Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/54. The contractual start date was in January 2015. The draft report began editorial review in May 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ray Fitzpatrick is a member of the Health Technology Assessment Priority Research Advisory Methods Group. Richard Hindley has received payments for lecturing and proctoring for SonaCare Medical (Charlotte, NC, USA; high-intensity focused ultrasound treatment). Hashim U Ahmed reports grants and personal fees from SonaCare Medical and grants from Trod Medical (Heverlee, Belgium) and Sophiris Bio Inc. (La Jolla, CA, USA) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Hamdy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Research objectives
-
To assess the feasibility of a randomised controlled trial (RCT) comparing high-intensity focused ultrasound (HIFU) with radical prostatectomy (RP) for intermediate-risk clinically localised prostate cancer (PCa) by recruiting and randomising 80 participants.
-
To undertake a QuinteT Recruitment Intervention (QRI) to understand recruitment challenges for this trial and inform optimal recruitment strategies for a main RCT.
-
To collect data on quality of life and resource use to inform power calculations for the proposed main trial.
-
To explore data capture methods and the feasibility of such methods to inform power calculations and a health economic evaluation for a main RCT.
Scientific background
Prostate cancer prevalence and incidence in the UK
In the UK, PCa is the most common cancer in men and the second most common cause of cancer deaths in males (accounting for 13% of such deaths) after lung cancer. 1 In 2014, 46,690 new cases of PCa were diagnosed, and 11,287 men died from the disease. 1 The lifetime risk of being diagnosed with PCa is one in eight. 1 Incidence is increasing with wider use of prostate-specific antigen (PSA) testing in asymptomatic men in the community setting and an ageing UK population.
Diagnosis of prostate cancer
Prostate cancer is currently diagnosed following serum PSA testing, imaging in the form of multiparametric magnetic resonance imaging (mpMRI) scans and prostate biopsies.
Although PCa can be lethal, most men who are diagnosed with PCa will not suffer clinically significant consequences from the disease during their lifetime. Currently, opportunistic PSA testing leads to overdetection and overtreatment and places an increasing burden on the NHS.
The risk of progression to metastases in intermediate-risk, clinically localised PCa and the need for accurate targeting and imaging modalities to direct minimally invasive interventions have precluded RCTs from being undertaken; however, mpMRI technology and dissemination is reaching a level at which accurate assessment of the location and grade of PCa using imaging and biopsies is possible, as demonstrated in the recently published National Institute for Health Research (NIHR) Health Technology Assessment (HTA) PROstate MRI Imaging Study (PROMIS),2 a definitive validating cohort study evaluating mpMRI as a triage test. PROMIS demonstrated higher levels of accuracy in detection of clinically important disease, and found that transrectal ultrasound (TRUS)-guided biopsy performs poorly as a diagnostic test for clinically significant prostate cancer. mpMRI, used as a triage test before first prostate biopsy, could identify one-quarter of men who might safely avoid an unnecessary biopsy and might improve the detection of clinically significant cancer. mpMRI is being increasingly adopted by many centres in the pre-biopsy diagnostic pathway.
Treatment options for localised prostate cancer
Conventional treatment options for men with clinically localised PCa include active monitoring (AM) (also known as active surveillance); radical prostatectomy (RP), now most commonly performed as robot-assisted laparoscopic procedures; intensity-modulated radiotherapy (IMRT); and brachytherapy. These treatment options appear to have similar short- to medium-term oncological outcomes in non-randomised studies.
Active monitoring
Active monitoring protocols involve regular clinical examination, PSA measurements, mpMRI and repeat biopsies. If these parameters suggest the risk of progression, men are offered radical treatment. A number of Phase II studies have shown that signs of progression lead to intervention within 5 years of diagnosis in approximately one-third of patients undergoing AM. 3,4 For those with intermediate-risk disease, AM has been reported as conferring an 84% 5-year metastasis-free survival rate. 5 However, observational strategies can lead to significant anxiety. 6
Active monitoring has been tested in clinically localised PCa in the ProtecT (Prostate testing for cancer and Treatment) trial,7 which reported that, although RP and radiotherapy were associated with lower rates of disease progression, 44% of men assigned to AM did not receive radical treatment and avoided side effects. Men with newly diagnosed, localised PCa therefore need to consider the critical trade-off between the short- and long-term effects of radical treatments on urinary, bowel and sexual function and the higher risks of disease progression with AM, as well as the effects of each of these options on quality of life (QoL).
Radical prostatectomy
Radical prostatectomy involves total open, laparoscopic or robot-assisted surgery to remove the entire prostate gland and seminal vesicles. The proportion of PCa patients receiving surgery varies with age; 8% of PCa patients receive a major surgical resection as part of their cancer treatment, with fewer resections in the oldest age group (0% in those aged ≥ 85 years) than in the youngest group (29% in those aged 15–54 years). 8
Radical radiotherapy
Radical radiotherapy in the form of external beam radiotherapy (EBRT) is a common treatment in the UK for men diagnosed with localised PCa. It is usually preceded by 3–6 months of neoadjuvant androgen suppression, and is given in daily fractions over 4–8 weeks on an outpatient basis. In large reported series, EBRT conferred a 5-year disease-free survival of between 78% and 80%, or 88% and 94% in combination with hormone therapy. 9–12 IMRT, an optimised form of EBRT, is delivered in some centres. 13
Brachytherapy
Brachytherapy can be given either as permanent radioactive seed implantation or as high-dose brachytherapy using a temporary source. For localised PCa, the 5-year biochemical failure rates are similar for permanent seed implantation, high-dose (> 72 Gy) external radiation, combination seed/external beam irradiation and RP. 14
Radical, extensive treatments carry the potential for significant short-, medium- and long-term morbidity such as urinary leakage, erectile dysfunction and radiotherapy toxicity. At present, there is little difference between RP and radical radiotherapy in terms of cancer control in the short to medium term; much of the decision-making process that governs treatment allocation is based on the differences in the side-effect profiles associated with the various interventions. 13
The recently published clinical- and patient-reported outcomes from the ProtecT trial7 demonstrate that each treatment option has a particular pattern of adverse effects on QoL in the short term: urinary incontinence and sexual dysfunction are worst after surgery, followed by recovery by a number of men but persistent difficulties for some, and bowel problems are worst after radiation, with sexual dysfunction mostly related to neoadjuvant androgen deprivation therapy. Although adverse effects of interventions can be avoided initially with AM, there is a natural decline in urinary and sexual function symptoms over time, and the adverse impacts of radical treatments will be experienced when such treatments are received. 15,16 These side-effect profiles have been described consistently, even with more modern and contemporary radical treatment options, such as robot-assisted surgery and different forms of radiation, including brachytherapy. It is, therefore, true that contemporaneous men who are treated with current forms of radical therapy will continue to suffer from the now well-described and well-documented side-effect profile patterns related to these treatments.
Alternatives to conventional therapies
Alternative, targeted focal ablative therapies are being developed in an attempt to reduce treatment burden, improve QoL and reduce adverse events (AEs) associated with radical treatment, while retaining at least equivalent cancer control. Focal therapies should minimise morbidity by lowering the chance of damage to the neurovascular bundles responsible for erectile function and the urinary continence mechanism, and may help to avoid the psychological morbidity associated with surveillance, but their long-term oncological effectiveness remains untested.
These alternative technologies are being used as primary ablative therapies in a number of centres worldwide, but have been introduced without robust Phase III RCT validation. Examples include HIFU, cryotherapy, vascular-targeted photodynamic therapy (VTP), radiofrequency interstitial tumour ablation (RITA), laser photocoagulation and irreversible electroporation. Each one is at a different stage in its evaluation and application to clinical practice. The current evidence for focal therapy for PCa is mainly from non-randomised Phase I and II trials in single centres and from case series with small numbers of patients. A previous Phase I/II study demonstrated that as few as 5% of men suffer from genitourinary side effects after focal therapy and also demonstrated no detectable clinically significant cancer in all treated patients. 17–19 A manufacturer-sponsored Phase III RCT of VTP versus AM in 413 men with very low-risk disease has been published recently with 2-year follow-up data. 20 This is discussed below.
High-intensity focused ultrasound
High-intensity focused ultrasound uses ultrasound energy focused by an acoustic lens to cause tissue damage as a result of thermal coagulative necrosis and acoustic cavitation. The procedure is performed using a transrectal approach and may be performed under general or spinal anaesthesia as a day-case procedure.
Four systematic reviews of HIFU in the treatment of prostate cancer have been published. 21–24 Warmuth et al. 21 identified 20 uncontrolled prospective case series, totalling 3021 patients (2794 primary therapy and 227 salvage therapies). They concluded that, for all HIFU procedures, the biochemical disease-free rate at 1, 5 and 7 years was 78–84%, 45–84% and 69%, respectively. The negative biopsy rate was 86% at 3 months and 80% at 15 months. Overall survival rates and PCa-specific survival rates were 90% and 100% at 5 years and 83% and 98% at 8 years, respectively. AEs included complications of the urinary tract (1–58%), erectile function (1–77%) and the rectum (0–15%), and pain (1–6%). Lukka et al. 22 found that there were no adequate RCTs or meta-analyses. They concluded that the current evidence on HIFU use in PCa patients is of low quality, rendering it difficult to draw conclusions about its efficacy. Veereman et al. 23 found very low-quality evidence in case series of patients who received HIFU treatment with no comparison with AM or radical treatment. These suggested an overall survival rate of ≤ 89% and a PCa survival rate of ≤ 99% after 5 years, but these numbers vary depending on the patient’s risk category. Longer-term effects on QoL are unknown. Kuru et al. 24 conclude that HIFU treatment, and especially focal ablation of tumour foci, seems to be a safe alternative to standard treatment, with fewer side effects. The oncological results seem satisfactory but need further follow-up to validate this practice of PCa control. 24
Cryotherapy
Cryotherapy is the localised destruction of tissue by extremely cold temperatures followed by thawing, and may be performed under general or regional anaesthesia. Cryoneedles or probes are inserted into the prostate via the perineum, using image guidance. Argon gas is circulated through needles or probes, generating very low temperatures and causing the formation of ice around the prostate with profound tissue destruction. Newer cryotherapy techniques allow these needles to be removed or repositioned so that the frozen zone conforms to the exact size and shape of the target tissue.
In 2008, a Cochrane systematic review25 of prostate cryotherapy as a primary treatment was published, and recommended that RCTs comparing cryotherapy with established treatments for early PCa be conducted. All eight studies identified were case series (two of which were retrospective), with a total of 1483 patients. At 5 years, overall survival was reported as 89–92% in two studies and disease-specific survival was 94% in one study. The major complications observed in all studies included impotence (47–100%), incontinence (1.3–19.0%) and urethral sloughing (3.9–85.0%), with less common complications of fistula (0–2%), bladder neck obstruction (2–55%), stricture (2.2–17.0%) and pain (0.4–3.1%). Most patients were discharged the following day (range 1–4 days). Since 2008, one Canadian RCT,26 comparing cryotherapy with EBRT as primary therapy for localised PCa in > 200 men, has been reported. With a median follow-up period of 100 months, no significant difference in disease progression was observed between the two arms. 26 However, because of recruitment difficulties, the study was closed before the target accrual was reached.
Vascular-targeted photodynamic therapy
Vascular-targeted photodynamic therapy uses light to activate a photosensitising drug, administered intravenously, to produce instantaneous vessel occlusion and subsequent tissue necrosis. The light is delivered by optical fibres placed transperineally under transrectal ultrasound guidance. VTP is given under general anaesthesia and can be carried out on a day-case basis.
Small case series of VTP have been reported in groups receiving primary therapy and those receiving salvage therapy. The initial drug-dose escalation studies, followed by a light-dose escalation study, were performed in men who progressed following EBRT. 27–30 These studies showed that around 60% of men receiving whole-gland treatment at the maximal and light drug doses had a complete response to treatment. Side effects included two rectourethral fistulae, one of which required surgical intervention. Urinary side effects tended to last for ≤ 6 months. In a study of 40 men with no previous treatment for PCa, early results showed no significant side effects. 31 Recently, a manufacturer-sponsored Phase III RCT comparing VTP with AM in 413 men with very low-risk disease has been published with 2-year follow-up data. 20 It found VTP to be safe and effective, with a 66% reduced risk of treatment failure (adjusted hazard ratio 0.34, 95% confidence interval 0.24 to 0.46) compared with AM; however, the VTP group experienced more frequent and severe side effects, although these were mostly mild and of short duration. The most common side effect was difficulty passing urine, and in all cases this resolved within 2 months of treatment. Outcomes for 5- and 10-year progression and survival are not known. The major limitation of this study20 is that the cohort of men investigated had low-risk disease; active treatment is currently not recommended for low-risk PCa by most international guidelines, including the National Institute for Health and Care Excellence (NICE) recommendations, which advocate that such men are offered primarily AM because of the insignificant risk of disease progression.
Other ablative technologies
Other ablative technologies are currently under evaluation, but there is not sufficient evidence to be used within the context of a RCT. Examples include radiofrequency ablation, which acts by converting radiofrequency waves to heat, resulting in thermal damage. RITA has recently been proposed as a treatment for PCa. 32–35 Interstitial laser photocoagulation was reported by Amin et al. ,36 who described a percutaneous technique for local ablation. Irreversible electroporation is a new non-thermal ablation method that uses short pulses of direct current (DC) to create irreversible pores in the cell membrane, thus causing cell death. 37,38 This technology is in the early clinical phases of development, and a RCT is currently under way. 39
These newer techniques have been evaluated in a systematic review by Ramsay et al. ,40 who conclude that they have not been evaluated with sufficient reliability to inform their utilisation within the NHS. There is, however, some evidence that cancer-specific outcomes in the short term are either better than or equivalent to either EBRT or RP, with comparable adverse effect profiles; however, there is a possible increased risk of dysuria and urinary retention. Valerio et al. 41 undertook a more recent systematic review and concluded that there is evidence that focal therapy is safe and has low detrimental impact on continence and potency, but they note that the oncological outcome has yet to be evaluated against the standard of care. Combined with the increasing incidence of clinically localised PCa, demand for these less aggressive, organ-sparing treatments is expected to increase. 40 A further important consideration is the rapid evolution of the technologies over time, with constant new developments to improve energy delivery, targeting, safety and imaging. Focal therapy relies on accurately assessing the status of the disease in the prostate, using imaging and biopsies for which the technology is improving and becoming more widely disseminated. Transperineal prostate mapping (TPM) biopsies provide the optimal biopsy strategy for accurately mapping disease within the prostate with sensitivity of > 90% for 0.2-cc and 0.5-cc lesions. 42
Ongoing and recently completed studies clearly indicate that the evidence base for partial ablation (PA) therapies – in particular, focal ablative therapies – is increasing. However, the quality of the evidence base will not improve substantially given that the majority of these studies are case series. Research efforts in the use of ablative therapies in the management of PCa should focus on conducting more rigorous, high-quality studies. Ramsay et al. 40 identify HIFU and brachytherapy as the most promising newer interventions, but these lack high-quality evaluation. They recommended evaluation by multicentre RCTs, with long-term follow-up, to include predefined assessment of cancer-specific, dysfunction and health-related quality of life (HRQoL) measures, as well as economic evaluations to inform economic modelling.
Implications for research
The lack of RCT-based evidence means that the true clinical effectiveness and cost-effectiveness of focal treatment have not been established; this lack of evidence highlights the need for robust primary research.
An important strategy, which remains untested, is to use novel technologies to target and treat all clinically significant cancers in the gland focally, with careful follow-up and repeat treatments as necessary, particularly for emerging new lesions detected by biopsy. The strategy may obviate the need for any radical therapies.
The current NIHR HTA feasibility study was therefore developed and conducted in order to inform a main, definitive RCT. RP was selected in the radical treatment arm to reflect true pathological staging and grading of the randomised cohort, and ease of determination of treatment failure. HIFU was selected as the partial ablation comparator in the feasibility stage. At the time of the design of this study, a HTA systematic review40 suggested that HIFU was the most likely treatment to be considered cost-effective when assessed against threshold values for a cost per quality-adjusted life-year (QALY) that society might be willing to pay.
Recruitment to RCTs is often slower or more difficult than expected,43 and recruiting to surgical RCTs is particularly challenging. 44 Although the communication style of a doctor or nurse explaining the study is one of the key factors that exerts a considerable influence on patients’ preparedness to accept or decline participation,45 research has shown that recruiters can experience emotional and intellectual challenges related to their roles as researchers and clinicians. 46,47 Many recruiters find it difficult to accept the possibility that their usual preferred treatment is no more effective than the comparator. 48 In particular, surgeons often have to take decisive action during operations (often with incomplete information), which can make it difficult to be certain which treatment is best for patients. 44 In addition, research has shown that recruiters can find concepts such as randomisation challenging to explain to patients. 49 Taken together, these factors highlight the need for training and support for recruiters.
Systematic reviews have identified only a few programmes that provided training to those recruiting patients into RCTs. 50,51 The majority of these workshops provide general information about the key principles of RCTs without covering issues specific to a particular trial. Few interventions to improve recruitment have been shown to be effective;52 the most successful have been studies that used qualitative research to identify key issues and then developed interventions based on these issues to improve recruitment. 50
A feasibility trial was conducted to assess rates of recruitment and randomisation, identify barriers to recruitment and devise strategies to overcome them, and test the trial processes and data collection methods to inform the design and conduct of a main RCT.
Chapter 2 Methods
Study design
This study aimed to evaluate the feasibility of a prospective, multicentre, parallel-group (1 : 1) RCT to assess the clinical effectiveness and cost-effectiveness of PA (using HIFU) or RP in patients with intermediate-risk, unilateral clinically localised PCa. The study flow chart and visit schedule are shown in Appendices 1 and 2, respectively.
The full trial protocol can be accessed in the NIHR Journals Library: www.journalslibrary.nihr.ac.uk/programmes/hta/123554/#/ (accessed 13 November 2017).
QuinteT Recruitment Intervention methods
A QRI was embedded into the feasibility study with the aim of understanding the recruitment process and to identify clear obstacles and hidden challenges to recruitment. 46,47,53 The methods were developed initially in the ProtecT trial by the applicants involved in the Partial prostate Ablation versus Radical prosTatectomy (PART) trial,54 and have subsequently been used and refined in other RCTs. 55
The QRI involved two iterative phases: phase I (January to November 2015) sought to identify and understand recruitment difficulties through the use of multiple qualitative methods, and phase II (December 2015 to March 2017) developed and implemented strategies to optimise recruitment and informed consent.
Phase I: understanding recruitment issues
Phase I of the QRI sought to understand recruitment in each clinical centre, with the intention of subsequently developing interventions to improve recruitment and informed consent. The approaches used to identify recruitment difficulties followed principles of ethnography: data collection and analysis were supplemented by observations of recruitment appointments and meetings, monitoring screening log data and examining study documentation.
Sampling and recruitment
In-depth interviews
Scene-setting interviews were conducted with members of the Trial Management Group (TMG) [including the chief investigator (CI) and those most closely involved in the design, management, leadership and co-ordination of the PART trial overall and in each of the clinical centres]. Snowball sampling was subsequently used, in which TMG members provided the names of colleagues whom they considered it would beneficial for the QRI researcher to talk to. These informants were invited via e-mail to take part in an interview with the QRI researcher at a mutually convenient time and date. E-mail reminders were sent 1 week after dispatch of the original invitation. Informants were purposefully selected, so as to build a sample of maximum variation on the basis of professional role (e.g. surgeons, research nurses) and recruitment centre. Characteristics were assessed as the study progressed and some individuals were subsequently selected on the basis of emerging issues that warranted further investigation (i.e. the evidence for HIFU) or were approached as new centres opened throughout the course of the study (i.e. Southampton and Basingstoke).
Recorded recruitment consultations
All health-care professionals recruiting to the trial were requested to audio-record all appointments in which they provided information to eligible patients about the study and treatment options, until a decision was reached. To facilitate this, the QRI team provided each participating centre with a ‘Recruiter Pack’ with detailed guidance on the process of obtaining informed consent, the operation of digital recorders and how to record, name and transfer audio-files and documents to ensure that information from the QRI remained secure and confidential. In addition, site visits were conducted to ensure that each recruiter had the appropriate equipment and felt comfortable with recording consultations.
Data collection
In-depth interviews
Written consent for the QRI component of the PART trial was obtained at the start of the interview. A digital voice-recorder was used to record the discussions. Interviews followed topic guides that had been designed and piloted in several other trials. Separate topic guides were developed for members of the TMG and those recruiting to the study to ensure that discussions covered the same basic issues but with sufficient flexibility to allow new issues, of importance to the informants, to emerge (see Report Supplementary Material 1). Key topics included perspectives on the trial design and protocol, views about the evidence on which the trial was based, perceptions of uncertainty/equipoise in relation to the treatment arms, views about how the arms/protocol were delivered in the relevant clinical centre, methods for identifying eligible patients, views on eligibility and examples of actual recruitment successes and difficulties. The interview schedule was adapted as analysis progressed to enable exploration of emerging themes, such as how clinicians interpreted the impact of the publication of the ProtecT trial findings.
Recorded recruitment consultations
Eligible patients were sent information about the QRI before their surgical consultation. This allowed the patient sufficient time to carefully read the information sheet and decide whether or not they were comfortable with their initial consultation being recorded. Written consent was obtained from each patient before the start of the consultation. If consent was provided, the recruitment consultation – and any subsequent discussions – were recorded on an encrypted audio-recorder. A research nurse at each centre regularly uploaded all recordings from the device on to an encrypted memory stick and securely posted them to the QRI researcher.
Patient pathway through eligibility and recruitment
All study centres were asked to maintain detailed screening logs, capturing details of patients who were screened for the PART trial, reasons for ineligibility and details of eligible patients who did not consent to trial participation. These logs were returned to the trial manager on a monthly basis. Unclear or absent information was checked and queried. The QRI researcher also conducted regular site visits to understand patient pathways and to discuss and observe how the PART trial was integrated into clinical practice.
Observation of study meetings
In addition to these data collection methods, the QRI researcher attended as many study meetings as possible to gain an overview of trial conduct and overarching challenges, including ‘core’ TMG meetings that took place every few months (consisting of the CI and those with the greatest responsibility for trial oversight), wider monthly study meetings (for the core TMG and those recruiting to the study), and collaborators’ meetings (attended by all involved in the management of and recruitment to the study).
Data analysis
In-depth interviews
Interview recordings were transcribed verbatim or in selected parts, whichever was necessary to conduct a sufficiently detailed analysis. Transcripts were imported into NVivo version 10 (QSR International, Warrington, UK), in which data were analysed sentence by sentence and similarities and differences were coded. Data were analysed using techniques of constant comparison56 and emerging themes and codes within transcripts and across the data set were then compared with each other, to look for shared or disparate views among patients. Emerging themes were discussed within the QRI team with reference to the raw data. Data collection and analysis continued until the point of data saturation, that is, the point at which no new themes emerged.
Recorded recruitment consultations
Recordings of consultations were also transcribed verbatim or in selected parts, whichever was necessary to conduct a sufficiently detailed analysis. These were analysed as described above for interviews, with the addition of some of the techniques of focused conversation analysis. The delivery of information during the recruitment appointments was investigated, with a particular interest in the interaction between the recruiter and the patient to identify and document aspects of informed consent and information provision that were unclear, or disrupted or hindered recruitment.
Patient pathway through eligibility and recruitment
Detailed eligibility and recruitment pathways were compiled for each clinical centre, noting the point at which patients received information about the trial, which members of the clinical team they met and the timing and frequency of appointments. Recruitment pathways were compared with details specified in the trial protocol and pathways from other centres to identify practices that were more efficient and those that were less efficient. Logs of eligible and recruited patients were collated using simple flow charts and counts to display the numbers and percentages of patients at each stage of the eligibility and recruitment processes. These insights were considered alongside data emerging from interviews and audio-recorded consultations.
Sample size
The sample size for the feasibility study was determined pragmatically, based on the number of patients that it was considered feasible to recruit within the given time frame. In the grant application, we proposed to randomise between 80 and 100 men, to assess willingness to participate in and be randomised to the PART trial. The sample size was set at 100 participants in the trial protocol; this was revised to 80 participants, as discussed and agreed with the NIHR HTA Monitoring Committee following a monitoring hub meeting on 26 November 2015 and formalised in the PART trial contract variation, which was approved by the NIHR HTA programme in September 2016.
Outcomes
-
Recruitment and randomisation of men to RP or HIFU (the target was set at 80 randomised participants).
-
Findings of the QRI.
-
Assessment of data capture methods including, collection and completeness of case report forms (CRFs), Patient Reported Outcome Measures (PROMs) and resource use diaries.
Patients were asked to complete the following PROMs:
-
International Index of Erectile Function – 15 items (IIEF-15)57
-
International Prostate Symptom Score (IPSS)58
-
EuroQoL-5 Dimensions, five-level version (EQ-5D-5L)59
-
Functional Assessment of Cancer Therapy – Prostate (FACT-P)60
-
University of California, Los Angeles – Expanded Prostate Cancer Index Composite (EPIC)61
-
Memorial Anxiety Scale for Prostate Cancer (MAX-PC). 62
They were also asked to complete a self-reported resource use diary (see Report Supplementary Material 2), in which they were asked to identify and record items relating to utilisation of the health-care resources mentioned and any other relevant health-care resources. These were completed at baseline and at 3, 6, 9, 12, 18, 24, 30 and 36 months, in line with routine clinical follow-up schedules.
Patient selection and recruitment
Patients must have been diagnosed with intermediate-risk, unilateral, clinically localised PCa and be fit for either RP or PA of the prostate.
Inclusion criteria
Men were eligible if they had unilateral, clinically significant intermediate-risk PCa or dominant unilateral clinically significant intermediate-risk and small contralateral low-risk disease and met all of the following criteria:
-
Gleason score of 7(3+4 or 4+3) or
-
a high-volume Gleason score of 6 (a cancer core length of > 4 mm)
-
a PSA level of ≤ 20 ng/ml
-
a clinical stage ≤ T2b disease
-
a life expectancy of ≥ 10 years
-
be fit, eligible and normally destined for radical surgery
-
have no concomitant cancer
-
have no previous treatment of their PCa
-
have sufficient proficiency in the English language to understand written and verbal information about the trial, its consent process and the study questionnaires.
Exclusion criteria
-
Unfit for radical surgery.
-
Significant bilateral disease.
-
Low-risk disease (i.e. a Gleason score of ≤ 6, PSA level of 10 ng/ml).
-
High-risk disease (i.e. a Gleason score of ≥ 8, PSA level of > 20 ng/ml).
-
Clinical T3 disease (extracapsular locally advanced).
-
Men who have received previous active therapy for PCa.
-
Men with evidence of extraprostatic disease.
-
Men with an inability to tolerate a TRUS.
-
Men with a latex allergy.
-
Men who had undergone a transurethral resection of the prostate (TURP) for symptomatic lower urinary tract symptoms in the previous 6 months.
-
Metal implants/stents in the urethra.
-
Prostatic calcification and cysts that interfere with effective delivery of HIFU.
-
Men with renal impairment and a glomerular filtration rate (GFR) of < 35 ml/minute/1.73 m2.
-
Unable to give consent to participate in the trial, as judged by the attending clinicians.
Appropriate patients were identified by the dedicated research nurse or clinician. Potential patients were identified at the multidisciplinary team (MDT) meeting, during which histopathological data were discussed. Men with a histological diagnosis of intermediate-risk PCa following a biopsy (see Trial management processes) and mpMRI (see Safety), and evidence of unilateral, clinically localised disease, were assessed for the PART trial. Some patients required a targeted or template biopsy to confirm unilateral disease. Patients who were ineligible for the PART trial were informed by their doctor, who discussed their treatment options with them.
Once confirmed as eligible for the study, patients were approached at urology/oncology clinics in the centres and provided with a patient information leaflet (PIL) (see Report Supplementary Material 3), detailing the exact nature of the trial, what it would involve for the participant, the implications and constraints of the protocol and the known side effects and any risks involved in taking part. Patients who fulfilled all the entry criteria for the trial were invited to attend an information appointment. Patients were given ample time to discuss the trial with family, friends and their general practitioner (GP) (there were typically around 6 weeks between MDT discussion, counselling and then randomisation). If the patient agreed to take part, they were asked to sign an informed consent form (ICF) (see Report Supplementary Material 4).
It was clearly stated that the participant was free to withdraw from the trial at any time for any reason, without prejudice to future care and with no obligation to give the reason for withdrawal. In the event of withdrawal, the choice of treatment was decided on by the patient and his clinical team.
Randomisation
Participants were randomised on a 1 : 1 basis to receive either RP or HIFU. A secure web-based system [Registration/Randomisation and Management of Product (RRAMP)] was provided by the Oxford Clinical Trials Research Unit (OCTRU). Randomisation was undertaken using the method of minimisation by a member of the research team or the PART trial office and participants were notified immediately of their treatment allocation. Participants were stratified by the following factors: age (< 60, 60–64, 65–70 or > 70 years), PSA level (< 3.0, 3.0–4.9 , 5.0–9.9 or 10.0–20.0 ng/ml), Gleason score (3 + 4, 4 + 3 or high-volume score of 6) and whether participants were considered to have unilateral clinically localised intermediate-risk disease or dominant unilateral clinically significant intermediate-risk and small contralateral low-risk disease. Owing to the nature of the interventions, it was not possible to blind participants, clinical staff or outcome assessors to treatment allocation.
Trial interventions
Once participants accepted their allocation, treatment was delivered within 8 weeks of randomisation, when possible. Participants randomised to RP were listed for surgery, optimally within 2 weeks of randomisation. This was co-ordinated by local investigators and study research nurses.
Conventional open, laparoscopic or robot-assisted RP is carried out under general anaesthesia in accordance with local centre expertise and clinical judgement, to remove the entire prostate gland and seminal vesicles. Lymphadenectomy was performed at the discretion of the operating surgeon in discussion with the participant, and conducted as standard (obturator nodes), or extended (to the bifurcation of the common iliac vessels), based on disease extent, PSA level and Gleason grade. Nerve-sparing surgery was usually carried out on the contralateral side of the existing tumour, and bilaterally if judged to be appropriate by the operating surgeon. Full operative and postoperative details were recorded for each participant.
Partial ablation is performed using HIFU (defined in Chapter 1). Patients randomised to PA underwent a mandatory rest period of 4–6 weeks following biopsies to allow swelling and inflammation to settle in the prostate, thereby lowering the risk of side effects such as rectal damage. Patients were admitted on the day of the procedure or the evening before, as appropriate. A phosphate enema was administered on the morning of surgery to ensure an empty rectum. The type of anaesthesia (regional/general) was discussed with the participant and depended on the opinion of the anaesthetist. A catheter is inserted before the proposed HIFU treatment. This can either be a urethral or suprapubic catheter depending on surgeon preference.
The HIFU probe is introduced into the rectum with as little trauma as possible. The treatment is then planned using the pre-operative imaging and biopsy data. The area of cancer is targeted and treated with a safe margin; this usually constituted a hemiablation, that is, treating approximately the 50% of the prostate containing the significant disease. Other treatment protocols include a quadrant ablation and true focal ablation of the tumour. There is an additional trial-specific instruction (TSI) relating to the HIFU treatment/re-treatment strategy and training of HIFU clinicians (see Report Supplementary Material 5).
The patients go home on the day of the procedure with the catheter remaining in situ.
Follow-up
Patients randomised to radical prostatectomy
-
Routine removal of catheter at 10–14 days.
-
Follow-up in the clinic at approximately 6 weeks post surgery as per routine NHS care. Patients will have had a PSA test prior to their follow-up appointment, the result of which should be available.
-
Follow-up in the clinic approximately every 3 months post surgery in the first year and then approximately every 6 months, as per routine NHS care, for 3 years (see Appendix 2). Patients will have had a PSA test prior to their follow-up appointment, the result of which should be available. If at any point disease progression is suspected (i.e. a PSA level rising to ≥ 0.2 µg/ml) the patient will be restaged.
Patients randomised to partial ablation
-
Routine removal of catheter at 7 days.
-
For centres new to performing HIFU, a study-specific mpMRI was performed on the first five patients at 2 weeks post HIFU.
-
Followed up routinely at approximately 6 weeks post surgery as per routine NHS care. Patients will have had a PSA test prior to their follow-up appointment, the result of which should be available.
-
Followed up in the clinic approximately every 3 months post surgery for the first year and then approximately every 6 months, as per routine NHS care, for 3 years. Patients will have had a PSA test prior to their follow-up appointment, the result of which should be available.
-
Standard care included a mpMRI at 12 months.
-
Standard care included transrectal biopsies at 12 months.
-
Standard care included a mpMRI at 3 years.
-
Standard care included transrectal biopsy at 3 years.
Trial completion and exit
The end of the trial would be when the last patient was contacted to arrange their final follow-up visit, whether or not the visit took place at 3 years from receiving their treatment.
Statistical analysis
Statistical analyses are mostly descriptive because this was a feasibility study. The number and percentage of patients screened, consented and randomised are summarised in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (see Figure 2). Baseline and outcome variables are described by treatment arm, continuous variables are described using mean and standard deviation or median and interquartile range (IQR), and categorical variables are summarised as percentages. The percentage of missing data is summarised but imputation was not performed.
For disease-specific HRQoL outcomes, mixed models for repeated measures (MMRMs) were used to evaluate differences over time by treatment arm. MMRMs were adjusted for treatment, baseline score, visit and a treatment-by-visit interaction as fixed-effect terms with patient included as a random effect and reported as mean difference with 95% confidence intervals. All HRQoL outcomes were analysed up to the 12-month postprocedure assessment, because most patients in the study had not reached further than this visit at the time of analysis. HRQoL forms returned partially complete were analysed in accordance with the scoring instructions for each questionnaire.
All statistical analyses are in accordance with the intention-to-treat (ITT) principle, apart from procedural and postprocedural complication rates, serious adverse events (SAEs) and treatment-related details, which are summarised according to the treatment the patient received. Analyses were performed using SAS® version 9.3 (SAS Institute Inc., Cary, NC, USA) and Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
Health economic methods
The objectives of the exploratory health economics data collection alongside the PART feasibility study were to:
-
evaluate the response and completion rates of the EuroQol-5 Dimensions, five-level version (EQ-5D-5L) instrument for assessing generic QoL, as well as the response and completion rates of the trial-specific patient resource use diary
-
conduct a preliminary exploration of reported generic HRQoL using the EQ-5D-5L utility scores and EuroQol Visual Analogue Scale (EQ-VAS) scores and reported resource use across trial arms, which may inform the design of a main RCT.
At the randomisation visit, generic and disease-specific HRQoL were assessed in the format of a PROMs survey pack that was given to each participant to fill out at each follow-up appointment. The EQ-5D-5L instrument (included in the PROMs survey pack), used to inform the health economic analysis, was completed at baseline, 6 weeks and 3, 6, 9, 12, 18, 24, 30 and 36 months post procedure. The completed survey pack was expected to be returned to the trial nurse prior to the participant attending the follow-up visit with the consultant.
A trial-specific patient resource use diary was developed using feedback from clinicians and nurses. This assessed the following four types of resource use.
-
Section 1: how many times the participant had to see or talk to a doctor or nurse or other health-care professional in relation to PCa, and what for. This included hospital admissions.
-
Section 2: what special medications, aids and adaptations the participant bought or had prescribed to help them with PCa and treatment-related symptoms.
-
Section 3: how many days the participant felt too unwell to engage in normal activities because of PCa-related symptoms.
-
Section 4: details of any travel made for the participant’s health-care appointments.
The diary was given to each participant by the trial nurse after the initial treatment and at each follow-up visit (i.e. at 6 weeks and 3, 6, 9, 12, 18, 24, 30 and 36 months). The participant was reminded to keep track of all health-care resources used and any time off work or usual activities between visits.
The completeness assessment for the exploratory health economics data collection differed from the ITT cohort used in the statistical analysis, and comprised patients meeting the following criteria: those who were randomised and received treatment by 16 March 2017 as per their initial treatment allocation (per-protocol analysis). The generic HRQoL instrument, EQ-5D-5L, was evaluated at the individual domain level [the five domains captured in the EQ-5D-5L are (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression, with five possible response levels, 1 being no problems and 5 being severe problems] and these were summarised along with the EQ-VAS scores. 63 Completeness levels are presented and patient/environment factors, including age and trial centre, were assessed to identify any drivers of incomplete data. For complete cases, EQ-5D-5L profiles were constructed for each time point and transformed into utility scores using the EQ-5D-5L UK population-based preference weights. 64 The baseline differences for the utility and EQ-VAS scores were assessed, along with changes from baseline for the follow-up period. These are presented by trial arm. Similar to the statistical analysis of disease-specific HRQoL, differences in utility were analysed up to 12 months post procedure, because most patients in the study had not reached further than this visit at the time of analysis.
Self-reported resource use from the patient diary was assessed by section and subsection for completeness levels and descriptive statistics were presented by trial arm across all follow-up time points up to the 12-month assessment. Units of resource use were aggregated across the follow-up period and presented by trial arm.
Resource use following treatment, including inpatient length of stay, intensive care unit (ICU) bed-days and high-dependency unit bed-days, was collected in the trial CRFs and is reported in the statistical analysis (see Table 7). Resource use associated with SAEs and complications was also analysed in the statistical analysis (see Tables 13 and 14). The resource use diary also collected self-reported data on accident and emergency (A&E) visits and inpatient days in the follow-up period. For this feasibility stage, the cost implications of trial procedures (i.e. HIFU and RP), inpatient bed-days and SAEs are informed by the literature and NHS reference costs;65,66 costs are expressed in 2015 Great British pounds.
The exploratory analysis was performed in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) and Stata.
Although not undertaken within this feasibility trial, a full costing and cost-effectiveness analysis would be required in a main RCT. A full RCT would include a complete cost-effectiveness analysis and a budget impact model with the cost of all trial procedures estimated using a microcosting approach.
Safety
Adverse event reporting was undertaken in accordance with the National Research Ethics Service (NRES) guidelines for reporting in non-CTIMPs (Clinical Trial of an Investigational Medicinal Product).
Details of recurrence and any subsequent re-treatment were captured on a ‘Treatment Failure’ and a ‘For cause HIFU treatment’ worksheet as appropriate, and entered remotely to the trial database, or returned to the trial office for central data entry.
Treatment failure
Radical prostatectomy
In patients receiving RP, primary treatment failure was defined as a rising serum PSA level reaching ≥ 0.2 ng/ml following initial reduction to < 0.1 ng/ml after surgery; or a failure of serum PSA to reach the level of < 0.1 ng/ml after surgery; or clinical progression to local recurrence/systemic disease.
Partial ablation
In patients receiving PA, primary treatment failure was determined by a combination of repeat prostate biopsies, serum PSA level and clinical appearance of symptoms/signs suggesting disease progression. Prostate biopsies following PA would determine one of five defined scenarios:
-
negative biopsies, in which case the patient would continue to be followed up as described
-
positive biopsy in the originally treated area, in which case the patient would be allowed one additional HIFU treatment before the treatment is classed as having failed
-
positive biopsy in the untreated area demonstrating a new focus of intermediate-risk disease, in which case the patient would be offered additional PA in this area
-
positive biopsies in any area of the prostate demonstrating low-risk, low-volume disease, in which case the patient will not require additional treatment and follow-up will continue as described
-
positive biopsy following two consecutive treatments in any area of the prostate, in which case the treatment will be deemed as having failed.
If follow-up biopsies demonstrate high-risk disease at any point, this will be considered as primary treatment failure and patients will be offered whole-gland treatment in accordance with standard of care.
In the presence of primary treatment failure as described above, the patient would be re staged using pelvic cross-sectional imaging and bone imaging. Patients would be fully informed of their disease status, grade, clinical stage and the treatment options, such as salvage EBRT. Regardless of decisions about additional interventions, patients would continue to be followed up and analysed within the trial in accordance with ITT, with full documentation of subsequent treatments.
Trial management processes
The PART trial was run within the Surgical Intervention Trials Unit (SITU) and the OCTRU, which is registered with the UK Clinical Research Collaboration (UKCRC). This trial was given ethics approval for conduct in the NHS by the South Central – Berkshire Ethics Committee (see Report Supplementary Material 6).
The PART trial was conducted in accordance with OCTRU standard operating procedures (SOPs) and a number of complementary TSIs on performing HIFU procedures, proctoring of HIFU cases and histopathology reporting. The aim of these documents was to ensure quality assurance (QA) and quality control (QC) among participating clinicians. Clinicians new to performing HIFU were proctored by a HIFU expert and a competency form was required after each proctoring session. All documentation was saved on a secure Oxford University drive in the e-Trial Master File.
The PART trial management team were all trained in Good Clinical Practice (GCP) and an in-depth risk assessment was carried out. Central Monitoring and Data Management plans were finalised. PART trial data were entered into the OCTRU in-house OpenClinica (OpenClinica, Waltham, MA, USA) database system. Central monitoring activities were conducted by the trial manager; no on-site monitoring took place.
The trial manager conducted site initiation visits at all participating centres, and each centre received an investigator site file. The trial manager trained the local research teams in OpenClinica, Registration/Randomisation and Management of Product (RRAMP; the in-house randomisation system) and all study documentation. Ad hoc training was delivered both face to face and by telephone throughout the duration of the study. Screening logs were requested and collated on a monthly basis. To aid centres, the PART trial office pre-printed all CRFs, PROM questionnaire packs and resource use diaries and produced individual participant packs.
The CI, trial manager and core study staff met regularly to discuss the progress at all centres, and the following oversight meetings took place:
-
monthly minuted TMG and recruiter teleconferences
-
two full face-to-face TMG meetings, on 9 December 2015 and 28 March 2016
-
two Trial Steering Committee (TSC) meetings, on 9 June 2015 and 24 January 2017
-
four independent safety data reviewer meetings, on 20 May 2015, 25 September 2015, 13 April 2016 and 14 December 2016
-
one investigator’s meeting (open to all PART trial collaborators), on 9 December 2015.
Quality assurance and quality control
Radiology
All the mpMRI was performed in accordance with European Society of Urogenital Radiology (ESUR) guidelines, using both 1.5- and 3-tesla magnetic field strengths and a pelvic phased-array coil. Standard T1, T2, diffusion-weighted and dynamic contrast-enhanced mpMRI sequences were performed.
The pre-treatment scans were reported using the standardised reporting Prostate Imaging and Data Reporting System (PI-RADS) scoring system approved by ESUR, with a local T stage also reported. Additionally, it was agreed that the degree of capsular abutment of identified tumour would be recorded to the nearest millimetre, and patients with abutment of > 15 mm were regarded as ineligible for the study.
The post-HIFU treatment scans were performed at three time points: within the first month post HIFU for centres new to performing HIFU, and at 12 and 36 months. The early scans focused on assessing technical treatment success and complications, whereas the later scans focused on identification of disease, particularly the position of the tumour on the initial scan.
We aimed to establish the ability of early post-HIFU mpMRI to determine whether or not treatment has been adequate and the likelihood of local relapse, by reviewing the non-enhancing volume on initial scans against appearances at 12 and 36 months.
The reports were provided on a standard template across all centres, with the prostate divided into anterior and posterior, right and left, and three sectors: base, mid and apex.
Pathology
Biopsies and RP specimens were reported at the local trial centres to the requirements set in Dataset for Histopathology Reports for Prostatic Carcinoma. 67 Gleason grading was undertaken using the 2005 International Society of Urological Pathology’s modified Gleason grading system. 68 Pathological tumour, node, metastasis (TNM) staging was undertaken using TNM Classification of Malignant Tumours. 69
Biopsy reporting
Biopsies were reported at the centres by local pathologists. Biopsies were not routinely double reported or reviewed before completion of the CRF.
These were not double reported or centrally reviewed for the feasibility study.
Postprocedure biopsies can often be difficult, and immunohistochemistry may be required to distinguish posttreatment glands from residual adenocarcinoma. Cases are reported as negative for tumour, prostatic intraepithelial neoplasia (PIN), atypical small acinar proliferation (ASAP) or residual invasive adenocarcinoma. Data on histopathological features of post-HIFU changes are limited, but some guidance is provided by Ryan et al. 70 Difficult cases can be reviewed by the pathology group. Stromal fibrosis is the commonest finding post HIFU, with necrosis in a smaller number of cases. Treatment effects that preclude the ability to identify or Gleason grade post HIFU prostatic adenocarcinoma have not been identified. 70
All study pathologists were required to report histology specimens as detailed in the PART trial histopathology TSIs (see Report Supplementary Material 7). There was a lead pathologist at each recruiting centre; these pathologists formed the PART trial pathology group and were invited to participate in the PART trial monthly teleconferences. Members of the PART trial pathology group attended the update meeting on 9 December 2015 in Oxford, at which the lead PART trial pathologist gave a pathology update talk.
Radical prostatectomy reporting
Radical prostatectomy specimens were reported in each centre by the local pathologists. Oxford cases were centrally reviewed in Oxford by the PART trial lead pathologist, to record additional parameters that would not otherwise have been routinely reported and to allow future correlation of tumour topography with the mpMRI. Additional parameters recorded on central review were the diagnosis within each of the 12 PI-RADS zones A–L (benign, malignant, atypical), Gleason grade grouping71 and whether or not there was inflammation or fibrosis present.
Tumour location on the RP was summarised as follows:
-
unilateral clinically significant intermediate-risk disease – defined as a Gleason score of 7 (3 + 4 or 4 + 3) or a high-volume Gleason score of 6 (any tumour dimension of ≥ 4 mm)
-
dominant (defined as the largest tumour, which is usually the tumour with the highest stage and grade)68 unilateral clinically significant intermediate-risk disease and small contralateral low-risk disease – defined as a Gleason score of 7 in the dominant tumour (3 + 4 or 4 + 3) with a Gleason score of ≤ 3 + 3 on the contralateral side (any tumour dimension of ≤ 4 mm)
-
bilateral/high-risk disease – bilateral disease is defined as a Gleason score of 7 (3 + 4 or 4 + 3) on the dominant side and a Gleason score of 7 (3 + 4 or 4 + 3) or a high-volume Gleason score of 6 on the contralateral side (any tumour dimension of > 4 mm); high-risk disease is defined as a Gleason score of ≥ 8.
Partial ablation
As HIFU is a relatively new technology, proctoring of clinicians new to performing the procedure is important. A PA working group was established, chaired by a HIFU expert, and two TSIs were written detailing the training and delivery of HIFU (see Report Supplementary Material 5). Proctoring was performed by HIFU experts, and training and competency forms were completed for each proctored case. mpMRI scans at 2 weeks post HIFU were also requested for the first five patients in centres new to performing HIFU, to be centrally reviewed by the chairperson of the PA working group.
Patient and public involvement
Strong links with the Oxfordshire Prostate Cancer Support Group (OPCSG) were maintained throughout the feasibility study. The previous OPCSG chairperson and secretary were involved in the development of the original HTA funding application. The NIHR Research Design Service South Central patient and public involvement (PPI) officer facilitated a meeting to discuss the research idea, the proposed design and opportunities for involvement throughout the duration of the study, as well as in the dissemination process. This was attended by an independent patient representative, who had recently been treated with HIFU for localised PCa in a clinical trial. A lay summary was produced for Cancer Research UK’s website and permission was granted by Cancer Research UK to use its logo on our website.
The lead PART trial research nurse in Oxford presented the PART trial to the OPCSG in January 2016. The PART trial was well received by the committee, and all members of the committee agreed on the importance of the study and the need for a larger RCT.
The new OPCSG chairperson continued PPI involvement as a lay member on the TSC, in addition to another patient representative, who had previously received RP. The OPCSG chairperson acted as a link to the OPCSG members and attended the PART trial investigator’s meeting, at which he participated in the discussion about how QoL is being measured in the PART trial, a question that had previously been raised by the OPCSG members. OPCSG members reviewed the PIL to ensure that it was a clear and understandable document from a patient’s perspective. Two queries arose regarding the risk of leaking urine and the risk of problems with sexual activity; these were addressed in the PIL, which was amended and approved by the Research Ethics Committee (REC) in February 2016.
Protocol and study documentation changes
Research Ethics Committee approval was obtained on 22 December 2014 for all PART study documentation. The protocol and PIL underwent substantial amendments and approvals were obtained on 18 January 2016 and 10 February 2016, respectively.
Protocol version 3.0, dated 2 November 2015
Further detail/clarification was included on:
-
the pre-randomisation diagnostic (imaging/biopsy) pathway for PCa patients
-
QA and QC information (for HIFU delivery, radiology and pathology)
-
the definition of treatment failure in patients receiving HIFU.
Consent process
There were no changes to the consent process.
Inclusion and exclusion criteria
‘Clinical T3 disease’ was added as an exclusion criterion.
Participant documentation
Following on from the PPI review of the PIL (now version 4.0 dated 21 January 2016), the ‘Possible disadvantages following RP’ column in table 2 was amended to read:
-
The risk of severe urine leaking is about 1%. The risk of moderate urine leaking is about 10%.
-
The risk of problems with sexual activity is around 50%.
Two clinic posters received Research Ethics Committee approval
-
One for clinicians, itemising the inclusion and exclusion criteria.
-
One promoting the study to patients [with a Quick Response (QR) code added so that patients could navigate directly to the PART trial website].
New centre recruitment
Originally, four recruiting centres were planned, including North Bristol, which was never opened because of unforeseen circumstances. Southampton General Hospital was opened to replace North Bristol. Basingstoke and North Hampshire Hospital opened as a fifth recruiting centre.
Project timetable and milestones
The recruitment period was originally projected to end on 30 June 2016 but was extended until 31 March 2017.
Chapter 3 Trial results
Recruitment and screening
Five UK NHS centres were opened in the PART study and recruitment took place in four of these five centres: (1) Churchill Hospital (Oxford), (2) Royal Hallamshire Hospital (Sheffield), (3) Southampton General Hospital, (4) Basingstoke and North Hampshire Hospital and (5) University College Hospital (UCH), London (no patient recruitment). The first patient was consented on 27 January 2015 and randomised on 3 February 2015. Quarterly randomisation by centre is shown in Table 1 for both the initial recruitment period (up to 30 June 2016) and the period of extension (up to 31 March 2017).
| Centre | Recruitment period | Grand total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | ||||||||
| January–March | April–June | July–September | October–December | January–March | April–June | July–September | October–December | January–March | ||
| Churchill Hospital, Oxford | 4 | 5 | 2 | 4 | 4 | 2 | 5 | 2 | 9a | 37 |
| Royal Hallamshire Hospital, Sheffield | R&D 16 November 2015 | 2 | 0 | 7 | 4 | 2 | 10b | 25 | ||
| Basingstoke and North Hampshire Hospital | R&D 1 June 2015 | 0 | 1 | 2 | 5 | 1 | 0 | 1 | 10 | |
| Southampton General Hospital | R&D 7 October 2015 | 1 | 0 | 2 | 0 | 3 | 4 | 10 | ||
| UCH, London | R&D 29 May 2015 | No patients recruited | 0 | |||||||
| Quarterly total | 4 | 5 | 2 | 8 | 6 | 16 | 10 | 7 | 24 | 82 |
The cumulative consent and randomisation rate is shown in Figure 1. The rate of recruitment was slower than expected, with one centre (UCH, London) unable to recruit any patients. The number of patients recruited per month ranged between 1 and 12, with an average of three per month throughout the 27-month period.
FIGURE 1.
Cumulative recruitment rate (consented and randomised patients).

Eligibility and randomisation
A total of 329 patients were screened across the five centres. The CONSORT flow diagram is presented in Figure 2, summarising the overall number of patients screened, considered eligible, consented and randomised. Of the patients screened, 93 were found to be ineligible and 149 declined consent. As the study had only just completed recruitment at the time of writing this report, data were pending for a number of patients. To allow for analysis to be undertaken on the available data, a database lock and extraction took place on 10 October 2017 (to include all data received up to and including 10 October 2017) when 82 participants had been randomised. See Table 5 for a summary of the data received by this time point.
FIGURE 2.
The CONSORT flow diagram. a, Incomplete screening data received on 72 patients. b, Consent data included up to and including 31 March 2017. c, Randomisation data included up to and including 4 May 2017. d, Data included up to and including October 2017.

Of the 87 patients who consented by 31 March 2017, 82 were randomised by 4 May 2017. Five patients were not randomised after giving consent: three patients failed imaging assessment post consent and two patients preferred not to be randomised. Of the 82 participants randomised, 41 were allocated to RP and 41 to HIFU. One participant randomised to RP withdrew from the study before receiving treatment. Within the RP group, eight participants rejected their treatment allocation: four participants crossed treatment groups to receive HIFU, one participant chose brachytherapy, two participants chose radiotherapy and one participant chose AM. One participant in the RP group was deemed unfit to receive surgery on clinical grounds, and crossed over to receive HIFU. In the HIFU group, two participants received brachytherapy treatment as a result of clinical judgement and one participant crossed over to receive RP. All randomised participants were included in analyses apart from the health economic evaluation, for which patient selection is described in Chapter 2, Study design.
QuinteT Recruitment Intervention results
In the presentation of the findings in the following sections, quotations are provided to support the results, and distinctions are made between data from interviews and those from recorded consultations. Quotations are anonymised to ensure confidentiality.
Data
Interviews
The QRI researcher approached 23 staff members to take part, and a total of 13 one-to-one interviews were conducted between July and November 2015. Interviews were conducted by the QRI researcher. Eight interviews were conducted face to face and five were conducted by telephone. The final sample of interviewees consisted of the trial manager, 11 recruiting clinicians (four of whom were members of the TMG) and one research nurse. At least one representative from each of the recruiting centres was interviewed. Interviews lasted an average of 43 minutes (range 31–53 minutes).
Recorded consultations
A total of 64 recruitment appointments, with 54 different patients, were obtained (in the case of five patients, two consultations were recorded). The first recording was received in September 2015. A total of 22 recordings were made before any QRI feedback had been provided and 45 were made after QRI feedback had been provided. Consultations lasted an average of 27 minutes (range 10–42 minutes). Twelve different recruiters (11 consultant urologists and one research nurse) led the consultations. Audio-recordings were obtained from four recruiting centres. One centre did not any provide recordings, another provided two recorded consultations (although both of these patients were ineligible), two centres provided 14 recordings and one centre provided 34 recordings.
Phase I: understanding recruitment challenges
The aim of phase I of the QRI was to understand recruitment difficulties as quickly as possible, so that they could be promptly addressed in phase II. The difficulties tended to be related to organisational arrangements or more complex issues about aspects of the design of the trial and intervention – so-called hidden challenges associated with perceptions of evidence, aspects of patient eligibility and the existence (or not) of equipoise.
Support for the study
Those involved in the study were asked about the existing evidence and the rationale for the study. HIFU was described as an ‘exciting’ and ‘promising’ alternative to radical procedures. Most of the recruiters described findings from observational studies, which suggested that HIFU had a lower side-effect profile in terms of erectile dysfunction or incontinence and acceptable outcomes in terms of cancer control. Recruiters described how much of the research had been conducted within UCH, and several commented that Oxford running the study was a strength as it was considered an ‘impartial’ centre:
I think this trial is needed because there has been a lot of hype or buzz about HIFU and focal therapy for some years now.
Interview, recruiter
I think having a study that is being led by a sort of equivocal centre is probably going to be very good for the study.
Interview, recruiter
Overall, there was strong commitment to a randomised study comparing RP with HIFU:
It’s an important study, I think everyone would like it to succeed.
Interview, recruiter
We don’t have any high-quality, randomised, comparative trial results, so it’s crying out really for a randomised trial and a good comparator would be radical prostatectomy.
Interview, recruiter
However, the QRI identified a number of issues that affected recruitment. These are outlined in the following section.
Organisational challenges
Several logistical obstacles disrupted the anticipated recruitment timelines, including the delivery of HIFU equipment in two centres (Sheffield and Bristol). In January 2015, it became evident that if the centre did not own a HIFU machine, the cost of hiring one was not covered in the original costing tariff. The TMG approached the manufacturers of the HIFU device to determine if the machine could be rented for free, which was not possible. The TMG then approached both Basingstoke and Southampton centres that were offering HIFU routinely, in May 2016. In Sheffield, an excess treatment cost (ETC) bid was submitted and successfully awarded so that part of the shortfall was covered by the Clinical Commissioning Group. However, ensuring the ETC took nearly 10 months and, as a result, recruitment in Sheffield (a high-yielding recruitment centre) was severely delayed:
HIFU is a new technology and costings in the NHS in terms of R&D [research and development] finance and the way they are costing it is quite difficult. What it meant was that the cost of hiring a HIFU machine if the site didn’t own it wouldn’t have been covered in the original tariff, so there would be a shortfall to the trust essentially. This had quite a severe knock-on effect in two of the original sites, Sheffield and Bristol, because they don’t own a HIFU machine and HIFU wasn’t routinely offered there. I think that might have been the biggest delay.
Interview, TMG
Obtaining research and development (R&D) approval in all centres, apart from Oxford, took significantly longer than hoped. In some institutions, it took as long as 6 months from submitting the site-specific information (SSI) form to centre R&D approval being issued. Many staff involved in recruitment to the study left their roles. This included the proposed PI for Bristol, and, in December 2015, the TMG made the decision not to pursue activating this centre. Research nurses from two active centres also left their roles (November 2015 in Basingstoke, April 2016 in Southampton and December 2016 again in Basingstoke), meaning that all new staff had to be recruited and subsequently trained:
We’re 6 months in and we’ve not even hit the start button for our R&D approval. That’s the biggest problem.
Interview, recruiter
Table 2 provides an overview of the organisational issues encountered. Although it was anticipated that all five centres would begin recruitment in January 2015, delays in activating the centres meant that, combined, a total of 30 recruiting months were lost.
| Issue | Impact on recruitment |
|---|---|
| Delay getting centres active | Obtaining R&D approval in all centres meant that four centres began recruitment significantly later than anticipated:
|
| Availability of HIFU machine | Not all centres owned a HIFU machine and this had not been covered in the original costing tariff. An ETC was awarded to Sheffield, although this meant that there was an 11-month delay in getting the centre activated |
| High staff turnover | Many staff involved in recruitment left their roles, including the PI for Bristol, and several research nurses in Basingstoke and Southampton. Consequently, new staff had to be identified and trained |
Hidden challenges
The organisational issues meant that data collection for the QRI was also delayed in most centres. For instance, the first recording of a consultation was only obtained in September 2015. Once the centres began recruiting and sufficient QRI data became available, analyses demonstrated that there were a number of intellectual and emotional challenges to recruiting to the PART trial. These are outlined in the following sections.
Previous randomised controlled trial experience
Before the PART trial, many of those interviewed had not been involved in recruiting to randomised studies and had therefore not received any support or training for their role as recruiters. The exception to this was several recruiters from Oxford and Sheffield, who had received recruitment training for the ProtecT trial. Analysis of the data showed that, overall, these recruiters appeared more comfortable with the concept of uncertainty, with the need for pragmatic inclusion criteria and with explaining trial concepts to patients. This is further discussed throughout this chapter, but is exemplified by the following quotations:
I have seen patients that have been recruited to different trials through my training, but I haven’t, personally, been responsible for recruiting to trials [. . .] I have no idea whether HIFU’s going to work or not, so it makes it very difficult to know how much of that information to tell patients. The problem with HIFU is that I have no idea whether, in a year’s time or whatever it is, 20% of all the people that are having HIFU need a further treatment, or whether it’s 2% or whether it’s 50%. Therefore, it’s very difficult to then talk to patients about it. Whereas, if they opt for surgery, I can say to them, based on their disease parameters, that their chance of having a recurrence is something like 5% to 10% in 10 years.
Interview, recruiter
I think we need to be confident on our uncertainty, and you know, I’ve learned a lot by being involved in ProtecT [. . .] We have a national reputation for being good with prostate cancer, and we acknowledge that there are uncertainties in the decision-making, which is why we run clinical trials.
Interview, recruiter
Discomfort regarding the inclusion criteria
The method of determining suitability for the various treatment options was a complex process that took into consideration a large number of factors. All recruiters explained that the Gleason score was a key factor in determining whether or not the patient was regarded as being at ‘intermediate risk’ – a key eligibility criterion. The study specified a Gleason score of 7 as the inclusion criterion, but there was considerable concern about perceptions of differential levels of risk depending on whether the patient’s score was a ‘4 + 3’ or ‘3 + 4’ dominant pattern. As one recruiter remarked, ‘4 + 3 is just not the same as 3 + 4, they’re very different’. Some recruiters expressed concerns about whether or not men with a score of 4 + 3 were suitable for HIFU and instead felt that a prostatectomy was more appropriate. Equally, there was uncertainty about whether or not a patient with a 3 + 4 score would be ‘overtreated’ with prostatectomy. These patients were considered to be more suitable for HIFU or AM. The following quotations highlight the different responses to the two scores:
3 + 4’s, I think they are maybe the best ones to treat with HIFU, where they’ve got mainly pattern 3, but a bit of pattern 4. I think you can be pretty confident that you’re going to wipe out that pattern 4 when you do the treatment.
Interview, recruiter
I was just marginally uncomfortable because he had a 4 + 3 and he was 50 years old and it was quite a significant volume of tumour. I just found myself thinking, ‘Do you know what? I wonder if you’d be better off having a radical prostatectomy’.
Interview, recruiter
A further aspect of the inclusion criteria was the cancer core length. In the protocol, this was stated as > 4 mm. However, there was a feeling that patients who had larger core lengths – although technically eligible for the PART trial – would be more suited to RP:
If there’s a younger man with more volume of disease, longer cores and such. It’s a bigger lump on MRI. I might be tempted to, though I’d be reluctant to put him through HIFU, he needs surgery.
Interview, recruiter
Recruiters also described how the protocol stated that patients eligible for the PART trial must be expected to live for 5–10 years. Generally, most clinicians appeared to feel uncomfortable with treating men > 70 years old with a prostatectomy. There was also a feeling that patients who were younger would benefit more from the prostatectomy rather than HIFU.
Consultation:
What would be your advice? Which treatment, in this particular case?
I think, for someone who is fitter and younger like you, that surgery offers more benefits at this stage.
[Patient opts for radical prostatectomy.]
Alongside these clinical factors, patients’ personal circumstances were carefully taken into account. For example, recruiters emphasised the difference between curing and treating cancer. For instance, they described scenarios in which a patient might opt for a treatment that they did not necessarily feel was the ‘best’ but that suited their personal circumstances:
We know with prostate cancer it is not just which treatment is best of getting rid of cancer; it is a whole load of other stuff that they have to consider. We know that some people will choose a less good cancer operation or cancer treatment. Say, in my opinion, radiotherapy is not as good as surgery, but for some people choosing radiotherapy is the best for them because of all of the other concerns they have about surgery.
Interview, recruiter
Recruiter bias towards a particular arm
During the interviews, many of the recruiters expressed clear preferences for a particular treatment option and explained that this made it difficult to be in equipoise and to randomise to the PART trial:
What you may find is that for every single patient you could have a strong opinion that takes you away from equipoise. The trouble is the real life situation will have all of those details. Every single man will have some sort of thing . . . This all factors in to the consultation and what you recommend to someone. To have equipoise and randomise in this I think is very challenging.
Interview, recruiter
Those who favoured prostatectomy expressed concerns that HIFU would not remove all of the cancer. Conversely, advocates of HIFU expressed concerns that a prostatectomy would be overtreating cancer and compromising QoL. These recruiters questioned whether or not prostatectomy was a ‘cure’ for cancer at the cost of the patient’s sexual function and continence, whereas HIFU offered an opportunity to ‘control’ the disease while maintaining these functions:
So I have complete confidence that HIFU has fewer side effects [. . .] But they definitely, they are compromising their cancer treatment by taking the risks that we’re only treating one part of the prostate. And so there might be another part of the prostate which has some prostate cancer in. So that they [patients] understand after I’ve told them.
Interview, recruiter
I’m wondering if we are overtreating those men [with intermediate-risk disease] though, by doing radical prostatectomy.
Interview, recruiter
The consultations demonstrated that these beliefs were conveyed to the patients. Loaded terminology was frequently adopted, such as describing ‘conventional surgery’ and an ‘experimental’, ‘alternative’ and ‘novel’ HIFU. There were many consultations in which the concept of uncertainty was not introduced to the patient. Instead, recruiters often provided treatment recommendations:
I think that surgery or radiotherapy, for someone who is young and fit like you, with slightly more bulky disease, would be more appropriate. So, I don’t think the trial is right for you.
Consultation, recruiter
[Patient opts for radical prostatectomy.]
Consultation:
If you have surgery, with the kind of disease you have, you’d almost certainly not die of prostate cancer.
I think I’d prefer to be alive.
[Patient opts for radical prostatectomy.]
Discomfort with exploring patient preferences
In the consultations recorded, treatment options described usually included prostatectomy, EBRT and AM. In addition, cryotherapy, permanent seed brachytherapy, temporary brachytherapy and CyberKnife® (Accuray, Sunnyvale, CA, USA) were occasionally discussed. Analysis of the consultations suggested that several recruiters struggled with outlining all the options that were available to patients in a concise manner, and seemed unclear when and how to mention the PART trial without bombarding the patient with too much information:
There are a lot of things to discuss in that 20-minute consultation. At that point, they don’t know whether their cancer needs treating or doesn’t need treating. They don’t know whether they can be watched safely or whether they need a form of radical treatment. They don’t know the side effects of surgery, the side effects of radiotherapy, what kind of surgery they would get, or whether they would have conventional radiotherapy or whacky therapy. There are so many options.
Interview, recruiter
There are already choices, and then you throw at them, ‘Oh, by the way, there’s a randomised trial. That’s another thing we can offer to you’.
Interview, recruiter
There was some evidence to suggest that patients appeared overwhelmed by their options:
The options are the problem. There’s just so much to choose from.
Consultation, patient
I asked my GP about this, because I said that, ‘One of the problems I’m having is that there are so many options open to me and I’m not being given clear advice on one rather than any of the others’.
Consultation, patient
After receiving their diagnosis, most patients sought information from a variety of sources, including other health-care professionals, the internet and friends or relatives; therefore, all recruiters explained that most patients often came to consultations with preconceptions about the treatment options. These preferences were constructed as ‘fixed’, and most recruiters expressed discomfort with discussing these:
Sometimes patients just come in and say, ‘I don’t want radiotherapy. I have googled it and it is not for me. I don’t want brachytherapy. I have googled it and it is not for me. I want surgery.’ Patients are now empowered with knowledge so I think most of the time once they have been given the diagnosis they are googling. I think they know. It’s their decision, and I think it is the right decision.
Interview, recruiter
When patients did express preferences, most recruiters did not discuss these further. Consequently, the study was often not discussed. This meant that, in such cases, there was insufficient evidence to demonstrate that patients fully understood the key information about each treatment and were basing their decision on correct information.
Consultation:
I think I would prefer to have it zapped [HIFU].
Sure. That’s absolutely fine, no, totally understand.
[PART trial not discussed in consultation.]
In contrast, those who had been involved in the ProtecT trial emphasised the importance of exploring patient views and understanding:
Usually, a patient decision is often based on misinformation rather than a personal decision . . . There is a fine line, we must not cross the line, but I think if they have misunderstandings about the disease or the trial, it’s our duty to clarify that, and then the decision is theirs. It’s always about bouncing the decision to them and making sure they understand.
Interview, recruiter
Difficulty explaining randomisation
Many recruiters felt that the concept of randomisation was a key reason why patients did not want to join the study. However, analysis of consultations showed that very few explained the rationale for randomisation. Instead, recruiters tended to focus on the process of randomisation and use metaphors such as ‘lucky dip’ and ‘toss of a coin’. In addition, there was a tendency to explain randomisation in a negative manner (i.e. focusing on the lack of control/choice):
The downside is you’re going into it not knowing which one you’re going to go for.
Consultation, recruiter
Consultation:
It is asking men to agree to have the choice taken away from them.
You don’t have a choice in that?
You don’t have a choice.
[Reason for randomisation not discussed, patient declined participation in the PART trial.]
The consultations suggested that these explanations were off-putting for patients, and there was little evidence to show that patients understood why randomisation was necessary:
Well I don’t want to be like a contestant on a quiz show.
Consultation, patient
Hmmm . . . But I don’t get a choice with the study, do I?
Consultation, patient
Phase I: summary of key recruitment difficulties
Recruiters expressed strong support for a high-quality study comparing RP with HIFU and expressed their commitment to the study. However, the findings from the QRI have highlighted a number of challenges that were limiting recruitment. These included serious difficulties in activating the centres because of delays to the availability of HIFU equipment, R&D delays, and staff departures and arrivals – meaning that a combined total of 30 months was lost. This also meant that data collection for the QRI was delayed in most centres. For instance, the first recording of a consultation was not obtained until September 2015.
Once the centres began recruiting and sufficient QRI data were available, analyses demonstrated that there were a number of intellectual and emotional challenges to recruiting to the PART trial. Many recruiters had not been involved in randomised studies and had therefore not received any support or training for their role as recruiters. Consequently, they expressed some discomfort with key trial concepts, such as the pragmatic inclusion criteria. For instance, when determining suitability for the different treatments options, a range of clinical – and personal – factors were taken into account, and clinicians often held strong preconceptions about which treatment was best for a patient. This made it difficult for several clinicians to accept the concept of randomisation. The data also demonstrated that there were difficulties with maintaining equipoise in practice among many recruiters, even though they supported the conduct of the PART trial. Recruiters expressed particular difficulty in accepting that surgery and HIFU were equivalent. Some recruiters appeared to favour prostatectomy for its oncological outcomes, whereas advocates of HIFU argued that prostatectomies were overtreating patients and HIFU had fewer side effects. These beliefs were sometimes conveyed to patients with loaded terminology, unbalanced information about the treatment arms and treatment recommendations. It was not surprising that, following this, many patients expressed preferences for a particular treatment. When these reflected the views of recruiters, they were often promptly accepted without further exploration. Although these issues had a substantial impact on recruitment, they are not unique to the PART trial. 46,47,54,55
Table 3 shows recruitment during phase I of the QRI (January–November 2015). By November 2015, only 15 men had been recruited. Furthermore, the lead centre (Oxford) had recruited the majority of these, whereas some centres had not recruited any patients at all.
| Site | Recruitment month | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | |
| Churchill Hospital | 0 | 4 | 0 | 1 | 3 | 1 | 0 | 1 | 1 | 3 | 0 |
| UCH | Not activated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Basingstoke and North Hampshire Hospital | Not activated | 0 | 0 | 0 | 0 | 1 | |||||
| Royal Hallamshire Hospital | Not activated | ||||||||||
| Southampton General Hospital | Not activated | 0 | 0 | ||||||||
| Total | 0 | 4 | 0 | 1 | 3 | 1 | 0 | 1 | 1 | 3 | 1 |
Phase II: optimising recruitment and informed consent
In November 2015, the QRI team and the CI held a meeting to discuss strategies to improve recruitment and informed consent. An outline of the subsequent steps taken are presented below (summarised in Figure 3). It should be noted that it was not possible to organise any group or individual feedback sessions with one centre (UCH) as sufficient data through audio-recordings and screening logs were not provided.
FIGURE 3.
Recruitment interventions and randomisations by month.
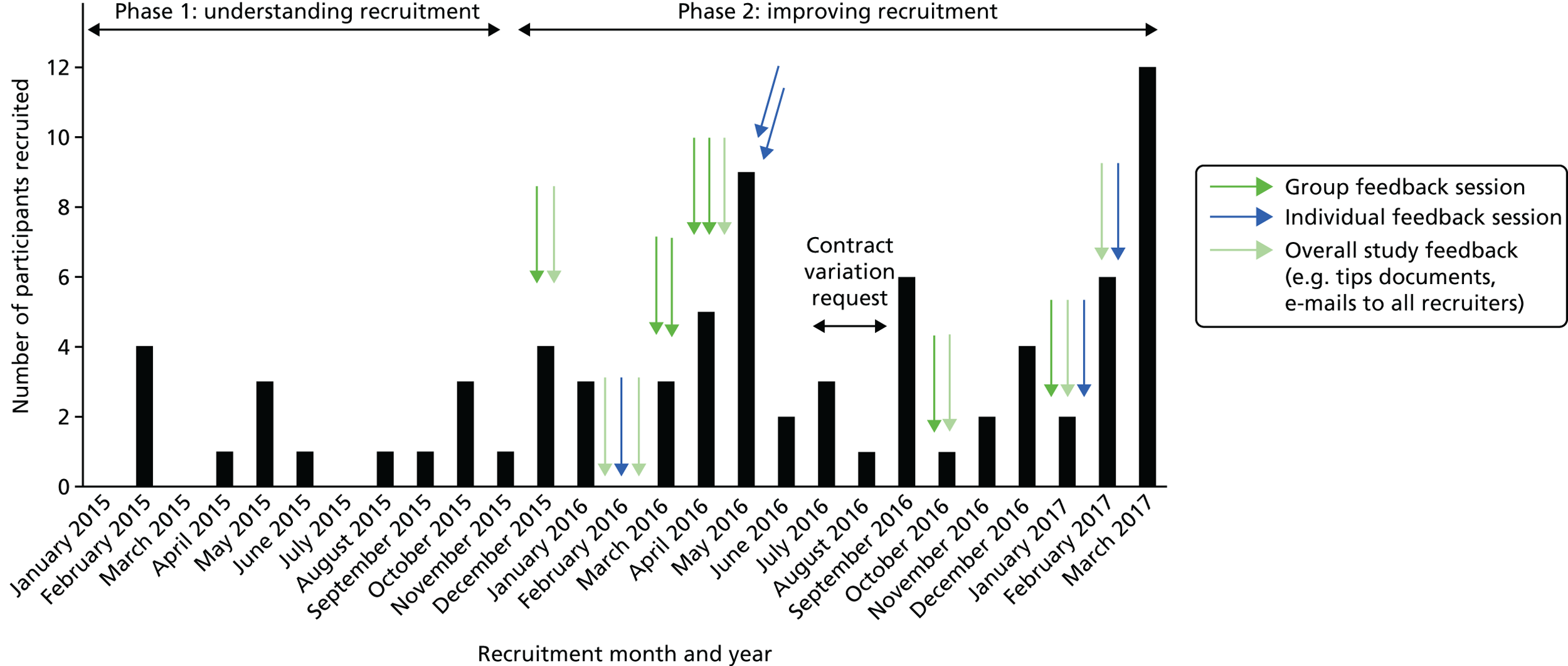
Recruitment session at collaborators’ meeting
In December 2015, a collaborators’ meeting was hosted in Oxford with 30 key centre staff in attendance from all active centres. The day included a two-part session on recruiting to the PART trial, led by the QRI researcher. This featured a presentation of the findings of phase I, which were supplemented with anonymised quotations to illustrate the key issues. Overall, the session aimed to improve recruiter engagement with the study. Because the interviews highlighted the lack of individual equipoise between the recruiters, the session primarily focused on the lack of randomised evidence comparing prostatectomy with HIFU, the extent to which there was community equipoise (i.e. by demonstrating the conflicting biases for the treatment arms), and examples of how recruiter beliefs could influence patient preferences. Furthermore, because many recruiters had not been involved in randomised studies before the PART trial, the session emphasised the ways in which recruiting to RCTs differed from standard practice, with the aim of encouraging recruiters to feel more comfortable with the concept of uncertainty and refrain from providing treatment recommendations. Alongside this, the researcher discussed the importance of approaching all eligible patients so that all men had the opportunity to consider the study. Recruiters were encouraged to explore patient preferences in future discussions to ensure that men were making a fully informed decision. The session was interactive, with honest and open discussions being encouraged.
Recruiter tips and guidance
The challenges associated with structuring consultations and explaining trial-specific processes (i.e. randomisation) were addressed through circulation of a ‘tips sheet’ in February 2016 (see Report Supplementary Material 8). This two-page document contained advice for structuring the consultation and presenting the treatment options in a balanced manner. The document also featured guidance on introducing the study and explaining randomisation. This was placed on the study website and laminated copies were posted to 22 recruiters from all five centres. The key points were summarised in monthly recruitment newsletters that were circulated to all recruiters. Those involved in the trial were also sent several recruitment e-mails. These provided guidance for specific issues, such as exploring patient preferences and optimising recruitment in centres offering HIFU outside the PART trial, in addition to outlining how the rationale for the PART trial was strengthened by the publication of the ProtecT trial findings.
Site recruitment reviews
Between December 2015 and January 2017, group feedback sessions at each recruiting centre were conducted. In addition to the QRI researcher, the principal investigator (PI) and lead research nurse, all centre staff involved in the trial were encouraged to join the meeting so that the team could discuss recruitment to date, areas of difficulty and plans for improving recruitment. Sessions often began with an overview of the screening log data and discussion of patient pathways. These sessions were another opportunity to reinforce the generic key points from the sessions at the collaborators’ meeting, and there was also a focus on specific issues that had emerged from the analysis of the appointment recordings in each clinical centre. For instance, the sessions covered a variety of issues, such as discussing competing research studies, clarifying particular roles within the team and introducing the treatments that were available at each centre. A total of seven recruitment reviews were conducted.
Individual feedback
Recruiters who had provided at least three recorded consultations were provided with individual feedback by the QRI researcher. This was presented and discussed in a supportive and confidential manner, which was supplemented with a written summary. The feedback highlighted instances of good practice in terms of shared decision-making and informed consent, and made suggestions as to what might be improved. In total, individual feedback was provided to three recruiting surgeons.
Impact of the QuinteT Recruitment Intervention
Pre- and post-feedback recruitment rates
Between January and November 2015, 15 patients were recruited. In this time, the average number of patients agreeing to be randomised was 1.4 per month. In addition, the conversion rate (the numbers of eligible men invited to join the PART trial who then went on to be randomised) was 20% (15/75). Phase II of the QRI began in December 2015, and continued for the duration of the study until recruitment ended in March 2017. During this time, 67 patients were recruited. On average, this equated to 4.5 patients per month. In addition, the conversion rate increased to 37%. Furthermore, after the initial intervention in December 2015, several centres (rather than predominantly Oxford) began recruiting more consistently. Although it is not possible to determine the precise impact that the QRI had on recruitment, this suggests that it had a positive impact.
Changes in information provision
Conveying equipoise
Although feedback was targeted in relation to the requirements of individual recruiters and centres, the main issues consistently focused on conveying equipoise to the patient, exploring preferences and discussing the study concepts (including randomisation) in a clear manner. Analysis of the recordings available post feedback highlighted clear changes to the way that recruiters discussed the study and engaged with patients during consultations. For instance, before feedback, the majority of recruiters did not explain that there was uncertainty as to what treatment was best for patients with intermediate-risk, unilateral PCa. Consequently, the PART trial had not been discussed in detail and tended to be mentioned towards the end of the consultation (if it was mentioned at all). After feedback, many recruiters began to present the study enthusiastically (‘We are involved in an exciting study’). In addition, they often mentioned the PART trial very early in the consultation (often at the beginning of the discussion about treatment options). The following quotation illustrates how recruiters acknowledged very early in the discussion that there was uncertainty:
[After discussion about the diagnosis, patient asks what treatment he should have.]
I’ve been a consultant for years and I sit here telling men what’s going on, telling them they’ve got cancer, they ask me what treatment we go for, and I try and help them and steer them in the direction, but, at the end of the day, I have to sometimes stop and say, ‘Actually, there isn’t really any evidence that this treatment is better than that one because there have never been any proper trials comparing properly treatment A with treatment B. To get proper results, you have to actually do it in a randomised way.’ There is a study called PART, that we’re very much involved with . . .
Consultation, recruiter
There were also several examples to suggest that recruiters had refrained from providing treatment recommendations. For instance, the following quotations highlight an emphasis on shared decision-making when the patient asked what treatment would be best.
Consultation:
What would you recommend?
Well, we just don’t know. I’m here to help and guide you.
[Discussion of positives and negatives of each treatment.]
The QRI had emphasised the need to present the treatment options in a more neutral manner. Post feedback, there were many more instances in which recruiters outlined the advantages and disadvantages of each treatment. Many recruiters also explained that they would be comfortable with the patient having either procedure:
Now, we’re involved with a trial at the moment of two surgical treatments for prostate cancer . . . I guess what we don’t know is because the HIFU treatment has not been used focally to partially treat the gland for long enough. How good is it in the longer term? What proportion of men does it cure? Is there a trade-off between a few percentage points for a likelihood of cure versus a better side-effect profile?
Consultation, recruiter
We feel equally good about both treatments; they’re both good treatments.
Consultation, recruiter
Exploring patient preferences
Recruiters had often accepted patient preferences without discussing these further. Therefore, feedback had encouraged recruiters to elicit the reasons underlying patient preferences to ensure that these were based on accurate and balanced information. The consultations after feedback suggested that recruiters appeared more comfortable exploring patients’ perspectives and concerns.
Consultation:
The way I’m going, brachytherapy sounds good to me at the moment.
OK. Why do you like the sound of that?
Two recruiters also then went on to tailor information to each patient. For instance, the following quotation is from a consultation in which a patient expressed a preference for HIFU so that he would be able to return to work sooner. The recruiter responded by explaining a possible disadvantage to HIFU:
Well, yes, but there are patients who after HIFU have a higher risk of having blockage issues afterwards. There is a possibility that you would end up having to cancel work because we have to come and do a cystoscopy or pass catheters and teach you how to do that yourself, I’d say the risk of that is slightly higher with you.
Consultation, recruiter
It should be noted that some recruiters still had a tendency to present the trial as an option for those who did not have a preference, indicating that further feedback would be needed:
If you’ve got a strong feeling one way or the other about them [treatments], but if you haven’t, I would ask you to take away and read this leaflet that talks in detail about the trial.
Consultation, recruiter
Explaining randomisation
Post feedback, most recruiters stopped using metaphors to describe randomisation. Instead, there were many more instances in which recruiters explained why this method was necessary.
One of the ways to overcome that bias is with a large group of people in that situation, have a random assignment of each treatment, so that at the end, you can look at both groups and say, ‘These two comparable groups of people, who’ve had the same situation and dealt with in two different ways: what did it look like at the end? Which group had the best outcome?’.
Consultation, recruiter
As a result, patients appeared to be more receptive to the prospect of being randomised:
My concern is that one is better than the other. But I understand what you’re saying, that no one knows what’s best to do. It’s toss a coin time, I have nothing to lose.
Consultation, patient
I think I’d probably rather go on the trial, so I don’t have to choose.
Consultation, patient
Post feedback: why did some patients continue to decline?
Although there were clear improvements in the way that recruiters described the study and engaged in patient discussions, the conversion rate was still lower than the target of 50%. There were a number of reasons for this. For instance, there were several consultations in which, even though the recruiter balanced the treatment options and explained the study well, patients did not want their treatment to be allocated. The following consultation provides an example of this.
Consultation:
How do you feel about being allocated a decision?
Not my style really. I’m the boss, I make the decisions.
Can you understand the philosophy of that though?
Yes I absolutely understand the philosophy, yes, it’s research, I do that as well, so I have to understand it because it’s got to be ran– [. . .] I’m torn actually because I sense that going for the trial is a good thing generally because this is something we should try and do.
Since we don’t know, since this is a difficult choice, entering a trial allows you to be allocated one of two very good treatments. One thing we know is that people who are managed in trials generally do better than people who aren’t in trials.
Yes, I know, my medical help at the hospital have been telling me exactly that, but I’m a risk taker.
Although recruiters began to engage in discussions about preferences, patient preferences were still the main reason why patients declined to participate in the trial. For instance, several patients declined to participate in the study because they wanted other forms of radical treatments (such as radiotherapy) or PA (such as cryotherapy) that were not available in the trial. These treatments would, however, be included in a main study comparing radical treatments with ablative therapies, and so removing this barrier to recruitment would probably lead to more recruits.
I’m going towards the radiotherapy, purely because I thought it was, well, painless. I thought it wasn’t going to hurt me. As much as I’m a man, you don’t want to put yourself through anything you don’t really have to unless you have to.
Consultation, patient
Some patients declined to participate in the study because they did not want the entire prostate removed. These men expressed concerns about the impact that radical treatments would have on their erectile functioning and wanted to pursue a treatment that would give them a greater chance of maintaining an active sex life.
Consultation:
I am not really in favour of having the whole lot out.
Basically he doesn’t want to lose his sex life yet. That is the one of the main . . .
Too many side effects and I have got an active sex life.
You can give me the options, but I understand why you can’t advise me. But as I said to my friend here, that I see this as a house. And we’ve got a bit of dry rot in one of the bedrooms. And I don’t see that as a reason to knock the house down. We treat the dry rot . . . Because I’m very sexually active. And I want to be for the next few years.
Conversely, other patients stated that they wanted a RP because it gave them the greatest chance of removing all the cancer. These patients stated that they were prepared to accept the higher risk of side effects of radical treatment:
If you’ve got a problem, get rid of the damn thing. You’ve got gangrene in your finger, cut your finger off, don’t try and cure it.
Patient, consultation
I just want surgery. I just want it out of my body [. . .] To be quite honest with you, a physical relationship, it wouldn’t be the be-all and end-all of it all.
Patient, consultation
Variation between centres
It is important to acknowledge that there was also considerable variation between the centres regarding conversion rates, that is, the proportion of eligible patients consenting to randomisation (Table 4).
| Centre | Conversion rate | |
|---|---|---|
| n/N | % (95% CI) | |
| Sheffield | 27/40 | 68 (60.1 to 74.9) |
| Oxford | 40/89 | 45 (39.7 to 50.3) |
| Basingstoke | 10/47 | 21 (15.4 to 27.2) |
| Southampton | 10/47 | 21 (15.4 to 27.2) |
| UCH | 0/13 | 0 (0 to 0) |
Although NICE guidelines72 state that HIFU is not recommended other than in the context of controlled clinical trials comparing HIFU with established interventions (such as surgery), HIFU was openly available on the NHS at three of the five participating centres (Basingstoke, Southampton and UCH). Two of these centres had the lowest conversion rates (21%) and one did not recruit any participants (UCH). UCH was anticipated to be a strong recruiter owing to the high number of procedures that are performed at the centre each year. However, as it became clear that this centre was recognised nationally as a specialist centre for HIFU, this meant that patients were referred to it from surrounding and distant hospitals specifically for HIFU treatment. These patients expressed strong preferences for HIFU, and none agreed to be participants in the PART trial.
Both Southampton and Basingstoke found recruitment challenging because HIFU was available locally, outside the PART trial. For instance, Basingstoke was active for 4 months before it randomised a participant. Before QRI feedback, seven patients were approached. Although one participant was randomised, four patients opted for HIFU and two had surgery. In Southampton, in the 2 months before QRI feedback, seven patients also were approached. Only one was randomised, whereas three declined the study because they did not want surgery and instead wanted HIFU. The remaining three participants chose radiotherapy (which would be included in a main study). Therefore, before QRI support, both centres had a conversion rate of 14% each.
Following on from QRI feedback, the consultations provided from Southampton and Basingstoke showed considerable improvements in how the recruiters discussed the PART trial. However, there were still a number of patients who declined to participate in the study and opted for HIFU. In the following consultation, the recruiter introduced the concept of uncertainty early in the consultation, conveyed enthusiasm for the study and balanced the treatment options well. Nonetheless, the patient states that he would prefer to have HIFU.
Consultation:
At the moment we’re involved in, what we think, is an exciting trial, looking at and comparing the two surgical treatment options. I spend a lot of my time telling them they’ve got prostate cancer and they say to me, ‘What’s the right treatment? What should I go for?’. Sometimes I can give them a steer. But the more I have these discussions and the more I reflect on it all, it does make me think, ‘You know what [name], a lot of what you say is based on very little evidence’. We don’t really know whether treatment A is better than treatment B. In the long term, because there are no proper randomised trials comparing the options. I just want to tell you a little bit about the trial that we’re involved with. It’s called PART. It stands for partial ablation versus radical therapy, radical prostate removal. It’s the two surgical options being compared with each other. The good thing about the trial is, we know that if any man goes into a trial, the results of the treatments within the trial tend to be good. The outcomes are good. So that we do know. The only way, in the future, that we’re going to be able to properly compare whether the right treatment is complete removal or partial treatment using ultrasound – well, not whether it’s right or wrong and which is better, but when to use one or the other and how they compare – is by doing a randomised proper trial. That is the only way we’re ever going to get useful information that we can help other men in the future with their decision-making.
Well HIFU sounds better to me.
[Discussion about side effects and oncological outcomes, recruiter balances treatment options.]
From my perspective, with yours being a 4 plus 3, whatever way we were going to treat it, my preference would be that you were within the trial.
I want, really, what is going to be best for my situation, including the after effects . . . What’s the best solution for me?
I think the short answer is, if I’m being honest, I don’t know which the best option is. That’s why we’re doing the trial.
Looking at factors I think, what we’ve just talked about and discussed. I’d rather keep a part of it, if it was possible, part of the prostate and also a rapid solution. I would prefer a rapid solution. It seems that the HIFU is probably the best option, like I say, because if it doesn’t work or anything like that, then you just cut it out anyway and have surgery and you do the other thing anyway. You might just as well do the HIFU for that reason.
You understand that . . .
I know nothing is guaranteed.
This meant that recruitment was straightforward in the only two remaining centres (Oxford and Sheffield):
A lot of patients are a mixture of private and potentially NHS patients who have been diagnosed with prostate cancer intermediate risk. They have done their research and they have decided they don’t want any of the standard treatments, so radical prostatectomy or radical radiotherapy. They will have potentially taken a long time trying to get to UCL [University College London] so they can have HIFU.
Interview, TMG
Completeness of data
Once participants were randomised, it was expected, when possible, that allocated treatment would be delivered within 8 weeks. All participants had a follow-up clinic visit at 6 weeks post procedure (as per routine NHS care), then every 3 months for the first year and, subsequently, every 6 months up to 3 years.
The overall return rate of the follow-up CRFs was 95% (Table 5).
| Assessment time point | Forms | |
|---|---|---|
| Expecteda (n) | Received, n (%) | |
| Baseline | 82 | 81 (98.8) |
| 6 weeksb | 74 | 74 (100.0) |
| 3 months | 69 | 66 (95.7) |
| 6 months | 55 | 48 (87.3) |
| 9 months | 44 | 44 (100.0) |
| 12 months | 36 | 32 (88.9) |
| 18 months | 19 | 15 (78.9) |
| 24 months | 8 | 8 (100.0) |
Demographic data
Table 6 presents a comparison of the key baseline participant characteristics, taken from the 82 baseline CRFs received and analysed, which were well balanced across the two treatment groups, given the small sample of participants. The median age of participants randomised to the study was 66 years (range 48.4–78.2 years) with an average PSA level of 7.2 ng/ml (range 2.4–17.1 ng/ml). Most participants (78%) had a Gleason score of 3 + 4 at randomisation and were clinical stage T2a (59%). One-quarter of participants had a family history of PCa.
| Characteristics | Trial arm | |
|---|---|---|
| RP (N = 41) | HIFU (N = 41) | |
| Age at randomisation (years), median (range) | 65.5 (48.4–76.9) | 66.4 (54.2–78.2) |
| BMI (kg/m2), median (range) | 26.7 (22.0–61.6) | 27.3 (22.2–39.6) |
| Height (m), median (range) | 1.77 (1.1–2.0) | 1.74 (1.5–1.9) |
| Weight (kg), median (range) | 81.5 (72.0–111) | 82.5 (64.0–114) |
| PSA level (ng/ml), median (range) | 6.90 (2.4–16.2) | 7.70 (2.5–17.1) |
| Family history of prostate cancer, n (%) | ||
| No | 13 (31.7) | 19 (46.3) |
| Yes | 11 (26.8) | 10 (24.4) |
| Do not know | 16 (39.0) | 12 (29.3) |
| Missing | 1 (2.4) | 0 (0.0) |
| Gleason score, n (%) | ||
| 3 + 4 | 32 (78.0) | 32 (78.0) |
| 4 + 3 | 8 (19.5) | 8 (19.5) |
| High-volume 6 | 1 (2.4) | 1 (2.4) |
| Clinical stage, n (%) | ||
| T1c | 1 (2.4) | 0 (0.0) |
| T2 | 12 (29.3) | 11 (26.8) |
| T2a | 22 (53.7) | 26 (63.4) |
| T2b | 5 (12.2) | 2 (4.9) |
| T2c | 1 (2.4) | 1 (2.4) |
| Missing | 0 (0.0) | 1 (2.4) |
| Ethnicity, n (%) | ||
| White British | 40 (97.6) | 39 (95.1) |
| Caribbean | 1 (2.4) | 1 (2.4) |
| Other black background (not Caribbean or African) | 0 (0.0) | 1 (2.4) |
| Biopsy and mpMRI show, n (%) | ||
| Unilateral clinically significant intermediate-risk disease | 32 (78.0) | 34 (82.9) |
| Dominant unilateral clinically significant intermediate-risk disease and small risk of contralateral low-risk disease | 8 (19.5) | 7 (17.1) |
| Missing | 1 (2.4) | 0 (0.0) |
| History of PCa diagnosis: raised PSA level, n (%) | 36 (87.8) | 34 (82.9) |
| History of PCa diagnosis: abnormal DRE, n (%) | 4 (9.8) | 6 (14.6) |
| History of PCa diagnosis: incidental finding, n (%) | 4 (9.8) | 6 (14.6) |
| Prostate dimensions taken, n (%) | 15 (36.6) | 17 (41.5) |
| Transverse (mm), median (range) | 39.7 (3.9–60.0) | 46.6 (4.5–62.9) |
| Anteroposterior (mm), median (range) | 30.1 (3.1–55.7) | 35.7 (2.5–51.0) |
| Longitudinal (mm), median (range) | 46.0 (1.7–61.3) | 49.4 (4.5–74.1) |
| Prostate volume (cc), median (range) | 33.1 (15.0–64.0) | 43.0 (14.0–74.0) |
Treatment details
Tables 7–9 give details of the treatment received by study participants. All participants except one were discharged to home post procedure; RP participants spent longer in hospital than participants who received HIFU, with 62% of HIFU participants not spending any nights in hospital. One participant in the RP group required a night in an ICU. Median time to treatment from randomisation was longer in the HIFU group (54 vs. 35 days); this delay was because participants randomised to HIFU were treated in batches in order to mitigate the cost of renting the HIFU equipment. This only applied to participants treated in Oxford and Sheffield.
| Treatment details | Trial arm | |
|---|---|---|
| RP (N = 32) | HIFU (N = 39) | |
| Discharged to, n (%) | ||
| Home | 31 (96.9) | 39 (100.0) |
| Other | 1 (3.1) | 0 (0.0) |
| Blood transfusion received, n (%) | ||
| No | 32 (100.0) | 39 (100.0) |
| Analgesia, n (%) | ||
| None | 0 (0.0) | 9 (23.1) |
| Oral | 21 (65.6) | 27 (69.2) |
| Other | 0 (0.0) | 1 (2.6) |
| Patient-controlled analgesia | 11 (34.4) | 1 (2.6) |
| Missing | 0 (0.0) | 1 (2.6) |
| Nights spent on ward, n (%) | ||
| 0 | 0 (0.0) | 24 (61.5) |
| 1 | 20 (62.5) | 14 (35.9) |
| 2 | 9 (28.1) | 1 (2.6) |
| 3 | 2 (6.3) | 0 (0.0) |
| 6 | 1 (3.1) | 0 (0.0) |
| Nights spent on ICU, n (%) | ||
| 0 | 31 (96.9) | 39 (100.0) |
| 1 | 1 (3.1) | 0 (0.0) |
| Time to treatment from randomisation (days), median (range) | 35.0 (9.0–228) | 54.0 (12.0–398a) |
| Time spent in hospital (days), median (range) | 1.0 (1.0–7.0) | 0.0 (0.0–2.0) |
| RP treatment details | Participants, n (%) |
|---|---|
| Operation performed (N = 32) | |
| Laparoscopic prostatectomy | 1 (3.1) |
| Open prostatectomy | 1 (3.1) |
| Robotic prostatectomy | 30 (93.8) |
| LND performed | 6 (18.8) |
| LND type performed | |
| Extended | 3 (50.0) |
| Standard | 3 (50.0) |
| Postoperative destination | |
| Ward | 30 (96.9) |
| HDU | 1 (3.1) |
| Wound drain left in situ | 14 (43.8) |
| HIFU treatment details | Participants, n (%) |
|---|---|
| Treatment received (N = 39)a | |
| Right | 15 (38.5) |
| Left | 21 (53.8) |
| Hemiablation | 25 (64.1) |
| ‘Dog-leg’ ablation | 2 (5.1) |
| Quadrant ablation | 4 (10.3) |
| Anterior | 1 (2.6) |
| Posterior | 3 (7.7) |
| Treatment different from that intended | 7 (17.9) |
| Reason for difference | |
| Gland size | 2 (28.6) |
| Technical issue | 1 (14.3) |
| Otherb | 3 (42.9) |
| Subsequent HIFU treatment required | 2 (5.1) |
| Reason for subsequent treatment | |
| Second HIFU to treated area | 1 (50.0) |
| First HIFU treatment to untreated area | 1 (50.0) |
| Average time from first treatment (months) | 17.5 |
The majority of participants who received RP (93.8%) underwent a robotic prostatectomy procedure (see Table 8). One participant required a hernia repair at surgery that was not clinically considered to be a procedural complication, although it was recorded as such on the CRF. In the HIFU-treated group (see Table 9), most participants (82.1%) received their planned treatment; however, two were not treated because of gland size, one because of a technical failure and three for other reasons. Two participants required re-treatment at an average of 17.5 months following their original HIFU treatment.
Treatment failure
Treatment failure for both HIFU and RP is described in Chapter 2, Safety. As of 10 October 2017, there were two treatment failures. One of the treatment failures occurred 7.5 months post randomisation in a participant randomised to RP and was attributable to the participant’s PSA level reaching ≥ 0.2 ng/ml following an initial reduction to < 0.1 ng/ml after surgery.
A second treatment failure occurred at 12 months post randomisation in a participant randomised to HIFU and was as a result of a rising PSA level.
Change in prostate-specific antigen levels
Figure 4 shows the mean and standard error of the mean for the change in PSA levels by randomised treatment group. See Report Supplementary Material 9 for change in PSA levels in participants randomised either to RP or HIFU.
FIGURE 4.
Change in PSA levels over time.
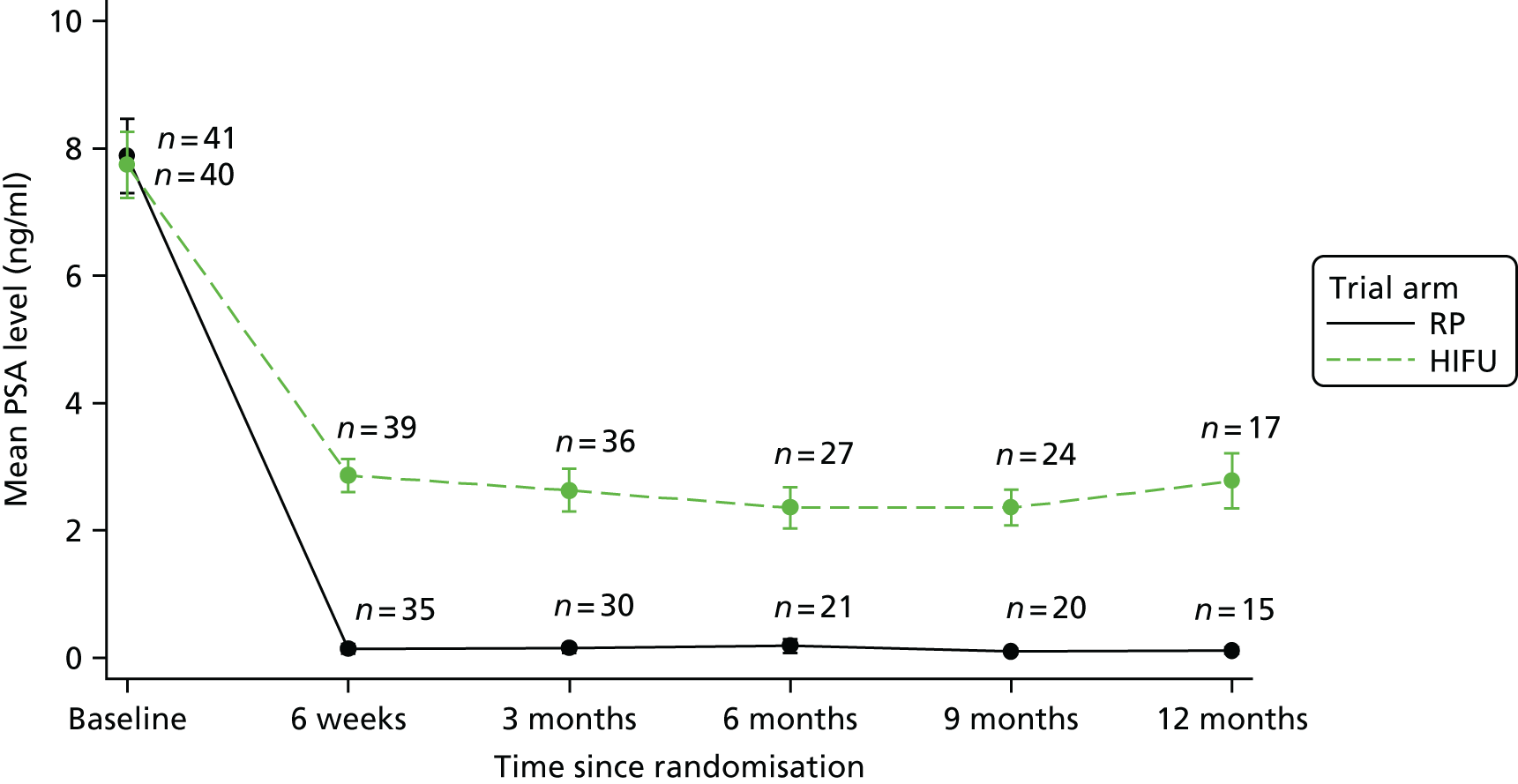
Radiology results
All reporters had been previously trained in mpMRI reporting and were familiar with the PI-RADS scoring system; there was no difficulty identified in image analysis and reporting.
Multiparametric MRI was performed in all centres without difficulty. Reporters experienced no problems in using the template and recording PI-RADS scores, or in measuring the degree of tumour abutting the capsule. Post-HIFU mpMRI could be performed, and it was possible to record the degree of non-enhancing tissue. The posttreatment scans identified enhancement at the edge of the HIFU treatments and mild diffuse enhancement on the untreated side.
Multiparametric MRI was performed in all participants before treatment, but was reportable only in 50 participants (in some participants, mpMRI was carried out post biopsy): 25 in the RP arm and 25 in the HIFU arm. Table 10 shows the baseline mpMRI characteristics and Table 11 shows the 2-week postprocedure mpMRI characteristics, which were available for seven participants in the HIFU arm (2-week mpMRI scan was mandated only in sites new to performing HIFU).
| mpMRI characteristics | Trial arm | |
|---|---|---|
| RP (N = 25) | HIFU (N = 25) | |
| Prostate volume (cc), median (range) | 36.0 (19.3–123.0) | 45.9 (16.7–74.0) |
| mpMRI clinical stage, n (%) | ||
| T1c | 3 (12.0) | 4 (16.0) |
| T2a | 14 (56.0) | 14 (56.0) |
| T2b | 1 (4.0) | 2 (8.0) |
| Missing | 7 (28.0) | 5 (20.0) |
| mpMRI characteristics | HIFU (N = 7) |
|---|---|
| Size of prostate: prostate volume (cc), median (range) | 49.6 (37.4–72.4) |
| Treated volume: prostate volume (cc), median (range) | 14.8 (11.8–37.8) |
| Likelihood of residual disease, n (%) | |
| Highly unlikely | 3 (42.9) |
| Unlikely | 3 (42.9) |
| Equivocal | 1 (14.3) |
Pathology results
Pathology reports were available for 25 out of the 32 participants treated with RP (Table 12). Eleven participants (44.0%) were found to have bilateral and/or high-risk disease based on pathology; in five of these participants (20.0%) the primary tumour (pT) was classified as stage 3a and in one (4.0%) it was classified as stage 3b. In eight participants (32.0%), the pT had positive margins. Bilateral or high-risk disease was an exclusion criterion, and it was expected that significant lesions would have been picked up by mpMRI before entry to the trial. Although this rate appears relatively high, determining the proportion of participants with bilateral or high-risk disease on the final RP histology was not an objective of the trial. A sample size of 25 RP histology results is too small to draw any meaningful conclusions about the likely proportion in a larger study.
| Pathology details | RP (N = 25), n (%) |
|---|---|
| Primary Gleason score | |
| 3 | 20 (80.0) |
| 4 | 5 (20.0) |
| Secondary Gleason score | |
| 3 | 1 (4.0) |
| 4 | 22 (88.0) |
| 5 | 2 (8.0) |
| Gleason score | |
| 7 (3 + 4) | 20 (80.0) |
| 7 (4 + 3) | 1 (4.0) |
| 9 (4 + 5) | 2 (8.0) |
| Missing | 2 (8.0) |
| Nodal status | |
| Nx: the lymph nodes cannot be checked | 22 (88.0) |
| N0: there are no cancer cells in lymph nodes close to the prostate | 3 (12.0) |
| Metastatic stage | |
| Mx: distant metastasis cannot be evaluated | 8 (32.0) |
| M0: no cancer has spread outside the pelvis | 11 (44.0) |
| Missing | 6 (24.0) |
| pT stage | |
| pT2: organ confined | 7 (28.0) |
| pT2a: unilateral up to half of one side | 3 (12.0) |
| pT2b: unilateral, involving more than half of one side but not both sides | 2 (8.0) |
| pT2c: bilateral disease | 7 (28.0) |
| pT3a: extraprostatic extension of microscopic invasion of bladder neck | 5 (20.0) |
| pT3b: seminal vesicle invasion | 1 (4.0) |
| Tumour location | |
| Unilateral clinically localised intermediate-risk disease | 10 (40.0) |
| Dominant unilateral clinically significant intermediate-risk and small contralateral low-risk disease | 3 (12.0) |
| Bilateral and/or high-risk disease | 11 (44.0) |
| Uncertain | 1 (4.0) |
| Margin status | |
| pT2: positive | 4 (16.0) |
| pT2: negative | 15 (60.0) |
| pT3: positive | 4 (16.0) |
| pT3: negative | 2 (8.0) |
Complication rates and serious adverse events
Table 13 summarises the 10 SAEs reported in the trial: six in participants who received RP and four in participants who received HIFU treatment. Six SAEs were considered to be related to treatment and four were considered unrelated, or unlikely to be related, to treatment; all were fully resolved. The two suspected unexpected serious adverse reactions were reported to the sponsor and the REC. Table 14 lists the complication rates, of which there were 54 at the time of data extraction: four procedural complications and 50 postprocedural complications (15 in the RP group and 35 in the HIFU group). Most complications occurred at 6 weeks post procedure, with self-catheterisation the most common, followed by urinary tract infections and urine leaks that did not require further intervention. Two strictures were reported in the RP group at 18 months post surgery.
| Treatment received | Related to treatment | Expected | SAE |
|---|---|---|---|
| HIFU | Definitely | No | Right-sided epididymo-orchitis and inflamed bladder |
| HIFU | Probably | Yes | Suspected urosepsis |
| HIFU | Probably | Yes | Retention followed by bleeding via penis post self-catheterisation |
| HIFU | Unlikely | N/A | Non-ST-elevation myocardial infarction |
| RP | Definitely | Yes | Urosepsis |
| RP | Definitely | Yes | Pain when passing urine; temperature |
| RP | Probably | No | Constipation |
| RP | Unlikely | N/A | Non-ST-elevation myocardial infarction |
| RP | Not related | N/A | Irreducible right inguinal hernia |
| RP | Not related | N/A | Drop in O2 saturation and unresponsive |
| Total complications | Trial arm (n) | Total (n) | |
|---|---|---|---|
| RP | HIFU | ||
| Total procedural complication rate | 3 | 1 | 4 |
| Other complicationsa | 3 | 1 | 4 |
| Total postprocedural complication rate | 15 | 35 | 50 |
| Immediate postprocedural complications | 2 | 0 | 2 |
| Respiratory problems | 1 | 0 | 1 |
| Other complicationsb | 1 | 0 | 1 |
| Total complications at 6 weeks | 7 | 14 | 21 |
| Urinary tract infection | 3 | 4 | 7 |
| Persistent dysuria (with a negative urine culture) | 0 | 1 | 1 |
| Urine leak not requiring further intervention | 1 | 1 | 2 |
| Myocardial infarction | 0 | 1 | 1 |
| Other complicationsc | 3 | 7 | 10 |
| Total complications at 3 months | 1 | 3 | 4 |
| Urinary tract infection | 0 | 1 | 1 |
| Urine leak requiring further intervention | 0 | 1 | 1 |
| Other complicationsd | 1 | 1 | 2 |
| Total complications at 6 months | 1 | 6 | 7 |
| Urinary tract infection | 0 | 1 | 1 |
| Urine leak requiring further intervention | 0 | 1 | 1 |
| Other complicationse | 1 | 4 | 5 |
| Total complications at 9 months | 1 | 2 | 3 |
| Other complicationsf | 1 | 2 | 3 |
| Total complications at 12 months | 1 | 0 | 1 |
| Urine leak requiring further intervention | 1 | 0 | 1 |
| Total complications at 18 months | 2 | 0 | 2 |
| Stricture | 2 | 0 | 2 |
Health-related quality of life
Health-related quality-of-life outcomes were split into four domains:
-
urinary function and its effect on HRQoL
-
sexual function and its effect on HRQoL
-
bowel function and its effect on HRQoL
-
overall HRQoL.
Study questionnaires were completed at baseline, at 6 weeks post procedure and at 3, 6, 9, 12, 18, 24, 30 and 36 months post procedure; Table 15 shows the interpretation of questionnaires. Return rates and completeness of questionnaires are described in Tables 16 and 17.
| Questionnaire | Interpretation of score | |
|---|---|---|
| EPIC (51-item scale) | Higher score → better HRQoL | |
| FACT-P (39-item scale) | Higher score → better HRQoL | |
| IPSS (8-item scale) | Higher score → worse HRQoL | |
| IIEF-15 (15-item scale) | Higher score → better HRQoL | |
| MAX-PC (18-item scale) | Higher score → worse HRQoL | |
| Questionnaire | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline (n = 82 expected) | 6 weeks (n = 72 expected) | 3 months (n = 70 expected) | 6 months (n = 56 expected) | 9 months (n = 44 expected) | 12 months (n = 36 expected) | |
| EPIC (51-item scale) | 78 (95.1) | 72 (100.0) | 64 (70.0) | 54 (96.4) | 42 (95.5) | 31 (86.1) |
| FACT-P (39-item scale) | 78 (95.1) | 72 (100.0) | 64 (70.0) | 54 (96.4) | 42 (95.5) | 31 (86.1) |
| IPSS (8-item scale) | 78 (95.1) | 72 (100.0) | 64 (70.0) | 54 (96.4) | 42 (95.5) | 31 (86.1) |
| IIEF-15 (15-item scale) | 78 (95.1) | 72 (100.0) | 64 (70.0) | 54 (96.4) | 42 (95.5) | 31 (86.1) |
| MAX-PC (18-item scale) | 78 (95.1) | 72 (100.0) | 64 (70.0) | 54 (96.4) | 42 (95.5) | 31 (86.1) |
| Questionnaire | Trial point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 weeks | 3 months | 6 months | 9 months | 12 months | |||||||
| n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | |
| EPIC (51-item scale) | 20 (25.6) | 0 (0–51) | 26 (36.1) | 0 (0–51) | 20 (31.3) | 0 (0–51) | 16 (29.6) | 0 (0–51) | 9 (21.4) | 0 (0–51) | 7 (22.6) | 0 (0–51) |
| FACT-P (39-item scale) | 21 (26.9) | 0 (0–39) | 24 (33.3) | 0 (0–39) | 18 (28.1) | 0 (0–39) | 11 (20.4) | 0 (0–39) | 6 (14.3) | 0 (0–39) | 6 (19.4) | 0 (0–39) |
| IPSS (8-item scale) | 13 (16.7) | 0 (0–8) | 16 (22.2) | 0 (0–8) | 16 (25.0) | 0 (0–8) | 5 (9.3) | 0 (0–8) | 4 (9.5) | 0 (0–8) | 3 (9.7) | 0 (0–8) |
| IIEF-15 (15-item scale) | 17 (21.8) | 0 (0–15) | 23 (31.9) | 0 (0–15) | 17 (26.6) | 0 (0–15) | 7 (13.0) | 0 (0–15) | 5 (11.9) | 0 (0–15) | 6 (19.4) | 0 (0–15) |
| MAX-PC (18-item scale) | 17 (21.8) | 0 (0–18) | 23 (31.9) | 0 (0–18) | 17 (26.6) | 0 (0–18) | 7 (13.0) | 0 (0–18) | 5 (11.9) | 0 (0–18) | 6 (19.4) | 0 (0–18) |
Urinary function and effect on health-related quality of life
Men randomised to RP experienced negative effects on their HRQoL in terms of urinary function. Men in the HIFU group reported a decrease in urinary-related HRQoL on most domains at 6 weeks, but in most men, HRQoL scores had returned to their baseline level or were even higher by 3 months post procedure. Overall, urinary QoL was lower in men randomised to RP than in those randomised to HIFU (Figure 5). Urinary function and urinary incontinence were also worse in these men (Figures 6 and 7). There was an increase, from baseline, in the need to use an absorbent pad at least once a day for men randomised to RP, with 84.6% of men in the RP group using pads by 6 weeks post procedure (0% of men reported use at baseline) compared with 17.6% of men in the HIFU group (209% of men reported use at baseline). By 6 months, no men in the HIFU group were reporting the need to use pads (Figure 8). There were no observed differences between the two groups in urinary irritation or urinary bother (Figures 9 and 10).
FIGURE 5.
Mean EPIC urinary QoL score.
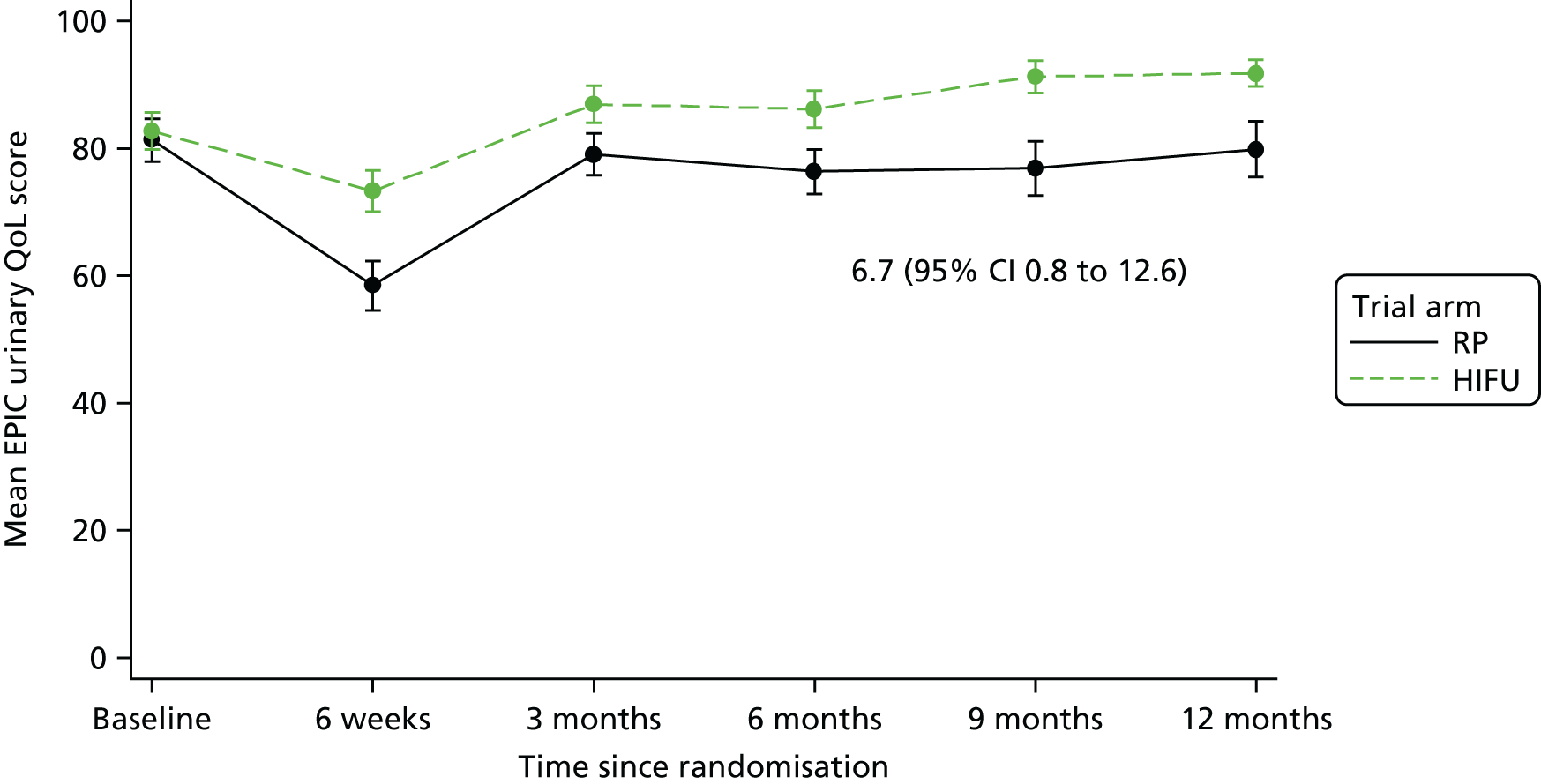
FIGURE 6.
Mean EPIC urinary function score.
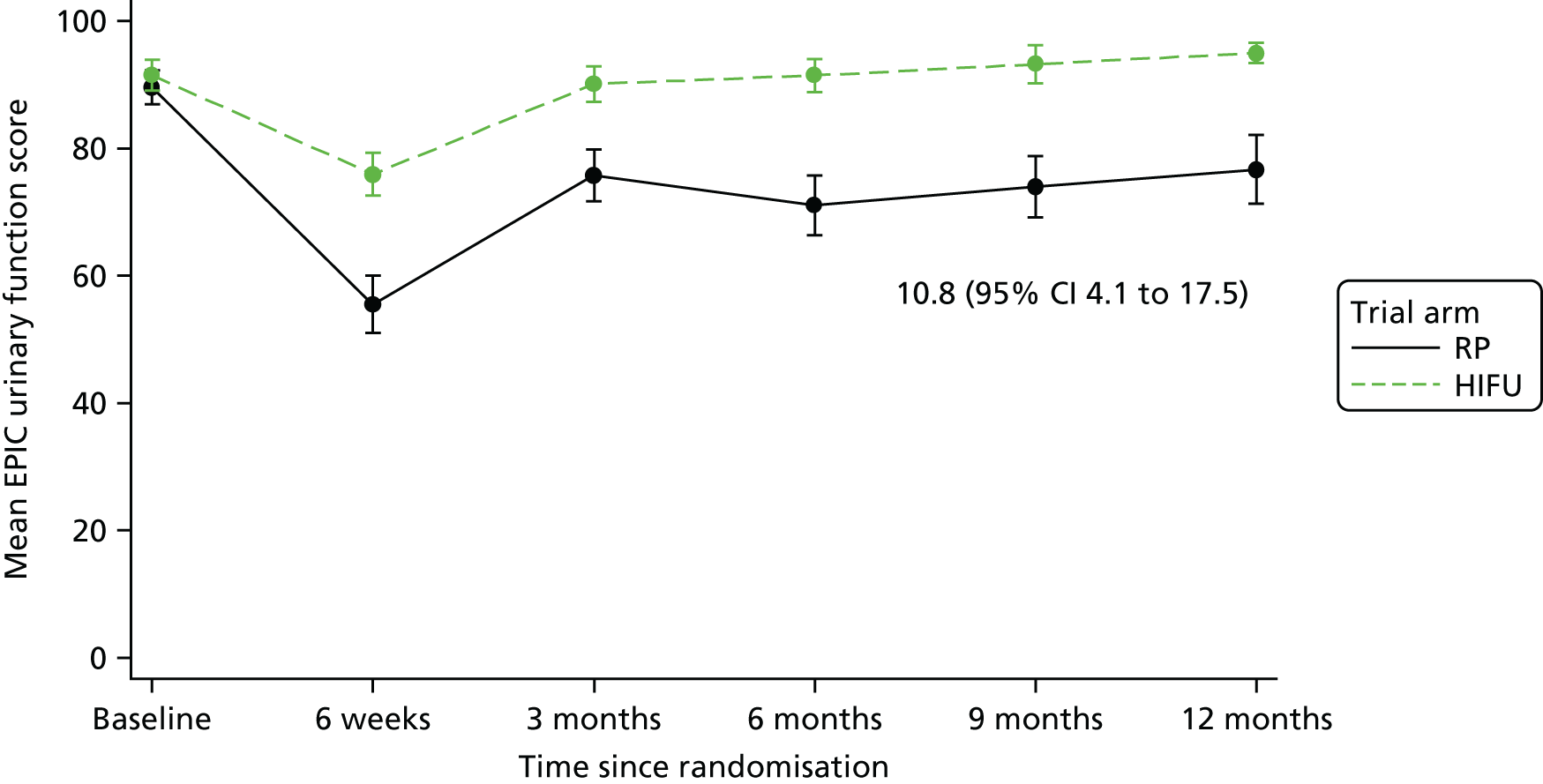
FIGURE 7.
Mean EPIC urinary incontinence score.

FIGURE 8.
Men using EPIC item: one or more pads per day.

FIGURE 9.
Mean EPIC urinary irritation/obstruction score.
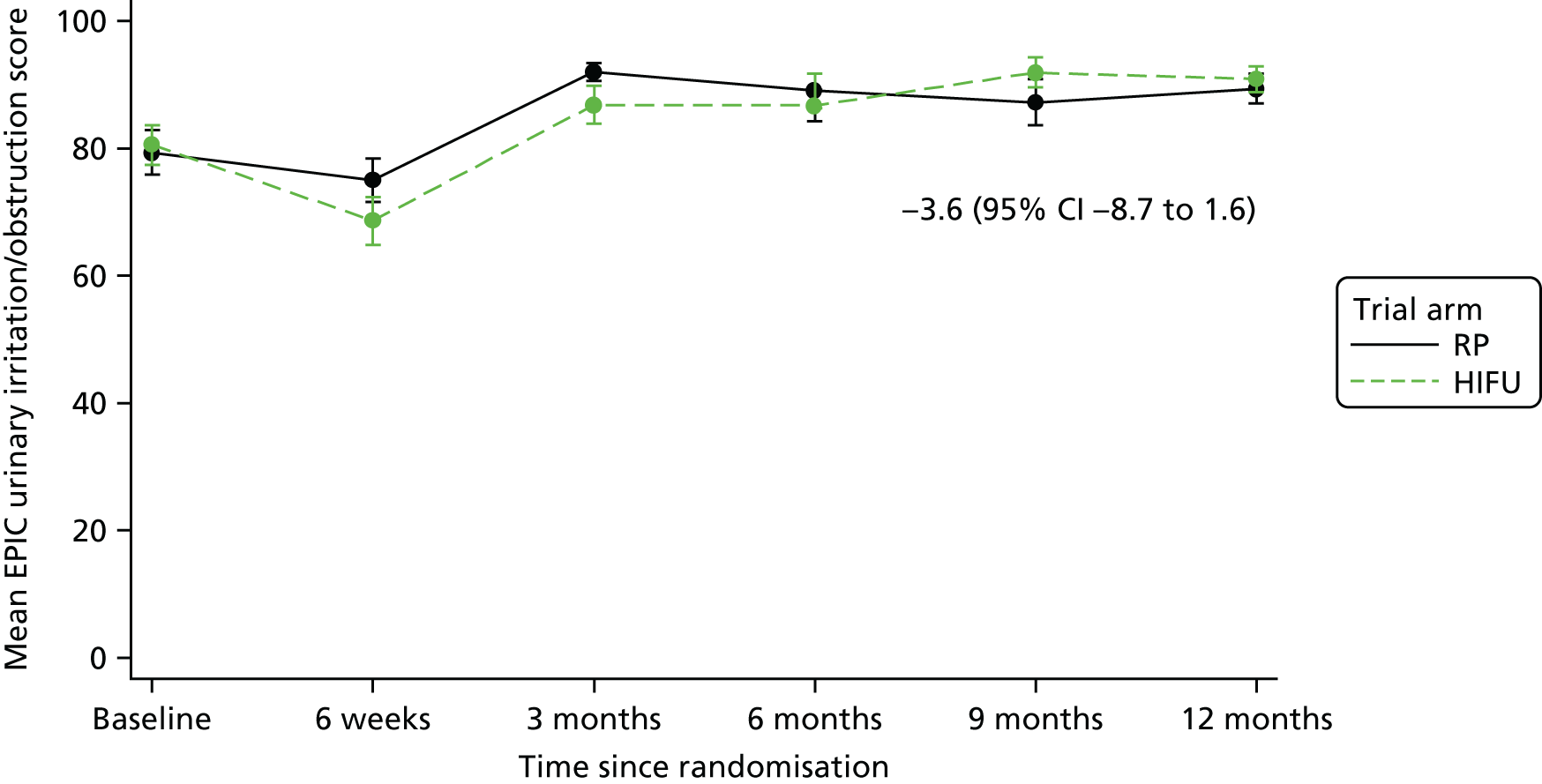
FIGURE 10.
Mean EPIC urinary bother score.

Sexual function
Sexual function and related effect on HRQoL was reduced in both groups from baseline to 6 weeks post procedure, but increased after this time point (Figure 11). The proportion of men reporting problems with erectile dysfunction at baseline was higher in the RP group than in the HIFU group (37% vs. 18%) (Figure 12). Men in the HIFU group appeared to report better sexual function and orgasmic function than men in the RP group (Figures 13–15). There were no observed statistical differences in sexual desire or the number of men reporting erections firm enough for intercourse (Figures 16 and 17).
FIGURE 11.
Mean EPIC sexual QoL score.

FIGURE 12.
Men reporting EPIC problem with erectile dysfunction.

FIGURE 13.
Mean EPIC sexual function score.
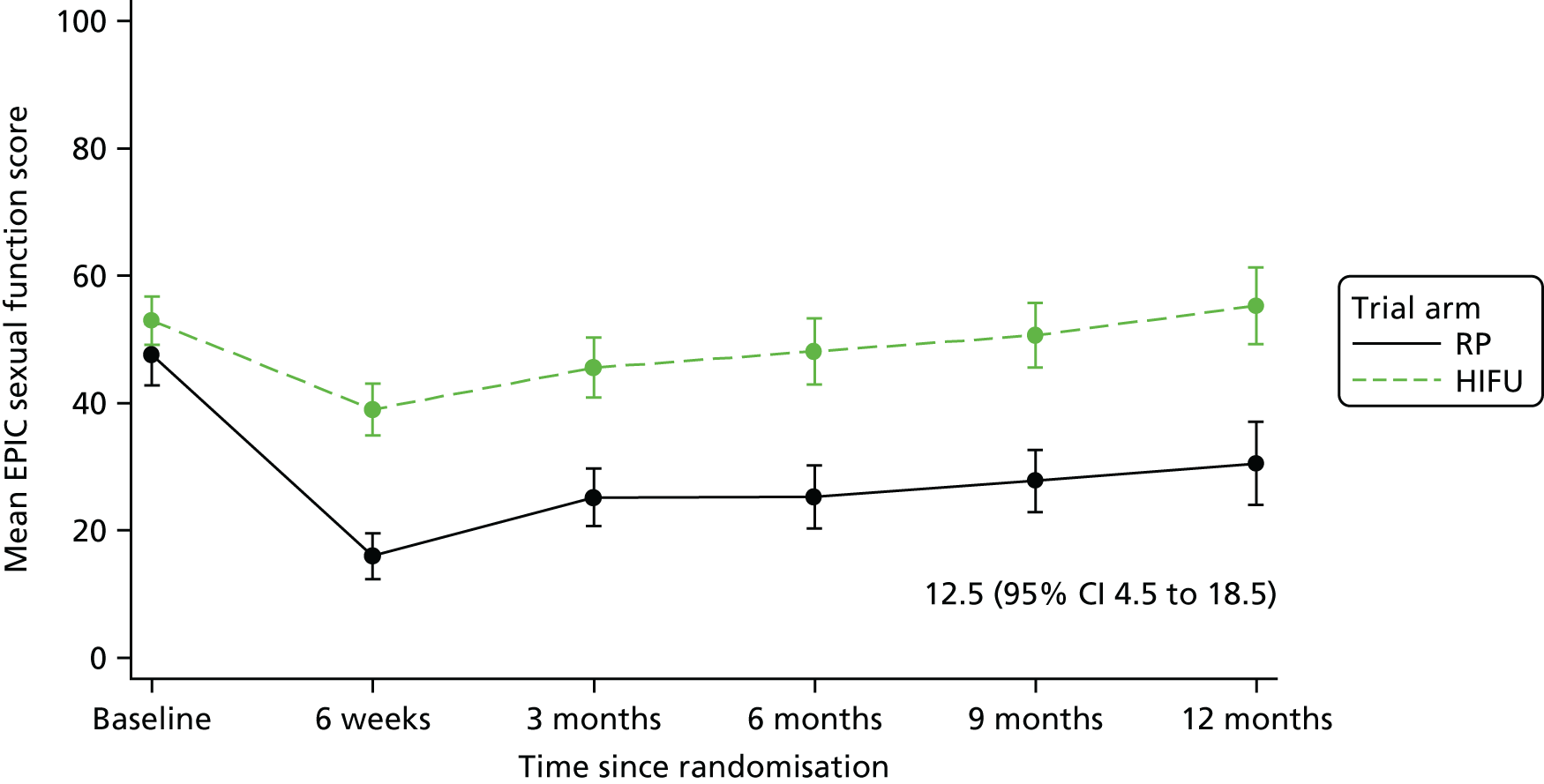
FIGURE 14.
Mean EPIC sexual bother score.

FIGURE 15.
Mean IIEF-15 intercourse satisfaction score.
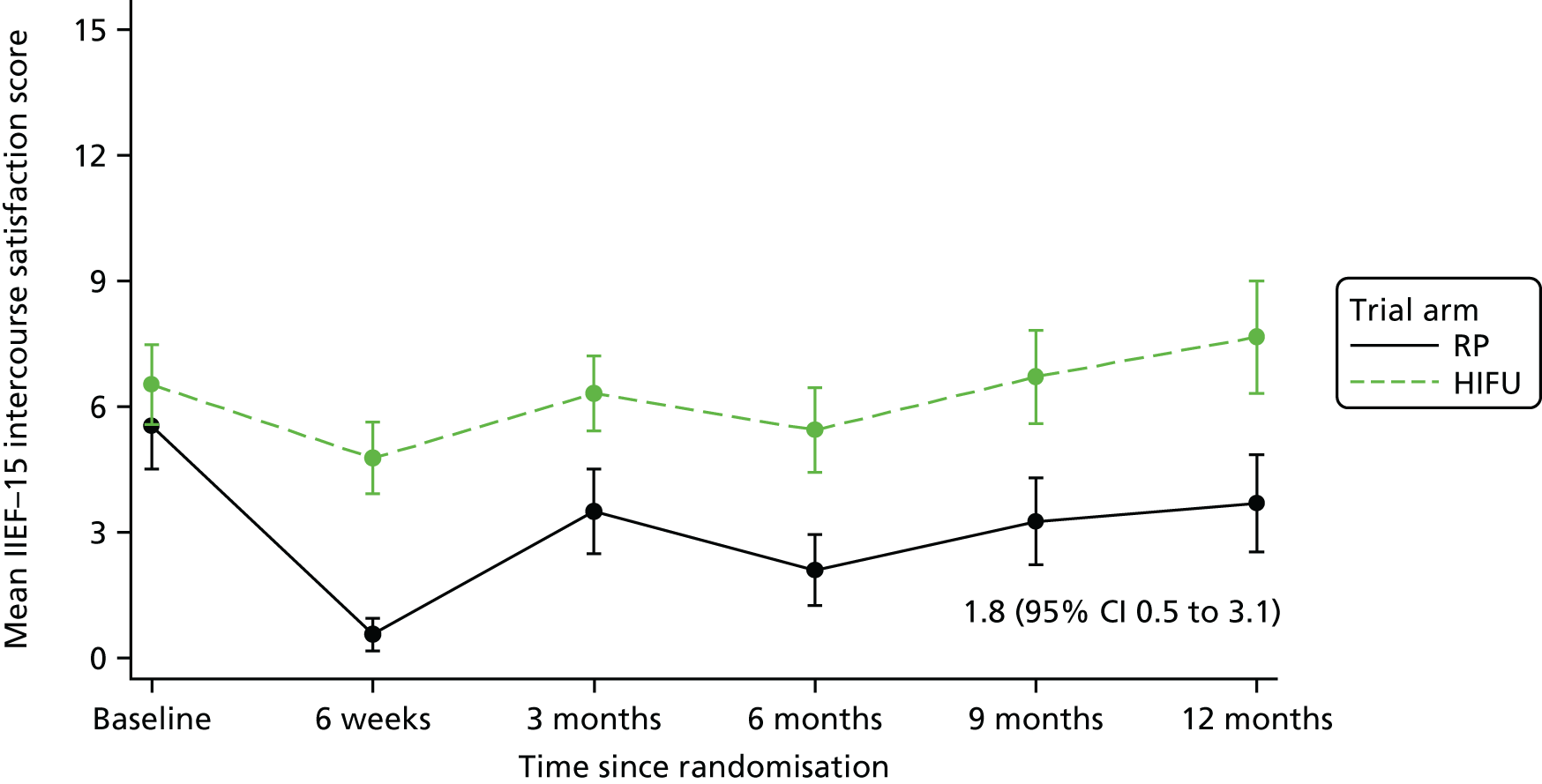
FIGURE 16.
Mean EPIC erection firmness score.
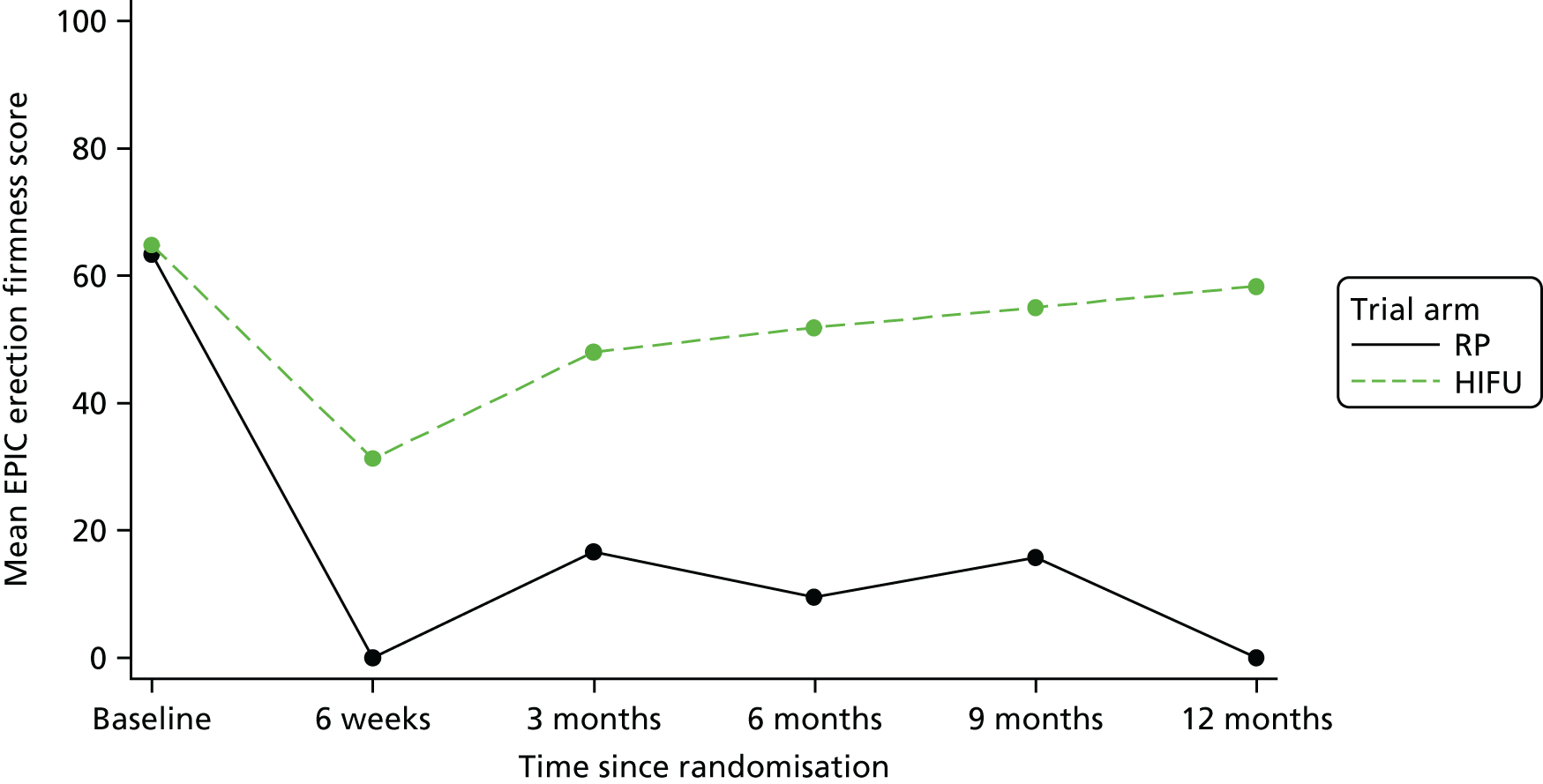
FIGURE 17.
Mean IIEF-15 sexual desire score.

Bowel function and effect on health-related quality of life
Bowel function and the effect on HRQoL did not appear to differ between the RP and HIFU groups for any of the domains, including function, overall QoL and bother (Figures 18–20). The percentage of men reporting faecal incontinence for half of the time or more (within the last 4 weeks at the questionnaire) was higher at all time points in the RP group (Figure 21).
FIGURE 18.
Mean EPIC bowel function score.

FIGURE 19.
Mean EPIC bowel QoL score.
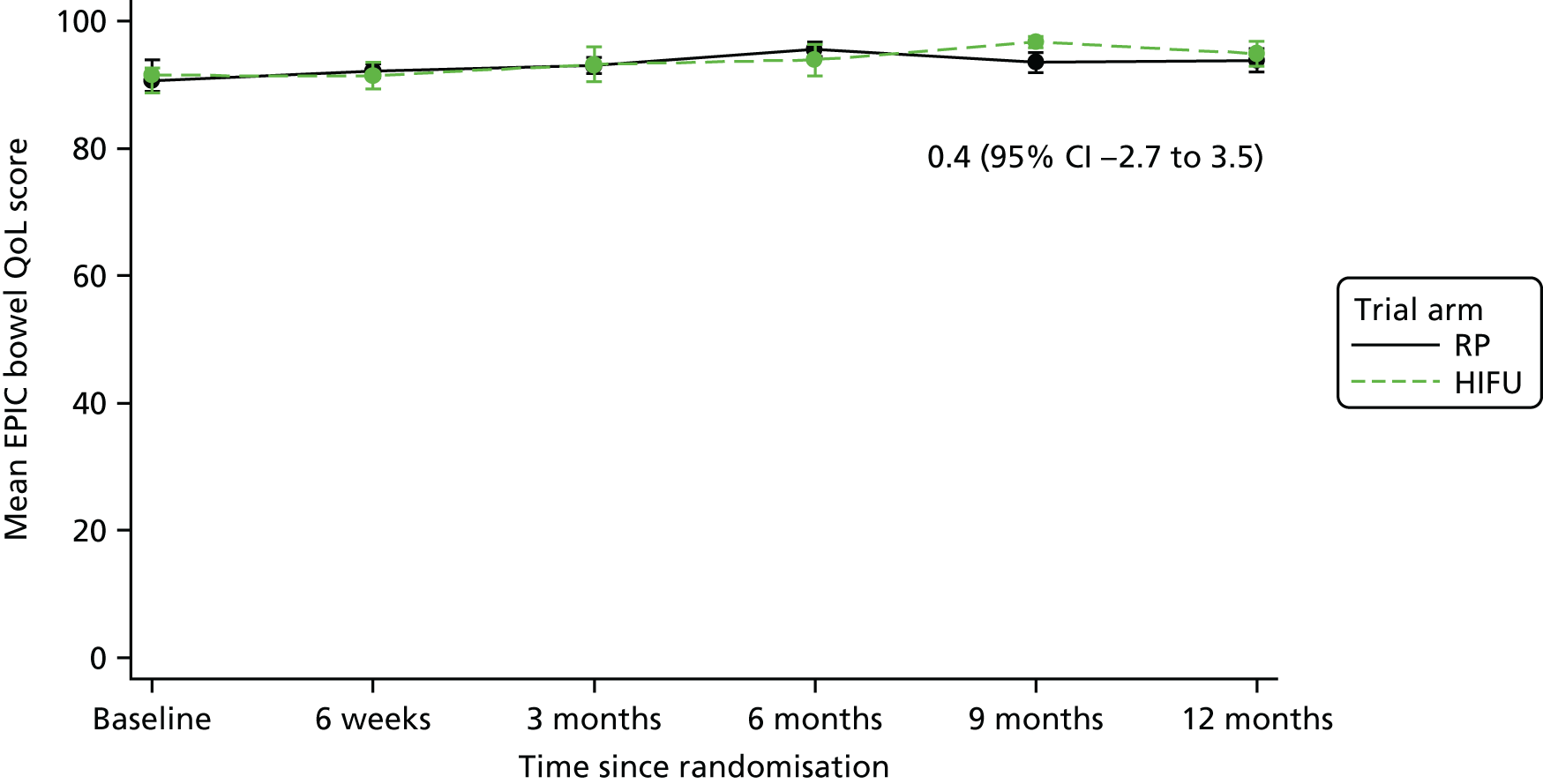
FIGURE 20.
Mean EPIC bowel bother score.

FIGURE 21.
Men reporting EPIC loose stools.

Overall health-related quality of life
There do not appear to be any significant differences in overall HRQoL for any of the domains, despite differences observed in urinary- and sexual function-related HRQoL (Figures 22–31).
FIGURE 22.
Mean FACT-P social/family well-being score.
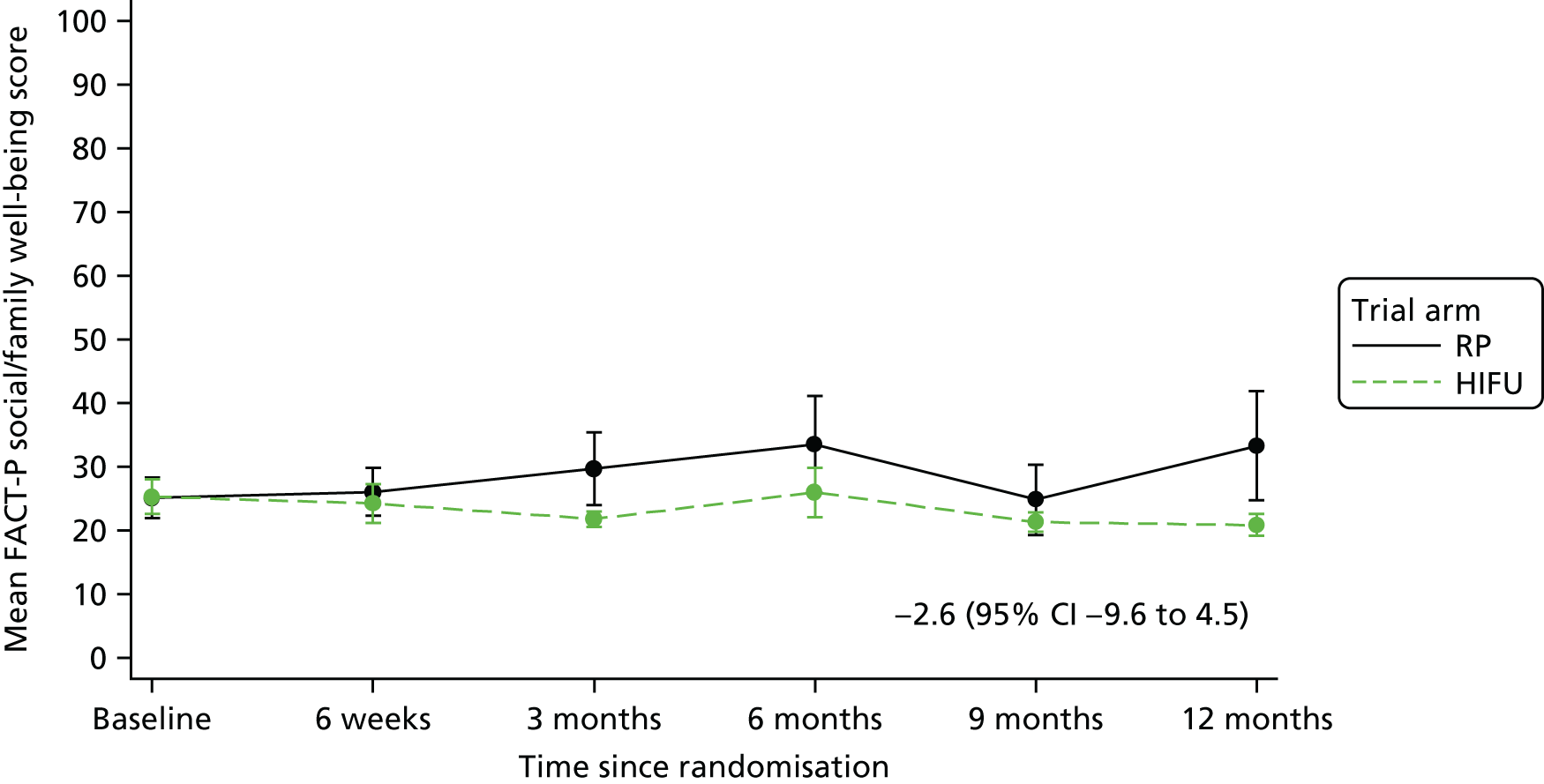
FIGURE 23.
Mean FACT-P physical well-being score.
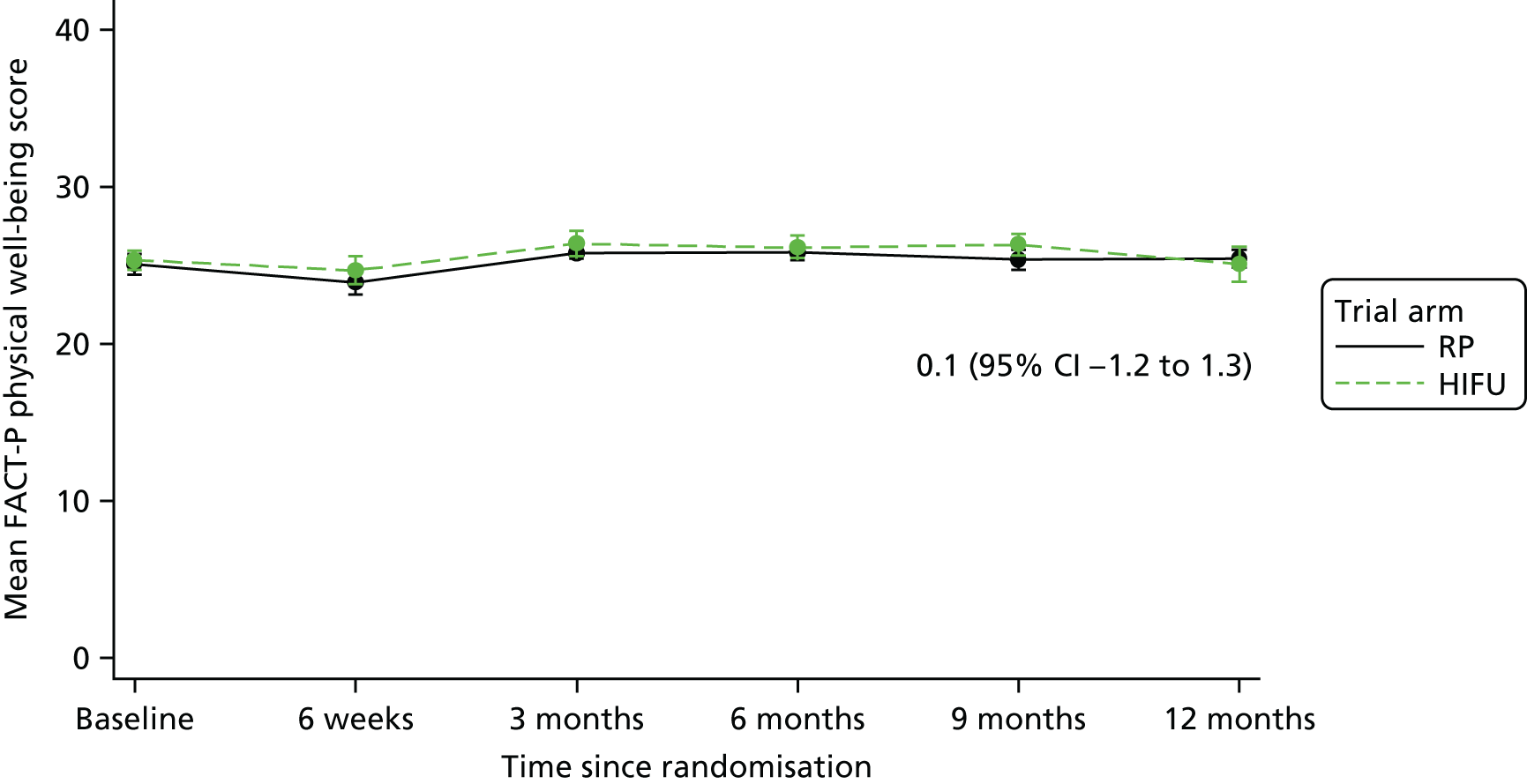
FIGURE 24.
Mean FACT-P emotional well-being score.

FIGURE 25.
Mean FACT-P functional well-being score.

FIGURE 26.
Mean IIEF-15 overall satisfaction score.
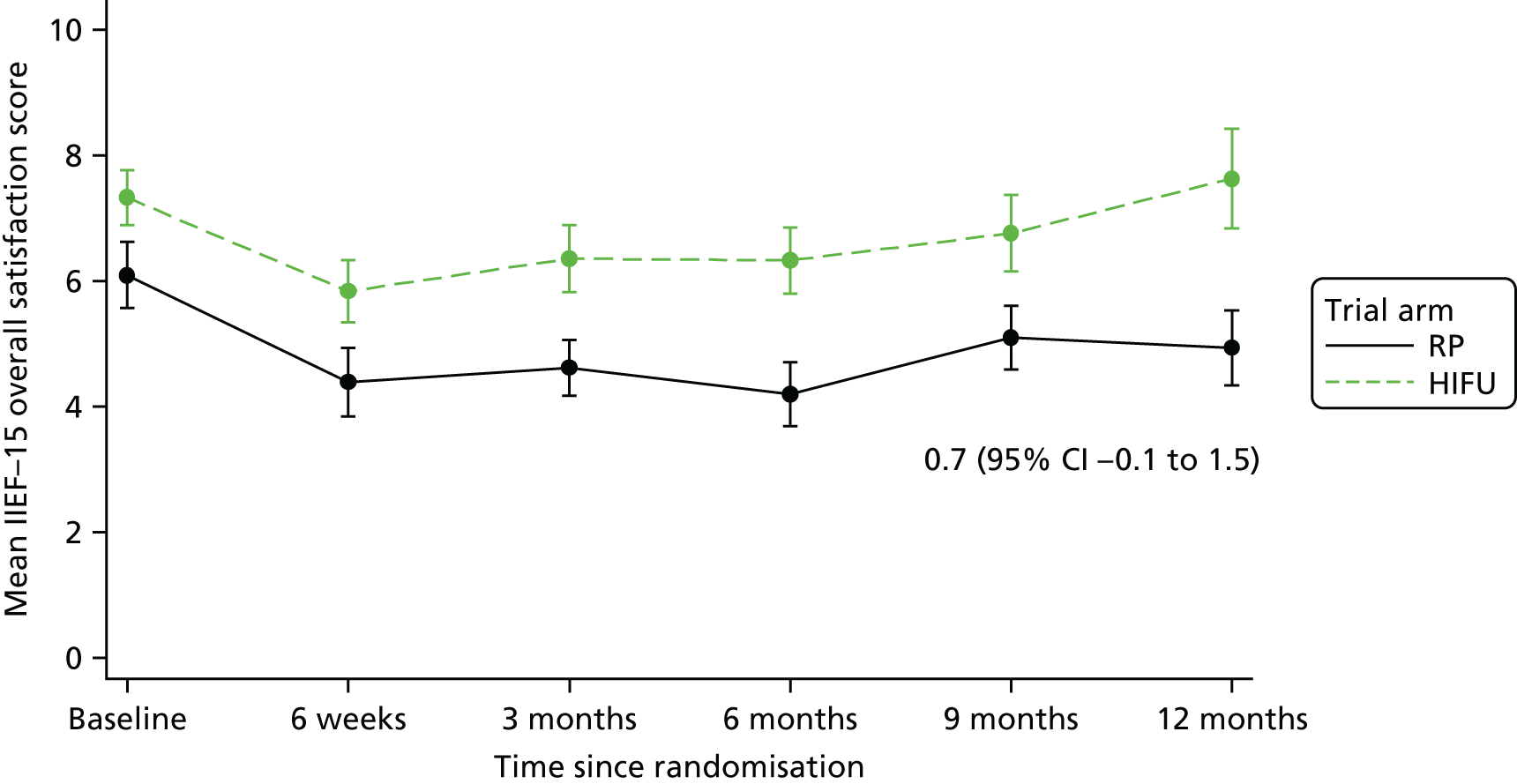
FIGURE 27.
Mean MAX-PC summary score.

FIGURE 28.
Mean MAX-PC PCa anxiety score.

FIGURE 29.
Mean MAX-PC PSA anxiety score.

FIGURE 30.
Mean MAX-PC fear of recurrence score.

FIGURE 31.
Mean IPSS total score.

Results of health economics data collection
This section reports on the results from the exploration of the feasibility and completeness of data collection for the purpose of designing the economic evaluation for a full RCT. Although not undertaken at this stage, a full costing and cost-effectiveness analysis would be performed in a main RCT and unit costs would be updated accordingly.
The total number of participants meeting the inclusion criteria for the completeness assessment and exploratory health economics data collection was 44 (as of 31 March 2017). The assessment of missing EQ-5D-5L data (i.e. data that were missing, not reported/collected or not updated in the database by the follow-up milestone) is presented in Table 18. Levels of EQ-5D-5L completeness were assessed using age and trial centre as potential confounders. No significant patient-level or centre-level factors contributed to missingness; however, there was an increased likelihood of missing follow-up data (at 6 weeks, 3 months and 6 months post procedure) if baseline data were missing. On average, 25% of the data for the EQ-5D-5L were missing.
| Data collection period | Expected (PPA) (n) | Actuala (n) | Completeness | ||
|---|---|---|---|---|---|
| Incompleteb (n) | Not completedc (n) | Missing/incomplete (%) | |||
| Baseline | 44 | 38 | 0 | 6 | 14 |
| 6-week follow-up | 41 | 36 | 0 | 5 | 12 |
| 3-month follow-up | 41 | 28 | 1 | 12 | 32 |
| 6-month follow-up | 33 | 23 | 2 | 8 | 30 |
| 9-month follow-up | 25 | 16 | 0 | 9 | 36 |
| 12-month follow-up | 16 | 10 | 0 | 6 | 38 |
| 18-month follow-up | 8 | 6 | 0 | 2 | 25 |
| Total responses | 208 | 157 | 3 | 48 | 25 |
Analysis of the individual domains at each follow-up period for both trial arms was undertaken (see Report Supplementary Material 10). There were no missing data at the domain level for those completing the EQ-5D-5L instrument. Baseline variation in reported domain-level QoL was higher in the HIFU (intervention) trial arm with responses ranging from 1 to 3, whereas responses in the RP (control) arm ranged from 1 to 2; no responses at baseline scored 4 or 5. The anxiety and depression domain at baseline across both arms indicated the largest impact on HRQoL, with 10 out of 17 participants in the RP arm and 4 out of 21 participants in the HIFU arm being ‘slightly anxious or depressed’ and 5 out of 21 participants in the HIFU arm being ‘moderately anxious or depressed’. At the 6-week follow-up, variation in reported anxiety and depression persisted; however, the proportion of participants who highlighted more issues in mobility and pain discomfort was also higher in the RP arm than in the HIFU arm. At the 3-month follow-up, variation across domains was similar for both arms; however, levels of ‘no problems’ across domains were slightly higher in the HIFU arm than in the RP arm. From the 6-month follow-up, trends were similar for both arms.
In the assessment of the EQ-5D-5L utility and EQ-VAS scores, presented in Table 19, the baseline differences observed were minimal. Although a higher overall HRQoL was reported for the HIFU arm when using the estimation of utilities from the EQ-5D-5L, a higher overall mean score was reported for the RP arm when using the EQ-VAS. Sample sizes were too small to robustly assess significance; however, HIFU appears to have a larger positive effect on HRQoL than does RP. The utility decrement post procedure is more than double in the RP arm at both 6 weeks’ and 3 months’ follow-up; mean utility using the EQ-5D-5L and EQ-VAS improves from baseline averages for the HIFU arm only.
| Utility measure | EQ-5D-5L | EQ-VAS | ||||
|---|---|---|---|---|---|---|
| Trial arm | Δ (HIFU – RP) | Trial arm | Δ (HIFU – RP) | |||
| RP | HIFU | RP | HIFU | |||
| Baseline (n = 38: RP = 17, HIFU = 21) | ||||||
| Mean score | 0.916 | 0.933 | 0.017 | 83.53 | 81.38 | –2.15 |
| 6 weeks (n = 36: RP = 14, HIFU = 2) | ||||||
| Mean score | 0.848 | 0.902 | 0.054 | 78.21 | 80.68 | 2.47 |
| % change from baseline | –7.4 | –3.3 | –6.4 | –0.9 | ||
| 3 months (n = 28: RP = 14, HIFU = 14) | ||||||
| Mean score | 0.907 | 0.945 | 0.038 | 82.93 | 87.64 | 4.71 |
| % change from baseline | –1.0 | 1.3 | –0.7 | 7.7 | ||
| 6 months (n = 23: RP = 13, HIFU = 10) | ||||||
| Mean score | 0.937 | 0.939 | 0.002 | 84.62 | 82.70 | –1.92 |
| % change from baseline | 2.3 | 0.6 | 1.3 | 1.6 | ||
| 9 months (n = 16: RP = 8, HIFU = 8) | ||||||
| Mean score | 0.897 | 0.939 | 0.042 | 77.13 | 83.50 | 6.37 |
| % change from baseline | –2.1 | 0.6 | –7.7 | 2.61 | ||
| 12 months (n = 10: RP = 5, HIFU = 5) | ||||||
| Mean score | 0.93 | 0.872 | –0.058 | 87.00 | 79.80 | –7.20 |
| % change from baseline | 1.5 | –6.5 | 4.2 | –1.9 | ||
| QALYs at 12 months’ follow-upa (n = 8) | 0.895 | 0.919 | 0.024 | |||
In the complete-case analysis for 12 months’ follow-up, the QALY scores indicate a higher benefit from HIFU, relative to RP, equivalent to a health gain of 8.8 days. Figure 32 highlights mean utility across the 12 months’ follow-up for the complete cases (n = 8). Although a small sample size, it appears that a gradual and steady improvement in HRQoL is attributable to HIFU, up to and including 9 months’ follow-up.
FIGURE 32.
Complete-case analysis of EQ-5D-5L utility scores for 12 months’ follow-up.
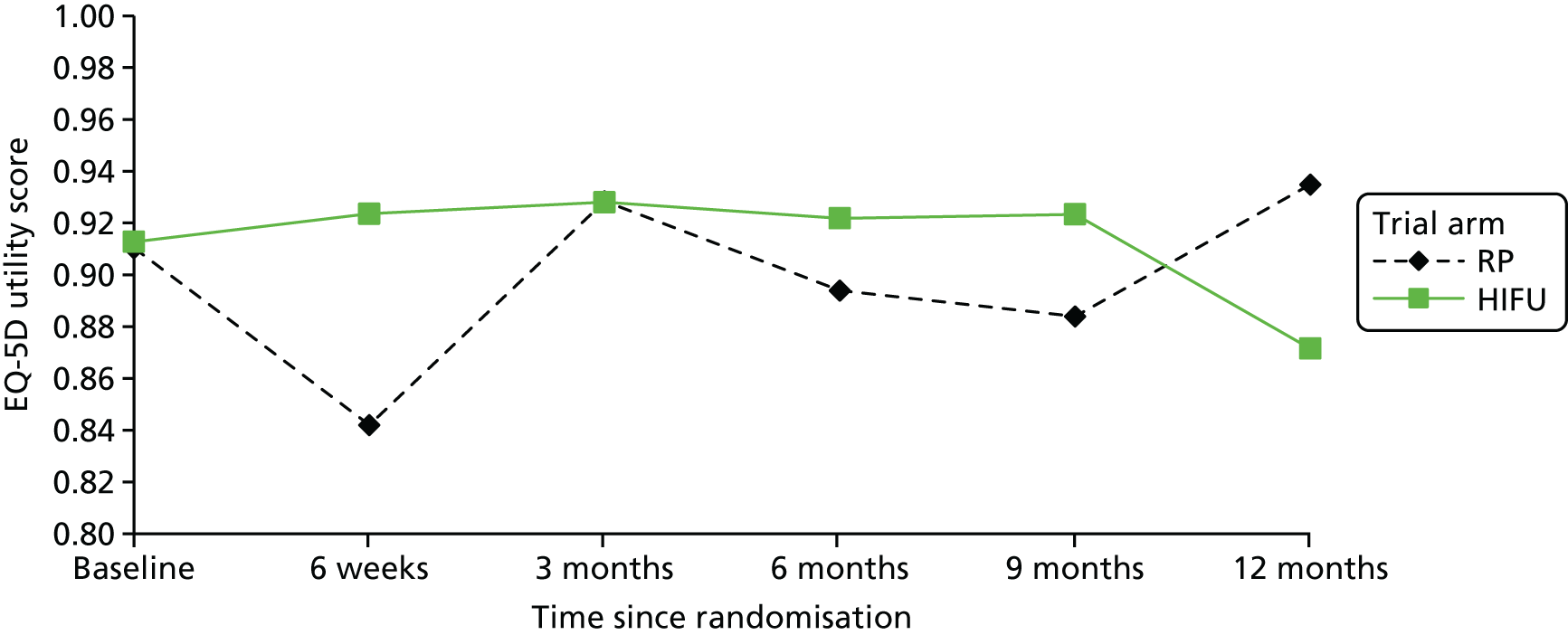
As shown in Table 20, 30% of patient resource use diaries were not returned. Completeness levels across the four sections in the resource use diary varied considerably, with the highest levels of incomplete responses among returned diaries occurring in section 3 (record of days unable to carry out usual activities), at 59% (67/114). Sections 1 (reporting health-care resource use) and 2 (medications taken/devices used in follow-up period) were well completed. Completeness of reported travel costs associated with resource use (section 4) was good, with only 23% (26/114) incomplete. The overall variation in completeness of the diaries suggests that participants were not comfortable with completing all sections. Trends in non-completion that have been identified, for example days off usual activities, require further refinement in the questionnaire and data collection process to improve completion rates.
| Follow-up time point | Patient resource use diary | Section | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1: contacts with health-care professionals reported, n (%) | 2: medication and medical devices reported, n (%) | 3: days unable to undertake usual activities, n (%) | 4: travel time and costs, n (%) | |||||||
| Expected (PPA) (n) | Not returned, n (%) | Returned and relevant section completed | Incompletea | Returned and relevant section completed | Incompletea | Returned and relevant section completed | Incompletea | Returned and relevant section completed | Incompletea | |
| 6 weeks | 41 | 10 (24) | 31 (76) | 0 (0) | 13 (32) | 18 (44) | 29 (71) | 2 (5) | 25 (61) | 6 (15) |
| 3 months | 41 | 15 (37) | 25 (61) | 1 (2) | 13 (32) | 13 (32) | 9 (22) | 17 (41) | 15 (37) | 11 (27) |
| 6 months | 33 | 13 (39) | 20 (61) | 0 (0) | 3 (9) | 17 (52) | 4 (12) | 16 (49) | 15 (45) | 5 (15) |
| 9 months | 25 | 7 (28) | 18 (72) | 0 (0) | 7 (28) | 11 (44) | 2 (8) | 16 (64) | 14 (56) | 4 (16) |
| 12 months | 16 | 3 (19) | 12 (75) | 1 (6) | 8 (50) | 5 (31) | 0 (0) | 13 (81) | 13 (81) | 0 (0) |
| 18 months | 8 | 2 (25) | 6 (75) | 0 (0) | 0 (0) | 6 (75) | 3 (38) | 3 (38) | 6 (75) | 0 (0) |
| Total | 164 | 50 (30) | 112 (68) | 2 (1) | 44 (27) | 70 (43) | 47 (29) | 67 (41) | 88 (54) | 26 (16) |
In Table 21, completeness by section is presented for returned resource use diaries (see Chapter 4 for more details about how we plan to reduce the proportion of incomplete responses).
| Resource use diary section | Completeness | |
|---|---|---|
| Complete (n) | Incomplete, n (%) | |
| 1: contacts with health-care professionals | 112 | 2 (2) |
| 2: medication and medical devices reported | 70 | 44 (39) |
| 3: days unable to undertake usual activities | 47 | 67 (59) |
| 4: travel time and costs | 88 | 26 (23) |
The analysis of self-reported resource use is presented in Table 22. Rates of reported contacts with health-care professionals in primary and secondary care, of all types, were higher in the RP arm. Reported A&E admissions were similar across both arms; however, more inpatient events were reported in the RP arm. Little variation in medication and device usage across the two trial arms was evidenced, apart from higher reported use of medication for sexual dysfunction in the RP arm. This is consistent with the statistical analysis, which showed that men in the HIFU group reported better sexual function and orgasmic function outcomes than men in the RP group (see Sexual function and effect on health-related quality of life). The number of participant-reported days unable to carry out usual activities is higher in the RP arm: the mean number of days lost overall in the RP arm (45 days) was nearly double that of the HIFU arm (25 days). Similarly, travel costs associated with contact visits in primary and secondary care were higher overall in the RP arm (£2546) than in the HIFU arm (£2249). As the sample sizes are small and the proportion of missing data is high in sections on productivity losses and travel costs, caution is needed in interpreting these results.
| Resource use type | Trial arm | Δ (HIFU – RP) | |
|---|---|---|---|
| RP | HIFU | ||
| Total events recorded (n) | |||
| GP visit (N = 24) | 52 | 33 | –19 |
| Practice nurse visit (N = 23) | 37 | 31 | –6 |
| District nurse (N = 8) | 14 | 2 | –12 |
| Macmillan nurse (N = 0) | 0 | 0 | 0 |
| Physiotherapist (N = 9) | 13 | 4 | –9 |
| Occupational therapist (N = 2) | 2 | 0 | –2 |
| Counsellor (N = 0) | 0 | 0 | 0 |
| Dietitian (N = 0) | 0 | 0 | 0 |
| Hospital doctor (N = 29) | 97 | 33 | –64 |
| Hospital nurse (N = 28) | 31 | 29 | –2 |
| Other contact (N = 19) | 23 | 11 | –12 |
| A&E admissions (N = 6) | 3 | 3 | 0 |
| Hospital inpatient events (N = 14) | 12 | 5 | –7 |
| Number of participants reporting medication/device use | |||
| Reported using medication or devices for | RP (N = 15) | HIFU (N = 12) | Δ (HIFU – RP) |
| Erection problems | 11 | 3 | –8 |
| Urinary problems | 15 | 10 | –5 |
| Treatment-related problems | 7 | 7 | 0 |
| Other problems | 3 | 2 | –1 |
| Productivity losses | |||
| Mean days lost | |||
| Days reported unable to undertake usual activities (N = 29) | 45 | 25 | –20 |
| Days lost for men who stated employed status (N = 14) | 48 | 31 | –17 |
| Days lost for men who stated unemployed status (N = 13) | 17 | 5 | –12 |
| Total days lost | |||
| Days reported unable to undertake usual activities (N = 29) | 578 | 376 | –202 |
| Travel time reported | |||
| Mean contacts reported | RP (N = 14) | HIFU (N = 14) | Δ (HIFU – RP) |
| GP contact | 3.43 | 1.79 | –1.64 |
| Inpatient contact | 1.36 | 0.50 | –0.86 |
| Outpatient contact | 5.14 | 4.71 | –0.43 |
| A&E contact | 0.14 | 0 | –0.14 |
| Travel costs reported (£) | |||
| Total travel costs | RP (N = 14) | HIFU (N = 14) | Δ (HIFU – RP) |
| GP contact | 277.50 | 124.90 | –152.60 |
| Inpatient contact | 558.00 | 166.40 | –391.60 |
| Outpatient contact | 1646.50 | 1957.80 | 311.30 |
| A&E contact | 64.00 | 0.00 | –64.00 |
| Total reported travel costs | 2546.00 | 2249.10 | –296.90 |
Chapter 4 Summary and discussion of main findings
The PART feasibility study was designed to address the unmet need for evidence of the role of PA technologies for the treatment of unilateral, intermediate-risk PCa, and based on information provided by the commissioned NIHR HTA evidence synthesis published by Ramsay et al. 40 The study demonstrated that a main RCT of radical treatment versus PA is feasible in this cohort of men, and that the majority of men are willing to participate and comply with the return of the important PROM questionnaire packs, which allow precise measurement of QoL and health economics of the treatments allocated.
Several lessons were learnt from the feasibility phase to inform the design of a main RCT, including the addition of radiotherapy and brachytherapy as alternatives to the radical treatment options and broadening the minimally invasive intervention available in the UK in the PA arm to avoid polarisation on the single method of HIFU. Training and monitoring recruiters to consolidate equipoise and overcome avoidable hurdles to recruitment will be used. Centre selection will be critical, particularly taking into account the availability of PA treatments under NHS providers, which appeared to slow down recruitment. It is therefore planned to recruit the centres that were involved in the ProtecT trial, where minimally invasive technologies are not offered routinely, and to expand the number of centres as necessary. Another important consideration will be coverage of the NHS ETCs associated with the delivery of minimally invasive therapies.
QuinteT Recruitment Intervention
Key findings from the QuinteT Recruitment Intervention
The QRI adopted a range of qualitative data collection methods to gain an in-depth understanding of recruitment processes, how the trial was presented and how patients responded to the information about the trial. Although there was strong support for a high-quality RCT comparing radical RP with HIFU, clinicians often held preconceptions about which treatment was best for patients and found it difficult to maintain a position of equipoise between the two treatment arms. For example, some recruiters appeared to favour RP for its oncological outcomes, whereas advocates of HIFU argued that prostatectomies are overtreating patients and that HIFU has a better side-effect profile. These beliefs were often conveyed to patients unwittingly through the use of loaded terminology, the provision of unbalanced information about the treatment arms and sometimes direct treatment recommendations. It is therefore unsurprising that, as a result, patients often expressed clear preferences for a particular treatment and, when these appeared to be sensible, they were promptly accepted without further exploration. Taken together, these findings provided important insights into the complexities of recruiting to a trial comparing different surgical treatments. The issues identified are common within many RCTs,47 and highlight the need to support clinicians to feel more comfortable with their role as recruiters.
Having identified the key issues that were affecting recruitment, there were a number of opportunities to optimise recruitment and informed consent. The collaborators’ meeting in December 2015 provided an initial opportunity to facilitate discussion of the key issues identified. A short ‘tips’ document was then sent to all recruiters, which contained guidance on structuring the consultations and presenting the treatment arms in a balanced way. Group feedback sessions in each of the participating centres provided an opportunity to address specific issues within each centre, clarify each team member’s role and discuss issues of eligibility, equipoise and the patient pathway. Several recruiters were also provided with supportive and confidential individual feedback. Overall, a number of key points were emphasised. Recruiters were encouraged to approach all eligible patients about the study and to mention it early in the appointment. They were also advised to refrain from making treatment recommendations, and instead to ensure that the patient was aware of the uncertainty regarding treatments. The QRI analysis also emphasised the need to present the treatment arms in a balanced manner, and to gently elicit any concerns and preferences to ensure that patients were fully informed about the options available. In addition, support through a ‘tips’ document was provided to describe the process and rationale for randomisation.
Recruitment to the PART trial improved as the study progressed, with notable increases after the first wave of QRI feedback in December 2015. Several centres (rather than predominantly Oxford) began recruiting more consistently. Indeed, between January and November 2015, the average number of patients agreeing to be randomised was 1.4 per month. In addition, the conversion rate (the numbers of eligible men invited to join the PART trial who then went on to be randomised) was 20% (15/75). Phase II of the QRI began in December 2015, and continued for the duration of the trial until recruitment ended in March 2017. During this time, an average of 4.5 participants per month were randomised and the conversion rate increased to 37% after the initial intervention in December 2015. There was qualitative evidence to suggest that the QRI had an impact on the way that clinicians communicated about the trial. Recordings demonstrated that there were notable changes in how the recruiters conveyed equipoise, explored preferences and explained the concept of randomisation. Although this suggests that the QRI had a positive impact, it is important to acknowledge that causality cannot be determined owing to the plethora of variables that could have influenced recruitment at any given time.
Strengths and limitations
According to de Salis et al. ,73 ‘each RCT has a unique – and uniquely complex – recruitment pathway and its own set of issues that need to be resolved’. The QRI adopted a range of qualitative data collection methods to gain an in-depth understanding of recruitment processes, how the trial was presented and how patients were responding to the trial. We were also able to compare and contrast data; for instance, interviews showed clinicians’ intention to recruit, but the consultations demonstrated ways in which they unintentionally steered patients towards particular treatments. Furthermore, analysis of the audio-recorded consultations highlighted variation in how the trial was presented.
The QRI was limited in a number of respects. Research nurses cited pressure of work as a reason why they were unavailable for interviews. Clinical centres were often unresponsive to the QRI researcher. Some centres, notably UCLH, provided few or no recordings. Even those centres that did provide recordings did not regularly record all consultations. For instance, the screening logs showed that, although 329 patients were approached about the study, discussions were recorded with only 54 patients (16%). This sometimes made it difficult to provide tailored and specific feedback. In one centre there was no feedback session, limiting the potential impact the QRI might have had.
The QRI has now been applied in over 25 RCTs,55 often showing improvements in recruitment. However, it is difficult to evaluate its precise contribution. Other factors that might have led to improvements in recruitment include recruiters perhaps becoming more comfortable with the recruitment process as they became more familiar with the study design in terms of the inclusion criteria74 and study processes,75 or gaining confidence with communicating about the trial with patients. 76 The trial team also provided regular recruitment updates and support, which recruiters have previously reported to be an incentive to improve recruitment. 77,78 However, these contributions are also difficult to identify. The QRI has consistently contributed to improvements in recruitment in RCTs identified to be particularly challenging for participation55 or to the maintenance of recruitment. 53 In the PART feasibility study, several recruiters acknowledged the contribution of the QRI to improvements in their approach to recruitment. A more formal evaluation of the effectiveness of QRIs implemented to date is under way [Rooshenas L, Scott L, Blazeby J, Rogers C, Tilling K, Husbands S, et al. (University of Bristol), 2018].
Site selection
Four centres were originally proposed: Oxford, Bristol, Sheffield and UCH. However, there were individual problems at some of the centres (Bristol’s PI left the UK, and the NHS trust declined to support the trial, and UCH encountered insurmountable challenges to recruitment). We therefore added two new centres during the course of the study (Basingstoke and Southampton), which caused delays in optimising our recruitment strategy and necessitated an extension to the projected recruitment period. In addition, reimbursement of the HIFU ETCs was extremely difficult to secure in the centres; it required considerable efforts to secure these costs from other sources.
Quality-of-life assessments
The study was designed to assess the feasibility of assessing the various dimensions of HRQoL that may be affected by PA therapies or RP in a main RCT. The results of the study were encouraging, particularly with postprocedure follow-up, with favourable response rates overall and instruments being completed with few missing items. The exception was the somewhat less complete data provided for FACT-P, a much longer questionnaire than others in the required battery. There may be some element of redundancy in the battery of questionnaires that could be addressed to reduce respondent burden and rate of missing data.
The pattern of differences in QoL between treatments observed, particularly for urinary and sexual function, is consistent with other recent studies of focal therapies and provides positive evidence for the construct validity of instruments selected for the PART study. 79,80 Only a main RCT can provide clear evidence of short- and long-term effects on QoL of alternative treatments, which can be judged in conjunction with reliable evidence of disease and survival results in order to inform patients’ and clinicians’ judgements about trade-offs and treatment choices. 15
Health economics
Completeness levels across the health economic instruments were good, and trends in incomplete data were examined. In order to strengthen a main RCT, the following concerns have been identified from the data audit alongside the health economic analysis:
-
Missing data in relation to the EQ-5D-5L instrument may be a result of patients requesting to take the PROMs survey pack home with them. There were no missing data at the domain level for patients who completed the EQ-5D-5L instrument. Discussion with the study nurses highlighted the fact that the patients did not have the time or physical space necessary to sit down and complete the survey pack. For the substantive phase of the trial, it would be more efficient if the EQ-5D-5L instrument (on its own and not as part of a PROMs survey pack) was presented to the patient on arrival at all follow-up visits and a request for immediate completion was made by the trial nurse. Further training and supportive material will also be supplied to trial nurses highlighting the importance of the EQ-5D-5L instrument in the overall assessment of whether or not the proposed intervention is cost-effective.
-
The completeness level of the patient resource use diary was good, with 31 out of 41 (76%) participants fully completing the diary; however, completeness by section varied considerably. The rationale for the full completion of the patient diaries between each follow-up may need to be further explained through supplementary material and further discussion with the trial nurse. Issues with the interpretation of what items to include were also highlighted. For example, patients included initial procedure details given at the treatment visit in the diary; therefore, it will be necessary, for the substantive phase of the trial, to explain that the diaries are for resources used between, but not including, initial or subsequent follow-up visits. We will also explore redesigning data capture methods for days unable to undertake usual activities.
-
The structure of the patient diary will be optimised for the main RCT to reduce incomplete sections. Certain items have been identified that were incomplete for most participants and solutions have been identified through consultation with the trial nurses to enhance the diary. The addition of ‘not-applicable’ tick boxes for each subsection is necessary for distinguishing between categories of missingness. Add-on checklists can also be included as reference material for patients. For example, the diary categorises medication and device usage into four subsections: (1) drugs or devices for erection problems, (2) medicines or devices for managing urinary problems, (3) other medicines or therapy for treatment-related problems (e.g. for anxiety or depression, pain, etc.) and (4) other aids or devices for treatment-related problems. A small sample of participants appeared to not have full knowledge of which medication was for which health problem, and so medications in particular were reported in incorrect sections. A comprehensive list of potential medications for sexual dysfunction and urinary problems may reduce the potential for mismatch in completing this section of the survey. A third defined category for digestive/bowel issues may be relevant to the disease area and further enhance the patient diary.
-
In the patient diary, men were asked to report days during which health status affected their ability to carry out usual activities. Of those who completed this section, the mean number of days reported in suboptimal health was 32.9 (range 3–154 days). Currently, the patient diary does not request information on informal care; given the variation in days reported unable to carry out usual activities across trial arms, the addition of a further section on informal care may highlight a further economic benefit from the intervention.
The analysis of EQ-5D-5L utility scores highlights potential health gains for patients receiving HIFU compared with RP, with evidence suggesting that HIFU is unlikely to cause a loss in health benefit relative to RP. This exploration of the EQ-5D-5L data adds to the clear need to undertake a larger-scale trial assessing HIFU against other therapies for localised PCa.
Reported resource use in terms of contacts with health-care professionals and inpatient events was higher in the RP arm than in the HIFU arm. Whether or not this would persist in a larger-scale study is unknown; however, at baseline, both cohorts of participants exhibited similar clinical profiles and, for this reason, variation in resource use evidenced across follow-up is at least partially attributable to the trial procedures.
The usefulness of a patient diary versus retrieving data from electronic patient records/notes was assessed in this feasibility stage of the trial. Return rates were good for the patient diary, at 70%, and a wider range of health-care contact at primary and secondary care settings, as well as medication and device usage, can be captured in one data collection tool, aiding more robust analysis of resource use data.
Cost per treatment arm was not analysed. The overall objective of the feasibility study was to assess the feasibility and completeness of data collection regarding HRQoL and patient-reported resource use. Median time spent in hospital after treatment was 1 bed-day (IQR 1–4 bed-days) in the RP arm and 0 bed-days (IQR 0–2 bed-days) in the HIFU arm (see Table 7); one participant in the RP arm also spent a night in an ICU following treatment. The cost implications of the variation in resource use across trial arms suggest that there are potential savings from both a NHS perspective and a societal perspective. This needs to be examined alongside a full RCT. Furthermore, the cost of the HIFU treatment compared with RP is substantially lower. The 2014/15 unit costs66 for HIFU and robotic RP were £2848 and £7317, respectively; therefore, based on NHS reference costs alone, the cost to the NHS of RP is 2.6 times higher than the cost of HIFU, suggesting a potential for cost saving. However, NHS reference costs reflect a national average and, as each trust negotiates the cost and availability of the devices (long-term lease, purchase, or ad hoc hire) based on capacity/usage as well as other factors, these unit costs will vary considerably from centre to centre. To address this, in the full RCT, a full micro-costing would need be undertaken to provide a more robust unit cost estimate of HIFU and RP to improve precision in the comparison.
The use of patient diaries improves the level of resource use detail reported and reduces the potential for missing data, which are usually an issue with electronic patient records. In the PART trial, there was a good response rate to the patient resource use diary; however, certain sections need refinement, and further supplementary guidance for trial nurses and patients is needed in the main RCT.
Imaging
In the feasibility protocol, patients needed to have received a mpMRI before confirmation of eligibility for randomisation. Although in some of the centres, pre-biopsy imaging was routine practice, in others mpMRI was performed post diagnosis; therefore, a delay of approximately 3 months was incurred to obtain images void of postbiopsy artefacts. The need for this important component of the evaluation for eligibility was explained to participants, in order to avoid using PA in the presence of significant disease on the contralateral side of the prostate. This delay of 3 months represented an acceptable formal period of AM for the participant, following which mpMRI and further biopsies, as necessary, would determine eligibility. During the course of the study, urological practice has changed considerably in most centres, particularly after the publication of the NIHR HTA PROMIS, and the majority of UK urological departments now include mpMRI before biopsies, which will resolve the challenge encountered in the feasibility phase.
Pathology
During the feasibility study, a new national initiative, the National Cancer Research Institute – Cellular Molecular Pathology (NCRI CM-Path), was formed. One of its aims is to improve the quality of pathology input to clinical trials. The PART trial lead pathologist is a member of the NCRI CM-Path Workstream 2 (clinical trials) and is chairperson for the Tissue Access and Quality Assurance in Trials subgroup. Information gained from this initiative would be utilised to maximise a main RCT.
The period of the feasibility study was a time of great change for urological pathology, with new reporting guidelines introduced. Although pathology specimens were processed and reported to standards set in the Royal College of Pathologists’ Dataset for Histopathology Reports for Prostatic Carcinoma, second edition,67 a third edition has now been published. 81 It was not feasible to change to the newer version of this data set part-way through this feasibility study, as this would have led to inconsistency.
It was intended that central review of RP specimens would happen from other recruiting centres, but logistically this proved to be difficult. Any cases with bilateral or high-risk disease in the main RCT will be scanned and shared between the PART trial pathology working group. The purpose of this would be to verify the overall categorisation either as dominant unilateral intermediate-risk and small contralateral low-risk disease or bilateral/high-risk disease. The borderline between Gleason patterns 3 and 4 is not always entirely clear, and the distinction between these two categories may vary depending on interpathologist interpretation of Gleason pattern 3 versus Gleason pattern 4. Central review ensures standardisation.
Data collection tools
The PART feasibility study allowed a mixture of remote and central data entry at the outset. Centres were initially requested to enter data remotely; however, it became apparent that centres were struggling to manage data entry, owing to the large number of CRFs, PROMs questionnaire packs and resource use diaries being tested. To support centres, the PART trial office took over all data entry, apart from at the Royal Hallamshire Hospital, Sheffield, where a dedicated data entry clerk had already been identified to enter data.
Interventions
A main RCT would be opened up to additional PA modalities to reflect the available range of treatments that are currently being evaluated, as well as offering brachytherapy and IMRT in the radical ablation arm. We believe that this would enhance recruitment as patients may be more willing to participate when there is a wider range of treatment options available to them.
Impact of patient and public involvement
Patient representatives attended all TSC meetings and were supportive of the study’s progress and the importance of a larger RCT. The proposed main RCT would open the treatment arms up, and compare partial versus radical treatments. The patient representatives did not feel that there would be concerns from a patient perspective about which PA therapy they received, as long as they received assurances about the efficacy of the technique. Online completion of PROMs questionnaires was considered but as ≈10% of the OPCSG members did not have internet access, a paper option would remain necessary. Patients were sent the validated questionnaire pack and resource utilisation diary for review. The diary was deemed fairly clear and helpful feedback was received on the questionnaire pack, including:
-
whether or not patients routinely weighed themselves, as a question in the pack relates to weight gain/loss
-
the size of the questionnaire pack
-
the layout of some of the questionnaires
-
the repetition of some questions within the pack.
These comments will help to inform the design of the questionnaire pack for the main RCT.
We will involve the OPCSG in the dissemination of the feasibility results, and draw heavily on their views and support for the funding application for a main RCT.
Chapter 5 Conclusion
The PART feasibility study has demonstrated that it is possible to recruit and randomise patients to a trial of RP versus PA in men with unilateral intermediate-risk PCa. A full RCT is now warranted, using all radical treatment options as a comparator for several minimally invasive ablative technologies available and used in the UK. A full RCT will provide much needed evidence of comparative treatment effectiveness and cost-effectiveness of radical versus PA treatments in order to inform NICE and future recommendations of adoption by the NHS as routine practice.
Chapter 6 Further research and plans for a main randomised controlled trial
Recent evidence, including the publication of the ProtecT trial results, confirms the need to seek evidence of the effectiveness of alternative, less invasive methods with fewer side effects to treat patients with unilateral intermediate-risk PCa. Should there be demonstrable equivalence in oncological outcomes between the treatments, it is likely that this will produce a fundamental paradigm shift in the ‘trade-off’ considerations by patients between long-term benefits and side-effect profiles, with profound health economic implications. From our literature review (see Chapter 1, Scientific background), it is evident that there are still no robust primary research studies comparing PA with radical treatment, and hence a lack of information on their long-term clinical effectiveness and cost-effectiveness. Having demonstrated the feasibility of a PART trial, a full RCT is now warranted. The NIHR HTA programme has recommended funding for the full trial, subject to final revisions and contract arrangements. This section of our report describes the lessons learnt from the feasibility phase that will be applied to the design of the full RCT.
Recruitment strategies for the main randomised controlled trial
Many of the challenges identified in phase I of the QRI are commonly reported barriers, including lack of clinician equipoise,46,47,82 variability in the inclusion criteria,83 and difficulties with exploring patient preferences84 and explaining randomisation to patients. 49 Recruitment is recognised to be a complex and fragile process whereby recruiters experience emotional and intellectual challenges related to their dual roles of researcher and clinician. 46,47 The QRI highlighted the complexity of the recruitment process and demonstrated a number of ways in which recruiters required training and support. It is likely that new centres will experience similar difficulties to those encountered in the feasibility study, particularly if they are new, or relatively new, to trial recruitment. These issues could be pre-empted by undertaking thorough screening of proposed centres and providing focused training sessions, based on the issues identified, with ongoing monitoring to identify additional opportunities to improve recruitment. The results of the QRI conducted in the feasibility study will be applied in the main RCT to ensure that new centres are aware of the issues of eligibility, equipoise and patient preferences, so as to reach optimal levels of recruitment without delay. Including other minimally invasive techniques within the PA arm, extending to centres with HIFU available only in the trial, or including radiotherapy in the radical treatment arm may also. As patient preferences are likely to remain a key challenge, recruiters to the main RCT will continue to need support with recruitment. Given that the context of a trial can change during its lifespan,44,55 the dynamic and flexible nature of the QRI will also enable the study team to detect – and respond accordingly – to any emerging recruitment issues as they arise in a main RCT.
Site selection
In selecting centres for the main trial, there will be several factors to take into account. First, the centre should have experience in recruiting to surgical RCTs, for example the ProtecT trial centres. Second, although having an established focal therapy programme should be an advantage, the access to the treatment should be as per NICE guidelines and within a clinical trial, such that surgeon equipoise is maintained. It would be preferable to have a centre new to focal therapy and establish a new programme within the trial, rather than the situation in which patients and surgeons are presented with the option to receive the study treatment outside the study. Third, it is important that each centre has an established funding stream to fund any treatment within the trial (robotic surgery or focal therapy). Finally, there should be adequate research nurse support to facilitate recruitment in each centre. UCH, a tertiary referral centre where patients have already had a number of clinical consultations and have been referred for a specific treatment before hearing about the study, was unable to recruit any patients, and we would therefore consider carefully whether or not tertiary centres should be included in a main RCT.
High-intensity focused ultrasound was openly available on the NHS at Basingstoke and Southampton, and these centres had lower conversion rates (21% and 20%, respectively). Because these centres were recognised nationally as specialist centres for HIFU, patients were referred to them from surrounding and distant hospitals specifically for this procedure. Although there are clear advantages in using centres that have an established expertise in the new technologies, investment in centres without an existing regional referral network for HIFU would ensure that patients are more likely to participate in the PART trial. The QRI will work with potential new centres for the main RCT to understand their patient pathways and investigate referral patterns and numbers of eligible patients.
Interventions
One of the barriers to recruitment was that the only radical treatment option was surgery, and the only PA option was HIFU. Participation in the trial was declined by 63% of patients because they wanted other forms of radical treatment (such as radiotherapy) or PA (such as cryotherapy). These treatments were not available in the feasibility trial; therefore, we will be expanding the radical options to include radiotherapy (which we have demonstrated has equivalence to surgery in terms of oncological outcomes in the ProtecT trial) and brachytherapy. In the PA arm, in addition to HIFU, we will add cryotherapy, VTP and focal brachytherapy. All of these technologies have been demonstrated to ablate tissue successfully, and will be offered depending on availability and expertise in individual centres. Excess NHS costs will need to be calculated and agreed in advance.
The proposed outcome measures for a main RCT are outlined in the following sections.
Primary outcomes
-
Primary treatment failure as demonstrated by the need for whole-gland ablation and/or secondary radiotherapy.
-
Quality of life using conventional questionnaires to assess sexual outcomes, urinary continence and other functional outcomes.
Secondary outcomes
-
Short-, medium- and long-term AEs related to treatments.
-
Disease progression, including development of metastases.
-
Resource utilisation and health economic evaluation in terms of cost per QALY.
-
The role of imaging by mpMRI and biopsy protocols in determining suitability of patients for PA therapy.
-
Disease-specific and all-cause mortality.
Sample size
Preliminary evidence from the feasibility study suggests that HRQoL is better in patients undergoing ablative therapy than in those undergoing RP, particularly for functional outcomes such as urinary incontinence and erectile dysfunction. Given that functional outcome appears substantially worse with radical rather than ablative therapy, the primary treatment failure rate might need to be > 10% lower (i.e. 20% vs. 30%) for radical therapy to replace ablative therapy as recommended standard clinical practice. Therefore, allowing for a 10% drop-out rate, an estimated sample of 800 patients randomised on a 1 : 1 basis would provide 90% power at p < 0.05 to detect a difference in treatment failure rate of ≥ 10% in favour of RP rather than ablative therapy. The statistical power would not be much affected if the failure rate in the radical treatment arm was somewhat lower, or somewhat higher, than 20%. For example, 800 participants (with 10% drop-out) would provide 95% power at p < 0.05 to detect a difference of 10% versus 20% in failure rates in the RP versus HIFU arms and 80% power to detect a 25% versus 35% failure rate in the RP versus HIFU arms. The drop-out rate would also not affect statistical power much: if, improbably, the drop-out rate was as high as 20%, then statistical power to detect a 20% versus 30% difference in failure rate in the RP versus HIFU arms would be 80%.
A projection of recruitment was undertaken, based on the performance of the four recruiting centres in the later part of the feasibility study, and is summarised in Table 23 and Figure 33.
| Recruitment centre | Recruitment performance | Projected recruitment (n) | ||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||
| 1 | 10 patients per month (n = 4 centres) (based on March 2017 recruitment number) | 106 | 136 | 196 |
| 2 | 8 patients per month (n = 4 centres) (target: two patients per site per month) | 100 | 124 | 172 |
| 3 | 6 patients per month (n = 4 centres) (based on last 3 months of actual recruitment data) | 94 | 112 | 148 |
| 4 | 4.2 patients per month (n = 4 centres) (based on last 6 and 12 months of actual recruitment data) | 88.6 | 101.2 | 126.4 |
FIGURE 33.
Graph of recruitment projections.

If we base our calculation on a conservative average of recruiting one or two participants per month in each centre, we will be able to recruit up to 840 men in 14 recruiting centres over a period of 5 years.
Data collection tools
The CRFs need to be streamlined in order to reduce the number of incomplete or missing data in some cases, participants had insufficient time to complete the PROMs packs and resource use diaries in clinic, and had to take them home to complete. Sometimes they were not returned despite telephone reminders. Issues vocalised by the trial nurses in relation to the data collection tools and protocols at follow-up visits are being systematically assessed via a PART Study Research Nurses’ Survey, constructed by the health economist (see Report Supplementary Material 11). We have also asked patient representatives on the TSC for their opinions. If funding is secured for a main RCT, the information will be collated and used to inform redesign of the health economic component of the trial. We would aim for all PROMs and resource use diaries to be completed electronically by the participants (co-ordinated by the clinical trials units, therefore avoiding the need for the research nurses to send these out). A main RCT would require a full-time data manager, who would be responsible for central data entry, which would facilitate entry of CRFs onto the database and permit data to be queried and missing CRFs to be requested in a systematic and timely manner.
Pathology
For a main RCT, the third edition of the Dataset for Histopathology Reports for Prostatic Carcinoma81 will be used for reporting. For similar reasons, despite publication of an International Society of Urological Pathology (ISUP) consensus conference on Gleason grading in 2014,85 the histopathology SOP was not amended, but a main RCT would utilise the recommendations in the ISUP Gleason 2014 publication. It is mandatory from 1 January 2018 to use the eigth edition86 of TNM Classification of Malignant Tumours (TNM), rather than the seventh edition,69 which was in use for the duration of the feasibility study. The American Joint Committee on Cancer version of the eigth edition of TNM,87 rather than the Union for International Cancer Control version, will be used. 86 This contains the recommendation that pT2 disease is no longer substaged but that all are categorised as pT2 without subcategorising into pT2a/2b/2c; thus, the CRFs for the main study will be amended to clarify this. 87
Double reporting of RP specimens in real time would not be utilised in a main RCT as this is logistically very difficult and would slow entry into the trial and compromise recruitment. For a main RCT, ideally a comprehensive library of images would be created to enable central review of original diagnostic biopsies by two expert urological pathologists before final publication of results.
There were no problems with local reporting and filling in of CRFs in the feasibility study. However, it would be optimal for a main RCT to amend the PIL and ICF to explicitly allow scanning of all histology slides and long-term storage of the images. This will include original diagnostic biopsies at trial entry, post-HIFU biopsies and RP slides, with the following objectives:
-
to create a comprehensive library of slide images for future reference and academic interest – the post-HIFU biopsies would be of particular interest
-
to allow central review by the PART trial pathology group.
General principles that will be adopted for a main RCT include strengthening of the PART trial pathology working group and establishing central review of cases. Before a main RCT commences, the current members of the PART trial pathology study working group will develop the histopathology SOP and guidance on requirements and training needed for pathologists participating in the trial. Digital pathology will form a larger component of the main RCT to overcome difficulties with sharing slides across a multicentre RCT and create a long-term record of the slides to avoid slides fading and so forth. This record is not just photographed images of the slides, but scans of the entire slide to enable them to be viewed digitally in a similar way to looking through a microscope. This will be flexible depending on local slide-scanning facilities. An ideal scenario would be scanning at the local centre and uploading to a secure cloud-based trial slide repository. As a minimum, slides can be sent to Oxford, where slide scanning facilities exist. A secure slide-hosting site would be created for the main RCT.
Pathology and radiology diagnostic pathway protocols would be standardised in a main RCT; this would be the remit of the pathology and radiology working groups, which have been established in the context of the feasibility study.
Health economic considerations for a main randomised controlled trial
Completeness of the EQ-5D-5L was good in the feasibility study but could be improved. For a main RCT, we propose collecting the EQ-5D-5L alongside the patient resource use diary, rather than with other disease-specific QoL instruments. It is also vital to ensure that, at each follow-up visit, the EQ-5D-5L questionnaire is completed before the patient sees the consultant, when possible, to reduce bias. If the patient needs to take home the EQ-5D-5L questionnaire to complete, this needs to be coded, and structured timings of prompts from research nurses to complete and return questionnaires would be required.
Although not undertaken at this stage, a full costing and cost-effectiveness analysis would be performed in a main RCT and unit costs would be updated accordingly. A main RCT would include a full cost-effectiveness analysis with the adapted resource use and EQ-5D-5L data collected in this feasibility stage being collected alongside a full trial with longer participant follow-up. Long-term patient costs attributable to AEs, complications, treatment side effects and recurrences, and the related impact on QoL beyond the length of the trial, would be explored by developing a cost-effectiveness model to provide an estimate of cost-effectiveness extrapolated beyond the full within-trial analysis. A budget impact model with the cost of all trial procedures estimated using a micro-costing approach would be undertaken. The unit costs of a number of different delivery mechanisms for focal therapy would be estimated and used in a scenario analysis to explore the cost-effectiveness of different potential focal therapy delivery mechanisms: for example, comparing provision of the therapy by a local centre using owned equipment, a local centre hiring equipment, focal therapy delivered at specialised hubs and equipment purchased by one centre and taken to each centre.
Acknowledgements
We would like to give our sincere thanks to:
-
the study research nurses for recruiting patients and providing ongoing feedback on local trial processes and progress, and without whom the study would not have been possible –
-
Shelagh Lovell and Jane Niederer (Churchill Hospital, Oxford)
-
Louise Goodwin, Marie Marshall, Louise Weatherley and Elizabeth Bell (Royal Hallamshire Hospital, Sheffield)
-
Caroline Andrews, Yanli Li and Aneta Zahorska (Southhampton General Hospital)
-
Abby Edwards and Philippa Aslet (Basingstoke and North Hampshire Hospital)
-
Marjorie Otieno (UCH, London)
-
-
the members of the trial support team – Bojana Selensek, Beth Delaplain and Akiko Greshon, for trial data entry and daily administration
-
the urologists who contributed to patient recruitment at local centres – Brian Birch, Richard Lockyer, Jonathan Dyer, Simon Bott and Christopher Eden
-
the oversight committee members for expert advice and support throughout the study – Dr David Bottomley (TSC Chairperson), Professor Rob Pickard (former TSC Chairperson), Professor Luke Vale (Independent Member), Dr Peter Albertsen (Independent Member), Nick Stogdon (Patient Representative) and John Grundy (Patient Representative), in addition to Professor Howard Kynaston (Independent Safety Data Reviewer) and Professor Duncan Young (Nominated Clinician) for reviewing SAEs
-
David Beesley (Lay Representative), for reviewing the original grant application, and OPCSG, for reviewing the patient-facing documents.
The QRI team wish to thank Paul Whybrow for conducting a subset of PART trial interviews.
Contributions of authors
Freddie C Hamdy (PART trial Chief Investigator, Professor of Urology) was responsible for the conception and design of the work, acquisition of funding, interpretation of data and drafting, reviewing and final approval of the report.
Daisy Elliott (Senior Research Associate in Qualitative Health Services Research) conducted data collection and analysis for the QRI and delivered recruiter training.
Steffi le Conte (PART trial Clinical Trial Manager) developed and implemented trial management processes and was responsible for data acquisition and drafting of the final report.
Lucy C Davies (Statistician) was responsible for statistical analysis and interpretation of data and drafting of the final report.
Richéal M Burns (Senior Researcher in Health Economics) conducted the health economic analysis and interpretation and drafted the health economics section of the final report.
Claire Thomson (Clinical Trials Development Lead) co-ordinated drafting and collating of the final report.
Richard Gray (Professor of Medical Statistics) provided guidance on statistical issues of study design and data analysis.
Jane Wolstenholme (Associate Professor of Health Economics) was responsible for the conception and design of the health economic component and drafted and edited health economics section of the final report.
Jenny L Donovan (Professor of Social Medicine) was responsible for the conception and design of QRI and supervised the senior research associate.
Ray Fitzpatrick (Professor of Public Health and Primary Care) was responsible for the conception and design of the QoL component and commented on the HRQoL analysis.
Clare Verrill (Senior Clinical Lecturer in Pathology) was the head of the PART trial pathology working group, was responsible for central review and reporting of pathology slides and drafted the pathology section of the final report.
Fergus Gleeson (Consultant Radiologist and Professor of Radiology) was the head of the PART trial radiology working group.
Surjeet Singh (Surgical Intervention Trials Unit Operational Lead) supervised the involvement of the Surgical Intervention Trials Unit, oversight of PART trial management and operations and drafted the final report.
Derek Rosario (PART trial Principal Investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
James WF Catto (PART trial Co-investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
Simon Brewster (PART trial Co-investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
Tim Dudderidge (PART trial Principal Investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
Richard Hindley (PART trial Principal Investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
Amr Emara (PART trial Co-investigator, Consultant Urologist) contributed to the study design and oversaw delivery of the intervention.
Prasanna Sooriakumaran (PART trial Co-investigator, Consultant Urologist) was head of the PART trial surgery working group, contributed to the study design and oversaw delivery of the intervention.
Hashim U Ahmed (Consultant Urologist) was head of the PART trial PA working group, contributed to the study design and oversaw delivery of the intervention.
Tom A Leslie (PART trial Principal Investigator, Consultant Urologist) was responsible for the conception and design of the work, acquisition of funding, acquisition and interpretation of data and drafting and reviewing the final report.
Publication
Elliott D, Hamdy FC, Leslie TA, Rosario D, Dudderidge T, et al. Overcoming difficulties with equipoise to enable recruitment to a randomised controlled trial of partial ablation versus radical prostatectomy for unilateral localised prostate cancer [published online ahead of print June 11 2018]. BJU Int 2018.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Prostate Cancer Statistics Table 2014. London: Cancer Research UK; n.d.
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. https://doi.org/10.1016/S0140-6736(16)32401-1.
- Klotz L. Active surveillance for prostate cancer: trials and tribulations. World J Urol 2008;26:437-42. https://doi.org/10.1007/s00345-008-0330-8.
- van As NJ, Parker CC. Active surveillance with selective radical treatment for localized prostate cancer. Cancer J 2007;13:289-94. https://doi.org/10.1097/PPO.0b013e318156ff65.
- Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med 1994;330:242-8. https://doi.org/10.1056/NEJM199401273300403.
- van den Bergh RC, Essink-Bot ML, Roobol MJ, Wolters T, Schröder FH, Bangma CH, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer 2009;115:3868-78. https://doi.org/10.1002/cncr.24446.
- Klotz L. Active surveillance with selective delayed intervention: a biologically nuanced approach to favorable-risk prostate cancer. Clin Prostate Cancer 2003;2:106-10. https://doi.org/10.3816/CGC.2003.n.017.
- Major Resections by Cancer Site, in England; 2006 to 2010. London: Cancer Research UK; 2015.
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233-9. https://doi.org/10.1001/jama.294.10.1233.
- D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004;292:821-7. https://doi.org/10.1001/jama.292.7.821.
- Speight JL, Roach M. Radiotherapy in the management of clinically localized prostate cancer: evolving standards, consensus, controversies and new directions. J Clin Oncol 2005;23:8176-85. https://doi.org/10.1200/JCO.2005.03.4629.
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a Phase III randomised trial. Lancet 2002;360:103-6. https://doi.org/10.1016/S0140-6736(02)09408-4.
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011;59:61-7.
- Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy ≥ 72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:25-33. https://doi.org/10.1016/S0360-3016(03)00784-3.
- Hamdy FC, Donovan JL. Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA 2017;317:1121-3. https://doi.org/10.1001/jama.2017.1703.
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425-37. https://doi.org/10.1056/NEJMoa1606221.
- Muto S, Yoshii T, Saito K, Kamiyama Y, Ide H, Horie S. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol 2008;38:192-9. https://doi.org/10.1093/jjco/hym173.
- Ahmed HU, Sahu M, Govindaraju SK, Arumainayagam N, Scott R, Illing RO, et al. High intensity focused ultrasound (HIFU) hemiablation trial in localised unilateral prostate cancer: interim results. Eur Urol Suppl 2009;8. https://doi.org/10.1016/S1569-9056(09)60842-3.
- Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622-32. https://doi.org/10.1016/S1470-2045(12)70121-3.
- Kim K, Zhang H, La Rosa S, Jebiwott S, Desai P, Kimm S, et al. Bombesin antagonist-based radiotherapy of prostate cancer combined with WST-11 vascular targeted photodynamic therapy. Clin Cancer Res 2017;23:3343-51. https://doi.org/10.1158/1078-0432.CCR-16-2745.
- Warmuth M, Johansson T, Mad P. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol 2010;58:803-15. https://doi.org/10.1016/j.eururo.2010.09.009.
- Lukka H, Waldron T, Chin J, Mayhew L, Warde P, Winquist E, et al. High-intensity focused ultrasound for prostate cancer: a systematic review. Clin Oncol 2011;23:117-27. https://doi.org/10.1016/j.clon.2010.09.002.
- Veereman G, Jonckheer P, Desomer A, Van Brabandt H, D’Hont C, Van Velthoven R, et al. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for localised prostate cancer. Eur Urol Focus 2015;1:158-70. https://doi.org/10.1016/j.euf.2015.04.006.
- Kuru TH, van Essen J, Pfister D, Porres D. Role of focal therapy with high-intensity focused ultrasound in the management of clinically localized prostate cancer. Oncol Res Treat 2015;38:634-8. https://doi.org/10.1159/000441600.
- Shelley M, Wilt TJ, Coles B, Mason MD. Cryotherapy for localised prostate cancer. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD005010.pub2.
- Donnelly BJ, Saliken JC, Brasher PM, Ernst SD, Rewcastle JC, Lau H, et al. A randomized trial of external beam radiotherapy versus cryoablation in patients with localized prostate cancer. Cancer 2010;116:323-30. https://doi.org/10.1002/cncr.24779.
- Haider MA, Davidson SR, Kale AV, Weersink RA, Evans AJ, Toi A, et al. Prostate gland: MR imaging appearance after vascular targeted photodynamic therapy with palladium-bacteriopheophorbide. Radiology 2007;244:196-204. https://doi.org/10.1148/radiol.2441060398.
- Trachtenberg J, Bogaards A, Weersink RA, Haider MA, Evans A, McCluskey SA, et al. Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. J Urol 2007;178:1974-9. https://doi.org/10.1016/j.juro.2007.07.036.
- Trachtenberg J, Weersink RA, Davidson SR, Haider MA, Bogaards A, Gertner MR, et al. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int 2008;102:556-62. https://doi.org/10.1111/j.1464-410X.2008.07753.x.
- Weersink RA, Forbes J, Bisland S, Trachtenberg J, Elhilali M, Brún PH, et al. Assessment of cutaneous photosensitivity of TOOKAD (WST09) in preclinical animal models and in patients. Photochem Photobiol 2005;81:106-13. https://doi.org/10.1562/2004-05-31-RA-182.
- Arumainayagam N, Allen C, Moore CM, Barber N, Hindley R, Muir G, et al. 936 TOOKAD® Soluble (Padeliporfin) second generation targeted photodynamic therapy (VTP) for prostate cancer: safety and feasibility. Eur Urol Suppl 2010;9. https://doi.org/10.1016/S1569-9056(10)60916-5.
- Zlotta AR, Djavan B, Matos C, Noel JC, Peny MO, Silverman DE, et al. Percutaneous transperineal radiofrequency ablation of prostate tumour: safety, feasibility and pathological effects on human prostate cancer. Br J Urol 1998;81:265-75. https://doi.org/10.1046/j.1464-410X.1998.00504.x.
- Djavan B, Zlotta AR, Susani M, Heinz G, Shariat S, Silverman DE, et al. Transperineal radiofrequency interstitial tumor ablation of the prostate: correlation of magnetic resonance imaging with histopathologic examination. Urology 1997;50:986-92. https://doi.org/10.1016/S0090-4295(97)00540-2.
- Tucker RD, Huidobro C, Larson T. Ablation of stage T1/T2 prostate cancer with permanent interstitial temperature self-regulating rods. J Endourol 2005;19:865-7. https://doi.org/10.1089/end.2005.19.865.
- Shariat SF, Raptidis G, Masatoschi M, Bergamaschi F, Slawin KM. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate 2005;65:260-7. https://doi.org/10.1002/pros.20242.
- Amin Z, Lees WR, Bown SG. Technical note: interstitial laser photocoagulation for the treatment of prostatic cancer. Br J Radiol 1993;66:1044-7. https://doi.org/10.1259/0007-1285-66-791-1044.
- Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007;6:295-300. https://doi.org/10.1177/153303460700600405.
- Rubinsky J, Onik G, Mikus P, Rubinsky B. Optimal parameters for the destruction of prostate cancer using irreversible electroporation. J Urol 2008;180:2668-74. https://doi.org/10.1016/j.juro.2008.08.003.
- Scheltema MJ, van den Bos W, de Bruin DM, Wijkstra H, Laguna MP, de Reijke TM, et al. Focal vs extended ablation in localized prostate cancer with irreversible electroporation; a multi-center randomized controlled trial. BMC Cancer 2016;16. https://doi.org/10.1186/s12885-016-2332-z.
- Ramsay CR, Adewuyi TE, Gray J, Hislop J, Shirley MD, Jayakody S, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19490.
- Valerio M, Cerantola Y, Eggener SE, Lepor H, Polascik TJ, Villers A, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol 2016;71:17-34. https://doi.org/10.1016/j.eururo.2016.08.044.
- Bostwick DG, Waters DJ, Farley ER, Meiers I, Rukstalis D, Cavanaugh WA, et al. Group consensus reports from the Consensus Conference on Focal Treatment of Prostatic Carcinoma, Celebration, Florida, February 24, 2006. Urology 2007;70:42-4. https://doi.org/10.1016/j.urology.2007.07.037.
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- Blencowe NS, Cook JA, Pinkney T, Rogers C, Reeves BC, Blazeby JM. Delivering successful randomized controlled trials in surgery: methods to optimize collaboration and study design. Clin Trials 2017;14:211-18. https://doi.org/10.1177/1740774516687272.
- Albrecht TL, Eggly SS, Gleason ME, Harper FW, Foster TS, Peterson AM, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol 2008;26:2666-73. https://doi.org/10.1200/JCO.2007.14.8114.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-5.
- Realpe A, Adams A, Wall P, Griffin D, Donovan JL. A new simple six-step model to promote recruitment to RCTs was developed and successfully implemented. J Clin Epidemiol 2016;76:166-74. https://doi.org/10.1016/j.jclinepi.2016.02.002.
- Krieger JL. Last resort or roll of the die? Exploring the role of metaphors in cancer clinical trials education among medically underserved populations. J Health Commun 2014;19:1161-77. https://doi.org/10.1080/10810730.2013.801537.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Townsend D, Mills N, Savović J, Donovan JL. A systematic review of training programmes for recruiters to randomised controlled trials. Trials 2015;16. https://doi.org/10.1186/s13063-015-0908-6.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002360.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29-36. https://doi.org/10.1016/j.jclinepi.2008.02.010.
- Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789-98. https://doi.org/10.1016/j.eururo.2017.04.036.
- Strauss A, Corbin J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. London: Sage; 1998.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. https://doi.org/10.1016/S0090-4295(97)00238-0.
- Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549-57. https://doi.org/10.1016/S0022-5347(17)36966-5.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997;50:920-8. https://doi.org/10.1016/S0090-4295(97)00459-7.
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899-905. https://doi.org/10.1016/S0090-4295(00)00858-X.
- Roth AJ, Rosenfeld B, Kornblith AB, Gibson C, Scher HI, Curley-Smart T, et al. The Memorial Anxiety Scale for Prostate Cancer: validation of a new scale to measure anxiety in men with prostate cancer. Cancer 2003;97:2910-18. https://doi.org/10.1002/cncr.11386.
- Devlin NJ, Krabbe PF. The development of new research methods for the valuation of EQ-5D-5L. Eur J Health Econ 2013;14:1-3. https://doi.org/10.1007/s10198-013-0502-3.
- Devlin NJ, Shah K, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: Office of Health Economics; 2016.
- Ramsay C, Pickard R, Robertson C, Close A, Vale L, Armstrong N, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16410.
- NHS Reference Costs 2014 to 2015. London: Department of Health and Social Care; 2015.
- Dataset for Histopathology Reports for Prostatic Carcinoma. London: Royal College of Pathologists; 2009.
- Epstein JI, Allsbrook WC, Amin MB, Egevad LL. International Society of Urological Pathology (ISUP) Grading Committee . The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005;29:1228-42. https://doi.org/10.1097/01.pas.0000173646.99337.b1.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Chichester: Wiley-Blackwell; 2016.
- Ryan P, Finelli A, Lawrentschuk N, Fleshner N, Sweet J, Cheung C, et al. Prostatic needle biopsies following primary high intensity focused ultrasound (HIFU) therapy for prostatic adenocarcinoma: histopathological features in tumour and non-tumour tissue. J Clin Pathol 2012;65:729-34. https://doi.org/10.1136/jclinpath-2011-200460.
- Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int 2013;111:753-60. https://doi.org/10.1111/j.1464-410X.2012.11611.x.
- High-intensity Focused Ultrasound for Prostate Cancer. Interventional Procedures Guidance [IPG118]. London: National Institute for Health and Care Excellence; n.d.
- de Salis I, Tomlin Z, Toerien M, Donovan J. Qualitative research to improve RCT recruitment: issues arising in establishing research collaborations. Contemp Clin Trials 2008;29:663-70. https://doi.org/10.1016/j.cct.2008.03.003.
- Lawton J, Kirkham J, White D, Rankin D, Cooper C, Heller S. Uncovering the emotional aspects of working on a clinical trial: a qualitative study of the experiences and views of staff involved in a type 1 diabetes trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-3.
- Horwood J, Johnson E, Gooberman-Hill R. Understanding involvement in surgical orthopaedic randomized controlled trials: a qualitative study of patient and health professional views and experiences. Int J Orthop Trauma Nurs 2016;20:3-12. https://doi.org/10.1016/j.ijotn.2015.05.002.
- Stein RC, Dunn JA, Bartlett JM, Campbell AF, Marshall A, Hall P, et al. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20100.
- Langley C, Gray S, Selley S, Bowie C, Price C. Clinicians’ attitudes to recruitment to randomised trials in cancer care: a qualitative study. J Health Serv Res Policy 2000;5:164-9. https://doi.org/10.1177/135581960000500307.
- Lawton J, Snowdon C, Morrow S, Norman JE, Denison FC, Hallowell N. Recruiting and consenting into a peripartum trial in an emergency setting: a qualitative study of the experiences and views of women and healthcare professionals. Trials 2016;17. https://doi.org/10.1186/s13063-016-1323-3.
- Yap T, Ahmed HU, Hindley RG, Guillaumier S, McCartan N, Dickinson L, et al. The effects of focal therapy for prostate cancer on sexual function: a combined analysis of three prospective trials. Eur Urol 2016;69:844-51. https://doi.org/10.1016/j.eururo.2015.10.030.
- Hatiboglu G, Popeneciu IV, Deppert M, Nyarangi-Dix J, Hadaschik B, Hohenfellner M, et al. Quality of life and functional outcome after infravesical desobstruction and HIFU treatment for localized prostate cancer. BMC Urol 2017;17. https://doi.org/10.1186/s12894-017-0198-2.
- Oxley J, Varma M, Berney D. Dataset for Histopathology Reports for Prostatic Carcinoma. London: Royal College of Pathologists; 2016.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Briel M, Olu KK, von Elm E, Kasenda B, Alturki R, Agarwal A, et al. A systematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol 2016;80:8-15. https://doi.org/10.1016/j.jclinepi.2016.07.016.
- Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-323.
- Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. Grading Committee . The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016;40:244-52. https://doi.org/10.1097/PAS.0000000000000530.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Chichester: Wiley-Blackwell; 2017.
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. Basel: Springer International Publishing; 2017.
Appendix 1 Trial flow diagram
FIGURE 34.
Trial flow diagram.

Appendix 2 Trial visit schedule
| Task | Visits | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 (procedure) | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Day/month | |||||||||||||
| Day 0 | Days 7–14 | 6 weeks | 3 months | 6 months | 9 months | 12 months | 18 months | 24 months | 30 months | 36 months | |||
| Give patient PART trial PIL | ✗ | ||||||||||||
| Give patient QRI PIL and consent form | ✗ | ||||||||||||
| Add patient to screening log | ✗ | ||||||||||||
| Targeted biopsy (if needed) | ✗ | ||||||||||||
| PART trial PIL and consent form given again (if necessary) | ✗ | ||||||||||||
| Informed consent | ✗ | ||||||||||||
| Inclusion criteria | ✗ | ||||||||||||
| Exclusion criteria | ✗ | ||||||||||||
| Patient history | ✗ | ||||||||||||
| Examination | ✗ | ||||||||||||
| Routine blood tests | ✗ | ||||||||||||
| PSA blood test | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Randomisation | ✗ | ||||||||||||
| Screening log updated | ✗ | ||||||||||||
| PA (arm 1) or RP (arm 2) | ✗ | ||||||||||||
| Catheter removal | ✗ | ||||||||||||
| mpMRI (HIFU only) | ✗a | ✗ | ✗ | ||||||||||
| Biopsy (HIFU only) | ✗ | ✗ | |||||||||||
| AE reporting | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| IIEF-15 questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| IPSS questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| EPIC questionnaire | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| EQ-5D-5L | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| FACT-P version 4 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| MAX-PC (18 terms) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Resource utilisation questionnaire (patient diary) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
Glossary
- Active monitoring/active surveillance
- Protocols that involve regular clinical examination, imaging in the form of multiparametric magnetic resonance imaging, prostate-specific antigen measurements and repeat biopsies. Men in whom these parameters suggest a risk of progression are offered radical treatment.
- Focal therapy
- A general term for a variety of non-invasive techniques for destroying small tumours inside the prostate while leaving the remaining gland intact and sparing most of its normal tissue.
- Gleason grade
- When cells are seen under the microscope, they have different glandular patterns, depending on how qucikly they are likely to grow. This pattern is given a grade (called the Gleason grade) from 1 to 5: the higher the grade, the more likely the cancer is to spread outside the prostate.
- Gleason score
- Obtained by adding together two Gleason grades. Different biopsy samples may have a different Gleason grade. This score is calculated by adding the grade that is most commonly found in the samples and the second most prevalent grade seen in the tissue examined.
- Minimally invasive technique
- A type of treatment intended to minimise the effects on non-cancerous tissue. A variety of energy sources and advanced imaging technology are used to ablate prostate tissue and, together with the use of protective devices, minimise treatment-related morbidity.
- Partial ablation
- Treatment that destroys only the area of the prostate affected by cancer, preserving the rest of the gland. Techniques used include high-intensity focused ultrasound and cryotherapy.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AM
- active monitoring
- CI
- chief investigator
- CRF
- case report form
- EBRT
- external beam radiotherapy
- EPIC
- Expanded Prostate Cancer Index Composite
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-VAS
- EuroQol Visual Analogue Scale
- ESUR
- European Society of Urogenital Radiology
- ETC
- excess treatment cost
- FACT-P
- Functional Assessment of Cancer Therapy – Prostate
- GP
- general practitioner
- HIFU
- high-intensity focused ultrasound
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICF
- informed consent form
- ICU
- intensive care unit
- IIEF-15
- International Index of Erectile Function – 15 items
- IMRT
- intensity-modulated radiation therapy
- IPSS
- International Prostate Symptom Score
- IQR
- interquartile range
- ISUP
- International Society of Urological Pathology
- ITT
- intention to treat
- MAX-PC
- Memorial Anxiety Scale for Prostate Cancer
- MDT
- multidisciplinary team
- MII
- minimally invasive intervention
- MMRM
- mixed model for repeated measures
- mpMRI
- multiparametric magnetic resonance imaging
- MRI
- magnetic resonance imaging
- NCRI CM-Path
- National Cancer Research Institute – Cellular Molecular Pathology
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OCTRU
- Oxford Clinical Trials Research Unit
- OPCSG
- Oxfordshire Prostate Cancer Support Group
- PA
- partial ablation
- PART
- Partial prostate Ablation versus Radical prosTatectomy
- PCa
- prostate cancer
- PI
- principal investigator
- PIL
- patient information leaflet
- PI-RADS
- Prostate Imaging and Data Reporting System
- PPA
- per-protocol analysis
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PROMIS
- PROstate MRI Imaging Study
- ProtecT
- Prostate testing for cancer and Treatment
- PSA
- prostate-specific antigen
- pT
- primary tumour
- QA
- quality assurance
- QALY
- quality-adjusted life-year
- QC
- quality control
- QoL
- quality of life
- QRI
- QuinteT Recruitment Intervention
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RITA
- radiofrequency interstitial tumour ablation
- RP
- radical prostatectomy
- SAE
- serious adverse event
- SOP
- standard operating procedure
- TMG
- Trial Management Group
- TNM
- tumour, node, metastasis
- TRUS
- transrectal ultrasound
- TSC
- Trial Steering Committee
- TSI
- trial-specific instruction
- UCH
- University College Hospital
- VTP
- vascular-targeted photodynamic therapy
