Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/09/04. The contractual start date was in July 2016. The draft report began editorial review in July 2018 and was accepted for publication in October 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alison Avenell, Clare Robertson and Graeme MacLennan acknowledge funding from the National Institute for Health Research Health Technology Assessment programme for projects outside this work. Paul Aveyard was an investigator on an investigator-initiated trial funded by Cambridge Weight Plan, and has done half a day's consultancy for Weight Watchers. These activities led to payments to the University of Oxford for his time but no payments to him personally.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Avenell et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
There has been a continued increase in severe obesity {denoted in this report by a body mass index (BMI) [weight in kg/(height in m)2] of ≥ 35 kg/m2} in adults in the UK. As BMI increases, the likelihood of obesity-related comorbidities and social, psychological and economic consequences increases, as may the need for greater support for help with weight loss. Current National Institute for Health and Care Excellence (NICE)1,2 and Scottish Intercollegiate Guidelines Network guidance on weight management for adults with obesity does not distinguish between the BMI range of 30 to < 35 kg/m2 and severe obesity. 3 In the UK, having severe obesity, with or without comorbidities, may be a referral criterion for Tier 3 specialist weight-management services in the obesity pathway, prior to Tier 4 services for bariatric surgery. 2,4 Most patients attending Tier 3 services are not seeking surgery. This project seeks to evaluate the evidence base for the feasibility, acceptability, effectiveness and cost-effectiveness of interventions for people with severe obesity.
Epidemiology
Data from the 2013 Health Survey for England (HSE)5 showed that 5% of women and 6% of men had a BMI of ≥ 35 to < 40 kg/m2, and 2% of men and 4% of women had a BMI of ≥ 40 kg/m2 (so-called morbid obesity). In 2016 in England, the prevalence of people with a BMI of ≥ 40 kg/m2 was similar to that reported in 2013, but figures for severe obesity were not presented. 6 BMIs of ≥ 40 kg/m2 were most prevalent among people aged 35–44 years. 6 Six per cent of women in the most deprived population group had a BMI of ≥ 40 kg/m2, compared with 2% in the least deprived group. 6 For men, 1% of the least and 2% of the most deprived group had a BMI of ≥ 40 kg/m2.
In Scotland in 2016,7 3% of men and 4% of women had a BMI of ≥ 40 kg/m2, with the greatest prevalence, of 8%, in women aged 35–44 years. Data on BMIs of ≥ 35 to < 40 kg/m2 were not presented. Data on BMIs of ≥ 40 kg/m2 from Wales8 and Northern Ireland9 were similar to those from England and Scotland. Data from the English National Obesity Observatory10 are not comparable with data for severe obesity in ethnic communities, making allowance for different BMI cut-off points impossible.
The Global BMI Mortality Collaboration11 found that the hazard ratio (HR) for all-cause mortality increased by 1.31 [95% confidence interval (CI) 1.29 to 1.33] for each 5 kg/m2 above a BMI of 25 kg/m2. Significant increases were also seen for cardiovascular disease (CVD), stroke, respiratory disease and cancer. For people with a BMI of 40–45 kg/m2, median survival may be reduced by 8–10 years compared with those with a BMI of 22.5–25 kg/m2. 12 Using 2003–10 National Health and Nutrition Examination Survey data, Grover et al. 13 found that healthy life-years lost were at least 2–4 times greater than years of life lost for each category of body weight and age.
The increasing trend in obesity has significant implications for health services because of the much greater risk of type 2 diabetes mellitus. UK health service costs in 2010–11 were already £8.8B per year, with indirect costs of £13B for type 2 diabetes mellitus in England. 14
In the UK, the Million Women Study15 found significant increases in the risk, and length, of hospital admission with increasing BMI. Women with a BMI of ≥ 35 kg/m2 had the most marked increase in hospital admissions compared with those with a BMI of ≤ 25 kg/m2 [knee replacement, relative risk (RR) 7; venous thromboembolism, RR 3; atrial fibrillation, RR 3; gallbladder disease, RR 2; and hip replacement, RR 2]. Korda et al. 16 reported that a BMI of 35–50 kg/m2 was associated with twice the risk of hospitalisation compared with the risk for men and women aged 45–64 years with normal weights.
Compared with people of normal weight (BMI 18.5–24.9 kg/m2), people with a BMI of ≥ 35 kg/m2 have the greatest decrements in health-related quality of life, as determined by the Short Form questionnaire-12 items and the EuroQol-5 Dimensions (EQ-5D). 17 Depressive and anxiety disorders are particularly associated with a BMI of ≥ 35 kg/m2, especially in women. 18
Costs
The costs to the UK economy of an increasingly overweight and obese population are substantial. The estimated costs of people being overweight and obese to society and the economy as a whole amounted to almost £16B per year in 2007, representing > 1% of total gross domestic product. 19 Some estimates suggest that if the trend of obesity remains unchecked, economic costs could increase to £50B per year by 2050. 19 Morbid obesity represents a particularly high health-care and economic cost burden. A 2013 systematic review20 found that people with a BMI of ≥ 40 kg/m2 had 1.5–3.9 times higher direct health-care costs and 1.7–8.0 times higher lost productivity costs than people with a BMI of 18.5–24.9 kg/m2. Costs grew exponentially as the level of obesity increased. The McKinsey Global Institute21 found that UK medical costs were 80% higher in people with a BMI of ≥ 35 kg/m2 than in people with a BMI of < 25 kg/m2.
Current evidence
Existing guidance and systematic reviews
The NICE public health and clinical guidance evidence syntheses2,22,23 do not examine behavioural interventions in accordance with the severity or complexity (presence of comorbidities) of obesity. Very low-calorie diets (VLCDs) (usually defined as < 800 kcal/day) may be of particular interest for people with severe obesity to provide greater and more rapid weight loss. Current NICE obesity guidelines provide VLCD guidance only in the context of a need to rapidly lose weight (e.g. joint replacement surgery or fertility services). 2,22,23
The evaluation for NICE public health guidance excluded randomised controlled trials (RCTs) in people with obesity and related comorbidities, making that guidance less likely to be applicable to people with higher BMIs, who are more likely to have comorbidities. 23 The NICE Public Health Guidance 53 economic model does suggest that behavioural interventions were found to be most cost-effective with increasing age and in groups in which the initial BMI lies between 30 and 40 kg/m2. 23
A systematic review and commissioning guidance for weight assessment and management clinics (Tier 3) in adults and children with severe complex obesity (defined as a BMI of ≥ 35 kg/m2 with complications or ≥ 40 kg/m2 without complications) has been published and accredited by NICE. 4,24 This focused on pathways for referral (including assessment and onward referral for bariatric surgery), organisation and staffing of services, but provided little detail on the actual interventions to be delivered for people with obesity. The Royal College of Physicians’ working party provided guidance25 on organising hospital-based obesity management, but undertook no evidence-based reviews of what interventions to provide. In the obesity pathway, patients in Tier 3 may not necessarily desire to move on to Tier 4 and bariatric surgery. Effective weight loss from attending Tier 3 services might also reduce the numbers of patients moving on to Tier 4, or contribute to subsequent effectiveness of bariatric surgery.
Brown et al. 26 undertook a review of Tier 3 weight-management interventions, including for adults, in the UK or Ireland for 2005–16, commissioned by Public Health England. Eight out of fourteen studies had follow-up durations of ≥ 12 months. Most studies had drop-out rates of 43–62% over 6–24 months. Six studies reported mean BMI changes ranging from –1.4 to –3.1 kg/m2, and most studies reported weight loss of 2–6 kg. In 2017, Public Health England27 published a qualitative evaluation of stakeholders’ and users’ experiences of Tier 3 services, which found the importance of social support, for example including groups of participants with common characteristics, such as age and sex. Empathy and clarity of purpose, psychological support, participants setting own milestones, self-monitoring, learning to take accountability, flexibility in services, including individual and group support, and helping people become independent at the end of the service were described as important features for services.
There have been several recent systematic reviews of the use of VLCDs. 28–32 These have either focused on a particular patient group, such as people with type 2 diabetes mellitus,31,32 or included people with BMIs of < 30 kg/m2,28–32 studies with very short follow-up,28,31,32 or non-randomised evidence. 28,29,31,32 There is a need to examine long-term RCT evidence for the use of VLCDs in people with severe obesity.
Hassan et al. 33 undertook a systematic review of 17 RCTs that included multicomponent lifestyle interventions (diet, exercise and behavioural therapy) of ≥ 12 weeks for people with a BMI of ≥ 40 or > 35 kg/m2 with comorbidities. The differences at 3–24 months between the intervention and control groups ranged from −1.0 to −11.5 kg.
Should more bariatric surgery be offered instead of more lifestyle weight-management programmes (WMPs) for people with severe obesity? Several systematic reviews of RCTs of bariatric surgery compared with WMPs/usual care/control interventions have been published. 34–36 NICE undertook economic evaluations of bariatric surgery in 2009 and 2012. 37,38 Since the NICE evaluations, more RCTs of bariatric surgery and WMPs, and longer-term RCT data on bariatric surgery and WMPs, have become available, allowing further economic evaluations of WMPs in comparison with bariatric surgery.
Long-term randomised trial evidence
Having a BMI of ≥ 35 kg/m2 has the potential to have an impact on health and quality of life. Is there evidence that effective WMPs can improve long-term weight, health and quality of life? There is clear systematic review evidence that the greater the weight loss, the greater the improvement in cardiovascular risk factors in obesity. 39,40 The largest long-term randomised trial of weight loss in people with type 2 diabetes mellitus conducted in the USA, the Look AHEAD study,41 examined an intensive diet, exercise and behavioural weight-loss intervention compared with a control intervention in > 5000 participants with type 2 diabetes mellitus with a mean BMI of 36 kg/m2. Although an effect on CVD outcomes was not demonstrated, numerous other beneficial outcomes have been reported. Mean weight loss was still 5% after 8 years in the intervention group,42 with no reduction in effectiveness of the intervention in people with a BMI of ≥ 40 kg/m2. 43 With the intensive lifestyle intervention (ILI), the incidence of severe chronic kidney disease,44 non-alcoholic fatty liver disease (NAFLD),45 knee pain and reduced mobility,46 depression47 and urinary incontinence in women48 was reduced. The lifestyle intervention also reduced the symptoms of incontinence in men,49 erectile dysfunction50 and sleep apnoea. 51
The Look AHEAD ILI in people with type 2 diabetes mellitus was more likely to produce remission of type 2 diabetes mellitus52 and preserve physical health-related quality of life. 47 Over 10 years’ follow-up, the lifestyle intervention reduced hospital admission days by 15% compared with the control group. 53
Why this research is needed now
In the 2015 The Lancet series on obesity,54 it was pointed out that ‘policy and environmental changes are unlikely to achieve substantial weight loss in patients with severe obesity’. As ‘obesity already poses an enormous clinical burden, innovative treatment and care-delivery strategies are needed. Alignment of the intensity of therapy with the severity of the disease is necessary to improve care for obesity’. 54 Hence, evaluating the long-term evidence on what constitutes effective and cost-effective weight-loss support for adults with a BMI of ≥ 35 kg/m2 may assist the development of WMPs for people with severe obesity.
Planned investigation
Aim
The aim was to systematically review the evidence base for bariatric surgery, behavioural and pharmacotherapy interventions for weight loss and weight maintenance for adults with obesity (BMI ≥ 35 kg/m2), and to evaluate their feasibility, acceptability, effectiveness and cost-effectiveness.
Research objectives
The overarching objective was to integrate the quantitative, qualitative and economic evidence base for the management of severe obesity by weight-loss and weight maintenance services, researching concurrently to systematically review:
-
the effectiveness of interventions for weight loss and maintenance for people with a BMI of ≥ 35 kg/m2
-
the qualitative and mixed-methods evidence relating to –
-
the acceptability, feasibility and appropriateness of interventions for adults with a BMI of ≥ 35 kg/m2
-
the feasibility of delivering services
-
-
the cost-effectiveness of interventions for weight loss and maintenance for people with a BMI of ≥ 35 kg/m2.
Research design
Systematic reviews
We undertook four systematic reviews, which were of:
-
RCTs of weight-loss or weight-maintenance programmes (including orlistat or comparisons between usual care/controls or WMPs and bariatric surgery) for adults with obesity (with a BMI of ≥ 35 kg/m2) with follow-up durations of ≥ 1 year, in any setting (see Chapter 3)
-
UK interventions of weight loss or weight maintenance of any study design with people with a BMI of ≥ 35 kg/m2 with follow-up durations of ≥ 1 year, in any setting (see Chapter 4)
-
qualitative and mixed-methods research exploring adults’ experiences of living with obesity and receiving weight-loss or maintenance interventions for obesity (BMI of ≥ 35 kg/m2) (including research exploring the views of professionals involved in their care) (see Chapter 5)
-
economic evaluations of weight-loss and weight maintenance interventions for adults with a BMI of ≥ 35 kg/m2 (see Chapter 6).
Economic evaluation
We used data from the systematic reviews to populate a microsimulation model predicting lifetime costs, outcomes and cost-effectiveness of the most promising effective weight-loss interventions, including bariatric surgery (see Chapter 7).
Integration of findings
We undertook a realist mixed-methods synthesis to produce a detailed summary of the effectiveness, acceptability, appropriateness and cost-effectiveness of weight-loss and weight maintenance interventions for people with a BMI of ≥ 35 kg/m2, and to understand how the content and processes of the interventions affect participant behaviour to achieve their outcomes (see Chapter 8).
Chapter 2 Methods for systematic reviews
We undertook four systematic reviews, which were of:
-
RCTs of weight-loss or weight maintenance programmes (including orlistat or comparisons between usual care/controls or WMPs and bariatric surgery) for adults with obesity (with BMIs of ≥ 35 kg/m2) with follow-up durations of ≥ 1 year, in any setting (see Chapter 3)
-
UK interventions of weight loss or weight maintenance of any study design with participants with a BMI of ≥ 35 kg/m2 with follow-up durations of ≥ 1 year, in any setting (see Chapter 4)
-
qualitative and mixed-methods research exploring adults’ experiences of living with obesity and receiving weight-loss or weight maintenance interventions for obesity (BMI ≥ 35 kg/m2) (including research exploring the views of professionals involved in their care) (see Chapter 5)
-
economic evaluations of weight-loss and weight maintenance interventions, including comparisons with bariatric surgery, for adults with a BMI of ≥ 35 kg/m2 (see Chapter 6).
We prepared an a priori protocol detailing the objectives, types of study design, participants, interventions and outcomes considered, and the inclusion and exclusion criteria for all reviews. For quantitative reviews, we followed methodological guidance recommended by The Cochrane Collaboration55 and the Centre for Reviews and Dissemination. 56 Details of the methods used for the economic evaluations are provided in Chapter 7. Systematic reviews 1 and 5 from the protocol (available at www.journalslibrary.nihr.ac.uk/programmes/hta/150904) were combined to become systematic review 1, for ease of searching, analysis and presentation of results.
Inclusion criteria
Types of studies
Systematic review 1 included full-text reports of long-term RCTs or quasi-randomised trials (including trials with a cluster design) with mean or median follow-up durations of ≥ 12 months.
Systematic review 2 included full-text reports of UK WMPs of any study design with mean or median follow-up durations of ≥ 12 months.
For systematic review 3 (studies providing qualitative data), the focus was on understanding the feasibility and acceptability of WMPs for adults with a BMI of ≥ 35 kg/m2 and intervention providers. To understand this, we also examined wider themes relating to adults’ experiences of living with obesity. We therefore included reports from three categories:
-
Qualitative and mixed-methods studies linked to eligible RCTs, including any qualitative data reported as part of papers reporting quantitative outcomes.
-
Qualitative and mixed-methods studies linked to ineligible RCTs and identified non-randomised intervention studies including any reported qualitative data.
-
UK-based qualitative studies not linked to any specific interventions, drawing on the experiences and perceptions of adults with a BMI of ≥ 35 kg/m2 (and/or providers involved in their care). Within this category, we focused on only those studies that explicitly state that they included the views of participants with a BMI of ≥ 35 kg/m2.
For systematic review 4, we included economic evaluations (trial analyses and decision modelling studies) undertaken for weight-loss and weight maintenance programmes (after weight loss) for people with BMIs of ≥ 35 kg/m2. Studies that compared both costs and outcomes for interventions for the management of obesity in adults with a BMI of ≥ 35 kg/m2 were also included.
Types of participants
Studies included adult groups with mean or median ages of ≥ 16 years (≥ 18 years for systematic reviews of economic evaluations), with no upper age limit. All groups of participants in studies had to have mean or median BMIs of ≥ 35 kg/m2 (for weight maintenance interventions, BMIs had to be ≥ 35 kg/m2 at the start of the weight-loss phase).
Types of interventions and comparators
For systematic reviews 1 and 2, we included interventions in the form of diets (including VLCDs and meal replacements), physical activity, types of counselling, orlistat or a combination of these. In our protocol, it was specified that drugs other than orlistat would be included in the review if they had a product licence from the European Medicines Agency and were prescribed in the UK. We interpreted this as meaning that drugs for weight management also had to possess approval from NICE, so we included orlistat only. We defined VLCDs as meal replacement products with ≤ 800 kcal/day (± 10%). Interventions had to assist weight loss or prevent weight regain after weight loss. We included newer modes of delivery (e.g. web-based, e-mail and mobile phone support). Studies examining the effect of different intervention providers, frequency of contact, mode of delivery (e.g. group vs. individual) and the use of incentives were included.
For systematic review 1, we included RCTs of lifestyle interventions compared with bariatric surgery. Forms of bariatric surgery examined were gastric banding (GB), gastric bypass (GBP) and sleeve gastrectomy (SG).
Comparators were alternative interventions, or control interventions, recognising that often control interventions also have the potential to change behaviour but vary widely in content and intensity between trials. 57,58
Weight loss or weight gain prevention had to be explicitly stated as the outcome of the studies.
Setting
All settings for interventions were included: secondary care (including residential courses), primary care, community (including community pharmacy), commercial organisations, voluntary sector, leisure centres, workplaces, the internet and other digital domains (e.g. mobile phone networks).
Types of outcome measures
The quantitative outcomes reported in systematic reviews 1and 2 are reported in the following sections.
Primary outcome
The primary outcome was weight change.
Secondary outcomes
The secondary outcomes were:
-
change in BMI
-
waist circumference
-
cardiovascular risk factors [total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, fasting glucose, glycated haemoglobin (HbA1c) and systolic and diastolic blood pressure]
-
disease-specific outcome measures (e.g. development of type 2 diabetes mellitus and reduction in sleep apnoea)
-
changes in medication and adverse events
-
psychological well-being
-
adverse events
-
quality of life
-
process outcomes (e.g. staff involvement, setting, type of intervention, timing, frequency, individual and/or group setting, couple or family setting, proportion recruited and dropping out and participants’ evaluations)
-
costs and economic evaluations.
Exclusion criteria
Complementary therapy (e.g. acupuncture) and non-diet products promoted for weight loss available solely over the counter were not included.
Literature searching
Literature searches, using both controlled vocabulary, when available, and text word terms, were undertaken on 14 databases. Initial searching was undertaken in June 2016 and updated during April/May 2017. With the exception of systematic review 2 (UK studies), no language restrictions were used, but reports published only as abstracts were excluded. The following databases were searched:
-
MEDLINE – all systematic reviews
-
MEDLINE Epub Ahead of Print and MEDLINE In-Process & Other Non-Indexed Citations – all systematic reviews
-
EMBASE – all systematic reviews
-
PsycINFO – systematic reviews 1, 2 and 3
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) – systematic reviews 1 and 3
-
Science Citation Index (SCI) – systematic reviews 1 and 3
-
Social Science Citation Index (SSCI) – systematic review 3
-
Cochrane Central Register of Controlled Trials (CENTRAL) – systematic review 1
-
CAB Abstracts – systematic review 3
-
NHS Economic Evaluation Database (NHS EED) – systematic review 4
-
Health Technology Assessment (HTA) database – systematic review 4
-
Cost-effectiveness Analysis Registry – systematic review 4
-
Research Papers in Economics (RePEc) – systematic review 4
-
ClinicalTrials.gov – systematic reviews 1 and 2.
Searches were tailored to the scope of each review. See Report Supplementary Material 1, Section 1, for details of the search strategies.
Report Supplementary Material 5, Table E1, details the databases that were searched for each review, along with the number of reports retrieved from each search.
Systematic review 1
Randomised controlled trials were restricted to publications after 1990 to reflect current practice, and therefore would possess additional materials we required for data extraction and coding (see below). Ovid MEDLINE and EMBASE autoalerts have been in place since 2002 and results are screened regularly for long-term RCTs on obesity management in adults (≥ 1 year of follow-up). 39,59 Copies of relevant reports are retained and the reviewers hand-searched these reports for systematic review 1. Therefore, search strategies for MEDLINE and EMBASE excluded the results of the autoalert search (using the Boolean operator NOT). A supplementary search of MEDLINE was undertaken to identify systematic reviews of severe or morbid obesity, and reference lists were scrutinised for additional studies.
Systematic review 2
Searches for systematic reviews 1 and 3 were considered likely to identify some UK studies and these were flagged for systematic review 2 at the screening stage. Therefore, specific searches for systematic review 2 aimed to find additional non-randomised studies in the major databases. The strategies combined the facets severe or morbid obesity, a UK location and observational or comparative studies. A fourth facet containing RCT and qualitative terms was then added and combined using NOT to exclude studies that had already been identified by the searches for systematic reviews 1 and 3.
Systematic review 3
The search strategies comprised two facets: severe or morbid obesity and qualitative studies. No date restrictions were imposed.
Systematic review 4
The search strategies for MEDLINE and EMBASE comprised three facets: (1) severe or morbid obesity, (2) dietary, pharmacological, lifestyle or surgical interventions and (3) economic evaluations. The databases with economic content only (HTA database, NHS EED, Cost-Effectiveness Analysis Registry and RePEc) were searched with only obesity terms.
Additional searches
In addition, ClinicalTrials.gov was searched for any ongoing studies, and reference lists of all included studies were scanned to identify additional potentially relevant studies. Nineteen relevant NHS and commercial organisations, including Dietitians in Obesity Management, and the REBALANCE (REview of Behaviour And Lifestyle interventions for severe obesity: AN evidenCE synthesis) Advisory Group, were contacted and requested to provide information about any studies relevant to our systematic reviews. Report Supplementary Material 5, Table E1, details the databases that were searched for each review, with the number of reports retrieved from each search.
Quantitative reviews of randomised controlled trials and other intervention studies
Data extraction strategy
Three reviewers (MAM, CR and FS) independently screened titles and abstracts of all identified items from database searching, checking the initial 10% for agreement. Full-text copies of all potentially relevant reports were obtained and assessed for eligibility. Differences in opinion were resolved by consensus or by discussion with a third member of the team (AA) if required. Reviewers flagged studies for the qualitative and economic reviews as they searched. The reviewers extracted details of study design, methods, participants, interventions and outcomes and TIDieR (Template for Intervention Description and Replication)60 using our online form. Numerical data extraction was then checked by a second reviewer (AA). Numerical outcome data were checked by a third reviewer (DC) if required. References were stored using EndNote version X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA].
Quality assessment strategy
The methods used for assessing quality in studies reporting quantitative data, including RCTs, were based on those used by the Health Services Research Unit, University of Aberdeen, for technology assessment reviews for NICE. The Cochrane risk-of-bias tool was used to assess the risk of bias in RCTs,55 and a 17-question checklist for non-randomised comparative studies and case series was used. 59 The development of the latter checklist was led by the Health Services Research Unit in partnership with the Review Body for Interventional Procedures for NICE. This checklist rates bias, generalisability, sample definition and selection, description of the intervention, outcome assessment, adequacy of follow-up and performance of the analysis. We used an adapted version of the Campbell and Cochrane Equity Methods Group checklist61 to assess the effect of interventions on disadvantaged groups and/or their impact on reducing socioeconomic inequalities. Three reviewers (MAM, CR and FS) conducted double, blinded assessment of the quality of primary studies. Differences in opinion were resolved by consensus or by discussion with a third member of the team (AA) if required.
Coding interventions and comparison group support
We used the TIDieR 12-item checklist to guide data collection on the content, context and intensity of the weight-management support delivered to intervention and control groups. 60 TIDieR includes the collection of information on materials used in interventions and the active ingredients of the intervention, details of the provider delivering the intervention, modes of delivery (e.g. face to face, telephone, internet, individual and/or group), location for the intervention and the infrastructure required, when and how much was delivered (e.g. number of sessions, duration and intensity), tailoring of the intervention, modifications made to the intervention during the trial and assessment of intervention fidelity on actual exposure. One reviewer extracted data and a second reviewer checked data.
After extensive training, three reviewers (MAM, CR and FS) undertook double, blinded coding of the active ingredients of the behavioural support provided in all intervention and control arms of the RCTs, using the consensus-based behaviour change technique (BCT) taxonomy. 62 Differences were resolved by discussion or by reference to a third reviewer (AA or ED).
When we could identify author e-mail addresses, we contacted the first, second and last authors of the main publications for each of the trials included in the review of RCTs in order to identify additional materials (e.g. protocols, trial materials and diet books) that would assist our data extraction, quality assessment and coding of BCTs. Two reminder e-mails were sent over a period of 2 months, if required.
Report Supplementary Material 1, Section 2, details all data items extracted in an online form used for the quantitative systematic reviews.
Data analysis
Statistical analysis
Means or changes in means or proportions between groups were collected. For continuous outcomes, we reported the mean difference or standardised mean difference (different scales for the same outcome), and risk ratio for dichotomous data, with 95% CIs.
For each study, we extracted weight change data and denominators, when these were presented by investigators. Our weight change analyses used data for all participants randomised if presented by investigators. If data were presented for completers only, we used a correction utilising baseline observation carried forward (BOCF). 63 When standard deviations (SDs) were not presented, we calculated them from 95% CIs or standard errors, or from a previous regression equation. 39 When weight change data were not available, the weight change was obtained using either the difference from baseline or the difference from the BMI data. When possible, we made reasonable assumptions to calculate these data and note these assumptions in the evidence tables. We contacted authors if data were missing. Therefore, for mean differences in weight (in kg), we present tabulated data in two forms (unless stated): with and without imputed data for dropouts. BMI change data, adjusted for dropouts, are presented in Chapter 7 for interventions used for economic modelling. Waist circumference data were rarely presented and are presented in full with BMI data in Report Supplementary Material 2.
Meta-analysis tables of risk factor data (cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, systolic and diastolic blood pressure, HbA1c and fasting glucose) are presented in the main text for comparisons of particular interest, using available data for the greatest number of participants (i.e. orlistat, low-carbohydrate diets vs. low-fat diets, higher-protein diets vs. lower-protein diets and bariatric surgery vs. WMPs). All risk factor data are presented in Report Supplementary Material 3.
We used Stata® version 14 (StataCorp LP, College Station, TX, USA) for data synthesis for RCTs. Previous experience of reviewing trials of obesity interventions has revealed considerable heterogeneity in the studies assessed. 39 For this reason, we used random-effects meta-analyses. In studies with data at multiple follow-up times, we reported data in meta-analyses with time periods aggregated to the nearest 6 months.
The large number of comparisons, with both adjusted and unadjusted data, and multiple time points, are summarised in tables for ease of interpretation.
When we were unable to combine data in meta-analysis, particularly for systematic review 2, in which most studies were not randomised, a narrative synthesis of data is provided.
We intended to undertake subgroup analyses for the effectiveness of interventions in accordance with whether all participants were selected on the basis of newly diagnosed or pre-existing obesity-related comorbidities (e.g. diabetes mellitus and hypertension). If sufficient data were available, we intended to explore the effect of BMI category (e.g. < 40 kg/m2 vs. ≥ 40 kg/m2), sex, deprivation, age and ethnicity on effectiveness. We planned to explore the effect of assumed values for weight outcomes on meta-analyses. These subgroup analyses did not prove possible in meta-analyses, owing to insufficient numbers of studies. However, we have provided narrative discussion of these subgroups throughout the text and exploration in metaregression.
We used visual inspection and the I2 statistic to assess heterogeneity in meta-analyses. 55 We undertook funnel plots to analyse reporting biases.
In an initial exploration of predictors of weight change, we used (bivariate) mixed-effects metaregression64 to examine which intervention, comparison group, study and sample characteristics helped to explain variation in intervention effect size. We started by exploring the effects of treatment arm characteristics and intervention characteristics (different diets, the presence of a physical activity programme to attend or the drug orlistat) on weight loss in lifestyle WMPs. Further detailed analyses incorporating moderator terms (e.g. number and duration of sessions, BCTs, group or individual delivery and in-person or remote delivery) in both the intervention group and the control group are planned, but were outside the timescale of this report.
Systematic review of qualitative and mixed-methods reports from randomised controlled trials, other intervention studies and other relevant data (systematic review 3)
A priori research questions
The broad initial research questions for this review included ‘What is it like to engage with (or be a provider of) weight-loss interventions for adults with BMI ≥ 35 kg/m2?’ and ‘What is it about interventions for adults with BMI ≥ 35 kg/m2 that make them helpful or unhelpful?’. As our analysis was conducted iteratively, our review also considered issues around what might motivate people to decide to engage in such programmes.
Searching for and identification of relevant studies
A systematic search was conducted for published papers that contained qualitative data from adults with BMIs of ≥ 35 kg/m2 (and/or the views of providers involved in their care) and considered issues relating to weight management. Studies conducted in developing countries were included if they were relevant to the UK context. Two researchers independently screened titles and abstracts and selected full-text papers. When consensus could not be reached regarding eligibility, a discussion took place at a research team meeting.
Analysis and synthesis
Synthesis of qualitative studies is an emerging methodology and there are many approaches that can be used. 65,66 Although aware of the differing philosophical stances underlying various approaches to qualitative synthesis, we chose to adopt a pragmatic approach to our work in this area, which specifically aims to synthesise data that are relevant to informing policy and practice. 59 Our pragmatic approach corresponded most closely to a ‘realist’ perspective66,67 as we were concerned with trying to find out not only ‘what works’ in terms of weight management for this group of adults and intervention providers, but also ‘for whom, and under what circumstances?’. At the same time, our approach was informed by and used aspects of review methods, such as meta-ethnography68 and thematic synthesis,69,70 and analytical approaches developed from methods of inquiry, such as grounded theory. 70
To collate and synthesise the available primary research, two authors (ZS and MAM) each read and systematically extracted data from the included papers, shared notes and discussed study findings and interpretations during a series of group meetings. The papers were initially organised in accordance with the categories described above but, as inductive analysis progressed, papers were grouped and compared and contrasted in accordance with emerging issues and themes. We used a standard data extraction form, which summarised the main themes, information regarding aims and methods and any other important information relating to the context of the research within each study in the qualitative systematic review.
Quality assessment strategy
The retrieved publications were appraised for methodological rigour and theoretical relevance by two reviewers using the criteria for quality in relation to meta-ethnography by Toye et al. ,71 who suggest including two core facets of quality in syntheses of qualitative evidence, namely (1) conceptual clarity (how clearly has the author articulated a concept that facilitates theoretical insight?) and (2) interpretive rigour (what is the context of the interpretation, how inductive are the findings and has the interpretation been challenged?). Two reviewers made notes regarding quality and the results were compared and discussed.
Systematic review of economic evaluations (systematic review 4)
The health economics component of the project had two parts. The first was a systematic review of all of the cost-effectiveness literature and the second was a decision analysis model to address some of the evidence gaps on cost-effectiveness from a UK perspective. The following sections outline the review’s methods. The modelling methods can be found in Chapter 7.
Inclusion and exclusion criteria
Studies that compared both costs and outcomes for interventions for the management of obesity in adults aged ≥ 18 years with a BMI of ≥ 35 kg/m2 were included. Studies were excluded if they did not attempt to relate cost to outcome data [e.g. with a cost-effectiveness or cost–utility analysis (CUA) framework]. Methodological studies, reviews of economic evaluations (although their reference lists were checked for additional papers to include), discursive analysis of costs/benefits, partial evaluation studies (such as cost analysis, efficacy or effectiveness evaluations) and cost-of-treatment/burden-of-illness papers were all excluded from formal review. In addition, studies comparing different types of surgery were excluded.
Data extraction strategy
One health economist (EJ) assessed all retrieved abstracts for inclusion. Full texts were retrieved and assessed against the inclusion and exclusion criteria. All full-text articles were assessed against the explicit inclusion and exclusion criteria. Economic evaluations were defined using the NHS EED guidelines for reviewers, which address and outline the key components for conducting economic evaluations. A second health economist (DB) checked the inclusion of studies at each stage. Decisions on the inclusion of studies were reached by consensus between the health economics reviewers. Any further disagreements, particularly regarding the definition of included interventions, were discussed at regular meetings of the review team to maintain consistency across reviews.
Data extraction and synthesis
Data were extracted into a bespoke online form by Elisabet Jacobsen and were checked by Dwayne Boyers. Important extraction items included study details (e.g. population details, setting, health system, interventions and comparators), general (e.g. perspective, time horizon, costing details, outcome measures and analysis framework) and obesity-specific (e.g. weight regain assumptions) methodological approaches and study results.
Extracted data on study results included intervention and obesity-related disease costs, outcomes in terms of weight loss, mortality, quality-adjusted life-years (QALYs), incremental outcomes and incremental cost per treatment effect per QALY gained. Data on results of any sensitivity analyses (deterministic and probabilistic) and subgroup analyses were recorded and reported narratively.
Quantitative synthesis of the data was not attempted because of significant heterogeneity in study interventions, comparators, health systems, methodological variability and quality. Instead, data from included studies were summarised narratively, by modality of intervention (WMP, drug therapy or surgery), to identify common results across broad intervention groups that may be of interest to policy-makers.
Quality assessment
Common strengths and weaknesses were identified through a quality assessment of included studies by Elisabet Jacobsen and Dwayne Boyers independently. This followed the British Medical Journal guidelines72 for reviewers of economic evaluations for studies conducted alongside RCTs and using the criteria for appraisal of decision analysis models by Philips et al. 73 The results were used to assess the quality of the current evidence base, but also to develop recommendations for future economic evaluation studies of weight-loss interventions more generally.
Integrating qualitative, quantitative and economic evaluation evidence synthesis (see Chapter 8)
All systematic reviews, metaregression analyses and economic evaluations provided evidence on features of effective interventions and their limitations for transfer into practice (e.g. what works, for whom and how can this be delivered?).
Drawing on a realist approach, we integrated (by combining and juxtaposing) the qualitative, quantitative and cost-effectiveness evidence to produce a narrative summary of what weight-management interventions work, with which adults and under what circumstances, and which effective interventions offer value for money for the NHS.
The integration of data was facilitated by weekly team meetings in Aberdeen involving systematic reviewers, health economists, a social scientist, a clinician, a health psychologist, statisticians and an information scientist. Monthly teleconferences were held between the study team in Aberdeen, a general practitioner (GP) (who was also a systematic reviewer and triallist) and the modelling team from the UK Health Forum (UKHF). Four meetings were held with the advisory group, consisting of lay representatives, a NHS dietitian leading weight-management services, a bariatric surgeon, a NHS consultant in metabolic medicine and representatives from Public Health England.
From a realist perspective, we conceptualised any intervention intended to improve health by considering:
-
the context that an intervention/programme will be situated within so that factors that might inhibit or enhance its effectiveness can be identified
-
mechanisms of the intervention/programme and how the intended programme beneficiaries will interact and react to the intervention processes and mechanisms
-
outcomes, both positive and negative, that may arise from an individual’s engagement with the proposed intervention.
A priori research questions
The primary aim of the evidence synthesis was to uncover how effective interventions work and to describe key intervention ingredients, processes and environmental and contextual factors that contribute to effectiveness. We also aimed to identify the barriers to and facilitators of engaging with WMPs experienced by people with a mean or median BMI of ≥ 35 kg/m2. Both deductive and inductive analytical approaches were employed throughout the review process and, for this reason, the following a priori research questions were developed to guide our initial investigation. Individual chapters covering these questions are given below, and the overall summary of findings is in Chapter 8:
-
What are the best evidence-based and cost-effective weight-management strategies for adults with obesity with a BMI of ≥ 35 kg/m2? (See Chapters 3–8.)
-
How can people with severe obesity best be engaged with weight management and weight-management services? (See Chapters 3–8.)
In addition to these a priori research questions, we developed a series of more-detailed research questions that emerged inductively from the initial findings of the review of RCTs and the expertise, knowledge and previous research of the project team and advisory group. Generating inductive research questions in this way is an inherent property of qualitative research, and particularly of a grounded theory approach in which data collection and analysis proceed iteratively to confirm or refute an emerging theory.
Organisational issues
-
What interventions are effective in increasing uptake of weight-loss services? (See Chapters 4 and 5.)
-
What factors seem to affect people’s choices of programme? (See Chapters 3–5.)
-
Does the effectiveness of programmes vary by socioeconomic status, ethnic group (including south-east Asian), BMI or other patient group? Is uptake related to these factors? (See Chapters 3, 4 and 6.)
-
Do programmes incorporate users in design, evaluation or delivery, and how do they do this? (See Chapters 3–5.)
-
What do people think about commercial weight-loss organisations? What is the influence of paying for their services? (See Chapter 5.)
-
What is the effectiveness of weight maintenance interventions? (See Chapter 3.)
-
Is an intensive/inpatient programme of value at the start of the WMP, particularly for people with very high BMI? (See Chapter 3.)
Diets
-
Which factors (type of diet, interventions for adherence, types of classes and characteristics of the participants) help explain weight loss and maintenance? (See Chapters 3–5.)
-
Does having a choice of reducing diet matter? Do people pick diets that are more effective for them? (See Chapters 4 and 5.)
Physical activity
-
Which factors (type of physical activity programme, interventions for adherence, types of classes and characteristics of the participants) help explain weight loss and maintenance? (See Chapters 3 and 4.)
-
Should physical activity classes and programmes designed specifically for people with a BMI of ≥ 35 kg/m2 be provided? (See Chapter 5.)
Intervention characteristics
-
Are certain psychological theories and BCTs more useful to people with severe obesity for losing weight and maintaining that weight loss? (See Chapters 3 and 5.)
-
Are group-based interventions more effective for weight loss than those delivered to individuals? (See Chapters 3, 5 and 6.)
-
What is the effect of financial/other incentives on participation, completion and weight loss? (See Chapters 3 and 4.)
-
Are programmes involving partners/families/friends more effective? (See Chapters 3 and 5.)
-
Do people say who they prefer to be the best person(s) to help with/deliver the weight-loss programme? Does this agree with what the interventions show? (See Chapter 5.)
-
How often should people be seen/contacted, and for how long? (See Chapters 3 and 5.)
-
What role does information technology have, particularly for monitoring and long-term follow-up? (See Chapters 3, 5 and 6.)
Drug therapy
-
Is current drug treatment(s), including long-term results post treatment, useful/cost-effective for people with severe obesity? (See Chapters 3–6.)
Surgery
-
Should more bariatric surgery be offered at the expense of lifestyle WMPs, if no extra funding is available? (See Chapters 3, 6 and 7.)
Chapter 3 Systematic review of randomised controlled trials
In this chapter, we present the results of the systematic review of RCTs. We cover RCTs of lifestyle WMPs, the drug orlistat and comparisons of bariatric surgery with WMPs/usual care or control interventions. The chapter starts with an overview of all trials, followed by assessments of effectiveness. We provide most detail for the interventions that are used to inform economic modelling in Chapter 7. In order of presentation, the interventions covered are VLCDs (including weight maintenance after their use), bariatric surgery, other interventions for weight maintenance after weight loss, other comparisons of diets, orlistat, variations in other components of WMPs and lifestyle WMPs versus control/usual care. Details of the characteristics of the included studies, assessments of risk of bias and equity, risk factor data and waist circumference and BMI data for all trials are provided in the appendices.
When possible, we present meta-analyses for mean weight change in two forms: unadjusted for dropouts and adjusted for dropouts (if this is required). Meta-analyses for cardiovascular risk factors are presented unadjusted. We provide summaries of findings for BCT coding, particularly for the interventions informing economic modelling.
Quantity of evidence
A total of 1174 reports retained from autoalerts were hand-searched, and primary literature searches identified 3052 potentially relevant titles and abstracts, of which 141 were selected for full-text screening. Of the full-text reports, 131 RCTs were identified as eligible for inclusion. 41,74–203 See Report Supplementary Material 1, Section 3, for a list of included studies and Report Supplementary Material 1, Section 4, for a list of excluded studies. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart is presented in Report Supplementary Material 4, Figure E1.
Characteristics of the studies
See Report Supplementary Material 1, Section 5, for full details of included studies. The majority (81/131, 61.8%) of included studies were set in North America (80 in the USA and 1 in the USA and Canada), 41 out of 131 (31.3%) were in Europe (two in multiple countries, one in the Czech Republic, two in Denmark, five in Finland, one in France, three in Germany, five in Italy, one in Norway, one in Scandinavia, two in Spain, nine in Sweden, one in the Netherlands and eight in the UK), 8 out of 131 (6.1%) were set in the Southern Hemisphere (six in Australia, one in New Zealand and one in Australia and New Zealand) and one study was set in Brazil.
Just under half (62/131, 47.3%) of the studies were published between 2011 and 2017, 11 of which were published between 2016 and 2017. Fifty studies were published between 2000 and 2010 and 19 studies were published between 1990 and 1999.
More than half of the trials (71/131, 54%) recruited participants either solely or partially through a health service provider. Recruitment methods were unclear or not reported in 18 trials (13%). Recruitment methods for the other trials were mainly advertisements in local newspapers or other media.
Characteristics of the participants
The majority of trials included participants with group mean ages in the 40–49 years and 50–59 years age categories. The youngest reported mean age of all the trials was 26.9 years, reported by Ebbeling et al. 115 Only two trials189,190 had a reported mean age of 70–79 years. The majority of trials recruited women. Women represented 5.4%129 to 96.1%155 of trials including both sexes. Twenty-four trials exclusively recruited women77,78,81,82,85,112,118,141,146,151,156,159,162,164,165,167,179,188,191–194,197,204 and three exclusively recruited men. 116,128,169
Eighty trials (61.0%) included participants with baseline comorbidities, including CVD, sleep apnoea, knee osteoarthritis, renal disease and asthma. Twenty-six trials exclusively recruited participants with type 2 diabetes mellitus and a further 28 trials included some participants with type 2 diabetes mellitus. Three trials exclusively recruited people with metabolic syndrome,84,119,196 two exclusively recruited people with knee osteoarthritis,93,99 one exclusively recruited women with breast cancer,112 two exclusively recruited people with asthma144,180 and one trial each exclusively recruited people with the following conditions: erectile dysfunction,116 stage 3/4 chronic kidney disease145 and urinary incontinence. 197
The lowest reported106,130,132,149,159,164 mean group BMI was 35.0 kg/m2 and the highest was 55.7 kg/m2. 169 Only five trials, all of surgery, targeted recruitment at people with a BMI of ≥ 35 kg/m2,111,145,153,154,169 and one of these169 targeted people with a BMI of ≥ 40 kg/m2.
Quality of the evidence
Risk of bias
Risk-of-bias items in RCTs were often poorly reported, so many risk-of-bias items were judged as unclear. Half of the trials were judged to have adequately performed and described how the randomisation sequence was generated and 66.9% described how allocation was concealed. Given the nature of the interventions, blinding of participants and personnel was not possible in the majority of trials, but 23.3% reported blinding outcome assessors. Just over half (51.5%) of the trials were judged to be at low risk of bias for selective outcome reporting and 40.8% were judged to be at low risk of incomplete outcome data or other biases. A high risk of other biases, such as conflicts of interest, was identified in 31.3% of trials (see Report Supplementary Material 4, Figure E2, and Report Supplementary Material 1, Section 6, for a full description of items and results for all RCTs).
Assessment of equity
Poor reporting also hindered assessment of equity. Just under half of trials (43.9%) were judged to have targeted or excluded specific populations, the majority (89.5%) of which targeted people of a specific sex, socioeconomic status, ethnic group or comorbidity. Almost half (48.5%) were judged to have included participants representative of people with severe obesity, based on comorbid conditions or broad inclusion criteria, and the majority (87.7%) reported participant baseline data for at least one PROGRESS (Place of residence, Race/ethnicity/culture/language, Occupation, Gender/sex, Religion, Education, Socioeconomic status, Social capital) category, usually sex. Few trials considered or reported on the remaining checklist items. Author conflict was considered possible in 33 RCTs, mainly owing to financial or employment links between authors and companies involved in the manufacture or delivery of intervention materials (e.g. manufacturers of weight-loss drugs or formula diets, or because the company supplied trial materials) (see Report Supplementary Material 4, Figure E3, and Report Supplementary Material 1, Section 7, for a full description of items and results for all RCTs).
Heterogeneity and small study bias
In general, statistical heterogeneity was high in meta-analyses (denoted by I2 > 50%). We did not find evidence of small study bias, as assessed by funnel plots for meta-analyses for weight change with ≥ 10 RCTs (data not shown).
Description of behaviour change techniques used
Besides dietary and physical activity recommendations, people in weight-loss trials usually receive behavioural support. This behavioural support was often very poorly reported. Detailed guidance for reporting is not yet available. Our protocol stipulated that we contact study authors for additional details (additional publications, protocols, trial materials, manuals, etc.). We found contact details for authors of 125 out of 131 trials (95.4%) for additional materials for BCT coding. The authors of 69 of these trials replied and additional materials were obtained for 41 trials. BCTs could target weight loss, weight maintenance and engagement in the WMP. For each intervention and control/usual care group, we coded the first instance that a BCT targeting one of these three aspects was identified. On average, intervention groups received 11.3 (SD 8.1) BCTs targeting weight loss, 7.6 (SD 7.4) BCTs targeting weight maintenance and 0.5 (SD 1.5) BCTs targeting participant engagement in the WMP. Control/usual care groups also received BCTs: 7.3 (SD 6.7) BCTs targeting weight loss, 5.2 (SD 6.1) BCTs targeting weight maintenance and 0.3 (SD 0.6) BCTs targeting participant engagement.
As expected, intervention groups received more BCTs for weight loss than control/usual care groups (this could also be attributable to better reporting), although the BCTs used most frequently in the intervention arms and control/usual care arms were almost identical. In both the treatment and the control/usual care arms, the four most common BCTs were receiving information from a credible source in favour of weight loss, setting a goal, receiving advice on how to lose weight and self-monitoring success in losing weight and/or following a diet and physical activity regimen. These four BCTs were applied in 84% of the intervention groups and 72% of the control/usual care groups. Additional common BCTs targeting weight-loss maintenance were similar, and also included problem-solving and general social support techniques.
Regarding attempts to engage people in WMPs, very few BCTs were identified. The typical BCT used the provision of material incentives and rewards, and a few trials reported discussion of the pros and cons of following the WMP or behavioural contracting. The differences in type of BCT used for promoting weight loss/maintenance (e.g. education, planning and self-management) versus engagement (i.e. external rewards and contracts for engagement) suggest that researchers’ perceptions of what drives these behaviours is very different.
Very low-calorie diets
Overview
We defined VLCDs as meal replacement products providing ≤ 800 kcal/day (± 10%). Nineteen RCTs76,82,93,99,107,139,150,155,161,166,171–173,180,184,186,193,198,200 included a VLCD as part of the intervention, usually compared with another intervention. Only one trial180 compared a VLCD intervention with usual care/control. If details of the content of the VLCD were unclear, we contacted manufacturers, two of which provided the nutritional information requested. Details of the VLCD product information are presented in Table 1.
| Study (first author and year) | VLCD commercial brand | Kilocalories provided | Nutritional breakdown | VLCD duration |
|---|---|---|---|---|
| Agras 199682 | Optifast 800 (Sandoz Nutrition, Minneapolis, MN, USA) | 800 kcal/day | No information available | 12 weeks |
| Bliddal 201193 | Speasy (Dansk Droge A/S, Ishøj, Denmark) | 810 kcal/day | Per cent energy:
|
12 weeks (first 8 weeks then for another 4 weeks during weeks 32–36) |
| Christensen 201399 | Speasy (Dansk Droge A/S, Ishøj, Denmark) | 810 kcal/day | Per cent energy:
|
12 weeks (first 8 weeks then for another 4 weeks during weeks 32–36) |
| Delbridge 2009107 | Optifast (Nestlé Nutrition, Frankfurt, Germany) | 500–550 kcal/day | No information available | 12 weeks |
| Lantz 2003139 | Modifast (Novartis Nutrition, Bern, Switzerland) | 450 kcal/day | No information available | 16 weeks |
| Melin 2003150 | No information reported |
200–800 kcal/day Instructed to decrease energy intake during 3 days from 800 to 200 kcal/day, and maintain this intake for 19 days. Energy intake then increased to 800 kcal/day during last 3 days of VLCD period |
No information reported | 25 days |
| Moreno 2014155 | PronoKal® Method (Protein Supplies S.L., Barcelona, Spain) | 600–800 kcal/day (each preparation provided 90–100 kcal). Protein 0.8–1.2 g/kg of ideal body weight | Each preparation contained:
|
VLCD ‘active phase’ continued until participants reached 80% of their weight-loss target |
| Pekkarinen 2015161 | Nutrilett (Nycomed Pharma, Oslo, Norway), Nutrifast, or Dietta Mini | 525–560 kcal/day |
52–58 g of protein 52–64 g of carbohydrate 8–13 g of fat |
9 weeks |
| Purcell 2014166 | Optifast (Nestlé Nutrition, Vevey, Switzerland) | 450–800 kcal/day | No information available | 12 weeks |
| Richelsen 200776 | Modifast (Novartis, Basel, Switzerland) or Nutrilett (Nycomed Pharma, Oslo, Norway) | 600–800 kcal/day | No information available | 8 weeks |
| Rössner 1997171 | Nutrilett (Nycomed Pharma, Oslo, Norway) |
Nutrilett: 420 kcal/day Other VLCD: 530 kcal/day |
No information available | 6 weeks |
| Ryttig 1997172 | Nutrilett (Nycomed Pharma, Oslo, Norway) | 420 kcal/day | Each sachet:
|
8 weeks |
| Ryttig 1995173 | Cambridge Weight Plan® (Corby, UK) | 330 kcal/day | Each sachet:
|
12 weeks |
| Stenius-Aarniala 2000180 | Nutrilett (Nycomed Pharma, Oslo, Norway) | 420 kcal/day | Each sachet:
|
8 weeks |
| Torgerson 1999184 | Modifast (Novartis Nutrition, Bern, Switerland) | 456–608 kcal/day | Three sachets every day. No further information available | 16 weeks |
| Torgerson 1997186 | Modifast (Novartis Nutrition, Bern, Switzerland) | 456–608 kcal/day | No information available | 12 weeks |
| Wadden 1994193 | Optifast 70 (Sandoz Nutrition, Minneapolis, MN, USA) | 420 kcal/day |
70 g of protein 30 g of carbohydrate 2 g of fat |
15 weeks |
| Wing 1994198 | Optifast 70 (Sandoz Nutrition, Minneapolis, MN, USA) | 400–500 kcal/day | No information available | 24 weeks (weeks 1–12 and weeks 24–36) |
| Wing 1991200 | Optifast 70 (Sandoz Nutrition, Minneapolis, MN, USA) | 420 kcal/day | No information available | 8 weeks |
Quality assessment of all very low-calorie diet randomised controlled trials
Risk of bias
It was unclear whether randomisation and allocation concealment were adequate for the majority of the 19 studies, or whether health-care providers and outcome assessors were blinded to treatment allocation. Only one trial was judged to be at a low risk of bias owing to blinding of participants receiving orlistat or placebo for weight maintenance following a VLCD weight-loss period. Only three trials were judged to be at low risk of bias owing to blinding of outcome assessors. Just over half of the trials were considered to be at either a high or an unclear risk of bias for incomplete outcome data or selective reporting. Nine studies were judged to be at high risk of bias, mainly owing to financial associations with the manufacturers of the VLCD products used in the trials (see Report Supplementary Material 4, Figure E4).
Equity
Four out of the nineteen trials targeted a specific population: two150,200 targeted people with diabetes mellitus or other obesity-related comorbidities and two161,172 targeted people receiving outpatient care. Just over half of the trials were considered representative of people with severe obesity. Those that were considered unrepresentative mainly excluded potential participants with obesity-related comorbid conditions. Most trials (79%) reported one or more PROGRESS characteristic. None of the studies clearly reported strategies to address diversity or disadvantage. Three studies107,161,186 reported whether or not there were sociodemographic differences between trial completers and those who withdrew/were excluded. Only one trial161 reported conducting a fidelity check and only two82,200 reported collecting process measure data. Sustainability, partnerships and political or organisational contexts of the interventions were generally poorly reported. Only eight trials76,93,155,166,171–173,184 reported harms or unintended effects, and only one trial161 was considered to have no potential for author conflict (see Report Supplementary Material 4, Figure E5).
Very low-calorie diets and dietary interventions versus dietary interventions
Risk of bias
Seven RCTs93,155,172,186,193,198,200 examined adding a VLCD to a dietary intervention. The method of randomisation was unclear for all trials, and adequacy of allocation concealment was unclear for all but one study. 186 Four93,155,172,186 out of the seven RCTs were considered to be at high risk of bias owing to financial associations with the manufacturers of the products used (see Report Supplementary Material 4, Figure E6).
Equity
The trials mainly recruited women, who represented 55.8%172 to 100%193 of participants. The trials did not report on ethnicity or socioeconomic status. Torgerson et al. 186 reported the educational attainment of participants, with the majority having attained intermediate or higher education. Just over half of the trials93,155,172,186 were conducted in a hospital outpatient setting, with the remainder conducted in university settings. 198,200 The weighted mean age of all participants in the trials was 49.9 years. The youngest reported mean age of the trial groups was 36.8 years (SD 8.9 years) and the oldest was 64.1 years (SD 10.5 years). Participants had a weighted mean weight of 105.9 kg and a weighted mean BMI of 37.9 kg/m2. The lowest and highest mean weights for the groups were 92.1 kg and 116.6 kg, respectively, and the lowest and highest mean BMIs were 35.1 kg/m2 and 40.5 kg/m2, respectively (see Report Supplementary Material 4, Figure E7).
Descriptions of the trials
Bliddal et al. 93 evaluated a VLCD for improving the symptoms of primary knee osteoarthritis. Participants were randomised to receive either dietary instruction with fewer contacts or a VLCD for 12 weeks over 1 year with frequent consultations with a dietitian. Participants randomised to the VLCD received a formula diet [Speasy (Dansk Droge, Ishoj, Denmark)] dissolved in water and taken as six daily meals, providing 810 kcal/day for 8 weeks. Participants then received group dietary guidance with the aim of achieving a 1200 kcal/day intake for 24 weeks. Following this, a period of weight maintenance was reinforced by the same VLCD for 4 weeks, followed by group instruction for 16 weeks. Participants in the control group received nutritional advice from a dietitian at baseline and at weeks 8, 32, 36 and 52. Participants received a total of 66 hours of instruction in the intervention group and 10 hours of instruction in the control group. The primary outcome was the mean group difference in total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC®) score, a validated, disease-specific questionnaire addressing the severity of joint pain, stiffness and limitation of physical function using a visual analogue scale. The mean BMI was 35.6 kg/m2 (SD 5.0 kg/m2). The most frequent adverse events reported by the intervention group were constipation (five participants, 11%), increased flatulence (four participants, 9%), dizziness (two participants, 5%) and heightened sensitivity to cold (two participants, 5%). Both groups showed improvement in reported WOMAC scores but there was no reported statistically significant difference in total WOMAC scores between groups (reported p = 0.11), although the VLCD intervention resulted in less WOMAC pain, with a reported group mean difference of 7.2 mm (reported 95% Cl 1.0 to 13.4 mm; p = 0.022). By trial completion, two of the participants in the control group were diagnosed with type 2 diabetes mellitus and one had died from a heart condition. The authors93 concluded that weight loss is effective at reducing symptoms of pain in knee osteoarthritis but there was no indication of clinical improvement in joint stiffness.
Moreno et al. 155 compared a very low-calorie ketogenic diet, usually followed for 30–45 days followed by a low-calorie diet, with a low-calorie diet alone. The mean BMI of participants was 35.4 kg/m2 (SD 4.9 kg/m2). Both groups received behavioural support and counselling. The control low-calorie diet was calculated to have a caloric value of 10% below the total metabolic expenditure of each individual (range 1400–1800 kcal/day). The very low-calorie ketogenic diet followed a commercial weight-loss programme (PronoKal® Method). The VLCD programme had three stages: active, re-education and maintenance. The active stage provided 600–800 kcal/day. In phase 1, participants consumed protein preparations five times a day as well as vegetables with a low glycaemic index. In phase 2, one of the protein servings was replaced with natural protein (e.g. meat and fish). In phase 3, a second serving of protein that was low in fat replaced the second biological protein preparation. The active stage was maintained until the participant achieved most (ideally 80%) of their weight-loss target. The VLCD induced more frequent asthenia, fatigue, headache, muscle weakness, constipation, hyperuricamia and nausea than the low-calorie diet. The authors reported that these effects were mild and transient.
Ryttig et al. 172 compared a low-fat diet of 1600 kcal/day for 26 months with a VLCD (Nutrilett®, Nycomed Pharma, Oslo, Norway) of 420 kcal/day for an initial 2 months. The VLCD participants were then randomised to the 1600-kcal/day diet with or without 1 mJ (240 kcal) of VLCD formula taken as three sachets daily for 26 months of weight maintenance. Participants received help with behaviour modification, including strategies to prevent relapse, dietitian-led training in low-energy cooking, a group or individual physiotherapist-led exercise programme and other activities, such as swimming and shopping exercises. The initial BMIs for the groups ranged from 37.6 kg/m2 (SD 5.7 kg/m2) to 37.7 kg/m2 (SD 3.9 kg/m2).
Torgerson et al. 186 randomised participants to receive a VLCD for 12 initial weeks plus regular dietary and behavioural support over 2 years, or 2 years of the same supportive programme only. The initial mean BMI was 40.2 kg/m2 (SD 3.3 kg/m2) for the VLCD group, and 40.5 kg/m2 (SD 4.3 kg/m2) for the non-VLCD group. Participants in the non-VLCD group were advised to consume an individualised diet aiming for 1200–1400 kcal/day for women or 1400–1800 kcal/day for men, with 25–30% of energy from fat. Participants in the VLCD group were provided with Modifast (Novartis Nutrition, Bern, Switzerland) and were recommended to consume 456–608 kcal/day (a higher level was recommended to men with high energy expenditure). Following 12 weeks of the VLCD, the hypocaloric diet was gradually introduced. Participants received 6-monthly individual nutritional counselling sessions with a dietitian and a physical examination with a physician, plus minor visits with a dietitian/nurse for support and further nutrition education weekly for weeks 1–2, fortnightly for weeks 2–8 and monthly thereafter. VLCD participants received three additional visits to facilitate refeeding following the 12-week VLCD. Participants were taught behavioural control techniques and attempts were made to identify risk circumstances for overeating and strategies to avoid these situations. Group swimming and physical training sessions were offered and all participants were encouraged to take part in physical activity.
Wadden et al. 193 compared a 1200-kcal/day diet (≤ 30% of energy as fat) and behavioural therapy with and without a VLCD in women. The mean BMI of groups ranged from 38.8 kg/m2 (SD 5.4 kg/m2) to 40 kg/m2 (SD 5.7 kg/m2). Following this, participants adjusted caloric intake during a 26-week maintenance programme, depending on their desired weight change (the minimum intake was 1200 kcal/day). Group sessions provided instruction on recording eating behaviour, stimulus control, modifying self-defeating thoughts and emotions and eliciting social support. An incremental walking-based exercise programme began at week 8, leading to 3–5 times/week at 60–70% of the maximum heart rate by 12 months. Biweekly ‘upkeep’ sessions were provided during the 26-week weight maintenance period to prevent and reverse weight gains and dietary lapses. Women in the VLCD group received a 1200-kcal/day diet for the first week and then a liquid VLCD formula (Optifast 70, Sandoz Nutrition, Minneapolis, MN, USA) during weeks 2–17. Conventional foods were gradually reintroduced during weeks 8–23, so that by week 23 participants were consuming a 1000-kcal/day diet of conventional food. Refeeding was supervised by the dietitian who co-led groups from weeks 18–27 and provided further information on food preparation and nutrition (also provided for the comparison group). VLCD participants received the same exercise and cognitive–behavioural treatment programme as the comparison group throughout the study, but materials were presented in a different order in the first 26 weeks.
Wing et al. 200 investigated a VLCD in people with type 2 diabetes mellitus. Participants were randomised to a low-fat diet (calorie goal of 1000–1500 kcal/day) and behaviour therapy for 20 weeks, or to the same behavioural therapy for 20 weeks with a VLCD of 400 kcal/day of lean meat, fish or fowl, and the option of using Optifast 70 for occasional meals for 8 weeks. The mean BMI of the participant groups ranged from 37.3 kg/m2 (SD 4.7 kg/m2) to 38.1 kg/m2 (SD 5.7 kg/m2). After 8 weeks on the VLCD, other foods were gradually reintroduced to reach 1000–1500 kcal/day by week 17. Behaviour modification strategies included self-monitoring behaviour, stimulus control, removing food cues from the environment, separating eating from other activities and modifying cognitions for relapse prevention and self-reinforcement. Participants were taught to increase their walking and were given weekly exercise goals. All participants deposited US$150 at the start of the programme, which was earned back weekly for meeting homework goals.
Wing et al. 198 evaluated an initial VLCD in people with type 2 diabetes mellitus compared with a low-fat 1000- to 1200-kcal/day diet, both with behaviour change support. Participants in the VLCD group were prescribed a diet of 400–500 kcal/day for weeks 1–12 and 24–36. The mean BMI of the participant groups ranged from 37.4 kg/m2 (SD 6.1 kg/m2) to 38.3 kg/m2 (SD 6.52 kg/m2). During VLCD periods, participants were instructed to consume no more than 500 kcal/day, either as liquid formula (Optifast 70) or lean meat, fish and fowl, with most participants choosing combinations of the food and liquid formula. After 12 weeks on the VLCD, other foods were gradually reintroduced, and the prescribed calories gradually increased over 4 weeks until participants were consuming the 1000–1200 kcal/day. Participants were restarted on the VLCD at week 24 unless their ideal body weight had been reached. Behavioural therapy focused on self-monitoring, goal-setting (including exercise goals), stimulus control, preplanning, relapse prevention and modifying cognitions during weekly group meetings conducted by a multidisciplinary team of therapists. All participants deposited US$150 at the start of the programme, which was refunded for reaching behavioural goals and attending assessment sessions. Diabetes mellitus medications were stopped at baseline and a survival analysis was used to compare the number of weeks that participants maintained blood sugar of < 13.3mmol/l without restarting medication. Fasting glucose remained below the cut-off level for a significantly longer time in the VLCD than in comparison group (reported p = 0.05) and the number of participants remaining on the diet without medication was significantly greater in the VLCD group (55%) than in the comparison group (31%) at 2 years (reported p = 0.01).
A summary of the delivery format of the interventions for these trials is provided in Report Supplementary Material 5, Table E2.
Meta-analyses
The results of the meta-analysis of the mean difference in weight change for the VLCD and dietary intervention versus dietary intervention are presented in Table 2. There was a difference in weight change between the VLCD intervention and control treatments at 12 months in favour of the VLCD for both adjusted and unadjusted data; however, at subsequent time points there is no evidence that VLCD treatments are effective and CIs rule out worthwhile weight loss.
| Type of analysis | Mean weight change (kg) | |||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | 48 months | |
| Adjusted |
Mean –4.41 (95% CI –5.93 to –2.88) p < 0.001 I2 = 81 Trials = 5(Favours VLCD) |
Mean –0.29 (95% CI –4.11 to 3.52) p = 0.880 I2 = 0 Trials = 2(Favours VLCD) |
Mean –0.56 (95% CI –2.33 to 1.20) p = 0.530 I2 = 0 Trials = 4(Favours VLCD) |
Mean –0.82 (95% CI –3.80 to 2.15) p = 0.588 I2 = N/A Trials = 1(Favours VLCD) |
| Unadjusted |
Mean –5.18 (95% CI –6.77 to –3.59) p < 0.001 I2 = 70 Trials = 5(Favours VLCD) |
Mean –0.16 (95% CI –3.91 to 3.59) p = 0.934 I2 = 0 Trials = 2(Favours VLCD) |
Mean –1.21 (95% CI –3.13 to 0.71) p = 0.215 I2 = 0 Trials = 3(Favours VLCD) |
Mean –1.30 (95% CI –5.16 to 2.56) p = 0.509 I2 = N/A Trials = 1(Favours VLCD) |
The weight change in the VLCD arms alone at 12 months was –9.69 kg (95% CI –12.55 to –6.82 kg).
Overall, the direction of the effects of the intervention on risk factors tended to favour VLCD groups. However, there were only reductions in diastolic blood pressure (–5.00 mmHg, 95% CI –8.66 to –1.34 mmHg; p = 0.007) at 12 months, and reductions in HbA1c (–2.60%, 95% CI –4.44% to –0.76%; p = 0.006) and glucose (–4.50 mmol/l, 95% CI –6.88 to –2.12 mmol/l; p = 0.000) at 18 months (the HbA1c and glucose results mainly reflecting participants with type 2 diabetes mellitus). No changes were found at 24 months’ follow-up. Table 3 provides further details.
| Risk factor | Mean risk factor change | ||
|---|---|---|---|
| 12 months | 18 months | 24 months | |
| Total cholesterol (mmol/l) |
Mean 0.11 (95% CI –0.23 to 0.44) p = 0.534 I2 = 36 Trials = 3(Favours dietary intervention alone) |
Mean –0.02 (95% CI –0.76 to 0.72) p = 0.958 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Mean –0.30 (95% CI –1.13 to 0.53) p = 0.478 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
| LDL cholesterol (mmol/l) |
Mean 0.04 (95% CI –0.22 to 0.29) p = 0.786 I2 = 78 Trials = 2(Favours dietary intervention alone) |
||
| HDL cholesterol (mmol/l) |
Mean 0.09 (95% CI –0.01 to 0.19) p = 0.088 I2 = 0 Trials = 2(Favours VLCD and dietary intervention) |
Mean 0.09 (95% CI –0.11 to 0.29) p = 0.373 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
|
| Triglycerides (mmol/l) |
Mean –0.08 (95% CI –0.38 to 0.22) p = 0.589 I2 = 42 Trials = 3(Favours VLCD and dietary intervention) |
Mean 0.16 (95% CI –0.50 to 0.82) p = 0.632 I2 = N/A Trials = 1(Favours dietary intervention alone) |
Mean –0.25 (95% CI –0.99 to 0.49) p = 0.506 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
| Systolic blood pressure (mmHg) |
Mean –3.00 (95% CI –8.61 to 2.61) p = 0.294 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Mean –8.63 (95% CI –18.38 to 1.12) p = 0.083 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
|
| Diastolic blood pressure (mmHg) |
Mean –5.00 (95% CI –8.66 to –1.34) p = 0.007 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Mean –3.79 (95% CI –10.16 to 2.58) p = 0.244 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
|
| HbA1c (%) |
Mean –0.27 (95% CI –1.19 to 0.65) p = 0.563 I2 = 0 Trials = 2(Favours VLCD and dietary intervention) |
Mean –2.60 (95% CI –4.44 to –0.76) p = 0.006 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Mean –0.17 (95% CI –1.23 to 0.89) p = 0.753 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
| Glucose (mmol/l) |
Mean –0.51 (95% CI –1.70 to 0.68) p = 0.402 I2 = 0 Trials = 2(Favours VLCD and dietary intervention) |
Mean –4.50 (95% CI –6.88 to –2.12) p < 0.001 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Mean –1.61 (95% CI –3.73 to 0.51) p = 0.136 I2 = N/A Trials = 1(Favours VLCD and dietary intervention) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Suboptimal reporting of the behavioural support made coding BCTs difficult. Generally, the same BCTs were provided to both arms in trials (particularly goal-setting), but descriptions were provided for few BCTs. VLCD and dietary intervention arms had a mean of 8.7 BCTs (range 4–13 BCTs) and dietary intervention only arms had a mean of 7.5 BCTs (range 1–12 BCTs).
Very low-calorie diet versus control
Descriptions of the trials
Stenius-Aarniala et al. 180 randomised people with asthma to either an initial VLCD treatment or control. The initial mean BMIs for groups ranged from 35.8 kg/m2 (range 31.3–39.4 kg/m2) to 36.7 kg/m2 (range 32.8–41.8 kg/m2). The VLCD (Nutrilett, Nycomed Pharma, Oslo, Norway) was taken for 8 weeks (420 kcal/day). The VLCD programme also included 12 group sessions on behaviour change over 14 weeks. The control group had sessions at the same intervals as the active group on education about asthma and allergy. After 1 year, the mean weight change was –13.40 kg (95% CI –17.21 to –9.59 kg; p < 0.001). The per cent of predicted forced expiratory volume in 1 second increased more in the active group than in the control group after the treatment period and at 1 year (mean difference 7.6%, 95% CI 1.5% to 13.8%; reported p = 0.02). Similarly, the per cent of predicted forced vital capacity was different between groups at 1 year (mean difference 7.6%, 95% CI 3.5% to 11.8%; reported p = 0.001). There was no evidence of differences in peak expiratory flow between the groups. After 1 year, the mean differences in the changes in the St George’s Respiratory Questionnaire between the two groups were –12 for symptom scores (range –1 to –22; reported p = 0.04) and –10 for total scores (–18 to –1; reported p = 0.02). During the 1-year follow-up period, the median number of exacerbations was 1 (range 0–4) in the treatment group and 4 (range 0–7) in the control group (reported p = 0.001). Thirteen control participants and 10 treatment group participants needed a course of oral steroids (reported p = 0.07) (Table 4).
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) at 12 months | |||
|---|---|---|---|---|---|
| VLCD | Control | Effect size (95% CI); p-value | |||
| Stenius-Aarniala 2000180 (trial of VLCD vs. control; all participants completed the intervention) | 12 | –11.10 (5.35) [n = 19] | 2.30 (6.57) [n = 19] |
–13.40 (–17.21 to –9.59); p < 0.001 (Favours VLCD) |
|
| Intensively treated | Less intensively treated | Effect size (95% CI); p-value | |||
| Melin 2003150 (trial of VLCD, intensively treated vs. less intensively treated) | 12 (adjusted analysis) | –5.86 (4.80) [n = 22] | –4.57 (4.78) [n = 21] |
–1.29 (–4.15 to 1.58); p = 0.379 (Favours intensively treated) |
|
| 12 (unadjusted analysis) | –7.58 (4.04) [n = 17] | –6.40 (4.49) [n = 15] |
–1.18 (–4.16 to 1.80); p = 0.437 (Favours intensively treated |
||
| 24 (adjusted analysis) | –5.25 (5.82) [n = 22] | –6.14 (6.53) [n = 21] |
0.89 (–2.82 to 4.59); p = 0.638 (Favours less intensively treated) |
||
| 24 (unadjusted analysis) | –6.80 (5.77) [n = 17] | –8.60 (6.19) [n = 15] |
1.80 (–2.36 to 5.96); p = 0.397 (Favours less intensively treated) |
||
| Rapid weight loss | Gradual weight loss | Effect size (95% CI); p-value | |||
| Purcell 2014166 (trial of rapid vs. gradual weight loss) | 36 (adjusted analysis) | –2.58 (6.16) [n = 97] | –1.80 (4.79) [n = 103] |
–0.78 (–2.32 to 0.75); p = 0.317 (Favours gradual weight loss) |
|
| 36 (unadjusted analysis) | –4.10 (7.37) [n = 61] | –4.30 (6.69) [n = 43] |
0.20 (–2.52 to 2.92); p = 0.886 (Favours rapid weight loss) |
||
| 420 kcal/day | 530 kcal/day | 880 kcal/day | Test of differences | ||
| Rössner 1997171 (trial of VLCDs) | |||||
| Adjusted | 13 | –14.80 (12.30) [n = 21] | –15.40 (9.90) [n = 19] | –12.10 (10.00) [n = 17] | p = 0.631 |
| Unadjusted | 13 | –10.36 (12.33) [n = 30] | –9.14 (10.77) [n = 32] | –6.64 (9.53) [n = 31] | p = 0.399 |
| VLCD-plus | VLCD-strict | VLCD-mw | Test of differences: one-way analysis of variance | ||
| Torgerson 1999184 (trial of different approaches to using a VLCD) | |||||
| Adjusted | 12 | –5.99 (7.61) [n = 41] | –5.84 (7.57) [n = 41] | –6.31 (7.81) [n = 39] | p = 0.962 |
| Unadjusted | 12 | –9.10 (8.49) [n = 27] | –12.60 (9.48) [n = 19] | –10.70 (7.50) [n = 23] | p = 0.390 |
Soenen et al. 177 compared four energy-restricted diets in a factorial design: high versus low protein and low versus normal carbohydrate. Initial energy intake was restricted to 33% of energy requirements during the weight-loss phase and, therefore, this could be classed as a VLCD. For further details, see Comparisons of diets.
Other trials of very low-calorie diets
Descriptions of the trials
Melin et al. 150 examined a VLCD with varying intensities of behavioural therapy. Participants had a mean group BMI of 35.2 kg/m2 (SD 4.6 kg/m2) to 35.6 kg/m2 (SD 4.5 kg/m2). The intensive treatment group received group meetings every fortnight for the first year and six meetings during the second year. Participants in the control group had group meetings every third month and less contact with supervisors, fewer repetitions with self-monitoring and less opportunity for nutrition counselling. Both groups underwent two VLCD periods, lasting 25 days each, in between following a diet with a 600-kcal/day deficit. Although favouring more meetings, there were no significant differences in weight change between the groups (see Table 4).
Purcell et al. 166 recruited volunteers without comorbidities, with a mean BMI of 35.3 kg/m2 (SD 3.8 kg/m2), and investigated the effect of rapid weight loss with a VLCD and gradual weight loss without a VLCD. In the rapid weight-loss programme, participants consumed OPTIFAST® (Nestlé Nutrition, Vevey, Switzerland) meal replacements of between 450–800 kcal/day, producing approximately 15% weight loss over 12 weeks. In the gradual weight-loss programme, participants followed a 400–500-kcal/day deficit low-fat diet, including one to two Optifast meal replacements daily, with the aim of 15% weight loss over 36 weeks. After 36 months, there was no evidence that rapid weight loss led to more weight being regained. Attrition in the weight-loss phase was significantly less with the VLCD (reported p = 0.002), and did not differ between groups in the weight maintenance phase. Two participants in the VLCD group developed cancer (see Table 4).
Rössner and Flaten171 compared three VLCDs of 420 (Nutrilett), 530 and 880 kcal/day for 6 weeks and a 2-week booster at 26 weeks. Between weeks 6 and 26, participants followed a diet of 1600 kcal/day and received sessions with a research nurse or dietitian. The initial group mean BMIs ranged from 38.4 kg/m2 (SD 4.3 kg/m2) to 39 kg/m2 (SD 5.2 kg/m2). At 1 year, weight loss was similar in the three groups (see Table 4).
Torgerson et al. 184 compared three approaches to VLCD use over 16 weeks in a 1-year programme in groups with mean BMIs of 37.7 kg/m2 (SD 4.3 kg/m2) to 38.5 kg/m2 (SD 4.5 kg/m2). All participants consumed three sachets of Modifast, providing 456 kcal/day. The VLCD-strict group was prescribed a strict outpatient VLCD for 16 weeks, followed by a 36-week hypocaloric diet. The metabolic ward (VLCD-mw) participants received the same treatment, but were hospitalised in a metabolic ward for the first week. The VLCD-plus participants were allowed two small meals weekly, but otherwise received the same recommendations as the VLCD-strict group. At 12 months, there were no significant differences in weight loss between the groups (see Table 4).
Weight maintenance interventions after very low-calorie diets
Eight trials investigated interventions to promote weight maintenance after a VLCD. 76,82,99,107,139,161,172,173 One of these trials, Ryttig et al. ,172 is described in Very low-calorie diets and dietary interventions versus dietary interventions. Details of the trials are provided in Report Supplementary Material 5, Table E3.
Descriptions of the trials
Details of the mean weight change in the trials are presented in Table 5. In the Ryttig et al. 172 trial, the VLCD followed by meal replacement group lost significantly more weight than the VLCD plus diet group at 12 months (p = 0.037). However, another trial from this group173 did not find as marked an effect from meal replacements (see Randomised controlled trials of meal replacements for weight loss and weight maintenance). In the trial by Christensen et al. ,99 a dietary programme was more successful at preventing weight regain than exercise or usual care. In the trial by Richelsen et al. ,76 new cases of type 2 diabetes mellitus were reported to have been significantly reduced with orlistat (8 cases out of 153 participants) versus placebo (17 cases out of 156 participants) (reported p = 0.041). Varying protein content107 or regular intermittent VLCD use compared with VLCD use on demand139 did not appear to affect weight regain. In one trial, Pekkarinen et al. 161 found little added benefit from a specific weight maintenance programme versus no follow-up.
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) | ||||
|---|---|---|---|---|---|---|
| Time-dependent regular food | Weight-dependent regular food | Time-dependent stimulus narrowing | Weight-dependent stimulus narrowing | Test of differences | ||
| Agras 199682 (trial of time- and weight-dependent reintroduction of food for weight maintenance; unadjusted data only) | 18 | –8.20 (12.30) [n = 45] | –8.60 (11.40) [n = 41] | –6.00 (11.10) [n = 34] | –2.80 (18.30) [n = 42] | p = 0.195 |
| Usual care | Exercise | Diet | Test of differences | |||
| Christensen 201399 (trial of usual care vs. exercise vs. diet for weight maintenance) | 12 | 5.00 (7.33) [n = 64] | 6.70 (7.81) [n = 64] | 1.00 (6.20) [n = 64] |
p < 0.001 (Favours diet) |
|
| High-carbohydrate diet | High-protein diet | Effect size (95% CI) | ||||
| Delbridge 2009107 (trial of higher-protein vs. lower-protein diet for weight maintenance) | 12 (adjusted analysis) | 4.30 (8.85) [n = 40] | 3.00 (7.13) [n = 42] |
1.30 (–2.19 to 4.79); p = 0.465 (Favours high protein) |
||
| 12 (unadjusted analysis) | 2.46 (6.99) [n = 70] | 1.77 (5.66) [n = 71] |
0.68 (–1.42 to 2.78); p = 0.524 (Favours high protein) |
|||
| Intermittent VLCD | On-demand VLCD | Effect size (95% CI) | ||||
| Lantz 2003139 (trial of intermittent vs. on-demand VLCD for weight maintenance) | 24 (adjusted analysis) | –2.48 (7.32) [n = 161] | –3.16 (7.15) [n = 173] |
0.68 (–0.88 to 2.23); p = 0.393 (Favours on-demand VLCD) |
||
| 24 (unadjusted analysis) | –7.00 (11.00) [n = 57] | –9.10 (9.70) [n = 60] |
2.10 (–1.67 to 5.87); p = 0.274 (Favours on-demand VLCD) |
|||
| Intervention without follow-up | 1-year maintenance programme | Effect size (95% CI) | ||||
| Pekkarinen 2015161 (trial of no follow-up vs. a weight maintenance programme) | 16 (adjusted analysis) | 8.80 (8.41) [n = 99] | 7.43 (8.02) [n = 100] |
1.38 (–0.91 to 3.66); p = 0.238 (Favours weight maintenance) |
||
| 16 (unadjusted analysis) | 8.80 (8.41) [n = 99] | 7.54 (8.04) [n = 99] |
1.30 (–0.99 to 3.59); p = 0.266 (Favours weight maintenance) |
|||
| 30 (adjusted analysis) | 11.60 (9.20) [n = 99] | 10.39 (8.85) [n = 100] |
1.21 (–1.30 to 3.72); p = 0.344 (Favours weight maintenance) |
|||
| 30 (unadjusted analysis) | 11.60 (9.20) [n = 99] | 10.60 (8.91) [n = 98] |
1.00 (–1.53 to 3.53); p = 0.438 (Favours weight maintenance) |
|||
| Intervention | Control | Effect size (95% CI) | ||||
| Richelsen 200776 (trial of orlistat and lifestyle counselling vs. placebo and lifestyle counselling for weight maintenance) | 18 | –11.70 (10.40) [n = 153] | –9.60 (8.40) [n = 156] |
–2.10 (–4.21 to 0.01); p = 0.051 (Favours intervention) |
||
| 36 | –9.40 (8.30) [n = 153] | –7.20 (6.30) [n = 156] |
–2.20 (–3.85 to –0.55); p < 0.01 (Favours intervention) |
|||
| Intervention with meal replacement | Control | Effect size (95% CI) | ||||
| Ryttig 1995173 (trial of diet and meal replacements vs. diet for weight maintenance) | 12 (adjusted analysis) | 5.94 (7.87) [n = 31] | 9.33 (9.96) [n = 29] |
–3.40 (–7.96 to 1.17); p = 0.145 (Favours meal replacement) |
||
| 12 (unadjusted analysis) | 8.00 (8.20) [n = 23] | 12.30 (9.70) [n = 22] |
–4.30 (–9.56 to 0.96); p = 0.109 (Favours meal replacement) |
|||
| Meal replacement | Diet | Effect size (95% CI) | ||||
| Ryttig 1997172 (trial of diet and meal replacements vs. diet for weight maintenance) | 12 (adjusted analysis) | –8.57 (8.34) [n = 27] | –4.17 (7.10) [n = 27] |
–4.39 (–8.52 to –0.26); p = 0.037 (Favours meal replacement) |
||
| 12 (unadjusted analysis) | –15.42 (10.28) [n = 15] | –10.24 (8.81) [n = 11] |
–5.18 (–12.54 to 2.18); p = 0.168 (Favours meal replacement) |
|||
| 26 (adjusted analysis) | –2.40 (6.60) [n = 27] | –3.17 (6.81) [n = 27] |
0.76 (–2.81 to 4.34); p = 0.676 (Favours diet) |
|||
| 26 (unadjusted analysis) | –5.90 (7.58) [n = 11] | –5.70 (7.53) [n = 15] |
–0.20 (–6.08 to 5.68); p = 0.947 (Favours meal replacement) |
|||
Bariatric surgery versus weight-management programmes/usual care or control interventions
Eleven RCTs compared bariatric surgery with a non-surgical intervention for weight loss. 100,101,109–111,126,145,153,154,169,174 The trials mainly recruited middle-aged participants. The youngest reported group mean age was 42.2 years and the oldest was 54.6 years. The weighted mean age of participants was 48.1 years. The participants had a weighted mean weight of 115.8 kg (the lowest reported mean weight was 99.5 kg and the highest was 168.6 kg) and a weighted mean BMI of 40.3 kg/m2 (the lowest reported mean BMI was 35.5 kg/m2 and the highest was 55.7 kg/m2). Women represented ≥ 50% of the recruited participants in every trial, with the exception of the trial conducted by Reis et al. ,169 which exclusively recruited men, and one111 in which women represented 40% of the recruited participants. Seven trials100,101,110,111,145,154,174 targeted their recruitment at people with type 2 diabetes mellitus, and one trial145 recruited people with stage 3/4 chronic kidney disease. Participants in all trials had a range of comorbidities: hypertension, dyslipidaemia, arthritis, sleep apnoea, gastro-oesophageal reflux and depression. Brief details of the interventions are presented in Report Supplementary Material 5, Table E4.
Quality assessment
Risk-of-bias assessment
Most trials (10/11, 90.9%) were considered to have adequate randomisation sequence generation, although it was unclear whether or not allocation concealment was adequate in seven trials (63.6%). Owing to the nature of the interventions, blinding of participants and personnel was not possible. Only one trial101 reported blinding outcome assessment. Four trials were rated as being at risk of bias owing to author links with surgical manufacturers (see Report Supplementary Material 4, Figure E8). 110,111,154,174
Equity assessment
Trials failed to report information for the majority of items on the Cochrane Public Health Equity assessment checklist. 61 Fewer than half of the trials were considered to have included a representative sample and none reported sociodemographic details of participants. Trials did not report information concerning diversity or disadvantage in their intervention design or delivery, nor did they discuss the sustainability of interventions. Trials did not report process measures or details of any fidelity checks (see Report Supplementary Material 4, Figure E9).
Roux-en-Y gastric bypass surgery
Five trials100,101,126,154,174 compared Roux-en-Y gastric bypass (RYGB) surgery with a lifestyle WMP versus a lifestyle WMP alone. Three trials100,154,174 also included a second surgical comparison, laparoscopic adjustable gastric banding (LAGB), biliopancreatic diversion (BPD) or SG. One trial169 compared RYGB and a lifestyle WMP with a control group in which participants received minimal education only.
The weighted mean age for all participants was 48.1 years and the weighted mean BMI was 39.2 kg/m2. All trials, except that conducted by Reis et al. ,169 recruited people with type 2 diabetes mellitus.
A brief description of the delivery format of the interventions for the trials is provided in Report Supplementary Material 5, Table E5.
Descriptions of the trials
The trial conducted by Courcoulas et al. 100 compared RYGB, LAGB and an intensive lifestyle WMP (ILI). Participants in the ILI [mean BMI 35.7 kg/m2 (SD 3.3 kg/m2)] followed an adapted 12-month programme based on the Diabetes Prevention Program (DPP)205 and the Look AHEAD trial. 41 ILI participants were prescribed a 1200- to 1800-kcal/day diet. Moderate intensity exercise was for prescribed 5 days per week, beginning at 20 minutes per day and progressing to ≥ 60 minutes per day. Surgical participants underwent clinical follow-up assessments at 2 weeks and 3, 6, 9 and 12 months postoperatively in the RYGB group and at 2 weeks and 2, 4, 6, 8, 10 and 12 months or more frequently, as necessary, for band adjustment in the LAGB group. The RYGB group had a mean initial BMI of 35.5 kg/m2 (SD 2.6 kg/m2). RYGB was performed with a standard retrocolic–retrogastric technique using a linear stapled and hand-sewn gastrojejunal anastomosis. Participants were counselled on a diet programme consistent with postbariatric surgery recommendations and were encouraged to exercise for a minimum of three or four times each week. LAGB participants had mean BMI of 35.5 kg/m2 (SD 3.4 kg/m2).
After 3 years, RYGB was the most successful treatment for achieving partial or complete remission of type 2 diabetes mellitus. Partial/complete remission was achieved by 40%, 29% and no participants in the RYGB, LAGB and ILI groups, respectively, and complete remission was achieved by 15%, 5% and no participants, respectively. The number of participants in remission declined in the RYGB group, from 60% at year 1 to 45% at year 2 and 40% at year 3, whereas remission remained stable at 29% for LAGB participants and none for ILI participants over the 3 years. The RYGB group had the greatest reductions in HbA1c and fasting glucose from baseline to 3 years (reported p < 0.0013 for RYGB vs. ILI, and reported p < 0.05 for RYGB vs. both LAGB and ILI, for HbA1c and fasting plasma glucose, respectively). No adverse events were reported for the ILI group during the 3 years. One serious adverse event (anastomotic ulcer) was reported for the RYGB group. One case of nausea and emesis requiring intravenous hydration and one case of renal lithiasis were reported for the RYGB group.
The CROSSROADS (Calorie Reduction Or Surgery: Seeking to Reduce Obesity And Diabetes Study) trial, conducted by Cummings et al.,101 randomised people to receive RYGB or an ILI. The ILI group [mean BMI 37.1 kg/m2 (SD 3.5 kg/m2)] had a 12-month in-person and telephone-based intervention. A dietitian delivered nutrition sessions based on the DPP205 with modifications for people with diabetes mellitus. Reduced calorie intake and weight loss were encouraged but participants were not given weight-loss goals. Participants attended physiologist-led supervised exercise sessions at least three times a week and were asked to exercise on ≥ 2 additional days per week at home for the first 6 months. Participants were then asked to attend the supervised exercise sessions for 1 day per week and to exercise on ≥ 4 days per week at home for the remaining 6 months. Participants in the RYGB group [initial mean BMI 38.3 kg/m2 (SD 3.7 kg/m2)] underwent laparoscopic RYGB and a 4-week preoperative and 10-month postoperative behavioural programme, which focused on diet and nutrition counselling, behaviour modification and exercise recommendations. Participants received weekly telephone-based appointments and attended two or three bariatric support group meetings during the preoperative phase. Telephone appointments with the health educator continued during the postoperative phase.
Diabetes mellitus remission was achieved by 60% of RYGB participants and 5.9% of ILI participants (reported p = 0.002). There were 64 adverse events reported in the ILI group and 31 adverse events reported in the RYGB group. There were no reported deaths or serious surgical adverse events for either group. More hypoglycaemic events were reported in the ILI group than in the RYGB group (43 vs. 16). One serious adverse event involving a hospital emergency room visit for acute alcohol intoxication was reported for a RYGB participant. Minor musculoskeletal complaints were reported by seven ILI and two RYGB participants. The authors reported that there was no difference between groups for other minor adverse events.
The trial by Halperin et al. 126 compared RYGB with a 12-month intensive diabetes mellitus medical and WMP: the Weight Achievement and Intensive Treatment (Why WAIT) programme. Why WAIT included a diet of 1500–1800 kcal/day and was delivered by a multidisciplinary team in 2-hour weekly group sessions during the first 12 weeks, focusing on diabetes mellitus medication adjustments, cognitive–behavioural therapy (CBT), group education and supervised exercise. Participants received individual monthly counselling thereafter. The initial mean BMI of Why WAIT participants was 36.5 kg/m2 (SD 3.4 kg/m2), and it was 36.0 kg/m2 (SD 3.5 kg/m2) for RYGB participants. RYGB participants showed greater improvements in quality of life, measured by the Impact of Weight on Quality of Life (IWQOL)-Lite, than Why WAIT participants at 12, 18 and 24 months, but there were no between-group differences for other quality-of-life measures.
The trial by Mingrone et al. 154 randomised people to RYGB, BPD or a diet and lifestyle modification. Participants in the lifestyle intervention group had an initial mean BMI of 45.6 kg/m2 (SD 6.2 kg/m2). Participants were advised to reduce their overall energy and fat intake (< 30% total fat and < 10% saturated fat) and increase exercise (approximately 30 minutes/day of brisk walking with additional moderate intensity aerobic activity twice a week). Participants in the surgical arms had an initial mean BMI of 44.5 kg/m2.
At 5 years, 37% of participants in the RYGB group and 63% in the BPD group achieved and maintained diabetes mellitus remission, compared with none of the medically treated participants. Five major complications of diabetes mellitus (including one fatal myocardial infarction) were reported in four participants (27%) in the medical group, compared with only one complication in the RYGB group and no complications in the BPD group. Two participants (13%) in the medical treatment group reported persistent diarrhoea associated with metformin. One intestinal obstruction requiring reoperation occurred 6 months after RYGB. The incidence of metabolic adverse events was higher in the surgical groups than in the medical treatment group. No late complications or deaths were reported in the surgery groups.
The STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial, conducted by Schauer et al.,174 randomised people to receive intensive medical therapy or medical therapy plus RYGB or SG. Participants in all groups received lifestyle counselling and weight-management support. Participants were encouraged to participate in the Weight Watchers® (New York, NY, USA) programme. Surgical participants were evaluated by a psychologist. The primary outcome of the trial was HbA1c of ≤ 6.0% with or without the use of diabetes mellitus medications. The initial mean BMI was 37 kg/m2 (SD 3.3 kg/m2) for GBP participants, 36.2 kg/m2 (SD 3.9 kg/m2) for SG participants and 36.8 kg/m2 (SD 3.0 kg/m2) for intensive medical therapy participants. At 5 years, the primary end point was reported by 2 out of 38 participants (5%) who received intensive medical therapy, 14 out of 49 GBP participants (29%) (reported unadjusted p = 0.01, reported adjusted p = 0.03 and p = 0.08 in the intention-to-treat analysis) and 11 out of 47 participants (23%) who underwent SG (reported unadjusted p = 0.03, adjusted p = 0.07 and p = 0.17 in the intention-to-treat analysis). Quality of life, as measured by the RAND 36-Item Health Survey, was significantly better in the RYGB group than in the intensive medical treatment group for general health scores and bodily pain scores (reported p < 0.05 for both). Four surgical participants required subsequent surgical interventions, one intensive medical therapy participant died as a result of myocardial infarction and one participant in the SG group had a stroke.
Meta-analyses
The five trials100,101,126,154,174 were included in a meta-analysis of weight change and used to inform the economic evaluation of RYGB in Chapter 7. The results of the meta-analysis of the mean difference in weight change between the intervention and control treatments are presented in Table 6. At all time points, up to 60 months following surgery, there is evidence of a difference in weight loss in favour of RYGB for both adjusted and unadjusted data.
| Type of analysis | Mean weight change (kg) | ||||
|---|---|---|---|---|---|
| 12 months | 24 months | 36 months | 48 months | 60 months | |
| Adjusted |
Mean –23.28 (95% CI –25.82 to –20.74) p < 0.001 I2 = 56 Trials = 4(Favours surgery) |
Mean –23.42 (95% CI –26.33 to –20.51) p < 0.001 I2 = 67 Trials = 4(Favours surgery) |
Mean –21.14 (95% CI –24.49 to –17.79) p < 0.001 I2 = 0 Trials = 2(Favours surgery) |
Mean –20.00 (95% CI –24.00 to –16.00) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
Mean –20.23 (95% CI –23.75 to –16.71) p < 0.001 I2 = 71 Trials = 2(Favours surgery) |
| Unadjusted |
Mean –22.56 (95% CI –25.21 to –19.90) p < 0.001 I2 = 47 Trials = 4(Favours surgery) |
Mean –23.56 (95% CI –26.64 to –20.49) p < 0.001 I2 = 69 Trials = 4(Favours surgery) |
Mean –21.30 (95% CI –24.59 to –18.01) p < 0.001 I2 = 0 Trials = 2(Favours surgery) |
Mean –19.30 (95% CI –23.38 to –15.23) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
Mean –19.70 (95% CI –23.29 to –16.11) p < 0.001 I2 = 74 Trials = 2(Favours surgery) |
A further RYGB trial was identified for inclusion in our review. 169 This trial compared RYGB and behavioural and psychological counselling with a control group who received only written and oral information about a healthy lifestyle, but not a diet and lifestyle WMP. It was not included in the weight change or risk factor meta-analyses. Reis et al. 169 examined the effects of weight loss on erectile function in men. Men in the RYGB group had a mean BMI of 55.7 kg/m2 (SD 7.8 kg/m2). Those in the control group had an initial mean BMI of 54.0 kg/m2 (SD 6.1 kg/m2). None of the men was taking phosphodiesterase type-5 inhibitors at baseline. At 24 months, men in the RYGB group had significantly greater reduction in weight and BMI than men in the control group (reported p = 0.0006 for both). Surgery increased erectile function quality, measured by the International Index of Erectile Function (IIEF)-5 questionnaire (reported p = 0.0224), and increased total testosterone (TT) (reported p = 0.0043) and free testosterone (FT) (reported p = 0.0149) levels.
Over 5 years, RYGB consistently improved HbA1c, glucose and HDL cholesterol. Triglycerides and systolic blood pressure were also improved at 3 years by RYGB, but were not improved at 5 years. LDL cholesterol was lower with lifestyle interventions, and other results were mixed (Table 7 provides more information).
| Risk factor | Meta-analysis of mean difference in risk factors | |||
|---|---|---|---|---|
| 12 months | 24 months | 36 months | 60 months | |
| Total cholesterol (mmol/l) |
Mean –0.18 (95% CI –0.50 to 0.15) p = 0.289 I2 = 0 Trials = 3(Favours surgery) |
Mean 0.54 (95% CI 0.04 to 1.04) p = 0.033 I2 = 0 Trials = 2(Favours diet and lifestyle) |
Mean –0.04 (95% CI –0.70 to 0.62) p = 0.905 I2 = N/A Trials = 1(Favours surgery) |
Mean 1.11 (95% CI 0.38 to 1.84) p = 0.003 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| LDL cholesterol (mmol/l) |
Mean –0.10 (95% CI –0.38 to 0.17) p = 0.466 I2 = 24 Trials = 3(Favours surgery) |
Mean 0.38 (95% CI –0.01 to 0.77) p = 0.053 I2 = 0 Trials = 2(Favours diet and lifestyle) |
Mean 0.16 (95% CI –0.12 to 0.44) p = 0.266 I2 = 0 Trials = 2(Favours diet and lifestyle) |
Mean 0.34 (95% CI 0.08 to 0.61) p = 0.011 I2 = 73 Trials = 2(Favours diet and lifestyle) |
| HDL cholesterol (mmol/l) |
Mean 0.23 (95% CI 0.16 to 0.30) p < 0.001 I2 = 0 Trials = 4(Favours surgery) |
Mean 0.29 (95% CI 0.16 to 0.42) p < 0.001 I2 = 0 Trials = 2(Favours surgery) |
Mean 0.31 (95% CI 0.21 to 0.41) p < 0.001 I2 = 0 Trials = 2(Favours surgery) |
Mean 0.27 (95% CI 0.16 to 0.37) p < 0.001 I2 = 3 Trials = 2(Favours surgery) |
| Triglycerides (mmol/l) |
Mean –0.44 (95% CI –0.64 to –0.23) p < 0.001 I2 = 48 Trials = 4(Favours surgery) |
Mean –0.23 (95% CI –0.66 to 0.19) p = 0.286 I2 = 42 Trials = 2(Favours surgery) |
Mean –0.44 (95% CI –0.77 to –0.11) p < 0.01 I2 = 71 Trials = 2(Favours surgery) |
Mean 0.02 (95% CI –0.32 to 0.37) p = 0.902 I2 = 0 Trials = 2(Favours diet and lifestyle) |
| Systolic blood pressure (mmHg) |
Mean –6.47 (95% CI –10.75 to –2.19) p = 0.003 I2 = 70 Trials = 4(Favours surgery) |
Mean –2.16 (95% CI –8.41 to 4.09) p = 0.498 I2 = 92 Trials = 2(Favours surgery) |
Mean –4.21 (95% CI –11.45 to 3.04) p = 0.255 I2 = 67 Trials = 2(Favours surgery) |
Mean 8.48 (95% CI 5.21 to 11.75) p < 0.001 I2 = 69 Trials = 2(Favours diet and lifestyle) |
| Diastolic blood pressure (mmHg) |
Mean –2.36 (95% CI –4.69 to –0.03) p = 0.047 I2 = 62 Trials = 4(Favours surgery) |
Mean –1.55 (95% CI –5.57 to 2.47) p = 0.450 I2 = 64 Trials = 2(Favours surgery) |
Mean 0.06 (95% CI –3.53 to 3.65) p = 0.973 I2 = 41 Trials = 2(Favours diet and lifestyle) |
Mean 1.22 (95% CI –2.54 to 4.98) p = 0.525 I2 = 63 Trials = 2(Favours diet and lifestyle) |
| HbA1c (%) |
Mean –1.69 (95% CI –2.13 to –1.25) p < 0.001 I2 = 0 Trials = 4(Favours surgery) |
Mean –0.82 (95% CI –1.64 to 0.01) p = 0.052 I2 = 0 Trials = 3(Favours surgery) |
Mean –1.57 (95% CI –2.33 to –0.81) p < 0.001 I2 = 0 Trials = 2(Favours surgery) |
Mean –1.54 (95% CI –2.29 to –0.80) p < 0.001 I2 = 57 Trials = 2(Favours surgery) |
| Glucose (mmol/l) |
Mean –2.77 (95% CI –3.66 to –1.89) p < 0.001 I2 = 34 Trials = 4(Favours surgery) |
Mean –1.29 (95% CI –2.68 to 0.11) p = 0.070 I2 = 0 Trials = 2(Favours surgery) |
Mean –3.18 (95% CI –4.33 to –2.04) p < 0.001 I2 = 56 Trials = 2(Favours surgery) |
Mean –2.27 (95% CI –3.53 to –1.02) p < 0.001 I2 = 81 Trials = 2(Favours surgery) |
Sleeve gastrectomy
Two trials145,174 compared SG plus a diet and lifestyle intervention with a diet and lifestyle intervention alone.
Descriptions of the trials
Details of the earlier of these trials, by Schauer et al. ,174 were reported in Roux-en-Y gastric bypass surgery and details of the interventions for all surgery trials are presented in Report Supplementary Material 5, Table E5.
The trial conducted by MacLaughlin et al. 145 compared laparoscopic SG with a lifestyle intervention in people with obesity and stages 3/4 chronic kidney disease to determine the effect on kidney function. The medical care group had an initial mean BMI of 37.4 kg/m2 (range 35.8–40.0 kg/m2). The intervention was delivered by an experienced renal dietitian and physiotherapist, supported by a nephrologist and renal pharmacist. Participants were prescribed a 1400- to 1800-kcal/day low-fat, renal diet; regular exercise; CBT; and 120 mg of orlistat three times daily. Dietary protein intake was 0.8–1.0 g/kg of ideal body weight. Participants randomised to the SG group [initial mean BMI of 40.3 kg/m2 (range 37.3–43.0 kg/m2)], received dietary and renal information. Energy intake was restricted to approximately 1000 kcal/day following surgery. Protein intakes were similar to those of the medical care group. The SG group showed greater improvement in quality of life than the medical care group, measured by Hospital Anxiety and Depression Scale scores and increased Short Form questionnaire-36 items (SF-36) physical domain scores (reported p < 0.05 for both). There were no significant differences in unadjusted body surface estimated glomerular filtration rate (eGFR). There was no change in diabetes mellitus prevalence throughout the study. One participant on insulin therapy in the SG group decreased their insulin dose by 59%, and four participants requiring insulin in the lifestyle group decreased their median insulin dose by 7% over 12 months.
Meta-analyses
The results of the meta-analysis of SG versus lifestyle intervention, presented in Table 8, show that, for data adjusted and unadjusted for participant withdrawal, SG produces greater weight loss than lifestyle intervention at all time points.
| Type of analysis | Mean weight change (kg) | ||||
|---|---|---|---|---|---|
| 12 months | 24 months | 36 months | 48 months | 60 months | |
| Unadjusted |
Mean –22.01 (95% CI –24.99 to –19.02) p < 0.001 I2 = 87 Included studies:(Favours surgery) |
Mean –17.90 (95% CI –22.07 to –13.73) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –17.00 (95% CI –20.85 to –13.15) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –17.36 (95% CI –21.55 to –13.16) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –13.30 (95% CI –17.35 to –9.25) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
| Adjusted |
Mean –22.24 (95% CI –25.13 to –19.34) p < 0.001 I2 = 85 Included studies:(Favours surgery) |
Mean –18.48 (95% CI –22.42 to –14.54) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –17.43 (95% CI –21.00 to –13.86) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –17.20 (95% CI –21.01 to –13.39) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Mean –13.46 (95% CI –17.03 to –9.88) p < 0.001 I2 = N/A Included studies:(Favours surgery) |
Favourable changes from SG were seen for all risk factors, with the exception of total cholesterol, LDL cholesterol and diastolic blood pressure (Table 9).
| Risk factors | Meta-analysis of mean difference | ||
|---|---|---|---|
| 12 months | 36 months | 60 months | |
| Total cholesterol (mmol/l) |
Mean 0.12 (95% CI –0.34 to 0.58) p = 0.610 I2 = N/A Trials = 1(Favours diet and lifestyle) |
||
| LDL cholesterol (mmol/l) |
Mean 0.17 (95% CI –0.25 to 0.59) p = 0.422 I2 = N/A Trials = 1(Favours diet and lifestyle) |
Mean 0.10 (95% CI –0.21 to 0.41) p = 0.526 I2 = N/A Trials = 1(Favours diet and lifestyle) |
Mean 0.38 (95% CI 0.06 to 0.70) p = 0.019 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| HDL cholesterol (mmol/l) |
Mean 0.19 (95% CI 0.09 to 0.29) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
Mean 0.34 (95% CI 0.22 to 0.46) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
Mean 0.27 (95% CI 0.15 to 0.39) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
| Triglycerides (mmol/l) |
Mean –0.42 (95% CI –0.82 to –0.02) p = 0.039 I2 = N/A Trials = 1(Favours surgery) |
Mean –0.14 (95% CI –0.54 to 0.26) p = 0.494 I2 = N/A Trials = 1(Favours surgery) |
Mean –0.04 (95% CI –0.45 to 0.37) p = 0.849 I2 = N/A Trials = 1(Favours surgery) |
| Systolic blood pressure (mmHg) |
Mean –1.20 (95% CI –7.75 to 5.35) p = 0.720 I2 = N/A Trials = 1(Favours surgery) |
Mean –5.06 (95% CI –14.16 to 4.04) p = 0.276 I2 = N/A Trials = 1(Favours surgery) |
Mean –4.30 (95% CI –12.95 to 4.35) p = 0.330 I2 = N/A Trials = 1(Favours surgery) |
| Diastolic blood pressure (mmHg) |
Mean 0.90 (95% CI –2.84 to 4.64) p = 0.637 I2 = N/A Trials = 1(Favours diet and lifestyle) |
Mean 0.21 (95% CI –5.13 to 5.55) p = 0.939 I2 = N/A Trials = 1(Favours diet and lifestyle) |
Mean –3.90 (95% CI –9.45 to 1.65) p = 0.168 I2 = N/A Trials = 1(Favours surgery) |
| HbA1c (%) |
Mean –1.50 (95% CI –2.18 to –0.82) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
Mean –1.70 (95% CI –2.83 to –0.57) p = 0.003 I2 = N/A Trials = 1(Favours surgery) |
Mean –1.80 (95% CI –2.71 to –0.89) p < 0.001 I2 = N/A Trials = 1(Favours surgery) |
| Glucose (mmol/l) |
Mean –1.95 (95% CI –3.40 to –0.50) p = 0.008 I2 = N/A Trials = 1(Favours surgery) |
Mean –1.94 (95% CI –3.40 to –0.48) p = 0.009 I2 = N/A Trials = 1(Favours surgery) |
Mean –1.90 (95% CI –3.39 to –0.41) p = 0.013 I2 = N/A Trials = 1(Favours surgery) |
Gastric band
Four trials100,109–111 compared gastric band surgery plus lifestyle interventions with lifestyle-only interventions.
Descriptions of the trials
Details of the Courcoulas et al. 100 trial are provided in Roux-en-Y gastric bypass surgery and details of the interventions of all surgery trials are presented in Report Supplementary Material 5, Table E5. Participants in the LAGB group followed the same diet and lifestyle programme as participants in the RYGB group.
Ding et al. 109 randomised people with type 2 diabetes mellitus to receive LAGB or the same Why WAIT intensive medical and WMP delivered by Halperin et al. ,126 described previously in Roux-en-Y gastric bypass surgery. The initial mean BMI was 36.7 kg/m2 (SD 4.2 kg/m2) for the Why WAIT participants and 36.4 kg/m2 (SD 3.0 kg/m2) for the LAGB participants. At 12 months, both groups showed similar improvements in health and quality of life.
In the 2008 trial by Dixon et al. ,110 participants with type 2 diabetes mellitus were randomised to conventional therapy and had meetings with a physician, a dietitian, a nurse and a diabetes mellitus educator every 6 weeks for 2 years. Participants were encouraged to reduce their fat intake to < 30%, and to consume low glycaemic index foods. VLCDs and drug treatment were used. Participants were encouraged to engage in 200 minutes per week of structured physical activity. Surgical participants followed conventional therapy and underwent the LAGB procedure. The mean BMI was 37 kg/m2 (SD 2.7 kg/m2) for participants in the LAGB group, and 37.2 kg/m2 (SD 2.5 kg/m2) for conventional treatment participants. After 2 years, more LAGB participants (73%) than conventional therapy participants (13%) had achieved type 2 diabetes mellitus remission (reported p < 0.001).
The 2012 trial by Dixon et al. 111 compared LAGB with conventional weight-loss therapy for obstructive sleep apnoea (OSA) in participants with an apnoea–hypopnoea index of ≥ 20 events per hour. Participants in the conventional therapy group [mean BMI 43.8 kg/m2 (SD 4.9 kg/m2)] were encouraged to engage in 200 minutes per week of structured physical activity and have a daily energy deficit of 500 kcal/day. Both groups saw a bariatric physician, a sleep physician and a dietitian, and were reviewed every 4–6 weeks for 2 years. LAGB participants reportedly had greater reduction in apnoea–hypopnoea index than conventional therapy participants (a decrease of 25.5 events/hour vs. a decrease of 14.0 events/hour), but the difference between groups was not significant. The LAGB group showed greater improvement in SF-36 physical activity scores than the conventional therapy participants (reported p = 0.04). The authors reported that 20 participants had type 2 diabetes mellitus at baseline (10 in each group) and two participants in the conventionally treated group developed type 2 diabetes mellitus during the study; however, there were no within- or between-group differences in the use of diabetes mellitus medication.
Meta-analyses
The results of the meta-analysis of gastric band and lifestyle intervention versus lifestyle intervention only, presented in Table 10, show that gastric band produces greater weight loss than lifestyle intervention at all time points.
| Type of analysis | Mean weight change (kg) | ||
|---|---|---|---|
| 12 months | 24 months | 36 months | |
| Unadjusted |
Mean –5.75 (95% CI –9.42 to –2.07) p = 0.002 I2 = 0 Trials = 2(Favours surgery) |
Mean –17.91 (95% CI –21.11 to –14.71) p < 0.001 I2 = 67 Trials = 3(Favours surgery) |
Mean –9.87 (95% CI –16.25 to –3.49) p < 0.01 I2 = N/A Trials = 1(Favours surgery) |
Risk factor data show no consistent benefit at each time point over 3 years with bariatric surgery, although HbA1c is lower at 3 years (Table 11 provides more details).
| Risk factors | Meta-analysis of the mean difference | ||
|---|---|---|---|
| 12 months | 24 months | 36 months | |
| Total cholesterol (mmol/l) |
Mean –0.06 (95% CI –0.47 to 0.34) p = 0.754 I2 = 0 Trials = 2(Favours surgery) |
Mean –0.06 (95% CI –0.39 to 0.28) p = 0.744 I2 = 0 Trials = 3(Favours surgery) |
Mean 0.36 (95% CI –0.30 to 1.02) p = 0.284 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| LDL cholesterol (mmol/l) |
Mean –0.13 (95% CI –0.49 to 0.23) p = 0.465 I2 = 73 Trials = 2(Favours surgery) |
Mean 0.35 (95% CI –0.33 to 1.03) p = 0.314 I2 = N/A Trials = 1(Favours diet and lifestyle) |
Mean 0.37 (95% CI –0.26 to 1.00) p = 0.247 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| HDL cholesterol (mmol/l) |
Mean 0.08 (95% CI –0.02 to 0.18) p = 0.128 I2 = 0 Trials = 2(Favours surgery) |
Mean 0.15 (95% CI 0.07 to 0.24) p < 0.001 I2 = 45 Trials = 3(Favours surgery) |
Mean 0.17 (95% CI –0.01 to 0.35) p = 0.067 I2 = N/A Trials = 1(Favours surgery) |
| Triglycerides (mmol/l) |
Mean –0.11 (95% CI –0.54 to 0.33) p = 0.633 I2 = 73 Trials = 2(Favours surgery) |
Mean –0.48 (95% CI –0.76 to –0.19) p = 0.001 I2 = 18 Trials = 3(Favours surgery) |
Mean –0.36 (95% CI –0.94 to 0.22) p = 0.227 I2 = N/A Trials = 1(Favours surgery) |
| Systolic blood pressure (mmHg) |
Mean 6.20 (95% CI 0.25 to 12.14) p = 0.041 I2 = 0 Trials = 2(Favours diet and lifestyle) |
Mean –3.01 (95% CI –7.78 to 1.77) p = 0.218 I2 = 0 Trials = 3(Favours surgery) |
Mean 2.96 (95% CI –8.76 to 14.68) p = 0.620 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| Diastolic blood pressure (mmHg) |
Mean 1.30 (95% CI –2.03 to 4.62) p = 0.444 I2 = 0 Trials = 2(Favours diet and lifestyle) |
Mean 0.96 (95% CI –2.08 to 4.00) p = 0.536 I2 = 0 Trials = 3(Favours diet and lifestyle) |
Mean 5.52 (95% CI 0.34 to 10.70) p = 0.037 I2 = N/A Trials = 1(Favours diet and lifestyle) |
| HbA1c (%) |
Mean –0.50 (95% CI –1.17 to 0.17) p = 0.143 I2 = 0 Trials = 2(Favours surgery) |
Mean –0.64 (95% CI –1.14 to –0.14) p = 0.012 I2 = 35 Trials = 3(Favours surgery) |
Mean –1.01 (95% CI –2.01 to –0.01) p = 0.049 I2 = N/A Trials = 1(Favours surgery) |
| Glucose (mmol/l) |
Mean –1.16 (95% CI –2.52 to 0.19) p = 0.093 I2 = 34 Trials = 2(Favours surgery) |
Mean –0.91 (95% CI –1.68 to –0.14) p = 0.020 I2 = 0 Trials = 3(Favours surgery) |
Mean –0.37 (95% CI –2.17 to 1.43) p = 0.687 I2 = N/A Trials = 1(Favours surgery) |
Biliopancreatic bypass
Two trials conducted by Mingrone et al. 153,154 examined the effect of biliopancreatic bypass surgery.
Descriptions of the trials
Details of the Mingrone et al. 154 2012 trial are presented in Roux-en-Y gastric bypass surgery and details of the interventions in the Mingrone et al. 153 2002 trial are presented in Report Supplementary Material 5, Table E5. In both trials, participants in the surgical groups lost more weight than those in the diet groups.
Behaviour change technique coding summary for all trials of bariatric surgery (see Report Supplementary Material 1, Section 8)
Surgical interventions were compared with lifestyle interventions, which usually included a dietary component and – in all but one trial – physical activity ‘advice’ or supervised physical activity. Control groups received, on average, 4.9 BCTs (range 1–10 BCTs) and intervention (surgery) groups received slightly fewer BCTs (average 2.6 BCTs, range 1–6 BCTs) targeting these behaviours. The most commonly used BCTs were setting a behavioural goal and a respected person explaining the importance of diet/physical activity. Comparator group support varied substantially between trials, with the least supported comparator group receiving only advice from a credible person, versus the most extensively supported comparator group, in which participants also received instructions, goal-setting, graded tasks, self-monitoring and problem-solving BCTs, as well as adding/removing objects from the environment, help with reducing negative emotions and social support. These results must be interpreted with caution, however, as intervention and control group support was generally described very poorly (only two trials were rated as adequately described and none was rated as well described) (see Report Supplementary Material 1, Section 8).
Weight maintenance interventions without a very low-calorie diet weight-loss phase
Eight trials105,137,140,143,162,164,176,197 evaluated interventions for weight maintenance. These trials are presented separately from those trials that evaluated weight maintenance following VLCDs, which are discussed under VLCDs. Owing to the heterogeneous nature of the interventions, it was not possible to conduct meta-analyses.
Orlistat weight maintenance trials
Two trials105,176 evaluated orlistat in weight maintenance. Both trials reported double-blinding, although authors105 note that participants might have suspected their allocation by the presence or absence of gastrointestinal (GI) adverse events specific to orlistat.
Descriptions of the trials
In the study by Davidson et al. ,105 all participants received placebo plus a controlled-energy diet in a 4-week lead-in phase. After 4 weeks, the diet was continued and participants were randomised to receive placebo three times daily or 120 mg of orlistat three times a day, for 12 months, after which time participants began a weight maintenance diet. The placebo group [mean BMI 36.5 kg/m2 (SD 2.4 kg/m2)] continued to receive placebo. Orlistat-treated participants [mean BMI 36.2 kg/m2 (SD 2.6 kg/m2)] were rerandomised to receive placebo, 60 mg of orlistat or 120 mg of orlistat three times a day for an additional 12 months. Participants who received 120 mg of orlistat during year 2 regained significantly less of their first-year weight loss (35.2% regain) than those who received 60 mg of orlistat (51.3% regain) or placebo (63.4% regain) during the second year (reported p < 0.001).
In the trial by Sjöström et al. ,176 participants who completed a 4-week placebo lead-in period on a 600-kcal/day deficit diet, followed by randomisation to double-blind treatment with 120 mg of orlistat or placebo three times a day in conjunction with the hypocaloric diet for a 1-year period, were then rerandomised to orlistat or placebo with a weight maintenance diet for a second period of 12 months. Mean BMI ranged from 36.0 to 36.1 kg/m2. At the end of the weight maintenance phase, participants who continued with orlistat regained half as much weight as those participants who were switched to placebo (reported p < 0.001). Participants who switched from placebo to orlistat lost an additional 0.9 kg during year 2, compared with a mean regain of 2.5 kg in participants who continued on placebo (reported p < 0.001). As shown in Table 12, weight loss was significantly greater in the orlistat group than in the placebo group at 24 months.
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) | Effect size (95% CI); p-value | ||
|---|---|---|---|---|---|
| Orlistat | Placebo | ||||
| Sjöström 1998176 (trial of orlistat vs. placebo throughout) | 24 | –7.90 (8.14) [n = 256] | –5.27 (7.45) [n = 261] |
–2.63 (–3.97 to –1.29); p < 0.001 (Favours orlistat) |
|
| Self help | Group help | ||||
| Kumanyika 2005137 (trial of self-help vs. group help for weight maintenance) | 18 | 1.10 (3.78) [n = 28] | 0.02 (4.72) [n = 28] |
1.08 (–1.16 to 3.32); p = 0.345 (Favours group help) |
|
| Continuing care | Standard care | ||||
| Latner 2013140 (trial of continuing vs. standard care for weight maintenance) | 24 | –2.43 (6.60) [n = 52] | –3.27 (6.84) [n = 38] |
0.84 (1.96 to 3.64); p = 0.557 (Favours standard care) |
|
| ED+ | ED– | ||||
| Lowe 2014143 (trial of meal replacements and energy-reducing diet for weight maintenance) | 24 | 1.65 (6.45) [n = 72] | 2.38 (6.60) [n = 60] | –0.73 (–2.96 to 1.50); p = 0.522 | |
| 36 | 2.70 (6.96) [n = 72] | 3.82 (7.08) [n = 60] |
–1.12 (–3.52 to 1.28); p = 0.361 (Favours ED+) |
||
| MR+ | MR– | ||||
| Lowe 2014143 (trial of meal replacements and energy-reducing diet for weight maintenance) | 24 | 2.16 (6.51) [n = 66] | 1.81 (6.54) [n = 66] | 0.35 (–1.88 to 2.58); p = 0.758 | |
| 36 | 3.63 (6.99) [n = 66] | 2.79 (7.06) [n = 66] |
0.84 (–1.56 to 3.24); p = 0.492 (Favours MR–) |
||
| Education | Telephone | Face to face | |||
| Perri 2008162 (trial of telephone and face-to-face counselling vs. education only for weight maintenance) | 12 | 3.70 (6.22) [n = 79] | 1.20 (5.94) [n = 72] | 1.20 (5.47) [n = 83] |
p = 0.010 (Less weight gain from telephone and face to face) |
| Relapse prevention or problem-solving | Standard behavioural therapy | ||||
| Perri 2001164 (trial of relapse prevention or problem-solving therapy vs. standard behavioural therapy for weight maintenance) | 17 | Unadjusted | –8.51 (8.00) [n = 43] | –4.14 (4.86) [n = 15] | –4.37 (–8.69 to –0.05); p = 0.047 |
| 17 | Adjusted | –5.90 (7.72) [n = 62] | –3.45 (4.69) [n = 18] |
–2.45 (–6.21 to –1.31); p = 0.202 (Favours relapse prevention or problem-solving) |
|
| Skill-based maintenance | Motivation-based maintenance | ||||
| West 2011197 (trial of skill-based or motivation-based weight maintenance programmes) | 12 | –7.50 (8.04) [n = 113] | –7.22 (7.96) [n = 113] |
–0.28 (–2.37 to 1.81); p = 0.792 (Favours skill-based maintenance) |
|
| 18 | –5.22 (10.96) [n = 113] | –5.34 (8.73) [n = 113] |
0.12 (–2.46 to 2.70); p = 0.927 (Favours motivation-based maintenance) |
||
Other weight maintenance trials
Six other trials137,140,143,162,164,197 investigated interventions targeted at weight maintenance.
Descriptions of the trials
Participants in the Kumanyika et al. 137 trial received a 10-week group counselling Healthy Eating and Lifestyle Programme (HELP) adapted for African American participants. Participants were then randomised to receive further group counselling, staff-assisted self-help or clinic visits only. The mean BMI of participants was 37.0 kg/m2. At 18 months, weight loss was greater in the group help condition but there was no evidence of an important difference between the two conditions, as shown in Table 12.
Latner et al. 140 compared standard care and self-help continuing care for weight maintenance. The mean BMI of participants was 35.8 kg/m2. At 24 months, weight maintenance was more successful in the standard care group than in the self-help group, but there was no evidence of a difference between groups (see Table 12).
Participants in the trial by Lowe et al. 143 were randomised to one of four treatment groups involving the presence or absence of meal replacements and a low-fat energy-reducing diet: MR+/ED+ (meal replacements and an energy-reducing diet), MR–/ED+ (energy-reducing diet only), MR+/ED– (meal replacements only) and MR–/ED– (healthy eating advice control group). The mean BMI of participants was 39.5 kg/m2 (SD 6.6 kg/m2). Table 12 shows the combined data for participants in the groups that did/did not follow the energy-reducing diet (ED+ and ED–) and in those that did/did not receive meal replacements (MR+ and MR–) at 24 and 36 months. Weight regain did not differ between the interventions.
In the trial by Perri et al. ,162 women from rural communities who had completed an initial 6-month weight-loss programme were randomised to two extended-care counselling interventions delivered by telephone or face to face, or to an education control group for a further 6 months. The participant group mean BMI ranged from 36.2 kg/m2 (SD 4.3 kg/m2) to 37.1 kg/m2 (SD 4.5 kg/m2). As shown in Table 12, participants receiving the telephone and face-to-face interventions regained less weight than those in the education control group at 12 months.
In an earlier trial conducted by Perri et al. ,164 two 12-month extended behavioural therapy treatments were compared with a standard behavioural therapy with no further contact. The two extended behavioural therapy conditions used different approaches to weight maintenance. The first focused on relapse prevention training and the second focused on problem-solving. The control group received standard behavioural therapy. The mean BMIs of the groups ranged from 35.0 to 36.4 kg/m2. Table 12 shows weight change at 17 months. When missing data were ignored, relapse prevention or problem-solving resulted in a weight change of –4.37 kg (95% CI –8.69 to –0.05 kg), but when accounting for missing data this was reduced to –2.45 kg (95% CI –6.21 to –1.31 kg).
West et al. 197 randomised African American women with urinary incontinence to a WMP or education control. Women received the WMP for 6 months and were than randomised to standard skill-based or motivation-based weight maintenance programmes for 12 months. The mean BMI was 36.0 kg/m2 (SD 6.0 kg/m2). Table 12 shows weight change at 12 and 18 months. The skill-based programme produced more favourable results at 12 months, whereas the motivation-based programme was more successful at 18 months. There is no evidence of a difference between groups at either time point.
Comparisons of diets
Low-carbohydrate diets (≤ 40 g of carbohydrate per day) versus other diets
Five trials89,106,120,129,181 compared a low-carbohydrate diet (classed as an intake of ≤ 40 g/day) with a low-fat diet (≤ 30% of daily energy from fat). All but one trial89 had an energy reduction for the low-fat diet. None of the low-carbohydrate diets specified an energy intake. The lowest reported mean BMI was 35.0 kg/m2 and the highest was 42.9 kg/m2. In two trials,106,129 all participants had type 2 diabetes mellitus, whereas in the third trial,181 participants had a high prevalence of diabetes mellitus (40.9%) or metabolic syndrome (41.7%). Other comorbid conditions in trials included congestive heart failure, coronary artery disease, depression, hyperlipidaemia, hypertension and sleep apnoea. Brief details of the interventions are provided in Report Supplementary Material 5, Table E6.
Risk-of-bias assessment
Random sequence generation was considered to be adequate for the majority (80%) of the trials, although details of allocation concealment were unclear for most (80%). Only one trial89 clearly reported blinding of outcome assessors. Most trials were rated as being at low risk of other types of bias (see Report Supplementary Material 4, Figure E10).
Equity assessment
Factors relevant for assessing equity were poorly reported in trials. All trials reported one or more PROGRESS category at baseline, usually sex, and most trials (80%) reported unintended effects or harms associated with the intervention. The remaining equity checklist items either were not considered or were so poorly reported that they were judged as unclear (see Report Supplementary Material 4, Figure E11).
Descriptions of the trials
Bazzano et al. 89 examined the effects of a low-carbohydrate diet versus a low-fat diet for 12 months; both groups received regular dietary counselling throughout. No serious adverse events were reported. The incidence of minor adverse events was similar between groups, with the exception of headaches at 3 months, which were reported by more participants in the low-fat diet group (reported p = 0.030).
Foster et al. 120 compared low-carbohydrate and low-fat dietary interventions with the same group behavioural intervention weekly for 20 weeks, every other week for 20 weeks and then every other month over 24 months. All participants were given the same instructions for increasing their levels of physical activity.
Davis et al. 106 compared low-carbohydrate and low-fat diets for people with type 2 diabetes mellitus over 12 months. Participants initially underwent a 3- to 4-week pre-randomisation phase, during which participants were asked to self-monitor their diet and blood glucose levels. During the pre-randomisation phase, 31.8% discontinued the study. The low-carbohydrate diet was based on the Atkins diet and the low-fat diet was based on the DPP intervention. 205 Both groups received booklets detailing the carbohydrate or fat content of common goods and were given general recommendations to increase physical activity. All participants received six counselling sessions with a dietitian.
Iqbal et al. 129 randomised people with type 2 diabetes mellitus to a low-carbohydrate or low-fat diet. All participants received dietitian sessions, which were weekly for the first month and monthly thereafter for 24 months. All participants were given educational handouts specific to their diet and were encouraged to engage in ≥ 30 minutes of moderate physical activity at least five times per week. Two people in the low-carbohydrate group and three in the low-fat group died during the course of the trial. One participant in each group died of myocardial infarction. The authors stated that the cause of death for the other three participants was not available. One participant was diagnosed with breast cancer (group unknown). No severe hypoglycaemic episodes were reported.
Participants in the trial by Stern et al. 181 received a low-carbohydrate or low-fat diet with weekly counselling sessions for 4 weeks followed by 11 monthly sessions. Participants were given diet handouts. Two people in the low-carbohydrate group and four people in the low-fat group developed diabetes mellitus at 1 year (reported p > 0.2). One person on the low-carbohydrate diet was reported to have been hospitalised with non-cardiac chest pain. Two people in the low-carbohydrate group died: one died of complications of hyperosmolar coma 5 months into the study and another had severe ischaemic cardiomyopathy and died suddenly 10 months after study enrolment.
In an additional study by Soenen et al. ,177 a low-carbohydrate diet (5% of energy from carbohydrate) was compared with a normal-carbohydrate diet (35% energy from carbohydrate) as part of a factorial design to also investigate high- versus low-protein diets. The Soenen et al. 177 trial is also discussed in Higher-protein diets versus lower-protein diets. The authors concluded that weight loss and weight maintenance depend on protein but not on the low-carbohydrate component of the diet.
Meta-analyses
The results of the meta-analyses of the low-carbohydrate versus low-fat diets are presented in Table 13. The meta-analysis of weight loss favoured the low-carbohydrate intervention for both adjusted and unadjusted data, with evidence of a benefit from low-carbohydrate diets at 12 months only. Subgroup analyses were conducted for interventions with and without a specified energy goal for the low-fat diets (see Table 13). In the Bazzano et al. 89 trial, participants were not given a specified energy goal for the low-fat diet, whereas the other four trials had an energy goal. Both subgroup analyses favoured a low-carbohydrate diet, with the trial by Bazzano et al. ,89 with no energy restriction for the low-fat diet, having a much larger effect.
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 24 months | |
| Unadjusted |
Mean –1.16 (95% CI –2.13 to –0.19) p = 0.019 I2 = 57 Trials = 5(Favours low-carbohydrate diet) |
Mean –0.00 (95% CI –1.82 to 1.81) p = 0.996 I2 = 36 Trials = 2(Favours low-carbohydrate diet) |
| Adjusted |
Mean –1.03 (95% CI –1.96 to –0.10) p = 0.029 I2 = 61 Trials = 5(Favours low-carbohydrate diet) |
Mean –0.07 (95% CI –1.77 to 1.63) p = 0.935 I2 = 34 Trials = 2(Favours low-carbohydrate diet) |
| Subgroup analysis of low-carbohydrate vs. low-fat diets with no energy goal | ||
|
Mean –3.5 (95% CI –5.40 to –1.60) p < 0.001 I2 = N/A Trials = 1(Favours low-carbohydrate diet) |
||
| Subgroup analysis of low-carbohydrate vs. low-fat diets with a specified energy goal | ||
| Unadjusted |
Mean –0.33 (95% CI –1.47 to 0.80) p = 0.564 I2 = 0 Trials = 4 |
|
| Adjusted |
Mean –0.26 (95% CI –1.33 to 0.80) p = 0.631 I2 = 0 Trials = 4(Favours low-carbohydrate diet) |
|
Participants randomised to low-carbohydrate diets had lower triglyceride levels and higher HDL cholesterol concentrations after 12 months. Only increased HDL cholesterol and decreased diastolic blood pressure were present at 24 months. None of the other risk factor changes, including HbA1c and glucose levels, was different between the groups (Table 14).
| Risk factors | Meta-analysis of mean difference | |
|---|---|---|
| 12 months | 24 months | |
| Total cholesterol (mmol/l) |
Mean 0.14 (95% CI –0.02 to 0.30) p = 0.080 I2 = 0 Trials = 4(Favours low-fat diet) |
Mean 0.03 (95% CI –0.44 to 0.50) p = 0.901 I2 = N/A Trials = 1(Favours low-fat diet) |
| LDL cholesterol (mmol/l) |
Mean 0.05 (95% CI –0.06 to 0.15) p = 0.379 I2 = 0 Trials = 5(Favours low-fat diet) |
Mean 0.08 (95% CI –0.06 to 0.21) p = 0.277 I2 = 0 Trials = 2(Favours low-fat diet) |
| HDL cholesterol (mmol/l) |
Mean 0.10 (95% CI 0.07 to 0.14) p < 0.001 I2 = 13 Trials = 5(Favours low-carbohydrate diet) |
Mean 0.06 (95% CI 0.01 to 0.10) p = 0.025 I2 = 54 Trials = 2(Favours low-carbohydrate diet) |
| Triglycerides (mmol/l) |
Mean –0.16 (95% CI –0.26 to –0.07) p = 0.001 I2 = 0 Trials = 5(Favours low-carbohydrate diet) |
Mean –0.01 (95% CI –0.17 to 0.15) p = 0.898 I2 = 0 Trials = 2(Favours low-carbohydrate diet) |
| Systolic blood pressure (mmol/l) |
Mean –0.01 (95% CI –1.96 to 1.94) p = 0.991 I2 = 0 Trials = 5(Favours low-carbohydrate diet) |
Mean –0.85 (95% CI –4.10 to 2.40) p = 0.609 I2 = 38 Trials = 2(Favours low-carbohydrate diet) |
| Diastolic blood pressure (mmol/l) |
Mean –0.45 (95% CI –1.83 to 0.94) p = 0.527 I2 = 0 Trials = 5(Favours low-carbohydrate diet) |
Mean –2.33 (95% CI –4.39 to –0.26) p = 0.027 I2 = 0 Trials = 2(Favours low-carbohydrate diet) |
| HbA1c (%) |
Mean –0.19 (95% CI –0.52 to 0.13) p = 0.243 I2 = 49 Trials = 3(Favours low-carbohydrate diet) |
Mean 0.10 (95% CI –0.02 to 0.22) p = 0.099 I2 = N/A Trials = 1(Favours low-fat diet) |
| Glucose (mmol/l) |
Mean 0.12 (95% CI –0.06 to 0.29) p = 0.194 I2 = 0 Trials = 3(Favours low-fat diet) |
Mean 0.14 (95% CI –1.39 to 1.67) p = 0.858 I2 = N/A Trials = 1(Favours low-fat diet) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Participants in low-carbohydrate diet groups received, on average, 7.2 BCTs (range 3–12 BCTs) and those in comparator groups received 6.6 BCTs (range 3–10 BCTs). Three BCTs were used routinely, namely setting a behavioural goal, providing instruction on how to perform the behaviour and a respected person explaining the importance of diet/physical activity. Comparator group support varied between trials, with the least supported comparator receiving instructions, goal-setting and advice from a credible person versus the most extensively supported comparator group additionally receiving feedback on and a review of behaviour goals, self-monitoring and social support BCTs, as well as additions to the environment and information about health consequences. These results must be interpreted with caution as group support was generally poorly described in both arms; only one trial was rated as well described and another was rated as adequately described.
Higher-protein diets versus lower-protein diets
Six trials102,107,119,134,160,177 compared diets with higher protein contents and diets with lower protein contents. The diet energy from protein varied from 30%107,134,160 to 34%102 in the groups with higher-protein diets, compared with < 20% in the comparison groups. Flechtner-Mors et al. 119 prescribed 1.3 g/kg of protein to participants in the intervention group and 0.8 g/kg of protein to those in the control group. Soenen et al. 177 prescribed 1.2 g/kg of protein to participants in the intervention group and 0.8 g/kg of protein to those in the control group. All seven RCTs matched the energy prescription in groups.
Trial groups had mean BMIs ranging from 35.4 to 45.8 kg/m2. One trial recruited people with diabetes mellitus,134 one recruited participants with diabetes mellitus and microalbuminuria160 and another recruited participants with metabolic syndrome. 119 Brief details of the interventions are provided in Report Supplementary Material 5, Table E7.
Risk-of-bias assessment
Most trials (71.4%) were considered to have adequate randomisation sequence generation, although in 57.1% of trials it was unclear whether or not allocation concealment was adequate. Owing to the nature of the interventions, blinding of participants was not possible. Only two trials134,160 reported blinded outcome assessment, and one160 reported blinded personnel. Two trials107,119 were rated as being at risk of bias owing to author links with dietetic product manufacturers (see Report Supplementary Material 4, Figure E12).
Equity assessment
The trials did report some information for the majority of items on the Cochrane Public Health Equity assessment checklist. 61 Fewer than half reported the sociodemographic details of their participants. Similarly, most of the trials did not report information concerning diversity or disadvantage in their intervention design or delivery, nor did they discuss the sustainability of the interventions. The trials also did not report process measures or details of any fidelity checks (see Report Supplementary Material 4, Figure E13).
Descriptions of the trials
Dalle Grave et al. 102 randomised participants (mean BMI 45.6 kg/m2) to energy-restricted diets (both 1200–1500 kcal/day, with 20% of energy from fat): a higher-protein diet (34% of energy from protein) or a lower-protein diet (17% of energy from protein). The first stage was 3 weeks of inpatient treatment and the second stage was 48 weeks of outpatient treatment. All participants received a CBT manual. Participants in both groups received 55 sessions that included weekly CBT group sessions, aerobic exercise sessions and callisthenic sessions. Psychosocial outcomes (i.e. body image dissatisfaction, binge eating, depression and quality of life) were reported as not statistically significantly different between the groups. No adverse events were reported.
Delbridge et al. 107 investigated a 3-month intensive weight-loss period with a VLCD (Optifast, described in Very low-calorie diets) for all participants (mean BMI of 39 kg/m2). For the 12-month weight maintenance phase, participants were randomised to diets with either 30% or 15% of energy being from protein. Participants of both groups were recommended a low glycaemic index diet with fat intake providing < 30% of energy. Twelve monthly individual face-to-face sessions were provided.
Flechtner-Mors et al. 119 recruited participants meeting at least three of five criteria for metabolic syndrome. Participants (mean BMI of 36 kg/m2) were randomised to low-fat diets with either 30% or 15% of energy from protein. In both diets, 30% of energy was from fat and there was a 500-kcal/day deficit. In the first 3 months, participants in the higher-protein group consumed two protein-enriched meal replacements, one conventional meal and two snacks (either a protein bar or a low-fat curd with fruit). After these 3 months, participants consumed one protein-enriched meal replacement, two meals and two snacks. The conventional diet group consumed three meals and two snacks with no meal replacements for the first 3 months. After these 3 months, participants consumed one standard meal replacement, two meals and two snacks each day. Participants received 15 sessions in groups and individually. After 12 months, 64.5% of the participants in the higher-protein group and 34.8% in the lower-protein group no longer met three essential criteria for metabolic syndrome. The difference between the groups was statistically significant (reported p < 0.05). It was reported that adverse events could not be related to the diets.
Pedersen et al. 160 recruited participants with type 2 diabetes mellitus, microalbuminuria and an eGFR of > 40 ml/minute/1.73 m2 (mean BMI of 36 kg/m2). After a run-in phase, volunteers were randomly assigned to either a higher-protein (30% of energy) or a lower-protein (20% of energy) low-fat (30% of energy) weight-loss diet for 12 months. The energy content was approximately 1430 kcal/day. Participants attended a visit every 2 weeks during the 16-week weight-loss phase and then every month. Participants were provided with diet information booklets and a sample daily meal plan. Weight loss improved renal function, but there was no reported statistically significant difference between the diets.
Krebs et al. 134 recruited people with type 2 diabetes mellitus (mean BMI of 36.6 kg/m2), who were randomised to either a 30% protein and 30% fat energy diet or a 15% protein and 30% fat energy diet. Individuals participated in group sessions led by dietitians. Energy was reduced by approximately 500 kcal/day using an individualised dietary prescription based on estimated energy requirements. Portion charts and sample diet plans were provided. There was no difference in the number of adverse renal events between the groups. Two deaths (one in the higher-protein group and one in the lower-protein group) and health problems (two in the higher-protein group and three in the lower-protein group) were reported by 6 months; one more death (in the higher-protein group) and two more health problems (in the lower-protein group) were reported by 24 months.
Soenen et al. 177 recruited from an outpatient WMP and randomised participants to four groups: (1) a high-protein, low-carbohydrate diet (60% of energy from protein for weight loss and 30% of energy from protein for weight maintenance), (2) a high-protein, normal-carbohydrate diet (60% of energy from protein for weight loss and 30% of energy from protein for weight maintenance), (3) a normal-protein, low-carbohydrate diet (30% of energy from protein for weight loss and 15% of energy from protein for weight maintenance) and (4) a normal-protein, normal-carbohydrate diet (30% of energy from protein for weight loss and 15% of energy from protein for weight maintenance). For the weight-loss period, participants were prescribed 33% of their energy requirement. Participants had 19 sessions over 12 months.
Meta-analyses
Higher-protein diets produced favourable changes at 12 months102,107,119,134,160,177 but not in one trial at 24 months134 (Table 15). Consistent beneficial changes in risk factors at 12 and 24 months were not seen (Table 16).
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 24 months | |
| Unadjusted |
Mean –1.84 (95% CI –2.94 to –0.73) p = 0.001 I2 = 15 Trials = 6(Favours higher protein) |
Mean 2.10 (95% CI 0.43 to 3.77) p = 0.014 I2 = N/A Trials = 1(Favours lower protein) |
| Adjusted |
Mean –0.91 (95% CI –1.83 to 0.00) p = 0.051 I2 = 0 Trials = 6(Favours higher protein) |
Mean 1.53 (95% CI 0.21 to 2.85) p = 0.023 I2 = N/A Trials = 1(Favours lower protein) |
| Risk factors | Meta-analysis of mean difference | |
|---|---|---|
| 12 months | 24 months | |
| Total cholesterol (mmol/l) |
Mean 0.04 (95% CI –0.11 to 0.18) p = 0.613 I2 = 0 Trials = 6(Favours lower protein) |
Mean –0.07 (95% CI –0.32 to 0.18) p = 0.579 I2 = N/A Trials = 1(Favours higher protein) |
| LDL cholesterol (mmol/l) |
Mean –0.01 (95% CI –0.13 to 0.11) p = 0.865 I2 = 0 Trials = 5(Favours higher protein) |
Mean 0.03 (95% CI –0.14 to 0.20) p = 0.728 I2 = N/A Trials = 1(Favours lower protein) |
| HDL cholesterol (mmol/l) |
Mean 0.02 (95% CI –0.02 to 0.06) p = 0.279 I2 = 0 Trials = 6(Favours higher protein) |
Mean –0.03 (95% CI –0.10 to 0.04) p = 0.375 I2 = N/A Trials = 1(Favours lower protein) |
| Triglycerides (mmol/l) |
Mean –0.05 (95% CI –0.18 to 0.08) p = 0.465 I2 = 61 Trials = 6(Favours higher protein) |
Mean –0.03 (95% CI –0.25 to 0.19) p = 0.789 I2 = N/A Trials = 1(Favours higher protein) |
| Systolic blood pressure (mmHg) |
Mean –0.25 (95% CI –2.32 to 1.83) p = 0.816 I2 = 4 Trials = 5(Favours higher protein) |
Mean 1.20 (95% CI –1.70 to 4.10) p = 0.418 I2 = N/A Trials = 1(Favours lower protein) |
| Diastolic blood pressure (mmHg) |
Mean –1.04 (95% CI –2.34 to 0.27) p = 0.119 I2 = 62 Trials = 75(Favours higher protein) |
Mean 0.10 (95% CI –1.80 to 2.00) p = 0.918 I2 = N/A Trials = 1(Favours lower protein) |
| HbA1c (%) |
Mean 0.10 (95% CI –0.01 to 0.21) p = 0.081 I2 = 0 Trials = 3(Favours lower protein) |
Mean 0.00 (95% CI –0.62 to 0.62) p = 1.000 I2 = N/A Trials = 1(Favours lower protein) |
| Glucose (mmol/l) |
Mean 0.22 (95% CI –0.10 to 0.54) p = 0.171 I2 = 0 Trials = 5(Favours lower protein) |
Mean 0.30 (95% CI –0.50 to 1.10) p = 0.461 I2 = N/A Trials = 1(Favours lower protein) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Only one trial included supervised physical activity,102 and one provided physical activity advice. 107 The higher-protein diet groups received, on average, 7.2 BCTs (range 3–12 BCTs) and the comparator groups received, on average, 6.8 BCTs (range 4–12 BCTs). Two BCTs were used routinely and provided to both arms, namely setting a behavioural goal, and providing instruction on how to perform the behaviour. Dietary groups in the same trial largely received the same BCTs; however, in Soenen et al. ,177 the high-protein, normal carbohydrate group received three more BCTs than the comparator group (normal protein, normal carbohydrate): self-monitoring of outcomes, habit formation and information from a credible source. These results must be interpreted with caution, as group support was generally poorly described in both arms: only one trial was rated as well described134 and one was rated as adequately described. 102
Randomised controlled trials of meal replacements for weight loss and weight maintenance
Eight trials evaluated the use of meal replacements: four trials evaluated their use for weight loss98,119,168,175 and four evaluated their use for maintaining weight lost. 82,143,172,173 The comparisons referred to here are for equicaloric diets. There is considerable overlap with the previous section, Very low-calorie diets, so we focus here on the results for weight (see Report Supplementary Material 5, Table E8).
Descriptions of the trials
Cheskin et al. 98 randomised people with type 2 diabetes mellitus to receive a standard diet or a portion-controlled diet of Medifast® Plus for Diabetics (Medifast, Inc., Owings Mills, MD, USA) meal replacements. After 34 weeks, the standard diet group continued their diet at maintenance energy levels, whereas the portion-controlled participants were rerandomised to 26 weeks of meal replacements followed by the maintenance standard diet, or vice versa. The mean BMI was 36 kg/m2.
The trials by Reichard et al. 168 and Shikany et al. 175 randomised people to follow a programme with meal replacements [Modified Stop Light and Medifast 5 & 1 Plan (Medifast, Inc., Owings Mills, MD, USA), respectively] or a food-based diet. The group mean BMI ranged from 40.4 to 45.6 kg/m2.
The Flechtner-Mors et al. 119 trial is discussed in Higher-protein diets versus lower-protein diets.
Participants in all weight maintenance trials82,143,172,173 followed a VLCD for an initial weight-loss phase and were then randomised to weight maintenance conditions, in which VLCD products were used as meal replacements. These trials are discussed in Very low-calorie diets.
Meta-analyses
The meta-analyses for all studies and subgroups of studies reporting weight loss and weight maintenance are presented in Table 17. The analyses favoured meal replacements at 12 months but not thereafter.
| Type of analysis | Mean weight change (kg) | |||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | 36 months | |
| Unadjusted | ||||
| All studies |
Mean –2.75 (95% CI –4.01 to –1.48) p < 0.0010 I2 = 53 Trials = 7(Favours meal replacement) |
Mean 3.02 (95% CI –0.45 to 6.48) p = 0.088 I2 = 0 Trials = 2(Favours diet) |
Mean 0.45 (95% CI –1.61 to 2.52) p = 0.666 I2 = 39 Trials = 2(Favours diet) |
Mean 1.11 (95% CI –1.21 to 3.42) p = 0.348 I2 = 88 Trials = 1(Favours diet) |
| Weight-loss subgroup |
Mean –4.23 (95% CI –5.94 to –2.52) p < 0.001 I2 = 14 Trials = 4(Favours meal replacement) |
Mean 1.50 (95% CI –5.13 to 8.13) p = 0.658 I2 = N/A Trials = 1(Favours diet) |
||
| Weight maintenance subgroup |
Mean –0.95 (95% CI –2.83 to 0.93) p < 0.001 I2 = 24 Trials = 3(Favours meal replacement) |
Mean 3.59 (95% CI –0.47 to 7.65) p = 0.083 I2 = 0 Trials = 1(Favours diet) |
||
| Adjusted | ||||
| All studies |
Mean –1.80 (95% CI –2.88 to –0.71) p = 0.001 I2 = 17 Trials = 7(Favours meal replacement) |
Mean 1.66 (95% CI –0.01 to 3.33) p = 0.051 I2 = 80 Trials = 2(Favours diet) |
Mean 0.15 (95% CI –1.73 to 2.02) p = 0.879 I2 = 45 Trials = 2(Favours diet) |
Mean 1.11 (95% CI –1.21 to 3.42) p = 0.348 I2 = 88 Trials = 1(Favours diet) |
| Weight-loss subgroup |
Mean –2.12 (95% CI –3.52 to –0.72) p = 0.003 I2 = 0 Trials = 4(Favours meal replacement) |
Mean –0.70 (95% CI –3.03 to 1.64) p = 0.559 I2 = N/A Trials = 1(Favours meal replacement) |
||
| Weight maintenance subgroup |
Mean –1.31 (95% CI –3.04 to 0.42) p = 0.138 I2 = 37 Trials = 3(Favours meal replacement) |
Mean 4.13 (95% CI 1.74 to 6.52) p < 0.001 I2 = 54 Trials = 1(Favours diet) |
||
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Usually, each trial gave the same number and type of BCTs to both arms, with the exception of Shikany et al. ,175 which reported participants in the meal replacement group receiving 15 more BCTs than those in the diet group. Weight-loss arms were reported to receive fewer BCTs than weight maintenance arms. The meal replacement arms received an average of 9.5 BCTs, and the dietary comparators received an average of 5.25, compared with an average of 13.5 in the meal replacement arms and 13 in the diet arms of the weight maintenance trials. In all trials, instruction was given on how to perform a behaviour for all arms, as well as information from a credible source in all but the dietary arm of Shikany et al. 175 Other commonly used BCTs were goal-setting and adding objects to the environment. For weight loss, the number of BCTs given to the meal replacement arms varied greatly in number and type, ranging from 4 to 18 BCTs, and similarly in the dietary arms, ranging from 3 to 11 BCTs between trials. For weight maintenance, the use of BCTs was similarly varied, and ranged from 6 to 32 BCTs in the meal replacement arms and from 4 to 32 in the dietary arms. Only one trial aimed at weight loss and two aimed at weight maintenance reported providing physical activity advice to participants. The BCT use in the trials was well described in Shikany et al. ,175 adequately described in Lowe et al. 143 and poorly described in all other trials.
Other diet studies
Descriptions of the trials
Participants with type 2 diabetes mellitus were randomised to receive a high-monounsaturated-fat diet (40% fat) or a high-carbohydrate diet (25% fat), with a deficit of 200–300 kcal/day, for 1 year in the trial by Brehm et al. 94 The mean participant BMI was 35.9 kg/m2 (SD 3.3 kg/m2). At 12 months, weight loss was not different between the groups, as shown in Table 18.
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) | |||
|---|---|---|---|---|---|
| High-monounsaturated-fat diet | High-carbohydrate diet | Effect size (95% CI); p-value | |||
| Brehm 200994 (trial of high MUFA vs. high carbohydrate) | 12 | –4.00 (5.25) [n = 43] | –3.80 (4.33) [n = 52] |
–0.20 (–2.13 to 1.73); p = 0.839 (Favours high monosaturated fat) |
|
| Low glycaemic load diet | Low-fat diet | Effect size (95% CI); p-value | |||
| Ebbeling 2007115 (trial of a low glycaemic load diet vs. a low-fat diet) | 12 | –2.85 (6.41) [n = 36] | –2.36 (6.16) [n = 37] |
–0.49 (–3.37 to 2.39); p = 0.739 (Favours low glycaemic load) |
|
| Vegetarian diabetic diet | Conventional diabetic diet | Effect size (95% CI); p-value | |||
| Kahleova 2014130 (trial of a vegetarian diabetic diet vs. a conventional diabetic diet) | 12 (unadjusted analysis) | –4.50 (7.19) [n = 23] | –1.70 (6.40) [n = 24] |
–2.80 (–6.69 to 1.09); p = 0.158 (Favours vegetarian diabetic diet) |
|
| 12 (adjusted analysis) | –2.80 (6.71) [n = 37] | –1.10 (6.23) [n = 37] |
–1.69 (–4.64 to 1.25); p = 0.260 (Favours vegetarian diabetic diet) |
||
| 18 (unadjusted analysis) | –4.54 (7.20) [n = 21] | –2.19 (6.53) [n = 23] |
–2.35 (–6.41 to 1.71); p = 0.256 (Favours vegetarian diabetic diet) |
||
| 18 (adjusted analysis) | –2.58 (6.64) [n = 37] | –1.36 (6.30) [n = 37] |
–1.22 (–4.17 to 1.73); p = 0.419 (Favours vegetarian diabetic diet) |
||
| Formula low-calorie diet | High-protein, low-fat diet | Effect size (95% CI); p-value | |||
| Khoo 2011133 (trial of a formula low-calorie diet vs. a high-protein, low-fat diet) | 12 | –9.60 (8.63) [n = 9] | –9.00 (8.46) [n = 7] |
–0.60 (–9.05 to 7.85); p = 0.889 (Favours formula low-calorie diet) |
|
| 1000 kcal/day | 1500 kcal/day | Effect size (95% CI); p-value | |||
| Nackers 2013156 (trial of a 1000-kcal/day diet vs. a 1500-kcal/day diet) | 12 | –8.52 (9.43) [n = 65] | –5.84 (8.60) [n = 60] |
–2.68 (–5.85 to 0.49); p = 0.098 (Favours 1000 kcal/day) |
|
| CAL + FAT | CAL | Effect size (95% CI); p-value | |||
| Pascale 1995159 (trial of a calorie plus fat restriction diet vs. a calorie restriction diet only) | 12 | –4.06 (7.84) [n = 31] | –2.10 (5.70) [n = 29] |
–1.96 (–5.45 to 1.53); p = 0.271 (Favours CAL + FAT) |
|
| Lower carbohydrate | Lower fat | Usual care | Test of differences | ||
| Rock 2014170 (trial of a lower carbohydrate diet vs. a lower fat diet vs. usual care) | 12 | –9.70 (8.66) [n = 77] | –7.70 (8.09) [n = 74] | –2.70 (6.68) [n = 76] |
p < 0.001 (Lower carbohydrate or fat has increased weight loss) |
Ebbeling et al. 115 randomised young adults aged 18–35 years with group mean BMIs of 36.6–37.2 kg/m2 to a low glycaemic load (35% of energy from fat) or a 20% fat energy diet, with a deficit of 250–500 kcal/day. Both groups attended group workshops with one private counselling session and five motivational telephone calls with a dietitian over a 6-month intensive phase. As shown in Table 18, there was no difference in weight between the glycaemic load diet and the low-fat diet.
Participants with type 2 diabetes mellitus were randomised to receive a vegetarian or conventional diet with similar caloric restriction (500-kcal/day deficit) for 24 weeks. 130 All participants received instruction in nutrition and cooking and in aerobic exercise. Group mean BMIs ranged from 35.0 kg/m2 (SD 4.6 kg/m2) to 35.1 kg/m2 (SD 6.1 kg/m2). The vegetarian diet produced better weight loss at 12 and 18 months when data are both adjusted and unadjusted, but differences between groups were estimated with some uncertainty, as shown in Table 18.
Khoo et al. 133 randomised men with type 2 diabetes mellitus to a high-protein, low-fat, 600-kcal/day deficit diet for 12 months or to receive the same diet but with an initial 900-kcal/day formula diet (KicStart™; Pharmacy Health Solutions, Sydney, NSW, Australia) for 8 weeks. Mean BMIs of groups ranged from 35.1 kg/m2 (SD 4.3 kg/m2) to 35.6 kg/m2 (SD 4.8 kg/m2). At 12 months, there was no difference between groups, as shown in Table 18. Erectile function, sexual desire and urinary symptoms improved similarly in both groups.
Nackers et al. 156 randomised women to receive 1000- or 1500-kcal/day balanced diets, based on US guidelines. Both groups also received a standard behavioural lifestyle intervention over 12 months. The mean BMI of participants was 37.8 kg/m2 (SD 3.9 kg/m2). The 1000-kcal/day diet produced better weight loss at 12 months, but the difference between groups showed some uncertainty, as shown in Table 18.
Women with type 2 diabetes mellitus and women with obesity and a family history of type 2 diabetes mellitus (FH participants) were separately randomised to receive a calorie restriction diet of 1000–1500 kcal/day (CAL) or the same calorie restriction and < 30% fat energy (CAL + FAT) in the Pascale et al. 159 trial. The mean BMI in the groups of participants with type 2 diabetes mellitus ranged from 36.3 kg/m2 (SD 4.2 kg/m2) to 36.4 kg/m2 (SD 4.7 kg/m2). The mean group BMI of FH participants ranged from 35.0 kg/m2 (SD 4.4 kg/m2) to 36.1 kg/m2 (SD 5.6 kg/m2) in the CAL + FAT group. Weight loss was greater in the CAL + FAT group at 12 months, but the difference between groups showed some uncertainty (see Table 18).
Rock et al. 170 randomised participants with type 2 diabetes mellitus to a higher-carbohydrate and lower-fat diet plan (LF diet), to a lower-carbohydrate and higher-fat diet plan (LC diet) or to usual care. Both plans were provided by Jenny Craig, Inc. (Carlsbad, CA, USA) as pre-packaged foods with weight-loss counselling. Mean group BMIs ranged from 36.2 kg/m2 (SD 4.3 kg/m2) to 36.3 kg/m2 (SD 4.4 kg/m2). Data at 12 months are shown in Table 18. Weight loss was better for the lower-carbohydrate and lower-fat groups than for the controls, but did not differ between diet plans.
Orlistat
Twelve trials86,95,96,105,117,127,131,135,152,176,183,185 evaluated the effect of the drug orlistat on weight loss. More details on three trials from the UK are provided in Chapter 4. 95,96,117 The trials mainly recruited participants in their forties and fifties; the youngest reported mean age was 40 years135 and the oldest was 58 years. 131 The trials mainly recruited women. Only one trial recruited more men than women, with 48% of participants in the Miles et al. 152 trial being women. The lowest mean BMI was 35.2 kg/m2,152 and the highest was 38 kg/m2. 183 Half of the trials recruited participants with type 2 diabetes mellitus,86,95,105,131,152,183 and four trials included participants based on cardiovascular risk. 86,95,96,183
Most trials provided a 600-kcal/day deficit diet with no more than 30% of calories from fat, and physical activity advice. In some trials the prescription for calorie intake was sometimes reported as being adjusted later, in accordance with weight lost.
Quality assessment
Risk-of-bias assessment
The quality of randomisation sequence and allocation concealment were unclear in the majority of trials (83.3% and 91.7%, respectively). Most trials (83.3%) reported attempting to blind participants but were less clear on blinding personnel/health professionals delivering the intervention and outcome assessors. All trials were rated as being at high risk of bias owing to author links with companies that manufacture orlistat.
Eight trials96,105,117,127,135,152,176,183 included single-blind, placebo lead-in phases prior to randomisation of study participants, ranging from 2 to 4 weeks. The percentage of participants who did not continue to the randomised phase ranged from 4%183 to 28%. 96 Common reasons for not continuing included withdrawal of consent, loss to follow-up and adverse events. In two trials,105,117 only participants who achieved high pill-taking compliance (≥ 70%) were reported to be eligible for the randomised element of the trial. Participants who experienced difficulty with taking oral medication were therefore ineligible to be included in the randomised phases, or could have been more likely to withdraw prior to randomisation, thus limiting the generalisability of these trial results. The summary graph of the risk-of-bias assessments for the orlistat RCTs is presented in Report Supplementary Material 4, Figure E14.
Equity assessment
The trials either did not report or were considered to have not considered the majority of the equity assessment items, with the exception of unintended effects and harms, which were reported by all but one trial. 183 None of the trials reported sociodemographic differences between trial completers and participants who withdrew or were excluded, although all but one trial185 reported some baseline PROGRESS data, usually sex. Only one trial185 was considered to have excluded a specific population by excluding participants with diabetes mellitus and ongoing active CVD from the trial. As with the risk-of-bias assessment, all trials were considered to have a potential risk of author conflict. The summary of the equity assessment is presented in Report Supplementary Material 4, Figure E15.
Descriptions of the trials
In a 12-month trial of 120 mg of orlistat three times daily or placebo, Bakris et al. 86 reported no deaths in either group; 14 out of 267 participants (5.2%) in the control group and 15 out of 265 (5.7%) in the orlistat group reported serious adverse events requiring hospitalisation.
In a 12-month UK trial of 120 mg of orlistat three times daily or placebo, Broom et al. 96 reported serious adverse events in 13 out of 265 orlistat participants and in 17 out of 266 placebo participants, and one death attributable to cancer was reported in the orlistat group. In a second UK study, Broom et al. 95 randomised people with obesity and hypercholesterolaemia to receive 120 mg of orlistat or placebo three times daily for 24 weeks. Participants who completed the 24-week, double-blind phase were entered into a 28-week open-label phase and received 120 mg of orlistat three times daily and continued with the hypocaloric diet.
Davidson et al. 105 evaluated placebo or 120 mg of orlistat three times daily for 12 months. Participants receiving drug treatment for type 2 diabetes mellitus were not eligible to participate in the trial. Participants who had ≥ 70% compliance then entered a weight-maintenance phase for a further year. Participants treated with orlistat were rerandomised to receive placebo, 60 mg of orlistat or 120 mg of orlistat three times daily for the weight maintenance phase. Second year weight regain was reported to be significantly less with 120 mg of orlistat than with 60 mg of orlistat or placebo (reported p < 0.001). Participants who were originally randomised to receive placebo continued to receive placebo for the final year of the trial. One orlistat-treated woman was diagnosed with breast cancer during the first year and 1 out of 197 placebo-treated women and 3 out of 548 of women treated with 120 mg of orlistat were diagnosed with breast cancer during year 2.
Finer et al. 117 randomised participants to 120 mg of orlistat or placebo three times daily for 12 months in a UK trial.
Hauptman et al. 127 evaluated placebo, 60 mg of orlistat or 120 mg of orlistat treatment three times per day for 24 months. On four occasions during the first 12 months, participants viewed videos of behaviour modification techniques, and at four points during the second year, they followed the Live for Life programme (Johnson & Johnson Health Management Inc., New Brunswick, NJ, USA). With the exception of GI events, the incidence and types of adverse events were similar in all groups.
In the trial by Krempf et al. ,135 participants were randomised to 120 mg of orlistat or placebo three times daily for 18 months. People with type 1 or 2 diabetes mellitus were excluded from trial entry. Five serious adverse events were reported in the orlistat group and four were reported in the placebo group.
A 1-year trial by Miles et al. 152 allocated people with metformin-treated type 2 diabetes mellitus to either placebo or 120 mg of orlistat three times per day for 12 months. At 12 months, fasting serum glucose was reported as significantly decreased with orlistat compared with placebo (reported p = 0.001). Significantly more orlistat-treated participants than placebo-treated participants were reported to have discontinued treatment owing to an adverse event (reported p < 0.05), and more placebo group participants withdrew overall (reported p < 0.05).
Participants in the Sjöström et al. 176 trial were randomised to placebo or 120 mg of orlistat three times a day for 12 months. In year 2, participants were reassigned orlistat or placebo for a further 12 months (see Weight maintenance interventions without a very low-calorie diet weight-loss phase). The authors reported that, during year 2, participants on orlistat regained half as much weight as those who were changed to placebo (reported p < 0.001). In a subgroup of the trial, at 12 months, no significant difference was reported between groups for total body, forearm or lumbar spine bone mineral density. Only the urinary hydroxyproline-to-creatinine ratio was increased with orlistat compared with placebo, suggesting increased bone breakdown. Vitamin D status after 12 months did not differ between groups. Serious adverse events were reported by 24 participants in the placebo group and by 25 participants in the orlistat group during the first 12 months. One GI neoplasm occurred in a participant treated with placebo for 2 years.
The Swinburn et al. 183 trial sought to determine whether or not orlistat treatment could reduce the estimated absolute 10-year risk of having a CVD event versus placebo in people with type 2 diabetes mellitus in a trial of placebo or 120 mg of orlistat three times a day for 12 months. The authors reported that the baseline median 10-year risk of a CVD event was moderately low, at about 10% [0.089 (range 0.008–0.416) for the orlistat group and 0.105 (range 0.017–0.353) for the placebo group]. At 12 months, it was reported that, although there was no difference in the 10-year CVD risk between groups, the orlistat group had significant improvements in individual CVD risk factors compared with the placebo group. The orlistat group was also reported to have a significantly better score on the SF-36 vitality domain than the placebo group at 12 months (reported p = 0.006). Serious adverse events were reported for 9.4% of orlistat participants and 7.1% of placebo participants. The authors did not describe the nature of these events.
Torgerson et al. 185 randomised people with normal or impaired glucose tolerance to 120 mg of orlistat or placebo three times daily. Authors reported that at 4 years the cumulative incidence of diabetes mellitus was 9.0% with placebo and 6.2% with orlistat, corresponding to a risk reduction of 37.3% (reported p = 0.0032).
Meta-analyses
Table 19 shows the meta-analysis of 120 mg of orlistat versus placebo. For both unadjusted and adjusted data, there is evidence of a benefit favouring orlistat for weight loss at all time points. The results of the analysis of 60 mg of orlistat versus placebo, which also significantly favours orlistat for weight loss, are shown in Table 20. Table 21 shows the results of the comparison of 120 mg of orlistat with 60 mg of orlistat and showed no evidence of a difference in effect.
| Type of analysis | Mean weight change (kg) | |||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | 48 months | |
| Unadjusted |
Mean –3.41 (95% CI –3.72 to –3.10) p < 0.001 I2 = 62 Trials = 12(Favours orlistat) |
Mean –3.06 (95% CI –4.12 to –2.00) p < 0.001 I2 = 0 Trials = 2(Favours orlistat) |
Mean –3.40 (95% CI –4.72 to –2.09) p < 0.001 I2 = 0 Trials = 2(Favours orlistat) |
Mean –2.20 (95% CI –2.65 to –1.75) p < 0.001 I2 = N/A Trials = 1(Favours orlistat) |
| Adjusted |
Mean –3.43 (95% CI –3.60 to –3.01) p < 0.001 I2 = 69 Trials = 12(Favours orlistat) |
Mean –3.06 (95% CI –4.12 to –2.00) p < 0.001 I2 = 0 Trials = 2(Favours orlistat) |
Mean –3.40 (95% CI –4.72 to –2.09) p < 0.001 I2 = 0 Trials = 2(Favours orlistat) |
Mean –2.20 (95% CI –2.65 to –1.75) p < 0.001 I2 = N/A Trials = 1(Favours orlistat) |
| Study (first author and year) | Time point (months) | Weight change (kg), mean (SD) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| 60 mg of orlistat | Placebo | |||
| Hauptman 2000127 | 12 | –7.08 (7.88) [n = 213] | –4.14 (8.15) [n = 212] | –2.94 (–1.84 to –4.04); p < 0.001 |
| 18 | –5.78 (7.59) [n = 213] | –2.93 (8.3) [n = 212] | –2.85 (–1.98 to –3.72); p < 0.001 | |
| 24 | –4.46 (8.90) [n = 213] | –1.65 (9.03) [n = 212] | –2.81 (–2.17 to –3.45); p < 0.001 | |
| Study (first author and year) | Time point (months) | Weight change (kg), mean (SD) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| 120 mg of orlistat | 60 mg of orlistat | |||
| Hauptman 2000127 | 12 | –7.94 (8.26) [n = 210] | –7.08 (7.88) [n = 213] | –0.86 (–2.40 to 0.68); p = 0.273 |
| 18 | –6.22 (8.98) [n = 210] | –5.78 (7.59) [n = 213] | –0.44 (–2.03 to 1.15); p = 0.587 | |
| 24 | –5.02 (10.58) [n = 210] | –4.46 (8.90) [n = 213] | –0.56 (–2.42 to 1.30); p = 0.556 | |
For all trials, the numbers of reported GI events were greater for participants treated with orlistat than for those treated with placebo. Table 22 presents the reported incidence of GI events for orlistat and placebo groups for each of the trials.
| Study (first author and year) | Group, reported incidence (%) | ||
|---|---|---|---|
| Placebo | 60 mg of orlistat three times daily | 120 mg of orlistat three times daily | |
| Bakris 200286 | 43.6 | 72.5 | |
| Broom 200296 | 47 | 63 | |
| Davidson 1999105 | 59 | 79a | |
| Finer 2000117 | 56.4 | 82.1 | |
| Hauptman 2000127 | 59 | 72 | 79 |
| Krempf 2003135 | 36.3 | 63.3 | |
| Miles 2002152 | 62 | 83 | |
| Sjöström 1998176 | 10; highest reported adverse event in year 1 | 31; highest reported adverse event in year 1 | |
| Swinburn 2005183 | 60.4 | 82.4 | |
Orlistat treatment significantly improved all risk factors apart from triglyceride levels and HDL cholesterol at 12 months, with HDL cholesterol showing a fall (Table 23). At 24 and 48 months, risk factors, apart from HDL cholesterol, were still favoured by orlistat but with greater uncertainty around most estimates.
| Risk factors | Mean difference | ||
|---|---|---|---|
| 12 months | 24 months | 48 months | |
| Total cholesterol (mmol/l) |
Mean –0.31 (95% CI –0.38 to –0.25) p < 0.001 I2 = 0 Trials = 9(Favours orlistat) |
Mean –0.25 (95% CI –0.39 to –0.10) p = 0.001 I2 = 42 Trials = 3(Favours orlistat) |
|
| LDL cholesterol (mmol/l) |
Mean –0.26 (95% CI –0.31 to –0.21) p < 0.001 I2 = 0 Trials = 9(Favours orlistat) |
Mean –0.23 (95% CI –0.32 to –0.13) p < 0.001 I2 = 68 Trials = 3(Favours orlistat) |
|
| HDL cholesterol (mmol/l) |
Mean –0.03 (95% CI –0.04 to –0.01) p = 0.003 I2 = 0 Trials = 7(Favours placebo) |
Mean –0.01 (95% CI –0.05 to 0.03) p = 0.619 I2 = 0 Trials = 3(Favours placebo) |
|
| Triglycerides (mmol/l) |
Mean –0.01 (95% CI –0.08 to 0.06) p = 0.771 I2 = 80 Trials = 6(Favours orlistat) |
Mean –0.04 (95% CI –0.16 to 0.09) p = 0.566 I2 = 90 Trials = 3(Favours orlistat) |
|
| Systolic blood pressure (mmHg) |
Mean –2.14 (95% CI –2.77 to –1.52) p < 0.001 I2 = 0 Trials = 9(Favours orlistat) |
Mean –1.38 (95% CI –3.29 to 0.54) p = 0.158 I2 = 0 Trials = 2(Favours orlistat) |
Mean –1.50 (95% CI –2.85 to –0.15) p = 0.029 I2 = N/A Trials = 1(Favours orlistat) |
| Diastolic blood pressure (mmHg) |
Mean –1.66 (95% CI –2.07 to –1.24) p < 0.001 I2 = 34 Trials = 8(Favours orlistat) |
Mean –2.40 (95% CI –3.65 to –1.15) p < 0.001 I2 = 32 Trials = 2(Favours orlistat) |
Mean –0.70 (95% CI –1.58 to 0.18) p = 0.120 I2 = N/A Trials = 1(Favours orlistat) |
| HbA1c (%) |
Mean –0.25 (95% CI –0.35 to –0.15) p < 0.001 I2 = 12 Trials = 3(Favours orlistat) |
||
| Glucose (mmol/l) |
Mean –0.34 (95% CI –0.49 to –0.18) p < 0.001 I2 = 61 Trials = 8(Favours orlistat) |
Mean –0.10 (95% CI –0.63 to 0.42) p = 0.702 I2 = 0 Trials = 2(Favours orlistat) |
Mean –0.10 (95% CI –0.47 to 0.27) p = 0.597 I2 = N/A Trials = 1(Favours orlistat) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
The WMP component reported physical activity advice in 8 out of the 12 trials, with no trial providing supervised physical activity. The orlistat groups received, on average, 5.1 BCTs (range 3–8 BCTs) and the comparator groups received, on average, 4.2 BCTs (range 2–7 BCTs) targeting behaviours. Setting a behavioural goal was used in both arms of all trials except one,152 and the same BCTs were given to both arms in all trials with the exception of pharmacological support. These results must be interpreted with caution as behavioural support was described poorly in both arms of all trials.
Other orlistat trials
Orlistat trials are also covered in Chapter 3, Weight maintenance interventions after very low-calorie diets and Orlistat weight maintenance trials.
Descriptions of the trials
Mexican American women in the Poston et al. 165 trial were randomised to receive a 1-year culturally tailored lifestyle WMP with 120 mg of orlistat three times a day or a wait list control group. At 12 months, weight loss was greater in the orlistat group. Data are provided in Table 24.
| Study (first author and year) | Time point (months) | Mean (SD) weight change | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| 120 mg of orlistat plus lifestyle intervention | Control | |||
| Poston 2003165 | 12 | –5.60 (7.50) [n = 56] | –0.30 (6.00) [n = 52] |
–5.30 (–7.87 to –2.73); p < 0.001 (Favours orlistat) |
Trials examining variations in components of weight-loss interventions
Group programmes versus individual programmes
Six trials examined the effects of group delivery compared with individual delivery of interventions: four103,157,192,196 trials focused on weight loss and two137,140 focused on weight maintenance. A brief description of the trial interventions is provided in Report Supplementary Material 5, Table E9.
Descriptions of the weight-loss trials
Participants in the trial conducted by Damschroder et al. 103 were randomised to receive a standard group-based weight-loss programme or to a lifestyle intervention [Aspiring for Lifelong Health (ASPIRE)], which was delivered either as in-person group sessions (ASPIRE-Group) or to individuals by telephone (ASPIRE-Phone) over 12 months. The mean BMI was 36.5 kg/m2 (range 25.2–63.0 kg/m2).
Nilsen et al. 157 compared a WMP delivered to individuals by a physician with the same WMP combined with a group-based interdisciplinary programme. Participants had a mean BMI of 36.8 kg/m2 (SD 6 kg/m2) and all were identified as having a high risk of type 2 diabetes mellitus as measured by the Finnish Diabetes Risk Score. 206,207
Participants in the Wadden et al. 192 trial were randomised to receive a group WMP, based on the LEARN (lifestyle, exercise, attitudes, relationships, nutrition) manual208 and conducted by a nutritionist, or the same treatment conducted by a psychiatrist experienced in weight control delivered to individuals. Group mean BMIs ranged from 36.2 kg/m2 (SD 6.2 kg/m2) to 36.7 kg/m2 (SD 3.6 kg/m2).
The SHINE (Support, Health Information, Nutrition and Exercise) trial, conducted by Weinstock et al. ,196 randomised people with metabolic syndrome to receive a telephone adaptation of the DPP intervention,205 delivered as individual calls or to groups via conference calls. The initial mean BMI was 38.9 kg/m2 (SD 7.6 kg/m2) in the individual call group and 39.7 kg/m2 (SD 8.3 kg/m2) in the conference call group. Six participants were diagnosed with diabetes mellitus during the trial, but their allocation was not given.
Descriptions of the weight maintenance trials
Kumanyika et al. 137 recruited African American women to receive a 10-week culturally adapted group counselling Health Eating and Lifestyle Programme (HELP) of 1200- to 1500-kcal/day. The mean BMI of participants was 37.0 kg/m2. Following the weight-loss programme, participants were randomised to receive interventions designed to facilitate weight maintenance or additional weight loss [i.e. to HELP as group counselling classes, to individual self-directed HELP (Self-HELP) facilitated by HELP staff members (nutrition, exercise or behaviour change specialists) or to usual care, which involved semi-annual clinic visits with a physician but no further HELP intervention].
Latner et al. 140 evaluated a community WMP, randomly allocating participants to receive group-based continuing care following weight loss or to a standard individual-care condition. All participants then received a maintenance manual providing additional behavioural strategies and skills; however, the continuing care participants continued to meet in groups of 10–15 participants, with two participants volunteering to act as co-facilitators within each group. The mean BMI of participants was 35.8 kg/m2.
Meta-analyses
Table 25 shows the meta-analysis of the comparison of group and individual programmes. Three trials140,157,192 had many more contacts with those participants receiving the group intervention, which may have affected results. For the overall comparison of group versus individual weight loss and weight maintenance programmes, there was a benefit from group programmes at 12 months, in unadjusted and adjusted analyses, and results favoured group programmes at other time points. Subgroups showed no consistent favouring of group or individual programmes.
| Type of analysis | Mean weight change (kg) | |||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | 36 months | |
| Unadjusted | ||||
| Weight loss and weight maintenance |
Mean –1.29 (95% CI –2.55 to –0.03) p = 0.045 I2 = 0 Trials = 3(Favours group intervention) |
Mean –0.18 (95% CI –1.65 to 1.29) p = 0.811 I2 = 8 Trials = 2(Favours group intervention) |
Mean –1.08 (95% CI –3.27 to 1.12) p = 0.336 I2 = 78 Trials = 2(Favours group intervention) |
Mean –4.09 (95% CI –7.99 to –0.19) p = 0.040 I2 = N/A Trials = 1(Favours group intervention) |
| Weight-loss subgroup |
Mean 0.50 (95% CI –1.45 to 2.45) p = 0.615 I2 = 0 Trials = 1(Favours individual interventions) |
Mean –4.00 (95% CI –7.48 to –0.52) p = 0.024 I2 = N/A Trials = 1(Favours group intervention) |
||
| Weight maintenance subgroup |
Mean –1.08 (95% CI –3.32 to 1.16) p = 0.345 I2 = N/A Trials = 1(Favours group intervention) |
Mean 0.84 (95% CI –1.98 to 3.66) p = 0.559 I2 = N/A Trials = 1(Favours individual intervention) |
||
| Adjusted | ||||
| Weight loss and weight maintenance |
Mean –1.05 (95% CI –2.04 to –0.06) p = 0.038 I2 = 0 Trials = 3(Favours group intervention) |
Mean –0.18 (95% CI –1.65 to 1.29) p = 0.811 I2 = 8 Trials = 2(Favours group intervention) |
Mean –1.08 (95% CI –3.27 to 1.12) p = 0.336 I2 = 78 Trials = 2(Favours group intervention) |
Mean –4.09 (95% CI –7.99 to –0.19) p = 0.040 I2 = N/A Trials = 1(Favours group intervention) |
| Weight-loss subgroup |
Mean 0.50 (95% CI –1.45 to 2.45) p = 0.615 I2 = N/A Trials = 1(Favours individual intervention) |
Mean –4.00 (95% CI –7.48 to –0.52) p = 0.024 I2 = N/A Trials = 1(Favours group intervention) |
||
| Weight maintenance subgroup |
Mean –1.08 (95% CI –3.32 to 1.16) p = 0.345 I2 = N/A Trials = 1(Favours group intervention) |
Mean 0.84 (95% CI –1.98 to 3.66) p = 0.559 I2 = N/A Trials = 1(Favours individual intervention) |
||
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Interventions comparing the effect of group programmes with individual programmes involved an in-person group meeting component in all trials but one (which used telephone calls196). Trials generally provided the same BCTs to both arms, with the exception of the Nilsen et al. 157 trial, which reported the group arm receiving six BCTs and the individual arm receiving only one (information from a credible source). Weight-loss trials typically reported giving more BCTs to trial groups than those aiming for weight maintenance, with an average of 15.1 BCTs (range 1–29 BCTs) versus 10 BCTs (range 9–11 BCTs), respectively. Only one BCT was used routinely in both weight loss and weight maintenance trials: a credible source providing arguments for weight loss or maintenance. Goal-setting, problem-solving and self-monitoring techniques were frequently used. In general, group support was described well or adequately in both the weight-loss and weight maintenance trials, apart from in one trial. 157
In-person delivery with additional telephone or internet support versus in-person delivery only
Four trials compared in-person delivery of a WMP with in-person delivery plus additional support delivered via telephone or the internet. 108,114,178,203 Additional support led to more contacts with participants (see Report Supplementary Material 5, Table E10).
Descriptions of the trials
In the Weigh To Go trial, Dennison et al. 108 evaluated a computer-assisted nutrition programme to assist weight loss and weight maintenance in employees of a large US automobile manufacturing firm. The mean weight of participants was 100 kg, consistent with a BMI of ≥ 35 kg/m2. Participants were randomised to receive the 8-week Weigh To Go programme classes either with computerised food intake and activity analysis entered by the participants (hands-on) or as a condition without computer assistance. The materials and activities were otherwise the same in both conditions. A control group received no nutrition programme. At 12 months, the authors reported that differences in weight between groups were not significant.
In the Choose to Lose study,114 participants were randomised to an enhanced lifestyle intervention or to a standard intervention, both including in-person counselling on a 500- to 1000-kcal/day deficit diet and physical activity based on the DPP. 205 Participants had a mean BMI of 37.8 kg/m2 (SD 6.6 kg/m2). During the first 12 months, enhanced intervention participants received monthly telephone calls from the counsellor, and received weekly mailings of tailored and non-tailored videos and printed materials that focused on weight loss. In the second year, enhanced intervention participants received tailored and non-tailored materials for weight maintenance biweekly for the first 6 months and monthly thereafter. Enhanced intervention participants also received exercise feedback reports.
The trial by Spring et al. 178 evaluated the effectiveness of adding an electronic mobile personal digital assistant (+ mobile group) and telephone coaching to a group-based WMP. Participants had an initial mean BMI of 36.3 kg/m2 (SD 4.6 kg/m2).
The trial conducted by Wylie-Rosett et al. 203 evaluated a WMP delivered via a workbook only, a workbook plus additional computerised assistance, and the addition of computers and staff consultations, including up to 18 telephone or face-to-face consultations with a registered dietitian and/or a cognitive–behavioural therapist. The mean BMI of participants was 35.6 kg/m2 (SD 6.5 kg/m2).
Meta-analyses
Table 26 shows that adding telephone or internet support to an in-person WMP favoured weight loss at 12 months, but not at later follow-up times in the one trial114 longer than 12 months.
| Type of analysis | Mean weight change (kg) | ||
|---|---|---|---|
| 12 months | 18 months | 24 months | |
| Unadjusted |
Mean –2.05 (95% CI –3.77 to –0.33) p = 0.020 I2 = 39 Trials = 4(Favours additional telephone or internet) |
Mean –0.10 (95% CI –2.49 to 2.29) p = 0.935 I2 = N/A Trials = 1(Favours additional telephone or internet) |
Mean –0.10 (95% CI –2.69 to 2.49) p = 0.940 I2 = N/A Trials = 1(Favours additional telephone or internet) |
| Adjusted |
Mean –1.88 (95% CI –3.55 to –0.21) p = 0.027 I2 = 49 Trials = 4(Favours additional telephone or internet) |
Mean –0.06 (95% CI –1.85 to 1.73) p = 0.948 I2 = N/A Trials = 1(Favours additional telephone or internet) |
Mean –0.10 (95% CI –1.95 to 1.76) p = 0.919 I2 = N/A Trials = 1(Favours additional telephone or internet) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
The in-person only groups received, on average, 12.8 BCTs (range 3–18 BCTs) and the enhanced intervention groups received, on average, 16.3 BCTs (range 8–24 BCTs). Only one BCT was used routinely and provided to both arms, namely using a credible source. The WMPs in both arms of the trials were the same. Eaton et al. 114 gave additional information about antecedents, demonstration of the behaviour, grade tasks, body changes, framing/reframing and self-talk to the enhanced intervention group. Spring et al. 178 also gave more BCTs to the enhanced intervention group, namely goal-setting, feedback on and self-monitoring of the behaviour, and graded tasks. Wylie-Rosett et al. 203 used the greatest number of BCTs of the four trials, with 24 in the additional support arm (providing extra goal outcome, action-planning and verbal persuasion BCTs) and 21 in the comparator arm. BCTs were well described in two trials,114,203 and adequately described in the remaining two. 108,178
In-person delivery versus telephone- or internet-only delivery of an intervention
Five trials compared in-person delivery with delivery via telephone or internet only. Four of these trials83,84,103,142 compared interventions targeted at weight loss, whereas one162 focused on weight maintenance. A brief description of the weight-loss trials is provided in Report Supplementary Material 5, Table E11. The numbers of contacts for participants were generally similar across intervention arms of trials.
Descriptions of the weight-loss trials
Appel et al. 83 compared delivery of a WMP by remote support via telephone, a study website and e-mail with remote support and in-person support during group and individual sessions over 24 months. The initial mean BMI of participants was 36.6 kg/m2 (SD 5.0 kg/m2). A third self-directed control group received brochures and a list of websites promoting weight loss at baseline only.
The E-LITE (Evaluation of Lifestyle Interventions to Treat Elevated Cardiometabolic Risk in Primary Care) study84 compared usual care, usual care plus in-person, coach-led support and individual self-directed remote support WMPs in people with prediabetes and/or metabolic syndrome (mean BMI ≥ 35 kg/m2).
Participants in the trial by Damschroder et al. 103 (see earlier description in Group programmes versus individual programmes) were randomised to the Veterans’ Affairs standard MOVE! weight-loss programme delivered by expert clinicians, or to two ASPIRE programmes delivered by non-clinician lifestyle coaches.
Little et al. 142 randomised participants with hypertension, hypercholesterolaemia or diabetes mellitus to receive a weight-loss programme: Positive Online Weight Reduction (POWeR+), delivered via the internet with either remote (POWeR+ R) or face-to face (POWeR+ F) nurse support. The mean group BMIs ranged from 36.3 kg/m2 (SD 5.7 kg/m2) to 37.1 kg/m2 (SD 6.0 kg/m2). In addition to the web-based intervention, POWeR+ F participants received three scheduled and four optional face-to-face sessions, plus 6-monthly weighing sessions. Face-to-face contact was replaced with three scheduled telephone or e-mail contacts and two optional telephone or e-mail contacts for the POWeR+ R participants. A control group received 6-monthly nurse follow-up sessions only. The POWeR trial is also discussed in Chapter 4.
Description of the weight maintenance trial
In the Perri et al. ,162 women with from rural communities who had completed a 6-month weight-loss programme were randomised to two extended-care counselling interventions, delivered by telephone or face to face, or to an education control group for a further 6 months. The participants’ mean BMI was 36.8 kg/m2 (SD 4.9 kg/m2).
Meta-analyses
Table 27 shows the meta-analysis of in-person versus telephone or internet delivery of a WMP. The trial by Azar et al. 84 was excluded from this analysis because it was not possible to identify the numbers of participants with a BMI of > 35 kg/m2, but it reported a greater reduction in BMI and body weight in the coach-led intervention than in the self-directed intervention (p < 0.05). For the meta-analysis of interventions targeted at both weight loss and weight maintenance, in-person intervention delivery was favoured at 12 and 24 months for adjusted and unadjusted data, with evidence of a small effect.
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 24 months | |
| Unadjusted | ||
| All studies |
Mean –0.62 (95% CI –1.38 to 0.14) p = 0.109 I2 = 0 Trials = 4(Favours in-person delivery) |
Mean –0.60 (95% CI –2.68 to 1.48) p = 0.573 I2 = N/A Trials = 1(Favours in-person delivery) |
| Weight-loss subgroup |
Mean –0.76 (95% CI –1.60 to 0.08) p = 0.077 I2 = 3 Trials = 3(Favours in-person delivery) |
|
| Weight maintenance subgroup |
Mean 0.00 (95% CI –1.81 to 1.81) p = 1.000 I2 = 0 Trials = 1 |
|
| Adjusted | ||
| All studies |
Mean –0.59 (95% CI –1.25 to 0.06) p = 0.076 I2 = 0 Trials = 4(Favours in-person delivery) |
Mean –0.64 (95% CI –2.65 to 1.37) p = 0.531 I2 = N/A Trials = 1(Favours in-person delivery) |
| Weight-loss subgroup |
Mean –0.68 (95% CI –1.38 to 0.02) p = 0.057 I2 = 0 Trials = 3(Favours in-person delivery) |
|
| Weight maintenance subgroup |
Mean 0.00 (95% CI –1.81 to 1.81) p = 1.000 I2 = 0 Trials = 1 |
|
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Interventions comparing the effects of in-person support programmes with those delivered remotely by telephone or via the internet generally provided the same BCTs to both comparisons, with one exception84 in which the in-person group received a demonstration of the behaviour and the remote group did not. Weight-loss trials gave an average of 13.6 BCTs (range 1–17 BCTs), and the one weight maintenance trial162 gave 20 BCTs. Several BCTs were used routinely in weight loss and weight maintenance trials, namely goal-setting, problem-solving, self-monitoring and social support. BCT support was well described in all trials.
Fitness club trainer (In SHAPE programme) versus fitness club membership
Descriptions of the trials
Bartels et al. 87,88 compared a 12-month fitness and weight-loss programme tailored for people with serious mental illnesses (In SHAPE) with a 12-month membership of a fitness club in two trials. The In SHAPE intervention was delivered in weekly sessions by a certified fitness trainer/health coach who received instruction on healthy eating and nutrition training in tailoring individual plans for people with serious mental illness. The intervention included healthy eating discussions with a registered dietitian, including group cooking classes. The control group participants received 12-month membership to the same local fitness club and educational materials on the benefits of exercise and healthy eating. In SHAPE participants received 56 intervention sessions, whereas control participants received four. From the two trials, the mean group BMI ranged from 36.2 kg/m2 (SD 7.6 kg/m2) to 38.3 kg/m2 (SD 8.5 kg/m2).
Meta-analyses
Table 28 shows the meta-analysis of fitness club trainer (In SHAPE programme) versus fitness club membership. The results show no differences at either time point.
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 18 months | |
| Unadjusted |
Mean –0.12 (95% CI –1.64 to 1.39) p = 0.873 I2 = 95 Trials = 2(Favours In Shape programme) |
Mean 0.77 (95% CI –1.20 to 2.74) p = 0.444 I2 = N/A Trials = 1(Favours fitness club membership) |
| Adjusted |
Mean –0.10 (95% CI –1.44 to 1.23) p = 0.881 I2 = 94 Trials = 2(Favours In Shape programme) |
Mean 0.62 (95% CI –1.08 to 2.33) p = 0.475 I2 = N/A Trials = 1(Favours fitness club membership) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Fitness trainer groups in both trials reported receiving six BCTs and the comparator groups reported receiving three or four BCTs. Both trials gave demonstration of the behaviour and a material reward to both arms of the trial, and the fitness trainer arms of both trials additionally received goal-setting, social support, and instruction on how to perform the behaviour and use a credible source. The comparator groups of both trials received information about health consequences, which was not reported for the fitness trainer groups. The BCTs were adequately described across both trials.
Inpatient session versus none
Three trials124,125,184 compared WMPs with an initial inpatient programme with WMPs that did not provide this. Brief details of how the interventions were delivered are presented in Report Supplementary Material 5, Table E12.
Descriptions of the trials
Hakala et al. 125 compared a 12-month group weight-loss programme, comprising an initial 2-week intensive weight reduction period in an inpatient rehabilitation setting and then a community programme, with an individual, community-based programme. Mean group BMIs ranged from 41.7 kg/m2 (SD 3.1 kg/m2) to 43.6 kg/m2 (SD 4.8 kg/m2). The inpatient intervention comprised intensive behavioural and educational group sessions along with a prescribed physical activity programme and occupational therapy, as well as individual nutritionist (1200 kcal/day) and physician counselling. The community intervention involved the same dietary intervention and individual physician counselling.
In a second trial, Hakala124 conducted a similar comparison between an initial 3-week intensive inpatient rehabilitation programme followed by a community programme, and a community-based weight-loss programme delivered over 2 years. The mean group BMI ranged from 37.7 kg/m2 (SD 2.3 kg/m2) to 40.5 kg/m2 (SD 3.9 kg/m2).
The trial by Torgerson et al. 184 (see Very low-calorie diets) compared delivery of a 456-kcal/day VLCD weight-loss programme starting with a week as a hospital inpatient (VLCD – metabolic ward) with the same VLCD treatment delivered without the hospital stay (VLCD – strict). A third group followed the VLCD treatment but participants were allowed two small meals weekly (VLCD – plus). All participants underwent VLCD for 16 weeks, followed by a 500-kcal/day deficit diet for 36 weeks.
Meta-analyses
Table 29 shows evidence that favoured inpatient sessions at 12 months for unadjusted data, but did not favour inpatient sessions thereafter, with evidence against provision at 24 months only. When data were adjusted for dropouts and withdrawals, meta-analysis did not favour inpatient sessions at any time point, again with evidence against provision at 24 months.
| Type of analysis | Mean weight change (kg) | ||
|---|---|---|---|
| 12 months | 24 months | 60 months | |
| Unadjusted |
Mean –2.05 (95% CI –5.18 to 1.08) p = 0.200 I2 = 86 Trials = 2(Favours inpatient session) |
Mean 8.91 (95% CI 3.09 to 14.74) p = 0.003 I2 = 54 Trials = 1(Favours no inpatient session) |
Mean 0.01 (95% CI –4.23 to 4.24) p = 0.997 I2 = 27 Trials = 2(Favours no inpatient session) |
| Adjusted |
Mean 0.26 (95% CI –2.36 to 2.87) p = 0.848 I2 = 82 Trials = 2(Favours no inpatient session) |
Mean 8.91 (95% CI 3.09 to 14.74) p = 0.003 I2 = 54 Trials = 1(Favours no inpatient session) |
Mean 0.14 (95% CI –4.51 to 4.80) p = 0.952 I2 = 25 Trials = 2(Favours no inpatient session) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
The inpatient groups received, on average, 8.7 BCTs (range 7–12 BCTs) and the comparator groups received, on average, 6 BCTs (range 3–10 BCTs). All trials used a credible source in both arms and gave instruction on how to perform the behaviour. The 1993 trial by Hakala et al. 125 gave additional social support, behavioural rehearsal and restructuring of the physical environment to the inpatient group, and the 1994 trial in the same group of participants124 gave additional behavioural rehearsal, restructuring of the social and physical environments and additional environmental object BCTs to the inpatient group. The trials used largely the same BCTs in the inpatient arms. Torgerson et al. 184 gave five additional BCTs targeting goal-setting, action-planning, problem-solving and feedback on and self-monitoring of behaviour to the inpatient arm. However, these results should be interpreted with caution because group support was poorly described across all three trials.
Adding or comparing types of counselling
Nine trials74,75,77–81,141,146 evaluated different types of counselling as part of a weight-loss programme.
Mindfulness
Daubenmier et al. 75 evaluated adding mindfulness techniques to a WMP compared with a WMP-only intervention. The authors reported that mindfulness techniques were based on stress reduction,209 raising awareness about eating210–212 and walking. 213 Both intervention arms attended 16 sessions lasting 2–2.5 hours and one day session spread over 5.5 months. Content in the control intervention was reduced by 30 minutes in sessions 9–16. Mindfulness was delivered by meditation instructors and one dietitian. The control intervention was run by dietitians. The group mean BMI ranged from 35.4 kg/m2 (SD 3.5 kg/m2) to 35.6 kg/m2 (SD 3.5 kg/m2). At both 12 and 18 months, adjusted and unadjusted data favour the mindfulness intervention (Table 30).
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| Mindfulness and WMP | Active control and WMP | |||
| Daubenmier 201675 (trial of mindfulness and WMP vs. an active control and WMP) | 12 (unadjusted analysis) | –5.10 (7.11) [n = 79] | –3.00 (6.69) [n = 70] | –2.10 (–4.33 to 0.13); p = 0.064 |
| 12 (adjusted analysis) | –4.03 (6.65) [n = 100] | –2.23 (5.91) [n = 94] | –1.79 (–3.57 to –0.02); p = 0.047 | |
| 18 (unadjusted analysis) | –5.00 (8.10) [n = 81] | –3.20 (8.06) [n = 65] | –1.80 (–4.44 to 0.84); p = 0.181 | |
| 18 (adjusted analysis) | –4.05 (7.54) [n = 100] | –2.21 (6.85) [n = 94] |
–1.84 (–3.87 to 0.19); p = 0.076 (Favours mindfulness) |
|
| Skill-based intervention | Counselling-based intervention | |||
| Yeh 200377 (trial of skill-based vs. standard counselling) | 12 (unadjusted analysis) | –0.77 (5.08) [n = 14] | –1.81 (4.63) [n = 14] | 1.04 (–2.56 to 4.64); p = 0.571 |
| 12 (adjusted analysis) | –0.31 (3.16) [n = 35] | –0.68 (2.92) [n = 37] | 0.38 (–1.03 to 1.78); p = 0.599 | |
| 24 (unadjusted analysis) | –0.59 (3.30) [n = 13] | –1.09 (5.76) [n = 14] | 0.50 (–3.08 to 4.08); p = 0.784 | |
| 24 (adjusted analysis) | –0.22 (1.98) [n = 35] | –0.41 (3.50) [n = 37] |
0.19 (–1.13 to 1.52); p = 0.775 (Favours counselling) |
|
| Motivational interviewing and WMP | Attention control and WMP | |||
| West 200778 (trial of motivational interviewing and WMP vs. an attention control and WMP) | 12 | –4.80 (6.15) [n = 109] | –2.70 (6.44) [n = 108] | –2.10 (–3.78 to –0.42); p = 0.014 |
| 18 | –3.50 (6.44) [n = 109] | –1.70 (6.55) [n = 108] |
–1.80 (–3.53 to –0.07); p = 0.041 (Favours motivational interviewing) |
|
Skill-based counselling
Yeh et al. 77 compared skill-based counselling with standard counselling. Skill-based counselling aimed to provide skills to support overcoming barriers to healthy eating. Both interventions were delivered by a dietitian [it was unclear if the same dietitian(s) were involved in both], with skill-based counselling participants having 19 contacts and standard counselling participants having 10 contacts during the first 12 months. Participants were contacted once in the second year. The mean BMIs of women in the trial arms ranged from 36.3 kg/m2 (SD 5.4 kg/m2) to 37.9 kg/m2 (SD 6.7 kg/m2). Data at 12 and 24 months are shown in Table 30. There was little difference between types of counselling.
Motivational interviewing
West et al. 78 investigated adding motivational interviewing to a group-based behavioural WMP in women with type 2 diabetes mellitus. All participants received the WMP, which was delivered in 42 sessions by a team including a behaviourist, a nutritionist, an exercise physiologist and a diabetes mellitus educator. The mean BMI of participants was 36.5 kg/m2 (SD 5.5 kg/m2). Weight loss was emphasised for the first 6 months and weight maintenance was emphasised for the following 12 months. All participants were prescribed a low-fat diet of 1200–1500 kcal/day and were encouraged to achieve a goal of ≥ 150 minutes per week of physical activity. Motivational interviewing was delivered in five sessions by a counsellor who focused on eliciting change talk, commitment language and how to motivate change. Individual health education sessions acted as an attention placebo and matched motivational interviewing sessions for frequency and duration. Data for 12 and 18 months are shown in Table 30. Motivational interviewing was superior to the attention control at 12 and 18 months.
Cognitive–behavioural therapy
Two trials141,146 investigated CBT compared with standard behavioural therapy for women. Participants in the Linde et al. 141 trial had a group mean BMI of 39.5 kg/m2 (SD 7.7 kg/m2) to 38.6 kg/m2 (SD 6.8 kg/m2). Manzoni et al. 146 had groups with mean BMIs from 39.2 kg/m2 (SD 5.3 kg/m2) to 41.8 kg/m2 (SD 6.3 kg/m2). Manzoni et al. 146 also investigated using virtual reality enhanced CBT (CBT + VR), in which participants were able to develop and practise weight-management techniques in response to trigger situations within virtual environments. The authors reported that weight-loss results from CBT + VR did not differ from those from CBT. In one trial,141 women had clinical depression. Both trials included a prescribed calorie diet (1200–1500 kcal/day) and physical activity goals. A brief description of the interventions is provided in Report Supplementary Material 5, Table E13. Meta-analysis favoured CBT at 12 months for both unadjusted and adjusted data (Table 31).
| Type of analysis | Mean weight change (kg) |
|---|---|
| 12 months | |
| Unadjusted |
Mean –3.11 (95% CI –5.03 to –1.19) p = 0.001 I2 = 96 Trials = 2(Favours CBT) |
| Adjusted |
Mean –3.01 (95% CI –4.68 to –1.33) p < 0.000 I2 = 94 Trials = 2(Favours CBT) |
Psychodynamic approach
Beutel et al. 79 compared a psychodynamic approach with a behavioural approach, both delivered in an inpatient rehabilitation setting to mostly female (85%) participants with psychiatric and somatic comorbidities over 7 weeks. In both arms, the therapists were psychological psychotherapists or psychiatrists. Both approaches used group therapy. The psychodynamic approach had more emphasis on individual therapy. Trial participants had a mean BMI of 44.3 kg/m2 (range 35.1–73.5 kg/m2). Adjusted and unadjusted data at 12 and 26 months favoured the psychodynamic treatment (mostly at 12 months) (Table 32).
| Study (first author and year) | Time point (months) | Weight change (kg), mean (SD) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| Psychodynamic approach | Behavioural approach | |||
| Beutel 200679 (trial of psychodynamic approach vs. behavioural approach) | 12 (unadjusted analysis) | –6.93 (7.88) [n = 63] | –4.72 (7.25) [n = 60] | –2.20 (–4.88 to 0.47); p = 0.107 |
| 12 (adjusted analysis) | –3.19 (6.82) [n = 137] | –2.18 (6.53) [n = 130] | –1.01 (–2.61 to 0.60); p = 0.219 | |
| 36 (unadjusted analysis) | –4.78 (7.27) [n = 63] | –4.12 (7.08) [n = 60] | –0.66 (–3.19 to 1.88); p = 0.612 | |
| 36 (adjusted analysis) | –2.20 (6.54) [n = 137] | –1.90 (6.45) [n = 130] |
–0.29 (–1.85 to 1.26); p = 0.711 (Favours psychodynamic treatment) |
|
| Weight management plus | Weight management | |||
| Østbye 201580 (trial of weight management plus vs. WMPs) | 14 (unadjusted analysis) | –0.94 (6.18) [n = 215] | –0.66 (6.10) [n = 220] | –0.29 (–1.44 to 0.87); p = 0.624 |
| 14 (adjusted analysis) | –0.74 (6.12) [n = 275] | –0.52 (6.06) [n = 275] |
–0.21 (–1.23 to 0.80); p = 0.681 (Favours weight management plus) |
|
| ABBI | SBT | |||
| Lillis 201674 (trial of an ABBI vs. SBT) | 12 | –8.92 (9.45) [n = 81] | –9.70 (9.27) [n = 81] |
0.78 (–2.10 to 3.66); p = 0.596 (Favours SBT) |
| 18 | –7.30 (9.36) [n = 81] | –7.40 (9.27) [n = 81] |
0.10 (–2.77 to 2.97); p = 0.946 (Favours SBT) |
|
| 24 | –4.29 (8.01) [n = 81] | –2.65 (7.92) [n = 81] |
–1.64 (–4.09 to 0.81); p = 0.190 (Favours ABBI) |
|
| Experimental | Control | |||
| Annesi 201781 (trial of an experimental vs. acontrol intervention) | 12 | –5.57 (4.64) [n = 53] | –1.70 (4.80) [n = 54] | –3.87 (–5.66 to –2.08); p < 0.001 |
| 24 | –4.95 (8.18) [n = 53] | –1.11 (4.93) [n = 54] |
–3.84 (–6.39 to –1.29); p = 0.003 (Favours experimental) |
|
Counselling
The Steps to Health study80 evaluated two existing employee health programmes. Employees were randomised to an intensive behavioural intervention (weight management plus) or to a healthy lifestyle educational programme (weight management). In addition to the different emphasis on behaviour versus education, the programmes differed in intensity, frequency and resource requirements. Weight management included one face-to-face meeting with a counsellor in the first month and two telephone counselling and feedback sessions at months 6 and 12, with monthly mailings of educational material. Weight management plus participants were offered monthly counselling sessions (face to face for months 1, 4, 8 and 12, with the rest by telephone), met with an exercise physiologist for months 2 and 5 and received quarterly biometric feedback and educational materials. Weight management plus participants were also encouraged to use eHealth trackers for diet and weight. Weight management plus participants received an additional 10 sessions during the first 12 months. The mean BMI of the trial participants was 37.2 kg/m2 (SD 6.4 kg/m2). Table 32 shows that the weight management plus programme was superior when data were adjusted and unadjusted at 14 months, but the differences were small and rule out any meaningful difference between the strategies.
Acceptance-based counselling
Lillis et al. 74 randomised people to an acceptance-based behavioural (counselling) intervention (ABBI) or standard behavioural treatment. Psychologists, exercise physiologists and nutritionists delivered both programmes in 32 group sessions over 12 months. Participants received the same diet of 1200–1800 kcal/day and exercise goal of 250 minutes per week. All participants were taught standard behavioural strategies. ABBI was based on Acceptance and Commitment Therapy214 and used acceptance and mindfulness techniques to promote detachment from problematic thoughts and commitment to values consistent with the target behaviour. Standard behavioural treatment used cognitive and emotional control strategies to address negative thoughts and emotions. The mean BMI of trial participants was 37.6 kg/m2 (SD 5.3 kg/m2). Data for 12, 18 and 24 months are shown in Table 32. Standard behavioural treatment was favoured at 12 and 18 months, and ABBI was favoured at 24 months.
Social cognitive theory-based programme
The trial by Annesi81 recruited women who were physically inactive to two different WMPs based on social cognitive theory or self-efficacy theory. 215,216 The social cognitive theory programme had group sessions, emphasising self-regulatory skills for weight loss and weight maintenance. Assigned daily energy intake was based on an individual’s weight (e.g. 79–99 kg = 1500 kcal/day).
Participants in the self-efficacy group reviewed chapters in the LEARN manual,208 and were recommended to limit energy intake to 1200 kcal/day. Lessons were followed by 15-minute telephone support sessions with a wellness leader. Both interventions were delivered over 6 months and addressed the concept of body image and its role in weight management. All participants were recommended to do 150 minutes per week of moderate exercise. The mean BMI of trial participants was 35.4 kg/m2 (SD 3.3 kg/m2). Data for 12 and 24 months are shown in Table 32. The social cognitive theory-based programme was favoured at both time points.
Family and social support
Two trials138,199 examined the effect of family and social support on weight loss.
Descriptions of the trials
The SHARE (Supporting Health Activity and eating Right Everyday) trial conducted by Kumanyika et al. 138 with African American men and women (90% were women) chose to enrol participants alone (individual stratum) or with one or two family members or friends (family stratum). Participants were then randomised to high or low social support within each stratum. Participants’ mean group BMIs ranged from 38.4 kg/m2 (SD 6.2 kg/m2) to 39.3 kg/m2 (SD 6.2 kg/m2). The trial by Wing et al. 199 randomised people with type 2 diabetes mellitus, and their spouses, also with obesity with/without diabetes mellitus, to attend a 20-week behavioural weight-loss programme together with their spouses or alone. Group mean BMIs ranged from 35.7 kg/m2 (SD 5.8 kg/m2) to 36.6 kg/m2 (SD 5.8 kg/m2). Details of the interventions for both trials are presented in Report Supplementary Material 5, Table E14.
Meta-analyses
Table 33 shows that there was no evidence of a difference between groups at any time point for either adjusted or unadjusted data. In the Kumanyika et al. 138 trial, partner success with weight loss was associated with greater participant weight loss. Spouses of both sexes lost more weight in the together group than in the alone group in the Wing et al. 199 trial (reported p < 0.05).
| Type of analysis | Mean weight change (kg) | ||
|---|---|---|---|
| 12 months | 18 months | 24 months | |
| Unadjusted |
Mean –1.07 (95% CI –3.16 to 1.02) p = 0.315 I2 = 4 Trials = 1(Favours high support) |
Mean 0.00 (95% CI –2.35 to 2.34) p = 0.997 I2 = 0 Trials = 2 (Favours low support) |
Mean 0.31 (95% CI –1.99 to 2.62) p = 0.789 I2 = 0 Trials = 1(Favours low support) |
| Adjusted |
Mean –0.59 (95% CI –1.80 to 0.61) p = 0.334 I2 = 0 Trials = 1(Favours high support) |
Mean –0.14 (95% CI –1.30 to 1.02) p = 0.811 I2 = 0 Trials = 2 (Favours high support) |
Mean –0.02 (95% CI –1.62 to 1.57) p = 0.978 I2 = 0 Trials = 1(Favours high support) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
Both the high-support and low-support groups received, on average, 13 BCTs in the trial by Kumanyika et al. ,138 which used four more BCTs in its groups than the trial by Wing et al. 199 The BCTs provided were largely similar in both treatment arms, with the exceptions of practical social support to the high-support group, and information about health consequences to the low-support group in the Kumanyika et al. 138 trial. Goal-setting, problem-solving, self-monitoring of the behaviour, social support, instruction on how to perform the behaviour, use of a credible source and a material reward were used in all trials. These results should be interpreted with caution as support was poorly described in one trial138 and adequately described in the other. 199
Weight-loss versus weight-neutral interventions
Descriptions of the trials
Three trials85,151,167 compared interventions emphasising weight loss with interventions that were weight neutral with an emphasis on healthy lifestyles rather than weight loss. In two of these trials,85,151 the WMPs were both based on the LEARN programme, and a standard cognitive–behavioural programme was delivered in the remaining trial. 167 The weight-neutral groups in all trials employed a range of behavioural and counselling techniques. All three trials recruited women and excluded those with obesity-related comorbidities (e.g. diabetes mellitus and hypertension). Mean BMIs in the groups in the trials ranged from 35.2 kg/m2 (SD 6.1 kg/m2) to 38.6 kg/m2 (SD 3.9 kg/m2). Details of the interventions are presented in Report Supplementary Material 5, Table E15.
Meta-analysis
Meta-analysis favoured weight-loss interventions, with evidence of a difference at all time points (unadjusted and adjusted data) (Table 34).
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 24 months | |
| Unadjusted |
Mean –3.81 (95% CI –6.12 to –1.51) p = 0.001 I2 = 71 Trials = 2(Favours weight loss) |
Mean –3.18 (95% CI –6.14 to –0.23) p = 0.035 I2 = 0 Trials = 2(Favours weight loss) |
| Adjusted |
Mean –3.20 (95% CI –5.33 to –1.07) p = 0.003 I2 = 80 Trials = 2(Favours weight loss) |
Mean –2.09 (95% CI –4.19 to 0.01) p = 0.051 I2 = 0 Trials = 2(Favours weight loss) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
The BCTs provided in the trials varied between the arms, both in type and in number, with the weight-loss arms typically receiving more BCTs than those aiming for weight neutrality [i.e. an average of 14 BCTs (range 5–28 BCTs) vs. seven BCTs (range 5–9 BCTs), respectively]. In one trial,151 the weight-loss group received 28 BCTs and the comparator group received only five. Two BCTs were used routinely in weight-loss and weight-neutral groups, namely instruction on how to perform a behaviour and information from a credible source. In general, group support was described well or adequately across all trial arms.
Intensity of counselling
Five trials evaluated WMPs with differing intensities of counselling. In four of these trials, the intervention was based on the Look AHEAD/DPP205 interventions. 136,148,163,195 Three of these trials recruited from hard-to-reach or rural populations, whereas one was set in urban and suburban primary care practices that served a racially and economically diverse population.
Descriptions of the trials
The Think Health! study136 was set in health-care practices that reached African American and Hispanic people in the USA. Participants had an initial mean BMI of 37.2 kg/m2 (SD 6.4 kg/m2) and were randomised to receive counselling from a primary care provider and lifestyle coach or to receive counselling from a primary care provider only.
In the Pounds Off With Empowerment (POWER) trial,148 people with diabetes mellitus living in medically underserved rural communities received an ILI delivered primarily via individual counselling or a condensed ‘reimbursable’ intervention delivered in group and individual sessions. The initial mean BMI of participants was 36.7 kg/m2.
The rural LITE (Lifestyle Intervention Treatment Effectiveness) trial163 was set in Cooperative Extension Offices in rural communities of the USA and randomised participants to low, moderate or high doses of behavioural treatment or control. Participants had a mean BMI of 36.3 kg/m2 (SD 4.0 kg/m2).
Practice based Opportunities for WEight Reduction trial at the University of Pennsylvania (POWER-UP)195 randomised people with at least two out of five components of the metabolic syndrome to receive brief or enhanced brief lifestyle counselling. In both conditions, participants received counselling from primary care providers and lifestyle coaches; in the enhanced arm, participants were also able to choose sibutramine, orlistat or meal replacements to increase weight loss (data without sibutramine used here).
In the remaining LITE trial,132 participants were randomised to receive intensive counselling from a nutritionist over 10 visits or to short-term counselling over two visits. The initial mean BMI of participants was 35 kg/m2 (SD 5 kg/m2). Brief details of the interventions are presented in Report Supplementary Material 5, Table E16.
Meta-analyses
When data are unadjusted, meta-analysis favours intensive counselling for weight loss for all studies, and the subgroup of studies based on the Look AHEAD/DPP-based interventions, with evidence of an effect at all available time points. When data are adjusted, meta-analysis also favours intensive counselling at 12 and 24 months for all studies and the Look AHEAD/DPP-based interventions (Table 35).
| Type of analysis | Mean weight change (kg) | ||
|---|---|---|---|
| 12 months | 18 months | 24 months | |
| Unadjusted | |||
| All studies |
Mean –1.51 (95% CI –2.43 to –0.59) p = 0.001 I2 = 0 Trials = 4(Favours intensive) |
Mean –2.23 (95% CI –3.93 to –0.53) p = 0.010 I2 = 0 Trials = 2(Favours intensive) |
Mean –2.62 (95% CI –3.80 to –1.45) p < 0.001 I2 = 49 Trials = 2(Favours intensive) |
| Subgroup of DPP/Look AHEAD-based interventions |
Mean –1.50 (95% CI –2.44 to –0.57) p = 0.003 I2 = 0 Trials = 3(Favours intensive) |
Mean –2.40 (95% CI –4.48 to –0.32) p = 0.024 I2 = N/A Trials = 1(Favours intensive) |
Mean –2.62 (95% CI –3.80 to –1.45) p < 0.001 I2 = 49 Trials = 2(Favours intensive) |
| Other |
Mean –1.74 (–6.40 to 2.92) p = 0.464 I2 = N/A Trials = 1(Favours intensive) |
Mean –1.90 (–4.82 to 1.02) p = 0.202 I2 = N/A Trials = 1(Favours intensive) |
|
| Adjusted | |||
| All studies |
Mean –0.83 (95% CI –1.55 to –0.10) p = 0.026 I2 = 0 Trials = 4(Favours intensive) |
Mean –0.81 (95% CI –2.05 to 0.43) p = 0.200 I2 = 0 Trials = 2(Favours intensive) |
Mean –1.90 (95% CI –3.00 to –0.79) p = 0.001 I2 = 88 Trials = 2(Favours intensive) |
| Subgroup of DPP/Look AHEAD-based interventions |
Mean –0.82 (95% CI –1.58 to –0.07) p = 0.033 I2 = 0 Trials = 3(Favours intensive) |
Mean –0.56 (95% CI –2.33 to 1.21) p = 0.537 I2 = N/A Trials = 1(Favours intensive) |
Mean –1.90 (95% CI –3.00 to –0.79) p = 0.001 I2 = 88 Trials = 2(Favours intensive) |
| Other |
Mean –0.91 (95% CI –3.68 to 1.86) p = 0.521 I2 = N/A Trials = 1(Favours intensive) |
Mean –1.05 (95% CI –2.79 to 0.68) p = 0.234 I2 = N/A Trials = 1(Favours intensive) |
|
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
The intensive trial arms received, on average, 13.4 BCTs (range 6–19 BCTs), and the less intensive arms received 11.6 BCTs on average (range 4–19 BCTs). The BCTs routinely used in both arms of the trials were goal-setting, social support, instruction on how to perform the behaviour and information from a credible source. In the trial by Keränen et al. ,132 the more intensive intervention arm received six more BCTs than the less intensive arm. However, in general, the trials provided the same BCTs to both arms but had more sessions in the more intensive arm. All trials except Keränen et al. 132 provided physical activity advice to both trial arms. BCTs were described well in three of five trials;136,163,195 the remaining two were described poorly. 132,148
The effects of adding exercise to diet
Three trials189,194,201 investigated the effectiveness of adding an exercise component to a diet. All trials included participant groups for diet only, exercise only and diet plus exercise. Brief details of the interventions are reported in Report Supplementary Material 5, Table E17. All of the physical activity interventions involved group attendance at classes 1–3 times per week for 60–90 minutes, involving aerobic and/or resistance exercise training. Only the trial by Wing et al. 201 used a walking programme in isolation.
Descriptions of the trials
In the Villareal et al. 189 trial, participants’ group mean BMIs ranged from 36.9 kg/m2 (SD 5.4 kg/m2) to 37.3 kg/m2 (SD 4.7 kg/m2). Bone loss at the total hip was relatively less in the diet plus exercise group (–1.1%) than in the diet group (–2.6%), and bone mineral density increased in the exercise group (reported p < 0.001). The authors also reported that cognitive function, as measured by the Modified Mini-Mental State Examination, word fluency test and IWQOL, improved more in the active interventions than in the control interventions (reported p = 0.0001 to 0.04) and improved more in the diet plus exercise group than in the diet group, but not more than the exercise group.
Wadden et al. 194 randomised participants to a diet-only group, a diet plus aerobic exercise group, a diet plus strength training group and a diet plus aerobic and strength training group. The mean BMI of participants was 36.5 kg/m2 (SD 5.7 kg/m2).
Wing et al. 201 recruited people without type 2 diabetes mellitus [mean BMI 35.9 kg/m2 (SD 4.3 kg/m2)] who had one or both parents with diabetes mellitus. The authors reported that, at 24 months, 21 participants (17%) developed diabetes mellitus over the course of the study. In the control group, 7% (n = 2) developed type 2 diabetes mellitus, compared with 30.3% of diet (n = 10), 14% of exercise (n = 4) and 15.6% of diet plus exercise conditions (n = 5) (reported p = 0.079).
Meta-analyses
The results presented in Table 36 show that our meta-analyses favour diet and exercise at 12 and 24 months when data are both adjusted and unadjusted.
| Type of analysis | Mean weight change (kg) | |
|---|---|---|
| 12 months | 24 months | |
| Unadjusted |
Mean –1.27 (95% CI –3.38 to 0.84) p = 0.238 I2 = 91 Trials = 2(Favours adding exercise) |
Mean –2.88 (95% CI –5.35 to –0.41) p = 0.022 I2 = 0 Trials = 2(Favours adding exercise) |
| Adjusted |
Mean –1.42 (95% CI –3.37 to 0.52) p = 0.152 I2 = 90 Trials = 2(Favours adding exercise) |
Mean –2.59 (95% CI –4.77 to –0.41) p = 0.020 I2 = 0 Trials = 2(Favours adding exercise) |
Behaviour change technique coding summary (see Report Supplementary Material 1, Section 8)
All diet plus exercise arms of the trials provided supervised physical activity, and the comparator groups were typically instructed not to change their activity levels. The diet plus exercise groups received 16 BCTs on average (range 11–25 BCTs) and the comparator groups received 13.3 BCTs on average (range 7–25 BCTs). Goal-setting, self-monitoring of behaviour and information about antecedents were used in both arms of all trials, as was the use of a credible source. Wing et al. 201 provided the same BCTs to both groups; however, Villareal et al. 189 and Wadden et al. 194 provided fewer BCTs to their diet-only groups. BCTs were adequately described in two trials189,201 and poorly described in the third. 194
Other comparisons
Descriptions of the trials
Following an initial 12-week VLCD phase, women in the Agras et al. 82 trial were randomised to receive one of four weight maintenance conditions, which evaluated time-dependent or weight-dependent reintroduction of solid foods over 9 months. The first time-dependent condition allowed gradual replacement of VLCD with meals and increases in energy intake at regular time intervals. A similar time-dependent, stimulus-narrowing condition provided participants with pre-packaged meals. The weight-dependent conditions allowed progress to the next stage of food reintroduction depending on participants’ weight either with or without (stimulus-narrowing) pre-packaged meals. The mean BMI was 36.6 kg/m2 (SD 4.4 kg/m2). Data at 18 months are shown in Table 37. Participants in the time-dependent condition were more successful at maintaining weight loss than those in the weight-dependent group.
| Study (first author and year) | Time point (months) | Mean (SD) weight change (kg) | Effect size (95% CI); p-value | |
|---|---|---|---|---|
| Time-dependent regular food | Weight-dependent regular food | |||
| Agras 199682 (trial of time-dependent vs. weight-dependent reintroduction of regular food) | 18 | –7.25 (11.78) [n = 79] | –5.67 (15.47) [n = 83] |
–1.58 (–5.83 to 2.67); p = 0.466 (Favours time dependent) |
| Three meals plus snacks | Three meals only | |||
| Bertéus Forslund 200892 (trial of three meals plus snacks vs. three meals only) | 12 (unadjusted analysis) | –1.10 (6.23) [n = 44] | –3.00 (6.76) [n = 49] | 1.90 (–0.75 to 4.55); p = 0.160 |
| 12 (adjusted analysis) | –0.69 (6.11) [n = 70] | –2.10 (6.51) [n = 70] |
1.41 (–0.68 to 3.50); p = 0.187 (Favours three meals only) |
|
| ILI | Conventional obesity therapy | |||
| Burguera 201597 (trial of an ILI vs. conventional obesity therapy) | 12 (unadjusted analysis) | –14.11 (9.91) [n = 35] | –0.48 (6.05) [n = 36] | –13.63 (–17.44 to –9.82); p < 0.001 |
| 12 (adjusted analysis) | –8.23 (8.24) [n = 60] | –0.38 (6.02) [n = 46] | –7.86 (–10.68 to –5.03); p < 0.001 | |
| 24 (unadjusted analysis) | –12.70 (9.51) [n = 18] | –0.90 (6.17) [n = 29] | –11.80 (–16.27 to –7.33); p < 0.001 | |
| 24 (adjusted analysis) | –3.81 (6.99) [n = 60] | –0.57 (6.08) [n = 46] | –3.24 (–5.78 to –0.70); p = 0.012 | |
| 30 (unadjusted analysis) | –18.50 (11.15) [n = 14] | –0.90 (6.17) [n = 19] | –17.60 (–23.55 to –11.65); p < 0.001 | |
| 30 (adjusted analysis) | –4.32 (7.14) [n = 60] | –0.37 (6.02) [n = 46] |
–3.94 (–6.51 to –1.38); p = 0.003 (Favours ILI) |
|
| ASPIRE group | MOVE – usual care | |||
| Damschroder 2014103 (trial of ASPIRE vs. MOVE!) | 12 (unadjusted analysis) | –2.11 (5.37) [n = 242] | –1.40 (5.29) [n = 119] | –0.71 (–1.88 to 0.46); p = 0.235 |
| 12 (adjusted analysis) | –1.58 (4.74) [n = 322] | –1.05 (4.61) [n = 159] |
–0.53 (–1.42 to 0.36); p = 0.243 (Favours ASPIRE) |
|
| Spirituality plus dietitian counselling | Dietitian counselling only | |||
| Djuric 2009112 (trial of spirituality plus dietitian counselling vs. dietitian counselling only) | 18 (unadjusted analysis) | –1.41 (6.31) [n = 11] | –1.80 (6.43) [n = 11] | 0.40 (–4.93 to 5.72); p = 0.884 |
| 18 (adjusted analysis) | –1.29 (6.28) [n = 12] | –1.65 (6.38) [n = 12] |
0.36 (–4.70 to 5.43); p = 0.888 (Favours dietitian only) |
|
| Small group | Large group | |||
| Dutton 2014113 (trial of small- vs. large-group treatment) | 12 | –7.03 (7.90) [n = 35] | –1.70 (6.62) [n = 31] |
–5.33 (–8.87 to –1.79); p = 0.003 (Favours small groups) |
| Delayed physical activity | Initial physical activity | |||
| Goodpaster 2010121 (trial of delayed vs. initial physical activity) | 12 | –9.90 (7.49) [n = 63] | –12.10 (8.77) [n = 67] |
2.20 (–0.61 to 5.01); p = 0.125 (Favours initial physical activity) |
| BWL + H | BWL | |||
| Gorin 2013122 [trial of a behavioural weight-loss programme with home environment modification (BWL + H) vs. a behavioural weight-loss (BWL) programme only] | 18 | –7.30 (10.10) [n = 102] | –5.50 (9.95) [n = 99] |
–1.80 (–4.57 to 0.97); p = 0.203 (Favours BWL + H) |
| Telephone aftercare | Usual care | |||
| Ströbl 2013182 (trial of telephone aftercare vs. usual care) | 12 | –4.40 (7.16) [n = 228] | –3.90 (7.02) [n = 239] |
–0.50 (–1.79 to 0.79); p = 0.446 (Favours telephone aftercare) |
The effect of snacking on weight loss was evaluated in the Bertéus Forslund et al. 92 trial. Participants were randomised to two groups with different eating frequencies: three meals daily or three meals plus three snacks daily. Both groups received regular individual counselling from dietitians over 12 months. Participant group mean BMIs ranged from 38.3 kg/m2 (SD 5.3 kg/m2) to 38.4 kg/m2 (SD 6.0 kg/m2). The 12-month data are shown in Table 37. Weight loss was favoured by the three meals only group.
Participants in the Burguera et al. 97 trial were randomised to an ILI involving group behavioural therapy, physical activity advice and nutritional counselling on a Mediterranean diet with no calorie restriction or to conventional obesity therapy (COT) providing standard medical treatment for weight loss for 24 months in accordance with the Spanish Endocrine Society protocol. The mean BMI of participants in the ILI group was 45.8 kg/m2 (SD 5.0 kg/m2) and the group mean BMI in the COT group was 46.8 kg/m2 (SD 4.6 kg/m2). Data at 12, 24 and 30 months are shown in Table 37. Weight loss was greater in the ILI group at all time points.
Participants from two Mid-western USA Veterans Affairs (VA) Medical Centres were randomised to receive a weight-loss programme delivered to individuals over the telephone (ASPIRE-PHONE) or in-person group sessions (ASPIRE-group), or to the VA’s standard MOVE! WMP. 103 The ASPIRE programmes focused on small but cumulative changes in lifestyle, based on personal goals. The comparison of individual treatment with group treatment in this trial has previously been discussed in Group programmes versus individual programmes. Table 37 shows the comparison of both ASPIRE groups with the MOVE! programme. Data at 12 months favour the ASPIRE-group condition compared with the MOVE! condition when data are both adjusted and unadjusted, but between-group differences were small to moderate and estimated with uncertainty.
In the trial by Djuric et al. ,112 African American women who had survived breast cancer were randomised to receive spirituality counselling or not for 12 months in addition to a standard dietitian-led programme, delivered in person and by telephone, with free Weight Watchers® coupons. The mean BMI of participants was 36.0 kg/m2 (SD 4 kg/m2). Table 37 shows that, at 18 months, the dietitian-only group was more successful, but the difference between the groups was < 1 kg, with considerable uncertainty.
African American people were randomised to receive a behavioural weight-loss programme, delivered in either a large or a small group, in the Dutton et al. 113 trial. The large-group condition comprised 30 participants per group, whereas small-group treatment comprised 12 members per group. Treatment lasted 12 months. The mean BMI of trial participants was 36.5 kg/m2 (SD 5.7 kg/m2). Small-group treatment produced greater weight loss at 12 months, as shown in Table 37.
Participants in the Goodpaster et al. 121 trial were randomised to an intensive WMP with diet and physical activity advice for 12 months, or to the same dietary intervention but with the physical activity component delayed for 6 months. The mean BMIs of the initial physical activity group and the delayed physical activity group were 43.5 kg/m2 (SD 5.4 kg/m2) and 43.7 kg/m2 (SD 5.5 kg/m2), respectively. Table 37 shows the data at 12 months. Participants in the initial physical activity group lost 2.2 kg more than participants in the delayed physical activity group, but there was some uncertainty around this estimate.
Participants in the Gorin et al. 122 trial were randomly assigned to a standard behavioural weight-loss programme (BWL) based on Look AHEAD41 or to BWL with modifications made to the participants’ home environments (BWL + H) for 18 months. In the BWL group, only participants received treatment, whereas both participants and their support partners (resident household members willing to participate) received the intervention in the BWL + H group (e.g. monthly cupboard clearouts of high-calorie foods, provision of exercise equipment for the home and restriction of television time). The mean BMI of participants was 36.4 kg/m2 (SD 6.1 kg/m2), respectively. The BWL + H intervention favoured better weight loss at 18 months, but differences were uncertain, as shown in Table 37.
Ströbl et al. 182 randomised participants to a 3-week inpatient obesity rehabilitation programme or the same obesity rehabilitation plus a combined planning and telephone aftercare intervention. Participants in the intervention group planned individual physical activities that they intended to perform after hospital discharge and were followed up by six telephone calls from a sports therapist over 6 months. The participants’ mean BMI was 36.3 kg/m2 (SD 3.5 kg/m2). Participants in the telephone aftercare condition were more successful than the usual care participants but the between-group difference was small and the estimated CIs rule out a worthwhile difference (see Table 37).
Weight loss versus control
The Look AHEAD study
The US Look AHEAD (Action for Health in Diabetes) study41,217 was a multicentre RCT that was intended to determine whether or not weight loss reduced cardiovascular morbidity and mortality in individuals with type 2 diabetes mellitus. Sixteen centres enrolled 5145 participants, who were randomised to an ILI (intervention group, n = 2570) or diabetes mellitus support and education (control group, n = 2575). Participants in the intervention group were encouraged to lose > 10% of their body weight, with the expectation that a greater number of participants would achieve a minimum of 7% weight loss.
The intervention was modelled on group behavioural programmes and included components from the DPP and the National Heart, Lung, and Blood Institute’s clinical guidelines. 217 The ILI had a calorie goal of 1200–1800 kcal/day with < 30% of calories from fat and > 15% of calories from protein. Initially, participants chose a liquid meal replacement (substituting two meals and snacks each day) or a structured meal plan.
Tailored, home-based exercises, including walking, were prescribed, with a gradual progression towards a goal of 175 minutes of moderate-intensity exercise per week. Sites had the option of providing weekly supervised exercise classes. The intervention included training in cognitive–behavioural strategies, such as stimulus control techniques, problem-solving and relapse prevention. Individual sessions focused primarily on goal-setting, problem-solving and motivational interviewing.
The ILI included group meetings, individual sessions, telephone calls or e-mails from interventionists, optional monthly follow-up group meetings and data collection sessions over 8 years; there was a total of 204 sessions or professional contacts in this period. The intervention sessions were typically led by registered dietitians or exercise specialists.
Participants assigned to diabetes mellitus support and education (control group) were invited to attend three group educational/social support sessions each year for 4 to 6.5 years. These sessions did not teach behavioural skills. One optional educational or social support session annually was offered from year 5 until the end of the trial. Regularly scheduled clinic visits for annual assessment and telephone calls for data collection and safety monitoring were provided, totalling 27 sessions or professional contacts. Sessions were led by a registered dietitian, an exercise specialist and a nurse educator or behaviour therapist.
The effect of Look AHEAD on body weight
Long-term weight differences between the ILI group and diabetes mellitus support and education are presented in Table 38. Absolute weight loss in both groups is likely to be less than in participants without type 2 diabetes mellitus, particularly because some medicines for type 2 diabetes mellitus (e.g. sulphonylureas and insulin) promote weight gain.
| Time (months) | Differences in weight change (kg) | 95% CI (kg) |
|---|---|---|
| 12 | –7.76 | –8.16 to –7.36 |
| 24 | –5.20 | –5.58 to –4.82 |
| 36 | –3.97 | –4.34 to –3.60 |
| 48 | –3.43 | –3.80 to –3.07 |
| 96 | –2.39 | –2.76 to –2.02 |
| 115 | –2.53 | –2.94 to –2.11 |
Mean weight loss from baseline was 6.0% in the intervention group and 3.5% in the control group. Participants with BMIs of 35 to < 40 kg/m2 or ≥ 40 kg/m2 had similar percentage weight losses to participants with BMIs of 30 to < 35 kg/m2. 43 The authors discussed whether or not the educational sessions in the control group may have lessened differences between groups. Statins were used more often in the control group. 41 Of the 10% of Look AHEAD participants reporting recent binge eating at recruitment, two-thirds did not report binge eating after 1 year, and 3% reported new binge eating at 1 year. Participants who continued to binge eat or started to binge eat had significantly less weight loss at 1 year. 218
Gorin et al. 218 reported that, at 1 year, partners of participants in the intensive lifestyle arm lost 2.2 kg (SD 4.5 kg), compared with partners in the control arm, who lost 0.2 kg (SD 3.3 kg) (reported p = 0.012).
The effect of Look AHEAD on cardiovascular disease
The trial was stopped early at a median follow-up of 9.6 years on the basis of a futility analysis for the primary composite cardiovascular outcome.
Occurrence of death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke or hospitalisation for angina was reported in 403 participants in the intervention group and 418 in the control group, with no between-group difference (HR 0.95, 95% CI 0.83 to 1.09; p = 0.51). 41 The intervention did not affect the incidence of atrial fibrillation (HR 0.99, 95% CI 0.77 to 1.28). 219
Differences in cardiovascular risk factors diminished over time, with HbA1c and systolic blood pressure showing the most beneficial sustained difference. LDL was lower in the control group. 41
The effect of Look AHEAD on type 2 diabetes mellitus
After a median of 8 years, the incidence of very-high-risk chronic kidney disease was lower in the intervention group than in the control group, with incidence rates of 0.91 cases per 100 person-years in the control group and 0.63 cases per 100 person-years in the intervention group (difference of 0.27 cases per 100 person-years, HR 0.69, 95% CI 0.55 to 0.87; reported p = 0.0016). 44 Over 4 years, 3.5% (95% CI 2.7% to 4.3%) of intervention participants had a continuous remission of type 2 diabetes mellitus, compared with 0.5% (95% CI 0.2% to 0.8%) of control group participants (reported p = 0.02). 52
Participants were followed up for neuropathy over 9–11 years. There was no reported significant between-group difference for the Michigan Neuropathy Screening Instrument physical examination score (≥ 2.5 being indicative of diabetic neuropathy). 220
The effect of Look AHEAD on obstructive sleep apnoea
A total of 264 participants were recruited at four Look AHEAD sites to study the intervention effects on OSA. Remission of OSA at 48 months was reported to be five times greater in the intervention group (20.7%) than in the control group (3.6%). 51
The effect of Look AHEAD on hepatic steatosis
A substudy examined weight-loss effects on hepatic steatosis and incident NAFLD in one centre. Proton magnetic resonance spectroscopy was performed in 151 participants at baseline, and 96 (46 in the intervention group and 50 in the control group) were evaluated at 12 months, after dropouts and exclusions. The median per cent decrease in steatosis was –50.8% in the intervention group and –22.8% in the control group (reported p = 0.04). At 12 months, 1 out of 31 intervention participants (3%) and 6 out of 23 control participants (26%) without NAFLD at baseline developed NAFLD [reported odds ratio (OR) 0.07, 95% CI 0.007 to 0.71]. 45
The effect of Look AHEAD on knee pain and physical function
A subsample of 2889 participants who reported no knee pain at baseline were followed up for 48 months. Pain was assessed with the WOMAC questionnaire. At 48 months, knee pain was newly reported in 25.9% of the intervention participants and 27.2% of the control participants (reported RR 0.95, 95% CI 0.84 to 1.07). 46 At all annual assessments to year 8, intervention participants reported less knee pain than controls, but more pulled or strained muscles. 221
Physical function, assessed by the Medical Outcomes Survey Short-Form (SF-36), led to a significant improvement in the intervention group compared with the controls at 1 year, which was maintained for 8 years, with greater benefit for older participants. 221
The effect of Look AHEAD on bone loss and fractures
After 4 years, there was a small increase in bone loss at the hip from the intervention in men but not in women. 222 After a median follow-up of 11.3 years, a total of 731 participants had a confirmed incident fracture: 358 in the control group and 373 in the intervention group. Compared with the control group, the intervention group had a reported statistically significant 39% increased risk of a frailty fracture (HR 1.39, 95% CI 1.02 to 1.89), although this difference was reported as not statistically different for all fractures (HR 1.03, 95% CI 0.89 to 1.19) or hip fractures (HR 1.69, 95% CI 0.93 to 3.07). 223
The effect of Look AHEAD on urinary incontinence and erectile dysfunction
In a substudy, 1910 men were examined for urinary incontinence at 1 year. The intervention group had an increased odds of incontinence resolving (OR 1.93, 95% CI 1.04 to 3.59) and reduced new-onset urinary incontinence (OR 0.66, 95% CI 0.42 to 1.02). 49 In a substudy at five sites, there was evidence after 1 year of less worsening in erectile dysfunction. 50
In another substudy, 2739 women were examined for urinary incontinence at 1 year. The intervention was associated with a statistically significant reduction in having urinary incontinence at 1 year (OR 0.80, 95% CI 0.65 to 0.98), but not of resolution of urinary incontinence (OR 1.20, 95% CI 0.97 to 10.21). 48
The effect of Look AHEAD on depression and cognitive function
At 10–13 years after randomisation, the intervention was not significantly associated with overall or domain-specific cognitive function. 224 Using the Beck Depression Inventory over 8 years, the intervention reduced the incidence of mild or more serious depression (HR 0.85, 95% CI 0.75 to 0.97). 41
Other weight-management programmes compared with usual care or attention control
Thirty-two RCTs compared a WMP with usual care or an attention control, including Look AHEAD, involving 13,126 adults. Two were men-only trials,116,128 and five were women-only trials. 118,179,188,191,197 Ten trials recruited participants with no reported existing medical conditions or no reported increased risk of developing obesity-related comorbidities. 91,108,118,128,147,163,179,187,188,201 Other trials recruited participants with type 2 diabetes mellitus, hypertension, psychiatric illnesses, cognitive impairment, osteoarthritis of the knee, coronary heart disease (CHD) or urinary incontinence.
Two trials were undertaken in a mainly black or African American population,118,187 one in a low-income Hispanic population,188 seven in Caucasian populations87,97,123,128,189,191,202 and 16 with mixed ethnic groups. 41,83,88,90,91,97,104,108,144,148,149,158,163,170,195,197 Six trials did not report the ethnicity of the population involved in the trials. 116,142,147,179,190,201
Most trials recruited predominantly middle-aged adults. Twenty-six trials (81.2%) had a mean or median age of groups of 41–59 years41,83,87,88,90,97,104,108,116,123,128,142,144,147,163,170,179,180,187,188,191,195,197,201,202,225 and four trials (12.5%) included groups with baseline ages of ≥ 60 years. 148,149,189,190 One (3.1%) had a mean or median age of < 40 years91 and one (3.1%) did not report the age of the included participants. 158 Sixteen trials (50%) followed up participants for 12 months,87,108,128,142,144,148,149,158,170,180,187–191,202 seven (21.8%) for 18 months,88,91,104,118,147,179,197 seven (21.8%) for 24 months,83,90,116,123,163,195,201 one (3.1%) for 30 months97 and one (3.1%) for 115 months. 41 In 10 trials (31.2%), the drop-out rate was > 20% at trial completion. 87,88,97,108,123,147–149,179,202
Sixteen trials (50%) provided little description of the dietary intervention,87,88,90,91,97,104,108,118,123,147–149,188,191,197,202 Ten of the trials (31.2%) included a prescribed calorie intake diet (which varied from 800 to 1800 kcal/day) in at least one of the interventions. 41,83,116,163,170,179,180,187,195,201 Three163,187,195 provided a caloric prescription in accordance with the participants’ initial weight. The macronutrient components of the diets varied, usually providing 20% to < 30% of energy as fat. Six of the trials (18.7%) included a calorie restriction from calculated energy requirements (which varied from 500 to 1000 kcal/day). 142,144,158,189,190,226
Meta-analyses
Subgroup analyses were performed in accordance with the physical activity and diet prescription. The three major categories refer to (1) trials in which participants were provided with a physical activity programme to attend, (2) trials that provided physical advice only and (3) trials that did not report providing either. Within these three groups, trials were classified in accordance with their dietary prescription into the following subgroups: (1) trials in which a specific number of calories was prescribed, (2) trials in which a caloric deficit was prescribed or (3) trials in which the caloric deficit or calorie intake was not described.
Results for the meta-analyses are presented for weight data adjusted for dropouts at 12 months and 24 months (Figures 1 and 2). Only Look AHEAD provided data after 30 months. In both meta-analyses, the marked heterogeneity of weight loss is very apparent (I2 = 96% in both cases). Funnel plots did not suggest small-study bias.
FIGURE 1.
Meta-analysis of weight change (kg) in trials of WMP vs. usual care or attention control at 12 months. WMD, weighted mean difference.
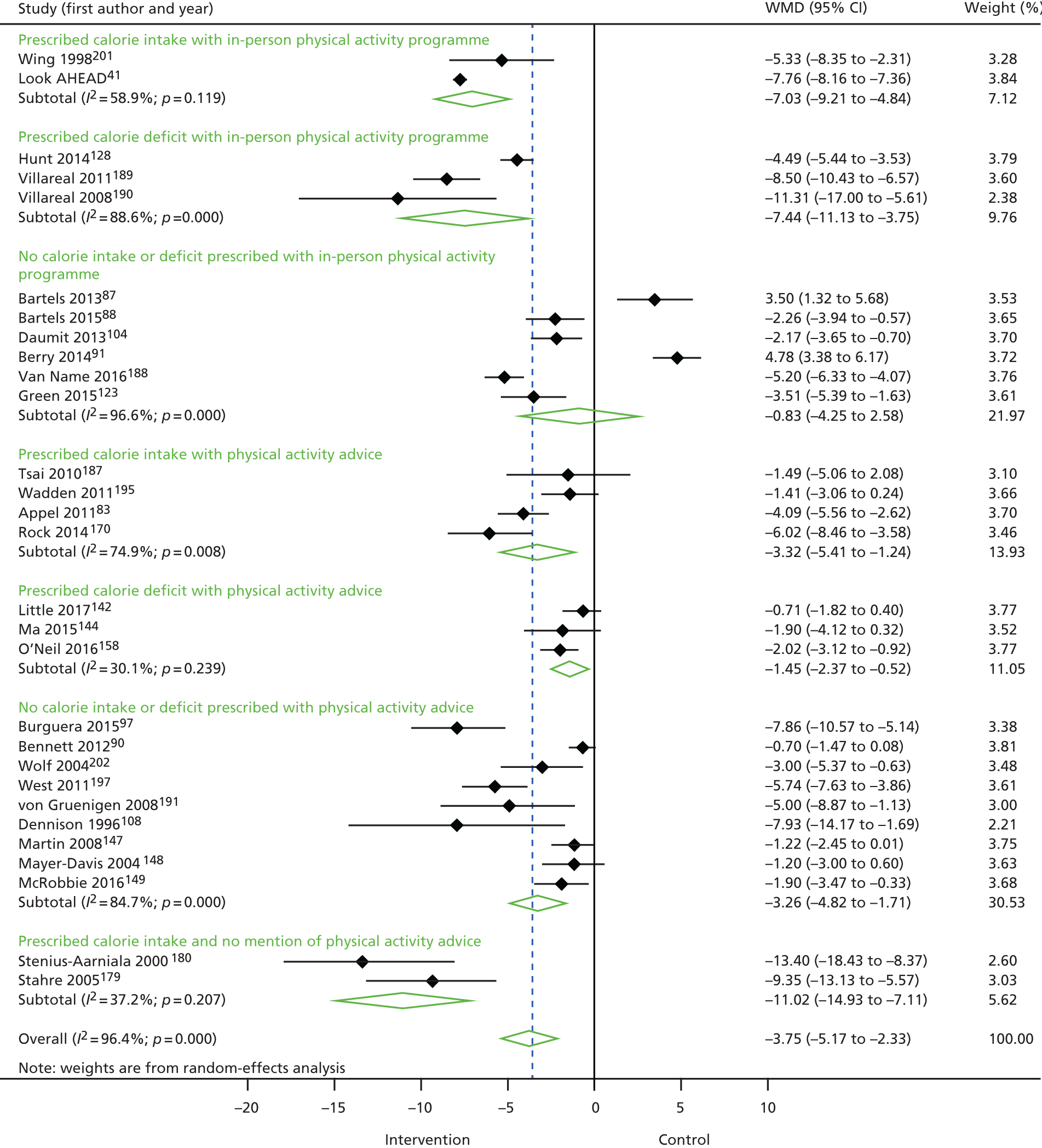
FIGURE 2.
Meta-analysis of weight change (kg) in trials of WMP vs. usual care or attention control at 24 months. WMD, weighted mean difference.

At 12 months, those trials with a physical activity programme providing a specific calorie intake (n = 2 trials)41,201 had a mean difference weight change of –7.03 kg (95% CI –9.21 to –4.84 kg), mainly influenced by Look AHEAD. Trials with a prescribed calorie deficit and physical activity programme had a mean difference weight change of –7.44 kg (95% CI –11.13 to –3.75 kg).
Trials with physical activity advice only generally had lower weight loss. Two small trials that did not describe providing exercise advice, prescribing a VLCD180 or a 1200- to 1300-kcal/day diet with a CBT programme179 (n = 2 trials, mean difference –11.02 kg, 95% CI –14.93 to –7.11 kg) had the greatest effect on weight.
To provide sufficient data for meta-analysis, data from 18 and 24 months of follow-up were combined in Figure 2 (whichever was the later available time point for each trial). At 24 months, Look AHEAD41 continued to show good weight loss. In addition, the trial by Esposito et al. ,116 with a calorie intake of 1700–1900 kcal/day for men, with supervised, individually tailored physical activity sessions, demonstrated very good weight loss. The small trial by Stahre and Hällström179 also continues to demonstrate effective weight loss.
Metaregression
The heterogeneity in weight loss from the 32 trials led us to begin an exploration of predictors of weight loss in all arms from all non-surgical RCTs in this chapter. We focused initially on demographic characteristics (age, sex and body weight) and treatment variables (physical activity programme to attend, VLCD, other prescribed calorie intake, prescribed calorie deficit, calorie deficit or intake not described, orlistat).
The metaregression controls for age and sex and fits dummy variables for the different intervention characteristics. ‘Active component’ in Tables 39 and 40 indicates the arm of the trial had that particular component. In many of the trials included in the metaregression, all arms have active components. Results are presented for weight change in kg at 12 and 24 months, again using 18-month data for trials that had no 24-month data. Adding in a dummy variable for a control arm intervention had little influence on these results (see Tables 39 and 40).
| Intervention characteristics | Estimate | 95% CI | p-value |
|---|---|---|---|
| Active component present | –1.1313 | –2.50 to 0.24 | 0.1046 |
| Physical activity programme to attend | –0.5501 | –2.00 to 0.90 | 0.4573 |
| VLCD | –6.5867 | –9.58 to –3.59 | < 0.0001 |
| Prescribed calorie intake | –4.4699 | –6.68 to –2.26 | < 0.0001 |
| Calorie deficit prescribed | –3.0189 | –5.29 to –0.75 | 0.0092 |
| Other diets | –0.3826 | –2.60 to 1.83 | 0.7351 |
| Orlistat | –0.5997 | –2.61 to 1.42 | 0.5596 |
| Intervention characteristics | Estimate | 95% CI | p-value |
|---|---|---|---|
| Active component present | –0.1738 | –1.68 to 1.33 | 0.8212 |
| Physical activity programme to attend | 0.9226 | –0.39 to 2.23 | 0.1667 |
| VLCD | –5.5437 | –8.72 to –2.37 | 0.0006 |
| Prescribed calorie intake | –4.0291 | –6.38 to –1.68 | 0.0008 |
| Calorie deficit prescribed | –2.8319 | –5.38 to –0.29 | 0.0293 |
| Other diets | –1.1663 | –3.54 to 1.21 | 0.3363 |
| Orlistat | –1.4179 | –4.00 to 1.16 | 0.2816 |
All of the interventions have the effect of reducing weight. The largest effect sizes and significant effects are seen for VLCDs, and diets with a specified calorie intake, or diets with calorie deficit with decreasing size of effect.
The results at 24 months are similar to the 12-month results, but with less weight loss.
Further detailed exploration is required to examine the influence of moderators (group/individual, in-person/remote delivery, expertise of staff, BCTs used and intervention intensity) but is outside the timescale of this report.
Discussion
Effective interventions used for our economic modelling
Although we identified 131 RCTs with follow-up of ≥ 12 months, trials of WMPs other than surgery were remarkable for their lack of longer term follow-up. Only the Look AHEAD trial41 and two small trials from Finland124,125 had follow-up of ≥ 5 years. The lack of long-term data, and thus the necessary assumptions made about weight regain, make economic modelling of WMP very challenging. As a result, the Look AHEAD trial intervention was chosen to model because this trial reported very long-term weight-loss data in people with type 2 diabetes mellitus, which might have underestimated weight loss from the same intervention in people without diabetes mellitus, and it described including many of the components that we found increased effectiveness in our systematic review. Look AHEAD was an intensive group and individual intervention with a calorie goal of 1200–1800 kcal/day (< 30% of calories from fat and > 15% of calories from protein). Initially, participants chose a liquid meal replacement or a structured meal plan.
Tailored, home-based exercises were prescribed, and some trial sites provided exercise classes. The intervention included training in cognitive–behavioural strategies, such as stimulus control techniques, problem-solving and relapse prevention. Individual sessions focused primarily on goal-setting, problem-solving and motivational interviewing.
Look AHEAD had a beneficial impact on many obesity-related comorbidities, many of which are in addition to those used in our economic modelling. The Look AHEAD intervention reflects intensive WMPs (with long-term support), had a large sample size, and was well conducted and described, including its costing. Variants of this intervention tested in trials in the USA have also demonstrated effectiveness.
The choice made was to model RYGB bariatric surgery, which produced greatest long-term weight loss from current types of surgery, and its comparator interventions from RCTs. In most cases, the comparator interventions were similar to, or based on, short-term, less-intense versions of the DPP/Look AHEAD intervention. 41 It is difficult to relate the comparator interventions in RYGB trials to existing Tier 3 services in the UK, which have no standardised approach. One RYGB trial had many more contacts with participants than would be likely in Tier 3 services,101 but others were more comparable. We did not include the RYGB trial by Reis et al. 169 in our modelling because it gave only a minimal intervention to the comparator group. RYGB provided data from the most RCTs, and had the longest reported follow-up (5 years), including the large trial by Schauer et al. 174 RYGB is presently the most common procedure undertaken in the UK. 227
There is increasing interest in the use of VLCDs, such as in the recent DiRECT (Diabetes Remission Clinical Trial) trial showing that a VLCD-based programme of support can lead to regression of type 2 diabetes mellitus at 12 months. 228 Presently, data are available only from the first 12 months of this trial, and the mean BMI of participants was lower than that in trials included in our review. The large trial in our review by Purcell et al. 166 found that a rapid weight-loss programme with a VLCD did not lead to less weight being regained over 3 years compared with a gradual weight-loss programme with a deficit of 400–500 kcal/day. The VLCD arm had significantly lower attrition. In our review we looked at the effect of adding a VLCD to an existing WMP, which gave an additional mean weight loss of 4.41 kg (95% CI 5.93 to 2.88 kg) at 12 months, but with very little difference in weight loss after this time point. We undertook an economic evaluation of the effect of adding a VLCD to an existing WMP, modelling both arms of trials, which was an important question for weight-management services to have answered. It is essential to examine much longer-term outcomes from adding VLCDs, not just for weight, but also clinical outcomes and adverse events, including the best weight maintenance interventions after these diets. Eight RCTs examined different approaches to weight maintenance after VLCDs.
Orlistat76 and dietary counselling99 (as opposed to an exercise programme to attend) showed effects in preventing weight regain. There was also some evidence to suggest that using VLCD products as meal replacements after weight loss with a VLCD could help with weight maintenance. 172,173
Effects of intervention components in weight-management programmes
Our review of other aspects of WMPs is summarised here, focusing on the results for adjusted weight outcome data. Not surprisingly, more intensive interventions, often with more contacts with WMP personnel (in person or remotely), were usually associated with greater weight loss and better weight maintenance, but starting with an inpatient programme was not associated with greater weight loss. Long-term weight maintenance, after weight-loss programmes that did not incorporate VLCDs, was improved by the use of orlistat,105,176 providing telephone or face-to-face follow-up,162 training in relapse prevention or problem-solving. 164
We were able to compare some differences in reducing diets. Data favoured increased weight loss only at 12 months for low-carbohydrate diets (≤ 40 g/day) compared with low-fat diets, but most effect was seen in one trial89 that did not have an energy prescription for the low-fat diet. Similarly, higher-protein diets compared with lower-protein diets (generally 30–34% vs. 15–20% of energy from protein) were favoured at 12 months, but not longer term. Effects for each were of the order of 1 kg of extra weight loss at 12 months. Consistent beneficial effects on all cardiovascular risk factors were not seen with either of these two diets producing more weight loss. The use of meal replacements, such as giving component parts of VLCDs (e.g. shakes to replace meals or the provision of ready-made meals), was also associated with greater weight loss at 12 months.
Three trials looked at the benefit of adding an intensive physical activity programme to attend. Weight change was greater with the exercise programme, particularly at 24 months, suggesting benefits to weight-loss maintenance (mean difference at 24 months –2.59 kg, 95% CI –5.35 to –0.41 kg). 189,194,201 In one trial,121 delaying the introduction of the physical activity programme for 6 months did not appear beneficial (mean difference 2.20 kg, 95% CI –0.61 to 5.01 kg). Adding a brief planning intervention and six telephone calls to support physical activity showed little effect on weight change at 12 months (mean difference –0.50 kg, 95% CI –1.79 to 0.79 kg). Thus, it appears that providing intensive exercise sessions for people as part of a weight-loss programme may enhance weight maintenance, but brief behavioural support to encourage home-based self-guided physical activity does not. 182
Orlistat in doses of 180 mg per day or 360 mg per day was associated with greater weight loss and weight-loss maintenance of about 2–3 kg for the duration of the RCTs, which was up to 4 years, at the expense of GI adverse events. Cardiovascular risk factors were beneficially changed at 12 months with the higher dose, apart from HDL cholesterol.
Group-delivered programmes were more effective than individually delivered programmes. They appeared to lead to greater weight loss at 12 months (mean difference –1.05 kg, 95% CI –2.04 to –0.06 kg), and were mostly favoured up to 36 months, but most trials in the comparison had more participant contacts for the group programmes than for the individual programmes. Only one small trial investigated the size of groups113 to find that weight change at 12 months was favoured by having a group size of 12 compared with 30 (mean difference –5.33 kg, 95% CI –8.87 to –1.79 kg).
For participants attending programmes in person, additional contact by telephone or internet support favoured greater weight change at 12 months only (mean difference –1.88 kg, 95% CI –3.55 to –0.21 kg). However, trials that compared a programme delivered in person to the same programme delivery remotely, with similar numbers of participant contacts, showed little difference in effectiveness. However, this evidence is clouded because two in-person-delivered programmes103,142 were not particularly effective for either means of delivery. As a consequence, it presently appears that delivery of a whole programme remotely has little weight outcome evidence to support it, but additional telephone and internet contacts beyond the end of the programme may be modestly effective.
There were many small RCTs individually examining different approaches to behavioural counselling. One trial75 favoured better weight change from adding mindfulness to a WMP at 12 months (mean difference –1.79 kg, 95% CI –3.57 to –0.02 kg), with similar results at 18 months. Another trial78 favoured motivational interviewing as part of a WMP over an attention control with WMP for up to 18 months (mean difference –1.80 kg, 95% CI –3.53 to –0.07 kg). Two trials of CBT versus standard behaviour therapy favoured greater weight change at 12 months from CBT (mean difference –3.01 kg, 95% CI –4.68 to –1.33 kg). 141,146
Two small trials138,199 examined the added benefit of enrolling members of the participant’s family for support. Although favouring such support, differences were minimal at 12 months (mean difference –0.59 kg, 95% CI –1.80 to 0.61 kg) and later time points. In one trial Gorin et al. 122 examined the added effect of modifying the home environment on weight change from a WMP at 18 months (mean difference –1.80 kg, 95% CI –4.57 to 0.97 kg). Thus, the evidence suggests that incorporating family members or modifying the home environment can have modest additional effects at best, but there is currently little evidence that they are effective.
Interventions that supported participants to improve their mental health and diet through healthy eating approaches, without a focus on a prescribed calorie intake or deficit, led to less weight loss than weight-loss interventions with this focus. Moreover, there was no evidence that they improved psychological health.
Weight-management programmes compared with usual care or attention control
Around one-quarter of the RCTs compared a non-surgical WMP with usual care or an attention control. The two meta-analyses graphs (see Figures 1 and 2) illustrate the marked heterogeneity in weight loss at 12 and 24 months compared with usual care or attention control. From metaregression for 12- and 24-month weight loss, the use of VLCDs, providing a prescribed calorie intake or a prescribed calorie deficit were important predictors, with a prescribed calorie intake appearing to have modestly more effect than a calorie deficit. Providing an additional in-person physical activity programme to attend, or orlistat, were not significant predictors.
Of note is that NICE guidelines22 generally recommend a 600-kcal/day deficit diet and state:
Consider low-calorie diets (800–1600 kcal/day), but be aware these are less likely to be nutritionally complete.
Guidelines in the USA229 include calorie deficit prescribing or prescribing a calorie intake for weight reduction of 1200–1500 kcal/day for women and 1500–1800 kcal/day for men, adjusted for body weight and energy requirements, without qualification. Prescribing calorie intakes could be simpler to implement and follow, particularly given the known difficulties in estimating energy requirements230 and, therefore, in calculating a deficit, particularly for people with higher BMIs.
Meta-analyses of WMPs versus usual care or attention controls are likely to underestimate weight loss from WMPs, because usual care or attention themselves produce weight loss of around 1 kg after 12 months. 231–233 Further work is needed to explore predictors of weight loss in trials, particularly the influence of BCTs, beyond our preliminary metaregression.
Strengths and weaknesses
How generalisable are the results from these 131 RCTs to people in the UK with a BMI of ≥ 35 kg/m2 or likely recipients of Tier 3 WMPs, given that RCTs already involve selection processes unlike clinical care? Apart from trials of orlistat, three trials were set in UK general practice142,149,234 and none took place in UK services equivalent to Tier 3 services. Although these three trials included participants, on average, with severe obesity, the services delivered were similar to Tier 2 services, being without intensive multidisciplinary care for diet and physical activity changes.
Trials involving orlistat often included run-in periods before randomisation, which would influence the interpretation of trials’ results through attrition in the run-in, but also by appearing to reduce the weight loss after randomisation. 235
Only one WMP had a focus on younger age groups115 and only two WMPs focused on groups aged > 60 years. 189,190 The majority of RCTs reported including participants with comorbidities related to their obesity, but trials for participants with particular obesity-related conditions, such as non-alcoholic fatty liver disease, polycystic ovary disease or infertility, were not found to be included in this review. Mean BMIs in trials were generally lower than those for participants for Tier 3 services in the UK, who usually have reported mean BMIs of ≥ 40 kg/m2. Only 13 of the non-surgical RCTs had mean BMIs of ≥ 40 kg/m2. Many trials excluded participants with eating disorders, mental health issues or a history of addiction.
In general, RCTs tended to be small and underpowered, with poor reporting of items for risk of bias, equity and BCT coding (despite requests for additional information). However, we did not identify clear evidence of small-study bias for RCTs of WMPs versus control/usual care.
We attempted to give an indication of the weight changes from interventions by presenting data corrected for dropouts as well as data presented by the investigators. The real weight outcomes are likely to lie somewhere between the two.
There is a growing literature on the need to improve the quality of the design, conduct, analysis and reporting of weight-loss studies. Issues that have been highlighted that need addressing include selective reporting of outcomes,236 infrequent fidelity reporting237 and statistical errors238 including, but not limited to, mishandling of clustering, mishandling missing data and p-value hacking. 239
Overall summary
We considered network meta-analyses for some comparisons in the report, but the heterogeneity of interventions and study population made this inappropriate. We summarise below the main points that have arisen from the review in this chapter. It is clear from the RCTs that sustainable long-term weight loss for people with severe obesity may require intensive interventions. Such interventions are most likely to succeed when the obesogenic environment is also able to support long-term behaviour change.
General issues relating to methodology
-
Randomised controlled trials should report in greater detail items needed to assess risk of bias, equity (PROGRESS items), TIDieR checklist, coding for BCTs and fidelity data. Provision of protocols and WMP materials would contribute to the evidence base for improving the design of WMPs, and allow assessment of replicability.
-
Randomised controlled trials should have adequate statistical power and attempt much longer-term follow-up for weight, comorbidities and adverse event data, ideally for ≥ 5 years.
-
From the evidence here, we were unable to determine whether hard-to-reach or disadvantaged groups, younger or older people, and groups with higher BMIs and/or eating disorders were adequately represented and not more likely to discontinue WMPs and achieve the same weight loss and health outcomes. Economic evaluations should explore outcomes for these subgroups. It is important to address this issue before designing particular specialist services.
-
Randomised controlled trials should evaluate different approaches for participants with higher BMIs than examined here (i.e. BMIs of ≥ 40 kg/m2), and should take into account the higher prevalence of eating disorders and histories of various forms of abuse in participants with very high BMIs. Evaluations of interventions are particularly needed in younger adults, as young women from disadvantaged backgrounds are particularly over-represented, and older adults.
-
A systematic review of weight-management approaches for people with severe obesity and eating disorders, who are disproportionately represented in Tier 3 services, is required. This may require or benefit from individual participant data meta-analysis.
-
Randomised controlled trials to examine different approaches in the setting of Tier 3 weight-loss services would be valuable, particularly to examine whether or not VLCDs should be used routinely.
-
Randomised controlled trials comparing calorie-prescribed diets with diets with a prescribed deficit would be useful to see if the former are easier to prescribe and adhere to, and are, therefore, more effective for weight loss.
-
There has been a focus on achieving 5% or 10% weight loss for cardiovascular benefit in WMPs. However, people with severe obesity are at risk of many equally important comorbidities, such as osteoarthritis, sleep apnoea and non-alcoholic fatty liver disease. It is important to assess whether 5% or 10% long-term weight loss is sufficient to have important benefits for these comorbidities.
-
There is a pressing need to agree common methods of analysis and reporting for weight outcome data to allow comparisons to be made across RCTs (e.g. whether or not to present data for completers and all participants as last observation carried forward and BOCF).
-
Analysing the later stages of weight-loss programmes (e.g. after 6 months) would be useful to examine which components of interventions lead to better weight-loss maintenance.
-
It was often difficult to interpret and analyse data from trials that were badged as ‘weight maintenance’, because data for the weight-loss period and the period of the randomised evaluation of alternative weight maintenance interventions were often combined. It would be helpful if investigators could provide data separately for each stage for participants as randomised.
Indicators for effective interventions
-
For severe obesity, effective long-term weight loss from a lifestyle WMP has been demonstrated for people with type 2 diabetes mellitus in the Look AHEAD trial. This intensive intervention’s diet incorporated < 30% from fat, a calorie goal of 1200–1800 kcal/day, initial meal replacements or diet plans, tailoring of exercise and the option of supervised classes, cognitive–behavioural strategies, group and individual in-person contact and follow-up by telephone or e-mail.
-
Bariatric surgery produces greater long-term weight loss than any present lifestyle WMP, with RYGB producing the greatest weight loss from current surgical techniques. Long-term remission of type 2 diabetes mellitus and other comorbidities has been clearly demonstrated in RCTs. Improved collection and reporting of long-term adverse event data from RCTs of bariatric surgery are required.
-
The addition of VLCDs to existing dietary approaches produces greater weight loss at 1 year, and may reduce dropouts from WMPs and increase motivation, particularly for people with severe obesity, who may have tried many other approaches to weight loss. All of the RCTs utilised VLCDs in structured behavioural support programmes with trained personnel. One RCT of a VLCD versus control demonstrated much greater weight loss at 12 months than any other WMP.
-
Diets with very low-carbohydrate or higher protein content than is typical of the Western diet lead to slightly greater weight loss at 12 months.
-
Adding the use of meal replacements to a behavioural weight-loss programme leads to slightly greater weight loss than the same programme without support.
-
Adding intensive supervised physical activity sessions to a behavioural weight-loss programme leads to modestly greater weight loss at 24 months than the weight-loss programme alone.
-
Prescribing orlistat to people following a behavioural weight-loss programme, including for weight maintenance, leads to additional weight loss compared with the behavioural weight-loss programme alone.
-
There is evidence that adding behavioural components to a standard behavioural weight-loss programme is more effective than the behavioural weight-loss programme alone. The components that may be effective include telephone or internet support after the end of the programme, group support, CBT, motivational interviewing and mindfulness. These intervention components generally produce longer-term weight-loss changes than 12 months.
-
Weight-neutral interventions did not appear to be generally helpful in the context of the RCTs examined here.
Chapter 4 Results for UK studies
In this chapter, we provide the results of the systematic review of UK non-surgical interventions for people with severe obesity (BMI of ≥ 35 kg/m2), taking place in any setting, with any interventional study design with a minimum duration of 12 months of follow-up.
Quantity of evidence
Our primary literature searches identified 3373 potentially relevant titles and abstracts (the PRISMA flow chart is presented in Report Supplementary Material 4, Figure E16). We contacted 18 commercial organisations and the specialist interest group for obesity of the British Dietetic Association for details of any other studies. For our review of UK studies of any design, we selected 79 reports for full-text assessment. We identified 26 eligible reports of participants with severe obesity (see Report Supplementary Material 1, Section 9, for a list of included studies, and Section 10 for a list of excluded studies).
Characteristics of the studies
Randomised controlled trials constituted 30.7% of the 26 included reports,95,96,117,128,142,149,167,234 including a 9-month RCT after a 3-month non-randomised screening period; 7.6% were non-randomised comparative studies240,241 and 57.7% were non-comparative studies. 241–256 Sixteen per cent were published before or during 2000,117,167,243,244 44% were published between 2001 and 201095,96,234,240,241,245–250 and 40% were published from 2011 onwards. 128,142,149,242,251–257
Fifteen studies had follow-up of 12 months,95,96,117,128,142,149,167,241,243,245,246,249,251,253,255 one had follow-up of 15 months,247 two had follow-up of 18 months,234,244 six had follow-up of 24 months,242,248,250,252,254,256 one had follow-up of 36 months257 and one of 60 months. 240
One study was a community-based intervention (Scottish Premier League football clubs),128 40% took place in primary care,142,149,167,234,243,246,249–251,253 12% took place in both primary and secondary care investigating orlistat,95,96,117 11 took place in secondary care (one took place in a nephrology clinic,252 three took place at diabetes mellitus clinics,240,245,248 seven took place at specialist weight-management clinics241,242,244,247,254–256 and one took place in a commercial setting257). In terms of location, 61.5% took place in England,142,149,167,234,240,242–249,254,256 15.4% took place in Scotland128,251,253,255 and 15.4% took place in more than one country of the UK. 95,96,117,250
Characteristics of the participants
A total of 22,093 participants started interventions, and 8552 were included in analyses at final follow-up, but numbers were sometimes unclear. Two studies included only women167,247 and one included only men. 128 Participant numbers varied from 26243 to 6715. 251 Women represented 68.5% of the total population. The age (weighted mean) was 48.4 years. The youngest reported mean age was 39.9 years241 and the oldest was 55.8 years. 249 The BMI (weighted mean) of all participants was 38.3 kg/m2, the lowest149 mean BMI was 35 kg/m2 and the highest256 mean BMI was 50 kg/m2. Fifteen of the studies reported including participants with baseline comorbidities, including angina,246 asthma,256 CVD,149,242,250 depression,242,254 type 2 diabetes mellitus,96,142,149,240,242,245,246,250,252,254,256 dyslipidaemia,95,244 hypertension,96,242,244,246,250,252,254,256 impaired fasting glucose,95,249,250 metabolic syndrome,252 osteoarthritis,256 sleep apnoea242,254 and stroke. 256 Two studies exclusively recruited participants with type 2 diabetes mellitus,240,245 one recruited participants with type 1 and type 2 diabetes mellitus,248 one recruited participants with chronic kidney disease252 and one recruited participants with hypercholesterolaemia. 95
Risk of bias
Seven RCTs are also discussed in Chapter 3;95,96,117,128,142,149,167 these studies’ risk of bias was judged by domains of The Cochrane Collaboration’s risk-of-bias tool (see Report Supplementary Material 4, Figure E17). Of these, 57.1% were judged to be at high risk for blinding of health-care providers and participants and 57.1% were judged to be unclear for blinding of outcome assessment and allocation concealment; 42.8% were judged to be at low risk of bias for sequence generation, selective reporting and other types of bias. Quality assessment and equity assessment are presented in Report Supplementary Material 1, Sections 11 and 12.
The remaining 18 studies were assessed using the ReBIP quality assessment tool for non-randomised comparative and case series studies. Report Supplementary Material 4, Figure E18, summarises the risk-of-bias assessment for these studies (see Report Supplementary Material 1, Section 11, for a full description of items and results; items in italics are valid for comparative studies only). The majority of studies (77.8%) were judged to have included a representative participant sample, 83.3% provided the intervention with an experienced person and used an objective outcome measurement, 66.7% collected data prospectively, 11.1% did not collect data prospectively and in 22.2% it was unclear how data collection was undertaken. Only half of the studies provided information on participants dropping out.
Assessment of equity and sustainability
Results for equity and sustainability for all studies are detailed in Report Supplementary Material 4, Figure E19 (see Report Supplementary Material 1, Section 12, for a full description of items and results). Just under half of the studies (42.3%) were conducted in settings that might exclude specific populations. 128,167,240,241,243,245,247–249,251,254 A high proportion of studies (80.8%) did not report sociodemographic differences between those completing and withdrawing, although 65.3% reported details for some PROGRESS categories. Few (19.2%) considered sustainability, but half (50%) discussed their interventions in political or other organisational contexts. One of the studies251 reported a partnership with the Scottish Government. Lean et al. 253 reported a partnership between the NHS and the Counterweight Programme, and Read et al. 246 reported partnership between the NHS, local authorities, community groups and voluntary and business sectors.
Adverse events were reported in 24% of studies. Some studies (30.8%) reported potential conflicts of interest. Orlistat studies95,96,117,248 were funded by the drug manufacturer. Lean et al. 253 reported that some authors received departmental research funding, lecture fees and support from the Cambridge Weight Plan® and others as part of the Counterweight Company and/or scientific board. For the LighterLife study by Rolland et al. ,241 some authors acted as LighterLife consultants and research funding was provided by the LighterLife Company.
Although Jennings et al. 254 declared that they had no conflict of interest, the Cambridge Weight Plan® donated products and one of the authors reported receiving grants from LighterLife and personal fees from Novo Nordisk, Merck Sharp & Dohme Limited and Boehringer Ingelheim/Eli Lilly and Company Alliance outside the submitted work. In 16% of the studies, the conflict of interest description was unclear.
Fidelity overview
There was a fidelity assessment of delivery in only two studies. 128,149 Adherence to the intervention was measured in seven studies. 95,96,128,142,149,249,252 Studies reported weight data in many different formats (e.g. change in kg or BMI or percentage weight change, or start and finish weight or BMI), meaning that data manipulation was required to allow some comparability.
Assessment of effectiveness
Because of the heterogeneity of the included studies, their presentation of results and missing denominators, we could not carry out a quantitative synthesis of the results. Instead, a narrative overview is presented. Characteristics of the included studies are presented in Report Supplementary Material 5, Table E18.
Interventions in community settings
One intervention was developed solely in a community setting. The Football Fans in Training (FFIT) RCT128 assessed a WMP delivered to football fans under the auspices of the Scottish Professional Football League. The researchers deliberately used a football club-based nutritional advice and exercise programme for men only (aged 35–65 years). Participants were recruited via clubs (e.g. club websites or in-stadium publicity) and media [e.g. newspapers, BBC (British Broadcasting Corporation) and independent radio]. Men were randomised to the FFIT programme or a waiting list control, which may have influenced weight management in the control group. There were 90-minute sessions weekly for 12 weeks, followed by a weight maintenance phase with six post-programme e-mails over 9 months and a group reunion at the club 6 months after the end of the programme, resulting in 22 sessions. Community coaching staff employed by clubs and trained by research staff delivered the intervention combining healthy eating advice, which was designed to provide a calorie deficit of around 600 kcal/day with the physical activity programme.
The FFIT men (mean BMI of 35.5 kg/m2) achieved significant weight loss, reduction of waist circumference and blood pressure (Table 41), compared with the waiting list men. The attrition rate was very low, with 89% of the intervention group returning at 12 months.
| Outcome | FFIT, mean (SD) | Waiting list, mean (SD) | ||
|---|---|---|---|---|
| Baseline (N = 374) | 12 months’ follow-up (N = 333) [% dropouts] | Baseline (N = 374) | 12 months’ follow-up (N = 355) [% dropouts] | |
| Baseline weight and change at follow-up (kg) | 110.3 (17.9) | –5.6 (8.1) [11.0] | 108.7 (16.6) | –0.6 (5.2) [5.1] |
| Baseline BMI and change at follow-up (kg/m2) | 35.5 (5.1) | –1.8 (2.6) | 35.1 (4.8) | –0.2 (1.7) |
| Weight change from baseline (%) | – | –5.0 (7.0) | – | –0.5 (4.7) |
| Baseline waist circumference and change at follow-up (cm) | 118.7 (12.3) | –7.3 (7.7) | 118.0 (11.1) | –2.0 (5.6) |
| Baseline systolic blood pressure and change at follow-up (mmHg) | 139.4 (17.6) (n = 374) | –7.9 (15.0) (n = 318) | 141.2 (14.9) (n = 371) | –6.6 (12.8) (n = 351) |
| Baseline diastolic blood pressure and change at follow-up (mmHg) | 88.2 (10.3) (n = 374) | –4.6 (9.2) (n = 318) | 89.5 (10.1) (n = 371) | –3.8 (8.5) (n = 351) |
Five serious adverse events were reported in the intervention group, and three were reported in the control group. Only two of these were reported as related to participation in the programme: one participant ruptured an Achilles tendon while playing football during the FFIT programme; another had abdominal pain from gallstones and cholecystectomy, which could have been aggravated or caused by weight loss.
NHS primary care
We identified 10 WMPs delivered in NHS primary care. 142,149,167,234,243,246,249–251,253 One142 was partly internet based, one234 was a weight-loss training programme delivered to general practice teams and two of the studies243,253 included VLCDs in the intervention. Four were RCTs142,149,167,234 and the rest were non-comparative studies. Overall weight change, percentage weight change and BMI change are presented in Table 42.
| Study (first author and year) | Baseline, outcome (SD); n | 12 months, outcome (SD) [% dropouts] | 24 months, outcome (SD) [% dropouts] |
|---|---|---|---|
| Jackson 2007249 | |||
| Weight (kg) | 103.2 (16.9); n = 89 | –11.6 [Unclear]a | – |
| Weight change (%) | – | –11.2 | – |
| BMI (kg/m2) | 37.4 (5.9) | –4.3 | – |
| Lean 2013 (VLCD)253 | |||
| Weight (kg) | 131.1 (25.2); n = 91 | –12.4 (11.4) [25.3]a | – |
| Weight change (%) | – | –9.1 (8.2) | – |
| BMI (kg/m2) | 48 (7.6) | –4.5 | – |
| Little 2017 (POWeR+ face to face)142 | |||
| Weight (kg) | 102.4 (16.9); n = 269 | –3.8 [17.8]a | – |
| Weight change (%) | – | –3.7 | – |
| BMI (kg/m2) | 36.7 (5.4) | –1.4 | – |
| Little 2017 (POWeR+ remote group)142 | |||
| Weight (kg) | 102.9 (18.3); n = 270 | –3.2 [19.3]a | – |
| Weight change (%) | – | –3.1 | – |
| BMI (kg/m2) | 36.3 (5.7) | NR | – |
| McRobbie 2016 (Weight Action Programme)149 | |||
| Weight (kg) | 95.5 (15.8); n = 221 | –4.2 (7.3) [32.5]b | – |
| Weight change (%) | – | –4.4 | – |
| BMI (kg/m2) | 35.0 (4.2) | –1.5 (2.6) | – |
| Molokhia 1998 (VLCD)243 | |||
| Weight (kg) | 102.9 (19.1); n = 25 | –15 (9.6) [3.8]a | – |
| Weight change (%) | – | –14.5 | – |
| BMI (kg/m2) | 38.7 (6.6) | –6.1 (3.7) | – |
| Read 2004246 | |||
| Weight (kg) | 108 (20); n = 216 | –11.5 [66.2]a | – |
| Weight change (%) | – | –10.6 | – |
| BMI (kg/m2) | 39.7 (6.9) | –4.2 | – |
| Counterweight 2008250 | |||
| Weight (kg) | 101.1 (NR); n = 1906 | –3.0 (6.6) [54.8]a | –2.3 (8.7) [56.7] |
| Weight change (%) | – | –2.9 | –2.3 |
| BMI (kg/m2) | 37.1 (6.0) | –1.1 (2.4) | NR |
| Counterweight 2012251 | |||
| Weight (kg) | NR; n = 6715 | –3.7 (12.2) [72]a | – |
| Weight change (%) | – | NR | – |
| BMI (kg/m2) | 37.0 (6.2) | NR | – |
| Rapoport 2000167 | |||
| Weight (kg) | 94.0 (16.1); n = 37 | –1.9 [18.9]a | – |
| Weight change (%) | – | –2 | – |
| BMI (kg/m2) | 35.4 (6.3) | –0.9 | – |
Weight-management programmes delivered in person
Jackson et al. 249 implemented a non-comparative prospective (before-and-after) study of a specialist health visitor-led and non-pharmacological WMP. The study focused on the creation of a therapeutic connection with the participant rather than on weight loss. Participants were referred by primary care staff, and the study was provided by the specialist health visitor in a health centre in a moderately deprived area. Each participant received 32 individual face-to-face sessions, the average consultation time of which was 20 minutes. Information about healthy eating and physical activity was provided, including 5-day menu plans (developed with the dietetics department). Participants were encouraged to share their knowledge and experiences with the specialist health visitor as reflective and learning opportunities. The attrition rate was unclear, as the length of follow-up for all participants was uncertain; from 89 participants initially recruited, weight data for 29 (33%) were provided at 12 months. Authors reported that, at baseline, the mean weight of participants (n = 86) was 103.2 kg (SD 16.9 kg). After 12 months, the weight change was –11.6 kg (SD unclear, n = 29). The mean BMI was 37.4 kg/m2 (SD 5.8 kg/m2, n = 86) at baseline and 33.1 kg/m2 (SD 5.7 kg/m2, n = 28) at 12 months. The authors reported significantly decreased weight, BMI, systolic blood pressure, diastolic blood pressure and fasting blood sugar, but not cholesterol, for those reported on at 12 months. Approximately 1 in 10 of the participants was identified as having undiagnosed diabetes mellitus.
Read et al. 246 evaluated a non-comparative pilot, dietitian-run WMP. Seven 2-hour education and face-to-face group sessions were run by a dietitian at intervals of 2 weeks. Further 2-hour sessions were at 4, 6, 9 and 12 months, totalling 13 sessions. Each session concluded with participants devising personal aims. No details were provided on calorie content. Physical activity advice was provided. Each participant was given a personal folder for information handouts and progress sheets. Only 33.8% (73/216) of the participants completed the 12-month follow-up. The initial mean weight of participants (n = 216) was 108 kg (SD 20 kg), and at 12 months the weight change was –11.5 kg (SD 16.9 kg, n = 73). The initial mean BMI was 39.7 kg/m2 (SD 6.9 kg, n = 216) and at 12 months it was 35.5 kg/m2 (SD 5.5 kg, n = 73). Statistical analysis of changes in weight and risk factors over 12 months was not presented.
In the Weight Action Programme (WAP),149 RCT participants were recruited mainly from two general practices. Participants were randomised to the WAP group intervention or to a control group receiving care from a practice nurse. The WAP group received group support targeted to individual needs. The use of any other weight-loss intervention (including pharmacological treatment) was allowed in this group. Participants received 28 sessions, including individual face-to-face sessions, peer support group sessions and weighing sessions, delivered weekly initially, and then monthly. Nurses supplied participants with tools (e.g. self-monitoring techniques, pedometers, food diaries, motivational techniques, dietary advice and non-judgemental support). No details on the calorie contents of diets were provided in the report. The control group attended standard care, including six face-to-face individual sessions with a nurse. In the WAP group, 67.4% of the participants (149/221) completed the 12 months of follow-up. The initial mean weight of the WAP participants (n = 221) was 95.5 kg (SD 15.8 kg) and the mean BMI was 35.0 kg/m2 (SD 4.2 kg/m2); the initial mean weight in the control group (n = 109) was 98.3 kg (SD 16.6 kg) and the BMI was 35.7 kg/m2 (SD 4.3 kg/m2). After 12 months, and using the analysis of all randomised participants, weight loss was greater in the WAP group (–4.2 kg, SD 7.3 kg) than in the control group (–2.3 kg, SD 6.6 kg), with a difference of –1.9 kg (95% CI –3.7 to –0.1 kg). Adverse events were reported by 25 participants (11%) in the WAP group and six (6%) in the control group. There were three serious adverse events (all in the WAP arm), but none appeared related to study procedures. Participants in the WAP group were significantly more likely to use orlistat (31%) than those in the control group (6%).
Rapoport et al. 167 undertook a RCT of modified CBT versus standard CBT to evaluate psychosocial outcomes, cardiovascular risk factors and long-term weight control. Only women were recruited and both treatment programmes involved weekly 2-hour group meetings over a 10-week period, led by a dietitian. A clinical psychologist and an exercise scientist also provided specialist sessions. In total, participants received 15 sessions. The modified CBT programme used motivational techniques. No physical activity programme was delivered as part of the intervention, but participants were encouraged to use a pedometer. The standard CBT programme aimed to achieve healthy weight loss with a diet of 1200 kcal/day, aided by cognitive and behavioural methods. The modified CBT programme focused on healthy eating without active calorie restriction. In the modified CBT group, 81% of participants (30/37) completed the 12-month intervention; in the standard CBT group, 73% of participants (28/38) completed the 12-month intervention. The mean BMI of the modified CBT group was 35.4 kg/m2 (SD 6.3 kg/m2) and the mean BMI of the standard CBT group was 35.3 kg/m2 (SD 5.6 kg/m2). At 12 months, weight change in the standard CBT group (n = 28) was –3.3 kg; it was –1.9 kg in the modified CBT group (n = 30). Weight and risk factor changes were reported as not statistically significant between groups at 12 months.
Counterweight programme
The Counterweight programme evaluation took place in 65 general practices from seven UK regions. 250 Dietitians trained GPs for 1 hour and practice nurses for 8 hours to deliver the programme with ongoing peer support. Practice nurses merged the programme into usual care appointments, which were usually spent managing comorbidities. Participants were recruited by GPs and practice nurses during normal appointments. Participants were requested to commit to nine appointments over 12 months after initial screening. This included six individual appointments (10–30 minutes each) or six group sessions (1 hour each) over 3 months, and then individual follow-up sessions at 6, 9, 12 and 24 months. First-line interventions were a prescribed eating plan, a goal-setting approach or a group intervention (exclusively based on goal-setting). These were aimed at achieving an energy deficit of ≥ 500 kcal/day. The intervention included diaries, healthy eating quizzes, a prescribed number of specific food group servings, personal weight-loss plans, advice for reading food labels, meal planning, eating out, emotional eating and social pressure to eat, reshaping negative thoughts, preventing relapse and physical activity advice. Participants were considered for pharmacotherapy if they did not achieve ≥ 5% weight loss at 3 months.
From 1419 participants’ data initially recorded (mean BMI of 37.1 kg/m2, SD 6.0 kg/m2), data were available for 642 participants (45.2%) at 12 months and for 357 participants (43.3%) at 24 months. Authors reported that the baseline mean weight was 101.1 kg (SD not reported, n = 1906) and the mean weight change was –3.0 kg (SD 6.6 kg) at 12 months and –2.3 kg (SD 8.7 kg) at 24 months. Some of the participants (19%) received anti-obesity drugs at some point (but no separate weight change information is provided for this subgroup). There were reported benefits at 12 months for all participants with data for total cholesterol, LDL cholesterol, systolic blood pressure and diastolic blood pressure, but not for HDL cholesterol, fasting glucose or HbA1c in participants with diabetes mellitus.
This same programme was later implemented in 13 health boards in Scotland251 and compared with previously published Counterweight data. 250 This analysis included 184 general practices, 16 pharmacies and one centralised community-based service. Eight hours of training was provided to primary care nurses, pharmacy assistants, health-care assistants and health coaches, followed by mentoring. Participants attended biweekly for the first 3 months, followed by three quarterly support visits, totalling nine appointments in 12 months. From the 6715 participants’ data initially recorded (mean BMI of 37.0 kg/m2), data were available for 1880 participants (28%) at 12 months. The mean weight change at 12 months was –3.7 kg (SD 12.2 kg).
Internet-based weight-management programme
Little et al. 142 evaluated the Positive Online Weight Reduction (POWeR+) programme, a RCT of an internet-based behavioural intervention. This intervention taught participants self-regulation and cognitive–behavioural techniques to improve eating patterns and physical activity. Fifty-six general practices participated and participants were randomised to dietetic advice and six monthly nurse follow-ups (control group), web-based intervention and face-to-face nurse support (POWeR+ F group) or web-based intervention and remote support (POWeR+ R group). Those randomised to the POWeR+ groups received 24 web-based sessions and e-mail reminders over 6 months. POWeR+ F participants had access to POWeR + and (up to) seven subsequent face-to-face nurse support sessions, totalling 34 sessions.
The POWeR+ R participants had access to POWeR+ and remote individual nurse support of (up to) five brief e-mail or telephone contacts, totalling 27 sessions. Participants assigned to the control group were directed to web-based pages with brief structured advice on a healthy diet and physical activity. POWeR groups could choose from a 600-kcal/day deficit diet or a low-carbohydrate diet (lowest carbohydrate 50 g/day), and had physical activity advice including a pedometer, if requested. Mean baseline BMIs of groups ranged from 36.3 kg/m2 to 37.1 kg/m2. Of the participants, 81.4% (227/279) of the control group, 82.1% (221/269) of the POWeR+ F group and 80.7% (218/270) of the POWeR+ R group participants completed 12 months’ follow-up. The control group achieved a mean reduction of 2.7 kg. It was reported that, compared with the control group, the POWeR+ F group achieved a weight reduction of 1.5 kg (95% CI 0.6 to 2.4 kg) averaged over 12 months, and the POWeR+ R group achieved a weight reduction of 1.3 kg (95% CI 0.34 to 2.2 kg) averaged over 12 months. Among the participants, 20.8% of the control group, 29.2% of the POWeR+ F group and 32.4% of the POWeR+ R group maintained a clinically important 5% weight loss. There were no adverse events reported by participants. HDL cholesterol was reported to be the only risk factor that was significantly improved for both intervention groups compared with the control group.
Very low-calorie diets in primary care
Two primary care studies included VLCDs. 243,253
Molokhia243 attempted to achieve a BMI of 25 kg/m2 in participants through Lipotrim (Howard Foundation Research Ltd, Cambridgeshire, UK). Once this was achieved, the VLCD was stopped and improvement in eating patterns based on a healthy diet was attempted. Participants were weighed weekly by practice nurses and had monthly visits to their GP. Advice on exercise was given. From 26 participants initially recruited, data for 25 (96.1%) were available at 12 months. The baseline mean weight of participants was 102.9 kg (SD 19.1 kg), and the mean BMI was 38.7 kg/m2 (SD 6.6 kg/m2) (n = 25). At 12 months, participants’ mean weight change was –15 kg (SD 9.6 kg, n = 25). Three participants reached a BMI of 25 kg/m2 after 12 months. Common minor side effects included headache, constipation, nausea and diarrhoea. No serious adverse events were reported.
Lean et al. 253 evaluated a VLCD in general practices delivering the Counterweight programme (predominantly rural or small-town settings). The aim was to achieve ≥ 15 kg of weight loss at 12 months. Nurses and dietitians mainly provided the intervention (after 8 hours of training). There was an initial VLCD stage (12 weeks or 20 kg of weight loss, whichever was sooner), a food reintroduction stage (6–8 weeks) with a low-calorie diet (360–400 kcal/meal) and reduction of the VLCD. The VLCD was milk- and fruit-juice based (811 kcal/day) with a multivitamin/mineral supplement, or the Cambridge Weight Plan® (832 kcal/day). Orlistat (120 mg/meal) was optional in the second phase. Participants (mean BMI of 48.0 kg/m2, SD 7.6 kg/m2) attended weekly, then fortnightly, then monthly appointments, totalling 18 sessions. They received telephone support and printed materials on weight loss and maintenance, nutrition, physical activity, goal-setting, energy requirements, portion control and orlistat. Mean weight change was –12.4 kg (SD 11.4 kg) for the 74.7% of participants (68/91) who completed 12 months. Of participants who started the food reintroduction stage, 29% (16/54) used orlistat, but it was prescribed at some point to 48.3% of participants (44/91) for 26 weeks. Adverse effects included constipation and dizziness; the number of participants reporting adverse events was not provided. The estimated cost per participant entered was £861.
Delivering a training programme to general practice teams
Moore et al. 234 undertook a cluster RCT to evaluate 4.5 hours of training for treatment of obesity, versus no training, delivered to 44 general practice teams. Practice staff invited patients with obesity to be part of the study. The intervention used an individual face-to-face approach, with a moderate energy deficit diet and physical activity advice, which was brief enough for primary care staff to provide during regular appointments. Participants were seen regularly (about every 2 weeks) until they had lost 10% of their body weight, and then less frequently (about once every 1–2 months) for maintenance of weight. Mean baseline BMI was 37.0 kg/m2. Of the 843 participants who were initially recruited, 565 (67%) completed the 12-month evaluation and 531 (62.9%) completed 18 months. At 18 months, it was reported that participants in the intervention group practices were 1.3 kg (95% CI –1.8 to 4.4 kg) heavier than participants in the control practices.
NHS primary and secondary care (orlistat prescription)
We identified three RCTs of orlistat delivered in NHS primary and secondary care. 95,96,117 Overall weight change, percentage of weight change and BMI change are presented in Table 43.
| Study (first author and year) | Baseline, outcome (SD); n | 12 months, outcome (SD) [% dropouts] |
|---|---|---|
| Broom 200296 | ||
| Weight (kg) | 100.9 (20.5); n = 265 | –5.8 (8.5) [29.8]a |
| Weight change (%) | – | –5.8 (7.8) |
| BMI (kg/m2) | 37.1 (6.4) | –2.1 |
| Broom 200295 | ||
| Weight (kg) | 100.6 (18.1); n = 71 | –5.0 (5.4) [52.1]b |
| Weight change (%) | – | –4.9 (4.9) |
| BMI (kg/m2) | 36.5 (5.5) | –1.8 |
| Finer 2000117 | ||
| Weight (kg) | 97.9 (12.9); n = 114 | –3.3 [35.9]b |
| Weight change (%) | – | NR |
| BMI (kg/m2) | 36.8 (3.6) | –1.2 |
Broom et al. 96 evaluated orlistat in a double-blind, randomised, placebo-controlled trial for weight and cardiovascular risk factor reduction. This 54-week study was conducted at 54 GP surgeries and 12 hospital clinics. Participants with a mean BMI of 37.1 kg/m2 (SD 6.4 kg/m2) had a 2-week run-in period before randomisation during which they received a placebo and a mildly hypocaloric diet. A 600-kcal/day deficit was prescribed. After 6 months, the energy content of the diet was reduced by a further 300 kcal/day to account for the expected reduction in energy requirement. Physical activity advice was not described. Participants were randomised to receive 120 mg of orlistat or a placebo three times daily with main meals. Participants visited the clinic monthly for individual face-to-face sessions. The health professional in charge of delivering the interventions was not specified. Overall, 70.1% of participants (186/265) completed 12 months.
After 12 months, the intervention group had significantly greater mean weight change (–5.8 kg, SD 8.5 kg) than the control group (–2.3 kg, SD 6.4 kg). More than twice as many participants in the orlistat group as in the control group achieved weight loss of ≥ 5% (55.6% vs. 24.3%). Weight reduction of ≥ 10% was achieved by more orlistat participants than placebo participants (19.7% vs. 11.0%). At 52 weeks, total cholesterol, LDL cholesterol, fasting glucose, systolic and diastolic blood pressure were reported as significantly improved in the intervention group compared with the placebo group (p < 0.05), but not triglycerides. Data for HDL cholesterol were not provided. Adverse effects reported were GI events (47% in the placebo group and 63% in the intervention group). Six participants from the placebo group and 13 participants from the orlistat group withdrew owing to the GI events. One death attributable to cancer occurred in the orlistat group.
Broom et al. 95 also investigated orlistat use for participants with hypercholesterolemia. Participants (n = 522, group mean BMI 36.5 kg/m2 to 37.1 kg/m2) were recruited from 12 obesity and/or dyslipidaemia outpatient clinics. Participants were randomised to 24 weeks of treatment with orlistat or placebo, and those who completed the double-blind phase entered a 28-week open-label phase with orlistat. All received a hypocaloric diet (30% fat energy, 600-kcal/day deficit, supervised by a dietitian) and advice on physical activity. Participants attended 11 individual, face-to-face sessions. Of the intervention participants, 47.9% (34/71) completed 12 months. The mean weight change was –5.0 kg (SD 5.4 kg, n = 34) in the intervention group and –4.3 kg (SD 5.9 kg, n = 43) in the control group. LDL cholesterol was reported to have decreased with orlistat compared with control treatment (reported p = 0.025), but no other cardiovascular risk factors differed between groups. Seven participants in the control group and eight in the intervention group reported at least one serious adverse event. Three participants in the control group and seven in the intervention group withdrew owing to GI adverse events.
Finer et al. 117 assessed orlistat over 12 months in participants with a mean starting BMI of 36.8 kg/m2 (SD 3.6 kg/m2). Five centres in the UK recruited participants through advertisements or GPs’ referral. A 4-week run-in, single-blind placebo period was followed by a 52-week, double-blind treatment period for participants with > 75% compliance in the run-in period. Participants were randomised to 120 mg of orlistat or placebo three times daily with meals. All participants were instructed on a 600-kcal/day deficit diet (and further reduction by 300 kcal/day at week 24), with 30% fat energy. Physical activity advice was not described. Medical staff, research nurses and dietitians delivered 17 sessions. Of the intervention participants, 64% (73/114) completed the follow-up. After 12 months, mean weight change was –3.3 kg for the intervention group and –1.3 kg for the control group; this difference was reported as statistically significant (p = 0.016). There were reported significant improvements at 12 months for total cholesterol and LDL cholesterol from orlistat. Changes in HDL cholesterol were not significant, and other risk factor data were not provided in sufficient detail to assess. GI events occurred more frequently in the intervention group (82.1%) than in the placebo group (56.4%).
Secondary care
We identified 10 studies in NHS secondary care: one252 in a nephrology clinic, three240,245,248 in clinics for people with diabetes mellitus and six241,244,247,254–256 in specialist weight-management clinics.
Nephrology clinics
MacLaughlin et al. 252 used a prospective cohort study with 135 participants to evaluate a WMP in a tertiary hospital. Participants with chronic kidney disease were recruited (mean BMI of 36.4 kg/m2, SD 5.6 kg/m2). During the study, 32 patients were on dialysis. The programme consisted of nine individual face-to-face sessions with a renal dietitian and a renal physiotherapist over 12 month, with additional measurement sessions at baseline and at 12, 18 and 24 months. This included a low-fat, energy-reduced renal diet prescription, a physical activity prescription, behaviour change counselling and 120 mg of orlistat three times daily. Of the participants, 73.5% (100/135) completed follow-up. The authors reported that the mean weight change from baseline was –4.3% (SD 5.5%) at 12 months, –4.1% (SD 5.8%) at 18 months and –4.0% (SD 5.8%) at 24 months (Table 44). Of the participants, 22.1% achieved > 10% weight loss and a further 20.6% achieved 5–10% weight loss after 24 months. Medication for lipids and blood pressure did not change in the first 12 months, and no other data are presented. The eGFR fell in non-dialysis participants at 24 months (–2.8 ml/minute/1.73 m2, SD 7.0 ml/minute/1.73 m2).
| Study (first author and year) | Baseline, outcome (SD); n | Outcome (SD) [% dropouts] | ||||
|---|---|---|---|---|---|---|
| 12 months | 18 months | 24 months | 36 months | 60 months | ||
| MacLaughlin 2012252 | ||||||
| Weight (kg) | 103.6 (21.0); n = 135 | NR [26.5]a | NR [26.5]a | NR [26.5]a | – | – |
| Weight change (%) | – | –4.3 (5.5) | –4.1 (5.8) | –4 (5.8) | – | – |
| BMI (kg/m2) | 36.4 (5.6) | –1.7 (2.3) | –1.6 (2.3) | –1.5 (2.3) | – | – |
| Dhindsa 2003 (VLCD)245 | ||||||
| Weight (kg) | 115 (15); n = 40 | –5.5 [9]b | – | – | – | – |
| Weight change (%) | – | –4.8 | – | – | – | – |
| BMI (kg/m2) | 40 (9.4) | –3 | – | – | – | – |
| Paisey 2002 (ICD group)240 | ||||||
| Weight (kg) | NR; n = 12 | –2.0 (NR) [20]b | –3.0 (NR) [20] | –6.1 (NR) [20] | –8.9 (4.0) [20] | |
| Weight change (%) | – | NR | NR | NR | ||
| BMI (kg/m2) | 35.9 (5.4) | NR | NR | –3.2 | ||
| Paisey 2002 (VLCD group)240 | ||||||
| Weight (kg) | NR; n = 13 | –14.2 (NR)b [13.3] | – | –9.2 (NR) [13.3] | –8.1 (NR) [13.3] | –4.8 (6.0) [13.3] |
| Weight change (%) | – | NR | – | NR | NR | NR |
| BMI (kg/m2) | 37.7 (9.9) | – | NR | NR | –1.6 | |
| Rowe 2005248 | ||||||
| Weight (kg) | 110.7 (25.0); n = 100 | – | – | –11.0 [77]b | – | – |
| Weight change (%) | – | – | – | NR | – | – |
| BMI (kg/m2) | 39.5 (6.5) | – | – | –3.9 | – | – |
Diabetes mellitus clinics
All of the populations in these studies were people with type 2 diabetes mellitus, except for one248 that also included people with type 1 diabetes mellitus (9%). Two studies used VLCDs240,245 and one included orlistat. 248 For the nutritional characteristics of the VLCDs, see below.
Dhindsa et al. 245 evaluated a VLCD (750 kcal/day) for people with type 2 diabetes mellitus and symptomatic hyperglycaemia, and a BMI of 40 kg/m2, taking combination oral antidiabetic therapy or insulin and metformin. This study included 8 weeks of VLCD with evaluation by the dietitian after 2, 4 and 8 weeks, and a follow-up to week 52, with a low-calorie diet (no further details provided) and advice about exercise in individual face-to-face sessions, at bimonthly visits to a diabetes mellitus centre, totalling nine sessions. Of the participants, 90.9% (40/44) completed 12 months. All withdrawals were in the first 5 days of starting the VLCD, mainly because of distaste and poor compliance. Participants had a mean baseline weight of 115 kg; after 12 months, the mean weight change was –5.5 kg. The authors reported significant (all p < 0.01) improvements at 12 months for weight, total cholesterol, fructosamine (a marker of glycaemic control) and blood pressure. Regarding diabetes mellitus management, glycaemic control tended to deteriorate at 12 months compared with baseline (no further details provided).
Paisey et al. 240 evaluated an intensive WMP with 5 years of follow-up. Participants [group mean BMI of 35.9 kg/m2 (SD 5.4 kg/m2) to 37.7 kg/m2 (SD 9.9 kg/m2)] were asked to choose the strategy of their preference: VLCD or intensive conventional diet and exercise programme (ICD). Fifteen participants chose VLCD, 15 chose ICD and 15 declined further involvement. The VLCD group attended weekly group sessions with a nurse, a counsellor and initially a doctor to initiate the VLCD for ≥ 6 weeks. Antidiabetic and antihypertensive medications were stopped during the first week. Participants who chose ICD had weekly sessions run by two dietitians based on healthy eating in which recommendations for change were made and agreed by participants. Diet and exercise sessions were held for approximately 2 hours. Both groups had 30 face-to-face individual or group sessions (including weighing sessions) over the year. Of VLCD participants, 86.6% (13/15) completed the 12 months; of ICD group participants, 80% (12/15) completed the 12 months. Two participants died in the ICD group.
Table 44 provides weight change data. The mean weight change was –4.8% (SD 6.0%) in the VLCD group and –8.9% (SD 4.0%) in the ICD group after 60 months. The authors reported that psychological well-being scores did not change significantly in either group. There were significant within-group improvements over 60 months for diastolic blood pressure and HDL cholesterol in the ICD group, and for cholesterol in the VLCD group.
Rowe et al. 248 evaluated 100 people with type 1 or type 2 diabetes mellitus treated with orlistat in a routine clinical setting. Participants attended a group education session run by the dietitian on how to estimate fat intake and were advised to restrict fat intake to < 50 g per day. Motivation was determined by a 2.5-kg reduction in the 4 weeks prior to the introduction of orlistat. Mean weight fell significantly (–11.0 kg) for those who accomplished the 24 months’ follow-up (23%). Mean HbA1c change over 24 months was not reported. Two participants reported acute illness with hospital admission, and six reported diarrhoea.
Specialist weight-management clinics
Seven studies evaluated specialist weight-management clinics;241,242,244,247,254–256 three included VLCDs. 241,244,247 Overall weight changes are presented in Table 45. The nutritional characteristics of the VLCDs are presented below.
| Study (first author and year) | Baseline, outcome (SD); n | Outcome (SD) [% dropouts] | ||
|---|---|---|---|---|
| 12 months | 18 months | 24 months | ||
| Barrett 1999 (VLCD)244 | ||||
| Weight (kg) | 119.8 (23.2); n = 115 | –13.4 (10.0) [Unclear]a | –7.8 (9.8) [Unclear]a | – |
| Weight change (%) | – | –10.9 (NR) | –6.6 (NR) | – |
| BMI (kg/m2) | 43.9 (7.5) | –4.9 | –2.8 | – |
| Cartwright 2014242 | ||||
| Weight (kg) | 132.1 (24.7); n = 262 | –7 (10.8) [67.9]a | –10.5 (18.7) [88.2] | –13.4 (15.2) [91] |
| Weight change (%) | – | –5 (8.0) | –7.2 (10.9) | –10.2 (11.8) |
| BMI (kg/m2) | 47 (7.9) | –2.6 (4.0) | –3.5 (5.6) | –4.8 (5.6) |
| Rolland 2009 (energy deficient diet)241 | ||||
| Weight (kg) | NR | –17.5 (6.4) [Unclear]a | – | – |
| Weight change (%) | – | NR | – | – |
| BMI (kg/m2) | NR | NR | – | – |
| Rolland 2009 (low-carbohydrate/high-protein diet)241 | ||||
| Weight (kg) | NR | –3.0 (6.7) [Unclear]a | – | – |
| Weight change (%) | – | NR | – | – |
| BMI (kg/m2) | NR | NR | – | – |
| Rolland 2009 (LighterLife)241 | ||||
| Weight (kg) | NR | –16.1 (19.0) [Unclear]a | – | – |
| Weight change (%) | – | NR | – | – |
| BMI (kg/m2) | NR | NR | – | – |
| Jennings 2014254 | ||||
| Weight (kg) | 124.4 (27.3); n = 230 | –10.2 (8.1) [45]a | –9.6 (12.8) [Unclear] | –5.9 (10.7) [Unclear] |
| Weight change (%) | – | –8.0 (6.0) | –7.1 (9.0) | –5.1 (9.1) |
| BMI (kg/m2) | 44.1 (7.8) | –2.1 | –1.7 | –0.9 |
| Logue 2014255 | ||||
| Weight (kg) | 118.1 (range 52.6–244.8); n = 1838 | –1.6 (5.5) [78.3] BOCF,b –3.6 LOCF | – | – |
| Weight change (%) | – | NR | – | – |
| BMI (kg/m2) | 43.3 (NR) | NR | – | – |
| Packianathan 2005247 | ||||
| Weight (kg) | 95.1 (13.2); n = 150 | –5.1 [69.3]a | – | – |
| Weight change (%) | – | –5.3 | – | – |
| BMI (kg/m2) | 36.1 (5.6) | –2.2 | – | – |
| Wallace 2016256 | ||||
| Weight (kg) | 139.4 (28.6); n = 489 | –11.8 (7.3) [Unclear]a | –14.9 (8.7) [Unclear] | –18.2 (8.7) [Unclear] |
| Weight change (%) | – | –8.5 | –10.7 | –13 |
| BMI (kg/m2) | 50 (7.9); n = 487 | –4.2 (2.5) | –5.2 (2.6) | –6.2 (2.7) |
Barrett et al. 244 evaluated a 12-week group programme providing a VLCD and behavioural and cognitive therapy to participants with a mean BMI of 43.9 kg/m2 (SD 7.5 kg/m2). A clinic was held fortnightly, delivered by a consultant physician, a clinical psychologist and a dietitian. Participants attended seven closed-group behavioural therapy sessions. For the first 6 weeks, the participants consumed only the VLCD (600–800 kcal/day), followed by food reintroduction. After completing the 12-week programme, participants returned at 3-monthly intervals, totalling nine sessions. Weight data were available for only 8% of participants (9/115) at 18 months, but whether or not follow-up data were variable is unclear; the mean weight change was –7.8 kg (SD 9.8 kg).
Cartwright242 evaluated the efficacy of a specialist weight-management service within the Heart of England NHS Foundation Trust. Participants’ mean BMI was 47 kg/m2 (SD 7.9 kg/m2). Visits were every 3 months, delivered by a physician, a psychologist or a dietitian (in accordance with the participants’ needs). Participants attended 5–13 appointments. Weight data were available for 32.1% of participants (84/262) at 12 months, 11.8% (31/262) at 18 months and 8.4% (22/262) at 24 months. Their mean weight change was –7 kg (SD 10.8 kg) at 12 months, –10.5 kg (SD 18.7 kg) at 18 months and –13.4 kg (SD 15.2 kg) at 24 months.
Rolland et al. 241 assessed the effectiveness of a low-carbohydrate diet (≤ 40 g/day) with 40% energy as protein (LCHP diet), a commercial VLCD (LighterLife; LL) or a 600-kcal deficit (CDD) diet. There was a 3-month run-in during which participants were assigned to a CDD with the aim of achieving 5% weight loss; those who lost > 5% of their body weight were maintained on this diet for an additional 3 months. If weight loss was > 10% at this time, the CDD was sustained for an additional 6 months. At 3 months, those who did not achieve this goal were randomised to LCHP or LL for 9 months. The LL group had 65 sessions (including screening sessions, weekly group meetings and regular weighing); the LCHP participants received 13 sessions. It is unclear how many sessions the CDD group received, and who delivered the interventions. Of all of the participants, 43.3% (52/120) completed follow-up. At 12 months, the mean weight change was –17.5 kg (SD 6.4 kg) for the CDD group, –3.0 kg (SD 6.7 kg) for the LCHP group and –16.1 kg (SD 19.0 kg) for the LL group. In the LCHP group, one participant was diagnosed with breast cancer and one became pregnant. In the CDD group, one participant was diagnosed with breast cancer and one became pregnant.
Packianathan et al. 247 evaluated a programme with two phases: a 16-week weight-loss phase of 900 kcal/day including SlimFast® (Palm Beach Gardens, FL, USA), and a weight-maintenance regimen including some use of SlimFast® meal replacements, a low-fat meal of 300 kcal, three portions of fruit and two portions of vegetables and advice on increasing physical activity. In the weight-loss phase, participants (mean BMI of 36.1 kg/m2, SD 5.6 kg/m2) attended a 1-hour biweekly group session for dietetic and CBT delivered by a dietitian. Only participants who achieved ≥ 10% weight loss after 16 weeks entered the second phase. Participants attended weighing and group sessions monthly for the remainder of the first 6 months and bimonthly for the next 6 months, and sessions were planned quarterly for the second year. Only 30.7% of the participants (46/150) completed 15 months. Weight change at 15 months was –5.1 kg for an initial weight of 95.1 kg (n = 150). There were reported statistically significant improvements in HDL cholesterol and triglycerides at 12 months (n = 46) (p < 0.05).
Jennings et al. 254 assessed their Fakenham weight-management service, a Tier 3 service, a flexible programme delivered in individual monthly sessions using motivational interviewing, a diet diary for agreeing change, starting with a 600-kcal/day deficit diet and physical activity advice with a programme to attend. Psychological therapies were offered in addition to, if clinically appropriate, VLCDs, pharmacotherapy or bariatric surgery. No nutritional information about the VLCD or commercial brand was provided. The programme was delivered by a GP with additional training as a bariatric physician, obesity specialist nurses, a dietitian, a psychological therapist, an exercise professional and health trainer and supported by a consultant endocrinologist and a public health consultant. Some group sessions for exercise and as patient-led follow-up support were available.
Participants had a mean BMI of 44.1 kg/m2 (SD 7.8 kg/m2). Of the participants, 55% completed 12 months. Nine participants used VLCDs and 36 were prescribed orlistat. The authors reported that mean weight change was –10.2 kg (SD 8.1 kg) for 117 participants who completed 12 months, –9.6 kg (SD 12.8 kg) for 58 participants who completed 18 months and –5.9 kg (SD 10.7 kg) for 29 participants who completed 24 months. BOCF data for participants, which also included some participants on a 6-month programme, were –5.9 kg (SD 7.8 kg) at 12 months, –4.7 kg (SD 9.7 kg) at 18 months and –2.6 kg (SD 7.4 kg) at 24 months.
The authors reported improvements at 12 months for systolic and diastolic blood pressure, HbA1c for people with diabetes mellitus and quality of life assessed by the EQ-5D (all reported as p < 0.01).
Logue et al. 255 evaluated the NHS Greater Glasgow and Clyde Weight Management Service. This was a time-limited structured educational lifestyle programme employing CBT techniques alongside a 600-kcal/day deficit diet and physical activity advice. In the first stage, nine 90-minute sessions were delivered fortnightly over 16 weeks. After this, participants could choose to enter the second stage of three 1-hour sessions at monthly intervals including further lifestyle advice, a prescribed low-calorie diet or orlistat prescription. At the end of the second stage, or directly from the end of first stage (depending on choice), participants entered a weight maintenance programme (third stage) of 12 1-hour sessions at monthly intervals. Those participants who failed to achieve their target weight loss could repeat the second stage once more. Dietitians, clinical psychologists, psychology assistants and physiotherapists delivered this programme. On average, participants received 28 individual face-to-face sessions. Of the participants, 399 out of 1838 (21.7%) completed 12 months. Using BOCF, the mean weight change for participants was –1.6 kg (SD 5.5 kg) after 12 months; using last observation carried forward, the mean weight change was –3.6 kg (SD 6.6 kg) after 12 months.
Wallace et al. 256 evaluated the effectiveness of the ‘Live Life Better’ service, a multicomponent WMP, which was part of the Derbyshire Obesity Referral Pathway service, but not part of the bariatric surgery pathway. This programme provided psychological support, behaviour change strategies, physical activity advice, dietetic advice and occupational therapy, as required. All participants (mean BMI of 50.0 kg/m2, SD 7.9 kg/m2) received a first assessment appointment with a clinical psychologist and a weight reduction support worker. Afterwards, all participants had routine follow-up dietetic and physiotherapy assessment as needed and were invited to attend routine weekly or fortnightly support worker-led clinic appointments during the first 12 weeks. Participants received individual face-to-face sessions, but the total number of sessions was not clear. It is unclear how many people dropped out from the programme. The mean weight change from baseline was –11.8 kg for 79 participants at 12 months, –14.9 kg for 41 participants at 18 months and –18.2 kg for 20 participants at 24 months. Mental health was assessed with the clinical outcomes of routine evaluation-outcome measure (CORE-OM) and was reported to be significantly improved at all time points. A survey of non-respondents indicated issues with being unable to attend a commercial weight-loss service simultaneously, and dissatisfaction with referral and appointment processes.
Commercial setting
Rolland et al. 257 assessed the effect of LighterLife Total (VLCD) with group-based behaviour therapy by means of retrospective database analysis. The nutritional characteristics of the VLCD used are presented in Table 46.
| Study (first author and name) | Baseline, outcome (SD); n | Outcome (SD) [% dropouts] | ||
|---|---|---|---|---|
| 12 months | 24 months | 36 months | ||
| Rolland 2014 (VLCD)257 | ||||
| Weight (kg) | 99.1 (16.6); n = 5965 | –18 (11.4) [Unclear] | –14.9 (11.4) [Unclear] | –12.9 (11.3) [Unclear] |
| Weight change (%) | – | –17.6 (9.5) | –14.7 (10) | –12.9 (10) |
| BMI (kg/m2) | 36.3 (5.1) | –6.6 | –5.4 | –4.7 |
The nutritional characteristics from VLCDs used in UK studies are presented in Table 47.
| Nutritional information | Non-commercial VLCDa | Cambridge Weight Plan® Liquid Dieta | Lipotrimb | SlimFast®c | LighterLife®a | Optifast™ | ||
|---|---|---|---|---|---|---|---|---|
| Study | ||||||||
| Lean 2013253 | Lean 2013253 | Molokhia 1998,243 Paisey 2002240 | Dhindsa 2003245 | Rolland 2009,241 Rolland 2014257 | Barrett 1999244 | |||
| Year | ||||||||
| 2013 | 2013 | 1998–2002 (women) | 1998–2002 (men) | 2017 | 2009–14 | 1994 (Sandoz Nutrition)d | 2017 (Nestlé)b | |
| Energy (kcal/day) | 811 | 832 | 417.8 | 530.6 | 690 | 550 | 420 | 624 |
| Fat (g/day) | 6 | 12 | 5.8 | 7.4 | 8.7 | 17 | 2 | 13.5 |
| % of total ingested energy | 6.7 | 13.0 | 12.5 | 12.6 | 11.3 | 27.8 | 4.3 | 19.5 |
| Carbohydrates (g/day) | 132 | 120 | 43 | 59.1 | 114 | 50 | 30 | 67.5 |
| % of total ingested energy | 65.1 | 57.7 | 41.2 | 44.6 | 66.1 | 36.4 | 28.6 | 43.3 |
| Protein (g/day) | 64 | 87 | 45 | 55.1 | 45.6 | 50 | 70 | 52.5 |
| % of total ingested energy | 31.6 | 41.8 | 43.1 | 41.5 | 26.4 | 36.4 | 66.7 | 33.7 |
| Sugars (g/day) | – | – | 43 | 42.1 | 62.7 | – | – | 55.5 |
| Fibre (g/day) | – | – | 5.1 | 6.4 | 12 | – | – | 10.8 |
Data relate to self-referred participants (mean BMI of 36.3 kg/m2, SD 5.1 kg/m2) who embarked on the LighterLife Total programme between 2007 and 2010, and whose weights at baseline and after a minimum of 12 months were available. The initial weight-loss phase could vary from several weeks to several months. Then participants continued to attend weekly group meetings to encourage long-term behaviour modification. Data for participants who finished the first weight-loss phase were available for 3921 participants at 12 months, 1464 participants at 24 months and 580 participants at 36 months. The mean weight change from baseline was –12.9 kg (SD 11.3 kg) at 36 months for 580 participants. Over 50% of participants returned to the weight-loss phase for a second attempt during this 36-month period.
Discussion
We identified 26 UK studies. Limitations in evaluation and reporting, particularly for denominators, and differences between participant groups in terms of comorbidities and likely social and psychological characteristics made comparisons challenging. There is one previous review of Tier 3 weight-loss services for adults by Brown et al. ,26 which included 14 studies with wider BMI inclusion criteria and studies with shorter follow-up delivered by multidisciplinary teams. Our focus was somewhat different, looking at longer-term outcome data from any service.
Only nine studies had data after 12 months. 240,242,244,248,250,252,254,256,257 The lack of long-term data after VLCDs is problematic. We also do not know what proportions of these populations would be interested in using them. VLCD reports showed mean weight change of –12.4 kg253 to –15 kg243 at 12 months, with reported drop-out rates of up to 25.3%,253 with generally fewer dropouts than comparable studies with other diets. This could suggest that better weight loss with these diets provides more motivation. Only the very small trial by Paisey et al. ,240 recruiting participants from primary and secondary care with type 2 diabetes mellitus, had very long-term follow-up with weight loss from a VLCD superior to an intensive conventional diet at 36 months, but not at 60 months. Unclear denominators in studies with the LighterLife VLCD make comparisons difficult at 12 months, but weight loss appears comparable. 241,244
Seven studies included participants with a mean group BMI of ≥ 40 kg/m2,241,242,244,245,253–255 mostly in specialist weight-management clinics. Of note, Rolland et al. 241 started all participants on a 600-kcal/day deficit diet, before randomising those doing less well to LighterLife or an Atkins-type diet at 3 months; the mean BMI was > 40 kg/m2 at the point of randomisation. This was the only study that reported changing participants’ dietary advice in accordance with response. Follow-up data from the report of a specialist weight-management service by Jennings et al. 254 show a mean weight change of –5.9 kg (SD 10.7 kg) at 24 months for those who completed the 12-month programme, or of –2.6 kg (SD 7.4 kg) at 24 months (BOCF), including some participants who undertook a 6-month programme.
The Counterweight reports in primary care250,251 were the only multicentre evaluations we found. Otherwise, cross-service evaluation was not reported. Developing networks for obesity services for training and evaluation, including conducting clinical trials, could be beneficial.
More women (68.5%) were involved than men. All studies included both sexes, with the exception of one for men128 and two for women. 167,247 After extensive developmental work, Hunt et al. 128 used strategies to attract and engage men in the FFIT programme. Exceptionally, this trial showed little evidence of weight regain by 12 months. The evidence from this chapter was insufficient to assess whether or not specific services for men or women were more effective in the UK.
Only three trials128,142,240 described expressly considering participants’ choices or motivations for improving engagement with starting or continuing services. Two studies allowed participants to choose their diets. 142,240 Dietary interventions were poorly described, which makes programme reproduction difficult. No study reported participants’ eating behaviours prior to enrolment. In some cases,149,244 authors reported weight-loss history (including number of past weight-loss attempts, methods used and average weight lost). Important features of the diets (e.g. availability, affordability, preferences and behavioural, social and economic costs for participants) were not described. These are important factors that need to be explored further. The extent to which diets are tailored may influence not only their success, but also the ease of delivery.
Only seven interventions were delivered in group sessions,128,149,167,240,244,246,257 14 were delivered in individual sessions,95,96,142,149,234,243,245,249,251–254,256 two were delivered in both group and individual sessions240,250 and in three cases the delivery was unclear. 117,241,248 Most of the sessions were face to face, with providers including dietitians,95,117,167,240,242,244,251–254 physicians,95,242,253,254 nurses,117,142,149,234,240,243,250,251,253 psychologists167,242,244,253–255 and football coaches. 128
Scaling up interventions to reach more participants is important. Little et al. 142 showed that remote delivery produced much the same 12-month weight change as face-to-face delivery with a drop-out rate of < 20% (mean –3.2 kg and –3.8 kg, respectively). This is comparable with the 12-month weight loss in the Counterweight evaluations,250,251 which had drop-out rates of 54.8% to 72%. However, these are smaller weight losses than found in UK RCTs of commercial WMPs in primary care with lower starting BMIs. 258,259
Many studies restricted calories using predictive formulae, usually a deficit from estimated requirements. 95,96,117,128,234,241,250–252,254,255 Studies did not often report adjusting energy allowances for sex or for physical activity. Two117,252 included sex in the estimation. MacLaughlin et al. 252 included an adjustment for activity of between 20% and 40%. The studies that included orlistat added a 1.3 factor for physical activity. 96,117 Whether or not weight loss is influenced by prescribing a lower calorie intake, or a deficit from estimated calorie requirements, is discussed in Chapter 3.
Physical activity interventions were poorly reported. The only exceptions were the FFIT programme, the MacLaughlin et al. 252 individualised progressive aerobic and muscular endurance exercises and the Little et al. 242 walking plan (with a pedometer supplied) or a participant–selected mixture of physical activities. 128,142,252 None of the studies provided information about prior physical activity from participants. Some studies described providing physical activity advice. 95,243,245–249,255,256
Some studies added devices (e.g. pedometers)128,142,149,167 or new technologies (i.e. picture-based food knowledge assessment or an internet-based intervention)142,149 to increase adherence and goal-setting.
Specialist weight-management services produced weight losses of 3.6% (100% of participants)255 to 8% (45.0% drop-out)254 after 12 months. By comparison, a VLCD supported in primary care led to 9.1% weight loss (25.3% drop-out) and a WMP in community football clubs led to 5% weight loss (11% drop-out) over the same time period. It is unclear whether or not the type of participant differed greatly between these settings. Given that primary care referral to a commercial provider for participants with a mean BMI of 34.6 kg/m2 demonstrated a weight loss of 4.9% from 12 weeks of the programme (100% of participants) and 7.1% from 52 weeks of the programme (100% of participants) at 12 months,258 a comparison of Tier 3 services with commercial WMPs would be of value. However, attendees at Tier 3 services may have tried commercial WMPs and may have more complex obesity.
There is a pressing need for guidance on weight data collection to allow comparisons across studies and services. Guidance particularly needs to focus on how to describe weight loss, the handling of dropouts and the need for long-term follow-up. Data on quality of life, clinical outcomes, adverse events, costs and economic outcomes would be valuable.
Overall summary
The following sections summarise the main points that have arisen from the review in this chapter.
General issues relating to methodology
-
We found no studies specifically evaluating alternatives to improving uptake or retention in services in the UK setting.
-
Potential participants were rarely consulted about the design of services or asked for their views about WMPs they used (see also Chapter 5).
-
Few study reports had participants with mean BMIs of > 40 kg/m2; therefore, participants are likely to have had lower BMIs than participants in Tier 3 services. Few studies reported consideration of how to engage hard-to-reach or disadvantaged groups (e.g. different ethnic groups, people with disabilities and younger or older people). Guidance is needed on basic demographic data to collect at WMP start.
-
Guidance on the content of WMPs to be reported would be very valuable, aiding with replication and evaluation.
-
Assessment of the fidelity of service delivery was rarely mentioned.
-
Standardisation of data collection for weight outcomes and dropouts, and their timing, is needed to allow evaluations across and within services. Services should collect data for > 1 year. Public Health England has guidance for the evaluation of weight-loss services,260 and a core outcome set is being developed in the UK using consensus methods. 261
-
Long-term UK data are needed from commercial providers for participants with severe obesity [e.g. LighterLife, Cambridge Weight Plan®, Weight Watchers® and Slimming World® (Afreton, UK)] for weight outcomes and dropouts. Randomised evaluations of comparisons with other approaches, including Tier 3 specialist weight-management services, including allowance for choice of reducing diet, would be valuable.
-
Whether physical activity programmes, as opposed to physical activity advice, should be provided should be evaluated. Programmes may be of particular interest to men.
-
A clinical research network for service development and evaluation and randomised trials would be helpful, and could help to expand on the few NHS centres with expertise.
Indicators for effective interventions
-
In primary care, VLCDs such as the Counterweight programme using the Cambridge Weight Plan® are effective at producing large weight loss at 12 months. Weight outcomes and improvements in comorbidities after 12 months are unclear.
-
A weight loss of around 3 kg at 12 months with the standard Counterweight programme appears comparable with the remotely delivered POWeR+ programme, which also recruited from general practice. A RCT of both programmes, including a comparison with commercial organisations, for outcomes and an economic evaluation would be useful.
-
Group-based programmes, including physical activity programmes, appear underutilised in UK practice, and may enhance motivation and weight loss (see Chapters 3 and 5).
-
For a discussion of other components that may enhance weight loss, see Chapter 3, Indicators for effective interventions, and Chapter 5, Indicators for effective interventions.
Chapter 5 Systematic review and synthesis of qualitative research
Qualitative studies have a key role to play in understanding how factors facilitate or hinder the effectiveness of interventions, and how the processes of interventions are perceived and implemented by users. The focus of this review was understanding the feasibility and acceptability of lifestyle WMPs for adults with a BMI of ≥ 35 kg/m2 and programme providers. We did not review bariatric surgery studies, which were outside the scope of our protocol. The broad initial research questions for this review included ‘What is it like to engage with (or be a provider of) weight-loss interventions for adults with BMI ≥ 35 kg/m2?’ and ‘What is it about interventions for adults with BMI ≥ 35 kg/m2 that make them helpful or unhelpful?’. As our analysis was conducted iteratively, our review also considered issues around what might motivate people to decide to engage in such programmes.
Methods
Searching and identification of relevant studies
A systematic search was conducted for published papers that contained qualitative data from adults with a BMI of ≥ 35 kg/m2 (and/or views of providers of their care) and considered issues relating to weight management. Details are described in Chapter 2, and included studies, excluded studies and data extraction details are given in Report Supplementary Material 1, Sections 13–15.
We included studies that fitted into the following broad categories:
-
qualitative and mixed-methods studies linked to eligible RCTs, including any qualitative data reported as part of papers reporting quantitative outcomes
-
qualitative and mixed-methods studies linked to ineligible RCTs and identified non-randomised intervention studies including any reported qualitative data
-
UK-based qualitative studies not linked to specific interventions that drew on the experiences and perceptions of adults with a BMI of ≥ 35 kg/m2 (and/or providers involved in their care) providing that they reported data specifically relating to views/experiences of strategies for weight loss.
Findings
Description of studies
The database search produced 4710 abstracts. Four additional papers were identified from included RCTs. In all, 33 papers met our inclusion criteria. 142,149,204,225,226,249,254,262–287 The focus and key study characteristics of the 33 papers are outlined in Report Supplementary Material 1, Section 16. The identified papers reported research conducted in seven countries (the USA, n = 12; the UK, n = 11; Norway, n = 3; Spain, n = 1; Canada, n = 2; Australia, n = 3; and Mexico, n = 1), published between 2007 and 2017. Seven papers were linked to broader intervention studies: the POWER study,268,274 the 5AsT study265,266 and The Change Program. 281–283 Seven papers were classed as category A, 24 as category B and two as category C. As can be seen in Report Supplementary Material 1, Section 16, the studies had varying aims, but all offered insights into stakeholders’ perceptions of weight-loss strategies and programmes (see Report Supplementary Material 4, Figure E20).
Although all the included papers provided some qualitative data for analysis, five of these provided qualitative data in the form of responses to open-ended survey questions within structured questionnaires. 149,225,249,267,287 Of those studies that used qualitative methods to collect their data, findings were presented from a total of 644 participants and 153 programme providers (mostly from interviews or focus group sessions).
Fourteen papers specifically stated that study participants had a range of additional physical and/or serious mental health problems (e.g. osteoarthritis, chronic pain, schizophrenia and post-traumatic stress disorder). It was also apparent across other included papers from quotations and/or author comments that many participants had a range of comorbidities.
Across the 33 papers, specific participant characteristics were inconsistently and poorly reported (if at all). Only 16 out of 33 papers provided any details. In terms of sex, information for 588 participants (out of 644 of those who specifically took part in qualitative evaluations) was provided: 372 females and 216 males. Age was reported across 15 papers, with the range being 19–88 years. Six of these papers provided mean age, with the range being 40.2–67 years. BMI for those involved in qualitative evaluations was reported in nine papers. Of those that provided a mean, this ranged from 36.8 to 44.7 kg/m2. Only four papers gave details of participants’ ethnicities: of 188 participants, 35 were reported as being from ethnic or racial minorities.
Although no included papers provided qualitative data from those who had been invited to join a programme but had declined to take part at recruitment stage, some papers reported including participants who had not fully engaged with programme activities (being described as ‘low users’, ‘quitters’ or ‘drop outs’). 142,204,262,269
The WMPs varied in terms of the types and formats of support offered. Some programmes involved predominantly face-to-face interaction and activities with other participants and/or programme staff,204,226,249,254,271,273,275,276,279,284,285 whereas others involved more remote forms of support (e.g. e-mail, telephone or text contact). 225,280 Other studies included and evaluated a mix of formats that also varied in intensity. 142,149,262,264,268,269,274,281–283,286,287
Programmes incorporated a variety of tools and techniques designed to support behaviour change and to help people lose weight (e.g. tools such as diet diaries,149,204 workbooks,281–283 pedometers,142,149,286 food logs,262,285 conversation maps,267 interactive telemonitoring devices,225 social media group interaction,264 daily text messages280 and buddying149) and a range of BCTs and/or psychological support,265,266,270 such as goal-setting,142,249,254 motivational interviewing,254 mindfulness,276 self-determination theory-based support,204 regulatory focus theory,280 self-regulation and cognitive–behavioural techniques,142,226,254,262,268,271,274,281–283 readiness to change and self-monitoring and feedback,285 psychotherapeutic sessions,275 emotional freedom therapy,254 neurolinguistic programming,254 solution-focused therapy254 and social learning theories. 279
Findings from the synthesis: participants
Motivating factors for engagement in weight-management programmes
Several papers provided insights into what apparently motivated prospective participants to take part in a specific WMP. 204,226,254,270,271,276,285 Important ‘push’ factors were sometimes apparently internal to participants, for example expressing a desire to do something about their weight/poor physical fitness for themselves (e.g. as a result of growing health concerns and/or recent personal health scares), and also feelings of accountability to their families (e.g. stating that they wanted to be more engaged in activities with family members, as well as being there for family for as long as possible). Others recounted familial past experiences of health problems owing to obesity or their own sudden and rapid weight gain attributable to mental health medication, as shown in the following quotations.
Recent personal health scares
I was told I was at risk of becoming diabetic.
No sample characteristics provided254
Feelings of accountability to their families
I’ve had two kids in the last 3 years . . . that was part of the motivation . . . just getting fitter for my kids . . . I need to be aboot [about] for as long as possible.
Male226
Familial past experiences of health problems owing to obesity
My dad was a big guy and he developed diabetes, and he had to have surgeries and all kinds of stuff. I don’t want to do that later in life.
Intervention arm; no other sample characteristics provided285
Sudden and rapid weight gain due to mental health medication
When I went on Zyprexa [olanzapine; Eli Lily and Company UK] I gained a hundred pounds, very quickly. And that was really frustrating for me.
Control arm; no other sample characteristics provided285
In addition to describing motivating factors that could be classed as somehow internal, some participants described motivators that were apparently related to certain aspects of the programme intervention itself, for example because it was perceived as being endorsed as credible by health professionals, because it was perceived as being novel and exciting in some key way and also because it provided an opportunity to engage with the intervention in a place that was valued:226,270,271
When I first went in there I thought this is great. I am going to diet at my doctor’s surgery. Knowing that it was at my doctor’s surgery gave me a big ‘oof’.
No sample characteristics provided270
Although one paper highlighted that decisions to join a WMP were sometimes difficult and that some participants had expressed initial apprehension and reservations around taking part,226 no included studies provided data about those who were invited to join but declined to take part at the recruitment stage.
Components of lifestyle programmes that participants described liking or valuing
We examined the various aspects of WMPs that participants described liking or valuing. In doing so, we were interested in the range of factors that might motivate those participants to join in the first place and continue to stay in the programme, and also the factors that they described as having assisted them to change aspects of their behaviour or ways of thinking. All but two papers were set within the context of a WMP. The two included papers that were not linked to a specific intervention277,278 also provided data regarding perceptions of weight-loss strategies and engagement in diet and lifestyle programmes and were useful in this context. Perhaps unsurprisingly, there was variation in terms of what participants described as liking or valuing within their WMP, demonstrating that a ‘one size fits all’ approach is unlikely to be appropriate. We noted some key recurring themes in terms of what participants valued, and we grouped these around aspects that relate to (1) the overall setting or context of the programme, (2) the people (both other participants and health professionals/support staff) within the programme setting, (3) the type of interaction/support offered, (4) dietary elements, (5) physical activities and (6) programme tools and techniques designed to support behaviour change. These are discussed in the following sections.
The overall setting or context of the programme
The overall setting or context of the programme was important for motivating people to decide to engage and was apparently important for motivating them to stay in and keep going with the various intervention activities. Some participants described their programmes as being exciting or novel in the sense that they perceived them to be different from interventions they had tried previously (e.g. being focused on physical activity rather than dieting204 or being focused on changing overall attitudes towards eating rather than dieting per se276,282), and an important consideration was the extent to which they could ‘relate’ to the nature of the programme (including how it was presented to them at recruitment) and how well it appeared to match with their own identities and values:204,226,276,278
. . . the main thing that drew us to it was because it’s [at a football club].
Male226
I always think somebody approaching you one on one is better. They can post all the weight loss you know pamphlets out there . . . I was hooked right away because somebody took the time to really explain it and take her time to do that.
Female276
Several participants also positively contrasted their overall perceptions of the WMPs with previous negative views towards other WMPs (e.g. WMPs that were perceived as being too ‘feminine’ or in some ways humiliating and embarrassing, or being perceived to be overly preoccupied with dieting):204,249,254,269,273,278
If you go to a slimming class you feel that you’ve made a fool of yourself or you get weighed and you’ve put on half a pound or a pound, and then you don’t want to go back the next week so you don’t go back.
Coaching group arm; no other sample characteristics provided269
Well, I think it’s [WHEEL] appealed to me because I won’t be dieting . . . I am obsessed with dieting me.
Female204
. . . spent many useless years at Weight Watchers with various leaders but never felt confident and in control or had the motivation I have now.
No sample characteristics provided249
The importance of the people within the programme setting (for fostering a sense of accountability)
A strong recurring theme was the value participants placed on perceiving themselves to be part of a like-minded group of individuals – individuals who faced similar issues and who had similar physiques and personalities. 142,149,204,226,264,267,269,273,275,276,279,285 For example:
I do not feel so ashamed of my body here. We are all in the same situation, you see, which is really nice.
Female273
These perceptions seemed to foster a strong group identity and related ‘accountability’ in participants, something that was apparently important for people in terms of motivating them to stick with the programmes and to not let their fellows down by dropping out or not sustaining behaviour changes:142,149,204,226,262,264,269,276,285
. . . you didn’t want to disappoint your . . . friends . . . either.
Female276
Many participants also discussed the importance of their interactions with health-care staff within the programmes. 149,204,249,254,262,269,271,273,275,276,279,282,284,287 They seemed to value the positive (and friendly, non-judgemental) encouragement received and they also discussed feeling accountable to programme staff, which helped with motivation. These aspects seemed to act as positive ‘pulls’ in terms of staying in the intervention and helping to sustain behaviour change:
I think I just like talking to you [programme leader]. And I suppose I feel that if I don’t do it [the programme] then I’m letting you down.
Female204
She is my motivator . . . And she makes me keep a record of my diet.
Female273
The type of interaction/support offered
Although not universally valued by all participants across papers (discussed later in this chapter), many described particularly valuing the social interactivity of group-based programme activities and also fairly intensive support from/interaction with programme staff. 142,204,226,249,262,264,269,272,275,276,279,285,286 Again, this appeared to function strongly as a motivator to maintain engagement with the programmes by fostering feelings of accountability and by helping to ensure the achievement of pre-set goals:
Oh god I haven’t done what I should of done and I promised to do it and I know that isn’t what’s supposed to spur you on but it I think it does.
Regular support group; no other sample characteristics provided269
[Discussing feedback from programme staff] . . . great encouragement when the results are positive and a way to improve if the results are not so good.
No sample characteristics provided249
Participants discussed appreciating when the timing of support offered was flexible and could fit around their needs,149,269,276 and several wanted more support (e.g. more frequent contact and for longer) than was offered within the programmes. 142,225,269,287 Many also expressed concern about support ending post intervention,204,269,273,276,280,285 with the suggestion that waning intensity of programme activities and/or programme cessation could cause problems for maintaining behaviour change patterns if group interaction and support was a key part of it:
I cannot do it without her support, it just wouldn’t work.
Female273
The WMPs varied in terms of the types and formats of support offered to participants. Some programmes involved predominantly face-to-face interaction and activities with other participants and/or programme staff,204,226,249,254,271,273,275,276,279,284,285 whereas others involved more remote forms of support (e.g. e-mail, telephone and text contact). 225,280 Some studies included and evaluated a mix of formats that also varied in intensity. 142,149,262,264,268,269,274,281–283,286,287 As discussed above, many participants discussed valuing the social interactivity of the group-based activities142,204,226,264,269,276,285 and, when it was discussed and compared, participants tended to value and desire human contact over more remote forms of support. 142,225 This preference seemed to be linked to incentivising people to stay committed to the various programmes and was also apparently important in terms of making participants feel accountable to a like-minded group of individuals.
Dietary elements
All of the programmes reflected on in the included studies incorporated attention to dietary aspects. Some provided specific dietary advice regarding food choices, whereas others specifically described interventions as ‘non-dietary’ (but nevertheless incorporated behavioural change techniques to support attitudinal changes towards food and eating patterns). We examined data that were available from participants and/or programme staff relating to the perceived usefulness or otherwise of these dietary aspects (see later in this chapter for reflections from programme staff). Although views were sometimes mixed, participants tended to describe valuing the flexibility and variety of diet format. 142,204,276,279 This seemed important in terms of helping them to ‘normalise’ and stabilise their eating habits, particularly as many had attempted diets over a period of many years (without success), leading them to develop negative and unhealthy relationships towards food:142,204,276,279
The other programmes told you not to eat this or that and you were afraid to go back if you hadn’t lost weight and . . . they tell you that you can eat everything but you yourself have to control the amount . . . You make up the diet every day and that’s very motivating.
Female279
Physical activities
All of the programmes incorporated some attention to increasing physical activity (of varying natures and to varying degrees of intensity). We examined data that were available from the papers from participants and/or programme staff relating to the perceived usefulness or otherwise of these components (see later in this chapter for reflections from programme staff). Although some participants clearly described struggling to engage in exercise for a variety of reasons, many participants described the positive psychological and physical benefits they experienced from exercising:204,254,264,273,285
When I first started I could hardly walk . . . now I can walk 300–400 yards . . . if this project has done nothing else it has helped me to walk.
No sample characteristics provided254
When it was offered as part of the programme, participants also discussed valuing the flexibility of being able to choose from a variety of exercise formats and approaches. 142,204
Programme tools and behaviour change techniques designed to support behaviour change
Several programmes incorporated tools and techniques designed to support behaviour change and to help people lose weight. For example, as described by the authors, the tools and techniques that were used included diet diaries,149,204 workbooks,281–283 pedometers,142,149,286 food logs,262,285 conversation maps,267 interactive telemonitoring devices,225 social media group interaction,264 daily text messages280 and a range of BCTs and/or psychological support, such as goal-setting,142,249,254 motivational interviewing,254 mindfulness,276 self-determination theory-based support,204 regulatory focus theory,280 self-regulation and cognitive–behavioural techniques,142,226,254,262,268,271,274,281–283 readiness to change and self-monitoring and feedback,285 psychotherapeutic sessions,275 emotional freedom therapy,254 neurolinguistic programming,254 solution-focused therapy254 and social learning theories. 279
We examined data that were available from participants and/or programme staff relating to the perceived usefulness or otherwise of these various aspects. Although not universally popular, participants described the incorporation of tools, such as food logs, goal-setting, regular text messages, telemonitoring devices and conversation maps as being motivating142,204,225,262,285 and also helpful for the purposes of education and learning, describing how they helped to facilitate self-awareness of and reflection on eating and other behaviour patterns:142,149,225,262,267,280,285–287
I found it to be very enlightening. It made me start to look at foods differently. It has given me a more conscious outlook on how to control my diabetes and the importance of exercise.
No sample characteristics provided267
What really helped me was having somebody go over the food log every day. That was the big thing.
No sample characteristics provided262
Participants discussed the positive psychological changes they experienced regarding their relationship to food/body image, which seemed to relate to the BCTs employed within some of the programmes (e.g. mindfulness and self-determination theory-based support). 204,262,271,276
We examined any qualitative data that were available from participants or programme staff relating to more critical reflections and experiences with various aspects of WMPs. Although the bulk of available data focused on positive reflections (i.e. what people stated they had enjoyed about participating), several papers also offered insights into some of the general challenges people faced with engagement (i.e. challenges not necessarily related to the programme itself) and also specific programme components that were described as being in some way problematic or perhaps not universally valued by all participants. 142,149,204,262,264,277,278,282,285 These are discussed in turn below.
General challenges for engagement in weight-management programmes
Despite the numerous positive comments from the data with regard to programme engagement, participation was not straightforward for everyone who took part. General challenges resulting in decreased engagement (or success) related to a number of factors. Sometimes these involved the timing of clinic appointments,149 cost of travel to appointments,254,286 general low self-efficacy270 or family members not being on board such that behavioural changes were difficult to sustain. 275,285 Others described factors that could be described as life getting in the way (e.g. holidays, social events and bad weather as a disincentive to exercise). 285
Across many papers, it was apparent that participants experienced a range of comorbidities, including some serious mental health issues. 142,149,225,263,264,277,278,285,286 Sometimes these specific illnesses presented challenges for motivation and continuing engagement, for example feeling too ill to focus on weight/feeling too ill to care or to be motivated:142,254,278,279,285
Because of the ME [myalgic encephalopathy] I’m sleeping 15 or more hours a day, and so exercise is out of the question because I can’t even walk to the end of the road.
Female277
Critical reflections on specific components of weight-management programmes
The type of interaction/support offered
As discussed above, a recurring theme was that many participants described particularly valuing the social interactivity of group-based programme activities. However, this was not universally valued by all, with some describing a reluctance to discuss issues within a group setting. 264,271,272,279,284,286 This was perhaps particularly pertinent in studies in which participants had additional mental health issues:
I know the importance of the programme is to be together, but at the beginning you don’t know these people, some of us have problems interacting with people we don’t know.
No sample characteristics provided264
It’s just I don’t like to be around people.
No sample characteristics provided286
I prefer to talk in private as I suffer from panic attacks.
No sample characteristics provided284
One study282 also included data that suggested that some participants felt guilty using up what they perceived to be too much of their health-care provider’s time (in an intervention involving regular GP visits):
I must admit I felt frequently embarrassed that I was taking up a lot of my GP’s time.
No sample characteristics provided282
Dietary elements and physical activities
Although the majority of participants tended to describe valuing the flexibility and variety of the diet formats offered within programmes,142,204,279,287 views were sometimes mixed with regard to diets, with a few wanting more prescriptive and structured eating plans than were offered:
I think [having a set meal plan to follow] would have been to a certain extent easier at the beginning, but I don’t think it would of actually adjusted my attitudes and thinking which it [POWeR+] has done.
Male; 64 years; face-to-face support; high user142
The above quotation illustrates that participants often discussed appreciating when programmes apparently emphasised changing attitudes towards food and eating over promoting a specific diet per se. However, sometimes participants did feel that their programme (or their primary care providers) tended to overemphasise diet rather than, for example, addressing issues around exercise, sleep or addiction problems:278,285
. . . there was no support counselling-wise as to why I have the issues I have with food . . .
Male278
Although many participants described the positive psychological and physical benefits they experienced from exercising,204,264,285 others described struggling to engage in exercise. Some described disliking the perceived high intensity of the exercises (e.g. feeling uncomfortable with sweating),204,272,273 whereas others discussed how their various physical or mental health comorbidities could prohibit them from full engagement in activities:142,149,204,263,272,273,277,278,285
Exercise is the best [to lose weight] and I get all this physical therapy exercise and all of that just increases my pain, which reduces my desire to have any exercise.
No sample characteristics provided263
I think for me, with my disability it was difficult to engage with some of the activities recommended.
No sample characteristics provided149
Programme tools and behaviour change techniques designed to support behaviour change
Several programmes incorporated tools and techniques designed to support behaviour change and to help people lose weight. Participants suggested that many of these tools and techniques were helpful for them in terms of reflecting on their habits and behaviours and for helping them to positively change their attitudes. However, some participants described these tools as being somewhat intrusive and sometimes inflexible in nature. For example, some participants described disliking food logs and found food diaries/goal-setting/daily self-weighing and the monitoring of exercise as excessive and too confrontational. 142,204,225,285 Others felt that programme staff did not appropriately monitor and feed back on progress:262
I mean no one ever looked at it [food diary]. No one ever asked for it. I just did all the work, like, for nothing because no one ever asked me for it.
No sample characteristics provided262
Others expressed frustration with the perceived inflexibility of tools designed to record behaviour and activities and to support behaviour change, for example not being able to record life events and/or comorbidities that might help to explain lack of achievement regarding weight loss:142,280
I thought that might be useful [to] have something [to] explain why things are going as they are going.
Female; 59 years; remote support; high user142
I would want to tailor the messages [daily text messages] to the things that I was most struggling with.
No sample characteristics provided280
With regard to psychological support, two papers highlighted that some people wanted more counselling for non-direct weight issues, such as mental health, recognising that these additional problems had implications for weight management. 225,278 By contrast, although many participants discussed the various positive psychological changes they experienced that seemed to relate to the BCTs/counselling employed within some of the programmes, others found personal development classes challenging and confrontational and questioned their appropriateness:271
I cannot benefit from it [the personal development classes]. I will never open up in that room and talk among others.
Male271
Findings from the synthesis: provider participants
Ten of the included papers provided qualitative data from a range of WMP providers about aspects relating to the programmes and/or their interactions with people with obesity more generally. 142,265,266,268,270,271,274,281–283 Seven of these papers were linked to one of three of the same interventions. Programme providers who provided qualitative data were described as primary care providers;268,274 nurses;142 GPs and consumer representatives;282 GPs;281,283 mental health-care workers, dietitians and nurses;265,266 GPs, weight-management advisors and practice nurses;270 and key personnel working at a residential weight-loss centre. 271
General impressions of being involved in weight-management programmes
With the exception of one study, in which some GPs (but not all) were reportedly less enthusiastic,270 views about being involved in a WMP were generally very positive, with health professionals acknowledging that engagement was potentially very useful for them in terms of facilitating a conversation around weight loss with patients – recognising that this could often be challenging in their everyday practices. 142,281–283
However, the authors of one study265 noted that discussions about weight tend to be embedded within the context of conversations about other health issues (rather than being discrete or standalone) and argued that this could act as a potential barrier regarding the implementation of WMPs within primary care:
I don’t have patients that come to see me just for obesity or . . . just one thing . . . yes they’re one of my diabetic patients but . . . we’re talking about their cholesterol today or their blood pressure and their weight another day.
Nurse265
Motivating factors for participant/provider engagement in weight-management programmes
One paper included some insights from the perspectives of programme providers about what apparently motivated prospective participants to take part in a WMP. 268 Health-care providers involved in the delivery of the programmes described how they regarded patients’ perceptions of their professional ‘buy in’ to the intervention study (i.e. endorsement) as important and influential regarding their decisions to take part. 268 One study (linked to two papers)268,274 also reported unusual success with enrolling men, which programme providers attributed to their endorsing it as a ‘medical’ programme:
I think that [our affiliation with a research institution] helped make it into a legitimate type of programme that [our patients] would have confidence in, not just one of these wild watermelon diets or things like that.
Primary care provider268
In terms of disincentives towards retention in such WMPs, some providers reported that some participants could have unrealistic expectations about weight loss, not fully understanding programme goals and commitment and wanting a ‘quick fix’:
What they wanted was a quick fix . . . They want to lose pounds very quickly. And it doesn’t happen . . .
GP270
Only one study270 provided data around apparent barriers to and facilitators of health professionals’ own engagement with a specific WMP. They described how clinicians’ preconceived beliefs and attitudes towards integrating WMPs into primary care settings were important and they noted that engaged practices (as opposed to less engaged practices) were characterised by active GP participation and ‘buy in’.
The importance of the people within the programme setting (for fostering a sense of accountability)
In keeping with some key findings from participants across the included papers, programme providers reflected on the importance of WMPs for creating a sense of accountability both for themselves as professionals (in terms of increasing their responsiveness and sensitivity to their participants’ weight-management plans and needs) and in terms of their continued engagement, motivation and success:268,281
. . . I think it just made me be more sensitive . . . I’ve been kinda tryin’ to dial it [being tough on the patients] down a little bit.
Primary care provider268
Programme providers also recognised and reflected on what they regarded to be the importance of establishing and maintaining good relationships and of giving positive reinforcement and encouragement and being supportive of their weight-loss efforts:142,265,268,274
‘You’ve lost 6 pounds since you were here last.’ [Patients] really need that positive feedback that we’re paying attention to what they’re doing.
Primary care provider274
However, some commented that as the frequency and intensity of professional contact seemed to be particularly important to many participants (which was supported by participants’ own observations), this could become problematic for sustaining behaviour change patterns post intervention.
The types of interaction/support offered
Several health-care providers recognised that the intensity of interactions between programme staff and patients was important for motivating the latter to stay engaged and to sustain behaviour changes. 268,274 However, several provider participants raised concerns about the reality of this for their everyday clinical practice when time constraints were a real issue. 265,266,282 Other health-care providers raised concerns around a lack of interdisciplinary working within clinic settings, which could inhibit their abilities to support weight loss, as well as lack of clarity with regard to professional role remits within teams:
I work with our RN all the time so on a daily basis we talk about things going back and forth but the others [referring to dietitian and mental health workers] I don’t really see to be honest.
Nurse266
Although providers in the above study266 raised broad issues in their interviews relating to these barriers, they reflected positively on the study WMP in terms of facilitating interdisciplinary collaboration.
Views about mode of support
In terms of views about mode of support, health providers in one primary care study90 argued that telephone-delivered weight counselling was the most convenient for patients. By contrast, providers in another study (one that involved a residential WMP)271 argued that face-to-face group interaction is essential and particularly useful for participants with severe obesity who often experience social isolation. In another primary care study,142 views regarding mode of delivery of support were more mixed. Although recognising the practicalities of remote forms of support, programme providers (in this case nurses) argued that face-to-face interactions worked best in terms of helping them connect more effectively and facilitating participant engagement and motivation. Some even stated that they did not regard remote support as support at all.
Views about levels of provider engagement
Health-care providers in one study268 stated that they played a fairly peripheral role in aspects of programme delivery and that sometimes this made it difficult for them to fully engage with their patient and to assess their progress. They suggested that individualised feedback from other professionals involved in programme delivery (e.g. in this case, weight-loss health coaches) would have been helpful. However, the study also reported that the majority of health-care providers valued the fact that they played a limited role in the WMP, with time constraints and specific skill sets being raised as issues. Another study142 raised related issues around level of provider engagement with aspects of the programme intervention. In this case, nurses discussed a potential issue around not being able to view the information provided to patients on the study website. Some felt that viewing this information would have allowed them to understand more fully what patients were referring to in consultations. In one study,282 GPs commented on and seemed to value the relatively ‘loose’ nature of the intervention design (in this case a weight-management toolkit) as they considered it offered scope to enable them to tailor it to the individual and their community.
Similarly, nurses in another study142 expressed frustration with the lack of flexibility of their intervention, both in terms of how they were supposed to behave (i.e. by not being directive) and in terms of the scope within the website to document individual issues. This was also a concern raised by the patients themselves. Although providers in these two studies142,282 apparently appreciated interventions that were more flexible in nature (and, therefore, could be tailored more appropriately to individual care), personnel in a residential WMP271 specifically designed for people with severe obesity seemed to value having a very strict programme structure (in this case, participants had to attend morning meetings, group activities and eat six meals a day at fixed times). The general feeling among staff was that instilling this strictness in participants would facilitate behaviours that they would then seek to maintain at home.
Views about intervention content
As discussed in the previous section, WMPs incorporated BCTs and other forms of psychological support to help support weight loss. Although some (but not all) participants in one study271 found personal development classes challenging and confrontational, providers in the same study consistently argued that personal development (in this case, focusing on internal factors such as self-knowledge and self-acceptance) was essential and crucially important for maintaining lifestyle changes longer term:
It is important that they become aware of what in their life makes a difference in being obese or not.
Personnel271
Discussion
This review synthesised findings from qualitative papers (or papers that provided some qualitative data) relating to the views of adults with a BMI of ≥ 35 kg/m2 (and/or their health-care providers) about engaging with WMPs and strategies. Many of our findings echo qualitative research undertaken for Public Health England, although that research included relatively few participants in Tier 3 services for adults. 27
In summary, although there was variation expressed in views about the acceptability of various programme components (indicating the likely inappropriateness of a ‘one size fits all’ approach), there were nevertheless recurring themes around what both participant and programme providers described valuing and enjoying. Some of these key findings resonate with previous qualitative research with people with less severe obesity about the critical features of WMPs. 288
Participants in our review described being attracted to WMPs that were perceived to be novel or exciting in some key way (e.g. being different from programmes that they had tried previously), as well as perceived to have been endorsed by their health-care providers (a view supported by programme providers themselves).
A recent systematic review by Dewhurst et al. 289 has highlighted that physicians find raising issues of weight management challenging, particularly for patient relationships, and that physicians lacked training and were sceptical of WMP’s efficacy. The clinicians’ more positive views in the papers examined by us are likely to reflect bias in the sample. A trial of very brief opportunistic advice by primary care physicians, or advice and 12 free weight-loss sessions at a commercial weight-loss organisation, draws on physician endorsement, and provides external accountability by asking the patient to return to see the GP. 290 This mechanism of referral, endorsement and providing accountability could also work with more intensive programmes, and address issues raised by Dewhurst et al. 289
The sense of belonging to a group of people who shared similar issues relating to weight and food, and who had similar physiques and personalities, was described as being particularly important to many participants and seemed to foster a strong group identity and related ‘accountability’, which seemed to help with motivation and continuing engagement. Good relationships with programme providers were described as being highly valued, with ongoing encouragement and monitoring apparently important for facilitating motivation and behaviour change (a view also endorsed by the programme providers themselves).
Group-based programme activities were apparently enjoyed by many participants along with fairly intensive support from programme providers. Although these were described by both participants and programme providers as being important for supporting engagement and positive behaviour changes, concerns were raised about the availability of continuing support post intervention, and similarly by providers, who questioned the practicalities and logistics of integrating such intense support into their everyday clinical practices once the studies were completed.
On the whole, both participants and programme provider participants valued some choice and flexibility. For example, participants valued when face-to-face coaching sessions were scheduled, some flexibility around diet choices and choice of exercises, and also flexibility within, for example, ehealth interventions to personalise their input. Similarly, some programme providers perceived the lack of flexibility with various intervention components frustrating and prohibitive in terms of supporting individualised care.
Those participants who described engaging in group discussions/therapy sessions (with other participants and/or providers) and also those who discussed engaging in exercises were mainly positive about their perceived benefits. For example, when it was discussed, participants very much valued the psychological input integrated into many interventions. This is a view supported in a study of user experiences of both Tier 2 and Tier 3 weight-management services. 27 However, it is worth noting that our review also highlighted that some patients did describe struggling with these aspects, with some describing them as particularly challenging. Some participants described struggling with the various physical activities (because of a range of physical comorbidities)142,204,263,272,273,277,278,285 and not everyone enjoyed group interaction and discussions with others (sometimes apparently because they suffered from various mental health comorbidities). 264,271,272,279,284,286 For intervention developers, this is worthy of note, particularly as people with very severe obesity might be especially vulnerable to both physical and mental comorbidities. These could inhibit engagement with much fitter peers with fewer weight-related issues, or restrict ability to undertake certain intervention components – an observation that is perhaps less apparent in research with people with less severe obesity (e.g. Sutcliffe et al. 288).
Methodological limitations
In this review, we were interested in ascertaining the views of participants with severe obesity (people with a BMI of ≥ 35 kg/m2). Therefore, our inclusion criterion was that papers needed to state that participants in their respective studies (i.e. either in their qualitative evaluations or in the intervention studies to which their qualitative evaluations were linked) had a mean BMI of ≥ 35 kg/m2. Of those papers that only considered programme provider views, these had to be linked to intervention studies in which we could establish that included participants had a mean BMI of ≥ 35 kg/m2. Only two papers stated that their respective WMPs were designed specifically for people with a BMI of ≥ 35 kg/m2. 271,284 Thus, across the papers some people with a BMI of < 35 kg/m2 would have been included (see Report Supplementary Material 1, Section 16). Quotations from participants were not linked by authors to specific detail regarding BMI status, and so we cannot be certain that findings reflect exclusively the views of those with severe obesity.
Only nine papers linked participant quotations to sex,128,142,204,271,273,276–279 only one to age status142 and none to socioeconomic/demographic characteristics, making it hard for us to consider whether any issues raised were particularly sensitive or pertinent to these aspects.
We know from a recent review of Tier 3 weight-management interventions for adults with severe obesity that drop-out rates are very high (43–63%). 26 Only four of our included papers stated that some of the participants in their qualitative evaluations had been ‘low users’, ‘quitters’ or ‘drop-outs’,142,204,262,269 and only one of these papers linked quotation data directly to intervention usage status. 142 Although our findings highlighted a range of views with regard to the usefulness or otherwise of various intervention components, it is worth noting that participant sample characteristics within the included papers are skewed towards those who had chosen to engage and who had completed the various intervention activities.
We included 33 papers that each reported some qualitative data that we felt were of use in terms of addressing our key research questions. Although all papers included qualitative data, in terms of ‘quality’, some were deemed richer than others in terms of data and insights, some ranged from being exclusively qualitative studies providing fairly rich data in our areas of interest through to mixed-methods studies, through to studies that were actually primarily quantitative with responses to open-ended survey questions (see Report Supplementary Material 1, Section 16). Despite this variation in the overall level of quality, we felt that it was more important to retain any relevant findings rather than disregard based on study quality. In doing so, we would argue that all 33 papers contributed useful elements to the collective whole and enabled us to develop our understanding of the issues of importance to people with a BMI of ≥ 35 kg/m2. Nevertheless, it is clear that the qualitative research literature focusing specifically on lifestyle WMPs for people with very high BMIs is limited, particularly for people who are low users or do not wish to engage with such services.
Overall summary
We summarise below the main points that have arisen from the review in this chapter.
General issues relating to methodology
-
Although 33 papers were included in this review, 21 of these could be described as qualitative studies exploring the views of those with severe obesity. Seven considered the views of programme providers and five were primarily quantitative with responses to structured questionnaires as open-ended survey questions.
-
For the qualitative data collected, specific participant characteristics were inconsistently and poorly reported (if at all). This made it difficult to disentangle and comment on any apparent variations in views in accordance with, for example, sex, age and ethnicity.
-
We only included papers in which we could establish that participants had a mean BMI of ≥ 35 kg/m2. BMIs for those involved in qualitative evaluations were reported in only nine papers. Of those that provided a mean, this ranged from 36.8–44.7 kg/m2, likely to be lower BMIs than participants in Tier 3 services. However, no quotations from participants in any of the included papers were linked to specific detail regarding BMI status. We cannot be certain that our key findings exclusively reflect the views of those with a BMI of ≥ 35 kg/m2.
-
No included papers provided qualitative data from those who had been invited to join a WMP but who had declined to take part at the recruitment stage, and only four papers reported including participants who had not fully engaged with all programme activities to varying degrees. Therefore, in terms of indicators for effective interventions, it is worth acknowledging that key findings will be skewed towards those who had chosen to engage and who had completed the various intervention activities.
Indicators for effective interventions
-
Weight-management programmes that are perceived to be novel or exciting and WMPs that are perceived to be endorsed by health-care providers tend to be valued.
-
The sense of belonging to a group of people who share similar issues and characteristics seems particularly important, helping to foster a strong group identity and related ‘accountability’, which aids motivation and continuing engagement.
-
Ongoing encouragement and monitoring by programme providers seems important for facilitating motivation and behaviour change.
-
Group-based programme activities tend to be valued, along with fairly intensive support from programme providers.
-
Both participants and programme providers tend to value some choice and flexibility within various intervention components.
-
Participants seem to value the psychological input integrated into many interventions.
-
People with very severe obesity might be especially vulnerable to both physical and mental comorbidities, which could inhibit engagement with certain intervention components (e.g. group-based interaction and physical activities).
Chapter 6 Systematic review of economic evaluations
This chapter reports the findings of the review of economic evaluation studies of interventions for people with severe obesity (see Chapter 2 for methods). The chapter begins with an overview of the principles of economic evaluation before describing the findings of the systematic review and quality assessment of the included studies. The chapter concludes with a discussion of the key findings for policy-makers and makes recommendations about the conduct of future economic evaluations, with a focus on delivering estimates of cost-effectiveness that are of high quality and of relevance particularly to UK policy-makers.
Overview of the principles of economic evaluation
Economic evaluation supports health-care decision-making, and is a necessary requirement for the reimbursement of drugs and adoption of new interventions in many countries. When deciding on public funding for new health-care interventions, agencies such as NICE, the Scottish Medicines Consortium (SMC) and the Canadian Coordinating Office for Health Technology Assessment (CADTH) require economic evaluation as an integral part of the decision-making process. This requirement to evaluate new interventions is driven by the fact that health-care budgets are fixed and there is limited availability of scarce resources (money) for investment in new interventions. The economic concept of opportunity cost means that by investing limited resources in health-care programme A, society cannot invest that same money in any alternative programme B. The opportunity cost is the value of the highest valued alternative forgone (e.g. programme B). It is, therefore, essential to invest in the most efficient, cost-effective health-care programmes that generate the greatest health benefit for minimal cost. Economic evaluation is a tool that helps decision-makers balance the benefits and costs of alternative investment decisions to allocate resources in the most efficient manner.
Economic evaluation is the comparative analysis of the costs and benefits of alternative health-care interventions. By comparing different interventions (e.g. a new intervention vs. a control), one can calculate incremental costs and incremental effects that can be used in economic evaluations. Economic evaluations can take the form of cost-minimisation analysis, a cost–benefit analysis, a cost-effectiveness analysis (CEA) and a CUA. The primary differences between the methods lie in how benefits are defined and valued. For example, in a CEA, the outcome measurement of interest can be any clinically relevant outcome, measured in its natural units (e.g. life-years gained, incidence of diabetes mellitus avoided or kg of weight loss). NICE recommends the use of CUA when possible,291 when benefits are most commonly measured in terms of the QALY. QALYs provide a composite measure of length and quality of life, whereby each year of life is weighted by a health-related quality of life (or utility) score [e.g. 10 QALYs could be obtained by living 10 years in full quality of life (10 × 1) or 20 years in half of the full quality of life (20 × 0.5)].
To help decision-makers reach decisions, the incremental costs and incremental effects are compared in a single ratio, called the incremental cost-effectiveness ratio (ICER), the additional cost required to achieve one unit of benefit. In CEA, the ICER might be the additional cost required to achieve 1 kg of weight loss. In general, lower values of the ICER are preferred because the health-care system can achieve health benefit for lower cost. Conversely, if a new intervention saves money, but generates health decrements, society would prefer a higher value of the ICER (i.e. greater cost savings for each unit of health benefit lost). However, ICERs in CEA are difficult to interpret as we do not know how much society might be willing to pay to gain 1 kg of weight loss. For this reason, CUA (cost per QALY) is preferred by NICE. NICE tends to recommend new interventions that deliver 1 additional QALY at a cost that is < £20,000. Interventions may be recommended up to a cost of £30,000 per QALY (borderline cost-effective) if they deliver substantial clinical benefit, but would rarely be accepted at a cost of > £30,000. Interventions that are more costly and less effective are said to be dominated by the comparator and are highly inefficient. Conversely, interventions that deliver additional health benefit while also saving money (e.g. the long-term cost savings of avoiding myocardial infarction outweigh the initial intervention cost) offer a particularly cost-effective option and are said to be dominant over the comparator.
Economic evaluations can be further broken down by investigating the time horizon over which they report results. There are two main types of economic evaluation and both can use any of the frameworks described above. The first, economic evaluation alongside RCTs, use costs and outcomes measured over the trial follow-up to determine cost-effectiveness, benefiting from a high degree of internal validity, driven by the randomised nature of the study design. However, for conditions with long-term sequelae, such as obesity, these analyses rarely have sufficient follow-up to capture all the costs and benefits of an intervention of relevance to decision-makers and of importance to patients. For example, the benefits of losing weight now are not likely to be seen until long into the future (e.g. reduced risk of obesity-related diseases such as type 2 diabetes mellitus, and myocardial infarction). For this reason, economic evaluation of chronic disease often relies on decision modelling methods to extrapolate trial results over the longer term, often over a patient’s lifetime.
The studies in this review are categorised depending on the intervention of interest, the framework of analysis (CEA or CUA) and whether they use RCT-based or decision-model-based (including RCTs in which data are extrapolated over the longer term) methods to assess cost-effectiveness.
Review methods
Detailed methods for the search strategies, data extraction, inclusion criteria, exclusion criteria and quality assessment can be found in Chapter 2.
Number of studies retrieved from the searches
Full details of study identification are provided in Report Supplementary Material 4, Figure E21.
A total of 2826 studies were excluded. The primary exclusion reason is reported here, but studies could have been excluded for more than one reason.
A total of 2871 titles and abstracts were identified from the searches (see Report Supplementary Material 1, Section 17, for a list of included studies and Section 18 for a list of excluded studies). A further three studies were identified from inspection of reference lists of included studies,292–294 leading to 2874 potentially relevant studies. Of these, 270 studies were read in full text to assess whether or not they met the inclusion criteria. Forty-six included studies that were reported in 48 articles meeting all the inclusion criteria were included in the cost-effectiveness review. Most studies (38/46, 83%) were published in the past 10 years, with the remaining studies published between 1999 and 2007.
Description of comparisons in the included studies
Descriptions of the intervention, controls and comparisons undertaken across the included studies are provided in Report Supplementary Material 5, Table E19. The 46 included studies can be described in terms of three categories of evaluation: studies that evaluated the cost-effectiveness of WMPs,128,142,149,163,294–305 drug interventions306–308 and bariatric surgery. 37,38,292,293,309–331 The study by Finkelstein and Kruger295 is categorised here as a WMP, although it could equally be categorised under drug interventions. The WMPs were further categorised into five groups: lifestyle WMPs, VLCDs, meal replacements, group versus individually delivered interventions and remote delivery of programmes. Bariatric surgery was further categorised into four groups: GB, GBP, other and bariatric surgery (in which different types of surgery were grouped together and the evaluation was conducted for generic ‘bariatric surgery’).
Weight-management programmes
Lifestyle weight-management programmes
Three studies included interventions with physical activity and dietary advice. 149,299,305 The intervention evaluated by Meenan et al. 299 was designed for people with serious mental health illnesses. This was the only study in this systematic review to focus on this population. Participants were given professional advice on eating behaviour, given physical activity advice and provided with counselling by a mental health counsellor and nutritional interventionist. Four of the studies gave a calorie deficit142,163,226,303 or low-carbohydrate diet. 142,303 Finkelstein and Kruger295 included commercial WMPs in their analyses, specifically Weight Watchers® and Vtrim (Middlebury, VT, USA). Vtrim is an online support programme providing diet and exercise advice.
The Counterweight programme was evaluated by Trueman et al. 302 Slimming World® was compared with usual care (advice on diet and lifestyle changes) in one study. 298 The intervention groups in the trial by Tsai et al. 304 had quarterly visits with a primary care provider and monthly visits with a weight-loss coach. One of the intervention groups included the option of a meal replacement (SlimFast®) or weight-loss drugs. Participants were prescribed a 1200-kcal/day diet and given advice on physical activity in one study. 195
Very low-calorie diets
Only one study evaluated the cost-effectiveness of a VLCD as part of a WMP. 297 The VLCD provided 600 kcal/day. Alongside the VLCD, participants were counselled in CBT and addiction change theory at weekly group meetings. The participants continued to be a part of the programme for up to 3 years after the VLCD period. Many comparators were included in the analyses: two bariatric surgeries (GB and GBP), three WMPs (standard Counterweight Programme in general practice, Weight Watchers® and Slimming World®) and one with no treatment.
Meal replacements
The Jenny Craig programme evaluated by Finkelstein and Kruger295 consisted of a meal replacement programme (Jenny Craig food packs), with a prescribed calorie intake of 1200–2000 kcal/day. Participants were counselled once each week with follow-up via telephone/e-mail.
Group versus individual sessions
Only one study evaluated the cost-effectiveness of having a group versus an individually delivered WMP. The SHINE trial294 was a comparison of counselling provided in an individual call with counselling provided in a conference call.
Evaluations of remote delivery of programmes
Four studies142,296,300,301 evaluated the cost-effectiveness of remotely delivered WMPs compared with face-to-face contact. The motivation behind the comparisons was to investigate if a remotely delivered intervention could save scarce health-care resources while also achieving similar or superior benefit. These interventions were internet based. The interventions were compared with usual care, or a less intensive WMP. The control groups in Little et al. 142 and Ritzwoller et al. 301 consisted of brief advice on how to lose weight. The control in Krukowski et al. 296 was the same WMP, but it was conducted in person instead of remotely. Participants in the control group in Miners et al. 300 were given some printed information at baseline. Hollenbeak et al. 294 also evaluated remotely delivered interventions, with telephone contact via a conference call versus an individual call, but these were not compared with direct face-to-face contact.
Orlistat
Four studies assessed the cost-effectiveness of orlistat for people with a BMI of ≥ 35 kg/m2. 295,306–308 All compared orlistat (usually prescribed with a diet) with diet only/placebo plus diet/no intervention. Finkelstein and Kruger295 did not explicitly report giving a diet, although they may have done so. Typically, orlistat was given three times daily with food.
Bariatric surgery
In contrast to the evidence on clinical effectiveness in Chapter 3, the majority of economic evaluation studies included in the review (27/46 studies, 59%) assessed the cost-effectiveness of some form of bariatric surgery for severe obesity. 34,37,38,292,293,309–312,314–331 There was substantial overlap in terms of the types of surgery evaluated in the studies. Overall, the following types of surgeries were evaluated: GB, 16 studies;37,38,292,293,309,311,314,316,318,320,321,323,324,328,329,331 GBP, 18 studies;37,292,293,309,311,312,314–319,322,324,325,327,329,331 SG, two studies;318,324 and vertical banded gastroplasty (VBG), two studies. 314,330 Eleven studies37,292,293,309,311,314,316,318,324,329,331 included more than one type of surgery, enabling comparison across surgery types (although not the focus of our review). Eight studies37,292,293,309,311,316,329,331 evaluated two types of surgery, and three studies314,318,324 evaluated three types of surgery. Four studies310,313,324,326 evaluated the cost-effectiveness of generic bariatric surgery, allowing more than one surgical procedure to be evaluated together. Most of these studies included either GB or GBP, but one allowed BPD, which is not reviewed here as it is rarely used in the form evaluated in these studies.
As with studies evaluating WMPs, there was substantial heterogeneity in the definition of comparators in the surgery studies. Fourteen studies38,292,293,309,310,316–322,324,328 used standard care as the comparator of interest. However, the content of the ‘standard care’ varied substantially across studies, including medical treatment,38,292,293,309,316–318,320,321,328 professional consultations324 and WMPs. 310,319,322 In studies evaluating bariatric surgery in diabetic patients, standard of care generally followed country-specific best practice guidelines for type 2 diabetes mellitus. Some bariatric surgery studies (14 studies) compared the intervention with a ‘no treatment’ group. 37,311–315,322,323,325–327,329–331 The assumptions with regard to BMI progression were varied, particularly in the control groups, with some studies assuming that the comparator remained at a stable BMI/at baseline BMI for the duration of the model.
Quality of the included studies
This section summarises the quality assessment of the 46 included studies. Eleven economic evaluation studies142,149,163,187,294,295,299,301,304,320,330 conducted without long-term extrapolation were quality assessed using the Drummond and Jefferson332 checklist. The remaining 35 studies37,38,128,292,293,296–298,300,302,305–320,322–329,331 were quality assessed using the Philips et al. 73 checklist for decision modelling studies (see Report Supplementary Material 1, Section 19). For details of quality assessment, see Report Supplementary Material 1, Section 20.
Quality assessment of trial-based economic evaluations
The studies quality assessed using the Drummond and Jefferson332 checklist were often economic evaluations conducted directly using patient-level data on costs and outcomes collected within RCTs. When also included in our review of RCTs, a quality assessment is provided in Chapter 3. The quality assessment in accordance with the Drummond and Jefferson332 checklist revealed that the within-trial economic evaluations were generally of good quality. Studies usually clearly described their research question. The majority of the studies gave a detailed description of the interventions, but description of the control interventions was limited. For example, costing of controls sometimes only included partial delivery costs, or did not specify the resource use required in the control arm of trials, such as the cost of health-care professionals’ time. As a result, studies may have overestimated the ICER and underestimated the interventions’ true cost-effectiveness. In general, there was scope for studies to provide more detail on costs and resource use. This was a recurring issue among the within-trial economic evaluations. For economic evaluations alongside trials that reported CUA results, all calculated QALYs, and most were based on patient-reported EQ-5D. However, the tariffs used to value EQ-5D responses were not always clear.
Seven economic evaluations assessed using the Drummond and Jefferson332 checklist did not extrapolate beyond the trial time horizon. 142,149,163,294,299,301,304 The follow-up period for these studies ranged between 1 and 2 years only. Given the chronic nature and health risks of obesity, it is likely that the most important costs to health services/patients and impact on patients’ quality of life and premature mortality attributable to obesity-related diseases are not captured by these short time horizons. Most of these studies evaluated the costs and benefits of a WMP. Half of all identified WMPs were economic evaluations alongside clinical trials. Only one bariatric surgery study did not extrapolate beyond the trial time horizon. In addition, five decision analysis models were populated using meta-analysis of data from systematic reviews, but did not use the model to extrapolate costs and outcomes over a longer term (three WMPs295,296,303 and two bariatric surgery studies320,330). RCTs have a distinct advantage for providing patient-level data, as they control for bias and have a good degree of internal validity. Although studies may achieve a high-quality score on the Drummond and Jefferson332 checklist, their relevance for policy-making is questionable, given that they fail to include the relevant long-term costs and benefits of weight loss. For this reason, the majority of studies in the review use decision models to assess longer-term cost-effectiveness.
Quality assessment of decision models
Details of important study characteristics pertaining to decision modelling studies are provided in Report Supplementary Material 1, Table 27. In general, the decision models had a clear statement of the decision problem and objective of the evaluation. The model scope and perspective were generally clearly described. The time horizon and discount rate were reported in all studies and the discount rate was generally in line with national recommendations for economic evaluations.
However, the sources used for developing the structure of the models were lacking, with limited justification and transparency on the structural assumptions. This lack of transparency was also an issue in the data identification methods. Few of the decision modelling studies used systematic methods to identify their data inputs. When choices were made between different potential sources, these were rarely made clear and were often insufficiently justified. As a result there is potential for selective use of sources, particularly for the use of utilities in the models.
Model structure
The majority of the included studies were decision models projecting long-term costs and outcomes, capturing costs and benefits accrued over a lifetime. In addition, Wilson et al. 305 project long-term outcomes, but not costs. Five decision models38,128,297,306,320 incorporated evidence from RCTs included in Chapter 3 as the primary estimate of clinical effectiveness underpinning the model. The majority of decision models used a lifetime horizon, but for those that did not, the studies reported running the decision model for between 5292,309 and 40 years. 326 The differing time horizons limit the comparability of ICERs between the WMPs and bariatric surgery studies. As bariatric surgery is usually modelled over a longer time horizon, the intervention is more likely to capture long-term benefits of weight loss, and thus is more likely to be cost-effective than the WMPs evaluated over a shorter time horizon.
Disease health states
The majority of the decision models did not include most of the important relevant obesity-related disease health states. Fourteen decision models included more than one of the relevant disease health states. 37,38,128,293,297,298,300,302,305,310,312,313,323,327 The average number of disease health states was five, ranging from two to nine. Some studies included only type 2 diabetes mellitus as the modelled obesity-related disease. 292,306,309,314,316,321 The majority of UK decision models, 7 out of 10,37,38,128,297,298,300,302,328 included more than one of the relevant disease health states. The average number of disease health states was three, ranging from two to three. The remaining two UK studies309,314 included only type 2 diabetes mellitus as a health state. For studies only interested in diabetes mellitus as an outcome, this modelling approach would be suitable. However, for studies modelling interventions for people with severe obesity, other obesity-related diseases would be relevant. Excluding obesity-related diseases such as CHD, stroke and cancers would not present the true cost-effectiveness of the interventions.
Analysis framework
All studies reported incremental costs and outcomes as these were explicit inclusion criteria for our review. The majority (40/46, 87%) of studies (all Markov models) were CUAs and reported benefits in terms of either QALYs37,38,128,142,149,292–295,297,298,300,302–307,309–319,321,322,324–326,328–331 or disability-adjusted life-years (DALYs). 308,323 CUA (cost per QALY) is preferred by NICE. The cost per QALY can be compared with an explicit threshold, whereas most evaluations using CEA cannot. The QALY is routinely used as a measurement of health outcome and therefore has the advantage of allowing for comparisons across different areas of health care.
Weight regain assumptions
Assumptions surrounding the effect of a treatment once treatment has ended can have an important impact on cost-effectiveness. Study data often reported short follow-up, and the rate of weight regain after the end of a study can have an important impact on the occurrence of obesity-related disease events, with associated implications for costs, outcomes and cost-effectiveness.
Weight-loss data for WMPs, in particular, were often based on data with short follow-up, and assumptions were required to project weight regain after the final time point. To our knowledge, there are no very long-term, unbiased studies investigating extended weight loss from WMPs or drug therapies, apart from the Look AHEAD trial, which reported 9-year follow-up data. 220 However, to date, no economic evaluation of this study has been published. Only five of the included WMP studies297,298,300,302,305 attempted to extrapolate longer-term weight loss, with variable weight regain assumptions that rendered cross-study comparability difficult. The majority of these were UK based, and all assumed different weight regain rates. For example, Lewis et al. 297 evaluated the LighterLife intervention and used weight regain data from their own internal data set, with exclusion of dropouts. Weight regain for the other WMPs was in accordance with a background natural annual BMI change of 0.16 kg/m2. This probably biases in favour of LighterLife as it is unclear what the weight regain rate would have been in the whole study population. The weight regain assumption applied for surgery in the Lewis et al. 297 study was based on Swedish Obese Subjects (SOS) data333 using an annual percentage change in BMI (0.35% and 0.34% for GB and bypass, respectively). Overall, the inconsistent and poorly justified assumptions regarding weight regain between and within studies of WMPs was a recurring issue of concern across the studies. Understanding the true, longer-term trajectory of weight following WMPs is a key area of importance for future research, as it has a very significant impact on the projection of the timing of onset of obesity-related diseases, costs and negative quality-of-life implications. A lack of understanding of regain trajectories could potentially lead to incorrect cost-effectiveness conclusions, yet few studies explore the impact of this important assumption in sensitivity analysis.
The weight regain assumption made in the orlistat studies also had an impact on the cost-effectiveness results. Veerman et al. 308 used a different assumption regarding weight regain (0.385 kg/month) and included more disease health states in their model. In contrast to the other two studies,306,307 Veerman et al. 308 found orlistat not to be cost-effective in the long term.
For bariatric surgery, the best existing data on long-term weight loss come from SOS observational data over 20 years, but not all studies made use of these data. 333 One UK study37 assumed that patients would keep the weight off for 5 years post surgery, based on a systematic review reporting weight reduction for up to 5 years following surgery followed by a linear regain to weight loss of 17.7% at 10 years. Five other bariatric surgery studies310,312,319,324,331 extrapolated weight-loss data using different versions of SOS data for up to 10 years. An older US study334 that had 14 years of follow-up of patients having GBP surgery was used to model weight regain in one study. 315 Other studies assumed either that patients had permanent weight loss post surgery317,323,327 or that they reverted back to baseline after trial follow-up. 314 The long-term weight-loss data from the SOS study showed that people regain weight after surgery, although not all do. Therefore, assuming unchanged permanent weight loss probably overestimates the cost-effectiveness, whereas reverting back to baseline after trial follow-up might underestimate the cost-effectiveness of surgery.
Selection of interventions considered
There was limited justification for the choice of interventions compared in the studies. There was a wide variety of interventions to consider, and the studies focused on comparing bariatric surgery, WMPs or weight-loss drugs with usual care. The WMPs were all very different, even within categories of WMPs (e.g. remotely delivered interventions). For bariatric surgery, the selection of interventions was less problematic, with the majority of studies evaluating either GB or GBP. However, many studies allowed both or other surgical interventions as well. A greater issue for cross-study comparability of the surgical evaluations relates to the choice of controls.
Selection of controls considered
Across the WMPs, there was substantial heterogeneity in the content of the control comparator treatments, varying from different WMPs, including some or all of physical activity programmes or advice, healthy eating advice, prescribed calorie deficit and professional counselling, to no active intervention. Within the remotely delivered interventions, the comparator arm varied from provision of minimal intervention, such as verbal advice or self-help booklets, to more intensive behavioural WMPs. In the studies with a physical activity programme provided or with physical activity advice, prescribed deficit of calories/no calorie intake or deficit prescribed, the controls varied between having no intervention,299,335 advice on diet and exercise,142,149,163 prescribed calorie deficit303 and usual care (which was not clearly described). 305
In the bariatric surgery studies, 14 studies38,292,293,309,310,316–322,324,328 compared surgery with usual care, which ranged from medical management for type 2 diabetes mellitus, weight-loss drug therapies, and WMPs (consultations, education, meal replacement, VLCD, other diets and exercise). The choice of these comparators was often based on what was found through a literature review or methods were unspecified. The remaining bariatric surgery studies compared surgery with a ‘do nothing’ approach.
There was a lack of justification for the choice(s) of comparator(s) used for the economic evaluations, for example how standard or usual care was determined. The terms ‘usual care’ and ‘standard practice’ were often applied as description of the control without further detail. The term ‘medical management’ was used for both weight-loss drugs and type 2 diabetes mellitus medication. ‘Diet and exercise’ was often used to describe the control groups, which gives very limited information regarding the type of WMP used as a comparison.
The choice of comparator is important for two reasons. First, it enables a consistent assessment of the studies against each other to determine an overall picture of cost-effectiveness. Second, the choice can have an important impact on cost-effectiveness. For example, across the review, when WMPs were compared with no treatment, they were more likely to be reported as cost-effective or dominant. 297,299,302
Modelled populations
Populations considered included people with type 2 diabetes mellitus, metabolic syndrome, mental health illnesses, different socioeconomic statuses, knee osteoarthritis and non-alcoholic steatohepatitis. There was a distinct lack of evidence on the cost-effectiveness of different interventions in the overall population with severe obesity (i.e. including all people with and without comorbidities reflecting the population as a whole).
Incorporation of data into economic models
There was limited justification for choice of data sources used to populate the model. Sixteen292,293,296,297,302,305,306,308,309,311,316,319,321–323,325 (out of 35) quality-assessed studies using the Philips et al. 73 checklist were lacking transparency in the data identification methods and were not appropriate given the objective of the model [e.g. how surgery was costed (the breakdown of the accumulated annual cost incurred by surgery group), complication rates and transition probabilities]. In many cases, the methods for identifying data were not specified at all. In 27 studies292,293,296,297,300,302,305,307,309–312,315–319,321–329,331 (out of the 35), data were not appropriately quality assessed, there was often no discussion on their choice of data and why chosen data sources were preferred. Methods used for modelling data were often not specified. When expert opinion was obtained, justification for the methods for obtaining that information was rarely reported. Data quality is highly important for the confidence in the accuracy of health-care decision-making.
Cost data in the models
Most studies reported costs from a health-care payer perspective, and few included indirect patient or social care costs. Three studies293,322,326 that reported having a societal perspective for analyses did not seem to include any non-health costs, such as the costs to participants of time and travel to WMPs or surgery, or lost productivity associated with obesity-related diseases. In general, when studies reported a health-care payer perspective, the associated resource use and costs were appropriate for that perspective, although in many of the studies scope existed for clearer details of resource use used for intervention costing, particularly for the development and delivery of WMPs. There is a need for future studies to consider not just the health-care payer perspective, but also the social care cost of obesity-related disease, particularly in an ageing population. The lack of studies including patient and social care costs is worrying because it means that the full consequences for patients are not realised. None of the studies included a full societal perspective, meaning that the full economic burden of obesity-related disease to society (e.g. lost productivity and disability payments) is not captured in the models. It is likely that widening the scope and perspective of costs would improve the cost-effectiveness case of many obesity treatments.
Even among the studies adopting a health-care perspective of costs, it is questionable whether or not the full scope of relevant costs to health-care payers was incorporated. The completeness of costs was particularly worrying for bariatric surgery evaluations. The preoperative surgery resource use that occurs before the surgery includes an evaluation for eligibility for surgery, and additional sessions with a dietitian/psychologist/consultant to see whether or not bariatric surgery is appropriate (in accordance with NICE obesity guideline costing report336). Only six studies37,292,309,310,314,318 reported having included preoperative assessment as part of costing. The total preoperative cost varied from ≈£350 to ≈£1000. Picot et al. 37 included the breakdown of resource use for preoperative assessment. They included seven outpatient visits, eight dietitian consultations and one psychologist session.
The reporting of resource use for postoperative care was also often poorly reported, or not included at all. This consists of follow-up care with a consultant/dietitian/psychologist. Follow-up visits monitor people post surgery to test for any vitamin and mineral deficiencies and comorbidities, provide advice on diet and physical activity, and provide psychological support. In the UK, the British Obesity & Metabolic Surgery Society (BOMSS) guidelines337 provide recommendations on preoperative and postoperative nutritional assessments. Some studies include postdischarge costs only, whereas others also include annual follow-up visits continuing for years after the surgery. The NICE obesity guideline costing report336 recommends that people having bariatric surgery are appropriately followed up to ensure that they do not have vitamin and mineral deficiencies and other problems that might arise post surgery. Many studies attempted to account for the costs of complications following surgery (20/27 studies). 37,38,293,310,311,313–320,322–324,326–328,331 However, complications data available for parameterisation of decision models was of short duration, ranging between only 30 days331 and 2 years310 post surgery. Owing to a lack of high-quality, detailed data on long-term costs of complications following bariatric surgery, it is likely that these costs have been substantially underestimated. The implication is that many cost-effectiveness models may overestimate the cost-effectiveness of surgical treatment of obesity.
Effectiveness, treatment outcomes and linking of evidence
The effectiveness data were usually incorporated into a model that predicted the risk of cardiovascular events. The model predictions would depend on factors including BMI, systolic blood pressure, HbA1c and lipids to finally determine changes in RRs of disease events (type 2 diabetes mellitus, stroke, CHD, cancer, hypertension and other obesity-related disease events). Five studies37,38,310,314,316 applied a type 2 diabetes mellitus incidence/remission rate (which benefited the surgery group) based on the SOS study. Some studies were not clear in linking the clinical evidence to obesity-related diseases. Methods used for linking of evidence to obesity-related diseases could have an important impact on the cost-effectiveness results (e.g. they could bias the cost-effectiveness results in favour of the surgery group, as in the example above).
Utilities and quality-adjusted life-years
Mortality data were, in general, poorly described across the studies, and methods used to estimate life-years gained were not always clear in the decision analysis models. Utility weights were generally provided for health states included in the models, but the methods for obtaining those weights were poorly described and rarely based on systematic or structured literature reviewing. When utility weights were not available, many studies drew on assumptions/expert opinions, but justifications for chosen weights were rarely provided or tested in sensitivity analysis.
Utility decrements associated with bariatric surgery complications were not included in any study. Possible complications post surgery might include abdominal hernia, GI tract ulcers, gallstones, abdominal pain, wound infections, anaemia, iron/vitamin B12 deficiency or surgical mortality. Leaving out the utility decrements of surgical complications means that the study analyses would be overestimating the accrued benefits of having bariatric surgery, and underestimating the ICER.
Sensitivity analyses
Methodological uncertainty
Methodological uncertainty was not comprehensively addressed, although most studies varied the discount rate and four studies varied the model time horizon. As one might anticipate, the longer the time horizon, the more cost-effective an intervention tended to be. 38,310,317,328 This clearly emphasises the importance of having a model time horizon sufficiently long to capture all the long-term costs and consequences of weight loss. Although long-term modelling is widely advocated, it must also be acknowledged that such exercises also increase uncertainty in projected costs and benefits when extrapolations are based on limited evidence and modelling assumptions. Varying the discount rate generally did not change cost-effectiveness results. 298,300,310,315,317,323,328 In some cases,323 when increasing the discount rate, surgery was no longer cost-saving (although still cost-effective). A further example can be found in Miners et al. ,300 in which reducing the health benefit discount rate to 0% substantially reduced the ICER from base-case values. Other important methodological issues, such as adjustment of utility weights for general population age and sex norms, were not explored in the studies. A well-conducted economic evaluation should test the different types of uncertainty thoroughly.
Heterogeneity
Few studies conducted subgroup analyses. Faria et al. 293 found that GBP was more cost-effective in participants with type 2 diabetes mellitus, younger age groups (< 40 years) and those without comorbidities at the start of the model. Borisenko et al. 310 found that surgery was cost-effective overall, but was cost-saving for all groups with type 2 diabetes mellitus. Hoerger et al. 316 found that surgery was more cost-effective for patients with newly diagnosed diabetes mellitus than for patients with established diabetes mellitus. Wilson et al. 305 found that the WMP was more cost-effective in the group with a higher percentage weight-loss goal. Surgery was more cost-effective in higher-BMI groups. 322,323 Five studies300,311,315,325,329 found that the ICER was higher for men than for women. For example, McEwen et al. 325 found that the ICER for men was much higher (US$74,629) than for women, driven by higher costs from health plan administrative data for men. However, the intervention remained cost-effective for different BMIs and for those with/without type 2 diabetes mellitus. Conversely, James et al. 318 found no impact of sex on the ICERs. There might be a subgroup of patients who would benefit more from an intervention because of the heterogeneity of treatment effects and, therefore, be an important contribution to health-care decision-making.
Structural uncertainty
Nine studies298,310,311,317–319,323,324,327 varied the weight regain assumption in a sensitivity analysis. Eight of these were applied in evaluations of surgery, in which these assumptions are perhaps less important than WMPs, as good long-term weight-loss data exist for surgery. Those that did vary the weight regain rate reported that it had a substantial impact on the cost-effectiveness results. For example, Borisenko et al. 310 assumed that instead of following weight projections based on the Scandinavian Obesity Surgery Registry,338 the weight was assumed to revert to the same weight as in the control group after 15 years. Surgery remained cost-saving (although less so) and with fewer QALYs gained overall.
Wang et al. 331 used RCT data from people with a BMI of ≥ 35 kg/m2 to determine reductions in BMI over a 5-year period. A natural history, BMI trajectory model was then used to extrapolate beyond 5 years. The model predicted people’s BMIs over time, which was dependent on baseline (and 5-year) BMI, starting age and sex. The study explored the sensitivity of their weight regain assumptions in a scenario analysis. Assuming that weight was regained within 5 years resulted in a fourfold increase in the ICERs, clearly illustrating the importance of the assumptions applied on cost-effectiveness. Wang et al. 331 was the only study that attempted to comprehensively incorporate the impact of the weight regain assumptions on cost-effectiveness.
Only one WMP study varied the weight regain assumptions. Meads et al. 298 assumed that all weight would be regained within 2–3 years (compared with a constant annual rate in the base case). The assumption did not have an impact on the cost-effectiveness, with the WMP remaining dominant compared with control. It is concerning that none of the other WMP studies conducted sensitivity analyses around weight regain assumptions, as this is a key area of uncertainty in which limited long-term data exist. Therefore, studies that have failed to adequately test the impact of their weight regain assumptions on results are probably under-representing the true variability and sensitivity of the base-case ICER.
Parameter uncertainty
Most studies conducted deterministic sensitivity analyses focusing on cost or utility parameters used in the model. All were univariate, with none reporting bivariate or multivariate analyses, simultaneously varying multiple parameters together. Given the rarity of probabilistic sensitivity analysis across the studies, this is an important limitation common to many studies.
Varying the underlying effectiveness of an intervention (i.e. weight loss) is an important area of uncertainty to explore. Few studies varied the underlying effectiveness of an intervention. For example, Ackroyd et al. ,309 a UK study, reduced the BMI and type 2 diabetes mellitus remission by 20% for surgery and compared with watchful waiting. This changed the end result for the UK, in which GB and GBP were no longer cost-saving but remained cost-effective.
Only one study varied the cost of preoperative assessment in the sensitivity analyses. Picot et al. 37 increased the cost of preoperative assessment by 20%; however, this had little effect on the ICER. Comprehensive costs of follow-up care after bariatric surgery were only included in some studies and these generally failed to capture all the important and relevant costs. Even fewer studies varied these costs in sensitivity analysis. However, those studies that did found that although increasing the follow-up costs after surgery had an impact on the ICER, this was not a large enough effect to change the base-case cost-effectiveness conclusion. 37,310,311,314,316
Costs falling outside the health sector were rarely included in the studies. Borisenko et al. 310 included indirect (non-health) costs in their sensitivity analysis and found that cost savings from surgery doubled. Conversely, Lee et al. 323 found that including such costs did not change the overall results, with surgery remaining dominant in those with a BMI of > 40 kg/m2 and cost-effective for those with a BMI of > 35 kg/m2.
Varying the rate (or probability) of complications post surgery was explored in eight studies. These studies reported that these analyses did not change the cost-effectiveness results. 311,317,318,323,324,326,330,331 However, all analyses, including the sensitivity analyses, were based on short-term data. Owing to a lack of data, no studies were able to comprehensively assess the impact of complication/revision surgery rates on both costs and outcomes over a long-term time horizon.
A utility decrement from complications of bariatric surgery was not applied in any studies. However, some studies looked at including a utility decrement post surgery to reflect the recovery period. Borisenko et al. 310 applied a utility decrement for the month post surgery in the sensitivity analysis. This resulted in a QALY loss of 0.2 overall. It might be anticipated that combining sensitivity analyses around complication rates, the costs of these and the utility decrement in a multivariate sensitivity analysis might have a greater impact on cost-effectiveness and more accurately categorise the uncertainty across these important model parameters.
Six studies showed that varying the mortality rate post surgery had little impact on the ICER. 37,316,324,326,329,331
Only a few studies conducted a probabilistic sensitivity analysis. To address parameter uncertainty, a probabilistic sensitivity analysis is a useful tool. This constitutes the uncertainty surrounding their values, and allows for the presentation of decision uncertainty represented in cost-effectiveness acceptability curves, which can show the probability that the health technology is cost-effective.
Consistency and validation of the results
Consistency of model outputs and validation of the results were poorly developed, with little evidence of model quality assessment. Nine studies tested the mathematical logic of the model. 128,302,305,310,313,316,317,327,328 Seven models were established models that had been previously published and subjected to some degree of peer review, one study was said to be validated but did not provide any details on the validation methods and one model was validated by comparing the simulated life expectancy and lifetime risks for each of the comorbidities included with the results reported in the literature. Most studies compared their findings with other published literature; however, deviations from existing literature were poorly described, explained or justified.
Summary of the results of the included studies
For all studies, we report ICERs in the same currency and year as reported in the reviewed studies. When a threshold value of the ICER is reported in the studies, this is detailed in Table 48. When thresholds are not reported, we have assumed standard thresholds that are country specific. We have not undertaken a translation exercise to convert data to Great British pounds because doing so would require major assumptions about the comparability of the health-care systems across different countries. Directly converting results to Great British pounds would mask important differences in the way health-care resources are consumed (and reimbursed by health-care payers) in different countries. Direct conversion could increase the risk of misleading conclusions that may or may not be relevant to a UK decision-making context.
Weight-management programmes
Table 48 summarises the results from the included studies evaluating WMPs. The studies are in order of the economic evaluation approach taken (economic evaluation alongside clinical trial, other and decision model).
| Study (first author and year) | Control: brief description | Intervention: brief description | Economic evaluation type | Benefit measurement | Study type | ICER | Assessment of uncertainty (probability of cost-effectiveness) | Threshold |
|---|---|---|---|---|---|---|---|---|
| Lifestyle WMPs | ||||||||
| Little 2017142 (see Chapters 3 and 4) | Brief verbal and online healthy eating advice | E-learning with and without face-to face support. Physical activity advice with low carbohydrate (< 50 g/day) or deficit of 600 kcal/day | CEA/CUA | QALY | EE alongside RCT | POWeR+ F vs. control: £1203; POWeR+ R vs. control: –£966 (dominant) | NR | NR |
| McRobbie 2016149 (see Chapters 3 and 4) | Four practice nurse sessions over 8 weeks; follow-up at 6 and 12 months | WAP with healthy eating and physical activity advice | CUA | QALY | EE alongside RCT | £7742 |
68.26% 77.46% |
£20,000 £30,000 |
| Meenan 2016299 (see Green 2015123 in Chapter 3) | Usual care | STRIDE programme: diet DASH based (≤ 30% fat and ≤ 10% saturated fat calories, for 4.5–6.8 kg weight loss) and exercise programme | CEA | Cost per kg lost | EE alongside RCT | US$1224 | NR | NR |
| Perri 2014163 (see Chapter 3) | Sixteen nutrition education sessions | Intervention groups – initial weekly sessions (8 for low, 16 for moderate and 24 for high), 1200- to 1800-kcal/day, physical activity advice | CEA | Cost per kg lost per participant in each group (control, low, moderate and high) | EE alongside RCT | Control: US$28; low: US$33; moderate: US$22; high: US$25 | NR | NR |
| Tsai 2013304 | Refer to Chapter 3, Wadden 2011.195 Usual care (quarterly visits with their primary care provider) | Refer to Chapter 3, Wadden 2011.195 Brief lifestyle counselling on calorie restriction and physical activity advice (quarterly provider visits plus monthly weight-loss counselling visits) or Enhanced Brief Lifestyle Counselling (as above plus choice of meal replacements or weight-loss medication) | CUA/CEA | QALY | EE alongside RCT | Brief lifestyle counselling vs. usual care: –US$18,962 (dominated), enhanced brief lifestyle counselling vs. usual care: > US$115,397 | 20% (brief lifestyle counselling) and 47% (enhanced brief lifestyle counselling) | US$100,000 |
| aFinkelstein 2014295 | No treatment | Weight Watchers®, Vtrim, Jenny Craig and orlistat | CEA/CUA | QALY | Other | Weight Watchers® vs. control: US$34630; Qsymia vs. Weight Watchers®: US$54,130; Jenny Craig vs. Qsymia: US$377,760; orlistat: dominatedly, Vtrim: extendedly dominated | Weight Watchers® dominates in 46% of simulations; Vtrim in 20% of simulations | US$50,000/QALY |
| Tsai 2005303 (see Stern 2004181 in Chapter 3) | Low-fat reducing diet with energy reduction goal | < 30 g/day of carbohydrate, no energy reduction goal given | CUA | QALY | Other | US$1225 (dominant) |
78.6% 79.8% |
US$100,000 US$150,000 |
| Wyke 2016335 (see Chapters 3 and 4) | Given a booklet on losing weight. Waiting list (could do the programme 12 months later) | FFIT Group: the FFIT had pitch-side physical activity sessions led by club community coaching staff and an incremental pedometer-based walking programme. The dietary component of FFIT was designed to deliver a 600-kcal daily deficit | CUA | QALY | Decision model | £2810 | 100% | £20,000–30,000 |
| Lewis 2014297 (see Chapter 4) | No treatment, Counterweight, Weight Watchers®, Slimming World®, GB and GBP | LighterLife Total is a WMP with a VLCD (600 kcal) component and participants are provided with meal replacements, behaviour change therapy and group support | CUA | QALY | Decision model | Results were presented for two groups. All interventions were compared with no treatment. BMI of ≥ 30 kg/m2: Slimming World®: £5613; Counterweight: £2618; Weight Watchers®: dominant; LighterLife Total: £12,585. BMI of ≥ 40 kg/m2; LighterLife Total: £4356; GB: £20,505; GBP: £10,627 | NR | NR |
| Meads 2014298 | Information provision either verbally or printed material only | Referral by a health professional in primary care to a commercial WMP group (Slimming World®) for usually 12 weeks | CUA | QALY | Decision model | £6906 | 68% | £20,000 |
| Trueman 2010302 (see Chapter 4) | No treatment; followed an expected trajectory (broadly representative of the UK population) without the Counterweight intervention | Counterweight Programme in primary care. Delivered by a practice nurse in groups or individual sessions (nine over 12 months). Participant either chose a goal-setting approach or was prescribed a calorie deficit (≥ 500 kcal/day) | CUA | QALY | Decision model | £473 (dominant) | NR | NR |
| Wilson 2015305 | Usual care, not clearly described | 12-week community-based WMP (called Beyond Sabor) with a physical activity programme. Weekly 2-hour classes that included physical activity, and education (including cooking demonstration and group interaction) to promote a healthy diet | CUA | QALY | Decision model | Results for those with morbid obesity ranged from US$32,078 to US$335,952 | NR | NR |
| VLCD | ||||||||
| Lewis 2014297 (see Chapter 4) | No treatment, Counterweight, Weight Watchers®, Slimming World®, GB and GBP | LighterLife Total is a WMP with a VLCD (600 kcal/day) component and participants are provided with meal replacements, subject to behavioural change therapy and group support | CUA | QALY | Decision model | Results were presented for two groups. All interventions were compared with no treatment. BMI of ≥ 30 kg/m2: Slimming World®: £5613; Counterweight: £2618; Weight Watchers®: dominant; LighterLife Total: £12,585. BMI of ≥ 40 kg/m2: LighterLife Total: £4356; GB: £20,505; GBP: £10,627 | NR | NR |
| Meal replacement | ||||||||
| Finkelstein 2014295 (see Chapters 3 and 4) | The control arm was a combination of all the control arms of the RCTs included in the systematic review. For WMPs it was usual care, provision of a self-help booklet, or using eDiets (online support of eating habits). For orlistat it was placebo plus the same diet as the intervention group | WMPs: Weight Watchers® (WMP with weekly in-person or online group meetings), Vtrim (WMP with online group support). WMP with low-calorie meal replacements called Jenny Craig. Drug therapies: 120 mg of orlistat taken three times daily (plus a calorie reduction; most orlistat studies included in their systematic review reported reduction of ≈500–900 kcal/day) and 7.5 mg of Qsymia phentermine and 45 mg of topiramite combination taken once daily (plus a calorie reduction of 500 kcal/day, LEARN manual and monthly visits) | CEA/CUA | QALY | Other | Weight Watchers® vs. control: US$34,630; Qsymia vs. Weight Watchers®: US$54,130, Jenny Craig vs. Qsymia: US$377,760; orlistat: dominated; Vtrim: extendedly dominated | Weight Watchers® dominates in 46% of simulations; Vtrim in 20% of simulations | US$50,000 |
| Group vs. individual | ||||||||
| Hollenbeak 2016294 (see Weinstock 2013 in Chapter 3) | Refer to Chapter 3, Weinstock 2013.196 WMP based on DPP with individual telephone calls |
Refer to Chapter 3, Weinstock 2013196 WMP based on DPP with conference telephone calls |
CUA | QALY | EE alongside RCT | US$9249 (intervention is less costly and less effective) | 48% | US$100,000 |
| Evaluations of remote delivery of programmes | ||||||||
| Little 2017142 (see Chapters 3 and 4) | Brief verbal and online healthy eating advice | E-learning with and without face-to face support. Physical activity advice with low carbohydrate (< 50 g/day) or deficit of 600 kcal/day | CEA/CUA | QALY | EE alongside RCT | POWeR+ F vs. control: £1203; POWeR+ R vs. control: –£966 (dominant) | NR | NR |
| Ritzwoller 2013301 (see Bennett 201290 in Chapter 3) | Refer to Chapter 3, Bennett 2012. Self-help booklet | Refer to Chapter 3, Bennett 2012. Community healthy eHealth eating and physical activity advice WMP | CEA | Cost per unit change in weight (kg) and cost per unit change in blood pressure (mmHg) | EE alongside RCT | Weight: ranged from US$2040 to US$2204. Blood pressure: ranged from US$574 to US$621 | NR | NR |
| Krukowski 2011296 | Same WMP and weekly 1-hour face-to-face groups for 6 months | Weekly 1-hour online meetings via a synchronous chat group. Calorie restricted diet and dietary fat goal < 25% of calories from fat. Graded exercise goals. Internet condition met weekly in small groups of 15–20 individuals in a secure online chat room. Online database to help monitor calorie intake (Calorie King, Family Health Network, Costa Mesa, CA, USA) | CEA | Life-years gained | Other | Internet based: US$2160; in-person: US$3306 | NR | NR |
| Miners 2012300 | Individuals were given a small amount of printed information at baseline, reflecting primary care (McConnon 2007339) | The e-learning device (website) provided advice, tools and information to support behaviour change in terms of dietary and physical activity patterns, as required. Personalised motivational statements were provided, based on online questions (McConnon 2007). E-mail reminders were sent if individuals had not been active on the website | CUA | QALY | Decision model | Only 2 ICERs reported for subgroup with BMI ≥ 35 kg/m2: £151,142 (female without T2DM) and £232,911 (male with T2DM) | NR | NR |
| Summary of findings for pharmacotherapy studies | ||||||||
| Finkelstein 2014295 (see Chapters 3 and 4) | The control arm was a combination of all the control arms of the RCTs included in the systematic review. For WMPs it was usual care, provision of a self-help booklet, or using eDiets (online support of eating habits). For orlistat it was placebo plus the same diet as the intervention group |
WMPs: Weight Watchers® (WMP with weekly in-person or online group meetings), Vtrim (WMP with online group support). WMP with low-calorie meal replacements called Jenny Craig Drug therapies: 120 mg of orlistat taken three times daily (plus a calorie reduction; most orlistat studies included in their systematic review reported reduction of ≈500–900 kcal/day) and 7.5 mg of Qsymia phentermine and 45 mg of topiramite combination taken once daily (plus a calorie reduction of 500 kcal/day, LEARN manual and monthly visits) |
CEA/CUA | QALY | Other | Orlistat dominated by Weight Watchers® and Qsymia | Probability that orlistat is cost-effective is < 5% | US$50,000 |
| Hertzman 2005306 (see Davidson 1999, Finer 2000, Hauptman 2000 and Sjöström 1998 in Chapter 3)101,113,123,174 | Placebo plus a low-fat diet with calorie reduction | 120 mg of orlistat (up to three times/day) in addition to a low-fat diet with calorie reduction for 12 months | CUA | QALY | Decision model | €13,125 | 90% | €22,000 |
| Lacey 2005307 | Placebo and low-fat calorie-reduced diet | Orlistat and low-fat calorie-reduced diet | CUA | QALY | Decision model | Base-case: €16,900. Sensitivity analyses: €11,000–35,000 | NR | NR |
| Veerman 2011308 | Australian reference population based on existing levels of morbidity and mortality for 2003 | 15 mg of orlistat three times daily for 12 months and (on average) 1.6 medication-related follow-up visits per person to the GP | CUA | DALY | Decision model | AU$240,000 | NR | NR |
| Results for GBP | ||||||||
| Surgery vs. ‘do nothing’ | ||||||||
| Campbell 2010311 | No treatment; assumed to have a stable BMI | Patients underwent either laparoscopic GB or laparoscopic RYGB | CUA | QALY | Decision model | RYGB vs. control (base-case): US$5600. Subgroup/scenario analyses: ranges from dominating to US$15,500. RYGB vs. GB (base case): US$6200. Subgroup/scenario analyses; ranged from US$1700 to dominating (in the subgroup analysis of those with BMI of ≥ 50 kg/m2 and using alternative efficacy data) | 100% | US$50,000 |
| Castilla 2014312 | Assumed to have a stable BMI | Patients underwent GBP (no further details given) | CUA | QALY | Decision model | Dominating | ≈88% at 10 years, increasing to 100% over a lifetime horizon | €30,000 |
| Clegg 2002314 | Assumed the control group remained at 135 kg (BMI of 45 kg/m2). One year of VLCD in sensitivity analysis | Patients underwent RYGB, VBG or adjustable silicone GB | CUA | QALY | Decision model | GBP vs. control: £6289; with sensitivity analyses ranging from £7255 to £20,768. GBP vs. VBG: £742. GB vs. GBP: £256,856 | NR | NR |
| Craig 2002315 | Stable BMI; no treatment | Patients underwent GBP, and followed up three times per year for 3 years if had a successful surgery (no further details given) | CUA | QALY | Decision model | Ranges from US$35,600 to US$5400 | NR | NR |
| Hoerger 2010316 | Usual diabetes mellitus care, which included monitoring glycaemic levels similarly to the monitoring provided in the UK Prospective Diabetes Study 1998 | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | Patients with newly diagnosed diabetes mellitus: GBP vs. control: US$6882. Patients with established diabetes mellitus: GBP vs. control: US$12,000 | 95% of simulated values | Between US$2000 and US$23,000 |
| Klebanoff 2017322 | The no-treatment arm had no treatment and followed natural history probabilities. The intensive WMP was based on Look AHEAD for 4 years (see Chapters 3 and 7) and Vilar-Gomez 2015,340 in which the intensive WMP comprised a 750-kcal/day deficit low-fat diet combined with exercise advice | Laparoscopic RYGB | CUA | QALY | Decision model | Results presented for patients in all liver fibrosis stages. Surgery vs. no treatment (BMI 35–39.9 kg/m2): US$21,806. Surgery vs. no treatment (BMI > 40 kg/m2): US$17,460 | 100% | US$100,000 |
| McEwen 2010325 | Usual care. Costs and QALYs were projected in the scenario in which no patients had surgery | Laparoscopic or open RYGB | CUA | QALY | Decision model | US$1425 | NR | NR |
| Michaud 2012327 | No surgery in population cohort | RYGB | CEA | Life expectancy | Decision model | Ranges from US$8171 (including surgery costs) to US$123,260 (including pharmacotherapy costs) | NR | NR |
| Salem 2008329 | Non-surgical WMPs (assumed stable BMI) | Laparoscopic GB or laparoscopic RYGB | CUA | QALY | Decision model | Base-case: women (aged 35 years with BMI of 40 kg/m2): US$14,680. Men (aged 35 years with BMI of 40 kg/m2): US$18,543 | NR | NR |
| Wang 2014331 | The no surgery group accrued costs and outcomes derived from the natural history model | Patients underwent open or laparoscopic RYGB, or laparoscopic GB | CUA | QALY | Decision model | Laparoscopic RYGB: ranges from US$6000 to US$24,100. Open RYGB: ranges from US$15,600 to US$59,500 | NR | NR |
| Picot 200937 | Participants were monitored, and did not receive weight-loss treatment | Laparoscopic GBP or laparoscopic GB | CUA | QALY | Decision model | GBP vs. control: (using weight-loss estimates from Clegg 2002314): £3160; (using alternative weight-loss estimates from Angrisani 2007341): £4127. Sensitivity analyses ranged from £1833 to £8584. Ranges from £3075 to £9845 | NR | NR |
| Surgery vs. usual care | ||||||||
| Ackroyd 2006309 | One year of medical treatment, presumed with dieting, followed by annual follow-up for 4 years | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | UK: GBP vs. control: £1517 | NR | NR |
| Anselmino 2009292 | One year of medical treatment, presumed with dieting, followed by annual follow-up for 4 years | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | Austria: GBP vs. control: –€1447; Italy: GBP vs. control: €1246; Spain: GBP vs. control: €2664 | NR | NR |
| Faria 2013293 | Best medical management (no further details given) | Patients underwent GBP or GB (no further details given) | CUA | QALY | Decision model | Dominating | 95% cost-saving | NR |
| Ikramuddin 2009317 | The CORE diabetes mellitus model simulated changes in the control group from medical management of T2DM using standard algorithms defined in Palmer 2004342 | Patients underwent RYGB | CUA | QALY | Decision model | US$21,973 | 83.7% | US$50,000 |
| James 2017318 | Usual care consisting of pharmacotherapy, diet and exercise management; this included periodic outpatient visits to dietitians/nutritionists, an exercise physiologist and a psychologist | Patients underwent RYGB, GB or SG | CUA | QALY | Decision model | RYGB vs. control: AU$22,645 | 75% | AU$70,000 |
| Jensen 2005319 | Weight Watchers® followed for 2 years, with hour-long weekly meetings | The SOS study, in which patients underwent GBP | CUA | QALY | Decision model | US$7126 | NR | NR |
| Klebanoff 2017322 | The no-treatment arm had no treatment and followed natural history probabilities. The intensive WMP was based on Look AHEAD for 4 years (see Chapters 3 and 7) and Vilar-Gomez 2015,340 in which the intensive WMP comprised a 750-kcal/day deficit low-fat diet combined with exercise advice | Laparoscopic RYGB | CUA | QALY | Decision model | Results presented for patients in all liver fibrosis stages. Surgery vs. intensive WMP (BMI 35–39.9 kg/m2): US$18,662. Surgery vs. ILI (BMI > 40 kg/m2): US$14,843b | 100% | US$100,000 |
| Mäklin 2011324 | A range of interventions from brief advice given by physicians to intensive conservative treatment | GB, GBP or SG (no further details given) | CUA | QALY | Decision model | Dominating | NR | NR |
| Results for SG | ||||||||
| Surgery vs. usual care | ||||||||
| James 2017318 | Usual care consisting of pharmacotherapy, diet and exercise management; this included periodic outpatient visits to dietitians/nutritionists, an exercise physiologist and a psychologist | RYGB, GB or SG (no further details given) | CUA | QALY | Decision model | SG vs. control: AU$27,523 | 71% | AU$70,000 |
| Mäklin 2011324 | A range of interventions from brief advice given by physicians to intensive conservative treatment | GB, GBP or SG (no further details given) | CUA | QALY | Decision model | Dominant | NR | NR |
| Results for sleeve VBG | ||||||||
| Surgery vs. ‘do nothing’ | ||||||||
| Clegg 2002314 | Assumed the control group remained at 135 kg (BMI 45 kg/m2). One year of VLCD in sensitivity analysis | Patients underwent RYGB, VBG or adjustable silicone GB | CUA | QALY | Decision model | VBG vs. control: £10,237 | NR | NR |
| van Gemert 1999330 | Preoperative measurements were used as the control group, no treatment | Patients underwent VBG. At year 2, 90% of patients were no longer followed up medically | CEA | QALY | Other | ≈US$4000 | NR | NR |
| Results for GB | ||||||||
| Surgery vs. ‘do nothing’ | ||||||||
| Campbell 2010311 | No treatment; assumed to have a stable BMI | Patients underwent either laparoscopic GB or laparoscopic RYGB | CUA | QALY | Decision model | GB vs. control (base case): US$5400. Subgroup/scenario analyses ranges from dominating to US$13,900. RYGB vs. GB (base case): US$6200. Subgroup/scenario analyses; ranges from US$1700 to dominated (in the subgroup analysis of those with BMI of ≥ 50 kg/m2 and using alternative efficacy data) | 100% | US$50,000 |
| Clegg 2002314 | Assumed the control group remained at 135 kg (BMI 45 kg/m2). One year of VLCD in sensitivity analysis | Patients underwent RYGB, VBG or adjustable silicone GB | CUA | QALY | Decision model | Adjustable silicone GB vs. control: £8527 | NR | NR |
| Hoerger 2010316 | Usual diabetes mellitus care, which included monitoring glycaemic levels similarly to the monitoring provided in the UK Prospective Diabetes Study 1998 | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | Patients with newly diagnosed diabetes mellitus: GB vs. control: US$11,596. Patients with established diabetes mellitus: GB vs. control: US$13,000 | 95% | US$0–30,000/QALY |
| Lee 2013323 | Baseline cohort with trend applied for BMI changes over 20 years | Laparoscopic GB | CUA | DALYs | Decision model | Ranges from AU$2154 to dominant | 100% | AU$10,000/DALY |
| Salem 2008329 | Non-surgical WMPs (assumed stable BMI) | Laparoscopic GB or laparoscopic RYGB | CUA | QALY | Decision model | Base-case: women (aged 35 years with BMI of 40 kg/m2): US$8878. Men (aged 35 years with BMI of 40 kg/m2): US$11,604 | NR | NR |
| Wang 2014331 | The no surgery group accrued costs and outcomes derived from the natural history model | Patients underwent open or laparoscopic RYGB, or laparoscopic GB | CUA | QALY | Decision model | Ranges from US$6200 to US$26,700 | NR | NR |
| Surgery vs. usual care | ||||||||
| Ackroyd 2006309 | One year of medical treatment, presumed with dieting, followed by annual follow-up for 4 years | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | UK: GB vs. control: £1929 | NR | NR |
| Anselmino 2009292 | One year of medical treatment, presumed with dieting, followed by annual follow-up for 4 years | Patients underwent GB or GBP (no further details given) | CUA | QALY | Decision model | Austria: GB vs. control: dominating. Italy: GB vs. control: dominating. Spain: GB vs. control: dominating | NR | NR |
| Faria 2013293 | Best medical management (no further details given) | Patients underwent GBP or GB (no further details given) | CUA | QALY | Decision model | Dominating | NR | NR |
| James 2017318 | Usual care consisting of pharmacotherapy, diet and exercise management; this included periodic outpatient visits to dietitians/nutritionists, an exercise physiologist and a psychologist | RYGB, GB or SG | CUA | QALY | Decision model | GB vs. control: AU$24,454 | 64% | AU$70,000 |
| Keating 2009320 | Refer to Dixon 2008.110 Best medical practice including WMP with possibility of drug therapy and VLCDs | Laparoscopic GB. Refer to Dixon 2008110 | CEA | Per additional case of T2DM cases remitted | Other | AU$16,600 | NR | NR |
| Keating 2009321 | Refer to Dixon 2008.110 Best medical practice including WMP with possibility of drug therapy and VLCDs | Laparoscopic GB. Refer to Dixon 2008110 | CUA | QALY | Decision model | Dominating | 98% | AU$50,000 |
| Mäklin 2011324 | A range of interventions from brief advice given by physicians to intensive conservative treatment | GB, GBP or SG (no further details given) | CUA | QALY | Decision model | Dominating | NR | NR |
| Picot 200937 | Participants were monitored and did not receive weight-loss treatment | Laparoscopic GBP or laparoscopic GB | CUA | QALY | Decision model | GB vs. control: (using weight-loss estimates from Clegg 2002314) £1897; (using alternative weight-loss estimates from Angrisani 2007341) £3863. Sensitivity analyses ranged from £1833 to £8584 | NR | NR |
| Picot 201238 | Two studies were included in the economic analysis. Dixon 2008:110b best medical practice including WMP with possibility of drug therapy and VLCDs. A more intensive WMP was reported in O’Brien et al.,343 which included behaviour therapy, VLCD, and advice on eating and exercise | Patients underwent laparoscopic GB | CUA | QALY | Decision model | £1634 | 100% | £20,000 and £30,000 |
| Pollock 2013328 | Refer to Dixon 2008.110 Best medical practice including WMP with possibility of drug therapy and VLCDs | GB. Refer to Dixon 2008110 | CUA | QALY | Decision model | Base-case: £3602. Sensitivity analyses ranged from –£52 to £17,176 | 100% | £20,000 |
| Results for bariatric surgery | ||||||||
| Surgery vs. ‘do nothing’ | ||||||||
| Chang 2011313 | Control groups within the identified studies from their systematic review, assumed to have stable BMI | Surgery search terms used for identifying relevant studies included bariatric surgery, weight-loss surgery, GB or RYGB | CUA | QALY | Decision model | Ranged from US$1,853 to dominating | NR | NR |
| McLawhorn 2016326 | Immediate total knee arthroplasty alone | Bariatric surgery 2 years before the total knee arthroplasty | CUA | QALY | Decision model | US$13,910 | 98.80% | US$100,000 |
| Surgery vs. usual care | ||||||||
| Borisenko 2015310 | Control group in the SOS study (Sjöström 2012) | Patients underwent either GBP, SG or GB (no further details given) | CUA | QALY | Decision model | Dominating | 99.1% cost-saving | NR |
| Mäklin 2011324 | A range of interventions from brief advice given by physicians to intensive conservative treatment | Bariatric surgery (GB, GBP or SG) | CUA | QALY | Decision model | Dominating | NR | NR |
The majority of the WMPs were economic evaluations alongside RCTs. Many concluded that the WMP was cost-effective compared with no intervention/usual care, despite some studies reporting a high degree of uncertainty. 142 Tsai et al. 304 showed that enhanced brief lifestyle counselling (and brief lifestyle counselling) was unlikely to be cost-effective when compared with usual care. However, the cost and outcomes were not extrapolated over a longer time horizon. In the study by Finkelstein and Kruger,295 Weight Watchers® was cost-effective compared with the control group, but Vtrim was dominated (more costly and less effective than a comparator). Tsai et al. 303 found that a low-carbohydrate diet was dominant, with lower costs and higher QALYs than its comparator. The results in the studies by Finkelstein and Kruger295 and Tsai et al. 303 are based on only a single year of data, and exclude many important obesity-related diseases, such as type 2 diabetes mellitus, hypertension, cancer, CHD and stroke.
Decision models formed a minority of evaluations of WMPs. Wilson et al. 305 developed a decision model, based on a 20-year time horizon, finding that a WMP was not cost-effective with ICERs at a threshold of > US$50,000 per QALY and even less cost-effective over a shorter 5-year time horizon. Both Lewis et al. 297 and Trueman et al. 302 evaluated the cost-effectiveness of the Counterweight Programme. Both studies were decision models with 10-year and lifetime horizons, respectively. Compared with usual care, Counterweight was found to be cost-effective in both studies. Hunt et al. 128 evaluated the cost-effectiveness of a WMP delivered within a football club. The WMP was cost-effective compared with the comparator (being given a booklet) in the long term.
Two studies included an evaluation of Slimming World®. Both of the decision models reported in Lewis et al. 297 and Meads et al. 298 found similar ICERs (<£10,000 per QALY gained) compared with minimal or no intervention.
Very low-calorie diets
The study by Lewis et al. 297 was the only study that included evaluation of a VLCD. The LighterLife Total intervention was more costly and more effective than the ‘no treatment’ arm over a 10-year modelled time horizon in both BMI groups (BMI of ≥ 30 kg/m2 and BMI of ≥ 40 kg/m2). LighterLife Total was also reported to be cost-effective when compared with three other commercial WMPs in the lower BMI group; however, in the higher-BMI group (comparing no treatment, LighterLife Total, GB and GBP), surgery was more effective and cost-effective. Overall, there is a distinct lack of economic evaluations looking at VLCDs for people with severe obesity.
Meal replacements
One study (Finkelstein and Kruger295) looked at the provision of meal replacements (Jenny Craig) in addition to usual care/counselling. The Jenny Craig meal replacement was not cost-effective compared with the other commercial WMPs. In terms of weight loss, Jenny Craig performed best, but the costs of the meal replacement were too high to generate a cost-effective use of resources.
Group versus individual delivery
Only one WMP study could be categorised as comparing group delivery with individual delivery. Hollenbeak et al. 294 report a within-trial CUA of group-based conference calls versus individual calls for delivery of a WMP similar to the DPP. They found an ICER just under the US$100,000 threshold, but with substantial uncertainty in cost-effectiveness, with an approximately equal chance that either group or individual calls were the most efficient use of resources.
Evaluations of remote delivery of programmes
In addition to Hollenbeak et al. 294 (see above), three studies evaluated remote delivery of WMPs. The two UK studies142,300 reached different conclusions for the cost-effectiveness of remote delivery of similar WMPs compared with minimal advice. Little et al. 142 was a within-trial economic evaluation, showing remote delivery to be highly cost-effective, whereas Miners et al. 300 had a decision model that generated ICERs closer to the threshold value, with much greater uncertainty regarding the conclusions drawn. Miners et al. 300 report small incremental QALYs, and few cases of type 2 diabetes mellitus and CVD were avoided. 300 The study also went on to report the expected value of perfect information, indicating that further research to establish the cost-effectiveness of e-learning devices is required.
The study by Krukowski et al. 296 was a US study looking at online delivery of a WMP. The study reported results from a decision model but did not include relevant disease health states, such as CVD and type 2 diabetes mellitus. This seems to be a recurring issue within decision models for obesity, and, therefore, the decision models do not give the whole picture of the chronic nature of obesity-related diseases.
We can conclude that the current economic evidence for delivering WMPs via e-learning devices is mixed. The evidence is also highly uncertain owing to missing factors that have not been accounted for in the decision models and the lack of studies extrapolating evidence beyond the trial time horizon.
In summary, there was mixed evidence of remotely delivered (four studies)142,296,300,301 and group-delivered (one study)294 interventions being cost-effective. Most of the WMPs (13 studies)128,142,149,163,295–299,301–304 were found to be cost-effective; however, adding a meal replacement was not. A VLCD was found to be cost-effective in one study. The summary results should be interpreted with caution and in the light of the highly variable study quality. Overall, there is a lack of high-quality evidence to support the long-term cost-effectiveness of WMPs.
Drug therapy
Table 48 summarises the results from the studies evaluating pharmacotherapy interventions.
Four studies evaluated the cost-effectiveness of orlistat, none of which were from a UK perspective. Two European studies306,307 found orlistat to be cost-effective compared with placebo or no intervention, with base-case ICERs from decision models of ≈€13,000 and ≈€17,000 per QALY gained, respectively. However, Finkelstein and Kruger295 compared orlistat with more active comparators, including WMPs (Weight Watchers® and Vtrim), and another, currently unlicensed, drug in the UK (phentermine and topiramate; Qsymia®, VIVUS Inc., Campbell, CA, USA). They found that orlistat was a dominated strategy, as other WMPs (e.g. Vtrim) were less costly and more effective. Veerman et al. 308 found that the cost per DALY averted was over the commonly used threshold in Australia, and only 0.1% of the total burden of disease in Australia was averted and, therefore, orlistat was unlikely to be cost-effective. Therefore, there is mixed evidence to support the cost-effectiveness of orlistat, and the case is less convincing when orlistat is compared with a more intensive WMP.
Surgery
To help with interpretation of the large number of surgery studies, the findings are presented by type of surgery. The results tables are further categorised by comparator: (1) no intervention and (2) standard care including WMPs.
Gastric bypass
Table 48 reports the findings from 19 GBP studies (1137,311,312,314–316,322,325,327,329,331 vs. do nothing and 8292,293,309,317–319,322,324 vs. usual care). GBP was found to be cost-effective in all studies. Not all studies specified the type of GBP (e.g. RYGB or open/laparoscopic procedure). Only a few studies reported the probability of cost-effectiveness. Four studies293,316,318,322 found a high probability (ranging from 75% to 100%) of GBP being cost-effective. Faria et al. 293 found a high probability (95%) that GBP was also cost-saving in the long term. Across studies in which GBP was compared with a ‘do nothing’ approach, the probability of cost-effectiveness was 95% at very low thresholds and 100% at the higher end of the acceptable threshold range. The ICERs ranged from dominating (less costly and more effective) to an ICER of ≈US$25,000 in one study, still a cost-effective use of resources. RYGB was cost-effective compared with both no treatment and an intensive WMP. 322 Michaud et al. 327 explored the cost-effectiveness of extending the criteria for offering surgery from (1) a BMI of > 40 to > 35 kg/m2 for those without comorbidities and (2) a BMI of 35–40 kg/m2 to 30–35 kg/m2 for those with comorbidities. This was found to double the population suitable for surgery, resulting in higher life expectancy: a gain of 1.55 years compared with 1.08 years with no treatment. The cost per life-year gained was US$8171 and US$10,579 for current eligibility and extended eligibility, respectively. Overall, GBP was found to be highly cost-effective, with three of the included studies finding that, when compared with no treatment or usual care, GBP could actually save health services money in the longer term.
Sleeve gastrectomy
Table 48 reports the findings of two studies318,324 evaluating the cost-effectiveness of SG. The comparator for both studies was defined as usual care. Mäklin et al. 324 found SG to be less costly and more effective than usual care (i.e. dominant). James et al. 318 also found SG to be cost-effective (71% probability) with a 29% chance that the surgery is cost-saving overall to the Australian health services.
Vertical banded gastroplasty
Table 48 reports the findings of two studies314,330 evaluating the cost-effectiveness of VBG surgery. VBG was cost-effective compared with no treatment in both. van Gemert et al. 330 reported an even stronger case for cost-effectiveness when a reduction in productivity losses was accounted for. Clegg et al. 314 also found VBG to be cost-effective compared with no treatment, even under the pessimistic assumption that all weight was regained within 5 years post surgery.
Gastric banding
The results from 16 GB studies (six311,314,316,323,329,331 vs. doing nothing and 1037,38,292,293,309,318,320,321,324,328 vs. usual care) are reported in Table 48. All evaluations found GB to be cost-effective. Six UK studies37,38,293,309,314,328 all report low base-case ICERs. There was a high probability that GB was cost-effective in studies that reported probabilistic sensitivity analyses, ranging from 95% to 100% when compared with no treatment.
When compared with usual care, four studies conducted probabilistic sensitivity analyses around their models, with three studies316,320,323 finding a > 95% chance of cost-effectiveness. James et al. ,318 however, report a lower probability of only 64% from an Australian health-care perspective. All base-case ICERs were cost-effective and four studies also reported dominance.
Table 48 reports the findings of four additional studies310,313,324,326 in which the type of bariatric surgery was not specified or in which more than one type of surgery was allowed, but results were reported only for surgery as a whole. Unsurprisingly, the results echo those of the surgery comparisons and found bariatric surgery to be highly cost-effective, with ICERs ranging from dominant (less costly and more effective) to very low values, clearly within the threshold values of willingness to pay for a QALY gained typically adopted by decision-making bodies in the respective countries.
Discussion
A systematic review was conducted of cost-effectiveness studies evaluating interventions for people with severe obesity (BMI of ≥ 35 kg/m2). Systematic review evidence on interventions for people with severe obesity is lacking. To our knowledge, this is the only systematic review comparing different types of interventions (WMPs, drug therapies and bariatric surgery) for people with a BMI of ≥ 35 kg/m2.
A similar review has been published, synthesising evidence on bariatric surgery only, for people with obesity, not limiting the evidence synthesis to people with a BMI of ≥ 35 kg/m2, as was done for this review. 344 The two reviews included 21 of the same economic evaluations for bariatric surgery. The review by Campbell et al. 344 included both full and partial economic evaluations that were quality assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Our review only included full economic evaluations, which allows us to summarise the cost-effectiveness evidence available on interventions for people with severe obesity. The studies were quality assessed using the checklists by Philips et al. 73 and Drummond and Jefferson,332 which allow for an assessment of the methodological quality of the included studies in the review that could have an important impact on the cost-effectiveness results. The advantages of a systematic approach are that one can gather all evidence on the research question and get an overview of the literature on studies from various countries. This leads to identifying the evidence gap that calls for more research.
In total, 46 studies were identified in the review, comparing different types of interventions for people with severe obesity: 16 WMPs,128,142,149,163,294–305 three drug interventions306–308 and 27 bariatric surgery interventions. 37,38,292,293,309–331 The cost-effectiveness results for WMPs and orlistat were mixed, and bariatric surgery was deemed cost-effective in all studies but, in many cases, bariatric surgery was not cost-saving. The methodological quality plays a role in the interpretation of the studies. The quality assessment of the included studies revealed that the variability in quality of the study methodology, including the costing methodology, modelling methods, the inclusion of relevant disease health states and assumptions regarding continuation of treatment beyond trial time horizon (weight regain assumptions), was a factor influencing the cost-effectiveness of the WMPs and bariatric surgery. Owing to the poor study quality and study heterogeneity, it was not possible to synthesise the data from the review.
The majority of decision models did not include all of the relevant disease health states. The chronic nature of obesity requires all relevant obesity-related diseases to be accounted for in obesity models. Studies that included only a limited number of obesity-related diseases or none at all would bias the analysis against the intervention owing to cost savings of diseases avoided.
The weight regain rate assumed in decision models once the effect of a treatment had ended could have an important impact on the cost-effectiveness. The weight regain rate has an impact on the occurrence of obesity-related diseases because of higher BMIs are often associated with a higher risk of developing obesity-related diseases. Apart from Look AHEAD, there seemed to be no unbiased studies of the long-term impact on weight from WMPs or drug therapies, and Look AHEAD may have underestimated weight loss for the non-diabetic population. The majority of included WMPs were economic evaluations alongside clinical trials, with no extrapolation beyond the trial time horizon. The short-term follow-up suggests that the long-term effects of WMPs are unknown. The weight regain assumptions applied in the projection of weight in the bariatric surgery studies varied between the studies. The best existing data on long-term weight loss come from the SOS observational study with 20 years of data. 345 The weight regain assumptions applied in the decision models varied across the included studies. Some studies assumed that participants revert back to baseline weight over a period of time, whereas others assume a more stable BMI with long-term maintenance of weight loss. The different assumptions have an impact on cost-effectiveness. Assuming a stable BMI after the treatment has ended most probably overestimates the true ICER, whereas assuming that participants revert back to their baseline weight most probably underestimates the ICER.
The limited reporting of surgery complications within the studies has an impact on the cost-effectiveness of bariatric surgery. Surgery was often substantially undercosted in the included studies. The risk of having complications from bariatric surgery has been established in the literature. 346 The risk of complications was also recently examined in a study347 comparing bariatric surgery (the majority being GBP) with specialised medical treatment, focusing on disease risk factors, using data from the Norwegian Patient Registry with a follow-up period of 6.5 years. Patients having bariatric surgery did indeed have a lower risk of obesity-related diseases, but they had a higher risk of complications such as abdominal pain, gastroduodenal ulcers and iron deficiency anaemia. Reges et al. 348 found that patients who underwent surgery had more non-bariatric reoperations such as intestinal obstruction, abdominal hernia and gastric ulcer. This further emphasised the importance of including long-term risk of complications in the economic evaluations in order to reflect the true cost-effectiveness of bariatric surgery. Furthermore, no study in the review included utility decrements associated with surgery complications. There is currently no evidence of the impact on the quality of life of patients undergoing bariatric surgery. The quality-of-life impact of surgery complications would further improve the analyses on bariatric surgery by providing a more realistic estimate of the ICER. Patients undergoing bariatric surgery incur substantial weight loss and sometimes these patients are offered plastic surgery provided by the NHS. In the UK, NICE currently recommends that, as part of postsurgery care, people undergoing bariatric surgery should be informed about and given access to plastic surgery if appropriate. 22 Because only patients in exceptional circumstances are offered this surgery, only a few will actually be offered plastic surgery provided by the NHS. Carloni et al. 349 reported an overall complication rate of 37% for patients undergoing plastic surgery. Highton et al. 350 carried out a survey of bariatric surgeons in the UK. Of the surgeons, 38% said that the success of bariatric surgery was dependent on the patient having body contouring surgery. This suggests that bariatric surgery is further undercosted in the economic evaluations in the review.
Some new evidence on the cost-effectiveness of bariatric surgery has emerged since the completion of this review. Lucchese et al. 351 compared bariatric surgery with conservative management using SOS data. They developed a Markov model (the same Markov model as reported in Borisenko et al. 310) and included obesity-related disease health states and complications post surgery, and assumed that people regained their weight in accordance with the SOS study, thereafter remaining at a stable BMI. The surgery group had a lower risk of developing obesity-related diseases than the control group. They found that bariatric surgery was cost-saving, less costly and more effective over a lifetime horizon, and even cost-saving over a 10-year time horizon. However, it is unclear if this study adequately incorporated all of the relevant long-term follow-up costs required for surgery patients. These are similar results and assumptions as found in the review of the cost-effectiveness of bariatric surgery. As in the included studies, it is questionable whether or not the full scope of relevant costs to health-care payers has been incorporated. In the UK, there are guidelines for preoperative and postoperative care after bariatric surgery (BOMSS guidelines). 337 The pre-surgery resource use includes evaluation for eligibility for surgery and postoperative care includes follow-up care with a consultant/dietitian/psychologist. The purpose of follow-up visits is to test for vitamin and mineral deficiencies and comorbidities, and to provide advice on diet and exercise and some psychological support. However, the extent of preoperative and postoperative care is seldom reflected in the included studies (or in newly published evidence), meaning that it is likely that the cost-effectiveness of bariatric surgery is generally overstated.
Two studies reported on mortality rates in bariatric surgery patients. Reges et al. 348 reported that within a retrospective cohort study in Israel, the bariatric surgery group had a lower mortality rate than the non-surgical group (1.3% and 2.3%, respectively). Cardoso et al. 352 reported that in the long term, all-cause mortality was reduced by 41% compared with the non-surgical group.
A consensus on all of the relevant outcome measurements in a bariatric surgery trial was evaluated by Coulman et al. ,353 who created a survey asking patients and professionals about the most important outcomes from bariatric surgery. The patients and professionals mostly agreed on the relevant outcomes. The survey suggested that this could potentially improve the quality of outcome data. Improved data collection on, for example, cardiovascular risks, mortality, stroke and complications post surgery could help produce better cost-effectiveness estimates.
The timing of the provision of bariatric surgery has an impact on the cost-effectiveness of bariatric surgery. One study347 reported that, on average, the cohort waited 23 months before undergoing bariatric surgery (time from first consultation to surgery). However, Lucchese et al. 351 reported that this watchful waiting significantly decreased life-year expectancy and QALYs gained (if delayed for 1, 2 or 3 years). At 2 or 3 years, the cost of surgery also increased from the base case (immediate surgery). The cohort had a mean BMI of 46 kg/m2, and 20% of the cohort had type 2 diabetes mellitus. This suggests that delaying surgery for this group of people with these patient characteristics is not cost-effective.
Prioritisation of patients with certain patient characteristics for bariatric surgery has not been answered in the systematic review. Whitty et al. 354 found, by asking the public using a discrete choice experiment, that patients who had spent a longer time on the wait list for surgery should be prioritised. They conducted a discrete choice experiment, in which the public were asked questions, choosing between two alternatives, each with different attributes, such as a patient’s BMI, presence of comorbidities, age, family history, commitment to lifestyle change, time on the surgery wait list and chance of maintaining weight loss after surgery. The results of the discrete choice experiment identified a preference among the studied population to prioritise services for individuals who (1) are willing to maintain a healthy lifestyle, (2) have a BMI of ≥ 40 kg/m2, (3) have comorbidities, (4) have a family history of obesity and (5) have spent more time trying to lose weight. Future obesity decision models could potentially include the estimates from the discrete choice experiment as probabilities to account for the prioritisation of certain patient groups.
An improved quality assessment checklist focusing on important factors for obesity could potentially improve the methodological quality of obesity models. Some important considerations for a checklist for future economic evaluations in obesity include (1) the impact of weight regain assumptions on findings, (2) ensuring that all relevant health states are included in obesity models (i.e. CVD, diabetes mellitus, stroke, cancer and hypertension as a minimum) and (3) studies should be extrapolated well beyond trial time horizons. A comprehensive checklist emphasising these three points would be a more useful quality assessment tool for researchers developing economic evaluations of interventions for obesity. There is a need to standardise how quality assessment was carried out for obesity-related studies. A checklist specific to obesity models could potentially help improve the methodological quality of the models.
The 12 UK studies included in the review (seven128,142,149,297,298,300,302 WMPs and five bariatric surgery studies37,38,309,314,328) give an indication of the cost-effectiveness of different interventions for people with severe obesity in the UK. Bariatric surgery was found to be cost-effective in all of the studies. The remote delivery of WMPs showed mixed results; however, the other WMPs that included a VLCD, physical activity programmes, lifestyle counselling and a commercial WMP were all found to be cost-effective. However, not all studies were decision models and, therefore, did not extrapolate beyond the trial time horizon. The cost-effectiveness results from the decision models are also to be interpreted with care because of the methodological quality and study heterogeneity. Therefore, the cost-effectiveness results on interventions for severe obesity is not clear-cut. There is a need for more research to estimate the long-term impact of WMPs on weight. Decision models evaluating an intervention for obesity should ideally include all of the relevant disease health states and apply weight regain assumptions more likely to reflect the real world. Decision models are subject to model uncertainty and, therefore, it is important to include sensitivity analyses to assess the decision uncertainty by varying the key assumptions made in the model, and thus show the impact on the cost-effectiveness results. Key model inputs for obesity models could be, for example, the weight regain assumption, weight-loss data and model time horizon. Conducting a value-of-information analysis to identify the parameters with the highest uncertainty could help decision-makers to prioritise research on obesity.
Overall summary of the cost-effectiveness review
We summarise below the main points that have arisen from the review in this chapter:
-
Forty-six studies of economic evaluations of weight-loss interventions evaluated the cost-effectiveness of weight-loss interventions for severe obesity, including WMPs, drug therapies and bariatric surgery, with the majority being bariatric surgery.
-
The evidence on WMPs suggests that, in the UK, a VLCD, a physical activity programme (including a calorie deficit), lifestyle counselling and a lifestyle WMP were considered cost-effective. The evidence on remote delivery of WMPs was mixed (results from two UK studies). The cost-effectiveness results from the WMPs should be interpreted with care owing to the highly variable study quality.
-
There is mixed evidence to support the cost-effectiveness of orlistat, and the case is less convincing when orlistat was compared with a more intensive WMP.
-
There is evidence to suggest that bariatric surgery is cost-effective. This result was found in all included studies comparing different types of surgery (GB, GBP, SG and VBG) with usual care or a ‘do nothing’ approach. Bariatric surgery was more likely to be cost-effective when compared with ‘do nothing’ rather than with a more active control, such as a WMP.
-
There is limited evidence to suggest that bariatric surgery is more likely to be cost-effective in certain groups of people with severe obesity. Some studies suggested that surgery was more likely to be cost-effective in younger age groups (one study) and in patients without comorbidities (one study), and cost-saving for people with type 2 diabetes mellitus (one study).
-
The methodology was of varying quality. Modelling methods, the inclusion of relevant disease health states and assumptions regarding continuation of treatment beyond trial time horizon (weight regain assumptions) varied between studies.
-
No studies included utility decrements associated with complications from bariatric surgery. The duration of follow-up for costs associated with surgery complications tended to be short.
-
The methodological variability and study heterogeneity regarding the difference in comparators, interventions, costing methodology, linking of evidence to long-term outcomes and country of study all contribute to the difficulty of interpretation of the cost-effectiveness results because these are factors that determine cost-effectiveness.
General issues relating to methodology
-
The description and justification of the key model inputs, such as cost inputs and utility weights, tended to be of poor quality. There is a need for better reporting to increase study transparency.
-
Studies did not include all of the relevant costs. Some economic evaluations included only the intervention cost and not the cost of, for example, follow-up care. For bariatric surgery, the costs of preoperative and postoperative care were not adequately captured. This especially included the cost of surgery complications. The bariatric surgery complications data post surgery within the studies were of short duration. This suggests that bariatric surgery is substantially undercosted. The utility decrements of surgery complications were not included in any of the studies, meaning that bariatric surgery is even more overestimated in terms of cost-effectiveness.
-
The transparency in the modelling methodology was of a poor standard. Studies were generally not transparent in their model structure assumptions and in how evidence was linked to final health outcomes. These are important factors when determining cost-effectiveness.
-
The majority of WMP studies were of short duration. The follow-up period for these studies ranged between 1 and 2 years. These short time horizons fail to capture the long-term effects of obesity-related diseases given the chronic nature of obesity. Weight regain for WMPs is unknown because of the short-term data currently available (apart from the Look AHEAD trial in people with type 2 diabetes mellitus). Longer-term weight data have an impact on the projection of the timing of onset of obesity-related diseases, costs and associated utility decrements.
-
Weight regain assumptions in the included decision models varied. For bariatric surgery, the best long-term data are currently available are from the SOS study, with 20 years of weight data. However, the weight regain assumptions varied from assuming a stable BMI to reverting back to baseline BMI at the end of the trial (when extrapolating beyond the trial time horizon).
-
The majority of studies were decision models; however, the included disease health states were not sufficient to give a true picture of the health risks of obesity. For decision models examining the cost-effectiveness of interventions for people with severe obesity, included health states should be all relevant obesity-related diseases.
Indicators for cost-effective interventions
-
The cost-effectiveness evidence on WMPs suggests that, in the UK, a VLCD, a physical activity programme (including a calorie deficit), lifestyle counselling and a lifestyle WMP were considered cost-effective. The evidence on remote delivery of WMPs was mixed (results from two UK studies). The majority of WMPs were with short-term follow-up, indicating that the long-term impact of a WMP is unknown and suggesting that there is a need for evidence to support the long-term impact of a WMP for people with severe obesity. This evidence could inform a decision model and provide high-quality, long-term modelling of WMPs.
-
Bariatric surgery was deemed cost-effective in people with severe obesity, suggested by results from all included studies. The interpretation of the ICERs should be done with care because of study heterogeneity and methodological quality. A standardised quality assessment tool could potentially help to improve the methodological quality by identifying the most important factors that have an impact on the cost-effectiveness. Important factors are the inclusion of all the relevant disease health states (not doing so could bias against an effective intervention), considering the impact of weight regain assumptions on study findings (not doing so could bias in favour of an intervention) and extrapolation of costs and outcomes beyond the trial time horizon (not doing so could bias against an effective intervention).
-
Bariatric surgery was more cost-effective in younger patients and people without comorbidities. However, very few studies conducted subgroup analyses. There were no subgroup analyses in studies evaluating WMPs. There is a need for further evidence to assess whether or not a WMP or bariatric surgery is cost-effective only for certain groups of people.
-
To our knowledge, there is no high-quality economic evaluation with long-term extrapolation of costs and outcomes that has yet looked at comparing different types of interventions (WMPs and bariatric surgery) for people with severe obesity. The comparisons in the included studies in the systematic review were done separately for different interventions, and were not comparable owing to the study heterogeneity and different methodological quality. This gap in the evidence base is now addressed in the economic evaluation developed in Chapter 7.
Chapter 7 Economic model including methods
Background
The health and economic burden of obesity is substantial. Obesity increases the risk of many serious illnesses, such as type 2 diabetes mellitus, heart disease, osteoarthritis, breast cancer and bowel cancer. Current WMPs provided by the NHS, local authorities and commercial organisations may not take the severity of people’s weight into account. People who might need more support, or different kinds of support, to lose weight may not be offered more help or that help for longer. The systematic review of economic evaluations (see Chapter 6) identified a paucity of evidence on the cost-effectiveness of WMPs, in particular lifestyle interventions for severe obesity. Studies were of limited relevance to NHS decision-makers. Issues limiting the relevance for UK decision-making included variable methodological quality, poorly justified weight regain assumptions and a lack of transferability of resource use, costs and utilities to the UK NHS. To address the gaps in the evidence base, this study reports the findings of a comprehensive microsimulation model to determine the long-term cost-effectiveness of the most promising WMPs identified from the review of RCTs in Chapter 3.
Project aims and objectives
The aim of this project was to determine the long-term cost-effectiveness of the most promising WMPs, identified from a systematic review of RCTs, for adults (aged ≥ 18 years) with severe obesity (BMI of ≥ 35kg/m2) from a NHS perspective. The specific comparisons were selected following discussion with the project advisory group to determine the most policy-relevant questions to the NHS. The economic analysis sets to determine the cost-effectiveness of:
-
the Look AHEAD41 ILI versus baseline (UK representative) general population BMI trends
-
a VLCD component added to a WMP (WMP1: mainly dietary intervention) versus a WMP1 alone versus baseline (UK-representative) general population BMI trends
-
RYGB surgery versus a WMP (WMP2: similar to short Look AHEAD/DPP) versus baseline (UK-representative) general population BMI trends
-
all WMPs and surgery from research questions 1–3, compared against each other from a NHS perspective.
Research questions 1–3 are based on meta-analyses of RCT evidence. Research question 4 presents a comparison across all identified WMPs to determine the most cost-effective strategy overall.
The UK Health Forum microsimulation model
The research questions were addressed using the UKHF microsimulation model. The dual-module modelling process written in the C++ programming language was developed by the UK Foresight working group,355 and was further refined for this study. This microsimulation is advantageous over other Markov cohort models because it allows for the incorporation of previous obesity-related disease history on future risk and can incorporate the impact of comorbidities. A schematic of how the model works is provided in Report Supplementary Material 4, Figure E22, and further technical details of the modelling used by the UKHF can be found in the technical appendix on the UKHF website. 356 The population of interest was the adult population (aged ≥ 18 years) with a BMI of ≥ 35 kg/m2 in 2016, as matching the scope for the review project.
Module 1 of the model created longitudinal projections of future BMI [see Risk factor (body mass index) data and Report Supplementary Material 1, Section 21]. Module 2 used microsimulation as a tool for predicting disease burden using the longitudinal projections from module 1. A microsimulation is a computer model of a specified population that accurately reflects age profiles, births, deaths and health statistics to make future projections. To generate individual case histories, the microsimulation randomly samples from the cohort it is simulating. The simulations specifically target the relationship between individuals’ evolving risk factors (BMI) and disease incidence.
In this model, virtual individuals were aged 1 year at a time and progressed through the model over a 30-year time horizon: 2016 until 2046. Throughout the modelling, there were no new entries (births and immigration) and individuals left the simulation only by death (and not by emigration). This is a characteristic of cohort simulation models. The model used the future projections of BMI to predict the burden of diseases into the future. Disease events competed to occur in each simulated life and a random component embedded in the models ensured that not all individuals at risk of an event experienced it.
The microsimulation incorporated an economic module, employing Markov-type simulations of long-term health benefits (QALYs) and health-care costs of interventions and obesity-related disease events. Each WMP was modelled separately and the simulated costs and QALYs (aggregated over 30 years) were compared across programmes to obtain incremental costs and QALYs. The ICER was then calculated as the ratio of incremental costs over incremental QALYs. The ICER gives the additional cost of achieving a QALY gained and can be compared with a threshold value reflecting society’s willingness to pay for a QALY. 357 Most interventions with an ICER of < £20,000 per QALY are considered cost-effective. 291
Each individual in the microsimulation is assigned an age, exposure to a specific risk (e.g. BMI and sex) based on input from the population data, as well as a set of diseases of interest (see Disease data). Each disease is associated with a cost to the health services and a utility weight. An individual may undergo an intervention, with parameters that are defined in the intervention scenario.
Data collection
Report Supplementary Material 5, Table E20, provides a summary of the key parameters that were required for input into the UKHF model. Detailed information about the sources of model input parameters are summarised in the technical document hosted on the UKHF website. 356
Risk factor (body mass index) data
Body mass index (in kg/m2) data were extracted from the HSE using the UK Data Service database,358 and included the years 2003 to 2014. BMI was categorised in accordance with the World Health Organization BMI cut-off points of healthy weight (< 25 kg/m2), overweight (25–29.99 kg/m2), class I obesity (30–34.99 kg/m2), class II obesity (35–39.99 kg/m2) and class III obesity (≥ 40 kg/m2). 359,360 Module 1 of the model used a non-linear multivariate, categorical regression model fitted to the cross-sectional BMI data to create longitudinal projections of the future proportion of the population in each BMI category, defined by 5-year age groups and sex. Category membership was constrained to sum to 100% and only those categorised as class II and III obesity level were included in the model cohort.
Disease data
The model included the following disease states: CHD, stroke, hypertension, type 2 diabetes mellitus, knee osteoarthritis and BMI-related cancers, including breast, colorectal, endometrial, oesophageal, pancreatic and renal. The modelled cohort may be healthy or have one of the diseases of interest at the start of the model. They are then exposed to a risk of developing any of these diseases in each model cycle depending on their risk factors. These specific disease states were chosen for inclusion in the model because (1) they have relatively high incidence (e.g. many cancers have not been included because of very small incidence in this population) and (2) the included diseases have robust evidence of a link to obesity (i.e. there is a RR identifying the diseases as having a higher degree of incidence as a result of higher BMI). Diseases were included as binary states, and did not account for remission, meaning that once an individual developed a disease in the model, they were assumed to have it for the modelled time horizon (or until they died and exited the model simulation). The justification for this assumption is that the majority of the modelled diseases are chronic. Full details of the disease definitions and their use in the model can be found in the UKHF’s technical appendix. 356
Incidence, prevalence and mortality
The most recent incidence (number of new cases of disease) and mortality (number of deaths) data were included as a proportion of the population, and stratified by age and sex for each of the BMI-related diseases. Incidence, prevalence and mortality data for CHD, hypertension, stroke and type 2 diabetes mellitus were identified from the published literature through searches of the ScienceDirect and PubMed databases. These were supplemented with searches of Google Scholar (Google Inc., Mountain View, CA, USA) and relevant organisational websites (sources for these are available on the UKHF website356). Incidence and mortality data for cancers of interest were collected from the Office for National Statistics (ONS) cancer registration statistics. 361 All-cause mortality was incorporated for all simulated patients (including those with and without any of the modelled diseases) in accordance with English- and Welsh-specific age- and sex-adjusted general population mortality rates. 362
Survival
Survival rates are estimates of the percentage of people in a population still alive for a given period of time after diagnosis. Survival parameters were entered only for terminal diseases (CHD, stroke and cancers). Survival rates for CHD and stroke were calculated in the microsimulation programme using the latest incidence and mortality data based on World Health Organization DISease MODelling (DISMOD)-II equations363 because 1-year survival data for these diseases were not available. When available, the most recent 1-, 5- and 10-year cancer survival rates for England were obtained from the ONS. 364 These data were presented as a proportion of the disease prevalence, by age and sex, and were classified by anatomical site using codes in the International Classification of Diseases, Tenth Revision. 365 The formulae used to derive the survival parameters and background information for the DISMOD-II equations can be found on the UKHF website. 356
Relative risks
The BMI-related RRs for each disease were collected from a range of sources following a review of the available literature, and were based on BMI category. For CHD, stroke, type 2 diabetes mellitus and hypertension, RRs were extracted from the Dynamic Model for Health Impact Assessment (DYNAMO-HIA) and World Obesity Federation (formerly International Obesity Task Force) estimates presented by age and BMI groups. 366 These repositories provided RR data to a high level of detail, as required for input into the microsimulation programme, and were used for the majority of other diseases, including knee osteoarthritis. The model used mortality rates for pancreatic cancer and assumed that the RR of acquiring pancreatric cancer was equal to the RR of dying from it. This assumption was deemed appropriate based on literature indicating that the low survival rate for pancreatic cancer makes the RR of death comparable with the RR of acquiring the disease. 367 This assumption may have underestimated the effect of BMI on pancreatic cancer incidence as not all who acquired the disease would have died after 10 years. Further details can be found on the UKHF website. 356
Time lags
The RR data used in the model inherently incorporated time lag components because they were an average of risk across time. Given the lack of availability of time lag data for onset of all obesity-related diseases, and the nature of the RR data used in the model, it was not deemed appropriate to ‘force fit’ time lag data into the model. A literature review was undertaken to identify data on the latent period, or time lag, between ‘exposure’ to increased BMI and the appropriate increase in risk of cancers. However, time lag data were not available for all cancers. 368,369
Disease-specific utility weights and quality-adjusted life-years
Quality-adjusted life-years were calculated for every individual at the end of each simulation year in the model. Each year, an individual who was alive at the end of the year was assigned a QALY value (quality of life or utility weight) between 0 and 1 based on the diseases they experienced in that year. Quality-of-life data (on a 0 to 1 scale) used in the model were dependent on the obesity-related disease events experienced. 370 EQ-5D-based utilities for each obesity-related disease were obtained from the literature,371 following NICE recommendations. 291 It was assumed that individuals with no disease events had 1 QALY, and those who died had 0 QALYs. For those with more than one disease, we chose an independence assumption and applied a multiplicative utility.
Searches were made in MEDLINE using terms related to utility measures as well as disease-specific terms. When different plausible utility values were identified, the most recent/representative/largest sample size was selected. EQ-5D values for each obesity-related disease modelled were thus obtained. Table 49 reports all the disease-specific utilities used in the model and further details on methods can be found the UKHF website. 356 Table 50 presents the disease-specific costs.
| Disease | Utility weights | Source (year) | |
|---|---|---|---|
| Male | Female | ||
| CHD | 0.760 | 0.760 | Laires et al.372 (2015) |
| Stroke | 0.713 | 0.713 | Rivero-Arias et al.373 (2010) |
| Hypertension | 0.721 | 0.721 | Sullivan et al.374 (2011) |
| Diabetes mellitus | 0.661 | 0.661 | Sullivan et al.374 (2011) |
| Knee osteoarthritis | 0.490 | 0.460 | Conner-Spady et al.375 (2015) |
| Breast cancer | N/A | 0.749 | Sullivan et al.374 (2011) |
| Colorectal cancer | 0.676 | 0.676 | Sullivan et al.374 (2011) |
| Endometrial cancer | N/A | 0.598 | Sullivan et al.374 (2011) |
| Oesophageal cancer | 0.904 | 0.904 | Sullivan et al.374 (2011) |
| Ovarian cancer | N/A | 0.848 | Sullivan et al.374 (2011) |
| Pancreatic cancer | 0.790 | 0.790 | Romanus et al.367 (2012) |
| Renal cancer | 0.661 | 0.661 | Sullivan et al.374 (2011) |
| Disease | Cost (£) per case per year (inflated to 2016 values) | Method | Source (year) |
|---|---|---|---|
| Colorectal cancer | 13,563.22 | Bottom-up | Hall et al.376 (2015) |
| Oesophageal cancer | 9568.28 | Bottom-up | Agus et al.377 (2013) |
| Renal (kidney) cancer | 414.81 | NHS programme budget378 | |
| Ovarian cancer | 1408.94 | NHS programme budget378 | |
| Pancreatic cancer | 5735.93 | Bottom-up | Laudicello379 (2011) |
| CHD | 2838.70 | Top-down | NHS 2015 – Proposed National Standards,380 and Liu et al.381 (2002) |
| Stroke | 1627.26 | Bottom-up | Saka et al.382 (2009) |
| Type 2 diabetes mellitus | 672.28 | Top-down | Minassian et al.383 (2012) and Kanavos et al.384 (2012) |
| Hypertension | 493.15 | Bottom-up | Brilleman et al.385 (2013) |
| Knee osteoarthritis | 223.97 | Bottom-up | Chen et al.386 (2012) |
| Endometrial cancer | 2471.21 | Bottom-up | Pennington et al.387 (2016) |
| Breast cancer | 13,295.53 | Bottom-up | Hall et al.376 (2015) |
Disease-specific health-care costs
Only the direct health-care costs of obesity-related diseases were included. Costs are reported as cost per case per year. Indirect and social care costs were not included as there is large uncertainty in non-direct health-care costs, with different levels of data available across different obesity-related diseases. The exclusion of these costs will probably underestimate total costs of disease events overall, but provides an overview of NHS commissioned costs potentially avoided by adopting different WMPs.
Direct costs were based on health-care expenditure data obtained from the published literature. When possible, we avoided using NHS programme budgeting costs as experts have advised that these underestimate the costs of disease in some care settings and recommend using published literature (NHS England, November 2018, personal communication). When direct health-care costs were not available within the literature, 2012–13 NHS programme budgeting costs were used. 378 All costs used in the model were 2016 values, unless otherwise stated. When costs were sourced in earlier years, these were inflated to 2016 values using an online tool. 388
A mix of top-down and bottom-up methods was used for cost estimation across the included studies in the review of disease state costs. The following costs were included in the total cost of disease for each health state:
-
hospital – inpatient and outpatient
-
primary care
-
medication.
The total health-care expenditure figures for each disease were divided by prevalence data of the disease to obtain an estimate of the average health-care cost incurred per individual with an obesity-related disease. The average cost per prevalence of each obesity-related illness was calculated from the search for prevalence data for all the diseases. The total treatment cost was then divided by the prevalence of the diseases. This process was repeated for all of the obesity-related illnesses included in the model.
Discount rates and time horizon
All of the simulations were run for the same time horizon (30 years) to 2046, after which few individuals remained in the cohort to produce robust estimates. For this reason, modelling a full lifetime horizon was not possible. The cost year in the model was 2016. To translate future costs and health outcomes, discount rates at 1.5% per year were used in the base case to allow comparisons with previous and current Centre for Health Technology Evaluation and Centre for Clinical Practice guidance, in which this is based on 1.5% for costs and benefits. 291 Sensitivity analysis explored the impact of varying the discount rate between 0%, 3.5% and 6% on results.
Development of the intervention scenarios and simulation parameters
Intervention scenarios
The microsimulation programme enabled different policy intervention scenarios to be tested. For this project, a baseline scenario and three distinct comparisons were modelled with their respective controls, to answer key policy questions (see Report Supplementary Material 5, Table E21). Epidemiological and health economic outcomes of each scenario were compared with those of the baseline scenario to evaluate its long-term effectiveness and cost-effectiveness. In modelling each additional scenario, we incorporated the effect of the associated intervention on the risk factor level of each individual but kept the rest of the model unchanged. Therefore, the difference in outcomes represented the net effect of the intervention.
A further analysis was conducted in which all considered WMPs and RYGB surgery were compared with each other in a fully incremental analysis to determine the most cost-effective strategy overall to the NHS in a single framework. One limitation of the incremental analysis was that the BMI change data were not necessarily based on randomised comparisons. For example, comparisons of cost-effectiveness between a VLCD and RYGB would be based on naive comparisons between sets of RCTs to determine BMI change. Attempts were made to generate a network meta-analysis of different comparisons, but this was not feasible owing to significant heterogeneity in study comparisons.
Intervention quality-of-life implications
It was assumed that there were no negative utility implications associated with adverse events attributable to different WMPs. However, studies have shown that complications occur following bariatric surgery,346 but previous cost-effectiveness studies have failed to incorporate utility decrements for this, potentially overvaluing the quality-of-life benefit attributable to surgery (see Chapter 6 for further details). The present modelling work attempted to quantify the utility decrements of surgery, using the limited evidence available.
Complication rates were obtained from a systematic review of the literature. 346 The review identified 14 studies that reported postoperative complication rates for ≥ 2 years after surgery. The reported complications were internal and incisional hernia, marginal ulcer, anaemia, iron deficiency requiring transfusion, abdominal pain requiring surgery, non-healing ulcer requiring surgery, GI bleeding and vitamin B12 deficiency. When complication rates were available for different types of surgery, data on GBP were used for the model. The complication rate for cholecystectomy was obtained from a UK obesity decision model. 37
To our knowledge, no studies adequately reported the utility implications of bariatric surgery complications. Therefore, utility implications from surgery-related complications were obtained from four different sources considered to report EQ-5D utility for similar complications in other disease areas. 389–391 An irritable bowel symptoms study was used as a proxy for complications with similar pain levels to bariatric surgery (internal hernia, incisional hernia, marginal ulcer, abdominal pain and non-healing ulcer). 392 Utility associated with anaemia and iron deficiency requiring transfusion were obtained from NICE guidance. 391 Utilities for patients with GI bleeding were based on a UK cohort with acute upper GI bleeding. 389 For cholecystectomy, utility was based on reported data from a recent NIHR report on treatment for the disease. 390
The utility values for each complication were divided by the EQ-5D score for the UK general population (0.86), obtained from Campbell et al. 389 The utility weights were then multiplied by the different surgical complication rates. The sum of the utility weights from each surgical complication was applied to the model, across the different health states. The complication rates obtained were applied over 2 years. The average weighted utility value across all the health states was 0.7958 in year 1 and 0.7442 in year 2. More details of the calculation are provided in Report Supplementary Material 1, Section 22.
Intervention costing
The calculated intervention costs for each of the modelled scenarios are provided in Table 51, and are summarised briefly in this section. More detailed intervention cost calculations can be found in Report Supplementary Material 1, Section 22.
| Year | Cost (£) | ||||
|---|---|---|---|---|---|
| Look AHEAD scenario | VLCD scenario | Bariatric surgery scenario | |||
| Look AHEAD intervention | WMP1a | VLCD added to WMP1a | WMP2b | Bariatric surgery | |
| 1 | 21,890 | 619 | 1893 | 754 | 8253 |
| 2 | 1452 | 268 | 268 | 152 | 1559 |
| 3 | 1270 | 60 | 60 | 186 | 921 |
| 4 | 1092 | 9 | 9 | 204 | 659 |
| 5 | 760 | N/A | N/A | 111 | 663 |
| 6 | 760 | N/A | N/A | N/A | 619 |
| 7 | 760 | N/A | N/A | N/A | 619 |
| 8 | 760 | N/A | N/A | N/A | 619 |
| 9 | 760 | N/A | N/A | N/A | 619 |
| 10 | N/A | N/A | N/A | N/A | 619 |
| Annual costs from year 11 to 30 | N/A | N/A | N/A | N/A | 536 |
| Total (undiscounted) costs | 9804 | 956 | 2230 | 1407 | 25,862 |
Intervention costs for WMPs were derived using a component costing approach using, when possible, resource use data from the same studies that were used to derive BMI change data (see Chapter 3).
Detailed resource use data were available from the Look AHEAD study,41 and these were directly costed using UK tariffs. In cases in which a United States dollar budget was reported to pay for support items for weight loss [e.g. fitness aides, trackers, apps (applications), etc.], this budget was converted to Great British pounds using purchasing power parities from the Cochrane economic methods tool. 393
For the WMPs (WMP1 and WMP2) and the VLCD intervention, resource use included health-care professional time required to deliver the interventions, meal replacement costs, rental of venues and provision of materials. Resource use was often poorly and inconsistently reported across studies, and assumptions were required to complete the costing. For WMPs delivered in group sessions in which group size was not reported, the average group size from the other studies was assumed. When vitamins and minerals were provided, the cost of Forceval® (Alliance Pharmaceuticals Ltd, Chippenham, UK) was assumed. When fibre supplements were used, the cost of Fybogel® [Reckitt Benckiser Healthcare (UK) Ltd, Hull, UK] was assumed. These are commonly used products in the UK NHS.
All resource use was costed using UK-specific tariffs and reported in 2016 values. Personal Social Services Research Unit unit costs were used for the time of health-care professionals. 394 It was assumed that delivery of group-based interventions required hall rentals at a cost of £50 per hour. Drug costs were sourced from the British National Formulary when required. 395 Meal replacement costs were sourced from manufacturers’ websites when possible, and the cheapest version was used to provide a conservative estimate of cost.
Costing of RYGB surgery involved four resource use categories (preoperative, operative, postoperative and complications). Preoperative costs included (1) screening for surgery eligibility (e.g. in-person/telephone-based appointment) and (2) preoperative assessments that included consultations with a dietitian, outpatient visits and an appointment with a psychologist. Preoperative resource use was obtained from the trial studies, and, when necessary, supplemented with the most recent economic evaluation of bariatric surgery in the UK37 to best reflect the current resource allocation pre surgery in the UK NHS. The costing of the RYGB itself was obtained from NHS Reference Costs 2015–2016, Healthcare Resource Group (HRG) code FZ84Z. 396
The approach to costing the postoperative phase was similar to that of the preoperative phase. Initially, resource use data were obtained from the included RCT studies. When data were not available, data from Picot et al. 37 were used. Postdischarge costs in primary care included visits to a practice nurse, a district nurse and a GP. Annual follow-up care included outpatient visits and psychology sessions. An annual outpatient visit was applied to the surgery group for the whole duration of the model time horizon. Participants were assumed to have a blood test for vitamins and trace elements prior to surgery and at each annual follow-up visit,336 and to have vitamin and nutrient supplementation after surgery, in accordance with BOMSS guidelines. 337 A fixed annual cost based on the BOMSS guidelines and British National Formulary prices was applied for follow-up care over the full duration of the model time horizon. In addition, the cost of a vitamin B12 injection every 3 months for the full duration of the model was incorporated.
The cost of surgery complications were based on the complication rates reported in the previous section based on Puzziferri et al. 346 National reference costs were used for both elective care and day-case procedures, in accordance with the relevant HRGs. The number of elective care procedures and day cases were weighted in accordance with the number of finished consultant episodes in each HRG code to obtain the weighted total cost for each complication (e.g. to account for each group of patients with different complication scores). The costing for a blood transfusion, however, was based on NICE guidance. 397 The rate of revision surgery for bariatric surgery patients was obtained from the SOS study345 and applied over a 10-year time period. The cost of having a GBP procedure (same as intervention cost) was applied to the proportion of patients having revision surgery.
Body mass index data
Baseline body mass index (baseline scenario)
In the baseline scenario, a population of 50 million virtual individuals is generated. The population carries the statistical characteristics of the UK population (i.e. age and sex distribution, disease prevalence and disease incidence at the start year). We only allow the subset of the population with a BMI of ≥ 35 kg/m2 at the start year to enter the simulation. The future BMI distribution of this subset of the population is determined by that of the whole population, which is set to match the BMI distribution projection results. In other words, during the years that the mean BMI level of the population is predicted to increase, the BMI level of the subset of the population will increase.
The modelling first uses multivariate regression (age, sex and year) analysis of historical BMI data to forecast age–sex population BMI distributions in future years. The BMI of each virtual individual is initialised in the modelling start year. In future years, it is assumed that everyone stays at the same BMI percentile within their age–sex peer group as they age (the ‘constant BMI percentile assumption’). The BMI distribution of their age–sex peer group is assumed to follow the forecasted age–sex population BMI distributions (in 5-year age categories) as they age.
Body mass index drop (effectiveness of the interventions)
The BMI drop data for the Look AHEAD intervention were obtained directly from the trial publication. 41 For the remaining comparisons, BMI drop data were calculated for each modelled scenario using meta-analyses of RCTs (see Chapter 3). Seven RCTs were identified comparing a VLCD plus WMP1 with WMP1. 93,155,172,186,193,198,200 Five RCTs were identified comparing RYGB with WMP2. 100,101,126,154,174
Body mass index change at the various time points was obtained by meta-analysis of the relevant studies for each comparison, using the inverse variance weighting. In some trials, the change in BMI from baseline was reported, in which case this value was used. In some other trials, the BMI was reported at baseline and also at the relevant follow-up time point. In this case, the BMI change was the difference from baseline at the appropriate time point. Not all trials reported the BMI or the change in BMI and, instead, they reported the weight or the weight change. In these circumstances, the BMI was calculated from available data. Many trials reported the BMI at baseline in addition to weight and so it was possible to obtain the height from the BMI and weight. When only weight data were reported, it was possible to estimate the BMI change by using relevant population reported statistics for the mean height. In these circumstances, the proportions of males and females were used to estimate the mean height for participants in the trial. The BMI change obtained from the methods above is referred to as the unadjusted BMI change.
The adjusted BMI change is the value that was used in the economic modelling to account for study dropouts. The method used BOCF assuming that when the BMI or weight of an individual was missing it returned to its value at baseline. The adjustment used the method contained in the paper by Cresswell and Mander. 63 The adjusted BMI change used for the model was given as πx¯, where x¯ is the unadjusted BMI change and π is the within-study arm proportion of participants with BMI data at the specific time point.
Body mass index regain assumptions
Most studies with follow-up beyond the active period of intervention have shown that there is gradual weight regain after a lifestyle or dietary intervention. The meta-analysis of 46 trials by Dansinger et al. 398 concluded that, on average, participants regain 0.03 kg/m2 units per month during maintenance phases of dietary-based weight-loss interventions. At this rate, participants would return to their baseline values after approximately 5.5 years. 398 A similar conclusion was reached by the NICE review, which found that, on average, intervention groups regained 0.047 kg per month more than control groups in the maintenance phase, suggesting a return to baseline after approximately 4.6 years. 399 For the base-case analysis, we modelled a linear 100% weight regain over 5 years from the last time point with observed data for each non-surgical WMP. As the risk factor modelled in the microsimulation was BMI, assuming that baseline height remained constant, the 100% weight regain after 5 years assumption translated to 100% of baseline BMI regained after 5 years.
Regain assumptions for surgery were different. Evidence from the SOS study was used to supplement data from the RCTs to predict regain over 20 years following intervention delivery. 345 As the SOS study did not report specifically for RYGB, regain assumptions for the model used GBP (13% of the total cohort) as an appropriate proxy. Between years 20 and 30, linear extrapolation of the trend in weight data from years 1 to 20 was used. Patients receiving RYGB did not return to their original BMI over the 30-year modelled time horizon.
The BMI drop data, including the impact of the base-case BMI regain assumptions used for each modelled scenario, are described, by year, in Table 52.
| Time (years) | BMI changes (kg/m2) | ||||
|---|---|---|---|---|---|
| Look AHEAD scenario | VLCD scenario | Bariatric surgery scenario | |||
| Look AHEAD interventiona | WMP1b | VLCD added to WMP1b | WMP2c | Bariatric surgery added to WMP2d | |
| 1 | –2.679 | –2.389 | –3.644 | –2.305 | –10.207 |
| 2 | –1.857 | –1.440 | –1.330 | –1.649 | –9.744 |
| 3 | –1.411 | –1.312 | –1.319 | –1.248 | –8.732 |
| 4 | –1.270 | –1.184 | –1.307 | –0.988 | –8.036 |
| 5 | –1.256 | –0.947 | –1.046 | –2.025 | –9.218 |
| 6 | –1.245 | –0.568 | –0.627 | –1.620 | –9.234 |
| 7 | –1.230 | –0.227 | –0.251 | –1.215 | –9.251 |
| 8 | –1.212 | –0.045 | –0.050 | –0.810 | –9.267 |
| 9 | –1.779 | 0 | 0 | –0.405 | –9.284 |
| 10 | –1.423 | N/A | N/A | 0 | –9.300 |
| 11 | –0.854 | N/A | N/A | N/A | –9.449 |
| 12 | –0.341 | N/A | N/A | N/A | –9.598 |
| 13 | –0.068 | N/A | N/A | N/A | –9.746 |
| 14 | 0 | N/A | N/A | N/A | –9.895 |
| 15 | N/A | N/A | N/A | N/A | –10.044 |
| 16 | N/A | N/A | N/A | N/A | –9.970 |
| 17 | N/A | N/A | N/A | N/A | –9.895 |
| 18 | N/A | N/A | N/A | N/A | –9.821 |
| 19 | N/A | N/A | N/A | N/A | –9.746 |
| 20 | N/A | N/A | N/A | N/A | –9.672 |
| 21 | N/A | N/A | N/A | N/A | –9.645 |
| 22 | N/A | N/A | N/A | N/A | –9.618 |
| 23 | N/A | N/A | N/A | N/A | –9.592 |
| 24 | N/A | N/A | N/A | N/A | –9.565 |
| 25 | N/A | N/A | N/A | N/A | –9.538 |
| 26 | N/A | N/A | N/A | N/A | –9.511 |
| 27 | N/A | N/A | N/A | N/A | –9.485 |
| 28 | N/A | N/A | N/A | N/A | –9.458 |
| 29 | N/A | N/A | N/A | N/A | –9.431 |
| 30 | N/A | N/A | N/A | N/A | –9.404 |
Sample size
To increase power and reduce error in our estimations, each simulation randomly simulated a population from the characteristics of the HSE cohort (age, sex and BMI). The simulation ran without replacement. Scenarios 1 and 2 (non-surgical comparisons) used a sample of 50 million individuals (including 5.8 million individuals with a BMI of ≥ 35 kg/m2), whereas scenario 3 (surgery) simulated 100 million individuals (including 11.6 million individuals with a BMI of ≥ 35 kg/m2). Further sampling was needed for the RYGB scenario to ensure that the scenario could accurately model surgery complications.
Initialisation approach
In the start year of each simulation (2016), the individuals in the cohort were randomly assigned diseases based on the current prevalence rates in the UK. The intervention was applied only to adults aged ≥ 18 years with a BMI of ≥ 35 kg/m2.
Analyses and reporting of results
Model outputs
Epidemiological and economic outputs are presented as rates per 100,000 people with a BMI of ≥ 35 kg/m2. Report Supplementary Material 5, Table E22, describes the specific model outputs.
Imprecision
In this report, the 95% CIs {Monte Carlo errors [p(1 – p)/N]} have been reported for each result. The CI around each result was estimated based on the probability of the event occurring and the number of Monte Carlo trials. Each Monte Carlo trial represents an individual who is sampled from the population. Thus, the confidence limits that accompany the sets of output data represent the accuracy of the microsimulation (stochastic, or aleatoric uncertainty) as opposed to the confidence of the input data itself (parametric uncertainty). Further information is available in the technical appendix. 356
To note, the BMI prevalence figures in Results (base case) present outputs using extrapolated trends from cross-sectional HSE data (2003–14). These sets of results differ slightly from the results output from the microsimulation programme because the latter takes into account dynamic population changes over time.
Base-case cost-effectiveness analysis
Intervention delivery costs were combined with simulated cost savings from reduction in obesity-related disease for each modelled scenario and compared with the respective QALY gains to generate ICERs for each of the comparative scenarios described in Report Supplementary Material 5, Table E21. As described throughout the methods, the BMI drop data for each specific comparison were sourced from a meta-analysis of selected RCTs to generate the most internally valid cost-effectiveness results output from the model.
However, policy-makers may also be interested in understanding the comparative costs and effects across all of the simulated intervention scenarios. All modelled scenarios were ranked in ascending order of simulated QALYs and plotted on the cost-effectiveness plane to determine the interventions lying on the cost-effectiveness frontier. Interventions that were more costly and less effective than a comparator were excluded on the grounds of strict dominance. Extended dominance rules out any intervention that has an ICER that is greater than that of a more effective intervention. Incremental costs and incremental QALYs were then calculated for each remaining intervention, relative to the next best alternative (i.e. next best in terms of QALY gains). The intervention with the greatest QALY gain, at a price under the threshold value (i.e. £20,000 per QALY) represents the most cost-effective use of NHS resources. This process has the advantage of simultaneously comparing the cost-effectiveness of all modelled scenarios, addressing the widest scope possible. However, the analysis is limited in that it no longer benefits from the advantages of BMI drop data determined through unbiased meta-analysis of RCTs.
Sensitivity analyses
Varying weight regain assumptions
The base-case analysis assumed BMI regain over 5 years for WMPs. For the sensitivity analysis, BMI regain was calculated using a linear trend fitted to the available data from the respective studies. Once a person had regained their original weight, we updated their new BMI value every year based on the projected BMI trends. Weight regain data used for the sensitivity analyses are reported in Report Supplementary Material 5, Table E23.
For bariatric surgery, the weight regain rate was not explored in sensitivity analysis because the SOS long-term cohort data provide robust estimates of weight loss after bariatric surgery for 20 years. Assuming a linear regain trend would probably underestimate the true weight loss obtained from surgery. The long-term weight regain rates for Look AHEAD, VLCD, WMP1 and WMP2 are more uncertain and, therefore, it was considered important to explore the weight regain assumption in the sensitivity analysis.
Results (base case)
Cost-effectiveness results for each of the comparisons are reported in Tables 53–55.
| Look AHEAD intervention compared with natural progression from baseline | Base case | Regain sensitivity analysis |
|---|---|---|
| Additional intervention cost (£M per 100,000 people) | 889 | 889 |
| Reduced obesity-related disease cost (£M per 100,000 people) | 144 | 233 |
| Total net cost (£) | 745 | 657 |
| Total QALYs gained (per 100,000 people) | 31,139 | 43,809 |
| ICER (£) | 23,915 | 14,986 |
| Cost-effectiveness | WMP1a vs. baseline | VLCDs vs. baseline | VLCDs vs. WMP1a | |||
|---|---|---|---|---|---|---|
| Base case | Regain sensitivity analysis | Base case | Regain sensitivity analysis | Base case | Regain sensitivity analysis | |
| Additional intervention cost (£M per 100,000 people) | 93.51 | 93.51 | 219.73 | 219.73 | 126.21 | 126.22 |
| Reduced obesity-related disease cost (£M per 100,000) | 84.26 | 65.14 | 86.73 | 58.97 | 2.47 | –6.17 |
| Total net cost (£M per 100,000 people) | 9.25 | 25.38 | 133.00 | 160.76 | 123.74 | 132.38 |
| Total QALYs gained (per 100,000 people) | 18,981.67 | 15,149.56 | 20,000.29 | 14,287.84 | 1018.62 | –861.72 |
| ICER (£) | 487.50 | 1873.19 | 6649.56 | 11,251.46 | 121,477.81 | Dominated |
| Cost-effectiveness | WMP2a vs. baseline | Surgery vs. baseline | Surgery vs. WMP2a | |||
|---|---|---|---|---|---|---|
| Base case | Regain sensitivity analysis | Base case | Regain sensitivity analysis | Base case | Regain sensitivity analysis | |
| Additional intervention cost (£M per 100,000 people) | 134.91 | 134.92 | 2024.13 | N/A | 1889.22 | 1889.22 |
| Reduced obesity-related disease cost (£M per 100,000 people) | 99.93 | 158.05 | 602.87 | N/A | 502.94 | 444.82 |
| Total net incremental cost (£M per 100,000 people) | 34.99 | –23.14 | 1421.26 | N/A | 1386.27 | 1444.40 |
| Incremental QALYs gained (per 100,000 people) | 22,710 | 32,502 | 140,362 | N/A | 117,652 | 107,860 |
| ICER (£) | 1541 | Dominant | 10,126 | N/A | 11,783 | 13,391 |
Future body mass index projections by age and sex
Modelled projections of the future prevalence of different BMI categories (underweight, < 25 kg/m2; overweight, 25–29.99 kg/m2; class I and II obesity, 30–39.99 kg/m2; and class III obesity, ≥ 40 kg/m2) in the population are reported by age and sex in Report Supplementary Material 1, Section 21.
Cost-effectiveness of the Look AHEAD intervention versus baseline body mass index progression (comparison 1)
Obesity prevalence results
The Look AHEAD intervention reduced the proportion of individuals with class II and class III obesity (i.e. BMI of ≥ 35 kg/m2) in the simulated population from 12.1% to 7.1% in 2016. By 2046, compared with the natural progression scenario (baseline), the Look AHEAD intervention resulted in a similar prevalence of people with a BMI of ≥ 35 kg/m2 owing to the weight regain assumptions applied (see Report Supplementary Material 4, Figure E23).
Epidemiological results
In the UK, a total of 287,791 new cumulative cases of disease were estimated to be accrued over 30 years in a natural progression scenario between 2016 and 2046 (baseline scenario).
By 2046, compared with baseline (natural progression scenario), the Look AHEAD intervention resulted in an estimated 32,982 fewer cumulative cases of disease per 100,000 individuals in the subgroup of the UK general population with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figure E24). Type 2 diabetes mellitus was the greatest contributor to the total cases avoided (95% confidence level, Monte Carlo error), with 14,702 (± 86) per 100,000 individuals in the population with a BMI of ≥ 35 kg/m2. Hypertension resulted in 6234 (± 81) cases avoided and CHD accounted for 4631 (± 85) fewer cases (per 100,000 individuals in the population with a BMI of ≥ 35 kg/m2). Disease-specific results are provided in Report Supplementary Material 4, Figure E25.
Economic module results
By 2046, compared with the baseline scenario, the Look AHEAD intervention resulted in an estimated (95% confidence level, Monte Carlo error) £144.36M (± £2.75M) avoided in cumulative direct health-care costs and an additional 31,139 (± 488) additional QALYs per 100,000 individuals with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figures E26 and E27).
The Look AHEAD study cost, on average, £8890 per person (discounted) (equivalent to £889M per 100,000 people) delivered over 9 years. When combined with cost savings from reductions in obesity-related disease, and the additional QALY gains, the resultant ICER was £23,915 per QALY gained in the base-case analysis, assuming BMI regain over a 5-year period.
A sensitivity analysis considering a linear extrapolation of BMI regain, using data available over the 9-year Look AHEAD study, suggests that baseline weight would not be regained until year 27 (compared with year 14 in the base case). This leads to a longer period of net BMI drop, increasing cost savings and QALY gains associated with obesity-related disease avoided and decreasing the ICER to £14,986 per QALY gained (Table 53).
Cost-effectiveness of very low-calorie diets added to weight-management programme 1 versus weight-management programme 1 alone versus baseline body mass index progression (comparison 2)
Obesity prevalence results
The dietary intervention (WMP1) (i.e. control) and a VLCD added to a WMP1 (i.e. intervention) reduced the proportion of individuals with a BMI of ≥ 35 kg/m2 in the simulated population from 12.1% to 7.7% and 5.3%, respectively, by the end of the first year of the simulation. At the end of the 30-year time period (i.e. by year 2046), compared with the natural progression scenario (baseline), WMP1 and VLCD added to WMP1 both resulted in a similar prevalence of people with a BMI of ≥ 35 kg/m2 overall, owing to the 5-year regain assumptions applied for the base-case analysis (see Report Supplementary Material 4, Figure E28).
Epidemiological results
In the UK, a total of 287,791 (± 147) new cumulative cases of disease were estimated to be accrued over 30 years in a natural progression scenario between 2016 and 2046 (baseline scenario).
By 2046, compared with baseline (natural progression scenario), the WMP1 (control) and VLCD added to WMP1 (intervention) resulted in an estimated 19,053 (± 208) and 19,482 (± 208) fewer cumulative cases of disease per 100,000 individuals in the subgroup of the UK general population with a BMI of ≥ 35 kg/m2, respectively (see Report Supplementary Material 4, Figure E29). Diabetes mellitus was the greatest contributor to the total cases avoided, with 8381 (± 86) and 8509 (± 86) per 100,000 individuals in the control and intervention groups, respectively. Per 100,000 people, there were 3879 (± 81) and 3900 (± 81) cases of hypertension avoided in the control and intervention groups, respectively. The number of cases of CHD avoided was 2658 (± 86) (control) and 2718 (± 86) (intervention) compared with baseline (per 100,000 of the population) (see Report Supplementary Material 4, Figure E30).
Economic results
An intervention adding a VLCD increases the overall intervention delivery cost by approximately £1274 per individual. This additional cost is driven primarily by the addition of the VLCD products to the group receiving a VLCD.
By 2046, compared with the natural progression scenario, WMP1 and VLCD added to WMP1 resulted in an estimated £84.26M (± £2.77M) and £86.73M (± £2.77M) avoided in cumulative direct health-care costs per 100,000 individuals with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figure E31).
By 2046, compared with baseline, WMP1 resulted in an estimated 18,982 (± 488) and VLCD added to WMP1 resulted in an estimated 20,000 (± 488) additional QALYs gained per 100,000 individuals with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figure E32).
Table 54 reports results from the base case and a sensitivity analysis varying the weight regain assumption for three comparisons: (1) WMP1 versus baseline, (2) VLCD plus WMP1 versus baseline and (3) VLCD plus WMP1 versus WMP1 alone.
The base-case analysis was based on the same 5-year BMI regain assumptions used in the Look AHEAD comparison described above. WMP1 and VLCD plus WMP1 generated ICERs of £488 and £6650 per QALY gained, respectively, compared with the baseline general population trends. The ICERs reported were well below the £20,000 threshold typically considered to represent cost-effectiveness. However, the ICER for VLCD plus WMP1 compared with WMP1 (i.e. adding a VLCD component to a dietary intervention) generated a much higher ICER of > £120,000 per QALY gained. The high ICER for adding a VLCD component is driven by the particularly large additional intervention cost for only very modest (and unsustained) additional BMI change over the longer term.
A sensitivity analysis on the BMI regain trajectory, using linearly extrapolated data from study observed time points, indicated that although a VLCD plus WMP1 (i.e. intervention group) generated a greater initial weight loss, the rate of regain over time was steeper than that of the dietary intervention without a VLCD (i.e. WMP1, the control group). The sensitivity analysis showed that both WMP1 and VLCD plus WMP1 generated ICERs of < £20,000 per QALY gained compared with baseline general population BMI trends. However, when compared with each other, VLCDs added to a WMP (i.e. WMP1) were more costly and generated fewer QALYs overall than a WMP alone (i.e. WMP1), and were thus dominated.
Cost-effectiveness of Roux-en-Y gastric bypass surgery versus a weight-management programme versus baseline body mass index progression (comparison 3)
Obesity prevalence results
The control (WMP2, lifestyle intervention) and intervention (RYGB) reduced the proportion of people with a BMI of ≥ 35 kg/m2 in the simulated population from 12.1% to 7.8% and 2.5%, respectively, by the end of the first year of the simulation. At the end of the 30-year time horizon (i.e. by year 2046), compared with the natural progression scenario (baseline), WMP2 resulted in a similar prevalence of people with a BMI of ≥ 35 kg/m2 owing to the regain assumptions (see Report Supplementary Material 4, Figure E33). However, RYGB resulted in a consistent decrease of prevalence of BMIs of ≥ 35 kg/m2, maintained over the full time horizon.
Epidemiological results
In the UK, a total of 287,701 (± 324) new cumulative cases of disease were estimated to be accrued over 30 years in a natural progression scenario between 2016 and 2046. Note that the number is slightly different from that in the other baseline scenarios owing to the larger simulated cohort used for comparison 3 (owing to the further simulations needed to account for bariatric surgery complications).
By 2046, compared with baseline (‘general population trends’), the control (WMP2) and intervention (RYGB) resulted in an estimated 22,263 (± 147) and 140,740 (± 135) fewer cumulative cases of disease per 100,000 individuals in the subgroup of the UK general population with a BMI of ≥ 35 kg/m2, respectively (see Report Supplementary Material 4, Figure E34). Type 2 diabetes mellitus was the greatest contributor to the total cases avoided, with 9793 (± 61) and 50,152 (± 53) per 100,000 individuals in the control and intervention groups, respectively. There were 4296 (± 57) and 30,951 (± 58) cases of hypertension avoided in the control and intervention groups, respectively. The number of cases of CHD avoided was 3141 (± 60) (WMP2) and 18,891 (± 54) (RYGB) compared with baseline (see Report Supplementary Material 4, Figure E35).
Economic results
Use of RYGB to treat individuals with severe obesity was found to be costly, requiring long-term follow-up and intensive preparation of patients for surgery, in addition to the costs of surgery itself. Furthermore, surgery incurs a risk of costly complications. Therefore, the total costs to the NHS of providing surgery and following patients up over the full model time horizon was estimated to be substantial: approximately £20,000 per person (discounted costs). The surgery intervention was therefore substantially more costly to deliver than the comparator WMP, which had a total intervention cost of £1349 (discounted).
However, the additional intervention cost was partly offset by the cost savings accrued from a reduction in treatment of obesity-related disease. By 2046, compared with the natural progression scenario, cost savings associated with obesity-related disease avoided were estimated to be £99.93M (± £1.96M) in the WMP2. Savings were six times greater in the surgery group, with £602.87M (± £1.87M) avoided per 100,000 of individuals with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figure E36).
By 2046, compared with baseline, the WMP and surgery groups resulted in an estimated 22,710 (± 345) and 140,362 (± 348) additional QALYs gained per 100,000 individuals with a BMI of ≥ 35 kg/m2 (see Report Supplementary Material 4, Figure E37).
Table 55 reports the base-case cost-effectiveness results for (1) the WMP2 (control) versus baseline, (2) surgery (intervention) versus baseline and (3) surgery versus WMP2. Results of a sensitivity analysis using a linearly extrapolated weight regain trend for the control (WMP2), instead of the 5-year base-case assumption, are also reported. As long-term weight-loss data for surgery were sourced from the SOS study,345 it was not necessary to conduct sensitivity analysis.
The ICERs for both the WMP (£1541 per QALY) and surgery (£10,126 per QALY) compared with the natural progression scenario (baseline) were well below the threshold value typically considered to represent cost-effectiveness in the UK (£20,000). The ICER for surgery compared with a WMP was £11,783 per QALY gained, again below the threshold value. Application of linear trend regain assumptions to the WMP suggests that cost savings from obesity-related disease may outweigh the additional intervention costs. When comparing surgery with the WMP, the ICER increased slightly but remained below the £20,000 threshold.
Comparative cost-effectiveness of all interventions
All interventions were plotted on the cost-effectiveness plane to illustrate comparative cost and QALY data estimated from the microsimulation model (Figure 3). All interventions were then ranked in ascending order of QALYs gained, with the exclusion of strictly and extendedly dominated strategies. ICERs were reported for all remaining strategies versus a baseline ‘do nothing’ approach and incrementally against the next most effective alternative. The highest ICER under the threshold value of willingness to pay for 1 QALY gained can be determined to be the most efficient treatment strategy in the incremental analysis (Table 56).
FIGURE 3.
Cost-effectiveness frontier.

| Intervention | Intervention cost (£) | Disease cost (£) | Total cost (£) | QALYs | Analysis vs. baseline | Analysis of remaining interventions | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | ICER (vs. baseline) (£) | Incremental costs (£) | Incremental QALYs | ICER (vs. next best alternative) (£) | |||||
| Base-case analysis | ||||||||||
| Baseline | 0 | 2898 | 2898 | 1,135,676 | – | – | – | – | – | – |
| WMP1 | 94 | 2814 | 2909 | 1,154,944 | 11 | 19,269 | 557 | 11 | 19,269 | 557 |
| VLCD added to WMP1 | 220 | 2812 | 3032 | 1,155,963 | 134 | 20,287 | 6628 | Dominated | Dominated | Dominated |
| WMP2 | 135 | 2798 | 2933 | 1,158,386 | 35 | 22,710 | 1540 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| Look AHEAD | 889 | 2754 | 3643 | 1,167,101 | 746 | 31,426 | 23,725 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 2024 | 2295 | 4319 | 1,276,038 | 1421 | 140,362 | 10,126 | 1411 | 121,094 | 11,648 |
| BMI regain sensitivity analysis | ||||||||||
| Baseline | 0 | 2898 | 2898 | 1,135,676 | – | – | – | Dominated | Dominated | Dominated |
| VLCD added to WMP1 | 220 | 2840 | 3060 | 1,150,251 | 163 | 14,575 | 11,152 | Dominated | Dominated | Dominated |
| WMP1 | 94 | 2834 | 2928 | 1,151,112 | 30 | 15,436 | 1965 | Dominated | Dominated | Dominated |
| WMP2 | 135 | 2740 | 2875 | 1,168,178 | Dominant | Dominant | Dominant | – | – | – |
| Look AHEAD | 889 | 2666 | 3555 | 1,179,771 | 657 | 44,095 | 14,906 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 2024 | 2295 | 4319 | 1,276,038 | 1421 | 140,362 | 10,126 | 1444 | 107,860 | 13,392 |
| 0% discounting of costs and QALYs | ||||||||||
| Baseline | 0 | 3714 | 3714 | 1,345,761 | – | – | – | Dominated | Dominated | Dominated |
| WMP1 | 94 | 3603 | 3697 | 1,370,416 | Dominant | Dominant | Dominant | – | – | – |
| VLCD added to WMP1 | 220 | 3600 | 3820 | 1,371,637 | 106 | 25,876 | 4110 | Dominated | Dominated | Dominated |
| WMP2 | 137 | 3581 | 3718 | 1,374,914 | 4 | 29,153 | 146 | 21 | 4498 | 4708 |
| Look AHEAD | 928 | 3522 | 4450 | 1,386,615 | 736 | 40,854 | 18,014 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 2257 | 2899 | 5156 | 1,532,976 | 1442 | 187,215 | 7701 | 1437 | 158,062 | 9094 |
| 3.5% discounting of costs and QALYs | ||||||||||
| Baseline | 0 | 2151 | 2151 | 930,221 | – | – | – | – | – | – |
| WMP1 | 93 | 2091 | 2184 | 943,960 | 33 | 13,739 | 2379 | 33 | 13,739 | 2379 |
| VLCD added to WMP1 | 219 | 2089 | 2308 | 944,781 | 157 | 14,560 | 10,774 | Dominated | Dominated | Dominated |
| WMP2 | 132 | 2079 | 2211 | 946,423 | 60 | 16,202 | 3726 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| Look AHEAD | 842 | 2050 | 2892 | 952,437 | 741 | 22,216 | 33,354 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 1798 | 1736 | 3534 | 1,027,562 | 1383 | 97,341 | 14,207 | 1350 | 83,602 | 16,151 |
| 6% discounting of costs and QALYs | ||||||||||
| Baseline | 0 | 1556 | 1556 | 751,490 | – | – | – | – | – | – |
| WMP1 | 92 | 1515 | 1607 | 761,029 | 51 | 9539 | 5362 | 51 | 9539 | 5362 |
| VLCD added to WMP1 | 218 | 1513 | 1731 | 761,679 | 176 | 10,189 | 17,245 | Dominated | Dominated | Dominated |
| WMP2 | 129 | 1507 | 1636 | 762,706 | 80 | 11,216 | 7166 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| Look AHEAD | 790 | 1489 | 2279 | 766,619 | 723 | 15,129 | 47,801 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 1605 | 1285 | 2889 | 815,451 | 1334 | 63,961 | 20,851 | 1282 | 54,422 | 23,565 |
| 5-year time horizon | ||||||||||
| Baseline | 0 | 388 | 388 | 319,023 | – | – | – | – | – | – |
| WMP1 | 94 | 384 | 477 | 320,030 | 89 | 1007 | 88,689 | 89 | 1007 | 88,689 |
| WMP2 | 135 | 383 | 518 | 320,037 | 130 | 1014 | 128,511 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| VLCD added to WMP1 | 220 | 383 | 603 | 320,256 | 215 | 1233 | 174,209 | 125 | 226 | 555,265 |
| Look AHEAD | 641 | 383 | 1024 | 320,295 | 636 | 1272 | 500,024 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 1172 | 366 | 1537 | 320,665 | 1150 | 1642 | 700,171 | 935 | 409 | 2,285,770 |
| 10-year time horizon | ||||||||||
| Baseline | 0 | 804 | 804 | 575,958 | – | – | – | – | – | – |
| WMP1 | 94 | 791 | 884 | 579,578 | 80 | 3620 | 21,975 | 80 | 3620 | 21,975 |
| VLCD added to WMP1 | 220 | 789 | 1009 | 580,047 | 204 | 4089 | 50,000 | Dominated | Dominated | Dominated |
| WMP2 | 135 | 788 | 923 | 580,099 | 118 | 4141 | 28,551 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| Look AHEAD | 889 | 787 | 1676 | 580,650 | 871 | 4692 | 185,688 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 1421 | 732 | 2153 | 592,597 | 1348 | 16,639 | 81,029 | 1269 | 13,019 | 97,449 |
| 20-year time horizon | ||||||||||
| Baseline | 0 | 1732 | 1732 | 931,620 | – | – | – | – | – | – |
| VLCD added to WMP1 | 220 | 1686 | 1905 | 943,556 | 174 | 11,936 | 14,542 | Dominated | Dominated | Dominated |
| WMP1 | 94 | 1688 | 1781 | 943,732 | 49 | 12,112 | 4077 | 49 | 12,112 | 4077 |
| WMP2 | 135 | 1679 | 1814 | 944,768 | 82 | 13,148 | 6233 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| Look AHEAD | 889 | 1660 | 2549 | 949,072 | 818 | 17,452 | 46,854 | Extendedly dominated | Extendedly dominated | Extendedly dominated |
| RYGB surgery | 1767 | 1470 | 3237 | 1,000,384 | 1505 | 68,764 | 21,889 | 1456 | 56,652 | 25,697 |
Sensitivity analysis results
Sensitivity analyses around weight regain assumptions, discount rates and varying time horizons are reported in Table 56. All analyses are further repeated in a world without surgery. These results can be found in Report Supplementary Material 1, Section 23.
Summary of base-case and sensitivity analysis results
Obesity levels are rising around the UK and the REBALANCE project aims to identify the most cost-effective method of addressing class II and class III obesity (i.e. BMI of ≥ 35 kg/m2).
The epidemiological results show that all WMPs would help prevent new disease cases over the next 30 years, compared with a ‘no change’ baseline. By 2046, a dietary intervention (WMP1) will prevent 19,053 cases of disease, whereas bariatric surgery will increase prevention more than sevenfold to 140,740 cases of disease prevented. Type 2 diabetes mellitus represents the most prevalent cases that are estimated to be avoided by the various WMPs.
The results show that, compared with baseline with no WMPs, all interventions were cost-effective. The VLCD added to a dietary intervention scenario was not cost-effective when compared with a dietary intervention (i.e. WMP1). This is most likely to be attributable to the high costs associated with implementing the VLCD intervention. This was the only specific scenario modelled that was not cost-effective when using the upper bound of NICE’s cost per QALY threshold of £30,000. However, if the more conservative threshold of £20,000 per QALY is used, the Look AHEAD scenario is no longer cost-effective, with an ICER of £23,915 for the period of 2016–46 in the base case. When comparing all interventions together, surgery is the most cost-effective strategy in the base case.
Sensitivity analysis varying the weight regain assumption for WMPs has some important impacts on cost-effectiveness results. First, for the Look AHEAD study, assuming a longer period of regain lowers the ICER (compared with baseline, no change) below the £20,000 per QALY threshold. A dietary intervention (WMP1) becomes dominant over the same intervention with a VLCD added. Despite having less BMI reduction, the trajectory of regain is slower in the group without a VLCD. Surgery remains cost-effective under the different regain assumptions for WMPs, but with a slightly higher ICER.
Varying the discount rate for costs and QALYs from the base-case value of 1.5% has important implications for the most cost-effective strategy overall. Lowering the discount rate improves the cost-effectiveness case for surgery, lowering the ICER. Increasing the discount rate to 3.5% does not alter the overall cost-effectiveness conclusions, but increasing it further to 6% means that the ICER for the specific comparison of surgery with WMP2 (lifestyle intervention) increases to £23,756. In the incremental analysis of all interventions, surgery continues to have a higher ICER (£23,565 per QALY gained), just above NICE’s typically considered £20,000 per QALY threshold.
Over a short time horizon of 5 years, none of the interventions was cost-effective, with ICERs all being > £30,000 per QALY gained in the incremental analysis. Over 10 years, all ICERs were > £20,000 per QALY, and the ICER for surgery was > £90,000 per QALY gained. At a 20-year time horizon, considering a £20,000 per QALY threshold, the VLCD control group (i.e. WMP1) was the most cost-effective strategy with an ICER of only £4077. Over a 20-year time horizon, the ICER for surgery, compared with the next best, non-dominated alternative, was £25,697, compared with WMP1. ICERs for surgery, in particular, are sensitive to the time horizon because it is over the longer term when the additional intervention costs are offset by cost savings and QALY gains of fewer obesity-related diseases. However, it is also over the longer time horizons when the microsimulation projections become most uncertain.
Comparison of findings with the literature
The results presented in this chapter and the findings from the economic evaluations included in Chapter 6 differ in a number of ways. First, for Look AHEAD, there was no directly comparable evidence in the review and this is the first study to investigate the cost-effectiveness of the Look AHEAD intervention in a general population with severe obesity. The two modelled WMPs were both cost-effective compared with a ‘do nothing’ approach. When compared with the review findings, there is mixed evidence for the cost-effectiveness of WMPs (less intensive than Look AHEAD). Brief lifestyle counselling was not likely to be cost-effective compared with usual care. 304 Weight Watchers® was cost-effective compared with usual care but Vtrim (a WMP with online support) was dominated. 295 Finally, a low-carbohydrate diet was cost-saving (Tsai et al. 303). However, the majority of reviewed studies of WMPs were economic evaluations alongside RCTs, with no extrapolation of costs and outcomes. The microsimulation projecting long-term costs and QALYs associated with obesity-related diseases, such as type 2 diabetes mellitus, stroke, hypertension, CHD and cancer, probably gives a more accurate reflection of the true cost-effectiveness of WMPs in the UK.
Only one study evaluated a VLCD in the review. 297 The VLCD had extended dominance over no treatment, Slimming World®, Counterweight and Weight Watchers®. The ICER versus no treatment was £12,858. The ICER from our model for a VLCD compared with a ‘do nothing’ approach was £6650 per QALY gained in the base case (5-year regain assumption), increasing to £11,251 in a sensitivity analysis assuming linear weight regain trends. However, when adding a VLCD to a WMP (i.e. WMP1), our base-case ICER increased to £121,478 per QALY. The results indicate that although a VLCD intervention is cost-effective compared with doing nothing, it is not a cost-effective addition to a WMP. Under a sensitivity analysis of linear weight regain, adding a VLCD component to a WMP was even less cost-effective (being overall more costly and less effective than a WMP without a VLCD). It is unclear how this finding would compare with Lewis et al. ,297 as their study did not explore the cost-effectiveness of adding a VLCD component to an existing WMP.
Bariatric surgery in the review was considered cost-effective in all studies, with many studies indicating long-term cost savings and hence dominance of surgery. Conversely, the UKHF model showed no evidence of long-term cost savings over a 30-year time horizon. Compared with a lifestyle intervention (WMP2), the ICER for surgery was £11,783. The UKHF analysis included a wider range of postoperative costs associated with management of bariatric surgery patients over the longer term; for example, the analysis included costs associated with surgery complications, preoperative and lifetime postoperative care. In addition, an attempt was made to incorporate the utility weights associated with surgery complications, which also further affects the cost-effectiveness of bariatric surgery. This was not incorporated in any of the studies in the review. Although surgery remains cost-effective in our base-case analysis, many sensitivity analyses around discount rates and modelled time horizons change the conclusions, indicating that the cost-effectiveness case for surgery may not be as clear-cut as in some other published studies.
Strengths and limitations
A microsimulation is best suited to addressing questions that would be difficult to study empirically: in this case, the population effects of the long-term effects of various weight-management trials. The strengths of this study are the systematic approach taken, and the use of best available, nationally representative and recent data. This, however, results in a limitation, as there is a lack of available evidence, notably for disease costs.
The direct health-care approach did not include indirect social care costs as they were not available or reliable for a range of diseases; this may lead to a conservative estimate of cost savings. In Chapter 3, we show that the Look AHEAD intervention affects many more obesity-related comorbidities than modelled here, such as depression, incontinence or fractures. On the other hand, the model does not include costs associated with a healthier, longer-living population through increased risk of other diseases of old age. Indeed, this effect can be seen, with an increase in female cancers later in life attributable in part to women not acquiring and dying from fatal conditions earlier in life. Owing to limitations within the published data, CHD was only modelled using incidence from myocardial infarction (excluding angina and chronic heart failure); this will also underestimate the disease burden and, therefore, the cost-effectiveness of the respective interventions. There were several limitations regarding data on knee osteoarthritis: not including all BMI-related osteoarthritis, such as hip, may underestimate the cost-effectiveness. Indeed, the knee site represents only 46% of the total number of people in England aged > 45 years who have sought treatment for osteoarthritis. 400 The data on prevalence of knee osteoarthritis are limited: they are only for people aged 45 to ≥ 75 years and are not available at a very detailed level (e.g. by age, sex and BMI), so the shape of the distribution may not reflect the real distribution. These data were not optimal and may lead to bias in the evaluation of cost-effectiveness of the interventions.
This study used a variety of published literature for inputs and assumptions for the model. Thus, the quality of these inputs is dependent on the quality of published data, including weight regain and direct health-care costs. The model outputs provide estimates of the mean and 95% CIs based on the probability of the event occurring and the number of Monte Carlo trials. The review of economic evaluation studies in Chapter 6 identified a lack of probabilistic sensitivity analyses as a common cause for concern. Unfortunately, this limitation is also replicated in our study where the impact of parametric uncertainty on the model outputs has not been incorporated. The microsimulation model is highly complex and running a complete sensitivity analysis in these types of models is challenging owing to the computational power required, the number of model inputs and the time available for this project. We would expect the errors provided in this study to underestimate the total error around each estimate owing to parametric uncertainty (based on further, unpublished UKHF research).
The baseline comparator for the model is a natural history cohort using risk factors (BMI) to project the future obesity trends and development of obesity-related disease (including associated obesity-related disease costs, utilities and mortality) over the longer term in the general population. Best practice economic evaluation techniques suggest that interventions should be compared with standard care, rather than ‘do nothing’, because to compare against the latter could overstate incremental costs and understate the cost-effectiveness of new interventions. It could be suggested that our model excludes the intervention costs of currently provided interventions. However, we argue that any bias created is negligible, because, in reality, current UK practice for this patient group is very closely aligned with doing nothing at the population level. The availability of and access to Tier 3 and 4 services is limited, and in some areas services ‘simply do not exist’ (© House of Commons Health Select Committee, 2015. 401 This information is licensed under the Open Government Licence v3.0. To view this licence, visit www.nationalarchives.gov.uk/doc/open-government-licence/). Therefore, given the lack of coherent services, the comparator used in the model is a fair representation of current UK standard care for individuals with a BMI of ≥ 35 kg/m2.
For this project, type 2 diabetes mellitus was included as a single-stage disease. Although the UKHF model can model multistage diseases such as type 2 diabetes mellitus, this was not undertaken owing to resource and time limitations. This would mean that, instead of a person having a disease or not having a disease, they can pass through various stages of disease (e.g. in the case of diabetes mellitus, patients can progress from no disease to prediabetes, go into remission from prediabetes or diabetes mellitus, or progress to diabetes mellitus). Future work could involve diabetes mellitus as a multistage disease to show the sustainable costs of the prediabetes.
The overarching aim of this project was to determine cost-effective weight-loss strategies for the whole population with severe obesity, so without a focus on people with type 2 diabetes mellitus alone. However, weight-loss data used to populate the model are sourced from RCTs that often include only participants with diabetes mellitus. It is assumed that the weight loss observed in these studies would be replicated by the same interventions in the general population with severe obesity. It is unclear whether or not this biases our results and, if so, in what direction.
The outputs presented in the model are based on those people with a BMI of ≥ 35 kg/m2 in 2016, as opposed to those with a BMI of ≥ 35 kg/m2 at any time point in the model. This work illustrates the effect of intervening with one cohort rather than the whole population, so we cannot answer the question of what happens if interventions are offered to all individuals with a BMI of ≥ 35 kg/m2 no matter when they reach this threshold. Furthermore, we have reported results for a wide cohort and the results cannot be used to make recommendations about the most cost-effective interventions for specific subgroups of the population with a BMI of ≥ 35 kg/m2. The model’s functionality allows consideration of subgroup analyses (e.g. different age groups and BMI of ≥ 40 kg/m2). However, we have not reported these data owing to additional uncertainty in long-term BMI projections for specific subgroups. However, an interesting avenue of future research would be to consider such subgroup analyses further, perhaps over a shorter time horizon, in which BMI extrapolations are more robust.
Given that our review in Chapter 6 has criticised surgery studies for not incorporating the costs and utility decrements of complications, we have attempted to do so in this study. However, we are not confident that we have captured all the important implications of surgery complications for patients because of the lack of relevant available data. For example, we have not included hypoglycaemia, abdominal pain and its investigation or the need sometimes for iron infusions. Further research is required to address this important evidence gap. Therefore, it is likely that the model results are biased in favour of bariatric surgery. The magnitude of that bias is unknown and would become clear only once a comprehensive study of the cost and utility implications of surgery was conducted.
The model assumes that losing weight returns people with obesity to the same risk as those without obesity. It is likely that this overestimates the benefits of weight loss, and potentially biases in favour of interventions delivering the greatest weight loss.
We modelled weight loss in the general population with severe obesity, and for Look AHEAD this might have underestimated weight loss, as people with type 2 diabetes mellitus could find it harder to lose weight. Finally, we conducted two different types of analysis. Our specific comparisons (i.e. Look AHEAD vs. baseline, VLCD plus WMP1 vs. WMP1 and surgery vs. WMP2) are based on weight loss observed in RCTs. Our incremental multiway comparison is based on the same weight-loss data, but no longer benefits from the benefits of randomisation in the trials and is essentially a naive indirect comparison. The results of the multiway comparison, although important for policy-making, are less robust than the specific comparisons. Network meta-analyses were considered, but study heterogeneity meant that a robust network meta-analysis could not be conducted.
Conclusion
Overall summary
-
The REBALANCE project provides the first comprehensive decision analysis modelling comparing multiple potentially beneficial WMPs for adults with a BMI of ≥ 35 kg/m2 from a UK health services perspective.
-
The UKHF model estimates that, compared with a general population trajectory of BMI, WMPs are cost-effective when compared with a no change baseline (ICER < £20,000/QALY).
-
The Look AHEAD intervention is borderline cost-effective in the base-case analysis (5-year regain), but is more likely to be cost-effective if assuming a linear regain trend, assuming that when individuals have longer weight loss observed (i.e. over 9 years), it is unlikely that 5-year regain is valid.
-
Bariatric surgery is cost-effective compared with a lifestyle WMP.
-
Adding a VLCD to a dietary WMP is not cost-effective compared with a dietary WMP on its own. Indeed, when varying weight regain assumptions a VLCD is more costly and less beneficial than a dietary WMP.
-
Overall, surgery is the most cost-effective strategy in the base case and under a sensitivity analysis around regain assumptions. However, the results are sensitive to the assumed discount rate and time horizons. Shorter time horizons and higher discount rates reduce the potential benefits (reduced disease costs and increased QALYs) for surgery, meaning less potential to offset the costly procedure. In such scenarios, RYGB may not be the most cost-effective use of resources. However, it should be noted that economic evaluations should be conducted over a time horizon that is sufficient to capture all the costs and outcomes of importance. Shorter time horizons may not be appropriate for decision-making.
-
Modelled sensitivity analyses suggest that for scenarios in which RYGB may not be cost-effective (such as 10- and 20-year time horizons), a less intensive WMP, consisting of mainly dietary advice (as described in the control groups of the VLCD studies), may be a cost-effective alternative. However, these analyses require caution as they do not capture all the long-term benefits of surgery. Under some sensitivity analyses (in which surgery is not a comparator), a more active WMP (similar to the shortened Look AHEAD/DPP) may also be cost-effective.
General issues relating to methodology
-
There is a lack of data on complication rates following surgery and associated costs and utility implications. The lack of adequate data means that cost-effectiveness estimates for surgery may be biased.
-
Weight regain assumptions can have important implications, especially for WMPs that show some evidence of longer-term maintenance of weight loss (e.g. Look AHEAD). It is crucial that different assumptions are explored in economic models. This should be a key quality assessment criterion for all obesity-related models driven by BMI/weight data.
-
There is a distinct lack of data on long-term weight loss for VLCDs. Weight regain assumptions used in the model are based on short-term data and are probably highly uncertain. Further research is required to clearly identify long-term effectiveness of VLCDs.
-
In common with the majority of modelling studies, this project focuses on an intermediate outcome (BMI). This adds additional uncertainty to the modelling process, and studies are required to explore the impact on a range of other health indicators and obesity-related disease events.
-
Long-term cohort studies and long-term follow-up of RCTs are required to determine the true long-term costs and benefits of weight-loss interventions. There is potential to make use of routinely collected data to aid this research objective.
Indicators for cost-effective interventions
-
Surgery is costly to deliver and it is costly to maintain patient contact over the rest of patients’ lives; however, the benefits appear to offset the costs over the longer term, and surgery is most cost-effective over longer-term horizons.
-
Adding a VLCD to a WMP does not appear to be cost-effective, with insufficient benefit to justify the additional cost. However, long-term benefits are uncertain and future research may change these conclusions.
-
An ILI is costly to deliver but, assuming longer term weight-loss maintenance, would be cost-effective compared with doing nothing, with greater QALY gains than other less intensive WMPs.
Chapter 8 Discussion
The following a priori main research questions were developed to guide our initial investigation:
-
What are the best evidence-based and cost-effective weight-management strategies for adults with severe obesity (BMI of ≥ 35 kg/m2)?
-
How can people with severe obesity best be engaged with weight management and weight-management services?
In addition to these a priori research questions, we developed more detailed research questions that emerged inductively from initial findings and the knowledge of the project team. These were discussed, revised and finalised with the advisory group in a series of teleconferences.
Here we integrate the evidence from all of the systematic reviews and economic evaluations in an attempt to answer these questions. Further details are provided in the individual discussions of each preceding chapter. We begin by answering our first main question.
Main research questions
What are the best evidence-based and cost-effective weight-management strategies for adults with severe obesity (body mass index of ≥ 35 kg/m2)?
Roux-en-Y gastric bypass produced the greatest long-term weight loss of all interventions. VLCDs had greatest weight loss at least in the short term, and the Look AHEAD intervention had the best evidence for long-term sustained weight loss over 9 years. We drew on these interventions for our economic evaluation.
In the microsimulation modelling, we found that the comparator WMPs in randomised trials of VLCDs or RYGB surgery were generally cost-effective when compared with a baseline ‘do nothing’ approach. However, the addition of a VLCD to a WMP was not cost-effective. The cost-effectiveness of WMPs was sensitive to assumptions about weight regain. The Look AHEAD intervention was borderline cost-effective in the base-case analysis (weight regain over 5 years), with an improved case for cost-effectiveness under more realistic, longer-term linear trend weight regain assumptions. This intensive group-based programme was the only lifestyle WMP with really long-term weight change data. The intervention included a calorie goal of 1200–1800 kcal/day with < 30% of calories from fat and > 15% of calories from protein, the initial use of meal replacements or a structured meal plan, and a tailored exercise plan with the option of classes to attend in some centres. Individual counselling was also included, in addition to telephone calls or e-mails from programme staff, who were mainly registered dietitians or exercise specialists.
When determining the most cost-effective strategy overall, surgery is the favoured option, although suggestions of long-term cost savings from our systematic review were not replicated in our modelling. The systematic review revealed that, in many cases, bariatric surgery was cost-saving (less costly and more effective) or highly cost-effective with very low ICERs. However, the reviewed decision models evaluating surgery contained limited resource use for pre and post surgery (including complications, corrective procedures and long-term management). Therefore, the published literature undercosts surgery and overestimates cost-effectiveness. None of the published economic evaluations account for quality-of-life implications of surgical complications. Our modelling accounts for complications, postsurgery care and revision procedures, and attempts to account for quality-of-life implications of surgery complications. Our ICERs for surgery compared with baseline and WMP were £10,126 and £11,783, respectively; these were generally higher than in the published literature.
Results are sensitive to the model time horizon and discount rate applied. In an era of scarce NHS resource availability, the budget impact of the wide rollout of costly surgery must also be considered, although few people fitting the eligibility criteria may opt for surgery. 402
How can people with severe obesity best be engaged with weight management and weight-management services?
Our second main research question is addressed by our more detailed research questions below.
Organisational issues
What interventions are effective in increasing uptake of weight-loss services?
We found no studies that compared alternative approaches to increasing uptake of services. However, the UK-based FFIT128 was notable for its extensive qualitative work and piloting of an intervention that successfully engaged and retained men, who are usually much less likely to take part in WMPs. The intervention was cost-effective in long-term modelling. One other UK study of a health-visitor-led intervention took place in a health centre in a moderately deprived area,249 but details on the uptake and retention of hard-to-reach groups were not provided.
Qualitative study reports offered insights into what apparently motivated prospective participants to take part in a WMP. 204,226,254,270,271,276,285 ‘Push’ factors for joining were internal to participants, for example expressing a desire to do something as a result of growing health concerns and/or recent personal health scares. Feelings of accountability to their families were important, such as being able to be more engaged in activities with family members and being there for their family for as long as possible. ‘Pull’ factors included WMPs being perceived as being endorsed as credible by health professionals, perceived as being novel and exciting in some key way and also because there was an opportunity to engage with the intervention in a place that participants liked to attend. 226,270,271 People’s enrolment in services will also be affected by perceptions about the content of WMPs, which we discuss next.
Credibility and novelty were features that users valued, but we have very little evidence on what WMPs can do to increase uptake. The need to research factors influencing ‘opting in’ to WMPs, and how to engage hard-to-reach groups, was a key recommendation of the BOMSS commissioning guide for Tier 3 weight-management services. 4
What factors seem to affect people’s choices of programme?
Participants valued being part of a group of like-minded individuals facing similar issues. 142,204,226,264,267,269,273,275,276,279,285 Most participants valued the social interactivity of group-based programme activities and also fairly intensive support from programme staff. 142,204,226,249,262,264,269,272,275,276,279,285,286 Participants appreciated flexible timing of support,269,276 desiring human contact over more remote forms of contact. 142,225
In the 26 UK studies, nine excluded people with eating disorders, nine excluded participants with diabetes mellitus, hypertension or CVD and seven mentioned excluding people for mental health issues. Randomised trials were also not inclusive, excluding people with eating disorders (30/131, 22.9%) or mental health issues (65/131, 49.6%). In particular, one recent UK RCT in general practice excluded participants with a BMI of > 45 kg/m2,149 and one other recent UK RCT, based in general practice and also delivered remotely, excluded participants who could not walk 100 m. 142 It would be valuable to establish whether or not UK interventions could adapt to be more inclusive of potential recipients.
More detailed discussion of valued components of WMPs is provided in Diets, Physical activity and Intervention characteristics.
Taken together, the evidence indicates that users of services that involve group and in-person contact value this highly, but there is little evidence on what people would value given a choice of programme.
Does the effectiveness of programmes vary by socioeconomic status, ethnic group (e.g. south-east Asian population groups), body mass index or other patient group? Is uptake related to these factors?
We had insufficient information from RCTs and UK studies to examine whether uptake or effectiveness of WMPs varied for hard-to-reach or disadvantaged groups, age groups or categories of severe obesity (e.g. BMI of < 40 kg/m2 or ≥ 40 kg/m2). None of the UK studies, apart from those by Hunt et al. 128 and Jackson et al. ,249 appear to have made efforts to include harder-to-reach groups.
Fourteen RCTs set in the USA sought particularly to recruit participants from African American, Hispanic or Mexican ethnic groups or deprived communities. 90,91,112,118,136–138,147,148,162,163,165,168,188 Most of these RCTs had only modest long-term weight loss of 2–3 kg, apart from the more intensive intervention by Perri et al. 163 based on DPP, which had more than double this weight loss at 2 years.
Few economic evaluations conducted subgroup analyses to examine effects in under-represented groups. Borisenko et al. 310 found that surgery was cost-effective overall, but was cost-saving for all groups with type 2 diabetes mellitus, with severe obesity. One study293 found that bariatric surgery was more cost-effective in younger age groups (< 40 years). The evidence on sex affecting cost-effectiveness was mixed,318,325 with one study finding a higher ICER for men and the other finding no impact of sex on the ICER.
Overall, we found no programmes that had formally tested whether or not effectiveness varies by population subgroup and, thus, there is little evidence to answer this question. In a review of the wider evidence base, including observational data, Hillier-Brown et al. 403 found that tailored, primary-care-delivered WMPs targeted at low-income groups and community-based WMPs had some evidence of effectiveness among low-income women.
Do programmes incorporate users in design, evaluation or delivery, and how do they do this?
Very few studies reported incorporating users in the design, evaluation or delivery of interventions. The FFIT trial,128 undertaken in the UK, is notable for incorporating men in developing and piloting an intervention specifically for people unlikely to attend conventional weight-loss services. The good weight loss achieved and very low drop-out rate suggest that these processes could be useful for developing services for other groups.
Three recent UK trials128,142,149 reported process evaluations, as did three other UK WMPs. 254,270,284 Participants generally reported positive perceptions of these programmes, valuing the accessibility of health professionals or professional staff, particularly for their encouragement. One study149 reported using Public Health England’s guidance for process evaluations260 as part of its standard evaluation framework.
The three trials attempted to evaluate the views of low users or people who dropped out. Whether or not these process evaluations led to subsequent changes in WMPs was unclear. It is likely that we have been unable to identify process evaluations of NHS WMPs, which may not be publicly available. The recent Public Health England report of users’ experiences of Tier 2 and 3 weight-management services is valuable, with similar findings to our review, although few Tier 3 participants were included. 27
Latner et al. 140 recruited participants to co-facilitate and then lead groups for weight maintenance. The investigators did not report an evaluation of this study feature.
What do people think about commercial weight-loss organisations? What is the influence of paying for their services?
From our qualitative review, several participants positively contrasted their overall perception of the WMPs they were attending with previous negative perceptions of commercial WMPs (e.g. programmes that were perceived as being too ‘feminine’ or in some ways humiliating and embarrassing, or being perceived to be overly preoccupied with dieting). 204,249,254,269,273,278 We did not find any information from participants about paying for commercial weight-loss services. Current referral schemes from general practice are generally for 12 weeks, after which participants would generally have to pay to continue to attend. Given that group-based interventions are associated with better long-term weight loss, it is possible that longer-term funding of commercial WMPs may be of benefit; however, the additional costs would need to be traded against the potential benefits of the programmes. In a randomised trial of participants with lower mean BMIs than in studies included in our review, Ahern et al. 258 found that referral from primary care to a commercial organisation for 52 weeks led to around 2 kg more weight loss at 12 months than a 12-week programme. The ICER for the 52-week programme compared with the 12-week programme was £3804 per QALY. Participants of lower socioeconomic status may be particularly disadvantaged by only short-term free attendance to commercial organisations’ WMPs. However, there was no direct evidence to assess the socioeconomic equity of these schemes.
Following a standard evaluation framework for outcomes, as recommended by Public Health England,260 would greatly aid the economic evaluation, interpretation and comparison of outcome data from commercial weight-loss organisations, whether working with, or separate from, the NHS.
What is the effectiveness of weight maintenance interventions?
Participants in qualitative research reports clearly expressed concern that withdrawal of support after WMPs stopped would have an impact on their abilities to maintain behaviour change. None of the UK studies specifically described investigating different approaches to improving weight maintenance.
Four RCTs provided data to suggest approaches to improving weight maintenance after VLCDs. One trial assessed the impact of focusing on diet versus physical activity versus usual care and found that a continued focus on diet was more effective than the other two options at 12 months. 99 One trial investigated the effectiveness of orlistat76 and showed some evidence of effectiveness. Two trials incorporated intermittent use of meal replacement products as used in the preceding VLCD and there was some suggestion that this approach reduced weight regain. 172,173
Orlistat use also facilitated weight maintenance after other WMPs,105,176 although meal replacements did not appear to help weight maintenance. 143 Adding a weight maintenance programme generally reduced weight regain by 1–2 kg over 18 months.
We found no economic evaluations of weight maintenance interventions per se. Because the length and intensity of follow-up are important for participants and are important considerations for the cost of service delivery, it would be valuable to undertake economic evaluations of variations in weight maintenance intensity.
The current evidence suggests that orlistat, possibly the use of meal replacements, and prolonged behavioural support focusing on dietary restraint can enhance weight-loss maintenance, but there is no evidence on whether or not these approaches are cost-effective.
Is an intensive/inpatient programme of value at the start of the weight-management programme, particularly for people with a very high body mass index?
Three trials from Scandinavia124,125,184 examined whether or not interventions starting with a hospital inpatient programme of 1–3 weeks led to better long-term weight loss than those delivered in the community. There was no evidence that inpatient programmes produced greater long-term weight loss. The highest group BMI was 43.6 kg/m2 at commencement, and people with psychiatric illness were generally excluded.
Diets
Which factors (type of diet, interventions for adherence, types of classes and characteristics of the participants) help explain weight loss and maintenance?
Our evidence suggests better weight loss from group classes for delivering WMPs (although this may involve more contacts), with ongoing follow-up, including internet or telephone support. We did not find evidence to help with deciding what characteristics of participants predict their response to particular dietary interventions.
In primary care, VLCDs, such as the Counterweight Programme using the Cambridge Weight Plan®, produced the best weight loss at 12 months, but effects on longer-term weight loss are presently unclear. Data from UK secondary care studies were similar for VLCDs. It appears that specialist weight-management services do not currently offer VLCDs as part of routine care. Our modelling indicates that adding a VLCD to an existing WMP does not appear cost-effective, although a VLCD-inclusive WMP compared with no treatment appears cost-effective compared with a ‘do nothing’ approach. Rapid weight loss at the start of a WMP from use of a VLCD could help with motivation to start a programme and reduce losses to follow-up.
Higher protein intakes, very low-carbohydrate diets and meal replacements were associated with greater weight losses of 1–2 kg at 12 months, but evidence of longer-term benefits was not demonstrated.
Our evidence did tend to suggest that a prescribed calorie content for diets, rather than a deficit in calories, might produce better weight loss. This could relate to all or some of the following factors: prescribed calorie diets might have had lower calorie intakes than those that prescribed a deficit, diet plans were easier to counsel and follow, and inaccuracies in calculations of energy requirements. 230 From the qualitative research review, some participants did favour prescriptive and structured diets.
Does having a choice of reducing diet matter and do people pick diets that are more effective for them?
Some participants also described valuing the flexibility and variety of diet formats. So, having a choice could improve uptake of WMPs. 142,204,276,279 This seemed important in terms of helping people to ‘normalise’ and stabilise their eating habits, particularly as many had attempted diets over a period of many years (without success), leading them to develop negative and unhealthy relationships with food. 142,204,276,279
Two UK studies142,240 allowed participants to choose from alternative dietary approaches, but there is presently no randomised trial evidence on whether or not offering a choice of diet has long-term benefits on weight in the UK setting. Paisey et al. 240 found that those participants with type 2 diabetes mellitus who selected the intensive conventional diet, rather than an initial VLCD, had much better long-term weight loss at 5 years. Participants were also allowed to choose between low-calorie and low-carbohydrate diets in the trial by Little et al. ,142 although weight loss appeared not to differ in accordance with the chosen diet.
In a randomised trial, not included in our review, Yancy et al. 404 randomised participants to a choice of two diets (a low-carbohydrate diet with 20 g/day or a low-fat diet) or random assignment to one of the two diets. Participants could change diets at 12 weeks. At 48 weeks, there was reported to be no difference in weight loss between the two groups.
Allowing choice of dietary approach would be useful to explore further, including the ease of service delivery.
Physical activity
Which factors (type of physical activity programme, interventions for adherence, types of classes and characteristics of the participants) help explain weight loss and maintenance?
We found little evidence to help us answer this question. This may relate to the fact that we only examined physical activity interventions as part of a WMP with dietary counselling. We did not evaluate physical activity interventions given in isolation. We can draw some conclusions from the three trials189,194,201 that were associated with greater long-term weight loss at 2 years from adding a physical activity programme. These programmes involved intensive attendance at group sessions one to three times per week for aerobic and/or resistance training exercises in motivated trial participants. They might not be cost-effective or practicable in a less motivated population. Tailoring of interventions to people who could have been very unfit was not described. Only one of these trials201 described walking groups to help improve physical activity.
However, the trial by Hunt et al. 128 demonstrates how associating a WMP with a football club can be successful in attracting men who are unfit to take up exercise. The WMP was found to be cost-effective in the long term.
Should physical activity classes and programmes designed specifically for this group be provided?
All of the programmes contributing to our review of qualitative research incorporated increasing physical activity. Some participants described struggling to engage with exercise, but many described psychological and physical benefits. 204,254,264,273,285 Given that participants had a wide range of fitness, flexibility of physical activity and exercise formats was valued. 142,204 Some disliked high-intensity exercise for reasons such as feeling uncomfortable about sweating. 204,272,273 Physical or mental health comorbidities were reported to limit full engagement in physical activity programmes. 142,204,263,272,273,277,285 Given that participants with severe obesity are likely to be less physically fit than participants with lower BMIs, adaptation of activities and goals in accordance with ability appears desirable.
Intervention characteristics
Are certain psychological theories and behaviour change techniques more useful to people with severe obesity for losing weight and maintaining that weight loss?
Participants described the positive psychological changes they experienced with regard to food or body image, which seemed to relate to mindfulness or self-determination theory-based support. 204,262,271,276 However, this could reflect that these particular programmes sought and reported such data. Some people wanted more counselling for less directly related weight issues, such as mental health, recognising that these additional problems had implications for weight management. 225,278 Although many participants discussed positive psychological changes, others found personal development classes challenging and confrontational, and questioned their appropriateness. 271
Data from RCTs favoured adding mindfulness, motivational interviewing or CBT to a WMP as being of additional benefit for weight loss. Binge-eating disorder is more prevalent in severe obesity. In their systematic review of RCTs, Brownley et al. 405 found that CBT reduced the frequency of binge eating, increased the likelihood of its cessation and improved psychological outcomes related to eating. However, they were unable to demonstrate useful effects on participants’ weights.
Barnes and Ivezaj406 systematically reviewed the evidence for motivational interviewing for weight loss in primary care for adults who are overweight or obese, finding some evidence of potential, but they did not specifically evaluate adding motivational interviewing to WMPs or referral pathways. Several systematic reviews407–409 have examined mindfulness-based interventions for weight loss, although only the review by Ruffault et al. 409 focused on RCTs, in which they found no significant effect on weight loss.
Our effect estimates are wide for these behavioural interventions, so that further evidence and evaluation of cost-effectiveness is required before routinely introducing these practices, which require additional training and skills to deliver.
Samdal et al. 410 undertook a systematic review and metaregression analysis of BCTs in 48 short- and long-term RCTs of healthy eating and physical activity for adults who are overweight or obese. They found goal-setting and self-monitoring to be particularly helpful. It is our intention to extend our metaregression work conducted for this project on determinants of weight loss, incorporating TIDieR variables and BCT coding, but this is outside the timescale that this project allows.
Interventions that supported participants to change their diet by eating healthily without a focus on reducing calories and improving mental health did not lead to better weight loss, and did not appear to improve psychological health. However, in a systematic review of qualitative studies of self-directed weight loss, Hartmann-Boyce et al. 411 found that reframing a dietary regimen as healthy eating might make weight control seem less of a burden.
Are group-based interventions more effective for weight loss than those delivered to individuals?
Group-delivered programmes led to greater weight loss than individually delivered programmes, but had more participant contacts, with one study also suggesting that a smaller group size of 12, compared with 30, could be beneficial. Borek et al. 412 undertook a systematic review of RCTs of group-based weight-loss interventions, but not for people with severe obesity, and not comparing group delivery with delivery to individuals. They found that poor reporting by investigators greatly limited the evidence for optimising the effectiveness of group interventions.
In a qualitative comparative analysis, Melendez-Torres et al. 413 synthesised the views of WMP users and compared those with the most and least effective interventions. A key finding for effectiveness was the fostering of supportive relationships with either providers or peers such as would take place in groups, as well as clear directions from providers and fostering the development of self-regulation.
Only one economic evaluation, conducted in the USA, examined delivery of a WMP to a group compared with delivery to individuals. This was an evaluation of a DPP-based intervention delivered by conference or individual calls. 294 Group-based calls were borderline cost-effective in the US health system, but substantial uncertainty existed. It is unclear how transferable the findings are to a UK setting.
From qualitative research reports, many participants described valuing the social interactivity of group-based programme activities and also fairly intensive support from/interaction with programme staff. 142,204,226,249,262,264,269,272,275,276,279,285,286 The key importance of an initial high level of external support from peers and providers in WMPs is also the main finding in the systematic review of qualitative research by Sutcliffe et al. ,414 which did not have a focus on people with severe obesity. However, there may also be a reluctance to discuss more sensitive issues within a group setting, particularly in the setting of mental health. 264,271,272,279,284,286 The qualitative research review also showed that participants valued individual time with a health professional for sensitive matters. Thus, it would seem helpful if group-based programmes allow confidential one-to-one discussions with programme staff, if desired. The delivery of group-based WMPs requires skills that may require additional training, particularly in the NHS setting. 415
What is the effect of financial/other incentives on participation, completion and weight loss?
We found no qualitative reports or intervention studies eligible for our reviews that addressed this question. However, there is some suggestion that financial incentives can improve follow-up in clinical trials of weight-loss interventions. The UK FFIT trial128 offered participants who had dropped out £20 to attend measurement sessions at 12 weeks, and all participants were offered a £40 club voucher to attend the final follow-up session in the football club at 12 months, which may have helped to facilitate the particularly low drop-out rate in this trial (11% at 12 months).
The influence of financial incentives on weight loss in men (mean BMI of ≥ 35 kg/m2) is being investigated as part of the UK National Institute for Health Research funded Game of Stones trial. 416 The endowment incentive, from which men are rewarded in accordance with weight loss, was developed with potential participants and requires no financial expenditure from the men.
Are programmes involving partners/families/friends more effective?
Participants in two studies described difficulties with maintaining behavioural changes if family members were not on board. 275,285 However, in RCTs we found no strong evidence to support regularly involving family members or friends, but weight loss by participants did show some potential to lead to weight loss in partners. WMPs could discuss with participants how to engage family members and friends for their benefit, or how to cope with circumstances that undermine their attempts to change behaviour.
Do people say who they prefer to be the best person/persons to help with/deliver the weight-management programme? Does this agree with what the interventions show us?
From qualitative research reports, it was clear that participants were motivated by the need to be accountable to programme providers who were encouraging and supportive. Endorsement of programmes by health-care staff facilitated engagement with programmes. Health-care staff valued having a limited role in WMPs, as a result of an acknowledged lack of skill and time constraints. Feeding back participants’ progress in programmes to health-care providers was highlighted as being helpful, and could potentially help with motivation when participants are seen by health-care staff for other aspects of care.
How often should people be seen/contacted, and for how long?
For time-limited WMPs, participants sometimes expressed the need for more support (e.g. more frequent contact and for longer duration) than was offered within the programmes,142,225,269,287 particularly in the context of group activities. Our systematic review of RCTs found that more intensive interventions produced additional weight loss of 1–2 kg for up to 2 years. There were no economic evaluations that directly compared the same programme delivered with longer or more frequent contacts.
What role does information technology have, particularly for monitoring and long-term follow-up?
Although not universally popular, participants described the incorporation of tools such as food logs, regular text messages, telemonitoring devices and conversation maps as being motivating and also helpful for the purposes of education and learning, describing how they helped to facilitate self-awareness of, and reflection on, eating and other behaviour patterns. 142,225,262,267,280,285 The importance to participants of receiving feedback from the use of these tools was highlighted.
From RCTs, adding contact by telephone or internet to an existing in-person WMP favoured greater weight loss at least for 12 months. It was difficult to compare programmes wholly delivered in person with those delivered remotely, as some of the in-person programmes themselves were not particularly effective. 142
Sorgente et al. 417 undertook a systematic review of systematic reviews of web-based interventions for weight loss or maintenance for people who are overweight or obese. They concluded that effect sizes were small, with weight loss generally being 1–2 kg. They were perhaps of more use for weight maintenance. Since this publication, there have been newer systematic reviews that have examined electronic health interventions, including mobile phone apps and text messaging. 418–424 These have generally found similar effect sizes for body weight, with some evidence that personalised feedback may improve effectiveness. 423
The evidence from economic evaluations on interventions using information technology was mixed. Two UK studies142,300 reached different conclusions regarding cost-effectiveness. In the economic evaluation alongside the trial, Little et al. 142 showed that the remote delivery of the intervention (POWeR+) was highly cost-effective, but neither intervention produced good weight loss compared with the control group (around 1.5 kg extra). However, in a decision model, Miners et al. 300 reported much higher ICERs closer to the cost-per-QALY threshold when comparing an e-learning device with a control group, but with great uncertainty surrounding the results.
Thus, existing WMPs using information technology alone appear unlikely to provide useful weight loss for people with severe obesity, but may prove useful for enhancing other WMPs, including for weight maintenance.
Drug therapy
Is current drug treatment(s), including long-term results post treatment, useful/cost-effective for people with severe obesity?
Randomised controlled trials of orlistat, including three undertaken in the UK settings of primary and secondary care, demonstrated that orlistat in doses of 180 mg per day or 360 mg per day, mostly compared with placebo, was associated with greater weight loss and maintenance of about 2–3 kg for up to 4 years during the RCTs, at the expense of GI adverse events. Orlistat use was also reported as part of other UK evaluations,149,248,250,252–255 but it was unclear how beneficial this was.
Our qualitative research review did not find accounts of people’s views on the use of drug treatment or orlistat (including the benefits of weight loss compared with the risk of adverse events).
Four studies295,306–308 evaluated the cost-effectiveness of orlistat for people with severe obesity based on results from RCTs. Evidence on cost-effectiveness was mixed. The two European studies, comparing orlistat with a placebo or no intervention, found that orlistat was cost-effective. 306,307 By contrast, an Australian study308 found that orlistat was not cost-effective, with a cost per DALY averted over the commonly used threshold in Australia. The case for orlistat was even less convincing when compared with more active comparators, when orlistat was the dominated strategy (more costly and less effective than WMPs). 295
Data from UK general practice (100,701 patients, mean BMI of 37.2 kg/m2), using the Clinical Practice Research Datalink, show less weight loss (around 2.5 kg at 1 year) from the use of orlistat425 compared with a range of 3.3–10.3 kg (median 5.6 kg) at 1 year from intervention groups in trials we reviewed. The lesser weight loss in primary care could be related to the inability to provide the intensity of dietary counselling seen in the orlistat RCTs. There is evidence that some RCTs of orlistat under-reported adverse events. 426 This may also reflect the selection of motivated participants in RCTs, including the use of lead-in periods, which are associated with fewer dropouts. 427
Surgery
Should more bariatric surgery be offered at the expense of lifestyle weight-management services if no extra funding is available?
Although bariatric surgery is costly to deliver and follow up, because of marked long-term weight loss, the benefits appear to partially offset the costs over the longer term. Surgery is, therefore, more cost-effective than lifestyle WMPs over longer-term time horizons. In an era of heavily restricted NHS budgets, the feasibility and affordability of wide rollout of costly surgery has to be considered, particularly as ≥ 8% of the adult population would fall into this category. This only serves to emphasise the pivotal importance of preventing obesity and facilitating weight loss and maintenance through changes to the obesogenic environment.
Less intensive WMPs, which were comparison arms for bariatric surgery or WMPs with VLCDs, would be the most cost-effective strategy in the absence of surgery. This might apply, for example, to those who do not want surgery, or for commissioning groups that are unable to fund surgery. However, the results of our modelling are dependent on assumptions about longer-term weight regain following WMPs. Presently, owing to the availability of long-term weight-loss data, the Look AHEAD intervention has the most reliable long-term evidence on weight regain, yet cost-effectiveness results remain sensitive to extended regain assumptions beyond the 9-year follow-up. Look AHEAD is borderline cost-effective compared with a ‘do nothing’ scenario when baseline weight is regained between years 9 and 14. The case for cost-effectiveness is stronger when regain is assumed over a longer time horizon, based on linear trends fitted to observed weight-loss data from the study. However, there is no evidence from our modelling to suggest that Look AHEAD is cost-effective compared with other WMPs.
Limitations in the evidence
We found that the studies we reviewed often lacked generalisability to the UK setting. Participants in the studies often had lower BMIs and were less disadvantaged than many users of Tier 3 services. Included studies often excluded people with eating disorders or problems with mental health or addiction. Few included younger adults or older people. Studies were often more resource intensive than would probably be the case in the NHS (e.g. the Look AHEAD trial). We used Look AHEAD for modelling because very long-term weight data were available; however, the participants had type 2 diabetes mellitus, so this might not reflect people with severe obesity in the general population, as used in our model.
Most of the interventions we reviewed lacked long-term follow-up (particularly for complications for surgery), leading to unrealistic weight regain assumptions. Bariatric surgery studies did not cover all of the important diseases related to obesity. Economic evaluations lacked a uniform costing framework. We took the perspective of the NHS, so costs to social care were not included in our economic evaluations.
The views of potential and actual users of services were rarely reported as contributing to service design. We sometimes could not be certain if the views of participants in qualitative research were those of people with severe obesity.
Despite rigorous searching, we may particularly have failed to identify unpublished UK evaluations. Dual, blinded numerical data extraction was not undertaken. The results should, therefore, be interpreted with caution.
Conclusion
Although our systematic review and economic evaluation have provided some answers to the questions that were posed for adult weight management, prior chapters have highlighted questions that remain to be answered by further research, which we list in the next chapter, together with our review’s implications for health care.
Chapter 9 Conclusions
We summarise our conclusions for adult weight management for severe obesity in this chapter. We have not investigated prevention of severe obesity. Changes to the obesogenic environment will enhance the effectiveness of WMPs by supporting people in their behaviour change. For further details on our conclusions, please refer to the discussions and summary points at the ends of Chapters 3–7, and to our overall discussion in Chapter 8.
Our conclusions should be interpreted with caution. Reviewed studies often lacked generalisability to the UK setting, in terms of participants’ characteristics and available resources for implementation. Studies usually lacked long-term follow-up (particularly for complications for surgery) and had unrealistic weight regain assumptions. We may particularly have failed to identify relevant unpublished UK evaluations, and dual, blinded numerical data extraction was not undertaken.
Implications for health care
-
Roux-en-Y bariatric surgery was the most effective and cost-effective weight-loss strategy, favoured over lifestyle WMPs, in the base-case economic evaluations and under sensitivity analyses around regain assumptions.
-
However, shorter time horizons and higher discount rates reduced bariatric surgery cost-effectiveness, in which case RYGB surgery might not then be the most cost-effective use of resources. Less intensive lifestyle WMPs, such as the WMPs in the comparison groups of the trials evaluating the addition of VLCDs, might be short-term cost-effective alternatives.
-
Systematic review evidence suggested that bariatric surgery was more cost-effective in younger patients (aged < 40 years) and people without comorbidities. However, very few studies conducted these subgroup analyses. There were no comparable subgroup analyses in studies evaluating lifestyle WMPs.
-
Adding a VLCD to a WMP was not cost-effective (ICER > £20,000/QALY) compared with a WMP alone, using current evidence. However, a VLCD added to a WMP was cost-effective compared with population obesity trends. Lifestyle WMPs (without VLCDs) were cost-effective when compared with population obesity trends (ICER < £20,000/QALY). VLCDs might also reduce dropouts from WMPs and increase motivation, particularly for people with severe obesity who may have tried many other approaches to weight loss in the past. However, VLCDs may not suit everyone.
-
The Look AHEAD intervention (which incorporated most of the key features for improved weight loss found in our systematic reviews) was borderline cost-effective compared with a ‘do nothing’ approach in our base-case analysis (i.e. with weight regain over 5 years at the end of the 9-year programme). Look AHEAD produced sustained weight loss from an intensive intervention with a low-fat reducing diet, a calorie goal of 1200–1800 kcal/day, initial use of meal replacements or diet plans, a tailored exercise programme, cognitive–behavioural strategies, group and individual contact and follow-up by telephone or e-mail. It is more likely to be cost-effective extrapolating slower linear weight regain after the 9 years of the programme. However, the multiple treatment comparison provides little evidence to suggest that Look AHEAD is among the most cost-effective treatment strategies.
-
Weight-management programmes with VLCDs produced greatest weight loss at 12 months. In primary care, the Counterweight programme, using the Cambridge Weight Plan® VLCD, was more effective at producing weight loss than the conventional Counterweight Programme or the remote or in-person POWeR+ intervention.
-
Diets with low-carbohydrate (≤ 40 g carbohydrate/day, compared with low-fat diets) or higher protein contents (≥ 30% of energy as protein compared with < 20% of energy as protein), or with the addition of meal replacements, led to slightly greater weight loss, but only for 12 months.
-
Adding an intensive physical activity programme to a WMP increased weight loss longer term and had more effect on weight than changes to diet within WMPs (low-carbohydrate or higher-protein diets or the use of meal replacements). Whether less intensive physical activity programmes, as opposed to physical activity advice, have this effect was unclear. Physical activity programmes may be of particular interest to men.
-
Prescribing orlistat to people following a WMP, including for weight maintenance, led to additional weight loss over the WMP alone. There is mixed evidence to support cost-effectiveness of orlistat, and the case is less convincing when orlistat is compared with a more intensive WMP.
-
Certain characteristics of behavioural WMPs were related to increased effectiveness for weight, namely telephone or internet support after the end of the programme, group support, CBT, motivational interviewing and mindfulness. These intervention components generally produced longer-term weight-loss changes than over 12 months. Participants seemed to value the psychological input integrated into interventions.
-
Weight-management programmes that were perceived to be novel or exciting (dietary, physical activity or behavioural elements not experienced before) and endorsed by health-care providers tended to be valued most by participants. Both participants and programme providers tended to value some choice and flexibility within various intervention components.
-
Group-based programme activities tended to be valued, along with fairly intensive support from programme providers, who were encouraging and provided regular monitoring. The sense of belonging to a group of people who shared similar issues seemed particularly important, helping to foster a strong group identity and related ‘accountability’, which aided motivation and continuing engagement.
-
Weight-neutral interventions, which had a focus on healthy eating, without a prescribed calorie intake or reduction in calorie intake, did not appear to be helpful for weight loss.
Recommendations for research
Quantitative research
-
Randomised controlled trials should report, in greater detail, items needed to assess risk of bias, equity (PROGRESS items) and intervention characteristics for experimental and control groups (e.g. using the TIDieR checklist or intervention mapping framework). There is a pressing need to agree common methods of analysis and reporting for weight outcome data, to allow comparisons to be made across services and research studies (e.g. whether to present data for completers, for all participants with last observation carried forward for dropouts or BOCF for dropouts). Public Health England has provided guidance for the evaluation of weight-loss services,260 and a core outcome set is being developed in the UK using consensus methods. 261 The fidelity of intervention delivery should be evaluated and reported.
-
The provision of protocols and intervention materials would facilitate intervention replication, synthesis and implementation in practice. In addition, intervention developers could learn from these materials to improve the design of future interventions.
-
Randomised controlled trials should have adequate statistical power and attempt much longer-term follow-up for weight, comorbidities, quality of life (see Kolotkin and Andersen428 for a summary of recommendations for research on quality of life) and adverse event data, ideally for ≥ 5 years.
-
We were unable to determine whether hard-to-reach or disadvantaged groups, younger or older adults and people with very high BMIs and/or with eating disorders were adequately represented in RCTs or services. We could not determine whether or not these groups were as likely to be retained in WMPs and achieve similar weight loss and health outcomes. These data should be reported.
-
Randomised controlled trials and other evaluations of interventions should seek to be representative of their populations with severe obesity, have long follow-up durations (preferably ≥ 5 years) and collect data to allow economic evaluations.
-
Randomised controlled trials and economic evaluations are needed to examine different approaches to weight management for people eligible for Tier 3 weight-loss services: whether or not VLCDs should be routinely offered, how weight maintenance can be optimised after VLCDs and whether or not NHS Tier 3 services provide better outcomes than commercial programmes.
-
The effectiveness and cost-effectiveness of differing lengths, frequencies and methods of follow-up after weight loss for weight maintenance should be explored in RCTs.
-
Long-term UK weight outcome data are needed from commercial providers for participants with severe obesity (e.g. LighterLife, Cambridge Weight Plan®, Weight Watchers® and Slimming World®), taking account of dropouts.
-
Randomised controlled trials comparing calorie-prescribed diets versus diets with a prescribed deficit should examine the ease of adherence and effectiveness for weight loss.
-
Whether or not providing a physical activity programme to attend compared with physical activity advice improves outcomes with a reducing diet and is cost-effective in the UK setting should be evaluated.
-
Research should explore how to increase engagement with services (e.g. through endorsement by health-care staff).
-
There has been a focus on achieving 5% or 10% weight loss for cardiovascular benefit in WMPs. However, people with severe obesity are at risk of many equally important comorbidities, such as osteoarthritis, sleep apnoea and non-alcoholic fatty liver disease. It is important to assess in RCTs whether 5% or 10% long-term weight loss is sufficient to have important benefits on other comorbidities in relevant patient groups.
-
It is often difficult to interpret and analyse data from trials that were badged solely as ‘weight maintenance’, because data for the non-randomised weight-loss period and the period of the randomised evaluation of alternative weight maintenance interventions are often combined. It would be helpful if investigators could provide randomised data separately, and consider expanding the evidence for weight maintenance by situating weight maintenance randomised trials within weight-loss trials.
-
A clinical research network for weight-management service development and evaluation, promoting best research practice, including undertaking clinical trials, would be helpful.
-
Annual prevalence data for BMIs of 30 to < 35 kg/m2 and 35 to < 40 kg/m2 are not readily available for the countries of the UK. Provision of these data would aid service planning and allow assessment of changes to BMI distributions in the population.
Qualitative and mixed-methods research
-
There is a lack of published qualitative research on the views of potential users, participants, low users, people who drop out and providers of WMPs for people with severe obesity, including, but not limited to, Tier 3 services in the UK. Descriptions of participants’ characteristics in such research would help further analyses (e.g. descriptions of sex, age, ethnicity, BMI and disabilities).
-
Programmes and intervention studies should involve potential and actual participants, including those who drop out, in the design and development of services.
Health economics
Recommendations for analysis and reporting of economic models of weight-management programmes
-
There is a need to undertake economic evaluations of services that are being delivered for the NHS.
-
Improved description and justification of key model inputs, such as cost inputs and utility weights, are needed.
-
All relevant costs and utilities, including all preoperative and postoperative costs, long-term follow-up costs, and the costs of long-term complications after bariatric surgery, should be included.
-
Transparent presentation of assumptions in health economic models, particularly long-term weight regain assumptions preferably based on RCT or epidemiological data, is required.
-
Decision models should include the important disease health states sufficient to give a true picture of the health risks of obesity.
-
Further economic evaluations are required to clearly identify long-term effectiveness of VLCDs derived from RCTs.
-
There is a need for an agreed set of key components of economic evaluations of obesity interventions, focusing in particular around assumptions about weight regain, descriptions of comparator groups as well as interventions, inclusion of all relevant health states (particularly for general population models), and key required sensitivity analyses to assess uncertainty. Such work would improve cross-study comparability and provide more coherent evidence for decision-makers.
Acknowledgements
We thank the members of the REBALANCE Advisory Group for all of their advice and support during this project: Margaret Watson, Lorna Van Lierop, Richard Clarke, Jennifer Logue, Laura Stewart, Richard Welbourn, Jamie Blackshaw and Su Sethi.
We express particular thanks to the data programmers: Mark Forrest, Gary Hay, Tomas Pohl and Brian Taylor.
We also thank the many researchers who gave information, additional materials and data from their studies.
We particularly thank Lara Kemp for keeping us organised and preparing this report. We also thank Louise Cotterell, Anne Buckle, Kath Gibson, Glenys Milton, Juliette Snow, Rodolfo Hernández, Seonaidh Cotton, Roderick Delanougerede and Craig Ramsay for their support.
Contributions of authors
Alison Avenell (Clinical Chairperson in Health Services Research) co-ordinated the design of the study, oversaw all aspects of project management, and wrote the first drafts of Chapters 1, 8 and 9, and the discussion in Chapter 3.
Clare Robertson (Research Fellow) reviewed the quantitative evidence and wrote the first draft of the results section of Chapter 3.
Zoë Skea (Research Fellow) led the systematic review of qualitative evidence and wrote the first draft of Chapter 5.
Elisabet Jacobsen (Research Assistant and Health Economist) reviewed the cost-effectiveness evidence and wrote the first draft of Chapter 6.
Dwayne Boyers (Research Fellow and Health Economist) led the supervision of the review of cost-effectiveness and the economic modelling.
David Cooper (Research Fellow) conducted the statistical analyses.
Magaly Aceves-Martins (Research Fellow) reviewed the quantitative evidence, contributed to the systematic review of qualitative evidence and wrote the first draft of Chapter 4.
Lise Retat (Mathematical Modeller) contributed to the development of the economic modelling.
Cynthia Fraser (Information Specialist) developed and ran the search strategies for all systematic reviews and was responsible for reference management and providing the search flow charts.
Paul Aveyard (GP, Professor of Behavioural Medicine) provided clinical advice.
Fiona Stewart (Research Fellow) reviewed the quantitative evidence.
Graeme MacLennan (Senior Statistician) supervised the statistical analyses.
Laura Webber (Director of Public Health Modelling, UKHF) jointly led the supervision of the economic modelling.
Emily Corbould (Data Analyst) contributed to the development of the economic modelling.
Benshuai Xu (Mathematical Modeller) contributed to the development of the economic modelling.
Abbygail Jaccard (Deputy Director of Public Health Modelling, UKHF) jointly led the supervision of the economic modelling.
Bonnie Boyle (Medical Student) provided assistance for the quantitative systematic reviews.
Eilidh Duncan (Research Fellow and Health Psychologist) provided advice for conducting the coding of behavioural change techniques.
Michal Shimonovich (Research Fellow) provided assistance for the quantitative systematic reviews.
Marijn de Bruin (Personal Chairperson in Health Psychology) was the senior advising health psychologist.
All authors commented on draft versions of the manuscript.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Weight Management: Lifestyle Services for Overweight or Obese Adults. Public Health Guideline PH53. London: NICE; 2014.
- Obesity Prevention. Clinical Guideline CG43. London: NICE; 2015.
- Management of Obesity. Clinical Guideline 115. Edinburgh: SIGN; 2010.
- Commissioning Guide: Weight Assessment and Management Clinics (Tier 3). London: BOMSS; 2017.
- Health Survey for England – 2013. NHS Digital; 2014.
- Health Survey for England – 2016. NHS Digital; 2017.
- Scottish Health Survey 2016. Volume 1; Main Report. Edinburgh: Scottish Government; 2017.
- Welsh Health Survey 2013. Cardiff: Welsh Government; 2014.
- Health Survey Northern Ireland 2012–2013. Northern Ireland Statistics and Research Agency; 2014.
- Obesity and Ethnicity. Oxford: National Obesity Observatory; 2011.
- Global BMI. Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776-86. https://doi.org/10.1016/S0140-6736(16)30175-1.
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. https://doi.org/10.1016/S0140-6736(09)60318-4.
- Grover SA, Kaouache M, Rempel P, Joseph L, Dawes M, Lau DC, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people: a modelling study. Lancet Diabetes Endocrinol 2015;3:114-22. https://doi.org/10.1016/S2213-8587(14)70229-3.
- Adult Obesity and Type 2 Diabetes. London: Public Health England; 2014.
- Reeves GK, Balkwill A, Cairns BJ, Green J, Beral V. Million Women Study Collaborators . Hospital admissions in relation to body mass index in UK women: a prospective cohort study. BMC Med 2014;12. https://doi.org/10.1186/1741-7015-12-45.
- Korda RJ, Liu B, Clements MS, Bauman AE, Jorm LR, Bambrick HJ, et al. Prospective cohort study of body mass index and the risk of hospitalisation: findings from 246361 participants in the 45 and Up Study. Int J Obes 2013;37:790-9. https://doi.org/10.1038/ijo.2012.155.
- Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health 2005;27:156-64. https://doi.org/10.1093/pubmed/fdi025.
- Scott KM, Bruffaerts R, Simon GE, Alonso J, Angermeyer M, de Girolamo G, et al. Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes 2008;32:192-200. https://doi.org/10.1038/sj.ijo.0803701.
- Healthy Lives, Healthy People: a Call to Action on Obesity in England. London: Department of Health and Social Care; 2011.
- Grieve E, Fenwick E, Yang HC, Lean M. The disproportionate economic burden associated with severe and complicated obesity: a systematic review. Obes Rev 2013;14:883-94. https://doi.org/10.1111/obr.12059.
- Dobbs R, Sawers C, Thompson F, Manyika J, Woetzel J, Child P. Overcoming Obesity: An Initial Economic Analysis 2014. www.mckinsey.com/∼/media/mckinsey/business%20functions/economic%20studies%20temp/our%20insights/how%20the%20world%20could%20better%20fight%20obesity/mgi_overcoming_obesity_executive_summary.ashx (accessed April 2018).
- Obesity: Identification, Assessment and Management. Clinical Guideline CG189. London: NICE; 2014.
- Weight Management: Lifestyle Services for Overweight or Obese Adults. London: NICE; 2016.
- Welbourn R, Hopkins J, Dixon JB, Finer N, Hughes C, Viner R, et al. Guidance Development Group . Commissioning guidance for weight assessment and management in adults and children with severe complex obesity. Obes Rev 2018;19:14-27. https://doi.org/10.1111/obr.12601.
- Action on Obesity: Comprehensive Care for all. Report of a Working Party. London: Royal College of Physicians; 2013.
- Brown TJ, O’Malley C, Blackshaw J, Coulton V, Tedstone A, Summerbell C, et al. Exploring the evidence base for Tier 3 weight management interventions for adults: a systematic review. Clin Obes 2017;7:260-72. https://doi.org/10.1111/cob.12204.
- Guzelegun N, Sissoko F, Jeong Ko U, Harrington J, Lanford K, Coulton V, et al. Qualitative Opportunities into User Experiences of Tier 2 and Tier 3 Weight Management Services 2017. www.gov.uk/government/publications/weight-management-services-insights-into-user-experiences (accessed April 2018).
- Leslie WS, Taylor R, Harris L, Lean ME. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis. Int J Obes 2017;41:96-101. https://doi.org/10.1038/ijo.2016.175.
- Mulholland Y, Nicokavoura E, Broom J, Rolland C. Very-low-energy diets and morbidity: a systematic review of longer-term evidence. Br J Nutr 2012;108:832-51. https://doi.org/10.1017/S0007114512001924.
- Parretti HM, Jebb SA, Johns DJ, Lewis AL, Christian-Brown AM, Aveyard P. Clinical effectiveness of very-low-energy diets in the management of weight loss: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2016;17:225-34. https://doi.org/10.1111/obr.12366.
- Rehackova L, Arnott B, Araujo-Soares V, Adamson AA, Taylor R, Sniehotta FF. Efficacy and acceptability of very low energy diets in overweight and obese people with Type 2 diabetes mellitus: a systematic review with meta-analyses. Diabet Med 2016;33:580-91. https://doi.org/10.1111/dme.13005.
- Sellahewa L, Khan C, Lakkunarajah S, Idris I. A systematic review of evidence on the use of very low calorie diets in people with diabetes. Curr Diabetes Rev 2017;13:35-46. https://doi.org/10.2174/1573399812666151005123431.
- Hassan Y, Head V, Jacob D, Bachmann MO, Diu S, Ford J. Lifestyle interventions for weight loss in adults with severe obesity: a systematic review. Clin Obes 2016;6:395-403. https://doi.org/10.1111/cob.12161.
- Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149:275-87. https://doi.org/10.1001/jamasurg.2013.3654.
- Cheng J, Gao J, Shuai X, Wang G, Tao K. The comprehensive summary of surgical versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. Oncotarget 2016;7:39216-30. https://doi.org/10.18632/oncotarget.9581.
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f5934.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13410.
- Picot J, Jones J, Colquitt JL, Loveman E, Clegg AJ. Weight loss surgery for mild to moderate obesity: a systematic review and economic evaluation. Obes Surg 2012;22:1496-506. https://doi.org/10.1007/s11695-012-0679-z.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8210.
- Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society. Obesity 2014;22:S5-S39. https://doi.org/10.1002/oby.20821.
- The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145-54. https://doi.org/10.1056/NEJMoa1212914.
- The Look AHEAD Research Group . Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity 2014;22:5-13. https://doi.org/10.1002/oby.20662.
- Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, et al. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med 2013;126:236-42. https://doi.org/10.1016/j.amjmed.2012.10.010.
- Bahnson JL, Knowler WC, Bantle JP, Bertoni AG, Bray GA, Chen H, et al. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2014;2:801-9. https://doi.org/10.1016/S2213-8587(14)70156-1.
- Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156-63. https://doi.org/10.2337/dc10-0856.
- White DK, Neogi T, Rejeski WJ, Walkup MP, Lewis CE, Nevitt MC, et al. Can an intensive diet and exercise program prevent knee pain among overweight adults at high risk?. Arthritis Care Res 2015;67:965-71. https://doi.org/10.1002/acr.22544.
- The Look AHEAD Research Group . Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD trial. Diabetes Care 2014;37:1544-53. https://doi.org/10.2337/dc13-1928.
- Phelan S, Kanaya AM, Subak LL, Hogan PE, Espeland MA, Wing RR, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol 2012;187:939-44. https://doi.org/10.1016/j.juro.2011.10.139.
- Breyer BN, Phelan S, Hogan PE, Rosen RC, Kitabchi AE, Wing RR, et al. Look AHEAD Research Group . Intensive lifestyle intervention reduces urinary incontinence in overweight/obese men with type 2 diabetes: results from the Look AHEAD trial. J Urol 2014;192:144-9. https://doi.org/10.1016/j.juro.2014.02.036.
- Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano IINC, et al. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD Trial. J Sex Med 2010;7:156-65. https://doi.org/10.1111/j.1743-6109.2009.01458.x.
- Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep 2013;36:641-649A. https://doi.org/10.5665/sleep.2618.
- Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489-96. https://doi.org/10.1001/jama.2012.67929.
- Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care 2014;37:2548-56. https://doi.org/10.2337/dc14-0093.
- Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet 2015;385:2521-33. https://doi.org/10.1016/S0140-6736(14)61748-7.
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. http://handbook-5-1.cochrane.org/ (accessed March 2018).
- University of York . Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care 2009. www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed March 2018).
- de Bruin M, Viechtbauer W, Hospers HJ, Schaalma HP, Kok G. Standard care quality determines treatment outcomes in control groups of HAART-adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychol 2009;28:668-74. https://doi.org/10.1037/a0015989.
- de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: a meta-analysis of randomized controlled trials. Arch Intern Med 2010;170:240-50. https://doi.org/10.1001/archinternmed.2009.536.
- Robertson C, Archibald D, Avenell A, Douglas F, Hoddinott P, van Teijlingen E, et al. Systematic reviews of and integrated report on the quantitative, qualitative and economic evidence base for the management of obesity in men. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18350.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E. Campbell and Campbell and Cochrane Equity Methods Group . Equity Checklist for Systematic Review Authors. Version 2012-10-04 2009. http://methods.cochrane.org/sites/methods.cochrane.org.equity/files/public/uploads/EquityChecklist2012.pdf (accessed March 2018).
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Cresswell L, Mander AP. How to use published complete case results from weight loss studies in a missing data sensitivity analysis. Obesity 2014;22:996-1001. https://doi.org/10.1002/oby.20635.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software 2010;36:1-48. https://doi.org/10.18637/jss.v036.i03.
- Barnett-Page E, Thomas J. Methods for the synthesis of qualitative research: a critical review. BMC Med Res Methodol 2009;9. https://doi.org/10.1186/1471-2288-9-59.
- Spencer L, Ritchie J, Lewis J, Dillon L. Quality in Qualitative Evaluation: a Framework for Assessing Research Evidence. London: Cabinet Office; 2003.
- Pawson R. Evidence-Based Policy: A Realist Perspective. London: Sage; 2006.
- Noblit GW, Hare RD. Meta-ethnography: Synthesizing Qualitative Studies. Newbury Park, CA: Sage; 1988.
- Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8. https://doi.org/10.1186/1471-2288-8-45.
- Harden A, Brunton G, Fletcher A, Oakley A. Teenage pregnancy and social disadvantage: systematic review integrating controlled trials and qualitative studies. BMJ 2009;339. https://doi.org/10.1136/bmj.b4254.
- Toye F, Seers K, Allcock N, Briggs M, Carr E, Andrews J, et al. ‘Trying to pin down jelly’ – exploring intuitive processes in quality assessment for meta-ethnography. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-46.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Lillis J, Niemeier HM, Thomas JG, Unick J, Ross KM, Leahey TM, et al. A randomized trial of an acceptance-based behavioral intervention for weight loss in people with high internal disinhibition. Obesity 2016;24:2509-14. https://doi.org/10.1002/oby.21680.
- Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, et al. Effects of a mindfulness-based weight loss intervention in adults with obesity: a randomized clinical trial. Obesity 2016;24:794-80. https://doi.org/10.1002/oby.21396.
- Richelsen B, Tonstad S, Rössner S, Toubro S, Niskanen L, Madsbad S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 2007;30:27-32. https://doi.org/10.2337/dc06-0210.
- Yeh MC, Rodriguez E, Nawaz H, Gonzalez M, Nakamoto D, Katz DL. Technical skills for weight loss: 2-y follow-up results of a randomized trial. Int J Obes Relat Metab Disord 2003;27:1500-6. https://doi.org/10.1038/sj.ijo.0802430.
- West DS, DiLillo V, Bursac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care 2007;30:1081-7. https://doi.org/10.2337/dc06-1966.
- Beutel ME, Dippel A, Szczepanski M, Thiede R, Wiltink J. Mid-term effectiveness of behavioral and psychodynamic inpatient treatments of severe obesity based on a randomized study. Psychother Psychosom 2006;75:337-45. https://doi.org/10.1159/000095439.
- Østbye T, Stroo M, Brouwer RJ, Peterson BL, Eisenstein EL, Fuemmeler BF, et al. Steps to Health employee weight management randomized control trial: short-term follow-up results. J Occup Environ Med 2015;57:188-95. https://doi.org/10.1097/JOM.0000000000000335.
- Annesi JJ. Mediation of the relationship of behavioural treatment type and changes in psychological predictors of healthy eating by body satisfaction changes in women with obesity. Obes Res Clin Pract 2017;11:97-107. https://doi.org/10.1016/j.orcp.2016.03.011.
- Agras WS, Berkowitz RI, Arnow BA, Telch CF, Marnell M, Henderson J, et al. Maintenance following a very-low-calorie diet. J Consult Clin Psychol 1996;64:610-13. https://doi.org/10.1037/0022-006X.64.3.610.
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959-68. https://doi.org/10.1056/NEJMoa1108660.
- Azar KM, Xiao L, Ma J. Baseline obesity status modifies effectiveness of adapted diabetes prevention program lifestyle interventions for weight management in primary care. Biomed Res Int 2013;2013. https://doi.org/10.1155/2013/191209.
- Bacon L, Keim NL, Van Loan MD, Derricote M, Gale B, Kazaks A, et al. Evaluating a ‘non-diet’ wellness intervention for improvement of metabolic fitness, psychological well-being and eating and activity behaviors. Int J Obes Relat Metab Disord 2002;26:854-65. https://doi.org/10.1038/sj.ijo.0802012.
- Bakris G, Calhoun D, Egan B, Hellmann C, Dolker M, Kingma I. Orlistat and Resistant Hypertension Investigators . Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. J Hypertens 2002;20:2257-67. https://doi.org/10.1097/00004872-200211000-00026.
- Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, et al. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv 2013;64:729-36. https://doi.org/10.1176/appi.ps.003622012.
- Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Naslund JA, Wolfe R, et al. Pragmatic replication trial of health promotion coaching for obesity in serious mental illness and maintenance of outcomes. Am J Psychiatry 2015;172:344-52. https://doi.org/10.1176/appi.ajp.2014.14030357.
- Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med 2014;161:309-18. https://doi.org/10.7326/M14-0180.
- Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller DP, et al. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med 2012;172:565-74. https://doi.org/10.1001/archinternmed.2012.1.
- Berry DC, Schwartz TA, McMurray RG, Skelly AH, Neal M, Hall EG, et al. The family partners for health study: a cluster randomized controlled trial for child and parent weight management. Nutr Diabetes 2014;4. https://doi.org/10.1038/nutd.2013.42.
- Bertéus Forslund H, Klingström S, Hagberg H, Löndahl M, Torgerson JS, Lindroos AK. Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur J Clin Nutr 2008;62:1308-17. https://doi.org/10.1038/sj.ejcn.1602860.
- Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis 2011;70:1798-803. https://doi.org/10.1136/ard.2010.142018.
- Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, et al. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009;32:215-20. https://doi.org/10.2337/dc08-0687.
- Broom I, Hughes E, Dodson P, Reckless J. The role of orlistat in the treatment of obese patients with mild to moderate hypercholesterolaemia: consequences for coronary risk. Br J Cardiol 2002;9:460-8.
- Broom I, Wilding J, Stott P, Myers N. UK Multimorbidity Study Group . Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract 2002;56:494-9.
- Burguera B, Jesús Tur J, Escudero AJ, Alos M, Pagán A, Cortés B, et al. An intensive lifestyle intervention is an effective treatment of morbid obesity: The TRAMOMTANA study-A two-year randomized controlled clinical trial. Int J Endocrinol 2015;2015. https://doi.org/10.1155/2015/194696.
- Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educ 2008;34:118-27. https://doi.org/10.1177/0145721707312463.
- Christensen P, Frederiksen R, Bliddal H, Riecke BF, Bartels EM, Henriksen M, et al. Comparison of three weight maintenance programs on cardiovascular risk, bone and vitamins in sedentary older adults. Obesity 2013;21:1982-90. https://doi.org/10.1002/oby.20413.
- Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, et al. Surgical vs. medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014;149:707-15. https://doi.org/10.1001/jamasurg.2014.467.
- Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016;59:945-53. https://doi.org/10.1007/s00125-016-3903-x.
- Dalle Grave R, Calugi S, Gavasso I, El Ghoch M, Marchesini G. A randomized trial of energy-restricted high-protein versus high-carbohydrate, low-fat diet in morbid obesity. Obesity 2013;21:1774-81. https://doi.org/10.1002/oby.20320.
- Damschroder LJ, Lutes LD, Kirsh S, Kim HM, Gillon L, Holleman RG, et al. Small-changes obesity treatment among veterans: 12-month outcomes. Am J Prev Med 2014;47:541-53. https://doi.org/10.1016/j.amepre.2014.06.016.
- Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013;368:1594-602. https://doi.org/10.1056/NEJMoa1214530.
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235-42. https://doi.org/10.1001/jama.281.3.235.
- Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, et al. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009;32:1147-52. https://doi.org/10.2337/dc08-2108.
- Delbridge EA, Prendergast LA, Pritchard JE, Proietto J. One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: does diet composition matter?. Am J Clin Nutr 2009;90:1203-14. https://doi.org/10.3945/ajcn.2008.27209.
- Dennison KF, Galante D, Dennison D, Golaszewski T. A one year post-program assessment of a computer-assisted instruction weight management program for industrial employees: lessons learned. J Health Educ 1996;27:38-43. https://doi.org/10.1080/10556699.1996.10603162.
- Ding SA, Simonson DC, Wewalka M, Halperin F, Foster K, Goebel-Fabbri A, et al. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015;100:2546-56. https://doi.org/10.1210/jc.2015-1443.
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-23. https://doi.org/10.1001/jama.299.3.316.
- Dixon JB, Schachter LM, O’Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012;308:1142-9. https://doi.org/10.1001/2012.jama.11580.
- Djuric Z, Mirasolo J, Kimbrough L, Brown DR, Heilbrun LK, Canar L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J Natl Med Assoc 2009;101:552-64. https://doi.org/10.1016/S0027-9684(15)30940-8.
- Dutton GR, Nackers LM, Dubyak PJ, Rushing NC, Huynh TV, Tan F, et al. A randomized trial comparing weight loss treatment delivered in large versus small groups. Int J Behav Nutr Phys Act 2014;11. https://doi.org/10.1186/s12966-014-0123-y.
- Eaton CB, Hartman SJ, Perzanowski E, Pan G, Roberts MB, Risica PM, et al. A randomized clinical trial of a tailored lifestyle intervention for obese, sedentary, primary care patients. Ann Fam Med 2016;14:311-19. https://doi.org/10.1370/afm.1952.
- Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs. low-fat diet in obese young adults: a randomized trial. JAMA 2007;297:2092-102. https://doi.org/10.1001/jama.297.19.2092.
- Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004;291:2978-84. https://doi.org/10.1001/jama.291.24.2978.
- Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord 2000;24:306-13. https://doi.org/10.1038/sj.ijo.0801128.
- Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity Reduction Black Intervention Trial (ORBIT): 18-month results. Obesity 2010;18:2317-25. https://doi.org/10.1038/oby.2010.47.
- Flechtner-Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev 2010;26:393-405. https://doi.org/10.1002/dmrr.1097.
- Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 2010;153:147-57. https://doi.org/10.7326/0003-4819-153-3-201008030-00005.
- Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010;304:1795-802. https://doi.org/10.1001/jama.2010.1505.
- Gorin AA, Raynor HA, Fava J, Maguire K, Robichaud E, Trautvetter J, et al. Randomized controlled trial of a comprehensive home environment-focused weight-loss program for adults. Health Psychol 2013;32:128-37. https://doi.org/10.1037/a0026959.
- Green CA, Yarborough BJ, Leo MC, Stumbo SP, Perrin NA, Nichols GA, et al. Weight maintenance following the STRIDE lifestyle intervention for individuals taking antipsychotic medications. Obesity 2015;23:1995-2001. https://doi.org/10.1002/oby.21205.
- Hakala P. Weight reduction programmes at a rehabilitation centre and a health centre based on group counselling and individual support: short- and long-term follow-up study. Int J Obes Relat Metab Disord 1994;18:483-9.
- Hakala P, Karvetti RL, Rönnemaa T. Group vs. individual weight reduction programmes in the treatment of severe obesity – a five year follow-up study. Int J Obes Relat Metab Disord 1993;17:97-102.
- Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716-26. https://doi.org/10.1001/jamasurg.2014.514.
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160-7. https://doi.org/10.1001/archfami.9.2.160.
- Hunt K, Wyke S, Gray CM, Anderson AS, Brady A, Bunn C, et al. A gender-sensitised weight loss and healthy living programme for overweight and obese men delivered by Scottish Premier League football clubs (FFIT): a pragmatic randomised controlled trial. Lancet 2014;383:1211-21. https://doi.org/10.1016/S0140-6736(13)62420-4.
- Iqbal N, Vetter ML, Moore RH, Chittams JL, Dalton-Bakes CV, Dowd M, et al. Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity 2010;18:1733-8. https://doi.org/10.1038/oby.2009.460.
- Kahleova H, Hill M, Pelikanova T. Vegetarian vs. conventional diabetic diet – a 1-year follow-up. Cor Vasa 2014;56:e140-4. https://doi.org/10.1016/j.crvasa.2013.12.004.
- Kelley DE, Bray GA, Pi-Sunyer FX, Klein S, Hill J, Miles J, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 2002;25:1033-41. https://doi.org/10.2337/diacare.25.6.1033.
- Keränen AM, Savolainen MJ, Reponen AH, Kujari ML, Lindeman SM, Bloigu RS, et al. The effect of eating behavior on weight loss and maintenance during a lifestyle intervention. Prev Med 2009;49:32-8. https://doi.org/10.1016/j.ypmed.2009.04.011.
- Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 2011;8:2868-75. https://doi.org/10.1111/j.1743-6109.2011.02417.x.
- Krebs JD, Elley CR, Parry-Strong A, Lunt H, Drury PL, Bell DA, et al. The Diabetes Excess Weight Loss (DEWL) Trial: a randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes. Diabetologia 2012;55:905-14. https://doi.org/10.1007/s00125-012-2461-0.
- Krempf M, Louvet JP, Allanic H, Miloradovich T, Joubert JM, Attali JR. Weight reduction and long-term maintenance after 18 months treatment with orlistat for obesity. Int J Obes Relat Metab Disord 2003;27:591-7. https://doi.org/10.1038/sj.ijo.0802281.
- Kumanyika SK, Fassbender JE, Sarwer DB, Phipps E, Allison KC, Localio R, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity 2012;20:1249-57. https://doi.org/10.1038/oby.2011.329.
- Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: the Healthy Eating and Lifestyle Program (HELP) study. Prev Med 2005;41:488-502. https://doi.org/10.1016/j.ypmed.2004.09.049.
- Kumanyika SK, Wadden TA, Shults J, Fassbender JE, Brown SD, Bowman MA, et al. Trial of family and friend support for weight loss in African American adults. Arch Intern Med 2009;169:1795-804. https://doi.org/10.1001/archinternmed.2009.337.
- Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on-demand use of a very low calorie diet: a randomized 2-year clinical trial. J Intern Med 2003;253:463-71. https://doi.org/10.1046/j.1365-2796.2003.01131.x.
- Latner JD, Ciao AC, Wendicke AU, Murakami JM, Durso LE. Community-based behavioral weight-loss treatment: long-term maintenance of weight loss, physiological, and psychological outcomes. Behav Res Ther 2013;51:451-9. https://doi.org/10.1016/j.brat.2013.04.009.
- Linde JA, Simon GE, Ludman EJ, Ichikawa LE, Operskalski BH, Arterburn D, et al. A randomized controlled trial of behavioral weight loss treatment versus combined weight loss/depression treatment among women with comorbid obesity and depression. Ann Behav Med 2011;41:119-30. https://doi.org/10.1007/s12160-010-9232-2.
- Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ, et al. Randomised controlled trial and economic analysis of an internet-based weight management programme: POWeR+ (Positive Online Weight Reduction). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21040.
- Lowe MR, Butryn ML, Thomas JG, Coletta M. Meal replacements, reduced energy density eating, and weight loss maintenance in primary care patients: a randomized controlled trial. Obesity 2014;22:94-100. https://doi.org/10.1002/oby.20582.
- Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Wilson SR, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc 2015;12:1-11. https://doi.org/10.1513/AnnalsATS.201406-271OC.
- MacLaughlin HL, Hall WL, Patel AG, Blacklock RM, Swift PA, Phanish MK, et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3-4 CKD: a randomized controlled pilot study. Am J Kidney Dis 2014;64:660-3. https://doi.org/10.1053/j.ajkd.2014.06.011.
- Manzoni GM, Cesa GL, Bacchetta M, Castelnuovo G, Conti S, Gaggioli A, et al. Virtual reality-enhanced cognitive-behavioral therapy for morbid obesity: a randomized controlled study with 1 year follow-up. Cyberpsychol Behav Soc Netw 2016;19:134-40. https://doi.org/10.1089/cyber.2015.0208.
- Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity 2008;16:2462-7. https://doi.org/10.1038/oby.2008.399.
- Mayer-Davis EJ, D’Antonio AM, Smith SM, Kirkner G, Levin Martin S, Parra-Medina D, et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health 2004;94:1736-42. https://doi.org/10.2105/AJPH.94.10.1736.
- McRobbie H, Hajek P, Peerbux S, Kahan BC, Eldridge S, Trépel D, et al. Tackling obesity in areas of high social deprivation: clinical effectiveness and cost-effectiveness of a task-based weight management group programme – a randomised controlled trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20790.
- Melin I, Karlström B, Lappalainen R, Berglund L, Mohsen R, Vessby B. A programme of behaviour modification and nutrition counselling in the treatment of obesity: a randomised 2-y clinical trial. Int J Obes Relat Metab Disord 2003;27:1127-35. https://doi.org/10.1038/sj.ijo.0802372.
- Mensinger JL, Calogero RM, Stranges S, Tylka TL. A weight-neutral versus weight-loss approach for health promotion in women with high BMI: a randomized-controlled trial. Appetite 2016;105:364-74. https://doi.org/10.1016/j.appet.2016.06.006.
- Miles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002;25:1123-8. https://doi.org/10.2337/diacare.25.7.1123.
- Mingrone G, Greco AV, Giancaterini A, Scarfone A, Castagneto M, Pugeat M. Sex hormone-binding globulin levels and cardiovascular risk factors in morbidly obese subjects before and after weight reduction induced by diet or malabsorptive surgery. Atherosclerosis 2002;161:455-62. https://doi.org/10.1016/S0021-9150(01)00667-0.
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-85. https://doi.org/10.1056/NEJMoa1200111.
- Moreno B, Bellido D, Sajoux I, Goday A, Saavedra D, Crujeiras AB, et al. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 2014;47:793-805. https://doi.org/10.1007/s12020-014-0192-3.
- Nackers LM, Middleton KR, Dubyak PJ, Daniels MJ, Anton SD, Perri MG. Effects of prescribing 1,000 versus 1,500 kilocalories per day in the behavioral treatment of obesity: a randomized trial. Obesity 2013;21:2481-7. https://doi.org/10.1002/oby.20439.
- Nilsen V, Bakke PS, Gallefoss F. Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus – results from a randomised, controlled trial. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-893.
- O’Neil PM, Miller-Kovach K, Tuerk PW, Becker LE, Wadden TA, Fujioka K, et al. Randomized controlled trial of a nationally available weight control program tailored for adults with type 2 diabetes. Obesity 2016;24:2269-77. https://doi.org/10.1002/oby.21616.
- Pascale RW, Wing RR, Butler BA, Mullen M, Bononi P. Effects of a behavioral weight loss program stressing calorie restriction versus calorie plus fat restriction in obese individuals with NIDDM or a family history of diabetes. Diabetes Care 1995;18:1241-8. https://doi.org/10.2337/diacare.18.9.1241.
- Pedersen E, Jesudason DR, Clifton PM. High protein weight loss diets in obese subjects with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2014;24:554-62. https://doi.org/10.1016/j.numecd.2013.11.003.
- Pekkarinen T, Kaukua J, Mustajoki P. Long-term weight maintenance after a 17-week weight loss intervention with or without a one-year maintenance program: a randomized controlled trial. J Obes 2015;2015. https://doi.org/10.1155/2015/651460.
- Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the Treatment of Obesity in Underserved Rural Settings (TOURS) randomized trial. Arch Intern Med 2008;168:2347-54. https://doi.org/10.1001/archinte.168.21.2347.
- Perri MG, Limacher MC, von Castel-Roberts K, Daniels MJ, Durning PE, Janicke DM, et al. Comparative effectiveness of three doses of weight-loss counseling: two-year findings from the rural LITE trial. Obesity 2014;22:2293-300. https://doi.org/10.1002/oby.20832.
- Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol 2001;69:722-6. https://doi.org/10.1037/0022-006X.69.4.722.
- Poston WS, Reeves RS, Haddock CK, Stormer S, Balasubramanyam A, Satterwhite O, et al. Weight loss in obese Mexican Americans treated for 1-year with orlistat and lifestyle modification. Int J Obes Relat Metab Disord 2003;27:1486-93. https://doi.org/10.1038/sj.ijo.0802439.
- Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:954-62. https://doi.org/10.1016/S2213-8587(14)70200-1.
- Rapoport L, Clark M, Wardle J. Evaluation of a modified cognitive-behavioural programme for weight management. Int J Obes Relat Metab Disord 2000;24:1726-37. https://doi.org/10.1038/sj.ijo.0801465.
- Reichard A, Saunders MD, Saunders RR, Donnelly JE, Lauer E, Sullivan DK, et al. A comparison of two weight management programs for adults with mobility impairments. Disabil Health J 2015;8:61-9. https://doi.org/10.1016/j.dhjo.2014.08.002.
- Reis LO, Favaro WJ, Barreiro GC, de Oliveira LC, Chaim EA, Fregonesi A, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl 2010;33:736-44. https://doi.org/10.1111/j.1365-2605.2009.01017.x.
- Rock CL, Flatt SW, Pakiz B, Taylor KS, Leone AF, Brelje K, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care 2014;37:1573-80. https://doi.org/10.2337/dc13-2900.
- Rössner S, Flaten H. VLCD versus LCD in long-term treatment of obesity. Int J Obes Relat Metab Disord 1997;21:22-6. https://doi.org/10.1038/sj.ijo.0800355.
- Ryttig KR, Flaten H, Rössner S. Long-term effects of a very low calorie diet (Nutrilett) in obesity treatment. A prospective, randomized, comparison between VLCD and a hypocaloric diet+behavior modification and their combination. Int J Obes Relat Metab Disord 1997;21:574-9. https://doi.org/10.1038/sj.ijo.0800444.
- Ryttig KR, Rössner S. Weight maintenance after a very low calorie diet (VLCD) weight reduction period and the effects of VLCD supplementation. A prospective, randomized, comparative, controlled long-term trial. J Intern Med 1995;238:299-306. https://doi.org/10.1111/j.1365-2796.1995.tb01202.x.
- Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76. https://doi.org/10.1056/NEJMoa1200225.
- Shikany JM, Thomas AS, Beasley TM, Lewis CE, Allison DB. Randomized controlled trial of the Medifast 5 & 1 Plan for weight loss. Int J Obes 2013;37:1571-8. https://doi.org/10.1038/ijo.2013.43.
- Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HPF, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet 1998;352:167-72. https://doi.org/10.1016/S0140-6736(97)11509-4.
- Soenen S, Bonomi AG, Lemmens SG, Scholte J, Thijssen MA, van Berkum F, et al. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance?. Physiol Behav 2012;107:374-80. https://doi.org/10.1016/j.physbeh.2012.08.004.
- Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, DeMott A, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med 2013;173:105-11. https://doi.org/10.1001/jamainternmed.2013.1221.
- Stahre L, Hällström T. A short-term cognitive group treatment program gives substantial weight reduction up to 18 months from the end of treatment. A randomized controlled trial. Eat Weight Disord 2005;10:51-8. https://doi.org/10.1007/BF03353419.
- Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000;320:827-32. https://doi.org/10.1136/bmj.320.7238.827.
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med 2004;140:778-85. https://doi.org/10.7326/0003-4819-140-10-200405180-00007.
- Ströbl V, Knisel W, Landgraf U, Faller H. A combined planning and telephone aftercare intervention for obese patients: effects on physical activity and body weight after one year. J Rehabil Med 2013;45:198-205. https://doi.org/10.2340/16501977-1095.
- Swinburn BA, Carey D, Hills AP, Hooper M, Marks S, Proietto J, et al. Effect of orlistat on cardiovascular disease risk in obese adults. Diabetes Obes Metab 2005;7:254-62. https://doi.org/10.1111/j.1463-1326.2004.00467.x.
- Torgerson JS, Agren L, Sjöström L. Effects on body weight of strict or liberal adherence to an initial period of VLCD treatment. A randomised, one-year clinical trial of obese subjects. Int J Obes Relat Metab Disord 1999;23:190-7. https://doi.org/10.1038/sj.ijo.0800816.
- Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-61. https://doi.org/10.2337/diacare.27.1.155.
- Torgerson JS, Lissner L, Lindroos AK, Kruijer H, Sjöström L. VLCD plus dietary and behavioural support versus support alone in the treatment of severe obesity. A randomised two-year clinical trial. Int J Obes Relat Metab Disord 1997;21:987-94. https://doi.org/10.1038/sj.ijo.0800507.
- Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity 2010;18:1614-18. https://doi.org/10.1038/oby.2009.457.
- Van Name MA, Camp AW, Magenheimer EA, Li F, Dziura JD, Montosa A, et al. Effective translation of an intensive lifestyle intervention for Hispanic women with prediabetes in a community health center setting. Diabetes Care 2016;39:525-31. https://doi.org/10.2337/dc15-1899.
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218-29. https://doi.org/10.1056/NEJMoa1008234.
- Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab 2008;93:2181-7. https://doi.org/10.1210/jc.2007-1473.
- von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol 2008;109:19-26. https://doi.org/10.1016/j.ygyno.2007.12.026.
- Wadden TA, Berkowitz RI, Vogt RA, Steen SN, Stunkard AJ, Foster GD. Lifestyle modification in the pharmacologic treatment of obesity: a pilot investigation of a potential primary care approach. Obes Res 1997;5:218-26. https://doi.org/10.1002/j.1550-8528.1997.tb00296.x.
- Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol 1994;62:165-71. https://doi.org/10.1037/0022-006X.62.1.165.
- Wadden TA, Vogt RA, Foster GD, Anderson DA. Exercise and the maintenance of weight loss: 1-year follow-up of a controlled clinical trial. J Consult Clin Psychol 1998;66:429-33. https://doi.org/10.1037/0022-006X.66.2.429.
- Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969-79. https://doi.org/10.1056/NEJMoa1109220.
- Weinstock RS, Trief PM, Cibula D, Morin PC, Delahanty LM. Weight loss success in metabolic syndrome by telephone interventions: results from the SHINE Study. J Gen Intern Med 2013;28:1620-8. https://doi.org/10.1007/s11606-013-2529-7.
- West DS, Gorin AA, Subak LL, Foster G, Bragg C, Hecht J, et al. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes 2011;35:259-69. https://doi.org/10.1038/ijo.2010.138.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome?. Am J Med 1994;97:354-62. https://doi.org/10.1016/0002-9343(94)90302-6.
- Wing RR, Marcus MD, Epstein LH, Jawad A. A ‘family-based’ approach to the treatment of obese type II diabetic patients. J Consult Clin Psychol 1991;59:156-62. https://doi.org/10.1037/0022-006X.59.1.156.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991;151:1334-40. https://doi.org/10.1001/archinte.1991.00400070100012.
- Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350-9. https://doi.org/10.2337/diacare.21.3.350.
- Wolf AM, Conaway MR, Crowther JQ, Hazen KY, L Nadler J, Oneida B, et al. Improving Control with Activity and Nutrition (ICAN) Study . Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care 2004;27:1570-6. https://doi.org/10.2337/diacare.27.7.1570.
- Wylie-Rosett J, Swencionis C, Ginsberg M, Cimino C, Wassertheil-Smoller S, Caban A, et al. Computerized weight loss intervention optimizes staff time: the clinical and cost results of a controlled clinical trial conducted in a managed care setting. J Am Diet Assoc 2001;101:1155-62. https://doi.org/10.1016/S0002-8223(01)00284-X.
- Borkoles E, Carroll S, Clough P, Polman RC. Effect of a non-dieting lifestyle randomised control trial on psychological well-being and weight management in morbidly obese pre-menopausal women. Maturitas 2016;83:51-8. https://doi.org/10.1016/j.maturitas.2015.09.010.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40. https://doi.org/10.1056/NEJMoa012512.
- Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725-31. https://doi.org/10.2337/diacare.26.3.725.
- Schwarz PE, Li J, Lindstrom J, Tuomilehto J. Tools for predicting the risk of type 2 diabetes in daily practice. Horm Metab Res 2009;41:86-97. https://doi.org/10.1055/s-0028-1087203.
- Brownell KD. The LEARN Program for Weight Management. Dallas, TX: American Health; 2004.
- Kabat-Zinn J. Full Catastrophe Living. New York, NY: Dell Publishing; 1990.
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. J Obes 2011;2011. https://doi.org/10.1155/2011/651936.
- Kristeller J, Wolever RQ, Sheets V. Mindfulness-Based Eating Awareness Training (MB-EAT) for binge eating: a randomized clinical trial. Mindfulness 2014;5:282-97. https://doi.org/10.1007/s12671-012-0179-1.
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord 2011;19:49-61. https://doi.org/10.1080/10640266.2011.533605.
- Dreyer D, Dreyer K. Chi Walking: The Five Mindful Steps for Lifelong Health and Energy. New York, NY: Simon and Schuster; 2006.
- Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York, NY: Guilford Press; 1999.
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood, NJ: Prentice Hall; 1986.
- Bandura A. Self-efficacy: The Exercise of Control. New York, NY: Freeman; 1997.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) . Action for Health in Diabetes. Look AHEAD Clinical Trial Protocol 2009. www.div12.org/wp-content/uploads/2015/04/Look-AHEAD-Protocol.pdf (accessed April 2018).
- Gorin AA, Niemeier HM, Hogan P, Coday M, Davis C, DiLillo VG, et al. Binge eating and weight loss outcomes in overweight and obese individuals with type 2 diabetes: results from the Look AHEAD trial. Arch Gen Psychiatry 2008;65:1447-55. https://doi.org/10.1001/archpsyc.65.12.1447.
- Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J 2015;170:770-7.e5. https://doi.org/10.1016/j.ahj.2015.07.026.
- Look AHEAD Research Group . Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia 2017;60:980-8. https://doi.org/10.1007/s00125-017-4253-z.
- Rejeski WJ, Bray GA, Chen SH, Clark JM, Evans M, Hill JO, et al. Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci 2015;70:345-53. https://doi.org/10.1093/gerona/glu083.
- Lipkin EW, Schwartz AV, Anderson AM, Davis C, Johnson KC, Gregg EW, et al. The Look AHEAD Trial: bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care 2014;37:2822-9. https://doi.org/10.2337/dc14-0762.
- Johnson KC, Bray GA, Cheskin LJ, Clark JM, Egan CM, Foreyt JP, et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the Look AHEAD randomized clinical trial. J Bone Miner Res 2017;32:2278-87. https://doi.org/10.1002/jbmr.3214.
- Rapp SR, Luchsinger JA, Baker LD, Blackburn GL, Hazuda HP, Demos-McDermott KE, et al. Effect of a long-term intensive lifestyle intervention on cognitive function: Action for Health in Diabetes study. J Am Geriatr Soc 2017;65:966-72. https://doi.org/10.1111/jgs.14692.
- VanWormer JJ, Martinez AM, Cosentino D, Pronk NP. Satisfaction with a weight loss program: what matters?. Am J Health Promot 2010;24:238-45. https://doi.org/10.4278/ajhp.080613-QUAN-92.
- Hunt K, Gray CM, Maclean A, Smillie S, Bunn C, Wyke S. Do weight management programmes delivered at professional football clubs attract and engage high risk men? A mixed-methods study. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-50.
- Wellbourn R, Small P, Finlay I, Sareela A, Somers S, Mahawar K. National Bariatric Surgery Registry of the British Obesity &Amp; Metabolic Surgery Society. Second Registry Report 2014. https://hscn.e-dendrite.com/csp/bariatric/cdb/BAR/upload/2nd%20NBSR%20Report%202014.pdf (accessed April 2018).
- Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541-51. https://doi.org/10.1016/S0140-6736(17)33102-1.
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA ACC TOS Guideline for the Management of Overweight and Obesity in Adults. A Report of the American College of CardiologyAmerican Heart Association Task Force on Practice Guidelines and The Obesity Society. 2014;129:5102-38.
- Madden AM, Mulrooney HM, Shah S. Estimation of energy expenditure using prediction equations in overweight and obese adults: a systematic review. J Hum Nutr Diet 2016;29:458-76. https://doi.org/10.1111/jhn.12355.
- Dawson JA, Kaiser KA, Affuso O, Cutter GR, Allison DB. Rigorous control conditions diminish treatment effects in weight loss-randomized controlled trials. Int J Obes 2016;40:895-8. https://doi.org/10.1038/ijo.2015.212.
- Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Behavioural Weight Management Review Group . Weight change among people randomized to minimal intervention control groups in weight loss trials. Obesity 2016;24:772-80. https://doi.org/10.1002/oby.21255.
- Waters L, George AS, Chey T, Bauman A. Weight change in control group participants in behavioural weight loss interventions: a systematic review and meta-regression study. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-120.
- Moore H, Summerbell CD, Greenwood DC, Tovey P, Griffiths J, Henderson M, et al. Improving management of obesity in primary care: cluster randomised trial. BMJ 2003;327. https://doi.org/10.1136/bmj.327.7423.1085.
- Affuso O, Kaiser KA, Carson TL, Ingram KH, Schwiers M, Robertson H, et al. Association of run-in periods with weight loss in obesity randomized controlled trials. Obes Rev 2014;15:68-73. https://doi.org/10.1111/obr.12111.
- Rankin J, Ross A, Baker J, O’Brien M, Scheckel C, Vassar M. Selective outcome reporting in obesity clinical trials: a cross-sectional review. Clin Obes 2017;7:245-54. https://doi.org/10.1111/cob.12199.
- Lemon SC, Wang ML, Haughton CF, Estabrook DP, Frisard CF, Pagoto SL. Methodological quality of behavioural weight loss studies: a systematic review. Obes Rev 2016;17:636-44. https://doi.org/10.1111/obr.12412.
- Ioannidis JP. Biases in obesity research: Identify, correct, endorse, or abandon effort?. Obesity 2016;24:767-8. https://doi.org/10.1002/oby.21457.
- George BJ, Beasley TM, Brown AW, Dawson J, Dimova R, Divers J, et al. Common scientific and statistical errors in obesity research. Obesity 2016;24:781-90. https://doi.org/10.1002/oby.21449.
- Paisey RB, Frost J, Harvey P, Paisey A, Bower L, Paisey RM, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002;15:121-7. https://doi.org/10.1046/j.1365-277X.2002.00342.x.
- Rolland C, Hession M, Murray S, Wise A, Broom I. Randomized clinical trial of standard dietary treatment versus a low-carbohydrate/high-protein diet or the LighterLife Programme in the management of obesity. J Diabetes 2009;1:207-17. https://doi.org/10.1111/j.1753-0407.2009.00033.x.
- Cartwright A. An Investigation of Weight Management Interventions for Extreme Obesity 2014.
- Molokhia M. Obesity wars: a pilot study of very low calorie diets in obese patients in general practice. Br J Gen Pract 1998;48:1251-2.
- Barrett P, Finer N, Fisher C, Boyle G. Evaluation of a multimodality treatment programme for weight management at the Luton and Dunstable Hospital NHS Trust. J Hum Nutr Diet 1999;12:43-52. https://doi.org/10.1046/j.1365-277X.1999.00006.x.
- Dhindsa P, Scott AR, Donnelly R. Metabolic and cardiovascular effects of very-low-calorie diet therapy in obese patients with Type 2 diabetes in secondary failure: outcomes after 1 year. Diabet Med 2003;20:319-24. https://doi.org/10.1046/j.1464-5491.2003.00937.x.
- Read A, Ramwell H, Storer H, Webber J. A primary care intervention programme for obesity and coronary heart disease risk factor reduction. Br J Gen Pract 2004;54:272-8.
- Packianathan I, Sheikh M, Boniface D, Finer N. Predictors of programme adherence and weight loss in women in an obesity programme using meal replacements. Diabetes Obes Metab 2005;7:439-47. https://doi.org/10.1111/j.1463-1326.2004.00451.x.
- Rowe R, Cowx M, Poole C, McEwan P, Morgan C, Walker M. The effects of orlistat in patients with diabetes: improvement in glycaemic control and weight loss. Curr Med Res Opin 2005;21:1885-90. https://doi.org/10.1185/030079905X74943.
- Jackson C, Coe A, Cheater FM, Wroe S. Specialist health visitor-led weight management intervention in primary care: exploratory evaluation. J Adv Nurs 2007;58:23-34. https://doi.org/10.1111/j.1365-2648.2007.04226.x.
- Counterweight Project Team . Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548-54. https://doi.org/10.3399/bjgp08X319710.
- Counterweight Project Team . The implementation of the Counterweight Programme in Scotland, UK. Fam Pract 2012;29:i139-44. https://doi.org/10.1093/fampra/cmr074.
- MacLaughlin HL, Sarafidis PA, Greenwood SA, Campbell KL, Hall WL, Macdougall IC. Compliance with a structured weight loss program is associated with reduced systolic blood pressure in obese patients with chronic kidney disease. Am J Hypertens 2012;25:1024-9. https://doi.org/10.1038/ajh.2012.80.
- Lean M, Brosnahan N, McLoone P, McCombie L, Higgs AB, Ross H, et al. Feasibility and indicative results from a 12-month low-energy liquid diet treatment and maintenance programme for severe obesity. Br J Gen Pract 2013;63:e115-24. https://doi.org/10.3399/bjgp13X663073.
- Jennings A, Hughes CA, Kumaravel B, Bachmann MO, Steel N, Capehorn M, et al. Evaluation of a multidisciplinary Tier 3 weight management service for adults with morbid obesity, or obesity and comorbidities, based in primary care. Clin Obes 2014;4:254-66. https://doi.org/10.1111/cob.12066.
- Logue J, Allardice G, Gillies M, Forde L, Morrison DS. Outcomes of a specialist weight management programme in the UK National Health Service: prospective study of 1838 patients. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003747.
- Wallace D, Myles P, Holt R, Nguyen Van-Tam J. Evaluation of the ‘Live Life Better Service’, a community-based weight management service, for morbidly obese patients. J Public Health 2016;38:e138-49. https://doi.org/10.1093/pubmed/fdv103.
- Rolland C, Johnston KL, Lula S, Macdonald I, Broom J. Long-term weight loss maintenance and management following a VLCD: a 3-year outcome. Int J Clin Pract 2014;68:379-87. https://doi.org/10.1111/ijcp.12300.
- Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JCG, Mander AP, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet 2017;389:2214-25. https://doi.org/10.1016/S0140-6736(17)30647-5.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d6500.
- Public Health England . Protecting and Improving the Nation’s Health. Standard Evaluation Framework for Weight Management Interventions 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/685545/SEF_weight_management_interventions.pdf (accessed April 2018).
- Logue J, Simpson SA, Ells LJ. Developing a Core Outcome Set for Lifestyle Weight Management Programmes by Expert Consensus 2017. www.comet-initiative.org/studies/details/1056?result=true (accessed April 2018).
- Abildso C, Zizzi S, Gilleland D, Thomas J, Bonner D. A mixed methods evaluation of a 12-week insurance-sponsored weight management program incorporating cognitive–behavioral counseling. J Mix Methods Res 2010;4:278-94. https://doi.org/10.1177/1558689810376949.
- Amy Janke E, Kozak AT. ‘The more pain I have, the more I want to eat’: obesity in the context of chronic pain. Obesity 2012;20:2027-34. https://doi.org/10.1038/oby.2012.39.
- Aschbrenner KA, Naslund JA, Bartels SJ. A mixed methods study of peer-to-peer support in a group-based lifestyle intervention for adults with serious mental illness. Psychiatr Rehabil J 2016;39:328-34. https://doi.org/10.1037/prj0000219.
- Asselin J, Osunlana AM, Ogunleye AA, Sharma AM, Campbell-Scherer D. Missing an opportunity: the embedded nature of weight management in primary care. Clin Obes 2015;5:325-32. https://doi.org/10.1111/cob.12115.
- Asselin J, Osunlana AM, Ogunleye AA, Sharma AM, Campbell-Scherer D. Challenges in interdisciplinary weight management in primary care: lessons learned from the 5As Team study. Clin Obes 2016;6:124-32. https://doi.org/10.1111/cob.12133.
- Barham K, West S, Trief P, Morrow C, Wade M, Weinstock RS. Diabetes prevention and control in the workplace: a pilot project for county employees. J Public Health Manag Pract 2011;17:233-41. https://doi.org/10.1097/PHH.0b013e3181fd4cf6.
- Bennett WL, Gudzune KA, Appel LJ, Clark JM. Insights from the POWER practice-based weight loss trial: a focus group study on the PCP’s role in weight management. J Gen Intern Med 2014;29:50-8. https://doi.org/10.1007/s11606-013-2562-6.
- Bradbury K, Dennison L, Little P, Yardley L. Using mixed methods to develop and evaluate an online weight management intervention. Br J Health Psychol 2015;20:45-5. https://doi.org/10.1111/bjhp.12125.
- McQuigg M, Brown JE, Broom JI, Laws RA, Reckless JP, Noble PA, et al. Engaging patients, clinicians and health funders in weight management: the Counterweight Programme. Fam Pract 2008;25:i79-86. https://doi.org/10.1093/fampra/cmn081.
- Dahl U, Rise MB, Kulseng B, Steinsbekk A. Personnel and participant experiences of a residential weight-loss program. A qualitative study. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0100226.
- Danielsen KK, Sundgot-Borgen J, Rugseth G. Severe obesity and the ambivalence of attending physical activity: exploring lived experiences. Qual Health Res 2016;26:685-96. https://doi.org/10.1177/1049732315596152.
- Groven KS, Engelsrud G. Dilemmas in the process of weight reduction: exploring how women experience training as a means of losing weight. Int J Qual Stud Health Well-Being 2010;5. https://doi.org/10.3402/qhw.v5i2.5125.
- Gudzune KA, Clark JM, Appel LJ, Bennett WL. Primary care providers’ communication with patients during weight counseling: a focus group study. Patient Educ Couns 2012;89:152-7. https://doi.org/10.1016/j.pec.2012.06.033.
- Jiménez-López JL, Maldonado-Guzmán ME, Flores-Pérez Pastén L, Déciga-García E. Reasons for losing weight: Why have programed support?. Rev Med Inst Mex Seguro Soc 2012;50:407-12.
- Kidd LI, Graor CH, Murrock CJ. A mindful eating group intervention for obese women: a mixed methods feasibility study. Arch Psychiatr Nurs 2013;27:211-18. https://doi.org/10.1016/j.apnu.2013.05.004.
- Owen-Smith A, Donovan J, Coast J. ‘Vicious circles’: the development of morbid obesity. Qual Health Res 2014;24:1212-20. https://doi.org/10.1177/1049732314544908.
- Owen-Smith A, Donovan J, Coast J. Experiences of accessing obesity surgery on the NHS: a qualitative study. J Public Health 2016;31. https://doi.org/10.1093/pubmed/fdv209.
- Isla Pera P, Ferrér MC, Nuñez Juarez M, Nuñez Juarez E, Maciá Soler L, López Matheu C, et al. Obesity, knee osteoarthritis, and polypathology: factors favoring weight loss in older people. Patient Prefer Adherence 2016;10:957-65. https://doi.org/10.2147/PPA.S92183.
- Shaw RJ, Bosworth HB, Silva SS, Lipkus IM, Davis LL, Sha RS, et al. Mobile health messages help sustain recent weight loss. Am J Med 2013;126:1002-9. https://doi.org/10.1016/j.amjmed.2013.07.001.
- Sturgiss E, Haesler E, Elmitt N, van Weel C, Douglas K. Increasing general practitioners’ confidence and self-efficacy in managing obesity: a mixed methods study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014314.
- Sturgiss EA, Douglas K. A collaborative process for developing a weight management toolkit for general practitioners in Australia-an intervention development study using the Knowledge To Action framework. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0060-4.
- Sturgiss EA, Elmitt N, Haesler E, van Weel C, Douglas K. Feasibility and acceptability of a physician-delivered weight management programme. Fam Pract 2017;34:43-8. https://doi.org/10.1093/fampra/cmw105.
- Turner D, Haboubi N. Qualitative and quantitative outcomes of a 1:1 multidisciplinary weight management clinic. Healthcare 2015;3:429-51. https://doi.org/10.3390/healthcare3020429.
- Yarborough BJ, Stumbo SP, Yarborough MT, Young TJ, Green CA. Improving lifestyle interventions for people with serious mental illnesses: qualitative results from the STRIDE study. Psychiatr Rehabil J 2016;39:33-41. https://doi.org/10.1037/prj0000151.
- Young AS, Cohen AN, Goldberg R, Hellemann G, Kreyenbuhl J, Niv N, et al. Improving weight in people with serious mental illness: the effectiveness of computerized services with peer coaches. J Gen Intern Med 2017;32:48-55. https://doi.org/10.1007/s11606-016-3963-0.
- Zizzi S, Kadushin P, Michel J, Abildso C. Client experiences with dietary, exercise, and behavioral services in a community-based weight management program. Health Promot Pract 2016;17:98-106. https://doi.org/10.1177/1524839915610316.
- Sutcliffe K, Richardson M, Rees R, Burchett H, Melendez-Torres GJ, Stansfield C, et al. What are the Critical Features of Successful Tier 2 Weight Management Programmes for Adults? A Systematic Review to Identify the Programme Characteristics, and Combinations of Characteristics, That Are Associated With Successful Weight Loss. London: EPPI-Centre, Social Science Research Unit, UCL Institute of Education, University College London; 2016.
- Dewhurst A, Peters S, Devereux-Fitzgerald A, Hart J. Physicians’ views and experiences of discussing weight management within routine clinical consultations: a thematic synthesis. Patient Educ Couns 2017;100:897-908. https://doi.org/10.1016/j.pec.2016.12.017.
- Aveyard P, Lewis A, Tearne S, Hood K, Christian-Brown A, Adab P, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. Lancet 2016;388:2492-500. https://doi.org/10.1016/S0140-6736(16)31893-1.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Anselmino M, Bammer T, Fernández Cebrián JM, Daoud F, Romagnoli G, Torres A. Cost-effectiveness and budget impact of obesity surgery in patients with type 2 diabetes in three European countries(II). Obes Surg 2009;19:1542-9. https://doi.org/10.1007/s11695-009-9946-z.
- Faria G, Preto J, Da Costa EL, Almeida AB, Maia JC, Guimaraes JT, et al. Gastric bypass is a cost-saving procedure: results from a comprehensive Markov model. Obes Surg 2013;22.
- Hollenbeak CS, Weinstock RS, Cibula D, Delahanty LM, Trief PM. Cost-effectiveness of SHINE: a telephone translation of the Diabetes Prevention Program. Health Serv Insights 2016;9:21-8. https://doi.org/10.4137/HSI.S39084.
- Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity 2014;22:1942-51. https://doi.org/10.1002/oby.20824.
- Krukowski RA, Tilford JM, Harvey-Berino J, West DS. Comparing behavioral weight loss modalities: incremental cost-effectiveness of an internet-based versus an in-person condition. Obesity 2011;19:1629-35. https://doi.org/10.1038/oby.2010.341.
- Lewis L, Taylor M, Broom J, Johnston KL. The cost-effectiveness of the LighterLife weight management programme as an intervention for obesity in England. Clin Obes 2014;4:180-8. https://doi.org/10.1111/cob.12060.
- Meads DM, Hulme CT, Hall P, Hill AJ. The cost-effectiveness of primary care referral to a UK commercial weight loss programme. Clin Obes 2014;4:324-32. https://doi.org/10.1111/cob.12077.
- Meenan RT, Stumbo SP, Yarborough MT, Leo MC, Yarborough BJ, Green CA. An economic evaluation of a weight loss intervention program for people with serious mental illnesses taking antipsychotic medications. Adm Policy Ment Health 2016;43:604-15. https://doi.org/10.1007/s10488-015-0669-2.
- Miners A, Harris J, Felix L, Murray E, Michie S, Edwards P. An economic evaluation of adaptive e-learning devices to promote weight loss via dietary change for people with obesity. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-190.
- Ritzwoller DP, Glasgow RE, Sukhanova AY, Bennett GG, Warner ET, Greaney ML, et al. Economic analyses of the Be Fit Be Well program: a weight loss program for community health centers. J Gen Intern Med 2013;28:1581-8. https://doi.org/10.1007/s11606-013-2492-3.
- Trueman P, Haynes SM, Felicity Lyons G, Louise McCombie E, McQuigg MS, Mongia S, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract 2010;64:775-83. https://doi.org/10.1111/j.1742-1241.2010.02349.x.
- Tsai AG, Glick HA, Shera D, Stern L, Samaha FF. Cost-effectiveness of a low-carbohydrate diet and a standard diet in severe obesity. Obes Res 2005;13:1834-40. https://doi.org/10.1038/oby.2005.223.
- Tsai AG, Wadden TA, Volger S, Sarwer DB, Vetter M, Kumanyika S, et al. Cost-effectiveness of a primary care intervention to treat obesity. Int J Obes 2013;37:31-7. https://doi.org/10.1038/ijo.2013.94.
- Wilson KJ, Brown HS, Bastida E. Cost-effectiveness of a community-based weight control intervention targeting a low-socioeconomic-status Mexican-origin population. Health Promot Pract 2015;16:101-8. https://doi.org/10.1177/1524839914537274.
- Hertzman P. The cost effectiveness of orlistat in a 1-year weight-management programme for treating overweight and obese patients in Sweden: a treatment responder approach. PharmacoEconomics 2005;23:1007-20. https://doi.org/10.2165/00019053-200523100-00004.
- Lacey LA, Wolf A, O’Shea D, Erny S, Ruof J. Cost-effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes 2005;29:975-82. https://doi.org/10.1038/sj.ijo.0802947.
- Veerman JL, Barendregt JJ, Forster M, Vos T. Cost-effectiveness of pharmacotherapy to reduce obesity. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0026051.
- Ackroyd R, Mouiel J, Chevallier JM, Daoud F. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg 2006;16:1488-503. https://doi.org/10.1381/096089206778870067.
- Borisenko O, Adam D, Funch-Jensen P, Ahmed AR, Zhang R, Colpan Z, et al. Bariatric surgery can lead to net cost savings to health care systems: results from a comprehensive European decision analytic model. Obes Surg 2015;25:1559-68. https://doi.org/10.1007/s11695-014-1567-5.
- Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care 2010;16:e174-87.
- Castilla I, Mar J, Valcárcel-Nazco C, Arrospide A, Ramos-Goñi JM. Cost-utility analysis of gastric bypass for severely obese patients in Spain. Obes Surg 2014;24:2061-8. https://doi.org/10.1007/s11695-014-1304-0.
- Chang SH, Stoll CR, Colditz GA. Cost-effectiveness of bariatric surgery: should it be universally available?. Maturitas 2011;69:230-8. https://doi.org/10.1016/j.maturitas.2011.04.007.
- Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A. The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6120.
- Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med 2002;113:491-8. https://doi.org/10.1016/S0002-9343(02)01266-4.
- Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care 2010;33:1933-9. https://doi.org/10.2337/dc10-0554.
- Ikramuddin S, Klingman D, Swan T, Minshall ME. Cost-effectiveness of Roux-en-Y gastric bypass in type 2 diabetes patients. Am J Manag Care 2009;15:607-15.
- James R, Salton RI, Byrnes JM, Scuffham PA. Cost-utility analysis for bariatric surgery compared with usual care for the treatment of obesity in Australia. Surg Obes Relat Dis 2017;13:2012-20. https://doi.org/10.1016/j.soard.2016.12.016.
- Jensen C, Flum DR. 2004 ABS Consensus Conference . The costs of nonsurgical and surgical weight loss interventions: is an ounce of prevention really worth a pound of cure?. Surg Obes Relat Dis 2005;1:353-7. https://doi.org/10.1016/j.soard.2005.03.215.
- Keating CL, Dixon JB, Moodie ML, Peeters A, Bulfone L, Maglianno DJ, et al. Cost-effectiveness of surgically induced weight loss for the management of type 2 diabetes: modeled lifetime analysis. Diabetes Care 2009;32:567-74. https://doi.org/10.2337/dc08-1749.
- Keating CL, Dixon JB, Moodie ML, Peeters A, Playfair J, O’Brien PE. Cost-efficacy of surgically induced weight loss for the management of type 2 diabetes: a randomized controlled trial. Diabetes Care 2009;32:580-4. https://doi.org/10.2337/dc08-1748.
- Klebanoff MJ, Corey KE, Chhatwal J, Kaplan LM, Chung RT, Hur C. Bariatric surgery for non-alcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology 2017;65:1156-64.
- Lee YY, Veerman JL, Barendregt JJ. The cost-effectiveness of laparoscopic adjustable gastric banding in the morbidly obese adult population of Australia. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0064965.
- Mäklin S, Malmivaara A, Linna M, Victorzon M, Koivukangas V, Sintonen H. Cost-utility of bariatric surgery for morbid obesity in Finland. Br J Surg 2011;98:1422-9. https://doi.org/10.1002/bjs.7640.
- McEwen LN, Coelho RB, Baumann LM, Bilik D, Nota-Kirby B, Herman WH. The cost, quality of life impact, and cost-utility of bariatric surgery in a managed care population. Obes Surg 2010;20:919-28. https://doi.org/10.1007/s11695-010-0169-0.
- McLawhorn AS, Southren D, Wang YC, Marx RG, Dodwell ER. Cost-effectiveness of bariatric surgery prior to total knee arthroplasty in the morbidly obese: a computer model-based evaluation. J Bone Joint Surg Am 2016;98. https://doi.org/10.2106/JBJS.N.00416.
- Michaud PC, Goldman DP, Lakdawalla DN, Zheng Y, Gailey AH. The value of medical and pharmaceutical interventions for reducing obesity. J Health Econ 2012;31:630-43. https://doi.org/10.1016/j.jhealeco.2012.04.006.
- Pollock RF, Muduma G, Valentine WJ. Evaluating the cost-effectiveness of laparoscopic adjustable gastric banding versus standard medical management in obese patients with type 2 diabetes in the UK. Diabetes Obes Metab 2013;15:121-9. https://doi.org/10.1111/j.1463-1326.2012.01692.x.
- Salem L, Devlin A, Sullivan SD, Flum DR. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis 2008;4:26-32. https://doi.org/10.1016/j.soard.2007.09.009.
- van Gemert WG, Adang EM, Kop M, Vos G, Greve JW, Soeters PB. A prospective cost-effectiveness analysis of vertical banded gastroplasty for the treatment of morbid obesity. Obes Surg 1999;9:484-91. https://doi.org/10.1381/096089299765552792.
- Wang BC, Wong ES, Alfonso-Cristancho R, He H, Flum DR, Arterburn DE, et al. Cost-effectiveness of bariatric surgical procedures for the treatment of severe obesity. Eur J Health Econ 2014;15:253-63. https://doi.org/10.1007/s10198-013-0472-5.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56-65. https://doi.org/10.1001/jama.2011.1914.
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-52. https://doi.org/10.1097/00000658-199509000-00011.
- Wyke S, Hunt K, Gray A, Fenwick E, Bunn C, Donnan PT, et al. Football Fans in Training (FFIT): a randomized controlled trial of a gender sensitised weight loss and healthy living programme delivered to men aged 35–65 by Scottish Premier League (SPL) football clubs. Public Health Res 2015;3. https://doi.org/10.3310/phr03020.
- National Institute for Health and Care Excellence (NICE) . Costing Report: Obesity. Implementing the NICE Guideline on Obesity (CG189) 2014. www.nice.org.uk/guidance/cg189/resources/costing-report-pdf-193304845 (accessed May 2018).
- BOMSS Guidelines on Perioperative and Postoperative Biochemical Monitoring and Micronutrient Replacement for Patients Undergoing Bariatric Surgery. London: BOMSS; 2014.
- Uppsala Clinical Research Center . SOReg Scandinavian Obesity Surgery Registry 2011. www.ucr.uu.se/soreg/ (accessed April 2018).
- McConnon A, Kirk SF, Cockroft JE, Harvey EL, Greenwood DC, Thomas JD, et al. The Internet for weight control in an obese sample: results of a randomised controlled trial. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-206.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367-78.e5. https://doi.org/10.1053/j.gastro.2015.04.005.
- Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis 2007;3:127-32. https://doi.org/10.1016/j.soard.2006.12.005.
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20:5-26. https://doi.org/10.1185/030079904X1980.
- O’Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 2006;144:625-33. https://doi.org/10.7326/0003-4819-144-9-200605020-00005.
- Campbell JA, Venn A, Neil A, Hensher M, Sharman M, Palmer AJ. Diverse approaches to the health economic evaluation of bariatric surgery: a comprehensive systematic review. Obes Rev 2016;17:850-94. https://doi.org/10.1111/obr.12424.
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34. https://doi.org/10.1111/joim.12012.
- Puzziferri N, Roshek TB, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA 2014;312:934-42. https://doi.org/10.1001/jama.2014.10706.
- Jakobsen GS, Småstuen MC, Sandbu R, Nordstrand N, Hofsø D, Lindberg M, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA 2018;319:291-30. https://doi.org/10.1001/jama.2017.21055.
- Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs. usual care obesity management with all-cause mortality. JAMA 2018;319:279-90. https://doi.org/10.1001/jama.2017.20513.
- Carloni R, Naudet F, Chaput B, de Runz A, Herlin C, Girard P, et al. Are there factors predictive of postoperative complications in circumferential contouring of the lower trunk? A meta-analysis. Aesthet Surg J 2016;36:1143-54. https://doi.org/10.1093/asj/sjw117.
- Highton L, Ekwobi C, Rose V. Post-bariatric surgery body contouring in the NHS: a survey of UK bariatric surgeons. J Plast Reconstr Aesthet Surg 2012;65:426-32. https://doi.org/10.1016/j.bjps.2011.09.047.
- Lucchese M, Borisenko O, Mantovani LG, Cortesi PA, Cesana G, Adam D, et al. Cost-utility analysis of bariatric surgery in Italy: results of decision-analytic modelling. Obes Facts 2017;10:261-72. https://doi.org/10.1159/000475842.
- Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab 2017;19:1223-32. https://doi.org/10.1111/dom.12922.
- Coulman KD, Hopkins J, Brookes ST, Chalmers K, Main B, Owen-Smith A, et al. A core outcome set for the benefits and adverse events of bariatric and metabolic surgery: the BARIACT project. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002187.
- Whitty JA, Ratcliffe J, Kendall E, Burton P, Wilson A, Littlejohns P, et al. Prioritising patients for bariatric surgery: building public preferences from a discrete choice experiment into public policy. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008919.
- McPherson K, March T, Brown M. Foresight. Tackling Obesities: Future Choices – Modelling Future Trends in Obesity &Amp; Their Impact on Health. 2007. www.gov.uk/government/uploads/system/uploads/attachment_data/file/295149/07-1662-obesity-modelling-trends.pdf (accessed March 2018).
- UK Health Forum . REBALANCE Technical Appendix. 2018. www.ukhealthforum.org.uk (accessed September 2018).
- Vemer P, Rutten-van Mölken MP. Largely ignored: the impact of the threshold value for a QALY on the importance of a transferability factor. Eur J Health Econ 2011;12:397-404. https://doi.org/10.1007/s10198-010-0253-3.
- UK Data Service . Health Survey for England 2014. https://discover.ukdataservice.ac.uk/series/?sn=2000021 (accessed April 2018).
- World Health Organization (WHO) . Obesity and Overweight – Fact Sheet 2018. www.who.int/mediacentre/factsheets/fs311/en/ (accessed April 2018).
- Body Mass Index – BMI. Copenhagen: WHO; 2018.
- Office for National Statistics . Cancer Registration Statistics, England, 2015 2017. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2015 (accessed April 2018).
- Office for National Statistics . Deaths Registered in England and Wales: 2016 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2016 (accessed May 2018).
- World Health Organization (WHO) . Health Statistics and Information Systems: DISMOD II 2014. www.who.int/healthinfo/global_burden_disease/tools_software/en/ (accessed April 2018).
- Office for National Statistics . Geographic Patterns of Cancer Survival in England: Adults Diagnosed 2011 to 2015 and Followed Up to 2016 2018. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/geographicpatternsofcancersurvivalinengland/adultsdiagnosed2011to2015andfollowedupto2016 (accessed April 2018).
- World Health Organization (WHO) . International Statistical Classification of Diseases and Related Health Problems 10th Revision 2016. http://apps.who.int/classifications/icd10/browse/2016/en (accessed May 2018).
- Estimated Relative Risks of Disease per Unit of BMI Above 22kg/m2. London: World Obesity Federation; 2010.
- Romanus D, Kindler HL, Archer L, Basch E, Niedzwiecki D, Weeks J, et al. Cancer and Leukemia Group B . Does health-related quality of life improve for advanced pancreatic cancer patients who respond to gemcitabine? Analysis of a randomized phase III trial of the cancer and leukemia group B (CALGB 80303). J Pain Symptom Manage 2012;43:205-17. https://doi.org/10.1016/j.jpainsymman.2011.09.001.
- Pierce JP, Thurmond L, Rosbrook B. Projecting international lung cancer mortality rates: first approximations with tobacco-consumption data. J Natl Cancer Inst Monographs 1992;12:45-9.
- Colangelo LA, Gapstur SM, Gann PH, Dyer AR. Cigarette smoking and colorectal carcinoma mortality in a cohort with long-term follow-up. Cancer 2004;100:288-93. https://doi.org/10.1002/cncr.11923.
- NICE Glossary. London: NICE; 2015.
- EQ-5D. Rotterdam: EuroQol; 2017.
- Laires PA, Ejzykowicz F, Hsu TY, Ambegaonkar B, Davies G. Cost-effectiveness of adding ezetimibe to atorvastatin vs switching to rosuvastatin therapy in Portugal. J Med Econ 2015;18:565-72. https://doi.org/10.3111/13696998.2015.1031794.
- Rivero-Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. https://doi.org/10.1177/0272989X09349961.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Conner-Spady BL, Marshall DA, Bohm E, Dunbar MJ, Loucks L, Al Khudairy A, et al. Reliability and validity of the EQ-5D-5L compared to the EQ-5D-3L in patients with osteoarthritis referred for hip and knee replacement. Qual Life Res 2015;24:1775-84. https://doi.org/10.1007/s11136-014-0910-6.
- Hall PS, Hamilton P, Hulme CT, Meads DM, Jones H, Newsham A, et al. Costs of cancer care for use in economic evaluation: a UK analysis of patient-level routine health system data. Br J Cancer 2015;112:948-56. https://doi.org/10.1038/bjc.2014.644.
- Agus AM, Kinnear H, O’Neill C, McDowell C, Crealey GE, Gavin A. Description and predictors of hospital costs of oesophageal cancer during the first year following diagnosis in Northern Ireland. Eur J Cancer Care 2013;22:450-8. https://doi.org/10.1111/ecc.12046.
- NHS 2012 13 Programme Budgeting Data 2014. www.networks.nhs.uk/nhs-networks/health-investment-network/news/2012-13-programme-budgeting-data-is-now-available (accessed May 2018).
- Laudicello M. Pancreatic Cancer UK Policy Briefing: Every Life Matters: the Real Cost of Pancreatic Cancer Diagnsoses via Emergency Admission. London: Pancreatic Cancer UK; 2011.
- Proposed National Standards and Service Specifications for Congenital Heart Disease Services: Financial Impact Analysis. NHS; 2015.
- Liu JL, Maniadakis N, Gray A, Rayner M. The economic burden of coronary heart disease in the UK. Heart 2002;88:597-603. https://doi.org/10.1136/heart.88.6.597.
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27-32. https://doi.org/10.1093/ageing/afn281.
- Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol 2012;96:345-9. https://doi.org/10.1136/bjo.2011.204040.
- Kanavos P, van den Aardweg S, Schurer W. Diabetes Expenditure, Burden of Disease and Management in 5 EU Countries 2012. www.lse.ac.uk/business-and-consultancy/consulting/consulting-reports/diabetes-expenditure-burden-of-disease-and-management-in-5-eu-countries?from_serp=1 (accessed April 2018).
- Brilleman SL, Purdy S, Salisbury C, Windmeijer F, Gravelle H, Hollinghurst S. Implications of comorbidity for primary care costs in the UK: a retrospective observational study. Br J Gen Pract 2013;63:e274-82. https://doi.org/10.3399/bjgp13X665242.
- Chen A, Gupte C, Akhtar K, Smith P, Cobb J. The global economic cost of osteoarthritis: how the UK compares. Arthritis 2012;2012. https://doi.org/10.1155/2012/698709.
- Pennington M, Gentry-Maharaj A, Karpinskyj C, Miners A, Taylor J, Manchanda R, et al. Long-term secondary care costs of endometrial cancer: a prospective cohort study nested within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0165539.
- CCEMG – EPPI-Centre Cost Converter 2016. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed April 2018).
- Campbell HE, Stokes EA, Bargo D, Logan RF, Mora A, Hodge R, et al. Costs and quality of life associated with acute upper gastrointestinal bleeding in the UK: cohort analysis of patients in a cluster randomised trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007230.
- Brazzelli M, Cruickshank M, Kilonzo M, Ahmed I, Stewart F, McNamee P, et al. Clinical effectiveness and cost-effectiveness of cholecystectomy compared with observation/conservative management for preventing recurrent symptoms and complications in adults presenting with uncomplicated symptomatic gallstones or cholecystitis: a systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18550.
- National Institute for Health and Care Excellence . Chronic Kidney Disease: Managing Anaemia NICE Guideline NG8 2015. www.nice.org.uk/guidance/ng8 (accessed May 2018).
- Canavan C, West J, Card T. Change in quality of life for patients with irritable bowel syndrome following referral to a gastroenterologist: a cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0139389.
- CCEMG-EPPI-Centre Cost Converter v.1.5 2016. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed April 2018).
- Curtis L. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit; 2016.
- Medicines Complete . British National Formulary 2016. www.medicinescomplete.com/mc/bnf/current/index.htm (accessed May 2018).
- NHS Reference Costs 2015–2016. London: Department of Health and Social Care; 2016.
- National Institute for Health and Care Excellence (NICE) . Costing Report: Obesity. Implementing the NICE Guideline on Obesity (CG189) 2014. www.nice.org.uk/guidance/cg189/resources/costing-report-pdf-193304845 (accessed May 2018).
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med 2007;147:41-50. https://doi.org/10.7326/0003-4819-147-1-200707030-00007.
- Johns D, Hartmann-Boyce J, Aveyard P, Onakpoya I, Jebb S, Phillips D, et al. Weight Regain after Behavioural Weight Management Programmes. Evidence Review in Support of Public Health Guideline PH53. London: NICE; 2013.
- State of Musculoskeletal Health. Chesterfield: Arthritis Research UK; 2017.
- Impact of Physical Activity and Diet on Health HC 845: House of Commons Oral Evidence. London: UK Parliament; 2015.
- Welbourn R, le Roux CW, Owen-Smith A, Wordsworth S, Blazeby JM. Why the NHS should do more bariatric surgery; how much should we do?. BMJ 2016;353. https://doi.org/10.1136/bmj.i1472.
- Hillier-Brown FC, Bambra CL, Cairns JM, Kasim A, Moore HJ, Summerbell CD. A systematic review of the effectiveness of individual, community and societal-level interventions at reducing socio-economic inequalities in obesity among adults. Int J Obes 2014;38:1483-90. https://doi.org/10.1038/ijo.2014.75.
- Yancy WS, Mayer SB, Coffman CJ, Smith VA, Kolotkin RL, Geiselman PJ, et al. Effect of allowing choice of diet on weight loss: a randomized trial. Ann Intern Med 2015;162:805-14. https://doi.org/10.7326/M14-2358.
- Brownley KA, Berkman ND, Peat CM, Lohr KN, Cullen KE, Bann CM, et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med 2016;165:409-20. https://doi.org/10.7326/M15-2455.
- Barnes RD, Ivezaj V. A systematic review of motivational interviewing for weight loss among adults in primary care. Obes Rev 2015;16:304-18. https://doi.org/10.1111/obr.12264.
- Carrière K, Khoury B, Günak MM, Knäuper B. Mindfulness-based interventions for weight loss: a systematic review and meta-analysis. Obes Rev 2018;19:164-77. https://doi.org/10.1111/obr.12623.
- Rogers JM, Ferrari M, Mosely K, Lang CP, Brennan L. Mindfulness-based interventions for adults who are overweight or obese: a meta-analysis of physical and psychological health outcomes. Obes Rev 2017;18:51-67. https://doi.org/10.1111/obr.12461.
- Ruffault A, Czernichow S, Hagger MS, Ferrand M, Erichot N, Carette C, et al. The effects of mindfulness training on weight-loss and health-related behaviours in adults with overweight and obesity: a systematic review and meta-analysis. Obes Res Clin Pract 2017;11:90-111. https://doi.org/10.1016/j.orcp.2016.09.002.
- Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int J Behav Nutr Phys Act 2017;14. https://doi.org/10.1186/s12966-017-0494-y.
- Hartmann-Boyce J, Nourse R, Boylan AM, Jebb SA, Aveyard P. Experiences of reframing during self-directed weight loss and weight loss maintenance: systematic review of qualitative studies. Appl Psychol Health Well Being 2018;10:309-29. https://doi.org/10.1111/aphw.12132.
- Borek AJ, Abraham C, Greaves CJ, Tarrant M. Group-based diet and physical activity weight-loss interventions: a systematic review and meta-analysis of randomised controlled trials. Appl Psychol Health Well Being 2018;10:62-86. https://doi.org/10.1111/aphw.12121.
- Melendez-Torres GJ, Sutcliffe K, Burchett HED, Rees R, Richardson M, Thomas J. Weight management programmes: re-analysis of a systematic review to identify pathways to effectiveness. Health Expect 2018;21:574-84. https://doi.org/10.1111/hex.12667.
- Sutcliffe K, Melendez-Torres GJ, Burchett HED, Richardson M, Rees R, Thomas J. The importance of service-users’ perspectives: a systematic review of qualitative evidence reveals overlooked critical features of weight management programmes. Health Expect 2018;21:563-73. https://doi.org/10.1111/hex.12657.
- Allan K, Hoddinott P, Avenell A. A qualitative study comparing commercial and health service weight loss groups, classes and clubs. J Hum Nutr Diet 2011;24:23-31. https://doi.org/10.1111/j.1365-277X.2010.01110.x.
- Hoddinott P. Feasibility Study of How Best to Engage Obese Men in Narrative Sms (Short Message System) and Incentive Interventions for Weight Loss, to Inform a Future Effectiveness and Cost-Effectiveness Trial 2016. www.journalslibrary.nihr.ac.uk/programmes/phr/1418509/#/ (accessed May 2018).
- Sorgente A, Pietrabissa G, Manzoni GM, Re F, Simpson S, Perona S, et al. Web-based interventions for weight loss or weight loss maintenance in overweight and obese people: a systematic review of systematic reviews. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.6972.
- Hutchesson MJ, Rollo ME, Krukowski R, Ells L, Harvey J, Morgan PJ, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev 2015;16:376-92. https://doi.org/10.1111/obr.12268.
- Job JR, Fjeldsoe BS, Eakin EG, Reeves MM. Effectiveness of extended contact interventions for weight management delivered via text messaging: a systematic review and meta-analysis. Obes Rev 2018;19:538-49. https://doi.org/10.1111/obr.12648.
- Liu F, Kong X, Cao J, Chen S, Li C, Huang J, et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol 2015;181:337-48. https://doi.org/10.1093/aje/kwu260.
- Raaijmakers LC, Pouwels S, Berghuis KA, Nienhuijs SW. Technology-based interventions in the treatment of overweight and obesity: a systematic review. Appetite 2015;95:138-51. https://doi.org/10.1016/j.appet.2015.07.008.
- Riaz S, Sykes C. Are smartphone health applications effective in modifying obesity and smoking behaviours? A systematic review. Health Technol 2015;5:73-81. https://doi.org/10.1007/s12553-015-0104-4.
- Sherrington A, Newham JJ, Bell R, Adamson A, McColl E, Araujo-Soares V. Systematic review and meta-analysis of internet-delivered interventions providing personalized feedback for weight loss in overweight and obese adults. Obes Rev 2016;17:541-51. https://doi.org/10.1111/obr.12396.
- Flores Mateo G, Granado-Font E, Ferré-Grau C, Montaña-Carreras X. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4836.
- Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. The effectiveness of pharmaceutical interventions for obesity: weight loss with orlistat and sibutramine in a United Kingdom population-based cohort. Br J Clin Pharmacol 2015;79:1020-7. https://doi.org/10.1111/bcp.12578.
- Schroll JB, Penninga EI, Gøtzsche PC. Assessment of adverse events in protocols, clinical study reports, and published papers of trials of orlistat: a document analysis. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002101.
- Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Gravallese EA, Erondu NE, et al. Attrition from randomized controlled trials of pharmacological weight loss agents: a systematic review and analysis. Obes Rev 2009;10:333-41. https://doi.org/10.1111/j.1467-789X.2009.00567.x.
- Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes 2017;7:273-89. https://doi.org/10.1111/cob.12203.
Glossary
- Body mass index
- Weight in kg/(height in m)2.
- DISease MODelling (DISMOD)-II
- A software tool that may be used to check the consistency of estimates of incidence, prevalence, duration and case fatality for diseases.
- Dominant
- A health economics term. When comparing tests or treatments, an option that is both more effective and costs less is said to be dominant compared with the alternative.
- EuroQol-5 Dimensions questionnaire
- A standardised instrument for measuring generic health status.
- Morbid obesity
- A body mass index of ≥ 40 kg/m2.
- Quality-adjusted life-year
- A measure of the state of health of a person or group in which the benefits, in terms of length of life, are adjusted to reflect the quality of life.
- Run
- The execution of an application.
- Run time
- The period during which a computer program is executing.
- Setup
- The act of making the program ready for execution.
- Severe obesity
- A body mass index of ≥ 35 kg/m2.
- Tab-delimited text file
- A file format used by the microsimulation in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
List of abbreviations
- ABBI
- acceptance-based behavioural intervention
- ASPIRE
- Aspiring for Lifelong Health
- BCT
- behaviour change technique
- BMI
- body mass index
- BOCF
- baseline observation carried forward
- BOMSS
- British Obesity & Metabolic Surgery Society
- BPD
- biliopancreatic diversion
- CBT
- cognitive–behavioural therapy
- CEA
- cost-effectiveness analysis
- CHD
- coronary heart disease
- CI
- confidence interval
- CUA
- cost–utility analysis
- CVD
- cardiovascular disease
- DALY
- disability-adjusted life-year
- DISMOD
- DISease MODelling
- DPP
- Diabetes Prevention Program
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- EuroQol-5 Dimensions
- FFIT
- Football Fans in Training
- GB
- gastric banding
- GBP
- gastric bypass
- GI
- gastrointestinal
- GP
- general practitioner
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HSE
- Health Survey for England
- HTA
- Health Technology Assessment
- ICD
- intensive conventional diet and exercise programme
- ICER
- incremental cost-effectiveness ratio
- ILI
- intensive lifestyle intervention
- IWQOL
- Impact of Weight on Quality of Life
- LAGB
- laparoscopic adjustable gastric banding
- LDL
- low-density lipoprotein
- LEARN
- lifestyle, exercise, attitudes, relationships, nutrition
- LITE
- Lifestyle Intervention Treatment Effectiveness
- NAFLD
- non-alcoholic fatty liver disease
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- ONS
- Office for National Statistics
- OR
- odds ratio
- OSA
- obstructive sleep apnoea
- POWER
- Pounds Off With Empowerment
- POWeR+
- Positive Online Weight Reduction
- POWeR+F
- Positive Online Weight Reduction – face to face
- POWeR+R
- Positive Online Weight Reduction – remote
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROGRESS
- Place of residence, Race/ethnicity/culture/language, Occupation, Gender/sex, Religion, Education, Socioeconomic status, Social capital
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REBALANCE
- REview of Behaviour And Lifestyle interventions for severe obesity: AN evidenCE synthesis
- RR
- relative risk
- RYGB
- Roux-en-Y gastric bypass
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SG
- sleeve gastrectomy
- SHINE
- Support, Health Information, Nutrition, and Exercise
- SOS
- Swedish Obese Subjects
- TIDieR
- Template for Intervention Description and Replication
- UKHF
- UK Health Forum
- VBG
- vertical banded gastroplasty
- VLCD
- very low-calorie diet
- WAP
- Weight Action Programme
- WMP
- weight-management programme
- WOMAC
- Western Ontario and McMaster Universities Osteoarthritis Index
