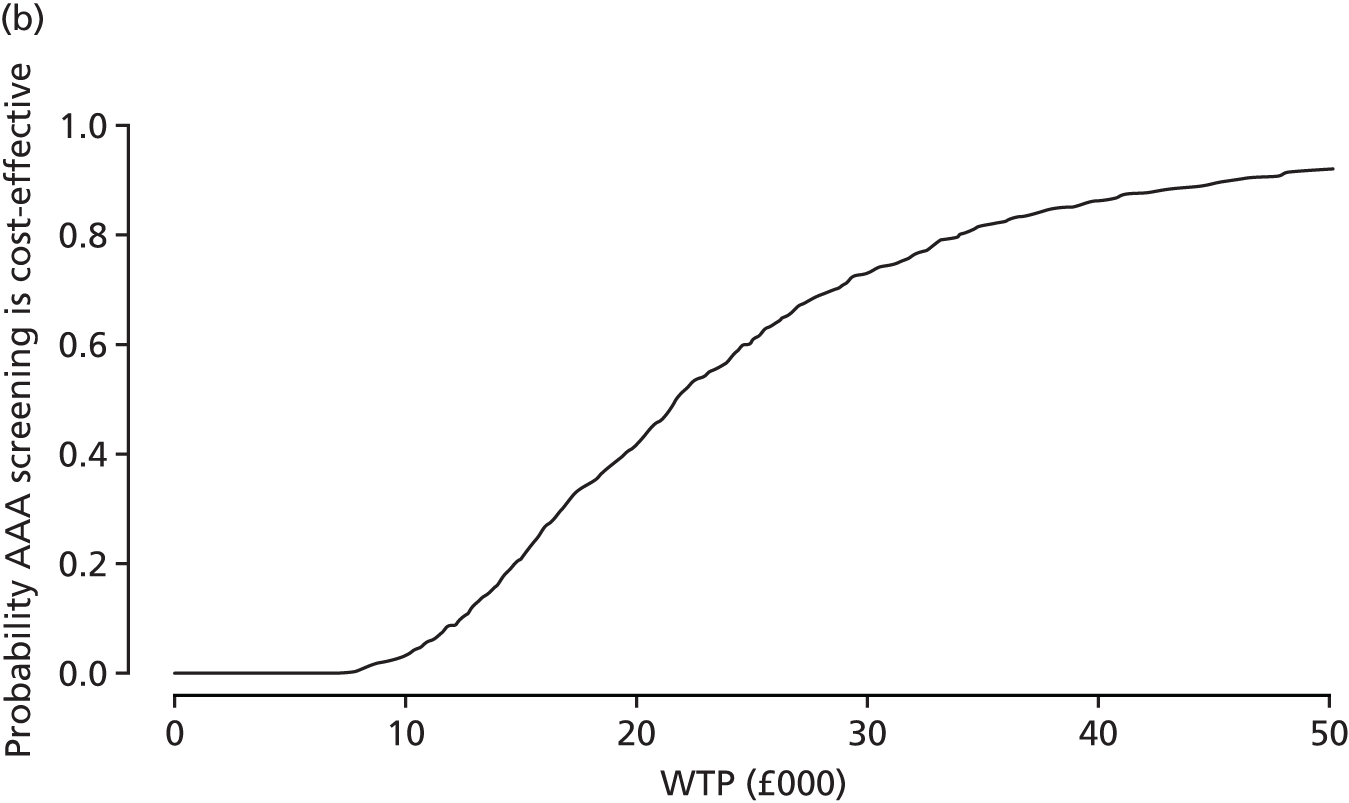
Conclusions
Screening women for AAAs might become more cost-effective if one moved away from the options adopted in NAAASP for men. Screening became more cost-effective if offered at age 70 years. Lowering the threshold for considering surgery, or lowering the threshold for defining an AAA to a diameter of 2.5 cm, also made AAA screening for women slightly more cost-effective, but none of these changes, when considered individually, brought the estimated ICER to below £20,000 per QALY gained.
The remaining possibility of finding a more cost-effective screening option for women was to combine the alternative screening options. All combinations relating to screening age (65 or 70 years), intervention threshold (4.5 cm, 5.0 cm or 5.5 cm) and diagnosis threshold (2.5 or 3.0 cm) have been investigated in this chapter. Although many combined options gave similar cost-effectiveness estimates, the numerically best option involved an invitation to screening at age 70 years, an aortic diameter of 2.5 cm defining an AAA (with a 5-year surveillance interval for AAAs with a diameter of 2.5- to 2.9-cm AAAs) and consideration for elective surgery at an AAA diameter of 5.0 cm. The estimated QALY gain was greater than the reference case analysis (0.00200 vs. 0.00112 QALYs per woman invited). Combined with a greater incremental cost than the reference case, principally due to an increased number of elective operations, the ICER was estimated as £23,000 per QALY gained. The corresponding INMB at a threshold of £20,000 per QALY gained was –£5.08 (95% uncertainty interval –£31.53 to £69.98); especially given the uncertainty in the cost-effectiveness estimate, this does not provide a sufficient basis to initiate such an AAA screening programme in women.
The results show that there is little difference, in terms of cost-effectiveness, if elective operations are considered at 4.5 cm, 5.0 cm or 5.5 cm (except when maintaining screening at age 65 years with a 3.0-cm diagnosis threshold). Lowering the intervention threshold increases both the QALYs gained and the incremental costs, but in almost equal proportions. This near equality in cost-effectiveness perhaps indicates the futility of arguments over which threshold is ‘better’. 7,107
Chapter 9 Discussion and conclusions
Nationwide screening programmes for AAA in 65-year old men in Sweden108 and the UK109 have been successfully introduced. Nevertheless, the death rate from AAAs has been much higher in England than in the USA,110 even though only a minority of eligible men in the USA are screened. 111 The comparison of AAA mortality in England and the USA did not provide sex-specific data, but, if aneurysm death rates are falling in English men, this higher overall mortality rate may imply an even greater disparity in AAA death rates for women between England and the USA. The prevalence of AAAs is much higher in smokers than never smokers. Historically, fewer women than men have smoked and women were also slower to take up smoking than men. Therefore, it is unsurprising that the prevalence of AAAs has been much lower in women than men. Up to now, this has been assumed to imply that screening women for AAA would not be cost-effective. However, women have a higher risk of a small AAA rupture than men, worse outcomes following a ruptured AAA and longer life expectancy; these factors would be favourable for a screening programme. On the other hand, together with the lower prevalence in women, their lower attendance rate in the Chichester trial33 and worse outcomes following elective AAA surgery would be unfavourable for a screening programme. The overall balance in favour or against screening women for AAA has, thus, been unclear.
Moreover, there may be screening options that would improve the cost-effectiveness of population screening for AAAs in women. These include increasing the yield of detected AAAs (by screening at a higher age, or lowering the aortic diameter threshold for defining an AAA in women), reducing the rates of AAA rupture while under surveillance (by lowering the diameter threshold for considering elective surgery) and reducing surveillance costs (by using longer surveillance intervals for the smallest AAAs). Another possibility for reducing AAA deaths in women would be selective screening, for example, of high-risk groups such as smokers, but this approach was not favoured by our PPI group.
The adoption of NAAASP for men was primarily based on the large MASS randomised screening trial,10 together with health economic modelling based on the trial. The MASS trial10 randomised 68,000 men aged 65–74 years and, ultimately, followed them up for an average of 13 years. The largest trial1 in women was much smaller (9300 women randomised with follow-up for 5 years). An adequately powered randomised trial to establish the value of AAA screening in women would have to be substantially larger than the MASS trial10 with similar follow-up. Such a trial would be enormously expensive to undertake, and is unlikely ever to be feasible. Given this, a detailed modelling study is the best way to address the uncertainties around AAA screening in women. Moreover, it provides a way to investigate the relative value of a variety of screening options, which would not be possible within the fixed protocol of a single randomised trial.
Development work
Discrete event simulation model
The DES model developed for this project was novel in a number of respects. First, in contrast to the previous multistate Markov model, it modelled the progress of individuals rather than groups. Aortic diameter expansion could, thus, be more precisely represented, allowing for the substantial heterogeneity between people in growth rates; avoiding the use of arbitrarily defined categories for aortic diameters and the awkward, but necessary, introduction of time-dependent transition rates in the multistate model;35 and better accounting for uncertainty. Moreover, and importantly in the context of investigating AAA screening for women, the modelling structure allowed the investigation of different screening options, which would not be feasible in a single multistate model. The downside of individual modelling is the computational requirements, as enough individuals have to be modelled to ensure that the results obtained are stable and reliable. The computational demands also increase substantially when undertaking PSAs. This problem was ameliorated to an important extent by appreciating that only those with an AAA had to be modelled in detail in order to obtain precise estimates of incremental effects.
The model developed also has potential for further use outside the present work. For example, with parameters reflecting those for men rather than women, it can be used to investigate alternative screening options for men, such as the choice of intervention threshold or the inclusion of subaneurysmal men (aortic diameters of 2.5–2.9 cm) within the surveillance programme. This could suggest ways in which NAAASP could be improved. The model could also be used for investigating selective screening, for example, of women smokers, if sufficiently robust data on the parameters for this group could be obtained. The model also has the potential to be used in other contexts, for example, internationally, by specifying relevant parameter inputs. In practice, the problem is less in adapting the model, but more in the likely unavailability of data on relevant parameters in new contexts and the assumptions that might have to be made.
Prevalence and definition of abdominal aortic aneurysms in women
The systematic review of AAA prevalence was of key importance to this project, but a number of limitations became apparent. First, the number of data in women was rather limited and complicated by studies including different age groups being undertaken in different calendar years (so any secular trends might influence prevalence estimates) and using different screening approaches (population based, self-referred or physician initiated, and free, self-purchased or reimbursed) and ultrasound measurement techniques (inner to inner aortic diameter, outer to outer, leading edge to leading edge or unstated), although nearly all used 3.0-cm diameter as the minimum diameter for definition of an AAA. Second, except for age and smoking status, rather few characteristics of the women invited (or screened) were available (e.g. body size or diabetes mellitus, which may influence baseline arterial diameters and AAA growth rates, respectively). However, it was clear that the prevalence of AAAs was much higher in current smokers. In the future, it is possible that AAA prevalence in women may increase because of the historical increase of smoking in women some decades ago.
The usual definition of an AAA in men is either a widest diameter > 50% greater than the suprarenal aortic diameter or the widely used pragmatic definition of ≥ 3.0 cm. Women have smaller-diameter aortas than men. 21 Most of the screening studies did not hold individual participant data, but these were available from two modestly sized studies (a Swedish study14 of 5140 women aged 70 years and a Danish study of 570 women aged 67 years), which we used to provide an estimate of the AAA diameter distribution in women. Analysis of these data indicated that 2.5 cm might be a better minimum threshold diameter for the definition of an AAA in women. The definition of an AAA in women had considerable impact on both prevalence and cost-effectiveness estimates.
Data sources analysed
As AAAs have traditionally been considered a male-dominated disorder, female-specific data or inputs required for the DES model were not readily available and had to be gathered for this project. Estimates of over 40 female-specific parameters were required (see Tables 1–3). We were fortunate to have access to several databases that could be explored, including those from the RESCAN study,35 EVAR-138 and other trials of EVAR versus open repair in the elective setting, and the IMPROVE trial30 for emergency repairs.
Other female-specific input parameters for the model came from exploration of local data sources (e.g. women dropping out of hospital-based surveillance programmes) or comparative reviews (e.g. proportion of women at any age accepting invitation to screening, and incidental detection rates). In addition, UK registries were explored for data about aneurysm repairs, particularly the NVR database and HES: analysis of such data indicates that EVAR is used preferentially in the older groups and that AAA repair in those aged > 80 years is now common. However, sadly, they showed that the mortality following elective repair in women was unacceptably high, particularly for open repair, for which the figure was 8%. We also obtained international data from the Vascunet collaboration. 112
Based on the individual data in the RESCAN study,35 the AAA rupture rate in women increased by about 30-fold as the AAA diameter increased from 3.0 to 5.5 cm, and the AAA rupture risk was about four-fold higher in women than men at the same AAA diameter. This might naively suggest that an AAA diameter threshold of 4.5 cm for considering elective surgery in women would correspond to the same balance of risks and benefits as the choice of a diameter of 5.5 cm in men. However, because of the worse elective surgery outcomes in women, there is a trade-off that comes into play, and this is reflected in the cost-effectiveness analyses.
The analysis of the individual data for women in the NVR26 was important in a number of respects. First, it substantiated in these recent UK data the overall higher mortalities in women than men for elective operations, both EVAR and open AAA repair, as found in the systematic review. Second, it showed that the proportion of patients actually receiving elective EVAR was lower in women than men, which paralleled the difference in the proportion suitable for EVAR found in the systematic review. Third, it provided reliable estimates of these parameters for emergency surgery for ruptured AAAs, while the literature review undertaken for emergency surgery was less detailed and the data sparser than for elective surgery. Fourth, it allowed the dependence of these parameters on age and AAA diameter for elective operations, and on age for emergency operations, to be modelled. This was especially important when considering screening options that changed the age at screening or intervention threshold; using overall figures in these analyses would have been unrealistic.
In summary, the derivation of the female-specific inputs for the model used a very wide array of sources and resources; it was a major task, taking over 1 year to complete.
Clinical effectiveness
The systematic reviews of elective AAA operations revealed that all relevant parameters were worse for women than men. The proportion anatomically suitable for EVAR was lower in women (a disadvantage as EVAR has a substantially lower operative mortality than open AAA repair), the non-intervention rate was higher (i.e. more women were either turned down for an operation by the multidisciplinary health-care team or refused an operation), and the operative mortalities associated with both EVAR and open AAA repair were higher.
An integral part of screening programmes is the availability of safe treatments to ameliorate the clinical course of the disease being screened for. In the present case, the aim of screening is to prevent the rupture of an AAA, which carries an overall mortality of ≥ 80%. 113 In men, the requirement for a safe treatment is met, since the 30-day mortality from either endovascular or open elective repair is < 1% in NAAASP. 109 These are excellent results, in part driven by a quality improvement programme for elective aneurysm repair in the UK, following the information from the Vascunet collaboration27 that operative mortality rates were higher in the UK than most other countries. Analysis of the Vascunet data112 did not identify sex as being significantly associated with perioperative mortality in either univariate or multivariate analyses. However, in this project three separate sources [namely (1) the systematic reviews, (2) NVR and (3) HES] identified operative mortality after repair of intact aneurysms as being higher in women than men, for both endovascular and open repair.
These results clearly show the particularly high operative mortality after open repair in women, where an elective operation kills about 1 in 12 women. This raises the question of whether or not women should be screened at all if the treatment is associated with such high mortality. There seems little to be gained from the detection of occult disease if the treatment offered is not as safe as it should be. The observed high mortality for AAA repair in women arguably fails to meet the criteria for an effective intervention that is required for the institution of a screening programme in the UK. However, it is notable that perioperative mortality is lower in men with screen-detected AAA than in men with incidentally detected AAA, and the same effect may hold for women. Thus, women with screen-detected AAA may have lower perioperative risk than that presented here, implying that screening should not be ruled out on the basis of perioperative risk alone.
Beyond screening, these findings raise important issues regarding AAA surgery. Given that the systematic review indicated that fewer than half of women evaluated for repair have aortic morphology suitable for EVAR,37 there currently is no safe treatment for more than half of women considered for elective repair. Improving endograft technology, such as low-profile device delivery systems, may allow higher proportions of women to be offered EVAR in the future and go some way to reduce the high non-intervention rate seen in women.
Overall, in the UK, it is unlikely that screening women for AAAs would be considered clinically effective, unless a new quality improvement programme can be successfully implemented to reduce the risks of surgery in women. A quality improvement programme was introduced earlier by the Vascular Society26 to reduce the operative mortality from elective AAA repair and has been successful in reducing the overall mortality to < 3.5%. This programme focused on centre volumes and standardised processes (e.g. use of red blood cell salvage systems) but there was never any attempt to look at sex-specific data. Given that our systematic review showed 30-day operative mortality as being almost double in women compared with men, and that the mortality difference between the sexes persisted in the NVR data97 after adjustment for age and AAA diameter, it might be hypothesised that standard operating processes (e.g. perioperative management of cardiovascular drugs) might have different physiological effects in older men and women. If this were true, the centre volume–outcome relationship might be different for women and men. Some of these considerations are included in the suggestions for further research.
Cost-effectiveness
Base-case and sensitivity analyses
The base-case cost-effectiveness analysis, which adopted the screening options used for men in NAAASP, showed that the estimated life-years gained per woman invited was very small: 0.00285 life-years or 1.04 life-days. A small average life-years gain is expected in population screening, as the vast majority of those invited are screened as normal and have no change in life expectancy. Nevertheless, the extremely small gain in life-years in this base-case analysis is the main reason for the unfavourable cost-effectiveness results. Using standard discounting for both costs and life-years, the ICER per QALY gained was estimated as £31,000. This is above the threshold of £20,000 generally used by NICE as a basis for accepting health interventions for use in the NHS. Moreover, there was considerable uncertainty in this cost-effectiveness estimate: in the PSA, the INMB estimate of –£12.03 (at a threshold of £20,000 per QALY) had a 95% uncertainty interval of –£27.88 to £22.12. The probability that AAA screening is cost-effective at this WTP threshold is < 20%.
The sensitivity analyses undertaken did not change this conclusion. These also underlined the pivotal role of AAA prevalence in determining the ICER. When the AAA prevalence was doubled, from 0.43% to 0.86%, the estimated ICER fell below £20,000 per QALY gained. A prevalence of 0.86% is beyond the upper limit (0.80%) of the 95% CI for women aged 65 years derived from the systematic review. So only an extreme change in prevalence, beyond what is likely to be compatible with the evidence, could lead to a conclusion that AAA screening is cost-effective using standard criteria. However, the cost-effectiveness is also sensitive to the exact shape of the distribution of aortic diameters (not just the prevalence), as shown by replacing NAAASP-based distribution with one based on the Uppsala14 distribution. This emphasises the need for better contemporary data on the distribution of aortic diameters in women at ages relevant to screening.
Screening options in women
Screening women for AAAs might become more cost-effective if one moved away from the options adopted in NAAASP for men. This was the purpose of the scenario analyses undertaken. As expected, screening became more cost-effective if offered at age 70 or 75 years. Perhaps surprisingly, it was more cost-effective at age 75 years than at age 70 years. Thus, the effect of increasing prevalence as age increased, from 0.43% at age 65 years to 0.70% at age 70 years and 1.15% at age 75 years, outweighed the more limited life expectancy remaining at older ages. Nevertheless, we adopted a screening age of 70 years rather than age 75 years in the final combined scenario investigated, for two reasons. First, the estimated non-intervention rate for elective surgery applied in the model was not dependent on age; this rate might become unrealistically low at higher ages as surgeons are likely to turn down many elderly women for elective surgery, especially open AAA repair, for which the risks are substantial. Second, the estimates for attendance at screening and AAA prevalence become more imprecise and potentially unreliable at older ages.
Lowering the threshold for defining an AAA to 2.5 cm, lengthening surveillance intervals somewhat for the smallest AAAs, or lowering the threshold for considering elective surgery to 5.0 or 4.5 cm, made AAA screening for women slightly more cost-effective, but these changes considered individually did not bring the ICER down below £20,000 per QALY gained.
The remaining possibility of finding a cost-effective screening option for women was to combine the alternative screening options. The best one investigated combined invitation to screening at age 70 years, an aortic diameter of 2.5 cm defining an AAA (with a 5-year surveillance interval for AAAs with a diameter of 2.5–2.9 cm), and consideration for elective surgery at an AAA diameter of 5.0 cm. The estimated QALY gain was greater than the base-case analysis (0.00200 vs. 0.00110 per woman invited). Combined with a greater incremental cost than the base case, principally due to an increased number of elective operations, the ICER was estimated as £23,000 per QALY gained. In the PSA, the corresponding INMB estimate of –£5.08, at a threshold of £20,000 per QALY, also had a substantial 95% uncertainty interval of –£31.53 to £69.98.
Conclusion
The conclusion of these analyses is that the accepted criteria for an AAA screening programme in women are not currently met with respect to either clinical effectiveness (low operative mortality rates) or cost-effectiveness. We also did not find a combination of screening options for women that would make population AAA screening cost-effective for the NHS at a WTP threshold of £20,000 per QALY.
Strengths and weaknesses
The study undertaken has a number of strengths. These include the use of individual simulation modelling, allowing evaluation of multiple screening options; modelling aortic diameter as a continuous variable; use of women-specific parameters whenever possible; systematic reviews undertaken for key parameters; and extensive data sources reanalysed, including RESCAN,35 NVR and HES.
The study had some general limitations, including ones shared with many other long-term health economic evaluations. These include the lack of any specific validation of the model for women against empirical data; the problem that some parameters were poorly estimated or not specifically available for women; and the fact that the relevance of some parameter values to current women in the UK was uncertain, this being an uncertainty that is not fully represented by the PSA.
There are also some specific limitations that can be noted. First, not only is AAA prevalence a key parameter, but the exact distribution of aortic diameters among women with an AAA is also important. There was a lack of data on this point. Second, non-AAA mortality was taken to be the same for women with an AAA as for women without an AAA, despite evidence that they may have a higher cardiovascular risk. 114 A higher cardiovascular risk would increase the effects of competing mortality in women for whom screening is most beneficial and, therefore, decrease the value of screening overall. However, modelling would be complex, as the dependence of cardiovascular mortality rates on aortic diameter would have to be estimated, and no obvious data are available for this. Third, the model assumed that once a decision for no elective intervention had been made, this would never be reversed in the future. This could be made more clinically realistic, either in terms of the decision changing as an AAA grew further, or in terms of the potential for intervention on adverse risk factors to reduce the risk of postoperative mortality so that an elective operation could take place later. These subtleties are difficult to model in the absence of relevant reliable data. Fourth, the QoL adjustment adopted just depended on age and was not based on contemporary data. More up-to-date information would have been preferable, QoL adjustments for a short period after surgery could also have been included, and the possible beneficial or deleterious effects on QoL of invitation to screening, and either subsequent reassurance or surveillance, have been ignored. Again, there is a lack of robust quantitative evidence on these points.
Research recommendations
Based on our research, we make the following recommendations for future research in priority order:
-
Undertake a large-scale empirical study of the current attendance rate at screening, AAA prevalence and exact aortic size distribution for women screened at relevant ages. This could include the investigation of whether or not AAA screening, and positive or negative results, influence QoL.
-
Adapt the DES model to evaluate screening options in men, to assess whether or not NAAASP could be improved.
-
Investigate why elective operative mortality for AAAs in women is so high and subsequently introduce any necessary quality improvement programme to lower operative mortality.
-
Make the modelling software program more accessible so that it can be adapted for other contexts, including internationally.
-
Undertake a comprehensive empirical study of current incidental AAA detection rates for women (and also for men).
-
Identify relevant parameter estimates specifically for women smokers, and model the cost-effectiveness of AAA screening in this group.
Acknowledgements
Funding
We have taken advantage of an opportunity to collaborate with Professor Jonathan A Michaels, as he had access to HES data that we could not obtain within the time scale of the project. The HES data were analysed by his team in Sheffield, only tabular data were shared with our research group and cells with fewer than five persons were suppressed (as required by HES to maintain confidentiality). The analysis of mortality and other outcomes and their relation to demographics and provider factors, as required for our project, were already part of the specified analysis for the NIHR programme that Professor Michaels is leading (NIHR Programme Grants for Applied Research project number RP-PG-1210-12009) and will also be included in the final report from that programme.
Study registration
The systematic reviews in Chapter 3 are registered at PROSPERO: CRD42015020444 for AAA prevalence and CRD42016043227 for parameters related to elective AAA operations.
Individual acknowledgements
We are grateful to a number of people who kindly provided input, data or analyses to help us with this project, including:
-
Professor Jonothan Earnshaw and Glenda Turton for data from the Gloucester Aneurysm Screening Programme relating to subaneurysmal aortas and AAA growth in men (see Chapter 2)
-
various individuals who provided extra unpublished details of their studies, contributing to our systematic reviews (see Chapter 3)
-
Professor Jonothan Earnshaw, Jo Jacomelli and Lisa Summers for data on screening, surveillance and referral to surgery for men in NAAASP (see Chapter 4)
-
Professor Martin Björck (Uppsala) for individual data on women screened for AAAs in Sweden (see Chapter 4)
-
Professor Jes Lindholt (Viborg) for individual data on women screened for AAAs in Denmark (see Chapter 4)
-
Professor Ray Ashleigh (Manchester) for data related to incidental AAA detection in women (see Chapter 4)
-
Dr David Sidloff (Leicester) for leading the paper on sex differences in mortality based on the NVR (see Chapter 5)
-
Dr David Epstein (Granada) for analyses of resource use and costs in the EVAR-1 trial38 (see Chapter 6)
-
Dr Manuel Gomes (London) for analyses of resource use and costs in the IMPROVE trial30 (see Chapter 6)
-
the members of the PPI group (see Appendix 1) – Heather Routledge, Alison Coates, Merle Payne, Wendy Saunders, Margaret Houghton, Patricia Eldridge, Angela Hardy, Daphne Baker, Norma Beck, Pat Mills, June McWatt, Patricia Heyden and Patricia Hulme
-
five reviewers and one editor for helpful comments on a previous draft of this report.
Contributions of authors
Simon G Thompson (Professor of Biostatistics) was the principal investigator and project leader.
Matthew J Bown (Professor of Vascular Surgery) was responsible for clinical input, the project website and the PPI group.
Matthew J Glover (Research Fellow in Health Economics) was responsible for costings and health economics input.
Edmund Jones (Research Fellow in Statistics) was responsible for the construction and programming of the DES model.
Katya L Masconi (Research Fellow in Statistics) was responsible for running the DES model.
Jonathan A Michaels (Professor of Clinical Decision Science) was responsible for the analysis of HES data.
Janet T Powell (Professor of Vascular Biology and Medicine) was responsible for clinical input and was the leader of the systematic reviews.
Pinar Ulug (Clinical Trial Manager) undertook the systematic reviews.
Michael J Sweeting (Senior Research Fellow in Statistics) was responsible for the leadership of the statistical and computational components of the project.
Publications
Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG, SWAN collaborative group. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg 2016;103:1097–104.
Sidloff DA, Saratzis A, Sweeting MJ, Michaels J, Powell JT, Thompson SG, Bown MJ. Sex differences in outcomes after AAA repair in the UK. Br J Surg 2017;104:1656–64.
Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT, SWAN collaborators. Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet 2017;389:2482–91.
Glover MJ, Jones E, Masconi KL, Sweeting MJ, Thompson SG; SWAN collaborative group. Discrete event simulation for decision modelling in health care: lessons from abdominal aortic aneurysm screening. Med Decis Mak 2018;38:439–51.
Sweeting MJ, Masconi KL, Jones E, Ulug P, Glover MJ, Michaels JA, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet 2018;392:487–95.
Data-sharing statement
Data used in this project were obtained from multiple sources (as outlined in Tables 1–3). The main sources of individual patient data were from individual investigators for a few studies within the systematic reviews/meta-analyses and for some clinical trials, HES and ONS (death registrations) data sets, and the NVR (vascular surgery operations and outcomes). Individual patient data from these sources cannot be shared further owing to conditions attached to release to the authors. Requests for access to the individual patient data should be submitted to the relevant data provider. All queries should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002;89:283-5. https://doi.org/10.1046/j.0007-1323.2001.02014.x.
- Office for National Statistics (ONS) . Deaths Registered in England and Wales 2015. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2015 (accessed 1 March 2017).
- Sweeting MJ, Thompson SG, Brown LC, Powell JT. RESCAN collaborators . Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655-65. https://doi.org/10.1002/bjs.8707.
- Wanhainen A, Lundkvist J, Bergqvist D, Björck M. Cost-effectiveness of screening women for abdominal aortic aneurysm. J Vasc Surg 2006;43:908-14. https://doi.org/10.1016/j.jvs.2005.12.064.
- LeFevre ML. US Preventive Services Task Force . Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:281-90. http://dx.doi.org/10.7326/M14-1204.
- Björck M, Bown MJ, Choke E, Earnshaw J, Flørenes T, Glover M, et al. International update on screening for abdominal aortic aneurysms: issues and opportunities. Eur J Vasc Endovasc Surg 2015;49:113-15. https://doi.org/10.1016/j.ejvs.2014.08.015.
- Bown MJ, Powell JT. Part two: against the motion. Evidence does not support reducing the threshold diameter to 5 cm for elective interventions in women with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2014;48:614-8. https://doi.org/10.1016/j.ejvs.2014.08.016.
- National Abdominal Aortic Aneurysm Screening Programme . 2015/16/AAA/Screening/Data 2016. http://aaa.screening.nhs.uk/ (accessed 1 March 2017).
- Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD002945.pub2.
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RA, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360:1531-9. https://doi.org/10.1016/S0140-6736(02)11522-4.
- Kim LG, Thompson SG, Briggs AH, Buxton MJ, Campbell HE. How cost-effective is screening for abdominal aortic aneurysms?. J Med Screen 2007;14:46-52. https://doi.org/10.1258/096914107780154477.
- Glover MJ, Kim LG, Sweeting MJ, Thompson SG, Buxton MJ. Cost-effectiveness of the National Health Service Abdominal Aortic Aneurysm Screening Programme in England. Br J Surg 2014;101:976-82. https://doi.org/10.1002/bjs.9528.
- Derubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, Faries PL, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007;46:630-5. https://doi.org/10.1016/j.jvs.2007.06.024.
- Svensjö S, Björck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg 2013;100:367-72. https://doi.org/10.1002/bjs.8984.
- Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population – a meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0081260.
- Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. RESCAN Collaborators . Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 2013;309:806-13. https://doi.org/10.1001/jama.2013.950.
- Egorova NN, Vouyouka AG, McKinsey JF, Faries PL, Kent KC, Moskowitz AJ, et al. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database. J Vasc Surg 2011;54:1-12.e6. https://doi.org/10.1016/j.jvs.2010.12.049.
- Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML. Vascular Study Group of New England . Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013;57:1261-8. https://doi.org/10.1016/j.jvs.2012.11.039.
- IMPROVE Trial Investigators . The effect of aortic morphology on peri-operative mortality of ruptured abdominal aortic aneurysm. Eur Heart J 2015;36:1328-34. http://dx.doi.org/10.1093/eurheartj/ehu521.
- Karthikesalingam A, Nicoli TK, Holt PJ, Hinchliffe RJ, Pasha N, Loftus IM, et al. The fate of patients referred to a specialist vascular unit with large infra-renal abdominal aortic aneurysms over a two-year period. Eur J Vasc Endovasc Surg 2011;42:295-301. https://doi.org/10.1016/j.ejvs.2011.04.022.
- Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, et al. Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol 2013;111:1510-16. https://doi.org/10.1016/j.amjcard.2013.01.306.
- Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. Multicentre Aneurysm Screening Study (MASS) Group . Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg 2012;99:1649-56. https://doi.org/10.1002/bjs.8897.
- De Rango P, Lenti M, Cieri E, Simonte G, Cao P, Richards T, et al. Association between sex and perioperative mortality following endovascular repair for ruptured abdominal aortic aneurysms. J Vasc Surg 2013;57:1684-92. https://doi.org/10.1016/j.jvs.2013.03.040.
- Powell JT, Sweeting MJ, Thompson MM, Ashleigh R, Bell R, Gomes M, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014;348. https://doi.org/10.1136/bmj.f7661.
- Grootenboer N, van Sambeek MR, Arends LR, Hendriks JM, Hunink MG, Bosch JL. Systematic review and meta-analysis of sex differences in outcome after intervention for abdominal aortic aneurysm. Br J Surg 2010;97:1169-79. https://doi.org/10.1002/bjs.7134.
- Vascular Services Quality Improvement Programme . NVR Annual Report 2015. www.vsqip.org.uk/reports/2015-nvr-annual-report/ (accessed 1 March 2017).
- Mitchell D, Venermo M, Mani K, Bjorck M, Troeng T, Debus S, et al. Quality improvement in vascular surgery: the role of comparative audit and Vascunet. Eur J Vasc Endovasc Surg 2015;49:1-3. https://doi.org/10.1016/j.ejvs.2014.08.026.
- NHS Digital . Hospital Episode Statistics 2016. http://content.digital.nhs.uk/hes (accessed 1 March 2017).
- Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. United Kingdom EVAR Trial Investigators . Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. https://doi.org/10.1056/NEJMoa0909305.
- IMPROVE Trial Investigators . Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-9. http://dx.doi.org/10.1093/eurheartj/ehv125.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Centre for Health Economics discussion paper 172. York: Centre for Health Economics, University of York; 1999.
- National Institute for Health Research (NIHR) . INVOLVE Library of Research Projects 1996. www.invo.org.uk/resource-centre/libraries/library-of-research-projects/ (accessed 1 March 2017).
- Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 1995;82:1066-70. https://doi.org/10.1002/bjs.1800820821.
- Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG. SWAN Collaborative Group . Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg 2016;103:1097-104. https://doi.org/10.1002/bjs.10225.
- Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, et al. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17410.
- Khashram M, Jones G, Roake J. Prevalence of abdominal aortic aneurysm (AAA) in a population undergoing computed tomography colonography in Canterbury, New Zealand. Eur J Vasc Endovasc Surg 2015;50:199-205. https://doi.org/10.1016/j.ejvs.2015.04.023.
- Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT. SWAN collaborators . Morphological suitability for endovascular repair, non-intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta-analysis. Lancet 2017;389:2482-91. https://doi.org/10.1016/S0140-6736(17)30639-6.
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators . Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. https://doi.org/10.1016/S0140-6736(16)31135-7.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 1 November 2016).
- Office for National Statistics . National Life Tables, United Kingdom: 2012–2014 2015. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2015-09-23 (accessed 1 November 2016).
- R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2008.
- Sweeting MJ, Masconi KL, Jones E, Ulug P, Glover MJ, Michaels JA, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet 2018;392:487-95. https://doi.org/10.1016/S0140-6736(18)31222-4.
- Davis S, Stevenson M, Tappenden P, Wailoo AJ. NICE DSU Technical Support Document No. 15. London: National Institute of Health and Care Excellence; 2014.
- Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-4. Med Decis Making 2012;32:701-11. http://dx.doi.org/10.1177/0272989x12455462.
- Darwood R, Earnshaw JJ, Turton G, Shaw E, Whyman M, Poskitt K, et al. Twenty-year review of abdominal aortic aneurysm screening in men in the county of Gloucestershire, United Kingdom. J Vasc Surg 2012;56:8-13. https://doi.org/10.1016/j.jvs.2011.12.069.
- Glover MJ, Jones E, Masconi KL, Sweeting MJ, Thompson SG. SWAN collaborative group . Discrete event simulation for decision modelling in health care: lessons from abdominal aortic aneurysm screening. Med Decis Mak 2017;38:439-51. https://doi.org/10.1177/0272989X17753380.
- PRISMA: Transparent Reporting of Systematic Reviews and Meta-analyses . Welcome to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Website n.d. www.prisma-statement.org (accessed 27 February 2017).
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses n.d. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 18 December 2015).
- Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994–2001. Circulation 2009;119:2202-8. https://doi.org/10.1161/CIRCULATIONAHA.108.817619.
- Palombo D, Lucertini G, Pane B, Mazzei R, Spinella G, Brasesco PC. District-based abdominal aortic aneurysm screening in population aged 65 years and older. J Cardiovasc Surg 2010;51:777-82.
- Ogata T, Arrington S, Davis PM, Sam AD, Hollier LH, Tromp G, et al. Community-based, nonprofit organization-sponsored ultrasonography screening program for abdominal aortic aneurysms is effective at identifying occult aneurysms. Ann Vasc Surg 2006;20:312-16. https://doi.org/10.1007/s10016-006-9056-5.
- Hupp JA, Martin JD, Hansen LO. Results of a single center vascular screening and education program. J Vasc Surg 2007;46:182-7. https://doi.org/10.1016/j.jvs.2007.04.042.
- Bulbulia R, Chabok M, Aslam M, Lewington S, Sherliker P, Manganaro A, et al. The prevalence of abdominal aortic aneurysm, carotid stenosis, peripheral arterial disease and atrial fibrillation among 280,000 screened British and Irish Adults. Vasc Med 2013;18.
- Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013;61:1736-43. https://doi.org/10.1016/j.jacc.2013.01.054.
- Jahangir E, Lipworth L, Edwards TL, Kabagambe EK, Mumma MT, Mensah GA, et al. Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18,782 persons aged above 65 years in the Southern Community Cohort Study. J Epidemiol Community Health 2015;69:481-8. https://doi.org/10.1136/jech-2014-204920.
- Kristmundsson T, Sonesson B, Dias N, Malina M, Resch T. Anatomic suitability for endovascular repair of abdominal aortic aneurysms and possible benefits of low profile delivery systems. Vascular 2014;22:112-15. https://doi.org/10.1177/1708538112473980.
- Hultgren R, Vishnevskaya L, Wahlgren CM. Women with abdominal aortic aneurysms have more extensive aortic neck pathology. Ann Vasc Surg 2013;27:547-52. https://doi.org/10.1016/j.avsg.2012.05.025.
- Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg 2011;54:931-7. https://doi.org/10.1016/j.jvs.2011.02.054.
- Park KH, Lim C, Lee JH, Yoo JS. Suitability of endovascular repair with current stent grafts for abdominal aortic aneurysm in Korean patients. J Korean Med Sci 2011;26:1047-51. https://doi.org/10.3346/jkms.2011.26.8.1047.
- Moise MA, Woo EY, Velazquez OC, Fairman RM, Golden MA, Mitchell ME, et al. Barriers to endovascular aortic aneurysm repair: past experience and implications for future device development. Vasc Endovascular Surg 2006;40:197-203. https://doi.org/10.1177/153857440604000304.
- Karthikesalingam A, Cobb RJ, Khoury A, Choke EC, Sayers RD, Holt PJ, et al. The morphological applicability of a novel endovascular aneurysm sealing (EVAS) system (Nellix) in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2013;46:440-5. https://doi.org/10.1016/j.ejvs.2013.06.017.
- Morrison TM, Yan X, Abel DB, Fairman RM, Glickman MH, Fillinger MF. Population-based study of age and gender effects in aneurysm anatomy. J Vasc Surg 2012;55. https://doi.org/10.1016/j.jvs.2012.03.096.
- Morrison T, Fillinger M, Meyer C, Abel D, Yan XS. Characterization of Human Aortic Anatomy Project (CHAP): Gender Disparities in Endovascular Treatment Options for Infrarenal Abdominal Aortic Aneurysms n.d. www.fda.gov/downloads/MedicalDevices/NewsEvents/WorkshopsConferences/UCM359044.pdf (accessed 1 March 2016).
- Scott SW, Batchelder AJ, Kirkbride D, Naylor AR, Thompson JP. Late survival in nonoperated patients with infrarenal abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2016;52:444-9. https://doi.org/10.1016/j.ejvs.2016.05.008.
- Gorst S, Drury D, Singh S, Cuschieri RJ, Tan PS, Macierewicz JA, et al. Outcomes for patients who do not undergo repair of their large aneurysms. Br J Surg 2012;99.
- Whittaker JD, Meecham L, Jennings A, Wall M, Newman J. Outcome After Turndown for Elective and Emergency Abdominal Aortic Aneurysm Surgery n.d.
- Nevidomskyte D, Shalhub S, Singh N, Farokhi E, Meissner MH. Influence of gender on abdominal aortic aneurysm repair in the community. Ann Vasc Surg 2017;39:128-36. https://doi.org/10.1016/j.avsg.2016.06.012.
- Ramanan B, Gupta PK, Sundaram A, Gupta H, Johanning JM, Lynch TG, et al. Development of a risk index for prediction of mortality after open aortic aneurysm repair. J Vasc Surg 2013;58:871-8. https://doi.org/10.1016/j.jvs.2013.03.024.
- Mani K, Björck M, Wanhainen A. Changes in the management of infrarenal abdominal aortic aneurysm disease in Sweden. Br J Surg 2013;100:638-44. https://doi.org/10.1002/bjs.9046.
- Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PS, Kreienberg PB, et al. Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg 2012;55:906-13. https://doi.org/10.1016/j.jvs.2011.11.047.
- Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O’Malley AJ, Cotterill P, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995–2008: a retrospective observational study. Ann Surg 2012;256:651-8. https://doi.org/10.1097/SLA.0b013e31826b4f91.
- Chung C, Tadros R, Torres M, Malik R, Ellozy S, Faries P, et al. Evolution of gender-related differences in outcomes from two decades of endovascular aneurysm repair. J Vasc Surg 2015;61:843-52. https://doi.org/10.1016/j.jvs.2014.11.006.
- Powell JT, Sweeting MJ, Ulug P, Blankensteijn JD, Lederle FA, Becquemin JP, et al. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br J Surg 2017;104:166-78. https://doi.org/10.1002/bjs.10430.
- Desai M, Choke E, Sayers RD, Nath M, Bown MJ. Sex-related trends in mortality after elective abdominal aortic aneurysm surgery between 2002 and 2013 at National Health Service hospitals in England: less benefit for women compared with men. Eur Heart J 2016;37:3452-60. https://doi.org/10.1093/eurheartj/ehw335.
- Lowry D, Singh J, Mytton J, Tiwari A. Sex-related outcome inequalities in endovascular aneurysm repair. Eur J Vasc Endovasc Surg 2016;52:518-25. https://doi.org/10.1016/j.ejvs.2016.07.083.
- Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group . Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005;352:2398-405. https://doi.org/10.1056/NEJMoa051255.
- Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT, Kohler TR, et al. OVER Veterans Affairs Cooperative Study Group . Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012;367:1988-97. https://doi.org/10.1056/NEJMoa1207481.
- Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P, et al. ACE trialists . A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low-to-moderate-risk patients. J Vasc Surg 2011;53:1167-73. https://doi.org/10.1016/j.jvs.2010.10.124.
- Heller JA, Weinberg A, Arons R, Krishnasastry KV, Lyon RT, Deitch JS, et al. Two decades of abdominal aortic aneurysm repair: have we made any progress?. J Vasc Surg 2000;32:1091-100. https://doi.org/10.1067/mva.2000.111691.
- Anjum A, von Allmen R, Greenhalgh R, Powell JT. Explaining the decrease in mortality from abdominal aortic aneurysm rupture. Br J Surg 2012;99:637-45. https://doi.org/10.1002/bjs.8698.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England, Wales and Scotland. Eur J Vasc Endovasc Surg 2012;43:161-6. https://doi.org/10.1016/j.ejvs.2011.11.014.
- Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet 2014;383:963-9. https://doi.org/10.1016/S0140-6736(14)60109-4.
- Reite A, Søreide K, Ellingsen CL, Kvaløy JT, Vetrhus M. Epidemiology of ruptured abdominal aortic aneurysms in a well-defined Norwegian population with trends in incidence, intervention rate, and mortality. J Vasc Surg 2015;61:1168-74. https://doi.org/10.1016/j.jvs.2014.12.054.
- Reimerink JJ, Hoornweg LL, Vahl AC, Wisselink W, van den Broek TA, Legemate DA, et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg 2013;258:248-56. https://doi.org/10.1097/SLA.0b013e31828d4b76.
- Desgranges P, Kobeiter H, Katsahian S, Boufi M, Gouny P, Favre J-P, et al. ECAR (Endovasculaire ou Chirurgie dans les Anévrysmes aorto-iliaques Rompus): a French randomized controlled trial of endovascular vs. open surgical repair of ruptured aorto-iliac aneurysms. Eur J Vasc Endovasc Surg 2015;50:303-10. https://doi.org/10.1016/j.ejvs.2015.03.028.
- Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT. Ruptured Aneurysm Trialists . Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg 2015;102:1229-39. https://doi.org/10.1002/bjs.9852.
- van Beek SC, Vahl AC, Wisselink W, Balm R. Amsterdam Acute Aneurysm Trial Collaborators . Fate of patients unwilling or unsuitable to undergo surgical intervention for a ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2015;49:163-5. https://doi.org/10.1016/j.ejvs.2014.10.007.
- Robinson WP, Schanzer A, Li Y, Goodney PP, Nolan BW, Eslami MH, et al. Derivation and validation of a practical risk score for prediction of mortality after open repair of ruptured abdominal aortic aneurysms in a US regional cohort and comparison to existing socring systems. J Vasc Surg 2013;57:354-61. https://doi.org/10.1016/j.jvs.2012.08.120.
- von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 2011;40:712-18. https://doi.org/10.1093/ije/dyr008.
- The Australian Bowel Cancer Screening Pilot Program and Beyond: Final Evaluation Report. Canberra, ACT: Australian Government Department of Health and Ageing; 2005.
- Deutekom M, van Rijn AF, Dekker E, Blaauwgeers H, Stronks K, Fockens P, et al. Uptake of faecal occult blood test colorectal cancer screening by different ethnic groups in the Netherlands. Eur J Public Health 2009;19:400-2. https://doi.org/10.1093/eurpub/ckp051.
- Lo SH, Waller J, Wardle J, von Wagner C. Comparing barriers to colorectal cancer screening with barriers to breast and cervical screening: a population-based survey of screening-age women in Great Britain. J Med Screen 2013;20:73-9. https://doi.org/10.1177/0969141313492508.
- Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118-23. https://doi.org/10.1161/CIRCULATIONAHA.111.030379.
- Kim LG, Thompson SG, Marteau TM, Scott RA. Multicentre Aneurysm Screening Study Group . Screening for abdominal aortic aneurysms: the effects of age and social deprivation on screening uptake, prevalence and attendance at follow-up in the MASS trial. J Med Screen 2004;11:50-3. https://doi.org/10.1177/096914130301100112.
- Singh K, Jacobsen BK, Solberg S, Kumar S, Arnesen E. The difference between ultrasound and computed tomography (CT) measurements of aortic diameter increases with aortic diameter: analysis of axial images of abdominal aortic and common iliac artery diameter in normal and aneurysmal aortas. The Tromsø Study, 1994–1995. Eur J Vasc Endovasc Surg 2004;28:158-67. https://doi.org/10.1016/j.ejvs.2004.03.018.
- Powell JT, Brown LC. The long-term results of the UK EVAR trials: the sting in the tail. Eur J Vasc Endovasc Surg 2010;40:44-6. https://doi.org/10.1016/j.ejvs.2010.04.020.
- Sidloff DA, Saratzis A, Sweeting MJ, Michaels J, Powell JT, Thompson SG, et al. Sex differences in mortality after abdominal aortic aneurysm repair in the UK. Br J Surg 2017;104:1656-64. https://doi.org/10.1002/bjs.10600.
- Multicentre Aneurysm Screening Study Group . Multicentre Aneurysm Screening Study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7373.1135.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16090.
- National Institute for Health and Care Excellence . Endovascular Stent–Grafts For The Treatment Of Abdominal Aortic Aneurysms 2009. www.nice.org.uk/guidance/ta167 (accessed 1 November 2016).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015 2015. www.pssru.ac.uk/project-pages/unit-costs/2015/ (accessed 1 November 2016).
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. https://doi.org/10.1136/bmj.316.7133.736.
- Coughlin PA, Jackson D, White AD, Bailey MA, Farrow C, Scott DJ, et al. Meta-analysis of prospective trials determining the short- and mid-term effect of elective open and endovascular repair of abdominal aortic aneurysms on quality of life. Br J Surg 2013;100:448-55. https://doi.org/10.1002/bjs.9018.
- Office for National Statistics . Deaths Registered in England and Wales (Series DR) 2016. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables (accessed 1 November 2016).
- Wanhainen A, Björck M, Boman K, Rutegård J, Bergqvist D. Influence of diagnostic criteria on the prevalence of abdominal aortic aneurysm. J Vasc Surg 2001;34:229-35. https://doi.org/10.1067/mva.2001.115801.
- Wild JB, Stather PW, Biancari F, Choke EC, Earnshaw JJ, Grant SW, et al. A multicentre observational study of the outcomes of screening detected sub-aneurysmal aortic dilatation. Eur J Vasc Endovasc Surg 2013;45:128-34. https://doi.org/10.1016/j.ejvs.2012.11.024.
- Vavra AK, Kibbe MR. Part one: for the motion. Evidence supports reducing the threshold diameter to 5 cm for elective interventions in women with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2014;48:611-14. https://doi.org/10.1016/j.ejvs.2014.08.014.
- Wanhainen A, Hultgren R, Linné A, Holst J, Gottsäter A, Langenskiöld M, et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation 2016;134:1141-8. https://doi.org/10.1161/CIRCULATIONAHA.116.022305.
- Jacomelli J, Summers L, Stevenson A, Lees T, Earnshaw JJ. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br J Surg 2016;103:1125-31. https://doi.org/10.1002/bjs.10173.
- Karthikesalingam A, Vidal-Diez A, Holt PJ, Loftus IM, Schermerhorn ML, Soden PA, et al. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med 2016;375:2051-9. https://doi.org/10.1056/NEJMoa1600931.
- Mell MW, Hlatky MA, Shreibati JB, Dalman RL, Baker LC. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg 2013;57:1519-23. https://doi.org/10.1016/j.jvs.2012.12.034.
- Mani K, Venermo M, Beiles B, Menyhei G, Altreuther M, Loftus I, et al. Regional differences in case mix and peri-operative outcome after elective abdominal aortic aneurysm repair in the Vascunet database. Eur J Vasc Endovasc Surg 2015;49:646-52. https://doi.org/10.1016/j.ejvs.2015.01.021.
- Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg 2013;100:1405-13. https://doi.org/10.1002/bjs.9235.
- Brady AR, Fowkes FG, Thompson SG, Powell JT. Aortic aneurysm diameter and risk of cardiovascular mortality. Arterioscler Thromb Vasc Biol 2001;21:1203-7. https://doi.org/10.1161/hq0701.091999.
- Oliver-Williams C, Sweeting MJ, Turton G, Parkin D, Cooper D, Rodd C, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg 2018;105:68-74. https://doi.org/10.1002/bjs.10715.
Appendix 1 Patient and public involvement
Introduction
The aims of our PPI activities in this study were to (1) establish a group of women to provide input into this specific project through reviewing project activities and aiding with the dissemination of project outputs and (2) ensure that this group became a resource for future research in this area. We chose to use traditional methods of PPI recruitment and supplement this with the use of social media to enhance PPI group recruitment, as this approach had proven successful in previous work within the NIHR Leicester Cardiovascular Biomedical Research Unit.
Generation of a subject-specific patient and public involvement group
Some of the key unknowns surrounding AAA screening for women relate to the acceptability of screening and likelihood of attending for screening. Although we had access to an existing PPI group with the NIHR Leicester Cardiovascular Biomedical Research Unit, this forum was deemed unsuitable for the purposes of this project as the majority of the members were male and, because the group had been established for nearly 8 years, many of the members were ‘lay experts’ whose opinions may not represent those of women to be invited for AAA screening. Therefore, we set out to recruit a new, research-naive, group of women.
Recruitment via abdominal aortic aneurysm patient forum
The Leicester Vascular Surgery Unit and the Leicester NAAASP run an annual patient education forum for men with small AAAs. The aim of this group is to provide information and advice to men with small AAAs and their partners in order to prepare them for the clinical decision-making process around surgery, and to provide general health advice. As part of this group, in June 2015, the issue of whether or not to screen women for AAAs was specifically discussed with the 42 men in attendance. All these men and their partners were asked if they would be willing to attend a meeting to discuss the issue of AAA screening for women in more detail. Four men and two women subsequently attended a PPI meeting in July 2015 (see Patient and public involvement meeting 24 July 2015).
Recruitment via media
Because of the poor representation of women in the group recruited via our male patient forum, it was decided that a more representative PPI group should be recruited. Initial efforts were based on social media (website, micro-blogging applications, Biomedical Research Unit newsletters) but generated no responses. Feedback from our existing Biomedical Research Unit PPI group and the PPI group recruited via our patient forum indicated that this was likely to be due to the limited use of social media in the target group. To address this, it was decided to engage traditional media outlets in a call for recruitment. Through direct contact with the Leicester British Broadcasting Corporation (BBC) office, the project was featured on the regional television news (BBC East Midlands Today) and on local radio (BBC Radio Leicester). The radio interview (two parts) is available on the project website (www.screeningaaawomen.com/; accessed 1 March 2017). In response to these activities, expressions of interest were received from 15 women, 11 of whom subsequently attended and formed the ongoing PPI group for the project.
Patient and public involvement meetings
Patient and public involvement meeting 24 July 2015
The initial PPI meeting for the project was held on 24 July 2015, facilitated by Matthew J Bown. The PPI group at this time consisted of the four men and two women recruited from the Leicester AAA patient forum. One of the women was the wife of one of the men, but otherwise the group members were unrelated.
At this meeting, a presentation was given to those attending detailing the background to the project. This included the evidence for screening in men and why this evidence was not available for women. Broad strategies regarding how this evidence may be gathered were discussed, including how to determine if screening in women would be cost-effective. The concept of asking lay people to assist with the research project was introduced, using the example of asking how women might respond to being asked to attend for screening.
The group were specifically asked the following questions:
-
Whether or not women would want to attend screening for AAAs?
-
Is it acceptable to invite only women in high-risk groups?
-
Do women want to be screened?
-
What would prevent them from attending?
-
Would women want an operation to correct an AAA if it was offered, bearing in mind that the operation is more risky in women than in men?
It was acknowledged by the group that only two women were present and all members had personal knowledge of the disease. This could lead to the answers not being generalisable to the wider population of women.
Following a general discussion on all of these points, the group felt that women were more health conscious and may be more inclined to go for screening if offered. However, there were also mixed feelings on whether or not women would want to be screened given that they have previously undergone other forms of screening (they might be ‘fed up’ with screening). Some felt that the detection of an AAA by screening may cause much anxiety among women, possibly because of the higher risks of treatment (surgery). The group stated that people generally do not want to know too much about their health, especially when there may not be a simple treatment for a condition detected by screening.
The group was also asked to consider:
-
What would the public want us to find out from this research project?
-
Is saving lives all that matters?
-
Should financial, personal, or psychological cost be a consideration in AAA screening?
There was a suggestion from the group that screening might be better added to well woman general practitioner (GP) visits (and health checks for men). Overall, the group felt that if providing screening saved lives at minimal psychological cost, it would be worthwhile. Financial and personal costs were not thought to be an issue (but the group stated that this was because AAA screening is so cheap and easy).
The question was raised regarding what is happening in other countries and why can we not simply see if their programmes work. It was pointed out that the UK is the only country with a national programme for men and there are not programmes for women anywhere.
Attendees were asked how they find out information on their health in general and AAAs in particular. Everyone agreed that websites were of some use, as was e-mail, but not many people in this age bracket would use social media to find out health information.
The general feeling was that there is no media coverage of AAAs and, until you are asked to go for a screening or are diagnosed with an AAA, you do not know what an aneurysm is. It was suggested that this be addressed by local media coverage (e.g. local radio or television, church groups, local newspapers).
Patient and public involvement meeting 12 January 2016
This meeting was the first meeting of the project-specific PPI group that had been recruited through the radio and television engagement programme. Eleven women attended the meeting, none of whom had previously been diagnosed with an AAA. Age information was not formally collected. One woman had a strong family history of AAAs (two first-degree relatives) and one woman’s husband had previously undergone an AAA repair. The majority (nine women) had family members who had been affected by AAAs.
The same information was presented to this group as had been presented to the previously convened PPI group and the group were asked the same set of questions.
The majority of the group thought that women would want to attend for AAA screening if invited. However, they recognised that this may be a biased response as they all had an interest in this area owing to having affected family members. The group thought that women were generally more accepting of screening because they had been used to being screening for other diseases during their lives. They noted that for the majority of those women screened the reassurance of a negative scan was very important and well worth the financial cost of screening and the cost to the individual.
The concept of screening only high-risk groups, using the example of tobacco smoking, was the most contentious area of discussion. Overall, the group thought that this would be unacceptable to the majority of women, largely because they knew of anecdotal cases in which women had been diagnosed with an AAA despite being non-smokers. Other high-risk groups, such as those with a family history of disease, were discussed. The group were surprised that the NHS does not record family history of diseases in any systematic manner and that this would be unavailable as a method to select women for screening.
The women in attendance were asked about age groups likely to attend for screening. It was proposed that women would probably be invited for screening between the ages of 65 and 75 years. The group wanted screening after age 75 years to still be done if the participant wanted this, although they did recognise that this may not be financially viable.
Barriers to screening were discussed. The group thought the location of screening (especially given the current model of community screening) was unlikely to have a negative influence on attendance. One barrier to screening was the lack of knowledge among NHS staff of the possibility of AAAs in women and that a reluctance of NHS staff to refer for screening, or to exclude women from screening, may have a negative effect on uptake.
The group were asked about whether or not they would want to undergo AAA repair if this were indicated, particularly with the knowledge that women have higher perioperative risk than men. The women thought that, providing the overall risks were considered, most women would want to undergo AAA repair. The effect of age on perioperative risk was raised by members of the group, who also suggested that older women may not want screening as they would not want to know or undergo surgery if diagnosed with an AAA.
Given the failure to engage a suitable group of women for PPI via social media, the use of social media for PPI and for patient information was discussed. The groups acknowledged that very few people in this age bracket would use social media to find out health information. There was an acknowledgement that public awareness of AAAs needs to be improved. The group identified the common scenario in which individuals know nothing about AAAs until the time of diagnosis. The best way of communicating with the likely target groups was thought to be through face-to-face meetings, traditional media (local radio/television) and community groups such as churches.
Patient and public involvement meeting 15 August 2016
At the time this meeting was held, the project was in a data gathering/processing phase and minimal PPI relevant updates had been made to project outputs. As one of the aims of the PPI activities for the project was to set up a PPI group specific to screening women for AAAs, this meeting was used to develop the discussion in this area. In order to provide a framework for this discussion, the main focus of the meeting was to discuss a separate study, the Female Aneurysm screening STudy (FAST). FAST is a NIHR Research for Patient Benefit (RfPB)-funded pilot of AAA screening for high-risk women with the main aims of determining screening attendance and disease prevalence.
The FAST was presented to the group. In brief, FAST will be based in Leicestershire, Rutland and parts of Northamptonshire, and will replicate the male screening programme processes. It will use the same invitation process and information sheets as the male programme. Women will be invited based on their risk of an AAA, with smokers, ex-smokers and those with a history of coronary artery disease forming the three groups being assessed. Information on whom to invite will be taken from GP records, but these records do not show women who have a first-degree relative with an AAA. However, accuracy of GP records will need to be ascertained, as extracting data from GP records is costly.
The group thought FAST represented the next step in establishing an evidence base for AAA screening in women, but were disappointed that the study was focused on high-risk groups. They felt that, as AAA screening was simple and cheap, it should be offered more widely. The group reiterated the positive psychological effects of a negative scan.
Patient and public involvement meeting 20 March 2017
In preparation for this final meeting, the main issues arising from the previous meetings were summarised. These were surrounding the acceptability of AAA screening, the positive effects of a negative screen and targeted screening. The following themes formed the basis for discussion:
-
The PPI group’s perception of AAA screening is that it is highly acceptable and there is a good likelihood that the uptake among women would be at least equivalent to that seen in men, or higher. Very few physical or logistical barriers to the uptake of screening exist, but the lack of public knowledge regarding AAAs is a significant area of need that should be addressed, preferably through traditional media outlets.
-
One of the main positive aspects of screening for AAAs in women may be the psychological benefit of a negative screening scan. Although no objective evidence for this effect exists (and, therefore, is a potential avenue for future investigation), our PPI group felt that this was important. Furthermore, one of the unifying motivations for the women to attend the PPI group was that they all wanted to be screened for AAA themselves to obtain this reassurance.
-
Targeted screening, particularly if the target group is smokers, is a contentious issue for the public. Given the simplicity and low cost of AAA screening and positive psychological effect of a negative screen, our PPI activities suggest that there is a public perception that there is no requirement to target screening. Key to improving public understanding of AAA screening and/or the acceptability of targeted screening at high-risk groups will be to provide information regarding the scale of AAA screening and the resultant effect this has on the overall cost, and cost-effectiveness of AAA screening.
Preliminary discussion of these themes did not raise any additional points. Following this discussion, the group was presented with the Plain English summary of the project results. The group provided feedback on the presentation of results and edited the Plain English summary (these edits are incorporated into the final version presented in this report). The group thought that the individual costs and the overall costs of screening were important. Comparison of costs for screening with other common NHS interventions would provide a good reference point for the public. After giving consent, all members present underwent an aortic screening scan to get direct experience of the procedure.
Following discussion of the project’s results and having undergone screening, the three themes were discussed again. In addition, the group were asked to give opinions on what future research should be performed and what further information the public would want.
All three themes were confirmed by the PPI group. Given the project’s results presented, some women present thought that targeted screening may be better than no screening at all for women but questioned the methods for identification of high-risk groups. Many of the women in the PPI group had an interest in AAA because of a family history or personal knowledge of disease. There was a majority view that screening based on family history of disease would be important. The group recognised the deficiencies in clinical systems for recording this type of data. The suggestion was made that when an AAA is diagnosed, NHS information systems should alert the relatives of the patient. The issues of confidentiality preventing such a process were discussed. The PPI group felt that alternatives may be to (1) improve public awareness of the increased risk associated with family history of AAAs and (2) provide information for patients with an AAA to encourage them to tell their relatives of the diagnosis. The importance of improved QoL was deemed to be extremely important for the women present. The group thought that the positive effects of a negative screening scan should be investigated as a research priority going forward.
Summary
The PPI activities for the project achieved their primary aims. A research-naive group of women were successfully recruited into a new PPI group and became fully engaged with the project. Direct and continual involvement of the PPI group over the course of the project was maintained, including input into the Plain English summary and prioritisation of future research. The group has now been established for the future and is already contributing to the FAST, a NIHR RfPB-funded project focused on AAA screening in women.
Appendix 2 Additional details for Chapter 2
Details of parameter estimates
Parameter estimates used in the 4-year validation model and 30-year contemporary model for men, and distributions used for a PSA, are shown in Table 27.
| Parameter | 4-year model | 30-year contemporary model | |||
|---|---|---|---|---|---|
| Estimate | Source | Estimate | PSA distribution | Source | |
| Baseline diameter distribution | N/A | NAAASP8 | N/A | N/A | NAAASP8 |
| Prevalence: Pr ≥ 3.0 cm at baseline | 0.0497 | MASS11 | 0.0134 | N/A | NAAASP8 |
| Growth model parameters | |||||
| β0 (log-cm) | 1.272 | MASS11 | 1.272 | Multivariate normala | MASS12 |
| β1 (log-cm per year) | 0.058 | 0.058 | |||
| σ0 | 0.176 | 0.176 | |||
| σ1 | 0.036 | 0.036 | |||
| ρ | 0.426 | 0.426 | |||
| σw | 0.075 | 0.075 | |||
| Rupture model parameters | |||||
| γ | –16.263 | MASS11 | –16.263 | Bivariate normalb | MASS12 |
| α | 7.210 | 7.210 | |||
| Probabilities | |||||
| Require reinvitation | 0.136 | MASS11 | 0.136 | Beta(4602,29237) | MASS12 |
| Attend screening | 0.802 | MASS11 | 0.750 | Beta(93170,31022) | NAAASP8 |
| Non-visualisation of aorta | 0.0121 | MASS11 | 0.0121 | Beta(329,26818) | MASS12 |
| Non-intervention (contraindicated) | 0.135 | MASS11 | 0.125 | Beta(69,481) | MASS12 |
| Proportion receiving elective open vs. EVAR | 1 | MASS11 | 0.298 | N/A | NVR26 |
| Elective open operative mortality | 0.0373 (0.0992c) | MASS11 | 0.0411 | Beta(24,560) | EVAR-138 |
| Elective EVAR operative mortality | N/A | N/A | 0.0161 | Beta(10,612) | EVAR-138 |
| Emergency surgery after rupture | 0.441 | MASS11 | 0.368 | Beta(193,331) | MASS12 |
| Emergency open operative mortality | 0.356 | MASS11 | 0.342 | Beta(66,127) | MASS12 |
| Rates per person-year | |||||
| Rate of postoperative AAA deaths following elective open | N/A | N/A | 0.0007 | Gamma(3,0.00023) | EVAR-138 |
| Rate of postoperative AAA deaths following elective EVAR | N/A | N/A | 0.0077 | Gamma(34,0.00023) | EVAR-138 |
| Dropout from surveillance | 0.082 | MASS11 | 0.057 | Gamma(330,0.00017) | MASS12 |
| Incidental detection | 0.0755 | MASS11 | 0.0459 | –4 log(1 – X) where X∼Beta(19.56,1695.95) | Glover et al.12 |
| Non-AAA death after contraindication | 0.234 | MASS11 | 0.247 | Gamma(41,0.006) | MASS12 |
| Non-AAA death | Age specific | MASS11 | Age specific | N/A | ONS2 |
| Other | |||||
| µCT (cm) | 0.2443 | RESCAN35 | 0.2443 | N/A | RESCAN35 |
| σCT | 0.190 | Singh et al.95 | 0.190 | N/A | Singh et al.95 |
| Delay from large AAA scan to consultation (days) | 71 | MASS11 | 71 | N/A | MASS12 |
| Delay from consultation to elective surgery | 59 | MASS11 | 59 | N/A | MASS12 |
Issues related to shrinkage in aortic growth estimates
The DES model has embedded within it a continuous-time linear mixed effects (LME) growth model to allow individual aortic diameters to grow over time. Parameter estimates for this model are obtained from suitable data sources (e.g. the MASS trial11,12 when validating the model in men). The LME model parameter estimates (such as the average log-diameter at baseline, β0, and the average rate of growth on the log-diameter scale, β1) are relevant to the AAA data used to fit the model. The MASS data used for the validation model in men are restricted to individuals whose initial diameter is in the range 3.0–5.5 cm. 98
The DES model first samples individual observed baseline diameters, γi0, from a relevant population distribution (e.g. NAAASP) and then samples individual true baseline log-diameters, bi0, and rates of growth, bi1, conditional on the observed diameter. The individual true baseline log-diameters can either be directly set to the observed log-diameter (a non-shrunken estimate) or they can be drawn from a conditional normal distribution based on their posterior distribution (a shrunken estimate). In the latter case, the true diameter is, on average, shrunk towards the population mean baseline diameter estimated from the LME model, which will be towards a diameter of > 3.0 cm (for example, the average baseline diameter in the MASS98 AAA population used to fit the LME model described in Chapter 2, Modelling aortic growth and abdominal aortic aneurysm rupture, is 3.6 cm). The degree of shrinkage increases for observed baseline diameters that are further away from the population mean diameter, and so will affect diameters measured < 2.5 cm more than those that are in the diameter range 3.0–4.4 cm.
Validation of growth model against 4-year Multicentre Aneurysm Screening Study data
The consequences of using shrunken or non-shrunken baseline diameters in the DES are not entirely obvious, and investigations of these revealed advantages and disadvantages of both. Based on 105 pairs of individuals, a DES using non-shrunken baseline diameters resulted in poor 4-year validation performance (Table 28). This occurs because the DES model and the LME model are now discordant, with the LME model accounting for shrinkage in its parameter estimates whereas the DES simulation does not. This results in estimated growth rates (and consequently rupture rates) for diameters observed above the aneurysmal mean (3.6 cm) being too high. If shrunken estimates are used, then the 4-year validation results look more reasonable. A third DES model that was investigated shrinks baseline diameters that measure ≥ 3.0 cm at baseline but not those that measure < 3.0 cm. This model is seen to perform better than the non-shrunken model and only one key event (number of men contraindicated who are screen detected) has an E/O ratio of > ± 20%. The reason this third model was investigated is described next [see Validation of growth model in subaneurysmal (2.6–2.9 cm) diameters].
| Event | E/O ratio (% of MASS98) | ||
|---|---|---|---|
| Non-shrunken estimates | Shrunken estimates | Shrunken estimates if γi0 ≥ 3.0Non-shrunken estimates if γi0 < 3.0 | |
| No screening invitation | |||
| Elective operation | 106 | 98 | 106 |
| Emergency operation | 142 | 111 | 109 |
| Rupture | 136 | 114 | 106 |
| AAA death | 124 | 108 | 102 |
| Non-AAA death | 99 | 99 | 99 |
| Invited to screening | |||
| Elective operation | |||
| Resulting from screen detection | 121 | 113 | 118 |
| Resulting from incidental detection | 98 | 87 | 93 |
| Emergency operation | 109 | 109 | 102 |
| Rupture | 107 | 105 | 96 |
| Contraindicated | |||
| Resulting from screen detection | 116 | 131 | 143 |
| AAA death | 106 | 100 | 96 |
| Non-AAA death | 101 | 101 | 100 |
Validation of growth model in subaneurysmal (2.6–2.9 cm) diameters
The next step was to investigate how different variations of the AAA growth model affected the growth rates of individuals who have subaneurysmal diameters (2.6–2.9 cm) at baseline, for whom model extrapolated estimates are used. Data from the Gloucestershire surveillance study115 were available on 1233 individuals with aortic diameters of 2.6–2.9 cm at screening. Figure 15 shows the cumulative incidence of these individuals progressing to AAAs (diameter of ≥ 3.0 cm) over a 15-year period. Superimposed on the plot are estimates of the number reaching the diagnosis threshold from the three DES models: (1) using shrunken estimates of baseline diameters, (2) using non-shrunken estimates and (3) using shrunken estimates only for baseline diameters measuring ≥ 3.0 cm and non-shrunken estimates otherwise. The model using shrunken estimates can clearly be seen to overestimate the number of subaneurysmal individuals who progress to the diagnosis threshold within the first 10 years, with the upwards shrinkage particularly evident at screening, where > 20% are already presumed to be above the threshold. Meanwhile, the models with no shrinkage (either for AAAs or aortic diameters measuring < 3.0 cm) give a much better fit to the Gloucestershire data. 115 Owing to the poor 4-year validation results of the model that uses non-shrunken estimates throughout, it was decided to progress with the model that only shrinks baseline diameters that measure ≥ 3.0 cm.
FIGURE 15.
Progression of subaneurysmal (2.6–2.9 cm at baseline) individuals to the diagnosis threshold of 3.0 cm over a 30-year time horizon, and comparison with data from the Gloucestershire study. 115
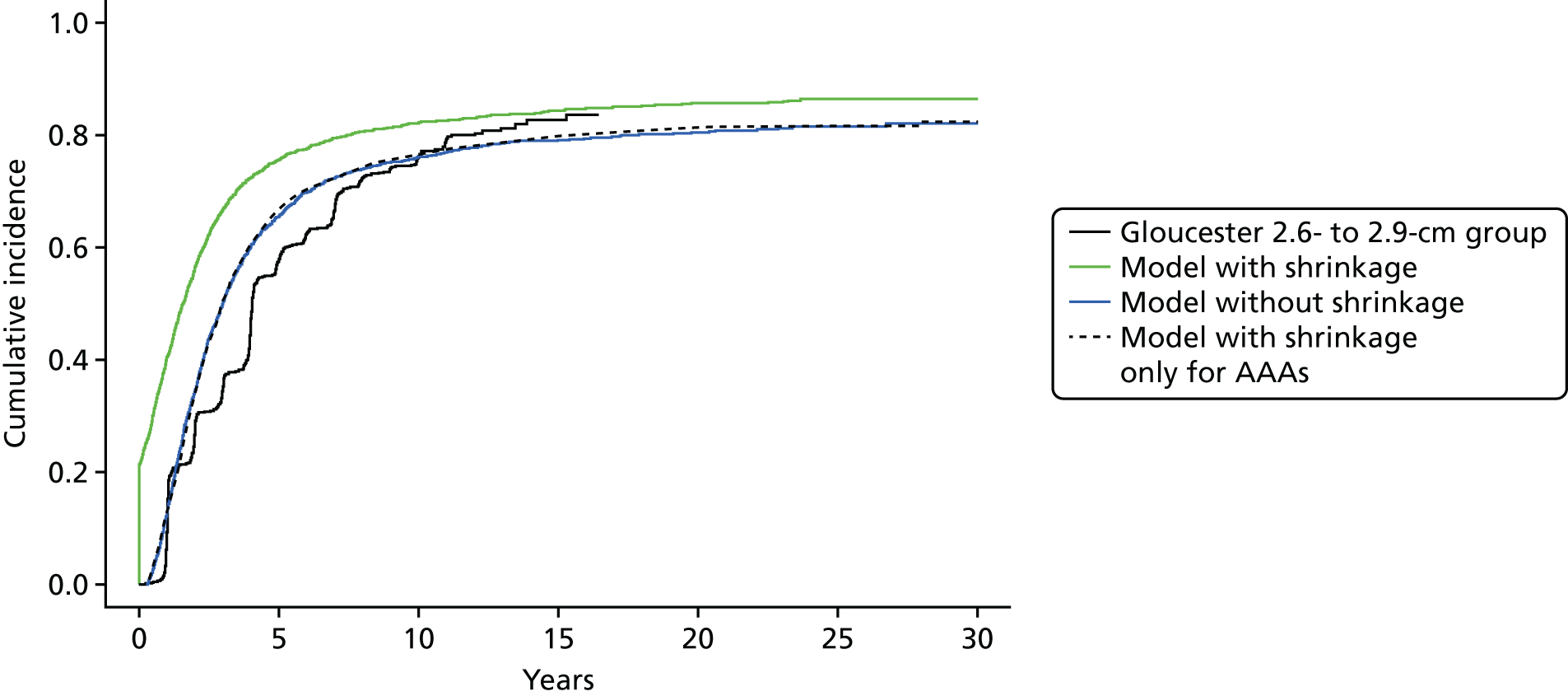
Validation of growth model in all screened normal individuals
A further consideration is the growth of aortic diameters for all screened normal individuals (measuring < 3.0 cm). Although no direct evidence regarding the progression of these individuals exists, the rupture rates in screened normal individuals in the MASS trial12 can be used to provide a comparison against the outputs of the DES model. Figure 16 shows the empirical rupture rates from the MASS trial12 alongside those estimated from two DES models: (1) a DES model with non-shrunken estimates < 3.0 cm with growth allowed < 2.0 cm and (2) a DES model with non-shrunken estimates < 3.0 cm with no growth allowed < 2.0 cm. The model that does not allow aortic diameters to grow < 2.0 cm gives a better fit to the rupture rates seen in the MASS trial. 12 The use of this model is further supported by the cumulative incidence of those screened as normal progressing to the diagnosis threshold of 3.0 cm (both diagnosed and undiagnosed AAAs) shown in Figure 17. The model that allows growth < 2.0 cm estimates that 19% of all 65-year-old men will have an AAA within their lifetimes, compared with 10% using the model that limits growth. This latter estimate appears to be more reasonable based on prevalence estimates seen in the literature.
FIGURE 16.
Rupture rates in those screened normal and compared with MASS trial10 data. Two DES models are investigated: (1) with growth allowed < 2.0 cm; and (2) with no growth allowed < 2.0 cm.
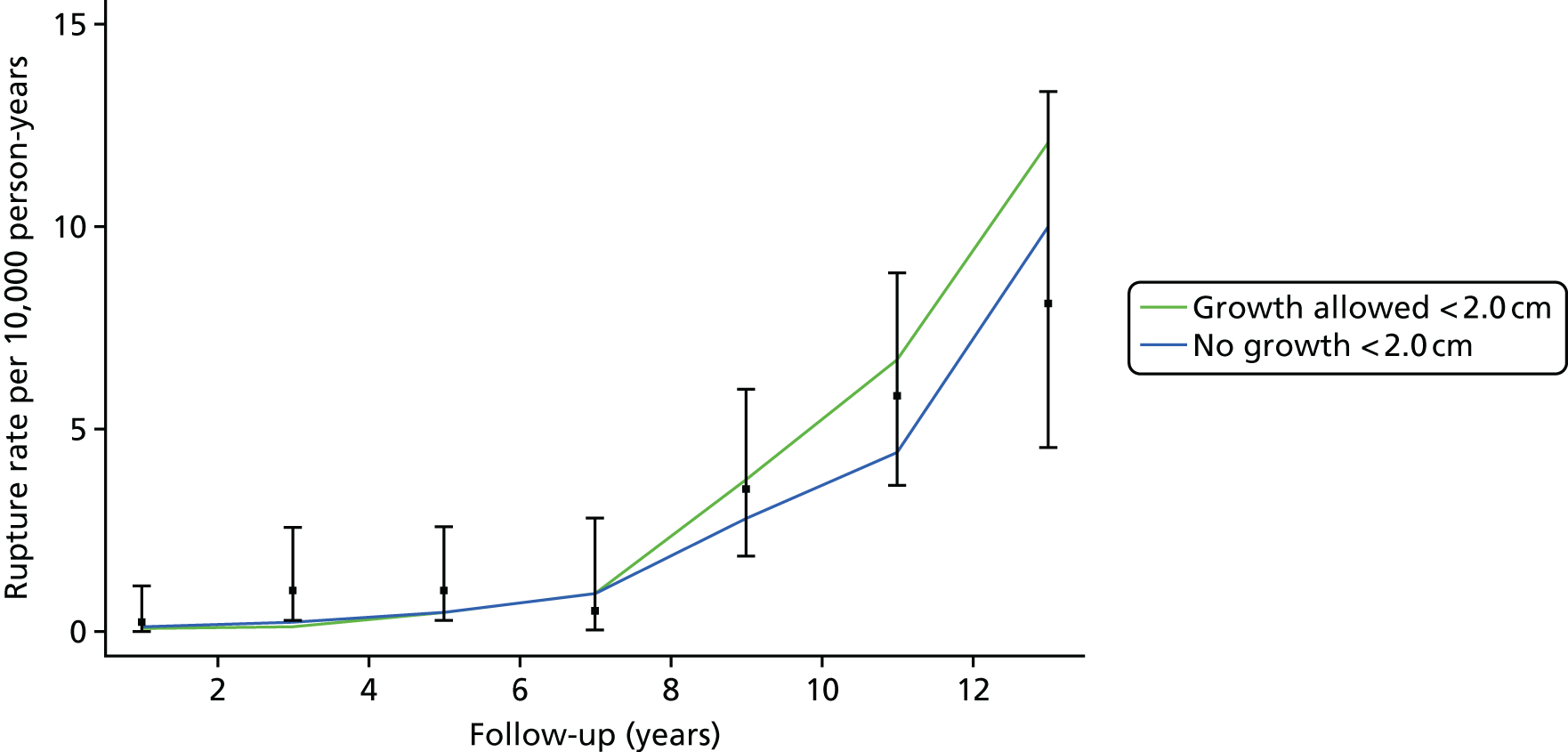
FIGURE 17.
Cumulative incidence of progressing to ≥ 3.0 cm for screened normal individuals using a DES model with and without growth for individuals who initially measure < 2.0 cm.
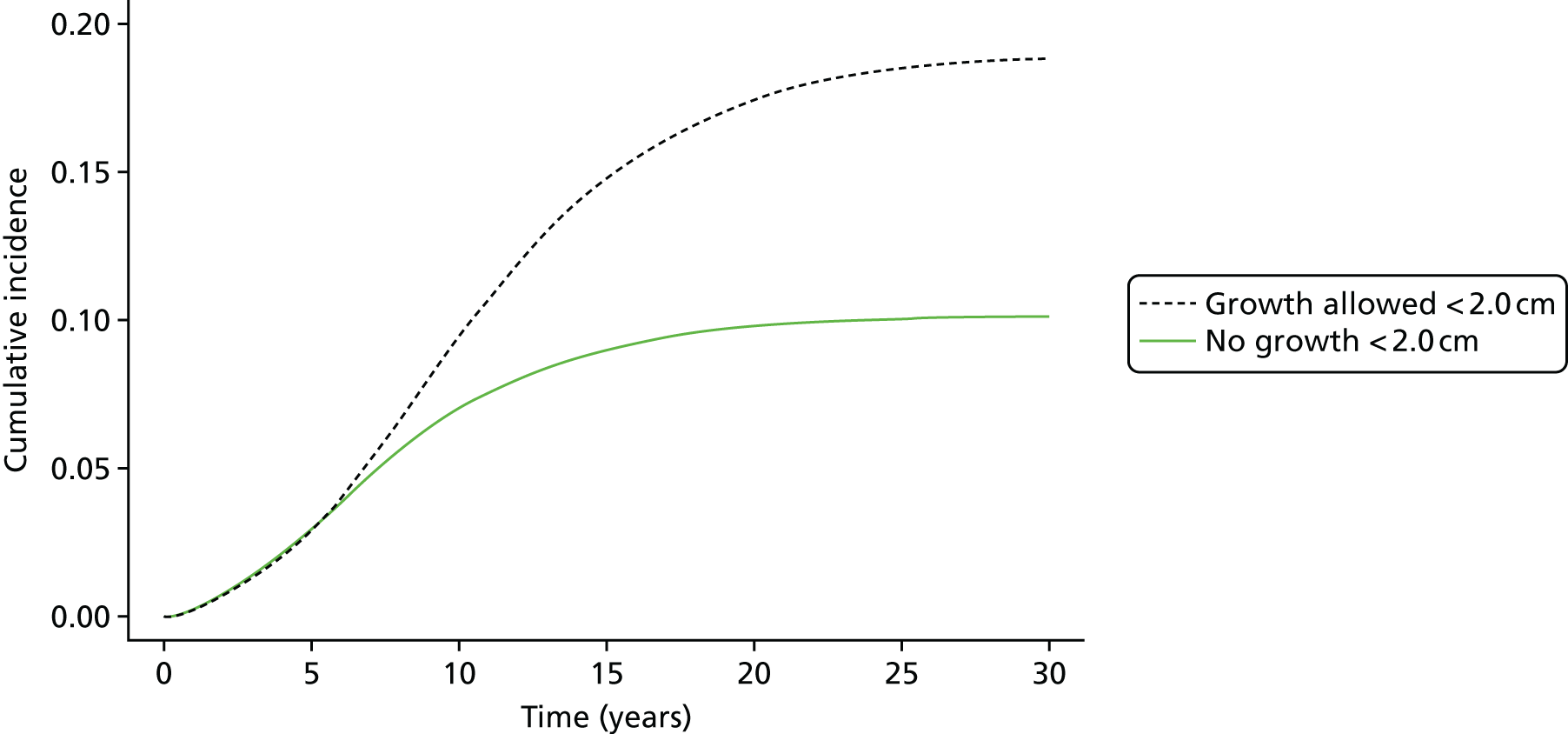
Convergence of the incremental cost-effectiveness ratio using selective versus non-selective sampling
As described in Chapter 2, Convergence, accurate estimates of incremental effects and costs and the derived ICER and INMB can be obtained by selectively sampling only individuals above the diagnosis threshold. Figure 18 shows how well this strategy performs. The green line shows the cumulative mean ICER for a DES model run on 10 million pairs of individuals (using input parameters for women as described in Chapter 7). Even after 10 million pairs of individuals, the ICER has not converged sufficiently to suggest accuracy of more than approximately £5000 per QALY. Conversely, the DES model that samples only individuals above the diagnosis threshold (3.0 cm in this case) converges to within £1000 per QALY after only 1 million pairs of individuals. Therefore, the decision was taken to run all models described in Chapters 7 and 8 for 10 million pairs of individuals using the selective sampling approach to get accurate estimates of incremental effects and costs. When conducting the PSA, it was considered that 500,000 pairs of individuals for each PSA iteration would be sufficient based on the trace plot shown in Figure 18.
FIGURE 18.
Convergence of the ICER in a DES with (black line) and without (green line) selective sampling. The selective sampling model is run for a total of 1 million pairs of individuals while the no selective sampling model is run for 10 million pairs of individuals.

Appendix 3 Additional figures and tables for Chapter 3
FIGURE 19.
Prevalence review: PRISMA flow chart.
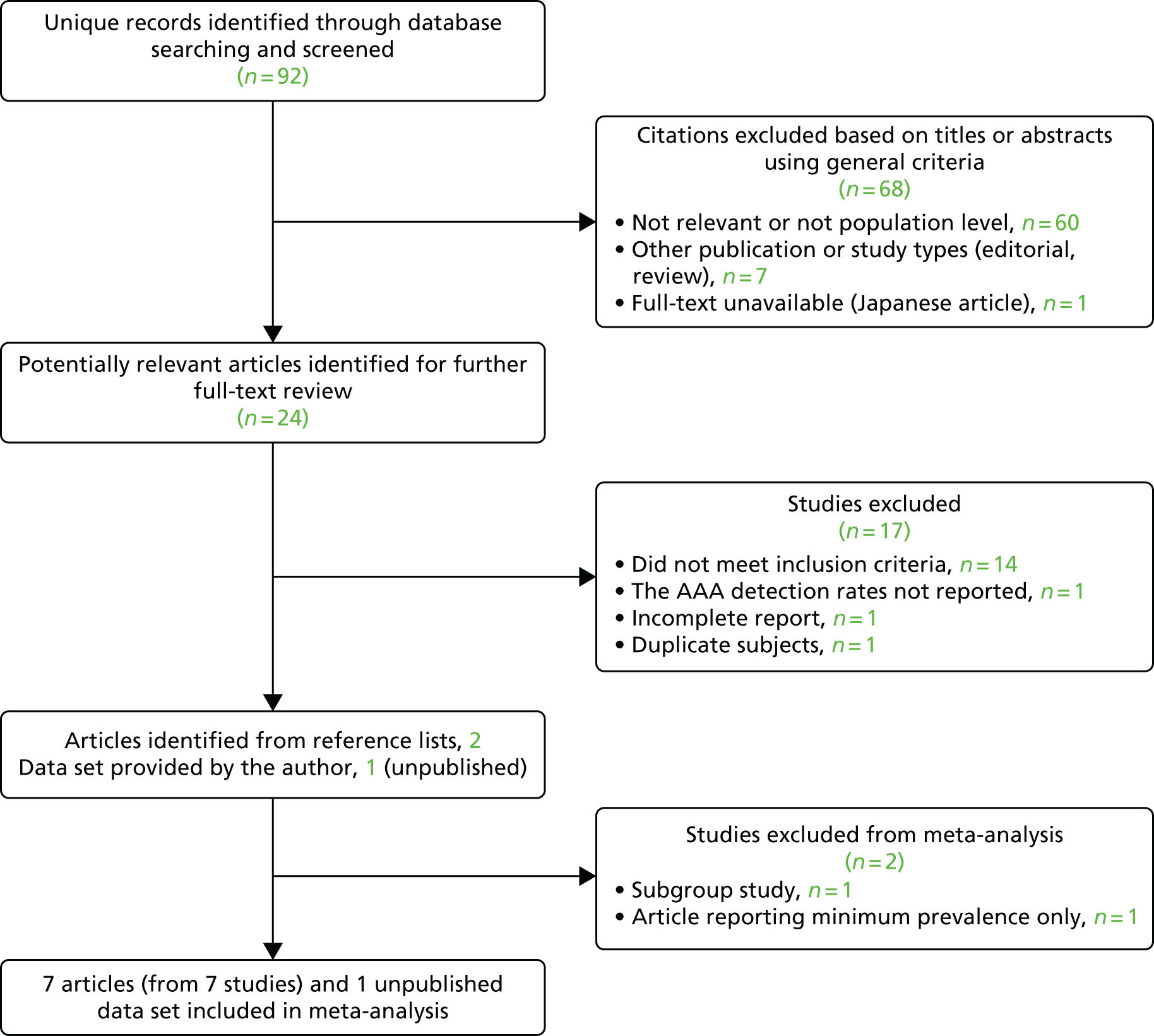
FIGURE 20.
Endovascular aneurysm repair suitability review: PRISMA flow chart.
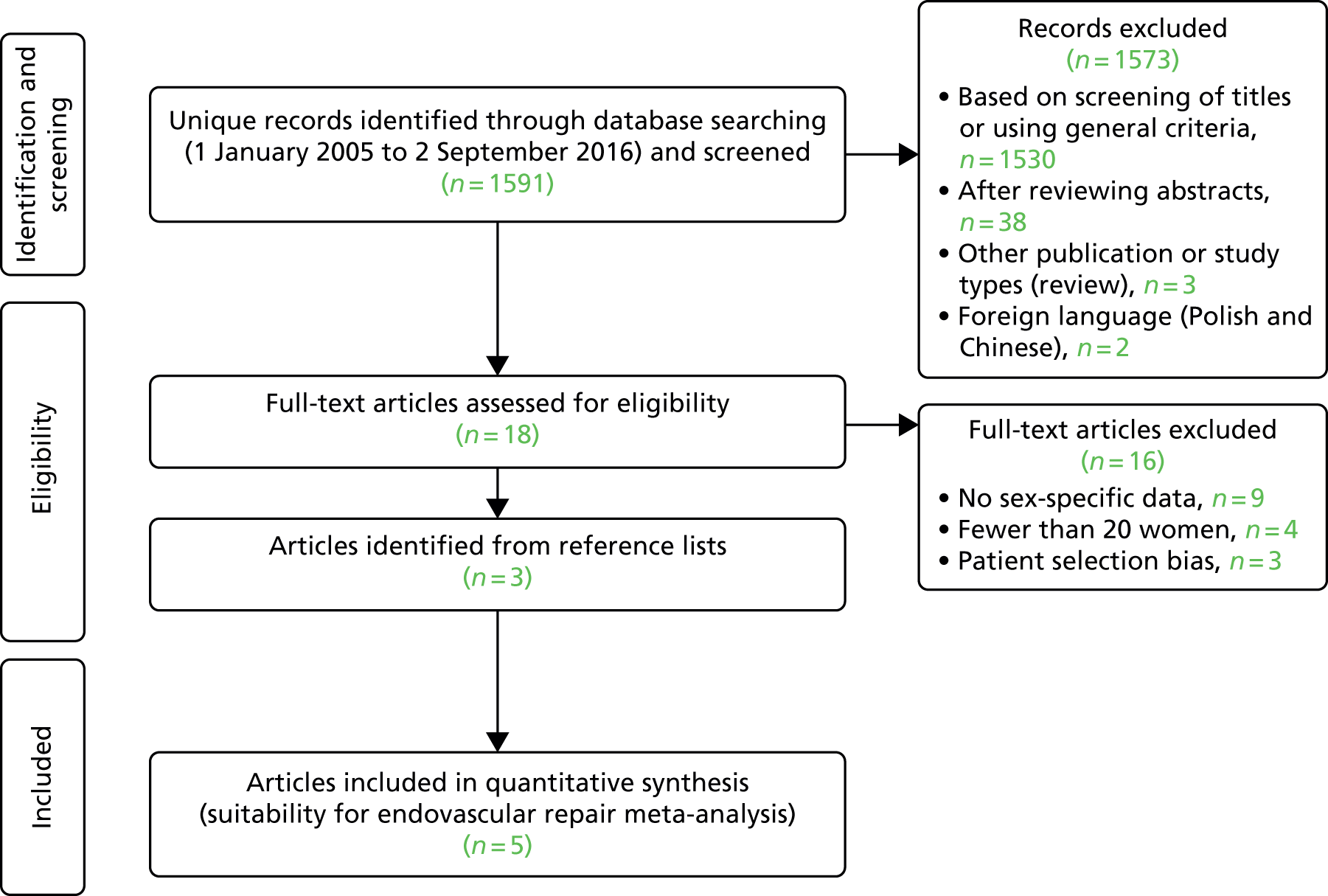
FIGURE 21.
Non-intervention review: PRISMA flow chart. a, One publication65 only available in abstract form.

FIGURE 22.
Thirty-day operative mortality review: PRISMA flow chart.

FIGURE 23.
Prevalence of AAAs in women aged ≥ 60 years: eight studies with screening performed between 2001 and 2012.

FIGURE 24.
Prevalence of AAAs in women aged ≥ 60 years by smoking status. Derubertis et al. 13 reported data on smoking status in a subgroup of patients from the study by Savji et al. ;54 the analysis included 10,012 women, with a mean age of 69 years, with at least one cardiovascular risk factor screened between 2004 and 2006.
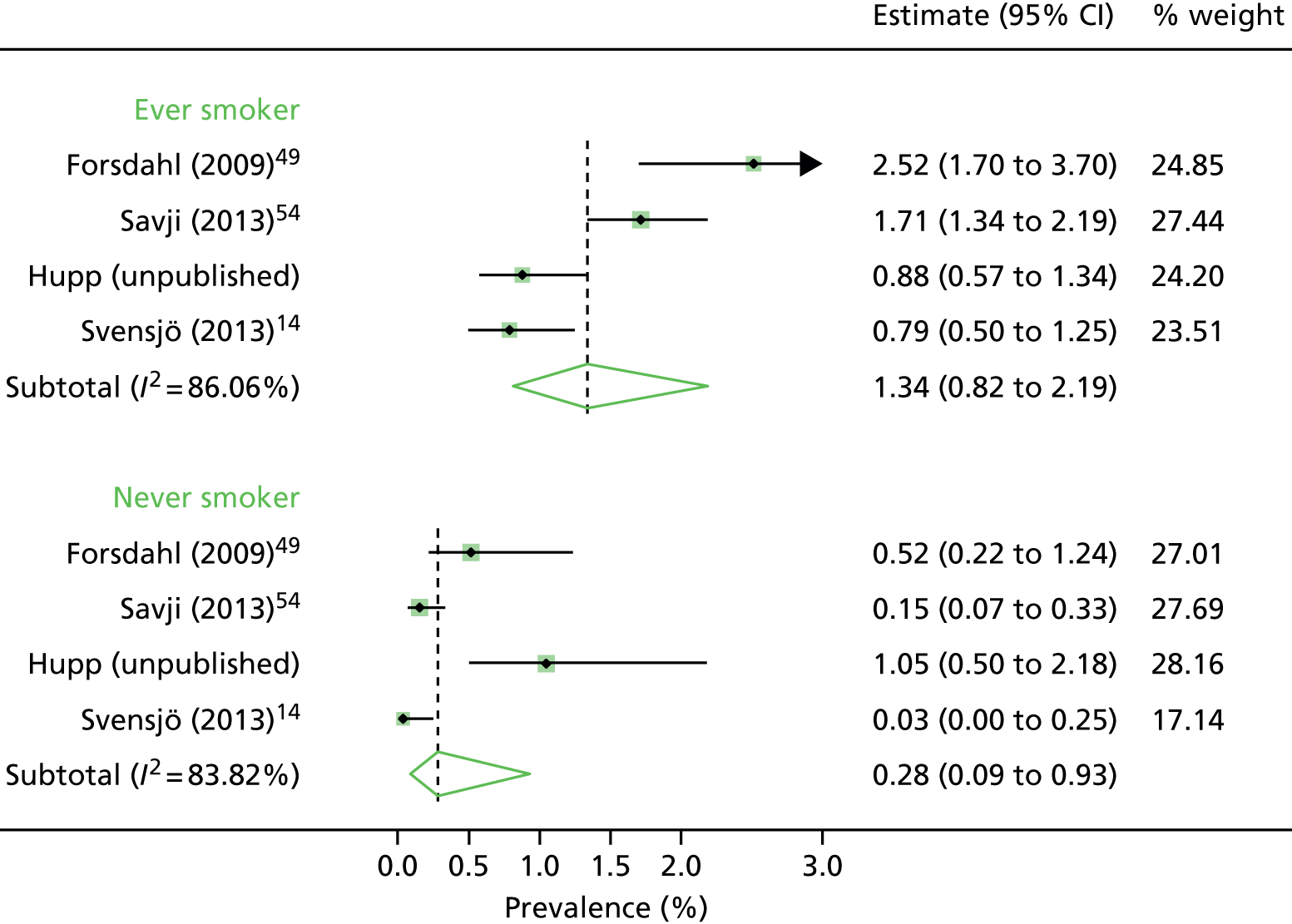
ti,ab(prevalence OR incidence OR occurence OR frequency)
ti,ab(screening)
MESH.EXACT(“Aortic Aneurysm, Abdominal”)
EMB.EXACT(“abdominal aorta aneurysm”)
ti,ab(abdom[*6] near/5 aort[*2] near/5 (aneurysm[*1] or aneurism[*1]))
MESH.EXACT(“Female”) OR MESH.EXACT.EXPLODE(“Women”) OR MESH.EXACT.EXPLODE(“Women’s Health”)
EMB.EXACT.EXPLODE(“female”) OR EMB.EXACT(“women’s health”)
ti,ab(female or females or woman or women)
MESH.EXACT(“Sex Factors”) OR MESH.EXACT(“Sex Distribution”) OR MESH.EXACT(“Sex Ratio”) OR MESH.EXACT(“Sex Characteristics”)
EMB.EXACT(“sex difference”) OR EMB.EXACT(“gender and sex”) OR EMB.EXACT(“gender”) OR EMB.EXACT(“sex ratio”)
ti,ab(gender or genders or sex)
Limits: start 1 January 2000; end 13 January 2016.
MESH.EXACT.EXPLODE(“Endovascular Procedures”) OR MESH.EXACT.EXPLODE(“Stents”) OR MESH.EXACT(“Vascular Surgical Procedures”) OR MESH.EXACT(“Blood Vessel Prosthesis”) OR MESH.EXACT(“Blood Vessel Prosthesis Implantation”) OR MESH.EXACT(“Vascular Grafting”)
EMB.EXACT(“endovascular aneurysm repair”) OR EMB.EXACT(“aortic aneurysm endovascular graft”) OR EMB.EXACT(“endovascular surgery”) OR EMB.EXACT.EXPLODE(“stent”) OR EMB.EXACT.EXPLODE(“blood vessel graft”) OR EMB.EXACT(“endoprosthesis”) OR EMB.EXACT.EXPLODE(“vascular stent”) OR EMB.EXACT(“aneurysm surgery”) OR EMB.EXACT(“vascular surgery”) OR EMB.EXACT.EXPLODE(“blood vessel prosthesis”) OR EMB.EXACT.EXPLODE(“blood vessel transplantation”)
ti,ab(endovascular or endostent[*4] or stent[*4] or evar or fevar or pevar or endoprosthe[*4] or endograft[*4] or graft[*4])
incraft or palmaz or zenith or dynalink or hemobahn or luminex* or memotherm or wallstent or viabahn or nitinol or intracoil or tantalum or powerlink or talent or excluder or aorfix or endologix or anaconda or triascular or cordis or endurant or quantum or aneurx or ancure or ankura or “e vita” or “e xl” or “endomed endofit” or fortron or hercules or lifepath or ovation or treovance or ventana or nellix
MESH.EXACT(“Aortic Aneurysm, Abdominal”)
EMB.EXACT(“abdominal aorta aneurysm”)
ti,ab(abdom[*6] near/5 aort[*2] near/5 (aneurysm[*1] or aneurism[*1]))
ti,ab(aaa or aaas or iaaa or iaaas)
ti,ab(abdom[*6] near/5 aort[*2] near/5 (balloon[*3] or dilat[*6] or bulg[*4] or expan[*6]))
MESH.EXACT(“Female”) OR MESH.EXACT.EXPLODE(“Women”) OR MESH.EXACT.EXPLODE(“Women’s Health”)
EMB.EXACT.EXPLODE(“female”) OR EMB.EXACT(“women’s health”)
ti,ab(female or females or woman or women)
MESH.EXACT(“Sex Factors”) OR MESH.EXACT(“Sex Distribution”) OR MESH.EXACT(“Sex Ratio”) OR MESH.EXACT(“Sex Characteristics”)
EMB.EXACT(“sex difference”) OR EMB.EXACT(“gender and sex”) OR EMB.EXACT(“gender”) OR EMB.EXACT(“sex ratio”)
ti,ab(gender or genders or sex)
MESH(ah) OR MESH(anatom[*6]) OR MESH(morpholog[*6]) OR MESH.EXACT(“Iliac Artery”) OR MESH(calcification)
EMB(anatom[*6]) OR EMB(morpholog[*6]) OR EMB.EXACT.EXPLODE(“pathological anatomy”) OR EMB.EXACT(“neck circumference”) OR EMB.EXACT(“artery diameter”) OR EMB.EXACT(“blood vessel diameter”) OR EMB.EXACT.EXPLODE(“iliac artery”) OR EMB.EXACT(“artery calcification”) OR EMB.EXACT(“calcification”) OR EMB.EXACT(“blood vessel calcification”)
ti,ab(anatom[*6] or morpholog[*6] or diameter[*1] or circumference[*1] or size[*1] or calcif[*8] or angle[*1] or angulat[4] or tortuous or tortuosit[*3] or calibre[*1] or calibre[*1] or “access vessel[*1]” or “iliac arter[*3]” or “ileal arter[*3]” or “ilial arter[*3]” or aortoiliac or “aorto iliac”)
ti,ab(neck[*2] near/5 (aneurysm[*2] or aneurism[*2] or infrarenal or “infra renal” or aortic or proximal or short or shorten[*2] or favourable or unfavourable or challenging or length[*1] or shape[*1] or hostile)) or ti,ab(funnel or conical)
ti,ab(“instructions for use” or ifu or ifus)
Limits: start 1 January 2005; end 2 September 2016.
MESH.EXACT.EXPLODE(“Endovascular Procedures”) OR MESH.EXACT.EXPLODE(“Stents”) OR MESH.EXACT(“Vascular Surgical Procedures”) OR MESH.EXACT(“Blood Vessel Prosthesis”) OR MESH.EXACT(“Blood Vessel Prosthesis Implantation”) OR MESH.EXACT(“Vascular Grafting”)
EMB.EXACT.EXPLODE(“blood vessel prosthesis”) OR (EMB.EXACT(“aorta graft”) OR EMB.EXACT(“blood vessel transplantation”)) OR repair OR (endovascular surgery) OR (EMB.EXACT(“endovascular aneurysm repair”) OR EMB.EXACT.EXPLODE(“aortic aneurysm endovascular graft”) OR EMB.EXACT.EXPLODE(“endovascular surgery”)) OR (open surgery)
MESH.EXACT(“Aortic Aneurysm, Abdominal”)
EMB.EXACT(“abdominal aorta aneurysm”)
ti,ab(abdom[*6] near/5 aort[*2] near/5 (aneurysm[*1] or aneurism[*1]))
ti,ab(aaa or aaas or iaaa or iaaas)
ti,ab(abdom[*6] near/5 aort[*2] near/5 (balloon[*3] or dilat[*6] or bulg[*4] or expan[*6]))
MESH.EXACT(“Female”) OR MESH.EXACT.EXPLODE(“Women”) OR MESH.EXACT.EXPLODE(“Women’s Health”)
EMB.EXACT.EXPLODE(“female”) OR EMB.EXACT(“women’s health”)
ti,ab(female or females or woman or women)
MESH.EXACT(“Sex Factors”) OR MESH.EXACT(“Sex Distribution”) OR MESH.EXACT(“Sex Ratio”) OR MESH.EXACT(“Sex Characteristics”)
EMB.EXACT(“sex difference”) OR EMB.EXACT(“gender and sex”) OR EMB.EXACT(“gender”) OR EMB.EXACT(“sex ratio”)
ti,ab(gender or genders or sex)
(treatment refusal) OR (MESH.EXACT(“Refusal to Treat”)) OR (MESH.EXACT(“Patient Selection”))
COMORBIDITY AND MESH.EXACT(“Comorbidity”) OR (MESH.EXACT(“Risk Factors”)) AND (MESH.EXACT(“Risk Assessment”))
(MESH.EXACT(“Elective Surgical Procedures”)) or ti,ab(“elective”)
(ti,ab(“treatment refusal” or “undergo treatment”)) OR (MESH.EXACT(“Refusal to Treat”)) OR (MESH.EXACT(“Patient Selection”)) OR (“turn down” or “turndown”) OR (MESH.EXACT(“Palliative Care”)) OR palliat[*3] OR (ti,ab(“nonoperated” or “non-operated”))
Limits: start 1 January 2005; end 2 September 2016.
MESH.EXACT.EXPLODE(“Aortic Aneurysm, Abdominal”) OR (abdominal aort*) AND aneurysm*
MESH.EXACT(“Blood Vessel Prosthesis”) OR MESH.EXACT(“Blood Vessel Prosthesis Implantation”) OR MESH.EXACT(“Vascular Grafting”) OR repair OR (endovascular surgery) OR (open surgery) OR MESH.EXACT(“Aortic Aneurysm, Abdominal -- surgery”)
MESH.EXACT(“Aortic Aneurysm, Abdominal -- mortality”) OR MESH.EXACT(“Aortic Aneurysm, Abdominal -- complications”) OR MESH.EXACT(“Hospital Mortality”) OR MESH.EXACT(“Minimally Invasive Surgical Procedures -- mortality”) OR MESH.EXACT(“Vascular Surgical Procedures -- mortality”) OR MESH.EXACT.EXPLODE(“Vascular Surgical Procedures : E.04.100.814 -- adverse effects”) OR mortality
MESH.EXACT.EXPLODE(“Treatment Outcome”)
EMB.EXACT.EXPLODE(“abdominal aorta aneurysm”) OR (ti,ab(abdominal aort*) AND aneurysm)
EMB.EXACT.EXPLODE(“blood vessel prosthesis”) OR (EMB.EXACT(“aorta graft”) OR EMB.EXACT(“blood vessel transplantation”)) OR repair OR (endovascular surgery) OR (EMB.EXACT(“endovascular aneurysm repair”) OR EMB.EXACT.EXPLODE(“aortic aneurysm endovascular graft”) OR EMB.EXACT.EXPLODE(“endovascular surgery”)) OR (open surgery)
ti,ab(female or females or woman or women)
MESH.EXACT(“Sex Factors”) OR MESH.EXACT(“Sex Distribution”) OR MESH.EXACT(“Sex Ratio”) OR MESH.EXACT(“Sex Characteristics”)
EMB.EXACT(“sex difference”) OR EMB.EXACT(“gender and sex”) OR EMB.EXACT(“gender”) OR EMB.EXACT(“sex ratio”)
ti,ab(gender or genders or sex)
(EMB.EXACT(“cardiovascular mortality”) OR EMB.EXACT.EXPLODE(“surgical mortality”)) OR EMB.EXACT.EXPLODE(“mortality”) OR mortality
EMB.EXACT(“treatment outcome”)
Limits: start 1 January 2009; end 26 August 2016.
| Reference, country | Patient base | Suitability criteria | N–O scorea | N | Mean age (years) | Mean aneurysm morphology | EVAR suitable, n/N (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAA diameter (mm) | Neck diameter (mm) | Neck length (mm) | Neck angle (α)a | Iliac or access artery diameter (mm) | |||||||
| Kristmundsson 2014,56 Sweden | All AAAs with CT scans 2006–7 |
Within any IFU for excluder, endurant or zenith flex grafts ND 18–32 mm NL ≥ 10 mm NA ≤ 75° IAD ≥ 7.5 mm |
3 | 41 women | N/A | 58.6 | 26.8 | 16.9 | 30.0 | 6.4 | 11/41 (27) |
| 200 men | N/A | 64.9 | 27.0 | 22.8 | 46.3 | 8.2 | 108/200 (54) | ||||
| Hultgren 2013,57 Sweden | All elective repairs in one clinic 2006–8 |
ND ≤ 32 mm NL ≥ 15 mm NA ≤ 60° ≤ 7.5 mm IAD ≤ 20 mm |
5 | 32 women | 72 | 56 | – | – | – | – | 15/32 (47) |
| 140 men | 72 | 65 | – | – | – | – | 80/140 (57) | ||||
| Sweet 2011,58 USA | All AAAs of > 4.0 cm with CT scans 1997–2009 |
18 mm ≤ ND ≤ 32 mm NL ≥ 15 mm NA < 60° |
5 | 251 women | 77 | 58 | 24 | 15 | 28 | 5.6 | 63/251 (25) |
| 812 men | 74 | 59 | 25 | 19 | 20 | 7.0 | 374/812 (46) | ||||
| Park 2011,59 Korea | All AAAs of > 4.0 cm with CT scan between 2003 and 2010 |
Within any IFU for AneuRx, excluder, talent, or zenith grafts ND ≤ 32 mm NL ≥ 10 mm NA ≤ 60° IAD≥ 8 mm |
4 | 35 women | 73 | – | – | – | – | – | 15/35 (43) |
| 156 men | 73 | – | – | – | – | – | 74/156 (47) | ||||
| Moise 2006,60 USA | Patients evaluated for EVAR between 2000 and 2003 |
ND ≤ 29 mm NL ≥ 15 mm NA ≤ 60° IAD ≥ 7 mm |
4 | 41 women | N/A | – | – | – | – | – | 15/41 (37) |
| 199 men | N/A | – | – | – | – | – | 128/199 (64) | ||||
| First author, time period | N–O scorea (n/8 points) | Age information | Non-intervention rate for men (%) | Non-intervention rate for women (%) |
|---|---|---|---|---|
| Whittaker,66 January 2013 to December 2015 | 5 | N/A | 83/389 (21) | 24/65 (37) |
| Scott,64 January 2006 to April 2012 | 5 | Median overall 73 years | 123/516 (24) | 15/59 (25) |
| Gorst,65 July 2007 to May 2011 | 5 | Mean overall 82 years | 58/254 (23) | 29/78 (37) |
| Karthikesalingam,20 January 2008 to December 2009 | 5 | Mean overall 75 years | 16/206 (8) | 16/45 (41) |
| First author, country | Repair date(s) | Derivation of cohort | Intervention | N–O score (n/10 points) | Women/men (N) | Mean age (years) | 30-day mortality, EVAR (%) | 30-day mortality, open repair (%) |
|---|---|---|---|---|---|---|---|---|
| Nevidomskyte,67 USA | July 2010 to September 2013 | State-wide registry VI-SCOAP | EVAR, open repair | 6 | 216 women | 73.1 | 5/160 (3.1) | 5/56 (8.9) |
| 848 men | 73.0 | 4/696 (0.6) | 4/152 (2.6) | |||||
| aChung,72 USA | June 2003 to July 2012 | Single centre | EVAR | 5 | 121 women | N/Ab | 2/121 (1.7) | N/A |
| 617 men | 11/617 (1.8) | |||||||
| Lo,18 USA | 2003–11 | VSGNE | EVAR, open repair | 7 | 820 women | 75d | 5/408 (1.2) | 15/412 (3.6) |
| 2777 men | 72d | 15/1660 (0.9) | 19/1117 (1.7) | |||||
| Mani,69 Sweden | 2006–10 | Swedvasc | EVAR, open repair | 9 | 765 women | N/A | 10/329 (3) | 17/436 (3.9) |
| 3367 men | 39/1669 (2.3) | 23/1698 (1.4) | ||||||
| Ramanan,68 USA | 2007–9 | ACS NSQIP | Open repair | 7 | 728 women | N/A | N/A | 34/728 (4.7) |
| 2117 men | 61/2117 (2.9) | |||||||
| Mehta,70 USA | 2002–9 | Single centre | EVAR, open repair | 7 | 553 women | N/A | 11/344 (3.2) | 12/209 (5.7) |
| 1827 men | 12/1248 (1.0) | 27/579 (4.7) | ||||||
| aPowell,73 five countriesc | 2000–9 | EVAR-1,38 ACE,78 DREAM,76 OVER77 RCTs | EVAR, open repair | 9 | 148 women | 75.2 | 1/77 (1.3) | 5/71 (6.9) |
| 2545 men | 71.3 | 15/1312 (1.1) | 35/1233 (2.8) | |||||
| Schermerhorn,71 USA | 2008 only | Medicare | EVAR, open repair | 6 | 5421 women | N/A | 77/3657 (2.1) | 123/1764 (7.0) |
| 19,705 men | 203/15590 (1.3) | 214/4115 (5.2) |
Appendix 4 Additional tables for Chapter 4
| Parameter | Source | Estimate | Distribution |
|---|---|---|---|
| Reinvitation | NAAASP8 | 0.239 | None |
| Attendance | Chichester33 | 0.727 | Beta(218,82) |
| Non-visualisation | NAAASP8 | 0.0035 | None |
| Prevalence | Systematic review 60–69 year olds34 | 0.0043 | LogN(–5.451,0.3232) |
| Aortic size distribution | NAAASP (reweighted) | See Aortic diameter distribution | N/A |
| AAA growth rates | RESCAN (11 studies)35 | N(µ,Σ)a | |
| Slope (β1) | 0.052 | ||
| Intercept (β0) | 1.33 | ||
| Slope log-SD [log(σ1)] | –3.28 | ||
| Intercept log-SD [log(σ0)] | –1.99 | ||
| Arctanh correlation [atanh(ρ)] | 0.41 | ||
| Residual log-SD [log(σW)] | –2.96 | ||
| AAA rupture rates | RESCAN35 | ||
| Association with diameter (γ1) | 5.47 | (1.5892−2.2178−2.21783.1406) | |
| Intercept (γ0) | –12.40 | ||
| Dropout from surveillance | NAAASP8 | 0.0546 | Gamma(1072,19650) |
| Incidental detection | New Zealand study,36 population data and prevalence estimate | 0.0293 | Gamma(40,1364) |
| Time from referral scan to consultation (days) | NAAASP8 | 10.6 | N/A |
| Mean difference in CT vs. ultrasound scan measurement (mm) | RESCAN (4 studies)35 | 2.44 | N/A |
| Measurement error SD for a CT scan (mm) | Singh et al.95 | 1.9 | N/A |
| Decision at consultation: proportion contraindicated | Four UK hospitals37 | 0.342 | Logit(p) ≈ normal(–0.654, 0.1352) |
| Time from consultation to elective surgery (days) | NAAASP8 | 70.8 | N/A |
| Number | 2013/14 | 2014/15 | Total |
|---|---|---|---|
| Eligible men | 304,381 | 294,253 | 598,634 |
| Offered an appointment | 300,667 | 293,709 | 594,376 |
| Declined screening | 8738 | 8620 | 17,358 |
| Attended after first invite | 210,845 | 205,294 | 416,139 |
| Who did not attend first appointment | 80,463 | 79,022 | 159,485 |
| Who attended following DNA | 31,829 | 31,642 | 63,471 |
| Conclusively tested | 235,339 | 232,183 | 467,522 |
| With at least one cancelled appointment | 621 | 773 | 1394 |
| Reinvited | 71,725 | 70,402 | 142,127 |
| Proportion reinvited | 0.2386 | 0.2397 | 0.2391 |
| Number | 2013/14 | 2014/15 | Total |
|---|---|---|---|
| Men visualised at first screen | 232,546 | 231,203 | 463,749 |
| Men non-visualised at first screen | 3803 | 2979 | 6782 |
| Men subsequently visualised | 2878 | 2252 | 5130 |
| Total non-visualised | 925 | 727 | 1652 |
| Proportion non-visualised | 0.0039 | 0.0031 | 0.0035 |
| Study | Mean calendar year at baseline | Threshold for intervention (cm) | Measurement modalities used | Internal/external diameter measured | Number, men/women | Mean follow-up, men/women (years) | Number of small AAA ruptures, men/women | Crude rupture rate (per 1000 person-years), men/women |
|---|---|---|---|---|---|---|---|---|
| Chichester, UK | 1999 | 6.0 (later 5.5) | Ultrasound only | Internal | 1405/99 | 4.45/4.42 | 43/8 | 6.88/18.26 |
| Edinburgh, UK | NAa | 5.5 | Ultrasound only | External | 670/382 | 2.89/2.42 | NA/NA | NA/NA |
| Leeds, UK | 2004 | 5.5 | Ultrasound and CT | External | 220/47 | 3.27/3.14 | NA/NA | NA/NA |
| Manchester, UK | 2005 | 5.5 | Ultrasound only | External | 837/258 | 2.41/2.41 | 6/5 | 2.97/8.03 |
| Tromsø, Norway | 1995 | 5.5 | Ultrasound only | External | 179/45 | 8.59/8.16 | 2/2 | 1.30/5.45 |
| PIVOTAL, USA | 2007 | 5.0 | Ultrasound and CT | External | 619/96 | 0.92/0.96 | 0/1 | 0.00/10.84 |
| Propranolol, Canada | 1996 | 5.0 or 5.5 by centre | Ultrasound only | External | 460/88 | 2.47/2.39 | 3/0 | 2.64/0.00 |
| Galdakao, Spain | 2001 | 5.0 | Ultrasound and CT | External | 859/64 | 3.93/2.55 | 5/1 | 1.47/6.14 |
| Stirling, UK | 2003 | 5.5 | Ultrasound and CT | No set protocol | 331/125 | 3.08/3.34 | 4/5 | 3.92/11.98 |
| Gävle, Sweden | 2003 | 5.0 or 5.5 by centre | Ultrasound only | External | 184/59 | 2.46/2.52 | 1/0 | 2.21/0.00 |
| UKSAT, UK | 1993 | 5.5 | Ultrasound and CT | External | 1747/480 | 2.38/2.65 | 32/28 | 7.68/22.00 |
| Study | β0 | β1 | log(σ0) | log(σ1)] | atanh(ρ) | log(σW) |
|---|---|---|---|---|---|---|
| Chichester, UK | 1.22 | 0.035 | –1.68 | –2.73 | 1.00 | –2.21 |
| Edinburgh, UK | 1.34 | 0.074 | –1.79 | –3.27 | 0.14 | –3.05 |
| Leeds, UK | 1.31 | 0.061 | –1.86 | –3.92 | 0.76 | –2.43 |
| Manchester, UK | 1.37 | 0.049 | –1.84 | –3.35 | 0.49 | –3.15 |
| Tromsø, Norway | 1.19 | 0.046 | –2.12 | –3.54 | 0.47 | –2.94 |
| PIVOTAL, USA | 1.47 | 0.033 | –2.98 | –3.89 | 0.76 | –3.17 |
| Propranolol, Canada | 1.32 | 0.045 | –2.07 | –2.97 | –0.05 | –3.01 |
| Galdakao, Spain | 1.32 | 0.058 | –1.82 | –3.18 | 0.74 | –2.84 |
| Stirling, UK | 1.33 | 0.054 | –1.66 | –3.40 | 0.25 | –2.75 |
| Gävle, Sweden | 1.37 | 0.055 | –2.15 | –3.26 | 0.13 | –3.49 |
| UKSAT, UK | 1.42 | 0.062 | –1.96 | –3.11 | 0.39 | –3.46 |
| Pooled (two-stage multivariate meta-analysis) | 1.33 (0.02) | 0.052 (0.004) | –1.99 (0.11) | –3.28 (0.10) | 0.41 (0.11) | –2.96 (0.12) |
| I2 (%) | 98 | 87 | 97 | 85 | 77 | 99 |
| Baseline size (cm) | Proportion reaching 5.5 cm (%) | ||||
|---|---|---|---|---|---|
| Observed range | Diameter for prediction | Observed in 5 years (95% CI) | Predicted in 5 years | Observed in 10 years (95% CI) | Predicted in 10 years |
| 3.0–3.4 | 3.25 | 2.1 (0.7 to 6.5) | 4.5 | 33.5 (22.3 to 48.3) | 35.6 |
| 3.5–3.9 | 3.75 | 18.6 (12.4 to 27.4) | 25.2 | 58.7 (42.4 to 75.8) | 63.4 |
| 4.0–4.4 | 4.25 | 48.7 (37.9 to 60.9) | 59.2 | 77.6 (55.1 to 93.8) | 84.4 |
| 4.5–4.9 | 4.75 | 89.1 (77.3 to 96.4) | 84.2 | a | 94.1 |
| 5.0–5.4 | 5.25 | a | 95.9 | a | 98.1 |
| Item | Females | Males | Total |
|---|---|---|---|
| Number of individuals | 1071 | 5358 | 6429 |
| Number of contributing studies | 6 | 6 | 6 |
| Number of ruptures (occurring before 5.5-cm threshold) | 49 | 92 | 141 |
| Length of follow-up to rupture event/censoring date, mean (SD) (years) | 3.1 (3.0) | 3.4 (3.3) | 3.4 (3.3) |
| Parameter | Estimate (SE) | I 2 | Interpretable parameter | Value |
|---|---|---|---|---|
| γ0 | –12.40 (1.77) | 75% (43, 89) | Baseline hazard, per 100 person-years (for a 5.0-cm AAA female) | 2.74 (0.94); p = 0.004 |
| γ1 | 5.47 (1.26) | 82% (61, 91) | Hazard ratio per 2% increase in AAA diameter | 1.11 (0.03); p < 0.001 |
| γ2 | –1.46 (0.23) | 25% (0, 68) | Hazard ratio for males vs. females (reference) | 0.23 (0.05); p < 0.001 |
Appendix 5 Additional figures and tables for Chapter 5
FIGURE 25.
Cumulative hazard of a reintervention after elective EVAR or open AAA repair, based on the EVAR-1 trial:38 men and women combined. Nelson–Aalen estimates of cumulative hazard, with exponential piecewise-constant survival model fits where time period is split into three epochs (0–30 days, 31–120 days and > 120 days), with censoring for mortality.

FIGURE 26.
Sex differences in first reintervention rates by operation received, based on the EVAR-1 trial. 38 Kaplan–Meier plots with censoring for mortality.
FIGURE 27.
Rate of AAA-related deaths after successful elective EVAR or open repair, based on the EVAR-1 trial:38 men and women combined. Kaplan–Meier plots (95% CIs) with exponential survival model fits, with censoring for mortality from other causes.
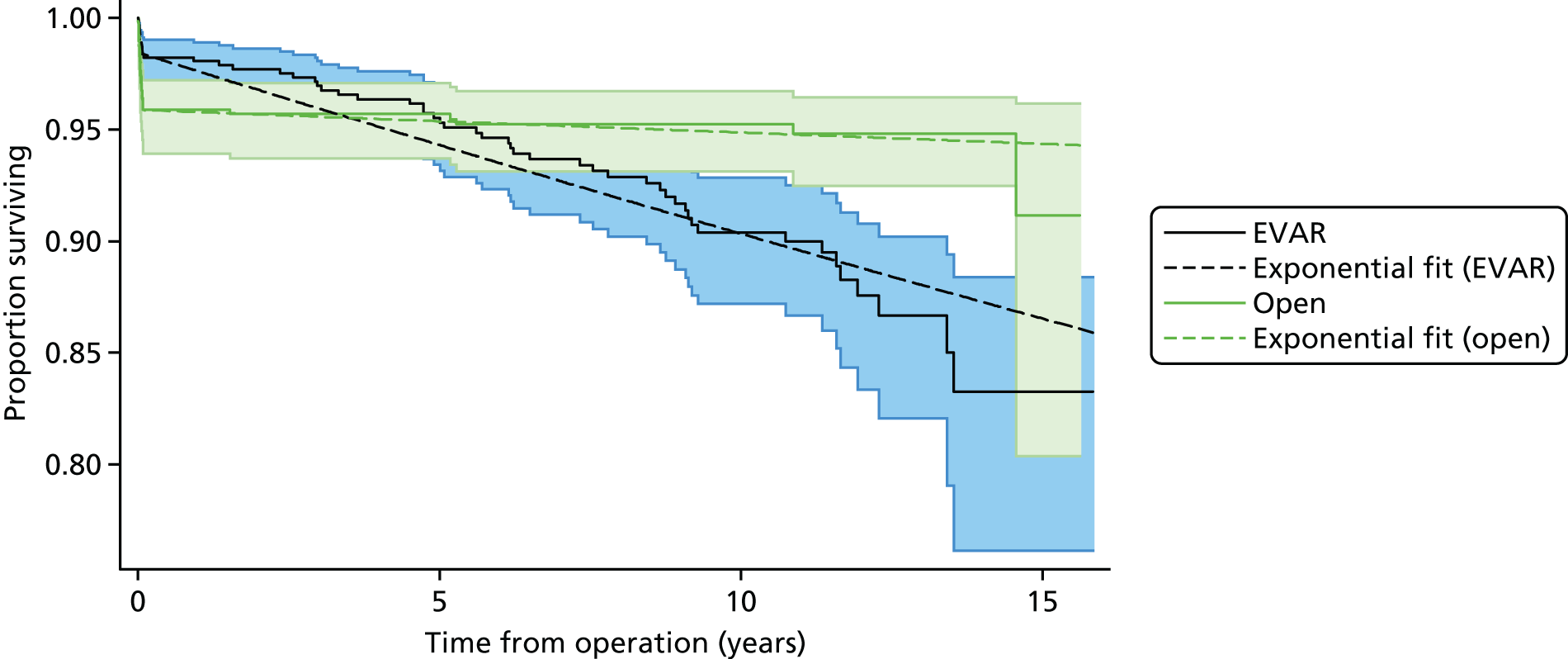
| Parameter | Proportion receiving EVAR | In-hospital operative mortality after EVAR | In-hospital operative mortality after open repair |
|---|---|---|---|
| Model (Equation 26), use of the NVR data as a source of parameter estimates in women | |||
| Sample size | 18,693 | 11,758 | 6935 |
| aF | 0.348 (0.043) | –4.022 (0.210) | –2.596 (0.130) |
| aM | 0.205 (0.046) | –0.922 (0.241) | –0.572 (0.145) |
| Model (Equation 27), use of the NVR data as a source of parameter estimates in women | |||
| Sample size | 17,062 | 10,590 | 6472 |
| aF | 0.702 (0.056) | –3.910 (0.217) | –2.336 (0.165) |
| aM | 0.529 (0.062) | –1.125 (0.264) | –0.385 (0.192) |
| b1F | 0.095 (0.007) | 0.002 (0.032) | 0.064 (0.022) |
| b1M | –0.001 (0.008) | 0.089 (0.037) | 0.024 (0.024) |
| b2F | –0.303 (0.053) | –0.028 (0.257) | 0.077 (0.128) |
| b2M | 0.002 (0.055) | 0.310 (0.276) | 0.043 (0.138) |
| Parameter | Proportion receiving EVAR | In-hospital operative mortality after EVAR | In-hospital operative mortality after open repair |
|---|---|---|---|
| Model (Equation 26), use of the NVR data as a source of parameter estimates in women | |||
| Sample size | 4552 | 862 | 3690 |
| aF | –1.599 (0.095) | –1.099 (0.201) | –0.413 (0.080) |
| aM | 0.173 (0.104) | –0.245 (0.221) | –0.124 (0.088) |
| Model (Equation 27), use of the NVR data as a source of parameter estimates in women | |||
| Sample size | 4549 | 861 | 3688 |
| aF | –1.548 (0.096) | –1.150 (0.211) | –0.343 (0.084) |
| aM | 0.289 (0.106) | –0.116 (0.231) | 0.103 (0.095) |
| b1F | 0.041 (0.014) | 0.061 (0.027) | 0.033 (0.012) |
| b1M | 0.003 (0.015) | –0.011 (0.030) | 0.031 (0.013) |
List of abbreviations
- AAA
- abdominal aortic aneurysm
- ACE
- French Anevrysme de l’aorte abdominale: Chirurgie versus Endoprothese
- BBC
- British Broadcasting Corporation
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CT
- computerised tomography
- DES
- discrete event simulation
- DREAM
- Dutch Randomised Endovascular Aneurysm Management
- DSA
- deterministic sensitivity analysis
- E/O
- expected to observed
- EQ-5D
- EuroQol-5 Dimensions
- EVAR
- endovascular aneurysm repair
- EVAR-1
- endovascular aneurysm repair trial 1
- FAST
- Female Aneurysm screening STudy
- FEL
- future event list
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IFU
- instructions for use
- IMPROVE
- Immediate Management of Patients with Ruptured aneurysm: Open versus Endovascular Repair
- INMB
- incremental net monetary benefit
- LME
- linear mixed effects
- LOS
- length of stay
- MASS
- Multicentre Aneurysm Screening Study
- MeSH
- medical subject heading
- NAAASP
- NHS Abdominal Aortic Aneurysm Screening Programme
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NVR
- National Vascular Registry
- ONS
- Office for National Statistics
- OVER
- US Open Versus Endovascular Repair
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
- International prospective register of systematic reviews
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RfPB
- Research for Patient Benefit
- SD
- standard deviation
- SE
- standard error
- VAS
- visual analogue scale
- WTP
- willingness to pay

