Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/30/02. The contractual start date was in December 2016. The draft report began editorial review in May 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Objective
The overall objective of this assessment was to summarise the evidence on the clinical effectiveness and cost-effectiveness of using alternative risk scores, which includes measuring the levels of cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) or morphological features seen on ultrasound (detailed in Chapter 2, Intervention technologies), to guide referral decisions for people with suspected ovarian cancer in secondary care. The current National Institute for Health and Care Excellence (NICE) guidance (CG122)1 recommends that the levels of serum CA125 should be measured in secondary care, in all people with suspected ovarian cancer for whom serum CA125 levels have not already been measured in primary care. CA125 levels are a component of secondary care investigation and are not used in isolation; the CG122 specifically recommends the calculation of a Risk of Malignancy Index 1 (RMI 1) score, which includes the measurement of CA125 levels, and referral to a specialist gynaecological oncology multidisciplinary team (MDT) for people with a RMI 1 score of ≥ 250. The CG122 does not currently include any recommendations on HE4 testing or alternative methods of risk-scoring. An evaluation of current evidence was needed to assess the clinical utility and cost-effectiveness of alternative methods of risk-scoring. The following research questions were defined to address the objectives of this assessment:
-
What are the performance characteristics of alternative risk scores (including alternative RMI 1 score thresholds), which include HE4 or CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice), for which the target condition is histologically confirmed ovarian cancer?
-
What are the effects of using alternative risk scores (including alternative RMI 1 score thresholds), which include measuring HE4 or CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice), on clinical management decisions and clinical outcomes?
-
What is the cost-effectiveness [incremental cost per quality-adjusted life-year (QALY)] of alternative risk scores (including alternative RMI 1 score thresholds), which include HE4 or CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice), when routinely used, in secondary care, to guide decisions about referral to a specialist multidisciplinary team (SMDT), for women with suspected ovarian cancer?
Chapter 2 Background and definition of the decision problem(s)
Population
The primary indication for this assessment was optimisation of the routine secondary care assessment of women with suspected ovarian cancer, to decide whether or not a patient should be referred to a SMDT. The assessment was conducted in the context of an update to the current guidance (CG122). 1 The relevant population was women of any age, including premenopausal and postmenopausal women, who had been referred to secondary care for the investigation of suspected ovarian cancer. This assessment includes data from women of any age, but no cost-effectiveness modelling was undertaken for the population aged < 18 years owing to a lack of data on the performance of risk scores in this age group. Women with a previous history of ovarian cancer who were being monitored for possible recurrence, and those referred directly from primary care to a SMDT, were outside the scope of this assessment.
Target condition
The target condition for this assessment was ovarian cancer. Ovarian cancer is a term describing a group of cancers arising from cells in or near the ovaries. Ovarian cancers can be classified based on tissue type (epithelial ovarian tumours, sex cord–stromal tumours and germ cell tumours), with epithelial carcinomas being the most common type (90%) of primary ovarian cancers; non-epithelial ovarian cancers are more common in premenopausal women. 2 The target conditions covered by the CG122 were epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer;1 excluded target conditions were pseudomyxoma peritonei, relapsed ovarian, fallopian tube or peritoneal cancer, germ cell tumour of the ovary and sex cord–stromal tumours of the ovary. This assessment was not limited to any particular type of ovarian cancer.
Ovarian cancers are staged using the four-stage International Federation of Gynecology and Obstetrics (FIGO) system:3
-
stage I – confined to the organ of origin (ovaries or fallopian tubes)
-
stage II – invasion of the surrounding organs or tissues [pelvic extension or primary peritoneal cancer (below the pelvic brim)]
-
stage III – spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes
-
stage IV – distant metastases, excluding peritoneal (e.g. lungs, liver, spleen).
Ovarian cancer can also be graded based on how differentiated cells appear:
-
grade 1 – well differentiated
-
grade 2 – moderately differentiated
-
grade 3 – poorly differentiated/undifferentiated.
Ovarian cancer is the sixth most common cancer in women in the UK (as of 2013), accounting for 4% of all new cases of cancer in females. 4,5 In 2013, there were 7284 new cases of ovarian cancer in women in the UK, giving an age-standardised incidence rate of 23.3 per 100,000. 4,5 Ovarian cancer accounts for around 5% of cancer deaths in women in the UK; 2014 statistics recorded 4100 ovarian cancer deaths. 6 The incidence of ovarian cancer is strongly related to age, with 2011–13 data indicating that approximately half (53%) of new cases of ovarian cancer were diagnosed in women > 65 years of age. 4,5 Ovarian cancer mortality is also strongly related to age at diagnosis. 6
Data from the Office for National Statistics, published by Cancer Research UK,7 indicate that, although ovarian cancer incidence rates have increased overall since the 1970s, the UK age-standardised incidence rates decreased by 6% in the decade between 2002–4 and 2011–13. However, it remains the case that a high proportion of women (58%) are diagnosed at an advanced stage (stage III or IV), and 21% have metastases at diagnosis. 8 Ovarian cancer survival is strongly related to stage at diagnosis; 2012 data6 showed that the 1-year and 5-year survival rates for women diagnosed at stage I were 97% and 90%, respectively, versus 53% and 4%, respectively, for women diagnosed at stage IV. Improving early diagnosis is therefore a priority, and variation in the performance of testing strategies for the detection of different stages of ovarian cancer should be considered. The majority of studies about ovarian cancer diagnosis concern epithelial carcinomas; however, there is some evidence to indicate that the diagnostic performance of tumour markers and risk scores may vary between tumours of different tissue types;9 the possible effects of tumour tissue type on estimates of test performance should also be considered.
It has been suggested that CA125 results should be interpreted cautiously in premenopausal women because of the high rate of false-positive (FP) diagnoses resulting from various non-malignant conditions (e.g. fibroids, endometriosis, adenomyosis, pelvic infection). 10 It is therefore important to consider the effects of the menopausal status of women on the performance of testing strategies, either by stratification of data from test accuracy studies or by including menopausal status in risk models (as in the RMI 1).
Intervention technologies
Serum tumour markers are used in the secondary care investigation of people with suspected ovarian cancer; these are not considered to be ‘stand-alone’ diagnostic tests, but are used in conjunction with other tests, signs and symptoms to assess the risk of malignancy. An estimate of an individual’s risk of malignancy can inform decisions about specialist referral, further testing and treatment. It is anticipated that these risk assessment tools will be used in secondary care, for people in whom ultrasound imaging suggests confined disease or a low volume of disease outside the pelvis (stages I–IIIb).
An optimised risk assessment that reduces the number of women with ovarian cancer who are not referred for further specialist care [i.e. those with a ‘false-negative’ (FN) risk assessment] has the potential to improve prognosis, be cost-saving in terms of unnecessary further investigations and reduce associated anxiety. Prognosis may be adversely affected by a failure to refer women to a SMDT and specialist surgery. In particular, it is likely that women who are believed to have a benign explanation for any pelvic mass will be operated on in secondary care. If they actually have ovarian cancer, then the prognosis might be worse than if they had been operated on by a specialist gynaecological oncology surgeon. Indeed, there is evidence of up to a 45% difference in the median overall survival between a set of regional centres in the UK and the UK as a whole. 11
The current standard assessment (RMI 1) has been reported as having poor sensitivity – approximately 63% at an operating threshold of 200. 12 If referral decisions are based on the RMI 1 score at this threshold, there remains the potential for significant numbers of people with ovarian cancer to remain unreferred, and to experience consequential delays in diagnosis and detrimental effects on prognosis. A systematic review of studies comparing HE4, CA125 and the Risk of Ovarian Malignancy Algorithm (ROMA) score reported similar overall sensitivity estimates for HE4 and CA125 (76% and 79%, respectively) and a higher sensitivity (85%) for the ROMA score. 9 Sensitivity estimates were lower for early-stage cancer (55% for both HE4 and CA125 levels, and 74% for the ROMA score). 9 Risk scores with higher sensitivity are needed to facilitate prompt referral of the appropriate patient group.
The Risk of Ovarian Malignancy Algorithm score
The ROMA score uses serum HE4 and serum CA125 levels, along with menopausal status, to generate an individualised estimate of the risk that a person has ovarian cancer. Initially, a predictive index (PI) value is calculated using a formula that differs depending on whether the woman is premenopausal or postmenopausal (Equations 1 and 2 in Box 1). This PI value can then be used to calculate the ROMA score (Equation 3 in Box 1). 13 The ROMA score is intended for use in women who present with an adnexal mass (i.e. following ultrasound examination). Manufacturers of HE4 assays recommend the use of these assays, in the context of a ROMA score, in combination with a specific CA125 assay or assays; if a CA125 level has been obtained in primary care, using a different assay, this will need to be repeated in secondary care before a ROMA score can be calculated.
Cut-off values for the ROMA scores are used to classify individuals as having a low or high risk of developing epithelial ovarian cancer. Recommended thresholds can differ depending on the tumour marker assays used, as described below.
There are currently three commercial HE4 assays for use with automated immunoassay analysers that are available for use in the UK NHS; a summary of the key technical characteristics of these assays is provided below (Table 1).
| Name of assay (manufacturers’ details) | Company | Detection | Assay time | |
|---|---|---|---|---|
| Limit | Range | |||
| ARCHITECT HE4 (Abbott Diagnostics, Abbott Park, IL, USA) | Abbott Diagnostics | 15 pmol/l | 20–1500 pmol/l | 28 minutesa |
| LUMIPULSE® G HE4 (Fujirebio Diagnostics, Gothenburg, Sweden) | Fujirebio Diagnostics | 3.5 pmol/l | 20–1500 pmol/l | 35 minutesb |
| Elecsys® HE4 (Roche Diagnostics, Rotkreuz, Switzerland) | Roche Diagnostics | 15 pmol/l | 15–1500 pmol/l | 18 minutesc |
The ARCHITECT human epididymis protein 4 assay (Abbott Diagnostics)
The ARCHITECT HE4 assay (Abbott Diagnostics, Abbott Park, IL, USA) is a chemiluminescent microparticle immunoassay for the quantitative determination of HE4 levels in human serum. The assay is designed for use on an immunoassay analyser, specifically the ARCHITECT i2000SR or the ARCHITECT i1000SR analysers. Additional materials required to run the assay are the ARCHITECT HE4 assay software file, ARCHITECT HE4 calibrators, ARCHITECT HE4 controls, ARCHITECT multiassay manual diluent, ARCHITECT pre-trigger solution, ARCHITECT trigger solution, ARCHITECT wash buffer, ARCHITECT reaction vessels, ARCHITECT sample cups, ARCHITECT septum and ARCHITECT replacement caps.
The results of the assay are intended to be used in conjunction with the ARCHITECT CA125 II assay, as an aid in estimating the risk of epithelial ovarian cancer in women presenting with a pelvic mass who will undergo surgical intervention. The company recommends that the HE4 assay results are used in the calculation of the ROMA scores, using the following cut-off values for ROMA scores, based on obtaining a specificity of 75%:
-
in premenopausal patients, a ROMA value of ≥ 7.4% indicates a high risk of finding epithelial ovarian cancer
-
in premenopausal patients, a ROMA value of < 7.4% indicates a low risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of ≥ 25.3% indicates a high risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of < 25.3% indicates a low risk of finding epithelial ovarian cancer.
These results must be interpreted in conjunction with other methods and clinical data (e.g. symptoms and medical history), in accordance with standard clinical management guidelines. The company states that additional testing should be done if the HE4 results are inconsistent with the clinical evidence.
LUMIPULSE G human epididymis protein 4 (Fujirebio Diagnostics)
The LUMIPULSE G HE4 (Fujirebio Diagnostics, Gothenburg, Sweden) is a chemiluminescent enzyme immunoassay (CEIA) for the quantitative measurement of HE4 levels in human serum. The assay is designed for use on the LUMIPULSE G system (either the LUMIPULSE G1200 or the LUMIPULSE G600 immunoassay analysers). Samples are run using immunoreaction cartridges, which contain reagents and into which samples are added. Further materials required for the assay are LUMIPULSE G HE4 calibrators, LUMIPULSE G substrate solution, LUMIPULSE G wash solution, LUMIPULSE G specimen diluent I, sampling tips for the LUMIPULSE system, soda lime for the LUMIPULSE system and LUMIPULSE G dilution cartridges.
The assay is intended for use in conjunction with CA125 levels (measured using the LUMIPULSE G CA125 II assay) as an aid in estimating the risk of epithelial ovarian cancer in premenopausal and postmenopausal women presenting with a pelvic mass who will undergo surgical intervention.
The company recommends that the HE4 results are used in the calculation of the ROMA scores, and suggests the following cut-off values for the ROMA scores, based on obtaining a specificity of 75%:
-
in premenopausal patients, a ROMA value of ≥ 13.1% indicates a high risk of finding epithelial ovarian cancer
-
in premenopausal patients, a ROMA value of < 13.1% indicates a low risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of ≥ 27.7% indicates a high risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of < 27.7% indicates a low risk of finding epithelial ovarian cancer.
Results should be interpreted in conjunction with further methods and clinical data (e.g. clinical findings, age, family history and imaging results), in accordance with standard clinical management guidelines.
A further HE4 assay is also available from Fujirebio Diagnostics: the HE4 enzyme immunoassay (EIA), a manual, enzyme immunometric assay for the quantitative determination of HE4 in human serum. Clinical experts commented that manual kits would be unlikely to be used in routine practice in the NHS; therefore, this assay has not been included in the scope of this assessment.
Elecsys human epididymis protein 4 immunoassay (Roche Diagnostics)
The Elecsys HE4 (Roche Diagnostics, Rotkreuz, Switzerland) is an immunoassay that uses Roche Diagnostics’ electrochemiluminescence detection technology to quantify HE4 levels. The assay uses anti-HE4 mouse monoclonal antibodies to capture HE4 in a serum sample and label it with a ruthenium complex. The application of a voltage to the samples then induces chemiluminescent emissions, which are measured by a photomultiplier.
The assay is designed for use on an immunoassay analyser, specifically the following analysers: modular analytics E170, cobas e 411, cobas e 601/e 602 and cobas e 801. Additional materials required for the HE4 assay are the HE4 CalSet (Roche Diagnostics, Rotkreuz, Switzerland), PreciControl HE4 (Roche Diagnostics, Rotkreuz, Switzerland) and Diluent MultiAssay (Roche Diagnostics, Rotkreuz, Switzerland). Further materials are also required for the general running of analysers, such as wash and cleaning solutions and disposable consumables.
The assay is intended to be used in conjunction with the Elecsys CA125 II assay as an aid in estimating the risk of epithelial ovarian cancer in premenopausal and postmenopausal people with a pelvic mass. The company recommends that the HE4 and CA125 assay results are used in the calculation of the ROMA scores. The company suggests the following cut-off values for the ROMA scores, based on obtaining a specificity of 75%:
-
in premenopausal patients, a ROMA value of ≥ 11.4% indicates a high risk of finding epithelial ovarian cancer
-
in premenopausal patients, a ROMA value of < 11.4% indicates a low risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of ≥ 29.9% indicates a high risk of finding epithelial ovarian cancer
-
in postmenopausal patients, a ROMA value of < 29.9% indicates a low risk of finding epithelial ovarian cancer.
The company states that additional testing should be done if the HE4 results are inconsistent with the clinical evidence.
Simple Rules ultrasound classification system (International Ovarian Tumour Analysis group)
Simple Rules is a morphological scoring system based on the presence of ultrasound features (described as rules) to characterise an ovarian mass as benign or malignant, and was developed by the International Ovarian Tumour Analysis (IOTA) group. The system uses a morphological scoring system based on the presence of ultrasound features to characterise an ovarian mass as benign or malignant, and requires the use of transvaginal sonography (TVS), which may be supplemented with abdominal ultrasound for larger masses. There are five ‘rules’ describing the features of malignant tumours (M-rules) and five rules that describe benign tumours [B-rules (see Table 2)]. 14,15 Because the use of the Simple Rules system requires specialist training in interpreting real-time ultrasound images in relation to these rules, it is assumed that using the Simple Rules system in the specified population will require a secondary care ultrasound examination (i.e. a repeat examination in which the ultrasound has been conducted in primary care).
| M-rules (rules for predicting a malignant tumour) | B-rules (rules for predicting a benign tumour) |
|---|---|
|
|
The M-rules and B-rules can be combined to aid classification:
-
If any M-rules (and no B-rules) apply, the mass is classified as malignant.
-
If any B-rules (and no M-rules) apply, the mass is classified as benign.
-
If both M-rule and B-rule (or neither) apply, the mass is unclassifiable, and the IOTA group states that there are then a number of options:
-
classify the mass as malignant
-
refer the patient to an expert ultrasound operator for a second opinion
-
use alternative imaging techniques
-
use the Simple Rules risk model16 to calculate risk of malignancy using the morphological features seen on ultrasound described in the Simple Rules model.
-
No specific make or model of ultrasound device is required for the model inputs. A transvaginal probe is required, and the image must be of sufficient quality to allow the ultrasound features specified by the model to be seen. The IOTA group states that the approach to evaluating masses required by the classification system is not more time-consuming than a standard ultrasound scan.
The IOTA group organises 1-day courses that teach the techniques for classifying masses required by the system, with participants assessed by a multiple-choice test. An online training tool, which will be freely accessible to NHS practitioners, is also being developed. In addition to this training, the IOTA group also recommends that practitioners should have completed 300 gynaecological scans. Software is not required to run the Simple Rules model.
The Simple Rules model is not recommended for use with women who are pregnant. Physiological changes during pregnancy can alter the appearance of ovarian masses, which can affect the classification made using the Simple Rules model, and the model has not been validated in this group.
The Assessment of Different NEoplasias in the adneXa model (International Ovarian Tumour Analysis group)
The Assessment of Different NEoplasias in the adneXa (ADNEX) model was developed by the IOTA group to aid preoperative discrimination between benign, borderline, stage I invasive, stages II–IV invasive and secondary metastatic ovarian tumours in women with an ovarian (including paraovarian and tubal) mass. 17 The model uses nine predictors, three clinical variables [age, serum CA125 level and type of referral centre (oncology or other)] and six ultrasound variables (maximal lesion diameter, proportion of solid tissue, > 10 cyst locules, number of papillary projections, acoustic shadows and ascites). The IOTA group have produced iPhone, Android and web applications for calculating the ADNEX risk score (www.iotagroup.org/adnexmodel/). Guidance has also been published on the application of the ADNEX model in clinical practice, and the selection of risk cut-off values for risk stratification and choice of clinical management. 18 The IOTA group notes that, as with other diagnostic prediction models (other IOTA group models, ROMA scores, the RMI 1), the ADNEX model cannot be applied to women with conservatively treated adnexal tumours.
Overa (multivariate index assay, second generation)
The Overa [multivariate index assay, second generation (MIA2G); Vermillion, Inc., Austin, TX, USA] assay is a Conformité Européenne (CE)-marked qualitative serum test that combines the results of five immunoassays into a single numeric result [i.e. the Overa (MIA2G) risk score]. The five biomarkers included in the test are:
-
follicle-stimulating hormone (FSH)
-
HE4
-
apolipoprotein A-1 (apo A-1)
-
transferrin (TRF)
-
CA125.
The levels of these biomarkers present in serum are determined using immunoassays run on the Roche Diagnostics’ cobas® 6000 system (Roche Diagnostics, Rotkreuz, Switzerland). The Overa (MIA2G) risk score is generated by the company’s OvaCalc software, with the results ranging between 0.0 and 10.0. A risk score of < 5.0 is indicative of a low probability of malignancy and a score of ≥ 5.0 indicates a high probability of malignancy.
The assay is indicated for use in people > 18 years with a pelvic mass in whom surgery may be considered. It is intended for use as part of a preoperative assessment to help decide if a person presenting with a pelvic mass has a high risk or a low risk of ovarian malignancy.
The company states that the test results must be interpreted in conjunction with an independent clinical and imaging evaluation, and that the test is not intended for use in screening or as a stand-alone assay.
The Risk of Malignancy Index 1
The RMI 1, used at thresholds other than those currently recommended in the NICE clinical guidelines (see Comparator), was considered as an alternative intervention technology.
Comparator
The comparator for this assessment is the RMI 1, using the referral thresholds that best reflect current UK clinical practice (≥ 250), recommended in NICE clinical guideline CG122. 1 The RMI 1 score uses three components (measured serum CA125 levels, ultrasound imaging and menopausal status) to calculate a risk score:
where:
-
U is the ultrasound score – 1 point scored for the presence of each of the following features: multilocular cysts, solid areas, metastases, ascites, bilateral lesions. U = 0 (0 points), U = 1 (1 point) or U = 3 (2–5 points)
-
M is the menopause score – M = 1 (premenopausal) or M = 3 (postmenopausal); a ‘postmenopausal’ woman is one who has had no period for more than 1 year or a woman aged > 50 years who has had a hysterectomy
-
CA125 is the serum CA125 concentration – measured in international units (IU)/ml.
Notably, because the ultrasound score component of this equation is zero, if none of the specified features is present on an ultrasound scan, RMI 1 scores above zero are possible only if ultrasound scans identify features indicative of ovarian cancer.
The NICE clinical guideline CG1221 recommends that people with a RMI 1 score of ≥ 250 should be referred to a specialist gynaecological oncology MDT. However, this guideline also includes a research recommendation that states that further research should be undertaken to determine the optimum RMI 1 threshold that should be applied in secondary care to guide the management of people with suspected ovarian cancer. The guideline notes that there was variation in the evidence base at that time with regard to the optimum RMI 1 threshold to use in secondary care, and that the value used will have implications for the management options considered, and the number of women who will be referred for specialist treatment.
The Scottish Intercollegiate Guidelines Network (SIGN)’s guideline on the management of epithelial ovarian cancer (SIGN 135)19 recommends referring women with a RMI 1 score of > 200 to a gynaecological oncology MDT. In addition, the Royal College of Obstetrics and Gynaecology (RCOG)’s guideline on ovarian cysts in postmenopausal women recommends the use of 200 as a threshold to predict the likelihood of ovarian cancer, although it notes that the threshold of 250 is also acceptable; in the current literature,10 a score of 200 is often used as a cut-off value.
Reference standard
Histopathology is the reference standard for assessing the accuracy of tests to identify people at a high risk of developing epithelial ovarian cancer. In addition to distinguishing between malignant and benign tumours, this testing can also determine the type of ovarian cancer present. Tissue samples used to confirm diagnosis can be obtained by biopsy or during surgery; however, for the population of interest (people in whom imaging suggests a confined disease or a low volume of disease outside the pelvis), it is expected that pre-surgery biopsy would not routinely occur. When tissue samples are not taken, clinical follow-up (ideally for a minimum of 12 months) may be required to determine the presence, or absence, of ovarian cancer.
Care pathway
Primary care assessment and criteria for referral to secondary care
The 2011 NICE clinical guideline CG1221 provides recommendations about the assessment of people with suspected ovarian cancer in primary care.
These recommendations include information about signs and symptoms (e.g. abdominal bloating, feelings of satiety or loss of appetite, pelvic or abdominal pain, changes in bowel habit and urinary frequency/urge) as well as information about the use of CA125 testing.
More recent guidance about cancer diagnoses, the NICE guidance NG12,20 published in 2015, reproduces the recommendations from the CG1221 with no update.
The more recent (2013) guidance, from SIGN19 provided recommendations covering similar topic areas.
Establishing a diagnosis in secondary care
The 2011 NICE clinical guideline CG1221 also includes recommendations about testing following referral to secondary care. These recommendations cover the use of various blood tests [alpha-fetoprotein (AFP), beta-human chorionic gonadotrophin (beta-hCG) and CA125], risk scoring using the RMI 1 score, imaging (ultrasound and CT) and the role of the MDT.
Those secondary care recommendations that refer to CA125 consider its use in a clinical context, particularly in relation to the calculation of the RMI 1 score. 1
The SIGN guideline (SIGN 135)19 includes similar recommendations about the RMI 1 score and further imaging investigations.
The RCOG and the British Society for Gynaecological Endoscopy have produced a joint guideline about the management of suspected ovarian masses in premenopausal women. This guideline aimed to clarify when ovarian masses can be managed in a ‘benign’ gynaecological service and when referral to a gynaecological oncological service is needed. 10 The guideline notes the importance of thorough history-taking, including risk factors, and careful physical examination, including abdominal and vaginal examination and the determination of the presence or absence of local lymphadenopathy.
The Royal College of Radiologists iRefer radiological investigation guidelines tool21 recommends that CT of the abdomen and pelvis has a role in identifying women who may benefit from chemotherapy or cytoreductive surgery. MRI of the abdomen and pelvis is recommended for specialised investigation when enhanced CT is contraindicated, or for problem-solving. PET-CT is indicated as a specialised investigation for difficult management situations.
Management of early (stage I) ovarian cancer
National Institute for Health and Care Excellence guideline CG1221 includes recommendations about the overall management of women with suspected early (stage I) ovarian cancer, and NICE Technology Appraisal (TA) guidance TA5522 provides recommendations about first-line chemotherapy regimens.
Management of advanced (stage II to IV) ovarian cancer
National Institute for Health and Care Excellence guideline CG1221 includes recommendations about the management of women with advanced (stages II–IV) ovarian cancer, and NICE TA guidance (TA55 and TA284)22,23 provides recommendations about first-line chemotherapy regimens.
Further recommendations about chemotherapy regimens for women with recurrent ovarian cancer can be found in NICE TA guidance documents TA389, TA381 and TA285. 24–26
Summary of the decision problem
The current guidance, NICE clinical guideline CG122,1 recommends that serum CA125 levels should be measured in secondary care in all women with suspected ovarian cancer in whom serum CA125 levels have not already been measured in primary care. CA125 levels can inform clinical decision-making in secondary care and are not used in isolation; CG1221 specifically recommends the calculation of a RMI 1 score, which includes CA125 level. CG122 does not currently include any recommendations on HE4 levels, risk scores or testing algorithms (other than RMI 1 score). An update to the section of CG1221 that deals with establishing a diagnosis in secondary care is planned in order to assess the potential role of alternative risk scores in assessing women with suspected ovarian cancer for possible referral to a SMDT and to consider the best way to incorporate tumour markers and other tests in the decision-making process.
This assessment systematically reviews the evidence about the comparative performance of alternative risk scores that include CA125 levels, HE4 levels or ultrasound (detailed in Intervention technologies) to guide referral decisions for women with suspected ovarian cancer in secondary care. The assessment focuses on direct comparisons between the interventions described and the RMI 1 score, using the referral threshold of ≥ 250 (current practice as indicated in CG1221). However, assessments of the accuracy of individual risk scores have also been included. Data were collected on the accuracy and comparative accuracy of different risk scores, alternative cut-off values and risk scores used in combination in order to determine the best way to incorporate tumour markers and ultrasound findings in the diagnostic process. Prediction-modelling studies have also been included, which report the development and validation of multivariable prediction models intended to be used to guide individual patient care.
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of different risk scores, used as a triage step to guide referral decisions for women with suspected ovarian cancer in secondary care, compared with the RMI 1 score, as recommended in CG122. 1 Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination’s (CRD’s) guidance27 for undertaking reviews in health care and NICE’s diagnostics assessment programme manual. 28
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Systematic review methods
Search strategies
Search strategies were based on the specified risk scores [the ROMA score, the IOTA group’s simple ultrasound rules, the ADNEX score, Overa (MIA2G) score and the RMI 1 score] and the target condition (ovarian cancer), as recommended in the CRD’s guidance27 for undertaking reviews in health care and the Cochrane’s handbook for diagnostic test accuracy reviews. 29,30
Candidate search terms were identified from target references, browsing database thesauri (e.g. MEDLINE MeSH and EMBASE Emtree), and from existing reviews identified during the initial scoping searches. These scoping searches were used to generate test sets of target references, which informed the text-mining analysis of high-frequency subject indexing terms, using EndNote X6 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] reference management software. Strategy development involved an iterative approach, testing candidate text and indexing terms across a sample of bibliographic databases and aiming to reach a satisfactory balance of sensitivity and specificity. Search strategies were developed specifically for each database.
No restrictions on language, publication status or date of publication were applied. Searches took into account generic and other product names for the intervention. The main EMBASE strategy for each search was independently peer reviewed by a second information specialist, using the Canadian Agency for Drugs and Technologies in Health’s peer review checklist. 31 Identified references were downloaded in EndNote X6 software for further assessment and handling. References in retrieved articles were checked for additional studies. The final list of included papers were also checked on PubMed for retractions, errata and related citations. 32–35
The following databases were searched for relevant studies:
-
MEDLINE (via Ovid) – 1946 to week 2 November 2016
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) – to 22 November 2016
-
MEDLINE Daily Update (via Ovid) – to 22 November 2016
-
MEDLINE Epub Ahead of Print (via Ovid) – to 22 November 2016
-
EMBASE (via Ovid) – 1974 to 23 November 2016
-
Cochrane Database of Systematic Reviews (via Wiley Online Library) – to issue 11 of 12, November 2016
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library) – to issue 10 of 12, October 2016
-
Database of Abstracts of Reviews of Effects (via Wiley Online Library) – to issue 2 of 4, April 2015
-
Health Technology Assessment (HTA) Database (via Wiley Online Library) – to issue 4 of 4, October 2016
-
International Network of Agencies for HTA publications (via the internet: www.inahta.org/publications/) – to 25 November 2016
-
National Institute for Health Research (NIHR) HTA programme (via the internet: www.nets.nihr.ac.uk/programmes/hta) – to 25 November 2016
-
Aggressive Research Intelligence Facility database (via the internet: www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx) – to 25 November 2016
-
PROSPERO (international prospective register of systematic reviews; via the internet: www.crd.york.ac.uk/prospero/) – to 25 November 2016.
Completed and ongoing trials were identified by searches of the following resources:
-
National Institutes of Health ClinicalTrials.gov (via the internet: www.clinicaltrials.gov/) – to 24 November 2016
-
European Union Clinical Trials Register (via the internet: www.clinicaltrialsregister.eu/ctr-search/search) – to 25 November 2016
-
World Health Organization’s International Clinical Trials Registry Platform (via the internet: www.who.int/ictrp/en/) – 24 November 2016.
The following key conference proceedings were identified in consultation with clinical experts and were screened for the last 3 years:
-
Radiological Society of North America
-
American Society of Clinical Oncology Annual Conference
-
Society of Gynecologic Oncology
-
The National Cancer Research Institute
-
European Society of Radiology.
Full search strategies are presented in Appendix 1.
Inclusion and exclusion criteria
Inclusion criteria for each of the clinical effectiveness questions are summarised in Table 3. Studies that fulfilled these criteria were eligible for inclusion in the review.
| Criteria | Question | |
|---|---|---|
| What are the performance characteristics of alternative risk scores (including alternative RMI 1 score thresholds), which include HE4 levels, CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice1), for which the target condition is histologically confirmed ovarian cancer? | What are the effects of using alternative risk scores (including alternative RMI 1 score thresholds), which include HE4 levels, CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice1), on clinical management decisions and clinical outcomes? | |
| Participants | Women of any age with suspected ovarian cancer, who have not previously been treated for ovarian cancer and are not currently receiving chemotherapy | |
| Setting | Secondary carea | |
| Interventions (index test) | Alternative methods of risk-scoring or RMI 1 used at thresholds other than 250, as described in Chapter 2, Intervention technologiesb | |
| Comparators | RMI 1 scorec | |
| Reference standard | Histological examination of surgically resected tissue sampled | NA |
| Outcomes | Diagnostic accuracy (the numbers of TP, FN, FP and TN test results), whereby the target condition is histologically confirmed ovarian cancer | Diagnosis of ovarian cancer confirmed by pathological examination of a biopsy, or prognostic outcomes for ovarian cancer (e.g. stage at diagnosis, differentiation status, suitability for surgical intervention/curative treatment, overall survival, progression-free survival) |
| Study designc | Diagnostic cohort studies directly comparing one or more interventions (index tests) with the comparatore | Prediction-modelling studies, randomised and non-RCTs |
Inclusion screening and data extraction
Two reviewers (MW and SL or SD) independently screened the titles and abstracts of all reports identified by searches, and any discrepancies were discussed and resolved by consensus. Full copies of all studies deemed potentially relevant were obtained, and the same reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of the studies excluded at the full-paper screening stage are presented in Appendix 2.
When studies reported insufficient information (e.g. tumour marker assay details not specified, incomplete accuracy data), the authors were contacted by e-mail to request additional information.
Studies cited in materials provided by the manufacturers of HE4 assays, the manufacturer of the Overa (MIA2G) multiple-marker test and the IOTA group were first checked against the project reference database in EndNote X6; any studies not already identified by our searches were screened for inclusion, following the aforementioned process.
Data were extracted on the following: study design/details; participant characteristics (age, pre- or post-menopause, presenting symptoms, tumour marker levels and other risk factors, when these were reported); details of the risk score and its component tests [manufacturer, antibody, detection method (including analyser used), ultrasound method and definition of a positive risk score]; details of the reference standard (details of the methods used, when these were reported, definition of disease positive and details of the final histopathological diagnoses of study participants, when these were reported); and test performance outcome measures. Data were extracted by one reviewer, using a piloted, standard data extraction form, and checked by a second reviewer (MW and SL or SD); any disagreements were resolved by consensus.
Quality assessment
The methodological quality of included test accuracy studies was assessed using the quality assessment of diagnostic accuracy studies 2 (QUADAS-2) tool,36 and the methodological quality of prediction model studies was assessed using the PROBAST (Prediction model study Risk Of Bias Assessment Tool). 37 Quality assessment was undertaken by one reviewer and checked by a second reviewer (MW and SL or SD); any disagreements were resolved by consensus or discussion with a third reviewer.
The results of the quality assessments are summarised in tables and graphs in the results of the systematic review (see Study quality) and examples of full quality assessments (QUADAS-2 and PROBAST) are provided in Appendix 3; full quality assessments for all included studies can be obtained from the authors.
Methods of analysis/synthesis
Sensitivity and specificity were calculated for each set of 2 × 2 data. All meta-analyses estimated separate pooled estimates of sensitivity and specificity using random-effects logistic regression. 24 The bivariate/hierarchical summary receiver operating characteristic model38–40 could not be applied because the data sets were too small and/or homogeneous. Heterogeneity was assessed visually, using summary receiver operating characteristic plots or receiver operating characteristic space plots. Analyses were performed in MetaDisc (Hospital Universitario Ramón y Cajal, Madrid, Spain). 41
The differences between the sensitivity and specificity estimates for different risk scores were described as statistically significant when the 95% confidence intervals (CIs) did not overlap.
Studies were grouped by risk score, manufacturer of the tumour marker assays (when appropriate), definition of disease positive (target condition) and menopausal status. Stratified results tables and forest plots were used to illustrate the variation of test performance by threshold.
Results of the assessment of clinical effectiveness
The searches of bibliographic databases identified 2456 records after deduplication. Following the initial screening of titles and abstracts, 241 publications were considered to be potentially relevant and ordered for full-paper screening; of these, 64 were included in the review. 17,42–103
In addition, one set of slides from a conference presentation was provided, through NICE, by the manufacturer of Overa (MIA2G),104 and an unpublished interim report of phase 5 of the IOTA study was provided (confidential information has been removed) personal communication: e-mail via Frances Nixon, Technical Advisor, NICE Diagnostic Assessment Programme to Marie Westwood, Project Lead, Kleijnen Systematic Reviews Ltd, 1 March 2017. All potentially relevant studies cited in other documents supplied by the test manufacturers had already been identified through other sources. Figure 1 shows the flow of studies through the review process, and Appendix 2 provides details, with reasons for exclusions, of all publications excluded at the full-paper screening stage. In total, there were 51 included studies, reported in 65 publications, and one unpublished interim report.
FIGURE 1.
Flow of studies through the review process. a, One study reported data for both the ROMA score using Roche Diagnostics’ Elecsys tumour marker assays and Overa (MIA2G); and b, two studies reported data for both the ADNEX model and IOTA group’s simple ultrasound rules.

A total of 165 publications were excluded after full-text screening. Six articles could not be obtained,105–110 and a further three ongoing studies, reported in four references, were identified as potentially relevant to future updates of this assessment. 111–114 Of particular note is Refining Ovarian Cancer Test accuracy Scores (ROCkeTS),112,113 a large prospective Phase III study, which was funded by NIHR and which is due to report in 2019/2020. The ROCkeTS study is evaluating the clinical utility, as well as the accuracy, of the RMI 1, ROMA scores, IOTA group’s simple ultrasound rules and other models and novel models not included in the scope of this assessment, and will consider the delivery of tests in the NHS (in which an imaging service is predominantly delivered by sonographers, rather than expert gynaecologists or radiologists). Trial registry entries for two additional diagnostic test accuracy studies were identified: one ongoing study is comparing the diagnostic performance or IOTA group’s simple ultrasound rules with that of ultrasound pattern recognition in women undergoing surgery for adnexal mass (the reference standard is the histopathological diagnosis) and the estimated completion date is September 2017;111 the second trial registry entry referred to a study assessing the diagnostic performance of a two-step triage process involving RMI 1 (threshold of 200) and IOTA group’s simple ultrasound rules, which has been terminated without publication. 114
The authors of 11 studies that were reported as conference abstracts with insufficient detail were contacted to determine whether or not the studies met our inclusion criteria, or when the outcomes were unclearly reported in the full paper;45,53,60,83,84,90,94,115–118 four authors provided additional information that allowed the study to be included in this review. 83,84,90,94
Overview of included studies
Details of the 51 included studies and their associated references are provided in Table 4. The following sections of this report cite studies using the primary publication and, when this is different, the publication (shown in bold in Table 5) in which the referenced data were reported.
| Details | Country | n | Main target condition reported |
|---|---|---|---|
| ROMA score | |||
| Abbott Diagnostics | |||
| ARCHITECT | |||
| Karlsen et al. (2012)83 | Denmark | 579 | All ovarian malignancies, excluding borderline |
| Al Musalhi et al. (2016)103 | Oman | 213 | All malignant tumours, including borderline |
| Chan et al. (2013)82 | Multinational (Asia) | 387 | All epithelial ovarian malignancies, including borderline |
| Clemente et al. (2015)90 | The Philippines | 62 | Ovarian malignancies (undefined – not clear whether or not borderline tumours were included) |
| Li et al. (2016)96 | China | 917 | Ovarian malignancies (undefined – not clear whether or not borderline tumours were included) |
| USA | 450 | All epithelial ovarian malignancies, including borderline | |
| Novotny et al. (2012)86 | Czech Republic | 277 | All malignant tumours, including borderline |
| Presl et al. (2012)81 | Czech Republic | 552 | Ovarian malignancies (undefined – not clear whether or not borderline tumours were included) |
| Winarto et al. (2014)99 | Indonesia | 128 | All epithelial ovarian malignancies, including borderline |
| Fujirebio Diagnostics | |||
| Langhe et al. (2013)94 | NR | 377 | All malignant tumours, including borderline |
| Belgium | 374 | All malignant tumours, including borderline | |
| Roche Diagnostics | |||
| Janas et al. (2015)97 | Poland | 259 | All malignant tumours, including borderline |
| Shulman et al. (2016)104 | USA | 993 | All malignant tumours, including borderline |
| Xu et al. (2016)95 | China | 521 | All epithelial ovarian malignancies, excluding borderline |
| Yanaranop et al. (2016)89 | Thailand | 260 | All malignant tumours – borderline tumours classified as disease negative |
| China | 612 | All epithelial ovarian malignancies | |
| Simple ultrasound rules (IOTA group) | |||
| Poland | 87 | All malignant tumours, including borderline | |
| Alcázar et al. (2013)52 | Spain | 340 | All malignant tumours, including borderline |
| Baker et al. (2013)66 | UK | 28 | All ovarian malignancies |
| Multinational (worldwide) | 2445 | All malignant tumours, including borderline | |
| Fathallah et al. (2011)63 | France | 109 | All malignant tumours, including borderline |
| IOTAa | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Poland | 226 | All malignant tumours, including borderline | |
| Meys et al. (2016)44 | The Netherlands | 326 | All malignant tumours, including borderline |
| Murala et al. (2014)60 | UK | 51 | All malignant tumours (undefined – not clear whether or not borderline tumours were included) |
| Italy | 391 | All malignant tumours, including borderline | |
| Ruiz de Gauna et al. (2015)64 | Spain | 154 | All malignant tumours, including borderline |
| Sayasneh et al. (2013)62 | UK | 255 | All malignant tumours, including borderline |
| Silvestre et al. (2015)55 | Brazil | 75 | All malignant tumours, including borderline |
| Tantipalakorn et al. (2014)51 | Thailand | 319 (masses) | All malignant tumours, including borderline |
| Testa et al. (2014)50 | Multinational (Europe) | 2403 | All malignant tumours, including borderline |
| Multinational (worldwide) | 1938 | All malignant tumours, including borderline | |
|
Tinnangwattana et al. (2015)47 Tongsong et al. (2016)59 |
Thailand | 94 | All malignant tumours, including borderline |
| Weinberger et al. (2013)53 | NR | 347 | All ovarian malignancies, including borderline |
| ADNEX model | |||
| IOTAa | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Joyeux et al. (2016)43 | France | 284 | Ovarian malignancies, including borderline |
| Meys et al. (2016)44 | The Netherlands | 326 | All malignant tumours, including borderline |
| Moffatt et al. (2016)45 | UK | 81 | Ovarian malignancies (undefined – not clear whether or not borderline tumours were included) |
| Sayasneh et al. (2016)46 | UK and Italy | 610 | All malignant tumours, including borderline |
| Szubert et al. (2016)42 | Poland and Spain | 327 | All ovarian malignancies, including borderline |
| Van Calster et al. (2014)17 | Multinational (Europe) | 2403 | All malignant tumours, including borderline |
| Overa (MIA2G) | |||
|
Coleman et al. (2016)70 Wolf et al. (2015)69 |
USA | 493 | All malignant tumours, including borderline |
| Shulman et al. (2016)104 | USA | 993 | All malignant tumours, including borderline |
| Zhang et al. (2015)68 | USA | 305 | All malignant tumours, including borderline |
| RMI 1 threshold variation | |||
| Aktürk et al. (2011)71 | Turkey | 100 | All ovarian malignancies, excluding borderline |
| Asif et al. (2004)77 | Pakistan | 100 | All malignant tumours (undefined – not clear whether or not borderline tumours were included) |
| Davies et al. (1993)79 | UK | 124 | All malignant tumours, including borderline |
| Jacobs et al. (1990)78 | UK | 139 | All malignant tumours, including borderline |
| Lou et al. (2010)73 | China | 223 | All malignant tumours, including borderline |
| Manjunath et al. (2001)75 | India | 148 | All malignant tumours, excluding borderline |
| Morgante et al. (1999)80 | Italy | 124 | All malignant tumours, including borderline |
| Tingulstad et al. (1996)76 | Norway | 173 | All malignant tumours, including borderline |
| Ulusoy et al. (2007)74 | Turkey | 296 | All malignant tumours, including borderline |
| Yamamoto et al. (2009)72 | Japan | 253 | All ovarian malignancies, including borderline |
All studies included in our systematic review were diagnostic cohort studies that reported data on the diagnostic accuracy of one or more ovarian cancer risk scores [the ROMA score, IOTA group’s simple ultrasound roles, the ADNEX model or Overa (MIA2G)], or that provided data on the accuracy of the RMI 1 at different decision thresholds (including 250, as specified in the current NICE guidelines1). Although 10 studies reported an age range that included women aged < 18 years,42,44,48,51,52,61,64,65,83,103 no study reported separate test performance data for this age group or indicated how many women were aged < 18 years. Sixteen studies reported data on the accuracy of the ROMA score,81–83,86,89,90,94–99,101–104 five of which reported data to support a direct comparison of the ROMA score to the RMI 1 score, using a decision threshold of 200. 83,89,98,99,103 There were no studies that reported comparative accuracy data for the ROMA score versus the RMI 1, using a decision threshold of 250. Seventeen published studies reported data on the accuracy of the IOTA group’s simple ultrasound rules,44,47–53,55,58,60–66 six of which reported data to support a direct comparison of the IOTA group’s simple ultrasound rules with the RMI 1 score, using a decision threshold of 200. 44,48,50,61,62,65 One study compared the IOTA group’s simple ultrasound rules with the RMI 1 score, using a decision threshold of 250, but this study was reported only as a conference abstract and the results were incomplete. 60 Six published studies reported data on the accuracy of the ADNEX model,17,42–46 one of which reported data to support a direct comparison of the ADNEX model with the RMI 1, using a decision threshold of 200. 44 The unpublished interim report (Frances Nixon, personal communication) provided data to support a direct comparison between the IOTA group’s simple ultrasound rules, the ADNEX model and the RMI 1 at both decision thresholds (200 and 250). Three studies reported data on the accuracy of Overa (MIA2G),68,70,104 one of which also provided comparative accuracy data for Overa (MIA2G) versus the ROMA score. 104 There were no studies comparing the accuracy of Overa (MIA2G) with the RMI 1, at any decision threshold. Finally, 10 studies provided data on the accuracy of the RMI 1 at different decision thresholds. 71–80
No randomised controlled trials (RCTs) or controlled clinical trials (CCTs) were identified; no studies provided data on patient-relevant outcomes following different risk assessment strategies.
Approximately half of the included published studies (25/51) were conducted in Europe,17,42–46,48–50,52,58,60,62–64,66,78–81,83,86,97,98,119 six of which were conducted solely in the UK45,60,62,66,78,79 and a further two were multinational studies that included a UK centre. 17,42 The unpublished interim analysis (Frances Nixon, personal communication) (confidential information has been removed). There were two multinational, worldwide studies, both of which included UK centres. 61,65 Four studies were conducted in the USA,68,70,101,104 13 were conducted in Asia,47,51,72,73,75,77,82,89,90,95,96,99,102 two were conducted in Turkey,71,74 one was conducted in Oman103 and one was conducted in Brazil. 55 Two studies, which were published only as conference abstracts, did not report information about geographic location. 53,94
Seventeen published studies,17,44,46,47,50,51,61,62,65,78,81,86,95–98,102 and the unpublished study for which an interim report was provided (confidential information has been removed) (Frances Nixon, personal communication), were publicly funded, and four studies reported receiving some funding from manufacturers (including a supply of test kits, reagents and analysers). 70,82,83,101 The remaining 29 included studies either did not report any information about funding42,43,45,48,49,52,53,55,58,60,63,64,66,68,71–77,79,80,89,90,94,99,104 or stated that they were unfunded. 103
All studies included women with an adnexal/ovarian mass; however, studies frequently reported analyses that excluded some women based on their final histopathological diagnosis (information that could not be known at the point of presentation); hence, only those studies that reported data for the target condition ‘all malignant tumours, including borderline’ could be considered to have evaluated risk scores in a population similar to those in whom these scores would be applied in practice. Full study details [inclusion and exclusion criteria, baseline characteristics of study participants and details of the risk score(s) (index test) evaluated are provided in Appendix 4 (Tables 34 and 35)].
Study quality
All studies included in this systematic review were diagnostic cohort studies. The methodological quality of these studies was assessed using the QUADAS-2 tool (summarised in Table 5 and Figure 2). One of these studies17 reported the development and validation of the ADNEX model, in addition to the test accuracy results. This study was assessed using PROBAST, a tool specifically developed to assess the methodological quality of prediction-modelling studies, (Table 6) as well as the QUADAS-2. Examples of full QUADAS-2 and PROBAST assessments are provided in Appendix 3, and full assessments for each included study are available on request.
| Study (year of publication) | Risk of bias | Applicability | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Abdalla et al. (2013)48 | ? | + | ? | + | – | – | – |
| Aktürk et al. (2011)71 | ? | + | ? | ? | + | + | + |
| Al Musalhi et al. (2016)103 | ? | + | ? | ? | + | + | – |
| Alcázar et al. (2013)52 | ? | + | ? | + | + | + | + |
| Asif et al. (2004)77 | + | ? | ? | ? | + | + | + |
| Baker et al. (2013)66 | – | ? | ? | – | + | – | ? |
| Chan et al. (2013)82 | + | + | + | – | + | + | + |
| Clemente et al. (2015)90 | ? | ? | + | + | ? | + | + |
| Coleman et al. (2016)70 | + | + | + | + | + | + | + |
| Davies et al. (1993)79 | + | + | – | – | + | + | ? |
| Di Legge et al. (2012)61 | + | + | ? | + | – | – | ? |
| Fathallah et al. (2011)63 | + | + | + | – | ? | + | + |
| IOTA5 (2017)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Jacobs et al. (1990)78 | + | + | – | – | + | + | ? |
| Janas et al. (2015)97 | ? | + | ? | + | ? | + | + |
| Joyeux et al. (2016)43 | ? | ? | ? | + | + | + | + |
| Karlsen et al. (2012)83 | ? | + | ? | – | + | + | + |
| Knafel et al. (2016)49 | + | + | ? | + | ? | – | – |
| Langhe et al. (2013)94 | ? | + | ? | – | ? | – | ? |
| Li et al. (2016)96 | + | + | ? | ? | ? | + | ? |
| Lou et al. (2010)73 | ? | ? | + | ? | + | + | – |
| Manjunath et al. (2001)75 | + | + | + | – | + | + | + |
| Meys et al. (2016)44 | + | – | + | + | ? | – | + |
| Moffatt et al. (2016)45 | ? | ? | + | – | + | – | ? |
| Moore et al. (2011)101 | ? | + | ? | – | – | + | + |
| Morgante et al. (1999)80 | + | ? | ? | ? | + | + | ? |
| Murala et al. (2014)60 | + | – | ? | – | + | ? | ? |
| Novotny et al. (2012)86 | ? | + | ? | ? | + | + | ? |
| Piovano et al. (2016)58 | + | + | + | + | ? | + | – |
| Presl et al. (2012)81 | ? | ? | ? | ? | + | ? | ? |
| Ruiz de Gauna et al. (2015)64 | + | + | + | + | + | + | + |
| Sayasneh et al. (2013)62 | + | + | ? | + | – | + | – |
| Sayasneh et al. (2016)46 | + | ? | + | + | + | + | – |
| Shulman et al. (2016)104 | ? | ? | ? | ? | + | + | – |
| Silvestre et al. (2015)55 | + | + | + | + | + | + | – |
| Szubert et al. (2016)42 | ? | + | ? | + | + | – | – |
| Tantipalakorn et al. (2014)51 | ? | + | ? | – | + | + | – |
| Testa et al. (2014)50 | + | + | + | + | – | – | – |
| Timmerman et al. (2010)65 | + | + | + | – | – | – | – |
| Tingulstad et al. (1996)76 | – | + | ? | ? | + | + | – |
| Tinnangwattana et al. (2015)47 | + | + | + | + | + | + | – |
| Ulusoy et al. (2007)74 | + | + | ? | + | – | – | – |
| Van Calster et al. (2014)17 | + | + | + | ? | + | + | – |
| Van Gorp et al. (2012)98 | + | + | ? | – | ? | – | + |
| Weinberger and Minar (2013)53 | ? | ? | ? | ? | ? | – | ? |
| Winarto et al. (2014)99 | ? | + | ? | + | ? | + | + |
| Xu et al. (2016)95 | – | + | ? | + | ? | + | + |
| Yamamoto et al. (2009)72 | ? | + | ? | + | + | + | + |
| Yanaranop et al. (2016)89 | ? | + | + | + | + | + | + |
| Zhang et al. (2015)68 | ? | ? | ? | ? | + | + | ? |
| Zhang et al. (2015)102 | – | + | ? | – | ? | + | + |
FIGURE 2.
Summary of QUADAS-2 results for accuracy studies of risk scores.

| Study (year of publication) | Risk of bias | Applicability concerns | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant selection | Predictors | Outcome | Analysis | Overall judgement | Participant selection | Predictors | Outcome | Overall judgement | ||||||||
| Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | |||
| Van Calster et al. (2014)17 | + | + | + | + | ? | ? | ? | + | ? | – | – | + | + | + | + | + |
Eight studies were reported only as conference abstracts or meeting slides, with limited descriptions of the methods used,45,53,60,66,68,90,94,104 and study methods were generally poorly reported. Thirty-seven studies (73%) were rated as having an ‘unclear’ risk of bias on at least one QUADAS-2 domain, and 24 studies (47%) were rated as being ‘unclear’ for applicability on at least one domain.
Two studies64,70 were rated as having a ‘low’ risk of bias and ‘low’ concerns regarding applicability for all domains, and four further studies were rated low for all risk-of-bias domains. 47,50,55,58 In total, 11 studies (22%) were rated as having ‘low’ concerns regarding all applicability domains. 43,52,64,70–72,75,77,82,83,89
Nineteen studies (37%) were rated as having a ‘high’ risk of bias on at least one QUADAS-2 domain, whereas 26 studies (51%) were rated as ‘high’ for applicability on at least one domain.
The main potential sources of bias across the included published studies concerned flow and timing. Fifteen studies (30%) were rated as having a ‘high’ risk of bias on the flow and timing domain. For most of these studies (13/1545,47,51,60,63,65,66,78,82,94,98,101,102), this was because not all included patients were included in the analysis. In five studies51,75,78,79,83 the included patients did not all receive the same reference standard.
The main areas of concern regarding applicability were in relation to how the index test was applied and whether or not this could be considered to be representative of routine practice, and how the reference standard positive (target condition) was defined. Fourteen studies (28%) were rated as having ‘high’ concerns regarding the applicability of the index test; for six studies,48,50,61,65,94,98 this was because all or part of the index test was performed before referral; in three studies,45,53,66 the index test was applied retrospectively to existing patient data; and in seven studies,42,44,49,50,53,65,74 the index test was performed by practitioners whose level of experience was judged to be higher than that likely to be routinely available in secondary care settings. Eighteen studies (35%) were rated as having ‘high’ concerns regarding the applicability of the reference standard because malignancy was defined as ‘any malignant tumour’, which could include non-ovarian cancers and metastases, whereas the scope of this assessment defined the target condition as ovarian cancer. However, it should be noted that, in order for a study to report risk score performance data for the specific target condition of ovarian cancer, study participants found to have non-ovarian cancers and metastases would need to be excluded from the analysis. Studies that excluded patients with non-ovarian cancers and metastases were rated as having a ‘high’ risk of bias on the flow and timing domain, because post hoc exclusion of these patients may result in overestimation of test performance. Appendix 4, Table 36 lists the final histological diagnoses (where reported) of the study participants. These data illustrate the between-study variations in the definitions of disease positive used, which could include borderline, non-ovarian cancers, metastatic cancers and non-ovarian metastatic cancers. To take into account as much of this heterogeneity as possible, the results were analysed according to whether or not disease positive (target condition) was defined as ‘ovarian malignancy’ or ‘any malignant tumour’, and whether or not this definition included borderline tumours.
(Confidential information has been removed.)
Overall, more than half of the included studies were rated as having a high or unclear risk of bias for patient selection, the reference standard and flow and timing. More than half of the studies were rated as having a high level of, or unclear, concern for the applicability of the reference standard.
The PROBAST prediction score (see Table 6) for Van Calster et al. 17 indicated that there was a high risk of bias for the applicability of patient selection. The high risk of bias was attributable to the selection of women from a mixture of secondary and tertiary care centres, which is not a complete match for the scope of this assessment. However, the ADNEX model adjusts for study setting and, therefore, the overall concern regarding applicability is low. The overall risk of bias was judged to be unclear, as not all aspects of the model development were clearly described.
Clinical effectiveness of risk scores
No RCTs or CCTs were identified; no studies provided data on patient-relevant outcomes following different risk assessment strategies.
Diagnostic performance of the Risk of Ovarian Malignancy Algorithm score
Details of Risk of Ovarian Malignancy Algorithm studies
Sixteen diagnostic cohort studies,81–83,86,89,90,94–99,101–104 reported in 24 publications,56,81–83,85–104 provided data on the diagnostic performance of the ROMA score for identifying women who have an adnexal mass and are at a high risk of developing ovarian cancer. Nine studies81–83,86,90,96,99,101,103 used a ROMA score based on Abbott Diagnostics’ ARCHITECT tumour marker assays, of which six81,82,86,96,99,103 evaluated a decision threshold for the ROMA score that was consistent with the manufacturer’s recommendations. None of the included studies used the Fujirebio Diagnostics’ LUMIPULSE G automated CEIA system. For information, two studies94,98 that used a ROMA score based on manual Fujirebio Diagnostics’ tumour marker EIAs (see Appendix 5, Tables 41 and 42) were included, both using the manufacturer’s recommended decision threshold for the ROMA score; however, it should be noted that the manual assays are not specified interventions for this assessment. Finally, five studies89,95,97,102,104 used a ROMA score based on Roche Diagnostics’ Elecsys tumour marker assays, all of which used the manufacturer’s recommended decision threshold for the ROMA score.
None of the ROMA score studies that used Abbott Diagnostics’ ARCHITECT tumour marker assays was conducted in the UK; three studies81,83,86 were conducted in European countries, four82,90,96,99 were conducted in Asia, one101 was conducted in the USA and one103 was conducted in Oman. None of the ROMA score studies that used Roche Diagnostics’ Elecsys tumour marker assays was conducted in the UK, and only one97 was conducted in a European country. Three89,93,95 of the remaining studies were conducted in Asia and one104 was conducted in the USA.
This assessment is primarily concerned with providing a comparison between the RMI 1,78 used with a decision threshold of 250 (current standard practice in the NHS1), and the specified alternative risk-scoring methods (see Chapter 2, Intervention technologies). No studies were identified that reported a direct comparison (both tests used to assess the same patient cohort) between the ROMA score and the RMI 1, used with a decision threshold of 250. Five studies reported direct comparisons between the ROMA score and the RMI 1, used with a decision threshold of 200; three studies used Abbott Diagnostics’ ARCHITECT tumour marker assays;83,99,103 one study used Roche Diagnostics’ Elecsys assays;89 and one study used Fujirebio Diagnostics’ manual EIAs. 98 The following sections report all available data from direct comparison studies, as well as non-comparative data on the accuracy of the ROMA score, when decision thresholds that were consistent with the manufacturers’ recommendations were used. Additional accuracy data for alternative decision thresholds are reported in Appendix 5, Table 37.
The target condition for this assessment is ovarian cancer, including conditions covered by the NICE clinical guideline CG1221 (i.e. epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer). All studies in this section included women with one or more adnexal mass. The definition of reference standard positive ‘ovarian cancer’ varied between studies, with borderline tumours being most frequently classified as positive or excluded from analyses. In addition, some studies included patients with non-ovarian primary cancers/metastases to the ovary97,98,103 and germ cell tumours. 103 When the target condition was described as ‘all ovarian malignancy’, those women whose postoperative histological diagnosis was identified as non-ovarian primary were excluded from the estimates of test performance. Conversely, when the target condition was described as ‘all malignant tumours’, women with a non-ovarian primary were not excluded and were classified as being disease positive; this could potentially include women with any tumour on the ovaries that has metastasised from another primary [e.g. colorectal cancer (CRC)], and/or women with an adnexal/pelvic mass that turns out to be non-ovarian (not clearly specified by the included studies). Full details of the final histopathological diagnoses of study women who had a malignant mass are reported in Appendix 4, Table 36.
Accuracy of the Risk of Ovarian Malignancy Algorithm score using Abbott Diagnostics’ ARCHITECT tumour marker assays
Three83,99,103 of the nine81–83,86,90,96,99,101,103 ROMA score studies that used Abbott Diagnostics’ ARCHITECT tumour marker assays, reported a direct comparison of the ROMA score with the RMI 1. Only one study included all participants the analysis, regardless of their final histopathological diagnosis (target condition: all malignant tumours, including borderline) and this study used different thresholds from those recommended by the manufacturer (13.1% in premenopausal women and 27.7% in postmenopausal women, as opposed to the manufacturer’s recommendation of 7.4% and 25.3%). 103 One study was a retrospective study, which excluded women with histopathological diagnoses other than epithelial ovarian cancer. 99 A second study excluded from the analysis nine women (1%) with non-epithelial ovarian cancer, 69 women (6%) with non-ovarian cancers and 252 women (21%) with borderline tumours;83 the distribution of positive and negative ROMA score results in these women was not reported.
The sensitivity estimate for the ROMA score was highest (96.4%, 95% CI 93.6% to 98.2%) when the analyses excluded women with borderline tumours and those with malignancies other than epithelial ovarian cancer, and lowest (75.0%, 95% CI 60.4% to 86.4%) when all women were included in the analysis, regardless of their final histopathological diagnosis (Table 7). Conversely, the specificity estimate for the ROMA score was highest (87.9%, 95% CI 81.9% to 92.4%) in the study that included all participants,103 and lowest (53.3%, 95% CI 50.0% to 56.7%) when the analyses excluded women with borderline tumours and those with malignancies other than epithelial ovarian cancer (see Table 7). When women with borderline tumours and/or those with malignancies other than epithelial ovarian cancer were excluded from the analyses, the sensitivity estimates for the ROMA score were not significantly different from those for the RMI 1 (threshold 200), whereas the specificity estimates were significantly lower (see Table 7). In contrast, the study that included all participants in the analysis reported similar sensitivity and specificity estimates for the ROMA score and the RMI 1, with a sensitivity of 75% (95% CI 60.4% to 86.4%) versus 77.1% (95% CI 62.7% to 88.0%), and a specificity of 87.9% (95% CI 81.9% to 92.4%) versus 81.8% (95% CI 75.1% to 87.4%), respectively. 103 This study also reported lower sensitivity and higher specificity estimates, for both the ROMA score and the RMI 1, in premenopausal women than those in postmenopausal women (see Table 7).
| Study (year of publication) | Subgroup | ROMA threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | RMI 1 | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours, including borderline | |||||||||||||||||
| Al Musalhi et al. (2016)103 | All women | 13.1%/27.7% | 36 | 12 | 20 | 145 | 213 | 75.0 (60.4 to 86.4) | 87.9 (81.9 to 92.4) | 200 | 37 | 11 | 30 | 135 | 213 | 77.1 (62.7 to 88.0) | 81.8 (75.1 to 87.4) |
| Premenopausal women | 13.1% | 11 | 10 | 14 | 127 | 162 | 52.4 (29.8 to 74.3) | 90.1 (83.9 to 94.5) | 200 | 12 | 9 | 21 | 120 | 162 | 57.1 (34.0 to 78.2) | 85.1 (78.1 to 90.5) | |
| Postmenopausal women | 27.7% | 25 | 2 | 5 | 19 | 51 | 92.6 (75.7 to 99.1) | 79.2 (57.8 to 92.9) | 200 | 22 | 2 | 9 | 18 | 51 | 91.7 (73.0 to 99.0) | 66.7 (46.0 to 83.5) | |
| Target condition: epithelial ovarian malignancies, including borderline | |||||||||||||||||
| Winarto et al. (2014)99 | All women | 7.4%/25.3% | 61 | 6 | 35 | 26 | 128 | 91.0 (81.5 to 96.6) | 42.6 (30.0 to 55.9) | 200 | 54 | 13 | 21 | 40 | 128 | 80.6 (69.1 to 89.2) | 65.6 (52.3 to 77.3) |
| Target condition: epithelial ovarian malignancies, excluding borderline | |||||||||||||||||
| Karlsen et al. (2012)83 | All women | 7.4%/25.3% | 244 | 8 | 371 | 438 | 1061 | 96.8 (93.8 to 98.6) | 54.1 (50.6 to 57.6) | 200 | 238 | 14 | 150 | 659 | 1061 | 94.4 (90.9 to 96.9) | 81.5 (78.6 to 84.1) |
| Winarto et al. (2014)99 | All women | 7.4%/25.3% | 47 | 3 | 35 | 26 | 111 | 94.0 (83.5 to 98.7) | 42.6 (30.0 to 55.9) | 200 | 44 | 6 | 21 | 40 | 111 | 88.0 (75.7 to 95.5) | 65.6 (52.3 to 77.3) |
| Summary estimates | 96.4 (93.6 to 98.2) | 53.3 (50.0 to 56.7) | 93.4 (90.0 to 95.9) | 80.3 (77.5 to 82.9) | |||||||||||||
| Karlsen et al. (2012)83 | Premenopausal women | 7% | 46 | 3 | 251 | 279 | 579 | 93.9 (83.1 to 98.7) | 52.6 (48.3 to 57.0) | 200 | 41 | 8 | 42 | 488 | 579 | 83.7 (70.3 to 92.7) | 92.1 (89.4 to 94.2) |
| Postmenopausal women | 25.3% | 198 | 5 | 120 | 159 | 482 | 97.5 (94.3 to 99.2) | 57.0 (51.0 to 62.9) | 200 | 196 | 7 | 108 | 171 | 482 | 96.6 (93.0 to 98.6) | 61.3 (55.3 to 67.0) | |
One study reported test performance estimates calculated both with and without the inclusion of participants with borderline tumours. 99 Although the number of participants involved was small, these data indicated that around half of the FN risk scores were accounted for by women with borderline tumours, 3 out of 6 (50%) using the ROMA score and 7 out of 13 (54%) using the RMI 1 (threshold of 200). 99 Approximately 13% (17/128) of the women in this study had borderline tumours, whereas 39% (50/128) had malignant tumours [i.e. a higher proportion of women with borderline tumours had a negative ROMA score – 17.6% (3/17) – than was the case for women with malignant tumours – 6% (3/50)]. 99 A similar pattern was observed for the RMI 1; the proportion of women with borderline tumours who had a negative RMI 1 was approximately 41% (7/17), compared with 12% (6/50) for those with malignant ovarian tumours. 99
One additional study reported performance estimates for the ROMA score, excluding women with borderline tumours and those with non-ovarian malignancies, without a comparison with the RMI 1. 82 When data from this study were combined with the ROMA data from the two similar comparative accuracy studies,83,99 the summary estimates of sensitivity did not change significantly [95.1%, 95% CI 92.4% to 97.1%, based on three studies (Table 8), vs. 96.4%, 95% CI 93.6% to 98.2%, based on two studies; see Table 7]. The summary estimate of specificity, based on all three studies (62.5%, 95% CI 59.7% to 65.3%; see Table 8), was higher than that derived from the two comparative accuracy studies alone (53.3%, 95% CI 50% to 56.7%; see Table 7). There were no additional studies that evaluated the performance of the ROMA score alone, and included all participants in the analysis (target condition: all malignant tumours, including borderline).
| Study (year of publication) | Subgroup | Threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: epithelial ovarian malignancies, including borderline | |||||||||
| Winarto et al. (2014)99 | All women | 7.4%/25.3% | 61 | 6 | 35 | 26 | 128 | 91.0 (81.5 to 96.6) | 42.6 (30.0 to 55.9) |
| Target condition: epithelial ovarian malignancies, excluding borderline | |||||||||
| Karlsen et al. (2012)83 | All women | 7.4%/25.3% | 244 | 8 | 371 | 438 | 1061 | 96.8 (93.8 to 98.6) | 54.1 (50.6 to 57.6) |
| Chan et al. (2013)82 | All women | 7.4%/25.3% | 58 | 7 | 41 | 281 | 387 | 89.2 (79.1 to 95.6) | 87.3 (83.1 to 90.7) |
| Winarto et al. (2014)99 | All women | 7.4%/25.3% | 47 | 3 | 35 | 26 | 111 | 94.0 (83.5 to 98.7) | 42.6 (30.0 to 55.9) |
| Summary estimates | 95.1 (92.4 to 97.1) | 62.5 (59.7 to 65.3) | |||||||
| Karlsen et al. (2012)83 | Premenopausal women | 7% | 46 | 3 | 251 | 279 | 579 | 93.9 (83.1 to 98.7) | 52.6 (48.3 to 57.0) |
| Chan et al. (2013)82 | Premenopausal women | 7% | 18 | 4 | 34 | 235 | 291 | 81.8 (59.7 to 95.9) | 87.4 (82.8 to 91.1) |
| Summary estimates | 90.1 (80.7 to 95.9) | 64.3 (60.9 to 67.7) | |||||||
| Karlsen et al. (2012)83 | Postmenopausal women | 25.3% | 198 | 5 | 120 | 159 | 482 | 97.5 (94.3 to 99.2) | 57.0 (51.0 to 62.9) |
| Chan et al. (2013)82 | Postmenopausal women | 25.3% | 40 | 3 | 7 | 46 | 96 | 93.0 (80.9 to 98.5) | 86.8 (56.3 to 67) |
| Summary estimates | 96.7 (93.7 to 98.6) | 61.7 (56.3 to 67.0) | |||||||
| Target condition: epithelial ovarian malignancies (stage III/IV) – borderline and stage I/II tumours excluded | |||||||||
| Chan et al. (2013)82 | All women | 7.4%/25.3% | 35 | 3 | 41 | 281 | 360 | 92.1 (78.6 to 98.3) | 87.3 (83.1 to 90.7) |
| Premenopausal women | 7% | 10 | 2 | 34 | 235 | 281 | 83.3 (51.6 to 97.9) | 87.4 (82.8 to 91.1) | |
| Postmenopausal women | 25.3% | 24 | 1 | 7 | 46 | 78 | 96.0 (79.6 to 99.9) | 86.8 (74.7 to 94.5) | |
| Target condition: epithelial ovarian malignancies (stage I/II) – borderline and stage III/IV tumours excluded | |||||||||
| Chan et al. (2013)82 | All women | 7.4%/25.3% | 19 | 4 | 41 | 281 | 345 | 82.6 (61.2 to 95.0) | 87.3 (83.1 to 90.7) |
| Premenopausal women | 7.4% | 6 | 2 | 34 | 235 | 277 | 75.0 (34.9 to 96.8) | 87.4 (82.8 to 91.1) | |
| Postmenopausal women | 25.3% | 12 | 2 | 7 | 46 | 67 | 85.7 (57.2 to 98.2) | 86.8 (74.7 to 94.5) | |
| Target condition: ovarian borderline tumours – higher-stage tumours excluded | |||||||||
| Chan et al. (2013)82 | All women | 7.4%/25.3% | 9 | 7 | 41 | 281 | 338 | 56.3 (29.9 to 80.2) | 87.3 (83.1 to 90.7) |
| Premenopausal women | 7.4% | 6 | 2 | 34 | 235 | 277 | 75.0 (34.9 to 96.8) | 87.4 (82.8 to 91.1) | |
| Postmenopausal women | 25.3% | 12 | 2 | 7 | 46 | 67 | 85.7 (57.2 to 98.2) | 86.8 (74.7 to 94.5) | |
One study82 assessed the variation in the performance of the ROMA score with different stages of epithelial ovarian cancer (see Table 8). The sensitivity estimate was highest (92.1%, 95% CI 78.6% to 98.3%) when the target condition was stage III/IV epithelial ovarian cancer, and women with stage I/II and borderline disease were excluded from the analysis. 82 There was a small, but non-significant, fall in sensitivity (82.6%, 95% CI 61.2% to 95.0%) when the target condition was stage I/II epithelial ovarian cancer and women with borderline and higher-stage disease were excluded from the analysis. 82 When the target condition was borderline epithelial tumours and all women with higher-stage disease were excluded from the analysis, the sensitivity estimate was significantly lower (56.3%, 95% CI 29.9% to 80.2%). 82 These data are consistent with the observation that the proportion of women with a negative ROMA score is higher among those women with borderline disease than among those with ovarian malignancies, and may also be higher among those with lower-stage epithelial ovarian cancer than those with higher-stage epithelial ovarian cancer.
Two studies81,96 reported accuracy data for ovarian malignancy, but without clarifying whether or not the definition of malignancy included borderline tumours (see Appendix 5, Table 43). Accuracy data for thresholds other than those recommended by the manufacturer (7.4% in premenopausal women and 25.3% in postmenopausal women) are reported in Appendix 5, Table 37; no study reported accuracy data at an alternative threshold for the inclusive target condition of all malignant tumours, including borderline, and no alternative threshold offered a clear performance advantage.
Accuracy of the Risk of Ovarian Malignancy Algorithm score using Fujirebio Diagnostics’ tumour marker assays
None of the included studies used the Fujirebio Diagnostics’ LUMIPULSE G automated CEIA system; hence, there were no studies of the ROMA score, using Fujirebio Diagnostics’ assays, that met the inclusion criteria for this assessment. Two studies94,98 that evaluated a ROMA score based on manual Fujirebio Diagnostics’ tumour marker EIAs have been included in this report. These studies are included for information only. Both of these studies included all women in the analysis, regardless of their final histopathological diagnosis (target condition: all malignant tumours, including borderline). One study98 reported a direct comparison of the ROMA score with the RMI 1 (threshold of 200). The results of these studies are provided in Appendix 5, Tables 41 and 42.
Accuracy of the Risk of Ovarian Malignancy Algorithm score using Roche Diagnostics’ tumour marker assays
Only one89 of the five89,95,97,102,104 ROMA score studies, which used Roche Diagnostics’ Elecsys tumour marker assays, reported a direct comparison of the ROMA score with the RMI 1 (threshold of 200). This study classified women found to have borderline ovarian tumours as disease negative and included women whose final histopathological diagnoses were epithelial ovarian cancer, non-epithelial ovarian cancer and metastases from non-ovarian primaries (target condition: all malignant tumours). 89 This study may be considered to be more applicable to clinical practice if it is considered to be preferable to manage women with borderline tumours in non-specialist settings. In these women, the sensitivity estimate for the ROMA score appeared to be slightly higher than that for the RMI 1 (83.8%, 95% CI 73.4% to 91.3%, vs. 78.4%, 95% CI 67.3% to 87.1%), and the specificity estimate for the ROMA score appeared to be slightly lower than that for the RMI 1 (68.8%, 95% CI 61.6% to 75.4%, vs. 79.6%, 95% CI, 73.1% to 85.1%), but neither difference was statistically significant. 89 A similar pattern was observed when data were stratified by menopausal status (Table 9). The same study also reported test performance data, whereby eight women (3%) with non-epithelial ovarian cancer and non-ovarian primaries were excluded from the analysis. This exclusion did not significantly change the test performance estimates for either the ROMA score or the RMI 1 (see Table 9). Although the number involved was small, it should be noted that women with malignancies other than epithelial ovarian cancer accounted for four (50%) of the FN results, using the ROMA score, and for three (37.5%) of the results, using the RMI 1. 89
| Study (year of publication) | Subgroup | ROMA threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | RMI 1 | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours – borderline tumours classified as disease negative | |||||||||||||||||
| Yanaranop et al. (2016)89 | All women | 11.4%/29.9% | 62 | 12 | 58 | 128 | 260 | 83.8 (73.4 to 91.3) | 68.8 (61.6 to 75.4) | 200 | 58 | 16 | 38 | 148 | 260 | 78.4 (67.3 to 87.1) | 79.6 (73.1 to 85.1) |
| Premenopausal women | 11.4% | 24 | 4 | 35 | 85 | 148 | 85.7 (67.3 to 96.0) | 70.8 (61.8 to 78.8) | 200 | 21 | 7 | 23 | 97 | 148 | 75.0 (55.1 to 89.3) | 80.8 (72.6 to 87.4) | |
| Postmenopausal women | 29.9% | 38 | 8 | 23 | 43 | 112 | 82.6 (68.6 to 92.2) | 65.2 (52.4 to 76.5) | 200 | 37 | 9 | 15 | 51 | 112 | 80.4 (66.1 to 90.6) | 77.3 (65.3 to 86.7) | |
| Target condition: epithelial ovarian malignancies – borderline tumours classified as disease negative | |||||||||||||||||
| Yanaranop et al. (2016)89 | All women | 11.4%/29.9% | 58 | 8 | 58 | 128 | 252 | 87.9 (77.5 to 94.6) | 68.8 (61.6 to 75.4) | 200 | 53 | 13 | 38 | 148 | 252 | 80.3 (68.7 to 89.1) | 79.6 (73.1 to 85.1) |
| Target condition: epithelial ovarian malignancies (stage I) – borderline tumours classified as disease negative and higher-stage tumours excluded | |||||||||||||||||
| Yanaranop et al. (2016)89 | All women | 11.4%/29.9% | 23 | 7 | 58 | 128 | 216 | 76.7 (57.7 to 90.1) | 68.8 (61.6 to 75.4) | 200 | 21 | 9 | 38 | 148 | 216 | 70.0 (50.6 to 85.3) | 79.6 (73.1 to 85.1) |
| Target condition: epithelial ovarian malignancies (stages II–IV) – borderline tumours classified as disease negative and stage I tumours excluded | |||||||||||||||||
| Yanaranop et al. (2016)89 | All women | 11.4%/29.9% | 35 | 1 | 58 | 128 | 222 | 97.2 (85.5 to 99.9) | 68.8 (61.6 to 75.4) | 200 | 32 | 4 | 38 | 148 | 222 | 88.9 (73.9 to 96.9) | 79.6 (73.1 to 85.1) |
The aforementioned comparative accuracy study89 also assessed the variation in the performance of the ROMA score with different stages of epithelial ovarian cancer (see Table 9). The sensitivity estimate was highest for both the ROMA score (97.2%, 95% CI 95.5% to 99.9%) and the RMI 1 score (88.9%, 95% CI 73.9% to 96.9%), for which the target condition was stages II–IV epithelial ovarian cancer; women with stage I disease were excluded from the analysis. 89 As with the ROMA score using Abbott Diagnostics’ ARCHITECT tumour marker assay, sensitivity estimates were lower for both the ROMA score (76.7%, 95% CI 57.7% to 90.1%) and the RMI 1 score (70.0%, 95% CI 50.6% to 85.3%), for which the target condition was stage I epithelial ovarian cancer; women with higher-stage disease were excluded from the analysis. 89 This indicates that the proportion of women with a negative ROMA score may be higher among those with lower-stage epithelial ovarian cancer than those with higher-stage epithelial ovarian cancer.
Two97,104 of the four95,97,102,104 additional studies that evaluated the performance of the ROMA score but did not provide a comparison with the RMI 1 score included all study participants in the analysis regardless of their final histopathological diagnoses [target condition: all malignant tumours including borderline (Table 10)]. The summary estimate of the sensitivity of the ROMA score (79.1%, 95% CI 74.2% to 83.5%), derived from these two studies, was lower than that reported by the comparative accuracy study89 described earlier, in which women with borderline tumours were classified as disease negative, and the summary specificity estimate was also lower (79.1%, 95% CI 76.3% to 81.6%), but these differences were not statistically significant. Two studies95,97 reported test performance data for the ROMA score, in which women found to have borderline tumours and those with non-ovarian primaries were excluded from the analyses. The sensitivity estimates derived from these two studies were very different (see Table 10) hence, no summary estimates were calculated. One of these studies97 reported test performance estimates calculated both with and without the inclusion of women with borderline tumours and those with non-ovarian primaries. As with the ROMA score, using Abbott Diagnostics’ ARCHITECT tumour marker assays, these data indicated that women with borderline tumours and those with non-ovarian primaries accounted for a high proportion (12/14; 86%) of the FN risk scores observed. 97
| Study (year of publication) | Subgroup | Threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity %, (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||
| aJanas et al. (2015)97 | All women | 11.4%/29.9% | 52 | 14 | 39 | 154 | 259 | 78.8 (67.0 to 87.9) | 79.8 (73.4 to 85.2) |
| Shulman et al. (2016)104 | All women | 11.4%/29.9% | 194 | 51 | 158 | 590 | 993 | 79.2 (73.7 to 83.8) | 78.9 (75.8 to 81.7) |
| Summary estimates | 79.1 (74.2 to 83.5) | 79.1 (76.3 to 81.6) | |||||||
| aJanas et al. (2015)97 | Premenopausal women | 11.4% | 9 | 1 | 22 | 100 | 132 | 90.0 (55.5 to 99.7) | 82.0 (74.0 to 88.3) |
| aJanas et al. (2015)97 | Postmenopausal women | 29.9% | 44 | 12 | 17 | 54 | 127 | 78.6 (65.6 to 88.4) | 76.1 (64.5 to 88.4) |
| Target condition: ovarian malignancies excluding borderline | |||||||||
| aJanas et al. (2015)97 | All women | 11.4%/29.9% | 42 | 2 | 39 | 154 | 237 | 95.5 (84.5 to 99.4) | 79.8 (73.4 to 85.2) |
| Xu et al. (2016)95 | All women | 11.4%/29.9% | 113 | 97 | 39 | 272 | 521 | 53.8 (46.8 to 60.7) | 87.5 (83.3 to 90.9) |
| aJanas et al. (2015)97 | Premenopausal women | 11.4% | 6 | 0 | 22 | 100 | 128 | 100 (54.1 to 100) | 82.0 (74.0 to 88.3) |
| Xu et al. (2016)95 | Premenopausal women | 11.4% | 56 | 51 | 38 | 226 | 371 | 54.9 (42.5 to 62.1) | 85.6 (80.8 to 89.6) |
| aJanas et al. (2015)97 | Postmenopausal women | 29.9% | 36 | 2 | 17 | 54 | 109 | 94.7 (82.3 to 99.4) | 76.1 (64.5 to 85.4) |
| Xu et al. (2016)95 | Postmenopausal women | 29.9% | 57 | 46 | 1 | 46 | 150 | 53.3 (45.2 to 65.1) | 97.9 (88.7 to 99.9) |
One study102 provided performance estimates for the ROMA score, using Roche Diagnostics’ Elecsys tumour marker assays, for the target condition ‘ovarian malignancy’, when it was not clear whether or not the definition of malignancy included borderline tumours (see Appendix 5, Table 44). Accuracy data for thresholds other than those recommended by the manufacturer (11.4% in premenopausal women and 29.9% in postmenopausal women) are reported in Appendix 5, Table 37; no study reported accuracy data at an alternative threshold for the inclusive target condition of all malignant tumours including borderline, and no alternative threshold offered a clear performance advantage.
Between-assay comparisons
No study assessed variation in the performance of the ROMA score with the use of different manufacturers’ tumour marker assays. However, between-study comparisons indicate that, when all study participants were included in the analyses regardless of final histopathological diagnosis (target condition: all malignant tumours including borderline), the estimates of sensitivity did not differ significantly between the two manufacturers’ assays for which data were available (Figure 3). The sensitivity estimate for the ROMA score using Abbott Diagnostics’ ARCHITECT tumour marker assay was 75.0% (95% CI 60.4% to 86.4%), derived from one study,103 compared with 79.1% (95% CI 74.2% to 83.5%) using Roche Diagnostics’ Elecsys tumour marker assay, derived from two studies. 97,104 However, the specificity estimate for Abbott Diagnostics’ ARCHITECT tumour marker assay (87.9%, 95% CI 81.9% to 92.4%) was higher than that for Roche Diagnostics’ Elecsys tumour marker assay (79.1%, 95% CI 76.3% to 81.6%). There were no studies of the ROMA score using Fujirebio Diagnostics’ tumour marker assays that met the inclusion criteria for this assessment. There were insufficient data to compare the performance of the ROMA score with the use of different manufacturers’ tumour marker assays for detecting different stages of disease.
FIGURE 3.
Comparison of the accuracy of the ROMA score using Abbott Diagnostics’ ARCHITECT vs. the ROMA score using Roche Diagnostics’ Elecsys (target condition: all malignant tumours including borderline). (a) Sensitivity estimate; and (b) specificity estimate.

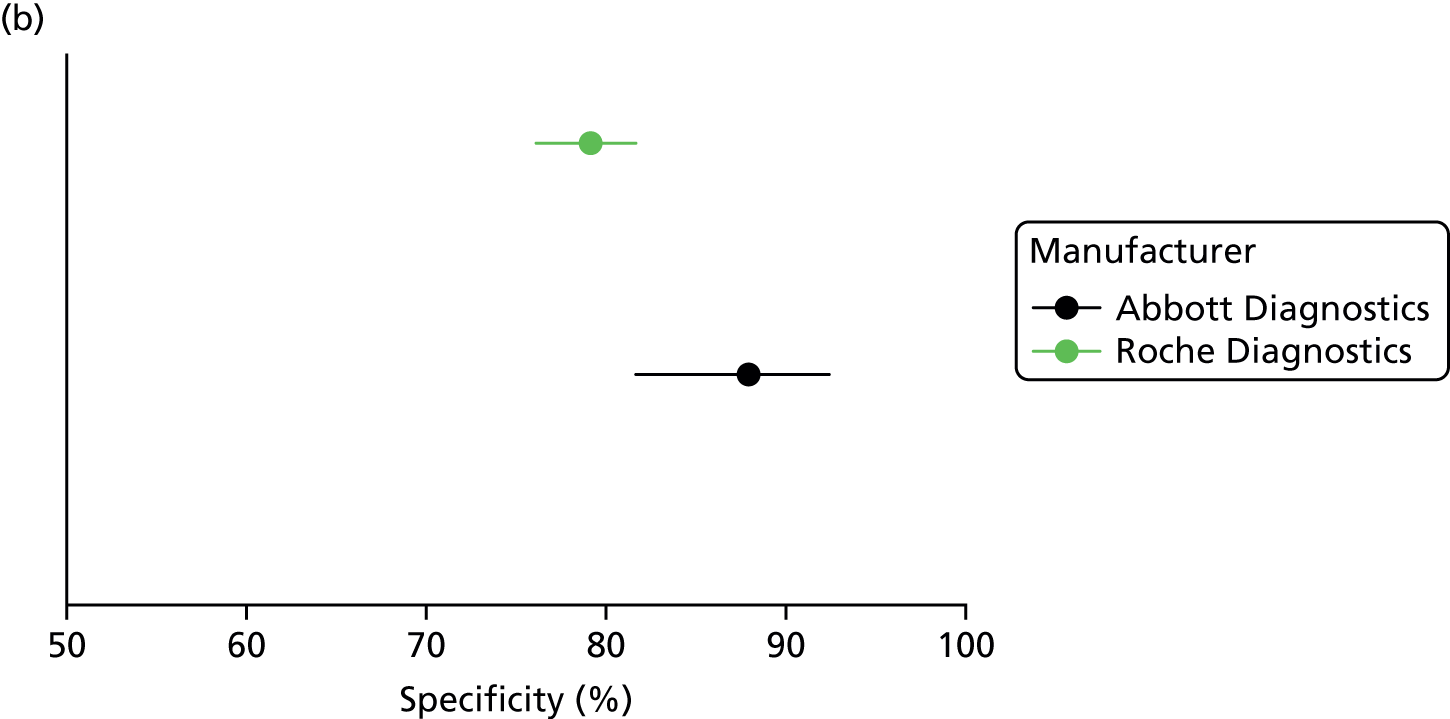
Diagnostic performance of the International Ovarian Tumour Analysis group’s simple ultrasound rules and the Assessment of Different NEoplasias in the adneXa model
Details of the Assessment of Different NEoplasias in the adneXa studies
Six published studies17,42–46 and one unpublished interim report (Frances Nixon, personal communication) provided data on the diagnostic performance of the ADNEX scores at different thresholds. All studies reported accuracy data for the validated 10% decision threshold to identify women with an adnexal mass who were at a high risk of developing ovarian cancer and used a version of the ADNEX model that included a measurement of CA125 level. Four of the six published studies did not report any details of the experience of those performing the ultrasound examinations. 42–45 One study46 reported that ultrasound examinations were performed by European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) level 2 ultrasound examiners (non-consultant gynaecology specialist, gynaecology trainee doctors and gynaecology sonographers), and the remaining study17 used EFSUMB level 2/3 practitioners with 8–20 years’ experience in gynaecological sonography. (Confidential information has been removed.)
This section reports accuracy data for only the 10% threshold. Three studies17,43,46 provided accuracy data for additional thresholds and these are reported in Appendix 5, Table 38. All studies in this section were conducted in Europe; one study45 was conducted solely in the UK, and two were multicentre studies17,46 that included UK participants.
The target condition for this assessment is ovarian cancer, including conditions covered by the NICE clinical guideline CG1221 (i.e. epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer). All studies in this section include women with one or more adnexal mass, and all but one study45 included borderline tumours in their definition of malignancy; the study that did not was reported only as a conference abstract and it was not clear whether or not any borderline tumours were included (see Appendix 5, Table 45). Three published studies17,44,46 and the unpublished interim report (Frances Nixon, personal communication) included participants with ‘other malignancies’, metastases from non-ovarian sites and ‘non-ovarian cancers’. When the target condition was described as ‘all ovarian malignancy’, those participants whose postoperative histological diagnosis identified a non-ovarian primary were excluded from the estimates of test performance. Conversely, when the target condition was described as ‘all malignant tumours’, participants with a non-ovarian primary were not excluded and were classified as disease positive; this could potentially include participants with any tumour on the ovaries that has metastasised from another primary (e.g. CRC) and/or participants with an adnexal/pelvic mass that turns out to be non-ovarian (not clearly specified by the included studies). Full details of the final histopathological diagnoses of study participants who had a malignant mass are reported in Appendix 4, Table 36.
Accuracy of the Assessment of Different NEoplasias in the adneXa model for determining high risk of ovarian cancer
Three published studies17,44,46 and the unpublished interim report (Frances Nixon, personal communication) included all participants in the analysis, regardless of their final histopathological diagnosis (target condition: all malignant tumours including borderline). The summary estimate of sensitivity derived from these studies was 96.3% (95% CI 95.3% to 97.1%) and the summary estimate of specificity was 69.1% (95% CI 67.4% to 70.8%; Table 11). These estimates did not differ significantly from those calculated from only those studies of the ADNEX model that reported a direct comparison with the RMI 1 score (at a threshold of 200 or 250; see Table 14). Two further studies,42,43 reporting three data sets, excluded women with histopathological diagnoses other than primary ovarian cancer. The summary estimate of sensitivity (94%, 95% CI 88.6% to 97.4%) derived from these studies did not differ significantly from that derived from the studies that included all participants in their analyses. However, the summary estimate of specificity (77.6%, 95% CI 73.6% to 81.2%) was higher. One study,42 which reported results from two separate cohorts (Spain and Poland), also reported accuracy data stratified by menopausal status. Menopausal status did not significantly affect sensitivity; however, the specificity estimate was significantly higher in premenopausal women than in postmenopausal women (see Table 11).
| Study (year of publication) | Subgroup | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||
| IOTA5 (2017)a,b | All women | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Meys et al. (2016)44 | 113 | 2 | 80 | 131 | 326 | Calculated | 98.0 (93.0 to 100) | 62.0 (55.0 to 68.0) | |
| Sayasneh et al. (2016)46 | 177 | 5 | 138 | 290 | 610 | Calculated | 97.3 (93.5 to 98.9) | 67.7 (63.0 to 72.0) | |
| bVan Calster et al. (2014)17 | 946 | 34 | 408 | 1015 | 2403 | Calculated | 96.5 (95.2 to 97.6) | 71.3 (68.9 to 73.7) | |
| Summary estimates | 96.3 (95.3 to 97.1) | 69.1 (67.4 to70.8) | |||||||
| Meys et al. (2016)44 | Premenopausal women | 31 | 0 | 28 | 69 | 128 | Calculated | 100 (86.0 to 100) | 71.0 (61.0 to 80.0) |
| Meys et al. (2016)44 | Postmenopausal women | 82 | 2 | 52 | 62 | 198 | Calculated | 98.0 (91.0 to 100) | 54.0 (44.0 to 63.0) |
| Target condition: ovarian malignancies including borderline | |||||||||
| Joyeux et al. (2016)43 | All women | 27 | 3 | 48 | 206 | 284 | Calculated | 90 (73.5 to 97.9) | 81.1 (75.7 to 85.7) |
| Szubert et al. (2016)42 | All women – Poland | 66 | 4 | 37 | 97 | 204 | Reported | 94.3 (88.5 to 98.7) | 72.4 (65.1 to 79.7) |
| All women – Spain | 33 | 1 | 22 | 67 | 123 | Reported | 97.1 (89.7 to 100) | 75.3 (65.2 to 84.7) | |
| Summary estimates | 94 (88.6 to 97.4) | 77.6 (73.6 to 81.2) | |||||||
| Szubert et al. (2016)42 | Premenopausal women – Poland | 29 | 3 | 23 | 83 | 138 | Calculated | 90.6 (77.0 to 100) | 78.3 (70.7 to 85.9) |
| Premenopausal women – Spain | 15 | 0 | 11 | 51 | 66 | Calculated | 100 (78.2 to 100) | 82.3 (71.6 to 91.1) | |
| Summary estimates | 93.6 (82.5 to 98.7) | 79.8 (72.9 to 85.6) | |||||||
| Szubert et al. (2016)42 | Postmenopausal women – Poland | 37 | 1 | 14 | 14 | 77 | Calculated | 97.4 (91.7 to 100) | 50.0 (32.1 to 69.8) |
| Postmenopausal women – Spain | 18 | 1 | 11 | 16 | 46 | Calculated | 95.8 (85.7 to 100) | 59.3 (41.5 to 77.6) | |
| Summary estimates | 96.5 (87.9 to 99.6) | 54.5 (40.6 to 68) | |||||||
Accuracy data for thresholds other than the 10% validated threshold (1%, 3%, 5%, 15%, 20% and 30%) are reported in Appendix 5, Table 38. As might be expected, sensitivity estimates increase and specificity estimates decrease with decreasing threshold.
Details of the International Ovarian Tumour Analysis group’s simple ultrasound rules studies
Seventeen published studies44,47–52,55,58–65,67 and the unpublished interim report (Frances Nixon, personal communication) provided data on the diagnostic performance of the IOTA group’s simple ultrasound rules, for the identification of women with an adnexal mass who are at a high risk of developing ovarian cancer. The majority (11/17) of the published studies44,48–50,52,58,60,62–64,66 were conducted in Europe; three of the studies were conducted in the UK. 60,62,66 Two further studies61,65 were worldwide, with multinational studies including UK participants. Two of the remaining studies were conducted in Thailand47,51 and one was conducted in Brazil. 55 One study did not report sufficient detail to determine the geographic location. 53 (Confidential information has been removed.)
Three published studies50,61,65 were clearly conducted by the IOTA study core group, using data from various phases of the IOTA study; only one report was included for each phase of the IOTA study. Phase 5 of the IOTA study is ongoing and an interim report was supplied to this assessment (confidential information has been removed) (Frances Nixon, personal communication).
Ten published studies,44,48–50,52,55,58,61,62,65 as well as the unpublished interim report (Frances Nixon, personal communication), included all participants in the analysis; participants with inconclusive IOTA group’s simple ultrasound rules assessments were either assumed to have malignant tumours or classified by subjective assessment of ultrasound images. This section reports data for studies in which all participants were included in the analysis. Six further studies47,51,53,63,64,66 excluded participants with inconclusive IOTA group’s simple ultrasound rules assessments from their analyses. The results of these studies are provided in Appendix 5, Table 39. One study60 did not report sufficient information to determine how participants with inconclusive IOTA group’s simple ultrasound rules assessments were handled.
Accuracy of the International Ovarian Tumour Analysis group’s simple ultrasound rules for determining a high risk of developing ovarian cancer
All studies in this section included all participants in their analyses, regardless of their final histopathological diagnosis (target condition: all malignant tumours including borderline). Eight published studies44,48–50,52,55,62,65 and the unpublished interim report (Frances Nixon, personal communication) provided accuracy data for the IOTA group’s simple ultrasound rules, whereby women with inconclusive assessments were assumed to have malignant tumours. The summary estimate of sensitivity derived from these studies was 94.2% (95% CI 93.3% to 95.1%) and the summary estimate of specificity was 76.1% (95% CI 74.9% to 77.3%). These estimates did not differ significantly from those calculated from only those studies of the IOTA group’s simple ultrasound rules, in which participants with inconclusive assessments were assumed to have malignant tumours, which reported a direct comparison with the RMI 1 score (at a threshold of 200 or 250; see Tables 14 and 15). Four studies44,49,50,62 of these studies reported accuracy data stratified by menopausal status. Menopausal status did not significantly affect sensitivity; however, the specificity estimate was significantly higher in premenopausal women than in postmenopausal women (Table 12).
| Threshold | Study (year of publication) | Subgroup | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | ||||||||||
| Malignant (inconclusive results were treated as malignant) | Adballa et al. (2013)48 | All women | 16 | 1 | 7 | 63 | 87 | Reported | 94.1 (71.3 to 99.9) | 90.0 (80.5 to 95.9) |
| Alcazar et al. (2013)52 | 51 | 4 | 54 | 231 | 340 | Reported | 92.7 (82.4 to 98.0) | 81.1 (76.0 to 85.4) | ||
| IOTA5 2017a,b | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Knafel et al. (2016)49 | 78 | 4 | 15 | 129 | 226 | Reported | 95.1 (88.0 to 98.7) | 89.6 (83.4 to 94.1) | ||
| Meys et al. (2016)44 | 107 | 8 | 67 | 144 | 326 | Calculated | 93.0 (86.0 to 97.0) | 68.0 (61.0 to 70.0) | ||
| Sayasneh et al. (2013)62 | 67 | 7 | 24 | 157 | 255 | Calculated | 91.0 (82.0 to 95.0) | 87.0 (82.0 to 91.0) | ||
| Silvestre et al. (2015)55 | 32 | 0 | 17 | 26 | 75 | Reported | 100 (89.1 to 100) | 60.5 (44.4 to 75.0) | ||
| bTesta et al. (2014)50 | 934 | 46 | 369 | 1054 | 2403 | Calculated | 95.3 (93.1 to 96.19) | 74.1 (67.7 to 79.7) | ||
| bTimmerman et al. (2010)65 | 515 | 27 | 307 | 1089 | 1938 | Calculated | 95.0 (92.0 to 96.0) | 78.0 (75.0 to 80.0) | ||
| Summary estimates | 94.2 (93.3 to 95.1) | 76.1 (74.9 to 77.3) | ||||||||
| Knafel et al. (2016)49 | Premenopausal women | 32 | 1 | 9 | 101 | 143 | Calculated | 96.9 (84.2 to 99.9) | 91.9 (85.0 to 96.2) | |
| Meys et al. (2016)44 | 29 | 2 | 23 | 74 | 128 | Calculated | 94.0 (77.0 to 99.0) | 76.0 (66.0 to 84.0) | ||
| Sayasneh et al. (2013)62 | 24 | 4 | 16 | 121 | 165 | Calculated | 86.0 (69.0 to 94.0) | 88.0 (83.0 to 93.0) | ||
| bTesta et al. (2014)50 | 359 | 19 | 225 | 751 | 1354 | Calculated | 95.0 (91.0 to 97.0) | 77.0 (70.0 to 83.0) | ||
| Summary estimates | 94.5 (92.0 to 96.4) | 79.3 (77.0 to 81.5) | ||||||||
| Knafel et al. (2016)49 | Postmenopausal women | 46 | 3 | 6 | 28 | 83 | Calculated | 94 (83.1 to 98.7) | 81.8 (65.5 to 93.2) | |
| Meys et al. (2016)44 | 78 | 6 | 44 | 70 | 198 | Calculated | 93.0 (85.0 to 97.0) | 61.0 (52.0 to 70.0) | ||
| Sayasneh et al. (2013)62 | 43 | 3 | 7 | 37 | 90 | Calculated | 93.0 (82.0 to 98.0) | 84.0 (71.0 to 92.0) | ||
| bTesta et al. (2014)50 | 578 | 24 | 152 | 295 | 1049 | Calculated | 96.0 (93.0 to 97.0) | 66.0 (59.0 to 73.0) | ||
| Summary estimates | 95.4 (93.7 to 96.8) | 67.3 (63.5 to 70.9) | ||||||||
| Malignant (inconclusive results were classified by expert SA) | Alcázar et al. (2013)52 | All women | 49 | 6 | 11 | 274 | 340 | Reported | 89.1 (77.8 to 95.9) | 96.1 (93.2 to 98.1) |
| Malignant (inconclusive results were classified by level 2 or level 3 by expert SA) | Knafel et al. (2016)49 | 78 | 4 | 9 | 135 | 226 | Calculated | 95.1 (88.0 to 98.7) | 93.8 (88.5 to 97.1) | |
| Meys et al. (2016)44 | 102 | 13 | 21 | 190 | 326 | Calculated | 89.0 (81.0 to 94.0) | 90.0 (85.0 to 94.0) | ||
| Piovano et al. (2016)58 | 69 | 15 | 23 | 284 | 391 | Calculated | 82.1 (72.3 to 89.6) | 92.5 (89 to 95.2) | ||
| Sayasneh et al. (2013)62 | 64 | 10 | 11 | 170 | 255 | Calculated | 86.0 (77.0 to 92.0) | 94.0 (90.0 to 97.0) | ||
| bTesta et al. (2014)50 | 900 | 80 | 157 | 1266 | 2403 | Calculated | 91.8 (89.1 to 93.9) | 89.0 (85.2 to 92.0) | ||
| bTimmerman et al. (2010)65 | 494 | 102 | 48 | 1294 | 1938 | Calculated | 91.0 (88.0 to 93.0) | 93.0 (91.0 to 94.0) | ||
| Summary estimates | 88.4 (86.9 to 89.8) | 92.5 (91.6 to 93.4) | ||||||||
| Knafel et al. (2016)49 | Premenopausal women | 32 | 1 | 5 | 105 | 143 | Calculated | 96.9 (84.2 to 99.9) | 95.5 (89.7 to 98.5) | |
| Meys et al. (2016)44 | 27 | 4 | 4 | 93 | 128 | Calculated | 87.0 (69.0 to 96.0) | 96.0 (89.0 to 99.0) | ||
| Piovano et al. (2016)58 | 18 | 3 | 6 | 194 | 221 | Calculated | 86.0 (71.0 to 100) | 97.0 (94.0 to 99.0) | ||
| Sayasneh et al. (2013)62 | 23 | 5 | 5 | 132 | 165 | Calculated | 82.0 (64.0 to 92.0) | 96.0 (91.0 to 98.0) | ||
| bTesta et al. (2014)50 | 348 | 24 | 88 | 888 | 1354 | Calculated | 92.0 (86.0 to 95.0) | 91.0 (87.0 to 94.0) | ||
| Summary estimates | 92.4 (89.6 to 94.6) | 92.9 (91.5 to 94.1) | ||||||||
| Knafel et al. (2016)49 | Postmenopausal women | 46 | 3 | 1 | 33 | 83 | Calculated | 94.0 (83.1 to 98.7) | 97.9 (84.7 to 99.9) | |
| Meys et al. (2016)44 | 75 | 9 | 39 | 75 | 198 | Calculated | 89.0 (80.0 to 95.0) | 85.0 (77.0 to 91.0) | ||
| Piovanono et al. (2016)58 | 51 | 12 | 17 | 90 | 170 | Calculated | 81.0 (71.0 to 91.0) | 84.0 (77.0 to 91.0) | ||
| Sayasneh et al. (2013)62 | 41 | 5 | 4 | 40 | 90 | Calculated | 89.0 (77.0 to 95.0) | 91.0 (79.0 to 96.0) | ||
| bTesta et al. (2014)50 | 560 | 42 | 76 | 371 | 1049 | Calculated | 93.0 (90.0 to 95.0) | 83.0 (78.0 to 87.0) | ||
| Summary estimates | 91.6 (89.5 to 93.4) | 81.6 (78.7 to 84.4) | ||||||||
Seven studies44,49,50,52,58,62,65 provided accuracy data for the IOTA group’s simple ultrasound rules, whereby participants with inconclusive assessments were classified by an expert subjective assessment. In this analysis, only studies in which the subjective assessment was done by experts or by level 2/3 examiners as per the EFSUMB classification system have been included. The summary estimates of sensitivity and specificity derived from these studies were 88.4% (95% CI 86.9% to 89.8%) and 92.5% (95% CI 91.6% to 93.4%), respectively (see Table 12). One of these studies49 also assessed the effect of the training level of examiners on the diagnostic performance of the IOTA group’s simple ultrasound rules and found no significant differences in test performance between EFSUMB level 2 examiners (see Table 12) and EFSUMB level 1 examiners (Table 13). However, it should be noted that all examiners received 1 half-day of practical training in the IOTA group’s Simple Rules before the study.
| Threshold | Study (year of publication) | Subgroup | Index test variations | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||||
| Malignant (inconclusive were treated as malignant) | Knafel et al. (2016)49 | All women | Level 1 examiner | 79 | 3 | 26 | 118 | 226 | Reported | 96.3 (89.7 to 99.2) | 81.9 (74.7 to 87.9) |
| Knafel et al. (2016)49 | Postmenopausal women | Level 1 examiner | 46 | 3 | 12 | 22 | 83 | Calculated | 94 (83.1 to 98.7) | 63.6 (46.5 to 80.3) | |
| Premenopausal women | Level 1 examiner | 33 | 0 | 14 | 96 | 143 | Calculated | 100 (89.4 to 100) | 87.4 (79.6 to 92.9) | ||
| Malignant (inconclusive were classified by expert SA) | Knafel et al. (2016)49 | All women | Level 1 examiner | 79 | 3 | 7 | 137 | 226 | Calculated | 96.3 (89.7 to 99.2) | 95.1 (90.2 to 98.0) |
| Postmenopausal women | Level 1 examiner | 46 | 3 | 3 | 31 | 83 | Calculated | 93.9 (83.1 to 98.7) | 90 (76.3 to 98.1) | ||
| Premenopausal women | Level 1 examiner | 33 | 0 | 4 | 106 | 143 | Calculated | 100 (89.4 to 100) | 96.4 (91.0 to 99.0) | ||
Five of these studies44,49,50,58,62 reported accuracy data stratified by menopausal status. Menopausal status did not significantly affect sensitivity; however, the specificity estimate was significantly higher in premenopausal women than in postmenopausal women (see Table 12). One study58 (see Appendix 5, Table 39) also assessed whether or not the addition of biomarkers to the IOTA group’s simple ultrasound rules could improve the diagnostic performance. When a positive index test was defined as a malignant classification by the IOTA group’s simple ultrasound rules (with subjective assessment of inconclusives) and a ROMA score of > 11.4% out of 29.9%, the sensitivity and specificity estimates were 90.5% (95% CI 82.1% to 95.8%) and 80.1% (95% CI 75.2% to 84.4%), respectively. When a positive index test was defined as a malignant classification by the IOTA group’s simple ultrasound rules (with subjective assessment of inconclusives) and a HE4 level of ≥ 70 out of 140, the sensitivity and specificity estimates were 86.9% (95% CI 77.8% to 93.3%) and 86.3% (95% CI 82% to 90%), respectively. Neither the addition of the ROMA score nor the addition of HE4 level alone significantly affected estimates of test performance. Finally, when a positive index test was defined as a malignant classification by the IOTA group’s simple ultrasound rules (with subjective assessment of inconclusives) and a CA125 level of ≥ 35, the sensitivity estimate was similar to that for the IOTA group’s simple ultrasound rules (90.5%, 95% CI 82.1% to 95.8%); however, the specificity estimate was significantly lower (68.1%, 95% CI 62.5% to 73.3%).
Comparison of the different methods of operationalising the IOTA group’s simple ultrasound rules (i.e. ‘inconclusive results treated as malignant’ vs. ‘inconclusive results were classified by an expert’) indicates that sensitivity estimates were significantly higher when inconclusive results were treated as malignant, whereas specificity was significantly higher when patients with inconclusive results were classified by an expert. Thus, as might be expected, applying the assumption that all patients with an inconclusive result have a malignant tumour is likely to result in fewer patients with ovarian cancer being missed (FNs), whereas expert reassessment of inconclusive results is likely to result in fewer unnecessary referrals (FPs).
Comparisons between the International Ovarian Tumour Analysis group’s Simple Rules, the Assessment of Different NEoplasias in the adneXa and the Risk of Malignancy Index 1
This assessment is primarily concerned with providing a comparison between the RMI 1,78 used with a decision threshold of 250 (the current standard practice in the NHS1) and the specified alternative risk-scoring methods (see Chapter 2, Intervention technologies). Our searches did not identify any studies that reported a direct comparison (both tests were used to assess the same patient cohort) between the ADNEX model or the IOTA group’s simple ultrasound rules and the RMI 1, used with a decision threshold of 250. One published study44 and the unpublished interim report (Frances Nixon, personal communication) reported direct comparisons between the ADNEX model at the 10% threshold, the IOTA group’s simple ultrasound rules (whereby patients with an inconclusive assessment were assumed to have malignant tumours) and the RMI 1, used with a decision threshold of 200 (Table 14). Both of these studies included all participants in the analysis, regardless of their final histopathological diagnosis (target condition: all malignant tumours including borderline). The summary estimates of sensitivity derived from these two studies were slightly higher for the ADNEX model (96%, 95% CI 94.5% to 97.1%) than for the IOTA group’s simple ultrasound rules (92.8%, 95% CI 90.9% to 94.3%). The summary estimates of specificity were similar (67%, 95% CI 64.2% to 69.6%, and 71.6%, 95% CI 68.9% to 74.1%) for the ADNEX model and the ultrasound Simple Rules, respectively. The summary estimate of sensitivity for the RMI 1 at a decision threshold of 200 (66%, 95% CI 62.9% to 69%) was significantly lower than that for both the ADNEX model estimate and the IOTA group’s simple ultrasound rules estimate. Conversely, the specificity estimate for the RMI 1 at a decision threshold of 200 was significantly higher (89%, 95% CI 87% to 90.7%) than that for both the ADNEX model estimate and the IOTA group’s simple ultrasound rules estimate (Figure 4). The unpublished interim report (Frances Nixon, personal communication) also reported direct comparisons between the ADNEX model at the 10% threshold, the IOTA group’s simple ultrasound rules (whereby patients with an inconclusive assessment were assumed to have malignant tumours) and the RMI 1, used with a decision threshold of 250. The comparative accuracy estimates at this threshold did not differ from those at 200 (see Table 14).
| Study (year of publication) | Subgroup | Index test | Threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | RMI threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | ||||||||||||||||||
| Meys et al. (2016)44 | All women | ADNEX model | ≥ 10% | 113 | 2 | 80 | 131 | 326 | 98.0 (93.0 to 100) | 62.0 (55.0 to 68.0) | 200 | 82 | 33 | 44 | 167 | 326 | 71.0 (62.0 to 79.0) | 79.0 (72.0 to 84.0) |
| IOTA5 2017a,b | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 200 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||
| Summary estimates | 96.0 (94.5 to 97.1) | 67.0 (64.2 to 69.6) | Summary estimates | 66.0 (62.9 to 69.0) | 89.0 (87.0 to 90.7) | |||||||||||||
| IOTA5 2017a | All women | ADNEX model | ≥ 10% | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 250 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Meys et al. (2016)44 | All women | IOTA group’s simple ultrasound rules | Inconclusive = malignant | 107 | 8 | 67 | 144 | 326 | 93.0 (86.0 to 97.0) | 68.0 (61.0 to 70.0) | 200 | 82 | 33 | 44 | 167 | 326 | 71.0 (62.0 to 79.0) | 79.0 (72.0 to 84.0) |
| IOTA5 2017a,b | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 200 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||
| Summary estimates | 92.8 (90.9 to 94.3) | 71.6 (68.9 to 74.1) | Summary estimates | 66.0 (62.9 to 69.0) | 89.0 (87.0 to 90.7) | |||||||||||||
| IOTA5 2017a | All women | IOTA group’s simple ultrasound rules | Inconclusive = malignant | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 250 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Meys et al. (2016)44 | All women | IOTA group’s simple ultrasound rules | Inconclusive = SA | 102 | 13 | 21 | 190 | 326 | 89.0 (81.0 to 94.0) | 90.0 (85.0 to 94.0) | 200 | 82 | 33 | 44 | 167 | 326 | 71.0 (62.0 to 79.0) | 79.0 (72.0 to 84.0) |
| Premenopausal women | ADNEX model | ≥ 10% | 31 | 0 | 28 | 69 | 128 | 100 (86.0 to 100) | 71.0 (61.0 to 80.0) | 200 | 13 | 18 | 6 | 91 | 128 | 42.0 (25.0 to 61.0) | 94.0 (86.0 to 97.0) | |
| IOTA group’s simple ultrasound rules | Inconclusive by SA | 27 | 4 | 4 | 93 | 128 | 87.0 (69.0 to 96.0) | 96.0 (89.0 to 99.0) | ||||||||||
| Inconclusive = malignant | 29 | 2 | 23 | 74 | 128 | 94.0 (77.0 to 99.0) | 76.0 (66.0 to 84.0) | |||||||||||
| Postmenopausal women | ADNEX model | ≥ 10% | 82 | 2 | 52 | 62 | 198 | 98.0 (91.0 to 100) | 54.0 (44.0 to 63.0) | 200 | 69 | 15 | 39 | 75 | 198 | 82.0 (72.0 to 89.0) | 66.0 (56.0 to 74.0) | |
| IOTA group’s simple ultrasound rules | Inconclusive = SA | 75 | 9 | 39 | 75 | 198 | 89.0 (80.0 to 95.0) | 85.0 (77.0 to 91.0) | ||||||||||
| Inconclusive = malignant | 78 | 6 | 44 | 70 | 198 | 93.0 (85.0 to 97.0) | 61.0 (52.0 to 70.0) | |||||||||||
FIGURE 4.
Comparison of the accuracy of the ADNEX model, the IOTA group’s simple ultrasound rules and the RMI 1. (a) Sensitivity estimate and; (b) specificity estimate.


Only the published study44 reported accuracy data stratified by menopausal status. In premenopausal women, the ADNEX model at the 10% threshold and the IOTA group’s simple ultrasound rules had similar sensitivities of 100% (95% CI 86% to 100%) and 94% (95% CI 77% to 99%), respectively, in comparison with the overall population. The specificities were significantly lower at 71% (95% CI 61% to 80%) and 76% (95% CI 66% to 84%), respectively. In postmenopausal women, the ADNEX model at the 10% threshold and the IOTA group’s simple ultrasound rules had similar sensitivities of 98% (95% CI 91% to 100%) and 93% (95% CI 85% to 97%), respectively, in comparison with the overall population. The specificities of 54% (95% CI 44% to 63%) and 61% (95% CI 52% to 70%), respectively, were significantly lower than those of the overall population. The RMI 1, using a decision threshold of 200, had a significantly lower sensitivity of 42% (95% CI 25% to 61%) and a significantly higher specificity of 94% (95% CI 86% to 97%) in premenopausal women. Conversely, the sensitivity estimate was higher at 82% (95% CI 72% to 89%) and the specificity estimate was lower at 66% (95% CI 56% to 74%) in postmenopausal women (but this was not significant).
Four published studies44,48,50,62 and the unpublished interim report (Frances Nixon, personal communication) reported direct comparisons between the IOTA group’s simple ultrasound rules (whereby patients with an inconclusive assessment were assumed to have malignant tumours) and the RMI 1 used with a decision threshold of 200 (Table 15). All of these studies included all participants in the analysis, regardless of their final histopathological diagnosis (target condition: all malignant tumours including borderline). The summary estimate of sensitivity for the IOTA group’s simple ultrasound rules (93.9%, 95% CI 92.8% to 94.9%) was significantly higher than that for the RMI 1 (66.9%, 95% CI 64.8% to 68.9%). Conversely, the summary estimate of specificity for the IOTA group’s simple ultrasound rules (74.2%, 95% CI 72.6% to 75.8%) was significantly lower than that for the RMI 1 (90.1%, 95% CI 88.9% to 91.2%).
| Study (year of publication) | Subgroup | IOTA group’s simple ultrasound rules threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | RMI threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||||||||||
| Adballa et al. (2013)48 | All women | Inconclusive = malignant | 16 | 1 | 7 | 63 | 87 | 94.1 (71.3 to 99.9) | 90.0 (80.5 to 95.9) | 200 | 15 | 2 | 8 | 62 | 87 | 88.2 (63.6 to 98.5) | 88.6 (78.7 to 94.9) |
| IOTA5 2017a,b | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 200 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Meys et al. (2016)44 | 107 | 8 | 67 | 144 | 326 | 93.0 (86.0 to 97.0) | 68.0 (61.0 to 70.0) | 200 | 82 | 33 | 44 | 167 | 326 | 71.0 (62.0 to 79.0) | 79.0 (72.0 to 84.0) | ||
| cSayasneh et al. (2013)62 | 67 | 7 | 24 | 157 | 255 | 91.0 (82.0 to 95.0) | 87.0 (82.0 to 91.0) | 200 | 53 | 21 | 11 | 170 | 255 | 72.0 (60.0 to 81.0) | 94.0 (90.0 to 97.0) | ||
| b,cTesta et al. (2014)50 | 934 | 46 | 369 | 1054 | 2403 | 95.3 (93.1 to 96.19) | 74.1 (67.7 to 79.7) | 200 | 657 | 323 | 134 | 1289 | 2403 | 67.1 (61.4 to 72.4) | 90.6 (87.3 to 93.1) | ||
| Summary estimates | 93.9 (92.8 to 94.9) | 74.2 (72.6 to 75.8) | Summary estimates | 66.9 (64.8 to 68.9) | 90.1 (88.9 to 91.2) | ||||||||||||
| Meys et al. (2016)44 | All women | Inconclusive = SA | 102 | 13 | 21 | 190 | 326 | 89.0 (81.0 to 94.0) | 90.0 (85.0 to 94.0) | 200 | 82 | 33 | 44 | 167 | 326 | 71.0 (62.0 to 79.0) | 79.0 (72.0 to 84.0) |
| cSayasneh et al. (2013)62 | 64 | 10 | 11 | 170 | 255 | 86.0 (77.0 to 92.0) | 94.0 (90.0 to 97.0) | 200 | 53 | 21 | 11 | 170 | 255 | 72.0 (60.0 to 81.0) | 94.0 (90.0 to 97.0) | ||
| b,cTesta et al. (2014)50 | 900 | 80 | 157 | 1266 | 2403 | 91.8 (89.1 to 93.9) | 89.0 (85.2 to 92) | 200 | 657 | 323 | 134 | 1289 | 2403 | 67.1 (61.4 to 72.4) | 90.6 (87.3 to 93.1) | ||
| Summary estimates | 91.2 (89.4 to 92.8) | 89.6 (88.1 to 91) | Summary estimates | 67.8 (65.0 to 70.4) | 98.5 (98.3 to 98.7) | ||||||||||||
| Meys et al. (2016)44 | Premenopausal women | Inconclusive = malignant | 29 | 2 | 23 | 74 | 128 | 94.0 (77.0 to 99.0) | 76.0 (66.0 to 84.0) | 200 | 13 | 18 | 6 | 91 | 128 | 42.0 (25.0 to 61.0) | 94.0 (86.0 to 97.0) |
| cSayasneh et al. (2013)62 | 24 | 4 | 16 | 121 | 165 | 86.0 (69.0 to 94.0) | 88.0 (83.0 to 93.0) | 200 | 15 | 13 | 5 | 132 | 165 | 54.0 (36.0 to 70.0) | 96.0 (92.0 to 98.0) | ||
| b,cTesta et al. (2014)50 | 359 | 19 | 225 | 751 | 1354 | 95.0 (91.0 to 97.0) | 77.0 (70.0 to 83.0) | 200 | 200 | 178 | 59 | 917 | 1354 | 53.0 (45.0 to 61.0) | 94.0 (92.0 to 96.0) | ||
| Summary estimates | 94.3 (91.7 to 96.3) | 78.2 (75.7 to 80.5) | Summary estimates | 52.2 (47.4 to 56.9) | 94.2 (92.7 to 95.5) | ||||||||||||
| Meys et al. (2016)44 | Inconclusive = SA | 27 | 4 | 4 | 93 | 128 | 87.0 (69.0 to 96.0) | 96.0 (89.0 to 99.0) | 200 | 13 | 18 | 6 | 91 | 128 | 42.0 (25.0 to 61.0) | 94.0 (86.0 to 97.0) | |
| cSayasneh et al. (2013)62 | 23 | 5 | 5 | 132 | 165 | 82.0 (64.0 to 92.0) | 96.0 (91.0 to 98.0) | 200 | 15 | 13 | 5 | 132 | 165 | 54.0 (36.0 to 70.0) | 96.0 (92.0 to 98.0) | ||
| b,cTesta et al. (2014)50 | 348 | 24 | 88 | 888 | 1354 | 92.0 (86.0 to 95.0) | 91.0 (87.0 to 94.0) | 200 | 200 | 178 | 59 | 917 | 1354 | 53.0 (45.0 to 61.0) | 94.0 (92.0 to 96.0) | ||
| Summary estimates | 92.3 (89.4 to 94.7) | 92 (90.3 to 93.5) | Summary estimates | 52.2 (47.4 to 56.9) | 94.2 (92.7 to 95.5) | ||||||||||||
| Meys et al. (2016)44 | Postmenopausal women | Inconclusive = malignant | 78 | 6 | 44 | 70 | 198 | 93.0 (85.0 to 97.0) | 61.0 (52.0 to 70.0) | 200 | 69 | 15 | 39 | 75 | 198 | 82.0 (72.0 to 89.0) | 66.0 (56.0 to 74.0) |
| cSayasneh et al. (2013)62 | 43 | 3 | 7 | 37 | 90 | 93.0 (82.0 to 98.0) | 84.0 (71.0 to 92.0) | 200 | 38 | 8 | 5 | 39 | 90 | 83.0 (69.0 to 91.0) | 89.0 (76.0 to 95.0) | ||
| b,cTesta et al. (2014)50 | 578 | 24 | 152 | 295 | 1049 | 96.0 (93.0 to 97.0) | 66.0 (59.0 to 73.0) | 200 | 470 | 132 | 85 | 362 | 1049 | 78.0 (72.0 to 83.0) | 81.0 (76.0 to 85.0) | ||
| Summary estimates | 95.5 (93.7 to 96.9) | 72.3 (68.9 to 75.5) | Summary estimates | 78.8 (75.7 to 81.7) | 78.7 (75.2 to 81.9) | ||||||||||||
| Meys et al. (2016)44 | Postmenopausal women | Inconclusive = SA | 75 | 9 | 39 | 75 | 198 | 89.0 (80.0 to 95.0) | 85.0 (77.0 to 91.0) | 200 | 69 | 15 | 39 | 75 | 198 | 82.0 (72.0 to 89.0) | 66.0 (56.0 to 74.0) |
| cSayasneh et al. (2013)62 | 41 | 5 | 4 | 40 | 90 | 89.0 (77.0 to 95.0) | 91.0 (79.0 to 96.0) | 200 | 38 | 8 | 5 | 39 | 90 | 83.0 (69.0 to 91.0) | 89.0 (76.0 to 95.0) | ||
| b,cTesta et al. (2014)50 | 560 | 42 | 76 | 371 | 1049 | 93.0 (90.0 to 95.0) | 83.0 (78.0 to 87.0) | 200 | 470 | 132 | 85 | 362 | 1049 | 78.0 (72.0 to 83.0) | 81.0 (76.0 to 85.0) | ||
| Summary estimates | 92.3 (90.2 to 94.2) | 80.3 (76.9 to 83.4) | Summary estimates | 78.8 (75.7 to 81.7) | 78.7 (75.2 to 81.9) | ||||||||||||
| bDi Legge et al. (2012)61 | Tumour size of < 4 cm | Inconclusive = malignant | 42 | 9 | 13 | 332 | 396 | 82.0 (69.0 to 92.0) | 96.0 (94.0 to 98.0) | 200 | 29 | 22 | 16 | 329 | 396 | 56.0 (43.0 to 70.0) | 95.0 (93.0 to 98.0) |
| Tumour size of ≥ 10 cm | 281 | 23 | 66 | 222 | 592 | 92.0 (89.0 to 95.0) | 77.0 (72.0 to 82.0) | 200 | 224 | 80 | 38 | 250 | 592 | 74.0 (69.0 to 79.0) | 87.0 (83.0 to 91.0) | ||
| Tumour size of 4–9.9 cm | 303 | 27 | 60 | 1067 | 1457 | 92.0 (88.0 to 95.0) | 95.0 (93.0 to 96.0) | 200 | 220 | 110 | 68 | 1059 | 1457 | 67.0 (62.0 to 72.0) | 94.0 (92.0 to 95.0) | ||
| Target condition: ovarian borderline tumours – higher-stage malignancies excluded | |||||||||||||||||
| b,cTesta et al. (2014)50 | All women | Inconclusive = malignant | 133 | 20 | 367 | 1056 | 1576 | 87.5 (79.3 to 92.8) | 74.2 (66.5 to 80.7) | 200 | 45 | 108 | 134 | 1289 | 1576 | 29.6 (21.2 to 39.7) | 90.6 (87.1 to 93.2) |
| Inconclusive = SA | 121 | 32 | 152 | 1271 | 1576 | 79.5 (70.8 to 86.1) | 89.3 (84.7 to 92.7) | 200 | 45 | 108 | 134 | 1289 | 1576 | 29.6 (21.2 to 39.7) | 90.6 (87.1 to 93.2) | ||
Three of the above studies44,50,62 also reported comparative accuracy data for the IOTA group’s simple ultrasound rules versus the RMI 1 (threshold of 200), whereby participants with inconclusive IOTA assessments were classified by an expert subjective assessment of the ultrasound images (see Table 15). The summary estimate of sensitivity for the IOTA group’s simple ultrasound rules (91.2%, 95% CI 89.4% to 92.8%) was significantly higher than that for the RMI 1 (67.8%, 95% CI 65% to 70.4%). Conversely, the summary estimates of specificity were significantly lower for the IOTA group’s simple ultrasound rules (89.6%, 95% CI 88.1% to 91%) than for the RMI 1 (98.5%, 95% CI 98.3% to 98.7%). These three studies also reported accuracy data stratified by menopausal status, and the comparative accuracy estimates for both subgroups followed the pattern observed for all participants (see Table 15).
In premenopausal women, using the IOTA group’s simple ultrasound rules whereby participants with an inconclusive assessment were assumed to have a malignant tumour, the summary estimates of sensitivity and specificity were 94.3% (95% CI 91.7% to 96.3%) and 78.2% (95% CI 75.7% to 80.5%), respectively. These estimates were not significantly different from those for postmenopausal women (95.5%, 95% CI 93.7% to 96.9%, and 72.3%, 95% CI 68.9% to 75.5%, respectively). When participants with inconclusive IOTA group’s Simple Rules assessments were classified by an expert subjective assessment, the summary estimates of sensitivity were similar (for both premenopausal women and postmenopausal women) to those obtained when inconclusive assessments were assumed to be malignant (see Table 15). However, in both premenopausal women and postmenopausal women, the use of a subjective assessment significantly increased the summary estimate of specificity to 92% (95% CI 90.3% to 93.5%) and 80.3% (95% CI 76.9% to 83.4%), respectively. In premenopausal women, the summary sensitivity estimate for the RMI 1 (52.2%, 95% CI 47.4% to 56.9%) was significantly lower than that for the IOTA group’s simple ultrasound rules, and the summary specificity estimate (94.2%, 95% CI 92.7% to 95.5%) was significantly higher. In postmenopausal women, the summary sensitivity estimate for the RMI 1 (78.8%, 95% CI 75.7% to 81.7%) was also significantly lower than that for the IOTA group’s simple ultrasound rules; however, there were no significant differences between the specificity estimates (see Table 15).
One further study,60 which was reported as only a conference abstract, did not report sufficient information to determine how participants with inconclusive IOTA group’s simple ultrasound rules assessments were handled or how the target condition was defined (whether or not non-ovarian and borderline tumours were included in the definition of disease positive). This study reported sensitivity estimates of 94% for the IOTA group’s simple ultrasound rules and 72% for the RMI 1, used at a decision threshold of 250. The corresponding specificity estimate was 80% for both tests.
Diagnostic performance of Overa (multivariate index assay, second generation)
Details of Overa (multivariate index assay, second-generation) studies
Three diagnostic cohort studies reported in four publications68–70,104 provided data on the diagnostic performance of the Overa (MIA2G) score, for the identification of women with an adnexal mass who are at a high risk of developing ovarian cancer. All the studies were conducted in the USA. Only one study70 was reported as a full paper; the remaining two studies were reported in the form of meeting slides104 and a conference abstract. 68
One study70 used an Overa (MIA2G) score based on Roche Diagnostics’ assays and a Roche Diagnostics analyser; the other two studies68,104 did not report assay details.
The target condition for this assessment is ovarian cancer (i.e. epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer). All studies in this section included women with one or more adnexal mass and used a definition of malignancy that included borderline cancers. Histopathology indicated that all of the studies also included some women with non-ovarian malignancies and non-ovarian metastases. Full details of the final histopathological diagnoses of study participants who had a malignant mass are reported in Appendix 4, Table 36.
Accuracy of Overa (MIA2G) for determining a high risk of developing ovarian cancer
No studies were identified that directly compared Overa (MIA2G) to the RMI 1 at either decision threshold (200 or 250).
One study104 reported comparative accuracy data for Overa (MIA2G) versus the ROMA score, using Roche Diagnostics’ Elecsys tumour marker assays (Table 16). This study included all participants in the analysis, regardless of their final histopathological diagnosis (target condition: all malignancies including borderline). At a 29.9%) was 79.2% (95% CI 73.7%/29.9%) was 79.2% (95% CI 73.7% to 83.8%) and the specificity estimate was 78.9% (95% CI 75.8% to 81.7). These data indicate that the sensitivity of the Overa (MIA2G) score was significantly higher than that of the ROMA score, whereas the specificity of the Overa (MIA2G) score was significantly lower than that of the ROMA score (Figure 5).
| Study (year of publication) | Index test | Threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||
| Shulman et al. (2016)104 | Overa (MIA2G) | 5 units | 223 | 22 | 258 | 490 | 993 | 91.0 (86.8 to 94.0) | 65.5 (62.0 to 68.8) |
| ROMA score using Roche Diagnostics’ tumour marker assay | 11.4%/29.9% | 194 | 51 | 158 | 590 | 993 | 79.2 (73.7 to 83.8) | 78.9 (75.8 to 81.7) | |
FIGURE 5.
Comparison of the summary estimates for Overa (MIA2G) and ROMA score using Roche Diagnostics’ tumour marker assay (all malignant tumours plus borderline). (a) Sensitivity estimate; and (b) specificity estimate.

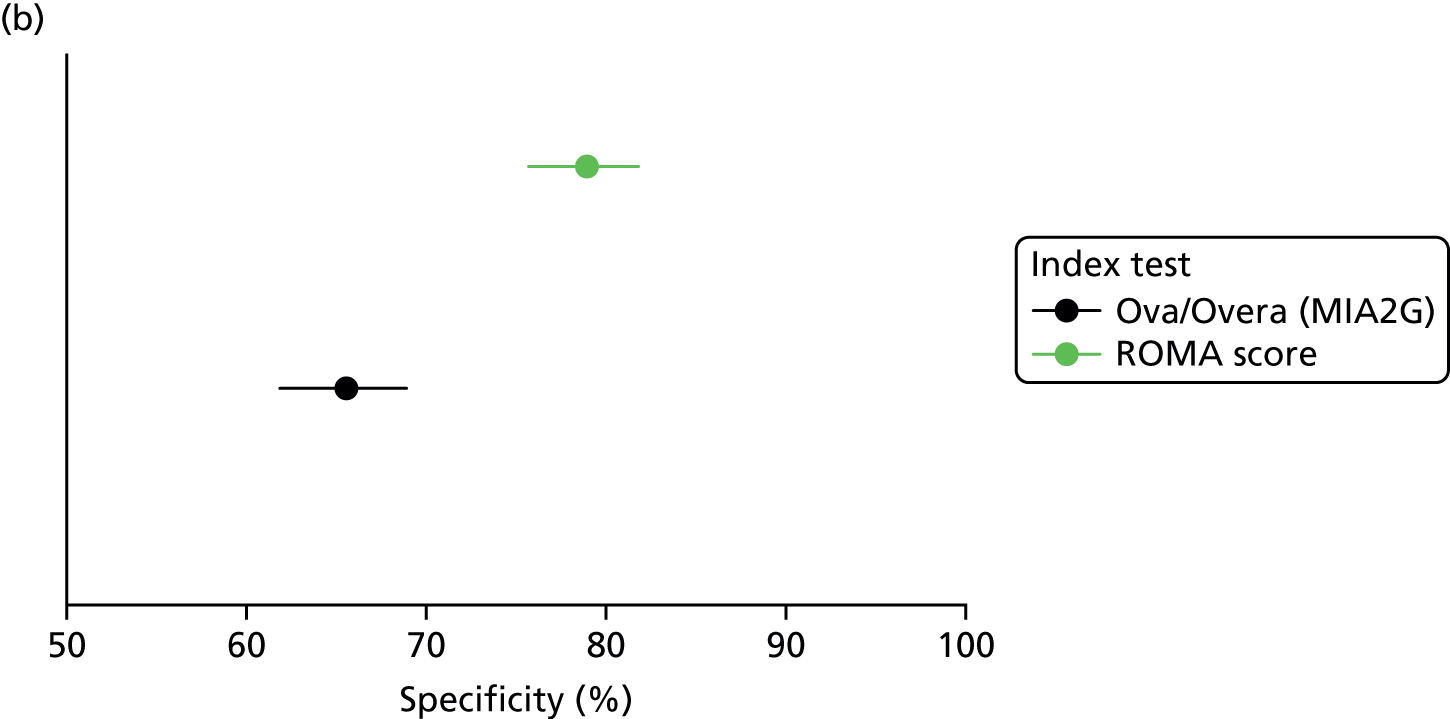
The two remaining studies68,70 reported data on the accuracy of Overa (MIA2G) without comparison with the RMI 1 or any other risk score and analysed data for any malignant tumour plus borderline (Table 17). At a threshold of 5 units, the pooled sensitivity estimate was 90.2% (95% CI 84.6% to 94.3%) and the pooled specificity estimate was 65.8% (95% CI 61.9% to 69.5%); these estimates were similar to those reported by the comparative accuracy study. One study stratified data by menopausal status and found no significant variation in test performance (see Table 17). 70
| Study (year of publication) | Subgroup | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||
| Coleman et al. (2016)70 | All women | 84 | 8 | 124 | 277 | 493 | Reported | 91.3 (83.8 to 95.5) | 69.1 (64.4 to 73.4) |
| Zhang et al. (2015)68 | All women | 64 | 8 | 93 | 140 | 305 | Reported | 88.9 (79.3 to 95.1) | 60.1 (53.5 to 66.4) |
| Summary estimates | 90.2 (84.6 to 94.3) | 65.8 (61.9 to 69.5) | |||||||
| Coleman et al. (2016)70 | Premenopausal women | 28 | 3 | 70 | 175 | 276 | Reported | 90.3 (75.1 to 96.7) | 71.4 (65.5 to 76.7) |
| Postmenopausal women | 56 | 5 | 54 | 102 | 217 | Reported | 91.8 (82.2 to 96.4) | 65.4 (57.6 to 72.4) | |
Diagnostic performance of the Risk of Malignancy Index 1 using decision thresholds other than 250
Details of Risk of Malignancy Index 1 studies
Ten diagnostic cohort studies,71–80 reported in 10 full-paper publications, provided data comparing the diagnostic performance of the RMI 1 at multiple decision thresholds, including a decision threshold of 250, for the identification of women with an adnexal mass who were at a high risk of developing ovarian cancer.
Two studies78,79 specifically included women from the UK, two studies were European (from Italy and Norway)76,80 and six studies were from five non-European countries (Turkey, Pakistan, China, India and Japan). 71–75,77
Three studies75,76,78 used a RMI 1 score based on an Abbott Diagnostics’ CA125 assay, three studies71,72,74 used a Roche Diagnostics assay, one study77 used an IMMULITE® assay, one study79 used CIS Bioindustries, one study80 used a commercial kit by Centocor (Malvern, PA, USA) and one study73 did not report the CA125 assay used.
This assessment is primarily concerned with providing a comparison between the RMI 1,78 used with a decision threshold of 250 (current standard practice in the NHS1) and the specified alternative risk-scoring methods (see Chapter 2, Intervention technologies). The identified studies for the RMI 1 reported test performance data for multiple thresholds, and full data are reported in Appendix 5, Table 40. All of the identified studies that provided comparative accuracy data for alternative risk-scoring methods versus the RMI 1 used a decision threshold of 200. In order to assess the applicability of these data to the stated objective of this assessment, this section therefore focuses on the comparative accuracy of the RMI 1, using decision thresholds of 200 and 250.
The target condition for this assessment is ovarian cancer (i.e. epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer), defined as those conditions covered by the NICE clinical guideline CG122. 1 All studies in this section included women with one or more adnexal mass. Seven studies72–74,76,78–80 used a definition of malignancy that included borderline tumours, two studies71,75 excluded women found to have borderline tumours from the analyses and, in the remaining study,77 it was unclear whether or not women with borderline tumours were included in the analysis (no histopathology was reported with which to confirm the tumour type). Six studies73,74,76,78–80 included all study participants in the analyses and included some women with ‘other malignancies’, metastases from non-ovarian sites and ‘non-ovarian cancers.’ Full details of the final histopathological diagnoses of study participants who had a malignant mass are reported in Appendix 4, Table 26.
Accuracy of Risk of Malignancy Index 1 for determining a high risk of developing ovarian cancer using different decision thresholds
Six studies73,74,76,78–80 included all study participants in the analyses, regardless of final histopathological diagnosis [target condition: all malignant tumours including borderline (Table 18)]. At the decision threshold of 200, the summary estimate of sensitivity derived from these studies was 70.8% (95% CI 65.6% to 75.6%) and the summary estimate of specificity was 91.2% (95% CI 88.9% to 93.1%). At the decision threshold of 250, the summary estimate of sensitivity was 69% (95% CI 63.7% to 73.9%) and the summary estimate of specificity was 91.6% (95% CI 89.3% to 93.5%). The sensitivity and specificity estimates did not differ significantly between the two decision thresholds [200 and 250 (Figure 6)]. Studies compared multiple thresholds (between 25 and 500); as would be expected, the sensitivity estimate for the RMI 1 increased and the specificity estimate decreased with a decreasing threshold (see Appendix 5, Table 40).
| Study (year of publication) | Threshold | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 250 | |||||||||||||
| TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |
| Target condition: all malignant tumours including borderline | ||||||||||||||
| Davies et al. (1993)79 | 33 | 4 | 11 | 76 | 124 | 89.2 (74.6 to 97.0) | 87.4 (78.5 to 93.5) | 34 | 3 | 21 | 66 | 124 | 91.9 (78.1 to 98.3) | 75.9 (65.5 to 84.4) |
| Jacobs et al. (1990)78 | 35 | 6 | 3 | 95 | 139 | 85.4 (70.8 to 94.4) | 96.9 (91.3 to 99.4) | 32 | 9 | 1 | 97 | 139 | 78.0 (62.4 to 89.4) | 99.0 (94.5 to 100) |
| Lou et al. (2010)73 | 34 | 27 | 5 | 157 | 223 | 55.7 (42.4 to 68.5) | 96.9 (92.9 to 99.0) | 35 | 26 | 3 | 159 | 223 | 57.4 (44.1 to 70.0) | 98.1 (94.7 to 99.6) |
| Morgante et al. (1999)80 | 18 | 13 | 5 | 88 | 124 | 58.1 (39.1 to 75.5) | 94.6 (87.9 to 98.2) | 17 | 14 | 4 | 89 | 124 | 54.8 (36.0 to 72.7) | 95.7 (89.4 to 98.8) |
| Tingulstad et al. (1996)76 | 40 | 16 | 5 | 112 | 173 | 71.4 (57.8 to 82.7) | 95.7 (90.3 to 98.6) | 38 | 18 | 5 | 112 | 173 | 67.9 (54.0 to 79.7) | 95.7 (90.3 to 98.6) |
| Ulusoy et al. (2007)74 | 75 | 31 | 37 | 153 | 296 | 71.1 (62.1 to 80) | 80.5 (74.2 to 85.9) | 73 | 33 | 29 | 161 | 296 | 68.9 (59.1 to 77.5) | 84.7 (78.8 to 89.5) |
| Summary estimates | 70.8 (65.6 to 75.6) | 91.2 (88.9 to 93.1) | Summary estimates | 69.0 (63.7 to 73.9) | 91.6 (89.3 to 93.5) | |||||||||
| Target condition: ovarian malignancies including borderline | ||||||||||||||
| Yamamoto et al. (2009)72 | 32 | 8 | 29 | 184 | 253 | 80.0 (65.2 to 89.5)a | 86.4 (81.8 to 89.9)a | 29 | 11 | 24 | 189 | 253 | 72.5 (57.2 to 83.9)a | 88.7 (84.4 to 92)a |
| All malignant tumours excluding borderline | ||||||||||||||
| Aktürk et al. (2011)71 | 15 | 5 | 9 | 71 | 100 | 75.0 (50.9 to 91.3) | 88.8 (79.7 to 94.7) | 13 | 7 | 4 | 76 | 100 | 65.0 (40.8 to 84.6) | 95.0 (87.7 to 98.6) |
| Manjunath et al. (2001)75 | 68 | 25 | 5 | 50 | 148 | 73.1 (62.9 to 81.8) | 90.9 (80.0 to 97.0) | 62 | 31 | 5 | 50 | 148 | 66.7 (56.1 to 76.1) | 90.9 (80.0 to 97.0) |
| Summary estimates | 73.5 (64.3 to 81.3) | 89.6 (83.2 to 94.2) | Summary estimates | 66.4 (56.9 to 75.0) | 93.3 (87.7 to 96.9) | |||||||||
FIGURE 6.
Comparison of the summary estimates for the RMI 1 at thresholds of 200 and 250 (all malignant tumours plus borderline). (a) Sensitivity estimate; and (b) specificity estimate.

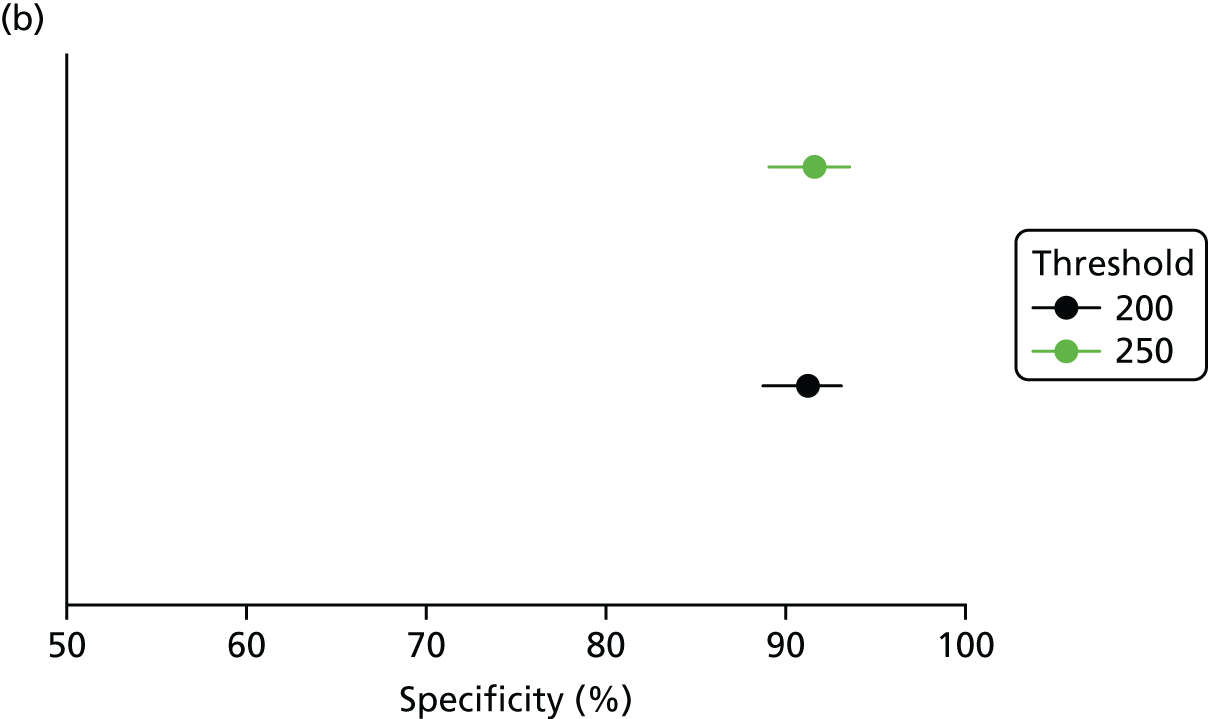
One study72 reported a direct comparison of the RMI 1 at decision thresholds of 250 and 200 and excluded women with a final histopathological diagnosis other than primary ovarian cancer from the analysis [target condition: ovarian malignancies including borderline (see Table 18)]. At the decision threshold of 200, the sensitivity estimate was 80% (95% CI 65.2% to 89.5%) and the specificity estimate was 86.4% (95% CI 81.8% to 89.9%). At the decision threshold of 250, the sensitivity estimate was 72.5% (95% CI 57.2% to 83.9%) and the specificity estimate was 88.7% (95% CI 84.4% to 92.0%). Although the sensitivity estimate was higher for the 200 threshold and the specificity estimate was higher for the 250 threshold, these differences were not significantly different. In addition, the sensitivity and specificity estimates from this study did not differ significantly from the summary estimates described earlier.
Two further studies71,75 excluded participants found to have borderline tumours from the analysis (target condition: all malignant tumours including borderline). At the decision threshold of 200, the summary estimate of sensitivity was 73.5% (95% CI 64.3% to 81.3%) and the summary estimate of specificity was 89.6% (95% CI 83.2% to 94.2%). At the decision threshold of 250, the summary estimate of sensitivity was 66.4% (95% CI 56.9% to 75.0%) and the summary estimate of specificity was 93.3% (95% CI 87.7% to 96.9%). The sensitivity and specificity estimates did not differ significantly between the two decision thresholds (200 and 250). In addition, these summary sensitivity and specificity estimates did not differ significantly from those derived from the six studies that included all participants in their analyses.
One study77 included participants with malignant tumours, but it was unclear whether or not borderline tumours were included (see Appendix 5, Table 43).
Selection of diagnostic performance estimates for inclusion in cost-effectiveness modelling
Data for the target condition ‘all malignant tumours including borderline’ were prioritised. This is because the scope and protocol for this assessment specified that the definition of ovarian cancer should include borderline tumours. In addition, the population in which risk-scoring would be applied in practice is likely to include some women who will ultimately be found to have a non-ovarian primary and some who will have cancers that fall outside the scope of conditions covered in CG1221 (e.g. germ cell tumours and sex cord–stromal tumours of the ovary); therefore, it was considered that studies that include all participants in their analysis, irrespective of final histological diagnosis, are more likely to produce estimates of risk score performance that are representative of what might be expected in clinical practice.
Comparative accuracy data were available for the risk scores ROMA, IOTA group’s simple ultrasound rules and the ADNEX model versus the RMI 1 (i.e. studies evaluated the diagnostic performance both of the risk score and the RMI 1 in the same patient cohort). No studies were identified that provided a direct comparison of Overa (MIA2G) with the RMI 1. Summary estimates of the diagnostic performance of risk scores, calculated using all available data sets for a given target condition, did not differ significantly from those calculated from only those studies that reported a direct comparison with the RMI 1. Cost-effectiveness modelling therefore used the summary estimates of diagnostic performance of these larger data sets, making maximum use of the available data.
Estimates of the diagnostic performance of the comparator, the RMI 1 with a decision threshold of 250, were derived from a meta-analysis of all available RMI 1 data sets with the corresponding target condition (e.g. all malignant tumours including borderline or all ovarian tumours including borderline) and population (e.g. all participants, premenopausal women or postmenopausal women). When no data were available for the RMI 1 with a decision threshold of 250, data for a decision threshold of 200 were used; the analysis reported in Diagnostic performance of the Risk of Malignancy Index 1 using decision thresholds other than 250 indicated no significant difference in the performance of the RMI 1 at these two thresholds.
Chapter 4 Assessment of cost-effectiveness
This chapter examines the cost-effectiveness of alternative risk scores, which include HE4 levels, CA125 levels or ultrasound, compared with the RMI 1 score as used in current practice for women with suspected ovarian cancer in secondary care, to guide decisions about referral to a SMDT. More specifically, the following research question is addressed:
-
What is the cost-effectiveness of alternative risk scores (including alternative RMI 1 score thresholds), which include HE4 levels, CA125 levels or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current practice), when routinely used in secondary care to guide decisions about referral to a SMDT, for people with suspected ovarian cancer?
Review of economic analyses of ovarian cancer risk scores
Search strategy
Searches were undertaken to locate relevant economic evaluations of the target condition (ovarian cancer) and diagnosis with ultrasound, CA125 levels, HE4 levels or biomarkers.
Methodological study design filters were included in the search strategy when relevant. No restrictions on language or publication status were applied. The main EMBASE strategy was independently peer reviewed by a second information specialist using the Canadian Agency for Drugs and Technologies in Health Peer Review checklist. 31 Identified references were downloaded in EndNote X6 software for further assessment and handling. References in retrieved articles were checked for additional studies.
The following databases were searched for relevant studies:
-
MEDLINE (via Ovid) – 1946 to week 2 November 2016
-
MEDLINE In-Process Citations (via Ovid) – to 22 November 2016
-
MEDLINE Daily Update (via Ovid) – to 22 November 2016
-
MEDLINE Epub Ahead of Print (via Ovid) – to 23 November 2016
-
EMBASE (via Ovid) – 1974 to 22 November 2016
-
NHS Economic Evaluation Database (via Wiley Online Library) – to Issue 2 of 4, April 2015
-
EconLit (via EBSCOhost) – 1966 to 25 November 2016
-
Cost-Effectiveness Analysis Registry (via the internet: http://www.cearegistry.org) – to 25 November 2016
-
Research Papers in Economics (via the internet: http://repec.org/) – to 25 November 2016.
The full search strategies are presented in Appendix 1.
Inclusion criteria
Studies reporting outcomes of a full cost-effectiveness analysis, examining QALYs, with (at least) one of the comparators, were eligible for inclusion. Studies conducted in primary care settings and screening studies were included to ensure that no potentially relevant information on costs or health-related quality of life was missed.
Quality assessment
Included studies are appraised using a quality checklist based on Drummond et al. 120
Results
The literature search identified 749 records from bibliographic database searches and supplementary searching (e.g. reference/citation checking and additional database searches, including the database search for the assessment of clinical effectiveness). After title and abstract screening, 10 records were considered to be potentially relevant; after full-text screening, five studies121–125 (five publications, including one abstract) were considered to be eligible for inclusion (Figure 7). These studies are described in more detail below and summarised in Table 19. The results of the quality assessment are shown in Table 20.
FIGURE 7.
Flow chart (review of economic analyses). a, Reasons for exclusion: did not report outcomes of a full cost-effectiveness analysis (n = 2) and did not report quality-adjusted LYs or LYs as an outcome (n = 3).
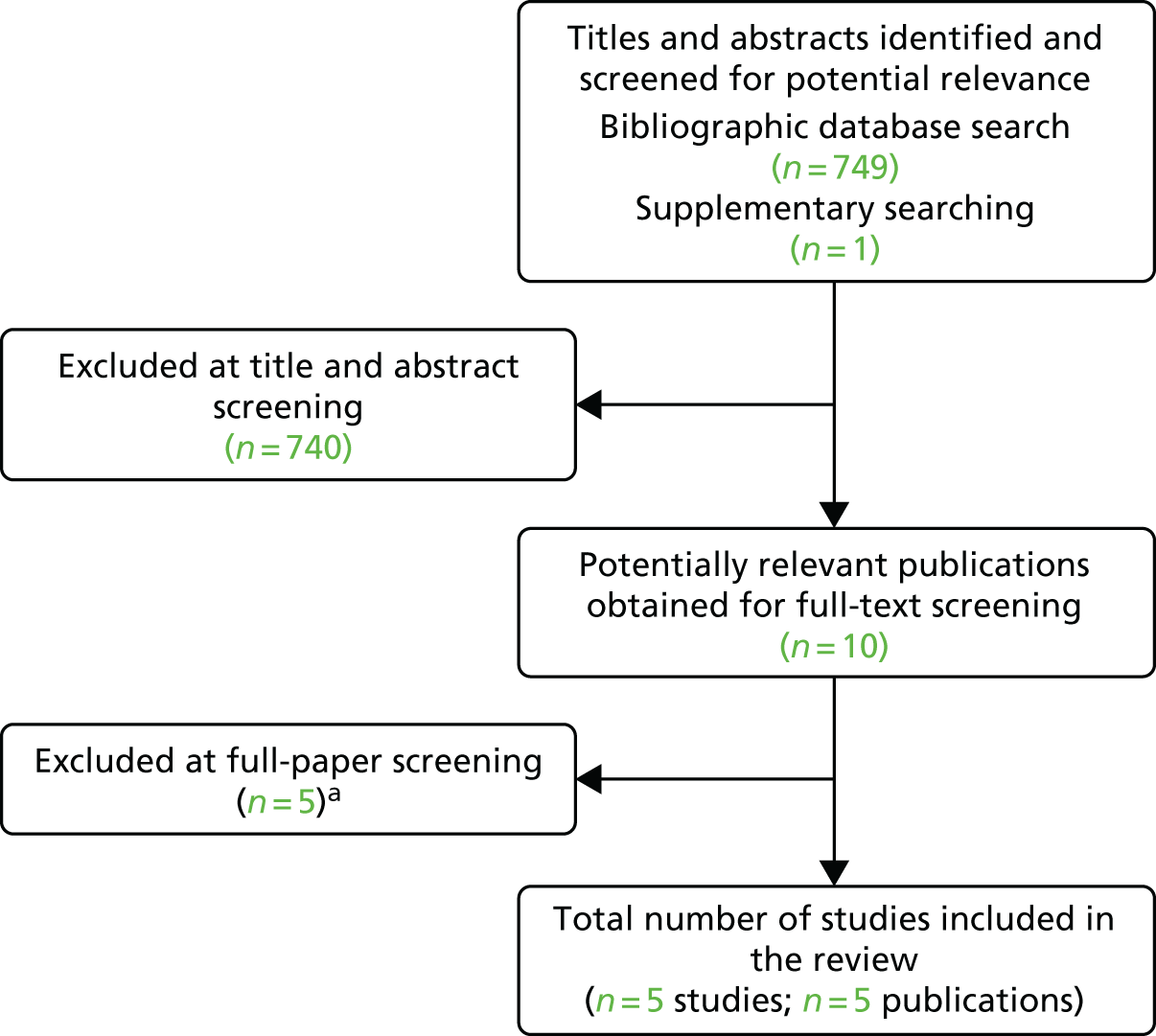
| Study characteristics | Study (year of publication) | ||||
|---|---|---|---|---|---|
| Havrilesky et al. (2015)123 | Drescher et al. (2012)124 | Kearns et al. (2016)125 | Forde et al. (2016)122 | Ding et al. (2010)121 | |
| Population | Women with an adnexal mass | Women aged 45–85 years | Postmenopausal women aged 50–74 years in the UK | Women with adnexal masses | Postmenopausal females aged 65–69 years |
| Setting | At generalist obstetrician–gynaecologist (decision to refer to a subspecialist) | First-line screening | Secondary care | Secondary care | Screening |
| Time horizon | NR | NR | Lifetime | Lifetime | Lifetime |
| Objective | To compare the estimated costs and outcomes of five strategies to help clinicians decide which women with an adnexal mass requiring surgery would most benefit from subspecialist referral | To estimate the mortality reduction, years of life saved, and cost-effectiveness of epithelial ovarian cancer screening protocols in a hypothetical cohort of women aged 45–85 years | To evaluate the potential cost-effectiveness of screening for ovarian cancer in the UK and to estimate the value of further research into ovarian cancer screening | To evaluate the cost-effectiveness of the MIA for use in triaging women with an adnexal mass | To assess the cost-effectiveness of annual MMS vs. no screening for postmenopausal females aged 65–69 years |
| Source of effectiveness information | Literature | Literature | UKTOCS study and extrapolation of mortality data | Published data on survival, prognostic factors, effectiveness of surgical cytoreduction | NCT00058032 clinical trial |
| Comparators |
|
|
|
|
|
| Costs items |
|
|
Multimodal and USS dropouts and complete screening, screening invitation, diagnosis and treatment of borderline or stages I–IV ovarian cancer, end-of-life costs | Chemotherapy with different cycle lengths and for CRC; diagnosis-related group costs and professional fees for surgery for malignancy, non-malignancy, staging surgery; CT scan; CA125 level; modified ACOG guidelines; MIA | Not stated |
| Main measure of benefit | LY | LY | QALY | QALY | QALYs |
| Main assumptions |
|
|
Log-normal for modelling survival in screening arms and Weibull in no-screening arm; disutility associated with diagnosis relates to treatment and lasts for only 1 year; no disutility associated with screening, use of ROCA does not increase costs | Major treatment-related costs occur during the first year of treatment; quality-of-life utility weights change with disease progression and differ by stage and type of cancer | Not stated |
| Perspective | Societal perspective | NR | NHS and Personal Social Services | Public payer | US societal perspective |
| Discount rate | 3% | 3% | 3.5% for costs and QALYs | 3% for costs and QALYs | 3% for costs and QALYs |
| Uncertainty around cost-effectiveness ratio expressed | Yes | Yes | Yes, EVPI and EVPPI was also performed | Yes, ICERs with one-way sensitivity analysis are given | No, but stated that cancer incidence rates and time required for screening exhibited substantial impact in sensitivity analyses |
| Sensitivity analysis | PSA | Threshold and scenario analyses | Yes, one-way sensitivity analysis and PSA | Yes, one-way sensitivity analysis | Yes |
| Monetary outcomes | 2013 US$ | 2010 US$ | £ | 2014 US$ | 2009 US$ |
| Outcomes per comparator | Postmenopausal (costs; LYs):
|
|
MMS vs. USS vs. no screening:
|
|
NR |
| Summary of incremental analysis | CA125 level is cost-effective for willingness-to-pay thresholds below US$9423 and US$10,644 per LY gained for postmenopausal women and premenopausal women, respectively. Refer all is cost-effective above these thresholds. The other strategies are dominated | CA125 level and TVS led to 1.68 more LYs than no screening, resulting in an ICER of US$88,993 per LY gained (see results section of the paper for the results of hypothetical strategies) | MMS and USS are likely to be associated with benefits for patients, but also with additional costs. The ICER of MMS vs. no screening was £8864 and USS was dominated by MMS | MIA and referral to a gynaecologic oncologist (instead of surgery by a gynaecologist) for all patients are the most cost-effective triage strategies for women with adnexal masses | MMS resulted in additional costs and QALYs of US$820 and 0.0037, respectively vs. no screening. This resulted in an ICER of US$226,622 per QALY gained |
| Study details | Study (year of publication) | ||||
|---|---|---|---|---|---|
| Havrilesky et al. (2015)123 | Drescher et al. (2012)124 | Kearns et al. (2016)125 | Forde et al. (2016)122 | Ding et al. (2010)121 | |
| Study design | |||||
| The research question is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The economic importance of the research question is stated | ✓ | ✓ | ✓ | ✓ | ✗ |
| The viewpoint(s) of the analysis are clearly stated and justified | ✓ | ✗ | ✓ | ✓ | ✓ |
| The rationale for choosing alternative programmes or interventions compared is stated | ✓ | ✓ | ✓ | ✓ | ✗ |
| The alternatives being compared are clearly described | ✓ | ✓ | ✓ | ✓ | ✗ |
| The form of economic evaluation used is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | ✗ | ✓ | ✓ | ✓ |
| Data collection | |||||
| The source(s) of effectiveness estimates used are stated | ✓ | ✓ | ✓ | ✓ | NA |
| Details of the design and results of the effectiveness study are given (if based on a single study) | ✗ | ✗ | ✓ | ✗ | ✗ |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | NA | NA | NA | NA | ✗ |
| The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods to value benefits are stated | NA | NA | ✓ | ✗ | ✗ |
| Details of the subjects from whom valuations were obtained were given | NA | NA | ✗ | ✗ | ✗ |
| Productivity changes (if included) are reported separately | NA | NA | NA | ✓ | ✗ |
| The relevance of productivity changes to the study question is discussed | NA | NA | NA | ✓ | ✗ |
| Quantities of resource use are reported separately from their unit costs | ✗ | ✗ | ✓ | ✗ | ✗ |
| Methods for the estimation of quantities and unit costs are described | ✗ | ✗ | ✓ | ✗ | ✗ |
| Currency and price data are recorded | ✓ | ✓ | ✓ | ✓ | ✓ |
| Details of currency of price adjustments for inflation or currency conversion are given | ✓ | ✓ | ✓ | ✓ | ✗ |
| Details of any model used are given | ✓ | ✗ | ✓ | ✓ | ✗ |
| The choice of model used and the key parameters on which it is based are justified | ✗ | ✗ | ✓ | ✓ | ✗ |
| Analysis and interpretation of results | |||||
| Time horizon of costs and benefits is stated | ✗ | ✗ | ✓ | ✓ | ✓ |
| The discount rate(s) is stated | ✓ | ✓ | ✓ | ✓ | ✓ |
| The choice of discount rate(s) is justified | ✗ | ✗ | ✓ | ✓ | ✗ |
| An explanation is given if costs and benefits are not discounted | NA | NA | NA | NA | NA |
| Details of statistical tests and CIs are given for stochastic data | ✓ | ✗ | ✓ | ✗ | ✗ |
| The approach to sensitivity analysis is given | ✓ | ✓ | ✓ | ✓ | ✓ |
| The choice of variables for sensitivity analysis is justified | ✗ | ✓ | ✓ | ✓ | ✗ |
| The ranges over which the variables are varied are justified | ✗ | ✓ | ✓ | ✓ | ✗ |
| Relevant alternatives are compared | ✓ | ✓ | ✓ | ✓ | ✓ |
| Incremental analysis is reported | ✓ | ✓ | ✓ | ✓ | ✓ |
| Major outcomes are presented in a disaggregated, as well as aggregated form | NA | NA | ✓ | ✓ | ✗ |
| The answer to the study question is given | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions follow from the data reported | ✓ | ✓ | ✓ | ✓ | ✓ |
| Conclusions are accompanied by the appropriate caveats | ✓ | ✗ | ✓ | ✓ | ✓ |
Havrilesky et al. (2015)
Havrilesky et al. 123 constructed a Markov model [in TreeAge Pro 2013 (TreeAge Software, Inc., Williamstown, MA, USA)] using alive and death health states. From a societal US perspective, the authors estimated the costs and outcomes of five strategies to help clinicians to decide which women with an adnexal mass requiring surgery would most benefit from subspecialist referral:
-
American Congress of Obstetricians and Gynecologists (ACOG)’s guidelines
-
multivariate index assay (MIA) algorithm
-
ROMA
-
CA125 level alone with lowered cut-off values to prioritise test sensitivity over specificity (15 U/ml for postmenopausal and 22 U/ml for premenopausal women)
-
referral of all women.
The analyses indicated that CA125 level is a cost-effective test for willingness-to-pay thresholds below US$9423 and US$10,644 per LYs gained for postmenopausal women and premenopausal women, respectively. The refer-all strategy was cost-effective above these thresholds. The other strategies are dominated. Therefore, it was concluded that referral of all women to a subspecialist is a cost-effective strategy for managing women with adnexal masses requiring surgery. However, if a test-based triage strategy is needed (e.g. because of capacity constraints), CA125 level with lowered cut-off values should be considered.
Drescher et al. (2012)
Drescher et al. 124 used an unspecified model type and structure to estimate the cost-effectiveness of first-line testing of women with an adnexal mass, TVS and CA125 level, in women aged 45–85 years (US setting, perspective not stated). The following multimodal testing strategies were considered:
-
no primary care testing
-
CA125 level followed by TVS
-
CA125 level followed by hypothetical imaging with 50% improvement in sensitivity compared with TVS
-
hypothetical biomarker with twofold greater sensitivity followed by TVS
-
hypothetical biomarker and hypothetical imaging (as above).
The analysis indicated that CA125 level and TVS led to 1.68 more LYs than no primary care testing, resulting in an incremental cost-effectiveness ratio (ICER) of US$88,993 per LY gained. Moreover, it was concluded that testing outcomes are relatively insensitive to second-line test performance and costs. Identification of a first-line test that does substantially better than CA125 level and has similar costs is required for primary care testing to reduce ovarian mortality by at least 25% and be reasonably cost-effective.
Kearns et al. (2016)
Using the NHS Personal Social Services perspective, Kearns et al. 125 developed a Markov model to estimate the cost-effectiveness of different screening strategies in postmenopausal women and to estimate the value of further research. The following screening strategies were considered:
-
multimodal screening (MMS): first-line screening with CA125 level interpreted with risk of ovarian cancer algorithm, followed by TVS performed by senior staff
-
ultrasound screening (USS): first-line screening with TVS performed by less-experienced staff, followed by TVS performed by more-experienced staff
-
no screening.
Results indicated that USS was dominated by MMS, being both more costly and less effective. Compared with no screening, MMS cost £419 more and generated 0.047 additional QALYs, resulting in an ICER of £8864 per QALY gained, but alternative mortality extrapolation methods increased the ICER. The conclusion was that MMS for ovarian cancer is both more effective and more expensive than no screening, but that substantial uncertainty remains regarding the extrapolated long-term effectiveness.
Forde et al. (2016)
From the perspective of the public payer, Forde et al. 122 developed a Markov model to evaluate the cost-effectiveness of different strategies for use in triaging women with an adnexal mass. The following triage strategies were considered:
-
the MIA (Ova1; Vermillion, Inc., Austin, TX, USA) based on five biomarkers, including CA125 level
-
the modified ACOG (mACOG) referral guidelines
-
CA125 level testing alone.
The MIA resulted in fewer reoperations and pretreatment CT scans, and was cost-effective compared with the ACOG referral guidelines, with an ICER of US$35,094 per QALY gained. The MIA dominated CA125 level alone, by being cost-saving and QALY-increasing. The MIA is expected to increase the percentage of women with ovarian cancer referred to gynaecological oncologists, thereby improving clinical outcomes.
Ding and Hay (2010)
Ding and Hay121 assess the cost-effectiveness of annual MMS (with CA125 marker, followed by TVS for those women at an increased risk of developing ovarian cancer according to their CA125 level) versus no screening for postmenopausal females aged 65–69 years from a US societal perspective. It should be noted that the available information for this assessment is restricted to one abstract (despite efforts to contact the authors). The incremental analysis indicated that, over a lifetime, MMS was both more costly (incremental costs of US$820) and more effective (incremental QALY of 0.004), resulting in an ICER of US$221,622 per QALY gained compared with no screening.
Quality assessment and summary of studies in the cost-effectiveness review
In total, three121,122,125 out of the five included studies reported QALYs as the outcome. Of these studies, two121,125 considered population screening, whereas the remaining study122 considered the assessment of women referred to secondary care from the US perspective. The last study was of reasonable quality (see Table 20). The UK screening study125 indicated that multimodal triage consisting of CA125 level followed by TVS could be cost-effective compared with ultrasound only and no triage. The two studies considering MIA, both from the US perspective, provided conflicting results; one122 indicated that MIA might be cost-effective, whereas the other indicated that it was dominated by other strategies (when considering LYs). 123 This latter study123 was the only one to consider the ROMA score, and also indicated that this strategy would be dominated by other strategies (when considering LYs). Moreover, this study indicated that a refer-all strategy is cost-effective for thresholds above US$10,644 per LY gained. 123 In conclusion, there is limited and conflicting evidence regarding the cost-effectiveness of alternative risk scores, which include HE4 level, CA125 level or morphological features seen on ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current UK practice1) for women with suspected ovarian cancer in secondary care. The population screening studies were included as a potential source of information for our cost-effectiveness analysis in case all data gaps could not be filled with the more relevant second-line studies. However, because all data gaps could be addressed with the more relevant studies, the population screening studies were not used.
Model structure and methodology
Interventions and comparators
The health economic analysis estimates the cost-effectiveness of different risk scores to estimate an individual’s risk of malignancy. This risk score can inform decisions about SMDT referral. The following risk scores are considered in the model:
-
RMI 1 score (at a threshold of 200)
-
RMI 1 score (at a threshold of 250)
-
ROMA score using Abbott Diagnostics’ ARCHITECT
-
ROMA score using Roche Diagnostics’ Elecsys
-
Overa (MIA2G) from Vermillion (at a threshold of 5 units)
-
IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant)
-
IOTA group’s ADNEX model (threshold most commonly used in studies: 10%).
An optimised risk assessment that reduces the number of women with ovarian cancer who are not referred for further specialist care (i.e. those with a FN risk assessment) has the potential to improve prognosis, be cost-saving in terms of avoiding unnecessary further investigations and optimising staging and surgical treatment, and to reduce associated anxiety. It is likely that women who are believed to have a benign explanation for any pelvic mass will be operated on in secondary care. If they actually have ovarian cancer, then the prognosis might be worse than if they had been operated on by a specialist gynaecological oncology surgeon.
The current standard assessment (RMI 1 score at a decision thresholds of ≥ 250) has been reported as having poor sensitivity (69%) for the prediction of malignancy (see Table 18). If referral decisions are based on the RMI 1 score at this threshold, there remains the potential for significant numbers of women with ovarian cancer to remain unreferred and experience consequential delays in diagnosis and detrimental effects on prognosis. This risk score was used as reference strategy. Alternative risk scores evaluated in the model are the ROMA score [ARCHITECT tumour marker assays (CA125 and HE4 levels) from Abbott Diagnostics; Elecsys tumour marker assays (CA125 and HE4) from Roche Diagnostics], the simple ultrasound rules classification system from the IOTA group, the ADNEX model from the IOTA group and the Overa [(MIA2G) from Vermillion], and alternative decision thresholds for the RMI 1. The model does not include LUMIPULSE G HE4 (Fujirebio Diagnostics), as no studies of this technology were identified, or LUMIPULSE HE4 EIA (Fujirebio Diagnostics), as this test was outside the scope of our assessment (see Accuracy of the Risk of Ovarian Malignancy Algorithm score using Fujirebio Diagnostics’ tumour marker assays). Alternative threshold values for the IOTA group’s ADNEX model were not considered, as the 10% threshold is the most commonly studied and has been used in model validation studies. 42,46
For the IOTA group’s simple ultrasound rules risk score, it was assumed that inconclusive assessments would be classified as malignant, as this was assumed to be most representative of what would be available in secondary care (no additional input from a specialist ultrasonographer needed). Concerning the alternative decision thresholds for the RMI 1, a threshold of ≥ 200 (used in the original publication78) was used in the base-case analysis and other RMI 1 thresholds were considered in scenario analyses.
Model structure
This assessment uses the economic model from CG1221 as a starting point. CG1221 reviewed clinical and economic questions that involve the detection in primary care, diagnosis in secondary care and initial management of early- and advanced-stage ovarian cancer (AOC). The CG1221 model consisted of a decision tree outlining the assessment strategies, and a Markov process to model the progression and survival of women with ovarian cancer based on the results of the diagnostic tests and the subsequent treatment of women presenting with symptom(s) of ovarian cancer. The CG1221 model was constructed using TreeAge Pro [(2009) TreeAge Software Inc., Williamstown, MA, USA] software. The assessment group used the description of this model as a starting point to develop a de novo model [in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA)], adapted to better fit the scope of the current assessment. Consistent with the CG1221 model, the population age in the base case was assumed to be 40 years. In the subgroup analysis, different ages were used to reflect the premenopausal (mean age of 38 years) and postmenopausal (mean age of 68 years) groups of women. The mean age for both groups was estimated based on information on the distribution of ovarian cancer patients pre- and post-age 50 years from Cancer Research UK, assuming that menopause occurs approximately at the age of 50 years. 126
In the de novo health economic model, the mean expected costs and QALYs were calculated for each alternative risk score. These long-term consequences were estimated based on the accuracy of the different risk scores to predict ovarian cancer, followed by referral to and treatment by a SMDT, or no tertiary care referral. It was also taken into account that a small proportion of the women with pelvic masses, who tested positive based on the risk score, were ultimately diagnosed with CRC (consistent with CG122). These women were therefore included in the model and the prognosis for women with CRC was included in the Markov model.
A decision tree and a Markov model were developed. The decision tree was used to model the short-term (up to 30 days after surgery) outcomes. It was assumed that women who receive a high-risk test result (either true or false) are referred to a SMDT, and women who receive a low-risk test result (either true or false) are not referred to a SMDT. After the risk assessment and referral decision, women in the decision tree are allocated to ‘early ovarian cancer’, ‘AOC’, ‘benign mass’, ‘colorectal cancer’ and ‘death’. Death was included as an outcome in the decision tree to account for 30 days’ post-surgery mortality. Women referred to a SMDT receive surgery by gynaecological oncology specialists that has shown to achieve better patient outcomes than those for patients not referred to a SMDT and, for a proportion of patients, this surgery is extensive. Women not referred to a SMDT receive surgery by secondary care gynaecologists. In the case of a FN diagnosis (i.e. when a woman has a malignancy and is incorrectly classified as having a low risk score), there is an increased risk of progressing to AOC and/or death. This increased risk is likely to be the result of a combination of factors, such as a delay in appropriate treatment, because the woman would be operated on and then referred to a SMDT for another surgery (based on clinical experts’ feedback). Women with a benign mass who are incorrectly classified as being at a high risk of developing ovarian cancer and referred to a SMDT receive surgery and have their benign mass removed. This incorrect referral has only cost implications, as women are identified as having a benign mass at surgery. Alternatives in the women’s care pathway are explored in scenario analyses. The decision tree is shown in Figure 8.
FIGURE 8.
Decision tree structure.

The long-term consequences in terms of costs and QALYs were estimated using a Markov cohort model (Figure 9) with a lifetime time horizon. The cycle time was 1 year. The following health states were included:
-
benign mass
-
early ovarian cancer, not referred to a tertiary care SMDT
-
early ovarian cancer, referred to a tertiary care SMDT
-
AOC, not referred to a tertiary care SMDT
-
AOC, referred to a tertiary care SMDT
-
colorectal cancer Dukes’ stage A
-
colorectal cancer Dukes’ stage B
-
colorectal cancer Dukes’ stage C
-
colorectal cancer Dukes’ stage D
-
death.
FIGURE 9.
Markov process structure.
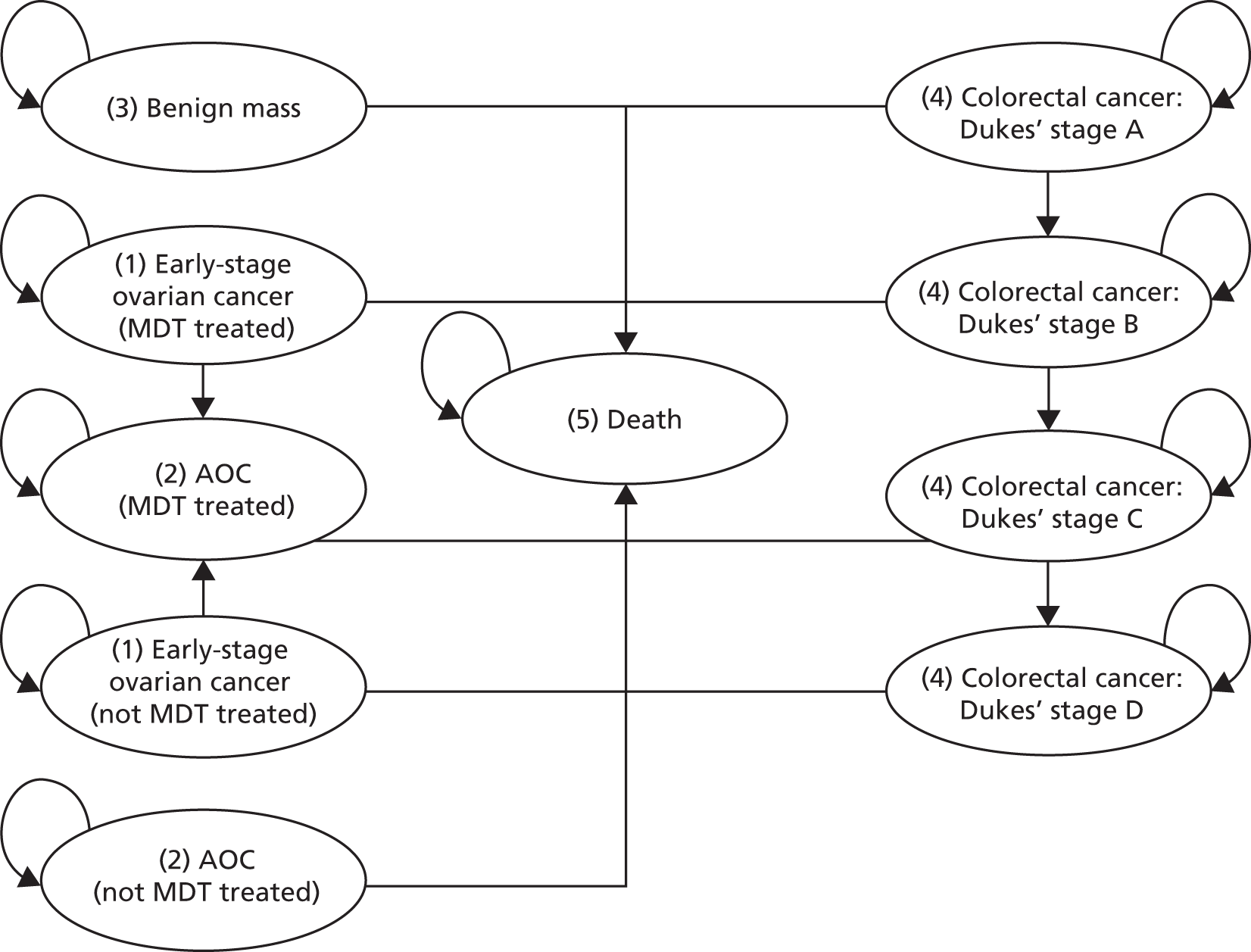
A distinction between the decision to refer to a SMDT or not was made only for ‘early-stage ovarian cancer’ and ‘AOC’. This was done as it was assumed that a referral to the SMDT would have an impact on the long-term outcomes in terms of LYs and QALYs for women with ovarian cancer only.
Model parameters
Estimates for the model input parameters were retrieved from the literature and by consulting experts for unpublished data. For consistency, and when the same parameters were required, the same sources were used as those used in CG122. 1 Accuracy estimates were derived from the systematic review component of this assessment (see Results of the assessment of clinical effectiveness). In case empirical estimates of standard errors were unavailable, it was assumed that the standard error would be equal to 20% of the expected values.
Probabilities not related to the risk scores
An overview of the disease-related (ovarian cancer, CRC and benign mass) probabilities for both the decision tree and the Markov model is provided in Table 21. It was assumed that all patients are female (used for utility estimation).
| Estimate | SE | Distribution | Source (year of publication) | |
|---|---|---|---|---|
| Decision tree (short term) | ||||
| Prevalence (all malignancies) | 21.3% | 1.0% | Beta | Aktürk et al. (2011),71 Colombo et al. (2009),127 Coleman et al. (2016),70 Di Legge et al. (2012),61 Jacobs et al. (1990),78 Janas et al. (2015),97 Knafel et al. (2016),49 Lou et al. (2010),73 Meys et al. (2016),44 Moore et al. (2011),101 Morgante et al. (1999),80 Sayasneh et al. (2016),46 Shulman et al. (2016),104 Testa et al. (2014),50 Timmerman et al. (2010),65 van Gorp et al. (2012),98 Xu et al. (2016)95 and Yanaranop et al. (2016)89 |
| Prevalence (all malignancies) – premenopausal women | 16.2% | 2.0% | Beta | Al Musalhi et al. (2016),103 Coleman et al. (2016),70 Janas et al. (2015),97 Knafel et al. (2016),49 Meys et al. (2016),44 Piovano et al. (2016),58 Sayasneh et al. (2013),62 Testa et al. (2014),50 van Gorp et al. (2012)98 and Yanaranop et al. (2016)89 |
| Prevalence (all malignancies) – postmenopausal women | 45.9% | 3.3% | Beta | Al Musalhi et al. (2016),103 Coleman et al. (2016),70 Janas et al. (2015),97 Knafel et al. (2016),49 Meys et al. (2016),44 Piovano et al. (2016),58 Sayasneh et al. (2013),62 Testa et al. (2014),50 van Gorp et al. (2012)98 and Yanaranop et al. (2016)89 |
| Prevalence non-ovarian malignancies (colorectal) within malignancies | 2.9% | 0.3% | Beta | Aktürk et al. (2011),71 Colombo et al. (2009),127 Coleman et al. (2016),70 Di Legge et al. (2012),61 Jacobs et al. (1990),78 Janas et al. (2015),97 Knafel et al. (2016),49 Lou et al. (2010),73 Meys et al. (2016),44 Moore et al. (2011),101 Morgante et al. (1999),80 Sayasneh et al. (2016),46 Shulman et al. (2016),104 Testa et al. (2014),50 van Gorp et al. (2012),98 Xu et al. (2016)95 and Yanaranop et al. (2016)89 |
| Advanced stage if ovarian malignancya | 75% | Fixed | Bell et al. (1998)128 | |
| If CRC, proportion of Dukes’ stage A | 13.2% | 0.1% | Dirichlet | National Cancer Registration and Analysis Service (2010)129 |
| If CRC, proportion of Dukes’ stage B | 36.9% | 0.1% | Dirichlet | |
| If CRC, proportion of Dukes’ stage C | 35.9% | 0.1% | Dirichlet | |
| If CRC, proportion of Dukes’ stage D | 14.0% | Dirichlet | ||
| If FN result, proportion of ovarian cancer | 100.0% | Fixed | Assumption | |
| If FP result, proportion having benign mass | 100.0% | Fixed | Assumption | |
| If TN result, proportion having benign mass | 100.0% | Fixed | Assumption | |
| 30-day post-surgery mortality, early-stage ovarian cancer | 1.1% | 0.5% | Beta | National Collaborating Centre for Cancer (2011),1 and Venesmaa and Ylikorkala (1992)130 |
| 30-day post-surgery mortality, AOC | 2.9% | 0.3% | Beta | National Collaborating Centre for Cancer (2011)1 and Gerestein et al. (2009)131 |
| 30-day post-surgery mortality related to benign surgery | 0.2% | 0.0% | Beta | National Collaborating Centre for Cancer (2011),1 Loft et al. (1991)132 |
| Markov model (long-term) | ||||
| 10-year progression-free survival for early-stage ovarian cancer | 70.0% | 4.7% | Beta | ICON Group study 1, Collinson et al. (2014)133 |
| 10-year overall survival for early-stage ovarian cancer | 73.0% | 4.0% | Beta | ICON Group study 1, Collinson et al. (2014)133 |
| 2-year overall survival for AOC | 62.6% | 1.8% | Beta | ICON Group study 3 (2002)134 |
| HR overall survival with SMDT treatment vs. no SMDT treatment | 0.900 | 0.048 = SE ln(HR) | Log-normal | Woo et al. (2012)135 |
| HR progression-free survival with SMDT treatment vs. no SMDT treatment | Assumed equal to HR for overall survival given that overall survival and progression-free survival HRs for teaching vs. general hospitals are very similar135 | |||
| Annual progression for Dukes’ stage A to B | 58.3% | 0.5% | Beta | Westwood et al. (2016)136 and Tappenden et al. (2007)137 |
| Annual progression for Dukes’ stage B to C | 65.6% | 0.8% | Beta | |
| Annual progression for Dukes’ stage C to D | 86.7% | 0.8% | Beta | |
| Mortality CRC | Time dependent, see Appendix 7 in Westwood et al.136 for more details | |||
The prevalence of malignancies (all, including borderline and non-ovarian malignancies) as well as the proportion of women diagnosed with other malignancies (assumed to be CRC) were obtained using a random-effects meta-analysis (with log-transformation) of diagnostic cohort studies, included in our systematic review, which reported data for the relevant target condition and subgroup. The following parameters were estimated as in CG122:1
-
percentage of early-stage ovarian cancer versus AOC
-
30 days post-surgery ovarian cancer mortality
-
10-year overall survival and progression-free survival of ovarian cancer [using updates of the same trials: the International Collaborative Ovarian Neoplasm (ICON) Group’s study 1 was used to model progression-free survival and overall survival for early ovarian cancer;133 and the ICON Group’s study 3 was used to model these outcomes for AOC134].
The following parameters were estimated as in the most recent diagnostic appraisal review (DAR) in CRC:136
-
percentage in each of the Dukes’ stages
-
annual progression between Dukes’ stages
-
mortality by Dukes’ stage.
The effect of SMDT treatment (i.e. with gynaecological oncologists on site) versus women treated in secondary care was estimated from Woo et al. ,135 who reported a hazard ratio (HR) of 0.90 (95% CI 0.820 to 0.990) for the overall survival of women with ovarian cancer treated in teaching hospitals versus general hospitals. This HR was also assumed for progression-free survival, as the analyses by Woo et al. 135 indicated that the HR for overall and progression-free survival for teaching hospitals versus general hospitals is very similar. This study was obtained from a focused literature search, which was pragmatic in design. For this, the following resources were searched:
-
MEDLINE (via Ovid): 1946 to week 3 January 2017
-
MEDLINE In-Process Citations (via Ovid): to 30 January 2017
-
MEDLINE Daily Update (via Ovid): to 30 January 2017
-
MEDLINE Epub Ahead of Print (via Ovid): to 30 January 2017
-
EMBASE (via Ovid): 1974 to 30 January 2017
-
Cochrane Database of Systematic Reviews (via Wiley Online Library): to Issue 1 of 12, January 2017
-
Database of Abstracts of Reviews of Effects (via Wiley Online Library): to Issue 2 of 4, April 2015
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library): to Issue 11 of 12, November 2016
-
NHS Economic Evaluation Database (via Wiley Online Library): to Issue 2 of 4, April 2015.
Full search strategies are presented in Appendix 1.
Finally, age-dependent mortality from the general population was used for women with a benign mass, after the 30-day post-surgery period. All input parameters for the Markov model are reported in Table 21.
Risk score accuracy parameters
The proportions of women testing positive (and thus referred to a SMDT) or negative were based on the estimated accuracy of the risk scores considered (see Chapter 3, Selection of diagnostic performance estimates for inclusion in cost-effectiveness modelling and Table 22) and the estimated prevalence of all malignancies detected in this population (21.3% with a standard error of 1.0%). The proportions of true positives (TPs), FPs, FNs and true negatives (TNs) were calculated as follows:
| Risk score | Sensitivity (SE) | Specificity (SE) | Source (systematic review; see Chapter 3) |
|---|---|---|---|
| RMI 1 (threshold of 250) | 64.4% (1.4%) | 91.8% (0.7%) | Summary estimate derived from all studies, six published studies73,74,76,78–80 and one unpublished studya that reported data for RMI 1 (at a threshold of 250) and the target condition ‘all malignant tumours’ |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 75.0% (6.6%) | 87.9% (2.7%) | Al Musalhi et al. (2016)103 (see Table 7) |
| ROMA score using Roche Diagnostics’ Elecsys | 79.1% (2.4%) | 79.1% (1.4%) | Summary estimate derived from two studies97,104 (see Table 10) |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 90.2% (2.5%) | 65.8% (1.9%) | Summary estimate derived from two studies68,70 (see Table 17) |
| IOTA group’s Simple Rules (inconclusive, assumed to be malignant) | 94.2% (0.5%) | 76.1% (0.6%) | Summary estimate derived from eight published studies44,48–50,52,55,62,65 and one unpublished study (see Table 12) |
| IOTA group’s ADNEX model (threshold of 10%) | 96.3% (0.5%) | 69.1% (0.9%) | Summary estimate derived from three published studies17,44,46 and one unpublished study (see Table 11) |
| RMI 1 (threshold of 200) | 68.1% (0.9%) | 90.1% (0.5%) | Summary estimate derived from all studies, 12 published studies44,48,50,62,73,74,76,78–80,98,103 and one unpublished study that reported data for the RMI 1 (threshold of 200) and the target condition ‘all malignant tumours’ |
Subsequently, the proportions of women who are referred to a SMDT (TPs and FPs), and who are not referred to a SMDT (TNs and FNs) were calculated. The results are listed in Table 23.
| Risk score | TP (%) | FP (%) | FN (%) | TN (%) | PPV | NPV | LR+ | LR– |
|---|---|---|---|---|---|---|---|---|
| RMI 1 (at a threshold of 250) | 13.7 | 6.5 | 7.6 | 72.2 | 0.68 | 0.90 | 7.85 | 0.39 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 16.0 | 9.5 | 5.3 | 69.2 | 0.63 | 0.93 | 6.20 | 0.28 |
| ROMA score using Roche Diagnostics’ Elecsys | 16.9 | 16.4 | 4.5 | 62.2 | 0.51 | 0.93 | 3.78 | 0.26 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 19.2 | 26.9 | 2.1 | 51.8 | 0.42 | 0.96 | 2.64 | 0.15 |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 20.1 | 18.8 | 1.2 | 59.9 | 0.52 | 0.98 | 3.94 | 0.08 |
| IOTA group’s ADNEX model (at a threshold of 10%) | 20.5 | 24.3 | 0.8 | 54.4 | 0.46 | 0.99 | 3.12 | 0.05 |
| RMI 1 (at a threshold of 200) | 14.5 | 7.8 | 6.8 | 70.9 | 0.65 | 0.91 | 6.88 | 0.35 |
After the risk assessment and referral decision, women in the decision tree were allocated to ‘early-stage ovarian cancer’, ‘AOC’, ‘benign mass’, ‘CRC’ and ‘death’. One of the main assumptions in the decision tree was that the women categorised as testing FN all had early-stage disease based on expert opinion (i.e. the value of 25% for early-stage ovarian cancer refers only to the TPs).
Health state utilities
For women with a benign mass, age-dependent general population utility estimates were used. 138 The utilities for early-stage and AOC were taken from Havrilesky et al. 139 and Grann et al. ,140 respectively. These estimates were also used in the economic model in CG122. 1 As in the latest CRC DAR, the study by Ness et al. 136,141 was used to inform utilities for the four stages of CRC. Utility estimates and sources are summarised in Table 24.
| Population | Estimate (SE) | Distribution | Source (year of publication) |
|---|---|---|---|
| Benign mass (assumed to be equal to the general population) | Age dependent | Ara et al. (2010)138 | |
| Early-stage ovarian cancer treated by a SMDT | 0.830 (0.063) | Beta | Havrilesky et al. (2009)139 |
| Early-stage ovarian cancer not treated by a SMDT | Assumed to be equal to treatment by a SMDT | ||
| AOC treated by a SMDT | 0.630 (0.247) | Beta | Grann et al. (1998)140 |
| AOC not treated by a SMDT | Assumed to be equal to treatment by a SMDT | ||
| CRC Dukes’ stage A | 0.740 (0.023) | Beta | Ness et al. (1999)141 |
| CRC Dukes’ stage B | 0.670 (0.026) | Beta | |
| CRC Dukes’ stage C | 0.500 (0.031) | Beta | |
| CRC Dukes’ stage D | 0.250 (0.028) | Beta | |
Resource use and costs related to the risk scores
Risk score costs are listed in Table 25.
| Risk score | Estimate (£) | SE (£) | Distribution | Source |
|---|---|---|---|---|
| RMI 1 | 102 | 20 | Gamma | More detail on the calculation of these costs is provided in the following sections and in Appendix 6 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 130 | 26 | Gamma | |
| ROMA score using Roche Diagnostics’ Elecsys | 126 | 25 | Gamma | |
| Overa (MIA2G) from Vermillion | 176 | 35 | Gamma | |
| IOTA group’s simple ultrasound rules | 77 | 15 | Gamma | |
| IOTA group’s ADNEX model | 102 | 20 | Gamma |
To derive the costs associated with these risk scores, several assumptions were made. These pertained to the different components of the RMI 1 score, the ROMA score, Overa (MIA2G), the IOTA group’s simple ultrasound rules and the ADNEX model, and are summarised in the following sections.
Ultrasound costs
All risk scores entail, or are intended to be derived partly from, TVS scans. The costs for these were informed by the costs for TVS scans used in CG122,1 and inflated to 2015–16 Personal Social Services Research Unit (PSSRU) values, resulting in a cost of £77.
Cancer antigen 125 test costs
The RMI 1, the ROMA score, Overa (MIA2G) from Vermillion and the IOTA group’s ADNEX model risk scores all require an estimated cost for CA125 tests. These can differ in practice depending on which company’s test is used. Only Roche Diagnostics made CA125 costs available. However, these costs referred to only the cost of the kit, not to the overall CA125 test cost. The cost used here was therefore taken from CG1221 and estimated to be £26 (adjusted for inflation).
Risk of Malignancy Index 1 costs
The RMI 1 entails ultrasound scans and CA125 testing. RMI 1 costs were therefore the sum of the costs of ultrasound scans and CA125 testing (£102).
International Ovarian Tumour Analysis group’s simple ultrasound rules
The IOTA group’s simple ultrasound rules entail the costs of ultrasound scans only (£77). The IOTA group stated that using the simple rules algorithm would not add to the time needed to conduct the scan or interpret the results (Thomas Walker, Maastricht University, 2016, personal communication).
The International Ovarian Tumour Analysis group’s Assessment of Different NEoplasias in the adneXa model
The IOTA group’s ADNEX model consists of the costs of ultrasound scans and CA125 testing, and was therefore estimated to be £102.
Vermillion’s Overa (MIA2G)
Vermillion provided a cost estimate of £99 chargeable for the provision of its test. It was unclear whether or not this cost included all materials. The cost of ultrasound was added to it, resulting in £176. This was added because the manufacturer recommends the use of Overa (MIA2G) alongside clinical and radiological evaluation and states that the product is not recommended as a stand-alone screening or diagnostic test.
Risk of Ovarian Malignancy Algorithm (Abbott Diagnostics’ ARCHITECT and Roche Diagnostics’ Elecsys)
The estimation of costs related to HE4 testing relied on the information provided by the different manufacturers. The cost of ultrasound was added to both of the ROMA risk scores as determined in the final scope based on clinical expert opinion. Manufacturers of both tests stated that final costs may be subject to volume-based discounts. Not all companies provided the same cost items and assumptions were made in order to fill in data gaps. The following is a list of these assumptions:
-
Cost per HE4 test kit:
-
The Abbott Diagnostics’ ARCHITECT HE4 test kit cost provided by the manufacturer was £21.33 per single test (e-mail from Abbott Diagnostics to Thomas Walker, NICE, 2017, personal communication).
-
The Roche Diagnostics’ Elecsys HE4 test kit cost provided by the manufacturer was £1594.65, with each kit containing 100 tests, resulting in a cost per test of £15.95.
-
-
Capital costs:
-
Analyser equipment costs were assumed to be the same for Fujirebio Diagnostics’ LUMIPULSE G HE4, Abbott Diagnostics’ ARCHITECT HE4 and Roche Diagnostics’ Elecsys HE4, and these were based on the average analyser equipment cost of Fujirebio Diagnostics’ LUMIPULSE G1200 and G600II.
-
These analyser equipment costs were annuitised with an assumed lifetime of 10 years and using a discount rate of 3.5%, resulting in an annuity factor of 8.32.
-
To calculate the analyser equipment capital cost per each test, an average of 253 working days per year with 7 work hours per day was assumed, and it was also assumed that two tests on average would be run per hour. This resulted in 3542 HE4 tests run on one analyser per year. However, many tests were run at the same time and some laboratories would only run these tests weekly, whereas some others would run these daily, resulting in a relatively crude estimate of numbers of tests per year. The resulting capital cost per test might, therefore, be an overestimate. However, it amounted to only £1.92 per test and, therefore, did not significantly affect the model outcomes.
-
-
Maintenance costs:
-
Only Fujirebio Diagnostics provided cost estimates for maintenance. The average of the maintenance costs for LUMIPULSE G1200 and G600 II were assumed to be representative of the maintenance costs for all of the analysers of the different manufacturers. The two options of maintenance cover (fully comprehensive and preventative) were assumed to be adopted in equal proportions.
-
-
Quality control:
-
Abbott Diagnostics and Roche Diagnostics provided cost estimates for their quality control. These were applied for each.
-
-
Calibration:
-
Calibration costs were provided by Abbott Diagnostics and Roche Diagnostics, but it was not clear how often the calibration costs provided by Abbott Diagnostics were going to be incurred. The Roche Diagnostics calibration costs were therefore applied to both tests.
-
-
Shipping:
-
Only Fujirebio Diagnostics provided costs for shipping each month. These were assumed to apply to Roche Diagnostics. Abbott Diagnostics stated that one shipment per month was free of charge and that further shipments were unlikely, so no further shipment costs were added to the Abbott Diagnostics’ test costs.
-
-
Personnel costs:
-
An estimate of 0.05 hours to prepare and perform one test was used. Given that many tests can be performed at the same time, this is likely to be an overestimate. The personnel cost was assumed to be that of a health-care scientist derived from Curtis (Unit Costs of Health and Social Care 2014)142 at £2.76 per test. Given that these costs are still relatively low, the potential overestimation of personnel costs was not likely to affect the model outcomes. These costs were therefore added to both Roche Diagnostics and Abbott Diagnostics’ tests.
-
The Roche Diagnostics’ Elecsys HE4 test costs amounted to £23.75 and the Abbott Diagnostics’ ARCHITECT HE4 test costs amounted to £27.97. The main difference in costs between Roche Diagnostics’ Elecsys HE4 and Abbott Diagnostics’ ARCHITECT HE4 thus stemmed from the cost per kit; other differences were caused by shipping costs and quality control costs.
Resource use and costs not related to the risk scores
All women with a high-risk test result are assumed to be referred to the SMDT. Based on expert opinion, the additional resource use for this is assumed to be the cost of a SMDT meeting, assuming no additional cost of the surgery or other investigations. The cost of this meeting (£116) is estimated to be that of the SMDT meetings, from the 2015–16 NHS reference costs. 143
The treatment of ovarian cancer may consist of surgery and/or chemotherapy or supportive care, depending on the disease stage. As assumed in CG122,1 chemotherapy consists of six cycles of carboplatin for women with early-stage ovarian cancer and six cycles of carboplatin/paclitaxel for women with advanced-stage disease. 1 Surgery costs were also based on CG122,1 and calculated as a weighted average of the relevant NHS reference costs, taking into account the probability of complications and the underlying disease (early-stage or AOC or benign mass). 143 The proportions of women receiving each type of care were based on CG122,1 and the unit costs of care from the latest PSSRU publication. 144 The frequency of follow-up costs for women with ovarian cancer was based on CG122,1 and the unit costs were based on the PSSRU publication. 144 Annual costs for the CRC states were estimated from the lifetime costs of CRC and mean survival as was done in the most recent DAR in CRC136,137 (Table 26). Women with a high risk score but a benign mass would be identified at the SMDT meeting to be FP cases, and would undergo SMDT surgery without any further costs incurred in the tertiary care setting.
| Health state or event | Estimate | SE | Distribution | Source |
|---|---|---|---|---|
| Health state costs of CRC | ||||
| CRC Dukes’ stage A (lifetime costs) | £10,683 | £3959 | Gamma | Westwood et al. (2016)136 and Tappenden et al. (2007)137 |
| CRC Dukes’ stage B (lifetime costs) | £18,015 | £6677 | Gamma | |
| CRC Dukes’ stage C (lifetime costs) | £29,141 | £10,800 | Gamma | |
| CRC Dukes’ stage D (lifetime costs) | £19,392 | £7187 | Gamma | |
| CRC Dukes’ stage A (annual costs) | £264 | Calculated (using mean survival as in the previous DAR); Westwood et al. (2016)136 | ||
| CRC Dukes’ stage B (annual costs) | £609 | |||
| CRC Dukes’ stage C (annual costs) | £2039 | |||
| CRC Dukes’ stage D (annual costs) | £8391 | |||
| SMDT visit | ||||
| SMDT visit (necessary or unnecessary for benign mass) | £116 | Fixed | Calculated | |
| Treatment costs of ovarian cancer/benign mass | ||||
| Chemotherapy for early-stage ovarian cancer (six cycles of carboplatin) | £1898 | £380 | Gamma | Calculated |
| Unit costs of one cycle of carboplatin | £316.29 | Fixed | National Collaborating Centre for Cancer (2011)1 and the British National Formulary (2016)145 | |
| Chemotherapy administration for early-stage ovarian cancer | £1417 | £283 | National Collaborating Centre for Cancer (2011)1 | |
| Simple parenteral chemotherapy administration (per cycle) | £236 | Fixed | NHS Reference Costs 2015-2016– SB12Z143 | |
| Chemotherapy for AOC (six cycles of carboplatin and paclitaxel) | £5905 | £1118 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Unit costs of paclitaxel | £667.88 | National Collaborating Centre for Cancer (2011)1 and the British National Formulary (2016)145 | ||
| Chemotherapy administration for AOC | £1918 | £384 | Calculated | |
| More complex parenteral chemotherapy administration (per cycle) | £320 | Fixed | NHS Reference Costs 2015-2016 – SB13Z143 | |
| Laparotomy malignancy without complication | £3615 | £723 | Gamma | NHS Reference Costs 2015-2016 – M06C143 |
| Laparotomy malignancy with complication | £4566 | £913 | Gamma | NHS Reference Costs 2015-2016 – MA06A and MA06B143 |
| Laparotomy benign mass without complication | £3.301 | £660 | Gamma | NHS Reference Costs 2015-2016 – MA07G and MA08B143 |
| Laparotomy benign mass with complication | £4112 | £822 | Gamma | NHS Reference Costs 2015-2016 – MA07E, MA07F and MA08A143 |
| Proportion complication laparotomy benign mass | 5.0% | 1.0% | National Collaborating Centre for Cancer (2011)1 | |
| Proportion complication laparotomy early ovarian cancer | 5.0% | 1.0% | ||
| Proportion complication laparotomy AOC | 12.5% | 2.5% | ||
| Number of hospital specialist care support visits | 14.0 | 2.8 | Gamma | |
| Unit costs of hospital specialist care support | £100 | Fixed | NHS Reference Costs 2015-2016 – SD03A143 | |
| Number of hospital specialist care visits | 14.0 | 2.8 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Unit costs of hospital specialist care visit | £396 | Fixed | NHS Reference Costs 2015-2016 – SD01A143 | |
| Number of GP visits | 1.0 | 0.2 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Unit costs of GP visits | £76 | Fixed | PSSRU144 | |
| Number of district nurse visits | 4.0 | 0.8 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Unit costs of district nurse visit | £42 | Fixed | PSSRU144 | |
| Number of nurse specialist visits | 2.0 | 0.4 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Unit costs of nurse specialist visits | £50 | Fixed | PSSRU144 | |
| Total costs of supportive care | £7290 | Calculated | ||
| Proportion of chemotherapy administered for early-stage ovarian cancer | 50% | 10% | Beta | National Collaborating Centre for Cancer (2011)1 |
| Proportion chemotherapy AOC | 95% | (100% minus the proportion of supportive care) | ||
| Proportion surgery early ovarian cancer | 100% | fixed | ||
| Proportion surgery AOC | 85% | 17% | Beta | |
| Proportion supportive care early ovarian cancer | 0% | Fixed | ||
| Proportion supportive care AOC | 5% | 1% | Beta | |
| Total treatment costs for benign mass | £3342 | Calculated | ||
| Total treatment costs for early-stage ovarian cancer | £5320 | |||
| Total treatment costs for AOC | £10,606 | |||
| Follow up costs | ||||
| Annual number of follow-up visits (years 1–3) | 4 | 0.8 | Gamma | National Collaborating Centre for Cancer (2011)1 |
| Annual number of follow-up visits (> year 3) | 1 | 0.2 | Gamma | |
| Unit costs of follow-up visits | £92 | Fixed | PSSRU144 | |
| Total annual follow-up costs (years 1–3) | £398 | Calculated | ||
| Total annual follow-up costs (> year 3) | £92 | |||
Overview of main model assumptions
The main assumptions in the health economic analyses were:
-
All non-ovarian malignancies are CRC malignancies.
-
False-negative tests are more likely to be early-stage ovarian cancer than AOC.
-
For the IOTA group’s simple ultrasound rules, inconclusive assessments would be assumed to be malignant.
-
Carboplatin costs (six cycles) for early ovarian cancer and carboplatin plus paclitaxel costs (six cycles) for AOC [without bevacizumab (Avastin®; Roche Diagnostics, Hertford, UK)].
-
List prices are used for carboplatin and paclitaxel.
-
The HR of 0.900 retrieved from the Cochrane review by Woo et al. ,135 which focused on the comparison of institutions with gynaecologic oncologists on site versus community or general hospitals, is representative of the relative progression-free survival and overall survival for SMDT treatment versus no SMDT treatment.
-
All FP and FN patients will be operated on for a benign mass.
-
No disutility is incorporated for FP women (i.e. women who are incorrectly told that they have ovarian cancer).
The impact of all of the assumptions listed above on the model outcomes is explored in the scenario analyses.
Model analyses
Expected costs, LYs and QALYs were estimated for all risk scores from the perspective of the NHS. Discount rates of 3.5% and a half-cycle correction were applied for both costs and effects. Incremental costs and QALYs for each strategy versus the RMI 1 at a decision threshold of 250 and versus the next best alternative were calculated (full incremental analysis). The ICER was then calculated by dividing the incremental costs by the incremental QALYs. Probabilistic sensitivity analyses (PSAs; 15,000 simulations) were performed and cost-effectiveness acceptability curves were constructed.
Sensitivity analyses
Deterministic one-way sensitivity analyses were performed, using all input parameters incorporated as stochastic parameters in the PSAs as well as the discount rates, to assess the impact input parameters on the estimated outcomes. The results of these analyses are presented using tornado diagrams.
Scenario analyses
Various scenario analyses were performed to assess the impact of the assumptions on the estimated outcomes:
-
Assuming a prevalence of 20% for all malignancies.
-
Assuming a prevalence of 30% for all malignancies.
-
Assuming a 0% prevalence of non-ovarian (CRC) malignancies.
-
Assuming an equal proportion of early-stage versus AOC in the FN and TP groups (in the base case, it was assumed that FN women would all have early-stage ovarian cancer).
-
Assuming that, for the IOTA group’s simple ultrasound rules, subjective assessment would be used for inconclusive assessments (instead of being assumed to be malignant). A subjective assessment of the ultrasound images was done by experts or by level 2/3 examiners as per the EFSUMB classification system.
-
Assuming equal test costs for all risk scores.
-
Assuming that no ultrasound is performed in conjunction with the ROMA and Overa (MIA2G) risk scores, thus reducing the costs of these risk scores.
-
Assuming additional costs for the FP group (surgery costs with malignancy instead of without) and additional costs for the FN group (additional costs of benign surgery).
-
Assuming additional costs for the FP group (surgery costs with malignancy instead of without) and additional costs for the FN group (additional costs of benign surgery and SMDT costs).
-
Assuming a discount of 92% for carboplatin (CG122:1 a discount in England of 91.8%; and a discount in Wales of 92.1%).
-
Assuming a discount of 95% for paclitaxel (CG122:1 a discount in England of 91.0%; and a discount in Wales of 95.4%).
-
Assuming an alternative HR for progression-free and overall survival for SMDT treatment versus no SMDT treatment (of 0.808). 146 This study was selected because it was not included in the Cochrane review that was used in the base case, and it provided an alternative HR (n = 275, n = 238 for this comparison).
-
Assuming an alternative HR for progression-free and overall survival for SMDT treatment versus no SMDT treatment (of 0.990; the upper bound of the CI used in the base case).
-
Assuming that the proportion of women receiving supportive care (for advanced-stage cancer) is 10% (instead of 5%).
-
Assuming an alternative TVS cost of £142.46 [(MA36Z) instead of £76.75 based on CG122]. 1
-
Assuming an alternative TVS cost of £142.46 [(MA36Z) instead of £76.75 based on CG122]1 and increasing the TVS cost for the IOTA groups’ risk scores by 20% (to reflect the potential training costs).
-
Assuming an additional SMDT cost of £2500 to reflect higher surgery costs, given that, according to expert opinion, 1 in 3 or 4 patients referred to a SMDT may receive extensive surgery for ovarian cancer (IPG 470), for which the price is unknown.
-
Assuming 90% of the non-malignancy surgery and complication costs for TN, which reflects a scenario wherein 90% of the TN group are operated on (instead of all).
-
Assuming Avastin for advanced-stage cancer. Assuming an additional treatment cost of £17,760 per treated woman147 and assuming a median survival rate of 39.7 months (95% CI 36.0 to 44.2 months), derived from a clinically predefined high-risk subgroup of the ICON7 trial. 148 This subgroup was used because an overall survival benefit was recorded in poor-prognosis patients, in contrast with the study population as a whole, providing further evidence towards the optimum use of bevacizumab in the treatment of ovarian cancer. 148
-
Assuming a disutility for the FP group during the first year in a state-transition model of 0.100.
-
Assuming a disutility for the FP group during the first year in a state-transition model of 0.010.
-
Using the optimal RMI 1 threshold (i.e. the RMI 1 threshold is cost-effective at £20,000 and/or £30,000 per QALY gained in the former scenario), based on a comparison of only different RMI 1 thresholds (see Appendix 7 for accuracy estimates).
Subgroup analyses
Various subgroup analyses were performed (if applicable, see Appendix 7 for accuracy estimates):
-
premenopausal women (mean age of 38 years, subgroup-specific accuracy data)
-
postmenopausal women (mean age of 68 years, subgroup-specific accuracy data)
-
using a baseline age of 50 years for the base case (instead of 40 years, no other changes)
-
early-stage disease only
-
advanced-stage disease only.
Results of the cost-effectiveness analyses
This section describes the results using probabilistic analyses for the base-case analysis. In addition, the sensitivity (deterministic), scenario (deterministic) and subgroup (probabilistic) analyses are described.
Base-case analysis
The base-case analysis included seven risk scores. Tables 27 and 28, as well as Figure 10, show the probabilistic results of this analysis. The RMI 1, with a threshold of 250, was the least effective (16.926 LYs, 13.820 QALYs) and the second cheapest (£5669). The IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant), was the cheapest (£5667) and the second most effective (16.954 LYs, 13.841 QALYs), thereby dominating the RMI 1 (at both the 200 and 250 thresholds). The IOTA group’s ADNEX model (at a threshold of 10%), costing £5699, was the most effective (16.957 LYs, 13.843 QALYs), and compared with the IOTA group’s simple ultrasound rules, resulted in an ICER of £15,304 per QALY gained. The remaining risk scores [ROMA score using Abbott Diagnostics’ ARCHITECT (at a threshold of 7.4%/25.3%); ROMA score using Roche Diagnostics’ Elecsys (at a threshold of 11.4%/29.9%); and Overa (MIA2G) from Vermillion (at a threshold of 5 units)] were dominated. As a result, the incremental analysis indicated that, up to thresholds of £15,304 per QALY gained, the IOTA group’s simple ultrasound rules are cost-effective, whereas the IOTA group’s ADNEX model (threshold of 10%) is cost-effective for higher thresholds.
| Risk score | LYs (95% CI) | Compared with the RMI 1 (at a threshold of 250) |
|---|---|---|
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 16.954 (16.651 to 17.247) | 0.029 |
| RMI 1 (at a threshold of 250) | 16.926 (16.619 to 17.223) | |
| RMI 1 (at a threshold of 200) | 16.928 (16.621 to 17.225) | 0.002 |
| IOTA group’s ADNEX model (at a threshold of 10%) | 16.957 (16.653 to 17.250) | 0.031 |
| ROMA score using Abbott Diagnostics’ ARCHITECT (at a threshold of 7.4%/25.3%) | 16.934 (16.627 to 17.229) | 0.008 |
| ROMA score using Roche Diagnostics’ Elecsys (at a threshold of 11.4%/29.9%) | 16.936 (16.631 to 17.231) | 0.011 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 16.950 (16.646 to 17.243) | 0.024 |
| Risk score | Costs, £ (95% CI) | QALYs (95% CI) | Compared with the RMI 1 (threshold of 250) | Full incremental analysis | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts/ΔQALYs | ΔCosts/ΔQALYs | |||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5667 (4551 to 6941) | 13.841 (13.477 to 14.154) | –2 | 0.021 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5669 (4553 to 6934) | 13.820 (13.456 to 14.134) | Dominated | |||
| RMI 1 (at a threshold of 200) | 5673 (4557 to 6939) | 13.821 (13.456 to 14.135) | 4 | 0.002 | £2483 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5699 (4585 to 6973) | 13.843 (13.480 to 14.155) | 30 | 0.023 | £1274 | £15,304 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5707 (4593 to 6976) | 13.825 (13.458 to 14.138) | 38 | 0.005 | £7506 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5713 (4597 to 6985) | 13.826 (13.461 to 14.141) | 44 | 0.007 | £6409 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5775 (4655 to 7049) | 13.837 (13.472 to 14.151) | 105 | 0.017 | £6038 | Dominated |
FIGURE 10.
Probabilistic results. CEF, cost-effectiveness frontier.
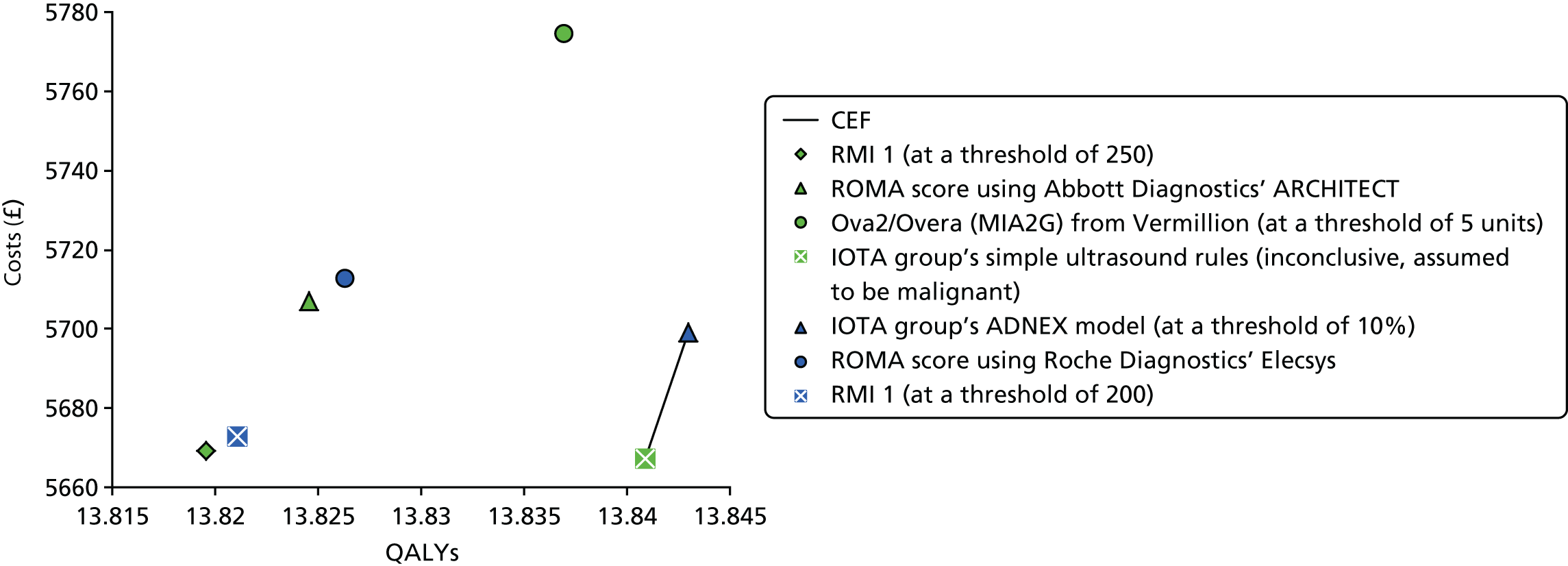
At willingness-to-pay thresholds of both £20,000 and £30,000 per QALY, the RMI 1 at a decision threshold of 250 had a probability of being cost-effective of 1%. For the IOTA group’s simple ultrasound rules and the IOTA group’s ADNEX model (threshold of 10%), this was 39% and 60%, respectively, at the £20,000 threshold, and 23% and 75%, respectively, at the £30,000 threshold. The probabilities for the other risk scores were < 1% for these thresholds (Figure 11).
FIGURE 11.
Cost-effectiveness acceptability curve for the base-case analysis.
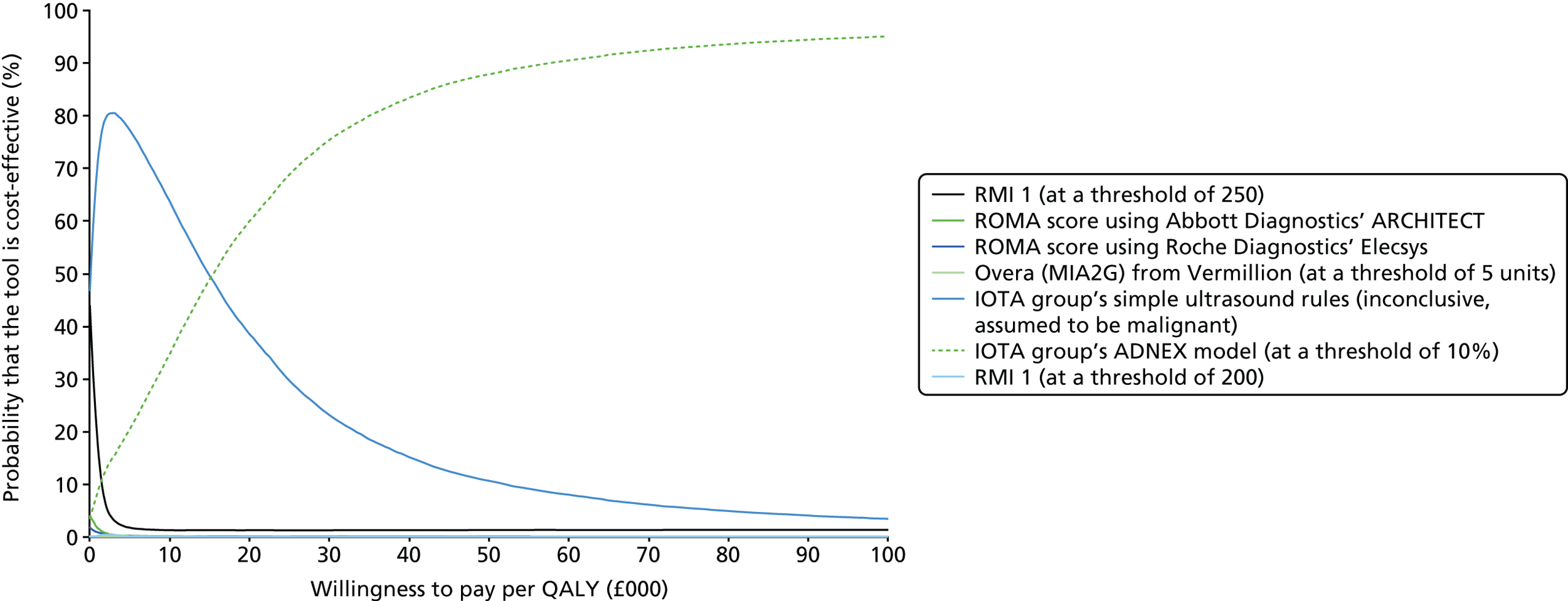
Sensitivity analyses
The deterministic one-way sensitivity analyses (conditional upon the base-case analysis) indicated that the following parameters were the most influential in regard to the impact on the ICER versus the RMI 1 at the 250 threshold:
-
progression-free and overall survival HRs for SMDT referral versus no SMDT referral
-
test costs
-
utility of AOC
-
specialist multidisciplinary team costs
-
test sensitivity
-
discount rate
-
test specificity.
When considering a threshold of £20,000 per QALY gained, the IOTA group’s ADNEX model (threshold of 10%) remained cost-effective, except in five sensitivity analyses wherein the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) became cost-effective:
-
upper bound (0.990) for the overall survival HR for SMDT referral versus no SMDT referral
-
upper bound (95.1%) for sensitivity for the IOTA group’s simple ultrasound rules
-
lower bound (95.3%) for sensitivity for the IOTA group’s ADNEX model
-
lower bound (£47) for the IOTA group’s simple ultrasound rules costs
-
upper bound (£142) for the IOTA group’s ADNEX model costs.
When considering a threshold of £30,000 per QALY gained, the IOTA group’s ADNEX model (threshold of 10%) remained cost-effective, except in two sensitivity analyses wherein the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) became cost-effective:
-
upper bound (0.990) for the overall survival HR for SMDT referral versus no SMDT referral
-
upper bound (£142) for the IOTA group’s ADNEX model costs.
These results are shown in the tornado diagrams in Appendix 8.
Scenario analyses
The scenario analyses included the same seven risk scores. The tabulated results are provided in Appendix 9. The scenario analyses indicated that at thresholds of £20,000 and £30,000 per QALY gained, the IOTA group’s ADNEX model (threshold of 10%) remained the most cost-effective strategy. This excludes the following scenarios:
-
assuming an equal proportion of early-stage ovarian cancer versus AOC in the FN and TP groups (in the base case it was assumed, based on expert opinion, that the FN group would have only early-stage ovarian cancer)
-
assuming 90% of the non-malignancy surgery and complications costs for the TN group, reflecting a scenario wherein 90% of the TN group are operated on (instead of all)
-
assuming a disutility for the FP group during the first year in the state-transition model of 0.010.
In these scenario analyses, the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) was cost-effective at a threshold of £20,000 per QALY gained, whereas again the IOTA group’s ADNEX model (threshold of 10%) was cost-effective at a threshold of £30,000 per QALY gained. Moreover, in the following scenario analyses, the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) became cost-effective at thresholds of £20,000 and £30,000 per QALY gained:
-
assuming an alternative HR for progression-free and overall survival for SMDT referral versus no SMDT referral (of 0.990; the upper bound of the CI used in the base-case analysis)
-
assuming a disutility for the FP group during the first year in the state-transition model of 0.100.
Finally, in the scenario with increased SMDT surgery costs, given that, according to expert opinion, 1 in 3 or 4 patients referred to a SMDT may receive extensive surgery for ovarian cancer149 for which the price is unknown, the RMI 1 (threshold of 250) was cost-effective at a threshold of £20,000 per QALY gained, whereas the IOTA group’s simple ultrasound rules were cost-effective at a threshold of £30,000 per QALY gained.
When comparing different RMI 1 thresholds only, it was found that the RMI 1 with a threshold of 25 would be cost-effective at all thresholds of £2890 per QALY gained or higher. However, when including this RMI 1 threshold with optimal sensitivity (instead of the RMI 1 with a threshold of 200) in the base-case analysis, the RMI 1 was still dominated.
Subgroup analysis
Premenopausal subgroup
The premenopausal subgroup analysis used a different mean starting age (38 years) and drew on subgroup-specific accuracy data (see Appendix 7). Tables 29 and 30, as well as Figure 12, show the probabilistic results of the subgroup analysis in premenopausal women. The ROMA score using Abbott Diagnostics’ ARCHITECT was the least effective (18.108 LYs, 14.927 QALYs) and the RMI 1 with a threshold of 200 was the cheapest (£5188), followed by the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) at £5189. The most effective options were the IOTA group’s ADNEX model (18.137 LYs, 14.948 QALYs) followed by the IOTA group’s simple ultrasound rules (18.135 LYs, 14.946 QALYs). Consequently, the incremental analysis indicated that between thresholds of £15 and £18,304 per QALY gained, the IOTA group’s simple ultrasound rules are cost-effective, whereas the IOTA group’s ADNEX model (threshold of 10%) is cost-effective for higher thresholds.
| Risk score | LYs (95% CI) | Compared with the RMI 1 (at a threshold of 250) |
|---|---|---|
| RMI 1 (at a threshold of 200) | 18.108 (17.435 to 18.720) | –0.006 |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 18.135 (17.470 to 18.740) | 0.021 |
| RMI 1 (at a threshold of 250) | ||
| ROMA score using Abbott Diagnostics’ ARCHITECT | 18.108 (17.434 to 18.720) | –0.006 |
| IOTA group’s ADNEX model (at a threshold of 10%) | 18.137 (17.472 to 18.741) | 0.024 |
| ROMA score using Roche Diagnostics’ Elecsys | 18.132 (17.461 to 18.737) | 0.018 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 18.131 (17.464 to 18.736) | 0.018 |
| Risk score | Costs, £ (95% CI) | QALYs (95% CI) | Compared with the RMI 1 (at a threshold of 250) | Full incremental analysis | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts/ΔQALYs | ΔCosts/ΔQALYs | |||
| RMI 1 (at a threshold of 200) | 5188 (4045 to 6510) | 14.927 (14.309 to 15.471) | –7 | –0.003 | £1954 | Cheapest |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5189 (4046 to 6515) | 14.946 (14.331 to 15.486) | –6 | 0.016 | Dominant | £15 |
| RMI 1 (at a threshold of 250) | 5195 (4051 to 6516) | 14.93 (14.311 to 15.473) | 0 | 0.000 | Dominated | |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5219 (4076 to 6542) | 14.927 (14.308 to 15.471) | 24 | –0.004 | Dominated | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5223 (4081 to 6549) | 14.948 (14.335 to 15.487) | 28 | 0.018 | £1564 | £18,466 |
| ROMA score using Roche Diagnostics’ Elecsys | 5235 (4090 to 6571) | 14.944 (14.329 to 15.484) | 40 | 0.013 | £2993 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5295 (4150 to 6617) | 14.943 (14.327 to 15.484) | 100 | 0.013 | £7748 | Dominated |
FIGURE 12.
Probabilistic results for the premenopausal subgroup. CEF, cost-effectiveness frontier.

At willingness-to-pay thresholds of both £20,000 and £30,000 per QALY gained, the RMI 1 (at a threshold of 250) had a probability of being cost-effective of < 1%. For the IOTA group’s ADNEX model (at a threshold of 10%), the IOTA group’s simple ultrasound rules and the ROMA score using Roche Diagnostics’ Elecsys, the probability of being cost-effective was 46%, 37% and 16%, respectively, (at the £20,000 threshold) and 52%, 27% and 19%, respectively (at the £30,000 threshold). The probabilities for the other risk scores were < 2% for these thresholds (Figure 13).
FIGURE 13.
Cost-effectiveness acceptability curve for the premenopausal subgroup analysis.

Postmenopausal subgroup
The postmenopausal subgroup analysis used a different mean starting age (68 years) and drew on subgroup-specific accuracy data (see Appendix 7). Tables 31 and 32, as well as Figure 14, show the probabilistic results of the subgroup analysis in postmenopausal women (mean age 68 years). The RMI 1, with a threshold of 250, was the least effective (8.031 LYs, 5.690 QALYs). The cheapest risk score was the IOTA group’s simple ultrasound rules (£7742), which was £1 cheaper than the RMI 1 with a threshold of 250. The most effective option was the IOTA group’s ADNEX model (8.076 LYs, 5.721 QALYs). The IOTA group’s simple ultrasound rules was the second most effective (8.072 LYs, 5.718 QALYs) and cost-effective up to a threshold of £12,876 per QALY gained; thereafter, the IOTA group’s ADNEX model was cost-effective. The other risk scores [the RMI 1 at a threshold of 200, ROMA score using Abbott Diagnostics’ ARCHITECT, ROMA score using Roche Diagnostics’ Elecsys and Overa (MIA2G) from Vermillion] were dominated.
| Risk score | LYs (95% CI) | Compared with the RMI 1 (at a threshold of 250) |
|---|---|---|
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 8.072 (7.623 to 8.505) | 0.042 |
| RMI 1 (at a threshold of 250) | 8.031 (7.57 to 8.472) | |
| RMI 1 (at a threshold of 200) | 8.052 (7.597 to 8.487) | 0.021 |
| IOTA group’s ADNEX model (at a threshold of 10%) | 8.076 (7.626 to 8.508) | 0.045 |
| ROMA score using Abbott Diagnostics’ ARCHITECT (at a threshold of 7.4%/25.3%) | 8.069 (7.618 to 8.502) | 0.038 |
| ROMA score using Roche Diagnostics’ Elecsys (at a threshold of 11.4%/29.9%) | 8.051 (7.597 to 8.487) | 0.020 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 8.068 (7.618 to 8.5) | 0.037 |
| Risk score | Costs, £ (95% CI) | QALYs (95% CI) | Compared with the RMI 1 (at a threshold of 250) | Full incremental analysis | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts/ΔQALYs | ΔCosts/ΔQALYs | |||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 7742 (6338 to 9281) | 5.718 (5.061 to 6.178) | –1 | 0.028 | Dominance | Cheapest |
| RMI 1 (at a threshold of 250) | 7743 (6334 to 9289) | 5.69 (5.035 to 6.153) | 0 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 7765 (6356 to 9309) | 5.703 (5.043 to 6.168) | 22 | 0.013 | £1746 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 7774 (6370 to 9318) | 5.721 (5.063 to 6.181) | 31 | 0.031 | £1013 | £12,876 |
| ROMA score using Abbott Diagnostics’ ARCHITECT (at a threshold 7.4%/25.3%) | 7788 (6381 to 9329) | 5.716 (5.059 to 6.177) | 45 | 0.026 | £1759 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys (at a threshold 11.4%/29.9%) | 7789 (6377 to 9334) | 5.702 (5.044 to 6.168) | 46 | 0.012 | £3738 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 7842 (6429 to 9396) | 5.715 (5.058 to 6.176) | 99 | 0.025 | £3992 | Dominated |
FIGURE 14.
Probabilistic results for the postmenopausal subgroup (mean age 68 years). CEF, cost-effectiveness frontier.

At willingness-to-pay thresholds of both £20,000 and £30,000 per QALY gained, the RMI 1 at the threshold of 250 had a probability of being cost-effective of < 2%. For the IOTA group’s simple ultrasound rules and the IOTA group’s ADNEX model (at a threshold of 10%), the probability of being cost-effective was 40% and 59%, respectively, at the £20,000 threshold, and 24% and 74%, respectively, at the £30,000 threshold. The probabilities for the other risk scores were < 1% for these thresholds (Figure 15).
FIGURE 15.
Cost-effectiveness acceptability curve for the postmenopausal subgroup analysis (mean age 68 years).

Additional subgroup analyses
The results for the subgroup analyses, for which only the mean age was changed, to a mean age of 50 years (instead of 40 years as in the base case), and consisting of early-stage ovarian cancer only, were similar to the base-case results; at the thresholds of £20,000 and £30,000 per QALY gained, the IOTA group’s ADNEX model (threshold of 10%) remained the most cost-effective strategy. In contrast, for the subgroup consisting of AOC only, the IOTA group’s simple ultrasound rules were cost-effective at the thresholds of £20,000 and £30,000 per QALY gained. The tabulated results are provided in Appendix 10.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
All of the studies included in our systematic review were diagnostic cohort studies that reported data on the diagnostic accuracy of one or more ovarian cancer risk scores [the ROMA score, the IOTA group’s simple ultrasound rules, the ADNEX model or Overa (MIA2G)] or provided data on the accuracy of the RMI 1 at different decision thresholds (including a threshold of 250, as specified in the current NICE guidelines1). With the exception of Overa (MIA2G), studies were identified that provided direct comparisons of the performance of each included risk score versus the RMI 1 (at a threshold of 200), that is, the performance of the intervention risk score and the performance of the RMI 1 (at a threshold of 200) were assessed in the same patient cohort. No study reported data to allow a direct comparison of all included index tests (risk scores) with each other and the RMI 1 in the same patient cohort. No RCTs or CCTs were identified, and no studies provided data on patient-relevant outcomes following different risk assessment strategies.
Studies evaluating the ROMA score used either the Roche Diagnostics Elecsys or the Abbott Diagnostics ARCHITECT tumour marker assays. None of the included studies used the Fujirebio Diagnostics LUMIPULSE G automated CEIA system. Two studies94,98 that used a ROMA score based on the manual Fujirebio Diagnostics tumour marker EIAs (see Appendix 5, Tables 41 and 42) were included. These studies are included for information only and it should be noted that the manual assays are not specified interventions for this assessment.
The target condition for this assessment is ovarian cancer, defined as those conditions covered by NICE clinical guideline CG122,1 namely epithelial ovarian cancer, fallopian tube carcinoma, primary peritoneal carcinoma and borderline ovarian cancer; excluded conditions are pseudomyxoma peritonei, relapsed cancers, germ cell tumours of the ovary and sex cord–stromal tumours of the ovary. All studies in our systematic review included women with one or more adnexal or pelvic masses. The definition of ovarian cancer varied between studies and did not always include borderline tumours. In addition, the definition of disease/reference standard positive could include all malignancies or only ovarian malignancies. Although studies that report the performance of risk scores for the specific target condition of ovarian cancer (as described in CG122)1 could be considered the most applicable to the scope of this assessment (and, accordingly, have been rated as giving rise to ‘low concerns regarding applicability’ in our QUADAS-2 assessments), it should be noted that the calculation of accuracy estimates for ovarian cancer or epithelial ovarian cancer requires the post hoc exclusion of women with other histological diagnoses from the analysis. In practice, such patients form part of the population in whom risk-scoring would be applied and, hence, their exclusion from the analyses may result in estimates of test performance that cannot be achieved in real-world clinical settings.
The majority of the studies that assessed the performance of the ROMA score used the Abbott Diagnostics ARCHITECT tumour marker assays. The summary sensitivity estimate for the ROMA score (using the manufacturer’s recommended cut-off values of 7.4% in premenopausal women and 25.3% in postmenopausal women) was highest (96.4%, 95% CI 93.6% to 98.2%) when analyses excluded women with borderline tumours and those with malignancies other than epithelial ovarian cancer. It was lowest (75.0%, 95% CI 60.4% to 86.4%) when all women were included in the analysis, regardless of their final histopathological diagnosis and if different cut-off values (13.1% and 27.7%) were used; non-exclusion is more likely to reflect the performance of the score in a clinical setting. The study that included all women in the analysis reported similar sensitivity and specificity estimates for the ROMA score and the RMI 1 [(at a threshold of 200) 75%, 95% CI 60.4% to 86.4% vs. 77.1%, 95% CI 62.7% to 88.0%; and 87.9%, 95% CI 81.9% to 92.4% vs. 81.8%, 95% CI 75.1% to 87.4%, respectively]. 103 By contrast, when women with borderline tumours and/or those with malignancies other than epithelial ovarian cancer were excluded from the analyses, the summary specificity estimate for the ROMA score (53.3%, 95% CI 50.0% to 56.7%) was significantly lower than that for the RMI 1 [(at a threshold of 200) 80.3%, 95% CI 77.5% to 82.9%]. The only study to report a direct comparison of the ROMA score using Roche Diagnostics’ Elecsys tumour marker assays (with the manufacturer’s recommended thresholds of 11.4% in premenopausal women and 29.9% in postmenopausal women) with the RMI 1 included all study participants in the analysis irrespective of final histological diagnosis, but classified women with borderline tumours as being disease negative. In this study, the sensitivity estimate for the ROMA score appeared to be slightly higher than that for the RMI 1 (83.8%, 95% CI 73.4% to 91.3% vs. 78.4%, 95% CI 67.3% to 87.1%), and the specificity estimate for the ROMA score appeared to be slightly lower than that for the RMI 1 (68.8%, 95% CI 61.6% to 75.4% vs. 79.6%, 95% CI 73.1% to 85.1%), but neither difference was statistically significant. 89 This study may be considered to be more applicable to clinical practice if it is considered preferable to manage women with borderline tumours in non-specialist settings. The summary estimates of sensitivity and specificity for the ROMA score, using Roche Diagnostics’ Elecsys tumour marker assays at the manufacturer’s recommended thresholds, derived from non-comparative accuracy studies in which all women were included in the analysis (target condition: all malignancy) were 79.1% (95% CI 74.2% to 83.5%) and 79.1% (95% CI 76.3% to 81.6%), respectively. In studies in which the manufacturers’ recommended cut-off values were used, the performance of the ROMA score did not differ significantly between premenopausal women and postmenopausal women.
When considering the risk-scoring methods produced by the IOTA group, our report focuses on data for the ADNEX model for which the validated 10% threshold is used and on data for the IOTA group’s simple ultrasound rules, for which all study participants have an index test-based classification (either by assuming that inconclusive classifications are malignant or by applying subjective judgement to inconclusive assessments). Accuracy data for studies in which women with an inconclusive IOTA group’s simple ultrasound rules assessment were not classified (excluded from the analyses) are reported in Appendix 5, Table 39. However, these results are considered to be of limited clinical value, as it is unclear which alternative methods might be used to select the most appropriate care pathway for these women. The majority of these studies included all participants in the analyses, irrespective of final histological diagnosis (i.e. the target condition was all malignant tumours including borderline). The summary estimates of sensitivity were high for both the ADNEX model (96.3%, 95% CI 95.3% to 97.1%) and the IOTA group’s simple ultrasound rules for which inconclusive results were assumed to be malignant (94.2%, 95% CI 93.3% to 95.1%); when subjective assessment was applied to inconclusive and IOTA group’s simple ultrasound rules results, the summary sensitivity estimate was significantly lower (88.4%, 95% CI 86.9% to 89.8%). Conversely, the summary estimates of specificity were low for both the ADNEX model (69.1%, 95% CI 67.4% to 70.8%) and the IOTA group’s simple ultrasound rules, for which inconclusive results were assumed to be malignant (76.1%, 95% CI 74.9% to 77.3%), and significantly higher (92.5%, 95% CI 91.6% to 93.4%) when subjective assessment was applied to inconclusive results and the IOTA group’s simple ultrasound rules results. Menopausal status did not significantly affect the performance of either the ADNEX model or the IOTA group’s simple ultrasound rules, but the specificity estimate was significantly higher in premenopausal women than in postmenopausal women for both instruments. One published study44 and one unpublished interim report (Frances Nixon, personal communication) provided comparative accuracy data for the ADNEX model, the IOTA group’s simple ultrasound rules, for which inconclusive results were assumed to be malignant, and the RMI 1, using a decision threshold of 200. The summary estimates of sensitivity derived from these two studies were slightly higher for the ADNEX model (96%, 95% CI 94.5% to 97.1%) than for the IOTA group’s simple ultrasound rules (92.8%, 95% CI 90.9% to 94.3%). Likewise, the summary estimates of specificity were similar (67%, 95% CI 64.2% to 69.6% and 71.6%, 95% CI 68.9% to 74.1%) for the ADNEX model and the IOTA group’s simple ultrasound rules, respectively. The summary estimate of sensitivity for the RMI 1 at a decision threshold of 200 (66%, 95% CI 62.9% to 69%) was significantly lower than both the ADNEX model and IOTA group’s simple ultrasound rules estimates. Conversely, the specificity estimate for the RMI 1 at a decision threshold of 200 was significantly higher (89%, 95% CI 87% to 90.7%) than both the ADNEX model and the IOTA group’s simple ultrasound rules estimates.
No studies were identified that directly compared Overa (MIA2G) with the RMI 1 at either decision threshold (200 or 250). One study104 reported comparative accuracy data for Overa (MIA2G) versus the ROMA score, using the Roche Diagnostics Elecsys tumour marker assays. This study included all participants in the analysis, regardless of their final histopathological diagnosis (target condition: all malignancies including borderline). At a threshold of 5 units, the sensitivity estimate for Overa (MIA2G) was 91% (95% CI 86.8% to 94%) and the specificity estimate was 65.5% (95% CI 62.0% to 68.8%). The sensitivity of the Overa (MIA2G) score was significantly higher than that of the ROMA score (79.2%, 95% CI 73.7% to 83.8%), whereas the specificity of the Overa (MIA2G) score was significantly lower than that of the ROMA score (78.9%, 95% CI 75.8% to 81.7%).
Summary estimates derived from studies that compared the diagnostic performance of different RMI 1 decision thresholds (between 25 and 500) and included all study participants in the analyses, regardless of final histopathological diagnosis (target condition: all malignant tumours including borderline), indicated that sensitivity and specificity estimates did not differ significantly between the two decision thresholds (200 and 250). At the decision threshold of 200, the sensitivity estimate was 70.8% (95% CI 65.2% to 75.6%) and the specificity estimate was 91.2% (95% CI 88.9% to 93.1%). At the decision threshold of 250, the sensitivity estimate was 69.0% (95% CI 63.7% to 73.9%) and the specificity estimate was 91.6% (95% CI 89.3% to 93.5%). The summary estimates of sensitivity for the RMI 1, derived from studies included in our systematic review, were lower than those reported in a recent systematic review150 [75%, 95% CI 72% to 74% (based on 14 studies)]; however, the difference was not statistically significant and the specificity estimate was similar (92%, 95% CI 88% to 94%). It should be noted that this systematic review150 included studies of women undergoing surgery for an adnexal mass and excluded any studies that selectively excluded some histopathological subtypes of ovarian cancer or classified borderline tumours as benign. As would be expected, the sensitivity estimate for the RMI 1 increased and the specificity decreased with decreasing threshold.
For both the IOTA group’s simple ultrasound rules and the ADNEX model, there was evidence that specificity can be significantly decreased in postmenopausal women in comparison with overall populations or premenopausal women. Neither of these risk scores incorporates menopausal status; preliminary evidence suggests that menopausal status should be taken into account when applying these tools in practice.
The base case for the cost-effectiveness analysis considers the target condition ‘all malignant tumours including borderline’. This is because the scope and protocol for this assessment specified that the definition of ovarian cancer should include borderline tumours. In addition, as previously outlined, the population in which risk-scoring would be applied in practice is likely to include some women who will ultimately be found to have a non-ovarian primary and some who will have cancers that fall outside the definition of ovarian cancer as used in CG1221 (e.g. germ cell tumours and sex cord–stromal tumours of the ovary); therefore, it was considered that studies that include all participants in their analysis, irrespective of the final histological diagnosis, are more likely to produce estimates of risk-score performance that are representative of what might be expected in clinical practice. For all index tests (risk scores), there were no significant differences between the summary performance estimates calculated from all available data and those that included only those studies reporting a direct comparison with the RMI 1 (see Chapter 3, Diagnostic performance of the Risk of Ovarian Malignancy Algorithm score, Diagnostic performance of International Ovarian Tumour Analysis group’s simple ultrasound rules and the Assessment of Different NEoplasias in the adneXa model and Diagnostic performance of Overa (multivariate index assay, second generation). Therefore, the cost-effectiveness modelling used summary estimates of the diagnostic performance of risk scores, calculated using all available data sets for a given target condition. The ROMA score is considered to be a separate intervention for each tumour marker manufacturer (Roche Diagnostics’ Elecsys and Abbott Diagnostics’ ARCHITECT; none of the included studies used the Fujirebio Diagnostics LUMIPULSE G automated CEIA system and, therefore, the ROMA score using this assay option is not included in the cost-effectiveness analysis). Estimates of the diagnostic performance of the comparator, the RMI 1 with a decision threshold of 250, were derived from a meta-analysis of all available RMI 1 data sets with the corresponding target condition (e.g. all malignant tumours including borderline or all ovarian tumours including borderline) and population (e.g. all participants, premenopausal women or postmenopausal women). When no data were available for the RMI 1 with a decision threshold of 250, data for a decision threshold of 200 were used; the analysis reported in Chapter 3, Diagnostic performance of the Risk of Malignancy Index 1 using decision thresholds other than 250 indicated no significant difference in the performance of the RMI 1 at these two thresholds.
Cost-effectiveness
The review of economic analyses examined studies reporting outcomes of a full cost-effectiveness analysis, examining QALYs, with at least one of the comparators. In total, five studies were included, of which three studies reported QALYs as an outcome. Of these studies, one considered screening, whereas the remaining two considered secondary care from the UK and US perspectives. The UK study indicated that MMS consisting of CA125 testing followed by TVS could be cost-effective compared with USS and no screening. The two studies considering MIA, both from the US perspective, provided conflicting results: one study indicated that MIA might be cost-effective, whereas the other indicated that it was dominated by other strategies (when considering LYs). This latter study was the only one considering the ROMA score and also indicated that this score would be dominated by other strategies (when considering LYs). Moreover, this study indicated that a ‘refer all’ approach is cost-effective for thresholds above US$10,644 per LY gained. In conclusion, there is limited and conflicting evidence regarding the cost-effectiveness of alternative risk scores, which include HE4 testing, CA125 testing and ultrasound, compared with the RMI 1 score with a referral threshold of ≥ 250 (current UK practice) for people with suspected ovarian cancer in secondary care.
In our health economic analysis, the cost-effectiveness of different risk scores, which include HE4 testing, CA125 testing and ultrasound, compared with the RMI 1 score, as used in current practice for patients with suspected ovarian cancer in secondary care, was assessed to guide decisions about referral to a SMDT. The base-case analysis included seven risk scores:
-
RMI 1 (at a threshold of 250)
-
ROMA score using Abbott Diagnostics’ ARCHITECT
-
ROMA using Roche Diagnostics’ Elecsys
-
Overa (MIA2G) from Vermillion (at a threshold of 5 units)
-
IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant)
-
IOTA group’s ADNEX model (at a threshold of 10%)
-
RMI 1 (at a threshold of 200).
In the base-case analysis, the RMI 1 with a threshold of 250 was the least effective (16.926 LYs, 13.820 QALYs) and the second cheapest (£5669). The IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) was the cheapest (£5667) and the second most effective (16.954 LYs, 13.841 QALYs), and thereby dominated the RMI 1 (at both the 200 and 250 thresholds). The IOTA group’s ADNEX model (threshold of 10%, cost of £5699) was the most effective (16.957 LYs, 13.843 QALYs), and compared with the IOTA group’s simple ultrasound rules, resulted in an ICER of £15,304 per QALY gained. The remaining risk scores [ROMA score using Abbott Diagnostics’ ARCHITECT, ROMA score using Roche Diagnostics’ Elecsys and Overa (MIA2G) from Vermillion] were dominated. As a result, the incremental analysis indicated that, up to thresholds of £15,304 per QALY gained, the IOTA group’s simple ultrasound rules are cost-effective, whereas the IOTA group’s ADNEX model (threshold of 10%) is cost-effective for higher thresholds. Consequently, at willingness-to-pay thresholds of both £20,000 and £30,000 per QALY, the RMI 1 at a threshold of 250 had a probability of being cost-effective of 1%. For the IOTA group’s simple ultrasound rules and IOTA group’s ADNEX model (threshold of 10%), this was 39% and 60%, respectively, at the £20,000 threshold, and 23% and 75%, respectively, at the £30,000 threshold. The probabilities for the other risk scores were < 1% for these thresholds.
The sensitivity and scenario analyses indicated that the HR for SMDT referral versus no SMDT referral (for women with ovarian cancer) was the most influential parameter in the model, and the results are reasonably robust. Most scenario analyses indicated that, at thresholds of £20,000 and £30,000 per QALY gained, the IOTA group’s ADNEX model (threshold of 10%) remained the cost-effective strategy. In two scenario analyses, the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) were considered to be cost-effective at a threshold of £20,000 and/or £30,000 per QALY gained. For the scenario comparing the optimal sensitivity RMI 1 threshold, which was found to be 25 (at all thresholds of £2890 per QALY gained or higher), the RMI 1 was still dominated.
For the premenopausal and postmenopausal subgroups, the IOTA group’s ADNEX model (threshold of 10%) was cost-effective at thresholds of £20,000 and £30,000 per QALY gained.
Strengths and limitations of the assessment
Clinical effectiveness
We are not aware of any previous systematic review that has considered the performance of both ultrasound-based risk scores, such as the IOTA group’s Simple Rules, and biomarker-based scores, such as the ROMA score and Overa (MIA2G). The most recent systematic review151 of the ROMA score completed searching in November 2014. In addition, previous systematic reviews9,151,152 of the ROMA score have focused on predicting ovarian cancer (no definition reported) or epithelial ovarian cancer and have combined data from studies using different manufacturers’ tumour marker assays and thresholds, and have not clearly described how study participants with borderline tumours and those with non-ovarian primaries were classified. A more recent systematic review150 (searches completed in July 2015) is available for ultrasound-based risk scores, but previous systematic reviews150,153 have tended to focus on comparing these scores with subjective ultrasound evaluation rather than with other types of risk-scoring. Risk-scoring for ovarian malignancy is a rapidly evolving field and it is believed that the full update, comparing all options currently available to the NHS, provided by this assessment, will be of value to clinicians and decision-makers. In addition, there is currently a large, ongoing Cochrane review, entitled ‘Symptoms, ultrasound imaging and biochemical markers alone or in combination for the diagnosis of ovarian cancer in women with symptoms suspicious of ovarian cancer’154 that will provide data on testing options that lie outside the scope of this assessment.
Extensive literature searches were conducted in an attempt to maximise the retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,30 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, relatively few of which met the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment (e.g. a significant difference between the treatment and control groups that favours treatment). This is not the case for test accuracy studies, which measure agreement between the index test and the reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often, but the relative priorities given to sensitivity and specificity estimates may vary depending upon the intended application of the test. In addition, test accuracy data are often collected as part of routine clinical practice or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear to be unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear, but simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 155 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic, and reliability is limited. 29 A statistical assessment of publication bias in this review was not undertaken. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts and an unpublished interim report (Frances Nixon, personal communication).
Despite our extensive searches, no studies were identified that assessed the diagnostic performance of the ROMA score using the Fujirebio Diagnostics’ LUMIPULSE G automated CEIA system. It is not considered appropriate to treat ROMA scores calculated using different manufacturers’ tumour marker assays as equivalent technologies, as each uses different thresholds and is CE marked for use with the specified tumour marker assays. Furthermore, no studies that reported a direct comparison of the diagnostic performance of the ROMA score using different manufacturers’ tumour marker assays in the same patient cohort were identified.
No studies were identified that directly compared the performance of the Overa (MIA2G) score with that of the RMI 1 score; the data included in the systematic review component of this assessment refer only to the performance of the Overa (MIA2G) score compared with that of the ROMA score (using Roche Diagnostics’ Elecsys tumour marker assays) and not to its performance in relation to the specified comparator, the RMI 1 score.
Clear inclusion criteria were specified in the protocol for this review, a copy of which is available online (www.nice.org.uk/guidance/GID-DG10012/documents/final-protocol, accessed 5 July 2018). The eligibility of studies for inclusion is therefore transparent. In addition, specific reasons for exclusion have been provided for all of the studies that were considered to be potentially relevant at the initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 2). The review process followed recommended methods to minimise the potential for error and/or bias;27 studies were independently screened for inclusion by two reviewers and data extraction and quality assessment were done by one reviewer and checked by a second reviewer (MW, SD or SL). Any disagreements were resolved by consensus.
All studies included in this review were assessed for risk of bias and applicability using the QUADAS-2 tool,36 which is recommended by the Cochrane Collaboration. 29 The QUADAS-2 tool is structured into four key domains, covering participant selection, index test, reference standard and the flow of patients through the study (including the timing of tests). Each domain is rated for risk of bias (low, high or unclear); the participant selection, index test and reference standard domain are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). The results of the QUADAS-2 assessment are reported, in full, for all included studies in Appendix 3 and are summarised in Chapter 3, Study quality. Those studies that reported the development of risk scores, in addition to test accuracy data, were also assessed using the PROBAST. 37 The PROBAST has been designed to assess both the risk of bias and concerns regarding applicability of a study that evaluates (develops and/or validates) a multivariable diagnostic or prognostic prediction model. It has a domain-based structure, similar to that of QUADAS-2, and is intended to be used for the assessment of primary studies included in a systematic review. The PROBAST is not yet published but has been used with the consent of the steering group, of which the lead author of this assessment report is a member.
The studies included in our systematic review used a variety of definitions of disease/reference standard positive. In order to facilitate clinically relevant comparisons, it was decided to group studies according to whether or not they included borderline tumours in their definition of malignancy and whether women found to have non-ovarian malignancies were included in the analyses or excluded post hoc. However, a detailed breakdown of histopathological diagnoses was not always reported (see Appendix 4, Table 36), and, hence, the within-group variation in the distribution of diagnoses cannot be fully quantified.
There remains a further question regarding the clinical applicability of the studies included in this assessment. All study participants underwent surgery (i.e. a histological confirmation of disease status was available). In practice, risk scores may be used in secondary care to triage women to surgery or surveillance/conservative management, as well as to guide decisions about when surgery should be undertaken (referral to a specialist gynaecological oncology unit). This potential mismatch between the study populations and real-world clinical practice is reflected in the relatively high estimate for the prevalence of malignancy (21.3%) derived from the studies included in our systematic review. It should be noted that a lower prevalence of malignancy may also affect risk score performance in practice.
Approximately half of the included published studies17,42–46,48–50,52,58,60,62–64,66,76,78–81,83,86,97,98 (25/49) were conducted in Europe, but only six studies45,60,62,66,78,79 were conducted solely in the UK, and a further two were multinational studies17,42 that included a UK centre. There were no studies of the ROMA score or Overa (MIA2G) that included UK participants. The data included in this report may therefore have limited applicability to UK settings, particularly in relation to the performance estimates for the ROMA score and Overa (MIA2G).
Although the sample sizes of studies included in our systematic review were generally large for diagnostic accuracy studies (median n = 277, range 48–2445), it should be noted that the largest data sets were derived from the various phases of the IOTA study, and these tended to dominate the analyses for the ADNEX model and the IOTA group’s simple ultrasound rules. Only one report per intervention (IOTA group’s simple ultrasound rules of the ADNEX model) was included for each phase of the IOTA study.
Cost-effectiveness
Our cost-effectiveness analysis is the most comprehensive to date in terms of the costs and consequences considered, as well as the number of relevant risk scores considered. Moreover, the de novo probabilistic model was based on a previously published model for CG122. 1 For the present analysis, a number of adjustments were made to the model, mostly to update cost estimates, and most of the assumptions were maintained.
The model was also informed by a comprehensive, high-quality systematic review of diagnostic test accuracy. Additional parameters were either those from the original CG122 model1 or any of the further assessments, or, when necessary, were based on a focused literature review, prioritising the key input parameters (e.g. the HR for SMDT referral vs. no SMDT referral). Such a review is standard practice in economic modelling, given the large number of parameters required.
As in any economic model, a number of major and minor assumptions had to be made (see Chapter 4, Model parameters). It is important to understand the impact of these assumptions in order to correctly interpret the results of the model. The impact of most assumptions has been explored in sensitivity and scenario analyses. These analyses underscored the robustness of the base-case results.
Uncertainties
Clinical effectiveness
There remain a number of areas of uncertainty in relation to the performance characteristics of risk scores for ovarian cancer in specific subgroups of women; no study reported data on the effects of other risk factors, such as family history of ovarian cancer, on the performance of any risk for ovarian malignancy.
There is uncertainty about the downstream consequences of using the various risk-scoring options available to select the most appropriate care pathway for women with an adnexal mass (management by a general gynaecologist or referral to a SMDT). The limited data available for the ROMA score do not suggest any substantial performance advantage over current practice (the RMI 1), particularly when the more inclusive definition of malignancy is used (target condition: all malignant tumours including borderline). Consideration of the data from studies that reported accuracy estimates for both the whole-study population (target condition: all malignant tumours including borderline) and for selected populations in which participants found to have borderline tumours and/or those with rare ovarian cancers or non-ovarian primaries were excluded, indicates that women with borderline tumours and those with rare ovarian cancers or non-ovarian primaries may be disproportionately represented among those with FN, low-risk ROMA scores. One comparative accuracy ROMA score study,99 using Abbott Diagnostics’ ARCHITECT tumour marker assays, reported test performance estimates for the target condition epithelial ovarian cancer, calculated both with and without the inclusion of participants with borderline tumours; these data indicated that around half of the FN risk scores were accounted for by women with borderline tumours, 3 out of 6 (50%) using the ROMA score and 7 out of 13 (54%) using the RMI 1. 99 Similarly, a comparative accuracy ROMA score study,89 using Roche Diagnostics’ Elecsys tumour marker assays, reported test performance estimates for the whole-study population and for a selected population in which eight (3%) women with non-epithelial ovarian cancer and non-ovarian primaries were excluded from the analysis; women with malignancies other than epithelial ovarian cancer accounted for four (50%) of the FN results using the ROMA score and three (37.5%) of the FN results using the RMI 1 score. The potential to detect non-epithelial ovarian cancers by including other tests (e.g. to measure AFP and beta-hCG, as recommended in CG1221 for women aged < 40 years with suspected ovarian cancer) in the standard work-up is unclear and was outside the scope of this assessment.
One further non-comparative ROMA score study,97 using Roche Diagnostics’ Elecsys tumour marker assays, reported test performance estimates calculated both with and without the inclusion of participants with borderline tumours and those with non-ovarian primaries; these data indicated that women with borderline tumours and those with non-ovarian primaries accounted for a high proportion, 12 out of 14 (86%), of the FN risk scores observed. 97 It should be noted that these observations are based on small numbers of women. Furthermore, although other risk scores [Overa (MIA2G), the IOTA group’s simple ultrasound rules and the ADNEX model] appear to offer increased sensitivity, data were not available to explore the distribution of histological diagnoses among those women with FN low-risk classifications. The downstream consequences of a FN low-risk classification are likely to differ between women with different histological cancer types and between those with borderline tumours and higher-stage malignancies. A more complete exploration of the types of women who are likely to be misclassified as being at a low risk of developing ovarian cancer, using the various risk-scoring options available, as well as an investigation of the downstream clinical consequences for these patients, is therefore needed.
The results of comparative accuracy studies, as noted in Statement of principal findings, Clinical effectiveness, indicate that both the ADNEX model and the IOTA group’s simple ultrasound rules (for which inconclusive results are assumed to be malignant) offer substantial increases in sensitivity for the prediction of malignancy relative to the RMI 1 at a decision threshold of 200 or 250. The introduction of these scores into routine practice would therefore be likely to reduce the numbers of women with malignancy who are falsely classified as being at low risk of developing ovarian cancer. However, this increased sensitivity is accompanied by a decrease in specificity, and, hence, an increase in the numbers of women with benign disease who would be unnecessarily referred to a specialist gynaecological oncology MDT, relative to the numbers associated with risk-scoring using the RMI 1. This trade-off can be illustrated using a hypothetical cohort of 1000 patients: assuming an overall prevalence of malignancy of 21.3% (the estimate used for the base case in our cost-effectiveness analysis), the numbers of women with malignancy who would not be referred to a SMDT would be 18, 33 and 154, based on the ADNEX model, the IOTA group’s simple ultrasound rules and the RMI 1, respectively, and, conversely, the corresponding numbers of ‘unnecessary’ referrals of women with benign disease would be 181, 155 and 60, respectively. To achieve a similar level of sensitivity to that of the ADNEX model or the IOTA group’s simple ultrasound rules, using the RMI 1 would require a very low decision threshold; for the same sample cohort of 1000 women, a RMI 1 threshold of 25 would result in 16 women with malignancy who would not be referred to a SMDT and 335 ‘unnecessary’ referrals of women with benign disease.
It should also be noted that the performance of risk-scoring tools that include morphological features seen on ultrasound is likely to be affected by the level of skill and experience of the ultrasonographers. This is particularly the case when the method of applying the score includes an unspecified element of subjective judgement (e.g. the IOTA group’s simple ultrasound rules with expert subjective assessment for inconclusive results). The effect of the ultrasonographer’s experience on measures of test performance was considered in our systematic review, but very few data were found to inform this question. The majority of the studies of the ADNEX model and the IOTA group’s simple ultrasound rules were derived from the IOTA cohort and tended to use experienced ultrasound examiners and/or provide tool-specific pre-study training. One study49 explicitly assessed the effect of the training level of examiners on the diagnostic performance of the IOTA group’s simple ultrasound rules and found no significant differences in test performance between EFSUMB level 2/3 examiners and EFSUMB level 1 examiners, but it should be noted that the information value of this study is limited, as all examiners received one half-day of practical training in the IOTA group’s simple ultrasound rules before the study. Perhaps more interestingly, two of the studies52,62 evaluating the IOTA group’s simple ultrasound rules explicitly reported using ultrasound operators with lower levels of experience: ‘63% of operators had performed fewer than 1000 scans, 24% were medical doctors and 76% were ultrasonographers’;62 ‘Ultrasound examinations were performed by a fourth year trainee and junior staff in obstetrics and gynaecology who had less than one year of ultrasound experience, under the supervision of an expert examiner’. 52 Test performance estimates from both of these studies were similar to the overall summary estimates (see Table 12 and Chapter 3, Diagnostic performance of the Risk of Ovarian Malignancy Algorithm score), providing some indication that the IOTA group’s simple ultrasound rules may remain effective in the hands of less experienced operators. A more complete assessment of the levels of training and experience needed to achieve the required levels of test performance would inform implementation considerations (e.g. training requirements for secondary care ultrasonographers, increases in specialist cancer centre workload arising from the use of triage methods with higher sensitivity and the introduction of routine TVS in secondary care assessment).
Risk-scoring tools that are based solely on ultrasound also carry the inherent limitation that they cannot be used to assess women who are symptomatic but do not have a mass that is large enough to be detected as abnormal on an ultrasound scan; none of the studies identified by this systematic review included women in this group.
The ideal method of comparing the downstream resource use and clinical consequences of using the various risk-scoring options available would be a RCT comparing treatment pathways and subsequent clinical outcomes following risk-scoring by different methods. No randomised or non-randomised controlled trials that met the inclusion criteria for our systematic review were identified. A recently published RCT,156 conducted in asymptomatic postmenopausal women with an incidentally detected adnexal mass on ultrasound, compared two risk assessment protocols based on the RMI 1 and on the IOTA group’s simple ultrasound rules. This study found that more of the women who were assessed using the RMI 1 protocol than those assessed using the IOTA group’s simple ultrasound rules protocol had surgery [18/68 (28.1%) vs. 7/68 (10.3%), with a relative risk of 2.57 (95% CI 1.15 to 5.76)]; there were no significant differences in rates of referral to a tertiary oncology unit or in delayed cancer diagnoses at 12 months. These findings are unlikely to be applicable to the population of interest in this assessment, as the prevalence of malignancy was much lower (2.7%) in this study population than in women referred to secondary care for the investigation of an adnexal mass, as seen in the studies included in our systematic review (median 29.9%, range 15–48.4%). The question of how different risk-scoring strategies affect referral rates and subsequent clinical outcomes in this population remains outstanding.
Cost-effectiveness
The economic analyses emphasise the importance of prioritising the sensitivity of the risk scores over the specificity. The benefits of referring as many women as possible or, if possible, all women with ovarian cancer to the SMDT outweigh the additional SMDT costs, even the additional SMDT costs related to unnecessary referrals (i.e. for FPs). More specifically, informal analyses using 100% sensitivity and 0% specificity indicate that a ‘refer all to SMDT’ strategy without risk scores might be cost-effective at the thresholds of £20,000 and £30,000 per QALY gained. It is, however, questionable as to whether or not such a strategy would be feasible for clinical practice, considering, among other factors, the potentially limited SMDT capacity. This is particularly because clinician opinion indicated that the real impact of FPs is probably not the additional cost but the time/resources taken away from TP women. This is an area of uncertainty, mainly because limited capacity is currently not considered in the economic model. Another logistic aspect that was not considered was a potential difference in time taken from entering the secondary care pathway to a confirmed diagnosis. Adding this might, for instance, favour the IOTA group’s simple ultrasound rules (given that these are ultrasound only) and/or the ROMA score (if an ultrasound scan has already been done in primary care). However, no evidence was found to inform this potential difference in time between the strategies or to inform any possible consequences (e.g. utility increment associated with earlier diagnosis).
Other areas of uncertainty were those relating to risk-score costs. However, scenario analyses using equal risk-score costs indicated that this would not alter the conclusions. Other potentially relevant scenarios, such as (1) excluding the cost of CA125 testing from the ADNEX model (as the test can be used without CA125) and (2) adding the cost of CA125 testing to the IOTA group’s simple ultrasound rules (in case the assay is still run in parallel), may be of interest. However, there are currently insufficient test accuracy data to support these analyses, and it is likely that these scenarios would result in improved cost-effectiveness for the ADNEX model. Furthermore, the handling of patients with malignancies other than ovarian cancer by assuming that all women have CRC in the model is a simplifying assumption made in line with CG1221 to avoid additional complexity in the modelling. This simplifying assumption was shown not to be influential in a scenario in which women with other malignancies were not modelled, which is explained by the same approach being adopted for all risk scores, thus not affecting the results of the incremental analyses.
The main driver of the model results is the progression-free and overall survival HRs for SMDT referral versus no SMDT referral. This HR was obtained from a Cochrane review. 135 Although this Cochrane review135 concluded that the evidence was consistent and stronger for ovarian cancer, it was stated to be low-quality evidence (because of the high risk of bias of the included retrospective observational studies). In addition, it is unclear whether or not this HR is representative of the difference between SMDT referral and no SMDT referral in the UK; this HR might be country specific as a result of differences between health systems. It is, however, reassuring that the review135 included only studies that were performed in developed countries (Canada, the Netherlands, the UK and the USA). Nevertheless, given the aforementioned considerations, this HR should be considered an area of uncertainty. Furthermore, scenario analyses indicated that the SMDT (surgery) costs as well as a potential disutility for FP groups (favouring risk scores with higher specificity and hence informing the trade-off between sensitivity and specificity) are areas of uncertainty. With regard to the SMDT costs, feedback from clinicians highlighted that SMDT costs used in the model (obtained from NHS reference costs) were likely to be an underestimate and did not appropriately reflect the high costs associated with extensive surgery, which is performed in a proportion of women undergoing surgery in this setting. It should therefore be borne in mind that the RMI 1 (threshold of 250) was cost-effective at a threshold of £20,000 per QALY gained, whereas the IOTA group’s simple ultrasound rules were cost-effective at a threshold of £30,000 per QALY gained in the scenario when higher SMDT costs were used. Unfortunately, there was no evidence to justify this increased cost of surgery.
Chapter 6 Conclusions
Implications for service provision
There is evidence to suggest that using either the IOTA group’s ADNEX model or the IOTA group’s simple ultrasound rules to assess the risk of malignancy in women with an adnexal mass may offer increased sensitivity relative to current practice (the RMI 1 at a decision threshold of 250 or 200), that is, a higher proportion of those women who have a malignant tumour would be referred to a SMDT. Both the IOTA group’s ADNEX model and IOTA group’s simple ultrasound rules have a lower specificity than the RMI 1 at a decision threshold of 250 or 200 and, hence, if the RMI 1 were replaced with either of these methods, it is also likely that more women with benign tumours would be ‘unnecessarily’ referred to a SMDT, with the attendant implications for an increased workload. However, to achieve a similar sensitivity using the RMI 1 would require a very low decision threshold (25) and hence a lower specificity and a greater number of unnecessary referrals than that achievable using either the IOTA group’s ADNEX model or the IOTA group’s simple ultrasound rules. The limited available evidence suggested that the ROMA score does not offer any clear performance advantage over the RMI 1. Although Overa (MIA2G) appeared to have a higher sensitivity than the ROMA score, there were no data to support a direct comparison between Overa (MIA2G) and the RMI 1.
In the base-case analysis, the IOTA group’s ADNEX model (threshold of 10%) was considered to be cost-effective at thresholds of £20,000 and £30,000 per QALY gained. However, both cost and QALY differences between the strategies were small. This means that ICERs can change substantially, especially with small changes in either costs or QALYs. Therefore, it is difficult to be confident that other strategies, particularly the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant), which was cost-effective in some scenario analyses, might not be cost-effective. This is illustrated in the probabilities of being cost-effective for the IOTA group’s simple ultrasound rules and the IOTA group’s ADNEX model (threshold of 10%), which was 39% and 60%, respectively, at the £20,000 threshold and 23% and 75%, respectively, at the £30,000 threshold.
For the premenopausal and postmenopausal subgroups of women, the IOTA group’s ADNEX model (threshold of 10%) remained cost-effective at the thresholds of £20,000 and £30,000 per QALY gained.
Overall, the model does provide evidence to strongly prioritise sensitivity over specificity. As a result, the IOTA group’s ADNEX model (threshold of 10%), which had the highest sensitivity (96.3%) was considered to be cost-effective.
Suggested research priorities
In addition to information about the diagnostic performance of different risk-scoring methods, it is important to understand the consequences of applying these scores in practice:
-
Further studies are required to explore the distribution of histological diagnoses among patients with FN, low-risk classifications. A more complete exploration of the types of women who are likely to be misclassified as having a low risk of developing ovarian cancer, using the various risk-scoring options available, as well as an investigation of the downstream clinical consequences for these women is required. If one or more of the risk scores evaluated in this assessment is introduced into routine practice, a postimplementation audit would be informative.
-
Studies designed to capture the downstream resource use and clinical consequences of using the various risk-scoring options are likely to be informative. An example of such a study might be a cluster RCT, in which general gynaecology departments are randomised to use different risk-scoring methods to inform decisions about referral to a SMDT; outcomes could include rates of referral, staging investigations, surgery in a specialist setting (gynaecological oncologist), postsurgical outcomes and survival measures. Such a study could also inform issues around the costs and logistics of delivering various strategies in the NHS.
There are a number of areas that require further investigation if the introduction of ultrasound-based risk-scoring systems is being considered:
-
An assessment of the levels of training and experience needed to achieve the required levels of test performance when using risk scores that include morphological features observed on ultrasound examination is required.
-
Given the implementation issues around the use of risk-scoring systems that require ultrasound examination by expert or specifically trained personnel, research should consider whether or not implementation could be better delivered through one-stop clinics, similar to those used to assess postmenopausal bleeding. Such one-stop clinics, in which women could be seen by specialist gynaecologists and scanned by IOTA-trained personnel, may overcome some of the potential hurdles of implementing an imaging-based approach and interpreting imaging information in the context of other observations.
-
Further studies or further analyses of the IOTA data set are needed to understand the role of menopausal status in the performance of both the IOTA and ADNEX model tests.
-
Studies on the acceptability (the likely uptake) of TVS for women being assessed in general gynaecology (secondary care) settings may also be useful.
Further large diagnostic cohort studies are needed to fully evaluate the performance of the ROMA score (using different manufacturers’ tumour marker assays) and Overa (MIA2G) compared with that of the RMI 1 at a decision threshold of 250 or 200, or at a lower threshold(s) if this is considered to be appropriate or if current guidance changes. These studies should be conducted in a population that includes the full spectrum of differential diagnoses that are likely to be present in women referred to secondary care for the investigation of an adnexal mass.
Further diagnostic cohort studies or subgroup analyses of existing data sets are needed to fully explore the possible variation in the accuracy of all risk scores in relevant subgroups (e.g. menopausal status and family history of ovarian cancer).
Given the areas of uncertainty highlighted in the Chapter 5, Discussion, the feasibility of a ‘refer all to SMDT’ strategy should be considered. If this strategy is not deemed feasible, the thresholds of the risk scores should examined, bearing in mind that sensitivity should be prioritised over specificity, and also bearing in mind the available SMDT capacity.
Acknowledgements
The authors would like to acknowledge the clinical advice and expert opinion provided by specialist members of the assessment subgroup: Dr Cathie Sturgeon, Consultant Clinical Scientist, Royal Infirmary of Edinburgh; Mr Jed Hawe, Consultant Gynaecologist, Countess of Chester Hospital NHS Foundation Trust; Dr Jurjees Hasan, Consultant Medical Oncologist, The Christie Hospital, Manchester; Dr Michael Weston, Consultant Radiologist, St James’s University Hospital, Leeds; Professor Richard Edmonds, Professor of Gynaecological Oncology, University of Manchester; Dr Sudha Sundar, Senior Lecturer and Consultant in Gynaecological Oncology, City Hospital, Birmingham; and Dr Tracie Miles, Clinical Nurse Specialist, Royal United Hospital, Bath.
We are grateful to the following researchers for providing additional information and unpublished data to support this assessment: the IOTA study group; Mona Aarenstrup Karlsen, Herlev University Hospital; Dr Sharon O’Toole, University of Dublin; Dr Elisa Piovano, University Hospital, Turin; and Dr Julie Clemente, Department of Obstetrics and Gynaecology, Alfonso Specialist Hospital, Manila.
We would also like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Subgroup for providing input on the patients’ perspective at key stages of the assessment process.
Contributions of authors
Marie Westwood, Shona Lang and Sohan Deshpande planned and performed the systematic review and interpretation of the evidence.
Bram Ramaekers and Sabine Grimm planned and performed the cost-effectiveness analyses and interpreted the results.
Shelley de Kock devised and performed the literature searches and provided information support to the project.
Nigel Armstrong contributed to the planning and interpretation of the systematic review and cost-effectiveness analyses and the acquisition of input data, and conducted the model peer review.
Manuela Joore and Jos Kleijnen provided senior advice and support to the cost-effectiveness analyses and the systematic review, respectively.
All authors were involved in drafting and/or commenting on the report.
Data-sharing statement
All available data are contained within the report. All queries should be submitted to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. Manchester: National Institute for Health and Care Excellence; 2011.
- Myers ER, Bastian LA, Havrilesky LJ, Kulasingam SL, Terplan MS, Cline KE, et al. Management of adnexal mass. Evid Rep Technol Assess 2006;130:1-145.
- Prat J. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol 2015;26:87-9. https://doi.org/10.3802/jgo.2015.26.2.87.
- Cancer Registration Statistics, England: 2014. Cancer Diagnoses and Age-Standardised Incidence Rates for All Cancer Sites by Age, Sex and Region. Newport: ONS; 2016.
- Ovarian Cancer (C56-C57.4): 2013. Number of New Cases, Crude And European Age-Standardised (AS) Incidence Rates per 100,000 Population, Females, UK. London: Cancer Research UK; 2015.
- Ovarian Cancer (C56-C57.4): 2012–2014. Average Number of Deaths Per Year and Age-Specific Mortality Rates per 100,000 Population, UK. London: Cancer Research UK; 2015.
- Ovarian Cancer (C56-C57.4): 2011–2013. Average Number of New Cases Per Year and Age-Specific Incidence Rates per 100,000 Population, Females, UK. London: Cancer Research UK; 2015.
- Ovarian Cancer (C56): 2014. Proportion of Cancers Diagnosed at Each Stage, All Ages, England. London: Cancer Research UK; 2016.
- Wang J, Gao J, Yao H, Wu Z, Wang M, Qi J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol 2014;35:6127-38. https://doi.org/10.1007/s13277-014-1811-6.
- Management of Suspected Ovarian Masses in Premenopausal Women. London: RCOG; 2011.
- Khoja L, Nolan K, Mekki R, Milani A, Mescallado N, Ashcroft L, et al. Improved survival from ovarian cancer in patients treated in Phase III trial active cancer centres in the UK. Clin Oncol 2016;28:760-5. https://doi.org/10.1016/j.clon.2016.06.011.
- Anton C, Carvalho FM, Oliveira EI, Maciel GA, Baracat EC, Carvalho JP. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics 2012;67:437-41. https://doi.org/10.6061/clinics/2012(05)06.
- Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112:40-6. https://doi.org/10.1016/j.ygyno.2008.08.031.
- Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. International Ovarian Tumor Analysis (IOTA) Group . Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol 2000;16:500-5. https://doi.org/10.1046/j.1469-0705.2000.00287.x.
- Timmerman D, Testa AC, Bourne T, Ameye L, Jurkovic D, Van Holsbeke C, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008;31:681-90. https://doi.org/10.1002/uog.5365.
- Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W, et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol 2016;214:424-37. https://doi.org/10.1016/j.ajog.2016.01.007.
- Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, Van Holsbeke C, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014;349. https://doi.org/10.1136/bmj.g5920.
- Van Calster B, Van Hoorde K, Froyman W, Kaijser J, Wynants L, Landolfo C, et al. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn 2015;7:32-41.
- Management of Epithelial Ovarian Cancer. Edinburgh: SIGN; 2013.
- Suspected Cancer: Recognition and Referral. Manchester: NICE; 2015.
- About iRefer. London: RCR; 2016.
- Guidance on the Use of Palitaxel in the Treatment of Ovarian Cancer. London: NICE; 2005.
- Bevacizumab in Combination with Paclitaxel and Carboplatin for First-Line Treatment of Advanced Ovarian Cancer. London: NICE; 2013.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-3.
- Olaparib for Maintenance Treatment of Relapsed, Platinum-Sensitive, BRCA Mutation-Poisitive Ovarian, Fallopian Tube and Peritoneal Cancer after Response to Second-Line or Subsequent Platinum-Based Chemotherapy. London: NICE; 2016.
- Topotecan, Peylated Liposomal Doxorubicin Hydrochloride, Paclitaxel, Trabecteden and Gemcitabine for Treatment Recurrent Ovarian Cancer. London: NICE; 2016.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Diagnostics Assessment Programme Manual. Manchester: NICE; 2011.
- Handbook for DTA Reviews. London: The Cochrane Collaboration; 2009.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. https://doi.org/10.1016/j.jclinepi.2010.07.006.
- CADTH Peer Review Checklist for Search Strategies. Ottawa, ON: CADTH; 2013.
- Wright K, McDaid C. Is the Retraction of Journal Articles in Electronic Journals and Databases Consistent and Timely? A Case Study n.d.
- Wright K, McDaid C. Reporting of article retractions in bibliographic databases and online journals. J Med Libr Assoc 2011;99:164-7. https://doi.org/10.3163/1536-5050.99.2.010.
- Royle P, Waugh N. Should systematic reviews include searches for published errata?. Health Info Libr J 2004;21:14-20. https://doi.org/10.1111/j.1471-1842.2004.00459.x.
- Waffenschmidt S. Assessing the Completeness of Systematic Reviews via the ‘Related Articles’ Function and Or a Simple Structured Boolean Search in PubMed – A Pilot Study (B202) n.d.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Wolff R, Whiting P, Mallett S, Riley R, Westwood M, Kleijnen J. PROBAST: Prediction Model Risk of Bias Assessment Tool n.d.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103. https://doi.org/10.1016/j.jclinepi.2007.09.013.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-31.
- Szubert S, Wojtowicz A, Moszynski R, Zywica P, Dyczkowski K, Stachowiak A, et al. External validation of the IOTA ADNEX model performed by two independent gynecologic centers. Gynecol Oncol 2016;142:490-5. https://doi.org/10.1016/j.ygyno.2016.06.020.
- Joyeux E, Miras T, Masquin I, Duglet PE, Astruc K, Douvier S. Before surgery predictability of malignant ovarian tumors based on ADNEX model and its use in clinical practice. Gynecol Obstet Fertil 2016;44:557-64. https://doi.org/10.1016/j.gyobfe.2016.07.007.
- Meys EM, Jeelof LS, Achten NM, Slangen BF, Lambrechts S, Kruitwagen RF, et al. Estimating the risk of malignancy in adnexal masses: an external validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound Obstet Gynecol 2016;12.
- Moffatt J, Laver L, Dunford F, Cullimore J. Predicting the risk of malignancy of ovarian masses using the ADNEX (Assessment of Different NEoplasias in the adneXa) model: a retrospective study at the Great Western Hospital. BJOG 2016;123:103-4.
- Sayasneh A, Ferrara L, De Cock B, Saso S, Al-Memar M, Johnson S, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model: a multicentre external validation study. Br J Cancer 2016;115:542-8. https://doi.org/10.1038/bjc.2016.227.
- Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, Charoenratana C, Lerthiranwong T, Tongsong T. IOTA simple rules in differentiating between benign and malignant adnexal masses by non-expert examiners. Asian Pac J Cancer Prev 2015;16:3835-8. https://doi.org/10.7314/APJCP.2015.16.9.3835.
- Abdalla N, Bachanek M, Trojanowski S, Cendrowski K, Sawicki W. Diagnostic value of ultrasound indicators of neoplastic risk in preoperative differentiation of adnexal masses. J Ultrason 2013;13:145-54. https://doi.org/10.15557/JoU.2013.0015.
- Knafel A, Banas T, Nocun A, Wiechec M, Jach R, Ludwin A, et al. The prospective external validation of International Ovarian Tumor Analysis (IOTA) simple rules in the hands of level I and II examiners. Ultraschall Med 2016;37:516-23. https://doi.org/10.1055/s-0034-1398773.
- Testa A, Kaijser J, Wynants L, Fischerova D, Van Holsbeke C, Franchi D, et al. Strategies to diagnose ovarian cancer: new evidence from phase 3 of the multicentre international IOTA study. Br J Cancer 2014;111:680-8. https://doi.org/10.1038/bjc.2014.333.
- Tantipalakorn C, Wanapirak C, Khunamornpong S, Sukpan K, Tongsong T. IOTA simple rules in differentiating between benign and malignant ovarian tumors. Asian Pac J Cancer Prev 2014;15:5123-6. https://doi.org/10.7314/APJCP.2014.15.13.5123.
- Alcázar JL, Pascual MÁ, Olartecoechea B, Graupera B, Aubá M, Ajossa S, et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: prospective external validation. Ultrasound Obstet Gynecol 2013;42:467-71. https://doi.org/10.1002/uog.12485.
- Weinberger V, Minar L. Diagnostics of malign ovarian tumors by ultrosound and CA 125 – our experience. Int J Gynecol Cancer 2013;23.
- Knafel A, Nocun A, Banas T, Wiechec M, Jach R, Pietrus M, et al. IOTA simple ultrasound-based rules: why do we have inconclusive results?. Int J Gynecol Cancer 2013;23:155-6.
- Silvestre L, Martins WP, Candido-Dos-Reis FJ. Limitations of three-dimensional power Doppler angiography in preoperative evaluation of ovarian tumors. J Ovarian Res 2015;8. https://doi.org/10.1186/s13048-015-0174-y.
- Kaijser J, Van Gorp T, Van Holsbeke C, Sayasneh A, Vergote I, Bourne T, et al. IOTA simple descriptors (SD) or simple rules (SR) as a triage test in patients with ovarian tumours: subsequent value of CA125, HE4 or ROMA in clinical reality?. BJOG 2013;120.
- Abdalla N, Bachanek M, Kowalska J, Cendrowski K, Sawicki W. Role of HE4 and simple ultrasound rules proposed by iota group in preoperative evaluation of adnexal masses: a prospective study. Int J Gynecol Cancer 2013;23.
- Piovano E, Cavallero C, Fuso L, Viora E, Ferrero A, Gregori G, et al. Diagnostic accuracy and cost-effectiveness of different strategies to triage adnexal masses: a prospective study. Ultrasound Obstet Gynecol 2016;5.
- Tongsong T, Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, Charoenratana C, Lerthiranwong T. Comparison of effectiveness in differentiating benign from malignant ovarian masses between IOTA simple rules and subjective sonographic assessment. Asian Pac J Cancer Prev 2016;17:4377-80.
- Murala KS, Ma K, Henry RJW, Snape S, Dwivedi R. Performance of IOTA simple rules and RMI in preoperative classification of adnexal lesions in DGH setting. BJOG 2014;121:49-50.
- Di Legge A, Testa AC, Ameye L, Van Calster B, Lissoni AA, Leone FP, et al. Lesion size affects diagnostic performance of IOTA logistic regression models, IOTA simple rules and risk of malignancy index in discriminating between benign and malignant adnexal masses. Ultrasound Obstet Gynecol 2012;40:345-54. https://doi.org/10.1002/uog.11167.
- Sayasneh A, Wynants L, Preisler J, Kaijser J, Johnson S, Stalder C, et al. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer 2013;108:2448-54. https://doi.org/10.1038/bjc.2013.224.
- Fathallah K, Huchon C, Bats AS, Metzger U, Lefrère-Belda MA, Bensaid C, et al. External validation of simple ultrasound rules of Timmerman on 122 ovarian tumors. Gynecol Obstet Fertil 2011;39:477-81. https://doi.org/10.1016/j.gyobfe.2011.05.007.
- Ruiz de Gauna B, Rodriguez D, Olartecoechea B, Aubá M, Jurado M, Gómez Roig MD, et al. Diagnostic performance of IOTA simple rules for adnexal masses classification: a comparison between two centers with different ovarian cancer prevalence. Eur J Obstet Gynecol Reprod Biol 2015;191:10-4. https://doi.org/10.1016/j.ejogrb.2015.05.024.
- Timmerman D, Ameye L, Fischerova D, Epstein E, Melis GB, Guerriero S, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010;341. https://doi.org/10.1136/bmj.c6839.
- Baker C, Pasipanodya T, Dwivedi R. The management of suspected ovarian masses in premenopausal women in a DGH setting. BJOG 2013;120.
- Ameye L, Timmerman D, Valentin L, Paladini D, Zhang J, Van Holsbeke C, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012;40:582-91. https://doi.org/10.1002/uog.11177.
- Zhang Z, Pappas T, Sokoll LJ, Smith A, Chen L, Chan DW, et al. Derivation of a second generation multivariate index assay to improve specificity in presurgical evaluation of adnexal masses for risk of ovarian malignancy. J Clin Oncol 2015;33.
- Wolf J, Pappas T, Zhang Z, Smith A, Hudson T, Carpenter B, et al. Validation of a second-generation MIA (MIA2G) for triage of adnexal masses. J Clin Oncol 2015;33.
- Coleman RL, Herzog TJ, Chan DW, Munroe DG, Pappas TC, Smith A, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol 2016;215:82.e1-11. https://doi.org/10.1016/j.ajog.2016.03.003.
- Aktürk E, Karaca RE, Alanbay I, Dede M, Karaşahin E, Yenen MC, et al. Comparison of four malignancy risk indices in the detection of malignant ovarian masses. J Gynecol Oncol 2011;22:177-82. https://doi.org/10.3802/jgo.2011.22.3.177.
- Yamamoto Y, Yamada R, Oguri H, Maeda N, Fukaya T. Comparison of four malignancy risk indices in the preoperative evaluation of patients with pelvic masses. Eur J Obstet Gynecol Reprod Biol 2009;144:163-7. https://doi.org/10.1016/j.ejogrb.2009.02.048.
- Lou HY, Meng H, Zhu QL, Zhang Q, Jiang YX. Application values of four risk of malignancy indices in the preoperative evaluation of patients with adnexal masses. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010;32:297-302. https://doi.org/10.3881/j.issn.1000-503X.2010.03.013.
- Ulusoy S, Akbayir O, Numanoglu C, Ulusoy N, Odabas E, Gulkilik A. The risk of malignancy index in discrimination of adnexal masses. Int J Gynaecol Obstet 2007;96:186-91. https://doi.org/10.1016/j.ijgo.2006.10.006.
- Manjunath AP, Pratapkumar, Sujatha K, Vani R. Comparison of three risk of malignancy indices in evaluation of pelvic masses. Gynecol Oncol 2001;81:225-9. https://doi.org/10.1006/gyno.2001.6122.
- Tingulstad S, Hagen B, Skjeldestad FE, Onsrud M, Kiserud T, Halvorsen T, et al. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol 1996;103:826-31. https://doi.org/10.1111/j.1471-0528.1996.tb09882.x.
- Asif N, Sattar A, Dawood MM, Rafi T, Aamir M, Anwar M. Pre-operative evaluation of ovarian mass: risk of malignancy index. J Coll Physicians Surg Pak 2004;14:128-31. https://doi.org/03.2004/JCPSP.128131.
- Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990;97:922-9. https://doi.org/10.1111/j.1471-0528.1990.tb02448.x.
- Davies AP, Jacobs I, Woolas R, Fish A, Oram D. The adnexal mass: benign or malignant? Evaluation of a risk of malignancy index. Br J Obstet Gynaecol 1993;100:927-31. https://doi.org/10.1111/j.1471-0528.1993.tb15109.x.
- Morgante G, la Marca A, Ditto A, De Leo V. Comparison of two malignancy risk indices based on serum CA125, ultrasound score and menopausal status in the diagnosis of ovarian masses. Br J Obstet Gynaecol 1999;106:524-7. https://doi.org/10.1111/j.1471-0528.1999.tb08318.x.
- Presl J, Kučera R, Topolčan O, Novotný Z, Vrzalová J, Fuchsova R, et al. HE4 a biomarker of ovarian cancer. Ceska Gynekol 2012;77:445-9.
- Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol 2013;128:239-44. https://doi.org/10.1016/j.ygyno.2012.09.034.
- Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2012;127:379-83. https://doi.org/10.1016/j.ygyno.2012.07.106.
- Piovano E, Macchi C, Cavallero C, Fuso L, De Ruvo D, Viora E, et al. Preoperative management of the adnexal mass: a prospective multi-center study based on 391 patients. Int J Gynecol Cancer 2015;25.
- Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer 2011;104:863-70. https://doi.org/10.1038/sj.bjc.6606092.
- Novotny Z, Presl J, Kucera R, Topolcan O, Vrzalova J, Fuchsova R, et al. HE4 and ROMA index in Czech postmenopausal women. Anticancer Res 2012;32:4137-40.
- Moore R, Miller C, DiSilvestro P, Landrum L, Gajewski W, Renneisen P, et al. Evaluation of the risk of ovarian malignancy algorithm in women with a pelvic mass presenting to general gynecologists. Gynecol Oncol 2011;120. https://doi.org/10.1016/j.ygyno.2010.12.163.
- Moore R, Miller M, MacLaughlan S, Vucetic Z, Gajewski W, DiSilvestro P. The use of the Risk of Ovarian Malignancy Algorithm (ROMA) with clinical assessment improves ovarian cancer detection in women with a pelvic mass. Gynecol Oncol 2012;125. https://doi.org/10.1016/j.ygyno.2011.12.087.
- Yanaranop M, Anakrat V, Siricharoenthai S, Nakrangsee S, Thinkhamrop B. Is the Risk of Ovarian Malignancy Algorithm better than other tests for predicting ovarian malignancy in women with pelvic masses?. Gynecol Obstet Invest 2016;21.
- Clemente J, Benitez G. A retrospective cohort study to validate CA125 and the combination of CA125 and HE4 using the ROMA in assessing the risk for ovarian malignancy in women diagnosed with an adnexal mass in Makati Medical Centre. BJOG 2015;122:133-4.
- Kaijser J, Van Gorp T, Van Hoorde K, Van Holsbeke C, Sayasneh A, Vergote I, et al. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to assess the risk of malignancy in women with an adnexal mass. Gynecol Oncol 2013;129:377-83. https://doi.org/10.1016/j.ygyno.2013.01.018.
- Kaijser J, Van Gorp T, Van Holsbeke C, Sayasneh A, Vergote I, Bourne T, et al. Diagnostic test performance of CA125, HE4, ROMA and IOTA’s LR2 in adnexal tumours of different size. BJOG 2013;120:360-1.
- Chen X, Zhou H, Chen R, He J, Wang Y, Huang L, et al. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clin Chim Acta 2015;440:57-63. https://doi.org/10.1016/j.cca.2014.11.013.
- Langhe R, Norris L, Petzold M, Abu Saadeh F, Kamran W, Ibrahim N, et al. ROMA index improves specificity of ovarian cancer diagnosis compared to CA125 alone. Int J Gynecol Cancer 2013;23.
- Xu Y, Zhong R, He J, Ding R, Lin H, Deng Y, et al. Modification of cut-off values for HE4, CA125 and the ROMA algorithm for early-stage epithelial ovarian cancer detection: results from 1021 cases in South China. Clin Biochem 2016;49:32-40. https://doi.org/10.1016/j.clinbiochem.2015.07.029.
- Li L, Wan J, Cai G, Yuan L, Liang J, Song J, et al. Value of serum human epididymis secretory protein 4 as a marker for differential diagnosis of malignant and benign gynecological diseases of patients in southern China. Clin Chim Acta 2016;459:170-6. https://doi.org/10.1016/j.cca.2016.06.010.
- Janas L, Głowacka E, Wilczyński JR, Malinowski A, Nowak M. Evaluation of applicability of HE4 and ROMA in the preoperative diagnosis of adnexal masses. Ginekol Pol 2015;86:193-7. https://doi.org/10.17772/gp/2062.
- Van Gorp T, Veldman J, Van Calster B, Cadron I, Leunen K, Amant F, et al. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur J Cancer 2012;48:1649-56. https://doi.org/10.1016/j.ejca.2011.12.003.
- Winarto H, Laihad BJ, Nuranna L. Modification of cutoff values for HE4, CA125, the Risk of Malignancy Index, and the Risk of Malignancy Algorithm for ovarian cancer detection in Jakarta, Indonesia. Asian Pac J Cancer Prev 2014;15:1949-53. https://doi.org/10.7314/APJCP.2014.15.5.1949.
- Kaijser J, Van Gorp T, Smet ME, Van Holsbeke C, Sayasneh A, Epstein E, et al. Are serum HE4 or ROMA scores useful to experienced examiners for improving characterization of adnexal masses after transvaginal ultrasonography?. Ultrasound Obstet Gynecol 2014;43:89-97. https://doi.org/10.1002/uog.12551.
- Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol 2011;118:280-8. https://doi.org/10.1097/AOG.0b013e318224fce2.
- Zhang P, Wang C, Cheng L, Zhang P, Guo L, Liu W, et al. Comparison of HE4, CA125, and ROMA diagnostic accuracy: a prospective and multicenter study for Chinese women with epithelial ovarian cancer. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000002402.
- Al Musalhi K, Al Kindi M, Al Aisary F, Ramadhan F, Al Rawahi T, Al Hatali K, et al. Evaluation of HE4, CA-125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) in the preoperative assessment of patients with adnexal mass. Oman Med J 2016;31:336-44. https://doi.org/10.5001/omj.2016.68.
- Shulman LP, Smith A, Pappas T, Bonato V, Munroe D, Wolf J. Clinical Performance Comparison of Two IVDMIAs for Pre-Surgical Assessment of Ovarian Cancer Risk n.d. https://doi.org/10.1097/01.AOG.0000483382.09130.2b.
- Gizzo S, Berretta R, Noventa M, Nardelli GB, Silvio Patrelli T. Borderline ovarian tumors and diagnostic dilemma of intraoperative diagnosis: could preoperative HE4 assay and ROMA score assessment increase the frozen section accuracy? A multicenter case-control study. Int J Gynecol Cancer 2014;24. https://doi.org/10.1155/2014/803598.
- Ali M, Jabeen R, Khurshid M. Preoperative diagnosis of malignancy in ovarian masses: a comparison of risk of malignancy indices I and II. Med Forum Mon 2010;21:19-23.
- Abudia S. Evaluation of the risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic mass. Jamahiriya Med J 2010;10:286-9.
- Shahzad N, Rashid N, Zahra S, Malik A. Role of malignancy index in prediction of malignancy in ovarian masses preoperative. Med Forum Mon 2015;26:44-7.
- Rossi A, Forzano L, Romanello I, Ambrosini G, Iuri V, Marchesoni D. Comparison of pelvic masses score (PMS) and Risk of Malignancy Index (RMI 3) in the evaluation of pelvic masses. Eur J Gynaecol Oncol 2014;35:421-4.
- Kucera R, Topolcan O, Presl J, Novotny Z, Svobodova S, Vrzalova J, et al. Clinical benefit of ROMA index in the diagnosis of ovarian cancer. Klin Biochem Metabol 2012;20:248-51.
- Woman’s Health University Hospital, Egypt . IOTA Versus Pattern Recognition Method in Diagnosis of Ovarian Masses 2000. https://ClinicalTrials.gov/show/NCT02800031 (accessed 9 March 2017).
- Sundar S, Rick C, Dowling F, Au P, Snell K, Rai N, et al. Refining ovarian cancer test accuracy scores (ROCkeTS) trial update. BJOG 2016;123.
- Sundar S, Rick C, Dowling F, Au P, Snell K, Rai N, et al. Refining Ovarian Cancer Test accuracy Scores (ROCkeTS): protocol for a prospective longitudinal test accuracy study to validate new risk scores in women with symptoms of suspected ovarian cancer. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010333.
- Meys EM, Rutten IJ, Kruitwagen RF, Slangen BF, Bergmans MG, Mertens HJ, et al. Investigating the performance and cost-effectiveness of the simple ultrasound-based rules compared to the risk of malignancy index in the diagnosis of ovarian cancer (SUBSONiC-study): protocol of a prospective multicenter cohort study in the Netherlands. BMC Cancer 2015;15. https://doi.org/10.1186/s12885-015-1319-5.
- Ikiz N, Guvenal T, Taneli F, Koyuncu FM, Kandiloglu AR, Bilge S, et al. Comparison of ROMA (Risk of Ovarian Malignancy Algorithm), RMI (Risk of Malignancy Index) and OTI (Ovarian Tumor Index) in patients with adnexal mass. Int J Gynecol Cancer 2013;23.
- Keogh F, Tan AL, Eva LJ. HE4 as a tumour marker for the prediction of ovarian carcinoma. BJOG 2015;122:137-8.
- Romagnolo C, Leon AE, Fabricio ASC, Del Pup L, Papadakis C, Odicino FE, et al. HE4, CA125 and risk of ovarian malignancy algorithm (Roma) as diagnostic tools of ovarian cancer in patients with pelvic mass: an Italian multicenter prospective study. Int J Gynecol Cancer 2015;25:528-9.
- Martin Rodriguez S, Ascorbe Salcedo P, Jareno Blanco MS. Diagnostic accuracy of HE4, CA125 and Roma for women with suspected ovarian cancer. Clin Chem Lab Med 2015;53.
- Tingulstad S, Hagen B, Skjeldestad FE, Halvorsen T, Nustad K, Onsrud M. The risk-of-malignancy index to evaluate potential ovarian cancers in local hospitals. Obstet Gynecol 1999;93:448-52. https://doi.org/10.1097/00006250-199903000-00028.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Ding Y, Hay J. Cost-effectiveness analysis of multimodal screening for ovarian cancer. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(10)72162-8.
- Forde GK, Hornberger J, Michalopoulos S, Bristow RE. Cost-effectiveness analysis of a multivariate index assay compared to modified American College of Obstetricians and Gynecologists criteria and CA-125 in the triage of women with adnexal masses. Curr Med Res Opin 2016;32:321-9. https://doi.org/10.1185/03007995.2015.1123679.
- Havrilesky LJ, Dinan M, Sfakianos GP, Curtis LH, Barnett JC, Van Gorp T, et al. Costs, effectiveness, and workload impact of management strategies for women with an adnexal mass. J Natl Cancer Inst 2015;107. https://doi.org/10.1093/jnci/dju322.
- Drescher CW, Hawley S, Thorpe JD, Marticke S, McIntosh M, Gambhir SS, et al. Impact of screening test performance and cost on mortality reduction and cost-effectiveness of multimodal ovarian cancer screening. Cancer Prev Res 2012;5:1015-24. https://doi.org/10.1158/1940-6207.CAPR-11-0468.
- Kearns B, Chilcott J, Whyte S, Preston L, Sadler S. Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: a model-based economic evaluation. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0743-y.
- Ovarian Cancer Incidence Statistics. London: Cancer Research UK; 2017.
- Colombo N, Peiretti M, Castiglione M. ESMO Guidelines Working Group . Non-epithelial ovarian cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:24-6. https://doi.org/10.1093/annonc/mdp118.
- Bell R, Petticrew M, Luengo S, Sheldon T. Screening for ovarian cancer: a systematic review. Health Technol Assess 1998;2.
- Colorectal Cancer Survival by Stage – NCIN Data Briefing. London: National Cancer Intelligence Network; 2010.
- Venesmaa P, Ylikorkala O. Morbidity and mortality associated with primary and repeat operations for ovarian cancer. Obstet Gynecol 1992;79:168-72. https://doi.org/10.1016/0020-7292(92)90941-B.
- Gerestein CG, Damhuis RA, Burger CW, Kooi GS. Postoperative mortality after primary cytoreductive surgery for advanced stage epithelial ovarian cancer: a systematic review. Gynecol Oncol 2009;114:523-7. https://doi.org/10.1016/j.ygyno.2009.03.011.
- Loft A, Andersen TF, Brønnum-Hansen H, Roepstorff C, Madsen M. Early postoperative mortality following hysterectomy. A Danish population based study, 1977–1981. Br J Obstet Gynaecol 1991;98:147-54. https://doi.org/10.1111/j.1471-0528.1991.tb13360.x.
- Collinson F, Qian W, Fossati R, Lissoni A, Williams C, Parmar M, et al. Optimal treatment of early-stage ovarian cancer. Ann Oncol 2014;25:1165-71. https://doi.org/10.1093/annonc/mdu116.
- International, Collaborative Ovarian Neoplasm Group . Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet 2002;360:505-15. https://doi.org/10.1016/S0140-6736(02)09738-6.
- Woo YL, Kyrgiou M, Bryant A, Everett T, Dickinson HO. Centralisation of services for gynaecological cancer. Cochrane Database Syst Rev 2012;3. https://doi.org/10.1002/14651858.CD007945.pub2.
- Westwood M, Corro Ramos I, Lang S, Luyendijk M, Zaim R, Stirk L, et al. Faecal Immunochemical Tests to Triage Patients with Lower Abdominal Symptoms for Suspected Colorectal Cancer Referrals in Primary Care: A Systematic Review and Cost-Effectiveness Analysis. A diagnostic assessment report. York: Kleijnen Systematic Reviews; 2016.
- Tappenden P, Chilcott J, Eggington S, Patnick J, Sakai H, Karnon J. Option appraisal of population-based colorectal cancer screening programmes in England. Gut 2007;56:677-84. https://doi.org/10.1136/gut.2006.095109.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol 2009;113:216-20. https://doi.org/10.1016/j.ygyno.2008.12.026.
- Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol 1998;16:979-85. https://doi.org/10.1200/JCO.1998.16.3.979.
- Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650-7. https://doi.org/10.1111/j.1572-0241.1999.01157.x.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- NHS Reference Costs 2015–2016. London: DHSC; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Kumpulainen S, Sankila R, Leminen A, Kuoppala T, Komulainen M, Puistola U, et al. The effect of hospital operative volume, residual tumor and first-line chemotherapy on survival of ovarian cancer – a prospective nation-wide study in Finland. Gynecol Oncol 2009;115:199-203. https://doi.org/10.1016/j.ygyno.2009.07.011.
- Hinde S, Epstein D, Cook A, Embleton A, Perren T, Sculpher M. The cost-effectiveness of bevacizumab in advanced ovarian cancer using evidence from the ICON7 trial. Value Health 2016;19:431-9. https://doi.org/10.1016/j.jval.2016.01.013.
- Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015;16:928-36. https://doi.org/10.1016/S1470-2045(15)00086-8.
- Ultra-Radical (Extensive) Surgery for Advanced Ovarian Cancer. London: NICE; 2013.
- Meys EM, Kaijser J, Kruitwagen RF, Slangen BF, Van Calster B, Aertgeerts B, et al. Subjective assessment versus ultrasound models to diagnose ovarian cancer: a systematic review and meta-analysis. Eur J Cancer 2016;58:17-29. https://doi.org/10.1016/j.ejca.2016.01.007.
- Dayyani F, Uhlig S, Colson B, Simon K, Rolny V, Morgenstern D, et al. Diagnostic performance of risk of ovarian malignancy algorithm against CA125 and HE4 in connection with ovarian cancer: a meta-analysis. Int J Gynecol Cancer 2016;26:1586-93. https://doi.org/10.1097/IGC.0000000000000804.
- Li F, Tie R, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC Cancer 2012;12. https://doi.org/10.1186/1471-2407-12-258.
- Nunes N, Ambler G, Foo X, Naftalin J, Widschwendter M, Jurkovic D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstet Gynecol 2014;44:503-14. https://doi.org/10.1002/uog.13437.
- Rai N, Champaneria R, Snell K, Mallett S, Bayliss SE, Neal RD, et al. Symptoms, ultrasound imaging and biochemical markers alone or in combination for the diagnosis of ovarian cancer in women with symptoms suspicious of ovarian cancer. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.cd011964.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
- Nunes N, Ambler G, Foo X, Naftalin J, Derdelis G, Widschwendter M, et al. Comparison of two protocols for the management of asymptomatic postmenopausal women with adnexal tumours – a randomised controlled trial of RMI/RCOG vs Simple Rules. Br J Cancer 2017;116:584-91. https://doi.org/10.1038/bjc.2017.17.
- Search Strategies: NHS EED MEDLINE Using OvidSP (Economics Filter). York: CRD; 2014.
- Search Strategies: NHS EED EMBASE Using OvidSP (Economics Filter). York: CRD; 2014.
Appendix 1 Literature search strategies
Clinical effectiveness searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 2 November 2016.
Date searched: 24 November 2016.
Records found: 644.
Search strategy
-
exp Ovarian Neoplasms/ (79,417)
-
Fallopian Tube Neoplasms/ (2722)
-
Uterine Neoplasms/ (40,421)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (6088)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (104,209)
-
or/1-5 (151,393)
-
Peritoneal Neoplasms/ (13,589)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (337,303)
-
or/7-8 (348,068)
-
ovar$.ti,ab,ot. (225,015)
-
9 and 10 (23,572)
-
6 or 11 (155,473)
-
((risk adj4 malignan$ adj4 index) or (risk adj4 malignan$ adj4 indice$) or RMI).ti,ab,ot. (815)
-
(menopau$ or perimenopaus$ or premenopaus$ or postmenopaus$ or POF or climacteric or (change adj2 life)).ti,ab,ot. (91,426)
-
exp Menopause/ (55,959)
-
14 or 15 (102,167)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. (315,798)
-
Ultrasonography/ (67,496)
-
17 or 18 (333802)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (7731)
-
CA-125 Antigen/ (4333)
-
20 or 21 (8485)
-
16 and 19 and 22 (316)
-
13 or 23 (1059)
-
(ROMA or (Ovar$ adj5 Algor$)).ti,ab,ot. (1670)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (469)
-
16 and 22 and 26 (91)
-
25 or 27 (1704)
-
(IOTA or international ovarian tumo?r analysis).ti,ab,ot. (1360)
-
((Simple adj3 rules) or (simple adj3 descriptors) or SRrisk or b-rules or m-rules).ti,ab,ot. (1493)
-
19 or 29 (335,099)
-
30 and 31 (38)
-
(adnex$ adj8 (model$ or score$ or assess$)).ti,ab,ot. (287)
-
(ova2 or overa).ti,ab,ot. (25)
-
Follicle Stimulating Hormone/ (35,545)
-
(Follicle stimulat$ hormone$ or FSH or follitropin or fertiline fertinom p or follicotropin folliculostimulating hormone$ or follitrophin or follitropin$ or folltropin$ or 9002-68-0).ti,ab,ot,rn. (49,152)
-
35 or 36 (49,152)
-
Apolipoprotein A-I/ (8782)
-
(apolipoprotein A1 or apo a1 or apo hdl 3 or apo hdl iii or apo high density lipoprotein 3 or apolipoprotein a 1 or apolipoprotein a i or apoprotein a1 or apoprotein ai or apoprotein a 1 or apoprotein a i).ti,ab,ot. (8019)
-
38 or 39 (12379)
-
Transferrin/ (17,226)
-
(transferrin or siderophilin or transferrin?emia or transferrins or trf or 82030-93-1).ti,ab,ot,rn. (34,367)
-
41 or 42 (34,367)
-
22 and 26 and 37 and 40 and 43 (0)
-
34 or 44 (25)
-
24 or 28 or 32 or 33 or 45 (3019)
-
12 and 46 (644)
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), MEDLINE Daily Update (via Ovid), MEDLINE Epub Ahead of Print (via Ovid)
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid): searched to 22 November 2016.
MEDLINE Daily Update (via Ovid): searched to 22 November 2016.
MEDLINE Epub Ahead of Print (via Ovid): searched to 23 November 2016.
Date searched: 24 November 2016.
Records found: 83.
Search strategy
-
exp Ovarian Neoplasms/ (0)
-
Fallopian Tube Neoplasms/ (0)
-
Uterine Neoplasms/ (0)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (904)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (9696)
-
or/1-5 (9801)
-
Peritoneal Neoplasms/ (0)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (25,858)
-
or/7-8 (25,858)
-
ovar$.ti,ab,ot. (18,312)
-
9 and 10 (2204)
-
6 or 11 (10,115)
-
((risk adj4 malignan$ adj4 index) or (risk adj4 malignan$ adj4 indice$) or RMI).ti,ab,ot. (89)
-
(menopau$ or perimenopaus$ or premenopaus$ or postmenopaus$ or POF or climacteric or (change adj2 life)).ti,ab,ot. (7942)
-
exp Menopause/ (0)
-
14 or 15 (7942)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. (41,009)
-
Ultrasonography/ (0)
-
17 or 18 (41,009)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (849)
-
CA-125 Antigen/ (0)
-
20 or 21 (849)
-
16 and 19 and 22 (32)
-
13 or 23 (110)
-
(ROMA or (Ovar$ adj5 Algor$)).ti,ab,ot. (144)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (185)
-
16 and 22 and 26 (22)
-
25 or 27 (149)
-
(IOTA or international ovarian tumo?r analysis).ti,ab,ot. (93)
-
((Simple adj3 rules) or (simple adj3 descriptors) or SRrisk or b-rules or m-rules).ti,ab,ot. (347)
-
19 or 29 (41,084)
-
30 and 31 (11)
-
(adnex$ adj8 (model$ or score$ or assess$)).ti,ab,ot. (35)
-
(ova2 or overa).ti,ab,ot. (3)
-
Follicle Stimulating Hormone/ (0)
-
(Follicle stimulat$ hormone$ or FSH or follitropin or fertiline fertinom p or follicotropin folliculostimulating hormone$ or follitrophin or follitropin$ or folltropin$ or 9002-68-0).ti,ab,ot,rn. (2146)
-
35 or 36 (2146)
-
Apolipoprotein A-I/ (0)
-
(apolipoprotein A1 or apo a1 or apo hdl 3 or apo hdl iii or apo high density lipoprotein 3 or apolipoprotein a 1 or apolipoprotein a i or apoprotein a1 or apoprotein ai or apoprotein a 1 or apoprotein a i).ti,ab,ot. (440)
-
38 or 39 (440)
-
Transferrin/ (0)
-
(transferrin or siderophilin or transferrin?emia or transferrins or trf or 82030-93-1).ti,ab,ot,rn. (1465)
-
41 or 42 (1465)
-
22 and 26 and 37 and 40 and 43 (1)
-
34 or 44 (4)
-
24 or 28 or 32 or 33 or 45 (287)
-
12 and 46 (83)
EMBASE (via Ovid)
Date range searched: 1974 to 23 November 2016.
Date searched: 24 November 2016.
Records found: 1185.
Search strategy
-
exp ovary cancer/ (97,370)
-
uterine tube tumor/ (1263)
-
uterine tube carcinoma/ (1899)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (9245)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (136,192)
-
peritoneum cancer/ (3891)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (405888)
-
or/6-7 (408,204)
-
ovar$.ti,ab,ot. (278,995)
-
8 and 9 (30,110)
-
1 or 2 or 3 or 4 or 5 or 10 (168,296)
-
((risk adj4 malignan$ adj4 index) or (risk adj4 malignan$ adj4 indice$) or RMI).ti,ab,ot. (1394)
-
risk of malignancy index/ (46)
-
12 or 13 (1396)
-
(menopau$ or perimenopaus$ or premenopaus$ or postmenopaus$ or POF or climacteric or (change adj2 life)).ti,ab,ot. or menopause/ (136,468)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. or ultrasound/ or sonography/ (591,945)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (11,962)
-
CA 125 antigen/ (13,650)
-
17 or 18 (16,956)
-
15 and 16 and 19 (627)
-
14 or 20 (1886)
-
ovarian malignancy algorithm/ (1)
-
(ROMA or (Ovar$ adj5 Algor$)).ti,ab,ot. (2502)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (956)
-
human epididymis protein 4/ (507)
-
or/24-25 (1036)
-
15 and 19 and 26 (237)
-
22 or 23 or 27 (2593)
-
(IOTA or international ovarian tumo?r analysis).ti,ab,ot. (846)
-
((Simple adj3 rules) or (simple adj3 descriptors) or SRrisk or b-rules or m-rules).ti,ab,ot. (1796)
-
16 or 29 (592,674)
-
30 and 31 (66)
-
(adnex$ adj8 (model$ or score$ or assess$)).ti,ab,ot. (466)
-
(ova2 or overa).ti,ab,ot. (78)
-
follitropin/ (56,500)
-
(Follicle stimulat$ hormone$ or FSH or follitropin or fertiline fertinom p or follicotropin folliculostimulating hormone$ or follitrophin or follitropin$ or folltropin$ or 9002-68-0).ti,ab,ot,rn. (67,340)
-
or/35-36 (67,499)
-
apolipoprotein A1/ (16,294)
-
(apolipoprotein A1 or apo a1 or apo hdl 3 or apo hdl iii or apo high density lipoprotein 3 or apolipoprotein a 1 or apolipoprotein a i or apoprotein a1 or apoprotein ai or apoprotein a 1 or apoprotein a i).ti,ab,ot. (9160)
-
or/38-39 (18,737)
-
transferrin/ (27,791)
-
(transferrin or siderophilin or transferrin?emia or transferrins or trf or 82030-93-1).ti,ab,ot,rn. (42,260)
-
or/41-42 (42,344)
-
19 and 26 and 37 and 40 and 43 (3)
-
34 or 44 (81)
-
21 or 28 or 32 or 33 or 45 (4880)
-
11 and 46 (1185)
Cochrane Database of Systematic Reviews (via Wiley Online Library), Database of Abstracts of Reviews of Effects (via Wiley Online Library), Cochrane Central Register of Controlled Trials (via Wiley Online Library), Health Technology Assessment Database (via Wiley Online Library)
Cochrane Database of Systematic Reviews (via Wiley Online Library): Issue 11 of 12, November 2016.
Database of Abstracts of Reviews of Effects (via Wiley Online Library): Issue 2 of 4, April 2015.
Cochrane Central Register of Controlled Trials (via Wiley Online Library): Issue 10 of 12, October 2016.
Health Technology Assessment Database (via Wiley Online Library): Issue 4 of 4, October 2016.
Date searched: 24 November 2016.
Records found: 43.
-
Cochrane Database of Systematic Reviews: 1.
-
Database of Abstracts of Reviews of Effects: 5.
-
Cochrane Central Register of Controlled Trials: 37.
-
Health Technology Assessment: 0.
Search strategy
-
#1 MeSH descriptor: [Ovarian Neoplasms] explode all trees (1511)
-
#2 MeSH descriptor: [Fallopian Tube Neoplasms] this term only (45)
-
#3 MeSH descriptor: [Uterine Neoplasms] this term only (691)
-
#4 (AOSCa* or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA* or dysgerminom*):ti,ab,kw (231)
-
#5 ((ovar* or “high-grade serous” or “low-grade serous” or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) near/5 (cancer* or adenocarcin* or adeno-carcin* or tumo?r* or sarcoma* or neoplas* or metasta* or meta-sta* or carcino* or oncogenesis or malignan* or choriocarcinom* or teratoma* or cystadenocarcin* or rhabdomyosarcom* or rhabdo-myosarcom* or rhabdosarcom* or leiomyosarcoma* or leio-myosarcom* or androblastom* or arrhenoblastom* or adenoma* or lesion* or oncolo*)):ti,ab,kw (7371)
-
#6 #1 or #2 or #3 or #4 or #5 (7441)
-
#7 MeSH descriptor: [Peritoneal Neoplasms] this term only (213)
-
#8 (peritoneum or borderline or epithelial or primary peritoneal):ti,ab,kw (7882)
-
#9 #7 or #8 (8005)
-
#10 ovar*:ti,ab,kw (9974)
-
#11 #9 and #10 (1073)
-
#12 #6 or #11 (7490)
-
#13 ((risk near/4 malignan* near/4 index) or (risk near/4 malignan* near/4 indice*) or RMI):ti,ab,kw (53)
-
#14 (menopau* or perimenopaus* or premenopaus* or postmenopaus* or POF or climacteric or (change near/2 life)):ti,ab,kw (18,330)
-
#15 MeSH descriptor: [Menopause] explode all trees (6396)
-
#16 #14 or #15 (18,330)
-
#17 (ultraso* or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph* or doptone* or echograph* or echogram* or echosound*):ti,ab,kw (21,215)
-
#18 MeSH descriptor: [Ultrasonography] this term only (956)
-
#19 #17 or #18 (21,215)
-
#20 (CA125* or “CA 125*” or “CA 12-5*” or (antigen near/2 125) or (mucin near/1 16) or mucin16 or (muc near/1 16) or muc16):ti,ab,kw (473)
-
#21 MeSH descriptor: [CA-125 Antigen] this term only (157)
-
#22 #20 or #21 (473)
-
#23 #16 and #19 and #22 (21)
-
#24 #13 or #23 (73)
-
#25 (ROMA or (ovar* near/5 algor*)):ti,ab,kw (67)
-
#26 (“human epididymis protein 4” or “human epididymal protein 4” or “WAP four disulfide core domain protein 2” or “wap 4 disulfide core domain protein 2” or WFCD2 or EDDM4 or WAP5 or “wap four disulfide core domain 2” or “wap 4 disulfide core domain 2” or “HE 4” or HE4):ti,ab,kw (35)
-
#27 #16 and #22 and #26 (5)
-
#28 #25 or #27 (69)
-
#29 (IOTA or “international ovarian tumo?r analysis”):ti,ab,kw (22)
-
#30 ((simple near/3 rule*) or (simple near/3 descriptor*) or SRrisk or b-rule* or m-rule*):ti,ab,kw (44)
-
#31 #19 or #29 (21,234)
-
#32 #30 and #31 (2)
-
#33 (adnex* near/8 (model* or score* or assess*)):ti,ab,kw (17)
-
#34 (ova2 or overa):ti,ab,kw (4)
-
#35 MeSH descriptor: [Follicle Stimulating Hormone] this term only (1700)
-
#36 (“Follicle stimulat* hormone*” or FSH or follitropin or fertiline or “fertinom p” or follicotropin or “folliculostimulating hormone*” or follitrophin or follitropin* or folltropin* or 9002-68-0):ti,ab,kw (4010)
-
#37 #35 or #36 (4010)
-
#38 MeSH descriptor: [Apolipoprotein A-I] this term only (444)
-
#39 (“apolipoprotein A1” or “apo a1” or “apo hdl 3” or “apo hdl iii” or “apo high density lipoprotein 3” or “apolipoprotein a 1” or “apolipoprotein a I” or “apoprotein a1” or “apoprotein ai” or “apoprotein a 1” or “apoprotein a I”):ti,ab,kw (1334)
-
#40 #38 or #39 (1334)
-
#41 MeSH descriptor: [Transferrin] this term only (343)
-
#42 (transferrin or siderophilin or transferrin?emia or transferrins or trf or 82030-93-1):ti,ab,kw (1467)
-
#43 #41 or #42 (1467)
-
#44 #22 and #26 and #37 and #40 and #43 (0)
-
#45 #34 or #44 (4)
-
#46 #24 or #28 or #32 or #33 or #45 (155)
-
#47 #12 and #46 (44)
International Network of Agencies for Health Technology Assessment Publications (via the internet: www.inahta.org/publications/)
Date searched: 25 November 2016.
Records found: none.
Search strategy
(ovar* OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum) AND (RMI OR “risk of malignancy” OR ROMA OR “Ovarian Malignancy Algorithm” OR IOTA OR “International ovarian tumor analysis” OR “simple ultrasound rule” OR “simple rule” OR SRrisk OR b-rule OR m-rule OR Adnex OR OVA2 OR Overa OR HE4 OR “HE 4” OR epididy* OR “WAP 4” OR WAP4 OR “WAP four” OR WAP5 OR WFCD2 OR EDDM4 OR CA125 OR “CA 125” OR “CA 12-5” OR “antigen 125” OR “mucin 16” OR mucin16 OR “muc 16” OR muc16)
National Institute for Health Research Health Technology Assessment Journals Library (via the internet: www.journalslibrary.nihr.ac.uk/#/)
Date searched: 25 November 2016.
Records found: 23.
| Search terms | Journal reports, (n) | Research projects, (n) |
|---|---|---|
| ovarian | 12 | 44 |
| ovary | 0 | 5 |
| ovaries | 0 | 6 |
| fallopian | 0 | 7 |
| oviduct | 0 | 0 |
| Total | 12 | 62 |
| Total after removal of duplicates | 12 | 43 |
| Combined total | 55 | |
| Total after removal of irrelevant studies | 23 | |
Aggressive Research Intelligence Facility database (via the internet: www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx)
Date searched: 25 November 2016.
Records found: 25.
Advanced search.
All published libraries
| Search terms | Results |
|---|---|
|
Abstract: ovarian AND Abstract: rmi |
18 |
|
Abstract: ovarian AND Abstract: malignancy index |
2 |
|
Abstract: ovarian AND Abstract: malignancy indices |
0 |
|
Abstract: ovarian AND Abstract: ROMA |
3 |
|
Abstract: ovarian AND Abstract: malignancy algorithm |
0 |
|
Abstract: ovarian AND Abstract: IOTA |
1 |
| Abstract: International ovarian tumor analysis | 1 |
|
Abstract: ovarian AND Abstract: simple rule |
1 |
|
Abstract: SRrisk OR Abstract: b-rule OR Abstract: m-rule |
0 |
|
Abstract: ovarian AND Abstract: adnex |
4 |
|
Abstract: OVA2 OR Abstract: HE4 OR Abstract: human epididymis protein 4 OR Abstract: human epididymal protein 4 |
2 |
|
Abstract: WAP4 OR Abstract: WAP 4 OR Abstract: WAP four OR Abstract: WAP5 |
0 |
|
Abstract: CA125 OR Abstract: CA-125 OR Abstract: CA 12-5 OR Abstract: antigen 125 |
4 |
| Total | 36 |
| Total after removal of duplicates | 25 |
PROSPERO (via the internet: www.crd.york.ac.uk/prospero/)
Date searched: 25 November 2016.
Records found: four.
Search strategy
-
#1 MeSH DESCRIPTOR Ovarian Neoplasms EXPLODE ALL TREES (38)
-
#2 MeSH DESCRIPTOR Fallopian Tube Neoplasms EXPLODE ALL TREES (0)
-
#3 MeSH DESCRIPTOR Uterine Neoplasms EXPLODE ALL TREES (80)
-
#4 (ovar* or “high-grade serous” or “low-grade serous” or “sertoli-leydig cell” or fallopian or oviduct or uterine or uterus or tubal) near (cancer* or adenocarcin* or adeno-carcin* or tumor* or tumour* or sarcoma* or neoplas* or metasta* or meta-sta* or carcino* or oncogenesis or malignan* or choriocarcinom* or teratoma* or cystadenocarcin* or rhabdomyosarcom* or rhabdo-myosarcom* or rhabdosarcom* or leiomyosarcoma* or leio-myosarcom* or androblastom* or arrhenoblastom* or adenoma* or lesion* or oncolo*) (99)
-
#5 (AOSCa* or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA* or dysgerminom*) (9)
-
#6 #5 OR #4 OR #3 OR #2 OR #1 (160)
-
#7 MeSH DESCRIPTOR Peritoneal Neoplasms EXPLODE ALL TREES (4)
-
#8 peritoneum or borderline or epithelial or “primary peritoneal” (130)
-
#9 #7 OR #8 (134)
-
#10 ovar* (224)
-
#11 #9 AND #10 (24)
-
#12 #9 AND #10 (24)
-
#13 #6 OR #11 (164)
-
#14 (risk near malignan* near index) or (risk near malignan* near indice*) or RMI (7)
-
#15 menopau* or perimenopaus* or premenopaus* or postmenopaus* or POF or climacteric or (change near life) (372)
-
#16 MeSH DESCRIPTOR Menopause EXPLODE ALL TREES (30)
-
#17 #16 OR #15 (373)
-
#18 MeSH DESCRIPTOR Ultrasonography EXPLODE ALL TREES (98)
-
#19 (ultraso* or phonophoresis or sonication or sonification or “ultra sound” or ultrashell or sonograph* or doptone* or echograph* or echogram* or echosound*) (625)
-
#20 #19 OR #18 (653)
-
#21 MeSH DESCRIPTOR CA-125 Antigen EXPLODE ALL TREES (2)
-
#22 (CA125* or “CA 125*” or ca 12-5* or (antigen near “125”) or (mucin near “16”) or mucin16 or (muc near “16”) or muc16) (8)
-
#23 #22 OR #21 (9)
-
#24 #17 AND #20 AND #23 (4)
-
#25 #14 OR #24 (9)
-
#26 ROMA or (Ovar* near Algor*) (30)
-
#27 (“human epididymis protein 4” or “human epididymal protein 4” or “WAP four” or “wap 4” or wap4 or WFCD2 or EDDM4 or WAP5 or “HE 4” or HE4) (3)
-
#28 #17 AND #23 AND #27 (2)
-
#29 #26 OR #28 (32)
-
#30 IOTA or “international ovarian tumor analysis” or “international ovarian tumour analysis” (3)
-
#31 ((Simple near rules) or (simple near descriptors) or SRrisk or b-rules or m-rules) (3)
-
#32 #20 OR #30 (653)
-
#33 #32 AND #31 (2)
-
#34 (adnex* near (model* or score* or assess*)) (0)
-
#35 ova2 or overa (0)
-
#36 MeSH DESCRIPTOR Follicle Stimulating Hormone EXPLODE ALL TREES (2)
-
#37 (“follicle stimulat* hormone*” or FSH or follitropin or fertiline or “fertinom p” or follicotropin or “folliculostimulating hormone*” or follitrophin or follitropin* or folltropin*) (31)
-
#38 #37 OR #36 (32)
-
#39 MeSH DESCRIPTOR Apolipoprotein A-I EXPLODE ALL TREES (0)
-
#40 (“apolipoprotein A1” or “apo a1” or “apo hdl 3” or “apo hdl iii” or “apo high density lipoprotein 3” or “apolipoprotein a 1” or “apolipoprotein a i” or “apoprotein a1” or “apoprotein ai” or “apoprotein a 1” or “apoprotein a i”) (8)
-
#41 #40 OR #39 (8)
-
#42 MeSH DESCRIPTOR Transferrin EXPLODE ALL TREES (0)
-
#43 (transferrin or siderophilin or transferrinemia or transferrinaemia or transferrins) (21)
-
#44 #42 OR #43 (21)
-
#45 #23 AND #27 AND #38 AND #41 AND #44 (0)
-
#46 #35 OR #45 (0)
-
#47 #25 OR #29 OR #33 OR #34 OR #46 (40)
-
#48 #13 AND #47 (4)
ClinicalTrials.gov (via the internet: http://clinicaltrials.gov/ct2/search/advanced)
Date searched: 24 November 2016.
Records found: 269.
Expert search option.
Search strategy
(ovarian OR ovary OR ovaries OR “high-grade serous” OR “low-grade serous” OR “sertoli-leydig cell” OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum) AND (cancer OR adenocarcinoma OR tumor OR tumour OR sarcoma OR neoplasm OR neoplasia OR metastatic OR metastasis OR metastases OR carcinoma OR oncogenesis OR malignancy OR malignancies OR choriocarcinoma OR teratoma OR cystadenocarcinoma OR rhabdomyosarcoma OR rhabdosarcoma OR leiomyosarcoma OR androblastoma OR arrhenoblastoma OR adenoma OR lesion OR oncology OR oncologic) AND (RMI OR “risk of malignancy index” OR “risk of malignancy indices” OR ROMA OR “Risk of Ovarian Malignancy Algorithm” OR IOTA OR “International ovarian tumor analysis” OR “simple ultrasound rule” OR “simple rule” OR SRrisk OR b-rule OR m-rule OR Adnex OR OVA2 OR Overa OR HE4 OR “HE 4” OR “human epididymis protein 4” OR “human epididymal protein 4” OR “WAP 4” OR WAP4 OR “WAP four” OR WAP5 OR WFCD2 OR EDDM4 OR CA125 OR “CA 125” OR “CA 12-5” OR “antigen 125” OR “mucin 16” OR mucin16 OR “muc 16” OR muc16)European Union Clinical Trials Register (via the internet: www.clinicaltrialsregister.eu/ctr-search/search)
Date searched: 25 November 2016.
Records found: 122.
Search strategy
(ovarian OR ovary OR ovaries OR “high-grade serous” OR “low-grade serous” OR “sertoli-leydig cell” OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum) AND (cancer OR adenocarcinoma OR tumor OR tumour OR sarcoma OR neoplasm OR neoplasia OR metastatic OR metastasis OR metastases OR carcinoma OR oncogenesis OR malignancy OR malignancies OR choriocarcinoma OR teratoma OR cystadenocarcinoma OR rhabdomyosarcoma OR rhabdosarcoma OR leiomyosarcoma OR androblastoma OR arrhenoblastoma OR adenoma OR lesion OR oncology OR oncologic) AND (RMI OR “risk of malignancy index” OR “risk of malignancy indices” OR ROMA OR “Risk of Ovarian Malignancy Algorithm” OR IOTA OR “International ovarian tumor analysis” OR “simple ultrasound rule” OR “simple rule” OR SRrisk OR b-rule OR m-rule OR Adnex OR OVA2 OR Overa OR HE4 OR “HE 4” OR “human epididymis protein 4” OR “human epididymal protein 4” OR “WAP 4” OR WAP4 OR “WAP four” OR WAP5 OR WFCD2 OR EDDM4 OR CA125 OR “CA 125” OR “CA 12-5” OR “antigen 125” OR “mucin 16” OR mucin16 OR “muc 16” OR muc16)
World Health Organization International Clinical Trials Register Portfolio (via the internet: http://apps.who.int/trialsearch/)
Date searched: 24 November 2016.
Records found: 51.
| Advanced search option | Results |
|---|---|
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Intervention: risk of malignancy index |
(2 records for) 2 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Title: risk of malignancy index |
(5 records for) 5 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Intervention: Risk of Ovarian Malignancy Algorithm |
(0 records for) 0 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Title: Risk of Ovarian Malignancy Algorithm |
(1 records for) 1 trial found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Intervention: IOTA OR International ovarian tumor analysis OR simple ultrasound rule OR simple rule OR SRrisk OR b-rule OR m-rule |
(1 records for) 1 trial found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Title: IOTA OR International ovarian tumor analysis OR simple ultrasound rule” OR simple rule OR SRrisk OR b-rule OR m-rule |
(6 records for) 6 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Intervention: HE4 OR human epididymis protein 4 OR human epididymal protein 4 |
(4 records for) 4 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Title: HE4 OR human epididymis protein 4 OR human epididymal protein 4 |
(10 records for) 10 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Intervention: CA125 OR CA-125 |
(23 records for) 20 trials found |
|
Condition: ovarian OR ovary OR ovaries OR high-grade serous OR low-grade serous OR sertoli-leydig cell OR fallopian OR oviduct OR uterine OR uterus OR tubal OR peritoneal OR peritoneum Title: CA125 OR CA-125 |
(27 records for) 21 trials found |
| Standard search | Results |
| RMI AND ovarian | (4 records for) 4 trials found |
| ROMA AND ovarian | (2 records for) 2 trials found |
| adnex AND ovarian | (2 records for) 2 trials found |
| Total | 78 |
| Total after removal of duplicates | 51 |
Radiological Society of North America (via the Internet: www.rsna.org/Past_Meetings.aspx)
Plenary Sessions, Science Sessions.
Date searched: 2 February 2017.
Records found: 45.
Filters: Biomarkers/Quantitative Imaging; Obstetric/Gynecologic Radiology; Radiation Oncology; Genitourinary Radiology; Oncologic Imaging; Ultrasound.
2016
| Text words | Hits |
|---|---|
| Ovar* | 5 |
| Serous | 0/1 duplicate removed |
| Sertoli-leydig | 0 |
| Fallopian | 1 |
| Oviduct | 0 |
| Uterine | 10 |
| Uterus | 0 |
| tubal | 1 |
| Total | 17 |
2015
| Text words | Hits |
|---|---|
| Ovar* | 4 |
| Serous | 1/2 duplicates removed |
| Sertoli-leydig | 0 |
| Fallopian | 0 |
| Oviduct | 0 |
| Uterine | 7 |
| Uterus | 0 |
| tubal | 1 |
| Total | 6 |
2014
Filter: meeting program.
| Text words | Hits |
|---|---|
| Ovar* | 4 |
| Serous | 1/3 duplicates removed |
| Sertoli-leydig | 0 |
| Fallopian | 1 |
| Oviduct | 0 |
| Uterine | 16/17 duplicates removed |
| Uterus | 0 |
| Tub* | 0/2 duplicates removed |
| Total | 22 |
American Society of Clinical Oncology annual conference (via the internet http://meetinglibrary.asco.org/abstracts)
Date searched: 2 February 2017.
Records found: 603.
2016
| Text words | Hits |
|---|---|
| (ovar* OR fallopian OR uter* OR tubal) (diagnos* OR predict* OR sensitiv* OR specific* OR likel* OR accura*) | 217 |
2015
| Text words | Hits |
|---|---|
| (ovar* OR fallopian OR uter* OR tubal) (diagnos* OR predict* OR sensitiv* OR specific* OR likel* OR accura*) | 210 |
2014
| Text words | Hits |
|---|---|
| (ovar* OR fallopian OR uter* OR tubal) (diagnos* OR predict* OR sensitiv* OR specific* OR likel* OR accura*) | 176 |
Society of Gynecologic Oncology (via the internet)
Date searched: 2 February 2017.
Records found: 108.
2016 (www.sgo.org/2016-annual-meeting-archives/)
| Text words in title/abstract (abstracts scanned for ovarian or uterine or gynaecologic cancer) | Hits |
|---|---|
| Risk of malignancy | 2 |
| RMI | 0 |
| Ultrasound | 5 |
| Ultra sound | 0 |
| CA125 | 1 |
| CA 125 | 22/25 duplicates removed |
| ROMA | 0 |
| HE4 | 0/2 duplicates removed |
| Human epididymis protein 4 | 0/1 duplicate removed |
| Iota | 0/1 duplicate removed |
| International ovarian | 0 |
| Simple rules | 0 |
| SRrisk | 0 |
| Ova2 | 0 |
| Overa | 0 |
| Adnex | 0/1 duplicate removed |
| Total | 30 |
2015 (www.gynecologiconcology-online.net/issue/S0090–8258(15)X0005–9)
| Text words | Hits |
|---|---|
| “Risk of malignancy” | 5 |
| RMI | 0 |
| Ultrasound | 14 |
| “Ultra sound” | 0 |
| CA125 | 29/30 duplicates removed |
| “CA 125” | 0/30 duplicates removed |
| ROMA | 0/1 duplicates removed |
| HE4 | 1/3 duplicates removed |
| Human epididymis protein 4 | 0/2 duplicates removed |
| Iota | 0 |
| International ovarian | 0 |
| Simple rules | 0 |
| SRrisk | 0 |
| Ova2 | 0 |
| Overa | 0 |
| Adnex | 0 |
| Total | 49 |
2014 (www.sgo.org/wp-content/uploads/2014/07/YGYNO_133_S1_compressed.pdf)
| Text words in title/abstract (abstracts scanned for ovarian or uterine or gynaecologic cancer) | Hits |
|---|---|
| Risk of malignancy | 3 |
| RMI | 0/3 duplicates removed |
| Ultrasound | 2/4 duplicates removed |
| Ultra sound | 0 |
| CA125 | 0 |
| CA 125 | 22/25 duplicates removed |
| ROMA | 0 |
| HE4 | 2/4 duplicates removed |
| Human epididymis protein 4 | 0 |
| Iota | 0 |
| International ovarian | 0 |
| Simple rules | 0 |
| SRrisk | 0 |
| Ova2 | 0 |
| Overa | 0 |
| Adnex | 0 |
| Total | 29 |
The National Cancer Research Institute (via the internet)
Date searched: 2 February 2017.
Records found: 132.
2016 (http://abstracts.ncri.org.uk/year_published/2016/)
| Text words: abstracts scanned for ovarian/uterine/gynaecological cancer | Hits |
|---|---|
| Ovar* | 25 |
2015 (http://abstracts.ncri.org.uk/year_published/2015/)
| Text words: abstracts scanned for ovarian/uterine/gynaecological cancer | Hits |
|---|---|
| Ovar* | 61 |
2014 (http://abstracts.ncri.org.uk/year_published/2014/)
| Text words: abstracts scanned for ovarian/uterine/gynaecological cancer | Hits |
|---|---|
| Ovar* | 46 |
European Society of Radiology
Date searched: 2 February 2017.
Records found: 25.
2016: Scientific sessions and clinical trials in radiology (www.myesr.org/congress/about-ecr/past-congresses/ecr-2016)
| Text words | Hits |
|---|---|
| Ovar* | 5 |
2015: scientific sessions and late-breaking clinical trials (www.myesr.org/congress/about-ecr/past-congresses/ecr-2015)
| Text words | Hits |
|---|---|
| Ovar* | 13 |
2014: scientific sessions (www.myesr.org/congress/about-ecr/past-congresses/ecr-2014)
| Text words | Hits |
|---|---|
| Ovar* | 7 |
Cost-effectiveness searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 2 November 2016.
Date searched: 23 November 2016.
Records found: 370.
Search strategy
-
exp Ovarian Neoplasms/ (79,388)
-
Fallopian Tube Neoplasms/ (2721)
-
Uterine Neoplasms/ (40,416)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (6079)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (104,167)
-
or/1-5 (151,338)
-
Peritoneal Neoplasms/ (13,578)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (337,128)
-
or/7-8 (347,883)
-
ovar$.ti,ab,ot. (224,941)
-
9 and 10 (23,562)
-
6 or 11 (155,417)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. (315,645)
-
Ultrasonography/ (67,487)
-
13 or 14 (333,646)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (7726)
-
CA-125 Antigen/ (4329)
-
16 or 17 (8480)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (467)
-
Biomarkers, Tumor/ (118,213)
-
(Tumo?r marker$ or biomarker$ or bio-marker$ or cancer marker$ or neoplasm marker$).ti,ab,ot. (159,924)
-
20 or 21 (246,574)
-
15 or 18 or 19 or 22 (580,167)
-
12 and 23 (20,146)
-
economics/ (28,593)
-
exp “costs and cost analysis”/ (216,876)
-
economics, dental/ (1917)
-
exp “economics, hospital”/ (23,025)
-
economics, medical/ (9388)
-
economics, nursing/ (4000)
-
economics, pharmaceutical/ (2804)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (541,112)
-
(expenditure$ not energy).ti,ab. (21,940)
-
(value adj1 money).ti,ab. (29)
-
budget$.ti,ab. (20,740)
-
or/25-35 (682,801)
-
((energy or oxygen) adj cost).ti,ab. (3151)
-
(metabolic adj cost).ti,ab. (1034)
-
((energy or oxygen) adj expenditure).ti,ab. (20,576)
-
or/37-39 (23,929)
-
36 not 40 (677,656)
-
letter.pt. (943,994)
-
editorial.pt. (416,892)
-
historical article.pt. (507,294)
-
or/42-44 (1,844,260)
-
41 not 45 (644,021)
-
24 and 46 (370)
Economics terms based on costs filter. 157
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), MEDLINE Daily Update (via Ovid) and MEDLINE Epub Ahead of Print (via Ovid)
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid): to 22 November 2016.
MEDLINE Daily Update (via Ovid): to 22 November 2016.
MEDLINE Epub Ahead of Print (via Ovid): to 23 November 2016.
Date searched: 24 November 2016.
Records found: 31.
Search strategy
-
exp Ovarian Neoplasms/ (0)
-
Fallopian Tube Neoplasms/ (0)
-
Uterine Neoplasms/ (0)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (904)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (9696)
-
or/1-5 (9801)
-
Peritoneal Neoplasms/ (0)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (25,858)
-
or/7-8 (25,858)
-
ovar$.ti,ab,ot. (18,312)
-
9 and 10 (2204)
-
6 or 11 (10,115)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. (41,009)
-
Ultrasonography/ (0)
-
13 or 14 (41,009)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (849)
-
CA-125 Antigen/ (0)
-
16 or 17 (849)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (185)
-
Biomarkers, Tumor/ (0)
-
(Tumo?r marker$ or biomarker$ or bio-marker$ or cancer marker$ or neoplasm marker$).ti,ab,ot. (32,099)
-
20 or 21 (32,099)
-
15 or 18 or 19 or 22 (73,106)
-
12 and 23 (1440)
-
economics/ (0)
-
exp “costs and cost analysis”/ (1)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (0)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (0)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (91,939)
-
(expenditure$ not energy).ti,ab. (2877)
-
(value adj1 money).ti,ab. (5)
-
budget$.ti,ab. (3723)
-
or/25-35 (95,781)
-
((energy or oxygen) adj cost).ti,ab. (467)
-
(metabolic adj cost).ti,ab. (159)
-
((energy or oxygen) adj expenditure).ti,ab. (2166)
-
or/37-39 (2715)
-
36 not 40 (95,025)
-
letter.pt. (38,204)
-
editorial.pt. (27,650)
-
historical article.pt. (0)
-
or/42-44 (65,854)
-
41 not 45 (94,317)
-
24 and 46 (31)
Economics terms based on costs filter. 157
EMBASE (via Ovid)
Date range searched: 1974 to 23 November 2016.
Date searched: 24 November 2016.
Records found: 665.
Search strategy
-
exp ovary cancer/ (97,370)
-
uterine tube tumor/ (1263)
-
uterine tube carcinoma/ (1899)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (9245)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (136,192)
-
peritoneum cancer/ (3891)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (405,888)
-
or/6-7 (408,204)
-
ovar$.ti,ab,ot. (278,995)
-
8 and 9 (30,110)
-
1 or 2 or 3 or 4 or 5 or 10 (168,296)
-
(Ultraso$ or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph$ or doptone$ or echograph$ or echogram$ or echosound$).ti,ab,ot. or ultrasound/ or sonography/ (591,945)
-
(CA125$ or CA 125$ or ca 12-5$ or (antigen adj2 “125”) or (mucin adj1 “16”) or mucin16 or (muc adj1 “16”) or muc16).ti,ab,ot. (11,962)
-
CA 125 antigen/ (13,650)
-
13 or 14 (16,956)
-
(human epididymis protein 4 or human epididymal protein 4 or WAP four disulfide core domain protein 2 or wap 4 disulfide core domain protein 2 or WFCD2 or EDDM4 or WAP5 or wap four disulfide core domain 2 or wap 4 disulfide core domain 2 or HE 4 or HE4).ti,ab,ot. (956)
-
human epididymis protein 4/ (507)
-
or/16-17 (1036)
-
tumor marker/ (62,368)
-
(Tumo?r marker$ or biomarker$ or bio-marker$ or cancer marker$ or neoplasm marker$).ti,ab,ot. (260,389)
-
19 or 20 (294,218)
-
12 or 15 or 18 or 21 (887,954)
-
11 and 22 (25,903)
-
health-economics/ (37,185)
-
exp economic-evaluation/ (262,667)
-
exp health-care-cost/ (249,052)
-
exp pharmacoeconomics/ (184,843)
-
or/24-27 (566,720)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (778,313)
-
(expenditure$ not energy).ti,ab. (30,211)
-
(value adj2 money).ti,ab. (1844)
-
budget$.ti,ab. (29,697)
-
or/29-32 (806,888)
-
28 or 33 (1,107,372)
-
letter.pt. (963,779)
-
editorial.pt. (523,590)
-
note.pt. (663,117)
-
or/35-37 (2,150,486)
-
34 not 38 (1,007,210)
-
(metabolic adj cost).ti,ab. (1124)
-
((energy or oxygen) adj cost).ti,ab. (3573)
-
((energy or oxygen) adj expenditure).ti,ab. (25,235)
-
or/40-42 (29,019)
-
39 not 43 (1,001,250)
-
exp animal/ (22,704,681)
-
exp animal-experiment/ (2,060,346)
-
nonhuman/ (4,993,357)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,418,904)
-
or/45-48 (24,242,905)
-
exp human/ (18,234,517)
-
exp human-experiment/ (393,716)
-
50 or 51 (18,236,045)
-
49 not (49 and 52) (6,007,829)
-
44 not 53 (926,312)
-
23 and 54 (665)
Economics terms based on costs filter. 158
NHS Economic Evaluation Database (via Wiley Online Library)
Issue 2 of 4, April 2015.
Date searched: 24 November 2016.
Records found: 11.
Search strategy
-
#1 MeSH descriptor: [Ovarian Neoplasms] explode all trees (1511)
-
#2 MeSH descriptor: [Fallopian Tube Neoplasms] this term only (45)
-
#3 MeSH descriptor: [Uterine Neoplasms] this term only (691)
-
#4 (AOSCa* or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA* or dysgerminom*):ti,ab,kw (231)
-
#5 ((ovar* or “high-grade serous” or “low-grade serous” or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) near/5 (cancer* or adenocarcin* or adeno-carcin* or tumo?r* or sarcoma* or neoplas* or metasta* or meta-sta* or carcino* or oncogenesis or malignan* or choriocarcinom* or teratoma* or cystadenocarcin* or rhabdomyosarcom* or rhabdo-myosarcom* or rhabdosarcom* or leiomyosarcoma* or leio-myosarcom* or androblastom* or arrhenoblastom* or adenoma* or lesion* or oncolo*)):ti,ab,kw (7371)
-
#6 #1 or #2 or #3 or #4 or #5 (7441)
-
#7 MeSH descriptor: [Peritoneal Neoplasms] this term only (213)
-
#8 (peritoneum or borderline or epithelial or primary peritoneal):ti,ab,kw (7882)
-
#9 #7 or #8 (8005)
-
#10 ovar*:ti,ab,kw (9974)
-
#11 #9 and #10 (1073)
-
#12 #6 or #11 (7490)
-
#13 (ultraso* or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph* or doptone* or echograph* or echogram* or echosound*):ti,ab,kw (21,215)
-
#14 MeSH descriptor: [Ultrasonography] this term only (956)
-
#15 #13 or #14 (21,215)
-
#16 (CA125* or “CA 125*” or “CA 12-5*” or (antigen near/2 125) or (mucin near/1 16) or mucin16 or (muc near/1 16) or muc16):ti,ab,kw (473)
-
#17 MeSH descriptor: [CA-125 Antigen] this term only (157)
-
#18 #16 or #17 (473)
-
#19 (“human epididymis protein 4” or “human epididymal protein 4” or “WAP four disulfide core domain protein 2” or “wap 4 disulfide core domain protein 2” or WFCD2 or EDDM4 or WAP5 or “wap four disulfide core domain 2” or “wap 4 disulfide core domain 2” or “HE 4” or HE4):ti,ab,kw (35)
-
#20 MeSH descriptor: [Biomarkers, Tumor] this term only(1866)
-
#21 (“tumo?r marker*” or biomarker* or bio-marker* or “cancer marker*” or “neoplasm marker*”):ti,ab,kw (19,067)
-
#22 #20 or #21 (19,071)
-
#23 #15 or #18 or #19 or #22 (40,201)
-
#24 #12 and #23 (708)
EconLit (via EBSCOhost)
Date range searched: 1966 to 25 November 2016.
Date searched: 25 November 2016.
Records found: 1.
Search strategy
| S10 | S4 AND S9 | 1 |
|---|---|---|
| S9 | S5 OR S6 OR S7 OR S8 | 144 |
| S8 | ((tumo?r N3 marker*) or biomarker* or bio-marker* or (cancer N3 marker*) or (neoplas* N3 marker*)) | 92 |
| S7 | (“human epididymis protein 4” or “human epididymal protein 4” or “WAP four disulfide core domain protein 2” or “wap 4 disulfide core domain protein 2” or WFCD2 or EDDM4 or WAP5 or “wap four disulfide core domain 2” or “wap 4 disulfide core domain 2” or “HE 4” or HE4) | 0 |
| S6 | (CA125* or CA 125* or ca 12-5* or (antigen N2 “125”) or (mucin N1 “16”) or mucin16 or (muc N1 “16”) or muc16) | 4 |
| S5 | (ultraso* or phonophoresis or sonication or sonification or ultra sound or ultrashell or sonograph* or doptone* or echograph* or echogram* or echosound*) | 48 |
| S4 | S1 or S2 or S3 | 32 |
| S3 | (peritoneum or epithelial or primary peritoneal) | 2 |
| S2 | (AOSCa* or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA* or dysgerminom*) | 10 |
| S1 | (ovar* or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) N5 (Cancer* or adenocarcin* or adeno-carcin* or tumo?r* or sarcoma* or neoplas* or metasta* or meta-sta* or carcino* or oncogenesis or malignan* or choriocarcinom* or teratoma* or cystadenocarcin* or rhabdomyosarcom* or rhabdo-myosarcom* or rhabdosarcom* or leiomyosarcoma* or leio-myosarcom* or androblastom* or arrhenoblastom* or adenoma* or lesion* or oncolo*) | 21 |
Cost-Effectiveness Analysis Registry (via the internet: www.cearegistry.org)
Date searched: 25 November 2016.
Records found: two.
Search strategy
| Search terms | Results |
|---|---|
| CA125 | 0 |
| CA-125 | 1 |
| CA 125 | 1 |
| Antigen 125 | 1 |
| Mucin 16 | 0 |
| Mucin16 | 0 |
| epidiymis | 0 |
| epididymal | 0 |
| HE4 | 0 |
| HE-4 | 0 |
| WAP 4 | 0 |
| WAP4 | 0 |
| WAP four | 0 |
| WAP5 | 0 |
| EDDM4 | 0 |
| WFCD2 | 0 |
| Ovarian ultrasound | 0 |
| Ovarian ultrasonography | 0 |
| Ovarian biomarker | 0 |
| Ovarian biomarkers | 0 |
| tumor marker | 0 |
| tumour marker | 0 |
| tumor markers | 0 |
| tumour markers | 0 |
| Total | 3 |
| Total after removal of duplicates | 2 |
Research Papers in Economics (via the internet: http://repec.org/)
Date searched: 25 November 2016.
Records found: six.
IDEAS search interface
Search strategy
(ovarian | ovary | ovaries | “high-grade serous” | “low-grade serous | sertoli-leydig cell” | fallopian | oviduct | uterine |uterus | tubal | peritoneum | borderline | epithelial | “primary peritoneal” | AOSCa | HGSC | EOC | HGSOC | LGSC | LGSOC | OVCA | dysgerminoma) + (ultrasound | phonophoresis | sonication | sonification | “ultra sound” | ultrashell | sonograph | doptone | echograph | echogram | echosound)
Records retrieved: one.
Search strategy
(ovarian | ovary | ovaries | “high-grade serous” | “low-grade serous | sertoli-leydig cell” | fallopian | oviduct | uterine |uterus | tubal | peritoneum | borderline | epithelial | “primary peritoneal” | AOSCa | HGSC | EOC | HGSOC | LGSC | LGSOC | OVCA | dysgerminoma) + (CA125 | “CA 125” | “CA 12-5” | “antigen 125” | “mucin 16” | mucin16 | “muc 16” | muc16)
Records retrieved: three.
Search strategy
(ovarian | ovary | ovaries | “high-grade serous” | “low-grade serous | sertoli-leydig cell” | fallopian | oviduct | uterine |uterus | tubal | peritoneum | borderline | epithelial | “primary peritoneal” | AOSCa | HGSC | EOC | HGSOC | LGSC | LGSOC | OVCA | dysgerminoma) + (“human epididymis” | “human epididymal” | WAP4 | “WAP 4” | “WAP four” | WFCD2 | EDDM4 | WAP5 | “HE 4” | HE4)
Records retrieved: none.
Search strategy
(ovarian | ovary | ovaries | “high-grade serous” | “low-grade serous | sertoli-leydig cell” | fallopian | oviduct | uterine |uterus | tubal | peritoneum | borderline | epithelial | “primary peritoneal” | AOSCa | HGSC | EOC | HGSOC | LGSC | LGSOC | OVCA | dysgerminoma) + (“tumor marker” | “tumor markers” | “tumour marker” | “tumour markers” | biomarker | biomarkers | bio-marker | bio-markers | “cancer marker” | “cancer markers | “neoplasm marker” | “neoplasm markers”)
Records retrieved: three.
Records retrieved in total: seven.
Records retrieved after duplicates: six.
Key
| OR
+ AND
“...” phrase search
Focused outcomes searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 January 2017.
Date searched: 31 January 2017.
Records found: 205.
Search strategy
-
Specialization/ (22,763)
-
Surgical Oncology/ (9)
-
((medical or surg$ or gyn?ecolog$ or physician$) adj1 (speciali$ or oncolog$)).ti,ab,ot. (18,188)
-
((special$ or tertiary) adj5 (hospital$ or care$ or healthcare or centre$ or center$ or facility or facilities)).ti,ab,ot. (102,854)
-
(central$ adj5 (hospital$ or care$ or healthcare$ or facility or facilities)).ti,ab,ot. (11,444)
-
exp Tertiary Healthcare/ (601)
-
or/1-6 (148,856)
-
((general$ or obstetric$ or secondary or regular) adj1 (care or healthcare or surg$ or gyn?ecolog$)).ti,ab,ot. (30,791)
-
exp Secondary Care/ (274)
-
or/8-9 (30,863)
-
7 and 10 (3252)
-
exp Gynecologic Surgical Procedures/ (74,324)
-
(gyn?ecolog$ adj2 surger$).ti,ab,ot. (5371)
-
or/12-13 (77,049)
-
exp Ovarian Neoplasms/ (73,422)
-
Fallopian Tube Neoplasms/ (2583)
-
Uterine Neoplasms/ (38,852)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (5388)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (96,213)
-
or/15-19 (141,283)
-
Peritoneal Neoplasms/ (13,029)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (298,409)
-
or/21-22 (308,780)
-
ovar$.ti,ab,ot. (208,481)
-
23 and 24 (21,354)
-
20 or 25 (145,013)
-
exp Cystadenoma/ (5907)
-
(cystadenoma$ or cystoma$ or cyst$ adenoma$).ti,ab,ot. (5325)
-
Fibroma/ (11,012)
-
(Fibroma$ or acrochordon$ or fibroepithelial or fibrous tumo?r$).ti,ab,ot. (12,675)
-
exp Teratoma/ (19,759)
-
(teratoma$ or dermoid$ or dentigerous cyst$ or dysembryoplastic anomal$ or goiter$ or goitre$ or struma$ or sacrococcygeal fistle$ or teratodermoid cyst$ or teratoid tumo?r$).ti,ab,ot. (34,781)
-
or/27-32 (68,835)
-
Pelvis/ (20,123)
-
exp Adnexa Uteri/ (96,410)
-
(pelvi$ or ovar$ or adnexa$).ti,ab,ot. (312,267)
-
or/34-36 (357,008)
-
33 and 37 (8928)
-
((pelvi$ or adnexa$ or ovar$) adj6 (mass or masses)).ti,ab,ot. (7720)
-
14 or 26 or 38 or 39 (213,422)
-
11 and 40 (205)
MEDLINE Epub Ahead of Print (via Ovid), MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE Daily Update
MEDLINE Epub Ahead of Print (via Ovid): to 30 January 2017.
MEDLINE In-Process & Other Non-Indexed Citations: to 30 January 2017.
MEDLINE Daily Update: to 30 January 2017.
Date searched: 31 January 2017.
Records found: 29.
Search strategy
-
Specialization/ (12)
-
Surgical Oncology/ (2)
-
((medical or surg$ or gyn?ecolog$ or physician$) adj1 (speciali$ or oncolog$)).ti,ab,ot. (3288)
-
((special$ or tertiary) adj5 (hospital$ or care$ or healthcare or centre$ or center$ or facility or facilities)).ti,ab,ot. (20,479)
-
(central$ adj5 (hospital$ or care$ or healthcare$ or facility or facilities)).ti,ab,ot. (1681)
-
exp Tertiary Healthcare/ (4)
-
or/1-6 (24,851)
-
((general$ or obstetric$ or secondary or regular) adj1 (care or healthcare or surg$ or gyn?ecolog$)).ti,ab,ot. (4328)
-
exp Secondary Care/ (2)
-
or/8-9 (4328)
-
7 and 10 (484)
-
exp Gynecologic Surgical Procedures/ (87)
-
(gyn?ecolog$ adj2 surger$).ti,ab,ot. (683)
-
or/12-13 (766)
-
exp Ovarian Neoplasms/ (331)
-
Fallopian Tube Neoplasms/ (5)
-
Uterine Neoplasms/ (57)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (1232)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (12,027)
-
or/15-19 (12,208)
-
Peritoneal Neoplasms/ (41)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (28,926)
-
or/21-22 (28,956)
-
ovar$.ti,ab,ot. (20,892)
-
23 and 24 (2883)
-
20 or 25 (12,554)
-
exp Cystadenoma/ (7)
-
(cystadenoma$ or cystoma$ or cyst$ adenoma$).ti,ab,ot. (486)
-
Fibroma/ (5)
-
(Fibroma$ or acrochordon$ or fibroepithelial or fibrous tumo?r$).ti,ab,ot. (1688)
-
exp Teratoma/ (31)
-
(teratoma$ or dermoid$ or dentigerous cyst$ or dysembryoplastic anomal$ or goiter$ or goitre$ or struma$ or sacrococcygeal fistle$ or teratodermoid cyst$ or teratoid tumo?r$).ti,ab,ot. (3571)
-
or/27-32 (5709)
-
Pelvis/ (13)
-
exp Adnexa Uteri/ (82)
-
(pelvi$ or ovar$ or adnexa$).ti,ab,ot. (33,945)
-
or/34-36 (33,961)
-
33 and 37 (792)
-
((pelvi$ or adnexa$ or ovar$) adj6 (mass or masses)).ti,ab,ot. (1272)
-
14 or 26 or 38 or 39 (14,145)
-
11 and 40 (29)
EMBASE (via Ovid)
Date range searched: 1974 to 30 January 2017.
Date searched: 31 January 2017.
Records found: 524.
Search strategy
-
medical specialist/ (102,067)
-
((medical or surg$ or gyn?ecolog$ or physician$) adj1 (speciali$ or oncolog$)).ti,ab,ot. (40,274)
-
((special$ or tertiary) adj5 (hospital$ or care$ or healthcare or centre$ or center$ or facility or facilities)).ti,ab,ot. (189,219)
-
(central$ adj5 (hospital$ or care$ or healthcare$ or facility or facilities)).ti,ab,ot. (18,866)
-
exp tertiary healthcare/ (71,024)
-
or/1-5 (326,375)
-
((general$ or obstetric$ or secondary or regular) adj1 (care or healthcare or surg$ or gyn?ecolog$)).ti,ab,ot. (47054)
-
exp secondary healthcare/ (4685)
-
or/7-8 (48,456)
-
6 and 9 (7200)
-
(gyn?ecolog$ adj2 surger$).ti,ab,ot. (8507)
-
exp gynecologic surgery/ (132,958)
-
or/11-12 (135,722)
-
exp ovary cancer/ (99,193)
-
uterine tube tumor/ (1280)
-
uterine tube carcinoma/ (1938)
-
(AOSCa$ or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA$ or dysgerminom$).ti,ab,ot. (9541)
-
((ovar$ or high-grade serous or low-grade serous or sertoli-leydig cell or fallopian or oviduct or uterine or uterus or tubal) adj5 (Cancer$ or adenocarcin$ or adeno-carcin$ or tumo?r$ or sarcoma$ or neoplas$ or metasta$ or meta-sta$ or carcino$ or oncogenesis or malignan$ or choriocarcinom$ or teratoma$ or cystadenocarcin$ or rhabdomyosarcom$ or rhabdo-myosarcom$ or rhabdosarcom$ or leiomyosarcoma$ or leio-myosarcom$ or androblastom$ or arrhenoblastom$ or adenoma$ or lesion$ or oncolo$)).ti,ab,ot. (138,404)
-
exp peritoneum cancer/ (13,274)
-
(peritoneum or borderline or epithelial or primary peritoneal).ti,ab,ot. (412,063)
-
or/19-20 (422,066)
-
ovar$.ti,ab,ot. (283,143)
-
21 and 22 (31,658)
-
14 or 15 or 16 or 17 or 18 or 23 (171,059)
-
cystadenoma/ (7719)
-
(cystadenoma$ or cystoma$ or cyst$ adenoma$).ti,ab,ot. (7325)
-
fibroma/ (11,802)
-
(Fibroma$ or acrochordon$ or fibroepithelial or fibrous tumo?r$).ti,ab,ot. (16,228)
-
teratoma/ (25,747)
-
ovary teratoma/ (2771)
-
(teratoma$ or dermoid$ or dentigerous cyst$ or dysembryoplastic anomal$ or goiter$ or goitre$ or struma$ or sacrococcygeal fistle$ or teratodermoid cyst$ or teratoid tumo?r$).ti,ab,ot. (43,888)
-
or/25-30 (58,645)
-
pelvis/ (67,918)
-
ovary/ (67,924)
-
(pelvi$ or ovar$ or adnexa$).ti,ab,ot. (445,224)
-
or/33-35 (462,883)
-
32 and 36 (10,871)
-
((pelvi$ or adnexa$ or ovar$) adj6 (mass or masses)).ti,ab,ot. (12,936)
-
13 or 24 or 37 or 38 (295,584)
-
10 and 39 (524)
Cochrane Database of Systematic Reviews (via Wiley Online Library), Database of Abstracts of Reviews of Effects (via Wiley Online Library), Cochrane Central Register of Controlled Trials (via Wiley Online Library), Health Technology Assessment Database (via Wiley Online Library) and NHS Economic Evaluation Database (via Wiley Online Library)
Cochrane Database of Systematic Reviews (via Wiley Online Library): Issue 1, January 2017.
Database of Abstracts of Reviews of Effects (via Wiley Online Library): Issue 2, April 2015.
Cochrane Central Register of Controlled Trials (via Wiley Online Library): Issue 11, November 2016.
Health Technology Assessment Database (via Wiley Online Library): Issue 4 of 4, October 2016.
NHS Economic Evaluation Database (via Wiley Online Library): Issue 2 of 4, April 2015.
Date searched: 31 January 2017.
Records found: 23.
Cochrane Database of Systematic Reviews: six.
Database of Abstracts of Reviews of Effects: none.
Cochrane Central Register of Controlled Trials: 17.
NHS Economic Evaluation Database: none.
Health Technology Assessment Database: none.
Search strategy
-
#1 MeSH descriptor: [Specialization] explode all trees (107)
-
#2 ((medical or surg* or gynaecolog* or gynecolog* or physician*) near/1 (speciali* or oncolog*)):ti,ab,kw (2745)
-
#3 ((special* or tertiary) near/5 (hospital* or care* or healthcare or centre* or center* or facility or facilities)):ti,ab,kw (8821)
-
#4 (central* near/5 (hospital* or care* or healthcare* or facility or facilities)):ti,ab,kw (1006)
-
#5 MeSH descriptor: [Tertiary Healthcare] explode all trees (7)
-
#6 #1 or #2 or #3 or #4 or #5 (12,086)
-
#7 ((general* or obstetric* or secondary or regular) near/1 (care or healthcare or surg* or gynaecolog* or gynecolog*)):ti,ab,kw (3877)
-
#8 MeSH descriptor: [Secondary Care] explode all trees (22)
-
#9 #7 or #8 (3877)
-
#10 #6 and #9 (306)
-
#11 MeSH descriptor: [Gynecologic Surgical Procedures] explode all trees (4254)
-
#12 ((gynaecolog* or gynecolog*) near/2 surger*):ti,ab,kw (1907)
-
#13 #11 or #12 (5608)
-
#14 MeSH descriptor: [Ovarian Neoplasms] explode all trees (1513)
-
#15 MeSH descriptor: [Fallopian Tube Neoplasms] explode all trees (45)
-
#16 MeSH descriptor: [Uterine Neoplasms] explode all trees (3024)
-
#17 (AOSCa* or HGSC or EOC or HGSOC or LGSC or LGSOC or OVCA* or dysgerminom*):ti,ab,kw (235)
-
#18 ((ovar* or high-grade-serous or low-grade-serous or sertoli-leydig-cell or fallopian or oviduct or uterine or uterus or tubal) near/5 (Cancer* or adenocarcin* or adeno-carcin* or tumor* or tumour* or sarcoma* or neoplas* or metasta* or meta-sta* or carcino* or oncogenesis or malignan* or choriocarcinom* or teratoma* or cystadenocarcin* or rhabdomyosarcom* or rhabdo-myosarcom* or rhabdosarcom* or leiomyosarcoma* or leio-myosarcom* or androblastom* or arrhenoblastom* or adenoma* or lesion* or oncolo*)):ti,ab,kw (7517)
-
#19 #14 or #15 or #16 or #17 or #18 (7909)
-
#20 MeSH descriptor: [Peritoneal Neoplasms] explode all trees (213)
-
#21 (peritoneum or borderline or epithelial or primary peritoneal):ti,ab,kw (7962)
-
#22 #20 or #21 (8085)
-
#23 ovar*:ti,ab,kw (10,032)
-
#24 #22 and #23 (1080)
-
#25 #19 or #24 (7947)
-
#26 MeSH descriptor: [Cystadenoma] explode all trees (4)
-
#27 (cystadenoma* or cystoma* or cyst* adenoma*):ti,ab,kw (101)
-
#28 MeSH descriptor: [Fibroma] explode all trees (8)
-
#29 (Fibroma* or acrochordon* or fibroepithelial or fibrous-tumour* or fibrous-tumor*):ti,ab,kw (57)
-
#30 MeSH descriptor: [Teratoma] explode all trees (29)
-
#31 (teratoma* or dermoid* or dentigerous-cyst* or dysembryoplastic-anomal* or goiter* or goitre* or struma* or sacrococcygeal-fistle* or teratodermoid-cyst* or teratoid-tumour* or teratoid-tumor*):ti,ab,kw (580)
-
#32 #26 or #27 or #28 or #29 or #30 or #31 (728)
-
#33 MeSH descriptor: [Pelvis] explode all trees (815)
-
#34 MeSH descriptor: [Adnexa Uteri] explode all trees (1277)
-
#35 (pelvi* or ovar* or adnexa*):ti,ab,kw (17,164)
-
#36 #33 or #34 or #35 (17,379)
-
#37 #32 and #36 (55)
-
#38 ((pelvi* or adnexa* or ovar*) near/6 (mass or masses)):ti,ab,kw (268)
-
#39 #13 or #25 or #37 or #38 (12,832)
-
#40 #10 and #39 (23)
Appendix 2 Excluded studies
To be included in the review, studies had to fulfil the following criteria:
-
population: people of any age with suspected ovarian cancer
-
setting: secondary care
-
index test: ROMA score, simple ultrasound rules (IOTA group), ADNEX model (IOTA group), Overa (MIA2G), RMI 1 (using decision thresholds other than 250)
-
reference standard: histological examination of a surgically resected of biopsy sample; studies that used follow-up as the reference standard for some or all test negative patients were also eligible for inclusion
-
outcome: sufficient data to construct 2 × 2 table of test performance or clinical outcomes.
The following table summarises the studies that were screened for inclusion based on full-text publication, but did not fulfil one or more of the above criteria. Studies were assessed sequentially against the criteria; the first criterion failed is classified as the reason for exclusion. The table shows which of the criteria each study fulfilled (‘Yes’) and on which items it failed (‘No’), as well as any that were ‘Unclear’. Articles that did not report primary research were not assessed further. Any criteria that are not applicable to a study are marked as NA.
Details of excluded studies with rationale for exclusion
| Study | Criteria | Reason for exclusion | |||||
|---|---|---|---|---|---|---|---|
| Primary study | Population | Setting | Index test | Reference standard | Outcome | ||
| Abbott Laboratories (Singapore). Evaluation of HE4 and CA125 Serum Markers to Improve the Risk Determination of Ovarian Cancer in Malaysian women. In WHO International Clinical Trials Registry Platform (ICTRP) [Internet]. Geneva: World Health Organization; 2014. URL: http://isrctn.com/ISRCTN45238573 (accessed 24 November 2016) | No | Yes | Yes | No | Unclear | No | Trial registry entry for completed study, no results or publications posted |
| Abdalla N, Bachanek M, Winiarek J, Cendrowski K, Sawicki W. Analysis of the diagnostic value of logistic regression model and HE4 in the presurgical assessment of adnexal masses. Int J Gynecol Cancer 2015;25(Suppl. 1):379 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention IOTA group’s regression model (not the IOTA group’s Simple Rules or the ADNEX model) |
| Abdulrahman GO Jr, McKnight L, Lutchman Singh K. Risk of malignancy index in women with adnexal masses – comparing RMI 1, 2 and 3 in the Welsh population. Int J Gynecol Cancer 2012;22:E411 | Yes | Yes | No | No | Unclear | Yes |
No relevant intervention Accuracy of RMI 1 at a threshold of 200, in a tertiary care setting |
| Abdulrahman GO Jr, McKnight L, Lutchman Singh K. The risk of malignancy index (RMI) in women with adnexal masses in Wales. Taiwan J Obstet Gynecol 2014;53:376–81 | Yes | Yes | No | No | No | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200, in a tertiary care setting |
| Akdeniz N, Kuyumcuoglu U, Kale A, Erdemoglu M, Caca F. Risk of malignancy index for adnexal masses. Eur J Gynaecol Oncol 2009;30:178–80 | Yes | Yes | Unclear | No | Yes | No |
No relevant outcomes Insufficient information to calculate sensitivity and specificity |
| Alanbay I, Akturk E, Coksuer H, Ercan M, Karasahin E, Dede M, et al. Comparison of risk of malignancy index (RMI), CA125, CA 19–9, ultrasound score, and menopausal status in borderline ovarian tumor. Gynecol Endocrinol 2012;28:478–82 | Yes | No | Yes | No | Unclear | Yes |
Case–control study (benign vs. borderline) No relevant intervention RMI 4 |
| Alcazar JL, Pascual MA, Graupera B, Auba M, Errasti T, Olartecoechea B, et al. External validation of IOTA simple descriptors and simple rules for classifying adnexal masses. Ultrasound Obstet Gynecol 2016;48:397–402 | Yes | No | Yes | No | Yes | Yes |
No relevant intervention IOTA group’s simple ultrasound rules in combination with other rules not included in this assessment Selected population (not classifiable using the IOTA group’s simple descriptors) |
| Al Musalhi K, Al-Kindi M, Ramadhan F, Al-Rawahi T, Al-Hatali K, Mula-Abed WA. Validity of cancer antigen-125 (CA-125) and risk of malignancy index (RMI) in the diagnosis of ovarian cancer. Oman Med J 2015;30:428–34 | Yes | Yes | Yes | No | No | No |
No relevant intervention Study of RMI 2 |
| Andersen ES, Knudsen A, Rix P, Johansen B. Risk of malignancy index in the preoperative evaluation of patients with adnexal masses. Gynecol Oncol 2003;90:109–12 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Anton C, Carvalho FM, Oliveira EI, Maciel G, Baracat EC, Carvalho JP. Comparison of four methods for classification of ovarian masses using CA125, HE4, risk of malignancy index, and ROMA. Int J Gynecol Cancer 2011;21(Suppl. 3):658 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention ROMA score using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Anton C, Carvalho FM, Oliveira EI, Maciel GA, Baracat EC, Carvalho JP. A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (São Paulo) 2012;67:437–41 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA score using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Antovska V, Dimitrov G, Aleksioska N. Our modification of risk of malignancy index in patients with ovarian malignancy. Int J Gynecol Cancer 2011;21(Suppl. 3):820 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Araujo KG, Jales RM, Pereira PN, Yoshida A, de Angelo Andrade L, Sarian LO, et al. Performance of the IOTA ADNEX model in the preoperative discrimination of adnexal masses in a gynecologic oncology centre. Ultrasound Obstet Gynecol 2016;19:19 | Yes | Yes | No | Yes | Yes | Yes |
Tertiary care gynaecological oncology centre Threshold optimisation study |
| Arun-Muthuvel V, Jaya V. Pre-operative evaluation of ovarian tumors by risk of malignancy index, CA125 and ultrasound. Asian Pac J Cancer Prev 2014;15:2929–32 | Yes | Yes | No | Yes | Yes | Yes | Tertiary care setting (women scheduled for surgery in a gynaecological oncology department) |
| Ashrafgangooei T, Rezaeezadeh M. Risk of malignancy index in preoperative evaluation of pelvic masses. Asian Pac J Cancer Prev 2011;12:1727–30 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Threshold optimisation study for RMI, data for cut-off value of 238 |
| Ashrafganjooei T. Risk of malignancy index in evaluation of pelvic masses. Int J Gynecol Cancer 2011;21(Suppl. 3):673 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Optimised RMI threshold (238) |
| Ashrafganjooei T. Risk of malignancy index in evaluation of pelvic masses. Int J Gynecol Cancer 2011;21(Suppl. 2):96 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention RMI (unspecified threshold) |
| Aslam N, Banerjee S, Carr JV, Savvas M, Hooper R, Jurkovic D. Prospective evaluation of logistic regression models for the diagnosis of ovarian cancer. Obstet Gynecol 2000;96:75–80 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Validation of regression models (not interventions included in this assessment) |
| Auge JM, Molina R, Escudero JM, Foj L, Filella X, Fuste P. HE-4 utility to increase efficiency in patients with abdominal masses. Clin Chem Lab Med 2014;52:S365 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Bailey J, Tailor A, Naik R, Lopes A, Godfrey K, Hatem HM, et al. Risk of malignancy index for referral of ovarian cancer cases to a tertiary centre: does it identify the correct cases? Int J Gynecol Cancer 2006;16(Suppl. 1):30–4 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 in a tertiary care setting |
| Bensaid C, Le Frere Belda MA, Metzger U, Larousserie F, Clement D, Chatellier G, et al. Performance of laparoscopy in identifying malignant ovarian cysts. Surg Endosc 2006;20:1410–4 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 in a tertiary care setting |
| Braicu E, Torsten U, Richter R, Zimmermann M, Chekerov R, Kronenberger C, et al. Value of biomarkers and sonography in predicting malignancy in pelvic mass patients. Preliminary results from prospective, multicentric, ongoing study. Int J Gynecol Cancer 2014;24(Suppl. 4):366–7 | Yes | Yes | Unclear | Yes | Yes | No |
No relevant outcomes Insufficient information to calculate sensitivity and specificity |
| Braicu EI, Torsten U, Mecke H, Richter R, Ames K, Hellmeyer L, et al. Role of HE4, CA125, and ultrasound in risk assessment in pelvic mass patients: results from a prospective, multicentric study. J Clin Oncol 2015;33:5535 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Braicu EI, Torsten U, Mecke H, Richter R, Hellmeyer L, Nohe G, et al. HE4 performs better than CA125 as a diagnostic biomarker in premenopausal pelvic mass patients. Final results from a prospective, multicentric study. Int J Gynecol Cancer 2016;26:21–2 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Blontzos N, Vorgias G, Papatheodorou D, Vylliotou V, Novkovic N, Diakosavas M, et al. The clinical value of adding HE4 and ROMA index to CA-125 in the preoperative workout of adnexal masses. Int J Gynecol Cancer 2016;26:172 | Yes | Yes | Unclear | No | Yes | No |
No relevant intervention ROMA assays and threshold NR |
| Bristow RE, Hodeib M, Smith A, Chan DW, Zhang Z, Fung ET, et al. Impact of a multivariate index assay on referral patterns for surgical management of an adnexal mass. Am J Obstet Gynecol 2013;209:581 | Yes | Yes | Unclear | No | Unclear | Yes | No relevant intervention |
| Cacho R, Sia Su L. Distinguishing the benign and malignant adnexal mass: a prospective external validation of a risk of malignancy index (RMI) based on intra-operative features. Int J Gynaecol Obstet 2009;107:S136 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Validation of an unspecified RMI scoring system |
| Campos C, Sarian LO, Jales RM, Hartman C, Araujo KG, Pitta D, et al. Performance of the Risk of Malignancy Index for Discriminating Malignant Tumours in Women With Adnexal Masses. J Ultrasound Med 2016;35:143–52 | Yes | Yes | No | Yes | Yes | Yes | Accuracy of RMI 1 in a tertiary care setting |
| Chia YN, Marsden DE, Robertson G, Hacker NF. Triage of ovarian masses. Aust N Z J Obstet Gynaecol 2008;48:322–8 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200, in a tertiary care setting |
| Chopra S, Vaishya R, Kaur J. An Evaluation of the applicability of the risk of malignancy index for adnexal masses to patients seen at a tertiary hospital in Chandigarh, India. J Obstet Gynaecol India 2015;65:405–10 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI 2 |
| Chudecka-Glaz A, Cymbaluk-Ploska A, Jastrzebska J, Menkiszak J. Can ROMA algorithm stratify ovarian tumor patients better when being based on specific age ranges instead of the premenopausal and postmenopausal status? Tumour Biol 2016;37:8879–87 | Yes | Yes | Unclear | No | Unclear | Accuracy |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Chudecka-Glaz A, Cymbaluk-Ploska A, Luterek-Puszynska K, Menkiszak J. Diagnostic usefulness of the Risk of Ovarian Malignancy Algorithm using the electrochemiluminescence immunoassay for HE4 and the chemiluminescence microparticle immunoassay for CA125. Oncol Lett 2016;12:3101–14 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Clarke SE, Grimshaw R, Rittenberg P, Kieser K, Bentley J. Risk of Malignancy Index in the Evaluation of Patients With Adnexal Masses. J Obstet Gynaecol Can 2009;31:440–5 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 120, in a tertiary care setting |
| Daemen A, Valentin L, Fruscio R, Van Holsbeke C, Melis GB, Guerriero S, et al. Improving the preoperative classification of adnexal masses as benign or malignant by second-stage tests. Ultrasound Obstet Gynecol 2011;37:100–6 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention IOTA group data set used to evaluate performance of different regression models |
| Dasari P, Pannirselvan PCL, Sridhar MG. Ultrasonographic scoring and risk of malignancy index in preoperative prediction of ovarian malignancy. J Gynecol Surg 2013;29:61–4 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI 2 |
| Ellerbrock J, Mertens H, Engelen M, Bergmans M, Nolting E, Kruitwagen R. Evaluation of the risk of malignancy index performance for referral in the south-eastern part of the Netherlands. Int J Gynecol Cancer 2011;21(Suppl. 3):1269 | Yes | Yes | No | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200, in a tertiary care setting |
| Elsawy MM, Meleiss M, Abdel Sattar HR, Abo Ollo M. Prospective study using the risk of ovarian malignancy algorithm for detection of ovarian cancer in Egypt. Int J Gynecol Cancer 2012;22:E317 | Yes | No | Unclear | No | Unclear | Yes |
Case–control study No relevant intervention ROMA assays and threshold NR |
| Enakpene CA, Omigbodun AO, Goecke TW, Odukogbe AT, Beckmann MW. Preoperative evaluation and triage of women with suspicious adnexal masses using risk of malignancy index. J Obstet Gynaecol Res 2009;35:131–8 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 250, in a tertiary care setting |
| Ertas S, Vural F, Vural F, Tufekci EC, Ertas AC, Kose G, et al. Predictive value of malignancy risk indices for ovarian masses in premenopausal and postmenopausal women. Asian Pac J Cancer Prev 2016;17:2177–83 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Evelyne M, Jeroen K, Roy K, Arnold-Jan K, Brigitte S, Ben Van C, et al. Subjective assessment of grey scale and colour Doppler ultrasound features versus the International Ovarian Tumour Analysis (IOTA) logistic regression (LR2) model versus simple ultrasound rules versus Risk of Malignancy Index (RMI) for diagnosing ovarian cancer in women with an adnexal mass. 2013 | No | Yes | PROSPERO registration for a relevant systematic review | ||||
| Farzaneh F, Honarvar Z, Yaraghi M, Yaseri M, Arab M, Hosseini M, et al. Preoperative evaluation of risk of ovarian malignancy algorithm index in prediction of malignancy of adnexal masses. Iran Red Crescent Med J 2014;16:e17185 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA score using different manufacturers’ assays for CA125 and HE4 measurements measurements (not a valid CE-marked intervention) |
| Froyman W, Landolfo C, Bourne T, Cock BD, Testa A, Valentin L, et al. Performance of the RMI and IOTA ADNEX and simple rules risk model in the evaluation of adnexal masses not classifiable using the easy descriptors as first step. BJOG 2016;123:83–4 | Yes | No | Unclear | Yes | Yes | No |
Selected, ‘difficult to diagnose’ tumours No relevant outcomes Senisitivity and specificity data not fully reported |
| Fujirebio Diagnostics I. New Biomarkers Evaluating Ovarian Cancer. 2014. URL: https://ClinicalTrials.gov/show/NCT01466049 (accessed 5 July 2018) | No | Yes | Yes | No | Unclear | No | Trial registry entry for completed study, no results or publications posted |
| Gasparov AS, Zhordania, Paianidi Iu G, Dubinskaia ED. [Oncogynecological aspects of adnexal masses.] Vestn Ross Akad Med Nauk 2013;8:9–13 | Yes | Yes | Unclear | No | Unclear | No | No relevant outcomes |
| Gramellini D, Fieni S, Sanapo L, Casilla G, Verrotti C, Nardelli GB. Diagnostic accuracy of IOTA ultrasound morphology in the hands of less experienced sonographers. Aust N Z J Obstet Gynaecol 2008;48:195–201 | Yes | Yes | Yes | No | Yes | Yes | No relevant intervention |
| Grenache DG, Vucetic Z. Comparison of two multimarker serum tests for the prediction of ovarian cancer in women with a pelvic mass. J Clin Oncol 2013;31(Suppl.):A5555 | Yes | No | Unclear | Yes | Yes | No | Not patients with suspected ovarian cancer |
| Grenache DG, Heichman KA, Werner TL, Vucetic Z. Clinical performance of two multi-marker blood tests for predicting malignancy in women with an adnexal mass. Clin Chim Acta 2015;438:358–63 | Yes | No | Unclear | Yes | Yes | No | Not patients with suspected ovarian cancer |
| Guerriero S, Saba L, Ajossa S, Peddes C, Sedda F, Piras A, et al. Assessing the reproducibility of the IOTA simple ultrasound rules for classifying adnexal masses as benign or malignant using stored 3D volumes. Eur J Obstet Gynecol Reprod Biol 2013;171:157–60 | Yes | No | No | Yes | NA | Yes |
Not a clinical study in patients with suspected ovarian cancer IOTA group’s training study, using video clips |
| Gulati A, Sharma A, Suneja A, Vaid NB, Sharma S, Yadav P. Comparison of ovarian crescent sign & risk of malignancy index in prediction of ovarian malignancy. Int J Gynecol Cancer 2011;21(Suppl. 2):117 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI (unspecified threshold) |
| Hagen B, Tingulstad S, Onsrud M, Moen M, Kiserud T, Eik-Nes S, et al. [Preoperative identification of malignancy among women with a pelvic mass. Evaluation of a risk index based on ultrasound findings. CA 125 in serum and menopausal status.] Tidsskr Nor Laegeforen 1995;115:820–2 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Harry VN, Narayansingh GV, Parkin DE. The risk of malignancy index for ovarian tumours in Northeast Scotland – a population based study. Scott Med J 2009;54:21–3 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI 2 |
| He G, Holcroft CA, Beauchamp MC, Yasmeen A, Ferenczy A, Kendall-Dupont J, et al. Combination of serum biomarkers to differentiate malignant from benign ovarian tumours. J Obstet Gynaecol Can 2012;34:567–74 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Regression model, including multiple biomarkers and RMI |
| Hodeib M, Bristow RE, Smith A, Zhang Z, Chan DW, Fung ET, et al. Impact of a multivariate index assay on referral patterns for surgical management of an adnexal mass. Gynecol Oncol 2013;131:258 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Multivariate index assay (MIA), Overa 1 |
| Hogdall E, Karlesn MA, Christensen IJ, Lundvall L, Engelholm SA, Nedergaard L, et al. Diagnostic value of HE4, CA125 and the ROMA index in ovarian cancer patients from a tertiary centre. Int J Gynecol Cancer 2012;22:S42 | Yes | Yes | No | No | Unclear | No |
No relevant intervention Tertiary care, ROMA assays and threshold NR, RMI threshold NR |
| Ikiz N, Guvenal T, Taneli F, Koyuncu FM, Kandiloglu AR, Bilge S, et al. Comparison of ROMA (risk of ovarian malignancy algorithm), RMI (risk of malignancy index) and OTI (ovarian tumour index) in patients with adnexal mass. Int J Gynecol Cancer 2013;23(Suppl. 1):905 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Imperial NA, Angeli N, Rivera W, Wilhelmina. Risk of malignancy index in the preoperative evaluation of patients with adnexal masses. J Obstet Gynaecol Res 2015;41:77 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Optimised RMI threshold (273) |
| Imperial NA, Rivera W. Risk of malignancy index in the preoperative evaluation of patients with adnexal masses. BJOG 2015;122:137 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 and optimised threshold (273) |
| Imperial NA, Rivera W. Risk of malignancy index in the preoperative evaluation of patients with adnexal masses. Int J Gynaecol Obstet 2015;131:E412 | Yes | Yes | No | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Irshad F, Irshad M, Naz M, Asim Ikram M. Accuracy of ‘risk of malignancy index’ in the preoperative diagnosis of Zovarian malignancy in post menopausal women. Rawal Med J 2013;38:266–70 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 250 |
| Jabeen R, Khan SA, Naveed S. Risk of Malignancy Index in the preoperative evaluation of patients with ovarian masses. Rawal Med J 2015;40:78–80 | Yes | Yes | Yes | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, et al. No benefit from combining HE4 and CA125 as ovarian tumour markers in a clinical setting. Gynecol Oncol 2011;121:487–91 | Yes | No | Unclear | Yes | Yes | Yes | Not patients with suspected ovarian cancer |
| Jarvis S. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Ann Clin Biochem 2011;48:392 | No | Yes | Not a primary study | ||||
| Javdekar R, Maitra N. Risk of Malignancy Index (RMI) in evaluation of adnexal mass. J Obstet Gynaecol India 2015;65:117–21 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI 2 |
| Kaijser J, Van Gorp T, Van Hoorde K, Van Holsbeke C, Bourne T, Vergote I, et al. Serum CA-125 and HE-4 versus an ultrasound based predictive model to assess risk of malignancy in women with adnexal masses. Int J Gynecol Cancer 2012;22:E149–50 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Kaijser J, Van Gorp T, Sayasneh A, Vergote I, Bourne T, Van Calster B, et al. Differentiating stage I epithelial ovarian cancer from benign disease in women with adnexal tumors using biomarkers or the ROMA algorithm. Gynecol Oncol 2013;130:398–9 | No | Yes | Not a primary study | ||||
| Kalapotharakos G, Asciutto C, Henic E, Casslen B, Borgfeldt C. High preoperative blood levels of HE4 predicts poor prognosis in patients with ovarian cancer. J Ovarian Res 2012;5:20 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA score using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Kader Ali Mohan GR, Jaaback K, Proietto A, Robertson R, Angstetra D. Risk Malignancy Index (RMI) in patients with abnormal pelvic mass: Comparing RMI 1, 2 and 3 in an Australian population. Aust N Z J Obstet Gynaecol 2010;50:77–80 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Kadija S, Stefanovic A, Jeremic K, Radojevic MM, Nikolic L, Markovic I, et al. The utility of human epididymal protein 4, cancer antigen 125, and risk for malignancy algorithm in ovarian cancer and endometriosis. Int J Gynecol Cancer 2012;22:238–44 | Yes | Yes | Yes | No | Yes | No |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Karimi-Zarchi M, Mojaver SP, Rouhi M, Hekmatimoghaddam SH, Moghaddam RN, Yazdian-Anari P, et al. Diagnostic value of the Risk of Malignancy Index (RMI) for detection of pelvic malignancies compared with pathology. Electron Physician 2015;7:1505–10 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 70 |
| Karlsen MA, Hogdall EV, Christensen IJ, Borgfeldt C, Kalapotharakos G, Zdrazilova-Dubska L, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer – an international multicenter study in women with an ovarian mass. Gynecol Oncol 2015;138:640–6 | Yes | Unclear | No | No | No | No |
No relevant intervention Risk model development (Copenhagen Index) using data from existing studies and stored blood samples |
| Keogh F, Tan AL, Eva LJ. HE4 as a tumour marker for the prediction of ovarian carcinoma. BJOG 2015;122:137–8 | Yes | Yes | Unclear | No | Yes | No |
No relevant intervention ROMA assays and threshold NR |
| Kho CZB, Chong YW, Lee YT, Krishnaswamy G, Ong CL, Lam SL, et al. Preoperative evaluation of paediatric adnexal masses with paediatric risk of malignancy index improves ovarian conservation and surgical morbidity. Pediatr Blood Cancer 2015;62:S187 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Paediatric version of RMI (not a specified intervention) |
| Ko HS, Kim N, Park YG. Re: interobserver agreement in describing adnexal masses using the International Ovarian Tumour Analysis simple rules in a real-time setting and using three-dimensional ultrasound volumes and digital clips. Ultrasound Obstet Gynecol 2015;45:238 | No | Yes | Not a primary study | ||||
| Kondalsamy-Chennakesavan S, Obermair A. Differentiating stage I epithelial ovarian cancer from benign disease in women with adnexal tumours using biomarkers or the ROMA algorithm. Gynecol Oncol 2013;130:400 | No | Yes | Not a primary study | ||||
| Kondalsamy-Chennakesavan S, Hackethal A, Bowtell D, Australian Ovarian Cancer Study Group, Obermair A. Differentiating stage 1 epithelial ovarian cancer from benign ovarian tumours using a combination of tumour markers HE4, CA125, and CEA and patient’s age. Gynecol Oncol 2013;129:467–71 | Yes | No | Unclear | Yes | Yes | Yes | Diagnostic case–control study |
| Lasho MA, Algeciras-Schimnich A. Determination of ROMA score performance using the roche elecsys HE4 and CA 125 immunoassays. Clin Chem 2014;60(Suppl. 1):S12–13 | Yes | Yes | Unclear | Yes | Unclear | Yes | Reference standard unspecified |
| Leelahakorn S, Tangjitgamol S, Manusirivithaya S, Thongsuksai P, Jaroenchainon P, Jivangkul C. Comparison of ultrasound score, CA125, menopausal status, and risk of malignancy index in differentiating between benign and borderline or malignant ovarian tumors. J Med Assoc Thai 2005;88(Suppl. 2):22–30 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Not RMI 1 |
| Li AJ. New biomarkers for the diagnosis of ovarian carcinoma: OVA1 and ROMA. [Italian.] G Ital Ostet Ginecol 2012;34:409–14 | No | Yes | Not a primary study | ||||
| Li ZQ, Smalley RJ, Glover CL, Raju S, Falcone K, Fegely M, et al. Comparison of serum CYFRA 21–1 and ROMA in distinguishing ovarian cancer from benign pelvic masses. J Clin Oncol 2012 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Loh AHP, Ong CL, Lam SL, Chua JHY, Chui CH. Risk of malignancy index for preoperative evaluation of paediatric ovarian tumors. Pediatr Blood Cancer 2010;55:785 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Development of new paediatric risk indices |
| Lokich E, Palisoul M, Romano N, Craig Miller M, Robison K, Stuckey A, et al. Assessing the risk of ovarian malignancy algorithm for the conservative management of women with a pelvic mass. Gynecol Oncol 2015;139:248–52 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Lokich E, Palisoul M, Romano N, Stuckey AR, Robison KM, DiSilvestro PA, et al. ROMA guided conservative management for women diagnosed with an ovarian cyst or pelvic mass. Gynecol Oncol 2015;137:21 | Yes | Yes | No | No | Unclear | Yes |
No relevant intervention Tertiary care, ROMA assays not specified |
| Longoria T, Ueland F, Zhang Z, Chan D, Smith A, Fung E, et al. Clinical performance of a multivariate index assay for detecting early-stage ovarian cancer. Gynecol Oncol 2013;131:259 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention MIA, Overa 1 |
| Ma S, Shen K, Lang J. [Effect of a risk of malignancy index in preoperative diagnosis of ovarian cancer.] Zhonghua Fu Chan Ke Za Zhi 2001;36:162–4 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Ma S, Shen K, Lang J. A risk of malignancy index in preoperative diagnosis of ovarian cancer. Chin Med J 2003;116:396–9 | Yes | Yes | Yes | No | Unclear | Yes |
No relevant intervention Data for various RMI thresholds (50 to 1000, not including 250) |
| Maitra NK, Javadekar R. Risk of malignancy index in the evaluation of adnexal mass. BJOG 2014;121:206 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI 2 |
| Manegold-Brauer G, Schoetzau A, Hacker N, Lapaire O, Heinzelmann-Schwarz V. Proposal of a new two-step use of the risk of malignancy index in a general gynecological outpatient setting as compared to a gynecological cancer center. Int J Gynecol Cancer 2015;25(Suppl. 1):223 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Mansour GM, El-Lamie IK, El-Sayed HM, Ibrahim AM, Laban M, Abou-Louz SK, et al. Adnexal mass vascularity assessed by 3-dimensional power Doppler: does it add to the risk of malignancy index in prediction of ovarian malignancy?: four hundred-case study. Int J Gynecol Cancer 2009;19:867–72 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention RMI threhold optimisation in a tertiary care setting |
| Martin Rodriguez S, Ascorbe Salcedo P, Jareno Blanco MS. Diagnostic accuracy of HE4, CA125 and Roma for women with suspected ovarian cancer. Clin Chem Lab Med 2015;53:S424 | Yes | Yes | Unclear | Yes | Unclear | No |
No relevant outcomes Insufficient data to determine accuracy measures |
| Martra F, Tripodi E, Modaffari P, Zanfagnin V, Fuso L, De Sanso G, et al. Ultrasound score versus experienced ultrasound examiner interpretation: are both necessary to improve the management of ovarian masses? Int J Gynecol Cancer 2011;21(Suppl. 3):385 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention IOTA score (no details) |
| Meray O, Turkcuoglu I, Meydanli MM, Kafkasli A. Risk of malignancy index is not sensitive in detecting non-epithelial ovarian cancer and borderline ovarian tumor. J Turkishgerman Gynecol Assoc 2010;11:22–6 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Mills P, Court S, Giamougiannis P, Daines L. Is the risk of malignancy (RMI) score useful in deciding management when below 250? A 2-year retrospective surgical study. BJOG 2015;122:144–5 | Yes | Yes | Unclear | No | Unclear | No | No relevant intervention |
| Mohammed ABF, Ahuga VK, Taha M. Validation of the Risk of Malignancy Index in primary evaluation of ovarian masses. Middle East Fertil Soc J 2014;19:324–8 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Study of RMI 3 and 4 |
| Mol BW, Boll D, De Kanter M, Heintz AP, Sijmons EA, Oei SG, et al. Distinguishing the benign and malignant adnexal mass: an external validation of prognostic models. Gynecol Oncol 2001;80:162–7 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Validation study of 21 published models (not interventions included in this assessment) |
| Molina R, Escudero JM, Fuste P. HE-4 levels in gynaecological patients undergoing surgical treatment for suspected malignancies. Systems to increase efficiency. Tumor Biol 2014;35:S9 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Molina R, Escudero JM, Fuste P. HE-4 utility to increase efficiency in patients with abdominal masses. Tumor Biol 2014;35:S6 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Moolthiya W, Yuenyao P. The risk of malignancy index (RMI) in diagnosis of ovarian malignancy. Asian Pac J Cancer Prev 2009;10:865–8 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller CM, Allard JW, et al. Comparison of a novel multiple marker assay versus the risk of malignancy index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112(Suppl. 1):25–6 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention RMI (unspecified threshold) |
| Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol 2010;203:228 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Moore EK, Iavazzo C, Argent V, Leung E, Pitkin S, Benton S, et al. Does the risk of malignancy algorithm have a role in triaging symptomatic women for further investigation? Results of a pilot ‘real world’ study. Int J Gynecol Cancer 2013;23(Suppl. 1):64 | Yes | Yes | No | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Moore RG, Hawkins DM, Miller MC, Landrum LM, Gajewski W, Ball JJ, et al. Combining clinical assessment and the Risk of Ovarian Malignancy Algorithm for the prediction of ovarian cancer. Gynecol Oncol 2014;135:547–51 | Yes | Yes | Yes | No | Unclear | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Moszynski R, Zywica P, Wojtowicz A, Szubert S, Sajdak S, Stachowiak A, et al. Menopausal status strongly influences the utility of predictive models in differential diagnosis of ovarian tumors: an external validation of selected diagnostic tools. Ginekol Pol 2014;85:892–9 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) The IOTA data are for models other than simple ultrasound rules or ADNEX model |
| Nahar S, Shamsuddin L, Faruqui M, Ara G. Sonographic prediction of ovarian malignancy in adnexal mass. Bangladesh J Obstet Gynecol 2012;27:67–71 | Yes | Yes | Yes | No | Yes | Yes | No relevant intervention |
| Numanoglu C, Kuru O, Sakinci M, Akbayir O, Ulker V. Ovarian fibroma/fibrothecoma: retrospective cohort study shows limited value of risk of malignancy index score. Aust N Z J Obstet Gynaecol 2013;53:287–92 | Yes | Yes | Unclear | No | Yes | Yes | RMI at a threshold of 200, data for a small subgroup of patients with fibroma/fibrothecoma |
| Ong C, Biswas A, Choolani M, Low JJ. Comparison of risk of malignancy indices in evaluating ovarian masses in a Southeast Asian population. Singapore Med J 2013;54:136–9 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Data for various RMI thresholds (from 50 to 1000, not including 250) |
| Ozbay PO, Ekinci T, Caltekin MD, Yilmaz HT, Temur M, Yilmaz O, et al. Comparative evaluation of the risk of malignancy index scoring systems (1–4) used in differential diagnosis of adnexal masses. Asian Pac J Cancer Prev 2015;16:345–9 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 250 |
| Park JY, Park YR, Choe JW, Chun SI, Kim DY, Suh DS, et al. Human epididymis secretory protein 4 (HE4) versus cancer antigen 125 (CA125) in the diagnosis of malignant ovarian tumor. Int J Gynecol Cancer 2015;25(Suppl. 1):511 | Yes | No | Unclear | Yes | Unclear | No | Diagnostic case–control study |
| Partheen K, Kristjansdottir B, Sundfeldt K. Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass. J Gynecol Oncol 2011;22:244–52 | Yes | Yes | No | Yes | Yes | Yes | Tertiary care setting gynaecologic oncology surgery |
| Peces Rama A, Llanos Llanos MC, Sanchez Ferrer ML, Alcazar Zambrano JL, Martinez Mendoza A, Nieto Diaz A. Simple descriptors and simple rules of the International Ovarian Tumour Analysis (IOTA) Group: a prospective study of combined use for the description of adnexal masses. Eur J Obstet Gynecol Reprod Biol 2015;195:7–11 | Yes | No | Yes | Yes | Yes | No |
No relevant outcomes Selected population (unclassifiable using IOTA group’s simple descriptors) |
| Pineda L, Salcedo E, Vilhena C, Juez L, Alcazar JL. Interobserver agreement in assigning IOTA colour score to adnexal masses using three-dimensional volumes or digital videoclips: potential implications for training. Ultrasound Obstet Gynecol 2014;44:361–4 | Yes | No | No | No | NA | No |
Not a clinical study in patients with suspected ovarian cancer IOTA training study, using video clips |
| Pitta Dda R, Sarian LO, Barreta A, Campos EA, Andrade LL, Fachini AM, et al. Symptoms, CA125 and HE4 for the preoperative prediction of ovarian malignancy in Brazilian women with ovarian masses. BMC Cancer 2013;13:423 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention ROMA score using different manufacturers’ assays for CA125 and HE4 measurements (not a valid CE-marked intervention) |
| Putri I, How JA, Marino J, Villegas R, McNally O, Grover S, et al. A 32 year review of clinical presentation and the use of risk of malignancy index (RMI2) in diagnosis of ovarian malignancies in children and adolescents. Int J Gynecol Cancer 2014;24(Suppl. 4):211–12 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI 2 |
| Ratnavelu N, Founta C, Addison C, Bradbury M, Handley G, Das M, et al. The role of adding HE4 to CA125 for women referred to secondary care with suspected ovarian cancer in facilitating management decision making: a prospective pilot study. Int J Gynecol Cancer 2014;24(Suppl. 4):486–7 | Yes | Yes | Unclear | No | No | Yes |
No relevant intervention ROMA assays and threshold NR |
| Raza A, Mould T, Wilson M, Burnell M, Bernhardt L. Increasing the effectiveness of referral of ovarian masses from cancer unit to cancer center by using a higher referral value of the risk of malignancy index. Int J Gynecol Cancer 2010;20:552–4 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 450 |
| Richards A, Herbst U, Pather S, Saidi S, Tejada-Berges T, Williams P, et al. HE4, CA125, the Risk of Malignancy Algorithm (ROMA) and the Risk of Malignancy Index (RMI) and complex pelvic masses – a prospective comparison in the preoperative evaluation of adnexal and pelvic masses in an Australian population. BJOG 2015;122:150 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Assay details NR |
| Richards A, Herbst U, Manalang J, Pather S, Saidi S, Tejada-Berges T, et al. HE4, CA125, the Risk of Malignancy Algorithm and the Risk of Malignancy Index and complex pelvic masses – a prospective comparison in the pre-operative evaluation of pelvic masses in an Australian population. Aust Z J Obstet Gynaecol 2015;55:493–7 | Yes | Yes | No | Yes | Yes | No | Tertiary care setting |
| Rogulski L, Strzelczyk J. Simple ultrasound rules used by general gynecologists supplemented with ROMA assessment in differentiating malignant and benign adnexal masses. Int J Gynecol Cancer 2015;25(Suppl. 1):1479 | Yes | Yes | Unclear | Yes | Unclear | No | No relevant outcomes |
| Romagnolo C, Leon AE, Fabricio ASC, Del Pup L, Papadakis C, Odicino FE, et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools of ovarian cancer in patients with pelvic mass: an Italian multicenter prospective study. Int J Gynecol Cancer 2015;25(Suppl. 1):528–9 | Yes | Yes | Unclear | Yes | Unclear | No | No relevant outcomes |
| Rossi A, Braghin C, Soldano F, Isola M, Capodicasa V, Londero AP, et al. A proposal for a new scoring system to evaluate pelvic masses: Pelvic Masses Score (PMS). Eur J Obstet Gynecol Reprod Biol 2011;157:84–8 | Yes | Yes | Yes | No | Unclear | Yes | No relevant intervention |
| Ruiz de Gauna B, Sanchez P, Pineda L, Utrilla-Layna J, Juez L, Alcazar JL. Interobserver agreement in describing adnexal masses using the International Ovarian Tumor Analysis simple rules in a real-time setting and using three-dimensional ultrasound volumes and digital clips. Ultrasound Obstet Gynecol 2014;44:95–9 | Yes | No | No | Yes | No | No |
Not a clinical study in patients with suspected ovarian cancer IOTA training study, using video clips |
| Sandri MT, Bottari F, Franchi D, Boveri S, Candiani M, Ronzoni S, et al. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol Oncol 2013;128:233–8 | Yes | Yes | Yes | Yes | Yes | No |
No relevant outcomes Data for specificity at a fixed sensitivity |
| Sayasneh A, Kaijser J, Preisler J, Johnson S, Stalder C, Husicka R, et al. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol Oncol 2013;130:140–6 | Yes | No | Unclear | No | Yes | Yes |
No relevant intervention No data for IOTA group’s simple ultrasound rules alone Selected population (unclassifiable using the IOTA group’s simple descriptors) |
| Sayasneh A, Preisler J, Stlader C, Husicka R, Naji O, Kaijser J, et al. A randomised controlled trial to compare the clinical impact of RMI versus LR2 to characterise adnexal masses: interim analysis of phase 4 IOTA study. BJOG 2013;120:357–358 | Yes | Yes | No | No | NA | Yes |
No relevant intervention IOTA group’s regression model (not simple ultrasound rules or ADNEX model) |
| Sayasneh A, Kaijser J, Preisler J, Smith AA, Raslan F, Johnson S, et al. Accuracy of ultrasonography performed by examiners with varied training and experience in predicting specific pathology of adnexal masses. Ultrasound Obstet Gynecol 2015;45:605–12 | Yes | Yes | No | No | Yes | Yes | No relevant intervention |
| Senel SA, Ozcam H, Ateser GB, Vatansever D. Risk of malignancy indices in differentiation of malignant adnexal masses from the benign adnexal masses. Int J Gynecol Cancer 2015;25(Suppl. 1):1006 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Accuracy of RMI 4 |
| Shimada K, Matsumoto K, Mimura T, Ishikawa T, Hirose Y, Shimizu H, et al. Ultrasound-based logistic regression modelling versus magnetic resonance imaging for discriminating between benign and malignant adnexal masses: a prospective study. Int J Gynecol Cancer 2016;26:820 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention IOTA group’s regression model (not simple ultrasound rules or ADNEX model) |
| Simsek HS, Tokmak A, Ozgu E, Doganay M, Danisman N, Erkaya S, et al. Role of a risk of malignancy index in clinical approaches to adnexal masses. Asian Pac J Cancer Prev 2014;15:7793–7 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Optimised RMI threshold (163.5) in a tertiary care setting |
| Simsek S, Tokmak A, Ozgu E, Doganay M, Danisman N, Erkaya S, et al. The role of risk of malignancy index (RMI) in clinical approach to adnexial masses. Int J Gynecol Cancer 2014;24(Suppl. 4):348 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Optimised RMI threshold (163.85) |
| Sladkevicius P, Valentin L. Intra- and interobserver agreement when describing adnexal masses using the International Ovarian Tumour Analysis terms and definitions: a study on three-dimensional ultrasound volumes. Ultrasound Obstet Gynaecol 2013;41:318–27 | Yes | Yes | Unclear | No | Yes | No |
No relevant intervention IOTA group models (not simple ultrasound rules or ADNEX model) |
| Sole-Sedeno J, Agramunt S, Mancebo G, Rueda C, Sastre M, Alameda F, et al. Risk malignancy index in the evaluation of the adnexal masses. Int J Gynecol Cancer 2012;22:E967–8 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention Unspecified RMI threshold |
| Stiekema A, Van De Vrie R, Lok C, Van Driel W, Korse T, Buist M, et al. Serum HE4 as additional step to the RMI 1 improves the diagnosis of patients with a pelvic mass. Int J Gynecol Cancer 2016;26:169 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 and HE4 |
| Sumpaico WW. Comparison of ROMA to RMI for ovarian carcinoma in Asia. Int J Gynaecol Obstet 2012;119:S248–9 | Yes | Yes | Unclear | No | Unclear | No |
No relevant intervention ROMA assays and threshold NR |
| Tanriverdi HA, Sade H, Akbulut V, Barut A, Bayar U. [Clinical and ultrasonographic evaluation of pelvic masses.] J Turk German Gynecol Assoc 2007;8:67–70 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Terzic M, Dotlic J, Ladjevic IL, Atanackovic J, Ladjevic N. Evaluation of the risk malignancy index diagnostic value in patients with adnexal masses. Vojnosanit Pregl 2011;68:589–93 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Terzic M, Dotlic J, Likic I, Brndusic N, Pilic I, Ladjevic N, et al. Risk of malignancy index validity assessment in premenopausal and postmenopausal women with adnexal tumours. Taiwan J Obstet Gynecol 2013;52:253–7 | Yes | Yes | Yes | No | Yes | No | No relevant intervention |
| Thompson R, Dempsey A, Abdel-Aty M. Which risk of malignancy index (RMI) calculation is a better predictor of malignancy, and at what level should we refer to the cancer centre? A retrospective observational study conducted at East Lancashire Hospitals NHS Trust. BJOG 2014;121:9 | Yes | No | Unclear | Yes | No | Yes | Diagnostic case–control study |
| Timmerman D, Verrelst H, Bourne TH, De Moor B, Collins WP, Vergote I, et al. Artificial neural network models for the preoperative discrimination between malignant and benign adnexal masses. Ultrasound Obstet Gynecol 1999;13:17–25 | Yes | Yes | Unclear | No | Unclear | Accuracy | No relevant intervention |
| Timmerman D, Testa AC, Bourne T, Ferrazzi E, Ameye L, Konstantinovic ML, et al. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery: a multicenter study by the International Ovarian Tumor Analysis Group. J Clin Oncol 2005;23:8794–801 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention IOTA group models (not simple ultrasound rules or ADNEX model) |
| Timmerman D, Van Calster B, Jurkovic D, Valentin L, Testa AC, Bernard JP, et al. Inclusion of CA-125 does not improve mathematical models developed to distinguish between benign and malignant adnexal tumours. J Clin Oncol 2007;25:4194–200 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention IOTA group model (not simple ultrasound rules or ADNEX model) |
| Timmerman D, Van Calster B, Testa AC, Guerriero S, Fischerova D, Lissoni AA, et al. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: a temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol 2010;36:226–34 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Validation of other IOTA group’s models (not simple ultrasound rules or ADNEX model) |
| Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W, et al. Predicting the risk of malignancy in adnexal masses based on the simple rules from the International Ovarian Tumour Analysis group. Am J Obstet Gynecol 2016;214:424–37 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Development and validation of the IOTA group’s simple ultrasound rules risk model |
| Tingulstad S, Hagen B, Skjeldestad FE, Halvorsen T, Nustad K, Onsrud M. The risk-of-malignancy index to evaluate potential ovarian cancers in local hospitals. Obstet Gynecol 1999;93:448–52 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention RMI 2 |
| Toledo KL, Audifred JR, Topete RE, Niebla DC, Hernandez SE, Morales L. Comparison between histopathological results and malignancy index risk in adnexal complex cysts treated by laparoscopic surgery. J Minim Invasive Gynecol 2016;23(Suppl. 1):217–18 | Yes | Yes | Yes | No | Yes | No | No relevant outcomes |
| Torres JC, Derchain SF, Faundes A, Gontijo RC, Martinez EZ, Andrade LA. Risk-of-malignancy index in preoperative evaluation of clinically restricted ovarian cancer. São Paulo Med J 2002;120:72–6 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Not RMI 1 |
| Trevino-Baez JD, Cantu-Cruz JA, Medina-Mercado J, Abundis A. [Diagnostic accuracy of malignancy risk index II in post-menopausal women with adnexal tumour.] Cir Cir 2016;84:109–14 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Study of RMI 2 |
| University of South F, Universitaire Ziekenhuizen L. International Ovarian Tumour Analysis (IOTA) Phase 5. URL: https://ClinicalTrials.gov/show/NCT01698632 (accessed 5 July 2018) | No | Yes | Unclear | No | NA | No | Trial registry entry |
| Valentin L, Hagen B, Tingulstad S, Eik-Nes S. Comparison of ‘pattern recognition’ and logistic regression models for discrimination between benign and malignant pelvic masses: a prospective cross validation. Ultrasound Obstet Gynecol 2001;18:357–65 | Yes | Yes | Unclear | No | Yes | Yes | No relevant intervention |
| Valentin L, Ameye L, Savelli L, Fruscio R, Leone FP, Czekierdowski A, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of grey-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011;38:456–65 | Yes | No | Unclear | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200, for masses unclassifiable by CA125 |
| Valentin L, Ameye L, Savelli L, Fruscio R, Leone FP, Czekierdowski A, et al. Unilocular adnexal cysts with papillary projections but no other solid components: is there a diagnostic method that can classify them reliably as benign or malignant before surgery? Ultrasound Obstet Gynecol 2013;41:570–81 | Yes | Yes | Unclear | No | Yes | No |
No relevant intervention Development of an IOTA group model to predict malignancy in unilocular cysts with papillations |
| Van Calster B, Timmerman D, Bourne T, Testa AC, Van Holsbeke C, Domali E, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Natl Cancer Inst 2007;99:1706–14 | Yes | Yes | Unclear | No | Yes | Yes | No relevant intervention |
| Van Calster B, Timmerman D, Valentin L, McIndoe A, Ghaem-Maghami S, Testa AC, et al. Triaging women with ovarian masses for surgery: observational diagnostic study to compare RCOG guidelines with an International Ovarian Tumour Analysis (IOTA) group protocol. BJOG 2012;119:662–71 | Yes | Yes | Yes | No | Yes | No |
No relevant intervention IOTA group model (not simple ultrasound rules or ADNEX model) |
| van den Akker PA, Aalders AL, Snijders MP, Kluivers KB, Samlal RA, Vollebergh JH, et al. Evaluation of the Risk of Malignancy Index in daily clinical management of adnexal masses. Gynecol Oncol 2010;116:384–8 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Van Holsbeke C, Van Calster B, Valentin L, Testa AC, Ferrazzi E, Dimou I, et al. External validation of mathematical models to distinguish between benign and malignant adnexal tumors: a multicenter study by the International Ovarian Tumor Analysis Group. Clin Cancer Res 2007;13:4440–7 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Van Holsbeke C, Van Calster B, Testa AC, Domali E, Lu C, Van Huffel S, et al. Prospective internal validation of mathematical models to predict malignancy in adnexal masses: results from the international ovarian tumour analysis study. Clin Cancer Res 2009;15:684–91 | Yes | Yes | Unclear | No | Yes | Yes |
No relevant intervention Validation of IOTA group models (not simple ultrasound rules or ADNEX model) |
| Van Holsbeke C, Van Calster B, Bourne T, Ajossa S, Testa AC, Guerriero S, et al. External validation of diagnostic models to estimate the risk of malignancy in adnexal masses. Clin Cancer Res 2012;18:815–25 | Yes | Yes | Unclear | No | Yes | No |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Villiotou V, Vorgias G, Lekka I, Karampelas A, Dertimas V. Evaluation of HE4, CA 125 and ROMA predictive index in patients with gynaecological diseases. Clin Chem Lab Med 2014;52:S479 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention ROMA assays and threshold NR |
| Wang LM, Song H, Song X, Zhou XB. An improved risk of malignancy index in diagnosis of adnexal mass. Chin Med J 2012;125:533–5 | Yes | Yes | Yes | No | Yes | No |
No relevant intervention Unspecified RMI threshold |
| Wilailak S, Chan KK, Chen CA, Nam JH, Ochiai K, Aw TC, et al. Distinguishing benign from malignant pelvic mass utilizing an algorithm with HE4, menopausal status, and ultrasound findings. Journal of Gynecologic Oncology 2015;26:46–53 | Yes | Yes | Unclear | No | Unclear | Yes | No relevant intervention |
| Winarto H, Bismarck JL, Purbadi S, Nuranna L. Is ROMA scoring systems really better than RMI for indonesian patients, in DR. ciptomangunkusumo hospital. Int J Gynecol Cancer 2011;21(Suppl. 3):S403 | Yes | Yes | Unclear | No | Unclear | No | No relevant intervention |
| Yamamoto Y, Tsuchida A, Ushiwaka T, Nagai R, Matsumoto M, Komatsu J, et al. Comparison of 4 risk-of-malignancy indexes in the preoperative evaluation of patients with pelvic masses: a prospective study. Clin Ovarian Other Gynecol Cancer 2014;7:8–12 | Yes | Yes | Yes | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 |
| Yavuzcan A, Caglar M, Ozgu E, Ustun Y, Dilbaz S, Ozdemir I, et al. Should cut-off values of the risk of malignancy index be changed for evaluation of adnexal masses in Asian and Pacific populations? Asian Pac J Cancer Prev 2013;14:5455–9 | Yes | No | Unclear | No | Yes | Yes | Not patients with suspected ovarian cancer |
| Yazbek J, Aslam N, Tailor A, Hillaby K, Raju KS, Jurkovic D. A comparative study of the risk of malignancy index and the ovarian crescent sign for the diagnosis of invasive ovarian cancer. Ultrasound Obstet Gynecol 2006;28:320–4 | Yes | Yes | No | No | Yes | Yes |
No relevant intervention Accuracy of RMI at a threshold of 200 in a tertiary care setting |
| Yoshida A, Derchain SF, Pitta DR, Andrade LA, Sarian LO. Comparing the Copenhagen Index (CPH-I) and Risk of Ovarian Malignancy Algorithm (ROMA): two equivalent ways to differentiate malignant from benign ovarian tumors before surgery? Gynecol Oncol 2016;140:481–5 | Yes | Yes | No | Yes | Yes | No | Tertiary care setting |
| Zannoni L, Savelli L, Jokubkiene L, Di Legge A, Condous G, Testa AC, et al. Intra- and interobserver agreement with regard to describing adnexal masses using International Ovarian Tumour Analysis terminology: reproducibility study involving seven observers. Ultrasound Obstet Gynecol 2014;44:100–8 | No | No | No | No | NA | No |
Not a clinical study in patients with suspected ovarian cancer IOTA group’s training study, using video clips |
| Zhang S. Performance of ovarian malignancy algorithm in predicting pelvic mass in patients at risk of ovarian cancer. Chin J Clin Oncol 2014;41:513–17 | Yes | Yes | Unclear | No | Unclear | Yes |
No relevant intervention ROMA assays and threshold NR |
Appendix 3 Example assessments of study quality
Example QUADAS-2 assessment
Van Calster et al. (2014)17
Domain 1: patient selection
| A. Risk of bias | |
|---|---|
| Consecutive patients with at least one adnexal mass selected for surgical intervention, referred for IOTA group phase 3 study | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | Risk: low |
| B. Applicability | |
| Women referred for evaluation of an adnexal mass. Secondary or tertiary care referral, but ADNEX model includes a term for type of referral centre | |
| Do the included patients match the question? | Concerns: low |
Domain 2: index test(s)
| A. Risk of bias | |
|---|---|
| ADNEX validation data set. No details regarding who performed tests, whether or not they were blind, or when they were performed | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | Risk: low |
| B. Applicability | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: low |
Domain 3: reference standard
| A. Risk of bias | |
|---|---|
| Histology of resected mass (no further details). Performed without knowledge of ultrasound | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Risk: low |
| B. Applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concerns: high |
Domain 4: flow and timing
| A. Risk of bias | |
|---|---|
| Calculation of 2 × 2 data from reported sensitivity and specificity values resulted in non-whole numbers for some analyses. The time from index test to surgery was ≤ 120 days | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | Yes |
| For comparative accuracy studies, did all patients receive all index tests? | NA |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | Risk: unclear |
Example PROBAST assessment
Van Calster et al. (2014)17
Domain 1: participant selection
| A. Risk of bias | |||
|---|---|---|---|
|
Describe the sources of data and criteria for participant selection: Data were derived from an international, multicentre, prospective cohort study (the IOTA study) of consecutive women with at least one adnexal mass that was clinically judged to require surgery. Participants were excluded if they refused transvaginal ultrasonography, were pregnant at the time of presentation or received surgery > 120 days after the ultrasound examination. The IOTA group was established to develop and validate diagnostic models for adnexal masses, based on large multicentre data sets, using a standardised ultrasound examination protocol, terms and definitions The ADNEX model was developed using data collected in IOTA study phases 1, 1b and 2 (1999–2007) and validated using data collected in phase 3 (2009–12); inclusion criteria remained the same throughout |
|||
| Dev | Val | ||
| 1.1 Were participant selection criteria similar to the model development study? |

|
Yes | |
| 1.2 Were appropriate data sources used (e.g. cohort, RCT or nested case–control study data?) | Yes | Yes | |
| 1.3 Were all inclusions and exclusions of participants appropriate? | Yes | Yes | |
| Risk of bias introduced by selection of participants | Risk: (low/high/unclear) | Low | Low |
| Rationale of bias rating: | |||
| B. Applicability | |||
|
Describe included participants, setting and dates: Women with at least one adnexal mass requiring surgery. Women were evaluated in a mixture of secondary care settings and gynaecological oncology tertiary referral centres |
|||
| Concern that the included participants and setting do not match the review question | Concern: (low/high/unclear) | High | High |
|
Rationale of applicability rating: The study setting is not a complete match for that specified in the scope for this assessment |
|||
Domain 2: predictors
| A. Risk of bias | |||
|---|---|---|---|
|
List and describe predictors included in the final model (e.g. definition and timing of assessment): Age, serum CA125 level (log-transformed), type of centre (tertiary referral gynaecological oncology centre vs. other centres), maximum diameter of the lesion (log-transformed), proportion of solid tissue (with quadratic term), number of papillary projections, > 10 cyst locules, acoustic shadows and ascites were included in the final ADNEX model. Family history of ovarian cancer was dropped by the variable selection analysis. Predictors were assessed prior to surgery and histological evaluation. Participating centres used one of four manufacturers’ immunoradiometric assay kits to measure CA125; all kits used the OC125 antibody |
|||
| Dev | Val | ||
| 2.1 Were predictors defined and assessed in a similar way for all participants? | No | No | |
| 2.2 Were predictors defined and assessed in a similar way to predictors in the development model? |

|
Yes | |
| 2.3 Were predictor assessments made without knowledge of outcome data? | Yes | Yes | |
| 2.4 Are all predictors available at the time the model is intended to be used? | Yes | Yes | |
| Risk of bias introduced by predictors or their assessment | Risk: (low/high/unclear) | Low | Low |
|
Rationale of bias rating: Study centres used different CA125 assays; however, all assays used the same antibody and, therefore, the effects of this variation are likely to be minimal |
|||
| B. Applicability | |||
| Concern that the definition, assessment or timing of predictors in the model do not match the review question | Concern: (low/high/unclear) | Low | Low |
|
Rationale of applicability rating: The inclusion of CA125 assays from a variety of manufacturers reflects the reality of clinical practice |
|||
Domain 3: outcome
| A. Risk of bias | |||
|---|---|---|---|
|
Describe the outcome, how it was defined and determined, and the time interval between predictor assessment and outcome determination: The outcome was determined by histopathological analysis of the mass after surgical removal by laparotomy or laparoscopy (as considered appropriate by the surgeon). The stage of malignant tumours was recorded using the FIGO classification system. Excised tissue was examined locally at each study centre. The histological classification was performed without knowledge of the ultrasound results, but it was not clear whether or not the pathologists were aware of other predictor information. The final diagnosis was divided into five types: benign, borderline, stage I invasive, stages II–IV invasive and secondary metastatic cancer |
|||
| Dev | Val | ||
| 3.1 Was the outcome determined appropriately? | Yes | Yes | |
| 3.2 Was a prespecified or standard outcome definition used? | Yes | Yes | |
| 3.3 Were predictors excluded from the outcome definition? | Yes | Yes | |
| 3.4 Was the outcome defined and determined in a similar way for all participants? | Yes | Yes | |
| 3.5 Was the outcome defined and determined in a similar way to the outcome in the model development study? |

|
Yes | |
| 3.6 Was the outcome determined without knowledge of predictor information? | Unclear | Unclear | |
| 3.7 Was the time interval between predictor assessment and outcome determination appropriate? | Yes | Yes | |
| Risk of bias introduced by the outcome or its determination | Risk: (low/high/unclear) | Unclear | Unclear |
|
Rationale of bias rating: It was not clear whether or not pathologists were blinded to the CA125 results |
|||
| B. Applicability | |||
|
At what time point was the outcome determined: All surgery was performed within 120 days of ultrasound examination If a composite outcome was used, describe the relative frequency/distribution of each contributing outcome: NA |
|||
| Concern that the outcome, its definition, timing or determination do not match the review question | Concern: (low/high/unclear) | Low | Low |
| Rationale of applicability rating: | |||
Domain 4: analysis
| Risk of bias | |||
|---|---|---|---|
|
Describe numbers of participants, number of candidate predictors, outcome events and events per candidate predictor: The development data set included 3506 women and the validation data set included 2403 women. There were 10 candidate predictors. The development data set included 949 (27%) women with malignancies (including borderline tumours) and the validation data set included 980 (41%) women with malignancies (including borderline tumours) |
|||
|
Describe how the model was developed (predictor selection, optimism, risk groups, model performance): To avoid overfitting, 10 candidate predictors were selected; selection was based on topic expertise and stability of predictors across centres. Furthermore, data-driven selection used a method based on multivariable fractional polynomials; the variable selection procedure is a variant of the standard backward selection procedure. Age and type of centre were forced into the model To acknowledge the variability between centres, multinomial logistic regression with random centre intercepts was used to construct a polytomous model. Predictor coefficients were multiplied with uniform shrinkage factors to avoid exaggerated model coefficients |
|||
|
Describe whether and how the model was validated, either internally (e.g. bootstrapping, cross-validation, random split sample) or externally (e.g. temporal validation, geographical validation, different setting, different type of participants): The model was validated using data collected, by the same criteria, in a later phase of the IOTA study (temporal validation). Discriminatory performance was assessed using diagnostic accuracy measures, with histological diagnosis as the reference standard and by calculating a polytomous discrimination index. Calibration of predicted probabilities was assessed using calibration plots showing the relationship between predicted and observed probabilities for each type of tumour. The plots were based on a parametric, multinomial logistic n calibration analysis, using random centre intercepts |
|||
|
Describe the performance measures of the model [e.g. (re)calibration, discrimination, (re)classification, net benefit]: Discrimination measures and calibration plots were reported |
|||
|
Describe any participants who were excluded from the analysis: All participants who met the study inclusion criteria appear to have been included in the analysis |
|||
|
Describe missing data on predictors and outcomes as well as methods used for missing data: The CA125 data were missing for 31% of participants. Predictive mean-matching regression, using variables that were related to either the level of CA125 itself or to the unavailability of CA125 (i.e. a binary indicator indicating for each woman whether or not CA125 was missing) was used to estimate missing values. This was repeated 100 times to generate multiple imputations of the missing values, resulting in 100 completed data sets |
|||
| Dev | Val | ||
| 4.1 Were there a reasonable number of participants with the outcome? | Yes | Yes | |
| 4.2 Were continuous and categorical predictors handled appropriately? | Yes |

|
|
| 4.3 Were all enrolled participants included in the analysis? | Yes |

|
|
| 4.4 Were participants with missing data handled appropriately? | Yes |

|
|
| 4.5 Was selection of predictors based on univariable analysis avoided? | Yes |

|
|
| 4.6 Were important complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | Yes | Yes | |
| 4.7 Were relevant model performance measures evaluated (e.g. calibration and discrimination)? | Yes | Yes | |
| 4.8 Was model overfitting and optimism in model performance accounted for? | Unclear |

|
|
| 4.9 Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | Unclear |

|
|
| Risk of bias introduced by the analysis | Risk: (low/high/unclear) | Unclear | Low |
|
Rationale of bias rating: Some aspects of model development were not fully reported |
|||
Overall judgement about risk of bias and applicability of the prediction model evaluation
| Overall judgement of risk of bias | Risk: (low/high/unclear) | Unclear |
|
Summary of sources of potential bias: Some aspects of model development were not fully reported |
||
| Overall judgement of applicability | Concern: (low/high/unclear) | Low |
|
Summary of applicability concerns: The study setting is not a complete match for that specified in the scope for this assessment; however, the final ADNEX model includes a variable for centre type (general secondary care vs. tertiary referral gynaecological oncology setting); the model should therefore be usable in either setting |
||
Appendix 4 Full study details
| Study details | Selection criteria | Participant details | Test(s) |
|---|---|---|---|
|
Abdalla et al. (2013)48 Country: Poland Funding: NR Recruitment start–end: January 2011–December 2011 |
Inclusion criteria: women admitted with adnexal mass Exclusion criteria: ultrasound examination > 90 days before surgery; no CA125 level Study setting: mixed Point in care pathway at which index test is given: following referral to the Department of Clinical Obstetrics, Women’s Diseases and Gynaecological Oncology, in a university hospital |
All
|
IOTA group’s simple ultrasound rules, and RMI 1 |
|
Aktürk et al. (2011)71 Country: Turkey Funding: NR Recruitment start–end: October 2008–February 2010 |
Inclusion criteria: women with pelvic masses scheduled for laparotomy or laparoscopy Exclusion criteria: NR Study setting: secondary care (Department of Obstetrics and Gynaecology) Point in care pathway at which index test is given: following referral to secondary care (Department of Obstetrics and Gynaecology) |
Benign
|
RMI 1 threshold comparison |
|
Al Musalhi et al. (2016)103 Country: Oman Funding: other (unfunded) Recruitment start–end: March 2014–April 2015 |
Inclusion criteria: women attending a gynaecology department for investigation of an ovarian mass Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to a gynaecology department |
Benign
|
ROMA score using Abbott Diagnostics’ tumour marker assay and RMI 1 |
|
Alcázar et al. (2013)52 Country: Spain Funding: NR Recruitment start–end: January 2011–June 2012 |
Inclusion criteria: women with an adnexal mass, referred to one of two Spanish university centres (Clinica Universidad de Navarra, Pamplona or Spain and Institut Dexeus, Barcelona) Exclusion criteria: pregnancy, spontaneous resolution of the mass by the time of a 2- to 3-month follow-up scan, surgery not performed because of physician’s and/or patient’s decision at follow-up, or surgery performed in another centre Study setting: secondary care (Department of Obstetrics and Gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
All
|
IOTA group’s simple ultrasound rules |
|
Asif et al. (2004)77 Country: Pakistan Funding: NR Recruitment start–end: January 2001–January 2002 |
Inclusion criteria: consecutive women admitted to the Department of Gynaecology and Obstetrics (Military Hospital and Combined Military Hospital Rawalpindi) for elective surgical exploration and resection of proven ovarian mass Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Baker et al. (2013)66 Country: UK Funding: NR Recruitment start–end: NR |
Inclusion criteria: premenopausal women with ovarian masses Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to a district general hospital |
All
|
IOTA group’s simple ultrasound rules |
|
Chan et al. (2013)82 Country: Hong Kong, Taiwan, Republic of Korea, Japan, Thailand and the Philippines Funding: industry (Abbott Diagnostics) Recruitment start–end: NR 2009–NR 2010 |
Inclusion criteria: consecutive women (aged > 18 years) diagnosed with an adnexal mass by ultrasound scan, CT, PET or MRI scan Exclusion criteria: previous history of ovarian cancer, primary peritoneal or any known malignancy; or previous bilateral oophorectomy Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to one of six obstetrics and gynaecology departments |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Clemente and Benitez (2015)90 Country: the Philippines Funding: NR Recruitment start–end: October 2010–December 2013 |
Inclusion criteria: women with an adnexal mass who underwent surgery Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral to a tertiary care hospital (unclear whether or not referral was to a specialist gynaecological oncology department) |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Coleman et al. (2016)70 Country: USA Funding: industry (Vermillion Inc.) Recruitment start–end: August 2010–December 2011 |
Inclusion criteria: women ≥ 18 years with a documented pelvic mass who were scheduled for surgical intervention within 3 months of imaging, and who agreed to phlebotomy Exclusion criteria: diagnosis of malignancy in the previous 5 years (except of non-melanoma skin cancers) or enrolment by a gynaecologic oncologist Study setting: secondary care Point in care pathway at which index test is given: following referral to secondary care |
All
|
Overa (MIA2G) |
|
Davies et al. (1993)79 Country: UK Funding: NR Recruitment start–end: NR |
Inclusion criteria: retrospective review of women admitted consecutively to a gynaecology department for surgical investigation of an adnexal mass Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Di Legge et al. (2012)61 Country: Sweden, Belgium, Italy, Poland, UK, Czech Republic, China and Canada Funding: government (Swedish Research Council and the Research Foundation of Flanders) Recruitment start–end: NR |
Inclusion criteria: women with an adnexal mass recruited from 11 oncology referral centes, five general hospitals and three referral centres for ultrasonography Exclusion criteria: surgical removal of the mass > 120 days after ultrasound, pregnancy or inability to tollerate transvaginal ultrasonography Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care |
Tumour size ≤ 4 cm
|
IOTA group’s simple ultrasound rules; and RMI 1 |
|
Fathallah et al. (2011)63 Country: France Funding: NR Recruitment start–end: January 2002–December 2005 |
Inclusion criteria: women who had undergone surgery and histological analysis, following observation of at least one persistent ovarian cyst on two consecutive ultrasound examinations Exclusion criteria: NR Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care |
All
|
IOTA group’s simple ultrasound rules |
|
IOTA5 2017 Confidential information has been removed |
Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
|
Jacobs et al. (1990)78 Country: UK Funding: charity (Gynaecology Cancer Research Fund, Cancer Research Campaign) Recruitment start–end: NR |
Inclusion criteria: women admitted consecutively for surgical investigation of an adnexal mass Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Janas et al. (2015)97 Country: Poland Funding: government (MNISW) Recruitment start–end: NR 2011–NR 2014 |
Inclusion criteria: women referred for surgery for an adnexal mass Exclusion criteria: NR Study setting: mixed Point in care pathway at which index test is given: following referral to gynaecology or gynaecological oncology clinic |
All
|
ROMA score using Roche Diagnostics’ tumour marker assay |
|
Joyeux et al. (2016)43 Country: France Funding: NR Recruitment start–end: January 2013–December 2015 |
Inclusion criteria: women aged 14–100 years, received or referred with an adnexal mass (detected on ultrasound) requiring surgery Exclusion criteria: the absence of TVS, pregnancy, an echographic aspect of functional ovarian cyst or the lack of a CA125 level Study setting: secondary care (Department of Obstetrics and Gynaecology or Gynaecological Surgery) Point in care pathway at which index test is given: following referral to secondary care (Department of Obstetrics and Gynaecology) |
Malignant
|
ADNEX model |
|
Karlsen et al. (2012)83 Country: Denmark Funding: industry (Abbott Diagnostics provided assay reagents) Recruitment start–end: September 2004–January 2010 |
Inclusion criteria: women admitted to the gynaecology clinic for surgery because of a pelvic mass or pelvic pains potentially caused by a malignant disease or endometriosis Exclusion criteria: preoperative-known relapse of a previous cancer or active cancer other than ovarian cancer Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to a gynaecology clinic |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay; and RMI 1 |
|
Knafel et al. (2015)49 Country: Poland Funding: NR Recruitment start–end: January 2011–October 2012 |
Inclusion criteria: women, aged ≥ 18 years with an adnexal tumour requiring surgery Exclusion criteria: pregnancy, lack of a histopathology result or surgery performed > 90 days after diagnosis Study setting: unclear Point in care pathway at which index test is given: following referral to a university hospital, Department of Oncology and Gynaecology |
All
|
IOTA group’s simple ultrasound rules |
|
Langhe et al. (2013)94 Country: NR Funding: NR Recruitment start–end: NR |
Inclusion criteria: women scheduled for surgery for invasive, borderline and benign ovarian disease Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral to hospital |
All
|
ROMA score using Fujirebio Diagnostics’ tumour marker assay |
|
Li et al. (2016)96 Country: China Funding: government (Specialised Research Fund for the Doctoral Programme of Higher Education of China; Science and Technology Department, Guangdong province; Natural Science Foundation, Guangdong province; and the Science and Technology Department of Guangzhou City) Recruitment start–end: September 2012–April 2014 |
Inclusion criteria: women with gynaecological diseases, diagnosed by ultrasound, CT, PET-CT or MRI Exclusion criteria: previous or concomitant history of malignant disease; bilateral oophorectomy Study setting: unclear Point in care pathway at which index test is given: following referral to a university hospital |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Lou et al. (2010)73 Country: China Funding: NR Recruitment start–end: June 2008–December 2008 |
Inclusion criteria: women with an adnexal mass Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to an obstetrics and gynaecology department |
All
|
RMI 1 threshold comparison |
|
Manjunath et al. (2001)75 Country: India Funding: NR Recruitment start–end: January 1997–August 1999 |
Inclusion criteria: retrospective study of women admitted for surgical exploration of pelvic masses Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Meys et al. (2016)44 Country: the Netherlands Funding: government (Academic fund of the University of Maastricht) Recruitment start–end: July 2011–July 2015 |
Inclusion criteria: consecutive women with adnexal pathology Exclusion criteria: no pathology result obtained, pathology result known before the ultrasound scan, pathology > 120 days after ultrasound or previous oophorectomy Study setting: secondary Point in care pathway at which index test is given: following referral to an obstetrics and gynaecology department |
Metastases
|
ADNEX model; IOTA group’s simple ultrasound rules and RMI 1 |
|
Moffatt et al. (2016)45 Country: UK Funding: NR Recruitment start–end: January 2014–September 2015 |
Inclusion criteria: women with excised adnexal masses that had been sent for histological analysis Exclusion criteria: ectopic pregnancy, no ultrasound available or no CA125 level Study setting: unclear Point in care pathway index test is given: following referral to secondary care |
All
|
ADNEX model |
|
Moore et al. 2011101 Country: USA Funding: mixed (Fujirebio Diagnostics and the National Cancer Institute) Recruitment start–end: October 2009–August 2010 |
Inclusion criteria: women (aged ≥ 18 years) presenting to a generalist (general gynaecologist, internist, family practitioner, gastroenterologist or general surgeon) with an ovarian cyst or adnexal mass and subsequently scheduled to undergo surgery Exclusion criteria: NR Study setting: mixed Point in care pathway at which index test is given: following referral to a general or specialist hospital |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Morgante et al. (1999)80 Country: Italy Funding: NR Recruitment start–end: January 1995–December 1997 |
Inclusion criteria: women aged > 30 years admitted consecutively for surgical excision of ovarian masses Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Murala et al. (2014)60 Country: UK Funding: NR Recruitment start–end: September 2012–September 2013 |
Inclusion criteria: women referred to Poole District General Hospital or the Royal Bournmouth District General Hospital, with suspected adnexal pathology Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care |
All
|
IOTA group’s Simple Rules and RMI 1 |
|
Novotny et al. (2012)86 Country: the Czech Republic Funding: government (Ministry of Health, the Czech Republic) Recruitment start–end: NR |
Inclusion criteria: women with abnormalities of the pelvis Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to a gynaecology and obstetrics department |
Malignant
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Piovano et al. (2016)58 Country: Italy Funding: NR Recruitment start–end: February 2013–January 2015 |
Inclusion criteria: consecutive women (aged ≥ 18 years), with an adnexal mass, who were candidates for surgery Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral to hospital |
All
|
IOTA group’s simple ultrasound rules |
|
Presl et al. (2012)81 Country: the Czech Republic Funding: government Recruitment start–end: June 2010–January 2011 |
Inclusion criteria: women with abnormalities in the pelvis Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to a university hospital’s Department of Obstetrics and Gynaecology |
All
|
ROMA score using Abbott Diagnostics’ tumour marker assay |
|
Ruiz de Gauna et al. (2015)64 Country: Spain Funding: NR Recruitment start–end: June 2012–December 2013 |
Inclusion criteria: women diagnosed with a persistent adnexal mass evaluated in one of two Spanish centres Exclusion criteria: pregnant women, masses with spontaneous resolution or masses removed surgically in another centre from recruitment Study setting: mixed Point in care pathway at which index test is given: following referral to secondary care |
All
|
IOTA group’s simple ultrasound rules |
|
Sayasneh 201362 Country: UK Funding: government (NHS, NIHR and Imperial College London) Recruitment start–end: September 2010–September 2012 |
Inclusion criteria: women with at least one adnexal mass, who underwent TVS examination at one of the participating centres Exclusion criteria: surgical removal of the mass > 120 days after ultrasound, refusal to undergo TVS, pregnancy, examined by a consultant with a specialist interest in gynaecological malignancy, or cytology rather than histology used to establish diagnosis Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care |
All
|
IOTA group’s simple ultrasound rules; and RMI 1 |
|
Sayasneh et al. (2016)46 Country: UK and Italy Funding: charity (NIHR; FWO grants; and a KU Leuven grant) Recruitment start–end: September 2010–February 2015 |
Inclusion criteria: women presenting ≥ 1 adnexal mass who underwent transvaginal ultrasonography Exclusion criteria: pregnancy, women examined by a consultant, refusal of TVS, cytology rather than histology as an outcome and failure to undergo surgery within 120 days of the ultrasound examination Study setting: tertiary care (cancer centres) Point in care pathway at which index test is given: referral to tertiary care |
All
|
ADNEX model |
|
Shulman et al. (2016)104 Country: USA Funding: NR Recruitment start–end: NR |
Inclusion criteria: two published registries of women undergoing surgery for an adnexal mass Exclusion criteria: NR Study setting: secondary care Point in care pathway at which index test is given: following referral to secondary care |
All
|
Overa (MIA2G) and ROMA score using Roche Diagnostics’ tumour marker assay |
|
Silvestre et al. (2015)55 Country: Brazil Funding: NR Recruitment start–end: September 2008–December 2010 |
Inclusion criteria: women who were consecutively scheduled for surgery to remove adnexal masses Exclusion criteria: NR Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care (Department of Obstetrics and Gynaecology) |
Malignant
|
IOTA group’s simple ultrasound rules |
|
Szubert et al. (2016)42 Country: Poland and Spain Funding: NR Recruitment start–end: December 2012–April 2015 |
Inclusion criteria: women requiring surgery for an ovarian tumour, who had complete data required for the ADNEX calculation and who were evaluated between 1 and 5 days before surgery Exclusion criteria: NR Study setting: secondary care (Department of Obstetrics and Gynaecology or Gynaecological Surgery) Point in care pathway at which index test is given: following referral to secondary care (Division of Gynaecological Surgery or Department of Obstetrics and Gynaecology) |
Poland
|
ADNEX model |
|
Tantipalakorn et al. (2014)51 Country: Thailand Funding: government (Faculty of Medicine Research, Fund of Chiang Mai University and the National Research University Project, Thailand) Recruitment start–end: April 2007–March 2012 |
Inclusion criteria: women scheduled for surgery because of the detection of an adnexal mass (by pelvic examination, previous ultrasonography or both) Exclusion criteria: known diagnoses of adnexal masses, ovarian cancers scheduled for second-look operation or endometrioma diagnosed by previous laparoscopy, etc.; or patients undergoing surgery > 24 hours after ultrasound examination Study setting: secondary care Point in care pathway at which index test is given: following referral to secondary care |
All
|
IOTA group’s simple ultrasound rules |
|
Testa et al. (2014)50 Country: Sweden, Belgium, Italy, Poland, Spain and the Czech Republic Funding: government (Swedish Research Council and the UK’s NIHR) Recruitment start–end: October 2009–May 2012 |
Inclusion criteria: women with at least one adnexal mass (ovarian, paraovarian or tubal), who underwent TVS examination by a principal investigator at one of the participating centres Exclusion criteria: surgical removal of the mass > 120 days after ultrasound, pregnancy at ultrasound, unresolved data inconsistencies or incomplete final histology Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care |
All
|
IOTA group’s simple ultrasound rules and RMI 1 |
|
Timmerman et al. (2010)65 Country: Sweden, Belgium, Italy, Poland, UK, the Czech Republic, China and Canada Funding: government (Swedish Research Council and Research Council KU Leuven) Recruitment start–end: NR 2005–NR 2007 |
Inclusion criteria: women with at least one adnexal mass, who underwent TVS examination by a principal investigator at one of the participating centres Exclusion criteria: surgical removal of the mass > 120 days after ultrasound, refusal to undergo TVS, or pregnancy Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care |
All
|
IOTA group’s Simple Rules and RMI 1 |
|
Tingulstad et al. (1996)76 Country: Norway Funding: NR Recruitment start–end: February 1992–February 1994 |
Inclusion criteria: women with a pelvic mass, who were scheduled for laparotomy and who were at least 30 years old Exclusion criteria: NR Study setting: secondary care (Department of Gynaecology and Obstetrics) Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
RMI 1 threshold comparison |
|
Tinnangwattana et al. (2015)47 Country: Thailand Funding: government (Office of the Higher Education Commission) Recruitment start–end: March 2014–December 2014 |
Inclusion criteria: women scheduled for surgery because of an adnexal mass either detected by pelvic examination or previous ultrasound examination Exclusion criteria: known diagnoses or surgery > 24 hours after ultrasound examination Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to secondary care (Department of Obstetrics and Gynaecology) |
All
|
IOTA group’s simple ultrasound rules |
|
Ulusoy et al. (2007)74 Country: Turkey Funding: NR Recruitment start–end: September 2002–November 2004 |
Inclusion criteria: consecutive women undergoing surgery for an adnexal mass Exclusion criteria: known ovarian malignancy or pregnancy Study setting: mixed Point in care pathway at which index test is given: following referral to secondary or tertiary care (Department of Gynaecology and Obstetrics and Gynaecological Oncology Clinic) |
Malignant
|
RMI 1 threshold comparison |
|
Van Calster et al. (2014)17 Country: Belgium, Sweden, Italy, the Czech Republic, Poland and the UK Funding: government (FWO) Recruitment start–end: NR 1999–NR 2012 |
Inclusion criteria: consecutive women with ≥ 1 adnexal mass (judged not to be a physiological cyst), examined with TVS and selected for surgical intervention Exclusion criteria: refusal of TVS, pregnancy or surgical removal of the mass > 120 days after ultrasound Study setting: mixed (oncology centres, general hospitals and gynaecology units) Point in care pathway at which index test is given: following referral to secondary or tertiary care |
All
|
ADNEX model |
|
Van Gorp et al. (2012)98 Country: Belgium Funding: government (Belgian Federation against Cancer and FWO) Recruitment start–end: August 2005–March 2009 |
Inclusion criteria: women with a pelvic mass, suspected to be of ovarian origin, who were scheduled to undergo surgery Exclusion criteria: prior bilateral oophorectomy Study setting: unclear Point in care pathway at which index test is given: following referral to a university hospital for resection of a pelvic mass |
Malignant
|
ROMA score using Fujirebio Diagnostics’ tumour marker assay and RMI 1 |
|
Weinberger and Minar (2013)53 Country: NR Funding: other (NR) Recruitment start–end: NR 2010–NR 2012 |
Inclusion criteria: women with suspiscious adnexal mass Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral (unclear whether secondary or tertiary care) |
All
|
IOTA group’s simple ultrasound rules |
|
Winarto et al. (2014)99 Country: Indonesia Funding: NR Recruitment start–end: November 2010–May 2011 |
Inclusion criteria: women diagnosed with an ovarian tumour, by physical examination and TVS Exclusion criteria: unresectable tumour, non-epithelial histopathological results, history of oophorectomy, ovarian cancer treatment or pregnancy Study setting: unclear Point in care pathway at which index test is given: following referral to hospital |
Malignant
|
ROMA score using Abbott Diagnostics’ tumour marker assay and RMI 1 |
|
Xu et al. (2016)95 Country: China Funding: government (Guangdong Natural Science Foundation, Guangdong Province Science and Technology Project Plan and Social Development Foundation, and the Medical Science and Technology Research Foundation of Guangdong Province) Recruitment start–end: July 2013–November 2014 |
Inclusion criteria: retrospective study of women with an ovarian mass Exclusion criteria: missing tumour marker data or women with non-epithelial ovarian cancer Study setting: unclear Point in care pathway at which index test is given: following referral to secondary care |
Malignant
|
ROMA score using Roche Diagnostics’ tumour marker assay |
|
Yamamoto et al. (2009)72 Country: Japan Funding: NR Recruitment start–end: January 2002–April 2005 |
Inclusion criteria: women with a pelvic mass scheduled for laparotomy and laparoscopy at the Department of Obstetrics and Gynaecology, Kochi Medical School Exclusion criteria: NR Study setting: secondary care (Department of Gynaecology and Obstetrics) Point in care pathway at which index test is given: following referral to secondary care (Department of Obstetrics and Gynaecology) |
Malignant
|
RMI 1 threshold comparison |
|
Yanaranop et al. (2016)89 Country: Thailand Funding: NR Recruitment start–end: January 2012–December 2012 |
Inclusion criteria: women, aged ≥ 18 years, undergoing elective surgery for clinically diagnosed pelvic or adnexal mass Exclusion criteria: pregnancy, previous history of ovarian cancer, any known malignancy, previous history of adnexal surgery, incomplete ultrasound or biomarker results, or cancelled surgery Study setting: secondary care (general gynaecology) Point in care pathway at which index test is given: following referral to an obstetrics and gynaecology department |
All
|
ROMA score using Roche Diagnostics’ tumour marker assay and RMI 1 |
|
Zhang et al. (2015a)68 Country: USA Funding: NR Recruitment start–end: NR |
Inclusion criteria: women with a documented pelvic mass scheduled for surgery Exclusion criteria: NR Study setting: unclear Point in care pathway at which index test is given: following referral (unclear whether secondary or tertiary care) |
All
|
Overa (MIA2G) |
|
Zhang et al. (2015)102 Country: China Funding: government (National High Technology Research and Development Programme, China Postdoctoral Science Special Foundation, National Science and Technology Infrastructure, and the National Science Foundation of China) Recruitment start–end: October 2012–February 2013 |
Inclusion criteria: women with a pelvic mass, suspected to be of ovarian origin, who were to undego surgery Exclusion criteria: aged < 18 years; missing clinical examination results; blood sample of < 0.5 ml, stored or transported at > 0 °C, lipaemic or haemolytic appearance; pregnancy; family history of ovarian cancer; or receiving chemotherapy, radiotherapy and other treatments Study setting: unclear Point in care pathway at which index test is given: following referral to one of nine centres |
Malignant
|
ROMA score using Roche Diagnostics’ tumour marker assay |
Index test details
Risk of Ovarian Malignancy Algorithm score using Abbott Diagnostics’ tumour marker assay
| Study (year of publication) | ROMA score using Abbott Diagnostics’ tumour marker assay details | ||
|---|---|---|---|
| Analyser, manufacturer of CA125 and HE4 assays | Sample collection, storage | Time from test to surgery | |
| Al Musalhi et al. (2016)103 | ARCHITECT I2000, Abbott Diagnostics |
Samples collected using serum separator tubes and centrifuged immediately Serum samples were stored at –20 °C |
NR |
| Chan et al. (2013)82 | ARCHITECT, Abbott Diagnostics |
Blood samples were collected after pelvic mass was confirmed and surgery scheduled, to minimise the time between testing and surgery. Samples were centrifuged and serum separated within 4 hours of collection Serum samples were stored at –20 °C |
NR |
| Chan et al. (2013)82 | ARCHITECT, Abbott Diagnostics |
Blood samples were collected after pelvic mass was confirmed and surgery scheduled, to minimise the time between testing and surgery. Samples were centrifuged and serum separated within 4 hours of collection Serum samples were stored at –20 °C |
NR |
| Clemente et al. (2015)90 | NR, Abbott Diagnostics | NR | NR |
| Karlsen et al. (2012)83 | ARCHITECT I 2000sr, Abbott Diagnostics | Blood samples were collected within 2 weeks prior to surgery | NR |
| Samples were centrifuged within 6 hours of collection. After centrifugation, serum samples were stored at –80 °C until analysis | |||
| Li et al. (2016)96 | ARCHITECT, Abbott Diagnostics |
Blood samples were collected on the day of surgery, before anaesthesia. Samples were centrifuged and serum separated Serum samples were stored at –80 °C |
< 1 day |
| Moore et al. (2011)101 | ARCHITECT i2000, Abbott Diagnostics |
Blood samples were collected within 30 days prior to surgery and before induction of anaesthesia Samples were collected into a serum separator tube and centrifuged after clotting Serum samples were stored at –20 °C |
≤ 30 days |
| Novotny et al. (2012)86 | ARCHITECT 1000i, Abbott Diagnostics |
Blood samples were collected prior to surgery or treatment and centrifuged Serum samples were stored at –80 °C |
NR |
| Presl et al. (2012)81 | ARCHITECT 1000, Abbott Diagnostics |
Blood samples were centrifuged immediately or within 24 hours of collection Serum samples were stored at –80 °C |
NR |
| Winarto et al. (2014)99 | NR, Abbott Diagnostics | NA | Blood samples collected 1 day before surgery, time from ultrasound to surgery unclear |
Risk of Ovarian Malignancy Algorithm score using Roche Diagnostics’ tumour marker assays
| Study (year of publication) | ROMA score using Roche Diagnostics’ tumour marker assay details | ||
|---|---|---|---|
| Analyser, manufacturer of CA125 and HE4 assays | Sample collection, storage | Time from test to surgery | |
| Janas et al. (2015)97 | NR, Roche Diagnostics | NR | NR |
| Shulman et al. (2016)104 | NR | NR | NR |
| Xu et al. (2016)95 | Cobas E170, Roche Diagnostics |
Blood samples were collected before surgery and centrifuged within 3 hours Serum samples were stored at –80 °C |
NR |
| Yanaranop et al. (2016)89 | Cobas 6000, Roche Diagnostics |
Samples were collected within 48 hours prior to surgery and centrifuged immediately Serum samples were stored at –20 °C |
Within 6 weeks before surgery |
| Zhang et al. (2015)102 | Cobas 601, Roche Diagnostics |
Blood samples were collected into a tube containing a clot activator and centrifuged Serum samples were stored at –80 °C |
NR |
Risk of Ovarian Malignancy Algorithm score using Fujirebio Diagnostics’ tumour marker assays
| Study (year of publication) | ROMA score using Fujirebio Diagnostics’ tumour marker assay details | ||
|---|---|---|---|
| Analyser, manufacturer of CA125 and HE4 assay | Sample collection, storage | Time from test to surgery | |
| Langhe et al. (2013)94 | NR, Fujirebio Diagnostics | NR | Collected before surgery |
| Van Gorp et al. (2012)98 | NR, Fujirebio Diagnostics |
Blood samples were collected in clotting tubes, immediately before surgery After centrifugation, serum samples were stored at –80 °C until analysis |
Time from ultrasound to surgery NR |
Assessment of Different NEoplasias in the adneXa
| Study (year of publication) | ADNEX model test details | |||
|---|---|---|---|---|
| Analyser, manufacturer of CA125 assay | Ultrasound details | Sample collection storage | Time from test to surgery | |
| IOTA5 (2017)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Joyeux et al. (2016)43 | NR | TVS could be complemented by another imaging technique (abdominal ultrasound, CT scan, MRI) | NR | NR |
| Meys et al. (2016)44 | NR | TVS or transrectal ultrasound with transabdominal ultrasound for larger masses | NA | ≤ 120 days |
| Meys et al. (2016)44 | NR | Transvaginal or transrectal ultrasound with transabdominal ultrasound for larger masses | NA | ≤ 120 days |
| Moffatt et al. (2016)45 | NR | NR | NR | NR |
| Sayasneh et al. (2016)46 | NR |
TVS examinations performed by EFSUMB level 2 ultrasound examiners (non-consultant gynaecology specialist, gynaecology trainees doctors and gynaecology sonographers) The ultrasound examiners were blind to the results of the reference test TVS was performed using the standardised approach previously published by the IOTA group14 Transabdominal ultrasonography was undertaken for a large mass |
NR | NR |
| Szubert et al. (2016)42 |
Unclear Immunoenzymatic test (ST AIA-PACK OVCATosoH Bioscience) and Cobas-Core CA-125-II (Roche Diagnostics) Roche Diagnostics and Tosoh Bioscience |
Poland: Aloka Alpha 10 (3.75–7.5 MHz) endovaginal probe and Aloka 3500 (7.5 MHz) endovaginal probe (Hitachi Aloka) Spain: TVS or transrectal ultrasound Voluson E8 (RIC5–9 MHz) endovaginal probe (GE Healthcare) A transabdominal probe was used for large tumours Tumours were ultrasonographically assessed according to the 2000 IOTA criteria 14 |
Poland: assessed 1–5 days before surgery Spain: assessed 1–5 days before ultrasound No further details reported |
1–5 days |
| Van Calster et al. (2014)17 |
Immunoradiometric assay kits for CA-125 II Roche Diagnostics, Centocor, Cis-Bio, Abbott Laboratories, Bayer Diagnostics, bioMérieux, DiaSorin, Siemens and Beckman Coulter |
Standardised TVS examination (additional transabdominal sonography for women with large masses) All ultrasound examinations were performed by one of three experienced practitioners [with 8–20 years’ experience in gynaecological sonography and EFSMUB level 2 (Poland) and level 3 (Spain)] |
NR | NR |
International Ovarian Tumour Analysis group’s simple ultrasound rules
| Study (year of publication) | Ultrasound details | Time from test to surgery |
|---|---|---|
| Abdalla et al. (2013)48 | TVS with transabdominal ultrasound for tumours larger than 5 cm and extended beyond the pelvis minor. Morphology, echostructure and vascularisation were assessed by Doppler examination. Ultrasound examinations were performed by the attending physician (various levels of experience) prior to referral to the hospital | ≤ 90 days |
| Alcazar et al. (2013)52 | Transvaginal colour Doppler ultrasound (5- to 9-MHz transducers), Voluson E8 or 730 machine (GE Healthcare, Chicago, IL, USA). Transabdominal scanning was also performed in large masses. Ultrasound was performed by a trainee or junior staff under the supervision of an expert | Surgery was performed within 3 weeks after ultrasound examination |
| Baker et al. (2013)66 | Retrospective review of ultrasound scan reports | NR |
| DiLegge et al. (2012)61 | High-frequency transvaginal probe with transabdominal ultasonography for large masses that could not be entirely visualised using a transvaginal probe | ≤ 120 |
| Fathallah et al. (2011)63 | Endovaginal | NR |
| IOTA5 (2017)a | Confidential information has been removed | Confidential information has been removed |
| Knafel et al. (2015)49 | Transvaginal (5–9 MHz) ultrasound with transabdominal (2–5 MHz) ultrasound for larger tumours. Examinations were performed by both EFSUMB level 1 and level 2 examiners. All examiners received 1 half-day of practical training in the IOTA group’s simple ultrasound rules before the study | ≤ 90 days |
| Meys et al. (2016)44 | Transvaginal or transrectal ultrasound with transabdominal ultrasound for larger masses | ≤ 120 days |
| Murala et al. (2014)60 | Scan images were analysed by non-expert gynaecology trainees and masses were classified as benign, malignant or inconclusive | NR |
| Piovano et al. (2016)58 | Greyscale and Doppler TVS, performed by a trainee who had undergone IOTA group’s Simple Rules training and was supervised by an experienced examiner. A transabdominal probe was used for large masses that could not be entirely visualised transvaginally. All inconclusive masses were re-evaluated by a consultant expert (EFSUMB level) | ≤ 30 days |
| Ruiz de Gauna et al. (2015)64 | Transvaginal colour Doppler ultrasound (5- to 9-MHz transducers), Voluson E8 machine. Transabdominal scanning was also performed in large masses. In Centre A, ultrasound scanning was performed by an expert, and in centre B, ultrasound scanning was performed by a trainee. In both centres, masses classified as inconclusive by the IOTA group’s simple ultrasound rules were given a classification of benign, malignant or uncertain, based on the subjective assessment of an expert examiner; patients with a final classification of malignant or uncertain were referred to specialist gynaecological oncology services | Surgery was performed within 3 weeks after ultrasound examination |
| Sayasneh et al. (2013)62 | Standardised ultrasound conducted at one of the participating centres. Transabdominal ultasonography was used for large masses that could not be entirely visualised using a transvaginal probe | ≤ 120 days |
| Silvestre et al. (2015)55 | The descriptions of the masses were interpreted based on the IOTA group’s simple ultrasound rules15 to characterise whether the features were malignant or benign. Vascular power Doppler score is included in the IOTA group’s simple ultrasound rules as one variable: a score of 1 is given when no blood flow is found in the tumour, a score of 2 when only minimal flow is detected, a score of 3 when moderate flow is present and a score of 4 when the tumour presents marked blood flow | 7 days |
| Tantipalakorn et al. (2014)51 | Transabdominal (3.5- to 5-MHz curvilinear transducer) or transvaginal (real-time 5–7.5 MHz) or both, connected to Aloka model SSD alpha-10 (Tokyo, Japan). IOTA group’s simple ultrasound rules15 were applied to determine whether there were malignant (M) features or benign (B) features. If one or more M-rules apply in the absence of a B-rule, the mass is classified as malignant. If one or more B-rules apply in the absence of an M-rule, the mass is classified as benign. If both M-rules and B-rules apply, the mass cannot be classified or inconclusive. Likewise, if no rule applies, the mass cannot be classified or inconclusive | All participants underwent ultrasound examination within 24 hours of operation |
| Testa et al. (2014)50 | Standardised TVS by examiners experienced in gynaecological ultrasound (Level III Education, Practical Standards Committee, EFSUMB), grey scale and Doppler imaging; when there was more than one adnexal mass, the mass with the most complex morphology was assessed and analysed | ≤ 120 days |
| Timmerman et al. (2010)65 | Standardised ultrasound conducted by a principal investigator at one of the participating centres. All principal investigators were fully trained gynaecologists or radiologists with a special interest in gynaecological ultrasound and at least 5 years’ experience. Transvaginal probe frequencies ranged from 5 to 12 MHz and transabdominal ultasonography was used for large masses that could not be entirely visualised using a transvaginal probe. Doppler ultrasound images were used to obtain morphological and blood-flow variables | ≤ 120 days |
| Tinnangwattana et al. (2015)47 | All examinations were done with either transabdominal or transvaginal approach as suitable, using real-time 5- to 7.5-MHz transvaginal or 2.5- to 5-MHz transabdominal curvilinear transducer connected to a machine (Hitachi Aloka model ProSound37) | ≤ 24 hours |
| Weinberger and Minar (2013)53 | Retsopective analysis by an experienced sonographer | NR |
Overa (MIA2G)
| Study (year of publication) | Overa (MIA2G) test details | ||
|---|---|---|---|
| Analyser, manufacturer of assays | Sample collection, storage | Time from test to surgery | |
| Coleman et al. (2016)70 |
Roche Diagnostics’ Cobas 6000 clinical analyser (c501 and e601 modules) Roche Diagnostics’ Cobas assays for apo A-1, TRF (immunoturbidimetric assays), CA125-II, HE4 and FSH (electrochemiluminescent detection) |
A preoperative blood sample of 80 ml was processed within 1–6 hours of collection, and serum was frozen at the collection site Frozen and stored at –65 to –85 °C. No sample had undergone > 2 or < 2 freeze–thaw cycles |
Median 1 week (range 0–11) |
| Shulman et al. (2016)104 | NR | NR | NR |
| Zhang et al. (2015)68 | NR | NR | NR |
Risk of Malignancy Index 1
| Study (year of publication) | RMI test details | |||
|---|---|---|---|---|
| Analyser, manufacturer of CA125 assay | Ultrasound details | Sample collection, storage | Time from test to surgery | |
| Abdalla et al. (2013)48 | NR |
TVS with transabdominal ultrasound for tumours larger than 5 cm and extended beyond the pelvis minor Morphology, echostructure and vascularisation were assesed by Doppler examination Ultrasound examinations were performed by the attending physician (various levels of experience) prior to referral to the hospital |
NR | ≤ 90 days |
| Aktürk et al. (2011)71 | Electrochemiluminescence immunoassay, Roche Diagnostics | Siemens transvaginal 7.5-MHz transducer, with transabdominal ultrasound if the mass was too large for complete visualisation transvaginally |
Serum samples were collected preoperatively NR |
NR |
| Al Musalhi et al. (2016)103 | ARCHITECT I2000, Abbott Diagnostics | Pelvic ultrasonography by specialist gynaecologists |
Samples were collected using serum separator tubes and centrifuged immediately Serum samples were stored at –20 °C |
NR |
| Asif et al. (2004)77 | IMMULITE-Automated Analyser DPC-U5 A (CA125), Siemens Healthineers, Erlangen, Germany | Score based on presence of multilocular cystic lesion, solid areas, bilateral lesions, ascites and abdominal metastasis: 0 = no positive factor; 1 = single positive factor; 3 = two–five positive factors |
Venous blood was collected in a plain tube, avoiding haemodialysis. Serum was isolated by centrifugation Serum was stored at –20 °C |
NR |
| Davies et al. (1993)79 | Radioimmunoassay, CIS bioindustries | One point score was assigned for ultrasound investigation for each of the following: multilocular cyst, evidence of solid areas, evidence of metastases, presence of ascites and bilateral lesions |
Peripheral venous blood samples were drawn from each patient before surgery Blood was allowed to clot at room temperature then centrifuged at 3000 r.p.m. for 10 minutes and serum was separated and stored at –20 °C |
NR |
| Di Legge et al. (2012)61 |
Centocor or Cis-Bio or Abbott Diagnostics’ Axsym system or Immuno-l-analyser or Vidas Centocor or Cis-Bio or Abbott Diagnostics’ or Bayer or Vidas |
High-frequency transvaginal probe with transabdominal ultasonography for large masses that could not be entirely visualised using a transvaginal probe | NR | ≤ 120 days |
| IOTA5 (2017)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Jacobs et al. (1990)78 | Radioimmunoassay, Abbott Laboratories CA125 | One point score was assigned for ultrasound investigation for each of the following: multilocular cyst, evidence of solid areas, evidence of metastases, presence of ascites and bilateral lesions |
Peripheral venous blood samples were drawn from each patient before surgery Blood was allowed to clot at room temperature then centrifuged at 3000 r.p.m. for 10 minutes and serum was separated and stored at –20 °C |
NR |
| Karlsen et al. (2012)83 | ARCHITECT I 2000sr, Abbott Diagnostics | No details reported |
Blood samples were collected within 2 weeks prior to surgery Samples were centrifuged within 6 hours of collection. After centrifugation, serum samples were stored at –80 °C until analysis |
Time from ultrasound to surgery, NR |
| Lou et al. (2010)73 | NR | NR | NR | NR |
| Manjunath et al. (2001)75 | Microparticle EIA, Abbott Diagnostics’ AXSYM System | The ultrasound was performed vaginally by a 5-MHz transducer (Ultramark 4 PLUS, Advanced Technology Laboratories) and extended to the transabdominal approach with full bladder if the mass was huge. A score (1 point each) was assigned for the following morphological features seen on ultrasound, suggestive of malignancy: the presence of a multilocular cystic lesion, solid areas, bilateral lesions, ascites and intra-abdominal metastases |
Serum samples were collected preoperatively No details of storage were reported |
NR |
| Meys et al. (2016)44 | NR | Transvaginal or transrectal ultrasound with transabdominal ultrasound for larger masses | NR | ≤ 120 days |
| Morgante et al. (1999)80 | NA, Centocor | Siemens Somoline SL2 with transabdominal probe (3.5 MHz) and transvaginal probe (5–7.5 MHz). One point score was assigned for ultrasound investigation for each characteristics: presense of multilocular cystic lesions, solid areas, bilateral lesions, ascites and intra-abdominal metastates |
Peripheral venous blood samples were drawn from each patient before surgery Serum stored at –15 °C |
NR |
| Sayasneh et al. (2013)62 |
Abbott Diagnostics’ ARCHITECT CA125 II immunoassay kit, ADVIA Centaur XP Immunoassay System, UniCel DxI Immunoassay System Beckman; Abbott Diagnostics; Siemens |
Transabdominal ultasonography was used for large masses that could not be entirely visualised using a transvaginal probe | NR | NR |
| Testa et al. (2014)50 | NR |
Standardised TVS by examiners experienced in gynaecological ultrasound (EFSUMB level 3) Greyscale and Doppler imaging When there was more than one adnexal mass, the mass with the most complex morphology was assessed and analysed |
NR | ≤ 120 days |
| Timmerman et al. (2010)65 | NR | Standardised ultrasound conducted by a principal investigator at one of the participating centres. All principal investigators were fully trained gynaecologists or radiologists with a special interest in gynaecological ultrasound and at least 5 years’ experience. Transvaginal probe frequencies ranged from 5 to 12 MHz and transabdominal ultasonography was used for large masses that could not be entirely visualised using a transvaginal probe. Doppler ultrasound images were used to obtain morphologiocal and blood flow variables | NR | NR |
| Tingulstad et al. (1996)76 | NR, Abbott Diagnostics | TVS with transabdominal ultrasound as needed | NR | NR |
| Ulusoy et al. (2007)74 | Roche Diagnostics–Hitachi Modular E170 Immunologic Analyser System, NR | Ultrasound examinations performed with Toshiba Sonolayer SSA-270A and/or a Siemens Sonoline G50 (abdominal convex transducers and/or endovaginal probes). Gynaecological oncologists evaluated all patients | NR | NR |
| Van Gorp et al. (2012)98 | NR, Fujirebio Diagnostics | Standardised TVS performed by an experienced examiner or a trainee supervised by an experienced examiner. Transabdominal ultrasound was added for large masses that could not be visualised completely using a transvaginal probe |
Blood samples were collected in clotting tubes immediately before surgery After centrifugation, serum samples were stored at –80 °C until analysis |
Time from ultrasound to surgery NR |
| Winarto et al. (2014)99 | NR, Abbott Diagnostics | TVS | NR | Samples collected 1 day before surgery, time from ultrasound to surgery unclear |
| Yamamoto et al. (2009)72 | ECLusys CA125 II assay, Roche Diagnostics | Transvaginally with a 6.0 MHz transducer (an abdominal scan was also conducted when indicated) |
Blood samples were taken pre-operatively No further details reported |
NR |
| Yanaranop et al. (2016)89 | Cobas 6000, Roche Diagnostics |
Pelvic (transabdominal or transvaginal) ultrasound using a Voluson E8 Examiner blinded to clinical information and serum biomarkers Morphological features noted (wall structure and thickness, echogenicity, multiocularity, solid areas, bilaterality, ascites and intra-abdominal metastases) |
Samples were collected within 48 hours prior to surgery and centrifuged immediately Serum samples were stored at –20 °C |
Within 6 weeks to surgery |
| Study (year of publication) | Test | Histology details for malignancy (n) |
|---|---|---|
| Abdalla et al. (2013)48 | IOTA group’s simple ultrasound rules; and RMI 1 | Malignancy included serous cystadenocarcinoma (n = 7), metastatic tumours from the gastrointestinal tract (n = 3), borderline tumours (n = 3), mucinous cystadenocarcinoma (n = 1), fallopian tube carcinoma (n = 1), mixed carcinoma (n = 1) and undifferentiated carcinoma (n = 1) |
| Aktürk et al. (2011)71 | RMI 1 threshold comparison | Malignancy included primary ovarian cancer (n = 19) and metastases (n = 1) |
| Al Musalhi et al. (2016)103 | ROMA score using Abbott Diagnostics’ tumour marker assay and RMI 1 | Malignancy included serous adenocarcinoma (n = 20), mucinous adenocarcinoma (n = 1), endometrial adenocarcinoma (n = 3), undifferentiated (n = 1), borderline epithelial (n = 7), granulosa (n = 5), yolk sac cancer (n = 1), teratoma (n = 2), secondaries (n = 7) and lymphoma (n = 1) |
| Alcázar et al. (2013)52 | IOTA group’s simple ultrasound rules | Malignancy included invasive epithelial ovarian cancer (n = 29), borderline ovarian cancer (n = 16) and other malignancies (n = 7) |
| Asif et al. (2004)77 | RMI 1 threshold comparison | No histology details |
| Baker et al. (2013)66 | IOTA group’s simple ultrasound rules | No histology details |
| Chan et al. (2013)82 | ROMA score using Abbott Diagnostics’ tumour marker assay |
Malignancy included epithelial ovarian carcinoma only (n = 65), serous adenocarcinoma (n = 30), mucinous adenocarcinoma (n = 14), endometrial adenocarcinoma (n = 7), clear cell (n = 8), mixed (n = 5) and poorly differentiated (n = 1) Subgroups looked at stages I–IV and borderline |
| Clemente et al. (2015)90 | ROMA score using Abbott Diagnostics’ tumour marker assay | No histology details |
| Coleman et al. (2016)70 | Overa (MIA2G) | Malignancy included epithelial ovarian cancer (n = 60), non-epithelial ovarian cancer (n = 5), borderline ovarian cancer (n = 17), metastases to the ovaries (n = 6) and other non-ovarian malignancies (n = 4) |
| Davies et al. (1993)79 | RMI 1 threshold comparison | Malignancy included epithelial ovarian cancer (n = 28), borderline ovarian cancer (n = 7) and other malignancies (n = 2) |
| Di Legge et al. (2012)61 | IOTA group’s simple ultrasound rules; and RMI 1 | Malignancy included primary invasive (n = 476), borderline (n = 128) and metastatic tumours (n = 78) |
| Fathallah et al. (2011)63 | IOTA group’s simple ultrasound rules | Malignancy included primary ovarian cancer (n = 8) and borderline ovarian cancer (n = 6) |
| IOTA5 (2017)a | ADNEX model, IOTA group’s simple ultrasound rules and RMI 1 | Confidential information has been removed |
| Jacobs et al. (1990)78 | RMI 1 threshold comparison | Malignancy included primary invasive epithelial ovarian malignancies (n = 36), dysgerminoma (n = 1), metastatic bowel adenocarcinoma (n = 1) and borderline malignancy (n = 4) |
| Janas et al. (2015)97 | ROMA score using Roche Diagnostics’ tumour marker assay |
Malignancy included primary ovarian cancer (n = 44), metastases to the ovary (n = 14) and borderline tumours (n = 8) Subgroups looked at ovarian cancer alone |
| Joyeux et al. (2016)43 | ADNEX model | Malignancy included primary ovarian cancer (n = 25) and borderline ovarian cancer (n = 5) |
| Karlsen et al. (2012)83 | ROMA score using Abbott Diagnostics’ tumour marker assay; and RMI 1 | No histology details |
| Knafel et al. (2015)49 | IOTA group’s simple ultrasound rules |
Malignancy included ovarian carcinoma (n = 60: serous, clear cell, endometrioid, mucinous, undifferentiated, carcinosarcoma), borderline (n = 7), sex cord–stromal tumours (n = 2), germ cell tumours (n = 5) and metastases (n = 8) Note that 15 out of 82 malignancies were not classed as ovarian |
| Langhe et al. (2013)94 | ROMA score using Fujirebio Diagnostics’ tumour marker assay | Malignant included 53 borderline tumours. No further details reported |
| Li et al. (2016)96 | ROMA score using Abbott Diagnostics’ tumour marker assay | Ovarian malignancy included serous (n = 80), mucinous (n = 42), endometrioid (n = 40), clear cell (n = 21) and undifferentiated (n = 7) |
| Lou et al. (2010)73 | RMI 1 threshold comparison | Ovarian malignancy included epithelial ovarian cancer (n = 50), non-epithelial ovarian cancer (n = 8) and metastatic carcinoma (n = 3) |
| Manjunath et al. (2001)75 | RMI 1 threshold comparison | Malignancy included primary ovarian malignancies (n = 88), germ cell tumours (n = 3) and metastases (n = 2) |
| Meys et al. (2016)44 | ADNEX model, IOTA group’s simple ultrasound rules and RMI 1 | Malignancy included epithelial ovarian cancer (n = 70), borderline (n = 27), granulosa cell carcinoma (n = 3), yolk sac tumour (n = 1), metastatic tumour (n = 10) and non-primary ovarian carcinoma (n = 4) |
| Moffatt et al. (2016)45 | ADNEX model | No histology details |
| Moore et al. (2011)101 | ROMA score using Abbott Diagnostics’ tumour marker assay |
Epithelial ovarian cancer (n = 43) and low malignant potential tumours (n = 14) – non-epithelial tumours and other gynaecological cancers, other cancers and metastatic cancers excluded Subgroups looked at stages I–IV |
| Morgante et al. (1999)80 | RMI 1 threshold comparison | Malignancy (n = 31) included serous cystadenocarcinoma (n = 14), mucinous cystadenocarcinoma (n = 6), borderline (n = 2), clear cell carcinoma (n = 2) undifferentiated carcinoma (n = 2), granulosa cell carcinoma (n = 1) Kruckenberg (n = 1), immature teratoma (n = 1), endometrioid adenocarcinoma (n = 1) and metastatic carcinoma (n = 1) |
| Murala et al. (2014)60 | IOTA group’s simple ultrasound rules; and RMI 1 | No histology details |
| Novotny et al. (2012)86 | ROMA score using Abbott Diagnostics’ tumour marker assay | No histology details |
| Piovano et al. (2016)58 | IOTA group’s simple ultrasound rules | Ovarian malignancy included epithelial ovarian cancer (n = 45), non-epithelial ovarian cancer (n = 8), borderline (n = 22) and metastatic carcinoma (n = 9) |
| Presl et al. (2012)81 | ROMA score using Abbott Diagnostics’ tumour marker assay | No histology details |
| Ruiz de Gauna et al. (2015)64 | IOTA group’s simple ultrasound rules | Malignancy included primary ovarian cancer, borderline ovarian cancer and metastases; no numbers reported |
| Sayasneh et al. (2013)62 | IOTA group’s simple ultrasound rules and RMI 1 | Malignancy included borderline (n = 18), serous cyst/adenocarcinoma (n = 26), mucinous cyst/adenocarcinoma (n = 7), endometrioid carcinoma (n = 6), clear cell carcinoma (n = 5), granulosa cell tumour (n = 1), transitional cell tumour (n = 1), signet ring cell adenocarcinoma (n = 1), peritoneal serous adenocarcinoma (n = 1), gastrointestinal adenocarcinomas (n = 5), malignant mixed Mullerian tumour (n = 1), large cell neuroendocrine carcinoma (n = 1) and endocrine tumour (n = 1) |
| Sayasneh et al. (2016)46 | ADNEX model | Malignancy included primary ovarian cancer (n = 116), borderline ovarian cancer (n = 42) and metastatic ovarian cancer (n = 24) |
| Shulman et al. (2016)104 | Overa (MIA2G) and ROMA score using Roche Diagnostics’ tumour marker assay | Malignancy included epithelial ovarian cancer (n = 150), non-epithelial ovarian cancer (n = 16), borderline ovarian cancer (n = 42), metastases (n = 23) and non-ovarian malignancies (n = 14) |
| Silvestre et al. (2015)55 | IOTA group’s simple ultrasound rules | Malignancy included primary ovarian malignancies (n = 15), borderline ovarian malignancy (n = 5), metastases (n = 5) and other malignancies (n = 7) |
| Szubert et al. (2016)42 | ADNEX model | Spain. Malignancy included primary ovarian cancer (n = 26), borderline ovarian cancer (n = 3) and metastatic ovarian cancer (n = 5) |
| Poland. Malignancy included primary ovarian cancer (n = 53), borderline ovarian cancer (n = 12) and metastatic ovarian cancer (n = 5) | ||
| Tantipalakorn et al. (2014)51 | IOTA group’s simple ultrasound rules | Malignancy included primary ovarian cancer (n = 62), borderline ovarian cancer (n = 12), germ cell tumours (n = 9), sex cord–stromal tumour (n = 6), metastatic adenocarcinoma (n = 10) and other malignant tumours (n = 8) |
| Testa et al. (2014)50 | IOTA group’s simple ultrasound rules and RMI 1 | Malignancy included primary invasive ovarian cancer (n = 633), borderline ovarian tumours (n = 153), metastatic ovarian cancer (n = 126) and rare primary invasive (e.g. dysgerminom granulosa cell tumour, yolk sac tumour or malignant treatoma) (n = 68) |
| Timmerman et al. (2010)65 | IOTA group’s Simple Rules and RMI 1 | Malignancy included borderline (n = 111), primary invasive, stages I–IV and rare (n = 373) and metastatic ovarian cancer (n = 58) |
| Tingulstad et al. (1996)76 | RMI 1 threshold comparison | Malignancy included ovarian cancer (n = 51), neurosarcoma (n = 1), leiomyosarcoma (n = 1), lymphoma (n = 1), Kruckenberg tumour (n = 1) and rectal cancer (n = 1) |
| Tinnangwattana et al. (2015)47 | IOTA group’s simple ultrasound rules | Malignancy included primary ovarian malignancies (n = 13), borderline (n = 8), metastases (n = 5) and other malignancies (n = 3) |
| Ulusoy et al. (2007)74 | RMI 1 threshold comparison | Malignancy included primary ovarian cancers (n = 84), borderline ovarian cancers (n = 15) and metastases (n = 7) |
| Van Calster et al. (2014)17 | ADNEX model | Malignancy included primary ovarian cancer (n = 701), borderline ovarian cancer (n = 153) and metastases (n = 126). IOTA phase 3 – validation data set |
| Van Gorp et al. (2012)98 | ROMA score using Fujirebio Diagnostics’ tumour marker assay and RMI 1 | Malignancy included epithelial ovarian cancer stages I–IV (n = 120), non-epithelial ovarian cancer (n = 4) and metastatic ovarian cancer (n = 25) |
| Weinberger and Minar (2013)53 | IOTA group’s simple ultrasound rules | Malignancy included all invasive ovarian cancers and borderline tumours. No further details reported |
| Winarto et al. (2014)99 | ROMA score using Abbott Diagnostics’ tumour marker assay and RMI 1 | Malignancy included serous cystadenocarcinoma (n = 19), endometrioid (n = 14), mucinous (n = 8), clear cell (n = 7), carcinosarcoma (n = 2) and borderline (n = 17) |
| Xu et al. (2016)95 | ROMA score using Roche Diagnostics’ tumour marker assay | Malignancy was described as epithelial ovarian cancer (n = 210), endometrioid (n = 80), serous (n = 59), papillary serous (n = 15), mucinous (n = 6), seromucinous (n = 2), clear cell (n = 12) and adenocarcinoma (n = 36) |
| Yamamoto et al. (2009)72 | RMI 1 threshold comparison | Malignancy included primary ovarian cancer (n = 29), borderline ovarian cancer (n = 8) and tubal cancer (n = 3) |
| Yanaranop et al. (2016)89 | ROMA score using Roche Diagnostics’ tumour marker assay; and RMI 1 |
Malignancy included epithelial ovarian carcinoma (n = 66) and non-epithelial ovarian cancer (n = 8) Subgroups looked at epithelial and stages I–IV |
| Zhang et al. (2015)68 | Overa (MIA2G) | Malignancy (n = 72) included stage I/II (n = 19) and LMP (n = 13) |
| Zhang et al. (2015)102 | ROMA score using Roche Diagnostics’ tumour marker assay |
Malignancy was described as epithelial ovarian cancer (n = 264), serous (n = 170), mucinous (n = 20), endometrioid (n = 25), other kinds (n = 13) and unknown (n = 36) Subgroups looked at stages I–IV |
Appendix 5 Additional results
| Test | Study (year of publication) | Subgroup | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| ROMA score using Abbott Diagnostics’ tumour marker assay | Target condition: ovarian malignancies (undefined – not clear whether or not borderline tumours were included) | |||||||||
| aClemente and Benitez (2015)90 | All women | NR | 4 | 5 | 14 | 39 | 62 | 44.4 (13.7 to 78.8) | 73.6 (59.7 to 84.7) | |
| Premenopausal women | NR | 2 | 3 | 11 | 32 | 48 | 40.0 (5.3 to 85.3) | 74.4 (58.8 to 86.5) | ||
| Postmenopausal women | NR | 2 | 2 | 3 | 7 | 14 | 50.0 (6.8 to 93.2) | 70.0 (34.8 to 93.9) | ||
| aNovotny et al. (2012)86 | Postmenopausal women | 37.7 | 18 | 3 | 13 | 243 | 277 | 85.7 (63.7 to 97.0) | 94.9 (91.5 to 97.3) | |
| Target condition: epithelial ovarian malignancies including borderline | ||||||||||
| Moore et al. (2011)101 | All women | 13.1/27.7 | 59 | 8 | 96 | 287 | 450 | 88.1 (77.8 to 94.7) | 74.9 (70.3 to 79.2) | |
| Premenopausal women | 13.1 | 13 | 3 | 60 | 173 | 249 | 81.3 (54.4 to 96.0) | 74.2 (68.1 to 79.7) | ||
| Postmenopausal women | 27.7 | 46 | 5 | 36 | 114 | 201 | 90.2 (78.6 to 96.7) | 76.0 (68.4 to 82.6) | ||
| Target condition: epithelial ovarian malignancies (stage III/IV) – borderline and stage I/II tumours excluded | ||||||||||
| Moore et al. (2011)101 | All women | 13.1/27.7 | 34 | 0 | 96 | 287 | 417 | 100 (89.7 to 100) | 74.9 (70.3 to 79.2) | |
| Premenopausal women | 13.10 | 5 | 0 | 60 | 173 | 238 | 100 (47.8 to 100) | 74.2 (68.1 to 79.7) | ||
| Postmenopausal women | 27.7 | 29 | 0 | 36 | 114 | 179 | 100 (88.1 to 100) | 76.0 (68.4 to 82.6) | ||
| Target condition: epithelial ovarian malignancies (stage I/II) – borderline and stage III/IV tumours excluded | ||||||||||
| Moore et al. (2011)101 | All women | 13.1/27.7 | 9 | 3 | 96 | 287 | 395 | 75.0 (42.8 to 94.5) | 74.9 (70.3 to 79.2) | |
| Premenopausal women | 13.1 | 3 | 0 | 60 | 173 | 236 | 100 (29.2 to 100) | 74.2 (68.1 to 79.7) | ||
| Postmenopausal women | 27.7 | 6 | 3 | 36 | 114 | 159 | 66.7 (29.9 to 92.5) | 25.3 (21.4 to 29.6) | ||
| ROMA score using Roche Diagnostics’ tumour marker assay | Target condition: epithelial ovarian malignancies excluding borderline | |||||||||
| Xu et al. (2016)95 | Premenopausal women | 13.40 | 58 | 49 | 23 | 241 | 371 | 54.2 (44.3 to 63.9) | 91.3 (87.2 to 94.4) | |
| Postmenopausal women | 18.70 | 69 | 34 | 6 | 41 | 150 | 67.0 (57.0 to 75.9) | 87.2 (74.3 to 95.2) | ||
| Target condition: epithelial ovarian malignancies (stage III/IV) – borderline and stage I/II tumours excluded | ||||||||||
| aZhang et al. (2015)102 | All women | 11.4–29.9 | 143 | 16 | 72 | 276 | 507 | 89.9 (84.2 to 94.1) | 79.3 (74.7 to 83.4) | |
| Premenopausal women | 11.4 | 40 | 10 | 58 | 227 | 335 | 80.0 (66.3 to 90.0) | 79.6 (74.5 to 84.2) | ||
| Postmenopausal women | 29.9 | 103 | 6 | 14 | 49 | 172 | 94.5 (88.4 to 98.0) | 77.8 (65.5 to 87.3) | ||
| Target condition: epithelial ovarian malignancies (stage I/II) – borderline and stage III/IV tumours excluded | ||||||||||
| Zhang et al. (2015)102 | All women | 11.4–29.9 | 49 | 15 | 72 | 276 | 412 | 76.6 (64.3 to 86.2) | 79.3 (74.7 to 83.4) | |
| Premenopausal women | 11.4 | 21 | 9 | 58 | 227 | 315 | 70.0 (50.6 to 85.3) | 79.6 (74.5 to 84.2) | ||
| Postmenopausal women | 29.9 | 28 | 6 | 14 | 49 | 97 | 82.4 (65.5 to 93.2) | 77.8 (65.5 to 87.3) | ||
| Study (year of publication) | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All malignant tumours including borderline | |||||||||
| Sayasneh et al. (2016)46 | 1 | 182 | 0 | 377 | 51 | 610 | Calculated | 100 (97.4 to 100) | 11.9 (9.1 to 15.5) |
| 3 | 182 | 0 | 297 | 131 | 610 | Calculated | 100 (97.4 to 100) | 30.6 (26.3 to 35.3) | |
| 5 | 180 | 2 | 200 | 228 | 610 | Calculated | 99 (94.9 to 99.8) | 53.2 (48.2 to 58.1) | |
| 15 | 172 | 10 | 106 | 322 | 610 | Calculated | 94.4 (90 to 97) | 75.2 (70.7 to 79.2) | |
| 20 | 165 | 17 | 89 | 339 | 610 | Calculated | 90.6 (85.2 to 94.1) | 79.3 (75.1 to 83) | |
| 30 | 157 | 25 | 69 | 359 | 610 | Calculated | 86.3 (80.4 to 90.6) | 83.9 (80.1 to 87.2) | |
| Van Calster et al. (2014)17 | 3 | 969 | 11 | 760 | 663 | 2403 | Calculated | 98.9 (98 to 99.4) | 46.6 (44 to 49.2) |
| 5 | 964 | 16 | 578 | 845 | 2403 | Calculated | 98.4 (97.4 to 99.1) | 59.4 (56.8 to 62) | |
| 15 | 913 | 67 | 324 | 1099 | 2403 | Calculated | 93.2 (92.5 to 95.6) | 77.2 (74.9 to 79.3) | |
| Ovarian malignancies including borderline | |||||||||
| Joyeux et al. (2016)43 | 3 | 30 | 0 | 134 | 120 | 284 | Calculated | 100 (88.4 to 100) | 47.2 (41 to 53.6) |
| 5 | 29 | 1 | 78 | 176 | 284 | Calculated | 96.6 (82.8 to 99.9) | 69.2 (63.2 to 74.9) | |
| 15 | 26 | 4 | 38 | 216 | 284 | Calculated | 86.6 (69.3 to 96.2) | 85 (80 to 89.2) | |
| Study (year of publication) | Threshold | Subgroup | Index test variations | TP, n | FN, n | FP, n | TN, n | Total, n | 2 × 2 data | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||||
| Alcazar et al. (2013)52 | Malignant (inconclusives were excluded) | All women | 29 | 4 | 6 | 231 | 270 | Reported | 87.9 (72.4 to 95.2) | 97.5 (94.6 to 98.8) | |
| Ruiz de Gauna et al. (2015)64 | Centre A | 27 | 0 | 4 | 62 | 93 | Reported | 100 (87.5 to 100) | 93.9 (85.4 to 97.6) | ||
| Centre B | 11 | 2 | 4 | 92 | 109 | Reported | 84.6 (57.8 to 95.7) | 95.8 (89.8 to 98.4) | |||
| Silvestre et al. (2015)55 | All women | 32 | 0 | 8 | 26 | 66 | Reported | 100 (89.1 to 100) | 76.5 (58.8 to 89.3) | ||
| Tantipalakorn et al. (2014)51 | All women | 88 | 19 | 10 | 202 | 319 (masses) | Reported | 82.2 (75 to 89.5) | 95.3 (92.4 to 98.1) | ||
| Tinnangwattana et al. (2015)47 | All women | 25 | 3 | 11 | 55 | 94 | Reported | 89.3 (77.8 to 100) | 83.3 (74.3 to 92.3) | ||
| Piovano et al. (2016)58 | Malignant (inconclusives were classified by expert SA) and ROMA score of > 11.4%/29.9% | All women | + ROMA | 76 | 8 | 61 | 246 | 391 | Calculated | 90.5 (82.1 to 95.8) | 80.1 (75.2 to 84.4) |
| Postmenopausal women | + ROMA | 58 | 5 | 25 | 82 | 170 | Calculated | 92.0 (85.0 to 99.0) | 77.0 (69.0 to 85.0) | ||
| Premenopausal women | + ROMA | 18 | 3 | 36 | 164 | 221 | Calculated | 86.0 (71.0 to 100) | 82.0 (77.0 to 87.0) | ||
| Malignant (inconclusives were classified by expert SA) and HE4 level of ≥ 70/140 pmol/l | All women | + HE4 | 73 | 11 | 42 | 265 | 391 | Calculated | 86.9 (77.8 to 93.3) | 86.3 (82.0 to 90.0) | |
| Postmenopausal women | + HE4 | 55 | 8 | 18 | 89 | 170 | Calculated | 87.0 (79.0 to 96.0) | 83.0 (76.0 to 90.0) | ||
| Premenopausal women | + HE4 | 18 | 3 | 24 | 176 | 221 | Calculated | 86.0 (71.0 to 100) | 88.0 (83.0 to 92.0) | ||
| Malignant on IOTA group’s simple ultrasound rules (inconclusives were classified by expert SA) and CA125 level of ≥ 35 U/ml | All women | + CA125 | 76 | 8 | 98 | 209 | 391 | Calculated | 90.5 (82.1 to 95.8) | 68.1 (62.5 to 73.3) | |
| Postmenopausal women | + CA125 | 58 | 5 | 26 | 81 | 170 | Calculated | 92.0 (85.0 to 99.0) | 76.0 (68.0 to 84.0) | ||
| Premenopausal women | + CA125 | 18 | 3 | 72 | 128 | 221 | Calculated | 86.0 (71.0 to 100) | 64.0 (57.0 to 61.0) | ||
| Ruiz de Gauna et al. (2015)64 | Malignant (inconclusives were classified by expert SA; final ratings of unclassifiable were treated as malignant) | Centre A | 31 | 0 | 9 | 74 | 114 | Reported | 100 (88.8 to 100) | 89.2 (80.4 to 94.9) | |
| Centre B | 13 | 2 | 13 | 105 | 133 | Reported | 86.7 (59.5 to 98.3) | 89 (81.9 to 94.0) | |||
| Target condition: ovarian malignancies including borderline | |||||||||||
| Fathallah et al. (2011)63 | Malignant (inconclusives were excluded) | All women | 8 | 3 | 3 | 95 | 109 | Reported | 73.0 (45.0 to 100) | 97.0 (94.0 to 100) | |
| Weinberger and Minar (2013)53 | Malignant (handling of inconclusives was unclear) | All women | 118 | 7 | 16 | 206 | 347 | Calculated | 94.0 (88.8 to 97.7) | 93.0 (88.6 to 95.8) | |
| Target condition: ovarian malignancies excluding borderline | |||||||||||
| Weinberger et al. (2013)53 | Malignant (handling of inconclusives was unclear) | All women | 99 | 2 | 16 | 222 | 323 | Calculated | 98.0 (93.0 to 99.8) | 93.0 (89.3 to 96.1) | |
| Target condition: ovarian malignancies (undefined – not clear whether borderline tumours were included) | |||||||||||
| Baker et al. (2013)66 | Malignant (inconclusives were excluded) | Premenopausal women | 2 | 0 | 5 | 21 | 28 | Calculated | 100 (15.8 to 100) | 80.8 (60.6 to 93.4) | |
| Target condition: ovarian borderline tumours | |||||||||||
| Weinberger et al. (2013)53 | Malignant (handling of inconclusives was unclear) | All | 19 | 5 | 16 | 222 | 262 | Calculated | 79.2 (57.8 to 92.9) | 93.3 (89.3 to 96.1) | |
| Study (year of publication) | CA125 assay | Ultrasound details | Threshold | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| All malignant tumours including borderline | ||||||||||
| Davies et al. (1993)79 | RIA (CIS Bioindustries) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 25 | 36 | 2 | 39 | 48 | 124 | 94.7 (82.3 to 99.4) | 55.2 (44.1 to 65.9) |
| Jacobs et al. (1990)78 | RIA (Abbott Diagnostics) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 41 | 0 | 37 | 61 | 139 | 100 (91.4 to 100) | 62.2 (51.9 to 71.8) | |
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 30 | 1 | 37 | 56 | 124 | 96.8 (83.3 to 99.9) | 60.2 (49.5 to 70.2) | |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 51 | 5 | 37 | 80 | 173 | 91.1 (80.4 to 97.0) | 68.4 (59.1 to 76.7) | |
| Ulusoy et al. (2007)74 | NR | Toshiba Sonolayer SSA-270A and/or a Siemens Sonoline G50 with 3.75-MHz and 5-MHz abdominal convex transducers and/or 5-MHz and 9-MHz endovaginal probes | 100 | 6 | 136 | 54 | 296 | 94.3 (88.1 to 97.9) | 28.4 (22.1 to 35.4) | |
| Summary estimates | 94.9 (91.5 to 97.2) | 51.1 (47.0 to 55.2) | ||||||||
| Davies et al. (1993)79 | RIA (CIS Bioindustries) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 50 | 36 | 2 | 28 | 59 | 124 | 94.7 (82.3 to 99.4) | 67.8 (56.9 to 77.4) |
| Jacobs et al. (1990)78 | RIA (Abbott Diagnostics) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 39 | 2 | 23 | 75 | 139 | 95.1 (83.5 to 99.4) | 76.5 (66.9 to 84.5) | |
| Lou et al. (2010)73 | NR | NR | 49 | 12 | 36 | 126 | 223 | 80.3 (68.2 to 89.4) | 77.8 (70.6 to 83.9) | |
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 29 | 2 | 23 | 70 | 124 | 93.5 (78.6 to 99.2) | 75.3 (65.2 to 83.6) | |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 49 | 7 | 22 | 95 | 173 | 87.5 (75.9 to 94.8) | 81.2 (72.9 to 87.8) | |
| Ulusoy et al. (2007)74 | NR | Toshiba Sonolayer SSA-270A and/or a Siemens Sonoline G50 with 3.75-MHz and 5-MHz abdominal convex transducers and/or 5-MHz and 9-MHz endovaginal probes | 96 | 10 | 106 | 84 | 296 | 90.6 (83.3 to 95.4) | 44.2 (37 to 51.6) | |
| Summary estimates | 89.5 (85.7 to 92.6) | 68.1 (64.7 to 71.5) | ||||||||
| Davies et al. (1993)79 | RIA (CIS Bioindustries) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 75 or 80 | 33 | 4 | 18 | 69 | 124 | 89.2 (74.6 to 97.0) | 79.3 (69.3 to 87.3) |
| Jacobs et al. (1990)78 | RIA (Abbott Diagnostics) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 38 | 3 | 15 | 83 | 139 | 92.7 (80.2 to 98.5) | 84.7 (76 to 91.2) | |
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 25 | 6 | 19 | 74 | 124 | 80.6 (62.5 to 92.5) | 79.6 (69.9 to 87.2) | |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 44 | 12 | 14 | 107 | 173 | 78.6 (65.6 to 88.4) | 88.4 (81.3 to 93.5) | |
| Summary estimates | 84.8 (78.5 to 89.9) | 83.5 (79.4 to 87.0) | ||||||||
| Davies et al. (1993)79 | RIA (CIS Bioindustries) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 100 | 32 | 5 | 13 | 74 | 124 | 86.5 (71.2 to 95.5) | 85.1 (75.8 to 91.8) |
| Jacobs et al. (1990)78 | RIA (Abbott Diagnostics) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 35 | 6 | 12 | 86 | 139 | 85.4 (70.8 to 94.4) | 87.8 (79.6 to 93.5) | |
| Lou et al. (2010)73 | NR | NR | 45 | 16 | 18 | 144 | 223 | 73.8 (60.9 to 84.2) | 88.9 (83.0 to 93.3) | |
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 24 | 7 | 9 | 84 | 124 | 77.4 (58.9 to 90.4) | 90.3 (82.4 to 95.5) | |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 44 | 12 | 13 | 108 | 173 | 78.6 (65.6 to 88.4) | 89.3 (82.3 to 94.2) | |
| Summary estimates | 79.6 (73.8 to 84.7) | 88.4 (85.5 to 90.9) | ||||||||
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 120 or 125 | 23 | 8 | 7 | 86 | 124 | 74.2 (55.4 to 88.1) | 92.5 (85.1 to 96.9) |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 44 | 12 | 12 | 109 | 173 | 78.6 (65.6 to 88.4) | 90.1 (83.3 to 94.8) | |
| Ulusoy et al. (2007)74 | NR | Toshiba Sonolayer SSA-270A and/or a Siemens Sonoline G50 with 3.75-MHz and 5-MHz abdominal convex transducers and/or 5-MHz and 9-MHz endovaginal probes | 86 | 20 | 59 | 131 | 296 | 81.1 (72.4 to 88.1) | 68.9 (61.8 to 75.4) | |
| Summary estimates | 79.3 (72.9 to 84.8) | 80.7 (76.5 to 84.4) | ||||||||
| Davies et al. (1993)79 | RIA (CIS Bioindustries) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 150 | 30 | 7 | 13 | 74 | 124 | 81.1 (64.8 to 92.0) | 85.1 (75.8 to 91.8) |
| Jacobs et al. (1990)78 | RIA (Abbott Diagnostics) | Diasonics DS 1 sector scanner with a 3.5-MHz transducer | 35 | 6 | 6 | 92 | 139 | 85.4 (70.8 to 94.4) | 93.9 (87.2 to 97.7) | |
| Lou et al. (2010)73 | NR | NR | 37 | 24 | 8 | 154 | 223 | 60.7 (47.3 to 72.9) | 95.1 (90.5 to 97.8) | |
| Morgante et al. (1999)80 | RIA (Centocor) | A Siemens Sonoline SL2 was used with a 3.5-MHz transabdominal sectorial probe and a 5.0- to 7.5-MHz transvaginal probe | 20 | 11 | 6 | 87 | 124 | 64.5 (45.4 to 80.8) | 93.5 (86.5 to 97.6) | |
| Tingulstad et al. (1996)76 | IMx™ (Abbott Diagnostics) | Transvaginal with transabdominal as needed | 43 | 13 | 7 | 110 | 173 | 76.8 (63.6 to 87.0) | 94.0 (88.1 to 97.6) | |
| Ulusoy et al. (2007)74 | NR | Toshiba Sonolayer SSA-270A and/or a Siemens Sonoline G50 with 3.75-MHz and 5-MHz abdominal convex transducers and/or 5-MHz and 9-MHz endovaginal probes | 81 | 25 | 42 | 148 | 296 | 76.4 (67.2 to 84.1) | 77.9 (71.3 to 83.6) | |
| Summary estimates | 74.1 (69.0 to 78.7) | 89.0 (86.6 to 91.2) | ||||||||
| Lou et al. (2010)73 | NR | NR | 300 | 33 | 28 | 2 | 160 | 223 | 54.1 (40.8 to 66.9) | 98.8 (95.6 to 99.9) |
| Ulusoy et al. (2007)74 | NR | Toshiba Sonolayer SSA-270 A and/or a Siemens Sonoline G50 with 3.75-MHz and 5-MHz abdominal convex transducers and/or 5-MHz and 9-MHz endovaginal probes | 500 | 57 | 49 | 12 | 178 | 296 | 53.8 (43.8 to 63.5) | 93.7 (89.2 to 96.7) |
| All malignant tumours excluding borderline | ||||||||||
| Aktürk et al. (2011)71 | Electrochemiluminescence immunoassay (Roche Diagnostics) | Siemens transvaginal 7.5-MHz transducer | 50 | 17 | 3 | 33 | 47 | 100 | 85 (62.1 to 96.8) | 58.8 (47.2 to 69.6) |
| 100 | 15 | 5 | 13 | 67 | 100 | 75 (50.9 to 91.3) | 83.8 (73.8 to 91.1) | |||
| 150 | 15 | 5 | 12 | 68 | 100 | 75 (50.9 to 91.3) | 85.0 (75.3 to 92.0) | |||
| 300 | 9 | 11 | 2 | 78 | 100 | 45 (23.1 to 68.5) | 97.5 (91.3 to 99.7) | |||
| 350 | 9 | 11 | 2 | 78 | 100 | 45 (23.1 to 68.5) | 97.5 (91.3 to 99.7) | |||
| 400 | 6 | 14 | 2 | 78 | 100 | 30 (11.9 to 54.3) | 97.5 (91.3 to 99.7) | |||
| Manjunath et al. (2001)75 | Microparticle EIA (Abbott Diagnostics) | The ultrasound was performed vaginally by a 5-MHz transducer (Ultramark 4 PLUS, Advanced Technology Laboratories, Signal Hill, CA, USA) and extended to the transabdominal approach with full bladder if the mass was large | 25 | 85 | 8 | 27 | 28 | 148 | 91.4 (83.8 to 96.2) | 50.9 (37.1 to 64.6) |
| 50 | 75 | 18 | 21 | 34 | 148 | 80.6 (71.1 to 88.1) | 61.8 (47.7 to 74.6) | |||
| 80 | 74 | 19 | 18 | 37 | 148 | 79.6 (69.9 to 87.2) | 67.3 (53.3 to 79.3) | |||
| 100 | 74 | 19 | 14 | 41 | 148 | 79.6 (69.9 to 87.2) | 74.5 (61.0 to 85.3) | |||
| 125 | 73 | 20 | 11 | 44 | 148 | 78.5 (68.8 to 86.3) | 80.0 (67.0 to 89.6) | |||
| 150 | 72 | 21 | 9 | 46 | 148 | 77.4 (67.6 to 85.4) | 83.6 (71.2 to 92.2) | |||
| 300 | 60 | 33 | 3 | 52 | 148 | 64.5 (53.9 to 74.2) | 94.5 (84.9 to 98.9) | |||
| 350 | 58 | 35 | 3 | 52 | 148 | 62.4 (51.7 to 72.2) | 94.5 (84.9 to 98.9) | |||
| 400 | 57 | 36 | 3 | 52 | 148 | 61.3 (50.6 to 71.2) | 94.5 (84.9 to 98.9) | |||
| All malignant tumours (undefined – not clear whether or not borderline tumours were included) | ||||||||||
| Asif et al. (2004)77 | IA (IMMULITE) | NR | 25 | 54 | 1 | 15 | 30 | 100 | 98.2 (90.3 to 100) | 66.7 (51.0 to 80.0) |
| 50 | 53 | 2 | 10 | 35 | 100 | 96.4 (87.5 to 99.6) | 77.8 (62.9 to 88.8) | |||
| 75 | 52 | 3 | 8 | 37 | 100 | 94.5 (84.9 to 98.9) | 82.2 (67.9 to 92.0) | |||
| 100 | 49 | 6 | 7 | 38 | 100 | 89.1 (77.8 to 95.9) | 84.4 (70.5 to 93.5) | |||
| 125 | 48 | 7 | 5 | 40 | 100 | 87.3 (75.5 to 94.7) | 88.9 (75.9 to 96.3) | |||
| 150 | 47 | 8 | 4 | 41 | 100 | 85.5 (73.3 to 93.5) | 91.1 (78.8 to 97.5) | |||
| 175 | 47 | 8 | 4 | 41 | 100 | 85.5 (73.3 to 93.5) | 91.1 (78.8 to 97.5) | |||
| 190 | 47 | 8 | 4 | 41 | 100 | 85.5 (73.3 to 93.5) | 91.1 (78.8 to 97.5) | |||
| 300 | 40 | 15 | 0 | 45 | 100 | 72.7 (59.0 to 83.9) | 100 (92.1 to 100) | |||
| Ovarian malignancies including borderline | ||||||||||
| Yamamoto et al. (2009)72 | Elecsys CA125 II | Transvaginal (6.0-MHz transducer), with transabdominal as indicated | 100 | 39 | 1 | 65 | 148 | 253 | 97.5 (86.8 to 99.9) | 69.5 (62.8 to 75.6) |
| 150 | 34 | 6 | 36 | 177 | 253 | 85.0 (70.2 to 94.3) | 83.1 (77.4 to 87.9) | |||
| 300 | 27 | 13 | 18 | 195 | 253 | 67.5 (50.9 to 81.4) | 91.5 (87.0 to 94.9) | |||
| Study (year of publication) | Subgroup | ROMA threshold (%) | TP | FN | FP | TN | Total | Sensitivity, % (95% CI) | Specificity, % (95% CI) | RMI 1 | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||||||||||
| Van Gorp et al. (2012)98 | All women | 12.5/14.4 | 127 | 23 | 52 | 172 | 374 | 84.7 (77.9 to 90) | 76.8 (70.7 to 82.2) | 200 | 114 | 36 | 17 | 207 | 374 | 76.0 (68.4 to 82.6) | 92.4 (88.1 to 95.5) |
| Premenopausal women | 12.5 | 26 | 13 | 17 | 122 | 178 | 66.7 (49.8 to 80.9) | 87.8 (81.1 to 92.7) | 200 | 25 | 14 | 6 | 133 | 178 | 64.1 (47.2 to 78.8) | 95.7 (90.8 to 98.4) | |
| Postmenopausal women | 14.4 | 101 | 10 | 35 | 50 | 196 | 91 (84.1 to 95.6) | 58.8 (47.6 to 69.4) | 200 | 89 | 22 | 11 | 74 | 196 | 80.2 (71.5 to 87.1) | 87.1 (78 to 93.4) | |
| Study (year of publication) | Subgroup | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: all malignant tumours including borderline | |||||||||
| aLanghe et al. (2013)94 | All women | 12.5/14.4 | 129 | 47 | 31 | 170 | 377 | 73.3 (66.1 to 79.7) | 84.6 (78.8 to 89.3) |
| Van Gorp et al. (2012)98 | All women | 12.5/14.4 | 127 | 23 | 52 | 172 | 374 | 84.7 (77.9 to 90.0) | 76.8 (70.7 to 82.1) |
| Summary estimates | 78.5 (73.7 to 82.9) | 80.5 (76.4 to 84.1) | |||||||
| aLanghe et al. (2013)94 | Premenopausal women | 12.5 | 23 | 22 | 6 | 81 | 132 | 51.1 (35.8 to 66.3) | 93.1 (85.6 to 97.4) |
| Van Gorp et al. (2012)98 | Premenopausal women | 12.5 | 26 | 13 | 17 | 122 | 178 | 66.7 (49.8 to 80.9) | 87.8 (81.1 to 92.7) |
| Summary estimates | 58.3 (47.1 to 69.0) | 89.8 (85.1 to 93.4) | |||||||
| aLanghe et al. (2013)94 | Postmenopausal women | 14.4 | 105 | 26 | 25 | 89 | 245 | 80.2 (72.3 to 86.6) | 78.1 (69.4 to 85.3) |
| Van Gorp et al. (2012)98 | Postmenopausal women | 14.4 | 101 | 10 | 35 | 50 | 196 | 91 (84.1 to 95.6) | 58.8 (47.6 to 69.4) |
| Summary estimates | 85.1 (80.0 to 89.4) | 69.8 (63.0 to 76.1) | |||||||
| Study (year of publication) | Subgroup | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: ovarian malignancies (undefined – not clear whether or not borderline tumours were included) | |||||||||
| Li et al. (2016)96 | All women | 7.4/25.3 | 166 | 24 | 141 | 586 | 917 | 87.4 (81.8 to 91.7) | 80.1 (77.0 to 83.0) |
| aPresl et al. (2012)81 | All women | 7.3/26.3 | 25 | 5 | 72 | 450 | 552 | 83.3 (65.3 to 94.4) | 86.2 (82.9 to 89.0) |
| Summary estimates | 86.8 (81.6 to 91.0) | 82.7 (80.5 to 84.8) | |||||||
| Li et al. (2016)96 | Premenopausal women | 7.4 | 96 | 12 | 136 | 501 | 745 | 88.9 (81.4 to 94.1) | 78.6 (75.3 to 81.8) |
| aPresl et al. (2012)81 | Premenopausal women | 7.30 | 5 | 4 | 44 | 243 | 296 | 55.6 (21.2 to 86.3) | 84.7 (80.0 to 88.6) |
| Summary estimates | 86.3 (78.7 to 92.0) | 80.5 (77.8 to 83.0) | |||||||
| Li et al. (2016)96 | Postmenopausal women | 25.3 | 70 | 12 | 5 | 85 | 172 | 85.4 (75.8 to 92.2) | 94.4 (87.5 to 98.2) |
| Novotny et al. (2012)86,a | Postmenopausal women | 26.3 | 20 | 1 | 31 | 225 | 277 | 95.2 (76.2 to 99.9) | 87.9 (83.3 to 91.6) |
| aPresl et al. (2012)81 | Postmenopausal women | 26.3 | 20 | 1 | 28 | 207 | 256 | 95.2 (76.2 to 99.9) | 88.1 (83.2 to 91.9) |
| Summary estimates | 88.7 (81.8 to 93.7) | 89.0 (86.2 to 91.4) | |||||||
| Study (year of publication) | Subgroup | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: ovarian malignancies (undefined – not clear whether or not borderline tumours were included) | |||||||||
| aZhang et al. (2015)102 | All women | 11.4/29.9 | 224 | 40 | 73 | 275 | 612 | 84.8 (79.9 to 88.9) | 79.0 (74.4 to 83.2) |
| Premenopausal women | 11.4 | 70 | 25 | 59 | 226 | 380 | 73.7 (63.6 to 82.2) | 79.3 (74.1 to 83.9) | |
| Postmenopausal women | 29.9 | 154 | 15 | 14 | 49 | 232 | 91.1 (85.8 to 94.9) | 77.8 (65.5 to 87.3) | |
| Study (year of publication) | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Moffatt et al. (2016)45 | 10 | 4 | 2 | 29 | 46 | 81 | 66.7 (22.3 to 95.7) | 61.3 (64.4 to 81.6) |
| Study (year of publication) | Total number | Threshold | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 250 | ||||||||||||
| TP, n | FN, n | FP, n | TN, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | TP, n | FN, n | FP, n | TN, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
| All malignant tumours (undefined – not clear whether or not borderline tumours were included) | |||||||||||||
| Asif et al. (2004)77 | 100 | 47 | 8 | 3 | 42 | 85 (NR) | 93 (NR) | 40 | 15 | 2 | 43 | 72 (NR) | 95 (NR) |
| Study (year of publication) | Subgroup | Threshold (%) | TP, n | FN, n | FP, n | TN, n | Total, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Target condition: ovarian malignancies (undefined – not clear whether or not borderline tumours were included) | |||||||||
| aZhang et al. (2015)102 | All women | 11.4/29.9 | 224 | 40 | 73 | 275 | 612 | 84.8 (79.9 to 88.9) | 79.0 (74.4 to 83.2) |
| Premenopausal women | 11.4 | 70 | 25 | 59 | 226 | 380 | 73.7 (63.6 to 82.2) | 79.3 (74.1 to 83.9) | |
| Postmenopausal women | 29.9 | 154 | 15 | 14 | 49 | 232 | 91.1 (85.8 to 94.9) | 77.8 (65.5 to 87.3) | |
Appendix 6 Cost calculations for risk scores
Risk assessment tool cost calculations
| Test | Cost (£) | ||||
|---|---|---|---|---|---|
| Test cost per kit | Sum of HE4 test-related costs (capital, other, personnel as per below) | Ultrasound | CA125 | Total cost for risk score | |
| ROMA score using Abbott Diagnostics’ ARCHITECTa | 21.33 | 6.64 | 76.75 | 25.58 | 130.31 |
| ROMA score using Roche Diagnostics’ Elecsysa | 15.95 | 7.81 | 76.75 | 25.58 | 126.09 |
| Vermillion Overa (MIA2G)a | 99.00 | – | 76.75 | – | 175.80 |
| IOTA group’s simple ultrasound rules | – | – | 76.75 | – | 76.75 |
| IOTA group’s ADNEX model | – | – | 76.75 | 25.58 | 102.34 |
| RMI 1 | – | – | 76.75 | 25.58 | 102.34 |
Risk assessment tool components: cost breakdown
| Risk assessment tool component | Cost per test kit/ultrasound (£) |
|---|---|
| Serum CA125 | 25.58 |
| TVS | 76.75 |
| Abbott Diagnostics’ ARCHITECT HE4 | 21.33 |
| Roche Diagnostics’ Elecsys HE4 | 15.95 |
| Vermillion Overa (MIA2G) | 99.00 |
| Capital cost calculation items for Abbott Diagnostics and Roche Diagnostics’ HE4 test | Capital cost items for HE4 tests | Per year (annuitised) | Cost per test (annuitised) |
|---|---|---|---|
| Costs of LUMIPULSE (average of G1200 and G600II) | £56,432.00 | £6785.46 | £1.92 |
| Resale value | 0 | – | – |
| Lifetime of analyser equipment | 10 years | – | – |
| Number of tests per year on one analyser (full capacity) | 3542 | – | – |
| Other cost items for Abbott Diagnostics and Roche Diagnostics’ HE4 tests | Cost (£) | ||
|---|---|---|---|
| Item | Per year | Per test | |
| Quality control for Abbott Diagnostics’ HE4 test (1.5 times per year) | 87.52 | 131.28 | 0.04 |
| Quality control for Roche Diagnostics’ HE4 test (12 times per year) | 354.37 | 4252.44 | 1.20 |
| Maintenance (per year, but not in the first year), taken from Fujirebio Diagnostics and assumed to be the same for Abbott Diagnostics and Roche Diagnostics in the absence of other information | 3819.51 | 3437.56 | 0.97 |
| Calibration (six times per year) Abbott Diagnostics and Roche Diagnostics, taken from Roche Diagnostics, assumed to be the same for Abbott Diagnostics in the absence of other information | 566.98 | 3401.88 | 0.96 |
| Shipment (per month), taken from Fujirebio Diagnostics and assumed to be the same for Roche Diagnostics in the absence of other information | 0.26 | 3.12 | 0.001 |
| Personnel cost items for Abbott Diagnostics and Roche Diagnostics’ HE4 tests | Personnel cost | |
|---|---|---|
| Item | Per test | |
| Personnel time to prepare and perform test | 0.05 hours | – |
| Personnel costs to prepare and perform test (per hour) | £55.16 | £2.76 |
Appendix 7 Test accuracy estimates used for scenario and subgroup analyses
Comparison of different Risk of Malignancy 1 thresholds
| Threshold | Sensitivity, % (SE) | Specificity, % (SE) | Source (systematic review, see Appendix 5) |
|---|---|---|---|
| 250 | 64.4 (1.4) | 91.8 (0.7) | Summary estimate derived from all studies, six published studies73,74,76,78–80 and one unpublished studya that reported data for the RMI 1 (threshold of 250) and the target condition ‘all malignant tumours’ |
| 200 | 68.1 (0.9) | 90.1 (0.5) | Summary estimate derived from all studies, 12 published studies44,48,50,62,73,74,76,78–80,98,103 and one unpublished study that reported data for the RMI 1 (threshold of 200) and the target condition ‘all malignant tumours’ |
| 25 | 94.9 (1.5) | 51.1 (2.1) | Summary estimate derived from all RMI 1 threshold comparison studies that reported data for the relevant threshold74,76,78–80 |
| 50 | 89.5 (1.8) | 68.1 (1.7) | Summary estimate derived from all RMI 1 threshold comparison studies that reported data for the relevant threshold73,74,76,78–80 |
| 100 | 79.6 (2.8) | 88.4 (1.4) | Summary estimate derived from all RMI 1 threshold comparison studies that reported data for the relevant threshold73,76,78–80 |
| 150 | 73.0 (3.1) | 92.8 (1.1) | Summary estimate derived from all RMI 1 threshold comparison studies that reported data for the relevant threshold74,76,78–80 |
| 300 | 53.8 (5.0) | 98.8 (1.1) | Estimate from one RMI 1 threshold comparison study that reported data for this threshold73 |
Premenopausal subgroup
| Test | Sensitivity, % (SE) | Specificity, % (SE) | Source (systematic review, see Chapter 3) |
|---|---|---|---|
| RMI 1 threshold of 250 | 64.4 (1.4) | 91.8 (0.7) | No data available (sensitivity and specificity estimates for all participants used) |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 52.4 (11.4) | 90.1 (2.7) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’103 (see Table 7) |
| ROMA score using Roche Diagnostics’ Elecsys | 90.0 (11.3) | 82.0 (3.6) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’97 (see Table 10) |
| Overa (MIA2G) from Vermillion | 90.3 (5.5) | 71.4 (2.9) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’70 (see Table 17) |
| IOTA group’s simple ultrasound rules (inconclusive results treated as malignant) | 94.5 (1.1) | 79.3 (1.1) | Summary estimate derived from the four studies that reported subgroup data for the target condition ‘all malignant tumours’44,49,50,62 (see Table 12) |
| IOTA group’s ADNEX model | 97.0a (2.9) | 71.0 (4.8) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’44 (see Table 11) |
| RMI 1 threshold of 200 | 53.3 (2.3) | 93.5 (0.7) | Summary estimate derived from all five studies that reported subgroup data for the target condition ‘all malignant tumours’44,50,62,98,103 |
Postmenopausal subgroup
| Test | Sensitivity, % (SE) | Specificity, % (SE) | Source (systematic review, see Chapter 3) |
|---|---|---|---|
| RMI 1 threshold 250 | 64.4 (1.4) | 91.8 (0.7) | No data available (sensitivity and specificity estimates for all participants used) |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 92.6 (6.0) | 79.2 (9.0) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’103 (see Table 8) |
| ROMA score using Roche Diagnostics’ Elecsys | 78.6 (5.8) | 76.1 (6.1) | Sensitivity and specificity estimates taken form the only study to report subgroup data for the target condition ‘all malignant tumours’97 (see Table 11) |
| Overa Vermillion (MIA2G) | 91.8 (3.6) | 65.4 (3.8) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’70 (see Table 18) |
| IOTA group’s simple ultrasound rules (inconclusive results treated as malignant) | 95.4 (0.8) | 67.3 (1.9) | Summary estimate derived from the four studies that reported subgroup data for the target condition ‘all malignant tumours’44,49,50,62 (see Table 13) |
| IOTA group’s ADNEX model | 98.0 (2.3) | 54.0 (4.8) | Sensitivity and specificity estimates taken from the only study to report subgroup data for the target condition ‘all malignant tumours’44 (see Table 12) |
| RMI threshold 200 | 79.4 (1.4) | 79.2 (1.5) | Summary estimate derived from all five studies that reported subgroup data for RMI 1 (threshold of 200) and the target condition ‘all malignant tumours’44,50,62,98,103 |
Appendix 8 Deterministic one-way sensitivity analyses
FIGURE 16.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with ROMA score using Abbott Diagnostics’ ARCHITECT. AOC_OS2y, the overall survival estimate for patients with AOC at 2 years; disc_QALY, the discount rate used for QALYs/costs; HR_OS_MDT_noMDT, the overall survival HR for women referred to SMDT compared with those women not referred to SMDT; U_AOC_MDT, the utility associated with advanced/early ovarian cancer when the woman had been referred to SMDT.

FIGURE 17.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with ROMA score using Roche Diagnostics’ Elecsys. AOC_OS2y, the overall survival estimate for women with AOC at 2 years; disc_QALY, the discount rate used for QALYs/costs; OS, overall survival.
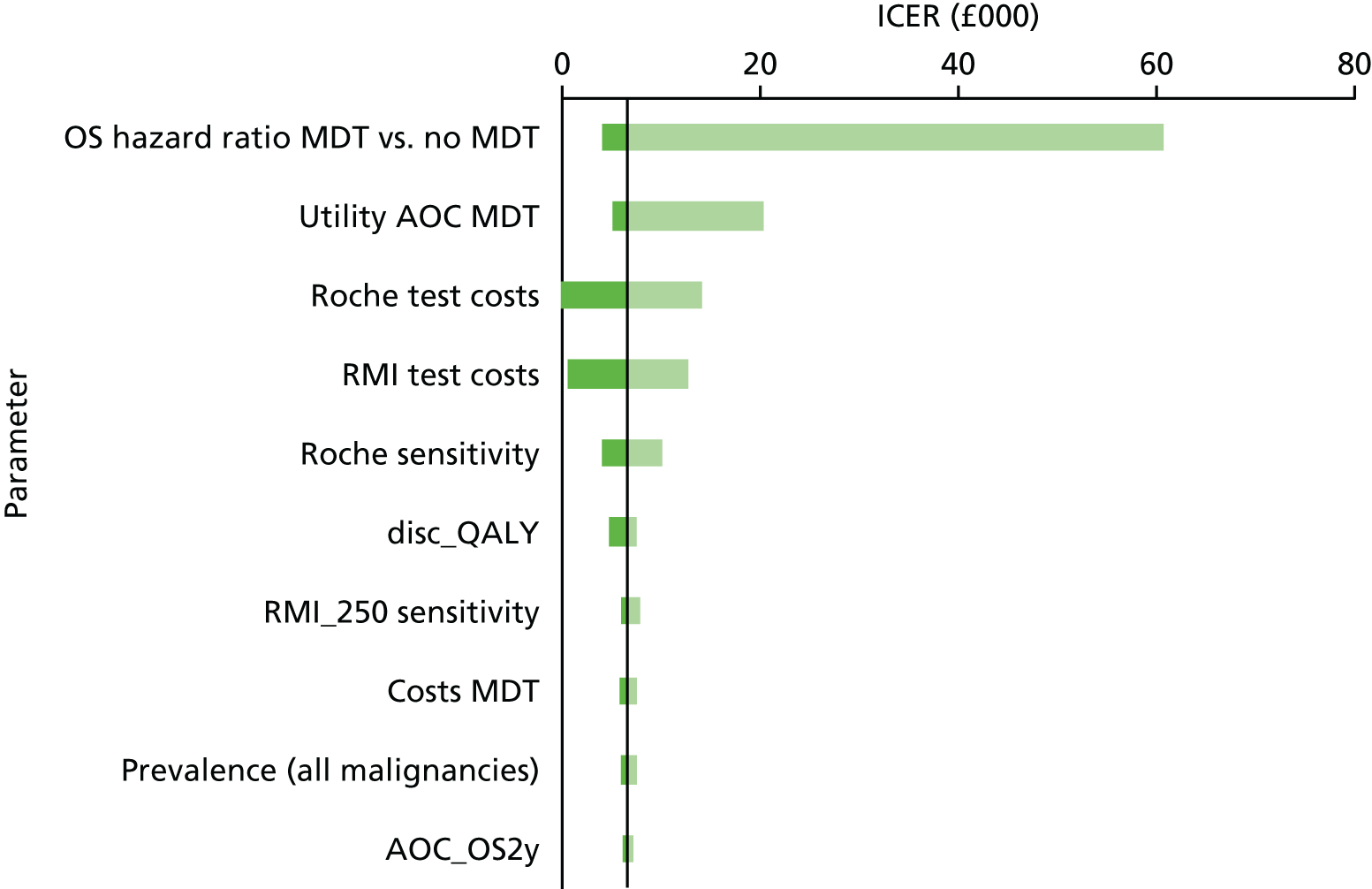
FIGURE 18.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with Overa [MIA2G (threshold of 5 units)]. C_MDT, costs associated with SMDT; disc_QALY, the discount rate used for QALYs/costs; HR_OS_MDT_noMDT, the overall survival HR for women referred to SMDT compared with those women not referred to SMDT; U_AOC_MDT/U_EOC_MDT, the utility associated with advanced/early ovarian cancer when the woman had been referred to SMDT.

FIGURE 19.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with the IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant). C_MDT, costs associated with SMDT; disc_QALY, the discount rate used for QALYs/costs; EOC_OS10y, the overall survival estimate for women with early ovarian cancer at 10 years; HR_OS_MDT_noMDT, the overall survival HR for women referred to SMDT compared with those women not referred to SMDT; Prop_AOC_supp, proportion of women with AOC receiving only supportive care (and no surgery).
FIGURE 20.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with the IOTA group’s ADNEX model (threshold of 10%). C_MDT, costs associated with SMDT; disc_QALY, the discount rate used for QALYs/costs; EOC_OS10y, the overall survival estimate for women with early ovarian cancer at 10 years; HR_OS_MDT_noMDT, the overall survival HR for women referred to SMDT compared with those women not referred to SMDT; U_AOC_MDT/U_EOC_MDT, the utility associated with advanced/early ovarian cancer when the woman had been referred to SMDT.
FIGURE 21.
Top 10 influential parameters in the comparison of the RMI 1 (threshold of 250) with the RMI 1 (threshold of 200). C_MDT, costs associated with SMDT; disc_QALY, the discount rate used for QALYs/costs; HR_OS_MDT_noMDT, the overall survival HR for women referred to SMDT compared with those women not referred to SMDT; Prop_AOC_supp, proportion of women with AOC receiving only supportive care (and no surgery); U_AOC_MDT/U_EOC_MDT, the utility associated with advanced/early ovarian cancer when the woman had been referred to SMDT.

Appendix 9 Scenario analyses (deterministic)
Assuming a prevalence of 20% for all malignancies
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5540 | 14.026 | 17.156 | –2 | 0.020 | 0.027 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5542 | 14.006 | 17.129 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5546 | 14.007 | 17.131 | 4 | 0.001 | 0.002 | £2511 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5572 | 14.028 | 17.158 | 30 | 0.022 | 0.029 | £1333 | £16,137 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5580 | 14.010 | 17.136 | 37 | 0.004 | 0.007 | £9008 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5586 | 14.012 | 17.138 | 43 | 0.006 | 0.009 | £7169 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5648 | 14.022 | 17.151 | 106 | 0.017 | 0.022 | £6385 | Dominated |
Assuming a prevalence of 30% for all malignancies
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 6432 | 12.709 | 15.626 | –1 | 0.032 | 0.041 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 6433 | 12.677 | 15.585 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 6438 | 12.679 | 15.589 | 4 | 0.002 | 0.003 | £2059 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 6464 | 12.712 | 15.630 | 31 | 0.035 | 0.045 | £888 | £10,504 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 6473 | 12.683 | 15.595 | 40 | 0.006 | 0.010 | £6408 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 6478 | 12.687 | 15.600 | 45 | 0.010 | 0.015 | £4466 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 6539 | 12.703 | 15.619 | 106 | 0.026 | 0.034 | £4105 | Dominated |
Assuming 0% prevalence of non-ovarian malignancies
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5339 | 13.920 | 16.995 | –4 | 0.024 | 0.031 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5343 | 13.896 | 16.964 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5347 | 13.898 | 16.967 | 4 | 0.002 | 0.002 | £2427 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5371 | 13.922 | 16.997 | 28 | 0.026 | 0.033 | £1083 | £15,094 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5381 | 13.901 | 16.971 | 38 | 0.004 | 0.007 | £8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5385 | 13.905 | 16.976 | 42 | 0.009 | 0.012 | £4876 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5447 | 13.916 | 16.990 | 104 | 0.020 | 0.026 | £5259 | Dominated |
Assuming an equal proportion of early-stage ovarian cancer versus advanced-stage ovarian cancer in the false-negative and true-positive groups (in the base case it was assumed that false negatives would predominantly/all be early stage)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 250) | 5652 | 13.838 | 16.933 | 0 | 0.000 | 0.000 | Cheapest | |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5655 | 13.855 | 16.957 | 2 | 0.017 | 0.024 | £147 | £147 |
| RMI 1 (at a threshold of 200) | 5656 | 13.840 | 16.936 | 3 | 0.002 | 0.003 | £1552 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5688 | 13.856 | 16.959 | 35 | 0.018 | 0.026 | £1958 | £27,656 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5689 | 13.844 | 16.942 | 36 | 0.006 | 0.009 | £6057 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5694 | 13.847 | 16.945 | 42 | 0.008 | 0.012 | £5052 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5761 | 13.853 | 16.954 | 109 | 0.015 | 0.021 | £7451 | Dominated |
Assuming for International Ovarian Tumour Analysis group’s simple ultrasound rules that subjective assessment would be used for inconclusive assessments (instead of assumed to be malignant)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5642 | 13.847 | 16.948 | –17 | 0.016 | 0.022 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5663 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | £2427 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.855 | 16.957 | 30 | 0.024 | 0.031 | £1249 | £5922 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5697 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | £8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5703 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | £6625 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5765 | 13.849 | 16.950 | 106 | 0.018 | 0.024 | £5949 | Dominated |
Assuming equal test costs for all risk scores
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (a threshold 250) | 5676 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Cheapest | |
| RMI 1 (a threshold 200) | 5680 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | £2427 | Extendedly dominated |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5686 | 13.835 | 16.933 | 10 | 0.004 | 0.007 | £2227 | Extendedly dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5696 | 13.837 | 16.936 | 20 | 0.007 | 0.010 | £3025 | Extendedly dominated |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5699 | 13.853 | 16.955 | 23 | 0.022 | 0.029 | £1073 | £1073 |
| IOTA group’s ADNEX model (a threshold 10%) | 5706 | 13.855 | 16.957 | 30 | 0.024 | 0.031 | £1249 | £3057 |
| Overa (MIA2G) from Vermillion (a threshold 5 units) | 5708 | 13.849 | 16.950 | 33 | 0.018 | 0.024 | £1832 | Dominated |
Assuming no ultrasound is performed in conjunction with Risk of Ovarian Malignancy Algorithm and Overa (MIA2G) risk scores, thus reducing the costs of these risk scores
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5621 | 13.835 | 16.933 | –39 | 0.004 | 0.007 | –£8759 | Cheapest |
| ROMA score using Roche Diagnostics’ Elecsys | 5626 | 13.837 | 16.936 | –33 | 0.007 | 0.010 | –£5006 | Extendedly dominated |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5657 | 13.853 | 16.955 | –2 | 0.022 | 0.029 | –£96 | £2109 |
| RMI 1 (at a threshold of 250) | 5659 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | £0 | Dominated |
| RMI 1 (at a threshold of 200) | 5663 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | £2427 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5689 | 13.849 | 16.950 | 29 | 0.018 | 0.024 | £1645 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.855 | 16.957 | 30 | 0.024 | 0.031 | £1249 | £15,094 |
Assuming additional costs for false positives (surgery costs with malignancy instead of without) and additional costs for false negatives (additional costs of benign surgery)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5759 | 13.853 | 16.955 | –174 | 0.022 | 0.029 | –£7986 | Cheapest |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5793 | 13.855 | 16.957 | –140 | 0.024 | 0.031 | –£5829 | £16,372 |
| ROMA score using Roche Diagnostics’ Elecsys | 5904 | 13.837 | 16.936 | –29 | 0.007 | 0.010 | –£4384 | Dominated |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5905 | 13.835 | 16.933 | –28 | 0.004 | 0.007 | –£6261 | Dominated |
| RMI 1 (at a threshold of 200) | 5915 | 13.832 | 16.928 | –18 | 0.002 | 0.002 | –£11,809 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5921 | 13.849 | 16.950 | –12 | 0.018 | 0.024 | –£675 | Dominated |
| RMI 1 (at a threshold of 250) | 5933 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | £0 | Dominated |
Assuming additional costs for false positives (surgery costs with malignancy instead of without) and additional costs for false negatives (additional costs of benign surgery and specialist multidisciplinary team costs)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5760 | 13.853 | 16.955 | –182 | 0.022 | 0.029 | –£8322 | Cheapest |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5794 | 13.855 | 16.957 | –147 | 0.024 | 0.031 | –£6158 | £16,128 |
| ROMA score using Roche Diagnostics’ Elecsys | 5909 | 13.837 | 16.936 | –32 | 0.007 | 0.010 | –£4935 | Dominated |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5911 | 13.835 | 16.933 | –30 | 0.004 | 0.007 | –£6851 | Dominated |
| RMI 1 (at a threshold of 200) | 5923 | 13.832 | 16.928 | –19 | 0.002 | 0.002 | –£12,400 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5923 | 13.849 | 16.950 | –18 | 0.018 | 0.024 | –£1033 | Dominated |
| RMI 1 (at a threshold of 250) | 5942 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Dominated |
Assuming a discount of 92% for carboplatin (CG122: discount in England of 91.8%; discount in Wales of 92.1%)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5363 | 13.853 | 16.955 | –1 | 0.022 | 0.029 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5364 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5368 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | £2427 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5395 | 13.855 | 16.957 | 31 | 0.024 | 0.031 | £1280 | £15,136 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5402 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | £8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5408 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | £6629 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5470 | 13.849 | 16.950 | 106 | 0.018 | 0.024 | £5977 | Dominated |
Assuming a discount of 95% for paclitaxel (CG122: discount in England of 91.0%; discount in Wales of 95.4%)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5102 | 13.853 | 16.955 | –1 | 0.022 | 0.029 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5103 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5107 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | 2427 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5134 | 13.855 | 16.957 | 32 | 0.024 | 0.031 | 1318 | £15,186 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5141 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | 8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5146 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | 6635 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5210 | 13.849 | 16.950 | 107 | 0.018 | 0.024 | 6010 | Dominated |
Assuming an alternative hazard ratio of 0.808 for progression-free and overall survival for specialist multidisciplinary team referral versus no specialist multidisciplinary team referral
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5658 | 13.847 | 16.948 | 0 | 0.043 | 0.057 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.804 | 16.891 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5664 | 13.807 | 16.896 | 5 | 0.003 | 0.005 | 1664 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5690 | 13.851 | 16.953 | 31 | 0.047 | 0.062 | 660 | 7464 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5700 | 13.812 | 16.905 | 41 | 0.009 | 0.014 | 4812 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5707 | 13.816 | 16.911 | 48 | 0.013 | 0.020 | 3760 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5767 | 13.839 | 16.938 | 108 | 0.035 | 0.047 | 3078 | Dominated |
Assuming an alternative hazard ratio of 0.990 for progression-free and overall survival for specialist multidisciplinary team referral versus no specialist multidisciplinary team referral
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5656 | 13.858 | 16.961 | –4 | 0.002 | 0.003 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5660 | 13.856 | 16.958 | 0 | 0.000 | 0.000 | Dominated | |
| RMI 1 (at a threshold of 200) | 5663 | 13.856 | 16.959 | 3 | 0.000 | 0.000 | £16,921 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.858 | 16.961 | 29 | 0.002 | 0.003 | £12,374 | £158,980 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5694 | 13.856 | 16.959 | 34 | 0.000 | 0.001 | £78,602 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5699 | 13.856 | 16.959 | 39 | 0.001 | 0.001 | £60,637 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5763 | 13.858 | 16.961 | 103 | 0.002 | 0.002 | £60,005 | Dominated |
Assuming that the proportion of patients receiving supportive care (for advanced-stage ovarian cancer) is 10% (instead of 5%)
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 250) | 5780 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Cheapest |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5781 | 13.853 | 16.955 | 1 | 0.022 | 0.029 | 37 | £37 |
| RMI 1 (at a threshold of 200) | 5784 | 13.832 | 16.928 | 5 | 0.002 | 0.002 | 3005 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5813 | 13.855 | 16.957 | 33 | 0.024 | 0.031 | 1368 | £15,066 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5820 | 13.835 | 16.933 | 40 | 0.004 | 0.007 | 9106 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5827 | 13.837 | 16.936 | 47 | 0.007 | 0.010 | 7134 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5889 | 13.849 | 16.950 | 109 | 0.018 | 0.024 | 6119 | Dominated |
Assuming an alternative transvaginal sonography cost of £142.46 (MA36Z) (instead of £76.75 based on CG122)1
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5723 | 13.853 | 16.955 | –2 | 0.022 | 0.029 | –96 | Cheapest |
| RMI 1 (at a threshold of 250) | 5725 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Dominated |
| RMI 1 (at a threshold of 200) | 5729 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | 2427 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5755 | 13.855 | 16.957 | 30 | 0.024 | 0.031 | 1249 | £15,094 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5763 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | 8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5768 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | 6625 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5831 | 13.849 | 16.950 | 106 | 0.018 | 0.024 | 5949 | Dominated |
Assuming an alternative transvaginal sonography cost of £142.46 (MA36Z) (instead of £76.75 based on CG122)1 and increasing the transvaginal sonography for the International Ovarian Tumour Analysis group’s risk scores by 20% (to reflect potential training costs)
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 250) | 5725 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Cheapest |
| RMI 1 (at a threshold of 200) | 5729 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | 2427 | Extendedly dominated |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5751 | 13.853 | 16.955 | 26 | 0.022 | 0.029 | 1206 | £1206 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5763 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | 8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5768 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | 6625 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5783 | 13.855 | 16.957 | 58 | 0.024 | 0.031 | 2435 | £15,094 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5831 | 13.849 | 16.950 | 106 | 0.018 | 0.024 | 5949 | Dominated |
Assuming additional costs of specialist multidisciplinary team referral of £2500 to reflect higher surgery costs
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 250) | 6162 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Cheapest |
| RMI 1 (at a threshold of 200) | 6219 | 13.832 | 16.928 | 57 | 0.002 | 0.002 | 36,724 | Extendedly dominated |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 6333 | 13.835 | 16.933 | 171 | 0.004 | 0.007 | 38,526 | Extendedly dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 6533 | 13.837 | 16.936 | 371 | 0.007 | 0.010 | 56,351 | Extendedly dominated |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 6627 | 13.853 | 16.955 | 464 | 0.022 | 0.029 | 21,275 | £21,275 |
| IOTA group’s ADNEX model (at a threshold of 10%) | 6807 | 13.855 | 16.957 | 645 | 0.024 | 0.031 | 26,929 | £85,145 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 6915 | 13.849 | 16.950 | 753 | 0.018 | 0.024 | 42,337 | Dominated |
Assuming 90% of the non-malignancy surgery and complications costs for true negatives reflecting a scenario wherein 90% of the true negatives are operated on (instead of all)
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 250) | 5419 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | 0 | Cheapest |
| RMI 1 (at a threshold of 200) | 5427 | 13.832 | 16.928 | 8 | 0.002 | 0.002 | 5311 | Extendedly dominated |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5458 | 13.853 | 16.955 | 39 | 0.022 | 0.029 | 1791 | £1791 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5467 | 13.835 | 16.933 | 48 | 0.004 | 0.007 | 10,837 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5496 | 13.837 | 16.936 | 77 | 0.007 | 0.010 | 11,685 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5508 | 13.855 | 16.957 | 89 | 0.024 | 0.031 | 3735 | £23,755 |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5593 | 13.849 | 16.950 | 174 | 0.018 | 0.024 | 9783 | Dominated |
Assuming Avastin for advanced-stage ovarian cancer
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 8412 | 13.887 | 17.010 | –9 | 0.022 | 0.029 | –406 | Cheapest |
| RMI 1 (at a threshold of 250) | 8421 | 13.865 | 16.980 | 0 | 0.000 | 0.000 | 0 | Dominated |
| RMI 1 (at a threshold of 200) | 8425 | 13.867 | 16.983 | 4 | 0.002 | 0.003 | 2284 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 8443 | 13.889 | 17.012 | 22 | 0.024 | 0.032 | 904 | £14,728 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 8459 | 13.870 | 16.988 | 38 | 0.005 | 0.008 | 7894 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 8464 | 13.872 | 16.991 | 44 | 0.007 | 0.011 | 6151 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 8522 | 13.883 | 17.005 | 101 | 0.018 | 0.024 | 5526 | Dominated |
Assuming a disutility of 0.100 for false positives during the first year in the state-transition model
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5657 | 13.835 | 16.955 | –2 | 0.010 | 0.029 | –207 | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.825 | 16.926 | 0 | 0.000 | 0.000 | 0 | Dominated |
| RMI 1 (at a threshold of 200) | 5663 | 13.825 | 16.928 | 4 | 0.000 | 0.002 | 13,465 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.832 | 16.957 | 30 | 0.007 | 0.031 | 4257 | Dominated |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5697 | 13.826 | 16.933 | 38 | 0.002 | 0.007 | 24,820 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5703 | 13.822 | 16.936 | 44 | –0.003 | 0.010 | –15,114 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5765 | 13.823 | 16.950 | 106 | –0.002 | 0.024 | –66,380 | Dominated |
Assuming a disutility of 0.010 for false positives during the first year in the state-transition model
| Discounted | LYs | Compared with standard RMI 1 | ICER (£) | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5657 | 13.851 | 16.955 | –2 | 0.021 | 0.029 | –102 | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.830 | 16.926 | 0 | 0.000 | 0.000 | 0 | Dominated |
| RMI 1 (at a threshold of 200) | 5663 | 13.832 | 16.928 | 4 | 0.001 | 0.002 | 2644 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.852 | 16.957 | 30 | 0.022 | 0.031 | 1344 | £20,023 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5697 | 13.834 | 16.933 | 38 | 0.004 | 0.007 | 9126 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5703 | 13.836 | 16.936 | 44 | 0.006 | 0.010 | 7738 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5765 | 13.846 | 16.950 | 106 | 0.016 | 0.024 | 6676 | Dominated |
Comparison of different Risk of Malignancy Index 1 thresholds
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| RMI 1 (at a threshold of 300) | 5647 | 13.826 | 16.919 | –13 | –0.004 | –0.007 | 2865 | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Extendedly dominated | |
| RMI 1 (at a threshold of 200) | 5663 | 13.832 | 16.928 | 4 | 0.002 | 0.002 | 2427 | Extendedly dominated |
| RMI 1 (at a threshold of 150) | 5664 | 13.834 | 16.932 | 4 | 0.004 | 0.006 | 1172 | Extendedly dominated |
| RMI 1 (at a threshold of 100) | 5671 | 13.838 | 16.937 | 11 | 0.007 | 0.011 | 1619 | Extendedly dominated |
| RMI 1 (at a threshold of 50) | 5690 | 13.848 | 16.949 | 30 | 0.017 | 0.023 | 1783 | £2006 |
| RMI 1 (at a threshold of 25) | 5706 | 13.853 | 16.956 | 46 | 0.023 | 0.030 | 2051 | £2890 |
Using the most optimal Risk of Malignancy Index 1 threshold (i.e. Risk of Malignancy Index 1 threshold cost-effective at £20,000 and/or £30,000 per quality-adjusted life year gained in former scenario)
| Discounted | LYs | Compared with standard RMI 1 | ICER | Full incremental | ||||
|---|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ΔCosts (£) | ΔQALYs | ΔLYs | ||||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5657 | 13.853 | 16.955 | –2 | 0.022 | 0.029 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5659 | 13.831 | 16.926 | 0 | 0.000 | 0.000 | Dominated | |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5689 | 13.855 | 16.957 | 30 | 0.024 | 0.031 | £1249 | £15,094 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5697 | 13.835 | 16.933 | 38 | 0.004 | 0.007 | £8527 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5703 | 13.837 | 16.936 | 44 | 0.007 | 0.010 | £6625 | Dominated |
| RMI 1 (at a threshold of 25) | 5706 | 13.853 | 16.956 | 46 | 0.023 | 0.030 | £2051 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5765 | 13.849 | 16.950 | 106 | 0.018 | 0.024 | £5949 | Dominated |
Appendix 10 Additional subgroup analyses (probabilistic)
| Risk scores | Costs (£) (95% CI) | QALYs (95% CI) | Compared with RMI 1 at a threshold of 250 | Full incremental | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts (£)/ΔQALYs | ΔCosts/ΔQALYs | |||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5652 (4544 to 6922) | 11.640 (11.306 to 11.911) | –3 | 0.020 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5654 (4542 to 6924) | 11.621 (11.287 to 11.891) | Dominated | |||
| RMI 1 (at a threshold of 200) | 5658 (4545 to 6929) | 11.622 (11.287 to 11.893) | 4 | 0.001 | 2561 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5684 (4574 to 6958) | 11.642 (11.308 to 11.912) | 29 | 0.021 | 1371 | £17,212 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5691 (4584 to 6962) | 11.625 (11.291 to 11.897) | 37 | 0.005 | 7719 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5698 (4588 to 6979) | 11.627 (11.291 to 11.899) | 43 | 0.007 | 6657 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5760 (4638 to 7035) | 11.637 (11.302 to 11.907) | 106 | 0.016 | 6602 | Dominated |
| Risk scores | Costs (£) (95% CI) | QALYs (95% CI) | Compared with RMI 1 at a threshold of 250 | Full incremental | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts (£)/ΔQALYs | ΔCosts/ΔQALYs | |||
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5460 (4364 to 6710) | 14.711 (14.363 to 15.018) | –10 | 0.029 | Dominant | Cheapest |
| RMI 1 (at a threshold of 250) | 5470 (4372 to 6724) | 14.681 (14.333 to 14.987) | Dominated | |||
| RMI 1 (at a threshold of 200) | 5471 (4373 to 6726) | 14.685 (14.337 to 14.991) | 2 | 0.004 | 480 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5492 (4387 to 6750) | 14.713 (14.365 to 15.019) | 22 | 0.031 | 715 | £15,631 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5501 (4403 to 6754) | 14.692 (14.343 to 14.999) | 32 | 0.010 | 3052 | Dominated |
| ROMA score using Roche Diagnostics’ Elecsys | 5506 (4404 to 6757) | 14.696 (14.348 to 15.003) | 36 | 0.014 | 2501 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5568 (4469 to 6825) | 14.707 (14.359 to 15.013) | 98 | 0.025 | 3897 | Dominated |
| Risk scores | Costs (£) (95% CI) | QALYs (95% CI) | Compared with RMI 1 at a threshold of 250 | Full incremental | ||
|---|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ΔCosts (£)/ΔQALYs | ΔCosts/ΔQALYs | |||
| RMI 1 (at a threshold of 250) | 5719 (4582 to 6995) | 13.556 (13.138 to 13.906) | 0 | 0.000 | 0 | Cheapest |
| RMI 1 (at a threshold of 200) | 5712 (4579 to 6988) | 13.544 (13.133 to 13.890) | 4 | 0.002 | Extendedly dominated | |
| IOTA group’s simple ultrasound rules (inconclusive, assumed to be malignant) | 5716 (4583 to 6992) | 13.545 (13.134 to 13.893) | 7 | 0.012 | 571 | £571 |
| ROMA score using Abbott Diagnostics’ ARCHITECT | 5752 (4621 to 7024) | 13.556 (13.138 to 13.906) | 38 | 0.004 | 8837 | Dominated |
| IOTA group’s ADNEX model (at a threshold of 10%) | 5750 (4623 to 7024) | 13.548 (13.134 to 13.897) | 40 | 0.013 | 3104 | £39,171 |
| ROMA score using Roche Diagnostics’ Elecsys | 5757 (4623 to 7028) | 13.549 (13.135 to 13.898) | 45 | 0.006 | 7495 | Dominated |
| Overa (MIA2G) from Vermillion (at a threshold of 5 units) | 5824 (4693 to 7105) | 13.554 (13.137 to 13.904) | 112 | 0.010 | 10,748 | Dominated |
Glossary
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- False negative
- An incorrect negative test result – the number of diseased persons with a negative test result.
- False positive
- An incorrect positive test result – the number of non-diseased persons with a positive test result.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test of which the performance is being evaluated.
- Markov model
- An analytic method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- A statistical technique used to combine the results of two or more studies and obtain a combined estimate of effect.
- Metaregression
- A statistical technique used to explore the relationship between the study characteristics and the study results.
- Negative predictive value
- The probability of non-disease among persons with a negative test result.
- Opportunity cost
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Positive predictive value
- The probability of disease among persons with a positive test result.
- Probabilistic sensitivity analysis
- A method of quantifying the uncertainty in a mathematical model, such as a cost-effectiveness model.
- Publication bias
- The bias arising from the preferential publication of studies with statistically significant results.
- Regression analysis
- A statistical method for estimating relationships among variables.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Quality of life
- An individual’s emotional, social and physical well-being, and their ability to perform the ordinary tasks of living.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity, which result from varying the diagnostic threshold.
- Reference standard
- The best currently available method for diagnosing the target condition. The index test is compared against this to allow for the calculation of estimates of accuracy.
- Sensitivity
- The proportion of people with the target disorder who have a positive test result.
- Specificity
- The proportion of people without the target disorder who have a negative test result.
- True negative
- A correct negative test result – the number of non-diseased persons with a negative test result.
- True positive
- A correct positive test result – the number of diseased persons with a positive test result.
List of abbreviations
- ACOG
- American Congress of Obstetricians and Gynecologists
- ADNEX
- Assessment of Different NEoplasias in the adneXa
- AFP
- alpha-fetoprotein
- AOC
- advanced-stage ovarian cancer
- apo A-1
- apolipoprotein A-1
- beta-hCG
- beta-human chorionic gonadotrophin
- CA125
- cancer antigen 125
- CCT
- controlled clinical trial
- CE
- Conformité Européenne
- CEIA
- chemiluminescent enzyme immunoassay
- CI
- confidence interval
- CRC
- colorectal cancer
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- DAR
- diagnostic appraisal review
- EFSUMB
- European Federation of Societies for Ultrasound in Medicine and Biology
- EIA
- enzyme immunoassay
- FIGO
- International Federation of Gynecology and Obstetrics
- FN
- false negative
- FP
- false positive
- FSH
- follicle-stimulating hormone
- GP
- general practitioner
- HE4
- human epididymis protein 4
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IBS
- irritable bowel syndrome
- ICER
- incremental cost-effectiveness ratio
- ICON
- International Collaborative Ovarian Neoplasm
- IOTA
- International Ovarian Tumour Analysis
- IU
- International Unit
- LY
- life-year
- MDT
- multidisciplinary team
- MIA
- multivariate index assay
- MIA2G
- multivariate index assay, second generation
- MMS
- multimodal screening
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PET
- positron emission tomography
- PET-CT
- positron emission tomography computed tomography
- PI
- predictive index
- PROBAST
- Prediction model study Risk Of Bias Assessment Tool
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS-2
- quality assessment of diagnostic accuracy studies 2
- RCOG
- Royal College of Obstetrics and Gynaecology
- RCT
- randomised controlled trial
- RMI 1
- Risk of Malignancy Index 1
- ROCkeTS
- Refining Ovarian Cancer Test accuracy Scores
- ROMA
- Risk of Ovarian Malignancy Algorithm
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMDT
- specialist multidisciplinary team
- TA
- Technology Appraisal
- TN
- true negative
- TP
- true positive
- TRF
- transferrin
- TVS
- transvaginal sonography
- USS
- ultrasound screening
This monograph is based on the Technology Assessment Report produced for the National Institute for Health and Care Excellence (NICE). The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.