Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/69/19. The protocol was agreed in June 2016. The assessment report began editorial review in June 2017 and was accepted for publication in January 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
John Ramage reports research grants to King’s College Hospital from Novartis Pharmaceuticals UK Ltd (Frimley, UK) and Imaging Equipment Ltd (Radstock, UK) and a research grant to Hampshire Hospitals NHS Foundation Trust from Pfizer UK (Tadworth, UK), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Mujica-Mota et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
‘Neuroendocrine tumours’ (NETs) is the overarching term for the group of heterogeneous cancers that develop in cells in the diffuse neuroendocrine system. The diffuse neuroendocrine system is made up of neuroendocrine cells found in the respiratory and digestive tracts. As these cancers share common clinical features, they are considered under the same group of neoplasms. 1 Most commonly, NETs are found in the lungs, pancreas or gastrointestinal (GI) system. NETs also encompass carcinoids and may be referred to as neuroendocrine carcinoids, which leads to substantial confusion over their name. 2
Aetiology, pathology and prognosis
The aetiology of NETs is poorly understood. 1 Predominantly, NETs are sporadic in nature (i.e. they arise from de novo changes); however, there is a small genetic risk associated with familial endocrine cancer syndromes. Neuroendocrine cells are present throughout the gut and are the largest group of hormone-producing cells in the body. 2 NETs develop slowly and may remain undetected over a number of years. Therefore, it is common for NETs to be diagnosed when they have already metastasised (i.e. spread to other organs or tissues in the body).
Characteristics of neuroendocrine tumours
The characteristics of a NET will determine the methods of treatment and the impact on prognosis. Important characteristics include the tumour location, tumour grade and differentiation, tumour stage and secretory profile of the tumour. There are, however, inconsistencies in the reproducibility of diagnoses between pathologists and institutions, which has been suggested to be caused by the use of a variety of different classification systems and a lack of adherence to them. 2
Location
Most NETs have been generally classified as foregut (including those in the lungs), midgut or hindgut, as it was thought that they were derived from embryonic neural crest cells. However, this theory is not now accepted and classification should be based on the site of origin of the tumour, that is, pancreas, lung, stomach, small bowel or large bowel (colon). The term ‘carcinoid’ is outdated but colloquially refers to NETs of the small bowel that secrete 5-hydroxytryptamine; the term ‘carcinoid’ is also still in common usage for NETs of the lung. ‘NET’ is the preferred term for all of these tumours. NET tumours may be grouped together as gastroenteropancreatic (GEP) NETs. Typically, the locations of these tumours are as follows:1
-
foregut tumours: develop in the bronchi, stomach, gallbladder, duodenum and pancreas
-
midgut tumours: develop in the jejunum, ileum, appendix and right colon
-
hindgut tumours: develop in the left colon and rectum.
Prognosis can be dependent on where a tumour is located. An analysis of 13,715 carcinoid tumours over a 5-decade period in the USA found that the best 5-year survival rates were found in patients with rectal (88.3%), bronchopulmonary (73.5%) and appendicle (71.0%) NETs. 3 The lowest 5-year survival rates were found in patients with pancreatic NETs (pNETs) (37.5%). 3
Neuroendocrine tumours from the pancreas may also be called endocrine tumours of the pancreas and include insulinomas (which produce the hormone insulin), gastrinomas (which produce the hormone gastrin), glucagonomas (which produce the hormone glucagon), VIPomas (which produce the hormone vasoactive intestinal peptide) and somatostatinomas (which produce the hormone somatostatin). However, the majority of pNETs are non-functioning and do not produce measurable levels of hormones that give symptoms.
Other, rarer locations for NETs include the thyroid gland (medullary thyroid tumours), skin (Merkel cell cancer), pituitary gland, parathyroid gland and adrenal gland.
This assessment report focuses on the tumours of the pancreas, GI tract and lung as these are locations for which the interventions of interest are licensed.
Tumour grade/degree of differentiation
A NET can be defined as grade 1, 2 or 3. The grade relates to an estimation of how fast the cells are dividing to form new cells and is based on the histological assessment and the mitotic count of the tumour. The grade of a tumour is also related to its differentiation. Differentiation relates to how well/little the tumour looks like the normal tissue/tissue of origin. Well-differentiated and low-grade cancer cells look more like normal cells and tend to grow and spread more slowly than poorly differentiated cells. High-grade tumours have cells that look very abnormal and are likely to grow and spread rapidly.
In 2010, the World Health Organization (WHO) introduced a new system for grading cancer tumours. 4 This grading system is also endorsed by the European Neuroendocrine Tumor Society (ENETS) grading schemes. 5,6 The grading scheme is as follows:
-
NET grade 1 (low grade)
-
well-differentiated tumour with a low number of cells actively dividing
-
Ki-67 index of ≤ 2%
-
mitotic count of < 2 per 10 high-power field (HPF)
-
-
NET grade 2 (intermediate grade)
-
well-differentiated tumour, but with a higher number of cells actively dividing
-
Ki-67 index of 3–20%
-
mitotic count of 2–20 per 10 HPF
-
-
neuroendocrine carcinoma (NEC) grade 3 (high grade)
-
poorly differentiated, malignant carcinoma (most aggressive form of NET)
-
Ki-67 index of > 20%
-
mitotic count of > 20 per 10 HPF.
-
Stage of tumour (see Appendix 20)
Determination of the size of a tumour and whether or not it has spread beyond its original site is known as staging of the tumour. Tumour staging is performed according to a system of site-specific criteria. There are two main systems for staging NETs: the Union for International Cancer Control’s (UICC’s) TNM Classification of Malignant Tumours, Seventh Edition7 (see Appendix 19, Table 133) and the ENETS staging system5,6 (see Appendix 20, Table 134). The Royal College of Pathologists8 recommended both the WHO and the ENETS systems for assessing the grade/stage of a NET. In current practice, both systems are used together with the UICC grading system above. The difference between the UICC grading system and the ENETS grading systems is not great and would not affect outcomes relating to this report.
Secretory profile
A tumour that is releasing above-typical levels of hormones is known as a functioning tumour. The increase in hormone release will often cause symptoms that may themselves need treating in addition to treating the cancer. For example, hormones released by a pNET include insulin, glucagon and pancreatic polypeptide, whereas hormones released by an appendix NET include serotonin and somatostatin. Tumours that are not releasing hormones, and, therefore, that have no hormone-related clinical features, are known as non-functioning tumours.
Epidemiology
Incidence and prevalence
In October 2016, Public Health England (PHE)9 published the first data briefing on the incidence and survival in NETs and NECs in England. In 2013 and 2014, 8726 neoplasms were diagnosed, equating to 4000 per year or an approximate rate of 8 per 100,000 people per year (not age standardised). Although the annual incidence of NETs is low, because of the long survival of individuals with NETs, the prevalence is much greater and has been calculated as 35 per 100,000 people. 10
Incidence trends for NETs were compared between a Norwegian registry and an American registry. 11 From the time period 1993–7 to 2000–4, there was an incidence rate increase of 72% for NETs in Norway (from 2.35 to 4.06 per 100,000 people). Over the same time period in America, the increase was 37% (from 4.22 to 5.79 per 100,000 people) for the white population and 40% (from 5.48 to 7.67 per 100,000 people) for the black population. In a Canadian population, between 1994 and 2009, the incidence rates for NETs at all locations increased by 138% (from 2.46 to 5.86 per 100,000 people). 12
More specifically, for the subgroup of GI NETs, Ellis et al. 13 reviewed incidence rates in the UK between 1971 and 2006. In this time period, 10,324 cases of GI NETs were identified from the national population-based cancer registry. They report an overall increase per 100,000 people from 0.27 in men and 0.35 in women (1971–8) to 1.32 in men and 1.33 in women (2000–6). This is equivalent to an increase in incidence rates for GI NETs from 1971 to 2006 of 392% for men and 282% for women. 13
However, these incidence rates for the diagnosis of NETs do not account for the overall prevalence of NETs. As the delay in diagnosis is typically 5–7 years after the appearance of the first symptoms, many cases of NETs are undiagnosed. 1
Public Health England9 produced a diagram depicting the morphology (form of the neuroendocrine neoplasms) and topography (location of the neuroendocrine neoplasms) of 8726 individuals diagnosed with NETs and NECs in 2013 and 2014. Low-grade (grade 1) NETs and not-otherwise-specified NECs make up the predominant morphology of neuroendocrine neoplasms in England. PHE went on to describe some characteristics of the cohort:
-
almost an exact 50 : 50 male-to-female ratio
-
no obvious variation with geographical region
-
no obvious variation by ethnicity
-
distribution of age similar to that of other malignant cancers combined
-
higher incidence of patients from the most affluent population quintile (20.2%) than from the most deprived population quintile (18.6%; p = 0.011).
Risk factors
As NETs are sporadic in nature; there are very few factors known to determine susceptibility to developing a NET.
In the USA, African American males have a higher overall incidence rate of NETs than other demographic groups. 3 Following an epidemiological review of NETs in Japan, the authors compared the distribution of the origin of NETs between European and US populations and Asian populations. 14 In the former countries, a midgut origin represented 30–60% of new NETs, whereas in Asian populations the midgut was the origin of < 10% of new NETs. In a parallel way, the hindgut was the origin of a higher proportion of new NETs in Asian populations. 14 A case–control study of risk factors for NETs of the small intestine, stomach, lung, pancreas and rectum in 740 individuals with NETs and 924 healthy control subjects in the USA indicated an increased risk for women with a family history of cancer and diabetes mellitus. 15 In contrast, in the UK, PHE9 found no association of ethnicity and sex with NET prevalence.
There are some suggestions that individuals suffering from rare family syndromes may have a higher risk of developing NETs. These family syndromes include multiple endocrine neoplasia type 1 (MEN1), neurofibromatosis type 1 and von Hippel–Lindau (VHL) syndrome.
Survival
Although prognosis is generally better with an early diagnosis, the majority of NETs are diagnosed at a later stage when the tumour has already metastasised.
In data collected between 1986 and 1999 for 4104 cases of malignant digestive endocrine tumours in England and Wales, overall 5-year and 10-year survival was reported to be 45.9% and 38.4% respectively. 16 Well-differentiated tumours had a higher 5-year survival rate (56.8%), whereas small-cell tumours had the lowest 5-year survival rate (5.2%). Survival rates were higher for women and young people (15–54 years vs. 55–74 years and 75–99 years) and the overall prognosis was dependent on the features (e.g. tumour differentiation, anatomical site, histological type) of the NET. 16
Although it is impossible to compare accurately different countries with the data available, median 5-year survival varied from 38% to 61% across Europe,17 Taiwan18 and Canada. 12 Whether or not survival has improved over time remains debated. Korse et al. 19 reported in the Netherlands an ongoing improvement in survival for well-differentiated NETs and suggested that the introduction of somatostatin analogues (SSAs) and their long-acting forms may explain this change in survival over time. However, other research groups in the USA and France have not confirmed this trend. 20,21
Impact of the health problem
Significance for patients in terms of ill health (burden of disease)
Although prognosis is better with an early diagnosis, NETs are generally diagnosed at a late stage when the tumour has already metastasised. In such cases, treatment is rarely curative, although individuals can live and maintain a good quality of life for a number of years (e.g. 68–77% of people diagnosed with a carcinoid tumour will survive for ≥ 5 years22). The primary management strategy for NETs is managing symptoms originating from the tumour. The onset of symptoms, however, may take between 3 and 5 years from the development of the tumour. Symptoms can vary widely and some patients may have no symptoms or non-specific and vague symptoms (often leading to a delay in diagnosis).
Most individuals with NETs will experience non-specific symptoms such as pain, nausea and vomiting and, in some cases, anaemia, because of intestinal blood loss. Most GEP NETs are non-functioning and present predominantly with mass effects of the primary tumour or metastases (usually liver). 1 Symptoms are more common with functioning pNETs, in which hormones are significantly elevated.
In total, 20% of well-differentiated endocrine tumours of the jejunum or ileum (midgut NETs) will have carcinoid syndrome. Carcinoid syndrome consists of (usually) dry flushing (without sweating; 70% of cases) with or without palpitations, diarrhoea (50% of cases) and intermittent abdominal pain (40% of cases). 1 The metastases in the liver release vasoactive compounds, including biogenic amines (e.g. serotonin and tachykinins), into the systemic circulation, which cause the carcinoid syndrome. Direct retroperitoneal involvement with venous drainage bypassing the liver may also cause carcinoid syndrome (i.e. it is not dependent on liver metastases). 1
Carcinoid crisis may also occur in individuals with NETs. Symptoms include profound flushing, bronchospasm, tachycardia and widely and rapidly fluctuating blood pressure. It is usually linked to an anaesthetic induction for an operation or other invasive therapeutic procedure and is thought to be linked to the release of mediators leading to high levels of serotonin and other vasoactive peptides. 1
Measurement of disease
A number of outcomes can be measured in clinical trials or as part of the management of disease:
-
Overall survival (OS), defined as the time from randomisation to death from any cause.
-
Progression-free survival (PFS), defined as the time from randomisation until disease progression or death.
-
Objective response rate (ORR), defined as either a partial response or a complete response:
-
Complete response – all detectable tumour has disappeared.
-
Partial response – roughly corresponds to a ≥ 50% decrease in the total tumour volume but with evidence of some residual disease still remaining.
-
Stable disease – includes either a small amount of growth (typically < 20% or < 25%) or a small amount of shrinkage.
-
Progressive disease – means that the tumour has grown significantly or that new tumours have appeared. The appearance of new tumours is always defined as progressive disease regardless of the response of other tumours. Progressive disease normally means that the treatment has failed.
-
-
Health-related quality of life (HRQoL): how a person’s well-being is affected by treatment. HRQoL is a key measure for the treatment of NETs as this captures changes in symptom control. It is the control of the symptoms that has the most impact on a patient’s day-to-day life.
Current service provision
Management of disease
Diagnosis
Diagnosis of NETs can be difficult as they are often small tumours (some may be < 1 cm in size), they can occur almost anywhere in the body and they can result in a vast array of symptoms or no symptoms at all. NETs are slow-growing tumours and may be present for many years without recognisable symptoms. Therefore, diagnosis often occurs at quite a late stage in the disease.
Typical symptoms in the early stages include vague abdominal pain and potential changes in bowel habits, which primarily are diagnosed as irritable bowel syndrome. 23 More progressive symptoms include shortness of breath, loss of appetite and weight loss. 24 Diagnosis primarily occurs following detailed histology. Other tests may include urine tests, blood tests, ultrasound scans, computed tomography (CT) scans, magnetic resonance imaging (MRI) scans, radioactive scans and positron emission tomography (PET)/CT scans. Diagnosis is also dependent on the clinical manifestations, peptide and amine secretions and specialised radiological and nuclear imaging of the NETs. 1 Being able to determine the secretory products of a NET is helpful for diagnosis, to assess the efficacy of subsequent treatment and to assess changes in prognosis. 1 Similarly, imaging is used not only for detecting the primary tumour, but also for screening at-risk populations, assessing the extent of the disease and assessing the response to treatment at follow-up. 1
Treatment
The aim of treatment, when realistically possible, should always be curative. However, in the majority of cases it is most likely to be palliative (i.e. aimed at symptom control). As metastatic disease is common for individuals with NETs, often improving quality of life is the primary aim of treatment (as opposed to curing the disease). 1 Individuals with NETs can maintain a good quality of life for a long period of time. 1 Quality of life is therefore assessed regularly throughout treatment.
There is a vast array of treatment options for treating NETs. The initial treatment often starts with surgery and symptom management. Surgery is the only curative treatment for NETs. Symptom treatment, particularly with hormonal hypersecretion in functional NETs, can have a significant impact on an individual’s quality of life as the symptoms themselves, as opposed to the cancer, may be life-threatening (e.g. severe diarrhoea and hypokalaemia). 1 Symptom control is often manged with a SSA, for example octreotide (Sandostatin®; Novartis International AG, Basel, Switzerland) or lanreotide (Somatuline Autogel®; Ipsen, Paris, France). Available treatments that follow surgery and initial symptom control include:
-
liver transplant
-
interferon alpha (Roferon-A, Roche Products Ltd)
-
chemotherapy
-
ablation therapies
-
targeted radionuclide therapy, including one of the interventions of interest in this assessment report: lutetium-177 DOTATATE [(177Lu-DOTATATE) Lutathera®; Imaging Equipment Ltd, Radstock, UK]
-
transhepatic artery embolisation/chemoembolisation
-
external-beam radiotherapy
-
emerging therapies, including two of the interventions of interest in this assessment report: everolimus (Afinitor®; Novartis) and sunitinib (Sutent®; Pfizer Inc., New York, NY, USA).
Describing an overarching treatment pathway for NETs is challenging as there are many different options depending on the characteristics of the NET (e.g. location, grade, differentiation, secretory profile).
Current service cost
The economic burden to the NHS of health-care provision for people with NETs is not well documented. This may be partly because of the rarity and heterogeneity of the disease, but also because significant new therapeutic options have only recently been introduced.
Public Health England9 has reported that approximately 4000 new cases of neuroendocrine neoplasms are diagnosed each year. From a budgetary perspective this is a small subgroup of the 300,000 new cancer diagnoses registered annually in England,25 but with the arrival of new high-cost targeted therapeutic treatments, the cost-effectiveness of disease management is now a relevant area for scrutiny through secondary research.
The main costs involved in current service provision for people with inoperable progressive NETs can be divided into the cost of diagnosis and monitoring of disease (e.g. measurement of blood markers and CT, MRI and PET), the cost of acquiring and administering active and supportive treatments (in particular long-acting repeat SSA therapy but also chemotherapy), the cost of managing symptoms (if the tumour is functioning), the cost of managing adverse events (AEs) and the cost of human resources for patient consultation, multidisciplinary team meetings and hospitalised care.
Relevant national guidelines, including National Service Frameworks
Guidelines for the management of GEP NETs (including carcinoid tumours) were published in 2012 by a group of authors who are members of the UK and Ireland Neuroendocrine Tumour Society (UKINETS). 1
There is a related guideline from the National Institute for Health and Care Excellence (NICE) from 2010 that focuses on tumours of unknown origin26 that fall under the NETs umbrella. This guideline is distinct from the work in this report, which focuses on pancreatic, lung and GI NETs.
Description of the technology under assessment
Summary of interventions
The scope of this review is to ascertain the clinical effectiveness and cost-effectiveness of three interventions for unresectable or metastatic NETs with disease progression. These interventions are everolimus, 177Lu-DOTATATE and sunitinib.
Everolimus27
Everolimus is an orally active agent that is able to slow down the growth and spread of a tumour. It acts by binding to FK506-binding protein-12 (FKBP-12) to form a complex, which is able to block the mammalian target of rapamycin (mTOR) protein. Division of tumour cells and growth of blood vessels require mTOR and it is through the blocking of mTOR that everolimus is able to slow down the growth and spread of the tumour.
Everolimus has a marketing authorisation for tumours of pancreatic origin:
Afinitor is indicated for the treatment of unresectable or metastatic, well- or moderately-differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease.
It also has a marketing authorisation for NETs of GI or lung origin:
Afinitor is indicated for the treatment of unresectable or metastatic, well-differentiated (Grade 1 or Grade 2) non-functional neuroendocrine tumours.
Everolimus is an oral drug that is typically given at a dose of 10 mg a day. It is recommended that treatment is continued for as long as benefits are observed or an unacceptable level of side effects occur. The dose of everolimus may be reduced or stopped in an effort to minimise side effects. Tablets should be taken every day at the same time of day.
The most common side effects of everolimus (affecting > 1 in 10 people) are rash, pruritus (itching), nausea, decreased appetite, dysgeusia (taste disturbances), headache, decreased weight, peripheral oedema (swelling, especially of the ankles and feet), cough, anaemia (low red blood cell count), fatigue (tiredness), diarrhoea, asthenia (weakness), infections, stomatitis (inflammation of the lining of the mouth), hyperglycaemia (high blood glucose level), hypercholesterolaemia (high blood cholesterol level), pneumonitis (inflammation of the lungs) and epistaxis (nosebleeds). Everolimus is not suitable for people who are hypersensitive to rapamycin derivatives.
Everolimus was removed from the Cancer Drugs Fund (CDF) on 12 March 2015; it was previously available for the treatment of progressive unresectable or metastatic well-differentiated NETs of the pancreas.
177Lu-DOTATATE28
Lutetium-177 DOTATATE is a radiolabelled SSA. It is made up of a radionuclide (177Lu) and the peptide–chelator complex [DOTA0, Tyr3-]-octreotate (DOTATATE). The (Tyr3)-octreotate binds to malignant cells that overexpress somatostatin receptors [specifically the somatostain receptor type 2 (SSTR2)]. Once bound, the 177Lu-DOTATATE accumulates within the tumour cells, delivering cytotoxic radiation that kills them.
As of December 2016, 177Lu-DOTATATE does not have marketing authorisation in the UK for any indication.
Administration of 177Lu-DOTATATE is through an intravenous infusion and involves 3 days of hospital appointments, including an overnight stay. Typically, four cycles are administered over a total of 8 to 10 months.
There are two main types of side effects from 177Lu-DOTATATE: those relating to the therapy and those relating to the radiation dose given. Side effects related to the therapy include nausea, pain, flushing, sweating, palpitations, wheezing, diarrhoea, hair loss and fatigue. Side effects relating to the radiation dose include the effects on bone marrow production and kidney function, which in turn may increase the number of infections experienced by patients.
Lutetium-177 DOTATATE was removed from the CDF on 4 November 2015. It was previously available for the treatment of advanced NETs after sunitinib/chemotherapy, pNETs and midgut carcinoid tumours (after octreotide/somatostatin therapies).
Sunitinib29
Sunitinib is a protein kinase inhibitor that is able to reduce the growth and spread of cancer and cut off the blood supply that enables cancer cell growth. Sunitinib works by blocking enzymes known as protein kinases that are found in some receptors at the surface of cancer cells. The development of new blood vessels and the growth and spread of cancer cells requires protein kinases and it is through the blocking of these enzymes that sunitinib is able to slow the growth and spread of the tumours.
Sunitinib has a marketing authorisation for tumours of pancreatic origin:
SUTENT is indicated for the treatment of unresectable or metastatic, well-differentiated pNETs with disease progression in adults.
Sunitinib is an oral drug and is typically given at a dose of 37.5 mg a day. Treatment is recommended to be continued for as long as benefits are observed or an unacceptable level of side effects occurs. The dose of sunitinib may be reduced or stopped in an effort to minimise side effects.
The most common side effects of sunitinib are fatigue (tiredness), GI disorders (such as diarrhoea, feeling sick, inflammation of the lining of the mouth, indigestion and vomiting), respiratory disorders (such as shortness of breath and cough), skin disorders (such as skin discoloration, dryness of the skin and rash), hair colour changes, dysgeusia (taste disturbances), epistaxis (nosebleeds), loss of appetite, hypertension (high blood pressure), palmar–plantar erythrodysaesthesia syndrome (rash and numbness on the palms and soles), hypothyroidism (an underactive thyroid gland), insomnia (difficulty falling and staying asleep), dizziness, headache, arthralgia (joint pain), neutropenia (low levels of neutrophils, a type of white blood cell), thrombocytopenia (low blood platelet count), anaemia (low red blood cell count) and leukopenia (low white blood cell count).
Sunitinib is available on the CDF for the treatment of pNETs when all of the following criteria are met:
-
application made, and first cycle of systemic anticancer therapy to be prescribed, by a consultant specialist specifically trained and accredited in the use of systemic anticancer therapy
-
biopsy-proven well-differentiated pNET
-
first-line indication or second-line indication or third-line indication
-
no previous vascular endothelial growth factor (VEGF)-targeted therapy.
Identification of important subgroups
From the NICE scope,30 the following subgroups were identified as being important in the treatment of NETs:
-
location of tumour
-
grade/degree of differentiation
-
stage of tumour
-
secretory profile
-
number of previous treatments.
Further information on these subgroups can be found in Characteristics of neuroendocrine tumours.
Current usage in the NHS
It was difficult to ascertain the current usage of everolimus, sunitinib and 177Lu-DOTATATE in the NHS. In its submission, Advanced Accelerator Applications (AAA) Ltd31 (Saint-Genis-Pouilly, France) reported that, although unlicensed, 177Lu-DOTATATE has been used to treat patients in England through the CDF (confidential information has been removed). Likewise, Pfizer32 report that sunitinib is also available through the CDF and is used in the NHS in England for the treatment of patients with pNET (52 requests were made in the 12 months ending March 2015). Novartis33 did not report in its submission the estimated use of everolimus within the NHS in England; however, our clinicians suggest that the rate of use of everolimus is higher than that of sunitinib.
Anticipated costs associated with the interventions
The cost of treating a patient with everolimus or sunitinib varies from one patient to the next because the duration of treatment with these oral preparations is continuous and largely dependent on effectiveness for the individual. The mean duration of treatment with everolimus in the RADIANT-3 (RAD001 in Advanced Neuroendocrine Tumors, Third Trial)34 and RADIANT-4 (RAD001 in Advanced Neuroendocrine Tumors, Fourth Trial)35 trials of NET patients is about 9 months, with a range of 1 week to 2 years. 34 In practice, it is disease stability and drug tolerability that trigger the decision to purchase the next month of therapy. Everolimus and sunitinib are normally self-administered and so the cost of drug delivery is limited to the time needed by hospital pharmacy staff to dispense the drugs. In contrast, the drug acquisition cost of 177Lu-DOTATATE is less variable between patients because its delivery is fixed to a maximum of four treatment cycles. In addition, in comparison to the oral preparations of everolimus and sunitinib, the delivery of 177Lu-DOTATATE is more resource intensive. 177Lu-DOTATATE is a radiolabelled intravenous preparation and so administration involves careful handling, specialist staff and post-administration observation, which for most patients means an overnight hospital stay. Beyond acquisition and administration, the remaining treatment-related costs arise from disease monitoring and the medical management of AEs, which will of course differ across treatments but which are less substantial components of the overall cost.
We expect that all of these cost components will vary between individuals and hence they were subject to modelling, but the acquisition costs of treatments are presented in Table 23 for simple comparative purposes. 36
Chapter 2 Changes to the project scope
During the course of this review, NICE consulted on amendments to the original project scope. The revised scope was agreed on 18 August 201630 and the differences between the original and the revised scope are provided in Table 1.
| Characteristic | Old scope | New scope |
|---|---|---|
| Intervention(s) |
|
|
| Population(s) |
People with progressed unresectable or metastatic NETs According to the specific locations covered by the marketing authorisations of the interventions |
People with progressed unresectable or metastatic NETs According to the specific locations covered by existing and anticipated marketing authorisations of the interventions |
| Comparators |
|
|
| Outcomes | The outcome measures to be considered include:
|
The outcome measures to be considered include:
|
| Other considerations | If the evidence allows the following subgroups will be considered:
|
If the evidence allows the following subgroups will be considered:
|
| Economic analysis |
The reference case stipulates that the cost-effectiveness of treatments should be expressed in terms of incremental cost per quality-adjusted life-year The reference case stipulates that the time horizon for estimating clinical effectiveness and cost-effectiveness should be sufficiently long to reflect any differences in costs or outcomes between the technologies being compared Costs will be considered from a NHS and Personal Social Services perspective The use of 177Lu-DOTATATE is conditional on the presence of somatostatin receptor-positive GEP NETs. The economic modelling should include the costs associated with diagnostic testing for somatostatin receptor-positive GEP NETs in people who would not otherwise have been tested. A sensitivity analysis should be provided without the cost of the diagnostic test. See section 5.9 of the Guide to the Methods of Technology Appraisals37 |
The reference case stipulates that the cost-effectiveness of treatments should be expressed in terms of incremental cost per quality-adjusted life-year The reference case stipulates that the time horizon for estimating clinical effectiveness and cost-effectiveness should be sufficiently long to reflect any differences in costs or outcomes between the technologies being compared Costs will be considered from a NHS and Personal Social Services perspective |
What is the impact of the changes in scope?
-
The population and outcomes under review were unchanged from the original scope.
-
The following intervention was removed: lanreotide (NETs of mid-gut, pancreatic or unknown origin).
-
The following comparator was removed: octreotide (long-acting release formulation).
Chapter 3 Definition of the decision problem
Decision problem
Population
The population specified in the final scope issued by NICE30 is people with progressed unresectable or metastatic NETs. In addition, the population must be in accordance with the specific locations covered by the existing and anticipated marketing authorisations of the interventions.
Subgroups of interest based on the NICE scope include:
-
location of the tumour
-
grade/degree of differentiation of the tumour
-
stage of the tumour
-
secretory profile of the tumour
-
number of previous treatments.
Interventions
-
Everolimus – for NETs of GI, pancreatic or lung origin.
-
Lutetium-177 DOTATATE – for NETs of GI or pancreatic origin.
-
Sunitinib – for NETs of pancreatic origin.
Comparators
Interventions should be compared with each other and with:
-
interferon alpha
-
chemotherapy regimes (including but not restricted to combinations of streptozocin, fluorouracil (5-FU), doxorubicin, temozolomide and capecitabine)
-
best supportive care (BSC).
The Assessment Group (AG) noted, following consultation with our clinicians, that interferon alpha was not commonly used within UK clinical practice.
Outcomes
The outcomes of interest based on the NICE scope include:
-
OS
-
PFS
-
response rates (RRs: including complete response, partial response, stable disease, progressive disease, tumour shrinkage and ORR)
-
symptom control
-
AEs
-
HRQoL.
Key issues
The primary factors that may influence the clinical effectiveness of treatment for individuals with NETs are predominantly covered within the population subgroups listed in Population.
In addition to the number of previous treatments, covered as a population subgroup, the use of concomitant treatment (primarily SSA use) while taking part in clinical trials may also be a key issue. This is because the administration of SSAs as a concomitant treatment is not uniform in the treatment of NETs.
Treatment switching from placebo to the active treatment is also another key issue for consideration in terms of how the switching may confound the outcomes reported for the placebo arm.
Overall aims and objectives of the assessment
The aim of this report was to review the clinical effectiveness and cost-effectiveness of everolimus, 177Lu-DOTATATE and sunitinib for treating unresectable or metastatic NETs with disease progression in a multiple technology appraisal (MTA). We carried out a systematic review of clinical effectiveness studies to assess the medical benefit and risks associated with these treatments, and compared the treatments against available alternative standard treatments. We also assessed whether or not these drugs are likely to be considered good value for money for the NHS using a model-based economic evaluation.
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 4 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Evidence for the clinical effectiveness of everolimus, 177Lu-DOTATATE and sunitinib within their marketing authorisations for treating unresectable or metastatic NETs with disease progression was obtained by conducting a systematic review of published and unpublished research evidence. This review was undertaken following the methodological guidance published by the Centre for Reviews and Dissemination (CRD). 38
Changes to the protocol
As discussed in Chapter 2, NICE issued a revised scope for this project on 18 August 2016. 30 The revised scope necessitated a change to our published protocol39 as lanreotide was removed as an intervention and octreotide was removed as a comparator. A revised protocol was drafted (PROSPERO CRD42016041303); there were no other changes to the published protocol.
Identification of studies
The literature search aimed to systematically identify studies relating to the clinical effectiveness of everolimus, 177Lu-DOTATATE and sunitinib in the treatment of unresectable or metastatic NETs with disease progression. The search strategy was developed in MEDLINE (Ovid) and then adapted for use in the other resources searched.
The bibliographic literature search was undertaken in May 2016 and the search was further updated in September 2016.
Searching of bibliographic databases and databases of ongoing trials
The following bibliographic databases were searched: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), MEDLINE Daily (Ovid), MEDLINE Epub Ahead of Print (Ovid), EMBASE (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Wiley Interface) and Web of Science (including Conference Proceedings Citation Index) (Thomson Reuters).
The search syntax took the following form: (search terms for neuroendocrine tumours) AND (search terms for the interventions under review). The searches were not limited by study design, language or date.
The following trial registries were handsearched: Current Controlled Trials, ClinicalTrials.gov, the US Food and Drug Administration (FDA) website and the European Medicines Agency (EMA) website40 [including European Public Assessment Reports (EPARs)].
The full search strategies are provided in Appendix 1.
Website searching
The following websites were searched:
-
ENETS [www.enets.org/ (accessed 19 May 2016)]
-
UKINETS [www.ukinets.org/ (accessed 19 May 2016)].
Deduplication
All references were exported into EndNote X7 (Clarivate Analytics, Philadelphia, PA, USA), where automatic and manual deduplication was performed.
Screening
Title and abstracts were independently double-screened by two reviewers. Studies meeting the inclusion criteria at the title and abstract stage were ordered as full texts, which were independently double-screened by three reviewers.
Citation searching, appraisal of company submissions and identification of systematic reviews of randomised controlled trials
All studies meeting the full-text inclusion criteria were citation chased. Forwards citation searching was conducted in Web of Science (Thomson Reuters) and backwards citation searching was conducted manually through the appraisal of the bibliographies of included studies. Citation searching is reported in Appendix 1.
Included randomised controlled trials (RCTs) from identified systematic reviews were checked against the table of included studies for this review. Studies included in the clinical effectiveness sections of company submissions were also checked against the table of studies included in this review.
Inclusion and exclusion criteria
Inclusion and exclusion criteria for the selection of clinical effectiveness studies were defined exactly as per the decision problem (see Chapter 3). Studies identified prior to the publication of the revised scope30 were rechecked for inclusion against this revised scope.
The inclusion and exclusion criteria for the original and revised scope are summarised in Table 1. Studies were also required to be in the English language.
The systematic review of clinical effectiveness focused only on RCTs. When no RCTs were identified for an intervention of interest, a non-systematic review of non-randomised evidence was conducted (see Appendix 2). Non-randomised evidence included prospective observational cohort studies (both comparative and single-arm studies). Case reports were not included.
In addition to identifying RCTs, systematic reviews of RCTs, although not formally included in the systematic review, were used as potential sources of additional references providing efficacy evidence.
Studies published as abstracts or conference presentations were included if they linked to included full-text papers, in which appraisal of the methodology and an assessment of the results could be undertaken.
Data extraction and management
Studies included at the full-text stage were shared between three reviewers for the purposes of data extraction. A standardised data specification form was used and extracted data were double-checked by a second reviewer. When multiple publications of the same study were identified, data were extracted and reported as if a single study.
Information sourced for extraction and tabulation included the study design and methodology, the baseline characteristics of participants and the following outcomes; PFS, OS, RRs, AEs and HRQoL. Definitions of the outcomes are provided in Chapter 1 (see Measurement of disease).
When information on key data was incomplete, we attempted to contact the study author(s). In addition, the companies were approached through NICE and asked to provide missing data and supplementary individual patient data.
Assessment of risk of bias
The methodological quality of each included study was assessed by one reviewer and checked by a second reviewer, using criteria based on those proposed by the CRD for RCTs. 38 An additional question (question 10; see Table 4) was added to assess the applicability of the study to the NHS in England.
Methods of data analysis/synthesis
Data were tabulated and narratively synthesised. If sufficient evidence was available and study designs were homogeneous, meta-analysis would be performed. In addition, when the data allowed, an indirect treatment comparison (ITC) would be performed.
The study design and baseline characteristics for all included studies are presented, followed by the outcome results. Outcomes from the studies are reported by tumour location, first for pNETs and then for GI and lung NETs combined, as this was how the included studies were published. Additional data were subsequently made available so that GI NETs and lung NETs could be assessed as isolated tumour locations.
Indirect treatment comparisons
When data were available, the Bucher et al. 41 method was used for an ITC for the outcomes of PFS, OS, RR and AEs. This method was best suited for this assessment given that the available data were only in summary form and were limited to a handful of studies. More sophisticated methods were explored in the economic analysis described in Chapter 6 using individual patient data, which highlight the limitations of the Bucher et al. 41 method, but resource limitations prevented this evidence being incorporated in the clinical effectiveness review. Further details can be found in Indirect treatment comparison: pancreatic neuroendocrine tumours.
Results
The results of study identification in accordance with the updated NICE scope are discussed first in this section, followed by a description of the quality of the evidence and overview tables of the included trials. When available, outcomes (PFS, OS, RRs, HRQoL and AEs) are then reported by tumour location and by subgroup. The subgroups considered were based on the NICE scope (see ‘Other considerations’ in Table 1).
When non-randomised evidence was sought, details are presented after the RCT evidence. These data are tabulated and narratively discussed in brief.
Quantity and quality of research available (randomised controlled trial evidence)
Studies identified
Titles and abstracts were screened for 6209 unique references identified by the searches, after which 273 full-text papers were retrieved for detailed consideration. A total of 217 full texts were excluded (a table of these excluded references, along with the reasons for exclusion can be found in Appendix 3). The Cohen’s kappa for full-text screening was 0.579 [standard error 0.045, 95% confidence interval (CI) 0.491 to 0.667].
Update searches were conducted in September 2016. A total of 645 references were identified and 25 were selected for full-text retrieval. Of these, six citations were formally included in the review.
Six systematic reviews42–47 were retained for scrutiny and three trials were included in the review: RADIANT-3 (four full texts34,48–50 and 22 abstracts51–72), RADIANT-4 (one full text35 and eight abstracts73–80) and A6181111 (one full text,81 19 abstracts82–100 and erratum to the full text81). Following scrutiny of the included studies from the six systematic reviews, no further evidence was identified. A table of all of the included citations is provided in Appendix 4.
Of note, two citations related to a study by Yao et al. 101 This study met our inclusion criteria; however, it was excluded as the paper was retracted because ‘the authors discovered statistical errors which need further validation’. The study compared everolimus (n = 44) with placebo (n = 35) in Chinese patients with pNETs.
No randomised studies were identified that met the inclusion criteria of the systematic review for clinical evidence for the following interventions and comparators:
-
177Lu-DOTATATE compared with any of the included comparators
-
everolimus compared with interferon alpha or chemotherapy
-
sunitinib compared with interferon alpha or chemotherapy.
The AG ran an additional search (see Appendix 5 for the search strategy) with the aim of identifying any RCTs that compared chemotherapy with BSC or placebo in the NETs population. Identified studies would help inform discussions around the clinical effectiveness of the interventions in comparison to chemotherapy through an ITC. Following the screening of 850 citations, no studies were identified. The AG, on the advice from our clinicians, did not search for RCTs comparing interferon alpha with BSC or placebo, as interferon alpha is not commonly used in UK clinical practice.
Neuroendocrine Tumors Therapy trial
The NETTER-1 (Neuroendocrine Tumors Therapy) trial102 was identified as an includable trial through four published abstracts103–106 identified in the systematic review in accordance with the original NICE scope. However, it was not included in this systematic review as it did not meet the revised inclusion criteria of the updated scope issued by NICE on 18 August 2016. 30
The NETTER-1 trial is a RCT that compares 177Lu-DOTATATE plus 30 mg of octreotide long-acting release (LAR) with 60 mg of octreotide LAR. Whether or not octreotide LAR could be deemed a concomitant treatment, as the doses were different in each treatment arm, was explored.
The AG sought consultation from our clinicians, who were unable to confirm whether or not the different dosing of octreotide LAR would result in different clinical effectiveness results. Therefore, the AG searched for RCT dosing studies (see Appendix 5 for the search strategy) to ascertain whether or not 30 mg of octreotide LAR had the same clinical effectiveness as 60 mg of octreotide LAR in the NETs population. Following screening of 180 citations, no studies were identified.
As the AG could not verify with any certainty that 30 mg of octreotide LAR had the same clinical effectiveness as 60 mg of octreotide LAR, and octreotide LAR was not a comparator within the scope, this study was excluded from the review.
Taken from the company submission,31 AAA reports that the rationale for treating the comparator arm with a high dose of octreotide (60 mg) was as follows:
A higher dose was required by the regulatory authorities at the time of the parallel scientific advice meeting with the FDA and EMA considering that the patients enrolled in the trial had have progressive disease following 20 or 30 mg octreotide LAR, and it was not ethical to maintain them on the same dose regimen. Consequently, 60 mg octreotide LAR at 4-week intervals dose was agreed for the control arm in the absence of an alternative efficacious treatment approved for this type of tumour.
p. 4431
The AG appreciate that, as the only RCT of 177Lu-DOTATATE identified, this trial may be of interest to the committee and so the main outcomes are presented in Appendix 6 along with the results of an ITC with everolimus from RADIANT-4. 35
The study selection process is outlined in Figure 1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Ongoing trials
The following trials registries were handsearched for ongoing trials: Current Controlled Trials, ClinicalTrials.gov; the FDA website and the EMA website (including EPARs) (see Appendix 1 for the search strategy). All searches were carried out in May 2016. Three trials were considered relevant to this review:
-
NCT02687958: Study of Everolimus as Maintenance Therapy for Metastatic NEC with Pulmonary or Gastroenteropancreatic Origin. N = 30, currently recruiting, sponsored by the Gruppo Oncologico Italiano di Ricerca Clinica.
-
NCT02358356: Capecitabine ON Temozolomide Radionuclide Therapy Octreotate Lutetium-177 NeuroEndocrine Tumours Study (CONTROL NETs). N = 165, currently not open for recruitment, sponsored by the Australasian Gastro-Intestinal Trials Group.
-
NCT02230176: Antitumor Efficacy of Peptide Receptor Radionuclide Therapy With 177Lutetium -Octreotate Randomized vs Sunitinib in Unresectable Progressive Well-differentiated Neuroendocrine Pancreatic Carcinoma: First Randomized Phase II (OCCLURANDOM). N = 80, currently recruiting, sponsored by Gustave Roussy, Cancer Campus, Grand Paris.
As two of the trials were investigating 177Lu-DOTATATE, the intervention that we were unable to provide relevant RCT evidence for, we contacted the study organisers and received replies from both. The CONTROL NETs trial is in progress and data are not expected until 2018/19. The OCCLURANDOM study has recruited a total of 13 individuals and data are not expected to be ready before submission of this assessment report.
Study design and participant characteristics: pancreatic neuroendocrine tumours
This review includes the two trials that evaluated treatments in pNETs (RADIANT-334 – everolimus; A618111181 – sunitinib). The characteristics of the study designs are summarised in Table 2. In both trials, participants were randomised 1 : 1 to the intervention or placebo and BSC was given in both the intervention arm and the placebo arm. Both trials measured the following outcomes: PFS, OS, RR (to include complete response, partial response, stable disease, progressive disease and ORR) and AEs. A618111181 also reported HRQoL.
| Study ID | ITT population (n) | Intervention | Tumour locations included | Inclusion criteria | Randomisation stratification factor | Primary end point | Secondary end points | Median (range) treatment duration (months) | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| RADIANT-333,34 (NCT00510068) | 207 | Everolimus + BSC | Pancreas | Low or intermediate grade, advanced (unresectable or metastatic), disease progression in previous 12 months | Stratified according to status with respect to previous chemotherapy (receipt vs. no receipt) and according to WHO PS (0 vs. 1 or 2) at baseline | PFS | OS, ORR, duration of response, safety | (Confidential information has been removed | 17 |
| 203 | Placebo + BSC | (Confidential information has been removed | |||||||
| A618111181 (NCT00428597) | 86 | Sunitinib + BSC | Pancreas | Pathologically confirmed, well differentiated, advanced and/or metastatic, disease progression in previous 12 months | NR | PFS | OS, ORR, time to response, duration of response, safety, patient-reported outcomes | 4.6 (0.4–17.5) | 34.1 |
| 85 | Placebo + BSC | 3.7 (0.03–20.2) | |||||||
| RADIANT-435 (NCT01524783) | 205 | Everolimus + BSC | Lung + GI (Ileum, rectum, unknown origin, jejunum, stomach, duodenum, colon, other, caecum, appendix) | Pathologically confirmed, advanced (unresectable or metastatic), non-functional, well differentiated (grade 1 or 2), disease progression in previous 6 months | Stratified by previous SSA treatment, tumour origin and WHO PS (0 vs. 1) | PFS | OS, ORR, disease control rate, HRQoL, WHO PS, safety, pharmacokinetics, changes in chromogranin A and neuron-specific enolase levels | 9.3 (0.1–27.7)a | 21 |
| 97 | Placebo + BSC | 4.5 (0.9–30.0)b | |||||||
| Study ID | ITT (n) | Interventions evaluated (dose) | Mean relative dose intensityc | Dose reductions/interruptions, n/N (%) | Treatment switching, n/N (%) | SSA use during study | |||
| RADIANT-334 (NCT00510068) | 207 | 10 mg of oral everolimus once daily; BSC (confidential information has been removed)a | 0.86 | (59) | NA | Approximately 40% of individuals: everolimus arm 37.7%,d placebo arm 39.9%e | |||
| 203 | Matching placebo; BSC (includes SSAs)a | 0.97 | (28) | 148/203 (73) | |||||
| A618111181 (NCT00428597) | 86 | 37.5 mg of oral sunitinib once daily;f BSC | 0.91 | (30) | NA | n = 23 (n = 22 were already on SSAs; n = 1 started following study enrolment) | |||
| 85 | Matching placebo; BSC | 1.01 | (12) | 59/85 (69)g | n = 25 (n = 20 were already on SSAs; n = 5 started following study enrolment) | ||||
| RADIANT-435 (NCT01524783) | 205 | 10 mg of oral everolimus once daily; BSC | 0.90h | 135/202 (67) | NA | NRi | |||
| 97 | Matching placebo; BSC | 1.00j | 29/98 (30) | Not permitted | |||||
The primary end point was the same (PFS) for both trials. Median treatment duration was 4.6 months in the treatment arm in A618111181 and (confidential information has been removed) in RADIANT-334 for the treatment arm and (confidential information has been removed) for the placebo/BSC arm. Median follow-up was reported to be 17 months for RADIANT-334 and 34.1 months for A6181111. 81
A summary of information relating to drug administration is also provided in Table 2. The mean relative dose intensity (RDI) of the active treatment was slightly lower in the everolimus studies (0.86 in RADIANT-334) than in the sunitinib study (0.91 in A618111181). The use of SSAs was permitted in both treatment arms in both trials. Treatment switching after disease progression (from placebo to active treatment) was allowed in both trials.
The A6181111 trial was discontinued early following a recommendation from the safety monitoring committee:
because of the greater number of deaths and serious adverse events in placebo group and the difference in progression-free survival favouring sunitinib.
Raymond et al. 81
The statistical power of the study was reduced because of the early termination of the trial. Only 171 individuals were randomised rather than the target of 340.
To achieve sufficient statistical power in RADIANT-3,34 it was estimated that 392 individuals would need to be randomised to detect a clinically meaningful improvement in PFS. This target was reached as 410 patients were recruited and randomised to the study.
Population characteristics: pancreatic neuroendocrine tumours
The baseline demographic and disease characteristics for RADIANT-334 and A618111181 are reported in Table 3.
| Study ID | Intervention | Tumour location | n | Age (years), median (range) | Male, n/N (%) | Tumour functioning, n/N (%) | Tumour differentiation, n/N (%) | WHO PS, n/N (%) | Previous treatments, n/N (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Well differentiated | Moderately differentiated | Unknown | ||||||||
| RADIANT-334 | Everolimus + BSC | Pancreas | 207 | 58 (23–87) | 110/207 (53) | NR | NR | 170/207 (82) | 35/207 (17) | 2/207 (1) | 0: 139/207 (67); 1: 62/207 (30); 2: 6/207 (3) | Radiotherapy: 47/207 (23); chemotherapy: 104/207 (50); SSAs: 101/207 (49) |
| Placebo + BSC | 203 | 57 (20–82) | 117/203 (58) | NR | NR | 171/203 (84) | 30/203 (15) | 2/203 (1) | 0: 133/203 (66); 1: 64/203 (32); 2: 6/203 (3) | Radiotherapy: 40/203 (20); chemotherapy: 102/203 (50); SSAs: 102/203 (50) | ||
| A618111181 | Sunitinib + BSC | Pancreas | 86 | 56 (25–84) | 42/86 (49) | 25/86 (29) | 42/86 (49) | 86/86 (100)a | (0) | ECOG PS: 0: 53/86 (62); 1: 33/86 (38); 2: 0/86 (0) | Surgery: 76/86 (88); radiation therapy: 9/86 (10); chemoembolisation: 7/86 (8); radiofrequency ablation: 3/86 (3); percutaneous ethanol injection: 1/86 (1); SSAs: 30/86 (35) | |
| Placebo + BSC | 85 | 57 (26–78) | 40/85 (47) | 21/85 (25) | 44/85 (52) | 85/85 (100)a | (0) | ECOG PS: 0: 41/85 (48); 1: 43/85 (51); 2: 1/85 (1)b | Surgery: 77/85 (91); radiation therapy: 12/85 (14); chemoembolisation: 14/85 (16); radiofrequency ablation: 6/85 (7); percutaneous ethanol injection: 2/85 (2); SSAs: 32/85 (38) | |||
| RADIANT-435 | Everolimus + BSC | Lung, GI | 205 | 65 (22–86) | 89/205 (43) | 0/205 (0) | 205/205 (100) | 205/205 (100)a | (0) | 0: 149/205 (73); 1: 55/205 (27) | Surgery: 121/205 (59); chemotherapy: 54/205 (26); radiotherapy: 44/205 (22); locoregional + ablative therapy: 23/205 (11); SSAs: 109/205 (53) | |
| Placebo + BSC | 97 | 60 (24–83) | 53/97 (55) | 0/97 (0) | 97/97 (100) | 97/97 (100)a | (0) | 0: 73/97 (75); 1: 24/97 (25) | Surgery: 70/97 (72); chemotherapy: 23/97 (24); radiotherapy: 19/97 (20); locoregional + ablative therapy: 10/97 (10); SSAs: 54/97 (56) | |||
RADIANT-334 and A618111181 recruited participants of a similar age (median age ranged from 56 to 58 years). There was a slightly higher proportion of men recruited to RADIANT-334 (53% to the everolimus arm and 58% to the placebo arm) than to A618111181 (49% to the sunitinib arm and 47% to the placebo arm). Both studies recruited individuals with pNETs only.
The functionality of the tumour was not reported in RADIANT-3,34 whereas A618111181 recruited a mixture of functioning (> 30%) and non-functioning (≈50%) individuals (the functionality of the remaining ≈20% was not clarified).
A618111181 recruited individuals with well-defined or moderately defined tumours, whereas RADIANT-334 recruited around 80% of individuals with well-defined tumours, with the remainder having moderately defined tumours.
RADIANT-334 measured performance status (PS) using the WHO PS score system, whereas A618111181 measured PS using the Eastern Cooperative Oncology Group (ECOG) PS system. Our clinicians suggested that there is little difference between PS measured by either system. The majority of individuals had a PS score of 0 in RADIANT-334 (66–67%), with the majority of the remaining individuals having a PS score of 1 (30–32%) and the remainder having a PS score of 2 (3%). A618111181 had a lower proportion of individuals with a PS score of 0 (62% in the sunitinib arm and 48% in the placebo arm) and a higher proportion of individuals with a PS score of 1 (38% in the sunitinib arm and 51% in the placebo arm) than RADIANT-3. 34 One individual was recruited with a PS score of 2 in the placebo arm; this was a protocol deviation for A6181111. 81
The proportions of individuals who had received previous treatments were variable between RADIANT-334 and A618111181 (see Table 3). Of particular note, SSA use prior to treatment was around 50% in RADIANT-334 and between 35% and 38% in A6181111. 81
Study design and participant characteristics: gastrointestinal and lung neuroendocrine tumours
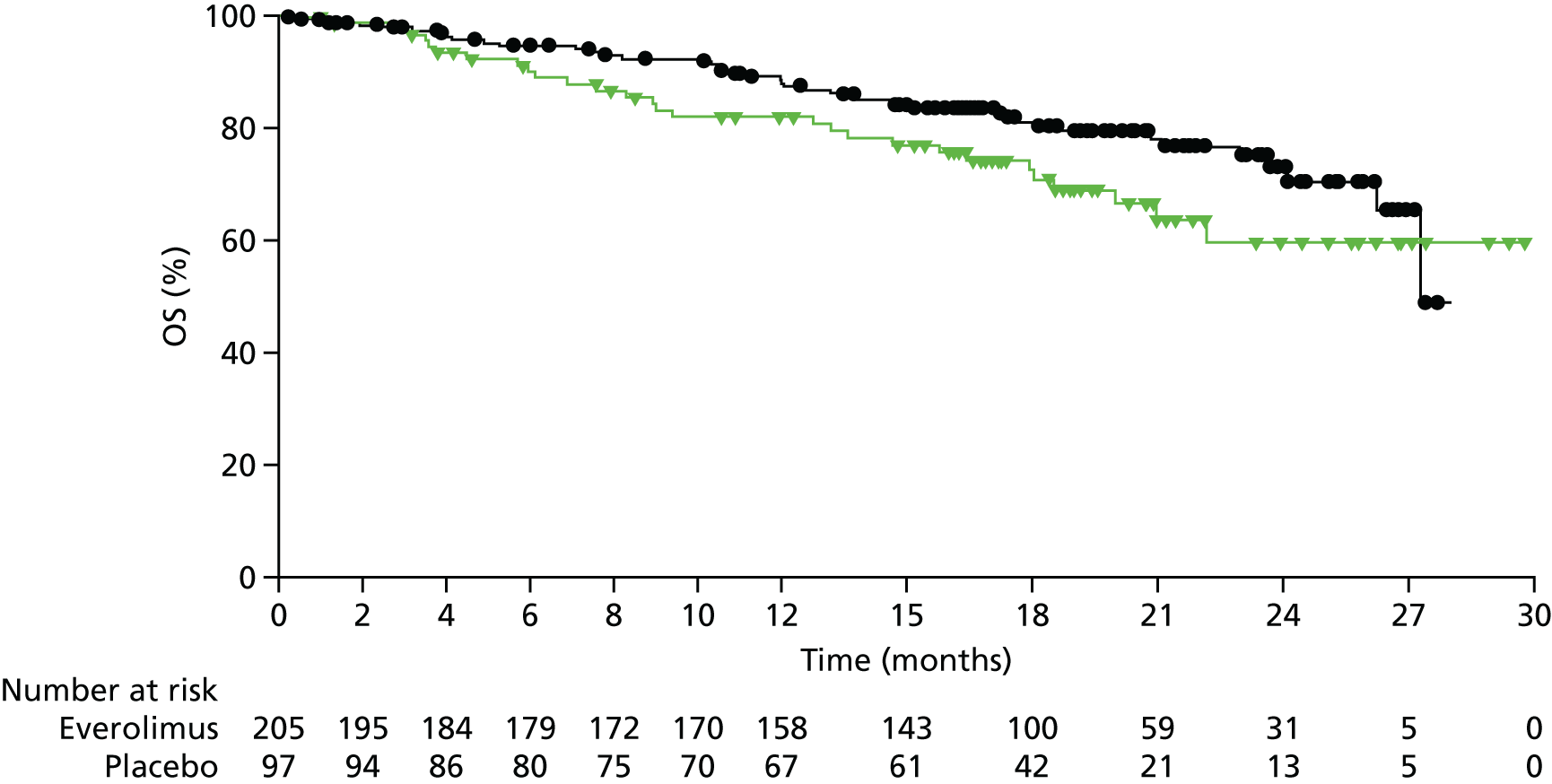
This review includes RADIANT-4,35 which evaluated everolimus in individuals with GI and lung NETs. The characteristics of the study design are summarised in Table 2. Participants were randomised 2 : 1 to everolimus or placebo. BSC was given in both the intervention (everolimus) arm and the placebo arm. RADIANT-435 measured the following outcomes: PFS, OS, RR (to include complete response, partial response, stable disease, progressive disease and ORR) and AEs. The primary end point was PFS. Median treatment duration was 9.3 months in the everolimus arm and 4.5 months in the placebo arm. Median follow-up was 21 months.
A summary of information relating to drug administration is provided in Table 2. The use of SSAs was permitted in both treatment arms. Treatment switching (from placebo to active treatment) was not allowed in RADIANT-4. 35
For RADIANT-435 it was estimated that 285 individuals would need to be randomised at a ratio of 2 : 1. This requirement was met as 302 individuals were randomised.
Population characteristics: gastrointestinal and lung neuroendocrine tumours
Baseline demographic and disease characteristics for RADIANT-435 are reported in Table 3.
The median age of participants ranged from 60 to 65 years in RADIANT-4. 35 There was a slightly lower proportion of men recruited to the everolimus arm (43%) than to the placebo arm (55%). Only individuals with non-functioning, well-defined tumours were recruited to RADIANT-4. 35 PS was measured using the WHO PS scoring system. The majority of individuals had a PS score of 0 (73–75%), with the remaining having a PS score of 1 (27–25%). The proportions of individuals who had received previous treatments were variable between the arms (see Table 3).
Quality appraisal
The three identified RCTs were appraised for quality (Table 4). When necessary for clarification purposes, published protocols available as online supplementary material for each of the main citations for the three studies were referred to. For each trial, data from all publications for that trial contributed to the quality appraisal.
| Item | RADIANT-334 | A618111181 | RADIANT-435 |
|---|---|---|---|
|
1. Was the assignment to the treatment groups really random? |
Low risk | Low risk | Low risk |
|
2. Was treatment allocation concealed? |
Low risk | Low risk | Low risk |
|
3. Were the groups similar at baseline in terms of prognostic factors? |
Unclear riska | Unclear riskb | Unclear riskc |
|
4. Were the care providers blinded to the treatment allocation? |
Low risk | Low risk | Low risk |
|
5. Were the outcome assessors blinded to the treatment allocation? |
Low risk | Low risk | Low risk |
|
6. Were the participants blinded to the treatment allocation? |
Low risk | Low risk | Low risk |
|
7. Were all a priori outcomes reported? |
Low risk | Low risk | Low risk |
|
8. Were complete data reported [e.g. was attrition and exclusion (including reasons) reported for all outcomes]? |
Low risk | Low riskd | Low risk |
|
9. Did the analyses include an ITT analysis? |
Low risk | Low risk | Low risk |
|
10. Are there any specific limitations that might limit the applicability of this study’s findings to the current NHS in England? |
Unclear riske | Unclear riskd | Unclear risk |
Treatment allocation
Overall, the risk of bias was found to be the same in the three trials with regard to selection, performance, detection, attrition and reporting bias. It was assessed that these trials demonstrated a low risk of bias.
RADIANT-3,34 A618111181 and RADIANT-435 all used a centralised internet or telephone registration system for determining treatment allocation. RADIANT-334 and RADIANT-435 based their stratification on prognostic factors (tumour location,35 WHO PS,34,35 previous chemotherapy use34 and previous SSA use35). A618111181 stratified by country/region only.
Similarity of groups
Baseline characteristics were predominantly similar between the two arms for all of the trials. However, there were some differences between arms in the trials:
-
RADIANT-334 – participants in the everolimus arm tended to have a shorter time from initial diagnosis at baseline than participants in the placebo arm (6 months to < 2 years: 31% vs. 21% respectively; 2 years to ≤ 5 years: 26% vs. 40% respectively). The proportion of individuals with two disease sites was higher in the everolimus arm than in the placebo arm (41% vs. 32%).
-
A618111181 – there was a higher proportion of participants with an ECOG PS of 0 in the sunitinib arm than in the placebo arm (62% vs. 48%), whereas the proportion of participants with a PS of 1 was lower in the sunitinib arm than in the placebo arm (38% vs. 51%).
-
RADIANT-435 – there was a higher proportion of women in the everolimus arm than in the placebo arm (57% vs. 45%). In addition, fewer individuals had been treated with surgery prior to entering the study in the everolimus arm than in the placebo arm (59% vs. 72%).
The difference in ECOG PS between arms in A618111181 is the difference most likely to affect the clinical effectiveness results, with those receiving sunitinib having a proportionally better PS than those receiving placebo. Otherwise, it was considered by the clinicians that these baseline differences between the treatment arms were unlikely to affect significantly the clinical effectiveness outcomes reported in the trials.
Implementation of masking
RADIANT-3,34 A618111181 and RADIANT-435 were all double-blind trials and, as such, the participants, investigators, site personnel and trial teams were blinded to the allocated treatment. In addition, central reviews of tumour progression were carried out in both RADIANT-334 and RADIANT-4;35 these outcome assessors were also blinded to treatment allocation. Information obtained from the protocols indicated that both everolimus and placebo had an identical appearance, identical packaging and labelling and an identical scheduling of administration in both RADIANT-334 and RADIANT-4. 35 Information on the appearance of the placebo medication was not provided for A6181111. 81
It was assessed that there was a low risk of bias with regard to the blinding of outcome assessors, participants and care providers.
Completeness of trial data
All a priori outcomes reported in the protocols were reported in the trial publications. 34,35,81 Intention-to-treat (ITT) analysis was carried out in each of the trials. Discrepancies in participant numbers in reports of AEs were poorly explained in all three trials. Both RADIANT-334 and A618111181 included fewer participants in their AE outcomes than the number of participants recruited, whereas RADIANT-435 included an additional participant in the placebo arm who was not accounted for (n = 97 randomised and n = 98 reported for AEs).
It was assessed that there was a low risk of bias for the completeness of trial data from all three trials.
Generalisability
The populations evaluated by RADIANT-3,34 A618111181 and RADIANT-435 were all in line with the licensed indication for each treatment and with the final scope issued by NICE. 30 All of the studies were multicentre studies including centres in both the UK and Europe. In total, 38% of the population in RADIANT-334 were European, whereas 67% of the population in A618111181 were European. It was not reported in RADIANT-435 what proportion of the population was European.
To assess the generalisability of the trials to the UK setting, the AG sought data on the prevalence of NETs in the UK. There is limited information available on the current prevalence of NETs in the UK. In October 2016, PHE9 published the first documentation of the incidences of and survival in NETs, based on a cohort of 8726 neoplasms diagnosed in England in 2013–14. 9
In the UK, NETs were described by PHE as having a 50 : 50 male-to-female ratio, with no obvious variation by geographical region or ethnicity. 9 In the three trials included here there was an average split for male-to-female ratio, with the percentage of males recruited ranging from 43%35 to 58%. 34
Public Health England9 deemed the age at which NETs are most prevalent to be similar to that for all other malignant cancers. The age range of participants in the three trials appears to be younger (median age ranging between 56 and 65 years) than the age range of the typical population with NETs in the UK.
Based on the very limited data available on what the UK demographic for people with NET constitutes, it was assessed that all three trials had an unclear risk for the applicability of their results to the UK.
Assessment of effectiveness: randomised controlled trial evidence
The following outcomes were assessed:
-
PFS
-
OS
-
RRs: complete response, partial response, stable disease, progressive disease, ORR and tumour shrinkage
-
AEs
-
HRQoL.
Outcomes from randomised controlled trial evidence for pancreatic neuroendocrine tumours
Progression-free survival
In RADIANT-334 and A618111181 the primary outcome was PFS. Disease progression was defined by both trials as the time from randomisation to the first evidence of progression or death from any cause. 34,81 Both trials used the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0107 to define disease progression. In RADIANT-3,34 PFS was obtained from central radiology review and also local investigator review, whereas in A618111181 PFS was obtained only from local investigator review in the published paper. 81 PFS from the assessment of an independent review was available from the company submission. 33
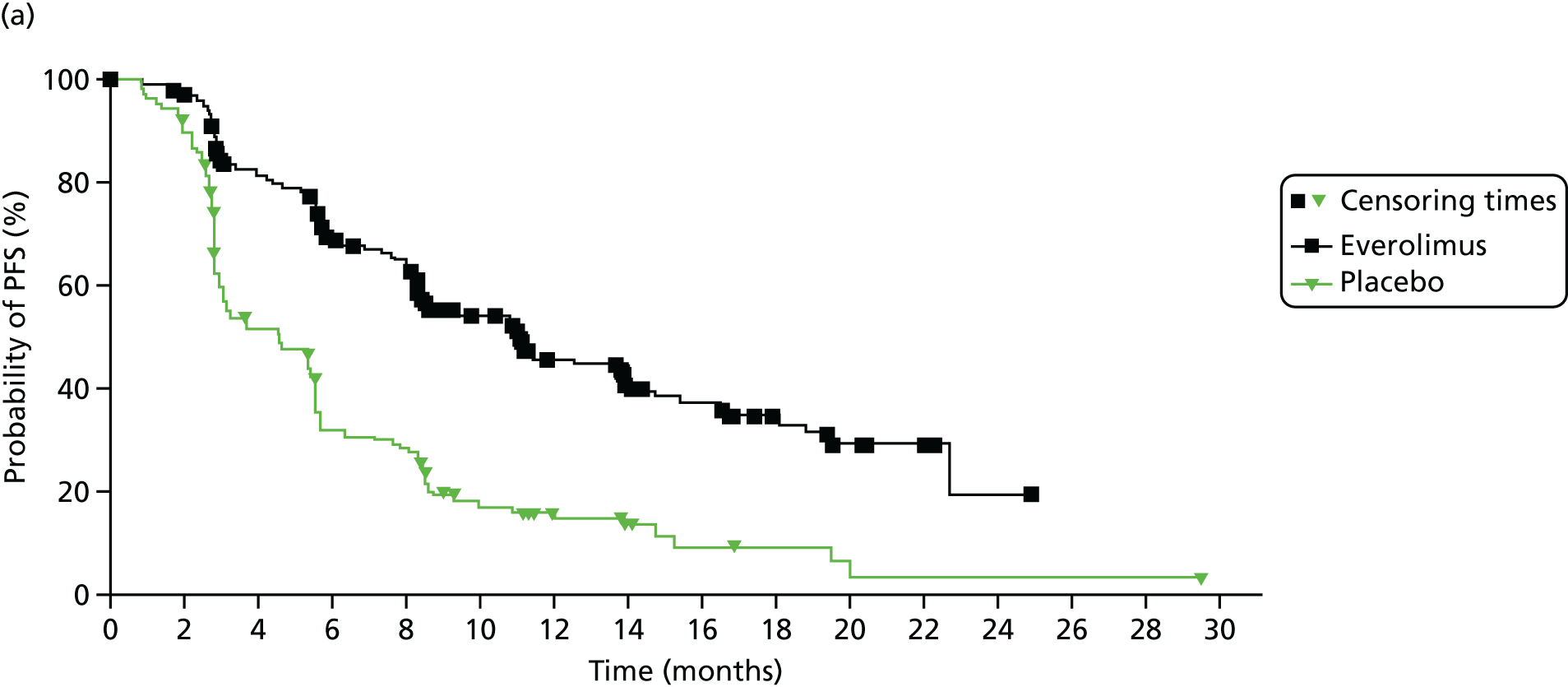
In RADIANT-3,34 median PFS as assessed by central review was 11.4 months (95% CI 10.8 to 14.8 months) in the everolimus plus BSC arm and 5.4 months (95% CI 4.3 to 5.6 months) in the placebo plus BSC arm. Everolimus was associated with a 66% reduction in the risk of disease progression or death for people with pNETs compared with placebo [hazard ratio (HR) 0.34, 95% CI 0.26 to 0.44; Table 5].
| Outcome | RADIANT-334 | A618111181 | ||||
|---|---|---|---|---|---|---|
| Everolimus + BSC (n = 207) | Placebo + BSC (n = 203) | HR (95% CI) | Sunitinib + BSC (n = 86) | Placebo + BSC (n = 85) | HR (95% CI) | |
| Tumour location | Pancreas | Pancreas | ||||
| PFS (central radiology review) (months), median (95% CI) | (n = 95/207) 11.4 (10.8 to 14.8) | (n = 142/203) 5.4 (4.3 to 5.6) | 0.34 (0.26 to 0.44), p < 0.001 | 12.6 (11.1 to 20.6) | 5.8 (3.8 to 7.2) | 0.32 (0.18 to 0.55), p < 0.001 |
| PFS (local review) (months), median (95% CI) | (n = 109/207) 11.0 (8.4 to 13.9) | (n = 165/203) 4.6 (3.1 to 5.4) | 0.35 (0.27 to 0.45), p < 0.001 | (n = 30/86) 11.4 (7.4 to 19.8) | (n = 51/85) 5.5 (3.6 to 7.4) | 0.42 (0.26 to 0.66), p < 0.001 |
| OS (months), median (95% CI) | Not reached | Not reached | 1.05 (0.71 to 1.55),a p = 0.59 | (n = 77/86) 92.6 (86.3 to 98.9)b | (n = 64/85) 85.2 (77.1 to 93.3)b | 0.41 (0.19 to 0.89),c p = 0.02 |
| Final OS (months), median (95% CI) | (n = 81/207)d 44.0 (35.6 to 51.8) | (n = 73/203)d 37.7 (29.1 to 45.8) | 0.94 (0.73 to 1.20), p = 0.30 | (n = 31/86)d 38.6 (range 25.6–56.4) | (n = 27/85)d 29.1 (range 16.4–36.8) | 0.73 (0.50 to 1.06), p = 0.094 |
| Complete response, n/N (%) | 0/207 (0) | 0/203 (0) | 2/86 (2) | 0/85 | ||
| Partial response, n/N (%) | 10/207 (5) | 4/203 (2) | 6/86 (7) | 0/85 | ||
| Stable disease, n/N (%) | 151/207 (73) | 103/203 (51) | 54/86 (63) | 51/85 (60) | ||
| Progressive disease, n/N (%) | 29/207 (14) | 85/203 (42) | 12/86 (14) | 23/85 (27) | ||
| Tumour shrinkage, n/N (%) | 123/191e (64) | 39/189e (21) | 12/86 (14) | 11/85 (13) | ||
| ORR, n/N (%) | 10/207 (5) | 4/203 (2) | 9.3 (3.2 to 15.4), p = 0.007 | 8/86 (9.3)f | 0/85 (0)f | |
In the submission from Pfizer,32 PFS from the assessment of an independent review was 12.6 months (95% CI 11.1 to 20.6 months) for sunitinib plus BSC and 5.8 months (95% CI 3.8 to 7.2 months) for placebo plus BSC. Sunitinib was associated with a 68% reduction in the risk of disease progression or death for people with pNETs compared with placebo (HR 0.32, 95% CI 0.18 to 0.55; see Table 5).
Locally assessed PFS in RADIANT-334 was 11.0 months (95% CI 8.4 to 13.9 months) in the everolimus plus BSC arm compared with 4.6 months (95% CI 3.1 to 5.4 months) in the placebo plus BSC arm. Everolimus was associated with a reduction (65%) in the risk of disease progression or death for people with pNETs compared with placebo (HR 0.35, 95% CI 0.27 to 0.45; see Table 5). The A618111181 trial reported locally assessed PFS to be 11.4 months (95% CI 7.4 to 19.8 months) in the sunitinib plus BSC arm and 5.5 months (95% CI 3.6 to 7.4 months) in the placebo plus BSC arm. Sunitinib was associated with a 58% reduction in the risk of disease progression or death for people with pNETs compared with placebo (HR 0.42, 95% CI 0.26 to 0.66; see Table 5). Both trials reported a shorter time for PFS in both arms for locally assessed PFS than for central/independent review.
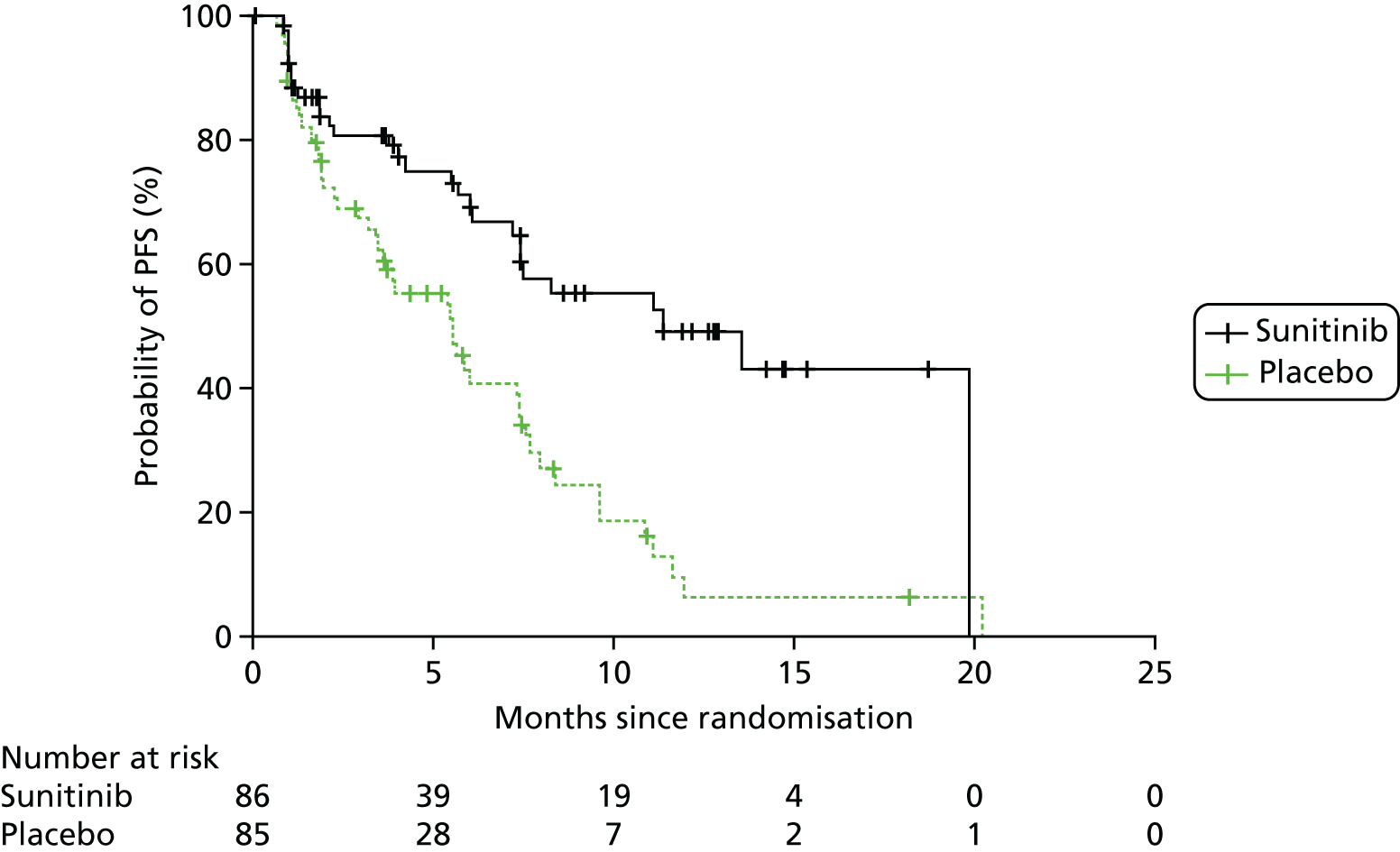
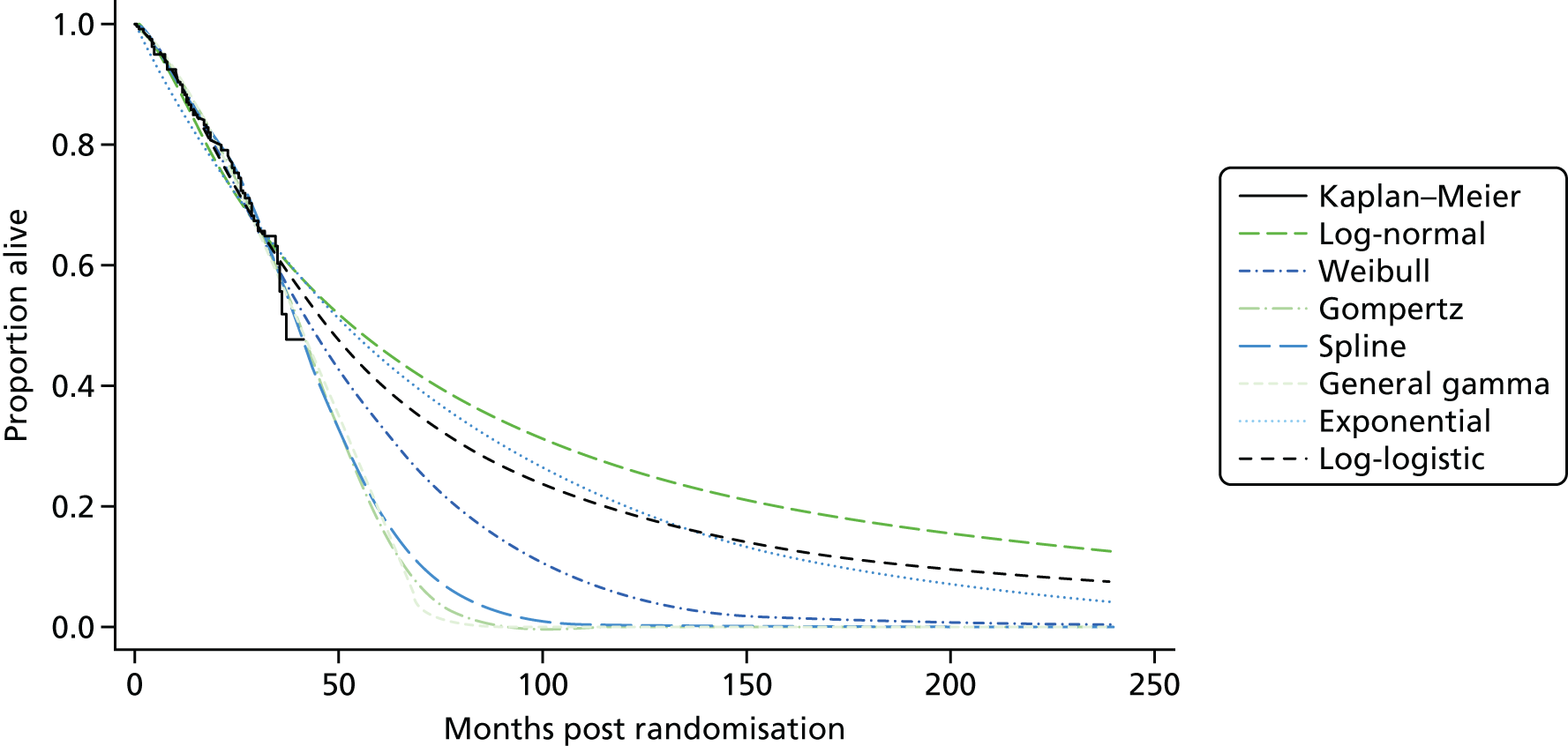
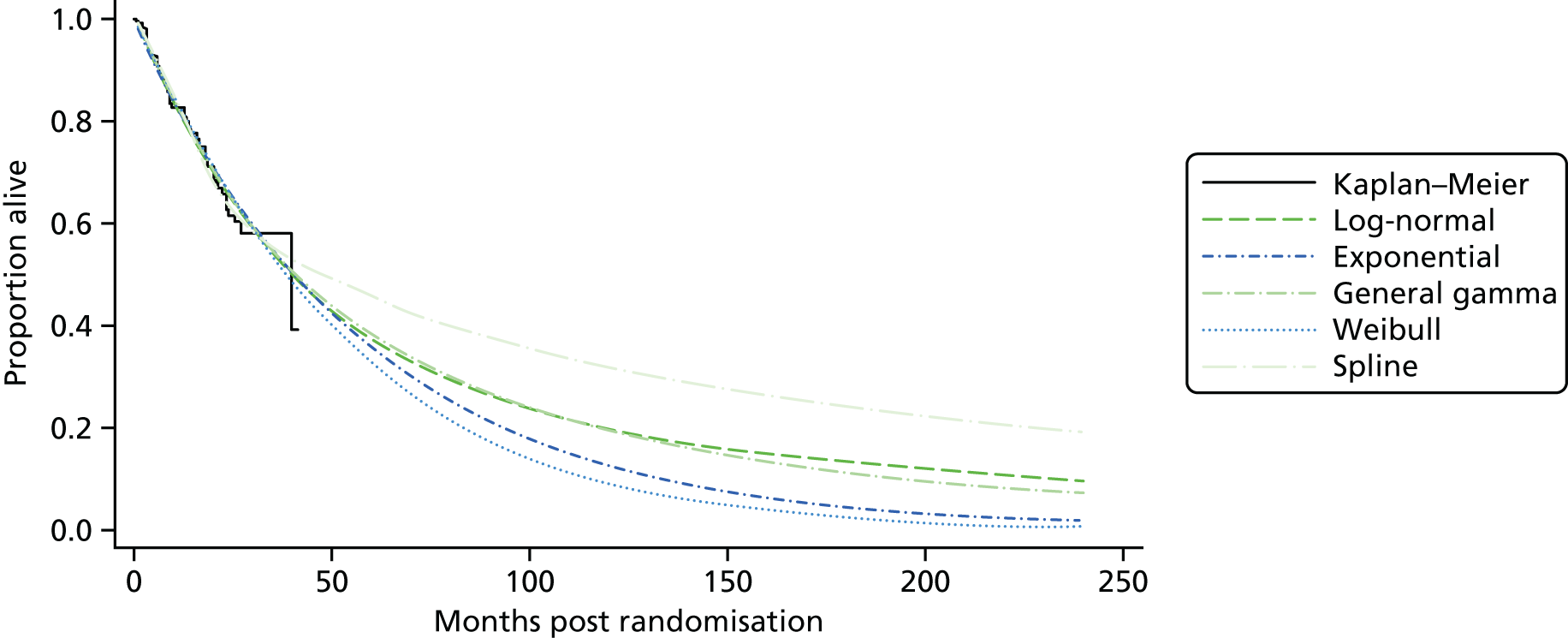
Kaplan–Meier curves are presented for RADIANT-334 (local and central review) and A618111181 (local review) in Figures 2 and 3 respectively.
FIGURE 2.
Kaplan–Meier plots for PFS in RADIANT-3. 34 (a) PFS assessed by local review – Kaplan–Meier medians: everolimus 11.0 months, placebo 4.6 months; HR 0.35 (95% CI 0.27 to 0.45; p < 0.001 by one-sided log-rank test); (b) central review – Kaplan–Meier median: everolimus 11.4 months, placebo 5.4 months; HR 0.34 (95% CI 0.26 to 0.44; p < 0.001 by one-sided log-rank test). Source: figure 4.3 (p. 39) of the Novartis submission. 33

Overall survival
Both of the pNET studies (RADIANT-334 and A618111181) reported some data relating to OS.
It was reported for RADIANT-334 that the OS data were immature: ‘median overall survival was not reached at the time of this analysis . . . final analysis of overall survival will be performed once approximately 250 deaths have occurred’. 34 In addition, of the 203 people initially assigned to receive placebo in RADIANT-3,34 172 (85%) received open-label everolimus and 148 (73%) crossed over from placebo to everolimus following disease progression. By individuals crossing over from placebo to everolimus, the detection of a treatment-related survival benefit is confounded in ITT analysis. In RADIANT-334 the HR for OS was 1.05 (95% CI 0.71 to 1.55; see Table 5).
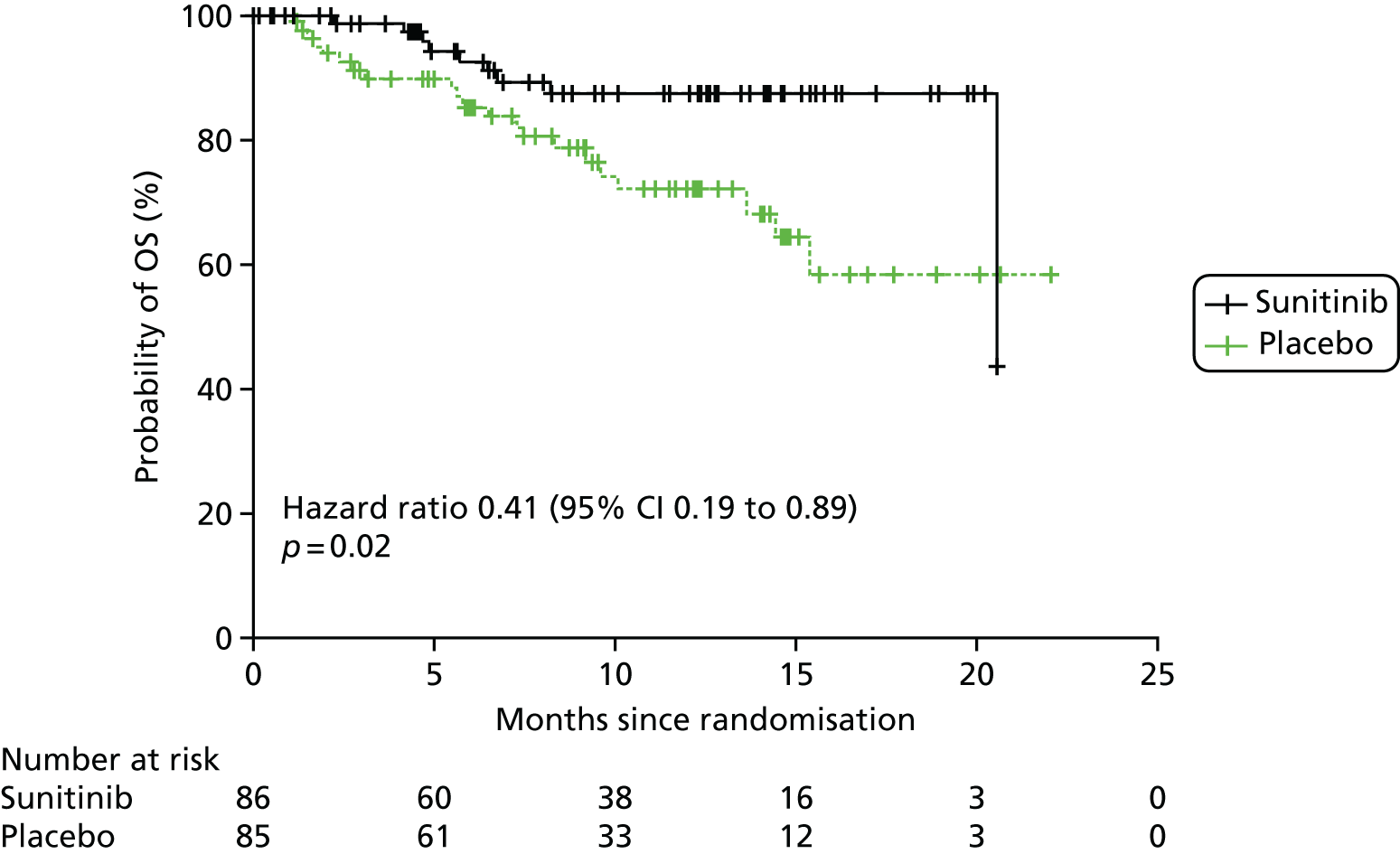
As it had not been reached, median OS was not reported for A6181111;81 instead, survival probability at month 6 was reported. Survival was predicted to be higher in the sunitinib arm (92.6%, 95% CI 86.3% to 98.9%) than in the placebo arm (85.2%, 95% CI 77.1% to 93.3%). Survival was improved by 59% following sunitinib treatment compared with placebo (HR 0.41, 95% CI 0.19 to 0.89; see Table 5).
Both companies [Novartis33 for everolimus (RADIANT-334) and Pfizer32 for sunitinib (A618111181)] presented updated OS data in their submission.
For everolimus, the initial data presented in Yao et al. 34 were analysed on 28 February 2010. In its submission, Novartis33 presented interim OS analysis from 23 February 2011 and final OS analysis from 5 March 5. The final OS data are also available in the published paper by Yao et al. 48 At the interim time point, median OS was still not reached in the everolimus plus BSC arm, but it was 36.63 months for the placebo plus BSC arm (HR 0.89, 95% CI 0.64 to 1.23). At the final OS time point, the median OS for everolimus plus BSC was 44.0 months (95% CI 35.6 to 51.8 months) and for placebo plus BSC was 37.68 months (95% CI 29.1 to 45.8 months), indicating an overall improvement in median OS of 6.3 months (HR 0.94, 95% CI 0.73 to 1.20; p = 0.30; see Table 5). In its submission, Novartis33 commented that ‘the results may be confounded due to the high level of treatment switching from placebo to everolimus and the receipt of subsequent anti-neoplastic therapies’ (p. 43). Novartis accounted for the treatment switching from placebo to everolimus using the rank-preserving structural failure time (RPSFT) model. The RPSFT results are shown in Appendix 7 (see Table 49) and suggest a 40% reduction in OS with everolimus compared with placebo (HR 0.60, 95% CI 0.09 to 3.95).
Additional OS data were also available in the submission from Pfizer32 (pp. 45–9). Pfizer performed final OS analysis in A618111181 after 5 years of follow-up post study closure. From the ITT population, median OS was 38.6 months (range 25.6–56.4 months; n = 55 deaths) in the sunitinib plus BSC arm and 29.1 months (range 16.4–36.8 months; n = 58 deaths) in the placebo plus BSC arm, an improvement of 9.5 months (HR 0.73, 95% CI 0.50 to 1.06; p = 0.094; see Table 5). After accounting for treatment switching using the RPSFT method, median OS in the placebo group (if the 69% of patients who crossed over to sunitinib had remained on placebo) was estimated as 13.2 months (range 11.3–16.5 months) (HR 0.34, 95% CI 0.14 to 1.28; p = 0.094) (it should be noted that the 95% CI was also reported as 0.15 to 1.28 in the company submission).
Kaplan–Meier curves for OS and RPSFT-adjusted OS were presented for both RADIANT-334 (see Appendix 7, Figure 29, and Figure 4 respectively) and A618111181 (see Appendix 7, Figure 30, and Figure 5 respectively).
FIGURE 4.
Kaplan–Meier plot of the final OS analysis from RADIANT-334 adjusted using RPSFT (everolimus vs. placebo). Kaplan–Meier medians (95% CI): everolimus 44.02 (35.61 to 51.57) months, placebo 37.68 (29.14 to 45.77) months, placebo RPSFT (reconstructed placebo data as if the treatment crossover to open-label everolimus had not occurred): not available (20.61 to not available) months. Source: figure 4.7 (p. 45) of the Novartis submission. 33

Response rate
Both studies used RECIST version 1.0107 to assess tumour response. Response rate was assessed by local investigators in RADIANT-3. 34 It was unclear whether response rate was assessed locally or centrally in A6181111;81 however, as PFS was assessed only locally,81 it might be assumed that the response rate was also assessed locally. In A618111,81 clinical assessments were performed at screening, during weeks 5 and 9 and every 8 weeks thereafter, whenever progression was suspected and at the end of treatment or withdrawal from the study, whereas in RADIANT-334 assessments were performed at baseline and every 12 weeks thereafter.
Complete response, partial response, stable disease and progressive disease were reported by both studies (see Table 5). In RADIANT-334 tumour shrinkage was also reported, whereas in A618111181 the proportion of individuals who could not be evaluated and ORR were also reported (see Table 5). Complete response was achieved by only two individuals in A618111181 following treatment with sunitinib and BSC. Complete response was not achieved by any participants receiving placebo (both trials), nor following treatment with everolimus (RADIANT-334). The numbers of individuals achieving a partial response or stable disease were higher in the treatment arms (everolimus and sunitinib) than in the placebo arms in both trials. Likewise, there were higher proportions of individuals with progressive disease in the placebo arms than in the treatment arms.
Novartis33 reported in its submission that (confidential information has been removed). Novartis33 also reported central investigator RRs and adjudicated central investigator RRs from RADIANT-3. 34
Although relevant outcome data were reported, this information was designated as commercial-in-confidence.
Adverse events
In both RADIANT-334 and A618111181 AEs were assessed in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. 108
Treatment-related AEs (all grades and grades 3 and 4 combined) are reported for both trials in Table 6. In RADIANT-3,34 AEs were reported that occurred in at least 10% of the safety population, whereas, in A6181111,81 AEs were reported that occurred in > 15% of the safety population. AEs were more commonly reported following treatment with everolimus and sunitinib than following treatment with placebo. The five most common all-grade AEs following treatment with everolimus (RADIANT-334) were stomatitis (64%), rashes (49%), diarrhoea (34%), fatigue (31%) and infections (23%). Following treatment with sunitinib (A618111181), the five most common all-grade AEs were diarrhoea (59%), nausea (45%), vomiting (34%), asthenia (34%) and fatigue (32%).
| AE | All grades | Grades 3 + 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| aRADIANT-334 | b,cA618111181 | aRADIANT-334 | b,cA618111181 | |||||
| Everolimus + BSC, n/N (%) | Placebo + BSC, n/N (%) | Sunitinib, n/N (%) | Placebo + BSC, n/N (%) | Everolimus + BSC, n/N (%) | Placebo + BSC, n/N (%) | Sunitinib, n/N (%) | Placebo + BSC, n/N (%) | |
| On-treatment deaths | 12/204 (6) | 4/203 (2) | 5/83 (6) | 9/82 (11) | ||||
| Treatment discontinuation | 13/204 (6) | 2/203 (1) | NR | NR | ||||
| Abdominal pain | NR | NR | 23/83 (28) | 26/82 (32) | NR | NR | 4/83 (5) | 8/82 (10) |
| Anaemia | 35/204 (17) | 6/203 (3) | NR | NR | 12/204 (6) | 0/203 (0) | NR | NR |
| Asthenia | 26/204 (13) | 17/203 (8) | 28/83 (34) | 22/82 (27) | 2/204 (1) | 2/203 (1) | 4/83 (5) | 3/82 (4) |
| Back pain | NR | NR | 10/83 (12) | 14/82 (17) | NR | NR | 0/83 (0) | 4/82 (5) |
| Constipation | NR | NR | 12/83 (14) | 16/82 (20) | NR | NR | 0/83 (0) | 1/82 (1) |
| Cough | 22/204 (11) | 4/203 (2) | NR | NR | 0/204 (0) | 0/203 (0) | NR | NR |
| Decreased appetite | 40/204 (20) | 14/203 (7) | 18/83 (22) | 17/82 (21) | 0/204 (0) | 2/203 (1) | 2/83 (2) | 1/82 (1) |
| Decreased weight | 32/204 (16) | 9/203 (4) | 13/83 (16) | 9/82 (11) | 0/204 (0) | 0/203 (0) | 1/83 (1) | 0/82 (0) |
| Diarrhoea | 69/204 (34) | 20/203 (10) | 49/83 (59) | 32/82 (39) | 7/204 (3) | 0/203 (0) | 4/83 (5) | 2/82 (2) |
| Dry skin | 21/204 (10) | 9/203 (4) | NR | NR | 0/204 (0) | 0/203 (0) | NR | NR |
| Dysgeusia | 35/204 (17) | 8/203 (4) | 17/83 (20) | 4/82 (5) | 0/204 (0) | 0/203 (0) | 0/83 (0) | 0/82 (0) |
| Epistaxis | 35/204 (17) | 0/203 (0) | 17/83 (20) | 4/82 (5) | 0/204 (0) | 0/203 (0) | 1/83 (1) | 0/82 (0) |
| Fatigue | 64/204 (31) | 29/203 (14) | 27/83 (32) | 22/82 (27) | 5/204 (2) | 1/203 (< 1) | 4/83 (5) | 7/82 (8) |
| Hair colour change | NR | NR | 24/83 (29) | 1/82 (1) | NR | NR | 1/83 (1) | 0/82 (0) |
| Headache | 39/204 (19) | 13/203 (6) | 15/83 (18) | 11/82 (13) | 0/204 (0) | 0/203 (0) | 0/83 (0) | 1/82 (1) |
| Hyperglycaemia | 27/204 (13) | 9/203 (4) | NR | NR | 11/204 (5) | 4/203 (2) | NR | NR |
| Hypertension | NR | NR | 22/83 (26) | 4/82 (5) | NR | NR | 8/83 (10) | 1/82 (1) |
| Infections | 46/204 (23) | 12/203 (6) | NR | NR | 5/204 (2) | 1/203 (< 1) | NR | NR |
| Insomnia | NR | NR | 15/83 (18) | 10/82 (12) | NR | NR | 0/83 (0) | 0/82 (0) |
| Mucosal inflammation | NR | NR | 13/83 (16) | 6/82 (7) | NR | NR | 1/83 (1) | 0/82 (0) |
| Nail disorder | 24/204 (12) | 2/203 (1) | NR | NR | 1/204 (< 1) | 0/203 (0) | NR | NR |
| Nausea | 41/204 (20) | 37/203 (18) | 37/83 (45) | 24/82 (29) | 5/204 (2) | 0/203 (0) | 1/83 (1) | 1/82 (1) |
| Neutropenia | NR | NR | 24/83 (29) | 3/82 (4) | NR | NR | 10/83 (12) | 0/82 (0) |
| Palmar–plantar erythrodysaesthesia | NR | NR | 19/83 (23) | 2/82 (2) | NR | NR | 5/83 (6) | 0/82 (0) |
| Peripheral oedema | 41/204 (20) | 7/203 (3) | NR | NR | 1/204 (< 1) | 0/203 (0) | NR | NR |
| Pneumonitis | 35/204 (17) | 0 | NR | NR | 5/204 (2) | 0/203 (0) | NR | NR |
| Pruritus | 30/204 (15) | 18/203 (9) | NR | NR | 0/204 (0) | 0/203 (0) | NR | NR |
| Pyrexia | 22/204 (11) | 0/203 (0) | NR | NR | 0/204 (0) | 0/203 (0) | NR | NR |
| Rash | 99/204 (49) | 21/203 (10) | 15/83 (18) | 4/82 (5) | 1/204 (< 1) | 0/203 (0) | 0/83 (0) | 0/82 (0) |
| Stomatitis | 131/204 (64) | 34/203 (17) | 18/83 (22) | 2/82 (2) | 14/204 (7) | 0/203 (0) | 3/83 (4) | 0/82 (0) |
| Thrombocytopenia | 27/204 (13) | 1/203 (< 1) | 14/83 (17) | 4/82 (5) | 8/204 (4) | 0/203 (0) | 3/83 (4) | 0/82 (0) |
| Vomiting | 31/204 (15) | 13/203 (6) | 28/83 (34) | 25/82 (30) | 0/204 (0) | 0/203 (0) | 0/83 (0) | 2/82 (2) |
Treatment-related AEs occurring in ≥ 20% of patients in RADIANT-334 at the latest cut-off (5 March) were presented in the company submission from Novartis33 (see Appendix 7, Table 50). These AE rates are different (predominantly higher) from the rates published by Yao et al. 34 The AEs published in the paper by Yao et al. 34 were coded using the Medical Dictionary for Regulatory Activities, version 16.1. 109
Health-related quality of life
IN A618111181 the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) version 3.0 was used to measure HRQoL. The EORTC QLQ-C30 was available for 73 out of 86 (85%) individuals treated with sunitinib and 71 out of 85 (84%) individuals treated with placebo. The EORTC QLQ-C30 includes one global health scale, five functional scales (physical, role, emotional, cognitive and social), three symptom scales (fatigue, nausea/vomiting and pain) and six single-item scales (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties). High scores are better for the global and functioning scales, whereas low scores are better for the symptom items/scales. The questionnaire was administered at baseline, at every cycle (4 weeks) and at the end of treatment. There were no overall differences observed between study groups for any of these measures except for diarrhoea (21.4-point difference; p < 0.001) and insomnia (7.8-point difference; p = 0.04), which were higher in the sunitinib arm than in the placebo arm.
More detailed results were available for HRQoL from Pfizer’s submission. 32 The mean baseline global HRQoL score was 67.0 (95% CI 62.0 to 72.0) in the sunitinib plus BSC arm compared with 64.0 (95% CI 58.4 to 69.6) in the placebo plus BSC arm. Post-baseline scores were 62.44 for sunitinib and 61.28 for placebo. Overall differences in the EORTC QLQ-C30 for each item on the scale between the two arms are shown in Table 53 (see Appendix 7). Changes in global HRQoL scores over time in the two arms are shown in Figure 31 (see Appendix 7).
Subgroup analysis
A618111181 reported Cox proportional hazards analysis of PFS for the subgroups tumour functioning, number of previous systemic regimes and previous use of SSAs (see Appendix 7, Table 54), whereas RADIANT-334 reported PFS for subgroups based on tumour grade, previous chemotherapy use and previous long-acting SSA use (see Appendix 7, Table 55).
Novartis (A618111181) also reported covariate analysis of OS using a Cox’s proportional hazards model for previous use of SSAs and previous use of chemotherapy (see Appendix 7, Table 56).
Indirect treatment comparison: pancreatic neuroendocrine tumours
Two RCTs were used to compare everolimus with sunitinib: RADIANT-334 (everolimus plus BSC vs. placebo plus BSC) and A618111181 (sunitinib plus BSC vs. placebo plus BSC; Figure 6).
FIGURE 6.
Diagram of the ITC for pNETs.

The Bucher method41 was used to indirectly compare everolimus with sunitinib in individuals with pNETs for the following outcomes: PFS, OS, RR (not including complete response as there were zero responses in both treatment arms of RADIANT-334 and a zero response in the placebo arm of A618111181) and various AEs. Because there were only two relevant trials for this synthesis we could not undertake any analyses for heterogeneity between the trials or inconsistency in the network. As we used only aggregate summary data, other more complex methods of indirect comparison were not considered [e.g. matched adjusted indirect comparisons (MAICs) using individual patient data]. After we had conducted these analyses, the AG requested individual patient data from the companies, and obtained data on the A618111181 trial, for the purposes informing de novo economic analysis. However, limited resources prevented us from updating the indirect comparison reported in this section using matched adjusted Bucher methods.
Results for PFS and OS are reported in terms of HRs and 95% CIs, whereas results for RR and AEs are reported as odds ratios (ORs) and 95% CIs. For some AEs, when there were zero events in one of the arms, a continuity correction of 0.5 was added to each of the 2 × 2 cells to allow calculation of the ORs.
An assessment of the characteristics of the two trials (RADIANT-334 and A618111181) suggested that they were comparable to allow an ITC. Both trials compared the active treatment with placebo plus BSC and included only participants with advanced or metastatic disease. A slightly higher proportion of participants used SSAs in RADIANT-334 (≈40%) than in A618111181 (≈28%); however, it was not thought that this would affect the relative effectiveness of the treatments.
Treatment switching from the active arm to the placebo arm was permitted in both trials after disease progression. For OS, ITCs were conducted using ITT analyses and analyses adjusted for treatment switching (using the RPSFT method). Methods other than the RPSFT were considered by one of the companies A6181111,81 including the inverse probability of censoring weights. The RPSFT method relies on the common treatment effect assumption, that is, that the effectiveness of targeted treatment is the same in patients in the targeted intervention arm and patients in the control arm who switch over to the targeted treatment on disease progression. This assumption was considered to pose less practical problems than the inverse probability of censoring weights method, which requires censoring of the data at the time of crossover and adjusting the remaining data using all relevant confounders affecting both the decision to switch and the outcome (PFS, OS).
Progression-free survival
Table 7 shows the evidence used from RADIANT-334 and A618111181 to inform the indirect comparison between everolimus and sunitinib for PFS assessed by local review. The analysis suggests that everolimus is associated with a 17% decrease in disease progression or death compared with sunitinib (HR 0.83, 95% CI 0.49 to 1.42). The 95% CI is wide and includes the null hypothesis that there is no difference in PFS effectiveness between everolimus and sunitinib.
| AE | Everolimus + BSC vs. placebo + BSC | Sunitinib + BSC vs. placebo + BSC | Everolimus + BSC vs. sunitinib + BSC | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | Data source | HR (95% CI) | Data source | HR (95% CI) | Data source | |
| Disease progression or death (local radiology review) | 0.35 (0.27 to 0.45) | RADIANT-334 | 0.42 (0.26 to 0.66) | A618111181 | 0.83 (0.49 to 1.42) | Calculated by AG |
| Disease progression or death (central radiology review) | 0.34 (0.26 to 0.44) | RADIANT-334 | 0.32 (0.18 to 0.55) | From Pfizer submission32 (A618111181) | 1.06 (0.57 to 1.97) | Calculated by AG |
| OS | 1.05 (0.71 to 1.55) | RADIANT-334 | 0.41 (0.19 to 0.89) | A618111181 | 2.56 (1.08 to 6.08) | Calculated by AG |
| Final OS | 0.94 (0.73 to 1.20) | RADIANT-334 | 0.73 (0.50 to 1.06) | From Pfizer submission32 (A618111181) | 1.26 (0.82 to 2.02) | Calculated by AG |
| OS accounting for treatment switching | 0.60 (0.09 to 3.95) | RADIANT-334 | 0.34 (0.14 to 3.95) | From Pfizer submission32 (A618111181) | 1.76 (0.20 to 15.78) | Calculated by AG |
| Partial response | OR 2.53 (0.78 to 8.19) | RADIANT-334 | OR 13.81 (1.65 to 115.85) | A618111181 | OR 0.18 (0.02 to 2.08) | Calculated by AG |
| Stable disease | OR 2.62 (1.73 to 3.95) | RADIANT-334 | OR 1.13 (0.61 to 2.07) | A618111181 | OR 2.33 (1.11 to 4.86) | Calculated by AG |
| Progressive disease | OR 0.23 (0.14 to 0.37) | RADIANT-334 | OR 0.44 (0.20 to 0.95) | A618111181 | OR 0.52 (0.21 to 1.30) | Calculated by AG |
Further data available from the company submissions32,33 enabled an indirect comparison for PFS assessed by central radiology review to be carried out. The ITC between everolimus and sunitinib for PFS based on central radiology review suggests no difference between the treatments (see Table 7).
Overall survival
Table 7 shows the evidence used to inform the indirect comparison between everolimus and sunitinib for OS. The analysis suggests that there is a 2.56 times greater hazard of dying associated with treatment with everolimus than treatment with sunitinib, which is statistically significant. However, as these analyses are based on published HRs from RADIANT-334 and A6181111,81 which were not adjusted for treatment switching after disease progression, these results should not be relied on.
Final follow-up data for OS available from the company submissions32,33 enabled an indirect comparison between everolimus and sunitinib for this outcome to be carried out, which suggests a lower hazard of death associated with sunitinib than with everolimus. However, the 95% CI includes the null effect, suggesting that this is not a statistically significant effect (see Table 7). This analysis does not account for the fact that approximately 70% of participants in the placebo plus BSC arms of these two trials switched to receive the active treatment after disease progression and so it should be interpreted with caution.
The indirect comparison of everolimus with sunitinib for OS in which the companies have used the RPSFT method to adjust for treatment switching suggests a lower hazard of death associated with sunitinib than with everolimus (as in the ITT analyses above); however, the 95% CI is very wide and includes the null effect (see Table 7).
Response rate
All ORs reported in Table 7 for the intervention compared with placebo were calculated by the AG, based on the numbers of individuals reported to have experienced the different outcomes in RADIANT-334 and A6181111. 81 The ITCs for everolimus and sunitinib were based on the AG-calculated ORs from RADIANT-334 and A6181111. 81 The indirect analysis suggests that there is an 82% increase in the odds of a partial response in individuals treated with sunitinib compared with everolimus. However, sunitinib was associated with a 52% increase in the odds of progressive disease compared with everolimus. Everolimus was associated with a 2.3 times greater odds for disease stability than sunitinib. However, all of these ITCs were associated with wide 95% CIs, suggesting that there is little evidence of a difference in RRs between everolimus and sunitinib.
Adverse events
An ITC was completed only for those AEs for which data were available from both trials. All ORs reported in Appendix 7 (see Table 57 for all grades of AE and Table 58 for grade 3/4 AEs) were calculated by the AG based on the numbers of participants experiencing these AEs reported in A618111181 and RADIANT-3. 34 For all grades of AE, the ITC suggests that there is a 19% increase in the odds of experiencing stomatitis and a 42% increase in the odds of experiencing nausea associated with sunitinib compared with everolimus. For the other AEs (all grades), the evidence suggests an increase in the odds of experiencing the AE with everolimus compared with sunitinib. However, except for decreased appetite, all of these ITCs were associated with wide 95% CIs that included the null hypothesis of no difference, suggesting that there is little evidence of a difference in AEs between everolimus and sunitinib. For all grades of decreased appetite, there was a statistically significant increase in the odds of experiencing the event with everolimus compared with sunitinib.
For the grade 3/4 AEs, the ITC could consider only seven AEs based on the data available from the two trials. The evidence suggests an increased odds of experiencing grade 3/4 stomatitis, fatigue, diarrhoea, nausea and thrombocytopenia with everolimus compared with sunitinib and an increased odds of experiencing decreased appetite and asthenia with sunitinib compared with everolimus. However, all of the ITCs for grade 3/4 AEs were associated with wide 95% CIs that included the null hypothesis of no difference, suggesting that there is little evidence of a difference in AEs between everolimus and sunitinib.
Subgroup analysis
Subgroup analysis based on whether or not participants had previously received SSAs suggests very little difference in time to disease progression or death for everolimus compared with sunitinib (see Appendix 7, Table 59).
Outcomes from randomised controlled trial evidence for gastrointestinal and lung neuroendocrine tumours
Progression-free survival
In RADIANT-4,35 PFS was the primary outcome, with disease progression defined as ‘the time from randomisation to death or progression as per modified RECIST version 1.0 criteria’. 35 In RADIANT-4,35 PFS was reported according to both central radiology review and local investigator review.
Median PFS in RADIANT-4,35 assessed by central review, was 11.0 months (95% CI 9.2 to 13.3 months) in the everolimus plus BSC arm and 3.9 months (95% CI 3.6 to 7.4 months) in the placebo plus BSC arm. Everolimus was associated with a 52% reduction in the risk of disease progression or death for people with lung and GI NETs compared with placebo (HR 0.48, 95% CI 0.35 to 0.67; Table 8).
| Outcome | RADIANT-435 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Everolimus + BSC (n = 205) | Placebo + BSC (n = 97) | HR (95% CI) | Everolimus + BSC (n = 118) | Placebo + BSC (n = 57) | HR (95% CI) | Everolimus + BSC (n = 118) | Placebo + BSC (n = 57) | HR (95% CI) | |
| Tumour location | Lung and GI | GI only | Lung only | ||||||
| PFS (central radiology review) (months), median (95% CI) | (n = 113/205) 11.0 (9.2 to 13.3) | (n = 65/97) 3.9a (3.6 to 7.4) | 0.48 (0.35 to 0.67), p < 0.00001 | (n = NR/118) 13.1 (9.2 to 17.3) | (n = NR/57) 5.4 (3.6 to 9.3) | 0.56 (0.37 to 0.84) | n = 42/63 | n = 18/27 | 0.50 (0.28 to 0.88) |
| PFS (local review) (months), median (95% CI) | (n = 98/205) 14.0 (11.2 to 17.7) | (n = 70/97) 5.5 (3.7 to 7.4) | 0.39 (0.28 to 0.54), p < 0.00001 | NR | NR | NR | NR | NR | NR |
| OS (primary cut-off point) (months), median (95% CI) | (n = 42/205) 23.7 (17.6 to 27.3)b | (n = 28/97) 16.5 (9.0 to 21.0)b | 0.64 (0.40 to 1.05),c p = 0.037 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| OS (secondary cut-off point) (months), median (95% CI) | (n = 66/205) 25.7 (18.4 to 28.6) | (n = 35/97) 16.5 (9.0 to 20.2)d | 0.73 (0.48 to 1.11), p = 0.071 | NR | NR | NR | NR | NR | NR |
| Complete response, n/N (%) | 0 | 0 | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | NR | |
| Partial response, n/N (%) | 4/205 (2) | 1/97 (1) | NR | Confidential information has been removed | Confidential information has been removed | NR | |||
| Stable disease, n/N (%) | 165/205 (80) | 62/97 (64) | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | NR | |
| Progressive disease, n/N (%) | 19/205 (9) | 26/97 (27) | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | NR | |
| Unknown response, n/N (%) | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | NR | |||
| ORR, n/N (%) [95% CI] | 4/205 (2) [0.5 to 4.9] | 1/97 (1) [0.0 to 5.6] | NR | NR | NR | Confidential information has been removed | Confidential information has been removed | NR | |
| Disease control rate, n/N (%) [95% CI] | 169/205 (82.4) [76.5 to 87.4] | 63/97 (64.9) [54.6 to 74.4] | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | NR | |
| Tumour shrinkage, n/N (%) | 117/184 (64) | 22/85 (26) | Confidential information has been removed | Confidential information has been removed | NR | NR | NR | ||
In RADIANT-4,35 locally assessed PFS was longer in duration in both arms than PFS assessed by central review, at 14.0 months (95% CI 11.2 to 17.7 months) in the everolimus plus BSC arm compared with 5.5 months (95% CI 3.7 to 7.4 months) in the placebo plus BSC arm. Everolimus was associated with a reduction (61%) in the risk of disease progression or death for people with lung and GI NETs compared with placebo (HR 0.39, 95% CI 0.28 to 0.54; see Table 8).
The company submission from Novartis33 made available secondary analysis of PFS (1 year and 2 days after the PFS analysis presented in the published paper35). Median PFS from central review was 14.39 months (95% CI 11.24 to 17.97 months) for the everolimus plus BSC arm and 5.45 months (95% CI 3.71 to 7.39 months) for the placebo plus BSC arm. Everolimus was associated with a 59% reduction in the risk of disease progression or death for people with lung and GI NETs compared with placebo (HR 0.41, 95% CI 0.30 to 0.56).
Kaplan–Meier curves were produced for PFS in RADIANT-435 from both central review and local review (Figure 7) with data from the primary cut-off point.
FIGURE 7.
Kaplan–Meier plots for PFS (primary cut-off point) in RADIANT-435 (everolimus vs. placebo). (a) PFS assessed by central review – Kaplan–Meier medians: everolimus 11.0 (95% CI 9.2 to 13.3) months, placebo 3.9 (95% CI 3.6 to 7.4) months; HR 0.48 (95% CI 0.35 to 0.67; p < 0.00001 by stratified one-sided log-rank test); and (b) PFS assessed by local review – Kaplan–Meier medians: everolimus 14.0 (95% CI 11.2 to 17.7) months, placebo 5.5 (95% CI 3.7 to 7.4) months; HR 0.39 (95% CI 0.28 to 0.54; p < 0.00001 by stratified one-sided log-rank test). Source: figures 5.3 (p. 67) and 5.4 (p. 68) of the Novartis submission. 33
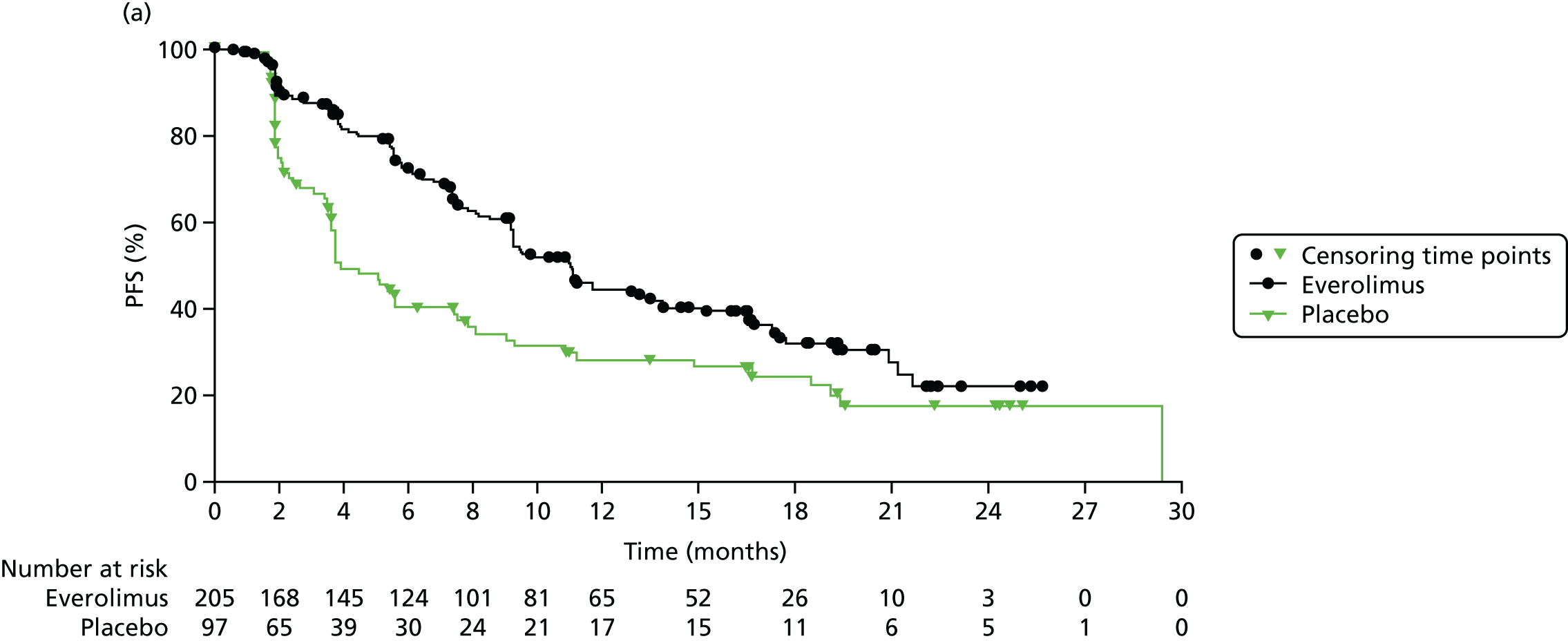
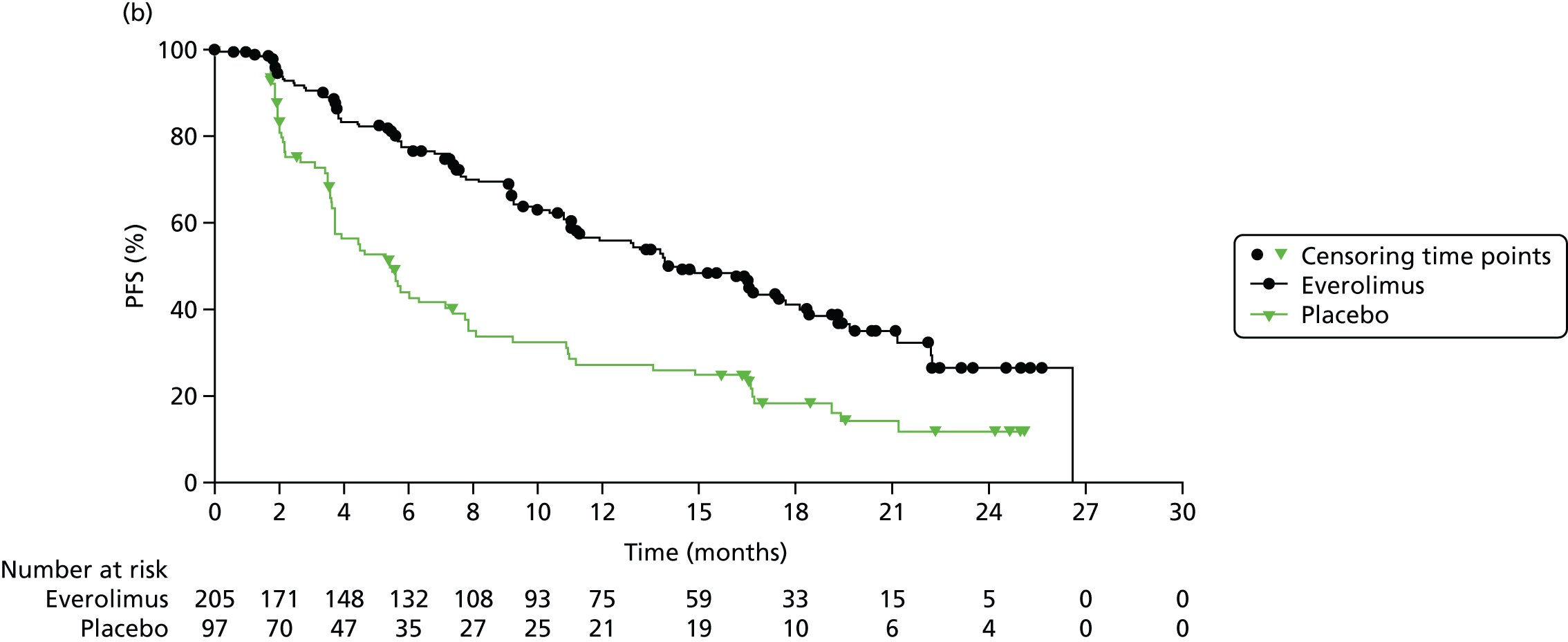
Overall survival
Interim OS analysis was carried out for RADIANT-435 once 70 deaths had been reached. Data were not sufficiently mature to provide an estimation of median OS. In individuals with lung and GI NETs, Kaplan–Meier estimates for OS at the 25th percentile – 25% of individuals having died – were 23.7 months (95% CI 17.6 to 27.3 months) for everolimus and 16.5 months (95% CI 9.0 to 21.0 months) for placebo. Everolimus was associated with a 36% improvement in OS for individuals with lung and GI NETs compared with placebo (HR 0.64, 95% CI 0.40 to 1.05; see Table 8).
In its company submission, Novartis33 presented secondary analysis of OS from RADIANT-4,35 which was performed 1 year and 2 days after the published analysis presented by Yao et al. 35 This analysis was based on 101 deaths, corresponding to a 52.9% information fraction, and the median duration of follow-up was 33.4 months. Everolimus was associated with a 27% improvement in OS for individuals with lung and GI NETs compared with placebo (HR 0.73, 95% CI 0.48 to 1.11).
A Kaplan–Meier plot was produced for OS in RADIANT-435 at both the primary data cut-off point (see Appendix 7, Figure 32) and the secondary data cut-off point (Figure 8).
FIGURE 8.
Kaplan–Meier plot for OS estimates in RADIANT-4:35 secondary data cut-off point. Kaplan–Meier medians: everolimus + BSC 37.16 (95% CI 35.35 to not evaluable) months, placebo + BSC 39.56 (95% CI 23.46 to not evaluable) months; HR 0.73 (95% CI 0.48 to 1.11; log-rank p-value = 0.071). p-value is obtained from the stratified log-rank test. Source: figure 5.11 (p. 75) of the Novartis submission. 33
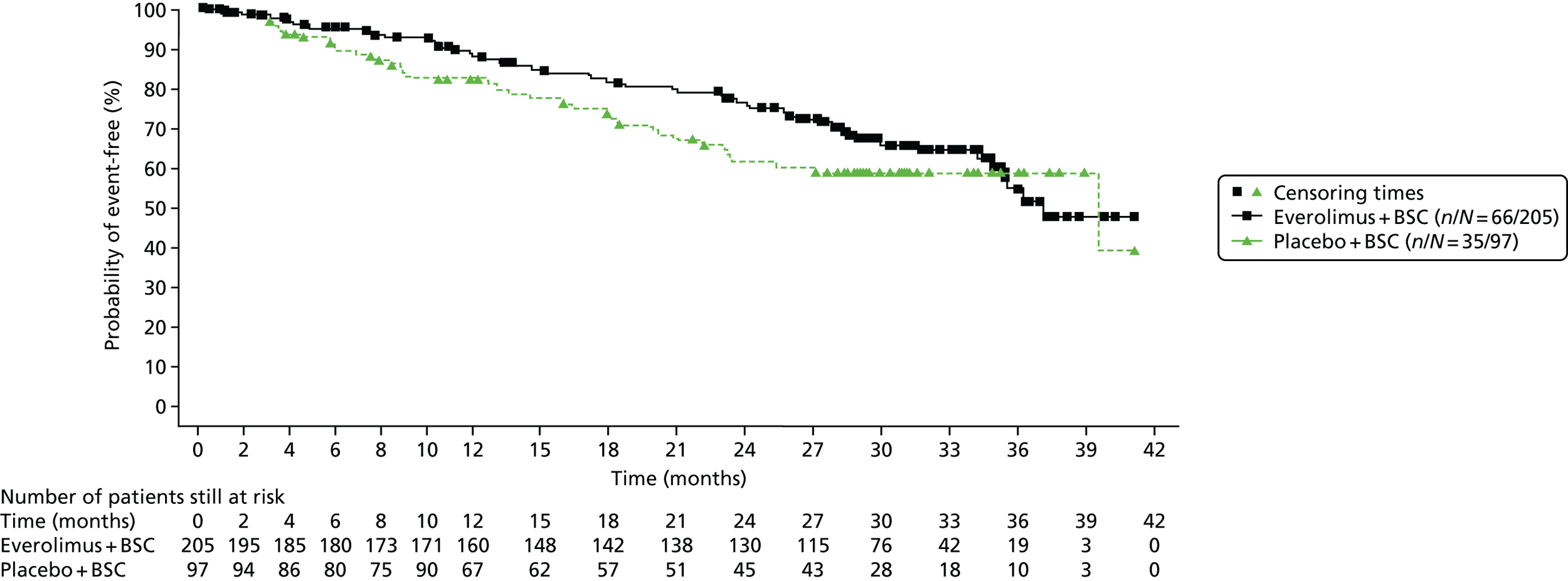
Response rate
In RADIANT-4,35 a modified version of RECIST version 1.0107 was used to assess tumour response by central radiology review. Efficacy was assessed every 8 weeks following randomisation for the first 12 months and then every 12 weeks thereafter.
Complete response, partial response, stable disease, progressive disease, ORR, disease control rate and tumour shrinkage, following central radiology review, were reported (see Table 8). For all RR outcomes, treatment with everolimus for lung and GI NETs resulted in a favourable response compared with treatment with placebo, except for complete response, which was not achieved in either arm.
Adverse events
In RADIANT-435 AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. 111 Treatment-related AEs (all grades and grades 3 and 4 combined), reported in ≥ 10% of the safety population, are presented in Table 9. AEs were more commonly reported following treatment with everolimus than following treatment with placebo. The five most common all-grade AEs following treatment with everolimus were stomatitis (63%), diarrhoea (31%), fatigue (31%), infections (29%) and rash (27%).
| AE | Grades, n/N (%) | |||
|---|---|---|---|---|
| All | 3 + 4 | |||
| Everolimus + BSC | Placebo + BSC | Everolimus + BSC | Placebo + BSC | |
| On-treatment deaths | 7/202 (4) | 3/98 (3) | ||
| Treatment discontinuation | 59/202 (29)a | 7/98 (7)b | ||
| Any AE | 193/202 (96) | 67/98 (68) | 106/202 (53) | 13/98 (13) |
| Anaemia | 33/202 (16) | 2/98 (2) | 8/202 (4) | 1/98 (1) |
| Asthenia | 33/202 (16) | 5/98 (5) | 3/202 (3) | 0/98 (0) |
| Cough | 26/202 (13) | 3/98 (3) | 0/202 (0) | 0/98 (0) |
| Decreased appetite | 32/202 (16) | 6/98 (6) | 1/202 (< 1) | 0/98 (0) |
| Diarrhoea | 63/202 (31) | 16/98 (16) | 15/202 (7) | 2/98 (2) |
| Dysgeusia | 30/202 (15) | 4/98 (4) | 1/202 (< 1) | 0/98 (0) |
| Dyspnoea | 21/202 (10) | 4/98 (4) | 2/202 (1) | 1/98 (1) |
| Fatigue | 62/202 (31) | 24/98 (24) | 7/202 (3) | 1/98 (1) |
| Hyperglycaemia | 21/202 (10) | 2/98 (2) | 7/202 (3) | 0/98 (0) |
| Infections | 59/202 (29) | 4/98 (4) | 14/202 (7) | 0/98 (0) |
| Nausea | 35/202 (17) | 10/98 (10) | 3/202 (1) | 0/98 (0) |
| Non-infectious pneumonitis | 32/202 (16) | 1/98 (1) | 3/202 (1) | 0/98 (0) |
| Peripheral oedema | 52/202 (26) | 4/98 (4) | 4/202 (2) | 1/98 (1) |
| Pruritus | 26/202 (13) | 4/98 (4) | 1/202 (< 1) | 0/98 (0) |
| Pyrexia | 22/202 (11) | 5/98 (5) | 4/202 (2) | 0/98 (0) |
| Rash | 55/202 (27) | 8/98 (8) | 1/202 (< 1) | 0/98 (0) |
| Stomatitis | 127/202 (63) | 19/98 (19) | 18/202 (9) | 0/98 (0) |
In its submission, Novartis33 also reported AEs in patients regardless of the study drug relationship of the safety population. This table can be found in Appendix 7 (see Table 51).
Health-related quality of life
In its submission, Novartis33 presented data on HRQoL from RADIANT-435 using the Functional Assessment of Cancer Therapy – General (FACT-G) questionnaire. 112 The FACT-G is based on 27 items in four domains: physical well-being, social/family well-being, emotional well-being and functional well-being. Participant completion rates for the FACT-G questionnaire in RADIANT-4 are provided in Table 60 (see Appendix 7).
Mean total score over time for the FACT-G questionnaire is presented in Figure 34 (see Appendix 7). The HR for definitive deterioration of the total FACT-G score was (confidential information has been removed) Figure 34 (see Appendix 7).
In its submission, Novartis33 reported that the ‘scores were well-balanced between the two arms and never exceeded the threshold of 7 points, defined as the minimal clinically important difference between treatment arms’ (p. 77).
Subgroup analysis
Progression-free survival (based on central review) in RADIANT-435 was reported for everolimus compared with placebo based on treatment naivety, previous chemotherapy use and previous long-acting SSA use (see Appendix 7, Table 61). There is little evidence of a difference in PFS within subgroups.
Outcomes from randomised controlled trial evidence for gastrointestinal neuroendocrine tumours
Following a data request to Novartis, some of the outcomes from RADIANT-435 were provided for individuals with GI NETs alone and for individuals with lung NETs alone. The following sections report the baseline characteristics and outcomes provided by Novartis for individuals with GI NETs from RADIANT-4. 35 Tumour locations included under the GI umbrella were stomach, colon, rectum, appendix, caecum, ileum, duodenum, jejunum and small intestine.
Baseline characteristics
Baseline characteristics for individuals with GI NETs only are presented in Table 62 (see Appendix 7).
Progression-free survival
Novartis provided data for PFS from the RADIANT-435 trial for patients with GI NETs. Median PFS assessed by central review was 13.1 months (95% CI 9.2 to 17.3 months) for the everolimus plus BSC arm and 5.4 months (95% CI 3.6 to 9.3 months) for the placebo plus BSC arm. Everolimus was associated with a 44% reduction in the risk of disease progression or death for people with GI NETs compared with placebo (HR 0.56, 95% CI 0.37 to 0.84; see Table 8).
Overall survival
Novartis provided data for OS and Kaplan–Meier estimates of OS at the 25th percentile from the RADIANT-435 trial for patients with GI NETs (see Table 8).
Response rate
Novartis provided data for RRs from RADIANT-435 for patients with GI NETs (see Table 8).
For all RR outcomes, treatment with everolimus plus BSC for GI NETs resulted in a favourable response compared with treatment with placebo plus BSC.
Adverse events
Novartis provided data for AEs from RADAINT-4 for individuals with GI NETs. AEs were more commonly reported following treatment with everolimus than following treatment with placebo (Table 10). The five most common all-grade AEs following treatment with everolimus were stomatitis (71.8%), infections (59%), diarrhoea (44.4%), peripheral oedema (40.2%) and fatigue (36.8%).
| AE | All grades | Grades 3 + 4 | ||
|---|---|---|---|---|
| Everolimus + BSC (%) (n = 117a) | Placebo + BSC (%) (n = 58a) | Everolimus + BSC (%) (n = 117a) | Placebo + BSC (%) (n = 58a) | |
| Abdominal pain | 19.7 | 27.6 | 5.1 | 6.9 |
| Anaemia | 23.9 | 12.1 | 6.8 | 1.7 |
| Arthralgia | 16.2 | 10.3 | 0.9 | 0 |
| Asthenia | 21.4 | 10.3 | 2.6 | 0 |
| Cough | 26.5 | 22.4 | 0 | 0 |
| Decreased appetite | 21.4 | 22.4 | 1.7 | 1.7 |
| Diarrhoea | 44.4 | 43.1 | 11.1 | 3.4 |
| Dysgeusia | 22.2 | 5.2 | 0.9 | 0 |
| Dyspnoea | 16.2 | 8.6 | 1.7 | 0 |
| Fatigue | 36.8 | 41.1 | 5.1 | 1.7 |
| Headache | 17.1 | 17.2 | 0 | 0 |
| Hypertension | 15.4 | 8.6 | 6.8 | 1.7 |
| Infectionsb | 59.0 | 22.4 | 12.8 | 3.4 |
| Nausea | 28.2 | 17.2 | 3.4 | 1.7 |
| Non-infectious pnuemonitisc | 19.7 | 1.7 | 0.9 | 0 |
| Peripheral oedema | 40.2 | 6.9 | 2.6 | 1.7 |
| Pruritus | 18.8 | 10.3 | 0 | 0 |
| Pyrexia | 22.2 | 8.6 | 1.7 | 0 |
| Rash | 29.1 | 10.3 | 0.9 | 0 |
| Stomatitisd | 71.8 | 22.4 | 7.7 | 0 |
| Weight decrease | 18.8 | 10.3 | 0 | 0 |
Outcomes from randomised controlled trial evidence for lung neuroendocrine tumours
Following a data request to Novartis, some of the outcomes from RADIANT-435 were provided for individuals with lung NETs alone. The following sections report the baseline characteristics and outcomes provided by Novartis for the individuals with lung NETs only from RADIANT-4. 35
Baseline characteristics
Baseline characteristics for individuals with lung NETs only are reported in Table 63 (see Appendix 7).
Progression-free survival
Novartis provided data for PFS from the RADIANT-435 trial for patients with lung NETs. There were 42 progression events for 63 individuals assigned to everolimus plus BSC compared with 18 events for 27 individuals in the placebo plus BSC arm. Everolimus was associated with a 50% reduction in the risk of disease progression or death for people with lung NETs compared with placebo (HR 0.50, 95% CI 0.28 to 0.88; see Table 8).
Overall survival
Novartis provided data for OS from the RADIANT-435 trial for patients with lung NETs (see Table 8). HRs were obtained from the unstratified Cox model.
Response rate
Novartis provided data for RRs from RADIANT-435 for patients with lung NETs (see Table 8).
For all RR outcomes, treatment with everolimus plus BSC for lung NETs resulted in a favourable response compared with treatment with placebo plus BSC.
Adverse events
Novartis provided data for AEs from RADIANT-4 for individuals with lung NETs. AEs were more commonly reported following treatment with everolimus than following treatment with placebo (see Appendix 7, Table 64). The five most common all-grade AEs following treatment with everolimus (RADIANT-435) (confidential information has been removed).
Summary
Summary of the clinical effectiveness systematic review
-
Of 6209 titles/abstracts screened, three trials met the inclusion criteria for the clinical effectiveness systematic review.
-
The three trials were reported in 56 citations (six full texts, one errata and 49 conference abstracts).
-
The risk of bias within the trials was low and was found to be the same within the three studies with regard to selection, performance, detection, attrition and reporting bias.
Pancreatic neuroendocrine tumours
-
Two trials provided evidence on the effectiveness of everolimus (RADIANT-332) and sunitinib (A618111145) in the treatment of pNETs. Both interventions were compared with placebo. BSC was also given in both the intervention arm and the placebo arm in both trials.
-
Median PFS, assessed by central review, was 11.4 months for everolimus34 and 12.6 months for sunitinib81 compared with 5.4 months and 5.8 months, respectively, in the placebo arms of the trials. Locally assessed PFS was also reported.
-
In the first publication for each trial, median OS was not reached or data were immature. Longer follow-up data were available from company submissions. 32,33 Median OS was 44.0 months for everolimus34 and 38.6 months for sunitinib81 compared with 37.7 months and 29.1 months, respectively, in the placebo arms. Treatment switching from the placebo arm to the treatment arm (73% in RADIANT-334 and 69% in A618111181) significantly compromises the OS results.
-
Tumour response rates were assessed locally for RADIANT-334 and were assumed to be locally assessed in A6181111. 81 Complete response was achieved by only two individuals receiving sunitinib;81 complete response was not achieved in any of the other arms. Both trials reported higher rates for partial response and stable disease and lower rates for progressive disease in the treatment arms (everolimus and sunitinib) than in the placebo arms.
-
Overall, AEs were more commonly reported following treatment with everolimus and sunitinib than following with placebo. The five most common all-grade AEs following treatment with everolimus34 were stomatitis (64%), rashes (49%), diarrhoea (34%), fatigue (31%) and infections (23%). Following treatment with sunitinib,81 the five most common all-grade AEs were diarrhoea (59%), nausea (45%), vomiting (34%), asthenia (34%) and fatigue (32%).
-
Health-related quality of life was assessed in A618111181 (sunitinib) using the EORTC QLQ-C30. There were no overall differences between study groups, except for diarrhoea (21.4-point difference) and insomnia (7.8-point difference), which occurred with a higher frequency in the sunitinib arm than in the placebo arm. HRQoL data were not collected in RADIANT-3. 34
Indirect treatment comparison: pancreatic neuroendocrine tumours
-
RADIANT-334 and A618111181 were used to compare everolimus with sunitinib in an ITC using the Bucher method.
-
The ITC for PFS from central radiology review suggests no difference between the treatments (HR 1.06, 95% CI 0.57 to 1.97).
-
The ITC for PFS from local review suggests that everolimus is associated with a 17% decrease in disease progression or death compared with sunitinib (HR 0.83, 95% CI 0.49 to 1.42). The 95% CI is wide and includes the null hypothesis that there is no difference in PFS effectiveness between everolimus and sunitinib.
-
For OS, the ITC suggests that there is a 2.56 times greater hazard of dying associated with treatment with everolimus than with sunitinib, which is statistically significant. However, as these analyses are based on published HRs from RADIANT-334 and A6181111,81 which were not adjusted for treatment switching after disease progression, these results should not be relied on.
-
The ITC for OS, in which the companies have used the RPSFT method to adjust for treatment switching, suggests a lower hazard of death associated with sunitinib than with everolimus (HR 1.76, 95% CI 0.20 to 15.78). However, the 95% CI is very wide and includes the null effect.
-
For RRs, the ITC suggests that there is an 82% increase in the odds of a partial response in individuals treated with sunitinib compared with individuals treated with everolimus. However, sunitinib was associated with a 52% increase in the odds of progressive disease compared with everolimus. Everolimus was associated with a 2.3 times greater odds of disease stability than sunitinib. However, all of these ITCs were associated with wide 95% CIs, suggesting that there is little evidence of a difference in RRs between everolimus and sunitinib.
-
An ITC was completed only for those AEs for which data and events were available from both trials. For all grades of AE, the ITC suggests a 19% increase in the odds of experiencing stomatitis and a 42% increase in the odds of experiencing nausea with sunitinib compared with everolimus. For rash, fatigue, diarrhoea, dysguesia, epistaxis, loss of weight, thrombocytopenia, decrease in appetite, headache, vomiting and asthenia (all grades), the evidence suggests an increase in the odds of experiencing the AE with everolimus compared with sunitinib. However, except for decreased appetite, all of these ITCs were associated with wide 95% CIs that included the null hypothesis of no difference, suggesting that there is little evidence of a difference in AEs between everolimus and sunitinib. For all grades of decreased appetite, there was a statistically significant increase in the odds of experiencing the event with everolimus compared with sunitinib. For the grade 3/4 AEs, the ITC could consider only seven AEs based on the data that were available from the two trials. The evidence suggests an increased odds of experiencing grade 3/4 stomatitis, fatigue, diarrhoea, nausea and thrombocytopenia with everolimus compared with sunitinib and an increased odds of experiencing decreased appetite and asthenia with sunitinib compared with everolimus. However, all of the ITCs for grade 3/4 AEs were associated with wide 95% CIs that included the null hypothesis of no difference, suggesting that there is little evidence of a difference in AEs between everolimus and sunitinib.
Gastrointestinal and lung neuroendocrine tumours
-
One trial, RADIANT-4,35 provided evidence for the effectiveness of everolimus for treating GI and lung NETs. The intervention was compared with placebo and both arms received BSC.
-
Median PFS for RADIANT-435, assessed by central review, was 11.0 months for treatment with everolimus and 3.9 months for treatment with placebo. Locally assessed PFS was also reported.
-
Median OS was not reached. However, Kaplan–Meier estimates for OS at the 25th percentile were 23.7 months (95% CI 17.6 to 27.3 months) in the everolimus arm and 16.5 months (95% CI 9.0 to 21.0 months) in the placebo arm. In longer follow-up analysis of OS from the Novartis submission, OS was 25.7 months in the everolimus arm compared with 16.5 months in the placebo arm. 33 Treatment switching was not permitted in RADIANT-4. 35
-
Tumour RRs were assessed by central radiology review. No arm achieved a complete response. Individuals receiving everolimus had a favourable response for partial disease, stable disease, progressive disease and tumour shrinkage compared with those in the placebo arm.
-
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo. The five most common all-grade AEs following treatment with everolimus35 were stomatitis (63%), diarrhoea (31%), fatigue (31%), infections (29%) and rash (27%).
-
HRQoL was reported in the company submission from Novartis33 for RADIANT-4. The FACT-G questionnaire was used. Confidential information has been removed.
Gastrointestinal neuroendocrine tumours
-
Following a data request from the AG to Novartis, results from RADIANT-435 were provided for individuals recruited with only GI NETs (n = 118 for everolimus vs. n = 57 for placebo). 33
-
Median PFS for participants with GI NETs from RADIANT-435 was 13.1 months for everolimus and 5.4 months for placebo.
-
Median OS estimated from a Kaplan–Meier curve at the 25th percentile was (confidential information has been removed) in the everolimus arm compared with (confidential information has been removed) in the placebo arm.
-
Confidential information has been removed. Individuals receiving everolimus (confidential information has been removed) response for stable disease, progressive disease and tumour shrinkage compared with those in the placebo arm.
-
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo for individuals with GI NETs. The five most common all-grade AEs following treatment with everolimus were stomatitis (71.8%), infections (59%), diarrhoea (44.4%), peripheral oedema (40.2%) and fatigue (36.8%).
Lung neuroendocrine tumours
-
Following a data request from the AG to Novartis, results from RADIANT-435 were provided for individuals recruited with only lung NETs (n = 62 for everolimus vs. n = 27 for placebo).
-
There were (confidential information has been removed) assigned to everolimus compared with (confidential information has been removed) for the placebo arm. Everolimus was associated with a (confidential information has been removed) in the risk of disease progression compared with placebo.
-
There were (confidential information has been removed) assigned to everolimus arm compared with (confidential information has been removed) for the placebo arm. Survival was (confidential information has been removed) following everolimus treatment compared with placebo.
-
Rates of stable disease and progressive disease (confidential information has been removed) with everolimus.
-
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo. The five most common all-grade AEs following treatment with everolimus35 (confidential information has been removed).
Summary of the non-randomised 177Lu-DOTATATE studies
-
Thirty-two non-randomised single-arm trials were identified.
-
There was a wide variation in outcome measures, which is likely to be the result of factors inherent in the single-arm study design and compounded by wide variations in participant characteristics, for example tumour sites, with outcomes often reported for mixed tumour locations, for example data for gut, pancreas and lung NETs being grouped together.
Chapter 5 Critical appraisal of the company submissions
Two companies submitted economic models to NICE: AAA and Novartis. Novartis submitted an economic evaluation in patients with pNETs and, separately, in patients with GI and lung NETs. AAA presented an economic evaluation in pNETs and another in midgut carcinoid tumours. The background safety and efficacy evidence supporting the companies’ model submissions detailed model characteristics, and results are presented in Appendix 8. Pfizer did not submit a cost-effectiveness model; it stated that:
Previous technology appraisals (SMC [Scottish Medicines Consortium] and AWMSG [All Wales Medicines Strategy Group]) have challenged the data limitations relating to uncertainty associated with modelling the clinical OS benefits of sunitinib in PNET due to the extensive treatment switching of patients in study A6181111. Because A6181111 is the only clinical trial for sunitinib in this indication, the limitations in the data for certain key model attributes remain. For these reasons, any cost-effectiveness evidence submitted would continue to be associated with considerable uncertainty.
Pfizer submission, p. 8432
Novartis submission33
Economic evaluation of everolimus in pancreatic neuroendocrine tumours
Overview
In pNETs, the company evaluated everolimus plus BSC compared with sunitinib plus BSC. The company based this analysis on an ITC of the results of RADIANT-3,34 a Phase III pivotal trial of 10 mg of everolimus once daily plus BSC compared with placebo plus BSC, and A6181111,81 a Phase III RCT of sunitinib (confidential information has been removed) plus BSC compared with placebo plus BSC. These RCTs were the only available relevant evidence identified from a systematic review of RCTs and non-RCTs of everolimus, sunitinib and 177Lu-DOTATATE for treating patients with advanced, metastatic or inoperable pNETs, and 177Lu-DOTATATE for treating advanced, metastatic or unresectable GEP NETs. The company did not provide the reasons for omitting BSC from the analysis, for which head-to-head trial data were available compared with each of the targeted treatments from RADIANT-334 and A6181111. 81
The ITC of everolimus and sunitinib found no statistically significant differences in PFS and OS outcomes, with estimated differences having wide CIs. The company found that everolimus was associated with a lower frequency of grade 3/4 AEs and a different tolerability profile (see Chapter 4, Results).
Everolimus was found to dominate sunitinib. It generated lower discounted costs and more discounted quality-adjusted life-years (QALYs), which were calculated on the assumption that the two treatments produced the same mean PFS and OS. As the model assumed only two disease states, stable disease and disease progression, and utilities in the latter state were assumed to be the same across treatments, the QALY differences rested on the health state utility in stable disease under the two treatments, which in turn reflected their differences in toxicity and AEs. Critically, these utility values were based on clinical experts’ valuations of HRQoL descriptors (vignettes) of stable disease in general and the impact of treatment-specific AEs, as opposed to HRQoL outcomes in actual patients.
Economic evaluation by the company
Novartis evaluated the costs and health benefits of everolimus plus BSC relative to sunitinib plus BSC in advanced, well-differentiated or moderately differentiated pNETs patients with progressive disease from a NHS or Personal Social Services (PSS) perspective. A semi-Markov model of monthly health state cycles experienced by a patient cohort was used to synthesise the evidence on effectiveness, resource use, costs and health state utilities, over a period of 20 years following the start of treatment. The main source of evidence was an ITC of PFS, OS, concomitant SSA use, treatment duration and grade 3/4 AEs in the A618111181 trial of sunitinib and the RADIANT-334 trial of everolimus (see Chapter 4, Indirect treatment comparison: pancreatic neuroendocrine tumours, and Appendix 8, Novartis). The model consisted of three health states, representing stable disease, disease progression and death, each associated with different costs and utilities, as illustrated in Figure 9.
FIGURE 9.
Model structure in pNETs. Source: reproduced from figure 6.1 of the Novartis submission. 33
To allocate the distribution of patients across the health states for each treatment at a given point in the modelled time, the rate of survival in the patient cohort as obtained from a parametric OS curve fitted to the trial data was partitioned between the two alive health states using a parametric PFS curve also estimated from the trial data for each treatment arm. Thus, in each treatment cycle the residual between the OS and the PFS rates was used as the estimator for the proportion of patients in the progressive disease phase.
The resource utilisation data were obtained from a survey of 32 clinical experts in the UK. 113 Health state utility values elicited for a set of vignettes describing health states in pNETs from a sample of members of the general public were used for health states with and without AEs, as no HRQoL or utility data were recorded in RADIANT-3. 34 Sensitivity analyses adopted alternative values for the sunitinib arm derived from HRQoL outcomes measured in the A618111181 trial of sunitinib compared with placebo.
The model accounts for the costs of subsequent treatments after disease progression by including a fixed cost of radiotherapy, chemoembolisation and chemotherapy in the first cycle of the progressive disease state of the model. Similarly, a fixed cost of end-of-life care is included on transition to the death state.
Results
In the advanced pNETs patients with progressive disease, the base-case analysis found a life expectancy of 4.17 years over a 20-year time horizon after the start of treatment with everolimus or sunitinib in patients with a median age of 58 years (as in the everolimus arm of RADIANT-334). Everolimus produced more QALYs than sunitinib (2.73 vs. 2.71, a difference of 0.02 QALYs discounted at 3.5%) (Table 11). As this analysis assumed equal PFS and OS outcomes between the two treatments, the QALY difference was purely the result of the impact on HRQoL of treatment differences in AEs.
| Treatment | Life-years (undiscounted) | QALYs (discounted) | Costs (£) | ICER |
|---|---|---|---|---|
| Everolimus | 4.17 | 2.73 | 36,933 | Dominant |
| Sunitinib | 4.17 | 2.71 | 38,569 | |
| Difference | 0 | 0.02 | 1636 |
Everolimus was associated with discounted health-care costs of £36,933 per patient, that is, an overall saving of £1636 relative to sunitinib, which had discounted health-care costs of £38,569 per patient. With a (confidential information has been removed) Patient Access Scheme (PAS) discount applied to the list price of everolimus and reimbursement of the first cycle of sunitinib, the total cost to the NHS of using everolimus was reduced to (confidential information has been removed) and of using sunitinib was reduced to £36,247, that is, a saving of (confidential information has been removed) per patient for everolimus. As PFS, OS and costs in the progressive disease state were the same across the two arms, the cost differences were the result of differences in the drug acquisition costs of the targeted therapies in the stable disease period. As a result, everolimus was found to be dominant over sunitinib.
Novartis [table 6.18 ( p. 112) of the Novartis submission33] reports that the total cumulative time spent per patient in the stable disease health state is 0.899 years under everolimus and 0.878 years under sunitinib. This must be an error as the base-case model assumes equal PFS outcomes for both treatments and there is no accounting in the model for differences in grade 5 AEs (deaths).
Probabilistic sensitivity analyses (PSAs) of drug acquisition prices unadjusted for the PAS produced a mean cost saving estimate for everolimus of £2055 per patient and a QALY gain of 0.002. With the PAS, the mean cost saving with everolimus was increased to (confidential information has been removed). This result suggests that the main determining factor is costs, as the mean QALY difference of 0.002 is not considered to be clinically significant. 115 However, these PSAs by Novartis were not adequately performed as the PFS and OS and SSA use (set at zero) parameter values were assumed to be the same rather than differ between the treatments according to their mean estimates in the trial data and their associated sampling uncertainty. Therefore, the claim by the company that ‘at the £30,000/QALY threshold, there is (confidential information has been removed) probability of everolimus being cost-effective when compared to sunitinib at their respective PAS prices’ (Novartis submission, p. 11433) should be considered with these reservations in mind.
Consequently, deterministic sensitivity analysis performed by Novartis showed that the most influential parameters are the relative treatment effects on PFS and OS, and the treatment duration, RDI and costs of AEs. However, the company states that the incremental difference in terms of the incremental cost-effectiveness ratio (ICER) is ‘so marginal, it does not materially affect the model results’ (Novartis submission, p. 11633). Similarly, positive rates of SSA use, use of PFS data assessed by local experts compared with centrally assessed data and choice of PFS distribution had a marginal impact. In scenario analyses, exploring the effects of changing clinical effectiveness, utility and cost parameter values, as well as structural assumptions about OS and PFS outcomes, everolimus was still dominant, except for the case in which the relative treatment effect on PFS was set to favour sunitinib according to estimates derived from the Bucher indirect comparison (HR: PFS 0.93, OS 0.72). In this instance, everolimus had lower costs and produced fewer QALYs than sunitinib and the ICER was found to be (confidential information has been removed). The interpretation of the ICER in this case reverses, that is, the higher the value the more cost-effective everolimus is; it may be reasonable to consider (confidential information has been removed) in this patient population. It must be noted that this is the result of everolimus saving £5963 (confidential information has been removed) at the expense of having 0.685 fewer QALYs per patient [table 6.23 (p. 117) of the Novartis submission33].
The scenarios explored by Novartis do not cover some major areas of uncertainty, such as variation in the relative probability of AEs, nor structural uncertainty associated with different functional forms for the different treatment arms in each of the progressive disease and stable disease phases. Nevertheless, given the possibly clinically insignificant utility differences discussed earlier, or that Novartis may produce fewer QALYs than sunitinib in exchange for a small reduction in NHS costs, the dominance of everolimus over sunitinib in the Novartis base-case analysis is not robust to the sources of uncertainty investigated by Novartis.
Strengths and weaknesses of Novartis’s evaluation of pancreatic neuroendocrine tumours
The model by Novartis follows the NICE reference case37 (see Appendix 9) except for one major aspect, which was the lack of inclusion of BSC as a comparator. This omission is at odds with the fact that the two RCTs from which the effectiveness data were obtained for the model compared targeted therapy (everolimus or sunitinib) plus BSC with placebo plus BSC. There was no justification given by Novartis for omitting the BSC-only treatment option in its cost-effectiveness analysis. However, in the opinion of our clinical experts, BSC is a relevant initial treatment option for patients with advanced, progressive pNETs and small or asymptomatic tumours, in whom active treatment may be considered on disease progression.
This was a complex area to analyse because of limited data on advanced pNETs patients with progressive disease, which is a natural result of the small incidence of this disease. Evidence on resource use was particularly limited, especially for the progressive disease phase of the model, for which the quantities used in the model were based on expert opinion. Another major uncertainty in the evidence base is the lack of HRQoL data for everolimus in the patient population of interest here. This ultimately led Novartis to base the comparison of HRQoL and, given the base-case assumptions of equal OS and PFS outcomes between the treatments, QALY outcomes between the two treatments on their relative impact on AE incidence and severity. Thus, the difference in QALYs was based on values derived from actual patient AE outcomes complemented by clinical experts’ views of quality of life in stable disease under different AEs.
A critical feature of the OS data used in the Novartis economic model was the adjustment for crossover from placebo to active treatment in the RCT data. In particular, the method used for such adjustment, the RPSFT model, relied on the assumption that the benefit derived by patients from receiving targeted treatment was the same whether they were given it as initial treatment or subsequently on disease progression. This assumption may be questionable and it is therefore natural to expect that, in the present case, sensitivity analyses allowing for a reduction in the benefit conferred by targeted treatment received after disease progression should have been performed. Although other methods are available to adjust for treatment crossover, such as inverse probability of censoring weight116 and censoring at crossover,117 they are clearly inferior as the majority of cases of crossover in RADIANT-334 and A618111181 occurred following study termination, making the key assumption underlying these methods, either that crossover is random or that patients who did not cross over may be representative of those who did, unlikely to hold true.
The Bucher indirect comparison from which Novartis derived estimates of relative effectiveness and side effects for populating its economic model was based on data that appear to be outdated on three fronts. First, Novartis estimated relative OS effectiveness using an indirect comparison (Bucher method) of placebo-adjusted HRs that corrected for crossover from the placebo to the active treatment arms in the two RCTs used in the indirect comparison. The crossover-adjusted OS HR estimate used for sunitinib compared with placebo in the indirect comparison by Novartis is lower than that in the final published results for OS for A6181111. 81 With these new data, we obtained a crossover-adjusted OS HR for everolimus compared with sunitinib of 0.51, instead of the OS HR of 0.72 derived by Novartis. Second, our updated searches of the literature conducted in September 2016 identified a forthcoming publication48 providing grade 3/4 AE counts that differ in some instances from those used for the everolimus and placebo arms in the Bucher indirect comparison submitted as evidence to NICE by Novartis. Third, some of the grade 3/4 AE data used in the Novartis Bucher indirect comparison for the sunitinib and placebo arms were different from the corresponding data submitted by Pfizer32 to NICE. When the Pfizer data are used instead, the pooled AE OR estimated by Novartis as 4.47 changes to 1.37, thus reducing the differences in costs and disutilities of AEs between sunitinib and everolimus.
The way that Novartis synthesised the effectiveness and safety evidence in its cost-effectiveness model inadequately reflected the available information. The company’s base-case assumption was that the PFS and OS outcomes of sunitinib and everolimus were the same, on the basis of wide CIs around the point estimates of relative effectiveness. This practice is clearly inadequate because it misrepresents the level of uncertainty in the data as evidence of lack of effect. This issue is made more serious in view of the direction and extent of possible bias resulting from the use of inadequate data, discussed in the previous paragraph.
In terms of model implementation, the limitations of the Novartis model analysis include the assumption of the same treatment duration for everolimus and sunitinib. As pointed out earlier, the mean number of treatment cycles of sunitinib is likely to be lower than that of everolimus, based on the Pfizer data32 submitted to NICE and comparison of median treatment durations in the main publications of A618111181 and RADIANT-3. 34 On the other hand, Novartis did not account for the fact that the mean PFS (area under the Kaplan–Meier curve) in the placebo arm of the A618111181 trial of sunitinib was lower than the mean PFS of the placebo arm of the RADIANT-334 trial of everolimus. If treatment duration is proportional to PFS, a fair indirect comparison with everolimus would require an increase in sunitinib treatment duration in proportion to the magnitudes of PFS in the placebo arm of RADIANT-334 and PFS of the placebo arm of A6181111. 81
Another limitation was the implementation of subsequent treatment costs. In the partitioned survival model used by Novartis the number of people who transitioned into progressive disease and who were eligible to receive subsequent treatment was not obtainable from the model output and had to be approximated using summary information reported in the trial about the number of people who were censored, died before progression and experienced a PFS event. This approximation involved the strong assumption of a constant relative frequency of these events throughout the PFS horizon.
Despite its limitations, the evidence presented by Novartis suggests that, with the currently available information, the choice between sunitinib and everolimus hinges on their relative effects on PFS and OS and drug acquisition costs and is subject to high levels of uncertainty related to clinical effectiveness. The disutility of AEs is unlikely to be a significant factor in that choice and determining its importance is hampered by the lack of data of sufficient quality for meaningful assessment.
Economic evaluation of everolimus in gastrointestinal and lung neuroendocrine tumours
Overview
The economic evaluation by Novartis of everolimus plus BSC compared with BSC alone in advanced, progressive, well-differentiated, non-functioning GI and lung NETs was based on the effectiveness, safety and quality of life evidence reported from a Phase III RCT, RADIANT-4. 35 As in its economic evaluation in pNETs, discussed in the previous section, Novartis relied on data from a resource use survey,113 which was validated for the UK.
Economic evaluation by the company
Novartis evaluated the cost-effectiveness of 10 mg of everolimus daily plus BSC relative to BSC alone in patients with advanced, progressive, well-differentiated, non-functional GI and lung NETs. This evaluation assessed costs, life-years and QALYs over a 30-year time horizon. For this purpose a three-health-state, semi-Markov model of monthly cycles was used, populated with data from a partitioned survival analysis of data from the RADIANT-435 Phase III trial of everolimus plus BSC compared with placebo plus BSC. The model represented the disease course experience of a cohort of patients from the start of treatment, starting from a stable disease phase, moving to a progressive disease phase at the time of disease progression and, at any time in the disease course, facing the risk of death from any cause. The structure was the same as that described in the Novartis pNETs model and that used by Ayyagari et al. 118 in GI locations (see Figure 9).
Results
Novartis reports that the life expectancy over a 30-year period for patients with advanced, progressive, well-differentiated, non-functional GI and lung NETs is 5.79 life-years for 10 mg of everolimus daily plus BSC compared with 4.77 life-years for BSC alone (Table 12). The respective total discounted costs are expected to be £59,720 and £25,817 and the total QALYs produced are 4.28 and 3.51. This results in an ICER of £43,642 per QALY gained for everolimus plus BSC compared with BSC alone. Under the (confidential information has been removed) PAS discount on the everolimus price, the cost per QALY gained with everolimus would be reduced to (confidential information has been removed). In total, 71% of the QALY gain for everolimus plus BSC compared with BSC takes place in the stable disease state and the share of the cost falling during the stable disease state is 98% at list prices. The costs of active initial treatment with everolimus and BSC costs represent 79% and 8%, respectively, of the total incremental costs of treatment with everolimus plus BSC. The mean PSA ICER for everolimus plus BSC compared with BSC was £45,385 without the PAS and (confidential information has been removed) with the PAS.
| Treatment | Life-years (undiscounted) | QALYs (discounted) | Costs (£) | ICER (£) |
|---|---|---|---|---|
| Everolimus | 5.79 | 4.28 | 59,720 | 43,642 |
| BSC | 4.77 | 3.51 | 25,817 | |
| Difference | 1.02 | 0.77 | 33,903 |
Univariate deterministic sensitivity analyses found that the ICER was most sensitive to the choice of distribution for extrapolating OS. The ICER varied from £39,571 to £59,832 with different OS distributions and (confidential information has been removed) with the PAS. When treatment-specific utility values were applied, the ICER increased to £56,385 and (confidential information has been removed) under the PAS. The results were also sensitive to the RDI. Novartis also reports how extending the lifetime horizon and extrapolating outcomes beyond the trial period improved the cost-effectiveness of everolimus.
From the results reported by Novartis it is evident that, in the analysis of the PAS, the (confidential information has been removed) PAS discount was applied only to everolimus given as initial treatment, not as subsequent treatment. In principle, the discount should have been applied to subsequent treatment too.
The company concluded that the ICER (confidential information has been removed). The company states that the results should be considered within the context of unmet medical need for effective treatment options in this heterogeneous and small patient population, with there being approximately 936 patients in England across the two indications (pNETs and GI/lung) [table 3.1 (p. 27) of the Novartis submission33].
Strengths and weaknesses of Novartis’s evaluation of gastrointestinal and lung neuroendocrine tumours
The economic evaluation by Novartis of everolimus in GI and lung NET patients relies on the quality of RADIANT-4,35 which was the source of the effectiveness, AE and treatment duration and intensity data for the model and rates of subsequent treatment use. A major limitation is the omission of relevant active comparators, such as 177Lu-DOTATATE, in the analysis. Another limitation of the study design is the lack of a separate analysis of lung and GI patients.
In terms of data, the main limitation is the lack of resource use data measured in a sample of patients. It is not clear how robust the estimated costs of subsequent treatment use are likely to be, with issues such as administrative censoring (i.e. from termination of the study), and, indeed, whether or not the differences captured may have been an artefact of the length of follow-up.
In terms of evidence synthesis, the decision analysis relied on applying the same parametric survival distributions for extrapolation to both arms, which may have unnecessarily restricted the modelling capabilities in this study. Another issue was that, although crossover from everolimus to placebo was not permitted in the trial, 10% (10/97) of patients in the placebo arm did cross over (four before and six after unmasking). In spite of this, the analysis of OS data in RADIANT-435 did not adjust for such treatment crossover. This limitation may slightly bias the results if the analysis is intended to inform the evaluation of two alternative states of the world, one in which everolimus is provided as initial treatment and another in which it is not provided at all. If instead the NICE decision is between choosing everolimus as initial treatment or everolimus at the discretion of the physician as a potential subsequent treatment, the lack of adjustment for crossover by Novartis may not need to be a source of bias per se.
Another minor limitation is the inaccuracy in estimating the costs of subsequent treatments in the Novartis GI and lung model. This issue is the same as that discussed in the previous section for the Novartis model in pNETs and is not repeated here.
On the other hand, data on BSC treatment use, everolimus treatment duration and intensity and the incidence of grade 3/4 AEs in RADIANT-435 are detailed and Novartis describes important sources of uncertainty in the evidence base.
Advanced Accelerator Applications submission31
Overview
In anticipation of European market authorisation, the company’s submission considers the use of the radiolabelled SSA 177Lu-DOTATATE (7.4 GBq, equivalent to 200 mCi) for people with inoperable progressive somatostatin receptor-positive GEP NETS. The company separates the GEP NETs population into two subpopulations in the model:
-
(confidential information has been removed)
-
(confidential information has been removed).
Separation into these subpopulations was seen by AAA as appropriate as pNETs and GI NETs have different clinical profiles and undergo different management. It is also important to note that with the selection of this trial102 the population considered in the economic evaluations was further restricted to the subpopulation of somatostatin receptor-positive (SSTR+) patients.
The comparators in the pNETs evaluation were everolimus (10 mg per day) and sunitinib (37.5 mg per day). The comparator in the GI NETs evaluation was everolimus (10 mg per day) only. BSC was not offered as a comparative strategy.
In the base-case analysis of pNETs the reported ICERs favoured 177Lu-DOTATATE over both included comparators. The estimated cost per QALY gained for 177Lu-DOTATATE compared with everolimus was (confidential information has been removed). In the comparison with sunitinib, 177Lu-DOTATATE was estimated to be less costly and produce more QALYs and therefore dominated sunitinib.
In the base-case analysis of GI NETs, the reported ICER also favoured 177Lu-DOTATATE. The cost per QALY gained compared with everolimus was estimated to be (confidential information has been removed).
Supportive arguments for these findings are not discussed except that it was stated that the result is driven by superior survival with 177Lu-DOTATATE. The company did not draw comparisons with existing published economic evidence as this is the first cost-effectiveness analysis of 177Lu-DOTATATE.
Economic evaluation by the company
The decision-analytic model is structured using a partitioned survival (‘area under the curve’) approach based on a parametric extrapolation of Kaplan–Meier curves for baseline PFS and OS and HRs applied proportionally through a 20-year time horizon. The evaluation utilises a three-health state cohort transition model to simulate survival and progression (Figure 10). The selected cycle length is 1 month. Costs and benefits were discounted at 3.5% in future years and are reported from the NHS/PSS and patient’s perspective respectively.
Advanced Accelerator Applications conducted two mixed treatment comparisons (MTCs) for the outcomes of PFS and OS: one for pNETs comparing 177Lu-DOTATATE with everolimus and sunitinib, and one for GI NETs comparing 177Lu-DOTATATE with everolimus (see Appendix 8, Advanced Accelerator Applications).
The sources of evidence of health effects in the pNETs evaluation were the NETTER-1 study102 for octreotide LAR (baseline reference) and 177Lu-DOTATATE;102 RADIANT-334 for everolimus; and A618111181 for sunitinib. The sources of evidence of health effects in the GI NETs evaluation were the ERASMUS study for 177Lu-DOTATATE (Dutch pNETs subgroup) and RADIANT-435 for everolimus.
Result
Over a time horizon of 20 years, for people with inoperable progressive SSTR+ pNETs the use of 177Lu-DOTATATE was found to be cost-effective compared with both everolimus and sunitinib at a threshold of £20,000 per QALY gained (Table 13).
| Outcome | 177Lu-DOTATATE | Everolimus | Sunitinib |
|---|---|---|---|
| PFS at 5 years (%) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| OS at 5 years (%) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Life-years (discounted) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| QALYs PFS (discounted) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| QALYs PPS (discounted) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Total QALYs | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Drug cost | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Drug administration | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Disease monitoring | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| AE management | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Total costs | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Incremental cost vs. 177Lu-DOTATATE (£) | 21,489 | –6648 | |
| Life-years gained by 177Lu-DOTATATE | 2.75 | 0.07 | |
| QALYs gained by 177Lu-DOTATATE | 2.18 | 0.10 | |
| ICER (£) (177Lu-DOTATATE vs. treatment in the respective column heading) | 9847 | Dominant (–68,916) |
Over a time horizon of 20 years, for people with inoperable progressive SSTR+ functional and non-functional carcinoid midgut NETs (GI NETs) the use of 177Lu-DOTATATE was found to be cost-effective compared with everolimus at a threshold of £20,000 per QALY gained (Table 14).
| Outcome | 177Lu-DOTATATE | Everolimus |
|---|---|---|
| PFS at 5 years (%) | Confidential information has been removed | Confidential information has been removed |
| OS at 5 years (%) | Confidential information has been removed | Confidential information has been removed |
| Life-years (discounted) | Confidential information has been removed | Confidential information has been removed |
| QALYs PFS (discounted) | Confidential information has been removed | Confidential information has been removed |
| QALYs PPS (discounted) | Confidential information has been removed | Confidential information has been removed |
| Total QALYs | Confidential information has been removed | Confidential information has been removed |
| Drug cost | Confidential information has been removed | Confidential information has been removed |
| Drug administration | Confidential information has been removed | Confidential information has been removed |
| Disease monitoring | Confidential information has been removed | Confidential information has been removed |
| AE management | Confidential information has been removed | Confidential information has been removed |
| Total costs | Confidential information has been removed | Confidential information has been removed |
| Incremental cost vs. 177Lu-DOTATATE (£) | 28,099 | |
| Life-years gained by 177Lu-DOTATATE | 1.77 | |
| QALYs gained by 177Lu-DOTATATE | 1.42 | |
| ICER (£) (177Lu-DOTATATE vs. treatment in the respective column heading) | 19,816 |
In a one-way deterministic sensitivity analysis in which individual parameter point estimates were varied to their upper and lower 95% CI or interquartile range boundaries (or, when not available, ±20% of the mean):
-
the 177Lu-DOTATATE acquisition cost and RDI adjustment were identified as highly sensitive model input parameters
-
PFS and post-progression survival (PPS) utility scores were identified as moderately sensitive input parameters.
Uncertainty around the point estimates of input parameters in the deterministic analysis was explored in a PSA. In 5000 iterations selected parameters were varied using conventional distributions. Parameters not included in the PSA were relative treatment effect (PFS, OS), drug acquisition costs and drug administration costs. The results revealed that PSA ICERs were consistently lower than deterministic ICERs (Table 15). AAA offered no explanation for this discrepancy, which in theory may be due to an error in the PSA build or the inclusion of one or more non-linear parameters in the model.
| Comparison | ICER (£) | |
|---|---|---|
| Deterministic result | Probabilistic result | |
| pNETS: 177Lu-DOTATATE vs. everolimus | 9847 | Confidential information has been removed |
| pNETS: 177Lu-DOTATATE vs. sunitinib | –68,916 (dominant) | Confidential information has been removed |
| GI NETS: 177Lu-DOTATATE vs. everolimus | 19,816 | Confidential information has been removed |
Summary results of scenario analyses
-
Shortening the time horizon to 5 or 10 years reduced the ICERs, except for the 5-year comparison with everolimus in GI NETs, in which the ICER increased to £23,334.
-
Discounting of costs and benefits to 6% and 1%, respectively, decreased all ICERs.
-
Increasing the 177Lu-DOTATATE dose intensity to 100% increased all ICERs (pNETs: vs. everolimus £14,206 per QALY gained; pNETs: vs. sunitinib £29,686 per QALY gained; GI NETs: vs. everolimus £26,386 per QALY gained).
-
Using alternative sources of utility for pre-progression in GI NETs:
-
using the NETTER-1 utility value (mean all patients: 0.750) rather than the utility value from Guy’s and St Thomas’ NHS Foundation Trust (0.793) resulted in an ICER of £21,295 for 177Lu-DOTATATE compared with everolimus
-
using the ERASMUS utility value (GI NETs subgroup: 0.773) rather than the utility value from Guy’s and St Thomas’ NHS Foundation Trust (0.793) resulted in an ICER of £20,931 for 177Lu-DOTATATE compared with everolimus (not £20,136 as reported by AAA).
-
-
Including palliative care costs and an end-of-life utility decrement in the last 4 weeks of life has a negligible effect on ICERs in both the pNET and the GI NET evaluations.
-
The inclusion of background mortality has a negligible effect on findings.
Strengths and weaknesses of Advanced Accelerator Applications’s evaluation
Strengths
-
The analysis separated the evaluation of pNETs from the evaluation of GI NETs.
-
The structural methodology followed recommended approaches and was implemented correctly. The model was well presented, transparent and generally straightforward to understand.
-
Serious AEs were incorporated, albeit poorly.
-
The model was found to contain only minor errors in wiring, which could effectively be overlooked.
Weaknesses
-
No comparison was made with a strategy of BSC.
-
The MTCs used to inform the relative treatment effect in each of the evaluations were premised on crucial yet unjustified assumptions:
-
that 60 mg of octreotide can be assumed to be equivalent to placebo and placebo plus 30 mg of octreotide in the GI NETs network
-
that 60 mg of octreotide is equivalent to placebo and placebo plus BSC in the pNETs network
-
that data from the NETTER-1 trial102 can be used to inform the network for pNETs even though no participants in the NETTER-1 trial had pNETs.
-
-
The MTCs used to inform the relative treatment effect in each of the evaluations were premised on weak methodology:
-
RADIANT-2119 should be not have been included in the GI NETs MTC as the population in this trial all had functioning tumours, which is outside the marketing licence for everolimus for GI NETs
-
NETTER-1 should not have been included in the pNETs MTC as this trial did not include any patients with pNETs, meaning that AAA’s pNETs evaluation is tenuous
-
there was no consideration of the extent of treatment switching in RADIANT-2 (58% switched to active treatment), RADIANT-334 (73% switched to active treatment) and A618111181 (69% switched to active treatment), which limits the interpretation of the results for OS.
-
-
Treatment after progression was oversimplified to octreotide across all strategies, which was continued until death.
-
Treatment with everolimus and sunitinib was assumed to continue until disease progression. This is an overestimate of usage and therefore cost as the average duration of treatment in trials was a fraction of this period.
-
The pNETs evaluation relied on non-randomised evidence for baseline estimates of PFS and OS.
-
The resource requirement for the administration of 177Lu-DOTATATE was underestimated given that current practice as described by the AG clinical experts is for an overnight stay rather than day case administration and that there is a greater time requirement from clinical specialists. No alternative estimates of drug administration costs were tested. Exploratory univariate variations in the cost of 177Lu-DOTATATE administration carried out by the AG revealed that this may be an important area for scrutiny.
-
The costing of serious adverse events (SAEs) was implemented poorly. On the one hand, costs were underestimated because of an overly low unit costing of SAEs, most of which require attention in the hospital setting, and, on the other hand, they were overestimated by their application well beyond the expected mean duration of treatment.
Chapter 6 Independent economic assessment
Methods
Model structure
Structure of relevant published models
In Table 16 we present the key aspects of published models from the studies included in our systematic review of the cost-effectiveness of drugs for treating NETs (see Appendix 10 for details). For comparison, we include characteristics of the Peninsula Technology Assessment Group (PenTAG) model.
| Model attribute | Casciano et al.120 | Mucino Ortega et al.121 | Johns et al.122 | Walczak et al.123 | PenTAG |
|---|---|---|---|---|---|
| Model type | Partitioned survival | Markov | Markov | Markov | Partitioned survival |
| Patient population | Advanced progressive pNETs | Non-resectable pNETs | Advanced or metastatic pNETs | Patients with unresectable or metastatic well-differentiated pNETs with disease progression | People with progressed unresectable or metastatic NETs of pancreatic, GI or lung origin |
| Initial treatments | Everolimus vs. sunitinib (including SSAs) | Sunitinib + BSC vs. placebo + BSC | Sunitinib + BSC vs. placebo + BSC | Sunitinib + BSC vs. placebo + BSC (including SSAs) | For pNETs: everolimus vs. BSC; everolimus vs. sunitinib; sunitinib vs. BSC. For GI NETs: everolimus vs. BSC; everolimus vs. 177Lu-DOTATATE; 177Lu-DOTATATE vs. BSC. For GI and lung NETs: everolimus vs. BSC |
| Health states | ‘Stable disease with no AEs’, ‘stable disease with AEs’, ‘disease progression’ and ‘death’ | ‘Pre progression’, ‘post progression’ and ‘death’ | ‘Progression free’, ‘post progression’ and ‘death’ | ‘Initial state’, ‘disease progression’ and ‘death’ | ‘Pre progression’, ‘post progression’ and ‘death’ |
| PFS and drug costs | PFS estimates were obtained from the indirect analysis (Signorovich et al.124) based on data from RADIANT-334 and A6181111.81 Initial treatment assumed up to progression | PFS data used in the analysis were from A6181111.81 Costs included the costs of drug acquisition and medical management, including specialist consultations, laboratory and imaging tests, pain management and palliative care | PFS data were from A6181111.81 Cost components were not reported | PFS data from A618111181 were extrapolated using the Weibull and RPSFT methods (to allow for treatment switching between the arms of the clinical trial). Cost components were not reported | Initial treatment assumed up to progression |
| Subsequent treatments | BSC | BSC | BSC | BSC | BSC |
| Method of estimating OS | OS estimates were obtained from the indirect analysis (Signorovich et al.124) based on data from RADIANT-334 and A618111181 | OS data used in the analysis were from A618111181 | OS data from A618111181 were adjusted for treatment switching using the RPSFT method | OS data from A618111181 were extrapolated using the Weibull and RPSFT methods (to allow for treatment switching between the arms of the clinical trial) | Extrapolation of OS data from RCTs |
| Patient age at model entry (years) | Not reported | Not reported | Not reported | Not reported | 60 |
| Cycle length | 30.4 days | 2 weeks | Not reported | 4 weeks | 28 days |
| Time horizon | 20 years | 20 years | Lifetime | Lifetime | 40 years |
Structure of the Peninsula Technology Assessment Group model
The majority of the studies, selected during the systematic review of cost-effectiveness (see Chapter 5, Novartis submission), reported models with three health states. The study by Casciano et al. 120 reported a four-state model, distinguishing patients with and without symptoms in the stable disease state. However, in this publication there is inconsistency between the graphical representation of the model and the model description. We believe that the reported model had only three states: stable disease, disease progression and death.
In our analysis, we adopted the three-state model structure shown in Figure 11.
FIGURE 11.
Structure of the PenTAG cost-effectiveness model.

The model health states are defined as follows:
-
pre progression
-
post progression
-
death.
The PenTAG cost-effectiveness model, implemented in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA), simulates a hypothetical cohort of 1000 patients with progressed unresectable or metastatic NETs. At the beginning of the simulation, all patients start in the pre-progression state; they then transition to the post-progression and death states according to PFS and OS estimates. At the end of each cycle, they can either remain in their current health state (which is denoted by bent arrows) or move to other states (which is depicted by straight arrows). Death is the absorbing state in this model. Health state membership is defined using the partitioned survival approach, which estimates the mean time spent in each health state from the area under the relevant survival curve. Therefore, the transitions in Figure 11 are not modelled explicitly. Costs and utilities are estimated for each health state and model cycle and aggregated over the modelled time horizon to estimate the total per-patient costs and QALYs for each treatment. The economic outcome in the model is the ICER. A model half-cycle correction has been applied.
The structure of the PenTAG model, informed by a cost-effectiveness systematic review and the opinions of our clinical experts, is very similar to the structures of the models submitted by the companies (see Table 19 for a detailed description of the submitted models).
In the model we assumed that:
-
patients receive active treatment until disease progression or earlier treatment discontinuation because of the onset of serious AEs or other reasons as observed in the RCT sources of effectiveness data
-
on progression of disease, patients are treated with BSC.
See Interventions and comparators for further details of the treatments and comparators considered in our analysis.
Population
In line with the NICE scope, we considered people with progressed unresectable or metastatic NETs from three different patient populations according to tumour location:
-
patients with NETs of pancreatic origin
-
patients with GI and lung NETs
-
patients with GI (midgut) NETs.
The choice of these particular patient populations was determined by the available clinical effectiveness RCT data. Specifically, GI (midgut) NETs were assessed as the population recruited to the single RCT for 177Lu-DOTATATE (NETTER-1)102 included only patients with midgut NETs. We did not consider any other subgroups in our analysis as no relevant clinical evidence was identified during the clinical effectiveness systematic review (see Chapter 4, Outcomes for randomised controlled trial evidence for pancreatic neuroendocrine tumours, Subgroup analysis, and Outcomes for randomised controlled trial evidence for gastrointestinal and lung neuroendocrine tumours, Subgroup analysis, for further details).
Interventions and comparators
Clinical data identified during the systematic literature review allowed the analyses shown in Table 17 to be carried out. The treatments included in the model were:
-
everolimus
-
sunitinib
-
177Lu-DOTATATE (in scenario analyses only)
-
BSC.
| Tumour location | Treatment | Treatment or comparator | Type of data | Source of data |
|---|---|---|---|---|
| pNETs | Everolimus | BSC | Head-to-head RCT | RADIANT-334 |
| Everolimus | Sunitinib | Indirect comparison | RADIANT-3,34 A618111181 | |
| Sunitinib | BSC | Head-to-head RCT | A618111181 | |
| GI NETs | Everolimus | BSC | Head-to-head RCT | RADIANT-435 |
| Everolimus | 177Lu-DOTATATE | Indirect comparison | RADIANT-4,35 NETTER-1102 | |
| 177Lu-DOTATATE | BSC | Head-to-head RCT | NETTER-1102 | |
| GI and lung NETs | Everolimus | BSC | Head-to-head RCT | RADIANT-435 |
All included treatments are in the NICE scope.
All treatments included in the model are used in NHS clinical practice in England and Wales. Chemotherapy and interferon alpha were both considered as comparators in the NICE scope. However, no evidence on the clinical effectiveness of chemotherapies listed in the scope was identified during the clinical effectiveness systematic literature review (see Chapter 4, Results). Therefore, chemotherapy was not included in our analysis. Following the advice from our clinical experts, the AG did not consider interferon alpha in the economic analysis as it is not commonly used in UK clinical practice.
Perspective, time horizon and discounting
The model perspective was that of the NHS and PSS, in accordance with the NICE reference case. 37 In the base-case analysis, the model time horizon was 40 years, which reflects the lifetime horizon of patients with advanced NETs. Costs and benefits were discounted at 3.5% per annum. The model used a 4-weekly cycle length to facilitate the implementation of the costs of drug acquisition and administration for 177Lu-DOTATATE as these costs were incurred every 4 weeks.
A number of scenario analyses were performed to estimate the effect on outcomes of different survival model structures and data and assumptions around the discount rate and model time horizon (see Appendix 11, Scenario analyses, for further details).
Model parameters
Population characteristics
Mean age
We assumed that all patients were aged 60 years at the start of treatment. The estimate of the mean age of patients in the model population was based on the patient characteristics from the clinical trials used in our analysis. In the model, this affects only age-related utilities and background mortality. Mean age estimates from the companies’ models varied from 61.7 to 63.7 years.
Sex composition
All analyses were performed assuming that the proportion of male patients is 53% (as in RADIANT-334), which affects only background mortality (see Appendix 11, Table 98). The models in the company submissions used proportions of 50–51%.
Background mortality
In the base case, we did not incorporate background mortality in all analyses; we accounted for this in scenario analyses related to 177Lu-DOTATATE. This was because the PFS and OS curves on which the partitioned survival in the model was based were expected to account for background mortality. However, background mortality rises as the modelled cohort ages and, as in some analyses OS data were immature, in those cases the effect of background mortality was taken into account using data for the years 2012–14 from the Office for National Statistics. 125
Models submitted by AAA for GEP NETs allow estimation of the ICER with and without general mortality. When general mortality was taken into account, it was modelled in the subpopulation of patients with stable disease; in the subpopulation of patients whose disease has progressed, background mortality was not modelled. Therefore, death events are double-counted during the stable disease stage and may be underestimated in the progressive disease subpopulation.
In contrast, none of the model-based analyses submitted by Novartis, which considered pancreatic and GI/lung locations, separately modelled background mortality.
Treatment effectiveness and extrapolation
Baseline randomised controlled trials
RADIANT-334 was chosen as the baseline trial for the pNETs population, whereas RADIANT-435 was chosen as the baseline trial for the GI (midgut) NETs and GI/lung NETs analyses. The size of the study populations in these trials was larger and the data were more mature than in A618111181 and NETTER-1. 102 In addition, the control arm in NETTER-1102 received 60 mg of octreotide daily plus BSC, which is outside the NICE scope for this review.
Modelled progression-free survival and overall survival
A partitioned survival approach was used to populate the parameters of the semi-Markov model. PFS Kaplan–Meier curves for the trial arms of the main RCTs informing the company submissions on pNETs (including A6181111 and RADIANT-3), GI/lung NETs (RADIANT-4) and GI (midgut) NETs (RADIANT-4 midgut subgroup and NETTER-1) were extracted from graphs in the latest available source for each trial [peer-reviewed publications for A6181111,81 RADIANT-334 and RADIANT-4;35 a published conference abstract for the RADIANT-4 midgut subgroup;74 and an industry submission (NETTER-1)] using digitising software (DigitizeIt version 2.2.2; Braunschweig, Germany). The extracted data were used to recreate the associated original individual patient data using the Guyot algorithm126 implemented in R (The R Foundation for Statistical Computing, Vienna, Austria).
A range of parametric curves from the proportional hazards (Weibull, exponential and Gompertz), piecewise proportional hazards (restricted cubic splines with five pieces or knots) and accelerated failure time (AFT) (log-normal, log-logistic and generalised gamma) families were fit to the recreated individual patient data for each arm separately and evaluated for use in the base-case analysis according to goodness of fit criteria [Akaike information criterion (AIC) and Bayesian information criterion (BIC)], visual fit to the empirical data (i.e. the instantaneous probability of event occurrence and Kaplan–Meier curve), plausibility of long-term extrapolation and consistency between PFS and OS (i.e. no crossing of PFS and OS curves of the same trial arm). We also consulted our clinical experts for their opinion about the plausibility of the long-term extrapolations associated with candidate functions. Finally, for our base-case analysis, we adopted the recommended practice that the same parametric function be used to extrapolate trial data for all arms in a comparison, to avoid introducing subjective assumptions in the long-term effectiveness estimates. 127 We relaxed this restriction in scenario analyses. The following is a summary of the main results of the two time-to-event outcomes in each of the three locations analysed.
In the RCT sources of effectiveness data for the pNETs model, treatment switching from the placebo arm to the active treatment arm was observed after disease progression (89% in RADIANT-334 and 65% in A618111181). Therefore, the following analyses for pNETs are based on Kaplan–Meier OS curves adjusted for crossover, using the RPSFT method (Robins and Tsiatis128). This approach affects only the placebo arm of each trial and produces a counterfactual placebo Kaplan–Meier curve, that is, the curve that would have occurred had no patient switched to the active arm.
In contrast, the analysis of GI or lung NETs is based on RADIANT-4,35 in which 6% of placebo patients switched to the active arm after disease progression. As no RADIANT-4 OS data were identified in which treatment switching in the placebo arm was adjusted for, the following analyses are based on the most recent ITT OS curves reported for RADIANT-435 (cut-off date 20 November 2015). As for the PFS Kaplan–Meier curves used in our analysis, switching to new active antineoplastic therapy before disease progression occurred in (confidential information has been removed) of patients in the everolimus arm and (confidential information has been removed) in the placebo arm (central radiological review), and these cases were censored from the analysis at the time of switch [RADIANT-435 final clinical study report (CSR), p. 71].
The statistical analysis used to select parametric survival functions for modelling PFS and OS outcomes and to extrapolate beyond the trial follow-up periods is presented next.
Pancreatic neuroendocrine tumours
In general, accelerated failure time models had a better fit than other models to the observed PFS data from the RADIANT-334 treatment arms, but their advantage was not significant (i.e. BIC difference of < 5 points between the Weibull and the log-logistic functions) in the everolimus arm (Table 18). The fact that the best model for the placebo arm of RADIANT-334 was the restricted cubic spline function (with six segments) suggests that the other models may not be valid representations of the trial data for that arm. The exponential function was the model with the best (i.e. lowest) goodness of fit statistic for sunitinib in A618111181 (see Table 18), although no significant differences were found between models. On the basis of this and the available evidence discussed in the following section, and for consistency across arms, in the base-case analysis the model adopted the Weibull function for the everolimus plus BSC and BSC-only arms and the exponential function (i.e. Weibull with the shape parameter set to the value of 1) for the sunitinib plus BSC arm.
| Model | Everolimus plus BSCa(n = 207) | Sunitinib plus BSCb(n = 86) | Placebo plus BSCa(n = 203) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of parameters | AIC | BIC | Number of parameters | AIC | BIC | Number of parameters | AIC | BIC | |
| PFS | |||||||||
| Weibull | 2 | 416 | 422 | 2 | 178 | 183 | 2 | 465 | 472 |
| Exponential | 1 | 424 | 428 | 1 | 176 | 179 | 1 | 488 | 492 |
| Gompertz | 2 | 422 | 429 | 2 | 177 | 182 | 2 | 485 | 491 |
| Log-normal | 2 | 413 | 420 | 2 | 174 | 179 | 2 | 440 | 447 |
| Log-logistic | 2 | 412 | 419 | 2 | 176 | 181 | 2 | 443 | 450 |
| Gamma | 3 | 414 | 424 | 3 | 172 | 180 | 3 | 440 | 450 |
| Spline | 6 | 419 | 439 | 6 | 169 | 183 | 6 | 374 | 394 |
| OS | |||||||||
| Weibull | 2 | 554 | 561 | 86 | 237 | 242 | 203 | 396 | 403 |
| Exponential | 1 | 555 | 558 | 86 | 235 | 238 | 203 | 399 | 402 |
| Gompertz | 2 | 555 | 561 | 86 | 237 | 242 | 203 | 401 | 407 |
| Log-normal | 2 | 560 | 567 | 86 | 233 | 238 | 203 | 387 | 394 |
| Log-logistic | 2 | 557 | 563 | 86 | 234 | 239 | 203 | 393 | 399 |
| Gamma | 3 | 556 | 566 | 86 | 235 | 242 | 203 | 385 | 395 |
| Spline | 6 | 560 | 580 | 86 | 238 | 252 | 203 | 390 | 410 |
In contrast, proportional hazard models had a better fit to the OS data, with the exception of OS in the placebo arm of RADIANT-3,34 which was best represented by the log-normal function according to the BIC statistic (see Table 18). The model adopted the Weibull function for the everolimus plus BSC arm and exponential functions for the sunitinib plus BSC and BSC-only arms in the base-case analysis.
Progression-free survival
Our base-case analysis adopted the Weibull function for PFS outcomes for the everolimus plus BSC and BSC-only arms and the exponential function for the sunitinib plus BSC arm. In scenario analyses we adopted the log-logistic, exponential and log-normal functions for the everolimus plus BSC, sunitinib plus BSC and BSC-only arms respectively.
Everolimus plus best supportive care
The log-logistic model has the most favourable goodness-of-fit results (i.e. lowest value of information criteria) for the data from the everolimus arm of RADIANT-3,34 although its advantage over the log-normal and Weibull models is not statistically significant (see Appendix 12, Figure 38). The log-logistic model also follows the shape of the instantaneous risk (hazard) of progression or death (see Appendix 12, Figure 38).
The log-logistic and log-normal models perform similarly to each other in fitting the Kaplan–Meier PFS curve, with the Weibull model fitting the data almost as well as the log-logistic and log-normal models. However, by the end of the observed follow-up period, the risk of progression or death with the Weibull model is increasing, whereas the risk of progression or death with the log-normal and log-logistic models is declining (see Appendix 12, Figure 38).
By the end of the observation period, almost 2 years after randomisation, 20% of patients in the everolimus arm are alive and their disease has not progressed (Figure 12). Thus, adopting the log-logistic or log-normal model has noticeable implications for the long-term modelling of life free of disease progression. By the end of a 10-year follow-up, 3.5% of patients would be alive and progression free with the log-logistic or log-normal (not shown) model, whereas according to the Weibull model all patients would have progressed or died by 6 years after randomisation (Figure 13).
FIGURE 13.
Progression-free survival in the everolimus arm in RADIANT-3:34 extrapolation to 10 years.
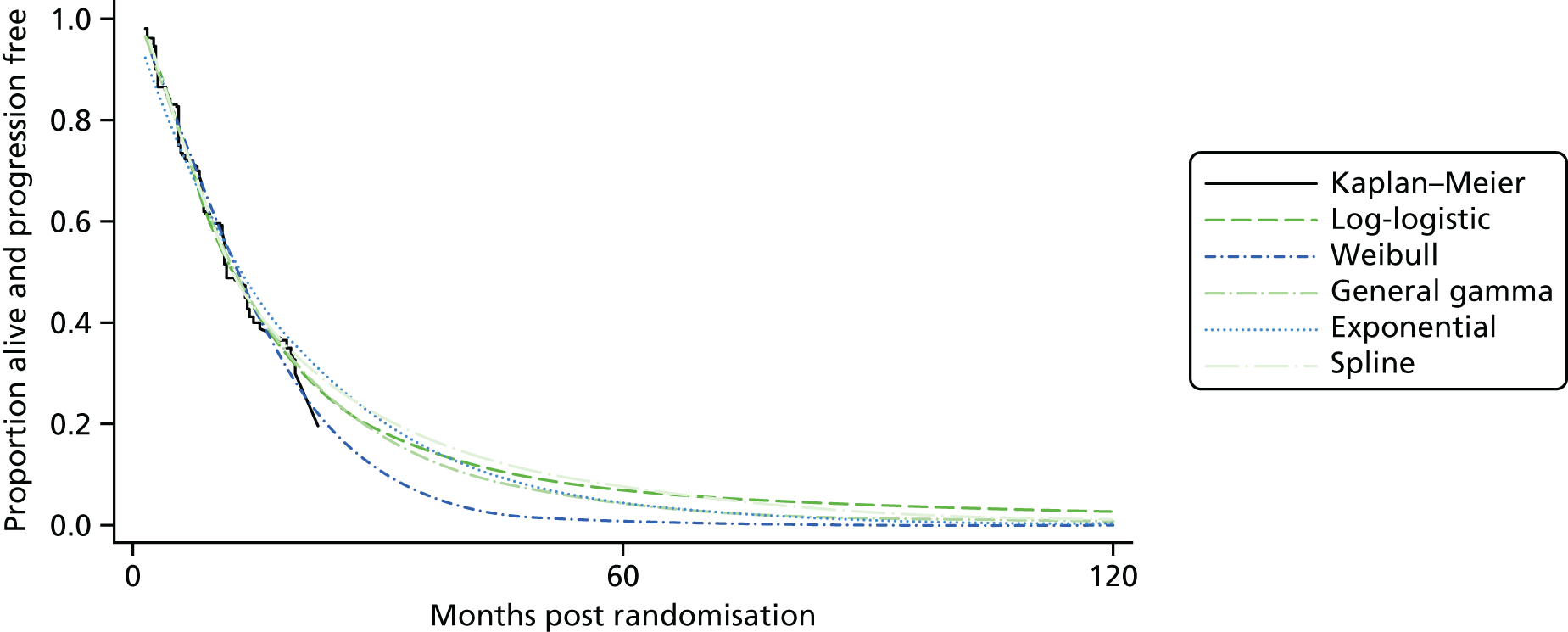
Best supportive care alone
The BSC-only arm was modelled from data from the placebo arm in RADIANT-3. 34 The information criteria favour the log-normal, log-logistic and generalised gamma models over the rest (see Table 21). The AIC and BIC statistics, however, do not discriminate between the favoured models (their magnitudes differ by < 5 points).
The hazard function is non-constant and non-monotonic, which suggests that the Weibull and exponential models are inappropriate models for these data (see Appendix 12, Figure 39). The information criteria statistics are consistent with this observation and suggest that the log-normal or gamma model fit the data best.
Consistent with the model diagnostics of Table 21, the generalised gamma model is a closer match to the hazard function (see Appendix 12, Figure 39). The log-normal model approximates the smoothed hazard in Figure 39 (see Appendix 12), except for the drop between week 20 and week 40.
As illustrated in Figure 14, the Weibull model underestimates PFS early on, overestimates it in the medium term and underestimates it in the final part of the analytical horizon. The log-normal and generalised gamma models differ only in the final part; the log-normal model appears to fit better the final part of the Kaplan–Meier curve. Nevertheless, the choice of curve has little impact on mean PFS in this case.
Sunitinib plus best supportive care
As presented in Figure 15, for the PFS data from the sunitinib arm of A6181111,81 the generalised gamma function provides the best diagnostic results, although the differences between the generalised gamma model and the exponential and log-normal models are not significant.
As depicted in Figure 40 (see Appendix 12), the generalised gamma model consistently underestimates the risk of progression, whereas the exponential model, with its constant risk, fits the pattern of risk up to approximately week 30 and underestimates it thereafter. The log-logistic model seems to follow the shape of the hazard function for longer periods than the other models.
The implications of adopting one of these curves to extrapolate outcomes for cost-effectiveness analysis is illustrated in Figure 16. The generalised gamma model’s parametric flexibility appears to produce an overly optimistic forecast of approximately 35% of patients still alive and not experiencing disease progression after 10 years. The exponential model, in contrast, predicts that by 5 years 95% of people have experienced progression or died. The predictions of the log-logistic model fall in between the predictions of the generalised gamma model and the exponential model, but much closer to the exponential forecast than the generalised gamma forecast.
FIGURE 16.
Progression-free survival in the sunitinib arm: extrapolation to 10 years.

The apparent contradiction between the diagnostic results, which suggest that the generalised gamma function fits the data best, and the hazard and survival function fits, which suggest that the log-logistic form is superior, appears to be determined by the ability of the gamma form to fit the data better in the early follow-up period, when more observations are available (e.g. number at risk: 85 at baseline vs. 39 at 22 weeks). Its poor ability to match the risk (hazard) profile and the latter part of the survival curve suggests, however, that it overfitted the data. The exponential curve was thus selected for the base-case analysis.
Adjustment for indirect comparisons
To derive estimates of PFS time for the sunitinib arm that were comparable to the PFS estimates in RADIANT-3,34 the sunitinib parametric PFS distribution was adjusted by the ratio of the area under the non-parametric Kaplan–Meier curve of the placebo arm in A618111181 to the area under the non-parametric Kaplan–Meier curve of the placebo arm in RADIANT-334 at the shortest of the maximum follow-up times across the two placebo arms. This method was preferred to the common alternative approach of using the extrapolated means, which by definition are affected by the choice of parametric function as opposed to being determined solely by the observed data, as in our ‘restricted means’ approach. Thus, in the base case, in which the sunitinib PFS parametric distribution was the exponential, the sunitinib PFS parametric distribution function was adjusted according to the equation:
where λ^s is the adjusted hazard function of the exponential time-to-disease progression or death distribution, λs is the hazard function of the exponential distribution estimated from the sunitinib arm of A618111181 and the AUC(tmin{TAp,TRp)Ap and AUC(tmin{TAp,TRp)Rp functions are the area under the Kaplan–Meier curve of the placebo arms of A618111181 and RADIANT-3,34 respectively, evaluated at the shortest of the maximum observation times in the placebo arms of the two trials (i.e. 65 weeks). This is illustrated in Figure 17, where the vertical discontinuous line denotes the 65th-week time point. At this point, the mean PFS in the placebo arm is 25.69 weeks in A618111181 and 28.85 weeks in RADIANT-3. 34 Thus, the PFS distribution of the sunitinib arm in the base case is (t)=exp(−λ^t)=exp(−0.01197*t). The same approach was used for OS.
FIGURE 17.
Progression-free survival in the placebo + BSC arms of pNETs trials.
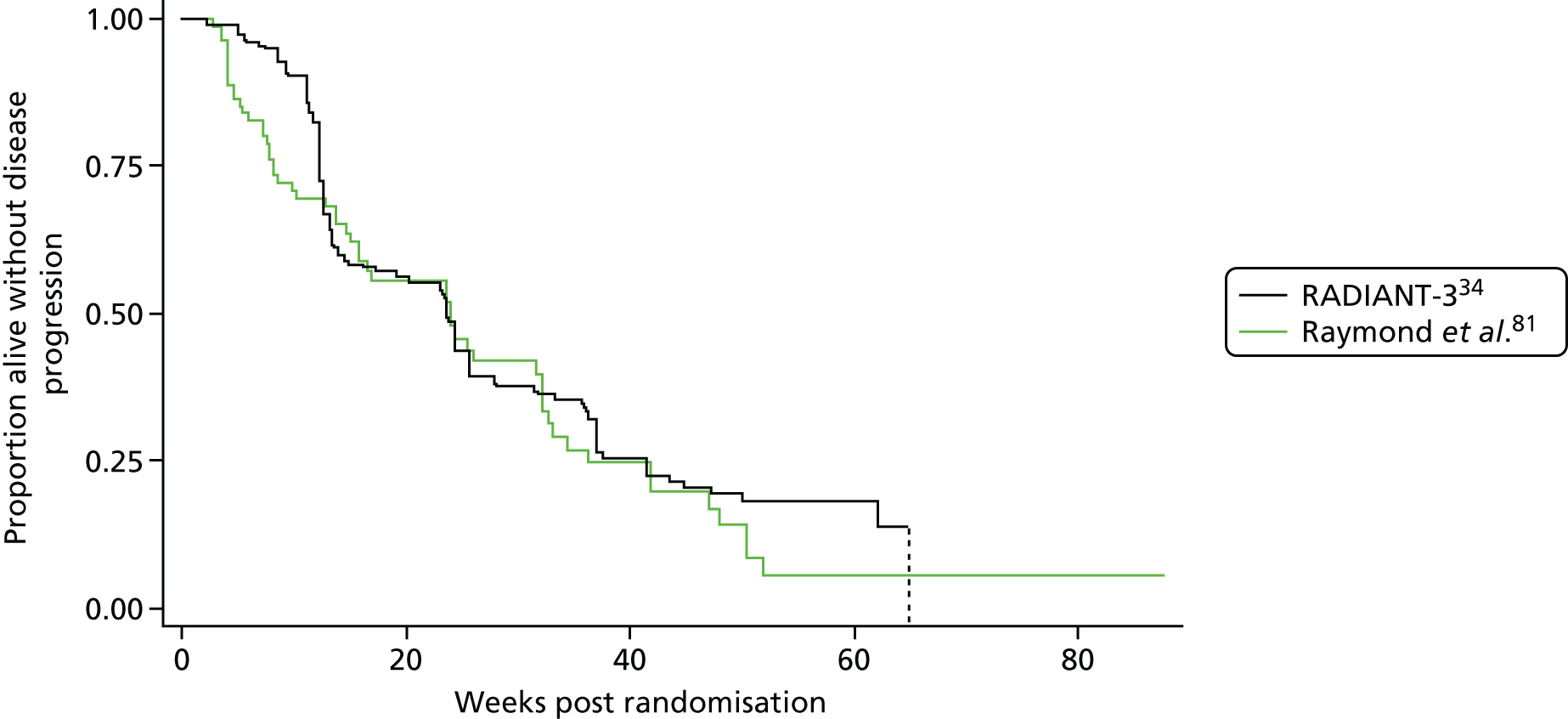
Individual patient data provided by Pfizer to the AG for this assessment allowed us to investigate the robustness of indirect comparisons of PFS outcomes between the sunitinib arm in A618111181 and the everolimus and placebo arms in RADIANT-3. 34 We conducted a MAIC following the methods described in a previous study by Signorovitch et al. 129 We obtained similar results (which are academic-in-confidence) to those reported here.
Overall survival
Using similar criteria to those described for the analysis of PFS, the diagnostics information in Table 18 and visual fit in Figures 41–43 (see Appendix 12), and extrapolated survival rates in Figures 18–20, resulted in the exponential model being chosen for the everolimus arm, the exponential for the sunitinib and the exponential for the BSC-only arm of RADIANT-3. 34 The resulting equation for sunitinib was then matched to the RADIANT-334 arms using the same scaling method as described for the PFS time-to-event analysis. For scenario analyses, the OS function for the BSC-only arm was changed to the log-normal function while keeping the exponential function for the active treatment arms.
Everolimus plus best supportive care
As illustrated in Figure 18, according to the exponential function, by 15 years (180 months) 4% of patients initially treated with everolimus remain alive, whereas according to the log-normal survival curve, 10% of patients would be alive at that time point. In its submission, Novartis33 cites an estimated 15-year survival rate of 6% for patients with advanced pNETs from the Surveillance, Epidemiology, and End Results Program (SEER) database, and considers as plausible extrapolating only those curves predicting survival rates above that level. However, there is the caveat that:
the RADIANT-3 trial only included patients with well- or moderately-differentiated tumours with radiologic progression within the 12 months prior to entry in the study. (Confidential information has been removed.) In contrast, patients in the SEER registry are followed from diagnosis, and are therefore likely to be treatment-naïve. Also SEER includes all advanced patients regardless of tumour grade and SEER data were derived over a relatively long period beginning in 1973 whereas follow-up in RADIANT-3 began in 2009.
Novartis submission, p. 9333
[In English life tables, the 15-year survival rate in the general population aged 60 years increased from 56% in males and 74% in females in 1980–2, to 75% and 83%, respectively, in 2006–8 (Office for National Statistics130)]. The company states that the net impact of these differences in patient characteristics is unknown. It concludes that ‘it is reasonable to assume that survival in the everolimus arm of the RADIANT-334 trial would not be substantially less than that for patients with advanced pNETs in the SEER registry’ (Novartis submission, p. 9333).
Best supportive care alone
The log-normal function had the best fit to the data of the placebo plus BSC arm in RADIANT-3. 34 The generalised gamma function had a good visual fit to the risk of death observed in the trial period (see Appendix 12, Figure 42) but an overly optimistic 20-year OS rate of 20% (Figure 19). The exponential function underestimated the hazard risk throughout the trial period (see Appendix 12, Figure 42), but had a 20-year PFS in the middle of those depicted in Figure 19.
FIGURE 19.
Rank-preserving structural failure time (RPSFT)-adjusted OS in the placebo arm of RADIANT-3:34 extrapolation to 20 years.
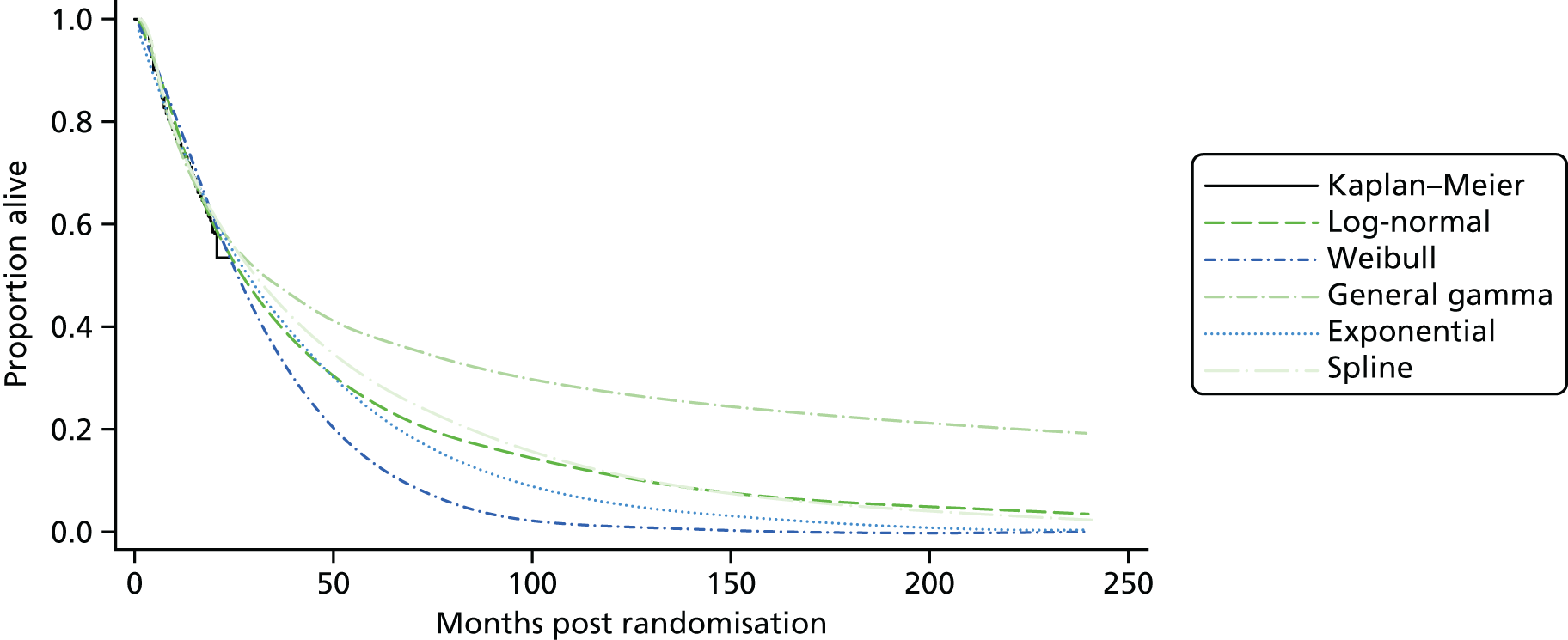
Sunitinib plus best supportive care
The log-normal and exponential functions had the best fit to the OS data for the sunitinib arm in A618111188 (Table 21), although the log-normal function tracked the risk of death (hazard) observed in the trial better than the exponential function (see Appendix 12, Figure 43). The projected 15-year survival rate with the log-normal function is > 10%, whereas with the exponential function it is 4.7% (see Figure 20). However, the 15-year OS rate used in the model was higher than that seen with the exponential function as, after adjusting the exponential function for the difference in the placebo arms of the A618111181 and RADIANT-334 trials (see the following section), the OS rate was 9.7%.
Adjustment for indirect comparison
As before, to derive comparable OS estimates for the treatments in RADIANT-3,34 the OS exponential curve for sunitinib was adjusted to reflect the difference in RPSFT-adjusted OS between the placebo arm of A618111181 and the placebo arm of RADIANT-3. 34 Figure 21 illustrates the difference, and the vertical discontinuous line marks the point at which the area under the curve calculation was restricted for both arms (24 months).
FIGURE 21.
Kaplan–Meier RPSFT-adjusted OS in the placebo + BSC arms of the pNETs trials.
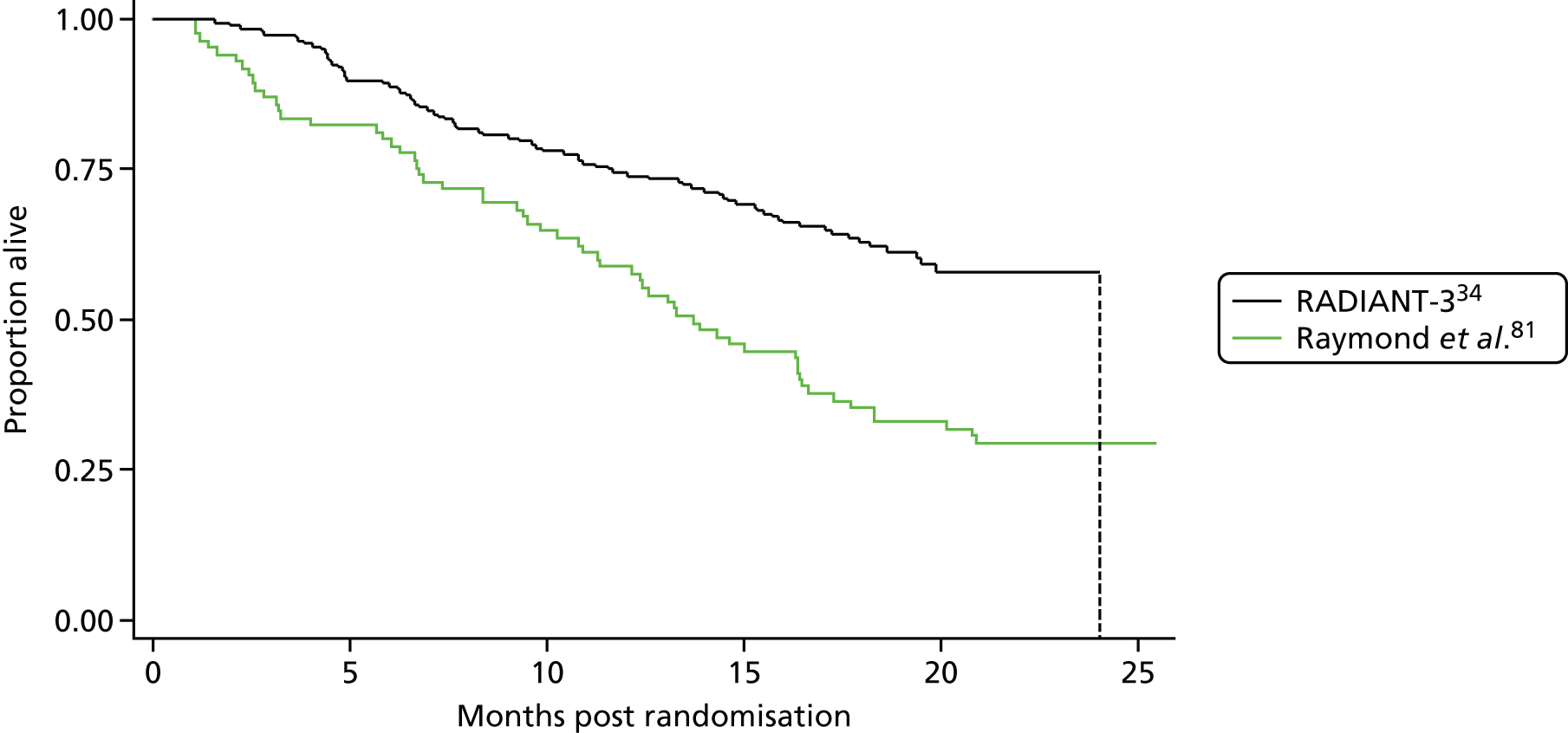
Gastrointestinal and lung neuroendocrine tumours
In gastrointestinal and lung NETs, the model diagnostics suggested that the log-normal model had the best fit to the PFS data in the everolimus arm of RADIANT-4,35 whereas the two-parameter models were inferior to the three-parameter gamma or six-parameter spline model were the best fit to the PFS data in the placebo arm of RADIANT-435 (Table 19). In contrast, the exponential, Gompertz and Weibull models fitted the OS data as well as or better than other models.
| Model | Everolimus + BSCa (n = 205) | Placebo + BSCa (n = 97) | ||||
|---|---|---|---|---|---|---|
| Number of parameters | AIC | BIC | Number of parameters | AIC | BIC | |
| PFS | ||||||
| Weibull | 2 | 456 | 463 | 2 | 258 | 263 |
| Exponential | 1 | 461 | 465 | 1 | 256 | 259 |
| Gompertz | 2 | 462 | 469 | 2 | 253 | 258 |
| Log-normal | 2 | 446 | 453 | 2 | 237 | 242 |
| Log-logistic | 2 | 450 | 456 | 2 | 240 | 246 |
| Gamma | 3 | 447 | 457 | 3 | 211 | 218 |
| Spline | 6 | 449 | 469 | 6 | 198 | 213 |
| OS | ||||||
| Weibull | 2 | 340 | 347 | 2 | 194 | 199 |
| Exponential | 1 | 346 | 350 | 1 | 192 | 195 |
| Gompertz | 2 | 338 | 345 | 2 | 194 | 199 |
| Log-normal | 2 | 348 | 355 | 2 | 193 | 198 |
| Log-logistic | 2 | 342 | 349 | 2 | 193 | 198 |
| Gamma | 3 | 341 | 351 | 3 | 195 | 202 |
| Spline | 6 | 344 | 364 | 5 | 198 | 210 |
Progression-free survival
In the base-case analysis, the Weibull function was chosen for the everolimus plus BSC arm and the BSC-only arm based on data from RADIANT-4. 35 In scenario analyses, the generalised gamma function was used instead for both model arms. See Appendix 13 for details.
Overall survival
The base-case analysis adopted exponential distributions separately fitted to OS data in the everolimus arm and the placebo arm of RADIANT-4. 35 The Gompertz and Weibull distributions had the best goodness of fit statistics and seemed to provide the best fit to the everolimus hazard rates (see Appendix 12, Figure 46) and Kaplan–Meier curves (Figure 22), whereas the exponential function seemed to be the best fit to the placebo data (see Figures 23 and 47). However, only the extrapolations of the exponential and log-logistic distributions seemed plausible, as discussed in the following two sections. In scenario analyses, log-logistic distributions separately estimated for the two trial arms were adopted.
Everolimus plus best supportive care
To reflect the uncertainty resulting from immature data, Figure 22 presents the OS extrapolations for all available parametric curves. The exponential function appears to overestimate the risk of death (see Appendix 12, Figure 46) and underestimate the Kaplan–Meier OS curve (see Figure 22) in the early part of the trial observation period35 (data cut-off 30 November 2015), although the discrepancy is within the sampling error (95% CI, not presented). The exponential curve crosses the log-logistic curve twice, once during the interpolation (within-trial) period and once in the late extrapolation (beyond-trial) period. In its submission to NICE, Novartis33 turns to external data to inform its choice of survival curves. In particular, it states that:
Analysis of distant NET cases diagnosed between 1997 and 2012 in the SEER database (a large population-based registry in the USA) suggests that survival for patients with distant disease at diagnosis at 15 years is approximately 10% (Data unpublished). Although it is difficult to make comparisons between the RADIANT-4 trial population and the available SEER data (see Appendix 9 of Novartis submission for further details), it is reasonable to assume that survival in the placebo plus BSC arm of the RADIANT-4 trial is likely to be no less than that of patients with distant disease in the SEER database given improvements in survival over time in patients with NET.
Novartis submission (p. 137)33
In appendix 9 of the Novartis submission, Novartis33 reports the data and methods used to obtain its 10% survival benchmark at 15 years; Kaplan–Meier survival curves by location from SEER were weighted according to the distribution of patients by location in RADIANT-4. 35 Novartis also acknowledges the limitations of these SEER data, as discussed earlier for pNETs.
Gastrointestinal (midgut) neuroendocrine tumours
The base-case analysis of the GI (midgut) location was populated with data from the head-to-head comparison of everolimus plus BSC and placebo plus BSC in RADIANT-435 for the GI (midgut) population subgroup73 (Table 20).
| Model | Everolimus plus BSCa (n = 80) | 177Lu-DOTATATE plus BSCb (n = 116) | Placebo plus BSCa (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of parameters | AIC | BIC | Number of parameters | AIC | BIC | Number of parameters | AIC | BIC | |
| PFS | |||||||||
| Weibull | 2 | 149 | 154 | 2 | 139 | 145 | 2 | 87 | 90 |
| Exponential | 1 | 151 | 153 | 1 | 140 | 143 | 1 | 85 | 87 |
| Gompertz | 2 | 150 | 155 | 2 | 141 | 146 | 2 | 87 | 90 |
| Log-normal | 2 | 149 | 154 | 2 | 138 | 143 | 2 | 83 | 86 |
| Log-logistic | 2 | 150 | 154 | 2 | 139 | 144 | 2 | 85 | 88 |
| Gamma | 3 | 151 | 158 | 3 | 139 | 148 | 3 | 82 | 87 |
| Spline | 6 | 151 | 158 | 6 | 140 | 149 | 6 | 87 | 96 |
| OS | |||||||||
| Weibull | 2 | NA | NA | 2 | 99 | 105 | 2 | NA | NA |
| Exponential | 1 | NA | NA | 1 | 98 | 101 | 1 | NA | NA |
| Gompertz | 2 | NA | NA | 2 | 99 | 105 | 2 | NA | NA |
| Log-normal | 2 | NA | NA | 2 | 99 | 105 | 2 | NA | NA |
| Log-logistic | 2 | NA | NA | 2 | 99 | 105 | 2 | NA | NA |
| Gamma | 3 | NA | NA | 3 | 101 | 109 | 3 | NA | NA |
| Spline | 6 | NA | NA | 6 | 102 | 119 | 6 | NA | NA |
Progression-free survival
In the base-case analysis the exponential distribution was adopted to model PFS outcomes in the everolimus arm and the placebo arm of RADIANT-4. 73 PFS in the 177Lu-DOTATATE arm of NETTER-1102 was also modelled with an exponential distribution (see Appendix 11 for scenario analyses).
Everolimus plus best supportive care
The exponential function, which was the function with the best statistical fit (Figure 24), appeared to have a poor fit to the hazard rates for everolimus in RADIANT-473 (see Appendix 12, Figure 48). However, this is caused by the small sample available in the latter part of the RADIANT-473 follow-up period. Of the candidate functions, the log-normal function has the longest PFS duration, followed by the exponential and the Weibull functions (see Figure 24).
FIGURE 24.
Progression-free survival in the everolimus arm of RADIANT-435 [GI (midgut)]: extrapolation to 20 years.
Best supportive care alone
The diagnostic statistics (see Table 20) did not discriminate between the [accelerated failure time (AFT) or proportional hazards] models available to represent the PFS data in the placebo arm of RADIANT-4,73 although the generalised gamma and log-normal functions displayed PFS hazard rates that were similar to those observed during the trial (see Appendix 12, Figure 49). When turning to the extrapolation of PFS, the generalised gamma function seems to result in overly optimistic disease PFS rates (see Figure 25). In scenario analyses, the log-normal distribution was used instead of the exponential to model the PFS experience of patients in the placebo arm.
FIGURE 25.
Progression-free survival in the placebo arm of RADIANT-435 [GI (midgut)]: extrapolation to 20 years.

Overall survival
In the absence of data, the base-case analysis currently assumes that the OS curve for everolimus in the GI (midgut)-only location is the exponential OS curve estimated in the GI or lung patient population in the everolimus arm of RADIANT-4,35 discussed earlier, adjusted by the proportional difference in mean PFS in the everolimus arm of RADIANT-435 between the overall GI/lung patient population and the subgroup of GI (midgut) patients. Likewise, the OS curve for the BSC-only arm of RADIANT-435 in the GI (midgut)-only population is derived from adjusting the exponential function fitted to the OS data from the everolimus arm of RADIANT-435 in the GI/lung population considered above by the proportional difference in mean PFS between the overall GI/lung and the GI (midgut) patient groups. These derivations follow the same steps as in Equation 1. We requested OS data for the GI (midgut)-only location from Novartis, but the company did not provide them to us.
Adverse events
The probabilities of AEs were used to estimate costs in the stable disease state. For the economic evaluation of treatments for pNETs, probabilities of AEs were derived from rates estimated from our indirect comparison of treatment-related grade 3/4 AEs of ≥ 2% incidence in any active treatment arm (see Chapter 4, Indirect treatment comparison: pancreatic neuroendocrine tumours; Appendix 21, Table 136 and Appendix 9). We updated these analyses with data provided in the Pfizer submission to NICE. 32 For the GI/lung analysis, the AG model adopted the probabilities in the Novartis model submitted to NICE,33 as these were calculated with individual patient data not available to the AG. For everolimus plus BSC and BSC only in the GI (midgut) evaluation, we adopted the grade 3/4 AE rates for the everolimus and placebo arms reported in a recent conference poster by RADIANT-4 investigators;73 for 177Lu-DOTATATE we used the grade 3/4 AE rates reported in the AAA submission to NICE. 31
The AE probabilities for the pNETs model were obtained by assuming that patients had no multiple instances of the same AE type and that AEs lasted for only one cycle. This seems a reasonable assumption in the light of the evidence in the CSR for A6181111,81 which reports the actual duration of the grade 3/4 AEs recorded in the trial (tables 5, 24, 26 and 29 in the full CSR of A61811181). For GI/lung and GI (midgut) NETs the same assumption was adopted.
Based on calculations by the AG the measured differences in grade 3/4 AEs between everolimus and sunitinib in patients with pNETs considered by Novartis in its submission,33 and for which disutility values are available (see Utility values for the Peninsula Technology Assessment Group model for details), are associated with negligible differences in utility, equal to a 0.002 quality-adjusted months, and were therefore not used in calculating utility values for stable disease in the pNETs model. For GI/lung and GI (midgut) analyses, the available utility values, which were derived from patient-reported outcomes in RADIANT-435 and evidence from the ERASMUS study31 submitted to NICE by AAA, were assumed to capture the impact of AEs.
Modelling post progression
Based on data from RADIANT-334 for pNETs and RADIANT-435 for GI and lung NETs, which we assumed applied to GI (midgut) NETs, in the base-case analysis we assumed that all patients have BSC after progression on initial treatment. This consists of palliative care and 30 mg of octreotide for symptomatic treatment, with no active drug treatment.
Subsequent treatments were allowed in the post-progression phase and were applied as a fixed cost in the first cycle after disease progression. The frequency of subsequent treatment use was assumed to be zero in the base-case analysis; scenario analyses considered applying the same costs of subsequent treatments as in the pNETs and GI and lung models from Novartis. 33 This choice of base case reflected the fact that (1) the A618111181 trial of sunitinib did not collect information on subsequent treatments, which led Novartis to apply the same costs of subsequent treatments to both arms, and (2) the way that Novartis implemented subsequent treatment costs in its models is unreliable (see Chapter 5, Critical appraisal of the company submissions). In the GI and lung and GI (midgut)-only analyses, we adopted the same costs and implementation of subsequent treatments as in the GI and lung model of Novartis, which used detailed information on differences between trial arms in the frequency of treatment use post progression in RADIANT-4. 35 As we had no information for 177Lu-DOTATATE, we assumed that it had the same subsequent treatment costs as applied to everolimus.
The costs of disease monitoring in our model were obtained from the pNETs and GI and lung models from Novartis. 33 Based on the opinion of our clinical experts, we adopted a smaller number of visits for the GI and lung evaluation than were used by Novartis in its model of the same location (see Costs of medical management and disease monitoring).
Systematic review of utilities
The methods used to identify utility parameter values for the model are reported in Appendix 15.
Utility values for the Peninsula Technology Assessment Group model
Of the eight independent sources of data identified through the systematic search of utility studies, only a limited proportion of evidence was suitable to populate the PenTAG cost-effectiveness model. This was mainly because of the differences in the treatments being evaluated and the discrepancies between the definitions of HRQoL outcome measures in the studies and the model’s health states.
The utility values used in the PenTAG cost-effectiveness model and their sources are presented by tumour location, in accordance with the NICE scope. No utility values for HRQoL outcomes measured in pNET patients under treatment with everolimus were found (Table 21). The only available estimates for everolimus in pNETs were those reported by the preference elicitation study of Swinburn et al. ,131 who asked approximately 100 members of the general public to assess descriptors (vignettes) of pre-progression and post-progression health states of patients with GI NETs and pNETs, using the time trade-off method. This study also elicited utility decrements resulting from the occurrence of some of the most common AEs associated with treatment therapies (see Appendix 10, Pancreatic studies, for details; AEs considered include neutropenia, hypertension, palmar–plantar erythrodysaesthesia syndrome, leukopenia, diarrhoea, stomatitis, thrombocytopenia, anaemia, hyperglycaemia, fatigue, infections, pneumonitis and nausea). In the base-case analysis submitted to NICE, Novartis33 used the utility value reported by this source for the pre-progression health state, adjusted for the disutility associated with the incidence of the common types of grade 3 or 4 AEs in RADIANT-3. 34 As these values do not meet the NICE reference case37 we considered them only in scenario analyses.
| Pre progression | Post progression | |||||
|---|---|---|---|---|---|---|
| Everolimus + BSC | Sunitinib + BSC | Placebo | Everolimus | Sunitinib | Placebo | |
| n | NA | 86 | 85 | NA | 86 | 85 |
| Mean utility | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| SE | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Source | Assumed to be equal to that for sunitinib + BSC | Analysis by the AG of individual patient data from A618111181 provided by the manufacturer | Analysis by the AG of individual patient data from A618111181 provided by manufacturer | Assumed to be equal to that for sunitinib + BSC | Analysis by the AG of individual patient data from A618111181 provided by manufacturer | Analysis by the AG of individual patient data from A618111181 provided by manufacturer |
| Alternative values | 0.749 | 0.749 | 0.771 | 0.612 | 0.612 | 0.612 |
| Source | Value from Swinburn et al.131 times the ratio between the value for sunitinib and that for BSC in A618111181 | Assumed to be the same as that for everolimus | Value from Swinburn et al.131 – AE adjusted | Value from Swinburn et al.131 | Assumed to be the same as that for everolimus | Value from Swinburn et al.131 |
In the absence of HRQoL outcomes measured in pNET patients treated with everolimus, the base-case value was assumed to be the same as for sunitinib (see Table 21 and its description). This assumption was adopted after calculating the net difference in disutility from grade 3/4 AEs between everolimus and sunitinib according to the disutility values reported by Swinburn et al. ,131 which was equal to 0.002 quality-adjusted months. Giving the uncertainty associated with other parameters in the model, including the limited quality of AE data available for economic evaluation purposes, we considered such a difference insignificant.
Estimates of the utility of pNET patients undergoing treatment with sunitinib in both the pre-progression and the post-progression health states (see Table 21) were obtained by mapping individual patient data onto responses to the EORTC QLQ-C30 from A618111181 provided by the manufacturer (Pfizer, data request through NICE) using the algorithm of McKenzie and van der Pol. 132 Using these data the utility values that the company used in the model-based cost-effectiveness evidence submitted to NICE were validated. Although the methods used by the company were not properly described in the submission itself,32 the values used and the methods had been reported in the study by Mucino Ortega et al. 121 The validation exercise therefore sought to replicate the utility values submitted to NICE by following the methods described by Mucino Ortega et al. 121 This consisted of fitting a linear mixed-model equation to the EORTC QLQ-C30 data mapped to the EuroQol-5 Dimensions (EQ-5D) using the algorithm developed by McKenzie and van der Pol132 to estimate the effect of random group allocation (sunitinib vs. placebo) on EQ-5D utilities, adjusting for baseline EQ-5D score and treatment cycle (information available from the authors). Confidential information has been removed. Nonetheless, the estimate obtained employing the model is an approximation, as the estimate of utility in progressive disease was based only on data for the end-of-treatment follow-up time point relating to the placebo arm. This approximation relies on the fact that > 90% of patients in the placebo arm had progressed by the end of the study.
Confidential information has been removed. This difference is the result of the incorrect use of the data in the analysis conducted by the manufacturer of sunitinib (Pfizer) in its submission to the Scottish Medicines Consortium (SMC)133 and used by Novartis in its sensitivity analysis of the economic evaluation submitted to NICE. 33 The company incorrectly used baseline utility as the utility for the pre-progression health state, thus omitting the effects of treatment on patients’ utility during stable disease. In contrast, our replication of the utility values in Mucino Ortega et al. ’s121 study led us to the following estimated linear mixed-model equation for stable disease:
where EQ-5Dm is the mapped utility score from the EORTC QOLQ-C30 in A6181111,81 using the algorithm from McKenzie and van der Pol. 132 To estimate utilities for the two trial arms in the stable disease state, this model was fitted to data excluding the last follow-up, that is, the end-of-treatment follow-up observations, when some patients may have experienced disease progression, resulting in their withdrawal from treatment.
For GI and lung NETs patients under treatment with everolimus (Table 22), we used unpublished treatment-specific utility values, which were presented by Novartis as part of the evidence submitted to NICE and used by the company in sensitivity analyses of its economic evaluation. These data were preferred to published estimates from pooled analysis74 (i.e. utilities by health state regardless of treatment arm) used by the company in its base-case economic model submission, as they incorporate the impact of treatment-specific AEs and comorbidities on HRQoL. The treatment-specific utilities in the company’s submission were based on unpublished individual patient data from RADIANT-435 that were not available to us for review. To validate such estimates, we mapped mean FACT-G scores in RADIANT-4,35 reported by Singh et al. 74 in a poster also reporting pooled utilities by health state, using a linearised version of the algorithm from Longworth et al. 134 (see Appendix 15). The values that we obtained were approximately equal to those produced by the company from individual patient data in the pooled analysis with the original, non-linear mapping algorithm (see Appendix 22).
| Pre progression | Post progression | |||||
|---|---|---|---|---|---|---|
| Everolimus + BSC | Placebo + BSC | 177Lu-DOTATATE | Everolimus + BSC | Placebo + BSC | 177Lu-DOTATATE | |
| n | 837 | 281 | 227 | 238 | 143 | 111 |
| Mean utility | 0.767 | 0.807 | 0.77 | 0.725 | 0.725 | 0.725 |
| SE | 0.010 | 0.015 | 0.005 | 0.010 | 0.010 | 0.010 |
| Source | Treatment arm analysis using individual patient data from RADIANT-435 (Novartis33) | ERASMUS study (AAA31) | Pooled analysis of individual patient data from RADIANT-435 (Novartis33) | Assumed to be the same as for everolimus | ||
| Alternative values | 0.779 | 0.79 | 0.714 | 0.747 | 0.740 | |
| Source | Pooled analysis (Novartis33) | Guy’s and St Thomas’ (UK) hospital registry (AAA31) | Treatment arm-specific analysis (Novartis33) | ERASMUS study (AAA31) | ||
In the absence of data specific to lung or to GI-only patients for everolimus plus BSC and BSC only, we assumed the same utility values for these subgroups as for the overall RADIANT-435 population.
No data on HRQoL for patients treated with 177Lu-DOTATATE were identified through the systematic review. For this reason, we had to rely on unpublished data submitted by AAA. 31 The utility values in the PenTAG base-case model are based on a Dutch single-arm, uncontrolled study that has not been published at the time of writing. Utility values for pre-progression GI NET patients (see Table 22) were measured in the ERASMUS study, a single-centre, non-controlled, Phase I/II open-label study, conducted in 810 Dutch patients with different somatostatin receptor-positive tumour types. These data were used to estimate the utility of GI NET patients in the pre- and post-progression health states in the base-case cost-effectiveness model. Empirical data on HRQoL associated with 177Lu-DOTATATE in GI NET patients obtained from the Guy’s and St Thomas’ (UK) hospital registry were used by the company to estimate the utility of pre-progression patients. As no justification for this inconsistency in the choice of sources between the two health states was provided, the evidence from the ERASMUS study was preferred.
The utilities adopted for the de novo model by the AG, presented in Tables 21 and 22, were further adjusted for the effect of ageing in the model using the following linear equation, which was estimated by the AG from EQ-5D data in the Health Surgery for England 2012135 following the approach of Ara and Brazier:136
This adjustment was applied to all utilities irrespective of health state or treatment arm.
Summary
There is a lack of published evidence on HRQoL, especially for progressive disease in pNETs. In addition, for one of the comparators evaluated in this location, everolimus, there is no evidence available on the HRQoL outcomes in actual patients. In contrast, the present review benefited from access to individual patient data on HRQoL outcomes in patients from one of the main trials in the assessment, A6181111,81 provided by Pfizer through a NICE request.
The AG was able to validate the utilities derived by Novartis for the progressive disease and stable disease states in the GI location from RADIANT-435 data, without having access to the individual patient data but only aggregate HRQoL domain scores, by a linear approximation to the best-fitting algorithm from Longworth et al. ,134 used by Novartis to map individual FACT-G scores onto the EQ-5D. Linearising the best-fitting non-linear algorithm using first-order approximations enabled the successful validation of published mapped utilities.
In the absence of data specific to lung or to GI-only patients for everolimus plus BSC and BSC only, we assumed the same utility values for these subgroups as for the overall RADIANT-435 population.
The analyses of individual patient data highlighted the importance of requesting such data from the trial sponsors. This allowed the identification of fundamental errors in the interpretation of the data contained in the submissions to the three bodies responsible for making resource allocation decisions for England, Wales and Scotland.
Resources and costs
Cost parameters and assumptions
The unit costs of treatments and resources were sourced according to the NICE Guide to the Methods of Technology Appraisal 2013. 37 Only costs that relate to the included interventions for the treatment of NETs, and to resources under the control of the NHS and PSS, were included. Value-added tax was excluded. Costs that were common and equal in all treatment strategies over the time horizon of the analysis were excluded. The cost-effectiveness results reflect the present value of costs and benefits accruing over the time horizon of the analysis.
We modelled the following costs, which were inflated to the cost year 2016, and used an annual discount rate of 3.5%:
-
drug acquisition
-
drug administration
-
medical management and disease monitoring
-
SAE management
-
end-of-life care.
Costs of drug acquisition
Table 23 presents the unit costs of comparator treatments. The unit costs of everolimus and sunitinib were sourced from the British National Formulary (BNF) in September 2016. 36 The cost per unit of 177Lu-DOTATATE was provided by AAA as commercial-in-confidence information.
| Comparator | Unit size | Unit cost (list price) (£)a | PAS agreementa |
|---|---|---|---|
| Everolimus | 5-mg tablets, 30-tablet pack | 2250.00 | Confidential information has been removed |
| 10-mg tablets, 30-tablet pack | 2673.00 | ||
| Sunitinib | 12.5-mg capsules, 28-capsule pack | 784.70 | Confidential information has been removed |
| 25-mg capsules, 28-capsule pack | 1569.40 | ||
| 50-mg capsules, 28-capsule pack | 3138.80 | ||
| 177Lu-DOTATATE | 7.4-GBq single cycle | Confidential information has been removed | NA |
The base-case analyses used list prices. The results were also run using PAS prices, which can be found in the confidential appendix. This appendix is not included here as it contained commercial information provided by a company in confidence, as part of the NICE assessment process.
The recommended dosing for everolimus and sunitinib was sourced from their Summary of Product Characteristics (10 mg daily and 37.5 mg daily, respectively). The dose and administration schedule for 177Lu-DOTATATE (four administrations at intervals of 8 ± 1 week) was provided by AAA.
The base case used recommended dosing, adjusted for treatment interruptions and dose modifications as observed during the clinical trials. These RDIs were 85.9%34 and 91.3%81 for everolimus and sunitinib, respectively, in pNETs and 79.4%35 for everolimus in GI and lung NETs. 177Lu-DOTATATE in GI (midgut) NETs had a RDI of 86.4%.
Table 24 presents the median unadjusted durations of treatment observed in the trials. We used median values to create exponential distributions for sunitinib to estimate the proportion of patients with stable disease remaining on treatment and thereby estimate the average cost of a course of treatment. As a conservative approach, for everolimus we adopted the mean values used by Novartis33 in its pNETs and GI/lung economic evaluations. Had we used exponential extrapolations fitted to the median treatment durations, the mean values for everolimus would have been 12.68 and 13.40 months for pNETs and GI/lung NETs, respectively, instead of the base case values of 9.41 and 11.54 months respectively. Time on treatment in the AG model was then assumed to follow an exponential distribution using the mean values in Table 124.
| Comparator | Trial | Evaluation | Median treatment duration in the trial | Mean treatment duration in the AG model |
|---|---|---|---|---|
| Everolimus | RADIANT-334 | pNETs | Confidential information has been removed | (Confidential information has been removed) monthsa |
| RADIANT-435 | GI and lung NETs | 9.29 months | 11.54 monthsb | |
| RADIANT-435 | GI (midgut) | Not available | 13.99 monthsc | |
| Sunitinib | A618111181 | pNETs | 4.64 months | 7.51 monthsd |
| 177Lu-DOTATATE | NETTER-1102 | GI (midgut) NETs | NAe |
Table 25 presents the acquisition costs of the comparator treatments per 28-day Markov cycle. These are calculated by multiplying the unit cost for 28 days of treatment by the RDI.
| Treatment | Unit size | Unit cost (£) | Cost per 28 days (£) | Source |
|---|---|---|---|---|
| Everolimus (pNETs) | 10 mg | 2143.03 | BNF36 | |
| Everolimus (GI and lung NETs) | 10 mg | 1980.87 | BNF36 | |
| Sunitinib | 37.5 mg | 2148.93 | BNF36 | |
| 177Lu-DOTATATE [GI (midgut) NETs] | Four administrations, one per 8 ± 1-week interval | Confidential information has been removed | Confidential information has been removed | Price provided by the company to NICE for this evaluation |
| Octreotide | 20-mg depot preparation | 632.40 | 632.40 | eMIT137 |
| 30-mg depot preparation | 806.42 | 806.42 | eMIT137 | |
| 500-mc SC PFS | 5.02 | 140.56 | eMIT137 | |
| Lanreotide | 90-mg prefilled syringe | 736.00 | 736.00 | BNF36 |
| Granisetron | 1-mg tablets, 10-tablet pack | 50.38 | 50.38 | BNF36 |
| Vamin 18 | 1 l premixed | 26.70 | 26.70 | BNF36 |
| 5-fluorouracila | 250-mg vial | 4.00 | 52.00 | BNF36 |
| 500-mg vial | 6.40 | |||
| Capecitabineb | 500-mg tablets, 120-tablet pack | 225.72 | 158.00 | BNF36 |
| Doxorubicinc | 50-mg vial | 100.12 | 200.24 | BNF36 |
| Streptozocin | NA | Nild | Nil | |
| Interferon alphae | 5 million IU | 28.37 | 397.32 | BNF36 |
| Temozolomidef | 180-mg capsules, 5-capsule pack | 296.48 | 762.38 | BNF36 |
| Lidocaineg (Ralvo, Grunenthal Ltd) | 50 mg/g plasters, 30 | 72.40 | 67.57 | BNF36 |
| Dexamethasone (Aspen Pharma Trading Ltd)h | 2-mg tablets, 100-tablet pack | 78.00 | 131.04 | BNF36 |
| Prednisone (Lodotra; Napp Pharmaceuticals Ltd)i | 5-mg tablets, 100-tablet pack | 89.00 | 74.76 | BNF36 |
| Prochlorperazine (Actavis UK Ltd)i | 5-mg tablets, 84-tablet pack | 2.09 | 2.09 | BNF36 |
The unit cost of SSAs, drugs administered as an adjunct to 177Lu-DOTATATE, chemotherapies and supportive treatments are also presented in the table.
The proportions of patients using SSAs for tumour suppression in stable disease were based on the proportions reported in clinical trials, adjusted in an ITC conducted by Novartis (section 4.7.2 of the Novartis submission33). The adjusted rates are presented in Table 26. We assumed that the SSA usage was equally split between octreotide and lanreotide and that SSA usage following sunitinib would be the same as that for everolimus.
| Comparator | Trial | Arm | Proportion using SSAs (%)a |
|---|---|---|---|
| Everolimus | RADIANT-334 | Active | 37.7 |
| Placebo | 39.9 | ||
| Sunitinib | A618111181 | Active | 36.8b |
The proportions of patients using SSAs for tumour suppression post progression were based on targeted treatment utilisation (proportions) following progression in the RADIANT-334 trial (data provided on request by Novartis). The proportion using targeted treatments following progression after everolimus was 23%; in the absence of a better source, this was assumed to be a fair estimate of the proportion of patients using targeted treatments following progression after sunitinib. In the BSC arm of RADIANT-3,34 19.2% of patients were treated with targeted treatments following progression. In both the active and the BSC strategies, target treatments were assumed to be 20 mg of 50% octreotide and 90 mg of 50% lanreotide.
Somatostatin analogues were not used in stable disease for symptom control; however, we did include this resource in progressive disease. The proportion of patients using SSAs for symptom control was the same across the active treatment and BSC strategies; these were sourced from the unpublished UK utilisation survey presented in the Novartis submission. 33 The average number of SSA administrations at 500 µg was 1.9 per patient per cycle.
The proportions of patients using SSAs for tumour suppression were based on octreotide utilisation in RADIANT-4. 35 The estimates used are unpublished but are reported in the CSR. 138 The estimates used in the model are presented in Table 27. We made the assumption that SSA utilisation concurrent and following sunitinib treatment would be the same as observed for everolimus.
| Comparator | Disease | Proportion using SSAs (%) |
|---|---|---|
| Active treatment | Stable | 1.95 |
| BSC | Stable | 1.03 |
| Active treatment | Progressed, initial cycle | 29.80 |
| Progressed, subsequent cycles | 1.95 | |
| BSC | Progressed, initial cycle | 22.74 |
| Progressed, subsequent cycles | 1.03 |
Advice from expert clinicians is that use of antiemetics and parenteral amino acids should be standard practice in support of treatment with 177Lu-DOTATATE. Similar to the approach adopted by AAA, we assumed that every treatment cycle of 177Lu-DOTATATE (adjusted for RDI) was accompanied by a 5-day course of an antiemetic {2 mg of granisetron [Granisetron hydrochloride, Alliance Healthcare (Distribution) Ltd]; unit cost £50.38) and an amino acid supplement [an intravenous infusion of Vamin 18 (Amino-Acid electrolyte-free, Fresenius Kabi Ltd, Runcorn, UK); unit cost £26.70].
For the pNETs evaluation we adopted unpublished chemotherapy utilisation rates from RADIANT-3,34 provided by Novartis33 and presented in Appendix 15, Table 111. Slightly different data were also provided in table 3.7 of appendix 3 of the Novartis evidence submission to NICE on the rate of subsequent treatments in RADIANT-333,34 for patients who progress following active treatment (29.4%) and BSC (29.1%)]. In the absence of post-progression treatment information for patients in A618111,81 we assumed the same chemotherapy utilisation rates for people who progressed following sunitinib as was observed for everolimus.
For the GI and lung NETs evaluation and the GI (midgut) evaluation, we adopted unpublished chemotherapy utilisation rates from RADIANT-435 (supplied in the Novartis evidence submission33), presented in Appendix 16, Table 113.
Other therapies are used to support patients with NETs in addition to the use of SSAs, including analgesics, antiemetics, and antidiarrhoeals. We included the cost of these therapies in the GI and lung NETs evaluation and the GI (midgut) NETs evaluation using the unpublished utilisation rates supplied in the Novartis submission,33 which are based on data from RADIANT-4. 35 These are presented in Table 114 (see Appendix 16). No equivalent utilisation estimates were identified for other supportive therapies for patients with pNETs and so no costs of this type were included.
Costs of drug administration
There is significant variation across the comparator treatments in the resource requirements for their administration. Everolimus and sunitinib are ingested orally as a tablet and capsule, respectively, and are usually self-administered, whereas 177Lu-DOTATATE is administered in the secondary care setting by intravenous infusion over 20–30 minutes. 139,140 The cost of hospital pharmacy dispensing, applied at each outpatient clinic visit, was included in our costing for the oral preparations. This required 12 minutes of hospital pharmacist time, equating to £14.40.
In contrast, the administration of 177Lu-DOTATATE is resource intensive. As a radiolabelled and intravenously delivered drug, it requires specialist oversight and a hospital setting. AAA31 concurs in its data submission (section 2, p. 25) by stating that its expectation of routine treatment is delivery ‘in a nuclear medicine department within a secondary care hospital as an outpatient appointment’. However, we are guided by expert clinical opinion (consultants in nuclear medicine) that current standard practice is to admit patients overnight. We understand that selected patients at a single specialist centre in England are managed as day cases and this approach may be expanded in the future.
Table 28 presents the cost of drug administration for 177Lu-DOTATATE. The estimated quantity of resource required is the average elicited from two NHS consultants in nuclear medicine. Unit costs were obtained from standard sources. 141,142
| Resource | Quantitya | Unit cost (£) | Cost of resource type (£) |
|---|---|---|---|
| Hospital admission | 90% | 586.93b | 528.24 |
| Day case | 10% | 720.78b | 72.08 |
| Nuclear medicine consultant | 2.5 hours | 137.00c | 342.50 |
| General medicine consultant | 0.25 hours | 137.00c | 34.25 |
| Radiographer | 1.5 hours | 40.00c | 60.00 |
| Physicist (band 7) | 0.5 hours | 52.00c | 26.00 |
| Total | 1063.07 |
The costs associated with administering supportive treatments are presented in Table 29. Unit costs were obtained from standard sources. 141,142 Supportive treatments in Tables 25 and 114 but not listed here did not attract an administration cost; the cost of dispensing was not included for any supportive treatment.
| Administration | Visit | Treatments | Unit cost (£) |
|---|---|---|---|
| Intravenous/intramuscular injection | Firsta | 5-fluorouracil, doxorubicin, streptozocin, lanreotide | 239.12 |
| Subsequentb | 326.46 | ||
| Subcutaneous injection | Any | Octreotide | 22.00c |
Costs of medical management and disease monitoring
Medical management and disease monitoring resource estimates include the following categories of resource:
-
hospitalisation: general and emergency
-
outpatient clinic consultation
-
procedures and tests
-
other supportive procedures.
In the absence of published disease-specific detailed estimates of NHS resourcing, we relied on a source of unpublished evidence supplied by Novartis to tell us which resources are used and what the expected rates of utilisation are. In an industry-sponsored survey, nine physicians from seven UK centres were asked in 2016 to confirm the nature of NETs resourcing (type and rate) from a previous resource use survey of 32 clinicians in England in 2011. The validation process was framed in the context of personal practice in the previous year, across various disease stages and primary tumour locations.
The physicians were asked to list the various types of resource that a patient with NETs requires during the course of the disease and estimate the number of times that a patient would see each physician each month. The resource use for the given NETs patient population was then calculated as a weighted average of the annual number treated by each clinician relative to the total number treated annually across all clinicians. These estimates were then weighted according to the respective proportions of patients with pNETs in RADIANT-334 and GI NETs and lung NETs in RADIANT-4,35 to determine resource use for the overall trial populations.
For the BSC strategy in the pNETs evaluation, which was not modelled by Novartis, the resource utilisation of patients with stable disease was assumed to be the same as that presented for patients on active treatment. For patients with pNETs who progressed on BSC, resource utilisation was assumed to be equal to that for those who progressed on active treatment.
In some instances we modified the raw survey findings for our modelling:
-
The frequency of resource use for people who progressed following active treatment was adjusted downwards according to the proportion of people who resided ‘in observation’ in clinical trials (32.7% following progression on active treatment, 33.3% following BSC), which was effectively BSC.
-
The frequencies of consultations and procedures and tests for people with stable pNETs receiving BSC were reduced to below the estimates for active treatment. The reductions were proportionate to the differences observed between active treatment and BSC in GI and lung and GI (midgut) NETs (approximately 4 : 1 for consultations and 2 : 1 for tests/procedures).
-
The frequencies of consultation between people with GI and lung NETs/GI (midgut) NETs and the medical oncologist were adjusted downwards to the average of estimates from our expert clinicians. Utilisation survey estimates gathered by Novartis appeared high in absolute terms but also compared with frequencies in people with pNETs.
We used standard sources for the unit costing of disease management and monitoring. 141 These unit costs are presented in Table 30.
| Resource | Unit | Unit cost (£) |
|---|---|---|
| Hospitalisation | Per admission | |
| General admission | 586.93 | |
| Emergency admission | 147.30 | |
| Outpatient clinic consultations | Per consultation | |
| Medical oncologist | 158.54 | |
| Surgeon | 132.95 | |
| Palliative care | 185.92 | |
| Respirologist | 156.29 | |
| Nurse specialist | 37.26 | |
| Dietitian | 69.64 | |
| Primary physician | 37.26 | |
| Other physician | 69.64 | |
| Procedures and tests | Per procedure/test | |
| Abdominal ultrasound | 55.17 | |
| Echocardiography | 81.48 | |
| CT scan: chest, abdominal, pelvic; conventional | 124.53 | |
| CT scan: conventional | 111.61 | |
| Pulmonary angiogram: conventional | 238.25 | |
| CT scan: chest, abdominal, pelvic; helical/spiral | 124.53 | |
| CT scan: head; helical/spiral | 111.61 | |
| Pulmonary angiogram: helical/spiral | 238.25 | |
| MRI | 181.76 | |
| Chest radiography | 42.12 | |
| Octreoscan/SRS | 806.32 | |
| I-131 mIBG scan | 348.54 | |
| FDG PET | 492.51 | |
| Pro-BNP | 20.37 | |
| Standard blood test: biomarkers | 1.19 | |
| Special blood test: other | 3.01 |
Rates of hospital resource use are presented in Table 31 for the pNETs evaluation and Table 32 for the GI and lung NETs and GI (midgut) NETs evaluations.
| Resource | Stable disease | Progressive disease | Stable disease | Progressive disease |
|---|---|---|---|---|
| Active treatment | BSC | Active treatment | BSC | |
| Hospitalisation | ||||
| General admission | 0 | 0 | 0.0577 | 0.0577 |
| Emergency admission | 0 | 0 | 0 | 0 |
| Outpatient clinic consultations | ||||
| Primary physician: initial cycle | 0.2737 | 0.0690 | 0.7437 | 74.37 |
| Primary physician: subsequent cycles | 0.2737 | 0.0690 | 0.3380 | 33.80 |
| Another physician: initial cycle | 0.0805 | 0.0203 | 1.3846 | 1.3846 |
| Another physician: subsequent cycles | 0.0805 | 0.0203 | 0.3776 | 0.3776 |
| Procedure or test | ||||
| Abdominal ultrasound | 0.0241 | 0.0252 | 0.0321 | 0.0321 |
| CT scan: chest, abdominal, pelvic; conventional | 0.1449 | 0.0713 | 0.1731 | 0.1731 |
| Octreoscan/SRS | 0.0080 | 0.0081 | 0 | 0 |
| MRI | 0.0241 | 0.0159 | 0.0192 | 0.0192 |
| Chest radiography | 0 | 0 | 0.0128 | 0.0128 |
| Standard blood test: biomarkers | 0.2254 | 0.0660 | 0.3333 | 0.3333 |
| Special blood test: other | 0.5715 | 0.4280 | 0.7051 | 0.7051 |
| Resource | Stable disease | Progressive disease | Stable disease | Progressive disease |
|---|---|---|---|---|
| Active treatment | BSC | Active treatment | BSC | |
| Hospitalisation | ||||
| General admission | 0.0357 | 0.0357 | 0.0005 | 0.0052 |
| Emergency admission | 0.0357 | 0.0357 | 0.0350 | 0.0348 |
| Outpatient clinic consultations | ||||
| Medical oncologist | 0.4137 | 0.1041 | 0.3977 | 0.3958 |
| Surgeon | 0.0463 | 0.0477 | 0.0182 | 0.0182 |
| Palliative care | 0 | 0.2295 | 0.1022 | 0.1041 |
| Respirologist | 0 | 0.0172 | 0.0189 | 0.0189 |
| Nurse specialist | 0.0750 | 0.0226 | 0 | 0 |
| Dietitian | 0.0444 | 0.0462 | 0.0127 | 0.0129 |
| Procedure or test | ||||
| Abdominal ultrasound | 0.0073 | 0.0076 | 0.0091 | 0.0091 |
| Echocardiography | 0.0176 | 0 | 0.0162 | 0.0160 |
| CT scan: chest, abdominal, pelvic; conventional | 0.1166 | 0.0573 | 0.0453 | 0.0452 |
| CT scan: head; conventional | 0 | 0 | 0.0006 | 0.0006 |
| Pulmonary angiogram: conventional | 0 | 0 | 0.0006 | 0.0006 |
| CT scan: chest, abdominal, pelvic; helical/spiral | 0.2009 | 0.0573 | 0.1754 | 0.1741 |
| CT scan: head; helical/spiral | 0 | 0 | 0.0001 | 0.0001 |
| Pulmonary angiogram: helical/spiral | 0 | 0 | 0.0028 | 0.0028 |
| MRI | 0.0989 | 0.0653 | 0.0902 | 0.0894 |
| Chest radiography | 0.0065 | 0.0067 | 0.0052 | 0.0053 |
| Octreoscan/SRS | 0.0771 | 0.0780 | 0.0375 | 0.0372 |
| I-131 mIBG scan | 0.0022 | 0.0022 | 0 | 0 |
| FDG PET | 0.0009 | 0 | 0 | 0 |
| Pro-BNP | 0.0278 | 0.0278 | 0 | 0 |
| Standard blood test: biomarker | 3.4318 | 1.0060 | 2.6808 | 2.6592 |
| Special blood test: other | 0.8824 | 0.6610 | 1.3154 | 1.3107 |
Additional supportive procedures were included in the pNETs evaluation based on patient-level data from RADIANT-334 supplied by Novartis in its evidence submission. 33
Similarly, supportive treatments were included in the costing for the GI and lung NETs and GI (midgut) NETs evaluations. These rates of utilisation were based on patient-level data from RADIANT-435 supplied by Novartis in its evidence submission. 33
Costs of adverse event management
Adverse events experienced by patients on treatment attract additional health-care resources. To approximate the cost of managing those treatment-related AEs that could influence the cost-effectiveness of included treatments, we included only grade 3 and 4 AEs (SAEs) occurring in ≥ 2% of patients in either arm of the trials (National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03111).
In the evaluation of pNETs this included SAEs reported in RADIANT-334 and A6181111. 81 In the evaluation of GI and lung NETs, as well as GI (midgut) NETs, this included SAEs reported in RADIANT-435 and NETTER-1. 102
In pNETs, an ITC was conducted to match the trial populations of A618111181 and NETTER-1102 to the trial populations of the respective RADIANT trials (see Table 135). For each active treatment in the three evaluations, an OR was applied to the weighted average rate to give a relative rate by strategy for each event type (see Table 115). In GI and lung NETs (see Table 116), and GI (midgut) NETs (see Table 117), the unadjusted proportions of patients experiencing a SAE as reported in RADIANT-435 and NETTER-1102 were used.
Based on the assumption that no patient would report more than one SAE of any specific type during their time on treatment, we applied the costs of SAE management only to the initial Markov cycle in the progression-free health state.
Cost of end-of-life care
On the basis that the average cost of health resource use in the final weeks of the life of a cancer patient is a reasonable surrogate for patients with a NET, we used an estimate from the literature for cancer patients in England and Wales (£4346.19). This includes elective and non-elective inpatient admissions, outpatient appointments, accident and emergency visits and district nurse and general practitioner (GP) visits from the point at which a strong opioid is first used. 143
Checking the model for wiring errors
The economic model was checked in three ways. First, all calculations in the model were performed by one person and checked by another person. Second, the results of the model were checked by construction of an independent simplified model. Third, the reasonableness of outputs given extreme input values was checked. For example, total mean life-years are expected to be equal to total mean QALYs when all utilities are set to 1.
Cost-effectiveness results
Base-case results
In this section, we report the outputs of our base-case analysis on a per-tumour location basis assuming list prices for everolimus and sunitinib.
Pancreatic neuroendocrine tumours
According to the model predictions, the highest mean survival time is expected in patients with pNETs treated with sunitinib (6.39 years); an intermediate mean survival time (4.69 years) is predicted in patients treated with everolimus and the lowest mean survival time is expected in patients treated with BSC (3.46 years). Similarly, the highest mean QALYs are produced in patients treated with sunitinib followed by patients treated with everolimus and BSC only. The highest costs are predicted in patients in the sunitinib arm, followed by patients in the everolimus and BSC-only arms, with the costs of drug acquisition being the major driver of the total costs.
The resulting mean ICER for everolimus compared with BSC is £45,493 per QALY. As this figure is higher than the ICER for sunitinib compared with everolimus, sunitinib and BSC extendedly dominate everolimus, so that, ultimately, the relevant comparison is sunitinib compared with BSC, for which the ICER is £20,717.
The breakdown of life-year, QALY and cost outcomes is presented in Table 33. It may be noted that, although sunitinib incurs higher incremental per-patient drug acquisition costs compared with BSC than everolimus does (£27,431 vs. £26,885), sunitinib more than compensates for the excess in costs through the larger corresponding incremental gain in QALYs compared with BSC (1.32 years vs. 0.59 years). The majority of the difference in QALY outcomes originates from survival time in the post-progression health state (1.89 years vs. 0.52 years), which has the same associated HRQoL under both treatment options.
| Outcome or cost measure/health state | Sunitinib | Everolimus | BSC | Sunitinib vs. everolimus | Everolimus vs. BSC | Sunitinib vs. BSC |
|---|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | ||||||
| Pre progression | 1.60 | 1.28 | 0.57 | 0.32 | 0.71 | 1.03 |
| Post progression | 4.79 | 3.41 | 2.89 | 1.37 | 0.52 | 1.89 |
| Total | 6.39 | 4.69 | 3.46 | 1.70 | 1.23 | 2.93 |
| QALYs (mean, discounted) | ||||||
| Pre progression | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.18 | 0.43 | 0.62 |
| Post progression | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.55 | 0.16 | 0.71 |
| Total | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 0.73 | 0.59 | 1.32 |
| Costs (mean, discounted) (£) | ||||||
| Pre progression | ||||||
| Drug acquisition | 22,216 | 25,547 | 2003 | –3331 | 23,544 | 20,213 |
| Drug administration | 1308 | 1104 | 510 | 204 | 594 | 798 |
| Medical management | 952 | 776 | 184 | 176 | 592 | 768 |
| AEs | 89 | 132 | 15 | –43 | 117 | 74 |
| Total (pre progression) | 24,566 | 27,559 | 2712 | –2994 | 24,847 | 21,853 |
| Post progression | ||||||
| Drug acquisition | 8120 | 6113 | 4660 | 2006 | 1453 | 3460 |
| Drug administration | 1949 | 1468 | 1106 | 482 | 361 | 843 |
| Medical management | 4993 | 3759 | 3394 | 1234 | 365 | 1599 |
| End-of-life care | 3565 | 3747 | 3889 | –182 | –142 | –324 |
| Total (post progression) | 18,627 | 15,087 | 13,049 | 3540 | 2038 | 5578 |
| Total | 43,192 | 42,646 | 15,761 | 546 | 26,885 | 27,431 |
| ICER (cost/QALY) (£) | 745 | 45,493 | 20,717 | |||
Gastrointestinal and lung neuroendocrine tumours
The comparison between treatment with everolimus and treatment with BSC for the GI and lung NETs patient subpopulation yielded an ICER of £44,557 per QALY, exceeding the upper bound of NICE’s threshold range. Treatment of these patients with everolimus results in better survival (6.21 years vs. 4.82 years for BSC). Likewise, the treatment costs in the everolimus arm are higher, driven by the drug acquisition costs in the pre- and post-progression health states (Table 34).
| Outcome or cost measure/health state | Everolimus | BSC | Everolimus vs. BSC |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| Pre progression | 1.42 | 0.83 | 0.59 |
| Post progression | 4.79 | 3.99 | 0.80 |
| Total | 6.21 | 4.82 | 1.39 |
| QALYs (mean, discounted) | |||
| Pre progression | 1.04 | 0.65 | 0.38 |
| Post progression | 2.70 | 2.39 | 0.31 |
| Total | 3.74 | 3.05 | 0.69 |
| Costs (mean, discounted) (£) | |||
| Pre progression | |||
| Drug acquisition | 26,054 | 376 | 25,679 |
| Drug administration | 147 | 2 | 144 |
| Medical management | 4141 | 2038 | 2102 |
| AEs | 171 | 34 | 137 |
| Total (pre progression) | 30,513 | 2450 | 28,063 |
| Post progression | |||
| Drug acquisition | 4331 | 2511 | 1820 |
| Drug administration | 21 | 10 | 11 |
| Medical management | 8886 | 7822 | 1064 |
| End-of-life care | 3583 | 3732 | –149 |
| Total (post progression) | 16,822 | 14,076 | 2746 |
| Total | 47,334 | 16,526 | 30,809 |
| ICER (cost/QALY) (£) | 44,557 | ||
Gastrointestinal (midgut) neuroendocrine tumours
In our analysis, treatment of patients from the GI (midgut) NETs subpopulation with everolimus and BSC results in survival times of 7.5 years and 7.05 years respectively. Predicted QALYs are slightly higher in patients treated with everolimus than in patients treated with BSC (4.37 vs. 4.12).
A mean cost of £55,842 per patient was incurred in the everolimus arm, whereas the mean cost in the BSC arm was £21,119 per patient. Drug acquisition was the major cost component in this analysis (Table 35). The resulting ICER for everolimus compared with BSC was £199,233 per QALY.
| Outcome or cost measure/health state | Everolimus | BSC | Everolimus vs. BSC |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| Pre progression | 2.08 | 1.44 | 0.65 |
| Post progression | 5.42 | 5.62 | –0.20 |
| Total | 7.50 | 7.05 | 0.44 |
| QALYs (mean, discounted) | |||
| Pre progression | 1.49 | 1.10 | 0.38 |
| Post progression | 2.88 | 3.09 | –0.21 |
| Total | 4.37 | 4.19 | 0.17 |
| Costs (mean, discounted) (£) | |||
| Pre progression | |||
| Drug acquisition | 31,805 | 635 | 31,170 |
| Drug administration | 178 | 4 | 174 |
| Medical management | 5945 | 3449 | 2495 |
| AEs | 287 | 105 | 182 |
| Total (pre progression) | 38,215 | 4194 | 34,021 |
| Post progression | |||
| Drug acquisition | 4637 | 3260 | 1377 |
| Drug administration | 23 | 13 | 10 |
| Medical management | 9515 | 10,155 | –640 |
| End-of-life care | 3452 | 3497 | –45 |
| Total (post progression) | 17,627 | 16,925 | 702 |
| Total | 55,842 | 21,119 | 34,723 |
| ICER (cost/QALY) (£) | 199,233 | ||
In Table 36 we present the results for the treatment of GI (midgut) NETs with 177Lu-DOTATATE. This analysis incorporates background mortality as explained in Background mortality.
| Outcome or cost measure/health state | Everolimus | 177Lu-DOTATATE | BSC | Everolimus vs. BSC | 177Lu-DOTATATE vs. everolimus | 177Lu-DOTATATE vs. BSC |
|---|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | ||||||
| Pre progression | 2.07 | 5.41 | 1.43 | 0.63 | 3.35 | 3.98 |
| Post progression | 3.68 | 1.25 | 3.46 | 0.22 | –2.43 | –2.22 |
| Total | 5.75 | 6.66 | 4.90 | 0.85 | 0.91 | 1.76 |
| QALYs (mean, discounted) | ||||||
| Pre progression | 1.48 | 3.51 | 1.10 | 0.38 | 2.03 | 2.41 |
| Post progression | 2.09 | 0.68 | 2.01 | 0.08 | –1.41 | –1.33 |
| Total | 3.57 | 4.19 | 3.11 | 0.45 | 0.63 | 1.08 |
| Costs (mean, discounted) (£) | ||||||
| Pre progression | ||||||
| Drug acquisition | 31,786 | Confidential information has been removed | 633 | 31,152 | Confidential information has been removed | Confidential information has been removed |
| Drug administration | 178 | Confidential information has been removed | 4 | 174 | Confidential information has been removed | Confidential information has been removed |
| Medical management | 5904 | Confidential information has been removed | 3437 | 2466 | Confidential information has been removed | Confidential information has been removed |
| AEs | 287 | Confidential information has been removed | 105 | 182 | Confidential information has been removed | Confidential information has been removed |
| Total (pre progression) | 38,155 | Confidential information has been removed | 4180 | 33,975 | Confidential information has been removed | Confidential information has been removed |
| Post progression | ||||||
| Drug acquisition | 3349 | 1093 | 2117 | 1232 | –2256 | –1024 |
| Drug administration | 16 | 5 | 8 | 8 | –11 | –3 |
| Medical management | 6871 | 2242 | 6595 | 276 | –4629 | –4353 |
| End-of-life care | 3627 | 3522 | 3728 | –101 | –105 | –206 |
| Total (post progression) | 13,863 | 6862 | 12,448 | 1415 | –7001 | –5586 |
| Total | 52,018 | 83,667 | 16,628 | 35,390 | 31,649 | 67,039 |
| ICER (cost/QALY) (£) | 78,330 | 50,499 | 62,158 | |||
Sensitivity analyses are presented in Appendix 18. Subgroup analyses and scenario analyses are presented in Appendix 11.
Discussion
In patients with NETs of pancreatic origin, sunitinib plus BSC was estimated to incur a cost per QALY gained compared with BSC alone of £17,890. Everolimus was found to be an inefficient treatment option as it achieves QALY gains compared with BSC at a higher average cost than sunitinib (i.e. it is ‘extendedly dominated’) in this patient population. Therefore, sunitinib is cost-effective in the NHS at the upper NICE threshold range of £30,000 per QALY.
As discussed in Chapter 7, sunitinib also meets the end-of-life criteria from NICE in the patient population of A6181111,81 as it extends mean OS in patients with pNETs by > 3 months relative to placebo plus BSC, for which life expectancy is not significantly different from 24 months at conventional levels of statistical significance (i.e. p < 0.05).
These results are based on an indirect comparison of two RCTs in different patient populations. Assessment of the extent of heterogeneity across the trials and relative effectiveness between treatments is complicated by the fact that there was substantial treatment crossover from the placebo arms to the active arms in these trials. The companies sponsoring the two treatments have conducted statistical analyses that seek to adjust for such crossover. The AG asked the companies to provide the codes and data to be able to replicate their crossover-adjusted analyses of OS and understand whether the methods are likely to be comparable. The sponsor of everolimus provided such information too close to the end of the reviewing period to allow the AG to review and incorporate the evidence in this report. The sponsor of sunitinib provided the trial data but not the code to replicate the results of its crossover-adjusted analysis of OS. This leaves a crucial source of uncertainty unaddressed, as the available crossover-adjusted OS curves from published reports, which we used to inform our base-case analysis, suggest that life expectancy in the placebo arm of the everolimus trial (RADIANT-334) is 30% higher than life expectancy in the placebo arm of the sunitinib trial (A6181111;81 18 vs. 14 months).
Our analyses extend the evaluation of pNETs submitted by the companies to include the BSC-only arm, in line with the NICE scope for this assessment. There is no clear justification for excluding this treatment option from the analysis, especially as the RCTs in this patient population have themselves included this treatment option as the control arm. More importantly, advice from our clinical experts suggests that, in advanced, unresectable or metastatic patients with progressive disease who are asymptomatic, giving no active initial treatment is a treatment option in practice.
In the GI or lung NETs patient population, the available head-to-head trial evidence from the Phase III RADIANT-435 trial suggests that everolimus is not cost-effective at the upper NICE threshold of £30,000 per QALY, even after adjusting for the negotiated PAS discount. Contrary to the analysis submitted to NICE by the company sponsoring everolimus, we adopted different utility values in stable disease to acknowledge the effect of treatment on patient HRQoL. Whereas the company found that everolimus is (confidential information has been removed) after applying the PAS discount, we found that the ICER was £39,323 per QALY. Our analysis reveals that the company’s results were not robust to limited variations in the interpretation of the same available data (derived from the RADIANT-435 trial and the company’s resource use survey) used to populate model parameter values and specify the survival time structure in the model. We provide a detailed comparison of the analyses produced by the AG and those submitted to NICE by the companies in Appendix 23.
We have also extended the economic evaluation of everolimus to the GI (midgut) population based on subgroup analyses of PFS published by the company and found everolimus to have an ICER of £135,000 per QALY gained compared with BSC. This analysis is subject to high levels of uncertainty because of the lack of OS data specific to this patient subgroup, which was addressed by assuming that the OS treatment effect of everolimus was proportional to its PFS treatment effect in this population. Moreover, in RADIANT-4,35 the source of the effectiveness data for this analysis, randomisation was not stratified according to the midgut location and thus the resulting PFS evidence in the midgut subgroup is subject to a lower level of internal validity.
We conducted scenario analyses for the GI (midgut) location in which evidence from the NETTER-1102 trial for the 177Lu-DOTATATE arm was matched to the midgut population of RADIANT-435 by assuming that the control arm in NETTER-1102 represents the same treatment as that given in the placebo plus BSC arm in RADIANT-4. 35 As this assumption has been questioned by our clinical experts, we consider this analysis with reservation. Subject to these caveats, 177Lu-DOTATATE is associated with higher QALY benefits compared with BSC than everolimus compared with BSC, and achieves those benefits at a lower cost per QALY than everolimus (i.e. it extendedly dominates it), but its ICER of £74,000 relative to BSC is well above the NICE threshold.
Chapter 7 End of life
For each of the NET locations considered in our analyses, we estimated life expectancy as the area under the OS Kaplan–Meier curve of the placebo plus BSC arm, which was used as the source of data in the AG model. For pNETs the curve used in these analyses was the placebo Kaplan–Meier curve adjusted using the RPSFT method,48,69,122 whereas for GI/lung only, for which only unadjusted Kaplan–Meier data were available (crossover in the placebo arm was 6%35,88), the ITT placebo Kaplan–Meier curve was used. The results are presented in Table 37.
| Treatment | pNETs | GI/lung NETs | |
|---|---|---|---|
| aRADIANT-334 | A618111181 | RADIANT-435 | |
| Restricted mean (95% CI) at the end of follow-up (area under the Kaplan–Meier curve) (months) | |||
| Placebo + BSC | 18.3 (17.2 to 19.4) | 14.5 (12.6 to 16.3) | 29.1 (26.1 to 32.1) |
| Everolimus + BSC | 19.9 (19.0 to 20.9) | ||
| Sunitinib + BSC | 20.4 (18.9 to 22.0) | 31.7 (29.9 to 33.5) | |
| Treatment effect (active treatment arm – placebo arm) | 1.6 | 5.9 | 2.6 |
| Extrapolated mean (95% CI) using the exponential survival functionb (months) | |||
| Placebo + BSC | 41.6 (33.9 to 53.6) | 20.5 (16.4 to 27.4) | 57.9 (43.5 to 86.2) |
| Everolimus + BSC | 56.3 (48.2 to 67.7) | 74.5 (60.0 to 97.8) | |
| Sunitinib + BSC | 59.0 (55.8 to 80.0) | ||
| Treatment effect (active treatment arm – placebo arm) | 14.7 | 38.5 | 16.6 |
Mean survival estimates from head-to-head trials show that the null hypothesis that the pNETs population in A618111181 meets the life expectancy end-of-life criterion is not rejected by the data as the 95% CI of the extrapolated (to a maximum age of 100 years) mean survival in the placebo arm (95% CI 16 to 27 months) crosses the 24-month threshold. In other words, the data support the view that life expectancy with BSC only in A618111181 may be ≤ 2 years. In contrast, the pNETs population in RADIANT-334 has an extrapolated mean survival estimate in the placebo arm that is statistically significantly higher than 24 months (95% CI 34 to 54 months). The same result is obtained for GI/lung NETs, for which the data reject the null hypothesis that the life expectancy of the population is < 24 months (95% CI 44 to 86 months) at the 5% significance level.
Sunitinib is estimated to have a mean treatment effect of 5.9 months, using observed data from A618111181, or 38.5 months, using a parametric (exponential) survival curve fitted to the OS data of the two trials arms and extrapolated to 100 years of age. The treatment effect of everolimus in RADIANT-334 is 1.6 months using observed data and 14.7 months according to the extrapolated survival curve. The respective estimates for everolimus in GI or lung NETs are 2.6 and 16.6 months.
In conclusion, the end-of-life criteria may be met only by sunitinib in the pNETs population of A6181111. 81 In GI or lung NETs, life expectancy does not meet the end-of-life criteria set by NICE. 37
Chapter 8 Discussion
Aim
The key objectives of this technology assessment report, in keeping with the final NICE scope, were twofold: first, to estimate the clinical effectiveness of three interventions (everolimus, 177Lu-DOTATATE and sunitinib) for treating unresectable or metastatic NETs with disease progression and, second, to establish the cost-effectiveness of these interventions. The comparator treatments were chemotherapy, interferon alpha and BSC.
During the course of this review, NICE consulted on amendments to the original final NICE scope. Originally, lanreotide was included as an intervention and octreotide as a comparator. In the revised final scope, agreed on 18 August 2016,30 lanreotide and octreotide were dropped.
Clinical effectiveness evidence
The interventions of interest were everolimus (NETs of pancreatic, GI or lung origin), 177Lu-DOTATATE (NETs of pancreatic or GI origin) and sunitinib (pNETs).
Three trials, RADIANT-3,34 A618111181 and RADIANT-4,35 met the inclusion criteria for the clinical effectiveness systematic review.
The risk of bias within the trials was low and remained comparable between the three studies regarding selection, performance, detection, attrition and reporting bias.
Clinical effectiveness results: pancreatic neuroendocrine tumours
Key results only are given here. A fuller summary of the results is provided in Chapter 4, Summary.
Two trials provided evidence on the effectiveness of everolimus (RADIANT-334) and sunitinib (A618111181) for the treatment of pNETs. Both interventions were compared with placebo. In both trials, BSC was also given in both the intervention arm and the placebo arm.
Evidence consistently suggested a treatment effect in favour of both everolimus plus BSC and sunitinib plus BSC compared with placebo plus BSC for the outcomes of interest.
Treatment with everolimus was associated with a 66% reduction in the risk of progression or death (HR 0.34, 95% CI 0.26 to 0.44, by central review). Similarly, treatment with sunitinib was associated with a 68% reduction in the risk of progression or death (HR 0.32, 95% CI 0.18 to 0.55).
Treatment switching from the placebo arm to the treatment arm occurred in 73% of participants in RADIANT-334 and 69% of participants in A6181111. 81 The treatment switching significantly compromised the OS results. The HR for unadjusted OS was reported to be 0.94 (95% CI 0.73 to 1.20; p = 0.30) in RADIANT-334 and 0.73 (95% CI 0.50 to 1.06; p = 0.094) in A6181111. 81 Using the RPSFT model, the HR for OS was reported to be 0.60 (95% CI 0.09 to 3.95) in RADIANT-334 and 0.34 (95% CI 0.14 to 1.28; p = 0.094) in A6181111. 81
Overall, AEs were more commonly reported following treatment with everolimus and sunitinib than following treatment with placebo.
We compared everolimus with sunitinib in a simple ITC using the Bucher method.
Clinical effectiveness results: gastrointestinal/lung neuroendocrine tumours
One trial (RADIANT-435) provided evidence for the effectiveness of everolimus plus BSC in GI and lung NETs.
Evidence consistently suggested a treatment effect in favour of the use of everolimus plus BSC compared with placebo plus BSC for the outcomes of interest. Treatment with everolimus was associated with a 52% reduction in the risk of disease progression or death (HR 0.48, 95% CI 0.28 to 0.54). For OS, treatment with everolimus plus BSC was associated initially with a 36% improvement for individuals with lung and GI NETs compared with placebo (HR 0.64, 95% CI 0.40 to 1.05). However, in follow-up data from the company submission,33 a 27% improvement in OS following treatment with everolimus was reported (HR 0.73, 95% CI 0.48 to 1.11); however, this was unadjusted for crossover.
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo.
Clinical effectiveness results: gastrointestinal neuroendocrine tumours
Following a data request from us to Novartis, results from RADIANT-435 were provided for people recruited with only GI NETs.
Median PFS for those with GI NETs from RADIANT-435 was 13.1 months for treatment with everolimus and 5.4 months for placebo (HR 0.56, 95% CI 0.37 to 0.84). OS estimated from a Kaplan–Meier curve at the 25th percentile was (confidential information has been removed) in the everolimus arm compared with (confidential information has been removed) in the placebo arm.
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo for people with GI NETs.
Clinical effectiveness results: lung neuroendocrine tumours
Following a data request from us to Novartis, results from RADIANT-435 were provided for people recruited with only lung NETs.
Everolimus was associated with a 50% reduction in the risk of disease progression compared with placebo. Survival was improved by 44% following everolimus treatment compared with treatment with placebo.
Overall, AEs were more commonly reported following treatment with everolimus than following treatment with placebo.
Strengths and limitations of the clinical effectiveness review
Strengths of the clinical effectiveness review
A strength of this study is that a systematic review of RCTs of everolimus, 177Lu-DOTATATE and sunitinib in people with unresectable or metastatic NETs with disease progression was conducted to evaluate relative efficacy. In the absence of head-to-head RCTs, an ITC was conducted to assess the relative efficacy of everolimus and sunitinib for pNETs and everolimus and 177Lu-DOTATATE for GI NETs for the outcomes of PFS, OS, RRs and AEs. The strength of our review was the systematic nature of our search and review of published and unpublished data from the few available RCTs in this field, especially information held by sponsoring companies, which were willing to provide commercial-in-confidence information through the NICE process.
Limitations of the clinical effectiveness review
-
We were unable to compare 177Lu-DOTATATE with everolimus and sunitinib in individuals with pNETs, as the NETTER-1 RCT102 did not include patients with pNETs.
-
We were unable to compare any intervention with chemotherapy or interferon alpha, as there was no randomised evidence.
-
In several instances we were forced to rely on clinical results from the companies, rather than extracting the data from peer-reviewed publications.
-
We had to make many strong assumptions in the ITC comparing everolimus and 177Lu-DOTATATE in GI NETs, primarily that 30 mg of octreotide is equivalent to placebo plus BSC; therefore, these analyses should be treated with caution.
Cost-effectiveness
Systematic review of cost-effectiveness studies
We reviewed the cost-effectiveness literature according to the criteria set by the effectiveness review complemented with criteria for the inclusion of costing studies relevant to the UK, economic evaluations of interventions and modelling studies in this clinical area.
We identified three full economic evaluation studies, all relating to targeted treatments for advanced pNETs in patients with progressive disease in countries other than the UK (the USA,120 Mexico121 and Poland123,144). Two of these studies compared sunitinib plus BSC with BSC alone and one study compared everolimus with sunitinib. All of these studies were supported by the companies sponsoring the treatments in question.
One study conducted in the USA and sponsored by Novartis found that everolimus was cost-effective compared with sunitinib, based on an ICER for everolimus equivalent to £30,524 per QALY gained relative to sunitinib at 2015 UK prices. This study was based on an ITC of relative outcomes compared with placebo for RADIANT-334 and A6181111. 81 A strength of the study was its use of matching methods that acknowledge the heterogeneity in patient populations across trials. 129 A weakness was its omission of BSC alone as a comparator in its own right, especially as both RADIANT-334 and A618111181 included this treatment option as a control arm.
A second study, conducted in Mexico and sponsored by Pfizer, found that sunitinib was cost-effective based on an ICER equivalent to £32,842 per QALY gained compared with BSC alone at 2015 UK prices. The study was based on trial data from A6181111. 81 A strength of the study was its assessment of quality of life using patient-reported outcomes in the trial. A weakness of the study was its omission of an active treatment comparator in the economic evaluation. Another limitation is the fact that the study did not adjust for the effect on OS of treatment crossover from placebo to sunitinib in the open-label phase of A6181111,81 which results in an underestimation of health benefits and likely overestimation of the ICER of initial treatment with sunitinib.
A third study, conducted in Poland and also sponsored by Pfizer, found that sunitinib was cost-effective based on an ICER equivalent to £33,866 per QALY gained relative to BSC alone at 2015 UK prices. The study was based on trial data from A6181111. 81 A strength of the study was its adjustment for the effect of crossover from placebo to sunitinib in the open-label phase of A6181111. 81 One limitation was the lack of an active treatment comparator in the evaluation. Another limitation was its use of outdated data from the trial and limited reporting of methods used to measure utility values. This was the only identified full report of a study conducted in this clinical area in Europe.
A fourth study, the only one identified that was conducted in the UK,122 which was also sponsored by Pfizer, was reported as a conference poster. This summarised the evidence submitted to the SMC on sunitinib compared with placebo in Scotland, according to which sunitinib was cost-effective based on an ICER of £24,244 per QALY gained relative to placebo at 2015 UK prices. The strength of the study was its adjustment for the effect of treatment crossover from placebo to sunitinib on OS. The main limitation was the lack of adequate methodological detail available from the report.
Critique of the company model submissions
Of the three companies that submitted evidence to NICE, two included economic evaluations in their submission. Novartis33 evaluated treatments in pNETs and GI and lung NETs. AAA31 evaluated treatments in pNETs and GI NETs. Pfizer did not submit an economic evaluation.
The economic evaluation by Novartis used a partitioned survival model of sunitinib compared with everolimus in pNETs, based on an ITC of placebo-controlled outcomes in A618111181 and RADIANT-3. 34 The company found that sunitinib dominated everolimus as it had lower costs and produced more QALYs. This result was derived by assuming equal PFS and OS outcomes between treatments, which in turn was based on the CIs found in the ITC, that is, a PFS HR of 1.08 (95% CI 0.59 to 1.99) and a RPFST-adjusted OS HR of 1.39 (95% CI 0.17 to 11.72). As a result, the only health benefit on which treatments were compared was HRQoL (state utility values) before disease progression (utility values after progression were assumed to be the same between treatments). However, in the absence of utility data for everolimus from RADIANT-3,34 the company imputed treatment differences according to the incidence of AEs and values of their associated disutilities from a preference elicitation survey of the general public based on vignettes designed by clinical experts. The assumption of equal outcomes of PFS and OS and the poor quality of the utility data, which do not meet the NICE requirement that HRQoL data be derived from actual patient outcomes, hamper the value of this evidence for NICE decision-making. Furthermore, the data used in this evaluation by Novartis33 from A618111181 appear to be outdated.
A second evaluation by Novartis assessed everolimus plus BSC relative to BSC alone in the non-functional GI and lung NETs population using data from RADIANT-4. 35 Novartis found that everolimus had an ICER of £43,642 per QALY gained relative to BSC alone or an ICER of (confidential information has been removed) when a PAS discount of (confidential information has been removed) was applied to the list price. The main strength of this assessment was its use of data from RADIANT-435 to populate the model parameters. The main limitations were the immaturity of the OS data in the trial, the lack of adjustment for treatment crossover to targeted treatments and the lack of adjustment for treatment switching before disease progression (13% and 14% in the everolimus and placebo arms respectively), which was dealt with by censoring data for switching cases at the time of the switch. Finally, the study adopted a high frequency of oncologist visits. 33
Advanced Accelerator Applications submitted an evaluation in pNETs of 177Lu-DOTATATE compared with everolimus and sunitinib but the value of the resulting evidence is questionable as it was based on trial data from NETTER-1,102 which included only midgut NETs patients. Furthermore, the AAA evaluation lacked BSC as a relevant comparator. The company also submitted an assessment of 177-Lu-DOTATATE compared with everolimus in the GI NETs subpopulation of SSTR+ patients, which produced a base-case ICER of (confidential information has been removed). This evidence is also of limited quality as it involved an ITC of NETTER-1 outcome data with data from RADIANT-4,35 which included non-midgut GI and lung NETs. This analysis by AAA in the GI NET population also omitted BSC alone, a relevant comparator. There is also a limitation in that costs did not include resource use for disease monitoring, for example oncologist visits and the costs of 177Lu-DOTATATE administration were underestimated. 31
Strengths and limitations of the evidence from company model submissions
The company submissions benefit from having individual patients from the few trials available to inform their assessments. The main limitation with the submitted evidence is the lack of adequate comparators for the case of pNETs and the lack of adequate comparisons with 177Lu-DOTATATE in the NETTER-1 population of GI (midgut) NETs. In GI and lung NETs the main issue is the selective use of utility data from RADIANT-4,35 in particular the use of the same utility values in stable disease for the two treatment strategies, everolimus plus BSC and BSC alone, when treatment-specific values are available.
The available evidence from the submitted models provides cost information that is not found in the publicly available sources. In particular, details on the frequency of patients using medications or non-medical treatments in stable disease and disease progression in GI and lung NETs from RADIANT-435 are uniquely available from this source. On the other hand, the evidence on treatment regimens used and the frequency of contacts with health professionals was based on a validation of a previous expert survey, which provided limited data on resource use for these patients in progressive disease. Cost data on pNETs are limited, particularly for A6181111,81 with, for example, information on treatments used after disease progression not collected for this trial.
Given the limitations of the evidence from industry submissions and the literature, the AG requested from Novartis individual patient data from RADIANT-3 to replicate some of the ITCs (MAICs) and adjustment for treatment crossover in pNETs. The company declined to provide the MAIC analysis data, noting that the analyses in question did not inform its economic evaluation. However, the company did agree to provide data on its adjustment of OS outcomes for crossover in RADIANT-348,69 which the AG could use to replicate the company’s findings submitted to NICE. Pfizer also agreed to provide its individual patient data and code for its own crossover-adjusted OS results, but only individual data from an outdated data cut-off were provided, and in the absence of the code and updated OS data the AG could not replicate the company’s findings. We did manage, however, to conduct exploratory analyses for the matched ITC, matching the A618111181 sample of individual patient data to the RADIANT-435 baseline characteristics. This highlighted the limitations associated with simple standard Bucher-type comparisons underpinning the Novartis submission,33 originating from the small sample size of A618111181 and the consequent imbalance in key baseline characteristics between trial arms, that is, PS, time since diagnosis and number of disease sites.
In the light of the above limitations of the evidence base, development of an independent de novo economic model was undertaken by the AG.
Independent economic assessment
The AG built a three-health-state partitioned survival model in two NET patient populations. One was in patients with advanced pNETs and evaluated sunitinib plus BSC, everolimus plus BSC and BSC alone over a lifetime horizon. These analyses were based on Bucher-type indirect comparisons of outcomes from RADIANT-334 and A6181111. 81 The second evaluation compared everolimus plus BSC with BSC alone in patients with non-functional GI and lung NETs, based on RADIANT-435 data. In addition, we conducted a subgroup analysis of everolimus plus BSC, BSC alone and 177Lu-DOTATATE plus 30 mg of octreotide in the GI (midgut) population using PFS data for this subgroup from RADIANT-435 and indirectly comparative data on 177-Lu-DOTATATE from NETTER-1.
The models were populated with parameter estimates from time-to-event analyses of recreated individual patient OS and PFS survival data digitised from the latest OS and PFS Kaplan–Meier curves from published sources and industry submissions. Resource use model parameters were populated with data from the Novartis submission,33 with modifications to reflect our clinical experts’ opinions of resource use intensity associated with disease monitoring. Prices and other details adhered to the NICE reference case recommendations.
In the pNETs population, we found that sunitinib had an ICER of £20,717 per QALY gained relative to BSC alone, at the current list price. The corresponding figure for everolimus was £45,493 per QALY gained. These figures imply that sunitinib is superior to everolimus as it may achieve the same amount of benefit at a lower cost to the NHS. In the GI and lung population, everolimus had an ICER of £44,557 per QALY gained relative to BSC alone. In the GI (midgut) subgroup, the ICER for everolimus relative to BSC alone was £199,233 per QALY gained. It must be noted that the results in the GI (midgut) subgroup are affected by a high level of uncertainty because of PFS-based imputation of OS outcomes in the model, as we did not have available actual OS data on this subgroup of RADIANT-435 patients.
In our additional indirect comparison in the GI (midgut) population (adjusting for background mortality), for everolimus we found an ICER of £78,330 per QALY gained relative to BSC alone, at the current list price; the respective figure for 177Lu-DOTATATE plus 30 mg of octreotide was £62,158 per QALY gained. Both these results and the results from a scenario analysis based on outcomes up to disease progression produced lower ICERs relative to BSC alone for 177Lu-DOTATATE than for everolimus.
Our scenario analyses in the pNETs patient population show that the cost-effectiveness of targeted treatment relies critically on adjusting for the effects of crossover from placebo to sunitinib on OS. At current list prices, the ICERs for initial treatment with sunitinib and everolimus relative to BSC were £37,217 and £136,455 per QALY gained, respectively, without adjustment for crossover, that is, 1.5 and 3 times the base-case values. Our sensitivity analyses suggest that there is a high degree of uncertainty arising from the immaturity of OS data in GI and lung NETs and from NETTER-1 data. 102 In particular, there is evidence that the cost-effectiveness of everolimus in GI and lung NETs depends on benefits that arise in the later years of life, and it is thus sensitive to the discount rate.
The above figures appear to suggest that the data for sunitinib are more robust than those for everolimus in both pNETs and GI and lung NETs, which are more sensitive to adjustment for treatment crossover and the effects of the time horizon and discounting. In addition, 177Lu-DOTATATE was found to produce the largest health benefits of all treatment strategies investigated for GI (midgut) NETs, that is, 1.76 and 0.91 more years of life than the BSC alone and everolimus strategies, respectively. These figures are remarkable, especially because the fact that 60 mg of octreotide was given in the control arm would suggest that the health benefits of 177Lu-DOTATATE relative to other treatments are underestimated in our analysis.
On request from the NICE appraisal committee, we performed further analyses for lung NETs (see addendum in Appendix 17) and, separately, the overall GI location (see addendum in Appendix 19) using RADIANT-4 data on these populations extracted from survival curves provided in analyses performed by Novartis in response to our assessment herein. We found that in these two locations and at current list prices, everolimus has ICERs of > £20,000 per QALY relative to BSC (base case: overall GI location £26,383; lung £31,016).
Strengths and limitations of the independent economic assessment
Our analysis of pNETs was based on the most up-to-date effectiveness data from the RCT informing the ITC of the targeted treatments sunitinib and everolimus. (For the comparison of our results with the company results see Appendix 23.) However, the ITC underlying our economic analysis was of a simple Bucher type, unadjusted for any differences in the baseline characteristics across the two trials. Our cost-effectiveness results may thus be biased if indeed the patients in the two trials come from populations with different prognoses. Our comparison of the PFS curves of the BSC arms across trials suggests that the diseases of the two patient groups have different propensities to progress and, given the theoretical and empirical evidence linking PFS and OS outcomes, different associated death risks. In such a case the results would remain valid if the proportional effect of targeted treatments over BSC alone is constant across levels of baseline disease and death risks. Confidential information has been removed.
Nevertheless, caveats are due with respect to the small size of the A618111181 trial, which resulted in an imbalance in key baseline characteristics. A Bucher-type analysis does not adequately deal with bias arising from such an imbalance in baseline characteristics across arms within the same trial. Confidential information has been removed. Our findings on 177LU-DOTATATE are based on a limited quantity and quality of data available for the ITC with everolimus. The immaturity of the available effectiveness data from NETTER-1, and the fact that the control arm received 60 mg of octreotide and therefore was of a different nature to BSC in the RADIANT-435 GI (midgut) subgroup, suggests that these results need to be considered with caution. It is not clear, in particular, whether or not the GI (midgut) subgroup of RADIANT-435 represents a patient population with a similar prognosis to that in NETTER-1. Nevertheless, our scenario and sensitivity analyses, adjusting for the extent of optimism in our long-term survival projections, suggest that, based on the early evidence from NETTER-1, 177Lu-DOTATATE may produce at least as much value for money as everolimus does in the GI (midgut) NETs patient population.
Further research is required to investigate the robustness of the findings presented here. In particular, the availability of individual patient data from RADIANT-334 would allow the robustness of the findings to be tested and would be better suited for that task than the individual patient data from A618111181 made available to us by Pfizer. This is because RADIANT-334 was a larger trial and therefore is less subject to instability than A618111181 as a result of small effective sample sizes remaining after matching, Similar analyses will be required to assess the robustness of indirect comparisons with 177Lu-DOTATATE outcomes in the NETTER-1 trial102 using Bucher-type compared with more elaborate methods of indirect comparison, while adjusting for treatment crossover. Information on the quality-of-life outcomes measured in NETs patients receiving targeted treatments is also required, as such information is available only for patients in stable disease who are subject to high rates of missing data.
The analyses presented as addenda to this report, for the lung (see Appendix 17) and separately for the overall GI population (see Appendix 19) in RADIANT-4,35 are subject to the limitations already discussed for RADIANT-435 data. In addition, we could not verify the quality of the OS data for the overall GI group as the only information available was a single Kaplan–Meier curve provided by the company in response to our assessment. Further research is needed to confirm our findings in the overall GI location.
The current study seeks to provide evidence to inform the optimal choice of initial treatment in advanced, progressive pNETs and GI and lung NETs. The nature of the available evidence limited our analysis and the type of questions that we could address. Our assessment therefore provides very limited information on questions such as choice of treatment sequences. Another important question on which the present analysis may shed some light is whether targeted treatments may be given initially or after disease progression in patients who have progressive disease. The further availability of data on subsequent treatments after disease progression may allow more precise answers than those allowed by this assessment.
Acknowledgements
We are grateful for the excellent assistance of Sue Whiffin, Jenny Lowe and Kate Newell.
Contributions of authors
Ruben Mujica-Mota (Senior Lecturer, Economic Evaluation) led the economic evaluation and the systematic review of the cost-effectiveness literature, critiqued one of the industry model submissions, contributed to the review of the utility literature and performed the survival analysis to populate the de novo model and statistical analyses of individual patient data provided by the companies. He wrote the respective sections in the report, including the end-of-life criteria and the discussion of the economic evaluation sections, and contributed to writing the methods and results of the de novo model.
Jo Varley-Campbell (Research Fellow, Systematic Review) led the clinical effectiveness systematic review from August 2016, screened abstracts and papers, extracted the data and second-checked the data extraction. She wrote the results for the clinical effectiveness review, the background and the critique of the company submissions, contributed to the corresponding sections within the executive summary, discussion and appendices and contributed to the editing of the report and collation of the final report.
Irina Tikhonova (Associate Research Fellow, Economic Evaluation) contributed to the design and parameterisation of the PenTAG economic model, implemented the model in Microsoft Excel and wrote the sections on the design and the results of the model. She contributed to the cost-effectiveness systematic review by screening titles, abstracts and papers of published cost-effectiveness studies and contributed to the editing of the report.
Chris Cooper (Senior Research Fellow, Systematic Review and Information Specialist) provided guidance on the review of clinical effectiveness. He screened titles, abstracts and papers for inclusion in the systematic review, extracted data and second-checked the data extraction, led on contacting ongoing trial and study authors and checked and extracted studies from relevant systematic reviews. He wrote the methods sections [clinical effectiveness reviews (RCT and non-RCT)] and contributed to the writing of the clinical effectiveness section and corresponding sections within the executive summary and appendices and the editing of the report. He supported the clinical data extraction and carried out literature searches for clinical effectiveness, cost-effectiveness and HRQoL studies. He undertook separate searches to inform a network meta-analysis. He also advised on citation and supplementary searches and critiqued the company literature searches. He wrote the sections on literature searching and the appendices on the systematic searches of clinical effectiveness and cost-effectiveness and utilities and wrote the section on literature searching for the network meta-analysis. Finally, he undertook document retrieval.
Ed Griffin (Associate Research Fellow, Economic Evaluation) provided a critique of the submission by AAA and contributed to the development of the PenTAG independent economic assessment, including the analysis of costs and their methodological description.
Marcela Haasova (Research Fellow, Systematic Review) project managed and led the clinical effectiveness review for part of the duration of the project (until August 2016), wrote the project protocol and contributed to discussions about searches.
Jaime Peters (Senior Research Fellow, Statistician) carried out the ITC, wrote the critique of the company submissions of effectiveness evidence and contributed to the writing and editing of the report.
Stefano Lucherini (Student Intern, Economic Evaluation) reviewed the utility literature to populate the de novo economic model and wrote the corresponding section.
Juan Talens-Bou (Graduate Trainee, Systematic Review) was the third reviewer from August 2016 to the project completion. He screened the abstracts and papers and extracted data and undertook update searching, searching for background information and supplementary searching. He checked all data tables and calculations, contributed to the editing of the clinical effectiveness review and executive summary and contributed to the editing of the report.
Linda Long (Research Fellow, Systematic Review) contributed to the systematic review of clinical effectiveness, assessed and wrote the summary of non-RCTs and contributed to the writing of the background section.
David Sherriff (Consultant Oncologist) provided clinical advice and contributed to the editing of the report.
Mark Napier (Consultant Oncologist) provided clinical advice and contributed to the editing of the report.
John Ramage (Professor and Consultant Physician in Gastroenterology and Hepatology) provided clinical advice and contributed to the editing of the report.
Martin Hoyle (Associate Professor) was the project manager for part of the duration of the project (August–December 2016), contributed to the design of the economic model, checked the PenTAG economic model and contributed to the editing of the report. He was the director and guarantor of the report.
Data-sharing statement
Access to the executable economic model developed within this study can be requested and is subject to appropriate agreements being in place. All queries should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012;61:6-32. https://doi.org/10.1136/gutjnl-2011-300831.
- Modlin IM, Öberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. https://doi.org/10.1016/S1470-2045(07)70410-2.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. https://doi.org/10.1002/cncr.11105.
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer; 2010.
- Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007;451:757-62. https://doi.org/10.1007/s00428-007-0452-1.
- Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395-401. https://doi.org/10.1007/s00428-006-0250-1.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Oxford: Wiley-Blackwell; 2009.
- Stephenson TJ, Cross SS, Chetty R. Dataset for Neuroendocrine Tumours of the Gastrointestinal Tract Including Pancreas (3rd Edition). London: Royal College of Pathologists; 2012.
- Incidence and Survival in Neuroendocrine Tumours and Neuroendocrine Carcinomas (NETs/NECs) in England, 2013–2014. London: Public Health England; 2016.
- Öberg K, Akerström G, Rindi G, Jelic S. ESMO Guidelines Working Group . Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v223-7. https://doi.org/10.1093/annonc/mdq192.
- Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AK, et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 2008;113:2655-64. https://doi.org/10.1002/cncr.23883.
- Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589-97. https://doi.org/10.1002/cncr.29099.
- Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol 2010;105:2563-9. https://doi.org/10.1038/ajg.2010.341.
- Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010;45:234-43. https://doi.org/10.1007/s00535-009-0194-8.
- Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: a US-based case–control study. Int J Cancer 2008;123:867-73. https://doi.org/10.1002/ijc.23529.
- Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 2007;132:899-904. https://doi.org/10.1053/j.gastro.2007.01.006.
- Lepage C, Ciccolallo L, De Angelis R, Bouvier AM, Faivre J, Gatta G. EUROCARE working group . European disparities in malignant digestive endocrine tumours survival. Int J Cancer 2010;126:2928-34. https://doi.org/10.1002/ijc.24698.
- Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0062487.
- Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer 2013;49:1975-83. https://doi.org/10.1016/j.ejca.2012.12.022.
- Lepage C, Bouvier AM, Phelip JM, Hatem C, Vernet C, Faivre J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 2004;53:549-53. https://doi.org/10.1136/gut.2003.026401.
- Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer 2012;3:292-30. https://doi.org/10.7150/jca.4502.
- Cancer Research UK . Survival Statistics for Carcinoid Tumours 2014 2014. www.cancerresearchuk.org/about-cancer/type/carcinoid/treatment/statistics-and-outlook-for-carcinoid#is6ujgs6I1bPTZp2.99 (accessed December 2016).
- Vinik A, Moattari AR. Use of somatostatin analog in management of carcinoid syndrome. Dig Dis Sci 1989;34:14S-27S. https://doi.org/10.1007/BF01536042.
- Macmillian . Neuroendocrine Tumours 2016 2015. www.macmillan.org.uk/information-and-support/neuroendocrine-tumours-nets (accessed November 2016).
- Cancer Registration Statistics, England: 2014. Newport: ONS; 2016.
- Metastatic Malignant Disease of Unknown Primary Origin in Adults: Diagnosis and management. London: NICE; 2010.
- European Medicines Agency . Afinitor (Everolimus) 2016 2016. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001038/human_med_000633.jsp&mid=WC0b01ac058001d124 (accessed December 2016).
- Lutetium Therapy: Patient Information Fact Sheet. London: Guy’s and St Thomas’ NHS Foundation Trust; 2015.
- European Medicines Agency . Sutent (Sunitinib) 2016 2016. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000687/human_med_001069.jsp&mid=WC0b01ac058001d124 (accessed December 2016).
- Final Scope: Everolimus, Lanreotide, Lutetium-177 DOTATATE and Sunitinib for Treating Unresectable or Metastatic Neuroendocrine Tumours with Disease Progression. London: NICE; 2016.
- Advanced Accelerator Applications (AAA) SA . Evidence Submission for Lutetium-177 DOTATATE n.d.
- Pfizer Inc . Evidence Submission for Sunitinib n.d.
- Novartis International AG . Evidence Submission for Everolimus n.d.
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. https://doi.org/10.1056/NEJMoa1009290.
- Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-77. https://doi.org/10.1016/S0140-6736(15)00817-X.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Haasova M, Cooper C, Hoyle M, Lucherini S, Mujica-Mota R, Napier M, et al. The Effectiveness and Cost-Effectiveness of Everolimus, Lanreotide, Lutetium-177 DOTATATE and Sunitinib for Treating Unresectable or Metastatic Neuroendocrine Tumours With Disease Progression: A Systematic Review and Economic Evaluation 2016. www.nice.org.uk/guidance/GID-TA10024/documents/final-protocol (accessed 30 November 2016).
- European Medicines Agency . IntronA Summary of Product Characteristics n.d. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000281/WC500034679.pdf (accessed July 2018).
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. https://doi.org/10.1016/S0895-4356(97)00049-8.
- Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol 2011;29:3450-6. https://doi.org/10.1200/JCO.2010.34.4309.
- Valle JW, Eatock M, Clueit B, Gabriel Z, Ferdinand R, Mitchell S. A systematic review of non-surgical treatments for pancreatic neuroendocrine tumours. Cancer Treat Rev 2014;40:376-89. https://doi.org/10.1016/j.ctrv.2013.08.007.
- Massironi S, Conte D, Rossi RE. Somatostatin analogues in functioning gastroenteropancreatic neuroendocrine tumours: literature review, clinical recommendations and schedules. Scand J Gastroenterol 2016;51:513-23. https://doi.org/10.3109/00365521.2015.1115117.
- Walter T, Krzyzanowska MK. Quality of clinical trials in gastroenteropancreatic neuroendocrine tumours. Neuroendocrinology 2012;96:238-48. https://doi.org/10.1159/000337662.
- Lee A, Chan DL, Wong MH, Li BT, Lumba S, Clarke S, et al. Systematic review on the role of targeted therapy in metastatic neuroendocrine tumor (NET). Neuroendocrinology 2016;16.
- Roviello G, Zanotti L, Venturini S, Bottini A, Generali D. Role of targeted agents in neuroendocrine tumors: results from a meta-analysis. Cancer Biol Ther 2016;17:883-8. https://doi.org/10.1080/15384047.2016.1210735.
- Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. J Clin Oncol 2016;34:3906-13. https://doi.org/10.1200/JCO.2016.68.0702.
- Ito T, Okusaka T, Ikeda M, Igarashi H, Morizane C, Nakachi K, et al. Everolimus for advanced pancreatic neuroendocrine tumours: a subgroup analysis evaluating Japanese patients in the RADIANT-3 trial. Jpn J Clin Oncol 2012;42:903-11. https://doi.org/10.1093/jjco/hys123.
- Lombard-Bohas C, Yao JC, Hobday T, Van Cutsem E, Wolin EM, Panneerselvam A, et al. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas 2015;44:181-9. https://doi.org/10.1097/MPA.0000000000000262.
- Lombard-Bohas C, Yao JC, Hobday TJ, Van Cutsem E, Wolin EM, Panneerselvam A, et al. Efficacy and safety of everolimus in patients with advanced low-or intermediate-grade pancreatic neuroendocrine tumors previously treated with chemotherapy: RADIANT-3 subgroup analysis. J Clin Oncol 2013;31. https://doi.org/10.1200/jco.2013.31.4_suppl.224.
- Chambers J, Reed N, Mansoorc W, Ross P, Grossman A. Phase-3 randomized trial of everolimus (RAD001) vs. placebo in advanced pancreatic NET (RADIANT-3). Regul Pept 2010;164:6-7. https://doi.org/10.1016/j.regpep.2010.07.018.
- Hobday T, Pommier R, Van Cutsem E, Panneerselvam A, Saletan S, Winkler RE, et al. Analysis of progression-free survival (PFS) by prior chemotherapy use and updated safety in radiant-3: a randomized, double-blind, placebo-controlled, multicenter, Phase III trial of everolimus in patients with advanced low-or intermediate-grade pancreatic neuroendocrine tumors (PNET). Pancreas 2012;41.
- Hobday TJ, Capdevila J, Saletan S, Panneerselvam A, Pommier RF. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): multivariate analysis of progression-free survival from the RADIANT-3 trial. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.e21091.
- Horsch D, Lombard-Bohas C, Lincy J, Saletan S, Kocha W. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET) (RADIANT-3): updated safety results. Endocr Rev 2011;32:25-2.
- Ito T. Current status of mTOR inhibitor as a new therapeutic strategy for advanced pancreatic endocrine tumors. Ann Oncol 2011;22.
- Ito T, Okusaka T, Ikeda M, Tajima T, Kasuga A, Fujita Y, et al. Everolimus versus placebo in Japanese patients with advanced pancreatic neuroendocrine tumors (pNET): Japanese subgroup analysis of RADIANT-3. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.4_suppl.289.
- Lombard-Bohas C, Van Cutsem E, Capdevila J, De Vries EGE, Tomassetti P, Lincy J, et al. Updated survival and safety data from radiant-3 – a randomized, double-blind, placebo-controlled, multicenter, phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumours (pNET). Eur J Cancer 2011;47. https://doi.org/10.1016/S0959-8049(11)71872-X.
- Okusaka T, Ito T, Ikeda M, Igarashi H, Morizane C, Nakachi K, et al. Phase III trial of everolimus in advanced pancreatic neuroendocrine tumors (RADIANT-3): overall population and Japanese subgroup analysis. Ann Oncol 2012;23.
- Okusaka T, Ito T, Ikeda M, Tajima T, Kasuga A, Fujita Y, et al. Efficacy and safety of everolimus in Japanese patients with advanced pancreatic neuroendocrine tumors (pNET): Japanese subgroup analysis of RADIANT-3. Neuroendocrinology 2011;94:37-8.
- Pavel M, Unger N, Borbath I, Ricci S, Hwang TL, Brechenmacher T, et al. Quality-of-life (QoL) assessments in patients (pts) with pancreatic neuroendocrine tumors (pNET) enrolled in the open-label, phase 3b, multicenter, expanded access study of everolimus in pts with advanced NET. Eur J Cancer 2013;49.
- Pavel ME, Lombard-Bohas C, Van Cutsem E, Lam DH, Kunz T, Brandt U, et al. Everolimus in patients with advanced, progressive pancreatic neuroendocrine tumors: overall survival results from the phase III RADIANT-3 study after adjusting for crossover bias. J Clin Oncol 2015;33.
- Pommier R, Yao J, Hobday T, Van Cutsem E, Wolin E, Panneerselvam A, et al. Efficacy and safety of everolimus in patients with advanced low- or intermediate-grade pancreatic neuroendocrine tumors previously treated with chemotherapy: a subgroup analysis of the RADIANT-3 trial. Pancreas 2014;43.
- Pommier RF, Wolin EM, Panneerselvam A, Saletan S, Winkler RE, Van Cutsem E. Impact of prior chemotherapy on progression-free survival in patients (pts) with advanced pancreatic neuroendocrine tumors (pNET): results from the RADIANT-3 trial. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.4103.
- Shah MH, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, Sachs C, et al. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): updated results of a randomized, double-blind, placebo-controlled, multicenter phase III trial (RADIANT-3). J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.4_suppl.158.
- Shah MH, Öberg K, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, et al. Treatment of pancreatic neuroendocrine tumors (pNET) with everolimus: improved progression-free survival compared with placebo (RADIANT-3). Pancreas 2011;40:331-2.
- Strosberg JR, Lincy J, Winkler RE, Wolin EM. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): updated results of a randomized, double-blind, placebo-controlled, multicenter, phase III trial (RADIANT-3). J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.4009.
- Wolin E, Pommier R, Lincy J, Winkler R, Yao J. Updated results from the randomized, double-blind, placebo-controlled, multicenter, phase III trial (RADIANT-3) of everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET). Am J Gastroenterol 2011;106.
- Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for advanced, progressive pancreatic neuroendocrine tumors (PNET): final overall survival (OS) from a randomized, double-blind, placebo (PBO)-controlled, multicenter phase 3 RADIANT-3 study. Neuroendocrinology 2015;102.
- Yao JC, Pavel M, Lombard-Bohas C, van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (PNET): final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter phase III trial (RADIANT-3). Ann Oncol 2014;25. https://doi.org/10.1093/annonc/mdu345.1.
- Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (pNET): final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter phase 3 trial (RADIANT-3). Pancreas 2015;44.
- Yao JC, Shah MH, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, et al. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (PNET) (RADIANT-3). Ann Oncol 2010;21:viii4-5.
- Singh S, Carnaghi C, Buzzoni R, Pommier RF, Raderer M, Tomasek J, et al. Efficacy and safety of everolimus in advanced, progressive, nonfunctional neuroendocrine tumors (NET) of the gastrointestinal (GI) tract and unknown primary: a subgroup analysis of the phase III RADIANT-4 trial. J Clin Oncol 2016;34. https://doi.org/10.1200/jco.2016.34.4_suppl.315.
- Singh S, Pavel ME, Strosberg JR, Bubuteishvili-Pacaud L, Degtyarev E, Neary M, et al. Association of disease progression, health-related quality of life (HRQoL), and utility in patients (pts) with advanced, nonfunctional, well-differentiated gastrointestinal (GI) or lung neuroendocrine tumors (NET) in the phase 3 RADIANT-4 trial. J Clin Oncol 2016;34.
- Yao J, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: efficacy and safety results from the placebo-controlled, double-blind, multicenter, phase 3 RADIANT-4 study. Eur J Cancer 2015;51:S709-10. https://doi.org/10.1016/S0959-8049(16)31928-1.
- Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Safety and efficacy of everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: findings of the randomized, placebo-controlled, double-blind, multicenter, phase 3 RADIANT-4 study. Pancreas 2016;45.
- Yao JC, Singh S, Wolin E, Voi M, Pacaud LB, Lincy J, et al. RADIANT-4: efficacy and safety of everolimus in advanced, nonfunctional neuroendocrine tumors (NET) of the lung or gastrointestinal (GI) tract. Ann Oncol 2015;26. https://doi.org/10.1093/annonc/mdv522.01.
- Pavel ME, Strosberg JR, Bubuteishvili-Pacaud L, Degtyarev E, Neary M, Hunger M, et al. Health-related quality of life (HRQoL) in patients with advanced, nonfunctional, well-differentiated gastrointestinal (GI) or lung neuroendocrine tumors (NET) in the phase 3 RADIANT-4 trial. J Clin Oncol 2016;34.
- Anonymous . From ECC 2015 – neuroendocrine cancer: RADIANT-4 trial – NET improvement with everolimus?. Nat Rev Clin Oncol 2015;12. https://doi.org/10.1038/nrclinonc.2012.181.
- Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin EM, et al. Everolimus (EVE) in advanced, nonfunctional, well-differentiated neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin: second interim overall survival (OS) results from the RADIANT-4 study. J Clin Oncol 2016;34.
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. https://doi.org/10.1056/NEJMoa1003825.
- Faivre S, Niccoli P, Raoul JL, Bang Y, Borbath I, Valle JW, et al. Updated overall survival (OS) analysis from a phase III study of sunitinib vs placebo in patients (pts) with advanced, unresectable pancreatic neuroendocrine tumor (NET). Ann Oncol 2012;23.
- Hammel P, Castellano D, Van Cutsem E, Niccoli P, Faivre S, Patyna S, et al. Evaluation of progression-free survival by blinded independent central review in patients with progressive, well-differentiated pancreatic neuroendocrine tumors treated with sunitinib or placebo. Pancreas 2011;40.
- Ishak J, Valle J, Van Cutsem E, Lombard-Bohas C, Ruszniewski P, Sandin R, et al. Overall survival (OS) analysis of sunitinib (SU) after adjustment for crossover (CO) in patients with pancreatic neuroendocrine tumors (NET). Neuroendocrinology 2011;94:27-8.
- Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, Valle JW, et al. Updated safety and efficacy results of the phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET). J Clin Oncol 2010;28. https://doi.org/10.1200/jco.2010.28.15_suppl.4000.
- Raoul JL, Niccoli P, Bang YJ, Borbath I, Lombard-Bohas C, Metrakos P, et al. Sunitinib (SU) vs placebo for treatment of progressive, well-differentiated pancreatic islet cell tumours: results of a phase III, randomised, double-blind trial. Eur J Cancer Supp 2009;7. https://doi.org/10.1016/S1359-6349(09)71223-6.
- Raymond E, Harmon C, Niccoli P, Metrakos P, Borbath I, Bang Y, et al. Impact of baseline Ki-67 index and other baseline characteristics on outcome in a study of sunitinib (SU) for the treatment of advanced, progressive pancreatic neuroendocrine tumor (NET). Neuroendocrinology 2011;94.
- Raymond E, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, et al. Sunitinib (SU) in patients with advanced, progressive pancreatic neuroendocrine tumors (pNET): final overall survival (OS) results from a phase III randomized study including adjustment for crossover. J Clin Oncol 2016;34. https://doi.org/10.1200/jco.2016.34.4_suppl.309.
- Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Evidence of activity and clinical benefit with sunitinib in patients with pancreatic neuroendocrine tumors (NET). Ann Oncol 2010;21.
- Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Updated overall survival (OS) and progression-free survival (PFS) by blinded independent central review (BICR) of sunitinib (SU) versus placebo (PBO) for patients (Pts) with advance unresectable pancreatic neuroendocrine tumors (NET). J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.4008.
- Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Cox proportional hazard analysis of sunitinib (SU) efficacy across subgroups of patients (pts) with progressive pancreatic neuroendocrine tumors (NET). J Clin Oncol 2010;28. https://doi.org/10.1200/jco.2010.28.15_suppl.4031.
- Raymond E, Seitz JF, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, et al. Sunitinib for the treatment of advanced, progressive pancreatic neuroendocrine tumors. Neuroendocrinology 2010;92:54-5.
- Valle J, Faivre S, Raoul J, Bang Y, Patyna S, Lu DR, et al. Phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET): impact of somatostatin analogue (SSA) treatment on progression-free survival (PFS). Ann Oncol 2010;21.
- Valle J, Niccoli P, Raoul JL, Bang YJ, Borbath I, Van Cutsem E, et al. Updated overall survival data from a phase III study of sunitinib vs. placebo in patients with advanced, unresectable pancreatic neuroendocrine tumour (NET). Eur J Cancer 2011;47. https://doi.org/10.1016/S0959-8049(11)71880-9.
- Van Cutsem E, Dahan L, Patyna S, Klademenos D, Lu DR, Chao R, et al. Evaluation of progression-free survival (PFS) by blinded independent central review (BICR) in patients (pts) with progressive, well-differentiated pancreatic neuroendocrine tumours (NET) treated with sunitinib (SU) or placebo. Ann Oncol 2010;21.
- Van Cutsem E, Seitz JF, Raoul J, Valle JW, Faivre SJ, Patyna S, et al. Evaluation of progression-free survival by blinded independent central review in patients with progressive, well-differentiated pancreatic neuroendocrine tumors treated with sunitinib or placebo. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.4_suppl.249.
- Vinik A, Bang Y, Raoul J, Valle JW, Metrakos P, Horsch D, et al. Patient-reported outcomes (PROs) in patients (pts) with pancreatic neuroendocrine tumors (NET) receiving sunitinib (SU) in a Phase III trial. J Clin Oncol 2010;28. https://doi.org/10.1200/jco.2010.28.15_suppl.4003.
- Vinik A, Bang YJ, Raoul JL, Valle J, Metrakos P, Horsch D, et al. Sunitinib for treatment of pancreatic neuroendocrine tumors: patient-reported outcomes and efficacy across patient subgroups in a Phase III trial. Pancreas 2011;40:334-5.
- Vinik A, Cutsem EV, Niccoli P, Raoul JL, Bang YJ, Borbath I, et al. Progression-free survival (PFS) by blinded independent central review (BICR) and updated overall survival (OS) of sunitinib versus placebo for patients with progressive, unresectable, well differentiated pancreatic neuroendocrine tumor (NET). Pancreas 2012;41.
- Vinik A, Van Cutsem E, Niccoli P, Raoul JL, Bang YJ, Borbath I, et al. Updated results from a Phase III trial of sunitinib versus placebo in patients with progressive, unresectable, well-differentiated pancreatic neuroendocrine tumor (NET). J Clin Oncol 2012;30.
- Yao J, Wang JY, Liu Y, Wang B, Li YX, Zhang R, et al. A randomized Phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med Oncol 2014;31. https://doi.org/10.1007/s12032-014-0251-x.
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125-35. https://doi.org/10.1056/NEJMoa1607427.
- van Vliet EI, Krenning EP, Teunissen JJ, Bergsma H, Kam BL, Kwekkeboom DJ. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 2013;54:1689-96. https://doi.org/10.2967/jnumed.112.117408.
- van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL, et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 2015;56:1647-53. https://doi.org/10.2967/jnumed.115.158899.
- Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumours: results of the Phase III NETTER-1 trial. Eur J Cancer 2015;51. https://doi.org/10.1016/S0959-8049(16)31929-3.
- Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumors: results of the Phase III NETTER-1 trial. Pancreas 2016;45.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. https://doi.org/10.1093/jnci/92.3.205.
- National Cancer Institute Division of Cancer Treatment and Diagnosis . Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0 2006. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 9 May 2018).
- MedDRA . Introductory Guide. MedDRA Version 16.1 2013. www.meddra.org/sites/default/files/guidance/file/intguide_16_1_english.pdf (accessed 9 May 2018).
- Pfizer Global Research and Development . Protocol Number: A6181111. A Phase III Randomised, Double-Blind Study of Sunitinib (SU011248, Sutent®) Versus Placebo in Patients With Progressive Advanced Metastatic Well-Differentiated Pancreatic Islet Cell Tumors 2009.
- National Cancer Institute . Common Terminology Criteria for Adverse Events, Version 4.03 2010. www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed 9 May 2018).
- Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-79.
- Casciano R, Wang X, Stern L, Parikh R, Chulikavit M, Willet J, et al. International practice patterns and resource utilization in the treatment of neuroendocrine tumors. Pancreas 2013;42:339-47. https://doi.org/10.1097/MPA.0b013e31826707cc.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56:779-88. https://doi.org/10.1111/j.0006-341X.2000.00779.x.
- Latimer NR, Abrams KR, Lambert PC, Crowther MJ, Morden JP. Assessing Methods for Dealing with Treatment Crossover in Clinical Trials: A Follow-up Simulation Study. Sheffield: Health Economics and Decision Science, School of Health and Related Research (ScHARR), University of Sheffield; 2014.
- Ayyagari R, Neary M, Li S, Zhao C, Higuchi K, Xie JP, et al. Economic evaluation of octreotide LAR versus lanreotide depot in the treatment of metastatic gastrointestinal neuroendocrine tumors. Pancreas 2016;45:471-2.
- Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005-12. https://doi.org/10.1016/S0140-6736(11)61742-X.
- Casciano R, Chulikavit M, Perrin A, Liu Z, Wang X, Garrison LP. Cost-effectiveness of everolimus vs. sunitinib in treating patients with advanced, progressive pancreatic neuroendocrine tumors in the United States. J Med Econ 2012;15:55-64. https://doi.org/10.3111/13696998.2012.720319.
- Muciño Ortega E, Chi-Chan A, Peniche-Otero G, Gutierrez-Colin CI, Herrera-Rojas J, Galindo-Suarez RM. Cost-effectiveness of sunitinib in the treatment of non-resectable advanced pancreatic neuroendocrine tumors in Mexico. Value Health Reg Issues 2012;1:150-5.
- Johns A, Eatock M, Johal S. Cost–utility of sunitinib (SU) for treatment of advanced or metastatic pancreatic neuroendocrine tumors (PNET) in Scotland and Wales. Neuroendocrinology 2012;96.
- Walczak J, Garbacka M, Zawieja J, Przada-Machno P, Kroc J. Treatment of well-differentiated pancreatic neuroendocrine tumors with sunitinib in patients with disease progression: cost–utility and cost-effectiveness analysis. Value Health 2012;15. https://doi.org/10.1016/j.jval.2012.08.2133.
- Signorovitch J, Swallow E, Kantor E, Wang X, Klimovsky J, Haas T, et al. Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol 2013;2. https://doi.org/10.1186/2162-3619-2-32.
- National Life Tables: United Kingdom. Newport: ONS; 2015.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. https://doi.org/10.1177/0272989X12472398.
- Robins JM, Tsiatis AA. Correcting for non-compliance in randomized trials using rank preserving structural failure time models. Comm Stat Theor Meth 2007;20:2609-31. https://doi.org/10.1080/03610929108830654.
- Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health 2012;15:940-7. https://doi.org/10.1016/j.jval.2012.05.004.
- National Life Tables: England. Newport: ONS; 2016.
- Swinburn P, Wang J, Chandiwana D, Mansoor W, Lloyd A. Elicitation of health state utilities in neuroendocrine tumours. J Med Econ 2012;15:681-7. https://doi.org/10.3111/13696998.2012.670175.
- McKenzie L, van der Pol M. Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: the potential to estimate QALYs without generic preference data. Value Health 2009;12:167-71. https://doi.org/10.1111/j.1524-4733.2008.00405.x.
- Sunitinib 12.5 mg, 25 mg, 37.5 mg, 50 mg Hard Capsules (Sutent®). SMC No. (698/11). Glasgow: Scottish Medicines Consortium; 2011.
- Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18090.
- Craig R, Mindell J. Health Survey for England 2012: Health, Social Care and Lifestyles. Leeds: NHS Digital; 2013.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Drugs and Pharmaceutical Electronic Market Information Tool (eMit). London: DHSC; 2016.
- Novartis Pharmaceuticals UK Ltd . Summary of Product Characteristics: Everolimus (Afinitor®) 2015. www.medicines.org.uk/emc/medicine/22281/SPC/Afinitor+Tablets (accessed 22 April 2016).
- Pfizer Ltd . Summary of Product Characteristics: Sunitinib (Sutent®) 2016. www.medicines.org.uk/emc/medicine/18531 (accessed 22 April 2016).
- NHS Reference Costs 2014 to 2015. London: DHSC; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med 2015;29:899-907. https://doi.org/10.1177/0269216315595203.
- Walczak J, Garbacka M, Jarosz J, Zawieja J, Przada-Machno P, Kroc J. Clinical effectiveness and cost-utility analysis of sunitinib for the treatment of pancreatic neuroendocrine tumors. JHPOR 2012;2:48-63.
- Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD, et al. Peptide receptor radionuclide therapy with Lu-177-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas 2014;43:518-25. https://doi.org/10.1097/MPA.0000000000000113.
- Balter H, Victoria T, Mariella T, Javier G, Rodolfo F, Andrea P, et al. 177Lu-labeled agents for neuroendocrine tumor therapy and bone pain palliation in Uruguay. Curr Radiopharm 2016;9:85-93. https://doi.org/10.2174/1874471008666150313112620.
- Barber TW, Hofman MS, Thomson BN, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol 2012;38:64-71. https://doi.org/10.1016/j.ejso.2011.08.129.
- Basu S, Ranade R, Thapa P. Metastatic Neuroendocrine tumor with extensive bone marrow involvement at diagnosis: evaluation of response and hematological toxicity profile of PRRT with (177)Lu-DOTATATE. World J Nucl Med 2016;15:38-43. https://doi.org/10.4103/1450-1147.165353.
- Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I–II study. Eur J Nucl Med Mol Imaging 2011;38:2125-35. https://doi.org/10.1007/s00259-011-1902-1.
- Bodei L, Kidd M, Modlin IM, Severi S, Drozdov I, Nicolini S, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2016;43:839-51. https://doi.org/10.1007/s00259-015-3250-z.
- Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011;38:302-11. https://doi.org/10.1007/s00259-010-1631-x.
- Claringbold PG, Price RA, Turner JH. Phase I–II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm 2012;27:561-9. https://doi.org/10.1089/cbr.2012.1276.
- Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology 2016;103:432-9. https://doi.org/10.1159/000434723.
- Claringbold PG, Turner JH. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): a Phase I study. Cancer Biother Radiopharm 2015;30:261-9. https://doi.org/10.1089/cbr.2015.1876.
- Ezziddin S, Opitz M, Attassi M, Biermann K, Sabet A, Guhlke S, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2011;38:459-66. https://doi.org/10.1007/s00259-010-1610-2.
- Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, et al. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J Nucl Med 2011;52:1197-203. https://doi.org/10.2967/jnumed.111.090373.
- Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 2014;55:183-90. https://doi.org/10.2967/jnumed.113.125336.
- Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014;41:925-33. https://doi.org/10.1007/s00259-013-2677-3.
- Ilan E, Sandstrom M, Wassberg C, Sundin A, Garske-Roman U, Eriksson B, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using Lu-177-DOTATATE. J Nucl Med 2015;56:177-82. https://doi.org/10.2967/jnumed.114.148437.
- Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V, et al. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging 2014;41:1831-44. https://doi.org/10.1007/s00259-014-2788-5.
- Kunikowska J, Królicki L, Sowa-Staszczak A, Pawlak D, Hubalewska-Dydejczyk A, Mikołajczak R. Nephrotoxicity after PRRT – still a serious clinical problem? Renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATATE and 90Y/177Lu-DOTATATE. Endokrynol Pol 2013;64:13-20.
- Kwekkeboom DJ, Bakker WH, Kam BL, Teunissen JJM, Kooij PPM, Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue Lu-177-DOTA(0),Tyr(3) octreotate. Eur J Nucl Med Mol Imaging 2003;30:417-22. https://doi.org/10.1007/s00259-002-1050-8.
- Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog Lu-177-DOTA(0),Tyr(3) octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23:2754-62. https://doi.org/10.1200/JCO.2005.08.066.
- Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124-30. https://doi.org/10.1200/JCO.2007.15.2553.
- Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a Phase II study. Eur J Nucl Med Mol Imaging 2014;41:1845-51. https://doi.org/10.1007/s00259-014-2735-5.
- Sabet A, Khalaf F, Haslerud T, Al-Zreiqat A, Sabet A, Simon B, et al. Bone metastases in GEP-NET: response and long-term outcome after PRRT from a follow-up analysis. Am J Nucl Med Mol Imaging 2013;3:437-45.
- Sabet A, Khalaf F, Mahjoob S, Al-Zreiqat A, Biersack HJ, Ezziddin S. May bone-targeted radionuclide therapy overcome PRRT-refractory osseous disease in NET? A pilot report on (188)Re-HEDP treatment in progressive bone metastases after (177)Lu-octreotate. Am J Nucl Med Mol Imaging 2013;4:80-8.
- Sabet A, Khalaf F, Yong-Hing CJ, Sabet A, Haslerud T, Ahmadzadehfar H, et al. Can peptide receptor radionuclide therapy be safely applied in florid bone metastases? A pilot analysis of late stage osseous involvement. Nuklearmedizin 2014;53:54-9. https://doi.org/10.3413/Nukmed-0614-13-08.
- Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging 2015;42:1238-46. https://doi.org/10.1007/s00259-015-3041-6.
- Sansovini M, Severi S, Ambrosetti A, Monti M, Nanni O, Sarnelli A, et al. Treatment with the radiolabelled somatostatin analog Lu-177-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 2013;97:347-54. https://doi.org/10.1159/000348394.
- Severi S, Sansovini M, Ianniello A, Bodei L, Nicolini S, Ibrahim T, et al. Feasibility and utility of re-treatment with (177)Lu-DOTATATE in GEP-NENs relapsed after treatment with (90)Y-DOTATOC. Eur J Nucl Med Mol Imaging 2015;42:1955-63. https://doi.org/10.1007/s00259-015-3105-7.
- Soydal Ç, Peker A, Özkan E, Küçük ÖN, Kir MK. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turk J Med Sci 2016;46:409-13. https://doi.org/10.3906/sag-1412-11.
- van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging 2007;34:1219-27. https://doi.org/10.1007/s00259-006-0355-4.
- van Essen M, Krenning EP, Kam BL, de Herder WW, Feelders RA, Kwekkeboom DJ. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med 2010;51:383-90. https://doi.org/10.2967/jnumed.109.068957.
- Advanced Accelerator Applications . A Phase I II Single Arm Study to Evaluate the Efficacy of 177Lu-DOTA0-Tyr3-0ctreotate in Patients With Somatostatin Receptor Positive Tumors 2016.
- Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:1556-7. https://doi.org/10.1056/NEJMoa1316158.
- National Schedule of Reference Costs: The Main Schedule. London: Department of Health and Social Care; 2015.
- Everolimus, 5 mg, 10 mg Tablets (Afinitor®). SMC No. (777/12). Glasgow: Scottish Medicines Consortium; 2012.
- Sunitinib (Sutent®): Reference No. 390. Penarth: All Wales Medicines Strategy Group; 2011.
- Everolimus (Afinitor®): Reference No. 142. Penarth: All Wales Medicines Strategy Group; 2012.
- Anonymous . From ECC 2015 – neuroendocrine cancer: SSA therapies – 177Lu-DOTATATE is a better one in NETTER-1. Nat Rev Clin Oncol 2015;12.
- Mujica-Mota RE, Roberts M, Abel G, Elliott M, Lyratzopoulos G, Roland M, et al. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: evidence from a national survey. Qual Life Res 2015;24:909-18. https://doi.org/10.1007/s11136-014–-0820-7.
- Crathorne L, Huxley N, Haasova M, Snowsill T, Jones-Hughes T, Hoyle M, et al. The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20130.
- Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. PharmacoEconomics 2013;31:277-88. https://doi.org/10.1007/s40273-013-0033-x.
- Bagust A, Beale S. Survival analysis and extrapolation modeling of time-to-event clinical trial data for economic evaluation: an alternative approach. Med Decis Making 2014;34:343-51. https://doi.org/10.1177/0272989X13497998.
- Young TA, Mukuria C, Rowen D, Brazier JE, Longworth L. Mapping functions in health-related quality of life: mapping from two cancer-specific health-related quality-of-life instruments to EQ-5D-3L. Med Decis Making 2015;35:912-26. https://doi.org/10.1177/0272989X15587497.
- Advanced Accelerator Applications . A Multicentre, Stratified, Open, Randomized, Comparator-Controlled, Parallel-Group Phase III Study Comparing Treatment With 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in Patients With Inoperable, Progressive, Somatostatin Receptor Positive, Midgut Carcinoid Tumours 2016.
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0008933.
- Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Eastern Cooperative Oncology Group . Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897-904. https://doi.org/10.1200/JCO.2005.03.616.
- Yao JC, Ricci S, Winkler RE, Jehl V, Pavel ME. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors (NET): updated safety and efficacy results from RADIANT-2. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.4011.
- Strosberg JR, Wolin EM, Chasen B, Kulke MH, Bushnell DL, Caplin ME, et al. NETTER-1 phase III: progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-DOTATATE. J Clin Oncol 2016;34. https://doi.org/10.1200/jco.2016.34.4_suppl.194.
- Schonfeld WH, Eikin EP, Woltering EA, Modlin IM, Anthony L, Villa KF, et al. The cost-effectiveness of octreotide acetate in the treatment of carcinoid syndrome and VIPoma. Int J Technol Assess Health Care 1998;14:514-25. https://doi.org/10.1017/S0266462300011491.
- Takemoto ML, Fernandes RA, Chinen R, Alves MR. Cost-effectiveness of octreotide LAR in patients with metastatic neuroendocrine midgut tumours from the private payer perspective in Brazil. Value Health 2010;13. https://doi.org/10.1016/S1098-3015(11)71972-6.
- Kansal A, Chao R, Patyna S, Scheijbeler H, Klok R, Sandin R, et al. Cost-effectiveness of sunitinib in patients (pts) with advanced or metastatic pancreatic neuroendocrine tumors (P-NET) in the Netherlands. Neuroendocrinology 2012;96.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Marty R, Roze S, Kurth H. Decision-tree model for health economic comparison of two long-acting somatostatin receptor ligand devices in France, Germany, and the UK. Med Devices 2012;5:39-44. https://doi.org/10.2147/MDER.S30913.
- Soares M, Ines M, Contente M. Cost-effectiveness of sunitinib in patients with advanced or metastatic pancreatic neuroendocrine tumors in Portugal. Value Health 2011;14. https://doi.org/10.1016/j.jval.2011.08.1164.
- Roze S, Kurth H, Maury Le Breton A. Injection of long-acting somatostatin analogs: a cost consequence analysis in France, Germany, the United Kingdom and the United States. Value Health 2011;14. https://doi.org/10.1016/j.jval.2011.02.909.
- Swinburn P, Chandiwana D, Quadri N, Wang X, Lloyd AJ. Elicitation of health state utilities in neuroendocrine tumours. Value Health 2011;14. https://doi.org/10.1016/j.jval.2011.08.1226.
- Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. https://doi.org/10.1200/JCO.2009.22.8510.
- Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors – the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003;21:2689-96. https://doi.org/10.1200/JCO.2003.12.142.
- Meyer T, Qian W, Caplin ME, Armstrong G, Lao-Sirieix SH, Hardy R, et al. Capecitabine and streptozocin ± cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur J Cancer 2014;50:902-11. https://doi.org/10.1016/j.ejca.2013.12.011.
- Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519-23. https://doi.org/10.1056/NEJM199202203260804.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. https://doi.org/10.1177/0272989X12458724.
- Kulke MH, Ruszniewski P, Van Cutsem E, Lombard-Bohas C, Valle JW, De Herder WW, et al. A randomized open-label Phase II study of everolimus alone or in combination with pasireotide LAR in advanced, progressive pancreatic neuroendocrine tumors (pNET): COOPERATE-2 trial. Neuroendocrinology 2015;102.
- Salazar R, Verslype C, Baudin E, Libutti SK, Yao JC, Buzzoni R, et al. Phase II studies of BEZ235 in patients with advanced pancreatic neuroendocrine tumors (pNET). J Clin Oncol 2015;33.
- Yao J, Wang J, Liu Y, Wang B, Li YX, Zhang R, et al. Retraction note to: A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med Oncol 2015;32. https://doi.org/10.1007/s12032-015-0661-4.
- Bajetta E, Catena L, Fazio N, Pusceddu S, Biondani P, Blanco G, et al. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer 2014;120:2457-63. https://doi.org/10.1002/cncr.28726.
- Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment Phase II study of depot octreotide with bevacizumab and pegylated interferon alfa-2b. J Clin Oncol 2008;26:1316-23. https://doi.org/10.1200/JCO.2007.13.6374.
- Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. https://doi.org/10.1056/NEJMoa1316158.
- Jacobsen MB, Hanssen LE. Clinical effects of octreotide compared to placebo in patients with gastrointestinal neuroendocrine tumours: report on a double-blind, randomized trial. J Intern Med 1995;237:269-75. https://doi.org/10.1111/j.1365-2796.1995.tb01175.x.
- Arnold R, Müller H, Schade-Brittinger C, Rinke A, Klose K, Barth P, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol 2009;27.
- Wolin EM, Jarzab B, Eriksson B, Walter T, Toumpanakis C, Morse MA, et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther 2015;9:5075-86. https://doi.org/10.2147/DDDT.S84177.
- Castellano D, Bajetta E, Panneerselvam A, Saletan X, Kocha W, O’Dorisio T, et al. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the Phase III RADIANT-2 study. Oncologist 2013;18:46-53. https://doi.org/10.1634/theoncologist.2012-0263.
- Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, et al. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest 2013;143:955-62. https://doi.org/10.1378/chest.12-1108.
- Pavel M, Hainsworth JD, Baudin E, Peeters M, Hoersch D, Anthony L, et al. A randomized, double-blind, placebo-controlled, multicenter Phase III trial of everolimus + octreotide LAR vs placebo + octreotide LAR in patients with advanced neuroendocrine tumors (net) (RADIANT-2). Ann Oncol 2010;21.
- Ferolla P, Faggiano A, Grimaldi F, Ferone D, Scarpelli G, Ramundo V, et al. Shortened interval of long-acting octreotide administration is effective in patients with well-differentiated neuroendocrine carcinomas in progression on standard doses. J Endocrinol Invest 2012;35:326-31.
- Campana D, Nori F, Pezzilli R, Piscitelli L, Santini D, Brocchi E, et al. Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocr Relat Cancer 2008;15:337-42. https://doi.org/10.1677/ERC-07-0251.
- Grozinsky-Glasberg S, Kaltsas G, Gur C, Gal E, Thomas D, Fichman S, et al. Long-acting somatostatin analogues are an effective treatment for type 1 gastric carcinoid tumours. Eur J Endocrinol 2008;159:475-82. https://doi.org/10.1530/EJE-08-0420.
- Panzuto F, Di Fonzo M, Iannicelli E, Sciuto R, Maini CL, Capurso G, et al. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol 2006;17:461-6. https://doi.org/10.1093/annonc/mdj113.
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242-58. https://doi.org/10.1002/sim.5984.
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-139.
- Panobinostat for Treating Multiple Myeloma in People Who Have Received at Least One Prior Therapy. London: NICE; 2016.
- Mapi Research Trust . About ProQolid n.d. https://eprovide.mapi-trust.org/about/about-proqolid (accessed 18 July 2018).
- Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer 2005;41:2613-19. https://doi.org/10.1016/j.ejca.2005.05.017.
- Cornish D, Holterhues C, Van de Poll-Franse LV, Coebergh JW, Nijsten T. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 2009;20:vi51-8. https://doi.org/10.1093/annonc/mdp255.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ 2013;14:367-72. https://doi.org/10.1007/s10198-013-0471-6.
- Doble B, Lorgelly P. Mapping the EORTC QLQ-C30 onto the EQ-5D-3L: assessing the external validity of existing mapping algorithms. Qual Life Res 2016;25:891-91. https://doi.org/10.1007/s11136-015-1116-2.
- Wong SQ, Fellowes A, Doig K, Ellul J, Bosma TJ, Irwin D, et al. Assessing the clinical value of targeted massively parallel sequencing in a longitudinal, prospective population-based study of cancer patients. Br J Cancer 2015;112:1411-20. https://doi.org/10.1038/bjc.2015.80.
- Versteegh MM, Leunis A, Luime JJ, Boggild M, Uyl-de Groot CA, Stolk EA. Mapping QLQ-C30, HAQ, and MSIS-29 on EQ-5D. Med Decis Making 2012;32:554-68. https://doi.org/10.1177/0272989X11427761.
- Cramer B, Xing M, Webber G, Kim HS. Longitudinal quality of life assessment in patients with hepatic tumors treated with CT-guided radiofrequency ablation. J Vasc Interv Radiol 2014;25.
- Pavel M, Unger N, Borbath I, Ricci S, Hwang TL, Brechenmacher T, et al. Safety and QOL in patients with advanced NET in a phase 3b expanded access study of everolimus. Target Oncol 2016;11:667-75. https://doi.org/10.1007/s11523-016-0440-y.
- Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Clin Oncol 2004;22:2724-9. https://doi.org/10.1200/JCO.2004.10.016.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
Appendix 1 Literature search strategies
Literature searching was undertaken in May 2016, with update searches carried out in September 2016.
Searching of bibliographic and ongoing trials databases
The following search strategies were run on 19 May 2016 and rerun on 29 September 2016.
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 1334.
| # | Searches | Results |
|---|---|---|
| 1 | exp Neuroendocrine Tumors/ | 146,579 |
| 2 | Carcinoma, Neuroendocrine/ | 2939 |
| 3 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 46,552 |
| 4 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 52,214 |
| 5 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 70,454 |
| 6 | 1 or 2 or 3 or 4 or 5 | 292,693 |
| 7 | (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6).ti,ab,kw. or Everolimus/ | 4765 |
| 8 | (Lanreotide or Somatuline or ITM-014 or 108736-35-2).ti,ab,kw. | 701 |
| 9 | (Lutetium-177 DOTATATE or Lutetium or DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9).ti,ab,kw. | 969 |
| 10 | (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4).ti,ab,kw. | 4011 |
| 11 | 7 or 8 or 9 or 10 | 9910 |
| 12 | 6 and 11 | 1334 |
EMBASE
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 4863.
| # | Searches | Results |
|---|---|---|
| 1 | exp neuroendocrine tumor/ | 60,694 |
| 2 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 62,025 |
| 3 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 68,495 |
| 4 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 109,421 |
| 5 | 1 or 2 or 3 or 4 | 273,156 |
| 6 | (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6).ti,ab,kw. | 10,357 |
| 7 | everolimus/ | 18,280 |
| 8 | (Lanreotide or Somatuline or ITM-014 or 108736-35-2).ti,ab,kw. | 1072 |
| 9 | angiopeptin/ | 2770 |
| 10 | (Lutetium-177 DOTATATE or Lutetium or DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9).ti,ab,kw. | 2025 |
| 11 | lutetium 177/ | 1859 |
| 12 | (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4).ti,ab,kw. | 7888 |
| 13 | sunitinib/ | 16,334 |
| 14 | 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 37,159 |
| 15 | 5 and 14 | 4863 |
The Cochrane Library
Host: Wiley Online Library.
Data parameters: Database of Abstracts of Reviews of Effect, Issue 2 of 4, April 2015; Cochrane Central Register of Controlled Trials: Issue 4 of 12, April 2016; Health Technology Assessment database, Issue 2 of 4, April 2016; NHS Economic Evaluation Database (NHS EED), Issue 2 of 4, April 2015.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 247.
#1 MeSH descriptor: [Neuroendocrine Tumors] explode all trees (1523)
#2 MeSH descriptor: [Carcinoma, Neuroendocrine] this term only (10)
#3 (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs) (2515)
#4 ((neuro or endocrine or carcinoid* or carcinoma*) near/5 (tumour* or tumor*)) (1608)
#5 (((low* or intermediate) near/3 grade) or (“grade 1” or “grade 2”)) (6921)
#6 #1 or #2 or #3 or #4 or #5 (12,098)
#7 (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6) (1484)
#8 MeSH descriptor: [Everolimus] this term only (390)
#9 (Lanreotide or Somatuline or ITM-014 or 108736-35-2) (137)
#10 (Lutetium-177 DOTATATE or Lutetium or DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9) (105)
#11 (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4) (436)
#12 #7 or #8 or #9 or #10 or #11 (2097)
#13 #6 and #12 (251)
Web of Science
Host: Thomson Reuters.
Data parameters: Science Citation Index Expanded (SCIE),1900 to present; Social Sciences Citation Index (SSCI), 1956 to present; Conference Proceedings Citation Index – Science (CPCI-S), 1990 to present; Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH), 1990 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 1875.
| #10 | 1875 |
#9 AND #4 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #9 | 16,520 |
#8 OR #7 OR #6 OR #5 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #8 | 6271 |
TOPIC: (((Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #7 | 2331 |
TOPIC: (((Lutetium-177 DOTATATE or Lutetium or DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #6 | 1080 |
TOPIC: (((Lanreotide or Somatuline or ITM-014 or 108736-35-2))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #5 | 7488 |
TOPIC: (((everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #4 | 405,576 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #3 | 76,335 |
TOPIC: (((((low* or intermediate) near/2 grade) or (“grade 1” or “grade 2”)))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #2 | 41,490 |
TOPIC: ((((neuro or endocrine or carcinoid* or carcinoma*) near/2 (tumour* or tumor*)))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #1 | 296,189 |
TOPIC: (((Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Current Controlled Trials
Date searched: 25 May 2016.
Searched via www.isrctn.com/
Total studies identified: 24.
Duplicates removed: 0.
Unique studies to screen: 24.
| Field searched | Search terms | n identified | n for screening |
|---|---|---|---|
| Text Search | everolimus | 12 | 12 |
| Text Search | afinitor | 0 | 0 |
| Text Search | affinitor | 1 | 0 |
| Text Search | VOTUBIA | 9 | 1 |
| Text Search | Zortress | 0 | 0 |
| Text Search | CERTICAN | 0 | 0 |
| Text Search | xience | 3 | 0 |
| Text Search | RAD001 | 3 | 0 |
| Text Search | “RAD 001” | 0 | 0 |
| Text Search | SDZ RAD | 0 | 0 |
| Text Search | SDZRAD | 0 | 0 |
| Text Search | 159351-69-6 | 0 | 0 |
| Text Search | Lanreotide | 1 | 1 |
| Text Search | Somatuline | 0 | 0 |
| Text Search | ITM-014 | 0 | 0 |
| Text Search | 108736-35-2 | 0 | 0 |
| Text Search | Lutetium-177 | 0 | 0 |
| Text Search | Lutetium | 0 | 0 |
| Text Search | Lutathera | 0 | 0 |
| Text Search | Sunitinib | 9 | 9 |
| Text Search | Sutent | 4 | 1 |
| Text Search | SU 011248 | 0 | 0 |
| Text Search | 557795-19-4 | 0 | 0 |
ClinicalTrials.gov
Date searched: 26 May 2016.
Searched via https://clinicaltrials.gov/ct2/search/advanced
Total studies identified: 173.
Duplicates removed: 18.
Unique studies to screen: 155.
| Field searched | Search terms | n identified | n for screening |
|---|---|---|---|
| Text Search |
Conditions: Neuroendocrine Interventions: Everolimus |
85 | 85 |
| Text Search |
Conditions: NETs Intervention: Everolimus |
12 | 1 |
| Text Search |
Conditions: Neuroendocrine Intervention: afinitor |
85 | 7 |
| Text Search |
Conditions: NETs Intervention: afinitor |
12 | 0 |
| Text Search |
Population: Neuroendocrine Intervention: afinitor |
0 | 0 |
| Text Search |
Conditions: NETs Intervention: afinitor |
0 | 0 |
| VOTUBIA | |||
| Text Search |
Conditions: Neuroendocrine Intervention: Zortress |
85 | 3 |
| Text Search |
Conditions: NETs Intervention: Zortress |
12 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: CERTICAN |
85 | 0 |
| Text Search |
Conditions: NETs Intervention: CERTICAN |
12 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: xience |
0 | 0 |
| Text Search |
Conditions: NETs Intervention: xience |
0 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: RAD001 |
85 | 0 |
| Text Search |
Conditions: NETs Intervention: RAD001 |
12 | 0 |
| Text Search | “RAD 001” | 3 | 0 |
| Text Search | SDZ RAD | 0 | 0 |
| Text Search | SDZRAD | 0 | 0 |
| Text Search | 159351-69-6 | 1 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: Lanreotide |
17 | 17 |
|
Conditions: NETs Intervention: Lanreotide |
6 | 0 | |
| Text Search |
Conditions: Neuroendocrine Intervention: Somatuline |
17 | 0 |
|
Conditions: Neuroendocrine Intervention: Somatuline |
6 | 0 | |
| Text Search | ITM-014 | 0 | 0 |
| Text Search | 108736-35-2 | 0 | 0 |
| Text Search | Lutetium | 21 | 21 |
| Text Search | Lutathera | 1 | 1 |
| Text Search |
Conditions: Neuroendocrine Intervention: Sunitinib |
33 | 33 |
| Text Search |
Conditions: NETs Intervention: Sunitinib |
33 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: Sutent |
33 | 1 |
| Text Search |
Conditions: NETs Intervention: Sutent |
0 | 0 |
| Text Search |
Conditions: Neuroendocrine Intervention: SU 011248 |
33 | 1 |
| Text Search |
Conditions: Neuroendocrine Intervention: SU 011248 |
0 | 0 |
| Text Search | 557795-19-4 | 0 | 0 |
Web searching
US Food and Drug Administration website
Searched via www.fda.gov/Drugs/
Date searched: 6 July 2016.
| Search term | Hits | Included |
|---|---|---|
| Everolimus | 87 | 7 |
| Afinitor | 40 | 4 |
| lanreotide | 31 | 1 |
| Lutetium-177 | 0 | 0 |
| Lutetium | 3 | 0 |
| Dotatate | 4 | 0 |
| Sunitinib | 61 | 3 |
Drugs@FDA
Searched via www.accessdata.fda.gov/scripts/cder/drugsatfda/
Date searched: 6 July 2016.
| Search term | Hits | Included |
|---|---|---|
| Everolimus/ Zortess | 2 | 2 |
| Lanreotide | 0 | 0 |
| Lutetium-177 | 0 | 0 |
| Lutetium | 6 | 1 |
| Sunitinib | 2 | 2 |
European Medicines Agency
Searched via www.ema.europa.eu/ema/index.jsp?curl=pages/includes/medicines/medicines_landing_page.jsp
Date sarched: 6 July 2016.
| Search term | Hits | Included |
|---|---|---|
| Everolimus | 2 | 2 |
| Lanreotide | 0 | 0 |
| Lutetium-177 | 0 | 0 |
| Lutetium | 6 | 1 |
| Sunitinib | 2 | 2 |
Economic study searches
| Database | Hits |
|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 299 |
| EMBASE | 716 |
| NHS EED | 3 |
| Web of Science | 123 |
| EconLit | 1 |
| Total | 1143 |
| Duplicates | 247 |
| Unique records to screen | 896 |
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 299.
| # | Searches | Results |
|---|---|---|
| 1 | exp Neuroendocrine Tumors/ | 146,579 |
| 2 | Carcinoma, Neuroendocrine/ | 2939 |
| 3 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 46,552 |
| 4 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 52,214 |
| 5 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 70,454 |
| 6 | 1 or 2 or 3 or 4 or 5 | 292,693 |
| 7 | (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6).ti,ab,kw. or Everolimus/ | 4765 |
| 8 | (Lanreotide or Somatuline or ITM-014 or 108736-35-2).ti,ab,kw. | 701 |
| 9 | (Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9).ti,ab,kw. | 969 |
| 10 | (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4).ti,ab,kw. | 4011 |
| 11 | 7 or 8 or 9 or 10 | 9910 |
| 12 | exp Economics/ | 526,611 |
| 13 | ec.fs. | 363,988 |
| 14 | economics, medical/ | 8869 |
| 15 | Economics, Nursing/ | 3937 |
| 16 | Economics, Pharmaceutical/ | 2619 |
| 17 | Economics, Hospital/ | 10,680 |
| 18 | (economic* or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration* or expenditure or expenditures or budget* or afford* or pharmacoeconomic or pharmaco-economic*).tw. | 502,094 |
| 19 | (cba or cea or cua).ti,ab. | 29,189 |
| 20 | exp “Fees and Charges”/ | 28,197 |
| 21 | (fee or fees or charge* or preference*).tw. | 304,749 |
| 22 | (fiscal or funding or financial or finance).tw. | 102,276 |
| 23 | exp “Costs and Cost Analysis”/ | 197,689 |
| 24 | exp Health Care Costs/ | 52,041 |
| 25 | cost*.tw. | 433,649 |
| 26 | exp decision support techniques/ | 66,124 |
| 27 | exp Models, Economic/ | 11,688 |
| 28 | exp Statistical Model/ | 314,512 |
| 29 | markov*.tw. | 17,327 |
| 30 | markov chains/ | 11,224 |
| 31 | monte carlo.tw. | 36,521 |
| 32 | monte carlo method/ | 22,617 |
| 33 | (decision adj2 (tree* or analy* or model*)).tw. | 15,053 |
| 34 | (survival adj3 analys*).tw. | 34,011 |
| 35 | “Deductibles and Coinsurance”/ | 1525 |
| 36 | exp Health expenditures/ | 17,233 |
| 37 | uncertain*.tw. | 118,654 |
| 38 | uncertainty/ | 8052 |
| 39 | (quality adj3 life).tw. | 191,145 |
| 40 | quality of life/ | 137,192 |
| 41 | value of life/ | 5500 |
| 42 | Quality-adjusted life years/ | 8422 |
| 43 | (qol* or qoly or qolys or hrqol* or qaly or qalys or qale or qales).tw. | 41,092 |
| 44 | (sensitivity analys* or discrete event or “willingness to pay” or quality-adjusted life year* or quality adjusted life year* or quality-adjusted life expectanc* or quality adjusted life expectanc*).tw. | 28,468 |
| 45 | utilit*.tw. | 147,802 |
| 46 | valu*.tw. | 1,601,201 |
| 47 | exp hospitalization/ | 181,928 |
| 48 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 | 3,893,099 |
| 49 | 6 and 11 and 48 | 299 |
EMBASE
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 716.
| # | Searches | Results |
|---|---|---|
| 1 | exp neuroendocrine tumor/ | 60,694 |
| 2 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 62,025 |
| 3 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 68,495 |
| 4 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 109,421 |
| 5 | 1 or 2 or 3 or 4 | 273,156 |
| 6 | (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6).ti,ab,kw. | 10,357 |
| 7 | everolimus/ | 18,280 |
| 8 | (Lanreotide or Somatuline or ITM-014 or 108736-35-2).ti,ab,kw. | 1072 |
| 9 | angiopeptin/ | 2770 |
| 10 | (Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9).ti,ab,kw. | 2025 |
| 11 | lutetium 177/ | 1859 |
| 12 | (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4).ti,ab,kw. | 7888 |
| 13 | sunitinib/ | 16,334 |
| 14 | 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | 37,159 |
| 15 | exp Economics/ | 233,417 |
| 16 | exp health-economics/ | 690,449 |
| 17 | exp economic-evaluation/ | 242,008 |
| 18 | exp pharmacoeconomics/ | 178,599 |
| 19 | (economic* or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration* or expenditure or expenditures or budget* or afford* or pharmacoeconomic or pharmaco-economic*).tw. | 663,479 |
| 20 | Cost benefit analysis/ | 71,608 |
| 21 | Cost effectiveness analysis/ | 114,041 |
| 22 | Cost minimization analysis/ | 2798 |
| 23 | (cba or cea or cua).ti,ab. | 38,314 |
| 24 | “cost of illness”/ | 16,348 |
| 25 | exp “health care cost”/ | 233,023 |
| 26 | cost*.tw. | 561,500 |
| 27 | (fee or fees or charge* or preference*).tw. | 317,066 |
| 28 | (fiscal or funding or financial or finance).tw. | 127,595 |
| 29 | markov*.tw. | 20,044 |
| 30 | monte carlo.tw. | 32,840 |
| 31 | (decision adj2 (tree* or analy* or model*)).tw. | 20,035 |
| 32 | (survival adj3 analys*).tw. | 50,880 |
| 33 | (quality adj3 life).tw. | 283,909 |
| 34 | (sensitivity analys* or discrete event or “willingness to pay” or quality-adjusted life year* or quality adjusted life year* or quality-adjusted life expectanc* or quality adjusted life expectanc* or uncertain*).tw. | 182,839 |
| 35 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 | 2,444,303 |
| 36 | 5 and 14 and 35 | 716 |
NHS Economic Evaluation Database
Host: Wiley Online Library.
Data parameters: Issue 2 of 4, April 2015.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 3.
#1 MeSH descriptor: [Neuroendocrine Tumors] explode all trees (1523)
#2 MeSH descriptor: [Carcinoma, Neuroendocrine] this term only (10)
#3 (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs) (2515)
#4 ((neuro or endocrine or carcinoid* or carcinoma*) near/5 (tumour* or tumor*)) (1608)
#5 (((low* or intermediate) near/3 grade) or (“grade 1” or “grade 2”)) (6921)
#6 #1 or #2 or #3 or #4 or #5 (12,098)
#7 (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6) (1484)
#8 MeSH descriptor: [Everolimus] this term only (390)
#9 (Lanreotide or Somatuline or ITM-014 or 108736-35-2) (137)
#10 (Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9) (105)
#11 (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4) (436)
#12 #7 or #8 or #9 or #10 or #11 (2097)
#13 #6 and #12 (251)
Web of Science
Host: Thomson Reuters.
Data parameters: Science Citation Index Expanded (SCIE),1900 to present; Social Sciences Citation Index (SSCI), 1956 to present; Conference Proceedings Citation Index – Science (CPCI-S), 1990 to present; Conference Proceedings Citation Index – Social Science & Humanities (CPCI-SSH), 1990 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 123.
| #13 | 123 |
#12 AND #9 AND #4 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #12 | 3,777,475 |
#11 OR #10 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #11 | 52,810 | TOPIC: ((decision near/1 (model* or tree* or analy*)))Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years | Edit | Select to combine sets. □ | Select to delete this set. □ |
| #10 | 3,745,638 |
TOPIC: ((pharmacoeconomic* or pharmaco-economic* or economic* or price* or pricing* or discount or discounted or discounts or discounting or ration* or cost* or budget* or fiscal or funding or financial or finance* or expenditure* or afford* or cba or cea or cua or “health utilit*” or “value for money” or cba or cea or cua or fee or fees or charge* or preference* or fiscal or funding or financial or finance or monte carlo or markov or “resource* alloca*” or “resource* use”)) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #9 | 16,520 |
#8 OR #7 OR #6 OR #5 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #8 | 6271 |
TOPIC: (((Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #7 | 2331 |
TOPIC: (((Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #6 | 1080 |
TOPIC: (((Lanreotide or Somatuline or ITM-014 or 108736-35-2))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #5 | 7488 |
TOPIC: (((everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #4 | 405,576 |
#3 OR #2 OR #1 Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #3 | 76,335 |
TOPIC: (((((low* or intermediate) near/2 grade) or (“grade 1” or “grade 2”)))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #2 | 41,490 |
TOPIC: ((((neuro or endocrine or carcinoid* or carcinoma*) near/2 (tumour* or tumor*)))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Edit | Select to combine sets. □ | Select to delete this set. □ |
| #1 | 296,189 |
TOPIC: (((Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs))) Indexes = SCI-EXPANDED, SSCI, CPCI-S, CPCI-SSH Timespan = All years |
Ovid EconLit
Host: EBSCOhost.
Data parameters: 1886 to present.
Date searched: 19 May 2016.
Searcher: CC.
Hits: 1.
| # | Query | Limiters/expanders | Last run via | Results |
|---|---|---|---|---|
| S4 | TI ( (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4) ) OR AB ( (Sunitinib or sutent or SU011248 or “SU 011248” or SU11248 or SU 11248 or suo11248 or su010398 or “su 010398” or su010398 or pha 2909040ad or pha2909040ad or 557795-19-4) ) | Search modes - Boolean/Phrase |
Interface - EBSCOhost Research Databases Search Screen - Advanced Search Database - EconLit |
0 |
| S3 | TI ( (Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9). ) OR AB ( (Lutetium-177 DOTATATE or lutecium or Lutetium 177 or Lutetium-177 or Lutetium177 or 177LU or 177 LU or Lu177 or LU 177 or Lutathera or 14265-75-9). ) | Search modes - Boolean/Phrase |
Interface - EBSCOhost Research Databases Search Screen - Advanced Search Database - EconLit |
0 |
| S2 | TI ( (Lanreotide or Somatuline or ITM-014 or 108736-35-2) ) OR AB ( (Lanreotide or Somatuline or ITM-014 or 108736-35-2) ) | Search modes - Boolean/Phrase |
Interface - EBSCOhost Research Databases Search Screen - Advanced Search Database - EconLit |
0 |
| S1 | TI ( (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6) ) OR AB ( (everolimus or afinitor or affinitor or VOTUBIA or Zortress or CERTICAN or xience or RAD001 or “RAD 001” or SDZ RAD or SDZ-RAD or SDZRAD or 159351-69-6) ) | Search modes - Boolean/Phrase |
Interface - EBSCOhost Research Databases Search Screen - Advanced Search Database - EconLit |
1 |
Appendix 2 Review of effectiveness for non-randomised controlled trial evidence for 177Lu-DOTATATE
Methods
This section details the methods used in the identification and synthesis of studies reporting non-randomised 177Lu-DOTATATE data, as no relevant RCT data were available for 177Lu-DOTATATE.
Identification of studies
Study identification was undertaken in May 2016, with update searches carried out in September 2016. Our literature searches were not limited by study design and so the same searches were used to identify randomised and non-randomised studies.
The searches are reported in Chapter 4 and Appendix 1.
Inclusion and exclusion criteria
Non-randomised studies of individuals with pNETs or GI NETs receiving 177Lu-DOTATATE and reporting outcomes of interest were included in the review. Non-randomised evidence included prospective observational cohort studies, both comparative and single-arm studies. Case studies were excluded.
Screening
Titles and abstracts were independently double-screened by two reviewers. Full texts of studies meeting the inclusion criteria at title and abstract stage were double-screened by three reviewers.
Data extraction and management
A standardised data specification form was used and the data extracted were independently checked. When multiple publications of the same study were identified, data were extracted and reported as if a single study.
Extracted and tabulated information included country of study, number of participants, location of tumour, dose of 177Lu-DOTATATE, any additional drugs given, baseline characteristics of participants (age, percentage of male participants, tumour functionality, tumour differentiation and ECOG or WHO PS) and whether or not any previous treatments had been given. Outcomes extracted included follow-up duration, PFS, OS, RR, AEs and HRQoL.
Critical appraisal strategy
Included studies were not critically appraised.
Methods of data synthesis
Data are presented in summary tables. The following outcomes have been narratively synthesised in the following section: PFS, OS, RR, AEs and HRQoL.
Results for non-randomised controlled trial evidence
Quantity and quality of research available
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram is presented in Figure 26. Studies were coded separately in the first round of screening so that they could be reintroduced if (and in this case) necessary.
FIGURE 26.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for non-randomised studies of 177Lu-DOTATATE.
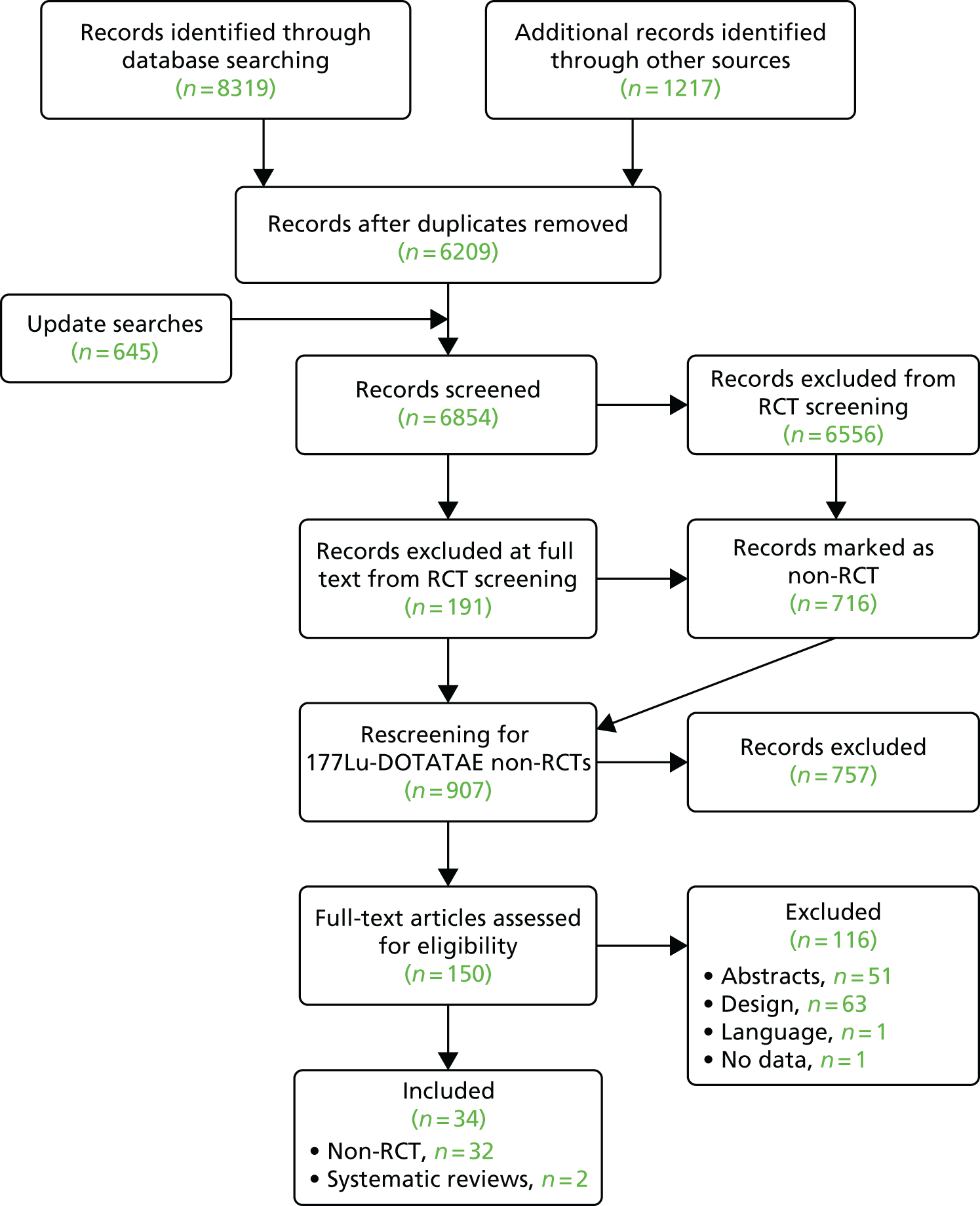
Overview of results
All 32 included studies103,104,145–174 were case series with no internal controls. There was a wide variation in the number of study participants (n = 5–310), with only four103,104,163,164 out of the 32 studies having > 100 study participants. Studies were conducted in participants with a wide range of baseline characteristics.
For outcome measures, following treatment with 177Lu-DOTATATE, PFS ranged from 10 to 40 months and OS ranged from 34.2 to 105 months. In terms of RRs, complete response ranged from 2% to 27% and partial response ranged from 12% to 100% [with a standard deviation (SD) range from 12% to 100%].
This wide variation in outcome measures is likely to be because of factors inherent in study design and compounded by wide variations in participant characteristics, for example tumour sites, with outcomes often reported for mixed tumour locations, for example data gut, pancreas and lung NETs grouped together.
Twenty-three143,145–152,155,158–168,170,171 of the 32 studies reported on AEs, whereas seven143,146,148,152,154,159,161 of the 32 studies reported on HRQoL outcomes.
The extreme sensitivity of outcomes to apparently small variations in study features, particularly case mix, illustrates the great importance of having studies with parallel control groups, ideally with participants being randomly allocated, to assess the effectiveness of treatments. Without controlled studies it is very difficult to determine whether differences in outcomes between case series for a new treatment (in this case 177Lu-DOTATATE) and case series for existing treatments are attributable to differences in treatments or differences in prognostic factors.
Non-randomised controlled trials of 177Lu-DOTATATE
No non-RCT comparative trials were identified; all 32 studies identified were single-arm trials. The baseline characteristics of these 32 trials are provided in Table 38 and the outcomes in the trials are provided in Table 39.
| Study | Country | n | Location of NETs | Lutetium dose | Other drugs | Age (years) | Male, n/N (%) | Tumour functioning, n/N (%) | Tumour differentiation, n/N (%) | Previous treatments, n/N |
|---|---|---|---|---|---|---|---|---|---|---|
| Balter 2016146 | Uruguay | 5 | Pancreas, n = 2; ileum, n = 2; bronchial, n = 1 | Cumulative dose of 4.44–22.2 GBq | NR | Range 51–79 | 4/5 (80) | NR | NR | NR |
| Barber 2012147 | Australiaa | 5 | Pancreas, n = 4; duodenum, n = 1 | 7.0–10.0 GBq (mean 8.6 GBq) | Premedication: granisetron (3 mg), dexamethasone (8 mg), amino acid solution. Concurrent: 5-FU chemotherapy (200 mg/m2/24 hours) | Range 55–72, mean 68 | 5/5 (100) | Non-functioning 5/5 (100) | 5/5 (100) well differentiated | Pancreatic 2/5; SSA 1/5; chemotherapy 1/5; incomplete resection duodenum 1/5 |
| Basu 2016148 | Indiaa | 5 | Lung, n = 1; bronchial carcinoid, n = 2; unknown, n = 1; duodenum, n = 1 | Cumulative dose of 16.1–25.6 GBq | NR | Range 26–62 | 3/5 (60) | NR | 3/5 (60) Well differentiated (n = 3 thoracic NETs) | NR |
| Bodei 2011149 | Italy | 51 | Bronchial, n = 5; appendix, n = 1; pancreatic, n = 14; duodenal, n = 3; ileum, n = 19; sigma-rectal, n = 2; unknown, n = 3; paraganglioma, n = 3; meningioma, n = 1 | Group 1: 3.7–5.18 GBq/cycle, median in six cycles 26.4 GBq; group 2: 5.18–7.4 GBq/cycle, median in four cycles 25.2 GBq | 100 ml of physiological saline, 25 g of lysine diluted in 1 l of normal saline, 12.5 g of lysine diluted in 500 ml of normal saline | Range 30–79, median 57 | 26/51 (51) | NR | 35/37 (94.6) well differentiated | SSA 43/51 |
| Bodei 2016150 | Italy | 54 | Bronchial, n = 13; GEP-NETs, n = 35; unknown, n = 6 | PRRT-naive patients (risk factors and no risk factors): 18.5 or 27.8 GBq in four cycles; PRRT pretreated: 14.8 in four cycles | NR | Range 43–83, median 66 | 37/54 (69) | NR | 6/35 (17.1) well differentiated (GEP NETs non-specified) | Surgery 32/54; SSA 44/54; chemotherapy 21/54; everolimus 5/54; sunitinib 1/54; interferon alpha 1/54; PRRT 16/54; radiotherapy 6/54; TACE 4/54 |
| Claringbold 2011151 | Italy | 33 | Pancreas, n = 10; small bowel, n = 13; large bowel, n = 2; lung, n = 2; unknown, n = 6 | 7.8 GBq | Amino acids (Synthamin; Baxter Healthcare Australia, Old Toongabbie, NSW, Australia): 11.6 g/l of lysine and 23 g/l of arginine at 250 ml/hour for 4 hours; 5 mg of tropisetron (Navoban; Novartis Pharmaceuticals Australia, Macquarie Park, NSW) and 2 mg of lorzaepam (Ativan; Sigma Pharmaceuticals, Watford, UK); 1650 mg/m2 of capecitabine. Of the 19 patients with carcinoid, 18 were receiving regular octreotide analogue therapy for symptom control | Range 32–82, median 60 | 21/33 (63) | Functioning 21/33 (64) | 33/33 (100) well or moderately well differentiated | Surgery 20/33; octreotide 18/33; chemotherapy 5/33 |
| Claringbold 2012152 | Australia | 34 | Bowel, n = 15; GEP NETs, n = 17; lung, n = 2 | 7.8 GBq | 1500 mg/m2 of capecitabine and 200 mg/m2 of temozolomide. Amino acids: 11.6 g/l of lysine and 23 g/l of arginine at 240 ml/hour | Range 33–81, median 63 | 24/35 (69) | Non-functioning: 16/35 (46); functioning 13/35 (37) | 35/35 (100) well differentiated | Octreotide LAR 12/35; chemotherapy 6/35; surgery 12/35 |
| Claringbold 2015154 | Australia | 16 | Pancreas, n = 5; small bowel, n = 11 | 7.8 GBq | 5, 7.5 and 10 mg daily of everolimus. Amino acids: 11.6 g/litre of lysine and 23 g/litre of arginine at 240 ml/hour. Intravenous tropisetron and dexamethasone and oral aprepitant | Range 43–72, median 63 | 9/16 (56) | NR | NR | Surgery 8/16; SSA 11/16; chemotherapy 6/16; PRRT 5/16; sunitinib 1/16;67 Y-microspheres 2/16 |
| Claringbold 2016153 | Australia | 30 | Pancreas | 7.9 GBq | 1500 mg/m2 of capecitabine and 200 mg/m2 of temozolomide. Amino acids: 11.6 g/l of lysine and 23 g/l of arginine at 240 ml/hour. Tropisetron and lorazepam | Range 38–78, median 60 | 18/30 (60) | Non-functioning 21/30; functioning 9/30 | 30/30 (100) well differentiated | Surgery 8/30; SSA 4/30; chemotherapy 3/30; targeted agents 3/30; radiopeptide 2/30 |
| Delpassand 2014145 | USA | 37 | Pancreas, n = 14; small bowel, n = 12; rectal, n = 3; large bowel, n = 1; unknown, n = 7 | 200 mCi (7.4 GBq ± 10%) administered up to cumulative dose of 800 mCi (29.6 GBq ± 10%) | Kidney-protecting agents, 15% Clinisol (1000 ml), mixture composed of positively charged amino acids | Range 43–86, median 64 | 16/37 (43) | NR | NR | Sandostatin 28/37 |
| Ezziddin 2011155 | Australia | 81 | Pancreas, n = 37; GEP NET, n = 44 (foregut, n = 5; midgut, n = 19; hindgut, n = 2; undetermined primary, n = 18) | Mean activity 7.9 GBq per cycle | NR | Range 33–83, mean 61 | 46/81 (57) | Non-functioning 63/81; functioning18/81 | 79/81 well-differentiated; 2/81 poorly differentiated | Previous treatments 63/81; octreotide 29/81; IFN 5/81; chemotherapy 23/81; ablative treatment 13/81; surgery 40/81 |
| Ezziddin 2011156 | Germany | 42 | Pancreas, n = 12; non-pancreatic GEP NETs, n = 30 | Mean activity 8.1 ± 0.98 GBq per cycle | NR | Range 44–88, mean 62 | 26/42 (70) | NR | 42/42 (100) well differentiated | Surgery 22/42; biotherapy 17/42; chemotherapy 11/42; locoregional treatment 2/42 |
| Ezziddin 2014157 | Germanya | 74 | Pancreas, n = 33; non-pancreatic GEP NETs, n = 41 | Mean activity 7.9 GBq per cycle | Standard amino acid co-infusion (2.5% lysine and 2.5% arginine in 1 l of 0.9% NaCl; infusion of 250 ml/hour) | Range 34–83, mean 62.5 | 42/74 (57) | Non-functioning 55/74; functioning 9/74 | 74/74 (100) well differentiated | Surgery 38/74; biotherapy 28/74; chemotherapy 18/74; locoregional treatment 13/74 |
| Ezziddin 2014158 | Germanya | 68 | Pancreas | Mean activity 8.0 GBq (216 mCi) per cycle | Nephroprotective: 2.5% lysine and 2.5% arginine in 1 l of 0.9% NaCl; infusion of 250 ml/hour | Range 37–82, mean 62 | 35/68 (52) | Non-functioning 50/68; functioning 18/68 | 68/68 (100) well differentiated | Surgery 30/68; biotherapy 20/68; chemotherapy 17/68; locoregional treatment 7/68 |
| Ilan 2015159 | Sweden | 24 | Pancreas | Range 4.0–7.9 GBq per cycle | Kidney protection: 2 l of mixed amino acids solution (Vamin, 14 g/l, without added electrolytes; Fresenius Kabi Ltd) | Range 43–78 | 13/24 (54) | NR | NR | NR |
| Kong 2014160 | Australia | 68 | Pancreas, n = 33; non-pancreatic NETs, n = 35 | Median cumulative dose 31 GBq (21–45.3 FBq) | Granisetron and dexamethasone with amino acid infusion (25 g of lysine and 25 g of arginine in 1 l of normal saline). 5-FU chemotherapy (200 mg/m2/24 hours) | Range 17–76, median 56 | 39/68 (57) | NR | NR | NR |
| Kunikowska 2013161 | Polanda | 28 | Foregut, n = 14; midgut, n = 9; hindgut, n = 1; unknown primary, n = 2; other, n = 2 | Total maximum dose of 7.4 GBq/m2, for an injected activity per course of 2.2–3.7 GBq, of 90Y/177Lu-DOTATATE |
Total maximum dose of 7.4 GBq/m2, for an injected activity per course of 2.2–3.7 GBq, of 90Y/177Lu-DOTATATE Amino acid infusion, consisting of 11.3 g of arginine and 9.0 g of lysine (1000 ml of Vamin 18) and Ringer’s solution (500 ml). Ondansetron (8 mg) (Zofran; Glaxo Wellcome, Atossa, Anpharm S.A.) |
Range 39–78, mean 55 ± 10.9 | 10/28 (36) | NR | NR | Chemotherapy 9/28 |
| Kwekkeboom 2003162 | Netherlands | 35 | Pancreas, n = 12; carcinoid, n = 12; unknown origin, n = 8; gastrinoma, n = 3 | 100, 150 or 200 mCi to a final cumulative dose of 600–800 mCi (27.8–29.6 GBq) | 3 mg of granisetron and amino acids (2.5% lysine and 2.5% arginine in 1 l of 0.9% NaCI infusion 250 ml/hour). Eight patients used sandostatin | Range 19–78, mean 54 | 14/35 (40) | NR | NR | Surgery 12/35; radiotherapy 1/35; chemotherapy 3/25; octreotide (Sandostatin; Novartis) 14/35 |
| Kwekkeboom 2005163 | Netherlands | 131 | Gastrinoma, n = 8; insulinoma, n = 2; non-functioning endocrine pancreatic tumours, n = 33; endocrine tumours of unknown origin, n = 18; carcinoid tumours, n = 70 | 600–800 mCi (22.2–29.6 GBq). Cycle dosages were 100 mCi (3.7 GBq), 150 mCi (5.6 GBq) and 200 mCi (7.4 GBq) | 3 mg of granisetron and amino acids (2.5% lysine and 2.5% arginine in 1 l of 0.9% NaCI infusion 250 ml/hour) | Range 19–83, mean 56 | 65/129 (50) | NR | NR | Surgery 63/129; external beam radiation 6/129; chemotherapy 20/129; SSA 66/129 |
| Kwekkeboom 2008164 | Netherlands | 310 | Carcinoid, n = 188; non-functioning pNETs, n = 72; unknown, n = 31; gastrinoma, n = 12; insulinoma, n = 5; VIPoma, n = 2 | 750–800 mCi (27.8–29.6 GBq). Cycle dosages were 100 mCi (3.7 GBq), 150 mCi (3.6 GBq) and 200 mCi (7.4 GBq) | 3 mg of granisetron or 8 mg of ondasentron and amino acids (2.5% lysine and 2.5% arginine in 1 l of 0.9% NaCI infusion 250 ml/hour) | Range 21–85, mean 59 | 164/310 (53) | NR | NR | Surgery 153/310; radiotherapy 16/310; chemotherapy 52/310; SSA168/310 |
| Paganelli 2014165 | Italy | 43 | Stomach, n = 2; appendix, n = 1; small intestine (midgut), n = 34; colon, n = 5; rectum, n = 1 | Cumulative dose 18.5 or 27.8 GBq, (cycle dosages of 3.7 or 5.5 GBq); 25 patients (58%) treated with a ‘standard’ Lu-PRRT full dose of 25.7 GBq (range 22.2–27.8 GBq), with a reduced dosage of 18.4 GBq for patients at risk. Some patients were treated with reduced dosage of 3.7 GBq per cycle | Amino acids [70 mEq of lysine in 500 ml of saline (250 ml in 30 minutes immediately before therapy, 250 ml during therapy), 70 mEq of lysine in 500 ml of saline in the first 3 hours after therapy and 60 mEq of lysine in 500 ml of saline over 1 hour twice the following day] | Range 44–82, median 65 | 28/43 (65) | NR | 49/49 (100) well differentiated | Surgery 35/43; SSA 34/43; chemotherapy 4/43; Y-PRRT 4/43; other treatments 13/43 |
| Sabet 2013166 | Germany | 68 | Pancreas, n = 23; non-pancreatic GEP NETs, n = 45 | 8.1 ± 0.76GBq | NR | Range 40–88, mean 63 | 39/68 (57) | NR | 68/68 (100) well differentiated | Surgery 35/68; biotherapy 30/68; chemotherapy 18/68; locoregional treatment 2/68 |
| Sabet 2013167 | Germanya | 6 | Pancreas, n = 2; non-pancreatic NETs, n = 4 | Mean cumulative dose 48.7 GBq (range 29.6–96.7 GBq) | 2.6–3.3 GBq of Re-HEDP, cumulative dose 5.9 GBq | Range 43–70 | 5/6 (83) | NR | NR | Radiation 1/6; chemotherapy 5/6; locoregional treatment 3/6; biotherapy 4/6; surgery 2/6 |
| Sabet 2014168 | Germany | 11 | Pancreas, n = 3; non-pancreatic GEP NETs, n = 8 | Mean dose of 6.95 GBq per cycle; aimed for four courses and standard intervals of 3 months | Amino acids were co-administered to reduce the absorbed dose to the kidneys | Range 40–78, mean 62 | 7/11 (64) | NR | 11/11 (100) well differentiated | Surgery 6/11; SSAs 6/11; chemotherapy 8/11; locoregional treatment 2/11; PRRT 4/11 |
| Sabet 2015169 | Germany | 61 | Advanced small intestinal NETs | Mean activity 7.9 GBq (214 mCi) per cycle (four cycles); mean cumulative activity per patient was 27.2 ± 5.9 GBq | Amino acids (2.5% lysine and 2.5% arginine in 110.9% NACI; infusion of 250 ml/hour) | Range 34–83, mean 62 | 34/61 (56) | Non-functioning 17/61; functioning 44/61 | 61/61 (100) well differentiated | Biotherapy 53/61; surgery 41/61; chemotherapy 9/61; locoregional treatment 10/61 |
| Sansovini 2013170 | Italy | 52 | Advanced pNETs | n = 26 received full dose of 25.5 GBq (range 20.7–27.8 GBq); n = 26 received reduced dose of 17.8 GBq (range 11.1–19.9 GBq) | Amino acids (70 mEq of lysine in saline) | Range 26–82, mean 61 | 30/52 (58) | NR | NR | Surgery 22/52; chemotherapy 14/52; SSA 34/52; Y-PRRT 14/52; other treatments 8/52 |
| Severi 2015171 | Italy | 26 | Pancreas n = 17; ileum, n = 5; appendix, n = 1; colon, n = 1; rectum, n = 1; unknown, n = 1 | Total activity 14.8–18.5 GBq in four or five cycles (median dose 16.5 GBq). Primary treatment: median dose 10.8 GBq in five cycles. Retreatment: median dose 16.5 GBq in five cycles | Amino acids [70 mEq of lysine in 500 ml of saline (250 ml over 30 minutes immediately before therapy, 250 ml during therapy), 70 mEq of lysine in 500 ml of saline during the first 3 hours after therapy and 60 mEq of lysine in 500 ml of saline over 1 hour twice the following day | Range 37–79, median 54 | 15/26 (58) | NR | NR | Surgery 13/26; chemotherapy 13/26; locoregional treatments 3/26; SSA 24/26 |
| Soydal 2016172 | Turkey | 29 | Pancreas, n = 9; unknown, n = 5; colon, n = 1; stomach, n = 2; lung, n = 2; retroperitoneum, n = 2; ovary, n = 2; thyroid, n = 2; ileum, n = 3; appendix, n = 1 | 7400 MBq each cycle | 100 MBq of Ga-68 DOTATATE, 50-g cocktail of 25 g of lysine and 25 g of arginine diluted in 2 l of normal saline | Range 19–76, mean 50.7 ± 14.6 | 12/29 (41) | NR | 24/27 well differentiated; 3/27 moderately differentiated | Surgery 16/29; chemotherapy 13/29; radiotherapy 3/29; SSA 19/29 |
| van Essen 2007173 | Netherlands | 16 | Bronchial, n = 9; gastric, n = 5; thymic carcinoids, n = 2 | Cumulative dose 22.2–29.6 GBq;. cycle doses were 7.4 GBq. Cumulative dose could be reduced to 22.2–27.8 GBq. Dose of the last cycle adjusted to 3.7 or 5.55 GBq | 3 mg of granisetron, amino acids (2.5% lysine, 2.5% arginine) | Range 37–76, median 57 | 10/16 (62) | NR | NR | Surgery 11/16; chemotherapy 4/16; radiotherapy 3/16 |
| van Essen 2010174 | Netherlands | 33 | Pancreas n = 8; unknown, n = 5; carcinoid, n = 20 (bronchial, n = 3; gastric, n = 1; rectal, n = 1; midgut, n = 15) | Intended cumulative dose of 14.8 GBq in two cycles; cycle dose of 7.4 GBq or occasionally 3.7 GBq | 3 mg of granisetron, amino acids (1 l of 2.5% arginine and 2.5% lysine) | Range 35–75, median 57 | NR | NR | NR | NR |
| van Vliet 2013103 | Netherlands | 268 | Pancreas, n = 72; GI or thoracic NETs, n = 178 (foregut, n = 22; midgut, n = 145; hindgut, n = 11); unknown, n = 18 | Cycle dose of 3.7 or 7.4 GBq, cumulative intended dose of 22.2–29.6 GBq. If dosimetric calculations indicated that the radiation dose to the kidneys would exceed 23 Gy with a dose of 29.6 GBq, the cumulative dose was reduced to 22.2–27.8 GBq | 3 mg of granisetron (Kytril; Roche), amino acids (1 l of 2.5% arginine and 2.5% lysine) | Range 23–83, mean 59 | 138/268 (52) | Non-functioning 61 (85); functioning 11 (15) | NR | Octreotide 142/268; surgery 118/268; chemotherapy 26/268; radiotherapy 10/268 |
| van Vliet 2015104 | Netherlands | 119 | Pancreas: G1 (n = 15): borderline or unresectable pNETs; G2 (n = 14): borderline or unresectable pNETs and oligometastatic disease (three or fewer liver metastases); G3 (n = 90): pNETS and more than three liver metastases or other distant metastases | Cycle dose of 7.4 GBq, cumulative dose of 22.2–29.6 GBq | 3 mg of granisetron, amino acids (1 l of 2.5% arginine and 2.5% lysine) | Range 23–85, mean 55 | 54/119 (45) | Non-functioning 119/119 (100) | NR | NR |
| Study | Follow-up (months) | PFS (months) | OS (months) | RR, n/N (%) | AEs, n/N (%) | HRQoL |
|---|---|---|---|---|---|---|
| Balter 2016146 | 3 | NR | NR | pNETs: PR 1/2; SD 1/2; ileal NETs: PR 2/2; bronchial NETs: PR 1/1 | NR | NR |
| Barber 2012147 | 12–48 | NR | n = 5/5 (100) | Radiological response: pNETs: PR 4/4; duodenal NETs: SD 1/1 | NR | NR |
| Basu 2016148 | 10–27 | Duodenum 27; unknown 10 | NR | Duodenum and unknown: PR 2/2 | PRRT well tolerated; no haematological toxicity | Improved symptomatic palliation/quality of life |
| Bodei 2011149 | 4–66 | Outcome not reported by tumour location | Outcome not reported by tumour location | Pancreas: PR 8/14, MR 1/14, SD 2/14, PD 3/14, duodenum: CR 1/3, PR 1/3, PD 1/3; ileum: PR 2/19, MR 6/19, SD 7/19, PD 4/19; sigma-rectum: PR 1/2, PD 1/2; unknown: MR 2/3, SD 1/3; appendix: SD 1/1; bronchial: PR 2/5, MR 2/5, SD 1/5; paraganglia: MR 2/3, SD 1/3; meninges: SD 1/1 | Outcome not reported by tumour location | Outcome not reported by tumour location |
| Bodei 2016150 | Median 16, range 1–33 | Median PFS was not achieved | NR | Responders (SD + PR + CR): 71% GI and 93% pancreas | No serous side effects with PRRT | NR |
| Claringbold 2011151 | 16, range 5–33 | Outcome not reported by tumour location; however, for the whole cohort, median PFS was not achieved at follow-up | Outcome not reported by tumour location | pNETs: PR 1/3, SD 1/3, PD 1/3; small bowel: PR 1/13, SD 12/13; colon: SD 2/2; lung: PR 1/2, SD 1/2; unknown: SD 6/6; pancreatic islet cell: PR 4/5, SD 1/5; insulinoma: PR 1/1 | Outcome not reported by tumour location | Outcome not reported by tumour location |
| Claringbold 2012152 | Median 18, range 12–33 | Outcome not reported by tumour location | Outcome not reported by tumour location | GEP NET: CR 3/17, PR 11/17, SD 2/17, PD 1/17; bowel NETs: CR 2/15, PR 2/15, SD 10/15, PD 1/15; lung: SD 1/2, PD 1/2 | Outcome not reported by tumour location | NR |
| Claringbold 2015154 | Median 34, range 18–42 | Outcome not reported by tumour location | Median OS was not reached at 34 months | pNETS: PR 4/5, SD 1/5; GI NETs: PR 3/11, SD 7/11, not assessable 1/11 | Outcome not reported by tumour location | NR |
| Claringbold 2016153 | Median 33, range 13–58 | Median 48 | Not reached after 33 months’ follow-up | ORR 80% (95% CI 66% to 93%); CR 4/30; PR 20/30; SD 6/30 | Thrombocytopenia (grade 3 severity) 3/30; myelodysplastic syndrome 1/30 | NR |
| Delpassand 2014145 | Average 14.26, median 16.11, range 0.3–26.87 | Median PFS not reached. GI: Kaplan–Meier survival estimate at 12 months 72.7% (95% CI 49.1% to 86.7%) and at 24 months 72.7% (95%CI 49.1% to 86.7%). Pancreas: Kaplan–Meier survival estimate at 12 months 79.5% (95% CI 39.3% to 94.5%) and at 24 months 63.6% (95% CI 22.2% to 87.3%) | Outcome not reported by tumour location | Outcome not reported by tumour location | Outcome not reported by tumour location | Outcome not reported by tumour location |
| Ezziddin 2011155 | NR | NR | NR | pNETs: PR 57%, MR 13.5%, SD 16%, PD 13.5%; GEP NETs: PR 23%, MR 13.5%, SD 45.5%, PD 18% | NR | NR |
| Ezziddin 2011156 | Median 32 (95% CI 29 to 35) | pNETS: median 29 (95% CI 18 to 40); other GEPNETs: median 35 (95% CI 16 to 54) | Outcome not reported by tumour location | Regression (CR, PR and MR): pNETS: 7/12; other GEP NETs: 14/30 | NR | Outcome not reported by tumour location |
| Ezziddin 2014157 | Median 47 (95% CI 44.5 to 49.5) | Outcome not reported by tumour location | pNETs: median 57 (95% CI 48 to 66); other GEP NETs: median 43 (95% CI 31 to 55) | pNETs: PR 54.5%, MR 18.2%, SD 18.2%, PD 9.1%; other GEP NETs: PR 22%, MR 17.1%, SD 48.8%, PD 12.2% | Outcome not reported by tumour location | NR |
| Ezziddin 2014158 | Median 58, range 4–112 | Median PFS 34 (95% CI 26 to 42) | Median 53 (95% CI 46 to 60) | PR 41/68, MR 8/68, SD 9/68, PD 10/68 | Reversible haematotoxicity (grade 3 or above) 4/68; no significant nephrotoxicity (grade 3 or above) | NR |
| aIlan 2015159 | 3 months after termination of treatment | NR | NR | In all 24 patients, there was a significant correlation between absorbed dose and best tumour response | NR | NR |
| Kong 2014160 | Median 60, range 5–86 | NR | Outcomes not reported by tumour location | Partial and minor responses: pNETs: 55%; non-pancreatic NETs: 81% (OR 0.28; 95% CI 0.08 to 0.94) | NR | NR |
| bKunikowska 2013161 | NR – other measures taken at 48 months | Event-free survival 24.3; TTP 24.3 | Median 49.8 | NR | Grade 1 + 2 nephrotoxicity 3/28; mild nausea in both groups (38% of entire population) | NR |
| Kwekkeboom 2003162 | Average 9 | NR | NR | pNETs: CR 1/12, PR 1/12, SD 7/12, PD 3/12; carcinoid: PR 4/12, SD 6/12, PD 2/12; unknown: PR 4/7, SD 1/7, PD 2/7; gastrinoma: PR 3/3 | Outcome not reported by tumour location | Outcome not reported by tumour location |
| Kwekkeboom 2005163 | Median 16, range 7–44 | Outcome not reported by tumour location | NR | pNETS: CR 3/32, PR 7/32, MR 7/32, SD 11/32, PD 4/32; carcinoid: PR 13/66, MR 13/66, SD 28/66, PD 12/66; unknown origin: PR 6/17, MR 2/17, SD 4/17, PD 5/17; gastrinoma: PR 5/8, MR 2/8, SD 1/8; insulinoma: PR 1/2, PD 1/2 | Outcome not reported by tumour location | NR |
| Kwekkeboom 2008164 | NR | Outcome not reported by tumour location | Outcome not reported by tumour location | Carcinoid: CR 1/188, PR 41/188, MR 31/188, SD 78/188, PD 37/188; pNETs: CR 4/72, PR 26/72, MR 13/72, SD 19/72, PD 10/72; unknown: PR 10/31, MR 3/31, SD 7/31, PD 11/31; gastrinoma: PR 5/12, MR 4/12, SD 2/12, PD 1/12; insulinoma: PR 3/5, SD 1/5, PD 1/5; VIPoma: PR 1/2, PD 1/2 | Outcome not reported by tumour location | Outcome not reported by tumour location |
| Paganelli 2014165 | Median 38, range 11–59 | Median PFS 36 (95% CI 24 to NR) | Mean OS not yet reached | Median duration objective response 25 (95% CI 7 to 50) months. CR 3/43; SD 33/43; PD 7/43. Disease control rate 84% (95% CI 73% to 95%) | No cases of major toxicity; most common side effects were nausea (maximum grade 2), asthenia and mild alopecia | NR |
| Sabet 2013166 | Median 48 (95% CI 39 to 54) | NR | pNETs: median 48 (95% CI 29 to 67); other GEP NETs: median 57 (95% CI 36 to 78) | Regression (CR, PR or MR): pNETs 14/23; other GEP NETs 19/45 | Outcome not reported by tumour location | NR |
| Sabet 2013167 | NR | NR | pNETs 5; GI NETs range 2–9 | Remission response for pNETs: SD 1/2, PD 1/2; Remission response for GI NETs: SD 1/4, PD 3/4 | Outcome not reported by tumour location | NR |
| Sabet 2014168 | NR | Outcome not reported by tumour location | NR | pNETs: PR 1/3, SD 2/3; GI NETs: PR 1/8, MR 1/8, SD 5/8, PD 1/8 | Outcome not reported by tumour location | NR |
| Sabet 2015169 | Median 62 (95% CI 57 to 67), range 4–102 | Median PFS 33 (95% CI 25 to 41) | Median 61 (95% CI NA) | PR 8/61, MR 19/61, SD 29/61, PD 5/61. Objective response was associated with longer survival (median OS not reached vs. 49 months) | Reversible haematotoxicity (grade 3 or above) 5/61; relevant haematotoxicity (grade 3/4) 5/61. No other relevant toxicities (including nephrotoxicity) or treatment-related deaths were observed | NR |
| Sansovini 2013170 | Median 25, range 9–39 | Median PFS for whole group 29 (95% CI 19 to 39); median PFS not reached in the full-dose group and was 20 months in the reduced-dose group | Median OS not reached | Whole group: CR 4/52, PR 11/52, SD 27/52, PD 10/52. Disease control rate 81% (95% CI 68% to 89%) | No major acute or delayed haematological toxicity. The most common minor side effects were nausea (maximum grade 2), asthenia and mild alopecia. One patient developed grade 3 renal toxicity | NR |
| Severi 2015171 | Median 36, range 4–58 | Outcome not reported by tumour location | Outcome not reported by tumour location | pNETS: PR 1/17, SD 14/17, PD 2/17; ileum: SD 3/5, PD 2/5; appendix: SD 1/1; colon SD 1/1; rectum CR 1/1; unknown SD 1/1 | Outcome not reported by tumour location | NR |
| Soydal 2016172 | NR | NR | NR | pNETs: PR 3/9, SD 5/9, PD 1/9; other NETs (unknown, stomach, colon, retroperitoneum, stomach, ileum, appendix): PR 3/14, SD 9/14, PD 2/14 | NR | NR |
| van Essen 2007173 | 18 and 21 | Gastric carcinoids: estimated median TTP 16 | NR | Gastric carcinoids: CR 1/5, MR 1/5, SD 2/5, PD 1/5 | Outcome not reported by tumour location | NR |
| van Essen 2010174 | Median 16, range 1–40 | Median TTP: pNETS (n = 8) 17; carcinoid NETs (n = 27) 20 | Outcome not reported by tumour location | pNETs: PD 5/8; carcinoid NETs: PD 12/27 | Treatment effects in patients with pNETs were similar to those in patients with other GEP NETs | NR |
| van Vliet 2013103 | NR | Outcome not reported by tumour location | Outcome not reported by tumour location | pNETS: Objective response (CR + PR + MR) 20/61, SD 22/61, PD 19/61; midgut: Objective response 31/138, SD 80/138, PD 27/138 | NR | NR |
| van Vliet 2015104 | NR | Median PFS (in 29 patients in groups 1 and 2) was 55 (95% CI 37 to 73) months; median PFS was 69 months for patients with successful surgery and 49 months for the other patients. Median PFS (in 90 other patients in group 3) was 25 months | Median OS (in 29 patients in groups 1 and 2) was > 105 months. Median OS was > 103 months for patients with successful surgery and 60 months for the other patients. Median OS (in 90 other patients in group 3) was 52 months | Tumour response (3 months after last treatment): Objective response (CR + PR + MR) in 72/119 (61%) patients; stable disease in 24/119 (20%) and progressive disease in 21/119 (18%) | NR | NR |
Appendix 3 Table of excluded studies with rationale
| Number | Reference | Reason for exclusion |
|---|---|---|
| 1 | Adlbrecht C, Wild C. Targeted Radionuclide Therapy with 90Y- and 177-Lu-DOTATOC in Patients with Neuroendocrine Tumors (Structured Abstract). 2007. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32008000054/frame.html | Design |
| 2 | Anonymous. Everolimus 10 mg and pancreatic neuroendocrine tumours: many adverse effects and uncertain benefit. Prescrire International 2012;21:234 | Design |
| 3 | Anonymous. Sunitinib and pancreatic neuroendocrine tumours. More assessment needed. Prescrire International 2012;21:123–5 | Design |
| 4 | Anonymous. Lanreotide slows growth of neuroendocrine cancer. Cancer Discovery 2014;4:OF3 | Design |
| 5 | Anonymous. Everolimus for advanced, progressive, nonfunctional neuroendocrine tumors (NET) of the gastrointestinal (GI) tract: efficacy and safety from a RADIANT-4 subgroup analysis. Clinical Advances in Hematology & Oncology 2016;14:11–13 | Design |
| 6 | Anonymous. NETTER-1 Phase III in patients with midgut neuroendocrine tumors treated with 177Lu-DOTATATE: efficacy and safety results. Clinical Advances in Hematology & Oncology 2016;14:8–9 | Design |
| 7 | Panzuto F, Rinzivillo M, Fazio N, de Braud F, Luppi G, Zatelli MC, et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 2014;19:966–74. [Erratum published in Oncologist 2015;20:570.] | No data |
| 8 | Anonymous. Retraction Note to: A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. [Retraction of Yao J, Wang JY, Liu Y, Wang B, Li YX, Zhang R, et al. Med Oncol 2014;31:251]. Med Oncol 2015;32:221 | Retracted |
| 9 | Anthony L, Bajetta E, Kocha W, Panneerselvam A, Saletan S, O’Dorisio T. Efficacy and safety of everolimus plus octreotide LAR in patients with colorectal neuroendocrine tumors (NET): subgroup analysis of the phase III RADIANT-2 trial. Am J Gastroenterol 2011;106:S154–5 | Design – RADIANT2 |
| 10 | Anthony L, Singh N, Passos VQ, Pavel M, Öberg K, Yao JC. Impact of prior somatostatin analog use on PFS in the phase III radiant-2 trial of everolimus + octreotide lar vs placebo + octreotide lar in patients with advanced neuroendocrine tumors. Pancreas 2012;41:342 | Design – RADIANT2 |
| 11 | Anthony LB, Pavel ME, Hainsworth JD, Kvols LK, Segal S, Hörsch D, et al. Impact of Previous Somatostatin Analogue Use on the Activity of Everolimus in Patients with Advanced Neuroendocrine Tumors: Analysis from the Phase III RADIANT-2 Trial. Neuroendocrinology 2015;102:18–25 | Design – RADIANT2 |
| 12 | Anthony LB, Peeters M, Hainsworth JD, Baudin E, Hoersch D, Klimovsky J, et al. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors (NET): effect of prior somatostatin analog therapy on progression-free survival in the RADIANT-2 trial. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29 | Design – RADIANT2 |
| 13 | Antonuzzo A, Ricci S, Galli L, Conte PF. Long-acting lanreotide in the treatment of neuroendocrine tumors (NETs). Ann Oncol 1998;9:175 | Design |
| 14 | Bajetta E, Guadalupi V, Procopio G. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2009;27:319–20 | Design |
| 15 | Barni S, Borgonovo KF, Ghilardi M, Cabiddu M, Maspero F, Cremonesi M, et al. The impact of anemia in advanced solid tumors treated with sorafenib (SO) and sunitinib (SU): A pooled analysis of 6 trials. Ann Oncol 2012;23:ix519 | Design |
| 16 | Baudin E, Castellano D, Kaltsas G, Gross D, Lebrec J, Tsuchihashi Z, et al. Correlation of PFS and chromogranin a and 5-hydroxyindoleacetic acid levels in patients with advanced neuroendocrine tumors: Phase III radiant-2 study results. Ann Oncol 2011;22:v20 | No data |
| 17 | Baudin E, Wolin E, Castellano D, Kaltsas G, Lebrec J, Tsuchihashi Z, et al. Effect of everolimus + octreotide lar treatment on 5-hydroxyindoleacetic acid levels in patients with advanced neuroendocrine tumors: Phase III radiant-2 study results. Neuroendocrinology 2011;94:16 | No data |
| 18 | Baudin E, Wolin E, Castellano D, Kaltsas G, Panneerselvam A, Tsuchihashi Z, et al. Correlation of PFS with early response of chromogranin a and 5-hydroxyindoleacetic acid levels in pts with advanced neuroendocrine tumours: Phase III radiant-2 study results. Eur J Cancer 2011;47:S460 | No data |
| 19 | Baudin E, Wolin EM, Castellano DE, Kaltsas G, Lebrec J, Tsuchihashi Z, et al. Effect of everolimus plus octreotide LAR treatment on chromogranin A and 5-hydroxyindoleacetic acid levels in patients with advanced neuroendocrine tumors: Phase III RADIANT-2 study results. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29 | No data |
| 20 | Bechter OE, Unger N, Borbath I, Ricci S, Hwang TL, Park YS, et al. Open-label, phase IIIb, multicenter, expanded access study of everolimus in patients with advanced neuroendocrine tumors (NET). Journal of Clinical Oncology Conference 2013;31 | Design |
| 21 | Berruti A, Pia A, Terzolo M. Advances in pancreatic neuroendocrine tumor treatment. N Engl J Med 2011;364:1871–2 | Design |
| 22 | Blumenthal GM, Cortazar P, Zhang JJ, Tang S, Sridhara R, Murgo A, et al. FDA approval summary: sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist 2012;17:1108–13 | Design |
| 23 | Bodei L, Bartolomei M, Cremonesi M, Rocca P, Ferrari M, Grana C, et al. Receptor radionuclide therapy with Lu-177-DOTA(0) -Tyr(3)-octreotate (Lu-177-DOTATATE) in endocrine tumors: preliminary results. Eur J Nucl Med Mol Imaging 2005;32:S100–S | Design |
| 24 | Bodei L, Cremonesi M, Grana C, Bartolomei M, Baio S, Bufi G, et al. Receptor radionuclide therapy with Lu-177-DOTATATE in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2006;33:S214–S | Design |
| 25 | Boussion H, Hammel P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. Oncologie 2015;17:59–60 | Language |
| 26 | Buil-Bruna N, Dehez M, Manon A, Nguyen TX, Trocóniz IF. Establishing the Quantitative Relationship Between Lanreotide Autogel®, Chromogranin A, and Progression-Free Survival in Patients with Nonfunctioning Gastroenteropancreatic Neuroendocrine Tumors. AAPS J 2016;18:703–12 | No data |
| 27 | Buil-Bruna N, Dehez M, Manon A, Nguyen TXQ, Troconiz IF. Relationship between lanreotide autogel, chromogranin a and progression-free survival in patients with gastroenteropancreatic neuroendocrine tumors. Pancreas 2016;45:472–3 | No data |
| 28 | Buil-Bruna N, Dehez M, Manon A, Thi Xuan QN, Troconiz I. Relationship between lanreotide autogel, chromogranin A and progression-free survival in patients with gastroenteropancreatic neuroendocrine tumours. Eur J Cancer 2015;51:S448 | No data |
| 29 | Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604–20 | Design |
| 30 | Caplin ME, Phan AT, Ruszniewski P, Pavel ME, Ćwikła JB, Raderer M, et al. Antitumor effects with lanreotide autogel/depot (LAN) in patients with metastatic enteropancreatic (EP) neuroendocrine tumors (NETs): interim results of the CLARINET extension study. Pancreas 2015;44:351–2 | Treatment – Clarinet |
| 31 | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–33 | Treatment – Clarinet |
| 32 | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer 2016;23:191–9 | Treatment – Clarinet |
| 33 | Caplin ME, Ruszniewski PB, Pavel ME, Ćwikła JB, Phan AT, Raderer M, et al. Progression-free survival (PFS) with lanreotide autogel/depot (LAN) in enteropancreatic NETs patients: the CLARINET extension study. J Clin Oncol 2014;32(15 Suppl. 1) | Design |
| 34 | Caplin M, Phan A, Liyanage N, Gomez-Panzani E, Blumberg J, Uk, et al. Lanreotide autogel significantly improves tumor progression-free survival in patients with non-functioning gastroenteropancreatic neuroendocrine tumors: results of the CLARINET study. Pancreas 2014;43:495 | Treatment – Clarinet |
| 35 | Caplin M, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Chromogranin A (CgA) and PFS outcomes in lanreotide autogel (LAN) in patients with metastatic enteropancreatic (EP-) NETs: data from the CLARINET study. Neuroendocrinology 2015;102:124 | Design |
| 36 | Caplin M, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Antitumor treatment with lanreotide autogel 120 mg (LAN) for enteropancreatic (EP-)NET: update from the CLARINET open-label extension (OLE) study. Neuroendocrinology 2015;102:123 | Design |
| 37 | Caplin M, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Health-related quality of life (HRQoL) with lanreotide autogel (LAN) 120 mg in patients with enteropancreatic (EP-)NETs: post hoc analyses from the CLARINET study. Neuroendocrinology 2015;102:123 | Treatment – Clarinet |
| 38 | Caplin M, Ruszniewski P, Pavel M, Ćwikła J, Phan A, Raderer M, et al. Progression-free survival (PFS) and tumor growth with lanreotide autogel (LAN) in patients (Pts) with enteropancreatic NETs: Results from clarinet, a randomized, double-blind, placebo (Pbo)-controlled study. Neuroendocrinology 2014;99:270 | Treatment – Clarinet |
| 39 | Caplin M, Ruszniewski P, Pavel M, Ćwikła J, Phan A, Raderer M, et al. A randomized, double-blind, placebo-Controlled study of lanreotide antiproliferative response in patients with gastroenteropancreatic neuroendocrine tumors (CLARINET). Eur J Cancer 2013;49:S3 | Treatment – Clarinet |
| 40 | Castellano D, Bajetta E, Panneerselvam A, Saletan S, Kocha W, O’Dorisio T, et al. Everolimus plus octreotide long-acting repeatable in patients with colorectal neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-2 study. Oncologist 2013;18:46–53 | Design – RADIANT2 |
| 41 | Castellano D, Bajetta E, Panneerselvam A, Saletan S, Kocha W. Subgroup analysis of patients with colorectal neuroendocrine tumors (NET) in the phase III radiant-2 study comparing everolimus plus octreotide LAR with placebo plus octreotide LAR. Ann of Oncol 2011;22:v11–2 | Design – RADIANT2 |
| 42 | Clark OH, Ajani JA, Benson IAB, Berlin JD, Blaszkowsky LS, Byrd D, et al. Neuroendocrine tumors. JNCCN 2009;7:712–47 | Design |
| 43 | Dasari A, Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, et al. Lanreotide depot/autogel (LAN) in midgut neuroendocrine tumors (NETs): A subgroup analysis from the CLARINET study. J Clin Oncol 2015;33(15 Suppl. 1) | Treatment – Clarinet |
| 44 | Dasari A, Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, et al. Lanreotide depot/autogel (LAN) in patients with neuroendocrine tumors (NETs) aged > 65 vs. >65 years: Subgroup analyses from the CLARINET study. J Clin Oncol 2015;33(15 Suppl. 1) | Treatment – Clarinet |
| 45 | de Herder WW. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Best Pract Res Clin Gastroenterol 2012;26:689–90 | Design |
| 46 | de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, et al. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology 2006;84:183–8 | Design |
| 47 | Deeks ED, Raymond E. Sunitinib: in advanced, well differentiated pancreatic neuroendocrine tumors. BioDrugs 2011;25:307–16 | Design |
| 48 | Delaunoit T, Neczyporenko F, Rubin J, Erlichman C, Hobday TJ. Medical management of pancreatic neuroendocrine tumors. Am J Gastroenterol 2008;103:475–83 | No data |
| 49 | Delavault P, Caplin ME, Liyange N, Blumberg J. The CLARINET study: assessing the effect of lanreotide autogel on tumor progression-free survival in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumors. J Clin Oncol 2012;30(15 Suppl. 1) | Treatment – Clarinet |
| 50 | Delpassand E, Mohammadali H, Thamake S, Broline S, Ranganathan D, Wagh N, et al. (177)Lutetium-DOTA-octreotate therapy in progressive somatostatin receptor-expressing neuroendocrine neoplasms. J Nucl Med 2015;56 | Design |
| 51 | Dreyer C, Hentic O, Zappa M, Hammel P, Bouattour M, Mateescu C, et al. Response evaluation using recist and choi criteria in patients with well-differentiated pancreatic neuroendocrine tumors (PNET) treated with sunitinib or everolimus. Ann Oncol 2012;23:379 | Design |
| 52 | Eberle AN, Beglinger C. Does 177Lu-labeled octreotate improve the rate of remission of endocrine gastroenteropancreatic tumors? Nat Clin Pract Endocrinol Metab 2005;1:20–1 | Design |
| 53 | Eriksson B, Klöppel G, Krenning E, Ahlman H, Plöckinger U, Wiedenmann B, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors – well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 2008;87:8–19 | Design |
| 54 | Eriksson B, Öberg K. Neuroendocrine tumours of the pancreas. Br J Surg 2000;87:129–31 | Design |
| 55 | Faggiano A, Malandrino P, Modica R, Agrimi D, Aversano M, Bassi V, et al. Efficacy and Safety of Everolimus in Extrapancreatic Neuroendocrine Tumor: A Comprehensive Review of Literature. Oncologist 2016;21:875–86 | Design |
| 56 | Faiss S, Riecken EO, Wiedenmann B, Int Lanreotide Interferon-alpha study g. Evaluation of the antiproliferative effect of lanreotide or interferon-alpha or the combination of both in the therapy of metastatic neuroendocrine tumors. Gastroenterology 1998;114:A593–A | Design |
| 57 | Faiss S, Scherübl H, Riecken EO, Wiedenmann B. Interferon-alpha versus somatostatin or the combination of both in metastatic neuroendocrine gut and pancreatic tumours. Digestion 1996;57(Suppl. 1):84–5 | Design |
| 58 | Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology 2006;84:196–211 | Design |
| 59 | Fazio N, Granberg D, Grossman A, Saletan S, Klimovsky J, Panneerselvam A, Wolin EM. Everolimus plus octreotide long-acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest 2013;143:955–62 | Design – RADIANT2 |
| 60 | Fazio N, Granberg D, Grossman A, Saletan S, Winkler RE, Panneerselvam A, et al. Effect of everolimus + octreotide LAR in patients with advanced lung neuroendocrine tumours - Analysis from RADIANT-2. European Journal of Cancer. 2011;47:S463 | Design – RADIANT2 |
| 61 | Fazio N, Öberg K. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors. J Clin Oncol 2004;22:573–4 | Design |
| 62 | Fisher Jr GA, Wolin EM, Kunz P, Liyanage N, Gomez-Panzani E, Lowenthal SP, et al. Safety and efficacy of lanreotide depot versus placebo in neuroendocrine tumor patients with a history of carcinoid syndrome and prior octreotide therapy. Am J Gastroenterol 2015;110:S1007 | Treatment |
| 63 | Fisher GA, Wolin EM, Kunz P, Liyanage N, Gomez-Panzani E, Lowenthal SP, et al. Efficacy and Safety of Lanreotide Depot vs. Placebo in Patients With Neuroendocrine Tumor and a History of Carcinoid Syndrome and Prior Octreotide Therapy. Pancreas. 2016;45:475 | Treatment |
| 64 | Freeman S. Lanreotide has benefit in nonfunctioning neuroendocrine tumors. Oncology Report 2013:7–8 | Design |
| 65 | Galli L, Ricci S, Antonuzzo A, Bengala C, Conte PF. The new long-acting somatostatin analogue Lanreotide in neuroendocrine tumors (NETs). Ann Oncol 1998;9:130 | Design |
| 66 | Gan HK, Seruga B, Knox JJ. Sunitinib in solid tumors. Expert Opin Investig Drugs 2009;18:821–34 | Design |
| 67 | Ganetsky A, Bhatt V. Gastroenteropancreatic neuroendocrine tumors: update on therapeutics. Ann Pharmacother 2012;46:851–62 | Design |
| 68 | Gilbert JA. Lanreotide delays progression of neuroendocrine tumours. Lancet Oncol 2014;15:e418 | Design |
| 69 | Goldstein R, Meyer T. Role of everolimus in pancreatic neuroendocrine tumors. Expert Rev Anticancer Ther 2011;11:1653–65 | Design |
| 70 | Gomez-Panzani E, Vinik A, Wolin E, Audry H. Elect: A phase 3 study of efficacy and safety of lanreotide autogel (LAN) treatment for carcinoid syndrome (CS) in patients (Pts) with gastroenteropancreatic neuroendocrine tumors (gep-NETs). Neuroendocrinology 2014;99:271 | Treatment |
| 71 | Gotthardt M, Librizzi D, Wolf D, Lalyko G, Behr TM, Behe M. Increased therapeutic efficacy through combination of Lu-177-DOTATOC and chemotherapy in neuroendocrine tumours in vivo. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:S115–S | Design |
| 72 | Granberg D, de Herder W, O’Toole D, Kvols L. Treatment of liver metastases in patients with neuroendocrine tumors. Int J Hepatol 2012;2012:790635 | Design – RADIANT2 |
| 73 | Granberg D, Fazio N, Grossman A, Saletan S, Pannnerselvam A, Wolin E. Everolimus plus octreotide LAR in patients with lung carcinoids. J Thorac Oncol 2014;9(9 Suppl. 3):S179 | Design |
| 74 | Grenader T, Ruszniewski P, Pavel M, Ćwikła J, Phan A, Raderer M, et al. Prognostic value of neutrophil/lymphocyte ratio in intestinal and pancreatic neuroendocrine tumors: Exploratory analysis of data from the CLARINET trial of lanreotide depot/autogel. Eur J Cancer 2015;51:S443 | No data |
| 75 | Gross D, Peeters M, Jehl V, Saletan S, Sideris L. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus + octreotide lar vs. Placebo + octreotide lar in patients with advanced neuroendocrine tumors (NET) (RADIANT-2): Updated safety results. Endocrine Reviews Conference: 93rd Annual Meeting and Expo of the Endocrine Society, ENDO. 2011;32 | Design – RADIANT2 |
| 76 | Gulenchyn KY, Yao X, Asa SL, Singh S, Law C. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol 2012;24:294–308 | Design |
| 77 | Guo LJ, Tang CW. Somatostatin analogues do not prevent carcinoid crisis. APJCP 2014;15:6679–83 | Design |
| 78 | Guo LJ, Tang CW. Somatostatin analogues for carcinoid syndrome. J Dig Dis 2014;15:62 | No data |
| 79 | Guo L, Tang C. Somatostatin analogues for carcinoid syndrome. Neuroendocrinology 2014;99:272 | Design |
| 80 | Guo L, Tang C. Somatostatin analogues for preventing carcinoid crisis. Neuroendocrinology 2014;99:271 | No data |
| 81 | Hobday T, Becerra C, Yalcin S, Panneerselvam A, Saletan S, Hainsworth J. Post-progression therapies in patients with advanced neuroendocrine tumors (NET): analysis from the RADIANT-2 trial. Am J Gastroenterol 2011;106:S85-6 | Design – RADIANT2 |
| 82 | Hofman MS, Hicks RJ. Peptide receptor radionuclide therapy for neuroendocrine tumours: standardized and randomized, or personalized? Eur J Nucl Med Mol Imaging 2014;41:211–13 | Design |
| 83 | Hofman MS, Kashyap R, Kong G, Akhurst T, Pattison D, Eu P, et al. Improved survival of poor prognosis fdg-avid neuroendocrine tumours with lu-177 octreotate peptide receptor chemoradionuclide therapy (PRCRT). Int Med J 2015;45:13 | Design |
| 84 | Horsch D, Schrader J. Molecular therapy of neuroendocrine neoplasia: sunitinib (Sutent) and everolimus (Afinitor). Verdauungskrankheiten 2014;32:147-–9 | Language |
| 85 | Hosking E, McKay E, Browne E, Thomas D, Liauw W, Morris D, et al. Lu-177 DOTATATE therapy in patients with NETS. Int Med J 2015;45:18 | Design |
| 86 | Hyrdel R, Nikou G, Krenning E, Vullierme MP, Ahlman H, Arnold R, et al. Rare functioning pancreatic endocrine tumors. Neuroendocrinology 2006;84:189–95 | Design |
| 87 | Iwasaki M, Phan A, Caplin M, Ruszniewski P, Pavel M, Gomez-Panzani E. Quality of life (QOL) with lanreotide depot (LAN) vs. placebo in patients with pancreatic and gastrointestinal neuroendocrine tumours: results from the clarinet Phase III study. Oncology Nursing Forum 2015;42:E144-E | Treatment – Clarinet |
| 88 | Iwasaki M, Wolin E, Dasari A, Liyanage N, Lowenthal SP, Phan A. Response rates in the phase 3 clarinet trial of lanreotide depot vs. placebo in patients with metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETS). Oncology Nursing Forum 2016;43:136 | Treatment – Clarinet |
| 89 | Jacobsen MB, deVries EGE, Eriksson B, Fiasse R, Wijmenga M, Salmela P, et al. Symptomatic effect of a long acting Lanreotide formulation in patients with gastrointestinal neuroendocrine tumours. Gastroenterology 1996;110:A535-A | Design |
| 90 | Joelle B, Nilani L, Martyn C. The clarinet study y assessing the effect of lanreotide autogel on tumor progression-free survival in patients with non-functioning gastroenteropancreatic neuroendocrine tumors (GEP-NETS). Pancreas 2012;41:343 | Treatment – Clarinet |
| 91 | Kaltsas G, Grossman AB. The expanding role of somatostatin analogues in the treatment of neuroendocrine tumours: the CLARINET study. Clin Endocrinol 2015;83:759–61 | Design |
| 92 | Kim SJ, Pak K, Koo PJ, Kwak JJ, Chang S. The efficacy of (177)Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging 2015;42:1964–70 | No data |
| 93 | Koussis H, Scola A, Cirillo F, Basso U, Ziampiri S, Casara D, et al. Alternating lanreotide and octreotide in the treatment of metastatic neuroendocrine tumors (NETs). J Clin Oncol 2008;26 | Design |
| 94 | Krampitz GW, Norton JA. Pancreatic neuroendocrine tumors. Curr Probl Surg 2013;50:509–45 | Design |
| 95 | Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin North Am 2014;94:689–708 | Design |
| 96 | Kwekkeboom DJ, Bakker WH, Teunissen JJ, Kooij PP, Krenning EP. Treatment with Lu-177-DOTA-Tyr3-octreotate in patients with neuroendocrine tumors: Interim results. J Nucl Med 2003;44:135P–6P | Design |
| 97 | Lahner H, Dorffel Y, Bojunga J. Health-related Quality-of-Life in everolimus-treated patients with advanced neuroendocrine tumors: Results from an open-label, phase IIIb, multicenter, expanded access program (EVIDENT). Oncol Res Treatment 2014;37:152 | Design |
| 98 | Lahner H, Rinke A, Poppel TD, Fuhrer D. Sunitinib in pancreatic neuroendocrine tumours. Exp Clin Endocrinol Diabetes 2013;121 | Design |
| 99 | Lamberts SW, van der Lely AJ, Hofland LJ. New somatostatin analogs: will they fulfil old promises? Eur J Endocrinol 2002;146:701–5 | Design |
| 100 | Lee YS, Park EJ. National formulary review of the drugs used in pancreatic neuroendocrine tumors in Korea. Value Health 2011;14:A465 | Design |
| 101 | Lu CC, Lin HF, Feng CC, Yu CY, Kao WY. Sunitinib malate as the salvage therapy in advanced rectal carcinoid tumor. Int J Colorectal Dis 2008;23:1265–6 | Design |
| 102 | Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res 2004;120:139–61 | Design |
| 103 | Massironi S, Conte D, Rossi RE. Somatostatin analogues in functioning gastroenteropancreatic neuroendocrine tumours: literature review, clinical recommendations and schedules. Scand J Gastroenterol 2016;51:513–23 | Design |
| 104 | Migliori M, Tomassetti P, Montini GC, Lalli S, Corinaldesi R. Treatment of gastro-entero-pancreatic (GEP) neuroendocrine tumors with lanreotide, a new-long-acting somatostatin analogue. Gastroenterology 1998;114:A643–A | Design |
| 105 | Mittendorf EA, Inabnet WB, Libutti SK, McHenry CR, Demeure MJ. Islet Cell Tumors. Current Problems in Surgery 2006;43:685–765 | Design |
| 106 | Modlin IM, Bodei L, Kidd M. A historical appreciation of bronchopulmonary neuroendocrine neoplasia: resolution of a carcinoid conundrum. Thorac Surg Clin 2014;24:235–55 | Design |
| 107 | Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother 2008;9:2617–26 | Design |
| 108 | Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 2010;31:169–88 | Design |
| 109 | Motylewska E, Gawronska J, Niedziela A, Melen-Mucha G, Lawnicka H, Komorowski J, et al. Somatostatin Analogs and Tumor Localization Do Not Influence Vitamin D Concentration in Patients with Neuroendocrine Tumors. Nutr Cancer 2016;68:428–34 | Design |
| 110 | Muniraj T, Vignesh S, Shetty S, Thiruvengadam S, Aslanian HR. Pancreatic neuroendocrine tumors. Dis Mon 2013;59:5–19 | Design |
| 111 | Niederhuber JE, Fojo T. Treatment of metastatic disease in patients with neuroendocrine tumors. Surg Oncol Clin N Am 2006;15:511–33, viii | Design |
| 112 | NIHR HSC. Everolimus (Afinitor) for advanced, unresectable or metastatic neuroendocrine tumours (Structured abstract). 2012. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32012000933/frame.html | Design |
| 113 | NIHR HSC. Lanreotide for unresectable, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumours – first line (Structured abstract). 2014. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32015000117/frame.html | Design |
| 114 | NIHR HSC. Lutetium-177 for inoperable gastroenteropancreatic neuroendocrine tumours (Structured abstract). 2014. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32014000670/frame.html | Design |
| 115 | Öberg K. Treatment of neuroendocrine tumors. Cancer Treat Rev 1994;20:331–55 | Design |
| 116 | Öberg K. Universal everolimus for malignant neuroendocrine tumours? Lancet 2016;387:924–6 | Design |
| 117 | Öberg K, Akerström G, Rindi G, Jelic S, ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl. 5):v223–7 | Design |
| 118 | Öberg K, Anthony L, Sideris L, Chen L, Cherfi A, Tsuchihashi Z, et al. Role of chromogranin a and neuron-specific enolase biomarkers in progression-free survival (PFS) with everolimus (EVE) versus placebo (PB) in patients with advanced pancreatic neuroendocrine tumors (pNET): Phase III radiant-3 results. Neuroendocrinology 2011;94:37 | No data |
| 119 | Öberg K, Hellman P, Ferolla P, Papotti M, ESMO Guidelines Working Group. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl. 7):vii120–3 | Design |
| 120 | Öberg K, Kulke M, Pavel M, Phan A, Hoosen S, St. Peter J, et al. Prognostic and predictive value of chromogranin a and neuron-specific enolase in patients (pts) with advanced pancreatic neuroendocrine tumors (pnet) treated with everolimus. Ann Oncol 2010;21:viii266 | Design |
| 121 | Öberg K, Kulke M, Pavel M, Phan A, Hoosen S, St. Peter J, et al. Evaluation of chromogranin a and neuron-specific enolase as predictors of response to everolimus therapy in patients with advanced pancreatic neuroendocrine tumors (pNET). Pancreas 2011;40:329–30 | Design |
| 122 | Öberg K, Knigge U, Kwekkeboom D, Perren A, ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl. 7):vii124–30 | Design |
| 123 | Ong SL, Garcea G, Pollard CA, Furness PN, Steward WP, Rajesh A, et al. A fuller understanding of pancreatic neuroendocrine tumours combined with aggressive management improves outcome. Pancreatology 2009;9:583–600 | Design |
| 124 | O’Toole D, Ducreux M, Bommelaer G, Wemeau JL, Bouché O, Catus F, et al. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000;88:770–6 | Design |
| 125 | Ozdemir N, Yazici O, Zengin N. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:1555–6 | Design |
| 126 | Pavel M, Hainsworth JD, Baudin E, Peeters M, Hoersch D, Anthony L, et al. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus 1 octreotide lar vs. placebo 1 octreotide lar in patients with advanced neuroendocrine tumors (net) (radiant-2). Ann Oncol 2010;21:viii4 | Design – RADIANT2 |
| 127 | Pavel M, Öberg KE, Hainsworth JD, Lam D, Stergiopolos SG, Rouyrre N, et al. Everolimus plus octreotide long-acting release (LAR) for the treatment of advanced neuroendocrine tumors (NET) associated with carcinoid syndrome (RADIANT-2): Updated overall survival results. Eur J Cancer 2013;49:S577 | Design – RADIANT2 |
| 128 | Pavel M, Peeters M, Hoersch D, Van Cutsem E, Öberg K, Jehl V, et al. Everolimus plus Octreotide LAR versus Placebo plus Octreotide LAR in Patients with Advanced NET: Results of a Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase 3 Trial (RADIANT-2). Neuroendocrinology 2011;94:38–9 | Design – RADIANT2 |
| 129 | Pavel M, Singh N, Passos V, Öberg K. Impact of prior somatostatin analog (SSA) use on PFS in the multicenter, phase III trial of everolimus + octreotide LAR vs. placebo + octreotide LAR in patients with advanced neuroendocrine tumours (radiant-2). European Journal of Cancer. 2011;47:S462–3 | Design – RADIANT2 |
| 130 | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011;378:2005–12 | Design – RADIANT2 |
| 131 | Pavel ME, Singh N, Passos V, Strosberg J. Progression-free survival by prior somatostatin analog use and primary tumor site and updated safety results in patients with advanced neuroendocrine tumors and a history of carcinoid syndrome: A radiant-2 analysis. Neuroendocrinology 2012;96:53–4 | Design – RADIANT2 |
| 132 | Pavel M, De Vries E, Öberg K, Lebrec J, Winkler R, Tsuchihashi Z, et al. Everolimus improves progression-free survival (PFS) regardless of baseline chromogranin a (CGA) and neuron-specific enolase (NSE) levels in patients with advanced pancreatic neuroendocrine tumors (pNET) (RADIANT-3). Ann Oncology 2011;22:v13–4 | No data |
| 133 | Pavel ME, Grosch K, Cheung W, Hasskarl J, Becerra C. Effect of everolimus on the pharmacokinetics of octreotide LAR in patients with advanced neuroendocrine tumors: A RADIANT-2 analysis. Endocrine Reviews Conference: 94th Annual Meeting and Expo of the Endocrine Society, ENDO. 2012;33 | Design – RADIANT2 |
| 134 | Pavel ME, Grosch K, Cheung W, Hasskarl J, Becerra C. Effect of everolimus on pharmacokinetics of octreotide LAR in patients with advanced neuroendocrine tumors: a radiant-2 analysis. Neuroendocrinology 2012;96:54 | Design – RADIANT2 |
| 135 | Pavel M, Grosch K, Cheung W, Becerra C, Yao J. Effect of everolimus on pharmacokinetics of octreotide LAR in patients with advanced neuroendocrine tumors: a RADIANT-2 analysis. Am J Gastroenterol 2012;107:S602 | Design – RADIANT2 |
| 136 | Pavel M, Grosch K, Cheung W, Becerra C, Yao J. Pharmacokinetics of octreotide lar when administered with everolimus in patients with advanced neuroendocrine tumors: a radiant-2 analysis. J Oncol Pharm Pract 2013;19:15–16 | Design – RADIANT2 |
| 137 | Pavel M, Raderer M. Antitumor activity of lanreotide autogel 120 mg in enteropancreatic Neuroendocrine Tumour (NET) patients: the clarinet open-label extension study. Oncol Res Treat 2015;38:151–2 | Design |
| 138 | Pavel M, Unger N, Borbath I, Ricci S, Hwang TL, Park YS, et al. Safety and quality-of-life (QOL) assessments in the open-label, multicenter, phase 3b, expanded access study of everolimus in patients with advanced neuroendocrine tumors (NET). Pancreas 2014;43:500–1 | Design |
| 139 | Peeters M, Becerra C, Panneerselvam A, Saletan S, Yalcin S. Post-study treatment options are limited in patients with advanced net: analysis of postprogression therapies from the radiant-2 trial. Ann Oncology 2011;22:v81–2 | Design |
| 140 | Phan A, Caplin M, Pavel M, Ćwikła J, Raderer M, Sedláčková E, et al. Relative risk analysis of safety profile of lanreotide autogel/depot vs. placebo in patients with pancreatic and intestinal neuroendocrine tumours. Eur J Cancer 2015;51:S460 | Treatment – Clarinet |
| 141 | Phan AT. Lanreotide depot/autogel in neuroendocrine tumors: subgroup analyses from the CLARINET study. Clin Adv Hematol Oncol 2015;13:6–8 | Design |
| 142 | Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, Sedláčková E, et al. Lanreotide depot/autogel (LAN) in pancreatic neuroendocrine tumors (pNETs): A subgroup analysis from the CLARINET study. J Clin Oncol 2015; 33(15 Suppl. 1) | Treatment – Clarinet |
| 143 | Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, Sedláčková E, et al. Effects of lanreotide autogel/depot (LAN) in patients with neuroendocrine tumors (NETs) age 65 or younger versus older than age 65: Subgroup analyses from the CLARINET study. Journal of Clinical Oncology Conference 2015;33 | Treatment – Clarinet |
| 144 | Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, Sedláčková E, et al. Relative risk of adverse events with lanreotide depot/autogel (LAN) vs. Placebo (PBO) in patients with intestinal and pancreatic neuroendocrine tumors (NETs). Journal of Clinical Oncology Conference 2015;33 | Treatment – Clarinet |
| 145 | Phan AT, Caplin ME, Pavel ME, Jaroslaw BC, Raderer M, Sedláčková E, et al. Effects of lanreotide autogel/depot (LAN) in pancreatic neuroendocrine tumors (pNETs): A subgroup analysis from the CLARINET study. Journal of Clinical Oncology Conference 2015;33 | Treatment – Clarinet |
| 146 | Phan AT, Dasari A, Liyanage N, Cox D, Lowenthal SP, Wolin EM. Tumor response in the CLARINET study of lanreotide depot vs. placebo in patients with metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Journal of Clinical Oncology Conference 2016;34 | Treatment – Clarinet |
| 147 | Pusceddu S, De Braud F, Festinese F, Bregant C, Lorenzoni A, Maccauro M, et al. Evolution in the treatment of gastroenteropancreatic-neuroendocrine neoplasms, focus on systemic therapeutic options: a systematic review. Future Oncol 2015;11:1947–59 | Design |
| 148 | Pusceddu S, De Braud F, Lo Russo G, Concas L, Femia D, Vernieri C, et al. How do the results of the RADIANT trials impact on the management of NET patients? A systematic review of published studies. Oncotarget 2016;7:44841–44847 | Design |
| 149 | Qi WX, Huang YJ, Yao Y, Shen Z, Min DL. Incidence and risk of treatment-related mortality with mTOR inhibitors everolimus and temsirolimus in cancer patients: a meta-analysis. PLOS ONE 2013;8:e65166 | Design |
| 150 | Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012;61:6–32 | Design |
| 151 | Ramundo V, Marciello F, Modica R, Marotta V, Pizza G, Camera L, et al. Efficacy of lanreotide versus follow-up in early-stage duodeno-pancreatic neuroendocrine tumors (NETs) related to multiple endocrine neoplasia type 1 (MEN1): Preliminary data. Neuroendocrinology 2015;102:125 | Treatment |
| 152 | Raut CP, Kulke MH, Glickman JN, Swanson RS, Ashley SW. Carcinoid Tumors. Current Problems in Surgery 2006;43:391–450 | Design |
| 153 | Regnault A, Ferrer L, Dinet J, Gabriel S, Pavel ME, Ruszniewski PB, et al. Health-related quality of life in CLARINET, a phase III trial of lanreotide autogel 120 mg in patients with non-functioning entero-pancreatic neuroendocrine tumour: analytical challenges and statistical solutions. Quality of Life Research. 2015;24:75 | Treatment – Clarinet |
| 154 | Ricci S, Ruszniewski P, Tomasetti P, Jehl V, Saletan S, Yao JC, et al. Updated safety and efficacy results from radiant-2 - A randomized, double-blind, multicenter, phase III trial of everolimus + octreotide LAR vs. placebo + octreotide LAR in pts with advanced neuroendocrine tumours (NET). Eur J Cancer 2011; 47:S461 | Design – RADIANT2 |
| 155 | Ricci S, Carnaghi C, Cirillo F, De Angelis C, Galli C, Iannopollo M, et al. Efficacy of 1-month lanreotide in patients with neuroendocrine tumours: Preliminary results of a Italian multicenter study. Ann Oncol 2000;11:61 | Design |
| 156 | Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, Choueiri TK. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol 2011;29:3450–6 | Design |
| 157 | Rindi G, Caplin M. mTOR inhibitor therapy for patients with carcinoid. Lancet 2011;378:1978–80 | Design |
| 158 | Rossi RE, Massironi S, Spampatti MP, Conte D, Ciafardini C, Cavalcoli F, Peracchi M. Treatment of liver metastases in patients with digestive neuroendocrine tumors. J Gastrointest Surg 2012;16:1981–92 | Design |
| 159 | Roth I. Phase III Data in neuroendocrine Tumors show: RAD001 as Monotherapy and in Combination with Sandostatin (c) LAR (c) effectively. Viszeralmedizin 2010;26:290 | Language |
| 160 | Ruszniewski P, Tomassetti P, Saletan S, Panneerselvam A, Yao JC. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients (pts) with advanced neuroendocrine tumors: Multivariate analysis of progression-free survival from the RADIANT-2 trial. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29 | Design – RADIANT2 |
| 161 | Ruszniewski P, Phan AT, Caplin ME, Pavel ME, Ćwikła JB, Raderer M, et al. Quality of Life (QoL) with Lanreotide Autogel/Depot (LAN) vs. Placebo in Patients with Enteropancreatic Neuroendocrine Tumors: Results From the CLARINET Core Study. Pancreas 2015;44:357 | Treatment – Clarinet |
| 162 | Ruszniewski P, Rougier P, Rampal P, Grange JD, Jian R, Treffot MJ, et al. Does lanreotide influence the growth of metastatic carcinoid-tumors. Gastroenterology 1994;106:A319-A | Design |
| 163 | Schnirer II, Yao JC, Ajani JA. Carcinoid – a comprehensive review. Acta Oncol 2003;42:672–92 | Design |
| 164 | Sciandivasci A, Correale P, Del Vecchio MT, Marsili S, Pascucci A, Petrioli R, et al. Chemo-hormone therapy of undifferentiated endocrine tumors from different anatomic sites with cisplatinum etoposide and long lasting release lanreotide. Ann Oncol 2004;15:88 | Design |
| 165 | Siddall R. Sunitinib use is justified for pancreatic neuroendocrine tumours. Br J Hosp Med 2010;71:610 | Design |
| 166 | Sidéris L, Dubé P, Rinke A. Antitumor effects of somatostatin analogs in neuroendocrine tumors. Oncologist 2012;17:747–55 | Design |
| 167 | Signorovitch J, Swallow E, Kantor E, Wang X, Klimovsky J, Haas T, et al. Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol 2013;2:32 | Design |
| 168 | Sjoquist KM. Control nets: Capecitabine on temozolomide radionuclide therapy octreotate lutetium-177 neuroendocrine tumours study. Asia-Pacific Journal of Clinical Oncology 2015;11:76 | No data |
| 169 | Slooter GD, Mearadji A, Breeman WA, Marquet RL, de Jong M, Krenning EP, van Eijck CH. Somatostatin receptor imaging, therapy and new strategies in patients with neuroendocrine tumours. Br J Surg 2001;88:31–40 | Design |
| 170 | Srirajaskanthan R, Toumpanakis C, Meyer T, Caplin ME. Review article: future therapies for management of metastatic gastroenteropancreatic neuroendocrine tumours. Aliment Pharmacol Ther 2009;29:1143–54 | Design |
| 171 | Strosberg J, Ricci S, Ruszniewski P, Tomassetti P, Jehl V, Saletan S, et al. Radiant-2: A randomized, double-blind, multicenter, phase III trial of everolimus + octreotide lar vs. placebo + octreotide lar in patients with advanced neuroendocrine tumors: Progression-free survival by primar tumor site and updated safety results. Pancreas 2012;41:349 | Design – RADIANT2 |
| 172 | Strosberg J, Anthony L, Sideris L, Lebrec J, Tsuchihashi Z, Winkler R, et al. Prognostic value of chromogranin a and neuron-specific enolase in patients with advanced pancreatic neuroendocrine tumors (pNET): Phase III RADIANT-3 study results 2011 ACG presidential poster. Am J Gastroenterol 2011;106:S58 | No data |
| 173 | Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177-Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumors: results of the Phase III NETTER-1 trial. Pancreas 2016;45:483 | Treatment – NETTER |
| 174 | Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177-Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumours: Results of the phase III NETTER-1 trial. Eur J Cancer 2015;51:S710 | Treatment – NETTER |
| 175 | Strosberg JR, Wolin EM, Chasen B, Kulke MH, Bushnell DL, Caplin ME, et al. NETTER-1 phase III: Progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-DOTATATE. Journal of Clinical Oncology Conference 2016;34 | Treatment – NETTER |
| 176 | Sutcliffe R, Maguire D, Ramage J, Rela M, Heaton N. Management of neuroendocrine liver metastases. Am J Surg 2004;187:39–46 | Design |
| 177 | Thompson LA, Kim M, Wenger SD, O’Bryant CL. Everolimus: a new treatment option for advanced pancreatic neuroendocrine tumors. Ann Pharmacother 2012;46:1212–19 | Design |
| 178 | Ujeyl M. Everolimus for the treatment of unresectable or metastatic neuroendocrine tumours of pancreatic origin (Structured abstract). 2011. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32012000299/frame.html | Design |
| 179 | Van Essen M, Krenning EP, Bakker WH, Kooij PP, Kwekkeboom DJ. Peptide receptor radionuclide therapy with Lu-177-octreotate in foregut carcinoid tumours. Eur J Nucl Med Mol Imaging 2006;33:S215–S | Design |
| 180 | Vinik AI. Advances in diagnosis and treatment of pancreatic neuroendocrine tumors. Endocr Pract 2014;20:1222–30. https://doi.org/10.4158/EP14373.RA | Design |
| 181 | Vinik A, Wolin EM, Audry H, Gomez-Panzani EL. ELECT: a phase 3 study of efficacy and safety of lanreotide autogel/depot (LAN) treatment for carcinoid syndrome in patients with neuroendocrine tumors (NETs). Journal of Clinical Oncology Conference 2014;32 | Treatment |
| 182 | Vinik A, Wolin EM, Audry H, Gomez-Panzani E, Fisher GA. Lanreotide depot/autogel (LAN) vs. placebo (PBO) for carcinoid syndrome (CS) in patients with neuroendocrine tumors (NETs): Subgroup analysis of the ELECT study. J Clin Oncol 2015;33(15 Suppl. 1) | Treatment |
| 183 | Völter V, Peschel C. Is lanreotide and/or interferon alfa an adequate therapy for neuroendocrine tumors? J Clin Oncol 2004;22:573 | Design |
| 184 | Vries E, Anthony LB, Sideris L, Chen L, Lebrec J, Tsuchihashi Z, et al. Effect of everolimus treatment on chromogranin A, neuron-specific enolase, gastrin, and glucagon levels in patients with advanced pancreatic neuroendocrine tumors (pNET): Phase III RADIANT-3 study results. J Clin Oncol 2011;29(15 Suppl. 1) | No data |
| 185 | Wachter K. Sunitinib doubles PFS of pancreatic neuroendocrine tumors. Oncol Report 2010:18 | Design |
| 186 | Walczak WJ, Jarosz J, Lipinska M, PrzaDa-Machno P, Kroc J. Clinical effectiveness analysis of sunitinib for the treatment of pancreatic neuroendocrine tumors. Value Health 2012;15:A411 | Design |
| 187 | Walker PR. Sunitinib in patients with advanced neuroendocrine tumors. Am J Hematol Oncol 2009;8 | Design |
| 188 | Wallace D, Morrissey S. Endocrine tumors of the pancreas. Practical Gastroenterology 2012;36:13–27 | Design |
| 189 | Wesolowski R, Abdel-Rasoul M, Lustberg M, Paskell M, Shapiro CL, Macrae ER. Treatment-related mortality with everolimus in cancer patients. Oncologist 2014;19:661–8 | Design |
| 190 | Williams D, Vinik A, Wolin E, Kunz P, Lowenthal SP, Fisher G. Safety and efficacy of lanreotide depot vs. placebo in neuroendocrine tumor patients with a history of carcinoid syndrome and prior octreotide therapy. Oncology Nursing Forum 2016;43:133–4 | Treatment |
| 191 | Wolin EM, Fazio N, Saletan S, Winkler RE, Panneerselvam A, Kvols L. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors: Analysis by primary tumor site from RADIANT-2. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29 | Design – RADIANT2 |
| 192 | Wolin E, Castellano D, Kaltsas G, Gross D, Panneerselvam A, Klimovsky J, et al. Correlation of progression-free survival (PFS) with early response of biomarkers chromogranin a (CGA) and 5-hhydroxyindoleacetic acid (5-HIAA) levels in patients with advanced neuroendocrine tumors: Phase III radiant-2 study results. Pancreas 2012;41:350–1 | No data |
| 193 | Wolin E, Caplin M, Pavel M, Ćwikła J, Phan A, Raderer M, et al. Multivariate analysis of progression-free survival in the CLARINET study of lanreotide Autogel/Depot vs. placebo identifies prognostic factors in neuroendocrine tumours. Eur J Cancer 2015;51:S461-S2 | Treatment – Clarinet |
| 194 | Wolin EM. Long-term everolimus treatment of patients with pancreatic neuroendocrine tumors. Chemotherapy 2014;60:143–50 | Design |
| 195 | Wolin EM, Caplin ME, Pavel ME, Ćwikła JB, Phan AT, Raderer M, et al. Prognostic factors for progression-free survival (PFS) in CLARINET study of lanreotide depot/autogel (LAN) vs. placebo (PBO) in neuroendocrine tumors (NETs). Journal of Clinical Oncology Conference 2015;33 | Treatment – Clarinet |
| 196 | Wolin EM, Caplin ME, Pavel ME, Ćwikła JB, Phan AT, Raderer M, et al. Lanreotide depot/autogel (LAN) in intestinal and pancreatic neuroendocrine tumors (NETs) according to body mass index (BMI): Subgroup analyses from the CLARINET study. Journal of clinical oncology [Internet]. 2015; 33(15 Suppl. 1) | Treatment – Clarinet |
| 197 | Wolin EM, Caplin ME, Pavel ME, Ćwikła JB, Phan AT, Raderer M, et al. Lanreotide Depot/Autogel in Intestinal and Pancreatic Neuroendocrine Tumors According to Body Mass Index: Subgroup Analyses From the CLARINET Study. Pancreas 2016;45:485 | Treatment – Clarinet |
| 198 | Wolin EM, Caplin ME, Pavel ME, Ćwikła JB, Phan AT, Raderer M, et al. Multivariate Analysis of Progression-Free Survival in the CLARINET Study of Lanreotide Autogel/Depot vs. Placebo Identifies Prognostic Factors in Neuroendocrine Tumors. Pancreas 2016;45:486 | Treatment – Clarinet |
| 199 | Yang F, Jin C, Fu D. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:1556 | Design |
| 200 | Yao JC, Hainsworth JD, Baudin E, Peeters M, Hoersch D, Anthony L, et al. Radiant-2: A phase III trial of everolimus + octreotide lar in patients with advanced neuroendocrine tumors (NET). Pancreas 2011;40:335 | Design – RADIANT2 |
| 201 | Yao JC, Hainsworth JD, Baudin E, Peeters M, Hoersch D, Anthony LB, et al. Everolimus plus octreotide LAR (E+O) versus placebo plus octreotide LAR (P+O) in patients with advanced neuroendocrine tumors (NET): Updated results of a randomized, double-blind, placebo-controlled, multicenter phase III trial (RADIANT-2). Journal of Clinical Oncology Conference 2011;29 | Design – RADIANT2 |
| 202 | Yao JC, Hainsworth JD, Wolin EM, Pavel ME, Baudin E, Gross D, et al. Multivariate analysis including biomarkers in the phase III RADIANT-2 study of octreotide LAR plus everolimus (E+O) or placebo (P+O) among patients with advanced neuroendocrine tumors (NET). Journal of Clinical Oncology Conference 2012;30 | Design – RADIANT2 |
| 203 | Yao JC, Öberg KE, Hainsworth JD, Lam D, Stergiopolos SG, Rouyrre N, et al. Everolimus Plus Octreotide Long-Acting Repeatable (LAR) for the Treatment of Advanced Neuroendocrine Tumors (NET) Associated with Carcinoid Syndrome: Updated Overall Survival Results from RADIANT-2 Study. Pancreas 2014;43:508-9 | Design – RADIANT2 |
| 204 | Yao JC, Ricci S, Winkler RE, Jehl V, Pavel ME. Everolimus plus octreotide LAR versus placebo plus octreotide LAR in patients with advanced neuroendocrine tumors (NET): Updated safety and efficacy results from RADIANT-2. Journal of Clinical Oncology Conference: ASCO Annual Meeting 2011;29 | Design – RADIANT2 |
| 205 | Yao JC, Buzzoni R, Carnaghi C, Fazio N, Singh S, Wolin EM, et al. Baseline demographics of the randomized, placebo-controlled, double-blind, phase III RADIANT-4 study of everolimus in nonfunctional gastrointestinal (GI) or lung neuroendocrine tumors (NET). J Clin Oncol 2015;33(3 Suppl. 1) | No data |
| 206 | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008;26:4311–18 | Design |
| 207 | Yao JC, Shah M, Panneerselvam A, Chen D, Stergiopoulos S, Ito T, et al. The VEGF pathway in pancreatic neuroendocrine tumors: Prognostic and predictive capacity of baseline biomarker levels on efficacy of everolimus analyzed from the RADIANT-3 study. Pancreas 2013;42:386–7 | No data |
| 208 | Yao JC, Shah M, Panneerselvam A, Stergiopoulos S, Chen D, Ito T, et al. The VEGF pathway in patients with pancreatic neuroendocrine tumors: Efficacy of everolimus by baseline marker level, and prognostic and predictive effect analyses from radiant-3. Ann Oncol 2012;23:ix376 | No data |
| 209 | Yao JC, Tsuchihashi Z, Panneerselvam A, Winkler RE, Bugarini R, Pavel M. Effect of everolimus treatment on markers of angiogenesis in patients with advanced pancreatic neuroendocrine tumours (pNET) - Results from the phase III RADIANT-3 study. Eur J Cancer 2011;47:S463 | No data |
| 210 | Yao J, Wang JY, Liu Y, Wang B, Li YX, Zhang R, et al. A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med Oncol 2014;31:251 | Retracted |
| 211 | Off-label uses of Sorafenib and Sunitinib (Structured abstract). 2007. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32010000210/frame.html | Design |
| 212 | Everolimus (RAD-001) for advanced gastroenteropancreatic neuroendocrine tumours (Structured abstract). 2008. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32010000614/frame.html | Design |
| 213 | Sunitinib for advanced and/or metastatic pancreatic neuroendocrine tumours (Structured abstract). 2010. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32010001219/frame.html | Design |
| 214 | Sunitinib (Sutent®) (Structured abstract). 2011. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32012000356/frame.html | Design |
| 215 | Everolimus (Afinitor®) (Structured abstract). 2012. URL: http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32012000603/frame.html | Design |
| 216 | Neuro-endocrine tumor. New study on tumor growth control with Sandostatin LAR and high efficacy or oral mTOR-Inhibitor RAD 001. Viszeralmedizin 2009;25:66 | Language |
| 217 | Advanced pancreatic Neuroendocrine Tumors: mTOR Inhibitor Afinitor (R) (Everolimus) receives EU Approval. Viszeralmedizin 2011;27:403 | Language |
Appendix 4 Included citations
| Reference | Type |
|---|---|
| RADIANT-334 | |
| Chambers J, Reed N, Mansoorc W, Ross P, Grossman A. Phase-3 randomized trial of everolimus (RAD001) vs. placebo in advanced pancreatic NET (RADIANT-3). Regul Pept 2010;164:6–752 | Abstract |
| Hobday T, Pommier R, Van Cutsem E, Panneerselvam A, Saletan S, Winkler RE, Yao JC. Analysis of progression-free survival (PFS) by prior chemotherapy use and updated safety in radiant-3: a randomized, double-blind, placebo-controlled, multicenter, Phase III trial of everolimus in patients with advanced low-or intermediate-grade pancreatic neuroendocrine tumors (PNET). Pancreas 2012;41:34553 | Abstract |
| Hobday TJ, Capdevila J, Saletan S, Panneerselvam A, Pommier RF. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): multivariate analysis of progression-free survival from the RADIANT-3 trial. J Clin Oncol 2011;29(15 Suppl. 1):e2109154 | Abstract |
| Horsch D, Lombard-Bohas C, Lincy J, Saletan S, Kocha W. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET) (RADIANT-3): updated safety results. Endocr Rev 2011;32:OR25–2(3 Meeting Abstracts)55 | Abstract |
| Ito T. Current status of mTOR inhibitor as a new therapeutic strategy for advanced pancreatic endocrine tumors. Ann Oncol 2011;22:ix3056 | Abstract |
| Ito T, Okusaka T, Ikeda M, Igarashi H, Morizane C, Nakachi K, et al. Everolimus for advanced pancreatic neuroendocrine tumours: a subgroup analysis evaluating Japanese patients in the RADIANT-3 trial. Jpn J Clin Oncol 2012;42:903–1149 | Full text |
| Ito T, Okusaka T, Ikeda M, Tajima T, Kasuga A, Fujita Y, Furuse J. Everolimus versus placebo in Japanese patients with advanced pancreatic neuroendocrine tumors (pNET): Japanese subgroup analysis of RADIANT-3. J Clin Oncol 2011;29(4 Suppl. 1):28957 | Abstract |
| Lombard-Bohas C, Van Cutsem E, Capdevila J, De Vries EGE, Tomassetti P, Lincy J, et al. Updated survival and safety data from radiant-3 – a randomized, double-blind, placebo-controlled, multicenter, phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumours (pNET). Eur J Cancer 2011;47:S45958 | Abstract |
| Lombard-Bohas C, Yao JC, Hobday TJ, Van Cutsem E, Wolin EM, Panneerselvam A, et al. Efficacy and safety of everolimus in patients with advanced low-or intermediate-grade pancreatic neuroendocrine tumors previously treated with chemotherapy: RADIANT-3 subgroup analysis. J Clin Oncol 2013;31:22451 | Abstract |
| Lombard-Bohas C, Yao JC, Hobday T, Van Cutsem E, Wolin EM, Panneerselvam A, et al. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas 2015;44:181–950 | Full text |
| Okusaka T, Ito T, Ikeda M, Igarashi H, Morizane C, Nakachi K, et al. Phase III trial of everolimus in advanced pancreatic neuroendocrine tumors (RADIANT-3): overall population and Japanese subgroup analysis. Ann Oncol 2012;23:xi1559 | Abstract |
| Okusaka T, Ito T, Ikeda M, Tajima T, Kasuga A, Fujita Y, et al. Efficacy and safety of everolimus in Japanese patients with advanced pancreatic neuroendocrine tumors (pNET): Japanese subgroup analysis of radiant-3. Neuroendocrinology 2011;94:37–860 | Abstract |
| Pavel M, Unger N, Borbath I, Ricci S, Hwang TL, Brechenmacher T, et al. Quality-of-life (QoL) assessments in patients (pts) with pancreatic neuroendocrine tumors (pNET) enrolled in the open-label, phase 3b, multicenter, expanded access study of everolimus in pts with advanced NET. Eur J Cancer 2013;49:S61961 | Abstract |
| Pavel ME, Lombard-Bohas C, Van Cutsem E, Lam DH, Kunz T, Brandt U, et al. Everolimus in patients with advanced, progressive pancreatic neuroendocrine tumors: overall survival results from the phase III RADIANT-3 study after adjusting for crossover bias. J Clin Oncol 2015;33(15 Suppl. 1):409162 | Abstract |
| Pommier R, Yao J, Hobday T, Van Cutsem E, Wolin E, Panneerselvam A, et al. Efficacy and safety of everolimus in patients with advanced low- or intermediate-grade pancreatic neuroendocrine tumors previously treated with chemotherapy: a subgroup analysis of the RADIANT-3 trial. Pancreas 2014;43:50163 | Abstract |
| Pommier RF, Wolin EM, Panneerselvam A, Saletan S, Winkler RE, Van Cutsem E. Impact of prior chemotherapy on progression-free survival in patients (pts) with advanced pancreatic neuroendocrine tumors (pNET): results from the RADIANT-3 trial. J Clin Oncol 2011;29:410364 | Abstract |
| Shah MH, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, Sachs C, et al. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): updated results of a randomized, double-blind, placebo-controlled, multicenter phase III trial (RADIANT-3). J Clin Oncol 2011;29(4 Suppl. 1):15865 | Abstract |
| Shah MH, Öberg K, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, et al. Treatment of pancreatic neuroendocrine tumors (pNET) with everolimus: improved progression-free survival compared with placebo (RADIANT-3). Pancreas 2011;40:331–266 | Abstract |
| Strosberg JR, Lincy J, Winkler RE, Wolin EM. Everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET): updated results of a randomized, double-blind, placebo-controlled, multicenter, phase III trial (RADIANT-3). J Clin Oncol 2011;29(15 Suppl. 1):400967 | Abstract |
| Wolin E, Pommier R, Lincy J, Winkler R, Yao J. Updated results from the randomized, double-blind, placebo-controlled, multicenter, phase III trial (RADIANT-3) of everolimus in patients with advanced pancreatic neuroendocrine tumors (pNET). Am J Gastroenterol 2011;106:S5968 | Abstract |
| Yao JC, Pavel M, Lombard-Bohas C, van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (PNET): final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter phase III trial (RADIANT-3). Ann Oncol 2014;25:iv39470 | Abstract |
| Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for advanced, progressive pancreatic neuroendocrine tumors (PNET): final overall survival (OS) from a randomized, double-blind, placebo (PBO)-controlled, multicenter phase 3 radiant-3 study. Neuroendocrinology 2015;102:13469 | Abstract |
| Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Lam D, Kunz T, et al. Everolimus (EVE) for the treatment of advanced pancreatic neuroendocrine tumors (pNET): final overall survival (OS) results of a randomized, double-blind, placebo (PBO)-controlled, multicenter phase 3 trial (RADIANT-3). Pancreas 2015;44:36271 | Abstract |
| Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol 2016;34:3906–1348 | Full text |
| Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–2334 | Full text |
| Yao JC, Shah MH, Ito T, Lombard-Bohas C, Wolin EM, Van Cutsem E, et al. A randomized, double-blind, placebo-controlled, multicenter phase III trial of everolimus in patients with advanced pancreatic neuroendocrine tumors (PNET) (RADIANT-3). Ann Oncol 2010;21:viii4–572 | Abstract |
| RADIANT-435 | |
| Anonymous. From ECC 2015 – neuroendocrine cancer: RADIANT-435 trial – NET improvement with everolimus? Nat Rev Clin Oncol 2015;12:68479 | Abstract |
| Pavel ME, Strosberg JR, Bubuteishvili-Pacaud L, Degtyarev E, Neary M, Hunger M, et al. Health-related quality of life (HRQoL) in patients with advanced, nonfunctional, well-differentiated gastrointestinal (GI) or lung neuroendocrine tumors (NET) in the phase 3 RADIANT-4 trial. J Clin Oncol 2016;34(Suppl. 15):e1565778 | Abstract |
| Singh S, Carnaghi C, Buzzoni R, Pommier RF, Raderer M, Tomasek J, et al. Efficacy and safety of everolimus in advanced, progressive, nonfunctional neuroendocrine tumors (NET) of the gastrointestinal (GI) tract and unknown primary: a subgroup analysis of the phase III RADIANT-4 trial. J Clin Oncol 2016;34:31573 | Abstract |
| Singh S, Pavel ME, Strosberg JR, Bubuteishvili-Pacaud L, Degtyarev E, Neary M, et al. Association of disease progression, health-related quality of life (HRQoL), and utility in patients (pts) with advanced, nonfunctional, well-differentiated gastrointestinal (GI) or lung neuroendocrine tumors (NET) in the phase 3 RADIANT-4 trial. J Clin Oncol 2016;34:409374 | Abstract |
| Yao J, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: efficacy and safety results from the placebo-controlled, double-blind, multicenter, phase 3 RADIANT-4 study. Eur J Cancer 2015;51:S709–1075 | Abstract |
| Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968–7735 | Full text |
| Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Safety and efficacy of everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: findings of the randomized, placebo-controlled, double-blind, multicenter, phase 3 RADIANT-4 study. Pancreas 2016;45:48776 | Abstract |
| Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin EM, et al. Everolimus (EVE) in advanced, nonfunctional, well-differentiated neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin: second interim overall survival (OS) results from the RADIANT-4 study. J Clin Oncol 2016;34(Suppl. 15):409080 | Abstract |
| Yao JC, Singh S, Wolin E, Voi M, Pacaud LB, Lincy J, et al. RADIANT-4: efficacy and safety of everolimus in advanced, nonfunctional neuroendocrine tumors (NET) of the lung or gastrointestinal (GI) tract. Ann Oncol 2015;26:ix4077 | Abstract |
| A618111181 | |
| Anonymous. From ECC 2015 – neuroendocrine cancer: SSA therapies – 177Lu-DOTATATE is a better one in NETTER-1. Nat Rev Clin Oncol 2015;12:684181 | Abstract |
| Faivre S, Niccoli P, Raoul JL, Bang Y, Borbath I, Valle JW, et al. Updated overall survival (OS) analysis from a phase III study of sunitinib vs placebo in patients (pts) with advanced, unresectable pancreatic neuroendocrine tumor (NET). Ann Oncol 2012;23:ix37682 | Abstract |
| Hammel P, Castellano D, Van Cutsem E, Niccoli P, Faivre S, Patyna S, et al. Evaluation of progression-free survival by blinded independent central review in patients with progressive, well-differentiated pancreatic neuroendocrine tumors treated with sunitinib or placebo. Pancreas 2011;40:32783 | Abstract |
| Ishak J, Valle J, Van Cutsem E, Lombard-Bohas C, Ruszniewski P, Sandin R, et al. Overall survival (OS) analysis of sunitinib (SU) after adjustment for crossover (CO) in patients with pancreatic neuroendocrine tumors (NET). Neuroendocrinology 2011;94:27–884 | Abstract |
| Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, Valle JW, et al. Updated safety and efficacy results of the phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET). J Clin Oncol 2010;28:400085 | Abstract |
| Raoul JL, Niccoli P, Bang YJ, Borbath I, Lombard-Bohas C, Metrakos P, et al. Sunitinib (SU) vs placebo for treatment of progressive, well-differentiated pancreatic islet cell tumours: results of a phase III, randomised, double-blind trial. Eur J Cancer Supp 2009;7:36186 | Abstract |
| Raymond E. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors (vol 364, pg 501, 2011). N Engl J Med 2011;364:108281 | Erratum to full text |
| Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13. [Erratum published in N Engl J Med 2011;364:1082]81 | Full text |
| Raymond E, Harmon C, Niccoli P, Metrakos P, Borbath I, Bang Y, et al. Impact of baseline Ki-67 index and other baseline characteristics on outcome in a study of sunitinib (SU) for the treatment of advanced, progressive pancreatic neuroendocrine tumor (NET). Neuroendocrinology 2011;94:4187 | Abstract |
| Raymond E, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, et al. Sunitinib (SU) in patients with advanced, progressive pancreatic neuroendocrine tumors (pNET): final overall survival (OS) results from a phase III randomized study including adjustment for crossover. J Clin Oncol 2016;34:30988 | Abstract |
| Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Evidence of activity and clinical benefit with sunitinib in patients with pancreatic neuroendocrine tumors (NET). Ann Oncol 2010;21:vi1389 | Abstract |
| Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Updated overall survival (OS) and progression-free survival (PFS) by blinded independent central review (BICR) of sunitinib (SU) versus placebo (PBO) for patients (Pts) with advance unresectable pancreatic neuroendocrine tumors (NET). J Clin Oncol 2011;29:400890 | Abstract |
| Raymond E, Niccoli P, Raoul J, Bang Y, Borbath I, Lombard-Bohas C, et al. Cox proportional hazard analysis of sunitinib (SU) efficacy across subgroups of patients (pts) with progressive pancreatic neuroendocrine tumors (NET). J Clin Oncol 2010;28:403191 | Abstract |
| Raymond E, Seitz JF, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, et al. Sunitinib for the treatment of advanced, progressive pancreatic neuroendocrine tumors. Neuroendocrinology 2010;92:54–592 | Abstract |
| Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177-Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumours: results of the phase III NETTER-1 trial. Eur J Cancer 2015;51:S710105 | Abstract |
| Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. 177-Lu-DOTATATE significantly improves progression-free survival in patients with midgut neuroendocrine tumors: results of the phase III NETTER-1 trial. Pancreas 2016;45:483106 | Abstract |
| Strosberg JR, Wolin EM, Chasen B, Kulke MH, Bushnell DL, Caplin ME, et al. NETTER-1 phase III: progression-free survival, radiographic response, and preliminary overall survival results in patients with midgut neuroendocrine tumors treated with 177-Lu-DOTATATE. J Clin Oncol 2016;34:194191 | Abstract |
| Valle J, Faivre S, Raoul J, Bang Y, Patyna S, Lu DR, et al. Phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET): impact of somatostatin analogue (SSA) treatment on progression-free survival (PFS). Ann Oncol 2010;21:viii26493 | Abstract |
| Valle J, Niccoli P, Raoul JL, Bang YJ, Borbath I, Van Cutsem E, et al. Updated overall survival data from a phase III study of sunitinib vs. placebo in patients with advanced, unresectable pancreatic neuroendocrine tumour (NET). Eur J Cancer 2011;47:S46294 | Abstract |
| Van Cutsem E, Dahan L, Patyna S, Klademenos D, Lu DR, Chao R, et al. Evaluation of progression-free survival (PFS) by blinded independent central review (BICR) in patients (pts) with progressive, well-differentiated pancreatic neuroendocrine tumours (NET) treated with sunitinib (SU) or placebo. Ann Oncol 2010;21:viii23595 | Abstract |
| Van Cutsem E, Seitz JF, Raoul J, Valle JW, Faivre SJ, Patyna S, et al. Evaluation of progression-free survival by blinded independent central review in patients with progressive, well-differentiated pancreatic neuroendocrine tumors treated with sunitinib or placebo. J Clin Oncol 2011;29:24996 | Abstract |
| Vinik A, Bang Y, Raoul J, Valle JW, Metrakos P, Horsch D, et al. Patient-reported outcomes (PROs) in patients (pts) with pancreatic neuroendocrine tumors (NET) receiving sunitinib (SU) in a phase III trial. J Clin Oncol 2010;28:400397 | Abstract |
| Vinik A, Bang YJ, Raoul JL, Valle J, Metrakos P, Horsch D, et al. Sunitinib for treatment of pancreatic neuroendocrine tumors: patient-reported outcomes and efficacy across patient subgroups in a phase III trial. Pancreas 2011;40:334–598 | Abstract |
| Vinik A, Cutsem EV, Niccoli P, Raoul JL, Bang YJ, Borbath I, et al. Progression-free survival (PFS) by blinded independent central review (BICR) and updated overall survival (OS) of sunitinib versus placebo for patients with progressive, unresectable, well differentiated pancreatic neuroendocrine tumor (NET). Pancreas 2012;41:35099 | Abstract |
| Vinik A, Van Cutsem E, Niccoli P, Raoul JL, Bang YJ, Borbath I, et al. Updated results from a phase III trial of sunitinib versus placebo in patients with progressive, unresectable, well-differentiated pancreatic neuroendocrine tumor (NET). J Clin Oncol 2012;30:4118100 | Abstract |
Appendix 5 Additional literature search strategies
Search 1: randomised controlled trials of octreotide
The first search attempted to identify studies reporting RCTs of octreotide.
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 30 September 2016.
Searcher: CC.
Hits: 72.
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | Octreotide/ | 6852 | |
| 2 | (Octreotide or Octreotida or Octreotidum or Octrotide or Sandostatin or sandostatina or sandostatine or longastatin or longastatina or OncoLar or samilstin or sandstatin or “SMS 201-995” or “sms201 995” or sms201995 or “sms 201995” or “sms 995” or “sdz 201995” or sdz201995 or “sms 995aaa” or “1607842-55-6” or “UNII-H92K6Q47Q9” or “H92K6Q47Q9”).ti,ab,kw. | 7788 | |
| 3 | 1 or 2 | 9404 | |
| 4 | exp Neuroendocrine Tumors/ | 149,135 | |
| 5 | Carcinoma, Neuroendocrine/ | 3058 | |
| 6 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 47,802 | |
| 7 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 53,142 | |
| 8 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 72,758 | |
| 9 | 4 or 5 or 6 or 7 or 8 | 299,124 | |
| 10 | 3 and 9 | 2558 | |
| 11 | randomized controlled trial.pt. | 428,443 | |
| 12 | 10 and 11 | 36 |
EMBASE
Host: Ovid.
Data parameters: 1946 to present.
Date search: 30 September 2016.
Searcher: CC.
Hits: 121.
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | exp neuroendocrine tumor/ | 62,334 | |
| 2 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 63,805 | |
| 3 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 69,708 | |
| 4 | (((low$ or intermediate) adj3 grade) or (“grade 1” or “grade 2”)).ti,ab,kw. | 112,582 | |
| 5 | 1 or 2 or 3 or 4 | 279,923 | |
| 6 | octreotide/ | 18,643 | |
| 7 | (Octreotide or Octreotida or Octreotidum or Octrotide or Sandostatin or sandostatina or sandostatine or longastatin or longastatina or OncoLar or samilstin or sandstatin or “SMS 201-995” or “sms201 995” or sms201995 or “sms 201995” or “sms 995” or “sdz 201995” or sdz201995 or “sms 995aaa” or “1607842-55-6” or “UNII-H92K6Q47Q9” or “H92K6Q47Q9”).ti,ab,kw. | 10,946 | |
| 8 | 6 or 7 | 20,348 | |
| 9 | 5 and 8 | 6168 | |
| 10 | randomized controlled trial/ | 416,370 | |
| 11 | 9 and 10 | 72 |
Cochrane Central Register of Controlled Trials (CENTRAL)
Host: Ovid.
Data parameters: 1946 to present.
Date search: 16 August 2016.
Searcher: CC.
-
#1 MeSH descriptor: [Octreotide] this term only (573)
-
#2 (Octreotide or Octreotida or Octreotidum or Octrotide or Sandostatin or sandostatina or sandostatine or longastatin or longastatina or OncoLar or samilstin or sandstatin or “SMS 201-995” or “sms201 995” or sms201995 or “sms 201995” or “sms 995” or “sdz 201995” or sdz201995 or “sms 995aaa” or “1607842-55-6” or “UNII-H92K6Q47Q9” or “H92K6Q47Q9”):ti,ab,kw (1067)
-
#3 #1 or #2 (1067)
-
#4 MeSH descriptor: [Neuroendocrine Tumors] explode all trees (1532)
-
#5 MeSH descriptor: [Carcinoma, Neuroendocrine] this term only (10)
-
#6 (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs):ti,ab,kw (1740)
-
#7 ((neuro or endocrine or carcinoid$1 or carcinoma$1) near/5 (tumour$ or tumor$)):ti,ab,kw (45)
-
#8 (((low$ or intermediate) near/3 grade) or (“grade 1” or “grade 2”)):ti,ab,kw (5575)
-
#9 #4 or #5 or #6 or #7 or #8 (8701)
-
#10 #3 and #9 (111)
-
#11 randomized controlled trial:pt (398,696)
-
#12 #10 and #11 (28)
Search 2: searches for dosing studies
The second search attempted to identify dosing or dose-ranging studies.
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 30 September 2016.
Searcher: CC.
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | Octreotide/ | 6855 | |
| 2 | (Octreotide or Octreotida or Octreotidum or Octrotide or Sandostatin or sandostatina or sandostatine or longastatin or longastatina or Oncolar or samilstin or sandstatin or “SMS 201-995” or “sms201 995” or sms201995 or “sms 201995” or “sms 995” or “sdz 201995” or sdz201995 or “sms 995aaa” or “1607842-55-6” or “UNII-H92K6Q47Q9” or “H92K6Q47Q9”).ti,ab,kw. | 7789 | |
| 3 | 1 or 2 | 9407 | |
| 4 | (dos* adj5 stud*).ti,ab,kw. | 72,876 | |
| 5 | 3 and 4 | 112 |
EMBASE
Host: Ovid.
Data parameters: 1974 to 17 August 2016.
Date searched: 30 September 2016.
Searcher: CC.
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | Octreotide/ | 18,635 | |
| 2 | (Octreotide or Octreotida or Octreotidum or Octrotide or Sandostatin or sandostatina or sandostatine or longastatin or longastatina or Oncolar or samilstin or sandstatin or “SMS 201-995” or “sms201 995” or sms201995 or “sms 201995” or “sms 995” or “sdz 201995” or sdz201995 or “sms 995aaa” or “1607842-55-6” or “UNII-H92K6Q47Q9” or “H92K6Q47Q9”).ti,ab,kw. | 10,948 | |
| 3 | 1 or 2 | 20,343 | |
| 4 | (dos* adj5 stud*).ti,ab,kw. | 103,372 | |
| 5 | 3 and 4 | 171 |
Chemotherapy
The third search attempted to identify RCTs of chemotherapy use in NETs.
The Cochrane Library
Host: Wiley Online Library.
Data parameters: not applicable.
Date searched: 5 September 2016.
Searcher: CC.
-
#1 MeSH descriptor: [Neuroendocrine Tumors] explode all trees (1553)
-
#2 MeSH descriptor: [Carcinoma, Neuroendocrine] this term only (10)
-
#3 (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETS or NF-NETS or NFNETs) (2590)
-
#4 ((neuro or endocrine or carcinoid* or carcinoma*) near/5 (tumour* or tumor*)) (1654)
-
#5 #1 or #2 or #3 or #4 (5557)
-
#6 (chemotherapy or chemo therap*) (45,476)
-
#7 #5 and #6 (1132) (Trials 802)
EMBASE
Host: Ovid.
Data parameters: 1974 to 2 September 2016.
Date searched: 5 September 2016.
Searcher: CC.
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | exp neuroendocrine tumor/ | 62,582 | |
| 2 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 64,123 | |
| 3 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 69,943 | |
| 4 | 1 or 2 or 3 | 172,631 | |
| 5 | chemotherapy/ | 119,461 | |
| 6 | (chemotherapy or chemo therap$).ti,ab,kw. | 443,631 | |
| 7 | 5 or 6 | 457,535 | |
| 8 | randomized controlled trial/ | 418,791 | |
| 9 | 4 and 7 and 8 | 106 |
MEDLINE Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Host: Ovid.
Data parameters: 1946 to present.
Date searched: 5 September 2016.
Searcher: CC
| # | Searches | Results | Annotations |
|---|---|---|---|
| 1 | exp Neuroendocrine Tumors/ | 149,452 | |
| 2 | Carcinoma, Neuroendocrine/ | 3074 | |
| 3 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 47,967 | |
| 4 | exp Neuroendocrine Tumors/ | 149,452 | |
| 5 | Carcinoma, Neuroendocrine/ | 3074 | |
| 6 | (Neuroendocrine or NETs or GEPNETs or GI NETs or pNETs or fNETs or f-NETs or NF-NETs or NFNETs).ti,ab,kw. | 47,967 | |
| 7 | ((neuro or endocrine or carcinoid$1 or carcinoma$1) adj5 (tumour$ or tumor$)).ti,ab,kw. | 53,239 | |
| 8 | 4 or 5 or 6 or 7 | 229,980 | |
| 9 | (chemotherapy or chemo therap$).ti,ab,kw. | 291,430 | |
| 10 | randomized controlled trial.pt. | 429,552 | |
| 11 | 8 and 9 and 10 | 251 |
Appendix 6 Additional evidence from the NETTER-1 trial
The NETTER-1 trial was identified through four published abstracts103–106 in accordance with the original NICE scope. The NETTER-1 trial was not included in the systematic review in this assessment report as it did not meet the revised inclusion criteria in the updated scope issued by NICE on 18 August 2016. 30 As the NETTER-1 trial is the only RCT to have assessed the effectiveness of 177Lu-DOTATATE, the AG has presented the findings from the trial here.
There are currently four published abstracts103–106 relating to the NETTER-1 trial in the public domain. Data provided on the NETTER-1 trial in this appendix are from the company submission (AAA)31 or are data given to the AG following a request to AAA. The data presented in the company submission are from taken from the CSR for the NETTER-1 trial. 175
Study design
The NETTER-1 trial compared treatment with 177Lu-DOTATATE plus BSC (30 mg of octreotide LAR) with treatment with high-dose octreotide LAR (60 mg). All participants had metastatic midgut NETs and were previously receiving octreotide LAR (20 or 30 mg) prior to randomisation to the trial. 31
Participants were recruited from 41 centres and were stratified by highest radiotracer uptake observed on planar somatostatin receptor scintigraphy and by the length of time on a constant dose of octreotide (≤ 6 and > 6 months).
177Lu-DOTATATE was administered in a dose of 7.4 GBq (200 mCi), over 8 ± 1-week intervals. For kidney protection, amino acid infusions (Vamin 18 in European centres and Aminosyn II 10% in the US centres) were given concomitantly with 177Lu-DOTATATE; for symptom control, 30 mg of octreotide LAR was given. For the comparator arm, 60 mg of octreotide LAR was given every 4 weeks. Additional octreotide subcutaneous rescue injections were allowed in either arm if clinical symptoms associated with the carcinoid tumour were experienced. Average dose intensity was 25.6 GBq overall and 7.2 GBq per cycle.
A sample size of 230 was calculated as being required for statistical significance for PFS and OS. A total of 229 patients were recruited to the trial. 31
The primary outcome was PFS. Secondary outcomes included ORR, OS and time to progression, safety, tolerability and HRQoL. Median treatment follow-up was (confidential information has been removed) for 177Lu-DOTATATE and (confidential information has been removed) for octreotide LAR. At the time of the primary end point analysis, (confidential information has been removed) of the safety population had been exposed to (confidential information has been removed) of 177Lu-DOTATATE. The study is still ongoing.
Rationale for the choice of comparator
In the company submission, AAA reported that:
The use of octreotide LAR in the control arm was appropriate in terms of both study design and ethical considerations as to provide patients of the control arm with the best standard of care. A higher dose was required by the regulatory authorities at the time of the parallel scientific advice meeting with the FDA and EMA considering that the patients enrolled in the trial had have progressive disease following 20 or 30 mg octreotide LAR, and it was not ethical to maintain them on the same dose regimen. Consequently, 60 mg octreotide LAR at 4-week intervals dose was agreed for the control arm in the absence of an alternative efficacious treatment approved for this type of tumour.
AAA submission (p. 44)31
Baseline characteristics in the NETTER-1 trial
The baseline characteristics of participants recruited to the NETTER-1 trial are presented in Table 40.
| Characteristic | 177Lu-DOTATATE + 30 mg of octreotide LAR (N = 116) | 60 mg of octreotide LAR (N = 113) |
|---|---|---|
| Male, n/N (%) | 63/116 (54.3) | 53/113 (46.9) |
| Age (years), median | 63.5 | 65 |
| Age (years), mean ± SD | 63.3 ± 9.4 | 64.1 ± 9.7 |
| ENETS grade 1 (≤ 2% positive tumour cells), n/N (%) | 76/116 (65.5) | 81/113 (71.7) |
| ENETS grade 2 (3–20% positive tumour cells), n/N (%) | 40/116 (34.5) | 32/113 (28.3) |
| Tumour functioning | Not available | Not available |
| Tumour differentiation | ||
| Well differentiated, n/N (%) | 76/116 (65.5) | 81/113 (71.7) |
| Moderately differentiated, n/N (%) | 40/116 (34.5) | 32/113 (28.3) |
| WHO PS score | Not available | Not available |
| Previous treatments, n/N (%) | ||
| Resection | 90/116 (77.6) | 93/113 (82.3) |
| Ablation | 6/116 (5.2) | 11/113 (9.7) |
| Chemoembolisation | 14/116 (12.1) | 11/113 (9.7) |
| Chemotherapy | 47/116 (40.5) | 51/113 (30.0) |
| Radiotherapy | 7/116 (6.0) | 8/113 (4.7) |
| SSAs | 116/116 (100) | 113/113 (100) |
| Other | 48/116 (41.4) | 40/113 (23.5) |
Outcomes in the NETTER-1 trial
Progression-free survival
Progression-free survival was reported as the primary outcome in the company submission31 and is defined as ‘the time from randomisation to documented, centrally assessed disease progression, as evaluated by the independent reading centre, or death due to any cause’. Progression was determined using RECIST version 1.1.
Confidential information has been removed.
Overall survival
Confidential information has been removed.
Response rate
Adverse events
| SOC | Patient | 177Lu-DOTATATE (N = 111) | Octreotide LAR (N = 110) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All grades | Grades 3–5 | All grades | Grades 3–5 | ||||||
| n | % | n | % | n | % | n | % | ||
| All SOCs | All patients | 105 | 94.6 | 46 | 41.4 | 92 | 83.6 | 36 | 32.4 |
| GI disorders | Nausea | 65 | 58.6 | 4 | 3.6 | 13 | 11.8 | 2 | 1.8 |
| Vomiting | 52 | 46.8 | 8 | 7.2 | 11 | 10.0 | 1 | 0.9 | |
| Diarrhoea | 32 | 28.8 | 3 | 2.7 | 21 | 19.1 | 2 | 1.8 | |
| Abdominal paina | 29 | 26.1 | 3 | 2.7 | 29 | 26.4 | 6 | 5.5 | |
| Abdominal distension | 14 | 12.6 | 0 | 0.0 | 15 | 13.6 | 0 | 0.0 | |
| General disorders and administration site conditions | Fatigueb | 44 | 39.6 | 2 | 1.8 | 28 | 25.5 | 2 | 1.8 |
| Oedema peripheral | 16 | 14.4 | 0 | 0.0 | 8 | 7.3 | 0 | 0.0 | |
| Musculoskeletal and connective tissue disorders | Musculoskeletal painc | 32 | 28.8 | 2 | 1.8 | 22 | 20.0 | 1 | 0.9 |
| Blood and lymphatic system disorder | Thrombocytopeniad | 28 | 25.2 | 2 | 1.8 | 1 | 0.9 | 0 | 0.0 |
| Lymphopeniae | 20 | 18.0 | 10 | 9.0 | 2 | 1.8 | 0 | 0.0 | |
| Anaemiaf | 16 | 14.4 | 0 | 0.0 | 6 | 5.5 | 0 | 0.0 | |
| Leukopeniag | 11 | 9.9 | 1 | 0.9 | 1 | 0.9 | 0 | 0.0 | |
| Metabolism and nutrition disorders | Decreased appetite | 20 | 18.0 | 0 | 0.0 | 9 | 8.2 | 3 | 2.7 |
| Nervous system disorders | Headache | 18 | 16.2 | 0 | 0.0 | 5 | 4.5 | 0 | 0.0 |
| Dizziness | 12 | 10.8 | 0 | 0.0 | 6 | 5.5 | 0 | 0.0 | |
| Vascular disorders | Flushing | 14 | 12.6 | 1 | 0.9 | 10 | 9.1 | 0 | 0.0 |
| Skin and subcutaneous tissue disorders | Alopecia | 12 | 10.8 | 0 | 0.0 | 2 | 1.8 | 0 | 0.0 |
| Respiratory, thoracic and mediastinal disorders | Cough | 12 | 10.8 | 0 | 0.0 | 6 | 5.5 | 0 | 0.0 |
Health-related quality of life
Confidential information has been removed.
Subgroup analysis
No subgroup analysis was carried out by AAA for the NETTER-1 trial. 31
Indirect treatment comparison
Methods: intended indirect treatment comparison
Data on the effectiveness of everolimus and 177Lu-DOTATATE in participants with GI NETs were identified from RADIANT-435 (everolimus + BSC vs. placebo + BSC) and NETTER-131 (177Lu-DOTATATE + 30 mg of octreotide vs. 60 mg of octreotide). The AG intended to indirectly compare everolimus with 177Lu-DOTATATE for GI NETs as shown in Figure 27.
FIGURE 27.
Intended ITC for 177Lu-DOTATATE and everolimus.
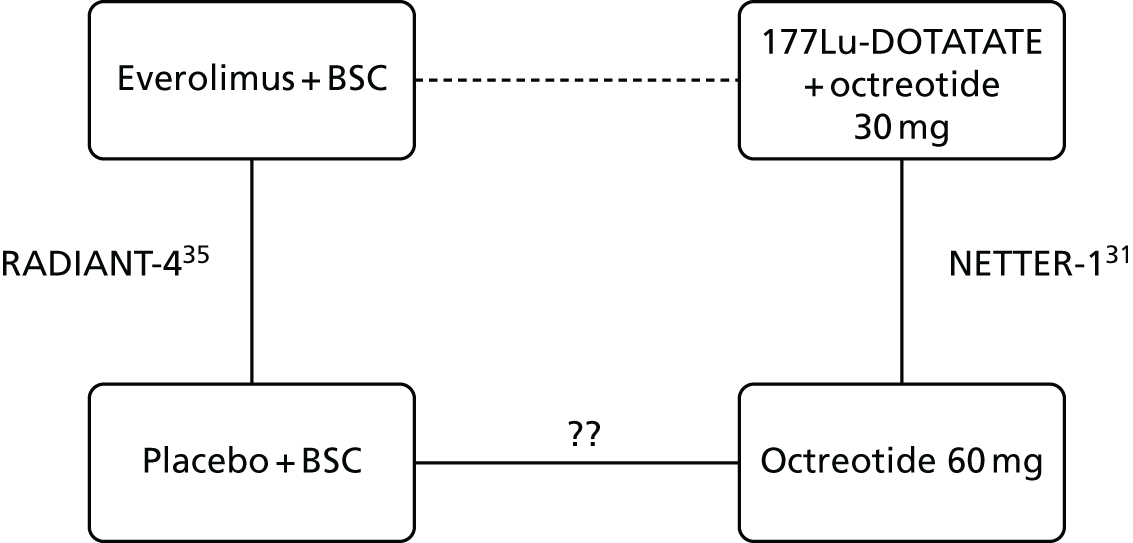
To enable an indirect comparison, a trial connecting placebo and BSC to 60 mg of octreotide was required. The AG found no such trial in the primary searches and so two supplementary bibliographic database searches were undertaken to find evidence to link these studies.
Search 1: randomised controlled trials of octreotide
The first search attempted to identify studies reporting RCTs of octreotide. The search syntax took the following form: ((search terms for neuroendocrine tumours) AND (search terms for octreotide (any dose) AND (a study design literature search filter for RCTs)).
This search was run in the following bibliographic databases: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid) and CENTRAL (The Cochrane Library, Wiley Interface).
Search 2: searches for dosing studies
The second search attempted to identify dosing or dose-ranging studies. The search syntax took the following form: ((search terms for Octreotide (any dose) AND (free text to capture reference to dosing studies)).
This search was run in the following bibliographic databases: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid) and EMBASE (Ovid).
The searches were not limited by language or date and both searches are fully reported in Appendix 5.
Results of searches
Search 1 (RCTs of octreotide) identified 83 citations for screening. Screening criteria were defined as RCTs, NETs population and octreotide given in doses of ≥ 30 mg. One study was eligible for inclusion in the review (PROMID),176 in which 30 mg of octreotide LAR was compared with placebo (n = 42 vs. n = 43 respectively). Individuals recruited to the PROMID study were treatment naive. Following consultation with our clinicians, it was considered by the AG that the population of treatment-naive patients was not comparable to the populations in RADIANT-435 and NETTER-1,31 with a minimum of 59% of the population in RADIANT-435 and 100% of the population in NETTER-131 having had at least one previous treatment.
Search 2 (dosing studies) identified 180 citations for screening. Screening criteria were defined as RCTs, NETs population and octreotide given in doses to include at least 30 mg or 60 mg in one arm. No studies were eligible for inclusion in the review.
Methods: actual indirect treatment comparison
As additional trials comparing placebo plus BSC with 60 mg of octreotide could not be found, the intended ITC in Figure 27 could not be performed. In consultation with clinical experts, and in the absence of evidence to suggest otherwise, the AG did not think that it was appropriate to link the RADIANT-435 and NETTER-131 trials by assuming that placebo plus BSC (as used in RADIANT-435) was equivalent to 60 mg of octreotide (as used in NETTER-1;31 Figure 28).
FIGURE 28.
Diagram of the ITC for GI NETs.

However, in a sensitivity analysis, the AG has made the strong assumption that placebo and BSC can be considered equivalent to 60 mg of octreotide, although this ITC should be interpreted with caution. Moreover, the data used for this network were obtained through a request for data by the AG to the companies as the NETTER-1 trial31 is currently unpublished and RADIANT-435 does not report outcomes for the subgroup of participants with GI NETs only (instead, RADIANT-435 reports outcomes for the combined group of GI + lung NETs).
In addition, a further caveat to this ITC is the different tumour locations included under the overarching term of GI in the two RCTs and hence included in the ITC. NETTER-131 recruited only individuals with midgut NETs, whereas RADIANT-435 recruited those with fore-, mid- and hind-gut NETs. Table 43 reports the tumour locations of the individuals recruited to NETTER-131 and RADIANT-4. 35
| Tumour location | NETTER-1,31 n/N (%) | RADIANT-4,35 n/N (%) | ||
|---|---|---|---|---|
| 177Lu-DOTATATE | Octreotide 60 mg | Everolimus + BSC | Placebo + BSC | |
| Jejunum | 6/116 (5.2) | 9/113 (8.0) | 16/142 (11.3) | 6/70 (8.6) |
| Ileum | 86/116 (74.1) | 82/113 (72.6) | 47/142 (33.1) | 24/70 (34.3) |
| Appendix | 1/116 (0.9) | 2/113 (1.8) | 1/142 (0.7) | 0/70 (0) |
| Right colon | 3/116 (2.6) | 1/113 (0.9) | NA | NA |
| Duodenum | 1/116 (0.9) | 1/113 (0.9) | 8/142 (5.6) | 2/70 (2.9) |
| Ileum + caecum | 1/116 (0.9) | 1/113 (0.9) | NA | NA |
| Ileum + caecum + colon | 0/116 (0) | 1/113 (0.9) | NA | NA |
| Mesentery | 5/116 (4.3) | 3/113 (2.7) | NA | NA |
| Midgut | 1/116 (0.9) | 1/113 (0.9) | NA | NA |
| Small bowel | 10/116 (8.6) | 11/113 (9.7) | NA | NA |
| Unknown | 2/116 (1.7) | 1/113 (0.9) | 23/142 (16.2) | 13/70 (18.6) |
| Rectum | NA | NA | 25/142 (17.6) | 15/70 (21.4) |
| Stomach | NA | NA | 7/142 (4.9) | 4/70 (5.7) |
| Colon | NA | NA | 5/142 (3.5) | 3/70 (4.3) |
| Other | NA | NA | 5/142 (4.2) | 2/70 (2.9) |
| Caecum | NA | NA | 4/142 (2.8) | 1/70 (1.4) |
The results reported for GI NETs only from RADIANT-435 in the clinical effectiveness section in Chapter 4 (see Outcomes for RCT evidence for GI NETs) include all of the tumour locations in Table 43 except for ‘unknown’ and one less participant in the everolimus group for ‘other’. This resulted in a total of n = 118 for everolimus plus BSC (down from n = 142) and n = 57 for placebo plus BSC (down from n = 70). The definition of GI NETs omitting the ‘unknown’ location was used by Singh et al. 73 in their published poster. The definition of GI used by Singh et al. 73 is the definition of GI that the AG used in its ITC for NETTER-1. 31
Despite the concerns raised above, the Bucher method41 was used to indirectly compare everolimus with 177Lu-DOTATATE in individuals with GI NETs for the following outcomes: central review of PFS, OS, RR and various AEs. Because there were only two relevant trials for this synthesis we could not undertake any analyses for heterogeneity between the trials or inconsistency in the network.
For AEs, instead of providing data on all grades of AE and grade 3 and 4 AEs as requested by the AG, AAA provided data on all grades of AEs and grade 3–5 AEs from NETTER-1;31 Novartis provided the requested data for all grades of AEs and for grade 3 and 4 AEs from RADIANT-4. 35 As grade 5 AEs are defined as death associated with an AE, the AG attempted to identify whether or not any deaths associated with AEs had occurred in RADIANT-4. 35 Confidential information has been removed. It was therefore assumed that data on the grade 3–5 AEs provided by AAA could be compared with data on the grade 3 and 4 AEs provided by Novartis.
Results
Two RCTs were used to compare everolimus with 177Lu-DOTATATE: RADIANT-435 (everolimus + BSC vs. placebo + BSC) and NETTER-131 (177Lu-DOTATATE + 30 mg of octreotide vs. 60 mg of octreotide) (see Figure 28).
For PFS, the ITC (Table 44) suggested that 177Lu-DOTATATE plus 30 mg of octreotide is associated with a statistically significant reduction of 63% in disease progression or death compared with everolimus plus BSC.
| Intervention | Comparator | Data source | HR (95% CI) |
|---|---|---|---|
| Everolimus + BSC | Placebo + BSC | RADIANT-435 (from AG data request to Novartis) | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60mg | NETTER-131 (from AG data request to AAA) | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Calculated by AG | 0.37 (0.19 to 0.69) |
The results of the ITC for OS (Table 45) suggest that a (confidential information has been removed) in the hazard for death with 177Lu-DOTATATE plus 30 mg of octreotide compared with everolimus plus BSC; however, this result is associated with a wide 95% CI (confidential information has been removed).
| Intervention | Comparator | Data source | HR (95% CI) |
|---|---|---|---|
| Everolimus + BSC | Placebo + BSC | RADIANT-435 (from AG data request to Novartis) | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | NETTER-131 (from AG data request to AAA) | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Calculated by AG | Confidential information has been removed |
From the available data on response rates (Table 46), the ITC results suggest that objective response and stable disease (confidential information has been removed) with everolimus plus BSC than 177Lu-DOTATATE plus 30 mg of octreotide: objective response (confidential information has been removed); stable disease (confidential information has been removed). However, the evidence suggests (confidential information has been removed) of progressive disease between 177Lu-DOTATATE plus 30 mg of octreotide and everolimus plus BSC (confidential information has been removed).
| Intervention | Comparator | Data source | Objective/overall response, OR (95% CI) | Stable disease, OR (95% CI) | Progressive disease, OR (95% CI) |
|---|---|---|---|---|---|
| Everolimus + BSC | Placebo + BSC | RADIANT-435 (from AG data request to Novartis)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | NETTER-131 (from AG data request to AAA)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Calculated by AG | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
For all grades, data on nine AEs could be compared between RADIANT-435 and NETTER-1. 31 Table 47 shows the ORs for the AEs from each study and the results of the ITC. The findings suggest that 177Lu-DOTATATE is generally associated with (confidential information has been removed) of experiencing AEs compared with everolimus plus BSC. This finding is statistically significant for the AEs of (confidential information has been removed) but not for (confidential information has been removed). The (confidential information has been removed) of experiencing fatigue associated with 177Lu-DOTATATE compared with everolimus plus BSC is (confidential information has been removed). For peripheral oedema, there is a (confidential information has been removed) of experiencing the AE with everolimus plus BSC than with 177Lu-DOTATATE: (confidential information has been removed).
| Outcome | Intervention | Comparator | OR (95% CI) |
|---|---|---|---|
| Abdominal pain | Everolimus + BSC | Placebo + BSC | 0.64 (0.31 to 1.33) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Anaemia | Everolimus + BSC | Placebo + BSC | 2.28 (0.95 to 5.47) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Cough | Everolimus + BSC | Placebo + BSC | 1.25 (0.60 to 2.60) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Decreased appetite | Everolimus + BSC | Placebo + BSC | 0.94 (0.45 to 2.00) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Diarrhoea | Everolimus + BSC | Placebo + BSC | 1.05 (0.56 to 1.98) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Fatigue | Everolimus + BSC | Placebo + BSC | 0.83 (0.44 to 1.58) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Headache | Everolimus + BSC | Placebo + BSC | 0.99 (0.44 to 2.26) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Nausea | Everolimus + BSC | Placebo + BSC | 1.89 (0.87 to 4.12) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Peripheral oedema | Everolimus + BSC | Placebo + BSC | 9.07 (3.24 to 25.38) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed |
Data on grade 3/4 AEs were available only for the ITC for five AEs: abdominal pain, decreased appetite, diarrhoea, fatigue and nausea. The ORs from the studies and those calculated in the ITC are shown in Table 48. For the grade 3/4 AEs, 177Lu-DOTATATE is associated with a (confidential information has been removed) of experiencing the AE compared with everolimus plus BSC, (confidential information has been removed) between the two treatments.
| Outcome | Intervention | Comparator | OR (95% CI) |
|---|---|---|---|
| Abdominal pain | Everolimus + BSC | Placebo + BSC | 0.73 (0.20 to 2.57) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Decreased appetite | Everolimus + BSC | Placebo + BSC | 1.00 (0.12 to 8.57) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Diarrhoea | Everolimus + BSC | Placebo + BSC | 3.55 (0.88 to 14.35) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Fatigue | Everolimus + BSC | Placebo + BSC | 3.11 (0.50 to 19.27) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed | |
| Nausea | Everolimus + BSC | Placebo + BSC | 2.04 (0.30 to 13.75) |
| 177Lu-DOTATATE + octreotide 30 mg | Octreotide 60 mg | Confidential information has been removed | |
| 177Lu-DOTATATE + octreotide 30 mg | Everolimus + BSC | Confidential information has been removed |
Appendix 7 Additional clinical effectiveness data
| Time point (months) | Survival rate (95% CI) | HR (95% CI) for everolimus vs. RPSFT-corrected placebo | ||
|---|---|---|---|---|
| Everolimus + BSC | Placebo + BSC | RPSFT-corrected placebo | ||
| 6 | 93.1 (88.6 to 95.9) | 91.6 (86.8 to 94.7) | 88.9 (83.6 to 92.5) | – |
| 12 | 82.6 (76.6 to 87.2) | 82.0 (75.9 to 86.7) | 74.9 (68.1 to 80.4) | – |
| 18 | 75.0 (68.3 to 80.4) | 74.3 (67.6 to 79.8) | 64.6 (57.4 to 71.0) | – |
| 24 | 67.7 (60.7 to 73.8) | 64.0 (56.8 to 70.2) | ≤ 55.6 (NA to NA) | 0.60 (0.09 to 3.95) |
| 36 | 56.7 (49.4 to 63.3) | 50.9 (43.6 to 57.7) | NA (NA to NA) | – |
| 48 | 46.9 (39.7 to 53.8) | 41.3 (34.3 to 48.1) | NA (NA to NA) | – |
| 60 | 34.7 (27.7 to 41.7) | 35.5 (28.7 to 42.4) | NA (NA to NA) | – |
| AE | Number of participants (%) | |||||
|---|---|---|---|---|---|---|
| Everolimus + BSC (N = 204) | Placebo + BSC (N = 203) | Open-label everolimus (N = 225) | ||||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| All or any AE | 203 (99.5) | 126 (61.8) | 198 (97.5) | 82 (40.4) | 221 (98.2) | 165 (73.3) |
| Abdominal pain | 49 (24.0) | 6 (2.9) | 49 (24.1) | 12 (5.9) | 63 (28.0) | 16 (7.1) |
| Anaemia | 49 (24.0) | 19 (9.3) | 19 (9.4) | 4 (2.0) | 56 (24.9) | 18 (8.0) |
| Asthenia | 38 (18.6) | 6 (2.9) | 41 (20.2) | 7 (3.4) | 45 (20.0) | 17 (7.6) |
| Cough | 46 (22.5) | 1 (0.5) | 22 (10.8) | 0 | 54 (24.0) | 0 |
| Decreased appetite | 61 (29.9) | 3 (1.5) | 37 (18.2) | 3 (1.5) | 66 (29.3) | 11 (4.9) |
| Diarrhoea | 98 (48.0) | 11 (5.4) | 48 (23.6) | 5 (2.5) | 98 (43.6) | 10 (4.4) |
| Dysgeusia | 38 (18.6) | 0 | 11 (5.4) | 0 | 46 (20.4) | 1 (0.4) |
| Epistaxis | 44 (21.6) | 0 | 3 (1.5) | 0 | 38 (16.9) | 0 |
| Fatigue | 91 (44.6) | 6 (2.9) | 54 (26.6) | 5 (2.5) | 74 (32.9) | 11 (4.9) |
| Headache | 62 (30.4) | 1 (0.5) | 30 (14.8) | 2 (1.0) | 52 (23.1) | 6 (2.7) |
| Hyperglycaemia | 41 (20.1) | 18 (8.8) | 22 (10.8) | 8 (3.9) | 61 (27.1) | 23 (10.2) |
| Nausea | 67 (32.8) | 5 (2.5) | 66 (32.5) | 4 (2.0) | 84 (37.3) | 4 (1.8) |
| Peripheral oedema | 76 (37.3) | 2 (1.0) | 23 (11.3) | 2 (1.0) | 66 (29.3) | 2 (0.9) |
| Pyrexia | 63 (30.9) | 2 (1.0) | 25 (12.3) | 1 (0.5) | 61 (27.1) | 2 (0.9) |
| Rash | 107 (52.5) | 1 (0.5) | 32 (15.8) | 0 | 90 (40.0) | 3 (1.3) |
| Stomatitis | 110 (53.9) | 10 (4.9) | 27 (13.3) | 0 | 105 (46.7) | 5 (2.2) |
| Vomiting | 61 (29.9) | 2 (1.0) | 42 (20.7) | 5 (2.5) | 74 (32.9) | 10 (4.4) |
| Weight decreased | 59 (28.9) | 1 (0.5) | 24 (11.8) | 0 | 72 (32.0) | 5 (2.2) |
| AE | n (%) | |||
|---|---|---|---|---|
| Everolimus + BSC (N = 202) | Placebo + BSC (N = 98) | |||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| All Aes | 200 (99.0) | 140 (69.3) | 87 (88.8) | 28 (28.6) |
| Stomatitisa | 111 (55.0) | 15 (7.4) | 19 (19.4) | 0 (0.0) |
| Diarrhoea | 83 (41.1) | 18 (8.9) | 30 (30.6) | 2 (2.0) |
| Peripheral oedema | 78 (38.6) | 6 (3.0) | 6 (6.1) | 1 (1.0) |
| Fatigue | 75 (37.1) | 9 (4.5) | 35 (35.7) | 1 (1.0) |
| Rash | 61 (30.2) | 1 (0.5) | 9 (9.2) | 0 (0.0) |
| Cough | 55 (27.2) | 0 (0.0) | 20 (20.4) | 0 (0.0) |
| Nausea | 53 (26.2) | 6 (3.0) | 17 (17.3) | 1 (1.0) |
| Asthenia | 47 (23.3) | 5 (2.5) | 8 (8.2) | 0 (0.0) |
| Pyrexia | 47 (23.3) | 4 (2.0) | 8 (8.2) | 0 (0.0) |
| Anaemia | 45 (22.3) | 11 (5.4) | 9 (9.2) | 2 (2.0) |
| Decreased appetite | 45 (22.3) | 2 (1.0) | 17 (17.3) | 1 (1.0) |
| Weight decreased | 44 (21.8) | 3 (1.5) | 11 (11.2) | 1 (1.0) |
| Dyspnoea | 40 (19.8) | 5 (2.5) | 11 (11.2) | 2 (2.0) |
| Abdominal pain | 39 (19.3) | 10 (5.0) | 19 (19.4) | 5 (5.1) |
| Dysguesia | 37 (18.3) | 1 (0.5) | 4 (4.1) | 0 (0.0) |
| Pruritus | 35 (17.3) | 1 (0.5) | 9 (9.2) | 0 (0.0) |
| Vomiting | 30 (14.9) | 7 (3.5) | 12 (12.2) | 2 (2.0) |
| Back pain | 27 (13.4) | 3 (1.5) | 14 (14.3) | 0 (0.0) |
| Pneumonitis | 27 (13.4) | 3 (1.5) | 2 (2.0) | 0 (0.0) |
| Epistaxis | 26 (12.9) | 1 (0.5) | 3 (3.1) | 0 (0.0) |
| Headache | 25 (12.4) | 0 (0.0) | 15 (15.3) | 0 (0.0) |
| Arthralgia | 24 (11.9) | 1 (0.5) | 8 (8.2) | 0 (0.0) |
| Hyperglycaemia | 24 (11.9) | 9 (4.5) | 3 (3.1) | 0 (0.0) |
| Hypertension | 24 (11.9) | 8 (4.0) | 8 (8.2) | 3 (3.1) |
| Urinary tract infection | 22 (10.9) | 4 (2.0) | 5 (5.1) | 0 (0.0) |
| Constipation | 21 (10.4) | 0 (0.0) | 18 (18.4) | 0 (0.0) |
| Upper abdominal pain | 19 (9.4) | 0 (0.0) | 11 (11.2) | 0 (0.0) |
| AE | n (%) | |||
|---|---|---|---|---|
| Sunitinib (N = 83) | Placebo (N = 82) | |||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Diarrhoea | 44 (53.0) | 4 (4.8) | 25 (30.5) | 1 (1.2) |
| Nausea | 32 (38.6) | 1 (1.2) | 18 (22.0) | 0 (0.0) |
| Asthenia | 26 (31.3) | 3 (3.6) | 18 (22.0) | 2 (2.4) |
| Fatigue | 24 (28.9) | 4 (4.8) | 14 (17.1) | 3 (3.7) |
| Hair colour changes | 24 (28.9) | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Neutropenia | 24 (28.9) | 10 (12.0) | 3 (3.7) | 0 (0.0) |
| Vomiting | 21 (25.3) | 0 (0.0) | 14 (17.1) | 0 (0.0) |
| Hypertension | 19 (22.9) | 8 (9.6) | 3 (3.7) | 0 (0.0) |
| Palmar–plantar erythordysaesthesia syndrome | 19 (22.9) | 5 (6.0) | 2 (2.4) | 0 (0.0) |
| Stomatitis | 18 (21.7) | 3 (3.6) | 2 (2.4) | 0 (0.0) |
| Anorexia | 17 (20.5) | 2 (2.4) | 11 (13.4) | 0 (0.0) |
| Dysgeusia | 16 (19.3) | 0 (0.0) | 3 (3.7) | 0 (0.0) |
| Epistaxis | 16 (19.3) | 1 (1.2) | 2 (2.4) | 0 (0.0) |
| Thrombocytopenia | 14 (16.9) | 3 (3.6) | 4 (4.9) | 0 (0.0) |
| Mucosal inflammation | 13 (15.7) | 1 (1.2) | 6 (7.3) | 0 (0.0) |
| Rash | 13 (15.7) | 0 (0.0) | 4 (4.9) | 0 (0.0) |
| Abdominal pain | 12 (14.5) | 1 (1.2) | 10 (12.2) | 3 (3.7) |
| Dyspepsia | 12 (14.5) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Weight decreased | 11 (13.3) | 1 (1.2) | 6 (7.3) | 0 (0.0) |
| Dry skin | 11 (13.3) | 0 (0.0) | 9 (11.0) | 0 (0.0) |
| Headache | 10 (12.0) | 0 (0.0) | 5 (6.1) | 1 (1.2) |
| Constipation | 8 (9.6) | 0 (0.0) | 8 (9.8) | 1 (1.2) |
| Leukopenia | 8 (9.6) | 5 (6.0) | 1 (1.2) | 0 (0.0) |
| Nail disorder | 8 (9.6) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Dry mouth | 7 (8.4) | 0 (0.0) | 4 (4.9) | 0 (0.0) |
| Erythema | 7 (8.4) | 0 (0.0) | 3 (3.7) | 0 (0.0) |
| Insomnia | 7 (8.4) | 0 (0.0) | 5 (6.1) | 0 (0.0) |
| Pain in extremity | 7 (8.4) | 0 (0.0) | 3 (3.7) | 0 (0.0) |
| Abdominal pain upper | 6 (7.2) | 1 (1.2) | 1 (1.2) | 0 (0.0) |
| Arthralgia | 6 (7.2) | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Dyspnoea | 6 (7.2) | 1 (1.2) | 8 (9.8) | 0 (0.0) |
| Yellow skin | 6 (7.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 5 (6.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Aphthous stomatitis | 5 (6.0) | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Decreased appetite | 5 (6.0) | 0 (0.0) | 3 (3.7) | 0 (0.0) |
| Dizziness | 5 (6.0) | 1 (1.2) | 3 (3.7) | 0 (0.0) |
| Eyelid oedema | 5 (6.0) | 1 (1.2) | 0 (0.0) | 0 (0.0) |
| Flatulence | 5 (6.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Gingival bleeding | 5 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 5 (6.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Variable | Sunitinib | Placebo | Difference | p-value |
|---|---|---|---|---|
| Global HRQoL | 62.44 | 61.28 | 1.15 | 0.6799 |
| Functional scales | ||||
| Cognitive functioning | 79.94 | 81.38 | –1.44 | 0.6058 |
| Emotional functioning | 72.59 | 76.15 | –3.56 | 0.3008 |
| Physical functioning | 78.92 | 76.13 | 2.79 | 0.3230 |
| Role functioning | 70.88 | 69.37 | 1.51 | 0.7113 |
| Social functioning | 74.44 | 76.11 | –1.67 | 0.6487 |
| Symptom items/scales | ||||
| Appetite loss | 24.95 | 23.07 | 1.88 | 0.6545 |
| Constipation | 10.70 | 14.70 | –4.00 | 0.1936 |
| Diarrhoea | 37.19 | 15.81 | 21.38 | < 0.0001 |
| Dyspnoea | 22.31 | 17.08 | 5.23 | 0.1339 |
| Fatigue | 40.52 | 38.74 | 1.78 | 0.6138 |
| Insomnia | 32.61 | 24.86 | 7.75 | 0.0372 |
| Nausea and vomiting | 14.29 | 13.15 | 1.15 | 0.6939 |
| Pain | 25.48 | 28.99 | –3.51 | 0.3711 |
| Financial difficulties | 17.28 | 17.00 | 0.28 | 0.9367 |
FIGURE 31.
Change scores and 95% CIs for EORTC QLQ-C30 global HRQoL scores by cycle: patient-reported outcome analysis set. Source: figure 7 (p. 52) of the Pfizer submission. 32

| Covariate | Subgroup | n | HR (95% CI) |
|---|---|---|---|
| Tumour functionality | Not functioning | 86 | 0.26 (0.13 to 0.54) |
| Functioning | 46 | 0.75 (0.30 to 1.84) | |
| Number of previous systemic regimens | 0 or 1 | 121 | 0.33 (0.19 to 0.59) |
| ≥ 2 | 50 | 0.61 (0.27 to 1.37) | |
| Previous use of SSAs | Yes | 68 | 0.43 (0.21 to 0.89) |
| No | 103 | 0.41 (0.22 to 0.75) |
| Covariate | Subgroup | n | HR (95% CI); p-value |
|---|---|---|---|
| Tumour grade | Well differentiated | 341 | 0.41 (0.31 to 0.53); < 0.001 |
| Moderately differentiated | 65 | 0.21 (0.11 to 0.42); < 0.001 | |
| Previous chemotherapy | Yes | 189 | 0.34 (0.24 to 0.49); < 0.001 |
| No | 221 | 0.41 (0.29 to 0.58); < 0.001 | |
| Previous long-acting SSA use | Yes | 203 | 0.40 (0.28 to 0.57); < 0.001 |
| No | 207 | 0.36 (0.25 to 0.51); < 0.001 |
| Covariate | Subgroup | n | HR (95% CI); p-value |
|---|---|---|---|
| Previous chemotherapy | Yes | 189 | |
| No | 221 | 0.78 (0.61 to 1.01); 0.056 | |
| Previous long-acting SSA use | Yes | 203 | |
| No | 207 | 1.15 (0.89 to 1.49); 0.288 |
| Outcome | Intervention | Comparator | OR (95% CI) |
|---|---|---|---|
| Stomatitis | Everolimus | Placebo | 8.92 (5.59 to 14.22) |
| Sunitinib | Placebo | 11.08 (2.84 to 43.26) | |
| Everolimus | Sunitinib | 0.81 (0.19 to 3.40) | |
| Rash | Everolimus | Placebo | 8.17 (4.82 to 13.86) |
| Sunitinib | Placebo | 4.30 (1.43 to 12.95) | |
| Everolimus | Sunitinib | 1.90 (0.56 to 6.45) | |
| Fatigue | Everolimus | Placebo | 2.74 (1.68 to 4.49) |
| Sunitinib | Placebo | 1.31 (0.68 to 2.56) | |
| Everolimus | Sunitinib | 2.09 (0.91 to 4.78) | |
| Diarrhoea | Everolimus | Placebo | 4.68 (2.71 to 8.07) |
| Sunitinib | Placebo | 2.25 (1.21 to 4.19) | |
| Everolimus | Sunitinib | 2.08 (0.91 to 4.74) | |
| Nausea | Everolimus | Placebo | 1.13 (0.69 to 1.85) |
| Sunitinib | Placebo | 1.94 (1.03 to 3.68) | |
| Everolimus | Sunitinib | 0.58 (0.26 to 1.30) | |
| Dysgeusia | Everolimus | Placebo | 5.05 (2.28 to 11.18) |
| Sunitinib | Placebo | 5.02 (1.69 to 14.93) | |
| Everolimus | Sunitinib | 1.01 (0.26 to 3.87) | |
| Epistaxis | Everolimus | Placebo | 83.88 (5.11 to 1377.99) |
| Sunitinib | Placebo | 5.02 (1.69 to 14.93) | |
| Everolimus | Sunitinib | 16.97 (0.84 to 341.97) | |
| Decreased weight | Everolimus | Placebo | 4.01 (1.86 to 8.64) |
| Sunitinib | Placebo | 1.51 (0.61 to 3.70) | |
| Everolimus | Sunitinib | 2.66 (0.82 to 8.67) | |
| Thrombocytopenia | Everolimus | Placebo | 30.81 (4.14 to 229.09) |
| Sunitinib | Placebo | 3.96 (1.30 to 12.01) | |
| Everolimus | Sunitinib | 7.79 (0.79 to 77.12) | |
| Decreased appetite | Everolimus | Placebo | 3.29 (1.73 to 6.27) |
| Sunitinib | Placebo | 1.06 (0.50 to 2.22) | |
| Everolimus | Sunitinib | 3.11 (1.16 to 8.30) | |
| Headache | Everolimus | Placebo | 3.45 (1.78 to 6.69) |
| Sunitinib | Placebo | 1.42 (0.62 to 3.29) | |
| Everolimus | Sunitinib | 2.43 (0.84 to 7.05) | |
| Vomiting | Everolimus | Placebo | 2.62 (1.33 to 5.17) |
| Sunitinib | Placebo | 1.16 (0.61 to 2.22) | |
| Everolimus | Sunitinib | 2.26 (0.88 to 5.78) | |
| Asthenia | Everolimus | Placebo | 1.60 (0.84 to 3.05) |
| Sunitinib | Placebo | 1.39 (0.72 to 2.70) | |
| Everolimus | Sunitinib | 1.15 (0.46 to 2.90) |
| Outcome | Intervention | Comparator | OR (95% CI) |
|---|---|---|---|
| Stomatitis | Everolimus | Placebo | 29.99 (1.77 to 507.09) |
| Sunitinib | Placebo | 6.19 (0.63 to 60.73) | |
| Everolimus | Sunitinib | 4.32 (0.12 to 159.36) | |
| Fatigue | Everolimus | Placebo | 5.08 (0.59 to 43.83) |
| Sunitinib | Placebo | 0.54 (0.15 to 1.90) | |
| Everolimus | Sunitinib | 9.36 (0.77 to 113.29) | |
| Diarrhoea | Everolimus | Placebo | 14.46 (0.82 to 256.56) |
| Sunitinib | Placebo | 2.03 (0.40 to 10.13) | |
| Everolimus | Sunitinib | 7.63 (0.28 to 204.92) | |
| Nausea | Everolimus | Placebo | 10.23 (0.56 to 188.42) |
| Sunitinib | Placebo | 0.99 (0.08 to 12.64) | |
| Everolimus | Sunitinib | 11.36 (0.24 to 540.30) | |
| Thrombocytopenia | Everolimus | Placebo | 16.61 (0.95 to 291.21) |
| Sunitinib | Placebo | 6.19 (0.63 to 60.73) | |
| Everolimus | Sunitinib | 2.45 (0.06 to 92.93) | |
| Decreased appetite | Everolimus | Placebo | 0.25 (0.01 to 5.48) |
| Sunitinib | Placebo | 2.00 (0.24 to 16.98) | |
| Everolimus | Sunitinib | 0.10 (0 to 4.06) | |
| Asthenia | Everolimus | Placebo | 1.00 (0.14 to 7.13) |
| Sunitinib | Placebo | 1.33 (0.31 to 5.79) | |
| Everolimus | Sunitinib | 0.75 (0.06 to 8.70) |
| Intervention | Comparator | Data source | HR (95% CI) | |
|---|---|---|---|---|
| Previous use | No previous use | |||
| Everolimus + BSC | Placebo + BSC | RADIANT-334 | 0.40 (0.28 to 0.57) | 0.36 (0.25 to 0.51) |
| Sunitinib + BSC | Placebo + BSC | A618111181 | 0.43 (0.21 to 0.89) | 0.41 (0.22 to 0.75) |
| Everolimus + BSC | Sunitinib + BSC | Calculated by the AG | 0.93 (0.42 to 2.08) | 0.88 (0.43 to 1.78) |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
|---|---|---|
| Confidential information has been removed | Confidential information has been removed | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
FIGURE 33.
Change from baseline in FACT-G total score over time (on treatment). Source: figure 5.13 (p. 77) of the Novartis company submission. 33 Confidential information has been removed.
FIGURE 34.
Kaplan–Meier plot of time to deterioration in FACT-G total score by at least 7 points (full analysis set). NA, not accessible. Source: figure 5.14 (p. 78) of the Novartis submission. 33 Confidential information has been removed.
| Covariate | Subgroup | n | HR (95% CI) |
|---|---|---|---|
| Treatment naive | Yes | 177 | 0.65 (0.39 to 1.08) |
| No | 185 | 0.51 (0.35 to 0.76) | |
| Previous chemotherapy | Yes | 77 | 0.35 (0.19 to 0.64) |
| No | 225 | 0.60 (0.42 to 0.86) | |
| Previous SSA treatment | Yes | 157 | 0.52 (0.34 to 0.81) |
| No | 145 | 0.60 (0.39a to 0.94) | |
| Tumour grade | 1 | 194 | 0.57 (0.39 to 0.84) |
| 0 | 107 | 0.49 (0.29 to 0.83) |
| Characteristic | Everolimus + BSC (n = 118) | Placebo + BSC (n = 57) |
|---|---|---|
| Age (years), median (range) | 63.0 (22–83) | 60.0 (33–83) |
| Male (%) | 40.7 | 54.4 |
| Tumour functioning | 100% non-functioning | 100% non-functioning |
| Tumour differentiation | Well differentiated: 50.8%; moderately differentiated: 5.1%; not defined: 44.1% | Well differentiated: 61.4%; moderately differentiated: 3.5%; not defined: 35.1% |
| WHO PS | 0: 75.4%; 1: 24.6% | 0: 84.2%; 1: 15.8% |
| Previous treatments | SSAs: 59.0%; chemotherapy: 18.6%; surgery: 69.5%; radiotherapy: (confidential information has been removed); locoregional + ablative therapy: (confidential information has been removed) | SSAs: 63.0%; chemotherapy: 12.3%; surgery: 84.2%; radiotherapy: (confidential information has been removed); locoregional + ablative therapy: (confidential information has been removed) |
| Characteristic | Everolimus + BSC (n = 63) | Placebo + BSC (n = 27) |
|---|---|---|
| Age (years), median (range) | Confidential information has been removed | Confidential information has been removed |
| Male, n/N (%) | Confidential information has been removed | Confidential information has been removed |
| Tumour functioning | 100% non-functioning | 100% non-functioning |
| WHO PS, n/N (%) | Confidential information has been removed | Confidential information has been removed |
| Previous treatments, n/N (%) | Confidential information has been removed | Confidential information has been removed |
| AE | n/N (%) | |||
|---|---|---|---|---|
| All grades | Grades 3 + 4 | |||
| Everolimus + BSC (N = 62) | Placebo + BSC (N = 27) | Everolimus + BSC (N = 62) | Placebo + BSC (N = 27) | |
| Abdominal pain (all) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Abdominal pain (upper) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Anaemia | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Asthenia | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cardiac disorder | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Cough | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Diarrhoea | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Dry mouth | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Dysgeusia | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Dyspnoea | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Ear and labyrinth disorders | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Eye disorders | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Nausea | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Peripheral oedema | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Stomatitis | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Vomiting | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
Appendix 8 Evidence informing the companies’ economic models
Novartis
The characteristics of the models submitted by Novartis are summarised in Table 65. See Table 76 for the results.
| Company | Population | Comparators | Horizon | Model structure | Health states/events modelled | Utilities | Costs | Key individual parameters (sensitivity analysis) | Source of effectiveness parameters | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | Advanced, progressive, well- or moderately differentiated pNETs | Everolimus vs. sunitinib | 20 years | Semi-Markov – partitioned survival with monthly cycles. Proportional hazards model of PFS and OS with baseline Weibull form | SD, PD, death | SD with no AEs; SD with AEs: everolimus; SD with AEs: sunitinib; PD; death (0). Source: vignettes (Swinburn et al.130) | Drug administration, drug acquisition, AEs, resource use (physician visits, procedures/tests and hospitalisations) and post-progression therapy costs. Source: Novartis data on file; NHS reference costs 2014–15 and PCTs combined177 | PFS HR, OS HR, RDI, treatment duration (use of PFS for treatment costs) | HR: indirect comparison of HRs of (updated) A618111181 (sunitinib) vs. RADIANT-334 (everolimus) outcome data (Bucher method). Parametric baseline function from company data on file for RADIANT-3 |
|
| Novartis | Advanced progressive, non-functional, GI/lung NETs; from Phase III RADIANT-435 trial | Everolimus + BSC vs. BSC | 30 years | Partitioned survival with monthly cycles (three states). Restricted log-normal distribution for PFS and OS (base case) | SD, PD, death | SD, PD, death | Active anti-tumour treatment, BSC, procedures/tests, physician visits, therapy administration costs and dispensing fees, hospitalisations, AEs, post-progression treatments, end-of-life care | Not presented in the submission (including appendices) | The most mature data from RADIANT-435 were used in the modelling of PFS (by central review) and OS. For PFS, the primary analysis data cut-off point (28 November 2014) was used and, for OS, data from the second OS interim analysis data cut-off point (30 November 2015) were used |
|
Pancreatic neuroendocrine tumours
Efficacy, effectiveness and safety evidence
The systematic review by Novartis involved searching major electronic libraries (see sections 4 and 5 of the company submission33) on 21 July 2016, as well as hand searches of conference proceedings on 8 August 2016. The two identified trials have been described earlier in this report (see Chapter 4, Results). Here, only the major results and design features for the purposes of economic analysis are summarised.
RADIANT-3,34 a Phase III double-blind RCT, assessed 10 mg of everolimus given orally plus BSC relative to matched placebo plus BSC in 410 adult, mTOR inhibitor-naive patients with progressive and advanced pNETs. Participants were randomised on a 1 : 1 ratio to the two treatments in a stratified fashion according to their baseline status in terms of prior chemotherapy (receipt vs. no receipt) and WHO PS (0 vs. 1/2).
According to the effectiveness section of the Novartis submission,33 the median follow-up period in RADIANT-334 was 17 months, with median treatment durations of (confidential information has been removed) for everolimus compared with 3.74 months for placebo. However, in the economic analysis section, the median treatment duration for everolimus is reported as 8.61 (Novartis submission, p. 10133). At the time that the primary publication was written (cut-off date of 28 February 2010), 32% of participants in the everolimus group and 13% of participants in the placebo group were still receiving the allocated treatment; 44% in the everolimus group had stopped because of disease progression and 80% in the placebo group had stopped for the same reason. 34 Participants in the placebo group whose disease subsequently progressed were eligible to cross over to open-label everolimus. Of those patients initially randomised to placebo, 85% received open-label everolimus. In addition, both groups received BSC, which involved SSA use in 37.7% and 39.9% of participants in the everolimus and placebo groups, respectively.
The primary analysis (based on assessment by a local investigator) found that median PFS was 11.0 months (95% CI 8.4 to 13.9 months) for everolimus compared with 4.6 months (95% CI 3.1 to 5.4 months) for placebo, with a HR for disease progression or death for everolimus of 0.35 (95% CI 0.27 to 0.45). The assessment by central review found a HR of 0.34 (95% CI 0.26 to 0.44). The final OS analysis, which was unadjusted for crossover to everolimus, performed with data available on 5 March 2014, produced a median OS of 44.0 months for the everolimus group and 37.7 months for the placebo group and a HR for everolimus of 0.94 (95% CI 0.73 to 1.20). In the Novartis submission33 it was acknowledged that these results ‘may be confounded due to the high level of cross-over from placebo to everolimus and the receipt of subsequent anti-neoplastic therapies’. In particular, (confidential information has been removed) of everolimus group received antineoplastic therapies after discontinuation of the study drug compared with (confidential information has been removed) of the placebo group. In total, 23% of patients in the everolimus group and 19.2% in the placebo group received a targeted therapy, whereas 29% of participants in each arm received chemotherapy.
The company conducted an OS analysis in which it adjusted for the effect of crossover from the placebo to the everolimus group, whether as a result of disease progression or after completing the core phase and entering the open-label phase of the study. The method used for this purpose was the RPSFT model, which assumes that the effect of treatment on OS is the same whenever the patient receives the treatment, for example at the start of the trial, after disease progression or after completing the core phase of the study. Although the duration of follow-up in RADIANT-334 was 72–78 months, the RPSFT analysis effectively required limiting the follow-up to 24 months after the start of treatment and produced a HR of 0.60 (95% CI 0.09 to 3.95). 62
The other RCT identified by the company’s systematic review was A6181111,81 which compared 37.5 mg of sunitinib daily plus BSC against placebo plus BSC. 81 As with RADIANT-3,34 A618111181 was conducted in patients with progressive, advanced and well- or moderately differentiated pNETs and measured the same primary outcome, PFS. Similarly, participants randomised to placebo were allowed to cross over to open-label active treatment, sunitinib, following disease progression. In total, 51% of participants in the sunitinib arm (n = 44/86) and 69% of participants in the placebo arm (n = 59/85) entered the open-label extension study. 81 The median treatment duration was 4.6 months for sunitinib and 3.7 months for placebo. The most common reasons for study discontinuation were disease progression (occurring in 22% of sunitinib and 55% of placebo cases), termination of the trial (48% and 19%) and AEs (17% and 8%). 81 In a separate report referred to by Pfizer in its submission, it is stated that 38, that is, two-thirds, of the placebo group who crossed over did so following disease progression, whereas 21 in the placebo group started sunitinib after the study closure88 (see Chapter 4, Results, for further details of A618111181).
In the absence of head-to-head RCT evidence, Novartis resorted to an indirect comparison of everolimus with sunitinib based on their respective relative outcomes compared with placebo in RADIANT-334 and A618111181 using the method of Bucher et al. 41 The relative effect of the treatments on OS was estimated using ITT analysis and, alternatively, RPSFT model-adjusted HRs. For the comparison based on the RPSFT model OS estimates, Novartis cites a HR for sunitinib of 0.43 (95% CI 0.17 to 1.20) without clear reference as to the source. In contrast, in its submission, Pfizer cites a HR for sunitinib relative to placebo of 0.34 (95% CI 0.14 to 1.28). 88
Novartis reported a PFS HR of 1.08 (95% CI 0.59 to 1.99; blinded independent review committee) and an ITT OS HR of 1.32 (95% CI 0.81 to 2.16) and a RPFST model-adjusted OS HR of 1.39 (95% CI 0.17 to 11.72).
The company also found that the HR for SSA use as part of BSC was 1.04 (95% CI 0.48 to 2.26) for everolimus relative to sunitinib. The company concluded that there was no significant difference between the treatments in terms of these outcomes.
In terms of AEs, the company’s Bucher indirect comparison analysis resulted in an overall OR of 4.47 (95% CI 0.5 to 39.4) for an overall rate of grade 3–4 AEs of 0.35 for everolimus compared with an indirectly estimated overall rate of 0.71 for sunitinib. These data were used by Novartis to estimate the relative incidence of AEs and associated costs and utilities in the economic model, by assuming that the AEs in question occurred only once for each individual. As the types of grade 3–4 AEs for which sunitinib had an excess risk compared with everolimus occurred less frequently (i.e. neutropenia, hypertension, leukopenia and palmar–plantar erythrodysaesthesia syndrome occurred in < 1% of participants) than those for which everolimus had worse outcomes (i.e. diarrhoea, stomatitis, thrombocytopenia, anaemia, hyperglycaemia, fatigue, infections, pneumonitis and nausea occurred in 3–7% of participants), the excess risks estimated by the Bucher method across individual AE categories added up to a larger total with everolimus than sunitinib. Thus, although sunitinib had a higher incidence of any of the 13 grade 3/4 AEs considered, the IC of individual categories produced absolute AE rates that implied the opposite, that is, that everolimus was associated with more of any of these events. As described in the critique of their submission (see Chapter 5), the company addressed this contradiction in an ad hoc manner in the economic evaluation.
In its submission, Novartis also included the results of a published indirect comparative study between everolimus and sunitinib that analysed placebo-controlled outcome data from RADIANT-334 and A618111181 using the MAIC method. 124 A detailed discussion of this evidence is presented in Chapter 4 (see Results). The MAIC PFS HR for everolimus compared with sunitinib was estimated to be 0.84 (95% CI 0.46 to 1.53). This estimate was smaller although statistically indistinguishable from the PFS HR estimate of 0.90 (0.53 to 1.53) before matching and the PFS HR of 1.08 from the Bucher analysis by Novartis, described above. Matching to the A618111181 sample reduced the relative effectiveness of everolimus compared with sunitinib from the unadjusted OS HR of 0.69 (0.46 to 1.05) to the MAIC OS HR of 0.81 (0.49 to 1.31).
The MAIC pooled OR for the subset of eight AEs (neutropenia, hypertension, palmar–plantar erythrodysaesthesia syndrome, diarrhoea, stomatitis, thrombocytopenia, anaemia and fatigue) was 1.16, which is smaller than the corresponding Bucher indirect comparison estimate of 1.37 (calculated by PenTAG from data in the Novartis submission; see Appendix 21). More importantly, unlike the Bucher IC estimates used by Novartis to populate the economic model, the MAIC AE rate estimates added up to similar totals for sunitinib and everolimus for the subset of common categories (0.38 vs. 0.37, respectively) (in contrast, the Bucher rates were 0.15 and 0.29, respectively; PenTAG calculations of Novartis data submitted to NICE33). In contrast, a MAIC pooled placebo-adjusted OR for sunitinib compared with everolimus of 1.37 (calculated by the AG) for the 14 grade 3/4 AEs had been reported previously by Signorovitch et al. 124 using older RADIANT-334 data.
The company concluded that there was no evidence of any difference between everolimus and sunitinib in terms of PFS, OS and SSA use. It also concluded that sunitinib led to a higher risk of grade 3/4 AEs, with a different tolerability profile between the two treatments. This led the company to adopt a base case in which both treatments had equal PFS and OS effectiveness. In addition, it cautioned about the potential bias as a result of heterogeneity between the populations in the trials (RADIANT-334 and A618111181) used in the Bucher indirect comparison, which was used as the source of the economic model parameters.
The AE estimates were obtained using the primary analysis cut-off date of 28 February 2010. In addition, Novartis presented updated results on AEs that occurred in the double-blind and extension phases of RADIANT-3,34 with a cut-off date of 5 March 2014. These are not discussed here as they were not used in the Novartis economic evaluation.
The company also presented commercial-in-confidence data from a non-randomised, unpublished study, the OBLIQUE trial, in advanced pNETs patients treated with 10 mg of everolimus in routine practice. The study involved 46 patients who were followed up for 6 months from treatment initiation, with HRQoL measured using the EORTC QLQ-C30, EORTC QLQ GINET21 (European Organisation for Research and Treatment of Cancer 21-item Quality of Life Questionnaire for gastrointestinal neuroendocrine tumours) and the EQ-5D. This study found that HRQoL was maintained over the observation period. In particular, the mean EQ-5D score was 0.72 (95% CI 0.67 to 0.77) at baseline, 0.67 (95% CI 0.61 to 0.73) at 3 months and 0.73 (95% CI 0.67 to 0.78) at 6 months. This study is important as it is the only source identified by the AG containing utility values for patients on everolimus in the advanced pNETs population (see Chapter 6, Systematic review of utilities, for the AG’s review of utilities). However, Novartis did not discuss how the population from which these utility values were measured differs from the trial populations of RADIANT-334 and A6181111. 81
Novartis review of economic models and their results
Novartis conducted a systematic literature review of economic evaluation studies, including studies on resource utilisation, costs and utilities. It identified two studies as being relevant to the NICE scope for this assessment, namely the economic evaluations of sunitinib plus BSC relative to BSC and of everolimus relative to sunitinib or BSC in progressive, advanced pNETs, as reported in the previous company submission to the SMC133,178 and All Wales Medicines Strategy Group (AWMSG). 179,180
One of the identified studies was the poster publication by Johns et al. ,122 reviewed in Appendix 10, which reported an ICER of £22,587 per QALY gained for sunitinib compared with placebo. The other study was the SMC178 and AWMSG180 submission on everolimus, which in the SMC analysis178 was found to have an ICER of £14,562 per QALY gained compared with sunitinib and £24,998 per QALY gained compared with BSC; in the analysis for the AWMSG,180 the respective values were £12,894 and £24,999.
The above evidence is likely to be outdated because of recently updated data and analyses, particularly in relation to evidence adjusted for treatment crossover, and therefore an updated review and analyses are warranted.
Data and methods
Efficacy and effectiveness data used in the model
The model used parametric curves fitted to the PFS individual patient data from the everolimus arm of RADIANT-334 to estimate the proportion of patients in stable disease during the observed period in the trial (up to 25 months) and to extrapolate beyond it up to 20 years. The sunitinib arm PFS was estimated by applying estimates of the PFS HR from the ITC analyses described earlier in this appendix (see also Chapter 4, Indirect treatment comparison: pancreatic neuroendocrine tumours). OS for both treatments was derived using the same approach as for PFS. The estimated PFS and OS curves were used to partition the total proportion of patients alive at any given time into the proportion in the progression-free state and the proportion in the progressive state. The former proportion was obtained directly from PFS and the latter indirectly, by subtracting the proportion of PFS from the corresponding OS proportion for the duration of the 74-month trial observation period and up to 20 years.
Novartis explored different parametric failure time distributions to model PFS and OS in the everolimus arm of RADIANT-3,34 including exponential, Weibull, Gompertz, log-normal and log-logistic models. To select the base case, three criteria were used: the BIC, as a measure of goodness of fit that penalises model complexity (i.e. the number of model parameters); the visual fit to the non-parametric Kaplan–Meier curves; and the visual fit to the empirical hazard rates (i.e. the instantaneous probability of failure). In addition, for the OS distributions, Novartis used external registry data from the SEER database to validate the candidate models; in particular, the 15-year survival rate of 6% after diagnosis in the SEER database was used to judge whether or not a model extrapolation beyond the end of the trial follow-up period was plausible. Finally, Novartis discarded PFS distributions that crossed the preferred OS parametric curve, which turned out to be the Weibull distribution. This led to the choice of the Weibull parametric distribution for PFS with everolimus in preference to the log-logistic and log-normal distributions, as these crossed the OS curve in the early part of the trial follow-up period. Other parametric functions not chosen for the base case were used in sensitivity analyses.
The adopted approach to derive the OS and PFS curves for sunitinib implied the assumptions of constant proportional hazards and, as the company acknowledged, that there were no confounders affecting the relative treatment effects between everolimus and sunitinib. In support of the second assumption, the submission states that the available subgroup analyses from both RADIANT-334 and A618111181 do not suggest that treatment effects relative to placebo are modified by measured characteristics. In support of the proportional hazards assumption, plots of the log cumulative hazard against the log of trial follow-up time (log-log plots) were presented, suggesting a parallel pattern between the active arm and the placebo arm in each trial.
The company warns that, because of crossover to the active treatment in the placebo arms of the two trials, the OS HR derived from the Bucher indirect comparison may be biased because of differences in the method used to adjust for treatment crossover in RADIANT-334 and A6181111,81 which was conducted by Pfizer and available only to Novartis from aggregate results submitted to the SMC181 and AWMSG. 124 In particular, Novartis argues that, as illustrated by the comparative log-log plots of the two analyses (see figure 6.2 in the Novartis NICE submission33), the extent of recensoring needed for valid adjustment of the placebo arm of RADIANT-334 using the RPSFT method produced a placebo OS curve (left-hand graph) that was much shorter than the corresponding curve for placebo (right-hand graph) in the Pfizer RPSFT analysis of A618111181 data. This led Novartis to propose that Pfizer may not have applied recensoring in its analyses, which is needed for a valid estimation of treatment effect. 128 The AG sought to verify this by requesting from Pfizer the individual patient data and statistical analysis code needed to replicate the company’s RPSFT analyses. In response, Pfizer provided the individual patient data without the analysis code, which prevented the AG from replicating the Pfizer RPFST analysis and determining whether or not the RPFST analyses by the two companies were comparable.
On the basis of the Bucher indirect comparison results showing that the HR for OS (RPSFT adjusted: 1.39, 95% CI 0.166 to 11.723) and PFS (local review: 0.833, 95% CI 0.490 to 1.417; central blinded investigator review: 1.079, 95% CI 0.586 to 1.990) for everolimus compared with sunitinib had wide CIs around 1, Novartis adopted a fixed HR value of 1 in the base case, that is, the assumption of no difference in effect between the two treatments in terms of both PFS and OS outcomes.
As discussed earlier, these OS figures and the log-log plots from A618111181 are the same as the latest OS results for that trial,88 which were presented in the Pfizer submission32 to NICE. Using the latest RPSFT-adjusted OS HR of 0.34 for sunitinib compared with placebo and the corresponding estimate for everolimus used by Novartis to derive the base-case (Bucher) HR of 0.60 for everolimus compared with sunitinib results in a (Bucher) HR of 1.76 for everolimus compared with sunitinib [Pfizer conducted a MAIC analysis of everolimus compared with sunitinib and found a MAIC OS HR of (confidential information has been removed), although this was derived by matching to the A618111181 population and therefore is not comparable to the figures in this section, which are matched to the RADIANT-334 population; Pfizer submission, p. 6832 and see earlier in this appendix].
Adverse events
The model measured only the costs and effect on HRQoL (disutility) of treatment-related grade 3/4 AEs as the ‘grade 1 and 2 events [observed in RADIANT-334] would not be associated with any meaningful management costs or impact on HRQoL’ (Novartis submission, p. 9733). Overall AE rates of 7% for the everolimus arm of the model and 26% for the sunitinib arm from cycle 0 to cycle 25, set to 0% thereafter, were applied (columns P and Q in ‘Survival’ sheet of Novartis’s pNETs Microsoft Excel model). The everolimus rate was obtained from RADIANT-334 data, whereas the sunitinib rate was obtained by scaling up this rate on the basis of the OR of 4.479 for sunitinib compared with everolimus for any grade 3/4 AEs from the Bucher indirect comparison conducted by Novartis (see Appendix 14, Novartis). However, new grade 3/4 data provided by Pfizer32 as part of its submission to NICE suggest that the rate for sunitinib is too high. Updating the Bucher analyses submitted by Novartis with the new Pfizer data results in a pooled grade 3/4 HR of 1.37 for everolimus. In any case, as Novartis acknowledges, the validity of this approach to estimate the economic impact of AEs hinges on the unverifiable and unlikely assumption that patients did not experience multiple instances of the same grade 3/4 AE.
Although the model does not explicitly account for the effect of AEs on treatment use, it included a measure of RDI for the targeted therapies recorded during the study period in RADIANT-334 and A6181111. 81
Similarly, the costs of AEs in the model were assumed to apply only to the first 25 cycles. The role of AEs in terms of treatment discontinuation was not explicitly modelled but accounted for independently by adjustments to the dose intensity and treatment duration.
Novartis presented additional published estimates of the relative incidence of AEs between sunitinib and everolimus from MAIC analysis of AE data from an earlier cut-off point124 than the data cut-off point in the Bucher indirect comparison analysis (see table 4.5 in the Novartis submission33) that informed its economic model. Although the two sources may refer to different populations, that is, the MAIC was adjusted to the A618111181 population and the Bucher indirect comparison analysis was adjusted to the RADIANT-334 population, comparison of the two sets of estimates suggests that the Bucher indirect comparison leads to misleading AE rate estimates for the cost-effectiveness analyses. However, the company decided not to use the MAIC estimates in the economic analysis because, in the previous submission to the SMC, the appraisal committee’s opinion was that the method was ‘non-standard with uncertainty as to the robustness of this type of analysis’ (Novartis submission, p. 4933). However, the overall balance of grade 3/4 AE risk implied by the individual AE rates obtained from the Bucher indirect comparison analysis was inconsistent with the pooled OR using the same method. Novartis used the Bucher AE rates adjusted for this inconsistency.
Since Novartis assumed that everolimus and sunitinib had equal PFS and OS outcomes, the resulting cost differences were driven, and the QALY differences completely determined, by the difference in the profile of AEs experienced under the two treatments. As discussed earlier in this appendix, the rates of individual types of AEs were determined by indirect comparison using the Bucher method. This led to differences in grade 3/4 AE rates between the two treatments that were inconsistent with the ranking of the two treatments in terms of pooled AEs: although the pooled OR indicated that sunitinib was associated with a higher incidence of AEs, the individual rates for the 13 AEs considered in the Novartis model combined implied the opposite. To address this contradiction, the company calculated costs and disutilities of AEs as weighted averages of the costs and disutilities associated with managing and experiencing individual AE types, using the Bucher-derived individual AE rates as weights. These weighted averages were then multiplied by the overall incidence of any grade 3/4 AE in RADIANT-334 for everolimus and by the Bucher pooled OR for sunitinib in its pNETs economic model. The same approach was used to estimate the costs of AEs.
Costs and benefits were discounted at 3.5%, as indicated in the NICE reference case37 (see Appendix 9).
Utility values
The utility values in the model were obtained using a time trade-off preference elicitation exercise conducted with 100 members of the general public. Individuals were asked to evaluate descriptors of health states previously designed by clinical experts as representative of those experienced by pNETs patients in routine practice. The analysis was generic in the sense that the vignettes assessed by participants in the exercise did not include details about any particular treatment but instead presented states that described stable disease (with and without AEs, including diarrhoea, palmar–plantar erythrodysaesthesia syndrome, hyperglycaemia, nausea/vomiting, pneumonitis, rash, stomatitis and thrombocytopenia) or progressive disease. 131 This study was sponsored by Novartis. A discussion of this study is presented in the review of utility values in Appendix 15 and Chapter 6, Systematic review of utilities.
The company used the disutilities estimated from the preference elicitation exercise to impute treatment-specific utility values for stable disease for the two treatments, after applying the weighted average method based on AE rates discussed in Chapter 6, Systematic review of utilities. The same progressive disease utility value was applied to both treatment arms. The average severity of events experienced with everolimus was marginally larger than that experienced with sunitinib (i.e. 0.647 vs. 0.656).
Some of the utility values for stable disease with AEs were based on assumption. These values are presented in Table 66. The assumption adopted by the company that hypertension, which was not measured in the preference elicitation study, had an average disutility equal to the average utility for all AEs in stable disease (0.128, = 0.771 – 0.643) is particularly implausible as national EQ-5D data in large samples suggest that the disutility of hypertension in patients with cancer in the last 5 years is negligible. 182 The disutility of anaemia, which was imputed in the same way as for hypertension, is higher than the 0.085 identified in previous reviews of chemotherapy-induced anaemia. 183 Likewise, the imputation of the 0.128 disutility value for all AEs for grade 3/4 fatigue by Novartis is questionable in view of lower time trade-off estimates found in previous studies. 183,184
| AE | Mean utility vale | SE | Reference/assumption |
|---|---|---|---|
| Neutropenia | 0.690 | 0.024 | Assumed similar to thrombocytopenia |
| Hypertension | 0.643 | 0.023 | Average of all AEs |
| Hand–foot syndromea | 0.583 | 0.023 | Swinburn et al.131 |
| Leukopenia | 0.690 | 0.024 | Assumed similar to thrombocytopenia |
| Diarrhoea | 0.600 | 0.025 | Swinburn et al.131 |
| Stomatitis | 0.557 | 0.024 | Swinburn et al.131 |
| Thrombocytopenia | 0.690 | 0.024 | Swinburn et al.131 |
| Anaemia | 0.643 | 0.023 | Average of all AEs |
| Hyperglycaemia | 0.771 | 0.020 | Higher than SD with no AEs, which is unlikely; thus, assumed similar to SD with no AEs |
| Fatigue | 0.643 | 0.023 | Average of all AEs |
| Infections | 0.612 | 0.026 | Assumed similar to pneumonitis |
| Pneumonitis | 0.612 | 0.026 | Swinburn et al.131 |
| Nausea | 0.710 | 0.021 | Swinburn et al.131 |
No disutility for end-of-life care costs was included in the analysis nor was any adjustment for the effect of age on background utility considered. The overall mean utility values after including treatment-specific AE profiles and the utility value for progressive disease used in the company’s analysis are presented in Table 67.
| Health state | Source | |||
|---|---|---|---|---|
| Swinburn et al.131 | A618111181 | |||
| Utility value, mean (SE) | 95% CI | Utility value, mean (SE)a | 95% CI | |
| SD without AEs | 0.771 | 0.731 to 0.810 | NA | NR |
| SD with AEs (everolimus) | 0.647 (0.023) | 0.601 to 0.693 | 0.730 (00.73) | NR |
| SD with AEs (sunitinib) | 0.656 (0.024) | 0.610 to 0.702 | NA | NR |
| PD | 0.612 | 0.564 to 0.659 | 0.596 (0.06) | NR |
Costs
Novartis included the costs of drug acquisition and administration (for first and subsequent treatments and treatments defined as BSC), disease monitoring, management of treatment-related grade 3/4 AEs and death.
The acquisition cost of everolimus of £2673.00 for 30 tablets of 10 mg adopted in the model was based on 2016 BNF prices. 36 The company presented analyses with and without a (confidential information has been removed) PAS discount, which reduced the drug acquisition cost to (confidential information has been removed). The cost of everolimus in each (monthly) cycle was calculated as the product of the monthly cost of everolimus acquisition and administration, that is, a dispensing fee, and the proportion of patients on treatment at each cycle in the everolimus arm of RADIANT-3,34 in which everolimus treatment was given for a median duration of 8.61 months and a mean duration of (confidential information has been removed). The cost of everolimus was adjusted by the RDI of 85.9% recorded in RADIANT-3,34 which accounted for everolimus treatment interruptions and dose reductions.
The acquisition cost of sunitinib was £2522.40 for 30 tablets of 37.5 mg, based on 2016 BNF prices. 36 The company assumed a PAS discount whereby sunitinib is given free of charge to the NHS for the first cycle, as is the case in Scotland, and adjusted costs by a RDI of 91.3%, as reported for sunitinib in A6181111. 81 In its base-case analysis, Novartis assumed that the cost of sunitinib acquisition and dispending was incurred for the same number of mean treatment cycles as for everolimus, on the basis that its ITC found no difference in PFS duration between the two treatments. This assumption seems untenable in the light of the available data on treatment duration from A618111181 and RADIANT-3. 34 The company performed a sensitivity analysis using an alternative figure of 9.66 months for sunitinib treatment duration, which the company attributed to the literature without providing a reference. It also cites a submission by Pfizer to the AWMSG in which Pfizer is reported to have assumed that ‘patients receive an average of 293 days of treatment per year’. 179 However, in its submission to NICE, Pfizer reported an average duration of sunitinib treatment of 8.3 months in clinical practice (253 days; Pfizer submission, p. 1732). In addition, the median treatment duration for sunitinib in A618111181 was 4.64 months as opposed to the (confidential information has been removed) of everolimus use in RADIANT-3. 34
The cost of drug administration involved a dispensing fee to cover a hospital pharmacist’s time required to dispense an oral medication, obtained from the Personal Social Services Research Unit,142 and the cost of delivering chemotherapies for intravenously administered drugs, obtained from NHS reference costs. 141 The same administration cost was thus applied to everolimus and sunitinib.
As for the costs of monitoring treatment and disease, data on 13 advanced pNETs (six well differentiated and seven moderately differentiated) patients, as provided by a survey of 34 UK clinicians, as part of a survey of 197 clinicians in six countries with experience of treating advanced NETs, were used in the company model. The main publication113 reporting the methods and findings of the survey is included in the systematic review of cost-effectiveness studies (see Appendix 10, Results). The survey asked clinicians about the treatment received by and health-care resources used for the two most recent patients they had treated, for each of three disease stages: a baseline period, a first progression period and a second progression period. The baseline period was described in the survey as the period following diagnosis with advanced pNETs, up to the first time that tumour progression was recorded. The first progression period followed the baseline period and ended when the patient was diagnosed with further measurable disease progression of advanced pNETs.
For the purposes of deriving data for its economic analysis, Novartis considered the first progression phase as the stable disease phase of the model and the second progression phase as the progressive disease phase of the model. The survey produced actual resource utilisation data for eight (60%) pNETs patients in the stable disease phase and no patients in the progressive disease phase. Because of limited data available on NETs in general in the progressive disease phase, the survey asked clinicians to provide hypothetical data on the 13 pNET patients. The majority of patients (n = 7; 54%) were in the 51–65 years age range and had an ECOG PS of 0–1 (n = 6; 46% – n = 4 had no recorded status).
Data were collected on resource use, including clinician visits, procedures and tests (e.g. CT scans, biomarker tests, including chromogranin A, and other tests) and hospitalisations. In addition, data on symptomatic (SSA and other) drug use in stable disease and symptomatic treatment and chemotherapy in progressive disease were collected. The clinicians were asked to estimate the duration spent in stable disease for patients, whereas for progressive disease they were asked to assume that patients would spend 12 months in that state. By dividing the reported amount of resource use for the whole stable disease and progressive disease periods by their respective duration, the average cost of a monthly cycle was derived for use in the model. As more clinician visits were obtained for the first cycle of the progressive disease phase, the Novartis model allowed for different health-care costs in the first and subsequent cycles after progression. Unit costs were obtained from NHS reference costs. 141
The resulting values used for populating the model are presented in Table 68, which is reproduced from the Novartis submission. 33 In total, 84% of the total health-care costs in stable disease (£87; physician visits plus tests plus hospitalisations) are associated with physician visits (£55 per month) and CT scans (£18 per month). The progressive disease state for the first month after disease progression generates total health-care costs of £376 (first cycle), with a cost of £170 per month subsequently; the difference is due to 0.4 additional visits to the primary physician and one additional visit to other physicians in the first month after progression. In subsequent months of the progressive disease state, 95% of the costs are incurred from physician visits (£106 per month), CT scans (£22 per month) and hospitalisations (£34 per month).
| Resource | Unit cost (£) | Unit | Stable disease | Progressive disease first cycle | Progressive disease subsequent cycle | |||
|---|---|---|---|---|---|---|---|---|
| Frequency per cycle | Cycle cost (£) | Frequency per cycle | Cycle cost (£) | Frequency per cycle | Cycle cost (£) | |||
| Physician visits | ||||||||
| Follow-up (primary physician) | 158.54 | Visit | 0.274 | 43.39 | 0.744 | 117.91 | 0.338 | 53.58 |
| Follow-up (another physician) | 139.99 | Visit | 0.080 | 11.27 | 1.385 | 193.83 | 0.378 | 52.86 |
| Subtotal | 54.65 | 311.74 | 106.44 | |||||
| Procedures and tests | ||||||||
| Ultrasound | 55.17 | Procedure | 0.024 | 1.33 | 0.032 | 1.77 | 0.032 | 1.77 |
| CT | 124.53 | Procedure | 0.145 | 18.04 | 0.173 | 21.55 | 0.173 | 21.55 |
| SRS | 806.32 | Procedure | 0.008 | 6.49 | 0.000 | 0.00 | 0.000 | 0.00 |
| MRI | 181.76 | Procedure | 0.024 | 4.39 | 0.019 | 3.50 | 0.019 | 3.50 |
| Chest radiography | 42.12 | Procedure | 0.000 | 0.00 | 0.013 | 0.54 | 0.013 | 0.54 |
| Neuron-specific enolase | 1.19 | Test | 0.000 | 0.00 | 0.006 | 0.01 | 0.006 | 0.01 |
| Chromogranin A | 1.19 | Test | 0.056 | 0.07 | 0.083 | 0.10 | 0.083 | 0.10 |
| Pancreatic hormone | 1.19 | Test | 0.024 | 0.03 | 0.026 | 0.03 | 0.026 | 0.03 |
| Plasma vasoactive intestinal peptide total | 1.19 | Test | 0.016 | 0.02 | 0.032 | 0.04 | 0.032 | 0.04 |
| Serum marker | 1.19 | Test | 0.016 | 0.02 | 0.026 | 0.03 | 0.026 | 0.03 |
| Ki-67 | 1.19 | Test | 0.016 | 0.02 | 0.026 | 0.03 | 0.026 | 0.03 |
| 5-hydroxyindoleacetic acid | 1.19 | Test | 0.064 | 0.08 | 0.083 | 0.10 | 0.083 | 0.10 |
| Plasma substance P | 1.19 | Test | 0.016 | 0.02 | 0.026 | 0.03 | 0.026 | 0.03 |
| Plasma vasoactive intestinal peptide total free T4 | 1.19 | Test | 0.016 | 0.02 | 0.026 | 0.03 | 0.026 | 0.03 |
| CBC blood test | 3.01 | Test | 0.121 | 0.36 | 0.179 | 0.54 | 0.179 | 0.54 |
| Blood urea nitrogen | 3.01 | Test | 0.121 | 0.36 | 0.141 | 0.42 | 0.141 | 0.42 |
| Serum glucose | 3.01 | Test | 0.121 | 0.36 | 0.128 | 0.39 | 0.128 | 0.39 |
| Serum creatinine | 3.01 | Test | 0.121 | 0.36 | 0.179 | 0.54 | 0.179 | 0.54 |
| Lipid profile | 3.01 | Test | 0.089 | 0.27 | 0.077 | 0.23 | 0.077 | 0.23 |
| Subtotal | 32.24 | 29.87 | 29.87 | |||||
| Hospitalisations | ||||||||
| General hospitalisation | 586.93 | Hospitalisation | 0.000 | 0.00 | 0.058 | 33.86 | 0.058 | 33.86 |
| Subtotal | 0.00 | 33.86 | 33.86 | |||||
The costs of managing AEs covered treatment-related grade 3/4 AEs that occurred in the stable disease phase of the model. Only AEs that were recorded in > 2% of patients in any of the active treatment arms of A618111181 and RADIANT-334 were accounted for. Details of the AE types, the methods used for deriving the AE probability estimates for the sunitinib arm, involving the Bucher indirect comparison analysis in Chapter 4 (see Indirect treatment comparison: pancreatic neuroendocrine tumours), and the duration for which AEs were measured in the model are described in Appendix 14 and Chapter 6 (see Utility values for the Peninsula Technology Assessment Group model). As described in Chapter 6, the AE probabilities were based on the assumption that the rates of individual types of AEs were constituted by single events per patient in each trial arm; this was a consequence of Novartis not having access to individual patient data from the A618111181 trial sponsored by Pfizer.
Moreover, as described earlier in the utility section, the Bucher indirect comparison produced sunitinib AE rates whose aggregate magnitude was smaller than the respective magnitude in the everolimus arm in RADIANT-3,34 whereas the opposite occurred when the count of different AEs was combined to derive an overall AE rate for sunitinib using the same Bucher method. This led Novartis to calculate the disutility and cost of a typical AE as a weighted average of the costs of the different AEs multiplied by the relative contribution to the overall sum of AE rates for each treatment arm in the model. The inputs into this weighted average are presented in Table 69. In line with the weighted average disutility of AEs used in the Novartis model, the cost of a typical AE in the everolimus arm is more expensive, by 15%, than the average cost of an AE under sunitinib treatment. Novartis estimated an overall AE OR of 4.47 for sunitinib compared with everolimus, but the data on AEs with sunitinib in the A618111181 trial that the company used were obtained from previous publications and, for certain AE types, differed from those reported by Pfizer in its submission to NICE; with the updated figures, the AG obtained a OR estimate of 1.37 instead. Based on the monthly (confidential information has been removed) probability of AEs with everolimus estimated by Novartis from RADIANT-334 individual patient data, which the company applied for the first 25 cycles of the stable disease model under everolimus (with a probability of 0 applied for subsequent cycles), the Bucher OR estimated by Novartis produced a monthly probability of AEs with sunitinib of 26% in those cycles; with the OR calculated by the AG, the monthly probability of AEs with sunitinib changes to 10%.
| Grade 3/4 AE | Everolimus | Sunitinib | |||||
|---|---|---|---|---|---|---|---|
| Unit cost (£) | AE ratea | Weighted frequency | AE cost (£) | AE rate from the ITCa,b | Weighted frequency | AE cost (£) | |
| Neutropenia | 127.70 | 0.002 | 0.007 | 0.83 | 0.055 | 0.224 | 28.60 |
| Hypertension | 736.89 | 0.002 | 0.007 | 4.81 | 0.044 | 0.179 | 131.56 |
| Hand–foot syndromec | 172.58 | 0.002 | 0.007 | 1.13 | 0.028 | 0.113 | 19.49 |
| Leukopenia | 1765.87 | 0.002 | 0.007 | 11.53 | 0.028 | 0.113 | 199.45 |
| Diarrhoea | 797.95 | 0.034 | 0.092 | 73.13 | 0.020 | 0.081 | 64.76 |
| Stomatitis | 431.84 | 0.071 | 0.189 | 81.78 | 0.017 | 0.071 | 30.58 |
| Thrombocytopenia | 643.48 | 0.042 | 0.111 | 71.43 | 0.017 | 0.071 | 45.57 |
| Anaemia | 777.82 | 0.061 | 0.163 | 126.98 | 0.002 | 0.010 | 7.70 |
| Hyperglycaemia | 1058.07 | 0.054 | 0.144 | 152.38 | 0.019 | 0.079 | 84.02 |
| Fatigue | 324.53 | 0.025 | 0.065 | 21.24 | 0.005 | 0.020 | 6.44 |
| Infections | 1080.69 | 0.025 | 0.065 | 70.74 | 0.005 | 0.020 | 21.45 |
| Pneumonitis | 1934.80 | 0.027 | 0.072 | 138.98 | 0.002 | 0.010 | 19.15 |
| Nausea | 79.47 | 0.027 | 0.072 | 5.71 | 0.002 | 0.010 | 0.79 |
| Total | 760.68 | 659.58 | |||||
This means that the costs of the additional risks of an AE with sunitinib are partly offset by the lower severity of these AEs relative to everolimus. Moreover, updating Novartis’s data on AEs with sunitinib with data submitted by Pfizer32 to NICE reduces the additional costs of AEs with sunitinib. These additional costs decline over time as fewer people in both arms remain in the stable disease state. Furthermore, in the base case they decline at the same rate in both arms, as Novartis assumed that PFS is the same across the two arms, until cycle 25 (at 2 years after treatment starts), after which no AE costs are incurred.
The use of symptomatic treatment, defined as SSA, in the stable disease phase was estimated from the proportion, 37.7%, of people who received the treatment before progression in the everolimus arm of RADIANT-334 and the Bucher indirect comparison OR of 1.04 (95% CI 0.478 to 2.262) estimated for everolimus compared with sunitinib (Novartis submission, section 4.7.2.3;33 see earlier in this appendix). This resulted in a SSA treatment rate with sunitinib of 36.8%, which was multiplied by the monthly cost of treatment with SSAs, as detailed in Table 70. The costs used by Novartis were based on BNF drug acquisition prices. 36 In contrast, average drug acquisition prices paid by hospitals, as recorded in the electronic market information tool (eMIT) database,137 are 8–26% lower for the symptomatic treatments considered by Novartis (final column of Table 70). As for symptomatic treatment in progressive disease, a rate of SSA use of 25% was assumed based on the results of the health-care resource use survey of UK experts113 described earlier, 1.9 administrations of octreotide per cycle, at a mean daily dose of 30 µg for the first 15 days and 450 µg thereafter, and 90% RDI.
| SSA | Costing assumption (£) | Administration cost (£) | Costing assumption based on eMIT prices (not considered in the Novartis model submission) (£) |
|---|---|---|---|
| Octreotide LAR (Sandostatin LAR)a | 799.33 | 239.12 | 632.40 |
| Octreotide (Sandostatin) – 500 mg | 14.12 | 239.12 | NA |
| Lanreotide (Somatuline Autogel)a | 736.00 | 239.12 | NA |
| Octreotide (Sandostatin) 500 µg/ml, 1-ml ampoule | 27.09 | 239.12 | 25.10 |
The costs of subsequent treatments following disease progression were included for chemotherapy, radiotherapy and chemoembolisation. Information on subsequent therapy used in the sunitinib arm of A618111181 was not recorded in the trial (Pfizer, response to AG data request, August 2016). Novartis stated that the rates of subsequent treatments used in its model were obtained from those recorded in the RADIANT-334 trial of everolimus compared with placebo; these were applied to both the sunitinib and the everolimus arms of the model. However, the rates used in the model (reproduced in Table 71) and those observed in RADIANT-334 and reported in the appendix of the Novartis submission33 do not seem to correspond.
| Treatment | Unit cost (£) | Initial drug administration cost (£) | Subsequent drug administration cost (£) | Number of cycles | Number of units adjusted by number of cycles | Proportion of use | Total |
|---|---|---|---|---|---|---|---|
| Radiotherapy | 2026.86 | 0.00 | 0.00 | 1.27 | 1.27 | 0.094 | 241.39 |
| Chemoembolisation | 3993.90 | 0.00 | 0.00 | 0.03 | 0.03 | 0.094 | 12.30 |
| 5-fluorouracil | 11.20 | 239.12 | 326.46 | 2.50 | 13.57 | 0.219 | 983.32 |
| Doxorubicin | 129.78 | 239.12 | 326.46 | 1.66 | 1.80 | 0.281 | 206.87 |
| Streptozocin | 0.00 | 239.12 | 326.46 | 2.14 | 11.61 | 0.313 | 1156.84 |
| Total | 2600.72 |
End-of-life care costs were included as a single fixed amount of £4346 occurring at the time that patients died in the model. This figure was obtained from a published study estimating the per-patient health-care costs observed in the terminal phase of life of cancer patients in England and Wales,143 measured from the time when strong opioids are used, and included the costs of elective and non-elective inpatient hospitalisations, outpatient visits, AE attendances and visits to district nurses and GPs.
Costs were expressed in 2015 prices.
Gastrointestinal and lung neuroendocrine tumours
Efficacy, effectiveness and safety evidence
In RADIANT-4,35 205 patients were randomised to 10 mg daily of everolimus plus BSC and 97 were randomised to placebo plus BSC. Randomisation was carried out with stratification by previous SSA use (continuous SSA for ≥ 12 weeks), tumour origin [better-prognosis stratum: appendix, caecum, jejunum, ileum, duodenum or NET of unknown primary origin; worse-prognosis stratum: lung, stomach, colon (other than caecum) or rectum] and WHO PS (0 vs. 1). The median follow-up period in the study was 21 months and the median duration of treatment with everolimus was 40.4 weeks. Median PFS was 11.0 months (95% CI 9.2 to 13.3 months) in the everolimus arm and 3.9 months (95% CI 3.6 to 7.4 months) in the placebo arm. A 52% reduction in the estimated risk of progression or death was observed in the everolimus arm (HR 0.48, 95% CI 0.35 to 0.67).
Participants in RADIANT-435 were not allowed to undergo treatment crossover after disease progression. Interim OS analysis found a reduction in the risk of death with everolimus of 36% (HR 0.64, 95% CI 0.40 to 1.05), but the data were not mature enough to estimate median OS in any arm. Grade 3/4 drug-related AEs observed in the trial included stomatitis, diarrhoea, infections, anaemia, fatigue and hyperglycaemia (see Chapter 4, Results for details).
Review of the economic models and their results in the submission
A systematic literature review was conducted by Novartis with the aim of identifying economic evaluations related to the use of everolimus in the GI and lung NETs patient population and resource utilisation or costing and utilities associated with health states or treatments in the GI/NETs patient population. No studies were found.
Data and methods
Efficacy and effectiveness data used in the model
A partitioned survival analysis method was used to derive the distribution of the patient cohort between health states in each cycle, using the same methods as described earlier for the Novartis model of pNETs and including PFS (cut-off date 28 November 2014) and OS (cut-off date 30 November 2015) data from RADIANT-4. 35 For this purpose and to extrapolate the OS and PFS outcome distributions beyond the end of the follow-up period in RADIANT-4,35 a parametric survival curve was chosen from six parametric distributions of time to event: exponential, Weibull, gamma, Gompertz, log-normal and log-logistic. Two variants of each of these survival distributions were estimated: an unrestricted variant, in which one distribution was estimated using data from both trial arms but different estimates for each parameter in the distribution were obtained for the two arms, and a variant in which the data from both arms were analysed with the same distribution but all but one of the parameters (i.e. the scale) were restricted to be the same for the everolimus and placebo arms.
More broadly, Novartis assessed the empirical adequacy of three classes of treatment effect models for PFS and OS data. One was the shifted failure time model, which assumes that treatment effects take place by displacing the survival curve to the right by a constant amount at each percentile of the cumulative survival distribution. The second model class is the proportional hazards model, which assumes that the treatment proportionally alters the (instantaneous) risk of the event occurring; this model class includes the exponential, Weibull and Gompertz models. The third model class considered was the AFT model, which assumes that treatment affects the survival time proportionally; this model class includes the log-logistic, log-normal and gamma models. By applying the counterfactual criteria of Bagust and Beale185 to model section, Novartis was able to discriminate the AFT model class as providing valid PFS model candidates, whereas the proportional hazards and AFT model classes were valid for modelling OS. The counterfactual criteria consist of obtaining for each candidate model the (predicted) survival curve of the placebo arm that would have occurred had the placebo patients been randomly allocated to the active treatment and comparing it with the actual survival curve of the active treatment arm; valid models are those for which the counterfactual placebo survival curve matches the survival curve of the active treatment arm.
Additional criteria were used to select survival distributions within model classes, including having a low BIC statistic (goodness of fit), and plausibility of long-term extrapolation. The latter had two requirements. One was having curves that were above the 15-year 5% survival rate, a criterion adopted by Novartis on the survival rate evidence from the SEER database, despite the caveats acknowledged by the company and discussed earlier with regard to the Novartis pNETs model. The other requirement was that curves did not cross, which, in the analysis of OS, was justified on the basis that ‘there is no reason to believe that the OS for everolimus plus BSC would be less than that for placebo plus BSC at any point in time’ (Novartis submission, p. 13733).
The PFS model selected for the base case was the (restricted) log-normal model, whereas the (restricted) gamma, (restricted) log-logistic and (unrestricted) log-normal models were used for sensitivity analyses. The (restricted) log-logistic distribution was chosen to model OS in the base-case analysis, with the (restricted and unrestricted) log-normal model used in sensitivity analysis. Novartis considered unrestricted variants as more flexible options than the restricted forms of each survival distribution but in practice their added flexibility ended up ruling them out as candidate models for PFS and OS data because of curve crossing between the everolimus arm and the placebo arm. Therefore, it is questionable whether or not the modelling approach adopted by Novartis may have imposed too straight a jacket by fitting a common survival distribution function to PFS and OS data from both arms of RADIANT-4,35 given that the more flexible (although potentially less efficient) modelling approach of separately modelling the data from each trial arm was not considered.
Although patients in RADIANT-435 were not allowed to cross over from placebo to open-label everolimus after disease progression, data on subsequent treatments submitted to NICE by Novartis as part of the economic model (table 7.13, p. 15433) show that 9% of patients in RADIANT-4 received everolimus as subsequent treatment after disease progression. It may be argued that, from the point of view of the NICE decision problem, the approach adopted by Novartis of modelling and extrapolating OS outcomes without adjustment for placebo crossover to everolimus (in spite of including, in the BSC arm of the model, the costs of subsequent everolimus treatment used by placebo arm participants) in RADIANT-435 may be invalid.
Adverse events
The model included the costs of grade 3/4 AEs reported in RADIANT-435 that had an incidence of ≥ 2%. This resulted in the inclusion of stomatitis, diarrhoea, fatigue, infections, peripheral oedema, anaemia, pyrexia and hyperglycaemia. The proportions of participants experiencing these events were used to calculate a weighted average cost of AEs for each of the two model arms, which was then multiplied by the probability of any such AEs in each cycle. Using individual patient data from the trial, the average AE rate per cycle was calculated to be 0.0625 from the first to the 26th cycle in the stable disease phase for everolimus and 0.0147 from the first to the 30th cycle in the SD phase for BSC. Other cycles were assigned AE probabilities of zero. Given that HRQoL outcomes that allowed derivation of health state utilities were measured in RADIANT-4,35 these AE probabilities were not used to calculate base-case utility values but they were used to calculate alternative utility values in sensitivity analysis following the approach described earlier for pNETs (see Chapter 5, Economic evaluation of everolimus in pancreatic neuroendocrine tumours).
Model implementation
In order to incorporate the costs of subsequent treatment, different costs were used in the model in the first and in subsequent cycles after disease progression. The costs of drug administration were applied to both initial and subsequent treatments. AE costs were applied only in the stable disease state. The costs of terminal care were also included as a single cost as participants die in the model. Costs and QALYs were discounted at a 3.5% annual rate.
Utility values
Health state utility values for stable disease and progressive disease were obtained from FACT-G outcome data collected in RADIANT-4. 35 This involved using the ordinary least squares mapping algorithm estimated by Longworth et al. 134 and Young et al. 186 This served to meet the requirements of the NICE reference case of using patient-reported HRQoL outcomes and valuing such outcomes using preferences from the general public. 37 The AG has been able to reproduce the utility estimates used in the base-case analysis from publicly available summary data on FACT-G domain scores reported by RADIANT investigators74 and a linearised version of the best-fitting non-linear algorithm, based on domain responses134 (see Chapter 6, Systematic review of utilities). The base-case analysis used the value of 0.779 for stable disease and 0.725 for progressive disease in both treatment arms, although the company also estimated treatment-specific stable disease utility values of 0.767 for everolimus plus BSC and 0.807 for placebo plus BSC and progressive disease values of 0.714 and 0.747, respectively, and used these values in scenario analyses. The stated reason for the choice of base-case values was that the differences in utilities between everolimus plus BSC and placebo plus BSC in RADIANT-435 ‘were not statistically significant or clinically meaningful’ (Novartis submission, p. 15933). As acknowledged by the company, the utility values for progressive disease are unlikely to be valid measures of the post-progression period as they are based on HRQoL outcomes of a subgroup of people who had progressed by the time that the study had ended and which covered only the early phase of the progressive disease state. This led the company to explore lower values in sensitivity analyses.
No adjustment was applied to utilities for end-of-life care or the effect of ageing on HRQoL.
Costs
The costs of drug acquisition, dispensation and administration associated with everolimus and BSC were included in the analysis. Analyses of costs using list prices and alternative potential PAS discounts were presented.
The cost of drug acquisition of 10 mg daily of everolimus, as given in RADIANT-4,35 was calculated using the 2016 BNF price of £2673 for 30 tables of 10 mg each. 36 Alternatively, the PAS discount of (confidential information has been removed) was applied to the list price of everolimus. This, plus the cost of oral drug administration, was multiplied by the proportion of patients remaining on everolimus at each cycle in the stable disease health state, which was derived from a time-on-treatment curve calculated from individual patient data from RADIANT-4. 35 The Kaplan–Meier median treatment duration was (confidential information has been removed) months and by month 38 (confidential information has been removed) remained on treatment. A RDI obtained from RADIANT-435 data of 79.4% was applied to the everolimus treatment costs.
The costs of orally administered treatment were based on hospital pharmacy staff time, with unit costs obtained from the Personal Social Services Research Unit. 142 The costs of intravenously administered therapies as part of BSC were applied using unit costs obtained from NHS reference costs. 141
Included in BSC were analgesics, antiemetics, antidiarrhoeals, external beam radiation therapy and SSAs, based on the views of key opinion leaders consulted by Novartis to validate an earlier resource survey of UK clinicians. The rates used in the model for each of these categories of BSC were derived from RADIANT-435 data. Novartis selected the most commonly observed specific treatment as a representative for each category for the purposes of calculating the costs of BSC in the model. The usage rates of therapies constituting BSC used in the model are presented in Table 72.
| BSC therapy | Usage rates (derived from RADIANT-435) (%) | |
|---|---|---|
| Everolimus + BSC | Placebo + BSC | |
| Analgesics: representative treatment lidocaine | 12.7 | 6.2 |
| Other pain medication: corticosteroids and glucocorticoids – representative treatments dexamethasone (corticosteroids) and prednisone (glucocorticoids)a | Corticosteroids 31.7, glucocorticoids 41.5 | Corticosteroids 10.3, glucocorticoids 11.3 |
| Antiemetics: representative treatment prochlorperazine | 2.9 | 3.1 |
| Antidiarrhoeals: representative treatments Biofermin, Saccharomyces boulardiib | 5.8 | 5.2 |
| EBRT | 1 | 0 |
| SSAs: representative treatment octreotide LAR | 2 | 1 |
Health-care resource use was estimated from a survey conducted in 2016 to validate the results of an earlier 2011 survey of 32 UK clinicians in England, relative to current practice. The original survey findings have been published for the combined GI and lung NETs location. 120 The methods used in the survey are described earlier for Novartis’s pNETs model. The validation exercise considered current management practice in non-functional GI and lung NETs separately and involved five clinicians from four centres, two of which were ENETS European centres of excellence. The results of the survey, by averaging the responses of the five clinicians according to the annual number of GI and lung patients they treated annually, are presented in Table 73, weighted in proportion to the mix of GI and lung participants in RADIANT-4. 35
| Item | Resource use in SD | Resource use in PD | Unit cost (£) | ||
|---|---|---|---|---|---|
| Everolimus + BSC | BSC alone | Everolimus + BSC | BSC alone | ||
| Physician visits | |||||
| Follow-up: medical oncologist | 0.843 | 0.273 | 0.745 | 0.531 | 158.54 |
| Follow-up: surgeon | 0.046 | 0.048 | 0.021 | 0.013 | 132.95 |
| Follow-up: palliative care | 0.000 | 0.230 | 0.000 | 0.313 | 185.92 |
| Follow-up: respirologist | 0.000 | 0.017 | 0.018 | 0.020 | 156.29 |
| Follow-up: nurse | 0.075 | 0.023 | 0.000 | 0.000 | 37.26 |
| Follow-up: dietitian | 0.044 | 0.046 | 0.000 | 0.039 | 69.64 |
| Procedures/tests | |||||
| Abdominal ultrasound | 0.007 | 0.008 | 0.010 | 0.006 | 55.17 |
| Echocardiography | 0.018 | 0.000 | 0.024 | 0.000 | 81.48 |
| Chest, abdominal and pelvic CT scan (conventional) | 0.117 | 0.057 | 0.048 | 0.039 | 124.53 |
| Chest, abdominal and pelvic CT scan (helical/spiral) | 0.201 | 0.057 | 0.251 | 0.020 | 124.53 |
| MRI | 0.099 | 0.065 | 0.131 | 0.006 | 181.76 |
| Octreoscan/SRS | 0.077 | 0.078 | 0.054 | 0.003 | 806.32 |
| Neuron-specific enolase | 0.056 | 0.055 | 0.106 | 0.000 | 1.19 |
| Chromogranin A | 0.277 | 0.146 | 0.344 | 0.130 | 1.19 |
| 5-hydroxyindoleacetic acid | 0.166 | 0.104 | 0.213 | 0.059 | 1.19 |
| CBC blood test | 0.805 | 0.271 | 1.038 | 0.211 | 3.01 |
| Blood urea nitrogen | 0.655 | 0.136 | 0.748 | 0.158 | 3.01 |
| Serum glucose | 0.805 | 0.271 | 1.038 | 0.211 | 3.01 |
| Serum creatinine | 0.805 | 0.271 | 1.038 | 0.211 | 3.01 |
| Lipid profile | 0.363 | 0.055 | 0.000 | 0.000 | 3.01 |
| Hospitalisations | |||||
| General hospitalisation | 0.036 | 0.036 | 0.000 | 0.015 | 586.93 |
| Emergency room visit | 0.036 | 0.036 | 0.045 | 0.015 | 147.30 |
The validation survey elicited the opinion from clinical experts that ‘patients on active treatment are more likely to receive follow-up care to monitor disease progression and toxicity’ than patients receiving BSC alone (Novartis submission, p. 15933). In addition, patients in the progressive disease state accrue costs depending on whether they are receiving active post-progression treatment or are under observation (32.7% of patients initially treated with everolimus and 33.3% of patients who started the model under BSC alone were under observation; see details in Table 74).
| Post-progression treatment | Central review C, % | |
|---|---|---|
| Everolimus + BSC | Placebo + BSC | |
| Octreotide LAR | 29.8 | 22.7 |
| Lanreotide | 8.5 | 8.0 |
| Everolimus | 4.3 | 9.1 |
| PRRT | 5.0 | 3.4 |
| IFN | 1.4 | 0.0 |
| Hepatic artery embolisation | 5.0 | 6.8 |
| Chemoembolisation | 0.7 | 3.4 |
| Radiofrequency ablation | 0.7 | 1.1 |
| SIRT | 0.0 | 2.3 |
| Temozolomide | 14.2 | 11.4 |
| Capecitabine | 14.2 | 11.4 |
| Streptozocin | 2.8 | 1.1 |
| Fluorouracil | 2.8 | 1.1 |
| Observation | 32.7 | 33.3 |
Novartis stated that ‘Although the Key Opinion Leaders (KOLs) did not indicate that there would be a significant difference in how patients who had previously received everolimus plus BSC alone would be treated after disease progression, the relative use of these post-progression therapies was calculated using the RADIANT-435 trial data’ (Novartis submission,33 section 7.5.5.1). Costs for post-progression treatments in the model were applied according to the number of treatment cycles that they were observed to be given for in RADIANT-4,35 assuming standard dosages rounded to the nearest dose that was consistent with no wastage (Table 77).
Octreotide LAR was applied a unit cost of £998.41 per month based on 2016 BNF prices. 35 This price is 20% higher than the £806.42 average price available to hospitals according to the eMIT database137 (accessed October 2016). Another aspect to note is the rate of use of everolimus in the placebo arm of 9.3% compared with a rate of 4.3% in the everolimus arm, both of which were given for a treatment duration of 6.18 cycles in the model (see Table 74). Clinical expert advice received by the AG suggests that there is currently no access to peptide receptor radionuclide therapy in England, although there was previously, and that chemotherapy would be used instead in most patients.
A fixed cost of £4346 was applied when patients died in the model to account for the costs of terminal care. This figure was derived from the literature. 143
To estimate the costs of AEs, the probability of any AE, derived from the pooled incidence of grade 3/4 AEs with an incidence of ≥ 2% in either arm of RADIANT-435 (see Chapter 4, Outcomes from randomised controlled trial evidence for gastrointestinal and lung neuroendocrine tumours, Adverse events), was multiplied by the weighted average cost of those specific AEs, according to the relative magnitude of each AE type in the sum of all rates. The unit costs of specific AEs were obtained from NHS reference costs. 141
Advanced Accelerator Applications
The main characteristics of the models submitted by the company are presented in Table 75. The main results are presented in Table 76.
| Company/indication | Population | Comparators | Horizon | Model structure | Health states/events modelled | Utilities | Costs | Key individual parameters (sensitivity analysis) | Source of effectiveness parameters | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| AAA, pNETs within GEP NETs | pNETs. Effectiveness evidence from only the Dutch population of the progressive pNETs subgroup of patients in the ERASMUS study – patients with inoperable SSTR+ GEP NETs | 177Lu-DOTATATE vs. everolimus and sunitinib (also octreotide LAR – out of scope) | 20 years (lifetime) | Cost–utility analysis, QALYs. Three-state Markov model with partitioned-survival. Four-week corrected cycles. Proportional hazards model PFS and OS with baseline Weibull form | Pre-progression survival (PFS), post-progression survival (PPS), death | EORTC QLQ-C30 mapped to EQ-5D: PFS 0.80, PPS 0.79. AE disutility from various literature sources (grade 3/4 only); applied per cycle | Drug acquisition, drug administration, monitoring, AE. Resource utilisation rates from the NETTER-1 CSR.175 Base case includes 177Lu-DOTATATE drug acquisition cost reduction for real-world dose intensity (86.4%). BSC = 30 mg of octreotide LAR | PFS HRs, OS HRs, PFS and PPS health state utilities, 177Lu-DOTATATE dose intensity, 177Lu-DOTATATE drug cost | ERASMUS CSR187 for baseline PFS and OS risk curves (direct extraction). PFS and OS adjusted for an extreme value. MTC for adjusted proportional hazards. RADIANT-334 for everolimus outcome data; Raymond et al.81 for sunitinib data; NETTER-1, RADIANT-334 and Raymond et al.81 for AE proportions. PFS utility sourced from the ERASMUS study |
|
| AAA, GI NETs within GEP NETs | Patients with inoperable, SSTR+ mid-gut carcinoid tumours (NETTER-1 study) | 177Lu-DOTATATE vs. everolimus (also octreotide LAR – out of scope) | 20 years (lifetime) | Cost–utility analysis, QALYs. Three-state Markov model with partitioned-survival. Four-week corrected cycles. Proportional hazards model of PFS and OS with baseline Weibull form | Pre-progression survival (PFS), post-progression survival (PPS), death | EORTC-QLQ-C30 mapped to EQ-5D: PFS 0.79, PPS 0.74. AE disutility from various literature sources (grade 3/4 only); applied per cycle | Drug acquisition, drug administration, monitoring; AEs. Resource utilisation rates from the NETTER-1 CSR. Base case includes 177Lu-DOTATATE drug acquisition cost reduction for real-world dose intensity (86.4%). BSC = 30 mg of octreotide LAR | PFS HRs, OS HRs, PFS and PPS health state utilities, 177Lu-DOTATATE dose intensity, 177Lu-DOTATATE drug cost (no PAS). Drug costs not included in sensitivity analysis | NETTER-1 CSR (v1) for baseline PFS and OS risk curves (direct extraction). MTC for adjusted proportional hazards. RADIANT-2 and -4 for everolimus outcome data;35,119 NETTER-1 and RADIANT-435 for AE proportions. PFS utility sourced from UK Trust registry; PPS sourced from the ERASMUS study |
|
| Study | Regimens compared | Patient characteristics | Time horizon | PFS (years) | Life-years (undiscounted unless otherwise stated) | Discounted (3.5%) incremental QALYs | Discounted (3.5%) incremental costs (£) | ICER (£) | Notes on the ICER |
|---|---|---|---|---|---|---|---|---|---|
| Novartis, pNETs | Everolimus, sunitinib | pNETs, as in RADIANT-334 (everolimus) and A618111181 (sunitinib) | 20 years | Everolimus 14.348; sunitinib 12.512 | Everolimus 3.298; sunitinib 2.85 | 0.021 | –1,635.86. (Confidential information has been removed) (PAS) | Dominant Dominant (PAS) | Costs and QALYs discounted by 3.5%. PAS with (confidential information has been removed) discount for everolimus |
| Novartis, GI NETs | Everolimus + BSC, BSC | Mean age 61.7 years (RADIANT-435) | 30 years | Everolimus 11.01; BSC 5.50 | Everolimus 5.793; BSC 4.775 | 0.777 | 33,902 | £43,642 (list price). (Confidential information has been removed) (PAS price) | Costs and QALYs discounted by 3.5% per year and are in 2014–15 prices |
| AAA, GI NETs within GEP NETs | 177Lu-DOTATATE, everolimus | From NETTER-1 CSR: mean age 63.7 years; weight 74.05 kg. Population: unresectable or metastatic GI NETs with disease progression | 20 years | 177Lu-DOTATATE: 1 year 80.84%, 5 years 11.58%, 10 years 0.34%; everolimus: 1 year 61.99%, 5 years 0.79%, 10 years 0.00% | Discounted: 177Lu-DOTATATE 4.26; everolimus 2.49 | 1.42 | Confidential information has been removed | Confidential information has been removed | Base case excludes cost of palliative care; includes concomitant drugs and a dose intensity adjustment for lutetium |
| AAA, pNETs within GEP NETs | 177Lu-DOTATATE, everolimus, sunitinib | From NETTER-1 CSR: mean age 63.7 years; weight 74.05 kg. Population: unresectable or metastatic pNETs with disease progression | 20 years | 177Lu-DOTATATE: 1 year 90.06%, 5 years 19.53%, 10 years 0.58%; everolimus: 1 year 83.97%, 5 years 6.56%, 10 years 0.02%; sunitinib: 1 year 78.53%, 5 years 3.80%, 10 years 0.00% | Discounted: 177Lu-DOTATATE 6.91; everolimus 4.16; sunitinib 6.84 | 2.18 | 177Lu-DOTATATE: vs. everolimus: (confidential information has been removed); 177Lu-DOTATATE vs. sunitinib: (confidential information has been removed) | 177Lu-DOTATATE vs. everolimus: (confidential information has been removed); 177Lu-DOTATATE vs. sunitinib: (confidential information has been removed) | Base case excludes cost of palliative care; includes concomitant drugs and a dose intensity adjustment for lutetium |
| Post-progression treatment | Unit cost (£) (unit) | Source | Treatment duration | Source | |||
|---|---|---|---|---|---|---|---|
| Everolimus + BSC | BSC alone | ||||||
| Number of units per cycle | Number of cycles | Number of units per cycle | Number of cycles | ||||
| Octreotide LAR – 30 mg | 998.41 (per month) | BNF 201636 | 1.087 | 4.06 | 1.087 | 4.46 | RADIANT-435 |
| Lanreotide – 120 mg | 937.00 (per month) | BNF 201636 | 1.087 | 1.80 | 1.087 | 2.27 | RADIANT-435 |
| Everolimus – 10 mg | 89.10 (per day) | BNF 201636 | 30.438 | 6.18 | 30.438 | 6.18 | RADIANT-435 |
| PRRT | 2247.10 (per procedure) | NHS Reference Cost 2014 to 2015 141 | 0.400 | 5.74 | 0.400 | 0.03 | RADIANT-435 |
| IFN – 5 million IU | 28.37 (per day) | BNF 2016;36 IntronA Summary of Product Characteristics | 13.045 | 3.84 | 13.045 | 3.84 | RADIANT-435 |
| Hepatic artery embolisation | 3993.90 (per procedure) | NHS Reference Cost 2014 to 2015 141 | 1.000 | 1.00 | 1.000 | 1.00 | RADIANT-435 |
| Chemoembolisation | 3993.90 (per procedure) | NHS Reference Cost 2014 to 2015 141 | 1.000 | 1.00 | 1.000 | 1.00 | RADIANT-435 |
| Radiofrequency ablation | 937.54 (per procedure) | NHS Reference Cost 2014 to 2015 141 | 1.000 | 1.00 | 1.000 | 1.00 | RADIANT-435 |
| SIRT | 2026.86 (per procedure) | NHS Reference Cost 2014 to 2015 141 | 1.000 | 1.00 | 1.000 | 1.00 | RADIANT-435 |
| Temozolomide – 360 mg | 152.40 (per day) | BNF 2016;36 Strosberg et al.67 Sacco et al.188 | 5.435 | 2.34 | 5.435 | 3.08 | RADIANT-435 |
| Capecitabine – 2650 mg | £6.42 (per day) | BNF 2016;36 Strosberg et al.67 Sacco et al.188 | 15.219 | 2.34 | 15.219 | 3.08 | RADIANT-435 |
| Streptozocin – 895 mg | 0.00 (per day) | Assumption that cost is covered by the CDF | 2.174 | 1.23 | 2.174 | 1.45 | RADIANT-435 |
| Fluorouracil – 750 mg | 10.40 (per day) | BNF 2016;36 Sun et al.;189 Sacco et al.188 | 4.348 | 1.23 | 4.348 | 1.45 | RADIANT-435 |
| Observation | 0.00 | NA | 0.000 | 0.00 | 0.000 | 0.00 | NA |
Efficacy and effectiveness evidence
Advanced Accelerator Applications conducted a systematic review of studies providing evidence on the clinical efficacy and safety of 177Lu-DOTATATE in patients with GEP NETs. Randomised and non-randomised studies were included. Only one RCT145 was included in the review, NETTER-1, which compared 177Lu-DOTATATE plus 30 mg of octreotide with 60 mg of octreotide in individuals with GI NETs.
Advanced Accelerator Applications conducted two MTCs for the outcomes of PFS and OS: one for pNETs comparing 177Lu-DOTATATE with everolimus and sunitinib and one for GI NETs to compare 177Lu-DOTATATE with everolimus. AAA considered the study and participant characteristics in all studies in the two networks to be comparable, including RADIANT-2,190 which was excluded from our systematic review (see Chapter 4) because all participants have functioning tumours, which is outside the licence for everolimus. These MTCs are illustrated in Figures 35 and 36.
FIGURE 35.
Indirect comparison network for PFS and OS in pNETs. Source: figure 12 (pp. 71–2) of the AAA submission. 31
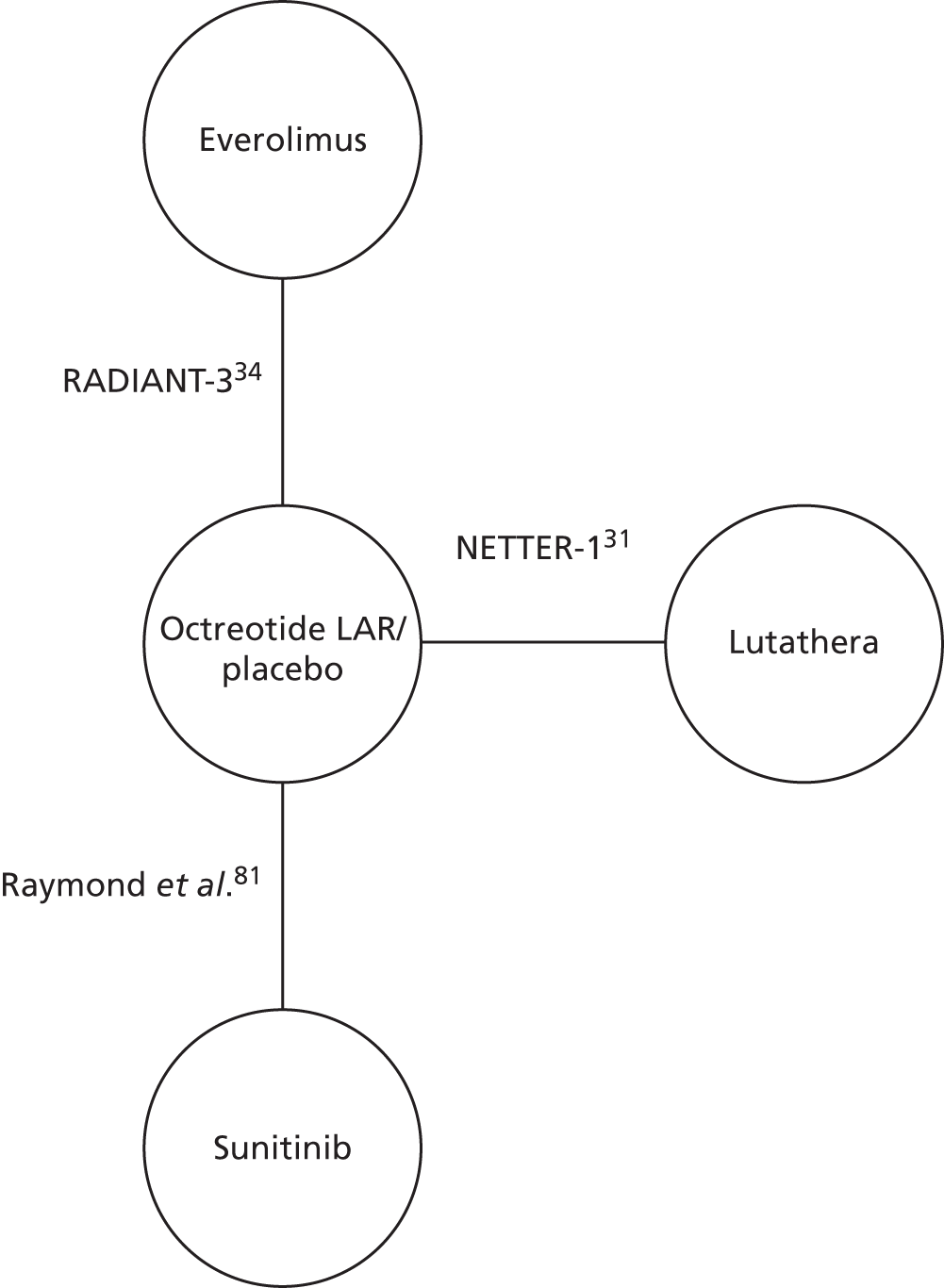
FIGURE 36.
Indirect comparison network for PFS and OS in GI NETs. Source: figure 13 (pp. 71–2) of the AAA submission. 31
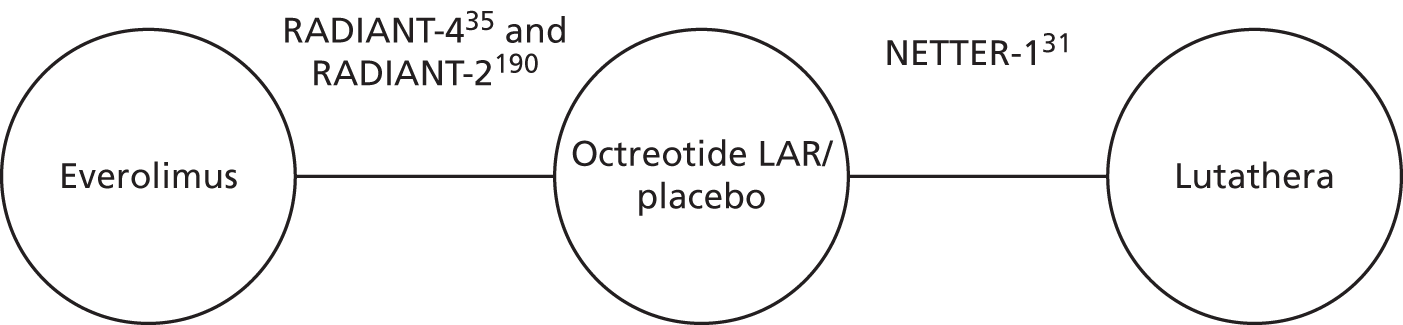
Advanced Accelerator Applications made three major assumptions in performing its MTC. These were that:
-
60 mg of octreotide can be assumed to be equivalent to placebo and placebo plus 30 mg of octreotide (in order to connect NETTER-1 to the other trials) in the GI NETs network
-
60 mg of octreotide is equivalent to placebo and placebo plus BSC (in order to connect NETTER-1 to the other trials) in the pNETs network
-
data from the NETTER-1 trial can be used to inform the network for pNETs even though no participants within the NETTER-1 trial had pNETs.
Advanced Accelerator Applications undertook a Bayesian analysis with Markov chain Monte Carlo (MCMC) simulation in R. It ran fixed- and random-effects models with HRs as response variables, using the poisson/log model and the binomial/cloglog model. AAA reported assessing convergence using trace plots, autocorrelations and ‘other standard convergence diagnostics’ (AAA submission, p. 21031), but did not state explicitly whether or not convergence was achieved in the models.
The results of the MTCs are shown in Tables 78 and 79.
| Intervention | PFS | OS |
|---|---|---|
| 177Lu-DOTATATE vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| Sunitinib vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DoTATATE vs. everolimus | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE vs. sunitinib | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. sunitinib | Confidential information has been removed | Confidential information has been removed |
| Intervention | PFS | OS |
|---|---|---|
| 177Lu-DOTATATE vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE vs. everolimus | Confidential information has been removed | Confidential information has been removed |
We have a number of reservations regarding the MTCs conducted by AAA:
-
RADIANT-2190 should be excluded from the MTC for GI NETs as the population in this trial all have functioning tumours, which is outside the marketing licence for everolimus in GI NETs.
-
NETTER-1 should be excluded from the pNETs network as this trial did not include any patients with pNETs
-
For the evaluation of GI NETs, the populations included for OS differ across the three studies (midgut NETs in NETTER-1, all NETs in RADIANT-2 and GI and lung-NETs in RADIANT-435).
-
There is no justification for the assumption that 60 mg of octreotide LAR is equivalent to placebo, placebo plus 30 mg of octreotide and placebo plus BSC.
-
There is no consideration of the extent of treatment switching within RADIANT-2190 (58% switched to active treatment), RADIANT-334 (73% switched to active treatment) and A618111181 (69% switched to active treatment), which limits the interpretation of the results for OS.
-
The 95% credibility intervals (CrIs) are very wide, indicating a great deal of uncertainty.
-
Results from the random-effects Poisson model and the fixed- and random-effects binomial model are not reported in the submission and so no comparison of any differences in point estimates or 95% CrIs between these models can be made.
For the non-randomised evidence, AAA identified four single-arm non-RCTs,145,162–165,170 yet focused on the Dutch subset of the ERASMUS study. 162–164 This is a single-centre Phase I/II open-label study of Dutch participants with GI NETs and pNETs (n = 810) administered 177Lu-DOTATATE. The primary outcomes of ORR, median PFS and OS by location are shown in Table 80.
| Outcome | Midgut | Hindgut | Foregut | Pancreas |
|---|---|---|---|---|
| ORR, % (95% CI) | 34 (28 to 41) | 46 (19 to 73) | 50 (22 to 78) | 56 (48 to 65) |
| Median PFS, months (95% CI) | 29.6 (24.8 to 34.4) | 29.3 (22.3 to 39.0) | NR | 30.5 (24.9 to 36.2) |
| Median OS, months (95% CI) | 55.4 (49.8 to 70.1) | NR | NR | 70.8 (63.2 to ND) |
Review of the economic models and their results in the submission
The submission details a systematic review of the economic literature, which identified 11 cost-effectiveness studies relevant to the decision problem.
Individual search strategies were developed for the following databases:
-
MEDLINE (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations and Daily Update (OvidSP)
-
EMBASE (OvidSP)
-
The Cochrane Library (Wiley): Cochrane Database of Systematic Reviews (CDSR), CENTRAL, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment database and NHS EED.
-
EconLit
The electronic database search was supplemented by hand searching the Cost-effectiveness Analysis (CEA) Registry, NICE HTA, PBAC (Pharmaceutical Benefits Advisory Committee) and CADTH (Canadian Agency for Drugs and Technologies in Health) submissions and conference proceedings.
In total, 597 records were retrieved, and after deduplication 533 individual titles and abstracts were screened; 16 records were retrieved for full review and 11 of these met the criteria for final inclusion. Three were from the UK; 10 were cost-effectiveness analysis studies and one was a budget impact study. All 11122,126,127,144,191–194 were assessed as being of good quality.
Seven cost-effectiveness analysis studies compared sunitinib and BSC with BSC; two compared octreotide and BSC with BSC; one compared everolimus with chemotherapy; and one compared everolimus with sunitinib.
Nine papers evaluated people with pNETs, one evaluated those with carcinoid syndrome and VIPoma and one evaluated GI (midgut) NETs.
The methodology used in the company’s systematic review of the economic literature was sound and comprehensive. However, the company did not include a description of the results or discuss the strengths or limitations of their review, nor were the findings of the included studies discussed alongside the findings of the company’s original economic evaluations.
Data and methods
Efficacy and effectiveness data used in the model
Baseline rates of progression and OS were estimated using Weibull parametric extrapolations of Kaplan–Meier curves from individual patient data, fitted using ordinary least squares regression methods. The Weibull function was selected based on goodness of fit using the AIC and BIC, combined with clinical plausibility on visual inspection.
A partitioned survival model was implemented in Microsoft Excel with Weibull coefficients for PFS and OS generated in Stata® (StataCorp LP, College Station, TX, USA) for each comparator.
The pNETs evaluation used the progressive pNETs subgroup of the Dutch population of patients treated with 177Lu-DOTATATE in the ERASMUS study for baseline risk (number at risk: PFS, n = 80; OS, n = 87; median PFS 35.6 months; median OS 80.7 months).
The GI NETs evaluation used patients treated with octreotide LAR in the NETTER-1 study for baseline risk (number at risk: PFS, n = 106; OS, n = 113; median PFS 8.4 months; median OS not reached).
Under an assumption of proportional hazards, HRs generated from the MTC were applied to the baseline survival curves (Table 81). Background mortality was not included in the base-case analysis but was included in scenario analysis.
| Intervention | PFS (95% CI) | OS (95% CI) |
|---|---|---|
| Everolimus pNETs vs. placebo | Confidential information has been removed | Confidential information has been removed |
| Sunitinib pNETs vs. placebo | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE GI NETs vs. placebo | Confidential information has been removed | Confidential information has been removed |
| Everolimus GI NETs vs. placebo | Confidential information has been removed | Confidential information has been removed |
Adverse events
Serious adverse events (grades 3 and 4) were incorporated into the model using incidence data from clinical trials (Table 82). For each treatment an AE profile was developed whereby each event, when appropriate, carried a cost of management and an associated utility decrement.
| SAE | 177Lu-DOTATATE (NETTER-1187) | Everolimus (pNETs) (RADIANT-334) | Sunitinib pNETs81 | Everolimus (GI NETs) (RADIANT-435) |
|---|---|---|---|---|
| Nausea | 4 | 2 | 1 | 2 |
| Vomiting | 7 | |||
| Diarrhoea | 3 | 3 | 5 | 7 |
| Abdominal pain | 3 | 5 | ||
| Thrombocytopenia | 2 | 4 | 4 | |
| Lymphopenia | 9 | |||
| Leukopenia | 1 | |||
| Stomatitis | 7 | 4 | 9 | |
| Flushing | 1 | |||
| Fatigue | 2 | 2 | 5 | 3 |
| Infections | 2 | 9 | ||
| Asthenia | 1 | 5 | 2 | |
| Anaemia | 6 | 4 | ||
| Pyrexia | 2 | |||
| Hyperglycaemia | 5 | 3 | ||
| Neutropenia | 12 | |||
| Hypertension | 10 | |||
| Musculoskeletal pain | 2 |
Treatment-specific adjustments to baseline PFS utility were calculated using decrements weighted according to event incidence in the trials (Table 83).
| Intervention | Utility decrement weighting for PFS |
|---|---|
| 177Lu-DOTATATE (pNETs) | 0.9725 |
| Everolimus (pNETs) | 0.9649 |
| Sunitinib (pNETs) | 0.9432 |
| Everolimus (GI NETs) | 0.9560 |
Decrements for AEs were applied to every cycle for which patients remained progression free, including post treatment for those patients administered 177Lu-DOTATATE.
The utility decrement weightings for SAEs followed the trend advised by the AG expert clinicians: BSC > 177Lu-DOTATATE > everolimus > sunitinib. 177Lu-DOTATATE dose adjustment as a result of AEs or missed treatments in NETTER-1 was incorporated into the costing of 177Lu-DOTATATE in the base case by applying a RDI reduction in mean drug acquisition cost. No such adjustment was made for everolimus or sunitinib. AE utility decrements were applied to all treatment strategies while patients were progression free. As these decrements should be applied to the treatment period only, this approach overestimates the period of disutility from AEs. Although the disutility penalty is greatest for everolimus and sunitinib, the mean duration of treatment of 177Lu-DOTATAE is less than that of everolimus and sunitinib in practice. In any case, the ICERs are insensitive to this limitation.
Utility values
A systematic literature search was conducted for relevant published HRQoL papers. Pragmatic searches were conducted for HRQoL mapping algorithms and utility decrements for SAEs. HRQoL scores were collected for stable and progressive disease in the base case from pNETs/GI NETs patients administered 177Lu-DOTATATE in the ERASMUS study (Table 84). However, the exception is the base-case estimate of stable disease in GI NETs, for which the estimate was sourced from a UK registry. The company did not explain why this approach was adopted.
| Site | PFS | PPS |
|---|---|---|
| pNETs | 0.80a (95% CI 0.79 to 0.81) | 0.79a (95% CI 0.76 to 0.82) |
| GI NETs | 0.79b (95% CI 0.77 to 0.82) | 0.74a (95% CI 0.72 to 0.76) |
Patients in the ERAMUS trial were Dutch and the number of participants and their characteristics were not stated. Responses were collected every 12 weeks from first treatment to 72 weeks. HRQoL scores from the EROTC QLQ-C30 questionnaire used in the ERASMUS trial were mapped to EQ-5D scores. 134 HRQoL scores from registry patients at Guy’s and St Thomas’ NHS Foundation Trust (UK) were attained directly using the EQ-5D questionnaire (58 patients; 57% male; 94% white; mean age 60 years). Overall, the synthesis of health state utility estimates is potentially weak because of the use of multiple sources, multiple quality-of-life assessment tools and cohorts from single-arm studies/registries rather than RCTs.
Adverse events were not modelled using an additional health state; instead, disutilities were applied to the baseline PFS estimates according to the incidence of AEs in the trial of each treatment (see Adverse events). Utility decrements were estimated for 19 event types, although 12 of these were assumptions based on the remaining seven for which sources were found. In nine cases the decrement was assumed to be equal to the worst value, which was incorrectly selected as 0.11 (thrombocytopenia) rather than 0.2 (fatigue). No adjustment were applied to utility accrual in the base case for end-of-life well-being; this was included in a univariate deterministic sensitivity analysis.
Costs
The included cost categories were drug acquisition, drug administration, disease monitoring and AE management. No provision was made for any hospitalisation of patients owing to deterioration of their condition. Patients with stable disease received continual drug treatment until the point of progression, except for the 177Lu-DOTATATE strategy, whereby therapy was limited to four treatment cycles. All patients received and incurred the cost of octreotide (30 mg) following progression and until death.
Drug acquisition prices and posology were sourced from the BNF36 (everolimus, sunitinib and octreotide), except for 177Lu-DOTATATE, for which information was supplied by AAA (Table 85).
| Treatment | Dose and frequency | Unit cost in base case |
|---|---|---|
| 177Lu-DOTATATE | Four administrations of 7.4 GBq (200 mCi), administered once every 8 weeks | 29.6 GBq (800 mCi) = (confidential information has been removed) |
| Everolimus | 10 mg administered once daily | 30 tablets, 10-mg pack = £2673 |
| Sunitinib | 37.5 mg administered once daily | 30 tablets, 12.5-mg pack = £784.70 |
| Granisetrona | 3 mg administered before administering 177Lu-DOTATATE | 10 tablets, 1-mg pack = £32.89 |
| Vamin 18 – 18%a | Given before administering 177Lu-DOTATATE | Vamin 18 (electrolyte free), net price 1 litre = £23.38 |
Drug prices were incorporated into the base case at the NHS list price; no discount or PAS prices were explored in sensitivity analyses. A RDI adjustment to reflect downwards dose modifications or skipped doses observed in the NETTER-1 trial (0.864) was applied to the acquisition cost of 177Lu-DOTATATE. No equivalent adjustments were made for everolimus or sunitinib. This reduced the cost of the 177Lu-DOTATATE strategy, which decreased the ICERs compared with everolimus/sunitinib.
The cost of 177Lu-DOTATATE administration was based on 15 minutes of pharmacist time, 1 hour of day ward nursing time and a 4-hour (one-third) day case attendance. This is inconsistent with the company’s statement in chapter 2 of its submission in which the involvement of nuclear medicine department resources are anticipated. It is also inconsistent with expert clinical opinion that we received, which indicated that specialist involvement and admission with an overnight stay is routine.
Everolimus and sunitinib are self-administered orally and therefore attracted a zero administration cost.
The resource utilisation for monitoring of disease was assumed to be the same for all treatment strategies. The unit costs are shown in Table 86.
| Resource use31 | Frequency31 | Unit cost in base case (£)137 |
|---|---|---|
| CT/MRI | Every 12 weeks | 124.10 |
| ECG | Every 8 weeks | 83.94 |
| CBC with differential | Every 4 weeks | 3.00 |
| Blood chemistry | Every 4 weeks | 3.00 |
| Urinalysis | Every 4 weeks | 1.19 |
Monitoring costs at baseline (screening) were not included because these costs are not influenced by choice of treatment and apply to all patients.
The unit costs of treatment were included for 18 separate SAE types. Seven were dedicated estimates taken from standard literature sources; eight were arbitrarily assigned a cost of £1, based on the presumption that the event would have little impact on NHS resources; and three were assumed to be equal to the cost of the highest cost event (stomatitis, £385.17). The cost of managing AEs was included for every cycle in stable disease, rather than while on treatment. Note the earlier assumption that everolimus and sunitinib treatment are continued until progression in all cases.
Additional costs relating to end-of-life care or palliative care were not included in the base-case analysis but were included in univariate deterministic sensitivity analysis.
The price year used in the analysis was not stated but it may reasonably be assumed to be 2015.
Appendix 9 Reference case
| Element of health technology assessment | Reference case | Novartis33 (pNETs) | Novartis33 (GI and lung NETs) | AAA31 (pNETs) | AAA31 (GI NETs) |
|---|---|---|---|---|---|
| Defining the decision problem | The scope developed by NICE | Yes | Yes | Yes | Yes |
| Comparator(s) | As listed in the scope developed by NICE | Yes | Yes | Yes | Yes |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | Yes | Yes | Yes | Yes |
| Perspective on costs | NHS and PSS | Yes | Yes | Yes | Yes |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes | Yes | Yes | Yes |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | Yes | Yes | Yes | Yes |
| Synthesis of evidence on health effects | Based on systematic review | Yes | Yes | Yes | Yes |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes | Yes | Yes | Yes |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | No | Yes | Yes | Yes |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Yes | Yes | Yes | Yes |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes | Yes | Yes | Yes |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | Yes | Yes | Yes | Yes |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | Yes | Yes | Yes | Yes |
Appendix 10 Review of cost-effectiveness evidence
The purpose of this appendix is to review existing evidence on the cost-effectiveness of sunitinib, everolimus and lutetium relative to chemotherapy or BSC in patients with unresectable or metastatic, progressive NETs.
Methods
Searches
Bibliographic literature searching was conducted on 19 May 2016 and forward citation searching was completed on 17 August 2016. The searches took the following form: (terms for neuroendocrine or pancreatic or GI or lung) AND (metastatic or unresectable or advanced) AND (terms for the interventions under review) AND (a costs or economic literature search filter). The searches were not date limited, were not limited by language and were not limited to human-only studies.
The following databases were searched: MEDLINE (Ovid), EMBASE (Ovid), NHS EED (Wiley Online Library), Web of Science (ISI – including conference proceedings) and EconLit (EBSCOhost). The search strategies are recorded in Appendix 1.
Screening
Inclusion and exclusion criteria were the same as for the clinical effectiveness systematic review (see Chapter 4, Inclusion and exclusion criteria), with the following exceptions (as specified in the appraisal protocol):
-
non-randomised studies were included (e.g. decision model-based analyses or analyses of patient-level cost and effectiveness data alongside observational studies)
-
full cost-effectiveness analyses, cost–utility analyses and cost–benefit analyses were included (economic evaluations that report only average cost-effectiveness ratios were included only if the incremental ratios could be easily calculated from the published data)
-
studies that measure only costs but not health benefits were excluded, except for stand-alone cost analyses from the perspective of the UK NHS.
Titles and abstracts were screened for relevance by two reviewers (RMM and IT), with disagreements resolved by discussion. Full texts were retrieved for references judged to be relevant and were screened for eligibility by the same reviewers, with disagreements resolved by discussion.
The bibliographies of included studies and review articles, which were not judged to be eligible for inclusion, were examined by one reviewer (RMM) to identify other potentially relevant references. These references were retrieved and checked for eligibility in the same way as for full texts identified from the database searches.
Quality assessment
Studies meeting the criteria for inclusion were assessed by one reviewer (RMM) using the checklist developed by Evers et al. 195 This checklist is intended as a criteria list for the quality assessment of economic evaluations in systematic reviews, thereby making these reviews transparent, informative and comparable. Studies based on decision models were further quality assessed using the checklist developed by Philips et al. 196
Synthesis
Economic studies were summarised and synthesised using tabulated data and narrative synthesis. The narrative synthesis described the major contributions of reviewed studies to the economic evidence on targeted treatments in patients with advanced, progressive NET and the methodological strengths and weaknesses. The findings were summarised with reference to the elements of the NICE reference case. 37
Results
Identified studies
The electronic database search for cost-effectiveness evidence identified 1143 records; six additional records were identified by other means. After deduplication, 896 records remained, all of which were screened by title and abstract. Of these, 30 full texts were assessed for eligibility. Eight of these were deemed to meet the eligibility criteria for the review. The study selection process is detailed in Figure 37.
FIGURE 37.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
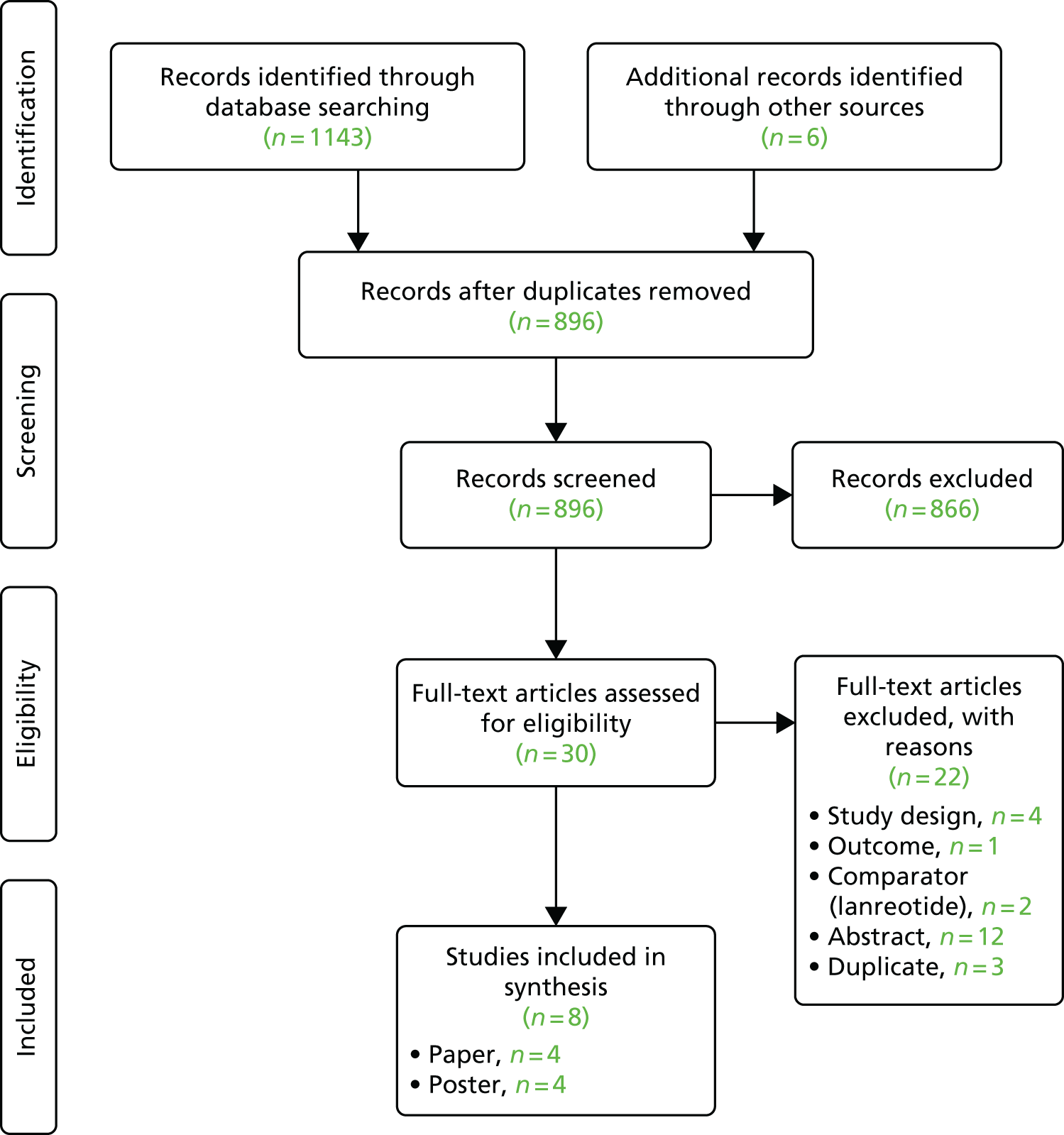
Four of the eight full texts were journal articles and the remaining four were posters presented at conferences. Three120,121,123 of the four articles were full economic evaluations and were included in the review. The remaining article197 was an analysis of the costs of administration of lanreotide and octreotide; because of the limited scope of this study, and as the revision of the NICE scope removed these two treatment options from the present technology assessment review, this study was excluded from the review. Of the four identified conference poster presentations, one described the only study conducted in the UK122 and provided detailed methods and results and was therefore included in the review; another reported limited methodological information and results and was conducted in the Portuguese health-care setting198 and was therefore excluded. The remaining two posters were evaluations of lanreotide and octreotide118,199 and were therefore excluded. Given the limited evidence found, and as no recent conference abstracts were found that reported economic evaluations, update searches were not conducted.
The three included studies reported in peer-reviewed journal article form were evaluations of treatments for pNETs. One study was an evaluation of sunitinib compared with everolimus in the US health-care setting,120 one was an evaluation of sunitinib compared with BSC in the Mexican health-care system121 and one was an evaluation of sunitinib compared with BSC in the Polish health-care system. The included study presented as a conference poster was an evaluation of sunitinib compared with BSC for pNETs patients in Scotland and Wales. 122 One of the excluded posters was the only report found, in poster or journal article form, that related to an economic evaluation of treatments for GI NETs. 118
Table 88 describes the characteristics of the included studies. All studies were sponsored by the industry or co-authored by an individual affiliated with a company manufacturing or commercialising one of the evaluated treatments.
| Author | Country | Regimens | Population | Study type | Perspective | Outcomes considered | Horizon | Model based? | Sponsor |
|---|---|---|---|---|---|---|---|---|---|
| Casciano 2012120 | USA | Sunitinib vs. everolimus | Advanced (i.e. unresectable and/or metastatic) progressive pNETs (mean years) | Cost–utility analysis | Hospital | Total health-care costs per patient, cost per QALY gained | 20 years | Yes | Funded by Novartis |
| Muciño Ortega 2012121 | Mexico | Sunitinib with BSC vs. BSC only | Advanced well-differentiated pNETs | Cost–utility analysis | Mexican (Public) Social Health Insurance Institution, IMSS | Total health-care costs per patient, cost per QALY gained | 10 years | Yes | Funded by Pfizer |
| Johns 2012122 | UK | Sunitinib with BSC vs. BSC only | Advanced pNETs | Cost–utility analysis | NHS | Total care costs, cost per QALY gained | 10 years | Yes | Funded by Pfizer |
| Walczak 2012123 | Poland | Sunitinib with BSC vs. BSC only | Advanced well-differentiated pNETs with disease progression | Cost–utility analysis | Public payer for health services, i.e. PNHF; additional analysis presented from the perspective of PNHF and the patient | Total health-care costs per patient, cost per QALY gained | Lifetime | Yes | Funded by Pfizer |
Pancreatic studies
Summary characteristics of the included studies are presented in Table 89. The main results of these studies are presented in Table 90. The critique of each of these studies is guided by the quality assessment reported in Table 91.
| Study | Population | Comparators | Horizon | Model structure | Health states/events modelled | Utilities | Costs | Key individual parameters (sensitivity analysis) | Source of effectiveness parameters | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Casciano 2012120 | Advanced pNETs, USA | Everolimus vs. sunitinib | 20 years | Semi-Markov – partitioned survival with monthly cycles. Proportional hazards model of PFS and OS with baseline Weibull form | SD without AEs, SD with AEs, PD, death | SD with no AEs, SD with AEs: everolimus, SD with AEs: sunitinib, PD, death (0) | Drug acquisition and administration. For each health state (entry and follow-up states): symptomatic care, procedures/tests, physician visits and hospitalisations. Also post-progression treatments and death (end-of-life care) | PFS HR, active treatment dose intensity, post-progression treatment costs, AE costs | HR: MAIC of HRs of (updated) A618111181 (sunitinib) vs. RADIANT-334 (everolimus) outcome data124 |
|
| Muciño Ortega 2012121 | Advanced well-differentiated pNETs, Mexico | Sunitinib + BSC vs. BSC | 10 years |
Markov – partitioned survival with 2-weekly cycles Proportional hazards model of PFS and OS with baseline Weibull form |
SD, PD, death | Treatment-specific SD values from mapping EORTC-QLQ-C30 data from A618111181,123 into EQ-5D scores with the McKenzie and van der Pol132 algorithm | Drug acquisition and AE management. For each health state: procedures/tests, physician visits and palliative care. The source of resource use data was a survey of 15 oncologists in public hospitals in four different cities | HR PFS, HR OS, cycle costs of routine care before progression, acquisition costs of sunitinib per cycle, utility of post progression | HRs for OS and PFS from A618111181 |
|
| Johns 2012122 | Advanced or metastatic pNETs | Sunitinib + BSC vs. BSC | 10 years (described as ‘lifetime’) | Markov – partitioned survival. Proportional hazards model of PFS and OS with baseline Weibull form | SD, PD, death | Treatment-specific SD values from mapping EORTC-QLQ-C30 data from A618111181 into EQ-5D scores using the McKenzie and van der Pol132 algorithm | Drug acquisition, grade 3/4 AE management, BSC patient management, outpatient visits, CT scans and end-of-life care | OS: ITT vs. crossover adjusted, utility PD, sunitinib drug acquisition, HR PFS | HRs from (updated) A618111181 (sunitinib) |
|
| Walczak 2012123 | Unresectable or metastatic, well-differentiated pNETs with disease progression, Poland | Sunitinib + BSC vs. BSC | Lifetime |
Markov – partitioned survival with 4 weekly cycles Proportional hazards model of PFS and OS with baseline Weibull form |
SD, PD, death | Treatment-specific SD values from mapping EORTC-QLQ-C30 data from A618111181 into EQ-5D scores | Direct medical costs: sunitinib, the administration of the drug, diagnostic and monitoring costs, SSAs, BSC, grade 3/4 severe AEs, palliative care and end-of-life care | Sunitinib treatment duration | HRs for OS and PFS from A618111181 (data at 2009 cut-off) |
|
| Study | Regiments compared | Patient characteristics | Time horizon | PFS (years) | Life-years (un-discounted) | Mean treatment duration (months) | Discounted incremental QALYs | Discounted incremental costs | ICER | Notes on ICER |
|---|---|---|---|---|---|---|---|---|---|---|
| Casciano 2012120 | Everolimus vs. Sunitinib | As in A618111181 (sunitinib) based on MAIC | 20 years | Everolimus 1.196; sunitinib 1.043 | Everolimus 3.29; sunitinib 2.85 | Everolimus 11.896; sunitinib 10.967 | 0.304 | US$12,673 | US$41,702 |
Costs, QALYs and ICERs are discounted at a 3% annual rate and prices are in 2010 US$ Was treatment duration also based on MAIC? |
| Muciño Ortega 2012121 | Sunitinib + BSC vs. BSC | As in A618111181 | 10 years | Sunitinib + BSC 1.02; BSC 0.52 | Sunitinib + BSC 2.76; BSC 1.58 (discounted) | NR | 0.70 | US$20,854 | US$29,807 | Costs, QALYs and ICER are discounted at 5% annual rate and prices are in 2011 US dollars |
| Johns 2012122 | Sunitinib + BSC vs. BSC | As in A618111181 | 10 years | TTP: sunitinib + BSC 1.10; BSC 0.57 (discounted) | Sunitinib + BSC 3.49; BSC 1.16 (discounted) | NR | 1.39 | £31,416 | £22,587 | Costs, QALYs and ICER are discounted at 3.5% annual rate and prices are in 2010 British pounds |
| Walczak 2012123 | Sunitinib + BSC vs. BSC | As in A618111181 | Lifetime | NR | NR | NR | 0.98 | €21,770 | €20,441 | Costs are discounted at 5% and QALYs are discounted at 3.5%. Prices according to the Polish National Health Fund regulations applicable in 2012 (exchange rate to euros from 2011). Median duration of drug use, accounting for discontinuation as a result of an AE, disease progression and death, was used to estimate the cost of sunitinib and SSAs |
| Criteria | Casciano 2012120 | Muciño Ortega 2012121 | Walczak 2014123 | Johns 2012126 | Ayaggari 2016118 |
|---|---|---|---|---|---|
| pNETs | pNETs | pNETs | pNETs | GI NETs | |
| 1. Is the study population clearly described? | No | Yes | Yes | Yes | Yes |
| 2. Are competing alternatives clearly described? | Yes | Yes | Yes | Yes | No |
| 3. Is a well-defined research question posed in an answerable form? | Yes | Yes | Yes | Yes | Yes |
| 4. Is the economic study design appropriate to the stated objective? | No | Yes | No | No | Yes |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | No | No | No | Yes | Yes |
| 6. Is the actual perspective chosen appropriate? | Yes | Yes | Yes | Yes | Yes |
| 7. Are all important and relevant costs for each alternative identified? | No | No | Yes | No | No |
| 8. Are all costs measured appropriately in physical units? | Yes | No | No | No | Yes |
| 9. Are costs valued appropriately? | Yes | Yes | Yes | No | Yes |
| 10. Are all important and relevant outcomes for each alternative identified? | No | No | No | No | No |
| 11. Are all outcomes measured appropriately? | No | No | No | No | No |
| 12. Are outcomes valued appropriately? | No | Yes | No | No | No |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | No | Yes | Yes | Yes |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | Yes | No | Yes | Yes |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes | Yes | Yes | No | Yes |
| 16. Do the conclusions follow from the data reported? | Yes | Yes | Yes | Yes | Yes |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | No | No | No | No |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | No | No | No | No | No |
| 19. Are ethical and distributional issues discussed appropriately? | No | No | No | No | No |
Casciano et al.120
The only study comparing targeted therapies evaluated everolimus and sunitinib in patients with advanced (unresectable or metastatic) progressive pNETs from a US health insurer perspective. In the absence of a head-to-head study of the two treatments, this study was based on the results of a previous indirect comparison of AE, PFS and OS outcomes with everolimus and sunitinib across their respective pivotal Phase III trials. 124 Data on outcomes with everolimus relative to placebo were those reported in RADIANT-3,34 whereas the sunitinib outcomes were obtained from A6181111. 81
The analysis modelled the experience of a cohort of patients who receive either everolimus plus BSC or sunitinib plus BSC, from the start of treatment until 20 years post treatment. Patients were assumed to be in an initial stable disease health state where they could remain until death or they experienced disease progression and moved to a deteriorated health state, progressive disease, with higher costs and lower utility values. In turn, those who experienced disease progression would, according to the model, remain there until death. This model was implemented as a semi-Markov model in which patients could move between the three health states (stable disease, progressive disease and death) at discrete time points every month. In each of these monthly cycles patients would accumulate costs and utilities specific to the health state; different costs and utilities were accumulated for stable disease between the two initial treatments (sunitinib and everolimus), whereas costs and utilities for progressive disease and death were the same under the two treatments (death incurred costs and utilities of zero). The study reported that four health states were used; however, two of these were stable disease states that were differentiated only by the presence or absence of AEs, which did not affect the transition probabilities to the other health states (progressive disease and death) but only the costs and utilities associated with the cycle. As the rate of AEs varied with each cycle, in effect this was a Markov model with three health states, with variable costs and utility pay-offs for the stable disease state.
The transition probabilities across states with each successive cycle were derived by partitioning survival into OS time and survival time free from disease progression. To estimate the PFS and OS curves for each treatment, the MAIC method was used. 124 In this application, this consisted of weighting individual patient data from one placebo-controlled trial (i.e. RADIANT-334 trial of everolimus) to match the distribution of summary baseline characteristics in the other trial (A618111181 trial of sunitinib, for which no individual patient data were available to the analysts). The resulting weighted placebo-controlled HRs for PFS and OS were applied to Weibull parametric curves of PFS and OS data from the everolimus arm of RADIANT-3. 34 For AEs, cycle-specific event rates were derived from the observed grade 3/4 AE rates for each successive cycle in the everolimus arm of RADIANT-3,34 scaled by the overall ratio of pre- to post-weighted rates of grade 3/4 AEs with everolimus and the ratio of sunitinib event rates to MAIC-weighted everolimus rates.
Data on resource utilisation were obtained from a survey of physicians with experience in the treatment of NETs in the USA, who were asked about the experiences of a total of 40 patients recently treated by them. 120 The survey differentiated between a baseline stable disease phase, the period following a first disease progression and the period after a second progression. Data collected covered actual patient management in the baseline and first post-progression periods, which were taken to reflect the stable disease health state in the model (as the patient population was defined as having advanced, progressive NETs), whereas the second progression period, which was assumed to correspond to the progressive disease heath state of the model, was mostly based on hypothetical treatment scenarios. 120
Drug acquisition costs for 10 mg/day of everolimus and 37.5 mg/day of sunitinib, which were given in RADIANT-334 and A618111181 until disease progression or dose reduction or discontinuation because of intolerance, were adjusted for dose intensities of 85.9% and 91.3% respectively. Other costs were related to BSC, which included SSA, physician visits, imaging and laboratory tests, hospitalised treatment for grade 3/4 AEs, post-progression therapy and end-of-life care.
Health state utility values were obtained from a time trade-off preference elicitation study in heathy individuals of health state descriptors (vignettes) constructed by physicians for the purpose of this economic model evaluation. Values for stable disease and progressive disease were elicited as well as disutilities of a selected number of AEs. 131 This was used to calculate a stable disease utility value constituted by an AE-free utility value common to both treatments from which a weighted average of disutilities according to their AE profiles in RADIANT-334 and A618111181 was subtracted, as well as a progressive disease utility value common to both treatment arms of the model.
Treatment with everolimus and sunitinib resulted in a mean PFS duration of 1.19 and 1.04 years, respectively (0.15-year difference), and 3.30 and 2.85 life-years, respectively (0.45-year difference). Everolimus increased the number of annually discounted (at 3%) QALYs relative to sunitinib by 0.304 and increased the discounted (at 3%) health-care costs by US$12,673 (in 2014 prices, purchasing power-adjusted price £9276) per patient, corresponding to a cost per QALY gained of US$41,702 (£30,524).
The results were most sensitive to the PFS HR, treatment dose intensity, costs of progressive disease and AE costs. PSA revealed a 69% probability that the ICER for everolimus was < US$100,000 and that the 95% CI ellipsoid covered all four possible combinations of outcomes (i.e. everolimus: increased costs and increased QALYs, decreased costs and increased QALYs, increased costs and decreased QALYs and decreased costs and decreased QALYs). Therefore, the study was inconclusive, although the authors argue that these results suggest that everolimus is cost-effective.
Critique
This study provided evidence on the costs and health benefits of choosing one of two targeted therapies available to treat advanced, progressive pNETs. It provides a comprehensive account of uncertainty in the available evidence, which emerges primarily from the fact that no direct head-to-head comparative studies of the two treatments exist and that, given the rare nature of the disease and treatment practice heterogeneity, standard methods of indirect comparison (e.g. Bucher et al. 41) are likely to lead to biased results. The results of this study thus suggest that any comparison between the two treatments is unlikely to lead to conclusive results and that measurement of cost and utility differences is crucial for informing treatment choice in this patient population.
The main limitation of this study is the omission of a BSC arm from the analysis. Another key limitation is the source of utility data, which were derived from time trade-off valuations of health state vignettes formulated by clinical experts as opposed to being derived from HRQoL measurements of patient-reported outcomes. Although the authors may have felt justified in their chose of values by the fact that their source of effectiveness data for everolimus, RADIANT-3,34 did not collect such HRQoL patient-reported outcomes, they could have employed the HRQoL outcomes reported in the A618111181 trial of sunitinib, with mapping algorithms used to derive EQ-5D values. Finally, the authors acknowledged the lack of adequate resource utilisation data for progressive disease.
Muciño Ortega et al.121
A study in Mexico compared sunitinib plus BSC with BSC alone, using the data from A6181111. 81 The study used the same three-state Markov model structure as in the study by Casciano et al. 120 but used a 2-week instead of a 4-week cycle length to model the costs and QALYs of each treatment over a 10-year period. The study adopted the perspective of the Mexican public health insurance system covering people with a current or past history of formal employment. At least one of the co-authors was affiliated to Pfizer, the sponsor of sunitinib.
The effectiveness data to populate the model parameters were obtained from a time-to-event analysis using Weibull parametric models of PFS and OS data in A6181111. 81 The relative treatment effects of sunitinib were estimated relative to these models as proportional hazards. AE rates in the model were obtained from the same trial.
The analysis included the costs of drug acquisition and medical management, including specialist consultations, laboratory and imaging tests, pain management and palliative care. The resource utilisation data were obtained from a survey of 15 clinical oncologists from institutions located in four large cities in the country. The unit costs were obtained from government procurement tariffs and public health insurer institution costs of services at the tertiary level.
Health state utility values were obtained from analysis of data collected in A6181111,81 using the EORTC QLQ-C30. Such data were mapped onto EQ-5D scores using the linear algorithm of McKenzie and van der Pol. 132 The mapped EQ-5D scores were then analysed in a linear mixed model with covariates for treatment allocation, cycle number and baseline mapped EQ-5D value.
The study found that sunitinib plus BSC resulted in an extra 1.18 (discounted) life-years over BSC alone and 0.70 extra (discounted) QALYs. Sunitinib plus BSC was also more expensive than BSC alone by US$20,854 (£12,925) in 2011 prices (£13,410 reflated to 2015 prices; adjusted for purchasing power parity £22,977). The corresponding incremental cost per QALY gained was US$29,808 (£18,475; £19,168 in 2015 prices; adjusted for purchasing power parity £32,842). The parameters that had the most influence on these results, in order of importance, were routine medical management costs before progression, the HR for PFS, the HR for OS, sunitinib acquisition costs, the utility of the post-progression health state and routine medical management costs after disease progression.
Critique
This study’s main strengths are its clarity of reporting, in defining the population, intervention and comparators and the institutional and medical practice context within which the treatments are given. The study clearly presents the derivation of resource use and cost parameters associated with AEs reported in A618111181 and heath state utility values from patients’ responses to a disease-specific (EORTC QLQ-C30) tool mapped onto the EQ-5D.
The main limitation of the study is its analysis of OS, in which crossover to sunitinib in patients in the placebo arm was not adjusted for. Crossover occurred in 69% of patients under placebo;122 as the relevant comparison in this analysis is between a situation in which sunitinib is available and an alternative situation in which it is not, the analysis is likely to underestimate life-year and QALY gains by sunitinib over BSC (provided that sunitinib extends life and disease progression-free life). The study did not account for subsequent treatments; this information was not collected in the trial [Pfizer (through NICE), October 2016, personal communication].
Another limitation of this study is its reliance on the opinions of a panel of oncologists to obtain resource use data on medical management, which turned out to be one of the most important sources of uncertainty in the study. The study did not analyse the extent of structural uncertainty in the results; there is no report of any testing of the proportional hazards assumptions on which the results rely heavily and no sensitivity analysis was performed using different parametric functions to extrapolate OS and PFS.
It is an open question, therefore, whether or not the estimated extension of life free of disease and overall life extension found by this study of 0.49 and 1.18 years, respectively, are robust to different parametric assumptions about the distribution of time to such events.
In summary, this study provides evidence on the potential cost-effectiveness of sunitinib relative to BSC in pNETs. However, the results on costs and, consequently, cost-effectiveness may be not generalisable to the UK setting because of marked differences in the cost of medical staff inputs relative to drug acquisition costs between Mexico and the UK. Nevertheless, the study provides valuable evidence on health state utility values in pancreatic patients.
Johns et al.122
The only published cost-effectiveness analysis in the UK is a conference poster by Johns et al. 122 presenting the evidence submitted by Pfizer to the SMC in January 2011 and to the AWMSG in March 2011, which resulted in a positive recommendation for the drug as treatment for advanced pNETs in Scotland and Wales. This analysis used the most recent data at the time from the Phase III A618111181 trial of sunitinib compared with placebo to model incremental costs and QALY gains using the same methods of Muciño Ortega,121 but with the addition of an adjustment to OS outcomes in the placebo arm for the effect of crossover to sunitinib in the blinded and extension phases of the study.
It was reported that adjustment of OS data for crossover using the RPSFT model resulted in a HR at the latest cut-off date of 0.499 (95% CI 0.351 to 0.947), citing a conference abstract source. 94 However, it was reported that the cost-effectiveness analysis was based on an estimate of the RPSFT model HR of 0.24 (95% CI 0.08 to 1.07) based on an ‘intermediate data cut’ dated December 2009 and citing ‘Pfizer data on file’. 122 This analysis used a proportional hazards model for both PFS and OS time-to-event analyses, in which PFS was analysed using a Weibull parametric regression form with a binary covariate indicating the randomly allocated treatment group and in which OS in the sunitinib arm was modelled using a Weibull form and the placebo OS outcomes were obtained from applying the HR from the RPSFT to the Weibull placebo distribution. However, the Weibull parameter values used to extrapolate PFS and OS rates in the model were not reported.
It was reported that the probabilities of AEs and treatment discontinuation were obtained from A6181111. 81 Resource utilisation data for BSC and AE management, with only grade 3/4 events considered, were obtained from UK clinical expert opinion, without citing any sources. Utilities were obtained from mapping the EORTC QLQ-C30 patient data from A618111181 onto the EQ-5D using the algorithm of McKenzie and van der Pol. 132 It was reported that ‘the mean baseline utility value was 0.73 for the sunitinib plus BSC and placebo plus BSC arms’ and that the value of 0.60 used for the progressive disease phase in both arms was the mean utility value measured during the last study cycle for patients who experienced disease progression. The quoted statement is unclear about the role that the baseline utility value played in the analysis, especially as it is utility values post baseline and before progression that are relevant for estimating utility in the stable disease phase.
The study reported that treatment with sunitinib resulted in an increase in discounted PFS years, discounted life-years and discounted QALYs by 0.53, 2.33 and 1.39, respectively, whereas it resulted in incremental costs of £31,416 (in 2010 prices), almost two-thirds of which was the cost of acquisition of sunitinib. The resulting ICER was £22,587 (£24,244 at 2015 prices). Besides the use of ITT values for the OS HR as opposed to crossover-adjusted values, which was most influential, the parameters to which the results were most sensitive included the post-progression utility, sunitinib acquisition cost and the PFS HR. The authors stated that the results were robust to variations in assumptions about concomitant SSA use and parametric forms used to extrapolate OS and PFS.
Critique
The limited information available on this study prevents an assessment of its quality. Indeed, it is not possible to replicate the results with the information presented in the poster, because, for example, the Weibull parameter values used to extrapolate the PFS and OS curves in the model were not reported. It is noteworthy that this study adjusted placebo OS outcomes for crossover to sunitinib, although the reported choice of data cut-off date appears to be different from the latest cut-off date. This study suggests that adjustment for crossover from placebo to the targeted treatment in RCTs in this area may determine whether or not a treatment is cost-effective.
On the other hand, the study suffers from the omission of everolimus as a competing treatment option. The methods used to estimate utilities were not clearly presented, although results presented in the tornado sensitivity analysis appear to imply that the authors followed the methods detailed in the study by Muciño Ortega et al. 121 In common with other studies, this analysis suffers from a lack of actual resource use data, as it was based entirely on expert opinions. According to these results, the cost per QALY gained for sunitinib may be between £20,000 and £30,000.
Walczak et al.123
The same study question was investigated from a Polish health payer’s perspective in a separate study by Pfizer. 123 Walczak et al. 123 provided a detailed account of the use of systematic methods to search for effectiveness evidence on sunitinib compared with BSC, following principles in the Cochrane Collaboration and the Polish Agency for Health Technology Assessment. The search was conducted in March 2012. Only one study, A6181111,81 met the inclusion criteria, which specified the reporting of effectiveness, safety and HRQoL outcomes in a head-to-head parallel-group RCT of the two treatment options for advanced, progressive pNETs.
The Polish study adopted the same Markov and partitioned survival analysis method of modelling the costs and health outcomes as in the analysis submitted to the SMC and the AWMSG in 2011 by Pfizer and reported by Johns et al. 122 In addition, as in the study by Johns et al. ,122 the Polish analysis adopted the estimates of OS effectiveness based on an interim data cut-off date of 15 April 2009,81,84 with a HR of 0.18 (95% CI 0.06 to 0.68), as opposed to the latest estimates available at the time, with a cut-off date of June 2010,94 which resulted in a HR of 0.49 (95% CI 0.35 to 0.94) based on the RPSFT model. The (inverse) HR was applied to extrapolated OS rates obtained from a parametric Weibull function fitted to the sunitinib arm, to derive the placebo curves that would have occurred in the absence of crossover (counterfactual placebo OS rates). The authors reported that a Weibull function with a binary treatment indicator was fitted to the PFS data from A618111181 to extrapolate PFS rates in the two trial arms, and ultimately partition OS time into stable disease and progressive disease, which equals the difference between extrapolated OS and extrapolated PFS rates (i.e. mean time in progressive disease for sunitinib: 2.98 – 1.89 = 1.09 years, and for BSC: 0.54 – 0.49 = 0.05 years; see Table 9.). However, the authors reported the parameter values in Table 92, which suggests that Weibull functions were fitted to each arm separately.
| Outcome | Parameter/summary statistic | Walczak et al.123 | Casciano et al.120 | ||
|---|---|---|---|---|---|
| Sunitinib | BCS | Sunitinib | Everolimus | ||
| PFS | Shape | 0.79 | 1.16 | 1.195 | 1.195 |
| Scale | 19.89b | 6.31b | 7.27c | 6.103c | |
| Predicted mean (years) | 1.89 | 0.49 | 0.97d | 1.15d | |
| OS | Shape | 1.63 | 1.63 | 1.379 | 1.379 |
| Scale | 40.04b | 7.20b | 5.88c | 7.263c | |
| Predicted mean (years) | 2.98 | 0.54e | 2.89f | 3.57f | |
The costs of drug acquisition, drug administration, diagnosis and monitoring (including a CT scan every 2 months for the first 6 months and every 3 months thereafter until disease progression), SSA use, BSC, grade 3/4 AE management and palliative care were measured. Sunitinib and SSA treatment costs were measured using the median treatment duration, with treatment stopped because of AEs, disease progression or death. Alternatively, treatment duration until disease progression was used in a scenario analysis. A compliance rate of 91.3%, obtained from the main trial report,81 was used, with compliance defined as the number of doses administered relative to the number of planned doses at 37.5 mg daily. End-of-life care was also measured using the costs of hospice-at-home care for the last week of life.
The study found that treatment with sunitinib plus BSC produced an extra 0.98 QALYs and had an ICER of €20,441 (£33,866 at purchasing power parity in 2005 prices) per QALY gained relative to BSC only. The parameter to which the results were most sensitive was the duration of sunitinib use.
Critique
Walczak et al. 123 documented a systematic search of the RCT literature comparing sunitinib with BSC, which identified only one study, the Phase III A618111181 trial. The authors reported a detailed assessment of the quality of this study and an account of the main findings.
A strength of this study is its application of a method to adjust OS outcomes for crossover of patients in the placebo arm to the targeted therapy arm in A6181111. 81 However, the authors reported the use of an estimate of OS effectiveness based on trial data from a cut-off date of 2009 when an updated 2010 estimate, which was less favourable to the targeted treatment, was available.
In common with other economic studies funded by Pfizer and reviewed here, this study omitted a relevant comparator, everolimus. RADIANT-334 may have been identified by the systematic search had it been designed to include such targeted therapy.
As with other studies in NETs, this evaluation suffered from its reliance on a Weibull function to extrapolate PFS and OS outcomes beyond the end of the trial. For the OS analysis this methodological choice may have been determined by the need to use a parametric extrapolating function that was consistent with the proportional hazards assumption so that the available estimate of effectiveness, which was in HR form, could be adopted in the analysis. For PFS however, no such justification existed, as there was no crossover adjustment to deal with, and the authors should have provided at least a sensitivity analysis of the parametric extrapolation functions used for each arm.
There was also inadequate reporting of model inputs for the duration of sunitinib treatment and of model outputs in terms of life-years and mean PFS time.
Overall, this is the only complete report of an economic evaluation in Europe. However, given the different country setting and relative prices, and the limitations of the report itself, the evidence provided by this study is of limited value to guide decisions in a UK NHS context.
Gastrointestinal studies
The only study identified in the GI location evaluated lanreotide relative to octreotide,118 both of which are outside the NICE scope for the current assessment. For the present purposes, it is relevant that this study adopted the same three-state semi-Markov structure as the analyses in pNETs reviewed in the previous sections, using a 3-year and lifetime time horizons. This study was presented in a conference poster format and, given the limited information thus provided, was not subject to any formal critique.
Appendix 11 Subgroup and scenario analyses
Subgroup analyses
The AG did not consider any other subgroups from the NICE scope apart from patients with pancreatic, GI and lung and GI (midgut) NETs.
Scenario analyses
A range of scenario analyses were conducted, including:
-
For pNETs and GI and lung NETs, using PFS data based on local investigator assessment for everolimus instead of the PFS data from central independent review used in the base case.
-
For pNETs, using OS data from ITT analysis instead of the RPSFT-adjusted OS data used in the base case.
-
Using an alternative set of utility values presented in Chapter 6, Tables 21 and 22.
-
Using an alternative set of OS and PFS curves, allowing for the parametric form of the best-fitting survival functions to differ across arms in a given comparison: for pNETs, the parametric PFS curve under everolimus and BSC alone was changed to the log-normal and log-logistic functions, respectively, whereas OS under everolimus was altered to the log-normal function; for GI and lung NETs, PFS under everolimus and BSC alone was changed to the log-normal function, whereas OS under the two strategies was changed to the log-logistic function. All other model specifications remained as in the base case.
-
Limiting the analysis to PFS, in recognition of the uncertainty associated with OS outcomes in this clinical area that arise from the immaturity of the OS data and crossover and active subsequent treatment use.
-
A scenario analysis including first-cycle drug acquisition costs, which were omitted in the base case.
-
A scenario analysis for GI (midgut) and GI and lung NETs with different costs of disease monitoring corresponding to the numbers of physician visits adopted in the Novartis model. These were larger than our base-case values, which reflected the opinion of our clinical experts. We altered values to be between 2 and 2.6 times the base-case value in stable disease and 1.5 times the base-case value in progressive disease.
-
Applying a 0% discount to costs and benefits.
Local assessment
When we changed the PFS data for everolimus from central review data to local investigator assessment data reported in the main study publications, we found that in pNETs the ICER for everolimus increased by £18 from the base-case value of £45,493 and that the ICER for sunitinib, which was affected indirectly through the Bucher-type adjustment to its PFS, decreased from the base-case value of £20,717 to £19,586. In GI and lung NETs the ICER changed from £44,557 to £44,252 (Table 93).
| Tumour location | Treatment | Treatment or comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 45,511 |
| Sunitinib | BSC | 19,586 | |
| GI and lung | Everolimus | BSC | 44,252 |
Intention-to-treat analysis for pancreatic neuroendocrine tumours
Using the ITT data from the A618111181 and RADIANT-334 trials of pNETs produced ICERs that were three times as high for everolimus (reaching an ICER of £136,000) and twice as high for sunitinib (£37,000) as their respective base-case values (Table 94). These changes reflect the influence of adjusting for the effects on OS of crossover to the targeted treatment in the placebo arms of both trials, which occurred in 69% of placebo arm participants in the sunitinib trial and 85% of participants in the everolimus trial.
| Treatment | Treatment or comparator | ICER (£) |
|---|---|---|
| Everolimus | BSC | 136,455 |
| Sunitinib | BSC | 37,217 |
Alternative set of utility values
Increasing the utility values for stable disease in pNETs by 0.09 and keeping the values in progressive disease practically unchanged, to correspond to the values in Swinburn et al. ,200 reduced the ICER for everolimus by 10% to £41,246, as expected given the larger quantity of life lived in stable disease under the everolimus strategy than under the BSC-only strategy. Similarly, the ICER for sunitinib was reduced by 6% to £19,411 (Table 95).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 41,246 |
| Sunitinib | BSC | 19,411 | |
| GI (midgut) | Everolimus | BSC | 352,801 |
| 177Lu-DOTATATE | BSC | 57,745 | |
| GI and lung | Everolimus | BSC | 49,949 |
Utility values for everolimus in GI and lung and GI (midgut) were increased by 0.01 in stable disease and reduced by 0.01 in progressive disease, while simultaneously reducing the utilities in stable disease with BSC alone by 0.03 and increasing utility in progressive disease under the BSC alone strategy by 0.02; for GI (midgut) these changes were applied at the same time as utilities under lutetium were increased by 0.02 in both disease states. These changes increased the ICERs of everolimus by 12% in GI and lung, and 7% in GI (midgut), and decreased the ICER of lutetium by 7%.
Alternative set of overall survival and progression-free survival curves
When the parametric survival models for everolimus and BSC alone were changed from proportional hazards to AFT forms, the ICER for sunitinib in pNETs remained more or less the same, whereas that for everolimus in pNETs decreased by 33% to £28,098 (Table 96). In contrast, everolimus in GI and lung NETs became less effective than BSC alone in terms of discounted QALYs, despite its larger life expectancy, that is, 7.11 compared with 6.84 years; this result is explained by the different periods of time over which quality of life benefits take place, so that, when the discount rate is switched to 0%, everolimus becomes the strategy with the highest number of QALYs (data not shown). Thus, the relative advantage of everolimus in terms of health outcomes tends to occur after an earlier disadvantage. At the 3.5% annual discount rate, such an advantage occurs too late in time and everolimus becomes inferior to BSC in GI and lung NETs.
| Tumour location | Treatment | PFS | OS | Comparator | PFS | OS | ICER (£) |
|---|---|---|---|---|---|---|---|
| Pancreas | Everolimus | Log-logistic | Log-normal | BSC | Log-normal | Exponential | 28,098 |
| Sunitinib | Exponential | Exponential | BSC | Log-normal | Exponential | 20,726 | |
| GI and lung | Everolimus | Lognormal | Loglogistic | BSC | Log-normal | Loglogistic | BSC dominant |
Analysis limited to progression-free survival
Because of the inherent uncertainty in the OS data caused by treatment crossover from placebo to targeted treatments and its immaturity in the GI and lung and GI (midgut) locations, alternative analyses that limit the measurement of costs and benefits until disease progression provide a good robustness test of our results. In this scenario, the ICER for sunitinib in pNETs increased by 75% to £35,448, whereas the ICER for everolimus in pNETs increased by 26% to £57,493 (Table 97). In GI and lung NETs, the ICER for everolimus increased from its base-case value of £44,557 to £73,086. Everolimus has an ICER in patients with midgut NETs that is 21% larger than that in GI and lung NETs, suggesting less value for money in this patient subgroup and higher cost-effectiveness in the non-midgut GI and lung NETs population. Furthermore, the ICER for 177Lu-DOTATATE is less than half that for everolimus at £30,115, which suggests that peptide receptor radionuclide therapy treatment may have better longer-term outcomes than everolimus.
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 57,493 |
| Sunitinib | BSC | 35,448 | |
| GI (midgut) | Everolimus | BSC | 88,801 |
| 177Lu-DOTATATE | BSC | 30,115 | |
| GI and lung | Everolimus | BSC | 73,086 |
Background mortality adjustments to the overall survival and progression-free survival curves
Adjusting for background mortality has a limited effect on the results in pNETs and GI and lung NETs. In GI (midgut) NETs, the ICER for everolimus declines from about £200,000 in the base case to £78,330 with background mortality adjustment (Table 98). This reflects the high degree of uncertainty in the extrapolation of survival outcomes in the GI (midgut) location, for which we did have access to OS but had to impute it from the available PFS data for this subgroup. In addition, in GI (midgut) NETs, the base-case analysis that includes 177-Lu-DOTATATE adopts a background mortality adjustment because of the immaturity of the OS data in NETTER-1, from which the effectiveness data for 177Lu-DOTATATE is derived. Thus, in Table 98 we present the ICER for this treatment without adjusting for background mortality, which reduces its ICER from £62,158 to £43,348.
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 44,032 |
| Sunitinib | BSC | 21,594 | |
| GI (midgut) | Everolimus | BSC | 78,330 |
| 177Lu-DOTATATE (no mortality adjustment) | BSC | 43,348 | |
| GI and lung | Everolimus | BSC | 46,687 |
First-cycle costs and disease monitoring
Accounting for first-cycle costs of subsequent treatments and disease monitoring intensity in GI and lung and GI (midgut) NETs has a minor effect on the results, as evidenced by results in Tables 99 and 100.
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 45,288 |
| Sunitinib | BSC | 20,624 | |
| GI (midgut) | Everolimus | BSC | 208,095 |
| 177Lu-DOTATATE | BSC | 61,619 | |
| GI and lung | Everolimus | BSC | 47,205 |
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| GI (midgut) | Everolimus | BSC | 205,437 |
| 177Lu-DOTATATE | BSC | 64,513 | |
| GI and lung | Everolimus | BSC | 46,249 |
Scenario analysis with a 0% discount rate
As evidenced previously by the results of the scenario analysis in which the parametric survival curves were altered to more optimistic forms, the discount rate has an influential effect on the results as treatments tend to yield significant benefits in the long term. This may be seen for both pNETs and GI and lung NETs in Table 101.
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 38,021 |
| Sunitinib | BSC | 17,605 | |
| GI (midgut) | Everolimus | BSC | 131,512 |
| 177Lu-DOTATATE | BSC | 49,907 | |
| GI and lung | Everolimus | BSC | 34,367 |
Appendix 12 Visual fit to (instantaneous) survival event risk
FIGURE 38.
Everolimus arm in RADIANT-3:34 observed and predicted PFS hazard functions by the best-fitting models.
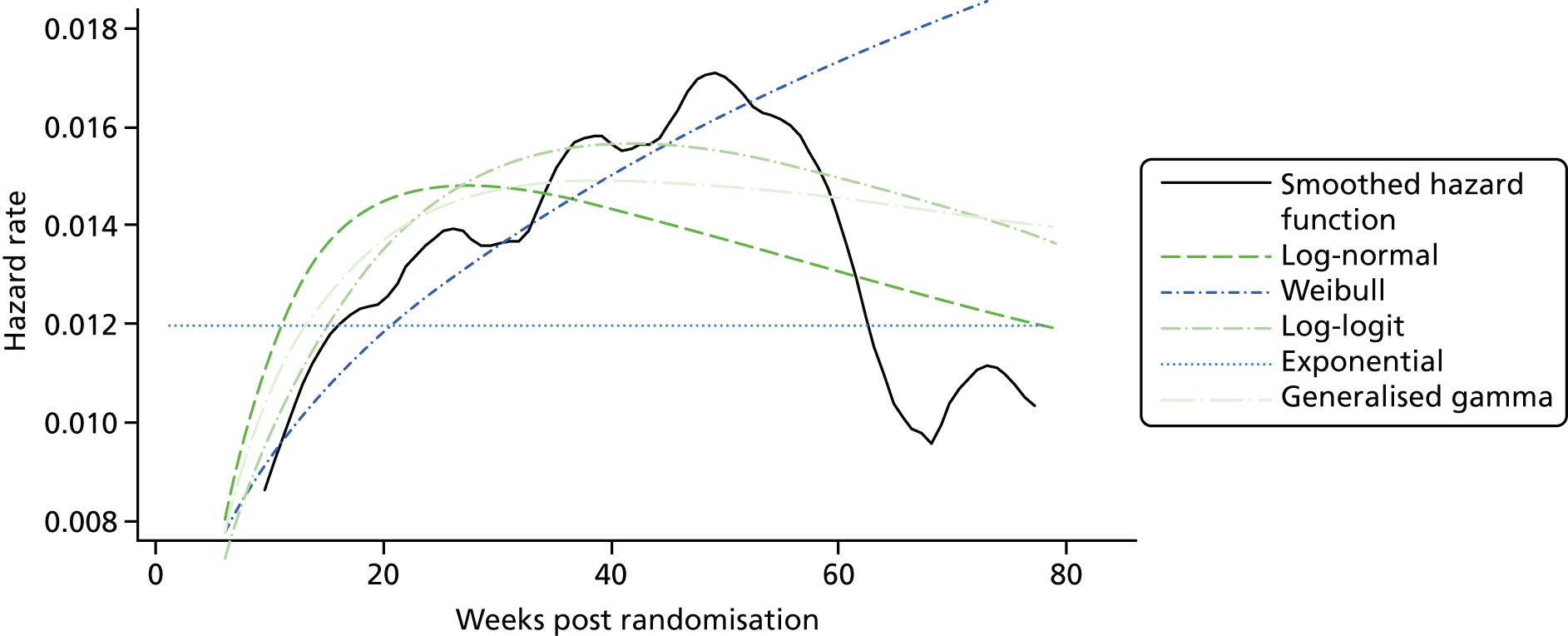
FIGURE 39.
Placebo arm: observed and predicted PFS hazard functions by the best-fitting models.
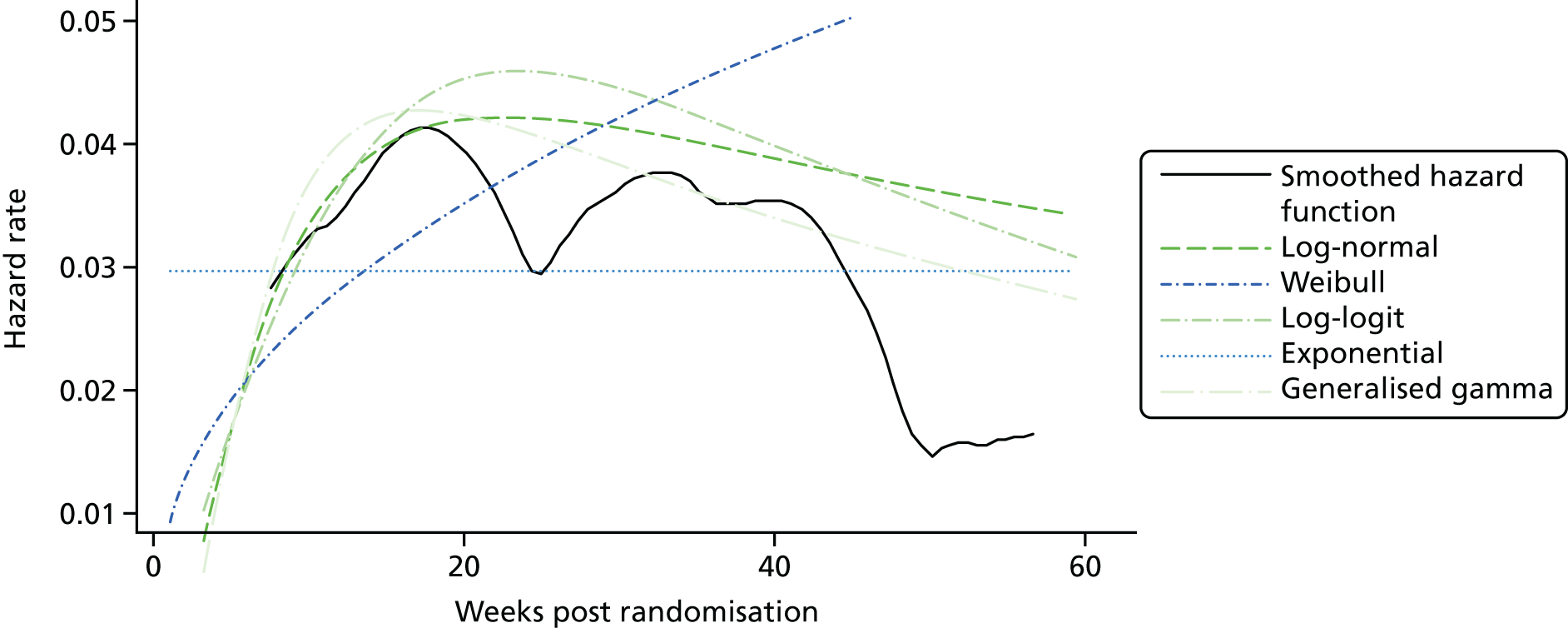
FIGURE 40.
Sunitinib arm: observed and predicted PFS hazard functions by the best-fitting models.

FIGURE 41.
Everolimus arm: observed and predicted PFS hazard functions by the best-fitting models.
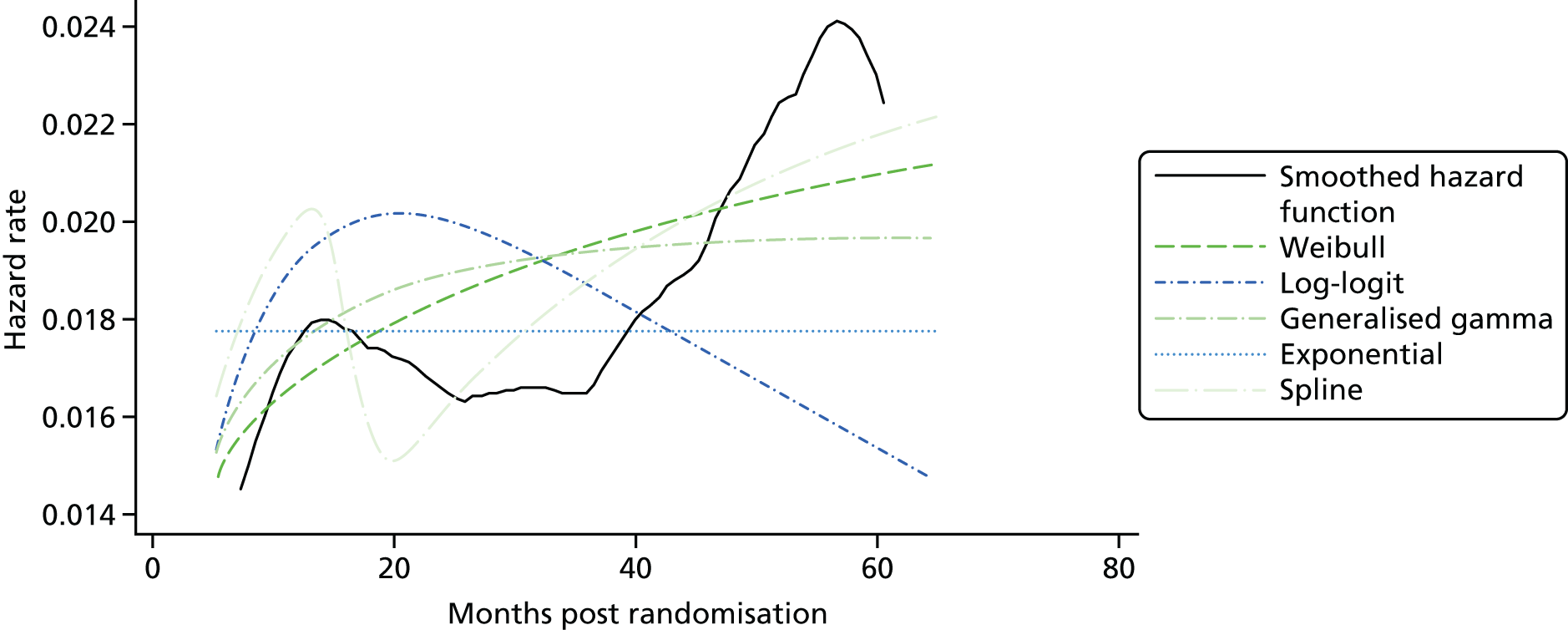
FIGURE 42.
Placebo arm in RADIANT-3:34 observed and predicted OS hazard functions by the best-fitting models.

FIGURE 43.
Sunitinib arm in A6181111:81 observed and predicted OS hazard functions by the best-fitting models.
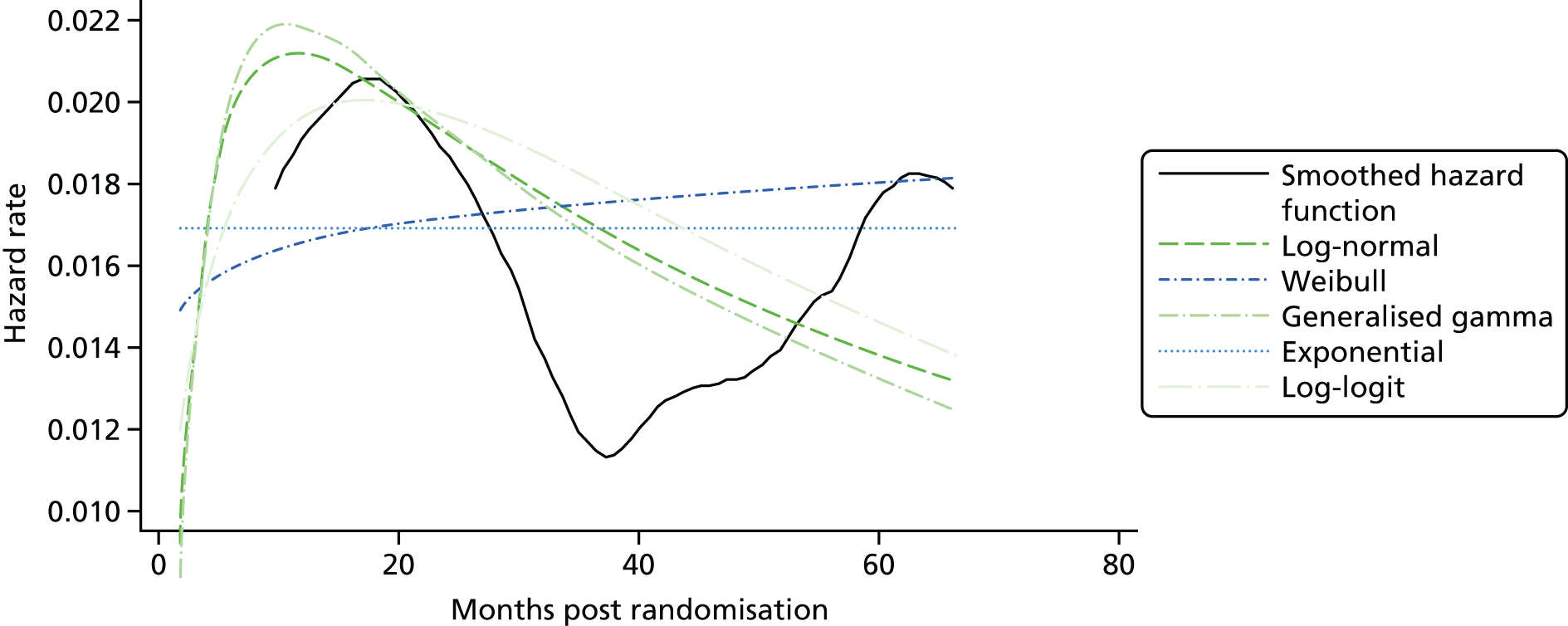
FIGURE 44.
Everolimus arm in RADIANT-4:35 observed and predicted PFS hazard functions by the best-fitting models.
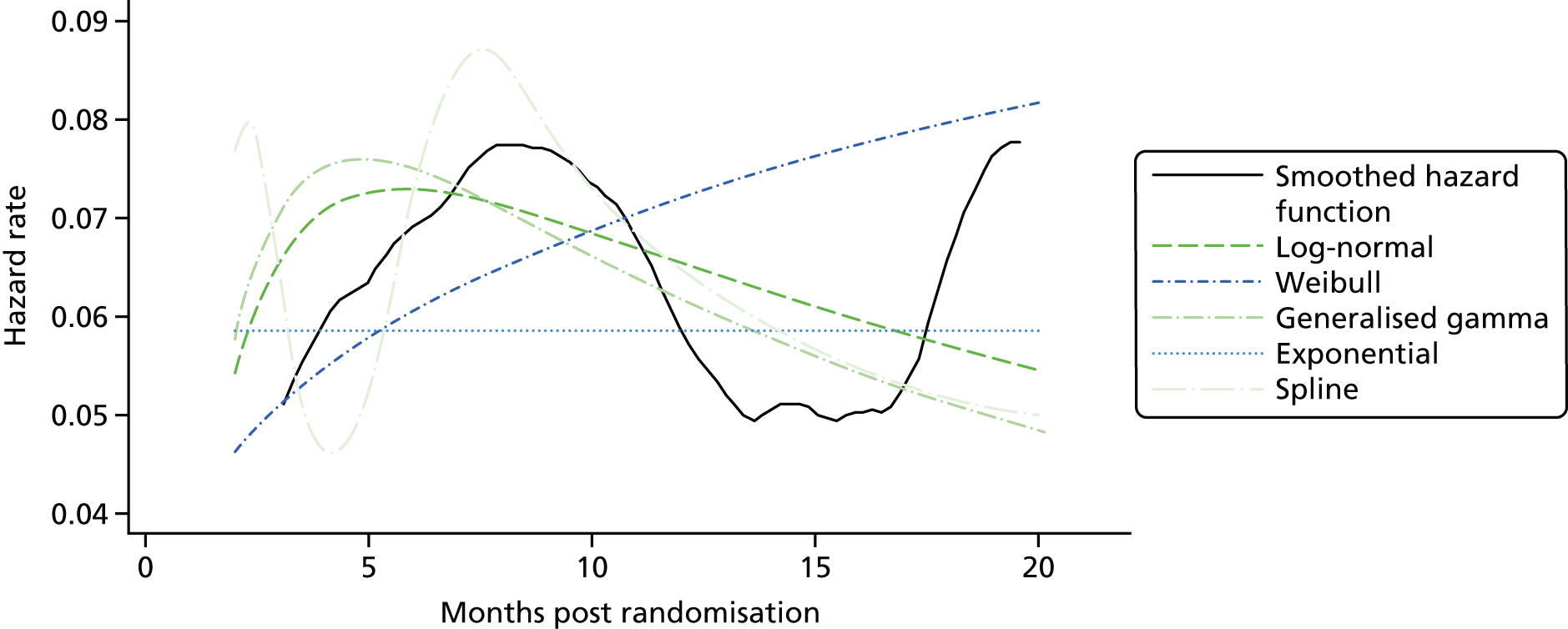
FIGURE 45.
Placebo arm in RADIANT-4:35 observed and predicted PFS hazard functions by the best-fitting models.

FIGURE 46.
Everolimus arm in RADIANT-4:35 observed and predicted OS hazard functions by the best-fitting models.
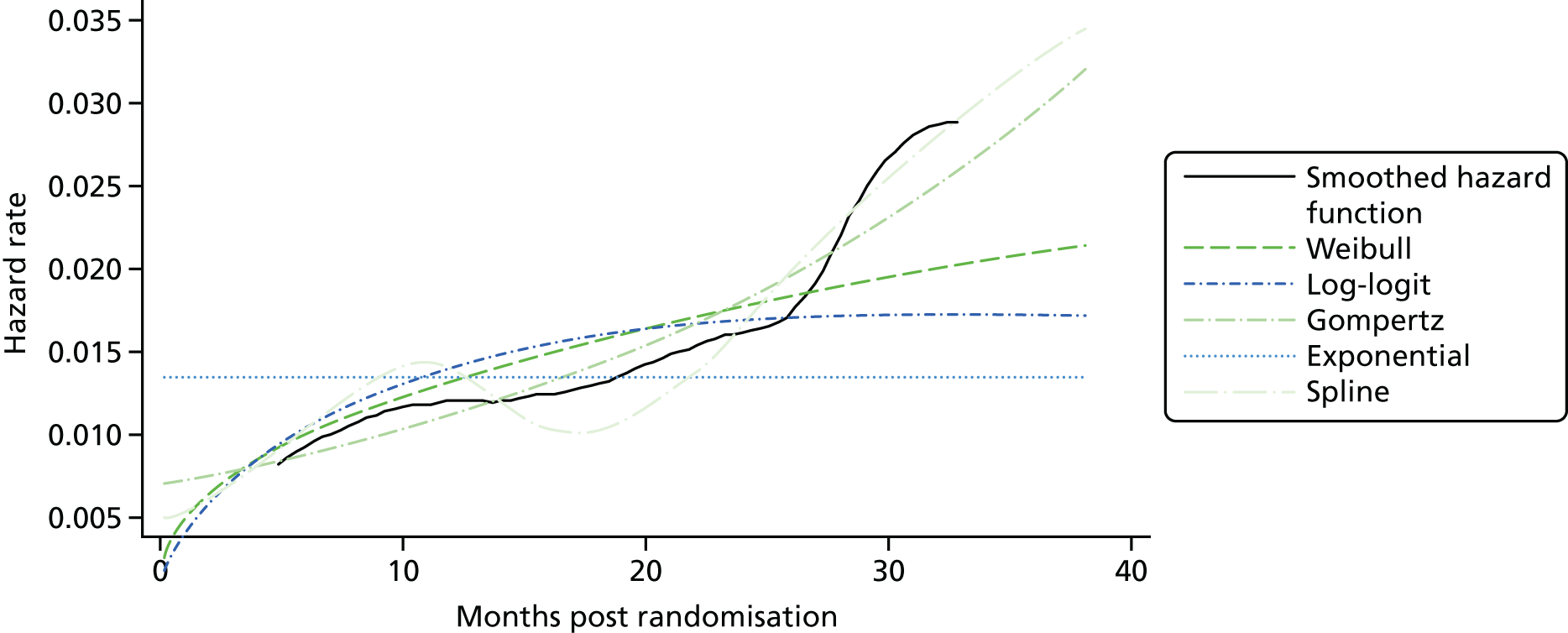
FIGURE 47.
Placebo arm in RADIANT-4:35 observed and predicted OS hazard functions by the best-fitting models.

FIGURE 48.
Everolimus arm in RADIANT-473 (midgut): observed and predicted PFS hazard functions by the best-fitting models.

FIGURE 49.
Placebo arm in RADIANT-473 (midgut): observed and predicted PFS hazard functions by the best-fitting models.
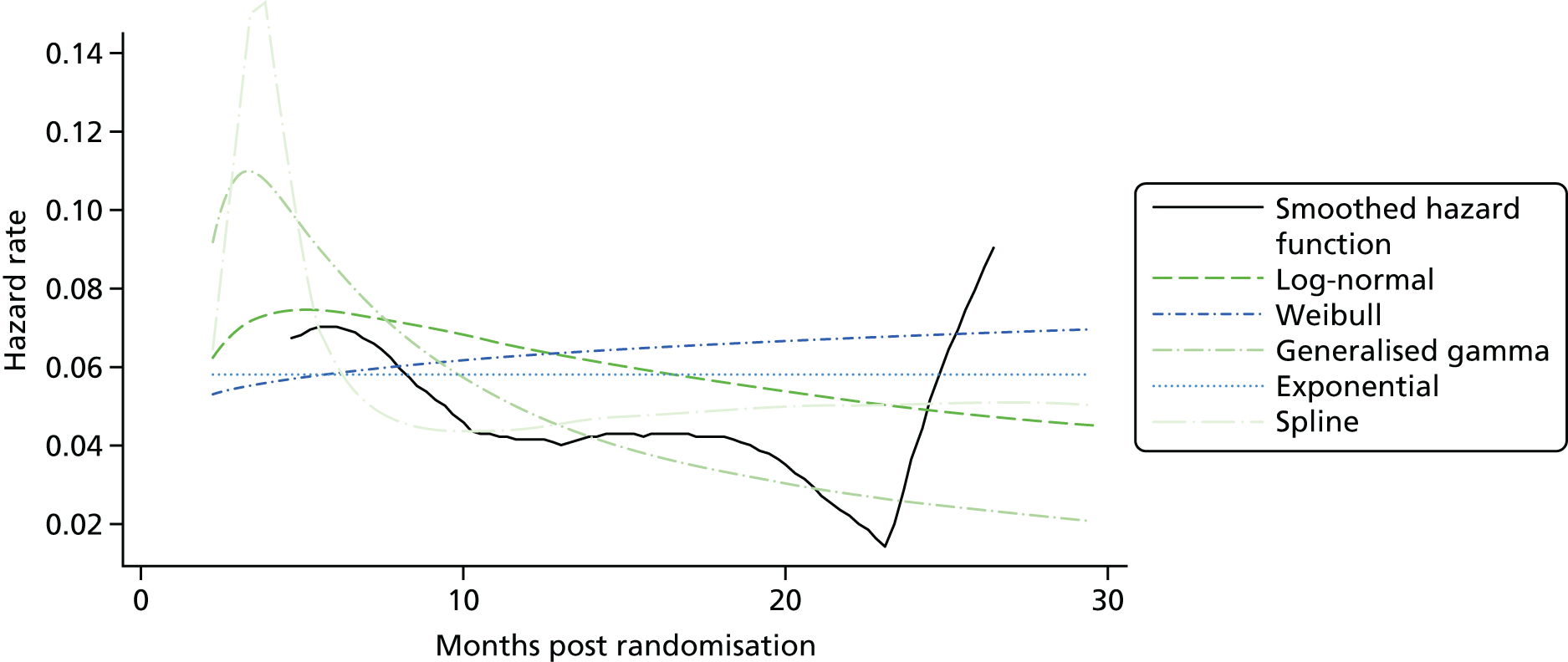
FIGURE 50.
177Lu-DOTATE arm in NETTER-1: observed and predicted PFS hazard functions by the best-fitting models.
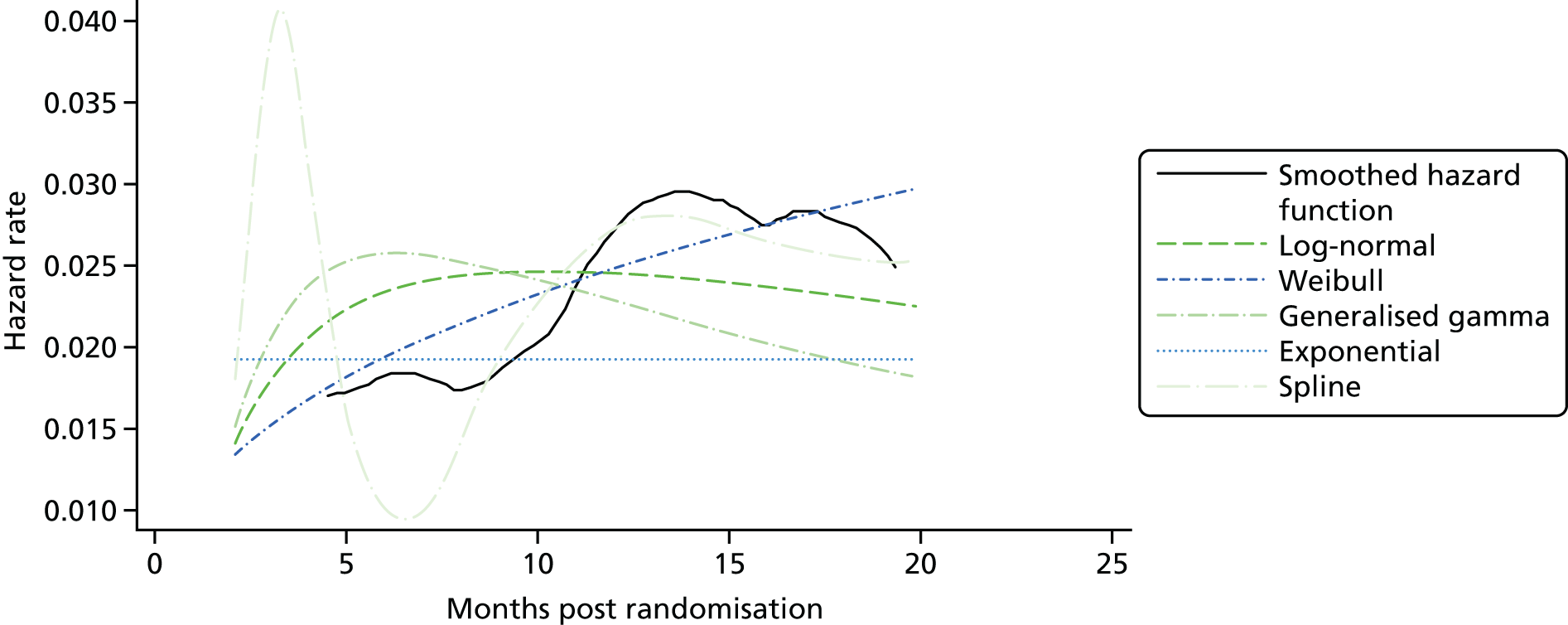
FIGURE 51.
177Lu-DOTATE arm in NETTER-1: observed and predicted OS hazard functions by the best-fitting models.

Appendix 13 Progression-free survival and overall survival: extrapolation of 177Lu-DOTATATE outcomes in the NETTER-1 trial
177Lu-DOTATATE in the NETTER-1 trial (Advanced Accelerator Applications submission to the National Institute for Health and Care Excellence)
Progression-free survival
Although the models did not differ in their goodness of fit to the PFS outcomes of 177Lu-DOTATE in NETTER-1 (Figure 52), the Weibull model exhibited the closest fit to its associated risk of death or disease progression (see Figure 50). The exponential fell in the middle of the range of PFS rates of the candidate distributions.
FIGURE 52.
Progression-free survival in the 177Lu-DOTATE arm of NETTER-1: extrapolation to 20 years.

The parameter of the PFS distribution was adjusted for the difference in expected PFS between the 60 mg of octreotide arm of NETTER-1 and the placebo arm of RADIANT-435 (midgut population), following the method described earlier for the analysis of pNETs (see Chapter 6, Model parameters). This indirect comparative analysis implicitly assumes that these two arms would be expected to produce the same PFS and OS outcomes and are thus subject to the reservations discussed in Chapter 6. Restricting the mean area under the Kaplan–Meier curve of the placebo arm in RADIANT-435 to the maximum length of follow-up of OS in the 60 mg of octreotide arm in NETTER-1 (which had a shorter follow-up than RADIANT-435), that is, 25.18 months, led to a restricted mean PFS of 9.97 months in the placebo arm of RADIANT-435 and 13.23 months in the 60 mg of octreotide arm in NETTER-1. In terms of Equation 1, the adjusted hazard and exponential survival functions with 177Lu-DOTATATE are:
and
The OS curve of the 177Lu-DOTATE arm of NETTER-1 adopted for the base case on the basis of the diagnostic results was the exponential (see Table 23). The parametric OS curve of 177Lu-DOTATE was adjusted for the 9.1%-shorter expected OS in the 60 mg of octreotide arm of NETTER-1 than in the placebo arm of RADIANT-435 (midgut population), using the methods described above for pNETs (see Chapter 6, Model parameters).
Overall survival
The 15-year OS rate with 177Lu-DOTATATE with the exponential distribution is 22%. After adjusting for the differences in the control arms of NETTER-1 and RADIANT-435 (midgut only), this rate becomes 25%. In contrast, the respective unadjusted rate for the Weibull function (Figure 53) is 3%. In any case, the available OS data from NETTER-1 is extremely immature, making the comparison of 177Lu-DOTATATE with everolimus very uncertain.
FIGURE 53.
Overall survival in the 177Lu-DOTATE arm of NETTER-1: extrapolation to 20 years.
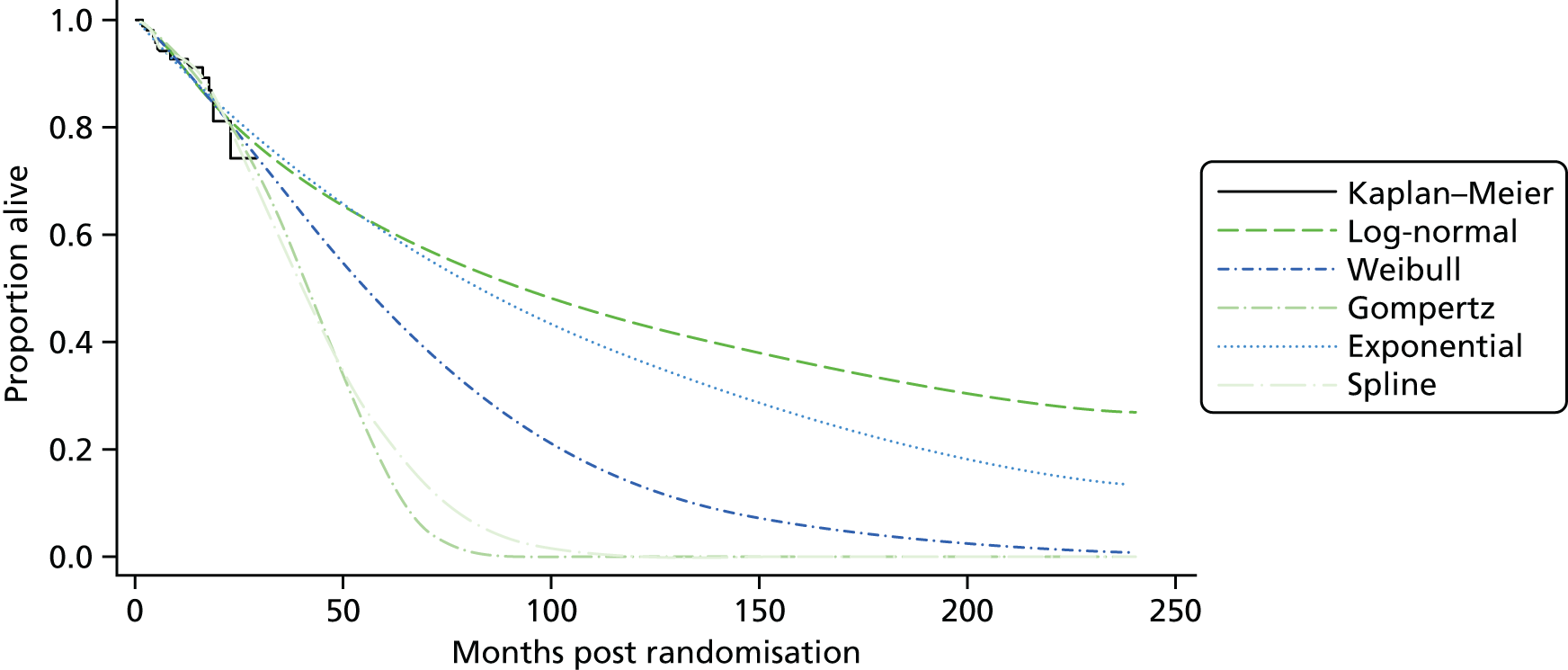
Appendix 14 Company reviews of clinical effectiveness
All three of the manufacturers – AAA, Novartis and Pfizer – submitted clinical evidence for consideration for this MTA.
Advanced Accelerator Applications
Advanced Accelerator Applications conducted a systematic literature review to ‘identify all studies that provide information on the clinical efficacy and safety of 177Lu-DOTATATE and relevant comparators in the treatment of patients with inoperable GEP-NETs’ (AAA submission to NICE,31 p.18). The literature searching carried out for this submission was sufficient, as were the inclusion/exclusion criteria used for screening. It is unclear whether or not title and abstract screening was completed in duplicate. Full-text screening was completed by two reviewers. As part of the inclusion criteria [table 16 of the AAA submission (p. 58)], AAA included SSAs (octreotide and lanreotide). SSAs were removed from the NICE scope on 18 August 2016. In its submission, AAA stated that conference abstracts were excluded (company submission, table 16, p. 58). It is unclear, therefore, why AAA included the NETTER-1 trial as, at the time, not only was it published only in abstract form (and so it would not be identified by AAA’s systematic review) but also its comparator was outside the NICE scope. AAA included non-RCT evidence in addition to RCT evidence for all interventions and comparators (everolimus, sunitinib, octreotide, chemotherapy, lanreotide, interferon and 177Lu-DOTATATE). The AG did not find any RCT evidence for 177Lu-DOTATATE (as NETTER-1 was excluded; see Chapter 4, Studies identified). The AG conducted a non-systematic review for non-RCT evidence for 177Lu-DOTATATE and identified 32 studies (see Appendix 2, Results for non-randomised controlled trial evidence). AAA identified four non-RCTs of 177Lu-DOTATE. 145,162–165,170 All four non-RCTs were included. It is unclear why AAA did not include the additional 28 studies that the AG had identified.
Network meta-analysis
Advanced Accelerator Applications did not undertake a meta-analysis as it found only one trial of 177Lu-DOTATATE. Instead, it performed an ITC for GI NETs, comparing everolimus with 177Lu-DOTATATE, and a MTC for pNETs, comparing everolimus, sunitinib and 177Lu-DOTATATE, for the outcomes of PFS and OS.
Five trials identified in the systematic review were excluded from the analyses by AAA for the following reasons: 96% of participants at baseline had stable disease (CLARINET),176 no data were provided on the number of participants with stable/progressive disease (PROMID)201 or the trials could not be connected to either the GI NETs or the pNETs network. 202–204
The three trials used in the ITC for GI NETs were RADIANT-2,190 RADIANT-435 and NETTER-131 (Figure 54). The three trials used in the MTC for pNETs were RADIANT-3,34 A618111181 and NETTER-131 (Figure 55).
FIGURE 54.
Gastrointestinal NETs network for the MTC conducted by AAA for PFS and OS. Source: figure 13 (pp. 71–72) of the AAA submission. 31

FIGURE 55.
Pancreatic NETs network for the MTC conducted by AAA for PFS and OS. Source: figure 12 (pp. 71–72) of the AAA submission. 31
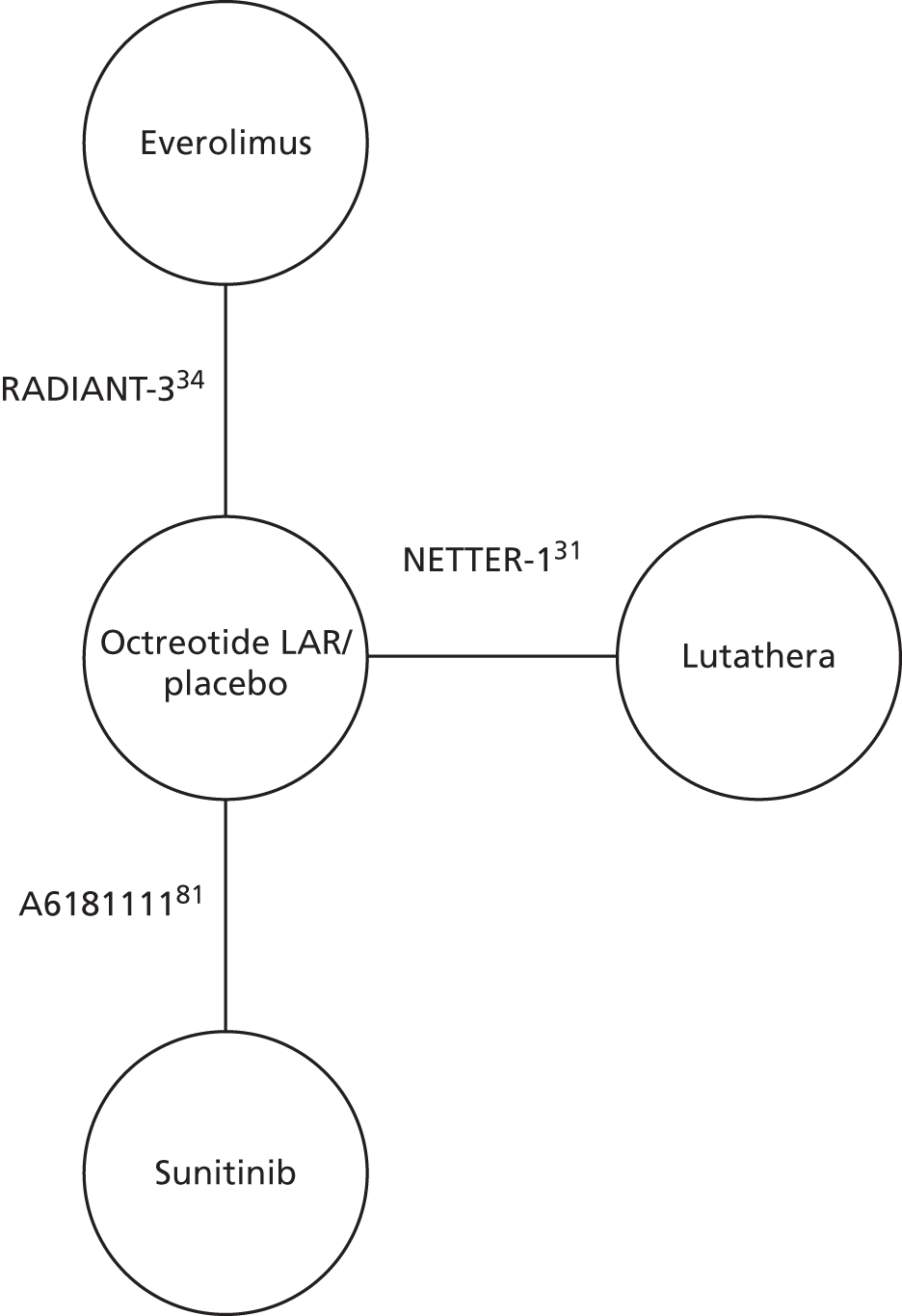
Study and participant characteristics were compared across studies by AAA for GI NETs and pNETs. For somatostatin receptor status, AAA stated that in NETTER-1 all participants were SSTR+, but reported that it was unable to obtain this information for RADIANT-2, RADIANT-3,34 RADIANT-435 and A6181111. 81 It was therefore assumed by AAA that the relative effectiveness between treatments does not alter according to somatostatin receptor status. As we do not know the somatostatin receptor status of participants in the other trials, we cannot be sure whether or not this assumption is correct.
For GI NETs, AAA highlighted that the tumour functioning status differs between participants in the RADIANT-2, RADIANT-435 and NETTER-1 trials. It stated that tumour function is not reported in RADIANT-2, that all participants in RADIANT-4 had non-functioning tumours and that, in NETTER-1, participants with functioning and non-functioning tumours were eligible. Based on a lack of evidence to suggest a difference in the relative effectiveness of everolimus and 177Lu-DOTATATE for participants with functioning and non-functioning tumours, AAA assumed that there is no difference. AAA stated that the participant populations from RADIANT-2, RADIANT-435 and NETTER-1 are aligned with each other and with the NICE scope in terms of disease progression. AAA noted that, although all patients in RADIANT-4 and NETTER-1 had received prior therapy, it was unclear whether or not this was the case in RADIANT-2. From expert clinical opinion, the AG notes that the prognosis for a patient differs depending on his or her tumour functioning status. As we do not know the different proportions or participants with functioning and non-functioning tumours in the different trials, we cannot be sure whether or not AAA’s assumption is correct regarding the relative effectiveness of everolimus and 177Lu-DOTATATE for participants with functioning and non-functioning tumours.
Advanced Accelerator Applications detailed the data used in the networks from each trial by NET location. For GI NETs, the company considered the populations to be in close alignment for PFS but commented that there are differences in the populations for OS (Table 102).
| Trial | PFS | OS |
|---|---|---|
| NETTER-1 | Midgut | Midgut |
| RADIANT-2119 | colorectal cancer | All NETs |
| RADIANT-435 | GI | Lung + GI |
For pNETs, AAA reported that, although NETTER-1 and A618111181 included participants with functioning and non-functioning tumours, RADIANT-334 did not report the tumour functioning status of their participants. As for GI NETs, AAA therefore assumed that the relative effectiveness of everolimus compared with 177Lu-DOTATATE does not differ by tumour functioning status. AAA stated that the participant populations in NETTER-1, RADIANT-334 and A618111181 had progressive disease, which was assumed to be aligned with the NICE scope. AAA noted that, although all patients in NETTER-1 had received prior therapy, it was unclear whether this was the case in RADIANT-334 and A6181111. 81
Advanced Accelerator Applications considered tumour location for RADIANT-334 and A618111181 to be aligned for PFS and OS, but that the population from NETTER-1 (GI NETs) was not aligned. Nevertheless, AAA included the GI NETs population from NETTER-1 in its MTC for pNETs (Table 103).
For both tumour locations, AAA noted that there was ‘considerable variation’ in the baseline characteristics across trials, yet considered the trials to be similar enough to synthesise the data.
Advanced Accelerator Applications made three major assumptions in performing their MTC: (1) that 60 mg of octreotide can be assumed to be equivalent to placebo and placebo plus 30 mg of octreotide (to connect the NETTER-1 trial to the other trials in the GI NETs network), (2) that 60 mg of octreotide is equivalent to placebo and placebo plus BSC (to connect the NETTER-1 trial to the other trials in the pNETs network) and (3) that data from the NETTER-1 trial can be used to inform the network for pNETs even though no participants in the NETTER-1 trial had pNETs. The third assumption is clearly untenable based on the difference in typical expected survival outcomes in pNETs and GI (midgut) NETs.
Advanced Accelerator Applications undertook a Bayesian analysis with MCMC simulation in R for both analyses using methods set out in Dias et al. 205 They ran fixed- and random-effects models using the Poisson/log model and the binomial/cloglog model. Prior distributions intended to be vague were used. A difference of > 5 for the deviance information criterion (DIC) was used to identify the most appropriate model of the four types run: fixed-effects Poisson/log model, random-effects Poisson/log model, fixed-effects binomial/cloglog model and random-effects binomial/cloglog model. For each analysis, AAA report simulating four MCMC chains, with a burn-in of 10,000 iterations. Results were based on 50,000 iterations and a thin rate of 4. AAA report assessing convergence using trace plots, autocorrelations and ‘other standard convergence diagnostics’ (AAA submission, p. 21031), but do not state explicitly whether or not convergence was achieved in the models. Consistency of the networks could not be assessed as there were no closed loops, meaning that direct evidence for treatments compared within a RCT could not be compared with indirect evidence for that treatment comparison.
Advanced Accelerator Applications reported very little difference between the DICs from the four models for each network [table 27 (p. 83) of the AAA submission31); therefore, the results from the random-effects Poisson model are presented for both tumour locations and outcomes. Point estimates and 95% CrIs are reported for all treatment comparisons in tables 23–26 (pp. 81–82) of the submission. 31 The main results are summarised in Tables 104 and 105.
| Intervention | PFS | OS |
|---|---|---|
| 177Lu-DOTATATE vs. octreotode/placebo | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE vs. everolimus | Confidential information has been removed | Confidential information has been removed |
| Intervention | PFS | OS |
|---|---|---|
| 177Lu-DOTATATE vs. octreotode/placebo | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| Sunitinib vs. octreotide/placebo | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE vs. everolimus | Confidential information has been removed | Confidential information has been removed |
| 177Lu-DOTATATE vs. sunitinib | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. sunitinib | Confidential information has been removed | Confidential information has been removed |
Limitations of Advanced Accelerator Applications’s mixed treatment comparison
We acknowledge the following important limitations of the MTC conducted by AAA, which limit the extent to which the findings can be relied on: (1) RADIANT-2 should be excluded from this MTA as the population in this trial all have functioning tumours, which is outside the marketing licence for everolimus for GI NETs, (2) NETTER-1 should be excluded from the pNETs network as it does not include any patients with pNETs, (3) for the evaluation of GI NETs, the populations differ across the three studies for OS (midgut NETs in NETTER-1, all NETs in RADIANT-2, GI and lung NETs in RADIANT-4), (4) there is no justification for the assumption that 60 mg of octreotide LAR is equivalent to placebo, placebo plus 30 mg of octreotide and placebo plus BSC, (5) there is no consideration of the extent of treatment switching within RADIANT-2 (58% switched to active treatment), RADIANT-3 (73% switched to active treatment) and A6181111 (69% switched to active treatment), which limits the interpretation of the results for OS, (6) the 95% CrIs are very wide, indicating a great deal of uncertainty, more so than the results from the RCTs suggest and (7) the results from the random-effects Poisson model and the fixed- and random-effects binomial model are not reported in the submission and so no comparison of any differences in point estimates or 95% CrIs between these models can be made.
Comparison with the Assessment Group’s indirect treatment comparison
For GI NETs, RADIANT-2 was excluded from the AG’s analysis as everolimus is not licensed for functioning tumours in GI and lung NETs and all participants in RADIANT-2 have functioning tumours. The AG did not identify any trials comparing placebo plus BSC with 60 mg of octreotide to allow RADIANT-435 and NETTER-1 to be linked in a network. In consultation with clinical experts, and in the absence of evidence to suggest otherwise, the AG did not think that it was appropriate to link the RADIANT-435 and NETTER-1 trials by assuming that placebo plus BSC (as used in RADIANT-435) is equivalent to 60 mg of octreotide (as used in NETTER-1). However, in a sensitivity analysis, the AG made the strong assumption that placebo plus BSC can be considered equivalent to 60 mg of octreotide, but this ITC should be interpreted with caution.
From data requests sent by the AG to AAA, the AG was able to obtain GI-only NETs data from RADIANT-435 (rather than GI plus lung NETS data, as used in AAA’s ITC), but only for PFS and some AEs. Therefore, the results of the ITC undertaken by the AG are different from those of the ITC undertaken by AAA, as RADIANT-2 was excluded from the AG analysis, only GI NETs data were included from RADIANT-435 and an ITC for OS was not conducted by the AG as these data were not received from the company.
For PFS, the HR for 177Lu-DOTATATE compared with everolimus was estimated as 0.37 (95% CI 0.19 to 0.69) by the AG and 0.43 (95% CrI 0.05 to 4.24) by AAA. The 95% CrI in AAA’s analysis is wide because a random-effects model was used. These findings are similar in magnitude; however, to accept these results it must be assumed that placebo plus BSC is equivalent to 60 mg of octreotide. The AG also conducted an indirect comparison of AEs, OS and RR.
For the pNETs network, the AG did not include data from NETTER-1 as none of the participants in this trial had pNETs. Therefore, only data from RADIANT-334 and A618111181 were included in the AG’s ITC between everolimus and sunitinib. As well as PFS and OS, the AG also reported ITC results for RR and AEs. AAA considered only PFS and OS. For the comparison of everolimus with sunitinib for pNETs, point estimates calculated from AAA’s MTC for PFS and OS are the same as those from the AG’s indirect comparison; however, the 95% CrIs from AAA’s analysis are much wider than the 95% CIs from the AG’s analysis. For example, the PFS HR for everolimus compared with sunitinib from AAA’s analysis is (confidential information has been removed), whereas the HR from the AG’s analysis is 0.83 (95% CI 0.49 to 1.42). It is likely that these differences in the widths of the 95% CrI and 95% CI occur because AAA reported the results from a random-effects model, whereas the analysis conducted by the AG assumed a fixed-effects model. As AAA did not report the results from a fixed-effects model it is not possible to check whether or not this is the reason for the uncertainty.
Novartis
Novartis conducted a systematic review aiming to identify ‘all relevant RCT and non-RCTs investigating everolimus, sunitinib or 177Lu-DOTATATE for the treatment of patients with advanced, metastatic or inoperable pNETs, and 177Lu-DOTATATE for advanced, metastatic or unresectable GEP-NETs’ (Novartis submission, p. 3333). The literature searching carried out for this submission was sufficient, although there were minor errors in one of the searches of The Cochrane Library. It is unlikely that any studies were excluded from the review because of this error. The review followed the CRD’s Guidance for Undertaking Reviews in Health Care. 38 The methods used by Novartis are described in brief and are adequate for the purpose of the submission. To minimise the risk of bias, it would have been preferable for two reviewers to have reviewed all titles and abstracts, rather than one reviewer screening them all and the second reviewer screening 10% and all included citations.
In relation to pNETs, Novartis identified five RCTs and 41 non-RCTs. Of the five RCTs, four evaluated everolimus (RADIANT-3,34 COOPERATE-2 (COmbination Of Pasireotide and evERolimus in Advanced Tumors of neuroEndocrine origin, second trial),206 Yao et al. ,107 NCT01628913207) and one evaluated sunitinib (A618111181). Of the four RCTs that evaluated everolimus, only RADIANT-334 was included in the submission as COOPERATE-2 and NCT01628913 compared everolimus with comparators outside the scope. The results from the fourth identified RCT, that by Yao et al. ,107 were retracted by the authors 6 months after their publication. 208 The inclusion of RADIANT-334 matches the RCT included by the AG for the assessment of everolimus in pNETs. RADIANT-334 is reported in detail in the main text of the company submission, with additional information presented in appendix 3 of the submission. 33 Novartis also refers to OBLIQUE, a currently unpublished Phase IV observational study, which assesses quality of life in individuals with pNETs receiving everolimus. Novartis also reports on non-RCTs of everolimus, which represent 16 or 17 [16 studies referred to in the main company submission document, whereas 17 studies are presented in the results table (see appendix 2 of the company submission33)] of the 41 identified non-RCTs. The non-RCT data were tabulated (see appendix 2 of the company submission33) and summarised in the main report (see chapter 4.8 of the company submission33). The AG did not assess any non-randomised evidence for everolimus. Novartis conducted two further systematic literature reviews aiming to identify ‘relevant clinical evidence on the efficacy and safety of everolimus for the treatment of GI NETs (SLR1) and lung NETs (SLR2) respectively’ (Novartis submission, p. 5933). The literature searching carried out was sufficient and the methods of the review were the same as those mentioned earlier. In terms of the GI NETs systematic review, eight RCTs and five non-RCTs were identified by Novartis, of which three RCTs and two non-RCTs also met the eligibility criteria for inclusion in the lung NETs systematic review. Of the eight RCTs and five non-RCTs, only one RCT and one non-RCT were deemed relevant by Novartis in its submission (RADIANT-435 and Bajetta et al. 209 respectively). Irrelevant RCTs were excluded based on the interventions not meeting the inclusion criteria in the scope (Yao et al. ;210 CLARINET;211 Faiss et al. ;201 Jacobsen et al. 212 PROMID;176,213 Wolin et al. 214) or the population recruited not being within the marketing authorisation for everolimus (RADIANT-2119,215–217). Irrelevant non-RCTs were all excluded based on the interventions not being within the scope (Ferolla et al. ,218 Campana et al. ,219 Grozinsky-Glasberg et al. 220 and Panzuto et al. 221). The AG did not include non-RCTs for everolimus. Consequently, from the two included studies from Novartis, the AG also identified RADIANT-435 (the RCT) but did not include Bajetta et al. 209 (the non-RCT). RADIANT-435 is reported in detail in the main text of the company submission, with additional information presented in Appendix 7 of the submission.
Network meta-analysis
Novartis did not conduct a meta-analysis, MTC or ITC for GI and/or lung NETs as it identified only the RADIANT-435 trial.
For pNETs, Novartis identified three trials in its systematic review that included everolimus (RADIANT-3,34 COOPERATE-2206 and NCT01628913207), stating that, because of the different comparators in these three trials, a meta-analysis was not undertaken. Instead, an indirect comparison between everolimus and sunitinib was carried out using RADIANT-334 and A6181111. 81 The network for Novartis’s pNETs MTC is shown in Figure 56.
FIGURE 56.
Pancreatic NETs network for the indirect comparison conducted by Novartis for PFS, OS, concomitant SSA use and AEs.

The network was used to compare PFS, OS, concomitant SSA use and 13 grade 3/4 AEs (for which there was an incidence of ≥ 2% in either trial) between everolimus and sunitinib. For PFS, Novartis conducted two indirect comparisons, one using PFS defined by local review and a second using PFS defined by a central blinded investigator review (referred to as BIRC in its submission). For OS, Novartis conducted an indirect comparison of OS based on ITT analysis and an indirect comparison of OS based on the RPSFT method, to account for treatment switching at disease progression that occurred in both trials.
A comparison of the study and participant characteristics between RADIANT-334 and A618111181 was conducted by Novartis, who deemed the trials to be similar enough to be combined. The outcomes from both trials contributing to the ITCs are presented in table 4.8 of Novartis’s submission. 33
Novartis also reported the results of a published MAIC,124 which used individual participant data from RADIANT-334 and aggregate data from A6181111. 81 This method was used to allow for matching of the characteristics of participants in RADIANT-334 with those in A618111181 and to help address the issue of approximately 70% of participants switching from the control arm to the active treatment arm in both trials after disease progression. However, Novartis argued (p. 49 of the submission33) that the limitations of the MAIC method, which include the inability to match on characteristics not accounted for in both trials, the unknown impact of unobserved differences in study and/or patient characteristics and the fact that the SMC, for which the MAIC had been included in a health technology assessment that it appraised on this clinical question, referred to the MAIC as ‘non-standard’, have led it to consider the more straightforward approach of Bucher et al. 41 for carrying out an indirect comparison between everolimus and sunitinib. Using the MAIC method partially corrects for some of the bias associated with comparing two different populations from the two trials, whereas there are no corrections for patient population differences in the Bucher et al. 41 method. In any case, the MAIC analysis serves as a robustness check of the Bucher results. The results of these analyses are summarised in Tables 106 and 107 (see tables 4.11–4.15 in Novartis’s submission33 for all data used and the MTC results).
| Outcome | Everolimus vs. placebo OR (95% CI) | Sunitinib vs. placebo OR (95% CI) | Everolimus vs. sunitinib OR (95% CI) |
|---|---|---|---|
| Local investigator-defined PFS | 0.35 (0.27 to 0.45) | 0.42 (0.26 to 0.66) | 0.83 (0.49 to 1.42) |
| Blinded independent review-defined PFS | 0.34 (0.26 to 0.44) | 0.32 (0.18 to 0.55) | 1.08 (0.59 to 1.99) |
| ITT OS | 0.94 (0.73 to 1.20) | 0.71 (0.47 to 1.09) | 1.32 (0.81 to 2.16) |
| RPSFT OS | 0.60 (0.09 to 3.95) | 0.43 (0.17 to 1.20) | 1.40 (0.17 to 11.72) |
| Concomitant SSA use | 0.91 (0.61 to 1.36) | 0.88 (0.45 to 1.71) | 1.04 (0.48 to 2.26) |
| Outcome | Sunitinib vs. everolimusa OR (95% CI) |
|---|---|
| Neutropenia | 23.71 (0.19 to 3037.28) |
| Hypertension | 18.68 (0.15 to 2414.14) |
| PPE syndrome | 11.62 (0.09 to 1540.16) |
| Leukopenia | 11.62 (0.09 to 1540.16) |
| Diarrhoea | 0.57 (0.03 to 12.113) |
| Stomatitis | 0.23 (0.01 to 14.06) |
| Thrombocytopenia | 0.41 (0.01 to 25.31) |
| Anaemia | 0.04 (0 to 4.76) |
| Hyperglycaemia | 0.35 (0.01 to 21.02) |
| Fatigue | 0.20 (0.01 to 17.25) |
| Infections | 0.20 (0.01 to 17.25) |
| Pneumonitis | 0.09 (0.01 to 11.67) |
| Nausea | 0.09 (0.01 to 11.67) |
| Sum | 4.48 (0.51 to 39.38) |
Novartis concluded that there are no significant differences in (locally and centrally defined) PFS, (ITT or RPSFT) OS and concomitant SSA use between everolimus and sunitinib. It reported that the indirect comparison for AEs suggests a higher odds of grade 3/4 neutropenia, hypertension, palmar–plantar erythrodysaesthesia syndrome and leukopenia events with sunitinib than with everolimus, whereas for the remaining AEs a higher odds is associated with everolimus than with sunitinib. However, none of the ORs is statistically significant and all have very wide 95% CIs.
Limitations of Novartis’s indirect treatment comparison
The AG notes the following limitations with the indirect comparison carried out by Novartis: (1) it is unclear where Novartis obtained the HR for blinded independent review-defined PFS for A618111181 as the AG was unable to identify this from the published literature and (2) the justification for using the Bucher et al. 41 method of indirect comparison is not clear when a MAIC analysis would have been possible. The AG notes that the conclusions of the published MAIC124 are similar to those reported by Novartis using the Bucher et al. 41 method, even though the methods used differ and the OS data used by Novartis are more mature than those used in the published MAIC analysis.
Comparison with the Assessment Group’s indirect treatment comparison
The AG identified the same two RCTs for pNETs and used the same method for the ITC41 as Novartis. The ITC results were exactly the same for local investigator-defined PFS between Novartis and the AG and were very slightly different for blinded independent review-defined PFS, even though the HRs and 95% CIs for RADIANT-334 and A618111181 were the same. The AG believes that this very slight difference is possibly related to Novartis using more precise data than the AG had access to. For OS, the AG used data for A618111181 from Pfizer’s submission32 rather than data from Raymond et al. ,81 which is what Novartis used. Therefore, there are some differences in the HRs and 95% CIs used between the AG and Novartis. However, both sets of results (the AG and Novartis) for PFS and OS indicate no statistically significant difference between everolimus and sunitinib. Similarly, the ITCs for grade 3/4 AEs from the AG and Novartis all show very wide 95% CIs, suggesting that there is no statistically significant difference between everolimus and sunitinib.
Pfizer
Pfizer did not conduct a systematic review to identify relevant trials for this decision problem as it was confident that the only trial conducted with sunitinib in its licensed indication for pNETs was the A618111181 trial. This matches the identification of A6181111 by the AG as the only relevant trial for the analysis of sunitinib. Pfizer reported data primarily from the principal study publication. 81 In addition, other data sources for the A618111181 trial included the CSR110 (dated 2009) and updated survival analysis from a conference abstract. 88 In its submission Pfizer reported incidence rates for AEs using the CSR as the source. The AE incidence rates published by Raymond et al. 81 are different and on average higher (by n = 1 or 2) for all grades of AEs.
Critique of the matched adjusted indirect comparison analyses by Pfizer
Pfizer presented a MAIC of everolimus and sunitinib using placebo-controlled treatment effects on PFS and OS from the A6181111 trial81 of sunitinib compared with placebo and the RADIANT-3 trial34 of everolimus compared with placebo. These analyses follow previously published work by Signorovitch et al. ,124 who first applied the MAIC method to this question. Pfizer used updated OS data and matched the sunitinib and placebo arms of A618111181 to the baseline characteristics in RADIANT-3. 34 The direction of matching, that is, of the A618111181 population to the RADIANT-334 population, was determined by the availability of individual patient data from the former trial and only summary data from the latter trial. In contrast, the study by Signorovitch et al. 124 was sponsored by Novartis and had available individual patient data from RADIANT-334 but only aggregate data from A6181111,81 which determined that matching was performed in the opposite direction, that is, from RADIANT-334 to the A618111181 population.
Briefly, a MAIC involves estimating sampling weights by regression analysis and applying these weights to data from individual patients to adjust their relative contribution to the analysis of outcome data from the ‘index’ trial, that is, A6181111;81 the weights reflect the likelihood that an individual with a mix of baseline characteristics is found in the population of a ‘target’ trial, that is, RADIANT-3. 34 In practice, logistic regression is used to obtain the weights, following the methodology of propensity score matching for observational data. 222 As a result, the weighted summary characteristics at baseline match the baseline characteristics of the target population in RADIANT-3. 34 In the present case, in which no individual patient data but only summary baseline characteristics are available for the target population, a modified approach using the method of moments was used by Pfizer to obtain the matching weights. 124
Pfizer’s justification for its use of MAIC, as opposed to simpler methods such as that by Bucher et al. , to indirectly compare sunitinib with everolimus is that simpler indirect methods based on a common comparator or anchor may fail to account for confounding when the populations of the trials in the network differ markedly or trial designs or implementation vary. The company acknowledges, however, that its MAIC analysis could not adjust for differences in study design across trials. The main justification offered by the company for its MAIC, however, is in relation to the effect on crossover in OS. As 69% and 85% of placebo patients in A618111181 and RADIANT–3,34 respectively, crossed over to the active treatment in open-label extension-phase studies, the OS outcomes in the placebo arm are ‘contaminated’ by the active treatments and would not serve as a common comparator or anchor. In contrast, by matching the sunitinib arm of A618111181 to the everolimus arm of RADIANT-3,34 the MAIC is feasible.
Although the two RCTs investigated patient populations with progressive, advanced, well- or moderately differentiated pNETs, important differences were noted between them. These included the early termination of A618111 because of improved PFS with sunitinib, the smaller size of this trial relative to RADIANT-334 and the more frequent imaging schedule in A618111181 (8 weeks vs. 12 weeks in RADIANT-334), which may result in earlier detection of disease progression. Unlike A6181111,81 RADIANT-334 included patients with a PS of 2, but as they constituted only 3% of the trial sample this had a limited effect on the results produced by Pfizer.
Although randomisation produced a balanced distribution of baseline characteristics in RADIANT-3,34 it (confidential information has been removed).
Two approaches were taken by Pfizer to the MAIC, one for PFS and another for OS. For PFS, for which a common BSC plus placebo comparator was available in both trials, the ‘comparator-based’ approach was followed, involving the following steps:
-
The sunitinib and BSC plus placebo arms of A618111181 were separately matched to the everolimus arm.
-
The HR for sunitinib compared with BSC plus placebo was estimated from the matched A618111181 individual patient data.
-
A Bucher indirect comparison of everolimus with sunitinib was estimated using the HR from the matched sunitinib compared with BSC plus placebo data from A618111181 and the reported HR for everolimus compared with BSC plus placebo in RADIANT-3. 34
The 95% CI for the resulting MAIC HR for PFS was calculated from the standard errors of the log HR of sunitinib compared with BSC plus placebo, adjusted for the effective sample size, and of the HR of everolimus compared with BSC plus placebo in RADIANT-3,34 as approximated from its reported point estimate and 95% CI (confidential information has been removed).
Because of contamination as a result of crossover, the MAIC for OS was conducted by directly matching the sunitinib arm to the everolimus arm. In this analysis, the following steps were followed:
-
The sunitinib arm of A61811114581 was matched to the everolimus arm.
-
The individual patient OS data for everolimus were recreated from digitised Kaplan–Meier curves using the algorithm of Hoyle and Henley. 223
-
The HR for sunitinib compared with everolimus was estimated from the matched individual patient data from the sunitinib arm and the recreated individual patient data in RADIANT-3. 34
The weights applied to the sunitinib arm for MAIC with everolimus were normalised (i.e. divided) by the effective sample size (defined as the ratio of the square of the sum of weights to the sum of the square of individual weights). The purpose of this adjustment was that the 95% CI associated with the hazard ratio in step 3 accounted for the reduced amount of information available from a sample of a given size after matching.
(Confidential information has been removed) Table 108. Confidential information has been removed.
| Comparison | PFS | OS | ||
|---|---|---|---|---|
| n | HR (95% CI) | n | HR (95% CI) | |
| Bucher IC | ||||
| Sunitinib vs. placebo | Confidential information has been removed | Confidential information has been removed | – | – |
| Everolimus vs. placebo | Confidential information has been removed | Confidential information has been removed | – | – |
| Sunitinib vs. everolimus | Confidential information has been removed | Confidential information has been removed | – | – |
| MAIC | ||||
| Sunitinib vs. placeboa | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Everolimus vs. placebob | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Sunitinib vs. everolimus | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Unmatched IC | ||||
| Sunitinib vs. everolimus | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
Confidential information has been removed. In particular, Pfizer compared the matching-adjusted Kaplan–Meier curve of the placebo arm in A618111181 with the respective curve from recreated individual patient data from the placebo arm of RADIANT-334 and (confidential information has been removed). However, given the differences in the timing of scheduled imaging assessments to determine disease status between the two trials, discussed in Critique of the matched adjusted indirect comparison analyses by Pfizer, adjusting for the placebo PFS outcomes in the common comparator approach seems warranted nonetheless.
In relation to its OS results, Pfizer acknowledged the limitation of the data available. In particular, the available sample for the sunitinib arm was small, especially after matching, which effectively halved its size. Confidential information has been removed.
Pfizer provided a clear justification for the MAIC evidence submitted to NICE. This was based on updating the previous analysis124 with new OS data and on methodological improvements on the previous work by adding more variables on which to match the two indirectly compared trials. The first argument is unquestionable given that final OS analyses have been published since the previous MAIC study. The second argument is, however, less firm, as discussed below.
The analysis by Pfizer provides a clear description and adequate details of the methods used in and results obtained from the MAIC. The discussion also acknowledges the main strengths and limitations of this analysis and provides an adequate explanation of the reasons for the (confidential information has been removed). This discussion provided the valuable insight that much of the (confidential information has been removed). This highlights the limitations associated with the small sample size in this trial.
The AG notes that MAICs in small samples have a difficult balance to strike between internally valid comparisons and generalisability to the relevant patient populations. We have discussed this issue before. 224 The estimates of relative effectiveness for sunitinib derived from this indirect comparative assessment by Pfizer may be relevant to a small group of patients, those who are represented in both A618111181 and RADIANT-3,34 but may not be generalisable to the subgroup of patients not represented in RADIANT-334 but present in A6181111. 81 Thus, although Pfizer presents its findings as improved evidence with regard to the previous study by Novartis on the basis of its use of additional variables for matching the samples from the two trials, any additional variable used for matching reduces the generalisability of the MIAC findings to the original A618111181 population. This is in addition to the limitations from increased sampling uncertainty, which, as Pfizer notes, increases as the effective sample size declines with increased variables on which to match.
As Pfizer acknowledges, the MAIC of OS between sunitinib and everolimus is affected by high levels of uncertainty because of the lack of a within-trial placebo control for indirect comparison and the problems of sample size. A further MAIC analysis is needed with a within-trial placebo control that is itself adjusted for crossover to active treatment. Because of the small sample size in A6181111,81 the most fruitful approach would be to match the sample of RADIANT-334 to the population of A6181111,81 as Signorovitch et al. 124 have done, rather than the other way around, which Pfizer has done. This would produce estimates of relative effectiveness with lower levels of uncertainty and risk of bias as a result of few observations.
Pfizer provided individual patient data on A618111181 to NICE, which the AG used to conduct some sensitivity analyses of its MAIC. Confidential information has been removed.
Appendix 15 Methods for systematic reviewing of utilities (health-related quality-of-life data)
A systematic review was conducted to identify, appraise and synthesise all available data on HRQoL of NETs patients, with the objective of estimating utility values for populating the ‘de novo’ PenTAG cost-effectiveness model.
Identification of studies
The systematic searches were conducted in MEDLINE (Ovid), EMBASE (Ovid), School of Health and Related Research Health Utilities Database (ScHARRHUD) [see www.scharrhud.org (accessed 15 May 2018)], the Health Economics Research Centre (HERC) database, the EQ-5D website [URL: https://euroqol.org (accessed 16 July 2018)], the ‘patient-reported outcome and quality of life instruments’ database225 and the Health Technology Assessment database and NHS EED. These searches were not limited by study design and language. A complete list of search strategies can be found in Appendix 1.
All references were exported into EndNote X7 where automatic and manual deduplication was performed.
Inclusion/exclusion criteria
Studies identified by the searches were screened for inclusion according to the criteria listed below. Abstracts were included conditional on their good reporting of the methods used and the outcomes obtained.
The population of interest consisted of patients with progressive, unresectable or metastatic NETs, irrespective of the tumour location. The following outcome was considered: HRQoL of health states relating to patients with progressive, unresectable and/or metastatic NETs. No exclusion criteria relating to the intervention, comparator or study design were used.
Screening
First, one researcher screened for inclusion on the titles and abstracts returned by the search strategy. All included records were then independently screened by a second researcher. Disagreements were resolved by discussion. Full texts of identified studies were obtained and screened in the same way.
Data extraction
Data extraction from included studies consisted of details of the study’s design and methodology, characteristics of the study population, the measure used to capture HRQoL outcomes, details on the outcomes measured, the time horizon of the study and the type of statistical analysis undertaken by the authors. Data were extracted by one reviewer (SL) and checked independently by a second reviewer (RMM). Disagreements were resolved by discussion.
Critical appraisal strategy
The quality of all studies included in the review was assessed by one reviewer. Owing to the lack of a standardised checklist for the quality appraisal of HRQoL studies, a set of criteria was formulated to critically appraise the studies included in the systematic review. The checklist used (Table 109) relies heavily on the 14-item checklist designed by Mols et al. 226 for the appraisal of quality-of-life studies in the area of breast cancer and later used by Cornish et al. 227 in the area of cutaneous melanoma. Compared with the original version outlined in Mols et al. ,226 three items were added and one was deleted in order to adapt the checklist to this specific disease area and types of studies. This version better captures the quality of HRQoL studies included in this review. Some changes in the formulation of a number of items were also made to clarify ambiguous language. Finally, the quality of reporting of two published economic evaluations included in this review was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist. 228
| Checklist question | Cramer et al. (2014)231 | Pavel et al. (2016)232 | Teunissen et al. (2004)233 | Swinburn et al. (2012)130 | Singh et al., (2016)73 | Cohen and Allred (2009) (as cited in the submission)32 |
|---|---|---|---|---|---|---|
| Are sociodemographic and medical data described? | No | Yes | Yes | No | No | Yes |
| Are inclusion/exclusion criteria formulated? | No | Yes | Yes | No | No | Yes |
| Was HRQoL a primary outcome of the study? | Yes | Yes | Yes | Yes | Yes | No |
| Is the process of data collection described? | No | No | Yes | Yes | No | Yes |
| Is the type of cancer described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Are results compared between two or more groups? | No | Yes | Yes | No | Yes | Yes |
| Are the mean (or median) and range of time since diagnosis given? | No | Yes | No | No | No | Yes |
| Are participation and response rates per group described and > 75%? | No | No | Yes | No | No | Yes |
| Is information on patient/disease characteristics of respondents and non-respondents presented? If there is no selective response, is this explained? | No | No | No | No | No | No |
| Is the use of a valid QoL questionnaire justified? | No | No | No | No | No | No |
| Are the mean (or median) and standard deviation for the HRQoL measures reported? | No | Yes | Yes | Yes | Yes | No |
| Are the results reported as item scores as opposed to summary scores? | No | No | Yes | No | Yes | Yes |
| Is an attempt made to identify a set of determinants with the highest prognostic value? | No | Yes | Yes | No | No | No |
| Is the degree of selection of the patient sample described? | No | Yes | Yes | No | No | Yes |
| Was it reported whether the changes in HRQoL measured were clinically statistically significant? | No | Yes | Yes | No | No | Yes |
Mapping
Mapping was performed to obtain utility values from the EORTC QLQ-C30 and FACT-G data identified in the literature review.
Mapping the EORTC QLQ-C30 to the EuroQol-5 Dimensions
Doble and Lorgelly229 conducted a comprehensive external validation study on the algorithms developed to map EORTC QLQ-C30 scores to the EuroQol-5 Dimensions three-level version (EQ-5D-3L). The data set that they used consisted of EORTC QLQ-C30 and EQ-5D values from a sample of 988 patients enrolled in the Cancer 2015 longitudinal study. 230 The patients involved were treatment naive and had been diagnosed with a variety of cancers. Different stages of disease were accounted for by dividing the patient sample into three groups according to disease severity and time to first follow-up.
Most mapping algorithms, particularly those relying on the ordinary least squares model and dummy variables, were found to perform inadequately. 229 Specifically, when tested using different tumour-specific samples, predictive accuracy was found to be higher, on average, in healthier patient samples and lower in patient samples with poorer health, corresponding to lower EQ-5D utility values. In general, the analysis concluded that most algorithms seemed to be insensitive to tumour location but very sensitive to disease severity. The algorithm by Versteegh et al. 231 and that by Longworth et al. 134 proved to perform particularly well on a range of different validation criteria, including the ability to predict extreme EORTC QLQ-C30 health states and make predictions consistent with the country-specific EQ-5D tariff range. Moreover, such algorithms showed a relatively small mean squared error when predicting EQ-5D values and corresponding QALYs. 229 Although the algorithm in Versteegh et al. 231 cannot be generalised easily as it can only provide utilities drawn from the Dutch value set, the algorithm developed by Longworth et al. 132 (see Appendix 22), although being computationally intensive, had the advantage of providing utility values for any country-specific tariff, making it more generalisable.
The algorithm developed by McKenzie and van der Pol,132 although not found to provide the highest accuracy in the review of algorithms by Doble and Lorgelly,229 has been widely used and cited in studies of cancer. The validation process showed that the McKenzie and van der Pol132 algorithm performs well in terms of predictive power, as all of the actual EQ-5D values were found to be in the 95% CIs of the mapped values. Even more importantly, the difference in QALYs between treatment arms calculated using mapped utilities was almost identical to the difference in QALYs calculated with the original EQ-5D utilities (–0.019 vs. –0.017 QALYs). Nonetheless, questions relating to the generalisability of such results remain unanswered, particularly in relation to the application of this algorithm to patient groups with widely different age ranges and health status from those in the oesophageal cancer patient group used to validate the algorithm.
As the impact of using different algorithms in cost–utility analysis could not be measured by Doble and Lorgelly,229 and a number of limitations in their analysis prevented the identification of a preferred algorithm beyond doubt, the authors recommend conducting sensitivity and scenario analysis to illustrate the impact of choosing different algorithms and corresponding sets of mapped utilities.
Mapping the FACT-G to the EuroQol-5 Dimensions
The mapping of FACT-G scores to EQ-5D utilities was performed based on the work by Longworth et al. ,134 who considered a wide range of models and tested their ability to predict EQ-5D utilities based on FACT-G values. In particular, models that employed item-level data were found to perform better than those using significant domain and total score models. The methods used to evaluate the algorithms presented in Longworth et al. 134 show that the best-fitting algorithm (see Appendix 22) used in this analysis achieves a high degree of accuracy in predicting EQ-5D values. Nevertheless, Young et al. 186 raise concerns regarding the generalisability of such results on the grounds that the patient sample used to test the algorithms in Longworth et al. 134 included a surprisingly low number of patients in poor health.
In this analysis, the best-fitting algorithm by Longworth et al. 134 (see Appendix 22) was linearised and used to map published FACT-G summary domain scores into EQ-5D values for the stable disease and disease progression health states for patients in RADIANT-4. 35 These utility values were the only empirical data available on EQ-5D health state utilities in patients treated with everolimus and, as we did not have access to the original individual patient data from the RADIANT-435 trial to replicate the analyses by Novartis, we validated their analyses by mapping published mean scores for FACT-G domains with linear Taylor series approximations to Longworth et al. ’s134 best-fitting algorithm. FACT-G scores mapped using the linearised algorithm were then compared with published EQ-5D utilities obtained from the corresponding FACT-G individual patient data mapped with Longworth et al. ’s134 non-linear, best-fitting algorithm (see Appendix 22).
Results
A total of 6792 records were identified. After deduplication, 5192 records were manually screened by two reviewers. After the screening process, eight studies were ultimately included in this review. 32,73,121,123,131,232–234 A modified PRISMA flow chart is provided in Figure 57.
FIGURE 57.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.

The main characteristics of the studies identified through the systematic search for data on HRQoL are summarised in Tables 109 and 110.
Evidence identified through the systematic search for studies on health-related quality of life
Of the included studies, data extraction on an initial set of six studies was undertaken (Table 110). Of the six studies, one was a conference abstract on a longitudinal study;232 one was a Phase III expanded-access study;233 one was a prospective cohort study;234 one was a preference elicitation study;131 and two were economic evaluations. 121,123
| Study | Study designa | n | Population | Intervention | Measure (typeb and name) | Outcomesc | Time period | Statistical method of analysis |
|---|---|---|---|---|---|---|---|---|
| Cramer 2014232 | U | 30 | US NETs patients with hepatic metastases | Y-90 radioembolisation | G, SF-36 | A | 24 months | |
| Pavel 2016233 | E | 246 | Patients with advanced NETs (pNETs vs. non-pNETS separately). Non-pNETs included small intestine, lung, colon and other | Everolimus | G, EQ-VAS, EQ-5D, EORTC QLQ-C30, EORTC QLQ-GI NET21 | A | 12 months | Last HRQoL value before treatment discontinuation was used |
| Teunissen 2004234 | U | 50 | Dutch metastatic GEP NETs | Lu-octreotide | D, EORTC QLQ-C30 | A | Until 6 weeks after last treatment | Variance analysis to compare before and after quality of life |
| Swinburn 2012131 | U | 100 | Bespoke health states were designed based on the literature and clinicians. States were valued by a sample of the English population | Unspecified | Vignettes | HS | One-off | NA |
| Walczak 2012123 | EE | NRd | Adults with unresectable or metastatic well-differentiated pNETs with disease progression | Sunitinib + BSC vs. placebo + BSC | G, mapped EORTC QLQ-C30 onto EQ-5D | HS | Patients’ lifetime | Markov model |
| Muciño Ortega 2012121 | EE | NRd | Mexican non-resectable pNETs patients | Sunitinib + BSC vs. placebo + BSC | G, mapped EORTC QLQ-C30 onto EQ-5D | HS | 10 years | Markov model |
Evidence obtained by contacting the authors or the sponsors of the studies
A second set of studies was identified in the systematic search for studies on HRQoL as abstracts only, with the study outcomes not available to the public. To obtain such data, the authors or the sponsors of the studies had to be contacted directly. Table 111 summarises the main characteristics of the studies included: a conference poster reporting HRQoL outcomes and some details on the methods used in a major clinical trial78 and a CSR provided by the sponsor of the study as part of the NICE appraisal process. 32
| Study | Study designa | n | Population | Intervention | Measure (typeb and name) | Outcomesc | Time period | Statistical method of analysis |
|---|---|---|---|---|---|---|---|---|
| Singh 201673 | E | 284 | Adults with advanced, progressive, non-functional GI or lung NETs | Everolimus | G, mapped FACT-G onto EQ-5D | HS | Unclear | Linear mixed models |
| Cohen 200932 | E | 144 | Adults with progressive, well differentiated pNETs | Sunitinib | D, EORTC QLQ-C30 | A | 21 months | Repeated measures mixed-effects model |
Appendix 16 Use of chemotherapy
The analysis by Novartis in patients with pNETs adopted the values for the use of chemotherapy after disease progression presented in Table 112. The corresponding values for the company’s evaluation of targeted therapy in GI or lung NETs are presented in Table 113. These values were used by the AG in the respective the novo model analyses. The frequency of use of other supportive drug therapies in the AG model-based analysis of GI and lung were obtained from RADIANT-4 data reported in the NICE submission by Novartis, as presented in Table 114. The frequencies of AEs in the AG analysis of pNETs, GI and lung and GI midgut patients, respectively, are presented in Tables 115–117.
| Treatment | Proportion of participants (%) | Number of cycles |
|---|---|---|
| 5-fluorouracil | 21.9 | 2.5 |
| Doxorubicin | 28.1 | 1.66 |
| Streptozocin | 31.3 | 2.14 |
| Treatment | Arm | Proportion of participants (%) | Number of cycles |
|---|---|---|---|
| 5-fluorouracil | Everolimus + BSC | 2.8 | 1.45 |
| BSC | 1.1 | ||
| Streptozocin | Everolimus + BSC | 2.8 | 1.45 |
| BSC | 1.1 | ||
| Temozolomide | Everolimus + BSC | 14.2 | 3.08 |
| BSC | 11.4 | ||
| Capecitabine | Everolimus + BSC | 14.2 | 3.08 |
| BSC | 11.4 |
| Treatment | Arm | Diseasea | Proportion (%) |
|---|---|---|---|
| Analgesic (lidocaine) | Everolimus + BSC | Stable | 12.7 |
| BSC | Stable | 6.2 | |
| Corticosteroid (dexamethasone) | Everolimus + BSC | Stable | 31.7 |
| BSC | Stable | 10.3 | |
| Glucocorticoid (prednisone) | Everolimus + BSC | Stable | 41.5 |
| BSC | Stable | 11.3 | |
| Antiemetic (prochlorperazine) | Everolimus + BSC | Stable | 2.9 |
| BSC | Stable | 3.1 | |
| Antidiarrhoeal (Biofermin/S. boulardii) | Everolimus + BSC | Stable | 5.8 |
| BSC | Stable | 5.2 |
| AE | Everolimus (%) | Sunitinib (%) | BSC (%) |
|---|---|---|---|
| Neutropenia | 0.2 | 5.5 | 0.2 |
| Hypertension | 0.2 | 4.4 | 0.2 |
| Palmar–plantar erythrodysaesthesia | 0.2 | 2.8 | 0.2 |
| Leukopenia | 0.2 | 2.8 | 0.2 |
| Diarrhoea | 3.4 | 2.0 | 0.5 |
| Stomatitis | 7.1 | 1.7 | 0.2 |
| Thrombocytopenia | 4.2 | 1.7 | 0.2 |
| Anaemia | 6.1 | 0.2 | 0.2 |
| Hyperglycaemia | 5.4 | 1.9 | 2.0 |
| Fatigue/lethargy | 2.5 | 0.7 | 0.5 |
| Infections | 2.5 | 0.5 | 0.5 |
| Pneumonitis | 2.7 | 0.2 | 0.2 |
| Nausea | 2.7 | 0.7 | 0.2 |
| Asthenia | 1.0 | 1.3 | 1.0 |
| Decreased appetite/anorexia | 0.2 | 1.2 | 1.2 |
| AE | Everolimus (%) | BSC (%) |
|---|---|---|
| Diarrhoea | 7.4 | 2.0 |
| Stomatitis | 8.9 | 0.0 |
| Anaemia | 4.0 | 1.0 |
| Hyperglycaemia | 3.5 | 0.0 |
| Fatigue/lethargy | 3.5 | 1.0 |
| Infections | 6.9 | 0.0 |
| Peripheral oedema | 2.0 | 1.0 |
| Pyrexia | 2.0 | 0.0 |
| AE | Everolimus (%) | 177Lu-DOTATATE (%) | BSC (%) |
|---|---|---|---|
| Hypertension | 6.8 | 1.7 | |
| Diarrhoea | 11.1 | 5.0 | 3.4 |
| Stomatitis | 7.7 | 0.0 | |
| Anaemia | 6.8 | 1.7 | 1.7 |
| Fatigue/lethargy | 5.1 | 1.7 | 1.7 |
| Infections | 12.8 | 3.4 | |
| Peripheral oedema | 2.6 | 1.7 | 1.7 |
| Pyrexia | 1.7 | 0.0 | |
| Abdominal pain | 5.1 | 3.4 | 6.9 |
Appendix 17 Addendum 1
Lung neuroendocrine tumours
The comparison between treatment with everolimus and treatment with BSC for lung NETs patients yielded an ICER of £31,016 (Table 118). Treatment of these patients with everolimus results in better survival (5.12 years vs. 2.96 for BSC). Likewise, the treatment costs in the everolimus arm are higher, driven by the drug acquisition costs in the pre-progression health state (Table 119).
| Outcome measure | Everolimus | BSC | Everolimus vs. BSC |
|---|---|---|---|
| Life-years (mean, undiscounted) | 5.12 | 2.96 | 2.16 |
| QALYs (mean, discounted) | 3.18 | 1.99 | 1.19 |
| Total costs (mean, discounted) (£) | 49,168 | 12,249 | 36,920 |
| ICER (cost per QALY) (£) | 31,016 |
| Outcome measure | Everolimus | BSC | Everolimus vs. BSC |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| Pre progression | 1.13 | 0.61 | 0.52 |
| Post progression | 3.98 | 2.35 | 1.64 |
| Total | 5.12 | 2.96 | 2.16 |
| QALYs (mean, discounted) | |||
| Pre progression | 0.84 | 0.48 | 0.35 |
| Post progression | 2.34 | 1.50 | 0.84 |
| Total | 3.18 | 1.99 | 1.19 |
| Costs (mean, discounted) (£) | |||
| Pre progression | |||
| Drug acquisition | 30,332 | 278 | 30,054 |
| Drug administration | 172 | 2 | 170 |
| Medical management | 3338 | 1509 | 1830 |
| AEs | 171 | 34 | 137 |
| Total (pre progression) | 34,013 | 1822 | 32,191 |
| Post progression | |||
| Drug acquisition | 3748 | 1572 | 2175 |
| Drug administration | 18 | 6 | 12 |
| Medical management | 7689 | 4898 | 2791 |
| End-of-life care | 3700 | 3950 | –250 |
| Total (post progression) | 15,155 | 10,426 | 4729 |
| Total | 49,168 | 12,249 | 36,920 |
| ICER (cost per QALY) | 31,016 | ||
It must be noted that these analyses were derived using:
-
mean treatment durations from exponential extrapolations of median treatment durations reported for the lung subgroup in the CSR appendices provided by Novartis138
-
exponential curves fitted to individual patient data derived from OS Kaplan–Meier curves and number of patients at risk data provided by Novartis for the lung subgroup of RADIANT-435
-
exponential curves fitted to individual patient data derived from lung PFS Kaplan–Meier curves presented in the American Society of Clinical Oncology (ASCO) poster by Singh et al. 73 [from PFS curves for all, non-prior treatment with SSA and prior treatment with SSA subgroups in RADIANT-435 and validated by comparing the resulting HR of 0.48 (95% CI 0.27 to 0.85) with the lung HR of 0.50 (95% CI 0.28 to 0.88) reported in the RADIANT-435 CSR]
-
all other parameters were assumed to be the same as for the GI/lung patient population in RADIANT-4. 35
Alternative set of utility values
When an alternative set of utility values was applied the ICER for sunitinib was £19,411 and for everolimus plus BSC was £41,246. In other locations the ICERs were > £30,000 (Table 120).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 41,246 |
| Sunitinib | BSC | 19,411 | |
| GI (midgut) | Everolimus | BSC | 352,801 |
| 177Lu-DOTATATE | BSC | 57,745 | |
| GI and lung | Everolimus | BSC | 49,949 |
| Lung | Everolimus | BSC | 32,413 |
Analysis limited to progression-free survival
When the analysis was limited to accounting for costs and benefits up to disease progression, in patients with GI (midgut) tumours 177Lu-DOTATATE had an ICER of £30,115 and everolimus had an ICER of £88,801. In other tumour locations targeted treatments had ICERs of ≥ £35,448 (Table 121).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 57,493 |
| Sunitinib | BSC | 35,448 | |
| GI (midgut) | Everolimus | BSC | 88,801 |
| 177Lu-DOTATATE | BSC | 30,115 | |
| GI and lung | Everolimus | BSC | 73,086 |
| Lung | Everolimus | BSC | 91,202 |
Background mortality adjustments to the overall survival and progression-free survival curves
Background mortality adjustment resulted in an ICER for sunitinib of £21,594. In all other locations targeted treatments resulted in ICERs of ≥£33,908 (Table 122) (the results for 177Lu-DOTATATE are presented in Table 122 without adjustment for background mortality as the respective base-case values included an adjustment for background mortality).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 44,032 |
| Sunitinib | BSC | 21,594 | |
| GI (midgut) | Everolimus | BSC | 78,330 |
| 177Lu-DOTATATE (no mortality adjustment) | BSC | 43,348 | |
| GI and lung | Everolimus | BSC | 46,687 |
| Lung | Everolimus | BSC | 33,908 |
First-cycle costs and disease monitoring
When the costs of treatment after disease progression were included (accounted for by adjusting the first-cycle costs of the progressive disease phase), the ICER for sunitinib plus BSC was £20,624 and for everolimus was £45,288. For targeted treatments in other locations, the ICERs were ≥ £32,744 (Table 123).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 45,288 |
| Sunitinib | BSC | 20,624 | |
| GI (midgut) | Everolimus | BSC | 208,095 |
| 177Lu-DOTATATE | BSC | 61,619 | |
| GI and lung | Everolimus | BSC | 47,205 |
| Lung | Everolimus | BSC | 32,744 |
The scenario using alternative costs for disease monitoring, as adopted by Novartis in its analysis submitted to NICE, resulted in ICERs of ≥ £32,221 across GI/lung tumour locations (Table 124).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| GI (midgut) | Everolimus | BSC | 205,437 |
| 177Lu-DOTATATE | BSC | 64,513 | |
| GI and lung | Everolimus | BSC | 46,249 |
| Lung | Everolimus | BSC | 32,221 |
Scenario analysis using a 0% discount rate
With no discounting for costs and QALYs, sunitinib plus BSC in pNETs is the only targeted treatment in the studied locations with an ICER of < £20,000. The only other treatment with an ICER of < £30,000 was everolimus pus BSC in patients with lung NETs (Table 125).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 38,021 |
| Sunitinib | BSC | 17,605 | |
| GI (midgut) | Everolimus | BSC | 131,512 |
| 177Lu-DOTATATE | BSC | 49,907 | |
| GI and lung | Everolimus | BSC | 34,367 |
| Lung | Everolimus | BSC | 26,114 |
Probabilistic sensitivity analyses
The sampling variation in model parameter data is consistent with everolimus having an ICER that is > £30,000 in lung pNETs (Figure 58).
FIGURE 58.
Cost-effectiveness plane: treatments in lung pNETs. WTP, willingness to pay.
For lung NETs, the probabilistic mean ICER is £31,987. The probability that everolimus is the most cost-effective treatment for lung NETs at the willingness-to-pay thresholds of £20,000 per QALY and £30,000 per QALY gained is 12.6% and 46.3%, respectively (Figure 59).
FIGURE 59.
Cost-effectiveness acceptability curves for treatments in lung NETs.

Appendix 18 Sensitivity analyses
Probabilistic sensitivity analyses
Pancreatic neuroendocrine tumours
The probabilistic analysis suggests that the ICER for sunitinic compared with everolimus is statistically significantly below the £20,000 incremental cost per QALY gained threshold (Figure 60).
FIGURE 60.
Cost-effectiveness plane: comparison of targeted treatments in pNETs. WTP, willingness to pay.

In contrast, the ICER for everolimus plus BSC compared with BSC alone was > £20,000 and the data may be consistent with it being > £30,000 (Figure 61).
FIGURE 61.
Cost-effectiveness plane: everolimus vs. BSC in pNETs. WTP, willingness to pay.

The sampling uncertainty in the data are consistent with sunitinib having an ICER that ranges from slightly > £30,000 to < £20,000 (Figure 62).
FIGURE 62.
Cost-effectiveness plane: sunitinib vs. BSC in pNETs. WTP, willingness to pay.

In line with the results illustrated in the previous figures, the probability that everolimus treatment for pNETs is the most cost-effective treatment at the willingness-to-pay thresholds of £20,000 per QALY and £30,000 per QALY gained is 0% and 0.6% respectively (Figure 63).
FIGURE 63.
Cost-effectiveness acceptability curves of treatments in pNETs.
The probability of sunitinib being the most cost-effective treatment for pNETs at a willingness-to-pay threshold of £20,000 per QALY is 21.2%; at £30,000 per QALY, sunitinib is the most cost-effective treatment, with a probability of 44.8% (see Figure 63).
Gastrointestinal and lung neuroendocrine tumours
The model parameter data are consistent with everolimus having an ICER of > £30,000 per QALY gained (Figure 64).
FIGURE 64.
Cost-effectiveness plane: everolimus vs. BSC in GI/lung NETs. WTP, willingness to pay.

The probability that everolimus for GI and lung NETs is the most cost-effective treatment at the willingness-to-pay thresholds of £20,000 per QALY and £30,000 per QALY gained is 0.9% and 10.5% respectively (Figure 65).
FIGURE 65.
Cost-effectiveness acceptability curves of treatments in GI/lung NETs.

Gastrointestinal (midgut) neuroendocrine tumours
The model parameter data are consistent with everolimus having an ICER of > £30,000 per QALY gained (Figure 66).
FIGURE 66.
Cost-effectiveness plane: everolimus vs. BSC in GI (midgut) NETs. WTP, willingness to pay.
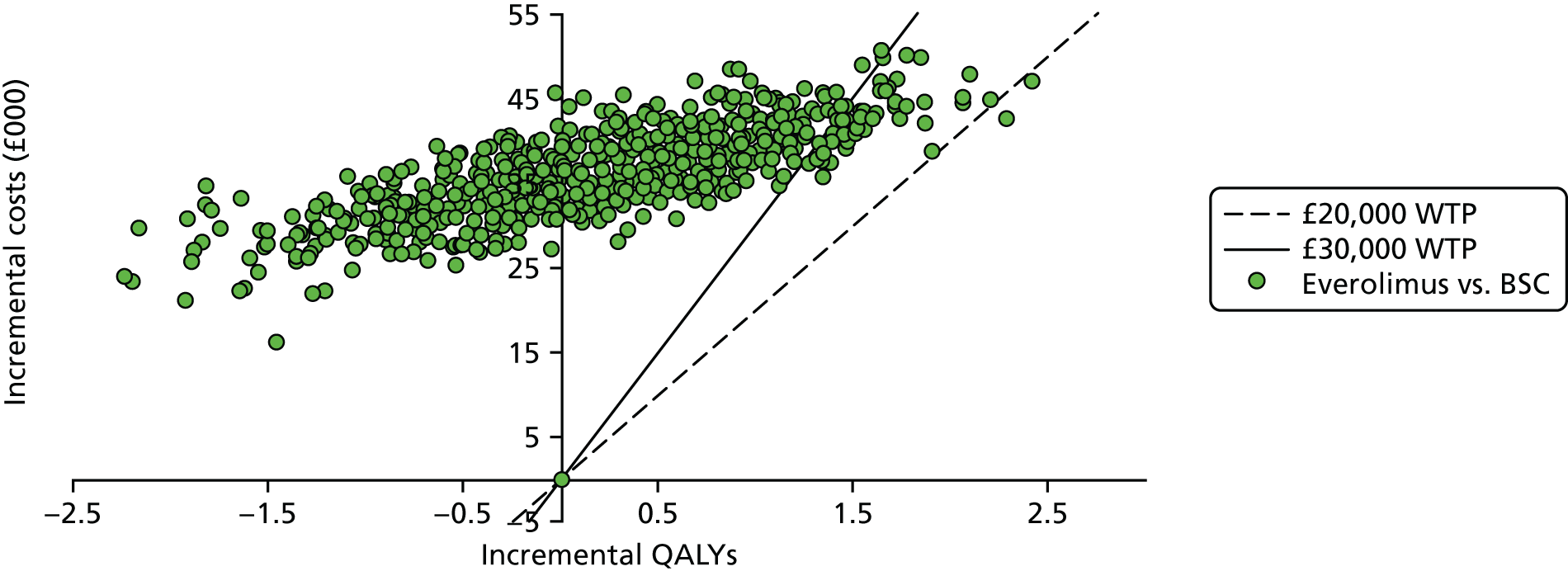
The probability that everolimus for GI (midgut) NETs is the most cost-effective treatment at the willingness-to-pay thresholds of £20,000 per QALY and £30,000 per QALY gained is 0.1% and 2.5% respectively (Figure 67).
FIGURE 67.
Cost-effectiveness acceptability curves of treatments in GI (midgut) NETs.
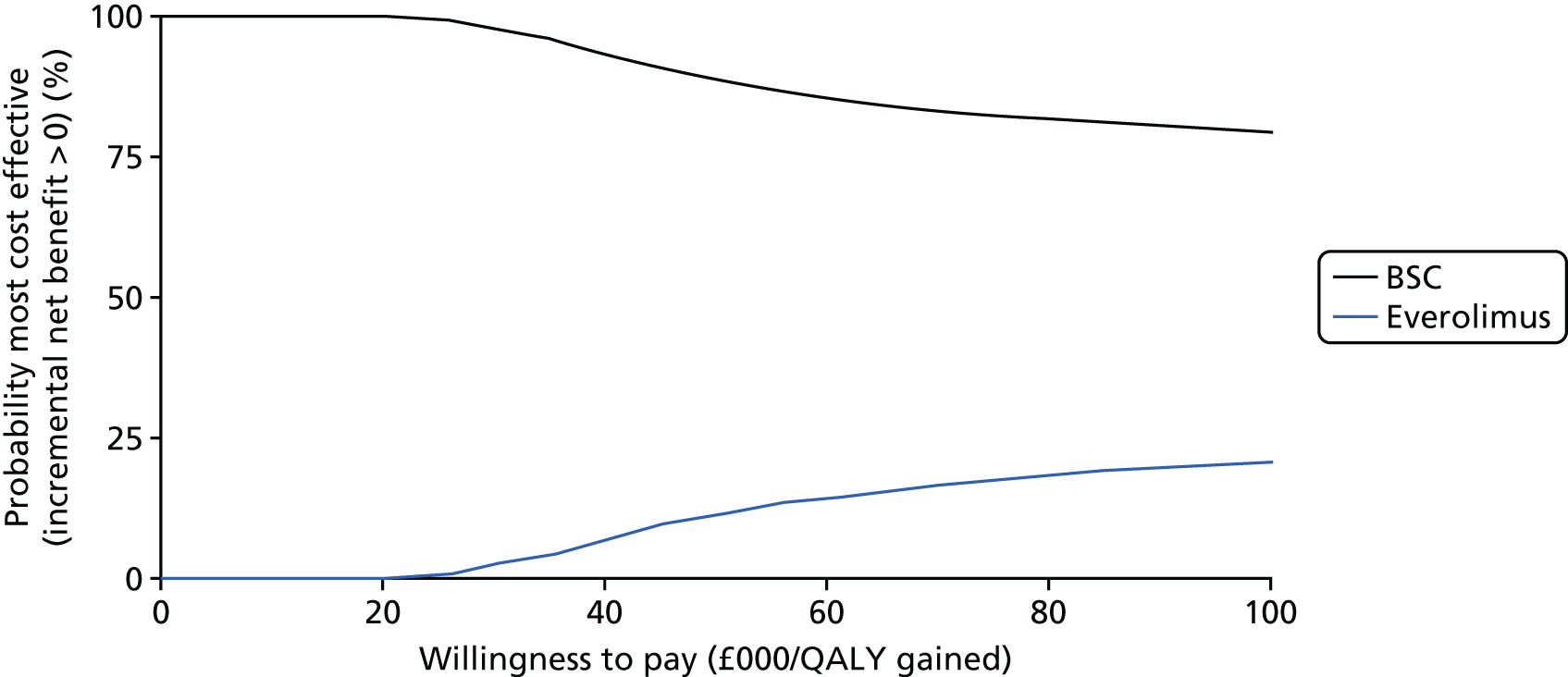
Deterministic sensitivity analyses
We varied parameters to either side of their point estimates by 20%, except for utility differences between stable disease and progressive disease, which were varied by 40%.
Pancreatic neuroendocrine tumours
In pNETs the OS HR is the most influential parameter in the model, particularly in relation to the ICER for everolimus, which varies from £25,000 to £105,000 with the treatment effect parameter variation of 20% around the mean point estimate (Figure 68). Other influential parameters include RDI and treatment duration. The utility of progressive disease and stable disease are much less influential and are ranked fourth on the list of most influential parameters in the model, with a larger influence on the ICER for sunitinib (Figure 69).
FIGURE 68.
Tornado analysis of everolimus treatment in pNETs. PD, progressive disease; SD, stable disease.
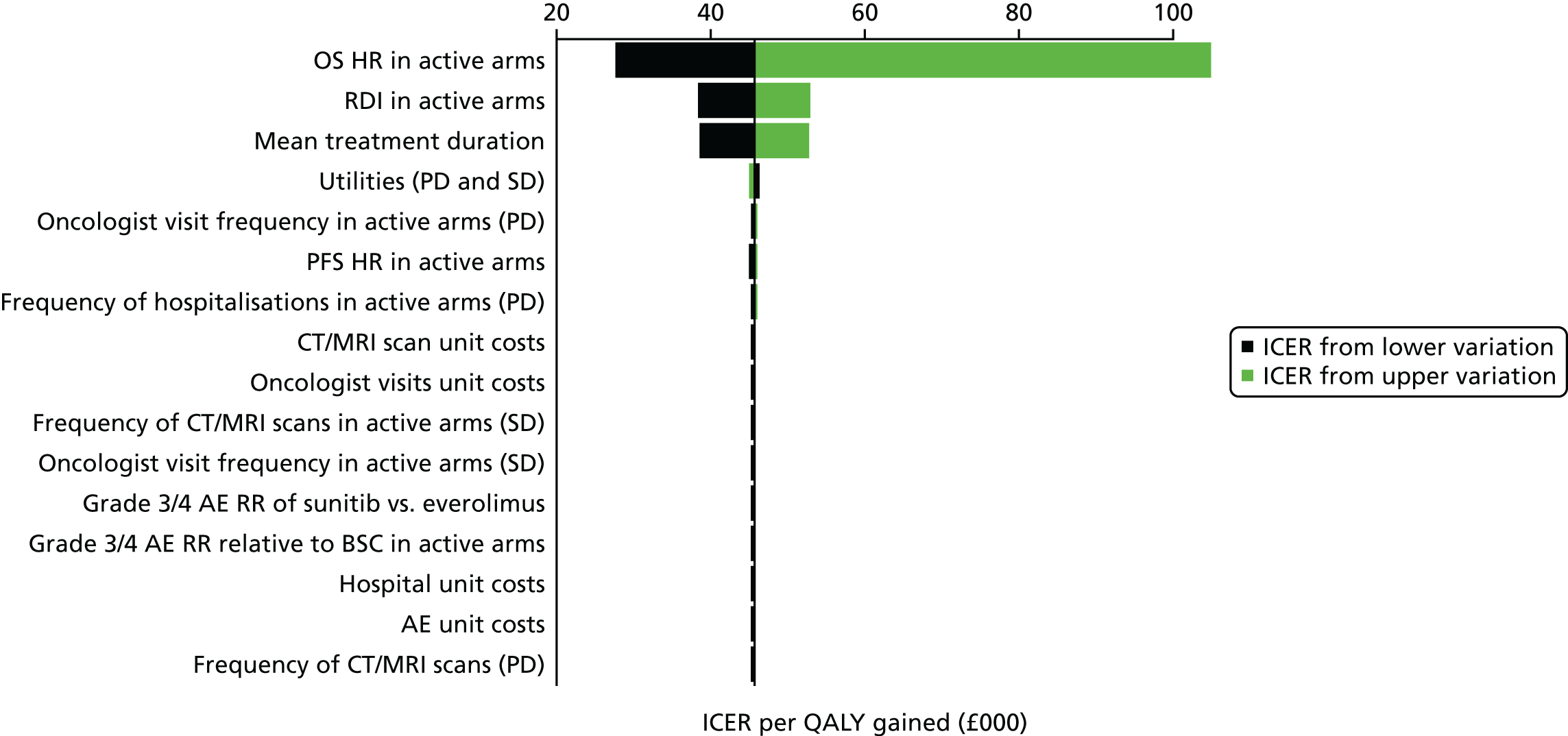
FIGURE 69.
Tornado analysis of sunitinib treatment in pNETs. PD, progressive disease; SD, stable disease.

Gastrointestinal and lung neuroendocrine tumours
Similar results to those for pNETs were found for GI and lung NETs, with variations around the point estimate of the OS HR of ±20% yielding an increase of 300% or a decrease of about 50% in the ICER for everolimus; RDI and mean treatment duration had smaller but significant effects (Figure 70).
FIGURE 70.
Tornado analysis of everolimus treatment in GI and lung NETs. PD, progressive disease; SD, stable disease.

Gastrointestinal (midgut) neuroendocrine tumours
The OS HR is still the most influential parameter; varying it by 20% above and below the base case produces an ICER that varies from £43,000 to a dominated value (shown in Figure 71 as a missing bar to the right-hand side of the point estimate). Unlike for treatments in other locations, the ICER for everolimus in GI (midgut) NETs was sensitive to the variation in the PFS HR. This, however, is partly an artefact of how OS was populated in the model; whereas for other locations OS data were available, for GI (midgut) NETs we did not have OS data available and thus had to rely on imputation based on PFS differences compared with the placebo plus BSC arm in RADIANT-4. 35 As a consequence, part of the effect of PFS depicted in Figure 71 is an indirect effect through OS.
FIGURE 71.
Tornado analysis of everolimus treatment in GI (midgut) NETs. PD, progressive disease; SD, stable disease.

The cost-effectiveness of 177Lu-DOTATATE is almost equally sensitive to the OS and PFS HRs, with utilities the third most influential parameter (Figure 72).
FIGURE 72.
Tornado analysis of 177Lu-DOTATATE treatment in GI (midgut) NETs. PD, progressive disease; SD, stable disease.
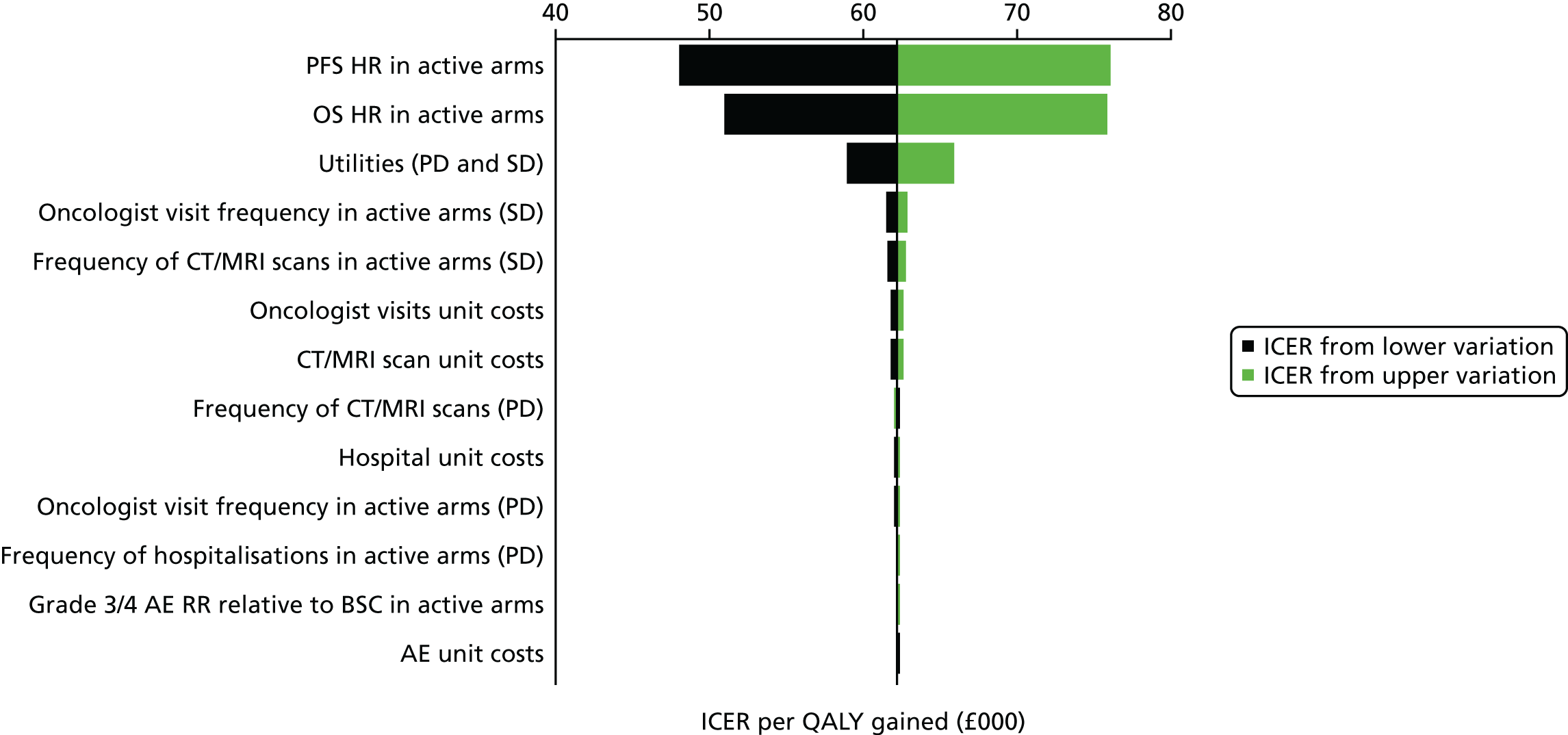
Appendix 19 Addendum 2
Gastrointestinal neuroendocrine tumours
The comparison between treatment with everolimus and treatment with BSC for the whole GI NETs patient subpopulation in RADIANT-435 yielded an ICER of £26,383 (Table 126). Treatment of these patients with everolimus results in better survival (8.25 years vs. 5.19 for BSC). Likewise, the treatment costs in the everolimus arm are higher; this is driven by the drug acquisition costs in the preprogression health state (Table 127).
| Everolimus | BSC | Everolimus vs. BSC | |
|---|---|---|---|
| Life-years (mean, undiscounted) | 8.25 | 5.19 | 3.06 |
| QALYs (mean, discounted) | 4.69 | 3.24 | 1.45 |
| Total costs (mean, discounted) (£) | 55,499 | 17,305 | 38,193 |
| ICER (cost per QALY) (£) | 26,383 |
| Everolimus | BSC | Everolimus vs. BSC | |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| Pre progression | 1.65 | 0.90 | 0.75 |
| Post progression | 6.59 | 4.28 | 2.31 |
| Total | 8.25 | 5.19 | 3.06 |
| QALYs (mean, discounted) | |||
| Pre progression | 1.20 | 0.71 | 0.49 |
| Post progression | 3.49 | 2.53 | 0.96 |
| Total | 4.69 | 3.24 | 1.45 |
| Costs (mean, discounted) (£) | |||
| Pre progression | |||
| Drug acquisition | 29,823 | 406 | 29,417 |
| Drug administration | 168 | 3 | 165 |
| Medical management | 4779 | 2202 | 2577 |
| AEs | 171 | 34 | 137 |
| Total (pre progression) | 34,940 | 2644 | 32,296 |
| Post-progression | |||
| Drug acquisition | 5621 | 2663 | 2958 |
| Drug administration | 27 | 10 | 17 |
| Medical management | 11,533 | 8296 | 3237 |
| End-of-life care | 3377 | 3692 | –315 |
| Total (post progression) | 20,558 | 14,661 | 5897 |
| Total | 55,499 | 17,305 | 38,193 |
| ICER (cost per QALY) | 26,383 | ||
It must be noted that these analyses were derived using:
-
mean treatment durations from exponential extrapolations of the median everolimus treatment duration (40 weeks) reported for the GI subgroup provided by Novartis on 11 November 2016 in response to a data request by the AG
-
exponential curves fitted to individual patient data derived from OS Kaplan–Meier curves and number of patients at risk data provided by Novartis for the GI subgroup of RADIANT-435 in response to the assessment report produced by the AG [data cut-off 30 November 2015, resulting in a HR of 0.65 (95% CI 0.37 to 1.13); the GI HR of 0.57 (95% CI 0.28 to 1.16) was provided by Novartis on 11 November 2016 in response to a data request by the AG]
-
exponential curves fitted to individual patient data derived from PFS Kaplan–Meier curves for the GI subgroup reported in the ASCO poster by Singh et al. 73 (data cut-off date 28 November 2014; from PFS curves for midgut and non-midgut subgroups in RADIANT-4,35 resulting in a HR of 0.54 (95% CI 0.36 to 0.82); the GI HR of 0.56 (95% CI 0.37 to 0.84) was provided by Novartis on 11 November 2016, in response to a data request by the AG)
-
all other parameters were assumed to be the same as for the GI/lung patient population in RADIANT-4. 35
Alternative set of utility values
With alternative utility values, sunitinib has an ICER of £19,411 in patients with pNETs and everolimus has an ICER of £28,063 in the overall GI NETs population. All other evaluated treatments have ICER values of > £30,000 (Table 128).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 41,246 |
| Sunitinib | BSC | 19,411 | |
| GI (midgut) | Everolimus | BSC | 352,801 |
| 177Lu-DOTATATE | BSC | 57,745 | |
| GI and lung | Everolimus | BSC | 49,949 |
| Lung | Everolimus | BSC | 32,413 |
| GI | Everolimus | BSC | 28,063 |
Analysis limited to progression-free survival
Limiting the analytical horizon to the time of disease progression results in 177Lu-DOTATATE having the lowest ICER (£30,115) of all of the evaluated treatments and tumour locations (Table 129).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 57,493 |
| Sunitinib | BSC | 35,448 | |
| GI (midgut) | Everolimus | BSC | 88,801 |
| 177Lu-DOTATATE | BSC | 30,115 | |
| GI and lung | Everolimus | BSC | 73,086 |
| Lung | Everolimus | BSC | 91,202 |
| GI | Everolimus | BSC | 65,775 |
Background mortality adjustments to overall survival and progression-free survival curves
After background mortality adjustment only sunitinib treatment in pNETs had an ICER of < £30,000. Everolimus treatment in the overall GI NETs population had an ICER of £31,353 (Table 130).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 44,032 |
| Sunitinib | BSC | 21,594 | |
| GI (midgut) | Everolimus | BSC | 78,330 |
| 177Lu-DOTATATE (no mortality adjustment) | BSC | 43,348 | |
| GI and lung | Everolimus | BSC | 46,687 |
| Lung | Everolimus | BSC | 33,908 |
| GI | Everolimus | BSC | 31,353 |
First-cycle costs and disease monitoring
When the costs of subsequent treatments are included, sunitinib had an ICER of £20,624 in pNETs and everolimus had an ICER of £27,834 in the overall GI NETs population (Table 131).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 45,288 |
| Sunitinib | BSC | 20,624 | |
| GI (midgut) | Everolimus | BSC | 208,095 |
| 177Lu-DOTATATE | BSC | 61,619 | |
| GI and lung | Everolimus | BSC | 47,205 |
| Lung | Everolimus | BSC | 32,744 |
| GI | Everolimus | BSC | 27,834 |
Everolimus had an ICER of £27,669 in the overall GI NETs population when the cost of disease monitoring that Novartis used in its model submitted to NICE was adopted (Table 132).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| GI (midgut) | Everolimus | BSC | 205,437 |
| 177Lu-DOTATATE | BSC | 64,513 | |
| GI and lung | Everolimus | BSC | 46,249 |
| Lung | Everolimus | BSC | 32,221 |
| GI | Everolimus | BSC | 27,669 |
Scenario analysis using a 0% discount rate
When no discounting is applied to costs and benefits the ICER for everolimus in the overall GI NETs population is £20,184 (Table 133).
| Tumour location | Treatment | Comparator | ICER (£) |
|---|---|---|---|
| Pancreas | Everolimus | BSC | 38,021 |
| Sunitinib | BSC | 17,605 | |
| GI (midgut) | Everolimus | BSC | 131,512 |
| 177Lu-DOTATATE | BSC | 49,907 | |
| GI and lung | Everolimus | BSC | 34,367 |
| Lung | Everolimus | BSC | 26,114 |
| GI | Everolimus | BSC | 20,184 |
Probabilistic sensitivity analyses
The model parameter data are consistent with everolimus having an ICER that is > £30,000 in the overall GI NETs population (Figure 73).
FIGURE 73.
Cost-effectiveness plane: treatments for the overall GI NETs population. WTP, willingness to pay.

For GI NETs, the probabilistic mean ICER is £27,582. The probability that everolimus is the most cost-effective treatment for GI NETs at the willingness-to-pay thresholds of £20,000 per QALY and £30,000 per QALY gained is 18.3% and 55.5%, respectively (Figure 74).
FIGURE 74.
Cost-effectiveness acceptability curve: treatments for overall GI NETs.

Appendix 20 Background tables
| Site | T stage | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Stomach | Invasion of the (sub)mucosa and size ≤ 1 cm | Invasion of the muscularis propria or size > 1 cm | Invasion of the subserosa | Perforation of the serosa or invasion of adjacent structures |
| Duodenum, ampulla, upper jejunum | Invasion of the (sub)mucosa and size ≤ 1 cm | Invasion of the muscularis propria or size > 1 cm | Invasion of the pancreas or retroperitoneum | Invasion of the peritoneum or other organs |
| Lower jejunum, Ileum | Invasion of the (sub)mucosa and size ≤ 1 cm | Invasion of the muscularis propria or size > 1 cm | Invasion of the subserosa | Invasion of the peritoneium or other organs |
| Colon/rectum | Invasion of the (sub)mucosa. T1a: size < 1 cm; T1b: size 1–2 cm | Invasion of the muscularis propria or size > 2 cm | Invasion of the subserosa/pericolic/perirectal fat | Invasion of the peritoneum or other organs/structures |
| Appendix | Size ≤ 2 cm. T1a: < 1 cm, T1b: > 1 cm to < 2 cm | Size ≥ 2 to ≤ 4 cm or extension to the caecum | Size > 4 cm or extension to the ileum | Perforation of the peritoneum or invasion of other organs |
| Pancreas | Limited to the pancreas and size < 2 cm | Limited to the pancreas and size > 2 cm | Outside the pancreas but no invasion of the coeliac axis/SMA of any size | Invasion of the coeliac axis/SMA |
| Site | T stage | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Stomach | Invasion of the (sub)mucosa and size < 1 cm | Invasion of the muscularis propria or subserosa or size > 1 cm | Penetration of the serosa | Invasion of adjacent structures |
| Appendix | Size < 1 cm and invasion of the submucosa or muscularis propria | Size < 2 cm and invasion of the submucosa, muscularis propria and/or invasion of < 0.3 cm into the subserosa/mesoappendix | Size > 2 cm and/or invasion of > 0.3 cm into the subserosa/mesoappendix | Invasion of the peritoneum or other organs |
| Pancreas | Limited to the pancreas and size < 2 cm | Limited to the pancreas and size 2–4 cm | Limited to the pancreas and size > 4 cm or invasion of the duodenum or bile duct | Invasion of the coeliac axis/SMA, stomach, spleen, colon or adrenal gland |
Appendix 21 Adverse events: indirect comparison
| AE | RADIANT-334 (everolimus, N = 204; placebo, N = 203) | A618111181 (sunitinib, N = 83; placebo, N = 82 ) | OR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Odds | n | % | Odds | Everolimus vs. placebo | Sunitinib vs. placebo | Bucher IC | |||||||
| Everolimus | Placebo | Everolimus | Placebo | Everolimus | Placebo | Sunitinib | Placebo | Sunitinib | Placebo | Sunitinib | Placebo | ||||
| Neutropenia | 0 | 0 | 0.25 | 0.25 | 0.002 | 0.002 | 10 | 0 | 12.70 | 0.60 | 0.145 | 0.006 | 1.00 | 23.61 | 23.723 |
| Hypertension | 0 | 0 | 0.25 | 0.25 | 0.002 | 0.002 | 8 | 0 | 10.20 | 0.60 | 0.114 | 0.006 | 1.00 | 18.60 | 18.689 |
| PPE syndrome | 0 | 0 | 0.25 | 0.25 | 0.002 | 0.002 | 5 | 0 | 6.60 | 0.60 | 0.071 | 0.006 | 1.00 | 11.57 | 11.625 |
| Leukopenia | 0 | 0 | 0.25 | 0.25 | 0.002 | 0.002 | 5 | 0 | 6.60 | 0.60 | 0.071 | 0.006 | 1.00 | 11.57 | 11.625 |
| Diarrhoea | 7 | 1a | 3.43 | 0.49 | 0.036 | 0.005 | 4 | 1 | 4.80 | 1.20 | 0.051 | 0.012 | 7.18 | 4.10 | 0.571 |
| Stomatitis | 14b | 0 | 7.11 | 0.25 | 0.077 | 0.002 | 3 | 0 | 4.20 | 0.60 | 0.044 | 0.006 | 30.99 | 7.18 | 0.232 |
| Thrombocytopenia | 8 | 0 | 4.17 | 0.25 | 0.043 | 0.002 | 3 | 0 | 4.20 | 0.60 | 0.044 | 0.006 | 17.61 | 7.18 | 0.408 |
| Anaemia | 12c | 0 | 6.13 | 0.25 | 0.065 | 0.002 | 0 | 0 | 0.60 | 0.60 | 0.006 | 0.006 | 26.44 | 0.99 | 0.037 |
| Hyperglycaemia | 11d | 4e | 5.39 | 1.97 | 0.057 | 0.02 | 0 | 0 | 0.60 | 0.60 | 0.006 | 0.006 | 2.84 | 0.99 | 0.348 |
| Fatigue | 5f | 1 | 2.45 | 0.49 | 0.025 | 0.005 | 4g | 3h | 4.80 | 3.70 | 0.051 | 0.038 | 5.08 | 1.33 | 0.263 |
| Infections | 5 | 1 | 2.45 | 0.49 | 0.025 | 0.005 | 0 | 0 | 0.60 | 0.60 | 0.006 | 0.006 | 5.08 | 0.99 | 0.195 |
| Pneumonitis | 5 | 0 | 2.70 | 0.25 | 0.028 | 0.002 | 0 | 0 | 0.60 | 0.60 | 0.006 | 0.006 | 11.22 | 0.99 | 0.088 |
| Nausea | 5i | 0 | 2.70 | 0.25 | 0.028 | 0.002 | 1j | 0 | 1.80 | 0.60 | 0.018 | 0.006 | 11.22 | 3.00 | 0.267 |
| Sum | 72 | 7 | 35.30 | 3.45 | 0.545 | 0.036 | 43k | 4l | 51.80 | 4.90 | 1.075 | 0.051 | 15.27 | 20.96 | 1.372m |
Appendix 22 Mapping FACT-G to the EuroQol-5 Dimensions
Linearised version of the best-fitting mapping algorithm of Longworth et al.134
The best-fitting mapping algorithm for the FACT-G estimated by Longworth et al. 134 (Table 137) maps the domain scores of that tool into each dimension of the EQ-5D-3L by fitting a multinomial logit model to response data for each dimension separately.
| Summary statistics and model performance test | Mobility | Self-care | Usual activities | Pain | Anxiety/depression | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Some problems | Extreme problems | Some problems | Extreme problems | Some problems | Extreme problems | Some problems | Extreme problems | Some problems | Extreme problems | |
| Physical | –0.111 (0.023)*** | NA | –0.100 (0.024)*** | –0.244 (–2.191) | –0.237 (0.044)*** | –0.285 (0.056)*** | –0.206 (0.030)*** | –0.319 (0.051)*** | ||
| Emotional | –0.331 (0.036)*** | –0.607 –5.147 | ||||||||
| Functional |
–0.074 (0.020)*** |
NA | –0.104 (0.027)*** | –0.307 (–6.663) | –0.124 (0.030)*** | –0.266 (0.053)*** | –0.057 (0.023)* | 0.01 (0.053) | –0.047 (0.021)* | –0.197 (1.465) |
| Constant |
3.089 (0.418)*** |
NA | 1.633 (0.427)*** | 2.017 (60.731) | 7.737 (0.895)*** | 8.239 (1.210)*** | 5.499 (0.574)*** | 3.51 (1.045)** | 6.773 (0.660)*** | 8.839 (47.729) |
| Log-likelihood | –310.22 | –189.70 | –338.3 | –346.92 | –302.08 | |||||
| Pseudo R2 | 0.132 | 0.151 | 0.263 | 0.191 | 0.263 | |||||
| AIC | 626.44 | 391.39 | 688.27 | 705.84 | 616.16 | |||||
| BIC | 639.26 | 417.03 | 713.91 | 731.48 | 641.8 | |||||
The EQ-5D index score equation (Dolan235) is:
where D1 = 1 if the person had any problems in any dimension and zero otherwise, and D2 = 1 if he had any severe problems in any dimension and zero otherwise; Mob is a binary indicator of reporting problems in the mobility dimension; Selfcare is a binary indicator of problems in self-care; Usualact is a binary indicator of problems with usual activities; Pain a binary indicator of problems in pain/discomfort; and Anx is an indicator of problems in anxiety/depression. Separate indicators are used for moderate and severe problems, denoted by the subscripts m and s respectively.
The mapping algorithm substitutes the binary indicators with the corresponding predicted probabilities of reporting the problem in question. As in the EQ-5D-3L, there are two levels of a problem that a person can choose as the response, the polytomous regression model is required to calculate the predicted probabilities of reporting a given level of problem (moderate or severe) for a given dimension. As for the predicted probabilities of reporting any problems across all dimensions (corresponding to D1) and of reporting any severe problems across all dimensions, these could be obtained by running separate regression analyses for dichotomous variables or by assuming that the probability of reporting problems in a dimension is independent of doing so in any other dimension. In the latter case, the predicted probability of reporting any severe problems (D2 = 1) is simply equal to:
where the hat symbols denote predicted probabilities of reporting the problem described by the respective variable. The value of D1^ is similarly obtained, except that the expressions within brackets in Equation 7 now include the predicted probability of reporting moderate problems:
The Longworth et al. 134 response mapping algorithm (p. 220) is then used to estimate the predicted probabilities of D1 and D2 and the 10 probabilities of reporting problem levels for the EQ-5D dimensions on the right-hand side of Equations 7 and 8. In the case of Mobm^ this is obtained by:
where Pm2j is the probability of a person j reporting ‘moderate problems’ for the EQ-5D dimension m (mobility) and the Greek symbols represent coefficients estimated by Longworth et al. 134 from a multinomial regression of a dependent variable D, taking the value of 1 for ‘no problems’, 2 for ‘moderate’ problems and 3 for ‘severe’ problems, against the three FACT-G domains of physical (PW), emotional (EW) and functional (FW) well-being. The predicted probability of choosing level 3 for the dimension m is given by the same formula but with the subscripts 2 and 3 reversed. In the same way, predicted probabilities for the other eight domain predictions in Equations 7 and 8 are obtained, based on the coefficients of the multinomial regression for the respective dimension.
With individual patient data on FACT-G available, the predicted probabilities for Equations 7 and 8, which are then used to derive EQ-5D utilities using Equation 6, are obtained by first substituting the FACT-G scores for each person in Equation 9 and then taking the mean of those predictions across the whole sample. Repeating this process using the corresponding equation of P for ‘severe’ mobility and all other eight possible responses provides the required probabilities to map FACT-G data into EQ-5D utilities using Equation 6.
When, as is common in multiple technology assessment reviews or economic modelling studies, only aggregate data are available in the form of mean FACT-G scores for a sample of patients, one cannot directly use those values in Equation 9 and the other nine multinomial equations for obtaining the required predicted response probabilities because their non-linear form means that the resulting predictions will have systematic errors. To solve this issue it is proposed that each of Equation 9 and the other nine multinomial probability equations be approximated using a first-order Taylor series expansion around the midpoint of the FCAT-G mean covariate scores that we had available for the two health states (before progression and after progression) in RADIANT-4,35 Thus, the linearised predictor of the probability of response in Equation 9 is:
where Pm2(PWo,EWo,FWo) represents Equation 9 evaluated at the midpoint value between the mean scores of the FACT-G domains PW, EW and FW for observations in the stable disease and disease progression states. (Here, the issue of missing data or information lost to follow-up is ignored but is a pertinent issue to address in further research). The derivatives of the Pm2 function are also evaluated at the midpoint and have the following expressions:
Similar expressions are used for the other derivatives in Equation 10 and in the corresponding equations for other EQ-5D dimensions and levels. Note that Equation 10 and the corresponding equations for other dimensions and levels are linear in the FACT-G domain scores, which is convenient as they may be used to approximate mean EQ-5D scores in a group of patients when only aggregate FACT-G data are available by substituting the mean FACT-G domain scores PW¯, EW¯ and FW¯.
Finally, substituting expressions such as Equation 10 (after substituting Equation 11 into Equation 10) for all arguments in Equation 6 leads to the linearised mapped EQ-5D score. This linearised mapped FACT-G function was used to approximate utilities for stable disease and disease progression using only data points for PW¯, EW¯ and FW¯. for the two phases, reported in Singh et al. ,73 to reproduce their reported mapped utilities, which used the original best-fitting response mapping non-linear Longworth et al. 134 algorithm with unpublished individual patient data. Table 138 presents the two sets of these estimates.
| Statistics | Unadjusted model including response status (pre- vs. post-progression) as a single categorical fixed-effects covariate | |||||
|---|---|---|---|---|---|---|
| Response status: pooled analysis | Response status: pooled analysis – linearised algorithm + summary domain scores | |||||
| Pre progression | Post progression | Pre progression | Post progression | |||
| Young mapping algorithm (n = 1499) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||
Appendix 23 Comparison of the Assessment Group results with the company results
Our model results can be compared with those of the companies in three areas:
-
everolimus, sunitinib and BSC in pNETs
-
everolimus and BSC in GI and lung NETs
-
everolimus, 177Lu-DOTATATE and BSC in GI (midgut) NETs.
In all analyses, drug list prices, not PAS prices, were assumed. Life-years were not discounted.
Everolimus, sunitinib and best supportive care in pancreatic neuroendocrine tumours
A compilatation of the main results for pNETs is presented in Table 139.
| Outcome | Everolimus | Sunitinib | BSC | ||||
|---|---|---|---|---|---|---|---|
| PenTAG | Novartis33 | AAA31 | PenTAG | Novartis33 | AAA31 | PenTAG | |
| Pre progression | |||||||
| Drug acquisition (£)a | 25,547 | 21,782 | Confidential information has been removed | 22,216 | 21,994 | 59,557 | 2003 |
| Drug administration (£) | 1104 | b | Confidential information has been removed | 1308 | b | 0 | 510 |
| Disease monitoring and management (£) | 776 | 5343 | Confidential information has been removed | 952 | 5242 | 2290 | 184 |
| SAE management (£) | 132 | 678 | Confidential information has been removed | 89 | 2101 | 91 | 15 |
| Post progression | |||||||
| Drug acquisition (£) | 6363 | 2216 | Confidential information has been removed | 8368 | 2216 | 56,667 | 4939 |
| Drug administration (£) | 1706 | b | Confidential information has been removed | 2187 | b | 965 | 1422 |
| Disease monitoring and management (£) | 3798 | 7206 | Confidential information has been removed | 5032 | 7206 | 5138 | 3447 |
| SAE management (£) | 0 | 0 | Confidential information has been removed | 0 | 0 | 205 | 0 |
| Death | |||||||
| End-of-life care (£) | 3747 | 3836 | Confidential information has been removed | 3565 | 3836 | 0 | 3889 |
| Total costs pre-progression (£) | 27,559 | 27,802 | Confidential information has been removed | 24,566 | 29,337 | 61,939 | 2712 |
| Total costs post-progression (£) | 11,867 | 9422 | Confidential information has been removed | 15,587 | 9422 | 62,976 | 9808 |
| Total costs (£) | 43,173 | 41,061 | Confidential information has been removed | 43,718 | 42,596 | 124,914 | 16,409 |
| Life-years pre progressionc | 1.28 | 1.18 | Confidential information has been removed | 1.60 | 1.18 | 2.22 | 0.57 |
| Life-years post progressionc | 3.41 | 3.44 | Confidential information has been removed | 4.79 | 3.44 | 5.98 | 2.89 |
| Total life-yearsc | 4.69 | 4.62 | Confidential information has been removed | 6.39 | 4.62 | 8.19 | 3.46 |
| QALYs pre progression | Confidential information has been removed | 0.89 | Confidential information has been removed | Confidential information has been removed | 0.87 | 1.60 | 0.38 |
| QALYs post progression | Confidential information has been removed | 1.84 | Confidential information has been removed | Confidential information has been removed | 1.84 | 3.74 | 1.53 |
| Total QALYs | Confidential information has been removed | 2.73 | Confidential information has been removed | Confidential information has been removed | 2.71 | 5.34 | 1.91 |
-
Life-years. The estimation of expected life-years in our model and the models of Novartis33 and AAA31 differed substantially for sunitinib but were consistent for everolimus. Novartis assumed no difference in PFS or OS between everolimus and sunitinib (mean of 4.62 life-years for both treatments), whereas we estimated a superior PFS and OS for people treated with sunitinib (OS of 6.39 years vs. 4.62 years). AAA’s OS estimate was significantly larger for sunitinib (8.19 years for sunitinib and 4.62 years for everolimus). The differences arise because of the adoption of three different methodological approaches, including differences in the parametric distribution selected for PFS/OS extrapolation. AAA did not adjust for treatment crossover in its MTC; Novartis used an assumption of no difference in OS from the outset; and we used publicly available survival curves with statistical adjustment for treatment crossover for each trial in our MTC.
-
QALYs. After adjusting for quality of life, our own and Novartis’s QALY estimates for everolimus remained similar [(confidential information has been removed) and 2.73], but AAA’s estimate of time with stable disease was higher, resulting in a higher estimate of total QALYs (3.25). Our estimate of QALYs with sunitinib was higher than that for everolimus, because of longer PFS and OS. Novartis estimated fewer QALYs with sunitinib than everolimus in spite of equal PFS and OS, because of differences in disutility from SAEs. From a MTC of the most up-to-date evidence submitted to NICE (see Appendix 21), we found the difference in the incidence of AEs between the two treatments to be unlikely to result in meaningful utility differences.
-
Costs. Treatment strategy estimates of total cost were consistent across models, including the within-model similarity between everolimus and sunitinib. Novartis found the cost of treatments to be less, but this is accounted for by its inclusion of other drug treatments under disease monitoring and management. The same methodological difference is behind the differences in component costing post progression. AAA’s estimate of everolimus and sunitinib strategy costs were significantly higher (227% and 289% respectively). In each case, this is accounted for by the overcosting of the acquisition of the active drug, because of the company’s assumption that treatment would continue until disease progression.
-
Incremental analysis compared with BSC. Given that neither Novartis nor AAA included a BSC strategy, it is not possible to compare our ICERs for everolimus compared with BSC and sunitinib compared with BSC with company estimates.
Everolimus and best supportive care in gastrointestinal and lung neuroendocrine tumours
Overall, there was consistency between the cost-effectiveness results produced by us and those produced by Novartis. A compilation of the main results is presented in Tables 139 and 140.
| Outcome | Everolimus | BSC | ||
|---|---|---|---|---|
| PenTAG | Novartis | PenTAG | Novartis | |
| Pre progression | ||||
| Drug acquisition (£)a | 26,054 | 26,881 | 376 | 0 |
| Drug administration (£) | 147 | b | 2 | b |
| Disease monitoring and management (£) | 4141 | 8583 | 2038 | 2799 |
| SAE management (£) | 171 | 601 | 34 | 87 |
| Post progression | ||||
| Drug acquisition (£) | 4331 | 2927 | 2511 | 3312 |
| Drug administration (£) | 21 | b | 10 | b |
| Disease monitoring and management (£) | 8886 | 17,205 | 7822 | 15,918 |
| SAE management (£) | 0 | 0 | 0 | 0 |
| Death | ||||
| End-of-life care (£) | 3583 | 3524 | 3732 | 3702 |
| Total costs pre progression (£) | 30,513 | 36,064 | 2450 | 2886 |
| Total costs post progression (£) | 13,238 | 20,132 | 10,343 | 19,230 |
| Total costs (£) | 47,334 | 59,720 | 16,526 | 25,817 |
| Life-years pre progressionc | 1.42 | 1.68 | 0.83 | 0.89 |
| Life-years post progressionc | 4.79 | 5.51 | 3.99 | 4.90 |
| Total life-yearsc | 6.21 | 7.19 | 4.82 | 5.79 |
| QALYs pre progression | 1.04 | 1.23 | 0.65 | 0.68 |
| QALYs post progression | 2.70 | 3.05 | 2.39 | 2.83 |
| Total QALYs | 3.74 | 4.28 | 3.05 | 3.51 |
| Outcome | PenTAG | Novartis |
|---|---|---|
| Pre progression | ||
| Drug acquisition (£)a | 25,679 | 26,881 |
| Drug administration (£) | 144 | b |
| Disease monitoring and management (£) | 2102 | 5784 |
| SAE management (£) | 137 | 513 |
| Post progression | ||
| Drug acquisition (£) | 1820 | –385 |
| Drug administration (£) | 11 | b |
| Disease monitoring and management (£) | 1064 | 1287 |
| SAE management (£) | 0 | 0 |
| Death | ||
| End-of-life care (£) | –149 | –178 |
| Total costs pre progression (£) | 28,063 | 33,178 |
| Total costs post progression (£) | 2895 | 902 |
| Total costs (£) | 30,809 | 33,903 |
| Life-years pre progressionc | 0.59 | 0.78 |
| Life-years post progressionc | 0.80 | 0.61 |
| Total life-yearsc | 1.39 | 1.40 |
| QALYs pre progression | 0.38 | 0.56 |
| QALYs post progression | 0.31 | 0.22 |
| Total QALYs | 0.69 | 0.78 |
| Cost per life-year gained (£) | 22,213 | 33,298 |
| Cost per QALY gained (£) | 44,557 | 43,642 |
-
Life-years. For people receiving BSC our own model and Novartis’s model33 found the number of expected life-years to be similar, at 4.82 and 4.77, respectively, with 0.83 and 0.87 years, respectively, of stable disease before progression. For people receiving everolimus we estimated life expectancy as 6.21 years, whereas Novartis estimated a value of 5.79 and we estimated a lower proportion with stable disease (23% vs. 27%). This was caused by our higher estimate of OS and lower estimate of PFS from our parametric extrapolation.
-
QALYs. For people receiving BSC we estimated a lower quality of life pre and post progression than Novartis, so, despite similar PFS and OS, slightly more QALYs for BSC were estimated by Novartis than by us (3.51 vs. 3.05). Similarly, for people receiving everolimus we found that our higher estimates of PFS and OS were more heavily adjusted for loss of quality of life than those of Novartis, so that our estimate of total QALYs for everolimus was lower than Novartis’s (3.74 vs. 4.28). This is because, although Novartis used the same utility values for stable disease and disease progression across arms, we adopted treatment arm-specific utility estimates for stable disease, with the utility estimate likely to be lower for everolimus than for BSC.
-
Costs. Our estimate of the cost of BSC was significantly less than that of Novartis (£16,526 vs. £25,817 per person) because the estimate of the cost of disease monitoring and management in the Novartis model was twice as high our estimate and also partly because we modelled fewer physician consultations. Our estimate of the cost of everolimus was also lower (£47,334 vs. £59,720). This was again because of our lower rate of resource utilisation for disease monitoring and management.
-
Incremental analysis. We estimated that the ICER for everolimus compared with BSC was £44,557 per QALY gained. Novartis found an ICER of £43,642 per QALY gained. We estimated that BSC was £30,809 less costly and provided 0.69 fewer QALYs. Novartis found that BSC was £33,903 less costly and provided 0.78 fewer QALYs.
Everolimus, 177Lu-DOTATATE and best supportive care in gastrointestinal (midgut) neuroendocrine tumours
For GI (midgut) NETs we modelled everolimus, 177Lu-DOTATATE and BSC, whereas AAA31 modelled only everolimus and 177Lu-DOTATATE. The main results are provided in Table 142.
| Everolimus | 177Lu-DOTATATE | BSC | |||
|---|---|---|---|---|---|
| PenTAG | AAA | PenTAG | AAA | PenTAG | |
| Pre progression | |||||
| Drug acquisition (£) | 31,786 | Confidential information has been removed | 59,187 | 59,633 | 633 |
| Drug administration (£) | 178 | Confidential information has been removed | 3482 | 1820 | 4 |
| Disease monitoring and management (£) | 5904 | Confidential information has been removed | 14,051 | 2702 | 3437 |
| SAE management (£) | 287 | Confidential information has been removed | 85 | 304 | 105 |
| Post progression | |||||
| Drug acquisition (£) | 3349 | Confidential information has been removed | 1093 | 21,235 | 2117 |
| Drug administration (£) | 16 | Confidential information has been removed | 5 | 723 | 8 |
| Disease monitoring and management (£) | 6871 | Confidential information has been removed | 2242 | 1925 | 6595 |
| SAE management (£) | 0 | Confidential information has been removed | 0 | 108 | 0 |
| Death | |||||
| End-of-life care (£) | 3627 | Confidential information has been removed | 3522 | – | 3728 |
| Total costs pre progression (£) | 38,155 | Confidential information has been removed | 76,805 | 64,459 | 4180 |
| Total costs post progression (£) | 13,863 | Confidential information has been removed | 6862 | 23,991 | 12,448 |
| Total costs (£) | 52,018 | Confidential information has been removed | 83,667 | 88,450 | 16,628 |
| Life-years pre progressiona | 2.07 | Confidential information has been removed | 5.41 | 2.66 | 1.43 |
| Life-years post progressiona | 3.68 | Confidential information has been removed | 1.25 | 2.13 | 3.46 |
| Total life-yearsa | 5.75 | Confidential information has been removed | 6.66 | 4.79 | 4.90 |
| QALYs pre progression | 1.48 | Confidential information has been removed | 3.51 | 1.97 | 1.10 |
| QALYs post progression | 2.09 | Confidential information has been removed | 0.68 | 1.31 | 2.01 |
| Total QALYs | 3.57 | Confidential information has been removed | 4.19 | 3.29 | 3.11 |
Our estimates of survival and costs for people who were treated with everolimus were significantly different from those reported by AAA. In contrast, there was some consistency in the cost results for the 177Lu-DOTATATE strategy produced by the AG and AAA.
-
Life-years. AAA’s31 estimates of OS for everolimus and 177-Lu-DOTATATE were substantially lower than our own. For 177Lu-DOTATATE the difference in life expectancy (4.79 life-years in AAA’s analysis vs. 6.66 life-years in the AG analysis) arose because of the different methods used for OS extrapolation: AAA used a proportional hazards treatment effect on a baseline Weibull distribution function, which showed an increasing trend in death risk, whereas we used an exponential distribution, which is characterised by a constant risk of death, supplemented by background mortality risk. AAA did not provide any statistical evidence in support of its proportional hazards model for 177Lu-DOTATATE in NETTER-1. We fitted separate parametric curves to 177Lu-DOTATATE in NETTER-1 and found that the exponential model was the model with the best goodness-of-fit statistics. The differences in survival time were most pronounced in the case of life-years post progression following everolimus, with AAA including lung and other non-midgut NETs patients from RADIANT-435 in its calculation, and a baseline risk of progression and death for both everolimus and 177Lu-DOATATE equal to that for people treated with 60 mg of octreotide; instead, we used the GI (midgut) subgroup of RADIANT-435 as the reference patient population, to which patients treated with 177Lu-DOTATATE were matched by a Bucher-type indirect comparison adjustment method.
-
QALYs. Our estimates of QALYs for everolimus and 177Lu-DOTATATE were also higher than AAA’s estimates (3.57 vs. 1.87 for everolimus; 4.19 vs. 3.29 for 177Lu-DOTATATE), although they were reduced by our lower estimate of utility for both pre and post progression. Therefore, the difference in total QALYs between the models was driven by the difference in life-year estimates. We found that BSC produced fewer QALYs (3.11) than the active treatments.
-
Costs. Similar to AAA, we found that totals costs for 177Lu-DOTATATE were higher than those for everolimus. However, our estimates were lower than those of AAA and there were significant differences in component costs. Comparing everolimus across models, the singular significant difference in costs was for drug acquisition; this is because AAA costed everolimus treatment until progression and did not adjust for RDI. In RADIANT-435 the median time to progression was 11 months, compared with a median time on treatment of 9.3 months, and the RDI was 79.4%. Comparing 177Lu-DOTATATE across models there was agreement on the total cost but notable differences in the costs of disease monitoring and management in stable disease, drug acquisition in progressive disease and end-of-life care. This is because AAA did not include the cost of hospital consultations, assumed that every patient was treated with octreotide from progression until death and opted not to include end-of-life/palliative care costs. In summation, these under- and overestimates were counterbalancing.
-
Incremental analysis. As AAA did not include a BSC strategy, there were no company figures with which to compare our estimated additional cost, QALY gains and ICER for 177Lu-DOTATATE versus BSC.
List of abbreviations
- 177Lu-DOTATATE
- lutetium-177 DOTATATE
- AAA
- Advanced Accelerator Applications
- AE
- adverse event
- AFT
- accelerated failure time
- AG
- Assessment Group
- AIC
- Akaike information criterion
- ASCO
- American Society of Clinical Oncology
- AWMSG
- All Wales Medicines Strategy Group
- BIC
- Bayesian information criterion
- BNF
- British National Formulary
- BSC
- best supportive care
- CDF
- Cancer Drugs Fund
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- COOPERATE-2
- COmbination Of Pasireotide and evERolimus in Advanced Tumors of neuroEndocrine origin, second trial
- CR
- complete response
- CRD
- Centre for Reviews and Dissemination
- CrI
- credibility interval
- CSR
- clinical study report
- CT
- computed tomography
- DIC
- deviance information criterion
- ECOG
- Eastern Cooperative Oncology Group
- EMA
- European Medicines Agency
- eMIT
- electronic market information tool
- ENETS
- European Neuroendocrine Tumor Society
- EORTC QLQ-C30
- European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
- EPAR
- European Public Assessment Report
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FACT-G
- Functional Assessment of Cancer Therapy – General
- FDA
- Food and Drug Administration
- GEP
- gastroenteropancreatic
- GI
- gastrointestinal
- HPF
- high-power field
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ITC
- indirect treatment comparison
- ITT
- intention to treat
- LAR
- long-acting release
- MAIC
- matched adjusted indirect comparison
- MCMC
- Markov chain Monte Carlo
- MRI
- magnetic resonance imaging
- MTA
- multiple technology appraisal
- MTC
- mixed treatment comparison
- mTOR
- mammalian target of rapamycin
- NEC
- neuroendocrine carcinoma
- NET
- neuroendocrine tumour
- NETTER-1
- Neuroendocrine Tumors Therapy
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- ORR
- objective response rate
- OS
- overall survival
- PAS
- Patient Access Scheme
- PenTAG
- Peninsula Technology Assessment Group
- PET
- positron emission tomography
- PFS
- progression-free survival
- PHE
- Public Health England
- pNET
- pancreatic neuroendocrine tumour
- PPS
- post-progression survival
- PS
- performance status
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RADIANT-3
- RAD001 in Advanced Neuroendocrine Tumors, Third Trial
- RADIANT-4
- RAD001 in Advanced Neuroendocrine Tumors, Fourth Trial
- RCT
- randomised controlled trial
- RDI
- relative dose intensity
- RECIST
- Response Evaluation Criteria in Solid Tumours
- RPSFT
- rank-preserving structural failure time
- RR
- response rate
- SAE
- serious adverse event
- SD
- standard deviation
- SEER
- Surveillance, Epidemiology, and End Results Program
- SMC
- Scottish Medicines Consortium
- SSA
- somatostatin analogue
- SSTR+
- somatostatin receptor positive
- SSTR2
- somatostatin receptor type 2
- UICC
- Union for International Cancer Control
- UKINETS
- UK and Ireland Neuroendocrine Tumour Society
- VIP
- vasoactive intestinal peptide
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.