Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 16/30/01. The protocol was agreed in January 2017. The assessment report began editorial review in August 2017 and was accepted for publication in March 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Peron et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
In 2014, 3224 people were diagnosed with cervical cancer in the UK, making it the 12th most common cancer in women, and 890 people died as a result of the disease. 1 More than 80% of people diagnosed with cervical cancer in England and Wales will survive for ≥ 1 year and almost 65% will survive for ≥ 10 years after their diagnosis. 1 The mortality rate is low because of the NHS Cervical Screening Programme (NHSCSP), and because cervical cancer is preventable if detected in its early stages. 2 However, mortality rates are higher for those living in the most deprived areas.
Detectable changes in the cervix develop many years before progressing to cancer. The cells lining the surface of the cervix may go through a series of changes called cervical intraepithelial neoplasia (CIN). The neoplasia is often harmless and may resolve without intervention; however, sometimes these changes can become cancerous. 3
Cervical intraepithelial neoplasia is classified as CIN 1, 2 or 3, depending on the depth of abnormal cells within the surface layer of the cervix observed on a diagnostic or excisional (treatment) biopsy:
-
CIN 1 – one-third of the thickness of the surface layer of the cervix is affected.
-
CIN 2 – two-thirds of the thickness of the surface layer of the cervix is affected.
-
CIN 3 – the full thickness of the surface layer of the cervix is affected.
Cervical intraepithelial neoplasia 1 is associated with benign viral replication, and in most cases will regress spontaneously. 4 CIN 3 is considered to be precancerous with the potential to progress to invasive cancer. 5 CIN 2 is also generally considered to be, and managed as, precancerous, although the average regression rate of CIN 2 to normal/negative high-risk human papillomavirus (hrHPV) in adult people is significant, with estimates of 21% over 12 months in a pooled analysis of three studies,6 and approximately 40% regression over 2 years in a large US trial. 7
Cervical cancer typically develops from precancerous changes over a period of 10–20 years. The most common types of cervical cancer cases are squamous cell carcinomas (approximately 90%) and adenocarcinomas. 8
One of the strongest risk factors for cervical cancer is hrHPV infections. There are around 13 types of hrHPV. 9,10 Of those, human papillomavirus (HPV) 16 and HPV 18 are associated with changes in the cervical cells leading to abnormalities (precancerous changes or CIN), which can progress into cervical cancer (around 70% of patients in the UK). However, most HPV infections will not progress to CIN, as the virus is usually cleared without any treatment. 11 Certain risk factors are associated with the progression of HPV infection to CIN, in particular the HPV genotype, smoking, other sexually transmitted infections, early age at first intercourse and a large number of different sexual partners. 12
There is evidence to suggest that cellular changes caused by HPV 16 may be more apparent on colposcopy examination than cellular changes caused by other hrHPV genotypes. 13 Therefore, the accuracy of colposcopy and the adjunctive technologies may differ in these subgroups.
Current service provision and care pathways
In England, women aged 25–49 years are offered screening every 3 years, and women aged 50–64 years are offered screening every 5 years under the NHSCSP. 14,15 Women are referred for colposcopy if cytological testing is abnormal or they have symptoms that are suggestive of cervical cancer.
Human papillomavirus immunisation
Since September 2008, all girls aged 12–13 years have been offered a HPV vaccination against the HPV 16 and 18 genotypes (a catch-up programme was initially implemented for girls aged between 14 and 18 years). 14 This cohort is now entering the NHSCSP but may not be fully protected against HPV 16 and 18. The relative sizes of subgroups with HPV 16 and 18 may change in the future, as people who are vaccinated enter the NHSCSP.
The full impact of HPV vaccination on the screening programme is therefore not fully understood at present, and the prevalence of disease is likely to change over time, as partially vaccinated and fully vaccinated cohorts enter screening and colposcopy services.
As HPV immunisation is new, very few immunised people will have entered the cervical screening programme or will have developed CIN or cervical cancer.
Cervical screening
Cervical screening is conducted by taking a sample of cells brushed from the cervix (liquid-based cytology). 14 These cells are tested for possible changes that may or may not develop into cancer. Cytological assessment is performed to detect nuclear abnormalities, referred to as dyskaryosis, which is graded on the basis of its severity. 15 Grading systems for cervical cytology differ by country, and the current system used in the NHS is shown in Smith and Patrick16 and Solomon and Nayar. 17
In 2015–16, a total of 4.21 million people aged 25–64 years were invited for screening, of whom 3.1 million (around 73%) attended, leading to the examination of 3.25 million samples. 18 Among those with an adequate test, a negative result was recorded in 94.5% and an abnormal result (from borderline change through to potential cervical cancer) in was recorded 5.5%; in 1.1% of those tested, a high-grade abnormality was detected.
High-risk human papillomavirus triage
The current HPV triage management protocols for cervical cytology and management options for patients are outlined in Table 1. Under the hrHPV triage protocol, people whose cervical samples show borderline change or low-grade dyskaryosis (abnormal cell changes) are given a reflex hrHPV test. If the test is HPV positive, the person will be invited to attend a colposcopy clinic. If the test is HPV negative, they will be returned to routine screening. People with high-grade dyskaryosis or worse are referred straight to colposcopy without a hrHPV test. 15 National implementation of hrHPV triage for people with borderline or low-grade cytology results and hrHPV test of cure was completed in 2013. From 1 April 2014, hrHPV triage has been implemented across England. 19
| Result | Management recommendation |
|---|---|
| Inadequate: insufficient cells were available for analysis | Repeat in 3 months, refer to colposcopy after three consecutive inadequate samples |
| Negative: adequate sample with no abnormal cells | Return to routine recall (3 or 5 years depending on age) |
| Borderline change in squamous cells |
Test residual sample for hrHPV: hrHPV detected – refer for colposcopy hrHPV not detected – return for routine recall |
| Borderline change in endocervical cells | |
| LG dyskaryosis | |
| HG dyskaryosis (moderate) | Refer for colposcopy |
| HG dyskaryosis (severe) | |
| HG dyskaryosis/suspected invasive squamous carcinoma | |
| Suspected glandular neoplasia of endocervical type | |
| Suspected glandular neoplasia (non-cervical) | Refer to gynaecology |
Human papillomavirus primary screening
Following the piloting of HPV primary screening, which commenced in six sites in England in 2013–14,20 the Department of Health and Social Care announced a change to the cervical screening process in July 2016. 21 In several sites in England where HPV primary screening was piloted, it has now been adopted as the standard of care.
In HPV primary screening, a cervical cytology sample is first tested for the presence of hrHPV, prior to cytology triage. The algorithm for the HPV primary screening pilots is shown in Figure 1. In general, primary screening with hrHPV testing detects over 90% of all cases of CIN 2, CIN 3 and invasive cancer. It is reported as being 25% more sensitive than liquid-based cytology in detecting borderline changes or worse, although it is approximately 6% less specific. 23
FIGURE 1.
The HPV primary screening algorithm (pilot sites). Adapted from: Public Health England. 22 Contains public sector information licensed under the Open Government Licence v3.0. See www.nationalarchives.gov.uk/doc/open-government-licence/version/3/. © Crown copyright, 2015.
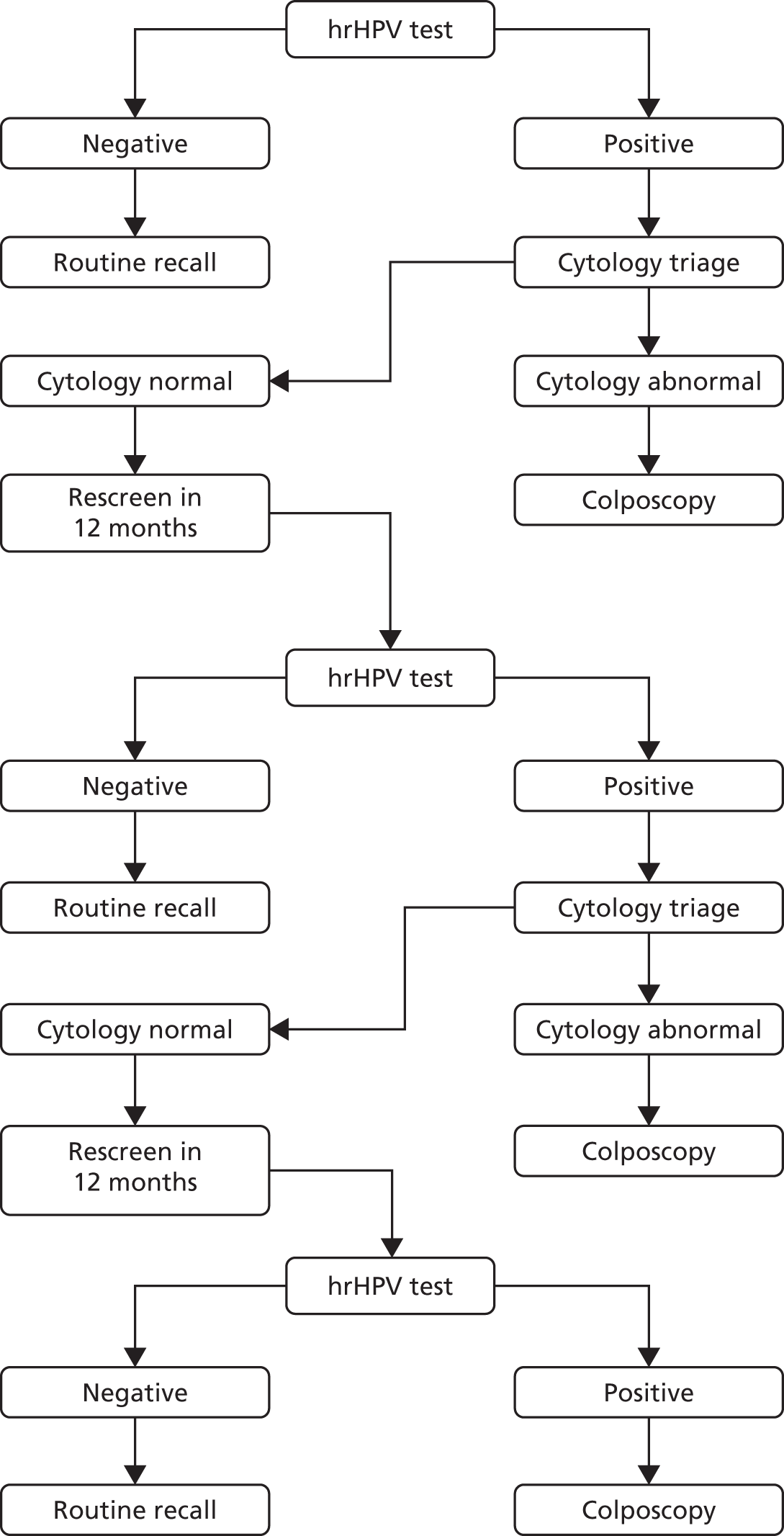
When genotyping tests are used, people testing HPV 16 or 18 positive and cytology normal at baseline and at their first 12-month follow-up test can be referred to colposcopy without further repeat tests.
The patient group of interest for this assessment is people referred for colposcopy through the NHSCSP under the HPV triage screening algorithm (with test of cure) or the HPV primary screening algorithm, as currently recommended for use in pilot sites (with test of cure). People referred because of symptoms indicative of cervical cancer (e.g. postcoital bleeding or appearance suggestive of cancer) are not of relevance to this assessment.
Colposcopy management and treatment
Standard binocular colposcopy, with directed biopsy/treatment when necessary, is the current usual management for people referred with abnormal cytology results. The colposcopist applies solutions such as acetic acid or Lugol’s iodine to the surface of the cervix. These help to highlight any areas of abnormality on the cervical epithelium. Video colposcopy may also be used, particularly for the Dynamic Spectral Imaging System [(DySIS) DySIS Medical Ltd, Edinburgh, UK] when the DySISmap is overlaid onto a video colposcopic image and no separate binocular colposcopy will be performed.
Colposcopy involves a significant amount of subjective assessment, and the final histological diagnosis depends on training, experience and the number of patients seen, as well as the ability of the colposcopist to identify the most appropriate sites for biopsies. 24–26 Details of the referral cytology results, HPV status, other clinical information, the type of management available and the number of biopsies taken may also be relevant when interpreting the results of colposcopy.
The 2015 NHSCSP publication19 recommends that, when a successful colposcopy has been performed, the positive predictive value (PPV) to detect high-grade lesions [CIN grade 2+ (CIN 2+)] should be at least 65%. It also recommends that treatment at first visit to colposcopy should not be offered to patients referred with borderline or low-grade dyskaryosis. It also recommends that, unless an excision is planned, a diagnostic biopsy should be performed when cytology results indicate high-grade dyskaryosis (moderate) or worse, and always when a recognisably atypical transformation zone is observed. In some circumstances, such as the presence of low-grade colposcopic change and high-grade dyskaryosis (severe), an excisional form of biopsy (rather than punch biopsy) is recommended.
The results of biopsies are used to guide treatment decisions. Typically, areas of CIN 2 or worse would usually be treated, although CIN 2 may be managed more conservatively if only part of the transformation zone is affected, and in younger women who have not completed their family. Treatment options during the colposcopy examination include excising the area of abnormal cells. If an abnormality is detected during the colposcopy examination, the colposcopist may treat an abnormality during the first clinic appointment (‘see and treat’) by excising the area of abnormal cells in which high-grade changes are suspected or, in rarer cases, by destroying them in situ (ablation). 15
The aim of excision is to remove all abnormal tissue. Excision is usually performed with a thin, electrically heated looped wire in a procedure called a large-loop excision of the transformation zone (LLETZ) under local anaesthesia. The excised tissue is sent to histopathology to confirm the extent of the abnormality and to inform further management. For some patients, notably when glandular abnormalities are present (Cervical Glandular Intraepithelial Neoplasia), a deeper excision (cone biopsy) is required, which is likely to be performed under general anaesthesia. The depth of the excision depends on the nature of the cervical transformation zone. 15
A number of ablative techniques exist, including laser ablation, cryocautery and cold coagulation. The NHSCSP publication21 recommends that ablative treatments are performed only when the entire transformation zone is visible, there is no evidence of glandular abnormality or invasive disease and there is no major discrepancy between cytology and histology.
If cervical cancer is identified, treatment options include cone biopsy (at a very early stage), trachelectomy, hysterectomy, radiotherapy and chemotherapy. Conservative treatment may also be offered. Further details are reported elsewhere. 27
NHS colposcopy and treatment
In 2015–16, 188,179 patients were referred for colposcopy: 65.6% as a result of screening, 23.1% were clinically indicated and 11.3% for other reasons (e.g. CIN treatment follow-up). 18 In the same period, 61% of all people referred to receive colposcopy in England underwent a procedure or treatment at their first appointment. Diagnostic biopsy was the most common procedure (47%), followed by an excision (12%). Only a small percentage underwent ablation (0.6%).
Treatment patterns vary significantly at the local and regional levels. In 2015–16, the percentage of all women receiving some treatment or procedure in England at their first appointment ranged from 53.5% in the North West of England to 70.5% in the North East of England. 18 Among those with high-grade abnormalities, the percentage who received a diagnostic biopsy ranged from 21.7% in the West Midlands to 71.1% in London; for low-grade abnormalities, the rates ranged from 51.6% in the East to 80.9% in the North East. The percentage of patients with high-grade abnormalities who underwent excision ranged from 11.6% in London to 65.4% in the North West. However, it is likely that most people presenting with high-grade abnormalities and reported as having either no treatment or a diagnostic biopsy at their first attendance went on to receive therapeutic treatment at a subsequent appointment.
Follow-up and test of cure
Post-colposcopy follow-up depends on whether treatment has been performed or surveillance has been recommended. Surveillance can be done within the colposcopy service or within the community.
The NHSCSP publication21 recommends that people referred with low-grade dyskaryosis or lower who are hrHPV positive and have a satisfactory and normal colposcopic examination can be returned to community-based recall. 15 People with a low-grade lesion based on colposcopy may be followed up at 12 months in the colposcopy clinic or the community. If the lesion has not resolved within 2 years of referral to colposcopy, a biopsy should be performed. For people referred with high-grade dyskaryosis who do not have treatment, surveillance with colposcopy and cytology at 6 months is recommended, even if no abnormality is seen with colposcopy. For patients who are not treated following a colposcopic diagnosis of a low-grade lesion, multiple directed biopsies should be performed. Treatment is recommended for people with high-grade cytology at follow-up.
When CIN 1 or lower is confirmed, colposcopy and cytology at 6 months is recommended. Follow-up for people referred under the HPV primary screening pilot algorithm is described in more detail elsewhere. 28
Under the hrHPV ‘test-of-cure protocol’, patients who have previously received treatment for CIN (all grades) are invited for screening 6 months after treatment for a repeat cervical sample in the community. Under HPV triage, a woman whose sample is reported as negative, borderline change or low-grade dyskaryosis is given a hrHPV test. If the HPV test result is negative, the woman is recalled for a screening test in 3 years (irrespective of age) and can be returned to routine recall if the subsequent cytology test result is negative. hrHPV-positive patients are referred back to colposcopy. People whose cytology is reported as high-grade dyskaryosis or worse are referred straight to colposcopy without a hrHPV test. 15 Under HPV primary screening, the test of cure differs and is described in the NHS Cancer Screening Programme pilot. 28
During 2015–16 in England, a total of 433,624 appointments were reported at colposcopy clinics, of which 163,859 (37.8%) were follow-ups.
Current service cost
Currently, the NHS spends around £21M per year on treating cervical cancer, mostly in women diagnosed at stage 2 (the cancer has grown beyond the cervix and uterus, but has not spread to the walls of the pelvis or the lower part of the vagina) or above. 29
Description of the technologies under assessment
Following a previous diagnostic assessment report (DG4),30 National Institute for Health and Care Excellence (NICE) diagnostics guidance (DG4)31 recommended using DySIS as an adjunct to colposcopy. ZedScan (Zilico Limited, Manchester, UK), previously known as APX100, was not included in the final guidance, as it had not received its Conformité Européenne (CE) mark prior to publication. Both DySIS and ZedScan are now being used in several hospitals in England and Wales.
Dynamic Spectral Imaging System with Dynamic Spectral Imaging System map (Dynamic Spectral Imaging System Medical)
Dynamic Spectral Imaging System is a high-resolution digital video colposcope. It also uses spectral imaging technology and an inbuilt algorithm to produce an adjunctive map of the cervical epithelium, which is known as the DySISmap (or pseudo-colour imaging). The DySISmap is intended to be used as an adjunct to colposcopy to assist clinicians in the diagnosis, biopsy and treatment of CIN.
The DySISmap maps the whitening effect following the application of acetic acid (acetowhitening) to the epithelium of the cervix in order to aid diagnosis, as well as selecting areas for biopsy and treatment. It does this by producing a quantitative measurement of the rate, extent and duration of acetowhitening, which is highly correlated with the altered structure and functionality of abnormal epithelial cells of the cervix. The DySISmap is produced during the period of the acetowhitening reaction. An inbuilt algorithm assigns each area of the cervix a colour on the DySISmap that corresponds to the likelihood of an abnormality being present. The DySISmap is displayed on the screen, overlaid on a live image of the cervix. The colour spectrum ranges from cyan, which represents weak acetowhitening, to white, which represents intense acetowhitening; the greater the intensity of the measured acetowhitening reaction, the greater the likelihood of an abnormality. Imaging typically takes 3 minutes, and the average duration of use per examination is < 15 minutes.
The manufacturer claims that new users can be trained to use DySIS in 2–4 hours (personal communication). Imaging takes 3 minutes and can be stopped manually; however, the company recommends at least 125 seconds of imaging to allow the system to calculate and display the DySIS map. 32 The list price for the latest version of DySIS (DySIS Touch colposcope) is £24,000 (personal communication). This is around twice the cost of a standard colposcope. The 5-year maintenance plan is an additional £6500, and the viewer licence is £650 in the first year and £500 per year in subsequent years. The DySIS includes a colposcope and no additional equipment is needed. The cost of specula is £3.50 per examination. 33
The DySIS is CE marked and is developed by DySIS Medical. The currently available version of DySIS is DySIS version 3, but the company intends that it will be superseded by the DySIS Touch and DySIS Ultra colposcopes in early 2017. Each updated version of the system has had modifications to both the hardware and the software, but the DySISmap algorithm has remained unchanged.
ZedScan (Zilico)
ZedScan is an electrical impedance spectroscopy (EIS) device. It is designed to be used as an adjunct to colposcopy to aid in the diagnosis, biopsy and treatment of high-grade CIN. It applies a small alternating current at different frequencies to the cells lining the cervix and measures the resulting voltage. By using EIS, one can measure the resistivity of cervical epithelial cells to distinguish between normal and abnormal tissue. Electrical impedance is measured at 14 different frequencies, producing a spectrum that varies depending on the structure and properties of the tissue. The degree of impedance is related to tissue structure, which is classed as normal, precancerous or cancerous. A handset displays a diagram of the measurement zone by coloured circles that indicate the location and results from each measurement point:34
-
clear/white – no reading
-
green – high-grade CIN is unlikely to be present
-
amber – high-grade CIN is likely to be present
-
red – the highest likelihood that high-grade CIN is present.
The results from each reading site are compared with reference spectra, derived from models of different cervical tissues, to calculate the probability of high-grade neoplasia. The device is also designed to indicate the location of high-grade CIN for biopsy. Further details on the ZedScan algorithm are reported in Appendix 1.
The manufacturer estimates that each cervical scan using the ZedScan takes 2–3 minutes. The device can also be used in a single-point mode to help to select sites for diagnostic biopsy after the initial 10–12 readings have been taken. The manufacturer states that it takes approximately 2 hours to train the new users. ZedScan is CE marked and is developed by Zilico Ltd. ZedScan was previously known as APX100, which was the name used in the previous assessment (DG4). 31 The ZedScan costs £3000, including computer software. The cost per case with the ZedScan is approximately £30 plus clinician time. There are no routine maintenance costs.
The previous assessment (DG4)30 found evidence to suggest that DySIS with DySISmap had higher sensitivity but lower specificity than colposcopy alone for detecting CIN 2+ disease, and it found limited evidence for other adjunctive technologies [LuViva Advanced Cervical Scan (developed by Guided Therapeutics, Norcross, GA, USA) and Niris Imaging System (developed by Imalux Corporation, Cleveland, OH, USA)].
Chapter 2 Definition of the decision problem
Women in England between the ages of 25 and 64 years are invited for regular cervical screening every 3–5 years in order to detect abnormal cells in the cervix. Screening is conducted using liquid-based cytology; women may also be tested for hrHPV.
Depending on the results of the cervical screening, people may be referred for a colposcopy examination. Colposcopy is largely a subjective examination, and diagnosis will partly depend on the opinion and expertise of the colposcopist. The DySIS digital video colposcope with DySISmap and the ZedScan device have been developed to be used alongside colposcopy. The aim of these adjunctive technologies is to help the colposcopist to find abnormal cells more accurately. The DySIS system provides a coloured map of the cervix on a computer screen, on which different colours represent different risks of abnormal cells. ZedScan uses an electrical current to distinguish between normal and abnormal cells, and shows coloured circles on a diagram ranging from green (low risk of abnormal cells) to red (high risk of abnormal cells).
The DySIS was previously reviewed in the DG4 assessment. 31 However, additional information on this technology, the development of ZedScan since that review and recent changes in the NHSCSP mean that the relative value of using these new tests is uncertain.
This report, undertaken for the NICE Diagnostics Assessment Programme, examines the clinical effectiveness and cost-effectiveness of DySISmap and ZedScan used adjunctively alongside regular colposcopy for women referred for colposcopy as part of the cervical cancer screening programme.
Decision problem in terms of participants, interventions, comparisons, outcomes, study design and other key issues
The primary population of interest is women referred for colposcopy as part of the NHSCSP under either:
-
the HPV triage screening algorithm (including test of cure)
-
the HPV primary screening algorithm (including test of cure).
All women who have been referred to colposcopy on the basis of a positive cytology test or because of the presence of hrHPV infection will be considered, bearing in mind that, outside the UK, the algorithms for deciding who should be referred for colposcopy may differ from those listed above.
The tests of interest are the DySISmap system, which generates a coloured map representing the level of acetowhitening of the cervix, and ZedScan, which uses EIS to detect abnormal cervical tissue. Both technologies should be used alongside standard colposcopy; DySIS video colposcopy is used with DySISmap and binocular colposcopy is used with ZedScan. The combination of tests is referred to as adjunctive colposcopy.
The key comparator of interest is standard colposcopy alone, whether using a binocular or video colposcope.
When assessing diagnostic accuracy, the accepted reference standard is histopathological diagnosis of CIN or cancer based on cells extracted from the cervix by punch biopsy or excision.
The key outcomes of interest were the diagnostic accuracy of adjunctive colposcopy (i.e. sensitivity, specificity and related measures), its broader clinical effects, ease of implementation and cost-effectiveness. Any prospective study reporting data on any of these outcomes was considered for inclusion in this review.
Overall aims and objectives of the assessment
The aim of the project was to determine the clinical effectiveness and cost-effectiveness of adjunctive colposcopy technologies (DySISmap and ZedScan) for assessing suspected cervical abnormalities in people who are referred for colposcopy through the NHSCSP under either HPV triage (including test of cure) or the HPV primary screening algorithm (including test of cure). To achieve this, the following objectives were proposed:
-
To perform a systematic review and meta-analysis of the diagnostic accuracy of adjunctive colposcopy technologies (DySISmap and ZedScan) in conjunction with standard colposcopy for the examination of the uterine cervix of the people who are referred for colposcopy.
-
To perform a systematic review of the clinical impact and implementation of adjunctive colposcopy. This would include an assessment of the associated mortality and morbidity, patient-centred outcomes, adverse events, acceptability to clinicians and patients, and compliance.
-
To perform a systematic review of published cost-effectiveness studies of adjunctive colposcopy technologies (DySISmap and ZedScan) for assessing suspected cervical abnormalities in people who are referred for colposcopy.
-
To develop a decision model to estimate the cost-effectiveness of adjunctive colposcopy technologies (DySISmap and ZedScan) for people who are referred for colposcopy through the NHSCSP under either HPV triage (including test of cure) or the HPV primary screening algorithm (including test of cure).
This report is in two parts: clinical effectiveness (covering objectives 1 and 2) is discussed in Chapter 3 and cost-effectiveness (covering objectives 3 and 4) is discussed in Chapters 4 and 5.
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
The review of clinical effectiveness of adjunctive colposcopy was broken down into the following three systematic reviews:
-
a review of the diagnostic accuracy (i.e. sensitivity/specificity and related statistics) of adjunctive colposcopy technologies (DySISmap and ZedScan) in conjunction with standard colposcopy for the examination of the uterine cervix of the people who are referred for colposcopy
-
a review of the broader clinical effects of adjunctive colposcopy technologies, including an assessment of the associated mortality and morbidity, patient-centred outcomes and adverse events
-
a review of the implementation of adjunctive colposcopy technologies, including their acceptability to patients and clinicians.
Throughout this report, diagnostic accuracy is taken to refer strictly to how well adjunctive colposcopy can diagnose CIN by distinguishing between women with CIN and women without CIN, measured using sensitivity, specificity and related statistics. Clinical effectiveness refers to the broader clinical impact that adjunctive colposcopy may have beyond altering diagnostic accuracy, such as its impact on biopsy rates, cancer rates and adverse events relating to the testing procedures. Implementation refers to practical issues relating to using the tests, such as ease of interpretation, compliance and acceptability to practitioners and patients.
The methodology of these reviews is now described.
Methodology of the clinical effectiveness review
The systematic reviews were conducted following the general principles recommended in the Centre for Reviews and Dissemination (CRD) guidance35 and the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. 36
Searches
The literature searches aimed to systematically identify research related to the clinical effectiveness of DySISmap and ZedScan.
The search strategy was developed in MEDLINE (via Ovid) and was based on the search strategy used for the previous Health Technology Assessment (HTA) review of adjunctive colposcopy by Wade et al. 30 The original strategy was checked and updated to reflect the changed scope of the current review. Updates were also necessary to account for changes to the database search interface or provider, and when new subject headings had been introduced or changed since the previous searches.
The strategy consisted of a set of terms for ‘cervix’, which were combined using the Boolean operator ‘AND’, with a set of terms for the two adjunctive colposcopy technologies. A date limit was applied to the search strategy to restrict retrieval to those studies published since 2000. No further limits relating to language or study design were applied. The MEDLINE strategy was adapted for use in all other resources searched.
The searches were carried out during January 2017, with a further updated search undertaken on 10 April 2017. The following databases were searched: MEDLINE (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Management Information Consortium, HTA Database, NHS Economic Evaluation Database (NHS EED), PubMed and Science Citation Index. In addition, ongoing studies and unpublished and grey literature were identified using the following resources: ClinicalTrials.gov, Conference Proceedings Citation Index – Science, European Union Clinical Trials Register, PROSPERO, World Health Organization (WHO)’s International Clinical Trials Registry Platform portal, technology manufacturer websites and NHS Digital data. Data were requested and obtained from the NHSCSP HPV screening pilot (sentinel sites). Data submitted to NICE by manufacturers as part of this assessment were also used. Abstracts from recent relevant conferences, including that of the British Society for Colposcopy and Cervical Pathology (BSCCP) and the International Federation for Cervical Pathology and Colposcopy, were also consulted.
Relevant guidelines were identified through searches of the following resources: NICE, NHS Evidence, National Guideline Clearinghouse, Scottish Intercollegiate Guidelines Network (SIGN), Public Health England, BSCCP, the Royal College of Obstetricians and Gynaecologists and the Turning Research Into Practice (TRIP) database. Search results were imported into EndNote X8 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated against the results from the previous 2013 HTA review of adjunctive colposcopy. 30 Full details of the search strategies can be found in Appendix 2.
Additional searches
Owing to the lack of evidence found in the review of clinical effectiveness, additional pragmatic PubMed searches were conducted to identify recent systematic reviews reporting on the adverse effects of CIN treatments on fertility, pregnancy and neonatal outcomes.
Selection criteria
Two researchers independently screened the titles and abstracts of all reports identified by the bibliographic searches and full-text papers were subsequently obtained for assessment and screened by at least two researchers. Disagreements were resolved by consensus.
Types of studies
Diagnostic accuracy
Prospective cohort studies in which the index test (DySISmap or ZedScan performed as an adjunct to colposcopy) and reference standard test (histopathology) were performed independently in the same group of participants, and which reported sufficient data to calculate diagnostic accuracy (sensitivity and specificity).
Effectiveness and implementation
Any experimental or observational study in which adjunctive DySIS and/or adjunctive ZedScan testing was used was included. As no studies included a parallel control group that underwent standard colposcopy alone, non-comparative studies that only recruited people who received adjunctive colposcopy were included.
The following types of report were excluded: editorials and opinions, case reports and reports focusing only on technical aspects of the technologies (such as technical descriptions of the testing process or specifications of machinery). When multiple reports for a particular study were identified, all studies were included, with the most recent or the most complete report included as the main study selected for inclusion. The authors of studies were contacted in cases in which it was unclear which was the most appropriate paper for inclusion.
Participants
Eligible studies included participants who were referred to colposcopy through a cervical screening programme as a result of a suspected abnormality identified via liquid-based cytology, Pap smear test or positive hrHPV test. People referred for colposcopy as a follow-up after a previous CIN diagnosis (including test of cure) were also eligible for inclusion.
Intervention
DySISmap (DySIS Medical) or ZedScan (Zilico Ltd) as an adjunct to binocular or video colposcopy used for the diagnosis of CIN or cervical cancer was the intervention of interest. Studies on all versions of these tools (including prototypes) were considered for inclusion.
Comparators
Standard colposcopy was the comparator of interest; however, data from standard colposcopy alone did not need to be reported in order for a paper to be eligible. Both binocular and video colposcopy were included.
Reference standard
The reference standard was histopathology based on excisional or treatment biopsies, used to classify samples into three CIN grades or cervical cancer. Studies that did not perform biopsies to confirm the absence of disease when colposcopic examination did not reveal any abnormalities were included.
Outcomes
The following outcomes were eligible for study inclusion:
-
diagnostic accuracy – including sensitivity and specificity, or sufficient data to calculate these
-
test failure rates (and reasons for test failure)
-
number of biopsies (and type) performed
-
diagnostic results of biopsies
-
number of treatments and treatment type
-
number of see-and-treat procedures
-
duration of colposcopy examination
-
number of people discharged from colposcopy.
Eligibility depended on the study reporting results from both the index test and the reference standard. Only studies that reported results in terms of graded CIN, differentiating between mild dysplasia or lower-grade dysplasia (≤ CIN 1, i.e. negative diagnostic result) and moderate dysplasia or worse (CIN 2 or higher-grade dysplasia, i.e. positive diagnostic result) were included.
The following clinical outcomes were also eligible for study inclusion:
-
morbidity and mortality associated with treatment and biopsies conducted as part of the colposcopy examination (including subsequent obstetric outcomes, such as miscarriage and infertility)
-
morbidity and mortality associated with cervical cancer (in studies of DySIS and ZedScan)
-
health-related quality of life (HRQoL)
-
pain and anxiety associated with colposcopic examination, biopsies, treatment and waiting for results
-
any other adverse event that may have an impact on resource use or quality of life (e.g. infection, infertility, miscarriage).
Outcomes related to the implementation of the interventions of interest and related practical issues were eligible for study inclusion:
-
acceptability of the adjunctive technologies (to clinicians and patients)
-
patient satisfaction
-
successful database and record management
-
training requirements
-
capacity to perform colposcopies
-
uptake and compliance.
Data extraction
A standardised data extraction form was designed, piloted and finalised to extract data relating to study design, patient characteristics, index, comparator and reference standard tests. Outcome data were extracted by one reviewer and the extracted data were independently checked for accuracy by at least one other reviewer. Duplicate data extraction was not performed. Disagreements were resolved through discussion until consensus was achieved, or with the involvement of an additional reviewer if necessary.
For studies reporting diagnostic accuracy data, the numbers of true-positive, true-negative, false-positive and false-negative results for each index test evaluated in each study were extracted to construct 2 × 2 tables. Otherwise, we calculated the number of true-positive, true-negative, false-positive and false-negative results from the summary estimates of sensitivity and specificity of the index test, if available. It should be noted that these values assume that the reference standard test (biopsy or excision) is assumed to be 100% accurate when extracting these data. This is not necessarily the case. In particular, cases of CIN 2 may be missed in women who do not receive any biopsy after a negative colposcopy result, and punch biopsies may not be as accurate as deeper excisions. 37
When available, the number of patients in the diagnostic categories (normal, CIN 1, CIN 2, CIN 3 or cancer) was also extracted. When only a subgroup of patients included in a study was eligible, we extracted, analysed and presented data for this subgroup only. Manufacturers and corresponding authors were contacted for all included studies to obtain additional data on diagnostic accuracy.
Diagnostic accuracy data were extracted using Microsoft Excel® software (Microsoft Corporation, Redmond, WA, USA). Data on study characteristics and results informing the reviews of clinical effectiveness and implementation were extracted using EPPI-Reviewer (Evidence for Policy and Practice Information and Co-ordinating Centre, Social Science Research Unit, Institute of Education, University of London, London, UK).
Additional data from manufacturers and study authors
For all studies, additional data on diagnostic accuracy were requested. Requests were made to the device manufacturers (DySIS Medical or Zilico) for studies in which they had direct involvement, or to the first author of the primary publication for those manufacturers that were not involved in the study.
Diagnostic accuracy data for both colposcopy and adjunctive colposcopy (with either DySISmap or ZedScan) were requested as a 5 × 5 table, with the results categorised as < CIN 1, CIN 1, CIN 2, CIN 3 and cancer. Also requested were 2 × 2 tables of diagnostic accuracy in the following participant subgroups:
-
participants with hrHPV infection (HPV 16 or 18)
-
participants with low-risk HPV or no HPV infection
-
participants referred to colposcopy with high-grade dyskaryosis or worse
-
participants referred to colposcopy with low-grade dyskaryosis or lower
-
participants with a previous history of CIN or cervical cancer (including test of cure).
Critical appraisal
Risk-of-bias assessments of all included studies included in the diagnostic accuracy review were performed using a modified version of the Quality Assessment tool of Diagnostic Accuracy Studies (QUADAS)-2 checklist. The modified version of the QUADAS-2 tool used in Wade et al. ,30 and further described elsewhere,38 to assess the risk of bias in comparative diagnostic accuracy studies (i.e. a comparison of the index test with both standard care and the gold standard) was used. Further questions were added to inform judgements about study quality in the following domains: index/comparator test, flow and timing, and other concerns. The quality of survey studies included in the implementation review was assessed using guidance from Burns et al. 39 and the Center for Evidence-Based Management. 40 Further details are presented in Appendix 3. Owing to the limited evidence, the quality of studies included in the clinical effectiveness review was not formally assessed.
The risk-of-bias assessments were performed by one reviewer and independently checked by a second reviewer. Disagreements were resolved through consensus and, if necessary, a third reviewer was consulted.
Methods of data synthesis
Statistical analyses
Estimates of sensitivity and specificity were calculated using diagnostic accuracy data from the constructed 2 × 2 tables or the 5 × 5 tables supplied by manufacturers, and presented both as forest plots and in the receiver operating characteristic (ROC) space to examine the within- and between-study variability of diagnostic test accuracy. PPVs and negative predictive values (NPVs) were also calculated, as were diagnostic odds ratios (DORs).
When equivalent clinical thresholds were used to diagnose CIN/cancer in three or more studies, the hierarchical bivariate model described by Reitsma et al. 41 was fitted, providing summary estimates of sensitivity and specificity, and associated 95% confidence intervals (CIs). The hierarchical summary ROC model42 was also fitted to provide summary ROC curves. As the bivariate model does not account for the fact that different diagnostic tests may be performed in the same study, a further logistic regression analysis43 was performed to meta-analyse sensitivity and specificity, accounting for the fact that standard colposcopy and adjunctive colposcopy were performed on the same participants.
Unless otherwise specified, all analyses used the threshold of CIN 2 or higher as the cut-off point for defining a positive diagnostic test. Data on other thresholds were generally too limited for analyses to be performed, and CIN 2 or higher is the standard threshold used in colposcopy in the NHS.
All statistical analyses assume that standard colposcopy and adjunctive colposcopy (DySISmap or ZedScan) are performed independently. This is not the case in practice, as the tests are performed together. It is therefore possible that knowledge of one test could bias interpretation of the other. For example, because it is known that a ‘second look’ will happen after standard colposcopy, the results may not be the same as if standard colposcopy had been performed without access to adjunctive tests. As this applies equally to all studies, it was not possible to investigate this possible bias further.
If at least two studies reported on the same clinical or implementation outcome, the results were pooled if reporting was consistent enough for feasible analysis; otherwise, the results were synthesised narratively. Meta-analyses were performed using standard random-effects DerSimonian and Laird methods.
Analyses were conducted in the R software package (The R Foundation for Statistical Computing, Vienna, Austria).
Investigation of heterogeneity and subgroup analyses
A visual inspection of forest plots and ROC space was performed to check for between-study heterogeneity of the diagnostic accuracy results. Sources of heterogeneity were investigated by performing meta-analyses of diagnostic accuracy within defined study subgroups and, when there were sufficient studies, by incorporating covariates in the logistic regression models of diagnostic accuracy. Heterogeneity was assessed using the I2-statistic and through visual inspection of forest plots. Subgroup analyses and metaregression were used when feasible. The following potential sources of heterogeneity were accounted for in the interpretation of the results:
-
presence of the hrHPV genotype, stratified by HPV 16, other hrHPV infection and no hrHPV infection
-
cytology results, stratified by low-grade dyskaryosis or lower and high-grade dyskaryosis (moderate) or worse
-
people with a previous diagnosis or history of CIN or cervical cancer.
Sensitivity analyses
Study quality based on the QUADAS-2 domain results was planned as a basis for conducting sensitivity analyses for diagnostic accuracy studies. This involved the exclusion of studies that were thought to be rated as having a high risk of bias in each particular domain, using this to explore the robustness of results. Results from the Cochrane risk-of-bias tool44 and study date (reflecting improvements in technology) were also used as a basis for the analyses.
The impact of excluding studies that performed biopsies only in patients with suspected high-grade lesions (rather than in all patients) was explored. Studies that were suspected of recruiting a substantial proportion of their population from another study cohort were excluded from the analysis to examine the effect of overlap on outcomes. Only the study with the most reliable or complete reporting was included in the main analyses.
Narrative and qualitative syntheses
Qualitative synthesis was performed for outcomes pertaining to implementation. Summary information relating to implementation outcomes, the conclusions of these studies, the consequences of colposcopy, recommendations for practice and suggested needs for further research were tabulated and summarised.
Narrative summaries were also performed for outcomes for which meta-analyses or other statistical analyses were not deemed feasible. This included tabulation or plotting of results as reported, which were then narratively described and compared.
Summary of clinical effectiveness evidence
This chapter is structured as follows. The next section provides information on the quantity of research available, including characteristics and the risk of bias of the included studies. This is then followed by the results sections with the diagnostic accuracy, clinical effectiveness and implementation of DySISmap and ZedScan as adjunctive technologies presented separately.
Number of studies included
The literature searches of bibliographic databases identified 3617 references. After the initial screening of titles and abstracts, 179 of these were considered to be potentially relevant and were ordered for full-paper screening. In total, 11 studies were included in the diagnostic review, three studies were included in the clinical effectiveness review and five studies were included in the review of implementation (from a total of 73 reports). Figure 2 shows a flow diagram outlining the screening process with reasons for the exclusion of full-text papers.
FIGURE 2.
Flow diagram: study selection process.

Most studies were reported in several papers and abstracts, with considerable overlaps in data and reporting. For each study and each review, we selected the paper with the most up-to-date and complete data, which was then treated as the main paper. Consequently, some papers were included in more than one review, and some papers (mostly conference abstracts with limited or outdated data) were not included in any analysis. Table 2 presents an overview of these studies, their included studies and how papers were included in each review.
| Study (country) | Number of full-text papers | Number of conference abstracts | References included in the review | |||
|---|---|---|---|---|---|---|
| Diagnostic accuracy (full/main paper) | Clinical effectiveness (full/main paper) | Implementation (full/main paper) | Linked conference abstracts | |||
| Budithi et al.45 (Wales) | 1 | 4 | Budithi et al.45 | None | Budithi et al.46 | Budithi et al.;47 Budithi et al.;48 and Budithi et al.49 |
| Coronado and Fasero50 (Spain) | 2 | 2 | Coronado and Fasero50 | None | Coronado and Fasero51 | Coronado et al.;52 and Coronado et al.53 |
| Founta et al.54 (England) | 1 | 5 | Founta et al.54 | None | None | Founta et al.;55 Founta et al.;56 Founta et al.;57 Founta et al.;58 and Founta et al.59 |
| Louwers et al.60 (the Netherlands) | 5 | 9 | Louwers et al.;60 Louwers et al.;61 Zaal et al.;62 and personal communication (A Zaal, University Medical Centre Utrecht, 2017) | Louwers et al.60 | Louwers et al.63 | Louwers et al.;64 Louwers;65 Louwers et al.;66 Louwers et al.;67 Louwers et al.;60 Louwers et al.;68 Zaal et al.;69 Louwers et al.;70 and Louwers et al.71 |
| Lowe et al.72 (England) | 0 | 3 | None | None | Lowe (2016)72 | Lowe et al.;72 and Brady et al.73 |
| Natsis et al.74 (England) | 0 | 5 | None | None | None | Natsis et al.;74 Founta et al.;75 Founta et al.;76 Founta et al.;77 and Natsis et al.78 |
| Roensbo et al.79 (Denmark) | 1 | 0 | Roensbo et al.79 | None | None | None |
| Salter and Livingston80 (USA) | 0 | 8 | None | None | None | Salter and Livingston;80 Salter et al.;81 Livingston and Salter;82 Papagiannakis et al.;83 Livingston and Papagiannakis;84 Weinberg et al.;85 Cholkeri-Singh et al.;86 and DySIS Medical87 |
| Soutter et al.88 (England) | 1 | 5 | Soutter et al.88 | Soutter et al.88 | None | Soutter et al.;89 Balas et al.;90 Soutter et al.;91 Soutter et al.;92 and Soutter et al.93 |
| Tidy et al.94 (England) | 4 | 5 | Tidy et al.;94 Macdonald et al.;95 Palmer et al.;96 and Zilico et al.97 | None | Palmer et al.96 | Tidy et al.;98 Macdonald et al.;99 Tidy et al.;100 Tidy et al.;101 and Tidy et al.102 |
| Tidy et al.103 (England and Ireland) | 2 | 7 | Tidy et al.;103 and (confidential information has been removed)104 | Tidy et al.103 | None | Tidy et al.;105 Tidy et al.;106 Tidy et al.;106 Tidy and Brown;107 Tidy et al.;108 Tidy et al.;109 and Tidy et al.110 |
| Tsetsa et al.111 (Greece) | 0 | 3 | None | None | None | Tsetsa et al.;111 Tsetsa et al.;112 and Tsetsa et al.113 |
Excluded studies
A list of full-text papers that were excluded, along with the reasons for their exclusion, is available on request. These papers were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study, participants, test, reference standard or outcomes reported. This includes four studies of EIS for the diagnosis of CIN,114–117 which were identified by the bibliographic database searches and were also submitted by Zilico. These studies were excluded because their focus was on demonstrating the potential of spectroscopy for detecting CIN and calculating the impedance levels that could be used to diagnose CIN 2+, rather than formal diagnostic accuracy assessment.
Results: assessment of diagnostic accuracy
Characteristics of the included studies
Table 3 presents the summary information of the characteristics of the included diagnostic accuracy studies. There were 11 studies included in the diagnostic review, including nine studies of DySIS45,50,54,60,74,79,80,88,111 and two studies of ZedScan. 94,103 A total of six studies were unpublished, including three full-text studies45,54,94 and three studies reported only as conference abstracts. 74,80,111 Two studies were ongoing but reported sufficient preliminary diagnostic accuracy data to be included in this review. 74,80 The manufacturers were involved in the design, conduct and/or interpretation of all ZedScan studies and all DySIS studies apart from two. 50,79
| Study | Country | Sample size (number of participants analysed) | Number of centres involved | Recruitment dates | Adjunctive technology | Age (years) | hrHPV prevalence | Reason for referral | LG dyskaryosis or less | HG dyskaryosis |
|---|---|---|---|---|---|---|---|---|---|---|
| Budithi et al.45 (unpublished) | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado and Fasero50 | Spain | 443 | 1 | March 2012 to February 2014 | DySIS (DySIS v3) | Mean 36, SD 10.9 | 37.5%a | Abnormal Pap smear | 82.9% | 17.1% |
| Founta et al.54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers et al.60 | The Netherlands | 239 | 3 | July 2008 to September 2009 | DySIS (DySIS v2.1) | Mean 36.7, median 35.3, range 18.7–62.6 | 66.1%b | Abnormal cytology: 91.6%; follow-up of untreated CIN 1–2: 8.4% | 66.1% | 33.9% |
| Natsis et al.74 (conference abstract, ongoing study) | England (Gateshead and Taunton) | 287 (and 948 in the parallel standard colposcopy control group) | 2 | NR | DySIS (DySIS v3) | NR | 100% | LG cytology and hrHPV | 100% | 0 |
| Roensbo et al.79 | Denmark | 239 | 1 | December 2013 to January 2014 | DySIS (version NR) | Mean 34.3, SD 11.5 | NR | Abnormal cytology | NR | NR |
| Salter and Livingston80 (conference abstract, ongoing study, IMPROVE-COLPO) | USA | 210 (and 1788 retrospective standard colposcopy control group)c | 2 | NR | DySIS (DySIS v3) | Median 31, range 21–62 | NR | Abnormal cytology/Pap test (99%), test of cure (1%) | 74%d | 25%e |
| Soutter et al.88 | England (London), Greece | 308 | 3 | May 2004 to July 2005 | DySIS (FPC-03 prototype) | Median 37, IQR 29–46 | NR | Abnormal Pap test: 96.1%; symptoms: 3.9% | NR | NR |
| Tidy et al.103 (Phase I) | England (Sheffield) | 214 (Phase I) | 2 | April 2009 to May 2011 | ZedScan (third-generation prototype) | Median 31.3, range 20–60 | NR | Abnormal cytology | 47.2% | 52.8% |
| Tidy et al.103 (Phase II) | England (Sheffield), Ireland | 196 (Phase II) | 3 | April 2009 to May 2011 | ZedScan (third-generation prototype) | Median 29.5, range 20–64 | NR | Abnormal cytology | 56.3% | 43.7% |
| Tidy et al.94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Macdonald et al.95 (linked to Tidy et al.94)f | England (Sheffield) | 839 | 1 | January 2014 to December 2015 | ZedScan (commercial version) | Mean 32.9, range 20.3–66.1 | 100% | Known hrHPV genotype (100%), abnormal cytology (73.1%), persistent hrHPV/negative cytology (22.3%), follow-up (4.2%), clinical indication (0.6%) | 49.0% | 24.1% |
| Tsetsa et al.111 (conference abstract, unpublished completed study) | Greece | 57 (54) | 1 | NR | DySIS (version unknown) | NR | NR | Abnormal cytology | NR | NR |
Risk of bias of the included studies
All included studies were conducted in hospital-based colposcopy clinics and used a prospective cohort design. All patients underwent colposcopy with an adjunctive colposcopy technology, except for participants included in two DySIS two-arm studies that included a separate parallel control group examined with colposcopy alone. 74,80 Six studies were conducted in more than one centre. 45,60,74,80,88,103
Five studies were conducted in England. 54,74,88,94,103 Of those studies, one also recruited patients in Greece88 and one involved a clinic in Ireland. 94 Other studies were conducted in Wales,45 the Netherlands,60 Spain,50 Denmark,79 the USA80 and Greece. 111
The sample size of studies (defined as the total number of participants analysed) ranged from 54 to 1237. The mean/median age of participants ranged from 29 to 37 years for studies in which this was reported. The prevalence of hrHPV was reported in only five studies and ranged from 37.5% to 100%,50,54,60,74,94 and three studies included patients with hrHPV exclusively. 54,74,94
The majority of patients included in the studies were referred to colposcopy because of an abnormal cytology/smear test, although one study included only test-of-cure patients referred with a negative cytology who tested positive for hrHPV either 6 months after LLETZ or in the context of the NHS catch-up programme. 54 All patients included in the study by Tidy et al. 94 were referred to colposcopy through the NHS HPV Primary Screening Pilot. 20 A substudy of Tidy et al. 94 included 613 patients with a known hrHPV genotype already included in Tidy et al. , as well as an additional 226 (26.9%) patients, of whom most (187 patients; 82.7%) had a persistent HPV test and cytology-negative result. 95 No other study included patients referred from HPV primary screening.
When reported, the percentage of low- and high-grade referrals varied widely across the studies. One study of test-of-cure patients reported a high prevalence of high-grade referral (84.7%),54 and another study included only patients with low-grade cytology and hrHPV. 74 In other studies, between 17.1% and 52.8% of participants were referred to colposcopy with high-grade dyskaryosis or worse and 9.5–82.9% of participants were referred with low-grade dyskaryosis or less severe disease. The prevalence of histology-confirmed CIN 2+ varied widely, from (confidential information has been removed)54 to 45.2%. Further details of histology-confirmed CIN and cancer prevalence are reported in Appendix 4.
One study excluded women with a type 3 transformation zone. 94 Five studies excluded pregnant women45,60,88,94,103 and two studies also excluded women with active menstruation. 94,103 Further details of the patient selection criteria and exclusions are reported in Appendix 5.
All but one of the nine DySIS studies evaluated DySISmap as an adjunct to colposcopy; the single exception reported only the diagnostic accuracy of DySISmap alone against colposcopy. 79 Four studies evaluated the accuracy of DySISmap both alone and as an adjunct to colposcopy. 50,80,88,95 Both ZedScan studies used ZedScan as an adjunct to colposcopy. All of the DySIS studies used a DySIS video colposcope and both ZedScan studies used a binocular colposcope.
Six studies evaluated a commercial version of DySISmap, of which five used DySIS v345,50,54,74,80 and one used DySIS v2.1. 60 One study evaluated a precommercial prototype version of DySISmap (FPC-03),88 and two studies did not report which version was used. 79,111 Most studies of DySIS reported using the upper end of the acetowhitening scale of the colour-coded DySIS map to identify predicted high-grade lesions (red/yellow/white). 50,54,60,80,88 One study also included areas with weaker acetowhitening (coloured as dark blue and green, in addition to the standard red, yellow and white) as potential high-grade lesions,79 and three studies did not report which part of the colour-coded scale was used to predict CIN 2+. 45,74,111 Following a request for information from NICE, the manufacturer stated that the DySISmap algorithm had not changed after the FPC-03 version, and that DySIS v3 had undergone improvements in the following areas compared with earlier versions: increased image resolution, ergonomic set-up to allow for flexible positioning, improved working distance to allow for easier biopsy and treatment, improved software usability and availability of single-use specula.
One ZedScan study was a two-phase study evaluating a precommercial version of the tool (third-generation prototype);103 in Phase I, 12 colposcopically guided ZedScan measurements were taken from the cervix and analysed from a group of 214 people on a per-point basis to determine the cut-off points for the detection of CIN 2+. The cut-off points were then used in the second phase to evaluate the diagnostic accuracy of adjunctive ZedScan with colposcopy alone and to conduct further analyses to test and determine further cut-off points.
The more recent ZedScan study, by Tidy et al. ,94 evaluated a commercial version of ZedScan. Clarification from the manufacturer indicated that (confidential information has been removed).
All 11 included studies were assessed for risk of bias and applicability using a modified version of the QUADAS-2 tool. Table 4 presents a summary of the results for the assessed risk of bias across all studies in the five main risk-of-bias domains: patient selection, index test, comparator test, reference standard and flow and timing. Appendix 3 presents the complete results of the quality assessment with the justifications for decisions.
| First author (year) | Risk of bias | Level of concern about applicability | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Comparator test | Reference standard | Flow and timing (including verification bias) | Patient selection | Index test | Comparator test | Reference standard | |
| Budithi (2018)45 | a | a | a | a | a | a | a | a | a |
| Coronado (2016)50 | + | + | ? | – | – | – | + | + | + |
| Founta (2018)54 | + | ? | + | – | – | + | + | + | ? |
| Louwers (2011)60 | + | + | + | – | + | – | + | – | + |
| Natsis (2016)74 | ? | ? | ? | – | – | + | ? | ? | ? |
| Roensbo (2015)79 | ? | – | – | – | –a | ? | – | – | + |
| Salter (2016)80 | ? | ? | ? | – | ? | ? | ? | ? | ? |
| Soutter (2009)88 | ? | + | + | – | –a | ? | – | – | + |
| Tidy (2013)103 | – | – | + | – | – | – | – | + | + |
| Tidy (2018)94 | a | a | a | a | a | a | a | a | a |
| Tsetsa (2012)111 | ? | ? | ? | – | ? | ? | – | – | ? |
Dynamic Spectral Imaging System studies
Only one study was rated as being at a low risk of bias overall,60 and the remaining eight studies were rated as being at a high risk of bias. Significant levels of concern regarding applicability were raised for five of the nine DySIS studies. 50,60,79,88,111 The main source of bias in DySIS studies was related to verification bias. Only three studies conducted biopsies in all patients analysed. 60,79,88 (Confidential information has been removed.) The remaining two DySIS studies were conference abstracts and did not report sufficient data to assess the risk of verification bias. 80,111
The DySIS technology used in the earlier study by Soutter et al. 88 was a precommercial model (FPC-03). The study reported technical issues relating to the software, speculum and a batch of faulty disposable nozzles, leading to the exclusion of a large proportion of eligible participants (31%) from the analyses. Therefore, the applicability of the results of this study may be limited.
ZedScan studies
Both studies of ZedScan were rated as having a high risk of bias overall,94,103 and significant levels of concern regarding applicability were raised for both studies.
Neither study conducted biopsies in participants with a normal cervical transformation zone to confirm the absence of CIN, and so both were rated as having a high risk of verification bias. Both studies were rated as having a high risk of study selection bias, notably because of the exclusion of patients with transformation zone type 3, in whom colposcopy may be harder to perform. 94
(Confidential information has been removed.) However, one study94 did collect data on whether or not biopsy would have been taken with colposcopy alone regardless of the ZedScan result, and the diagnostic accuracy results for standard colposcopy were reported in a linked substudy. 95 Therefore, the ZedScan results were rated as having a high risk of reporting bias.
(Confidential information has been removed) and most patients included in the study by Tidy et al. 103 were examined in a single centre (Sheffield), and the extent to which the results of this study are applicable to other settings is uncertain.
Risk of bias associated with the reference standard
In all included studies, nearly all histology was performed based on samples collected from punch biopsies rather than from deeper treatment biopsies. Although it is obviously unethical to perform treatment biopsies when this is not clinically indicated, samples from punch biopsies may be less accurate. 37 Therefore, all studies were rated as having a high risk of bias associated with the reference standard.
Additional data provided by the manufacturers
(Confidential information has been removed.)
In all analyses, these additional data were used in preference over published results. If additional data were not provided, the data extracted from publications were used.
We intended to further analyse the 5 × 5 diagnostic data provided. (Confidential information has been removed.) It was decided that a more detailed analysis of this additional analysis was not appropriate, as it may be biased by the availability and structure of the data provided.
Statistical synthesis of diagnostic accuracy
The initial meta-analyses of diagnostic accuracy were based on data presented in the publications listed in Table 2. Two studies were excluded from these analyses because they were conducted in very specific subpopulations: (confidential information has been removed). One other study74 was excluded because it was conducted only in women with both a hrHPV infection and low-grade cytology results. The statistical analyses therefore included eight studies, six of DySIS and two of ZedScan.
In performing the analyses, we made the following assumptions: we assumed that the DySIS video colposcope (used in DySIS studies) was equivalent, in diagnostic accuracy, to a binocular colposcope (used in ZedScan studies). DySIS was used in one study,80 but it was not clear whether this was DySISmap alone (without colposcopy) or DySISmap adjunctive to colposcopy. We have assumed the latter, as it is assumed that the colposcopists must have seen the video colposcopic image as part of the assessment. One study79 reported whether the colposcopists agreed or disagreed with the DySISmap result, rather than the result of adjunctive colposcopy. We have assumed that when either the colposcopists or the DySISmap result found CIN 2 (or greater) to be present, the test was taken to be positive for CIN 2. This differs from the interpretation in the original paper.
The threshold used for colposcopy in all publications was CIN 2 or greater, and that has been used in these analyses. Only one study50 reported diagnostic accuracy at CIN 1 or greater.
Only two ZedScan studies were available for analysis – one was of the current ZedScan device94 and the other was of a ZedScan prototype. 103 We have therefore not performed meta-analyses of these studies; instead, we report the diagnostic accuracy results on ROC plots without meta-analytic summary results.
Dynamic Spectral Imaging System forest plots of diagnostic accuracy
In this section we present diagnostic accuracy results from the studies of DySIS in the form of forest plots.
Figure 3 shows estimates of sensitivity and Figure 4 shows estimates of specificity. Colposcopy alone has moderate sensitivity (58.4%, 95% CI 50.3% to 66.5%) but high specificity (confidential information has been removed); colposcopy therefore misses many women who do have CIN of grade 2 or greater, but produces relatively few false-positive test results. DySISmap alone has similar performance (confidential information has been removed). For adjunctive DySIS use, the sensitivity rises to (confidential information has been removed), so using DySISmap in addition to colposcopy correctly identifies more CIN 2 cases, but with a higher false-positive rate, which may mean performing biopsies in a larger proportion of women who do not have CIN 2 (or greater).
FIGURE 3.
Forest plot of the diagnostic sensitivity of DySIS. (Confidential information has been removed.)
FIGURE 4.
Forest plot of the diagnostic specificity of DySIS. (Confidential information has been removed.)
Figure 17 in Appendix 6 presents the DORs for each study. The diagnostic ratio is a combination of sensitivity and specificity (formally, log-odds of sensitivity minus log-odds of specificity), which increases as the overall diagnostic accuracy of a test increases. The results show almost no difference between colposcopy and adjunctive DySIS (confidential information has been removed), suggesting that DySISmap does not improve the diagnostic accuracy of colposcopy when defined in terms of DORs.
Figures 18 and 19 (Appendix 6) show the studies’ PPVs and the NPVs, respectively. These are harder to interpret, as PPVs and NPVs vary with prevalence, which is different across the studies. The PPV for adjunctive colposcopy is lower than that for colposcopy alone (confidential information has been removed), so fewer than half of all women who receive a DySIS-guided biopsy will have high-grade CIN. The summary PPV, and the estimated PPV in most studies, is lower than the 65% level recommended by UK guidance. 15 The NPV is slightly higher with adjunctive DySIS (confidential information has been removed), so fewer high-grade CIN cases will be missed.
Heterogeneity was substantial in almost all meta-analyses. The I2 values are summarised in Table 5. All but one analysis had an I2 value above 60%.
| Variable | Technology | ||
|---|---|---|---|
| Colposcopy only | DySISmap only | DySISmap and colposcopy | |
| Sensitivity | 62.7 | 94.4 | 0 |
| Specificity | 90.2 | 91.5 | 94.5 |
| DOR | 78.6 | 92.7 | 74.4 |
| PPV | 64.9 | 79.4 | 88.4 |
| NPV | 94.8 | 97.7 | 89.8 |
Bivariate and regression models of diagnostic accuracy
The analyses presented so far have not accounted for the correlation between sensitivity and specificity. A formal bivariate meta-analysis of diagnostic accuracy should be used to account for this correlation. 41 The analyses have also not accounted for the fact that colposcopy and DySIS are performed in the same study on the same participants. Full individual-level data would be needed to properly account for the within-person correlation between test results. These were not available, but extensions to the bivariate model can account for the fact that the tests were compared within the same study. 43
Figure 5 shows the sensitivity and specificity for all included studies. It can be seen that, for all studies, adjunctive DySIS has higher sensitivity but lower specificity than using colposcopy alone. Using DySISmap alone generally falls somewhere between the two.
FIGURE 5.
Sensitivity and specificity for all DySIS studies in the ROC space. (Confidential information has been removed.)
Table 6 shows the results of the bivariate meta-analyses; Appendix 6, Figure 20, shows the results in the ROC space, including the 95% confidence regions (the ellipses) and the summary ROC curves. The results are consistent with those seen in the forest plots of diagnostic accuracy (see Figures 3 and 4), and show that using adjunctive DySIS increases sensitivity when compared with colposcopy alone, but at the cost of reduced specificity. As only three studies reported the use of DySISmap alone, no bivariate model was fitted for that test.
| Technology | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|
| Colposcopy alone | 57.74 (49.7 to 63.4) | 87.34 (79.7 to 92.4) |
| DySISmap and colposcopy | 80.97 (76.0 to 85.1) | 70.90 (60.8 to 79.3) |
The bivariate model analyses colposcopy and adjunctive DySIS separately, and does not account for the fact that these are measured in the same studies. To correct for this, we fitted logistic regression models, including study-level parameters, to account for a possible correlation between test results within studies (see Statistical synthesis of diagnostic accuracy for details).
The summary results for this regression model are shown in Table 7. The results are similar to the standard bivariate model in Table 6. This model also permits a direct comparison of colposcopy and adjunctive DySIS. This found evidence of a difference in specificity between the tests [difference in log-odds of specificity 1.33, standard error (SE) 0.33; p < 0.0001] but no evidence of a difference in diagnostic accuracy (difference in log-DORs 0.04, SE 0.20; p = 0.84). This suggests that using DySIS changes the test threshold for the diagnosis of CIN 2 such that more women go on to receive biopsy, but that it is not improving diagnostic accuracy (in terms of DOR) when compared with colposcopy alone.
| Technology | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|
| Colposcopy alone | 57.91 (47.2 to 67.9) | 87.41 (81.7 to 91.5) |
| DySISmap and colposcopy | 81.25 (72.2 to 87.9) | 70.40 (59.4 to 79.5) |
To confirm this, we also fitted a regression model that constrains adjunctive DySIS and colposcopy to have the same diagnostic accuracy (but which permits differences in specificity). The Bayesian information criterion (BIC) is used when comparing regression models; generally, a lower BIC suggests a better-fitting and more parsimonious model. This new model had a BIC of 198.3, which is lower than the previous model, which had a BIC of 201.5. This confirms that assuming that DySIS and colposcopy have the same diagnostic accuracy is reasonable.
ZedScan
Two studies of ZedScan are included in this analysis. The most recent study94 reported data for adjunctive ZedScan only, using the current ZedScan device, with no data on the performance of colposcopy alone; the other one103 was a study of a ZedScan prototype, which assessed the diagnostic accuracy at six different cut-off points of the ZedScan algorithm. This was compared with two colposcopy cut-off points: (1) ‘colposcopic impression’, whereby the colposcopy was considered to have a positive finding if it judged that high-grade CIN was present, and (2) ‘disease present’, whereby colposcopy was considered to have given a positive result if at least one measurement point was suggested for biopsy. The six ZedScan cut-off points were selected such that one had the same sensitivity as colposcopy (colposcopic impression or disease present), one had the same specificity as colposcopy and the third was a trade-off between sensitivity and specificity. Because only two studies were available, and there were the differences between the precommercial device and ZedScan, no meta-analysis was performed. Instead, the sensitivity and specificity data from the studies are shown in the ROC space in Figure 6. The black lines show summary ROC curves for adjunctive ZedScan and for colposcopy. The sensitivity and specificity results from the two studies are also presented in Table 8.
FIGURE 6.
Receiver operating characteristic presentation of results from the ZedScan studies. (Confidential information has been removed.)
| Study | Technology | |||||
|---|---|---|---|---|---|---|
| Colposcopy cut-off point | Colposcopy alone | ZedScan cut-off point | ZedScan and colposcopy | |||
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |||
| Tidy et al.94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tidy et al.103 | Colposcopic impression | 73.6 (64.3 to 82.8) | 83.5 (76.5 to 90.5) | 1.321 | 73.6 (64.3 to 82.8) | 90.8 (85.4 to 96.2) |
| 1.083 | 78.2 (69.5 to 86.8) | 83.5 (76.5 to 90.5) | ||||
| 1.568 | 62.1 (51.9 to 72.3) | 95.4 (91.5 to 99.3) | ||||
| Disease present | 88.5 (81.8 to 95.2) | 38.5 (29.4 to 47.7) | 0.768 | 88.5 (81.8 to 95.2) | 65.2 (56.2 to 74.1) | |
| 0.390 | 96.6 (92.7 to 100) | 38.5 (29.4 to 47.7) | ||||
| 0.568 | 92.0 (86.2 to 97.7) | 51.4 (42.0 to 60.8) | ||||
These results suggest that adjunctive ZedScan may have better diagnostic accuracy than colposcopy alone. In the prototype study, ZedScan had greater sensitivity for the same specificity as colposcopy or greater specificity for the same sensitivity. Greater diagnostic accuracy for ZedScan is also suggested by the fact that the summary ROC curve for ZedScan had greater sensitivity than that for colposcopy. However, the small size of the study, and the wide CIs, mean that it is uncertain if difference is clinically meaningful. Fitting a logistic regression model to the data from the prototype study found that the improvement in diagnostic accuracy was not quite statistically significant (difference in log-diagnostic accuracy 0.488, SE 0.28; p = 0.078).
(Confidential information has been removed.)
Test positive rates
Figure 21 in Appendix 6 shows the test positive rate (the proportion of women in whom colposcopy or adjunctive colposcopy suggests the presence of high-grade CIN) for each test in each study of DySIS. In every study, adjunctive use of DySIS increased the positive rate compared with colposcopy alone, often substantially. In the Louwers et al. 60 study, for example, the use of DySIS increased the positive rate from 33.1% to 55.5%. Hence, the use of DySIS will substantially increase the number of women who receive biopsies after colposcopy. The results for ZedScan are shown in Figure 22 in Appendix 6. These suggest that the positive rate for ZedScan is similar to that for colposcopy alone, regardless of the cut-off point used. The disease-present cut-off point, unsurprisingly, produces higher positive rates than the colposcopic impression cut-off point.
Subgroup analyses
Some of the studies provided diagnostic accuracy data for key identified subgroups, namely:
-
high-grade and low-grade cytology referral
-
high-risk HPV (including HPV 16) and low-risk HPV
-
the ‘test-of-cure’ population.
We include here data on these subgroups provided by the manufacturers or study authors, data from primary publications and data from secondary reports of primary studies when those papers included subgroup data not reported in the original study publication. Appendix 6, Table 44, provides an overview of the subgroups reported in each study.
Figure 7 shows sensitivity and specificity forest plots by subgroup for colposcopy alone, Figure 8 shows the same analyses for adjunctive DySIS and Figure 9 shows the same analyses for adjunctive ZedScan. Summary meta-analyses are not presented because of the small number of studies in each subgroup.
FIGURE 7.
Diagnostic accuracy of colposcopy by subgroup. (Confidential information has been removed.)
FIGURE 8.
Diagnostic accuracy of adjunctive DySIS by subgroup. (Confidential information has been removed.)
FIGURE 9.
Diagnostic accuracy of adjunctive ZedScan by subgroup. (Confidential information has been removed.)
Colposcopy appears to be less sensitive than average at detecting high-grade CIN in women with low-grade referrals. This may partly be a consequence of interpretation bias (the colposcopic results are analysed with more caution if a woman is known to have a high-grade cytology referral) or it may be because lesions are harder to detect in women with a low-grade cytology referral. The same applies to women who have low-grade referrals combined with hrHPV. This difference is not observed when using DySIS or ZedScan; in both cases, the sensitivity and the specificity are similar for both low- and high-grade referrals. This suggests that adjunctive colposcopy may improve the detection of high-grade CIN in women with a low-grade referral (i.e. mild dyskaryosis).
There is no convincing evidence that diagnostic accuracy differs between women who are infected with hrHPV and those who are not infected with hrHPV; however, data are limited. For women infected with hrHPV, both the sensitivity and the specificity are higher than average (see Figures 3 and 4) when using adjunctive DySIS, suggesting that high-grade CIN is easier to detect in women infected with hrHPV. The sensitivity appears to be higher among women with hrHPV when using adjunctive DySIS than with colposcopy alone. This suggests that adjunctive DySIS may improve the detection of high-grade CIN in women infected with hrHPV.
The results for ZedScan are more limited, with no apparent evidence of differences between subgroups.
We note that all of these conclusions are based on small subgroups, generally of only one to three studies, and so the results should be considered as speculative only.
Meta-analyses were generally not possible within subgroups, as only one or two studies reported data for each subgroup. For high- and low-grade referrals for DySIS, a logistic regression model (as shown in Table 7) was fitted in each subgroup to meta-analyse diagnostic accuracy within these subgroups, as there were two DySIS studies for each subgroup. The results of this analysis are shown in Table 9. These results suggest that, for colposcopy alone, the sensitivity to detect CIN 2 among low-grade referrals is much lower than that for high-grade referrals, for a similar specificity. This difference was not quite statistically significant (p = 0.072), owing to the limited data available. There was no evidence of any difference between types of referral when using adjunctive DySIS, suggesting that adjunctive DySIS may be preferable to colposcopy for women with a low-grade referral. Similar regression models did not find any evidence of differences between HPV subgroups.
| Technology | Referral grade | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Colposcopy alone | LG | 38.27 (18.9 to 62.4) | 75.57 (53.4 to 89.3) |
| HG | 64.06 (42.2 to 81.3) | 71.49 (50.5 to 86.0) | |
| DySISmap and colposcopy | LG | 80.53 (57.9 to 92.5) | 53.36 (28.6 to 76.3) |
| HG | 83.16 (58.4 to 94.6) | 57.00 (30.7 to 79.8) |
Sensitivity analyses
The study by Roensbo et al. 79 differed from the other included studies, as it did not assess DySIS as an adjunct to colposcopy directly, but only whether a colposcopist agreed or disagreed with the DySISmap result. As noted in Characteristics of the included studies, the study also used the DySISmap more conservatively than other DySIS studies, by interpreting areas with weaker acetowhitening on the coloured map (including dark blue and green) as being ‘suspicious for high-grade disease’.
We performed a sensitivity analysis of the logistic regression model in Table 7 for sensitivity and specificity, excluding this study. The results are shown in Table 10. There is little change when excluding Roensbo et al. ,79 with all estimates being similar to when the study was included.
| Technology | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|
| Colposcopy alone | 56.4 (47.5 to 64.9) | 90.2 (86.3 to 93.1) |
| DySIS and colposcopy | 82.9 (75.0 to 88.7) | 72.9 (63.3 to 80.7) |
A particular concern identified during the quality assessment was that different studies had different practices, when neither colposcopy nor adjunctive DySIS or ZedScan identified any abnormal areas requiring biopsy. In four studies45,50,74,80 no biopsy was performed in those women, in two studies60,88 a single biopsy was typically performed at a randomly location and in one79 study multiple biopsies were performed. In the case of one study, 80 insufficient information was provided. Table 11 shows the results of the meta-analyses categorised by the type of biopsy performed in the DySIS studies. These results suggest that specificity and sensitivity tend to decline as more biopsies are performed on colposcopy-negative women. This was observed for colposcopy alone and adjunctive DySIS. Despite these differences, the comparison between colposcopy and adjunctive DySIS is unchanged; using DySISmap as an adjunct to colposcopy increases sensitivity but decreases specificity in all cases.
| Additional biopsies | Technology | |||
|---|---|---|---|---|
| Colposcopy alone | Adjunctive colposcopy | |||
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | |
| No additional biopsy (four studies)45,50,74,80 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| One additional biopsy (two studies)60,88 | 50.27 (43.0 to 57.5) | 86.22 (79.1 to 93.3) | 78.7 (72.6 to 85.6) | 70.02 (57.9 to 82.2) |
| Multiple additional biopsies (one study)79 | 67.65 (56.5 to 78.8) | 67.25 (60.2 to 74.3) | 75.00 (64.7 to 85.3) | 57.31 (49.9 to 64.7) |
Comparison of Dynamic Spectral Imaging System and ZedScan
All analyses so far have considered DySIS versus colposcopy and ZedScan versus colposcopy. Here, we briefly compare all three technologies.
As no studies in the review included both DySIS and ZedScan, a direct comparison is not possible. Instead, we consider an indirect comparison of the technologies. This is less reliable than a direct comparison, as there are differences in the study populations and conduct that may alter diagnostic accuracy over and above the differences in the diagnostic technology used.
Table 12 presents what we consider to be the best estimates of diagnostic accuracy. For DySIS, these are sourced from the logistic regression model comparing adjunctive DySIS with colposcopy (see Table 7). (Confidential information has been removed.) When comparing ZedScan with colposcopy, the situation is more complex, as the most recent study did not report colposcopy diagnostic data. If we are willing to assume that binocular and video colposcopes have the same diagnostic performance, then the best evidence is that found from the DySIS studies. Only the ZedScan prototype study103 has reported diagnostic accuracy data for binocular colposcopy, so an alternative estimate is the ‘colposcopic impression’ cut-off point from that study, as that cut-off point is closest to that used to diagnose high-grade CIN.
| Technology | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Source |
|---|---|---|---|
| DySIS and colposcopy | 81.25 (72.2 to 87.9) | 70.40 (59.4 to 79.5) | Regression model (see Table 7) |
| ZedScan and colposcopy | Confidential information has been removed | Confidential information has been removed | Tidy et al.94 |
| Colposcopy (DySIS video colposcope) | 57.91 (47.2 to 67.9) | 87.41 (81.7 to 91.5) | Regression model (see Table 7) |
| Colposcopy (binocular colposcope) | 57.91 (47.2 to 67.9) or 73.56 (64.3 to 82.8) | 87.41 (81.7 to 91.5) or 83.49 (76.5 to 90.5) | Regression model (see Table 7) or Tidy et al. (2013) ZedScan prototype (colposcopic impression cut-off point) |
These results show that both adjunctive DySIS and adjunctive ZedScan substantially increase sensitivity, but also have substantially reduced specificity when compared with colposcopy. It would appear that adjunctive ZedScan more strongly favours high sensitivity, with a corresponding loss of specificity, when compared with adjunctive DySIS, but this is an indirect comparison.
We also performed a further logistic regression model to indirectly compare all three diagnostic tests. This model included all diagnostic data from all DySIS and ZedScan studies, and accounted for the fact that tests were conducted in the same studies and for the differing cut-off points used in the ZedScan prototype study. Compared with colposcopy alone, DORs were not higher with adjunctive DySIS (difference in log-DOR 0.06; p = 0.74), but were increased by adjunctive ZedScan (difference in log-DOR 0.84; p = 0.003). Hence, ZedScan had a greater DOR than DySIS (difference in log-DOR 0.59; p = 0.003), suggesting that ZedScan could have better diagnostic accuracy than DySIS, but the exact benefit would depend on the choice of cut-off point and the corresponding sensitivity and specificity values.
Narrative synthesis of further diagnostic accuracy results
Six studies reported diagnostic accuracy data that could not be included in the statistical synthesis: five DySIS studies54,62,74,83,111 and one ZedScan study. 95 Three of these studies were linked to studies also included in the meta-analyses62,83,95 and three studies could be reported only in the narrative synthesis. 54,74,111 Tables 45 and 46 in Appendix 7 report the results of the studies of DySIS and ZedScan, respectively.
Dynamic Spectral Imaging System
Overall, the results of the five DySIS studies included in this section confirm the results of the meta-analysis. Adjunctive DySIS improves sensitivity for detecting CIN 2+ compared with colposcopy alone, although this is associated with a reduction in specificity. (Confidential information has been removed.)
Louwers et al. 61 was a secondary analysis of Louwers et al. ,60 which aimed to reanalyse the performance of dynamic spectral imaging (DSI) and conventional colposcopy to determine the difference between low-grade cytology [borderline or mild dyskaryosis (BMD)] referrals and high-grade cytology referrals. The study also aimed to reanalyse the performance of DSI and conventional colposcopy by retrospectively assigning them to two referral strategies based on their initial cytology and hrHPV test results: (1) hrHPV testing as the primary screening test and cytology as triage (including patients with a positive hrHPV test and BMD or high-grade cytology), or (2) reflex hrHPV testing in patients with BMD cytology (including patients with BMD cytology and a hrHPV positive test or high-grade cytology, irrespective of the hrHPV test result). Compared with standard colposcopy, the sensitivity of adjunctive DySIS was higher and the specificity was lower in both referral strategies. Diagnostic accuracy estimates were similar between HPV primary and cytology primary referral strategies for adjunctive DySIS (sensitivity 81% vs. 80%, respectively; specificity 64% vs. 61%, respectively) and for colposcopy alone (sensitivity 53% vs. 54%, respectively; specificity 82% vs. 78%, respectively).
Natsis et al. 74 estimated the accuracy of adjunctive DySIS in a population of 287 hrHPV-positive patients with low-grade cytology. Initial colposcopy impression and potential biopsy sites were recorded before and after the appearance of the DySISmap. Colposcopy alone had low sensitivity (27%) but high specificity (91%) for detecting CIN 2+. Incorporation of DySISmap improved sensitivity (82%) but reduced specificity (36%).
Salter and Livingston80 reported some preliminary data from two colposcopy clinics as part of a large cohort of US community-based colposcopy clinics using adjunctive DySIS. Consistent with other studies, the addition of DySISmap increased sensitivity compared with colposcopy alone (83.9% with adjunctive DySIS compared with 61.3% for colposcopy alone), but was associated with a reduction in specificity (75.4% vs. 91.1%, respectively).
Tsetsa et al. 111 carried out a single-centre prospective diagnostic cohort study that assessed the performance of adjunctive DySIS in three different concentrations of acetic acid solution (3%, 4% and 5%). The study was reported only in conference abstracts and enrolled 57 patients with abnormal cytology, of whom 54 were analysed. Each patient was examined with DySIS colposcope and DySISmap in three successive examinations. Biopsies were collected from sites corresponding to the most atypical indications of the coloured map and sent for histology. The diagnostic performance of adjunctive DySIS was higher in examinations that used 3% acetic acid (sensitivity 86%, specificity 81%) than in those using 4% acetic acid (sensitivity 79%, specificity 77%) and 5% acetic acid (sensitivity 82%, specificity 77%); however, the study was small and it is not clear if these differences were statistically significant. The authors noted that morphological characteristics, such as mosaic pattern and atypical vessels, were better highlighted when 5% acetic acid was used.
Cervical cancer (> cervical intraepithelial neoplasia grade 3)
Six studies reported on the prevalence of cervical cancer [> CIN grade 3 (> CIN 3)]. (Confidential information has been removed.)
All of these were studies evaluating DySIS. Of those, three identified at least one histology-confirmed patient (a total of 15 patients) with the disease and reported sufficient data to evaluate the number of additional cases identified with DySISmap as an adjunct to colposcopy. (Confidential information has been removed.) Only one of these studies indicated that the addition of the DySISmap to colposcopy helped to identify additional cancer cases (two additional cases). 84 In Appendix 7, Table 47 summarises the ability of colposcopy and adjunctive DySIS to identify the cancer cases. There is insufficient evidence to suggest that adjunctive DySIS improves the detection of cancer cases.
ZedScan
(Confidential information has been removed.)
Macdonald et al. 95 evaluated the diagnostic accuracy of ZedScan in patients infected with known hrHPV and compared its performance between those with HPV 16 and those with other hrHPV genotypes. The study included 839 participants, of which 607 (72%) had abnormal cytology and were included in Tidy et al. 94 An additional 226 patients were included, of which most (82%) had a persistent HPV test and cytology-negative result. The sensitivity of adjunctive ZedScan was high (100%) regardless of the hrHPV genotype. The sensitivity of colposcopy alone was also high, and slightly higher in patients with the HPV 16 genotype (86.9%) than in those with other high-risk genotypes (79.7%), although this difference may not be significant as a result of overlapping CIs. Specificity estimates were not reported in this study.
Human papillomavirus primary screening
(Confidential information has been removed.)
Test failures
Appendix 7, Table 48, presents the rates of, and the reasons for, test failures. Reported rates of test failure varied widely across the studies. One study88 of a prototype version of DySIS reported a high rate of failure (31.4%), which was primarily attributable to unsatisfactory DySISmap image and issues with faulty disposable nozzles through which the acetic acid was delivered. Studies of a more recent version of DySIS reported lower failures rates, ranging from 2.9% to 16.7%, with a lack of/poor-quality imaging being the most common reason. The failure rates in the ZedScan and ZedScan prototype studies were (confidential information has been removed) and 13.4%, respectively. (Confidential information has been removed.)
Diagnostic and treatment biopsies
All included diagnostic accuracy studies reported some data on the diagnostic and treatment biopsies performed. However, owing to limited data, the impact of adjunctive technology on the rates of diagnostic biopsies and treatment (including unnecessary treatments) is uncertain.
Appendix 7, Table 49 presents the number of diagnostic biopsies and treatment biopsies performed in the diagnostic accuracy studies.
Three studies performed biopsies in all patients, as reported previously. 60,79,88 In other studies, the proportion of adjunctive colposcopy patients biopsied ranged from (confidential information has been removed). 80,94 The mean number of biopsies varied widely,60,83,88,103 (confidential information has been removed) to up to five in Roensbo et al. 79 when reported. Only four studies reported on the number of treatments performed during or after the examination, which were nearly always conducted as loop excisions. 60,79,94,103 See-and-treat cases ranged from 0 in Roensbo et al. 79 to (confidential information has been removed).
Only three studies provided data on the number of additional biopsies performed that were associated with the use of adjunctive colposcopy. However, these results are limited, as a result of the lack of randomised evidence comparing adjunctive colposcopy with standard colposcopy in parallel groups. (Confidential information has been removed.) Papagiannakis et al. 83 found that the addition of DySIS led to an increase of approximately one additional biopsy per five patients (from 1.03 biopsies per patient with standard colposcopy to 1.25 biopsies with adjunctive colposcopy), although this result is derived from a non-randomised comparison with a retrospective arm. Natsis et al. 74 reported that the proportion of patients undergoing biopsies was lower among patients undergoing colposcopy with adjunctive DySIS (80.8%) than in a parallel group of patients undergoing colposcopy alone (85.9%). (Confidential information has been removed.)
Results: assessment of clinical effectiveness
The review of clinical effectiveness aimed to evaluate the following outcomes in studies of DySIS and ZedScan:
-
morbidity and mortality associated with treatment and biopsies conducted as part of the colposcopy examination (including obstetric outcomes such as miscarriage and infertility)
-
morbidity and mortality associated with cervical cancer, and HRQoL
-
pain and anxiety associated with the colposcopy examination, biopsies, treatment and waiting for results
-
any other adverse event that may have an impact on resource use or quality of life.
Only three studies reported data on the clinical effectiveness outcomes. All three studies were also included in the review of diagnostic accuracy. 60,88,103 The characteristics of the studies are reported in Table 3.
Results of the studies on clinical effectiveness
Three studies reported data on adverse events. 60,88,103 One study of ZedScan103 reported one serious adverse event and two adverse events following colposcopy: one patient ‘felt unwell’ and two instances of bleeding after biopsies. It is not clear which of these three events was serious and whether or not any of these adverse events could be attributed to the use of adjunctive colposcopy. Both studies of DySIS reported that no patients experienced adverse events following colposcopy. 60,88 No further data on adverse events were reported.
No data were reported on morbidity and mortality associated with treatment and biopsies conducted as part of the colposcopy examination or associated with cervical cancer in studies of DySIS and ZedScan. No data on outcomes related to HRQoL were found. Data on pain and anxiety associated with colposcopy examination with DySIS were collected only in patient surveys using non-validated scales, and these results are reported in Systematic reviews of adverse outcomes of cervical intraepithelial neoplasia treatment.
Systematic reviews of adverse outcomes of cervical intraepithelial neoplasia treatment
Owing to the limited evidence identified by the review of clinical effectiveness, a pragmatic search for recent good-quality systematic reviews on the impact of CIN treatment on adverse fertility, pregnancy and obstetric outcomes was conducted. Two relevant systematic reviews with meta-analysis were identified. 118,119 This section provides a critical summary of these reviews.
Kyrgiou et al. (2015)118
Kyrgiou et al. 118 assessed the effect of excisional or ablative CIN treatment on fertility and early pregnancy (< 24 weeks’ gestation)-related outcomes. The review included studies that compared fertility and early pregnancy (< 24 weeks’ gestation)-related outcomes in women with a history of CIN treatment with the same outcomes in women who had not received treatment. Any types of excisional and ablative treatments were included.
The review included 15 studies (2,223,592 participants, of whom 25,008 received treatment for CIN). All included studies were non-randomised and heterogeneity was high. The quality of the evidence for early pregnancy-related outcomes was low [Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification]. The results of the meta-analysis showed that CIN treatment did not have an adverse effect on fertility outcomes. The overall pregnancy rate was higher for treated women than for those who were untreated [43% and 38%, respectively; relative risk (RR) 1.29, 95% CI 1.02 to 1.64; four studies]. There was no statistically significant difference in pregnancy rates in treated and untreated women with an intention to conceive and women taking more than 12 months to conceive. There was no increase in the total miscarriage rate or the first-trimester miscarriage rate between the treated and untreated groups; however, CIN treatment was associated with an increased risk of miscarriage in the second trimester (1.6% vs. 0.4%, RR 2.60, 95% CI 1.45 to 4.67; eight studies). Ectopic pregnancies (1.6% vs. 0.8%, RR 1.89, 95% CI 1.50 to 2.39; six studies) and terminations (12.2% vs. 7.4%, RR 1.71, 95% CI 1.31 to 2.22; seven studies) were also more frequent in treated women. The authors concluded that CIN treatment is unlikely to have an adverse effect on fertility and there was a slight increase in the risk of miscarriage in the second trimester associated with treatment; there was no stratification of risk by treatment type or magnitude. The authors also further emphasised the low quality of the evidence, but suggested that women should be advised that fertility is not compromised by treatment for CIN. The conclusions of this generally well-conducted review are likely to be reliable.
Kyrgiou et al. (2016)119
Kyrgiou et al. 119 focused on studies reporting obstetric (> 24 weeks’ gestation) and neonatal outcomes following local treatment for CIN or early-stage cervical cancer. The review included studies reporting on obstetric outcomes (> 24 weeks’ gestation) in women who had previously received local treatment for CIN or early-stage invasive cervical cancer compared with women with no history of treatment. All types of excisional and ablative treatments were included.
Outcomes pertaining to the risk of overall preterm birth were reported by 60 studies, with 25 reporting on ‘severe’ preterm birth (< 32–34 weeks’ gestation) and nine reporting on ‘extreme’ prematurity (< 28–30 weeks’ gestation). Other outcomes included the length of labour [precipitous or prolonged, use of analgesia (epidural, pethidine, other)], oxytocin use, cervical stenosis and haemorrhage. Neonatal outcomes were low birthweight, admission to neonatal intensive care, stillbirth, Apgar scores and perinatal mortality.
The review included 71 studies (6,338,982 participants, with 65,082 participants treated for CIN or early-stage invasive cancer). Nearly all of the studies were retrospective cohort studies and none was a randomised trial. Most studies were considered to be high-quality observational studies (Newcastle–Ottawa Scale scores 8–10 in all but two studies). For all treatment types, the risk of overall prematurity (< 37 weeks’ gestation) was increased in the treated group versus the untreated group (RR 1.78, 95% CI 1.60 to 1.98; 60 studies), as was severe prematurity (< 32–24 weeks’ gestation, RR 2.40, 95% CI 1.92 to 2.99; 25 studies) and extreme prematurity (< 28–30 weeks’ gestation, RR 2.54, 95% CI 1.77 to 3.63; nine studies). Compared with untreated women, all patients who were treated with LLETZ were at a higher risk of giving birth prematurely (RR 1.56, 95% CI 1.36 to 1.79; 26 studies), severely prematurely (RR 2.13, 95% CI 1.66 to 2.75; 11 studies) and extremely prematurely (RR 2.57, 95% CI 1.97 to 3.35; three studies). The meta-analysis showed that treatment for CIN increased the risk of preterm birth regardless of treatment method, with a higher magnitude of treatment effect associated with treatment techniques removing or ablating more tissue; deeper excisions were consistently associated with increased risk of preterm birth (≤ 10–12 mm: RR 1.54, 95% CI 1.09 to 2.18 mm; ≥ 10–12 mm: RR 1.93 mm, 95% CI 1.62 to 2.31 mm; ≥ 15–17 mm: RR 2.77 mm, 95% CI 1.95 to 3.93 mm; ≥ 20 mm: RR 4.91 mm, 95% CI 2.06 to 11.68 mm). There was also an association between the number of procedures undergone and the risk of preterm birth; pregnant women who had underone more than one treatment were significantly more likely to deliver prematurely (RR 3.78, 95% CI 2.65 to 5.39).
The risk of other adverse outcomes, such as spontaneous preterm birth, premature rupture of the membranes, chorioamnionitis, low birthweight, admission to neonatal intensive care and perinatal mortality, was also significantly increased after treatment. The authors concluded that any local treatment for preinvasive or early-stage invasive disease will increase the risk of preterm birth in a subsequent pregnancy and that the frequency and severity of adverse outcomes increases with greater cone depth and is higher for excision than for ablation. However, the authors noted that the risks associated with small excisions are likely to be smaller than those related to untreated CIN during pregnancy, which is itself linked to preterm birth (RR 1.24, 95% CI 1.14 to 1.35). The authors rightly noted the need to interpret the review results with caution, owing to the lack of prospective and randomised evidence, a high risk of confounding in the included studies and significant heterogeneity. Given this, the conclusions of this generally well-conducted review are likely to be reliable. However, as most of the studies included in these reviews were not recent UK cohort studies, the applicability of the conclusions of these reviews to the NHS context may be limited. 120–122
Results: assessment of implementation
The review of implementation aimed to evaluate the following outcomes in studies of DySIS and ZedScan: the acceptability of the adjunctive technologies (clinicians and patients), patient satisfaction, successful database and record management, training requirements, the capacity to perform colposcopies, and uptake and compliance.
As part of the wider database search for diagnostic accuracy studies and studies of clinical effectiveness, five studies were identified that reported data on any of these implementation outcomes. These included four DySIS studies46,51,63,72 and one ZedScan study. 96 All of these studies were linked to the diagnostic accuracy studies included in the review of diagnostic accuracy (see Table 2).
Characteristics of the included studies
Table 13 presents a summary of the characteristics of the five studies46,51,63,72,96 included in the review of implementation. Three studies were conducted in the UK,46,72,96 one study was conducted in Spain51 and one study was conducted in the Netherlands. 63 Three studies reported data on patient satisfaction with adjunctive DySIS,46,63,72 one study reported on the acceptability of DySIS to clinicians51 and one study reported views from both patients and colposcopists on DySIS. 46 One study reported data on the colposcopist training requirements associated with ZedScan.
| Study | Linked diagnostic accuracy study | Characteristics | |||||
|---|---|---|---|---|---|---|---|
| Location | Study dates | Population and sample size | Study design | Adjunctive colposcopy technology | Outcomes | ||
| Budithi et al.46 | Budithi et al.45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado and Fasero51 | Coronado and Fasero50 | San Carlos Hospital, Madrid | NR | 63 colposcopists | Survey questionnaires, retrospective case reviews | DySIS | Acceptability of the adjunctive technologies to colposcopists |
| Louwers et al.63 | Louwers et al.60 | Three Dutch hospitals | July 2008 to September 2009 | 239 participants | Survey questionnaires | DySIS | Patient satisfaction |
| Lowe et al.72 | None | Four NHS colposcopy clinics, UK | June 2015 to May 2016 | 763 patients | Survey questionnaires | DySIS | Acceptability of the adjunctive technologies to patients |
| Palmer et al.96 | Tidy et al.94 | Sheffield Teaching Hospitals | January 2014 to December 2015 | Five colposcopists | Observational, single arm | ZedScan | Training requirements |
Quality assessment of the implementation studies
The quality of the studies that used a survey questionnaire was limited overall. The validity and the reliability of the questionnaires were not established in any of the studies. Two studies included a sample that was considered likely to be representative of the population of interest. 46,63 Only two studies51,63 accounted for relevant confounding factors (e.g. age, education, number of pregnancies or colposcopist experience). The methods used to estimate the training requirements in the ZedScan study were limited, making the validity of this study uncertain. Further results of the quality assessment of the survey questionnaires are reported in Appendix 8.
Results of the implementation studies
Acceptability to patients and patient satisfaction
Lowe et al.72
Lowe et al. 72 conducted a survey in 763 patients referred to colposcopy clinics in four NHS hospitals to assess their experience with a DySIS colposcope with DySISmap. Two questionnaires were designed: one for patients undergoing their first colposcopy examination and one for patients with prior experience of colposcopy. The study was reported only as a conference abstract and the number of respondents for each questionnaire was not reported. Responses were given on a scale of 1–10, with higher scores indicating greater satisfaction/acceptability.
Both sets of participants, those receiving their first colposcopy and those with prior colposcopy experience, found that their examination did not take longer than their previous smear test or colposcopy. The level of anxiety for all patients dropped during and after the examination with DySIS colposcopy compared with the result of a prior examination, from a median score of 7 out of 10 before colposcopy to a median score of 4 during and 1 after the examination in patients undergoing their first colposcopy examination. The results were similar for patients with prior colposcopy experience (median scores of 6 before, 3 during and 1 after the examination).
All patients reported that they understood the DySIS colour-coded map and found the map reassuring. Finally, all patients with previous colposcopy experience declared that they preferred having their future colposcopies with DySIS and would recommend DySIS to family and friends requiring colposcopy.
The authors concluded that DySIS with DySISmap is very well received by patients and is not intimidating, and it does not require longer examination times. It also helped to improve patients’ experience and their understanding of their condition, which in turn improves their overall experience and may reduce non-attendance rates.
Louwers et al.63
Louwers et al. 63 was a substudy of the trial by Louwers et al. 60 and included 239 women who underwent colposcopy with DySIS and DySISmap. All participants were asked to complete a patient satisfaction questionnaire.
The results showed that 93.9% of the participants agreed or strongly agreed to have colposcopy with DSI if it assisted in locating cervical neoplasia, 29.5% agreed or strongly agreed that DySIS was less comfortable than a Pap smear, 16.5% reported that DySIS made them feel nervous during the examination colposcopy and only 6.5% of participants thought that DySIS colposcopy took too long.
When asked which test characteristics were considered to be most important, 88.3% of participants ranked test accuracy as the most important factor. Rapid testing was considered to be the second most important factor and comfort was considered to be the third most important factor. Quick turnaround on results was considered to be the second least important factor and cost was considered to be the least important.
Among a subset of 19 participants who had experienced colposcopy examination prior to the study, results were similar to those of participants who had never had colposcopy before. However, all participants disagreed or strongly disagreed with the statement ‘Colposcopy with DSI takes too much time’.
The authors concluded that women are willing to accept discomfort (in the form of an additional or longer test) if the test has clear clinical benefits.
Budithi et al.46
(Confidential information has been removed.)
Acceptability of the adjunctive technologies to clinicians
Coronado and Fasero51
Coronado and Fasero51 surveyed 63 medical practitioners with different levels of colposcopy experience to gather their views on using DySISmap images compared with conventional colposcopy alone. The study also included a retrospective review of colposcopy and DySISmap images to estimate the accuracy of conventional colposcopy and DySIS when diagnosing cervical pathology based on different levels of practitioners’ experience.
Images from 50 participants with normal and abnormal cervices collected during colposcopy examinations and the corresponding DySIS maps were projected consecutively to the colposcopists. For each case, participants were asked to select one of the following four probable results for that case: normal, low-grade lesion, high-grade lesion or cancer.
The study population comprised 27 practitioners with a low level of colposcopic experience (i.e. first- to third-year residents), 18 practitioners with a medium level of experience (fourth-year residents and gynaecologists with a low level of experience) and 18 practitioners with a high level of experience (experienced gynaecologists and accredited colposcopists). None of the participants had any previous experience with DySIS.
Correct diagnosis was more frequent with DySIS than with conventional colposcopy in the low-level experience group and the medium-level experience group, but not in the high-level experience group (Table 14).
| Group | Number of practitioners | Mean number of correct diagnoses by method, n (95% CI) | |
|---|---|---|---|
| DySIS | Colposcopy | ||
| Low level of colposcopic experience | 27 | 24.4 (22.7 to 26.2) | 20.4 (18.8 to 21.9) |
| Medium level of colposcopic experience | 18 | 26.0 (25.0 to 27.0) | 21.9 (20.4 to 23.4) |
| High level of colposcopic experience | 18 | 26.5 (24.4 to 28.7) | 24.8 (22.8 to 26.9) |
Table 15 presents the results of the survey. All experience groups agreed that DySIS was generally better than colposcopy at guiding biopsy site selection and tended to agree that DySIS allows practitioners to perform a colposcopy without experience.
| Question | Level of colposcopic experience, mean (95% CI) | |||
|---|---|---|---|---|
| Low (n = 27) | Medium (n = 18) | High (n = 18) | p-value | |
| Is the DySIS interpretation easier than conventional colposcopy? | 4.0 (3.6 to 4.4) | 4.2 (3.7 to 4.7) | 2.8 (2.2 to 3.5) | 0.001 |
| Did DySIS orient better my diagnosis? | 4.0 (3.6 to 4.4) | 3.9 (3.4 to 4.4) | 3.2 (2.6 to 3.8) | 0.028 |
| Did DySIS orient better my biopsy site? | 4.3 (4.0 to 4.5) | 4.1 (3.7 to 4.5) | 3.7 (3.2 to 4.3) | 0.127 |
| Do you believe that DySIS offers more information than conventional colposcopy? | 3.1 (2.6 to 3.6) | 3.5 (3.0 to 4.0) | 2.6 (2.1 to 3.1) | 0.074 |
| Do you believe that DySIS allows performing a colposcopy without experience? | 3.4 (2.9 to 3.9) | 3.4 (2.7 to 4.2) | 3.7 (3.2 to 3.8) | 0.731 |
| Do you believe that DySIS is better than conventional colposcopy? | 2.8 (2.3 to 3.3) | 2.9 (2.2 to 3.5) | 1.9 (1.5 to 2.4) | 0.030 |
Compared with the high-level experience group, the low-level and medium-level experience groups were more likely to agree that DySIS interpretation is easier than interpretation with conventional colposcopy, DySIS is better at directing diagnosis, DySIS provides more information than conventional colposcopy and DySIS is generally better than conventional colposcopy.
The authors concluded that adjunctive DySIS improves diagnostic accuracy compared with colposcopy alone, especially among less experienced colposcopists. The authors also stated that the inclusion of the DySIS map into colposcopy is an easy and intuitive way to improve a conventional colposcope, particularly for clinicians with limited colposcopic experience.
Budithi et al.46
(Confidential information has been removed.)
Training requirements
One study, which was conducted in a single centre in Sheffield that involved in-house colposcopists and was linked to Tidy et al. ,103 reported the time needed to train the colposcopists in using ZedScan for the first time. 96 The study reported that 5–10 minutes of additional time were needed for the initial training period and the colposcopists were able to complete the initial 10–20 ZedScan measurements within 2–3 minutes after examining 10–20 patients. No further details were reported.
The authors concluded that ZedScan as an adjunct to colposcopy has added minimal time to each appointment and exerted a negligible impact on the clinical output.
Other outcomes
No evidence was found for the following outcomes: successful database and record management, the capacity to perform colposcopies, and uptake and compliance.
Clinical effectiveness summary and conclusions
Diagnostic accuracy
Nine studies that evaluated adjunctive DySIS (DySISmap and DySIS colposcope) were identified. Adjunctive DySIS use was found to have a higher sensitivity (81.25%, 95% CI 72.2% to 87.9%) than standard colposcopy alone (57.91%, 95% CI 47.2% to 67.9%), but a lower specificity (70.40%, 95% CI 59.4% to 79.5%) than colposcopy alone (87.41%, 95% CI 81.7% to 91.5%). This difference appears to be primarily because adjunctive DySIS leads to more positive test results (i.e. more women are judged to have possible high-grade CIN); in all studies, the number of women with positive test results was higher with adjunctive DySIS than that with colposcopy alone. However, the summary PPV for colposcopy alone was only 55.8%, and so did not reach the recommended level of 65% for UK colposcopy. This may suggest that how colposcopy was used in the included studies may differ from UK practice. There was no evidence that DySIS improved diagnostic accuracy (in terms of DORs). There was insufficient evidence to assess whether or not adjunctive DySIS improves cancer detection.
Only two included studies investigated ZedScan. Both were led by the same researchers in Sheffield. (Confidential information has been removed.) The other was a study of a precommercial ZedScan prototype. These issues limited our ability to assess the diagnostic accuracy of ZedScan as an adjunct to colposcopy. The results from the prototype study suggested that adjunctive ZedScan could improve diagnostic accuracy when compared with colposcopy alone (i.e. it could increase sensitivity at the same specificity as colposcopy or vice versa). (Confidential information has been removed.)
Data on participant subgroups, including women infected with hrHPV or high-grade referrals, were limited. The results suggested that colposcopy alone has poor sensitivity to detect high-grade CIN in women with low-grade referrals (e.g. mild dyskaryosis). Adjunctive DySIS and ZedScan appeared to improve the diagnosis in low-grade referral cases. There was some limited evidence that the diagnostic accuracy of adjunctive DySIS may be greater in women infected with hrHPV.
The sensitivity analyses identified that the specificity of all methods was strongly dependent on what reference standard was used in women who were given a normal colposcopic evaluation result. Specificity was much higher when no biopsies were performed in those women, suggesting a possible verification bias caused by underdiagnosis of high-grade CIN. This verification bias could, in theory, also affect the estimates of sensitivity, although the evidence for this was less conclusive for standard colposcopy. This means that the actual specificity and sensitivity of colposcopy and adjunctive colposcopy are uncertain, as it depends on the use of the reference standard. Verification bias may not affect the relative differences in diagnostic accuracy between index and comparator tests in such studies, assuming that it will affect the accuracy of both tests equally.
Test failure rates ranged from 2.9% to 16.7% in studies evaluating a commercial version of DySIS. (Confidential information has been removed.)
There were limited data on diagnostic biopsies and treatments conducted during or following examination with adjunctive colposcopy. Owing to the lack of randomised evidence comparing adjunctive technology with colposcopy alone, evidence of the impact of adjunctive technology on the rates of diagnostic biopsies and treatment (including unnecessary treatments) is very limited.
Clinical effectiveness
Only three studies that reported data on our prespecified clinical effectiveness outcomes were included. One study of ZedScan reported three adverse events, of which one was serious, and two studies of DySIS with DySISmap reported that no adverse events occurred following colposcopy examination. No data were reported on mortality, morbidity and HRQoL in studies of DySIS and ZedScan.
Implementation
There is reasonable evidence that DySISmap as an adjunct to colposcopy is generally well received by patients referred for colposcopy and that patients are generally satisfied with the duration of examination (two studies). There is evidence to suggest that DySISmap was generally reassuring and that pre-examination levels of anxiety decreased significantly during and after the examination (one study), and that only a minority of patients (16.5%) felt nervous during the examination (one study).
There is evidence from two surveys that adjunctive DySIS was consistently perceived by clinicians to improve the accuracy of colposcopy and confidence in their diagnostic decisions and biopsy site selection. There is evidence that adjunctive DySIS was intuitive for clinicians with limited colposcopy experience and that it improved their evaluations (one study). There is evidence that the additional time required to use ZedScan is minimal for experienced colposcopists. All included studies had significant limitations; therefore, these findings need to be interpreted with caution.
No evidence was found for several of the prespecified outcomes (successful database and record management, the capacity to perform colposcopies and uptake and compliance). No evidence was found regarding the training requirements for DySIS. The limited evidence for ZedScan precludes conclusions for any of the implementation review prespecified outcomes.
Conclusions
The review of diagnostic accuracy found that adjunctive DySIS increased sensitivity, but decreased specificity, when compared with colposcopy alone. This appears to be because the number of women classed as having possible high-grade CIN is increased, rather than being attributable to any improvement in diagnostic accuracy per se. ZedScan also appears to be associated with a higher level of sensitivity and a lower level of specificity than colposcopy alone when compared with the evidence from other included studies. However, the evidence for the accuracy of ZedScan as an adjunct to colposcopy is limited, as it is based on a single study that was rated as being at a high risk of bias, and it also results from a lack of direct comparative evidence with standard colposcopy. This precludes any definitive conclusions regarding the diagnostic accuracy of ZedScan as an adjunct to colposcopy. There was insufficient evidence to directly compare DySIS with ZedScan.
There was too little evidence to assess whether or not the technologies have any adverse effects. DySIS appears to be well received by both patients and colposcopists.
Increased sensitivity should lead to more women with high-grade CIN being correctly diagnosed, but decreased specificity is also likely to lead to more unnecessary diagnostic biopsies and treatments in women without high-grade CIN. The clinical value of adjunctive colposcopy, whether with DySIS or ZedScan, therefore depends on whether or not the value of diagnosing more women with CIN outweighs the disadvantages of more unnecessary biopsies and treatment.
Chapter 4 Assessment of existing cost-effectiveness evidence
Relevant cost-effectiveness evidence of adjunctive colposcopy technologies (DySIS with DySISmap and ZedScan, hereafter referred to as ‘DySIS’ and ‘ZedScan’) was systematically identified, appraised for quality and summarised. The objectives of the review were to identify key structural assumptions, highlight areas of uncertainty and assess the generalisability of the results of existing models to the current decision problem. The findings from the review also informed the development of a new decision-analytic model reported in Chapter 5.
Methods
Searches
The literature search previously reported in Chapter 3, Methodology of the clinical effectiveness review was also used to identify studies relating to the cost-effectiveness of adjunctive colposcopy techniques.
Study selection
A broad range of studies were considered in the review, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (i.e. cost minimisation, cost-effectiveness and cost–utility and cost–benefit analyses) were included in the review.
Relevant studies were then selected in two stages. Titles and abstracts identified by the search strategy were examined and screened for possible inclusion. Full texts of the potentially relevant studies were obtained. Two researchers (MP and PM) examined these independently for inclusion or exclusion and disagreements were resolved by discussion.
A quality appraisal was carried out using the checklist of Drummond and Jefferson,123 which is available in Appendix 9. This checklist evaluates the extent to which each review result provides details on different aspects, such as study design, data collected, the use of these data in the economic evaluation and analysis, and interpretation of the results. The checklist was completed by one researcher and checked by another. Any disagreements were resolved by discussion.
Results of the review of existing cost-effectiveness evidence
The initial search of economic databases identified a total of 182 references. After the initial screening of titles and abstracts, only two studies30,124 were considered to be potentially relevant and were ordered for full-paper screening. Both studies met the selection criteria and were included in the review.
Of the two studies included, one was an independent assessment of the cost-effectiveness of DySIS developed for the previous NICE DG4 assessment31 (i.e. Wade et al. ). 30 The other study was a company-funded assessment of a prototype version of ZedScan (i.e. Whyte et al. ). 124 A summary and a critique of these studies are reported in the following sections.
Review of Wade et al.30
Decision problem/objective
A decision-analytic model was developed to assess the cost-effectiveness of adjunctive colposcopy technologies for assessing suspected cervical abnormalities in women referred for colposcopy as part of the NHSCSP under the HPV triage screening protocol.
Three technologies were initially considered: DySIS, LuViva Advanced Cervical Scan and Niris Imaging System. Owing to the lack of reliable data, LuViva Advanced Cervical Scan and Niris Imaging System were subsequently excluded from the base-case analysis.
The model evaluated costs from the perspective of the NHS and Personal Social Services, expressed in Great British Pounds (GBP) at a 2011 price base. The outcomes in the model were expressed in terms of quality-adjusted life-years (QALYs). The model employed a lifetime (50-year) horizon, and costs and outcomes were discounted at a rate of 3.5% per annum.
Strategies/comparators
The base-case economic evaluation compared the costs and health outcomes of DySIS, alone and as a colposopic adjunct, with standard colposcopy. The base-case results were presented by reason for referral (borderline and HPV-positive, mild and HPV-positive, moderate, severe, possible invasion, possible neoplasia, three times inadequate) and, for the whole population, were based on a weighted average of the results of each reason for referral. A separate indicative analysis was undertaken to test the sensitivity needed for Niris and LuViva to be considered to be cost-effective, given their reported costs and an assumed specificity.
Model structure
The model incorporated two elements: first, a decision tree to represent the initial diagnostic and treatment pathways for patients referred to colposcopy from the NHSCSP (under the HPV triage screening protocol), and, second, a Markov model that simulated the natural history of patients and captured future cytological screening and referrals to capture the long-term costs and outcomes of the initial diagnostic and treatment pathways.
The decision tree was specifically developed for the appraisal informing NICE DG4,31 whereas the Markov model was based on a revised model previously used by Hadwin et al. ,125 and was referred to as the ‘Sheffield model’. The decision tree had three main components: (1) diagnostic outcome, (2) treatment decision and (3) treatment outcome.
The diagnostic outcome depends on the diagnostic accuracy in relation to a patient’s true underlying health state. Specifically, diagnostic accuracy is modelled as the probability of being diagnosed with health state h, conditional on the true underlying health state h.
Treatment decisions depend on diagnostic outcome and the reason for referral. A patient may either not receive treatment or be referred for a diagnostic biopsy, a treatment biopsy (LLETZ) or a cancer treatment.
Treatment outcome has an impact on both the true underlying health state and subsequent screening. The impact on a patient’s health state depends on the probability of being cured. If patients have not been treated, they enter the natural history Markov model with their initial true underlying health state. In the case of patients with precancerous lesions who have been treated with excision biopsy, there is a probability that this will be cured. If treatment has been successful, patients enter the natural history model Markov in the ‘clear’ state; if not, they enter the natural history model with their initial health state.
As previously stated, a state-transition (Markov) cohort model is used to capture the long-term costs and outcomes of the initial diagnostic and treatment pathways by simulating the natural history of the patient and incorporating future cytological screening and referrals. Although the use of a cohort model was appropriate for the specific decision problem, the model itself subsequently required hundreds of separate states to be included in order to capture the complexity of screening pathways and the heterogeneity in treatment decisions and outcomes. This complexity arose because the screening pathways and treatment decisions depended on a patient’s characteristics (e.g. age, health state) and previous history (e.g. screening results, treatment outcomes, follow-up), and these also had an impact on the transitions between health states in the natural history model. With a cohort approach, the only way to account for individual heterogeneity and to build sufficient ‘memory’ to capture the complexities is to increase the number of states. Although the complexity could have been potentially more efficiently reflected by using a patient-level simulation, the final model structure appeared to rely heavily on the use of an existing natural history cohort model from Sheffield, which may have constrained the authors in terms of their chosen modelling approach.
The treatment pathways incorporated in the model were based on NHSCSP guidelines,15 which describe good practice in treatment decisions and the follow-up of precancerous lesions and invasive cancer. However, the authors identified an important discrepancy between guidelines and clinical practice and chose to use evidence from the latter based on data from Gateshead to capture heterogeneity in treatment pathways. For instance, national guidelines state that a patient with low-grade referral with normal colposcopy should be discharged without any treatment and returned to routine screening. Observational data from the Gateshead clinic suggested that 73.5% of these patients would receive a diagnostic biopsy and only 10.7% would be sent back to routine screening.
Adjunctive technologies to colposcopy were assumed to be used for the initial referral and for subsequent colposcopy appointments (treatment, follow-up). The model also assumed that there is no loss to follow-up and, hence, all women were assumed to attend subsequent appointments for colposcopy and cytology.
Main sources of data
The model incorporated three main sources of data input: (1) diagnostic accuracy, (2) natural history of cervical cancer and (3) characteristics of the population referred for colposcopy.
Diagnostic accuracy
The diagnostic accuracy of colposcopy and DySIS was based on the specificity and sensitivity reported by Louwers et al. 60 Louwers et al. 60 reported diagnostic accuracy based on a CIN 2+ cut-off point leading to a dichotomous classification of the performance of the diagnostic technologies. However, the economic model required the probability of the diagnoses of the different stages of disease, whether these were correct or incorrect, conditional on a patient’s true underlying health state (e.g. clear, HPV-positive, CIN 1 present). For example, a true ‘clear’ patient correctly classified as being below the CIN 2+ cut-off point (and hence defined as a true-negative patient based on the dichotomous cut-off point) could either have been correctly diagnosed as ‘clear’ or they could have been incorrectly diagnosed as having ‘CIN 1’. Hence, additional assumptions were employed in Wade et al. 30 to convert the diagnostic accuracy data into the probabilities required for the model.
The probabilities required for the model were calculated using two main steps. First, the probability of being diagnosed as true positive, true negative, false positive or false negative was derived from Louwers et al. 60 These probabilities depend on the devices’ diagnostic accuracy. Second, the probability of being diagnosed with a specific stage of the disease, conditional on a patient’s true underlying state and on being true positive, true negative, false positive or false negative was derived from a study by Gallwas et al. ,126 who reported the diagnostic accuracy of a different diagnostic device (the Niris Imaging System).
This approach relies on two strong assumptions, with the first being that the results reported by Gallwas et al. 126 are reliable and can be generalised for other types of devices. The study by Gallwas et al. 126 includes a 2 × 2 table with the outcome of colposcopy by patients’ health state, confirmed by histology. However, biopsies were performed only when precancerous lesions were suspected. The population targeted by the study therefore excludes false negatives. This was considered by Wade et al. 30 as a significant bias and motivated the decision to exclude the Niris Imaging System from the main analysis. The second main assumption is that the diagnostic accuracy of the Niris Imaging System, conditional on being true positive, true negative, false positive or false negative, is similar for colposcopy alone, DySIS alone and DySIS plus colposcopy.
Furthermore, the model aimed to capture subsequent referrals for colposcopy when patients are followed up or called for routine screening under the HPV triage scheme. Data on the performance of the cytology and HPV tests were derived from personal communication (S Eggington, University of Sheffield, 2017). The sensitivity and specificity of the HPV test given the patient’s age and her cytology result were derived from the TOMBOLA (Trial Of Management of Borderline and Other Low-grade Abnormal smears) study (Cotton et al. ). 127
Natural history of cervical cancer
The natural history model was based on the Sheffield model and simulated the progression of patients between nine mutually exclusive health states: clear, HPV, CIN 1, CIN 2/3, invasive cancer stages from 1 to 4 and death. Patients entered the natural history model in the health state based on screening and treatment outcomes. Patients were allowed to progress or regress between these states every 6 months. Age-related transition probabilities of progression and regression in the Sheffield model were derived from Myers et al. 128
The natural history model relies on three main structural assumptions: (1) the linear progression of patients between states, (2) a single combined state for CIN 2 and CIN 3 and (3) patients diagnosed with cancer who survive 5 years after treatment are cured and move to the ‘clear’ state. These assumptions are summarised below:
-
First, patients are allowed to progress stage by stage only. They are initially infected with HPV, then they develop CIN 1, CIN 2/3 and, finally, invasive cancer from stages 1 to 4. Cancer cannot regress without treatment. However, precancerous lesions can regress without intervention to an earlier stage or directly to the clear state.
-
Second, because the study by Myers et al. 128 did not provide separate transition probabilities for the CIN 2 and CIN 3 states, CIN 2 and CIN 3 were combined in the natural history model into a single combined health state. No additional mortality risk was assumed for precancerous lesions and HPV, CIN 1 and CIN 2/3, and these states were also assumed to be asymptomatic. Patients cured of CIN were assumed to have the same risk of future CIN as the general population.
-
Finally, the modelling of cervical cancer relies on a series of assumptions. The model uses different mortality rates for undiagnosed and treated cancer. Patients progressed between cancer stages only if they were not treated. Undetected cancer was assumed to be asymptomatic, with a probability of developing symptoms during each 6-month cycle that increased by stage. It was assumed that a patient who develops symptomatic cancer would be systematically diagnosed and treated within the next 6 months. When a patient was diagnosed with asymptomatic cancer during a routine screening, it was assumed that they would be diagnosed with stage 1 cancer. After being diagnosed with cancer, patients faced a 5-year elevated mortality risk. Patients who survived for 5 years were assumed to transition to the ‘clear’ state, and at this point were assigned a QALY decrement and cost associated with their previous cancer treatment. Patients who died from cancer immediately entered the death state, but were assigned a QALY decrement and cost associated with their previous cancer treatment.
Characteristics of the population referred for colposcopy
Estimating the characteristics of patients referred for colposcopy is critical to the cost-effectiveness assessment of adjunctive colposcopy technologies. Women entered the model with a ‘true underlying health state’ (e.g. clear, HPV, CIN 1) and a reason for referral (borderline changes, mild dyskaryosis, etc.). The reason for referral has an impact on treatment pathways and outcomes and the initial true underlying health state has an impact on colposcopy results and disease progression. To be representative of the population referred for colposcopy, the estimated joint distribution has to capture the impact of both the disease prevalence and the screening programme.
The joint distribution of the reason for referral and the true underlying health state in Wade et al. 30 was based on three sources of data: a study by Kelly et al.,129 data collected from sentinel sites for HPV triage implementation and data collected from Gateshead hospital. The distribution of patients by reason for referral, under the HPV triage protocol, was based on data from the NHSCSP, together with a study by Kelly et al. 129 Kelly et al. 129 reported the proportion of women with borderline or mild cytology results who tested positive for HPV. Data from sentinel sites for HPV triage implementation (10% of the English cervical screening programme) were used to estimate the impact of HPV triage on the distribution of women referred to colposcopy. The weighted population used in the base-case analysis was 38.52% of women infected with borderline HPV and HPV, 35.39% of women with mild dyskaryosis and infected with HPV, 11.51% of women with moderate dyskaryosis, 13.06% of women with severe dyskaryosis, 0.51% of women with possible invasion and 1.01% of women with possible glandular neoplasia.
The proportion of women with a true underlying health state h, given the reason for referral r, was based on a retrospective study conducted at the Northern Gynaecological Oncology Centre, Queen Elizabeth Hospital, Gateshead (hereafter referred to as the Gateshead data). The Gateshead data included 4533 patients who attended a colposcopy examination in 2008–9. Patients’ true health states were revealed by biopsy when available. The Gateshead data have two potential limitations. First, the data were collected from a single centre, whose patients may not be representative of the general population in terms of the prevalence of the disease. Second, the biopsies were not systematically performed. When a biopsy was not performed, the study reported the colposcopy diagnostic only. As colposcopy is not 100% sensitive and specific, the colposcopy outcome may not reflect the true prevalence of the disease. Hence, CIN 1 and ‘clear’ health states, which do not need to be confirmed by histopathology, may be over-represented. In an alternative scenario, the sample was restricted to diagnosis confirmed by histopathology. However, this approach tends to select more severe cases and therefore is likely to underestimate the prevalence of normal, HPV and CIN 1.
Resource use and costs
The resource use and cost estimates included the acquisition costs of the alternative technologies (including maintenance and use of disposables) and further treatments. The number of patients managed under a single colposcopy device was provided by clinical advisors and was used to calculate the average cost per procedure for each technology. To capture the additional costs of a colposcopy visit (e.g. diagnostic and treatment biopsy), treatment costs from the TOMBOLA study130 were used. Cancer costs by stage were derived from a UK-based study by Wolstenholme et al. 131
The cost per patient of colposcopy and adjunctive technologies has two main components: (1) a cost per patient, common to all devices, which includes costs related to the examination itself, such as facilities and staff, and (2) an additional cost that depends on the device and includes the cost of acquisition, maintenance and disposables. The UK-based TOMBOLA study was used to estimate the cost of colposcopy alone (including the price of the device and maintenance) as well as the per-patient costs of biopsy and excision treatment (LLETZ). 130 The examination with DySIS was assumed to be equivalent to colposcopy alone in terms of staff resources (i.e. the same length of consultation and negligible staff training). The additional cost includes the acquisition cost, annual maintenance costs and disposables, all provided by the manufacturer. It was assumed that clinics have the choice between investing in a binocular colposcope or the DySIS device (colposcope plus digital map). Therefore, the purchase price of a binocular colposcope was included in the cost of colposcopy alone, but not for DySIS. Another important assumption is the number of patients examined per year per colposcope. This is critical when estimating the cost per patient, especially if technologies differ in terms of the initial investment and throughput. An estimate of 1229 patients per year was assumed based on clinical advice. This was assumed to be exogeneous to the performance of the device.
The management costs of cervical cancer by stage at the initial diagnosis were derived from Wolstenholme et al. 131 The study was based on an audit of resources and costs over 5 years on 261 women in the Trent region of central England in 1990. Although these costs appear to be based on the best evidence available at the time, there exists uncertainty regarding how representative these estimates are, given the historic nature of the study and the need to inflate costs over a significant time period.
Quality of life/utilities
Within the model, colposcopy and DySIS were assumed to have an impact on health outcomes in two ways: (1) through the disutility associated with the examination itself and with subsequent treatment and tests, and (2) through the disutility associated with the development of invasive cancer.
The direct disutility associated with colposcopy alone and DySIS was not assumed to be different. However, because diagnostic accuracy has an impact on the number of subsequent treatments and follow-up, QALY decrements associated with colposcopy examination, cytology test and biopsy were important for the assessment of cost-effectiveness.
The disutility associated with cytology examination was assumed to be 0.02 (Insinga et al. 132) over 1 month (i.e. –0.0016 per cytology exam). The disutility of colposcopy and biopsies was derived from a time trade-off analysis by Birch et al. 133 The study reported HRQoL for three different scenarios: ‘three repeat Pap Smears’ (0.958), ‘immediate colposcopy with no pathology’ (0.927) and ‘cone biopsy after immediate colposcopy’ (0.922). The difference of 0.031 between Pap smears and immediate colposcopy was used as a QALY decrement applied for each colposcopy examination. The difference of 0.005 between colposcopy with no pathology and cone biopsy was used as a QALY decrement for diagnostic and treatment biopsies. As noted by the authors, this rather small decrement, which was similar for diagnostic and treatment biopsies, is a strong assumption and may underestimate the impact of biopsies on health. As noted in Chapter 3, Systematic reviews of adverse outcomes of cervical intraepithelial neoplasia treatment, recent evidence exists that indicates that treatment biopsies may increase the risk of adverse obstetric outcomes, and specifically preterm delivery. However, the evidence at the time at which the model was conducted was reported to be scarce and inconclusive. Given this uncertainty, the QALY decrement of a treatment biopsy on health outcomes was further explored in a separate scenario analysis. The HPV, CIN 1 and CIN 2/3 states were assumed to be asymptomatic and hence were assigned the same utility as the clear state, reported as 0.91.
Health utilities associated with the four stages of invasive cancer were derived from Chuck et al. 134 It was assumed that invasive cancer would be detected only at an asymptomatic stage through routine screening. Patients who subsequently developed symptoms were assumed to be immediately referred for colposcopy and were appropriately diagnosed and treated. The model thus made an important distinction in the health utilities between undiagnosed and diagnosed cancer. Specifically, the model incorporated much lower HRQoL scores for untreated (and therefore undiagnosed) cancer than for treated cancer. These estimates were reported to be based on estimates reported by Chuck et al. 134
In our review, we identified some important uncertainties surrounding the source and subsequent application of the estimates within the model by Wade et al. 30 The estimates reported by Chuck et al. 134 are actually referenced to a separate study by Goldie et al. 5 Goldie et al. 5 distinguished ‘quality weights’ for detected invasive cancer, by stage and quality weights after treatment for invasive cancer. The use of the estimates from Goldie et al. 5 and their application in the model by Wade et al. 30 raises several potential issues. First, it is unclear whether or not ‘detected invasive cancer’ as described by Goldie et al. 5 can be interpreted as cancer ‘without treatment’. This appears to be inconsistent with the fact that undiagnosed cancer is assumed to be asymptomatic. Our interpretation of the estimates reported by Goldie et al. 5 is that the lower weights of detected cancer might more appropriately indicate that disutility is higher at the time of diagnosis and initial treatment than ‘after treatment’. 5 The ‘detected invasive cancer’ stage might therefore be considered to capture the disutility associated with the initial period after diagnosis. This would appear to be more consistent with the initial treatment burden that patients treated for cervical cancer may face after being diagnosed, but who may subsequently recover relatively quickly, particularly when cancer is detected at an early stage. Second, the method to estimate these ‘quality weights’ reported by Goldie et al. 5 cannot be identified and hence the extent to which these appropriately represent health utilities is unclear. 5 Finally, there appears to be a reporting error in the Chuck et al. 134 study, and this was subsequently reproduced in the model by Wade et al. :30 the score associated with stage 2 cancer ‘without treatment’ is reported as 0.67 (higher than stage 1) in Chuck et al. 134 and 0.56 in Goldie et al. 5
Main results (including of the base-case and key sensitivity analyses)
The results of the economic evaluation compared DySIS plus colposcopy with colposcopy alone, for each reason for referral and for the whole population. In most instances, colposcopy alone was found to be dominated by DySIS plus colposcopy. That is, colposcopy alone had higher costs and lower health outcomes than DySIS plus colposcopy (Table 16).
| Technology | Costs (£) | QALYs | ICER |
|---|---|---|---|
| Colposcopy alone | 1313.59 | 20.41339 | |
| DySIS and colposcopy | 1254.00 | 20.42805 | Dominant |
We identified the potentially counterintuitive result that an increase in a diagnostic device’s specificity resulted in worse outcomes. In particular, it appeared to be better to falsely identify patients with CIN 1 as having CIN 2/3 than to find that they truly have CIN 1. According to the authors, this result suggested that the treatment of CIN 1 is more cost-effective than watchful waiting, because of the low cost and low QALY decrements associated with treatment biopsy. Further analyses subsequently determined the threshold of each input at which an increase in specificity would improve outcomes. The QALY decrement of treatment biopsy needed to be increased from 0.005 to 0.13 or its cost needed to be increased from £97 to £2758.
Assessment of uncertainty
Several scenario analyses were considered, with the following variations in the base-case assumptions: the patient’s age, duration of the HRQoL decrements as a result of cancer, cancer treatment costs, perfect health for the clear and HPV states, the QALY decrement associated with treatment biopsies and cytological screening, the alternative costs of a colposcope, alternative treatment probabilities, and the assumption that patients who received negative test results through the use of colposcopy or adjunct technologies would be diagnosed as ‘clear’. Overall, for all of the sensitivity analyses undertaken, colposcopy alone was dominated by DySIS plus colposcopy.
A secondary analysis considered a higher cost and QALY decrement associated with treatment biopsy (–0.13 from 0.005 in the base case). At this value, a higher level of specificity is expected to generate improved outcomes. The secondary analysis was also combined with the sensitivity analyses previously described. The results suggested that colposcopy alone was still dominated by DySIS plus colposcopy (Table 17).
| Technology | Costs (£) | QALYs | ICER |
|---|---|---|---|
| Colposcopy alone | 1313.59 | 20.33799 | |
| DySIS and colposcopy | 1254.00 | 20.34705 | Dominant |
A probabilistic sensitivity analysis (PSA) was not conducted and all of the results were based on deterministic estimates.
Review of Whyte et al.124
Decision problem/objective
The aim of Whyte et al. 124 was to assess the cost-effectiveness of a prototype version of ZedScan as an adjunct to colposcopy relative to standard colposcopy alone from a NHS perspective. The population included in the analysis was women referred to colposcopy from the NHSCSP (under the HPV triage screening protocol).
The analysis focused on assessing the impact of ZedScan on initial colposcopy appointments and colposcopy follow-up appointments. Outcomes were predicted for three different types of colposcopy clinic [‘see-and-treat’, ‘treat-later’ (watchful waiting) and ‘triage’ clinics], which allowed for the optimal screening pathway to be established, as well as the cost-effectiveness of ZedScan in each type of clinic. The different types of clinic were formally defined as follows:
-
See-and-treat clinic – women may receive treatment at their initial colposcopy appointment if the diagnosis suggests that this is appropriate.
-
Treat-later clinic – no treatment at the initial colposcopy appointment. Biopsy confirmation before treatment at a later colposcopy appointment.
-
Triage clinic – high-grade referral patients are seen in a see-and-treat clinic, so women may receive diagnosis and treatment at the same colposcopy appointment. Low-grade referral patients are seen in a treat-later clinic.
The analysis used a diagnostic threshold for ZedScan, which set the sensitivity at a similar level to that of standard colposcopy, resulting in the main difference between the two devices being their specificity rates. The analysis assessed the cost-effectiveness of ZedScan by assessing several outcomes, which included the number of colposcopy appointments, the number of biopsies conducted, the costs related to colposcopy and adverse events, and overtreatment rates. In addition, the analysis also considered the treatment rates of CIN and associated adverse event rates, as well as whether or not an increased sensitivity level with ZedScan might increase clinicians’ confidence to offer more ‘see-and-treat’ appointments as a result of reductions in the numbers of unnecessary treatments and the associated cost-effectiveness of such changes.
The time horizon of the evaluation was 3 years and was justified on the basis that most patients would be returned to routine screening within this time period. Costs and outcomes were discounted at a rate of 3.5% and a price year was not stated.
Strategies/comparators
Using ZedScan as an adjunct to colposcopy was compared with colposcopy alone. No attempt was made to compare ZedScan with alternative diagnostic tools that can be used as an adjunct to colposcopy.
Model structure
The economic evaluation modelled the natural history of women at risk of developing cervical cancer and the colposcopy diagnostic pathway. A patient-level model was constructed, allowing the model to track the underlying health state of patients and their location within the colposcopy pathway. After an initial colposcopy in the first cycle, patients were located in one of the following discrete locations within the colposcopy pathway: ‘low-grade CIN 1 follow-up’, ‘high-grade follow-up pathway’, ‘Test of cure’, ‘routine screening’, ‘refer to colposcopy following fail at test of cure’, and ‘cancer’. Following the diagnostic pathway, the model tracked patients’ natural history, with women able to progress or regress through the CIN stages. The discrete health states included in the analysis were ‘clear’, ‘HPV’, ‘CIN 1’, ‘CIN 2’, ‘CIN 3’ and ‘cancer’, with cancer divided into International Federation of Gynecology and Obstetrics (FIGO) stages of severity from I to IV. A cycle length of 6 months was selected, as this is the shortest length of time between repeat colposcopies.
The model estimated the number of colposcopy appointments and biopsies conducted at each of the clinic types, as well as the number of women who received treatment for CIN for each type of colposcopy. This allowed for the estimation of costs associated with each scenario, as well as the average utility decrements associated with colposcopy, biopsy and treatment. The model simulated 1,000,000 women for each diagnostic tool and each clinic type.
The distribution of the colposcopy population on the basis of the women’s health states and cytology grades was estimated using data on the outcome of colposcopy given the referral cytology. After patients received their initial cytology examination, there were assumed to be seven possible outcomes. If the result was negative, then patients were returned to routine screening, a mild or borderline outcome results in a HPV test being conducted, with a positive HPV test result leading to a low-grade referral to colposcopy, and a negative result leading to patients being returned to routine screening. If the cytology result was ‘moderate’, ‘severe’ or ‘invasive cancer’, then patients were sent to colposcopy as a high-grade referral. If the outcome of cytology was inadequate, then patients were sent for a repeat cytology test in 3 months’ time, with three consecutive inadequate results leading to a low-grade colposcopy referral. Patients’ referral type was tracked, as it was assumed that ZedScan would be used with a different threshold for low-grade and high-grade referrals, resulting in different sensitivities and specificities.
Whether or not patients received treatment and the timing of treatment was dependent on their referral grade, their colposcopy result and the clinic type. The three types of clinics that were considered in the analysis were ‘see-and-treat’, ‘treat-later’ and ‘triage’ clinics. At the ‘see-and-treat’ clinics, women could receive treatment at their initial colposcopy appointment if the diagnosis was clear that this was appropriate. ‘Treat-later’ clinics do not provide treatment at the initial colposcopy appointment; instead, patients receive a biopsy confirmation and are treated at a future appointment. ‘Triage clinics’ split patients by their referral grade, with patients with high-grade referrals being managed in a ‘see-and-treat’ clinic and those with low-grade referrals being managed in a ‘treat-later’ clinic. Women treated for CIN are assumed to be treated with LLETZ, although in practice a range of treatment options are available.
After colposcopy, patients follow one of six pathways. First, women with a low-grade referral who are identified as clear are sent back to routine screening in 3 years’ time. Second, patients who are identified with cancer are sent to oncology regardless of their referral grade. The third pathway results in women with a low-grade referral identified as having CIN 1 receiving a further colposcopy in 12 months’ time. If they are found to have CIN 1 at the follow-up colposcopy then they are sent for a further colposcopy 1 year later, but after a third CIN 1 result, they are treated. At the second or third colposcopy those who are identified as clear return to routine screening, women found to have CIN 2+ are sent for treatment and those identified with cancer are referred to oncology. The fourth pathway involves women with a high-grade referral who are identified as clear or having CIN 1 being referred to colposcopy in 6 months’ time, with a subsequent result of CIN 1+ resulting in treatment and a result of cancer leading to an oncology referral. Those who are identified as clear in the repeat colposcopy are sent back for a colposcopy in a further 6 months, with five consecutive clear colposcopy results resulting in patients being sent back to routine screening. The fifth pathway results in patients who are treated for CIN going on to receive a test of cure, which consists of a cytology test at 6 months and a subsequent HPV test if the cytology result is negative. The final pathway involves referring patients who receive a test of cure and have a positive cytology or a positive HPV test then patients to colposcopy.
Following completion of the screening phase, patients enter a natural history model. This model assumes that patients start with a high risk of HPV infection, which can either clear or progress to CIN, with patients starting with mild changes (CIN 1) and either progressing to more severe stages (CIN 2 and CIN 3) or regressing back to clear. If patients progress to cancer, then it is assumed that they can regress back to a non-cancer state with or without treatment. Progression and regression rates of CIN 2/3 to and from cancer were assumed to be similar to the rates from CIN 1 to CIN 2/3 and clear.
Owing to the analysis focusing on a time horizon of only 3 years, the natural history model is unable to capture the long-term effects of failing to identify and treat CIN caused by ZedScan having a lower sensitivity than colposcopy for low-grade referrals. In the short term, failing to treat those with CIN will reduce costs; however, there will be longer-term treatment cost, HRQoL and mortality impacts that are not captured within the time horizon.
Main sources of data
The methods used to identify appropriate parameter estimates were not transparently reported. The study makes reference to a review that was conducted in order to identify studies with information useful for understanding the natural history of HPV. However, the details of the search were not reported. No review was explicitly reported for other parameters.
Natural history/baseline data
The review conducted to identify publications reporting data on the natural history of HPV and CIN found eight studies135–142 of relevance. Data taken from a French prospective study conducted by Sastre-Garau135 were used to estimate the 6-month progression and regression rates between states in the natural history model. The study tracked 86 women with confirmed CIN 1 and observed how many patients progressed and regressed over a median follow-up time of 24 months. The transition probabilities utilised in the model were highly uncertain because of the small sample size of the study. In addition, strong assumptions were made that the progression and regression rates from CIN 2/3 to CIN 1 and cancer were the same as those from CIN 1 to clear/infected with HPV and CIN 2/3, with little justification provided for this assumption.
The proportions of the initial true underlying states of patients in the analysis and their reason for referral were estimated using data from a study published by Blanks and Kelly. 143 This study examined cytology data from 102 laboratories and presented findings for patients who had received a follow-up colposcopy. The assumption was therefore made that the result that patients received at colposcopy accurately determined their true underlying health status, which is a strong assumption, as the sensitivity of colposcopy is below 100%. In order to address this limitation, a sensitivity analysis was conducted in which the prevalence of CIN 2+ was increased by 10%.
Treatment effects
The performance of ZedScan and standard colposcopy in terms of their sensitivity and specificity rates was measured using two methods: colposcopic impression and disease present. Colposcopic impression measured performance by assessing whether or not a patient was correctly identified as either having CIN 2+ or having a normal result/having CIN 1 by clinical impression only. However, disease present measured performance by looking at whether or not a patient was correctly identified or scheduled to receive a biopsy, when a biopsy was assumed to have 100% sensitivity and specificity. The colposcopic impression method therefore results in a higher specificity value for the devices, whereas the disease present method results in the devices having a higher sensitivity.
The data on the sensitivity and specificity of standard colposcopy and ZedScan were taken from the EpiCIN trial (Tidy, 2013). 90 The study recruited 474 women who were referred to colposcopy with an abnormal cytology result and included two phases. The first phase involved using ZedScan to take EIS readings from different points on the cervix before and after the application of acetic acid and assessing its performance against colposcopic impression and disease present. This allowed for a probability index and a threshold value for the detection of CIN to be calculated, which indicated the sites for biopsy in the second phase. The second phase involved clinicians selecting biopsy sites before using ZedScan to identify additional sites based on whether the probability of high-grade CIN was greater than the selected threshold value. The sensitivity and specificity values for ZedScan and colposcopy used in the model were taken from Phase II of the trial. Different sensitivity and specificity values were estimated for high-grade and low-grade referrals, respectively.
A limitation of the analysis is that the sensitivity and specificity were assumed to be equal for clear/CIN 1 and for CIN 2/3, which is a simplification of reality. These sensitivity and specificity values are likely to differ, as, for example, patients with CIN 1 will be likely to have a greater chance of being incorrectly identified as having CIN 2+ than those who are clear. This is an issue, as the identification of CIN 1 patients is important because they will experience a different follow-up treatment pathway from those who are diagnosed as being clear.
Estimates of the frequency of biopsy at colposcopy were calculated from data found in Phases I and II of the EpiCIN trial and using several assumptions. The data used were estimated rather than being observed, which made the data susceptible to error, and the estimates did not come from a ‘see-and-treat’ clinic, so the generalisability of these may be limited.
Resource use and costs
The costs of colposcopy, biopsy and treatment were estimated using data from Sheffield Teaching Hospitals, and, therefore, it is unclear whether or not they are generalisable to the wider UK population. These costs were calculated using the price of components and staff time, with a fixed cost included for colposcopy.
The cost per use of the ZedScan device was assumed to be £31, which was stated to include the disposable tip, cost of device, training and maintenance. However, no calculations were reported to demonstrate how this cost had been derived. The ZedScan device was assumed to be used for all diagnostic colposcopies, but not for treatment colposcopies following biopsy confirmation of disease.
The costs of HPV testing and cytology were taken from the HPV sentinel sites. Adverse event costs were included for women experiencing severe bleeding or discharge (the TOMBOLA Group). 144
Quality of life/utilities
Quality-of-life decrements were included for bleeding, pain and discharge, with data on adverse event frequency and the duration of each event taken from the TOMBOLA trial, a large, multicentre, UK-based study. 144 Utility decrements were also included to capture patients’ preferences for the follow-up they receive after an abnormal cytology result, which were estimated for colposcopy, colposcopy with biopsy and colposcopy with LLETZ using the time trade-off method. 133 The potential cost and quality-of-life impact of the increased risk of preterm birth as a result of treatment or biopsy was not included in the model. This was justified based on conflicting results from the identified studies.
Main results (including base-case and key sensitivity analyses)
The base-case results reported a lower frequency of biopsy, as well as lower total costs and a lower cost per woman with CIN 2/3 treated for those diagnosed using ZedScan than for those treated with standard colposcopy for each clinic type. ZedScan was also reported to lower the rates of overtreatment, with 12% of the level of overtreatment occurring in ‘see-and-treat’ clinics, and 17% of the level of overtreatment occurring in ‘triage-by-cytology-result’ clinics compared with the level of overtreatment in women who received standard colposcopy.
The lower costs reported for ZedScan were also reported to be attributable to a reduction in the number of follow-up appointments for CIN 1. The authors concluded that using the disease present method resulted in fewer biopsies being taken following ZedScan and more women being followed up with a cytology test rather than a repeat colposcopy appointment. Importantly, the lower sensitivity of ZedScan for low-grade referrals also resulted in a lower number of women with CIN 2+ being diagnosed using ZedScan, as well as a minor reduction in the number of cancers identified at colposcopy across all clinic types. Hence, some of the reduction in total costs for ZedScan was as a result of undertreatment for CIN 2+.
The base-case results also found that ‘treat-later’ clinics had the lowest cost per woman treated for CIN 2/3, for both ZedScan and standard colposcopy. Based on their findings, the authors concluded that the results did not appear to support a move from a ‘treat-later’ clinic to a ‘see-and-treat’ clinic using ZedScan. This was because the cost per woman treated for CIN 2/3 was lower for a ‘treat-later’ clinic using standard colposcopy than for a ‘see-and-treat’ clinic using ZedScan.
The authors noted the limitations arising from restricting the scope of the model to initial colposcopy and follow-up for up to 3 years. Specifically, the long-term costs and consequences of failing to identify and treat CIN 2+ or the impact of identifying and treating CIN or cancer at an earlier stage were not captured. The authors noted that care should thus be taken regarding the interpretation of the results, as a reduction in the treatment of CIN 2+ or cancer will appear to be beneficial in the short time horizon of the model (reducing treatment costs), but would actually lead to higher costs and lower outcomes over a longer time horizon.
From a policy perspective, these limitations mean that it is not possible to determine whether or not the short-term benefits of ZedScan arising from a reduction in unnecessary treatments offset any potential reduction in benefits over a longer time horizon and, hence, whether over a more appropriate horizon in which this could be reflected ZedScan would be cost-effective or not.
Assessment of uncertainty
A range of sensitivity analyses were conducted in order to assess the uncertainty around particular parameters. For underlying disease prevalence, the proportion of CIN 2+ patients was increased by 10%, and for costs, the values used in a previous diagnostics assessment report (Wade et al. 30) were used as an alternative (with the authors noting an important disparity in the cost of biopsy). In addition, the threshold at which a biopsy was taken was lowered for ZedScan and colposcopy. Different values for the frequency of biopsy for patients who were diagnosed using ZedScan were also used: a low estimate that assumed that only one biopsy was required based on clinical guidance and a high estimate that assumed that clinicians could take additional biopsies. An analysis was also conducted in which ZedScan was assumed to have the same specificity as colposcopy, and therefore the only difference between the devices was their sensitivity.
A PSA was also conducted to assess the joint uncertainty of the parameter inputs. Distributions were assigned to the test characteristic parameters, the initial distribution of health states, utility decrements and transition probabilities. However, it appears that an arbitrary estimate of uncertainty (± 5% the base-case value) was used to inform the distributions for both the natural history transition probabilities and the utility decrements. No details were reported in terms of the number of simulations undertaken within the PSA.
The results of the sensitivity analyses demonstrated that the findings appeared to be robust to a range of alternative assumptions. However, the findings were reported to be particularly sensitive to assumptions surrounding the costs of colposcopy. When the estimates reported in Wade et al. 30 were applied to the model, standard colposcopy appeared to be cheaper than ZedScan, in terms of both total costs and the cost per woman with CIN 2/3 treated. The authors highlighted that the costs reported in Wade et al. 30 for both cervical biopsy and LLETZ appeared to be markedly lower than those based on the estimates derived from the Sheffield Teaching Hospitals.
The sensitivity analysis also found that setting the threshold in a way that resulted in the specificity of ZedScan being equal to that of colposcopy also increased the sensitivity and reduced the specificity of ZedScan. This resulted in an increase in the cost of using ZedScan and the number of patients treated, which reduced the benefits of the device in terms of cost per woman with CIN 2/3 treated. However, ZedScan was still reported to be cheaper than using colposcopy alone.
Using the higher estimate of the number of biopsies taken when patients were diagnosed using ZedScan had a small impact on the cost per woman with CIN 2/3 treated, but not enough to make ZedScan the more expensive option. Adjusting the disease prevalence data and the length of colposcopy appointments had a minimal impact on the results. The PSA results were reported to be comparable to the deterministic outcomes, demonstrating linearity in the parameter values.
Discussion of existing cost-effectiveness evidence and relevance to the current decision problem
Our review identified two published studies30,124 that reported the cost-effectiveness of DySIS and an earlier prototype version of ZedScan and that are partially relevant to the current decision problem. Although both studies evaluated the use of adjunctive colposcopy techniques for women referred for colposcopy only as part of the NHSCSP based on the current HPV triage screening protocol, only Whyte et al. 124 included a test of cure. As a result, neither study fully informs the current decision problem, which includes the current HPV triage protocol (including test of cure) and also the proposed HPV primary screening protocol.
Despite both studies considering a similar decision problem and referral population, there were important differences between the scope of the models and the analytic approaches employed. Only Wade et al. 30 attempted to capture the longer-term impacts of adjunctive colposcopy technologies in terms of the lifetime costs and QALYs. In contrast, the evaluation of ZedScan was restricted to a much shorter time horizon (3 years). The shorter time horizon precluded an overall assessment of the cost-effectiveness of ZedScan, as relevant longer-terms costs and outcomes were not quantified. However, one potential strength of the ZedScan study124 was that it provided a more granular assessment of the impact on a variety of different outcomes (including the rates of unnecessary treatment), as opposed to focusing on the final outcomes expressed in terms of life-years gained and QALY outcomes, as was the case in Wade et al. 30 From a policy perspective, the focus on final outcomes expressed in terms of life-years gained and QALYs is clearly not a limitation. However, the additional granularity in the reporting by Whyte et al. 124 provided greater transparency in relation to how costs and benefits manifest themselves with an adjunctive technology, which may be particularly informative in understanding how the trade-off between sensitivity and specificity has an impact on the intermediate outcomes, which then drive the estimates of life-years gained and QALY differences.
Structurally and conceptually, the models reflected similar pathways over the 3-year period, which was common to both models. The main difference was that Whyte et al. 124 included additional pathways for test of cure as part of the HPV triage screening protocol. Test of cure was not included by Wade et al. ,30 as this was not formally part of the HPV triage screening protocol at the time at which the study was conducted.
Although both models shared similar pathways over the initial 3-year period, there were important differences both in terms of the analytic approaches employed and in terms of how heterogeneity in the subsequent management of patients was characterised. In terms of the analytic approaches, Wade et al. 30 employed a cohort approach, utilising several hundred mutually exclusive states. The patient-level approach employed by Whyte et al. 124 resulted in a more efficient overall structure, albeit potentially at the computational expense of requiring 1,000,000 individual patients to be simulated to derive expected values for costs and outcomes. In terms of characterising the subsequent management of patients, the study by Wade et al. 30 assigned probabilities to different management strategies based on clinical practice reported in the Gateshead study. The study by Whyte et al. 124 characterised the different management strategies as a source of observable heterogeneity (as opposed to a source of uncertainty) and hence reported estimates to reflect three different types of clinical practice defined in accordance with clinic type (i.e. see-and-treat, treat-later/watchful-waiting and triage clinics). The approach by Whyte et al. 124 may confer potential advantages compared with treating treatment practice as an uncertain variable (and hence assigning a probability to subsequent management strategies), as it provides a basis both for determining how the cost-effectiveness of the adjunctive technologies might vary in accordance with different clinical practices, as well as potentially informing the efficiency of these practices.
The studies also differed in terms of several key inputs. The most notable differences were in the source of transition probabilities for the natural history model, which was derived from different studies, and particularly the costs of biopsy and LLETZ. The impact of the different natural history transition probabilities is not possible to determine, although the rationale for using a data set of only 84 patients was not clearly reported by Whyte et al. 124 In contrast, the impact of different costs for biopsies and LLETZ was identified as an important factor by Whyte et al. 124
Both studies also share two important and common limitations. First, both studies acknowledged a key assumption that followed from the use of diagnostic accuracy data based on a CIN 2+ cut-off point and the dichotomous classification of the performance of the diagnostic technologies; that is, the probability of a positive colposcopy result (CIN 2+) is assumed to be identical within the clear, HPV and CIN 1 states and within the CIN 2/3 and invasive cancer states. Second, both studies acknowledged that there may be additional risks of treatment (e.g. fertility and adverse obstetric outcomes), but neither study considered that these risks could be formally quantified, given the sparseness of, and conflicting results from, existing studies. To address the issues and uncertainties identified in the review, and particularly to inform the cost-effectiveness of both adjunctive technologies under both HPV triage and HPV primary screening protocols, a new independent model was developed.
Chapter 5 Independent economic assessment: York model
Overview
Chapter 4 identified a number of issues and uncertainties arising from previously published studies. A number of important limitations were also identified in relation to the current decision problem, specifically (1) the lack of any previously published studies reporting on the cost-effectiveness of the commercial version of ZedScan (ZedScan), (2) the lack of any attempt to formally compare different adjunctive technologies and (3) the absence of any studies evaluating the cost-effectiveness of either DySIS or ZedScan within a HPV primary screening protocol. For this reason, it has been necessary to develop a de novo decision model (hereafter referred to as the ‘York model’).
The York model was developed to estimate the cost-effectiveness of adjunctive colposcopy technologies (DySIS with DySISmap and ZedScan) for people who are referred for colposcopy through the NHSCSP, under either HPV triage (including test of cure) or the HPV primary screening protocol (including test of cure). The York model is implemented using a patient-level, state-transition modelling approach.
The model provides a link between diagnostic test accuracy and final health outcomes expressed in terms of QALYs. This is necessary in order to provide decision-makers with an indication of the health gain achieved by adjunctive colposcopy technologies, relative to their additional cost, in units that permit comparison with other uses of health service resources. This requires consideration of how each technology has an impact on the identification of cancerous and precancerous cervical tissue and linking this identification to treatment or monitoring options and their effect on disease progression. The model also includes the impact of the technologies on unnecessary biopsies and excisions, which may increase the risk of adverse obstetric outcomes.
The incremental cost-effectiveness of adjunctive colposcopy technologies (DySIS with DySISmap and ZedScan, hereafter referred to as ‘DySIS’ and ‘ZedScan’) compared with that of conventional colposcopy alone is determined based on an assessment of long-term NHS and Personal Social Services costs and QALYs. The time horizon of the model is a lifetime (60 years), costs and outcomes are discounted at 3.5% per annum, and a 2015–16 price year is used.
Contribution of the York model
Although the York model shares some of the assumptions and parameters from existing studies, it also provides a number of significant developments to existing cost-effectiveness analyses.
In the previous model used to inform DA4 (Wade et al. 30), the model structure required the probability of the diagnoses of the different stages of the disease, whether these were correct or incorrect, conditional on a patient’s true health state. Consequently, Wade et al. 30 used more granular data from Gallwas et al. ,126 which were based on another technology (Niris Imaging System), with the assumptions that the results from this study were reliable and generalisable to colposcopy alone and DySIS (S Eggington, personal communication). As previously highlighted in Chapter 4, the study by Gallwas et al. 126 was subject to a significant level of bias, and the use of data from another technology to inform the diagnosis accuracy of colposcopy and DySIS could be seen as an important limitation.
In the York model, the treatment pathways depend on the reason for referral (cytology result) and the dichotomous colposcopy result (CIN 2+) only. This allows for the use of diagnostic accuracy estimates with a CIN 2+ cut-off point. For patients referred for having low-grade dyskaryosis with a negative colposcopy result, the treatment pathway is identical whether the patients are diagnosed by the colposcopist as being clear, being infected with HPV or having CIN 1. For patients referred as having high-grade dyskaryosis and/or with a positive colposcopy result, a biopsy will be systematically performed, revealing the patient’s true underlying health state.
The York model still relies on two key assumptions: (1) the biopsy and the histopathology test are 100% accurate and (2) the probability of a positive colposcopy result (CIN 2+) is identical for patients who are clear, patients who are infected with HPV and patients with CIN 1, CIN 2/3 and invasive cancer. However, based on additional data provided by the DySIS manufacturer on the diagnostic accuracy of colposcopy alone and DySIS, we are able to explore the impact of the second assumption on the cost-effectiveness results.
An important limitation of the two cost-effectiveness studies identified (Wade et al. 30 and Whyte et al. 124) is that neither of the studies formally modelled the long-term adverse consequences of treatment excision. Based on more recent evidence of the impact of treatment on obstetric outcomes (Kyrgiou et al. 119), the York model includes the excess risk of preterm delivery for women who received LLETZ. We were therefore able to measure the consequences of an increase in treatments that arise from the higher sensitivity and lower specificity of adjunctive technologies than those of conventional colposcopy alone.
Finally, an important contribution of the York model is to inform the cost-effectiveness of adjunctive technologies under the HPV primary screening protocol. The implementation of HPV primary screening has two main consequences for the economic evaluation of adjunctive technologies: (1) the routine screening pathway is different and (2) the characteristics of the population referred for colposcopy are likely to be different. The impact of both of these issues is an important consideration regarding the possible differences in the cost-effectiveness of adjunctive colposcopy technologies under the current and potential future screening programmes.
Model structure
Choice of modelling approach
State-transition models can be used to conceptualise a decision problem in terms of the health states or conditions that individuals can be in (‘states’), how individuals move among these states (‘transitions’) and how likely such moves are [‘transition probabilities’ (Siebert et al. 145)]. State values (sometimes called ‘rewards’) are used to reflect the costs and HRQoL implications of residing in, or transitioning between, each health state. Estimates of expected costs and QALYs are derived by assigning state values to the time spent by patients in each health state.
A state-transition model is appropriate for modelling events, such as routine screening tests, that occur at fixed points of time and for conditions that have health states that may change repeatedly over time. Disease progression can be characterised in a state-transition model as a set of transitions among the states for time periods, typically of fixed duration (e.g. months, years).
State-transition models can use either a patient-level or a cohort-level (Markov) modelling approach to estimate the expected costs and outcomes across a particular population (Davis et al. 146). In a patient-level simulation, the costs and outcomes for individual patients are modelled and the expected (mean) values are derived from an average taken across the entire sample of patients. In a cohort approach, the expected cost and outcomes are estimated for an entire cohort and hence the costs and outcomes for individual patients are not explicitly modelled. The choice between using a patient-level simulation and a cohort approach requires careful consideration and appropriate justification (Davis et al. 146).
The challenge for a cohort approach arises from the complexity that comes from interactions between the natural history model (Figure 10) and the screening and treatment pathways (Figure 11). The natural history of cervical cancer can be schematically represented as an eight-state state-transition model (see Natural history model for further details). Such a model could be implemented using either a patient-level simulation or cohort approach. The main consideration in determining the choice of modelling approach is whether or not the ‘Markovian’ assumption (i.e. that transition probabilities do not depend on past history or time in state), which underpins the cohort approach, is appropriate. Inevitably, transitions between the natural history states will depend both on patient characteristics and also on patient history through previous screening outcomes (e.g. being referred to CIN 1 follow-up) or treatment outcomes (e.g. being cured of CIN 2/3). Appropriately accounting for patient heterogeneity and history using a cohort approach would require a series of subcohorts, which exponentially increases the required number of model states and would require hundreds of mutually exclusive states to be characterised. In this context, a cohort model becomes increasingly difficult to implement and manage. In contrast, patient-level state-transition models are not limited by the Markovian assumption, ensuring that transitions can appropriately reflect both individual patient characteristics and their history (using ‘tracker variables’). As a result, the complexities with the current decision problem can be more efficiently characterised using a patient-level simulation by significantly reducing the number of mutually exclusive states that are required.
FIGURE 10.
Natural history of cervical cancer.

FIGURE 11.
Links between screening, treatment pathways and the natural history model.
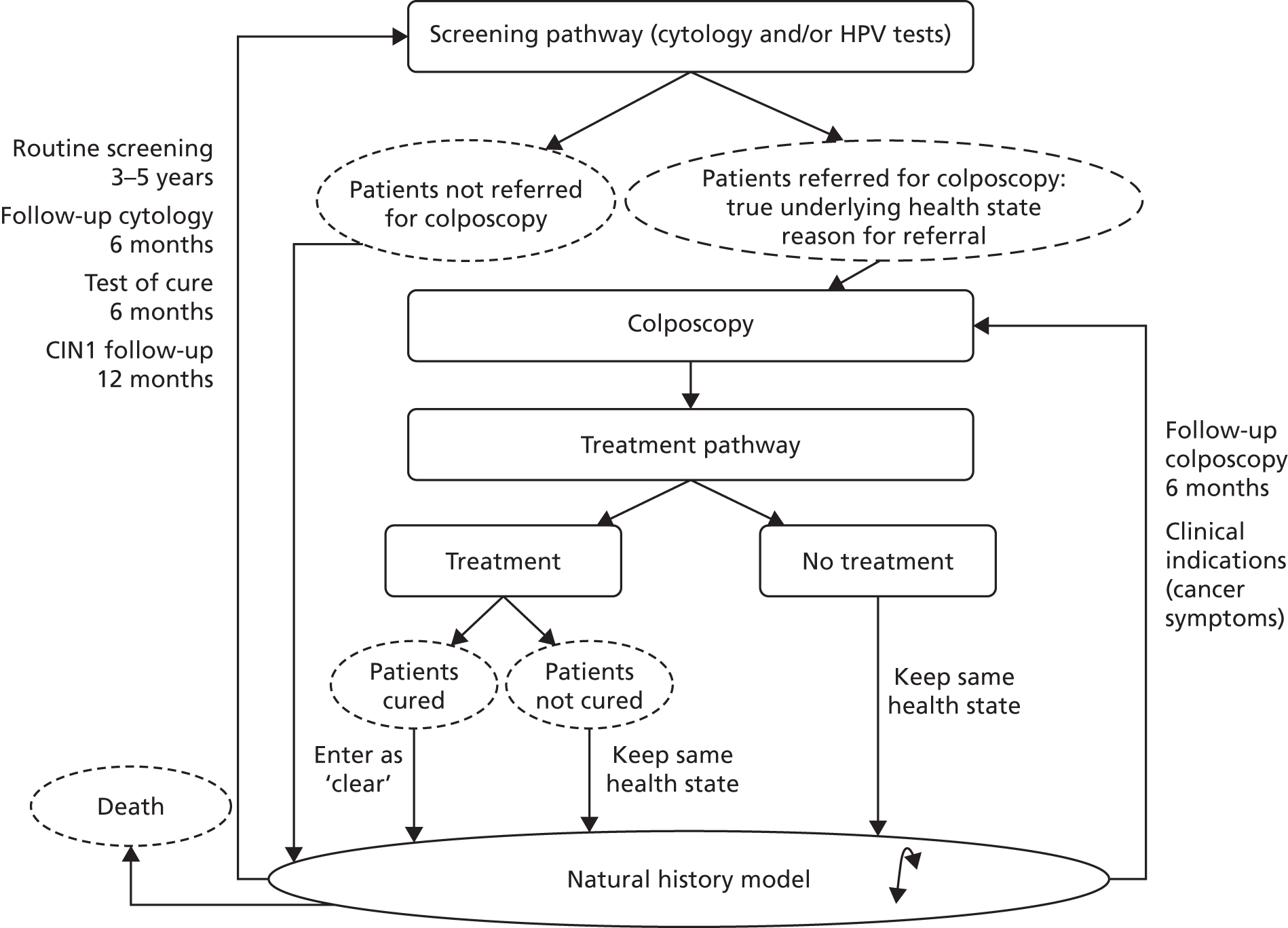
Patient-level Monte Carlo simulation
The model simulates individuals’ experiences with a Monte Carlo simulation and can be formally defined as a state-transition, patient-level Monte Carlo simulation. A patient-level model estimates the mean costs and benefits for a group of patients by considering the costs and benefits of each individual within the group. Each individual has specific characteristics that both affect and depend on the occurrence of events and associated transitions. In the present model, these characteristics are age, health state (clear, HPV, CIN 1, CIN 2/3, cancer), reason for referral to colposcopy (high-grade or low-grade dyskaryosis), next scheduled screening (routine call, 6-month cytology, 6-month colposcopy, test of cure, CIN 1 follow-up), time elapsed since last screening and type of clinic visited by the patient (see and treat or watchful waiting).
A cycle length of 6 months was selected, as this is the shortest time between repeat colposcopies. The cytology retesting (when inadequate) is the only event in the model that occurs after 3 months and can easily be taken into account in the 6-month probability of having a twice-inadequate cytology result. A 6-month cycle also avoids the computational burden of having to double the total number of cycles per individual simulation.
The decision-analytic model simulates for each patient the occurrence of uncertain events, such as disease progression, diagnostic results or treatment outcomes with a random walk (i.e. a series of uniform, pseudo-random numbers). A large number of simulations ensure that the proportion of patients in each state equals the individual probability. It is important to notice that a large number of simulations will appropriately characterise first-order uncertainty (i.e. the variability in the simulated experiences between patients), but not second-order uncertainty, which is linked to uncertainty around parameter values.
Implementation and schematics
The model is implemented with the TreeAge Pro 2016 software (TreeAge Software, Inc., Williamstown, MA, USA) and was run to simulate 500,000 women who were referred for an initial colposcopy appointment. The model has a decision tree structure, which represents events occurring sequentially as a pathway that is followed by individual patients. ‘Logic nodes’ (circles with an ‘L’ in the schematics) are used when the occurrence of the event is certain and depends on a patient’s characteristics only. ‘Chance nodes’ (an empty circle in the schematics) are used when the occurrence of the event is uncertain and depends on a probability. ‘Clones’ refer to subparts of the model that are common to different pathways (such as the natural history subpart) and therefore do not appear on the schematics for the sake of clarity. A triangle marks the end of a cycle; if the patient is still alive at the end of the cycle, they enter the model again; if they die, from cancer or other causes, they exit the model. The model structure is identical for the three strategies (colposcopy alone, DySIS and ZedScan), and only the input parameters vary (diagnostic accuracy and costs).
Main features of the model
The model can be separated into three main elements: (1) a natural history model, (2) screening and treatment pathways and (3) adverse obstetric outcomes. Although the time within each 6-month cycle is not explicitly modelled, screening events are assumed to occur before the natural history transitions, because treatment outcomes may affect a patient’s health state. The natural history model is derived from a widely used epidemiological model of cervical cancer and is discussed in more detail in Natural history model. Screening and treatment pathways are modelled based on NHSCSP guidelines15 and expert clinical opinion. 15
A patient enters the natural history model at each 6-month cycle. However, they do not necessarily experience a screening episode every 6 months. Indeed, the occurrence of screening events depends on the patient’s characteristics (age, cancer symptoms) and history (previous exams and treatment). A patient who received treatment faces a risk of adverse obstetric outcomes every year.
Natural history model
The natural history model (see Appendix 10, Figure 23) captures the progression of cervical cancer from the ‘clear’ state to stage 3 (distant) of invasive cancer. A patient enters the model with an initial health state and faces a 6-month transition probability to stay in the same state, progress or regress. The probability will determine the patient’s subsequent health state at the beginning of the next cycle.
The natural history model incorporates eight health states: clear, HPV infection without precancerous lesion, CIN 1, CIN 2/3, invasive cancer (local, regional and distant) and death. The structure of the natural history model is derived from Kulasingam et al. ,147 an update of Myers et al. ,128 which was used for the previous cost-effectiveness model by Wade et al. 30 Consequently, it relies on similar structural assumptions: HPV infection is a precondition to developing precancerous lesions, CIN 2 and CIN 3 are modelled as a single combined health state and patients are assumed to be cured from cancer if they survive for 5 years after treatment. The only update to the previous model is that HPV patients are allowed to develop CIN 2/3 within 6 months based on new evidence reported by Kulasingam et al. 147 on the possibility that women can progress in a short amount of time from being infected with HPV to developing CIN 2/3.
The modelling of cancer progression relies on several assumptions; most of these are similar to those in the previous model by Wade et al. :30 cancer cannot be cured without treatment and a patient progresses across stages until they receive treatment (i.e. while cancer remains undetected). Although Wade et al. 30 modelled cancer progression with four stages, our model considers only three stages (local, regional and distant). Indeed, the progression and mortality rates have been updated, based on a study by Campos et al. ,148 which relied on a three-stage model. Consistently with Wade et al. ,30 a patient with undetected cancer faces a 6-month probability to develop symptoms; the model assumes that a patient with symptoms is immediately referred for colposcopy, identified and treated. 30 When asymptomatic cancer is detected by the NHSCSP, it is assumed that the patient is in stage 1 of the model (local). A patient who survives for 5 years after treatment is assumed to be cured and is back to the ‘clear’ health state.
Screening and treatment pathways
At the beginning of each cycle, the patient follows one of four main screening and treatment pathways (which all end with the natural history model):
-
No screening – the patient directly enters the natural history model.
-
Colposcopy pathway – the patient is directly referred for colposcopy (first cycle and cancer symptoms).
-
Routine screening – the patient is recalled to routine screening every 3 or 5 years after their last test, depending on their age.
-
Follow-up pathways – after treatment, diagnosis or an inadequate result, the patient may be referred to follow-up, such as a test of cure (6 months after treatment), cytology and/or colposcopy (6 months after the initial test) or CIN 1 follow-up (12 months after diagnosis).
During the first cycle, all patients have a colposcopy examination with different outcomes depending on their health state, the reason for referral and the diagnostic accuracy of the colposcopy technologies. After the first cycle, subsequent examinations (follow-up or routine screening) depend on the patient’s characteristics and history.
Colposcopy pathway
All patients are directed to the colposcopy pathway during the initial cycle. Patients can also be referred for colposcopy subsequently after routine screening or follow-up tests, or directly, if they develop cancer symptoms. A patient who has a colposcopy is characterised by their health state, the reason for their referral (low-grade or high-grade dyskaryosis) and the type of clinic they visit (see-and-treat or watchful-waiting clinic). These characteristics affect the diagnostic and treatment outcomes (see Appendix 10, Figure 24).
The diagnostic outcome is modelled as the probability of being diagnosed after colposcopy as having CIN 2 or worse (‘colposcopy positive’). This probability depends on a patient’s health state and the diagnostic accuracy of colposcopy and adjunctive colposcopy technologies.
According to the NHSCSP guidelines15 and clinical experts, there are two possible types of management following a positive colposcopy result. 15 A patient with suspected CIN 2 or worse can be treated immediately after colposcopy during the same appointment. An excision sample is then sent for histopathology to confirm the initial diagnosis. The alternative is to perform a diagnostic biopsy, wait for histopathology to confirm CIN 2 or worse and treat the patient during a second colposcopy appointment. To take into account the heterogeneity in clinical practice and to analyse the cost-effectiveness of colposcopy devices in different settings, the model considers two types of clinics: ‘see-and-treat’ clinics and ‘watchful-waiting’ clinics. Modelling practice heterogeneity requires two further assumptions. First, the choice between see-and-treat clinics and watchful-waiting clinics is assumed to be independent of diagnostic accuracy. Instead, it is modelled as a patient characteristic; patients visit either a see-and-treat clinic or a watchful-waiting clinic. Second, based on the NHSCSP guidelines15 and usual practice reported by clinical experts, immediate treatment after colposcopy is performed only when both cytology and colposcopy indicate CIN 2 or worse (i.e. for patients with a referral based on high-grade dyskaryosis and a positive colposcopy result). 15 For all other patients (referral based on low-grade dyskaryosis with a positive colposcopy result and referral based on high-grade dyskaryosis with a negative colposcopy result), a diagnostic biopsy is performed and the patient is called again for colposcopy and a treatment biopsy if necessary. Patients with a referral based on low-grade dyskaryosis with a negative colposcopy result do not receive any diagnostic biopsy or treatment.
The diagnostic outcome, the reason for referral and a patient’s health state (revealed by diagnostic biopsy) determine future screening tests. Patients with a referral based on low-grade dyskaryosis and a normal colposcopy result or histopathology results are discharged from the colposcopy clinic and sent back to routine screening. Patients with a referral based on high-grade dyskaryosis but normal histopathology results are not discharged and are sent to a 6-month colposcopy follow-up. Based on the NHSCSP guidance, confirmed CIN 1 lesions are not treated, but patients are sent to receive a CIN 1 follow-up 12 months later. 15 Confirmed CIN 2/3 lesions are systematically treated and patients are sent to receive a test of cure 6 months later. A patient who has been treated for CIN lesions faces a probability to be cured. If the treatment is successful, they enter the natural history model in the ‘clear’ health state. If a patient has not received treatment or if the treatment has failed, they enter the natural history model with their initial health state. Patients who are diagnosed with cancer are assumed to be immediately treated and enter the natural history model with the ‘cancer/treated’ health state.
A key assumption is that diagnostic biopsy is assumed to be 100% specific and sensitive; it always reveals a patient’s true underlying health state. Consequently, watchful-waiting clinics never perform unnecessary treatments and patients referred for colposcopy with cancer symptoms, considered as having a referral for high-grade dyskaryosis, are always diagnosed and treated appropriately. The model does not consider conservative management for CIN 2 lesions.
Routine screening
The NHSCSP invites women aged 25–64 years to have a cervical screening test. Women aged < 50 years are invited 3 years after their last test, and women aged > 50 years are invited 5 years after their last test. Women who have been treated for precancerous lesions are invited 3 years after their last test, regardless of age. The model therefore keeps track of a patient’s age as well as the number of cycles elapsed since their last screening.
Cervical cytology test results are graded depending on the degree of abnormality. To be consistent with the data used for the diagnostic accuracy of cytology, the model refers to the previous NHS system terminology (British Society for Clinical Cytology 1986) and considers six possible cytology results: (1) negative, (2) inadequate, (3) borderline change, (4) mild dyskaryosis, (5) moderate dyskaryosis and (6) severe dyskaryosis. The current NHS system terminology (ABC3) is used in a second step to characterise patients referred for colposcopy: borderline change and mild dyskaryosis are defined as being low grade, whereas moderate or severe dyskaryosis is defined as being high grade.
The current management protocol is described in the NHSCSP colposcopy and programme management and referred to as ‘HPV triage’ (Figure 12). 15 An alternative protocol, known as ‘HPV primary’, has been implemented in pilot sites across England and is to be rolled out in the future within the NHSCSP (Figure 13). The cost-effectiveness analysis considers these two protocols using separate models.
FIGURE 12.
Human papillomavirus triage: routine screening and follow-up.
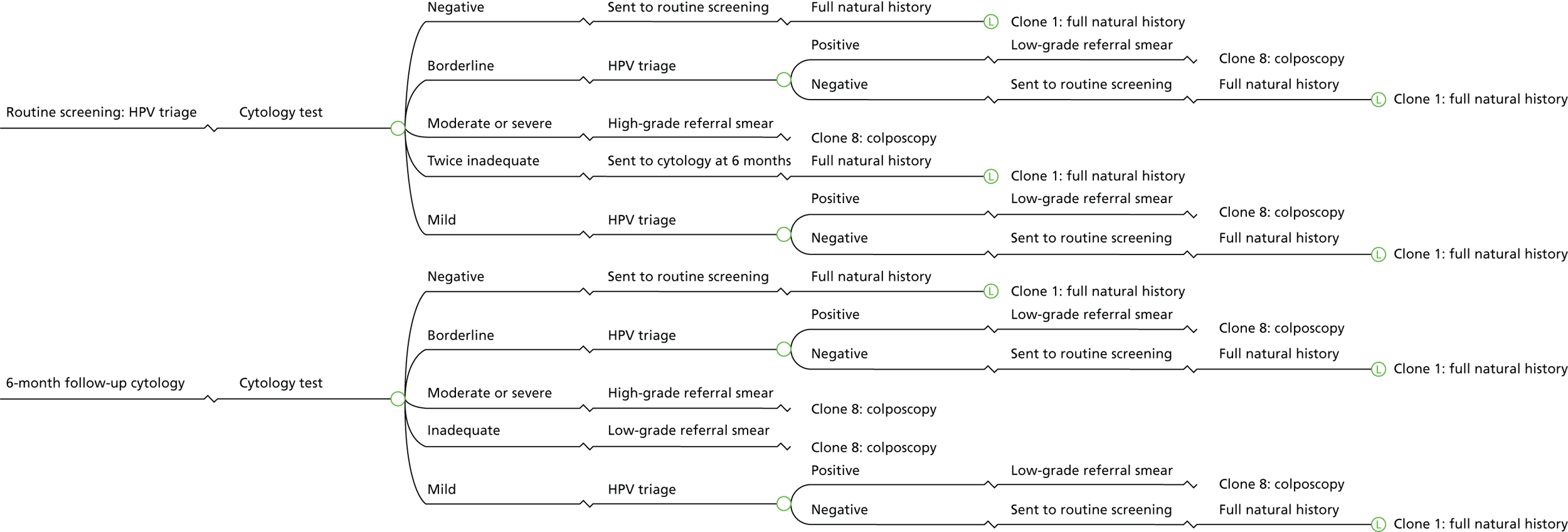
FIGURE 13.
Human papillomavirus primary: routine screening and follow-up.
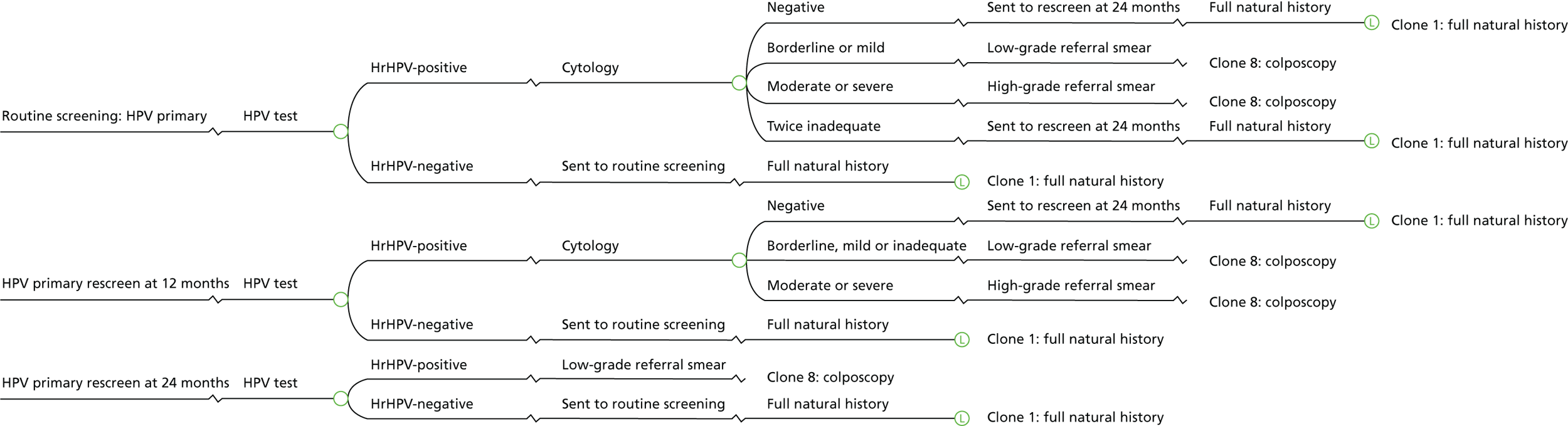
Under HPV triage, the patient is first tested with cytology. The diagnostic outcome is modelled as a probability of having a cytology result, r, given the underlying health state h. This probability depends on the cytology test performance. If the cytology is negative, the patient is sent back to routine screening and will be invited again 3 or 5 years later. When the cytology shows moderate or severe dyskaryosis, the patient is referred for colposcopy as a high-grade referral. When the cytology shows borderline changes or mild dyskaryosis, the sample collected during the cervical screening is used for hrHPV testing. The probability of being HPV-positive depends on the cytology result, the patient’s health state and the patient’s age. When HPV is detected, the patient is referred for colposcopy as a low-grade referral. Otherwise, she is sent back to routine screening. When the cytology is inadequate, the patient is tested again 3 months later. Because the cycle length is 6 months in the model, the probability of having a first inadequate result is included in the probability of having a negative, borderline, mild, moderate or severe cytology result. In the case of two consecutive inadequate results, the patient is invited 3 months later for another cytology test (i.e. 6 months after her initial cytology test). The protocol is similar to routine screening (under HPV triage), except that a patient with a third inadequate cytology result is referred for colposcopy. Although a patient with three inadequate cytology results is not defined as having low-grade dyskaryosis based on the ABC3 reporting terminology, clinical experts confirm that management after colposcopy is similar to that in low-grade dyskaryosis patients, hence the low-grade referral used in the model.
Under HPV primary screening, the patient is first tested for hrHPV. If the HPV test is negative, the patient is sent back to routine screening. If the HPV test is positive, a cytology test is used as a triage to refer patients for colposcopy. A patient with a borderline/mild or moderate/severe cytology result is immediately referred for colposcopy, as a low-grade referral or a high-grade referral, respectively. Similarly to the HPV triage, patients are tested again 3 months after a first inadequate cytology result. Consequently, in the model, the probability of having a first inadequate result is included in the probability of having a negative, borderline, mild, moderate or severe cytology result. If the cytology is inadequate or negative twice, the patient is rescreened 12 months later (‘HPV primary rescreen at 12 months’ in the model).The 12-month follow-up protocol is similar to the initial routine test, except that a patient with inadequate cytology is referred for colposcopy. Patients with a second consecutive HPV-positive/cytology-negative result are rescreened 12 months later (i.e. 24 months after the initial test (‘HPV primary rescreen at 24 months’ in the model). In this case, a HPV-positive test result is sufficient for a patient to be referred for colposcopy. A patient with a HPV-negative test result is sent back to routine screening. Because HPV primary screening has not been implemented yet, there are uncertainties regarding the post-colposcopy management of patients with two consecutive negative cytology results. Indeed, under the HPV triage protocol, these patients were not referred to colposcopy clinics. According to clinical experts, it is likely that these patients would be managed in the same way as those with low-grade referrals.
Follow-up: test of cure
Based on the NHSCSP guidelines,15 patients who receive treatment for CIN lesions are tested 6 months later (see Appendix 10, Figure 25). As the model assumes that screening occurs at the beginning of a 6-month cycle, patients who have been treated during a specific cycle will be sent to the test-of-cure pathway at the next cycle. First, the patient is tested with a cytology test. In the case of inadequate, moderate or severe results, the patient is directly referred for colposcopy (with either a low-grade referral or a high-grade referral). When the cytology results are negative, borderline or mild, the patient is tested for HPV and referred for colposcopy as a low-grade referral if the HPV test is positive. The patient then enters the colposcopy pathway as described previously. When the HPV test is negative, the patient is sent back to routine screening and enters the natural history model.
Follow-up: colposcopy at 6 months
The NHSCSP guidelines15 do not explicitly define the ‘colposcopy at 6 months’ pathway. However, clinical experts suggest that a patient with a referral for high-grade dyskaryosis and negative histopathology is usually not discharged from the colposcopy clinic. The patient will be tested again with cytology and colposcopy 6 months later (see Appendix 10, Figure 26). Given that high-grade dyskaryosis has been previously identified, a HPV test is unlikely.
Follow-up: cervical intraepithelial neoplasia 1 follow-up
The NHSCSP guidelines15 recommend conservative management for confirmed CIN 1 lesions. Instead of receiving treatment biopsy, the patient is tested 12 months after the first diagnosis. If CIN 1 is still present, she is tested again 12 months later. According to clinical experts, there is heterogeneity in the management of CIN 1 follow-up. It is most likely that the patient is sent back to the community and follows a similar pathway to routine screening (see Appendix 10, Figure 27). The model assumes that in the case of a positive colposcopy result and/or a high-grade referral, a diagnostic biopsy is systematically performed (no ‘see and treat’). Based on the NHSCSP guidelines,15 confirmed CIN 1 is treated only if lesions are persistent after 24 months. When the CIN 2/3 lesions or cancer are detected at any stage, the patient receives appropriate treatment.
Adverse obstetric outcomes
The adverse obstetric outcomes model (see Appendix 10, Figure 28) captures the impact of treatment for CIN on the adverse obstetric outcomes. The findings from two recent systematic reviews were previously summarised and discussed in Chapter 3, Systematic reviews of adverse outcomes of cervical intraepithelial neoplasia treatment. The two reviews reported results across a range of adverse fertility, pregnancy and obstetric outcomes. In the absence of robust evidence indicating an adverse treatment effect on fertility and early pregnancy (< 24 weeks’ gestation) outcomes, the adverse obstetric outcome model was limited to capturing the impact of an increased risk of preterm birth reported by Kyrgiou et al. 119
Following treatment for CIN, the adverse obstetric outcomes model captures the excess risk of treatment on preterm birth (< 37 weeks’ gestation) rates and applies a payoff to capture the associated costs and QALY decrements. As the model attempts to characterise the excess treatment risk only, only women who receive treatment for CIN enter the adverse obstetric outcome model. Within the model, a tracker variable is assigned to an individual patient following treatment with CIN. For each 12-month period following treatment (and up to the age of 45 years), the model captures the excess risk of preterm birth (< 37 weeks’ gestation) based on age-specific conception rates (adjusted for legal abortion), the risk of preterm birth for untreated women and the higher RR reported with treatment. The cost and QALY decrements capture the additional initial management costs associated with preterm birth and the increased probability of neonatal mortality, as well as the QALY decrements associated with higher risks of disability among survivors.
Model input parameters
Diagnostic accuracy
Colposcopy and adjunctive technologies
The sensitivity and specificity of colposcopy alone and the adjunctive use of DySIS and ZedScan are based on the best-evidence estimates of diagnostic accuracy reported in Chapter 3, Comparison of Dynamic Spectral Imaging System and Zedscan. An assumption is made that the binocular and video colposcopes have the same diagnostic performance and hence the diagnostic accuracy of colposcopy is based on the evidence reported in the DySIS studies. From the estimates of diagnostic accuracy, we derive the probability of a positive colposcopy result (i.e. CIN 2 or greater), given a patient’s true underlying health state. A key assumption is that the probability of a positive colposcopy result is similar for patients who are clear or have CIN 1 and for patients with CIN 2/3 or invasive cancer. A consequence of the CIN 2 threshold is that detecting CIN 1 during the colposcopy examination is not considered to be a positive colposcopy result. However, if the patient is referred for having high-grade dyskaryosis, a diagnostic biopsy will systematically reveal CIN 1 and the patient will be managed appropriately.
Based on the limitations identified in the clinical effectiveness review section, and particularly the lack of data on the diagnostic accuracy of colposcopy alone compared with the current version of ZedScan, separate pairwise analyses are presented comparing adjunctive DySIS with colposcopy alone, ZedScan with colposcopy alone and ZedScan with DySIS. Table 18 details the sensitivities and specificities used in the base-case analyses.
| Technology | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Source |
|---|---|---|---|
| Colposcopy alone | 57.91 (47.2 to 67.9) | 87.41 (81.7 to 91.5) | Regression model |
| DySIS | 81.25 (72.2 to 87.9) | 70.40 (59.4 to 79.5) | Regression model |
| ZedScan | 97.85 (96.5 to 99.2) | 58.63 (55.1 to 62.1) | Tidy et al.94 |
Cytology and human papillomavirus tests
The performance of cytology and HPV tests is also required for the model when patients are recalled for routine screening or follow-up (cytology at 6 months, test of cure, CIN 1 follow-up).
The performance of cytology test is modelled as the probability of having a certain cytology result (negative, borderline changes, mild dyskaryosis, moderate dyskaryosis, severe dyskaryosis), given a patient’s true underlying health state (Table 19). The diagnostic accuracy of cytology was derived from a published UK-based study on the cost-effectiveness of the NHSCSP [Hadwin et al. 125 – the probabilities were displayed in an unpublished document (S Eggington, personal communication)]. The probability of an inadequate cytology result was estimated to be 2.7%, based on Cervical Screening Programme, England – 2015–2016. 18 The probability of having an inadequate cytology result is assumed to be independent of a patient’s underlying health state and previous cytology results. These data were used for both HPV triage and the HPV primary screening protocol.
| Cytology result | Probability of cytology result by health state | Source | ||||
|---|---|---|---|---|---|---|
| Clear | HPV | CIN 1 | CIN 2/3 | Cancer | ||
| Inadequate | 0.027 | 0.027 | 0.027 | 0.027 | 0.027 | Cervical Screening Programme, England – 2015–2016 18 |
| Borderline | 0.002 | 0.266 | 0.262 | 0.086 | 0.082a | Hadwin et al.125 |
| Mild | 0.003 | 0.077 | 0.213 | 0.126 | 0.134a | Hadwin et al.125 |
| Moderate | 0.001 | 0.065 | 0.148 | 0.187 | 0.153a | Hadwin et al.125 |
| Severe | 0.000 | 0.025 | 0.056 | 0.228 | 0.604a | Hadwin et al.125 |
The performance of the HPV test is modelled as the probability of having a positive HPV test result given the patient’s true underlying health state, cytology result and age. The diagnostic accuracy of the HPV test under the HPV triage protocol (i.e. after a borderline or mild cytology result) was based on Cotton et al. 127 using data from the TOMBOLA trial. 130 The TOMBOLA trial aimed to compare different methods of management for women with low-grade cervical abnormalities under the NHSCSP in the UK. The study included 4439 women between 1999 and 2002, aged 20–59 years, with a cytology test showing borderline nuclear abnormalities or mild dyskaryosis. A cross-sectional analysis of trial data provided the sensitivity and specificity of a single HPV test in detecting CIN 2 or worse by cytology result and age (Table 20). Under the HPV primary screening protocol, the probabilities of a positive HPV test result by health state were derived from the ARTISTIC (A Randomised Trial in Screening to Improve Cytology) study, which based these estimates on extensive data in the literature on hrHPV testing [Kitchener et al. 149 (Table 21)].
Underlying health state and reason for referral
Women referred for colposcopy from the NHSCSP enter the model with two initial characteristics: a true underlying health state (clear, HPV, CIN 1, CIN 2/3 or cancer) and a reason for referral (low-grade or high-grade dyskaryosis). The joint distribution of health state and the reason for referral is linked to disease prevalence as well as routine screening diagnostic accuracy. For instance, the distribution under HPV triage is likely to be different from the distribution under HPV primary screening. For example, HPV primary screening is expected to be more sensitive (i.e. more CIN 2+ cases are likely to be referred for colposcopy) but less specific, leading to more low-grade referrals. Consequently, different estimates are used for the HPV triage and HPV primary screening protocols.
Human papillomavirus triage
Table 55 in Appendix 11 reports the joint distribution of the health state and the reason for referral in a HPV triage protocol. Because the HPV triage protocol is currently implemented in the NHSCSP in England, the joint distribution of the health state and the reason for referral was based on the outcomes of colposcopy referrals published in Cervical Screening Programme, England – 2015–2016. 18 From April 2015 to June 2015, all 55 operating laboratories in England collected outcomes of colposcopy referrals and linked these results to the reason for referral. Consistently with the model structure, women who were referred after non-negative samples showing either BMD with a positive HPV test or persistent inadequate results were considered as having a low-grade referral. Women who were referred after a potentially significant abnormality, including high-grade dyskaryosis (moderate or severe); high-grade, invasive squamous carcinoma; or glandular neoplasia of the endocervical type, were considered as having a high-grade referral. In total, 31,114 samples were collected. We excluded 570 cases whose results were unknown or showed non-cervical cancer. Of the remaining 30,544 women, 68.6% had a low-grade referral and 31.4% had a high-grade referral. The outcomes of colposcopy referral (cervical cancer, CIN 3, CIN 2, CIN 1, HPV only, no CIN/no HPV) were confirmed by biopsy when the colposcopy revealed an abnormality. When no abnormalities were detected at the colposcopy examination, patients were considered to be ‘clear’. However, because colposcopy is not 100% sensitive, an unknown proportion of patients with no abnormality detected might have been misdiagnosed. Consequently, the distribution may underestimate the proportion of CIN 2 and worse in the population.
Human papillomavirus primary screening
Table 56 in Appendix 11 reports the joint distribution of the health state and the reason for referral in a HPV primary screening protocol. The data came from unpublished preliminary results collected in the HPV primary pilot sites and included a total of 15,781 women referred for colposcopy. A total of 12,626 women were referred after the first round of routine screening (HPV-positive result and cytology result of borderline dyskaryosis or worse), 2628 women were referred after the second round of screening (12-month repeat) and 527 women were referred after the third round of screening (24-month repeat). As expected, the proportion of low-grade referrals under the HPV primary screening protocol was slightly higher than that under the HPV triage protocol (70.56% vs. 68.6%).
However, these preliminary results must be interpreted with caution. First, data collection is still incomplete, especially for women referred after the third round of screening. Second, around 70% of the data on HPV primary screening (n = 11,139) came from laboratories that were implementing the HPV genotyping triage. During the second round of screening, women detected as being infected with HPV 16/18 were immediately referred for colposcopy, even if their cytology test result was negative. The impact of genotyping on the characteristics of the population referred for colposcopy is hard to predict, with potentially more low-grade referrals (women with HPV-positive and cytology-negative results are referred sooner), but also more severe cases resulting from the presence of HPV 16/18. Finally, because pilot sites were not randomly selected, we can expect selection issues, and especially variability, in the prevalence of HPV and CIN lesions compared with that in the general population, regardless of the impact of a change in the routine screening protocol. For the analysis on the HPV primary screening protocol, we used data reported by all pilot sites (with and without genotyping) as a base case (see Table 56) and provided sensitivity analyses around the characteristics of the initial population using data from pilot sites that are and are not implementing genotyping (see Underlying health state and reason for referral).
Treatment probabilities
See-and-treat and watchful-waiting clinics
Treatment decisions after a positive colposcopy result (i.e. diagnostic biopsy or immediate treatment) vary considerably across England. Currently, for women referred with high-grade abnormalities, the most common treatment at first attendance is excision (53.2%), followed by diagnostic biopsy (35%). The use of excision at first attendance for women with high-grade referrals ranges from 11.6% in London to 65.4% in the North West of England (Cervical Screening Programme, England – 2015–2016). 18
Heterogeneity in treatment decisions after a positive colposcopy result is modelled as two different types of clinic a patient may visit, and is independent of health state or diagnostic accuracy. A patient may visit either a ‘see-and-treat’ clinic or a ‘watchful-waiting’ clinic. Because the NHSCSP guidance states that the PPV for CIN 2 or worse should be at least 90% to undergo ‘see and treat’, the model assumes that the use of excision at first attendance is possible for women with high-grade referrals with a positive colposcopy result only. When patients have a low-grade referral, CIN 2 or worse must be confirmed by the diagnostic biopsy result before being treated. This assumption has been validated by clinical experts.
As the cost-effectiveness of adjunctive colposcopy devices is likely to be driven by treatment practice, the results are presented separately for the two types of clinics. Indeed, a low specificity of colposcopy leads to overtreatment in a ‘see-and-treat’ clinic. In contrast, because biopsy is assumed to be 100% sensitive and specific, watchful-waiting clinics are assumed to never overtreat patients. Furthermore, watchful waiting requires two colposcopy examinations: a first one in which a diagnostic biopsy is performed and a second one in which CIN 2 lesions or worse are treated. However, ‘see-and-treat’ clinics require only one colposcopy.
Probability of cure from treatment biopsy
The probability of cure after treatment biopsy was derived from a study by Ghaem-Maghami et al. 150 that reported the failure rates for 2455 women treated for CIN for the first time between 1989 and 2004. Failure was measured by the detection of high-grade cervical disease after treatment, defined as the cytological findings of moderate dyskaryosis or more severe or histological findings of CIN 2 or worse. The median length of follow-up was 238 weeks. The authors reported that the cumulative failure rate at 10 years was 4.9% for CIN 1 (n = 570), 9.8% for CIN 2 (n = 886) and 10.3% for CIN 3 (n = 999). We calculated that the weighted excision failure rate of CIN 2/3 was 10.1% (see Appendix 11, Table 57).
Probabilities of adverse obstetric outcomes
For each 12-month period following treatment, the model captures the excess risk of preterm birth (< 37 weeks’ gestation) based on age-specific conception rates (adjusted for legal abortion), the risk of preterm birth for untreated women and the higher RR reported with treatment.
Age-specific conception rates were derived from national conception statistics reported by the Office for National Statistics (ONS). 151 The ONS statistics bring together records of birth registrations (including live births or stillbirths) and abortion notifications. Hence, the conception input data applied in the model do not include conceptions resulting in miscarriages or illegal abortions. The annual probability of conception (adjusted for legal abortion) applied in the model was derived from age-specific conception and legal abortion rates reported per 1000 women [England and Wales (see Appendix 11, Table 58)].
The excess risk of preterm birth was then estimated, based on applying a RR representing the additional risk following treatment for CIN to the probability of preterm birth without treatment. The increase in RR following treatment (LLETZ) was assumed to be 1.56. This was based on the results reported by Kyrgiou et al. 119 for all treatments in all preterm births (< 37 weeks’ gestation), when the external comparison includes the overall population.
The RR of preterm birth was then applied to the probability of preterm birth for untreated women. The probability of preterm birth for untreated women was assumed to be 7.3% based on data reported in the NICE guideline on preterm labour and birth. 152 Consequently, the excess risk of preterm delivery for women treated with LLETZ was estimated to be 4.09% [0.073 × (1.56 – 1); see Appendix 11, Table 59].
Natural history model
Precancerous lesions
The transition probabilities from the ‘clear’ state to CIN 2/3 were based on the transition probabilities reported by Kulasingam et al. ,147 an update of Myers et al. 128 used in Wade et al. 30 Kulasingam et al. 147 took into account recent evidence that, in young women (aged < 30 years), high-grade CIN may occur early in the course of a hrHPV infection. Recent studies also suggested that CIN may be more frequent in young women, but that progression to cancer from high-grade CIN is low. Compared with Myers et al. ,128 HPV and CIN incidence and regression estimates were higher, but progression rates between CIN states and from CIN 2/3 to cancer were lower. Transition probabilities were reported by the authors as annual probabilities and converted to 6-month probabilities in our model (see Appendix 11, Table 60).
Invasive cancer
Table 61 in Appendix 11 reports the parameters used to estimate the likelihood of symptoms of cervical cancer, progression across stages and cancer-related mortality. Women with undetected cancer may progress to a more severe stage (from local to regional to distant), with an increasing probability of developing symptoms. Once cancer is detected, the model assumes that patients will receive stage-specific treatment and face an excess risk of mortality as a result of having cervical cancer. Cancer-specific mortality depends on stage and decreases by time since diagnosis. After 5 years, excess mortality as a result of having cancer is assumed to be zero and patients are assumed to be cured. We assume that women with undetected cancer continually face the year 1 probability of cancer mortality until diagnosis.
Our estimates were based on Campos et al. ,148 which reported the monthly probability of symptoms, progression and mortality by stage (local, regional, distant). Cancer-specific mortality in Campos et al. 148 was derived from the Surveillance, Epidemiology, and End Results (SEER) Programme registry data from 2000 to 2009. 149 Data reported by Campos et al. ,148 which were based on US registry data, appeared to be the most recent, complete and consistent data on cancer progression and mortality. Transition probabilities (stage progression and mortality) when cancer is not detected should not be affected by differences in care between the USA and the UK. 148
All-cause mortality
Mortality rates from causes other than cervical cancer were calculated using data from the ONS in England and Wales in 2015. 153 Deaths resulting from cervical cancer were subtracted from the total number of deaths for each age group. The 2015 ONS data on the population for each age group were then used to calculate an annual mortality rate, converted to a 6-month probability in the model (see Appendix 11, Table 62).
Resource utilisation and cost data
Devices costs
The cost-effectiveness analysis requires an estimate of the average cost per procedure of each of the technologies being assessed. A colposcopy examination with DySIS or ZedScan is assumed to be equivalent to colposcopy alone in terms of staff resources and the length of consultation. The average cost of a procedure includes a set-up cost, annual recurring costs and per-patient costs. Information provided by the manufacturers has been used to estimate the costs of DySIS and ZedScan. For the purchase price and maintenance costs of colposcopy, we used estimates provided by clinical advisors for the previous diagnostics assessment report (Wade et al. 30) and inflated these to 2016 prices.
The set-up cost consists of the capital cost of the machine. The purchase price of each technology was annuitised over the expected lifetime of the technology. Consistent with Wade et al. ,30 the lifetime of a colposcopy was estimated to be 15 years. The lifetimes of DySIS and ZedScan were estimated to be 5 years each. The equivalent annual cost was calculated from the purchase price of the technology and the useful life of the equipment using the discount rate of costs of 3.5%.
The annual maintenance cost of the colposcope was estimated to be 10% of the purchase price and the cost of disposables was estimated to be equivalent to the cost of a speculum (£2.15). The annual maintenance costs and disposable costs of the adjunct technologies were provided by the manufacturers. For DySIS, the annual maintenance costs included the DySIS viewer licence registration and renewal as well as a 5-year service and maintenance plan. The price of a DySIS disposable speculum (£3.50) was added to the per-patient cost. The ZedScan manufacturer claimed no routine maintenance costs. However, a single-use EIS sensor (£30.00) is required for each patient examined. To estimate the total cost per patient, it is necessary to estimate the number of patients expected to be treated each year. We assumed that this number was independent of the type of devices and used the previous estimate of 1229 patients per device per year (see Appendix 11, Table 63). Sensitivity analyses were undertaken to test this particular assumption (see Underlying health state and reason for referral). As ZedScan uses a binocular colposcope to guide the probe or to confirm diagnosis, the cost of a colposcope was also added to its total cost. DySIS devices already include a colposcope and therefore do not require this additional cost.
In addition to the cost related to the device itself, the costs of a colposcopy visit, diagnostic biopsy and treatment (LLETZ) were estimated from NHS Reference Costs 2015 to 2016. 154 The NHS Reference Costs reported the costs (2016 prices) of a ‘diagnostic colposcopy’, a ‘diagnostic colposcopy with biopsy’ and a ‘therapeutic colposcopy’ (see Appendix 11, Table 64). The uncertainty surrounding whether or not the NHS reference costs accurately reflect resource use, and in particular if these include histology/pathology costs, is explored in a sensitivity analysis (see Underlying health state and reason for referral).
In the base case, we estimated the cost of a colposcopy visit to be £175 with a binocular colposcope, £180.49 with DySIS and £205.52 with ZedScan. The additional costs of a diagnostic biopsy and treatment were estimated to be £47 and £63, respectively. The costs of a cytology test and a HPV test were derived from the TOMBOLA study,130 inflated to 2016 prices, and were estimated to be £37.19 and £29.66, respectively (see Appendix 11, Table 65). 132
Cancer costs
Cancer costs by stage were taken from a UK-based study by Martin-Hirsch et al. ,155 which estimated the costs associated with the management of women with abnormal cervical cytology. The unit costs for cancer treatment (including chemotherapy, radiotherapy and inpatient care) were obtained by the authors from the national reference costs, the British National Formulary156 and from personal communications with the purchasing departments of clinics included in the study (six centres in England and Wales). The average costs per cancer treatment were reported in pounds sterling at 2006 prices by cancer stage, using the FIGO grading (stages I–IV). We assumed that the average cost of stage I and stage II refers to ‘local’ stage, the average cost of stage III refers to ‘regional’ stage and the average cost of ‘stage IV’ refers to ‘distant’ stage. In the model, all costs were inflated to 2016 prices (see Appendix 11, Table 66).
Costs associated with adverse obstetric outcomes
The additional costs associated with preterm birth were derived from the same source157 as the QALY decrements. The study by Lomas et al. 157 reported an expected incremental (discounted) lifetime cost of £24,071 per birth (inflated to £24,610 in 2016 prices). This estimate incorporates an estimate of the initial inpatient neonatal care and ongoing costs over the following 18 years of life in survivors, associated with higher rates of disability.
Health outcomes
Health utility values refer to the patient’s health measured on an interval scale, where 0 represents death and 1 represents perfect health. QALY estimates combine both the utility value of health states and the time spent in those health states, with 1 QALY representing a year in perfect health. A QALY decrement is the decrease in health utility over a set time period converted into lost QALYs.
Screening disutility
The disutility associated with screening and treatment was based on a recent study, especially designed to estimate utility values for HPV testing and cytology-based screening states among women targeted for cervical screening (Simonella et al. ). 158 A total of 43 women (mean age 49 years), living in Sydney, Australia, participated in the study. The participants were asked to state their preferences (rank and utility scores) for hypothetical states relating to cytology and HPV screening and precancerous lesions. The utility values were estimated via a two-stage standard gamble. The model uses utility values reported by Simonella et al. 158 for four types of screening episodes: (1) a routine screening episode with a normal cytology result, (2) a false-positive referral to colposcopy, (3) a colposcopy referral that leads to confirmed but not treated CIN 1 and (4) a colposcopy referral that leads to the treatment of CIN lesions. Each scenario was described in a narrative format in Simonella et al. 158 to characterise the screening process and possible adverse outcomes associated with examination and treatment. Consequently, this set of values captures the disutility associated with the screening process, from the experience of being screened (even if the test result is negative) to the possible short-term adverse outcomes of colposcopy and treatment.
Simonella et al. 158 reported the mean standard gamble utility values over a 12-month period. In the model, we converted these scores into QALY decrements (1 – mean utility value) of undergoing a screening episode (initial referral for colposcopy, follow-ups or routine screening). A screening episode with cytology and/or HPV test that did not result in a referral for colposcopy induced a QALY decrement of 0.0062. The QALY decrement associated with a false-positive referral for colposcopy (the cytology test and/or HPV test are positive, but the colposcopy or histopathology are negative) or a confirmed but not treated CIN 1 lesion was estimated to be 0.0276. Finally, a positive diagnosis followed by excision treatment of the CIN lesion induced a QALY decrement of 0.0296 (see Appendix 11, Table 67).
Health-related quality of life of underlying true health states
As HPV, CIN 1, CIN 2/3 and undetected cancer are considered to be asymptomatic, we applied age- and gender-specific utilities from the Measurement and Valuation of Health survey, a nationally representative interview survey of 3395 men and women living in the UK conducted in 1993. The objective of the survey was to collect data on the health state valuations using a time-trade-off procedure and on individuals’ self-reported health status using the EuroQol-5 Dimensions descriptive classification system (Kind et al. )159 (see Appendix 11, Table 68). The possible disutility that patients can experience once CIN lesions are identified is subsequently captured by screening disutility as outlined previously.
Quality-adjusted life-year decrements associated with invasive cancer were obtained from a published study (Goldie et al. 5). The authors reported the HRQoL for ‘detected invasive cancer’ and ‘after treatment for invasive cancer’ by stage (local, regional and distant). These weights were constructed based on expert elicitation. We considered the first set of HRQoL to be a utility score associated with the first year post diagnosis, during which period patients undergo treatment. We used the second set for the remaining 4 years, during which time patients are not yet considered to be cured from cancer but do not receive further treatments (see Appendix 11, Table 69).
Quality-adjusted life-year decrement associated with adverse obstetric outcomes
A QALY decrement was applied to capture the HRQoL and mortality consequences of the increased risk of preterm birth following treatment for CIN. We did not attempt to identify evidence for this parameter systemically. Instead, we restricted our search to the evidence reported in the NICE guideline on preterm labour and birth (NG25);152 although this guideline reports utility and QALY estimates, none of these directly provided the required estimates for our model (i.e. the QALY decrement associated with preterm birth at < 37 weeks’ gestation). Instead, we sourced estimates based on discussions with colleagues and identified a recent study by Lomas et al. 157 that reported cost and QALY decrements that matched the requirements of the model. The QALY decrement reported in Lomas et al. 157 was 1.3 QALYs and was derived from calculations based on the QALY loss associated with neonatal mortality and the discounted QALY loss associated with increased disability rates reported among survivors.
Analytic methods
A decision model was developed to estimate the cost-effectiveness of adjunctive colposcopy technologies (DySIS and ZedScan) for women referred for colposcopy through the NHSCSP under either HPV triage (including test of cure) or the HPV primary screening protocol (including test of cure).
The decision model is implemented using a patient-level state-transition modelling approach. The time horizon of the evaluation is 60 years, the costs and outcomes are discounted at a rate of 3.5% and a 2015–16 price year is used.
The model captures the long-term impact of standard colposcopy and adjunctive colposcopy technologies in terms of average cost and average QALYs per patient. The analysis compares colposcopy alone with DySIS, colposcopy alone with ZedScan and DySIS with ZedScan, based on incremental costs and QALYs and the incremental cost-effectiveness ratio (ICER). In addition, the model predicts several outcomes that include, for 1000 women referred for colposcopy, the number of CIN 2+ cases missed, the number of women who developed cancer, the number of women who died from cancer, the number of women who received treatment (LLETZ), the number of unnecessary treatments and diagnostic biopsies, and the number of women who experienced adverse obstetric outcomes (preterm delivery).
The model was run to simulate 500,000 women referred for colposcopy. This large number of iterations was necessary to ensure that the proportion of patients in each state equalled the individual probability. Unseeded simulations were undertaken for different sample sizes (from 1000 to 800,000). Based on the visual examination of the graphical representation of mean estimates of incremental QALYs, incremental costs and associated SEs, a sample of 500,000 women was considered to be sufficient to ensure the stability of the simulation results and therefore an appropriate characterisation of first-order uncertainty with a reasonable computation time.
In the base-case and scenario analyses, simulations were run separately for each routine screening model (HPV triage protocol and HPV primary protocol), each type of clinic (see and treat, watchful waiting) and for each reason for referral (all referrals, low grade and high grade). In order to obtain reproducible results and to limit statistical variation from one simulation to the next, the same number (or ‘seed’) was used to initialise the sequence of pseudo-random number.
Structural assumptions are identical for the three strategies (colposcopy alone, DySIS, ZedScan); only the diagnostic accuracy and the cost of the devices vary. The differences between HPV triage and HPV primary models are the characteristics of the population referred for colposcopy, the routine screening pathways and the diagnostic accuracy of the HPV test. We assumed that women who visit a see-and-treat clinic are treated at initial appointments only if the colposcopy result is positive (CIN 2+) and the cytology result is moderate or severe (high-grade referral smear). Women who visit watchful-waiting clinics are never treated at their initial appointment. A diagnostic biopsy is assumed to be systematically performed to confirm the diagnostic results. As we assumed 100% specificity and sensitivity for diagnostic biopsy, patients never received unnecessary treatment in a simulation with watchful-waiting clinics.
Structural assumptions that determine screening and treatment pathways are also identical for the three strategies. Indeed, the present assessment does not aim to evaluate a change in clinical practice as a result of the use of the adjuncts to colposcopy. Therefore, we use national guidelines to model treatment practice and rely on two structural assumptions to model diagnostic and treatment pathways. First, the decision to treat patients at first examination does not depend on the device used for colposcopy. The decision depends on the reason for referral (cytology result) and the dichotomous colposcopy result (CIN 2+) only; patients are treated at first examination if and only if the colposcopy result is positive and the cytology indicates high-grade lesions. Therefore, the fact that the ZedScan device can use different thresholds to influence the see-and-treat decision is not taken into account in the model. The second structural assumption is that patients with a high-grade referral smear are assumed to undergo diagnostic biopsy regardless of the colposcopy diagnostic result. Hence, the possible impact of ZedScan on the decision to discharge patients with a high-grade referral smear without a diagnostic biopsy is not modelled. Consequently, the main impact of ZedScan in the economic evaluation lies in the higher proportion of patients with CIN 2+ detected with ZedScan than with colposcopy alone, as reported in Tidy et al. 94
Finally, in the economic model, the performances of colposcopy alone, DySIS and ZedScan are assessed in the same way: the probability of a patient being diagnosed as having CIN 2+, given the patient’s true health state. This probability is estimated based on the proportion of patients with confirmed CIN 2+ detected by the device (sensitivity) and the proportion of patients with confirmed CIN 1 or less ‘truly’ diagnosed as having a negative result by the device (specificity). We believe that the sensitivity and specificity values reported in Tidy et al. 94 are the best-available evidence of the probability of a patient being diagnosed by ZedScan as having CIN 2+ and can be compared, within the limits identified in Chapter 3, Risk of bias of the included studies, with the diagnostic accuracy of colposcopy alone and that of DySIS reported in the meta-analysis studies.
Base-case analysis
The characteristics of the base-case analysis are summarised in Tables 70 and 71 in Appendix 11. Details on the structural assumptions and input parameters are provided in Contribution of the York model and Model structure.
Sensitivity and scenario analyses
To investigate the impact of parameter uncertainty on the results, several sensitivity analyses were undertaken, which focused on diagnostic accuracy; the costs of technologies, treatment and biopsies; and the initial characteristics of the population referred for colposcopy under the HPV primary protocol. In addition, three scenario analyses were undertaken to explore alternative structural assumptions: (1) restricting the analysis to a 3-year period to evaluate the costs and outcomes within a single screening interval, (2) excluding adverse obstetric outcomes and (3) assuming that ZedScan is used alongside colposcopy at all appointments. The sensitivity analyses and scenarios used the same assumptions and parameter values as the base-case analysis, unless stated otherwise.
Uncertainty around diagnostic accuracy
Sensitivity analysis 1: colposcopy alone and Dynamic Spectral Imaging System (Louwers et al.)64
In the first sensitivity analysis, the diagnostic accuracy for colposcopy alone and DySIS were based on a single study by Louwers et al. 64 This study was selected as it is the only study that was rated as being at a low risk of bias (see Chapter 3, Risk of bias of the included studies). It also provides a PPV of colposcopy alone above the NHS standard of 65% (PPV = 70.37; see Chapter 3, Dynamic Spectral Imaging System). Compared with the base-case analysis, the Louwers et al. 64 study reported slightly lower sensitivity and specificity values for both colposcopy alone and DySIS (Table 22). As similar data were not available for ZedScan, sensitivity analysis 1 compares DySIS with colposcopy alone only.
Sensitivity analysis 2: impact of a CIN 2+ cut-off point (colposcopy alone and DySIS)
Sensitivity analysis 2 explores the implications of using a CIN 2+ cut-off point (i.e. the assumption that specificity is independent of a patient being clear, being infected with HPV or having CIN 1 and sensitivity is independent of a patient having CIN 2/3 or cancer). Unpublished data provided by the DySIS manufacturer from the Louwers et al. 64 study were used to estimate the probability of a positive colposcopy result (i.e. detecting CIN 2 or worse) by the health state for colposcopy alone and DySIS (Table 23). As similar data were not available for ZedScan, sensitivity analysis 2 compares DySIS with colposcopy alone only.
Sensitivity analysis 3: diagnostic accuracy of colposcopy alone from Tidy et al.110
Sensitivity analysis 3 compared ZedScan with colposcopy alone using the diagnostic accuracy reported by Tidy et al. 110 for colposcopy alone and by Tidy et al. 94 for ZedScan. The former was a study of a ZedScan prototype, conducted in a similar context to the latter, and therefore provides an alternative estimate for colposcopy alone to inform the direct comparison between ZedScan and colposcopy (see Chapter 3, Comparison of Dynamic Spectral Imaging System and ZedScan). Tidy et al. 110 reported significantly higher sensitivity and specificity values for colposcopy alone than the meta-analysis results (Table 24).
Sensitivity analyses 4.1 and 4.2: lower and upper bounds of specificity and correlated sensitivity of Dynamic Spectral Imaging System
Sensitivity analyses 4.1 and 4.2 characterise the uncertainty around the estimates of specificity and sensitivity of DySIS compared with colposcopy alone (Table 25). Sensitivity analysis 4.1 used the lower bound of specificity from the 95% CI of the regression model estimates and the correlated sensitivity from the ROC curve (see Chapter 3, Dynamic Spectral Imaging System). Sensitivity analysis 4.2 used the upper bound of specificity from the 95% CI of the regression model estimates and the correlated sensitivity from the ROC curve. In both analyses, we used the base-case values for colposcopy alone (average point estimates of the regression model).
| Technology | Sensitivity (%) | Specificity (%) | Source |
|---|---|---|---|
| Colposcopy alone | 57.91 | 87.41 | Regression model (average point estimates) |
| Sensitivity analysis 4.1: lower-bound (2.5%) specificity | |||
| DySIS | 83.60 | 59.40 | Regression model |
| Sensitivity analysis 4.2: upper-bound (97.5%) specificity | |||
| DySIS | 78.50 | 79.50 | Regression model |
Sensitivity analyses 4.3 and 4.4: lower and upper bounds of specificity and sensitivity of ZedScan
Sensitivity analyses 4.3 and 4.4 characterise the uncertainty around the estimates of specificity and sensitivity of ZedScan compared with colposcopy alone (Table 26). As there was only one study available (Tidy et al. 94), we were not able to estimate the correlated sensitivity for extreme values of specificity. Instead, in order to reflect the negative correlation between specificity and sensitivity, sensitivity analysis 4.3 used the upper bound of specificity from the 95% CI reported in Tidy et al. 94 and the lower bound of sensitivity (from the 95% CI); sensitivity analysis 4.4 used the lower bound of specificity and the upper bound of sensitivity. In both analyses, we used the base-case values for colposcopy alone.
| Technology | Sensitivity (%) | Specificity (%) | Source |
|---|---|---|---|
| Colposcopy alone | 57.91 | 87.41 | Regression model (average point estimates) |
| Sensitivity analysis 4.3: 97.5% sensitivity – 2.5% specificity | |||
| ZedScan | 99.20 | 55.10 | Tidy et al.94 |
| Sensitivity analysis 4.4: 2.5% sensitivity – 97.5% specificity | |||
| ZedScan | 96.50 | 62.10 | Tidy et al.94 |
Uncertainty around costs
Sensitivity analysis 5.1 and 5.2: impact of throughput on the cost of devices
Sensitivity analyses 5.1 and 5.2 explore the impact of throughput on the cost of devices by assuming alternative estimates of the number of patients examined per colposcope per year. This parameter is used to estimate the cost per patient of DySIS and ZedScan. Sensitivity analysis 5.1 simulated a 50% decrease in the number of patients per colposcope per year (614 patients instead of 1229 patients), which drives up the costs of DySIS and ZedScan. Sensitivity analysis 5.2 simulated a 50% increase in the number of patients per colposcope per year (1844 patients instead of 1229 patients). Because the cost of a colposcopy examination with a binocular colposcope is based on the NHS Reference Costs,154 the cost of colposcopy alone remains unchanged. Given its higher purchase price, the cost of DySIS is more sensitive to a variation in the number of patients than the cost of ZedScan (Table 27).
| Cost (£) of colposcopy examination by technology | Lower bound of 614 patients per colposcope per year | Upper bound of 1844 patients per colposcope per year |
|---|---|---|
| Colposcopy alone | 175.00 | 175.00 |
| DySIS | 184.63 | 179.11 |
| ZedScan | 206.05 | 205.35 |
Sensitivity analysis 6: costs of diagnostic biopsy and large-loop excision of the transformation zone
We noted an important discrepancy in the estimates of the cost of a colposcopy with treatment (LLETZ) between the NHS reference cost (£238) and the cost reported by Whyte et al. 124 (£590). The costs for a LLETZ reported by Whyte et al. 124 included an estimated pathology cost of £407. The NHS Reference Costs should theoretically include all associated costs of a LLETZ (including associated histology/pathology costs). However, there is some uncertainty surrounding whether or not the reference costs accurately reflect actual resource use, and particularly histology/pathology costs.
Consequently, in sensitivity analysis 6, the cost estimates of histology/pathology for a diagnostic biopsy and a LLETZ reported in Whyte et al. 124 (£53 and £407, respectively, in 2011–12 prices) were added to the NHS Reference Costs154 (Table 28).
| Type of cost | Cost per treatment (£) | Source |
|---|---|---|
| NHS reference cost | ||
| Diagnostic biopsy | 47.00 | NHS Reference Costs 2015 to 2016154 |
| LLETZ | 63.00 | |
| Histology/pathology costs | ||
| Diagnostic biopsy | 55.72a | Whyte et al.124 |
| LLETZ | 427.89a | |
| Total cost (NHS reference cost and histology/pathology cost) | ||
| Diagnostic biopsy | 102.72 | |
| LLETZ | 490.89 | |
Population referred for colposcopy under the human papillomavirus primary screening protocol
Sensitivity analyses 7.1 and 7.2: alternative distributions of the health state and the reason for referral from human papillomavirus screening pilot sites
To address the uncertainty around the characteristics of the population referred for colposcopy under the HPV primary screening protocol (see Main features of the model), sensitivity analyses 7.1 and 7.2 used data from pilot sites without HPV 16/18 genotyping and pilot sites with HPV 16/18 genotyping, respectively. Although the proportion of low-grade and high-grade referrals appears to be very similar between the two types of pilot sites, the prevalence of HPV is higher in pilot sites that do not implement genotyping (see Appendix 11, Table 72). It is important to note that this sensitivity analysis is not intended to explore the impact of alternative HPV primary screening protocols, but rather to use the variation observed between different sites as a means of exploring the potential impact of uncertainty around the characteristics of the population referred.
Scenario analyses
Scenario analyses were undertaken to consider the impact of three key structural assumptions.
Scenario 1: time horizon of 3 years
In scenario 1, the model was run for only 3 years to evaluate the cost-effectiveness of adjunctive technologies with a short-term perspective. Indeed, a 3-year window captures the costs and health outcomes of the initial colposcopy appointment and immediate follow-ups (including test-of-cure or CIN 1 follow-up), but it does not capture the potential long-term consequences, such as future screenings, disease progression and adverse obstetric outcomes.
Scenario 2: adverse obstetric outcomes were excluded
Scenario 2 simulated the cost-effectiveness results of adjunctive technology without taking into account the potential adverse obstetric outcomes of treatment excision.
Scenario 3: ZedScan was used alongside colposcopy at all appointments
Although the base-case analysis assumed that ZedScan was alongside colposcopy only during the initial appointment (before histology results), scenario 3 was run with the assumption that ZedScan would be used at all appointments, including therapeutic colposcopies after confirmation by histology results. Note that, as is the case for DySIS, no additional benefit is associated with the use of adjunctive technologies during therapeutic colposcopies.
Model validation
The face validity of the model structure and the key assumptions were evaluated by our clinical advisors. A series of steps were undertaken to ensure the internal validity of the model, including (1) double-checking model input estimates with the original sources, (2) repeated testing of individual elements of the model and (3) extensive logical tests and sensitivity analyses to ensure that the model behaved as would be expected. The results of the model were cross-validated by comparing the results to the previous published studies to ensure that any possible differences were identified and could be explained.
Results of the independent economic assessment
The economic evaluation compares DySIS with colposcopy alone, ZedScan with colposcopy alone and ZedScan with DySIS. Tables 29–34 and Tables 73 and 74 in Appendix 12 display the average cost and QALYs per patient and the incremental cost and QALYs per patient, as well as the ICER and secondary outcomes for the base case. Figures 14, 15, 29 and 30 and Figures 31–38 in Appendix 13 graphically summarise the base-case results and the results of the sensitivity and scenario analyses, using a cost-effectiveness plane to plot the incremental costs and the incremental QALYs. Detailed results for the sensitivity and scenario analyses are given in Appendix 13.
Base-case results
The base-case results are presented separately for the HPV triage and HPV primary screening protocols. The results are presented based on clinical practice (see and treat, watchful waiting) and in accordance with the reason for referral (all referrals, high grade and low grade).
Human papillomavirus triage protocol: base-case results
The results for the base-case analysis under the HPV triage protocol are summarised in Figure 14 and presented in more detail in Tables 29–31 (costs and QALYs) and in Table 73 in Appendix 12 (secondary outcomes).
FIGURE 14.
Base-case analysis cost-effectiveness results: HPV triage protocol. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.
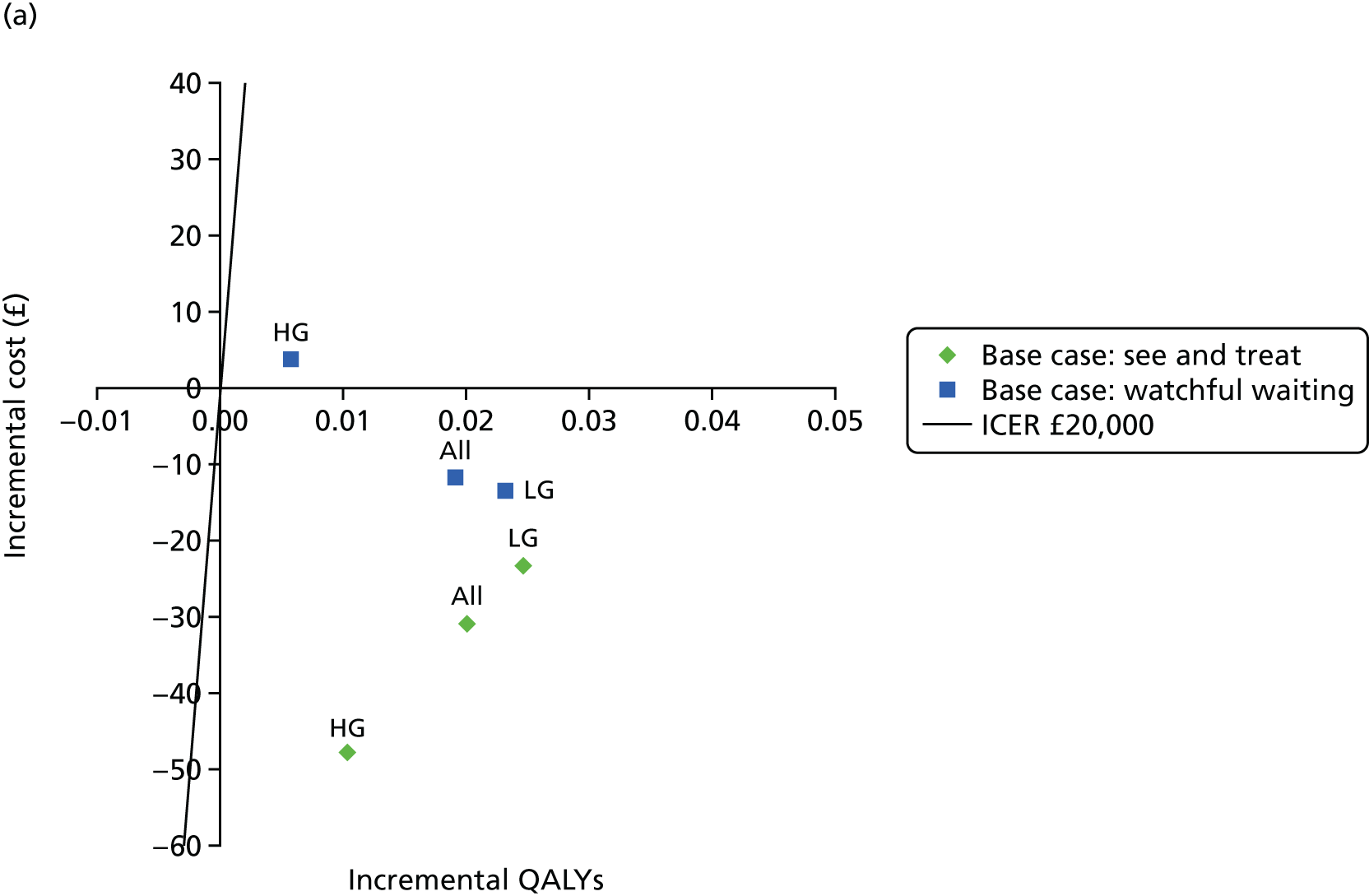
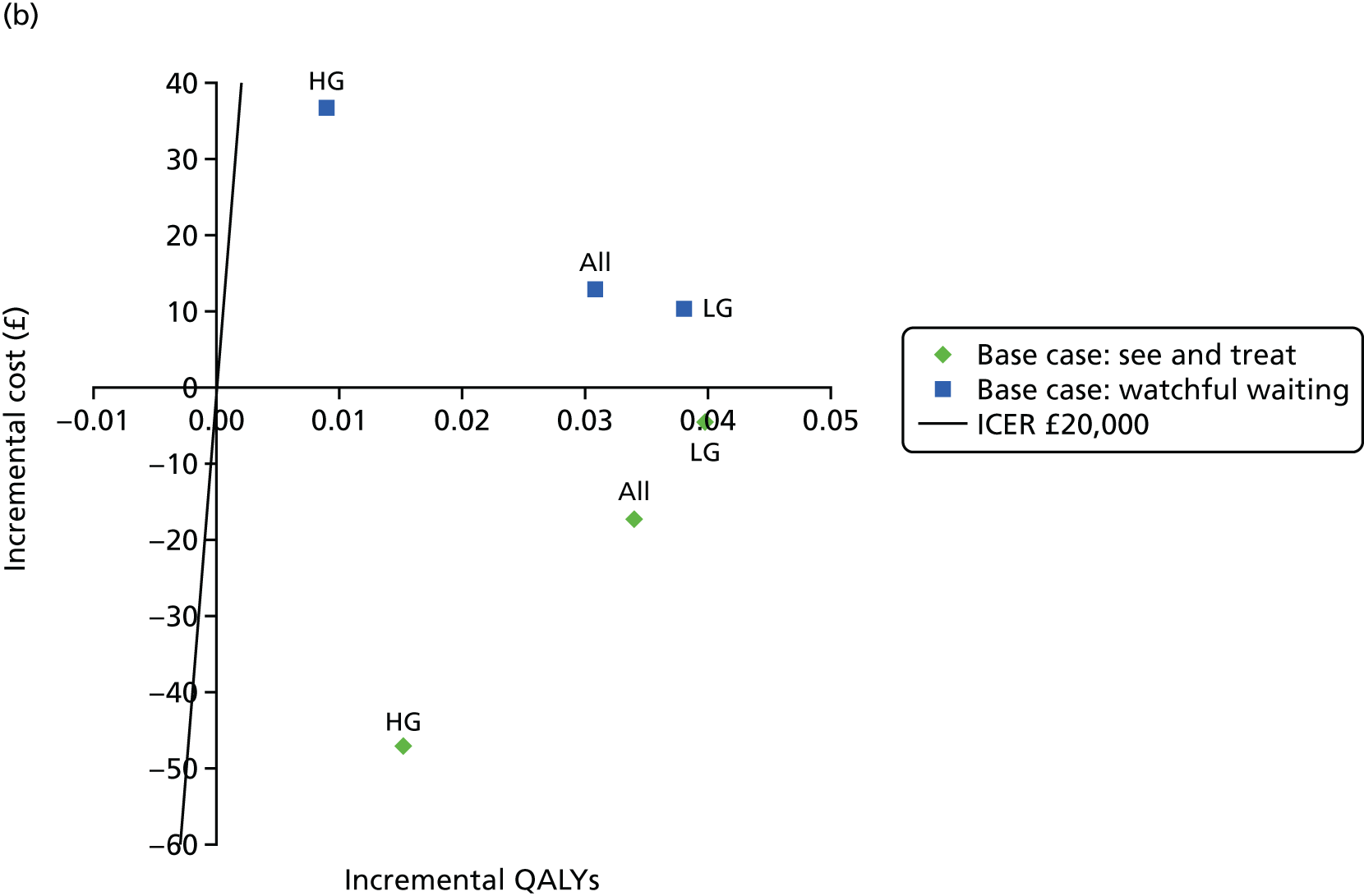

The main results of the base-case analysis under the HPV triage protocol can be summarised as follows:
-
Dynamic Spectral Imaging System routinely dominated colposcopy alone, regardless of the type of clinic or the reason for referral (Table 29). The only exception was for referrals for high-grade abnormalities in watchful-waiting clinic settings, in which DySIS was more costly and more effective, with an associated ICER of £675 per QALY.
-
ZedScan also dominated colposcopy alone in see-and-treat clinics (Table 30). However, in watchful-waiting clinics, ZedScan was always more effective than colposcopy alone, but was also more costly. The ICER for ZedScan in watchful-waiting clinics ranged from £272 (referrals for low-grade abnormalities) to £4070 per QALY (referrals for high-grade abnormalities).
-
The higher sensitivity of DySIS and ZedScan resulted in increased QALYs compared with conventional colposcopy alone in all sets of results. In addition, the incremental gain in QALYs always appeared to be higher for referrals for low-grade abnormalities. The higher incremental gain for referrals for low-grade abnormalities is attributable to the assumption that a diagnostic biopsy will not be performed if the colposcopy result is negative. Hence, false-negative results for referrals for low-grade abnormalities will therefore potentially be missed, making higher sensitivity a more critical consideration for referrals for low-grade abnormalities.
-
The impact on total cost appears to depend on the technology and clinic practice. Both adjunctive technologies generally decrease the average cost per patient in see-and-treat clinics. In watchful-waiting clinics, however, the average cost generally decreases to a smaller extent with DySIS and appears to increase with ZedScan compared with colposcopy alone.
-
The indirect comparison between ZedScan and DySIS showed that ZedScan routinely appears to be more effective but also more costly than DySIS (Table 31). The ICER for ZedScan ranged from £109 per QALY for referrals for high-grade abnormalities in see-and-treat clinics to £9918 per QALY for referrals for high-grade abnormalities in watchful-waiting clinics.
-
The secondary outcomes from the simulations show that a higher specificity (colposcopy alone) limits the number of unnecessary treatments and biopsies and consequently reduces the number of adverse obstetric outcomes (see Appendix 12, Table 73). In contrast, a higher sensitivity (adjunctive technologies) reduces the number of undetected CIN 2+, new cancer cases and deaths as a result of developing cancer.
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| DySIS | 872.34 | 19.18516 | –30.94 | 0.02016 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| DySIS | 770.65 | 19.18794 | –23.33 | 0.02464 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| DySIS | 1091.43 | 19.17156 | –47.70 | 0.01034 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| DySIS | 941.33 | 19.18194 | –11.69 | 0.01908 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| DySIS | 799.47 | 19.18601 | –13.38 | 0.02318 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| DySIS | 1255.93 | 19.16580 | 3.85 | 0.00571 | 675 |
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| ZedScan | 885.91 | 19.19901 | –17.37 | 0.03401 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| ZedScan | 789.30 | 19.20307 | –4.68 | 0.03978 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| ZedScan | 1091.97 | 19.17651 | –47.16 | 0.01529 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| ZedScan | 965.87 | 19.19363 | 12.85 | 0.03078 | 418 |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| ZedScan | 823.19 | 19.20082 | 10.34 | 0.03799 | 272 |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| ZedScan | 1288.82 | 19.16911 | 36.75 | 0.00903 | 4070 |
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 872.34 | 19.18516 | |||
| ZedScan | 885.91 | 19.19901 | 13.57 | 0.01385 | 980 |
| LG referrals | |||||
| DySIS | 770.65 | 19.18794 | |||
| ZedScan | 789.30 | 19.20307 | 18.65 | 0.01514 | 1232 |
| HG referrals | |||||
| DySIS | 1091.43 | 19.17156 | |||
| ZedScan | 1091.97 | 19.17651 | 0.54 | 0.00495 | 109 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 941.33 | 19.18194 | |||
| ZedScan | 965.87 | 19.19363 | 24.54 | 0.01170 | 2098 |
| LG referrals | |||||
| DySIS | 799.47 | 19.18601 | |||
| ZedScan | 823.19 | 19.20082 | 23.72 | 0.01481 | 1601 |
| HG referrals | |||||
| DySIS | 1255.93 | 19.16580 | |||
| ZedScan | 1288.82 | 19.16911 | 32.89 | 0.00332 | 9918 |
In Figure 14, the incremental costs and QALYs of DySIS versus colposcopy alone, ZedScan versus colposcopy alone and ZedScan versus DySIS are represented visually using separate cost-effectiveness planes. The horizontal axis divides the plane according to the incremental cost (positive above and negative below) and the vertical axis divides the plane according to the incremental QALYs (positive to the right and negative to the left). The cost-effectiveness plane is thus divided into four quadrants, with different implications for decision-making. If an intervention falls in the south-east quadrant, then it dominates the comparator technology (i.e. is less costly and more effective). Similarly, the intervention would be dominated by the comparator in the north-west quadrant. When non-dominance exists (north-east and south-west quadrants), the resulting ICER can be compared against the conventional cost-effectiveness threshold (£20,000–30,000 per QALY) to determine whether or not the intervention is cost-effective. In Figure 14, a £20,000 threshold is represented by the straight line that further divides the north-east and south-west quadrants. Points above and below the line indicate that the ICER of the intervention is higher or lower than the threshold, respectively. The results by type of clinic and the reason for referral are represented in each plane by distinct markers.
Human papillomavirus primary protocol: base-case results
The results for the base-case analysis under the HPV primary screening protocol are summarised in Figure 15 and presented in more detail in Tables 32–34 (costs and QALYs) and Appendix 12, Table 74 (secondary outcomes). The interpretation of the cost-effectiveness planes in Figure 15 is the same as for Figure 14.
FIGURE 15.
Base-case analysis cost-effectiveness results: HPV primary screening protocol. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.

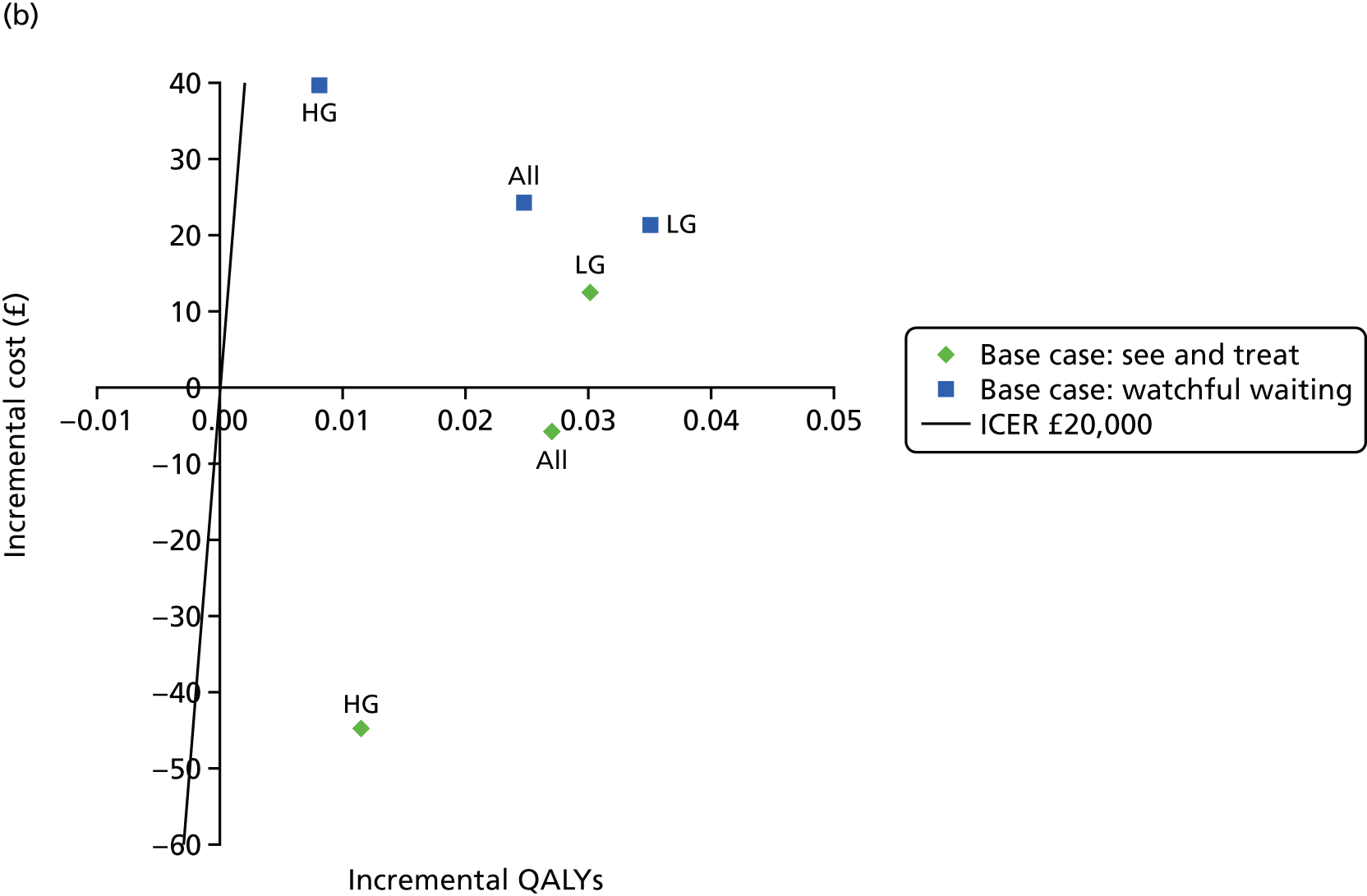
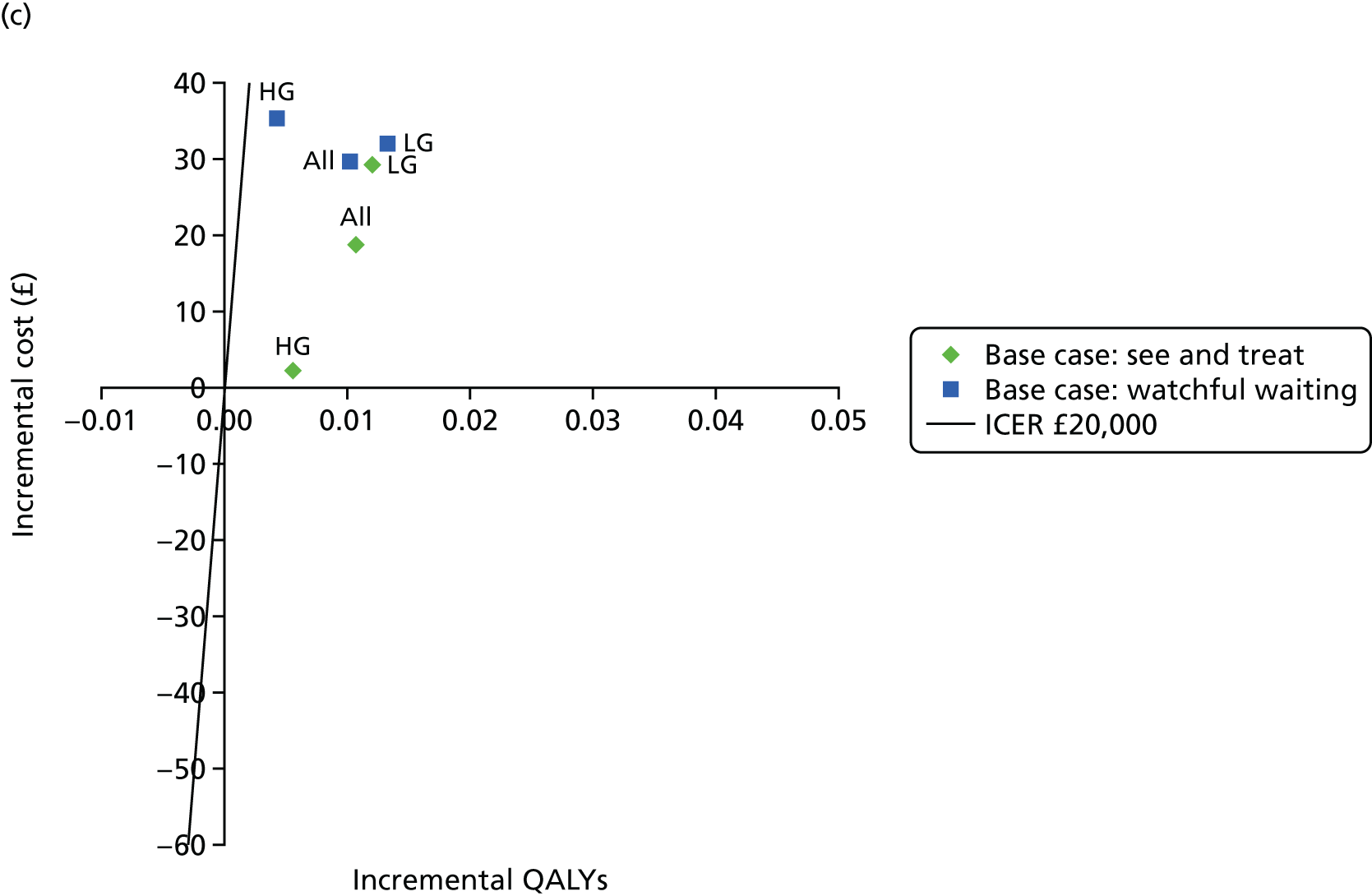
With regard to the cost-effectiveness of adjunctive technologies, the conclusions were quite similar under the HPV primary screening and HPV triage protocols:
-
In most instances, DySIS dominated colposcopy alone except for referrals for high-grade abnormalities in watchful-waiting clinics, for which the ICER was estimated to be £1095 per QALY (Table 32).
-
The results for ZedScan were more varied. ZedScan dominated colposcopy alone only for referrals for high-grade abnormalities in a see-and-treat clinic. In all other cases, ZedScan was more effective but also more costly than colposcopy alone. The ICER ranged from £417 per QALY for referrals for low-grade abnormalities in see-and-treat clinics to £4922 per QALY for referrals for high-grade abnormalities in watchful-waiting clinics (Table 33).
-
ZedScan was always more effective but also more costly than DySIS. The ICER ranged from £426 per QALY for referrals for high-grade abnormalities in see-and-treat clinics to £8190 per QALY for referrals for high-grade abnormalities in watchful-waiting clinics (Table 34).
-
Consistent with the findings reported in the ARTISTIC study149 simulations under the HPV primary screening protocol predicted higher health outcomes and a lower average cost per patient than under the HPV triage protocol. With regard to the cost-effectiveness of adjunctive technologies, the most significant impact of the HPV primary protocol was to reduce the incremental effect of the adjunctive technologies on health outcomes. Because HPV primary routine screening presents a higher sensitivity overall, cases of patients with CIN 2+ that were missed at the initial colposcopy appointment have a higher probability of being diagnosed 3 years later during routine screening, which would avoid the subsequent development of cancer. The lower sensitivity of colposcopy alone than that of adjunctive technologies therefore appears to be less critical in this context.
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| DySIS | 825.46 | 19.19120 | –24.62 | 0.01614 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| DySIS | 715.46 | 19.20787 | –16.87 | 0.01779 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| DySIS | 1079.83 | 19.16774 | –47.11 | 0.00581 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| DySIS | 889.04 | 19.18937 | –5.37 | 0.01426 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| DySIS | 738.10 | 19.20646 | –10.77 | 0.02150 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| DySIS | 1240.99 | 19.16234 | 4.06 | 0.00371 | 1095 |
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| ZedScan | 844.41 | 19.20206 | –5.67 | 0.02700 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| ZedScan | 744.85 | 19.22007 | 12.52 | 0.03000 | 417 |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| ZedScan | 1082.27 | 19.17347 | –44.66 | 0.01155 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| ZedScan | 918.78 | 19.19977 | 24.37 | 0.02466 | 988 |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| ZedScan | 770.26 | 19.21984 | 21.40 | 0.03487 | 614 |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| ZedScan | 1276.58 | 19.16668 | 39.64 | 0.00805 | 4922 |
| Grade of referral smear by clinic and technology | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER |
|---|---|---|---|---|---|
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 825.46 | 19.19120 | |||
| ZedScan | 844.41 | 19.20206 | 18.95 | 0.01085 | 1746 |
| LG referrals | |||||
| DySIS | 715.46 | 19.20787 | |||
| ZedScan | 744.85 | 19.22007 | 29.39 | 0.01220 | 2408 |
| HG referrals | |||||
| DySIS | 1079.83 | 19.16774 | |||
| ZedScan | 1082.27 | 19.17347 | 2.45 | 0.00574 | 426 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 889.04 | 19.18937 | |||
| ZedScan | 918.78 | 19.19977 | 29.74 | 0.01040 | 2860 |
| LG referrals | |||||
| DySIS | 738.10 | 19.20646 | |||
| ZedScan | 770.26 | 19.21984 | 32.16 | 0.01338 | 2404 |
| HG referrals | |||||
| DySIS | 1240.99 | 19.16234 | |||
| ZedScan | 1276.58 | 19.16668 | 35.58 | 0.00434 | 8190 |
Sensitivity analyses results
To investigate the impact of parameter uncertainty on the results, several sensitivity analyses were undertaken, which focused on diagnostic accuracy, costs and the initial characteristics of the population referred for colposcopy under the HPV primary screening protocol.
Uncertainty around diagnostic accuracy
Uncertainty around the diagnostic accuracy of colposcopy alone, DySIS and ZedScan was assessed with sensitivity analyses 1–4.4 (see 5.4.2.1 for a detailed description). Because the sensitivity analyses are different for DySIS and ZedScan, the results are presented separately in Figures 29 and 30 in Appendix 13. Detailed results for average and incremental costs and QALYs are presented in Appendix 13, Tables 75–88) for each sensitivity analysis, under the HPV triage and HPV primary screening protocols.
For DySIS compared with colposcopy alone, the results were globally unchanged compared with the base-case analysis:
-
Dynamic Spectral Imaging System dominated colposcopy alone in most instances except for referrals for high-grade abnormalities in watchful-waiting settings, in which the ICER ranged from £188 [sensitivity analysis 2 under the HPV triage protocol (see Appendix 13, Table 77)] to £1633 [sensitivity analysis 2 under the HPV primary screening protocol (see Appendix 13, Table 78)].
The results of sensitivity analyses comparing ZedScan with colposcopy alone were more varied:
-
The lower and upper bounds of ZedScan specificity and sensitivity (sensitivity analysis 4.3 and sensitivity analysis 4.4) had little impact on the results. ZedScan still dominated colposcopy alone in see-and-treat clinics and was more effective but also more costly than colposcopy alone in watchful-waiting clinics, under both the HPV triage and HPV primary screening protocols.
-
When ZedScan was compared with colposcopy alone based on diagnostic accuracy data that stemmed from a similar setting (sensitivity analysis 3), the incremental cost increased and the incremental QALYs decreased. Overall, ZedScan no longer dominated colposcopy alone in see-and-treat clinics, with an ICER ranging from £590 under the HPV triage protocol to £1457 under the HPV primary screening protocol. In watchful-waiting clinics, the ICER increased from £418 in the base case to £1910 in sensitivity analysis 3 under the HPV triage protocol and from £988 to £4023 under the HPV primary screening protocol. Under HPV primary screening, the ICER exceeded £20,000 per QALY for referrals for high-grade abnormalities in watchful-waiting clinics (see Appendix 13, Tables 79 and 80).
Uncertainty around costs
Sensitivity analyses results exploring the uncertainty around the costs of devices (sensitivity analyses 5.1 and 5.2) and the costs of diagnostic biopsy and LLETZ (sensitivity analysis 6) are summarised in Appendix 13, Figure 31, for the HPV triage protocol and Appendix 13, Figure 32, for the HPV primary screening protocol. As only the cost parameters were varied, the incremental QALYs are identical between the sensitivity analyses and the base case for each type of simulation. Note that, in Chapter 3, Figures 5 and 6, the scale of the vertical axis of the cost-effectiveness planes has been altered in order to represent higher incremental costs. Detailed results are presented in Appendix 13, Tables 89–106.
Logically, sensitivity analyses 5.1 and 6 increased the incremental cost for both technologies, but they did not appear to alter the base-case conclusions:
-
Owing to the higher purchase price, the results for DySIS appeared to be more sensitive to a decrease in the number of patients per colposcope per year (sensitivity analysis 5.1). DySIS no longer dominated colposcopy alone in watchful-waiting clinics under the HPV primary screening protocol, with an ICER of £270 for all referrals (see Appendix 13, Table 92).
-
Owing to the lower purchase price of ZedScan, the results did not appear to be sensitive to the assumed variation in throughput (sensitivity analyses 5.1 and 5.2). However, because of its high sensitivity and low specificity, the higher cost estimates for diagnostic biopsies and LLETZ (sensitivity analysis 6) had an impact on the results for ZedScan more significantly than the results for DySIS, especially for referrals for low-grade abnormalities in watchful-waiting clinics. ZedScan no longer dominated colposcopy alone, regardless of the type of clinics or the routine screening protocol. The ICER increased to £6709 for referrals for high-grade abnormalities in watchful-waiting clinics under the HPV primary screening protocol (see Appendix 13, Table 105).
-
The results on the indirect comparison between ZedScan and DySIS were unchanged.
Population referred for colposcopy under the human papillomavirus primary protocol
Sensitivity analyses 7.1 and 7.2 used the variation observed between pilot sites without and with HPV 16/18 genotyping, respectively, to explore the potential impact of uncertainty around the characteristics of the population referred for colposcopy under the HPV primary screening protocol. The results are summarised in Appendix 13, Figure 33, and presented in more detail in Appendix 13, Tables 107–112. Overall, the results are unchanged compared with those of the base case and the impact of a variation in population characteristics appears to be relatively small.
Scenarios
Three scenario analyses were undertaken to explore alternative structural assumptions: restricting the analysis to a 3-year period, excluding adverse obstetric outcomes and assuming that ZedScan was used alongside colposcopy at all appointments.
Scenario 1: time horizon of 3 years
The model was run for only 3 years to capture the cost and health outcomes of colposcopy and adjunctive technologies in the short term. Figures 31 and 32 in Appendix 13 summarise the results under the HPV triage and HPV primary screening protocols. The average and incremental costs and QALYs, as well as the secondary outcomes, are provided in Appendix 13 (see Tables 113–115).
The results were dramatically different when long-term costs and health outcomes were not taken into account in the evaluation:
-
In the short term, colposcopy alone routinely dominated DySIS and ZedScan (i.e. it was less costly and more effective). The higher specificity of colposcopy alone limited the number of treatments and therefore reduced the average cost compared with DySIS or ZedScan. Meanwhile, the lower specificity of colposcopy alone was less penalised, as most individuals with untreated CIN 2+ would not have developed cancer or died from cancer 3 years after their initial examination (see Appendix 13, Tables 113 and 114).
-
Colposcopy was not found to be dominant only for referrals for high-grade abnormalities in see-and-treat clinics. DySIS and ZedScan were less costly, but also less effective, than colposcopy alone, with ICERs, respectively, of £236,692 and £85,045 per QALY under the HPV triage protocol (see Appendix 13, Tables 113 and 114).
Scenario 2: adverse obstetric outcomes were excluded
Scenario 2 excluded from the analysis the adverse consequences of CIN treatment on obstetric outcomes. The results are summarised in Appendix 13, Figures 36 and 37 and presented in more detail in Tables 116–118.
When adverse obstetric outcomes were excluded, all technologies were found to be less costly than in the base-case scenario.
The cost-effectiveness results for DySIS and ZedScan compared with colposcopy alone were unchanged under both the HPV triage protocol and the HPV primary screening protocol:
-
Dynamic Spectral Imaging System routinely dominated colposcopy alone, regardless of the type of clinic, the reason for referral or the routine screening protocol.
-
ZedScan also dominated colposcopy alone in see-and-treat clinics. However, ZedScan was more effective but also routinely more costly than colposcopy alone in watchful-waiting clinics.
As in the base case, ZedScan was routinely more effective and more costly than DySIS:
-
Because ZedScan presents a lower specificity than DySIS, the ICER was lower when adverse obstetric outcomes were excluded than in the base-case scenario: £427 per QALY compared with £980 per QALY for see-and-treat clinics under the HPV triage protocol (all referrals) and £1476 per QALY compared with £1746 per QALY under the HPV primary screening protocol (all referrals).
Scenario 3: ZedScan was used alongside colposcopy at all appointments
Scenario 3 assumed that ZedScan was used alongside colposcopy at all appointments, including therapeutic colposcopies, after confirmation by histology results. As the costs and QALYs were not altered for colposcopy alone and DySIS compared with the base case, Appendix 13, Figure 38, presents the results for ZedScan compared with colposcopy alone, and for ZedScan compared with DySIS only, under the HPV triage and HPV primary screening protocols. Detailed results are given in Tables 119–122 in Appendix 13.
As no additional benefit is associated with the use of ZedScan during therapeutic colposcopies, the impact of scenario 3 is only an increase in the incremental cost of ZedScan compared with colposcopy alone and DySIS:
-
Overall, the conclusions remain unchanged compared with those of the base case.
However, the impact of using ZedScan alongside colposcopy at all appointments varies depending on the reason for referral and the type of clinic:
-
The increase in cost was higher for referrals for high-grade abnormalities in watchful-waiting clinics – when ZedScan was compared with colposcopy alone, the ICER was £7270 in the HPV triage protocol (see Appendix 13, Table 119) and £8557 under the HPV primary screening protocol (see Appendix 13, Table 121); when ZedScan was compared with DySIS, the ICER reached £18,628 under the HPV triage protocol (see Appendix 13, Table 120) and £14,928 under the HPV primary screening protocol (see Appendix 13, Table 122).
Discussion of the independent economic assessment
Only two studies that reported on the cost-effectiveness of DySIS and ZedScan were included. Neither study was considered to fully inform the stated decision problem, which includes the current HPV triage protocol (including test of cure) and also the proposed HPV primary screening protocol.
A de novo decision-analytic model (the ‘York model’) was developed using a patient-level state-transition modelling approach to estimate the cost-effectiveness of adjunctive colposcopy technologies (DySIS with DySISmap and ZedScan) for people who are referred for colposcopy through the NHSCSP under either HPV triage (including test of cure) or the HPV primary screening algorithm (including test of cure).
The York model was specifically developed to address the limitations of existing studies and concerns regarding the generalisability to both the HPV triage protocol and the HPV primary screening protocols. The main strength of the decision model is the linkage between the diagnostic accuracy of a given identification strategy, the impact on subsequent treatment decisions and the ultimate effect on health outcomes and costs. A potential limitation of the model is that the patient-level modelling approach precluded a probabilistic assessment of cost-effectiveness and hence decision uncertainty could also not be fully represented in our analyses. Although the inclusion of a probabilistic assessment was technically feasible, repeating simulations to appropriately represent second-order uncertainty was not considered to be feasible within the time frame. However, a broad range of scenario and sensitivity analyses were undertaken to address key assumptions and uncertainties.
The base-case cost-effectiveness results showed that DySIS routinely dominated colposcopy (i.e. it was less costly and more effective than standard colposcopy). The only exception was for referrals for high-grade abnormalities in a watchful-waiting clinic setting, in which the ICER of DySIS varied between £675 and £1095 per QALY under the HPV triage and primary screening protocols. ZedScan also dominated colposcopy alone for referrals for high-grade abnormalities in see-and-treat clinics. The ICER for ZedScan varied between £272 (referral for low-grade abnormality in a watchful-waiting clinic, HPV triage protocol) and £4922 per QALY (referral for high-grade abnormality in a watchful-waiting clinic, HPV primary screening). These findings appeared to be robust to a wide range of sensitivity and scenario analyses. Only in one of the analyses did the ICER exceed a £20,000-per-QALY threshold. This arose in a sensitivity analysis for ZedScan in which the diagnostic performance of colposcopy was derived from a separate study to the base-case analysis and only for referrals for high-grade abnormalities in a watchful-waiting clinic under the HPV primary screening protocol.
In the absence of a direct comparison between the alternative technologies, an indirect comparison was performed. However, these results should be considered to be exploratory in nature given the lack of a robust direct comparison and the challenges identified more generally, which arose from the limitations in the evidence base for ZedScan. The base-case cost-effectiveness results showed that ZedScan was always more effective but also more costly than DySIS. The ICER ranged from £109 per QALY for referrals for high-grade abnormalities in see-and-treat clinics under the HPV primary screening protocol to £9918 per QALY for referrals for high-grade abnormalities in watchful-waiting clinics under the HPV triage protocol. These findings appeared to be robust to a wide range of sensitivity and scenario analyses.
There remains uncertainty regarding the cost-effectiveness of ZedScan, given the challenges of comparing it with colposcopy and DySIS. Moreover, the cost-effectiveness results presented for the HPV primary screening protocols also require careful consideration. Our analysis is based on the current protocol and the assumption that the final HPV primary screening protocol may alter prior to HPV primary screening being rolled out nationally. Furthermore, key input data were derived from unpublished and preliminary results collected in the HPV pilot sites. Data collection is still ongoing and selection issues may limit the generalisability of the data used. Hence, the results under the HPV primary screening protocol should be considered to be exploratory and further analyses should ideally be undertaken when data collection has been completed and the implications of any selection effect is clearer.
Conclusions of the cost-effectiveness section
The cost-effectiveness of both adjunctive technologies compared with standard colposcopy, under both the HPV triage and HPV primary screening algorithms, appears to be favourable when compared with the conventional threshold used to determine value in the NHS. However, the limitations and uncertainties in the evidence base identified for ZedScan need to be carefully considered. The cost-effectiveness of both adjunctive technologies under the HPV primary screening protocol should also be reassessed when additional data become available from the pilot sites.
Chapter 6 Discussion
Statement of the principal findings
Diagnostic accuracy
Nine studies compared adjunctive DySIS (DySISmap and DySIS colposcope) with DySIS video colposcopy alone. Adjunctive DySIS use was found to have a higher sensitivity to detect CIN 2+ (81.25%, 95% CI 72.2% to 87.9%) than standard colposcopy alone (57.91%, 95% CI 47.2% to 67.9%), but a lower specificity (70.40%, 95% CI 59.4% to 79.5%) than colposcopy (87.41%, 95% CI 81.7% to 91.5%). This difference appears to be because adjunctive DySIS leads to more positive test results (i.e. more women are judged to have possible high-grade CIN).
Only two included studies investigated ZedScan, one of which was a study of a precommercial prototype. The results from the prototype study suggested that adjunctive ZedScan could improve diagnostic accuracy when compared with colposcopy alone (i.e. it could increase sensitivity at the same specificity as colposcopy or vice versa). (Confidential information has been removed.)
Data on participant subgroups, including women infected with hrHPV or referred with high-grade abnormalities, were limited. The results suggested that colposcopy alone has a poor sensitivity to detect high-grade CIN in women referred with low-grade abnormalities (e.g. mild dyskaryosis). Adjunctive DySIS and ZedScan appeared to improve diagnosis in women referred with low-grade abnormalities. There was some limited evidence that the diagnostic accuracy of adjunctive DySIS may be greater in women with hrHPV infection.
The sensitivity analyses suggested that the sensitivity and specificity of adjunctive and standard colposcopy were dependent on what reference standard was used in women with no colposcope-detected high-grade CIN. Both sensitivity and specificity tended to be higher when no biopsies were performed in those women, which suggests a possible verification bias. This suggests that the actual accuracy of colposcopy and adjunctive colposcopy is uncertain. However, the comparative results are valid, because any possible verification bias is likely to affect the results of adjunctive and standard colposcopy equally.
Clinical effectiveness
Only three studies that reported data on our prespecified clinical effectiveness outcomes were included. One study of ZedScan reported three adverse events, of which one was serious, and two studies of DySIS with DySISmap reported that no adverse events occurred following colposcopy examination. No data were reported on mortality, morbidity and HRQoL in studies of DySIS and ZedScan.
Implementation
Five studies reported data on our prespecified implementation outcomes, including four studies of DySIS and one of ZedScan.
There is reasonable evidence that DySISmap as an adjunct to colposcopy is generally well received by patients referred for colposcopy and that patients are generally satisfied with the duration of the examination. There is evidence that adjunctive DySIS was generally perceived by clinicians to improve the accuracy of colposcopy and confidence in their diagnostic decisions and biopsy site selection. There is also evidence that adjunctive DySIS was intuitive for clinicians with limited colposcopy experience and improved their evaluations. In addition, there is evidence that the additional time required to use ZedScan is minimal in experienced colposcopists. However, all included studies had significant limitations, and therefore these findings need to be interpreted with caution.
No evidence was found for several of the prespecified outcomes: successful database and record management, capacity to perform colposcopies, and uptake and compliance. No evidence was found regarding the training requirements for DySIS. The limited evidence for ZedScan precludes conclusions for any of the implementation review prespecified outcomes.
Cost-effectiveness
Only two studies that reported on the cost-effectiveness of DySIS and ZedScan were included. Neither study was considered to fully inform the stated decision problem, which includes the current HPV triage protocol (including test of cure) and also the proposed HPV primary screening protocol.
A de novo decision-analytic model (the ‘York model’) was developed using a patient-level state-transition modelling approach to estimate the cost-effectiveness of adjunctive colposcopy technologies (DySIS with DySISmap and ZedScan) for people who are referred for colposcopy through the NHSCSP under either HPV triage (including test of cure) or the HPV primary screening algorithm (including test of cure).
The York model provides a link between diagnostic test accuracy and final health outcomes expressed in terms of QALYs. It provides a quantitative framework, using the best available evidence, to determine how the diagnostic performance of both adjunctive colposcopy technologies is likely to affect subsequent treatment and/or monitoring options and their effect on disease progression. The model also captures the potential impact of the technologies on unnecessary biopsies and excisions, which may increase the risk of adverse obstetric outcomes.
The base-case cost-effectiveness results showed that adjunctive DySISmap routinely dominated (i.e. was less costly and more effective than) standard colposcopy. The only exception was for referrals for high-grade abnormalities in a watchful-waiting clinic setting, in which the ICER of DySISmap varied between £675 and £1095 per QALY under the HPV triage and HPV primary screening protocols. ZedScan also dominated colposcopy alone for referrals for high-grade abnormalities in see-and-treat clinics. The ICER for ZedScan varied between £272 (referral for low-grade abnormality in a watchful-waiting clinic, HPV triage protocol) and £4922 per QALY (referral for high-grade abnormality in a watchful-waiting clinic, HPV primary screening protocol). These findings appeared to be robust to a wide range of sensitivity and scenario analyses.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted with an attempt to maximise the retrieval of potentially relevant studies. These included electronic searches of a variety of bibliographic databases, as well as the screening of clinical trial registers and conference proceedings to identify unpublished studies. The search strategy did not restrict by study design. The device manufacturers and study authors were contacted to provide additional data, and the review includes additional data from published studies and data from as-yet-unpublished studies. The review process followed recommended methods to minimise the potential for error and/or bias. The quality of the included studies was assessed and accounted for when interpreting the review results. Appropriate synthesis methods were employed by taking into account the heterogeneity of the study characteristics.
One study of DySIS was rated as being at a low risk of bias, and all other included studies were rated as being at a high risk of bias. The evidence for ZedScan was particularly limited. Only one study of ZedScan was available, and there was no evidence directly comparing ZedScan with standard colposcopy. The evidence for ZedScan came mostly from a single centre and excluded relevant patient populations (including patients with transformation zone type 3), which limits the extent to which the evidence for ZedScan is applicable to the broader population of women referred through the NHSCSP.
No studies directly compared DySIS with ZedScan. Very few data on participant subgroups were available. In particular, there were few data on diagnostic accuracy in women referred through HPV primary screening.
There was very limited evidence relating to the clinical effectiveness of adjunctive DySIS or ZedScan, with little reporting of any potential adverse effects.
Cost-effectiveness
The York model was specifically developed to address the limitations of existing studies and concerns regarding the generalisability of the current decision problem under both the HPV triage and HPV primary screening protocols. The main strength of the decision model is that it directly addresses several of the key assumptions and areas of uncertainties identified in our review of previously published studies, including the consideration of the potential impact of unnecessary treatment on adverse obstetric outcomes.
A potential limitation of the model is that the patient-level modelling approach precluded a probabilistic assessment of cost-effectiveness, and hence decision uncertainty could also not be fully represented in our analyses. The decision to use a patient-level approach was taken with careful consideration and we consider that alternative modelling approaches were not appropriate, given the high level of complexity that arises from interactions between the natural history model and the screening and treatment pathways and the need to characterise two separate screening paradigms. Although the inclusion of a probabilistic assessment was technically feasible, each analysis (i.e. for each type of clinic and for each of the different reasons for referral) required 500,000 simulations, and took approximately 15 minutes to run. Repeating this simulation to appropriately represent second-order uncertainty was not considered to be feasible within the time frame. However, a broad range of scenario and sensitivity analyses were undertaken to address key assumptions and uncertainties.
Finally, given the complexity of the modelling and resource constraints, it was not possible to undertake systematic reviews for several of the model parameters (e.g. utilities, costs). Instead, estimates were sourced pragmatically based on sources used in other modelling studies.
Uncertainties
Clinical effectiveness
There were no data comparing ZedScan with colposcopy, so any improvement in diagnostic accuracy with ZedScan over colposcopy alone is uncertain. Owing to design limitations, the extent to which the evidence for ZedScan is applicable to the broader population of women referred through the NHSCSP is uncertain.
No studies compared DySIS with ZedScan directly, limiting the possibility of comparing the diagnostic accuracy of the technologies. Most studies were performed in women referred for colposcopy on the basis of cytology screening, so the diagnostic accuracy of all methods in women referred from HPV primary screening is uncertain, particularly as the data on the diagnostic accuracy in women infected with hrHPV were also limited.
The reference standard (histopathology of samples from punch biopsy or excision) was applied variably across studies. In particular, biopsies were not performed in women with normal colposcopy examination results in several DySIS studies and in all ZedScan studies. This may have led to positive bias in estimates of the diagnostic accuracy for both adjunctive colposcopy and colposcopy alone. Hence, the estimates of sensitivity and specificity reported may not be the same as the diagnostic accuracy that will be observed in the UK.
Cost-effectiveness
The uncertainties noted regarding the design limitations for the evidence of ZedScan also raise important uncertainties regarding the generalisability of the cost-effectiveness results for ZedScan to routine NHS usage.
The introduction of HPV primary screening will alter the population of women referred for colposcopy through the NHSCSP. However, the data are still incomplete, especially for women referred at the third round, and, because the pilot sites were not randomly selected, the data are subject to selection issues, especially variability in the prevalence of HPV and CIN lesions compared with the general population. The impact of these issues on the cost-effectiveness of the adjunctive technologies is not possible to determine. As a result, we would recommend that the cost-effectiveness analysis of the adjunctive technologies is updated when data collection from the HPV primary screening protocol has been completed and the implications of any selection effects are clearer.
Other relevant factors
The population of women referred for colposcopy is likely to change significantly in the future, as females who have received the HPV vaccine reach screening age. The implication of this for the cost-effectiveness of the adjunctive technologies has not been included in the current assessment.
Chapter 7 Conclusions
Implications for service provision
The use of adjunctive DySIS (DySISmap with DySIS video colposcope) increases sensitivity when compared with colposcopy alone, so it increases the number of women detected with high-grade CIN. However, it also reduces specificity when compared with colposcopy, so that more women with no or low-grade CIN will be incorrectly judged as possibly having high-grade CIN. This could lead to an increase in the number of unnecessary diagnostic biopsies, excisions and ‘see-and-treat’ cases, although the evidence on whether or not this is actually the case is limited. The use of adjunctive DySIS might therefore increase unnecessary anxiety and complications in subsequent pregnancies in women who did not require treatment. The use of DySIS is likely to be cost-saving when compared with standard colposcopy.
The limited evidence precludes any definitive conclusions regarding the diagnostic accuracy of ZedScan (confidential information has been removed). It is, therefore, also likely to be cost-saving compared with standard colposcopy. There is currently too little evidence to compare the relative diagnostic accuracy of ZedScan with that of DySIS.
The introduction of any of these adjunctive technologies may require additional staff training, which may impose additional costs that were not considered in the analysis.
Suggested research priorities
Given the limited evidence for ZedScan, further diagnostic accuracy studies of ZedScan are needed, particularly to compare its diagnostic accuracy with that of standard colposcopy, and in groups that are independent of the manufacturers. Diagnostic accuracy studies comparing both DySIS and ZedScan as adjunct to colposcopy directly and against colposcopy alone may also be useful.
As most current studies have been in women referred to colposcopy on the basis of cytology screening, diagnostic accuracy studies in women referred through HPV primary screening are needed to assess whether or not the new screening programme will alter diagnostic accuracy. Similarly, studies in women who have been vaccinated against HPV may be required in the future.
All future diagnostic accuracy studies should have robust designs with sufficient power, including consecutive patients from a representative population of NHS referrals, ensuring adequate blinding of all assessors and taking biopsies in all women, including those with no colposcopic evidence of CIN.
There is limited evidence on the clinical impact of using adjunctive colposcopy, such as its impact on biopsy rates or longer-term health. Appropriate audits of centres using adjunctive colposcopy should be used to gather evidence.
Acknowledgements
We would like to thank members of the NICE DG431 appraisal committee for providing clinical advice, including Dr Theresa Freeman-Wang, Consultant Gynaecologist, Whittington Hospital; Professor Jane Macnaughton, Professor of Medical Humanities (Durham University) and general practitioner colposcopist; and Miss Hema Nosib, Consultant in Obstetrics and Gynaecology, Hinchingbrooke Hospital. We would like to thank Dr Pluvio Coronado from Hospital San Carlos, Spain, and Matjeka Reboli, Senior Epidemiologist, Queen Mary University of London, for providing additional data. We thank Mr Philip Morgan, Health Economist at BresMed, for providing assistance with the review of economic evidence.
Contributions of authors
Mathilde Peron (Lecturer, Health Economist) contributed to the protocol, performed the economic analysis and wrote all sections relating to cost-effectiveness.
Alexis Llewellyn (Research Fellow, Systematic Reviewer) contributed to the protocol, performed the systematic review and wrote most of the sections on clinical effectiveness.
Thirimon Moe-Byrne (Research Fellow, Systematic Reviewer) contributed to the protocol, performed the systematic review and wrote the background section.
Simon Walker (Research Fellow, Health Economist) assisted in the economic analyses.
Matthew Walton (Master of Science Student) assisted in the systematic review and the writing of the clinical effectiveness sections.
Melissa Harden (Information Specialist) performed the database searches and maintained the review libraries.
Stephen Palmer (Professor of Health Economics) contributed to the protocol and oversaw the conduct and writing of the cost-effectiveness analyses and the report as a whole.
Mark Simmonds (Senior Research Fellow, Statistician) contributed to the protocol, performed the meta-analyses and oversaw the conduct and writing of the clinical effectiveness sections and the report as a whole.
Data-sharing statement
All available data are contained within the report. All queries should be submitted to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Cervical Cancer. Survival 2015. www.cancerresearchuk.org/about-cancer/cervical-cancer/survival (accessed 22 January 2017).
- Cancer Research UK . Cervical Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence (accessed 24 November 2016).
- NHS Choices . Cervical Cancer 2015. www.nhs.uk/Conditions/Cancer-of-the-cervix/Pages/Introduction.aspx (accessed 22 March 2017).
- Cox JT, Schiffman M, Solomon D. ASCUS-LSIL Triage Study (ALTS) Group . Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol 2003;188:1406-12. https://doi.org/10.1067/mob.2003.461.
- Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96:604-15. https://doi.org/10.1093/jnci/djh104.
- Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis 2009;9. https://doi.org/10.1186/1471-2334-9-119.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009;113:18-25. https://doi.org/10.1097/AOG.0b013e31818f5008.
- Stewart BW, Wild CW. World Cancer Report 2014. Geneva: World Health Organization; 2014.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Agents Classified by the IARC Monographs, Volumes 1–117. Lyon: IARC; 2016.
- HPV and Cancer. London: Cancer Research UK; 2017.
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008;26:K1-16. https://doi.org/10.1016/j.vaccine.2008.05.064.
- Cancer Research UK . Cervical Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer (accessed 24 November 2016).
- Kuroki LM, Bergeron LM, Gao F, Thaker PH, Massad LS. See-and-treat loop electrosurgical excision procedure for high-grade cervical cytology: are we overtreating?. J Low Genit Tract Dis 2016;20:247-51. https://doi.org/10.1097/LGT.0000000000000230.
- NHS Choices . Cervical Screening 2015. www.nhs.uk/conditions/Cervical-screening-test/Pages/Introduction.aspx (accessed 22 March 2017).
- NHS Cervical Screening Programme. Colposcopy and Programme Management. NHSCSP Publication Number 20. London: Public Health England; 2016.
- Smith JHF, Patnick J. Achievable Standards, Benchmarks for Reporting, and Criteria for Evaluating Cervical Cytopathology. Third Edition Including Revised Performance Indicators. Sheffield: NHS Cervical Screening Programme; 2013.
- Solomon D, Nayar R. The Bethesda System for Reporting Cervical Cytology. New York, NY: Springer-Verlag; 2004.
- Cervical Screening Programme, England – 2015–16. Leeds: NHS Digital; 2016.
- Cervical Screening Programme, England – 2014–15. Leeds: NHS Digital; 2015.
- Moss S, Gibney A. HPV Primary Screening Pilots: Evaluation Report to the National Screening Committee. February 2015. London: Public Health England; 2015.
- Changes to Cervical Cancer Screening. London: DHSC; 2016.
- HPV Primary Screening Pilot Protocol Algorithm. London: Public Health England; 2016.
- Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009;10:672-82. https://doi.org/10.1016/S1470-2045(09)70156-1.
- Ferris DG, Litaker M. ALTS Group . Interobserver agreement for colposcopy quality control using digitized colposcopic images during the ALTS trial. J Low Genit Tract Dis 2005;9:29-35. https://doi.org/10.1097/00128360-200501000-00007.
- Ferris DG, Litaker MS. Cervical biopsy sampling variability in ALTS. J Low Genit Tract Dis 2011;15:163-8. https://doi.org/10.1097/LGT.0b013e3181f2ddf3.
- Zuchna C, Hager M, Tringler B, Georgoulopoulos A, Ciresa-Koenig A, Volgger B, et al. Diagnostic accuracy of guided cervical biopsies: a prospective multicenter study comparing the histopathology of simultaneous biopsy and cone specimen. Am J Obstet Gynecol 2010;203:321.e1-6. https://doi.org/10.1016/j.ajog.2010.05.033.
- Management of Cervical Cancer. A National Clinical Guideline. Edinburgh: SIGN; 2008.
- HPV Primary Screening Pilot: Colposcopy Management Recommendations Algorithm. London: Public Health England; 2015.
- Salter J. Revealing the True Cost of Cervical Cancer. Behind the Screen. London: Demos; 2014.
- Wade R, Spackman E, Corbett M, Walker S, Light K, Naik R, et al. Adjunctive colposcopy technologies for examination of the uterine cervix – DySIS, LuViva Advanced Cervical Scan and Niris Imaging System: a systematic review and economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17080.
- Adjunctive Colposcopy Technologies for Examination of the Uterine Cervix – DySIS and the Niris Imaging System. London: NICE; 2012.
- Adjunctive Colposcopy Technologies for Assessing Suspected Cervical Abnormalities: The DYSIS colposcope with DYSISmap and the ZedScan I. London: NICE; 2018.
- Adjunctive Colposcopy Technologies for Examination of the Uterine Cervix – DySIS and the Niris Imaging System: Consultation Document. London: NICE; 2012.
- The ZedScan as an Adjunct to Colposcopy in Women with Suspected Cervical Intraepithelial Neoplasia. London: NICE; 2015.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339. https://doi.org/10.1136/bmj.b2700.
- Underwood M, Arbyn M, Parry-Smith W, De Bellis-Ayres S, Todd R, Redman CW, et al. Accuracy of colposcopy-directed punch biopsies: a systematic review and meta-analysis. BJOG 2012;119:1293-301. https://doi.org/10.1111/j.1471-0528.2012.03444.x.
- Wade R, Corbett M, Eastwood A. Quality assessment of comparative diagnostic accuracy studies: our experience using a modified version of the QUADAS-2 tool. Res Synth Methods 2013;4:280-6. https://doi.org/10.1002/jrsm.1080.
- Burns KE, Duffett M, Kho ME, Meade MO, Adhikari NK, Sinuff T, et al. ACCADEMY Group . A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008;179:245-52. https://doi.org/10.1503/cmaj.080372.
- Center for Evidence-Based Management . Critical Appraisal of a Cross-Sectional Study (Survey) 2014. www.cebma.org/wp-content/uploads/Critical-Appraisal-Questions-for-a-Cross-Sectional-Study-july-2014.pdf (accessed 15 March 2017).
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865-84. https://doi.org/10.1002/sim.942.
- Simmonds MC, Higgins JP. A general framework for the use of logistic regression models in meta-analysis. Stat Methods Med Res 2016;25:2858-77. https://doi.org/10.1177/0962280214534409.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Budithi S, Peevor RJ, Pugh D, Papagiannakis E, Durman A, Banu N, et al. Evaluating colposcopy with Dynamic Spectral Imaging during routine practice at five colposcopy clinics in Wales: clinical performance. Gynecol Obstet Invest 2018;83:234-40. https://doi.org/10.1159/000487319.
- Budithi S, Peevor R, Pugh D, Papagiannakis M, Leeson S. DySIS Service Evaluation in Wales. Patient and Colposcopist Feedback n.d.
- Budithi S, Peevor R, Pugh D, Papagiannakis M, Leeson S. DySIS Service Evaluation in Wales. Final Data from 5 Sites n.d.
- Budithi S, Peevor R, Pugh D, Papagiannakis M, Leeson S. DySIS Service Evaluation in Wales. A Report of Preliminary Data from 2 Sites n.d.
- Budithi S, Peevor R, Papagiannakis E, Leeson S. DySIS colposcopy service evaluation in Wales. A report of preliminary data from four sites. Int J Gynecol Cancer 2015;25.
- Coronado PJ, Fasero M. Colposcopy combined with dynamic spectral imaging. A prospective clinical study. Eur J Obstet Gynecol Reprod Biol 2016;196:11-6. https://doi.org/10.1016/j.ejogrb.2015.09.007.
- Coronado PJ, Fasero M. Correlating the accuracy of colposcopy with practitioner experience when diagnosing cervical pathology using the dynamic spectral imaging system. Gynecol Obstet Invest 2014;78:224-9. https://doi.org/10.1159/000365087.
- Coronado P, Fasero M, Papagiannakis M. Value of the Dynamic Spectral Imaging Map in the Diagnosis of Cervical Intraepithelial Neoplasia n.d.
- Coronado P, Fasero M, Rincon JA, Papagiannakis M, Herraiz M. Colposcopy Accuracy Using the Dynamic Spectral Imaging System (DySIS) by Colposcopist Experience n.d.
- Founta C, Papagiannakis E, Ratnavelu N, Feusi A, Natsis S, Bradbury M, et al. Diagnostic accuracy of colposcopy with dynamic spectral imaging for cytology-negative/high-risk HPV positive (failed test of cure) after large loop excision of the transformation zone (LLETZ) of the cervix: results of the DySIS colposcopy 1 study. Medicine (Baltimore) 2018;97. https://doi.org/10.1097/MD.0000000000009560.
- Founta A, Ang C, Biliatis I, Bradbury M, Fisher A, Kucukmetin A, et al. Dynamic Spectral Imaging for Post-Treatment Triage n.d.
- Founta C, O’Donnell R, Fisher A, Biliatis I, Ratnavelu N, Rangar R, et al. Dynamic Spectral Imaging for Post-Treatment Triage n.d.
- Founta C, Fisher A, Ratnavelu N, O’Donnell R, Feusi A, Bradbury M, et al. Dynamic Spectral Imaging for Post-Treatment Triage n.d.
- Founta C, Ratnavelu N, Bradbury M, Natsis S, O’Donnell R, Feusi A, et al. The role of dynamic spectral imaging (DySIS) in colposcopic assessment of cytology negative failed test of cure patients: final results of a pilot study. Int J Gynecol Cancer 2015;25.
- Founta C, Ratnavelu N, Bradbury M, Natsis S, O’Donnell R, Feusi A, et al. The Role of Dynamic Spectral Imaging (DySIS) in Colposcopic Assessment of Cytology Negative Failed Test of Cure Patients: Results of a Pilot Study n.d.
- Louwers J, Zaal A, Kocken M, Ter Harmsel W, Graziosi G, Spruijt J, et al. Dynamic spectral imaging colposcopy: higher sensitivity for detection of premalignant cervical lesions. BJOG 2011;118:309-18. https://doi.org/10.1111/j.1471-0528.2010.02806.x.
- Louwers JA, Zaal A, Kocken M, Berkhof J, Papagiannakis E, Snijders PJ, et al. The performance of Dynamic Spectral Imaging colposcopy depends on indication for referrals. Gynecol Oncol 2015;139:452-7. https://doi.org/10.1016/j.ygyno.2015.10.009.
- Zaal A, Louwers JA, Berkhof J, Kocken M, Ter Harmsel WA, Graziosi GC, et al. Agreement between colposcopic impression and histological diagnosis among human papillomavirus type 16-positive women: a clinical trial using dynamic spectral imaging colposcopy. BJOG 2012;119:537-44. https://doi.org/10.1111/j.1471-0528.2012.03280.x.
- Louwers JA, Zaal A, Kocken M, Papagiannakis E, Meijer CJ, Verheijen RH. Women’s preferences of dynamic spectral imaging colposcopy. Gynecol Obstet Invest 2015;79:239-43. https://doi.org/10.1159/000367921.
- Louwers JA, Zaal A, Berkhof J, Kocken M, Ter Harmsel WA, Graziosi GCM, et al. Performance of dynamic spectral imaging (DSI) colposcopy to detect high-grade cervical neoplasia in different referral groups, including reflex hrHPV-testing. BJOG 2013;120.
- Louwers J. Dynamic Spectral Imaging Colposcopy – Preliminary Results n.d.
- Louwers J, Zaal A, Kocken M, Balas C, Ter Harmsel B, Graziosi P, et al. Dynamic Spectral Imaging Colposcopy n.d.
- Louwers J, Zaal A, Kocken M, Ter Harmsel W, Graziosi G, Spruijt J, et al. Dynamic Spectral Imaging Colposcopy: Higher Sensitivity for Detection of Premalignant Cervical Lesions n.d.
- Louwers JA, Zaal A, Berkhof J, Kocken M, Papagiannakis E, Snijders PJF, et al. Performance of Dynamic Spectral Imaging Colposcopy in Patients With Abnormal Cytology or a Positive HRHPV Test n.d.
- Zaal A, Louwers J, Berkhof J, Kocken M, Ter Harmsel W, Graziosi G, et al. Agreement Between Colposcopic Impression and Histology Among hrHPV16 Positive Women n.d.
- Louwers J, Krocken M, Meijer CJLM, Snijders PJF, Verheijen RHM, Zaal A. Dynamic spectral imaging colposcopy. J Low Genit Tract Dis 2014;15:S1-2.
- Louwers JA, Zaal A, Kocken M, Papagiannakis E, Meijer CJLM, Verheijen RHM. Women’s Preferences of Dynamic Spectral Imaging Colposcopy n.d.
- Lowe G, Ogunremi A, Richardson D, Akpobome G, Allen E, Sathiyathasan S, et al. Assessing the Experience of the Patients and Their Sense of Reassurance After Having Their Colposcopy With the DySIS Digital Colposcope n.d.
- Brady J, Lowe G, Ogunremi A, Richardson D, Allen E, Sathiyathasan S, et al. Patient Experience and Reassurance After Colposcopy With DySIS n.d.
- Natsis S, Founta C, Papagiannakis E, Ratnavelu N, Plevris N, Ozkose B, et al. Dynamic Spectral Imaging (DySIS) for Low Grade Cytology High Risk HPV Positive Colposcopy Referrals: Up-To-Date Outcomes &Amp; Potential Role in Standardization Of Care n.d.
- Founta C, Ang C, Biliatis I, Bradbury M, Fisher A, Kucukmetin A, et al. Dynamic Spectral Imaging for Triage of Low-Grades n.d.
- Founta C, O’Donnell R, Fisher A, Biliatis I, Ratnavelu N, Rangar R, et al. Dynamic Spectral Imaging for Triage of Low-Grades n.d.
- Founta C, Fisher A, Natsis S, Ratnavelu N, O’Donnell R, Bradbury M, et al. Dynamic Spectral Imaging for Triage of Low Grades n.d.
- Natsis S, Founta C, Fisher A, Ratnavelu N, Metin A, Ang C, et al. The role of dynamic spectral imaging (DySIS) in triaging low grade cytology high risk HPV positive colposcopy referrals. Int J Gynecol Cancer 2015;25.
- Roensbo MT, Hammer A, Blaakaer J. Can dynamic spectral imaging system colposcopy replace conventional colposcopy in the detection of high-grade cervical lesions?. Acta Obstet Gynecol Scand 2015;94:781-5. https://doi.org/10.1111/aogs.12646.
- Salter WR, Livingston J. Dynamic Spectral Imaging Coloposcopy: First Results from Two IMPROVE-COLPO Study Community Clinics n.d.
- Salter W, Livingston J, Papagiannakis E. Dynamic spectral imaging colposcopy: prospective results from two IMPROVE-COLPO study community clinics. J Low Genit Tract Dis 2016;20.
- Livingston J, Salter WR. Dynamic spectral imaging colposcopy: first results from two IMPROVE-COLPO study community clinics. Obstet Gynecol 2016;127. https://doi.org/10.1097/01.AOG.0000483744.43232.df.
- Papagiannakis E, Cholkeri-Singh A, Olson CG. The IMPROVE-COLPO Study on USA Community-Based Colposcopy With Dynamic Spectral Imaging: Design and First Findings n.d.
- Livingston J, Papagiannakis E. Why cervical cancer is missed at colposcopy: to-date findings of the IMPROVE-COLPO community study. J Low Genit Tract Dis 2016;20.
- Weinberg L, Papagiannakis E, DeNardis S. CIN2+ Detection on ASC-US HrHPV+ Patients: Standard Colposcopy Vs Digital Colposcopy With Dynamic Spectral Imaging n.d.
- Cholkeri-Singh A, Olson CG, Papagiannakis E. IMPROVE-COLPO design and first findings. New Orleans, USA: 2016 ASCCP; n.d.
- Improved Practice Outcomes and Value Excellence in Colposcopy. Bethesda, MD: National Library of Medicine; 2017.
- Soutter WP, Diakomanolis E, Lyons D, Ghaem-Maghami S, Ajala T, Haidopoulos D, et al. Dynamic spectral imaging: improving colposcopy. Clin Cancer Res 2009;15:1814-20. https://doi.org/10.1158/1078-0432.CCR-08-1636.
- Soutter WP, Diakomanolis E, Lyons D, Ghaem-Maghami S, Ajala T, Haidopoulos D, et al. Colposcopy With DySIS Improves Remarkably the Detection Rate of HG Cases in the LG Smear Population: Clinical Implications n.d.
- Balas C, Pagoulatos N, Simos C, Skiadas Y, Kavagiou H, Papagiannakis E, et al. A New Optical Imaging System for the In Vivo Detection, Grading and Biopsy Sample Guiding of Cervical Neoplasia n.d.
- Soutter WP, Diakomanolis E, Lyons D, Ghaem-Maghami S, Ajala R, Haidopoulos D, et al. Dynamic Spectral Imaging – In Vivo Detection and Quantitative Grading of Cervical Neoplasia n.d.
- Soutter WP, Diakomanolis E, Lyons D, Haidopoulos D. Dynamic Spectral Imaging – In Vivo Detection and Quantitative Grading of Cervical Neoplasia n.d.
- Soutter WP, Diakomanolis E, Lyons D, Haidopoulos D, Balas C. Comparison of the diagnostic performance characteristics of dynamic spectral imaging and colposcopy in low and high grade referral smear populations, separately. J Low Genit Tract Dis 2010;14.
- Tidy JA, Brown BH, Lyon RE, Healey TJ, Palmer JE. Are colposcopy and electrical impedance spectroscopy complementary when used to detect high grade cervical neoplasia?. Eur J Gynaecol Oncol 2018;39:70-5.
- Macdonald MC, Brown BH, Lyon RE, Healey TJ, Palmer JE, Tidy JA. Influence of high risk HPV genotype on colposcopic performance: a large prospective study demonstrates improved detection of disease with ZedScan 1, particularly in non-HPV 16 patients. Eur J Obstet Gynecol Reprod Biol 2017;211:194-8. https://doi.org/10.1016/j.ejogrb.2017.02.020.
- Palmer JE, Lyon RE, Tidy JA. ZedScan Delivers Improvements in Clinical Performance and More Efficient Patient Management at Sheffield Teaching Hospitals NHS Foundation Trust. Manchester: Zilico Limited; 2016.
- Draft Clinical Evaluation Protocol . Clinical Evaluation of the Zilico ZedScan I As an Adjunct for the Detection of CIN in Women Referred to Colposcopy With Abnormal Cervical Cytology Version 2 2013.
- Tidy J, MacDonald M, Palmer J. The Increased Detection of CIN2+ by Electrical Impedance Spectroscopy (EIS) Is Independent of HR-HPV Genotype n.d.
- Macdonald MC, Lyon R, Palmer JE, Tidy JA. The Routine Use of ZedScan Within One Colposcopy Service in England n.d.
- Tidy J, Brown B, Healey J, Lyon R, Palmer J. The Utility of Electrical Impedance Spectroscopy (EIS) in the Detection of High Grade Glandular Neoplasia (HG-CGIN) n.d.
- Tidy J, Brown B, Healey J, Lyon R, Palmer J. Increased Detection of CIN2+ by Electrical Impedance Spectroscopy (ZedScan) Is Independent of HR-HPV Genotype n.d.
- Tidy J, Brown B, Palmer J. Performance of ZedScan in Different Colposcopy Clinics in England and Europe – An Evaluation of Over 1900 Women n.d.
- Tidy JA, Brown BH, Healey TJ, Daayana S, Martin M, Prendiville W, et al. Accuracy of detection of high-grade cervical intraepithelial neoplasia using electrical impedance spectroscopy with colposcopy. BJOG 2013;120:400-10. https://doi.org/10.1111/1471-0528.12096.
- Tidy J, Brown B, Healey J, Daayana S, Martin M, Prendiville W, et al. Improved detection of high-grade CIN using a hand held electrical spectrosopy device. Int J Gynecol Cancer 2012;22.
- Tidy J, Brown B, Healey J, Daayana S, Martin M, Prendiville W, et al. Improved Detection of HGCIN in Women Undergoing Colposcopy Using Electrical Impedance Spectroscopy. Manchester; 2011.
- Tidy JA, Brown B. Improved colposcopic performance using electrical impedance spectroscopy (APX100) as an adjunct. J Low Genit Tract Dis 2012;16.
- Tidy J, Brown B, Healey J, Daayana S, Kitchener H. Improved Detection of High Grade Cin In Women Undergoing Colposcopy Using Electrical Impedance Spectroscopy. Lisbon: European Research Organisation on Genital Infection and Neoplasia (EUROGIN); 2011.
- Tidy J, Brown B, Healey J, Daayana S, Martin M, Prendiville W, et al. Improved detection of high grade CIN in women undergoing colposcopy using electrical impedance spectroscopy. Int J Gynecol Cancer 2011;21.
- Tidy J, Healey J, Brown B. Accuracy of detection of high-grade cervical intra-epithelial neoplasia using electrical impedance spectroscopy with colposcopy. Gynecol Oncol 2013;130. https://doi.org/10.1016/j.ygyno.2013.04.151.
- Tsetsa P, Rodolakis A, Sakellaropoulos G, Haidopoulos D, Papagiannakis E, Skiadas Y, et al. Assessment of the performance of the dynamic spectral imaging system (DySIS) in three different concentrations of acetic acid solution. Int J Gynecol Cancer 2012;22.
- Tsetsa P, Skiadas Y, Haidopoulos D, Papagiannakis E, Rodolakis A, Sakellaropoulos G, et al. Assessment of the Dynamic Spectral Imaging System Performance in Three Different Concentrations of Acetic Acid Solution n.d.
- Tsetsa P, Skiadas Y, Papagiannakis E, Rodolakis A, Sakellaropoulos G, Haidopoulos D, et al. Assessment of the performance of the dynamic spectral imaging system (DySIS) in three different concentrations of acetic acid solution. Int J Gynecol Cancer 2011;21.
- Brown BH, Tidy JA, Boston K, Blackett AD, Smallwood RH, Sharp F. Relation between tissue structure and imposed electrical current flow in cervical neoplasia. Lancet 2000;355:892-5. https://doi.org/10.1016/S0140-6736(99)09095-9.
- Brown BH, Milnes P, Abdul S, Tidy JA. Detection of cervical intraepithelial neoplasia using impedance spectroscopy: a prospective study. BJOG 2005;112:802-6. https://doi.org/10.1111/j.1471-0528.2004.00530.x.
- Abdul S, Brown BH, Milnes P, Tidy JA. The use of electrical impedance spectroscopy in the detection of cervical intraepithelial neoplasia. Int J Gynecol Cancer 2006;16:1823-32. https://doi.org/10.1111/j.1525-1438.2006.00651.x.
- Balasubramani L, Brown BH, Healey J, Tidy JA. The detection of cervical intraepithelial neoplasia by electrical impedance spectroscopy: the effects of acetic acid and tissue homogeneity. Gynecol Oncol 2009;115:267-71. https://doi.org/10.1016/j.ygyno.2009.08.010.
- Kyrgiou M, Mitra A, Arbyn M, Paraskevaidi M, Athanasiou A, Martin-Hirsch PP, et al. Fertility and early pregnancy outcomes after conservative treatment for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD008478.pub2.
- Kyrgiou M, Athanasiou A, Paraskevaidi M, Mitra A, Kalliala I, Martin-Hirsch P, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ 2016;354. https://doi.org/10.1136/bmj.i3633.
- Castañon A, Brocklehurst P, Evans H, Peebles D, Singh N, Walker P, et al. Risk of preterm birth after treatment for cervical intraepithelial neoplasia among women attending colposcopy in England: retrospective-prospective cohort study. BMJ 2012;345. https://doi.org/10.1136/bmj.e5174.
- Castañon A, Landy R, Brocklehurst P, Evans H, Peebles D, Singh N, et al. Is the increased risk of preterm birth following excision for cervical intraepithelial neoplasia restricted to the first birth post treatment?. BJOG 2015;122:1191-9. https://doi.org/10.1111/1471-0528.13398.
- Castañon A, Landy R, Brocklehurst P, Evans H, Peebles D, Singh N, et al. Risk of preterm delivery with increasing depth of excision for cervical intraepithelial neoplasia in England: nested case-control study. BMJ 2014;349. https://doi.org/10.1136/bmj.g6223.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Whyte S, Bessey A, Chilcott J. Economic Evaluation of the ZedScan Electrical Impedance Spectroscopy (EIS) Device Used as an Adjunct to Colposcopy. Sheffield: University of Sheffield; 2013.
- Hadwin R, Eggington S, Brennan A, Walker P, Patnick J, Pilgrim H. Modelling the cost-effectiveness and capacity impact of changes to colposcopy referral guidelines for women with mild dyskaryosis in the UK Cervical Screening Programme. BJOG 2008;115:749-57. https://doi.org/10.1111/j.1471-0528.2008.01683.x.
- Gallwas J, Gaschler R, Stepp H, Friese K, Dannecker C. 3D optical coherence tomography of cervical intraepithelial neoplasia – early experience and some pitfalls. Eur J Gynaecol Oncol 2012;33:37-41.
- Cotton S, Sharp L, Little J, Cruickshank M, Seth R, Smart L, et al. The role of human papillomavirus testing in the management of women with low-grade abnormalities: multicentre randomised controlled trial. BJOG 2010;117:645-59. https://doi.org/10.1111/j.1471-0528.2010.02519.x.
- Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol 2000;151:1158-71. https://doi.org/10.1093/oxfordjournals.aje.a010166.
- Kelly RS, Patnick J, Kitchener HC, Moss SM, NHSCSP HPV. Special Interest Group. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the Sentinel Sites study. Br J Cancer 2011;105:983-8. https://doi.org/10.1038/bjc.2011.326.
- TOMBOLA Group . Options for managing low grade cervical abnormalities detected at screening: cost effectiveness study. BMJ 2009;339.
- Wolstenholme JL, Whynes DK. Stage-specific treatment costs for cervical cancer in the United Kingdom. Eur J Cancer 1998;34:1889-93. https://doi.org/10.1016/S0959-8049(98)00232-9.
- Insinga RP, Glass AG, Myers ER, Rush BB. Abnormal outcomes following cervical cancer screening: event duration and health utility loss. Med Decis Making 2007;27:414-22. https://doi.org/10.1177/0272989X07302128.
- Birch S, Melnikow J, Kuppermann M. Conservative versus aggressive follow up of mildly abnormal Pap smears: testing for process utility. Health Econ 2003;12:879-84. https://doi.org/10.1002/hec.783.
- Chuck A. Cost-effectiveness of 21 alternative cervical cancer screening strategies. Value Health 2010;13:169-79. https://doi.org/10.1111/j.1524-4733.2009.00611.x.
- Sastre-Garau X, Cartier I, Jourdan-Da Silva N, De Crémoux P, Lepage V, Charron D. Regression of low-grade cervical intraepithelial neoplasia in patients with HLA-DRB1*13 genotype. Obstet Gynecol 2004;104:751-5. https://doi.org/10.1097/01.AOG.0000139834.84628.61.
- Hording U, Junge H, Rygaard C, Lundvall F. Management of low-grade CIN: follow-up or treatment?. Eur J Obstet Gynecol Reprod Biol 1995;62:49-52. https://doi.org/10.1016/0301-2115(95)02139-X.
- Munk AC, Kruse AJ, van Diermen B, Janssen EAM, vanDiermen B, Skaland I, et al. Cervical intraepithelial neoplasia grade 3 lesions can regress. APMIS 2007;115:1409-15. https://doi.org/10.1111/j.1600-0463.2007.00769.x.
- Nobbenhuis MAE, Helmerhorst TJM, van den Brule AJC, Rozendaal L, Voorhorst FJ, Bezemer PD, et al. Cytological regression and clearance of high-risk human hapillomavirus in women with an abnormal cervical smear. Lancet 2001;358:1782-3. https://doi.org/10.1016/S0140-6736(01)06809-X.
- Pretorius RG, Peterson P, Azizi F, Burschette RJ. Subsequent risk and presentation of cervical intraepithelial neoplasia (CIN) 3 of cancer after a colposcopic diagnosis of CIN 1 or less. Am J Obstet Gynecol 2006;195:1260-5. https://doi.org/10.1016/j.ajog.2006.07.036.
- Smith M, Keech S, Perryman K, Soutter W. A long-term study of women with normal colposcopy after referral with low-grade cytological abnormalities. BJOG 2006;113:1321-8. https://doi.org/10.1111/j.1471-0528.2006.01065.x.
- Stoler HH, Vinchnin MD, Ferenczy A, Ferris DG, Perez G, Paavonen J, et al. The accuracy of colposcopic biopsy: analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer 2011;128:1354-62. https://doi.org/10.1002/ijc.25470.
- Trimble CL, Piantadosi S, Gravitt PP, Ronnett B, Pizer E, Elko A, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005;11:4717-23. https://doi.org/10.1158/1078-0432.CCR-04-2599.
- Blanks R, Kelly R. Comparison of cytology and histology results in English cervical screening laboratories before and after liquid-based cytology conversion: do the data provide evidence for a single category of high-grade dyskaryosis?. Cytopathology 2010;21:368-73. https://doi.org/10.1111/j.1365-2303.2010.00808.x.
- Sharp L, Cotton S, Cochran C, Gray N, Little J, . TOMBOLA (Trial Of Management of Borderline and Other Low-grade Abnormal smears) Group . After-effects reported by women following colposcopy, cervical biopsies and LLETZ: results from the TOMBOLA trial. BJOG 2009;116:1506-14. https://doi.org/10.1111/j.1471-0528.2009.02263.x.
- Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. ISPOR-SMDM Modeling Good Research Practices Task Force . State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 3. Value Health 2012;15:812-20. https://doi.org/10.1016/j.jval.2012.06.014.
- Davis S, Stevenson M, Tappenden P, Wailoo A. NICE DSU Technical Support Document 15: Cost-Effectiveness Modelling Using Patient-Level Simulation. Sheffield: School of Health and Related Research, University of Sheffield; 2014.
- Kulasingam SL, Havrilesky LJ, Ghebre R, Myers ER. Screening for cervical cancer: a modeling study for the US Preventive Services Task Force. J Low Genit Tract Dis 2013;17:193-202. https://doi.org/10.1097/LGT.0b013e3182616241.
- Campos NG, Burger EA, Sy S, Sharma M, Schiffman M, Rodriguez AC, et al. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol 2014;180:545-55. https://doi.org/10.1093/aje/kwu159.
- Kitchener H, Canfell K, Gilham C, Sargent A, Roberts C, Desai M, et al. The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18230.
- Ghaem-Maghami S, De-Silva D, Tipples M, Lam S, Perryman K, Soutter W. Determinants of success in treating cervical intraepithelial neoplasia. BJOG 2011;118:679-84. https://doi.org/10.1111/j.1471-0528.2010.02770.x.
- Conception Statistics, England and Wales. Newport: ONS; 2015.
- Preterm Labour and Birth. London: NICE; 2015.
- Death Registrations Summary Tables – England and Wales. Newport: ONS; 2016.
- NHS Reference Costs 2015 to 2016. London: DHSC; 2016.
- Martin-Hirsch P, Rash B, Martin A, Standaert B. Management of women with abnormal cervical cytology: treatment patterns and associated costs in England and Wales. BJOG 2007;114:408-15. https://doi.org/10.1111/j.1471-0528.2007.01261.x.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2005.
- Lomas J, Schmitt L, Jones S, McGeorge M, Bates E, Holland M, et al. A pharmacoeconomic approach to assessing the costs and benefits of air quality interventions that improve health: a case study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010686.
- Simonella L, Howard K, Canfell K. A survey of population-based utility scores for cervical cancer prevention. BMC Res Notes 2014;7. https://doi.org/10.1186/1756-0500-7-899.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Duffy A, Osman A. Colposcopy clinic non-attendance at Tallaght hospital. Ir J Med Sci 2016;185.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Tsetsa P. Assessment of the Performance of the Dynamic Spectral Imaging System (DySIS) in Three Different Concentrations of Acetic Acid Solution n.d.
Appendix 1 ZedScan algorithm
FIGURE 16.
ZedScan diagnostic flow chart. (Confidential information has been removed.)
Appendix 2 Literature search strategies
MEDLINE (via OvidSP: http://ovidsp.ovid.com/)
Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE (R) Daily and Ovid MEDLINE (R).
Date range searched: 1946 to present.
Date searched: 3 January 2017.
Records retrieved: 2505.
An updated search was carried out on 10 April 2017, retrieving 2436 records.
Search strategy
-
Cervix Uteri/ (27,749)
-
cervix.ti,ab. (44,380)
-
cervic$.ti,ab. (217,889)
-
(endocervix or endo-cervix).ti,ab. (1165)
-
(endocervic$ or endo-cervic$).ti,ab. (5220)
-
(ectocervix or ecto-cervix).ti,ab. (402)
-
(ectocervic$ or ecto-cervic$).ti,ab. (654)
-
((squamocolumnar or squamo-columnar) adj2 junction).ti,ab. (568)
-
transformation zone$.ti,ab. (1061)
-
or/1-9 (252,639)
-
Colposcopy/ (6321)
-
Colposcopes/ (193)
-
Spectrum Analysis/ (47,414)
-
Dielectric Spectroscopy/ (1674)
-
(colposcop$ adj4 (adjunct$ or digital$ or DSI or computer$ or video$ or alternative$ or conventional$)).ti,ab. (217)
-
(impedance adj2 spectroscop$).ti,ab. (5309)
-
(Dielectric adj2 Spectroscop$).ti,ab. (1232)
-
(impedance adj2 spectrometr$).ti,ab. (35)
-
(Dielectric adj2 Spectrometr$).ti,ab. (6)
-
(impedance adj2 spectrum analys$).ti,ab. (4)
-
(Dielectric adj2 Spectrum analys$).ti,ab. (0)
-
(telecolposcop$ or tele-colposcop$).ti,ab. (20)
-
(optical adj2 spectroscop$).ti,ab. (5149)
-
((point or pencil or impedance) adj2 probe$).ti,ab. (546)
-
(microcolposcop$ or micro-colposcop$).ti,ab. (20)
-
(dysis or dysismap).ti,ab. (31)
-
dynamic spectral imaging.ti,ab. (16)
-
Zilico.ti,ab. (0)
-
(ZedScan or Zed Scan).ti,ab. (0)
-
(APX 100 or APX100).ti,ab. (2)
-
EIS.ti,ab. (3007)
-
epitheliometer$.ti,ab. (1)
-
MKIII.ti,ab. (33)
-
or/11-33 (66,616)
-
10 and 34 (4876)
-
exp animals/ not humans/ (4,837,860)
-
35 not 36 (4845)
-
limit 37 to yr=“2000 -Current” (2505)
Key
-
/ = indexing term [medical subject heading (MeSH)]
-
exp = exploded indexing term (MeSH)
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
adj2 = terms within two words of each other (any order).
Cochrane Central Register of Controlled Trials (via Wiley Online Library: http://onlinelibrary.wiley.com/)
Issue 1 of 12, November 2016.
Date searched: 3 January 2017.
Records retrieved: 175.
An updated search was carried out on 10 April 2017, retrieving 183 records from CENTRAL.
Search strategy
-
#1. MeSH descriptor: [Cervix Uteri] this term only (1031)
-
#2. cervix:ti,ab,kw (4427)
-
#3. cervic*:ti,ab,kw (11,455)
-
#4. (endocervix or endo-cervix):ti,ab,kw (49)
-
#5. (endocervic* or endo-cervic*):ti,ab,kw (287)
-
#6. (ectocervix or ecto-cervix):ti,ab,kw (19)
-
#7. (ectocervic* or ecto-cervic*):ti,ab,kw (25)
-
#8. ((squamocolumnar or squamo-columnar) near/2 junction):ti,ab,kw (23)
-
#9. (transformation next zone*):ti,ab,kw (91)
-
#10. 156-#9 (12900)
-
#11. MeSH descriptor: [Colposcopy] this term only (353)
-
#12. MeSH descriptor: [Colposcopes] this term only (10)
-
#13. MeSH descriptor: [Spectrum Analysis] this term only (90)
-
#14. MeSH descriptor: [Dielectric Spectroscopy] this term only (11)
-
#15. (colposcop* near/4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*)):ti,ab,kw (35)
-
#16. (impedance near/2 spectroscop*):ti,ab,kw (35)
-
#17. (Dielectric near/2 Spectroscop*):ti,ab,kw (12)
-
#18. (impedance near/2 spectrometr*):ti,ab,kw (1)
-
#19. (Dielectric near/2 Spectrometr*):ti,ab,kw (0)
-
#20. (impedance near/2 (spectrum next analys*)):ti,ab,kw (0)
-
#21. (Dielectric near/2 (Spectrum next analys*)):ti,ab,kw (0)
-
#22. (telecolposcop* or tele-colposcop*):ti,ab,kw (2)
-
#23. (optical near/2 spectroscop*):ti,ab,kw (19)
-
#24. ((point or pencil or impedance) near/2 probe*):ti,ab,kw (44)
-
#25. (microcolposcop* or micro-colposcop*):ti,ab,kw (1)
-
#26. (dysis or dysismap):ti,ab,kw (5)
-
#27. (dynamic next spectral next imaging):ti,ab,kw (2)
-
#28. Zilico:ti,ab,kw (1)
-
#29. (ZedScan or Zed Scan):ti,ab,kw (0)
-
#30. (APX 100 or APX100):ti,ab,kw (0)
-
#31. EIS:ti,ab,kw (78)
-
#32. epitheliometer*:ti,ab,kw (0)
-
#33. MKIII:ti,ab,kw (3)
-
#34. 157-#33 (637)
-
#35. #10 and #34 (304)
-
#36. #10 and #34 Publication Year from 2000 to 2017 (229)
-
#37. #10 and #34 Publication Year from 2000 to 2017, in Cochrane Reviews (Reviews and Protocols) (2)
-
#38. #10 and #34 Publication Year from 2000 to 2017, in Trials (175)
Key
-
MeSH descriptor = indexing term (MeSH)
-
* = truncation
-
ti,ab,kw = terms in either title or abstract or keyword fields
-
near/2 = terms within two words of each other (any order)
-
next = terms are next to each other.
Cochrane Database of Systematic Reviews (via Wiley Online Library: http://onlinelibrary.wiley.com/)
Issue 1 of 12, January 2017.
Date searched: 3 January 2017.
Records retrieved: 2.
An updated search was carried out on 10 April 2017, retrieving two records from CDSR.
See Cochrane Central Register of Controlled Trials (via Wiley Online Library: http://onlinelibrary.wiley.com/) for the search strategy used.
Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus via EBSCOhost: www.ebscohost.com)
Date range searched: inception to 2 January 2017.
Date searched: 3 January 2017.
Records retrieved: 762.
An updated search was carried out on 10 April 2017, retrieving 786 records.
Search strategy
-
S1 (MH “Cervix”) (2037)
-
S2 TI cervix OR AB cervix (2536)
-
S3 TI cervic* OR AB cervic* (30,166)
-
S4 TI ( endocervix or endo-cervix ) OR AB ( endocervix or endo-cervix) (32)
-
S5 TI ( endocervic* or endo-cervic* ) OR AB ( endocervic* or endo-cervic* ) (339)
-
S6 TI ( ectocervix or ecto-cervix ) OR AB ( ectocervix or ecto-cervix ) (16)
-
S7 TI ( ectocervic* or ecto-cervic* ) OR AB ( ectocervic* or ecto-cervic* ) (28)
-
S8 TI ( (squamocolumnar or squamo-columnar) N2 junction ) OR AB ( (squamocolumnar or squamo-columnar) N2 junction ) (29)
-
S9 TI transformation N1 zone* OR AB transformation N1 zone* (101)
-
S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 (32,130)
-
S11 (MH “Colposcopy”) (1218)
-
S12 (MH “Spectrum Analysis”) (1861)
-
S13 TI ( colposcop* N4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*) ) OR AB ( colposcop* N4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*) ) (53)
-
S14 TI impedance N2 spectroscop* OR AB impedance N2 spectroscop* (80)
-
S15 TI dielectric N2 spectroscop* OR AB dielectric N2 spectroscop* (9)
-
S16 TI impedance N2 spectrometr* OR AB impedance N2 spectrometr* (3)
-
S17 TI dielectric N2 spectrometr* OR AB dielectric N2 spectrometr* (4)
-
S18 TI impedance N2 “spectrum analys*” OR AB impedance N2 “spectrum analys*” (2)
-
S19 TI dielectric N2 “spectrum analys*” OR AB dielectric N2 “spectrum analys*” (2)
-
S20 TI ( telecolposcop* or tele-colposcop* ) OR AB ( telecolposcop* or tele-colposcop* ) (7)
-
S21 TI optical N2 spectroscop* OR AB optical N2 spectroscop* (105)
-
S22 TI ( (point or pencil or impedance) N2 probe* ) OR AB ( (point or pencil or impedance) N2 probe* ) (37)
-
S23 TI ( microcolposcop* or micro-colposcop* ) OR AB ( microcolposcop* or micro-colposcop* ) (1)
-
S24 TI ( dysis or dysismap ) OR AB ( dysis or dysismap ) (9)
-
S25 TI “dynamic spectral imaging” OR AB “dynamic spectral imaging” (5)
-
S26 TI Zilico OR AB Zilico (0)
-
S27 TI ( ZedScan or Zed Scan ) OR AB ( ZedScan or Zed Scan ) (0)
-
S28 TI ( APX 100 or APX100 ) OR AB ( APX 100 or APX100 ) (1)
-
S29 TI EIS OR AB EIS (287)
-
S30 TI epitheliometer* OR AB epitheliometer* (0)
-
S31 TI MKIII OR AB MKIII (3)
-
S32 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 (3551)
-
S33 S10 AND S32 (838)
-
S34 S10 AND S32 Limiters - Published Date: 20000101-20170131 (762)
Key
-
MH = indexing term (CINAHL heading)
-
* = truncation
-
TI = terms in the title
-
AB = terms in the abstract
-
N2 = terms within two words of each other (any order).
Database of Abstracts of Reviews of Effects (via www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 4 January 2017.
Records retrieved: 16.
As DARE closed on 31 March 2015, an updated search was not carried out for this database.
Search strategy
-
(MeSH DESCRIPTOR Cervix Uteri) FROM 2000 TO 2017 (67)
-
(cervix) OR (cervic*) FROM 2000 TO 2017 (1302)
-
(endocervix) OR (endo-cervix) FROM 2000 TO 2017 (0)
-
(endocervic*) OR (endo-cervic*) FROM 2000 TO 2017 (28)
-
(ectocervix) OR (ecto-cervix) FROM 2000 TO 2017 (0)
-
(ectocervic*) OR (ecto-cervic*) FROM 2000 TO 2017 (0)
-
(((squamocolumnar or squamo-columnar) NEAR2 junction)) FROM 2000 TO 2017 (1)
-
((junction NEAR2 (squamocolumnar or squamo-columnar))) FROM 2000 TO 2017 (0)
-
(transformation zone*) FROM 2000 TO 2017 (14)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 (1308)
-
(MeSH DESCRIPTOR Colposcopy) FROM 2000 TO 2017 (55)
-
(MeSH DESCRIPTOR Colposcopes) FROM 2000 TO 2017 (3)
-
(MeSH DESCRIPTOR Spectrum Analysis) FROM 2000 TO 2017 (6)
-
(MeSH DESCRIPTOR Dielectric Spectroscopy) FROM 2000 TO 2017 (2)
-
((colposcop* NEAR4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*))) FROM 2000 TO 2017 (3)
-
(((adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*) NEAR4 colposcop*)) FROM 2000 TO 2017 (12)
-
(impedance NEAR2 spectroscop*) FROM 2000 TO 2017 (0)
-
(spectroscop* NEAR2 impedance) FROM 2000 TO 2017 (0)
-
(Dielectric NEAR2 Spectroscop*) FROM 2000 TO 2017 (2)
-
(Spectroscop* NEAR2 Dielectric) FROM 2000 TO 2017 (0)
-
(impedance NEAR2 spectrometr*) FROM 2000 TO 2017 (0)
-
(spectrometr* NEAR2 impedance) FROM 2000 TO 2017 (0)
-
(Dielectric NEAR2 Spectrometr*) FROM 2000 TO 2017 (0)
-
(Spectrometr* NEAR2 Dielectric) FROM 2000 TO 2017 (0)
-
(impedance NEAR2 spectrum analys*) FROM 2000 TO 2017 (0)
-
(spectrum analys* NEAR2 impedance) FROM 2000 TO 2017 (0)
-
(Dielectric NEAR2 Spectrum analys*) FROM 2000 TO 2017 (0)
-
(Spectrum analys* NEAR2 Dielectric) FROM 2000 TO 2017 (0)
-
(telecolposcop* or tele-colposcop*) FROM 2000 TO 2017 (1)
-
(optical NEAR2 spectroscop*) FROM 2000 TO 2017 (2)
-
(spectroscop* NEAR2 optical) FROM 2000 TO 2017 (0)
-
(((point or pencil or impedance) NEAR2 probe*)) FROM 2000 TO 2017 (0)
-
((probe* NEAR2 (point or pencil or impedance))) FROM 2000 TO 2017 (0)
-
(microcolposcop* or micro-colposcop*) FROM 2000 TO 2017 (0)
-
(dysis or dysismap) FROM 2000 TO 2017 (3)
-
(dynamic spectral imaging) FROM 2000 TO 2017 (0)
-
(Zilico) FROM 2000 TO 2017 (1)
-
(ZedScan or Zed Scan) FROM 2000 TO 2017 (0)
-
(APX 100 or APX100) FROM 2000 TO 2017 (0)
-
(EIS) FROM 2000 TO 2017 (3)
-
(epitheliometer*) FROM 2000 TO 2017 (0)
-
(MKIII) FROM 2000 TO 2017 (0)
-
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 (72)
-
#10 AND #43 (59)
-
(*) IN DARE FROM 2000 TO 2017 (43,354)
-
#44 AND #45 (16)
-
(*) IN NHSEED FROM 2000 TO 2017 (14,762)
-
#44 AND #47 (38)
-
(*) IN HTA FROM 2000 TO 2017 (14,138)
-
#44 AND #49 (5)
Key
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
NEAR2 = terms within two words of each other (order specified).
EMBASE (via Ovid: http://ovidsp.ovid.com/)
Date range searched: 1974 to 30 December 2016.
Date searched: 3 January 2017.
Records retrieved: 6177.
An updated search was carried out on 10 April 2017, retrieving 6300 records.
Search strategy
-
exp uterine cervix/ (27,722)
-
cervix.ti,ab. (48,465)
-
cervic$.ti,ab. (247,849)
-
(endocervix or endo-cervix).ti,ab. (1296)
-
(endocervic$ or endo-cervic$).ti,ab. (6123)
-
(ectocervix or ecto-cervix).ti,ab. (458)
-
(ectocervic$ or ecto-cervic$).ti,ab. (663)
-
((squamocolumnar or squamo-columnar) adj2 junction).ti,ab. (747)
-
transformation zone$.ti,ab. (1202)
-
or/1-9 (283,656)
-
colposcopy/ (11,114)
-
colposcope/ (251)
-
spectroscopy/ (95,966)
-
electrochemical impedance spectroscopy/ (5008)
-
(colposcop$ adj4 (adjunct$ or digital$ or DSI or computer$ or video$ or alternative$ or conventional$)).ti,ab. (281)
-
(impedance adj2 spectroscop$).ti,ab. (4587)
-
(Dielectric adj2 Spectroscop$).ti,ab. (843)
-
(impedance adj2 spectrometr$).ti,ab. (40)
-
(Dielectric adj2 Spectrometr$).ti,ab. (10)
-
(impedance adj2 spectrum analys$).ti,ab. (7)
-
(Dielectric adj2 Spectrum analys$).ti,ab. (0)
-
(telecolposcop$ or tele-colposcop$).ti,ab. (20)
-
(optical adj2 spectroscop$).ti,ab. (3982)
-
((point or pencil or impedance) adj2 probe$).ti,ab. (473)
-
(microcolposcop$ or micro-colposcop$).ti,ab. (31)
-
(dysis or dysismap).ti,ab,dv,dm. (111)
-
dynamic spectral imaging.ti,ab. (27)
-
Zilico.ti,ab,dm. (0)
-
(ZedScan or Zed Scan).ti,ab,dv. (3)
-
(APX 100 or APX100).ti,ab,dv. (4)
-
EIS.ti,ab. (3150)
-
epitheliometer$.ti,ab,dv. (1)
-
MKIII.ti,ab,dv. (48)
-
or/11-33 (117,271)
-
10 and 34 (8853)
-
(animal/ or nonhuman/) not exp human/ (5,039,945)
-
35 not 36 (8818)
-
limit 37 to yr=“2000 -Current” (6177)
Key
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
dm = terms in device manufacturer field
-
dv = terms in the device trade name field
-
adj2 = terms within two words of each other (any order).
Health Management Information Consortium (via Ovid: http://ovidsp.ovid.com/)
Date range searched: 1979 to November 2016.
Date searched: 3 January 2017.
Records retrieved: 19.
An updated search was carried out on 10 April 2017, retrieving 19 records.
Search strategy
-
cervix uteri/ (18)
-
cervix.ti,ab. (136)
-
cervic$.ti,ab. (1398)
-
(endocervix or endo-cervix).ti,ab. (1)
-
(endocervic$ or endo-cervic$).ti,ab. (17)
-
(ectocervix or ecto-cervix).ti,ab. (0)
-
(ectocervic$ or ecto-cervic$).ti,ab. (1)
-
((squamocolumnar or squamo-columnar) adj2 junction).ti,ab. (1)
-
transformation zone$.ti,ab. (6)
-
or/1-9 (1472)
-
colposcopy/ (49)
-
spectroscopy/ (20)
-
(colposcop$ adj4 (adjunct$ or digital$ or DSI or computer$ or video$ or alternative$ or conventional$)).ti,ab. (4)
-
(impedance adj2 spectroscop$).ti,ab. (0)
-
(Dielectric adj2 Spectroscop$).ti,ab. (0)
-
(impedance adj2 spectrometr$).ti,ab. (0)
-
(Dielectric adj2 Spectrometr$).ti,ab. (0)
-
(impedance adj2 spectrum analys$).ti,ab. (0)
-
(Dielectric adj2 Spectrum analys$).ti,ab. (0)
-
(telecolposcop$ or tele-colposcop$).ti,ab. (0)
-
(optical adj2 spectroscop$).ti,ab. (0)
-
((point or pencil or impedance) adj2 probe$).ti,ab. (0)
-
(microcolposcop$ or micro-colposcop$).ti,ab. (0)
-
(dysis or dysismap).ti,ab. (0)
-
dynamic spectral imaging.ti,ab. (0)
-
Zilico.ti,ab. (0)
-
(ZedScan or Zed Scan).ti,ab. (0)
-
(APX 100 or APX100).ti,ab. (0)
-
EIS.ti,ab. (26)
-
epitheliometer$.ti,ab. (0)
-
MKIII.ti,ab. (0)
-
or/11-31 (97)
-
10 and 32 (36)
-
limit 33 to yr=“2000 -Current” (19)
Key
-
/ = indexing term
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
adj2 = terms within two words of each other (any order).
Health Technology Assessment database (via www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 3 January 2017.
Date searched: 4 January 2017.
Records retrieved: 5.
See Database of Abstracts of Reviews of Effects (via www.crd.york.ac.uk/CRDWeb) for the search strategy used.
An updated search was carried out on 10 April 2017, retrieving five records.
NHS Economic Evaluation Database (via www.crd.york.ac.uk/CRDWeb)
Date range searched: inception to 31 March 2015.
Date searched: 4 January 2017.
Records retrieved: 38.
As NHS EED closed on 31 March 2015, an updated search was not carried out for this database.
See Database of Abstracts of Reviews of Effects (via www.crd.york.ac.uk/CRDWeb) for search strategy used.
PubMed (via www.ncbi.nlm.nih.gov/pubmed)
Date searched: 4 January 2017.
Records retrieved: 63.
An updated search was carried out on 10 April 2017, retrieving 59 records.
Search strategy
((((((((((((((((((((((((((((((“Colposcopy”[Mesh:NoExp]) OR “Colposcopes”[Mesh:NoExp]) OR “Spectrum Analysis”[Mesh:NoExp]) OR “Dielectric Spectroscopy”[Mesh:NoExp]) OR ((colposcop*[Title/Abstract]) AND (adjunct*[Title/Abstract] OR digital*[Title/Abstract] OR DSI[Title/Abstract] OR computer*[Title/Abstract] OR video*[Title/Abstract] OR alternative*[Title/Abstract] OR conventional*[Title/Abstract]))) OR ((impedance[Title/Abstract]) AND spectroscop*[Title/Abstract])) OR ((Dielectric[Title/Abstract]) AND Spectroscop*[Title/Abstract])) OR ((impedance[Title/Abstract]) AND spectrometr*[Title/Abstract])) OR ((impedance[Title/Abstract]) AND spectrum analys*[Title/Abstract])) OR ((Dielectric[Title/Abstract]) AND Spectrum analys*[Title/Abstract])) OR ((Dielectric[Title/Abstract]) AND Spectrometr*[Title/Abstract])) OR ((telecolposcop*[Title/Abstract] OR tele-colposcop*[Title/Abstract]))) OR ((optical[Title/Abstract]) AND spectroscop*[Title/Abstract])) OR (((point[Title/Abstract] OR pencil[Title/Abstract] OR impedance[Title/Abstract])) AND probe*[Title/Abstract])) OR ((microcolposcop*[Title/Abstract] OR micro-colposcop*[Title/Abstract]))) OR ((dysis[Title/Abstract] OR dysismap[Title/Abstract]))) OR dynamic spectral imaging[Title/Abstract]) OR Zilico[Title/Abstract]) OR ((ZedScan[Title/Abstract] OR Zed Scan[Title/Abstract]))) OR ((“APX 100”[Title/Abstract] OR APX100[Title/Abstract]))) OR EIS[Title/Abstract]) OR epitheliometer*[Title/Abstract]) OR MKIII[Title/Abstract])) AND ((((“Cervix Uteri”[Mesh:NoExp]) OR ((((((cervix[Title/Abstract]) OR cervic*[Title/Abstract]) OR (endocervix[Title/Abstract] OR endo-cervix[Title/Abstract])) OR (endocervic*[Title/Abstract] OR endo-cervic*[Title/Abstract])) OR (ectocervix[Title/Abstract] OR ecto-cervix[Title/Abstract])) OR (ectocervic*[Title/Abstract] OR ecto-cervic*[Title/Abstract]))) OR (((squamocolumnar[Title/Abstract] OR squamo-columnar[Title/Abstract])) AND junction[Title/Abstract])) OR ((“transformation zone”[Title/Abstract]) OR “transformation zones”[Title/Abstract])))) AND ((pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb])))) AND (“2000/01/01”[Date - Publication] : “3000”[Date - Publication]))) NOT ((animals [mh] NOT humans [mh]))
The above search strategy incorporates the following search line to limit it to studies found in PubMed but not available in Ovid MEDLINE: (pubstatusaheadofprint OR publisher[sb] OR pubmednotmedline[sb]). 160
Key
-
[Mesh] = exploded indexing term (MeSH)
-
[Mesh:noexp] = indexing term (MeSH) not exploded
-
* = truncation
-
‘‘ ’’ = phrase search
-
[Title/Abstract]) = terms in either title or abstract fields.
Science Citation Index (via Web of Science, Clarivate Analytics; http://thomsonreuters.com/thomson-reuters-web-of-science/)
Date range searched: 1900 to 2 January 2017.
Date searched: 3 January 2017.
Records retrieved: 279.
An updated search was carried out on 10 April 2017, retrieving 286 records.
Search strategy
-
#30. #28 AND #8 (279)
-
Indexes=SCI-EXPANDED Timespan=2000-2017
-
#29. #28 AND #8 (318)
-
#28. #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 (91,903)
-
#27. TS=MKIII (64)
-
#26. TS=epitheliometer* (2)
-
#25. TS=EIS (20,286)
-
#24. TS=(“APX 100” or APX100) (2)
-
#23. TS=(ZedScan or “Zed Scan”) (1)
-
#22. TS=Zilico (0)
-
#21. TS=“dynamic spectral imaging” (20)
-
#20. TS=(dysis or dysismap) (75)
-
#19. TS=(microcolposcop* or micro-colposcop*) (15)
-
#18. TS=((point or pencil or impedance) NEAR/2 probe*) (4324)
-
#17. TS=(optical NEAR/2 spectroscop*) (31,129)
-
#16. TS=(telecolposcop* or tele-colposcop*) (20)
-
#15. TS=(Dielectric NEAR/2 “Spectrum analys*”) (8)
-
#14. TS=(impedance NEAR/2 “spectrum analys*”) (35)
-
#13. TS=(Dielectric NEAR/2 Spectrometr*) (89)
-
#12. TS=(impedance NEAR/2 spectrometr*) (225)
-
#11. TS=(Dielectric NEAR/2 Spectroscop*) (7615)
-
#10. TS=(impedance NEAR/2 spectroscop*) (44,134)
-
#9. TS=(colposcop* NEAR/4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*)) (198)
-
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 (197,500)
-
#7. TS=“transformation zone*” (1431)
-
#6. TS=((squamocolumnar or squamo-columnar) NEAR/2 junction) (448)
-
#5. TS=(ectocervic* or ecto-cervic*) (457)
-
#4. TS=(ectocervix or ecto-cervix) (248)
-
#3. TS=(endocervic* or endo-cervic*) (3940)
-
#2. TS=(endocervix or endo-cervix) (754)
-
#1. TS=(cervix or cervic*) (194,743)
Key
-
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields
-
* = truncation
-
‘‘ ’’ = phrase search
-
NEAR/2 = terms within 2 words of each other (any order).
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov (https://clinicaltrials.gov)
Date searched: 4 January 2017.
Records retrieved: 173.
An updated search was carried out on 10 April 2017, retrieving eight new records.
Search strategy
A total of 169 studies found for (Cervix OR cervical) AND (Colposcopy OR spectroscopy OR spectrometry OR ‘spectrum analysis’).
Four studies found for dysis OR dysismap OR ‘dynamic spectral imaging’ OR Zilico OR ZedScan OR Zed Scan OR ‘APX 100’ OR APX100 OR epitheliometer OR MKIII.
Conference Proceedings Citation Index – Science (via Web of Science, Clarivate Analytics; http://thomsonreuters.com/thomson-reuters-web-of-science)
Date range searched: 1990 to 2 January 2017.
Date searched: 3 January 2017.
Records retrieved: 62.
An updated search was carried out on 10 April 2017, retrieving 63 records.
Search strategy
-
#30. #28 AND #8 (62)
-
Indexes=CPCI-S Timespan=2000-2017
-
#29. #28 AND #8 (67)
-
#28. #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 (20,223)
-
#27. TS=MKIII (27)
-
#26. TS=epitheliometer* (0)
-
#25. TS=EIS (3376)
-
#24. TS=(“APX 100” or APX100) (0)
-
#23. TS=(ZedScan or “Zed Scan”) (0)
-
#22. TS=Zilico (0)
-
#21. TS=“dynamic spectral imaging” (4)
-
#20. TS=(dysis or dysismap) (32)
-
#19. TS=(microcolposcop* or micro-colposcop*) (0)
-
#18. TS=((point or pencil or impedance) NEAR/2 probe*) (1606)
-
#17. TS=(optical NEAR/2 spectroscop*) (8132)
-
#16. TS=(telecolposcop* or tele-colposcop*) (3)
-
#15. TS=(Dielectric NEAR/2 “Spectrum analys*”) (3)
-
#14. TS=(impedance NEAR/2 “spectrum analys*”) (15)
-
#13. TS=(Dielectric NEAR/2 Spectrometr*) (19)
-
#12. TS=(impedance NEAR/2 spectrometr*) (51)
-
#11. TS=(Dielectric NEAR/2 Spectroscop*) (2063)
-
#10. TS=(impedance NEAR/2 spectroscop*) (7234)
-
#9. TS=(colposcop* NEAR/4 (adjunct* or digital* or DSI or computer* or video* or alternative* or conventional*)) (38)
-
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 (16,544)
-
#7. TS=“transformation zone*” (135)
-
#6. TS=((squamocolumnar or squamo-columnar) NEAR/2 junction) (60)
-
#5. TS=(ectocervic* or ecto-cervic*) (36)
-
#4. TS=(ectocervix or ecto-cervix) (20)
-
#3. TS=(endocervic* or endo-cervic*) (400)
-
#2. TS=(endocervix or endo-cervix) (54)
-
#1. TS=(cervix or cervic*) (16,171)
Key
-
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields
-
* = truncation
-
‘‘ ’’ = phrase search
-
NEAR/2 = terms within two words of each other (any order).
European Union Clinical Trials Register (www.clinicaltrialsregister.eu/ctr-search/search)
Date searched: 4 January 2017.
Records retrieved: 15.
An updated search was carried out on 10 April 2017, retrieving 16 records.
Search strategy
-
15 result(s) found for (Cervix OR cervical) AND (Colposcopy OR spectroscopy OR spectrometry OR “spectrum analysis”).
-
Dysis OR dysismap OR “dynamic spectral imaging” – 0 results.
-
Zilico OR ZedScan OR “Zed Scan” OR “APX 100” OR APX100 OR epitheliometer OR MKIII – 0 results.
PROSPERO (www.crd.york.ac.uk/PROSPERO)
Date searched: 4 January 2017.
Records retrieved: 4.
An updated search was carried out on 10 April 2017, retrieving three new records.
Search strategy
-
#1. MeSH DESCRIPTOR Cervix Uteri (10)
-
#2. cervix OR cervic* (399)
-
#3. endocervix OR endo-cervix (1)
-
#4. endocervic* OR endo-cervic* (4)
-
#5. ectocervix OR ecto-cervix (0)
-
#6. ectocervic$or ecto-cervic$ (0)
-
#7. ectocervic* OR ecto-cervic* (0)
-
#8. squamocolumnar OR squamo-columnar (1)
-
#9. transformation zone* (3)
-
#10. #1 OR #2 OR #3 OR #4 OR #5 OR #7 OR #8 OR #9 (400)
-
#11. MeSH DESCRIPTOR colposcopy (2)
-
#12. MeSH DESCRIPTOR Colposcopes (0)
-
#13. MeSH DESCRIPTOR Spectrum Analysis (1)
-
#14. MeSH DESCRIPTOR Dielectric Spectroscopy (0)
-
#15. (colposcop* AND (adjunct* OR digital* OR DSI OR computer* OR video* OR alternative* OR conventional*)) (3)
-
#16. ((impedance OR Dielectric) AND (spectroscop* OR spectrometr* OR spectrum analys*)) (1)
-
#17. telecolposcop* OR tele-colposcop* (0)
-
#18. telecolposcop* OR tele-colposcop* (0)
-
#19. optical AND spectroscop* (5)
-
#20. ((point OR pencil OR impedance) AND probe*) (16)
-
#21. microcolposcop* OR micro-colposcop* (1)
-
#22. dysis OR dysismap (2)
-
#23. dynamic spectral imaging (1)
-
#24. Zilico (1)
-
#25. ZedScan OR Zed Scan (0)
-
#26. APX 100 OR APX100 (1)
-
#27. EIS (1)
-
#28. epitheliometer* (0)
-
#29. MKIII (0)
-
#30. #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #19 OR #20 OR #21 OR #18 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 (26)
-
#31. #30 AND #10 (4)
Key
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation.
World Health Organization, International Clinical Trials Registry Platform (www.who.int/ictrp/search/en)
Date searched: 4 January 2017.
Records retrieved: 17.
An updated search was carried out on 10 April 2017, retrieving 15 records.
Search strategy
-
cervix OR cervical (Condition field) AND Colposcopy OR spectroscopy OR spectrometry OR spectrum analysis (Intervention field) – 16 records retrieved.
-
dysis OR dysismap OR dynamic spectral imaging OR Zilico OR ZedScan OR Zed Scan OR APX 100 OR APX100 OR epitheliometer OR MKIII (Intervention field) – 1 record retrieved.
Guideline searches
The following websites were all searched on 10 January 2017. An updated search was carried out on 10 April 2017; however, no new guidelines were identified.
Scottish Intercollegiate Guidelines Network (www.sign.ac.uk)
Searched the website using the terms ‘colposcopy’, ‘DySIS’, ‘ZedScan’, ‘Zed Scan’. Also browsed all guidelines. No new guidance found.
National Institute for Health and Clinical Excellence (www.nice.org.uk)
Searched the website using the terms ‘colposcopy’, ‘DySIS’, ‘ZedScan’, ‘Zed Scan’. Also browsed documents within the cervical cancer guidance section. Four relevant guidance documents were found.
National Guideline Clearinghouse (www.guideline.gov)
Searched using the terms ‘colposcopy OR DySIS OR ZedScan OR Zed Scan’, limited to publications from 2011 to 2017. A total of 19 results were browsed for relevance. Eight relevant guidelines were found.
NHS Evidence (www.evidence.nhs.uk)
Searched using the terms ‘colposcopy OR dysis OR ZedScan OR Zed Scan’. Filtered results by guidance and by date (1 January 2011 to 10 January 2017). A total of 40 records were retrieved and downloaded.
Turning Research into Practice database (www.tripdatabase.com)
Searched using the terms ‘colposcopy OR dysis OR ZedScan OR Zed Scan’. Filtered results by guidelines; 48 records were retrieved and browsed for relevance. One relevant record was found after duplicates were removed.
Public Health England (www.gov.uk/search)
Searched the website using the terms ‘colposcopy’, ‘DySIS’, ‘ZedScan’ and ‘Zed Scan’. Filtered by Public Health England. Nine results were retrieved and browsed for relevance. Seven relevant documents were found.
Royal College of Obstetricians and Gynaecologists (www.rcog.org.uk/en/guidelines-research-services/guidelines)
Searched all guidelines using the terms ‘colposcopy’, ‘DySIS’, ‘ZedScan’ and ‘Zed Scan’. Eight records were retrieved and browsed for relevance. One relevant report was found.
The British Society for Colposcopy and Cervical Pathology (www.bsccp.org.uk)
Searched the website using the terms ‘colposcopy’, ‘DySIS’, ‘ZedScan’ and ‘Zed Scan’, using the website general search box. A total of 110 results were returned and browsed for relevance. No guidelines were found.
Additional searches
The following search strategies were used to identify systematic reviews or meta-analyses examining the diagnostic test accuracy of cervical screening or HPV testing.
MEDLINE [Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R), via OvidSP: http://ovidsp.ovid.com]
Date range searched: 1946 to present.
Date searched: 29 March 2017.
Records retrieved: 267.
Search strategy
-
Uterine Cervical Neoplasms/ (67,631)
-
Cervical Intraepithelial Neoplasia/ (8926)
-
exp Uterine Cervical Dysplasia/ (4058)
-
Cervix Uteri/ (25,999)
-
((cervix or cervic*) adj3 (cancer* or neoplas* or carcinoma* or adenocarcinoma* or tumour* or tumor* dysplas* or dyskaryo* or precancer* or pre-cancer*)).ti,ab. (65,985)
-
((cervix or cervic*) adj3 (abnormal* or lesion* or atypical or squamous)).ti,ab. (13,430)
-
(cervix or cervic*).ti,ab. (227,836)
-
(LSIL or HSIL or ASCUS or ASC-US or ASC-H or ASC).ti,ab. (8776)
-
((intraepithelial or intra-epithelial) adj2 lesion$).ti,ab. (4844)
-
(atypical adj2 squamous).ti,ab. (1989)
-
8 or 9 or 10 (12,051)
-
7 and 11 (5015)
-
(CIN or CIN1* or CIN2* or CIN3* or CIN 1* or CIN 2* or CIN 3* or CIN I* or CIN II* or CIN III* or CINI* or CINII* or CINIII* or CGIN*).ti,ab. (10,256)
-
1 or 2 or 3 or 4 or 5 or 6 or 12 or 13 (114,337)
-
exp Papillomavirus Infections/ (28,658)
-
Papillomaviridae/ (21,795)
-
exp Alphapapillomavirus/ (6098)
-
(human* adj2 (papillomavirus* or papillomaviridae or papilloma virus*)).ti,ab. (33,953)
-
(HPV* or hrHPV* or hr-HPV*).ti,ab. (35,059)
-
or/15-19 (53,740)
-
Vaginal Smears/ (21,441)
-
Papanicolaou Test/ (5902)
-
Cytological Techniques/ (10,199)
-
Cytodiagnosis/ (15,335)
-
Mass Screening/ (92,108)
-
“Early Detection of Cancer”/ (15,713)
-
DNA Probes, HPV/ (1080)
-
Human Papillomavirus DNA Tests/ (340)
-
((vagina* or pap or papanicolaou) adj2 smear*).ti,ab. (9876)
-
((pap or papanicolaou) adj2 (test* or analys* or screen*)).ti,ab. (4980)
-
cytolog*.ti,ab. (85,141)
-
or/21-31 (214,406)
-
14 and 32 (26,028)
-
20 and 32 (10,837)
-
(screen* adj3 (cervic* or cervix)).ti,ab. (9587)
-
((cervic* or cervix) adj2 smear$).ti,ab. (4102)
-
((HPV* or hrHPV* or hr-HPV*) adj4 (screen* or test* or detect* or triage*)).ti,ab. (11,312)
-
(human* adj2 (papillomavirus* or papillomaviridae or papilloma virus*) adj2 (screen* or test* or detect* or triage*)).ti,ab. (3300)
-
35 or 36 or 37 or 38 (22,413)
-
33 or 34 or 39 (36,627)
-
systematic$ review$.ti,ab. (103,638)
-
meta-analysis as topic/ (15,759)
-
meta-analytic$.ti,ab. (5302)
-
meta-analysis.ti,ab,pt. (116,352)
-
metanalysis.ti,ab. (157)
-
metaanalysis.ti,ab. (1389)
-
meta analysis.ti,ab. (94,402)
-
meta-synthesis.ti,ab. (524)
-
metasynthesis.ti,ab. (231)
-
meta synthesis.ti,ab. (524)
-
meta-regression.ti,ab. (4549)
-
metaregression.ti,ab. (442)
-
meta regression.ti,ab. (4549)
-
(synthes$ adj3 literature).ti,ab. (2221)
-
(synthes$ adj3 evidence).ti,ab. (6509)
-
integrative review.ti,ab. (1692)
-
data synthesis.ti,ab. (9772)
-
(research synthesis or narrative synthesis).ti,ab. (1591)
-
(systematic study or systematic studies).ti,ab. (9808)
-
(systematic comparison$ or systematic overview$).ti,ab. (2592)
-
evidence based review.ti,ab. (1694)
-
comprehensive review.ti,ab. (10,438)
-
critical review.ti,ab. (13,445)
-
quantitative review.ti,ab. (583)
-
structured review.ti,ab. (641)
-
realist review.ti,ab. (158)
-
realist synthesis.ti,ab. (118)
-
or/41-67 (239,126)
-
review.pt. (2,263,518)
-
medline.ab. (84,579)
-
pubmed.ab. (65,446)
-
cochrane.ab. (51,941)
-
embase.ab. (54,367)
-
cinahl.ab. (17,283)
-
psyc?lit.ab. (937)
-
psyc?info.ab. (17,111)
-
(literature adj3 search$).ab. (41,423)
-
(database$ adj3 search$).ab. (39,606)
-
(bibliographic adj3 search$).ab. (1816)
-
(electronic adj3 search$).ab. (14,770)
-
(electronic adj3 database$).ab. (18,516)
-
(computeri?ed adj3 search$).ab. (3229)
-
(internet adj3 search$).ab. (2468)
-
included studies.ab. (13,602)
-
(inclusion adj3 studies).ab. (10,824)
-
inclusion criteria.ab. (57,533)
-
selection criteria.ab. (25,429)
-
predefined criteria.ab. (1537)
-
predetermined criteria.ab. (904)
-
(assess$ adj3 (quality or validity)).ab. (58,471)
-
(select$ adj3 (study or studies)).ab. (51,767)
-
(data adj3 extract$).ab. (43,710)
-
extracted data.ab. (10,058)
-
(data adj2 abstracted).ab. (4252)
-
(data adj3 abstraction).ab. (1226)
-
published intervention$.ab. (143)
-
((study or studies) adj2 evaluat$).ab. (145,298)
-
(intervention$ adj2 evaluat$).ab. (8561)
-
confidence interval$.ab. (314,381)
-
heterogeneity.ab. (125,402)
-
pooled.ab. (65,443)
-
pooling.ab. (9876)
-
odds ratio$.ab. (205,883)
-
(Jadad or coding).ab. (150,343)
-
or/70-104 (1,105,052)
-
69 and 105 (179,404)
-
review.ti. (35,4575)
-
107 and 105 (85,749)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$ or evaluation$)).ti,ab. (142,763)
-
68 or 106 or 108 or 109 (418,128)
-
letter.pt. (964,951)
-
editorial.pt. (434,093)
-
comment.pt. (685,970)
-
111 or 112 or 113 (1,570,204)
-
110 not 114 (408,039)
-
exp animals/ not humans/ (4,364,879)
-
115 not 116 (396,895)
-
40 and 117 (921)
-
limit 118 to yr=“2014 -Current” (267)
Key
-
/ = indexing term (MeSH)
-
exp = exploded indexing term (MeSH)
-
* = truncation
-
$ = truncation
-
? = optional wildcard – stands for zero or one character
-
.ti,ab. = terms in either title or abstract fields
-
.pt. = publication type
-
adj = terms next to each other (order specified)
-
adj2 = terms within two words of each other (any order).
Cochrane Database of Systematic Reviews (via Wiley Online Library: http://onlinelibrary.wiley.com)
Issue 3 of 12, March 2017.
Date searched: 29 March 2017.
Records retrieved: 20.
Search strategy
-
#1. MeSH descriptor: [Uterine Cervical Neoplasms] this term only (1975)
-
#2. MeSH descriptor: [Cervical Intraepithelial Neoplasia] this term only (518)
-
#3. MeSH descriptor: [Uterine Cervical Dysplasia] explode all trees (129)
-
#4. MeSH descriptor: [Cervix Uteri] this term only (1045)
-
#5. ((cervix or cervic*) near/3 (cancer* or neoplas* or carcinoma* or adenocarcinoma* or tumour* or tumor* dysplas* or dyskaryo* or precancer* or pre-cancer*)):ti,ab,kw (3701)
-
#6. ((cervix or cervic*) near/3 (abnormal* or lesion* or atypical or squamous)):ti,ab,kw (725)
-
#7. (cervix or cervic*):ti,ab,kw (13509)
-
#8. (LSIL or HSIL or ASCUS or ASC-US or ASC-H or ASC):ti,ab,kw (383)
-
#9. ((intraepithelial or intra-epithelial) near/2 lesion*):ti,ab,kw (217)
-
#10. (atypical near/2 squamous):ti,ab,kw (118)
-
#11. #8 or #9 or #10 (521)
-
#12. #7 and #11 (270)
-
#13. (CIN or CIN1* or CIN2* or CIN3* or CIN 1* or CIN 2* or CIN 3* or CIN I* or CIN II* or CIN III* or CINI* or CINII* or CINIII* or CGIN*):ti,ab,kw (1165)
-
#14. #1 or #2 or #3 or #4 or #5 or #6 or #12 or #13 (5623)
-
#15. MeSH descriptor: [Papillomavirus Infections] explode all trees (1107)
-
#16. MeSH descriptor: [Papillomaviridae] this term only (419)
-
#17. MeSH descriptor: [Alphapapillomavirus] explode all trees (220)
-
#18. (human* near/2 (papillomavirus* or papillomaviridae or papilloma next virus*)):ti,ab,kw (1353)
-
#19. (HPV* or hrHPV* or hr-HPV*):ti,ab,kw (1438)
-
#20. #15 or #16 or #17 or #18 or #19 (2137)
-
#21. MeSH descriptor: [Vaginal Smears] explode all trees (802)
-
#22. MeSH descriptor: [Papanicolaou Test] this term only (228)
-
#23. MeSH descriptor: [Cytological Techniques] this term only (82)
-
#24. MeSH descriptor: [Cytodiagnosis] this term only (120)
-
#25. MeSH descriptor: [Mass Screening] this term only (4758)
-
#26. MeSH descriptor: [Early Detection of Cancer] this term only (955)
-
#27. MeSH descriptor: [DNA Probes, HPV] this term only (16)
-
#28. MeSH descriptor: [Human Papillomavirus DNA Tests] this term only (8)
-
#29. ((vagina* or pap or papanicolaou) near/2 (smear*)):ti,ab,kw (1139)
-
#30. ((pap or papanicolaou) near/2 (test* or analys* or screen*)):ti,ab,kw (581)
-
#31. cytolog*:ti,ab,kw (2751)
-
#32. #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 (8679)
-
#33. #14 and #32 (1330)
-
#34. #20 and #32 (666)
-
#35. (screen* near/2 (cervic* or cervix)):ti,ab,kw (730)
-
#36. ((cervic* or cervix) near/2 smear*):ti,ab,kw (189)
-
#37. ((HPV* or hrHPV* or hr-HPV*) near/4 (screen* or test* or detect* or triage*)):ti,ab,kw (519)
-
#38. (human* near/2 (papillomavirus* or papillomaviridae or papilloma next virus*) near/2 (screen* or test* or detect* or triage*)):ti,ab,kw (240)
-
#39. #35 or #36 or #37 or #38 (1211)
-
#40. #33 or #34 or #39 (1740)
Key
-
MeSH descriptor = indexing term (MeSH)
-
* = truncation
-
ti,ab,kw = terms in either title or abstract or keyword fields
-
near/2 = terms within two words of each other (any order)
-
next = terms are next to each other.
Database of Abstracts of Reviews of Effects (www.crd.york.ac.uk/CRDWeb)
Date ranged searched: inception to 31 March 2015.
Date searched: 29 March 2017.
Records retrieved: 128.
Search strategy
-
MeSH DESCRIPTOR Uterine Cervical Neoplasms (540)
-
MeSH DESCRIPTOR Cervical Intraepithelial Neoplasia (136)
-
MeSH DESCRIPTOR Uterine Cervical Dysplasia EXPLODE ALL TREES (22)
-
MeSH DESCRIPTOR Cervix Uteri (89)
-
(((cervix or cervic*) ADJ3 (cancer* or neoplas* or carcinoma* or adenocarcinoma* or tumour* or tumor* dysplas* or dyskaryo* or precancer* or pre-cancer*))) (693)
-
(((cancer* or neoplas* or carcinoma* or adenocarcinoma* or tumour* or tumor* dysplas* or dyskaryo* or precancer* or pre-cancer*) ADJ3 (cervix or cervic*))) (168)
-
(((cervix or cervic*) ADJ3 (abnormal* or lesion* or atypical or squamous))) (60)
-
(((abnormal* or lesion* or atypical or squamous) ADJ3 (cervix or cervic*))) (43)
-
((cervix or cervic*)) AND (LSIL or HSIL or ASCUS or ASC-US or ASC-H or ASC) (41)
-
((cervix or cervic*)) (1481)
-
(((intraepithelial or intra-epithelial) ADJ2 lesion*)) (66)
-
((lesion* ADJ2 (intraepithelial or intra-epithelial))) (0)
-
((atypical ADJ2 squamous)) (36)
-
((squamous ADJ2 atypical)) (1)
-
#11 OR #12 OR #13 OR #14 (76)
-
#10 AND #15 (73)
-
((CIN or CIN1* or CIN2* or CIN3* or CIN 1* or CIN 2* or CIN 3* or CIN I* or CIN II* or CIN III* or CINI* or CINII* or CINIII* or CGIN*)) (111)
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #16 OR #17 (801)
-
MeSH DESCRIPTOR Papillomavirus Infections EXPLODE ALL TREES (283)
-
MeSH DESCRIPTOR Papillomaviridae (114)
-
MeSH DESCRIPTOR Alphapapillomavirus EXPLODE ALL TREES (54)
-
((human* ADJ2 (papillomavirus* or papillomaviridae or papilloma virus*))) (304)
-
(((papillomavirus* or papillomaviridae or papilloma virus*) ADJ2 human*)) (35)
-
(HPV* or hrHPV* or hr-HPV*) (259)
-
#19 OR #20 OR #21 OR #22 OR #23 OR #24 (409)
-
MeSH DESCRIPTOR Vaginal Smears EXPLODE ALL TREES (213)
-
MeSH DESCRIPTOR Papanicolaou Test (56)
-
MeSH DESCRIPTOR Cytological Techniques (34)
-
MeSH DESCRIPTOR Cytodiagnosis (40)
-
MeSH DESCRIPTOR Mass Screening (2100)
-
MeSH DESCRIPTOR Early Detection of Cancer (273)
-
MeSH DESCRIPTOR DNA Probes, HPV (6)
-
MeSH DESCRIPTOR Human Papillomavirus DNA Tests (6)
-
(((vagina* or pap or papanicolaou) ADJ2 smear*)) (258)
-
((smear* ADJ2 (vagina* or pap or papanicolaou))) (10)
-
(((pap or papanicolaou) ADJ2 (test* or analys* or screen*))) (128)
-
(((test* or analys* or screen*) ADJ2 (pap or papanicolaou))) (63)
-
(cytolog*) (483)
-
#26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 (2727)
-
#18 AND #39 (393)
-
#25 AND #39 (233)
-
((screen* ADJ3 (cervic* or cervix))) (142)
-
(((cervic* or cervix) ADJ3 screen*)) (264)
-
(((cervic* or cervix) ADJ3 smear*)) (81)
-
((smear* ADJ2 (cervic* or cervix))) (18)
-
(((HPV* or hrHPV* or hr-HPV*) ADJ4 (screen* or test* or detect* or triage*))) (135)
-
(((screen* or test* or detect* or triage*) ADJ4 (HPV* or hrHPV* or hr-HPV*))) (92)
-
((papillomavirus* or papillomaviridae or papilloma virus*) ADJ2 (screen* or test* or detect* or triage*)) (115)
-
(((screen* or test* or detect* or triage*) ADJ2 (papillomavirus* or papillomaviridae or papilloma virus*))) (75)
-
(human) (3164)
-
#48 OR #49 (144)
-
#50 AND #51 (123)
-
#42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #52 (392)
-
#40 OR #41 OR #53 (472)
-
(*) IN DARE (45,418)
-
#54 AND #55 (128)
-
(*) IN HTA (16,846)
-
#54 AND #57 (108)
Key
-
MeSH DESCRIPTOR = indexing term (MeSH)
-
* = truncation
-
ADJ2 = terms within two words of each other (order specified).
EMBASE (via Ovid: http://ovidsp.ovid.com)
Date range searched: 1974 to 28 March 2017.
Date searched: 29 March 2017.
Records retrieved: 676.
Search strategy
-
exp uterine cervix tumor/ (101,917)
-
exp uterine cervix dysplasia/ (5017)
-
exp uterine cervix/ (28,075)
-
((cervix or cervic*) adj3 (cancer* or neoplas* or carcinoma* or adenocarcinoma* or tumour* or tumor* dysplas* or dyskaryo* or precancer* or pre-cancer*)).ti,ab. (81,511)
-
((cervix or cervic*) adj3 (abnormal* or lesion* or atypical or squamous)).ti,ab. (17,047)
-
(cervix or cervic*).ti,ab. (279,094)
-
(LSIL or HSIL or ASCUS or ASC-US or ASC-H or ASC).ti,ab. (12,691)
-
((intraepithelial or intra-epithelial) adj2 lesion$).ti,ab. (6060)
-
(atypical adj2 squamous).ti,ab. (2491)
-
7 or 8 or 9 (16,616)
-
6 and 10 (6886)
-
(CIN or CIN1* or CIN2* or CIN3* or CIN 1* or CIN 2* or CIN 3* or CIN I* or CIN II* or CIN III* or CINI* or CINII* or CINIII* or CGIN*).ti,ab. (14,461)
-
1 or 2 or 3 or 4 or 5 or 11 or 12 (147,667)
-
exp papillomavirus infection/ (25,629)
-
papillomaviridae/ (816)
-
exp alphapapillomavirus/ (12,821)
-
wart virus/ (37,172)
-
(human* adj2 (papillomavirus* or papillomaviridae or papilloma virus*)).ti,ab. (39,987)
-
(HPV* or hrHPV* or hr-HPV*).ti,ab. (45,330)
-
14 or 15 or 16 or 17 or 18 or 19 (71,133)
-
vagina smear/ (10,593)
-
papanicolaou test/ (15,851)
-
uterine cervix cytology/ (12,091)
-
cytology/ (382,601)
-
cancer screening/ (66,243)
-
early cancer diagnosis/ (1191)
-
Human papillomavirus DNA test/ (1518)
-
DNA probe/ (27,048)
-
screening test/ (65,290)
-
diagnostic accuracy/ (217,693)
-
diagnostic test accuracy study/ (75,141)
-
((vagina* or pap or papanicolaou) adj2 smear*).ti,ab. (11,672)
-
((pap or papanicolaou) adj2 (test* or analys* or screen*)).ti,ab. (6474)
-
cytolog*.ti,ab. (105,880)
-
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 (854,789)
-
13 and 35 (36,343)
-
20 and 35 (17,008)
-
(screen* adj3 (cervic* or cervix)).ti,ab. (11,964)
-
((cervic* or cervix) adj2 smear$).ti,ab. (4794)
-
((HPV* or hrHPV* or hr-HPV*) adj4 (screen* or test* or detect* or triage*)).ti,ab. (14,930)
-
(human* adj2 (papillomavirus* or papillomaviridae or papilloma virus*) adj2 (screen* or test* or detect* or triage*)).ti,ab. (3861)
-
38 or 39 or 40 or 41 (28,042)
-
36 or 37 or 42 (49,894)
-
systematic$ review$.ti,ab. (127,491)
-
systematic$ literature review$.ti,ab. (9255)
-
“systematic review”/ (159,479)
-
“systematic review (topic)”/ (28,244)
-
meta analysis/ (161,820)
-
“meta analysis (topic)”/ (39,256)
-
meta-analytic$.ti,ab. (5990)
-
meta-analysis.ti,ab. (121,194)
-
metanalysis.ti,ab. (390)
-
metaanalysis.ti,ab. (5712)
-
meta analysis.ti,ab. (121,194)
-
meta-synthesis.ti,ab. (482)
-
metasynthesis.ti,ab. (226)
-
meta synthesis.ti,ab. (482)
-
meta-regression.ti,ab. (5717)
-
metaregression.ti,ab. (735)
-
meta regression.ti,ab. (5717)
-
(synthes$ adj3 literature).ti,ab. (2538)
-
(synthes$ adj3 evidence).ti,ab. (7290)
-
(synthes$ adj2 qualitative).ti,ab. (1370)
-
integrative review.ti,ab. (1388)
-
data synthesis.ti,ab. (11,134)
-
(research synthesis or narrative synthesis).ti,ab. (1581)
-
(systematic study or systematic studies).ti,ab. (10,580)
-
(systematic comparison$ or systematic overview$).ti,ab. (2810)
-
(systematic adj2 search$).ti,ab. (19,687)
-
systematic$ literature research$.ti,ab. (218)
-
(review adj3 scientific literature).ti,ab. (1419)
-
(literature review adj2 side effect$).ti,ab. (12)
-
(literature review adj2 adverse effect$).ti,ab. (2)
-
(literature review adj2 adverse event$).ti,ab. (12)
-
(evidence-based adj2 review).ti,ab. (3042)
-
comprehensive review.ti,ab. (12,025)
-
critical review.ti,ab. (14,564)
-
critical analysis.ti,ab. (7278)
-
quantitative review.ti,ab. (653)
-
structured review.ti,ab. (841)
-
realist review.ti,ab. (141)
-
realist synthesis.ti,ab. (95)
-
(pooled adj2 analysis).ti,ab. (13,908)
-
(pooled data adj6 (studies or trials)).ti,ab. (2176)
-
(medline and (inclusion adj3 criteria)).ti,ab. (17,624)
-
(search adj (strateg$ or term$)).ti,ab. (27,662)
-
or/44-86 (397,542)
-
medline.ab. (101,252)
-
pubmed.ab. (83,024)
-
cochrane.ab. (65,587)
-
embase.ab. (67,292)
-
cinahl.ab. (19,062)
-
psyc?lit.ab. (977)
-
psyc?info.ab. (15,707)
-
lilacs.ab. (5248)
-
(literature adj3 search$).ab. (51,495)
-
(database$ adj3 search$).ab. (48,637)
-
(bibliographic adj3 search$).ab. (2080)
-
(electronic adj3 search$).ab. (17,339)
-
(electronic adj3 database$).ab. (24,395)
-
(computeri?ed adj3 search$).ab. (3688)
-
(internet adj3 search$).ab. (3184)
-
included studies.ab. (16,745)
-
(inclusion adj3 studies).ab. (13,046)
-
inclusion criteria.ab. (94,570)
-
selection criteria.ab. (27,646)
-
predefined criteria.ab. (2021)
-
predetermined criteria.ab. (1098)
-
(assess$ adj3 (quality or validity)).ab. (74,945)
-
(select$ adj3 (study or studies)).ab. (65,709)
-
(data adj3 extract$).ab. (57,099)
-
extracted data.ab. (12,500)
-
(data adj2 abstracted).ab. (6653)
-
(data adj3 abstraction).ab. (1741)
-
published intervention$.ab. (167)
-
((study or studies) adj2 evaluat$).ab. (198,404)
-
(intervention$ adj2 evaluat$).ab. (11,277)
-
confidence interval$.ab. (367,946)
-
heterogeneity.ab. (154,328)
-
pooled.ab. (88,249)
-
pooling.ab. (12,583)
-
odds ratio$.ab. (252,412)
-
(Jadad or coding).ab. (172,491)
-
evidence-based.ti,ab. (104,203)
-
or/88-124 (1,482,382)
-
review.pt. (2,263,944)
-
125 and 126 (181,834)
-
review.ti. (404,112)
-
125 and 128 (105,785)
-
(review$ adj10 (papers or trials or trial data or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (413,152)
-
(retriev$ adj10 (papers or trials or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (21,339)
-
87 or 127 or 129 or 130 or 131 (776,417)
-
letter.pt. (983,216)
-
editorial.pt. (537,824)
-
133 or 134 (1,521,040)
-
132 not 135 (761,183)
-
(animal/ or nonhuman/) not exp human/ (5,103,479)
-
136 not 137 (735,678)
-
43 and 138 (2214)
-
limit 139 to yr=“2014 -Current” (676)
Key
-
/ = indexing term (Emtree heading)
-
exp = exploded indexing term (Emtree heading)
-
* = truncation
-
$ = truncation
-
ti,ab = terms in either title or abstract fields
-
adj = terms next to each other (order specified)
-
adj2 = terms within two words of each other (any order)
-
.pt. = publication type
-
? = optional wildcard – stands for zero or one character.
Health Technology Assessment database (www.crd.york.ac.uk/CRDWeb)
Date ranged searched: inception to 28 March 2017.
Date searched: 29 March 2017.
Records retrieved: 108.
See Database of Abstracts of Reviews of Effects (via www.crd.york.ac.uk/CRDWeb) for the search strategy used.
Appendix 3 Quality assessment of the diagnostic accuracy studies
An assessment relating to the risk of bias and concerns about the applicability of all studies included in the diagnostic accuracy review was performed using a modified version of the QUADAS-2 checklist. The modified version of the QUADAS-2 checklist used in Wade et al. 30 and further described elsewhere38 to assess the risk of bias in comparative diagnostic accuracy studies (i.e. a comparison of the index test with both standard care and the gold standard) was used. Further questions, presented in Table 35, were added to the following domains: index/comparator test (one question), flow and timing (two questions) and other concerns (three questions). A question about the predicted direction of bias, similar to that used in the Cochrane Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) tool161 for domains rated as being at a high risk of bias, was also added. The full results of the QUADAS-2 quality assessment are reported in Tables 36–41.
| Question | Domain | Difference with Wade et al.30 |
|---|---|---|
| Were the comparator test results interpreted and recorded without knowledge of the adjunctive technology results? | Index/comparator test | New |
| Were additional biopsies taken on random sites or sites with no apparent abnormality with colposcopy? | Flow and timing | New |
| Did all patients receive a reference standard? | Flow and timing | New |
| Any concerns about the size/power of the study? | Other concerns | New, replaced ‘Was a sample size calculation used?’ |
| Did the index test manufacturer have any involvement in the design, conduct of the study and/or in the interpretation of the results? | Other concerns | New |
| Was it a multicentre study and were several colposcopists involved? | Other concerns | New |
| First author of the study (year of publication) | Was a consecutive or random sample of patients enrolled? | Was a case–control design avoided? | Did the study avoid inappropriate exclusions? | Risk: could the selection of patients have introduced bias? | Is there concern that the included patients do not match the review question? |
|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Yes | Yes |
Yes 36 patients (8.1%) with DSI map not calculated because of excessive movement. No other exclusions |
Low |
High Low prevalence of hrHPV, referred following Spanish guidelines |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | Yes | Yes |
Yes Excluded current pregnancy and pregnancy in the last 3 months, previous cervix surgery or pelvic radiotherapy |
Low (ITT population) |
High Not HPV primary screening, 66.1% hrHPV positive |
| Natsis (2016)74 | Unclear | Yes | Unclear | Unclear conference abstract of ongoing study |
Low Relevant subgroup was women with LG cytology and infected with hrHPV in England |
| Roensbo (2015)79 | Unclear (NR) | Yes | Unclear if ‘sufficient view of the cervix’ was required, with no further details reported |
Unclear Unclear if consecutive patients were recruited and unclear definition of inclusion criterion |
Unclear No data on hrHPV prevalence or whether participants underwent hrHPV screening/triage |
| Salter (2016)80 | Unclear | Yes | Unclear | Unclear |
Unclear No data on hrHPV prevalence |
| Soutter (2009)88 | Yes | Yes |
Unclear Issues relating to the software, speculum and a batch of faulty disposable nozzles, leading to the exclusion of a large proportion of eligible participants (31%) |
Unclear Unclear if there were systematic differences in relevant baseline characteristics between included and excluded participants |
Unclear No data on hrHPV prevalence and cytology results |
| Tidy (2013)103 | No ‘non-consecutive’ | Yes | Unclear | High: non-consecutive selection of patients, exclusion of women with active menstruation | High: non-consecutive selection of patients |
| Predicted direction of bias: favours index test (menstruation affects spectroscopy) | |||||
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Unclear | Yes | Unclear | Unclear | Unclear |
| First author of study (year of publication) | Were the index test results interpreted without knowledge of the results of the reference standard? | Were the comparator test results interpreted and recorded without knowledge of the adjunctive technology results? | If a threshold was used, was it prespecified? | Were the colposcopists undertaking the tests experienced in colposcopy? | Were the colposcopists undertaking the new technologies given training/experience in the new technology? | Risk: could the conduct or interpretation of the index test have introduced bias? | Risk: could the conduct or interpretation of the comparator test have introduced bias? |
|---|---|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Yes |
Unclear Performed before DySIS map, but no reporting of measures to ensure that the two were recorded independently |
Yes | Yes | Unclear | Low |
Unclear Unclear if colposcopy results were interpreted and recorded independently of knowledge of index test results |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | Yes | Yes | Yes | Yes | Yes | Low | Low |
| Natsis (2016)74 | Yes | No | Unclear | Unclear | Unclear |
Unclear Insufficient information (conference abstract) |
Unclear Insufficient information (conference abstract) |
| Roensbo (2015)79 | Yes |
No Clinicians were not blinded to DySISmap results when performing colposcopy |
Unclear |
Partly Almost 50% of colposcopies were performed by colposcopists with a low level of experience (general practitioner residents), although one of two licensed and experienced nurses supervised all examinations. Licensed and experienced nurses performed all other colposcopies |
Unclear The DySIS colposcope had been in use in the outpatient clinic for 2 months before study initiation, but it is unclear whether or not all colposcopists had received sufficient training |
High 50% of colposcopies were performed by colposcopists with a low level of experience (although all were supervised by experienced and licenced nurses). Unclear whether or not colposcopists were sufficiently trained with adjunctive technology |
High Colposcopists were not blinded to DySISmap results, low level of experience of colposcopists who performed almost 50% of colposcopies |
| Salter (2016)80 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Soutter (2009)88 | Yes |
Yes Done independently by a separate blinded colposcopist |
Yes Specified following training on 82 patients, prior to starting test on actual study population. |
Yes UK colposcopists all experienced and accredited by BSCCP. Colposcopists in Greek clinic were similarly experienced |
Unclear Unclear if all colposcopists were involved in the training group |
Low | Low |
| Tidy (2013)103 | Unclear (Phase II); no (Phase I): colposcopic impression and histological data used concurrently |
Yes The colposcopist was blinded at all times to the EIS result to prevent bias |
No The cut-off points were further tested and refined in post-hoc analyses during Phase II ’on pragmatic grounds’ |
Yes | Yes |
High The cut-off points were further tested and refined in post-hoc analyses during Phase II ’on pragmatic grounds’ |
Low The colposcopist was blinded at all times to the EIS result to prevent bias |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Unclear | Unclear | Unclear | Unclear | Unclear |
Unclear Each patient was examined three times with three different concentrations of acetic acid (minimum of 45 minutes between examinations). It is not clear whether or not colposcopists were blinded to the results of examinations using different concentrations |
Unclear |
| First author of study (year of publication) | Were relevant clinical data available to the colposcopist during the examination (cytology/Pap smear and HPV test results)? | Was the execution of the intervention technology as it would be in practice? | Was the execution of the comparator technology as it would be in practice? | Applicability concern: is there concern that the index test or its conduct or interpretation differs from the review question? | Applicability concern: is there concern that the comparator test or its conduct or interpretation differs from the review question? |
|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Yes | Yes | Yes | Low | Low |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 |
Unclear Presumably yes |
Yes |
No Use of multiple/random biopsies in all patients |
Low | High |
| Natsis (2016)74 | Likely yes | Unclear | Unclear |
Unclear Insufficient information (conference abstract of ongoing study) |
Unclear Insufficient information (conference abstract of ongoing study) |
| Roensbo (2015)79 | Unclear |
No DySISmap not used as an adjunct to colposcopy. Areas with weaker acetowhitening (dark blue and green on the DySISmap results) were treated as ‘suspicious for HG disease’ |
No High number of biopsies performed (three to five in all participants) and use of random biopsies |
High DySISmap not used as an adjunct to colposcopy. Areas with less acetowhitening on the DySISmap treated as potential cases of CIN 2+ |
High Differs significantly from standard UK practice because of the number of biopsies performed (three–five in all participants) and the use of random biopsies |
| Salter (2016)80 | Unclear | Unclear | Unclear |
Unclear DySIS Medical played a role in study conduct, so the use of an index test likely to have been consistent with other trials, but information was too sparse |
Unclear |
| Soutter (2009)88 | Yes |
No Precommercial prototype, with different DySISmap algorithm |
No Biopsies performed in all patients, including those with normal transformation zone colposcopy result |
High Pre-commercial prototype, with different DySISmap algorithm |
High |
| Tidy (2013)103 | Yes |
No Prototype version with (confidential information has been removed) and with video display |
Yes |
High Prototype used with video display and different cut-off point from ZedScan |
Low |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Unclear |
No Patients were examined three times using different concentrations of acetic acid |
Unclear |
High Patients were examined three times using different concentrations of acetic acid |
High Patients were examined three times using different concentrations of acetic acid |
| First author of the study (year of publication) | Is the reference standard likely to correctly classify the target condition? | Were the reference standard results interpreted without knowledge of the results of the index test? | Risk: could the reference standard, its conduct or its interpretation have introduced bias? | Was the execution of the reference standard as it would be in practice (e.g. performed by experienced pathologists)? | Concern: is there concern that the target condition as defined by the reference standard does not match the review question? |
|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Noa | Unclear | Higha | Yes | Low |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | Noa |
Yes All histology was independently reviewed by a specialist pathologist. In case of disagreement between the original assessment and the review, a third expert reviewer graded the lesion (19.0% of all tissue samples), blinded to all previous results, and the majority decision determined the diagnosis |
Higha | Yes | Low |
| Natsis (2016)74 | Noa | Unclear | Higha | Unclear |
Unclear Insufficient information (conference abstract of ongoing study) |
| Roensbo (2015)79 | Noa | Unclear | Higha | Yes | Low |
| Salter (2016)80 | Noa | Unclear | Higha | Unclear | Unclear |
| Soutter (2009)88 | Noa |
Yes Reproduced from Soutter et al. 88 |
Higha | Yes | Low |
| Tidy (2013)103 | Noa | Unclear | Higha | Yes | Low |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Noa | Unclear | Higha | Unclear | Unclear |
| First author of the study (year of publication) | Was there an appropriate interval between index test(s) and reference standard? | Did all patients receive a reference standard? | Did the patients who received a reference standard all receive the same reference standard? (e.g. histology based on punch biopsy vs. LLETZ) | Were additional biopsies taken on random sites or seemingly normal sites? | Were all patients included in the analysis? | Risk: could the patient flow have introduced bias? |
|---|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Yes | No |
No An endocervical curettage was performed when the transformation zone was type 3. A LEEP was performed on all CIN 2+ cases diagnosed by a punch biopsy, in all women referred with a HSIL Pap smear that had transformation zone type 3 and on all cases of biopsy-confirmed CIN 1 that was persistent for > 2 years |
No |
No 8.1% excluded. Reasons for exclusion appeared to be appropriate |
High High risk of verification bias as a result of absence of biopsy for lower-risk patients. May positively bias sensitivity estimates |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | Yes | Yes |
No All histology, mostly through biopsy (89%), others via LLETZ |
Yes One additional control biopsy of apparently normal cervical tissue on the opposite side of abnormal looking lesion(s), or one biopsy at 12 o’clock if both colposcopy and DySIS found no abnormal sites |
No 9.5% excluded. Reasons for exclusion appeared to be appropriate |
Low |
| Natsis (2016)74 | Unclear |
No 80.8% in DySIS group, 85.9% in control group |
Unclear Unclear how many, if any, underwent LLETZ |
Unclear Unlikely (not UK practice) |
Unclear Insufficient information |
High Risk of verification bias |
| Roensbo (2015)79 | Yes | Yes | Yes |
Yes Biopsies taken in accordance with the clinician’s judgement/randomly. All participants received between three and five biopsies |
No 9.8% excluded. Reasons for exclusion appeared to be appropriate |
High Exclusion of significant proportion of enrolled participants |
| Salter (2016)80 | Unclear | No | Unclear | No | Unclear | Unclear |
| Soutter (2009)88 | Yes | Yes |
No Most from punch biopsies, others from treatment and follow-up biopsies |
Yes All received biopsies. Random biopsies taken from 115 sites thought by the colposcopist to be normal, metaplasia or HPV infection and 101 treatment or follow-up biopsies. The sensitivities of colposcopy and DySIS were 48.6% and 79.2%, respectively. If the cases of HG disease detected by biopsies taken to limit verification bias were excluded, the sensitivities would have seemed to be 55.6% and 83.8%, respectively |
No 31% excluded. Main reasons: unsatisfactory view (10%) and problem with acid-faulty acetic nozzles (8.3%) |
High High proportion of patients were included, although it is unclear whether or not there were any systematic differences in the baseline characteristics between patients included and patients excluded from the analyses |
| Tidy (2013)103 | Yes |
No Reproduced from Tidy et al. 103 |
No | No |
No 12 women were excluded in Phase II: nine had incomplete clinical data, one did not meet the inclusion criteria, one was unable to complete the colposcopic examination and one was excluded because of a protocol violation. For five women, the device exhibited technical problems that prevented the collection of EIS data. In addition, 110/7706 recorded measurements (1.4%) were unacceptable when the spectra were visually reviewed |
High Risk of verification bias: biopsies performed only in patients with suspected abnormalities based on examination |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Unclear | Unclear |
Unclear Potentially no: loop excisions and punch biopsies were taken |
No | No | Unclear |
| First author of the study (year of publication) | Were the data analysed by lesion, patient or both? | Were the results for all prespecified outcomes reported? | Did the index test manufacturer have any involvement in the design, conduct of the study and/or in the interpretation of the results? | Any concerns about the size/power of the study? | Was it a multicentre study, and were several colposcopists involved? | Overall quality |
|---|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | Patient |
Unclear No protocol found |
No | No |
No One centre, one colposcopist |
Unsound High risk of verification bias (no biopsy for all participants), limited applicability (population and single centre/colposcopist) |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | Patient |
Unclear No protocol found Sensitivity and specificity, number and reasons for exclusions were all reported |
Yes Role in the study design and critically appraised the manuscript |
No | Yes | Sound |
| Natsis (2016)74 | Patient |
Unclear No protocol found |
Yes | No | Yes |
Unsound Ongoing study, conference abstract, with significant proportion of patients (18.6%) who did not receive a biopsy |
| Roensbo (2015)79 | Patient |
Unclear No protocol found |
No | No |
No Single centre, multiple colposcopists with varying levels of experience |
Unsound (1) Almost 50% of colposcopies performed by colposcopists with a low level of experience (although supervised by experienced nurses), (2) lack of blinding of colposcopists to initial DySIS map results and (3) exclusion of 17% of participants following enrolment, including as a result of protocol failures |
| Salter (2016)80 | Patient | Yes |
Yes Specific role unclear |
No | Yes |
Unsound Conference abstract of ongoing study, limited data on diagnostic accuracy (full diagnostic accuracy data reported for only a small subgroup of two colposcopy clinics) |
| Soutter (2009)88 | Patient |
Unclear Protocol not found |
Yes Contributed to the study design and the writing of the report. The collection and collation of the data were supervised by the principal investigator and corresponding author. The analysis of data was undertaken by the principal investigator. Corresponding author is a member of the speakers bureau of Forth Photonics [(Edinburgh, UK) manufacturer]. Principal investigator has an ownership interest in Forth Photonics |
No | Yes | Unsound, owing to the exclusion of a large proportion of participants (31%). Significant levels of concern about the applicability of the study (FPC-03 pre-commercial prototype used) |
| Tidy (2013)103 | Patient |
Unclear No protocol found |
Yes First and second authors hold patents related to the technology. They are shareholders in Zilico Ltd and receive consultancy fees Another author is also a shareholder. A fourth author is a medical advisor to Zilico Ltd and receives consultancy fees |
No | Yes |
Unsound High risk of verification bias, selection bias, significant concerns about applicability (patient selection and use of precommercial prototype) |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | Patient |
Unclear No protocol found |
Yes No formal declaration, though E. Papagiannakis is an employee of DySIS Medical |
Yes Small study (n = 54) |
No |
Unsound Conference abstract, small study with little information available |
Appendix 4 Cervical intraepithelial neoplasia and cancer prevalence
| First author of the study (year of publication) | Number of participants | Normal, % | CIN grade | |||||
|---|---|---|---|---|---|---|---|---|
| CIN 1 prevalence, % | CIN 1 or lower prevalence, % | CIN 2 prevalence, % | CIN 2 or higher prevalence, % | CIN 3 prevalence, % | Worse than CIN 3, % | |||
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | 443 | 66.1 | 24.6 | 90.7 | 3.2 | 9.3 | 6.1 | 1.1 |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | 239 | NR | NR | NR | NR | 45.2 | NR | Confidential information has been removed |
| Natsis (2016)74 | 287 (+ 948 in the control group) | NR | NR | NR | NR | 17.1 | NR | NR |
| Roensbo (2015)79 | 239 | 71.5 | NR | 71.5 | NR | 28.5 | 0.0 | NR |
| Salter [2016 (IMPROVE-COLPO)]80 | 210 | 56.7 | 28.6 | 85.2 | 9.0 | 14.8 | 5.7 | 0.5a |
| Soutter (2009)88 | 308 | NR | NR | 76.6 | 8.4 | 23.4 | 14.9 | 1.0 |
| Tidy (2013)103 | 196 | NR | NR | 55.6 | NR | 44.4 | NR | NR |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | 54 | NR | NR | NR | NR | NR | NR | NR |
Appendix 5 Patient selection criteria and test failures in diagnostic accuracy studies
| First author of the study (year of publication) | Inclusion criteria | Exclusion criteria | Test failure rate, n (%) | Reasons for test failures |
|---|---|---|---|---|
| Budithi (2018)45 | Women referred to the colposcopy clinic because of abnormal cervical cytology, abnormal-appearing cervix or postcoital bleeding | Non-cervical disease (e.g. vulval and vaginal referrals) and pregnant women excluded from the analyses | 26 (6.2) | Missing colposcopic impression and DSI map (n = 25), missing histology data (n = 1) |
| Coronado (2016)50 | Women aged ≥ 18 years referred for colposcopy following Spanish national guidance | NR | 36 (8.1) | Excessive movements during the measurement |
| Founta (2018)54 | Women referred to the colposcopy clinic with negative cytology and testing positive for hrHPV either 6 months after treatment or in the context of the catch-up programme and who underwent DySIS colposcopy | NR | 3 (2.9) | Poor-quality imaging because of user errors |
| Louwers (2011)60 | Women aged ≥ 18 years with abnormal cervical cytology or follow-up of a CIN grade 1 or 2 lesion | Previous surgery on the cervix, pelvic radiotherapy. Current pregnancy and pregnancy in the last 3 months | 25 (9.5) | DySIS did not start (n = 7), no map (n = 9) and exam data not saved (n = 9) |
| Natsis (2016)74 | NR | NR | NR | NR |
| Roensbo (2015)79 | Women aged ≥ 18 years with adequate DySIS colposcopy (such as sufficient view of the cervix and no patient movement resulting in adequate DySIS analysis) | NR | 28 (9.8) | 48 women were excluded because biopsies were not sent separately (n = 28), it was not possible to classify the biopsy (n = 6), there were technical difficulties (n = 9) and other reasons (n = 5) |
| Salter (2016)80 | NR | NR | NR | NR |
| Soutter (2009)88 | Cervical smear showing squamous or glandular cell dyskaryosis or borderline nuclear change (ASCUS or AGUS), or symptoms of postcoital bleeding, postmenopausal bleeding or intermenstrual bleeding | Self-referring women without an abnormal smear, an inadequate or an inflammatory smear, any other clinical indication for referral to colposcopy, pregnancy, previous pelvic radiotherapy or any woman for whom any prolongation of the examination was thought to be inadvisable | 139 (31) | Software problems (n = 15), no biopsy (n = 23), unsatisfactory view (n = 45), not eligible (n = 6), 5% acetic acid (n = 1), lost data form (n = 1), lost biopsy slides (n = 5), blood or mucus (n = 1), biopsies from wrong point (n = 3), excessive movement (n = 2) and problem with acid-faulty acetic nozzles (n = 37) |
| Tidy (2013)103 | Women referred with abnormal cervical cytology | Type 3 transformation zone, pregnancy and active menstruation | Phase I: 33 (15.4); Phase II: 19 (8.8) |
Phase I: 31 women treated ‘as part of training’, two women with incomplete clinical data Phase II: biopsy not coincident with EIS reading or inadequate for histological examination (n = 14). Failure of EIS device (n = 12, including nine women who had incomplete clinical data), did not meet the inclusion criteria (n = 1), unable to complete the colposcopic examination (n = 1) and excluded because of a protocol violation (n = 1). In five cases, the device exhibited technical problems that prevented the collection of EIS data. In addition, 110/7706 recorded measurements (1.4%) were unacceptable when the spectra were visually reviewed |
| Tidy (2018)94 | Women referred to the colposcopy clinic with abnormal cervical cytology from the NHSCSP. Adequate colposcopic examination (i.e. type 1 or 2 transformation zone with the upper extent of the lesion seen) | Type 3 transformation zone, pregnancy | 73 (5.6) | 73 results were not considered to be analysable: 61 related to the use of ZedScan, mainly occurred in the early stages of adopting the device and were a combination of device failures and user errors, seven had problems unrelated to ZedScan (e.g. discomfort because of a speculum) and five had incomplete data or self-reported as being pregnant |
| Tsetsa (2012)111 | NR | NR | NR | NR |
Appendix 6 Meta-analysis of the diagnostic accuracy studies: additional figures and table
FIGURE 17.
Diagnostic odds ratios from the DySIS studies. (Confidential information has been removed.)
FIGURE 18.
Positive predictive values in the DySIS studies. (Confidential information has been removed.)
FIGURE 19.
Negative predictive values in the DySIS studies. (Confidential information has been removed.)
FIGURE 20.
Summary ROC plot from bivariate models.
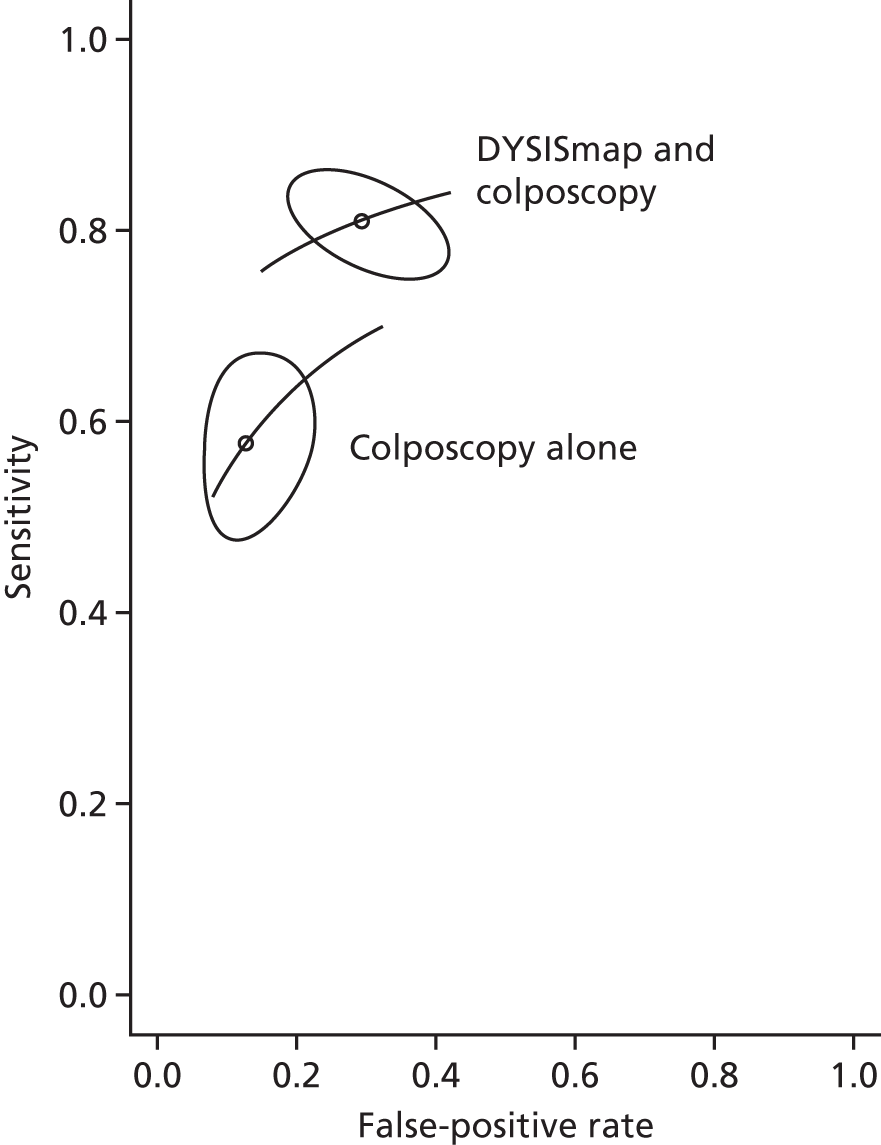
FIGURE 21.
Percentage of positive test results in DySIS studies. (Confidential information has been removed.)
FIGURE 22.
Percentage of positive test results in ZedScan studies. (Confidential information has been removed.)
| First author of the study (year of publication) | Comparisons | Subgroups reported | Primary source of data |
|---|---|---|---|
| DySIS studies | |||
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | DySISmap and colposcopy | hrHPV | Coronado and Fasero50 |
| DySISmap alone | hrHPV | Coronado and Fasero50 | |
| Colposcopy alone | hrHPV | Coronado and Fasero50 | |
| Founta (2018) DyS-CO154 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| IMPROVE-COLPO |
DySISmap alone Colposcopy alone (matched control) |
LG Pap smear result | Conference abstract: Papagiannakis et al.83 and Weinberg et al.85 |
| Louwers (2011)60 | DySISmap and colposcopy | Confidential information has been removed | Confidential information has been removed |
| DySISmap alone | Referral for LG cervical abnormalities | Linked publication (i.e. Louwers et al.)61 | |
| Colposcopy alone | Referral for HG cervical abnormalities | Linked publication (i.e. Louwers et al.)61 | |
| Positive hrHPV test and BMD, or HG cytology resulta | Linked publication (i.e. Zaal et al.)62 | ||
| BMD cytology and a hrHPV positive test or HG cytology result, irrespective of the hrHPV test resultb | Linked publication (i.e. Zaal et al.)62 | ||
| Natsis (2016)74 | DySISmap and colposcopy | hrHPV with a referral for LG cervical abnormalities | Conference abstract74 |
| Colposcopy alone | hrHPV with a referral for LG cervical abnormalities | Conference abstract74 | |
| Colposcopy alone (contemporaneous control group) | hrHPV with a referral for LG cervical abnormalities | Conference abstract74 | |
| Roensbo (2015)79 | DySISmap alone Colposcopy and random biopsies | None | Publication79 |
| Salter (2016)80 | DySISmap and colposcopy | Initial results from two clinics | Conference abstract80 |
| Soutter (2009)88 | DySIS and colposcopy | LG Pap smear result | Publication: Soutter et al.88 and Soutter et al.89 |
| Colposcopy alone | HG Pap smear result | ||
| DySISmap alone | |||
| Tsetsa (2012)111 | DySIS and colposcopy | 3% acetic acid treatment, 4% acetic acid treatment and 5% acetic acid treatment | Conference abstract111,162 |
| ZedScan studies | |||
| Tidy (2013)103 |
ZedScan and colposcopy Colposcopy alone |
Confidential information has been removed | Confidential information has been removed |
| Tidy (2018)94 | ZedScan and colposcopy | Confidential information has been removed | Confidential information has been removed |
| Colposcopy alone | Low-risk HPV | Linked manuscript95 | |
| hrHPV | Linked manuscript95 | ||
Appendix 7 Narrative synthesis of the diagnostic accuracy studies: additional tables
| First author of the study (year of publication) | Population | Number of participants | Comparisons | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI)a | NPV, % (95% CI)a |
|---|---|---|---|---|---|---|---|
|
Founta (2018)54 DySIS colposcopy 1 study |
Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||
| Louwers,61 subgroup of Louwers60 | Referral strategy 1: HPV primary screening with cytology triage (subgroup with a positive hrHPV test and BMD or HG cytology) | 165 | DySISmap and colposcopy | 81 (72 to 89) | 64 (53 to 74) | 71.7 (62.8 to 80.6) | 74.2 (63.7 to 84.8) |
| DySISmap alone | 68 (58 to 78) | 69 (58 to 79) | 71.4 (61.8 to 81.1) | 65.4 (55.1 to 75.8) | |||
| Colposcopy alone | 53 (43 to 64) | 82 (73 to 90) | 77.0 (66.5 to 87.6) | 60.6 (51.2 to 70.0) | |||
| Referral strategy 2: cytology primary with hrHPV triage (subgroup with BMD cytology and a hrHPV-positive test or HG cytology, irrespective of the hrHPV test result) | 186 | DySISmap and colposcopy | 80 (73 to 88) | 61 (51 to 71) | 69.0 (60.5 to 77.6) | 74.0 (63.9 to 84.0) | |
| DySISmap alone | 65 (55 to 74) | 69 (59 to 78) | 69.2 (59.7 to 78.7) | 64.2 (54.6 to 73.9) | |||
| Colposcopy alone | 54 (44 to 64) | 78 (69 to 86) | 72.2 (61.9 to 82.6) | 60.5 (51.6 to 69.5) | |||
| Natsis (2016)74 | LG cytology, hrHPV-positive result | 287 | DySISmap and colposcopy | 82 (71.2 to 92.8)a | 36 (29.9 to 42.1)a | 20.9 (15.1 to 26.6) | 90.7 (84.8 to 96.5) |
| Colposcopy alone | 27 (14.6 to 39.4)a | 91 (87.4 to 94.6)a | 38.2 (22.0 to 54.4) | 85.8 (81.5 to 90.1) | |||
| 814 | Colposcopy alone (contemporaneous control group) | 36 (28.5 to 43.5)a | 88 (85.7 to 90.3)a | 37.1 (29.4 to 44.8) | 87.5 (85.2 to 89.8) | ||
|
Salter (2016)80 Papagiannakis (2016)83 IMPROVE-COLPO |
Abnormal cytology/pap (99%), test of cure (1%) from two colposcopy clinics (subgroup) | 210 | DySISmap and colposcopy | 83.9 (70.9 to 96.8)a | 75.4 (69.1 to 81.7)a | 37.1 (25.8 to 48.5) | 96.4 (93.4 to 99.5) |
| DySISmap alone | 74.2 (58.8 to 89.6)a | 60.3 (53.1 to 67.5)a | 24.7 (16.0 to 33.5)b | 93.1 (88.5 to 97.7) | |||
| Colposcopy | 61.3 (44.1 to 78.4)a | 91.1 (86.9 to 95.2)a | 54.3 (37.8 to 70.8)b | 93.1 (89.4 to 96.9) | |||
| LG Pap smearc (subgroup), 44 colposcopy clinics | 1857 | DySISmap and colposcopy | NR | NR | 13.3 (11.4 to 15.1) | NR | |
| 1788 | Colposcopy (retrospective matched control) | NR | NR | 10.1 (8.4 to 11.7) | NR | ||
| Tsetsa (2012)111 | Abnormal cytology | 54 | DySIS and colposcopy (3% acetic acid) | 86 | 81 | NR | NR |
| DySIS and colposcopy (4% acetic acid) | 79 | 77 | NR | NR | |||
| DySIS and colposcopy (5% acetic acid) | 82 | 77 | NR | NR |
| First author of the study (year of publication) | Population | Number of participants | Comparisons | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % | NPV, % |
|---|---|---|---|---|---|---|---|
| Macdonald (2017),95 substudy of Tidy (2018)94 | All known hrHPV genotypes | 839 | ZedScan and colposcopy | 100 | NR | NR | NR |
| ZedScan alone | 96.2 (93.1 to 98.0) | NR | NR | NR | |||
| Colposcopy alone | 83.4 (78.4 to 87.4) | NR | NR | NR | |||
| All known HPV 16 | 303 | ZedScan and colposcopy | 100 | NR | NR | NR | |
| ZedScan alone | 95.6 (90.6 to 98.2) | NR | NR | NR | |||
| Colposcopy alone | 86.9 (80.1 to 91.6) | NR | NR | NR | |||
| All known hrHPV other than HPV 16 | 536 | ZedScan and colposcopy | 100 | NR | NR | NR | |
| ZedScan alone | 96.9 (91.9 to 99.0) | NR | NR | NR | |||
| Colposcopy alone | 79.7 (71.9 to 85.8) | NR | NR | NR | |||
| Tidy (2016),102 substudy of Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Study (first author) | Number of confirmed cases of cervical cancer | Number identified by DySISmap and colposcopy | Number identified by colposcopy alone | Number of additional cases identified by DySISmap |
|---|---|---|---|---|
| Coronado50 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Livingston84 (IMPROVE-COLPO)a | 7/1839 (0.4%) | 3 (of 5 recorded) | 1 (of 5 recorded) | 2 |
| Louwers60 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| First author of the study (year of publication) | Number of test failures, n (%) | Reasons for test failure |
|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | 36 (8.1) | A total of 36 excessive movements during the measurement |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | 33 (12.1) | DySIS did not start (n = 7), no map (n = 9), examination data not saved (n = 9), no available histology (n = 5) and no DySIS colposcopy after signing informed consent (n = 3) |
| Roensbo (2015)79 | 48 (16.7) | A total of 48 women were excluded because biopsies were not sent separately (n = 28), it was not possible to classify the biopsy (n = 6), technical difficulties (n = 9) and other reasons (n = 5) |
| Soutter (2009)88 | 139 (31.4) | Software problems (n = 15), no biopsy (n = 23), unsatisfactory view (n = 45) in 45 women, not eligible (n = 6), 5% acetic acid (n = 1), lost data form (n = 1), lost biopsy slides (n = 5), blood or mucus (n = 1), biopsies from wrong point (n = 3), excessive movement (n = 2) and problem with acid-faulty acetic nozzles (n = 37) |
| Tidy (2013)103 | Phase I: 33 (13.4); Phase II: 19 (13.6) |
Phase I: ‘as part of training’ (n = 31), with incomplete clinical data (n = 2) Phase II: biopsy not coincident with EIS reading or inadequate for histology (n = 14), failure of EIS device (n = 5) |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed |
| First author of the study (year of publication) | Total number of patient analysed | Patients receiving diagnostic/treatment biopsies, n (%) | Number of diagnostic biopsies (punch biopsies) performed | Patients receiving treatment biopsies, n (%) | Number of treatment biopsies performed | Mean number of biopsies per patient |
|---|---|---|---|---|---|---|
| Budithi (2018)45 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Coronado (2016)50 | 443 | 372 (84.0) | 332 | 59 (13.3) | NR | NR |
| Founta (2018)54 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Louwers (2011)60 | 239 | 239 (100) | NR (≥ 332)a | 84 | NR (≥ 84) | 2.27 |
| Natsis (2016)74 | DySIS and colposcopy: 287 | DySIS and colposcopy: 232 (80.8) | NR | NR | NR | NR |
| Colposcopy alone: 948 | Colposcopy alone: 814 (85.9) | NR | NR | NR | NR | |
| Roensbo (2015)79 | 239 | 239 (100) | NR | NR | NR | 3–5 |
| Salter (2016)80 | 210 | 173 (82.3) | NR | NR | 39 | NR |
| Papagiannakis (2016)83 | DySIS and colposcopy: 1857 | NR | DySIS and colposcopy: 2332 | NR | NR | DySIS and colposcopy: 1.26 |
| Colposcopy alone: 1788 | Colposcopy alone: 1846 | Colposcopy alone: 1.03 | ||||
| Soutter (2009)88 | 308 | 308 (100) | 603 | 86 (27.9) | 86 | 1.96 |
| Tidy (2013)103 | 196 (Phase II) | NR | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tidy (2018)94 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Tsetsa (2012)111 | 54 | NR | NR | NR | NR | NR |
Appendix 8 Quality assessment of the implementation studies
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| Was the objective clearly stated? | ✓ | ||
| Was the setting clearly described? | ✓ | ||
| Were the methods described clearly enough to permit other researchers to duplicate the study? | ✓ | ||
| Was the survey sample likely to be representative of the population to which the findings refer? | ✓ | ||
| Was the questionnaire described adequately? | ✓ | ||
| Have the validity and reliability of the questionnaire been established? | ✓ | ||
| Was the sample size based on prestudy considerations of statistical power? | ✓ | ||
| Was statistical significance assessed appropriately? | ✓ | ||
| Were all relevant confounding factors adjusted/accounted for? | ✓ | ||
| Did the results address the objective? | ✓ | ||
| Was a satisfactory response rate achieved? | ✓ | ||
| Were the results clearly and logically presented? | ✓ | ||
| Were the tables and figures appropriate? | ✓ | ||
| Were the numbers consistent in the text and the tables? | ✓a | ||
| Were CIs given for the main results? | ✓ |
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| Was the objective clearly stated? | ✓ | ||
| Was the setting clearly described? | ✓ | ||
| Were the methods described clearly enough to permit other researchers to duplicate the study? | ✓ | ||
| Was the survey sample likely to be representative of the population to which the findings are refer? | ✓a | ||
| Was the questionnaire described adequately? | ✓ | ||
| Have the validity and reliability of the questionnaire been established? | ✓b | ||
| Was the sample size based on prestudy considerations of statistical power? | ✓ | ||
| Was statistical significance assessed appropriately? | ✓ | ||
| Were all relevant confounding factors adjusted/accounted for? | ✓c | ||
| Did the results address the objective? | ✓ | ||
| Was a satisfactory response rate achieved? | ✓ | ||
| Were the results clearly and logically presented? | ✓ | ||
| Were the tables and figures appropriate? | ✓ | ||
| Were the numbers consistent in the text and the tables? | ✓ | ||
| Were CIs given for the main results? | ✓ |
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| Was the objective clearly stated? | ✓ | ||
| Was the setting clearly described? | ✓ | ||
| Were the methods described clearly enough to permit other researchers to duplicate the study? | ✓ | ||
| Was the survey sample likely to be representative of the population to which the findings refer? | ✓ | ||
| Was the questionnaire described adequately? | ✓ | ||
| Have the validity and reliability of the questionnaire been established? | ✓ | ||
| Was the sample size based on prestudy considerations of statistical power? | ✓ | ||
| Was statistical significance assessed? | ✓ | ||
| Were all relevant confounding factors adjusted/accounted for? | ✓a | ||
| Were the statistical methods used appropriately? | ✓ | ||
| Did the results address the objective? | ✓ | ||
| Was a satisfactory response rate achieved? | ✓ | ||
| Were the results clearly and logically presented? | ✓ | ||
| Were the tables and figures appropriate? | ✓ | ||
| Were the numbers consistent in the text and the tables? | ✓ | ||
| Were CIs given for the main results? | ✓ |
| Question | Yes | No | Cannot tell |
|---|---|---|---|
| Was the objective clearly stated? | ✓ | ||
| Was the setting clearly described? | ✓ | ||
| Were the methods described clearly enough to permit other researchers to duplicate the study? | ✓ | ||
| Was the survey sample likely to be representative of the population to which the findings refer? | ✓ | ||
| Was the questionnaire described adequately? | ✓ | ||
| Have the validity and reliability of the questionnaire been established? | ✓ | ||
| Was the sample size based on prestudy considerations of statistical power? | ✓ | ||
| Was statistical significance assessed? | ✓ | ||
| Were all relevant confounding factors adjusted/accounted for? | ✓ | ||
| Were the statistical methods used appropriately? | ✓ | ||
| Did the results address the objective? | ✓ | ||
| Was a satisfactory response rate achieved? | ✓ | ||
| Were the results clearly and logically presented? | ✓ | ||
| Were the tables and figures appropriate? | ✓ | ||
| Were the numbers consistent in the text and the tables? | ✓ | ||
| Were CIs given for the main results? | ✓ |
Appendix 9 Quality assessment of the cost-effectiveness studies
| Criteria | Study | |
|---|---|---|
| Wade et al.30 | Whyte et al.124 | |
| The research question is stated | Y | Y |
| The economic importance of the research question is stated | Y | Y |
| The viewpoint(s) of the analysis are clearly stated and justified | Y | Y |
| The rationale for choosing alternative programmes or interventions compared is stated | Y | Partially |
| The alternatives being compared are clearly described | Y | Y |
| The form of economic evaluation used is stated | Y | Y |
| The choice of form of economic evaluation is justified in relation to the question addressed | Y | N |
| The source(s) of effectiveness estimates used are stated | Y | Y |
| Details of the design and results of the effectiveness study are given (if based on a single study) | Partially | Partially |
| Details of the methods of synthesis or meta-analysis of estimates are given (if based on a synthesis of a number of effectiveness studies) | NA | NA |
| The primary outcome measure(s) for the economic evaluation are clearly stated | Y | Y |
| Methods to value benefits are stated | Y | Y |
| Details of the subjects from whom valuations were obtained are given | N | N |
| Productivity changes (if included) are reported separately | NA | NA |
| The relevance of productivity changes to the study question is discussed | NA | NA |
| Quantities of resource use are reported separately from their unit costs | Y | Y |
| Methods for the estimation of quantities and unit costs are described | Y | Y |
| Currency and price date are recorded | Y | N |
| Details of currency of price adjustments for inflation or currency conversion are given | Y | N |
| Details of any model used are given | Y | Y |
| The choice of model used and the key parameters on which it is based are justified | Y | Y |
| Time horizon of costs and benefits is stated | Y | Y |
| The discount rate(s) are stated | Y | Y |
| The choice of discount rate(s) is justified | Y | Y |
Appendix 10 Main features of the economic model
FIGURE 23.
Natural history model. HS, health state; SC, sypmtomatic cancer.
FIGURE 24.
Treatment pathway after colposcopy. HG, high grade; LG, low grade.
FIGURE 25.
Test of cure. HG, high grade; LG, low grade.
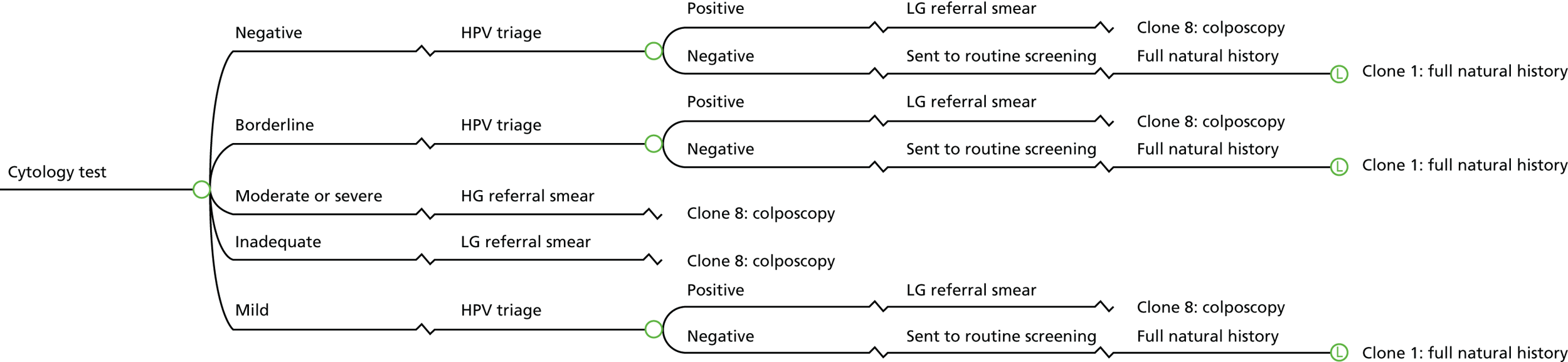
FIGURE 26.
Colposcopy at 6 months. HG, high grade; LG, low grade.

FIGURE 27.
Cervical intraepithelial neoplasia grade 1 follow-up.

FIGURE 28.
Adverse obstetric outcomes. #, no preterm birth because of treatment.
Appendix 11 Model input parameters
Underlying health state and reason for referral
| Health state; reason for referral | % | Source |
|---|---|---|
| Clear; LG result | 27.59 | NHSCSP18 |
| Clear; HG result | 1.20 | NHSCSP18 |
| HPV; LG result | 10.32 | NHSCSP18 |
| HPV; HG result | 0.89 | NHSCSP18 |
| CIN 1; LG result | 19.44 | NHSCSP18 |
| CIN 1; HG result | 2.53 | NHSCSP18 |
| CIN 2/3; LG result | 11.16 | NHSCSP18 |
| CIN 2/3; HG result | 25.97 | NHSCSP18 |
| Cancer; LG result | 0.07 | NHSCSP18 |
| Cancer; HG result | 0.82 | NHSCSP18 |
| Health state; reason for referral | % | Source |
|---|---|---|
| Clear; LG result | 32.07 | HPV primary screening pilot sites |
| Clear; HG result | 1.28 | HPV primary screening pilot sites |
| HPV; LG result | 12.6 | HPV primary screening pilot sites |
| HPV; HG result | 0.72 | HPV primary screening pilot sites |
| CIN 1; LG result | 15.14 | HPV primary screening pilot sites |
| CIN 1; HG result | 1.99 | HPV primary screening pilot sites |
| CIN 2/3; LG result | 10.68 | HPV primary screening pilot sites |
| CIN 2/3; HG result | 24.57 | HPV primary screening pilot sites |
| Cancer; LG result | 0.07 | HPV primary screening pilot sites |
| Cancer; HG result | 0.87 | HPV primary screening pilot sites |
Treatment probabilities
| Diagnosis | Failures | n | Probability of failure, % | Source |
|---|---|---|---|---|
| CIN 1 | 28 | 570 | 4.9 | Ghaem-Maghami et al.151 |
| CIN 2 | 87 | 886 | 9.8 | Ghaem-Maghami et al.151 |
| CIN 3 | 103 | 999 | 10.3 | Ghaem-Maghami et al.151 |
| CIN 2/3 | 190 | 1885 | 10.1 | Calculated from Ghaem-Maghami et al.151 |
| Age group (years) | Conception rate per 1000 women in age group | Percentage of conceptions leading to abortion | Annual probability of conception | Source |
|---|---|---|---|---|
| 25–29 | 127.2 | 18.2 | 0.1040a | ONS151 |
| 30–34 | 125.5 | 13.6 | 0.1084 | ONS151 |
| 35–39 | 68.9 | 16.7 | 0.0574 | ONS151 |
| ≥ 40 | 15.4 | 15.4 | 0.0111 | ONS151 |
Natural history model
| Parameters | Age (years) | Probability | ||
|---|---|---|---|---|
| Reported(annual unless statedˆ) | 6-month | Source | ||
| Clear to HPV | 24–29 | 0.05 | 0.0253 | Kulasingam et al.147 |
| 30–49 | 0.01 | 0.0050 | Kulasingam et al.147 | |
| ≥ 50 | 0.005 | 0.0025 | Kulasingam et al.147 | |
| HPV to clear | 15–24 | 0.7ˆ | 0.2081 | Kulasingam et al.147 |
| 25–29 | 0.5ˆ | 0.1535 | Kulasingam et al.147 | |
| 30–39 | 0.25ˆ | 0.0800 | Kulasingam et al.147 | |
| 40–49 | 0.15ˆ | 0.0488 | Kulasingam et al.147 | |
| ≥ 50 | 0.05ˆ | 0.0165 | Kulasingam et al.147 | |
| HPV to CIN 1 | – | 0.9 × 0.06 | 0.9 × 0.0305 | Kulasingam et al.147 |
| HPV to CIN 2/3 | – | 0.1 × 0.06 | 0.1 × 0.0305 | Kulasingam et al.147 |
| CIN 1 to clear | 15–34 | 0.9 × 0.1 | 0.9 × 0.0513 | Kulasingam et al.147 |
| ≥ 35 | 0.9 × 0.06 | 0.9 × 0.0305 | Kulasingam et al.147 | |
| CIN 1 to HPV | 15–34 | 0.1 × 0.1 | 0.1 × 0.0513 | Kulasingam et al.147 |
| ≥ 35 | 0.1 × 0.06 | 0.1 × 0.0305 | Kulasingam et al.147 | |
| CIN 1 to CIN 2/3 | 15–34 | 0.02 | 0.0101 | Kulasingam et al.147 |
| ≥ 35 | 0.06 | 0.0305 | Kulasingam et al.147 | |
| CIN 2/3 to clear | – | 0.5 × 0.06 | 0.5 × 0.0305 | Kulasingam et al.147 |
| CIN 2/3 to CIN 1 | – | 0.5 × 0.06 | 0.5 × 0.0305 | Kulasingam et al.147 |
| CIN 2/3 to cancer | 15–29 | 0.01 | 0.0050 | Kulasingam et al.147 |
| ≥ 30 | 0.04 | 0.0202 | Kulasingam et al.147 | |
| Parameters | Cancer stage | Reported value | 6-month probability | Source | |
|---|---|---|---|---|---|
| Probability of symptoms: undetected cancer | Local | 0.0174/1 month | 0.1000 | Campos et al.148 | |
| Regional | 0.0735/1 month | 0.3675 | Campos et al.148 | ||
| Distant | 0.1746/1 month | 0.6838 | Campos et al.148 | ||
| Progression: undetected cancer | Local to regional | 0.02/1 month | 0.1142 | Campos et al.148 | |
| Regional to distant | 0.025/1 month | 0.1409 | Campos et al.148 | ||
| Mortality: undetected cancer | Local | 0.0016/1 month | 0.0096 | Campos et al.148 | |
| Regional | 0.0095/1 month | 0.0557 | Campos et al.148 | ||
| Distant | 0.0293/1 month | 0.1634 | Campos et al.148 | ||
| Mortality: detected cancer | |||||
| 1 year post diagnosis | Local | 0.0016/1 month | 0.0096 | Campos et al.148 | |
| Regional | 0.0095/1 month | 0.0557 | Campos et al.148 | ||
| Distant | 0.0293/1 month | 0.1634 | Campos et al.148 | ||
| 2–3 years post diagnosis | Local | 0.0014/1 month | 0.0084 | Campos et al.148 | |
| Regional | 0.0078/1 month | 0.0459 | Campos et al.148 | ||
| Distant | 0.0195/1 month | 0.1114 | Campos et al.148 | ||
| 4–5 years post diagnosis | Local | 0.0009/1 month | 0.0054 | Campos et al.148 | |
| Regional | 0.0036/1 month | 0.0214 | Campos et al.148 | ||
| Distant | 0.0076/1 month | 0.0447 | Campos et al.148 | ||
| Age group (years) | Total number of deaths | Deaths attributable to cervical cancer | Deaths excluding cervical cancer | Population size | Annual mortality rate | 6-month probability of death | Source |
|---|---|---|---|---|---|---|---|
| 15–24 | 678 | 2 | 676 | 3,516,313 | 0.00019 | 0.00010 | ONS153 |
| 25–34 | 1368 | 54 | 1314 | 3,927,723 | 0.00033 | 0.00017 | ONS153 |
| 35–44 | 3265 | 92 | 3173 | 3,754,387 | 0.00085 | 0.00042 | ONS153 |
| 45–54 | 8438 | 119 | 8319 | 4,116,650 | 0.00202 | 0.00101 | ONS153 |
| 55–64 | 16,389 | 108 | 16,281 | 3,334,140 | 0.00488 | 0.00244 | ONS153 |
| 65–74 | 35,752 | 137 | 35,615 | 2,917,683 | 0.01221 | 0.00612 | ONS153 |
| 75–84 | 72,044 | 135 | 71,909 | 1,837,553 | 0.03913 | 0.01976 | ONS153 |
| ≥ 85 | 132,917 | 73 | 132,844 | 894,520 | 0.14851 | 0.07724 | ONS153 |
Resource utilisation and cost data
| Cost component | Technology | Source | ||
|---|---|---|---|---|
| Colposcopy alone | DySIS | ZedScan | ||
| Assumed useful life of equipment (years) | 15 | 5 | 5 | Clinical advisors |
| Purchase price (£) | 10,734 | 30,500 | 3000 | Manufacturers |
| Equivalent annual cost (£)a | 900 | 6527 | 642 | Calculations based on cost components |
| Annual maintenance cost (£) | 1073 | 530 | 0 | Manufacturers |
| Disposables (per patient, £) | 2.15 | 3.5 | 30 | Manufacturers |
| Total cost per patient (£)b | 3.75 | 9.24 | 30.52 | Calculations based on cost components |
| Treatment | Unit cost (£), 2016 prices | Source |
|---|---|---|
| Colposcopy examination only | 175 | NHS Reference Costs 2015 to 2016 154 |
| Colposcopy with biopsy | 222 | NHS Reference Costs 2015 to 2016 154 |
| Colposcopy with LLETZ | 238 | NHS Reference Costs 2015 to 2016 154 |
| Treatment | Technology | Cost (£) per treatment |
|---|---|---|
| Colposcopy examination only | Colposcopy alone | 175 |
| DySIS | 180.49 (175 – 3.75 ± 9.24) | |
| ZedScan | 205.52 (175 ± 30.52) | |
| Diagnostic biopsy | 47 (222 – 175) | |
| LLETZ | 63 (238 – 175) | |
| Cytology test | 37.19 | |
| HPV test | 29.66 | |
| Cancer treatment by stage | Cost (£) per event | Source | |
|---|---|---|---|
| 2006 | 2016 | ||
| Stage I | 2785 | 3434 | Martin-Hirsch et al.157 |
| Stage II | 4448 | 5484 | Martin-Hirsch et al.157 |
| Stage III | 12,562 | 15,487 | Martin-Hirsch et al.157 |
| Stage IV | 12,777 | 15,752 | Martin-Hirsch et al.157 |
| Local | – | 4459 | Calculated from Martin-Hirsch et al.157 |
| Regional | – | 15,487 | Calculated from Martin-Hirsch et al.157 |
| Distant | – | 15,752 | Calculated from Martin-Hirsch et al.157 |
Health outcomes
| Screening episode | QALY decrement | Source |
|---|---|---|
| Negative cytology and/or HPV | 0.0062 | Simonella et al.158 |
| False-positive referral for colposcopy | 0.0276 | Simonella et al.158 |
| Diagnosed CIN 1 | 0.0276 | Simonella et al.158 |
| Treatment of CIN | 0.0296 | Simonella et al.158 |
Characteristics of the base-case analyses
| Parameters | Value/source | Comment |
|---|---|---|
| Number of cycles | 120 (60 years) | |
| Discount rate | 3.5% | |
| Structure | ||
| Adverse obstetric outcomes | Yes | Excess risk of preterm delivery |
| See and treat for HG results only | Yes | |
| Use of ZedScan | Diagnostic colposcopy only | |
| Treatment pathways | NHSCSP guidelines15 and clinical experts | |
| Attendance rate | 100% | No patients were lost to follow-up |
| Input parameters | ||
| Diagnostic accuracy | ||
| Colposcopy | Regression | Cut-off CIN 2+ |
| DySIS | Regression | Cut-off CIN 2+ |
| ZedScan | Tidy et al.94 | Cut-off CIN 2+ |
| Cytology | S Eggington, personal communication | |
| HPV test (HPV triage) | Cotton et al.127 | |
| HPV test (HPV primary) | Kitchener et al.149 | |
| Initial population | ||
| Age at start | 36 | Average age under the NHSCSP guideline15 |
| HPV triage protocol | NHSCSP18 | |
| HPV primary screening protocol | Pilot sites | No genotyping of HPV, 16/18 |
| Treatment probabilities | ||
| Cured after LLETZ | Ghaem-Maghami et al.150 | |
| Adverse obstetric outcomes | Kyrgiou et al.119 | |
| Natural history | ||
| CIN | Kulasingam et al.147 | |
| Cancer | Campos et al.148 | |
| All-cause mortality | ONS data153 | |
| Costs | Value/source | Comment |
|---|---|---|
| Colposcopy alone | NHS Reference Costs 2015 to 2016 154 | |
| DySIS | Manufacturer | |
| ZedScan | Manufacturer | |
| Number of patients treated per colposcope per year | 1229, clinical advisors | |
| LLETZ and biopsy | NHS Reference Costs 2015 to 2016 154 | |
| Cytology and HPV tests | TOMBOLA study130 | |
| Cancer costs | Martin-Hirsch et al.155 | |
| Adverse obstetric outcomes | Lomas et al.157 | |
| Health outcomes | ||
| Screening disutility | Simonella et al.158 | Screening episodes |
| Baseline | Kind et al.159 | Age and gender specific |
| Cancer | Goldie et al.5 | 5-year decrement |
| Preterm births | Lomas et al.157 | |
| Health state; reason for referral | Pilot site | |
|---|---|---|
| No genotyping (%) | Genotyping (%) | |
| Clear; LG result | 25.42 | 34.84 |
| Clear; HG result | 1.27 | 1.28 |
| HPV; LG result | 18.07 | 10.32 |
| HPV; HG result | 0.99 | 0.61 |
| CIN 1; LG result | 16.85 | 14.43 |
| CIN 1; HG result | 2.18 | 1.91 |
| CIN 2/3; LG result | 9.63 | 11.12 |
| CIN 2/3; HG result | 24.71 | 24.52 |
| Cancer; LG result | 0.11 | 0.05 |
| Cancer; HG result | 0.78 | 0.92 |
Appendix 12 Base-case analysis: secondary outcomes
| Grade of referral smear, by clinic and technology | Secondary outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Missed CIN 2+ | Developed cancer | Died from cancer | LLETZ | Unnecessary treatment (clear, HPV) | Unnecessary treatment (CIN 1) | Unnecessary diagnostic biopsy | Preterm delivery | |
| See-and-treat clinics | ||||||||
| All referrals | ||||||||
| Colposcopy alone | 69 | 43 | 9 | 466 | 9 | 18 | 139 | 4.0 |
| DySIS | 30 | 34 | 7 | 501 | 22 | 39 | 229 | 4.4 |
| ZedScan | 3 | 29 | 6 | 524 | 30 | 52 | 291 | 4.8 |
| LG referrals | ||||||||
| Colposcopy alone | 91 | 51 | 10 | 276 | 6 | 18 | 131 | 1.4 |
| DySIS | 39 | 40 | 8 | 318 | 15 | 39 | 245 | 1.8 |
| ZedScan | 4 | 33 | 6 | 343 | 22 | 51 | 323 | 2.1 |
| HG referrals | ||||||||
| Colposcopy alone | 22 | 26 | 7 | 879 | 14 | 16 | 149 | 10.0 |
| DySIS | 9 | 22 | 6 | 902 | 34 | 36 | 192 | 10.3 |
| ZedScan | 1 | 20 | 6 | 916 | 49 | 50 | 220 | 10.5 |
| Watchful-waiting clinics | ||||||||
| All referrals | ||||||||
| Colposcopy alone | 69 | 44 | 9 | 449 | 0 | 0 | 147 | 3.9 |
| DySIS | 30 | 37 | 8 | 465 | 0 | 0 | 252 | 4.1 |
| ZedScan | 3 | 32 | 7 | 477 | 0 | 0 | 325 | 4.2 |
| LG referrals | ||||||||
| Colposcopy alone | 92 | 52 | 10 | 259 | 0 | 0 | 137 | 1.3 |
| DySIS | 39 | 43 | 8 | 283 | 0 | 0 | 260 | 1.7 |
| ZedScan | 4 | 37 | 7 | 299 | 0 | 0 | 347 | 2.0 |
| HG referrals | ||||||||
| Colposcopy alone | 22 | 27 | 7 | 862 | 0 | 0 | 164 | 9.5 |
| DySIS | 10 | 24 | 7 | 864 | 0 | 0 | 230 | 9.4 |
| ZedScan | 1 | 23 | 6 | 866 | 0 | 0 | 276 | 9.5 |
| Grade of referral smear, by clinic and technology | Secondary outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Missed CIN 2+ | Developed cancer | Died from cancer | LLETZ | Unnecessary treatment (clear, HPV) | Unnecessary treatment (CIN 1) | Unnecessary diagnostic biopsy | Preterm delivery | |
| See-and-treat clinics | ||||||||
| All referrals | ||||||||
| Colposcopy alone | 82 | 33 | 7 | 446 | 8 | 14 | 164 | 3.9 |
| DySIS | 34 | 25 | 5 | 478 | 20 | 30 | 296 | 4.2 |
| ZedScan | 4 | 20 | 4 | 498 | 28 | 40 | 386 | 4.5 |
| LG referrals | ||||||||
| Colposcopy alone | 103 | 38 | 7 | 263 | 6 | 14 | 164 | 1.3 |
| DySIS | 42 | 28 | 5 | 300 | 15 | 30 | 323 | 1.6 |
| ZedScan | 5 | 22 | 4 | 322 | 21 | 40 | 433 | 2.0 |
| HG referrals | ||||||||
| Colposcopy alone | 30 | 21 | 6 | 883 | 14 | 14 | 162 | 10.0 |
| DySIS | 12 | 18 | 5 | 904 | 33 | 31 | 231 | 10.2 |
| ZedScan | 1 | 16 | 5 | 917 | 46 | 42 | 276 | 10.4 |
| Watchful-waiting clinics | ||||||||
| All referrals | ||||||||
| Colposcopy alone | 82 | 34 | 7 | 432 | 0 | 0 | 172 | 3.8 |
| DySIS | 34 | 27 | 6 | 450 | 0 | 0 | 316 | 4.0 |
| ZedScan | 4 | 22 | 5 | 460 | 0 | 0 | 417 | 4.1 |
| LG referrals | ||||||||
| Colposcopy alone | 104 | 39 | 7 | 251 | 0 | 0 | 170 | 1.2 |
| DySIS | 43 | 30 | 6 | 273 | 0 | 0 | 337 | 1.6 |
| ZedScan | 5 | 24 | 5 | 288 | 0 | 0 | 454 | 1.9 |
| HG referrals | ||||||||
| Colposcopy alone | 30 | 22 | 6 | 869 | 0 | 0 | 177 | 9.6 |
| DySIS | 12 | 20 | 6 | 871 | 0 | 0 | 267 | 9.5 |
| ZedScan | 1 | 18 | 5 | 873 | 0 | 0 | 330 | 9.5 |
Appendix 13 Sensitivity and scenario analyses results
Scenario analysis 1: diagnostic accuracy from Louwers et al.60 for colposcopy and Dynamic Spectral Imaging System
FIGURE 29.
Uncertainty around diagnostic accuracy: DySIS vs. colposcopy alone. (a) HPV triage; and (b) HPV primary screening. HG, high grade; LG, low grade.
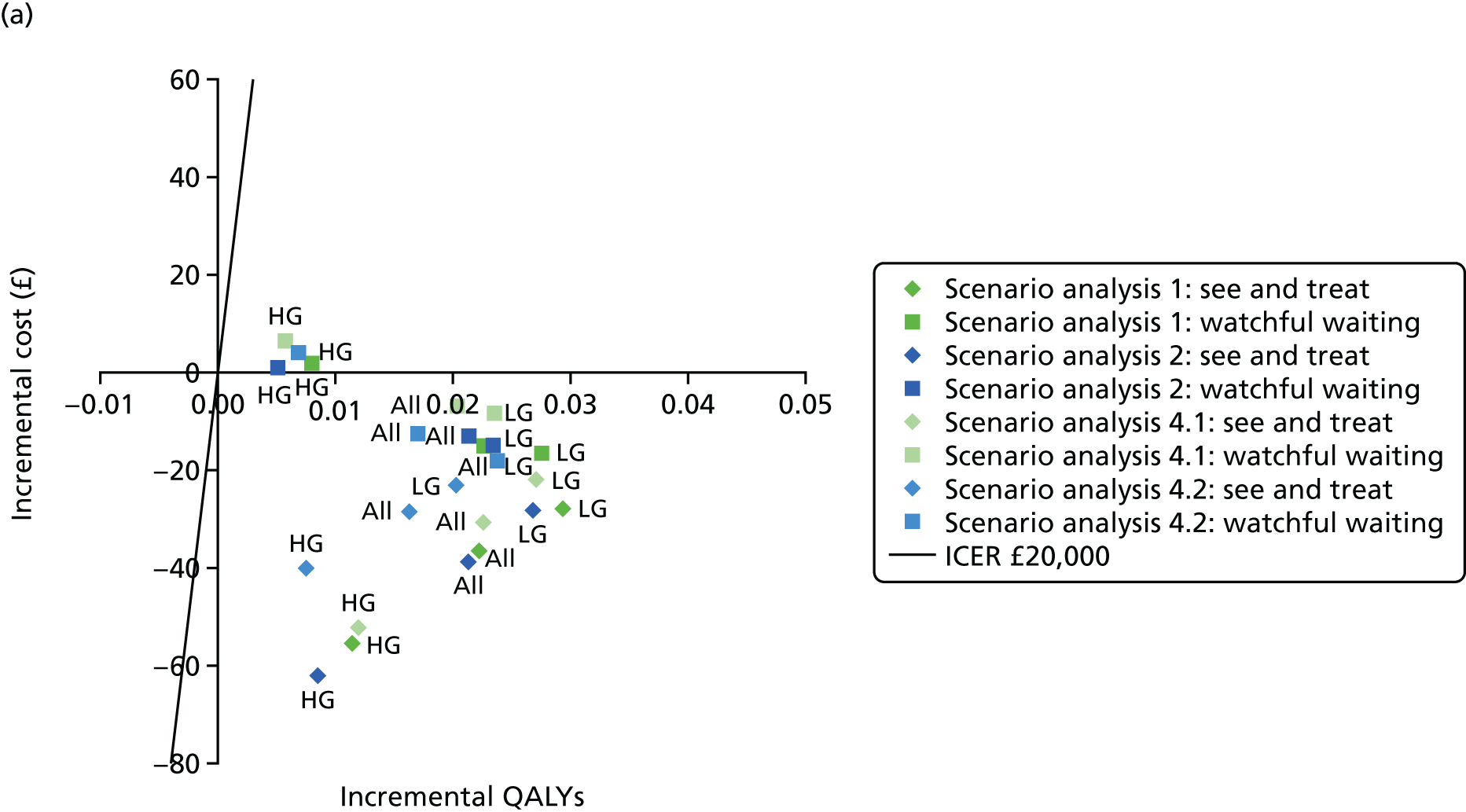
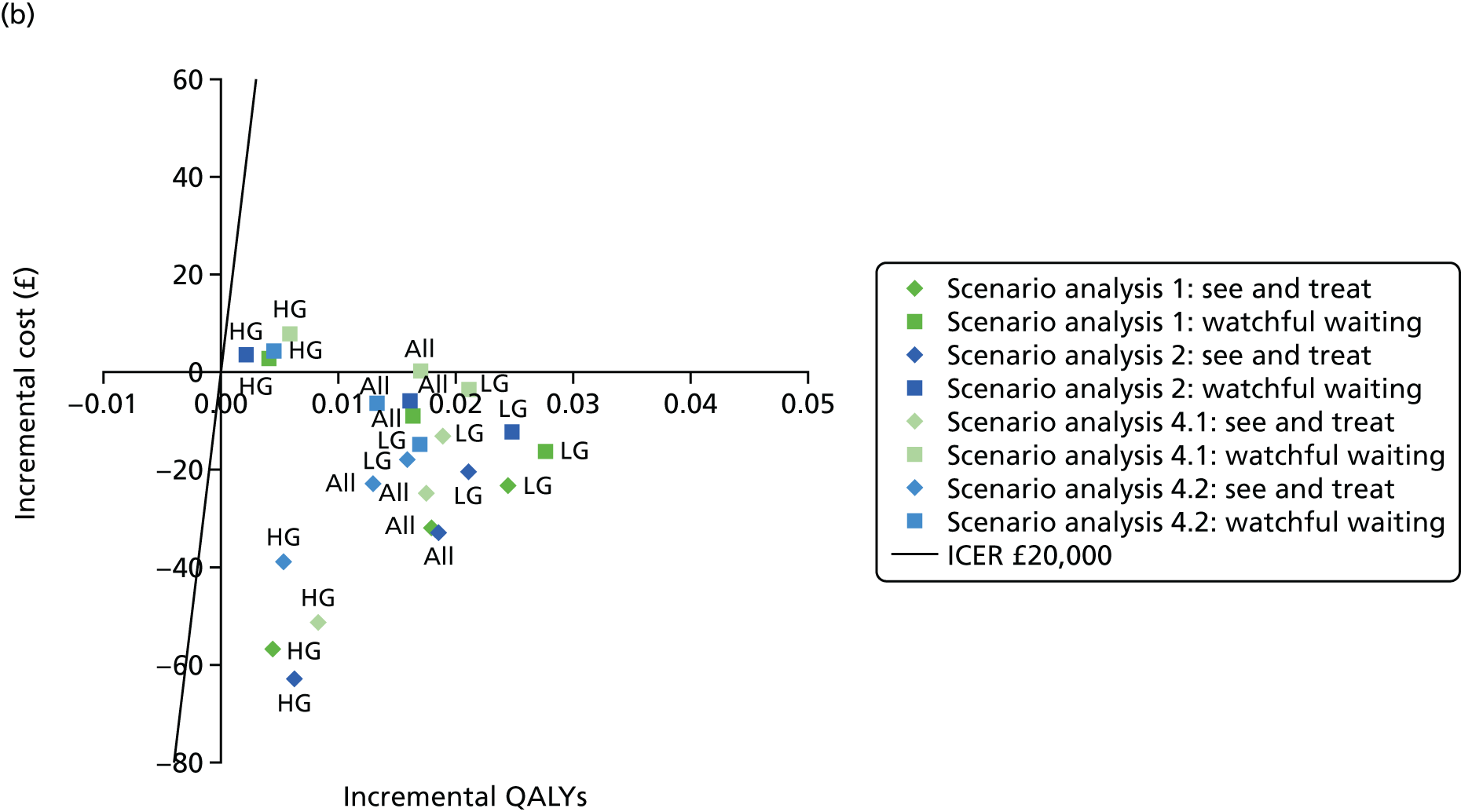
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 916.73 | 19.16145 | |||
| DySIS | 880.39 | 19.18359 | –36.35 | 0.02214 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 806.20 | 19.15627 | |||
| DySIS | 778.57 | 19.18554 | –27.63 | 0.02928 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1155.24 | 19.15946 | |||
| DySIS | 1099.83 | 19.17080 | –55.41 | 0.01134 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 964.91 | 19.15709 | |||
| DySIS | 949.98 | 19.17972 | –14.93 | 0.02263 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 826.11 | 19.15563 | |||
| DySIS | 809.60 | 19.18314 | –16.51 | 0.02751 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1257.65 | 19.15866 | |||
| DySIS | 1259.59 | 19.16660 | 1.94 | 0.00794 | 245 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 864.77 | 19.17079 | |||
| DySIS | 832.84 | 19.18872 | –31.93 | 0.01794 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 746.32 | 19.18168 | |||
| DySIS | 722.98 | 19.20610 | –23.34 | 0.02442 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1144.79 | 19.16330 | |||
| DySIS | 1087.97 | 19.16779 | –56.81 | 0.00448 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 906.82 | 19.17033 | |||
| DySIS | 897.72 | 19.18658 | –9.10 | 0.01625 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 764.55 | 19.17674 | |||
| DySIS | 748.24 | 19.20428 | –16.31 | 0.02754 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1243.12 | 19.15871 | |||
| DySIS | 1246.03 | 19.16275 | 2.92 | 0.00404 | 722 |
Scenario analysis 2: additional data from Louwers et al.60 for colposcopy and Dynamic Spectral Imaging System
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 915.59 | 19.16400 | |||
| DySIS | 877.11 | 19.18527 | –38.47 | 0.02126 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 803.82 | 19.15971 | |||
| DySIS | 775.53 | 19.18647 | –28.30 | 0.02676 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1158.06 | 19.16338 | |||
| DySIS | 1095.93 | 19.17186 | –62.14 | 0.00848 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 961.66 | 19.16057 | |||
| DySIS | 948.72 | 19.18188 | –12.94 | 0.02131 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 823.01 | 19.16003 | |||
| DySIS | 808.43 | 19.18335 | –14.59 | 0.02332 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1255.99 | 19.16118 | |||
| DySIS | 1256.93 | 19.16619 | 0.94 | 0.00501 | 188 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 862.25 | 19.17320 | |||
| DySIS | 829.31 | 19.19171 | –32.94 | 0.01850 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 741.00 | 19.18583 | |||
| DySIS | 720.31 | 19.20695 | –20.69 | 0.02112 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1146.90 | 19.16260 | |||
| DySIS | 1083.95 | 19.16881 | –62.95 | 0.00621 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 901.92 | 19.17230 | |||
| DySIS | 895.97 | 19.18840 | –5.95 | 0.01609 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 759.00 | 19.18034 | |||
| DySIS | 746.69 | 19.20509 | –12.31 | 0.02475 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1240.88 | 19.16197 | |||
| DySIS | 1244.50 | 19.16419 | 3.62 | 0.00222 | 1633 |
Scenario analysis 3: diagnostic accuracy from Tidy et al.110 for colposcopy
FIGURE 30.
Uncertainty around diagnostic accuracy: ZedScan vs. colposcopy alone. (a) HPV triage; and (b) HPV primary screening. HG, high grade; LG, low grade.
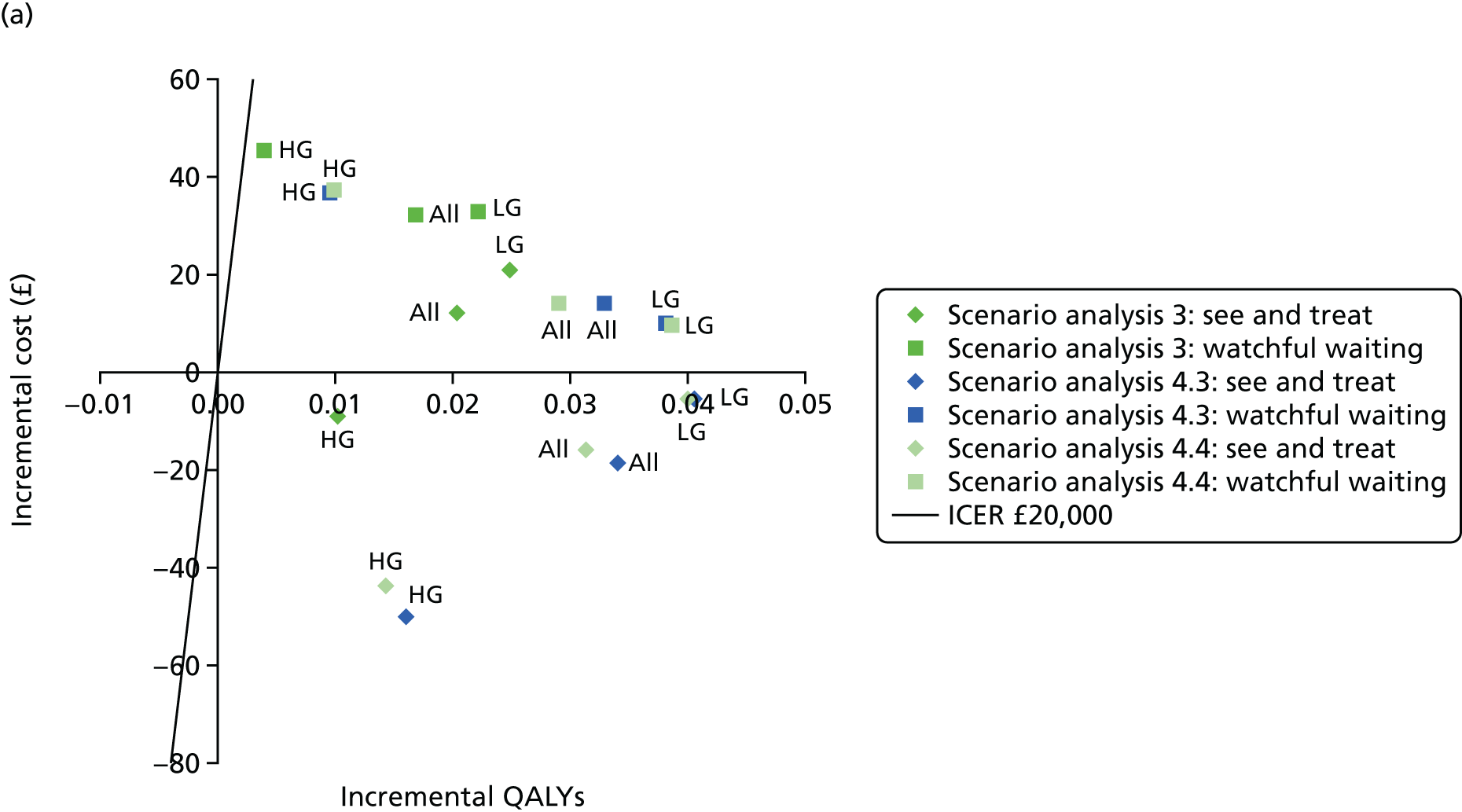

| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 873.87 | 19.17860 | |||
| ZedScan | 885.91 | 19.19901 | 12.04 | 0.02041 | 590 |
| LG referrals | |||||
| Colposcopy alone | 768.32 | 19.17826 | |||
| ZedScan | 789.30 | 19.20307 | 20.98 | 0.02482 | 845 |
| HG referrals | |||||
| Colposcopy alone | 1101.18 | 19.16626 | |||
| ZedScan | 1091.97 | 19.17651 | –9.22 | 0.01024 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 933.64 | 19.17676 | |||
| ZedScan | 965.87 | 19.19363 | 32.23 | 0.01687 | 1910 |
| LG referrals | |||||
| Colposcopy alone | 790.35 | 19.17872 | |||
| ZedScan | 823.19 | 19.20082 | 32.85 | 0.02210 | 1486 |
| HG referrals | |||||
| Colposcopy alone | 1243.40 | 19.16514 | |||
| ZedScan | 1288.82 | 19.16911 | 45.42 | 0.00397 | 11,448 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 822.80 | 19.18722 | |||
| ZedScan | 844.41 | 19.20206 | 21.61 | 0.01483 | 1457 |
| LG referrals | |||||
| Colposcopy alone | 708.37 | 19.20451 | |||
| ZedScan | 744.85 | 19.22007 | 36.49 | 0.01557 | 2344 |
| HG referrals | |||||
| Colposcopy alone | 1088.46 | 19.16641 | |||
| ZedScan | 1082.27 | 19.17347 | –6.19 | 0.00707 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 876.13 | 19.18917 | |||
| ZedScan | 918.78 | 19.19977 | 42.65 | 0.01060 | 4023 |
| LG referrals | |||||
| Colposcopy alone | 726.69 | 19.19959 | |||
| ZedScan | 770.26 | 19.21984 | 43.57 | 0.02025 | 2152 |
| HG referrals | |||||
| Colposcopy alone | 1228.18 | 19.16472 | |||
| ZedScan | 1276.58 | 19.16668 | 48.39 | 0.00196 | 24,686 |
Scenario analysis 4.1: Dynamic Spectral Imaging System – lower-bound specificity (2.5%) and correlated sensitivity
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.55 | 19.16672 | |||
| DySIS | 873.06 | 19.18922 | –30.48 | 0.02250 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.82 | 19.16515 | |||
| DySIS | 771.78 | 19.19215 | –22.03 | 0.02700 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.70 | 19.16221 | |||
| DySIS | 1087.56 | 19.17411 | –52.13 | 0.01190 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.16 | 19.16161 | |||
| DySIS | 946.17 | 19.18190 | –6.98 | 0.02029 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 812.81 | 19.16406 | |||
| DySIS | 804.49 | 19.18743 | –8.33 | 0.02337 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1252.23 | 19.16011 | |||
| DySIS | 1258.67 | 19.16576 | 6.44 | 0.00565 | 1140 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.17 | 19.17452 | |||
| DySIS | 825.20 | 19.19201 | –24.97 | 0.01749 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 731.84 | 19.18497 | |||
| DySIS | 718.51 | 19.20383 | –13.33 | 0.01885 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1126.46 | 19.16164 | |||
| DySIS | 1075.05 | 19.16989 | –51.41 | 0.00825 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.53 | 19.17496 | |||
| DySIS | 894.58 | 19.19188 | 0.05 | 0.01692 | 3 |
| LG referrals | |||||
| Colposcopy alone | 748.21 | 19.18388 | |||
| DySIS | 744.58 | 19.20499 | –3.63 | 0.02112 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1237.65 | 19.15840 | |||
| DySIS | 1245.44 | 19.16424 | 7.79 | 0.00584 | 1334 |
Scenario analysis 4.2: Dynamic Spectral Imaging System – upper-bound specificity (97.5%) and correlated sensitivity
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 902.94 | 19.16637 | |||
| DySIS | 874.62 | 19.18261 | –28.33 | 0.01624 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 794.28 | 19.16183 | |||
| DySIS | 771.24 | 19.18206 | –23.04 | 0.02023 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.09 | 19.16116 | |||
| DySIS | 1099.15 | 19.16860 | –39.94 | 0.00744 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 952.89 | 19.16303 | |||
| DySIS | 940.43 | 19.18000 | –12.46 | 0.01696 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 812.37 | 19.16024 | |||
| DySIS | 794.50 | 19.18385 | –17.87 | 0.02360 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1251.78 | 19.15972 | |||
| DySIS | 1256.10 | 19.16660 | 4.32 | 0.00688 | 628 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 849.66 | 19.17576 | |||
| DySIS | 826.70 | 19.18872 | –22.96 | 0.01296 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 733.04 | 19.19014 | |||
| DySIS | 715.06 | 19.20599 | –17.98 | 0.01585 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1126.83 | 19.16170 | |||
| DySIS | 1087.79 | 19.16707 | –39.04 | 0.00537 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.39 | 19.17731 | |||
| DySIS | 887.97 | 19.19055 | –6.42 | 0.01323 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 748.25 | 19.18760 | |||
| DySIS | 733.48 | 19.20442 | –14.77 | 0.01681 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1237.22 | 19.16004 | |||
| DySIS | 1241.84 | 19.16457 | 4.62 | 0.00453 | 1021 |
Scenario analysis 4.3: ZedScan – lower-bound specificity (2.5%) and upper-bound sensitivity (97.5%)
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.55 | 19.16672 | |||
| ZedScan | 885.01 | 19.20070 | –18.54 | 0.03398 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.82 | 19.16515 | |||
| ZedScan | 788.41 | 19.20559 | –5.40 | 0.04044 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.70 | 19.16221 | |||
| ZedScan | 1089.75 | 19.17831 | –49.95 | 0.01611 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.16 | 19.16161 | |||
| ZedScan | 967.10 | 19.19443 | 13.94 | 0.03282 | 425 |
| LG referrals | |||||
| Colposcopy alone | 812.81 | 19.16406 | |||
| ZedScan | 822.94 | 19.20219 | 10.12 | 0.03814 | 265 |
| HG referrals | |||||
| Colposcopy alone | 1252.23 | 19.16011 | |||
| ZedScan | 1289.12 | 19.16965 | 36.88 | 0.00954 | 3865 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.17 | 19.17452 | |||
| ZedScan | 843.97 | 19.20515 | –6.20 | 0.03063 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 731.84 | 19.18497 | |||
| ZedScan | 745.03 | 19.21610 | 13.19 | 0.03112 | 424 |
| HG referrals | |||||
| Colposcopy alone | 1126.46 | 19.16164 | |||
| ZedScan | 1079.65 | 19.17438 | –46.81 | 0.01274 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.53 | 19.17496 | |||
| ZedScan | 920.70 | 19.20378 | 26.17 | 0.02881 | 908 |
| LG referrals | |||||
| Colposcopy alone | 748.21 | 19.18388 | |||
| ZedScan | 770.96 | 19.21819 | 22.74 | 0.03431 | 663 |
| HG referrals | |||||
| Colposcopy alone | 1237.65 | 19.15840 | |||
| ZedScan | 1278.66 | 19.16758 | 41.00 | 0.00918 | 4466 |
Scenario analysis 4.4: ZedScan: upper-bound specificity (97.5%) and lower-bound sensitivity (2.5%)
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 902.94 | 19.16637 | |||
| ZedScan | 886.84 | 19.19761 | –16.10 | 0.03124 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 794.28 | 19.16183 | |||
| ZedScan | 788.79 | 19.20177 | –5.49 | 0.03994 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.09 | 19.16116 | |||
| ZedScan | 1095.33 | 19.17545 | –43.76 | 0.01429 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 952.89 | 19.16303 | |||
| ZedScan | 966.94 | 19.19209 | 14.05 | 0.02906 | 484 |
| LG referrals | |||||
| Colposcopy alone | 812.37 | 19.16024 | |||
| ZedScan | 822.06 | 19.19885 | 9.70 | 0.03861 | 251 |
| HG referrals | |||||
| Colposcopy alone | 1251.78 | 19.15972 | |||
| ZedScan | 1289.24 | 19.16956 | 37.46 | 0.00984 | 3805 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 849.66 | 19.17576 | |||
| ZedScan | 844.47 | 19.20290 | –5.20 | 0.02714 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 733.04 | 19.19014 | |||
| ZedScan | 745.03 | 19.21860 | 11.99 | 0.02846 | 421 |
| HG referrals | |||||
| Colposcopy alone | 1126.83 | 19.16170 | |||
| ZedScan | 1085.86 | 19.17054 | –40.97 | 0.00884 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.39 | 19.17731 | |||
| ZedScan | 918.89 | 19.20128 | 24.50 | 0.02396 | 1022 |
| LG referrals | |||||
| Colposcopy alone | 748.25 | 19.18760 | |||
| ZedScan | 768.47 | 19.22001 | 20.22 | 0.03241 | 624 |
| HG referrals | |||||
| Colposcopy alone | 1237.22 | 19.16004 | |||
| ZedScan | 1277.83 | 19.16834 | 40.61 | 0.00830 | 4891 |
Scenario analysis 5.1: number of patients per colposcope per year – 50% compared with the base case
FIGURE 31.
Uncertainty around costs: HPV triage. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.

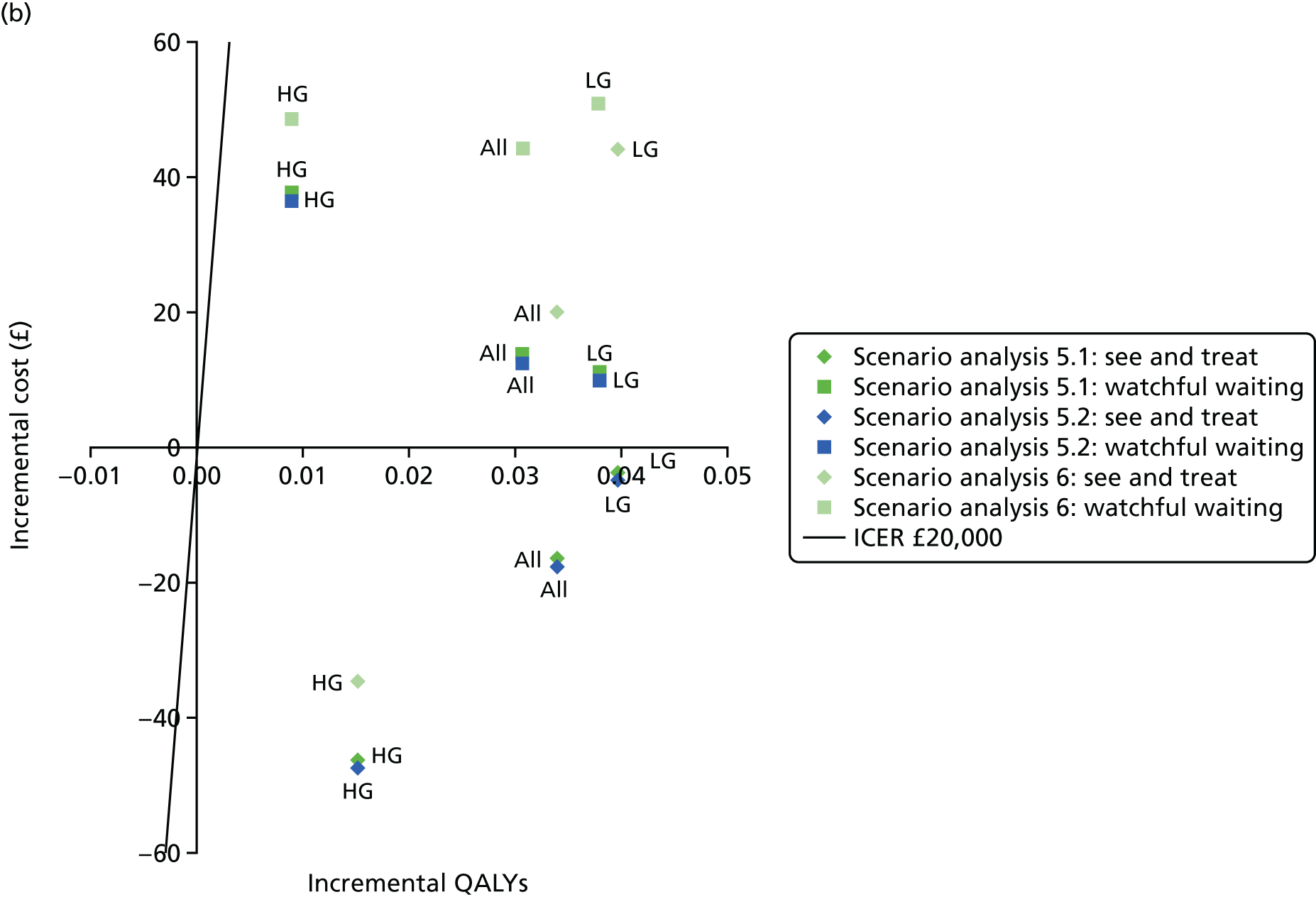

FIGURE 32.
Uncertainty around costs: HPV primary screening. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.
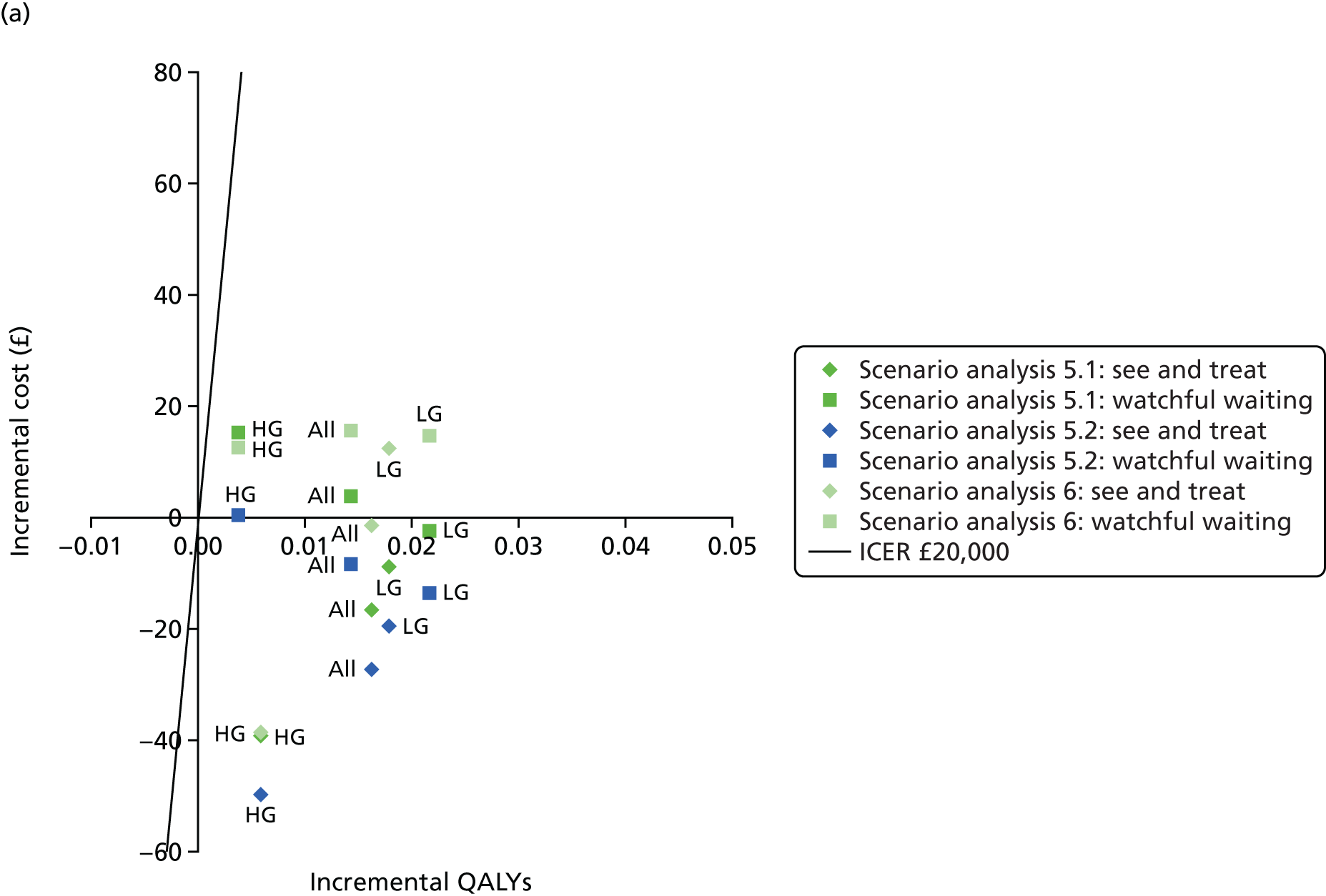
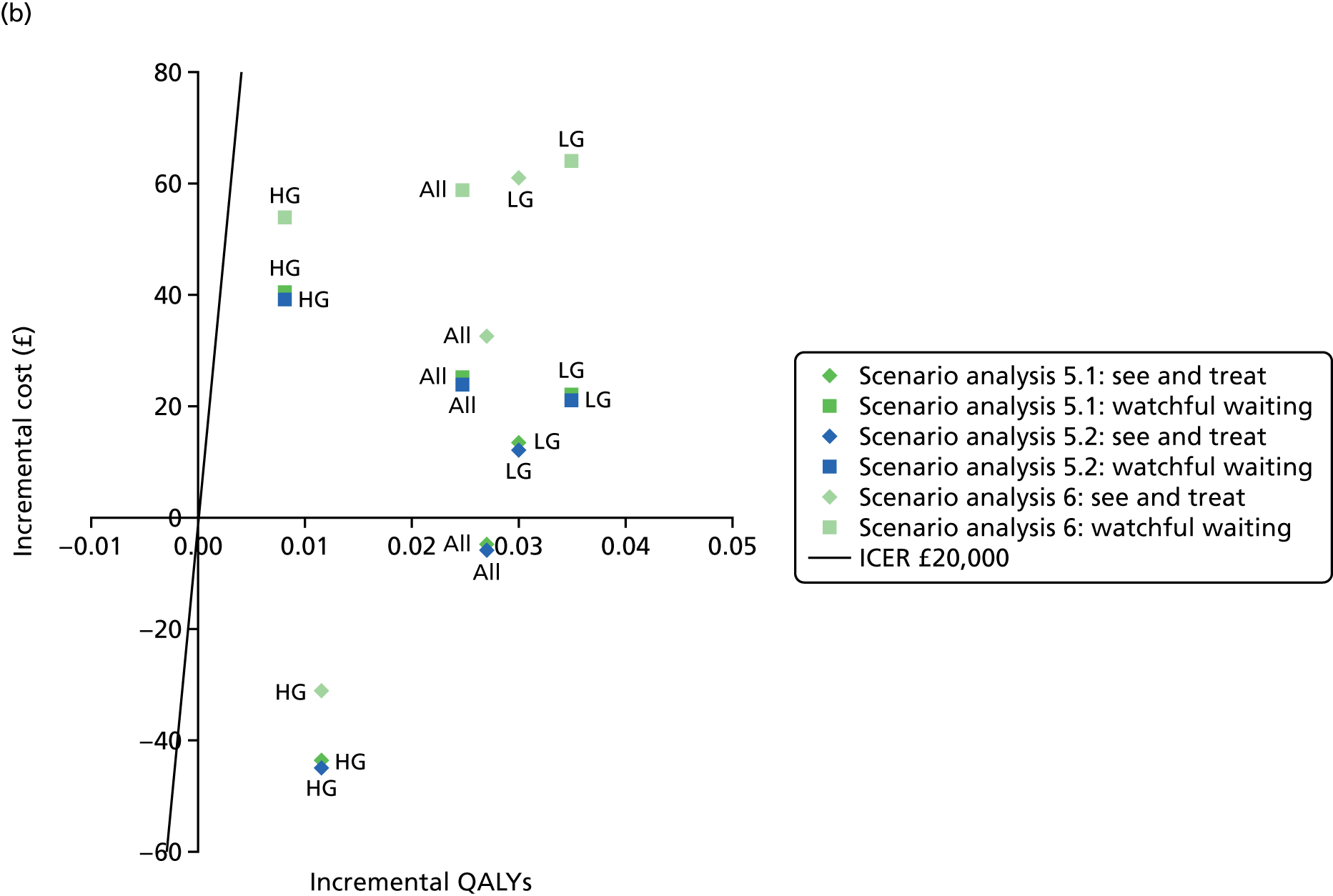

| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| DySIS | 879.49 | 19.18516 | –23.79 | 0.02016 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| DySIS | 777.71 | 19.18794 | –16.26 | 0.02464 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| DySIS | 1098.77 | 19.17156 | –40.36 | 0.01034 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| DySIS | 949.78 | 19.18194 | –3.24 | 0.01908 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| DySIS | 806.96 | 19.18601 | –5.89 | 0.02318 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| DySIS | 1266.47 | 19.16580 | 14.40 | 0.00571 | 2521 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| ZedScan | 886.72 | 19.19901 | –16.56 | 0.03401 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| ZedScan | 790.09 | 19.20307 | –3.88 | 0.03978 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| ZedScan | 1092.79 | 19.17651 | –46.34 | 0.01529 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| ZedScan | 966.69 | 19.19363 | 13.68 | 0.03078 | 444 |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| ZedScan | 824.01 | 19.20082 | 11.16 | 0.03799 | 294 |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| ZedScan | 1289.67 | 19.16911 | 37.60 | 0.00903 | 4164 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 879.49 | 19.18516 | |||
| ZedScan | 886.72 | 19.19901 | 7.22 | 0.01385 | 522 |
| LG referrals | |||||
| DySIS | 777.71 | 19.18794 | |||
| ZedScan | 790.09 | 19.20307 | 12.38 | 0.01514 | 818 |
| HG referrals | |||||
| DySIS | 1098.77 | 19.17156 | |||
| ZedScan | 1092.79 | 19.17651 | –5.99 | 0.00495 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 949.78 | 19.18194 | |||
| ZedScan | 966.69 | 19.19363 | 16.91 | 0.01170 | 1446 |
| LG referrals | |||||
| DySIS | 806.96 | 19.18601 | |||
| ZedScan | 824.01 | 19.20082 | 17.05 | 0.01481 | 1151 |
| HG referrals | |||||
| DySIS | 1266.47 | 19.16580 | |||
| ZedScan | 1289.67 | 19.16911 | 23.19 | 0.00332 | 6994 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| DySIS | 833.46 | 19.19120 | –16.62 | 0.01614 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| DySIS | 723.49 | 19.20787 | –8.85 | 0.01779 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| DySIS | 1087.72 | 19.16774 | –39.22 | 0.00581 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| DySIS | 898.26 | 19.18937 | 3.85 | 0.01426 | 270 |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| DySIS | 746.49 | 19.20646 | –2.37 | 0.02150 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| DySIS | 1252.13 | 19.16234 | 15.19 | 0.00371 | 4097 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| ZedScan | 845.31 | 19.20206 | –4.77 | 0.02700 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| ZedScan | 745.76 | 19.22007 | 13.43 | 0.03000 | 448 |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| ZedScan | 1083.15 | 19.17347 | –43.78 | 0.01155 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| ZedScan | 919.71 | 19.19977 | 25.30 | 0.02466 | 1026 |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| ZedScan | 771.19 | 19.21984 | 22.33 | 0.03487 | 640 |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| ZedScan | 1277.49 | 19.16668 | 40.56 | 0.00805 | 5036 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 833.46 | 19.19120 | |||
| ZedScan | 845.31 | 19.20206 | 11.85 | 0.01085 | 1092 |
| LG referrals | |||||
| DySIS | 723.49 | 19.20787 | |||
| ZedScan | 745.76 | 19.22007 | 22.28 | 0.01220 | 1825 |
| HG referrals | |||||
| DySIS | 1087.72 | 19.16774 | |||
| ZedScan | 1083.15 | 19.17347 | –4.56 | 0.00574 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 898.26 | 19.18937 | |||
| ZedScan | 919.71 | 19.19977 | 21.45 | 0.01040 | 2063 |
| LG referrals | |||||
| DySIS | 746.49 | 19.20646 | |||
| ZedScan | 771.19 | 19.21984 | 24.70 | 0.01338 | 1846 |
| HG referrals | |||||
| DySIS | 1252.13 | 19.16234 | |||
| ZedScan | 1277.49 | 19.16668 | 25.36 | 0.00434 | 5838 |
Scenario analysis 5.2: number of patients per colposcope per year – + 50% compared with base case
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| DySIS | 869.96 | 19.18516 | –33.32 | 0.02016 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| DySIS | 768.29 | 19.18794 | –25.68 | 0.02464 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| DySIS | 1088.98 | 19.17156 | –50.15 | 0.01034 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| DySIS | 938.51 | 19.18194 | –14.50 | 0.01908 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| DySIS | 796.97 | 19.18601 | –15.87 | 0.02318 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| DySIS | 1252.41 | 19.16580 | 0.34 | 0.00571 | 59 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| ZedScan | 885.66 | 19.19901 | –17.63 | 0.03401 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| ZedScan | 789.04 | 19.20307 | –4.93 | 0.03978 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| ZedScan | 1091.71 | 19.17651 | –47.42 | 0.01529 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| ZedScan | 965.60 | 19.19363 | 12.59 | 0.03078 | 409 |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| ZedScan | 822.93 | 19.20082 | 10.08 | 0.03799 | 265 |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| ZedScan | 1288.55 | 19.16911 | 36.47 | 0.00903 | 4040 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 869.96 | 19.18516 | |||
| ZedScan | 885.66 | 19.19901 | 15.70 | 0.01385 | 1134 |
| LG referrals | |||||
| DySIS | 768.29 | 19.18794 | |||
| ZedScan | 789.04 | 19.20307 | 20.75 | 0.01514 | 1371 |
| HG referrals | |||||
| DySIS | 1088.98 | 19.17156 | |||
| ZedScan | 1091.71 | 19.17651 | 2.72 | 0.00495 | 550 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 938.51 | 19.18194 | |||
| ZedScan | 965.60 | 19.19363 | 27.09 | 0.01170 | 2316 |
| LG referrals | |||||
| DySIS | 796.97 | 19.18601 | |||
| ZedScan | 822.93 | 19.20082 | 25.96 | 0.01481 | 1752 |
| HG referrals | |||||
| DySIS | 1252.41 | 19.16580 | |||
| ZedScan | 1288.55 | 19.16911 | 36.13 | 0.00332 | 10,896 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| DySIS | 822.80 | 19.19120 | –27.28 | 0.01614 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| DySIS | 712.78 | 19.20787 | –19.55 | 0.01779 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| DySIS | 1077.20 | 19.16774 | –49.74 | 0.00581 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| DySIS | 885.97 | 19.18937 | –8.44 | 0.01426 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| DySIS | 735.30 | 19.20646 | –13.56 | 0.02150 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| DySIS | 1237.28 | 19.16234 | 0.35 | 0.00371 | 94 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| ZedScan | 844.12 | 19.20206 | –5.96 | 0.02700 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| ZedScan | 744.56 | 19.22007 | 12.23 | 0.03000 | 408 |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| ZedScan | 1081.99 | 19.17347 | –44.94 | 0.01155 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| ZedScan | 918.48 | 19.19977 | 24.07 | 0.02466 | 976 |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| ZedScan | 769.96 | 19.21984 | 21.10 | 0.03487 | 605 |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| ZedScan | 1276.28 | 19.16668 | 39.35 | 0.00805 | 4886 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 822.80 | 19.19120 | |||
| ZedScan | 844.12 | 19.20206 | 21.33 | 0.01085 | 1965 |
| LG referrals | |||||
| DySIS | 712.78 | 19.20787 | |||
| ZedScan | 744.56 | 19.22007 | 31.78 | 0.01220 | 2604 |
| HG referrals | |||||
| DySIS | 1077.20 | 19.16774 | |||
| ZedScan | 1081.99 | 19.17347 | 4.79 | 0.00574 | 835 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 885.97 | 19.18937 | |||
| ZedScan | 918.48 | 19.19977 | 32.52 | 0.01040 | 3127 |
| LG referrals | |||||
| DySIS | 735.30 | 19.20646 | |||
| ZedScan | 769.96 | 19.21984 | 34.66 | 0.01338 | 2591 |
| HG referrals | |||||
| DySIS | 1237.28 | 19.16234 | |||
| ZedScan | 1276.28 | 19.16668 | 39.00 | 0.00434 | 8977 |
Scenario analysis 6: costs of diagnostic biopsy and large-loop excision of the transformation zone
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1142.55 | 19.16500 | |||
| DySIS | 1133.92 | 19.18516 | –8.64 | 0.02016 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 933.40 | 19.16330 | |||
| DySIS | 939.31 | 19.18794 | 5.91 | 0.02464 | 240 |
| HG referrals | |||||
| Colposcopy alone | 1595.81 | 19.16122 | |||
| DySIS | 1555.78 | 19.17156 | –40.03 | 0.01034 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1191.48 | 19.16286 | |||
| DySIS | 1198.24 | 19.18194 | 6.76 | 0.01908 | 355 |
| LG referrals | |||||
| Colposcopy alone | 945.18 | 19.16283 | |||
| DySIS | 955.93 | 19.18601 | 10.75 | 0.02318 | 464 |
| HG referrals | |||||
| Colposcopy alone | 1721.66 | 19.16008 | |||
| DySIS | 1732.53 | 19.16580 | 10.87 | 0.00571 | 1903 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1142.55 | 19.16500 | |||
| ZedScan | 1162.49 | 19.19901 | 19.94 | 0.03401 | 586 |
| LG referrals | |||||
| Colposcopy alone | 933.40 | 19.16330 | |||
| ZedScan | 977.37 | 19.20307 | 43.97 | 0.03978 | 1105 |
| HG referrals | |||||
| Colposcopy alone | 1595.81 | 19.16122 | |||
| ZedScan | 1561.11 | 19.17651 | –34.70 | 0.01529 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1191.48 | 19.16286 | |||
| ZedScan | 1235.83 | 19.19363 | 44.35 | 0.03078 | 1441 |
| LG referrals | |||||
| Colposcopy alone | 945.18 | 19.16283 | |||
| ZedScan | 996.19 | 19.20082 | 51.02 | 0.03799 | 1343 |
| HG referrals | |||||
| Colposcopy alone | 1721.66 | 19.16008 | |||
| ZedScan | 1770.28 | 19.16911 | 48.62 | 0.00903 | 5385 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 1133.92 | 19.18516 | |||
| ZedScan | 1162.49 | 19.19901 | 28.57 | 0.01385 | 2064 |
| LG referrals | |||||
| DySIS | 939.31 | 19.18794 | |||
| ZedScan | 977.37 | 19.20307 | 38.06 | 0.01514 | 2515 |
| HG referrals | |||||
| DySIS | 1555.78 | 19.17156 | |||
| ZedScan | 1561.11 | 19.17651 | 5.33 | 0.00495 | 1077 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 1198.24 | 19.18194 | |||
| ZedScan | 1235.83 | 19.19363 | 37.58 | 0.01170 | 3213 |
| LG referrals | |||||
| DySIS | 955.93 | 19.18601 | |||
| ZedScan | 996.19 | 19.20082 | 40.26 | 0.01481 | 2718 |
| HG referrals | |||||
| DySIS | 1732.53 | 19.16580 | |||
| ZedScan | 1770.28 | 19.16911 | 37.75 | 0.00332 | 11,383 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1080.43 | 19.17506 | |||
| DySIS | 1079.04 | 19.19120 | –1.39 | 0.01614 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 865.95 | 19.19008 | |||
| DySIS | 878.28 | 19.20787 | 12.33 | 0.01779 | 693 |
| HG referrals | |||||
| Colposcopy alone | 1587.75 | 19.16192 | |||
| DySIS | 1549.14 | 19.16774 | –38.61 | 0.00581 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1125.28 | 19.17511 | |||
| DySIS | 1140.86 | 19.18937 | 15.59 | 0.01426 | 1093 |
| LG referrals | |||||
| Colposcopy alone | 877.84 | 19.18496 | |||
| DySIS | 892.41 | 19.20646 | 14.57 | 0.02150 | 678 |
| HG referrals | |||||
| Colposcopy alone | 1712.09 | 19.15863 | |||
| DySIS | 1724.71 | 19.16234 | 12.62 | 0.00371 | 3402 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1080.43 | 19.17506 | |||
| ZedScan | 1113.02 | 19.20206 | 32.59 | 0.02700 | 1207 |
| LG referrals | |||||
| Colposcopy alone | 865.95 | 19.19008 | |||
| ZedScan | 927.00 | 19.22007 | 61.05 | 0.03000 | 2035 |
| HG referrals | |||||
| Colposcopy alone | 1587.75 | 19.16192 | |||
| ZedScan | 1556.56 | 19.17347 | –31.18 | 0.01155 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 1125.28 | 19.17511 | |||
| ZedScan | 1184.20 | 19.19977 | 58.92 | 0.02466 | 2390 |
| LG referrals | |||||
| Colposcopy alone | 877.84 | 19.18496 | |||
| ZedScan | 941.93 | 19.21984 | 64.09 | 0.03487 | 1838 |
| HG referrals | |||||
| Colposcopy alone | 1712.09 | 19.15863 | |||
| ZedScan | 1766.12 | 19.16668 | 54.03 | 0.00805 | 6709 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 1079.04 | 19.19120 | |||
| ZedScan | 1113.02 | 19.20206 | 33.98 | 0.01085 | 3131 |
| LG referrals | |||||
| DySIS | 878.28 | 19.20787 | |||
| ZedScan | 927.00 | 19.22007 | 48.72 | 0.01220 | 3992 |
| HG referrals | |||||
| DySIS | 1549.14 | 19.16774 | |||
| ZedScan | 1556.56 | 19.17347 | 7.43 | 0.00574 | 1295 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 1140.86 | 19.18937 | |||
| ZedScan | 1184.20 | 19.19977 | 43.34 | 0.01040 | 4168 |
| LG referrals | |||||
| DySIS | 892.41 | 19.20646 | |||
| ZedScan | 941.93 | 19.21984 | 49.52 | 0.01338 | 3701 |
| HG referrals | |||||
| DySIS | 1724.71 | 19.16234 | |||
| ZedScan | 1766.12 | 19.16668 | 41.41 | 0.00434 | 9531 |
Scenario analysis 7: alternative distributions of health state and reason for referral from human papillomavirus screening pilot sites
FIGURE 33.
Population referred for colposcopy under the HPV primary screening protocol. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.
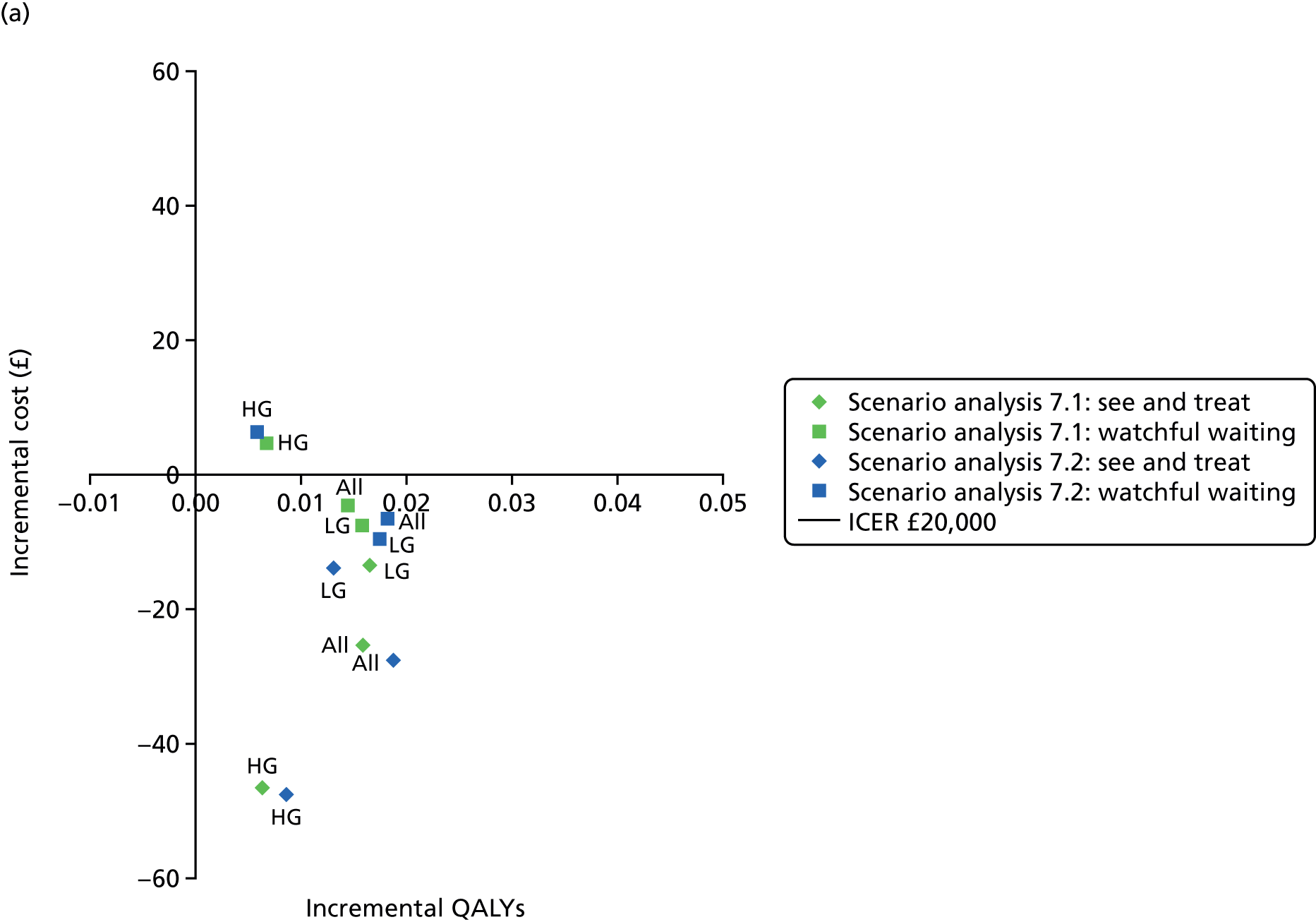

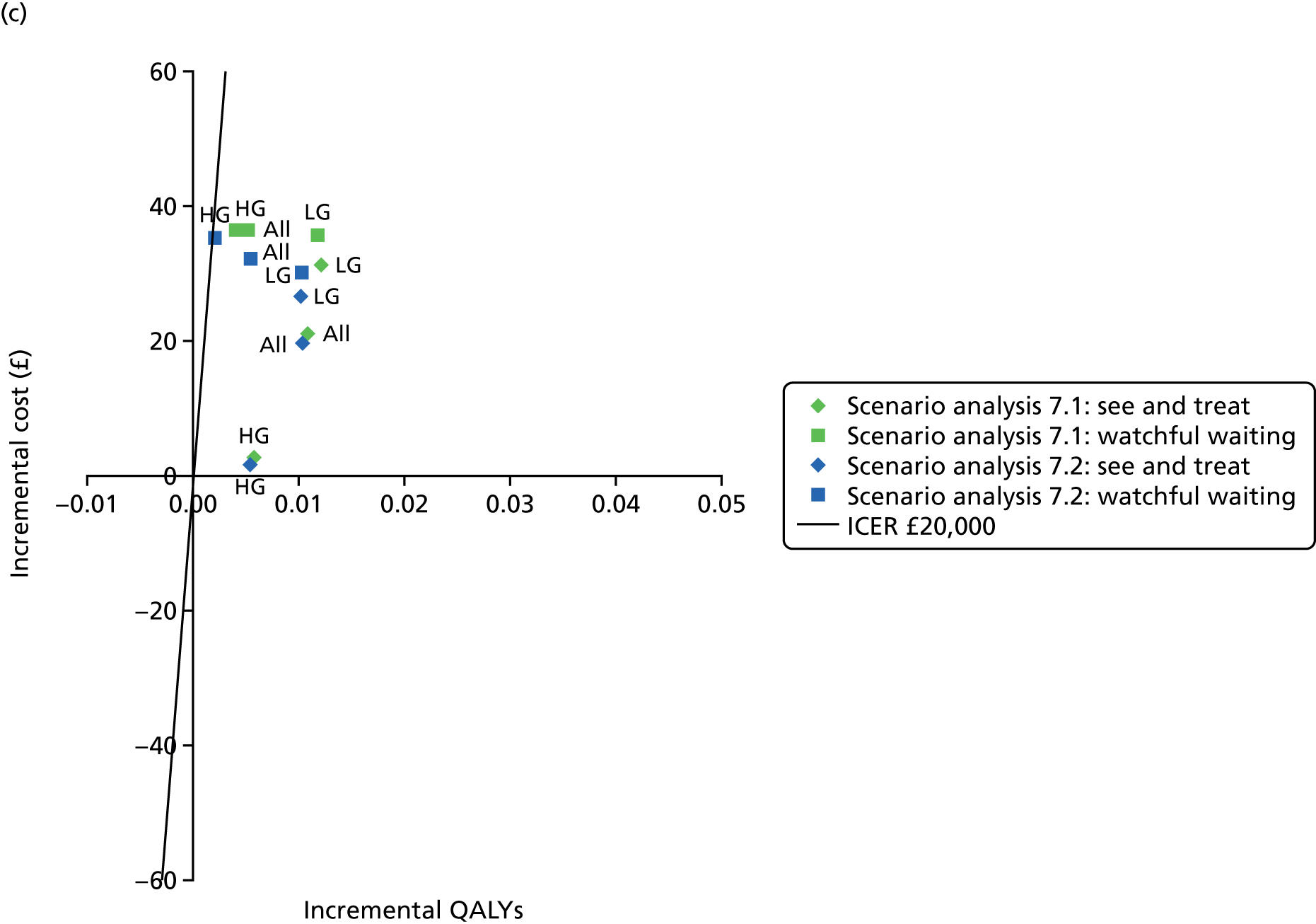
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 869.15 | 19.17797 | |||
| DySIS | 843.81 | 19.19374 | –25.34 | 0.01577 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 761.78 | 19.18360 | |||
| DySIS | 748.31 | 19.19999 | –13.47 | 0.01639 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1109.78 | 19.16614 | |||
| DySIS | 1063.28 | 19.17235 | –46.50 | 0.00622 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 916.54 | 19.17587 | |||
| DySIS | 911.90 | 19.19021 | –4.64 | 0.01433 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 780.26 | 19.18110 | |||
| DySIS | 772.76 | 19.19683 | –7.50 | 0.01573 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1224.74 | 19.15938 | |||
| DySIS | 1229.50 | 19.16602 | 4.75 | 0.00663 | 716 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 869.15 | 19.17797 | |||
| ZedScan | 864.85 | 19.20464 | –4.30 | 0.02668 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 761.78 | 19.18360 | |||
| ZedScan | 779.52 | 19.21213 | 17.74 | 0.02853 | 622 |
| HG referrals | |||||
| Colposcopy alone | 1109.78 | 19.16614 | |||
| ZedScan | 1065.96 | 19.17820 | –43.81 | 0.01206 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 916.54 | 19.17587 | |||
| ZedScan | 948.19 | 19.19542 | 31.64 | 0.01954 | 1619 |
| LG referrals | |||||
| Colposcopy alone | 780.26 | 19.18110 | |||
| ZedScan | 808.42 | 19.20864 | 28.16 | 0.02754 | 1023 |
| HG referrals | |||||
| Colposcopy alone | 1224.74 | 19.15938 | |||
| ZedScan | 1265.75 | 19.17010 | 41.00 | 0.01072 | 3826 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 843.81 | 19.19374 | |||
| ZedScan | 864.85 | 19.20464 | 21.03 | 0.01091 | 1929 |
| LG referrals | |||||
| DySIS | 748.31 | 19.19999 | |||
| ZedScan | 779.52 | 19.21213 | 31.21 | 0.01214 | 2571 |
| HG referrals | |||||
| DySIS | 1063.28 | 19.17235 | |||
| ZedScan | 1065.96 | 19.17820 | 2.69 | 0.00585 | 459 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 911.90 | 19.19021 | |||
| ZedScan | 948.19 | 19.19542 | 36.28 | 0.00521 | 6965 |
| LG referrals | |||||
| DySIS | 772.76 | 19.19683 | |||
| ZedScan | 808.42 | 19.20864 | 35.66 | 0.01181 | 3021 |
| HG referrals | |||||
| DySIS | 1229.50 | 19.16602 | |||
| ZedScan | 1265.75 | 19.17010 | 36.25 | 0.00408 | 8878 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 840.64 | 19.18448 | |||
| DySIS | 813.05 | 19.20309 | –27.59 | 0.01861 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 718.86 | 19.19501 | |||
| DySIS | 705.08 | 19.20804 | –13.78 | 0.01303 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1130.13 | 19.15967 | |||
| DySIS | 1082.72 | 19.16813 | –47.42 | 0.00846 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 882.93 | 19.17807 | |||
| DySIS | 876.39 | 19.19619 | –6.54 | 0.01812 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 735.43 | 19.19023 | |||
| DySIS | 725.96 | 19.20749 | –9.47 | 0.01726 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1243.76 | 19.16096 | |||
| DySIS | 1250.08 | 19.16665 | 6.31 | 0.00570 | 1109 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 840.64 | 19.18448 | |||
| ZedScan | 832.59 | 19.21357 | –8.05 | 0.02909 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 718.86 | 19.19501 | |||
| ZedScan | 731.56 | 19.21836 | 12.69 | 0.02336 | 543 |
| HG referrals | |||||
| Colposcopy alone | 1130.13 | 19.15967 | |||
| ZedScan | 1084.55 | 19.17361 | –45.59 | 0.01394 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 882.93 | 19.17807 | |||
| ZedScan | 908.53 | 19.20169 | 25.60 | 0.02362 | 1084 |
| LG referrals | |||||
| Colposcopy alone | 735.43 | 19.19023 | |||
| ZedScan | 756.10 | 19.21774 | 20.67 | 0.02751 | 751 |
| HG referrals | |||||
| Colposcopy alone | 1243.76 | 19.16096 | |||
| ZedScan | 1285.29 | 19.16882 | 41.53 | 0.00786 | 5285 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 813.05 | 19.20309 | |||
| ZedScan | 832.59 | 19.21357 | 19.54 | 0.01047 | 1866 |
| LG referrals | |||||
| DySIS | 705.08 | 19.20804 | |||
| ZedScan | 731.56 | 19.21836 | 26.47 | 0.01033 | 2564 |
| HG referrals | |||||
| DySIS | 1082.72 | 19.16813 | |||
| ZedScan | 1084.55 | 19.17361 | 1.83 | 0.00548 | 334 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 876.39 | 19.19619 | |||
| ZedScan | 908.53 | 19.20169 | 32.14 | 0.00550 | 5848 |
| LG referrals | |||||
| DySIS | 725.96 | 19.20749 | |||
| ZedScan | 756.10 | 19.21774 | 30.13 | 0.01025 | 2940 |
| HG referrals | |||||
| DySIS | 1250.08 | 19.16665 | |||
| ZedScan | 1285.29 | 19.16882 | 35.22 | 0.00216 | 16,277 |
Scenario 1: time horizon of 3 years
FIGURE 34.
Scenario analysis: 3-year time horizon – HPV triage. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.
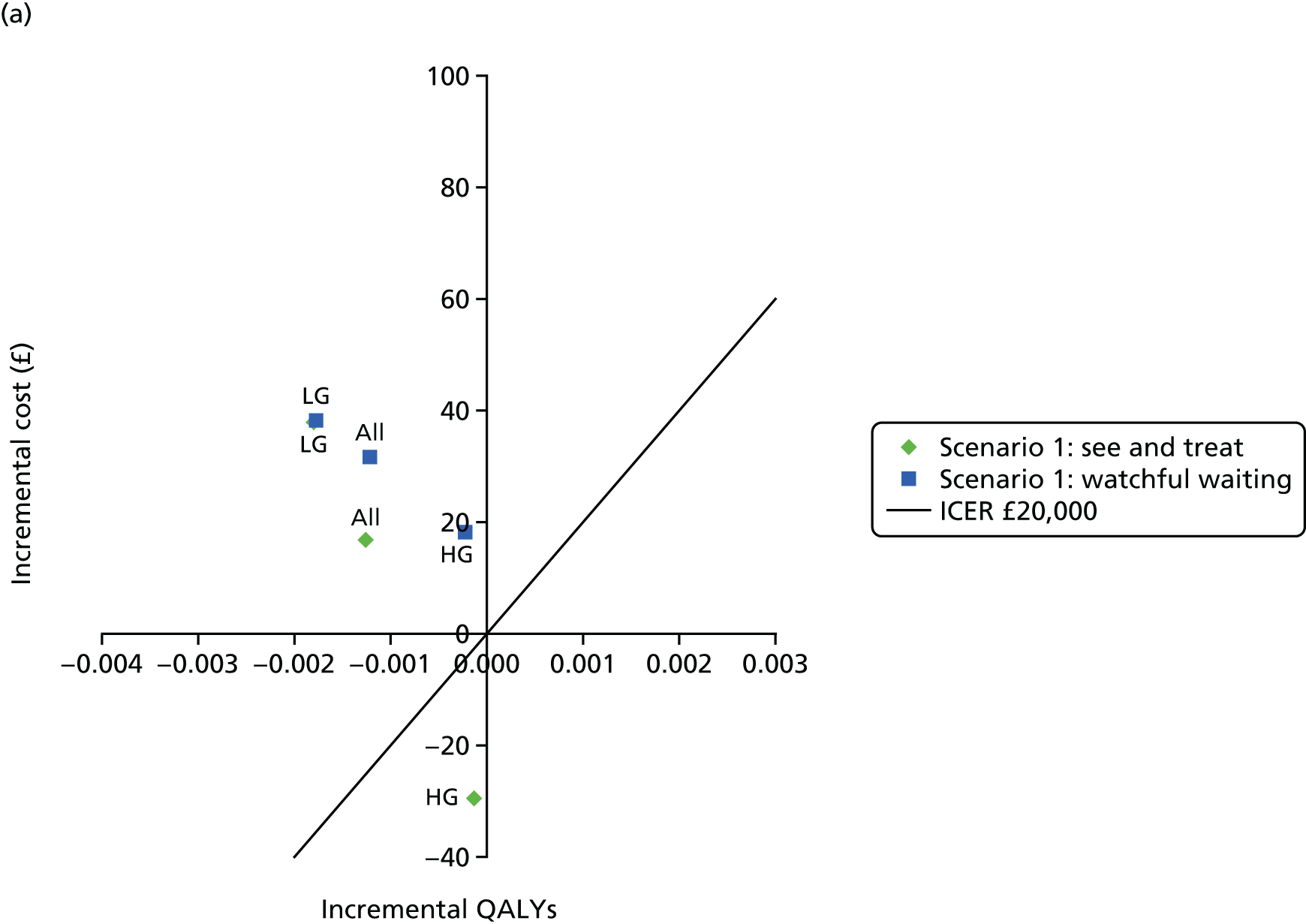
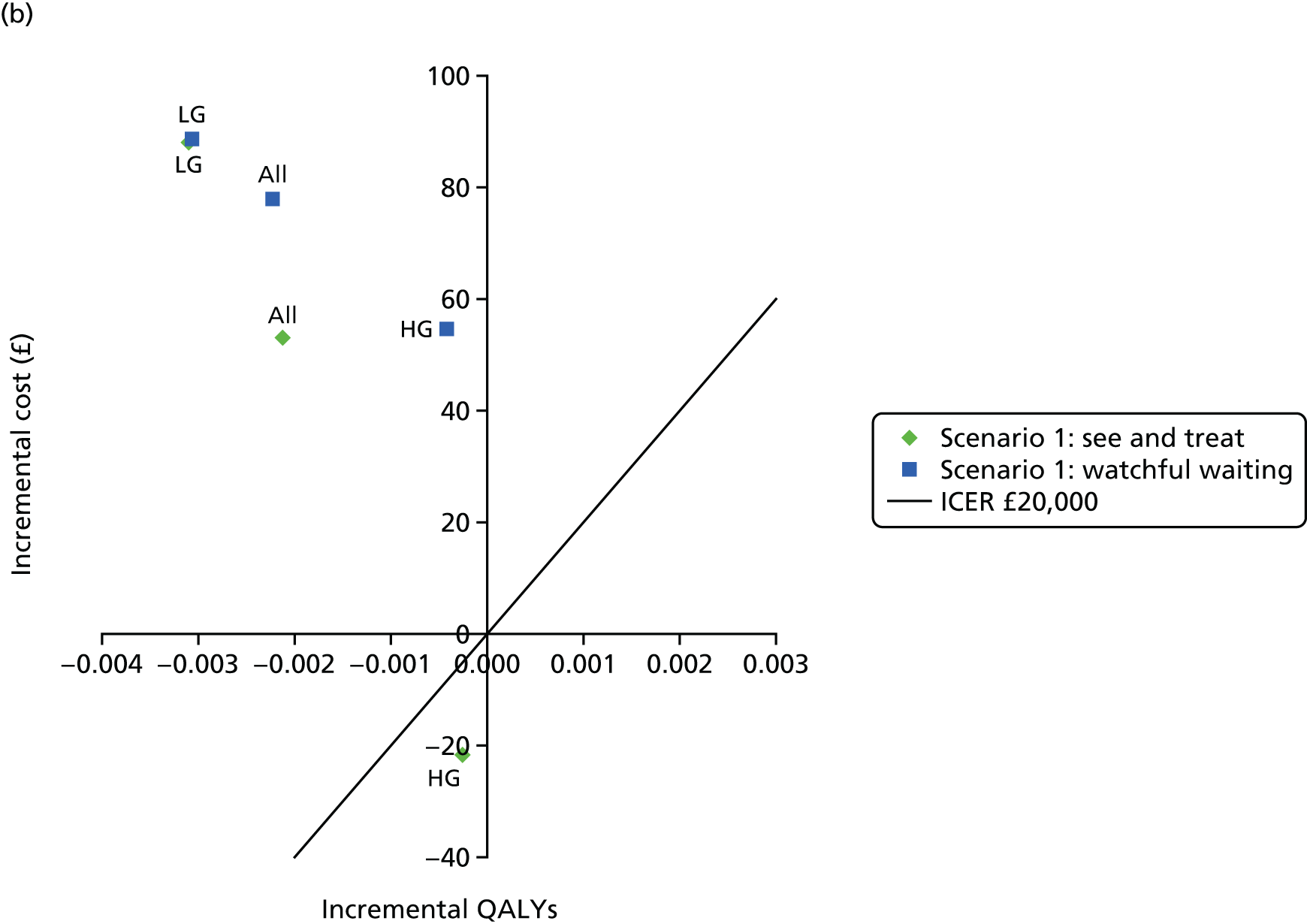

FIGURE 35.
Scenario analysis: 3-year time horizon – HPV primary screening. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.


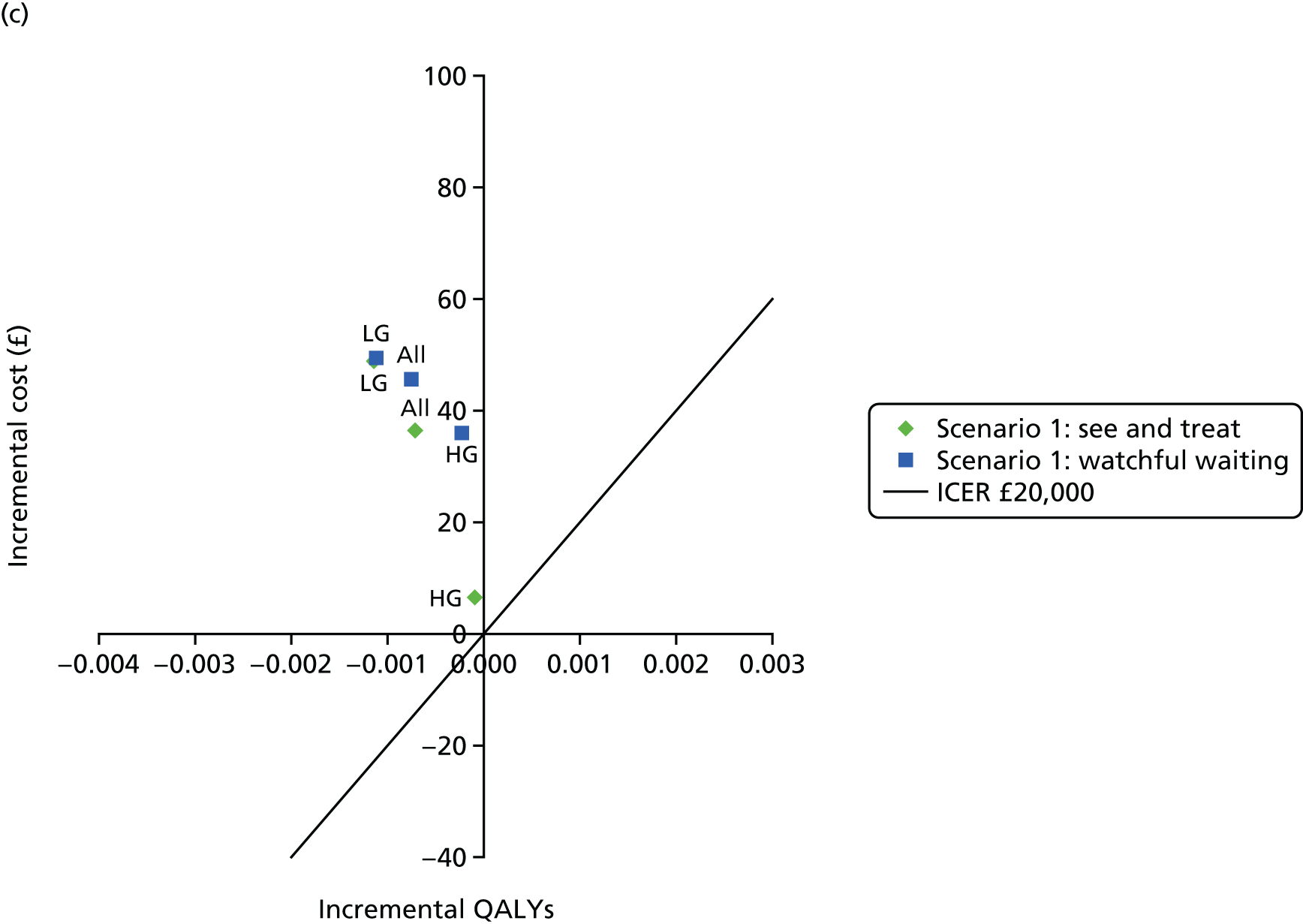
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 411.06 | 2.57359 | |||
| DySIS | 427.74 | 2.57234 | 16.69 | –0.00126 | Dominated |
| LG referrals | |||||
| Colposcopy alone | 265.59 | 2.58218 | |||
| DySIS | 303.39 | 2.58039 | 37.80 | –0.00180 | Dominated |
| HG referrals | |||||
| Colposcopy alone | 732.77 | 2.55474 | |||
| DySIS | 703.19 | 2.55461 | –29.58 | –0.00012 | 236,692 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 446.62 | 2.57361 | |||
| DySIS | 478.14 | 2.57239 | 31.53 | –0.00122 | Dominated |
| LG referrals | |||||
| Colposcopy alone | 265.84 | 2.58217 | |||
| DySIS | 303.98 | 2.58039 | 38.14 | –0.00178 | Dominated |
| HG referrals | |||||
| Colposcopy alone | 839.24 | 2.55471 | |||
| DySIS | 857.51 | 2.55450 | 18.27 | –0.00021 | Dominated |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 411.06 | 2.57359 | |||
| ZedScan | 463.86 | 2.57147 | 52.80 | –0.00213 | Dominated |
| LG referrals | |||||
| Colposcopy alone | 265.59 | 2.58218 | |||
| ZedScan | 353.48 | 2.57909 | 87.89 | –0.00310 | Dominated |
| HG referrals | |||||
| Colposcopy alone | 732.77 | 2.55474 | |||
| ZedScan | 710.95 | 2.55448 | –21.82 | –0.00026 | 85,045 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 446.62 | 2.57361 | |||
| ZedScan | 524.47 | 2.57138 | 77.85 | –0.00223 | Dominated |
| LG referrals | |||||
| Colposcopy alone | 265.84 | 2.58217 | |||
| ZedScan | 354.30 | 2.57911 | 88.47 | –0.00306 | Dominated |
| HG referrals | |||||
| Colposcopy alone | 839.24 | 2.55471 | |||
| ZedScan | 893.65 | 2.55427 | 54.41 | –0.00043 | Dominated |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 427.74 | 2.57234 | |||
| ZedScan | 463.86 | 2.57147 | 36.12 | –0.00087 | Dominated |
| LG referrals | |||||
| DySIS | 303.39 | 2.58039 | |||
| ZedScan | 353.48 | 2.57909 | 50.09 | –0.00130 | Dominated |
| HG referrals | |||||
| DySIS | 703.19 | 2.55461 | |||
| ZedScan | 710.95 | 2.55448 | 7.76 | –0.00013 | Dominated |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 478.14 | 2.57239 | |||
| ZedScan | 524.47 | 2.57138 | 46.33 | –0.00101 | Dominated |
| LG referrals | |||||
| DySIS | 303.98 | 2.58039 | |||
| ZedScan | 354.30 | 2.57911 | 50.32 | –0.00128 | Dominated |
| HG referrals | |||||
| DySIS | 857.51 | 2.55450 | |||
| ZedScan | 893.65 | 2.55427 | 36.14 | –0.00022 | Dominated |
Scenario 2: adverse obstetric outcomes excluded
FIGURE 36.
Scenario analysis: no adverse obstetric outcomes – HPV triage. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.

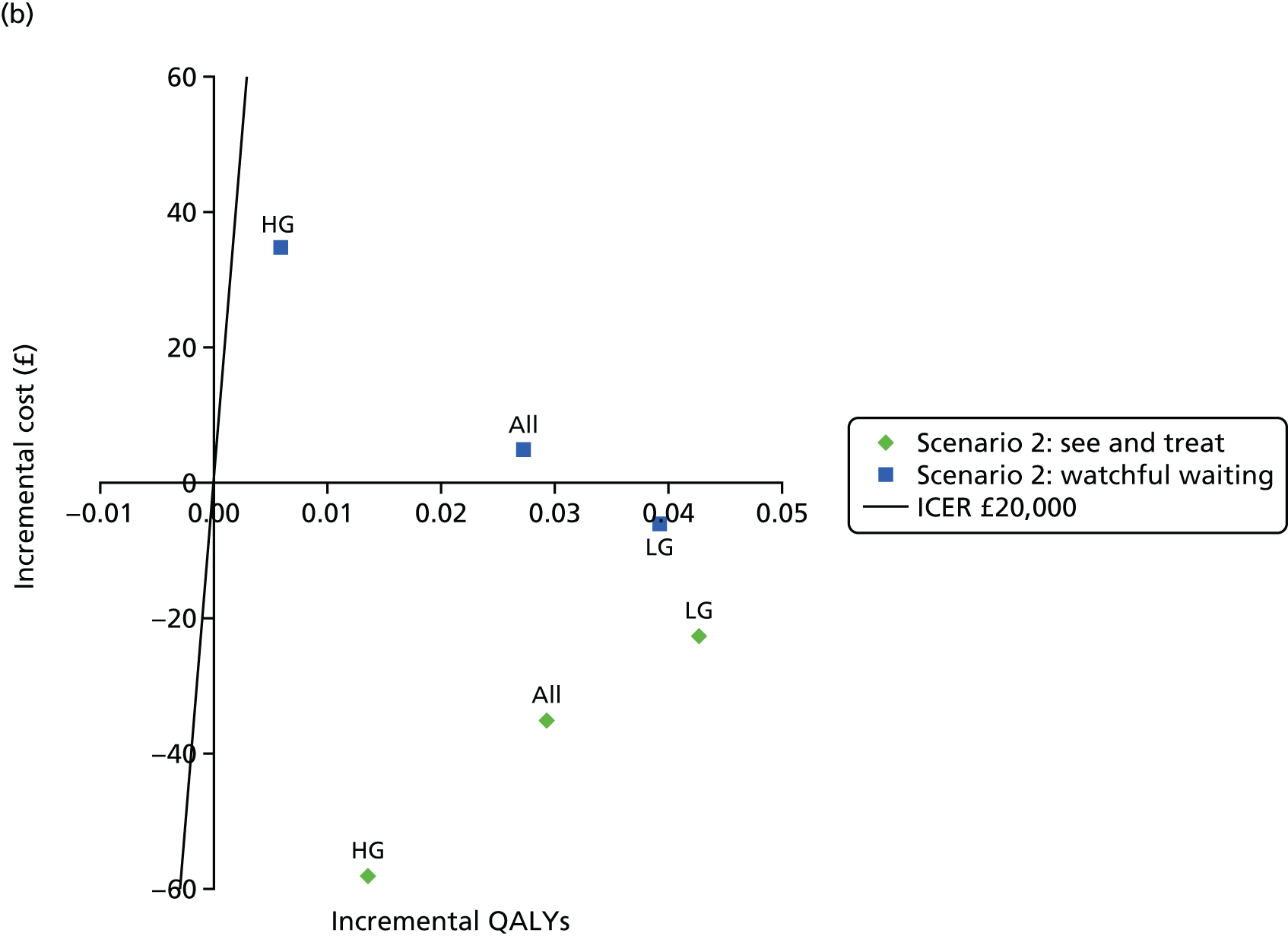

FIGURE 37.
Scenario analysis: no adverse obstetric outcomes – HPV primary screening. (a) DySIS vs. colposcopy alone; (b) ZedScan vs. colposcopy alone; and (c) ZedScan vs. DySIS. HG, high grade; LG, low grade.

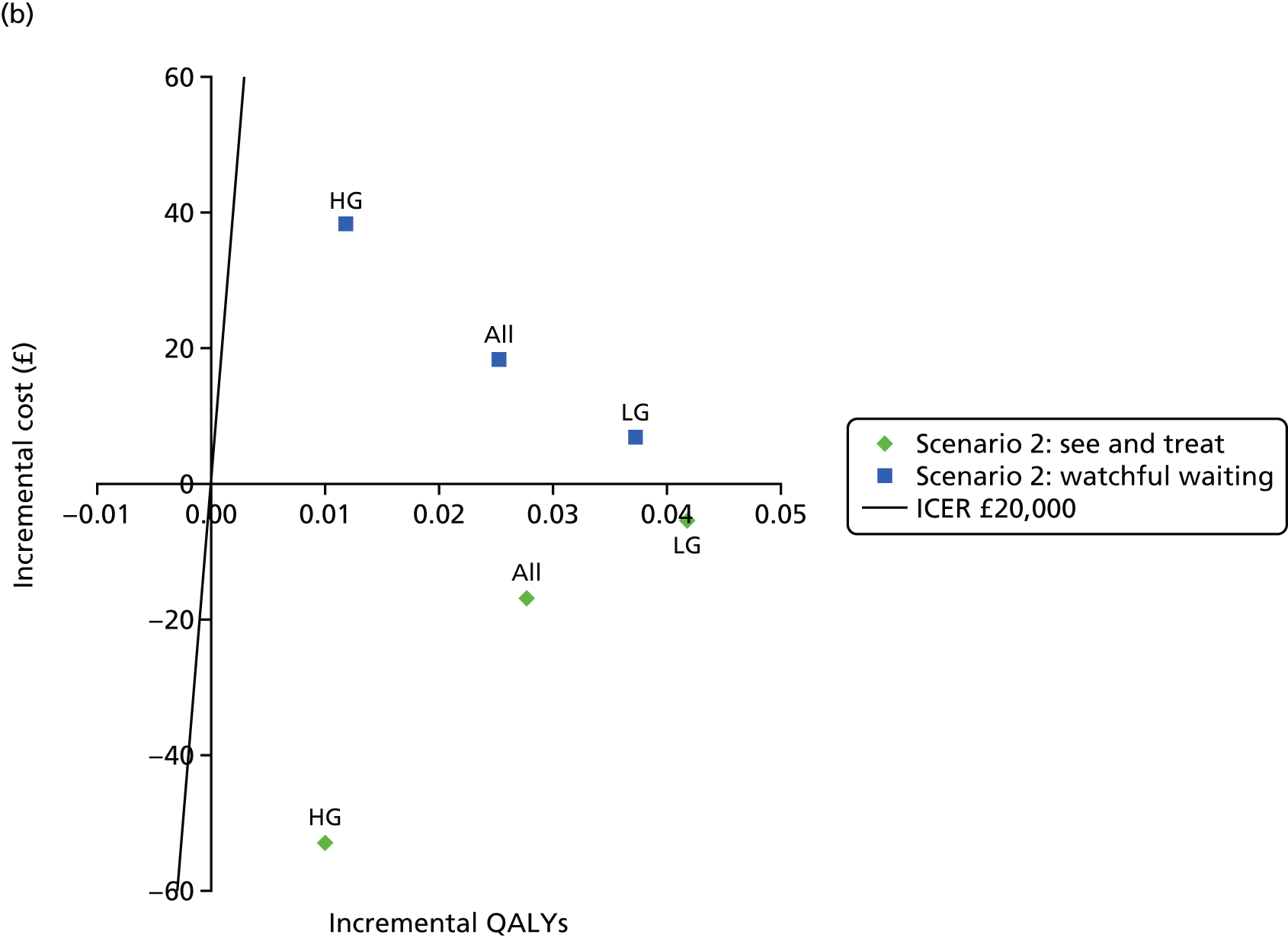
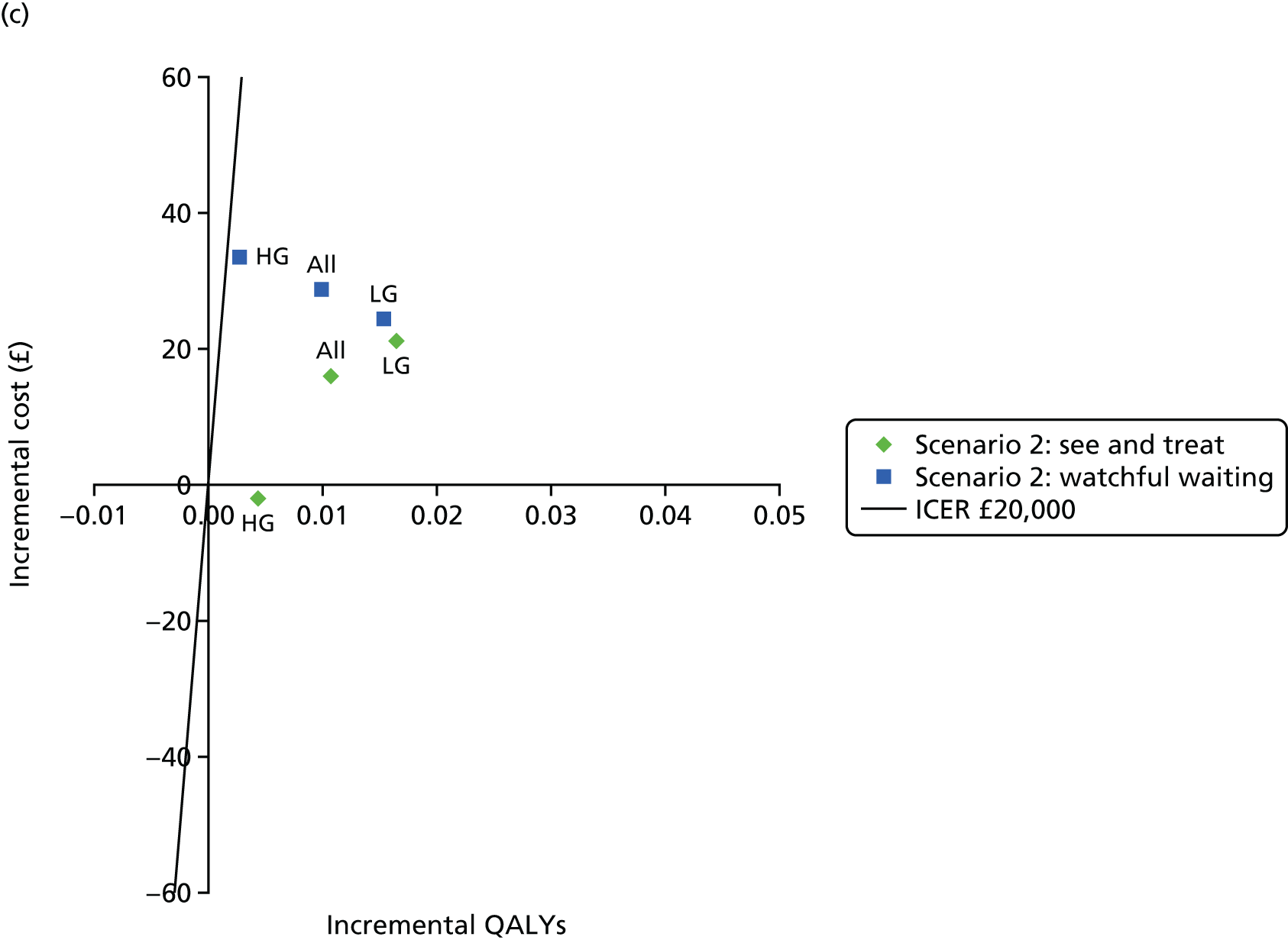
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 812.38 | 19.16794 | |||
| DySIS | 772.72 | 19.18643 | –39.66 | 0.01849 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 764.95 | 19.16568 | |||
| DySIS | 731.55 | 19.19163 | –33.40 | 0.02595 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 916.64 | 19.16996 | |||
| DySIS | 864.11 | 19.17924 | –52.53 | 0.00928 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 866.34 | 19.17183 | |||
| DySIS | 851.85 | 19.18963 | –14.49 | 0.01781 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 785.18 | 19.16212 | |||
| DySIS | 762.82 | 19.18799 | –22.36 | 0.02587 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1040.04 | 19.17105 | |||
| DySIS | 1045.25 | 19.17569 | 5.21 | 0.00464 | 1122 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 812.38 | 19.16794 | |||
| ZedScan | 777.24 | 19.19700 | –35.14 | 0.02906 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 764.95 | 19.16568 | |||
| ZedScan | 742.35 | 19.20822 | –22.60 | 0.04254 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 916.64 | 19.16996 | |||
| ZedScan | 858.46 | 19.18343 | –58.18 | 0.01348 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 866.34 | 19.17183 | |||
| ZedScan | 871.25 | 19.19886 | 4.91 | 0.02703 | 182 |
| LG referrals | |||||
| Colposcopy alone | 785.18 | 19.16212 | |||
| ZedScan | 778.96 | 19.20125 | –6.22 | 0.03912 | Dominant |
| HG referrals | |||||
| Colposcopy alone | 1040.04 | 19.17105 | |||
| ZedScan | 1074.92 | 19.17690 | 34.87 | 0.00585 | 5959 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 772.72 | 19.18643 | |||
| ZedScan | 777.24 | 19.19700 | 4.51 | 0.01057 | 427 |
| LG referrals | |||||
| DySIS | 731.55 | 19.19163 | |||
| ZedScan | 742.35 | 19.20822 | 10.80 | 0.01659 | 651 |
| HG referrals | |||||
| DySIS | 864.11 | 19.17924 | |||
| ZedScan | 858.46 | 19.18343 | –5.65 | 0.00419 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 851.85 | 19.18963 | |||
| ZedScan | 871.25 | 19.19886 | 19.40 | 0.00922 | 2104 |
| LG referrals | |||||
| DySIS | 762.82 | 19.18799 | |||
| ZedScan | 778.96 | 19.20125 | 16.14 | 0.01325 | 1218 |
| HG referrals | |||||
| DySIS | 1045.25 | 19.17569 | |||
| ZedScan | 1074.92 | 19.17690 | 29.66 | 0.00121 | 24,493 |
Scenario 3: ZedScan was used alongside colposcopy at all appointments
FIGURE 38.
Scenario analysis: ZedScan was used alongside colposcopy at all appointments. (a) ZedScan vs. colposcopy alone – HPV triage protocol; (b) ZedScan vs. DySIS – HPV triage protocol; (c) ZedScan vs. colposcopy alone – HPV primary screening protocol; and (d) ZedScan vs. DySIS – HPV primary screening protocol. HG, high grade; LG, low grade.


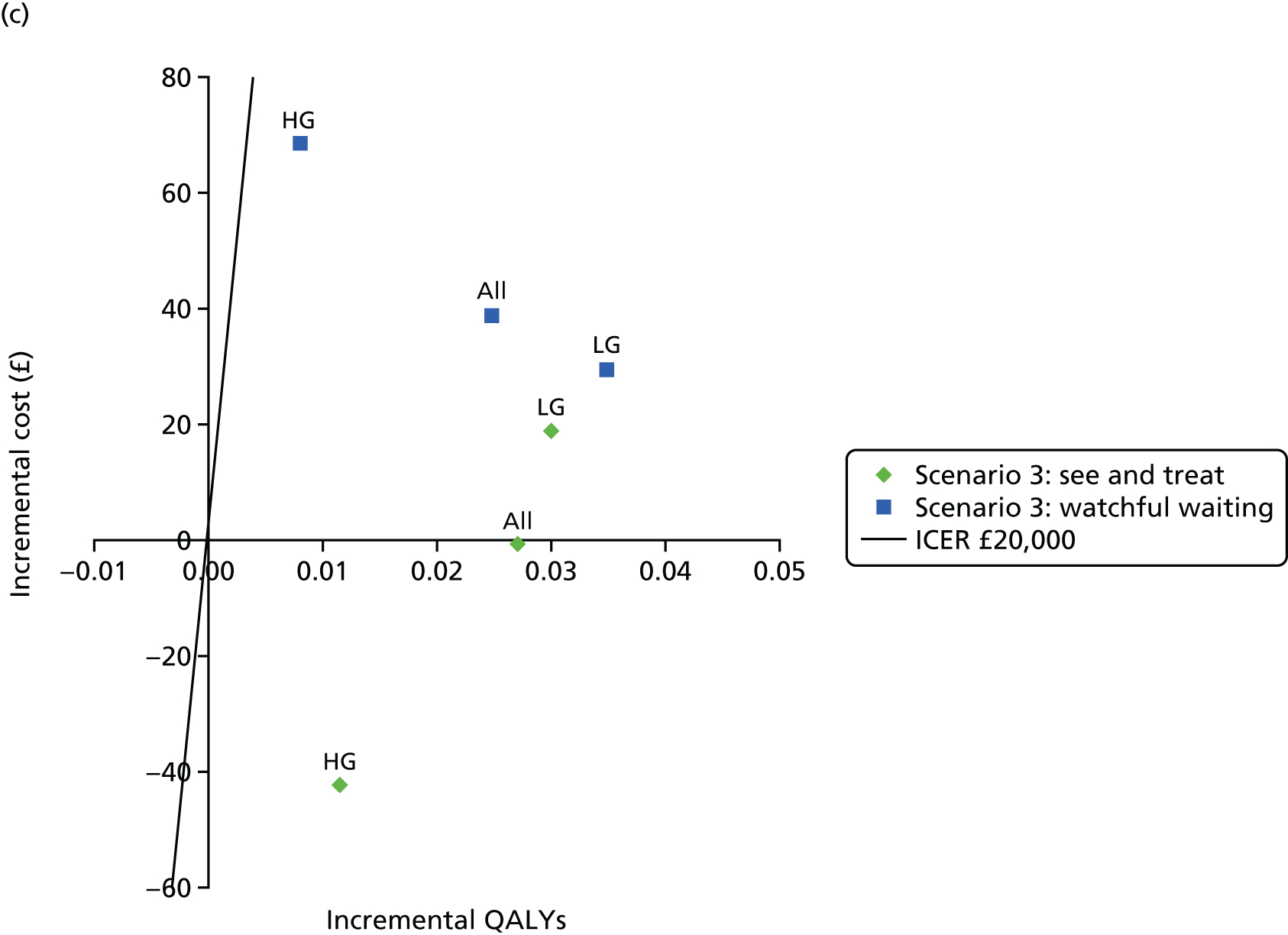
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 903.28 | 19.16500 | |||
| ZedScan | 890.89 | 19.19901 | –12.40 | 0.03401 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 793.97 | 19.16330 | |||
| ZedScan | 795.63 | 19.20307 | 1.65 | 0.03978 | 42 |
| HG referrals | |||||
| Colposcopy alone | 1139.13 | 19.16122 | |||
| ZedScan | 1094.01 | 19.17651 | –45.12 | 0.01529 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 953.02 | 19.16286 | |||
| ZedScan | 980.93 | 19.19363 | 27.92 | 0.03078 | 907 |
| LG referrals | |||||
| Colposcopy alone | 812.85 | 19.16283 | |||
| ZedScan | 831.92 | 19.20082 | 19.07 | 0.03799 | 502 |
| HG referrals | |||||
| Colposcopy alone | 1252.07 | 19.16008 | |||
| ZedScan | 1317.70 | 19.16911 | 65.63 | 0.00903 | 7270 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 872.34 | 19.18516 | |||
| ZedScan | 890.89 | 19.19901 | 18.54 | 0.01385 | 1339 |
| LG referrals | |||||
| DySIS | 770.65 | 19.18794 | |||
| ZedScan | 795.63 | 19.20307 | 24.98 | 0.01514 | 1651 |
| HG referrals | |||||
| DySIS | 1091.43 | 19.17156 | |||
| ZedScan | 1094.01 | 19.17651 | 2.58 | 0.00495 | 521 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 941.33 | 19.18194 | |||
| ZedScan | 980.93 | 19.19363 | 39.60 | 0.01170 | 3385 |
| LG referrals | |||||
| Colposcopy alone | 799.47 | 19.18601 | |||
| ZedScan | 831.92 | 19.20082 | 32.45 | 0.01481 | 2191 |
| HG referrals | |||||
| DySIS | 1255.93 | 19.16580 | |||
| ZedScan | 1317.70 | 19.16911 | 61.78 | 0.00332 | 18,628 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| Colposcopy alone | 850.08 | 19.17506 | |||
| ZedScan | 849.70 | 19.20206 | –0.38 | 0.02700 | Dominant |
| LG referrals | |||||
| Colposcopy alone | 732.33 | 19.19008 | |||
| ZedScan | 751.31 | 19.22007 | 18.98 | 0.03000 | 633 |
| HG referrals | |||||
| Colposcopy alone | 1126.93 | 19.16192 | |||
| ZedScan | 1084.58 | 19.17347 | –42.35 | 0.01155 | Dominant |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| Colposcopy alone | 894.41 | 19.17511 | |||
| ZedScan | 933.32 | 19.19977 | 38.91 | 0.02466 | 1578 |
| LG referrals | |||||
| Colposcopy alone | 748.86 | 19.18496 | |||
| ZedScan | 778.64 | 19.21984 | 29.78 | 0.03487 | 854 |
| HG referrals | |||||
| Colposcopy alone | 1236.94 | 19.15863 | |||
| ZedScan | 1305.85 | 19.16668 | 68.91 | 0.00805 | 8557 |
| Grade of referral smear, by clinic and technology | Cost (£) | QALYs | Incremental | ICER | |
|---|---|---|---|---|---|
| Cost (£) | QALYs | ||||
| See-and-treat clinics | |||||
| All referrals | |||||
| DySIS | 825.46 | 19.19120 | |||
| ZedScan | 849.70 | 19.20206 | 24.24 | 0.01085 | 2233 |
| LG referrals | |||||
| DySIS | 715.46 | 19.20787 | |||
| ZedScan | 751.31 | 19.22007 | 35.85 | 0.01220 | 2938 |
| HG referrals | |||||
| DySIS | 1079.83 | 19.16774 | |||
| ZedScan | 1084.58 | 19.17347 | 4.75 | 0.00574 | 829 |
| Watchful-waiting clinics | |||||
| All referrals | |||||
| DySIS | 889.04 | 19.18937 | |||
| ZedScan | 933.32 | 19.19977 | 44.28 | 0.01040 | 4259 |
| LG referrals | |||||
| DySIS | 738.10 | 19.20646 | |||
| ZedScan | 778.64 | 19.21984 | 40.54 | 0.01338 | 3030 |
| HG referrals | |||||
| DySIS | 1240.99 | 19.16234 | |||
| ZedScan | 1305.85 | 19.16668 | 64.85 | 0.00434 | 14,928 |
Glossary
- Acetowhitening
- A whitening effect following the application of acetic acid to epithelial tissue, used to identify zones of squamous cell change for biopsy.
- Adjunctive dynamic spectral imaging system
- A dynamic spectral imaging system map used as an adjunct to a dynamic spectral imaging system map colposcope.
- Adjunctive ZedScan
- A ZedScan used as an adjunct to a standard colposcope.
- Cervical intraepithelial neoplasia
- Abnormal changes in the squamous epithelial cells of the cervix. This precancerous disorder is graded in accordance with its pathological progress, from cervical intraepithelial neoplasia 1 to cervical intraepithelial neoplasia 3.
- Colposcope
- An instrument producing an illuminated, magnified view of cervical and vaginal tissues designed to facilitate visual inspection and biopsy of the cervix.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A theoretical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- Dynamic Spectral Imaging System
- A digital video colposcope using dynamic spectral imaging to assist in detecting cancerous and precancerous cervical tissue.
- Dynamic Spectral Imaging System map
- A colour-coded image of the cervix indicating the intensity of epithelial acetowhitening.
- Dyskaryosis
- Abnormal cytological changes of squamous epithelial cells. A synonym for dysplasia. Classified into degrees of severity: low grade (including borderline or mild cellular changes) and high grade (moderate and severe changes).
- Electrical impedance spectroscopy
- A form of spectroscopy assessing different patterns of electrical conductivity to assess tissue composition.
- False negative
- Incorrect negative test result – an affected individual with a negative test result.
- False positive
- Incorrect positive test result – an unaffected individual with a positive test result.
- Histology/histopathology
- The microscopic study of tissue samples to enable the diagnosis of cancerous and precancerous cells.
- Human papillomavirus
- A type of virus that can infect the skin and the mucous membranes. Some types of human papillomavirus can cause dyskaryosis in the cells of the cervix and are strongly associated with cancer.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test for which performance is being evaluated.
- Liquid-based cytology
- A method of preparation for microscopic examination of smear test samples. This method superseded Pap smear tests in the NHS cervical cancer screening programme.
- Markov model
- An analytical method particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Negative predictive value
- The probability that people with a negative test result truly do not have the disease.
- NHS Cervical Screening Programme
- The programme set up in the UK aimed at detecting and treating cervical abnormalities and high-risk human papillomavirus infection to prevent future cases of cervical cancer.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Positive predictive value
- The probability that people with a positive test result truly have the disease.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best currently available diagnostic test against which the index test is compared.
- See and treat
- The removal of an abnormal area during a colposcopy examination.
- Sensitivity
- The proportion of people with the target disorder who have a positive test result.
- Specificity
- The proportion of people without the target disorder who have a negative test result.
- Transformation zone
- An area of the cervix in which nearly all precancerous and cancerous changes occur.
- True negative
- A correct negative test result – an unaffected individual with a negative test result.
- True positive
- A correct positive test result – an affected individual with a positive test result.
- ZedScan
- A device that utilises electrical impedance spectroscopy to make judgements on the status of cervical tissue.
List of abbreviations
- ARTISTIC
- A Randomised Trial in Screening to Improve Cytology
- BIC
- Bayesian information criterion
- BMD
- borderline or mild dyskaryosis
- BSCCP
- British Society for Colposcopy and Cervical Pathology
- CDSR
- Cochrane Database of Systematic Reviews
- CE
- Conformité Européenne
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CIN
- cervical intraepithelial neoplasia
- CIN 2+
- cervical intraepithelial neoplasia grade 2+
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- DOR
- diagnostic odds ratio
- DSI
- dynamic spectral imaging
- DySIS
- Dynamic Spectral Imaging System
- EIS
- electrical impedance spectroscopy
- FIGO
- International Federation of Gynecology and Obstetrics
- HPV
- human papillomavirus
- hrHPV
- high-risk human papillomavirus
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- LLETZ
- large-loop excision of the transformation zone
- MeSH
- medical subject heading
- NHSCSP
- NHS Cervical Screening Programme
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NPV
- negative predictive value
- ONS
- Office for National Statistics
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- ROC
- receiver operating characteristic
- RR
- relative risk
- SE
- standard error
- TOMBOLA
- Trial Of Management of Borderline and Other Low-grade Abnormal smears
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.
