Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/151/07. The contractual start date was in June 2016. The draft report began editorial review in August 2017 and was accepted for publication in February 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Snowsill et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of the health problem
Lung cancer is malignant growth of cells in the lung. It typically affects older people1 who smoke or smoked in the past. Men are more likely than women to get lung cancer and are more likely to die from lung cancer. 1 Lung cancer is the leading cause of cancer death in men and women and is responsible for 22% of deaths from cancer. 1
Risk factors
Smoking
The main cause of lung cancer is smoking (mainly cigarettes). It is estimated that 85% of lung cancer is attributable to smoking. 2 Smoking cigars, pipes or waterpipes is also associated with lung cancer, as well as other health problems. 3,4
Smoking cessation leads to reduced risk of lung cancer, but the risk still remains higher than for those who have never smoked if there is a significant smoking history. 5 Generally, the risk of lung cancer increases with the number of cigarettes smoked and the duration of smoking, with the pack-year a commonly used measure for smoking history. One pack-year is the equivalent of smoking one pack (20 cigarettes) per day for 1 year (around 7300 cigarettes). Duration of smoking appears to be a more important factor than smoking intensity (the number of cigarettes per day), such that it is worse to smoke one pack per day for 40 years than two packs per day for 20 years. 6
Smoking rates have declined substantially and steadily over the previous 40 years, but there remains a significant proportion of adults who continue to smoke and who have quit smoking but remain at high risk of lung cancer. Since 2005, at least half of the people who were ever regular cigarette smokers have quit. 7
Cigarette smoking rates vary geographically within the UK. 8 Compared with other European Union (EU) countries in 2014, the UK had the second lowest rate of current smokers (17.3%), higher only than Sweden (16.7%). The UK also had a gender gap in the smoking rate of 3.1%, which was significantly below the EU average (9.2%). 9
Smoking is also strongly correlated with income and socioeconomic status (SES). Individuals with a gross personal income of < £20,000 are twice as likely to be current smokers compared with individuals with a gross personal income of ≥ £40,000 (22% vs. 11%). Employment is also significant: 36% of unemployed adults smoke compared with 20% of employed adults, and 15% of economically inactive adults (e.g. pensioners and students). 7
Efforts in the UK to reduce smoking include direct taxation of smoking (tobacco duty),10 smoking cessation services and campaigns (e.g. Smokefree,11 Stoptober12), standardised (plain) packaging (compulsory from May 2017)13 and restrictions on advertising. 14
Passive smoking
Passive smoking is when a person does not smoke themselves, but spends time in an environment where smoking occurs. Passive smoking causes lung cancer and other diseases, including respiratory infections and asthma. For example, women who never smoked but were exposed to second-hand smoke from their spouse face a 27% higher risk of lung cancer than similar women not exposed to second-hand spousal smoke. 15
Environmental risk factors
In addition to smoking, there are other environmental risk factors that, following exposure, have demonstrated an increased risk of lung cancer. These risk factors include asbestos, radon gas and air pollution. When these environmental risk factors are combined with smoking, the overall risk of lung cancer is exacerbated. 16
Pathology
Lung cancer is the term for a malignant neoplasm originating in the lung, typically developing from cells of the respiratory epithelium. 17
Lung cancer cell type
Lung cancer can be divided into two broad categories: (1) small cell lung cancer (SCLC) and (2) non-small cell lung cancer (NSCLC). SCLC is a highly malignant tumour accounting for 11% of all lung cancer cases in the UK, and is derived from cells exhibiting neuroendocrine characteristics. 18 NSCLC accounts for 88% of all lung cancer cases in the UK. NSCLC can further be divided into three major pathologic subtypes: (1) adenocarcinoma, (2) squamous cell carcinoma and (3) large cell carcinoma. Statistics from the National Lung Cancer Audit Annual Report 201618 report the distribution of NSCLC in the UK as follows: adenocarcinoma 36%, squamous 22%, other 11% and no pathology 31%. 18
Neuroendocrine tumours account for around one-quarter of primary lung neoplasms, including SCLC (20%), large cell neuroendocrine carcinoma (LCNEC) (a subtype of large cell carcinoma; 3%) and carcinoid tumours (typical and atypical carcinoid; 2%). 19 Lung neuroendocrine tumours arise from cells of the diffuse neuroendocrine system in the bronchial mucosa. 20
The World Health Organization (WHO) classification of lung tumours21 now recommends (2015 classification) greater restriction on the diagnosis of large cell carcinoma (former subtypes now reclassified), reclassification of adenocarcinoma subtypes, reclassification of squamous cell carcinoma subtypes and a number of smaller changes. 21
Grade
Grading of lung cancer for NSCLC distinguishes lung cancer cells into groups based on the cell’s appearance:22
-
Grade 1 – cells look normal, will grow slowly and are less likely to spread (may also be called ‘low grade’ or ‘well differentiated’).
-
Grade 2 – cells look abnormal and are more likely to spread (may also be called ‘moderate grade’ or ‘moderately well differentiated’).
-
Grades 3 and 4 – cells look very abnormal, will grow quickly and are more likely to spread (may also be called ‘high grade’ or ‘poorly differentiated’).
Neuroendocrine tumours are also graded:19
-
low grade (typical carcinoid)
-
intermediate grade (atypical carcinoid)
-
high grade (SCLC and LCNEC).
Stage
The stage of the lung cancer relates to the extent of the cancer and is currently classified by the NHS in the UK according to the international tumour, node, metastasis (TNM) system (Seventh Edition). 23,24 The Eighth Edition of the TNM system has been recently published25 and is due to be adopted in the UK. There are three components that are combined to make up the overall staging of lung cancer: (1) the size of the tumour (diameter in greatest dimension; TX, T0–T4), (2) whether or not it has spread to the lymph nodes (NX, N0–N3) and (3) whether or not it has distant metastasis (MX, M0, M1). 26 In the UK, the following percentages of cases recorded postoperatively by stage were reported by the National Lung Cancer Audit Annual Report 2016:18
-
stage IA, 11%
-
stage IB, 7%
-
stage IIA, 4%
-
stage IIB, 4%
-
stage IIIA, 12%
-
stage IIIB, 9%
-
stage IV, 53%.
Note that, at present, there is no national lung cancer screening programme, so the vast majority of these cases are either clinically presenting or incidental findings.
Diagnosis
Presentation
Diagnosis of lung cancer generally follows symptomatic presentation of (typically late-stage) lung cancer, but it can also be an incidental finding during other investigations.
Of the 73,063 lung cancer cases diagnosed in England (2012–13), 25,668 presented as an emergency, 20,420 were referred by a general practitioner (GP) through the 2-week wait pathway and 15,525 were otherwise referred by a GP. 27 The remaining cases presented through some other route or were identified only through a death certificate. Stages I–III were most often diagnosed following GP referral, whereas stage IV and unstaged cancers were most often diagnosed following emergency presentation. 27
The National Institute for Health and Care Excellence (NICE) guidelines on suspected cancer referral28 identify a number of key symptoms and findings that should prompt an urgent referral or chest X-ray (CXR), which include (not an exhaustive list):
-
unexplained haemoptysis (coughing blood or blood-stained mucus)
-
respiratory symptoms (cough, shortness of breath)
-
chest symptoms and signs (chest pain, persistent or recurring chest infection)
-
general symptoms and signs (fatigue, weight loss, appetite loss)
-
finger clubbing.
In addition, there are decision aids for GPs that can help to identify the significance of combinations of symptoms. 29
Investigation
The 2011 NICE guidelines on lung cancer diagnosis and management30 recommend an investigative pathway for suspected lung cancer that includes contrast-enhanced chest computed tomography (CT) to further the diagnosis and contribute to staging the disease. Positron emission tomography–computerised tomography (PET-CT) is recommended for patients who are potentially suitable for treatment with curative intent. Other investigations are recommended according to the location and spread of the disease; many of these are invasive and involve endoscopy (including bronchoscopy) and biopsy.
Prognosis
Overall, the prognosis for long-term survival with lung cancer is poor. Net survival for adults (aged 15–99 years) in England and Wales in 2010–11 was predicted as follows: 1-year survival, 32.1%; 5-year survival, 9.5%; and 10-year survival, 4.9%. 31 The 10-year survival for lung cancer ranks second lowest out of 20 common cancers in England and Wales. 31 However, prognosis is variable depending on a multitude of factors including:
-
type of lung cancer – survival is lower for those with SCLC than for those with NSCLC32
-
age – the prognosis for long-term survival with lung-cancer deteriorates with age32
-
stage – the higher the stage, the poorer the prognosis (e.g. the 1-year survival rate is > 80% when lung cancer is diagnosed in stage I, but < 20% when diagnosed in stage IV32)
-
sex – the prognosis for women is better than for men32
-
route of diagnosis – those presenting through the 2-week wait referral route have a better prognosis than those with emergency presentation33
-
geographical location. 34
Epidemiology
Incidence
Lung cancer is the most common cancer in the world, with 1.8 million new cases diagnosed in 2012. 35
In the UK in 2014, 46,400 new cases of lung cancer were diagnosed (130 new cases per day on average). Lung cancer is the third most common cancer in the UK and, in 2014, it accounted for 13% of all new cases of cancer in the UK. The directly age-standardised rates of lung cancer incidence in England (2014) were 91.6 and 65.2 per 100,000 person-years for men and women, respectively, but the incidence rate climbs with age. 1
The incidence of lung cancer has declined for men compared with historical levels, but has climbed for women. 1 Men continue to be at a higher risk of lung cancer than women. Across the UK, the age-standardised rate of lung cancer decreased from 2012 to 2014, to approximately 2006 levels.
Mortality
Lung cancer is the most common cause of deaths from cancer in the EU (20.8% of all cancer-related deaths). 36 Lung cancer accounted for 5.4% of the total number of deaths in the EU (2013), equating to more than one-quarter of a million people (268,744 people) or 55.2 deaths per 100,000 inhabitants. 36
There were 35,895 lung cancer deaths in the UK in 2014,37–39 accounting for 22% of all cancer deaths; lung cancer is the most common cause of cancer death. Crude mortality rates indicate that there are 62 lung cancer deaths for every 100,000 males and 50 for every 100,000 females. 37–40 In England in 2014, 15,856 men and 12,993 women died from lung cancer (19,563 and 16,332, respectively, for the UK). 37–39
Prevalence
The prevalence of lung cancer (i.e. the proportion of people previously diagnosed with lung cancer who are still alive at a given time) is relatively low because survival is generally poor. Worldwide, the overall ratio of mortality to incidence is 0.87, because of the high fatality rate associated with lung cancer.
In 2006, it was estimated that 38,141 people were alive in the UK who had been diagnosed with lung cancer within the previous 10 years, 15,802 of whom had been diagnosed within the previous year. 41
Impact of health problem
Significance for lung cancer patients
The prognosis for those diagnosed with lung cancer is disappointing despite recent advances in oncology and surgery. This is because lung cancer is typically diagnosed when the cancer is in the later stages, and in older people, who often have concomitant diseases that subsequently limit therapeutic options.
For individuals with NSCLC, the primary symptoms (or most frequent) are appetite loss (98%), fatigue (98%), shortness of breath (94%), cough (93%), pain (90%) and blood in sputum (70%). From the literature, it appears that fatigue is the primary symptom that has an impact on daily living for those with lung cancer, followed by pain (chest pain/pain swallowing). 42 Quality-of-life (QoL) scores deteriorate as the number of chemotherapy cycles increases (i.e. the longer the treatment lasts, the lower the QoL). 42 Being treated for lung cancer also has a detrimental impact on an individual’s social life, family life and ability to work. 42
Significance for the NHS
Luengo-Fernandez et al. 43 estimated that lung cancer had the highest economic cost of any cancer in the UK, but health-care costs are greater for colorectal cancer and breast cancer (lung cancer leads to significantly more productivity losses).
Measurement of disease
Treatment for lung cancer depends on where the cancer is within the lung, the tumour size, whether or not, and how far, it has spread, and the general health and fitness of the individual presenting. The main treatment options are chemotherapy, radiotherapy, surgery, chemoradiotherapy, control of symptoms and palliative care. These treatments may be offered in combination or in sequence. Typically, if the stage is low (I or II) and a patient is fit for surgery, this will generally be the treatment option. This may be followed by chemotherapy and radiotherapy depending on whether or not the patient has lymph node metastases. The intent will usually be curative. If the stage is high (III or IV), the intent of further treatment is typically palliative although it may be, in some cases, long-term survival.
Whether or not treatment is effective is measured by the following means:
-
Long-term survival (particularly for early-stage lung cancer in which treatment is with curative intent).
-
The size of the tumour (diameter, volume). With the intention of slowing progression of growth of the tumour, reducing it in size or removing it entirely. Typically, the RECIST 1.1 treatment response criteria44 can be used to assess this outcome.
-
Health-related quality of life (HRQoL). This can be measured using a variety of condition-specific tools including –
-
European Organisation for Research and Treatment of Cancer (EORTC) QLQ (Quality of Life Questionnaire)-C3045 (a 30-item patient questionnaire for use in cancer trials, including multi-item scales such as physical and role function, a global HRQoL scale and a number of single items) and QLQ-LC1346 (a 13-item patient module primarily consisting of symptoms of lung cancer and lung cancer treatments)
-
Lung Cancer Symptom Scale (LCSS)47 [a nine-item patient questionnaire using visual analogue scales (VASs), focusing particularly on physical and functional QoL]
-
Functional Assessment of Cancer Therapy – General (FACT-G)48 (a 27-item patient questionnaire for patients receiving cancer therapy covering physical, social/family, emotional and functional well-being) and Functional Assessment of Cancer Therapy – Lung (FACT-L)49 (a nine-item patient module primarily consisting of symptoms of lung cancer and lung cancer treatments).
-
In addition, generic, preference-based measures were used to measure treatment effectiveness, such as the EuroQoL 5-Dimensions (EQ-5D).
Current service provision
Management of disease
The normal clinical pathway in the absence of screening is that people will present with symptoms such as persistent cough, haemoptysis or persistent breathlessness that will then be investigated with CXR, CT, bronchoscopy and biopsy. The investigations not only potentially confirm lung cancer, but are also used for staging. Occasionally individuals will have a CXR or CT scan for another clinical investigation in which lung cancer is noted as an unexpected finding without any symptoms.
The National Lung Cancer Audit Annual Report 201618 reported that, in the UK, the following treatments were given for 20,323 males and 17,946 females: any anticancer treatment in 60% of males and 60% of females, surgery in 15% of males and 18% of females, chemotherapy in 32% of males and 31% of females and radiotherapy in 35% of males and 32% of females.
Current service cost
In 2009, it was estimated that cancer costs the EU €126B, of which €18.8B (or 15%) was specifically for lung cancer. 43 The total costs for lung cancer in the EU were made up from €4.23B for health-care costs, €9.92B from mortality losses, €8M from morbidity losses and €3.82B in informal care costs. 43 The same study reported that the proportion of health-care costs in 2009 for the EU were 12% on medicine, 68% on inpatient care, 1% on accident and emergency services, 13% on outpatient care and 6% on primary care. 43
Costs are generally concentrated around the time of diagnosis (when treatment is likely to be initiated) and the time of death (when significant palliative care and medical management costs accrue). The following estimates were obtained for the health economic model, and details of sources are given in Chapter 6, Resources and costs:
-
Costs in the first year following diagnosis range from approximately £8000 to £13,000 (depending on the stage).
-
Costs in subsequent years range from around £1000 to £1600.
-
The estimated cost at the end of life is approximately £4600.
Variation in services and/or uncertainty about best practice
It is evident that there is a variation in service across the UK. The National Lung Cancer Audit Annual Report 201618 reports the following ranges for different treatments: percentage of people who received any anticancer treatment ranged from 54% on the south-east coast to 64% in the London Cancer Alliance, surgery ranged from 13% on the south-east coast to 21% in the London Cancer Alliance, chemotherapy for NSCLC ranged from 58% in Cheshire and Merseyside to 78% in the London Cancer Alliance, and chemotherapy for SCLC ranged from 57% on the south-east coast to 78% in Wessex.
Relevant national guidelines, including National Service Frameworks
There are many potential relevant guidelines for the diagnosis, treatment and management of lung cancer. NICE has produced a significant amount of guidance through various programmes. The British Thoracic Society has also produced two relevant guidelines. 50,51
Description of technology under assessment
Over several decades, a number of potential screening tests for lung cancer have been investigated, including CXRs and sputum cytology. Neither of these has been found to be effective in randomised controlled trials (RCTs). As CT scanning has developed and offered progressively improved images at lower radiation dosage, so it has become the test offering the greatest potential for clinically effective and cost-effective screening for lung cancer, with much research devoted to investigating whether or not this is the case. 52
Summary of intervention
Computed tomography scanning makes use of computer-processed combinations of many X-ray images taken from different angles to produce cross-sectional (tomographic) images (virtual ‘slices’) of specific areas of a scanned object, allowing the user to see inside without cutting. CT scanning has developed in a number of ways, notably the number of detectors, the speed with which data can be acquired and the sophistication of the computer reconstruction techniques. The amount of radiation required to provide an acceptable image for initial diagnostic purposes has also reduced, so that a low-dose computed tomography (LDCT) scan requires an effective radiation dose of ≤ 1.6 mSv. In the UK, the average annual exposure, including background and medical applications, is about 2.7 mSv of radiation per year. 53 Training and quality control are critical in achieving high-quality images while minimising X-ray exposure.
Lung CT scans detect discrete pulmonary nodules as the most common abnormality that may be suggestive of malignancy, but abnormal scarring and ground glass opacities may also be seen as worrying features and potentially recognised as malignant changes. Nodules suspicious of malignancy are often referred to as non-calcified nodules, but calcification is not a guarantee that the nodule is not cancerous. Size is also important in determining the likelihood that a non-calcified nodule is malignant, and large lesions are more likely to be malignant than small ones. 54
An important issue is that LDCT screening for lung cancer is not a homogenous technology, so careful attention needs to be paid to the exact nature of the device being used, the protocol being used and precise criteria being employed to define an abnormality as potentially malignant, benign or indeterminate. In a screening programme, this needs to take into account the possibility that screening scans may be repeated and stability of abnormalities over time may be part of the criteria indicating a possible cancer. The further management of each category, particularly further investigation, also needs to be specified as part of the definition of the technology.
A major challenge in all screening is that virtually no test is completely accurate. As a consequence, the benefits flowing from earlier identification and treatment of disease in some individuals will need to be offset by the likelihood that there will be some who may be falsely reassured by false-negative results and a number found to be false positives who will require further investigations and possibly experience additional anxiety relative to the situation in which no screening takes place. The problem of false positives is frequently magnified in screening because the incidence of the cancer being detected is often still low in the screened population, so apparently accurate tests, particularly in terms of their specificity, generate large absolute numbers of false positives.
Some recent lung cancer screening trials [the UK Lung Cancer Screening Trial (UKLS)55 and the Dutch–Belgian Lung Cancer Screening trial, NEderlands Leuvens Longkanker Screenings ONderzoek (NELSON)56,57] have discriminated between positive and indeterminate findings, whereby positive findings require follow-up with technology other than LDCT, while indeterminate findings can be managed purely by LDCT follow-up. From an intention-to-diagnose perspective (for calculating measures of diagnostic accuracy), these are both counted as positive findings (and false positive if lung cancer is not actually present), although it is likely that management purely by LDCT follow-up will be less invasive and lead to less radiation exposure, [although other harms (e.g. anxiety) may not be lessened by an indeterminate classification].
Identification of important subgroups
The following subgroups present with variable risk profiles associated with the incidence of lung cancer:
-
smoking history
-
age
-
exposure to asbestos
-
history of respiratory disease
-
chronic obstructive pulmonary disease (COPD) link
-
socioeconomic group/income
-
urban or rural living.
Consideration as to how these subgroup risk profiles affect lung cancer risk will need to be considered for any screening programme.
Current usage in the NHS
In the UK, population-level lung cancer screening is currently not implemented by the NHS. This was based on the findings of the UK National Screening Committee (NSC) in July 2006 when they last assessed whether or not lung cancer screening should be recommended for adult cigarette smokers. They concluded that it should not be recommended but should be reviewed in 2015/16, which prompted the commissioning of this review.
Some screening pilots have gone ahead at local levels (without control arms), such as the Macmillan Cancer Improvement Partnership pilot in Manchester. 58
Anticipated costs associated with intervention
If a lung cancer screening programme is to be implemented, there are a multitude of costs that will need to be considered. These can be broadly categorised in accordance with the phase of implementation (setup, running, evaluation) and dependency on volume (fixed and variable costs). It is also likely that implementation would proceed initially with pilots before rollout, with pilots having their own evaluation costs. Furthermore, there would likely be societal costs and benefits of a screening programme, and impacts on other areas of government spending, though these would often not be considered by NHS policy-makers, such as NICE.
If lung cancer screening significantly has an impact on smoking behaviour, or if it is implemented with attached smoking cessation interventions, there could be significant impacts on the NHS, the government and society more widely.
Challenges of screening programmes
Implementation
When implementing a screening programme, of any nature, there will always be challenges in executing its delivery effectively. Predominantly all of these challenges fall around communication. First, how to identify and communicate with the target population so that they are eligible for the screening programme. Identification could be through GP records, questionnaires or self-referral, and they all come with their own limitations. For example, how accurate the GP records are, how honest will individuals be at responding to a questionnaire, would self-referral predominantly identify only the worried-well. Once the target population has been identified, the response to invitation needs to be considered. Again, will it disproportionately be the worried-well who respond and attend screening. Whether SES or income factors affect attendance and also whether or not the invitation itself causes a detrimental increase in anxiety for the individual invited need consideration. Communication regarding making and rescheduling appointments along with follow-up of missed appointments will all require careful administration along with the communication of results and any follow-up appointments required.
Value
Understanding the added value that a screening programme can offer will also be a challenge. Ensuring that public attitudes are acceptable and amenable to the implementation and value of the screening programme will be paramount. Also of importance will be ensuring that the value of the screening programme is understood by those selected for participation (individuals to be screened) and placating concerns surrounding whether or not accepting screening has an impact on health insurance. Beyond the added value of reducing costs of treating lung cancer early and improving outcomes for an individual, a screening programme can furthermore benefit individuals by prompting/motivating smoking cessation, give individuals with a lung cancer diagnosis more time with their loved ones and also give them time to put their affairs in order.
Assessment
Finally, when implementing a screening programme, there will be challenges in assessing whether or not the screening programme is clinically effective. Primarily these challenges hinge on the lack of an appropriate gold standard to compare against, particularly for negative results but also accounting for overdiagnosis, lead-time bias, length bias and other forms of bias such as differential efforts to achieve smoking cessation.
Chapter 2 Definition of the decision problem
The purpose of this work was to provide the NSC with the most up-to-date clinical effectiveness and cost-effectiveness evidence for the screening of lung cancer in the UK by LDCT.
Decision problem
Population
People identified as being at ‘high’ risk of lung cancer.
Intervention
Low-dose CT screening.
Comparators
No screening was set by the scope as the primary comparator. We have also included alternative screening programmes (e.g. CXR) for comparative purposes.
Outcomes
From the scope, the outcomes suggested were potential effect on mortality, QoL and cost-effectiveness. Additional outcomes that were deemed relevant following consultation with our advisory committee included lung cancer incidence, stage and morphology of lung cancer, follow-up investigations and treatments, smoking cessation, adherence to screening, diagnostic accuracy, radiation dose of screening and adverse psychological impacts.
Overall aims and objectives of assessment
In order to assess the clinical effectiveness and cost-effectiveness of lung cancer screening in a high-risk population using LDCT screening, the following key objectives were performed:
-
a systematic review of the clinical effectiveness
-
a systematic review of the cost-effectiveness
-
a de novo cost-effectiveness model.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
Identification of studies
The literature search aimed to systematically identify studies relating to the clinical effectiveness and cost-effectiveness of LDCT screening programmes in high-risk populations. For the identification of studies for clinical effectiveness, the following bibliographic databases were searched:
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
Health Management Information Consortium (via Ovid)
-
PsycINFO (via Ovid)
-
Web of Science (via Clarivate Analytics)
-
Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) and Health Technology Assessment (HTA) (all via The Cochrane Library)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost).
The search strategies were developed by an information specialist (SR) and were carried out in two stages. The first searches, from 2004 to January 2012, were intended to update the HTA by the Aberdeen HTA Group in 200654 and to supplement the Cochrane systematic review of 2013. 52 These comprised population terms for lung cancer and intervention terms for LDCT screening. Filters for diagnostic studies were not used to limit the study designs retrieved as these have not been found to be effective. Search results were limited to RCTs and English-language studies and were run in January 2017.
The second searches, from 2012 to January 2017, were more comprehensive in scope. These searches comprised population terms for lung cancer and intervention terms for CT screening (not restricted to low dose) and for CXR as a comparator. Searches were limited to RCTs and English-language studies. The search results were exported to EndNote X7 [Clarivate Analytics (formerly) Thomson Reuters, Philadelphia, PA, USA] and deduplicated using automatic and manual checking.
Systematic reviews identified by further bibliographic database searches were used to source other relevant studies. Items included after full-text screening were backward-citation chased using Scopus (via Elsevier) in order to identify additional relevant studies. A search for ongoing clinical trials was carried out in February 2017 in clinicaltrials.gov, the WHO registry, the EU clinical trials registry and the International Standard Randomised Controlled Trial Number (ISRCTN).
Reference lists of relevant systematic reviews were checked. We defined systematic reviews as those reviews in which systematic and reproducible search strategies were used, a well-defined research question (with clear inclusion and exclusion criteria) was addressed and either a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram or a sufficient description of the flow of studies that allows the construction of the flow diagram was included. A full search strategy for each database can be found in Appendix 1.
Inclusion and exclusion criteria
Inclusion criteria
Population
The eligible population was individuals at high risk of lung cancer. Any definitions of high-risk populations were eligible in order to facilitate exploration of risk as a particular feature by which clinical effectiveness and cost-effectiveness might vary.
Intervention(s)
Low-dose CT screening programmes, including both single and multiple rounds, were eligible for inclusion. We carefully investigated variations in the screening programme, not only in the techniques used to do the initial screen, but also the criteria used to define positive tests and how positive and indeterminate tests (when applicable) were followed up.
Comparator(s)
The eligible comparators were usual care (no screening) or other imaging technology screening programmes (such as CXR), including both single and multiple screening rounds.
Study design
The eligible study design was RCTs. The following types of report were excluded: editorials and opinions, case reports and reports focusing on only technical aspects of the CT technology (such as technical descriptions of the CT technology).
Outcomes
The following outcomes were included:
-
lung cancer mortality
-
all-cause mortality
-
stage distributions of lung cancers
-
number of lung cancers detected
-
number and type of follow-up investigations
-
number of patients who were more amenable to surgical treatment
-
surgical resection rate
-
any HRQoL
-
smoking cessation and patients’ smoking behaviour change
-
adherence rate to screening
-
diagnostic accuracy outcomes (including indeterminate results)
-
overdiagnosis
-
complications in those who underwent an invasive procedure
-
radiation dose of screening
-
radiation-related patient outcomes
-
adverse psychological impact.
Exclusion criteria
Studies were excluded if they did not match the inclusion criteria. In addition, certain studies were not considered, particularly:
-
animal models
-
preclinical and biological studies
-
non-systematic reviews, editorials, opinions
-
non-English language papers
-
reports published as meeting abstracts only, as there is unlikely to be sufficient methodological details to allow critical appraisal of study quality.
At least two reviewers independently screened the titles and abstracts (if available) of all reports identified by the search strategy. Full-text copies of all studies deemed to be potentially relevant were obtained and two reviewers independently assessed them for inclusion. Any disagreements were resolved by consensus or by a third reviewer.
Data abstraction strategy
We selected the most recent or most complete report in cases of multiple reports for a given study or when the possibility of overlapping populations could not be excluded.
The data extraction forms were developed and piloted. One reviewer independently extracted details from full-text studies of study design, participants, intervention, comparator and outcome data. The data extraction was checked by another reviewer. Any disagreements were resolved by consensus or by a third reviewer.
For studies reporting clinical outcomes, we extracted data on these as numbers of patients experiencing the specified outcome. Mean differences, relative risks (RRs) or odds ratios (ORs) [with 95% confidence intervals (CIs)] were extracted, when reported.
Critical appraisal strategy
One reviewer independently assessed the quality of all included studies using the Cochrane Risk of Bias tool. Other risks of bias include the following two items: (1) underpowered sample size for important outcomes and (2) significant baseline differences between study arms on important characteristics. The quality assessment was checked by another reviewer. Any disagreements were resolved by consensus or by a third reviewer if necessary.
Methods of data synthesis
All data were tabulated and primarily considered in a narrative review. When appropriate, DerSimonian and Laird random-effects models59 were performed to pool the estimates of effect size of clinical effectiveness data from included trials. A random-effects approach was prespecified as part of the protocol development process; a fixed approach was not favoured as it was thought highly unlikely that only random variation would account for differences between the results of included studies.
Statistical heterogeneity was assessed using the I2-statistic: 30–50% was considered as moderate heterogeneity and > 50% as substantial heterogeneity. We performed only statistical pooling of data for both lung cancer mortality and all-cause mortality with ≥ 5 years’ follow-up. The statistical analyses were performed in Stata® 14 (StataCorp LP, College Station, TX, USA).
We closely took into account any heterogeneity observed between studies. Particularly, we considered the following factors for the exploration of heterogeneity:
-
quality of trials (focusing on adequacy of randomisation to define the criteria)
-
characteristics of populations (e.g. different level of risk status of participants at baseline)
-
nature of interventions (e.g. frequency of LDCT screening)
-
characteristics of control groups (such as CXR screening or usual care).
Sensitivity analyses and subgroup analyses based on the above factors were performed to explore the potential sources of heterogeneity.
Changes to protocol
The methods for this review differ from the protocol prospectively registered on PROSPERO as described below.
Decision problem
The set of outcomes was expanded to include the following, after consultation with clinical experts:
-
number of patients who were more amenable to surgical treatment
-
surgical resection rate
-
smoking cessation and patients’ smoking behaviour change
-
adherence rate to screening
-
overdiagnosis
-
complications in those who underwent an invasive procedure
-
radiation dose of screening
-
radiation-related patient outcomes
-
adverse psychological impact.
Search methods
The following resources were not searched:
-
National Research Register
-
Food and Drug Administration website
-
European Medicines Agency website.
In addition, the WHO trial registry was searched.
Subgroups
The prospectively registered protocol indicated that heterogeneity would be explored through consideration of the study populations, methods and interventions. In the review, heterogeneity was specifically explored through consideration of study quality, which relates to study methods, but was not explicitly listed in the protocol.
Results
Quantity and quality of research available
The literature searches of bibliographic databases identified 7496 references. After initial screening of titles and abstracts, 380 were considered to be potentially relevant and were ordered for full-paper screening. In total, 12 RCTs were included for the systematic review of clinical effectiveness of lung cancer screening by LDCT scanning. All the included trials with linked citations are presented in Appendix 2. Figure 1 shows a flow diagram outlining the screening process with reasons for exclusion of full-text papers.
FIGURE 1.
The PRISMA flow diagram for systematic review of clinical effectiveness. SR, systematic review. Adapted from Moher et al. 60 © 2009 Moher et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
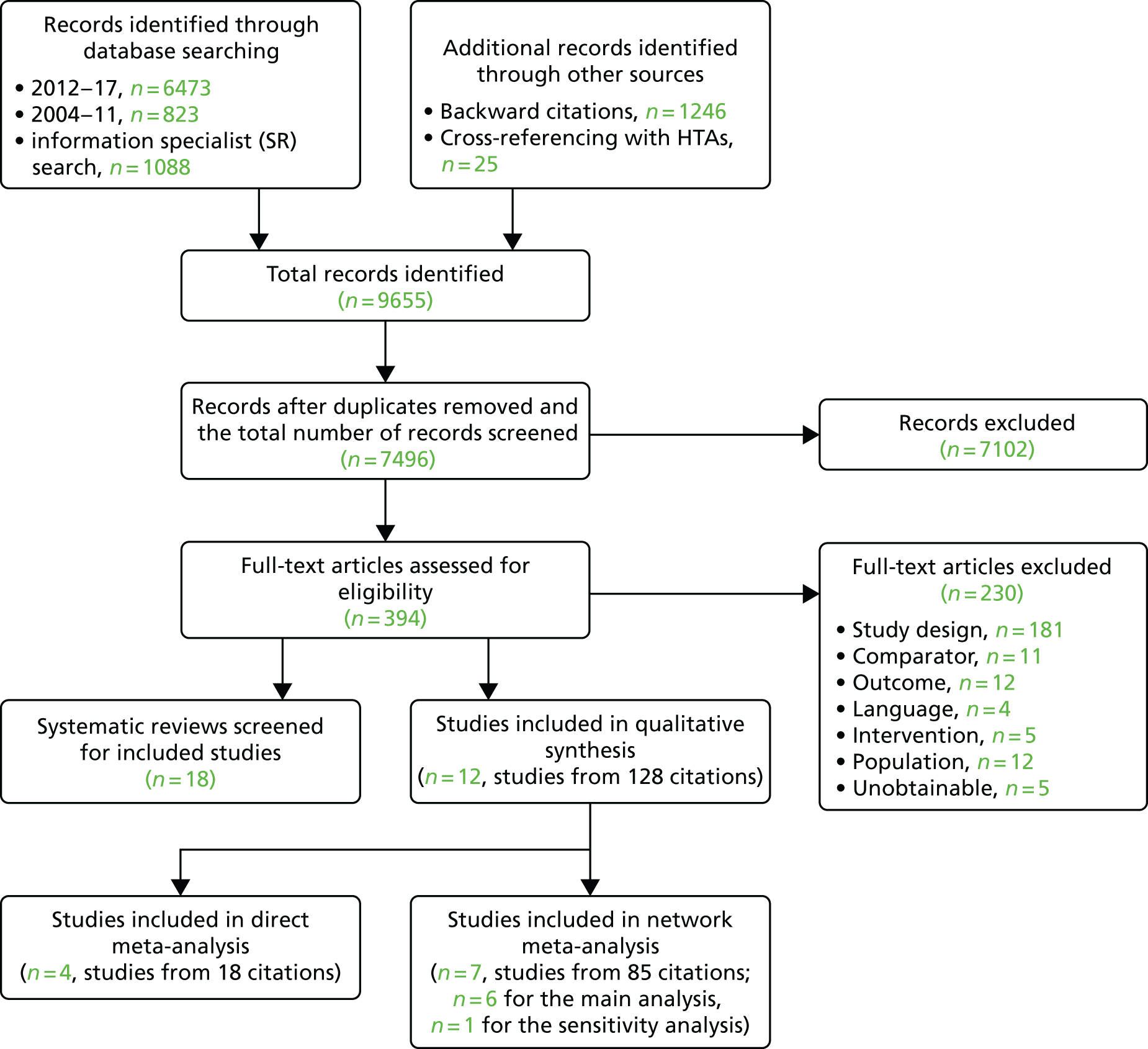
Most trials were reported in multiple papers and abstracts, with considerable overlaps in data and reporting. We selected the paper with the most up-to-date and complete data for the data extraction.
A list of full-text papers that were excluded along with the reasons for their exclusions is given in Appendix 3. These papers were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study design, participants, interventions or outcomes reported.
Assessment of clinical effectiveness
Characteristics of included studies
Table 1 presents the summary information of characteristics of included trials for the systematic review of clinical effectiveness. All of the included studies were RCTs. Nine studies were conducted in European countries and three studies were conducted in the USA. Two trials (one of which was a pilot trial) were conducted in the UK. Only a minority of included trials contributed to important comparative outcomes.
| Study identifier | Country | Recruitment time | Screening programme | Comparator | Sample size (n) | Age range, years (recruitment protocol) | Number of screening rounds | Screening times and interval (years) | Duration of follow-up (mean/median) |
|---|---|---|---|---|---|---|---|---|---|
| DANTE61 | Italy | March 2001 to February 2006 | LDCT, medical examination and one CXR | No screening, medical examination and one CXR | 2811 (2400 planned) | 60–74 | 5 | T0, T1, T2, T3, T4 (1-year interval) | At December 2012, median 6 years 3.5 months |
| Depiscan62 | France | NR | LDCT | CXR | 830 | 47–76 (protocol 50–75) | 3 | T0, T1, T2 (1-year interval) | NR |
| DLCST63 | Denmark | October 2004 to March 2006 | LDCT | No screening | 4104 | 50–70 | 5 | T0, T1, T2, T3, T4 (1-year interval) | Median: 9.47 years vs. 9.53 years (planned 10 years) |
| Garg et al.64 | USA | January 2001 to October 2001 | LDCT | No screening | 190 (400 planned) | 50–80 | 2 | T0, T1 (1-year interval) | NR (planned 2 years) |
| ITALUNG65 | Italy | NR | LDCT, smoking cessation programme | No screening, smoking cessation programme | 3206 | 55 –59 | 4 | T0, T1, T2, T3 (1-year interval) | NR |
| LSS-PLCO66 | USA | Randomisation from September 2000 to November 2000 or January 2001 (depending on source) | LDCT | CXR | 3318 (3000 planned) | 55–74 | 1 | T0, T1 (1-year interval) | NR |
| LungSEARCH67 | UK | August 2007 to March 2011 | Sputum surveillance, if abnormal sputum, LDCT and AFB | CXR at 5 years | 1568 (1300 planned) | Mean 63 | 5 | T0, T1, T2, T3, T4 (1-year interval) | NR (planned 5 years) |
| LUSI68 | Germany | September 2007 to April 2011 | LDCT, smoking counselling | No screening, smoking counselling | 4052 (4000 planned) | 50–69 | 5 | T0, T1, T2, T3, T4 (1-year interval) | NR |
| MILD69 | Italy | September 2005 to September 2011 | LDCT (annual and biannual), smoking cessation, pulmonary function test, blood sample | No screening, smoking cessation, pulmonary function test, blood sample | 4099 (10,000 planned) | > 49 | 10 | T0, T1, T2, T3, T4, T5, T6, T7, T8, T9, (1-year interval) vs. T0, T2, T4, T6, T8 (2-year interval) | Median 7.3 years |
| NELSON56,57 | The Netherlands/Belgium | Second half of 2003 to December 2006 | LDCT | No screening | 15,822 | 50–75 | 4 |
T0, T1, T2, T3, T0 to T1, 1 year; T0 to T2, 3 years; T0 to T3, 5.5 years |
NR (planned 10 years) |
| NLST70,71 | USA | August 2002 to April 2004 | LDCT | CXR | 53,454 | 55–74 | 3 | T0, T1, T2 (1-year interval) | Median 6.5 years |
| UKLS55 | UK | August 2011 to August 2012 | LDCT | No screening | 4061 (4000 planned) | 50–75 | 1 | T0 | NR (planned 10 years) |
Low-dose CT screening was a key component of the screening programmes. Most of the included trials used usual care as a comparator, whereas three trials used CXR as a comparator. The sample size of included trials ranged from 190 to 53,434. The included trials recruited participants with age ranging from 47 to 80 years. The number of screening rounds ranged from 1 to 10. Most trials adopted 1-year interval screening. However, one trial [Multicentric Italian Lung Detection (MILD)]69 used both annual and biennial screening and the NELSON trial56 performed screening at baseline, 1 year, 3 years and 5.5 years of follow-up. When reported, the duration of follow-up ranged from 2 to 9.53 years.
All included trials recruited high-risk populations. The characteristics of study populations are shown in Appendix 4. The percentage of male participants ranged from 32% to 100%. All the studies recruited current smokers and former smokers. Most trials recruited participants through targeted mailings of questionnaires via GPs and family doctors, media, internet and newspaper advertisements. The characteristics of recruitment methods are shown in Appendix 4.
As seen from this table, there were wide variations in definitions of high risk between trials. For example, the National Lung Screening Trial (NLST)71 (which was conducted in the USA) used two variables (age and smoking history) to define high risk:
-
aged 55–74 years
-
current smokers with at least a 30 pack-year smoking history
-
former smokers (who had quit within the previous 15 years) with at least a 30 pack-year smoking history.
However, UKLS55 used the Liverpool Lung Project lung cancer risk prediction algorithm to predict high risk. This risk prediction rule has been validated in three independent studies from Europe and North America and demonstrated its predictive benefit. The following variables were included in this risk prediction model:
-
age
-
sex
-
prior diagnosis of pneumonia
-
family history of lung cancer
-
smoking duration
-
prior diagnosis of malignant tumour
-
personal history of other cancer and non-malignant lung diseases
-
early onset (< 60 years of age) family history of lung cancer.
In this trial,55 participants were selected based on the prediction result (i.e. ≥ 5% risk of developing lung cancer in the next 5 years). It should be noted that such variations in the definition of ‘high-risk’ participants can lead to different prevalences of lung cancer being detected at baseline between different trials.
Furthermore, the Detection and Screening of Early Lung Cancer with Novel Imaging Technology and Molecular Essays (DANTE) trial61 defined high-risk participants as those smokers or former smokers (aged 60–74 years) of at least 20 pack-years who had quit < 10 years before recruitment. Similar criteria were adopted by the Italian lung cancer screening (ITALUNG) trial. 72 The Despiscan trial62 defined high-risk patients as those aged 50–75 years who were current or former smokers (having quit < 15 years from enrolment) with cigarette consumption of ≥ 15 cigarettes per day for ≥ 20 years. The Danish Lung Cancer Screening Trial (DLCST)63 defined high-risk participants as those current or previous smokers (aged 50–70 years) with ≥ 20 pack-years of smoking. Previous smokers had to have quit after the age of 50 years and < 10 years prior to the start of the study. Patients had to be able to climb 36 steps without pause. In this trial, forced expiratory volume in 1 second (FEV1) had to be at least 30% of predicted normal at baseline. It should be noted that the MILD trial69 recruited younger participants (≥ 49 years) who were current or former smokers (having quit smoking within 10 years of recruitment) with ≥ 20 pack-years of smoking. 69
The DANTE trial61 recruited only male participants, whereas most trials recruited both male and female participants. The NELSON trial57 recruited at first only men, and later also women (aged 50–75 years), who were current and former smokers with ≤ 10 years of cessation, who smoked > 15 cigarettes per day for > 25 years or > 10 cigarettes per day for > 30 years.
The characteristics of screening programmes are shown in Appendix 4. Most studies compared LDCT screening with usual care (no screening), whereas three studies compared LDCT screening with CXR screening. As seen in Appendix 4, Table 43, the definitions of a positive scan varied across studies in terms of nodule sizes. For example, the NELSON trial57 defined positive CT scans as those non-calcified nodules that had a solid component of > 500 mm3 (> 9.8-mm diameter) or volume-doubling time of < 400 days. If the volume of largest solid nodule or the solid component of a partially solid nodule was 50–500 mm3 (4.6–9.8 mm in diameter) or > 8 mm in diameter for non-solid nodules or volume-doubling time was 400–600 days, these results were treated as indeterminate test results. In NLST,71 any non-calcified nodule measuring ≥ 4 mm in any diameter and radiographic images were classified as positive, suspicious for lung cancer. Other abnormalities (e.g. adenopathy or effusion) could be positive or suspicious. As per the protocol, abnormal findings suspicious for lung cancer that were stable across the three screening rounds were classified as minor abnormalities rather than positive findings.
There were variations in imaging evaluation and interpretation strategy across included trials. When reported, two radiologists independently interpreted and reported the results in most trials. If there was disagreement, final interpretation was based on joint consensus.
The diagnostic follow-up strategies for suspicious abnormality findings varied between studies. When reported, most studies used further diagnostic imaging {e.g. high-resolution CT or chest fludeoxyglucose (18F) positron emission tomography ([18F] FDG-PET)} and/or invasive biopsy with rapid on-site examination.
Computed tomography parameters
The key CT technical specifications of the included studies are presented in Tables 2 and 3 (see the Glossary for definitions of CT parameters). Given the selective reporting of CT vendors, the available information suggests that the trials transition from single (single bank of detectors) to multislice (multiple banks of detectors) technology during a time of rapid CT development. The DANTE,61 Garg et al. ,64 ITALUNG72 and German Lung Cancer Screening Intervention (LUSI)68 trials have incorporated single-slice technology. The advantages of multislice over single-slice technology mainly include the same acquisition in shorter time, better z-axis resolution and the capacity to scan larger volumes in the same time. Specific to CT of the thorax, multislice technology allows for quicker scanning, resulting in reduced breathing artefact and better quantification of thoracic lesions where present.
| Study identifier | CT technology (vendor CT scanner) | Multi or single detector | Voltage (kV) | Tube current-time product (mAs) | Slice thickness (mm) | Volumetric analysis | Pitch | Estimated average effective dose (mSv) |
|---|---|---|---|---|---|---|---|---|
| DANTE61 | NR | Multi after 2003 and single before 2003 | 140 | 40 | 5 | NR | 1.25 | NR |
| DLCST63 | Philips Mx 8000 (Philips Medical Systems, Eindhoven, the Netherlands) | Multi (16 slice) | 120 | 40 | 1–1.5 | Philips evaluation semiautomated software | 1.5 | 1 |
| Garg et al.64 | NR | Single | 120 | 50 | NR | NR | 2 : 1 | NR |
| ITALUNG65 | NR | 1 × single and 4 × multi | 120–140 | 20–43 | 1–1.25 for multislice | NR | 1–2 | NR |
| LungSEARCH67 | NR | NR | NR | NR | NR | NR | NR | NR |
| LUSI68 | Unspecified Toshiba and Siemens scanners, (switch of technology at 2010) | Multi (16 and 128 slice) after 2010 and single before 2010 | NR | NR | 1 | Computer-aided detection (MEDIAN Technologies, Valbonne, France) with volumetric software | NR | 1.6–2 |
| MILD69 | Somatom Sensation 16, Siemens (Siemens Medical Solutions, Forchheim, Germany) | Multi (16 slice) | 120 | 30 | 1 | LungCare, Siemens, semi-automated software (Siemens Healthcare, Forchheim, Germany) | 1.5 | NR |
| NELSON56,57 | M×8000 IDT (Philips Medical Systems, Cleveland, OH, USA) or Brilliance 16P, Philips (Philips Medical Systems, Cleveland, OH, USA), or Sensation-16, Siemens (Siemens Medical Solutions, Forchheim, Germany) | Multi (16 slice) | 80–90 (< 50 kg) 100 (< 60 kg) 120 (60–80 kg) 140 (> 80 kg) | 20 | 1 | LungCare, Siemens, semi-automated software | 1.5 | < 0.4 (< 60 kg) < 0.8 (60–80 kg) < 1.6 (> 80 kg) |
| UKLS55 | Unspecified Siemens and Philips Brilliance 64 (Philips Medical Systems, Cleveland, OH, USA) | Multi (128 and 64 slice) | Automated based on BMI | Automated based on BMI | 1 | Siemens syngo LungCare, version Somaris/5 VB 10A, (Siemens Medical Solutions, Forchheim, Germany) | 0.9–1.1 | NR |
| Study | CT technology (vendor CT scanner) | Multi or single detector | Voltage (kV) | Tube current-time product (mAs) | Slice thickness (mm) | Volumetric analysis | Pitch | Estimated average effective dose (mSv) |
|---|---|---|---|---|---|---|---|---|
| Depiscan62 | NR | Multi | 100–140 automated based on BMI | 20–100 automated based on BMI | 1.25–3 | NR | NR | NR |
| LSS-PLCO66 | Variable and not specified | Multi (inclusion criteria said must have a history of a spiral/helical CT scan) | 120–140 | 60 | NR | NR | 2 | NR |
| NLST70,71 | 97 different scanners | Multi > 4 slices | 120–140 | 40–80 automated based on BMI | 1–2.5 | NR | 1.25–2 (typically 1.5) | 1.5 |
In general, the slice thickness ranges between 1 and 3 mm. These are generally considered ‘thin’ slices and were considered superior to ‘thick’ slices. Reconstructed slice thickness (which includes the consideration of pitch and collimation) is complicated in helical multidetector compared with single-detector scanning. In helical scanning, reducing slice thickness increases z-axis resolution but this results in a trade-off with increased image noise and possibly dose. The DANTE trial61 adopted a slice thickness of 5 mm, which is at odds with the other trials.
In the last decade, the development of multislice technology expanded the applications of CT, leading to the increased number of examinations and radiation exposure. Given the concern about the rise of medical radiation, automatic tube current modulation was designed to achieve the same image quality for individuals with different biological make-up/patient attenuation characteristics, reducing radiation exposure. In the setting of LDCT screening, there are two broad strategies in performing CT thorax, either (1) tube current modulation (as described) or (2) a fixed-tube current approach. In this report, three main strategies of dose reduction have been identified:
-
fixed-tube current and voltage regardless of the body mass index (BMI)
-
fixed-tube current and voltage depending on the BMI
-
automatic tube current and voltage depending on the BMI.
Considering the selective reporting, each LDCT thorax strategy results in different radiation output but the reported doses are generally lower than the doses of standard CT thorax in the literature.
Some trials conducted volumetric analysis using software mostly developed by the CT vendors. DLCST63 used semiautomated software designed by Philips, whereas MILD,69 NELSON56,57 and UKLS55 used semiautomated software made by Siemens. LUSI68 used computer-aided detection software made by MEDIAN. The comparability of volumetric measurements using different software is not known.
Estimation of radiation dose is achieved by multiplying the dose length product by a conversion factor; none of the studies reported whether or not the commonly used conversion factor (0.014) was used in estimating the average radiation dose. However, all studies used radiation doses that would be considered low by CT standards and are less than annual background radiation exposure. Image quality has not been considered as an outcome in the trials, which is influenced by patient demographics.
Ongoing studies
A total of 125 ongoing trials were identified in the search and investigated further. Of these, only two were considered relevant to this review. One is the MILD trial69 (NCT02837809), which is already included, and the second is a RCT based in China (NCT02898441), which is due for completion by December 2018. This study compares LDCT screening for lung cancer with usual care. The anticipated recruitment is 6000 participants and the primary outcome is lung cancer incidence. The 5-year follow-up is planned for lung cancer incidence, lung cancer mortality and all-cause mortality.
Outcome measures in included trials
In order to demonstrate the variability of reporting in the included trials, Table 4 displays which outcome is measured and whether or not it includes ≥ 5 years of follow-up. Additional information, when available, is given on whether or not the outcome was predefined in a protocol and, if so, whether it was categorised as a primary or secondary outcome.
| Study identifier (recruitment period) | Number of randomised participants | Mortality | Cancer incidence | Stage distribution | Complete resection | HRQoL | Smoking cessation | Additional information | |
|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | All-cause | ||||||||
| DANTE61,73 (March 2001 to February 2006) | 2450 | ≥ 5 years | ≥ 5 years | ≥ 5 years | ≥ 5 years | ≥ 5 years | NR | ≥ 5 years | NCT00420862
|
| Depiscan62 (October 2002 to December 2004) | 830 | NR | NR | NR | NR | NR | NR | NR | Pilot
|
| DLCST63 (October 2004 to March 2006) | 4104 | ≥ 5 years, 1°, 10 years | ≥ 5 years | ≥ 5 years, 2°, 5 years | ≥ 5 years, 2°, 5 years | NR | COS-LC 1–5 years | Annual smoking status 1–5 years | NCT00496977
|
| Garg et al.64 (January 2001 to October 2001) | 190 | NR | NR | NR | NR | NR | NR | NR | Feasibility study
|
| ITALUNG65 [March 2004 to September 2010 (end of last intervention scan at year 4)] | 3206 | NR, 1°, 8 years | NR, 2°, 8 years | NR, 2°, 8 yearsa | NR | NR | NR | NR | NCT02777996
|
| LSS-PLCO66 (September 2000 to November 2000 or January 2001)b | 3318 | NR | NR | NR | NR | NR | NR | NR | NCT00006382
|
| LungSEARCH67 (August 2007 to March 2011) | 1568 | NR, 1°, 15 years | NR | NR | NR, 2°, 5 years | NR | NR | NR | NCT00512746
|
| LUSI68 (September 2007 to April 2011) | 4052 | NR | NR | NR | NR | NR | NR | NR |
|
| MILD69 (September 2005 to September 2011) | 4099 | ≥ 5 years, 1°, 10 years | ≥ 5 years | ≥ 5 years | NR | NR | NR | NR, 2°, 10 years | NCT02837809
|
| NELSON56,57 (January 2004 to December 2006) | 15,822 | NR, 10 | NR | NR | NR | NR | < 5 yearsc 2° | < 5 yearsc | ISRCTN63545820
|
| NLST70,71,74 (August 2002 to April 2004) | 53,454 | ≥ 5 years,d 1° | ≥ 5 years,d 2° | ≥ 5 years,d 2° | ≥ 5 years | ≥ 5 years | < 1 years | NR | NCT00047385
|
| UKLS55 (August 2011 to August 2012) | 4061 | NR | NR | NR | NR | NR | < 5 years | NR | ISRCTN78513845
|
Of the 12 included studies, predefined outcomes could not be verified for five studies. 61,62,64,66,68 Only the ITALUNG trial65 had a full protocol published,65 whereas the remaining trials listed outcomes in relevant clinical trials registries. 55,57,63,67,69,70 Five studies62,64,66–68 have no data on the outcomes of interest. A sixth trial (ITALUNG65) has recently reported results for several outcomes; however, as they were published after the inclusion date for the current review, these results were not incorporated in the analyses.
Lung cancer mortality, all-cause mortality and cancer incidence with > 5 years’ follow-up are reported in four studies. 61,63,69,70 When lung cancer mortality is cited as a primary outcome, data for two studies57,65 were not identified. However, one of these is the ITALUNG trial,65 as previously mentioned, and the second is the more recent NELSON trial,57 for which results may be published imminently. As a secondary outcome in the LungSEARCH trial,67 data for lung cancer mortality remain unreported.
Cancer incidence is defined as a secondary outcome for three studies, with results available for two studies63,70 in this review and the third being the ITALUNG trial. 65
Data for stage distribution are provided for three studies61,63,70 with ≥ 5 years’ follow-up. Although the LungSEARCH trial67 has stage distribution defined as a primary outcome, no results were available.
Complete resection is the least reported outcome, with only two studies61,70 providing data for ≥ 5 years.
Smoking cessation was reported in three studies,56,61,63 with a fourth study defining this as a secondary outcome; however, the results have not been published. 69
With regard to HRQoL, this is reported for four studies;55,57,63,70 however, the follow-up is < 5 years and actually < 1 year for the NLST trial. Furthermore, the NELSON trial57 included only a subsample of participants for this outcome.
Risk of bias of included studies
All the studies were assessed for risk of bias using the Cochrane Risk of Bias tool. 76 However, as indicated in Table 4, only a minority of the included studies contributed data to the consideration of the main outcome measures assessed through a comparison of the intervention arm with the control. Thus, the reporting of quality focuses on the risk of bias for the studies contributing results of the main outcomes, particularly separating mortality, psychological consequences/HRQoL and smoking cessation, as we noted that not only were there different included studies for these outcomes, but also differing threats to validity depending on the outcome. This stemmed from variation in the objectivity of the outcome and different losses to follow-up between outcomes within a trial.
Risk of bias for lung cancer and overall mortality
There were four contributing included studies to mortality outcomes. As indicated in Table 5, according to the standard criteria for risk of bias, all but one of these trials were well conducted. All performed power calculations and met their sample size targets, but it should be noted that the anticipated effect on mortality was much more modest in NLST71 and, hence, the trial very much larger, with > 10 times the number of participants of DANTE61 and DLCST. 63 The only quality assessment issues identified for DANTE,61 DLCST63 and NLST71 were lack of demonstration of allocation concealment of randomisation and failure to blind study participants to allocation. Although allocation concealment is the most sensitive indicator of trial quality, it should be noted that failure to demonstrate allocation concealment is still common in trials, more so with RCTs designed several years ago. The great practical difficulty of achieving blinding in DANTE,61 DLCST63 and NLST71 was not felt to introduce bias in view of the relative objectivity of the outcome. This would have been reinforced further in the NLST trial because it employed an active control arm.
| Study identifier (country) | Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other risk of bias | |
| DANTE (Italy)61 | Low | Unclear | Low | Low | Low | Low | Low and low |
| DLCST (Denmark)63 | Low | Unclear | Low | Low | Low | Low | Low and low |
| MILD (Italy)69 | Unclear | Unclear | Low | Low | Low | Low | Inadequate and inadequate |
| NLST (USA)71 | Low | Unclear | Low | Low | Low | Low | Low and low |
Relative to the other trials measuring mortality, MILD69 appeared to be considerably more open to bias than DANTE, DLCST or NLST. 61,63,71 Like the other trials it did blind assessment of outcome, achieve complete follow-up and avoid selective reporting. Again, like the other trials, it did not achieve allocation concealment or blind participants. However, there were considerably greater concerns about the randomisation process, which were not true of the other trials. First, there was lack of detail about the process of randomisation. Second, there were marked differences in three of the baseline characteristics (participant sex, current smoking status and FEV1). This greatly challenges the assumption that randomisation achieved equivalence between the CT screening arm and the control arm, which is the fundamental premise of all RCTs. The comparison between the two intervention arms may not have been as badly affected.
An additional table illustrates the size of the imbalance in baseline characteristics between the four trials contributing results on mortality (Table 6).
| Study characteristics | Study (number of participants) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DANTE78 (n = 2450) | DLSCT63 (n = 4104) | MILD69 (n = 4099) | NLST71 (n = 53,456) | ||||||
| Trial arm | LDCT | Control | LDCT | Control | LDCT (biennial) | LDCT (annual) | Control | LDCT | Control |
| n (%) | 1264 (51.6) | 1186 (48.4) | 2052 (50) | 2052 (50) | 1186 (28.9) | 1190 (29.0) | 1723 (42.0) | 26723 (50) | 26733 (50) |
| Sex (% of n male) | NR | NR | 55.9a | 54.6a | 68.5 | 68.4 | 63.3 | 59.0 | 59.0 |
| Age (years), mean | 64.6 | 64.6 | 57.9a | 57.9a | 58.2a | 58.3a | 57.6a | 61.6a | 61.6a |
| Occupational exposure (%) | 31.3 | 34.1 | NR | NR | NR | NR | NR | 27.9 | 28.3 |
| Smoking | |||||||||
| Current smokers (%) | 56.5 | 57.4 | 75.3a | 76.9a | 68.3 | 68.9 | 89.7 | 48.2 | 48.3 |
| Pack-years (mean) | 47.3 | 47.2 | NR | NR | 39b | 39b | 38b | 56.0 | 55.9 |
| Smoking duration (years), mean | NR | NR | 38.5a | 38.6a | 38.4a | 38.3a | 38.5a | 43.1 | 43.1 |
| Cigarettes/day (mean) | NR | NR | 19.2a | 18.6a | 26.3a | 26.8a | 25.2a | 28.5 | 28.4 |
| Duration smoking cessation in former smokers (years), mean | NR | NR | 4.2a | 4.4a | NR | NR | NR | 7.7a | 7.7a |
| Comorbidities, n | |||||||||
| Respiratory | 35.3 | 31.2 | NR | NR | NR | NR | NR | NR | NR |
| Chronic bronchitis, emphysema or COPD | NR | NR | NR | NR | NR | NR | NR | 17.5 | 17.4 |
| Hypertension | 36.1 | 37.7 | NR | NR | NR | NR | NR | 35.1 | 35.7 |
| Cardiac | 12.6 | 13.9 | NR | NR | NR | NR | NR | NR | NR |
| Heart disease or heart attack | NR | NR | NR | NR | NR | NR | NR | 12.9 | 12.5 |
| Stroke | NR | NR | NR | NR | NR | NR | NR | 2.8 | 2.8 |
| PVD | 10.3 | 9.0 | NR | NR | NR | NR | NR | NR | NR |
| Diabetes | 8.3 | 8.4 | NR | NR | NR | NR | NR | 9.7 | 9.7 |
| Malignancies | NR | NR | NR | NR | NR | NR | NR | 4.0 | 4.5 |
| Lung function | |||||||||
| FEV1 (litres) | NR | NR | 2.9 | 2.9 | NR | NR | NR | NR | NR |
| FEV1 < 90% predicted | NR | NR | NR | NR | 27.7 | 28.2 | 19.2 | NR | NR |
| Other data available | Social status | Paper indicates that only ‘selected baseline characteristics’ were reported | Race; education; marital status; BMI categories | ||||||
In the MILD trial,69 percentage of male participants and smoking history were similar at baseline. However, there were more current smokers in the usual-care group (90%) than in the annual (69%) and biennial (68%) screening groups. There were more participants aged < 55 years in the usual-care group (38%) than in the annual (33%) and biennial (32%) screening groups. Furthermore, it should be noted that fewer participants in the usual-care group (19%) had FEV1 per cent predicted that was < 90%, compared with both the annual screening (28%) and biennial screening groups (28%). This indicated that the overall lung functions of patients in both annual and biennial screening groups were worse at baseline compared with the usual-care group, which could threaten the validity of the results.
The baseline imbalances in the other trials, DANTE,61 DLCST63 and NLST71 were much less common and, when they did occur, were much less marked in size.
Risk of bias for psychological consequences and health-related quality of life
Four included trials contributed information on psychological consequences and HRQoL. Two were common to mortality outcomes (DLCST and NLST)63,71 and two were trials which currently did not contribute evidence on mortality but are likely to do so in the future (NELSON and UKLS). 55,57 The risks of bias in the four RCTs, DLCST,63 NELSON,56,57 NLST71 and UKLS,55 contributing evidence on psychological consequences and HRQoL are shown in the Table 7.
| Study identifier (country) | Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other risk of bias | |
| DLCST (Denmark)63 | Low | Unclear | High | High | High | Low | Unclear and low |
| NELSON (Dutch-Belgian trial)57 | Unclear | Unclear | High | High | High | Low | Unclear and low |
| NLST (USA)71 | Unclear | Unclear | High | High | High | Low | Unclear and unclear |
| UKLS (UK)55 | Low | Low | High | High | High | Low | Unclear and low |
The most important feature is that, relative to mortality, the risk of bias for psychological consequences and HRQoL is much higher. This arises both because the outcomes are more subjective and, hence, susceptible to the lack of blinding that occurs across all the included trials, but also because losses to follow-up were often in excess of 10% and unequal between CT screening arms and the controls.
The UKLS was the only trial that demonstrated both good allocation sequence and allocation concealment. 55
None of the included trials was clear about whether or not it was adequately powered to assess the outcomes in question, which was often further complicated by the fact that samples of the whole-trial population were used to measure the effectiveness of screening on psychological consequences and HRQoL. UKLS was the largest study with respect to these outcomes, even though it was a pilot study. 55
There were no risk-of-bias issues with respect to baseline equivalence as there were for mortality. NLST did not demonstrate baseline equivalence, but it may be reasonable to assume this from the demonstration of baseline equivalence for the whole trial and that the sample of participants used for assessment of psychological consequences and HRQoL appeared to be random. 71
Risk of bias for smoking behaviour
Three included trials contributed information on smoking behaviour. Two were common to mortality outcomes (DLCST and NLST),63,71 and one was a trial that currently does not contribute evidence on mortality but is likely to do so in the future (NELSON). 57 The NLST reported its findings on smoking in two parts: one for each of its two contributing research networks, Lung Screening Study (LSS) and American College of Radiology Imaging Network (ACRIN). The risk of bias for each substudy was the same (Table 8). Evidence on smoking behaviour from the UKLS study was in press at time of writing, but has subsequently been published. 80 The risks of bias for the three RCTs, DLCST,63 NELSON56,57 and NLST71 contributing evidence on smoking behaviour are shown in Table 8.
| Study identifier (country) | Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Random-sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other risk of bias | |
| DLCST (Denmark)63 | Low | Unclear | High | High | High | Unclear | Unclear and low |
| NELSON (Dutch-Belgian trial)57 | Unclear | Unclear | High | High | High | Low | Low risk and low |
| NLST – LSS (USA)71 | Low | Unclear | High | High | Low | Unclear | Unclear and low |
| NLST – ACRIN (USA)70 | Low | Unclear | High | High | Low | Unclear | Unclear and low |
As for psychological consequences and HRQoL, the most important feature is that, relative to mortality, the risk of bias for smoking behaviour is much higher. This mainly arises because the outcomes are more subjective and, hence, susceptible to the lack of blinding that occurs across all the included trials, but also because of losses to follow-up, which were often > 10% and unequal between CT screening arms and the control arms. NLST performed best with respect to loss to follow-up, with levels well below 10%, but it did not report whether or not the levels were similar in both the CT screening and CXR screening arms. 71 However, despite this, it was categorised as being at a low risk of bias with respect to attrition bias. The risk of bias for NLST71 arising from lack of blinding may have been less than DLCST63 and NELSON57 because it had an active control arm rather than usual care. Across all trials, smoking behaviour was generally based on participant self-report with little or no confirmation of true smoking status using measurements such as exhaled carbon monoxide.
None of the included trials was clear about whether or not it was adequately powered to assess smoking behaviour. Given the nature and frequency of smoking behaviour, it seems likely that DLCST and NLST were adequately powered to assess it. 63,71 However, the smoking behaviour study for NELSON was undertaken on a very small subsample of the whole trial. 57 A power calculation was done to confirm that the sample size was sufficient to detect a 7% difference in quit rate.
There were no risk-of-bias issues with respect to baseline equivalence as there were for mortality.
Risk of bias for assessments of characteristics based on a single arm of a randomised controlled trial
Many studies provided information on outcomes measured in one arm of the study only, usually the intervention arm. These are not randomised comparisons and are hence open to the same biases as case series, particularly confounding. The data were summarised in the results sections for completeness. However, it should be clearly noted that they do not provide the same robustness of evidence as the randomised comparisons even though they are derived from RCTs, and they are clearly separated from the randomised comparisons in the results section as a consequence. The results from single arms of the RCTs have not been formally quality assessed, beyond noting that they are at very high risk of bias when making comparisons.
Results of clinical effectiveness
Comparative outcomes
Lung cancer mortality
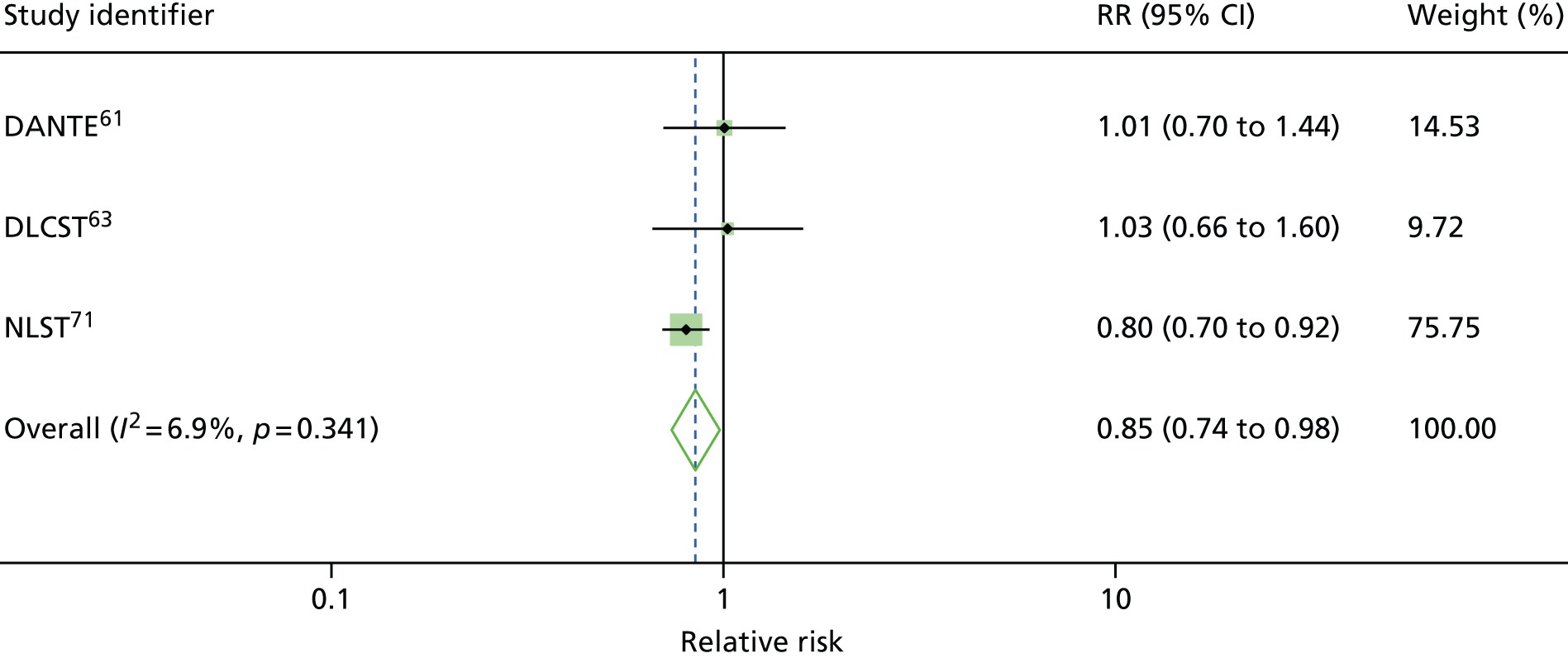
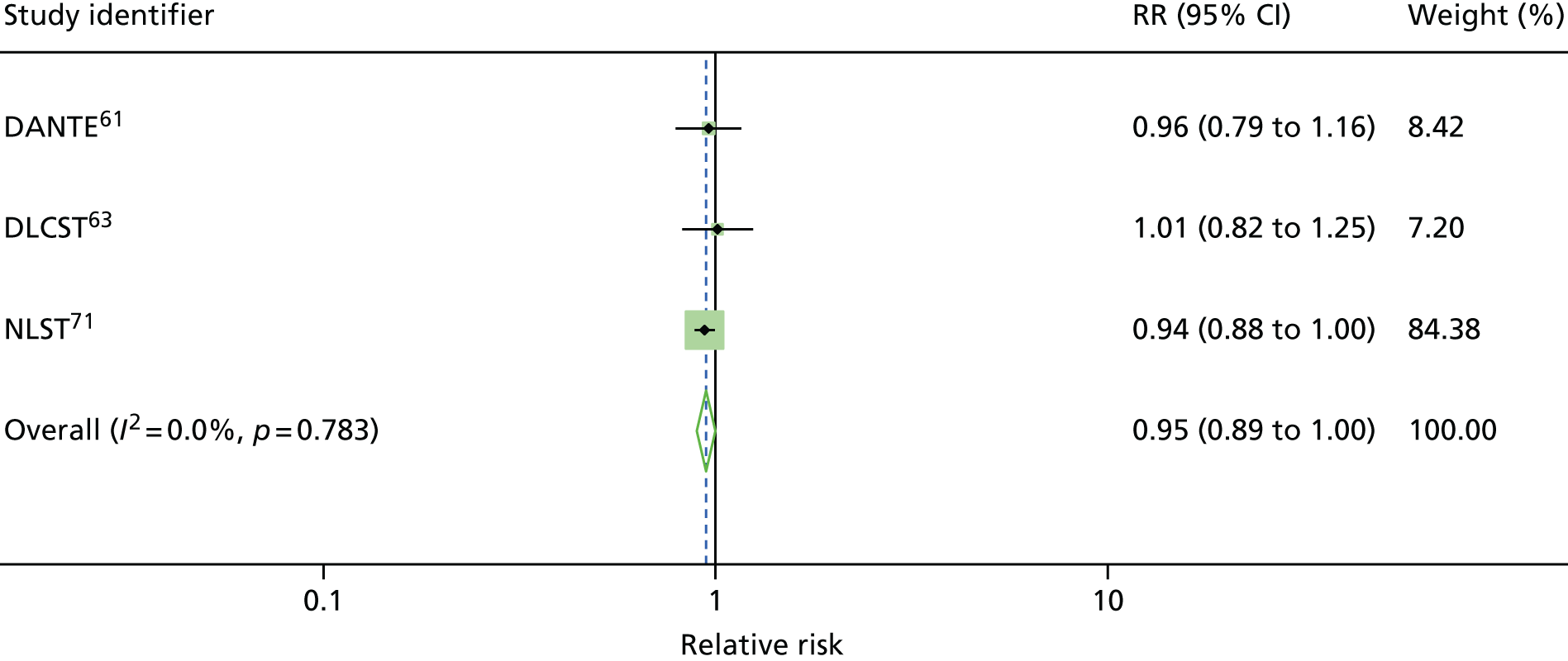
Four RCTs (DANTE, DLCST, MILD and NLST) assessed the effects of LDCT screening compared with either usual care (no screening) or the best available care (CXR screening), and reported lung cancer mortality at long-term follow-up. 61,63,69,71 Over the long-term follow-up, it was likely that CXR could be an element that constituted usual care for the early detection of lung cancer in a high-risk population. Therefore, usual care in this context was not dissimilar to the best available care when CXR was used in early detection of lung cancer. For this reason, we performed statistical pooling of all the four RCTs on mortality outcomes. All trials were conducted in participants at high risk for lung cancer. We only performed statistical pooling for trials that reported lung cancer mortality data with ≥ 5 years of follow-up, using the random-effects model.
Figure 2 shows the overall pooled result of four RCTs comparing LDCT screening with controls. When compared with controls (usual care/best available care), LDCT screening was associated with a non-statistically significant reduction in lung cancer mortality (pooled RR 0.94, 95% CI 0.74 to 1.19) with up to 9.80 years of follow-up. There was moderate heterogeneity in the magnitude of effects (I2 = 43.3%).
FIGURE 2.
Lung cancer mortality: overall results.
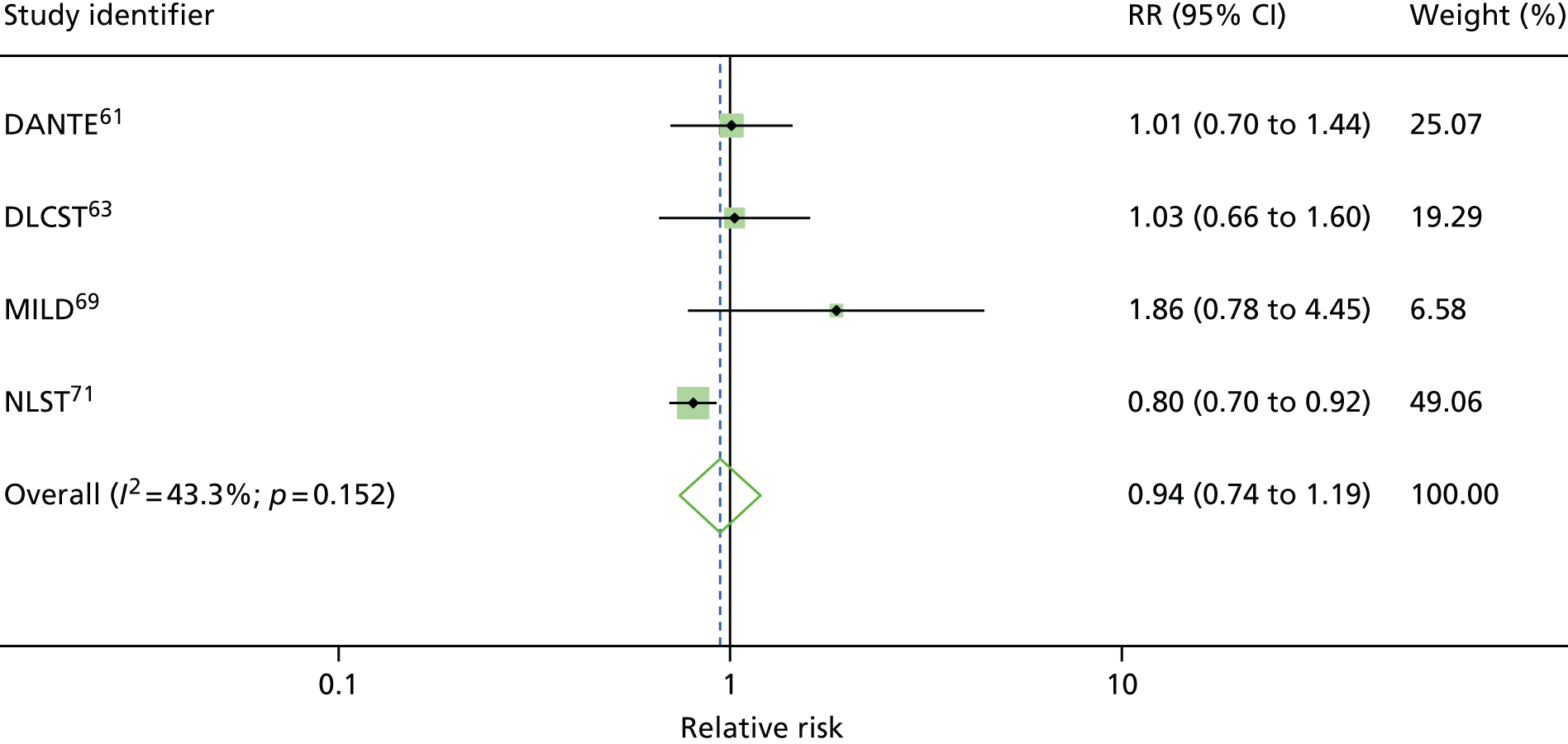
It is important to note that, given the moderate heterogeneity observed with this outcome (I2 = 43.3%), the pooled non-statistically significant decrease in lung cancer mortality (pooled RR 0.94, 95% CI 0.74 to 1.19) should be treated with caution.
Among these four RCTs, the MILD trial69 was judged to be of poor quality, whereas the remaining trials were judged to be of moderate to high quality (see Risk of bias for lung cancer and overall mortality). 55 We explored the impact of trial quality on the robustness of overall results.
Figure 3 presents the sensitivity analysis by excluding the poor-quality trial (MILD69). The results of the sensitivity analysis showed that, compared with controls (usual care/best available care), LDCT screening demonstrated a statistically significant reduction in lung cancer mortality (pooled RR 0.85, 95% CI 0.74 to 0.98). The level of heterogeneity was considerably reduced (I2 = 6.9%). This suggests that variation in trial quality could be a potential source of heterogeneity.
We further explored the heterogeneity on the basis of frequency of screening interventions (e.g. annual screening or biennial screening). We conducted a subgroup analysis based on the annual LDCT screening compared with usual care only. Figure 4 presents the pooled results of this subgroup analysis. When compared with usual care (no screening), LDCT screening programme demonstrated a non-statistically significant increase in lung cancer mortality outcome, with a pooled RR of 1.15 (95% CI 0.79 to 1.67) from three RCTs. The level of heterogeneity was reduced (I2 = 38.9%).
FIGURE 4.
Lung cancer mortality subgroup analysis: annual LDCT screening vs. usual care (no screening).

All-cause mortality
Four RCTs (DANTE, DLCST, MILD and NLST) assessed the effects of LDCT screening compared with either usual care (no screening) or the best available care (CXR screening), and reported all-cause mortality outcome with ≥ 5 years of follow-up (see Appendix 4). 61,63,69,71 Likewise, considering that CXR could be an element that constituted usual care for the early detection of lung cancer in high-risk populations, we performed statistical pooling of all these four RCTs on all-cause mortality outcome in a similar fashion.
Figure 5 shows the overall pooled result of four RCTs comparing LDCT screening with controls. When compared with controls (usual care/best available care), LDCT screening demonstrated no statistically significant difference in all-cause mortality outcome (pooled RR 1.00, 95% CI 0.87 to 1.16) with up to 9.80 years of follow-up. There was substantial heterogeneity associated with this outcome (I2 = 57.0%). Sensitivity analysis and subgroup analysis were also performed to explore the potential sources of heterogeneity.
FIGURE 5.
All-cause mortality: overall results.

Given the substantial heterogeneity detected between studies, the results from this pooled analysis should be treated with caution.
We assessed the impact of trial quality on the robustness of overall results as a means to explore heterogeneity. The details of trial quality are presented in Quantity and quality of research available, and Figure 6 shows the results of removing the low-quality trial (MILD). 55 Based on the pooled data, the LDCT screening programme demonstrated a non-statistically significant decrease in all-cause mortality (pooled RR 0.95, 95% CI 0.89 to 1.00) compared with controls (usual care/best available care).
It should be noted that the level of heterogeneity was considerably reduced (I2 = 0%), suggesting that variation in trial quality could be a potential source of heterogeneity between studies.
A subgroup analysis are performed on the basis of annual LDCT screening compared with usual care. Three trials evaluated the effect of annual LDCT screening versus usual care (no screening).
The pooled results of this subgroup analysis are shown in Figure 7. Annual LDCT screening showed a non-statistically significant increase in all-cause mortality compared with usual care, with a pooled RR of 1.15 (95% CI 0.84 to 1.58). There was substantial heterogeneity (I2 = 75.2%) observed in this outcome.
FIGURE 7.
All-cause mortality subgroup analysis: annual screening LDCT vs. usual care.

Cancer detection
We performed meta-analyses for three studies providing comparative cancer incidence data (with a control group) with ≥ 5-years’ follow-up, as this was judged sufficiently long to obtain mature data. 61,63,71 Compared with controls (usual care/best available care), LDCT screening was associated with a statistically significant increase in lung cancer detection rate (pooled RR 1.38, 95% CI 1.02 to 1.86; I2 = 79.7%) with ≥ 5 years’ follow-up (Figure 8). When compared with usual care (no screening) only, the sensitivity analysis demonstrated a consistent result (pooled RR 1.58, 95% CI 1.15 to 2.19) over 5 years after last screening, with a reduction in the level of heterogeneity (I2 = 54.6%).
FIGURE 8.
Cancer detection difference between LDCT and controls: overall results.

Stage distribution
We performed meta-analyses for trials that provided relevant data for ≥ 5 years’ follow-up as this was judged sufficiently long to obtain mature data. Figure 9 illustrates the change of lung cancer stage distribution between LDCT screening and control arms. When pooling data from three trials with ≤ 6 years’ follow-up, lung cancers detected in the LDCT screening arms (including interval cancers and cancers diagnosed after the final screening round) were more likely to be early stage (I and II) than those in the control arm, and this was statistically significant (RR 1.73, 95% CI 1.27 to 2.37, I2 = 61%). Correspondingly, these cancers were less likely to be late stage (RR 0.67, 97% CI 0.60 to 0.75, I2 = 22%).
FIGURE 9.
Early cancer stage (stage I and II) distribution between LDCT screening and control arms. M–H, Mantel–Haenszel.

We performed sensitivity analyses to explore the potential sources of heterogeneity. When pooling data of two trials comparing LDCT screening with usual care (no screening) only as part of investigation of heterogeneity, LDCT screening was associated with a statistically significant increase in early stage (I and II) cancer detection (RR 2.09, 95% CI 1.34 to 3.24) with ≤ 5 years’ follow-up. The level of heterogeneity was considerably reduced (from I2 of 61% to 39.1%).
The change in stage distribution in these analyses could arise through two mechanisms: (1) cancers that would have presented clinically as late stage are instead detected at the earlier stages (stage shift) and (2) cancers that would never have presented clinically are instead detected at the earlier stages (overdiagnosis). Figure 10 shows the impact of screening on the risk of late-stage lung cancer diagnosis. This is minimally affected by overdiagnosis because late-stage lung cancers are associated with poor survival. The results from three studies indicate that, on average, there is a risk reduction of 15% associated with LDCT screening, which is just statistically significant (RR 0.85, 95% CI 0.73 to 1.00; I2 = 17%).
FIGURE 10.
Late-stage lung cancer risk between LDCT screening and control arms. M–H, Mantel–Haenszel.

A sensitivity analysis comparing LDCT screening with usual care (no screening), that is, excluding NLST,71 indicated no statistically significant evidence of an impact on the late-stage lung cancer risk (RR 1.00, 95% CI 0.75 to 1.34; I2 = 0%).
Health-related quality of life and psychological consequences
Four trials (NELSON, NLST, DLCST and UKLS) evaluated the psychological consequences on patients of LDCT screening and patients’ HRQoL (Table 9). 55,57,63,71 Two trials (NELSON and NLST) assessed the impact of LDCT screening on patients’ HRQoL measures. 57,71 Both NELSON and NLST trials assessed this HRQoL outcome at short- and long-term follow-up. Four trials (DLCST, NELSON, UKLS and NLST) assessed adverse psychological impact associated with LDCT screening. 55,57,63,71
| Study identifier | Number of participants randomised to LDCT/control | Measures | Number of (LDCT/control); response rates | Result LDCT; mean (SD or 95% CI) | Result control | Difference | Notes | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLCST82,83 | 4104 (205/2052) | COS; COS-LC (results not included in this table) | Prevalence round (Y1)COS – high scores worse2052/1873A: 1.48 (2.20)B: 0.72 (1.78)D: 1.21 (1.99)S: 0.63 (1.56)A: 1.61 (2.31)B: 0.84 (2.08)D: 1.37 (2.17)S :0.70 (1.72) | Prevalence round (Y1) | COS – high scores worse | 2052/1873 |
A: 1.48 (2.20) B: 0.72 (1.78) D: 1.21 (1.99) S: 0.63 (1.56) |
A: 1.61 (2.31) B: 0.84 (2.08) D: 1.37 (2.17) S :0.70 (1.72) |
Prevalence round (Y1): reduced psychological consequences for LDCT. p-values: 0.07, 0.05, 0.03 and 0.20, respectively |
Screen positives (n = 179) not included in analysis Only results of four scales in COS reported in this table: ‘anxiety’ [A] (0–18), ‘negative impact on behaviour’ [B] (0–21), ‘sense of dejection’ [D] (0–18) and ‘negative impact on sleep’ [S] (0–12) Worsening psychological consequences noted in both groups between Y1 and Y2. The increases in the LDCT and control groups were not statistically significantly different in size |
|||||||||||||||||||||||||||||||||
| Prevalence round (Y1) | |||||||||||||||||||||||||||||||||||||||||||
| COS – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 2052/1873 |
A: 1.48 (2.20) B: 0.72 (1.78) D: 1.21 (1.99) S: 0.63 (1.56) |
A: 1.61 (2.31) B: 0.84 (2.08) D: 1.37 (2.17) S :0.70 (1.72) |
|||||||||||||||||||||||||||||||||||||||||
| Incidence round (prior to second screening round)1884/1817A: 1.50 (2.52)B: 1.76 (2.85)D: 1.61 (2.71)S: 1.64 (2.47)A: 1.71 (2.79)B: 2.02 (3.04)D: 1.88 (2.98)S: 1.79 (2.57) | Incidence round (prior to second screening round) | 1884/1817 |
A: 1.50 (2.52) B: 1.76 (2.85) D: 1.61 (2.71) S: 1.64 (2.47) |
A: 1.71 (2.79) B: 2.02 (3.04) D: 1.88 (2.98) S: 1.79 (2.57) |
Incidence round (Y2): reduced psychological consequences for LDCT. p-values: 0.03, 0.01, 0.01 and 0.10, respectively | ||||||||||||||||||||||||||||||||||||||
| Incidence round (prior to second screening round) | |||||||||||||||||||||||||||||||||||||||||||
| 1884/1817 |
A: 1.50 (2.52) B: 1.76 (2.85) D: 1.61 (2.71) S: 1.64 (2.47) |
A: 1.71 (2.79) B: 2.02 (3.04) D: 1.88 (2.98) S: 1.79 (2.57) |
|||||||||||||||||||||||||||||||||||||||||
| Y5a1825/1374 (some variation depending on outcomes)A: –0.26 (–0.39 to –0.13)B: 0.77 (0.63 to 0.91)D: 0.09 (0.04 to 0.22)S: 0.83 (0.71 to 0.95)A: 0.25 (0.04 to 0.46)B: 1.37 (1.13 to 1.60)D: 0.67 (0.44 to 0.90)S: 1.53 (1.31 to 1.74) | Y5a | 1825/1374 (some variation depending on outcomes) |
A: –0.26 (–0.39 to –0.13) B: 0.77 (0.63 to 0.91) D: 0.09 (0.04 to 0.22) S: 0.83 (0.71 to 0.95) |
A: 0.25 (0.04 to 0.46) B: 1.37 (1.13 to 1.60) D: 0.67 (0.44 to 0.90) S: 1.53 (1.31 to 1.74) |
Less worsening of psychological consequences in LDCT vs. control | Same pattern of results at Y5 | |||||||||||||||||||||||||||||||||||||
| Y5a | |||||||||||||||||||||||||||||||||||||||||||
| 1825/1374 (some variation depending on outcomes) |
A: –0.26 (–0.39 to –0.13) B: 0.77 (0.63 to 0.91) D: 0.09 (0.04 to 0.22) S: 0.83 (0.71 to 0.95) |
A: 0.25 (0.04 to 0.46) B: 1.37 (1.13 to 1.60) D: 0.67 (0.44 to 0.90) S: 1.53 (1.31 to 1.74) |
|||||||||||||||||||||||||||||||||||||||||
| NELSON84,85 | 15,822 |
Generic HRQoL (SF-12 and EQ-5D) Generic anxiety short form Spielberger STAI-6 (20–80; high scores indicating more anxiety) Lung-cancer-specific distress IES (0–75; higher score indicates worse distress) |
BaselineRandomised 733/733658 (89.8%)/630 (85.9%)EQ-5D – high scores better79.19 (78.02 to 80.36)78.50 (77.15 to 79.85)STAI-6 – high scores worse33.27 (32.51 to 34.03)33.75 (32.87 to 34.62)IES total – high scores worse4.05 (3.45 to 4.65)4.02 (3.33 to 4.71)Y2 (6 months post second CT scan in LDCT group)609 (89.3%)/322 (64.7%)EQ-5D – high scores better79.53 (78.35 to 80.71)77.45 (75.95 to 78.95)STAI-6 – high scores worse32.67 (31.91 to 33.43)33.42 (32.44 to 34.39)IES total – high scores worse3.72 (3.12 to 4.32)4.03 (3.24 to 4.81) | Baseline |
Randomised 733/733 658 (89.8%)/630 (85.9%) |
EQ-5D – high scores better | 79.19 (78.02 to 80.36) | 78.50 (77.15 to 79.85) | STAI-6 – high scores worse | 33.27 (32.51 to 34.03) | 33.75 (32.87 to 34.62) | IES total – high scores worse | 4.05 (3.45 to 4.65) | 4.02 (3.33 to 4.71) | Y2 (6 months post second CT scan in LDCT group) | 609 (89.3%)/322 (64.7%) | EQ-5D – high scores better | 79.53 (78.35 to 80.71) | 77.45 (75.95 to 78.95) | STAI-6 – high scores worse | 32.67 (31.91 to 33.43) | 33.42 (32.44 to 34.39) | IES total – high scores worse | 3.72 (3.12 to 4.32) | 4.03 (3.24 to 4.81) | No statistically significant differences were found in in any HRQoL scores or psychological consequence over time between the screen and control groups |
Study conducted on circa 10% random sample of whole-trial cohort Y2 questionnaire further restricted to random sample of original random sample in control group Only results for EQ-5D, STAI-6 and total IES included in this table to illustrate general findings Further questionnaire data in LDCT arm after baseline scan. Longitudinal trends available in HRQoL comparing indeterminate with negative screen findings. Results not included as not a randomised comparison |
||||||||||||||||
| Baseline | |||||||||||||||||||||||||||||||||||||||||||
|
Randomised 733/733 658 (89.8%)/630 (85.9%) |
EQ-5D – high scores better | ||||||||||||||||||||||||||||||||||||||||||
| 79.19 (78.02 to 80.36) | 78.50 (77.15 to 79.85) | ||||||||||||||||||||||||||||||||||||||||||
| STAI-6 – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 33.27 (32.51 to 34.03) | 33.75 (32.87 to 34.62) | ||||||||||||||||||||||||||||||||||||||||||
| IES total – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 4.05 (3.45 to 4.65) | 4.02 (3.33 to 4.71) | ||||||||||||||||||||||||||||||||||||||||||
| Y2 (6 months post second CT scan in LDCT group) | |||||||||||||||||||||||||||||||||||||||||||
| 609 (89.3%)/322 (64.7%) | EQ-5D – high scores better | ||||||||||||||||||||||||||||||||||||||||||
| 79.53 (78.35 to 80.71) | 77.45 (75.95 to 78.95) | ||||||||||||||||||||||||||||||||||||||||||
| STAI-6 – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 32.67 (31.91 to 33.43) | 33.42 (32.44 to 34.39) | ||||||||||||||||||||||||||||||||||||||||||
| IES total – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 3.72 (3.12 to 4.32) | 4.03 (3.24 to 4.81) | ||||||||||||||||||||||||||||||||||||||||||
| NLST74 | 53,454 |
Generic HRQoL (SF-36) Generic anxiety short form Spielberger STAI-Y1 (20–80; high scores indicating more anxiety; median norm for working adults aged 50–69 years is 34.51 for men and 32.20 for women) |
Baseline2812SF-36 physical – high scores better48.0748.89STAI-Y1 – high scores worseNRNR1 month2317(82.4%)SF-36 physical – high scores better47.5848.49STAI-Y1 – high scores worse33.5933.026 months1990(70.8%)SF-36 physical – high scores better47.3247.78STAI-Y1 – high scores worse33.3333.11 | Baseline | 2812 | SF-36 physical – high scores better | 48.07 | 48.89 | STAI-Y1 – high scores worse | NR | NR | 1 month |
2317 (82.4%) |
SF-36 physical – high scores better | 47.58 | 48.49 | STAI-Y1 – high scores worse | 33.59 | 33.02 | 6 months |
1990 (70.8%) |
SF-36 physical – high scores better | 47.32 | 47.78 | STAI-Y1 – high scores worse | 33.33 | 33.11 | No statistically significant differences between LDCT and CXR for any of the outcomes |
Study conducted on sample of whole-trial cohort Only results on physical subscale of SF-36 and STAI included in this table to illustrate results pattern Weighted average calculated from data in paper Comparison of trends over time in different screen categories (negative, significant incidental finding, false positives and true positives) also analysed |
||||||||||||||
| Baseline | |||||||||||||||||||||||||||||||||||||||||||
| 2812 | SF-36 physical – high scores better | ||||||||||||||||||||||||||||||||||||||||||
| 48.07 | 48.89 | ||||||||||||||||||||||||||||||||||||||||||
| STAI-Y1 – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| NR | NR | ||||||||||||||||||||||||||||||||||||||||||
| 1 month | |||||||||||||||||||||||||||||||||||||||||||
|
2317 (82.4%) |
SF-36 physical – high scores better | ||||||||||||||||||||||||||||||||||||||||||
| 47.58 | 48.49 | ||||||||||||||||||||||||||||||||||||||||||
| STAI-Y1 – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 33.59 | 33.02 | ||||||||||||||||||||||||||||||||||||||||||
| 6 months | |||||||||||||||||||||||||||||||||||||||||||
|
1990 (70.8%) |
SF-36 physical – high scores better | ||||||||||||||||||||||||||||||||||||||||||
| 47.32 | 47.78 | ||||||||||||||||||||||||||||||||||||||||||
| STAI-Y1 – high scores worse | |||||||||||||||||||||||||||||||||||||||||||
| 33.33 | 33.11 | ||||||||||||||||||||||||||||||||||||||||||
| UKLS55,86 | 4061 (2028/2027) |
Lung cancer distress (CWS); 6–24, > 12.5 indicates distress); Anxiety (HADS; 0–21 8–10 ‘mild’) Depression (HADS; 0–21 8–10 ‘mild’) Decision satisfaction; % very satisfied |
Baseline (T0)2018 (99.5%)/2019 (99.6%)CWS – high scores worse8.758.74HADS (anxiety) – high score worse3.723.67HADS (depression) – high score worse2.662.61Satisfaction – high score better39%42% | Baseline (T0) | 2018 (99.5%)/2019 (99.6%) | CWS – high scores worse | 8.75 | 8.74 | HADS (anxiety) – high score worse | 3.72 | 3.67 | HADS (depression) – high score worse | 2.66 | 2.61 | Satisfaction – high score better | 39% | 42% |
Only results for T0 scorers of < 12.5 included in table Results T0 > 12.5 were 326/3225 at T1 Differences in all outcomes between TP, FP, Incidental and TN also examined Size of differences noted to be small relative to clinically important changes in score |
|||||||||||||||||||||||||
| Baseline (T0) | |||||||||||||||||||||||||||||||||||||||||||
| 2018 (99.5%)/2019 (99.6%) | CWS – high scores worse | ||||||||||||||||||||||||||||||||||||||||||
| 8.75 | 8.74 | ||||||||||||||||||||||||||||||||||||||||||
| HADS (anxiety) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 3.72 | 3.67 | ||||||||||||||||||||||||||||||||||||||||||
| HADS (depression) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 2.66 | 2.61 | ||||||||||||||||||||||||||||||||||||||||||
| Satisfaction – high score better | |||||||||||||||||||||||||||||||||||||||||||
| 39% | 42% | ||||||||||||||||||||||||||||||||||||||||||
| 2-week post scan result (T1)1653 (84.1%)/1579 (78.3%)CWS – high scores worse8.54 (8.44 to 8.64)8.26 (8.16 to 8.36)HADS (anxiety) – high score worse3.67 (3.54 to 3.80)3.78 (3.64 to 3.92)HADS (depression) – high score worse2.53 (2.42 to 2.63)2.81 (2.70 to 2.92)Satisfaction – high score better42%34% | 2-week post scan result (T1) | 1653 (84.1%)/1579 (78.3%) | CWS – high scores worse | 8.54 (8.44 to 8.64) | 8.26 (8.16 to 8.36) | HADS (anxiety) – high score worse | 3.67 (3.54 to 3.80) | 3.78 (3.64 to 3.92) | HADS (depression) – high score worse | 2.53 (2.42 to 2.63) | 2.81 (2.70 to 2.92) | Satisfaction – high score better | 42% | 34% |
More distress in LDCT p ≤ 0.001 Less anxiety in LDCT, NS Less depression in LDCT p ≤ 0.001 More satisfaction in LDCT p ≤ 0.001 |
||||||||||||||||||||||||||||
| 2-week post scan result (T1) | |||||||||||||||||||||||||||||||||||||||||||
| 1653 (84.1%)/1579 (78.3%) | CWS – high scores worse | ||||||||||||||||||||||||||||||||||||||||||
| 8.54 (8.44 to 8.64) | 8.26 (8.16 to 8.36) | ||||||||||||||||||||||||||||||||||||||||||
| HADS (anxiety) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 3.67 (3.54 to 3.80) | 3.78 (3.64 to 3.92) | ||||||||||||||||||||||||||||||||||||||||||
| HADS (depression) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 2.53 (2.42 to 2.63) | 2.81 (2.70 to 2.92) | ||||||||||||||||||||||||||||||||||||||||||
| Satisfaction – high score better | |||||||||||||||||||||||||||||||||||||||||||
| 42% | 34% | ||||||||||||||||||||||||||||||||||||||||||
| 2 year (T2)1553 (82.3%)/1302 (65.3%)CWS – high scores worse8.15 (8.05 to 8.25)8.10 (7.99 to 8.25)HADS (anxiety) – high score worse3.66 (3.52 to 3.80)4.02 (3.86 to 4.19)HADS (depression) – high score worse2.77 (2.67 to 2.89)3.01 (2.89 to 3.14)Satisfaction – high score better40%26% | 2 year (T2) | 1553 (82.3%)/1302 (65.3%) | CWS – high scores worse | 8.15 (8.05 to 8.25) | 8.10 (7.99 to 8.25) | HADS (anxiety) – high score worse | 3.66 (3.52 to 3.80) | 4.02 (3.86 to 4.19) | HADS (depression) – high score worse | 2.77 (2.67 to 2.89) | 3.01 (2.89 to 3.14) | Satisfaction – high score better | 40% | 26% |
More distress in control, NS Less anxiety in LDCT p ≤ 0.001 Less depression in LDCT p ≤ 0.01 More satisfaction in LDCT p ≤ 0.001 |
||||||||||||||||||||||||||||
| 2 year (T2) | |||||||||||||||||||||||||||||||||||||||||||
| 1553 (82.3%)/1302 (65.3%) | CWS – high scores worse | ||||||||||||||||||||||||||||||||||||||||||
| 8.15 (8.05 to 8.25) | 8.10 (7.99 to 8.25) | ||||||||||||||||||||||||||||||||||||||||||
| HADS (anxiety) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 3.66 (3.52 to 3.80) | 4.02 (3.86 to 4.19) | ||||||||||||||||||||||||||||||||||||||||||
| HADS (depression) – high score worse | |||||||||||||||||||||||||||||||||||||||||||
| 2.77 (2.67 to 2.89) | 3.01 (2.89 to 3.14) | ||||||||||||||||||||||||||||||||||||||||||
| Satisfaction – high score better | |||||||||||||||||||||||||||||||||||||||||||
| 40% | 26% | ||||||||||||||||||||||||||||||||||||||||||
All included studies suffered from additional challenges to their validity relative to mortality outcomes, resulting from subjectivity of outcomes in trials that were not blinded and loss to follow-up. Three studies (NLST, NELSON and DLSCT) evaluated these outcomes in subsamples of their whole-trial populations. Hence, the size of the whole trial is not a good guide to quantity of evidence provided by each study on these outcomes. The sample size of each study was UKLS (n = 4061), DLSCT (n = 3929), NLST (n = 2812) and NELSON (n = 1466).
As a general comment, when normal values for psychological consequences and HRQoL were provided, the levels encountered were generally well outside the scale values, indicating marked psychological distress or QoL outside that which would be expected in the normal population.
UK Lung Cancer Screening Trial
The UKLS evaluated the psychological consequences on patients of LDCT screening and patients’ HRQoL in 4061 participants. 55 This trial assessed participants’ distress using the lung cancer distress (Cancer Worry Scale), assessed participants’ anxiety using the Hospital Anxiety and Depression Scale and assessed participants’ depression using the Hospital Anxiety and Depression Scale. Decision satisfaction was also assessed in this trial. The assessments were performed at baseline (T0), the 2-week post-scan result (T1) and the 2-year follow-up (T2).
At T1, distress scores were statistically significantly higher in the LDCT screening group (LDCT 8.54 vs. control 8.26; p ≤ 0.001), with a very small effect size. However, there was no statistically significant difference in the distress scores between the two groups at the 2-year follow-up (LDCT 8.15 vs. control 8.10).
Participants in the LDCT screening group had less anxiety compared with the control group at T1 (LDCT 3.67 vs. control 3.78), although this difference did not reach statistical significance. At the 2-year follow-up, participants in the LDCT screening group had statistically significantly less anxiety compared with the control group (LDCT 3.66 vs. control 4.02; p ≤ 0.001).
Furthermore, participants in the LDCT screening group had statistically significantly less depression than those in the control group at T1 (LDCT 2.53 vs. control 2.81; p ≤ 0.001) and at T2 (LDCT 2.77 vs. control 3.01, p ≤ 0.01).
At both T1 and T2, participants in the LDCT screening group had a statistically significantly higher satisfaction rate than the control group (T1: LDCT 42% vs. control 34%; T2: LDCT 40% vs. control 26%).
Danish Lung Cancer Screening Trial
In the DLCST,82 participants in the LDCT screening and usual-care groups were invited annually to the screening clinic to complete the validated lung cancer-specific questionnaire: consequences of screening lung cancer (COS-LC). In this questionnaire, higher scores indicate worse outcomes. COS-LC consists of two parts, but Part II is applicable only after a final diagnosis and was not used in the study. Part I contains nine psychosocial scales (anxiety, behavior, sense of dejection, negative impact on sleep, self-blame, focus on airway symptoms, stigmatization, introvert and harm of smoking) and two single items (busy to take mind off things and less interest in sex). In total, 4104 participants were randomised to either the LDCT screening arm or the control group. The completion rates for COS-LC for the LDCT group and usual-care group were 95.5% and 73.6%, respectively.
At the prevalence round, there was an effect of reduced psychological consequences in the LDCT screening arm compared with the control group (anxiety: LDCT 1.48 vs. control 1.61; negative impact on behaviour: LDCT 0.72 vs. control 0.84; sense of dejection: LDCT 1.21 vs. control 1.37; and negative impact on sleep: LDCT 0.63 vs. control 0.70). Of these, only the difference in sense of dejection between the two groups reached statistical significance (p = 0.03).
At the incidence round (prior to the second screening), an effect of reduced psychological consequences in the LDCT arm was also observed (anxiety: LDCT 1.50 vs. control 1.71; negative impact on behaviour: LDCT 1.76 vs. control 2.02; sense of dejection: LDCT 1.61 vs. control 1.88; and negative impact on sleep: LDCT 1.64 vs. control 1.79). The differences in anxiety, negative impact on behaviour and sense of dejection between the two groups reached statistical significance (p = 0.03, p = 0.01 and p = 0.01, respectively).
The results from the DLCST trial82 also showed a statistically significant increase in negative psychosocial consequences from baseline through rounds 2 to 5 for both the LDCT screening group and the control group (p < 0.0001 for three of four possible scales). It should be noted that, during rounds 2 to 5, participants in the control group experienced statistically significantly more negative psychosocial consequences in seven of nine scales than the LDCT screening group (p < 0.03). When evaluating the change from year 1 to 5, the data from Table 7 showed less worsening of psychological consequences in the LDCT screening group than in the control group. The differences seen were small and not likely to be clinically important.
National Lung Screening Trial
The NLST74 assessed the impact of LDCT screening on the HRQoL [Short Form questionnaire-36 items (SF-36)] measure in a sample of 2812 participants. A total of 2812 participants at 16 of 23 ACRIN sites who had completed baseline HRQoL assessments were asked to complete SF-36 questionnaires. There were no statistically significant differences in SF-36 (physical component score) between the LDCT screening and the CXR screening groups at baseline (LDCT 48.07 vs. CXR 48.89) or at the 1-month follow-up (LDCT 47.58 vs. CXR 48.49) or 6-month follow-up (LDCT 47.32 vs. CXR 47.78).
The NLST74 also assessed patients’ anxiety associated with screening using the Spielberger State–Trait Anxiety Inventory, (STAI-Y1) (20–80; high scores indicating more anxiety). The results showed no statistically significant differences between the LDCT and CXR screening groups for this outcome at the1-month follow-up (LDCT 33.59 vs. CXR 33.02) or 6-month follow-up (LDCT 33.33 vs. CXR 33.11).
NEderlands Leuvens Longkanker Screenings ONderzoek
The NELSON trial84 investigated whether or not HRQoL differed between groups in a random sample of 733 participants in each arm. A patient’s QoL in the NELSON trial84 was measured using the Short Form questionnaire-12 items (SF-12) and the EQ-5D questionnaire. Participants were asked to rate their own health on the VAS of EQ-5D, which ranged from 0 (worst imaginable health status) to 100 (best imaginable health status). The NELSON trial84 also assessed patients’ anxiety associated with screening using the STAI-6 and patients’ distress associated with screening using the lung cancer-specific distress impact of event scale (IES) (0–75; with higher scores indicating worse distress). The participants received questionnaires before randomisation (T0), 2 months after baseline screening (CT screen group only; T1) and at the 2-year follow-up (T2).
At baseline, there were no statistically significant differences in HRQoL measures and psychological consequences between the LDCT screening and control groups in terms of the mean scores of EQ-5D (LDCT 79.19 vs. control 78.50), STAI-6 (LDCT 33.27 vs. control 33.75) and IES (LDCT 4.05 vs. control 4.02) (see Table 9).
At the 2-year follow-up, as seen in Table 9, no statistically significant differences were found in mean EQ-5D scores over time between the LDCT screening group and the control group (LDCT 79.53 vs. control 77.45), indicating that there were no clinically relevant changes over time for the scores on EQ-5D. Likewise, there were no statistically significant differences in mean scores of STAI-6 over time between the LDCT screening group and the control group (LDCT 32.67 vs. control 33.43). Again, no statistically significant difference was observed in mean scores of IES over time at the 2-year follow-up between the LDCT screening group and the control group (LDCT 3.72 vs. control 4.03). The evidence from single-study arms for this outcome is presented in Appendix 4.
Conclusion
Overall, based on the randomised evidence from included trials, the majority of the data showed that there were no statistically significant differences in HRQoL or psychological consequences between the LDCT screening group and control group at any point in time. When there were statistically significant differences, these were generally of a size unlikely to be clinically important and were more commonly in a direction favouring LDCT screening rather than no screening.
Change in smoking behaviour
Lung cancer screening may have a potential impact on participants’ smoking behaviour, for example, providing a new opportunity for attempts to quit smoking. One potential drawback of lung cancer screening is that it may reduce smokers’ motivation to quit smoking by inducing a sense of assurance, thereby delaying smoking cessation. Three trials (DLCST, NELSON and NLST) reported relevant data on patients’ behaviour change in smoking within the trial period (Table 10). 57,63,71
| Study (balanced?) | LDCT smokers/ex-smokers | Missing | Control smokers/ex-smokers | Missing | Difference LDCT vs. control | Time | Other results | Notes | Number randomised |
|---|---|---|---|---|---|---|---|---|---|
|
DLCST87 Yes [five annual visits including brief (< 5 minutes) smoking cessation advice in both trial arms] |
1545/507 | 0 | 1579/473 No screening | 0 | Ex-smoker rate: 25% vs. 23% (p = 0.21) | Baseline | 4104 (2052/2052) | ||
| 1335/596 (calculated) | 121 | 1274/540 (calculated) | 238 | Quit rate: 11.3% vs. 10.4% (p = 0.47) | 1 year | Restart rates also noted to be similar | ITT analysis assuming missing info did not quit | ||
| 1051/806 | 195 | 937/713 | 402 | Ex-smoker rate: 42% vs. 40% (p = 0.075) | 5 years | 2-, 3-, 4-year results; similar results | Last observation carried forward used for missing data | ||
| MILD69 | NR | NR | NR | NR | NR | NR | NR | NR. Smoking a prespecified secondary outcome. Marked baseline imbalance in current smoker status likely to compromise results if published | 4099 |
|
Yes (standard brochure or a questionnaire by which people could ask for tailored smoking cessation information sent to both trial arms) |
NR | NR |
NR No screening |
NR | Ex-smoker rate: circa 45% | Baseline (all) | Within those screened, there was a positive relationship between indeterminate test result (as opposed to normal test result) and smoking cessation, but not statistically significant |
Analysis on sample of participants who smoked at baseline. 641 and 643 in LDCT and control groups, respectively. 581 (90.6%) and 503 (78.2%) responded in the LDCT and control groups, respectively Further analysis restricted to those screened, 550 testing negative and 440 with one or more indeterminate test results. Response rates 90.1% and 93.6%, respectively |
15,822 (7915/7453) |
| 641/0 | 0 | 643/0 | 0 | Baseline (sample) | |||||
| 493/88 | 60 | 404/99 | 140 | Quit rate (ITT non-responders assumed as continued smokers): 13.7% vs. 15.5% (p = 0.38) | 2 years | ||||
|
NLST89 Yes (just literature offered in both arms. No organised smoking cessation programmes) |
12,869/13,854 | 0 |
12,910/13,823 CXR screening |
0 |
Ex-smoker rate: 51.8% vs. 51.7% Quit rate: 23.8% vs. 23.2% (p = 0.38) |
Baseline (all) | Smoking cessation strongly associated with presence of abnormality at last scan | Analysis restricted to LSS centres. Analysis restricted to smokers at baseline who did not develop lung cancer (n = 15,489). Complete data available on 14661 (94.7%) (data from table used rather than paper text) |
53,456 (26,723/26,733) |
| 5618/1757 | NR | 5595/1691 | NR | Y3 | LSS 34,612 | ||||
|
NLST90 Yes (just literature offered in both arms. No organised smoking cessation programmes) |
12,869/13,854 | 0 |
12,910/13,823 CXR screening |
0 |
Ex-smoker rate: 51.8% vs. 51.7% HR of point abstinence in smokers: 1.07 (95% CI 1.00 to 1.15) HR of relapse in long-term abstinent former smokers: 0.96 (95% CI 0.79 to 1.11) |
Baseline (all) | Smoking cessation (point and sustained) associated with (false) positive result | Analysis restricted to ACRIN centres. Analysis restricted to those who did not develop lung cancer (n = 18,066). Complete data available on 16,964 (3.9%) |
53,456 (26,723/26,733) |
| NR | NR | NR | NR | Y5 | ACRIN 18840 | ||||
| UKLS55 | NR | NR | No screening | NR | NR | NR | NR | Results reported to be in press (Professor David Baldwin, Nottingham University Hospitals, 2017, personal communication) | 4061 |
The NLST71 and DLCST63 provided randomised evidence on all participants randomised (n = 4104 and n = 53,456, respectively). Evidence from NLST was reported for each of the two contributing research networks, LSS and ACRIN. The evidence for NELSON was on a much smaller scale (n = 1284) based on a sample of the whole trial. Additional evidence from UKLS is reported to be in the process of publication.
The randomised evidence for smoking behaviour was more open to bias than evidence on mortality. The absence of blinding would have influenced the validity of self-reported smoking status relative to more objectives. In addition, DLCST63 and NELSON56,57 were open to further bias through loss to follow-up, although this was greater in the no-screening arm. The fact that NLST71 was compared with an active intervention, CXR, rather than no screening in DLCST63 and NELSON56,57 is also noteworthy.
National Lung Screening Trial
The LSS centres of the NLST78 assessed the effect of LDCT screening on smoking cessation compared with CXR screening. There was no organised smoking cessation programme in either groups. At baseline, there was no statistically significant difference in ex-smoker rate between the two groups (LDCT 51.8% vs. CXR 51.7%). At the 3-year follow-up, no statistically significant difference in quit rate between the two groups was observed (LDCT 23.8% vs. CXR 23.2%; p = 0.38). Smoking cessation was strongly associated with the presence of abnormality at last scan.
The ACRIN centres of the NLST79 also reported that, at 5-year follow-up, there was borderline significance in point abstinence in smokers [hazard ratio (HR) 1.07, 95% CI 1.00 to 1.15] between the LDCT and CXR screening groups. Likewise, there was also no statistically significant difference in relapse among long-term abstinent former smokers (HR 0.96, 95% CI 0.79 to 1.11) between the two screening groups.
Danish Lung Cancer Screening Trial
The DLCST87 reported the effect of LDCT screening on smoking status and smoking cessation. There was a balance in support for smoking cessation between LDCT screening and control arms: five annual visits including < 5-minute brief for cessation advice in both trial arms. This trial reported that there was no statistically significant difference in ex-smoker rate at baseline between the screening group (25%) and the control group (no screening) (23%); p = 0.21. At the 1-year follow-up, no statistically significant difference in quit rate between the screening group (11.3%) and the control group (10.4%) was observed (p = 0.47). At the 5-year follow-up, there was no statistically significant difference in ex-smoker rate between the two groups (screening group 42% vs. control group 40%; p = 0.075). Similar results were observed at the 2-, 3- and 4-year follow-ups. The findings from the DLCST73 showed that screening with LDCT had no additional effect on participants’ smoking status compared with the control group (no screening).
NEderlands Leuvens Longkanker Screenings ONderzoek
The NELSON trial84 investigated the effect of lung cancer screening on smoking abstinence in two random samples of male smokers in the screening (n = 641) and control arm (no screening) (n = 643). In this trial, standard brochure or a questionnaire by which participants could ask for tailored smoking cessation information was sent to both trial arms. The point prevalence of smoking abstinence was 15.1% for the CT screening arm and 19.8% for the control arm (no screening). After 2 years’ trial participation, the findings showed that CT screening was associated with a lower prolonged abstinence rate (14.5%) than the control (no screening) (19.1%). However, there was no statistically significant difference in quit rate between the two groups at the 2-year follow-up (screening group 13.7% vs. control group 15.5%; p = 0.38).
The NELSON trial81,88 also reported that, within those who were screened, there was a positive relationship between indeterminate test results (as opposed to negative test results) and smoking cessation, but this not statistically significant. The results showed that the continued smoking abstinence rate was 8.9% among those with negative results; however, the continued smoking abstinence rate was higher among those with indeterminate results (11.5%).
In conclusion, the results on smoking behaviour are mixed but, on balance, slightly favour a positive effect on smoking cessation by virtue of a borderline statistically significant result in the largest of the three included studies, NLST (ACRIN). Uncertainty on this outcome may be resolved through ongoing studies in the process of publication.
Non-comparative outcomes
Nodule detection and lung cancer detection rate
Four trials57,61,63,71 reported the number of participants with non-calcified lung nodules identified on screening over the study period. Figure 11 presents the rate of participants with non-calcified lung nodules over the study threshold in the LDCT arm. As shown in this figure, there were wide variations in the percentages of participants with non-calcified lung nodules over the study threshold in the LDCT arm. It ranged from 11% to 69%, indicating that all these trials may have used different criteria to define a positive LDCT scan.
FIGURE 11.
Positive scans detected in the LDCT screening arm.
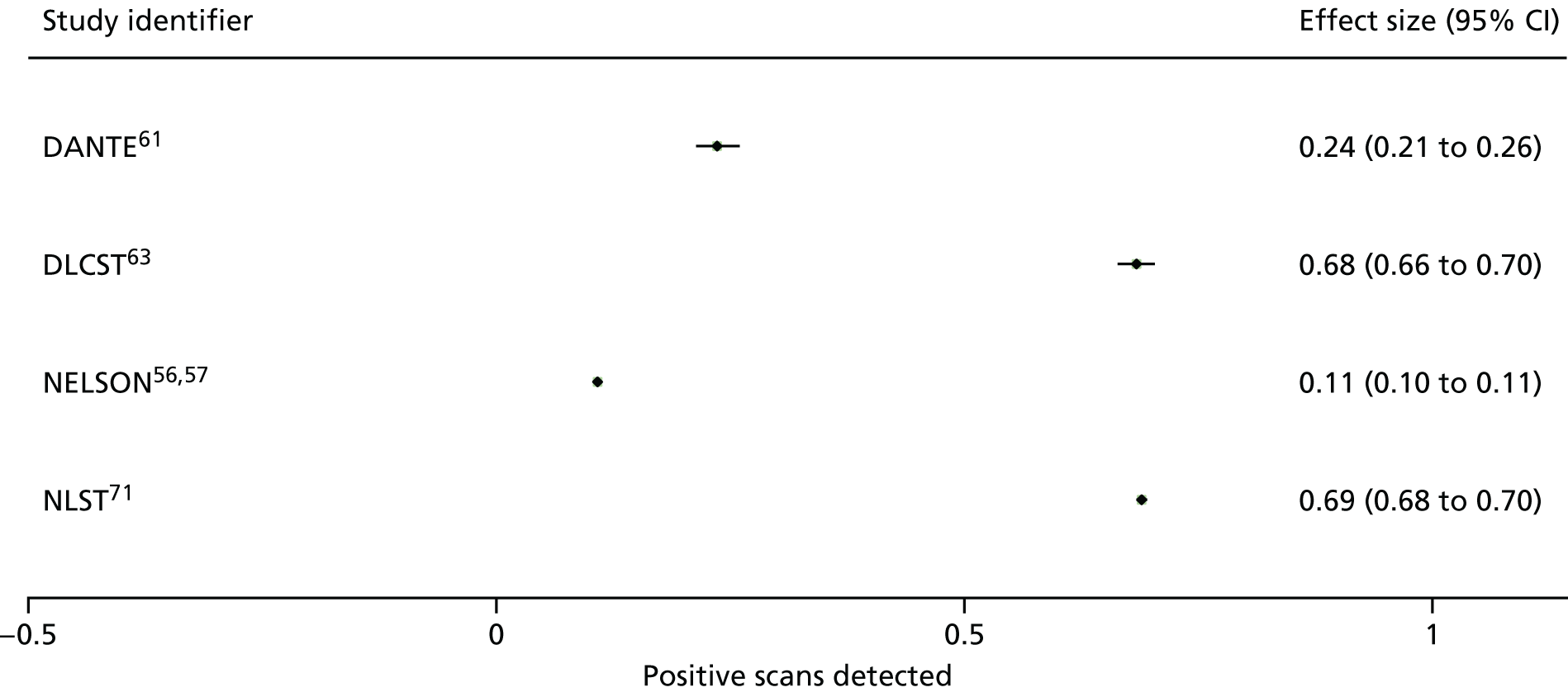
There were variations in the diagnostic methods used to follow nodules that were identified as ‘positive’ scans. Most positive scans were resolved by comparison with prior scans or further diagnostic imaging. Figure 12 presents the proportions of participants with additional diagnostic CT scans in the CT screening arm. It ranged from 5% to 52%. It should be noted that both NELSON and NLST trials had similar proportions (33% to 34%) of participants with additional diagnostic CT scans in the CT screening arm. However, UKLS achieved the lowest proportion (5%) of participants who underwent further diagnostic CT scans. This reflects the effective nodule management protocols being used in the UK setting.
FIGURE 12.
Proportion of participants with additional diagnostic CT scan.

Figure 13 presents the proportions of participants who underwent surgical biopsy or procedures for diagnosis in the CT screening arm. It ranged from 2% to 4%. This figure appears to be consistent across these trials.
FIGURE 13.
Proportion of patients undergoing surgical biopsy or procedures for diagnosis.
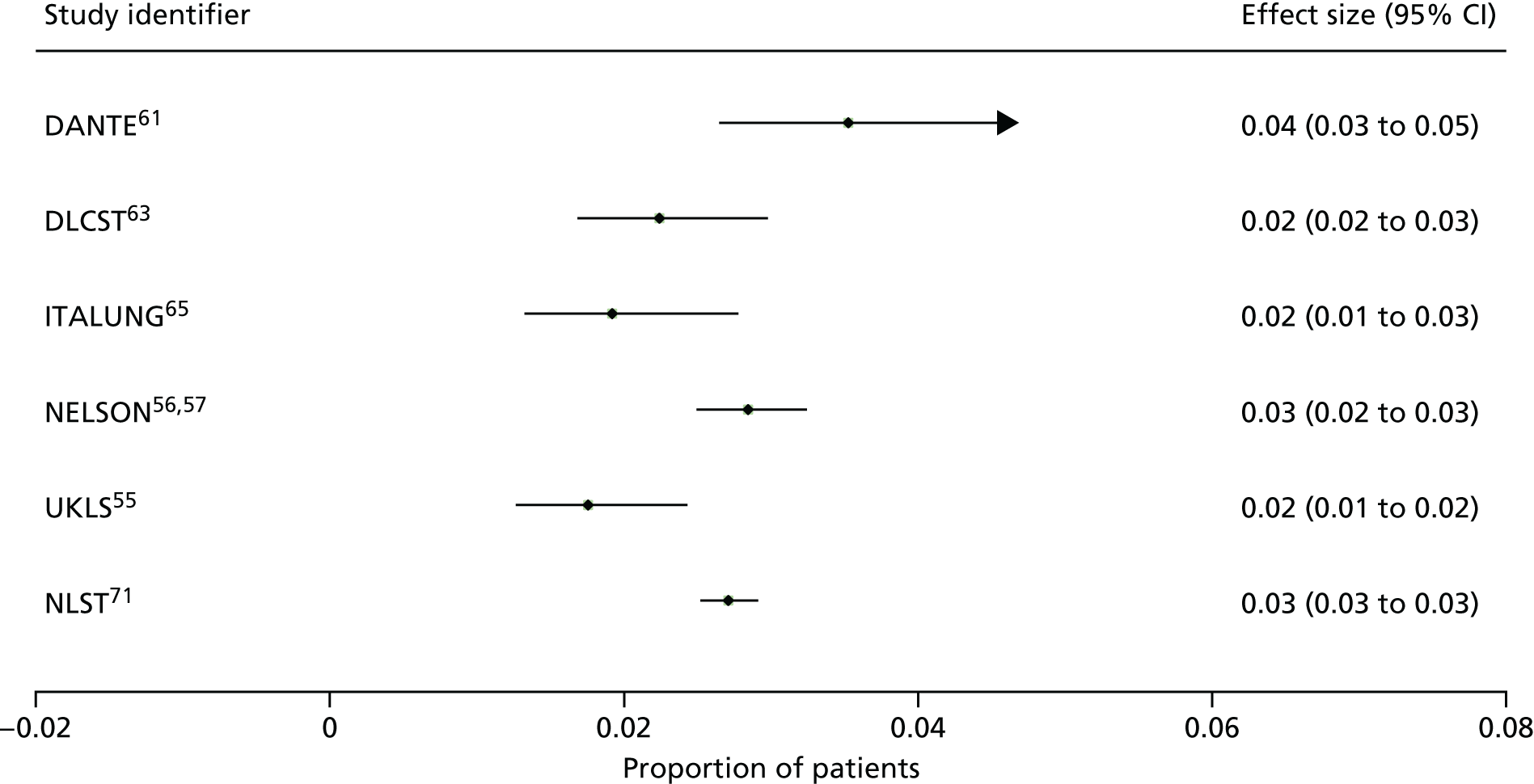
Figure 14 shows the lung cancer prevalence rate at baseline. The lung cancer prevalence rate ranged from 1% to 2% across different trials, indicating that included trials used different definitions of high-risk populations. For example, UKLS55 used a risk prediction model to define ‘high risk’, whereas both the NLST70 and the NELSON56 trial used only two variables (age and smoking status) to define ‘high risk’. We discuss this further in Discussion.
FIGURE 14.
Lung cancer prevalence rate at baseline.
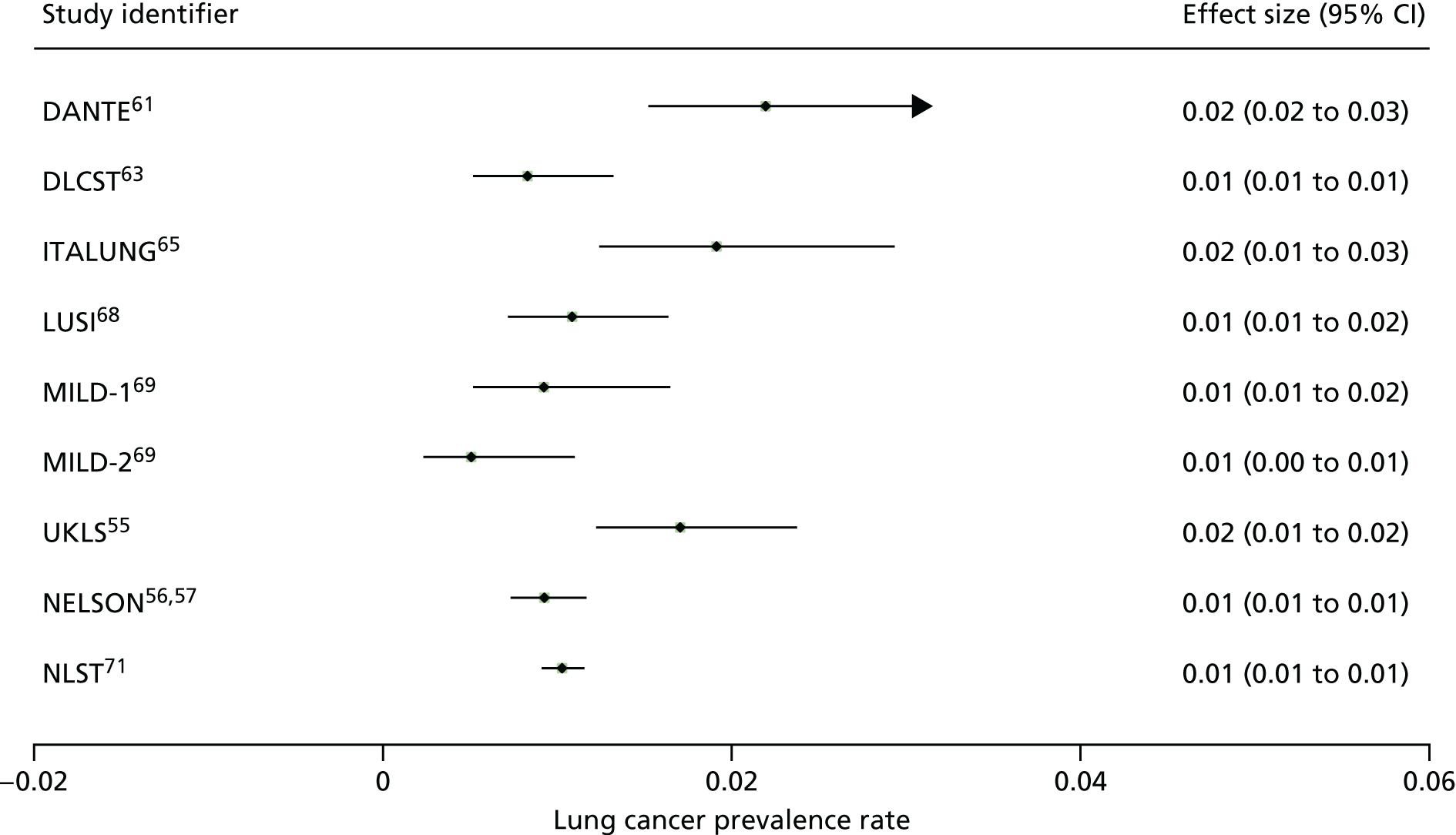
Figure 15 presents the cumulative lung cancer detection rates for LDCT screening. Six trials reported the cumulative lung cancer detection rates of all the screening rounds. 57,61,63,65,69,71 The cumulative lung cancer detection rate ranged from 1.7% to 5.2% for the LDCT screening arms.
FIGURE 15.
Cumulative lung cancer detection rate in CT screening.

As seen in the figure, the NELSON trial reported that the cumulative lung cancer detection rate of the three screening rounds was 2.6%. There was a relatively stable detection rate of lung cancer across the three screening rounds. NLST reported a detection rate of 2.5% across all three screening rounds. DLCST63 reported a detection rate of 3.4%. The ITALUNG trial65 reported that the cumulative lung cancer detection rate was 2.7%. The MILD trial69 reported that the cumulative lung cancer detection rate was 2.4% for annual screening and 1.7% for biannual screening. UKLS reported that the cumulative lung cancer detection rate was 2.1% for the pilot trial period. The DANTE trial61 reported that cumulative lung cancer detection rate was 5.2%.
Two trials reported interval cancer findings (i.e. those cancers were detected during the two rounds of screenings). One large US-based trial70 reported that, in the LDCT screening group, among 1060 total lung cancer cases detected, there were 367 (35%) participants who either missed the screening or received the diagnosis after their trial screening phase. In the CXR group, among 941 total lung cancer cases detected, there were 525 (56%) participants who either missed the screening or received the diagnosis after their trial screening phase.
Another large trial (NELSON) also reported that, among 200 total lung cancer cases detected, 52 (26%) cancers were detected between two different screening rounds (i.e. received the diagnosis of lung cancer after their trial screening phase). 56 The NELSON trial56 defined interval cancers as (1) lung cancers diagnosed after negative screening results, (2) lung cancers diagnosed after indeterminate screening results but without any follow-up LDCT investigations or diagnostic examinations in the screening arm or (3) lung cancers diagnosed after a positive screening test if the diagnostic investigation on the positive screening result did not lead to a diagnosis of lung cancer at that stage but a confirmed diagnosis was made later as patients’ symptoms triggered further diagnostic work-up that resulted in a diagnosis of lung cancer.
Number of participants who were more amenable to surgical treatment and surgical resection rate
No trials reported the number of participants who were more amenable to surgical treatment. The ITALUNG trial65 reported that 17 cancers in 16 subjects (81%) were surgically resected and one surgical resection was performed on a benign lesion. The LUSI trial68 reported that 19 out of 22 cancers were surgically resected. The NELSON trial91 reported 215 participants who had surgical work-up for treatment. The MILD trial69 reported that the rate of resectability was 84% overall and the vast majority of participants were treated with lobectomy. Similarly, the UKLS reported that 35 out of 42 participants (83.3%) who were diagnosed with lung cancer underwent surgical resection as their primary treatment. 55
False positives
False positives are one of the key outcomes for the evaluation of important adverse effects associated with LDCT screening for early detection of lung cancers. For those patients with positive scans, further evaluations often involve more non-invasive evaluations (e.g. imaging scans) and more invasive procedures (e.g. bronchoscopy, fine-needle biopsy and/or surgery). It is important to note that for patients with false positives, these further evaluations may be even harmful as a result of serious complications (e.g. serious infections and postoperative deaths associated with surgery).
Five trials55,57,61,63,69 reported the percentage of scans in the screening arm performed over the trial period that had a false positive result, ranging from 1.2% to 23% (Figure 16). It should be noted that such variations could be because of differences in the definition of a positive scan among included trials (e.g. different thresholds of lung nodule sizes being categorised as a positive scan).
FIGURE 16.
Proportion of scans in the screening arm with a false-positive result.

One large trial57 (NELSON with a total of 24,354 CT scans) reported that 59.4% (293 out of 493; 95% CI 54.8% to 63.9%) of positive screen results were false positive for a follow-up period of 5.5 years. The NELSON trial reported that the positive screenings had a predictive value of 40.6%. This trial reported that 55% to 65% of positive screen results were false positive across four screening arounds. A total of 493 positive results across three rounds of the NELSON trial led to a diagnosis of lung cancer in 200 patients.
In the NELSON trial,57 around 24.5% (n = 67) of those participants with false-positive screen results underwent an invasive procedure in the diagnostic work-up. A total of 91% (n = 61) of these invasive procedures were surgeries (including thoracotomies, mediastinoscopies, sternotomy and video-assisted thoracoscopies) and the remaining six procedures were transthoracic biopsies.
In NLST, across the three rounds, 96% of the positive scans in the LDCT group and 95% of those in the radiography group were false positive. These percentages differed little by screening round. The sensitivity of LDCT screening varied from 93% to 94%, whereas the sensitivity of CXR screening varied from 64% to 74% across three rounds. The specificity of LDCT screening varied from 73% to 84%, whereas the specificity of CXR screening varied from 91% to 95% across three rounds. The NLST reported complications from diagnostic procedures used to evaluate a positive LDCT scan. In this trial, around 1.4% of participants had at least one complication in the CT screening group and 1.6% in the CXR screening group.
However, the high positive rate in NLST reflects the fact that there was no distinction being made for those indeterminate findings or interval imaging findings from false positives.
It is important to note that recent trials (such as NELSON and UKLS) have made a distinction of the definitions of indeterminate findings or interval imaging rate from false positives. For example, in the NELSON trial, the LDCT screening result was indeterminate in 10.8% (2629 out of 24,354) of the all scans across three rounds of CT screening. A CT scan in the NELSON trial was considered indeterminate if the volume of the largest solid nodule or the solid component of a partially solid nodule was 50–500 mm3 or > 8 mm in diameter for non-solid nodule. Indeterminate results led to invitations for a repeat scan (after 6–8 weeks or after 12 months, depending on the nodule size and screening round) in order to determine the final result as positive or negative.
In UKLS,55 a false positive was counted if a participant did not have lung cancer but was referred to the multidisciplinary team (MDT) and/or was subjected to repeated imaging scans before 12 months had elapsed. The UKLS reported that the interval imaging rate for the category 3 (larger, potentially malignant) nodules was 23.2%.
False negatives
Another concern of using CT screening for lung cancer is the potential adverse effect associated with false-negative results. Four trials (DANTE,78 MILD,92 NLST93 and NELSON94) reported that the sensitivity of CT screening for the detection of lung cancer ranged from 69% to 94%. The data indicated that the false-negative examination rates ranged from 6% to 21%. Three trials57,61,69 reported the percentage of scans in the low-dose screening arm that had a false-negative result, ranging from 0.1% to 1.3% (Figure 17).
FIGURE 17.
Proportion of scans in the screening arm with a false-negative result.
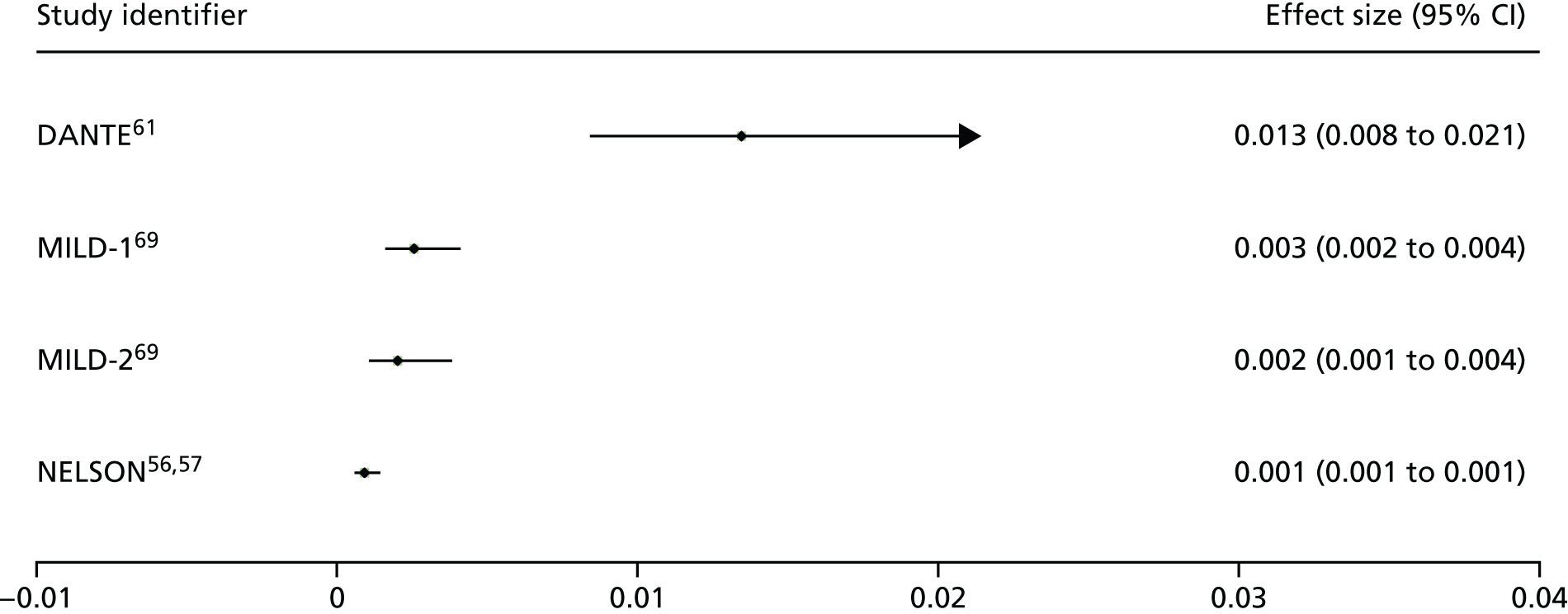
Most trials reported a false-negative result as a LDCT scan that was negative within 1 year. However, there were no studies evaluating the potential harm associated with false-negative examinations. One potential adverse effect associated with false reassurance of patients is that it can cause delayed examinations of future suspicious symptoms in patients. Given this consideration, participants should be aware of the importance of reporting symptoms (such as cough, haemoptysis or weight loss) even if they have a negative screening scan.
Overdiagnosis
Overdiagnosis is a common issue for all screening trials of cancer, and can lead to overtreatment. The treatment of an ‘overdiagnosed’ lung cancer would not extend a patient’s life, either because the disease itself would not have progressed or would have resolved spontaneously. Although we investigated this issue, most included trials did not report relevant data of overdiagnosed lung cancers attributable to screening. Overdiagnosis associated with LDCT screening was estimated at 18.5% (95% CI 5.4% to 30.6%) on the basis of detected cancers in NLST. 95
Adherence rate to screening
Ten trials report the adherence rate for LDCT screening,55,57,61–63,65,66,68–70 with four trials also reporting results for the control arm (DANTE, Depsican, ITALUNG and LUSI)61,63,65,68 and three trials reporting adherence to CXR [Depsican, Lung Screening Study as part of the Prostate, Lung, Colorectal and Ovarian cancer screening trial (LSS-PLCO) and NLST] (Table 11). 62,66,70
| Study identifier (country) | Time point, n/N (%) | Across all data points, n/N (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Round 2 | Round 3 | Round 4 | Round 5 | |||||||
| DANTE (Italy)61 | LDCT: 1264/1300 (97.2) | Control: 1186/1232 (96.3) | NR | NR | NR | NR | LDCT: 1184/1264a (93.7) | ||||
| Depiscan (France)62 | LDCT: 330/385 (85.7) | CXR: 291/380 (76.6) | NR | NR | NR | NR | NR | ||||
| DLCST (Denmark)63,82 | LDCT: 2047/2052 (99.8) | Control: 2052/2052 (100) | LDCT: 1976/2052 (96.3) | Control: 1516/2052 (73.9) | LDCT: 1944/2052 (94.7) | Control: 1388/2052 (67.6) | LDCT: 1982/2052 (96.6) | Control: 1179/2052 (57.5) | LDCT: 1851/2052 (90.2) | Control: 1414/2052 (68.9) | NRb |
| ITALUNG (Italy)65 | LDCT: 1406/1613 (87.2) | Control: 1593/1593 (100) | LDCT: 1356/1593 (85.1) | LDCT: 1308/1589 (82.3) | LDCT: 1263/1581 (79.8) | NA | LDCT: (79.0)c | ||||
| LSS-PLCO (USA)66 | LDCT: 1586/1660 (95.5) | CXR: 1550/1658 (93.5) | LDCT: 1398/1629 (85.8) | CXR: 1317/1648 (79.9) | NA | NA | NA | NR | |||
| LUSI (Germany)68,96 | LDCT: 2028/2029 (99.9) | Control: 2022/2023 (99.9) | LDCT: 1892/2000 (94.6) | Control: 1847/2018 (91.5) | LDCT: 1849/1978 (93.4) | Control: 1897/2005 (94.5) | LDCT: 1826/1958 (93.1) | Control: 1898/1988 (95.5) | LDCT: 1565/1656 (94.5) | Control: 1515/1599 (91.1) | NRd |
| MILD (Italy)69 | NR | NR | NR | NR | NR | Annual: 96.1e Biennial: 95.1e | |||||
| NELSON (The Netherlands and Belgium)56,57 | LDCT: 7582/7915 (95.8)f | LDCT: 7557/7915 (95.5)f | LDCT: 7295/7845 (93.0)f | LDCT: 6922/7790 (88.9)f | NR | NR | |||||
| NLST (USA)70,71 | LDCT: 26,309/26,715 (98.5) | CXR: 26,035/26,724 (97.4) | LDCT: 24,715/26,285 (94.0) | CXR: 24,089/26,410 (91.2) | LDCT: 24,102/25,942 (92.9) | CXR: 23,346/26,110 (89.4) | NR | NR | NRg | ||
| UKLS (UK)55 | LDCT: 1994/2028 (98.3) | NR | NR | NR | NR | NR | |||||
At baseline, the adherence rate for screening across the studies ranges from 85.7% to 99.9% for LDCT, from 76.6% to 97.4% for CXR and from 93.5% to 100% for the control arms.
The DLCST and LUSI trials provide data for five rounds of screening. 63,68 For the DLCST trial, the intervention group received annual LDCT screening and an invitation to a hospital screening clinic, where participants were offered spirometry and smoking counselling. Although the control group did not undergo LDCT screening, they did receive a similar invitation to the screening clinic. At baseline, adherence was 99.8% for the intervention group and 100% for the control group. At screening round 5, this decreased to 90.2% and 68.9%, respectively, indicating a high adherence rate for LDCT. 63 With regard to the LUSI trial,68 the control group were not offered additional spirometry or counselling. A slight decline in adherence is seen for both arms over time, with 99.9% adherence at baseline for both groups, falling to 94.5% for the LDCT group and 91.1% for the control group at screening round 5. The results from these two trials for LDCT screening are supported by the remaining studies, although with fewer screening rounds, in which the overall decrease in adherence is < 10%. 57,65,66,68,70
Three studies report adherence rates for LDCT and CXR (Depsican, LSS-PLCO and NLST). 62,66,70 In all cases and at all time points (maximum of three screening rounds), adherence rates were greater for those receiving LDCT, with a difference between arms ranging from 1.1% at baseline for NLST to 9.1% at baseline for the Depsican trial. 70
The Depsican trial reports the lowest adherence at baseline (LDCT, 85.7%; CXR, 76.6%) with 19% of participants overall refusing to participate after randomisation. 62 No clear explanation is given for this by the authors, other than a possible association with the motivation of GPs and trial participants.
Complications and postoperative deaths in those who underwent an invasive diagnostic procedure
Four trials (DANTE, DLCST, NELSON and NLST)56,57,61,63,70,71 reported major complications in those who underwent a surgical or other invasive diagnostic work-up procedure, although only two of these trials (DANTE and NLST)61,70,71 provided data for both the LDCT screening arm and the control arm. Two of the four trials also reported data on postoperative deaths (DLCST and NLST). 63,70,71 One of these trials (NLST) also provided data on major complications following non-surgical diagnostic procedures. 70,71
Across the four trials providing data, the proportion of participants in the LDCT screening arm who had major complications following invasive procedures ranged from 10.7% in the NELSON trial56,57 to 37.5% in DLCST. 63 It should be noted that, for DLCST, this was based on major complications following thoracotomy, and data were also provided following video-assisted thoracic surgery. 63 It is also important to note that NLST also reported a much smaller proportion of major complications following invasive diagnostic procedures among participants who did not have lung cancer confirmed (2.4%). 70,71
In both the DANTE trial61 and the NLST, there was a higher proportion of participants in the LDCT screening arm than in the control group who had major complications following invasive procedures [Table 12; DANTE trial,61 28.6% in the LDCT group, 19.3% in the usual-care group; NLST trial (among those with confirmed lung cancer), 12.0% in the LDCT group, 9.0% in the CXR group; NLST trial (among those with lung cancer not confirmed), 2.4% in the LDCT group, 0.9% in the CXR group]. 61,70,71
| Study identifier | Major complications following | Postoperative deaths following diagnostic work-up | |
|---|---|---|---|
| Surgical/invasive diagnostic work-up | Non-surgical work-up | ||
| DANTE61 |
LDCT: 22/77 (28.6%) Usual care: 6/31 (19.3%) |
NR | NR |
| DLCST63 | LDCT: 3/8 (37.5%);a 3/41 (7.3%)b | NR | 0c |
| NELSON56,57 | LDCT: 20/187 (10.7%) | NR | NR |
| NLST70,71 |
LDCT:d 73/618 (12.0%) CXR:d 23/264 (9.0%) LDCT:f 11/457 (2.4%) CXR:f 1/115 (0.9%) |
LDCT:d 2/31 (6.5%) CXR:d 1/15 (6.7%) LDCT:f 1/16,596 (< 0.1%) CXR:f 3/4559 (0.1%) |
|
In NLST, similar proportions of people experiencing major complications after non-invasive procedures (e.g. biopsy) were reported in the LDCT screening arm and the CXR arm (among those with confirmed lung cancer, 6.5% in the LDCT group and 6.7% in the CXR group; among those with lung cancer not confirmed, < 0.1% in the LDCT group and 0.1% in the CXR group). 70,71 It is important to note that, across groups, the number of people experiencing major complications after non-invasive procedures was very low (see Table 12).
With regard to postoperative deaths associated with surgery or an invasive diagnostic procedure (see Table 12), DLCST63 reported that, although one person died following surgical treatment for lung cancer, nobody died within 30 days of diagnostic surgery. The NLST reported that, of those with lung cancer confirmed, there were 10 participants (1.5%) in the CT screening arm and 11 participants (3.9%) in the CXR arm who died within 60 days of an invasive diagnostic procedure, and, of those with lung cancer not confirmed, there were 11 participants (0.1%) in the CT screening arm and three participants (0.1%) in the CXR arm who died within 60 days after an invasive diagnostic procedure. 70,71
Radiation dose/radiation exposure level for participants
Three trials (UKLS,55 ITALUNG65 and NLST71) reported radiation dose associated with LDCT screening. The ITALUNG trial reported that the mean collective effective dose among 1406 participants ranged from 8.75 to 9.36 mSv. This trial also reported that the mean effective dose to the single subject over 4 years’ follow-up was between 6.2 and 6.8 mSv (range 1.7–21.5 mSv) based on the cranial–caudal length of the LDCT volume. It was estimated that 77% of the radiation dose was attributable to annual LDCT scan and 23% of the radiation dose was attributable to further imaging investigations, including follow-up LDCT and FDG-PET scans.
A large US trial (NLST)97,98 reported that an average effective radiation dose was 1.4 mSv in the LDCT arm and 0.052 mSv in the CXR arm. The UKLS55 reported that the median radiation dose for baseline CT scans was 1.62 mGy (range 0.54–3.93 mGy). However, none of these studies reported radiation-related patient outcome (e.g. radiation-induced lung cancer) at long-term follow-up.
It should be noted that the radiation doses being measured in different trials were not comparable as the numbers of screening rounds were different. For example, the UKLS55 used one single-screening round but other trials, such as NLST, used multiple screening rounds. In addition, the collective effective dose from the ITALUNG trial was based on both LDCT scans and follow-up imaging investigations (including further LDCT and PDG-PET scans); however, other trials may not take into account the radiation exposure from further follow-up imaging investigations.
Discussion
Summary of findings
Twelve RCTs evaluated the clinical effectiveness of LDCT screening compared with no screening or CXR. The sample size of included trials ranged from 190 to 53,434.
The majority of included trials were judged to be of moderate to high quality; however, two trials (MILD69 and Garg et al. 64) were judged to be of poor quality. There were substantial differences between the LDCT screening and usual-care groups at baseline in both trials, raising concerns about the adequacy of randomisation. Both trials were also underpowered.
Key outcomes
Mortality
The LDCT screening was associated with a non-statistically significant decrease in lung cancer mortality (pooled RR 0.94, 95% CI 0.74 to 1.19) with up to 9.80 years of follow-up. However, there was moderate heterogeneity in the magnitude of effects (I2 = 43.3%).
We assessed the impact of trial quality on the robustness of overall results by excluding the poor quality trial (MILD69). The results demonstrated a statistically significant decrease in lung cancer mortality (pooled RR 0.85, 95% CI 0.74 to 0.98) in favour of LDCT screening. The statistical heterogeneity was considerably reduced (I2 = 6.9%), suggesting that variation in trial quality could be a potential source of heterogeneity.
A sensitivity analysis was performed including only annual LDCT screening. The result showed that LDCT screening demonstrated no statistically significant increase in lung cancer mortality compared with usual care. This result was generally consistent with the overall result.
The findings from this review also showed that, compared with controls (usual care/best available care), LDCT screening demonstrated no statistically significant increase on all-cause mortality outcome (pooled RR 1.01, 95% CI 0.87 to 1.16) with up to 9.80 years of follow-up. Similarly, given the substantial heterogeneity (I2 = 57.0%) detected between studies, the results from this pooled analysis should be treated with caution. We also investigated the potential sources of heterogeneity. When the low-quality trial (MILD69) was removed, sensitivity analysis showed that LDCT screening demonstrated a non-statistically significant decrease in all-cause mortality (pooled RR 0.95, 95% CI 0.89 to 1.00) compared with controls. The level of heterogeneity was also considerably reduced (I2 = 0%), suggesting that variation in trial quality could be a potential source of heterogeneity between studies.
Number of lung cancers and their stage distribution
The LDCT screening was associated with a statistically significantly increase in lung cancer detection rate (pooled RR 1.38, 95% CI 1.02 to 1.86) with ≥ 5 years’ follow-up. Although there was heterogeneity (I2 = 79.7%), all included studies individually showed statistically significant increases in the numbers of cancers detected in the LDCT group relative to the control group. This has implications for the possibility of overdiagnosis.
A shift in cancer stage distribution towards earlier stages is often considered as one of clinical benefits associated with LDCT screening. We assessed the results of lung cancer distribution difference between the screening and control arms with ≤ 6 years of follow-up. The results showed that LDCT screening was associated with statistically significantly increases in early stage (I and II) cancer detection (RR 1.73, 95% CI 1.27 to 2.37; I2 = 61%). When pooling data of trials comparing LDCT screening with no-screening only, the results were consistent with the overall results and the degrees of heterogeneities were also substantially reduced.
The LDCT screening was also associated with a statistically significant reduction in the risk of late-stage lung cancer compared with controls (RR 0.85, 95% CI 0.73 to 1.00), although this effect was not observed when only trials comparing LDCT to no screening were included (RR 1.00, 95% CI 0.75 to 1.34), despite these trials still finding an increased probability of lung cancers being early stage. This is consistent with overdiagnosis being a significant factor in the trials.
Psychological consequences and health-related quality of life
Based on the randomised evidence from four included trials (NELSON, NLST, DLCST and UKLS), the majority of the data demonstrated that there were no statistically significant differences in HRQoL or psychological consequences between the LDCT screening group and control group at any point in time. 55,57,63,71 When there were statistically significant differences between the two groups, these were generally of an effect size unlikely to be clinically important and were more commonly in a direction favouring LDCT screening rather than no screening. However, the validities of results from these outcomes were compromised by lack of blinding, loss to follow-up and the limitation of self-report data.
Impact on smoking behaviour
Lung cancer screening may have a potential impact on participants’ smoking behaviour as it can offer an opportunity for attempts to quit smoking because of increased awareness of risk associated with smoking. We found that the results on smoking behaviour were mixed. Overall, the results suggest that an introduction of LDCT screening in high-risk populations may have a positive impact on participants’ smoking behaviour change, given the borderline statistically significant result in the largest of the three included studies, NLST (ACRIN). Uncertainty on this effect may be resolved through ongoing studies in the process of publication.
Other outcomes
Lung cancer detection
The lung cancer prevalence rate ranged from 1% to 2% across included trials. This suggests that the different definitions for high risk of lung cancer (eligibility criteria) in different trials led to different included populations.
It should be noted that not all lung cancers were detected at the screening rounds and some lung cancers were detected between two different screening rounds (defined as interval cancers). Two large trials reported interval cancers that were detected between two different screening rounds. NLST70 reported that there were 367 (35%) participants who either missed the screening or received the diagnosis after their trial screening phase in the LDCT arm, and there were 525 (56%) participants who either missed the screening or received the diagnosis after their trial screening phase in the CXR group. 70 The NELSON trial reported that 52 (26%) lung cancers were detected between two different screening rounds (i.e. received a diagnosis of lung cancer after their trial screening phase). 57 These data suggest that a small proportion of lung cancers cannot be detected at any particular screening round or develop too fast to be identified through screening.
Positive scans and follow-up investigations
Positive scan findings at screening were high, and most patients who tested positive required further follow-up investigations including repeat CT scans, PET-CT scans, invasive biopsy or surgical procedures.
Our findings showed wide variations in the percentages of patients with non-calcified lung nodules over the study threshold. The included trials used different criteria to define a positive CT scan.
In terms of follow-up investigations for ‘positive’ nodules, although the diagnostic methods used differed between included trials, further diagnostic imaging was commonly used. There were considerable differences in the proportions of patients with additional diagnostic CT scans (5% to 52%). It should be noted that in both large trials (NELSON and NLST), similar proportions (33% to 34%) of participants undergoing additional diagnostic CT scans were observed. 57,71 This suggests that both trials may have used similar imaging follow-up protocols to resolve these scans that were identified as ‘positive’. In contrast, the proportions of patients undergoing surgical biopsy or procedures for diagnosis in the screening arm appeared to be low (ranging from 2% to 4%).
False-positive scans are one of the key concerns associated with the evaluation of adverse effects of screening, as they may be associated with an increased level of costs and more complications attributable to follow-up invasive investigations. The percentage of scans in the screening arm performed over the trial period that had a false-positive result ranged from 1.2% to 23%. The NELSON trial reported that 59.4% of positive screen results were false positive for a follow-up period of 5.5 years. 57 In particular, around 24.5% of those participants with false-positive screen results underwent an invasive procedure in the diagnostic work-up. Around 91% of these invasive procedures were surgeries (including thoracotomies, mediastinoscopies, sternotomy, video-assisted thoracoscopies), which were associated with risks of complications. The UKLS reported that, for further follow-up invasive investigations, only four participants had surgical biopsies or resections for benign disease. 55
The data from our review showed that a high proportion (96%) of positive CT screen results were false positives for the NLST trial, which required further investigations that may be harmful. 71 However, the high positive rate in NLST reflects the fact that there was no distinction being made for those indeterminate findings or interval imaging findings from false positives. It is important to note that more recent trials such as NELSON and UKLS have made such distinctions in their screening protocols in order to achieve better nodule detection management. 55,57 Limited data showed that a small proportion of participants who underwent invasive surgery procedures experienced major complications. For example, the NELSON trial reported that there were 187 participants who underwent thoracotomy in the screening arm. Among these participants, only 10.7% of participants who underwent surgical procedures had major complications. 57
Overdiagnosis
Overdiagnosis in lung cancer is one potential adverse effect associate with screening. Apart from detecting aggressive cancers, screening would also detect indolent tumours that may not cause clinical symptoms. In particular, slow-growing tumours are likely to be overdiagnosed. Therefore, it is plausible that there may be some harm to patients of identifying lung cancer nodules that are overdiagnosed, which would then lead to overtreatment (such as surgery). We found no direct data on the harms associated with overdiagnosis. Overdiagnosis associated with LDCT screening was estimated at 18.5% (95% CI 5.4% to 30.6%) on the basis of detected cancers in NLST. 95 However, the magnitude of overdiagnosed lung cancers attributable to screening is unknown, largely because the optimum duration of follow-up for measuring overdiagnosed lung cancers is not known. It should be noted that the adequate length of follow-up is of particular importance for quantifying the degree of overdiagnosis in cancer screening. 99
Radiation exposure
None of included studies reported radiation-related patient outcomes such as radiation-related lung cancer at long-term follow-up. It has been estimated that the lifetime attributable risk of major cancers due to LDCT screening ranged from 2.6 to 8.1 major cancers per 10,000 participants based on participants’ age and sex. 100
Based on a recent publication conducted in Plymouth (UK), approximately 1.5 mSv of additional radiation was given to subjects taking part in a low-dose chest CT diagnostic accuracy study. 101 This equates to an approximate 20% increase in radiation exposure when the consented individuals received the standard of care chest CT (4 mSv) and two low-dose chest CTs (0.9 and 0.48 mSv, respectively) at enrolment. This is equivalent to about 6 months of background radiation in the south-west of the UK (part of the region is designated a radon-affected area). The approximate lifetime risk for patients from 16 to 69 years old to develop fatal cancer as a result of receiving this additional radiation dose would be approximately 1 in 13,000. These risk levels represent very small additions to the 1 in 3 chance of developing cancer in the general population. Alternatively, the additional radiation exposure is the equivalent of receiving 15 extra chest radiographs. 101
Reliability of evidence
It is worth noting that the evidence for outcomes such as lung cancer and all-cause mortality is highly reliable, as such evidence is based on comparative data from RCTs. However, the evidence for other outcomes from single study arms such as positive scans and follow-up evaluations is less reliable, as this is not based on comparative data but primarily relying on data from a single arm of screening. Therefore, this limitation compromised the reliability of these findings.
Our results from this systematic review were consistent with recent systematic reviews on the effectiveness of LDCT screening. An overview of relevant systematic reviews is presented in Appendix 6. As seen in this appendix, most systematic reviews suggested that the evidence of effectiveness of LDCT was not yet conclusive. In our review, we have extended the analysis of the randomised evidence on HRQoL, psychological consequences and smoking behaviour. Our review appears to be the first review to notice the issues raised by quality assessment, particularly in relation to the MILD trial.
Generalisability of the results
Given that all included trials recruited high-risk participants, the findings from this review are generalisable to populations who are at high risk of lung cancer, despite variations in its definitions across trials. However, the generalisability of findings to those populations who are at low risk of lung cancer is very limited. The findings based on a large US-based trial (NLST) have limited generalisability to other settings (e.g. the UK setting) because of variations in health policies and differences in baseline risk profiles in the populations. In NLST, there is also concern about whether or not comparing LDCT screening with CXR screening gives a true indication of the effect of introducing LDCT where there is currently no screening. 71 This requires acceptance that CXR screening has similar effectiveness to no screening, an issue that is examined in more detail in the next chapter of the report.
It should be further noted that there were variations in the CT parameters and technology used between NLST and the European trials. For example, NLST did not use volumetric analysis but relied on utilising the CT nodule diameter in the measurement of positive nodules. 71 However, both NELSON and UKLS trials used volumetric analysis in their nodule measurement to minimise false-positive examinations. 55,57 Given this consideration, there is limited generalisability of NLST findings to the UK setting where volumetric analysis is routinely used in lung nodule detection.
Chapter 4 Network meta-analysis of lung cancer screening randomised controlled trials
The relative effectiveness of three screening strategies (LDCT, CXR and usual care) is unclear. To address this uncertainty, we performed a network meta-analysis for the primary outcome of lung cancer mortality to establish the relative effectiveness of different screening strategies between LDCT, CXR and usual care. The methods and results of the network meta-analysis are presented in this section.
Methods
Search strategy and selection criteria
The eligible population was individuals at high risk of lung cancer. Any definitions of high-risk populations were eligible for inclusion. RCTs comparing LDCT screening with usual care or CXRs were eligible for inclusion. In particular, RCTs comparing CXR with usual care were included in order to explore the possibility of network meta-analysis. RCTs that provided lung cancer mortality data were included. The search strategy has been described in Chapter 3, Identification of studies. A full search strategy can be found in Appendix 1.
Network meta-analysis methods
A network meta-analysis was performed to estimate the relative efficacy between different interventions among included trials. The relative efficacy in lung cancer mortality outcome was estimated between LDCT screening, CXR screening and usual care. We performed network meta-analysis in Stata® 14 (StataCorp LP, College Station, TX, USA).
We estimated the relative ranking probability of each intervention and obtained the treatment hierarchy of competing interventions using rankogram, surface under the cumulative ranking curve and mean ranks. 102 The probability was estimated using a Bayesian model with flat priors, under the assumption that the posterior distribution of the parameter estimates was approximated by a normal distribution with mean and variance equal to the frequentist estimates and variance–covariance matrix. 103
In order to assess the presence of inconsistency, both consistency and inconsistency models were fitted to data. We used the design-by-treatment model by Higgins et al. 104 to check the assumption of consistency in the entire network. This design-by-treatment model provides a robust approach to assess the consistency of the network being constructed.
Results
Characteristics of included studies
We identified six studies for the network meta-analysis. This network meta-analysis includes the following trials: three trials (DANTE,61 DLSCT63 and MILD69) comparing LDCT vs. usual care, one trial (NLST) comparing LDCT vs. CXR) and two trials105,106 comparing CXR vs. usual care. The characteristics of trial population and interventions for trials of LDCT screening have been described in Chapter 3,Characteristics of included studies. Figure 1 shows the flow of inclusion and exclusion of relevant trials.
Table 13 presents the summary information of characteristics for trials of CXRs for the network meta-analysis. Two trials105,106 assessing the effect of CXR screening compared with usual care were identified. The trial by Kubík and Haerting105 was conducted in the Czech Republic and the Mayo Clinic trial was conducted in the USA. The sample size ranged from 6346 to 10,933. The length of follow-up of included trials ranged from 6 to 15 years.
| Study identifier | Country | Recruitment time | Screening programme | Comparator | Sample size | Eligible age range per protocol (years) | Number of screening rounds | Screening times and interval (years) | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Kubík and Haerting105 | Czech Republic | June 1976 to June 1977 | CXR | Usual care | n = 6364 | 40–64 | Frequently = 9 vs. less frequently = 5 | Frequently: every 6 months for 3 years then once in years 4, 5 and 6 vs. less frequently: prior to randomisation, at 3, 4, 5 and 6 years | 15 years |
| Mayo106 | USA | August 1971 to NR (screening ended July 1976) | CXR, sputum cytology | Usual care (recommendation for an annual CXR and sputum cytology) | n = 11,001 (planned 10,000)a | > 45 | 18 | 4 months | 6 years |
| PLCO (for sensitivity analysis only)107 | USA | 1993 to 2001 | CXR | No screening | n = 154,901 (NLST eligible subgroup, n = 30,321) | 55–74 | 4 | Annually | 6 years (for NLST eligible subgroup) |
Both trials recruited high-risk populations. The characteristics of the study populations are shown in Appendix 5. The percentage of current smokers was 100% in the trial by Kubík and Haerting105 and 90% in the Mayo trial. 106 The percentage of former smokers was 10% in the Mayo trial. The percentage of males in both trials was 100%. The characteristics of the recruitment methods are shown in Appendix 5. The trial by Kubík and Haerting105 recruited participants from a general health preventative examination of the middle-aged male population. The Mayo trial106 recruited participants who underwent general medical examinations as outpatients at the Mayo Clinic.
There were some variations in the definitions of high risk between trials, but both trials mainly used age and smoking history to define high risk. For example, the trial by Kubík and Haerting105 used the following criteria to define high risk: (1) aged 40–64 years, (2) current smokers with an approximate lifetime consumption of ≥ 150,000 cigarettes and (3) participants who were smoking at time of enrolment with unknown pulmonary disease visible on the chest roentgenogram. The Mayo trial used the following criteria for the definition of high-risk participants: (1) aged ≥ 45 years and (2) participants who were smoking one packet or more of cigarettes each day (either at the time of entry into the screening programme or during the preceding year).
The characteristics of the screening programmes are shown in Appendix 5. In the trial by Kubík and Haerting105 CXR was performed at baseline, 6-monthly during years 1–3, and then at years 3–6 of follow-up. In the Mayo trial, CXR was conducted 4-monthly. This trial defined positive scans as those with an abnormality identified (it was at the discretion of the single radiograph reader whether or not further investigation was required, i.e. no second reading or central review). However, the Mayo trial106 did not report the definition of a positive scan. In terms of imaging evaluation and interpretation strategy, both trials reported that double-reading by chest physician and chest radiologist was used; if there was disagreement, the final decision was based on consensus (third experienced physician arbitrated disagreements).
We included the PLCO trial (NLST eligible subgroup only) for sensitivity analysis (see Table 13), as the PLCO trial was not an included trial because it did not meet inclusion criteria. This trial recruited the general population (including both high- and low-risk participants), with a sample size of 154,901. In our sensitivity analysis, we used only the data of the NLST eligible subgroup (n = 30,321) from the PLCO trial over the 6-year follow-up period because this subgroup (including high-risk participants) was more relevant to our research question. However, it should be noted that the results of this subgroup were based on post hoc analyses. This trial compared CXR screening with usual care (no-screening) only. The PLCO trial performed four screening arounds (annually), with duration of follow-up of 3 years. Further details of this trial are shown in Appendix 5.
Network meta-analysis results
Overall analysis
The overall network meta-analysis included six trials: three trials comparing LDCT with usual care, one trial comparing LDCT with CXR and two trials comparing CXR with usual care. Figure 18 presents the network of available intervention comparisons for lung cancer mortality.
FIGURE 18.
Network map. The size of nodes is proportional to the number of individuals randomised to each intervention and the thickness of each line is proportional to the number of direct comparisons in trials.
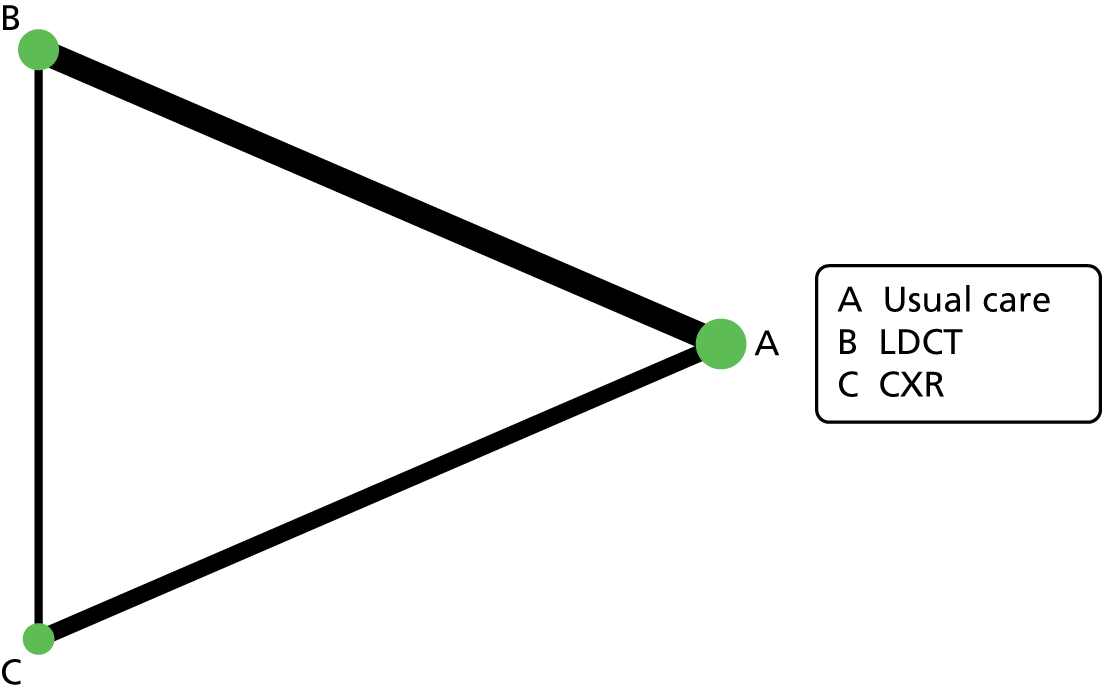
Figure 19 presents the cumulative probability of three screening strategies (LDCT, usual care and CXR) for the lung cancer mortality outcome. The estimated RR of LDCT compared with usual care was 0.95 (95% CI 0.82 to 1.11). LDCT was ranked first according to the estimated surface under the cumulative ranking curve values, with a 74.8% probability of being the best intervention in terms of lung cancer mortality reduction. Usual care had a 74.7% probability of being the second best strategy among the three interventions. However, CXR screening had a 99.7% probability of being the worst intervention in terms of lung cancer mortality reduction.
FIGURE 19.
Rankogram for the outcome of lung cancer mortality. Ranking indicates the probability of being the best treatment, the second best, and so on, among the different interventions under evaluation. Sensitivity analysis includes NLST-eligible subpopulation from PLCO. (a) Usual care; (b) LDCT; and (c) CXR.

Overall, the results of network meta-analysis showed that LDCT screening was ranked as the most effective intervention for the outcome of lung cancer mortality compared with other screening strategies (usual care and CXR).
Both consistency and inconsistency models were fit for lung cancer mortality data. By applying the design-by-treatment model, we did not find any evidence of inconsistency. The global test for inconsistency gives a p-value of 0.29, indicating no evidence of inconsistency.
Sensitivity analysis
Sensitivity analysis was performed by including data of the NLST eligible subgroup from another large trial (PLCO) comparing CXR with usual care. 107 The sensitivity analysis showed similar results to the overall analysis: LDCT was ranked as the most effective strategy and CXR screening was ranked as the worst strategy in terms of lung cancer mortality outcome.
Figure 19 presents the results of sensitivity analysis of cumulative probability of three screening strategies for the lung cancer mortality outcome. The estimated RR of comparing LDCT with usual care from the sensitivity analysis of network meta-analysis was 0.93 (95% CI 0.76 to 1.14). Based on the estimated surface under the cumulative ranking curve values, LDCT screening was ranked first: it had a 75.3% probability of being the best intervention in terms of lung cancer mortality reduction. Usual care had a 68.3% probability of being the second best strategy among the three interventions. Similarly, CXR screening had a 87.7% probability of being the worst intervention in terms of the lung cancer mortality outcome.
For sensitivity analysis, both consistency and inconsistency models were also fit for lung cancer mortality data. By applying the design-by-treatment model, we did not find any evidence of inconsistency. The global test for inconsistency gives a p-value of 0.18, suggesting that there was no evidence of inconsistency.
Discussion
The results of network meta-analysis showed that LDCT screening was ranked as the most effective intervention for the outcome of lung cancer mortality compared with both CXR screening and usual care, according to the estimated surface under the cumulative ranking curve values. The CXR was ranked as the worst screening strategy for the lung cancer mortality outcome. We performed sensitivity analysis by including data of the NLST eligible subgroup from the PLCO trial (comparing CXR with usual care). 107 This sensitivity analysis demonstrated consistent results. 107 Both consistency and inconsistency models were fit for data and we did not find any evidence of inconsistency.
To date, no research has been conducted to establish the relative efficacy between three screening strategies (LDCT, usual care and CXR screening). The current network meta-analysis provides insight into the relative effectiveness of LDCT, usual care and CXR screening for lung cancer mortality outcomes in high-risk populations. It suggests that it may not be appropriate to consider lung cancer screening with CXR as equivalent in effectiveness to usual care with no systematic screening. This reinforces concern about whether or not the largest of the RCTs in the systematic review, NLST, truly reflects the effectiveness of LDCT relative to no screening, the comparison best reflecting the policy decision in question in this report. If the effectiveness of CXR screening is truly worse than no screening, the results of NLST will overstate the magnitude of the effectiveness of LDCT screening. Whether or not this is true will be confirmed or refuted as new mortality data on RCTs that have compared LDCT with no screening data, such as NELSON, emerge.
The findings demonstrated that LDCT screening was ranked as the best screening strategy in terms of lung cancer mortality reduction. Compared with CXR screening, LDCT scans are able to detect smaller pulmonary abnormal nodules, thus leading to better precision of diagnosis. Therefore, the finding from the network meta-analysis may be partly explained by improved diagnostic abilities associated with LDCT screening.
In conclusion, the findings of this network meta-analysis provide robust evidence supporting LDCT to be the most effective screening strategy on lung cancer mortality reduction in high-risk populations. It also introduces uncertainty about whether or not the effectiveness of LDCT measured in NLST accurately captures the size of the effect of introducing LDCT screening relative to no screening. To date, no research has been conducted to establish the relative efficacy between three screening strategies (LDCT, usual care and CXR screening) (see Appendix 6 for an overview of relevant systematic reviews). The current network meta-analysis provides insight into the relative effectiveness of LDCT, usual care and CXR screening for lung cancer mortality outcomes in high-risk populations.
Individual participant data meta-analysis on this topic is a necessary next step to find target populations for different screening interventions and would allow a more personalised screening strategy for early lung cancer detection in high-risk populations.
Chapter 5 Systematic review of existing cost-effectiveness evidence
Methods
Identification of studies
The search strategy included searching the following electronic databases:
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
HMIC (Health Management Information Consortium) (via Ovid)
-
Web of Science (via Clarivate Analytics)
-
NHS EED (NHS Economic Evaluation Database) and HTA (via The Cochrane Library)
-
EconLit (via EBSCOhost).
The searches were developed and run by an information specialist (SR) in January 2017. Search filters were used to limit the searches to economic studies as appropriate. Searches for economic studies were limited to 2004 onwards and searches for health utilities studies were limited to the English language. An update search was carried out in April 2017 and limited to economic studies. The search strategies for each database are detailed in Appendix 1.
The database search results were exported to, and deduplicated using, EndNote (X7) [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA]. Deduplication was also performed using manual checking.
Records were screened for eligibility on the basis of title and abstract by one reviewer (TS). Full texts were retrieved for potentially eligible studies and were assessed for eligibility by one reviewer (TS). Studies included by any identified systematic reviews of economic evaluations were also retrieved as full texts and assessed for eligibility.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were decided before records were screened for eligibility and are shown in Table 14.
| Criteria | Include | Exclude |
|---|---|---|
| Screening population | People at risk of lung cancer |
People with existing cancer, including lung cancer People with clinically suspected lung cancer People with hereditary cancer syndromes |
| Target condition | Lung cancer | |
| Intervention(s) | LDCT (single or multiple screen) | |
| Comparator(s) | No screening or screening with another imaging modality (e.g. X-ray) |
No comparator Screening with non-imaging modality (e.g. sputum culture, breath analysis) |
| Study design |
Cost–utility analysis Cost-effectiveness analysis Cost–benefit analysis Cost–consequences analysis NHS cost analysis |
Cost-minimisation analysis Non-NHS cost analysis Studies not presenting incremental analyses or allowing for their calculation Non-systematic reviews Editorials/comments/letters |
| Methodology |
Trial based Model based Systematic review of economic evaluations |
|
| Other |
Abstracts (when not linked to an included full-text paper) Non-English-language papers without an official translation |
Data abstraction and quality assessment
Trial- and model-based evaluations
Simple data abstraction templates were developed in advance of study selection.
Data were abstracted by one reviewer (TS) based on main publications (i.e. without referring to any supplementary materials).
The quality of studies was assessed using the Consensus on Health Economic Criteria (CHEC)-list for economic evaluations,109 with certain modifications/guidelines for assessment (see Appendix 7).
Only the main publication was examined for quality assessment (i.e. supplementary materials were not checked).
Systematic reviews of economic evaluations
Simple data abstraction templates were developed in advance of study selection.
Data were abstracted by one reviewer (TS) based on main publications (i.e. without referring to any supplementary materials).
No quality assessment was conducted of systematic reviews of economic evaluations.
Methods of data synthesis
Synthesis was by tabulation of characteristics and results and narrative synthesis by one reviewer (TS).
Results
A total of 3004 citations were considered, which ultimately led to the identification of 19 trial- and model-based analyses (reported in 21 publications55,110–129) and five systematic reviews (reported in six publications54,130–134), as shown in Figure 20.
FIGURE 20.
The PRISMA flow diagram for systematic review of existing cost-effectiveness evidence.
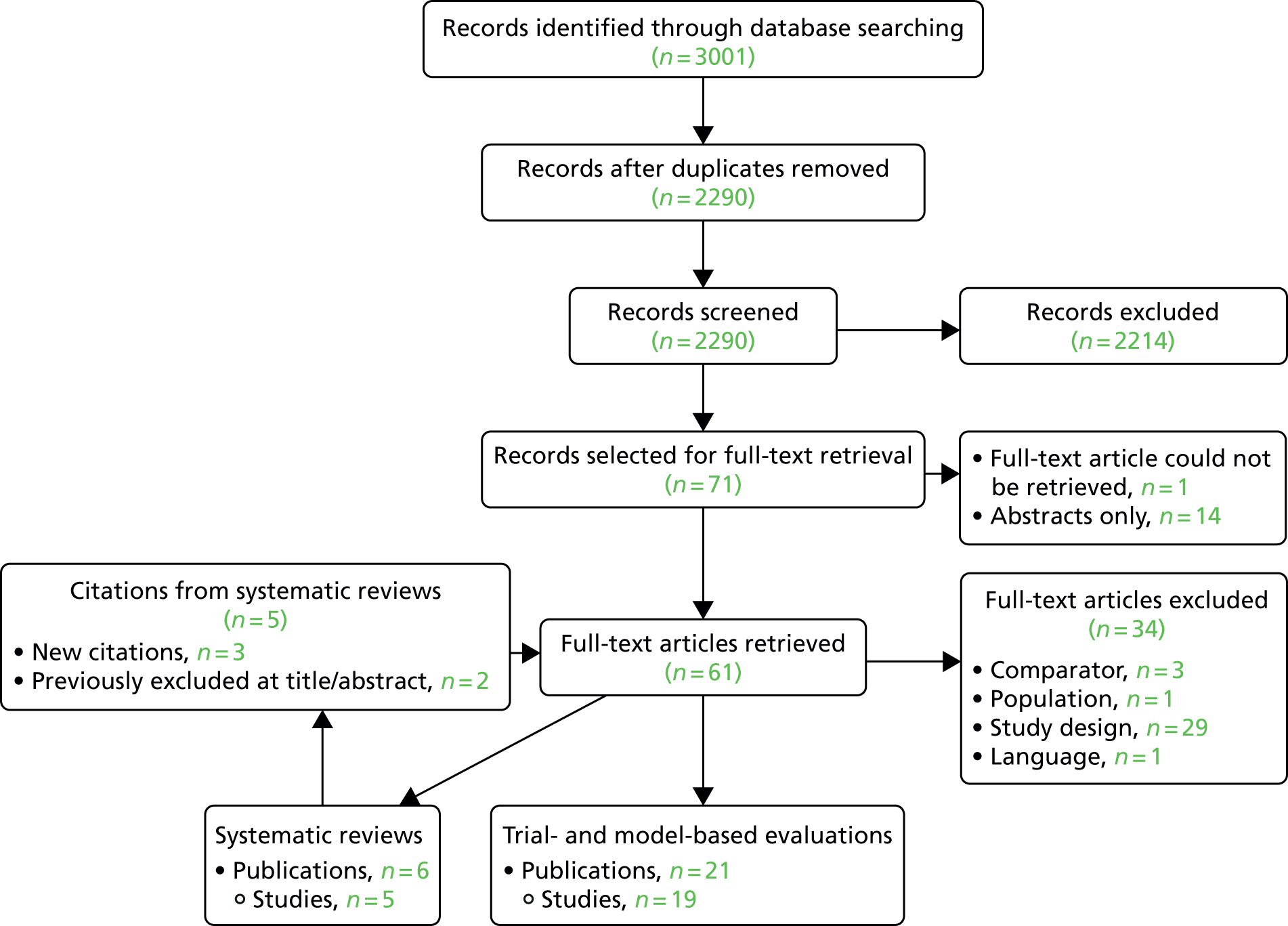
Characteristics of included studies
Tables 49 and 50, Appendix 7, present the characteristics and results of the included trial- and model-based studies, respectively.
Table 51, Appendix 7, presents the characteristics and results of the included systematic reviews.
Quality assessment
The results of the quality assessment are shown in Appendix 7.
Most studies failed to perform sufficient sensitivity analyses, and a significant number of studies did not estimate quality-adjusted life-years (QALYs). Only five studies (reported in six publications110–115) were judged to have used an economic study design appropriate to the stated objective. The other studies relied on sources at a high risk of bias (e.g. cohort studies) or unsupported assumptions.
No studies clearly failed to include the three necessary cost items (LDCT scans, follow-up tests and lung cancer costs), although some studies did not report adequately in the main publication to make a judgement.
Narrative synthesis
Trial- and model-based analysis
A common theme in the study results is that LDCT screening is more costly and more effective than no screening. Studies had sharply diverging conclusions about the cost-effectiveness of screening. There is some evidence that studies based on the Early Lung Cancer Action Project (ELCAP) cohort study135 predict improved cost-effectiveness for screening117–119,122,125,127,128 versus studies based on NLST110–113,115,124,127 or lung cancer natural history models.
Three different natural history models have been used to predict the cost-effectiveness of LDCT screening. The Lung Cancer Policy Model was used by McMahon et al. 114 and suggested that LDCT would not be cost-effective. The Cancer Risk Management Model (renamed OncoSim) was used by Goffin et al. 112,113 and suggested that biennial LDCT would be cost-effective. The Microsimulation Screening Analysis (MISCAN) – Lung model was used by ten Haaf et al. 115 and suggested that annual LDCT would be cost-effective.
Many studies identified that the cost of LDCT scans, the effectiveness of screening (identifying early-stage lung cancers) and the prevalence and incidence of lung cancer are key factors affecting the cost-effectiveness of screening. A number of studies considered age and smoking history and found these to be influential also.
Some studies incorporated smoking cessation as an adjunct intervention, but these studies did not generally consider the cost-effectiveness of screening versus smoking cessation, or screening with smoking cessation versus smoking cessation and, therefore, were not appropriate evaluations of lung cancer screening.
Systematic reviews of economic evaluations
Existing systematic reviews found significant variation in methodology and results of economic evaluations. Earlier economic evaluations did not have access to good-quality estimates of clinical effectiveness, and the studies that incorporated the results of NLST produced ICERs of < US$100,000 per QALY. More recent economic evaluations were generally more methodologically robust (see Table 51, Appendix 7).
The reviews concentrated on the cost-effectiveness of screening versus no screening, without any significant attention being paid to the impact of frequency of screening.
Discussion
Key findings
Although a number of economic evaluations of LDCT screening for lung cancer have been conducted, they have not produced consistent results in terms of the cost-effectiveness of screening, and few commented on the generalisability of their findings.
Previous systematic reviews have identified that there is significant heterogeneity in the results of economic evaluations of lung cancer screening, and that this makes it difficult to draw conclusions about its cost-effectiveness, particularly when considering an individual setting.
Two economic evaluations were conducted in the UK setting,55,116,117 both led by Professor David Whynes. Both concluded that LDCT screening could be cost-effective in the UK. The more recent of these evaluations55,116 included a comparison with an economic evaluation based on NLST,110,111 highlighting the likely reasons why the latter had found a less favourable estimate of the cost-effectiveness of LDCT screening. However, these UK-based economic evaluations have not been based on high-quality evidence (although they have produced somewhat consistent results in terms of incremental QALYs compared with studies that are based on high-quality evidence).
Certain factors regularly appeared as significant in determining cost-effectiveness:
-
the cost of a LDCT scan
-
the risk of lung cancer (prevalence, and incidence for studies evaluating more than a single screen) in the screened cohort
-
the effectiveness of LDCT screening in broad terms (e.g. achieving a stage shift without significant overdiagnosis, extending lung cancer survival beyond lead time, reducing lung cancer mortality).
The first of these points is specific to each setting and may also be a reasonable target for service delivery interventions (e.g. establishing specialist centres for high-throughput LDCT screening). The second and third points are intertwined, as the effectiveness as measured will depend on the population being investigated. Nevertheless, it is well within the bounds of possibility to estimate with some accuracy the risk of lung cancer, and to restrict screening to those with the greatest need or potential benefit.
Relation to existing work
Other systematic reviews of economic evaluations included six studies we have not included (three because they considered CXR rather than LDCT, one because it considered whole-body CT, two because they did not present an incremental analysis), as shown in Appendix 7, Table 53.
This review included 21 publications of trial- and model-based economic evaluations of lung cancer screening by LDCT, reporting results of 19 studies. Seven of these publications have not been identified by existing systematic reviews (see Appendix 7, Table 54).
Strengths and limitations
Comprehensive searches were designed and conducted by an experienced information specialist (SR). Other systematic reviews have not identified any studies eligible for inclusion that were not identified in these searches.
Other aspects of the review (study selection, data abstraction, quality assessment and narrative synthesis) were performed by a single reviewer with experience of reviews of economic evaluations (TS), but were not independently performed or checked by another reviewer. It is therefore possible that relevant studies could have been excluded (although no such studies were identified by other systematic reviews) and that errors could have been made in data abstraction or quality assessment.
Additionally, the review only included English-language publications and there was no quality assessment of systematic reviews.
Areas of uncertainty
Significant uncertainty remains as to the cost-effectiveness of LDCT screening for lung cancer. The wide range of results from existing studies makes it challenging to draw conclusions, and the evolving clinical effectiveness literature has significantly affected the economic evaluation literature. Many studies were conducted without access to high-quality estimates of the effectiveness of screening and most studies were conducted in countries other than the UK.
It is important to establish estimates for the cost-effectiveness of LDCT screening in the UK based on high-quality data, and for these estimates to cover a broad range of possible interventions to minimise the possibility of rejecting LDCT screening on cost-effectiveness grounds despite the existence of a cost-effective alternative configuration, which is not explored.
Chapter 6 Independent economic assessment
Owing to the lack of an existing economic evaluation in the UK that allows for exploration of multiple alternatives, an independent economic evaluation was conducted based on a new decision model. The layout of this chapter is based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist. 136
Methods
An individual patient simulation model was developed in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) using a discrete event simulation (DES) framework.
Individual patients were sampled across a spectrum of baseline characteristics and their outcomes concurrently simulated across different intervention strategies (i.e. the number of CT screening rounds and the time between rounds) and a control arm representing current practice (no screening). A large number of individual patients sampled together defined a cohort.
Different population strategies were modelled at the cohort level, which were defined in terms of age criteria for entry (minimum and maximum age) and a risk threshold. Simulated individuals meeting the criteria in the population strategy would receive an intervention strategy, while individuals not meeting the criteria would receive no screening.
Lifetime costs and QALYs were estimated for each combination of population and intervention strategy.
Modelling approach
Target population and subgroups
The target population was people who are at higher risk of lung cancer relative to the general population. Specifically, the model considered people aged between 55 and 80 years with a history of smoking (i.e. current or former smokers).
Subgroups in this population are identified by further restricting the age range and by imposing a minimum threshold on the predicted risk of lung cancer for an individual to be eligible for screening.
Setting and location
The setting was the NHS in the UK.
Initial invitations to screening may be sent from primary care (as initial identification of people who may be at high risk could be from primary care records). Screening CT scans are performed in secondary and tertiary settings. Cancer care is performed in secondary and tertiary settings. Palliative care may be delivered in secondary settings, in the community or in hospices.
Study perspective
The perspective on costs was NHS and Personal Social Services (PSS). The economic evaluation therefore did not include any effect on tax revenue, pensions, productivity or out-of-pocket expenses for affected individuals (e.g. transport).
Direct health effects on individuals who were contacted through a screening programme were included.
No direct health effects on family or carers were included as no good-quality evidence was found to support their inclusion.
Although some screening studies have shown an impact on the smoking behaviour of participants (e.g. Clark et al. 90), and despite it being very likely that increased quitting reduces the risk of lung cancer mortality, no attempt has been made to model smoking behaviour in the model.
Comparators
Four screening programme designs were compared with no screening in 12 population alternatives, thereby creating 48 intervention strategies and one control (no screening programme) strategy that represents current practice.
Chest X-ray was not considered as a comparator as it is not considered a relevant policy option (the briefing note for this project lists only no screening as a comparator) and because RCT evidence has shown that CXR is not expected to be clinically effective. 107
Discount rate
Costs and QALYs were discounted at 3.5% per year. These are the conventional discount rates for technology appraisal in England,137 Scotland138 and Wales139 (Northern Ireland typically endorses NICE technology appraisals) and are derived from the UK Treasury discount rate. 140
Time horizon
Individuals are modelled until death. Most simulated individuals are dead by 100 years of age (e.g. 98.9% of women simulated from the age of 80 years die before the age of 100 years).
Choice of health outcomes
The primary health outcomes were HRQoL and life-years attained in each strategy, expressed in QALYs, as is the preference in UK cost-effectiveness decision analyses. 137 In the incremental analysis, the outcome was QALYs gained versus no screening.
In addition, secondary health outcomes were:
-
screening programme sensitivity
-
number of lung cancers diagnosed per 100,000 entrants
-
interval cancers diagnosed per 100,000 entrants
-
mortality per 100,000 entrants
-
5-year survival from diagnosis of lung cancer
-
substage distribution
-
average age at diagnosis, death from lung cancer, death from other
-
lead time.
Analysis methods
The economic evaluation employed a cost-effectiveness (cost–utility) analysis, in which the costs and QALYs for each alternative (combination of population and intervention strategies) are estimated and then a cost-effectiveness frontier is constructed by eliminating strategies that are dominated or extendedly dominated. The cost-effectiveness of each alternative is then assessed using the incremental cost-effectiveness ratio (ICER), the ratio of incremental costs to incremental QALYs.
-
Main analysis: cost-effectiveness analysis of all alternatives, in which the ICER is calculated both versus the most effective alternative on the cost-effectiveness frontier that is less effective than the current option, and versus current practice (no screening).
-
Secondary analyses:
-
Cost-effectiveness analyses of intervention strategies for each choice of population strategy (e.g. a cost-effectiveness of four intervention strategies and no screening assuming a minimum age at entry of 60 years, a maximum age at entry of 75 years and a minimum risk of 4%).
-
Net monetary benefit (NMB) maximisation (at willingness to pay of £20,000 per QALY) conducted for each intervention strategy to identify the optimal choice of age limits and predicted risk thresholds.
-
These analyses are conducted in the base-case analysis (in which all parameters are fixed at their base-case values and a large cohort of patients is simulated).
Sensitivity analyses are also conducted:
-
Deterministic sensitivity analysis: main analysis conducted with deterministic changes to parameter values (the results remain stochastic as individual patients continue to be simulated).
-
Probabilistic sensitivity analysis (PSA): multiple cohorts simulated, each with a single set of parameter values sampled probabilistically from suitable distributions reflecting parameter uncertainty. A main analysis was conducted and cost-effectiveness acceptability curves were produced.
Software
The model was implemented in Microsoft Excel. Supporting analyses were conducted in Stata 14.2, R 3.3.3 (The R Foundation for Statistical Computing, Vienna, Austria), and JAGS 4.2 (Martyn Plummer, Lyon, France). 141
Model structure
Overview
A cohort of individuals is simulated with a range of baseline characteristics (including age and predicted risk of lung cancer). The age range of simulated individuals is 55–80 years, as no screening programmes are being evaluated that would include individuals outside this age range.
Each individual is concurrently simulated with four screening intervention arms and a control (no screening) arm. By simulating the individuals concurrently through all arms there is a reduction in stochastic variation.
The costs, QALYs and other outcomes for each full programme (combination of population strategy and intervention) are estimated using a decision tree. Costs of administering the screening programme (sending letters with questionnaires, analysing the questionnaires to estimate the lung cancer risk, sending invitations to those at high risk) are accumulated through the decision tree, and long-term costs and QALYs are estimated at the leaves of the decision tree by identifying appropriate individuals simulated in the cohort and assigning them appropriately either to the screening intervention (if they meet all criteria and join the screening programme) or to no screening.
It was assumed that the two uptake probabilities (relating to a person completing a risk questionnaire and agreeing to join the programme after being informed they are at high risk) are independent of age and sex, although gender has been identified as a potential factor influencing the decision to participate in a lung cancer screening programme (see Chapter 7).
Figure 21 shows how age thresholds and the risk threshold are used to identify individuals at high risk of lung cancer and map them to screening interventions.
FIGURE 21.
Population selection diagram.
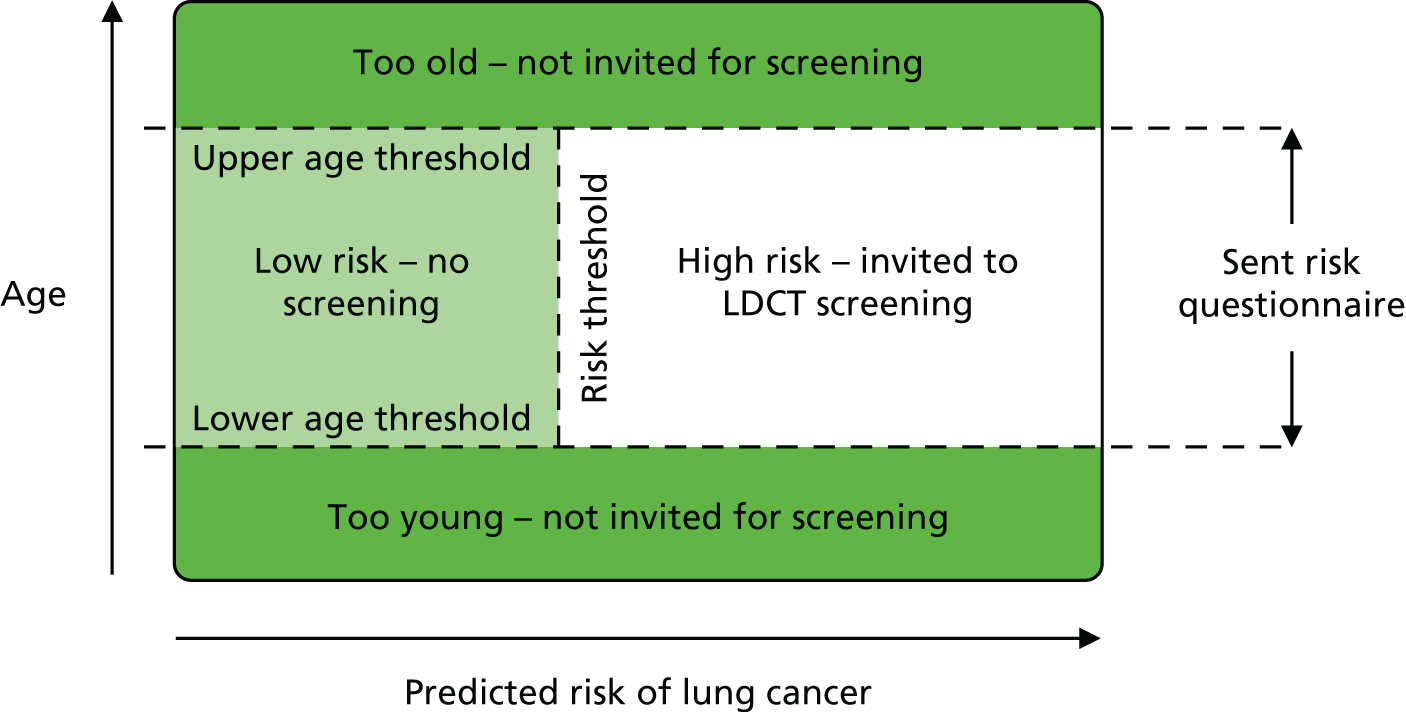
Simulating individuals
The DES modelling framework was employed, but without interactions between individuals (i.e. no queues or shared resources). This method of simulation involves sampling times to future events according to the current state of the individual (and any relevant history). The earliest of these events is modelled as occurring and the model ‘clock’ advances to that event. Times to events are then either reduced by the amount the clock has advanced or are resampled (as appropriate). Figure 22 provides a diagram of the model.
FIGURE 22.
Model diagram for simulating individuals. LC, lung cancer.

Individuals began the simulation without clinically diagnosed lung cancer, although they could have occult lung cancer.
A natural history model of lung cancer was utilised to generate outcomes for individuals in the absence of screening (see Natural history). The action of lung cancer screening is to identify preclinical (occult, asymptomatic) lung cancer earlier than it would be clinically diagnosed. The sensitivity of the screening test affects the likelihood that a cancer will be detected at the time of screening.
If a lung cancer is detected by screening in an earlier stage than it would have presented clinically, then the time to lung cancer mortality (i.e. survival) is extended, as survival is related to stage. If a lung cancer is detected by screening in the same stage as it would have presented clinically, the time to lung cancer mortality is extended by the lead time. In any case, the age at lung cancer mortality is modelled as never being earlier when a cancer is screen detected than if the cancer had presented clinically.
In the base case it is assumed that there is no heterogeneity between patients in the rate at which their cancers progress or present, but in a scenario analysis a random effect is included for each simulated individual across their rates of progression, as shown in Equation 1:
In this equation, λk→k + 1i is the rate of progression from state k to state k + 1 for individual i. ϵi is the error term for between-individual variability in the log-rate of progression, with variance σ2p. Note that the variance is assumed to be the same for all progression rates.
Note that in the base case there is no lung cancer mortality from the preclinical lung cancer states. This is justified in Natural history. Note also that there is no explicit modelling of cancer progression after diagnosis, as the costs and outcomes are intended to be averaged across lung cancers diagnosed in each stage.
The model does not include incidental findings resulting from screening. It is likely that such findings would lead to increased health-care resource use in the short term (although these could in some cases be offset by savings in the long term), and could lead to improved QoL if the disease has been causing reduced QoL and treatment is effective.
Five screening programme designs were developed following consultation with the expert advisory group. These varied according to the target number of screens, the interval time between screens and the duration of the programme (Table 15). UKLS55 adopted a single-screen design; the NLST and NELSON trials adopted a triple screen design, with increasing screening intervals in NELSON.
| Design name | Design features |
|---|---|
| No screening | Patients are not screened: diagnosis by clinical presentation only |
| Single screena | One-off screen shortly following entry into programme |
| Triple screenb | First screen shortly following entry, then a second at 12 months and a third at 24 months |
| Annual repeated screenc | First screen shortly following entry, then screens repeating 12-monthly from the date of entry until 80th birthday |
| Biennial repeated screen | First screen shortly following entry, then screens repeating 24-monthly from the date of entry until 80th birthday |
Twelve population subgroups combining age at entry (minimum ages 55 and 60 years, maximum ages 75 and 80 years) and predicted risk (minimum risk 3%, 4% and 5%) were specified. Only patients in the starting cohort whose age and risk profiles met the criteria of these populations were included in the respective strategy analyses.
Starting characteristics
Each individual was simulated with the following random starting characteristics:
-
age
-
sex
-
baseline disease state
-
risk score.
In the base case, the age distribution of individuals was estimated from UKLS for the participants returning a questionnaire. 55 Truncated normal regression was performed on the age of participants with cut-off points at 50 years and 76 years using the Stata command truncreg. The estimated age [mean and standard deviation (SD)] was 61.94 years (SD 9.00 years). The ages were sampled using a truncated normal distribution but with cut-off points at 55 years and 80 years to reflect the widest range of eligibility criteria.
A scenario analysis was conducted using an age distribution fitted to the approximate age distribution of smokers in the UK143,144 (aged 55–80 years) and using least squares regression to estimate an underlying normal distribution of 61.62 (SD 15.19) years. This scenario predicts a similar mean age but a greater spread.
The sex distribution of individuals was similarly estimated from UKLS participants returning a questionnaire, producing an estimate of 48.2% being men.
The baseline disease state was estimated by sampling the age at preclinical lung cancer incidence and the age at entry to the programme. If the age at preclinical lung cancer incidence was less than the age at entry to the programme, then the stage of preclinical lung cancer was estimated using probabilities inversely proportional to the expected time spent in each stage in the absence of screening. If the age at preclinical lung cancer incidence was greater than the age at entry to the programme, then the individual started with no lung cancer.
The risk score was estimated as detailed in Effectiveness estimates.
Model parameters
Details of model parameters are given throughout this chapter and Appendix 9 provides a full listing of model parameters, their base-case values and their PSA distributions.
Natural history
A Bayesian Markov chain Monte Carlo (MCMC) analysis was conducted to calibrate a natural history model of lung cancer to the NLST RCT71 and the incidence of lung cancer (International Classification of Diseases, Tenth Edition,145 C33–C34) in England in 2014 according to sex and age (5-year age groups),1 adjusted for the population attributable fraction due to smoking143 and the estimated smoking (current and former) population. 143
The analysis was performed using JAGS141 and the rjags and coda146 packages in R.
The natural history model assumes progression through the stages of lung cancer (IA, IB, . . ., IV) in the absence of treatment. Clinical presentation or identification through screening lead to clinical lung cancer.
A two-stage approach to calibration was used:
-
Natural history model fitted to NLST data.
-
Preclinical progression and clinical presentation parameters fixed to their expected values from step 1, and preclinical incidence parameters fitted to English estimates of the incidence of lung cancer among smokers.
A log-normal distribution was assumed for preclinical incidence of lung cancer. In step 1, this was assumed to be the same for men and women, whereas in step 2 it was assumed that the location (µ) parameter would vary between men and women.
The natural history model assumed exponential distributions for the time to preclinical progression (from stage IA to stage IB, from stage IB to stage IIA, etc.) and the time to clinical presentation (according to the stage).
In the base case, no heterogeneity in the overall speed of cancer progression was included, whereas in a scenario analysis, heterogeneity was included, as described in Equation 1.
As shown in Figure 23, the rate of clinical presentation is significantly lower at all stages than the expected time to progression, which is why most lung cancers are identified only in the latest stage. At the expected values of the parameters, the expected stage distribution is 10.3%, 4.7%, 10.4% and 74.6% for stages I, II, III and IV, respectively.
FIGURE 23.
Rates of progression and clinical presentation for each stage of lung cancer.

The calibrated incidence curves demonstrate somewhat higher incidence for men than for women, with very little incidence before the age of 50 years.
Further details of the calibration are given in Appendix 8, including example JAGS code and the model-run characteristics.
Lung cancer survival
Survival from lung cancer was estimated according to the stage of lung cancer at diagnosis. The key data source was the International Association for the Study of Lung Cancer (IASLC) study used to develop the TNM Seventh Edition,24 which provides mature survival estimates based on a significant number of patients. The drawback of this data set is that the patients are primarily not from the UK, although they were drawn from 46 sources across 19 countries.
Kaplan–Meier curves for survival according to clinical stage were extracted and annual values up to 10 years were isolated. A Weibull plot was constructed by plotting cumulative hazard (logarithmic scale) versus time (logarithmic scale). The lines were close to straight and close to parallel and, therefore, a proportional hazards Weibull model was judged to be appropriate. A weighted linear regression was performed on log(cumulative hazard) with log(time) and stage as independent variables. Each point was weighted by the number of patients diagnosed in the stage multiplied by Kaplan–Meier survival (as an approximation of the number of patients contributing to the data point).
The resulting survival curves closely match the extracted survival data, as shown in Figure 24.
FIGURE 24.
Comparison of survival data and Weibull fit. (a) Extracted Kaplan-Meier data; and (b) Weibull fit.

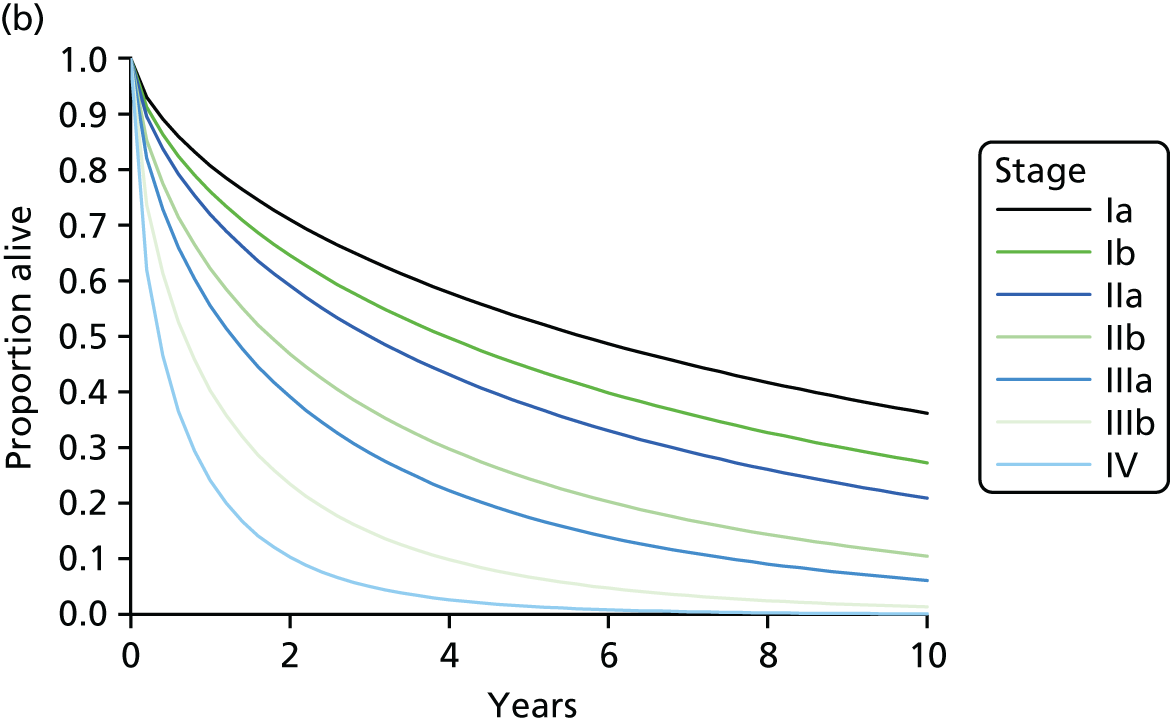
To reduce unnecessary variability between intervention arms and to achieve a consistent improvement in survival when a stage shift is achieved, a single quantile was sampled randomly per lung cancer and used to sample all survival times for the different stages of the lung cancer.
Mortality from undiagnosed lung cancer
The rate of death from preclinical lung cancer is a highly uncertain quantity, as it is believed that most individuals dying from lung cancer will do so with a diagnosis of lung cancer (i.e. they will have clinically presented). This suggests that the rate of death from preclinical lung cancer should be much lower than the rate of clinical presentation, even if this is significantly lower than the mortality rate for clinical lung cancer, or even close to zero. This appears paradoxical, as diagnosing lung cancer should not accelerate mortality, as the aim of treatment is (in many cases) to prolong life expectancy. The paradox is resolved by not assuming that the time to clinical presentation and lung cancer mortality are independent, but in fact that individuals would present shortly before dying from a previously undiagnosed lung cancer.
As our estimate of lung cancer survival should include all patients, even those with very limited survival post diagnosis, it is reasonable to estimate the rate of clinical presentation assuming a very low probability of dying from lung cancer prior to diagnosis.
In the base case it is assumed that there is no hazard of dying from preclinical lung cancer, but as soon as an individual is diagnosed with lung cancer, they then face the hazard of death from lung cancer.
Other cause mortality
Individuals at high risk of lung cancer are likely to be at a higher risk of mortality from other causes as well (e.g. cardiovascular disease, other respiratory disease, other cancers).
We estimated the risk of death from causes other than lung cancer for smokers in the following ways:
-
We adjusted the overall risk of death for smoking.
-
We estimated and removed the proportion of the mortality rate attributable to lung cancer.
-
We fitted a parametric (Gompertz) model to the resulting mortality profile from age 30 years.
The baseline risk of death was taken from the interim life tables for England and Wales based on data for the years 2010–12. 147
The adjustment for the risk of death caused by smoking was taken from the Institute and Faculty of Actuaries ‘00’ tables,148 which allow comparison of the mortality rates in male and female smokers versus males and females generally. The population here is individuals with permanent life assurance policies, which may mean that the data are not wholly representative, but we used the estimates after the ‘select period’ (during which individuals are at a lower risk of mortality because of selection bias), and these estimates were only used to adjust the life tables for England and Wales, which are based on national data. The adjustment was performed by estimating the mortality rate ratio between smokers and the general population for each year of age from 30 years, and then applying this to the England and Wales life tables.
The proportion of mortality caused by lung cancer was estimated using cause of death data from the Office for National Statistics,149 estimating the number of lung cancers attributable to smoking for each age group using population attributable fractions of 86.3% and 72.2% for men and women, respectively,143 using estimates of smoking prevalence (current smokers and former smokers) in each age group143 to estimate the population size in each age group, and finally dividing the number of lung cancers attributable to smoking by the population size of smokers in each age group to obtain a mortality rate from lung cancer in smokers.
The mortality rate from lung cancer in smokers was subtracted from the overall mortality rate in smokers to produce a final estimate of the risk of death from causes other than lung cancer (Figure 25).
FIGURE 25.
Survival rates for smokers, excluding lung cancer as a cause of death.

Graphical inspection of the mortality profiles (log-hazard versus time) demonstrated exponential growth in the mortality rate from age 30 years, suggesting that a Gompertz model would be appropriate. Gompertz models were fitted separately for men and women using least squares regression on log-hazard for ages 30–100 years.
In the model, the age at death from other causes is sampled conditionally on the individual being alive at the start of the simulation as shown in Equation 2, where λ and γ are the Gompertz parameters, U is a uniform random variable between 0 and 1 and t0 is the age of the individual at the start of the simulation:
Effectiveness estimates
Uptake of screening
The probability of an individual responding to an initial letter regarding lung cancer screening with a lung cancer risk questionnaire was estimated from UKLS,55 as this included contacting a significant number of people in two qualitatively different areas and included a risk questionnaire. In this trial, 247,354 individuals were sent invitation letters, of whom 75,958 responded positively with a completed questionnaire. Therefore, it was assumed that 30.7% of individuals would respond to an initial letter and complete a risk questionnaire.
The probability of an individual meeting all criteria for entry to a screening programme (having initially responded and completed a risk questionnaire) was also estimated from UKLS. 55 In this trial, 8729 individuals were classified as high risk, of whom 4061 individuals subsequently gave informed consent to participate in the trial. Therefore, it was assumed that 46.5% of individuals would join a programme if invited.
The model also assumes that once an individual takes up screening, that they are 100% compliant (i.e. there are no missed screens).
Risk prediction
To achieve a favourable balance of benefits, harms and costs, it is necessary to target screening towards individuals at high risk of lung cancer. The ability to accurately discriminate between individuals at low and high risk is a key component of the effectiveness of a screening programme.
In the model it is assumed that the Liverpool Lung Project (version 2) (LLPv2) risk prediction tool150 will be used, as this is the only risk prediction tool that has been used to select a population for a RCT of lung cancer screening (in UKLS55). Other risk prediction tools are available and may have different data requirements and different performance in terms of discriminatory ability. The choice of the LLPv2 tool does not represent an endorsement.
Rather than estimating the performance of LLPv2 from the case–control study in which it was developed150 or the validation studies,151 we instead considered its performance among responders to an invitation to screening, that is, in the UKLS population. 55
In UKLS, 41 lung cancers were detected in the baseline screen out of 1994 individuals scanned. 55 A further nine lung cancers were detected within 3 years of follow-up in these individuals (Professor John K Field and Dr Michael Marcus, University of Liverpool, 2017, personal communication).
However, the lung cancer outcomes are unknown in individuals:
-
meeting the risk threshold (4.5%) and being randomised but not receiving a CT scan (n = 2061)
-
not being randomised
-
not meeting the risk threshold
-
not replying to the questionnaire.
It is therefore not possible to directly estimate the sensitivity and specificity of the LLPv2 at any particular threshold, or to construct a receiver operating characteristic curve.
We were provided with data from UKLS on certain baseline characteristics, the risk prediction score and their lung cancer outcome (if known).
We then sought to estimate a statistical model for the risk score according to those baseline characteristics and the lung cancer outcome. As our model simulates individuals for their lifetime through different screening programmes, we are easily able to identify whether or not a simulated individual develops clinical lung cancer within a certain duration of follow-up, and then to ‘back-estimate’ their risk prediction score. In this way, simulated individuals who have a baseline lung cancer or develop clinical lung cancer early in the model will have a higher predicted risk score if the risk prediction tool is effective.
Equation 3 describes the statistical model we sought to fit, which is a linear regression on the logit of the risk score:
where r1 is the predicted risk for individual i, β(r)0 is the intercept term, β(r)x are the coefficients for baseline characteristics xi (age, sex, smoking status), β(r)y is the coefficient for the outcome yi (lung cancer or not) and ϵi is an error term with variance σ2r.
All analyses in this section were conducted using Stata version 14.2.
Fitting this model to only individuals for whom all data are available (i.e. individuals receiving a baseline screen) is expected to produce inefficient and biased estimates of the coefficients, because these individuals are not representative (they have been selected because of their high risk). The results of this regression are given in Table 16 and suggest that increasing age, being male, being a current or former smoker (vs. having never smoked) and developing lung cancer all predict a higher risk score, although the coefficient for smoking status is not statistically significant (as very few individuals received a CT scan who were not smokers, i.e. there is a lack of power).
| Coefficient | Estimate | 95% CI |
|---|---|---|
| Completed-case regressiona | ||
| Age (years) | 0.0267 | 0.0214 to 0.0320 |
| Male | 0.170 | 0.121 to 0.220 |
| Current/former smoker | 0.360 | –0.313 to 1.03 |
| Lung cancer | 0.157 | 0.0221 to 0.292 |
| (Intercept) | –4.74 | –5.50 to –3.98 |
| Root-mean-squared error | 0.485 | |
| Multiple imputation regressionb | ||
| Age (years) | 0.0899 | 0.0891 to 0.0906 |
| Male | 0.306 | 0.296 to 0.315 |
| Current/former smoker | 1.46 | 1.45 to 1.47 |
| Lung cancer | 0.335 | 0.0307 to 0.639 |
| (Intercept) | –11.4 | –11.4 to –11.4 |
| Root-mean-squared error | 0.629 | |
To account for the missing outcome (lung cancer) for the vast majority of individuals for whom data were available, multiple imputation was employed. The lung cancer outcome was imputed using logistic regression on the age, sex, logit risk score and smoking variables and 50 imputations were performed. The linear regression on logit risk score was then conducted again using the imputed data sets.
Table 16 gives the results of the multiple imputation regression. This shows stronger effects for all predictors, and they are all now statistically significant. The error term also now has increased variance. The central estimate for the lung cancer outcome coefficient is approximately half the root-mean-squared error, so there will be significant overlap in the predicted risks for individuals developing and not developing lung cancer.
Accuracy of low-dose computed tomography
In the model it was assumed that LDCT tests would be imperfect, and that sensitivity and specificity would both be < 100%.
Sensitivity was included in the model as the probability that an individual with preclinical lung cancer would be diagnosed with lung cancer following CT screening. If an individual is not diagnosed with lung cancer following CT screening, their preclinical disease may continue to progress until they present clinically or are diagnosed in a subsequent screening round.
Specificity was included in the model as the probability that an individual without preclinical lung cancer would receive a result that required further testing.
It was assumed that follow-up tests would be perfect (e.g. nobody receiving a false-positive result by LDCT screening would go on to receive treatment).
The sensitivity of LDCT screening was estimated in the calibration exercise described in Natural history as 70.9% in the base case, but 97.3% in the scenario analysis in which heterogeneity was included.
The specificity of LDCT screening was estimated from UKLS to be 62.4%. 55 This is lower than the 78% estimated in the calibration exercise based on NLST and, therefore, could be a conservative estimate, but it was judged that a UK-based estimate would be more relevant, as specificity can be relatively accurately estimated (compared with sensitivity) with reference standards that can be employed in trials.
Impact on survival
Numerous studies have shown that survival is higher for screen-detected cancers than non-screen-detected cancers, including those of the same stage (we have confirmed this for NLST using the data on which the natural history model is calibrated), but there are three significant reasons why these survival estimates would be biased:
-
Lead time bias – the screen-detected cancers are detected earlier and, therefore, even if the age at death is unchanged, the duration from date of diagnosis to date of death is extended.
-
Length bias – if some cancers are more aggressive than others, these are less likely to be detected by screening, as they spend less time in the preclinical stage before reaching advanced stages and being diagnosed.
-
Overdiagnosis (an extreme form of length bias) – some slow-growing cancers may never be clinically relevant for a patient in the absence of screening because the patient dies from another cause.
On this basis, it was decided that the model should assume the same survival for lung cancer in each stage, whether screen detected or clinically presenting, with the following caveat: the age of lung cancer mortality should not be brought forward by screening and, therefore, there is a lower bound on survival of A + B, where A is the expected survival in the later stage (in which the cancer would have presented absent screening) and B is the lead time. This also applies if the lung cancer is screen detected in the same state as it would have clinically presented.
Figure 26 illustrates the approach through three examples: (a) lung cancer is detected significantly earlier and in a substantially earlier stage in the screening arm (shown in green), resulting in a predicted age of lung cancer mortality beyond that in the control arm; (b) lung cancer is detected somewhat earlier and in a somewhat earlier stage in the screening arm – the predicted survival in the earlier stage would lead to an earlier age of mortality and, therefore, survival is extended in the screening arm to match the age of lung cancer mortality in the control arm; and (c) lung cancer is detected slightly earlier but in the same stage in the screening arm – the predicted survival is extended exactly by the lead time to match the age of lung cancer mortality in the control arm.
FIGURE 26.
Illustration of approach to modelling lung cancer survival.

This modelling approach directly translates a stage shift into a reduction in lung cancer mortality, but NLST is the only RCT thus far to report a positive result for this outcome. 70 To explore the possibility that lung cancer mortality may not be reduced (or may only be reduced a small amount), we also performed two scenario analyses. In the first, there was assumed to be no effect on lung cancer mortality from screening (i.e. the predicted age of lung cancer mortality is insensitive to whether or not patients receive screening). In this instance, it is still possible for screening to lead to benefits for patients, as HRQoL is better for earlier-stage detected cancers (see Measurement and valuation of preference-based outcomes). In the second scenario, the potential effect on lung cancer mortality is halved (i.e. if, in the base case, a lung cancer patient would be predicted to live x years longer in the screening arm than in the control arm, in the scenario they are modelled as living x/2 years longer).
Measurement and valuation of preference-based outcomes
A literature review was conducted to identify appropriate utility values. EQ-5D was the preferred tool to measure HRQoL, and the preferred valuation was the UK time trade-off (TTO) value set derived from a sample of the general population. 152 These were chosen as they are recommended in the NICE reference case for economic evaluations. 137 When such values were not available, generic health measures were preferred, with utility values either obtained by mapping to EQ-5D and valuing with the UK TTO value set, or obtained through TTO, standard gamble or discrete choice experiment. VASs were not accepted as valuations of health states.
Searches of the published literature were conducted for HRQoL and utility outcomes relating to lung cancer (see Appendix 1). The search terms were broad but the retrieved records were screened specifically for EQ-5D primary and secondary studies. Any primary studies in lung cancer patients measuring HRQoL with EQ-5D were eligible for inclusion unless they were lung cancer patients experiencing specific adverse events or symptoms. Secondary studies were only eligible if they were systematic reviews and included EQ-5D studies.
Screening
We identified two studies84,153 that reported EQ-5D measures relating to screening for lung cancer.
Mazzone et al. 153 reported on the impact of lung cancer screening by CXR with computer-aided detection versus placebo screening in a RCT. They used US population values elicited using the TTO method. 154 The authors’ key findings were that HRQoL was not affected by study arm (likely because patients were blinded) but that EQ-5D utility dropped significantly following notification that a lung nodule was detected (from 0.940 to 0.877).
Reporting results from the NELSON study, van den Bergh et al. 84 described patient-reported outcomes for individuals in the LDCT screening arm who did not receive a positive result (i.e. received negative or indeterminate results). The authors did not report EQ-5D utility values, but instead VAS scores and a number of anxiety and distress measures. These measures suggested that across all participants there was a worsening in patient-reported measures between giving consent and 1 week prior to CT scan, although this was reversed shortly after receiving their CT scan (prior to receiving results). They also suggest that participants receiving an indeterminate result had worse patient-reported outcomes after receiving their result and before receiving a follow-up scan. The generic QoL SF-12 showed no statistically significant difference over time (between those receiving an indeterminate and negative result) in the physical or mental component scales.
We judged that it was important to include some estimate of the impact of screening on HRQoL as there was some evidence to support it.
We therefore assumed that lung cancer screening itself would be associated with a small, temporary disutility of 0.01 (based on VAS drop from 79.3 to 78.8 in NELSON84) lasting for 2 weeks (i.e. a loss of 0.01 × 2/52 = 0.00038 QALYs). This is probably a negligible loss for a single participant, but considering that in screening very few patients benefit, the average benefit from screening may also be considered ‘negligible’.
We also assumed that receiving a false-positive result (the nearest representation of an indeterminate result in the model) would result in a temporary disutility of 0.063 (based on EQ-5D drop in Mazzone et al. 153) that would last for 3 months (it is anticipated that within 3 months an individual would have had some follow-up to give them reassurance). This corresponds to a loss of 0.063 × 3/12 = 0.0158 QALYs. As this represents a significant loss of utility (greater than the base-case disutility owing to stage IV lung cancer) and because the study is of CXR rather than LDCT, a scenario analysis was conducted in which this disutility is not included at all.
General (smokers)
All individuals included in the economic evaluation are current or former smokers. It is therefore important to note that such individuals are unlikely to be at perfect health, or even at the same average health of the population.
We estimated the impact of smoking (current or previously regular) on EQ-5D utility from the Health Survey for England 2014: Health, Social Care and Lifestyles. Summary of Key Findings,155 controlling for sex and age. Linear regression was conducted with appropriate weighting and stratification based on the survey design.
The effect of smoking was estimated to be –0.048. Men had slightly higher utility values (+0.029) and an age profile was observed in which utilities generally declined with age.
For the baseline age category (75–84 years), the estimated utility for female smokers was 0.753 and for male smokers was 0.782. These utility values were used for women and men in the model regardless of the current smoking status (which was not modelled) and age. It would have been possible to model utility as a function of age, but it was judged that this additional complexity would not greatly affect results and, therefore, it was decided to focus on other aspects of the economic evaluation.
Lung cancer
The ideal study to inform utility values relating to lung cancer would include lung cancer patients as well as matched patients without lung cancer (matched on at least age, sex and smoking history) and would estimate the effect of the stage of lung cancer as well as the effect of different treatments and time-dependent effects (e.g. time before death). In addition, EQ-5D utilities measured in patients and valued by a representative sample of the UK population using the TTO method would be preferred to be in line with the NICE reference case,137 which is used for the vast majority of economic evaluations of health technologies in the UK.
Unfortunately, no such study was found in our review of the literature.
It was decided, therefore, to focus on studies that gave evidence of the effect of lung cancer stage on utility values.
Only one such study, by Chouaid et al. ,156 explicitly measured HRQoL in UK patients (among patients from other countries) using EQ-5D and valued using a UK population TTO tariff. This study included 255 NSCLC patients with stage IIIB or stage IV lung cancer and produced utility estimates of 0.77 and 0.70 for these stages, respectively.
Another study, by Grutters et al. ,157 used the UK population TTO tariff but measured HRQoL in 245 NSCLC Dutch patients. Only two patients with stage IV lung cancer were included. The study estimated utility values of 0.77, 0.74, 0.70 and 0.86 for stages I, II, III and IV, respectively.
Three studies158–160 utilised a TTO tariff elicited from a US population sample. 154 Jang et al. 158 measured HRQoL in 172 NSCLC patients in Canada and produced utility estimates of 0.80, 0.78, 0.73 and 0.75 for stages I to IV, respectively. Yang et al. 160 measured HRQoL in 518 NSCLC patients in Taiwan and produced utility estimates of 0.85, 0.83 and 0.83 for operable stage I, II and III, respectively, and 0.72 and 0.75 for inoperable stage III and IV NSCLC. Tramontano et al. 159 measured HRQoL in 2396 lung cancer patients in the US and produced utility estimates of 0.81, 0.77, 0.77 and 0.76 for stages I to IV, respectively. This study also used the Short Form questionnaire-6 Dimensions (SF-6D) and UK population value set161 (derived using standard gamble) and estimated utility values of 0.71, 0.68, 0.67 and 0.66 for stages I to IV, respectively. In addition to differences in the population valuing the health states (USA vs. UK) and the valuation method (TTO vs. standard gamble), the EQ-5D asked patients to rate their health today, whereas the SF-6D asked patients to recall their health for the previous 4 weeks.
Of all these studies, the study by Tramontano et al. 159 is by far the largest and is the only study to not restrict to NSCLC. As such, we believe this is the best study with which to estimate lung cancer utility values according to stage, despite it using a US value set rather than a UK value set.
All of the studies identified will have been at some risk of bias for a number of reasons. First, as lung cancer can be associated with particularly poor HRQoL, it is possible that patients with worse HRQoL are underrepresented as they will be less likely to participate in studies or to be able to complete health questionnaires. Second, most of these estimates do not account for other differences between patients besides their cancer stage. It may be that late-stage cancer is associated with other factors that affect HRQoL, such as age, sex, income and other respiratory conditions.
It is also possible that generic health measures (such as EQ-5D and SF-6D) are not sensitive to aspects of lung cancer that negatively affect HRQoL, such as shortness of breath and fatigue.
It is notable that the identified studies did not produce a large difference in the utility of stage IV lung cancer versus earlier stages, the largest difference (0.07) being measured by Chouaid et al. 156 for stage IV versus stage IIIB NSCLC. This contrasts with a systematic review by Sturza that aimed to estimate utility values associated with lung cancer. 162 Using meta-regression across a large number of studies using a variety of HRQoL measures and valuation methods, Sturza estimated a difference in utility value of 0.25 between metastatic and non-metastatic lung cancer. There are a number of ways in which this estimate may be inflated, for example because it relies on meta-regression rather than investigation within a study population, because it includes estimates not derived using EQ-5D and because it includes multiple methods of valuation.
In the base-case analysis, it was decided that the utility values from Tramontano et al. 159 would be used to estimate the disutility for later stages of lung cancer versus stage I, whereas in a scenario analysis, a disutility of 0.252 for stage IV would be applied based on the estimate by Sturza. 162
It was further assumed that individuals with stage I cancers (mostly asymptomatic) would have the same utility as smokers without lung cancer. This was judged to be a pragmatic approach as the average utility for smokers without lung cancer estimated above is lower than the utility estimated for stage I lung cancer, and it would lack face validity to increase the utility for individuals with lung cancer versus individuals without lung cancer.
The utility values are applied for the remainder of the lung cancer patient’s life according to their stage at diagnosis. This is a simplifying assumption as lung cancer often progresses despite treatment, and HRQoL is likely to decline as patients approach death.
It was also assumed that individuals with preclinical lung cancer would experience some disutility as a result of lung cancer symptoms. The same utility values for clinical lung cancer of a particular stage were applied to the preclinical lung cancer stage also (i.e. it is assumed that a diagnosis of lung cancer does not intrinsically affect HRQoL).
Resources and costs
The approach to the measurement and costing of resource consumption followed that recommended by NICE in the Guide to the Methods of Technology Appraisal. 137 Costs to the NHS and PSS were included so as to assume the health and social care payer perspective, and NHS Reference Costs were the primary source of unit costs. 163 Reference costs for the financial year running 2015 to 2016 were standardised from financial returns from 237 NHS providers delivering £64.2B. 163 They used Healthcare Resource Group (HRG) currency version 4+ for NHS acute care in England, and include the direct, indirect and overhead costs for admitted patient care, outpatients and emergency care. Other unit costs were inflated when needed to the adopted price year, 2016.
Costs from outside the UK were converted using the Campbell and Cochrane Economics Methods Group (CCEMG) – Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) Cost Converter using International Monetary Fund (IMF) purchasing power parity data.
Health and social care resources were categorised according to whether they related directly to the screening programme, referral, diagnosis, treatment, follow-up (‘hospital costs’), or care at the end of life. Whenever possible, the estimates of resource utilisation were determined at the substage cancer level described by the Union for International Cancer Control Seventh Edition staging system. 23,24 The screening cost category considered the marginal cost of programme administration and, if selected, the LDCT examination(s) of programme joiners. Transactional costs relating to programme establishment were not included. The hospital costs category included referral, diagnosis, treatment and follow-up, although the relatively small cost of referral is borne by the primary care budget. The end-of-life cost category captured the palliative resource use in NHS and social care prior to lung cancer mortality, and did not include the contribution of charitable or informal care.
Directly applicable individual patient-level resourcing information was not available within the timeframe of the study, so a literature search of the MEDLINE database was conducted (January 2017) to identify resourcing and/or costing evaluations that might inform input parameters at summary level. The economic evaluation search criteria were adapted by excluding the screening, Markov model and cost-effectiveness search terms, and restricting to studies conducted in the UK setting from 2010 onwards. A total of 218 items were returned and a review of titles and abstracts identified six studies of interest, of which four were included for input parameter estimation. 115,164–166 Two studies were rejected on the grounds of limitations in outcomes reported167 and inferior applicability168 (Vinod et al. 168 reported estimates for a non-screening population). One additional study was identified from the clinical effectiveness systematic review and two reference texts were used for sourcing the unit costs of standard resources in England. 55,163,169
The included sources of evidence that inform the resource utilisation and cost parameters used in the model are given in Table 17.
| Study, year of publication | Resource category | Use in model |
|---|---|---|
| ten Haaf et al., 2017115 | Screening programme | Cost of invitations, questionnaires, scoring of questionnaires and follow-up administration. Based on the health-care system of ON, Canada |
| Field et al., 201655 | Screening programme, hospital costs | Rate of positive response to invitation to screening, and rate of programme uptake. Based on UK experience from the pilot screening programme. Resource use following a false-positive screen |
| Department of Health and Social Care, 2016163 | Screening programme, hospital costs | Unit cost estimates including LDCT and diagnostic procedures following a false-positive screen |
| Curtis and Burns, 2016169 | Screening programme, hospital costs | Cost of GP referral and clinical staffing of screening assessment |
| Kennedy et al., 2016164 | Hospital costs | Direct hospital costs in the first year. Based on the presentation of 3274 patients at a single large English teaching hospital between January 2008 and October 2013 |
| McGuire et al., 2015165 | Hospital costs | Direct hospital costs after the index year. Based on the 2-year treatment cost profile of NSCLC in England using Hospital Episode statistics for 20,081 patients in the index year 2007/8 |
| Round et al., 2015166 | End-of-life care | Cost of care at the end of life for patients with lung cancer. Based on the cost of health and social care for people with lung cancer in England and Wales from the start of strong opioid treatment to death, 2013/14 tariff |
Screening programme costs
Screening programme costs included programme administration around participation as well as LDCT examinations for subsequent joiners (Table 18). Administration comprised the postal invitation to self-assess to all potentially high-risk candidates, the scoring of responder questionnaires and a subsequent follow-up letter of invitation or decline. These costs were allocated appropriately using the decision tree described in Model structure and probability of uptake described in Effectiveness estimates. Since the probability of uptake (a product of the probability of responding and then the probability of joining the programme) in the base case was based on a trial population and may not accurately predict real-world uptake, scenario analyses tested lower and high rates of uptake, as described in Deterministic sensitivity analyses.
| Item | Base-case unit cost (£) | Scenario analysis |
|---|---|---|
| Initial postal invitation and questionnaire | 2.90 | |
| Scoring of questionnaire | 18.54 | |
| Follow-up letter | 1.74 | |
| Nurse consultation | 6.25 | |
| LDCT scan | 98.80 | 70.39, 120.76a |
The LDCT screening examinations were the same unit cost whether they were the first or subsequent examination, and were assumed to be of the kind directly accessed in NHS secondary and tertiary care settings (currency code RD20A). 163 In scenario analyses, the base-case unit cost of LDCT was varied up and down according to the upper and lower quartile values of the HRG currency code distribution. As it could be presumed direct access would be preceded by clinical consultation, as is not the case via invitation, this was added as an associated cost equating to 15 minutes with a band 5 hospital nurse. 169
Hospital costs
Following a referral, secondary care is the setting for most of the NHS expenditure on people with lung cancer (83% in 2012/13). 164 In order to incorporate the burden of referral and symptom management on primary care, an average of two GP consultations was assumed per clinical presentation (unit cost £36169) and included in this category. The cost of symptomatic referrals suspicious for lung cancer that are subsequently found to be negative for lung cancer was not included.
The rate of resource consumption for the hospital-based aspects of the care pathway, namely the diagnosis, treatment and clinical follow-up, was based on a retrospective 1-year cohort study of all emergency, inpatient and outpatient costs (not palliative) from the records of 3274 lung cancer patients between January 2008 and October 2013. 164 This study was limited to the experience of a single English teaching hospital, so may not reflect nationwide variation in disease management, but was not limited to NSCLC as was the study by McGuire et al. 165 Summary costs at 90 days were chart extracted by substage at diagnosis and attributed to all true diagnoses, whether clinical presentations or true-positive screen detections, irrespective of survival time with lung cancer as many of these costs are quickly accrued (more than half of first-year costs come in the first 90 days). People surviving beyond 90 days were attributed further costs in the first year proportionate to their survival up to a limit of 2 years. Second-year costs were adjusted downwards from index year costs using the rate of change observed between year 1 and year 2 in a separate retrospective cohort study of NHS lung cancer resource consumption. 165 Two years was judged a reasonable cut-off point for disease costs in the base case, given the front-loaded nature of resourcing following a lung cancer diagnosis. McGuire et al. 165 estimated that second-year costs were just 13% of first-year costs. However, in a scenario analysis the subsequent year costs were maintained at a flat rate for survivors up to 5 years.
A summary of hospital costs by substage of cancer and period post presentation is given in Table 19.
| Substage at presentation | Cost (£) | ||
|---|---|---|---|
| 90-days | Index yeara | Second yearb | |
| IA | 5558 | 11,406 | 1438 |
| IB | 6412 | 11,771 | 1484 |
| IIA | 7279 | 12,917 | 1628 |
| IIB | 6558 | 13,073 | 1648 |
| IIIA | 6512 | 11,927 | 1503 |
| IIIB | 6047 | 10,365 | 1306 |
| IV | 5442 | 8229 | 1037 |
People who received a false-negative screen were zero cost until a true diagnosis of lung cancer, whereupon costs accrued as described above. Those who received a false-positive screen were resourced as observed in UKLS. 55 Of 951 false-positive cases, there were 72 resultant cancer MDTs, 1466 outpatient CT scans, seven needle biopsies, 13 PET scans, one endobronchial ultrasound bronchoscopy and 21 follow-up outpatient consultations. The cost of investigative resourcing following a false-negative screen was £184.63. A breakdown of the unit costs and their consumption is given in Table 20.
| Resource item | Unit cost (£)163 | Unit consumption per FP case55 | Weighted cost (£) |
|---|---|---|---|
| Cancer MDT meeting | 107.35 | 0.0757 | 8.13 |
| Outpatient CT scan | 102.50 | 1.5415 | 158.00 |
| Elective inpatient percutaneous biopsy of lesion of, lung or mediastinum | 994.59 | 0.0074 | 7.32 |
| Outpatient endobronchial ultrasound examination of mediastinum | 562.87 | 0.0011 | 0.59 |
| Outpatient PET-CT scan | 573.91 | 0.0137 | 7.85 |
| Non-admitted face-to-face attendance, follow-up with clinical oncology | 124.01 | 0.0221 | 2.74 |
| Total cost of a FN screen | 184.63 |
End-of-life costs
In the literature search, we identified a recent and directly applicable study of the cost of caring for people with cancer in England and Wales. 166 In this study, the average per-patient cost of palliative resourcing over an average period of 180 days from the start of strong opioid treatment was £4589, based on 68,340 hospital episodes relating to lung cancer. The personalised social services component of this figure (£1380) was included in the base case but excluded in a scenario analysis in which the payer perspective was limited to health care. In a further scenario analysis, the end-of-life cost attributed to lung cancer mortality was excluded altogether in order to reflect the possibility that other deaths in this population could be equally costly owing to the preponderance of comorbidity in people with a history of smoking.
Key modelling assumptions
We have attempted to list the key assumptions in the model in Table 21, and to indicate (if possible) the anticipated impact of the assumptions made on the cost-effectiveness of screening programmes versus no screening, and to indicate whether the assumptions are explored in scenario or sensitivity analyses.
| Assumption | Anticipated impact (on cost-effectiveness of screening vs. no screening vs. likely true cost-effectiveness) | Explored in scenario or sensitivity analyses |
|---|---|---|
| Changes in smoking behaviour are not modelled | Possibly worsened; screening may encourage some to quit smoking, but evidence is mixed | No |
| Uptake will be similar in real life to that in UKLS | Unclear; on the one hand trials tend to recruit healthier volunteers, but on the other hand invitations to participate in a trial contain substantially different information to invitations to participate in a screening programme | Yes |
| Full concordance with screening programme (i.e. no missed appointments) | Improved; missed appointments lead to wasted resources and missed opportunities for patients to benefit from screening | No |
| HRQoL similar for preclinical and diagnosed lung cancer (stratified by stage) | Unclear; a diagnosis of lung cancer may lead to heightened anxiety, and treatments for lung cancer may lead to reduced HRQoL. However, with a diagnosis patients may also receive better support to manage their symptoms | No |
| HRQoL similar for clinically presenting and screen-detected lung cancer of the same stage | Unclear | No |
| HRQoL for diagnosed lung cancer is constant until death | Worsened; one would expect HRQoL to diminish over time as the disease progresses despite treatment, and this would be particularly acute in those dying from lung cancer (and there are more of these without screening) | No |
| Natural history of lung cancers is similar across all included individuals | Improved; length bias and overdiagnosis are not fully addressed in the base-case analysis, both of these phenomena undermine effectiveness | Yes |
| Lung cancers progress through stages in numerical order without skipping any stages | Unclear; if lung cancers do skip stages in significant proportion then the natural history model calibrated to NLST data may not be appropriate | No |
| Sensitivity of LDCT independent of patient and tumour characteristics | Unclear; sensitivity may be expected to be worse for earliest-stage cancers (people who could potentially benefit the most from screening), but this would also have a significant impact on overdiagnosis | No |
| Lung cancer mortality methodology | Unclear; the methodology establishes a lower bound on effectiveness (so that screening cannot be less effective than no screening), but it is possible that survival is underestimated when a stage shift is achieved, since there is some evidence (though at high risk of bias) that screen-detected cancers have improved survival vs. non-screen-detected cancers | Partially; there are scenario analyses in which the impact on mortality is eliminated or attenuated, but no scenario analysis in which the impact on mortality is strengthened |
| Mortality from preclinical lung cancer assumed to be negligible | Worsened; if there is significant mortality from preclinical (occult) lung cancer in the population, then screening would potentially be able to reduce this | No |
| Lung cancer incidence in participating population similar to incidence in general smoking (current and former) population | Possibly improved; respondents are likely to be healthier than general smoking population and, therefore, may have reduced incidence; however, the use of a risk prediction model should substantially mitigate this | Partially (through univariate sensitivity analyses) |
| Survival in participating population similar to survival in general population (stratified by stage) | Improved; it is more likely that a participating population would be healthier and less deprived than the general population of smokers and would therefore have improved survival, potentially benefiting less from screening | Partially (through univariate sensitivity analyses) |
| Incidental findings not modelled | Unclear; incidental findings may be of clinical value (i.e. it may be possible to offer treatment or management that improves patient outcomes) but may also significantly increase costs | No |
| True-positive results lead to immediate diagnosis and treatment | Improved; there is expected to be a delay between screening and diagnosis, during which lung cancer could progress further, thereby reducing the benefit of screen detection | No |
| False-positive and indeterminate results are treated equivalently | Unclear; indeterminate results typically result in less intensive follow-up than false-positive results, but the model assumes a weighted average of these results according to UKLS | No |
| Non-attendance of screening was not explicitly modelled | Unclear; NHS Reference Costs163 include costs of missed appointments as overheads within unit costs, but it is not clear whether or not the unit cost chosen will include a representative overhead for non-attendance in a hypothetical screening programme | Partially (through sensitivity analyses on the unit cost of LDCT) |
| Additional cancers caused by radiation exposure not modelled | Improved; additional cancers would lead to increased costs and decreased QALYs | No |
| Risk prediction is dependent only on prevalence of occult lung cancer or short-term incidence (within 3 years) | Worsened (especially for annual and biennial strategies); in the model the value of risk prediction is limited to the first 3 years, such that individuals who would develop lung cancer > 3 years later have no higher predicted risk on average than individuals who would not develop lung cancer beyond 3 years | No |
Of the 19 assumptions listed, nine have an unclear impact on cost-effectiveness, six may have improved cost-effectiveness (i.e. tended to be optimistic assumptions) and four may have worsened cost-effectiveness (i.e. been conservative assumptions). Thirteen are not explored in scenario or sensitivity analyses, while, of the six that are explored (or partially explored), three are assumptions with an unclear anticipated impact and the other three may have been optimistic assumptions.
Quality assurance
Quality assurance of the economic model was conducted by three modellers:
-
The lead modeller (EG) conducted developer testing and incorporated a number of automatic model checks to highlight any possible errors as the model was developed.
-
One member of the team (TS), who had contributed only a small quantity towards the implementation of the model itself, conducted two rounds of quality assurance on the model (one on the version for the draft report and one on the final version) and signed off corrections of errors identified through this quality assurance. Methods of quality assurance included formula review and parallel build.
-
A member of Peninsula Technology Assessment Group (PenTAG) not working on the project (Dr Irina Tikhonova, Research Fellow in Health Economic Modelling) conducted quality assurance on the version of the model used for the draft report.
Results
Throughout this section a naming convention is used for the different potential screening programmes (Box 1).
Where frequency is S (single screen), T (three screens), A (annual screening to age 80 years) or B (biennial screening to age 80 years).
For example, S–55–75–5% refers to a screening programme for which people aged 55–75 years are sent questionnaires, and those with a predicted risk of ≥ 5% are invited to a single CT screening round.
Base case
Forty-eight hypothetical screening programmes were modelled, as well as a no-screening comparator arm, representing current practice.
These analyses are conducted by simulating a cohort of 20,000 individuals, as cost and QALY predictions appear to be very stable after 15,000 simulations.
The different population selection criteria produced a wide range of proportions of smokers joining screening programmes (from 1.2% for 60–75–5% to 4.0% for 55–80–3%), as shown in Table 22. The predominant reasons for smokers not joining screening programmes were not responding to the initial invitation and not being invited (as outside the age limit).
| Population criteria | Proportion of smokers aged 55–80 years (%) | ||||
|---|---|---|---|---|---|
| Joiner | Non-joiner | ||||
| Decline | Low risk | No response | Not invited | ||
| No screening | – | – | – | – | 100.0 |
| 55–80–3% | 4.0 | 4.6 | 22.1 | 69.3 | – |
| 55–80–4% | 2.6 | 3.0 | 25.1 | 69.3 | – |
| 55–80–5% | 1.7 | 2.0 | 27.0 | 69.3 | – |
| 60–80–3% | 3.8 | 4.4 | 14.5 | 51.3 | 26.0 |
| 60–80–4% | 2.5 | 2.9 | 17.3 | 51.3 | 26.0 |
| 60–80–5% | 1.7 | 2.0 | 19.0 | 51.3 | 26.0 |
| 55–75–3% | 3.2 | 3.7 | 21.8 | 64.8 | 6.5 |
| 55–75–4% | 1.9 | 2.2 | 24.6 | 64.8 | 6.5 |
| 55–75–5% | 1.2 | 1.4 | 26.1 | 64.8 | 6.5 |
| 60–75–3% | 3.1 | 3.5 | 14.2 | 46.8 | 32.4 |
| 60–75–4% | 1.9 | 2.2 | 16.7 | 46.8 | 32.4 |
| 60–75–5% | 1.2 | 1.4 | 18.2 | 46.8 | 32.4 |
Main analysis
Cost-effectiveness
Four of the modelled screening strategies were on the cost-effectiveness frontier (i.e. strategies that can give the maximum NMB for at least one choice of the cost-effectiveness threshold) and ‘no screening’ was also on the frontier (the least costly and least effective option).
Table 23 gives key results for strategies on the cost-effectiveness frontier. In this analysis, none of the screening strategies would be considered cost-effective versus no screening at a threshold of £20,000 per QALY. At a threshold of £30,000 per QALY, S–60–75–3% would be cost-effective versus no screening (ICER £28,169 per QALY), as would S–55–75–3% (ICER £28,784 per QALY), but in a fully incremental analysis only S–60–75–3% would be cost-effective with a threshold of £30,000 per QALY.
| Strategy | Costs (£) | QALYs | ICER (vs. no screening) (£) | Incremental costs (vs. previous) (£) | Incremental QALYs (vs. previous) | ICER (vs. previous) (£) |
|---|---|---|---|---|---|---|
| No screening | 1103 | 8.502 | ||||
| S–60–75–3% | 1126 | 8.503 | 28,169 | 23 | 0.0008 | 28,169 |
| S–55–75–3% | 1129 | 8.503 | 28,784 | 3 | 0.0001 | 35,453 |
| S–55–80–3% | 1135 | 8.503 | 30,821 | 6 | 0.0001 | 44,087 |
| T–55–80–3% | 1151 | 8.503 | 40,034 | 17 | 0.0002 | 95,292 |
Figure 27 presents the cost-effectiveness plane with all strategies, and Figure 28 shows the strategies on the cost-effectiveness frontier.
FIGURE 27.
Cost-effectiveness plane for base-case results.

FIGURE 28.
Cost-effectiveness frontier for base-case results.
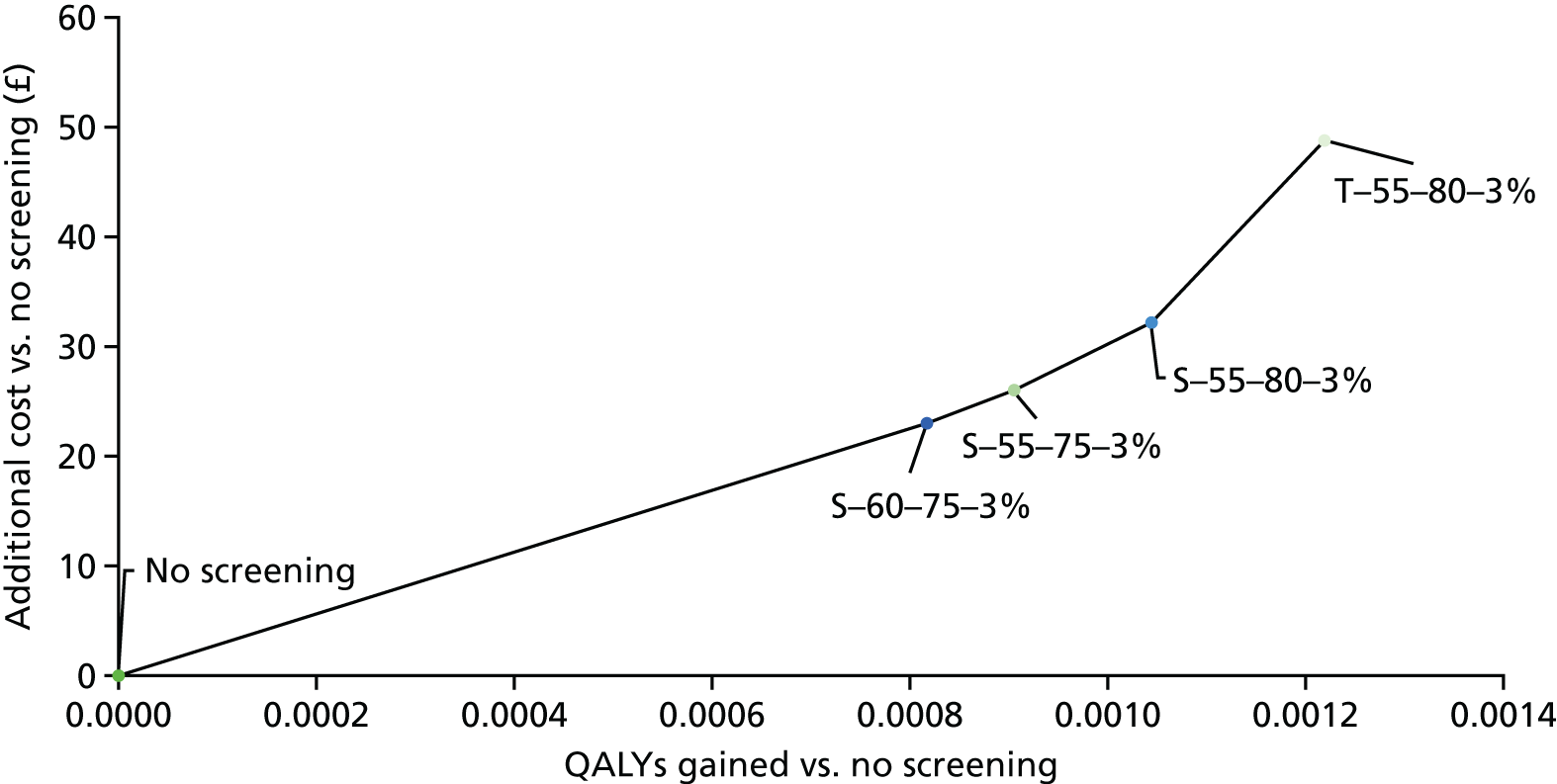
A summary of selected clinical outcomes is presented for the screening strategies on the cost-effectiveness frontier in Table 24.
| Screening programme strategy | Strategy | |||
|---|---|---|---|---|
| S–60–75–3% | S–55–75–3% | S–55–80–3% | T–55–80–3% | |
| Per participant | ||||
| Number of screens | 1.00 | 1.00 | 1.00 | 2.70 |
| Number of false positives | 0.33 | 0.33 | 0.33 | 0.95 |
| Lead time (years) | 0.299 | 0.299 | 0.295 | 0.395 |
| Life-years gained | 0.0537 | 0.0568 | 0.0524 | 0.0762 |
| Additional lung cancer survival (%) | 0.80 | 0.45 | 0.43 | 0.64 |
| Additional 5-year lung cancer survival (%) | 16.1 | 16.4 | 16.1 | 21.0 |
| Additional survival time with lung cancer (years) | 1.87 | 1.89 | 1.85 | 2.44 |
| Change in age at lung cancer diagnosis | –1.70 | –1.69 | –1.62 | –2.03 |
| Change in age at death from any cause | 0.05 | 0.06 | 0.05 | 0.08 |
| Change in age at death from lung cancer | 0.16 | 0.20 | 0.23 | 0.41 |
| Per 100,000 participants | ||||
| Proportion of diagnoses arising from screening (%) | 44.4 | 44.3 | 47.1 | 62.5 |
| Number of screen-detected cases | 1710 | 1785 | 2335 | 3185 |
| Number of interval cancers | 0 | 0 | 0 | 215 |
| Additional lung cancer diagnoses | 295 | 300 | 450 | 590 |
| Lung cancer deaths averted | 170 | 100 | 120 | 180 |
| Life-years gained | 0.2683 | 0.2839 | 0.2621 | 0.3809 |
Lung cancer mortality reduction
The average number of lung cancer deaths (per 100,000 participants) was 15,200, 15,100, 14,600 and 15,000 for the single, triple, annual and biennial strategies, respectively, with a comparable 15,800 for no screening.
Across the different screening programmes a reduction in lung cancer mortality of 2.9% to 8.7% was predicted (RR) among the participating individuals versus no screening. The results for the strategies on the cost-effectiveness frontier are shown in Table 25.
| Strategy | Lung cancer deaths in simulation (n) | Comparison (no screening in same individuals) (n) | RR |
|---|---|---|---|
| S–60–75–3% | 624 | 658 | 0.948 |
| S–55–75–3% | 669 | 689 | 0.971 |
| S–55–80–3% | 796 | 820 | 0.971 |
| T–55–80–3% | 780 | 820 | 0.951 |
The average lung cancer mortality reduction for single-screen strategies was 4.2%, while for triple screen strategies it was 4.4%, for annual strategies it was 7.7% and for biennial strategies it was 5.2%.
Lung cancer stage and survival
Screening strategies were associated with an increased probability of lung cancer being diagnosed in the early stages (I and II) versus later stages (III and IV). The average ORs of early diagnosis (geometric mean) were predicted to be 2.44, 3.29, 5.62 and 3.83 for single, triple, annual and biennial screening programmes, respectively.
Table 26 presents the average stage distributions for screening programmes by frequency of screening. As can be seen, the most significant impact is seen in the increase in lung cancers detected at stage IA and the decrease in lung cancers detected at stage IV.
| Screening programme | Lung cancer stage | ||||||
|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIIA | IIIB | IV | |
| No screening | 0.06 | 0.01 | 0.02 | 0.02 | 0.06 | 0.03 | 0.80 |
| Single | 0.13 | 0.03 | 0.03 | 0.03 | 0.06 | 0.03 | 0.69 |
| Triple | 0.17 | 0.04 | 0.03 | 0.03 | 0.06 | 0.03 | 0.64 |
| Annual | 0.27 | 0.05 | 0.03 | 0.04 | 0.05 | 0.02 | 0.54 |
| Biennial | 0.19 | 0.04 | 0.03 | 0.04 | 0.06 | 0.04 | 0.60 |
As would be expected, lung cancer survival was predicted to be higher in the screening arms. Lung cancer survival at 5 years was predicted to be 20.3%, 26.2%, 32.3% and 29.1% for single, triple, annual and biennial screening programmes (on average) versus a comparable average of 4.7% for no screening.
Lung cancer diagnoses
Lung cancer screening programmes led to increased lung cancer diagnoses across the lifetime of participants (i.e. what would be considered overdiagnosis) versus no screening. The average RRs of a lung cancer diagnosis were 1.11, 1.15, 1.20 and 1.18 for single, triple, annual and biennial screening programmes (geometric mean), respectively.
Per 100,000 participants, there were on average 19,200 lung cancers diagnosed in the single-screening arms, 19,700 in the triple screening arms, 20,600 in the annual screening arms and 20,300 in the biennial screening arms. The comparable figure for no screening was 17,200.
On average, 47.7% of lung cancer diagnoses in the single-screen programmes were screen detected, compared with 64.2%, 80.6% and 72.1% for triple, annual and biennial strategies, respectively (these could be considered the screening programme sensitivities). Interval cancers accounted for 3.9%, 5.6% and 11.8% of diagnoses in the triple, annual and biennial strategies, respectively.
Number of screening tests and false positives
Screening programmes were associated with an average of 1.00, 2.68, 8.03 and 4.55 LDCT screens per participant for single, triple, annual and biennial screening programmes, respectively, and with 0.32, 0.93, 2.96 and 1.60 false-positive or indeterminate results.
Average ages at events
The average age at diagnosis of lung cancer was lower in the screening arms (which would be expected unless there was significant overdiagnosis in older participants). The average ages at diagnosis were 74.6, 74.1, 73.6 and 73.9 years for single, triple, annual and biennial programmes, respectively, versus a comparable 76.2 years in the absence of screening.
The average age at death from lung cancer was higher in the screening arms. The average ages at death from lung cancer were 77.6, 77.8, 78.0 and 77.9 years for single, triple, annual and biennial programmes, respectively, versus a comparable average of 77.5 years for no screening.
The average age at death from other causes was not significantly affected (around 82 years), but was slightly higher in the screening arms. The only explanation for this in the model is that some lung cancer patients were dying from other causes in the screening arms, whereas they died from lung cancer in the no-screening arm, and that these patients were on average older at time of death than the people already dying from other causes.
Lead time is calculated in the model as the difference between the age at which an individual is diagnosed with lung cancer in the no-screening arm (or dies from other causes, whichever is earlier) and the age at which the individual is diagnosed with lung cancer in the screening arm. Lead time is therefore time spent by the individual with a known diagnosis of lung cancer that they would not have had in the absence of screening. The average lead time in the single-screening arms was 0.32 years, whereas it was 0.44 years in the triple screening arms. The average lead time in annual screening arms was 0.58 years, whereas it was 0.20 years in the biennial screening arms.
Costs
The costs per participant relating to LDCT screening ranged from £104 (single-screen programmes) to £603–794 (annual screening programmes).
Lung cancer costs (excluding end of life) also generally rose in line with the frequency of screening. If there are savings in the cost of treating some screen-detected cancers because they were detected at an earlier stage, these are outweighed by the increased number of lung cancers diagnosed (i.e. overdiagnosis).
The costs of end-of-life care are decreased as the frequency of screening increases, because there is a reduction in the number of people dying of lung cancer (the model assumes end-of-life costs only for individuals dying of lung cancer, not for those dying of other causes with lung cancer).
The costs for the screening programmes on the cost-effectiveness frontier are shown in Table 27. The programmes are predicted to lead to population lifetime cost increases of £299M to £634M for a relevant population of 13 million smokers aged 55–80 years. The costs of running the screening programme (invitations, risk scoring, LDCT scans) make up less than half of the increased cost, with the rest being attributable to increased costs associated with lung cancer (excluding end-of-life care).
| Costs | Strategy | ||||
|---|---|---|---|---|---|
| No screening | S–60–75–3% | S–55–75–3% | S–55–80–3% | T–55–80–3% | |
| Costs for each participant (£) | |||||
| LDCT screening | 104 | 104 | 104 | 275 | |
| Lung cancer costs (excluding end of life) | 1458 | 1445 | 1469 | 1724 | |
| End of life | 534 | 530 | 515 | 505 | |
| Total cost | 2097 | 2080 | 2088 | 2504 | |
| Population of 13 million smokers aged 55–80 years (lifetime costs, £M) | |||||
| Screening administration | 0 | 80.16 | 110.97 | 118.66 | 118.66 |
| LDCT screening | 0 | 41.42 | 43.53 | 54.06 | 142.48 |
| Lung cancer costs (excluding end of life) | 9355 | 9540 | 9547 | 9610 | 9742 |
| End of life | 4979 | 4972 | 4971 | 4970 | 4965 |
| Total cost | 14,334 | 14,633 | 14,673 | 14,753 | 14,968 |
| Additional cost vs. no screening | 299.1 | 338.8 | 418.5 | 634.2 | |
Secondary analyses
Cost-effectiveness of different screening frequencies in fixed populations
We evaluated the cost-effectiveness of the four different screening frequencies (and no screening) within the 12 fixed populations.
Table 28 gives the results of these analyses, and demonstrates the following:
-
Annual and biennial screening were dominated by triple screening in all populations.
-
Triple screening was always on the cost-effectiveness frontier and always gave the most QALYs.
-
The ICERs of triple screening were always in excess of £36,000 per QALY.
-
Single screening was sometimes on the cost-effectiveness frontier and was sometimes extendedly dominated by no screening and triple screening.
-
The ICERs of single screening (when not extendedly dominated) were < £30,000 (but well > £20,000) per QALY for 55–75–3% and 60–75–3%, and > £30,000 per QALY for other populations.
| Population | Screening frequency | Costs (£) | QALYs | ICER vs. no screening (£) | ICER (£) |
|---|---|---|---|---|---|
| 55–80–3% | No screening | 1103 | 8.50215 | ||
| Annual | 1188 | 8.50294 | 108,405 | D | |
| Biennial | 1164 | 8.50306 | 66,985 | D | |
| Single | 1135 | 8.50319 | 30,821 | 30,821 | |
| Triple | 1151 | 8.50337 | 40,034 | 95,292 | |
| 55–80–4% | No screening | 1103 | 8.50215 | ||
| Annual | 1160 | 8.50274 | 97,461 | D | |
| Biennial | 1145 | 8.50275 | 70,934 | D | |
| Single | 1128 | 8.50285 | 36,315 | 36,315 | |
| Triple | 1139 | 8.50309 | 38,574 | 45,121 | |
| 55–80–5% | No screening | 1103 | 8.50215 | ||
| Biennial | 1135 | 8.50256 | 79,242 | D | |
| Single | 1124 | 8.50261 | 46,378 | ED | |
| Annual | 1144 | 8.50264 | 85,031 | D | |
| Triple | 1131 | 8.50283 | 42,254 | 42,254 | |
| 60–80–3% | No screening | 1103 | 8.50215 | ||
| Annual | 1182 | 8.50290 | 104,759 | D | |
| Biennial | 1159 | 8.50301 | 65,627 | D | |
| Single | 1132 | 8.50311 | 30,485 | 30,485 | |
| Triple | 1148 | 8.50329 | 39,719 | 88,019 | |
| 60–80–4% | No screening | 1103 | 8.50215 | ||
| Biennial | 1142 | 8.50273 | 67,880 | D | |
| Annual | 1156 | 8.50274 | 91,335 | D | |
| Single | 1125 | 8.50281 | 34,432 | 34,432 | |
| Triple | 1136 | 8.50305 | 37,066 | 44,278 | |
| 60–80–5% | No screening | 1103 | 8.50215 | ||
| Biennial | 1132 | 8.50252 | 79,341 | D | |
| Single | 1121 | 8.50257 | 44,569 | ED | |
| Annual | 1141 | 8.50260 | 84,714 | D | |
| Triple | 1129 | 8.50279 | 40,615 | 40,615 | |
| 55–75–3% | No screening | 1103 | 8.50215 | ||
| Annual | 1178 | 8.50282 | 112,853 | D | |
| Biennial | 1155 | 8.50298 | 63,129 | D | |
| Single | 1129 | 8.50306 | 28,784 | 28,784 | |
| Triple | 1142 | 8.50318 | 38,375 | 106,423 | |
| 55–75–4% | No screening | 1103 | 8.50215 | ||
| Annual | 1151 | 8.50260 | 108,804 | D | |
| Biennial | 1138 | 8.50266 | 69,054 | D | |
| Single | 1123 | 8.50271 | 35,890 | 35,890 | |
| Triple | 1131 | 8.50289 | 38,131 | 44,930 | |
| 55–75–5% | No screening | 1103 | 8.50215 | ||
| Biennial | 1129 | 8.50251 | 73,225 | D | |
| Single | 1119 | 8.50251 | 45,239 | ED | |
| Annual | 1137 | 8.50253 | 89,986 | D | |
| Triple | 1125 | 8.50268 | 41,617 | 41,617 | |
| 60–75–3% | No screening | 1103 | 8.50215 | ||
| Annual | 1171 | 8.50278 | 108,778 | D | |
| Biennial | 1150 | 8.50293 | 61,342 | D | |
| Single | 1126 | 8.50297 | 28,169 | 28,169 | |
| Triple | 1139 | 8.50310 | 37,859 | 95,963 | |
| 60–75–4% | No screening | 1103 | 8.50215 | ||
| Annual | 1148 | 8.50260 | 100,793 | D | |
| Biennial | 1135 | 8.50264 | 65,367 | D | |
| Single | 1120 | 8.50267 | 33,475 | 33,475 | |
| Triple | 1128 | 8.50286 | 36,181 | 43,829 | |
| 60–75–5% | No screening | 1103 | 8.50215 | ||
| Biennial | 1126 | 8.50247 | 72,679 | D | |
| Single | 1117 | 8.50248 | 42,796 | ED | |
| Annual | 1134 | 8.50250 | 90,039 | D | |
| Triple | 1122 | 8.50264 | 39,437 | 39,437 |
Optimisation analysis
An optimisation analysis was performed for each screening frequency to identify the optimal choice of age limits and predicted risk thresholds. A simple grid optimisation approach was taken, in which minimum age was varied in 1-year steps from 55 to 75 years, maximum age was varied in 1-year steps from 65 to 80 years, and the risk threshold was varied in 1% steps from 0% to 10%. Grid points where the minimum age was not less than the maximum age were removed, and a pragmatic minimum of 200 patients (1% of the simulated cohort) had to meet all criteria. The quantity to be maximised was the incremental net monetary benefit (INMB) versus no screening with a willingness to pay of £20,000 per QALY.
The results of these analyses are shown in Table 29. They demonstrate that it is possible (post hoc) to identify an appropriate cohort for whom screening is cost-effective (vs. no screening) for any frequency except annual. The INMB for the single screen versus no screening is £3.24.
| Screening frequency | £20,000 per QALY | ||
|---|---|---|---|
| Age limit (years) | Risk threshold (%) | ICER vs. no screening (£) | |
| Single | 64 to 67 | 2 | 13,631 |
| Triple | 65 to 66 | 3 | 10,303 |
| Annual | 65 to 66 | 3 | 20,589 |
| Biennial | 65 to 66 | 3 | 17,291 |
The results of these analyses should be treated with significant caution. First, they are based on a particular microsimulation and results may change for newly simulated cohorts (although the number of simulations was chosen to give some degree of stability). Second, these are post hoc identified cohorts and the results may not generalise. Third, this is based only on the base-case analysis and optimal cohorts may differ substantially in a probabilistic analysis or under other scenario and sensitivity analyses.
Deterministic sensitivity analyses
Univariate sensitivity analyses
Univariate sensitivity analyses were conducted by rerunning the base case with a single parameter increased or decreased by 10% of its base-case value or by log(1.1) (≈0.095) depending on whether or not it could change sign and/or was estimated on a logarithmic scale.
Each run of the model simulated 6000 individuals and, therefore, it is possible that stability was not reached for strategy mean costs and QALYs; however, the results should still be indicative of the likely direction and magnitude of the impact on cost-effectiveness from changing each parameter.
The impact on cost-effectiveness was assessed by evaluating the INMB of the strategy S–60–75–3% (the optimal screening strategy in the base case, although not cost-effective) versus no screening (at £20,000 per QALY) and comparing it to the base-case value (–£7).
When the INMB is > £0, it indicates that S–60–75–3% is cost-effective with no screening at £20,000 per QALY.
The results of the univariate sensitivity analysis are presented in a tornado diagram in Figure 29.
FIGURE 29.
Tornado diagram for univariate sensitivity analyses. Parameters sorted by range of INMB produced (including the base-case value). See Appendix 9 for key to parameter names.
Four out of the five most influential parameters relate to the natural history for smokers (lung cancer survival, other cause mortality, preclinical lung cancer incidence). The cost of LDCT screens (c_LDCT; see Appendix 9 for parameter labels) is fairly influential and, as would be expected, screening is expected to be more cost-effective when the cost is lower.
There appears to be some asymmetry in the tornado diagram, which may suggest non-linearity in a number of the parameters. For some parameters, the resulting range of INMB does not include the base case. This may be due to Monte Carlo variation, or may be a result of non-linearities.
The specificity of screening (sens_LDCT) appears to be more influential than the sensitivity (spec_LDCT), but in both cases improved diagnostic performance leads to better cost-effectiveness. Likewise, the performance of the risk prediction (risk_lungcancer) positively affects cost-effectiveness, as would be expected.
It should be noted that parameters were all varied by approximately the same degree, regardless of how precisely they were estimated, and that no correlation between parameters has been incorporated. These issues are both addressed within the PSA (see Probabilistic sensitivity analysis).
Scenario analyses
A number of scenario analyses were conducted, in which changes to the structure or sets of parameter values were made. For each scenario analysis 10,000 individuals were simulated and the impact of the scenario analysis is assessed by presenting the INMB of S–60–75–3% versus no screening, as well as for up to two alternative screening strategies: (1) the strategy giving the highest INMB versus no screening of all screening strategies, and (2) the strategy on the cost-effectiveness frontier giving the highest INMB versus no screening of all screening strategies.
The results of these scenario analyses are presented in Table 30 and discussed in detail below.
| Scenario | INMB vs. no screening (£) | Alternative screening strategy | |||
|---|---|---|---|---|---|
| S–60–75–3% | 1 | 2 | 1: highest NMB | 2: highest NMB on frontier | |
| Base case | –7 | ||||
| Age distribution | –14 | –12 | –17 | S–60–75–5% | S–60–80–3% |
| Risk prediction accuracy | –9 | –5 | T–60–75–5% | ||
| Programme uptake (low) | –15 | –9 | –14 | S–60–80–5% | S–60–80–3% |
| Programme uptake (high) | –17 | –12 | –22 | S–60–75–5% | T–60–75–3% |
| Heterogeneity in tumour progression | –35 | –19 | –30 | S–60–75–5% | S–60–80–5% |
| Mortality impact (removed) | –46 | –28 | N/A | S–60–75–5% | N/A |
| Mortality impact (halved) | –20 | –10 | S–60–75–5% | ||
| Short-term impact on utility from lung cancer diagnosis | –8 | –1 | S–60–75–5% | ||
| Alternative (significantly higher) disutility for stage IV lung cancer | –3 | ||||
| No screening anxiety after first screen | –10 | –10 | S-60–75–5% | ||
| No change in HRQoL for false-positive result | 4 | ||||
| Follow-up care for up to 5 years | –10 | –5 | S–60–75–5% | ||
| PSS costs for end of life not included | –10 | –7 | –12 | S–60–75–4% | S–60–80–3% |
| End-of-life costs excluded | –9 | –6 | –8 | S–60–75–5% | S–60–80–4% |
| Lower unit cost of LDCT | –10 | –8 | S–60–75–5% | ||
| Higher unit cost of LDCT | –10 | –5 | –6 | S–60–75–5% | S–60–75–4% |
| 10-year time horizon | –9 | –5 | S–60–75–5% | ||
| No discounting | 2 | 8 | T–60–75–4% | ||
Very few scenario analyses led to any screening strategy being predicted to be cost-effective versus no screening at a threshold of £20,000 per QALY (this happened only if false-positive and indeterminate results were predicted to have no effect on HRQoL, or if there was no discounting).
Age distribution
In this scenario the age distribution of responders was presumed to match the age distribution of smokers in the UK population, as described in Model structure.
In this scenario, S–60–75–3% was extendedly dominated, and there were two screening strategies on the cost-effectiveness frontier: S–60–80–3% (ICER £36,526 per QALY) and T–60–80–3% (£69,956 per QALY).
Risk-prediction accuracy
The accuracy of risk prediction was increased by changing the risk_lungcancer parameter in the risk model.
In this scenario, S–60–75–3% was dominated and there were three screening strategies on the cost-effectiveness frontier: T–60–75–5% (£25,056 per QALY), T–60–75–3% (£51,077 per QALY) and T–55–75–3% (£13M per QALY).
Programme uptake
In one scenario analysis the uptake of the screening was halved. In this scenario, S–60–75–3% was dominated and there were three screening strategies on the cost-effectiveness frontier: S–60–80–3% (£41,040 per QALY), T–60–80–3% (£97,030 per QALY) and B–60–80–3% (£263,700 per QALY).
In another scenario analysis the uptake of the screening was increased. In this scenario, S–60–75–3% was dominated and there were three screening strategies on the cost-effectiveness frontier: T–60–75–3% (£49,409 per QALY), T–60–80–3% (£84,823 per QALY) and T–55–80–3% (£382,200 per QALY).
Incorporating heterogeneity in lung cancer progression
In this scenario analysis, the natural history model was recalibrated assuming heterogeneity between patients in the rate of lung cancer progression. This also affected the estimated sensitivity of LDCT screening (increasing it substantially).
In this scenario, S–60–75–3% was dominated and there was one screening strategy on the cost-effectiveness frontier: S–60–80–5% (£167,136 per QALY).
Impact on mortality
In one scenario analysis, the survival benefit from early detection was eliminated (i.e. the survival is extended only by the lead time because of screening). In this scenario all screening strategies were dominated by no screening.
In another scenario analysis, the survival benefit from early detection was halved. In this scenario, S–60–75–3% was dominated and there were two strategies on the cost-effectiveness frontier: S–60–75–5% (£74,157 per QALY) and T–60–75–5% (£121,200 per QALY).
Impact on health-related quality of life
In the first of these scenario analyses, it was assumed that there would be a short period of disutility following a lung cancer diagnosis. In this scenario, S–60–75–3% was dominated and there were four screening strategies on the cost-effectiveness frontier: S–60–75–5% (£22,190 per QALY), S–60–75–4% (£50,776 per QALY), S–55–75–3% (£58,040 per QALY) and S–55–80–3% (£113,800 per QALY).
In the second of these scenario analyses, a substantial disutility was assumed for stage IV lung cancer. In this scenario, five screening strategies were on the cost-effectiveness frontier: S–60–75–3% (£22,496 per QALY), S–60–80–3% (£34,254 per QALY), A–60–75–3% (£66,781 per QALY), A–60–80–3% (£93,480 per QALY) and A–55–80–3% (£120,200 per QALY).
In the third of these scenario analyses, it was assumed that there would be no impact on HRQoL in the run-up to subsequent screens (as participants would have ‘acclimatised’ to screening). In this scenario analysis, there were three screening strategies on the cost-effectiveness frontier: S–60–75–3% (£36,680 per QALY), S–60–80–3% (£44,784 per QALY) and S–55–80–3% (£1M per QALY).
In the fourth of these scenario analyses, it was assumed that there would be no impact on HRQoL as a result of false-positive or indeterminate results. In this scenario analysis there were four screening strategies on the cost-effectiveness frontier: S–60–75–3% (£16,759 per QALY), B–55–75–3% (£33,577 per QALY), A–55–75–3% (£63,262 per QALY) and A-55–80–3% (£105,800 per QALY).
Cost of follow-up care
In this scenario analysis, follow-up costs for lung cancer were included for up to 5 years (compared with 2 years in the base case). S–60–75–3% was extendedly dominated and there were three screening strategies on the cost-effectiveness frontier: S–60–75–5% (£31,086 per QALY), S–60–80–3% (£39,648 per QALY) and S–55–80–3% (£67,566 per QALY).
End-of-life costs
In the first of these scenario analyses, PSS costs were excluded from the cost of the end of life (reducing the cost from £4589 to £3209). S–60–75–3% was extendedly dominated and there were two screening strategies on the cost-effectiveness frontier: S–60–80–3% (£35,003 per QALY) and T–60–80–3% (£76,903 per QALY).
In the second of these scenario analyses, end-of-life costs were eliminated completely (which gives an approximation to the case in which end-of-life costs are included for other cause mortality at the same cost as for lung cancer mortality). In this scenario analysis S–60–75–3% was extendedly dominated and there were five screening strategies on the cost-effectiveness frontier: S–60–80–4% (£31,014 per QALY), S–55–80–4% (£31,485 per QALY), S–55–80–3% (£37,778 per QALY), T–55–80–4% (£244,500 per QALY) and T–55–80–3% (£751,000 per QALY).
Computed tomography screening costs
In the first of these scenario analyses, the cost of a LDCT scan was taken from the lower quartile cost across trusts in the NHS reference costs (£70 vs. £99 in the base case). 163 In this scenario, there were two screening strategies on the cost-effectiveness frontier: S–60–75–3% (£39,303 per QALY) and S–60–80–3% (£74,212 per QALY). These results appear anomalous, as it would be expected that cost-effectiveness would be improved by reducing the cost of LDCT (and this was confirmed in the univariate sensitivity analysis). The scenario analysis has not been reconducted as this would potentially introduce bias into the results.
In the second of these scenario analyses, the cost of a LDCT scan was taken from the upper quartile cost across trusts (£121 vs. £99 in the base case). In this scenario, S–60–75–3% was extendedly dominated and there were four screening strategies on the cost-effectiveness frontier: S–60–75–4% (£31,607 per QALY), S–60–80–4% (£45,210 per QALY), T–60–80–4% (£61,927 per QALY) and T–60–80–3% (£230,700 per QALY).
Time horizon
When a time horizon of 10 years was used (compared with a lifetime time horizon in the base case), there were five screening strategies on the cost-effectiveness frontier: S–60–75–5% (£31,290 per QALY), S–60–75–3% (£33,307 per QALY), S–60–80–3% (£45,075 per QALY), B–60–80–3% (£143,900 per QALY) and B–55–80–3% (£383,100 per QALY).
Discount rate
When costs and QALYs are not discounted, S–60–75–3% is dominated and there are five strategies on the cost-effectiveness frontier: T–60–75–5% (£15,001 per QALY), T–60–75–4% (£15,160 per QALY), T–60–75–3% (£63,533 per QALY), B–60–75–3% (£254,200 per QALY) and B–55–75–3% (£477,400 per QALY).
Probabilistic sensitivity analysis
A PSA was conducted with 200 separate samples of parameter values and cohorts of 3000 individuals sampled for each set of parameter values for a total of 600,000 simulations. With cohort sizes of 3000, it is likely that stability was not reached for strategy mean costs and QALYs for each parameter value, and that Monte Carlo variability affects the apparent variability in the PSA results. Nevertheless, there should be adequate exploration of the parameter space with 200 samples and, with a total of 600,000 simulations, the mean total costs and QALYs should be estimated with good precision.
Deterministic and probabilistic results were compared. As shown in Figure 30, there was very good agreement between deterministic and probabilistic costs (although probabilistic costs are slightly lower on average), which is expected as a large proportion of the costs relate to screening and are less affected by outcomes for individuals.
FIGURE 30.
Comparison of deterministic and probabilistic costs.
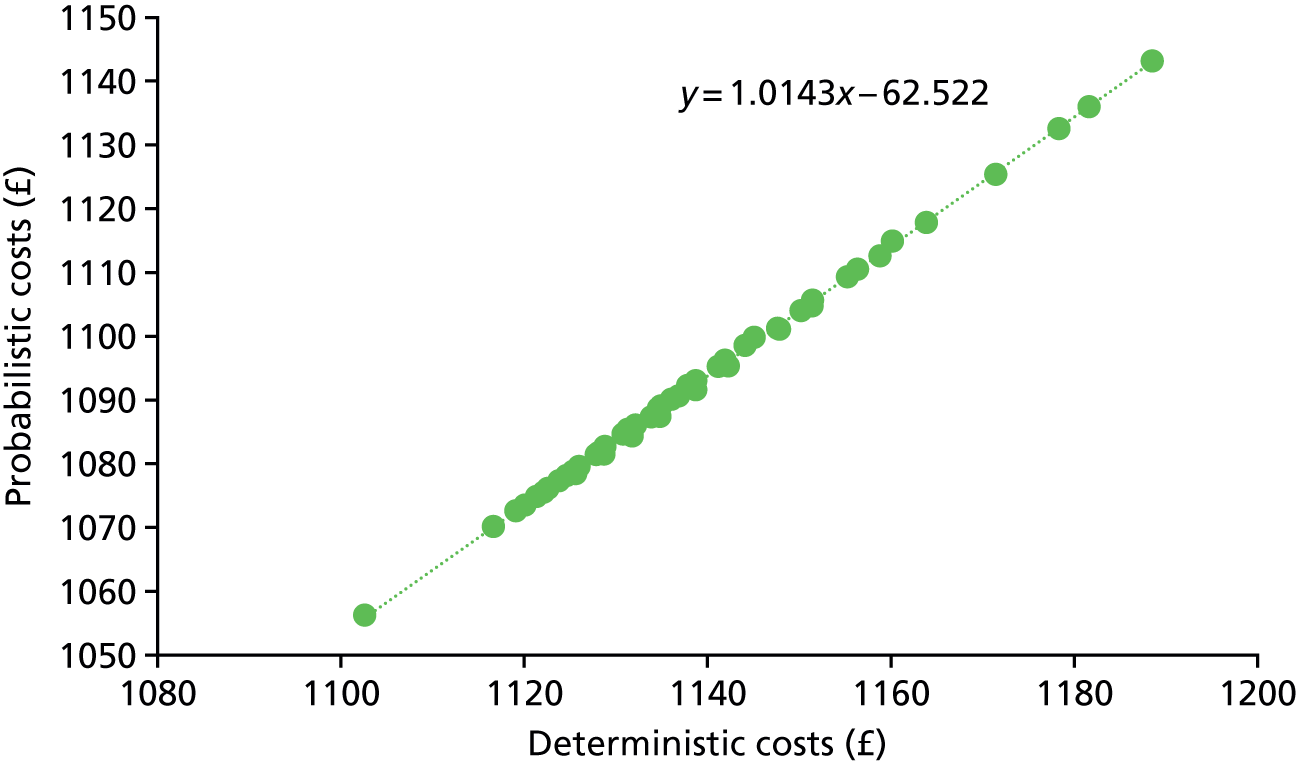
Figure 31 shows that there was less correlation between deterministic and probabilistic QALYs, with evidence that the range of probabilistic QALYs was compressed compared with deterministic QALYs.
FIGURE 31.
Comparison of deterministic and probabilistic QALYs.

The incremental economic value of the strategies was consistently estimated across the deterministic and probabilistic analyses (Figure 32).
FIGURE 32.
Comparison of deterministic and probabilistic INMB.

According to the mean costs and QALYs, there were three screening strategies on the cost-effectiveness frontier (S–60–75–3%, S–60–80–3% and S–55–80–3%) and no screening was also on the cost-effectiveness frontier (Table 31).
| Strategy | Costs (£) | QALYs | ICER vs. no screening, £ (95% CrI) | Full ICER, £ (95% CrI) |
|---|---|---|---|---|
| No screening | 1056 | 8.489 | ||
| S–60–75–3% | 1078 | 8.490 | 35,595 (12,000 to > 500,000) | 35,595 (14,000, NE) |
| S-60–80–3% | 1084 | 8.490 | 36,710 (13,000 to > 500,000) | 41,630 (16,000, NE) |
| S–55–80–3% | 1087 | 8.490 | 39,191 (14,000 to > 500,000) | 104,506 (46,000, NE) |
To determine whether the difference between the results in the base case and the PSA are genuine or a result of poor convergence, the INMB of S–60–75–3% at a willingness to pay of £28,169 per QALY (the base-case ICER) was plotted against PSA iteration number (Figure 33). This demonstrates that it is highly unlikely that the PSA and base-case results would converge with further iterations, and that the PSA results should be preferred as there is the suggestion of non-linearities.
FIGURE 33.
Convergence in PSA. Black dots represent INMBs calculated from individual PSA iterations; the green line represents the running mean INMB; the green ribbon represents the running 95% CI of the INMB; and the dashed black line represents the base-case INMB.

Decision uncertainty was characterised by the 95% credible interval (CrI) of the INMB for each strategy (vs. no screening) at a willingness to pay of £20,000 per QALY.
The 95% CrI of INMB was fully below £0 (i.e. unlikely to be cost-effective vs. no screening) for:
-
T–55–80–3%, A–55–80–3%, B–55–80–3%
-
T–55–80–4%, A–55–80–4%, B–55–80–4%
-
A–55–80–5%, B–55–80–5%
-
A–60–80–3%, B–60–80–3%
-
A–60–80–4%
-
A–60–80–5%
-
A–55–75–4%, B–55–75–4%
-
A–55–75–5%, B–55–75–5%
-
A–60–75–5%, B–55–75–5%
-
A–60–75–3%, B–60–75–3%
-
A–60–75–4%
-
A–60–75–5%.
The 95% CrI was not fully above £0 (i.e. likely to be cost-effective vs. no screening) for any screening strategies. For other strategies, the 95% CrI of INMB contained £0.
The screening strategy with the highest mean INMB was S–60–75–5% (mean –£7, 95% CrI –£18 to £7).
The strategy with the next highest probability of being cost-effective was S–60–75–4% (mean INMB –£8, 95% CrI –£20 to £9), followed by S–60–80–5% (mean INMB –£9, 95% CrI –£23 to £8).
Figure 34 shows the cost-effectiveness acceptability curves for four strategies on the cost-effectiveness frontier. As can be seen, no screening has a higher probability of being cost-effective than the other strategies for thresholds ≤ £50,000 per QALY, although S–60–75–3% and S–60–80–3% are expected to be cost-effective at thresholds below £50,000 per QALY.
FIGURE 34.
Cost-effectiveness acceptability curves.
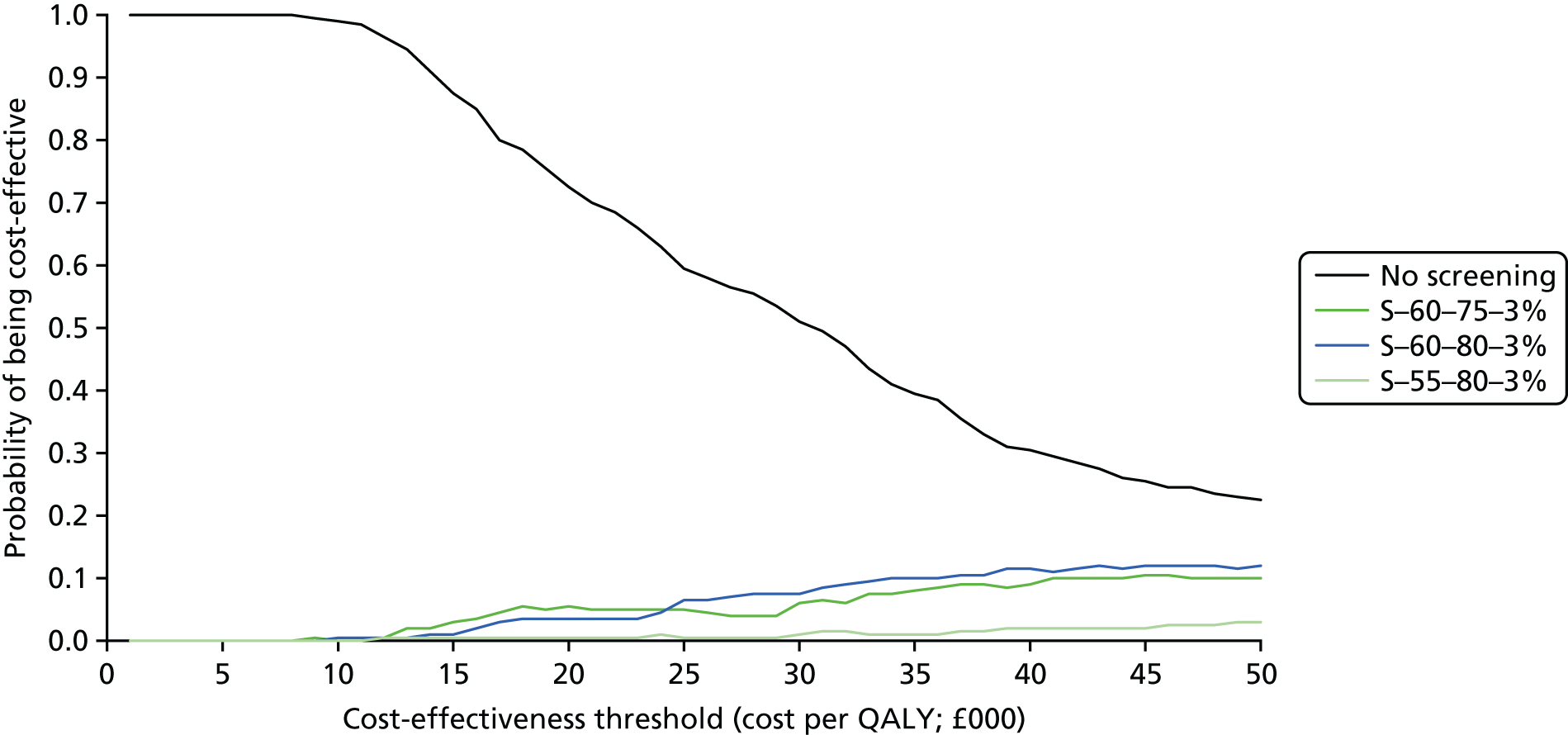
Discussion
Key findings
Lung cancer screening programmes are predicted to lead to health benefits for participants compared with no screening, but they are also predicted to lead to increased costs.
In the base-case analysis it is predicted that the lung cancer screening programmes modelled would not be considered a cost-effective use of limited NHS resources using a cost-effectiveness threshold of £20,000 per QALY.
If a higher cost-effectiveness threshold of £30,000 per QALY is used, then a single screen offered to people aged 60–75 years with a predicted risk of lung cancer ≥ least 3% is predicted to be cost-effective.
Lung cancer screening is estimated to lead to a reduction in mortality from lung cancer ranging from 4.2% to 7.7% depending on the frequency (this is in good agreement with the estimated 5% reduction in lung cancer mortality estimated in the network meta-analysis in Chapter 4), but also to result in increased lung cancer diagnoses (i.e. overdiagnosis) and increased costs relating to lung cancer.
A PSA showed that, at a threshold of £20,000 per QALY, no screening has a > 70% chance of being cost-effective, while it has a 50% chance of being cost-effective at a threshold of £30,000 per QALY. However, at £30,000 per QALY there are a number of LDCT screening strategies that could potentially be cost-effective and, therefore, the probability of any one strategy being cost-effective is low.
One-way sensitivity analyses showed that a 10% variation in any single parameter is unlikely to result in LDCT screening being cost-effective at £20,000 per QALY (this was the case for < 20% of parameters), and that the results were particularly sensitive to the natural history of lung cancer, the cost of treating lung cancer and the cost of LDCT scans.
Scenario analyses demonstrated that the impact of false-positive and indeterminate screening results on HRQoL was important in determining cost-effectiveness, as was the discount rate. Although anxiety and distress from screening results may be studied, as well as potentially affected by a variety of interventions, the discount rate is something that is considered across a range of interventions and there is little reason to believe that the discount rate that applies to lung cancer screening should be different from that applied to other screening interventions. The health effects of lung cancer screening do not lag significantly behind the costs, as survival is generally poor, so benefits are accrued relatively soon after costs are incurred.
Chapter 7 Public consultation and public involvement
A patient and public involvement (PPI) exercise was undertaken at the request of the report funders. In this chapter (and for the rest of the report) we use the term ‘patient’ to mean specific members of the public (smokers and former smokers) who might be invited to undertake the lung cancer screening technology assessed in this report. This term does not include general members of the public. We undertook workshop meetings with both patients and general members of the public and the views elicited in these consultations, that were judged to be relevant to this HTA, are presented and explored in relation to existing literature.
Methods
In response to a request to undertake PPI as part of feedback on the draft protocol for this HTA, we worked with our institution’s source of advice on PPI [the PPI team in Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC)] to design a feasible approach to collecting observations and views about the introduction of a possible lung cancer screening programme in the UK. In a joint strategy developed between PenTAG and the PPI team, we aimed to elicit views from a range of participants, with a particular focus on smokers/former smokers currently without symptoms of lung cancer who may be considered at ‘high risk’ of lung cancer and, hence, a potential priority target group for a UK national lung cancer screening programme. Given the high prevalence of ‘high-risk’ smokers in underprivileged populations of the UK, views were particularly sought from smokers/former smokers living in local deprived areas. Three workshop meetings were arranged targeting three different groups of smokers and former smokers. One group consisted of smokers/former smokers (and included close relatives of people dying from smoking-related conditions) from PenCLAHRC’s Peninsula Public Involvement Group (PenPIG), one group consisted of smokers/former smokers (and included one person whose father had died from a smoking-related condition) recruited through GP practices located in deprived areas and one group consisted of former smokers/non-smokers (and included people who had lost relatives to smoking-related conditions) recruited from Beacon Heath Community Centre/Food Bank located in the most deprived ward of Exeter. In addition to these workshop meetings, an unrestricted drop-in session at Beacon Heath community centre was conducted in order to capture a wide range of public views from people of a variety of ages and a range of smoking statuses.
All workshop meetings were specifically tailored to meet the needs of this lung cancer screening HTA, with each workshop meeting consisting of three sessions over 2 hours. One session consisted of a general introduction to HTA (using a video specifically designed for this purpose). A second session focused on the ‘impact’ from introducing or not introducing lung cancer screening. People were asked to discuss positive and negative aspects with each scenario (screening is/is not introduced). A third session focused specifically on the ‘impact’ of receiving an invitation to lung cancer screening. Workshop participants were asked to imagine how they (or smokers from deprived areas) might feel if they received an invitation to lung cancer screening and to consider the barriers and facilitators that may affect decisions to attend for lung cancer screening. At the end of the session, workshop participants were asked to comment on the findings of a recent study that investigated attitudes towards lung cancer screening in socioeconomically deprived and heavy-smoking communities. 170
The first workshop meeting, held at South Cloisters, University of Exeter Medical School, involved nine members of PenPIG comprising eight females and one male (four smokers and five ex-smokers).
The second workshop meeting, held at South Cloisters, University of Exeter Medical School, involved five members of the public consisting of three females and two males (two smokers and three ex-smokers) recruited from GP surgeries located in more deprived areas of Exeter.
The third workshop meeting, held in a private meeting space at The Beacon Community Centre and Food Bank Centre, involved four members of the public consisting of three males and one female (two ex-smokers and two non-smokers). Two of the meeting participants also attended the Food Bank Centre.
A drop-in session was conducted at Beacon Health during a community drop-in morning (unrestricted). In addition, a public representative who participated in the first PenPIG workshop meeting attended, and contributed to, the clinical experts’ meeting.
All three workshop meetings were audio-recorded and transcribed, with transcripts checked back against the original audio-recordings for accuracy and to aid familiarisation. NVivo software (version 11; QSR International, Warrington, UK) was used to analyse the transcripts. An inductive thematic analysis was performed across all workshop meetings, with analysis procedures used for coding and theme development following the approach proposed by Braun and Clarke. 171 Textual data, reduced into themes, were then interpreted into an analytical framework consisting of seven main categories. Views gathered from the unrestricted drop-in session that confirmed, refuted or extended the identified themes were included in the final analysis. The interpretive analytical framework was checked by PPI researchers, HTA researchers and a PPI representative involved in the workshop meetings, and transferability confirmed by clinical experts during a stakeholder meeting.
People’s views on impact from a lung cancer screening programme
The key themes arising from the qualitative thematic analysis of workshop transcripts are summarised in the seven categories in Table 32 and are expanded on in the full thematic analysis (see Appendix 11). Key associations and core dynamics between themes arising from public consultation in all three tailored workshop meetings, together with the community/food bank centre drop-in session, have been presented in Figure 35 as a concept map (with accompanying narrative summary) in order to provide an explanatory model of contextual ‘real-world’ perspectives through which to view our clinical effectiveness analysis and economic model. The chapter concludes with a reflection on the issues raised that reinforce those arising from other perspectives, but particularly issues that have not been raised elsewhere or where the views raised conflicted with generally accepted perspectives.
| Engagement/facilitators | Disengagement/barriers | Financial costs/funding | Culture/environment | Treatment/care pathway | QoL/mental well-being | Responsibility and risk |
|---|---|---|---|---|---|---|
|
Universal vs. targeted approach Other benefits of screening (detection of other lung diseases) Invitation to screening (content/format) Planning Work Motivation to quit smoking Support |
Poor access to screening/support Worry/denial/fear of knowing Fatalism; believe will die; ‘damage is done’ Invitation to screening (content/format) Work/lifestyle Gender Age Public stigma Blame from health professionals No motivation to quit smoking |
To NHS Insurance premiums Tax revenue from cigarettes Cost of travel to screening/additional tests Cost of travel to hospital for carers Cost of private screening Missed appointments |
Cultural norms Government and media influence Inequity/deprivation |
DetectionTreatment/no treatment Repeat testing |
Coping Anxiety/worry QoL and diagnosis QoL and treatment Reduced QoL attributable to effects of treatment Support (lack of) Empowerment Disempowerment |
Family history (of lung cancer) Smoking history Passive smoking Information (about risk) Environmental risk Exposure to radiation (technology) Perception of risk |
FIGURE 35.
Explanatory model of patient and public views arising from workshop meetings.
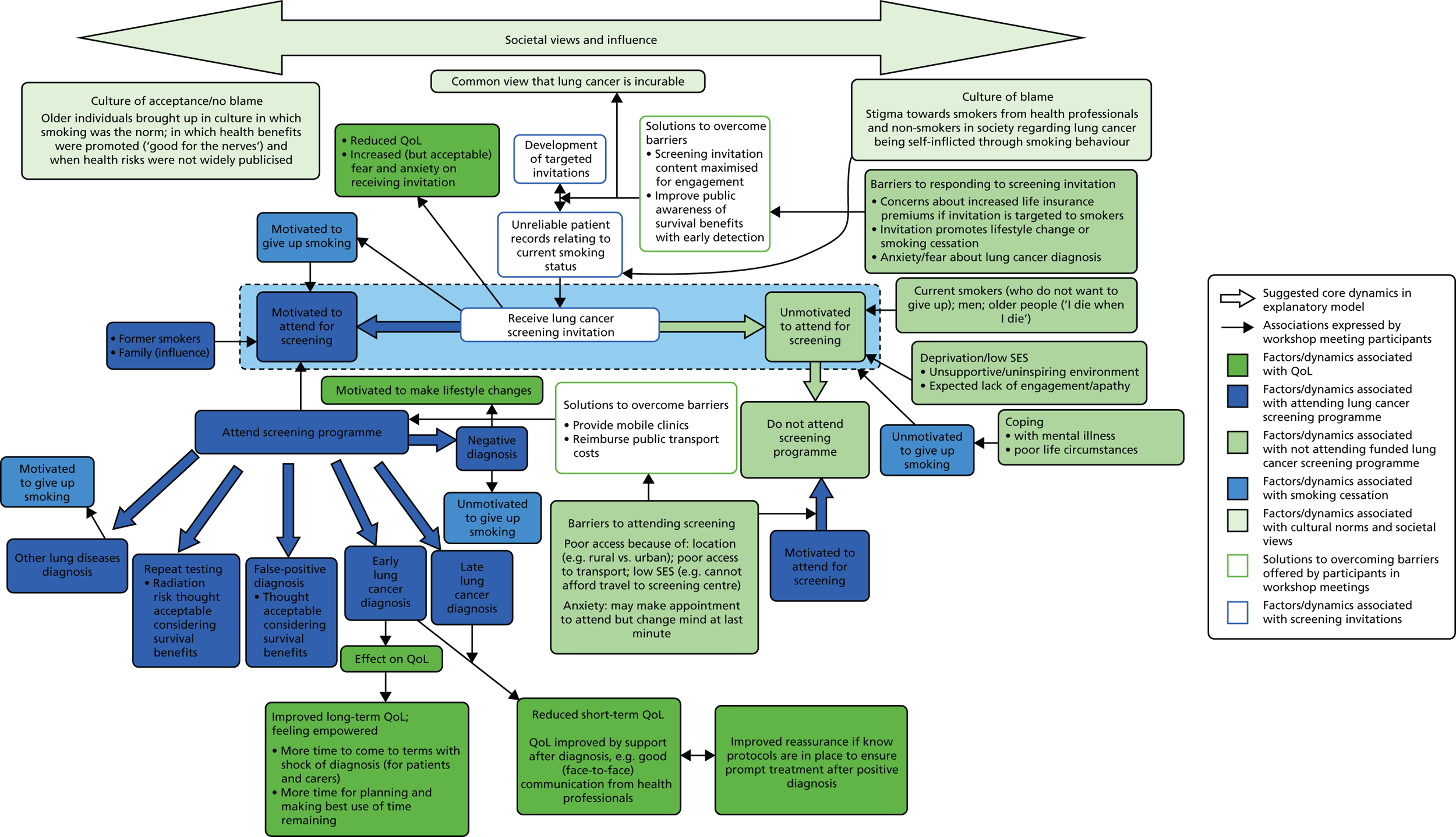
Narrative summary to accompany explanatory model of patient and public views
The explanatory model explores relationships between patient and public views expressed around decisions to attend a national lung cancer screening programme together with views on the broader cultural and societal influences that may influence such decisions.
At the centre of the explanatory model (highlighted in light blue in Figure 35) is the core dynamic arising from the consultation process that highlights the tension between feeling motivated/unmotivated to attend for screening and presents associated factors that may be influential in decisions to attend/not attend a national lung cancer screening programme. This core dynamic may be viewed within the context of current prevailing views and influences in the UK that were expressed during our PPI consultations (highlighted in mid-blue in Figure 35). In our PPI discussions, poor public awareness around potential treatments and survival benefits resulting from early detection as well as a culture of stigma and blame associated with smoking were acknowledged and thought to be influential on decisions to attend for screening.
Factors associated with poor motivation/barriers to attend for lung cancer screening are highlighted in mid-green on the right-hand side of Figure 35. Influences of poor life circumstances, gender, current smoking status and implications of being targeted as a smoker were discussed. It was thought that smokers may generally be less willing to engage with a lung cancer screening programme than former smokers because of the social stigma associated with smoking (from both members of the public and health professionals) and particularly unwilling to engage if they have no intention of giving up smoking. It was thought that people living with poorer life circumstances (e.g. people with low SES or people living with mental illness) may be more dependent on smoking in order to cope with general life stress and may be less willing to give up smoking/engage with a cancer screening programme. Participants said that some people would be cautious about accepting an invitation to attend for lung cancer screening as it would identify them as ‘high risk’ and may have implications for insurance (e.g. life insurance premiums). It was acknowledged that feelings of fear and anxiety associated with a potential positive diagnosis may be difficult to talk about and may cause some people (especially men) to avoid attending lung cancer screening. It was thought that smokers may be put off responding to an invitation to attend screening if the invitation promoted lifestyle change or smoking cessation. In addition, older people who consider themselves too old to benefit were thought less likely to be motivated to attend. Solutions offered in meetings to overcome such barriers included suggestions to modify invitation content in order to improve engagement (e.g. including information about ‘what to expect’ and safety information that encouraged informed choice) as well as increasing general public awareness of the benefits of lung cancer screening with regard to early detection and increased survival. It was thought that the use of GP records to identify and target smokers could be unreliable, as smokers may be less inclined to be honest about their smoking status in order to avoid public stigma/being judged and blamed by their GP. Participants acknowledged that some would hold the view that lung cancer was self-inflicted and should not be funded by the NHS. However, most participants felt that it was inappropriate to blame current/former smokers currently at high risk of lung cancer who began smoking when smoking was the cultural norm, when health benefits of smoking were promoted (smoking was ‘good for the nerves’) and when health risks were not widely publicised.
Factors associated with motivation for/facilitators to attend lung cancer screening are highlighted in Figure 35 in dark blue. Influences of smoking status, other family members and access to screening were discussed. It was suggested that wider family members may be influential in the decision to attend for lung cancer screening (e.g. unmotivated individuals may decide to attend for the sake of their children). It was recognised that some people may not be eligible to access a targeted national screening programme but might consider themselves at risk [e.g. because of environmental exposure (to passive smoking or asbestos) or because they have a family history of lung cancer]. Individuals eligible and motivated to attend lung cancer screening may be unable to do so because of poor access (e.g. as a consequence of living in a deprived or rural location with poor public transport or inability to afford public transport). Solutions to such barriers that were put forward by workshop meeting participants included provision of mobile screening clinics and reimbursement of public transport travel costs. It was also recognised that feelings of anxiety arising on receiving an invitation could mean that some motivated individuals may make an appointment, but then change their mind at the last minute and so fail to turn up.
Factors associated with treatment pathways for eligible motivated people able to access screening are highlighted in dark blue on the left-hand side of Figure 35. Implications of repeat testing and diagnosis were discussed. The radiation risk from repeat testing and potential anxiety caused by false-positive diagnosis were considered acceptable because of the potential survival benefits associated with early detection and successful treatment of lung cancer. It was thought that incidental findings of other lung diseases occurring during the screening process may motivate some people to give up smoking and that an invitation containing wording about lung screening (rather than lung cancer screening) would be less stigmatising and potentially more acceptable to smokers. Participants said that although receiving a negative lung cancer screening result may motivate some smokers to change their lifestyle, others receiving a negative screening result may become complacent and unmotivated to quit smoking.
Factors associated with QoL resulting from introducing a lung cancer screening programme are highlighted in dark green in Figure 35. Influences of receiving an invitation to attend lung cancer screening and influence of diagnosis were discussed. Given the survival benefits associated with lung cancer screening, participants thought that any feelings of anxiety and fear arising on receiving an invitation were acceptable. In addition, it was suggested that some people may become more motivated to give up smoking on receiving an invitation to attend lung cancer screening and that receiving a negative screening result may motivate individuals to make lifestyle changes. Although it was acknowledged that receiving a diagnosis of lung cancer would have an impact on QoL in the short term, it was suggested that this could be moderated by good face-to-face communication from health professionals. Reassurance could also be gained from knowing that protocols were in place to ensure prompt treatment after a positive diagnosis. A workshop participant who, as a child, lost his father (a smoker) to a lung disease (emphysema) suggested a link between lung cancer diagnosis and improved QoL for both patients and family/carers in the longer term. The view was expressed that an early diagnosis of untreatable lung cancer could lead to feelings of empowerment and increased QoL arising from having more time to deal with the shock of diagnosis (for both patients and family/carers) as well as more time to plan for the future (e.g. write a will) and make the best use of time remaining with family and friends.
Discussion
Lung cancer prevalence is higher in lower-SES communities, in which lifelong smokers are both over-represented172 and more tobacco dependent. 173 It is widely accepted that, for a lung cancer screening programme to be effective, it must achieve a positive benefit–harm ratio, which in turn depends on attracting the higher-risk and hard-to-reach lower-SES individuals in a population. Increasing the risk profile of participants has the potential to reduce avoidable invasive follow-up tests and the number needed to screen (NNS)174 and, hence, improves the cost-effectiveness and efficacy of a screening programme as well as reducing lung cancer inequalities. However, enrolment to screening programmes offered within the trial context has been extremely low and biased towards former smokers, rather than current smokers, and towards higher-SES individuals. 75,175 Lower-SES smokers are less likely to engage with an offer of screening or see it through, a tendency observed across other screening programmes176–178 and health-care services. 179,180
As part of our PPI workshop format, we invited workshop participants to comment on the findings of a recent study that investigated attitudes towards lung cancer screening in socioeconomically deprived and heavy-smoking communities. 170 The study found that, although participants were supportive of lung cancer screening in principle, many did not feel that screening could offer a long-term survival benefit for ‘heavy smokers’. Quaife et al. 181 found that a belief that lungs are not a treatable organ appeared to be a common lay explanation for poor survival and undermined the potential value of screening. Perceived blame and stigma around lung cancer as a self-inflicted smoker’s disease were described by study interviewees as important social deterrents of screening participation and this perspective also emerged during our consultations. The study suggested targeting invitation strategies to this high-risk SES group to achieve equitable participation in screening. Such invitation targeting is currently being investigated in the Lung Screen Uptake Trial (LSUT) (October 2013 to September 2017). 181 The study targets those aged 60–75 years who have been recorded as a smoker in the previous 5 years by their GP. Intervention invitation materials are targeted for (and in consultation with) high-risk and ‘hard-to-reach’ groups prior to the hospital appointment for assessment and LDCT screening (if eligible), with the primary outcome measure being uptake of hospital appointments for assessment/screening. Based on previously published research,182–186 targeted content was developed for this study (Box 2).
[. . .] to minimise fear [of a positive diagnosis], fatalism, stigma and blame around lung cancer by: i) emphasising a supportive and non-judgemental service, ii) providing a lay explanation for how early detection of lung cancer can work (using a diagram to illustrate that the lung is a treatable organ which need not be completely removed because early treatment can be focused within a lobe), iii) acknowledging that the invited generation were previously not informed of the risks of smoking, iv) avoiding mention of smoking, smoking cessation, and risk where possible at the screening invitation stage, v) emphasising the salience for older adults, and vi) normalising the offer so as to not implicate the reason for invitation as being that lung cancer is suspected or that the recipient is being singled out.
Reproduced from Quaife et al. 181
© Quaife et al. 2016. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Although most smoking/ex-smoking participants in all three of our PPI discussion workshops said that they would respond to a targeted invitation to lung cancer screening, some participants said that they would not respond to an invitation to lung cancer screening if it was specifically targeted at smokers/ex-smokers because of the stigma associated with smoking. They said that they would be more likely to respond to a universal invitation that did not specifically mention smoking and lung cancer, but that offered screening for general lung diseases or offered a universal programme based, for example, on age. A view was elicited relating to the unreliability of people’s willingness to be honest about their smoking status with their GP, so bringing into question the reliability of health records for targeting purposes, and this view was strongly expressed by our PPI representative during our initial HTA researcher/clinician stakeholder meeting.
Most participants in our workshop meetings who expressed a view said that risks to safety (from any intervention) were inevitable and that radiation risks from LDCT technology were acceptable considering the survival benefits resulting from early detection of lung cancer. Participants thought that raising public awareness about lung cancer screening and providing information about safety (radiation) and the likely physical sensations of the screening process in the screening invitation would be helpful to alleviate people’s worry and encourage participation in screening.
The research literature reports poorer HRQoL and increased anxiety among current smokers, those with a longer smoking history and individuals with a lower level of education in the NELSON84,85 and Pittsburgh Lung Screening Study (PLuSS)187 screening cohorts. Although it is recognised that there is potential for distress at any point along the lung cancer screening pathway, the psychological impact of screening may depend largely on the nature of the screening result that they receive (and any ensuing tests or surveillance) and psychosocial characteristics. 188 Higher perceptions of risk189 and guilt about smoking190 have been shown to predict higher screening-related distress. Research indicates screening-induced stress is short term (< 6 months). 191,192 However, it is recognised that there are important differences between trial and real-world contexts, and there is evidence from other screening programmes of long-term psychological distress associated with cancer screening. 193 Participants in our PPI workshop meetings recognised that smokers with low SES status, who would potentially benefit the most from a UK lung cancer screening programme, may face particular psychosocial challenges associated with poor life circumstances. Participants acknowledged the higher prevalence of smoking in these members of society, together with people living with mental illness, as a way of coping with general life pressures. During our PPI workshop meetings, participants recognised the importance of the impact of a lung cancer screening programme on QoL/mental well-being at all lung cancer screening stages. PPI workshop meeting participants said psychological/mental well-being would be affected just by the introduction of the screening programme, both positively and negatively, and that they thought psychological well-being should be an outcome assessed in this HTA review.
The importance of good communication from health professionals when delivering screening results, as reported in qualitative literature,194 arose as a point of discussion in our PPI workshop meetings. Brain et al. 86 and Quaife and Janes195 acknowledged the importance of preparing individuals psychologically for abnormal screening results so that they are fully informed, and the views elicited during our PPI workshop meetings are in agreement with this stance.
Some of our PPI workshop meeting participants described how important it would be to have access to information about the likelihood of survival after a diagnosis during the lung cancer screening process in order to minimise reduced QoL following a positive lung cancer diagnosis. This perspective is corroborated by a multicentre survey conducted in the USA that found that 23% of patients diagnosed with incidental pulmonary nodules who received information on lung cancer risk from their clinician provider were more likely to find this information reassuring than scary. 196
Incidental findings are often viewed as a negative aspect of CT screening, but their detection may also provide an opportunity to rectify other conditions that threaten QoL or survival. The NLST reported a 6.7% reduction in all-cause mortality, which may be explained by detection of clinical and radiological findings in the process of screening, and by the improved access to health care brought about by screening that may prompt intervention for non-lung cancer comorbidities. 197 During our PPI discussions, it was recognised that other diseases (e.g. pneumonia or fungal infections) may be detected incidentally during lung cancer screening and treated with consequent improvements in health.
The impact of lung cancer screening on smoking cessation remains unclear. Several studies have reported increased smoking cessation in trial participants compared with the background population. However, no statistically significant differences in outcomes between the screened and control groups have been noted, suggesting that trial participants may be a more motivated group. 73,81,88,198–201 During our PPI discussions, some participants said that they thought that receiving an invitation for screening would act as a motivator to quit smoking and that receiving a lung disease diagnosis could similarly motivate people to give up smoking. Quaife and Janes195 propose that similar positive responses could be researched and capitalised on to promote smoking cessation.
The use of mobile CT scanners versus dedicated screening centres was discussed in our PPI workshop meetings. It was acknowledged that, if screening was not offered at a local hospital, then certain members of society would find it more difficult to access screening because of their location, poor access to transport and caring responsibilities. It was thought that the impact from these factors would be accentuated if people had little support or low income or lived in more deprived areas. Participants in two meetings suggested that access might be improved by providing mobile screening clinics in the community, similar to those already provided for breast screening. Although the cost of various methodologies that can be used in the screening process, such as the use of mobile CT scanners versus dedicated screening centres, needs to be evaluated, during an initial researcher/clinician HTA stakeholder meeting the view was aired that the cost of funding mobile lung cancer screening clinics would be prohibitive. One low-SES community drop-in participant (who was accessing the food bank centre) expressed the view that people, including himself, would be willing to travel long distances using public transport to attend screening if they were financially reimbursed.
Summary
Views expressed during our PPI workshop meetings were broadly in agreement with currently accepted perspectives based on research findings from published trial and qualitative research literature. Our PPI participants discussed the challenges of engaging higher-risk individuals (low-SES smokers) and recognised that people living in deprived areas (as well as people living with mental health issues) are more likely to smoke in order to cope with poor life circumstance and associated stress. The importance of overcoming barriers to access screening, particularly for low-SES individuals who may not have their own transport, was discussed during our PPI workshop meetings and novel solutions offered. One low-SES community drop-in participant (who was accessing the food bank centre) expressed the view that people, including himself, would be willing to travel long distances using public transport to attend screening if they were financially reimbursed. Our PPI participants made some suggestions regarding screening invitation content to improve uptake [e.g. increase informed consent by providing information in screening invitation about safety (radiation) of LDCT screening technology and provide details of the likely physical experience of the screening process and subsequent potential treatment pathways]. PPI participants suggested that an invitation that did not specifically mention smoking and lung cancer, but that offered screening for general lung diseases, would be less stigmatising and more engaging for smokers. Incidental findings are often viewed as a negative aspect of CT screening, but it is currently recognised that their detection during lung cancer screening can reduce all-cause mortality.
Some PPI participants thought that a targeted screening invitation that promoted lifestyle change or offered help to give up smoking would be disengaging for smokers. A view was also expressed relating to smoking-related stigma and the unreliability of people’s willingness to be honest about their smoking status with their GP, thereby bringing into question the reliability of health records for targeting purposes.
During our PPI workshop discussions, participants recognised the potential impact of a lung cancer screening programme on QoL/mental well-being at all lung cancer screening stages and felt strongly that psychological well-being should be considered as an outcome in this HTA review. Many participants acknowledged that they would expect to experience feelings of fear on receiving an invitation to attend lung cancer screening but said that they would want to attend screening despite this. It was, however, acknowledged that people could change their mind at the last minute and fail to attend for screening because of anxiety, even after initially accepting the invitation. Interestingly, our PPI participants did not think that the distress and anxiety caused by false positives were problematic given the benefits of a lung cancer screening programme for survival. Participants expressed the view that, because all tests are fallible, some misdiagnosis is inevitable and it is an unfortunate but acceptable consequence of performing a test. Equally, PPI participants said that any anxiety experienced while waiting for test results was acceptable given the potential for early treatment and improved survival that lung cancer screening offers. Although the trial research literature reports short-term psychological distress associated with diagnosis during lung cancer screening, our PPI participants said that there may be some potential QoL gains in the longer term for people diagnosed with terminal lung cancer relating to empowerment, both for the patient (in planning and spending their remaining time wisely) but also for their family and carers (to plan for life after their loved one has died).
The impact of lung cancer screening on smoking cessation remains unclear in the research literature. During our PPI discussions, some participants said that they thought that receiving an invitation for screening would act as a motivator to quit smoking and that receiving a lung disease diagnosis could similarly motivate people to give up smoking. Quaife and Janes195 have proposed that similar positive responses could be researched further and capitalised on to promote smoking cessation.
Chapter 8 Assessment of factors relevant to the NHS and other parties
Resource implications
A lung cancer screening programme could potentially entail significant resource implications.
Although a significant number of costs were not included in the economic evaluation (particularly those related to the set-up and evaluation of a programme), it was still estimated that screening could result in additional costs on the order of hundreds of millions of pounds (see Table 27).
The current capacity of radiology departments will be of concern when considering the implementation of a lung cancer screening programme. If around 3.1% of 13 million smokers all received a single LDCT scan, this alone would represent 400,000 LDCT scans (not including a significant number of follow-up scans that would be necessary. The NHS Reference Costs 2015 to 2016163 (England) indicate that around two million CT scans are conducted per year (not including PET-CT), so that an additional 400,000 scans would represent a 20% increase in workload.
A lung cancer screening programme could be implemented incrementally by specifying a single year of age for screening eligibility, such that screening would be delayed for those younger than the year of age chosen, while those older than the year of age chosen would never become eligible. This would reduce the immediate burden on radiology services by a factor of 10–25, but would also mean significantly reduced benefits. Economic modelling should be capable of identifying the most cost-effective year of age for such a programme.
Identification of suitable populations
A key factor in the potential value of a lung cancer screening programme is the ability of a programme to effectively and efficiently identify individuals meeting the criteria for the programme. There are issues of sensitivity (minimising the number of people who should be included but are missed) and specificity (minimising the number of people contacted about screening who are in fact ineligible) to consider.
The economic analysis in Chapter 6 assumes that it is possible to identify all (or a clear majority of) smokers within a certain age range, and that this can be done at minimal cost compared with other costs associated with screening.
It is supposed that there are data of sufficient quality held by GPs to identify people with a history of ever smoking, although the data are not considered of suitable quality to estimate a more detailed smoking history (e.g. how long has each individual smoked, do they currently smoke, with what intensity have they smoked). A GP in the Expert Advisory Group for the project indicated that data on ever smoking would likely be of sufficient quality to accurately identify ever-smokers within a given age range, although concerns were raised about how time-consuming it would be to interrogate the data, whether or not GPs would be expected to improve the quality of these data using clinic time, and whether or not invitations would need to be sent by the GPs or if their names and reputations would be co-opted as part of a programme. On the other hand, views were raised in the PPI workshops that GP records may be unreliable as smokers may be less inclined to be honest about their smoking status in order to avoid stigma, judgement and blame.
The UK-based RCT of lung cancer screening (UKLS) did not use primary care-based recruitment of participants55 and so such a technique for recruitment to a screening programme may need to be demonstrated in a pilot study.
There is also a consideration to make that smokers are not the only group who are at risk of lung cancer, as there are certain occupations that increase the risk of lung cancer.
Equity, societal and ethics considerations
Lung cancer is a disease particularly affecting those with lower SES, with incidence being higher and survival poorer in the most deprived regions compared with the most affluent. 202 Data also demonstrate that men are at higher risk of lung cancer incidence and have poorer survival.
Interventions that reduce the morbidity and mortality from lung cancer have the potential to reduce health inequalities, if these interventions are equally available and taken up by individuals with lower SES.
A lung cancer screening programme based on risk stratification can arguably legitimately target screening more towards men and individuals with lower SES as these groups are more likely to be at high risk of lung cancer, as it will still target women and more affluent individuals if they are in fact at high risk.
Importantly, though, groups at a high risk of lung cancer also face significant barriers to screening. Transport and time off work to attend screening may be more difficult to obtain for such groups, and there may also be a higher risk of other significant health issues, including mental health issues, which may make screening attendance more challenging. Current smokers are at particular risk of lung cancer, but may be deterred from attending screening if they are resistant to lifestyle change and believe that screening is in part designed to get them to quit smoking.
There is a risk with screening that the worried-well will be disproportionately represented. An accurate risk prediction algorithm can partly counter this, but it will rely on accurate representations of smoking histories from potential participants.
The majority of people diagnosed with lung cancer are above retirement age. Although our review and economic evaluation have not considered costs and benefits from a societal (including productivity costs/gains) or governmental (including tax and pensions) perspective, nevertheless the potential for economic and patient benefit from a NHS perspective is concentrated in the lower ages at which people are routinely diagnosed with lung cancer (60–75 years). Two NICE Citizens Council meetings have considered the issue of age in terms of trading off equity (treating people of all ages the same) and efficiency (maximising the value obtained by spending money). 203,204 In one it was considered legitimate by most members to differentiate on the basis of age when age is an indicator of risk, and when certain age groups are more likely to benefit from a treatment,203 while in the other meeting it was considered that consideration of age is a circumstance when it is difficult to unpick the tensions between equity and efficiency.
Acceptability is an additional concern for mass screening programmes. In particular, here we can consider the acceptability to those receiving screening (including distress and anxiety, comfort during the scan, etc.), those deemed ineligible owing to low risk (potential to be reassured about risk level vs. concern about not receiving screening), and the wider public who are funding the screening. Concerns that there may be issues of public acceptance of a lung cancer screening programme on the basis that lung cancer is ‘self-inflicted’ can be somewhat allayed by the unanimous conclusion of a Citizens Council that such factors should not be considered when determining clinical need. 205
Chapter 9 Discussion
Statement of principal findings
Clinical effectiveness
Twelve RCTs were included in the systematic review of clinical effectiveness (see Table 1). Only six of these contributed to the key outcomes (see Table 4). Most studies were conducted in European countries but some studies were conducted in the USA (see Table 1), including by far the largest, NLST,70,71 with over 50,000 participants. One trial, UKLS,55 was conducted in the UK. Most RCTs started between 2001 to 2010 (see Table 4), and so many are just maturing. The majority of included trials were judged to be of moderate to high quality, but two trials64,69 were judged to be of poor quality including one that contributed mortality data. 69 There was variation between the LDCT programmes, but typically they involved three to five rounds of screening over 3 to 6.5 years. UKLS,55 a pilot trial, had only one screening round. The nature of high-risk participants also varied but was usually defined in terms of age and current and past smoking. Of the trials, NLST70,71 stands apart, not just in terms of size, but by being compared with CXR screening rather than no screening.
Concerning mortality, only four of the RCTs, including NLST, currently contribute (see Figure 2). Meta-analysis of these showed that LDCT screening was associated with a non-statistically significant decrease in lung cancer mortality (pooled RR 0.94, 95% CI 0.74 to 1.19) with up to 9.80 years of follow-up when compared with controls (usual care/best available care). A moderate level of heterogeneity was observed in the magnitude of effects (I2 = 43.3%), given which the results should be treated with caution.
A range of potential sources for heterogeneity was investigated. When removing the poor-quality trial (MILD69), sensitivity analysis demonstrated a statistically significant decrease in lung cancer mortality (pooled RR 0.85, 95% CI 0.74 to 0.98) in favour of LDCT screening compared with controls. A considerable reduction in heterogeneity was observed (I2 = 6.9%).
The findings from this review also showed that, compared with controls (usual care/best available care), LDCT screening demonstrated a non-statistically significant increase on all-cause mortality outcome (pooled RR 1.01, 95% CI 0.87 to 1.16) with up to 9.80 years of follow-up. Likewise, given the substantial heterogeneity (I2 = 57.0%) detected between studies, the results from this pooled analysis should be treated with caution. We also investigated the potential sources of heterogeneity. When removing the low-quality trial (MILD69), sensitivity analysis showed that LDCT screening demonstrated a non-statistically significant decrease in all-cause mortality (pooled RR 0.95, 95% CI 0.89 to 1.00) compared with controls. The level of heterogeneity was also considerably reduced (I2 = 0%), suggesting that variation in trial quality could be a potential source of heterogeneity between studies.
Number needed to screen is often advised as a way to improve the interpretation of clinical effectiveness data. This is more difficult for LDCT screening because of varying time periods over which events are taking place and variation in the baseline event rates. However, for a pooled RR for reducing lung cancer deaths of 0.94 (95% CI 0.74 to 1.19), we have cautiously calculated that this is equivalent to a NNS of 357 to avoid one lung cancer death [95% CI 82 to –113 (screening increases lung cancer deaths)]. This illustrates the impact of the very low frequency of the events in question even in a population selected to be at high risk. A number of assumptions for the NNS must be specified. First, it assumes a typical screening programme as used in LDCT-screening RCTs (five annual screens in a population of smokers and ex-smokers) over an 8-year period (5 years of screening followed by 3 further years of observation). Second, it assumes a baseline risk of lung cancer death without intervention of 4.7 lung cancer deaths per 100 persons over an 8-year period as found in the DANTE RCT,61 which identified the highest lung cancer risk of death in the RCTs contributing data on mortality. If lower baseline risks were used, the NNS would be higher.
Network meta-analysis (including six RCTs61,63,69,71,105,106) was performed to assess the relative effectiveness of LDCT, usual care and CXR screening. The results showed that LDCT was ranked as the best screening strategy, with a 74.8% probability of being the best intervention in terms of lung cancer mortality reduction. Usual care (no screening) had a 74.7% probability of being the second best strategy. However, CXR screening had a 99.7% probability of being the worst intervention on lung cancer mortality outcome.
Concerning numbers of lung cancers detected, compared with controls (usual care/best available care), LDCT screening was associated with a statistically significantly increase in lung cancer detection rate (pooled RR 1.38, 95% CI 1.02 to 1.86) with at least 5 years of follow-up. Although there was heterogeneity (I2 = 79.7%), all included studies individually showed statistically significant increases in the numbers of cancers detected in the LDCT group (see Figure 8).
Our findings further demonstrated a clear benefit of LDCT screening on the shift in stage distribution towards earlier stages for detection of lung cancers. LDCT screening was associated with statistically significantly increases in early stage (I and II) cancer detection (pooled RR 1.73, 95% CI 1.27 to 2.37), when compared with controls (usual care/best available care). LDCT screening was associated with a statistically significant reduction in the risk of late stage lung cancer compared with controls (RR 0.85, 95% CI 0.73 to 1.00), although this effect was not observed when only trials comparing LDCT to no screening were included (RR 1.00, 95% CI 0.75 to 1.34), despite these trials still finding an increased probability of lung cancers being early stage. This is consistent with overdiagnosis being a significant factor in the trials.
Based on the randomised data from four included trials (see Table 9), there were consistently no statistically significant differences in HRQoL or psychological consequences between the LDCT screening group and control groups during the trials.
The data from three included trials (one reported as two subcomponents) showed mixed results with regard to the effect of a LDCT screening programme on participants’ smoking behaviour (see Table 10). The data within trial arms sometimes indicated positive associations between smoking cessation and the presence of an abnormality on LDCT. However, this is inconsistent with the evidence comparing trial arms that did not show a consistent pattern favouring LDCT’s effect on smoking behaviour between trial arms.
Cost-effectiveness
Systematic review
Lung cancer screening programmes are predicted to lead to health benefits for participants compared with no screening, but also increased costs. Study estimates of cost-effectiveness differed substantially, and there are a number of key parameters in existing studies that may not be generalisable to the UK setting.
Independent economic evaluation
Forty-eight lung cancer screening strategies were considered, each representing a unique combination of frequency (single, triple, annual or biennial), minimum age for entry (55 or 60 years), maximum age for entry (75 or 80 years) and threshold for predicted risk (3%, 4% or 5%).
Lung cancer screening programmes are predicted to lead to health benefits for participants compared with no screening (including potential anxiety impacts as a result of screening), but they are also predicted to lead to increased costs (including increased costs associated with lung cancer).
Participants in lung cancer screening are estimated to have a reduction in lung cancer mortality of 4.2–7.7% (depending on the frequency of screening), but also to receive more lung cancer diagnoses than in the absence of screening (i.e. lung cancer is overdiagnosed).
It is expected that 1.2–4.0% of the approximately 13 million smokers aged 55–80 years would actually undergo screening (depending on the population criteria), as a significant number would not respond to an invitation to screening, and another significant proportion would not meet the threshold for lung cancer risk.
Lung cancer screening with a single LDCT scan for smokers aged 60–75 years with a ≥ 3% risk of lung cancer is predicted to be cost-effective at a threshold of £30,000 per QALY (the upper end of thresholds commonly considered in the UK for non-ultra-orphan, non-end-of-life treatments), but not at a threshold of £20,000 per QALY (the lower end of thresholds commonly considered in the UK). This screening programme was estimated to cost £28,000 per QALY gained. However, when the probable range of inputs is considered in a PSA, there were no screening strategies that were cost-effective at thresholds of £20,000 or £30,000 per QALY.
Annual and biennial screening programmes are not estimated to be cost-effective at any threshold, and this appears to be mostly as a consequence of the presumed detrimental impact of false-positive and indeterminate results on HRQoL.
Patient and public involvement
An explanatory model was constructed detailing the key contextual associations and core dynamics arising from our PPI meetings. The model details views expressed in our workshop meetings around smokers’/former smokers’ decisions to attend a potential national lung cancer screening programme together with views on the broader cultural and societal influences that may influence such decisions. Lung cancer prevalence is higher in lower-SES communities, where lifelong smokers are both over-represented172 and more tobacco dependent than higher-SES communities. 173 It is widely accepted that for a lung cancer screening programme to be effective, it must attract higher-risk and hard-to-reach lower-SES individuals in a population. Increasing the risk profile of participants has the potential to reduce avoidable invasive follow-up tests and the NNS174 and, hence, improve the cost-effectiveness and efficacy of a screening programme, as well as reduce lung cancer inequalities.
Our PPI participants discussed the challenges of engaging higher-risk individuals (low-SES smokers) and recognised that people living in deprived areas (as well as people living with mental health issues) are more likely to smoke in order to cope with poor life circumstance and associated stress. The importance of overcoming barriers (e.g. practical or financial) to access screening, particularly for low-SES individuals who may not have their own transport, was discussed. Our PPI participants made some suggestions regarding screening invitation content to improve uptake and discussed the potential impact of a lung cancer screening programme on QoL/mental well-being at all lung cancer screening stages, including empowerment at end of life. PPI participants held discussions around the impact of a lung cancer screening programme on smoking cessation as well as discussions about the psychological impact of false-positive results and radiation risks from screening. The importance of good communication from health professionals when delivering screening results arose as a point of discussion in our PPI workshop meetings.
In addition to personal views around uptake and implementation, the potential impact of wider societal and cultural contexts on decisions to attend for lung cancer screening was discussed. Poor public awareness about potential effective treatments for lung cancer and survival benefits resulting from early detection of lung cancer was acknowledged, as was a culture of stigma and blame associated with smoking. There was a general recognition that smokers aged ≥ 55 years, who would potentially be eligible for a national lung cancer screening programme, belonged to a generation that were previously ill-informed of the risks of smoking.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to identify potentially relevant studies. We performed electronic searches of a range of bibliographic databases as well as screening of clinical trial registers. Conference proceedings were also searched to identify unpublished studies. The review process followed recommended methods to minimise any potential errors and biases. The quality of included studies was assessed in detail at outcome level and accounted for when interpreting the findings. Appropriate synthesis approaches were employed by taking into account the heterogeneity of study characteristics, and the meta-analyses adhered to a pre-defined analytic strategy.
In terms of limitations, only English-language studies were included; therefore, some potentially relevant non-English-language studies may have been missed. There was some evidence of inconsistency in the meta-analysis of mortality outcomes. A range of potential sources of heterogeneity were further explored. The observed heterogeneity may be explained by variations in trial quality, different risk profiles of populations at baseline, and variations in the CT parameters used in included trials. In addition, there were wide variations in definitions of a positive scan on the lung nodule detection across trials.
Cost-effectiveness
This is a model developed independently by an experienced research group, free from potential conflicts of interest. It is also, we believe, the first economic evaluation of lung cancer screening to include a risk prediction component with a variable threshold (but, for example, ten Haaf et al. 115 have used risk proxies in the form of smoking histories).
The independent economic assessment evaluates the cost-effectiveness of a wide range of potential screening programmes, through the use of a natural history model (which allows for evaluation of hypothetical screening programmes that have not been evaluated in clinical trials). This natural history model is based on high-quality evidence from the large NLST RCT71 and UK national sources. 1 The assessment also includes recent estimates of the costs of screening, and somewhat recent estimates of the cost of lung cancer. A clear description of the assumptions underpinning the assessment has been given. The economic evaluation seems to suggest with some robustness that screening is unlikely to be cost-effective at a threshold of £20,000 per QALY.
A number of assumptions were made in the construction of the economic model and some of these were explored in scenario or sensitivity analyses. No modelling of smoking behaviour was included, and incidental findings were not modelled. By using the DES framework, there has been no need to artificially restrict the model states or distributions for event times, or to consider a homogeneous cohort.
The model does not take the impact on mortality as an input, but produces it as an output resulting from the natural history model and the screening programme design. If additional mortality benefit (above what the model currently predicts) needs to be incorporated in an economic evaluation (i.e. if it is demonstrated in future data from trials), new assumptions and parameters will need to be introduced. This could be based on, for example, an acceleration factor applied to lead time. The current model predicts that the cost-effectiveness of screening is closely linked to the RR of lung cancer mortality (Figure 36), which suggests that, with a RR of 0.935, single screening of individuals aged 60–75 years with ≥ 3% risk of lung cancer would become cost-effective at £20,000 per QALY (although this is based on extrapolation and is therefore subject to significant uncertainty).
FIGURE 36.
Impact of RR of lung cancer mortality on cost-effectiveness. INMB at £20,000 per QALY.
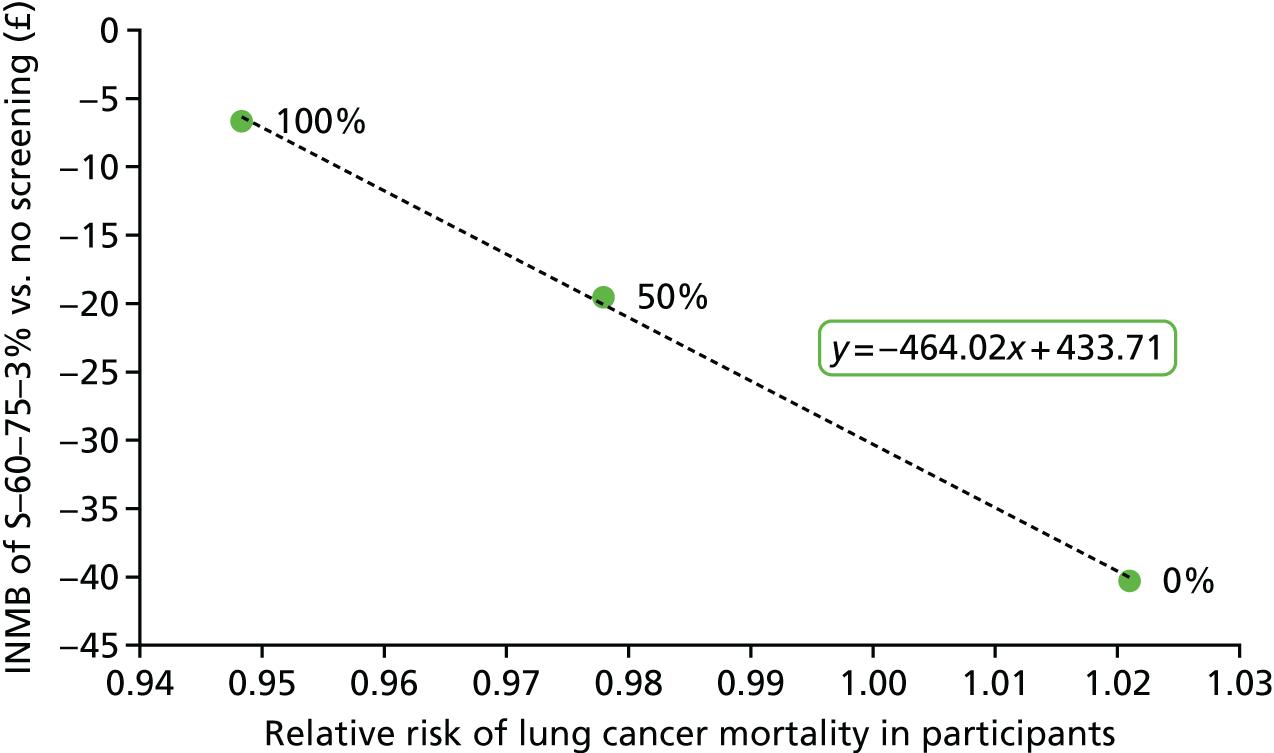
As a DES was used, greatly increasing the computational resource requirements for analyses, there is the risk that results are affected by Monte Carlo error because no inbuilt convergence checks were used, and certain analyses were conducted with a number of simulations known to be short of the apparent number required for stability.
Patient and public involvement
The diversity of perspectives and issues expressed by participants in discussions during our PPI consultations were audio-recorded and all meeting transcripts were analysed thematically to ensure an accurate and comprehensive record of our PPI consultation process for consideration in this and future related HTAs. A key strength of performing a thematic analysis of all workshop transcripts and constructing an explanatory model to reflect participant perspectives was that it ensured that these views were preserved throughout the research process and enabled efficient and accurate communication of PPI perspectives between PenTAG researchers, the majority of whom were not present at any of the PPI meetings. PenTAG researchers were consequently able to consider a variety of patient and public perspectives during the HTA process, particularly issues and concerns relevant to ‘at-risk’ asymptomatic smokers/former smokers recruited locally from deprived areas. Patient and public views relating to the psychological impact of screening and HRQoL were referred to by PenTAG modellers while running scenario analyses and provided further assurance that the economic model analysis had face validity.
Conducting PPI meetings with a tailored workshop format designed specifically for this HTA ensured that views expressed in our PPI meetings were relevant to our HTA and the variety of perspectives and issues discussed reflect the complexity of real-world situations that could potentially affect uptake and implementation of a potential UK lung cancer screening programme.
A key strength of our approach was that we were able to capture the perspectives of a range of patient and public members, with a particular focus on smokers/former smokers currently without symptoms of lung cancer who may be considered ‘high risk’ and, hence, a potential priority target group for a UK national lung cancer screening programme. Views were particularly sought from smokers/former smokers recruited from local deprived areas. Our consultations were extended to include the views of non-smokers during a visit to a community centre/food bank in the most deprived ward in Exeter. At the end of each PPI workshop meeting, all participants were asked to comment on the findings of a recent qualitative study that investigated attitudes towards lung cancer screening in socioeconomically deprived and heavy-smoking communities. 170 However, we acknowledge that many patients and members of the public have an interest in this HTA and we were not able to include everyone. Owing to practical limitations (e.g. a notable lack of local established support groups for lung cancer patients/carers in our local Exeter area), we took the decision not to specifically recruit lung cancer patients/carers. However, people who lost close relatives to cancer (including lung cancer) and other smoking-related diseases were involved in our workshop meetings, as were people who had a family history of lung cancer.
Areas of uncertainty
Clinical effectiveness
The overall effectiveness of LDCT screening depends on a complex interplay between its individual effects on screening programme participants, and it is often unclear how opposing effects interact. Thus, it can be difficult to judge, for example, how better outcomes arising from earlier treatment because a cancer is identified at stage I or II (as opposed to stage III or IV) are offset by the need for many participants to undergo further investigation of suspicious lung nodules that ultimately turn out to be benign. However, these are only two of many different effects that might be operating in LDCT screening for lung cancer. In this situation, when available, outcomes that capture the net effect of the screening programme are particularly helpful in gauging its overall clinical effectiveness. This is why emphasis has been placed on lung cancer mortality and overall mortality in this report.
The report shows that LDCT screening may be clinically effective in reducing lung cancer mortality but that there is considerable uncertainty. This arises from:
-
the imprecision of the pooled estimate with wide 95% CIs compatible with both an improvement and a worsening of lung cancer mortality
-
the heterogeneity between the results of the included studies, with different included studies indicating different effects on lung cancer mortality
-
the fact that the key RCT, NLST, compares LDCT against CXR screening rather than no screening
-
the finding from our network meta-analysis that screening with CXR may be associated with worse outcomes than no screening.
To these could be added concerns about whether or not results in the USA are generalisable to European health-care settings and whether or not newer LDCT techniques will help avoid unnecessary investigation without compromising the ability to identify lung cancers early. The strong possibility of overdiagnosis suggested by an excess of lung cancers identified by LDCT in the included RCTs is another very important uncertainty, and the nature of the additional cancers needs to be characterised.
Cost-effectiveness
A number of factors lead to uncertainty in the cost-effectiveness of lung cancer screening.
This economic evaluation assumes zero cost to identify individuals aged 55–80 years with a history of smoking from GP records.
The cost of lung cancer treatment is a critical factor in cost-effectiveness because of overdiagnosis and stage shift effects of screening, but has been estimated only from a single-centre study conducted in 2008–13 and, therefore, may not be fully generalisable to the whole of the UK at present (because of changes in clinical practice, the introduction of additional technologies and any significant changes in drug acquisition prices, e.g. expiry of market exclusivity). If the costs of lung cancer have increased since they were estimated for early lung cancer stages, then the estimated cost-effectiveness of screening will be biased in favour of screening, because the costs of overdiagnosis will not be fully represented. On the other hand, if costs have increased for later lung cancer stages then the estimates will be biased against screening because stage shift is then even more desirable because of the potential to avoid large costs. The National Lung Cancer Audit found that there was quite limited use of very expensive targeted treatments for patients diagnosed in 2015,18 but uptake may be expected to increase.
The economic evaluation assumes response rates to invitations observed in UKLS,55 but response rates may be lower outside the context of a clinical trial, or higher if the literature is able to claim significant benefits from lung cancer screening.
The HRQoL experienced by people with lung cancer is also critical. Our economic evaluation assumes a much smaller impact on HRQoL than other studies have, and, although we believe we have used the best-quality data available, it is possible that a high-quality mapping study could be conducted to incorporate QoL measurements using a number of different instruments. If HRQoL is significantly worse in reality than assumed in the model, then lung cancer screening will be more cost-effective than predicted.
The economic evaluation currently assumes that only the stage of lung cancer is clinically relevant, in that it affects survival. The model does not currently assume any relationship between the stage of lung cancer and the performance of LDCT (which may be unreasonable as small nodules in early-stage lung cancer are likely to be more challenging to identify). The model also does not consider whether or not lung cancer type (non-small-cell vs. small-cell vs. mixed) affects performance of screening, costs or survival. Likewise, the model does not consider the impact of location of the lung cancer (central vs. peripheral) on these aspects.
Patient and public involvement
The perspectives and views elicited during our PPI discussions are from one PPI exercise consisting of a number of people recruited from our local Exeter area and it is uncertain if different issues and perspectives would be represented if our PPI meetings were repeated with different people in a different geographical location. We specifically targeted certain groups most likely to be prioritised for a national lung cancer screening programme, with a strong focus on smokers/former smokers, especially from deprived areas, and it is uncertain whether or not the issues and perspectives represented in this report are generalisable to other potentially important groups, such as patients who have experienced treatment for lung cancer or people exposed to environmental risks such as asbestos. Given the uncertainties around the evidence for HRQoL experienced by people with lung cancer described in this report, and the implications for the economic evaluation outlined above, patient-based evidence (i.e. qualitative evidence synthesis of individual research studies conducted to understand the experience of lung cancer patients) could contribute scientific context-sensitive evidence to this area of uncertainty in a future report.
Other relevant factors
The presumed method of recruitment for a lung cancer screening programme – through primary care records – may not work as well in practice as assumed in the model. It may be inaccurate and carry significant costs.
Many individuals at high risk of lung cancer are also likely to face barriers to participating in screening (e.g. because of other health issues). It is possible that screening uptake would be disproportionately high in people with higher SES and at lower risk of lung cancer.
Chapter 10 Conclusions
The LDCT screening may be clinically effective in reducing lung cancer mortality but there is considerable uncertainty. This arises from the imprecision of the pooled estimate, the heterogeneity between the results of the included studies, the fact that the key RCT compares LDCT against CXR screening and the finding from our network meta-analysis that screening with CXR may be associated with worse outcomes than no screening.
Beyond mortality, the review confirms the theoretical basis of LDCT by showing that more lung cancers are diagnosed in the earlier stages and fewer in the later stages. However, it also confirms that more lung cancers are detected in the LDCT trial arms many years after completion of the screening programmes, indicating an element of overdiagnosis.
It seems unlikely that LDCT screening leads to major differences in psychological consequences and HRQoL, and the effect on smoking behaviour continues to be uncertain.
Evidence from economic modelling suggests that LDCT screening for lung cancer may not be cost-effective, depending on the cost-effectiveness threshold used. Thresholds of £20,000 to £30,000 per QALY are commonly used in the UK, and screening is estimated in the base-case analysis to be cost-effective with the higher threshold, but not with the lower. However, when the probable range of input values is considered, cost-effectiveness is no longer demonstrated at either threshold.
Economic modelling suggests that screening would result in a reduction in lung cancer mortality, but also an increase in lung cancer diagnoses. Lung cancer screening is predicted to be more effective than no screening on balance, but to result in additional costs. One screening strategy that was investigated provided a ratio of additional costs to benefits that was towards the upper limit of what would conventionally be considered cost-effective, while other screening strategies were outside the normal range of cost-effectiveness. Screening strategies with annual or biennial scans (once every 2 years) are not expected to be cost-effective, regardless of the amount one is willing to pay for benefits.
Implications for service provision
If lung cancer screening using LDCT is implemented as a national screening programme, evidence suggests that it may reduce the number of people dying from lung cancer and the number of people diagnosed in the latest stages of lung cancer. Evidence also suggests that more people overall will be diagnosed with lung cancer, and that NHS spending on lung cancer would increase overall. Lung cancer screening programmes would also result in a significant increase in workload for radiology services.
It is estimated that ≤ 4% of individuals contacted as part of a screening programme could end up participating. As there are an estimated 13 million smokers aged 55–80 years, this could result in an additional half a million CT screens per year compared with an estimated two million CT screens currently conducted each year (in England). It is unlikely that such an increase in the burden on radiography services would be accommodated without significant recruitment and/or service reconfiguration.
Suggested research priorities
Update assessment with anticipated future trial data
Clinical effectiveness and cost-effectiveness estimates should be updated with the anticipated results from several ongoing RCTs (particularly NELSON). This is likely to resolve many current uncertainties within a reasonable time.
In the longer term, another large trial of lung cancer screening is currently being conducted in Asia (based in Shanghai) that will further explore the generalisability of the initial trial results to populations with different ethnicities.
Overdiagnosis
Further investigation on the extent and nature of possible overdiagnosis in LDCT screening would be extremely helpful in elucidating the degree to which this may or may not influence the clinical effectiveness and cost-effectiveness of LDCT screening overall.
Quality of included randomised controlled trials
One included RCT, MILD,69 was revealed to have major problems with its randomisation on quality assessment (see Chapter 3, Risk of bias of included studies, and Table 8). These problems could be severe enough to challenge whether or not it was a true RCT. If it was not a true RCT it should not be included in the meta-analysis, with the effect of reducing the heterogeneity in the meta-analysis results. Further investigation of the quality of currently included trials would thus be useful. This could be achieved by further enquiry of the original investigators and should be done systematically and symmetrically across all included studies (to avoid introducing bias into a review). It should also include ongoing RCTs that may be included in systematic reviews of LDCT in future. Only if studies currently thought to be RCTs are not so on further investigation would it be appropriate to exclude them from future systematic reviews on the effectiveness of LDCT.
Detailed costing of lung cancer
The (lifetime) cost of lung cancer (diagnosis, staging, treatment, follow-up and palliative care) should be estimated according to key characteristics of the patient and tumour (e.g. age, sex, lung cancer stage and type), based on nationally representative data. This is because the estimates used in the economic modelling may be outdated and are from a single centre.
Health-related quality of life in lung cancer
Further consideration should be given to the HRQoL of patients with lung cancer. The economic modelling currently uses fixed disutilities according to the stage at diagnosis, taken from a single study. 159 Evidence from other studies could be considered to arrive at alternative parameter values, and structural changes could also be considered, such as estimating the HRQoL according to time since diagnosis, time before death, current treatment and current disease stage.
Further developments of economic model
We have acknowledged a number of assumptions in the economic model for this interim report that have not been explored through scenario or sensitivity analyses. In addition to collecting and incorporating better-quality parameters (the research recommendations above), some of these assumptions should be explored or relaxed through further developments of the economic model. A comparison of the methods and results of the economic model to other economic evaluations could also be made to identify common findings as well as discrepancies.
Acknowledgements
We are grateful to Sue Whiffin and Jenny Lowe (both of University of Exeter Medical School) for their administrative support during the project, and to Tracey Jones-Hughes and Irina Tikhonova (both of University of Exeter Medical School) for their assistance in preparing the report and quality assuring the economic model.
We are grateful to clinical radiologists Carl Roobottom and Chun Pang (Plymouth Hospitals NHS Trust) for their advice and contribution to the CT parameter and radiation dose sections of this report.
We are grateful to our External Advisory Group (membership below) for their comments on the protocol and the overall project design, their responses to questions and for reviewing a draft of this report.
We are grateful to NHS Digital and the UK Data Service for access to Health Survey for England data, which were used to derive health state utility values for smokers without lung cancer.
The authors thank the UKLS investigators, and in particular Dr Michael Marcus (Research Associate, University of Liverpool) for providing data from UKLS relating to the predicted risk scores.
The authors thank the National Cancer Institute (NCI) for access to NCI’s data collected by NLST. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
We thank Rosie Rowlands (Advanced Research Computing Support Officer, University of Exeter) and other staff maintaining the University of Exeter Isca High Performance Cluster, on which a number of calibration models were run.
Membership of External Advisory Group
-
David Baldwin (Consultant Respiratory Physician), Nottingham University Hospitals NHS Trust.
-
John Field (Clinical Professor of Medical Oncology), University of Liverpool.
-
Willie Hamilton (Professor of Primary Care Diagnostics), University of Exeter.
-
Julie Harvey (PPI representative).
-
Renee Manser (Respiratory Physician), Royal Melbourne Hospital and Peter MacCallum Cancer Centre.
-
Amelia Randle (Macmillan General Practitioner, Clinical Lead for Cancer), Somerset Clinical Commissioning Group.
-
Carl Roobottom (Professor of Radiology), Plymouth Hospitals NHS Trust.
-
Peter Sasieni (Professor of Biostatistics and Cancer Epidemiology), Queen Mary, University of London.
-
Kevin Smith (Deputy Director Healthcare), Public Health England, Yorkshire and the Humber.
-
Obi Ukoumunne (Associate Professor in Medical Statistics), University of Exeter.
Contributions of authors
Tristan Snowsill (Research Fellow, Health Economic Modelling) led the systematic review of existing cost-effectiveness studies and the independent economic evaluation and contributed to the writing of the relevant sections, as well as to the background, discussion, conclusions and various summaries. He contributed to the editing of the report and provided overall project management.
Huiqin Yang (Senior Research Fellow, Systematic Review) led the systematic review of clinical effectiveness and the writing of the clinical sections of the report, and also contributed to the writing and editing of the report.
Ed Griffin (Research Fellow, Health Economic Modelling) led the implementation of the economic model and the sourcing of costs, and contributed to the writing of the independent economic assessment section. He contributed to the editing of the report.
Linda Long (Research Fellow, Systematic Review) led the PPI, wrote the relevant section of the report and contributed to the writing of the discussion and various summaries. She compiled the summary table of overview of systematic reviews, wrote the accompanying narrative, contributed to the systematic review of screening effectiveness and contributed to the editing of the report.
Jo Varley-Campbell (Research Fellow, Systematic Review) contributed to the systematic review of screening effectiveness, writing of the background and editing of the report.
Helen Coelho (Research Fellow, Systematic Review) contributed to the systematic review of screening effectiveness and editing of the report.
Sophie Robinson (Information Specialist, Information Science) developed the literature search strategies and carried out the literature searches and citation-chasing searches. She also carried out the searches for ongoing clinical trials and contributed to the background section.
Chris Hyde (Professor of Public Health and Clinical Epidemiology) was the guarantor for the project and developed the protocol. He contributed to the design of the systematic reviews, the economic evaluation and the PPI, and contributed to the systematic review of clinical effectiveness and to the writing and editing of the report.
Data-sharing statement
There are no data available for further access or sharing owing to the nature of the study. All data presented and analysed in the systematic review are based on published accounts of studies and not owned by the authors. The independent economic assessment uses data from the UKLS, the Health Survey for England 2014: Health, Social Care and Lifestyles. Summary of Key Findings155 and the NLST, which are not owned by the authors and cannot be shared further. Data providers should be contacted directly for release of data sets. The economic model developed for this assessment has been deposited in the Open Research Exeter repository (https://ore.exeter.ac.uk/). It is available at https://doi.org/10.24378/exe.564 (under embargo until the date of monograph publication). All queries should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Registration Statistics, England: 2014. Newport: Office for National Statistics; 2016.
- Chyou PH, Nomura AM, Stemmermann GN. A prospective study of the attributable risk of cancer due to cigarette smoking. Am J Public Health 1992;82:37-40. https://doi.org/10.2105/AJPH.82.1.37.
- Chang CM, Corey CG, Rostron BL, Apelberg BJ. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-1617-5.
- Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol 2010;39:834-57. https://doi.org/10.1093/ije/dyq002.
- Pinsky PF, Zhu CS, Kramer BS. Lung cancer risk by years since quitting in 30+ pack year smokers. J Med Screen 2015;22:151-7. https://doi.org/10.1177/0969141315579119.
- Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev 2006;15:517-23. https://doi.org/10.1158/1055-9965.EPI-05-0863.
- Office for National Statistics . Adult Smoking Habits in Great Britain: 1974–2014 2016. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultsmokinghabitsingreatbritain (accessed 7 December 2016).
- Office for National Statistics . Adult Smoking Habits in the UK: 2015 2017. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2015 (accessed May 2017).
- Eurostat . European Health Interview Survey. Smoking of Tobacco Products by Sex, Age and Educational Attainment Level n.d. http://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_ehis_sk1e&lang=en (accessed 29 June 2018).
- Department of Health and Social Care . 2010 to 2015 Government Policy: Smoking n.d. www.gov.uk/government/publications/2010-to-2015-government-policy-smoking/2010-to-2015-government-policy-smoking (accessed 23 December 2016).
- NHS . Smokefree n.d. www.nhs.uk/smokefree (accessed 23 December 2016).
- Public Health England . Stoptober 2016. https://campaignresources.phe.gov.uk/resources/campaigns/6-stoptober/overview (accessed 23 December 2016).
- Perraudin F. MPs pass legislation to introduce standardised cigarette packaging. The Guardian 2015. www.theguardian.com/politics/2015/mar/11/mps-pass-legislation-introduce-standardised-cigarette-packaging (accessed 16 December 2016).
- Tobacco Advertising and Promotion Act 2002. London: The Stationery Office; 2002.
- Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol 2007;36:1048-59. https://doi.org/10.1093/ije/dym158.
- Cancer Research UK . Risk and Causes 2017. www.cancerresearchuk.org/about-cancer/lung-cancer/risks-causes (accessed May 2017).
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. https://doi.org/10.1016/j.ccm.2011.09.001.
- National Lung Cancer Audit Annual Report 2016 (for the Audit Period 2015). London: Royal College of Physicians; 2017.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. https://doi.org/10.1043/2009-0583-RAR.1.
- Bertino EM, Confer PD, Colonna JE, Ross P, Otterson GA. Pulmonary neuroendocrine/carcinoid tumors: a review article. Cancer 2009;115:4434-41. https://doi.org/10.1002/cncr.24498.
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. https://doi.org/10.1097/JTO.0000000000000630.
- Cancer Research UK . Stage and Grade of Lung Cancer 2017. www.cancerresearchuk.org/about-cancer/lung-cancer/stages-types-grades/stages-grades (accessed May 2017).
- TNM Classification of Malignant Tumours. New York, NY: Wiley-Liss; 2009.
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. https://doi.org/10.1097/JTO.0b013e31812f3c1a.
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, NJ: Wiley-Blackwell; 2016.
- Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004.
- National Cancer Intelligence Network . Routes to Diagnosis by Stage 2012–2013 Workbook 2015. www.ncin.org.uk/view?rid=3071 (accessed 16 January 2017).
- Suspected Cancer: Recognition and Referral. NICE Guideline NG12. London: NICE; 2015.
- Hamilton W, Peters TJ, Round A, Sharp D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 2005;60:1059-65. https://doi.org/10.1136/thx.2005.045880.
- Lung Cancer: Diagnosis and Management. Clinical Guideline CG121. London: NICE; 2011.
- Cancer Research UK . Lung Cancer Survival Statistics 2017. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/survival (accessed May 2017).
- Cancer Research UK . Survival 2017. http://about-cancer.cancerresearchuk.org/about-cancer/lung-cancer/survival (accessed May 2017).
- National Cancer Intelligence Network . Routes to Diagnosis 2006–2013 Workbook 2015. www.ncin.org.uk/view?rid=3053 (accessed 16 January 2017).
- Office for National Statistics . Geographic Patterns of Cancer Survival in England: Adults Diagnosed 2003 to 2010 and Followed Up to 2015 2017. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/geographicpatternsofcancersurvivalinengland/adultsdiagnosed2003to2010andfollowedupto2015 (accessed May 2017).
- World Cancer Research Fund International . Lung Cancer Statistics 2017. www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/lung-cancer-statistics (accessed May 2017).
- Eurostat . Cancer Statistics – Specific Cancers 2017. http://ec.europa.eu/eurostat/statistics-explained/index.php/Cancer_statistics_-_specific_cancers#Lung_cancer (accessed May 2017).
- Office for National Statistics . Deaths Registered in England and Wales (Series DR): 2014 2015. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregisteredinenglandandwalesseriesdr/2015-11-09 (accessed 18 May 2018).
- Information Services Division (ISD) Scotland . Cancer Mortality in Scotland (2014): Cancer Mortality by Year – Lung and Mesothelioma 2015. www.isdscotland.org/Health-Topics/Cancer/Publications/data-tables.asp (accessed 18 May 2018).
- Cancer Mortality Statistics for Northern Ireland: 1993–2016 – Lung Cancer (C33-C34). Newport: Office for National Statistics; 2018.
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland: Mid-2014 2016. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 18 May 2018).
- National Cancer Intelligence Network . One, Five and Ten Year Cancer Prevalence by Cancer Network, UK, 2006 2010. www.ncin.org.uk/view?rid=76 (accessed 12 December 2016).
- Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Oncotargets and Therapy 2016;9:1023-8. https://doi.org/10.2147/OTT.S100685.
- Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013;14:1165-74. https://doi.org/10.1016/S1470-2045(13)70442-X.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635-42. https://doi.org/10.1016/0959-8049(94)90535-5.
- Hollen PJ, Gralla RJ, Kris MG, Potanovich LM. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer 1993;29A:51-8. https://doi.org/10.1016/S0959-8049(05)80262-X.
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. https://doi.org/10.1200/JCO.1993.11.3.570.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. https://doi.org/10.1016/0169-5002(95)00450-F.
- Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-54. https://doi.org/10.1136/thoraxjnl-2015-207168.
- Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. https://doi.org/10.1136/thx.2010.145938.
- Manser R, Lethaby A, Irving LB, Stone C, Byrnes G, Abramson MJ, et al. Screening for lung cancer. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD001991.pub3.
- Public Health England . Guidance, Ionising Radiation: Dose Comparisons 2011. www.gov.uk/government/publications/ionising-radiation-dose-comparisons/ionising-radiation-dose-comparisons (accessed 24 May 2017).
- Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al. The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews. Health Technol Assess 2006;10. https://doi.org/10.3310/hta10030.
- Field JK, Duffy SW, Baldwin DR, Brain KE, Devaraj A, Eisen T, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20400.
- Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. https://doi.org/10.1016/S1470-2045(14)70387-0.
- Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659-67. https://doi.org/10.1183/09031936.00197712.
- McCartney M. Margaret McCartney: Why ask, if you ignore the answer?. BMJ 2017;357. https://doi.org/10.1136/bmj.j1824.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
- Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445-53. https://doi.org/10.1164/rccm.200901-0076OC.
- Blanchon T, Bréchot JM, Grenier PA, Ferretti GR, Lemarié E, Milleron B, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007;58:50-8. https://doi.org/10.1016/j.lungcan.2007.05.009.
- Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, et al. The Danish randomized lung cancer CT screening trial – overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. https://doi.org/10.1097/JTO.0b013e3181a0d98f.
- Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, et al. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225:506-10. https://doi.org/10.1148/radiol.2252011851.
- Lopes Pegna A, Picozzi G, Falaschi F, Carrozzi L, Falchini M, Carozzi FM, et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866-75. https://doi.org/10.1097/JTO.0b013e31828f68d6.
- Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9-15. https://doi.org/10.1016/j.lungcan.2004.06.007.
- Spiro SG, Hackshaw A. LungSEARCH Collaborative Group . Research in progress – LungSEARCH: a randomised controlled trial of surveillance for the early detection of lung cancer in a high-risk group. Thorax 2016;71:91-3. https://doi.org/10.1136/thoraxjnl-2015-207433.
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. https://doi.org/10.1007/s00432-012-1228-9.
- Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. https://doi.org/10.1097/CEJ.0b013e328351e1b6.
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. https://doi.org/10.1056/NEJMoa1102873.
- Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, . National Lung Screening Trial Research Team . Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. https://doi.org/10.1056/NEJMoa1209120.
- Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. https://doi.org/10.1016/j.lungcan.2008.07.003.
- Ashraf H, Saghir Z, Dirksen A, Pedersen JH, Thomsen LH, Døssing M, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax 2014;69:574-9. https://doi.org/10.1136/thoraxjnl-2013-203849.
- Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120:3401-9. https://doi.org/10.1002/cncr.28833.
- Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771-9. https://doi.org/10.1093/jnci/djq434.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- National Center for Health Statistics . National Death Index n.d. www.cdc.gov/nchs/ndi/index.htm (accessed 14 June 2018).
- Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015;191:1166-75. https://doi.org/10.1164/rccm.201408-1475OC.
- Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, et al. Results of the randomized Danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016;193:542-51. https://doi.org/10.1164/rccm.201505-1040OC.
- Brain K, Carter B, Lifford KJ, Burke O, Devaraj A, Baldwin DR, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK Lung Cancer Screening Trial. Thorax 2017;72:912-18. https://doi.org/10.1136/thoraxjnl-2016-209690.
- van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600-5. https://doi.org/10.1136/thx.2009.133751.
- Rasmussen JF, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer 2015;87:65-72. https://doi.org/10.1016/j.lungcan.2014.11.003.
- Aggestrup LM, Hestbech MS, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000663.
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, Th Scholten E, Prokop M, de Koning HJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27-34. https://doi.org/10.1038/sj.bjc.6605459.
- van den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, van Klaveren RJ, de Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J 2011;38:154-61. https://doi.org/10.1183/09031936.00123410.
- Brain K, Lifford KJ, Carter B, Burke O, McRonald F, Devaraj A, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax 2016;71:996-1005. https://doi.org/10.1136/thoraxjnl-2016-208283.
- Ashraf H, Tønnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Døssing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388-92. https://doi.org/10.1136/thx.2008.102475.
- van der Aalst CM, van Klaveren RJ, van den Bergh KA, Willemsen MC, de Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. Eur Respir J 2011;37:1466-73. https://doi.org/10.1183/09031936.00035410.
- Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106. https://doi.org/10.1093/jnci/dju084.
- Clark MA, Gorelick JJ, Sicks JD, Park ER, Graham AL, Abrams DB, et al. The relations between false positive and negative screens and smoking cessation and relapse in the National Lung Screening Trial: implications for public health. Nicotine Tob Res 2016;18:17-24. https://doi.org/10.1093/ntr/ntv037.
- van’t Westeinde S, Horeweg N, Leyn P, Groen H, Lammers J, Weenink C, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg 2012;42:420-9. https://doi.org/10.1093/ejcts/ezs081.
- Sverzellati N, Silva M, Calareso G, Galeone C, Marchianò A, Sestini S, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821-9. https://doi.org/10.1007/s00330-016-4228-3.
- Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920-31. https://doi.org/10.1056/NEJMoa1208962.
- van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. https://doi.org/10.1056/NEJMoa0906085.
- Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. https://doi.org/10.1001/jamainternmed.2013.12738.
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol 2015;10:890-6. https://doi.org/10.1097/JTO.0000000000000530.
- Larke FJ, Kruger RL, Cagnon CH, Flynn MJ, McNitt-Gray MM, Wu X, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 2011;197:1165-9. https://doi.org/10.2214/AJR.11.6533.
- Kruger R, Flynn MJ, Judy PF, Cagnon CH, Seibert JA. Effective dose assessment for participants in the National Lung Screening Trial undergoing posteroanterior chest radiographic examinations. AJR Am J Roentgenol 2013;201:142-6. https://doi.org/10.2214/AJR.12.9181.
- Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 2015;350. https://doi.org/10.1136/bmj.g7773.
- Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356. https://doi.org/10.1136/bmj.j347.
- Vardhanabhuti V, Pang CL, Tenant S, Taylor J, Hyde C, Roobottom C. Prospective intra-individual comparison of standard dose versus reduced-dose thoracic CT using hybrid and pure iterative reconstruction in a follow-up cohort of pulmonary nodules – Effect of detectability of pulmonary nodules with lowering dose based on nodule size, type and body mass index. Eur J Radiol 2017;91:130-41. https://doi.org/10.1016/j.ejrad.2017.04.006.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. https://doi.org/10.1016/j.jclinepi.2010.03.016.
- White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J 2011;11.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. https://doi.org/10.1002/jrsm.1044.
- Kubík A, Haerting J. Survival and mortality in a randomized study of lung cancer detection. Neoplasma 1990;37:467-75.
- Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-16. https://doi.org/10.1093/jnci/92.16.1308.
- Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. https://doi.org/10.1001/jama.2011.1591.
- Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis 1984;130:561-5.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Black WC. Computed tomography screening for lung cancer in the National Lung Screening Trial: a cost-effectiveness analysis. J Thorac Imaging 2015;30:79-87. https://doi.org/10.1097/RTI.0000000000000136.
- Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 2014;371:1793-802. https://doi.org/10.1056/NEJMoa1312547.
- Goffin JR, Flanagan WM, Miller AB, Fitzgerald NR, Memon S, Wolfson MC, et al. Cost-effectiveness of Lung Cancer Screening in Canada. JAMA Oncol 2015;1:807-13. https://doi.org/10.1001/jamaoncol.2015.2472.
- Goffin JR, Flanagan WM, Miller AB, Fitzgerald NR, Memon S, Wolfson MC, et al. Biennial lung cancer screening in Canada with smoking cessation-outcomes and cost-effectiveness. Lung Cancer 2016;101:98-103. https://doi.org/10.1016/j.lungcan.2016.09.013.
- McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6:1841-8. https://doi.org/10.1097/JTO.0b013e31822e59b3.
- ten Haaf K, Tammemägi MC, Bondy SJ, van der Aalst CM, Gu S, McGregor SE, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002225.
- Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. https://doi.org/10.1136/thoraxjnl-2015-207140.
- Whynes DK. Could CT screening for lung cancer ever be cost effective in the United Kingdom?. Cost Eff Resour Alloc 2008;6. https://doi.org/10.1186/1478-7547-6-5.
- Marshall D, Simpson KN, Earle CC, Chu CW. Economic decision analysis model of screening for lung cancer. Eur J Cancer 2001;37:1759-67. https://doi.org/10.1016/S0959-8049(01)00205-2.
- Marshall D, Simpson KN, Earle CC, Chu C. Potential cost-effectiveness of one-time screening for lung cancer (LC) in a high risk cohort. Lung Cancer 2001;32:227-36. https://doi.org/10.1016/S0169-5002(00)00239-7.
- Chirikos TN, Hazelton T, Tockman M, Clark R. Screening for lung cancer with CT: a preliminary cost-effectiveness analysis. Chest 2002;121:1507-14. https://doi.org/10.1378/chest.121.5.1507.
- Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 2003;289:313-22. https://doi.org/10.1001/jama.289.3.313.
- Wisnivesky JP, Mushlin AI, Sicherman N, Henschke C. The cost-effectiveness of low-dose CT screening for lung cancer: preliminary results of baseline screening. Chest 2003;124:614-21. https://doi.org/10.1378/chest.124.2.614.
- Manser R, Dalton A, Carter R, Byrnes G, Elwood M, Campbell DA. Cost-effectiveness analysis of screening for lung cancer with low dose spiral CT (computed tomography) in the Australian setting. Lung Cancer 2005;48:171-85. https://doi.org/10.1016/j.lungcan.2004.11.001.
- Goulart BH, Bensink ME, Mummy DG, Ramsey SD. Lung cancer screening with low-dose computed tomography: costs, national expenditures, and cost-effectiveness. J Natl Compr Canc Netw 2012;10:267-75. https://doi.org/10.6004/jnccn.2012.0023.
- Pyenson BS, Sander MS, Jiang Y, Kahn H, Mulshine JL. An actuarial analysis shows that offering lung cancer screening as an insurance benefit would save lives at relatively low cost. Health Aff 2012;31:770-9. https://doi.org/10.1377/hlthaff.2011.0814.
- Shmueli A, Fraifeld S, Peretz T, Gutfeld O, Gips M, Sosna J, et al. Cost-effectiveness of baseline low-dose computed tomography screening for lung cancer: the Israeli experience. Value Health 2013;16:922-31. https://doi.org/10.1016/j.jval.2013.05.007.
- Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0071379.
- Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits 2014;7:272-82.
- Tabata H, Akita T, Matsuura A, Kaishima T, Matsuoka T, Ohisa M, et al. Cost-effectiveness of the introduction of low-dose CT screening in Japanese smokers aged 55 to 74 years old. Hiroshima J Med Sci 2014;63:13-22.
- Klittich WS, Caro JJ. Lung cancer screening: will the controversy extend to its cost-effectiveness?. Am J Respir Med 2002;1:393-401. https://doi.org/10.1007/BF03257166.
- Murtagh J, Foerster V, Warburton RN, Lentle BC, Wood RJ, Mensinkai S, et al. Clinical and Cost Effectiveness of CT and MRI for Selected Clinical Disorders: Results of Two Systematic Reviews. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2006.
- Murtagh J, Warburton RN, Foerster V, Lentle BC, Wood RJ, Mensinkai S, et al. CT and MRI for Selected Clinical Disorders: A Systematic Review of Economic Evaluations. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2006.
- Puggina A, Broumas A, Ricciardi W, Boccia S. Cost-effectiveness of screening for lung cancer with low-dose computed tomography: a systematic literature review. Eur J Public Health 2016;26:168-75. https://doi.org/10.1093/eurpub/ckv158.
- Raymakers AJN, Mayo J, Lam S, FitzGerald JM, Whitehurst DGT, Lynd LD. Cost-effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy 2016;14:409-18. https://doi.org/10.1007/s40258-016-0226-5.
- Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. https://doi.org/10.1016/S0140-6736(99)06093-6.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9 (accessed 14 February 2017).
- Scottish Medicines Consortium . Guidance to Manufacturers for Completion of New Product Assessment Form (NPAF) 2016. www.webcitation.org/6oGzBw320 (accessed 14 February 2017).
- All Wales Medicines Strategy Group . Form B Guidance Notes 2016. www.webcitation.org/6oGzgKGKI (accessed 14 February 2017).
- The Green Book: Appraisal and Evaluation in Central Government: Treasury Guidance. London: HM Treasury; 2003.
- Plummer M. JAGS: Just Another Gibbs Sampler 2004. http://mcmc-jags.sourceforge.net/ (accessed 14 February 2017).
- Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. https://doi.org/10.7326/M13-2771.
- NHS Digital . Statistics on Smoking: England, 2016 2016. http://content.digital.nhs.uk/catalogue/PUB20781/stat-smok-eng-2016-tab.xlsx (accessed 5 May 2017).
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland: Mid-2015 2016. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2015 (accessed 15 June 2017).
- The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
- Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R News 2006;6:7-11.
- Office for National Statistics . Interim Life Tables: England and Wales, 2010–2012 2013. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/interimlifetables/englandandwales20102012 (accessed 21 June 2017).
- Working paper 21 – The Graduation of the CMI 1999–2002 Mortality Experience: Final ‘00’ Series Mortality Tables – Assured Lives. London: Institute and Faculty of Actuaries; 2006.
- Office for National Statistics . Deaths Registered in England and Wales, 2014: Table 5.2 Neoplasms 2015. www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables/2014table5/table5causeofdeath_tcm77-422538.xls (accessed 5 May 2017).
- Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270-6. https://doi.org/10.1038/sj.bjc.6604158.
- Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012;157:242-50. https://doi.org/10.7326/0003-4819-157-4-201208210-00004.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Mazzone PJ, Obuchowski N, Fu AZ, Phillips M, Meziane M. Quality of life and healthcare use in a randomized controlled lung cancer screening study. Ann Am Thorac Soc 2013;10:324-9. https://doi.org/10.1513/AnnalsATS.201301-007OC.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203-20. https://doi.org/10.1097/00005650-200503000-00003.
- Craig R, Mindell J. Health Survey for England 2014: Health, Social Care and Lifestyles. Summary of Key Findings. Leeds: NHS Digital; 2014.
- Chouaid C, Agulnik J, Goker E, Herder GJ, Lester JF, Vansteenkiste J, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol 2013;8:997-1003. https://doi.org/10.1097/JTO.0b013e318299243b.
- Grutters JP, Joore MA, Wiegman EM, Langendijk JA, de Ruysscher D, Hochstenbag M, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Thorax 2010;65:903-7. https://doi.org/10.1136/thx.2010.136390.
- Jang RW, Isogai PK, Mittmann N, Bradbury PA, Shepherd FA, Feld R, et al. Derivation of utility values from European Organization for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol 2010;5:1953-7. https://doi.org/10.1097/JTO.0b013e3181f77a6a.
- Tramontano AC, Schrag DL, Malin JK, Miller MC, Weeks JC, Swan JS, et al. Catalog and comparison of societal preferences (utilities) for lung cancer health states: results from the Cancer Care Outcomes Research and Surveillance (CanCORS) study. Med Decis Making 2015;35:371-87. https://doi.org/10.1177/0272989X15570364.
- Yang SC, Lai WW, Chang HY, Su WC, Chen HH, Wang JD. Estimation of loss of quality-adjusted life expectancy (QALE) for patients with operable versus inoperable lung cancer: adjusting quality-of-life and lead-time bias for utility of surgery. Lung Cancer 2014;86:96-101. https://doi.org/10.1016/j.lungcan.2014.08.006.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Sturza J. A review and meta-analysis of utility values for lung cancer. Med Decis Making 2010;30:685-93. https://doi.org/10.1177/0272989X10369004.
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 15 December 2016).
- Kennedy MP, Hall PS, Callister ME. Factors affecting hospital costs in lung cancer patients in the United Kingdom. Lung Cancer 2016;97:8-14. https://doi.org/10.1016/j.lungcan.2016.04.009.
- McGuire A, Martin M, Lenz C, Sollano JA. Treatment cost of non-small cell lung cancer in three European countries: comparisons across France, Germany, and England using administrative databases. J Med Econ 2015;18:525-32. https://doi.org/10.3111/13696998.2015.1032974.
- Round J, Jones L, Morris S. Estimating the cost of caring for people with cancer at the end of life: a modelling study. Palliat Med 2015;29:899-907. https://doi.org/10.1177/0269216315595203.
- Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012;13:353-65. https://doi.org/10.1016/S1470-2045(12)70041-4.
- Vinod SK, Sidhom MA, Gabriel GS, Lee MT, Delaney GP. Why do some lung cancer patients receive no anticancer treatment?. J Thorac Oncol 2010;5:1025-32. https://doi.org/10.1097/JTO.0b013e3181da85e4.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect 2017;20:563-73. https://doi.org/10.1111/hex.12481.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Dawe F. General Lifestyle Survey Overview – A Report on the 2011 General Lifestyle Survey. Newport: Office for National Statistics; n.d.
- Siahpush M, McNeill A, Borland R, Fong GT. Socioeconomic variations in nicotine dependence, self-efficacy, and intention to quit across four countries: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control 2006;15:iii71-5. https://doi.org/10.1136/tc.2004.008763.
- Baldwin DR, O’Dowd EL. Next steps and barriers to implementing lung cancer screening with low-dose CT. Br J Radiol 2014;87. https://doi.org/10.1259/bjr.20140416.
- Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011;73:325-31. https://doi.org/10.1016/j.lungcan.2010.12.018.
- Bryan L, Westmaas L, Alcaraz K, Jemal A. Cigarette smoking and cancer screening underutilization by state: BRFSS 2010. Nicotine Tob Res 2014;16:1183-9. https://doi.org/10.1093/ntr/ntu047.
- Byrne MM, Davila EP, Zhao W, Parker D, Hooper MW, Caban-Martinez A, et al. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol 2010;34:611-17. https://doi.org/10.1016/j.canep.2010.06.017.
- Vander Weg MW, Howren MB, Cai X. Use of routine clinical preventive services among daily smokers, non-daily smokers, former smokers, and never-smokers. Nicotine Tob Res 2012;14:123-30. https://doi.org/10.1093/ntr/ntr141.
- Hayton C, Clark A, Olive S, Browne P, Galey P, Knights E, et al. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med 2013;107:401-7. https://doi.org/10.1016/j.rmed.2012.11.016.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. https://doi.org/10.1093/pubmed/fdr034.
- Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer 2016;16. https://doi.org/10.1186/s12885-016-2316-z.
- Quaife SL, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening within socioeconomically deprived and heavy smoking communities: a qualitative study. Lancet 2014;384. https://doi.org/10.1016/S0140-6736(14)62142-5.
- Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax 2007;62:126-30. https://doi.org/10.1136/thx.2005.056036.
- Patel D, Akporobaro A, Chinyanganya N, Hackshaw A, Seale C, Spiro SG, et al. Lung-SEARCH Investigators . Attitudes to participation in a lung cancer screening trial: a qualitative study. Thorax 2012;67:418-25. https://doi.org/10.1136/thoraxjnl-2011-200055.
- Delmerico J, Hyland A, Celestino P, Reid M, Cummings KM. Patient willingness and barriers to receiving a CT scan for lung cancer screening. Lung Cancer 2014;84:307-9. https://doi.org/10.1016/j.lungcan.2014.03.003.
- Jonnalagadda S, Bergamo C, Lin JJ, Lurslurchachai L, Diefenbach M, Smith C, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer 2012;77:526-31. https://doi.org/10.1016/j.lungcan.2012.05.095.
- Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 2008;28:917-25. https://doi.org/10.1177/0272989X08322013.
- Wardle J, Pope R. The psychological costs of screening for cancer. J Psychosom Res 1992;36:609-24. https://doi.org/10.1016/0022-3999(92)90051-3.
- Bunge EM, van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. High affective risk perception is associated with more lung cancer-specific distress in CT screening for lung cancer. Lung Cancer 2008;62:385-90. https://doi.org/10.1016/j.lungcan.2008.03.029.
- Plank A, Nemesure B, Bilfinger T, Campolo-Athans S, Aleyas S. Lung cancer screening and self-reported distress. Chest 2014;146. https://doi.org/10.1378/chest.1991267.
- Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol 2014;9:927-34. https://doi.org/10.1097/JTO.0000000000000210.
- Wu GX, Raz DJ, Brown L, Sun V. Psychological burden associated with lung cancer screening: a systematic review. Clin Lung Cancer 2016;17:315-24. https://doi.org/10.1016/j.cllc.2016.03.007.
- Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, et al. Systematic review of the psychological consequences of false-positive screening mammograms. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17130.
- Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest 2013;143:672-7. https://doi.org/10.1378/chest.12-1095.
- Quaife SL, Janes SM. Lung cancer screening: improving understanding of the psychological impact. Thorax 2016;71:971-2. https://doi.org/10.1136/thoraxjnl-2016-208966.
- Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, et al. Patients’ knowledge, beliefs, and distress associated with detection and evaluation of incidental pulmonary nodules for cancer: results from a multicenter survey. J Thorac Oncol 2016;11:700-8. https://doi.org/10.1016/j.jtho.2016.01.018.
- Ruparel M, Navani N. Fulfilling the dream. Toward reducing inequalities in lung cancer screening. Am J Respir Crit Care Med 2015;192:125-7. https://doi.org/10.1164/rccm.201505-0897ED.
- MacRedmond R, McVey G, Lee M, Costello RW, Kenny D, Foley C, et al. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax 2006;61:54-6. https://doi.org/10.1136/thx.2004.037580.
- Schnoll RA, Miller SM, Unger M, McAleer C, Halbherr T, Bradley P. Characteristics of female smokers attending a lung cancer screening program: a pilot study with implications for program development. Lung Cancer 2002;37:257-65. https://doi.org/10.1016/S0169-5002(02)00106-X.
- Cox LS, Clark MM, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer 2003;98:2495-501. https://doi.org/10.1002/cncr.11813.
- Anderson CM, Yip R, Henschke CI, Yankelevitz DF, Ostroff JS, Burns DM. Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev 2009;18:3476-83. https://doi.org/10.1158/1055-9965.EPI-09-0176.
- Riaz SP, Horton M, Kang J, Mak V, Lüchtenborg M, Møller H. Lung cancer incidence and survival in England: an analysis by socioeconomic deprivation and urbanization. J Thorac Oncol 2011;6:2005-10. https://doi.org/10.1097/JTO.0b013e31822b02db.
- Are There Circumstances In Which the Age of a Person Should be Taken into Account when NICE is Making a Decision about How Treatments Should be Used in the NHS?. London: National Institute for Health and Clinical Excellence; 2003.
- What Are the Societal Values That Need to be Considered When Making Decisions About Trade-Offs Between Equity and Efficiency?. London: National Institute for Health and Care Excellence; 2014.
- Determining ‘Clinical Need’. London: National Institute for Health and Clinical Excellence; 2002.
- Usman Ali M, Miller J, Peirson L, Fitzpatrick-Lewis D, Kenny M, Sherifali D, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med 2016;89:301-14. https://doi.org/10.1016/j.ypmed.2016.04.015.
- Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20. https://doi.org/10.7326/0003-4819-159-6-201309170-00690.
- Bach PB. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;308. https://doi.org/10.1001/jama.2012.9005.
- Black C, de Verteuil R, Walker S, Ayres J, Boland A, Bagust A, et al. Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature. Thorax 2007;62:131-8. https://doi.org/10.1136/thx.2006.064659.
- Chang CY, Chang SJ, Chang SC, Yuan MK. The value of positron emission tomography in early detection of lung cancer in high-risk population: a systematic review. Clin Respir J 2013;7:1-6. https://doi.org/10.1111/j.1752-699X.2012.00290.x.
- Chien CR, Liang JA, Chen JH, Wang HN, Lin CC, Chen CY, et al. [(18)F]fluorodeoxyglucose-positron emission tomography screening for lung cancer: a systematic review and meta-analysis. Cancer Imaging 2013;13:458-65. https://doi.org/10.1102/1470-7330.2013.0038.
- Coureau G, Salmi LR, Etard C, Sancho-Garnier H, Sauvaget C, Mathoulin-Pélissier S. Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: a systematic methodological appraisal of published randomised controlled trials. Eur J Cancer 2016;61:146-56. https://doi.org/10.1016/j.ejca.2016.04.006.
- Fu CP, Liu ZL, Zhu F, Li SQ, Jiang LY. A meta-analysis: is low-dose computed tomography a superior method for risky lung cancers screening population?. Clin Respir J 2016;10:333-41. https://doi.org/10.1111/crj.12222.
- Gopal M, Abdullah SE, Grady JJ, Goodwin JS. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010;5:1233-9. https://doi.org/10.1097/JTO.0b013e3181e0b977.
- Piñeiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review. Lung Cancer 2016;98:91-8. https://doi.org/10.1016/j.lungcan.2016.05.028.
- Seigneurin A, Field JK, Gachet A, Duffy SW. A systematic review of the characteristics associated with recall rates, detection rates and positive predictive values of computed tomography screening for lung cancer. Ann Oncol 2014;25:781-91. https://doi.org/10.1093/annonc/mdt491.
- Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc 2014;11:619-27. https://doi.org/10.1513/AnnalsATS.201312-460OC.
- Wang Z, Hu Y, Wang Y, Han W, Wang L, Xue F, et al. Can CT screening give rise to a beneficial stage shift in lung cancer patients? Systematic review and meta-analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0164416.
- Yau G, Lock M, Rodrigues G. Systematic review of baseline low-dose CT lung cancer screening. Lung Cancer 2007;58:161-70. https://doi.org/10.1016/j.lungcan.2007.07.006.
- van Rosmalen J, Toy M, O’Mahony JF. A mathematical approach for evaluating Markov models in continuous time without discrete-event simulation. Med Decis Making 2013;33:767-79. https://doi.org/10.1177/0272989X13487947.
Appendix 1 Literature search strategies
Lung cancer screening searches: clinical effectiveness 1, 2004–January 2012, no comparator, low-dose computed tomography only, randomised controlled trial filter
First clinical effectiveness search, MEDLINE
Database: MEDLINE.
Host: Ovid.
Data parameters: 1946 to December week 1 2016.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 183.
Search strategy
-
exp Lung Neoplasms/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp Tomography, X-Ray Computed/
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8
-
((low$ adj3 dos$) or LDCT).ti,ab,kw,ot.
-
((ultralow$ or ultra-low$) adj3 dos$).ti,ab,kw,ot.
-
(low-dos$ or ultralow-dos$).ti,ab,kw,ot.
-
10 or 11 or 12
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
-
exp animals/ not humans.sh.
-
19 not 20
-
4 and 9 and 13 and 24
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 201201*).ed.
-
25 and 26
-
Limit 27 to English language and yr=”2004-“
First clinical effectiveness search, MEDLINE In-Process & Other Non-Indexed Citations
Database: MEDLINE In-Process & Other Non-Indexed Citations.
Host: Ovid.
Data parameters: December 30 2016.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 9.
Search strategy
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
4 or 5 or 6
-
((low$ adj3 dos$) or LDCT).ti,ab,kw,ot.
-
((ultralow$ or ultra-low$) adj3 dos$).ti,ab,kw,ot.
-
(low-dos$ or ultralow-dos$).ti,ab,kw,ot.
-
10 or 11 or 12
-
3 and 7 and 11
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 201201*).ed.
-
12 and 13
-
limit 14 to English language and yr=“2004-”
First clinical effectiveness search, EMBASE
Database: EMBASE.
Host: Ovid.
Data parameters: 1974 to 2016 December 30.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 82.
Search strategy
-
exp lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp computer assisted tomography/
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8
-
((low$ adj3 dos$) or LDCT).ti,ab,kw,ot.
-
((ultralow$ or ultra-low$) adj3 dos$).ti,ab,kw,ot.
-
(low-dos$ or ultralow-dos$).ti,ab,kw,ot.
-
10 or 11 or 12
-
4 and 9 and 13
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 201201*).dd.
-
14 and 15
-
limit 16 to english language and yr=“2004-”
First clinical effectiveness search, Health Management Information Consortium
Database: Health Management Information Consortium.
Host: Ovid.
Data parameters: 1979 to November 2016.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 15.
Search strategy
-
exp Lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).tw.
-
(NSLC or NSCLC or SLC or SCLC).tw.
-
1 or 2 or 3
-
((CT or CAT) adj3 (scan$ or screen$)).tw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).tw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).tw.
-
5 or 6 or 7
-
((low$ adj3 dos$) or LDCT).ti,ab,kw,ot.
-
((ultralow$ or ultra-low$) adj3 dos$).ti,ab,kw,ot.
-
(low-dos$ or ultralow-dos$).ti,ab,kw,ot.
-
10 or 11 or 12
-
4 and 8 and 12
-
limit 13 to yr=“2004 - 2011”
First clinical effectiveness search, PsycINFO
Database: PsycINFO.
Host: Ovid.
Data parameters: 1806 to December Week 4 2016.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 28.
Search strategy
-
exp Lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).tw.
-
(NSLC or NSCLC or SLC or SCLC).tw.
-
1 or 2 or 3
-
((CT or CAT) adj3 (scan$ or screen$)).tw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).tw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).tw.
-
5 or 6 or 7
-
((low$ adj3 dos$) or LDCT).ti,ab,kw,ot.
-
((ultralow$ or ultra-low$) adj3 dos$).ti,ab,kw,ot.
-
(low-dos$ or ultralow-dos$).ti,ab,kw,ot.
-
10 or 11 or 12
-
4 and 8 and 12
-
limit 13 to yr=“2004 – 2011”
First clinical effectiveness search, Web of Science
Database: Web of Science [Science Citation Index (SCI) and Conference Proceedings Citation Index – Science (CPCI-S)].
Host: Clarivate Analytics.
Data parameters: not applicable.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 332.
Search strategy
-
TS=((lung* or bronch* or pulmon*) near/2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TS=(NSLC or NSCLC or SLC or SCLC)
-
TS=((CT or CAT) near/2 (scan* or screen*))
-
TS=((computer* near/2 tomogram*) and (scan* or screen*))
-
TS=(tomogram* or helix or helical or spiral* or spiro*)
-
#1 or #2
-
#3 or #4 or #5
-
TS=(“low-dos*” or “ultralow-dos*”)
-
TS=((ultralow* or ultra-low*) near/2 dos*)
-
TS=((low* near/2 dos*) or LDCT)
-
#8 or #9 or #10
-
#6 and #7 and #11
Indexes=SCI-EXPANDED, CPCI-S Timespan=2004-2011.
First clinical effectiveness search, The Cochrane Library
Database: The Cochrane Library.
Host: Cochrane Collaboration.
Data parameters: CDSR: Issue 1 of 12, January 2017; HTA: Issue 4 of 4, October 2016; CENTRAL: Issue 11 of 12, November 2016.
Date searched: 9 January 2017.
Searcher: SR.
Hits: 56.
Search strategy
-
#1 MeSH descriptor: [Lung Neoplasms] explode all trees
-
#2 ((lung* or bronch* or pulmon*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,kw
-
#3 (NSLC or NSCLC or SLC or SCLC):ti,ab,kw
-
#4 #1 or #2 or #3
-
#5 MeSH descriptor: [Tomography, X-Ray Computed] explode all trees
-
#6 ((CT or CAT) near/3 (scan* or screen*)):ti,ab,kw
-
#7 ((computer* near/3 tomogra*) and (scan* or screen*)):ti,ab,kw
-
#8 (tomogram* or helix or helical or spiral* or spiro*):ti,ab,kw
-
#9 #5 or #6 or #7 or #8
-
#10 ((low* near/3 dos*) or LDCT):ti,ab,kw
-
#11 ((ultralow* or ultra-low*) near/3 dos*):ti,ab,kw
-
#12 (low-dos* or ultralow-dos*):ti,ab,kw
-
#13 #10 or #11 or #12
-
#14 #4 and #9 and #13 Publication Year from 2004 to 2011
First clinical effectiveness search, CINAHL
Database: CINAHL.
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 4 January 2017.
Searcher: SR.
Hits: 1275.
Search strategy
-
(MH “Lung Neoplasms+”)
-
TX (lung* or bronch* or pulmon*) N2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)
-
TX (NSLC or NSCLC or SLC or SCLC)
-
S1 OR S2 OR S3
-
(MH “Tomography, X-Ray Computed+”)
-
TX (CT or CAT) N2 (scan* or screen*)
-
TX (computer* N2 tomogra*) and (scan* or screen*)
-
TX (tomogram* or helix or helical or spiral* or spiro*)
-
S5 OR S6 OR S7 OR S8
-
((low* near/3 dos*) or LDCT):ti,ab,kw
-
((ultralow* or ultra-low*) near/3 dos*):ti,ab,kw
-
(low-dos* or ultralow-dos*):ti,ab,kw
-
S10 and S11 and S12
-
S4 AND S9 and S13
-
(MH “Clinical Trials+”)
-
PT Clinical Trial
-
TX clinic* n1 trial*
-
TX (singl* n1 blind*) or (singl* n1 mask*)
-
TX (doubl* n1 blind*) or (doubl* n1 mask*)
-
TX (tripl* n1 blind*) or (tripl* n1 mask*)
-
TX (trebl* n1 blind*) or (trebl* n1 mask*)
-
TX randomi* control* trial*
-
(MH “Random Assignment”)
-
TX random* allocat*
-
TX placebo*
-
(MH “Placebos”)
-
(MH “Quantitative Studies”)
-
TX allocat* random*
-
S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 or S25 or S26 or S27 or S28
-
S14 AND S29
-
Limit to 2004-2011
Summary
| Database | Hits |
|---|---|
| MEDLINE | 124 |
| MEDLINE in Process & Other Non-Indexed Citations | 9 |
| EMBASE | 82 |
| HMIC | 25 |
| PsycINFO | 28 |
| Web of Science (SCI and SCCI) | 332 |
| Cochrane | 56 |
| CINAHL | 167 |
| Total records | 823 |
| Duplicates | 230 |
| Total unique records | 593 |
Lung cancer screening searches: clinical effectiveness 2, 2012–current, all computed tomography scan doses, X-ray comparator, randomised controlled trial filter
Second clinical effectiveness search, MEDLINE
Database: MEDLINE.
Host: Ovid.
Data Parameters: 1946 to December week 1 2016.
Date Searched: 10 January 2017.
Searcher: SR.
Hits: 2074.
Search strategy
-
exp Lung Neoplasms/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp Tomography, X-Ray Computed/
-
exp Radiography, Thoracic/
-
(x ray or xray or x-ray or CXR or radiograph$).ti,ab,ot,kw.
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8 or 9 or 10
-
4 and 11
-
(2012* or 2013* or 2014* or 2015* or 2016* or 2017*).ed.
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
-
exp animals/ not humans.sh.
-
22 not 23
-
12 and 13 and 24
-
limit 25 to english language and yr=“2012-Current”
Second clinical effectiveness search, MEDLINE In-Process & Other Non-Indexed Citations
Database: MEDLINE In-Process & Other Non-Indexed Citations.
Host: Ovid.
Data parameters: 30 December 2016.
Date searched: 10 January 2017.
Searcher: SR.
Hits: nine.
Search strategy
-
1. ((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
2. (NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
3. 1 or 2
-
3. (x ray or xray or x-ray or CXR or radiograph$).ti,ab,ot,kw.
-
4. ((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
5. ((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
6. (tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
7. 4 or 5 or 6 or 7
-
8. 3 and 7
-
9. (2012* or 2013* or 2014* or 2015* or 2016* or 2017*).ed.
-
10. randomized controlled trial.pt.
-
11. controlled clinical trial.pt.
-
12. randomized.ab.
-
13. placebo.ab.
-
14. drug therapy.fs.
-
15. randomly.ab.
-
16. trial.ab.
-
17. groups.ab.
-
18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
-
19. exp animals/ not humans.sh.
-
20. 18 not 19
-
21. 8 and 9 and 20
-
22. limit 21 to english language and yr=“2012 - Current”
Second clinical effectiveness search, EMBASE
Database: EMBASE.
Host: Ovid.
Data parameters: 1974 to 30 December 2016.
Date searched: 10 January 2017.
Searcher: SR.
Hits: 2061.
Search strategy
-
exp lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp computer assisted tomography/
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
exp thorax radiography/
-
(x ray or xray or x-ray or CXR or radiograph$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8 or 9 or 10
-
4 and 11
-
Clinical trial/
-
Randomized controlled trial/
-
Randomization/
-
Single blind procedure/
-
Double blind procedure/
-
Crossover procedure/
-
Placebo/
-
Randomi?ed controlled trial$.tw.
-
Rct.tw.
-
Random allocation.tw.
-
Randomly allocated.tw.
-
Allocated randomly.tw.
-
(allocated adj2 random).tw.
-
Single blind$.tw.
-
Double blind$.tw.
-
((treble or triple) adj blind$).tw.
-
Placebo$.tw.
-
Prospective study/
-
or/13-30
-
Case study/
-
Case report.tw.
-
Abstract report/ or letter/
-
32 or 33 or 34
-
31 not 35
-
12 and 36
-
limit 37 to english language and yr=“2012 –Current”
Second clinical effectiveness search, Health Management Information Consortium
Database: Health Management Information Consortium.
Host: Ovid.
Data parameters: 1979 to November 2016.
Date searched: 10 January 2017.
Searcher: SR.
Hits: seven.
Search strategy
-
exp Lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).tw.
-
(NSLC or NSCLC or SLC or SCLC).tw.
-
1 or 2 or 3
-
((CT or CAT) adj3 (scan$ or screen$)).tw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).tw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).tw.
-
(x ray or xray or x-ray or CXR or radiograph$).tw.
-
5 or 6 or 7 or 8
-
4 and 9
-
limit 10 to yr=“2012 -Current” and english
Second clinical effectiveness search, PsycINFO
Database: PsycINFO.
Host: Ovid.
Data parameters: 1806 to December Week 4 2016.
Date Searched: 10 January 2017.
Searcher: SR.
Hits: 42.
Search strategy
-
exp Lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).tw.
-
(NSLC or NSCLC or SLC or SCLC).tw.
-
1 or 2 or 3
-
((CT or CAT) adj3 (scan$ or screen$)).tw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).tw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).tw.
-
(x ray or xray or x-ray or CXR or radiograph$).tw.
-
5 or 6 or 7 or 8
-
4 and 9
-
limit 10 to yr=“2012 -Current” and english
Second clinical effectiveness search, Web of Science
Database: Web of Science (SCI and CPCI-S).
Host: Clarivate Analytics.
Data parameters: not applicable.
Date searched: 10 January 2017.
Searcher: SR.
Hits: 1216.
Search strategy
-
TS=((lung* or bronch* or pulmon*) near/2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TS=(NSLC or NSCLC or SLC or SCLC)
-
TS=((CT or CAT) near/2 (scan* or screen*))
-
TS=((computer* near/2 tomogram*) and (scan* or screen*))
-
TS=(tomogram* or helix or helical or spiral* or spiro*)
-
TS=(x ray or xray or x-ray or CXR or radiograph*)
-
#1 or #2
-
#3 or #4 or #5 OR #6
-
#7 and #8
-
TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow-up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
-
#9 and #10
Indexes=SCI-EXPANDED, CPCI-S Timespan=2012–2017; Language: English
Second clinical effectiveness search, The Cochrane Library
Database: The Cochrane Library.
Host: Cochrane.
Data parameters: CDSR: Issue 1 of 12, January 2017; HTA: Issue 4 of 4, October 2016; CENTRAL: Issue 11 of 12, November 2016.
Date searched: 10 January 2017.
Searcher: SR.
Hits: 457.
Search strategy
-
#1 MeSH descriptor: [Lung Neoplasms] explode all trees
-
#2 ((lung* or bronch* or pulmon*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,
-
#3 (NSLC or NSCLC or SLC or SCLC):ti,ab,kw
-
#4 #1 or #2 or #3
-
#5 MeSH descriptor: [Tomography, X-Ray Computed] explode all trees
-
#6 ((CT or CAT) near/3 (scan* or screen*)):ti,ab,kw
-
#7 ((computer* near/3 tomogra*) and (scan* or screen*)):ti,ab,kw
-
#8 (tomogram* or helix or helical or spiral* or spiro*):ti,ab,kw
-
#9 MeSH descriptor: [Radiography, Thoracic] explode all trees
-
#10 (x ray or xray or x-ray or CXR or radiograph*):ti,ab,kw
-
#11 #5 or #6 or #7 or #8 or #9 or #10
-
#12 #4 and #11 Publication Year from 2012 to 2017
Second clinical effectiveness search, CINAHL
Database: CINAHL.
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 10 January 2017.
Searcher: SR.
Hits: 403.
Search strategy
-
(MH “Lung Neoplasms+”)
-
TX (lung* or bronch* or pulmon*) N2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)
-
TX (NSLC or NSCLC or SLC or SCLC)
-
S1 OR S2 OR S3
-
(MH “Tomography, X-Ray Computed+”)
-
TX (CT or CAT) N2 (scan* or screen*)
-
TX (computer* N2 tomogra*) and (scan* or screen*)
-
TX (tomogram* or helix or helical or spiral* or spiro*)
-
TX (x ray or xray or x-ray or CXR or radiograph*)
-
(MH “Radiography, Thoracic+”)
-
S5 and S6 and S7 and S8 and S9
-
S4 AND S11
-
(MH “Clinical Trials+”)
-
PT Clinical Trial
-
TX clinic* n1 trial*
-
TX (singl* n1 blind*) or (singl* n1 mask*)
-
TX (doubl* n1 blind*) or (doubl* n1 mask*)
-
TX (tripl* n1 blind*) or (tripl* n1 mask*)
-
TX (trebl* n1 blind*) or (trebl* n1 mask*)
-
TX randomi* control* trial*
-
(MH “Random Assignment”)
-
TX random* allocat*
-
TX placebo*
-
(MH “Placebos”)
-
(MH “Quantitative Studies”)
-
TX allocat* random*
-
S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 or S25 or S26 or S27 or S28
-
S12 AND S27
-
Limit to 2012-2017 and English
Summary
| Database | Hits |
|---|---|
| MEDLINE | 2074 |
| MEDLINE in Process & Other Non-Indexed Citations | 213 |
| EMBASE | 2061 |
| HMIC | 7 |
| PsycINFO | 42 |
| Web of Science (SCI and SCCI) | 1216 |
| Cochrane | 457 |
| CINAHL | 403 |
| Total records | 6473 |
| Duplicates | 1929 |
| Total unique records | 4544 |
Lung cancer screening cost-effectiveness searches
Cost-effectiveness search, MEDLINE
Database: MEDLINE.
Host: Ovid.
Data parameters: 1946 to December Week 1 2016.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 999.
Search strategy
-
exp Lung Neoplasms/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp Tomography, X-Ray Computed/
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8
-
4 and 9
-
exp Economics/
-
Economics, Medical/
-
Economics, Nursing/
-
Economics, Pharmaceutical/
-
exp Economics, Hospital/
-
(economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti,kf.
-
exp “Fees and Charges”/
-
(fee or fees or charge$ or preference$).tw.
-
(fiscal or funding or financial or finance).tw.
-
exp “Costs and Cost Analysis”/
-
exp Health Care Costs/
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
exp Decision Support Techniques/
-
exp Models, Economic/
-
economic model*.ab,kf.
-
markov$.tw.
-
Markov Chains/
-
monte carlo.tw.
-
Monte Carlo Method/
-
(decision adj2 (tree$ or analy$ or model$)).ti,ab,kf.
-
exp Decision Theory/
-
(survival adj3 analy$).tw.
-
“Deductibles and Coinsurance”/
-
exp Health Expenditures/
-
Uncertainty/
-
exp Budgets/
-
or/11-37
-
Animals/ not human.sh.
-
38 not 39
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).ed.
-
10 and 40 and 41
Cost-effectiveness search, MEDLINE In-Process & Other Non-Indexed Citations
Database: MEDLINE In-Process & Other Non-Indexed Citations.
Host: Ovid.
Data parameters: 30 December 2016.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 74.
Search strategy
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
4 or 5 or 6
-
3 and 7
-
(economic$ or price or prices or pricing or priced or discount or discounts or discounted or discounting or ration$ or expenditure or expenditures or budget$ or afford$ or pharmacoeconomic$ or pharmaco-economic$).ti,kf.
-
(fee or fees or charge$ or preference$).tw.
-
(fiscal or funding or financial or finance).tw.
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
economic model*.ab,kf.
-
markov$.tw.
-
monte carlo.tw.
-
(decision adj2 (tree$ or analy$ or model$)).ti,ab,kf.
-
(survival adj3 analy$).tw.
-
or/9-18
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).ed.
-
8 and 19 and 20
Cost-effectiveness search, EMBASE
Database: EMBASE.
Host: Ovid.
Data parameters: 1974 to 30 December 2016.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 1314.
Search strategy
-
exp lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
exp computer assisted tomography/
-
((CT or CAT) adj3 (scan$ or screen$)).ti,ab,ot,kw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).ti,ab,ot,kw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).ti,ab,ot,kw.
-
5 or 6 or 7 or 8
-
4 and 9
-
Economics/
-
Cost/
-
exp Health Economics/
-
Budget/
-
budget*.ti,ab,kw.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab. /freq=2
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw.
-
(value adj2 (money or monetary)).ti,ab,kw.
-
Statistical Model/
-
economic model*.ab,kw.
-
Probability/
-
markov.ti,ab,kw.
-
monte carlo method/
-
monte carlo.ti,ab,kw.
-
Decision Theory/
-
Decision Tree/
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kw.
-
or/11-28
-
10 and 29
-
(200407* or 200408* or 200409* or 200410* or 200411* or 200412* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).dd.
-
30 and 31
-
limit 32 to english language
Cost-effectiveness search, Health Management Information Consortium
Database: Health Management Information Consortium.
Host: Ovid.
Data parameters: 1979 to November 2016.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 22.
Search strategy
-
exp Lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).tw.
-
(NSLC or NSCLC or SLC or SCLC).tw.
-
1 or 2 or 3
-
((CT or CAT) adj3 (scan$ or screen$)).tw.
-
((computer$ adj3 tomogra$) and (scan$ or screen$)).tw.
-
(tomogra$ or helix or helical or spiral$ or spiro$).tw.
-
5 or 6 or 7
-
4 and 8
-
limit 9 to yr=“2004 - Current”
Cost-effectiveness search, Web of Science
Database: Web of Science (SCI and CPCI-S).
Host: Clarivate Analytics.
Data parameters: not applicable.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 302.
Search strategy
-
TS=((lung* or bronch* or pulmon*) near/2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous))
-
TS=(NSLC or NSCLC or SLC or SCLC)
-
TS=((CT or CAT) near/2 (scan* or screen*))
-
TS=((computer* near/2 tomogram*) and (scan* or screen*))
-
TS=(tomogram* or helix or helical or spiral* or spiro*)
-
#1 or #2
-
#3 or #4 or #5
-
#6 and #7
-
TS=((pharmacoeconomic* or socioeconomics or economic* or pric* or cost* or cba or cea or cua or “health utilit*” or “value for money”))
-
#9 and #8
Indexes=SCI-EXPANDED, CPCI-S Timespan=2004–2017.
Cost-effectiveness search, NHS Economic Evaluation Database and Health Technology Assessment
Database: NHS EED and HTA.
Host: The Cochrane Library.
Data parameters: HTA: Issue 4 of 4, October 2016; NHS EED: 2 of 4, April 2015.
Date searched: 5 January 2016.
Searcher: SR.
Hits: HTA: 17; NHS EED: 32 = total 49.
Search strategy
-
MeSH descriptor: [Lung Neoplasms] explode all trees
-
((lung* or bronch* or pulmon*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,kw
-
(NSLC or NSCLC or SLC or SCLC):ti,ab,kw
-
#1 or #2 or #3
-
MeSH descriptor: [Tomography, X-Ray Computed] explode all trees
-
((CT or CAT) near/3 (scan* or screen*)):ti,ab,kw
-
((computer* near/3 tomogra*) and (scan* or screen*)):ti,ab,kw
-
(tomogram* or helix or helical or spiral* or spiro*):ti,ab,kw
-
#5 or #6 or #7 or #8
-
#4 and #9 Publication Year from 2004 to 2017
Cost-effectiveness search, EconLit
Database: EconLit.
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 5 January 2017.
Searcher: SR.
Hits: 99.
Search strategy
-
TX (lung* or bronch* or pulmon*) N2 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)
-
TX (NSLC or NSCLC or SLC or SCLC)
-
S1 OR S2
-
Limit to 2004-2017
Summary
| Database | Hits |
|---|---|
| MEDLINE | 999 |
| MEDLINE in Process & Other Non-Indexed Citations | 74 |
| EMBASE | 1314 |
| HMIC | 22 |
| Web of Science (SCI and SCCI) | 358 |
| Cochrane – HTA and NHS EED | 49 |
| EconLit | 99 |
| Total records | 2915 |
| Duplicates | 692 |
| Total unique records | 2223 |
Lung cancer screening utilities searches
Utilities search, MEDLINE
Database: MEDLINE.
Host: Ovid.
Data parameters: 1946 to December Week 1 2016.
Date searched: 24 January 2017.
Searcher: SR.
Hits: 1102.
Search strategy
-
exp Lung Neoplasms/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
(HRQOL or HRQL or QOL or QALY$).tw.
-
(EQ-5D or EQ-5D-3L or EQ-5D-5L).tw.
-
quality-adjusted life years/
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
health utilit$.tw.
-
disutil$.tw.
-
5 or 6 or 7 or 8 or 9 or 10
-
4 and 11
-
limit 14 to english language
Utilities search, MEDLINE In-Process & Other Non-Indexed Citations
Database: MEDLINE In-Process & Other Non-Indexed Citations.
Host: Ovid.
Data parameters: January 20 2016.
Date searched: 24 January 2017.
Searcher: SR.
Hits: 258.
Search strategy
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2
-
(HRQOL or HRQL or QOL or QALY$).tw.
-
(EQ-5D or EQ-5D-3L or EQ-5D-5L).tw.
-
(euroqol or euro qol or eq5d or eq 5d).tw.
-
health utilit$.tw.
-
disutil$.tw.
-
4 or 5 or 6 or 7 or 8
-
3 and 9
-
limit 10 to english language
Utilities search, EMBASE
Database: EMBASE.
Host: Ovid.
Data parameters: 1974 to 2017 January 20.
Date searched: 10 January 2017.
Searcher: SR.
Hits: 709.
Search strategy
-
exp lung cancer/
-
((lung$ or bronch$ or pulmon$) adj3 (cancer$ or neopla$ or tumor$ or tumour$ or carcinoma$ or adenocarcinoma$ or small cell or squamous)).ti,ab,ot,kw.
-
(NSLC or NSCLC or SLC or SCLC).ti,ab,ot,kw.
-
1 or 2 or 3
-
(HRQOL or HRQL or QOL or QALY$).ti.
-
(EQ-5D or EQ-5D-3L or EQ-5D-5L).ti.
-
quality adjusted life year/
-
(euroqol or euro qol or eq5d or eq 5d).ti.
-
health utilit$.ti.
-
disutil$.ti.
-
5 or 6 or 7 or 8 or 9 or 10
-
4 and 11
-
limit 14 to english language
Utilities search, NHS Economic Evaluation Database
Database: NHS EED.
Host: The Cochrane Library.
Data parameters: NHS EED: 2 of 4, April 2015.
Date searched: 24 January 2017.
Searcher: SR.
Hits: 59.
Search strategy
-
MeSH descriptor: [Lung Neoplasms] explode all trees
-
((lung* or bronch* or pulmon*) near/3 (cancer* or neopla* or tumor* or tumour* or carcinoma* or adenocarcinoma* or “small cell” or squamous)):ti,ab,kw
-
(NSLC or NSCLC or SLC or SCLC):ti,ab,kw
-
#1 or #2 or #3
-
descriptor: [quality-adjusted life years] explode all trees
-
(HRQOL or HRQL or QOL or QALY*):ti,ab,kw
-
(EQ-5D or EQ-5D-3L or EQ-5D-5L):ti,ab,kw
-
(euroqol or euro qol or eq5d or eq 5d):ti,ab,kw
-
health utilit*:ti,ab,kw
-
disutil*:ti,ab,kw
-
#5 or #6 or #7 or #8 or #9 or #10
-
#4 and #11
Utilities search, School of Health and Related Research Utilities Database
Website: School of Health and Related Research Health Utilities Database (ScHARRHUD).
Date searched: 24 January 2017.
Searcher: SR.
Hits: nine.
Utilities search, Health Economics Research Centre
Website: Health Economics Research Centre (HERC) Oxford.
Date searched: 24 January 2017.
Searcher: SR.
Hits: seven.
Website: EQ-5D EuroQol.
Date searched: 24 January 2017.
Searcher: SR.
Hits: 60.
Summary
| Database | Hits |
|---|---|
| MEDLINE | 1102 |
| MEDLINE in Process & Other Non-Indexed Citations | 258 |
| EMBASE | 709 |
| Cochrane – NHS EED | 59 |
| HUD – ScHARR | 9 |
| HERC – Oxford | 7 |
| EQ-5D – EuroQol | 60 |
| Total records | 2210 |
| Duplicates | 588 |
| Total unique records | 1622 |
Backward citation chasing
Citation chasing yielded 1246 further references (after deduplicating and checking against already screened papers).
Update searches
The cost-effectiveness searches were updated on 11 April 2017 with following results.
| Database | Hits |
|---|---|
| MEDLINE | 7 |
| MEDLINE in Process & Other Non-Indexed Citations | 13 |
| EMBASE | 57 |
| HMIC | 0 |
| Web of Science (SCI and SCCI) | 9 |
| Cochrane – HTA and NHS EED | 0 |
| EconLit | 0 |
| Total records | 86 |
| Duplicates | 18 |
| Total unique records | 68 |
Ongoing trials
Registers searched: ClinicalTrials.gov and Controlled Trials (ISRCTN).
Search terms: Lung cancer AND screening.
Ongoing trials all phases.
Date limit was 2012 onwards.
Appendix 2 Included trials with linked citations
| Study | Extracted data | Citation |
|---|---|---|
| DANTE | Yes | Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med 2009;180:445–53 |
| Yes | Infante M, Chiesa G, Solomon D, Morenghi E, Passera E, Lutman FR, et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol 2011;6:327–35 | |
| Yes | Infante MV, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Passera E, et al. Preliminary five-year results from a randomized study of lung cancer screening with spiral CT (the DANTE trial). J Thorac Oncol 2011;6:S351–S352 | |
| Yes | Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015;191:1166–75. https://doi.org/10.1164/rccm.201408-1475OC | |
| Yes | Infante MV, Cavuto S, Lutman FR, Passera E, Chiesa G, Brambilla G, et al. The DANTE trial, a randomized study of lung cancer screening with spiral CT: 7-year results. J Thorac Oncol 2013;8:S147–S148 | |
| Yes | Infante MV, Fabio LR, Cavuto S, Brambilla G, Chiesa G, Passera E, et al. DANTE: a randomized study on lung cancer screening with low-dose spiral CT (LDCT): end of accrual and preliminary results. CHEST J 2006;130:114 | |
| Yes | Infante M, Lutman FR, Cavuto S, Brambilla G, Chiesa G, Passera E, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355–63 | |
| Depiscan | Yes | Blanchon T, Bréchot JM, Grenier PA, Ferretti GR, Lemarié E, Milleron B, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing Low Dose CT scan (LDCT) and Chest X-Ray (CXR). Lung Cancer 2007;58:50–8 |
| Repeat | Milleron B. Screening for Lung Cancer: Feasibility Study of a Randomized Trial Comparing Low Dose Spiral CT and Chest X-Ray. Chicago, IL: Annual Meeting Proceedings of the American Society of Clinical Oncology; 31 May–3 June 2014 | |
| Repeat | Milleron B. Screening for lung cancer: feasibility study of a randomized trial comparing low dose spiral CT and chest x-ray. J Clin Oncol 2004;22(Suppl. 14):7183 | |
| DLCST | Yes | Aggestrup LM, Hestbech MS, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open 2012;2:e000663. https://doi.org/10.1136/bmjopen-2011-000663 |
| Yes | Ashraf H, Saghir Z, Thomsen LH, Dirksen A, Dossing M, Pedersen JH, Tonnesen P. Smoking habits in the Danish Lung Cancer Screening Trial (DLCST): final results after 5-year screening program. Am J Respir Crit Care Med 2012;185:A2585 | |
| Yes | Ashraf H, Saghir Z, Dirksen A, Pedersen JH, Thomsen LH, Døssing M, Tønnesen P. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax 2014;69:574–9. https://doi.org/10.1136/thoraxjnl-2013-203849 | |
| No | Ashraf H, Tønnesen P, Holst Pedersen J, Dirksen A, Thorsen H, Døssing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388–92. https://doi.org/10.1136/thx.2008.102475 | |
| Yes | Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, et al. The Danish randomized lung cancer CT screening trial – overall design and results of the prevalence round. J Thorac Oncol 2009;4:608–14. https://doi.org/10.1097/JTO.0b013e3181a0d98f | |
| Yes | Pedersen JH, Wille MW, Dirksen A. The Danish lung cancer screening trial: results 5 years after last CT screening. J Thorac Oncol 2015;10:S191 | |
| Yes | Petersen RH, Hansen HJ, Dirksen A, Pedersen JH. Lung cancer screening and video-assisted thoracic surgery. J Thorac Oncol 2012;7:1026–31. https://doi.org/10.1097/JTO.0b013e31824fe942 | |
| Yes | Rasmussen JF, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer 2015;87:65–72. https://doi.org/10.1016/j.lungcan.2014.11.003 | |
| Yes | Saghir Z, Ashraf HG, Dirksen, Tønnesen P, Hansen H, Bach KS, et al. Danish Lung Cancer Screening Trial (DLCST): preliminary results after five annual screening rounds with low dose CT. Am J Respir Crit Car Med 2011;83:A6103 | |
| Yes | Saghir Z, Dirksen A, Ashraf HG, Tonnesen P, Bach KS, Hansen H, et al. CT screening of lung cancer brings forward early disease. The Danish Lung Cancer Screening Trial (DLCST): status after five years of CT screening. J Thorac Oncol 2011;6:S350–1 | |
| No | Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296–301. https://doi.org/10.1136/thoraxjnl-2011-200736 | |
| No data | Saghir Z, Dirksen A, Rasmussen JF, Heleno BM, Brodersen J, Pedersen JH. In lung cancer screening by CT incidental findings are frequent and often of clinical importance. Am J Respir Crit Car Med 2012;185:A5072 | |
| No data | Saghir Z, Dirksen A, Pedersen JH. Predictors of nodule malignancy in the Danish lung cancer screening trial (DLCST). J Thorac Oncol 2013;8:S678 | |
| Yes | Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016;193:542–51. https://doi.org/10.1164/rccm.201505-1040OC | |
| Garg | Yes | Garg K, Keith RL, Byers T, Kelly K, Kerzner AL, Lynch DA, Miller YE. Randomized controlled trial with low-dose spiral CT for lung cancer screening: feasibility study and preliminary results. Radiology 2002;225:506–10. https://doi.org/10.1148/radiol.2252011851 |
| ITALUNG | No | Conti B, Aquilini F, Pistelli F, Santis M, Tavanti L, Cini S. Lung function in a group of smokers or ex-smokers enrolled in a Randomized Controlled Trial (RCT) with low-dose Computed Tomography (CT) for lung cancer screening (ITALUNG-CT Study). Barcelona: European Respiratory Society Annual Congress;18–22 September 2010 |
| Yes | Gonfiotti A, Santini P, Pegna AL, Esposito I, Paci E, Mussi A, Janni A. Results of thoracic surgical operations in the italung trial for lung cancer screening. Interactive Cardiovascular and Thoracic Surgery 2009;9:S83 | |
| Yes | Lopes Pegna A, Picozzi G, Falaschi F, Carrozzi L, Falchini M, Carozzi FM, et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol 2013;8:866–75. https://doi.org/10.1097/JTO.0b013e31828f68d6 | |
| Yes | Mascalchi M, Mazzoni LN, Falchini M, Belli G, Picozzi G, Merlini V, et al. Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. Br J Radiol 2014;85:1134–9 | |
| Yes | Mascalchi M, Belli G, Zappa M, Picozzi G, Falchini M, Della Nave R, et al. Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trial. AJR Am J Roentgenol 2006;187:421–9 | |
| No | Mascalchi M, Picozzi G, Falchini M, Vella A, Diciotti S, Carrozzi L, et al. Initial LDCT appearance of incident lung cancers in the ITALUNG trial. Eur J Radiol 2014;83:2080–6. https://doi.org/10.1016/j.ejrad.2014.07.019 | |
| Yes | Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34–40. https://doi.org/10.1016/j.lungcan.2008.07.003 | |
| No | Picozzi G, Mascalchi M, Falaschi F, Paci E. Initial appearance of LDCT screen-detected lung cancers in the ITALUNG trial. J Thorac Imaging 2014;29:3 | |
| Yes | Picozzi G, Falaschi F, Mascalchi M, Paci E. Four years results of low dose CT screening and nodule management in the ITALUNG trial. J Thorac Imaging 2014;29:W26–W7 | |
| LSS-PCLO | Yes | Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the lung screening study of the National Cancer Institute. Chest 2004;126:114–21. https://doi.org/10.1378/chest.126.1.114 |
| Yes | Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9–15 | |
| Yes | Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Ann Intern Med 2010;152:505–12, W176–80. https://doi.org/10.7326/0003-4819-152-8-201004200-00007 | |
| lungSEARCH | Yes | Spiro SG, Hackshaw A, LungSEARCH Collaborative Group. Research in progress – LungSEARCH: a randomised controlled trial of surveillance for the early detection of lung cancer in a high-risk group. Thorax 2016;71:91–3. https://doi.org/10.1136/thoraxjnl-2015-207433 |
| Yes | Spiro SG, Hackshaw A, Shah P, Novelli M, Kocjan G, Shaw P, et al. Research in progress – LungSEARCH: a randomised controlled trial of surveillance for the early detection of lung cancer in a high-risk group. Thorax 2015;71:91–3 | |
| LUSI | Yes | Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized Study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol 2015;10:890–6. https://doi.org/10.1097/JTO.0000000000000530 |
| Yes | Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475–86. https://doi.org/10.1007/s00432-012-1228-9 | |
| Yes | Eigentopf A, Becker N, Motsch E, Gross ML. Interim results from the German randomized lung screening trial LUSI. Oncology Research and Treatment 2014;37:34–35 | |
| Yes | Eigentopf A, Motsch E, Gross ML, Becker N. Results of all 5 screening rounds of the randomized study on the early detection of lung cancer LUSI. Oncology Research and Treatment 2016;39:27–8 | |
| No | Sommer G, Tremper J, Koenigkam-Santos M, Delorme S, Becker N, Biederer J, et al. Lung nodule detection in a high-risk population: comparison of magnetic resonance imaging and low-dose computed tomography. Eur J Radiol 2014;83:600–5. https://doi.org/10.1016/j.ejrad.2013.11.012 | |
| MILD | Yes | Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308–15. https://doi.org/10.1097/CEJ.0b013e328351e1b6 |
| No | Pozzi P, Munarini E, Bravi F, Rossi M, La Vecchia C, Boffi R, Pastorino U. A combined smoking cessation intervention within a lung cancer screening trial: a pilot observational study. Tumori 2015;101:306–11. https://doi.org/10.5301/tj.5000282 | |
| No data | Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J 2011;38:392–400. https://doi.org/10.1183/09031936.00201809 | |
| No data | Sverzellati N, Cademartiri F, Bravi F, Martini C, Gira FA, Maffei E, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology 2012;262:460–7. https://doi.org/10.1148/radiol.11110364 | |
| No | Sverzellati N, Silva M, Calareso G, Galeone C, Marchianò A, Sestini S, et al. Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016;26:3821–9. https://doi.org/10.1007/s00330-016-4228-3 | |
| NELSON | No | Bunge EM, van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. High affective risk perception is associated with more lung cancer-specific distress in CT screening for lung cancer. Lung Cancer 2008;62:385–90. https://doi.org/10.1016/j.lungcan.2008.03.029 |
| Repeat | van de Wiel JC, Wang Y, Xu DM, van der Zaag-Loonen HJ, van der Jagt EJ, van Klaveren RJ, Oudkerk M, NELSON study group. Neglectable benefit of searching for incidental findings in the Dutch-Belgian lung cancer screening trial (NELSON) using low-dose multidetector CT. Eur Radiol 2007;17:1474–82. https://doi.org/10.1007/s00330-006-0532-7 | |
| No data | Gietema HA, Schilham AM, van Ginneken B, van Klaveren RJ, Lammers JW, Prokop M. Monitoring of smoking-induced emphysema with CT in a lung cancer screening setting: detection of real increase in extent of emphysema. Radiology 2007;244:890–7 | |
| No data | Gietema HA, Zanen P, Schilham A, van Ginneken B, van Klaveren RJ, Prokop M, Lammers JW. Distribution of emphysema in heavy smokers: impact on pulmonary function. Respir Med 2010;104:76–82. https://doi.org/10.1016/j.rmed.2009.08.004 | |
| No data | Heuvelmans MA, Oudkerk M, de Bock GH, de Koning HJ, Xie X, van Ooijen PM, et al. Optimisation of volume-doubling time cutoff for fast-growing lung nodules in CT lung cancer screening reduces false-positive referrals. Eur Radiol 2013;23:1836–45. https://doi.org/10.1007/s00330-013-2799-9 | |
| No data | Heuvelmans MA, Oudkerk M, de Jong PA, Mali WP, Groen HJ, Vliegenthart R. The impact of radiologists’ expertise on screen results decisions in a CT lung cancer screening trial. Eur Radiol 2015;25:792–9. https://doi.org/10.1007/s00330-014-3467-4 | |
| Yes (abstract) | Horeweg N, Van Der Aalst CM, Vliegenthart R, Zhao YR, Xie X, Scholten ET, et al. Participants’ results of three rounds of the randomised Dutch-Belgian lung cancer screening trial; a volumetry-based computer tomography screening strategy. Am J Respir Crit Care Med 2013;187:A2344 | |
| Yes | Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342–50. https://doi.org/10.1016/S1470-2045(14)70387-0 | |
| Yes | Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848–54. https://doi.org/10.1164/rccm.201209-1651OC | |
| Yes | Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J 2013;42:1659–67. https://doi.org/10.1183/09031936.00197712 | |
| No data | Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332–41. https://doi.org/10.1016/S1470-2045(14)70389-4 | |
| Yes | Oudkerk M, Heuvelmans MA. Screening for lung cancer by imaging: the NELSON study. J Belg Soc Radiol 2013;96:163–6 | |
| No data | Takx RA, Išgum I, Willemink MJ, van der Graaf Y, de Koning HJ, Vliegenthart R, et al. Quantification of coronary artery calcium in nongated CT to predict cardiovascular events in male lung cancer screening participants: results of the NELSON study. J Cardiovasc Comput Tomogr 2015;9:50–7. https://doi.org/10.1016/j.jcct.2014.11.006 | |
| No data | Takx RA, Vliegenthart R, Mohamed Hoesein FA, Išgum I, de Koning HJ, Mali WP, et al. Pulmonary function and CT biomarkers as risk factors for cardiovascular events in male lung cancer screening participants: the NELSON study. Eur Radiol 2015;25:65–71. https://doi.org/10.1007/s00330-014-3384-6 | |
| yes | van den Bergh KA, Essink-Bot ML, Bunge EM, Scholten ET, Prokop M, van Iersel CA, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113:396–404. https://doi.org/10.1002/cncr.23590 | |
| Yes | van den Bergh KA, Essink-Bot ML, Borsboom GJ, Th Scholten E, Prokop M, de Koning HJ, van Klaveren RJ. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27–34. https://doi.org/10.1038/sj.bjc.6605459 | |
| Yes | van den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, van Klaveren RJ, de Koning HJ. Long-term effects of lung cancer CT screening on health-related quality of life (NELSON). Eur Respir J 2010;38:154–61 | |
| No | van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. Eur Respir J 2009;34:711–20. https://doi.org/10.1183/09031936.00098908 | |
| Yes | van der Aalst CM, de Koning HJ, van den Bergh KA, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer 2012;76:204–10. https://doi.org/10.1016/j.lungcan.2011.10.006 | |
| No | van der Aalst CM, van den Bergh KA, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600–5. https://doi.org/10.1136/thx.2009.133751 | |
| Yes | van der Aalst CM, van Klaveren RJ, van den Bergh KA, Willemsen MC, de Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. Eur Respir J 2011;37:1466–73. https://doi.org/10.1183/09031936.00035410 | |
| Yes | van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120:868–74. https://doi.org/10.1002/ijc.22134 | |
| Yes | Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177–84 | |
| No | van Klaveren RJ, Oudkerk M, Prokop M, Yankelevitz DF, Reeves AP, Kostis WJ, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2010;362:757–9 | |
| Yes | van’t Westeinde SC, Horeweg N, De Leyn P, Groen HJ, Lammers JW, Weenink C, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J of Cardiothorac Surg 2012;42:420–9 | |
| No | Walter JE, Heuvelmans MA, de Jong PA, Vliegenthart R, van Ooijen PMA, Peters RB, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol 2016;17:907–16 | |
| Yes | Yousaf-Khan U, Horeweg N, van der Aalst C, ten Haaf K, Oudkerk M, de Koning H. Baseline characteristics and mortality outcomes of control group participants and eligible non-responders in the NELSON Lung Cancer Screening Study. J Thorac Oncol 2015;10:747–53. https://doi.org/10.1097/JTO.0000000000000488 | |
| Yes | Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Lammers JW, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48–56. https://doi.org/10.1136/thoraxjnl-2016-208655 | |
| Yes | Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11:S79–84. https://doi.org/10.1102/1470-7330.2011.9020 | |
| NLST | Yes | National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409 |
| Yes | Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771–9. https://doi.org/10.1093/jnci/djq434 | |
| Yes | Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31. https://doi.org/10.1056/NEJMoa1208962 | |
| No | Berg C. Screening with low-dose computed tomography reduced lung cancer mortality in high-risk patients. Annals of Clinical Outcomes Management 2011;155:5–6 | |
| No | Block JP. Screening for lung cancer with low-dose CT scans reduces lung cancer mortality. Journal of Clinical Outcomes Management 2011;18:343–5 | |
| No | Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology 2015;276:82–90. https://doi.org/10.1148/radiol.15142062 | |
| No | Chudgar NP, Bucciarelli PR, Jeffries EM, Rizk NP, Park BJ, Adusumilli PS, Jones DR. Results of the national lung cancer screening trial: where are we now? Thorac Surg Clin 2015;25:145–53. https://doi.org/10.1016/j.thorsurg.2014.11.002 | |
| No | Clark MA, Gorelick JJ, Sicks JD, Park ER, Graham AL, Abrams DB, Gareen IF. The relations between false positive and negative screens and smoking cessation and relapse in the National Lung Screening Trial: implications for public health. Nicotine Tob Res 2016;18:17–24. https://doi.org/10.1093/ntr/ntv037 | |
| No | Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative risk for a false-positive test using low-dose computed tomography in lung cancer screening. J Clin Oncol 2009;27(Suppl. 18):1502 | |
| No | Dillard TA, Patel RR, Schroeder C. Uneven Distribution of cancer histology in the National Lung Screening Trial. Am J Med Sci 2015;350:219–21. https://doi.org/10.1097/MAJ.0000000000000516 | |
| Yes | Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 2014;120:3401–9. https://doi.org/10.1002/cncr.28833 | |
| No | Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014;106:dju284. https://doi.org/10.1093/jnci/dju284 | |
| No | Horeweg N, Nackaerts K, Oudkerk M, de Koning HJ. Low-dose computed tomography screening for lung cancer: results of the first screening round. J Comp Eff Res 2013;2:433–6. https://doi.org/10.2217/cer.13.57 | |
| No | Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013;268:563–71. https://doi.org/10.1148/radiol.13120816 | |
| Yes | Larke FJ, Kruger RL, Cagnon CH, Flynn MJ, McNitt-Gray MM, Wu X, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 2011;197:1165–9. https://doi.org/10.2214/AJR.11.6533 | |
| No | Katki HA, Kovalchik SA, Tammemagi MC, Berg C, Caporaso N, Riley T, et al. Variation in the efficacy of low-dose computed tomographic lung screening based on risk of lung cancer mortality in the national lung screening trial. Am J Res Crit Car Med 2013;187:A6073 | |
| No | Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245–54. https://doi.org/10.1056/NEJMoa1301851 | |
| Yes | Kruger R, Flynn MJ, Judy PF, Cagnon CH, Seibert JA. Effective dose assessment for participants in the National Lung Screening Trial undergoing posteroanterior chest radiographic examinations. AJR Am J Roentgenol 2013;201:142–6. https://doi.org/10.2214/AJR.12.9181 | |
| No | Marcus PM, Doria-Rose VP, Gareen IF, Brewer B, Clingan K, Keating K, et al. Did death certificates and a death review process agree on lung cancer cause of death in the National Lung Screening Trial? Clin Trials 2016;13:434–8. https://doi.org/10.1177/1740774516638345 | |
| Yes | National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980–91 | |
| No | Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865–73. https://doi.org/10.1001/jama.2011.1591 | |
| No | Park ER, Gareen I, Rakowski W, Ostroff J, Perry K, Rigotti N. Risk perceptions among participants of the national lung cancer screening trial. Annals of Behavioral Medicine 2007;33:S138 | |
| No | Park ER, Gareen IF, Jain A, Ostroff JS, Duan F, Sicks JD, et al. Examining whether lung screening changes risk perceptions: National Lung Screening Trial participants at 1-year follow-up. Cancer 2013;119:1306–13. https://doi.org/10.1002/cncr.27925 | |
| Yes | Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägi MC, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269–74. https://doi.org/10.1001/jamainternmed.2013.12738 | |
| No | Patz EF, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol 2016;17:590–9. https://doi.org/10.1016/S1470-2045(15)00621-X | |
| No | Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013;119:3976–83. https://doi.org/10.1002/cncr.28326 | |
| No | Pinsky PF, Gierada DS, Nath H, Kazerooni EA, Amorosa J. ROC curves for low-dose CT in the National Lung Screening Trial. J Med Screen 2013;20:165–8. https://doi.org/10.1177/0969141313500666 | |
| No | Pinsky PF, Gierada DS, Nath PH, Kazerooni E, Amorosa J. National lung screening trial: variability in nodule detection rates in chest CT studies. Radiology 2013;268:865–73. https://doi.org/10.1148/radiol.13121530 | |
| No | Pinsky PF, Gierada DS, Hocking W, Patz EF, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med 2014;161:627–33. https://doi.org/10.7326/M14-1484 | |
| No | Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, Kazerooni E. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485–91. https://doi.org/10.7326/M14-2086 | |
| No | Pinsky PF, Nath PH, Gierada DS, Sonavane S, Szabo E. Short- and long-term lung cancer risk associated with noncalcified nodules observed on low-dose CT. Cancer Prev Res 2014;7:1179–85. https://doi.org/10.1158/1940-6207.CAPR-13-0438 | |
| No | Strauss GM, Dominioni L. Computed Tomography (CT) and Chest X-Ray (CXR) screening in the National Lung Screening Trial (NLST): do mortality differences provide an unbiased measure of the effectiveness of CT screening? Am J Res Crit Car Med 2012;185:A5063 | |
| No | Strauss GM, Dominioni L. Computed Tomography (CT) screening for lung cancer: does the mortality endpoint provide definitive evidence for CT superiority in the National Lung Screening Trial (NLST)? J Thorac Oncol 2013;8:S979–80 | |
| No | Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. https://doi.org/10.1093/jnci/dju084 | |
| No | Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial. Implications for widespread implementation. Am J Respir Crit Care Med 2015;192:200–8. https://doi.org/10.1164/rccm.201502-0259OC | |
| No | Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Halbert CH, Warren GW, et al. The association between smoking abstinence and mortality in the National Lung Screening Trial. Am J Respir Crit Care Med 2016;193:534–41. https://doi.org/10.1164/rccm.201507-1420OC | |
| No | Yip R, Henschke CI, Yankelevitz DF, Smith JP. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273:591–6. https://doi.org/10.1148/radiol.14132950 | |
| No | Yip R, Yankelevitz DF, Hu M, Li K, Xu DM, Jirapatnakul A, Henschke CI. Lung cancer deaths in the National Lung Screening Trial attributed to nonsolid nodules. Radiology 2016;281:589–96. https://doi.org/10.1148/radiol.2016152333 | |
| No | Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, et al. Airflow limitation and histology shift in the National Lung Screening Trial. The NLST-ACRIN cohort substudy. Am J Respir Crit Care Med 2015;192:1060–7. https://doi.org/10.1164/rccm.201505-0894OC | |
| UKLS | No | Ali N, Lifford KJ, Carter B, McRonald F, Yadegarfar G, Baldwin DR, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015;5:e008254. https://doi.org/10.1136/bmjopen-2015-008254 |
| Yes | Brain K, Lifford KJ, Carter B, Burke O, McRonald F, Devaraj A, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax 2016;71:996–1005. https://doi.org/10.1136/thoraxjnl-2016-208283 | |
| No | Field J, Baldwin D, Devaraj A, Brain K, Eisen T, Holemans J, et al. United Kingdom lung cancer screening trial (UKLS): First 88897 approaches. Cancer Res 2013;73(8 Suppl. 1):A3631 | |
| No | Field JK, Devaraj A, Baldwin DR, Holemans J, Screaton N, Ledson M, et al. UK Lung Cancer Screening Trial (UKLS): base line data. J Thorac Oncol 2013;8:S685 | |
| No | Field JK, Devaraj A, Baldwin DR, Holemans J, Screaton N, Ledson M, et al. 66 UK Lung Cancer Screening trial (UKLS): prevalence data at baseline. Lung Cancer 2014;83:S24–5 | |
| Yes | Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, et al. UK lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161–70 | |
| Yes | Field JK, Duffy SW, Baldwin DR, Brain KE, Devaraj A, Eisen T, et al. The UK lung cancer screening trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20(40). https://doi.org/10.3310/hta20400 | |
| No | Jones G, Komrower D, Murthy M, Hunt N, Holemans J, Field J, et al. S110 Experience with suspected cancer referrals from the UK lung screen trial. Thorax 2013;68(Suppl. 3):57–8 | |
| No | McRonald F, Baldwin DR, Devaraj A, Brain K, Eisen T, Holeman J, et al. The uniqueness of the United Kingdom Lung Cancer Screening trial (UKLS) – a population screening study. Lung Cancer 2013;79:S28–9 | |
| No | McRonald FE, Yadegarfar G, Baldwin DR, Devaraj A, Brain KE, Eisen T, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res 2014;7:362–71. https://doi.org/10.1158/1940-6207.CAPR-13-0206 | |
| No | Nair A, Gartland N, Barton B, Jones D, Clements L, Screaton NJ, et al. Comparing the performance of trained radiographers against experienced radiologists in the UK lung cancer screening (UKLS) trial. Br J Radiol 2016;89:20160301. https://doi.org/10.1259/bjr.20160301 |
| Study name | Linked citations |
|---|---|
| Czech | Kubík A, Haerting J. Survival and mortality in a randomized study of lung cancer detection. Neoplasma 1990;37:467–75 |
| Kubik A, Parkin DM, Khlat M, Erban J, Polak J, Adamec M. Lack of benefit from semiannual screening for cancer of the lung – follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer 1990;45:26–33. https://doi.org/10.1002/ijc.2910450107 | |
| Kubík A, Polák J. Lung cancer detection. Results of a randomized prospective study in Czechoslovakia. Cancer 1986;57:2427–37 | |
| Kubík AK, Parkin DM, Zatloukal P. Czech Study on Lung Cancer Screening: post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 2000;89(Suppl. 11):2363–8 | |
| Mayo | Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR. Lung cancer screening: the Mayo program. J Occup Med 1986;28:746–50 |
| Sanderson DR. Lung cancer screening. The Mayo study. Chest 1986;89(Suppl. 4):324 | |
| Flehinger BJ, Kimmel M, Polyak T, Melamed MR. Screening for lung cancer. The Mayo Lung Project revisited. Cancer 1993;72:1573–80 | |
| Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, Uhlenhopp MA. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis 1984;130:561–5. https://doi.org/10.1164/arrd.1984.130.4.561 | |
| Fontana RS, Sanderson DR, Woolner LB, Miller WE, Bernatz PE, Payne WS, Taylor WF. The Mayo Lung Project for early detection and localization of bronchogenic carcinoma: a status report. Chest 1975;67:511–22 | |
| Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, Prorok PC. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst 2000;92:1308–16 | |
| Marcus PM, Bergstralh EJ, Zweig MH, Harris A, Offord KP, Fontana RS. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst 2006;98:748–56 | |
| Marcus PM, Prorok PC. Reanalysis of the Mayo Lung Project data: the impact of confounding and effect modification. J Med Screen 1999;6:47–9. https://doi.org/10.1136/jms.6.1.47 | |
| Muhm JR, Miller WE, Fontana RS, Sanderson DR, Uhlenhopp MA. Lung cancer detected during a screening program using four-month chest radiographs. Radiology 1983;148:609–15. https://doi.org/10.1148/radiology.148.3.6308709 | |
| Sanderson D, Fontana R. Results of Mayo lung project: an interim report. Recent Results Cancer Res 1982;82:179–86 | |
| Taylor WF, Fontana RS, Uhlenhopp MA, Davis CS. Some results of screening for early lung-cancer. Cancer 1981;47(5):1114–20 | |
| Woolner LB, Fontana RS, Cortese DA, Sanderson DR, Bernatz PE, Payne WS, et al. Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-year period. Mayo Clin Proc 1984;59:453–66 | |
| Woolner LB, Fontana RS, Sanderson DR, Miller WE, Muhm JR, Taylor WF, Uhlenhopp MA. Mayo Lung Project: evaluation of lung cancer screening through December 1979. Mayo Clin Proc 1981;56:544–55 | |
| Mazzone | Mazzone PJ, Obuchowski N, Phillips M, Risius B, Bazerbashi B, Meziane M. Lung cancer screening with computer aided detection chest radiography: design and results of a randomized, controlled trial. PLOS ONE 2013;8:e59650. https://doi.org/10.1371/journal.pone.0059650 |
| Mazzone PJ, Obuchowski N, Fu AZ, Phillips M, Meziane M. Quality of life and healthcare use in a randomized controlled lung cancer screening study. Ann Am Thorac Soc 2013;10:324–9. https://doi.org/10.1513/AnnalsATS.201301-007OC | |
| LSS-PLCO | Croswell JM, Baker SG, Marcus PM, Clapp JD, Kramer BS. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Ann Intern Med 2010;152:505–12, W176–80. https://doi.org/10.7326/0003-4819-152-8-201004200-00007 |
| Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P, Writing Committee, Lung Screening Study Research Group. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs. chest radiograph: the lung screening study of the national cancer institute. Chest 2004;126:114–21. https://doi.org/10.1378/chest.126.1.114 | |
| Gohagan JK, Marcus PM, Fagerstrom RM, Pinsky PF, Kramer BS, Prorok PC, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47:9–15 |
Appendix 3 Excluded studies with reasons
| Number | Citation | Decision |
|---|---|---|
| 1 | Lung cancer trial results show benefit with low-dose CT. Coping With Cancer 2010;24:27 | Unobtainable |
| 2 | Giuliano DV. Screening of pulmonary neoplasia with low-dose spiral TC. Results of a triennial pilot study and design of the randomised clinical study ‘Italung CT’. Radiologia Medica 2005;109:575 | Unobtainable |
| 3 | HAYES, Inc. Low-dose helical (spiral) computed tomography for lung cancer screening. Lansdale, PA: HAYES, Inc.; 2013 | Unobtainable |
| 4 | Mascalchi M, Paci E. Screening of pulmonary neoplasia with low-dose spiral TC. Results of a triennial pilot study and design of the randomised clinical study ‘Italung CT’ – Dear director. Radiologia Medica 2005;109:575–6 | Unobtainable |
| 5 | Picozzi G, Paci E, Lopez Pegna A, Bartolucci M, Roselli G, De Francisci A, et al. Screening of lung cancer with low dose spiral CT: results of a three year pilot study and design of the randomised controlled trial ‘Italung-CT’. Radiol Med 2005;109:17–26 | Unobtainable |
| 6 | Anon. Lung cancer detection by chest X-rays at 6 monthly intervals. Nova Scotia Med Bull 1970;49:14–5 | Population |
| 7 | Brett GZ. The value of lung cancer detection by six-monthly chest radiographs. Thorax 1968;23:414–20 | Population |
| 8 | Brett GZ. Earlier diagnosis and survival in lung cancer. Br Med J 1969;4:260–2 | Population |
| 9 | Friedman GD, Collen MF, Fireman BH. Multiphasic health checkup evaluation: a 16-year follow-up. J Chronic Dis 1986;39:453–63 | Population |
| 10 | Gohagan JK, Prorok PC, Greenwald P, Kramer BS. The PLCO cancer screening trial: background, goals, organization, operations, results. Rev Recent Clin Trials 2015;10:173–80 | Population |
| 11 | Guldbrandt LM, Fenger-Grøn M, Rasmussen TR, Rasmussen F, Meldgaard P, Vedsted P. The effect of direct access to CT scan in early lung cancer detection: an unblinded, cluster-randomised trial. BMC Cancer 2015;15:934. https://doi.org/10.1186/s12885-015-1941-2 | Population |
| 12 | Hocking WG, Tammemagi MC, Commins J, Oken MM, Kvale PA, Hu P, et al. Diagnostic evaluation following a positive lung screening chest radiograph in the Prostate, Lung, Colorectal, Ovarian (PLCO) cancer screening trial. Lung Cancer 2013;82:238–44. https://doi.org/10.1016/j.lungcan.2013.07.017 | Population |
| 13 | Kvale PA, Johnson CC, Tammemägi M, Marcus PM, Zylak CJ, Spizarny DL, et al. Interval lung cancers not detected on screening chest X-rays: How are they different? Lung Cancer 2014;86:41–6. https://doi.org/10.1016/j.lungcan.2014.07.013 | Population |
| 14 | Meng LJ, Wang J, Pu XL, Xu JQ, Wang L, Yang S, et al. Clinical study of low-dose spiral ct used to opportunistically screen patients with solitary pulmonary nodule. J Am Geriatr Soc 2016;64:S337 | Population |
| 15 | Meng XX, Kuai XP, Dong WH, Jia NY, Liu SY, Xiao XS. Comparison of lung lesion biopsies between low-dose CT-guided and conventional CT-guided techniques. Acta Radiol 2013;54:909–15. https://doi.org/10.1177/0284185113485937 | Population |
| 16 | Pinsky PF, Zhu CS, Kramer BS. Lung cancer risk by years since quitting in 30+ pack year smokers. J Med Screen 2015;22:151–7. https://doi.org/10.1177/0969141315579119 | Population |
| 17 | Sagawa M. Screening in Japan – the JECS study. J Thorac Oncol 2015;10(Suppl. 2):S115 | Population |
| 18 | Blanchon T, Lukasiewicz-Hajage E, Lemarié E, Milleron B, Bréchot JM, Flahault A, Groupe Dépiscan. [DEPISCAN – a pilot study to evaluate low dose spiral CT scanning as a screening method for bronchial carcinoma.] Rev Mal Respir 2002;19:701–5 | Language |
| 19 | Kubík A. [Risk groups and effective x-ray screening frequency of bronchial cancer.] Z Erkr Atmungsorgane 1983;160:18–25 | Language |
| 20 | Pastorino U, Calabro E. The MILD project: prevention and early diagnosis of lung cancer. Multidisciplinary Respiratory Medicine 2007;2:52–7 | Language |
| 21 | Yang M, Wang J, Meng LJ, Wang Y, Xu L, Liu FY, Fan WF. Analysis of feasibility of lung cancer screening with low-radiation-dose spiral CT scan plus detection of p16 gene methylation in serum. Chinese Journal of Cancer Prevention and Treatment 2008;15:8–10 | Language |
| 22 | Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest 1984;86:44–53 | Comparator |
| 23 | Baker RR, Tockman MS, Marsh BR, Stitik FP, Ball WC, Eggleston JC, et al. Screening for bronchogenic carcinoma: the surgical experience. J Thorac Cardiovasc Surg 1979;78:876–82 | Comparator |
| 24 | Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis 1984;130:555–60. https://doi.org/10.1164/arrd.1984.130.4.555 | Comparator |
| 25 | Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, Uhlenhopp MA. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis 1984;130:561–5. https://doi.org/10.1164/arrd.1984.130.4.561 | Comparator |
| 26 | Levin ML, Tockman MS, Frost JK, Ball WC. Lung cancer mortality in males screened by chest X-ray and cytologic sputum examination: a preliminary report. Recent Results Cancer Res 1982;82:138–46 | Comparator |
| 27 | Melamed M, Flehinger B, Miller D, Osborne R, Zaman M, McGinnis C, Martini N. Preliminary report of the lung cancer detection program in New York. Cancer 1977;39:369–82 | Comparator |
| 28 | Melamed MR, Flehinger BJ, Zaman MB. Impact of early detection on the clinical course of lung cancer. Surg Clin North Am 1987;67:909–24 | Comparator |
| 29 | Martini N. Results of the Memorial Sloan-Kettering study in screening for early lung cancer. Chest 1986;89(Suppl. 4):325S | Comparator |
| 30 | Stitik FP, Tockman MS. Radiographic screening in the early detection of lung cancer. Radiol Clin North Am 1978;16:347–66 | Comparator |
| 31 | Tockman MS. Survival and mortality from lung-cancer in a screened population – the Johns-Hopkins study. Chest 1986;89:S324–5 | Comparator |
| 32 | Tockman MS, Frost JK, Stitik FP, Levin ML, et al. Screening and Detection of Lung Cancer. In Aisner J (ed). Contemporary Issues in Clinical Oncology. New York, NY: Churchill Livingstone; 1985 | Comparator |
| 33 | Hu P, Dai M, Shi J, Ren J, Li J, Liao X, et al. The Feasibility Study of a Randomized Cancer Screening Trial in China. American Association for Cancer Research (AACR), 107th Annual Meeting, 16–20 April 2016, New Orleans: Louisiana, abstract no. 1795 | Intervention |
| 34 | Marshall HM, Yang IA, Passmore L, McCaul EM, Bowman R, Fong KM. A randomized controlled trial of brief counselling intervention and audio materials for smoking cessation in a low-dose CT screening study. J Thorac Oncol 2013;8(Suppl. 2):S703 | Intervention |
| 35 | Navani N, Nankivell M, Lawrence DR, Lock S, Makker H, Baldwin DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282–9. https://doi.org/10.1016/S2213-2600(15)00029-6 | Intervention |
| 36 | Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol 2014;32:768–73. https://doi.org/10.1200/JCO.2013.50.4357 | Intervention |
| 37 | van der Aalst CM, de Koning HJ, van den Bergh KA, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: a randomised controlled trial. Lung Cancer 2012;76:204–10. https://doi.org/10.1016/j.lungcan.2011.10.006 | Intervention |
| 38 | Camiciottoli G, Cavigli E, Grassi L, Diciotti S, Orlandi I, Zappa M, et al. Prevalence and correlates of pulmonary emphysema in smokers and former smokers. A densitometric study of participants in the ITALUNG trial. Eur Radiol 2009;19:58–66. https://doi.org/10.1007/s00330-008-1131-6 | Outcome |
| 39 | Field J, Baldwin D, Stephen D, Devaraj A, Hansell D. (2012). UKLS – United Kingdom Lung cancer Screening trial. Cancer Research 2012;72(Suppl. 1) | Outcome |
| 40 | Gierada DS, Garg K, Nath H, Strollo DC, Fagerstrom RM, Ford MB. CT quality assurance in the lung screening study component of the National Lung Screening Trial: implications for multicenter imaging trials. AJR Am J Roentgenol 2009;193:419–24. https://doi.org/10.2214/AJR.08.1995 | Outcome |
| 41 | Hurt CN, Roberts K, Rogers TK, Griffiths GO, Hood K, Prout H, et al. A feasibility study examining the effect on lung cancer diagnosis of offering a chest X-ray to higher-risk patients with chest symptoms: protocol for a randomized controlled trial. Trials 2013;14:405. https://doi.org/10.1186/1745-6215-14-405 | Outcome |
| 42 | Hubers AJ, Heideman DAM, Duin S, Witte BI, de Koning HJ, Groen HJM, et al. DNA hypermethylation analysis in sputum of asymptomatic subjects at risk for lung cancer participating in the NELSON trial: argument for maximum screening interval of 2 years. J Clin Pathol 2017;70:250–4 | Outcome |
| 43 | Kaerlev L, Iachina M, Pedersen JH, Green A, Nørgård BM. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer 2013;12:188 | Outcome |
| 44 | Kanodra NM, Payne E, Gebregziabher M, Egede LE, Silvestri GA, Tanner NT. Mortality risk associated with smoking in the National Lung Screening Trial (NLST). Am J Res Crit Car Med 2015:191:A6116 | Outcome |
| 45 | Maldonado F, Duan F, Raghunath SM, Rajagopalan S, Karwoski RA, Garg K, et al. Noninvasive Computed Tomography-based risk stratification of lung adenocarcinomas in the National Lung Screening Trial. Am J Respir Crit Care Med 2015;192:737–44. https://doi.org/10.1164/rccm.201503-0443OC | Outcome |
| 46 | Marcus PM, Gareen IF, Miller AB, Rosenbaum J, Keating K, Aberle DR, Berg CD. The national lung screening trial’s endpoint verification process: determining the cause of death. Contemporary Clinical Trials 2011;32:834–40 | Outcome |
| 47 | Mario S, Nicola S, Carmelinda M, Giulio N, Alfonso M, Maurizio Z, et al. Long-term surveillance of ground-glass nodules: evidence from the MILD trial. J Thorac Oncol 2012;7:1541–6. https://doi.org/10.1097/JTO.0b013e3182641bba | Outcome |
| 48 | Patz EF. Lung cancer screening, overdiagnosis bias, and reevaluation of the Mayo Lung Project. J Natl Cancer Inst 2006;98:724–5 | Outcome |
| 49 | Zhao YR, Heuvelmans MA, Dorrius MD, van Ooijen PM, Wang Y, de Bock GH, et al. Features of resolving and nonresolving indeterminate pulmonary nodules at follow-up CT: the NELSON study. Radiology 2014;270:872–9. https://doi.org/10.1148/radiol.13130332 | Outcome |
| 50 | Aalst C, Iersel C, Klaveren R, Frenken F, Fracheboud J, Otto S, et al. Generalisability of the results of the Dutch-Belgian randomised controlled lung cancer CT screening trial (NELSON): does self-selection play a role? Lung Cancer 2012;77:51–7 | Design |
| 51 | Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243–53. https://doi.org/10.1148/radiol.10091808 | Design |
| 52 | Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243–53. https://doi.org/10.1148/radiol.10091808 | Design |
| 53 | Aberle DR, Abtin F, Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. J Clin Oncol 2013;31:1002–8. https://doi.org/10.1200/JCO.2012.43.3110 | Design |
| 54 | Incorrect table data in: Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;308:1324 | Design |
| 55 | Incorrect value in abstract in: Benefits and harms of ct screening for lung cancer: a systematic review. JAMA 2013;309:2212 | Design |
| 56 | Armato SG, Roy AS, Macmahon H, Li F, Doi K, Sone S, Altman MB. Evaluation of automated lung nodule detection on low-dose computed tomography scans from a lung cancer screening program(1). Acad Radiol 2005;12:337–46 | Design |
| 57 | Armstrong P, Husband JE, Holemans JA. Population screening for lung cancer. Hospital Medicine 2004;65:404–11 | Design |
| 58 | Asano F, Shinagawa N, Ishida T, Tsuzuku A, Tachihara M. Subgroup for which virtual bronchoscopic navigation is useful for endobronchial ultrasonography of peripheral pulmonary lesions. Respirology 2015;20:21 | Design |
| 59 | Asano F, Shinagawa N, Ishida T, Tsuzuku A, Tachihara M, Kanazawa K, et al. Virtual bronchoscopic navigation improves the diagnostic yield of radial-endobronchial ultrasound for peripheral pulmonary lesions with involved bronchi on CT. Intern Med 2015;54:1021–5. https://doi.org/10.2169/internalmedicine.54.3497 | Design |
| 60 | Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418–29. https://doi.org/10.1001/jama.2012.5521 | Design |
| 61 | Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 2011;66:308–13. https://doi.org/10.1136/thx.2010.152066 | Design |
| 62 | Becker N, Motsch E, Gross M-L, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475–86 | Design |
| 63 | Belani C, Fuhrman D, Wilson D, Fisher S, Sciurba F, Luketich J, et al. Two-year outcomes from lung cancer screening with low-dose helical computed tomography. Lung Cancer 2005;49:S177 | Design |
| 64 | Belvedere O, Grossi F, Meduri S, Barbone F, Zanin T, Pignata G, et al. Lung cancer and mesothelioma screening with low-dose spiral computed tomography (LDCT) in 1,000 asbestos-exposed workers: An Alpe-Adria Thoracic Oncology Multidisciplinary group study (ATOM 002). J Clin Oncol 2004;22:S628 | Design |
| 65 | Belvedere O, Grossi F, Rossetto C, Meduri S, Barbone F, Scogna A, et al. Final results of lung cancer and mesothelioma baseline screening with low-dose spiral computed tomography (LDCT) in 1000 asbestos-exposed workers: An Alpe-Adria Thoracic Oncology Multidisciplinary group study (ATOM 002). Lung Cancer 2005;49:S81 | Design |
| 66 | Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12 https://doi.org/10.1097/JTO.0b013e31815e6d6b | Design |
| 67 | Berlin NI, Buncher CR, Fontana RS, Frost JK, Melamed MR. The National Cancer Institute cooperative early lung cancer detection program. Results of the initial screen (prevalence). Early lung cancer detection: introduction. Am Rev Respir Dis 1984;130:545–9. https://doi.org/10.1164/arrd.1984.130.4.545 | Design |
| 68 | Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer (structured abstract). Annals of Thoracic Surgery 2005;79:375–82 | Design |
| 69 | Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al. The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews. Health Technol Assess 2006;10(3) | Design |
| 70 | Boiselle PM. Computed tomography screening for lung cancer. JAMA 2013;309:1163–70 | Design |
| 71 | Brewer BC, K, Keating K, Lawler-Heavner J, Payte N, Rosenbaum J, Ross W. Creating and supporting a productive environment for ancillary study development in a multicenter trial. Clinical Trials 2011;8:525–6 | Design |
| 72 | Cao JQ, Rodrigues GB, Louie AV, Zaric GS. Systematic review of the cost-effectiveness of positron-emission tomography in staging of non – small-cell lung cancer and management of solitary pulmonary nodules. Clin Lung Cancer 2012;13:161–70. https://doi.org/10.1016/j.cllc.2011.09.002 | Design |
| 73 | Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604–20. https://doi.org/10.1093/annonc/mdv041 | Design |
| 74 | Carozzi FM, Bisanzi S, Falini P, Sani C, Venturini G, Lopes Pegna A, et al. Molecular profile in body fluids in subjects enrolled in a randomised trial for lung cancer screening: Perspectives of integrated strategies for early diagnosis. Lung Cancer 2010;68:216–21. https://doi.org/10.1016/j.lungcan.2009.06.015 | Design |
| 75 | Cerello P. The Channeler Ant Model CAD performance on a heterogeneous lung CT dataset. Int J Computer Assisted Radiology and Surgery 2011;6:S195–6 | Design |
| 76 | Chien CR, Chen TH. Mean sojourn time and effectiveness of mortality reduction for lung cancer screening with computed tomography. Int J Cancer 2008;122:2594–9. https://doi.org/10.1002/ijc.23413 | Design |
| 77 | Chien CR, Kao CH, Wang HN, Liang JA. Positron emission tomography screening for lung cancer: a systematic review. Value in Health 2011;14:A80 | Design |
| 78 | Chien CR, Liang JA, Wang HN, Lin CC, Kao CH. Diagnostic performance of selective positron emission tomography for lung cancer computed tomography screening: a meta-analysis. European Journal of Cancer 2011;47:S211 | Design |
| 79 | Chiles C, Paul NS. Beyond lung cancer: a strategic approach to interpreting screening computed tomography scans on the basis of mortality data from the National Lung Screening Trial. J Thorac Imaging 2013;28:347–54. https://doi.org/10.1097/RTI.0000000000000052 | Design |
| 80 | Choi SH, Lee CH, Kwon SY. Lung cancer characteristics with low dose chest CT screening in Korea: comparison with korean general population. Respirology 2010;15:66 | Design |
| 81 | Church TR, National Lung Screening Trial Executive Committee. Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol 2003;10:713–15 | Design |
| 82 | Ciliberto M, Maggi F, Treglia G, Padovano F, Calandriello L, Giordano A, Bonomo L. Comparison between whole-body MRI and fluorine-18-fluorodeoxyglucose PET or PET/CT in oncology: a systematic review. Radiol Oncol 2013;47:206–18. https://doi.org/10.2478/raon-2013-0007 | Design |
| 83 | Clark K, Commean P, Rathmell J, Riley T, Jones G, Marquez G, Prior F. National Lung Screening Trial (NLST): post-trial data and image exploration with an open source query tool. Society for Clinical Trials 2013;10:S38–9 | Design |
| 84 | Clark KW, Gierada DS, Marquez G, Moore SM, Maffitt DR, Moulton JD, et al. Collecting 48,000 CT exams for the lung screening study of the National Lung Screening Trial. J Digit Imaging 2009;22:667–80. https://doi.org/10.1007/s10278-008-9145-9 | Design |
| 85 | Clark KW, Gierada DS, Moore SM, Maffitt DR, Koppel P, Phillips SR, Prior FW. Creation of a CT Image Library for the Lung Screening Study of the National Lung Screening Trial. J Digit Imaging 2007;20:23–31. https://doi.org/10.1007/s10278-006-0589-5 | Design |
| 86 | Clark MM, Jett JR. Change in smoking status after low-dose spiral chest CT screening for lung cancer: opportunity for smoking intervention. Thorax 2009;64:371–2. https://doi.org/10.1136/thx.2008.111039 | Design |
| 87 | Clingan K, Brewer B, Florczyk M, Lasrado M, Payte N, Rosenbaum J. A unique web-based tool for performing and tracking data quality assurance tasks in a multi-center study. Clinical Trials 2011;8:531–2 | Design |
| 88 | Dales LG, Friedman GD, Collen MF. Evaluating periodic multiphasic health checkups: a controlled trial. J Chronic Dis 1979;32:385–404 | Design |
| 89 | de Geus-Oei LF, van der Heijden HF, Corstens FH, Oyen WJ. Predictive and prognostic value of FDG-PET in nonsmall-cell lung cancer: a systematic review. Cancer 2007;110:1654–64. https://doi.org/10.1002/cncr.22979 | Design |
| 90 | de Gonzalez AB. Lung cancer mortality following low-dose helical CT screening: estimated radiation risks and mortality benefits. Cancer Epidemiol Biomarkers Prev 2006;15:410 | Design |
| 91 | DeFrank JT, Barclay C, Sheridan S, Brewer NT, Gilliam M, Moon AM, et al. The psychological harms of screening: the evidence we have versus the evidence we need. J Gen Intern Med 2015;30:242–8. https://doi.org/10.1007/s11606-014-2996-5 | Design |
| 92 | Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(Suppl. 5):e78–e92 | Design |
| 93 | DiGiacinto D, Bagley J, Garrison M. Comparison of endobronchial ultrasound and computed tomography with fluoroscopy in the guidance of lung cancer biopsies: a literature review. Journal of Diagnostic Medical Sonography 2012;28:224–30 | Design |
| 94 | Duffy SW, Field JK, Allgood PC, Seigneurin A. Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer 2014;110:1834–40. https://doi.org/10.1038/bjc.2014.63 | Design |
| 95 | Duke SL, Eisen T. Finding needles in a haystack: annual low-dose computed tomography screening reduces lung cancer mortality in a high-risk group. Expert Rev Anticancer Ther 2011;11:1833–6. https://doi.org/10.1586/era.11.185 | Design |
| 96 | Duranti L, Leo F, Pastorino U. PET scan contribution in chest tumor management: a systematic review for thoracic surgeons. Tumori 2012;98:175–84. https://doi.org/10.1700/1088.11927 | Design |
| 97 | Eberth JM. Lung Cancer Screening With Low-Dose CT in the United States. J Am Coll Radiol 2015;12:1395–402 | Design |
| 98 | Eckhart H, Langen A, Vleeming E, Lazarenko S, Gommans G, Knol R, Zant F. Comparison of PET/CT acquisition protocols in lung carcinoma: effective radiation dose and image quality. European Journal of Nuclear Medicine and Molecular Imaging 2013;40:S151 | Design |
| 99 | Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007;11:44 | Design |
| 100 | Faivre-Finn C, Lorigan P. Efficacy of positron emission tomography staging for small-cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol 2012;7:e25. https://doi.org/10.1097/JTO.0b013e318265a7c6 | Design |
| 101 | Field J. Follow-up on the national lung screening trial: the next steps. Cancer Prevent Res 2011;4(Suppl. 1) | Design |
| 102 | Field JK, Devaraj A, Baldwin DR, Duffy S. UKLS impact of utilization of risk assessment in trial selection. J Thorac Oncol 2015;2:S114–5 | Design |
| 103 | Field JK, Baldwin D, Devaraj A, Brain K, Eisen T, Holemans J, et al. United Kingdom lung cancer screening trial (UKLS): First 88897 approaches. American Association for Cancer Research Conference (AACR), 104th Annual Meeting, 6–10 April 2013, Washington, DC, abstract no. 3631 | Design |
| 104 | Flehinger BJ, Kimmel M. The natural history of lung cancer in a periodically screened population. Biometrics 1987;43:127–44 | Design |
| 105 | Flehinger BJ, Melamed MR. Current status of screening for lung cancer. Chest Surg Clin N Am 1994;4:1–15 | Design |
| 106 | Flores J, Moreno-Koehler A, Finkelman M, Caro J, Strauss G. Chest X-Ray (CXR) screening improves outcomes in Lung Cancer (LC) in the Prostate, Lung, Colon, and Ovary (PLCO) randomized controlled trial (RCT). Chest 2015;148 | Design |
| 107 | Flores JPE, Moreno-Koehler A, Finkelman M, Caro J, Strauss G. Effectiveness of lung cancer screening comparing Computed Tomography (CT) to Chest X-Ray (CXR) to no screening in PLCO and NLST randomized trials. J Thorac Oncol 2015;2:S733 | Design |
| 108 | Flores JP, Moreno-Koehler A, Finkelman M, Caro J, Strauss GM. Comparative effectiveness of lung cancer screening by computed tomography (CT) vs. chest x-ray (CXR) vs. no screening. J Clin Oncol 2016;34(Suppl. 15):e18246 | Design |
| 109 | Flores R, Bauer T, Aye R, Andaz S, Kohman L, Sheppard B, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619–26. https://doi.org/10.1016/j.jtcvs.2013.11.001 | Design |
| 110 | Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR, et al. Screening for lung cancer. A critique of the Mayo Lung Project. Cancer 1991;67(Suppl. 4):1155–64 | Design |
| 111 | Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc 2014;11:619–27. https://doi.org/10.1513/AnnalsATS.201312-460OC | Design |
| 112 | Ganti AK, Mulshine JL. Lung cancer screening. Oncologist 2006;11:481–7 | Design |
| 113 | National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243–53. https://doi.org/10.1148/radiol.10091808 | Design |
| 114 | Gerke O, Hermansson R, Hess S, Schifter S, Vach W, Høilund-Carlsen PF. Cost-effectiveness of PET and PET/computed tomography: a systematic review. PET Clin 2015;10:105–24. https://doi.org/10.1016/j.cpet.2014.09.008 | Design |
| 115 | Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration 2014;87:165–76. https://doi.org/10.1159/000355710 | Design |
| 116 | Gopal M, Abdullah SE, Grady JJ, Goodwin JS. Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of randomized controlled trials. J Thorac Oncol 2010;5:1233–9. https://doi.org/10.1097/JTO.0b013e3181e0b977 | Design |
| 117 | Gren LH, Lamerato LE, Wright P, Marcus PM. Validation of self-report of chest x-ray exam at a prostate, lung, colorectal and ovarian cancer screening trial center. Rev Recent Clin Trials 2015;10:194–9 | Design |
| 118 | Gross CP. Screening with low-dose computed tomography reduced lung cancer mortality in high-risk patients. Ann Intern Med 2011;155:JC5–6 | Design |
| 119 | Grossi F, Belvedere O, Meduri S, Barbone F, Zanin T, Follador A, et al. (2004). Final results of lung cancer and mesothelioma baseline screening with Low-Dose spiral Computed Tomography (LDCT) in 1,000 asbestos-exposed workers: an Alpe-adria Thoracic Oncology Multidisciplinary group study (ATOM 002). Annals of Oncology 2004;15:147 | Design |
| 120 | Grossi F, Fosola G, Meduri S, Barbone F, Rossetto C, Follador A, et al. Screening for mesothelioma and lung cancer with Low-Dose spiral Computed Tomography (LDCT) in asbestos-exposed subjects: an Alpe-Adria Thoracic Oncology Multidisciplinary group study (ATOM 002). Lung Cancer 2004;45:S69 | Design |
| 121 | Gu F, Katki H, Freedman N, Marcus P, Caporaso N. Time to First Morning Cigarette and Lung Cancer in National Lung Screening Trial. American Association for Cancer Research (AACR), 107th Annual Meeting, 16–20 April 2016, New Orleans, Louisiana, abstract no. 2596 | Design |
| 122 | Guldbrandt LM. The effect of direct referral for fast CT scan in early lung cancer detection in general practice. A clinical, cluster-randomised trial. Dan Med J 2015;62:B5027 | Design |
| 123 | Guldbrandt LM, Fenger-Grøn M, Folkersen BH, Rasmussen TR, Vedsted P. Reduced specialist time with direct computed tomography for suspected lung cancer in primary care. Dan Med J 2013;60:A4738 | Design |
| 124 | Guo B, Chojecki D, Bond K, Yan C, Chuck A. Low Dose Computed Tomography for the Screening of Lung Cancer in Adults. Edmonton, AB: Institute of Health Economics; 2014 | Design |
| 125 | Heelan RT, Flehinger BJ, Melamed MR, Zaman MB, Perchick WB, Caravelli JF, Martini N. Non-small-cell lung cancer: results of the New York screening program. Radiology 1984;151:289–93. https://doi.org/10.1148/radiology.151.2.6324279 | Design |
| 126 | Herrero JI, Zulueta JJ, D’Avola D, Pardo F, Montes U, Rotellar F, et al. Lung cancer screening with low-radiation dose computed tomography after liver transplantation. Hepatology 2011;54:659A | Design |
| 127 | Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011;73:325–31. https://doi.org/10.1016/j.lungcan.2010.12.018 | Design |
| 128 | Hocking WG, Tammemagi MC, Commins J, Oken MM, Kvale PA, Hu P, et al. Diagnostic evaluation following a positive lung screening chest radiograph in the Prostate, Lung, Colorectal, Ovarian (PLCO) cancer screening trial. Lung Cancer 2013;82:238–44. https://doi.org/10.1016/j.lungcan.2013.07.017 | Design |
| 129 | Howington, J. Early detection of lung cancer. Journal of Carcinogenesis 2011;10:S23 | Design |
| 130 | Iliopoulou SME, Kousoulis AA. Screening for lung cancer with low-dose computed tomography: a systematic review of the evidence. Tobacco Induced Diseases 2014;12 | Design |
| 131 | Infante M, Sestini S, Galeone C, Marchianò A, Lutman FR, Angeli E, et al. Lung cancer screening with low-dose spiral computed tomography: evidence from a pooled analysis of two Italian randomized trials. Eur J Cancer Prev 2017;26:324–9. https://doi.org/10.1097/CEJ.0000000000000264 | Design |
| 132 | The Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–71 | Design |
| 133 | Jacobs PC, Mali WP, Grobbee DE, van der Graaf Y. Prevalence of incidental findings in computed tomographic screening of the chest: a systematic review. J Comput Assist Tomogr 2008;32:214–21. https://doi.org/10.1097/RCT.0b013e3181585ff2 | Design |
| 134 | Jett JR, Midthun DE. Screening for lung cancer: for patients at increased risk for lung cancer, it works. Ann Intern Med 2011;155:540–2. https://doi.org/10.7326/0003-4819-155-8-201110180-00367 | Design |
| 135 | Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, Moriyama N. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798–802. https://doi.org/10.1148/radiology.201.3.8939234 | Design |
| 136 | Kaneko M, Kusumoto M, Kobayashi T, Moriyama N, Naruke T, Ohmatsu H, et al. Computed tomography screening for lung carcinoma in Japan. Cancer 2000;89(Suppl. 11):2485–8 | Design |
| 137 | Kaneko M, Ohmatsu H, Ohata M, Nakagawa T, Niitsuma S, Yoshikawa K, et al. 2005;49:S19 | Design |
| 138 | Kayyali A. Low-dose CT screening for lung cancer in high-risk patients. Am J Nurs 2011;111:60 | Design |
| 139 | Kessler L, Loggers E, Gatsonis C, Patrick D, Sullivan S, Hanash S. Surveillance trial to improve longevity in lung cancer patients (STILL). Am J Res Crit Car Med 2013;187 | Design |
| 140 | Kondo T, Mori K, Hagiwara Y, Kodama T. Results of low-dose multi-detector CT screening for lung cancer over a period of 4 years. Lung Cancer 2005;49:S182 | Design |
| 141 | Kramer BS, Berg CD, Aberle DR, Prorok PC. Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST). J Med Screen 2011;18:109–11. https://doi.org/10.1258/jms.2011.011055 | Design |
| 142 | Lacasse Y. Review: more frequent compared with less frequent chest radiographic screening may increase lung cancer mortality. ACP J Club 2004;140:48 | Design |
| 143 | Laschkolnig A, Pertl D. Quick assessment on lung cancer screening with low-dose computed tomography. Value in Health 2016;19:A686 | Design |
| 144 | Lathan C, Frank DA. Review: low-dose CT screening reduces lung cancer and mortality in current or former smokers. Ann Intern Med 2013;159 | Design |
| 145 | Lee EW, Kang SS, Lee YJ. Lung cancer screening with low dose spiral CT and autofluorescence bronchoscopy in high risk group. Am J Res Crit Care Med 2009;179:1 | Design |
| 146 | Li ZY, Luo L, Hu YH, Chen H, Den YK, Tang L, et al. Lung cancer screening: a systematic review of clinical practice guidelines. Int J Clin Pract 2016;70:20–30. https://doi.org/10.1111/ijcp.12744 | Design |
| 147 | MacRedmond R, McVey G, Lee M, Costello RW, Kenny D, Foley C, Logan PM. Screening for lung cancer using low dose CT scanning: results of 2 year follow up. Thorax 2006;61:54–6 | Design |
| 148 | Marshall HM, Bowman RV, Crossin J, Fuentes M, Slaughter R, Passmore, et al. Queensland lung cancer screening study: findings from the first 197 participants’ prevalence low dose CT scans. Respirology 2010;15:A14 | Design |
| 149 | Marshall HM, Bowman RV, Crossin J, Fuentes M, Slaughter R, Zimmerman P, et al. Low-dose CT screening for lung cancer in Australia: QLCSS project interim report. J Thorac Oncol 2009;4:S867 | Design |
| 150 | Mauri D, Kamposioras K, Proiskos A, Xilomenos A, Peponi C, Dambrosio M, et al. Old habits die hard: chest radiography for screening purposes in primary care. Am J Manag Care 2006;12:650–6 | Design |
| 151 | Mazzone PJ, Obuchowski N, Fu AZ, Phillips M, Meziane M. Quality of life and healthcare use in a randomized controlled lung cancer screening study. Ann Am Thorac Soc 2013;10:324–9. https://doi.org/10.1513/AnnalsATS.201301-007OC | Design |
| 152 | McCunney, RJ. Should we screen for occupational lung cancer with low-dose computed tomography? J Occup Environ Med 2006;48:1328–33 | Design |
| 153 | McMahon PM, Christiani DC. Computed tomography screening for lung cancer. BMJ 2007;334:271 | Design |
| 154 | McMahon PM, Meza R, Plevritis SK, Black WC, Tammemagi CM, Erdogan A, et al. Comparing benefits from many possible computed tomography lung cancer screening programs: extrapolating from the National Lung Screening Trial using comparative modeling. PLOS ONE 2014;9:e99978. https://doi.org/10.1371/journal.pone.0099978 | Design |
| 155 | Menezes RJ, Roberts HC, Paul NS, McGregor M, Chung TB, Patsios D, et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: the Toronto experience. Lung Cancer 2010;67:177–83. https://doi.org/10.1016/j.lungcan.2009.03.030 | Design |
| 156 | Midthun D, Jett J, Swensen S, Hartman T, Aughenbaugh G, Mandrekar S, et al. Screening for lung cancer with low-dose Spiral Computed Tomography (SCT): 5-year results of the mayo clinic trial. Lung Cancer 2005;49:S83 | Design |
| 157 | Miller A, Markowitz S, Manowitz A, Miller JA. Lung cancer screening using low-dose high-resolution CT scanning in a high-risk workforce: 3500 nuclear fuel workers in three US states. Chest 2004;125(Suppl. 5):152–3 | Design |
| 158 | Moore SM, Gierada DS, Clark KW, Blaine GJ, PLCO-NLST Quality Assurance Working Group. Image quality assurance in the prostate, lung, colorectal, and ovarian cancer screening trial network of the National Lung Screening Trial. J Digit Imaging 2005;18:242–50. https://doi.org/10.1007/s10278-005-5153-1 | Design |
| 159 | Mortani Barbosa EJ. Lung cancer screening overdiagnosis: reports of overdiagnosis in screening for lung cancer are grossly exaggerated. Acad Radiol 2015;22:976–82. https://doi.org/10.1016/j.acra.2014.10.011 | Design |
| 160 | Nackaerts K, Vansteenkiste J. Low-dose CT screening for lung cancer: trial and error? J Thorac Oncol 2009;4:563–4. https://doi.org/10.1097/JTO.0b013e3181a5db40 | Design |
| 161 | National Lung Screening Trial Research Team, Aberle Dr, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. The national lung screening trial: overview and study design. Radiology 2011;258:243–53 | Design |
| 162 | Neal RD, Hurt C, Roberts K, Rogers T, Hamilton W, Tudor Edwards R, et al. A feasibility randomised controlled trial looking at the effect on lung cancer diagnosis of giving a chest X-ray to smokers aged over 60 with new chest symptoms the ELCID trial. Lung Cancer 2014;83:S81–2 | Design |
| 163 | Neugut AI, Accordino MK. Review: CT screening for lung cancer reduced mortality in 1 large trial but not in 2 smaller trials. Ann Intern Med 2012;157 | Design |
| 164 | Novello S, Selvaggi G, Perotto F, Lausi P, Dogliotti L, Borasio R, et al. Three-year findings of an early lung cancer detection feasibility study with low-dose spiral computed tomography in heavy smokers. Lung Cancer 2005;49:S185 | Design |
| 165 | Novelle S, Fava C, Lausi P, Cardinale L, Brizzi M, Gollini P, et al. Extrapulmonary findings in a low-dose spiral CT scan early detection study for lung cancer in a high risk population. Lung Cancer 2005;49:S184–5 | Design |
| 166 | O’Grady TJ, Kitahara CM, DiRienzo AG, Boscoe FP, Gates MA. Randomization to screening for prostate, lung, colorectal and ovarian cancers and thyroid cancer incidence in two large cancer screening trials. PLOS ONE 2014;9:e106880. https://doi.org/10.1371/journal.pone.0106880 | Design |
| 167 | Ohmatsu H, Kaneko M, Kakinuma R, Moriyama N, Kusumoto M, Eguchi K, et al. Lung cancer screening using low-dose helical CT over 10 years. Lung Cancer 2005;49:S20. https//doi.org/10.1016/s0169-5002(05)80182-5 | Design |
| 168 | Ollier M, Chamoux A, Naughton G, Pereira B, Dutheil F. Chest CT scan screening for lung cancer in asbestos occupational exposure: a systematic review and meta-analysis. Chest 2014;145:1339–46 | Design |
| 169 | Lopes Pegna A, Picozzi G. Lung cancer screening update. Curr Opin Pulm Med 2009;15:327–33. https://doi.org/10.1097/MCP.0b013e32832b98be | Design |
| 170 | Pinsky P, Freedman M, Oken M, Kvale P, Caporaso N, Gohagan J. Prevalence of non-cancer-related abnormalities on low-dose spiral computer tomography versus chest radiograph in a screening population. Thorax 2007;62:190 | Design |
| 171 | Pinsky PF, Kramer BS. Lung Cancer Risk and Demographic Characteristics of Current 20-29 Pack-year Smokers: Implications for Screening. J Natl Cancer Inst 2015;107:djv226. https://doi.org/10.1093/jnci/djv226 | Design |
| 172 | Puggina A, Broumas A, Ricciardi W, Boccia S. Cost-effectiveness of screening for lung cancer with low-dose computed tomography: a systematic literature review. Eur J Public Health 2016;26:168–75. https://doi.org/10.1093/eurpub/ckv158 | Design |
| 173 | Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer 2016;16:281. https://doi.org/10.1186/s12885-016-2316-z | Design |
| 174 | Raymakers AJ, Whitehurst DG, FtizGerald JM, Lam S, Mayo J, Lynd L. Cost-effectiveness analyses of lung cancer screening strategies using low dose computed tomography – a systematic review. Value in Health 2015;18:A46 | Design |
| 175 | Raymakers AJN, Mayo J, Lam S, FitzGerald JM, Whitehurst DGT, Lynd LD. Cost-effectiveness analyses of lung cancer screening strategies using low-dose Computed Tomography: a systematic review. Appl Health Econ Health Policy 2016;14:409–18. https://doi.org/10.1007/s40258-016-0226-5 | Design |
| 176 | Riola-Parada C, García-Cañamaque L, Pérez-Dueñas V, Garcerant-Tafur M, Carreras-Delgado JL. Simultaneous PET/MRI vs PET/CT in oncology. A systematic review. Rev Esp Med Nucl Imagen Mol 2016;35:306–12. https://doi.org/10.1016/j.remn.2016.06.001 | Design |
| 177 | Roberts H, Walker-Dilks C, Sivjee K, Ung Y, Yasufuku K, Hey A, et al. Screening high-risk populations for lung cancer: guideline recommendations. J Thorac Oncol 2013;8:1232–7. https://doi.org/10.1097/JTO.0b013e31829fd3d5 | Design |
| 178 | Roberts HC, Patsios D, Paul NS, McGregor M, Weisbrod G, Chung T, et al. Lung cancer screening with low-dose computed tomography: Canadian experience. Can Assoc Radiol J 2007;58:225–35 | Design |
| 179 | Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging 2011;11:S79–84. https://doi.org/10.1102/1470-7330.2011.9020 | Design |
| 180 | Ruano-Ravina A, Pérez RM, Fernandez-Villar A. Lung cancer screening with low-dose computed tomography after the national lung screening trial. The debate is still open. Archivos de Bronconeumologia 2013;49:158–65 | Design |
| 181 | Saba L, Caddeo G, Mallarini G. Computer-aided detection of pulmonary nodules in computed tomography: analysis and review of the literature (structured abstract). Journal of Computer Assisted Tomography 2007;31:611–19 | Design |
| 182 | Sagawa M. Lung cancer screening in Japan. J Thorac Oncol 2012;7(Suppl. 5):S444. | Design |
| 183 | Sagawa M, Nakayama T, Tanaka M, Sakuma T, Sobue T, JECS Study Group. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of < 30 pack-years aged 50-64 years (JECS study): research design. Jpn J Clin Oncol 2012;42:1219–21. https://doi.org/10.1093/jjco/hys157 | Design |
| 184 | Saghir Z, Jacobs C, Ginneken B, Bruijne M, Dirksen A, Pedersen J. Probability of malignancy based on automatic segmentation and software measurements of nodules in the Danish Lung Cancer Screening Trial (DLCST). European Respiratory Journal 2012;40 | Design |
| 185 | Scagliotti GV, Borasio P, Dogliotti L, Fava C, Cortese G, Crida B, et al. Low-dose spiral CT for early diagnosis of lung cancer in a high risk population: 2-year results. Annals of Oncology 2004;15:29 | Design |
| 186 | Scheibler F, Zumbé P, Janssen I, Viebahn M, Schröer-Günther M, Grosselfinger R, et al. Randomized controlled trials on PET: a systematic review of topics, design, and quality. J Nucl Med 2012;53:1016–25. https://doi.org/10.2967/jnumed.111.101089 | Design |
| 187 | Schröer-Günther MA, Wolff RF, Westwood ME, Scheibler FJ, Schürmann C, Baumert BG, et al. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Syst Rev 2012;1:62. https://doi.org/10.1186/2046-4053-1-62 | Design |
| 188 | Seki N, Eguchi K, Kaneko M, Ohmatsu H, Kakinuma R, Matsui E, et al. Stage-size relationship in long-term repeated CT screening for lung cancer: anti-lung cancer association project. J Clin Oncol 2009;1:1540 | Design |
| 189 | Seki N, Eguchi K, Kaneko M, Ohmatsu H, Kakinuma R, Matsui E, et al. What size tumors should we detect as early-stage lung cancers in CT screening? Stage-size relationship in long-term repeated screening over 15 years. J Clin Oncol 2010;28(Suppl. 1) | Design |
| 190 | Shang W, Zhang H, Yang S, Zhang W, Huo S, Liu Y. Role of low-dose spiral CT scan in lung cancer screening: a meta-analysis (provisional abstract). Journal of Xi’an Jiaotong University 2011;32:38–42 | Design |
| 191 | Silvestri GA. Screening for lung cancer: it works, but does it really work? Ann Intern Med 2011;155:537–9. https://doi.org/10.7326/0003-4819-155-8-201110180-00364 | Design |
| 192 | Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer 2012;77:58–63. https://doi.org/10.1016/j.lungcan.2012.02.019 | Design |
| 193 | Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, et al. CT findings of early-stage small cell lung cancer in a low-dose CT screening programme. Lung Cancer 2007;56:207–15 | Design |
| 194 | Sone S, Nakayama T, Honda T, Tsushima K, Li F, Haniuda M, et al. Long-term follow-up study of a population-based 1996–1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329–41 | Design |
| 195 | Song J, Zhang A. Screening for lung cancer. Clin J Oncol Nurs 2014;18:601. https://doi.org/10.1188/14.CJON.601 | Design |
| 196 | Sox HC. Better evidence about screening for lung cancer. N Engl J Med 2011;365:455–7. https://doi.org/10.1056/NEJMe1103776 | Design |
| 197 | Stanbrook MB. ACP Journal Club. Low-dose computed tomography was associated with higher risk for false-positive lung cancer screening than chest radiography. Ann Intern Med 2010;153:JC2–5. https://doi.org/10.7326/0003-4819-153-4-201008170-02005 | Design |
| 198 | Sullivan DR, Pappas M, Humphrey LL, Slatore CG. Populations and individuals: a systematic review of the psychosocial impact of lung cancer screening with computed tomography on patients. Am J Res Crit Car Med 2014;189 | Design |
| 199 | Swensen SJ, Jett JR, Hartman TE, Midthun DE, Sloan JA, Sykes AM, et al. Lung cancer screening with CT: Mayo Clinic experience. Radiology 2003;226:756–61. https://doi.org/10.1148/radiol.2263020036 | Design |
| 200 | Tanner NT, Silvestri GA. Lung cancer screening with low-dose CT: benefits and potential risks. Evid Based Med 2013;18:108–9. https://doi.org/10.1136/eb-2012-100926 | Design |
| 201 | Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial. Implications for widespread implementation. Am J Respir Crit Care Med 2015;192:200–8. https://doi.org/10.1164/rccm.201502-0259OC | Design |
| 202 | Taylor WF, Fontana RS. Biometric design of the Mayo Lung Project for early detection and localization of bronchogenic carcinoma. Cancer 1972;30:1344–7 | Design |
| 203 | Taylor WF, Fontana RS. Biometric design of the Mayo Lung Project for early detection and localization of bronchogenic carcinoma. Cancer 1972;30:1344–7 | Design |
| 204 | ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol Biomarkers Prev 2015;24:154–61. https://doi.org/10.1158/1055-9965.EPI-14-0745 | Design |
| 205 | Tockman MS, Mulshine JL. Sputum screening by quantitative microscopy: a new dawn for detection of lung cancer? Mayo Clin Proc 1997;72:788–90 | Design |
| 206 | Toyoda Y, Nakayama T, Kusunoki Y, Iso H, Suzuki T. Sensitivity and specificity of lung cancer screening using chest low-dose computed tomography. Br J Cancer 2008;98:1602–7. https://doi.org/10.1038/sj.bjc.6604351 | Design |
| 207 | Treglia G, Giovannini E, Di Franco D, Calcagni ML, Rufini V, Picchio M, Giordano A. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: a systematic review. Ann Nucl Med 2012;26:451–61. https://doi.org/10.1007/s12149-012-0602-7 | Design |
| 208 | Treglia G, Sadeghi R, Annunziata S, Lococo F, Cafarotti S, Bertagna F, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis (provisional abstract). Academic Radiology 2014;21:11–20 | Design |
| 209 | Ung YC, Maziak DE, Vanderveen JA, Smith CA, Gulenchyn K, Lacchetti C, et al. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 2007;99:1753–67 | Design |
| 210 | Usman AM, Miller J, Peirson L, Fitzpatrick-Lewis D, Kenny M, Sherifali, D, Raina P. Screening for lung cancer: a systematic review and meta-analysis. Preventive Medicine 2016;89:301–14 | Design |
| 211 | Valente IR, Cortez PC, Neto EC, Soares JM, de Albuquerque VH, Tavares JM. Automatic 3D pulmonary nodule detection in CT images: a survey. Comput Methods Programs Biomed 2016;124:91–107. https://doi.org/10.1016/j.cmpb.2015.10.006 | Design |
| 212 | van der Aalst CM, van Iersel CA, van Klaveren RJ, Frenken FJ, Fracheboud J, Otto SJ, et al. Generalisability of the results of the Dutch-Belgian randomised controlled lung cancer CT screening trial (NELSON): does self-selection play a role? Lung Cancer 2012;77:51–7. https://doi.org/10.1016/j.lungcan.2012.02.021 | Design |
| 213 | van Klaveren RJ. Screening for lung cancer with low-dose spiral CT: the CONs. Lung Cancer 2004;45:S5 | Design |
| 214 | van’t Westeinde SC, de Koning HJ, Thunnissen FB, Oudkerk M, Groen HJM, Lammers JWJ, et al. The role of the (18)F-fluorodeoxyglucose-positron emission tomography scan in the Nederlands Leuvens Longkanker Screenings Onderzoek lung cancer screening trial. J Thorac Oncol 2011;6:1704–12 | Design |
| 215 | Veronesi G, Bellomi M, Scanagatta P, Preda L, Rampinelli C, Guarize J, et al. Difficulties encountered managing nodules detected during a computed tomography lung cancer screening program. J Thorac Cardiovasc Surg 2008;136:611–17. https://doi.org/10.1016/j.jtcvs.2008.02.082 | Design |
| 216 | Veronesi GB, Bellomi M, Spaggiari L, Pelosi G, Maisonneuve P, Paganelli G, et al. Low dose spiral computed tomography for early diagnosis of lung cancer. Results of baseline screening in 5,000 high-risk volunteers. J Clin Oncol 2006;24:371S | Design |
| 217 | Veronesi G, Bellomi M, Maisonneuve P, Pelosi G, Casiraghi M, Rampinelli C, Spaggiari L. Assessment of overdiagnosis in CT screening for lung cancer. J Clin Oncol 2010;28(Suppl. 1) | Design |
| 218 | Vidal SS, Mendez AL. CT screening for lung cancer: systematic review (provisional abstract). Medicina Clinica 2007;129:582–7 | Design |
| 219 | Walter SD, Kubik A, Parkin DM, Reissigova J, Adamec M, Khlat M. The natural history of lung cancer estimated from the results of a randomized trial of screening. Cancer Causes Control 1992;3:115–23 | Design |
| 220 | Wang CL, Tsai YH, Cheung YC, Ni YL, Feng PH, Yang PC, Chang GC. Low-dose spiral CT screen for lung cancer in patients with high family risk. J Thorac Oncol 2009;4:S512–3 | Design |
| 221 | Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956–61. https://doi.org/10.1164/rccm.200802-336OC | Design |
| 222 | Woo LE, Hwan LJ, Ryeol CD, Soo KS, Gik LY. Lung cancer screening with low dose spiral CT and autofluorescence bronchoscopy in high risk group. EJC Supplements 2009;7:517 | Design |
| 223 | Xu DM, Gietema H, de Koning H, Vernhout R, Nackaerts K, Prokop M, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer 2006;54:177–84 | Design |
| 224 | Xu G, Zhao L, He Z. Performance of whole-body PET/CT for the detection of distant malignancies in various cancers: a systematic review and meta-analysis. J Nucl Med 2012;53:1847–54. https://doi.org/10.2967/jnumed.112.105049 | Design |
| 225 | Yao YW, Yuan DM, Lü YL, Li YF, Song Y. [Screening of early lung cancer with low-dose computed tomography in high-risk populations: a meta-analysis.] Zhonghua Yi Xue Za Zhi 2011;91:2819–23 | Design |
| 226 | Yao YW, Yuan DM, Lv YL, Li YF, Song Y. Screening for early lung cancer detection with lowdose computed tomography in high-risk people: a systematic review and meta-analysis. Respirology 2011;16:154 | Design |
| 227 | Yip R, Islami F, Zhao S, Tao M, Yankelevitz DF, Boffetta P. Errors in systematic reviews: an example of computed tomography screening for lung cancer. Eur J Cancer Prev 2014;23:43–8. https://doi.org/10.1097/CEJ.0b013e3283616290 | Design |
Appendix 4 Clinical effectiveness outcome data
Characteristics of study populations
| Study identifier | Arm | Country and number of centres | Number of patients approached | Number of patients randomised | Number of patients screened at baseline (n/N, %) | Characteristics of patients at baseline | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median age (years), (range) | Male, n/N (%) | Current smokers, n/N (%) | Former smokers, n/N (%) | Family history of LC n/N (%) | ||||||
| DANTE61 | LDCT | Italy, 3 centres | 2811 | 2811 (1403 vs. 1408) | 1276 | 64.3 (64.0–64.7) | 1276/1276 (100) | 714/1276 (56) | 562/1276 (44) | |
| Control | 1196 | 64.6 (64.3–64.9) | 1196/1196 (100) | 681/1196 (56.9) | 515/1196 (43.1) | |||||
| DLCST63 | LDCT | Denmark, 1 centre | 561 | 4104 | 2052 | 57.9 ± 4.8 (49–71) | 1147/2052 (55.9) | 1545/2052 (75.3) | 507/2052 (24.7) | |
| Control | 2052 | 57.8 ± 4.8 (49–71) | 1120/2052 (54.6) | 1579/2052 (76.9) | 473/2052 (23.1) | |||||
| Garg et al. 200264 | LDCT | USA, 1 centre | 304 | 239 | 92 (55 high risk, 37 medium risk) | 68.1 ± 6.2 (high risk) 63.3 ± 6.6 (medium risk) | 185/190 | NR | NR | NR |
| Control | 98 (47 high risk, 51 medium risk) | 67.4 ± 8.2 (high risk) 62.1 ± 7.6 (medium risk) | NR | NR | NR | |||||
| ITALUNG72 | LDCT | Italy, 3 centres (urban) | 71,232 letters were sent. There were 17,055 (23.9%) responders | 3206 |
Recruited: 55–69 years 55–59 years, n = 734 60–65 years, n = 580 > 65 years, n = 299 |
1035/1613 (32.28) | 432/1406 (13.47) | 146/1406 (4.55) | ||
| Control |
Recruited: 55–69 years 55–59 years, n = 670 60–65 years, n = 626 > 65 years, n = 297 |
1039/1593 (32.41) | 406/1593 (12.66) | 148/1593 (4.62) | ||||||
| Lung SEARCH67 | LDCT | UK, 10 centres (urban) | NR | 785 | > 90% of screened subjects provided sputum in year 1 | 63 (mean age) | (52) | (56) | (44) | |
| Control | 783 | |||||||||
| LUSI68 | LDCT | Germany, (NR, but 5 study areas) | 292,440 | 4052 | 2029 |
50–54 years, n = 942 55–59 years, n = 518 60–64 years, n = 344 65–69 years, n = 225 |
1315/2029 (64.8) | 1259/2029 (62.1) | 770/2029 (37.9) | NR |
| Control | 2023 |
50–54 years, n = 932 55–59 years, n = 528 60–64 years, n = 341 65–69 years, n = 222 |
1307/2023 (64.6) | 1247/2023 (64.6) | 775/2023 (38.3) | NR | ||||
| MILD55 | LDCT (annual) | Italy, 1 centre | 4099 | 1190 | 1190 | 57 | 814/1190 (68.4) | 820/1190 (68.9) | 370/1190 (31.1) | |
| LDCT (biannual) | 1186 | 1186 | 58 | 813/1186 (68.5) | 810/1186 (68.3) | 376/1186 (31.7) | ||||
| Control | 1723 | 1723 | 57 | 1090/1723 (63.3) | 1546/1723 (89.7) | 177/1723 (10.3) | ||||
| NELSON57 | LDCT | The Netherlands and Belgium, 4 centres | 606,409 | 15,822 | 7915 | 58.0 (IQR 54.0–62.0) | 6328/7582 (83.5) | 4215/7582 (55.6) | 3367/7582 (44.4) | |
| Control | 7907 | 57.0 (IQR 8.0) | 6275/7453 (84.2%) | 4077/7434 (54.8) | 3357/7434 (45.2) | 377/7396 (4.7) | ||||
| UKLS55 | LDCT | UK, 2 centres | 247,354 sent questionnaire; 8729 eligible | 2028 | 1994 | 67 (67.1 ± 4.1) | 1529/2028 (75.4) | 777/2028 (38.3) | 1249/2028 (61.6) | 498/2028 (24.6) |
| Control | 2027 | 2027 | 67 (66.9 ± 4.1) | 1507/2027 (74.3) | 791/2027 (39.0) | 1236/2027 (61.0) | 554/2027 (27.3) | |||
| Study identifier | Arm | Country and number of centres | Number of patients approached | Number of patients randomised | Number of patients screened at baseline (n/N, %) | Characteristics of patients at baseline | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median age (years), (range) | Male, n/N (%) | Current smokers, n/N (%) | Former smokers, n/N (%) | Family history of lung canerr, n/N (%) | ||||||
| Depiscan62 | LDCT | France, 14 centres | 830 | 765 | 385 | 56 (47–75) | 274/385 (71) | 238/385 (65) | 129/385 (35) | NR |
| CXR | 380 | 56 (47–76) | 267/380 (70) | 224/380 (64) | 127/380 (36) | NR | ||||
| LSS-PLCO66 | LDCT | USA, 6 centres | 653,417 mailed; 12,270 contacted; 4828 eligible | 3318 | 1660 |
50–59 years, n = 616 60–64 years, n = 514 65–69 years, n = 337 70–74 years, n = 193 |
965/1660 (58.1) | 961/1660 (57.9) | 699/1660 (42.1) | NR |
| CXR | 1658 |
50–59 years, n = 624 60–64 years, n = 500 65–69 years, n = 3448 70–74 years, n = 186 |
965/1658 (59.0) | 947/1658 (57.1) | 711/1658 (42.9) | NR | ||||
| NLST71 | LDCT | USA, 33 centres | NR | 53,454 | 26,722 |
< 55 years, n = 2 55–59 years, n = 11,440 60–64 years, n = 8170 65–69 years, n = 4756 70–74 years, n = 2353 > 74 years, n = 1 |
15,770/26,722 (59.0) | 12,862/26,722 (48.1) | 13,860/26,722 (51.8) | 5815/26,723 (21.8) |
| CXR | 26,722 |
< 55 years, n = 4 55–59 years, n = 11,420 60–64 years, n = 8198 65–69 years, n = 4762 70–74 years, n = 2345 > 74 years, n = 3 Missing, n = 1 |
15,762/26,732 (59.0) | 12,900/26,722 (48.3) | 13,832/26,732 (51.7) | 5806/26,733 (21.7)a | ||||
Characteristics of recruitment and adherence
| Study identifier | Method of recruitment | Definition of high-risk individuals at baseline | Exclusion criteria | Initial adherence to screening |
|---|---|---|---|---|
| DANTE61 | Via family doctors, large-scale mailings, media, internet, hospital boards and leaflets. Only males recruited | Aged 60–74 years; current or former smokers (≥ 20 pack-years; quit < 10 years before recruitment) | Other disease with < 5 years’ life expectancy; < 5 years’ disease-free laryngeal and non-melanoma skin cancer; treatment of other cancer in the last 10 years; unable to engage with follow-up protocol |
Did not provide consent (post randomisation): 91/1403 vs. 166/1408 Non-adherence to baseline screening: 97% (1264/1300) vs. 96% (1186/1232) Proportion attending all five CT scans (of those with a baseline scan): 93% (1184/1264) |
| Depiscan62 | Via family and occupational doctors (selection and enrolment); information was provided, consent obtained and randomisation performed across two study appointments. Males and females recruited | Aged 50–75 years; current or former smokers (≥ 15 cigarettes per day for ≥ 20 years; quit < 15 years before recruitment) | History of other cancer; disease that would hinder or prevent thoracic surgery or diagnostic procedure, including pulmonary infections; congestive heart failure/recent myocardial infarction; heavy exposure to asbestos; prior disease that may look radiologically similar to lung cancer; current symptoms | Non-adherence to baseline screening: 144 (19%) across both arms, significantly lower in the CT arm (55/385 vs. 89/380) and in older participants |
| DLCST63 | Via local and regional media (free newspapers). Males and females recruited | Aged 50–70 years; current or former smokers (≥ 20 pack-years; quit at > 50 years of age and < 10 years before recruitment) | Other disease with < 10 years’ life expectancy; history of treatment for lung or breast cancer, malignant melanoma, or hypernephroma; disease-free < 5 years for other cancers and < 2 years for tuberculosis; CT scan ≤ 1 year ago; body weight > 130 kg; current symptoms; FEV2 of ≤ 30% of normal; not able to climb 36 steps without stopping | Non-adherence to baseline screening: low in both arms, higher in the CT arm (5/2052 vs. 0/2052). Mean participation rates across all study time-points: significantly higher in the CT arm (95.5% vs. 93.0%) |
| Garg et al. 200264 | Via medical centre for veterans and associated clinics. Mostly males recruited |
Aged 50–80 years; current or former smokers (≥ 30 pack-years) High-risk group also had airflow obstruction diagnosed in a sputum cytology cohort study. Moderate-risk group were randomly selected but met above risk criteria |
Other disease with < 6 months’ life expectancy; thoracic CT scan ≤ 3 years ago; pregnancy; not able to provide consent or engage with follow-up protocol Moderate-risk group only: symptomatic COPD; airflow obstruction; non-compliance with inhalers |
Adherence not reported |
| ITALUNG65 | Via letter from family doctors. Males and females recruited | Aged 55–69 years; current or former smokers (≥ 20 pack-years; quit < 10 years before recruitment) | History of other cancer (except non-melanoma skin cancer); unable to engage with follow-up protocol involving thoracic surgery | Adherence to baseline screening: 87% (1406/1613). Proportion attending four CT scans: 79% |
| LSS-PLCO66 | Via large-scale mailings, clinician recommendations, media adverts and posters. Males and females recruited | Aged 50–74 years; current or former smokers (≥ 30 pack-years; quit < 10 years before recruitment) | History of lung cancer; current treatment for other cancer (except non-melanoma skin cancer); thoracic or lung CT scan ≤ 2 years ago; previous lung resection; participation in other cancer trials (except smoking cessation) |
Adherence to baseline screening: higher in CT arm, 96% (1586/1660) vs. 93% (1550/1658) Proportion attending at 1 year: higher in CT arm, 85.8% vs. 79.9%; adherence significantly lower in those with positive screens at baseline |
| LungSEARCH67 | Via family doctors and hospital clinics. Males and females recruited | Current or former smokers (≥ 20 pack-years; smoked ≥ 20 years; quit < 8 years before recruitment); COPD | No history of cancer | Adherence to baseline screening not reported |
| LUSI68 | Via large-scale mailings to participants identified through population registers in the local area. Males and females recruited | Aged 50–69 years; current or former smokers (≥ 15 cigarettes per day for ≥ 25 years or ≥ 10 cigarettes per day for ≥ 30 years; quit < 10 years before recruitment) | Other disease with < 10 years’ life expectancy; cancer diagnosis ≤ 5 years ago, unable to engage with surgical treatment | Adherence to baseline screening: high (99.9%) in both arms 2028/2029 vs. 2022/2023, and similar in both arms across five screening rounds |
| MILD69 | Via media (newspaper, television) adverts. Males and females recruited | Aged ≥ 49 years; current or former smokers (≥ 20 pack-years; quit < 10 years before recruitment) | History of cancer ≤ 5 years ago |
Proportion attending at ≥ 1 CT scan: 97% in both screening groups (1149/1186 biennial; 1152/1190 annual) Proportion of participants adhering over the study: 96.1% annual 95.1% biennial |
| NELSON56,57 | Via population registries across two countries (the Netherlands and Belgium). Males only to start with, recruitment later expanded to females | Aged 50–75 years; current or former smokers (≥ 15 cigarettes per day for ≥ 25 years or ≥ 10 cigarettes per day for ≥ 30 years; quit < 10 years before recruitment) | Lung cancer diagnosis < 5 years ago or ≥ 5 years ago with current treatment; history of melanoma, hypernephroma, renal or breast cancer; history of other cancers (unless curatively treated > 5 years ago without recurrence); pneumonectomy; thoracic CT scan < 1 year ago; body weight ≥ 140 kg; moderate/bad health (self-report) and not able to climb two flights of stairs |
Adherence to first screening round: 95.5% (7557/7915) Proportion attending at ≥ 1 CT scan: 95.8% (7582/7915) |
| NLST70,71 | Via targeted mailings, media adverts (local radio and newspapers, television, websites, internet adverts), health fairs, unions, local branches of the American Cancer Society, and community groups. Recruitment included strategies to improve access to the study for minority groups. Males and females recruited | Aged 55–74 years; current or former smokers (≥ 30 pack-years; quit < 15 years before recruitment) | History of lung cancer; haemoptysis; thoracic CT scan < 18 months ago; unexplained weight loss (> 6.8 kg in last year) | Adherence to first screening round: high across both arms 98% (52,344/53,439), 98.5% (26,309/26,715) in the CT arm vs. 97.4% (26,035/26,724) in the control arm |
| UKLS55 | Via letter, sent by a data management company on behalf of the recipient’s PCT. Letter recipients of the correct age, living in six PCTs around Liverpool and Cambridgeshire, were randomly selected using NHS PCT records. Males and females recruited | Aged 50–75 years; using the LLPv2 risk prediction model, ≥ 5% 5-year risk of lung cancer | Other disease that would prevent screening or lung cancer treatment; thoracic CT scan < 1 year ago; not able to lie flat; not able to provide consent | Adherence to baseline screening: 98.3% (1994/2028) |
Characteristics of screening programmes
| Study identifier (country) | Screening programme comparison | Definition of a positive scan for lung cancer | Imaging evaluation and interpretation strategy | Diagnostic follow-up for suspicious abnormality finding |
|---|---|---|---|---|
| DANTE (Italy)61 |
LDCT (and baseline CXR and sputum cytology testing) vs. No screening (and baseline CXR and sputum cytology testing) |
LDCT Positive if one or more of the following was evident: ≥ 10-mm diameter non-calcified nodule, non-calcified nodule with speculated margins, hilar mass, focal ground glass opacities, major atelectasis, endobronchial lesions, mediastinal adenopathy, pleural effusion or mass Baseline CXR Positive if one or more of the following evident: non-calcified shadow, hilar mass, enlarged mediastinum, pleural effusion/thickening or lytic bone lesion |
LDCT Whole lungs scanned at full inspiration (following single breath hold) Independent double-reading of images by experienced chest radiologists. Decision based on consensus (local co-ordinator arbitrated disagreements) Baseline CXR Read by radiologists who were blind to the CT scan results |
LDCT Flexible follow-up protocol (guidance only) Step 1 (2–4 weeks): course of oral antibiotics followed by high-resolution CT scan Step 2 (dependent on results of step 1): |
| Depiscan (France)62 |
LDCT vs. CXR |
LDCT Positive (requiring follow-up) if non-calcified nodules evident CXR Positive (requiring follow-up) if non-calcified nodules evident |
LDCT Whole lungs scanned at full inspiration (following single breath-hold) Independent double-reading of images by radiologists. Decision based on consensus CXR Not reported |
LDCT Follow-up protocol (recommended)CXR Follow-up protocol |
| DLCST (Denmark)63 |
LDCT (and PFT and < 5-minute cessation counselling) vs. No screening (and PFT and < 5 minute cessation counselling) |
LDCT Positive if one or more of the following was evident: ≥ 5 mm diameter (note that nodules ≥ 5 mm but ≤ 15 mm indeterminate); nodules increasing in volume by 25%; nodules with suspicious morphology |
LDCT Whole ribcage and upper abdomen at full inspiration Read by two experienced chest radiologists. Decision based on consensus |
LDCT Follow-up protocol implemented after referral (decided by pulmonologist and radiologist) |
| Garg et al. (USA)64 |
LDCT vs. No screening |
LDCT Positive (requiring follow-up) if between one and six non-calcified nodules evident |
LDCT 30-cm scan of lungs and diaphragm inspiration at inspiration (during two breath-holds performed after hyperventilation) Read by one experienced chest radiologist; some systematically selected scans also read by a second experienced chest radiologist |
LDCT Follow-up protocol |
| ITALUNG (Italy)65 |
LDCT (and invitation to smoking prevention programme) vs. No screening (and invitation to smoking prevention programme) |
LDCT Positive if one or more of the following was evident: ≥ 5-mm diameter non-calcified nodule, ≥ 10-mm non-solid nodule, part-solid nodule, nodules increasing by ≥ 1-mm mean diameter, increase in solid part of a nodule from one scan to the next, several nodules indicative of inflammatory disease |
LDCT Independent double-reading of images by experienced radiologists. Decision based on consensus |
LDCT Follow-up protocol at each centre for positive LDCT scansIf FNAB indicated lung cancer, a staging CT scan was performed |
| LSS-PLCO (USA)66 |
LDCT vs. CXR |
LDCT Positive at T0 if one or more of the following was evident: ≥ 4 mm diameter non-calcified nodule, any other abnormality considered suspicious by radiologist After T0 the criteria changed so that results were considered positive if one or more of the following was evident: ≥ 4 mm diameter non-calcified nodule, ≤ 3 mm diameter spiculated nodule, focal parenchymal opacities, endobronchial lesions, other abnormality considered suspicious by radiologist CXR Positive if one or more of the following was evident (list not exhaustive): any nodule or mass, infiltrate/consolidation, alveolar opacity, enlargement of hilar or mediastinal lymph nodes (not calcified), lung/lobe collapse or closure |
LDCT Read by one radiologist; some scans also independently read by a second radiologist CXR Single, posteroanterior view CXR |
LDCT Follow-up (referral) for positive screening results were conducted on request of patients, according to clinic recommendations (i.e. no specific follow-up protocol and details at each centre not reported). Data on diagnoses were collected CXR As with LDCT |
| LungSEARCH (UK)67 |
LDCT (and sputum surveillance and AFB) vs. No screening (and exit CXR at 5 years) |
LDCT Positive if ≥ 9-mm abnormal nodule |
LDCT Not reported |
LDCT Follow-up of positive LDCT (or AFB) decided by clinicians, but includes an enhanced screening protocol (with more frequent AFB) for pre-invasive lesions |
| LUSI (Germany)68 |
LDCT (and cessation counselling) vs. No screening (and cessation counselling) |
LDCT Positive if one or more of the following was evident: new nodules ≥ 5 mm, nodules with VDT ≤ 600 days |
LDCT Read by radiologists, with special training for the study |
LDCT Follow-up protocol for positive LDCT results |
| MILD (Italy)69 |
LDCT – annual (and cessation programme, pulmonary function test and blood sample) vs. LDCT – biennial (and cessation programme, pulmonary function test and blood sample) vs. No screening (and cessation programme, pulmonary function test and blood sample) |
LDCT Positive if one or more of the following was evident: nodules of ≥ 60 mm3, i.e. approximately 5 mm diameter (note that nodules of ≥ 60 mm3 but ≤ 250 mm3 indeterminate), hilar/mediastinal lymphadenomegaly (non-calcified), atelectasis, consolidation, other indicative pleural findings, nodules increasing in volume by 25% in 3 months |
LDCT Whole lungs scanned at deep inspiration (following single breath hold, no use of contrast) Independent double-reading of images by trained radiologists (third radiologist arbitrated disagreements) |
LDCT Follow-up protocol for positive LDCT results |
| NELSON (the Netherlands and Belgium)56,57 |
LDCT vs. No screening |
LDCT Positive if one or more of the following was evident: solid nodules (or solid component) of ≤ 50 mm3, i.e. 4.6 mm diameter (note that nodules of ≥ 50 but ≤ 500 mm3 indeterminate), non-solid nodule of > 8 mm diameter, VDT of ≤ 600 days (≥ 400 days but ≤ 600 days indeterminate) |
LDCT Scan from posterior recess to apex of the lung, no use of contrast Independent double-reading of images by experienced radiologists, except the last two rounds (read by a single, experienced radiologist) |
LDCT Follow-up protocol for positive LDCT results |
| NLST (USA)70,71 |
LDCT vs. CXR |
LDCT Positive if one or more of the following was evident: non-calcified nodule ≥ 4 mm diameter, other abnormalities could be classified as positive or suspicious CXR Positive if any non-calcified nodule or mass was evident |
LDCT Read by experienced radiologists, images also compared with previous LDCT screens CXR Single-view posteroanterior X-rays, read by experienced radiologists |
LDCT Follow-up protocol for positive LDCT results (guidelines only, details not reported), radiologists could use discretion and make recommendations for diagnostic follow-up |
| UKLS (UK)55 |
LDCT vs. No screening |
LDCT Probably benign (‘negative’ with regard test accuracy; but requiring follow-up) if one or more of the following was evident: solid nodule of 15–49 mm3, solid or non-solid nodule of 3–4.9 mm diameter, part-solid nodule with solid component < 15 mm3 or < 3 mm diameter Positive (potentially malignant) if one or more of the following was evident: solid intraparenchymal nodule of 50–500 mm3, solid pleural/juxtapleural nodule of 5–9.9-mm diameter, non-solid or part-solid nodule with ground glass component of > 5 mm Positive (more likely malignant) if one or more of the following was evident: solid intraparenchymal nodule or ground glass component of non-solid or part-solid nodule of > 500mm3, solid pleural/juxtapleural nodule or ground glass component of non-solid or part-solid nodule of ≥ 10 mm, growth on follow-up CT |
LDCT Scan from lung apices to bases, suspended inspiration (following single breath hold), no use of contrast Double-reading of images by experienced chest radiologists. Decision based on consensus (third radiologist arbitrated disagreements) |
LDCT Follow-up protocol for LDCT results |
Lung cancer mortality and all-cause mortality data
| Study identifier | Comparator | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | All-cause | ||||||||
| Usual care | Number of events in the LDCT group | Total number of participants in the LDCT group | Number of events in the control group | Total number of participants in the control group | Number of events in the LDCT group | Total number of participants in the LDCT group | Number of events in the control group | Total number of participants in the control group | |
| DANTE78 | Usual care | 59 | 1264 | 55 | 1186 | 180 | 1264 | 176 | 1186 |
| DLCST79 | Usual care | 39 | 2052 | 38 | 2052 | 165 | 2052 | 163 | 2052 |
| MILD (annual)69 | Usual care | 12 | 1190 | 7 | 1723 | 31 | 1190 | 20 | 1723 |
| MILD (biannual)69 | Usual care | 6 | 1186 | 20 | 1186 | ||||
| NLST70 | CXR | 356 | 26,722 | 443 | 26732 | 1877 | 26,722 | 2000 | 26,732 |
Evidence from single study arms: health-related quality of life and psychological consequences
NEderlands Leuvens Longkanker Screenings ONderzoer
The NELSON trial84 evaluated longitudinal trends comparing indeterminate with negative screening results. These participants received four questionnaires at four time points: before randomisation (T0), 1 week before the baseline screening (T1), 1 day after the screening (T2) and 2 months after the screening results but before the 3-month follow-up CT (T3). At the time point of T3, there was a clinically relevant increase in the measure of lung-cancer-specific distress (the IES scores) but with a significant decrease in this measure among participants with a negative result. The results from this trial showed that trial participants receiving an indeterminate result experienced increased level of lung-cancer-specific distress compared with those who receiving a negative baseline screening result. However, these results were less robust as they were not based on randomised evidence.
Another NELSON trial85 reported that there was a temporary increase in lung-cancer-specific distress (the IES scores) after receiving an indeterminate baseline result at time point of T1 (mean score 7.8, 95% CI 6.5 to 9.0) compared with time point of T0 (mean score 4.0, 95% CI 2.8 to 5.3). 85 It should be noted that unfavourable short-term effects due to an indeterminate baseline screening result had resolved over long-term follow-up. The findings from this NELSON trial85 also showed that there was no significant impact of an indeterminate result at the second screening round on patients’ QoL measure at long-term follow-up.
The NELSON trials84,85 reported that generic anxiety scores (STAI-6) (mean score of 34.1 at baseline), which was measured in a subsample of 324 participants, were comparable to the Dutch general population. The results showed that STAI-6 scores were found to differ significantly over time between different rounds (p < 0.01). The mean score of STAI-6 was 34.1 measured at one day before screening, 32.7 measured 1 week after screening and 34.3 measured 6 months after screening. Furthermore, the NELSON trial also showed that waiting for the CT scan results was reported to be discomforting by approximately half of the participants. 84 Among a total of 351 participants who had an appointment for a baseline CT scan and were asked to complete questionnaires regarding their experienced discomfort, approximately half reported discomfort in connection with having to wait for the results of the CT scan and expressed feeling of anxiety at waiting for those results. Therefore, minimising waiting time for the screening test results is recommended.
National Lung Screening Trial
The NLST74 assessed the impact of lung cancer screening results (abnormal findings) on patients’ HRQoL among a total of 2812 participants. These participants were asked to complete the SF-36 to assess the effect of screening with LDCT or CXR in the short (1 month) and long term (6 months). False positives were defined as those who were lung cancer free at 1 year, whereas those with true-positive results were not. Of the total participants, there were 1024 (36.4%) participants receiving false-positive results, 63 (2.2%) receiving true-positive results, and 1381 (49.1%) receiving negative results.
The results from NLST74 showed that HRQoL measures at short- and long-term follow-up did not differ significantly between participants with false-positive and negative screens; participants receiving a false-positive screen result did not experience significant difference in HRQoL measures at the 1-month follow-up or at the 6-month follow-up compared with those receiving a negative result.
However, NLST74 showed that there were lower scores in HRQoL for participants receiving true-positive results at both short-term (1 month) and long-term (6 months) follow-up, compared with those participants receiving false-positive results and negative screening results. At 1 month post CT scan, the mean SF-36 physical component measure was 44.50 for those with true positive results, 47.7 for those with false-positive results and 47.6 for those with negative results. At 6 months post CT scan, the mean SF-36 physical component measure was 38.3 for those with true-positive results, 47.1 for those with false-positive results and 47.9 for those with negative results.
A similar pattern was also observed in SF-36 mental component score in the NLST. 74 At 1 month post CT scan, the mean SF-36 mental component measure was 44.1 for those with true-positive results 50.6 for those with false-positive results and 51.3 for those with negative results. At 6 months post CT scan, the mean SF-36 mental component measure was 46.3 for those with true-positive results, 50.4 for those with false-positive results and 51.4 for those with negative results.
UK Lung Cancer Screening Trial
The UKLS55 found statistically significant differences in T1 lung cancer distress among groups with different test results. Participants who were positive for MDT referral (major lung abnormality) were significantly more distressed than each of the other result groups [negative mean difference 0.36 (p < 0.001), positive for repeat scan mean difference 0.24 (p < 0.001)]. Lung cancer distress scores for the MDT group approached the thresholds of clinical significance. Participants who were positive for a repeat scan reported significantly greater T1 cancer distress than those receiving a normal (negative) result (mean difference 0.12; p < 0.001) in the UKLS. 55
In terms of T1 general anxiety, participants who were referred to MDT reported significantly greater anxiety than those receiving any other result [negative mean difference 0.36 (p < 0.001), positive for repeat scan mean difference 0.31 (p = 0.003)]. It should be noted that their scores were within the low/normal range. There were no statistically significant differences in depression scores between any of the screening result groups.
Summary of the evidence from single study arms
The evidence from single study arms showed that among participants randomised to LDCT, HRQoL or psychological consequences for participants receiving different test results may differ at both short- and long-term follow-up.
It is tempting to extrapolate these findings to suggest that HRQoL and psychological consequences might be greater in LDCT. However, this is inconsistent with direct evidence from the randomised comparisons that, as reported in the earlier section, show no difference between LDCT and no screening/CXR screening. The evidence from these randomised comparisons is less open to bias and includes all patients (as opposed to screening arm results subgroups, some of which were very small). So our conclusion remains that there are no differences between LDCT and no screening/CXR screening and were not modified by the evidence from the screening arms of the trials analysed in isolation.
Appendix 5 Network meta-analysis
Characteristics of study populations
| Study identifier | Arm | Country; number of centres | Number of patients approached | Number of patients randomised | Number of patients screened at baseline | Characteristics of patients at baseline | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Male, n/N (%) | Current smokers, n/N (%) | Former smokers, n/N (%) | Family history of LC, n/N (%) | ||||||
| Czech105 | CXR | Czech Republic; six districts | 6364 | 6346 | 3172 |
40–44: n = 487 45–49: n = 716 50–54: n = 923 55–59: n = 582 60–64: n = 464 |
3172/3172 (100) | 3172/3172 (100) | NR | NR |
| Control | 3174 |
40–44: n = 499 45–49: n = 710 50–54: n = 926 55–59: n = 584 60–64: n = 455 |
3174/3174 (100) | 3174/3174 (100) | NR | NR | ||||
| Mayo106 | CXR | USA; NR | NR | 10,933 screened; 9211 randomised | 4618 |
< 50: n = 1159 50 to < 55: n = 1102 55 to < 60: n = 1042 60 to < 65: n = 811 65 to < 70: n = 483 ≥ 70: n = 21 |
4618/4618 (100) | NR/NR (90) | NR/NR (10) | NR |
| Control | 4593 |
< 50: n = 1154 50 to < 55: n = 1135 55 to < 60: n = 1019 60 to < 65: n = 784 65 to < 70: n = 469 ≥ 70: n = 32 |
4593/4593 (100) | NR | ||||||
| PLCO (for sensitivity analysis only)107 | CXR | USA; 10 centres | 154,901 | 77,445 | 15,183 | NR | 9252/15,183 (60.9) | 6146 (40.5) | NR | NR |
| Control | 77,456 | 15,138 | NR | 9110/15,138 (60.2) | 6069/15,138 (40.1)a | NR | NR | |||
Characteristics of recruitment and adherence
| Study identifier | Method of recruitment | Definition of high-risk individuals at baseline | Exclusion criteria | Initial adherence to screening |
|---|---|---|---|---|
| Czech105 | Via general health examination of middle-aged males only | Aged 40–64 years; current smokers (approximate lifetime consumption > 150,000 cigarettes) | History of pulmonary disease. Likely inability to participate over 3 years due to serious disease or other reasons | Adherence to screening over 3 years in screening arm 92.5% vs. control arm 94.7% |
| Mayo106 |
Via ‘smoking survey’ completed by outpatients at a general medical examinations by the Mayo Clinic If questionnaire categorised as ‘high-risk’ males only were referred to the study |
Aged > 45 years; current or former smokers (at least one pack per day at time of recruitment or within previous year) | History of known or suspected cancer of the respiratory tract (except roentgenographically occult cancer); < 5 years’ life expectancy; unable to tolerate pulmonary resection; failure to complete general medical examination; insufficient mental capacity for study cooperation | Adherence to testing schedule over 6 years of screening averaged 75% |
| PLCO (for sensitivity analysis only)107 | Via mass mailing of general population. A subset of entire PLCO population in line with population characteristics of NLST were used for this analysis. Males and females recruited | Aged 55–74 years; current or former smokers (≥ 30 pack-years; quit < 15 years before recruitment) | History of prostate, lung, colorectal or ovarian cancer, or current cancer treatment or removal of one lung |
Adherence to baseline screening, screening arm 85.9% (13,035/15,183) Overall adherence to expected screens, screening arm 81.4% (48,330/15,183) |
Characteristics of screening programmes
| Study identifier(country) | Screening programme comparison | Definition of a positive scan for lung cancer | Imaging evaluation and interpretation strategy | Diagnostic follow-up for suspicious abnormality finding |
|---|---|---|---|---|
| Kubík et al. (Czech Republic)105 |
CXR (at baseline, 6-monthly during years 1–3, and then at years 3, 4 and 5 and 6, screening also included sputum cytology testing) vs. No screening (+ CXR at baseline, years 3, 4, 5 and 6, included sputum cytology testing at same times as CXR) |
CXR Positive if abnormality identified (reader decision whether or not further investigation was required) Other Also sent for further investigation if one or more of the following was evident: patient approached with symptoms, cancer or atypical cells from sputum testing, bloody sputum |
CXR Chest photofluorogram, posteroanterior view Double-reading by chest physician and chest radiologist. Decision based on consensus (third experienced physician arbitrated disagreements) |
CXR Follow-up protocol Positive CXR – referral to specialist diagnostic hospital ward (if sputum signs – recommendation for inpatient stay), fibre-optic animation, additional CXR, (including whole-lung CXR), otorhinolaryngological examination (for exclusion purposes) |
| Mayo (USA)106 |
CXR (4-monthly, screening also included sputum cytology testing, medical history review) vs. Usual care (annual CXR and sputum cytology testing) |
CXR Not clear |
CXR Stereo chest roentgenograms, standard size Double-reading by chest physician and radiologist. Decision based on consensus (another chest physician arbitrated disagreements) |
CXR Follow-up protocol Positive CXR, suggesting lung cancer – review of clinical data Positive CXR, new or growing abnormality – work-up could include additional CXR and sputum testing, bronchoscopy (with or without fluoroscopic guidance) |
| PLCO (USA)107 |
CXR (at baseline, annually up to 4 years) vs. No screening |
CXR Positive if the readers felt that one of the following was evident and suspicious: any nodule, mass, infiltrate or other abnormality |
CXR Posteroanterior CXR |
CXR No study follow-up protocol, positive CXR follow-up was decided by patients and their health-care providers |
Appendix 6 Overview of systematic reviews on clinical effectiveness
Usman Ali et al. 206 found that LDCT was more effective for reducing mortality (both lung-cancer-specific and all-cause mortality) than CXR (with or without smoking cessation) in one large RCT (NLST), whereas three smaller RCTs showed no benefit of screening in reducing mortality. However, LDCT screening was also found to be associated with important harms including overdiagnosis, false positives and consequences of false-positive screening results. Invasive follow-up procedures performed as a result of positive screening tests were associated with deaths and patients experiencing major complications. This reviews’ overall findings are similar to other systematic reviews evaluating the benefits of lung cancer screening (see Manser et al. 52 and Humphrey et al. 207).
Bach208 considered evidence from 21 studies (8 RCTs and 13 cohort trials) comparing LDCT with no screening/usual care and CXR for individuals at an elevated risk of developing lung cancer because of age and smoking history. One large RCT (NLST) showed a significant reduction in mortality using LDCT compared with CXR over 6.5 years of follow-up, with no benefit on mortality observed in two smaller RCTs comparing LDCT with usual care (DANTE61 and DLST). The authors208 concluded that LDCT screening can lead to harm. They report that screening identified a relatively high percentage of individuals with nodules (average of ≈20%), the vast majority of which are benign. The authors highlight the radiation exposure associated with additional imaging of these nodules. They also highlight the variability in the rates of surgical biopsy as well as surgical procedures performed for benign disease, although they also state that complications in those with benign lesions were rare.
Black et al. 54 performed a systematic review to assess effectiveness of CT screening for lung cancer in a mass population screening programme. The review’s narrative synthesis consisted of 12 studies of lung cancer CT screening, two of which were RCTs. Both RCTs were pilot studies and had too short a follow-up period (1 year) to assess lung cancer mortality and total mortality. None of the 12 studies reported disease-specific or total mortality compared with no screening and only one study provided survival data, leading the authors to conclude they were unable to assess whether or not CT screening for lung cancer is clinically effective in reducing mortality. Observational studies provided data on the screening process, the natural history of the detected nodules and survival. The authors concluded that their review found evidence that CT detects a greater number of non-calcified nodules and other suspicious chest abnormalities than screening with CXR, and that smaller non-calcified nodules are detected by CT than by CXR. Among the detected tumours, a high proportion were stage I (both baseline and incidence) or resectable. CT screening was associated with high false-positive rates. Reporting of adverse events, psychological effects of receiving false-positive test results or effects on QoL were universally poor across studies. The authors reported that receiving positive CT examinations were associated with a higher motivation to quit smoking.
It is likely that this systematic review by Black et al. 209 is linked to the systematic review by Black et al. 54 As above, this review reported that none of the 12 studies reported disease-specific or total mortality compared with no screening and the two pilot RCTs were of too short duration (1 year) to draw firm conclusions about the clinical effectiveness of CT screening for lung cancer in terms of a reduction in mortality. The authors reported that the proportion of people with abnormal CT findings varied between studies, as did the prevalence of lung cancer detected. The authors reported that incidence of lung cancer was lower with screening and that among the detected tumours, a high proportion were stage I or resectable tumours.
Chang et al. 210 performed a systematic review of seven studies (including two RCTs with short follow-up periods) to summarise findings on the early detection of lung cancer and the diagnostic accuracy of FDG-PET in high-risk individuals. The authors reported limited information regarding whole-body scanning using PET for lung cancer screening. The authors reported high sensitivity for the detection of T1 lung cancers, with detection being lower for carcinoid tumours, adenocarcinoma and bronchoalveolar cell carcinomas and concluded that a combination of FDG-PET and LDCT may improve screening for lung cancer in high-risk patients. The authors acknowledged and highlighted limitations in data pertaining to survival benefits from PET screening in high-risk individuals.
Chien et al. 211 provided a summary of evidence regarding the role of PET specifically for lung cancer screening. Identified papers were placed in two categories: studies that reported findings in lung cancer CT screening programmes with selective PET, and studies focusing on primary PET screening for cancer. The systematic review found high diagnostic performance (sensitivity and specificity) of selective PET screening, while the detection rate of lung cancer using primary PET was low (0.18%). The authors concluded that selective PET could be used as a selective screening modality (i.e. for diagnosing lung cancer in patients with a pulmonary nodule found using CT screening). None of included studies evaluated the efficacy of primary PET screening specific to lung cancer.
Coureau et al. 212 summarised evidence from 10 RCTs (completed and ongoing) on the impact of lung cancer screening by LDCT in populations highly exposed to tobacco. The review aimed to evaluate effectiveness and the disadvantages and risks associated with screening. Two of the 10 identified trials were pilot studies, one had recently started, five were ongoing and two had published main results. The authors noted the inconsistency in choice of control arm across trials and methods employed (e.g. trial participants, the definition of a positive screen and indeterminate nodules, number of screening rounds and follow-up durations differed between trials). Of the five trials that published results on mortality, only one (NLST) reported a significant 16% decrease in disease-specific mortality (with 6.5 years of median follow-up) and a 7% decrease in all-cause mortality with LDCT screening when compared with CXR screening. DANTE61 provided non-significant results in favour of LDCT screening. Two trials observed a higher mortality rate for subjects undergoing LDCT screening (significant for MILD69 and non-significant for DLCST63). The authors reported that harms of screening (e.g. secondary effects of false-positive results in the short term, psychological distress and the effect of repeated irradiation in the long term) were rarely reported, were inconsistent and that overdiagnosis was rarely addressed and used non-standardised calculation methods. The authors concluded that despite the positive effect on mortality found in NLST, no study identified in the review provides all elements necessary to document the risk–benefit balance.
Fu et al. 213 presented a systematic review and meta-analysis of nine RCTs comparing LDCT and CXR or usual care (no screening) in individuals at high risk of lung cancer (aged > 49 years and who had been exposed to smoking). The results from the review show LDCT screening is associated with detection of a significantly higher number of stage I lung cancers (OR 2.15, 95% CI 1.88 to 2.47) and a higher number of total lung cancers (OR 1.31, 95% CI 1.20 to 1.43) than the control. These results are generally similar to the meta-analysis of Gopal et al.,214 which showed that LDCT is more sensitive in discovering more stage I lung cancers and all cancers than CXR or no screening. The review findings, similar to Humphrey et al. ,207 also showed that LDCT screening could reduce lung-cancer specific mortality (OR 0.84, 95% CI 0.74 to 0.96) but not all-cause mortality (OR 0.96, 95% CI 0.90 to 1.02) compared with control. The meta-analysis by Fu et al. 213 indicated that screening with LDCT is associated with higher false-positive rates than the control.
Gopal et al. 214 presented findings from a meta-analysis for six RCTs of LDCT screening for lung cancer in a high-risk population. The authors reported a significantly higher number of stage I lung cancers, a higher number of total NSCLCs and higher total lung cancers. They also report that screening resulted in increased detection of false-positive nodules and an increased number of unnecessary thoracotomies for benign lesions. The authors concluded that their analysis offers no compelling evidence in favour of LDCT screening for lung cancer.
Humphrey et al. 207 performed a systematic review of RCTs and cohort studies in high-risk current/former smokers. The authors restricted their analysis of effectiveness of LDCT screening to for RCTs. The authors reported that one good-quality large RCT (NLST) was associated with significant reductions in lung cancer (20%) and all-cause (6.7%) mortality. The authors reported that three small European trials (DANTE,61 DLCST63 and MILD69) showed no benefit of screening in reducing mortality. The authors reported harms including radiation exposure, overdiagnosis and a high rate of false-positive findings that typically were resolved with further imaging. They also reported that smoking behaviour was not affected and that incidental findings were common.
Manser et al. 52 identified nine trials investigating LDCT screening, CXR screening or sputum testing to detect lung cancer. The authors reported that the evidence included in their review suggested that screening with annual plain chest radiography screening in smokers and ex-smokers is not effective at reducing lung cancer mortality. The authors identified a large trial (NLST) that showed LDCT screening reduced lung cancer mortality in high-risk current/former smokers when compared with usual care. They conclude that annual LDCT screening is associated with a reduction in lung cancer mortality in high-risk smokers. The authors also concluded that there is no evidence to support early screening for lung cancer with chest radiography or sputum cytology.
Piñeiro et al. 215 report findings of a narrative synthesis of three RCTs and three observational single-arm studies evaluating smoking cessation interventions for patients undergoing LDCT screening. The authors included two RCTs evaluating self-help smoking cessation interventions, and one pilot RCT evaluating the timing (before/after the LDCT scan) of a combined (counselling and pharmacotherapy) smoking cessation intervention. The authors reported that efficacy results across all studies were modest at best and that findings based on non-randomised and pilot studies in the review demonstrate that combined (counselling + pharmacotherapy) smoking cessation interventions can be successfully implemented in screening settings and that they may promote smoking cessation. The authors concluded that the review findings suggested that participation in LDCT screening promotes smoking cessation and may represent a teachable moment to quit smoking.
Seigneurin et al. 216 aimed to identify the LDCT technique associated with low recall rates, a low number of invasive procedures and high positive predictive values without substantially decreasing detection rate. They conducted a systematic review and meta-regression analysis of 10 RCTs and six observational studies to determine whether or not some characteristics of LDCT lung cancer screening programmes such as the number of readers, the use of a cut-off size to define the positive nodules that required further assessment and the use of volumetric assessment software could modify the operating characteristics (recall rates, detection rates and positive predictive values). Only lung cancer screening programmes based on slices that were < 5 mm thick were included because of the progression towards the standard use of multidetector CT scanners worldwide. The authors reported that the results of their meta-analysis highlighted the value of cut-off size at prevalent screens and confirm the relevance of the 5-mm value commonly used in LDCT screening programmes. They reported that volumetry software analysis at incident screens seems to decrease recall rates without a significant decrease in detection rates, whereas the number of readers did not have a great influence. The authors suggested the presence of PET in the work-up protocol may be associated with lower rates of surgical procedures for benign findings.
Slatore et al. 217 conducted a narrative systematic review of five RCTs (detailing DLCST,63 NELSON56,57 trials) and one cohort study (PLuSS) evaluating psychosocial consequences among asymptomatic adults at high risk of lung cancer undergoing LDCT lung cancer screening. The authors reported an association between lung cancer screening with LDCT and short-term psychological discomfort in many individuals, but did not find an association of LDCT screening with distress, worry or HRQoL. False-positive results were found to be associated with short-term increases in distress that returned to levels that were similar to those among people with negative results. Negative results were associated with short-term decreases in distress. The authors concluded that clinicians may want to consider tailoring communication strategies that can decrease the distress associated with these results.
Slatore et al. 217 conducted a narrative synthesis of two RCTs (DLCST63 and NELSON56,57 trials) and three cohort studies (ELCAP, Mayo CT and PLuSS trials) to evaluate the effect of LDCT screening on smoking abstinence. Overall, the authors reported that the results from the included RCTs suggested that LDCT screening itself does not influence smoking behaviours and that evidence from these trials and cohort studies suggest that participants who received positive results relating to lung cancer had higher abstinence rates than those with scans without such findings. The authors reported that this association between positive screening results and abstinence may have a dose–response relationship in terms of the number of abnormal CT scans as well as the seriousness of the finding. The authors concluded that clinicians should consider tailoring LDCT result communication in order to maximise the potential for smoking behaviour change and long-term abstinence.
In the systematic review and meta-analysis by Wang et al. ,218 the authors reported results showing that > 70% of NSCLC patients whose cancer was detected using CT were at pathological stage I, and that there is a tendency for this proportion to increase as screening continues. The authors reported that, relative to CXR screening and usual care, the proportion of stage I cancers detected using CT is higher by > 12% and 45%, respectively. The authors stated that almost all types of NSCLC can increasingly be detected at an early stage in a CT rather than a CXR screening, but acknowledge that evidence is lacking regarding this advantage in SCLC patients.
Wu et al. 192 performed a narrative synthesis of 13 studies, 10 of which were derived from three large RCTS assessing efficacy of lung cancer screening (NELSON,56,57 NLST71 and DLCST63) in order to evaluate the evidence pertaining to psychological burden associated with lung cancer screening. Three studies reported psychological outcomes in smaller cohorts who underwent lung cancer screening (PLuSS, asbestos workers, individuals with lung cancer family history). The authors reported that, taken collectively, the evidence suggests that lung cancer screening has the potential to cause short-term (< 6 months after screen) psychological burden in individuals with an indeterminate scan result but that effects do not appear to persist long term (> 6 months after screen). The authors concluded that lung cancer screening might be associated with short-term adverse psychological burden, particularly after a false-positive result, but that these adverse effects diminish over time.
Yau et al. 219 performed a systematic review to assess the operating characteristics (including sensitivity and specificity) associated with baseline LDCT screening for lung cancer. In addition, the stage distribution of LDCT-detected cancers from baseline scans was analysed. The authors reported that their review found that screening with LDCT detected a greater number of cancerous nodules. They also reported that, on average, 80% of lung cancers detected by baseline LDCT screening were categorised as stage I cancers. The authors conclude that, given the operating characteristics at baseline LDCT screening and the relatively high proportion of stage I cancers detected, screening for lung cancer with LDCT may potentially be effective. However, the authors acknowledged that the lack of established trial data for morbidity and mortality available to them preclude a full assessment of LDCT lung cancer screening. The authors concluded that one of the key findings from their review is that the clinical assessment of the false-negative rate is vital to the accurate determination of the operating characteristics of LDCT screening and has not been well reported among published trials.
| Author, year, title (number of included studies) | Participants (P), interventions (I) and comparators (C) | Outcomes | Design | Results | Comment |
|---|---|---|---|---|---|
|
Usman Ali, 2005,206 Screening for lung cancer: a systematic review and meta-analysis (34 RCT studies) |
P: adults, average to high risk of lung cancer, not suspected to have lung cancer I: CXR (with or without SC); LDCT C: no screening, usual care or head-to-head comparison |
Benefits of screening: lung cancer mortality, all-cause mortality, stage at diagnosis, smoking cessation rate, incidental findings Harms of screening and invasive follow-up testing: overdiagnosis, death, major complications or morbidity requiring hospitalisation or medical intervention, false positives and their consequences, negative consequences of incidental findings, anxiety, QoL, infection of bleeding from invasive follow-up testing |
RCTs |
Authors report no benefit of CXR screening (with or without SC) for lung cancer mortality. Three small trials (pooled) found no statistically significant benefits of LDCT for lung cancer mortality and all-cause mortality compared with usual care Authors report that one large trial found statistically significant reductions in lung cancer mortality (20%) for LDCT screening compared with CXR alone over a follow-up of 6.5 years and 15% over 7.4 years. The same trial found statistically significant relative reduction (6%) for all-cause mortality for LDCT over 7.4 years compared with CXR Authors conclude that there is no survival benefit when screening with CXR (with or without SC) but that in selected high-risk individuals, LDCT screening significantly reduced lung cancer mortality and all-cause mortality Authors note that LDCT was associated with overdiagnosis, deaths and major complications in patients undergoing invasive follow-up procedures |
Authors performed a meta-analysis if possible |
|
Bach, 2012,208 Benefits and harms of CT screening for lung cancer: a systematic review (21 studies, 8 RCTs and 13 cohort studies) |
P: people with elevated risk of developing lung cancer due to age and smoking history I: LDCT (screening in one arm of an RCT or non-comparative cohort of LDCT screening) C: NR |
Mortality (lung cancer and all-cause), nodule detection rate, frequency of additional imaging, frequency of invasive diagnostic procedures, complications from the evaluation of suspected lung cancer, and the rate of smoking cessation or reinitiation | RCTs and non-comparative cohort studies |
Authors report that LDCT was associated with a statistically significant reduction in mortality compared with control for one large RCT (NLST), with no benefit on mortality observed in for two smaller RCTs (DANTE61 and DLSCT63). Authors conclude that LDCT screening may benefit individuals at an elevated risk for lung cancer Authors conclude that LDCT screening can lead to harm with 20% diagnosed with nodules, but only 1% had lung cancer. They report marked heterogeneity in this finding and in the frequency of follow-up investigations, biopsies and the per cent of surgical procedures performed in those with benign lesions |
|
|
Black, 2006,54 The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews (12 studies, including 2 RCTs) |
P: no restriction based on age, sex or smoking history or risk status I: CT screening for lung cancer was the main theme of the paper C: no screening |
Lung cancer and all-cause mortality, positive CT examinations, investigations and follow-up, detection of lung cancer (prevalence and incidence, stage, resectability), lung cancer survival, follow-up requirements, QoL, adverse events | RCTs and cohort studies |
Authors report that none of the 12 studies provided data to assess lung-cancer-specific or total mortality compared with no screening and the two RCTs were too short in duration (1 year) to draw firm conclusions about the clinical effectiveness of CT screening Authors conclude there is no evidence that screening with LDCT improves survival or reduces mortality |
|
|
Black, 2007,209 Population screening for lung cancer using computed tomography, is there evidence of clinical effectiveness? A systematic review of the literature (12 studies, including 2 RCTs) |
P: no restriction based on age, sex or smoking history or risk status I: CT screening for lung cancer was the main theme of the paper C: no screening NR |
Lung cancer and all-cause mortality, nodule detection, histology, survival, follow-up requirements, QoL, adverse events | RCTs and cohort studies |
Authors report that none of the 12 studies provided data to assess lung-cancer-specific or total mortality compared with no screening and the two RCTs were too short in duration (1 year) to draw firm conclusions about the clinical effectiveness of CT screening Authors conclude that there is no evidence that screening with LDCT improves survival or reduces mortality |
|
|
Chang, 2013,210 The value of positron emission tomography in early detection of lung cancer in high-risk population: a systematic review [7 studies, 5 original articles (2 RCTs) and 2 SRs] |
P: high-risk patients (current/former smokers, > 50 years, > 20 pack-years smoking history) I: PET or PET + LDCT (PET/CT) C: observation |
Screening or early detection of lung cancer | RCTs, cohorts and case control studies | Authors conclude that PET or PET/CT may be useful for early detection of lung cancer tumours in high-risk individuals. Authors conclude that there is insufficient trial data to determine survival benefits | RCTs had small patient numbers and did not have long enough follow-up duration |
|
Chien, 2013,211 [(18)F]fluorodeoxyglucose-positron emission tomography screening for lung cancer: a systematic review and meta-analysis (12 studies, including 1 RCT) |
P: no restrictions I: studies focusing on PET screening (including PET/CT Screening) C: primary PET studies and selective PET studies |
Detection rates for all types of cancer including lung cancer | All single-arm studies, with exception of one RCT | Authors report that lung cancer screening programmes with selective PET have high sensitivity and specificity and could be used as a selective screening modality (i.e. for diagnosing lung cancer in patients with a pulmonary nodule found using CT screening) | All identified primary PET screening studies were conducted in Far East Asian countries (Japan, Taiwan, and Korea), whereas all the identified selective PET studies were conducted in Europe |
|
Coureau, 2016,212 Low-dose computed tomography screening for lung cancer in populations highly exposed to tobacco: a systematic methodological appraisal of published RCTs (10 RCT studies) |
P: individuals highly exposed to tobacco I: LDCT C: any other intervention |
Mortality, positive LDCTs and cancer incidence, false positives, surgery, complications, QoL, overdiagnosis and cost-effectiveness | RCTs | Authors report that from five RCTs reporting mortality results, only one (NLST) found a significant decrease of disease-specific and all-cause mortality with LDCT screening compared with CXR screening. None of the studies provided all the information needed to document the risk–benefit balance. Authors conclude that LDCT screening should not be recommended in subjects highly exposed to tobacco | |
|
Fu, 2016,213 A meta-analysis: is low-dose computed tomography a superior method for risky lung cancers screening population? (9 RCT studies) |
P: high-risk population for lung cancer. Smoking history > 15 years or ex-smokers with quit year < 10 years, average 50–60 years old and average smoking history 20–30 pack-years I: LDCT C: CXR and no screening |
Number of stage I lung cancers, number of total lung cancers, lung-cancer-specific or all-cause mortality, and false-positive rates | RCTs | Authors report using LDCT to screen for lung cancer (compared with CXR or no screening) in high-risk individuals resulted in a significantly higher number of stage I cancers, total lung cancers and lower lung-cancer-specific mortality. Authors report that LDCT screening does not decrease all-cause mortality and is associated with a higher false-positive rate | Authors present a funnel plot analysis, which showed no symmetry and suggest publication bias towards trials showing positive results for LDCT screening |
|
Gopal, 2010,214 Screening for lung cancer with low-dose computed tomography: a systematic review and meta-analysis of the baseline findings of RCTs (structured abstract) (6 RCT studies) |
P: high-risk population; average 50–60 years old, average smoking history 20–30 pack years I: LDCT C: CXR or no screening |
Number of stage I NSCLC, number of total NSCLC and total lung cancers, false-positive results and unnecessary treatments | RCTs | Authors report significantly higher number of stage I lung cancers, number of total NSCLC and higher total lung cancers. LDCT screening resulted in increased detection of false-positive nodules and number of unnecessary thoracotomies for benign lesions. Authors conclude that there is no compelling evidence in favour of LDCT screening for lung cancer | |
|
Humphrey, 2013,207 Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventative services task force recommendation [20 studies, including 7 RCTs and 13 cohort studies; (analysis limited to 4 RCTs)] |
P: asymptomatic current and former smokers I: LDCT C: CXR and usual care |
Lung cancer mortality and all-cause mortality Outcomes reported by all seven RCTs and cohort studies included radiation exposure, false-positive findings/follow-up evaluation; false-negative findings, overdiagnosis, psychological consequences, smoking behaviour, incidental findings |
RCTs or cohort studies |
Authors report from four RCTs, one good-quality, large RCT (NLST) was associated with significant reductions in lung cancer (20%) and all-cause (6.7%) mortality. Authors report that three smaller RCTs (DANTE61, DLCST63 and MILD69) showed no benefit of screening for reducing mortality Harms included radiation exposure, overdiagnosis and a high rate of false-positive findings that typically were resolved with further imaging. Authors report smoking behaviour was not affected and incidental findings were common |
Authors limited their analysis to four out of seven RCTs that reported results in the intervention and control groups. Authors present forest plots to display mortality findings and performed a narrative synthesis, but did not perform a meta-analysis because of heterogeneity, follow-up intervals and the quality of the trials |
|
Manser, 201352 Screening for lung cancer (9 studies, including 8 RCTs and 1 CCT) |
P: adult smokers, former smokers, non-smokers I: sputum examinations, CXR screening or CT screening C: not specified |
Disease-specific mortality, compliance, lung cancer incidence, 5-year survival, stage at diagnosis, resection rate, postoperative deaths, harms of screening, costs, all-cause mortality, QoL, test performance, smoking behaviour | RCTs and CCTs | Authors report their meta-analysis found no convincing evidence to support screening for lung cancer with chest radiography or sputum cytology. There was evidence of an association between annual LDCT screening and a reduction in lung cancer mortality in high-risk smokers | Authors performed an intention-to-screen meta-analysis |
|
Piñeiro, 2016,215 Smoking cessation interventions within the context of low-dose computed tomography lung cancer screening: a systematic review (6 studies including 3 RCTs) |
P: people undergoing LDCT I: smoking cessation intervention programmes C: not specified |
Reported smoking-related outcomes | RCTs and observational studies | Authors included two RCTs that evaluated self-help smoking cessation interventions, and one pilot RCT evaluating the timing (before/after the LDCT scan) of a combined (counselling and pharmacotherapy) smoking cessation intervention. Authors report efficacy results across all studies were modest at best. Authors report that findings based on non-randomised and pilot studies in the review demonstrate that combined (counselling + pharmacotherapy) smoking cessation interventions can be successfully implemented in screening settings and that they may promote smoking cessation | |
|
Seigneurin, 2014,216 A systematic review of the characteristics associated with recall rates, detection rates and PPVs of computed tomography screening for lung cancer (16 studies including 10 RCTs and 6 observational studies) |
P: smokers (current or former) or general population I: LDCT screening programmes based on slices < 5-mm thick C: not specified |
Number of positive LDCT results, cancers detected, number of surgical procedures for benign findings | RCTs and observational studies | Authors report that the results of their meta-analysis highlight the value of cut-off size at prevalent screens and confirm the relevance of the 5-mm value commonly used in LDCT screening programmes. Volumetry software analysis at incident screens decreased recall rates without a significant decrease in detection rates, whereas the number of readers (1 or ≥ 2) did not have a great influence. The authors suggest that presence of PET in the work-up protocol may be associated with lower rates of surgical procedures for benign findings | Authors carried out a metaregression analysis to relate the recall rate, detection rate and PPV to one or more characteristics of the studies involved |
|
Slatore, 2014217 Smoking behaviours among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventative services task force (5 studies including 2 RCTs and 3 cohort studies) |
P: adult asymptomatic current and former smokers I: LDCT lung cancer screening C: not specified |
Smoking behaviours (cessation; relapse; abstinence) | RCTs and cohort studies | Authors report that LDCT screening itself does not influence smoking behaviours however, participants who received positive lung cancer results had higher abstinence rates than those without. Authors suggest that this association between positive screening results and abstinence may have a dose–response relationship in terms of the number of abnormal CT scans as well as the seriousness of the finding | Authors report that trial results were not able to be pooled because of heterogeneity of outcome measures, hence a narrative synthesis was undertaken |
|
Slatore, 2014,191 Patient-centred outcomes among lung cancer screening recipients with computed tomography: a systematic review (6 studies including 2 RCTs (from 5 citations) and 1 cohort study) |
P: asymptomatic adults at a high risk of lung cancer because of smoking behaviours I: LDCT lung cancer screening C: no screening |
Patient-centred outcomes of QoL, distress and anxiety | RCTs and cohort studies | Authors report that following LDCT lung cancer screening there was an association with short-term psychological discomfort, but no effects for distress, worry or HRQoL. Short-term increases in distress followed false-positive results, which returned to levels similar to those among people with negative results. Negative results were associated with short-term decreases in distress | Authors report that trial results were not able to be pooled because of heterogeneity of outcome measures, hence a narrative synthesis was undertaken |
|
Wang, 2016218 Can CT screening give rise to a beneficial stage shift in lung cancer patients? Systematic review and meta-analysis (24 studies including 8 RCTs and 16 cohort studies) |
P: natural populations (wide geographical distribution) of any age I: CT lung cancer screening C: not specified |
Stage and pathology-specific stage information concerning both lung cancer patients and nodules | RCTs and cohort studies | Authors conclude that CT has superiority over CXR and usual care for detecting a higher proportion of early stage NSCLC, including a number of indolent cancers. Authors acknowledge that evidence is currently lacking for the same beneficial stage shift of the more aggressive SCLCs | Authors performed a meta-analysis. They state that their study focused on the topic of the first ‘detection step’ because of the current paucity of mortality outcome evidence in CT screening |
|
Wu, 2016192 Psychological burden associated with lung cancer screening: a systematic review (13 studies, including 3 RCTs (from 10 citations) and 3 cohort studies) |
P: not specified I: LDCT lung cancer screening C: not specified |
Patient self-report of psychological burden and related outcomes (e.g. HRQoL, psychological distress, depression) | RCTs and cohort studies | Authors report that lung cancer screening did not have substantial long-term (> 6 months) effects on psychological burden, but potential short-term (< 6 months) psychological burden |
Authors report heterogeneity in outcome measures used to capture psychological burden and conducted a narrative synthesis of the evidence Most outcome measures were general rather than condition specific |
|
Yau, 2007219 Systematic review of baseline low-dose CT lung cancer screening (15 studies, including 2 RCTs) |
P: former or current smokers I: LDCT screening C: not specified |
Number of lung cancers found | RCTs an cohort studies | Authors report that their initial assessment of LDCT screening demonstrated good sensitivity and specificity of the technology and a high proportion of early-stage lung cancer detection. However, the authors acknowledge that these findings are derived mainly from observational studies and that there is a lack of long-term mortality data |
Appendix 7 Methods and results of the systematic review of existing cost-effectiveness evidence
Methods
Quality assessment
The following modifications to the CHEC list and guidelines for assessment were used in the quality assessment:
-
General – ‘unclear’ was additionally allowed as a judgement for all items.
-
Study design – an appropriate study design was an economic evaluation principally based on a RCT of lung cancer screening without significant unsupported assumptions.
-
Perspective – health service and third-party payer perspectives were allowed as appropriate perspectives (in addition to a societal perspective), and other perspectives were allowed if appropriately justified.
-
Resource use identification – a full identification of all important and relevant costs required all of the following (note that there are other costs that may be considered important for an economic evaluation in this area, such as the cost of identifying an eligible population):
-
screening scan costs
-
costs of follow-up tests for all positive or indeterminate screening scans
-
costs of diagnosing, staging and treating lung cancer.
-
-
Resource use measurement – ‘lung cancer’ was judged to be an acceptable physical unit.
-
Resource valuation – sources should be clearly given and should be fair approximations of opportunity costs, well-established reference costs and tariffs with minimal inbuilt incentives are acceptable.
-
Outcome identification – since lung cancer is a disease with a significant effect on the quantity and QoL, a full identification of all important and relevant outcomes required all of the following:
-
lung cancer diagnoses
-
lung cancer deaths
-
life-years
-
QALYs.
-
-
Outcome valuation – valuation includes estimation of health state utility values (for calculation of QALYs) or determination of an appropriate cost-effectiveness threshold or willingness to pay for non-QALY outcomes.
-
Incremental analysis – all studies conducted at least a partial incremental analysis as this was an inclusion criterion for the review, so for this quality measure only a fully incremental analysis (including all relevant combinations of compatible interventions, e.g. screening with smoking cessation) was judged to be acceptable.
Results
Characteristics of included studies
| Study author and year of publication | Form of economic evaluation | Location, currency and price year | Population | Intervention(s) | Comparator(s) | Methodology |
|---|---|---|---|---|---|---|
| Marshall, 2001118 | CEA and CUA | USA, 1999 US$ | ‘High-risk’ adults aged 60–74 years | Annual LDCT for 5 years | No screening | Decision tree model |
| Marshall, 2001119 | CEA | USA, 1999 US$ | General smokers aged 60–74 years | Single LDCT screen | No screening | Decision tree model |
| Chirikos, 2002120 | CEA | USA, 2000 US$ | Adult smokers aged 45–74 years | Annual LDCT for 5 years | No screening | Cohort model |
| Mahadevia, 2003121 | CUA | USA, 2001 US$ | 60-year-old heavy smokers (current and former, > 20 pack-years) | Annual LDCT to age 80 years | No screening | Markov model |
| Wisnivesky, 2003122 | CUA | USA, 2000 US$ | Adults aged ≥ 60 years with ≥ 10 pack-year smoking history | Single LDCT screen | No screening | Decision tree model |
| Manser, 2005123 | CEA and CUA | Australia, 2002 AU$ | Male current smokers aged 60–64 years | Annual LDCT for 5 years | No screening | Markov model |
| Whynes, 2008117 | CUA | UK, 2004 GBP | Men aged 61 years at high risk | Single LDCT screen | No screening | Decision tree model |
| McMahon, 2011114 | CUA | USA, 2006 US$ | Current and former smokers ≥ 20 pack-years smoking history |
Annual LDCT screening Smoking cessation |
No intervention | Patient-level microsimulation model |
| Goulart, 2012124 | CEA | USA, 2011 US$ | Those eligible for NLST, i.e. smokers aged 55 to 74 years | LDCT screening (frequency unclear) | No screening | Decision tree model |
| Pyenson, 2012125 | CEA | USA, 2012 US$ | Current and former smokers aged 50 years with ≥ 30 pack-year smoking history | Annual LDCT from age 50–64 years | No screening | Cohort model |
| Shmueli, 2013126 | CUA | Israel, 2011 US$ | Adults aged ≥ 45 years with ≥ 10 pack-year smoking history | Single LDCT screen | No screening | Decision tree |
| Villanti, 2013127 | CUA | USA, 2012 US$ | High-risk adults aged 50 years |
Annual LDCT screening to age 64 years Screening plus smoking cessation |
No screening | Cohort model |
| Black, 2014111 and 2015110 | CEA and CUA | USA, 2009 US$ | NLST cohort (aged 55–74 years with ≥ 30 pack-year smoking history) |
Annual LDCT for 3 years Annual CXR for 3 years |
No screening | Decision tree model |
| Pyenson, 2014128 | CEA | USA, 2014 US$ | Adults aged 55–80 years with ≥ 30 pack-year smoking history | Annual LDCT | No screening | Cohort model |
| Tabata, 2014129 | CEA | Japan, JPY (¥; price year unclear) | Smokers aged 55–74 years | Annual LDCT | Annual CXR | Decision tree model |
| Goffin, 2015112 | CUA | Canada, 2008 CA$ | Smokers aged 55–74 years with ≥ 30 pack-year history |
Annual LDCT for 3 years with smoking cessation Annual LDCT to age 75 years with smoking cessation |
No intervention | Microsimulation model |
| Field, 2016,55 2016116 | CEA and CUA | UK, 2011–12 GBP | Adults aged 50–75 years | Risk prediction followed by single LDCT screen | No intervention | Decision tree model |
| Goffin, 2016113 | CUA | Canada, 2008 CA$ | Smokers aged 55–74 years with ≥ 30 pack-year history | Biennial LDCT screening for 20 years with/without smoking cessation | Annual LDCT screening for 20 years with/without smoking cessation | Microsimulation model |
| ten Haaf, 2017115 | CEA | Canada, 2015 CA$ | Adult smokers (current or former) aged 46–75 | Eligibility criteria and annual or biennial LDCT screening | No screening | Microsimulation model |
| Study author and year of publication | Source of effectiveness estimates | Measurement of health outcomes | Time horizon and discount rate | Base-case findings | Results of sensitivity and scenario analyses |
|---|---|---|---|---|---|
| Marshall, 2001118 | ELCAP (LDCT cohort study, n = 1000) | Life-years, QALYs | 5 years, 3% | LDCT more expensive and more effective than no screening, ICER US$19,533/QALY |
1-year decrease in assumed survival benefit → ICER US$50,783/QALY Also sensitive to incidence of lung cancer and cost of LDCT scan |
| Marshall, 2001119 | ELCAP | Life-years | 5 years, 3% |
LDCT more expensive and more effective than no screening, ICER US$23,100/LYG In ‘very high-risk’ cohort, ICER US$5940/LYG |
Cost-effectiveness improved as prevalence of lung cancer increased Cost-effectiveness worsened if adjusted for lead time bias Cost-effectiveness improved for higher specificity Cost-effectiveness sensitive to cost of CT scans |
| Chirikos, 2002120 | Hypothetical stage shift | Life-years | 15 years, 7.5% | LDCT more expensive and more effective than no screening, ICER US$33,557–90,022/LYG depending on achieved stage distribution |
Proportion diagnosed in earlier stage key factor of cost-effectiveness Higher discount factor leads to small improvement in cost-effectiveness |
| Mahadevia, 2003121 | Hypothetical stage shift | QALYs | 40 years (to age 100), 3% | LDCT more expensive and more effective than no screening, ICER US$116,300/QALY |
Degree of stage shift: 50% → 91% in current smokers results in ICER US$50,000/QALY No stage shift achieves ICER US$50,000/QALY for quitting and former smokers Favourable scenario ICER US$42,500/QALY |
| Wisnivesky, 2003122 | ELCAP | Life-years | Unclear, 3% | LDCT more expensive and more effective than no screening, ICER US$2,500/LYG | Sensitive to cost of LDCT, probability of overdiagnosis and prevalence of lung cancer |
| Manser, 2005123 | Diagnostic performance of LDCT based on ‘weighted averages of six studies’ | Life-years, QALYs | 15 years, 3% | LDCT more expensive and more effective than no screening, ICER AU$57,325/LYG or AU$105,090/QALY | Sensitive to performance of LDCT (proportion diagnosed in stage I, diagnostic performance and mortality impact), cost of LDCT, prevalence and incidence of lung cancer |
| Whynes, 2008117 | ELCAP | QALYs | Unclear (perhaps 40 years), 3.5% | LDCT more expensive and more effective than no screening, ICER £13,910/QALY (for men) |
If testing in women only, ICER £11,710/QALY Low prevalence significantly worsens cost-effectiveness |
| McMahon, 2011114 | Natural history model calibrated to tumour registry data and validated against screening studies | QALYs | Lifetime, 3% |
LDCT more expensive and more effective than no screening ICERs for screening consistently above US$100,000/QALY unless positive impact on smoking cessation included |
Screening dominated by smoking cessation if screening has no impact on quit rates |
| Goulart, 2012124 | NLST (LDCT RCT, n = 53,454) | Lung cancer deaths | Unclear (possibly 1 year), no discounting | LDCT more expensive and more effective than no screening, ICER US$240,000 per lung cancer death avoided | No sensitivity analyses of cost-effectiveness results |
| Pyenson, 2012125 | ELCAP | Life-years | 15 years, no discounting | LDCT more expensive and more effective than no screening, ICER US$18,862/LYG | Scenario analyses produced range of ICERs from US$11,708 to US$26,016/LYG |
| Shmueli, 2013126 | Single-centre Israeli cohort study | QALYs | Lifetime, 3% | LDCT more expensive and more effective than no screening, ICER US$1,464/QALY |
Sensitive to overdiagnosis, PPV of LDCT, cost of stage IV lung cancer, although ICERs ranged from US$155 to $3187/QALY PSA suggests that ICER very likely to be below US$20,000/QALY |
| Villanti, 2013127 | ELCAP and NLST | QALYs | 15 years, no discounting |
LDCT more expensive and more effective than no screening, ICER US$28,240/QALY (ELCAP) or US$47,115/QALY (NLST) Adding smoking cessation nearly doubled QALY gain from screening alone and had lower ICER |
Sensitive to screening costs and resulting stage distribution from screening |
| Black, 2014111 and 2015110 | NLST (assume same outcomes for no screening as CXR) | Life-years, QALYs | Lifetime, 3% |
LDCT screening more costly and more effective than CXR and no screening, ICER (vs. no screening) US$81,000/QALY CXR dominated by no screening |
Sensitive to sex (more cost-effective for women), age, smoking status, risk of lung cancer, cost of LDCT |
| Pyenson, 2014128 | ELCAP | Life-years | 20 years, no discounting | LDCT screening more costly and more effective than no screening, ICER US$18,452/LYG | Sensitive to sex (more cost-effective for women), cost of lung cancer treatment, cost of LDCT, effectiveness of stage shift |
| Tabata, 2014129 | Anti-Lung Cancer Association (ALCA), Japanese case–control study | Life-years | Unclear | LDCT screening more costly and more effective than CXR, ICERs ranging from ¥983,000 to ¥1942/LYG depending on sex and age | Sensitive to sex (more cost-effective for men) and age, the cost of LDCT, screening interval and proportion of cancer diagnosed in early stages |
| Goffin, 2015112 | Natural history model, partially calibrated to NLST | QALYs | 20 years (lifetime), 3% |
LDCT screening more costly and more effective than CXR ICER of triple screen (vs. no screening) CA$74,000/QALY ICER of annual screening (vs. no screening) CA$52,000/QALY ICER of annual screening vs. triple screen [estimated; not reported by authors] CA$21,000/QALY (triple screening extendedly dominated) |
Sensitive to smoking history of participants, impact on smoking and LDCT cost |
| Field, 2016,55 2016116 | UKLS and estimates of lead time | Life-years, QALYs | Lifetime, 3.5% | LDCT screening more costly and more effective than no screening, ICER £8466/QALY | If certain nodules not pursued further, cost-effectiveness is improved |
| Goffin, 2016113 | Natural history model partially calibrated to NLST | QALYs | Lifetime, 3% |
Biennial LDCT screening cheaper and less effective than annual LDCT screening ICER of annual vs. biennial ranged from CA$54,000 to CA$4.8M/QALY |
Sensitive to stage distributions |
| ten Haaf, 2017115 | Natural history model calibrated to NLST | Life-years | Lifetime, 3% |
576 screening scenarios evaluated LDCT screening more expensive and more effective than no screening 11 screening scenarios and no screening on the efficient frontier At CA$50,000/LYG threshold, it is cost-effective to screen annually in 55- to 75-year-olds with ≥ 40 pack-year smoking history (quit ≤ 10 years ago if former smoker), ICER CA$41,136/LYG |
Sensitive to cost of LDCT and smoking criteria |
| Study author and year of publication | Population, interventions and comparators | Eligible study designs | Date range for searches | Key findings |
|---|---|---|---|---|
| Klittich, 2002130 |
Any population Any lung cancer screening |
Costs, CUA | September 1998 to September 2001 | Seven studies included |
| Economic evaluations mostly ad hoc (adjunct to clinical trials) and/or methodologically weak | ||||
| Two studies methodologically robust and evaluated annual screening (one with CXR, one with low-dose CT). Both concluded screening would be cost-effective. One study found results were sensitive to the prevalence of lung cancer | ||||
| Black, 200654 | Population screening using CT | Full economic evaluations, CEA | 1994 to January 2005 | Six studies included |
| Lack of clinical effectiveness data made it difficult to assess suitability of approaches. Reporting was poor | ||||
| Interventions ranged from single CT screens to annual CT screens over 20 years. Four studies focused on high-risk individuals | ||||
| ICER (cost per QALY) ranged from US$19,500 to > US$2M | ||||
| Four studies concluded screening would be cost-effective, one study concluded insufficient evidence, another study concluded screening would not be cost-effective | ||||
| Several limitations were present across all studies | ||||
| CADTH, 2006131,132 | Population lung cancer screening by CTa | Full economic evaluations | 2000 to November 2005 | Five studies included |
| Studies ranged in quality. One study was higher quality than the rest, in which authors concluded screening is unlikely to be cost-effective. Other studies of lower quality concluded screening was likely to be or could be cost-effective | ||||
| Economic evaluations of lung cancer were most hypothetical of studies across all indications reviewed | ||||
| Puggina, 2016133 | LDCT screening for lung cancer in high-risk individuals (in terms of smoking history) | CEA (cost per life-year gained), CUA (cost per QALY) | To March 2015 | Nine studies included |
| Wide variation in results. With threshold of US$50,000 per QALY, five of nine studies found lung cancer screening to be cost-effective, increasing to seven and nine as the threshold is raised to US$100,000 and US$150,000 per QALY, respectively. Two studies found that smoking cessation rates strongly influence the cost-effectiveness of screening | ||||
| Most of the studies failed to include actual clinical evidence and failed to adopt a true societal perspective | ||||
| Raymakers, 2016134 | Lung cancer screening using LDCT | CEA | January 2000 to December 2014 | Thirteen studies included |
| Ten studies focused on high-risk populations. Most studies evaluated annual screening, four evaluated single screen. Four studies included smoking cessation with annual screening | ||||
| Cost-effectiveness estimates varied substantially. Costs per QALY varied from US$28,000 to US$243,000 for repeated screening. Cost per QALY of a single screen estimated in one study as US$1500 per QALY | ||||
| Results were particularly sensitive to the prevalence of lung cancer, cost of CT, achieved stage shift, lead time bias and smoking cessation |
Quality assessment
The results of quality assessment are shown in Table 52.
| Study author and year of publication | Question | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Marshall, 2001118 | N | Y | N | N | N | Y | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | N | N |
| Marshall, 2001119 | N | Y | N | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | N | Y | N | N | N |
| Chirikos, 2002120 | N | Y | N | N | Y | Y | Y | Y | Y | N | Y | N | Y | Y | N | Y | N | N | N |
| Mahadevia, 2003121 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | N |
| Wisnivesky, 2003122 | Y | Y | Y | N | U | Y | Y | Y | N | N | Y | N | Y | Y | N | Y | Y | N | N |
| Manser, 2005123 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | N | U | N |
| Whynes, 2008117 | Y | Y | Y | N | U | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | U | N |
| McMahon, 2011114 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | U | N |
| Goulart, 2012124 | Y | N | N | N | N | Y | Y | Y | N | N | Y | N | Y | N | N | Y | N | Y | N |
| Pyenson, 2012125 | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N | N | U | N | N | N |
| Shmueli, 2013126 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | U | N |
| Villanti, 2013127 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | N | N | N | N |
| Black, 2014111 and 2015110 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | N | N | N |
| Pyenson, 2014128 | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N | N | Y | N | N | N |
| Tabata, 2014129 | Y | Y | Y | N | U | U | U | U | U | N | Y | Y | Y | U | N | Y | N | N | N |
| Goffin, 2015112 | Y | Y | Y | Y | Y | Y | U | U | U | Y | Y | U | N | Y | N | Y | Y | N | N |
| Field, 2016,55 2016116 | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | U | N |
| Goffin, 2016113 | Y | Y | Y | Y | Y | U | U | U | U | Y | Y | U | N | Y | N | Y | N | U | N |
| ten Haaf, 2017115 | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | N | U | N |
Discussion
Relation to existing work
| Citation | Included in reviews | Reason for exclusion |
|---|---|---|
| Beinfeld MT, Wittenberg E, Gazelle GS. Cost-effectiveness of whole-body CT screening. Radiology 2005;234:415–22 | Raymakers134 | Intervention (whole-body CT) |
| Caro JJ, Klittich WS, Strauss G. Could chest X-ray screening for lung cancer be cost-effective? Cancer 2000;89:2502–5 | Klittich130 | Intervention (did not include LDCT) |
| Okamoto N. Cost-effectiveness of lung cancer screening in Japan. Cancer 2000;89:2489–93 | Klittich130; Black54; CADTH131,132; and Raymakers134 | Study design (did not include an incremental analysis) |
| Baba Y, Takahashi M, Tominguchi S, Kiyota S. Cost-effectiveness decision analysis of mass screening for lung cancer. Acad Radiol 1998;5(Suppl. 2):S344–6 | Klittich130 | Intervention (did not include LDCT) |
| Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, et al. Peripheral lung cancer: Screening and detection with low-dose spinal CT versus radiography. Radiology 1996;201:798–802 | Klittich130 | Study design (did not include an incremental analysis) |
| Eddy DM. Screening for lung cancer. Ann Intern Med 1989;111:232–7 | Klittich130 | Intervention (did not include LDCT) |
| Study author and year of publication | Study author and year of publication | ||||
|---|---|---|---|---|---|
| Klittich, 2002130 | Black, 200654 | CADTH, 2006131,132 | Puggina, 2016133 | Raymakers, 2016134 | |
| ten Haaf, 2017115 | |||||
| Goffin, 2016113 | |||||
| Field, 2016,55 2016116 | |||||
| Goffin, 2015112 | |||||
| Black, 2014111 and 2015110 | ✓ | ✓ | |||
| Tabata, 2014129 | |||||
| Pyenson, 2014128 | ✓ | ✓ | |||
| Villanti, 2013127 | ✓ | ✓ | |||
| Shmueli, 2013126 | ✓ | ✓ | |||
| Pyenson, 2012125 | ✓ | ||||
| Goulart, 2012124 | |||||
| McMahon, 2011114 | ✓ | ✓ | |||
| Whynes, 2008117 | |||||
| Manser, 2005123 | ✓ | ✓ | |||
| Wisnivesky, 2003122 | ✓ | ✓ | ✓ | ||
| Mahadevia, 2003121 | ✓ | ✓ | ✓ | ✓ | |
| Chirikos, 2002120 | ✓ | ✓ | ✓ | ||
| Marshall, 2001119 | ✓ | ✓ | ✓ | ✓ | |
| Marshall, 2001118 | ✓ | ✓ | ✓ | ✓ | |
Appendix 8 Details of natural history calibration
Methods
A natural history model was constructed as shown in Figure 22, with exponential time-to-event distributions (i.e. constant hazard) for all events except preclinical incidence of lung cancer, which had a log-normal time-to-event distribution.
This model was represented by 15 states (1, no cancer; 7, preclinical lung cancer; 7, clinical lung cancer) and a time-dependent transition rate matrix Q = qi,j, with the following properties:
-
qi,j is the rate of transitions from state i to state j
-
qi,i is equal to minus the sum of the transition rates qi,j, such that the row sums are all 0.
Of the elements in the matrix, only q1,2 varies with time (this represents the transition from no lung cancer to preclinical lung cancer, determined by a log-normal distribution). q1,2 was estimated as piecewise constant each year of age (x) using the following equation:
where δx = 1 year and:
(i.e. the cumulative hazard function for the log-normal distribution).
To fit to data from NLST, the participants of NLST were stratified into cohorts according to their age (to the nearest year) and each cohort’s membership over time was simulated using a continuous time Markov model approach,220 augmented with transition matrices applied at the time of screening (representing the sensitivity and specificity of screening). The cohort membership was then used as the vector of probabilities for a multinomial distribution, such that the number of cancer diagnoses in each stage for each year of the study (screen detected, interval and post-screening cancers) were the data inputs.
A total of 26,719 participants were included (those randomised to LDCT screening rather than CXR).
To fit to incidence data, a single cohort was modelled from birth using a Markov model approach. An additional parameter was included so that men and women could have a different location parameter for preclinical incidence. The rates of preclinical progression and clinical presentation were fixed to the expected values obtained by fitting to NLST data.
In the scenario analysis in which heterogeneity in the progression rate is included, this was achieved by running the continuous-time Markov model repeatedly with different values of the heterogeneity parameter each time. These values were sampled as evenly spaced quantiles of the distribution, with p = 0.025, 0.075, . . . , 0.975 (i.e. 20 quantile samples), as shown in Figure 37.
FIGURE 37.
Quantiles used to incorporate heterogeneity in progression rates.
The models were built and fitted in JAGS (software for Bayesian MCMC analysis, based on the BUGS language), called from R using the rjags package. Sample code is given in Sample code. Owing to the computational complexity of the model, it was not possible to run with extremely long burn-in periods or thinning, but visual inspection of traces and auto-correlation plots satisfied that convergence had probably been reached and adequate thinning had been conducted. Table 55 gives the setup parameters used in the analyses. Prior distributions for parameters are presented alongside the posterior distributions in Results.
| Analysis | Number of heterogeneity quantiles | Number of chains | Adaptation/burn-in/samples (per chain) | Sample thinning | Total samples |
|---|---|---|---|---|---|
| NLST without heterogeneity | N/A | 6 | 1000/0/5000 | 50 | 600 |
| Incidence without heterogeneity | N/A | 6 | 1000/4000/5000 | 50 | 600 |
| NLST with heterogeneity | 20 | 6 | 1000/0/5000 | 50 | 600 |
| Incidence with heterogeneity | 20 | 6 | 1000/4000/5000 | 50 | 600 |
Results
In this section we present the posterior distributions of parameters in the form of their mean, SD, median and 95% CrI.
Without heterogeneity
| Parameter name | Description | Prior distributiona | Posterior distribution, mean (SD) [median (95% CrI)] |
|---|---|---|---|
| Preclinical incidence | |||
| mu.p0_pIA | Log-normal distribution parameter (location) for preclinical incidence of lung cancer | Normal(3, 0.1) |
4.555 (0.020) [4.554 (4.515 to 4.597)] |
| sigma.p0_pIA | Log-normal distribution parameter (shape) for preclinical incidence of lung cancer | Uniform(0.001, 5) |
0.266 (0.017) [0.266 (0.232 to 0.302)] |
| Preclinical progression | |||
| lnlambda.pIA_pIB | Preclinical progression rate from IA to IB (log-scale) | Normal(0, 0.1) |
0.004 (0.141) [0.019 (–0.304 to 0.261)] |
| lnlambda.pIB_pIIA | Preclinical progression rate from IB to IIA (log-scale) | Normal(0, 0.1) |
1.645 (0.201) [1.643 (1.249 to 2.044)] |
| lnlambda.pIIA_pIIB | Preclinical progression rate from IIA to IIB (log-scale) | Normal(0, 0.1) |
1.801 (0.206) [1.792 (1.407 to 2.206)] |
| lnlambda.pIIB_pIIIA | Preclinical progression rate from IIB to IIIA (log-scale) | Normal(0, 0.1) |
1.626 (0.238) [1.646 (1.155 to 2.111)] |
| lnlambda.pIIIA_pIIIB | Preclinical progression rate from IIIA to IIIB (log-scale) | Normal(0, 0.1) |
1.080 (0.262) [1.090 (0.576 to 1.595)] |
| lnlambda.pIIIB_pIV | Preclinical progression rate from IIIB to IV (log-scale) | Normal(0, 0.1) |
2.080 (0.374) [2.053 (1.314 to 2.879)] |
| Clinical presentation | |||
| lnlambda.pIA_cIA | Clinical presentation rate in Stage IA (log-scale) | Normal(0, 0.1) |
–2.483 (0.175) [–2.469 (–2.802 to –2.111)] |
| lnlambda.pIB_cIB | Clinical presentation rate in Stage IB (log-scale) | Normal(0, 0.1) |
–1.873 (0.261) [–1.877 (–2.365 to –1.313)] |
| lnlambda.pIIA_cIIA | Clinical presentation rate in Stage IIA (log-scale) | Normal(0, 0.1) |
–1.651 (0.276) [–1.634 (–2.187 to –1.164)] |
| lnlambda.pIIB_cIIB | Clinical presentation rate in Stage IIB (log-scale) | Normal(0, 0.1) |
–2.136 (0.322) [–2.152 (–2.693 to –1.503)] |
| lnlambda.pIIIA_cIIIA | Clinical presentation rate in Stage IIIA (log-scale) | Normal(0, 0.1) |
–1.409 (0.272) [–1.418 (–1.930 to –0.866)] |
| lnlambda.pIIIB_cIIIB | Clinical presentation rate in Stage IIIB (log-scale) | Normal(0, 0.1) |
–0.881 (0.385) [–0.884 (–1.625 to –0.072)] |
| lnlambda.pIV_cIV | Clinical presentation rate in Stage IV (log-scale) | Normal(0, 0.1) |
–1.403 (0.196) [–1.415 (–1.780 to –0.993)] |
| Diagnostic performance | |||
| sensitivity | Probability that an individual with preclinical lung cancer (any stage) will be diagnosed with lung cancer as a result of screening | Uniform(0, 1) |
0.706 (0.055) [0.709 (0.578 to 0.806)] |
| specificity | Probability that an individual without preclinical lung cancer will receive a false-positive or indeterminate result from screening | Uniform(0, 1) |
0.777 (0.001) [0.777 (0.775 to 0.800)] |
With heterogeneity
| Parameter name | Description | Prior distributiona | Posterior distribution, mean (SD) [median (95% CrI)] |
|---|---|---|---|
| Preclinical incidence | |||
| mu.p0_pIA | Log-normal distribution parameter (location) for preclinical incidence of lung cancer | Normal(3, 0.1) |
4.550 (0.017) 4.545 (4.520 to 4.584) |
| sigma.p0_pIA | Log-normal distribution parameter (shape) for preclinical incidence of lung cancer | Uniform(0.001, 5) |
0.264 (0.016) 0.263 (0.237 to 0.297) |
| Preclinical progression | |||
| lnlambda.pIA_pIB | Preclinical progression rate from IA to IB (log-scale) | Normal(0, 0.1) |
0.447 (0.351) 0.403 (–0.165 to 1.206) |
| lnlambda.pIB_pIIA | Preclinical progression rate from IB to IIA (log-scale) | Normal(0, 0.1) |
–0.245 (0.502) –0.264 (–1.269 to 0.700) |
| lnlambda.pIIA_pIIB | Preclinical progression rate from IIA to IIB (log-scale) | Normal(0, 0.1) |
–1.260 (0.618) –1.248 (–2.535 to –0.094) |
| lnlambda.pIIB_pIIIA | Preclinical progression rate from IIB to IIIA (log-scale) | Normal(0, 0.1) |
–2.556 (0.775) –2.504 (–4.279 to –1.151) |
| lnlambda.pIIIA_pIIIB | Preclinical progression rate from IIIA to IIIB (log-scale) | Normal(0, 0.1) |
–4.498 (0.969) –4.444 (–6.570 to –2.799) |
| lnlambda.pIIIB_pIV | Preclinical progression rate from IIIB to IV (log-scale) | Normal(0, 0.1) |
–4.233 (1.161) –4.130 (–6.603 to –2.167) |
| sigma.lnlambda.p | Random effects on preclinical progression standard deviation | Exponential(1) |
5.611 (1.027) 5.540 (3.850 to 7.733) |
| Clinical presentation | |||
| lnlambda.pIA_cIA | Clinical presentation rate in Stage IA (log-scale) | Normal(0, 0.1) |
–2.583 (0.142) –2.591 (–2.852 to –2.301) |
| lnlambda.pIB_cIB | Clinical presentation rate in Stage IB (log-scale) | Normal(0, 0.1) |
–2.120 (0.276) –2.122 (–2.636 to –1.571) |
| lnlambda.pIIA_cIIA | Clinical presentation rate in Stage IIA (log-scale) | Normal(0, 0.1) |
–1.876 (0.293) –1.878 (–2.408 to –1.288) |
| lnlambda.pIIB_cIIB | Clinical presentation rate in Stage IIB (log-scale) | Normal(0, 0.1) |
–2.299 (0.314) –2.300 (–2.933 to –1.660) |
| lnlambda.pIIIA_cIIIA | Clinical presentation rate in Stage IIIA (log-scale) | Normal(0, 0.1) |
–1.302 (0.264) –1.296 (–1.810 to –0.776) |
| lnlambda.pIIIB_cIIIB | Clinical presentation rate in Stage IIIB (log-scale) | Normal(0, 0.1) |
–0.518 (0.423) –0.543 (–1.400 to 0.326) |
| lnlambda.pIV_cIV | Clinical presentation rate in Stage IV (log-scale) | Normal(0, 0.1) |
0.084 (0.198) 0.067 (–0.290 to 0.464) |
| Diagnostic performance | |||
| sensitivity | Probability that an individual with preclinical lung cancer (any stage) will be diagnosed with lung cancer as a result of screening | Uniform(0, 1) |
0.961 (0.028) 0.967 (0.895 to 0.999) |
| specificity | Probability that an individual without preclinical lung cancer will receive a false-positive or indeterminate result from screening | Uniform(0, 1) |
0.778 (0.001) 0.778 (0.775 to 0.780) |
Sample code
Appendix 9 Summary of economic model parameters
| Label | Description | Base-case value | PSA |
|---|---|---|---|
| Population | |||
| pop_size | Number of smokers aged 55–80 | 13,000,000 | Not varied |
| p_male | Proportion of those receiving risk prediction who are men | 0.482 | Beta(29393,31609) |
| pop_age_mean | Population mean age | 61.939 | N(61.939,0.048) |
| pop_age_sd | Population standard error | 9.000 | N(8.999,0.062) |
| pop_age_LL | Quantile for lower age limit of whole population and lower boundary of age at entry distribution | 0.220 | Not varied |
| pop_age_UL | Quantile for upper age limit of whole population and upper boundary of age at entry distribution | 0.978 | Not varied |
| Programme uptake | |||
| p_respond | Probability someone responds to the initial invite and returns the questionnaire | 0.307 | Beta(75958,171396) |
| p_join | Probability someone joins screening programme given they are eligible | 0.465 | Beta(4061,4668) |
| Natural history of disease | |||
| mu_AB | Lognormal parameter (location) for pre-clinical incidence of lung cancer | 4.7470 | Multivariate normal 1 |
| delta_mu_AB_F | Coefficient for women for lognormal parameter (location) for pre-clinical incidence of lung cancer | 0.0358 | Multivariate normal 1 |
| sigma_AB | Lognormal parameter (shape) for pre-clinical incidence of lung cancer | 0.3635 | Multivariate normal 1 |
| ln_lambda_pIA_pIB | Log rate of pre-clinical progression from stage Ia to Ib | 0.0035 | Multivariate normal 1 |
| ln_lambda_pIB_pIIA | Log rate of pre-clinical progression from stage Ib to IIa | 1.6451 | Multivariate normal 1 |
| ln_lambda_pIIA_pIIB | Log rate of pre-clinical progression from stage IIa to IIb | 1.8006 | Multivariate normal 1 |
| ln_lambda_pIIB_pIIIA | Log rate of pre-clinical progression from stage IIb to IIIa | 1.6258 | Multivariate normal 1 |
| ln_lambda_pIIIA_pIIIB | Log rate of pre-clinical progression from stage IIIa to IIIb | 1.0797 | Multivariate normal 1 |
| ln_lambda_pIIIB_pIV | Log rate of pre-clinical progression from stage IIIb to IV | 2.0803 | Multivariate normal 1 |
| ln_lambda_pIA_cIA | Log rate of clinical presentation at stage Ia | –2.4828 | Multivariate normal 1 |
| ln_lambda_pIB_cIB | Log rate of clinical presentation at stage Ib | –1.8726 | Multivariate normal 1 |
| ln_lambda_pIIA_cIIA | Log rate of clinical presentation at stage IIa | –1.6507 | Multivariate normal 1 |
| ln_lambda_pIIB_cIIB | Log rate of clinical presentation at stage IIb | –2.1362 | Multivariate normal 1 |
| ln_lambda_pIIIA_cIIIA | Log rate of clinical presentation at stage IIIa | –1.4088 | Multivariate normal 1 |
| ln_lambda_pIIIB_cIIIB | Log rate of clinical presentation at stage IIIb | –0.8811 | Multivariate normal 1 |
| ln_lambda_pIV_cIV | Log rate of clinical presentation at stage IV | –1.4027 | Multivariate normal 1 |
| Survival from diagnosis | |||
| lambda_lcs_Ia | Lambda constant for survival if diagnosed and treated from stage Ia | 0.214 | N(0.214,0.011) |
| lambda_lcs_Ib | Lambda constant for survival if diagnosed and treated from stage Ib | 0.274 | N(0.274,0.014) |
| lambda_lcs_IIa | Lambda constant for survival if diagnosed and treated from stage IIa | 0.330 | N(0.33,0.016) |
| lambda_lcs_IIb | Lambda constant for survival if diagnosed and treated from stage IIb | 0.475 | N(0.475,0.024) |
| lambda_lcs_IIIa | Lambda constant for survival if diagnosed and treated from stage IIIa | 0.588 | N(0.588,0.029) |
| lambda_lcs_IIIb | Lambda constant for survival if diagnosed and treated from stage IIIb | 0.909 | N(0.909,0.045) |
| lambda_lcs_IV | Lambda constant for survival if diagnosed and treated from stage IV | 1.423 | N(1.423,0.071) |
| gamma_lcs_all_stages | Gamma constant for survival if diagnosed and treated at any stage | 0.676 | N(0.676,0.034) |
| lambda_ocm_F | Lambda parameter (Gompertz distribution) for other cause mortality in women | 0.00019 | N(0.000195,0.00001) |
| gamma_ocm_F | Gamma parameter for above | 0.1018 | N(0.102,0.005) |
| lambda_ocm_M | Lambda as above for men | 0.00059 | N(0.00059,0.00003) |
| gamma_ocm_M | Gamma as above for men | 0.0917 | N(0.092,0.005) |
| Risk prediction | |||
| risk_age | Risk prediction coefficient for age (years) | 0.08985 | N(0.090,0.00038) |
| risk_male | Risk prediction coefficient for male sex | 0.30562 | N(0.306,0.005) |
| risk_smoker | Risk prediction coefficient for current/former smoker (vs. never smoker) | 1.45929 | N(1.459,0.005) |
| risk_lungcancer | Risk prediction coefficient for lung cancer (at baseline or within 3 years) | 0.33488 | N(0.335,0.152) |
| risk_intercept | Risk prediction intercept | –11.39758 | N(–11.398,0.024) |
| risk_SD | Risk prediction standard deviation (error term) | 0.62920 | Gamma(25,0.025) |
| Screening effectiveness | |||
| sens_LDCT | Sensitivity of LDCT test for lung cancer | 0.709 | Multivariate normal 1 |
| spec_LDCT | Specificity of LDCT test for lung cancer | 0.624 | Beta(740,445) |
| mu_ind_scrn_delay | Mean time to index screening examination | –2.823 | Multivariate normal 2 |
| sig_ind_scrn_delay | Standard deviation of time to index screening exam | 0.820 | Multivariate normal 2 |
| QoL | |||
| u_base_male | Utility of male smoker in the UK general population/occult lung cancer | 0.7816 | N(0.782,0.012) |
| u_base_female | Utility of female smoker in the UK general population/occult lung cancer | 0.7531 | N(0.753,0.11) |
| u_dis_sII | Disutility of second stage cancer vs. first stage | –0.04 | N(–0.04,0.013) |
| u_dis_sIII | Disutility of third stage cancer vs. first stage | –0.04 | N(–0.04,0.009) |
| u_dis_sIV | Disutility of fourth stage cancer vs. first stage | –0.05 | N(–0.05,0.01) |
| u_dis_fp | Disutility associated with a false-positive screen | –0.063 | N(–0.063,0.028) |
| u_dis_scr_anx | Disutility associated with anxiety of a screening event | –0.010 | N(–0.01,0.007) |
| t_dis_fp | Duration of disutility from false-positive screen | 3.00 | Gamma(4,0.75) |
| t_dis_scrn_anx | Duration of disutility from screening anxiety | 2.00 | Gamma(4,0.5) |
| Costs | |||
| c_invite | Cost of initial invite and questionnaire | £2.90 | Gamma(25,0.116) |
| c_score | Cost of scoring questionnaire and risk stratefication | £18.54 | Gamma(25,0.742) |
| c_letter | Cost of follow-up letter and (if applicable) LDCT appointment | £1.74 | Gamma(25,0.07) |
| c_gp_ref | Cost of GP consultations leading to lung cancer referral | £72.00 | Gamma(25,2.88) |
| c_LDCT | Cost of low-dose CT scan | £98.80 | Gamma(59.126,1.671) |
| c_scrn_nurse | Cost of nurse-led screening consultation | £6.25 | Gamma(25,0.25) |
| c_false_pos | Cost of resourcing following a false-positive screen | £184.63 | Gamma(25,7.385) |
| c_eol_lung | Cost of end-of-life care for lung cancer patient | £4589.04 | Gamma(3.329,1378) |
| c_rdtf_sIa_ini | Cost of initial diagnosis and treatment if dx stage Ia | £5558.14 | Gamma(25,222) |
| c_rdtf_sIb_ini | Cost of initial diagnosis and treatment if dx stage Ib | £6411.63 | Gamma(25,256) |
| c_rdtf_sIIa_ini | Cost of initial diagnosis and treatment if dx stage IIa | £7279.07 | Gamma(25,291) |
| c_rdtf_sIIb_ini | Cost of initial diagnosis and treatment if dx stage IIb | £6558.14 | Gamma(25,262) |
| c_rdtf_sIIIa_ini | Cost of initial diagnosis and treatment if dx stage IIIa | £6511.63 | Gamma(25,260) |
| c_rdtf_sIIIb_ini | Cost of initial diagnosis and treatment if dx stage IIIb | £6046.51 | Gamma(25,242) |
| c_rdtf_sIV_ini | Cost of initial diagnosis and treatment if dx stage IV | £5441.86 | Gamma(25,218) |
| c_rdtf_sIa_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage Ia | £5848.11 | Gamma(25,234) |
| c_rdtf_sIb_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage Ib | £5359.21 | Gamma(25,214) |
| c_rdtf_sIIa_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage IIa | £5637.60 | Gamma(25,226) |
| c_rdtf_sIIb_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage IIb | £6514.78 | Gamma(25,262) |
| c_rdtf_sIIIa_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage IIIa | £5415.46 | Gamma(25,217) |
| c_rdtf_sIIIb_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage IIIb | £4318.07 | Gamma(25,173) |
| c_rdtf_sIV_rem_ind_yr | Cost of index year diagnosis, treatment and follow-up if dx stage IV | £2787.31 | Gamma(25,111) |
| c_rdtf_sIa_subyrs | Cost of treatment and follow-up in subsequent years if dx stage Ia | £1437.79 | Gamma(25,58) |
| c_rdtf_sIb_subyrs | Cost of treatment and follow-up in subsequent years if dx stage Ib | £1483.75 | Gamma(25,59) |
| c_rdtf_sIIa_subyrs | Cost of treatment and follow-up in subsequent years if dx stage IIa | £1628.19 | Gamma(25,65) |
| c_rdtf_sIIb_subyrs | Cost of treatment and follow-up in subsequent years if dx stage IIb | £1647.88 | Gamma(25,66) |
| c_rdtf_sIIIa_subyrs | Cost of treatment and follow-up in subsequent years if dx stage IIIa | £1503.45 | Gamma(25,60) |
| c_rdtf_sIIIb_subyrs | Cost of treatment and follow-up in subsequent years if dx stage IIIb | £1306.49 | Gamma(25,52) |
| c_rdtf_sIV_subyrs | Cost of treatment and follow-up in subsequent years if dx stage IV | £1037.31 | Gamma(25,41) |
Appendix 10 Full listing of base-case cost-effectiveness results
| Strategy | Average costs per patient (£) | Average QALYs per patient |
|---|---|---|
| No screening | 1103 | 8.5021 |
| S–55–80–3% | 1135 | 8.5032 |
| T–55–80–3% | 1151 | 8.5034 |
| A–55–80–3% | 1188 | 8.5029 |
| B–55–80–3% | 1164 | 8.5031 |
| S–55–80–4% | 1128 | 8.5028 |
| T–55–80–4% | 1139 | 8.5031 |
| A–55–80–4% | 1160 | 8.5027 |
| B–55–80–4% | 1145 | 8.5027 |
| S–55–80–5% | 1124 | 8.5026 |
| T–55–80–5% | 1131 | 8.5028 |
| A–55–80–5% | 1144 | 8.5026 |
| B–55–80–5% | 1135 | 8.5026 |
| S–60–80–3% | 1132 | 8.5031 |
| T–60–80–3% | 1148 | 8.5033 |
| A–60–80–3% | 1182 | 8.5029 |
| B–60–80–3% | 1159 | 8.5030 |
| S–60–80–4% | 1125 | 8.5028 |
| T–60–80–4% | 1136 | 8.5031 |
| A–60–80–4% | 1156 | 8.5027 |
| B–60–80–4% | 1142 | 8.5027 |
| S–60–80–5% | 1121 | 8.5026 |
| T–60–80–5% | 1129 | 8.5028 |
| A–60–80–5% | 1141 | 8.5026 |
| B–60–80–5% | 1132 | 8.5025 |
| S–55–75–3% | 1129 | 8.5031 |
| T–55–75–3% | 1142 | 8.5032 |
| A–55–75–3% | 1178 | 8.5028 |
| B–55–75–3% | 1155 | 8.5030 |
| S–55–75–4% | 1123 | 8.5027 |
| T–55–75–4% | 1131 | 8.5029 |
| A–55–75–4% | 1151 | 8.5026 |
| B–55–75–4% | 1138 | 8.5027 |
| S–55–75–5% | 1119 | 8.5025 |
| T–55–75–5% | 1125 | 8.5027 |
| A–55–75–5% | 1137 | 8.5025 |
| B–55–75–5% | 1129 | 8.5025 |
| S–60–75–3% | 1126 | 8.5030 |
| T–60–75–3% | 1139 | 8.5031 |
| A–60–75–3% | 1171 | 8.5028 |
| B–60–75–3% | 1150 | 8.5029 |
| S–60–75–4% | 1120 | 8.5027 |
| T–60–75–4% | 1128 | 8.5029 |
| A–60–75–4% | 1148 | 8.5026 |
| B–60–75–4% | 1135 | 8.5026 |
| S–60–75–5% | 1117 | 8.5025 |
| T–60–75–5% | 1122 | 8.5026 |
| A–60–75–5% | 1134 | 8.5025 |
| B–60–75–5% | 1126 | 8.5025 |
Appendix 11 Thematic analysis of patient and public consultation workshop meetings
Engagement/facilitators
This category included themes relating to participants’ views on strategies most likely to improve lung cancer screening invitation uptake as well as factors or approaches that would potentially have an impact on willingness or capacity to engage with a lung cancer screening programme if offered.
Universal versus targeted approach
While acknowledging that it made sense to target screening to people most at risk of lung cancer (e.g. smokers), there was concern from participants in all three meetings that such an approach would exclude people who were also at (lower) risk of lung cancer (e.g. because of passive smoking or exposure to asbestos). Although most smoking/ex-smoking participants in all three workshops said that they would respond to a targeted invitation to lung cancer screening, some participants said that they would not respond to an invitation to lung cancer screening if it was specifically targeted at smokers/ex-smokers because of associated stigma. They said that they would be more likely to respond to a universal invitation that did not specifically mention smoking and lung cancer, but which offered screening for general lung diseases. A view was expressed that if lung cancer screening was the norm, more people would engage with it. A view was expressed relating to the unreliability of people’s willingness to be honest about their smoking status on health records, and implications for a screening programme targeted at smokers/ex -smokers as opposed to a universal programme based, for example, on age. One participant from the community drop-in session thought that people most at risk of lung cancer should be targeted for screening because of the risk of false positives and the associated anxiety.
Other benefits of screening
It was recognised that during lung cancer screening it was possible that other lung diseases may be identified. Views were expressed about the positives and negatives of this, with positives including the potential to detect treatable lung diseases (e.g. pneumonia or fungal infections with a consequent improvement in health), whereas negatives included the detection of more serious diseases with poor prognosis such as pulmonary fibrosis.
Invitation to screening
Wording of the screening invitation was discussed in two meetings and it was suggested that an approach similar to that taken in other national screening programmes should be taken. Participants in two meetings highlighted the importance of informed choice when deciding whether or not to accept an invitation to attend lung cancer screening. Participants thought it particularly important that the invitation addressed possible consequences of attending screening (e.g. acknowledging the potential impact of a positive screening result and providing details of potential treatments and treatment pathways that might then be offered). It was thought important to acknowledge in the invitation that people may be worried about attending lung cancer screening and that it would be beneficial to highlight any evidence of increased likelihood of survival with early detection and treatment.
Planning
It was recognised that family, especially reliant family members such as older relatives and children, could be influential on decisions whether or not to attend lung cancer screening, with participants from two meetings saying that they would go for screening for the sake of their family but would not go if they just had to consider themselves.
Work
One participant described how potentially influential the workplace could be for increasing engagement with lung cancer screening by illustrating how an older male relative went for a health check when instructed to by his workplace, despite resisting going for health checks for years before.
Motivation to quit smoking
Some participants described how receiving an invitation for screening could act as a motivator to quit smoking and thought that receiving a lung disease diagnosis could similarly motivate people to give up smoking.
Support
Participants at two meetings described how they thought that support from family and friends was important, both in aiding decisions about whether or not to attend for lung cancer screening as well as accompanying them to attend hospital appointments.
Disengagement/barriers
This category included themes relating to participants’ views on strategies likely to reduce lung cancer screening invitation uptake as well as factors or approaches that would potentially negatively affect willingness or capacity to engage with a lung cancer screening programme if offered.
Poor access to screening/support
It was thought that a combination of worry and poor access to screening would contribute to poor attendance for lung cancer screening. It was acknowledged that because screening probably would not be offered at a local hospital, certain members of society would find it more difficult to access screening because of their location, poor access to transport and caring responsibilities. It was thought that impact from these factors would be accentuated if people had little support or low income or lived in more deprived areas. It was also noted that people with caring responsibilities who had little support might struggle to cope if they had a positive diagnosis and underwent chemotherapy. Participants in two meetings suggested that access might be improved by providing mobile screening clinics in the community, similar to those already provided for breast screening. One community drop-in participant (who also accessed the food bank centre) thought that people on low incomes would be more likely to travel long distances to attend screening if they were financially reimbursed.
Worry/denial/fear of knowing
Participants at all three workshops imagined that they would have feelings of anxiety, fear and worry around obtaining results from a lung cancer screening test, and thought it likely that some people would experience denial and avoid attending for lung cancer screening. One participant expressed the view that fear of obtaining results may be more pronounced for people with a history of lung cancer in their family. Participants expressed the view that denial may increase with age, with older people more likely to agree that ‘ignorance is bliss’.
Fatalism
Participants thought that the general public perception of lung cancer was that it is incurable. Participants described their own experience of knowing the risks of smoking, but feeling that ‘it was too late anyway’.
Invitation to screening
Some participants thought that using the screening invitation to promote lifestyle change or offering help to give up smoking would act as a deterrent to smokers taking up the invitation to screening. Participants at two meetings discussed fear of pain that they associated with mammogram screening and noted how this affected their participation in the national breast cancer screening programme. One participant described feeling worried about having a magnetic resonance imaging (MRI) scan because they experienced claustrophobia. Participants thought that raising public awareness about lung cancer screening and providing information about safety (radiation) and the likely physical sensations of the screening process would be helpful to alleviate people’s worry and encourage participation in screening.
Work/lifestyle
Having to take time off work to attend screening was described as problematic both for people who were self-employed and employees. Issues included hours (and money if self-employed) lost during the working day, difficulties scheduling appointments outside working hours and experiencing docked pay if late for work because of hospital delays.
Gender
Views were expressed in two workshops that men may be less likely to respond to an invitation to lung cancer screening because they may feel unable to show or talk about experiences of fear relating to a potential lung cancer diagnosis.
Age
Some participants thought that it would be harder for older people who have been smoking for a long time to want or be able to quit smoking, making them less likely to engage with a lung cancer screening programme. The view was shared by a community drop-in participant (aged 86 years) who said that he would not engage with a lung cancer screening programme as he considered himself too old and would ‘die when I die’.
Public stigma
Participants from one meeting said that they would not feel judged for smoking if they received an invitation targeted at smokers for lung cancer screening, but were concerned that non-smokers might strongly feel that lung cancer screening should not be funded by the NHS, and the money allocated elsewhere, because of the self-inflicted link between smoking and lung cancer. A light ex-smoker attending the community centre drop-in session, who had family members employed by the NHS, described feeling worried about the future of the NHS and questioned whether or not a screening programme for a self-inflicted illness such as lung cancer should be funded by the NHS.
Blame from health professionals
Participants from two meetings described feeling blamed by a health-care professional for smoking. One participant described visiting a health-care professional for a health condition unrelated to smoking, only to be asked if they smoked and advised to give up. Another participant described feeling blamed by a lung specialist who told them that ‘the damage was done’ despite having given up smoking 26 years previously.
No motivation to quit smoking
Participants thought that a lack of readiness to give up smoking would result in smokers not accepting an invitation to lung cancer screening.
Financial costs/funding
This category included themes relating to participant’s views on costs and funding issues associated with providing a national lung cancer screening programme.
To NHS
Views were expressed in all three workshops around cost. Views were expressed that if lung cancer screening was offered, there would be more cases of treatable lung cancer detected and that this would save the NHS money in the long term, with fewer hospital stays and less medication needed. Views were expressed that if lung cancer screening was not offered by the NHS, money could be used elsewhere in the service and money would be saved through not offering early treatment (surgery). However, it was also acknowledged that not offering screening would result in more cases of late treatment of lung cancer, which might be more expensive than early treatment, with longer treatment times requiring more medical interventions and medications, more time in hospital and more incapacity. The issue of the cost of follow-on (palliative) care was also raised. A view was expressed by a community drop-in participant that money might potentially be saved by the NHS if the introduction of screening was staggered (i.e. targeted lung cancer screening was offered initially, and then, if successful, universal screening offered at a later date).
Insurance premiums
The fear of raised insurance premiums following participation in a national lung cancer screening programme targeting people most at risk of lung cancer was discussed in two meetings. One participant expressed concerns that people might feel that participating in a targeted lung cancer screening programme would jeopardise their life insurance, making them less likely to engage with it.
Tax revenue
It was acknowledged in two meetings that the government benefited from tax revenue derived from the sale of cigarettes, with some participants suggesting that explicit use of this revenue to fund a national lung cancer screening programme targeted at smokers/ex-smokers might help overcome any associated public stigma. One participant expressed the view that the association between smoking and lung cancer has been established for 40 years but noted that the government had not banned the sale of cigarettes. Another participant expressed the view that the government should take some responsibility for funding a lung cancer national screening programme now because they had not deterred people from smoking in the past and had benefited from cigarette tax revenue over many years.
Cost of travel to screening/additional tests
It was acknowledged by participants in two meetings that the cost of travel to hospital for screening and additional tests would hinder access for less affluent people and people from rural areas who were dependent on public transport.
Cost of travel to hospital for carers
Participants acknowledged the cost to carers of accompanying relatives to hospital for lung cancer screening tests and for visiting hospital to support relatives during stays for treatment.
Cost of private screening
It was acknowledged in one workshop that if a national lung cancer screening programme was not offered by the NHS, then the cost of private lung cancer screening would hinder access to lung cancer screening for less-affluent people.
Missed appointments
In one meeting, participants shared their observations of the high prevalence of missed appointments in primary care and their own struggles to make appointments to see their GP. It was acknowledged that people may change their mind at the last minute to attend for screening, even after initially accepting the invitation. One participant expressed the view that understanding the take-up rate and the reliability of people turning up was important in deciding if a national lung cancer screening programme was worth doing.
Culture/environment
This category included themes relating to participants’ views on cultural influences and social perceptions relating to smoking behaviour and the acceptability of a national lung cancer screening programme.
Cultural norms
There was acknowledgement in two meetings that it would be unfair to stigmatise smokers/ex-smokers who began smoking in a past era when smoking was the cultural norm, when benefits of smoking were promoted (e.g. smoking was ‘good for your nerves’) and health risks of smoking were not publicised. Meeting participants compared this with the high prevalence of diabetes and cultural norms of obesity occurring today despite public awareness of the associated health risks. A participant of the community centre drop-in compared the past with the current situation and expressed the view that enough information and public awareness now existed about the consequences of lifestyle choices, for example diabetes and eating sugar, for people to take responsibility for their health. She described eating too much sugar to deal with the stress of looking after young children even though she was informed about the health consequences of this behaviour and acknowledged that people living in more deprived areas may be exposed to more stress and that this would affect their behaviour in relation to smoking.
Government and media influence
Participants thought that raising public awareness before the introduction of a lung cancer screening programme would aid screening uptake. Acknowledgement of the strong cultural influence of the media in the glamorising of smoking in the past, and the resulting increase in uptake of smoking, was raised in two meetings.
Inequity/deprivation
In two meetings, participants said that they did not think lack of money affected people’s smoking behaviour, stating that smokers from more deprived areas prioritised purchasing cigarettes above other things. It was acknowledged that smoking for some people may be prioritised in order to cope with poor life circumstances. In both meetings, participants commented on lack of engagement/apathy of people living in more deprived areas to access services in general, and said that they expected a similar lack of engagement with a lung cancer screening programme.
Treatment/care pathway
This category included themes relating to participants’ views on the potential impact of a lung cancer screening programme relating to the screening process, lung cancer detection and potential treatment and pathways of care.
Detection
Early
Participants thought that an early diagnosis of lung cancer would mean that potentially more treatment options would be available and that the prognosis would be better.
Late
In one meeting, a view was elicited that treatment would be more intrusive if it was offered later.
Treatment/no treatment
The initial debilitating nature of treatment with chemotherapy was noted by participants in one meeting, as was the perceived importance of being able to make informed choices about treatment options based on prognosis and likelihood of survival. In one meeting, there were discussions around the use of protocols to ensure that people would feel reassured that they would receive a prompt referral to treatment or further tests following a positive lung cancer screening test result.
Repeat testing
In one meeting, the probable need for an increased frequency of repeat lung cancer screening compared with other screening programmes was discussed. One participant in the community drop-in session raised concerns about radiation exposure with repeat testing.
Quality of life/mental well-being
This category included themes relating to participants’ views on the potential impact of a lung cancer screening programme on QoL and mental well-being together with views on smoking behaviour in relation to QoL and mental well-being.
One participant expressed the view that she would worry if she attended for lung cancer screening and would worry if she did not. This view was shared by participants from two meetings who said that psychological/mental well-being would be affected just by the introduction of the screening programme, both positively and negatively, and that psychological well-being should be considered as an outcome in this HTA.
Coping
Participants in two workshops thought that many people smoked in order to cope with stress and general life pressures/poor life circumstances (e.g. caring for a partner who is an alcoholic). Participants from two meetings said that they thought that people with mental health issues were likely to be dependent on smoking as a way of coping with their illness.
Anxiety/worry
About the test
This was discussed in all three meetings. Participants thought that having information about the technology, particularly safety, would be helpful to alleviate people’s worry and facilitate informed choice. One participant described being unprepared for the physical sensations experienced when having a MRI scan and said that having had information about what to expect in advance would have helped reduce worry.
Misdiagnosis
Participants in two meetings discussed the possibility of misdiagnosis, and expressed the view that all tests were fallible and so some misdiagnosis was inevitable and that it was an unfortunate but acceptable consequence of performing a test.
On receiving screening invitation
Many participants acknowledged that they would expect to experience feelings of fear on receiving an invitation to attend lung cancer screening but that they would want to attend screening despite this.
Waiting for results and further tests
It was generally acknowledged that shorter waiting times between test/results was preferable, although one participant described not caring how long he would have to wait for results as long as he had access to lung cancer screening and the opportunity for early treatment of lung cancer that it might provide.
Quality of life and diagnosis
Participants from one meeting discussed how the benefits of early detection for self and family would include having more time to come to terms with a lung cancer diagnosis. However, another view was expressed that an early diagnosis of lung cancer, if not treatable, would result in a lowering of QoL. Other participants described how important it would be to have access to information about likelihood of survival after a positive diagnosis of lung cancer in order to ensure that QoL did not reduce further following a positive diagnosis.
Quality of life and treatment
It was recognised that treatment with chemotherapy/radiotherapy was debilitating, but that it affected individuals differently. It was also thought important to be informed of the likely chance of survival with such treatment, in order to make an informed decision about whether or not to have it.
Support (or lack of support)
At home
One participant described the importance of ensuring support at home for people with caring responsibilities undergoing treatment for lung cancer.
In care pathway
Participants from two meetings discussed the impact of receiving a positive diagnosis of lung cancer and the need for support to minimise the impact on QoL. One participant thought that the impact of a positive diagnosis on QoL would depend on how the news was communicated (face to face or by letter), the communication skills of the health-care staff and their ability to clearly communicate about what would happen next.
Empowerment
One participant described how she thought receiving an invitation to lung cancer screening would make her think about giving up smoking and invoke feelings of taking control of her life.
Participants from two meetings expressed views on the benefits of having a lung cancer diagnosis confirmed early. Participants thought an early diagnosis would provide time to adjust to the news and reduce shock, both for patients and for their family/carers. Others described how knowing that they had lung cancer would help them feel empowered to decide how to best spend their remaining time and potentially change their lifestyle. Participants spoke of feeling how they would feel more in control of their lives, how they could ‘put things in place’ (e.g. write wills and make funeral arrangements, spend time wisely and create memories with their loved ones and feel more empowered to help their children to come to terms with their diagnosis, e.g. make memory boxes). Participants described the benefits for family and carers of knowing a relative had lung cancer as this knowledge could help them plan for their own lives after their loved one had died. One participant described how such planning by carers/relatives could benefit patients as they could be reassured that their family would be alright after they have died.
Disempowerment
In one meeting, there was discussion about expected levels of apathy from people from more deprived areas regarding engaging with services such as a lung cancer screening programme. Participants felt that this apathy may be linked to identity and self-esteem issues, and be affected by how people viewed themselves/felt viewed by society. It was thought that such people may be experiencing higher levels of stress and/or living in an unsupportive or uninspiring environment (e.g. social housing that is perceived by others to be inhabited by ‘problem families’).
Responsibility and risk
This category included themes relating to participants’ views on risks from lung cancer screening and responsibility and risks associated with developing lung cancer.
Family history (of lung cancer)
Participants from two workshops described how their family history of lung cancer made them feel more at risk of lung cancer.
Smoking history
There was some deliberation between participants as to whether or not lung cancer screening should be offered based on age or smoking history.
Passive smoking
Participants describe being aware of increased risk from passive smoking from public places and if brought up in families in which either/both parents smoked.
Information (about risk)
In one meeting, a fatalistic view that once you have lung cancer ‘then that’s it’ was acknowledged as being commonly held in society. It was thought that this societal view might be counteracted by providing information in the screening invitation about how early detection of lung cancer can lead to successful treatment. Another participant thought that it would be useful to provide information about the risk of radiation during screening in case this would put some people off attending for lung cancer screening. One community drop-in participant thought that information about test accuracy and the risk of misdiagnosis was important.
Environmental risk
In two meetings, the topic of environmental risk, from asbestos and from vehicle fumes, was raised, with participants acknowledging passive risks of lung cancer from such exposure.
Exposure to radiation (from technology)
Although participants from two workshops were aware of some potential increased risk from radiation from the screening technology, none of these participants thought that the risk was unacceptably high or said that their concerns about radiation would prevent them having a scan. One participant from the community drop-in said that they would be anxious about participating in a lung cancer screening programme if they had to have a succession of tests involving radiation.
Perception of risk
Participants from one meeting discussed how non-smokers who were perceived as being at a low risk of lung cancer may still want to attend for lung cancer screening because of the risks from passive smoking, especially if they were exposed to smoking from one or both parents as well as in public spaces while they were growing up. Participants in one group discussed a feeling of a cultural shift when Roy Castle got cancer, that society changed as people became more aware of the risks of passive smoking. One participant expressed the view that it may send out mixed messages to the public about the risks of passive smoking if lung cancer screening was not offered to all members of the public as it would appear that the government/NHS thought that risks from passive smoking were sufficiently high for smoking to be banned in public spaces, but not high enough for people exposed to passive smoking to be screened for lung cancer.
Appendix 12 UK National Screening Criteria
Glossary
- Absorbed dose
- The physical amount of radiation absorbed by matter or tissue, without accounting for the biological impact of the radiation.
- Attenuation characteristics
- X-rays are normally fired from the source to the detectors. Each individual placed between the source and the detectors absorbs X-rays differently. The number of X-rays that finally reach the detectors affects the resultant images.
- Automatic tube current modulation
- The computed tomography machine changes the output of tube current based on the individual biological make-up (e.g. anteroposterior diameter of the thorax).
- Axial (non-helical/non-spiral) acquisition
- This technique is also known as the ‘step-and-shoot’ or ‘scan–move–scan’ technique. All data are collected in the z-axis direction of each slice before moving to the next position. There is a correlation between the z position, where data are collected, and reconstructed slices.
- Background radiation
- The combined ionising radiation from both natural and artificial sources. Some areas might have higher background radiation owing to environmental factors (e.g. radon-affected areas in Cornwall, UK).
- Baseline screening
- The first time screening is conducted in a population (it gives an estimate of the prevalence of the disease), also known as prevalence screening.
- Breathing artefact
- Imaging of the lung is susceptible to movement artefact due to respiration. The part of the lung near the diaphragm produces more movement artefact than the lung apices.
- Collimation
- In single-slice computed tomography, the X-ray beam collimation is the z-axis width of the X-ray beam at the centre of the gantry rotation, which is the same as slice thickness. This relationship is not true for multislice computed tomography. For dosimetry purpose, multislice computed tomography is assumed to behave like single-slice computed tomography.
- Computed tomography
- An imaging technology, usually based on X-rays, that reconstructs the density of cross-sectional slices of an object from a number of projections obtained from different angles.
- Confidence interval
- A range generated from a sample with a specified probability of containing the population statistic of interest (e.g. 95% of 95% confidence intervals for the mean are expected to contain the population mean).
- Conversion factor
- Tissue weighting factor is a measure of the stochastic risk of ionising radiation. It varies among different tissue and organs. Conversion factor is body-part specific and accounts for the variable radiosensitivities.
- Cost-effective
- An intervention is cost-effective compared with a comparator if it provides more health at an acceptable cost (according to a threshold) or provides significant savings with an acceptable loss of health; an intervention is cost-effective in a fully incremental analysis if it is cost-effective compared with all comparators.
- Credible interval
- A Bayesian equivalent of the confidence interval, which is a range containing the specified proportion of the probability mass of the posterior distribution.
- Dominated
- An intervention is dominated by a comparator if it is more costly and less effective.
- Dose length product
- An output of computed tomography radiation dose from a computed tomography machine. This is calculated using the volume of computed tomography dose index multiplied by scan length (slice thickness multiplied by number of slices) in centimetres.
- Effective dose
- A measure of the health effect of ionising radiation that accounts for the different health effects of different types of radiation and different tissues (if the whole body is not being uniformly irradiated).
- Equivalent dose
- A measure of the health effect of ionising radiation that accounts for the different health effects of different types of radiation, but not the variation of health effect according to the tissue being irradiated.
- Extendedly dominated
- An intervention is extendedly dominated by two comparators if it would be dominated by a linear combination of the two comparators.
- False-negative (test result)
- A negative test result when the disease was in fact present.
- False-positive (test result)
- A positive test result (leading to further testing or treatment) when the disease was in fact absent.
- Fixed-tube current
- The tube current is not adjusted according to biological factors for all individuals.
- Gray
- The SI-derived unit for the absorption of radiation energy; 1 gray = 1 joule of radiation energy per kilogram of matter.
- Helical/spiral acquisition
- Rotation and table movement occur at the same time with continuous data acquisition. In contrast to axial acquisition, there is no correlation between z positions, where the data are collected, and reconstructed slices.
- Image noise
- Computed tomography image noise depends on the number of X-rays contributing to the image. Low image noise enables the ability to resolve low-contrast structures.
- Incidence
- The rate at which members of a population are newly diagnosed with a disease, or newly develop a disease.
- Incidence screening
- Subsequent rounds of screening within a population; if the time between screening rounds is fixed, this can give an estimate of the incidence.
- Indeterminate
- When the test is unable to classify the disease as being present or absent, usually resulting in further testing in the future.
- Intention to treat
- A method of statistical analysis in which the groups for statistical comparison are defined by the intended (often randomised) treatment, even if a different treatment or no treatment was actually received.
- Interval cancer
- A cancer that is diagnosed after one or more screening rounds and before a scheduled screening round, but not as a consequence of screening.
- Lead-time bias
- Screening tends to diagnose disease earlier, which can appear to result in prolonged survival (or prolonged time to some event of interest), although the actual date of the event has not been delayed by screening.
- Length bias
- Screening tends to diagnose slower progressing, less aggressive disease, which leads to greater apparent survival compared with non-screen-detected disease.
- Meta-analysis
- A set of statistical methods for combining results from multiple studies (sophisticated averages).
- Multislice
- Other names include multidetector row computed tomography and multirow computed tomography. The design of the detector is in contrast to single-slice technology (see Single-slice computed tomography), where a long element is divided into several smaller detectors in the z-axis direction.
- Negative predictive value
- The probability that an individual does not have the target condition, given that the test is negative.
- Net monetary benefit
- The value of an intervention, estimated by valuing outcomes according to the willingness to pay and subtracting the costs of the intervention.
- Network meta-analysis
- Meta-analysis methods that can combine results from studies with different sets of interventions and comparators.
- Over-diagnosis
- When a disease is detected by screening that would not have clinically presented prior to death from other causes in the absence of screening (an extreme form of lead-time and length bias).
- Pack-year
- A composite measure of smoking history including the duration and intensity of smoking; one pack-year = one pack (20 cigarettes) per day for 1 year.
- Pitch (in axial scanning)
- Also known as detector pitch. This is the distance that the table travels in one full gantry rotation divided by beam collimation.
- Pitch (in helical scanning)
- Also known as beam pitch. Helical pitch is calculated as the movement of the table per rotation divided by slice thickness. Pitches of < 1 mean that X-ray beams overlap/double irradiation, whereas pitches of > 1 mean that there are gaps between the X-ray beams.
- Positive predictive value
- The probability that an individual has the target condition, given that the test result is positive.
- Prevalence
- The proportion of people in a population who have a disease.
- Prevalence screening
- See Baseline screening.
- Probabilistic sensitivity analysis
- A method for exploring the impact of parameter uncertainty, across all parameters, on decision uncertainty.
- Radiosensitivity
- The harmful effect of ionising radiation depends on the relative susceptibility of tissues and organs.
- Randomised controlled trial
- An experiment in which people are randomly assigned to receive one of two or more interventions, and are then followed up to see the effects of the treatment.
- Sensitivity
- The probability that a test conducted in an individual with the target condition will be positive.
- Sievert
- The SI-derived unit for the health effect of ionising radiation, the unit for equivalent dose and effective dose.
- Single-slice computed tomography
- Other names include single-detector computed tomography and single row computed tomography. The design of the detector array contains one long element in the z-axis direction.
- Slice thickness
- In multislice computed tomography, slice thickness is based on the detector configuration. In single-slice computed tomography, slice thickness is determined by beam collimators.
- Specificity
- The probability that a test conducted in an individual without the target condition will be negative.
- Test failure
- When a test is not completed as planned (e.g. as a result of operator failure or equipment failure).
- Tube current (in helical scanning)
- Indicates the number of electrons flowing from the cathode to anode. Milliamperes is the unit of measurement.
- Tube current time product (in helical scanning)
- The product of tube current and exposure time. Milliamperes-second (mAs) is the unit of measurement.
- Volumetric analysis
- Lung nodule on computed tomography thorax was traditionally measured axially to produce a two-dimensional measurement that subsequently served as the reference point for subsequent investigations. Different companies designed software to incorporate three-dimensional information to produce a volume measurement. Specifically, the doubling of volume measurement is being proposed as the indicator of significant change.
- Willingness to pay
- The rate at which the decision-maker is prepared to pay for some outcome, which may be related to the ability of the health service to disinvest from current technologies.
- z-axis resolution (in helical scanning)
- The z-axis is the longitudinal axis in helical computed tomography. Between the start and the end position of the data acquisition, the raw data can be reconstructed retrospectively at short or at longer intervals. A number of factors affect the resolution, including pitch, slice thickness and method of reconstruction.
List of abbreviations
- ACRIN
- American College of Radiology Imaging Network
- BMI
- body mass index
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHEC
- Consensus on Health Economic Criteria
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- COPD
- chronic obstructive pulmonary disease
- COS-LC
- consequences of screening lung cancer
- CPCI-S
- Conference Proceedings Citation Index – Science
- CrI
- credible interval
- CT
- computed tomography
- CXR
- chest X-ray
- DANTE
- Detection and Screening of Early Lung Cancer with Novel Imaging Technology and Molecular Essays
- DES
- discrete event simulation
- DLCST
- Danish Lung Cancer Screening Trial
- ELCAP
- Early Lung Cancer Action Project
- EQ-5D
- EuroQoL 5-Dimensions
- EU
- European Union
- [18F]FDG-PET
- fludeoxyglucose (18F) positron emission tomography
- FEV1
- forced expiratory volume in 1 second
- GP
- general practitioner
- HMIC
- Health Management Information Consortium
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IES
- impact of event scale
- INMB
- incremental net monetary benefit
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITALUNG
- Italian lung cancer screening
- LCNEC
- large cell neuroendocrine carcinoma
- LDCT
- low-dose computed tomography
- LLPv2
- Liverpool Lung Project version 2
- MCMC
- Markov chain Monte Carlo
- MDT
- multidisciplinary team
- MILD
- Multicentric Italian Lung Detection
- MRI
- magnetic resonance imaging
- NELSON
- NEderlands Leuvens Longkanker Screenings ONderzoek
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NLST
- National Lung Screening Trial
- NMB
- net monetary benefit
- NNS
- number needed to screen
- NSC
- National Screening Committee
- NSCLC
- non-small cell lung cancer
- OR
- odds ratio
- PenPIG
- Peninsula Public Involvement Group
- PenTAG
- Peninsula Technology Assessment Group
- PET-CT
- positron emission tomography–computerised tomography
- PLuSS
- Pittsburgh Lung Screening Study
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SCI
- Science Citation Index
- SCLC
- small cell lung cancer
- SD
- standard deviation
- SES
- socioeconomic status
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- STAI-6
- Spielberger State–Trait Anxiety Inventory, six-item Short Form
- TNM
- tumour, node, metastasis
- TTO
- time trade-off
- UKLS
- UK Lung Cancer Screening Trial
- VAS
- visual analogue scale
- WHO
- World Health Organization