Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/55/06. The contractual start date was in October 2011. The draft report began editorial review in November 2017 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Robert Howard reports membership of the Heath Technology Assessment (HTA) Commissioning Board. Peter Bentham reports grants from the HTA programme during the course of this study. Craig Ritchie reports grants and personal fees from Merck Sharp & Dohme Ltd (Kenilworth, NJ, USA), personal fees from Pfizer Inc. (New York City, NY, USA), Eisai Co. Ltd (Tokyo, Japan), Actinogen (Sydney, NSW, Australia), Kyowa Hakko Kirin (Tokyo, Japan), Eli Lilly and Company (Indianapolis, IN, USA), and F. Hoffmann-La Roche AG (Basel, Switzerland), grants from Biogen Inc. (Cambridge, MA, USA) and grants and non-financial support from Janssen EMEA (Beerse, Belgium) and Takeda Pharmaceutical Company Ltd (Osaka, Japan) during the conduct of the study. Craig Ritchie was also the co-coordinator and academic lead for the European Prevention of Alzheimer’s Dementia (EPAD) project, which has numerous commercial partners in keeping with the mechanisms of the European Union’s Innovative Medicine’s Initiative [i.e. Janssen, Eisai Co. Ltd, Pfizer, Eli Lilly and Company, Roche Diagnostics (Risch-Rotkreuz, Switzerland), Boehringher Ingelheim GmbH (Ingelheim am Rhein, Germany), Novartis International AG (Basel, Switzerland), AC Immune SA (Lausanne, Switzerland), IXICO (London, UK), Aridhia (Glasgow, UK), Amgen Inc. (Thousand Oaks, CA, USA), Berry Consultants (Abingdon, UK), H. Lundbeck A/S (Copenhagen, Denmark), Sanofi SA (Paris, France), IQVIA (formerly Quintiles IMS Holdings, Inc.) (Durham, NC, USA) and Takeda Pharmaceutical Company]. Andrew Sommerlad reports grants from the Wellcome Trust outside the submitted work. Ramin Nilforooshan reports personal fees from Eli Lilly and Company and non-financial support from Janssen outside the submitted work. Martin Knapp reports grants from Merck Sharp & Dohme outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Howard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter are reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
Very late-onset schizophrenia-like psychosis
Schizophrenia is a psychotic disorder affecting approximately 1% of the population and has typical onset of symptoms in early adulthood. Although a small number of schizophrenia patients have onset of illness in middle age, the first appearance of some of the symptoms of schizophrenia in people aged > 60 years has been reported since the 1950s,2 and the lifetime risk for the emergence of psychosis symptoms, particularly in women, may be at its highest in later life. 3
Because schizophrenia so commonly has onset in earlier life and because neurodegenerative disorders that are highly prevalent in older people may also be associated with psychosis symptoms, the diagnosis underlying cases of later-life-onset psychoses may be heterogeneous and their possible connection with schizophrenia has been controversial. The initial clinical description of these patients and use of the term late paraphrenia2,4 was chosen to emphasise both the apparent differences from people with chronic schizophrenia who had grown old and the apparent phenomenological similarity with Kraepelin’s schizophrenia subtype, which he had suggested could be distinguished from dementia praecox by an absence of affective blunting or personality deterioration. 5 However, a later age at onset was never part of Kraepelin’s description of paraphrenia and the diagnosis fell into disuse following later recognition that patients became indistinguishable from those with dementia praecox at follow-up. 6 Roth’s recycling of the term in the name he gave to these patients proved problematic. One suggested alternative, persistent persecutory states,7 never gained traction. Although the term ‘late paraphrenia’ was enthusiastically adopted by European psychiatrists and was included in International Classification of Diseases, ninth edition, it never gained acceptance in the USA, where a diagnosis of late-onset schizophrenia (LOS) was included in the Diagnostic and Statistical Manual of Mental Disorders, third edition, text revision, for cases with onset after the age of 45 years. Subsequent editions of both the International Classification of Diseases and Diagnostic and Statistical Manual of Mental Disorders have not included late paraphrenia or distinct later onset categories for schizophrenia.
Through an expert consensus process, the International Late-Onset Schizophrenia Group suggested that patients with onset of non-affective and non-organic psychosis between the ages of 40 and 60 years should be considered to have LOS, and those with onset after the age of 60 years should be considered to have very late-onset schizophrenia-like psychosis (VLOSLP). 8 Although a cumbersome term, VLOSLP captured the group’s consensus on the relationship between these patients and those with more typical schizophrenia. The term has subsequently gained international usage with researchers and clinicians but has not been included in official disease classification systems.
Epidemiology
The incidence, based on contacts with mental health services, of non-affective psychosis in later life has been reported to range between 14.3 people per 100,000 people per year in the > 65-year-old population in Northumberland9 and 31.4–39.9 people per 100,000 people per year in > 60-year-olds in Tower Hamlets and Camberwell. 10,11 Incidence is higher among women and migrant populations. 10,11
Symptoms
Paranoid (generally persecutory) delusions that are systematised and often fantastic are the hallmark of the presentation of VLOSLP. 4,7,12 Almost all of the symptoms of schizophrenia are seen in patients with VLOSLP, although formal thought disorder and negative symptoms are not found. 13 Partition delusions, a belief that something that normally acts as a physical barrier, such as the walls or ceiling of the patient’s home, has become permeable to the passage of objects or people, or transparent, are found in most patients. 14 Almost invariably, patients have little or no insight into the presence of illness or the potential benefits of antipsychotic treatment. 15 Anecdotal and clinical experience is that psychosis symptoms in this group are remarkably stable, often over several years or even decades.
Differential diagnoses for patients with onset of psychosis symptoms in later life
Early authors considered that patients with VLOSLP represented the expression of schizophrenia but with an onset delayed until later life. 2 Subsequent recognition of the high prevalence of delusions and hallucinations in people with dementia and delirium has led to suggestions that cases of late-life-onset psychosis invariably arise secondary to organic brain pathology. Distinguishing between psychosis symptoms that are associated with a dementia diagnosis and psychosis symptoms otherwise attributed to a functional disorder is complicated by the frequency of psychiatric symptoms in pre-dementia states, such as mild cognitive impairment (MCI),16 and a growing recognition that neuropsychiatric symptoms in cognitively normal individuals can be predictive of a later MCI diagnosis. 17 As research attention moves increasingly to earlier and pre-symptomatic stages of dementias, with recognition of neuropsychiatric symptoms as part of proposed pre-MCI conditions, such as mild behavioural impairment,18 the boundaries between organic and functional psychosis aetiologies will be further challenged. What follows in this section is a review of published investigations of the neuropsychological profile and cognitive prognosis of VLOSLP patients, applications of structural and functional brain imaging to this group and a small number of neuropathological studies that have looked for an underlying substrate for psychosis.
Alzheimer’s disease represents the most important differential diagnosis, as it is common in older people and delusions are often present. Indeed, Alzheimer’s first case, Auguste Deter, presented with delusions of her husband’s infidelity and hallucinations of the voices of her children. 19 Persecutory delusions of theft, harm or abandonment are common and may occur relatively early in Alzheimer’s disease. These may share common aetiological features with symptoms of schizophrenia, while misidentification symptoms are associated with more advanced limbic pathology and cognitive deficit. 20 Psychosis symptoms are also very common in dementia with Lewy bodies (DLB),21 with visual hallucinations reported in 78% of patients, misidentifications, including the phantom boarder and Capgras syndromes, in 56% of patients and delusions in 25% of patients. 22 In addition, DLB patients are exquisitely vulnerable to pareidolias (visual illusions within which figures and faces of people and animals are perceived from ambiguous forms). 23 Ten per cent of frontotemporal dementia (FTD) patients have psychosis symptoms,24 although a positive family history of dementia, progressive cognitive impairment and a lack of response to antipsychotic treatment, as well as the results of neuroimaging and genetic investigations, will point towards the underlying diagnosis. 25 The C9orf72 repeat expansion, which causes familial FTD and amyotrophic lateral sclerosis (ALS), is associated with persecutory or somatoform delusions in 38% of cases. 26 Psychosis in FTD is particularly common in patients with a 10-basepair deletion adjacent to the C9orf72 expansion. 27 Interestingly, this has also been described in three patients with schizophrenia or schizoaffective disorder who had no evidence of any features of FTD or ALS. 28 Just as dementias can present with psychosis, patients with what was later confirmed to be VLOSLP but who were initially misdiagnosed with FTD have been reported. 29
Attempts to understand the significance of cognitive deficits in VLOSLP, in terms of revealing the effects of potential underlying neurodegeneration, are complicated by consideration of the ubiquitous cognitive deficits seen in schizophrenia patients at all ages and a lack of application of standardised diagnostic criteria for dementia or failure to otherwise control for the presence of risk factors for cognitive impairment in studied patient groups. 30 Comparing the neuropsychological profile of LOS or VLOSLP with that of early-onset schizophrenia (EOS) represents one strategy to potentially control for some of these difficulties. LOS patients are less impaired than EOS patients on arithmetic, digit symbol coding and vocabulary, but are more impaired on executive functions, attention, fluency, global cognition and visuospatial construction. 31,32 However, patients with VLOSLP have similar age-corrected scores to EOS individuals on intelligence quotient (IQ), verbal memory, attention and executive functioning, and perform better on the Cambridge Cognition Examination than LOS patients. 33 Cluster analysis of clinical and cognitive features in VLOSLP patients generated two clusters: one characterised by a schizophrenia-like presentation with cognitive deficits restricted to executive functioning, and a cluster with less complex psychosis symptoms and generalised cognitive impairment. 34
Long-term cognitive follow-up of patients with LOS has generally suggested stability of cognitive and everyday functioning,35,36 in contrast to some reports of marked cognitive decline reported from older institutionalised people with chronic schizophrenia. 37 However, in an Australian study, 9 out of 27 patients with LOS had developed dementia after follow-up of 5 years. 38 It is worth noting that, although patients with known dementia were excluded from this cohort, the cognitive impairment threshold for inclusion was set very low at a Mini-Mental State Examination (MMSE)39 score of 20 points, so that individuals with mild dementia could have been included at the beginning of follow-up. A Danish register-based study, involving 7712 VLOSLP patients, and with more than 4 years’ follow-up, reported a relative risk of developing dementia of 2.21 [95% confidence interval (CI) 1.39 to 3.50]. 40
Brain imaging studies have indicated increased lateral ventricular volume, comparable to that seen in young-onset schizophrenia, in LOS41–43 and VLOSLP. 13,44 Both increases45 and no increases44,46 in areas of white matter magnetic resonance imaging signal hyperintensity have been reported in people with VLOSLP and diffusion tensor imaging has not suggested abnormalities within specific white matter tracts mediating frontal lobe connectivity. 47
Although based on only small numbers of patients, post-mortem reports of limbic tauopathy,48,49 a FTD-like pattern of dentate gyrus TAR-DNA binding protein50 and Lewy bodies and argyrophilic grain disease within the limbic system51 may suggest that some of these patients may have an as yet unrecognised, distinct neuropathology that may not proceed to diagnosable dementia. Although it is possible that some of the cases involved in these studies may have had unrecognised dementia pathologies, they suggest an important line for future research in the understanding of VLOSLP and potentially related disorders.
Treatment
Two Cochrane reviews have concluded that there is no available good-quality randomised clinical trial-based evidence on which to guide treatment of LOS or VLOSLP patients. 52,53
Most of what is known about the treatment received by VLOSLP patients and their responses and outcomes in practice is derived from register, case note and prescription review studies. Reviewing antipsychotic prescriptions from 30 sites in Tokyo, Uchida et al. 54 reported that LOS patients were typically prescribed half, and VLOSLP patients one-third, of the dose given to early-onset patients who had grown old. Case note review of VLOSLP patients who have been naturalistically treated with atypical antipsychotics has indicated response rates of 77% for inpatients and 38% for outpatients, although symptoms tended to be ameliorated rather than eradicated. 55 Male patients are more likely to be admitted compulsorily and to subsequently be lost to follow-up, and 38% of patients in contact with services continue to express paranoid delusions. 56 Patients typically have no insight into their illness or the potential value of treatment and consequent difficulties involved in engaging them in treatment and maintaining contact and compliance represent a huge challenge. In a UK specialist older adults’ mental health service, fewer than half of VLOSLP patients who had completed an assessment were successfully started on antipsychotic treatment and only 27% remained on treatment after 1 year or at the point of discharge from the service. 57 This reflects the way that mental health services currently fail to engage people with this diagnosis in treatment and the majority of patients are consequently not prescribed antipsychotic medication and discharged untreated back to primary care.
Comparisons of outcomes with patients with more typical early adult-onset schizophrenia who have grown old have indicated important differences. Although VLOSLP patients are more likely to be admitted to a psychiatric hospital than comparably aged patients with onset < 60 years, they are not more likely to transition to long-term residential care58 and have better social functioning as indexed by participation, network size and availability of confidantes. 59 Differences in standardised mortality ratios between VLOSLP (5.02) and early schizophrenia (2.93) are largely explained by increased physical comorbidity and accidents in VLOSLP patients. 60
There have been two published clinical trials of drug interventions in VLOSLP. The first61 was an open trial of 5 weeks’ treatment with 50–200 mg per day of amisulpride in 26 patients who met both criteria for VLOSLP8 and Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) criteria for schizophrenia. 62 Participants in this study had a MMSE39 score of > 26 points and comprised 17 women and nine men aged 65–85 years [mean 76.2 years, standard deviation (SD) 5.8 years]. The amisulpride dose was titrated up against symptoms and the emergence of side effects; by week 5 participants were taking a mean dose of 101 mg per day (SD 38.4 mg). Participants showed improvement on the primary outcome measure, the Brief Psychiatric Rating Scale (BPRS),63 with mean scores at baseline of 41.5 points (SD 8.9 points) and at 5 weeks of 27.2 points (SD 5.8 points). Amisulpride was well tolerated by participants, with no significant changes in overall cognitive function, sedation, weight or routine laboratory tests. Three participants reported the development of tremor during the study, but there was no significant overall worsening of extrapyramidal symptoms between baseline and study end.
The second reported trial in VLOSLP was an open trial of 4 weeks of yokukansan, a herbal medicine that is thought to be an antagonist at 5-hydroxytryptamine 2A receptors and to inhibit glutamate-mediated excitotoxicity. Forty patients (20 women and 20 men), also meeting criteria for both VLOSLP8 and DSM-IV-TR criteria for schizophrenia,62 showed reductions in BPRS scores from a mean of 36.7 points (SD 4.65 points) at baseline to 20.1 points (SD 1.6 points) after 4 weeks of treatment. 64 Treatment was well tolerated and no significant adverse effects were reported.
Meta-analyses have suggested that antipsychotic treatment shows only a small benefit over placebo in the treatment of psychosis symptoms in patients with dementia65 and that withdrawal of treatment is associated with worsening of symptoms. 66 However, antipsychotic treatment in people with dementia also carries significant risks, of both common and expected adverse effects such as extrapyramidal symptoms and falls,65 as well as stroke and death. 67 In the absence of data from controlled trials of the use of antipsychotic drugs in VLOSLP, clinicians have been unable to evaluate the potential risks and benefits of treatment for their patients. Awareness of the modest benefits of antipsychotics over placebo and the known serious risks of treatment in people with dementia have understandably led to caution in the use of these drugs in all older people with neuropsychiatric disorders. This caution, combined with the intrinsic failure of people with VLOSLP to appreciate that they have an illness and might benefit from treatment, has contributed to the very low current levels of treatment engagement discussed above. We conducted the Antipsychotic Treatment of very LAte-onset Schizophrenia-like psychosis (ATLAS) trial to provide evidence of the benefits and risks of antipsychotic drug treatment in this understudied patient group.
Chapter 2 Methods
Parts of this chapter are reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
Trial objectives
The ATLAS trial was a multicentre randomised controlled trial (RCT), conducted within NHS secondary care mental health services, for older people to evaluate the clinical effectiveness and cost-effectiveness of antipsychotic treatment in VLOSLP.
The primary objectives of the trial were:
-
To determine whether or not treatment with 100 mg/day of amisulpride was superior to placebo as measured by significant differences between amisulpride- and placebo-treated groups in changes in scores on the BPRS over 12 weeks. Given the nature of the symptoms of people with VLOSLP, we anticipated that any beneficial effects of treatment would be most apparent on the hostility, suspiciousness, hallucinations, tension, unco-operativeness and motor hyperactivity subscores of the BPRS.
-
To determine whether or not prolonging amisulpride treatment for a further 12 weeks after the initial 12-week treatment period conferred additional benefit, as measured by reduction in BPRS scores compared with placebo and fewer patients in the amisulpride than placebo groups being withdrawn to open treatment with amisulpride by their physicians.
In addition, there were a number of secondary objectives to determine the:
-
risks of side effects and serious adverse events (SAEs) associated with amisulpride treatment
-
compliance of VLOSLP participants with allocated trial treatment
-
effects of amisulpride treatment on quality of life
-
cost-effectiveness of amisulpride treatment.
Trial design
The ATLAS trial was a three-arm, parallel-group, randomised double-blind placebo-controlled trial with two stages.
-
Stage 1 consisted of an initial 12 weeks of double-blind placebo-controlled treatment to investigate the efficacy and tolerability of 100 mg/day of amisulpride (groups A and B) versus matched placebo (group C).
-
Stage 2 was a subsequent double-blind stage in order to investigate the effects of treatment continuation for a further 12 weeks with 100 mg/day of amisulpride (group A) versus switching to placebo (group B).
Outcome measures
The ATLAS trial had two primary outcome measures. The first was the BPRS, a widely used clinician-rated instrument for the assessment of positive, negative and affective symptoms in patients with psychotic disorders. 63 The BPRS assesses the severity of symptoms across 18 symptom constructs, each item being rated from 1 (not present) to 7 (extremely severe) and the total score obtained by summing scores from the 18 items. Scores therefore range from 18 to 126 points, with higher scores indicating greater levels of psychopathology. The BPRS was administered at baseline and then at weeks 4, 12, 16 and 24. Changes in BPRS score between baseline and the week 12 and week 24 assessments were coprimary outcomes.
The BPRS was chosen for the ATLAS trial, rather than alternative and more schizophrenia-specific symptom rating scales, for the following reasons:
-
The psychosis symptom profile of VLOSLP is different from that of schizophrenia, with more prominent persecutory delusions, hallucinations and hostility, and without affective blunting, negative symptoms or formal thought disorder.
-
The BPRS contains items that cover the most important symptoms seen in people with VLOSLP, in particular the hostility, suspiciousness, hallucinations, unusual thought content, tension and unco-operativeness items.
-
The BPRS had already been shown to be sensitive to improvements in symptoms with open-label amisulpride treatment in VLOSLP patients. Psarros et al. 61 reported a 30% reduction in BPRS scores in VLOSLP with 5 weeks of treatment with amisulpride.
-
Even clinically inexperienced raters can achieve high levels of test–retest reliability on the BPRS68 and clinicians can be trained to achieve high levels of reliability with the scale within a single day. The ATLAS trial assessments were carried out by NHS staff working within the older people’s mental health services where participants were recruited. In the ATLAS trial, the BPRS was administered by a suitably qualified and trained ATLAS trial team member: a consultant old age psychiatrist, a higher trainee in old age psychiatry, an associate specialist, a specialty doctor in old age psychiatry or an experienced and trained clinical trials nurse. We specified that raters should be individuals who were knowledgeable about psychotic disorders in older people, who were able to interpret the constructs used in administration and scoring of the assessment, and who had received appropriate training for the trial assessments. Whenever possible, the same rater was used for baseline and all subsequent follow-up ratings on each ATLAS participant.
The second primary outcome measure was the proportion of participants who were withdrawn by their physicians from allocated trial treatment to open-label amisulpride between weeks 13 and 24 because of perceived lack of treatment efficacy. This was compared between participants randomised to continue 100 mg/day of amisulpride (group A) and those randomised to switch to placebo (group B).
Secondary outcome measures were as follows:
-
Extrapyramidal symptoms were measured with a modified version of the Simpson–Angus Scale (SAS). 69 The modification involved omitting the head-dropping assessment as this was difficult to perform during home-based trial assessments. The modified SAS used for the ATLAS trial consequently measured nine extrapyramidal signs, all of which were assessed by direct examination: gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, glabellar tap, tremor and salivation. Each item is rated on a scale of 0 to 4, with higher scores indicating a greater severity of extrapyramidal symptoms. The scale range of the modified SAS was thus from 0 to 36. All trial staff were trained in the administration of the SAS and a standardised description of each item was given to increase reliability. The SAS was administered at baseline and weeks 4, 12, 16 and 24. The changes in SAS score between baseline and week 12 and between weeks 12 and 24 were compared between groups to assess extrapyramidal symptoms.
-
Compliance with medication, expressed as treatment discontinuation rates and percentages of prescribed trial medication taken between weeks 1 and 12 and between weeks 13 and 24, was compared between those allocated to receive amisulpride and placebo.
-
Quality of life, measured with the EuroQol-5 Dimensions (EQ-5D),70 to inform cost-effectiveness calculations, and with the self-rated 26-item World Health Organization’s quality-of-life scale (WHOQoL-BREF)71 at baseline, 12 and 24 weeks. The WHOQoL-BREF contains two items that ask about an individual’s overall perception of their quality of life and health, and questions that assess four domains: physical, psychological, social and environmental well-being. It has been used in studies of older people with schizophrenia. 72,73
-
Costs were calculated by attaching nationally applicable unit cost measures to health and social service use and medication data collected with a modified version of the Client Service Receipt Inventory (CSRI)74 at baseline, 12 and 24 weeks. The study also collected data on unpaid carer inputs and attached imputed values to these.
-
Pharmacokinetics of amisulpride, through serum levels of amisulpride and the hormone prolactin, were analysed during both stages of the study in participants who additionally consented to this. These data were combined with other clinical information to investigate variability and covariate effects on the relationship between serum amisulpride concentration and profiles of response and side effects.
Participants
Participants were patients with VLOSLP recruited from the community and inpatient teams of NHS older people’s mental health services. Inclusion criteria were as follows:
-
a diagnosis of VLOSLP meeting international consensus group criteria,8 including onset of delusions and/or hallucinations after the age of 60 years
-
a BPRS score of > 30 points or level of mental health symptoms consistent with this if BPRS was not routinely administered at the recruiting centre
-
capacity to give informed consent to participation in a clinical trial.
Exclusion criteria were any of the following:
-
Evidence of significant cognitive impairment and standardised MMSE score of < 25 points.
-
Uncontrolled serious physical illness.
-
Primary diagnosis of an affective disorder.
-
Prescribed amisulpride in the previous 28 days. Patients who were treated with other antipsychotic drugs in the previous 28 days but still satisfied the inclusion criteria, and for whom stopping their current antipsychotic and switching to trial medication was considered appropriate, could participate. This was included as a stratification factor at randomisation.
-
Contraindication to amisulpride (e.g. phaeochromocytoma, prolactin-dependent tumour) or potential drug interactions (e.g. with levodopa).
-
Participation in another clinical trial of an investigational medicinal product in the previous 28 days.
Screening and recruitment
Patients judged to have met eligibility criteria by their physicians had the potential benefits of antipsychotic treatment explained to them and participation in the ATLAS trial was introduced as one possible option at this stage. If patients were potentially interested in taking part in the study, they were given an information leaflet so that they could learn more about the trial. Once a potentially eligible patient was identified, they were registered with the ATLAS study office and an ATLAS patient treatment pack was sent to their local hospital pharmacy so that treatment could be given at the second appointment if they consented to be randomised. A second appointment was arranged at the clinic or in the patient’s home, after a delay of at least 24 hours, to discuss the trial information, answer any questions that the patient may have had about the study and to seek their consent to participate. At the second appointment, the patient was given a general outline of three possible options: (1) choice of treatment (i.e. amisulpride or no antipsychotic treatment), (2) participation in the ATLAS trial with the choice of amisulpride or placebo made by randomisation or (3) taking more time to consider. A checklist was provided in the ATLAS study folder to facilitate this information and consenting appointment. After a full explanation was given of all the treatment options and the manner of treatment allocation, all eligible patients were invited to participate in the trial. Written informed consent was sought from all of those who agreed to participate and, if the patient was dependent on a carer, assent was additionally obtained from the carer using the carer assent form in the study folder. If taking consent had been delegated to a non-physician, patients were offered the opportunity to speak with the study doctor, who would document that they had confirmed the patient’s diagnosis and eligibility.
After obtaining consent, baseline assessments (i.e. using BPRS, SAS, WHOQoL-BREF and EQ-5D) were undertaken. If the BPRS was used as part of the recruiting centre’s routine assessment and had been administered at screening, then this did not need to be repeated and the eligibility-confirming BPRS was used as the baseline measure. After completion of all baseline assessments, patients were randomly allocated. If a patient declined to participate, the ATLAS study office was notified so that they knew that the allocated treatment pack had not been taken from the hospital pharmacy. Reasons why eligible patients were not invited to participate or did not consent to take part were recorded on the screening log in the ATLAS study folder.
Randomisation
After informed consent was obtained and baseline assessments completed, randomisation was carried out centrally by the ATLAS randomisation service based at the Oxford Clinical Trials Service Unit (CTSU). The person randomising completed the ATLAS randomisation notepad before calling the CTSU so that they were prepared to answer the questions involved. Alternatively, randomisation forms could be faxed or scanned and e-mailed to the ATLAS randomisation service, which would call back with a treatment allocation. After the necessary details had been provided, the allocated treatment pack number was specified. The recruiting principal investigator (or other medically qualified doctor with a substantive or honorary contract with the recruiting NHS trust and who has signed the ‘Recruiting Investigator site delegation of authority’ form) completed an ATLAS prescription form. The first ATLAS treatment pack with the specified number was collected from the hospital pharmacy and given to the patient and a label containing instructions for the trial treatment was stuck in the patient’s clinical notes.
Trial treatment
Trial treatment was 100 mg/day of oral amisulpride or identically appearing overencapsulated placebo packed into treatment cartons of 12 weeks’ treatment in the form of 3 × 28 blister-packed capsules (for stages 1 or 2). Trial treatment was packed, labelled and released by a qualified person and dispatched to participating centres’ pharmacies by Sharp Clinical Services (Crickhowell, UK). Patients were allocated a treatment pack number at randomisation, which was also the patient’s unique identifying number. The initial (stage 1) treatment carton was obtained from the local hospital pharmacy using the ATLAS prescription form in the study folder. Treatment started as soon as possible and was continued for 24 weeks unless a definite contraindication was identified. If the participant was still compliant with trial treatment at the 12-week assessment (i.e. taking capsules sufficiently regularly that compliance with weeks 13 to 24 treatment seemed likely), the ATLAS study office was informed by telephone and the second ATLAS treatment pack was then allocated. This 12-week (stage 2) carton contained the participant’s treatment from weeks 13 to 24 in the same form of 3 × 28 blister-packed capsules (12 weeks’ treatment at one capsule per day). The second pack allocation was issued by the ATLAS trial office to ensure that participants were allocated the correct medication in stage 2. Pharmacies at each site maintained a study medication dispensing log (including date dispensed, batch number, expiry date and number of capsules prescribed).
The dosing regimens for the three treatment arms were:
-
Group A took one capsule containing 100 mg of amisulpride per day for 24 weeks.
-
Group B took one capsule containing 100 mg of amisulpride per day for 12 weeks, followed by one matched capsule containing placebo per day for a further 12 weeks.
-
Group C took one placebo capsule per day for 12 weeks, followed by one capsule containing 100 mg of amisulpride per day for a further 12 weeks.
Treatment compliance was monitored by capsule count. Patients were asked to bring any unused study medication at each follow-up visit and at the end of the trial. The local principal investigator or research worker logged study medication returns, return date and amount of study medication returned on the follow-up form. Once returned medication was logged, it was destroyed by the local pharmacy.
Arrangements for continued treatment at the end of the trial were made on an individual patient basis by the local principal investigator or other clinicians who were responsible for the patient’s care at that point. Responsible clinicians were asked to record on the last patient follow-up form what treatment plan was in place for the individual patient.
Other treatments
Treatment with any other antipsychotic drug was not permitted during the study period. Patients who were taking other antipsychotics at entry to the trial but still met inclusion criteria stopped their antipsychotic before commencing the ATLAS trial treatment. When prescribing concomitant medication, investigators were asked to take into consideration the potential for drug interactions with amisulpride. Apart from this, all other aspects of patient management were entirely at the discretion of their local doctors. Patients were managed in whatever way appeared best for them, with no special treatments, no additional laboratory or other investigations and no extra follow-up. Any concomitant medications were recorded on the ATLAS patient follow-up form. If patients agreed to have the optional blood test, these results were not used to inform management but were stored until the end of the trial to be analysed with other collated data.
Trial assessments
Assessments were undertaken prior to randomisation (baseline) and then at week 4 (±1 week), between weeks 10 and 12, week 16 (±1 week) and between weeks 22 and 24. The weeks 10–12 and 22–24 assessments were scheduled to take place during the last 2 weeks before completion of the first and second treatment stages to ensure that the patient would still be taking trial medication during the assessment, even if their appointment was delayed for any reason. The follow-up assessments were undertaken whether or not patients continued to be compliant with trial treatment. Table 1 shows the schedule of trial assessments.
| Assessment | Time point | |||||
|---|---|---|---|---|---|---|
| Eligibility screening | Information and consent | Week 4 (±1 week) | Weeks 10–12 | Week 16 (±1 week) | Weeks 22–24 | |
| Diagnosis (ICG criteria) | ✗ | |||||
| Standardised MMSE | ✗ | |||||
| BPRS | (✗)a | (✗)a | ✗ | ✗ | ✗ | ✗ |
| Inclusion and exclusion criteria | ✗ | |||||
| Capacity assessment | ✗ | ✗ | ||||
| Patient registration | ✗ | |||||
| Informed consent | ✗ | |||||
| Randomisation | ✗ | |||||
| SAS | ✗ | ✗ | ✗ | ✗ | ✗ | |
| EQ-5D | ✗ | ✗ | ✗ | ✗ | ||
| WHOQoL-BREF | ✗ | ✗ | ✗ | ✗ | ||
| Randomisation | ✗ | |||||
| Dispense medication | ✗ | ✗ | ||||
| Compliance check | ✗ | ✗ | ✗ | ✗ | ||
| Adverse events | ✗ | ✗ | ✗ | ✗ | ||
| Follow-up form | ✗ | ✗ | ✗ | ✗ | ||
| CSRI | ✗ | ✗ | ||||
Statistical considerations
As patients with VLOSLP have very rarely been recruited to RCTs, the ATLAS trial included an initial feasibility phase to assess recruitment and retention. Following this, the initial target sample size of 300 randomised participants was pragmatically reduced to 100 because of the practical difficulties involved in recruiting people with this diagnosis who are largely without insight into the presence of illness or the possible benefits of treatment.
Taking a 5-point improvement on the BPRS as a minimal clinically important difference (MCID)61 and assuming that the SD of BPRS measures would be 9 points, the trial was powered to detect a moderate treatment effect of 0.56 SDs. The initial target recruitment of 300 participants would have given > 90% power at 2p < 0.05 to detect the MCID of 5 points on the BPRS in stage 2 (75 participants completing 12 weeks of amisulpride and then randomised to 12 weeks further of amisulpride vs. 75 participants completing 12 weeks of amisulpride and then a further 12 weeks of placebo), and 90% power at 2p < 0.05 to detect the MCID after 12 weeks of stage 1 (150 participants receiving 12 weeks of amisulpride vs. 75 participants receiving 12 weeks of placebo). With 100 patients randomised and assuming that 90% of participants completed outcome assessments at 12 weeks, this would give 70% power at 2p < 0.05 to detect the 5-point MCID, and > 80% power to detect a 6-point difference in BPRS in stage 1.
Treatment packs were allocated centrally by the ATLAS study office. The minimised randomisation procedure aimed to balance treatment allocation (as far as possible given available packs at each centre) overall, and by six stratification variables: age (60–69, 70–79, ≥ 80 years), sex, home circumstances (living with spouse/partner, living alone, other), time since onset of symptoms (< 6, ≥ 6 months), previous antipsychotic treatment (no, yes > 1 month previously, yes ≤ 28 days ago) and BPRS score (30–39, 40–49, ≥ 50 points).
Analyses of each stage include all patients who have taken any study treatment in that stage. Participants who withdrew after pack allocation but before receiving any treatment can be safely excluded; as treatment allocation is double-blinded, it cannot influence the decision to withdraw and thus introduce selection bias. Analyses of this population were by intention to treat (i.e. all participants will be analysed in the treatment arm to which they were randomised, whether or not they adhered to the treatment). This is to avoid any potential bias in the analysis.
Primary analysis
The primary analysis was undertaken once all patients had reached 22–24 weeks from randomisation. To assess the efficacy of 12 weeks of amisulpride in stage 1, the primary outcome of BPRS score was compared using a repeated measures regression model. Data from weeks 4 and 12 were the outcome variable, baseline scores were entered into the model as a covariate. Time was modelled as a categorical variable. The comparison was between active amisulpride treatment (groups A and B together) and placebo (group C). The six minimisation factors (age, gender, home circumstances, BPRS score, time since onset of symptoms and previous antipsychotic treatment) were also included as covariates in the repeated measures model. A time-by-treatment interaction parameter was included to examine if there was any changing treatment effect over time. Treatment effects are presented with 95% CIs and associated p-values in each case. For these analyses, a p-value of 0.05 (5% level) is used to indicate statistical significance. This analysis uses all available visit data, which maximises statistical power to detect any difference at visits.
To assess the value of continuing treatment in stage 2, group A (amisulpride followed by amisulpride) was compared with group B (amisulpride followed by placebo). Most patients have only one outcome time point at week 24 or 36; the protocol was amended to shorten stage 2 to 12 weeks, with an additional assessment at week 16. An analysis of covariance was therefore carried out using the week 12 BPRS score as baseline into the model as a covariate. Confidence intervals for the difference in means were calculated. Participants who withdrew in stage 1 were not included in this analysis.
The BPRS covers the important symptoms elicited in VLOSLP patients. In particular, the hostility, suspiciousness, hallucinations, unusual thought content, tension and unco-operativeness items of the BPRS all assess important areas of psychopathology in these patients. The 7-point rating of the BPRS on each of these items generates a subscore for these six symptom domains that the protocol prespecified as those most likely to be affected by the disorder. Scores on the subset range from 6 to 42 points, with higher scores indicating greater levels of psychopathology. The subscore was analysed in the same way as the full BPRS analysis, for both stages 1 and 2.
Secondary analysis
The SAS was used to see if amisulpride had an effect on extrapyramidal side effects (EPSEs). The change in SAS scores was plotted over time to see if there were any increases. If the SAS scores remain constant over the assessment time points, it can be assumed that amisulpride is having no effect on EPSEs.
The EQ-5D is a standardised generic measure of health-related quality of life (HRQoL) applicable to a wide range of health conditions. It provides a simple descriptive profile. EQ-5D is made up of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension (in the version used by the ATLAS trial) has three levels: no problems, some problems and extreme problems. These five dimensions can be converted, using societal weights,75 into a single health utility value between –0.594 and 1.0, where 1.0 represents full health and values below 0 can be interpreted as representing health states worse than death. The change in EQ-5D health utility value was plotted over time to show any changes and analysed using analysis of covariance.
Quality of life is also measured with the self-rated, short, 26-item, WHOQoL-BREF at baseline, weeks 10–12, week 16 and the end of study. The WHOQoL-BREF includes two items about an individual’s overall perception of their quality of life and health and questions assessing four domains: physical, psychological, social and environmental well-being. Higher scores denote a better quality of life. The four domains were converted so they were all on a scale of 0 to 100, where higher scores denote better quality of life. These four separate domains were analysed using analysis of covariance.
Chapter 3 Results
Parts of this chapter are reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
Recruitment
Twenty-seven UK NHS mental health trusts in England and Scotland were opened to recruitment in the ATLAS trial.
The first patient was randomised on 27 September 2012, with recruitment ending on 6 June 2016. The ATLAS trial randomised over a period of 46 months, with an average recruitment rate of 2.2 participants per month. Yearly recruitment per site is shown in Table 2, and cumulative monthly recruitment is shown in Figure 1.
| Sites (NHS mental health trust) | Year (number of participants recruited) | Total number of participants recruited | ||||
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | ||
| Ayrshire and Arran | 0 | 2 | 0 | 0 | 1 | 3 |
| Birmingham and Solihull | 0 | 1 | 0 | 0 | 0 | 1 |
| Black Country Partnership | 0 | 0 | 1 | 0 | 1 | 2 |
| Bradford | 0 | 0 | 0 | 0 | 0 | 0 |
| Cambridge and Peterborough | 0 | 1 | 1 | 1 | 0 | 3 |
| Camden and Islington | 0 | 3 | 0 | 1 | 2 | 6 |
| Cheshire and Wirral | 0 | 3 | 3 | 0 | 0 | 6 |
| Coventry and Warwickshire | 0 | 0 | 1 | 0 | 0 | 1 |
| Derbyshire | 0 | 0 | 1 | 2 | 1 | 4 |
| Devon | 0 | 1 | 1 | 0 | 0 | 2 |
| East London | 0 | 3 | 1 | 2 | 1 | 7 |
| Forth Valley | 0 | 1 | 1 | 1 | 0 | 3 |
| Norfolk and Suffolk | 0 | 1 | 2 | 1 | 0 | 4 |
| North Staffordshire | 0 | 1 | 0 | 0 | 0 | 1 |
| Northamptonshire | 0 | 0 | 1 | 0 | 0 | 1 |
| Northumberland, Tyne and Wear | 0 | 1 | 1 | 2 | 0 | 4 |
| Nottinghamshire | 0 | 1 | 3 | 0 | 0 | 4 |
| Oxford | 0 | 1 | 0 | 0 | 0 | 1 |
| South London and Maudsley | 4 | 10 | 5 | 8 | 5 | 32 |
| South Staffordshire and Shropshire | 0 | 0 | 3 | 0 | 2 | 5 |
| South West Yorkshire | 0 | 2 | 0 | 0 | 0 | 2 |
| Southern Health | 0 | 0 | 0 | 0 | 0 | 0 |
| Surrey and Borders | 0 | 4 | 0 | 0 | 10 | 5 |
| Sussex Partnership | 0 | 1 | 0 | 0 | 0 | 1 |
| Tayside | 0 | 0 | 0 | 1 | 0 | 1 |
| West London | 0 | 1 | 0 | 0 | 0 | 1 |
| Worcestershire | 0 | 0 | 1 | 0 | 0 | 1 |
| Yearly total | 4 | 38 | 26 | 19 | 14 | 101 |
FIGURE 1.
Cumulative monthly recruitment.

A total of 122 patients were assessed for trial eligibility, with a total of 101 patients randomised across 25 sites. Thirty patients were allocated to group A, 36 to group B and 35 to group C. Figure 2 is the Consolidated Standards of Reporting Trials (CONSORT) flow chart of patients through the ATLAS trial, which summarises the patients through the two stages of the trial. Nine randomised patients did not start trial medication and, as prespecified in the protocol, are excluded from all analyses; one had been allocated to group A, four to group B and four to group C.
FIGURE 2.
The CONSORT flow chart. ITT, intention to treat. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
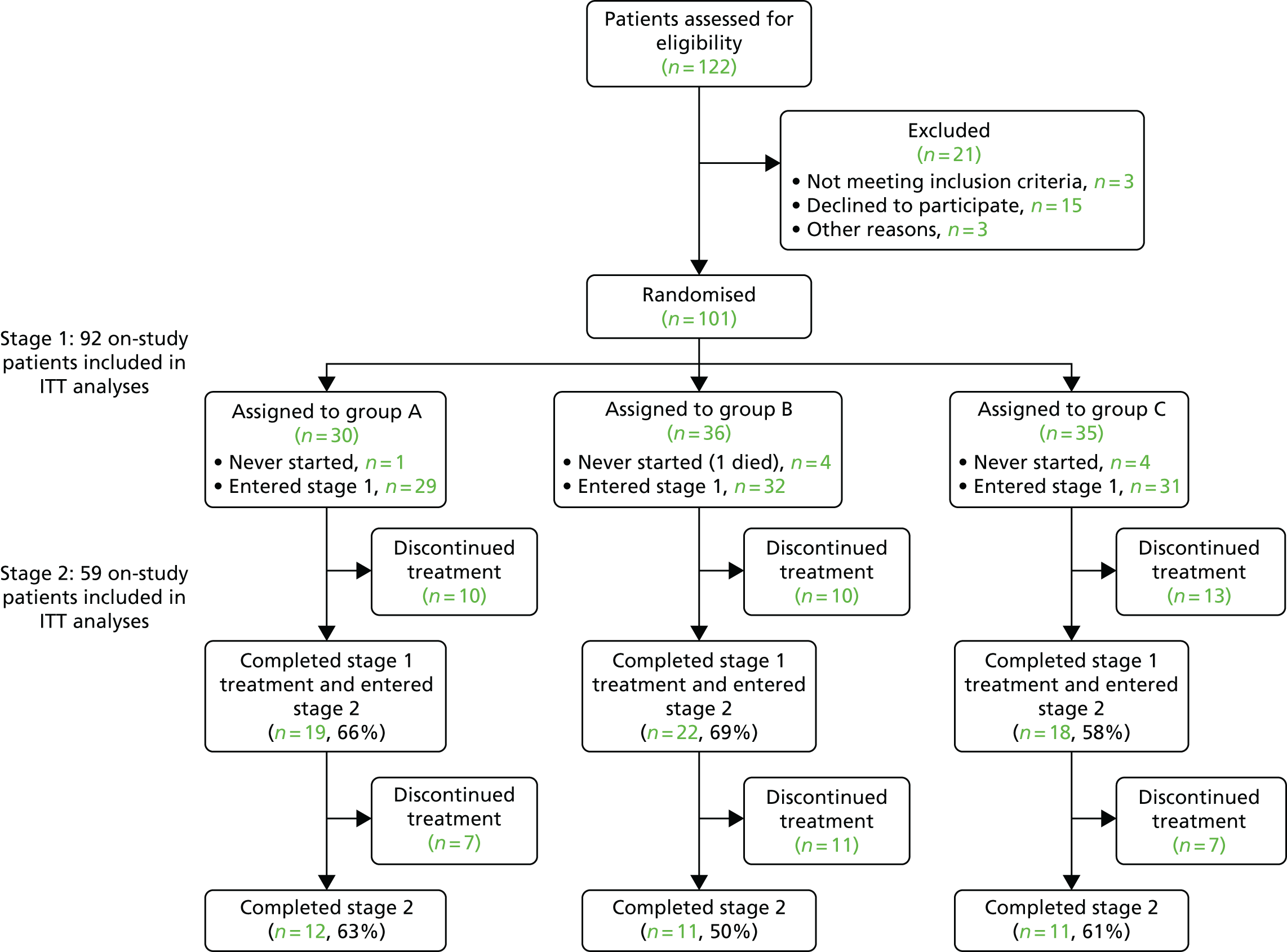
Of the 92 on-study patients, 15% (14/92) had stopped taking trial medication by 4 weeks and a further 21% (19/92) stopped between the 4-week and 12-week assessments. Of the remaining 59 patients who entered stage 2 (19 in group A; 22 in group B; and 18 in group C), 42% (25/59) stopped trial medication during the 24 weeks (or, later, 12 weeks). A total of 58% (34/59) of patients completed stage 2 treatment.
Completeness of data
Once patients were randomised, every effort was made to follow the patients through both stages of treatment to obtain all follow-up forms and outcome assessments. The return rates for each of the outcome measures are summarised in Tables 3–7, split by stage, and excluding those who withdrew prior to starting treatment (i.e. those not effectively randomised).
| Case report forms | Randomisation | Consent |
|---|---|---|
| Received (n) | 92 | 92 |
| Expecteda (n) | 92 | 92 |
| % | 100 | 100 |
| Case report forms | Stage | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| Baseline | 4 weeks | 12 weeks | 12 weeks | 16 weeks | 24 weeks | 36 weeks | |
| Received (n) | 92 | 87 | 83 | 58 | 12 | 14 | 40 |
| Expecteda (n) | 92 | 92 | 92 | 59 | 14 | 16 | 41 |
| % | 100 | 95 | 90 | 98 | 86 | 88 | 98 |
| Case report forms | Stage | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| Baseline | 4 weeks | 12 weeks | 12 weeks | 16 weeks | 24 weeks | 36 weeks | |
| Received (n) | 92 | 85 | 78 | 55 | 12 | 13 | 37 |
| Expecteda (n) | 92 | 92 | 92 | 59 | 14 | 16 | 41 |
| % | 100 | 92 | 85 | 93 | 86 | 81 | 90 |
| Case report forms | Stage | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| Baseline | 4 weeks | 12 weeks | 12 weeks | 16 weeks | 24 weeks | 36 weeks | |
| Received (n) | 90 | 80 | 57 | 11 | 14 | 40 | 40 |
| Expecteda (n) | 92 | 92 | 59 | 14 | 16 | 41 | 41 |
| % | 98 | 87 | 97 | 79 | 88 | 98 | 98 |
| Case report forms | Stage | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| Baseline | 4 weeks | 12 weeks | 12 weeks | 16 weeks | 24 weeks | 36 weeks | |
| Received (n) | 90 | 80 | 57 | 10 | 13 | 38 | 38 |
| Expecteda (n) | 92 | 92 | 59 | 14 | 16 | 41 | 41 |
| % | 98 | 87 | 97 | 71 | 81 | 93 | 93 |
Baseline data
Table 8 displays a comparison of the key patient characteristics of the 92 on-study patients in stage 1 and of the 59 on-study patients in stage 2.
| Characteristic | Stage | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| Group A (N = 29) | Group B (N = 32) | Group C (N = 31) | Group A (N = 19) | Group B (N = 22) | Group C (N = 18) | |
| Age (years), n (%) | ||||||
| 60–69 | 5 (16) | 4 (18) | ||||
| 70–79 | 13 (45) | 14 (44) | 12 (39) | 10 (53) | 10 (45) | 5 (28) |
| ≥ 80 | 16 (55) | 13 (41) | 19 (61) | 9 (47) | 8 (36) | 13 (72) |
| Mean age (years) (SD) | 81.2 (6.8) | 78.8 (8.3) | 80.6 (5.4) | 80.6 (7.4) | 77.6 (7.7) | 80.9 (5.3) |
| Gender | ||||||
| Male | 8 (28) | 7 (22) | 6 (19) | 5 (26) | 6 (27) | 4 (22) |
| Female | 21 (72) | 25 (78) | 25 (81) | 14 (74) | 16 (73) | 14 (78) |
| Ethnic group, n (%) | ||||||
| White | 22 (76) | 22 (71) | 22 (73) | 16 (84) | 15 (71) | 11 (65) |
| Black | 7 (24) | 7 (23) | 6 (20) | 3 (16) | 5 (24) | 4 (24) |
| Mixed | 1 (3) | |||||
| Other | 1 (3) | 2 (7) | 1 (5) | 2 (12) | ||
| Home circumstances, n (%) | ||||||
| Alone | 23 (79) | 20 (63) | 20 (65) | 14 (74) | 12 (55) | 12 (67) |
| With spouse/partner | 4 (14) | 6 (19) | 6 (19) | 4 (21) | 5 (23) | 2 (11) |
| Other | 2 (7) | 6 (19) | 5 (16) | 1 (5) | 5 (23) | 4 (22) |
| BPRS score, n (%) | ||||||
| 30–39 | 11 (38) | 13 (41) | 18 (58) | 6 (32) | 8 (36) | 12 (67) |
| 40–49 | 15 (52) | 12 (38) | 11 (35) | 12 (63) | 9 (41) | 6 (33) |
| ≥ 50 | 3 (10) | 7 (22) | 2 (6) | 1 (5) | 5 (23) | |
| Mean BPRS score (points) (SD) | 41.4 (7.2) | 43.5 (9.4) | 38.9 (6.2) | 41.8 (7.5) | 44.1 (9.4) | 37.7 (4.6) |
| Time with symptoms, n (%) | ||||||
| < 6 months | 10 (37) | 3 (10) | 8 (26) | 8 (44) | 1 (5) | 3 (17) |
| ≥ 6 months | 17 (63) | 28 (90) | 23 (74) | 10 (56) | 20 (95) | 15 (83) |
| Antipsychotics, n (%) | ||||||
| None previously | 13 (45) | 17 (53) | 15 (48) | 7 (37) | 12 (55) | 9 (50) |
| Yes, > 1 month previously | 2 (7) | 1 (3) | 6 (19) | 2 (11) | 1 (5) | 3 (17) |
| Yes, in last month | 14 (48) | 14 (44) | 10 (32) | 10 (53) | 9 (41) | 6 (33) |
| Standardised MMSE score, n (%) | ||||||
| 25–27 | 15 (52) | 15 (47) | 15 (48) | 9 (47) | 11 (50) | 11 (61) |
| 28–30 | 14 (48) | 17 (53) | 16 (52) | 10 (53) | 11 (50) | 7 (39) |
| Mean standardised MMSE score (SD) | 27.2 (1.5) | 27.6 (1.6) | 27.8 (1.7) | 27.4 (1.4) | 27.5 (1.7) | 27.4 (1.9) |
All characteristics appear to be reasonably well balanced across the three treatment arms, given the small sample of patients. The average age was 80.1 years, 77% were female, most (68%) lived alone, 74% had experienced symptoms for > 6 months, 51% had taken antipsychotic treatment previously and BPRS scores averaged 41.4 points.
Primary outcomes
Brief Psychiatric Rating Scale
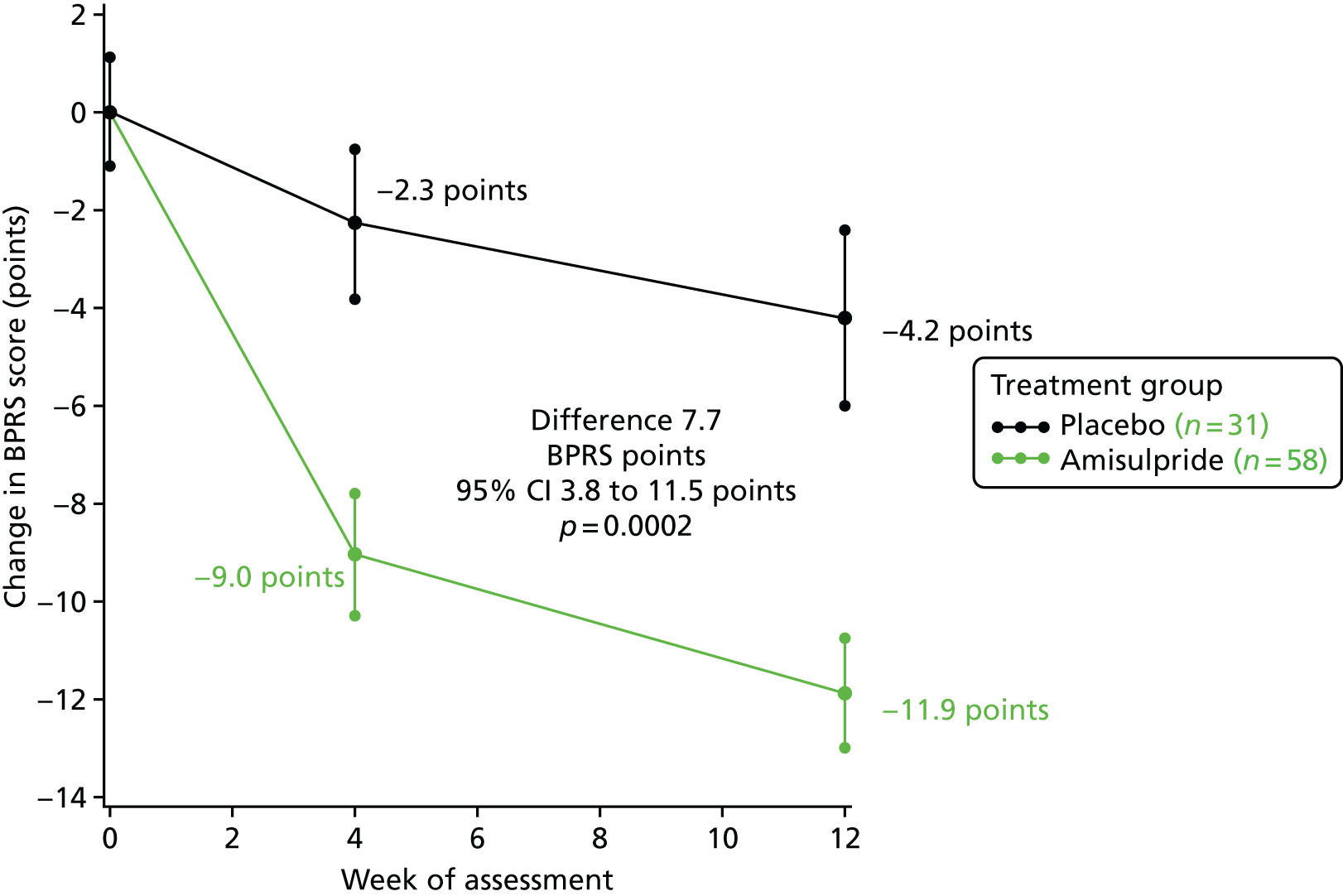
The BPRS scores were significantly lower at the 4- and 12-week assessments than at baseline in both the amisulpride- and placebo-treated groups, as shown in Figure 3. This figure shows the change in mean BPRS scores with standard errors. Improvements in BPRS scores over the 12-week stage 1 treatment period were, however, significantly larger with amisulpride (groups A and B) than placebo (group C). The difference in change in BPRS scores in favour of amisulpride over placebo was apparent by 4 weeks (6.7-point difference, 95% CI 3.1 to 10.3 points; p = 0.0004) and increased to 7.7 points (95% CI 3.8 to 11.5 points) at 12 weeks (1.9- vs. 4.2-point improvement; p = 0.0002). For six patients with 4- but not 12-week assessments, the 4-week assessment was carried forward for the 12-week comparisons of change from baseline.
FIGURE 3.
Change in BPRS score from baseline to 12 weeks in stage 1. Baseline scores: amisulpride 42.5 points and placebo 38.9 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
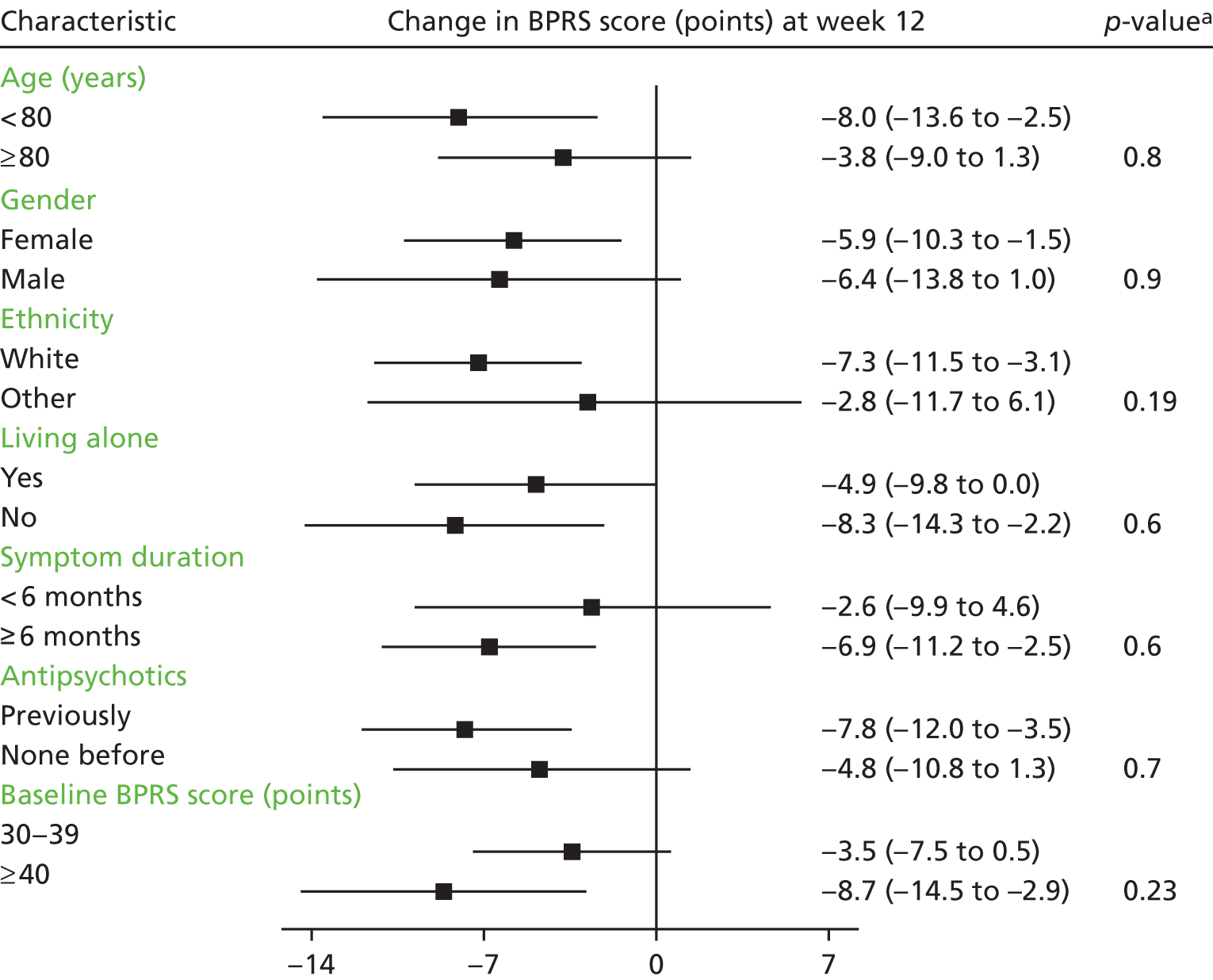
Treatment efficacy, as measured by BPRS score over the first 12 weeks, did not differ according to baseline characteristics (age, gender, ethnicity, residential status, duration of symptoms, previous antipsychotic use or severity of psychological symptoms); a subgroup plot for this analysis can be seen in Figure 4.
FIGURE 4.
Change in BPRS score from baseline to week 12: subgroup analyses by baseline characteristics. a, The p-value is derived from the test for differing treatment efficacy between subgroups in the repeated measures mode analyses. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
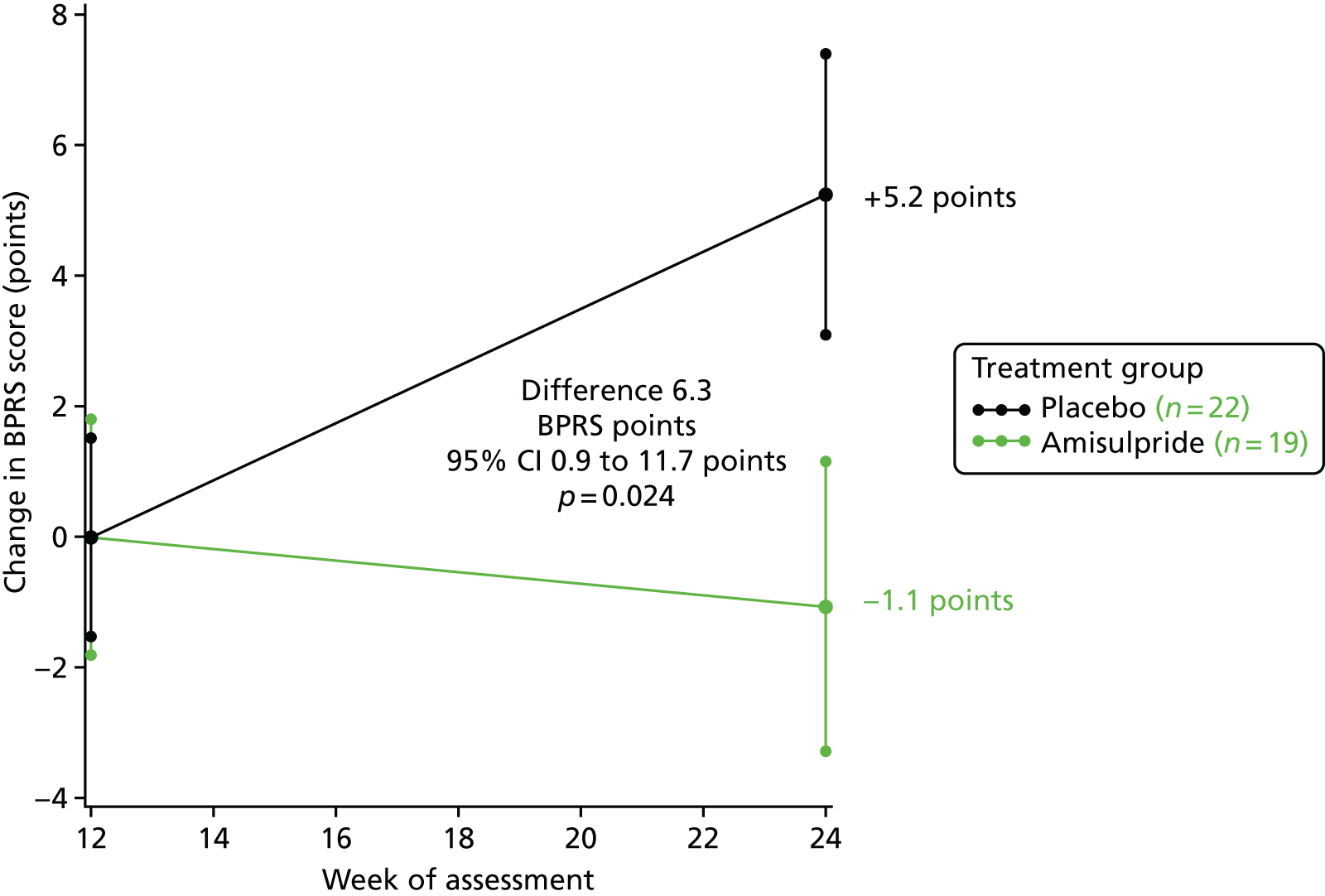
In stage 2, BPRS scores improved by 1.1 point from week 12 to the final assessment in those continuing amisulpride (group A), but deteriorated by 5.2 points in those who switched from amisulpride to placebo (group B) (6.3-point difference, 95% CI 0.9 to 11.7 points; p = 0.024). Figure 5 shows the mean BPRS scores in stage 2.
FIGURE 5.
Change in BPRS score from 12 weeks to final assessment in stage 2. Baseline scores: amisulpride 41.8 points and placebo 44.9 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
The BPRS covers the important symptoms elicited in VLOSLP patients. In particular, the hostility, suspiciousness, hallucinations, unusual thought content, tension and unco-operativeness items of BPRS all assess important areas of psychopathology in these patients. The 7-point rating of the BPRS on each of these generates a subset score for these six symptom domains that the protocol prespecified as those most likely to be affected by the disorder. Scores on the subset range from 6 to 42 points. Examination of change in the subscore indicated that most of the benefit from amisulpride was seen in these domains. In stage 1, the difference in the subset score between amisulpride and placebo was 4.1 points (95% CI 1.9 to 6.2 points; p = 0.0003) at 4 weeks and 5.3 points (95% CI 2.9 to 7.8 points; p < 0.0001) at 12 weeks (Figure 6).
FIGURE 6.
Change in BPRS six-item subset score from baseline to 12 weeks in stage 1. Baseline scores: amisulpride 20.2 points and placebo 18.6 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.

In stage 2, the difference in the subset score between continuing amisulpride past 12 weeks and stopping was 4.6 points (95% CI 1.3 to 8.0 points; p = 0.008), as shown in Figure 7.
FIGURE 7.
Change in BPRS six-item subset score from 12 weeks to final assessment in stage 2. Baseline scores: amisulpride 10.5 points and placebo 11.3 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.

Reasons for stopping trial treatment
A substantial level of treatment non-compliance was anticipated in the ATLAS trial. In total, 58 (63%) patients stopped taking trial medication: 17 in group A, 21 in group B and 20 in group C (see Figure 2). A somewhat higher proportion of patients allocated to the amisulpride group than the placebo group completed stage 1 treatment: 67% (41/61) versus 58% (18/31); however, this difference was not significant (p = 0.39; Table 9). Reasons for stopping treatment were broken down into no symptoms, treatment ineffective, side effects, patient decision and other health problem. However, when reasons for stopping treatment were compared, fewer patients allocated to the amisulpride group than the placebo group in stage 1 stopped because of non-efficacy: 7% (4/61) versus 26% (8/31) (p = 0.010). Similarly, fewer of those allocated to continue amisulpride in stage 2 stopped because of perceived non-efficacy than those who switched to placebo: 12% (2/9) versus 41% (9/22) (p = 0.031). The other reasons for stopping trial treatment showed no significant differences.
| Stage | Treatment group | ||
|---|---|---|---|
| Amisulpride (groups A and B) | Placebo (group C) | 2p-value | |
| Stage 1 | |||
| No symptoms, n | 3 | 0 | 0.21 |
| Treatment ineffective, n | 4 | 8 | 0.010 |
| Apparent side effects, n | 9 | 2 | 0.25 |
| Patient decision, n | 2 | 1 | 0.9 |
| Other health problem, n | 2 | 2 | 0.5 |
| Stage 1 subtotal, n (%) | 20/61 (33) | 13/31 (42) | 0.39 |
| Group | |||
| A | B | ||
| Stage 2 | |||
| No symptoms, n | 0 | 0 | |
| Treatment ineffective, n | 2 | 9 | 0.031 |
| Apparent side effects, n | 2 | 1 | 0.5 |
| Patient decision, n | 1 | 1 | 0.9 |
| Other health problem, n | 2 | 0 | 0.12 |
| Stage 2 subtotal, n (%) | 7/19 (37) | 11/22 (50) | 0.40 |
Secondary outcomes
Simpson–Angus Scale
The data presented in Figures 8 and 9 show that there were no significant differences between amisulpride- and placebo-treated patients in change in the SAS scores. In stage 1, SAS scores deteriorated by 0.05 points with amisulpride and improved by 0.47 points with placebo, but this difference was not significant: 0.52 points (95% CI –0.6 to 1.6 points; p = 0.4). In stage 2, SAS scores improved by 0.3 points for patients continuing amisulpride and deteriorated by 0.4 points in those stopping amisulpride, but again this difference was not significant: 0.7 points (95% CI –1.7 to 0.3 points; p = 0.1). Over time, there was no indication from the SAS scores that amisulpride was worsening EPSEs.
FIGURE 8.
Change in SAS score from baseline to 12 weeks. Baseline scores: amisulpride 2.3 points and placebo 2.4 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.
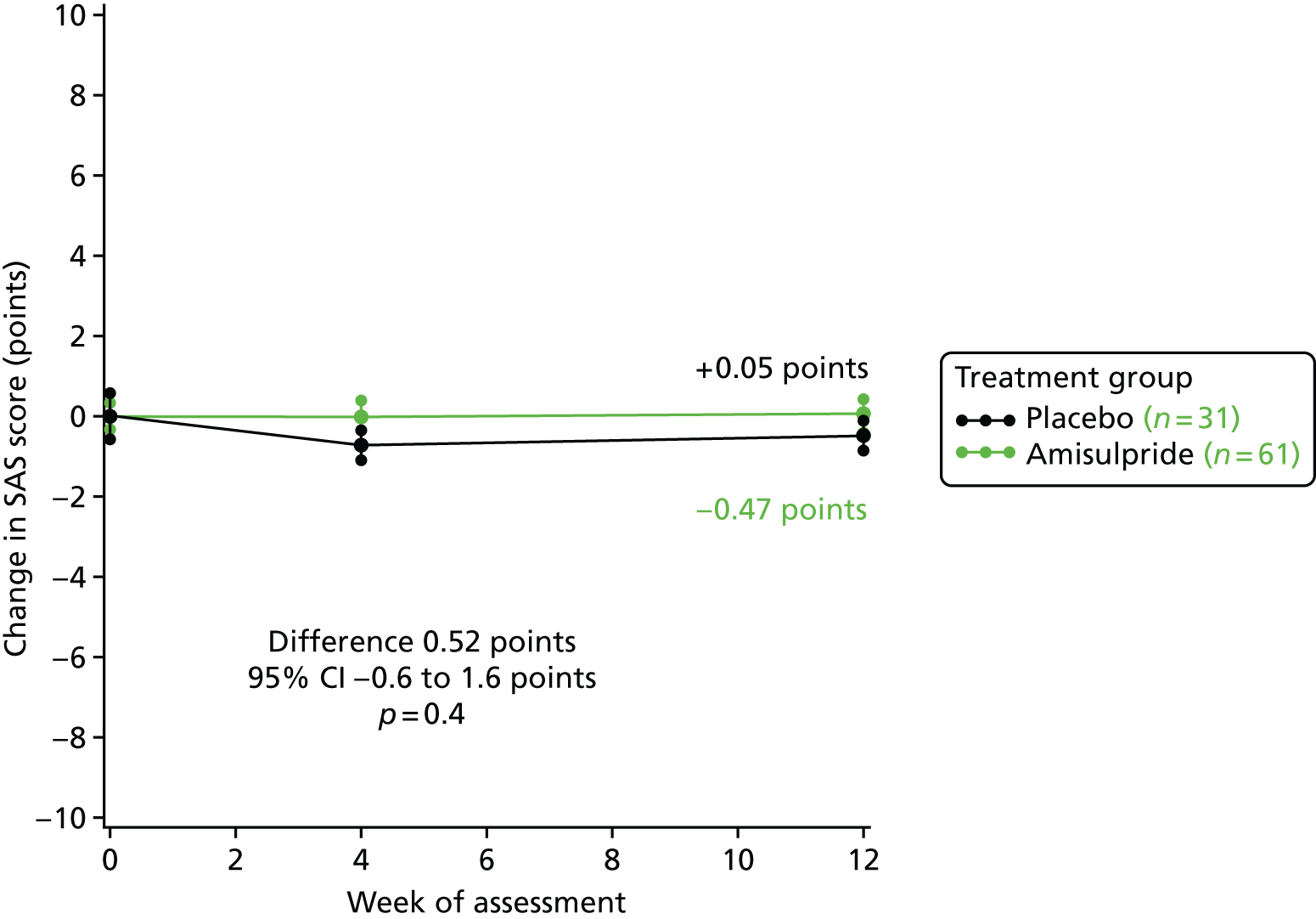
FIGURE 9.
Change in SAS score from week 12 to final assessment. Baseline scores: amisulpride 2.8 points and placebo 1.3 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.

Although no significant differences between amisulpride and placebo in the change in SAS score were seen over time, 11% (7/61) of patients allocated amisulpride developed clinically significant EPSEs (i.e. SAS score of ≥ 669) in stage 1, compared with none in the 31 placebo patients (p = 0.051).
EuroQol-5 Dimensions
There were no significant differences between the amisulpride and placebo groups in stages 1 and 2 for the EQ-5D utility score, which can be seen in Figures 10 and 11. In stage 1, EQ-5D utility scores improved by 0.027 points with amisulpride but deteriorated by 0.009 points with placebo, but this difference was not significant: 0.036 points (95% CI –0.060 to 0.133 points; p = 0.5).
FIGURE 10.
Change in EQ-5D utility score from baseline to 12 weeks. Baseline scores: amisulpride 0.711 points and placebo 0.755 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.

FIGURE 11.
Change in EQ-5D utility score from week 12 to final assessment. Baseline scores: amisulpride 0.788 points and placebo 0.743 points. Reprinted from Lancet Psychiatry, Vol. 5, Howard et al. ,1 Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial, pp. 553–63, copyright 2018, with permission from Elsevier.

WHOQoL-BREF
The WHOQoL-BREF assessment questionnaire is broken down into four domains, with higher scores denoting better quality of life: physical, psychological, social and environmental well-being. Table 10 shows the results from stages 1 and 2. There were no significant differences between amisulpride and placebo in any of the domains in either stage.
| Treatment group | Number of patients | Mean | 95% CI | SD | Standard error | t-statistic | p-value |
|---|---|---|---|---|---|---|---|
| WHOQoL-BREF stage 1 physical well-being (amisulpride vs. placebo) | |||||||
| Amisulpride | 46 | –0.8 | –5.6 to 4.0 | 16.1 | 2.38 | ||
| Placebo | 20 | 0.9 | –8.3 to 10.1 | 19.7 | 4.41 | ||
| Difference | –1.7 | –10.9 to 7.5 | 17.3 | 4.63 | –0.37 | 0.7 | |
| WHOQoL-BREF stage 1 psychological well-being (amisulpride vs. placebo) | |||||||
| Amisulpride | 51 | –0.1 | –4.9 to 4.7 | 17.0 | 2.38 | ||
| Placebo | 25 | 2.1 | –1.9 to 6.2 | 9.8 | 1.96 | ||
| Difference | –2.2 | –9.5 to 5.1 | 15.1 | 3.68 | –0.60 | 0.6 | |
| WHOQoL-BREF stage 1 social well-being (amisulpride vs. placebo) | |||||||
| Amisulpride | 52 | 4.1 | –1.2 to 9.4 | 19.1 | 2.65 | ||
| Placebo | 25 | 2.7 | –6.7 to 12.0 | 22.9 | 4.54 | ||
| Difference | 1.4 | –8.4 to 11. 3 | 20.3 | 4.95 | 0.29 | 0.8 | |
| WHOQoL-BREF stage 1 environmental well-being (amisulpride vs. placebo) | |||||||
| Amisulpride | 51 | 4.3 | 0.2 to 8.4 | 14.6 | 2.0 | ||
| Placebo | 25 | –1.3 | –8.8 to 6.1 | 18.1 | 3.6 | ||
| Difference | 5.6 | –2.0 to 13.3 | 15.8 | 3.9 | 1.46 | 0.15 | |
| WHOQoL-BREF stage 2 physical well-being (continuing amisulpride vs. placebo) | |||||||
| Amisulpride | 15 | –2.1 | –9.0 to –4.7 | 12.3 | 3.18 | ||
| Placebo | 20 | –0.6 | –7.7 to 6.6 | 15.3 | 3.42 | ||
| Difference | –1.5 | –11.3 to 8.2 | 14.1 | 4.81 | 0.32 | 0.75 | |
| WHOQoL-BREF stage 2 psychological well-being (continuing amisulpride vs. placebo) | |||||||
| Amisulpride | 15 | 2.7 | –4.8 to 10.2 | 13.5 | 3.49 | ||
| Placebo | 20 | 3.6 | –3.0 to 10.3 | 14.2 | 3.18 | ||
| Difference | –0.9 | –10.6 to 8.8 | 13.9 | 4.76 | –0.19 | 0.85 | |
| WHOQoL-BREF stage 2 social well-being (continuing amisulpride vs. placebo) | |||||||
| Amisulpride | 15 | 0.8 | –10.1 to 11.7 | 19.7 | 5.08 | ||
| Placebo | 16 | –4.7 | –13.5 to 4.1 | 16.4 | 4.11 | ||
| Difference | 5.5 | –7.8 to 18.8 | 18.1 | 6.50 | 0.85 | 0.40 | |
| WHOQoL-BREF stage 2 environmental well-being (continuing amisulpride vs. placebo) | |||||||
| Amisulpride | 15 | 1.7 | –4.8 to 8.1 | 11.6 | 3.00 | ||
| Placebo | 20 | 1.8 | –5.6 to 9.2 | 15.8 | 3.53 | ||
| Difference | –0.1 | –10.0 to 9.7 | 14.2 | 4.84 | –0.03 | 0.98 | |
Serious adverse events, side effects and deaths
Serious adverse events have been grouped into broad categories and summarised in Table 11. SAEs were reported more frequently in the amisulpride group than in the placebo group in both stage 1 [16% (10/61) vs. 3% (1/31); p = 0.057] and stage 2 [47% (9/19) vs. 27% (6/22); p = 0.19].
| Stage | Treatment group | 2p-value | |
|---|---|---|---|
| Stage 1 | Amisulpride (groups A and B) | Placebo (group C) | |
| Worsening EPSEs, n | 2 | 0 | 0.3 |
| Gastrointestinal, n | 1 | 0 | 0.47 |
| Infection, n | 4 | 1 | 0.48 |
| Cardiovascular, n | 1 | 0 | 0.47 |
| Falls, n | 4 | 0 | 0.14 |
| Genitourinary, n | 1 | 0 | 0.47 |
| Psychiatric symptoms, n | 2 | 0 | 0.3 |
| Stage 1 patients with SAE, n/N (%) | 10/61 (16) | 1/31 (3) | 0.057 |
| Stage 2 | Group A | Group B | |
| Worsening EPSEs, n | 1 | 0 | 0.3 |
| Infection, n | 1 | 2 | 0.64 |
| Cardiovascular, n | 0 | 1 | 0.35 |
| Falls, n | 2 | 1 | 0.47 |
| Genitourinary, n | 1 | 0 | 0.28 |
| Psychiatric symptoms, n | 1 | 1 | 0.92 |
| Other, n | 4 | 1 | 0.11 |
| Stage 2 patients with SAE, n/N (%) | 9/19 (47) | 6/22 (27) | 0.19 |
Three hospitalisations attributable to extrapyramidal symptoms were considered to be related to treatment, all in patients allocated amisulpride. Falls were more frequent in the amisulpride group in stage 1, but this difference was not significant (p = 0.14).
Side effects believed to be a result of trial treatment are summarised in Table 12. These side effects have been grouped into broad categories with a subtotal for extrapyramidal symptoms. More patients allocated to the amisulpride group reported side effects believed to be a result of trial treatment, with most of the excess attributable to potentially extrapyramidal symptoms (tremor, increased tone and increased salivation).
| Stage | Treatment group (n) | |
|---|---|---|
| Amisulpride (groups A and B) | Placebo (group C) | |
| Stage 1 | ||
| EPSEs | ||
| Tremor | 2 | 1 |
| Increased salivation | 4 | 0 |
| Increased muscle tone | 5 | 0 |
| EPSE subtotal | 11 | 1 |
| Dry mouth | 1 | 1 |
| Nausea or reduced appetite | 2 | 0 |
| Constipation | 3 | 0 |
| Urinary problems | 4 | 0 |
| Sleep disturbance | 3 | 2 |
| Worsening psychosis | 1 | 0 |
| Headache | 2 | 0 |
| Unsteadiness | 3 | 5 |
| Sedation | 5 | 1 |
| Confusion | 1 | 0 |
| Peripheral oedema | 1 | 0 |
| Total | 36 | 10 |
| Stage | Treatment group (n) | |
| Amisulpride (group A) | Placebo (group B) | |
| Stage 2 | ||
| EPSEs | ||
| Tremor | 1 | 0 |
| Increased salivation | 1 | 0 |
| Increased muscle tone | 2 | 0 |
| EPSE subtotal | 4 | 0 |
| Dry mouth | 0 | 2 |
| Nausea or reduced appetite | 1 | 0 |
| Constipation | 0 | 1 |
| Urinary problems | 1 | 0 |
| Worsening psychosis | 0 | 1 |
| Sedation | 1 | 1 |
| Total | 7 | 5 |
Five patients died during the study; the causes of death and the stage during which death occurred are shown in Table 13. The two patients who died while taking stage 2 treatment and the two patients who died in stage 1 stopped trial medication first and died several weeks later.
| Stage | Treatment group | Cause |
|---|---|---|
| Pre treatment | Group B | Gastric ulcer bleed |
| Stage 1 | Group B | Hypertensive disease |
| Stage 1 | Group C | Septicaemia |
| Stage 2 | Group A | Chest infection |
| Stage 2 | Group C | Myocardial infarction |
Chapter 4 Health economics evaluation
Economic evaluation overview
A prospective economic evaluation was conducted within the ATLAS trial to investigate the cost-effectiveness of oral amisulpride versus placebo over 12 weeks (stage 1) and the cost-effectiveness of continuation for a further 12 weeks versus discontinuation of amisulpride treatment at 12 weeks (stage 2). Cost-effectiveness was expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained. The analysis was based on the NHS and Personal Social Services (PSS) (social care) perspective as recommended by the National Institute for Health and Care Excellence (NICE) and a broader societal perspective that included the NHS/PSS perspective and unpaid carer costs. Two sensitivity analyses were conducted to explore the impacts on cost-effectiveness of changes in key parameters.
Economic evaluation methods
Measurement of medication, services and support
Medication costs for each participant in the study were estimated based on protocol-driven daily dosages. Data on services used and support received by each patient were collected using an adapted version of the Client Service Receipt Inventory (CSRI)74 retrospectively over 3 months at 12 weeks and 24 weeks. On each occasion, the patient was asked to record hospital service use and community-based service use over the previous 3 months. The CSRI collects data on the use of all hospital and community-based health services, social care and primary care services, including (but not limited to) accident and emergency attendances, day care visits, outpatient attendances, inpatient stays and hours spent in contact with community-based professionals, such as nurses (either district, practice, night or community psychiatric/community mental health), general practitioners, occupational therapists, physiotherapists, community psychologists, community psychiatrists, other community doctors, social workers, home care workers, paid carers and private home help or cleaners. Hospital and community-based service costs were assumed to be incurred by health and social care agencies, although it is likely that some community-based services may have been provided by the voluntary or private sectors. The study also collected data on unpaid carer support. Contacts and support made with unpaid carers were measured using days off work in the 3-month period when the patient’s behaviour or needs meant that the unpaid carer could not work as normal.
Costing of medication, services and support use
The NHS medication prices per tablet were obtained from the British National Formulary:76 £0.059 per 100-mg amisulpride tablet.
Service use data were converted into costs using NHS Reference Costs77 for accident and emergency attendances, and the Personal Social Services Research Unit (PSSRU)78 annual volume for all other services. Unit costs per minute for the use of psychologist and psychiatrist services were uprated from 2013/14 levels, and unit costs for district nurse and community psychiatric nurse were uprated from 2015 levels, all using the Hospital and Community Health Services index and prices inflator. 78 For those health professionals for whom unit costs at 2015/16 price levels were not available, these were derived using approaches consistent with those in the 2016 Unit Cost of Health and Social Care report. 78 The unit costs for night nurse, occupational therapist and physiotherapist were based on band 2 Agenda for Change79 from the PSSRU annual volume, and those for other community doctors were based on foundation doctor year 1. 78 The cost per hour of unpaid carer support was based on the hourly rate of a home care worker/paid carer. 78
The NHS Hospital and Community Health Services Pay and Prices Index was used to inflate cost, where appropriate, to 2015/2016 price levels. 80 There was no need to use time-discounting because the treatment phases were all contained within a 12-month period. Table 14 shows unit costs for 2015/16.
| Service use | Per unit | Price level (£) (2015/16) | Source |
|---|---|---|---|
| Hospital services | |||
| Accident and emergency | Per attendance | 138.00 | NHS Reference Costs (p. 10) 2015/1677 |
| Day hospital | Per day | 713.00 | UCHSC (p. 95), 201678 |
| Outpatient care | Per attendance | 135.00 | UCHSC (p. 95), 201678 |
| Inpatient care | Per day | 616.00 | UCHSC (p. 95), 201678 |
| Community-based services | |||
| Community/district nurse | Per minute | 0.75 | UCHSC (p. 169), 201678 |
| Practice nurse | Per minute | 0.60 | UCHSC (p. 143), 201678 |
| Night nurse | Per minute | 0.38 | UCHSC (p. 142), 201678 |
| Occupational therapist | Per minute | 0.38 | UCHSC (pp. 135–7), 201678 |
| Physiotherapist | Per minute | 0.38 | UCHSC (pp. 135–7), 201678 |
| General practitioner | Per minute | 3.30 | UCHSC (p. 145), 201678 |
| Other community doctor | Per minute | 0.40 | UCHSC (p. 191), 201678 |
| Social worker/care manager | Per minute | 0.65 | UCHSC (p. 156), 201678 |
| Home care worker/paid carer | Per minute | 0.33 | UCHSC (p. 160), 201678 |
| Private home help/cleaner | Per minute | 0.33 | UCHSC (p. 160), 201678 |
| Psychologist | Per minute | 1.07 | UCHSC (p. 183), 201678 |
| Psychiatrist | Per minute | 1.80 | UCHSC (p. 259), 201678 |
| Community psychiatric nurse/community mental health nurse | Per minute | 0.60 | UCHSC (p. 170), 201678 |
Cost estimation
Costs were estimated retrospectively for a 3-month period at 12 weeks and 24 weeks, from both NHS/PSS and societal perspectives. The NHS/PSS costs were derived for each stage of the analysis by summing the total treatment medication costs, total hospital services costs and total community-based health services costs. Societal costs included all NHS/PSS costs and unpaid carer costs.
To derive medication costs, it was assumed that patients did not miss any tablet during the study period and, thus, medication cost for each patient in the same allocation group was assumed not to vary (a fixed cost of £5.03 for patients in the amisulpride group, and no medication cost for patients in the placebo group).
The frequency and duration of service contacts for each resource use from the hospital services category and the community-based services category in the CSRI were multiplied by their respective unit costs to estimate total hospital services costs and total community-based health services costs.
Utilities and quality-adjusted life-years
We derived QALYs for patients in the trial using patient report of HRQoL assessed using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L),70 measured at baseline, 10–12 weeks and 22–24 weeks. The EQ-5D-3L comprises two components: (1) a visual analogue scale, which is not analysed further here; and (2) a descriptive system that defines HRQoL across five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety and depression. Responses in each dimension are categorised into three ordinal levels (no problems, some or moderate problems and severe or extreme problems) and weighted by applying population-based social tariffs75 to generate a preference-weighted utility score for each patient. The EQ-5D scores range from –0.594 to 1.0; 1.0 represents full health and any values below zero can be interpreted as representing health states worse than death.
Quality-adjusted life-years (QALYs) were calculated for the 3-month period over stages 1 and 2, the same period over which costs were estimated. There was no discounting of QALYs because the period of the analysis was < 1 year.
Missing data
Missing service use data were imputed from the within-treatment group median for participants with data for that service use item at that treatment stage if participants reported that they used a service or contacted a professional but did not report the number or duration of contacts. If the patient reported use of hospital services in general but did not report the number of attendances in any specific type of hospital services (i.e. no report on any of the four hospital services categories), then it was assumed that the patient used the lowest-cost hospital service (outpatient care) at least once in that stage. If there was no report on whether or not the individual used hospital services or community-based health services at all, then it was assumed that no use was made of services within that category and, thus, the total cost for that category of service use for that patient was assumed to be zero. If data were available at 10–12 weeks but missing at 22–24 weeks, or vice versa, it was assumed that services were used in the same way as the stage where data were missing.
Missing outcome data (QALYs) were imputed from within-treatment group median utility scores for participants with data in that stage. The QALYs were then calculated by the area-under-the-curve approach as defined by the imputed utility values.
Statistical analysis
The mean number of contacts and the number and percentage of the group who had at least one contact with a professional in that services category were presented by treatment group in each stage (group A + B vs. group C in stage 1; group A vs. group B in stage 2). Observed differences in service use patterns between the groups for a 3-month retrospective period at 12 weeks and at 24 weeks were reported descriptively but not statistically to avoid multiple testing.
All analyses were undertaken using Stata® (version 15; StataCorp LP, College Station, TX, USA). Intention-to-treat principles were applied to analyses as far as practically possible to preserve the unbiased distribution of factors in the groups produced by randomisation, given missing data.
The NHS/PSS costs, societal costs and QALYs at stages 1 and 2 were regressed on treatment allocation, age at baseline, gender, home circumstances, time since onset of symptoms, previous antipsychotic treatment and BPRS scores. Non-parametric bootstrapping was used to estimate 95% CIs around the mean costs and QALY outcomes. Between-group change scores were considered significant at a p-value of ≤ 0.05, where the bias-corrected CIs of between-group change scores excluded zero.
In each stage an incremental costs effectiveness ratio (ICER) was derived using estimates of bootstrapped mean costs and QALYs. 81 The ICER was calculated as follows:
If one treatment has both lower costs and better outcome than the other, then it is said to be dominant82 and the cost-effectiveness conclusion is straightforward. Difficulties arise when one treatment is either more costly and more effective, or less costly and less effective, than the other treatment, leaving decision-makers with the difficult task of judging whether or not the additional cost is justified by the outcome. To explore the uncertainty that exists around estimates of mean costs and outcomes (QALYs) and the uncertainty regarding the hypothesised maximum values of willingness to pay (λ), cost-effectiveness acceptability curves (CEACs) were plotted to show the probabilities that amisulpride would be seen as more cost-effective than placebo across a range of λ values placed on a unit improvement in effectiveness (QALY). Each CEAC was derived using the net benefit (NB) approach, in which monetary values of incremental costs and incremental effects were combined for each stage, and the NB was derived as:
A plausible range of λ values was explored for the outcome (QALYs). This approach allowed costs and QALYs to be considered on the same monetary scale, which was able to account for sampling uncertainty and make adjustments as necessary in the main and sensitivity analyses.
Sensitivity analysis
Two sensitivity analyses were undertaken to assess the impact on the main cost-effectiveness results if changes were made to key parameters and methodology. In the first sensitivity analysis, the three patients admitted to hospital for more than half (45 days) of the 3-month analysis period at stages 1 and 2 were included in the analyses, whereas they were excluded from the main analyses.
A second sensitivity analysis assumed that individuals who had missing service use data had at least used some hospital services or community-based services in the 3-month period. These patients’ total hospital services costs and total community-based services costs were imputed using within-treatment group median from patients who had data, instead of assuming the costs in these groups to be zero (as in the main analysis). Total NHS/PSS costs and total societal costs were then calculated.
Economic evaluation results
Data completeness
There were 89 patients who had primary outcome (BPRS) data at both baseline and 12 weeks in stage 1 and 41 who had data from 12 weeks to final assessment (22 weeks) in stage 2. In stage 1, service use data were available for 90% (80/89) of patients in stage 1 (amisulpride group, n = 53; placebo group, n = 27) and for 85.4% (35/41) of patients in stage 2 (amisulpride group, n = 14; placebo group, n = 21). Data on unpaid carer support were available for 89% (79/89) of patients in stage 1 (amisulpride group, n = 52; placebo group, n = 27), and for 84% (34/41) of patients in stage 2 (amisulpride group, n = 14; placebo group, n = 20). Service use and outcome data completeness are summarised in Table 15.
| Service useb | Stage, n (%) completeda | |||
|---|---|---|---|---|
| 1 (12 weeks) | 2 (24 weeks) | |||
| Low-dose amisulpride (N = 58) | Placebo (N = 31) | Amisulpride continuation (N = 19) | Placebo (N = 22) | |
| Hospital services | 53 (91.4) | 27 (87.1) | 15 (79.0) | 21 (95.5) |
| Community-based services | 55 (94.8) | 27 (87.1) | 15 (79.0) | 21 (95.5) |
| Unpaid carer support | 54 (93.1) | 27 (87.1) | 16 (84.2) | 20 (95.2) |
| Outcome (utility scores) | 52 (89.7) | 27 (87.1) | 16 (84.2) | 20 (95.2) |
There were 77 patients who had EQ-5D data at both baseline and 12 weeks in stage 1, and 34 patients who had data at both 12 weeks and final assessment (24 weeks) in stage 2.
At the 12-week assessment point in stage 1, EQ-5D data were available for 89% of patients (79/89) (amisulpride group, n = 52; placebo group, n = 27). At the 24-week assessment in stage 2, EQ-5D data were available for 89% of patients (36/41) (amisulpride group, n = 16; placebo group, n = 20).
Service use and support
Service contacts made by patients during stages 1 (12 weeks) and 2 (weeks 24) are shown in Table 16. The mean number of inpatient days in the amisulpride-treated group (12 days) was 12 times higher than in the placebo-treated group (1 days) in stage 1.
| Service use (attendances/contact) | Stage | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | |||||||
| Amisulpride (N = 58) | Placebo (N = 31) | Amisulpride continuation (N = 19) | Placebo (N = 22) | |||||
| n (%)a | Mean (SD)b | n (%)a | Mean (SD)b | n (%)a | Mean (SD)b | n (%)a | Mean (SD)b | |
| Hospital services | ||||||||
| Accident and emergency | 7 (12.1) | 1.7 (1.3) | 3 (9.7) | 1.0 (0) | 0 (0) | 0.0 (0) | 3 (13.6) | 1.0 (0) |
| Day hospital | 2 (3.4) | 1.0 (0) | 2 (6.5) | 1.5 (0.7) | 1 (5.3) | 2.0 (0) | 0 (0) | 0.0 (0) |
| Outpatient care | 16 (27.6) | 1.4 (0.7) | 12 (38.7) | 1.8 (1.3) | 4 (21.1) | 1.8 (1.0) | 4 (18.2) | 23.5 (27.0) |
| Inpatient care | 16 (27.6) | 11.9 (28.2) | 4 (12.9) | 1.0 (0) | 3 (15.8) | 1.0 (0) | 3 (13.6) | 17.3 (28.3) |
| Community-based services | ||||||||
| Community/district nurse | 8 (13.8) | 12.3 (29.0) | 2 (6.5) | 12.0 (14.1) | 3 (15.8) | 12.3 (11.5) | 4 (18.2) | 2.6 (2.4) |
| Practice nurse | 6 (10.3) | 2.3 (2.0) | 5 (16.1) | 1.8 (1.3) | 2 (10.5) | 2.0 (0) | 4 (18.2) | 2.5 (2.4) |
| Night nurse | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
| Occupational therapist | 2 (3.4) | 5.0 (1.4) | 2 (6.5) | 2.0 (0) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
| Physiotherapist | 2 (3.4) | 48.0 (50.9) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) | 2 (9.1) | 28.5 (33.2) |
| General practitioner | 30 (51.7) | 2.2 (1.3) | 16 (51.6) | 3.6 (5.6) | 11 (59.9) | 1.6 (1.1) | 11 (50.0) | 3.2 (5.6) |
| Other community doctor | 1 (1.7) | 1.0 (0) | 2 (6.5) | 1.5 (0.7) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
| Social worker/care manager | 7 (12.1) | 2.6 (1.1) | 5 (16.1) | 2.2 (1.1) | 2 (10.5) | 13.0 (15.6) | 1 (4.6) | 2.0 (0) |
| Home care/home help worker | 12 (20.7) | 87.5 (85.9) | 7 (22.6) | 145.3 (87.9) | 5 (26.3) | 87.0 (136.3) | 4 (18.2) | 98.3 (109.2) |
| Private home help/cleaner | 3 (5.2) | 1.0 (0) | 3 (9.7) | 10.0 (3.5) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
| Night sitter/paid carer | 2 (3.4) | 97.0 (52.3) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) | 0 (0) | 0.0 (0) |
| Psychologist | 3 (5.2) | 1.7 (1.2) | 3 (9.7) | 2.0 (0) | 0 (0) | 0.0 (0) | 4 (18.2) | 3.5 (1.3) |
| Psychiatrist | 24 (41.4) | 1.6 (0.9) | 11 (35.5) | 1.5 (0.5) | 6 (31.6) | 1.3 (0.5) | 12 (54.6) | 2.8 (3.3) |
| Community psychiatric nurse/community mental health nurse | 38 (65.6) | 6.8 (11.4) | 16 (51.6) | 4.56 (4.1) | 11 (59.9) | 7.09 (8.0) | 14 (63.6) | 5.9 (3.4) |
| n (%)a | n; mean number of days off (SD)c | n (%)a | n; mean number of days off (SD)c | n (%)a | n; mean number of days off (SD)c | n (%)a | n; mean number of days off (SD)c | |
| Unpaid carer support | 14 (24.1) | 2; 4.5 (0.7) | 11 (35.5) | 1; 11.0 (0) | 4 (21.1) | 1; 10.0 (0) | 5 (22.7) | 0; 0.0 (0) |
The mean number of contacts with the home care/home help worker was higher in the placebo group than in the amisulpride group in both stages, and in stage 1, the number of contacts in the placebo group was nearly twice that in the amisulpride group.
Incremental costs and outcomes
Two patients from the amisulpride group in stage 1 were recruited to the trial while they were inpatients and remained in hospital during the period of the stage 1 analyses. In stage 2, one patient who switched to the placebo group was hospitalised for 50 days. The inpatient care of these individuals contributed to the high mean total hospital services costs per person observed in the placebo group and, therefore, substantially increased the total NHS/PSS costs and total societal costs, with impacts for the ICER calculations. Cost data for these three patients with very high inpatient care service use were excluded from the main cost, QALY and cost-effectiveness analyses as intensive inpatient service use (and costs) were not typical for the majority of study participants and inclusion of such data would bias or influence mean values that are of substantive interest.
Unadjusted NHS/PSS costs were higher for people in the placebo group in stages 1 (£379) and 2 (£613) (Table 17). The higher medication and hospital services costs in the amisulpride group were offset by higher community-based service costs for people in the placebo group in stages 1 and 2.
| Treatment group, mean (SD) | Unadjusted mean difference | ||
|---|---|---|---|
| Amisulpride (n = 58) | Placebo (n = 31) | ||
| Costs (£) | |||
| Trial medication | 5.0 (0.0) | 0.0 (0.0) | 5.0 |
| Hospital services | 364 (578) | 258 (360) | 106 |
| Community-based services | 496 (792) | 986 (2106) | –490 |
| Total NHS/PSS costs (£) | 865 (1064) | 1244 (2311) | –379 |
| Unpaid carer support | 26 (136) | 57 (316) | –31 |
| Total societal costs (£) | 891 (1121) | 1301 (2341) | –410 |
| Outcome | |||
| QALYsa | 0.168 (0.073) | 0.161 (0.070) | 0.007 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | – | – | Amisulpride dominant |
| Societal perspective: costs (£) per QALY gain (ICER) | – | – | Amisulpride dominant |
| Treatment group, mean (SD) | |||
| Amisulpride continuation (n = 19) | Placebo (n = 21) | ||
| Costs (£) | |||
| Trial medication | 5.0 (0.0) | 0.0 (0.0) | 5.0 |
| Hospital services | 222 (373) | 683 (1913) | –461 |
| Community-based services | 501 (877) | 658 (892) | –157 |
| Total NHS/PSS costs (£) | 728 (980) | 1341 (2383) | –613 |
| Unpaid carer support | 84 (367) | 0 (0) | 84 |
| Total societal costs (£) | 812 (1032) | 1341 (2383) | –529 |
| Outcome | |||
| QALYsa | 0.18094 (0.063) | 0.18118 (0.063) | –0.00024 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | – | – | 255,417 |
| Societal perspective: costs (£) per QALY gain (ICER) | – | – | 2,204,166 |
Societal costs were highest for patients in the placebo group across both stages (stage 1, £410; stage 2, £529).
During stage 1, QALY gains were greater for the amisulpride group, but, at stage 2, greater QALY gains were evident in the group that switched to placebo.
These mean differences are not adjusted for baseline clinical or demographic characteristics.
After adjusting for age, gender, home circumstances, time since onset of symptoms, previous antipsychotic treatment and BPRS scores, total NHS/PSS costs and total societal costs in the amisulpride groups were lower than those in the placebo groups in both stages, but the differences in costs under either perspective in either stage did not achieve statistical significance [stage 1: NHS/PSS £502 (95% CI –£1651 to £318), societal £559 (95% CI –£1763 to £299); stage 2: NHS/PSS £811 (95% CI –£1976 to £130), societal £821 (95% CI –£1952 to £129) (Table 18)].
| Stage | Adjusted mean differencea | 95% CI |
|---|---|---|
| Stage 1 (n = 79)b | ||
| Incremental costs (£) | ||
| Trial medication | 5 | – |
| Hospital services | 114 | –104.5 to 363.5 |
| Community-based services | –621 | –1792.9 to 100.4 |
| Total NHS/PSS costs (£) | –502 | –1651.5 to 317.7 |
| Unpaid carer support | –57 | –230.3 to 66.4 |
| Total societal costs (£) | –559 | –1762.5 to 299.1 |
| Incremental outcome | ||
| QALYsc | –0.009 | –0.042 to 0.024 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | 55,773 | – |
| Societal perspective: costs (£) per QALY gain (ICER) | 62,119 | – |
| Stage 2 (n = 34)b | ||
| Incremental costs (£) | ||
| Trial medication | 5 | – |
| Hospital services | –232 | –1246.3 to 413.5 |
| Community-based services | –581 | –1318.5 to 73.9 |
| Total NHS/PSS costs (£) | –811 | –1975.6 to 129.5 |
| Unpaid carer support | –10 | –249.7 to 28.4 |
| Total societal costs (£) | –821 | –1952.3 to 128.9 |
| Incremental outcome | ||
| QALYsc | –0.019 | –0.076 to 0.049 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | 42,674 | – |
| Societal perspective: costs (£) per QALY gain (ICER) | 43,203 | – |
The QALY comparisons after adjustment for baseline clinical and demographic characteristics revealed no significant differences between groups in stage 1 (–0.009, 95% CI –0.042 to 0.024) or stage 2 (–0.19; 95% CI –0.076 to 0.049) (see Table 18).
Cost-effectiveness of amisulpride in stage 1
The incremental cost-effectiveness of amisulpride compared with placebo in stage 1 for patients with both costs and QALY data is shown in Table 18. The ICER was £55,773 per QALY under a NHS/PSS perspective and £62,119 under a societal perspective. We explored the probability that low-dose amisulpride would be cost-effective. Figure 12 shows 1000 bootstrapped replicates of incremental costs and incremental QALYs in stage 1 from a NHS/PSS perspective and a societal perspective. The corresponding CEAC (Figure 13) suggests that, at stage 1, the probability that amisulpride is more cost-effective than placebo (if decision-makers were willing to pay nothing for a QALY gain) is 85% under the NHS/PSS and 87% under the societal perspective. The probability of cost-effectiveness reduces as willingness to pay for a QALY gain increases. At £20,000, the probability of cost-effectiveness is 70% and 72% in the NHS/PSS and the societal perspectives, respectively, and falls to 65% (in the NHS/PSS perspective) and 68% (in the societal perspective) as the threshold increases to £30,000.
FIGURE 12.
Bootstrapped replicates of incremental cost and incremental QALYs for amisulpride vs. placebo over stage 1: (a) NHS/PSS perspective; and (b) societal perspective.

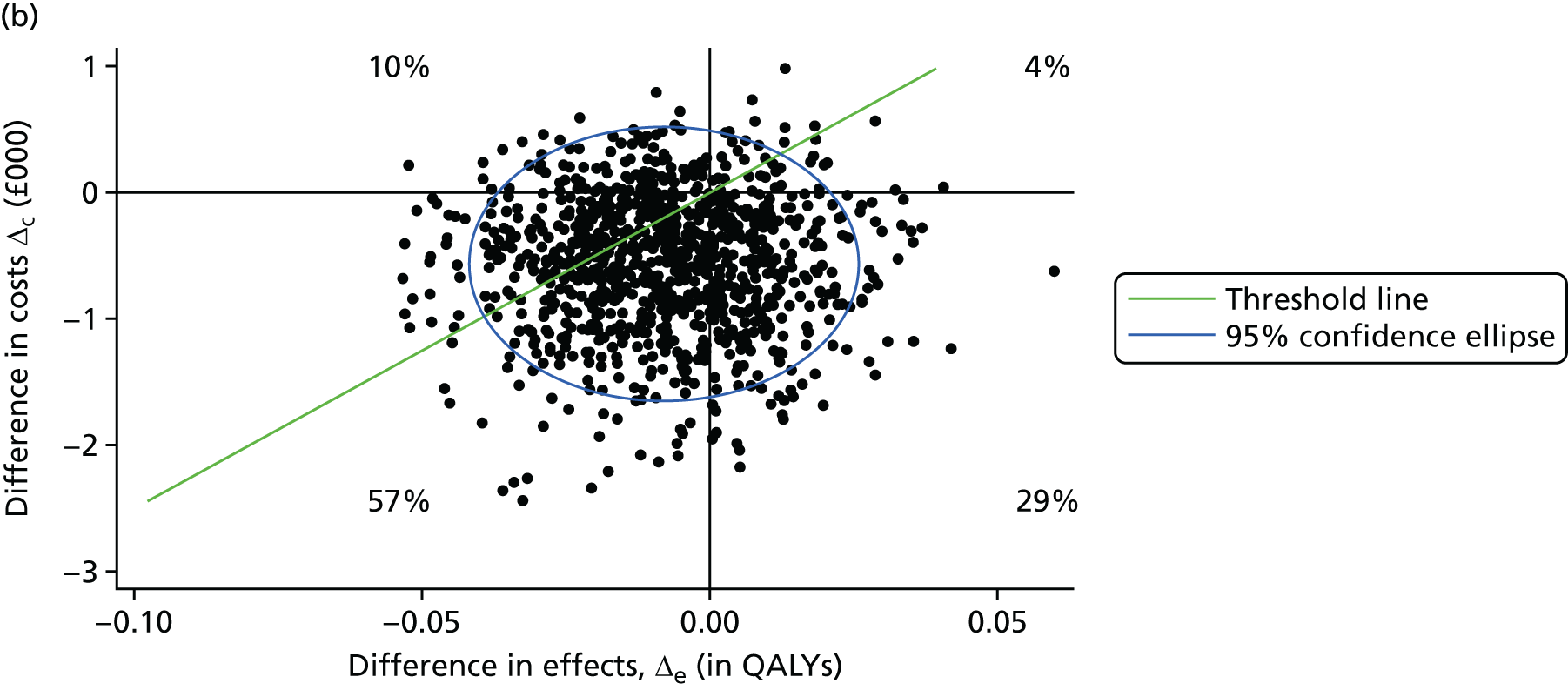
FIGURE 13.
Cost-effectiveness acceptability curve of amisulpride vs. placebo in stage 1 from NHS/PSS and societal perspectives, with effectiveness measured in QALYs.
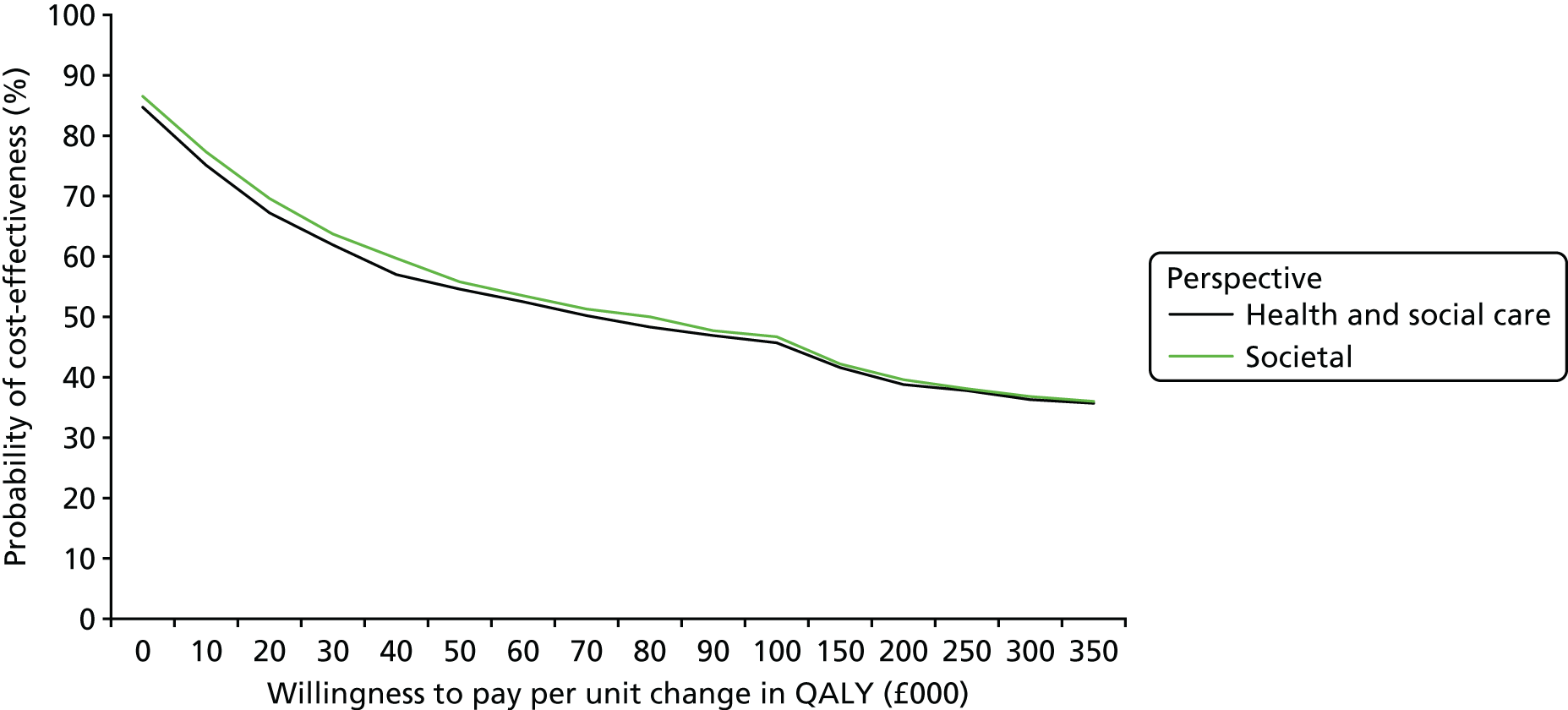
Cost-effectiveness of amisulpride continuation in stage 2
The analysis was repeated in stage 2 (see Table 18). A similar picture to stage 1 emerged: the ICER was £42,674 per QALY under a NHS/PSS perspective and £43,203 per QALY under a societal perspective. Figure 14 shows 1000 bootstrapped replicates of incremental costs and incremental QALYs in stage 2 from a NHS/PSS perspective and societal perspective. The corresponding CEACs to the scatterplots from a NHS/PSS and societal perspective suggest that, if decision-makers are willing to pay £20,000 for an additional improvement in QALY, there is an 84% and 85% probability, respectively, that amisulpride continuation would be cost-effective. This falls to 64% and 65% under NHS/PSS and societal perspectives, respectively, with a willingness-to-pay threshold of £30,000 (Figure 15) and continues to fall at higher values of willingness to pay for each additional QALYs.
FIGURE 14.
Bootstrapped replicates of incremental cost and incremental QALYs for amisulpride vs. placebo over stage 2: (a) NHS/PSS perspective; and (b) societal perspective.

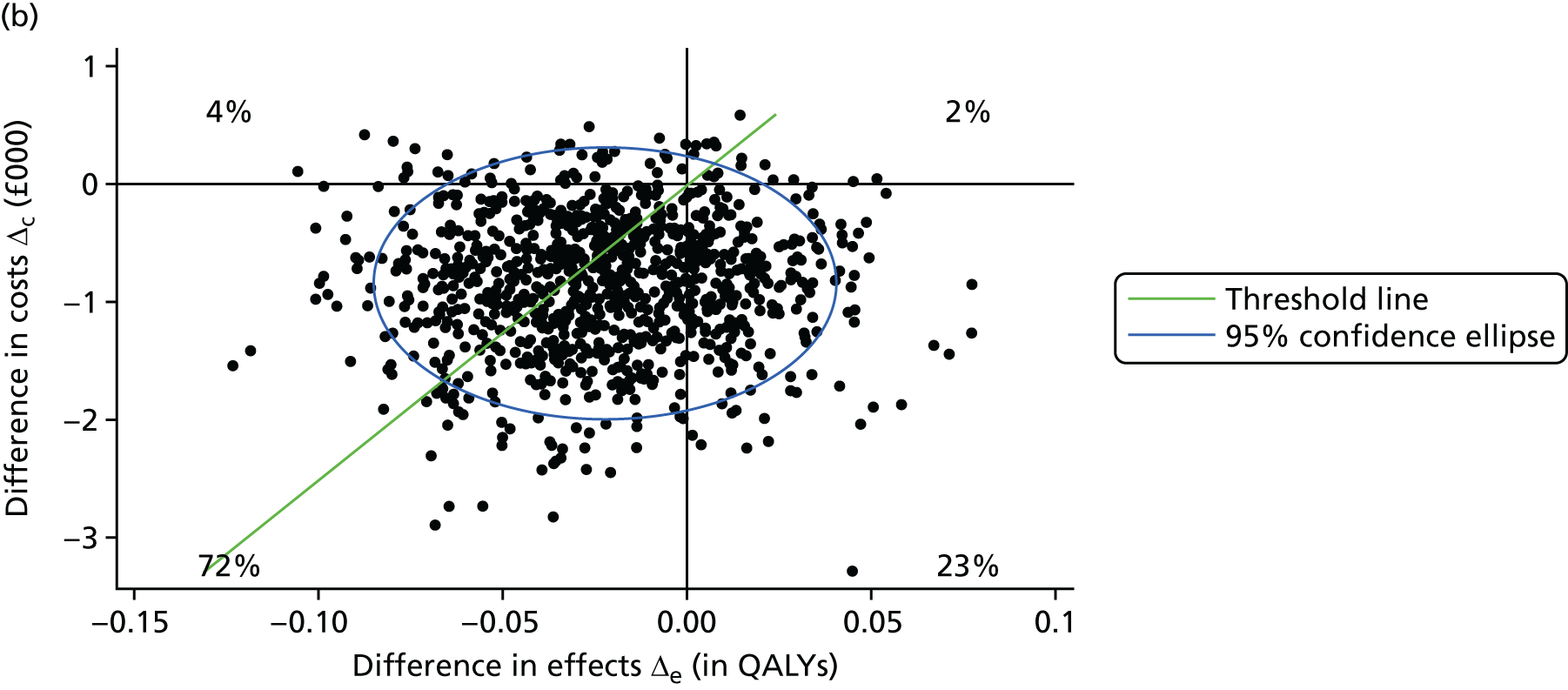
FIGURE 15.
Cost-effectiveness acceptability curve of amisulpride continuation vs. placebo discontinuation in stage 2, from the NHS/PSS and the societal perspectives, with effectiveness measured in QALYs.
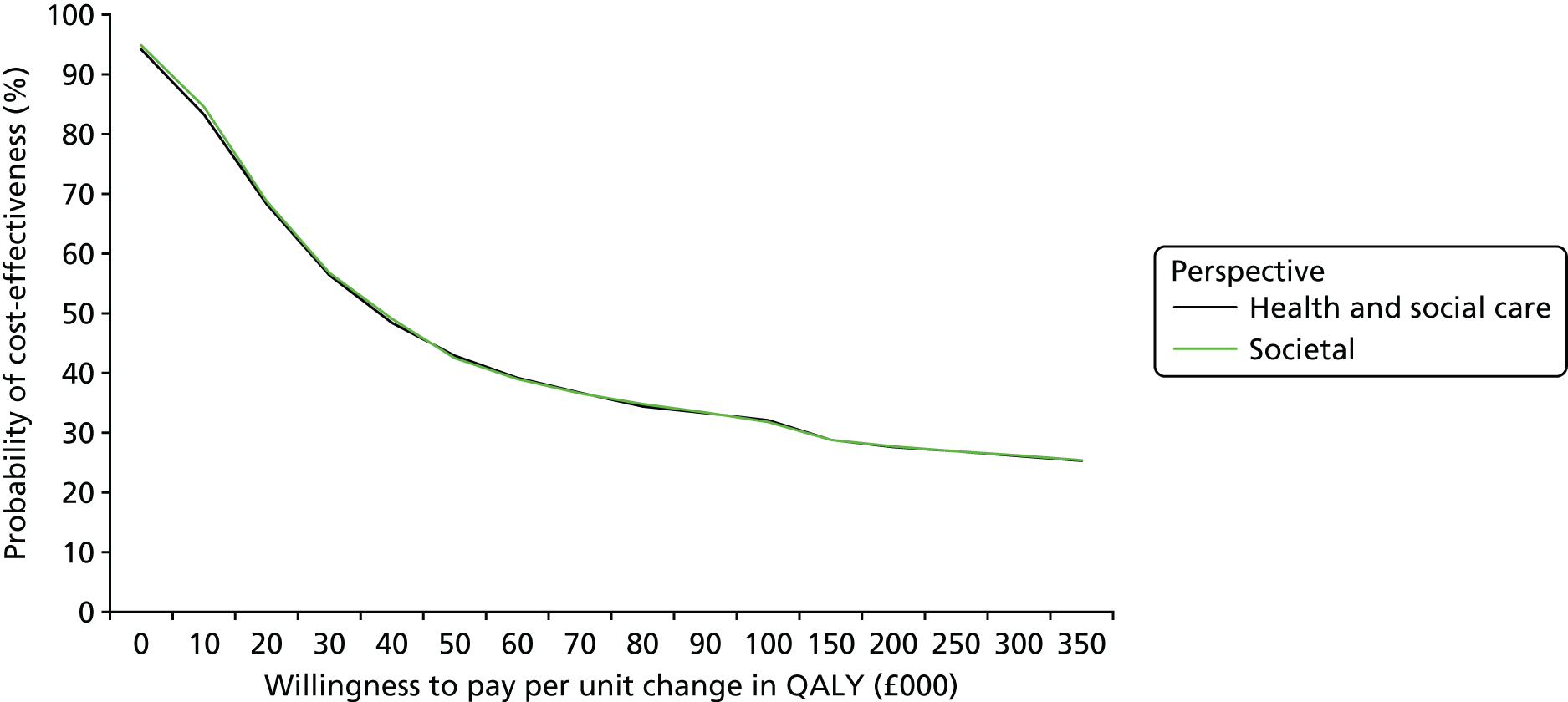
Sensitivity analyses
Two sensitivity analyses were undertaken to assess the impact of uncertainty surrounding key parameters or methodological features. In the first sensitivity analyses, we assessed whether or not there would be any effect on the main analyses if legitimate extreme service use and costs values for three patients were not removed. In stage 1, under both perspectives, placebo was dominant (Table 19), and in stage 2, NHS/PSS and societal costs were significantly lower in the amisulpride continuation group than for those who switched to placebo [NHS/PSS £2293 (95% CI –£8923 to –£122), societal £2306 (95% CI –£8985 to –£153)], but no significant differences were seen in QALYs (–0.014, 95% CI –0.069 to 0.052). The cost per QALY gain was £163,786 and £164,714 under the NHS/PSS and societal perspectives, respectively (Table 20).
| Perspective | Stage 1, adjusted mean differencea (95% CI) (n = 81) |
|---|---|
| NHS/PSS perspective: incremental costs and effect | |
| Costs (£) | 1401 (–773.7 to 5737.4) |
| QALY (EQ-5D)b | –0.010 (–0.044 to 0.020) |
| NHS/PSS perspective: cost (£) per QALY gain (ICER) | Placebo dominant |
| Societal perspective: incremental costs and effect | |
| Costs (£) | 1341 (–825.8 to 5785.6) |
| QALY (EQ-5D)b | –0.010 (–0.044 to 0.020) |
| Societal perspective: cost (£) per QALY gain (ICER) | Placebo dominant |
| Perspective | Stage 2, adjusted mean differencea (95% CI) (n = 35) |
|---|---|
| NHS/PSS perspective: incremental costs and effect | |
| Costs (£) | –2293 (–8924 to –122) |
| QALY (EQ-5D) | –0.014 (–0.069 to 0.052) |
| NHS/PSS perspective: cost (£) per QALY gain (ICER) | 163,786 |
| Societal perspective: incremental costs and effect | |
| Costs (£) | –2306 (–8986 to –153) |
| QALY (EQ-5D) | –0.014 (–0.069 to 0.052) |
| Societal perspective: cost (£) per QALY gain (ICER) | 164,714 |
In the second sensitivity analysis, we assessed the impact on findings at both stages of imputing missing data. In the main analysis, if there was no report on whether or not the individual used hospital services or community-based services at all, then it was assumed that no use was made of services within that category and, thus, the total cost for that category of service use for that patient was assumed to be zero. For this sensitivity analysis, when there was no report on whether or not the individual used hospital services or community-based services at all, missing cost data were imputed with the within-treatment group median values for that service cost. The effect of these changes, in both stages, did not change the result that differences in costs between amisulpride and placebo did not achieve statistical significance under either perspective, or that placebo was dominant (Table 21).
| Stage | Adjusted mean differencea | 95% CI |
|---|---|---|
| Stage 1 (n = 81)b | ||
| Incremental costs (£) | ||
| Trial medication | 5 | – |
| Hospital services | 1962 | 68 to 5680 |
| Community-based services | –576 | –1540 to 212 |
| Total NHS/PSS costs (£) | 1391 | –783 to 5723 |
| Unpaid carer support | –59 | –214 to 66 |
| Total societal costs (£) | 1332 | –846 to 5706 |
| Incremental outcome | ||
| QALYsc | –0.010 | –0.044 to 0.020 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | Placebo dominant | – |
| Societal perspective: costs (£) per QALY gain (ICER) | Placebo dominant | – |
| Stage 2 (n = 35)b | ||
| Incremental costs (£) | ||
| Trial medication | 5 | – |
| Hospital services | –1703 | –8287 to 284 |
| Community-based services | –542 | –1278 to 17 |
| Total NHS/PSS costs (£) | –2240 | –8981 to 16 |
| Unpaid carer support | –14 | –300 to 19 |
| Total societal costs (£) | –2254 | –8989 to 11 |
| Incremental outcome | ||
| QALYsc | –0.014 | –0.071 to 0.053 |
| NHS/PSS perspective: costs (£) per QALY gain (ICER) | 160,000 | – |
| Societal perspective: costs (£) per QALY gain (ICER) | 161,000 | – |
In stage 2, cost per QALY gained was very slightly lower, dropping from £163,786 to £160,000 in the NHS/PSS perspective and from £164,714 to £161,000 in the societal perspective (see Table 21). The probability that amisulpride was more cost-effective than placebo was similar to that for sensitivity analysis 1, with the probability of cost-effectiveness being slightly lower at the £20,000 and £30,000 thresholds in the societal perspective (see Appendix 1).
Economic evaluation discussion
A single daily dose of 100 mg of amisulpride over 12 weeks, or continuing the dosage for a further 12 weeks, in patients with VLOSLP did not lead to statistically significant differences in NHS/PSS costs, societal costs or QALY gains. There is little evidence that amisulpride improved QALYs as the bootstrapped replicates were scattered on either side of the line of zero for difference in QALYs. This could be because of the lack of sensitivity of a generic HRQoL measure to assess impacts on a patient’s well-being when they experience symptoms typical of VLOSLP such as hallucinations, suspiciousness, withdrawal of friends and sleep problems. Moreover, we also observed that, over both stages, most of the bootstrap replicates fell below the zero cost line, which would suggest that participants treated with amisulpride had incrementally lower NHS/PSS sector and caregiver costs than those treated with placebo. The position of the CEAC at £0 shows that, if society were unwilling to incur any additional costs to improve quality of life, that is, if society was interested only in reducing costs, then amisulpride for patients with VLOSLP is more likely to be acceptable because > 70% of replicates in stages 1 and 2 suggest cost savings. The curve slopes downwards because, as society values interventions that improve QALYs more, the apparent ineffectiveness of amisulpride to improve quality of life reduces the likelihood that it is cost-effective.
Strengths of approach
This is the first analysis that reports an economic analysis conducted alongside a RCT of amisulpride in patients with VLOSLP. It used data derived from the trial participants and assessed both clinical and economic measures of outcome (QALYs, service utilisation and costs). The study also explored both the NHS/PSS costs and impacts on unpaid carers as part of a wider societal perspective.
Limitations of analysis
The within-trial results highlighted the uncertainty around the costs and QALYs results using inferential statistics. This is a problem which can occur in trials that have been powered to detect significant difference in a clinical outcome and not economic end points. The economic analyses were limited by the small sample size.
Generalisability of the economic evaluation results
We are unable to compare the results of the current analysis with existing research, as there is no published economic research in this area.
There were three patients with high inpatient hospital service use during the study period. The main analysis excluded these individuals and found that those participants treated with amisulpride consistently (over both stages) had lower public sector and caregiver costs than participants treated with placebo. We have also shown in one of the sensitivity analyses that, when the service costs for these individuals are included, the results change from an economic advantage with amisulpride in stage 1 to amisulpride having a very low probability of cost-effectiveness, mainly attributable to the cost difference. However, these results should be treated with caution, taking into account issues related to sample size.
Future research
The economic evaluation results were based on a within-trial analysis that spanned 24 weeks. However, although the data can be used in longer-term modelling, the current analysis should not be used to extrapolate to a much longer time period. It would, therefore, be useful if further research was conducted that sought to replicate these analyses in a larger sample and over a longer time frame.
The current analyses were unable to detect significant differences in QALYs, which is obviously a key decision-making tool for NICE. The inability to detect differences in QALYs could have been attributable to the small sample size but also to the insensitivity of the measure of clinical changes following treatment in this patient group. Further research is needed to explore robust cross-walking of algorithms from clinical measures, such as the BPRS score to QALYs or use preference-based generic quality-of-life measures that are associated with BPRS scores, so that pharmacotherapies not traditionally licensed for use in certain patient groups could be more effectively assessed on clinical and economic grounds, following careful health and safety considerations.
Chapter 5 Discussion
Main outcomes
The ATLAS trial is the first randomised, parallel-group, placebo-controlled, double-blind trial of an antipsychotic drug to be conducted in patients with VLOSLP. Both the lack of clarity concerning this group’s relationship with schizophrenia and organic conditions, such as DLB, and the difficulties inherent in the conduct of gaining informed consent for participation in research from a patient group that resists any suggestion that they might benefit from treatment for a mental health disorder have contributed to the lack of such studies. There has also been a surprisingly small amount of research overall in this patient group, at least in part because the search for better understanding of disease mechanisms, treatments and ways of delivering care to people with dementia has become the overwhelming global public health and research priority. The relatively small size of the clinical population and difficulties in accessing and recruiting patients have also discouraged the pharmaceutical industry from targeting the treatment of people with VLOSLP in their trials.
Over the past 20 years, clinical trials of the use of antipsychotic drugs in people with dementia treated for a variety of behavioural and neuropsychiatric symptoms, including delusions and hallucinations, have generally reported only small treatment effect sizes (typically around 0.15 to 0.2 SDs),83,84 but significant increases in associated morbidity and mortality. 85 These results have undoubtedly dampened the enthusiasm of clinicians for prescribing antipsychotics to older people with all psychoses, although in the absence of clinical trial data from people with VLOSLP it has been difficult for prescribers to weigh up potential risks and benefits of treatment. This has resulted in very low rates of treatment, so that fewer than half of patients who complete an assessment in specialist secondary care services and are diagnosed with VLOSLP will be prescribed an antipsychotic, and only just over one-quarter will remain on treatment at 12 months or at discharge back to their general practitioner. 86
In the ATLAS trial, we established that a single daily dose of 100 mg of amisulpride was an effective and generally well-tolerated treatment for VLOSLP symptoms. We found a mean 12-point improvement in psychosis symptoms on the primary outcome measure, the BPRS, with amisulpride treatment, similar to the 14-point improvement reported by an earlier, open-treatment study. 61 We found a statistically significant 4-point BPRS score improvement in participants randomised to placebo, indicating that the net benefit of amisulpride, over and above the effect of placebo, was around 8 BPRS points. This change in BPRS scores attributable to amisulpride was close to 1 SD (8.8 points), indicating a large effect size. 87 This large treatment effect was achieved despite relatively poor compliance with trial medication and is larger than the moderate treatment effect sizes of around 0.5 SDs typically reported from meta-analysis of antipsychotic trials in younger patients with schizophrenia. 88
Although we used amisulpride in the ATLAS trial, this drug is not widely used by psychiatrists, and our results should be seen as supportive of the use of antipsychotic drugs as a class with this patient group. Our choice of amisulpride, which is a relatively limbic-selective D2 and D3 antagonist, was largely based on the fact that it is relatively non-sedating and we believed that this would help to maintain both compliance and blinding of treatment allocation. An open-treatment trial with dose escalation had already established that 100 mg per day of amisulpride had efficacy against psychosis symptoms in this patient group with low potential to induce extrapyramidal symptoms. 61 The data from the ATLAS trial supported this. Although the ATLAS trial may have been underpowered to detect significant differences in extrapyramidal symptoms between amisulpride and placebo treatment, the results suggested overall modest increases in extrapyramidal symptoms in a small number of participants, which did not appear to be of sufficient severity to affect compliance, even with 24 weeks of treatment. As the mean age of the ATLAS participants was around 80 years, participants would have been anticipated to have been at a high risk of developing extrapyramidal symptoms with antipsychotics. However, the dose chosen for the trial, 100 mg per day, is very low compared with recommended daily doses for younger adults with schizophrenia, which are typically 400–800 mg. We have recently shown in an open study that 50 mg per day of amisulpride is the minimum clinically effective and maximal tolerated dose for the treatment of psychosis symptoms in Alzheimer’s disease, due to 40–80% occupancy of striatal D2 and D3 receptors at this low dose, which leads to a high risk of extrapyramidal symptoms. 89,90 Patients with VLOSLP, in terms of the doses of antipsychotic necessary to treat their symptoms and their vulnerability to developing extrapyramidal symptoms, appear to occupy a position intermediate between people with schizophrenia and those with psychosis in dementia. Older people are generally more vulnerable to the adverse effects of treatment with antipsychotic drugs, and the ATLAS trial investigated only the benefits and risks of treatment over the 24-week duration of the trial. Safety monitoring for the emergence of extrapyramidal symptoms and cardiometabolic risk factors and side effects is important in clinical practice, particularly as patients may be prescribed treatment for several years.
The ATLAS trial was an extremely challenging trial to recruit to, and the original sample size of 300 participants was consequently reduced to 100 participants when it became apparent from the pilot trial phase that this larger number was not achievable. The revised recruitment target of 100 participants would have provided only 70% power to detect the 5-point BPRS MCID, and there was therefore a risk that the trial would have been underpowered to detect effects on the primary outcome measures, had the differences between amisulpride- and placebo-treated participants not been so large. But it is important to bear in mind that the trial could have been underpowered to detect differences between amisulpride and placebo treatment that might have been important for patients and prescribers. In particular, although there were no differences seen in the change in SAS scores between amisulpride- and placebo-treated participants, 11% of amisulpride participants and no placebo participants developed a clinically significant score of > 6 points on this scale during stage 1. This narrowly missed statistical significance (p = 0.051), and it is likely that, with a larger number of participants, more differences between amisulpride and placebo treatment would have been apparent.
Because VLOSLP patients only very rarely have insight into their illness or the potential benefits of antipsychotic treatment,15 they are a difficult group to engage in a clinical trial. Clinicians will generally avoid directly informing their patients that treatment is being prescribed for their symptoms of psychosis and will instead suggest that medication may help to improve sleep or reduce feelings of anxiety. The greater degree of transparency about the nature and purpose of treatment that was involved in gaining informed consent for participation in the ATLAS trial led many potential participants to decline involvement. Although the patient information and consent materials used for the ATLAS trial were carefully prepared in the light of this, with the assistance of the Service Users’ Research Enterprise at King’s College London, it would have been misleading and unethical to have avoided describing amisulpride within these as a drug that is used in the treatment of people with schizophrenia. Interestingly, some patients who were successfully recruited to the trial said that they had wanted to be included because they understood that their responsible clinical team were very keen for them to take antipsychotic medication and were consequently motivated to participate in the trial because of the one in three chance that they could be allocated to placebo.
Although amisulpride treatment improved participants’ psychosis symptoms as measured by the BPRS and the mean improvement compared with placebo (8 points) was greater than the MCID (5 points), such successful treatment was not accompanied by participant-rated improvements in HRQoL, as measured by the EQ-5D or WHOQoL-BREF. This was disappointing but probably reflects a lack of awareness and insight among participants that their psychotic symptoms have arisen as part of an illness. If the ATLAS trial participants could not recognise that they had a psychotic illness and that antipsychotic treatment might be able to improve their symptoms, it is unsurprising that they did not rate themselves or their situation as any better after successful treatment. It is also possible that the emergence of side effects experienced by participants during amisulpride treatment may have affected quality-of-life ratings. A further potential reason for the trial’s failure to demonstrate improvements in quality of life may have been our use of generic quality-of-life measurements. In the absence of validated measures that have been developed for this population, our results may also reflect an insensitivity of quality-of-life assessments that rely largely on changes in physical and mood difficulties that would have been unlikely to have occurred during treatment in the ATLAS trial.
The results of the ATLAS trial could encourage clinicians to be optimistic and more assertive with the use of antipsychotic treatment in this currently undertreated patient group. However, our demonstration that antipsychotic medication is effective and well tolerated in this patient population is only one component of their successful treatment. Engagement of patients with VLOSLP by specialist mental health services for older people is currently poor86 and falls below the standards of psychosis services provided for younger adults, within which very few actively psychotic patients would be discharged back to the care of their general practitioner without an ongoing prescription for antipsychotic treatment. In order to meet the needs of this patient group, older people’s mental health services will have to be prepared to more assertively engage with patients, if necessary through the use of the Mental Health Act 1983,91 and not allow patients to slip out of contact so that they remain actively psychotic and untreated for many years.
Study limitations
The ATLAS trial was a pragmatic trial, conducted with a clinical group who have not previously been studied as part of randomised clinical trials and who are not straightforward to involve in research. Our failure to recruit our original target of 300 participants increased the risk that the trial would have been underpowered to detect differences between amisulpride- and placebo-treated patients. Although we had sufficient power to detect the large effect size of amisulpride treatment on the primary outcome measure of the BPRS, it is possible that we were underpowered to see differences on other outcomes. The detection of differences in extrapyramidal symptoms between amisulpride and placebo treatment is the area where this was most likely and we acknowledge that these differences should be borne in mind when considering the risks and benefits of treatment.
The ATLAS protocol did not include collection of cognitive function data among the outcome measures. Although antipsychotic treatment might be expected to worsen cognitive functioning through sedation, amisulpride was chosen for the trial because it is relatively non-sedating and we decided that the costs of increased outcome measure burden involved in including a test of cognitive functioning did not exceed potential benefits.
Patients with VLOSLP often experience psychosis symptoms for many years and the 24-week duration of the trial cannot inform fully on the risks and benefits of longer-term treatment. It is possible, particularly for extrapyramidal symptoms, that the risk for their emergence may be greater with longer periods of treatment.
Future research
The conduct of the ATLAS trial demonstrates that it is possible, if challenging, to engage patients with VLOSLP in clinical trials. Future studies could investigate the longer-term benefits and risks of antipsychotic treatment because treatment in practice is given to this group for many years and the risks of side effects associated with prolonged treatment may be greater than indicated by the 24-week duration of the ATLAS trial. It would also be important to investigate the effectiveness of doses of amisulpride that are lower than the 100 mg per day used in the ATLAS trial, to see whether or not comparable improvement in psychosis symptoms with fewer side effects is achieved. The greatest barrier to successful treatment of people with VLOSLP is their successful engagement with services and willingness to accept that they have an illness that might respond to treatment. Future research could, therefore, explore the effectiveness of psychological therapy-based strategies to improve the engagement of patients with mental health services and treatment, as well as monitoring the safety of long-term antipsychotic use in this vulnerable population.
Chapter 6 Conclusions
The ATLAS trial showed that low-dose amisulpride, at 100 mg per day, is an effective and well-tolerated treatment for patients with VLOSLP and that symptomatic benefits are maintained by prolonging treatment to 24 weeks. The data could encourage psychiatrists to be more therapeutically optimistic and assertive in their treatment of VLOSLP patients. Future research should examine ways of improving the engagement of patients with VLOSLP in treatment, because their lack of insight into their illness or the potential for treatment to improve symptoms remains the most important factor limiting access to effective drug treatments.
Acknowledgements
Sadly, Professor Rob Jones died unexpectedly during the course of the ATLAS trial.
The ATLAS trial Triallists Group
Writing committee
Robert Howard (University College London, London, UK); Rosie Bradley (Medical Research Council Population Health Research Unit, University of Oxford, Oxford, UK); Elizabeth Cort (South London and Maudsley NHS Foundation Trust); Emma Harper (Nuffield Department of Population Health, University of Oxford, Oxford, UK); Linda Kelly (Nuffield Department of Population Health, University of Oxford, Oxford, UK); Craig Ritchie (University of Edinburgh, Edinburgh, UK); Robert Barber (Northumberland, Tyne and Wear NHS Trust); Waleed Fawzi (East London NHS Foundation Trust); Chris Fox (Norfolk and Suffolk NHS Foundation Trust); Rob Jones (Nottinghamshire Healthcare NHS Trust); Gill Livingston (Camden and Islington NHS Foundation Trust); Ajay Macharouthu (NHS Ayrshire and Arran); Ejaz Nazir (South Staffordshire and Shropshire Healthcare NHS Foundation Trust); Ramin Nilforooshan (Surrey and Borders Partnership NHS Foundation Trust); Sabu Oomman (Cheshire and Wirral Partnership NHS Foundation Trust); Vivek Pattan (NHS Forth Valley); Pranathi Ramachandra (Cambridgeshire and Peterborough NHS Foundation Trust); John Sykes (Derbyshire Healthcare NHS Foundation Trust); Peter Bentham (Birmingham and Solihull Mental Health NHS Foundation Trust); and Richard Gray (Medical Research Council Population Health Research Unit, University of Oxford, Oxford, UK).
Data monitoring committee
Brian Lawlor, Trinity College, Dublin (chairperson); Robert Hills, Cardiff University School of Medicine; and Alan Thomas, Wolfson Research Centre, Newcastle University.
Steering committee
Cornelius Katona, University College, London (chairperson); Lee Middleton, Birmingham Clinical Trials Unit; and Tom Dening, Institute of Mental Health, Nottingham.
Trial management
Emma Harper, Linda Kelly, Natalie Lam, Lynn Pank, Rosie Bradley and Richard Gray (Nuffield Department of Population Health, University of Oxford).
Nursing co-ordinators
Elizabeth Cort (South London and Maudsley NHS Foundation Trust), Analisa Smythe and Jan Wright (both Birmingham and Solihull Mental Health NHS Foundation Trust).
Health economics
Martin Knapp (London School of Economics and Political Science), Renee Romeo and Ching Yan Chung (both King’s College, London).
Pharmacy
Nigel Barnes (Birmingham and Solihull Mental Health NHS Foundation Trust).
Participating centres
(*Indicates principal investigator at that centre.)
Birmingham and Solihull Mental Health NHS Foundation Trust: Abdul Patel,* Peter Bentham, Analisa Smythe, Jan Wright, Nigel Barnes and Akram Ali.
Black Country Partnership NHS Foundation Trust: Valerie Curran,* Jay Viswanathan, Joanne Sawyer, Laura Lord and Amy Shipman.
Bradford District Care Trust: Gregor Russell,* Sushanth Kamath and Zarina Mirza.
Cambridgeshire and Peterborough NHS Foundation Trust: Pranathi Ramachandra,* Tesema Taye, Vanya Johnson, Katherine Cummergen, Sally-Anne Hurford, Lorna Jacobs, Inderpal Panesar, Senthil Soubramanian, Jacinto Almazan, Jane Chege, Rowan Simpson, Stephanie Williams, Clare Knight, Alison Stribling, Sara Williamson, George Griffiths, Chris Jenkins, Christine Rowe, Sue Coaker, Meriel Pope, Peter Dalrymple, Jacqueline Wood, Lorraine Carter, Rachel Carter, Bernice Gregory and Lauren Dawson.
Camden and Islington NHS Foundation Trust: Gill Livingston,* Amy Enfield-Bance, Andrew Sommerlad and Yvonne Foreshaw.
Cheshire and Wirral Partnership NHS Foundation Trust: Sabu Oomman,* Caroline Mogan, Lisa Douglas, Mark Theophanous and Mohan Kumar Choyi.
Coventry and Warwickshire Partnership NHS Trust: Rafi Arif.*
Derbyshire Healthcare NHS Foundation Trust: John Sykes,* Graham Spencer and Gemma Elliott.
Devon Partnership NHS Trust: Richard McCollum* and Jonathan Richards.
East London NHS Foundation Trust: Waleed Fawzi,* Sandra Evans, Emma Higgins, Mahmood Dillo, Azad Cadinouche, Cate Bailey, James Innes, Saira Doorjun, John Joseph and Emmanuel Quesie.
NHS Ayrshire and Arran: Ajay Macharouthu,* Paul Brown, Alistair Rennie and Mark Wilson.
NHS Forth Valley: Vivek Pattan* and Stuart Affleck.
NHS Tayside: Peter Connelly* and Ashleigh Duthie.*
Norfolk and Suffolk NHS Foundation Trust: Chris Fox,* Shamim Ruhi, Somaya Khalifa, Lauren Wright, Joanna Williams, Andrew Tarbuck, Robert Butler, Stephen Tucker, Catherine Porter, Silvia Ferrera, Louise Mc Carthy, Ben Underwood, Ruth Chipperfield, Carol Gregory and Judy Rubensztein Inderpal Panesar.
North Staffordshire Combined Healthcare NHS Trust: Bernard Udeze* and Kerri Mason.
Northamptonshire Healthcare NHS Foundation Trust: Paul Koranteng,* Leanne Holman and Abby Lovesy.
Northumberland, Tyne and Wear NHS Trust: Robert Barber,* Ginette Cass and June Pearson.
Nottinghamshire Healthcare NHS Foundation Trust: Rob Jones,* Sujata Das, Ramakrishnan Anandamandiram, David Kelly and Catherine Gordon.
Oxford Health NHS Foundation Trust: Rohan Van Der Putt* and Bobbie Sanghera.
South London and Maudsley NHS Foundation Trust: Robert Howard,* Suzanne Reeves, Suki Greaves, Shalini Sharma, Alan Smith, Liz Cort, Glynis Ivin, Martin Heasman and Mathew Francis.
South Staffordshire and Shropshire Healthcare NHS Foundation Trust: Ejaz Nazir,* Andy Taylor, Sajeev Kshemendra, Ayesha Bangash and Paula Dolby.
South West Yorkshire Partnership NHS Foundation Trust: Owais Sharif,* Wajid Khan, Stacey Phillips, Lubena Mirza and Ismail Patel.
Southern Health NHS Foundation Trust: Brady McFarlane* and Viv Hopkins.
Surrey and Borders Partnership NHS Foundation Trust: Ramin Nilforooshan* and Jane Gregg.
Sussex Partnership NHS Foundation Trust: Naji Tabet,* Sam Holden, Andrew Risbridger and Angela Ozduran.
West London Mental Health Trust: Craig Ritchie* and Sarah Gregory.
Worcestershire Health and Care NHS Trust: Dhanjeev Marrie,* Margaret Shannon, Sue Birch, Iona Brown, Sarah Dugan, Angela Hoadley, Tara Walker, Samantha Whitby and Mandy Wright.
Trial monitoring
Conducted by King’s Health Partners Clinical Trials Office: Ingrid Brumarescu, Hannah Mason, Kelly Gormley, Mohammed Hussain, Eve Wallace, Bina Patel and Aysar Al-Rawi.
Contributions of authors
Robert Howard, Peter Bentham, Craig Ritchie and Richard Gray designed the trial.
Robert Howard, Elizabeth Cort, Emma Harper, Linda Kelly and Richard Gray ran the trial.
Rosie Bradley and Richard Gray analysed the data.
Robert Howard, Elizabeth Cort, Peter Bentham, Craig Ritchie, Suzanne Reeves, Waleed Fawzi, Gill Livingston, Sabu Oomman, Ejaz Nazir, Ramin Nilforooshan, Robert Barber, Chris Fox, Ajay Macharouthu, Pranathi Ramachandra, Vivek Pattan, John Sykes, Valerie Curran, Cornelius Katona, Tom Dening, Martin Knapp and Renee Romeo contributed to the paper and all the authors assume responsibility for the accuracy and completeness of the data and for the overall content and integrity of the paper.
Robert Howard, Elizabeth Cort, Peter Bentham, Craig Ritchie, Waleed Fawzi, Gill Livingston, Andrew Sommerlad, Sabu Oomman, Ejaz Nazir, Ramin Nilforooshan, Robert Barber, Chris Fox, Ajay Macharouthu, Pranathi Ramachandra, Vivek Pattan, John Sykes and Valerie Curran recruited patients.
Robert Howard, Rosie Bradley and Richard Gray interpreted the data and wrote the initial paper draft.
Publications
Howard R, Cort E, Bradley R, Harper E, Kelly L, Bentham P, et al. Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial. Lancet Psychiatry 2018;5:553–63.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Howard R, Cort E, Bradley R, Harper E, Kelly L, Bentham P, et al. Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial. Lancet Psychiatry 2018;5:553-63. https://doi.org/10.1016/S2215-0366(18)30141-X.
- Roth M, Morrissey JD. Problems in the diagnosis and classification of mental disorder in old age; with a study of case material. J Ment Sci 1952;98:66-80. https://doi.org/10.1192/bjp.98.410.66.
- van Os J, Howard R, Takei N, Murray R. Increasing age is a risk factor for psychosis in the elderly. Soc Psychiatry Psychiatr Epidemiol 1995;30:161-4. https://doi.org/10.1007/BF00790654.
- Kay DW, Roth M. Environmental and hereditary factors in the schizophrenias of age (‘late paraphrenia’) and their bearing on the general problem of causation in schizophrenia. J Ment Sci 1961;107:649-86. https://doi.org/10.1192/bjp.107.449.649.
- Kraepelin E. Dementia Praecox and Paraphrenia. Malabar, FL: Krieger Publishing Company; 1971.
- Mayer W. Über paraphrene psychosen. Ze Neurol Psychiatr 1921;71:187-206. https://doi.org/10.1007/BF02897397.
- Post F. Persistent Persecutory States of the Elderly. Oxford: Pergamon Press; 1966.
- Howard R, Rabins PV, Seeman MV, Jeste DV. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry 2000;157:172-8. https://doi.org/10.1176/appi.ajp.157.2.172.
- Mitford E, Reay R, McCabe K, Paxton R, Turkington D. Ageism in first episode psychosis. Int J Geriatr Psychiatry 2010;25:1112-18. https://doi.org/10.1002/gps.2437.
- Mitter PR, Krishnan S, Bell P, Stewart R, Howard RJ. The effect of ethnicity and gender on first-contact rates for schizophrenia-like psychosis in Bangladeshi, Black and White elders in Tower Hamlets, London. Int J Geriatr Psychiatry 2004;19:286-90. https://doi.org/10.1002/gps.1084.
- Reeves SJ, Sauer J, Stewart R, Granger A, Howard RJ. Increased first-contact rates for very-late-onset schizophrenia-like psychosis in African- and Caribbean-born elders. Br J Psychiatry 2001;179:172-4. https://doi.org/10.1192/bjp.179.2.172.
- Herbert ME, Jacobson S. Late paraphrenia. Br J Psychiatry 1967;113:461-9. https://doi.org/10.1192/bjp.113.498.461.
- Howard RJ, Almeida O, Levy R, Graves P, Graves M. Quantitative magnetic resonance imaging volumetry distinguishes delusional disorder from late-onset schizophrenia. Br J Psychiatry 1994;165:474-80. https://doi.org/10.1192/bjp.165.4.474.
- Howard R, Castle D, O’Brien J, Almeida O, Levy R. Permeable walls, floors, ceilings and doors. Partition delusions in late paraphrenia. Int J Geriatr Psychiatry 1992;7:719-24. https://doi.org/10.1002/gps.930071006.
- Almeida OP, Levy R, Howard RJ, David AS. Insight and paranoid disorders in late life (late paraphrenia). Int J Geriatr Psychiatry 1996;11. https://doi.org/10.1002/(SICI)1099-1166(199607)11:7<653::AID-GPS380>3.0.CO;2-9.
- Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, et al. Behavioral symptoms in mild cognitive impairment. Neurology 2004;62:1199-201. https://doi.org/10.1212/01.WNL.0000118301.92105.EE.
- Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 2014;171:572-81. https://doi.org/10.1176/appi.ajp.2014.13060821.
- Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 2016;12:195-202. https://doi.org/10.1016/j.jalz.2015.05.017.
- Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet 1997;349:1546-9. https://doi.org/10.1016/S0140-6736(96)10203-8.
- Reeves SJ, Gould RL, Powell JF, Howard RJ. Origins of delusions in Alzheimer’s disease. Neurosci Biobehav Rev 2012;36:2274-87. https://doi.org/10.1016/j.neubiorev.2012.08.001.
- McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology 1999;53:902-5. https://doi.org/10.1212/WNL.53.5.902.
- Nagahama Y, Okina T, Suzuki N, Matsuda M, Fukao K, Murai T. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry 2007;15:961-7. https://doi.org/10.1097/JGP.0b013e3180cc1fdf.
- Uchiyama M, Nishio Y, Yokoi K, Hirayama K, Imamura T, Shimomura T, et al. Pareidolias: complex visual illusions in dementia with Lewy bodies. Brain 2012;135:2458-69. https://doi.org/10.1093/brain/aws126.
- Shinagawa S, Nakajima S, Plitman E, Graff-Guerrero A, Mimura M, Nakayama K, et al. Psychosis in frontotemporal dementia. J Alzheimers Dis 2014;42:485-99. https://doi.org/10.3233/JAD-140312.
- Galimberti D, Dell’Osso B, Altamura AC, Scarpini E. Psychiatric symptoms in frontotemporal dementia: epidemiology, phenotypes, and differential diagnosis. Biol Psychiatry 2015;78:684-92. https://doi.org/10.1016/j.biopsych.2015.03.028.
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9orf72 mutations. Brain 2012;135:693-708. https://doi.org/10.1093/brain/awr355.
- Snowden JS, Harris J, Adams J, Thompson JC, Richardson AM, Jones MS, et al. Psychosis associated with expansions in the C9orf72 gene: the influence of a 10 base pair gene deletion. J Neurol Neurosurg Psychiatry 2016;87:562-3. https://doi.org/10.1136/jnnp-2015-310441.
- Watson A, Pribadi M, Chowdari K, Clifton S, Wood J, Miller BL, et al. C9orf72 repeat expansions that cause frontotemporal dementia are detectable among patients with psychosis. Psychiatry Res 2016;235:200-2. https://doi.org/10.1016/j.psychres.2015.12.007.
- Pose M, Cetkovich M, Gleichgerrcht E, Ibáñez A, Torralva T, Manes F. The overlap of symptomatic dimensions between frontotemporal dementia and several psychiatric disorders that appear in late adulthood. Int Rev Psychiatry 2013;25:159-67. https://doi.org/10.3109/09540261.2013.769939.
- Shah JN, Qureshi SU, Jawaid A, Schulz PE. Is there evidence for late cognitive decline in chronic schizophrenia?. Psychiatric Q 2012;83:127-44. https://doi.org/10.1007/s11126-011-9189-8.
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry 2009;195:286-93. https://doi.org/10.1192/bjp.bp.108.060723.
- Girard C, Simard M, Noiseux R, Laplante L, Dugas M, Rousseau F, et al. Late-onset-psychosis: cognition. Int Psychogeriatr 2011;23:1301-16. https://doi.org/10.1017/S1041610211000238.
- Hanssen M, van der Werf M, Verkaaik M, Arts B, Myin-Germeys I, van Os J, et al. Comparative study of clinical and neuropsychological characteristics between early-, late and very-late-onset schizophrenia-spectrum disorders. Am J Geriatr Psychiatry 2015;23:852-62. https://doi.org/10.1016/j.jagp.2014.10.007.
- Almeida OP, Howard RJ, Levy R, David AS, Morris RG, Sahakian BJ. Clinical and cognitive diversity of psychotic states arising in late life (late paraphrenia). Psychol Med 1995;25:699-714. https://doi.org/10.1017/S0033291700034954.
- Mazeh D, Zemishlani C, Aizenberg D, Barak Y. Patients with very-late-onset schizophrenia-like psychosis: a follow-up study. Am J Geriatr Psychiatry 2005;13:417-19. https://doi.org/10.1097/00019442-200505000-00011.
- Palmer BW, Bondi MW, Twamley EW, Thal L, Golshan S, Jeste DV. Are late-onset schizophrenia spectrum disorders neurodegenerative conditions? Annual rates of change on two dementia measures. J Neuropsychiatry Clin Neurosci 2003;15:45-52. https://doi.org/10.1176/jnp.15.1.45.
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry 1999;45:32-40. https://doi.org/10.1016/S0006-3223(98)00273-X.
- Brodaty H, Sachdev P, Koschera A, Monk D, Cullen B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry 2003;183:213-19. https://doi.org/10.1192/bjp.183.3.213.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Kørner A, Lopez AG, Lauritzen L, Andersen PK, Kessing LV. Late and very-late first-contact schizophrenia and the risk of dementia – a nationwide register based study. Int J Geriatr Psychiatry 2009;24:61-7. https://doi.org/10.1002/gps.2075.
- Corey-Bloom J, Jernigan T, Archibald S, Harris MJ, Jeste DV. Quantitative magnetic resonance imaging of the brain in late-life schizophrenia. Am J Psychiatry 1995;152:447-9. https://doi.org/10.1176/ajp.152.3.447.
- Rabins PV, Aylward E, Holroyd S, Pearlson G. MRI findings differentiate between late-onset schizophrenia and late-life mood disorder. Int J Geriatr Psychiatry 2000;15:954-60. https://doi.org/10.1002/1099-1166(200010)15:10<954::AID-GPS224>3.0.CO;2-O.
- Sachdev P, Brodaty H, Rose N, Cathcart S. Schizophrenia with onset after age 50 years. 2: Neurological, neuropsychological and MRI investigation. Br J Psychiatry 1999;175:416-21. https://doi.org/10.1192/bjp.175.5.416.
- Symonds LL, Olichney JM, Jernigan TL, Corey-Bloom J, Healy JF, Jeste DV. Lack of clinically significant gross structural abnormalities in MRIs of older patients with schizophrenia and related psychoses. J Neuropsychiatry Clin Neurosci 1997;9:251-8. https://doi.org/10.1176/jnp.9.2.251.
- Sachdev P, Brodaty H. Quantitative study of signal hyperintensities on T2-weighted magnetic resonance imaging in late-onset schizophrenia. Am J Psychiatry 1999;156:1958-67. https://doi.org/10.1176/ajp.156.12.1958.
- Howard R, Cox T, Almeida O, Mullen R, Graves P, Reveley A, et al. White matter signal hyperintensities in the brains of patients with late paraphrenia and the normal, community-living elderly. Biol Psychiatry 1995;38:86-91. https://doi.org/10.1016/0006-3223(94)00248-2.
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, et al. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry 2005;13:1092-9. https://doi.org/10.1097/00019442-200512000-00009.
- Bozikas VP, Kövari E, Bouras C, Karavatos A. Neurofibrillary tangles in elderly patients with late onset schizophrenia. Neurosci Lett 2002;324:109-12. https://doi.org/10.1016/S0304-3940(02)00189-1.
- Casanova MF, Stevens JR, Brown R, Royston C, Bruton C. Disentangling the pathology of schizophrenia and paraphrenia. Acta Neuropathol 2002;103:313-20. https://doi.org/10.1007/s00401-001-0468-6.
- Velakoulis D, Walterfang M, Mocellin R, Pantelis C, Dean B, McLean C. Abnormal hippocampal distribution of TDP-43 in patients with late-onset psychosis. Aust N Z J Psychiatry 2009;43:739-45. https://doi.org/10.1080/00048670903001984.
- Nagao S, Yokota O, Ikeda C, Takeda N, Ishizu H, Kuroda S, et al. Argyrophilic grain disease as a neurodegenerative substrate in late-onset schizophrenia and delusional disorders. Eur Arch Psychiatry Clin Neurosci 2014;264:317-31. https://doi.org/10.1007/s00406-013-0472-6.
- Arunpongpaisal S, Ahmed I, Aqeel N, Suchat P. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev 2003;2. https://doi.org/10.1002/14651858.CD004162.
- Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev 2012;2. https://doi.org/10.1002/14651858.CD004162.pub2.
- Uchida H, Suzuki T, Mamo DC, Mulsant BH, Tanabe A, Inagaki A, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry 2008;16:584-93. https://doi.org/10.1097/JGP.0b013e318172b42d.
- Scott J, Greenwald BS, Kramer E, Shuwall M. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr 2011;23:742-8. https://doi.org/10.1017/S1041610210002188.
- Reeves S, Stewart R, Howard R. Service contact and psychopathology in very-late-onset schizophrenia-like psychosis: the effects of gender and ethnicity. Int J Geriatr Psychiatry 2002;17:473-9. https://doi.org/10.1002/gps.614.
- Sin Fai Lam CC, Reeves SJ, Stewart R, Howard R. Service and treatment engagement of people with very late-onset schizophrenia-like psychosis. Br J Psych Bull 2016;20:185-6.
- Talaslahti T, Alanen HM, Hakko H, Isohanni M, Kampman O, Häkkinen U, et al. Psychiatric hospital admission and long-term care in patients with very-late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry 2016;31:355-60. https://doi.org/10.1002/gps.4333.
- van Liempt S, Dols A, Schouws S, Stek ML, Meesters PD. Comparison of social functioning in community-living older individuals with schizophrenia and bipolar disorder: a catchment area-based study. Int J Geriatr Psychiatry 2017;32:532-8. https://doi.org/10.1002/gps.4490.
- Talaslahti T, Alanen HM, Hakko H, Isohanni M, Häkkinen U, Leinonen E. Patients with very-late-onset schizoprhenia-like psychosis have higher mortality rates than elderly patients with earlier onset schizophrenia. Int J Geriatr Psychiatry 2015;30:453-9. https://doi.org/10.1002/gps.4159.
- Psarros C, Theleritis CG, Paparrigopoulos TJ, Politis AM, Papadimitriou GN. Amisulpride for the treatment of very-late-onset schizophrenia-like psychosis. Int J Geriatr Psychiatry 2009;24:518-22. https://doi.org/10.1002/gps.2146.
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: APA; 2000.
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: ’The drift busters.’. Int J Meth Psych Res 1993;3:221-44.
- Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, et al. Yokukansan (TJ-54) for treatment of very-late-onset schizophrenia-like psychosis: an open-label study. Phytomedicine 2013;20:654-8. https://doi.org/10.1016/j.phymed.2013.01.007.
- Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191-210. https://doi.org/10.1097/01.JGP.0000200589.01396.6d.
- Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med 2012;367:1497-507. https://doi.org/10.1056/NEJMoa1114058.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934-43. https://doi.org/10.1001/jama.294.15.1934.
- Crippa JA, Sanches RF, Hallak JE, Loureiro SR, Zuardi AW. A structured interview guide increases Brief Psychiatric Rating Scale reliability in raters with low clinical experience. Acta Psychiatr Scand 2001;103:465-70. https://doi.org/10.1034/j.1600-0447.2001.00185.x.
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970;212:11-9. https://doi.org/10.1111/j.1600-0447.1970.tb02066.x.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Orley J. WHOQoL-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment. Geneva: Program on Mental Health, World Health Organization; 1996.
- Klug G, Hermann G, Fuchs-Nieder B, Stipacek A, Zapotoczky HG. Geriatric psychiatry home treatment (GHT): a pilot study on outcomes following hospital discharge for depressive and delusional patients. Arch Gerontol Geriatr 2008;47:109-20. https://doi.org/10.1016/j.archger.2007.07.002.
- Ritchie CW, Chiu E, Harrigan S, MacFarlane S, Mastwyk M, Halliday G, et al. A comparison of the efficacy and safety of olanzapine and risperidone in the treatment of elderly patients with schizophrenia: an open study of six months duration. Int J Geriatr Psychiatry 2006;21:171-9. https://doi.org/10.1002/gps.1446.
- Beecham J, Knapp M. Costing Psychiatric Interventions. London: Gaskell; 2001.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- Department of Health and Social Care . National Schedule of Reference Costs: The Main Schedule 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 5 November 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- NHS Employers . NHS Terms and Conditions of Service Handbook (Agenda for Change) 2016. www.nhsemployers.org/your-work/pay-and-reward/nhs-terms-and-conditions/nhs-terms-and-conditions-of-service-handbook (accessed 14 May 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Rascati K. Essentials of Pharmacoeconomics. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
- Rascati KL, Rascati KL. Essentials of Pharmacoeconomics. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
- Katz I, de Deyn PP, Mintzer J, Greenspan A, Zhu Y, Brodaty H. The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer’s disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry 2007;22:475-84. https://doi.org/10.1002/gps.1792.
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 2011;306:1359-69. https://doi.org/10.1001/jama.2011.1360.
- Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci 2006;7:492-500. https://doi.org/10.1038/nrn1926.
- Sin Fai Lam CC, Reeves SJ, Stewart R, Howard R. Service and treatment engagement of people with very late-onset schizophrenia-like psychosis. BJPsych Bull 2016;40:185-6. https://doi.org/10.1192/pb.bp.115.051599.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. London: Routledge; 1988.
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 2009;14:429-47. https://doi.org/10.1038/sj.mp.4002136.
- Reeves S, Eggleston K, Cort E, McLachlan E, Brownings S, Nair A, et al. Therapeutic D2/3 receptor occupancies and response with low amisulpride blood concentrations in very late-onset schizophrenia-like psychosis (VLOSLP). Int J Geriatr Psychiatry 2018;33:396-404. https://doi.org/10.1002/gps.4758.
- Reeves S, McLachlan E, Bertrand J, Antonio FD, Brownings S, Nair A, et al. Therapeutic window of dopamine D2/3 receptor occupancy to treat psychosis in Alzheimer’s disease. Brain 2017;140:1117-27. https://doi.org/10.1093/brain/aww359.
- Mental Health Act 1983. London: The Stationery Office; 1983.
Appendix 1
FIGURE 16.
Bootstrapped replicates of incremental cost and incremental QALYs for amisulpride vs. placebo over stage 2: (a) NHS/PSS perspective; and (b) societal perspective.
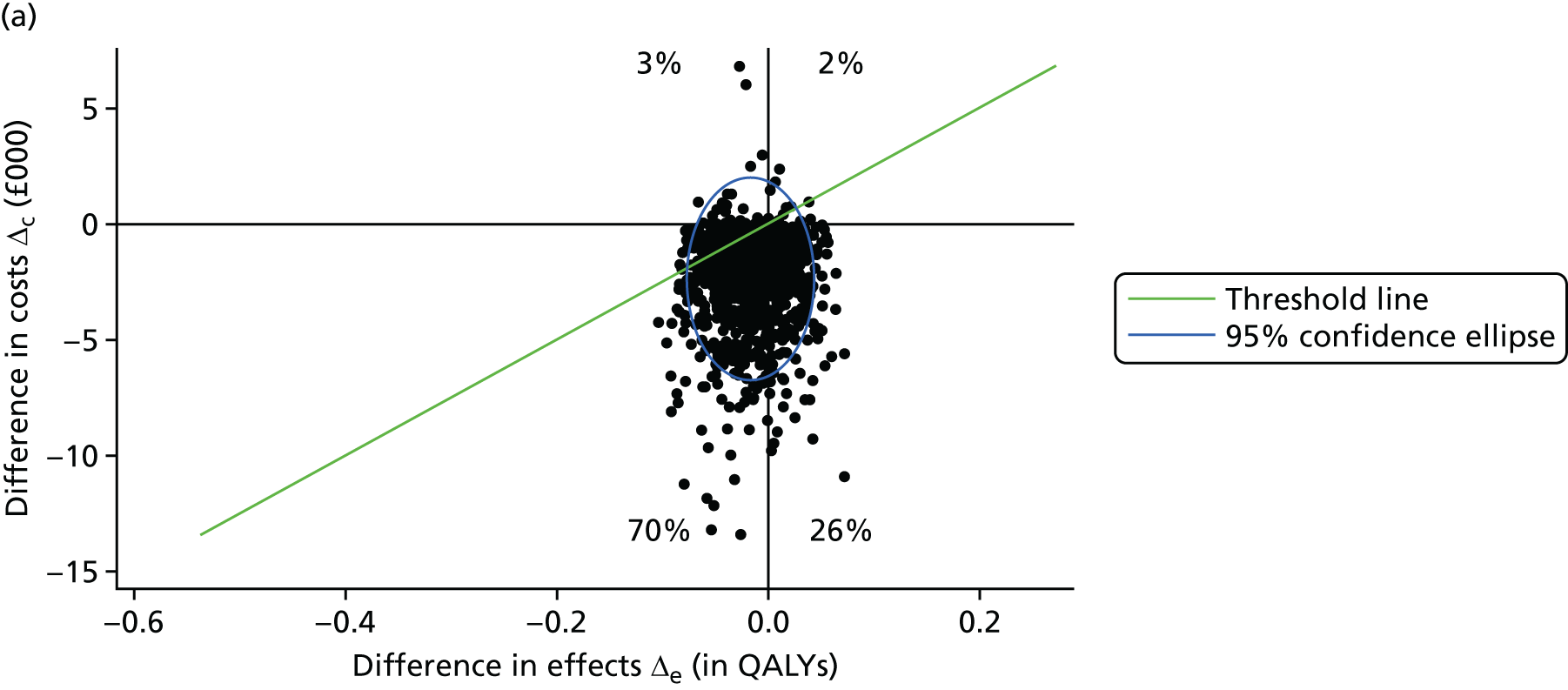

FIGURE 17.
Cost-effectiveness acceptability curve of sensitivity analysis 2: amisulpride continuation vs. placebo in stage 2 in the NHS/PSS perspective and the societal perspective, with effectiveness measured in QALYs. a, Adjusted for age, gender, home circumstances, time since onset of symptoms, previous antipsychotic treatment and BPRS score.
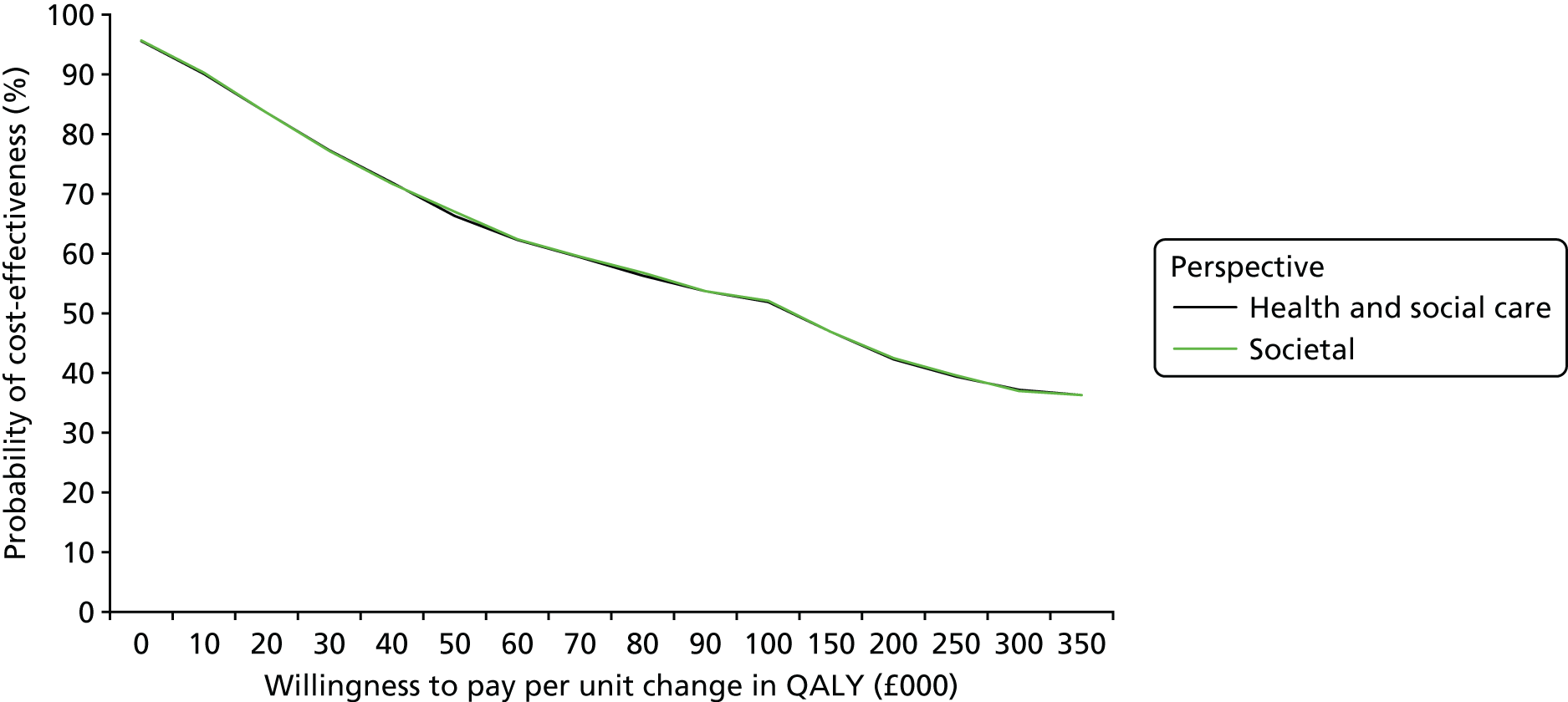
List of abbreviations
- ALS
- amyotrophic lateral sclerosis
- ATLAS
- Antipsychotic Treatment of very LAte-onset Schizophrenia-like psychosis
- BPRS
- Brief Psychiatric Rating Scale
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- CTSU
- Clinical Trials Service Unit
- DLB
- dementia with Lewy bodies
- DSM-IV-TR
- Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision
- EOS
- early-onset schizophrenia
- EPSE
- extrapyramidal side effect
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FTD
- frontotemporal dementia
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- LOS
- late-onset schizophrenia
- MCI
- mild cognitive impairment
- MCID
- minimal clinically important difference
- MMSE
- Mini-Mental State Examination
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAS
- Simpson–Angus Scale
- SD
- standard deviation
- VLOSLP
- very late-onset schizophrenia-like psychosis
- WHOQoL-BREF
- World Health Organization’s quality-of-life scale
