Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/220/06. The contractual start date was in February 2016. The draft report began editorial review in November 2017 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gill Livingston is on the National Institute for Health Research (NIHR) postdoctoral fellowship board. Gill Livingston and Penny Rapaport have grants from NIHR/Economic and Social Research Council, NIHR Health Technology Assessment (HTA) programme and the Alzheimer’s Society and are supported by North Thames NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC). Simon D Kyle reports grant funding from NIHR HTA, NIHR Oxford Biomedical Research Centre, Arthritis Research UK and the Dr Mortimer & Theresa Sackler Foundation. Claudia Cooper and Gill Livingston are supported by the University College London NIHR Biomedical Research Unit. James A Pickett is an employee of the Alzheimer’s Society. Colin A Espie reports grants from the NIHR HTA programme, NIHR Oxford Biomedical Research Centre, the Wellcome Trust, Education Endowment Foundation and the Dr Mortimer & Theresa Sackler Foundation. In addition, he has a patent US Patent Application Serial No. 14/172,347 INTERACTIVE SYSTEM FOR SLEEP IMPROVEMENT: Docket No. HAME-001 issued and is co-founder and Chief Medical Officer of Big Health Ltd (London, UK), which has developed Sleepio, an online digital cognitive–behavioural therapy intervention for insomnia. He is also a shareholder in Big Health Ltd.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Kinnunen et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
The prevalence of dementia and its impact, on both an individual level and a societal level, continue to increase. In 2015, there were 47 million people living with dementia worldwide. 1 It is estimated to cost US$818B and projected to become a trillion-dollar disease in 2018. 2 In the UK specifically, 850,000 people are living with dementia, a figure expected to rise to 1 million by 2021, and to double to 1.7 million over the next 30 years. 3 Currently, dementia care costs in the UK amount to approximately £26B, with the majority (£17.4B) falling on the people with dementia and their families. 3,4 Two-thirds of people with dementia live at home, where family members and informal carers provide most of the care. 3
Sleep disturbances are common in older people without dementia, as sleep may be adversely affected by physical health state and pain. In dementia, there are additional factors that cause poor sleep. The prevalence of sleep disturbances in those with neurodegenerative dementias is estimated to be 25–40%,5,6 with one meta-analysis of the prevalence of sleep disturbances in people with Alzheimer’s disease (AD) finding a pooled prevalence of 39%. 7
Sleep disturbances include reduced night-time sleep, fragmented sleep and wandering,8,9 and are correlated with excessive daytime sleepiness and depression. 10 They may be both caused by and worsen AD and other dementias. Recent research has suggested a correlation between sleep disturbances and amyloid beta (Aβ) deposits. 11 Dementia may also lead to impaired production of the hormone melatonin through structural and functional alterations to the suprachiasmatic nucleus (SCN). 12,13 The circadian rhythm of melatonin production, high levels at night and low levels during the day, helps control the sleep–wake cycle. 14 People with dementia may wake during the night and not be aware of the time, or be frightened because they do not know what is happening. Dementia can therefore lead to decreased regularity of sleep, impaired sleep initiation and difficulty maintaining sleep at night, and wakefulness during daylight hours. 15
Sleep acts as a restorative process for the brain and supports all aspects of mental and physical life, being a major determinant of day-to-day function and quality of life. 16 In turn, sleep disturbances reduce quality of life and are distressing for family members, whose sleep is often affected. Sleep disturbances in dementia increase family carer burden, predict their depressive symptoms and lead to care home admissions, elevating the individual, societal and economic costs associated with dementia. 10,17,18 Importantly, paid night-time care can be unaffordable or unfeasible for people who wish to continue caring at home.
Explanation of rationale
The causes of sleep disruption in individuals with dementia are multifactorial, and include physiological dementia-related changes, pain, environmental and behavioural factors, and medication side effects. There are currently no known effective treatments. Health teams use a mixture of sleep hygiene measures and psychotropic medication, extrapolated from other conditions. These confer limited benefit, and sedative medications may cause harm. A Cochrane review19 of studies examining the effects on actigraphy sleep measures of drugs found no definitive effectiveness evidence. These conclusions are supported by recent investigations20–23 (mostly in people with AD) that similarly found no efficacy evidence for any drugs or melatonin. Cholinesterase inhibitors and glutamate receptor antagonists, given particularly to people with AD or dementia with Lewy bodies, may increase wakefulness, but may hypothetically also support sleep through improved cognition. 24,25 However, there is no evidence of their efficacy. Overall, there is no conclusive evidence to suggest efficacy for any of these pharmacological options in the treatment of sleep disturbances in dementia.
In addition, people with dementia are often frail with multiple comorbidities, and non-pharmacological treatment options should therefore be the first line for sleep management. However, most evidence about non-pharmacological treatments comes from small-scale studies often lacking in methodological rigour, leading to insufficient, conflicting evidence. 26 The need for better research into such treatments for sleep disorders is mentioned specifically in the National Dementia Strategy,27 and the outputs of the 2010 Ministerial Dementia Research Summit and 2011 National Institute for Health Research (NIHR) Dementia Research Workshop, summarised in the Ministerial Advisory Group for Dementia Research (MAGDR) final report28 Priority Topics in Dementia Research in February 2011. As with many other problems, studies consistently indicate that patients and their doctors would prefer non-drug approaches for sleep problems. 29
One approach with suggested potential to improve sleep disturbances is bright light therapy. A systematic review and meta-analysis30 and a separate case series31 found that light therapy had small to medium effects on sleep disturbances in the general population.
There have also been recent small pilots of non-pharmacological interventions aiming to reduce sleep disturbances in people living with dementia. In one study, 7 out of 14 (50%) people with dementia and carer dyads completed a sleep education programme, which was delivered to the carers in a group setting. 32 In another pilot study, by Gibson et al. ,33 9 out of 15 (60%) dyads completed a programme incorporating light therapy, exercise and sleep education. Both studies were methodologically limited, with unsatisfactory completion rates, but the interventions showed some promise in alleviating sleep disturbances.
Neurodegenerative dementias are commonly characterised by circadian rhythm disruption attributable to progressive loss of SCN neurons. 34 Thus, strengthening circadian rhythmicity through bright light therapy is theoretically appealing. However, light therapy administered in a non-individualised way, for example on the wrong side of the phase–response curve, may exacerbate sleep disruption.
In the current study, the vision was to build on this contradictory, and as yet incomplete, evidence by bringing together expertise in clinical interventions in dementia and sleep, statistics and input from family carers, and to deliver the intervention individually to fit the specific problems of each participant in the study. The aim was to develop and test a manual to improve sleep and, therefore, quality of life for those with dementia and their families. Through our previous work with STrAtegies for RelaTives (START), a coping strategy-based manual for family carers of people with dementia, short-term and long-term clinical effectiveness and cost-effectiveness have successfully been demonstrated. 35,36 This provided an ideal platform on which to build our new manual: Dementia RElAted Manual for Sleep; STrAtegies for RelaTives (DREAMS START). It was planned that DREAMS START would be a manualised multicomponent intervention, delivered to carers by clinically supervised psychology graduates, comprising a cognitive–behavioural component, light therapy, behavioural activation, relaxation and coping skills for families. Details of its development and content are below.
Sleep disturbances are complex and their causes and best management strategies differ between individuals. It can also be difficult for family carers to attend a group at a specific or fixed time. Thus, our intervention was individually tailored. It used natural dusk and daylight (whenever feasible) as well as other light sources to manage endogenous melatonin production. In addition to bright light therapy and strategies to increase daytime activity, we planned the intervention to include cognitive–behavioural techniques for sleep management, which a Cochrane review had found effective in older adults without dementia and in family carers of people with dementia. 37,38 A collaborative, non-prescriptive model was central when this intervention was delivered. Family carers were encouraged to try different strategies and note in their manual what worked for them. Psychology graduates were trained as sleep therapists. The therapists had a similar skillset to Improving Access to Psychological Therapies (IAPT) practitioners, and psychology assistants in memory and mental health services. The intervention was designed to be delivered by therapists with this skill level so that it could be delivered by IAPT-based psychological well-being practitioners and secondary care psychology practitioners in the future. This research has the potential to improve sleep and quality of life of people with dementia and their family carers, in a feasible and scalable intervention without the side effects of medication. If found to be clinically effective in a future full-scale trial, this intervention should also be cost-effective: it is cheap and may delay care home admission.
Specific objectives
Our research question was: how feasible is a pragmatic, randomised study to investigate the clinical effectiveness and cost-effectiveness of a manualised intervention (DREAMS START) to manage NHS patients with dementia and significant sleep disturbance living in their own homes?
Aims
The aims were to develop a manualised behavioural intervention for sleep disturbances in dementia and examine the feasibility of a full-scale trial.
Objectives
The objectives were to:
-
obtain estimates of acceptability and feasibility that will inform continuation to the main trial, specifically to estimate [with 95% confidence intervals (CIs)] the proportion of eligible participants who agreed to participate in the trial and the proportion of participants offered the intervention that adhered to it
-
obtain estimates required for the main trial’s sample size calculation in relation to potential primary outcomes [standard deviations (SD); correlation between baseline and follow-up measurements and drop-out rate]
-
use qualitative interviews to assess acceptability of the intervention and to detail any required refinements.
Chapter 2 Methods
Ethics
The trial was approved by the London – Queen Square Research Ethics Committee (reference number 16/LO/0670; see Appendix 1).
Trial design
This was a cluster-randomised, parallel-group superiority trial, with blinded outcome assessment. Participants were allocated 2 : 1 to the intervention or treatment-as-usual (TAU) arm.
Participants: eligibility criteria
People with dementia and their family carer were included, and the inclusion criteria for such dyads are laid out in the feasibility study below. To help determine feasibility for a full trial, people with a range of types of dementia and living situations were included. We wanted to find out if there were obstacles in such wide inclusion criteria, to enable us to consider criteria for a full trial. In particular, people who had an alcohol-related dementia or lived alone or were looked after by paid carer were not excluded, although it was uncertain how this would affect delivery of the therapy.
People with dementia
Adults with dementia (any type and severity) and clinically significant sleep difficulties [Sleep Disorders Inventory (SDI)39 item score of ≥ 4 on screening] that were judged a problem by the person with dementia or their family were included. If the person with dementia did not have capacity to give consent, a consultee’s declaration was sought. Patients living in a care home were excluded. Those with a primary sleep disorder diagnosis (e.g. sleep apnoea), rather than dementia-related sleep problems, were also excluded.
Carers
Primary family carers of the person with dementia were included for quantitative assessment interviews and intervention delivery. When the carer who spent most time with the person with dementia was not a family carer, then the paid carer was able to participate in the intervention. The family carer, however, completed the carer assessment measures. All carers provided emotional or practical support to the person with dementia at least weekly. Carers who were unable to give informed consent, or with probable dementia, were excluded.
Study settings
NHS trusts
Participants were primarily recruited from two NHS trusts in London: Camden and Islington NHS Foundation Trust (site 1) and Barnet, Enfield and Haringey Mental Health NHS Trust (site 2). Both trusts provided funding to cover the excess costs resulting from training, intervention delivery and clinical supervision. Clinicians approached people with dementia and their family carers who were attending one of the five memory services: (1) Camden, (2) Islington, (3) Barnet, (4) Enfield or (5) Haringey. Those interested in the study were asked to give permission for the research team to approach them, and were given an information sheet. Following contact by our team, an appointment was arranged to obtain informed consent in the potential participants’ homes (or in the research team’s office if preferred). A baseline assessment was carried out after this. Intervention delivery and follow-up assessments also took place in the participants’ homes.
Join Dementia Research
In addition, the research team approached potential ‘matches’ through Join Dementia Research (JDR). This is an online and telephone service developed and launched in 2014/15 by the NIHR. It aims to make it easier for people with dementia and other interested members of the public to participate in dementia studies. People register their interest, providing basic or more detailed demographic and clinical information and their contact details (including their preferred means of contact). The registrant or a named representative can then be contacted by researchers to discuss the potential suitability of studies. To recruit from this site [site 3, University College London (UCL)/JDR], potential matches with any dementia diagnosis listed on JDR were identified. We ticked ‘yes’ to ‘Has next of kin?’, chose ‘Include empty or NULL values’, ticked ‘yes’ to ‘Has a carer?’, ticked ‘no’ to ‘Volunteer is a carer?’, ticked ‘no’ to ‘Volunteer cares/supports a person with dementia?’, and selected ‘More than 3 times per week’ for ‘Contact with carer’ (‘Include empty or NULL values’), and selected ‘Private Residence’ and ‘Assisted Living Accommodation’ for ‘Accommodation Type’ (‘Include empty or NULL values’). Owing to limited resources, we defined our search area based on proximity to UCL.
Data collection
Family carers were interviewed at screening/baseline (pre randomisation) and at the 3-month follow-up to collect data on the person with dementia and the carer. For around one-third of the participant dyads (22/62 randomised), each value entered into the participant database was double-checked; no systematic errors were found.
Patient measures
Demographic and clinical characteristics
-
Sociodemographic details (i.e. sex, date of birth, type of dementia diagnosed, age when left education, last occupation, current marital status, ethnicity) were collected at baseline. We had information on all potential participants’ sex, including those who did not provide consent to be screened, those who were not eligible and those who were not randomised.
-
Type of dementia was recorded from clinical information.
-
Severity of dementia was measured using Clinical Dementia Rating™ (CDR)40,41 through informant information at baseline. This is a reliable and valid instrument for rating the severity of dementia. 42 It is used to rate performance in memory, orientation, judgement and problem-solving, community affairs, home and hobbies, and personal care. This information was used to classify dementia severity of clinically diagnosed patients or those on JDR into very mild (0.5), mild (1), moderate (2) or severe (3).
-
Rescue medication’s role: all prescribed psychotropic medication and melatonin was measured at baseline and 3 months by completing the Client Service Receipt Inventory (CSRI),43 which incorporates a list of all medications in the previous 3 months. Use of the following was recorded:
-
anxiolytics and hypnotics
-
antipsychotics
-
antidepressants
-
other psychotropics.
-
-
Reported side effects: side effects were measured using a study-specific Safety and Tolerability Assessment (see Appendix 2) to record the occurrence of falls, dizziness, headaches and gastrointestinal symptoms (appetite or bowel symptoms) and any other side effects at baseline and 3 months.
Potential outcomes for the main trial
-
Sleep disturbance in the person with dementia was measured using the SDI39 at screening and 3 months. The SDI is a standalone tool for assessing sleep disorder symptoms in people with dementia, developed for use to measure outcomes in original melatonin trials. It has been used in pharmacological and non-pharmacological studies and validated against actigraphy and clinical variables. The SDI consists of the seven sleep subquestions of the sleep and night-time domain of the Neuropsychiatric Inventory (NPI). 44 These are: (1) difficulty falling asleep, (2) getting up at night, (3) wandering, pacing or conducting inappropriate activities at night, (4) awakening the carer at night, (5) awakening at night, dressing, planning to go out, thinking that it is morning, (6) awakening too early in the morning and (7) excessive daytime sleepiness. SDI item scores are based on the carer’s ratings on each of the seven symptoms separately. Each item is rated according to frequency (scale 0–4) and severity (scale 0–3) of sleep-disturbed behaviours and, when frequency and severity are multiplied, possible item scores range from 0–12. Those who scored ≥ 4 on any individual item were judged to have clinically significant sleep disturbance and were eligible for the study. The SDI mean global score, which is often reported, is the total frequency multiplied by severity, divided by 7.
-
Neuropsychiatric symptoms were measured using the NPI44 at baseline and 3 months. This is a validated instrument with 12 domains of neuropsychiatric symptoms. A single frequency and severity rating is given for all behaviour subquestions within a domain, with each domain scoring between 0 and 12; higher scores mean increasing severity. A summed score out of 144 captures total neuropsychiatric symptoms.
-
Daytime sleepiness was measured from the Epworth Sleepiness Scale (ESS)45 at baseline and 3 months. This is an eight-item measure assessing the tendency to sleep/doze in specific daily situations (possible score range 0–24; a score of > 10 indicates excessive sleepiness).
-
Quality of life was measured using the Dementia Quality of Life – Proxy (DEMQoL-Proxy)46 at baseline and 3 months. It is a 31-item interviewer-administered questionnaire answered by a carer. It is a responsive, valid and reliable measure of quality of life in people with dementia with satisfactory psychometric properties. 47
-
Services use was measured using the CSRI at baseline and 3 months. It is widely used for dementia trials and will delineate treatment as usual (TAU) as well as treatment for those in the active arm of the study.
Wrist-worn Actiwatches, worn 24 hours a day for 2 weeks at baseline pre randomisation and again 3 months after randomisation, provided estimates of rest–activity rhythms, light exposure and sleep. Actigraphy has theoretical advantages for patients who may not be able to remember well enough to accurately fill in questionnaires or sleep diaries. It has been validated against polysomnography (PSG) in populations including older adults without dementia (one study48 of 77 people, with a mean age of 35 years) for its accuracy at correctly detecting sleep, wakefulness and wakefulness after sleep onset. The Actiwatch detected sleep in the whole population with 96.5% accuracy, but was not as good at detecting wakefulness (32.9% accuracy), and this became worse with increasing age. Another study of older women without dementia (with a mean age of 69 years) found that wrist actigraphy detected sleep less accurately in this age group and was unacceptable for those with low measures of sleep efficiency. 49 However, it has been used in 10 nursing home residents with severe dementia to estimate sleep during night-time, and the measures significantly correlated with total sleep time from electroencephalographic recordings. 50 We used the MotionWatch 8 (herein referred to as ‘the Actiwatch’), a Conformité Européenne (CE)-marked class 1 (low-risk) medical device (CamNtech Ltd, Cambridge, UK). Such non-intrusive, reusable Actiwatches have been used successfully in previous trials of people with dementia and sleep disturbance. 51 The aim was to use a minimum of 1 week’s worth of data. The participants were given written instructions (see Appendix 3). It was ensured that that the person with dementia and their carer between them understood the instructions and had the opportunity to ask questions. Whenever possible, the watch was placed on the wrist of the participant’s non-dominant hand, and variability was controlled among the watches by ensuring that the participants wore the same watch at baseline and follow-up. The data were recorded in MotionWatch Mode 1, which uses a single-axis algorithm and peak detection, sampled at 50 Hz, and processed into 60-second epochs. Each recording was started in the evening on the day of the baseline or follow-up assessment (preferably at 17:00) and continued for a minimum of 14 days, after which the watch was collected. Carers were asked to use a sleep diary (see Appendix 3) to keep a record of the bedtimes and rise times of the person with dementia, or, as an alternative, to indicate these times by pressing the Actiwatch’s ‘event marker’ button. These were used to define each participant’s sleep analysis window. We also asked the carers to write down (e.g. in the sleep diary) any occurrences when the watch was not worn for > 1 hour. Participants were encouraged to contact the research team if they had any further questions about the watch.
-
Sleep measures:
-
Sleep efficiency – sleep time expressed as a percentage of time in bed. This captures both initiation and maintenance of sleep, reflecting the proportion of time in bed spent asleep, and has been found to be reliably impaired in actigraphy studies of people with dementia and sleep problems.
-
Sleep time (minutes) – the total time spent in sleep according to the epoch-by-epoch wake/sleep categorisation. As the Actiwatch infers, time asleep is not ‘actual sleep time’.
-
Wake time (minutes) – the total time spent awake according to the epoch-by-epoch wake/sleep categorisation. As the Actiwatch infers, time awake is not ‘actual wake time’ but it is the label used.
-
Time of ‘lights out’ or going to bed.
-
Time of falling asleep.
-
Time of waking up.
-
Time of getting up.
-
Time in bed (hours) – the total time between ‘lights out’ and getting up.
-
Fragmentation index – the degree of fragmentation of the sleep period, calculated by summing mobile time (%) and immobile bouts of ≤ 1 minute (%).
-
-
Circadian rest–activity rhythm metrics/non-parametric circadian rhythm analysis (NPCRA) measures to assess the timing, amplitude and stability of rest–activity rhythms:
-
Relative amplitude – the amplitude of circadian rhythm (range 0–1), calculated by dividing the difference between average activity in the most active (M10) and most restful (L5) periods by the sum of M10 and L5.
-
Interdaily stability – the degree of regularity in the activity–rest pattern, ranging from a total lack of rhythm (0) to a perfectly stable rhythm (1).
-
Intradaily variability – the degree of fragmentation of activity–rest periods (range 0–2), from prolonged periods of activity and rest over 24 hours to multiple short periods.
-
L5 – activity count for the 5 most restful hours.
-
L5 – start hour (of the 5 most restful hours).
-
M10 – activity count for the 10 most active hours.
-
M10 – start hour (of the 10 most active hours).
-
-
Core night-time analysis sleep measures:
-
Core night-time (00.00 to 06.00) sleep efficiency.
-
Core night-time (00.00 to 06.00) sleep time (minutes).
-
Core night-time (00.00 to 06.00) wake time (minutes).
-
Carer measures
Demographic characteristics
Sociodemographic details [sex, date of birth, current or last occupation, carer relationship to person with dementia, co-resident carer (yes/no), average number of visits per month (non-resident carer) and ethnicity] were collected at baseline. The details of a carer’s sex and relationship to the person with dementia were available for all potential participants referred to the study, including those who did not provide consent to be screened, those who were not eligible and those not randomised.
Potential outcomes for the main trial
-
Carer sleep quality was measured using the following:
-
The Pittsburgh Sleep Quality Index (PSQI)52 at baseline and 3 months. It is a validated, reliable instrument to measure the carer’s sleep (since it is commonly disrupted by sleep–wake patterns of the person with dementia).
-
The Sleep Condition Indicator (SCI)53 at baseline and 3 months (a new eight-item scale developed in the UK with data on tens of thousands of people of all ages). It characterises sleep both dimensionally (like PSQI) and against insomnia disorder criteria (which PSQI does not).
-
-
Mood disturbance was measured using the Hospital Anxiety and Depression Scale (HADS) at baseline and 3 months. 54,55 It is a validated, reliable measure of mood in carers throughout the age groups.
-
Subjective burden for carers was measured using the Zarit Burden Interview (ZBI) at baseline and 3 months. 56 It is the most commonly used and well-validated measure of burden for carers of people with dementia and was used in this report to indicate whether or not burden may be changed by the intervention.
-
A carer’s health-related quality of life was measured using the Health Status Questionnaire-12 (HSQ-12) at baseline and 3 months. 57 It is a 12-item quality-of-life scale validated throughout the age group.
Interventions
DREAMS START intervention development
Conceptualisation of manual elements
The DREAMS START intervention was developed using an iterative and collaborative coproduction process with the team that developed the START and Managing Agitation and Raising Quality of Life (MARQUE) manualised interventions for carers of people with dementia (GL, PR, CC), experts in manualised cognitive–behavioural interventions for sleep disorders (CAE, SDK), our collaborators from the Alzheimer’s Society (JAP, RH) and carers of people with dementia who have experienced sleep disturbances, and through practice sessions as part of therapist training. Figure 1 summarises the manual development process.
FIGURE 1.
Development process of the DREAMS START manual.

Development of the intervention began in February 2016 when the project management team met and agreed that DREAMS START would be a six-session, multicomponent intervention, based on the best existing evidence at the time,26,35,37,38,58–61 comprising a cognitive–behavioural component (including psychoeducation, coping skills for families, and activities for people living with dementia) and light therapy. Figure 1 provides an overview of this process.
The first stage of intervention development was to share the existing StrAtegies for RelaTives (START)35 and Oxford’s manuals on cognitive–behavioural therapy for insomnia62 within the team and to consider which elements to build into the DREAMS manual. One month later, Gill Livingston, Simon D Kyle and Penny Rapaport met face to face and, joined by Colin A Espie on the telephone, agreed the main initial structure and content of the six sessions. At this stage, it was agreed that the intervention would be both interactive and individualised, using the actigraphy data collected at baseline to inform a shared understanding of the person with dementia’s night-time settling and waking problems, and their daytime sleepiness/fatigue and to help generate an optimal ‘sleep window’. As in the team’s previous work, a collaborative, non-prescriptive model was adopted, explicitly encouraging family carers to build on their own experience of what they have found works and to develop and use new techniques and behavioural strategies, recording what works in their manual, and continuing to use successful strategies. A specific focus was incorporated within sessions on identifying and overcoming barriers to changing behaviours and routines of people with dementia and considering how to minimise the risk of harm when people with dementia are awake at night. In line with the form of the START intervention, each session included a stress reduction exercise, a between-session practice task, a recapitulation on the previous session and troubleshooting around putting strategies into practice. Five of the same stress reduction techniques from the START intervention were used in the DREAMS START manuals (sessions 1–5). All manuals from the START intervention are available freely online. 36,60
Session structure
Penny Rapaport and Simon D Kyle developed initial drafts of the six manual sessions (for further details, see Intervention content). These were as follows:
-
understanding sleep and dementia
-
making a plan
-
daytime activity and routine
-
difficult night-time behaviours
-
taking care of your own sleep
-
what works? Using strategies in the future.
Each session, after the first one, followed a similar structure. It began with a recapitulation of the previous week’s session and a discussion about what the carer had achieved since then. The new topic was then introduced, and the carer and therapist generated relevant, individually tailored, plans that the carer could implement in the coming week. At the end of the session, the therapist talked the carer through a new relaxation exercise.
Drafts of the manuals were initially circulated within the project management team and revised based on feedback on both form and content. The goal was for the manuals to present comprehensive information succinctly and in plain English, without jargon, in an interactive format that encouraged carers to actively engage in sessions. This was done by including both theoretical components and practical exercises that called on the carers’ own experiences and were discussed during the session. In addition, vignettes were used to involve the carer and to illustrate points, and to display diagrams and graphical information simply and clearly. We tried to maintain a balance between information provision and interactive exercises, to ensure that the carer participated in, rather than was lectured about, the manual. Large chunks of overly technical information were avoided and information was presented in a clear and engaging manner.
Patient and public involvement in the intervention development
After making initial revisions, family carers were invited, via the Alzheimer’s Society Research Network (ASRN), to attend a focus group (in May 2016) to provide feedback on the drafts and to share their experiences of what strategies they did and did not find useful in caring for a relative with sleep difficulties. The group was facilitated by Penny Rapaport and Kirsi M Kinnunen, based on a semistructured topic guide (see Appendix 4). All carers consented to the discussion being audio-recorded, and discussions were then externally transcribed and the data were entered into NVivo 11 software (QSR International, Warrington, UK) and analysed using thematic analysis. 63,64 Two researchers (BH and LW) independently coded the transcript for the main themes that occurred and each labelled the themes and generated a thematic framework. They then met with Penny Rapaport to discuss and agree a consensus on their coding and generate the final framework, according to which the data were organised. Two current and two former carers of people with dementia attended the focus group and, having further revised the manual based on this initial feedback, a virtual reference group of family carers and people living with dementia was consulted by e-mail to obtain further feedback on the draft manuals. The virtual reference group included the four focus group participants and three additional members of the ASRN, including one person living with dementia, one current carer and one former carer.
The key themes elicited from the focus group related to the sleep disturbances that people with dementia experienced and the explanations carers had for these difficulties, the effects that sleep disturbances had on the carers, what they found helpful, and suggestions that they had for the DREAMS START intervention.
The main difficulty that the carers described was their relative waking repeatedly in the night and getting out of bed. This often increased the risk of coming to harm, for example by falling down the stairs or by using electrical appliances unsafely. Carers attributed this night-time waking to a number of causes, mainly confusion, worrying about their symptoms, and dementia type and severity. Confusion meant their relative with dementia mixing up night and day, which one carer felt was exacerbated by being indoors and inactive during the day.
All four of the carers in the focus group spoke of the emotional, physical and practical effects that their relative’s sleep disturbance had on themselves, their relatives and other family members. The main effect on carers was that they themselves became sleep deprived. This happened because they were woken up directly by their relative, and also because they were kept awake by the worry that their relative might awaken, get up and come to harm. Carers described how the lack of sleep over time had negative physical effects on their health and affected their ability to work and function during the day. Carers highlighted various successful and unsuccessful strategies that they had used. Gentle touch and physical comfort had a relaxing effect on their relatives at bedtime and during the night, and keeping the evenings calm and winding down before bed was helpful. They also highlighted the importance of carers finding ways to manage their own stress and sleep problems. There was a common consensus between the carers that using medication to manage sleep problems in their relatives was not helpful. Some also found that there sometimes did not seem to be any pattern to the sleep problems and no helpful strategies.
These findings were used to further refine the manual, ensuring that the emergent themes were included and, in particular, that there was a greater focus on carers finding ways to minimise the impact of sleep disturbances on their own well-being and ensure that the person with dementia was physically and emotionally comfortable. At this point, direct quotations from the carers about their experiences were added to the manual to situate the content and privilege the experiences of family carers. In addition, both the focus group participants and the virtual reference group participants gave feedback and made suggestions about different aspects of the manual sessions. These are summarised in Table 1.
| Topic | Suggestions |
|---|---|
| Content | To include more on the impact of different dementia types on sleep |
| To make session 1 less didactic | |
| To explain the actigraphy data very simply | |
| To include a discussion of becoming more agitated or confused in the evening | |
| To include more on the physical causes of sleep problems, including medication | |
| To focus on increasing activities at times that the person is most likely to nap | |
| To mention the impact of nutrition on sleep | |
| Design | To reduce the number of words per page and overall |
| To include more pictures to make the manual more readable | |
| To ensure that any instruction to carers is very detailed and clear | |
| To simplify the diagrams delineating sleep processes | |
| To change specific pictures that were not felt to fit | |
| Formatting and typographical errors were identified | |
| Session delivery | To emphasise potential benefits of the intervention and how the intervention could help and make life easier |
| To ensure that the tone of this was as a partnership, working together with the carer rather than teaching them | |
| To respect the carer’s existing knowledge and experience | |
| To allocate more time for session 1 or reduce the content |
Finalising the intervention
Having further revised the manual based on feedback from family carers, Penny Rapaport drafted a therapist version of the manual that included additional prompts and guidance for the therapists. At this point, the team also checked the manuals for readability using the Flesch Reading Ease test,65 and all sessions were rated to be within the categories of ‘fairly easy’ or ‘easy’ to read. The research assistants who were going to be delivering the intervention then spent time practising each session in pairs, with Gill Livingston, Penny Rapaport, Claudia Cooper and Kirsi M Kinnunen making further revisions to timing, content and structure whenever it did not flow or was unclear, too long or repetitive. The research assistants also met in groups, practised delivering the sessions and provided oral and written feedback on how to improve the sessions and increase the accessibility and clarity of the session content. This process was repeated until it was agreed by the researchers and therapists that the sessions were ready for use. Box 1 shows the derivation of the final manual, including where each element of the intervention came from. Final versions of both the therapist and carer versions of the manual were then produced for use within the study (see Appendix 5).
Sleep and dementia – material provided by Simon D Kyle.
What is sleep? – material provided by Simon D Kyle.
What causes sleep problems in dementia? – written by Penny Rapaport, Simon D Kyle and Gill Livingston for DREAMS.
Making changes to improve sleep (lifestyle and bedroom factors) – adapted from CBT work by Colin A Espie.
Managing the stress that sleep problems can bring – adapted from START.
Managing stress: the signal breath – adapted from START.
Summary – adapted from START.
Putting it into practice – adapted from START.
Session 2Recapitulation on understanding sleep and dementia.
Light and sleep – material provided by Simon D Kyle.
Light, dementia and the body clock – material provided by Simon D Kyle.
Making a light therapy plan – developed for DREAMS.
Your relative’s sleep pattern – developed for DREAMS.
Making a new sleep routine: your relative’s plan – developed for DREAMS based on work on sleep efficiency by Colin A Espie and Simon D Kyle.
Managing stress 2: focused breathing – adapted from START.
Summary – adapted from START.
Putting it into practice – adapted from START.
Session 3Recapitulation on making a plan.
The importance of daytime activity and routine – adapted from START.
Planning daytime activity – adapted from START.
Sleep, exercise and physical activity – developed for DREAMS by Penny Rapaport, Gill Livingston and Simon D Kyle.
Establishing a good day and night routine – adapted from CBT work by Colin A Espie.
Managing stress 3: guided imagery – adapted from START.
Summary – adapted from START.
Putting it into practice – adapted from START.
Seated exercises visual guide – from NHS Choices website. 66
Session 4Recapitulation on daytime activity and routine.
Troubleshooting: putting plans into action – developed for DREAMS.
Managing night-time behaviour problems – adapted from MARQUE/START.
Describing and investigating behaviours – adapted from MARQUE/START.
Managing stress 4: stretching – adapted from START.
Summary – adapted from START.
Putting it into practice – adapted from START.
Session 5Recapitulation on night-time behaviour problems.
Creating strategies for managing behaviours – adapted from MARQUE.
Managing your own sleep – developed for DREAMS.
Managing thoughts and feelings – adapted from CBT work by Colin A Espie.
Challenging unhelpful thoughts and feelings – adapted from START.
Managing stress 5: guided imagery – ocean escape – adapted from START.
Summary – adapted from START.
Putting it into practice – adapted from START.
Session 6Overall structure based on that developed in START and refined in MARQUE.
Putting it all together.
What works? Light, sleep and dementia – written by Simon D Kyle for DREAMS.
What works? The importance of daytime activity – from START.
What works? Making a new sleep routine – based on the sleep manual by Colin A Espie and Simon D Kyle.
What works? Making changes to improve sleep – based on the sleep manual by Colin A Espie and Simon D Kyle.
What works? Managing night-time behaviours – based on MARQUE/START.
What works? Challenging unhelpful thoughts and feelings – based on START and CBT work by Colin A Espie.
What works? Relaxation – based on START.
Keeping it going – developing an action plan – developed for DREAMS.
Action plan for you and your relative – developed for DREAMS.
Summary – developed for DREAMS.
CBT, cognitive–behavioural therapy.
Adherence to the manual
To inform the calculation of adherence to the intervention, the number of sessions that would count as adherence was discussed in a project management meeting. It was agreed that this was a matter of clinical judgement. Penny Rapaport (psychologist) and Gill Livingston (psychiatrist) judged that, as this was a group of people who often had comorbidities, those who attended most of the sessions, that is four or more, could be judged as adhering to the manual. This was specified in the analytical plan before analysis.
Intervention delivery
The six-session DREAMS START intervention is manual based and was delivered by trained and clinically supervised psychology graduates to carers. They were sometimes accompanied by people with dementia who were able to participate or whom chose to be there. Each carer was given part of the manual specific to each session at the beginning of each meeting, for them to write in and keep. Generally, the sessions took place in the home of the carer or the home of the person with dementia for whom they were caring. Occasionally, carers chose to have sessions elsewhere (the team base, the premises of the memory service from which the participant had been referred to the trial) because it was easier for them to attend. Each session lasted ≈1 hour and took place approximately weekly, at a time convenient to the carer, including evenings. The intervention sessions were generally delivered to the family carer who had been recruited to the study. However, sometimes paid carers participated in the sessions if, for example, they were with the person around the clock who would be most likely to implement strategies. Generally, the carers were encouraged to have the sessions without the person with dementia in the room, so that they could talk freely about any difficulties and to reduce any potential distress to the person with dementia. If the person with dementia wanted to participate in the sessions, either alone or with their main carer, they were included in the sessions. However, carers were encouraged to also participate in the sessions to maximise the potential for new strategies to be developed and used.
Intervention content
The DREAMS START manual used in the trial was designed to optimise both the individual’s sleep at night and his/her wakefulness during the day. To optimise night-time sleep pressure and the circadian regulation of sleep, the intervention focused on supporting carers to use practical zeitgebers (cues that influence a person’s biological rhythms, e.g. regular timing of bed and rising, morning wake-up light, standardised mealtimes) and to establish adaptive stimulus control (e.g. pre-bed settling routine, management of wakeful episodes). The intervention introduces strategies to promote de-arousal at night (e.g. relaxation, bedroom comfort, no caffeine or alcohol intake before bed, no activities in bedroom) and behavioural activation during the day to maintain alertness, reduce daytime naps and increase engagement to strengthen both central and peripheral clock timing. The intervention also focuses on helping carers to develop coping strategies to manage concerns about their own sleep health.
Sessions 1–5 all included one or two key topics for discussion, a specific plan or goal to try out between sessions, a stress reduction exercise with an accompanying compact disc (CD)/MPEG-1 Audio Layer III (MP3) file and a sleep diary for the carer to fill in for monitoring progress between sessions. Although manualised, during each session the participants would develop specific goals, which were combined to make an individualised plan.
The six intervention sessions covered the following:
-
Understanding sleep and dementia – psychoeducation on the importance of sleep, sleep process, the impact of dementia on sleep and what causes sleep problems in people with dementia. This session also discussed lifestyle and bedroom environment factors that can affect sleep, and carers were encouraged to identify potential changes that they could try out before the next session. In addition, the impact that sleep problems can have on the person with dementia and their relative was discussed.
-
Making a plan – psychoeducation on the importance of light for sleep and the relationship between light, sleep and dementia. This session was built around the light and activity data collected by actigraphy at baseline, with the individual’s data inserted into the manual in advance of the session and an explanation given. The carers were given a ‘light box’ (Lumie Arabica SAD Light, Lumie, Cambridge, UK) to keep and it was switched on and left on during the sessions to encourage people to understand that they habituated to the bright light (10,000 lux at 25 cm). A time switch was provided if carers wanted it, so that it could be switched on when they were otherwise engaged. It was recommended that the light box be used for 30 minutes at the same time every morning. During the session, the participants made an individual plan for increasing natural and artificial light for the person with dementia. Based on the individual’s sleep data, a ‘sleep efficiency’ score (time asleep/time in bed) was calculated, and any suggested changes to an individual’s time to bed and rise and for reducing daytime naps was integrated into their individual action plan.
-
Daytime activity and routine – this session highlighted the importance of daytime activity and routine and focused on building pleasant activities and exercise/physical activity into the day. This session included a seated exercise video for less physically able individuals. It also included the importance of establishing a good day and night routine and ways to strengthen the link between bed and sleep. In addition, an exercise and activity plan were built into the individual plan.
-
Difficult night-time behaviours – this session began with troubleshooting around putting the individual plan into action and identifying potential solutions to any barriers. The rest of the session focused on describing and investigating difficult night-time behaviours that were specific to the individual with dementia.
-
Taking care of your own sleep – this session included using the information collected on difficult night-time behaviours to create strategies for managing these difficulties. The rest of the session focused on the carer managing their own sleep, including ways to challenge unhelpful thoughts and feelings and make time for themselves.
-
What works? Using strategies in the future – this session recapitulated on earlier sessions and focused on what carers found useful and what worked. From this, an individualised sleep action plan was finalised, which included strategies for both the person with dementia and the carer.
Treatment as usual
Participants received TAU for 6 weeks, delineated by the CSRI. This was expected to vary between trusts, and also according to individual patient needs, but was expected to be in line with the National Institute for Health and Care Excellence (NICE) pathways guidelines for dementia. 67 TAU varies according to the practices of the trust in which the person with dementia is treated and the person’s individual needs, but incorporates the NICE pathways guidelines67 for dementia and consists of assessment, diagnosis, symptomatic interventions, risk assessment and management and information.
The volunteers from JDR may not have been currently receiving professional care, but details of any services they were receiving were gathered. Services are based around the person with dementia. Treatment is medical, psychological and social. Thus, TAU consisted of assessment, diagnosis, risk assessment and information. These included referral to dementia navigators; medication; cognitive stimulation therapy; START (in some trusts); practical support (social services provided); risk plans, for example telecare, treatment of neuropsychiatric symptoms, driving information to the Driver and Vehicle Registry Agency, medical identification (ID) bracelets and advice regarding power of attorney and capacity assessment; and social services referral for personal care, day centre and financial advice, and carer support.
After the follow-up data had been collected (including the 2 weeks of follow-up actigraphy), the participants in the control group received a summary of the baseline Actiwatch data, with advice on improving sleep (see Appendix 6). To maintain the follow-up assessor’s blinding until the follow-up Actiwatch data had been analysed, the unblinded team posted this information to the participants once the follow-up actigraphy was complete (of which the assessor informed the unblinded team).
Both groups
After the follow-up assessor’s unblinding, the assessor sent the participants an end-of-involvement letter (see Appendix 7), enclosing a summary of the follow-up Actiwatch data. The referring clinician was sent a copy of the letter.
Details of how the interventions were standardised
The intervention was standardised primarily through being manualised. This allowed the research team to maintain tight control over how the intervention was delivered, and provided a clear and detailed structure for the graduate psychologist therapists to adhere to. It also meant that the carers could keep the manual and refer back to notes, plans and information between and after the sessions. Variation came with the introduction of personalised goals and strategies, and the use of individual actigraphy data. Delivery of the interventions as intended and in a standardised way was assured by training the therapists and ensuring that they were ‘signed off’ as individually competent in each session, and by offering regular and structured clinical supervision, as detailed in the following section.
Training the therapists
Four research assistants who were psychology graduates with no clinical training were employed to deliver the intervention and received in-depth training prior to delivering the intervention. The research assistants participated in five knowledge and skills-based sessions on the following topics:
-
Dementia (CC)
-
Sleep (SK)
-
Introducing DREAMS START (GL)
-
Clinical skills for delivering the intervention part 1 (PR)
-
Clinical skills for delivering the intervention part 2 (PR).
Training was delivered through a combination of seminars, discussion, reflective learning and guided reading. Skills-based competencies were learnt through role-play, small-group exercises and clinical simulation in pairs. Training drew on the curriculum for psychological therapists devised by the Department of Health and Social Care for its IAPT programme, and the successful training programme developed for the START intervention. There was a strong practical focus in the training programme on how to deliver the therapy, potential clinical dilemmas, collaborative goal-setting, managing sessions with more than one participant, working with interpreters, empathic listening skills, effective use of supervision, safe working practice, and when to ask for help. Throughout the training, an emphasis was placed on the researchers facilitating carers to develop and practise using their own strategies and finding their own solutions rather than feeling the need to instruct carers and provide solutions within the session. In addition to the knowledge and skills-based training, therapists were trained to adhere to the manual by practising repeatedly in pairs, and required to demonstrate, by role playing the entire intervention for one of the clinical members of the research team, competence in delivering each session of the intervention to an agreed standard.
Supervising the therapists
The process of formal clinical supervision of the therapists began at the start of the intervention delivery period and continued until the final sessions had been delivered. The clinical psychologist, Penny Rapaport, met with each of the two teams (two therapists in each team) for 1.5 hours of group supervision per fortnight. In addition to this group supervision, she was available for individual supervision, which was either requested by the psychology graduates on an ad hoc basis, or (on occasion) was initiated by the investigators. If they had any urgent clinical or procedural concerns or questions relating to their clients, the therapists could approach Penny Rapaport, Claudia Cooper or Gill Livingston at any time, for example if risk issues arose during a session.
The group supervision format was seen as the most effective use of available resources, with psychology graduates benefiting from both the professional expertise of their supervisor and the clinical experiences of their peers; it was successfully applied in the START randomised controlled trial (RCT). It was expected that the supervision format would also maximise peer support within and outside the supervision sessions, and facilitate effective teamworking. The format was tailored to reflect the specific needs of the present research study. During the course of the project, supervision performed a number of functions including case management, clinical skills development, ensuring safe practice with clients, and staff support. Each of these functions is explored in turn in the following sections.
Case management
An important function of supervision was to ensure that all of these interventions were being managed consistently, effectively and appropriately. Therefore, in every group supervision session, each therapist provided a brief overview of their caseload, ensuring that clients and any related issues or concerns did not get overlooked. This encouraged the therapists to be transparent about their work and to recognise when apparently simple or straightforward cases were more complex than initially perceived. As the cases for intervention were allocated and managed within the team, it was useful for the therapists to be aware of who their colleagues were seeing and who had space to take on new clients, developing a sense of shared responsibility. 61
Clinical skills development
Group and individual supervision sessions provided the therapist with the opportunity to develop their clinical skills via a range of approaches. In addition to giving a brief summary of their caseloads at the start of every supervision session, the therapists would identify a clinical challenge or dilemma that they wished to explore in more detail. 61 Although the intervention was manualised and psychology graduates were expected to adhere strictly to the manual, there was great variety in the dilemmas that they encountered in delivering the intervention. Various issues tended to emerge within supervision, for example how to keep carers focused on the manual and engaged in the process, and how to manage sessions when the person with dementia and the carer were both contributing. Using a combination of role play and reflection on extracts of the fidelity recordings, the therapists were able to enhance their skills in delivering the intervention in a safe environment.
Ensuring safe practice with clients
There was a written policy about lone working and safeguarding, to which the therapists were trained to adhere. The clinical team provided the therapists with specific training in how to respond to any risks either disclosed by carers or witnessed in interactions, in relation to harm to either themselves or the person for whom they were caring. If concerns were raised, a plan was made with one of the lead investigators about how to manage the risk, and information was shared with the local clinical teams. By ensuring that there was an opportunity for individual supervision on request, and that a senior member of the team was always available, a culture of transparency developed whereby the therapists felt comfortable raising concerns about clients with the clinical academics in the team. Time was also taken within supervision to highlight the importance of behaving ethically and safely in all aspects of clinical work, for example how to practise safely when working alone in people’s homes.
Staff support
Many of those receiving the intervention were experiencing high levels of emotional distress, often in the context of challenging social situations and physical environments. An important dimension of clinical supervision was to give the therapists an opportunity for self-reflection, making sense of their own responses to the people with whom, and situations in which, they were working. The combination of group and individual supervision meant that the psychology graduates benefited from the support of their peers and felt that their experiences were validated by their shared experiences.
Details of how adherence of care providers to the protocol was assessed or enhanced
Monitoring fidelity to the intervention
In addition to the close supervision and training identified in the previous section, a formal process for monitoring the fidelity of the therapists to the manualised intervention was instigated. Following a similar process to that used in the START RCT,35,61 therapists audio-recorded one session per participant. The session to be recorded was selected at random by the trial manager using a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) formula, before any interventions were carried out. Penny Rapaport devised a fidelity checklist for each session by considering the most important components of each session (see Appendix 8). To maintain the follow-up assessor’s blinding, this session was rated for fidelity to the manual by the other therapist in the same team (who was not involved in that participant’s intervention), using the checklist. For each recorded session, a fidelity score for four process factors was given by the rater, considering whether the therapist was (1) keeping the session to time, (2) keeping the carer focused on the manual, (3) keeping the carer engaged in the session and (4) managing the concerns of the carer. Possible scores ranged from 1, meaning ‘not at all’, to 5, meaning ‘very focused’, for each item. Ratings were out of a possible 5 points.
Outcomes
Primary outcomes
-
Feasibility of recruitment: this was assessed by (1) the proportion of participants consented out of those meeting the eligibility criteria at screening and (2) the proportion randomised after baseline assessment.
-
Feasibility of the intervention: this was assessed by recording the proportion of participants randomised into the intervention group who by the end of the trial had attended four or more of the six sessions.
-
Acceptability of the intervention: this was assessed through qualitative interviews with up to 20 intervention group participants after follow-up, post unblinding.
Therapist
Secondary outcomes
-
Referral rates from the recruitment period were measured from records about all and eligible referrals at the end of the recruitment period.
-
Follow-up rates were measured after the last follow-up visit, from records indicating which participants completed follow-up assessments at 3 months.
-
Reported side effects (patient falls and comorbid physical illnesses) were recorded using a study-specific DREAMS side-effects questionnaire at baseline and 3 months.
Acceptability of outcome measures for a future trial of clinical effectiveness and cost-effectiveness was assessed through recording the completion rates of instruments (see below) at baseline and 3 months, the acceptability of tools from the qualitative interviews post unblinding, and calculating the sample size that would be required to detect a clinically important difference in outcome.
Qualitative interviews
Sample recruitment and procedure
A purposive sample of carers who had received the intervention for qualitative interviews was recruited in order to assess acceptability of the intervention and whether or not there were groups that the intervention was unsuitable for, and to detail any required refinements in the intervention or the assessment procedure. They were invited to participate in the interview after follow-up assessment and unblinding.
Purposive sampling was used to ensure that people from a range of sociodemographic backgrounds were interviewed. 68 Included were people of differing ages and relationships to the person with dementia, family and paid carers, those living with the person with dementia and those living separately, people from varying ethnic backgrounds, those who had finished the intervention and one carer who had not finished the intervention. Carers were recruited until theoretical saturation was reached.
After this, Penny Rapaport and Lucy Webster met with the ASRN members who had been part of the development of the manual for a focus group to consider any changes post trial.
Qualitative interview content
A semistructured interview guide was developed for the carers who had been participants in the trial (see Appendix 9) of open-ended questions based on our study objectives. These explored the acceptability and practicality of both the intervention and the assessment measures, to gain suggestions for refining the trial. The interview guide was revised iteratively during the interview phase, adding themes as they were brought up by interviewees. All interviews were audio-recorded and recordings were destroyed after analysis.
After completing the trial and gaining qualitative feedback from the participants, the family carers that had contributed to the intervention development were invited to attend a focus group at the Alzheimer’s Society premises in September 2017. The aim of this focus group was to hear group members’ thoughts on the qualitative findings, the final version of the manual used in the trial and the materials being presented, and any suggestions for further refinement of the intervention.
Analysing actigraphy data
The actigraphy data were analysed using MotionWare Software 1.1.25 (CamNtech Ltd, Cambridge, UK), in accordance with a standard operating procedure (see Appendix 10). The analyses were performed as described below; baseline and follow-up measures were produced in the same way.
Sleep and non-parametric circadian rhythm analysis measures
The sleep analysis function of MotionWare was used to produce sleep measures from overnight actigraphy data. The NPCRA analysis in MotionWare is based on an approach that does not assume that the data fit any predefined distribution. 69 First, the whole recording period was highlighted, and the editing tool was used to remove recordings exceeding 14 days and periods of missing data (when the watch was off the wrist based on notes in the sleep diary or the carer’s verbal report on Actiwatch data collection, or the recording indicated no data). The maximum recording period used for the analysis was 14 days (in the majority of cases from 17.00 on the first day to 17.00 on day 14, but in some from 18.00/19.00 on the first day to 18.00/19.00 on day 14). Second, using the edited data, each sleep period was defined, choosing as the start and end points the bedtimes and rise times recorded in the sleep diary or by using event markers (showing as blue lines in the data). If the carer did not complete the sleep diary or use the event maker button, the bedtimes and rise times were defined based on the carer’s verbal report on Actiwatch data collection. In addition, the activity and light data were used as guidance for editing the sleep periods. When unsure, two researchers edited a sleep period and reached a consensus. Each sleep period was saved. The edited recording period was also saved to derive the NPCRA measures, and the summary option used to record average light (lux) over the same period. Finally, a report was produced, including all the sleep analyses and NPCRA measures evaluated in the feasibility trial as possible outcomes for the main trial. In the statistical analysis, sleep data based on fewer than seven nights were excluded. To produce NPCRA measures used in the statistical analysis, copies were made of each previously saved edited recording. The length of each missing data period was then checked, and any 24-hour periods with ≥ 3 hours of missing data were excluded. 70 Having saved the NPCRA period, a new report was produced containing the non-parametric measures only.
Core night-time measures
To produce sleep measures for what the research team defined as core night-time (0.00 to 06.00), a copy was created of the edited recordings previously saved. The existing sleep periods were removed and then the sleep summary table option was used to limit each new sleep period to start at 0.00 and end at 06.00. Having saved these periods, a report was produced containing the new sleep measures.
Sensitivity analysis
In a sensitivity analysis, only participants who lived with a family carer at baseline were included on the sleep and NPCRA measures. The sleep data that were based on seven or more nights and the NPCRA data without ≥ 3 hours of missing data for each 24-hour period were used.
Quantitative analysis
The flow of participants through the trial is described using a Consolidated Standards of Reporting Trials (CONSORT) diagram (see Figure 3). A patient’s recruitment site, sex of the person with dementia, sex of the carer and a carer’s relationship to the person with dementia were summarised as counts and proportions. Eligible patients and carers who consented were compared with those who were screened and did not consent. The following, with 95% CIs, were calculated:
-
proportion of screened patients who were eligible for the trial
-
proportion of eligible patients referred who consented to the trial
-
proportion of participants in each randomised group who dropped out or were lost to follow-up by 3 months (when available, reasons for losses will be summarised)
-
proportion of participants in the intervention group who adhered to the intervention (i.e. attended at least four of the six sessions)
-
median number of sessions attended by those in the intervention group.
Summary of baseline data
Baseline data (sociodemographic characteristics, actigraphy measures and other scores) for participants and carers were summarised by treatment group using means (with SDs), medians [with interquartile ranges (IQRs)], counts and proportions, as appropriate, to gauge the balance in characteristics between the randomised groups.
Three-month follow-up
Follow-up scores, actigraphy measures and other scores at 3 months were summarised using means (with SDs), medians (IQRs) and counts (%) as appropriate. For continuous measures, correlations between baseline and follow-up measurements were calculated. The number of participants with completed values was summarised for each outcome.
Measurements were compared between randomised groups using appropriate regression models to provide estimates of the effect of the intervention with 95% CIs (e.g. difference in means), adjusted for baseline score and site. For actigraphy data analyses, the following were focused on: sleep efficiency, relative amplitude, sleep fragmentation index, start hour of most restful hours, activity count for most restful hours, start hour of most active hours and activity count for most active hours for those with ≥ 7 days of data. Core sleep data between 0.00 and 06.00 were measured and a sensitivity analysis excluding participants who did not live with a carer at baseline was carried out.
Use of psychotropic medication
The frequency (%) of participants in each randomised group who had taken each type of medication (anxiolytics and hypnotics, antipsychotics, antidepressants, other psychotropics and melatonin) during the 3-month period prior to both the baseline and follow-up assessment was calculated.
Side effects
The frequencies (%) of comorbid physical illnesses and participant falls were summarised by randomised group at baseline and 3 months.
Qualitative analysis
Each interview with a carer who had participated in the trial was transcribed verbatim, with the transcription checked for accuracy by listening to the recording, and then anonymised. The transcribed interviews were entered into a software package for qualitative data analysis (NVivo 11). A thematic framework for analysis64 was created by displaying coding in matrices and diagrams until a comprehensive picture of all the phenomena was obtained, a standard, recommended method to ensure rigour. 71 This constant comparison method was used to identify similarities and differences in the data. To create the initial coding framework, trial manager Kirsi M Kinnunen and research assistants Brendan Hallam and Lucy Webster independently coded the first three interviews, identifying the main themes that occurred in line with the study’s objectives. They then met with investigators Gill Livingston and Penny Rapaport to discuss the themes and decide on a coding framework. If any discrepancies were identified, they met with Penny Rapaport and reached a consensus.
Using the initial framework, different pairs of researchers then independently coded all of the interviews and, as emerging themes and subthemes were identified, iteratively revised the framework. Each pair of researchers met to discuss discrepancies and agreed a consensus.
Similarly, in the focus groups of ASRN members, the carers consented to the discussion being audio-recorded. The recording was then externally transcribed and the data entered into NVivo 11 software and analysed using thematic analysis. 63,64
Sample size
It was estimated that with 40 intervention participants (a larger group than the control group, to allow a more precise estimate of the proportion adhering to the intervention) and 20 control participants, the following 95% CIs would be achieved for the expected adherence and participation estimates:
-
proportion of participants adhering to intervention – expected value 75%, 95% CI 59% to 87%
-
proportion of appropriate referrals consenting to the trial – expected value 50%, 95% CI 41% to 59%.
This sample size was also judged sufficient for estimating the SD required for the sample size calculation in the main trial. 72,73 The estimated recruitment referral rate was approximately six potential participants per week. Two out of these were expected to be suitable and agree to participate. The expected follow-up rate was approximately 80%.
It was anticipated that the 95% CI for the expected adherence and participation estimates would provide acceptable ranges to inform continuation to the main trial. Overall, it was expected that the ‘stop–go’ measures would be related to the proportion adhering:
-
≥ 70% – go to main trial
-
60–69% – consider a modified trial design to increase adherence
-
< 60% do not progress to main trial using this model.
Randomisation
Computer-generated randomisation lists were produced in Stata® Version 14 (StataCorp LP, College Station, TX, USA) by an independent statistician (not involved in data analysis). Randomisation was stratified by site and based on random permuted blocks of sizes three and six to allow 2 : 1 allocation to the intervention and TAU groups. The three site-specific lists produced were password protected, and could be accessed only by two members of a separate study team (randomisation allocator, as seen in Figure 2).
Blinding
Figure 2 shows the process from screening/baseline assessments (pre randomisation) through intervention delivery to blinded follow-up assessments. Three researchers [Assessors (A) 1 to 3 in Figure 2] screened the participants and carried out baseline assessments. The two researchers who were also therapists [Therapist (T) 1/A1 and T2/A2] asked for allocations from the randomisation allocators. They worked in two separate teams of two therapists each (Team A: T2 and T4; Team B: T1 and T3), and assessed outcomes only for those participants to whom the opposite team had delivered the intervention. The teams had their clinical supervision separately. Researcher A3 did not deliver any interventions and was kept blind to all participants’ allocations. The follow-up assessments were arranged by T1/A1 and T2/A2, for those participants they were unblinded to. Owing to the nature of the intervention, it was not possible to blind the trial participants. When arranging the appointment, participants were reminded that they should not disclose their allocation group to the assessor, and should hide anything related to the intervention from view (e.g. light box, manual). On arrival for the outcome assessment, the assessor also asked participants not to disclose their group. Two baseline assessments and one follow-up assessment took place in our team base at UCL; all others took place in the homes of the participants.
FIGURE 2.
Assessments, randomisation and intervention delivery. A, assessor; T, therapist.

Statistical methods
The co-applicant statistician led and supervised the analysis, planned and conducted according to International Conference on Harmonisation (ICH) E974 and following the standard operating procedures of the PRIMENT Clinical Trials Unit. A predefined statistical analysis plan described the analysis fully; the following sections are a summary of the methods used to assess the primary and secondary outcomes.
Primary outcomes
Feasibility of recruitment
The proportion of eligible patients consenting to the trial and the proportion randomised were calculated with exact 95% CIs. The characteristics of eligible patients and carers who consented and those who did not consent were summarised in terms of site, patient sex, carer sex and carer’s relationship to person with dementia. Comparisons between groups were made using chi-squared tests.
Treatment adherence
The proportion of participants in the intervention group that adhered to the treatment (i.e. attended at least four of the six sessions) was calculated with an exact 95% CI. The median number of sessions attended was also calculated.
Secondary outcomes
Referral rates
Weekly referral numbers were summarised from records kept over the 8 months of recruitment.
Follow-up rates
The proportion of participants lost to follow-up at 3 months in each randomised group was calculated with exact 95% CIs.
All psychotropic medication prescription
The proportion of participants receiving prescription for psychotropic medication was summarised by randomised group at baseline and follow-up. Logistic regression was used to compare prescription of psychotropic medication between the intervention and TAU groups, adjusting for site and baseline use.
Reported side effects: falls and comorbid physical illnesses
The frequency (%) of falls and comorbid physical illnesses are summarised by randomised group, at baseline and follow-up.
Potential outcomes for the main trial
Baseline data
Patient and carer sleep questionnaire scores and actigraphy measures at baseline and follow-up were summarised, by randomised group, using means (with SDs), medians (with IQRs), counts and proportions, as appropriate.
Follow-up data
Measurements were compared between randomised groups using appropriate regression models adjusted for baseline score and site. When normality assumptions of an ordinary least squares regression were met, these models were used to provide adjusted differences in means with 95% CIs. When distributional assumptions were violated, quantile regression was used to provide estimates of the adjusted median difference with a 95% CI. For continuous measures, Pearson correlation coefficients between baseline and follow-up measurements were calculated.
Chapter 3 Results: randomised controlled trial
Participant recruitment and flow
The flow of participants through each stage of the trial is shown in the CONSORT flow diagram75–78 (Figure 3). Four referrals per week were received, on average, and 123 people were referred through memory clinics from 4 August 2016 to 3 April 2017.
FIGURE 3.
The CONSORT flow diagram: summary of recruitment and follow-up.

The study was open on JDR to recruitment from 22 November 2016 to 24 March 2017. The first search for potential matches (22 November 2016) found 49 people. The search was then expanded, using all of the JDR eligibility criteria, but increasing distance from UCL from 5 miles to 10 miles, and found 140 people. The research team began to contact JDR volunteers on 5 December 2016 and stopped on 21 March 2017. Twenty-seven people were contacted using their preferred means of contact: e-mail (n = 25; mostly their carer’s e-mail) or telephone (n = 2). E-mails were followed up by telephone calls. Nineteen volunteers did not respond to contact from the research team; three were not eligible for the study (two carers reported that the person they cared for had no sleep problems and one carer reported that the person they cared for was moving out of London); in one case, only the person with dementia could be spoken to and so their eligibility could not be determined. Of the 120 people assessed for eligibility, 95 were eligible (79%, 95% CI 70% to 86%) and 25 did not meet inclusion criteria.
Comparison of those who consented with those who did not
Table 2 compares the known demographic details of those who consented with those who did not, and shows that the study sample had good external validity. The care recipients who did not consent were, however, more likely to be male.
| Characteristics | Participants, n (%) | p-value (χ2 test) | |
|---|---|---|---|
| Not consented (N = 33) | Randomised (N = 62) | ||
| Person with dementia | |||
| Site | |||
| Camden and Islington | 24 (73) | 40 (65) | |
| Barnet, Enfield and Haringey | 7 (21) | 20 (32) | |
| UCL/JDR | 2 (6) | 2 (3) | 0.460 |
| Sex | |||
| Male | 20 (61) | 19 (31) | |
| Female | 13 (39) | 43 (69) | 0.005 |
| Carer | |||
| Sex | |||
| Male | 11 (33) | 18 (29) | |
| Female | 22 (67) | 44 (71) | 0.665 |
| Relationship to person with dementia | |||
| Spouse | 9 (28) | 19 (31) | |
| Child/child-in-law | 22 (69) | 41 (66) | (Spouse vs. other) |
| Other | 1 (3) | 2 (3) | 0.800 |
Baseline demographic and diagnostic data
Table 3 compares the demographic and diagnostic details of the randomised groups. Overall, there was a good demographic mix, with recruitment from a range of ethnicities and age groups and a variety of relationships between the people with dementia and the carers. There was a range of diagnoses, but the majority of people had AD or a mixed dementia. Most (n = 45, 73%) of the primary carers lived with the person with dementia. Four had another member of the family living with them, so 49 out of 62 (79%) had family members living with them; six (10%) had paid carers living with them, and seven (11%) had family carers but lived alone. The intervention group included more women carers and carers were younger and less likely to be co-residents or spouses. The people with dementia in the intervention group were more likely to be women, and more had a diagnosis of AD than in the TAU group.
| Characteristics | Trial group | |
|---|---|---|
| Intervention (N = 42) | TAU (N = 20) | |
| Person with dementia | ||
| Site, n (%) | ||
| Camden and Islington | 27 (64) | 13 (65) |
| Barnet, Enfield and Haringey | 14 (33) | 6 (30) |
| UCL/JDR | 1 (2) | 1 (5) |
| Sex, n (%) | ||
| Male | 9 (21) | 10 (50) |
| Female | 33 (79) | 10 (50) |
| Age (years) | ||
| Mean (SD) | 80.4 (9.0) | 79.6 (7.0) |
| Lived with/alone, n (%) | ||
| Family carer | 28 (67) | 17 (85) |
| Another family member | 3 (7) | 1 (5) |
| Paid carer | 5 (12) | 1 (5) |
| Alone | 6 (14) | 1 (5) |
| Diagnosis, n (%) | ||
| Alzheimer’s disease | 22 (52) | 7 (35) |
| Dementia with Lewy bodies | 2 (5) | 3 (15) |
| Mixed | 8 (19) | 4 (20) |
| Vascular dementia | 8 (19) | 6 (30) |
| Alcohol-related dementia | 1 (2) | 0 (0) |
| Unspecified | 1 (2) | 0 (0) |
| Age left education (years) | ||
| Mean (SD) | 15.7 (3.7) (n = 40) | 16.4 (4.9) (n = 19) |
| Marital status, n (%) | ||
| Single | 1 (2) | 1 (5) |
| Married | 17 (40) | 11 (55) |
| Divorced | 1 (2) | 0 (0) |
| Widowed | 23 (55) | 8 (40) |
| Ethnic group, n (%) | ||
| White | 27 (64) | 13 (65) |
| Asian | 3 (7) | 3 (15) |
| Black | 7 (17) | 2 (10) |
| Other | 5 (12) | 2 (10) |
| CDR global, n (%) | ||
| Very mild | 5 (12) | 3 (15) |
| Mild | 16 (38) | 6 (30) |
| Moderate | 18 (43) | 9 (45) |
| Severe | 3 (7) | 2 (10) |
| Carer | ||
| Carer sex, n (%) | ||
| Male | 10 (24) | 8 (40) |
| Female | 32 (76) | 12 (60) |
| Carer age (years) | ||
| Mean (SD) | 56.15 (13.54) | 59.09 (12.22) |
| Carer co-resident, n (%) | ||
| Yes | 28 (67) | 17 (85) |
| Relationship to person with dementia, n (%) | ||
| Spouse | 10 (24) | 9 (45) |
| Child/child-in-law | 30 (72) | 11 (55) |
| Grandchild | 1 (2) | 0 (0) |
| Friend | 1 (2) | 0 (0) |
| Carer ethnicity, n (%) | ||
| White | 29 (69) | 14 (70) |
| Asian | 3 (7) | 3 (15) |
| Black | 7 (17) | 1 (5) |
| Other | 3 (7) | 2 (10) |
Primary outcomes
Of the eligible patients referred, 63 (65%, 95% CI 55% to 75%) consented: two from JDR and 61 from memory clinics. A total of 62 out of 95 eligible patients referred (65%, 95% CI 55% to 75%) were randomised. In eight cases, two carers were consented who were involved in the intervention (three people had two family carers; five had one family and one paid carer). A total of 42 people were randomised to the intervention. The median number of sessions attended was six; 37 participants (88%, 95% CI 75% to 96%) attended four or more sessions.
Secondary outcomes
Referral rates
An average of four potential participants were referred by the memory clinics weekly.
Follow-up rates
Of the 62 randomised participants, 57 (92%) were followed up at 3 months. The number lost to follow-up was 4 out of 42 participants (9.5%; exact 95% CI 3% to 23%) in the intervention group and 1 out of 20 (5%; exact 95% CI 0.1% to 25%) in the TAU group. Two people with dementia withdrew consent, two carers were uncontactable and one person with dementia died (see Figure 3).
All psychotropic medication prescription
Table 4 shows the number and frequency of psychotropic medication at baseline and 3 months, with 45% of each group receiving at least one psychotropic medication.
| Medication prescription at baseline | Trial group, n (%) | |
|---|---|---|
| Intervention (N = 42) | TAU (N = 20) | |
| Anxiolytics and hypnotics | 5 (12) | 2 (10) |
| Antipsychotics | 4 (10) | 1 (5) |
| Antidepressants | 14 (33) | 7 (35) |
| Melatonin | 0 (0) | 0 (0) |
| Other psychotropic | 1 (2) | 0 (0) |
| At least one prescription of above medications | 19 (45) | 9 (45) |
Table 5 shows the number of patients in each group who at the 3-month follow-up had a prescription for psychotropic medication or melatonin that had not been prescribed at baseline. The odds ratio of being prescribed at least one of these medications in the intervention group compared with the TAU group was 0.51 (95% CI 0.06 to 4.01; n = 55).
| Medication prescription at 3-month follow-up | Trial group, n (%) | |
|---|---|---|
| Intervention (N = 37) | TAU (N = 18) | |
| Anxiolytics and hypnotics | 2 (5) | 1 (6) |
| Antipsychotics | 3 (8) | 1 (6) |
| Antidepressants | 12 (32) | 7 (39) |
| Melatonin | 2 (5) | 1 (6) |
| Other psychotropic | 1 (3) | 0 (0) |
| At least one prescription of above medications | 16 (43) | 8 (44) |
Reported comorbid physical illnesses and side effects
Table 6 shows comorbid illnesses at baseline and comorbid illness and possible side effects at 3 months. At baseline, both groups reported similar rates of all symptoms. There is no clear pattern of difference in change over time between the two groups.
| Comorbid illness or side effect | Trial group, n (%) | |
|---|---|---|
| Intervention | TAU | |
| Baseline | (N = 42) | (N = 20) |
| Falls | 17 (40) | 7 (35) |
| Gastrointestinal | 21 (50) | 9 (45) |
| Neurological | 19 (45) | 10 (50) |
| Infections | 14 (34) | 6 (30) |
| Other | 6 (14) | 2 (11) |
| 3 months | (N = 38) | (N = 18) |
| Falls | 16 (42) | 7 (39) |
| Gastrointestinal | 16 (42) | 4 (22) |
| Neurological | 15 (39) | 10 (56) |
| Infections | 18 (47) | 5 (28) |
| Other | 9 (24) | 9 (50) |
Intervention delivery
Forty dyads started the intervention. Two dropped out after randomisation but before commencement of the intervention, because the carer cited lack of time or the person with dementia declined. Three (7%) attended one to three sessions. The median duration of intervention delivery was 49.5 days (IQR 43.0–64.5 days).
The intervention was delivered by four clinically trained and supervised psychology graduates, two women and two men, who were ethnically white British (n = 3) and white Asian British (n = 1). Their ages ranged from 23 to 33 years. The therapists visited most participants in their homes. Two were seen elsewhere: one for a single session at UCL and another for all six sessions at Islington Memory Service.
Twenty-one of the carers in the intervention group had sessions without the person with dementia. The sessions were delivered to family or paid carers. This included 18 interventions with the family carer alone, two interventions solely with paid carers (one with one paid carer and one with three paid carers), and one intervention delivered to a family carer and a paid carer together. For five of these interventions delivered to carers, the person with dementia was present in the same room, but did not participate as they could not do so (owing to auditory impairment or the severity of their dementia) or did not want to. In six cases, there was an additional carer or family member present in the room, who observed one session of the intervention.
For 13 dyads, the person with dementia and their carer attended every session together. For others, the person with dementia participated in some sessions, but the family carer also had sessions without them. This included one intervention with the person with dementia, their family carer and their paid carer, and 12 interventions with the person with dementia and their family carer. One person with dementia participated in all six sessions, although their family carer participated in only three sessions.
Altogether, this totalled 138 individual sessions just with carers (12 sessions with paid carers, six sessions with a paid carer and a family carer, and 120 sessions with family carers), 84 sessions with the person with dementia and their carer(s) (78 sessions with people with dementia and the family carer, and six sessions with the person with dementia and their family and paid carers) and three sessions with a person with dementia alone.
The therapists were flexible with timings and often met people in the evening or early morning to fit in with their work and other commitments. On average, each session took 69 minutes (means: session 1, 75 minutes; session 2, 71 minutes; session 3, 74 minutes; session 4, 66 minutes; session 5, 70 minutes; and session 6, 60 minutes).
Fidelity
One randomly selected intervention session was recorded for 34 out of 40 (85%) participants who started the intervention. Three participants refused the audio-recording, two did not continue the intervention up to the session randomised to be recorded and one recording was partial and could not be scored. Across all sessions (Figure 4), managing the carer’s concerns and keeping the carer engaged in the session was rated 5/5 (IQRs 4.00–5.00 and 4.25–5.00, respectively), while keeping the carer focused on the manual and keeping the session to time was rated 4.00 out of 5.00 (IQRs 4.00–5.00 and 3.25–5.00, respectively).
FIGURE 4.
Median scores from fidelity monitoring of all sessions of intervention delivery.
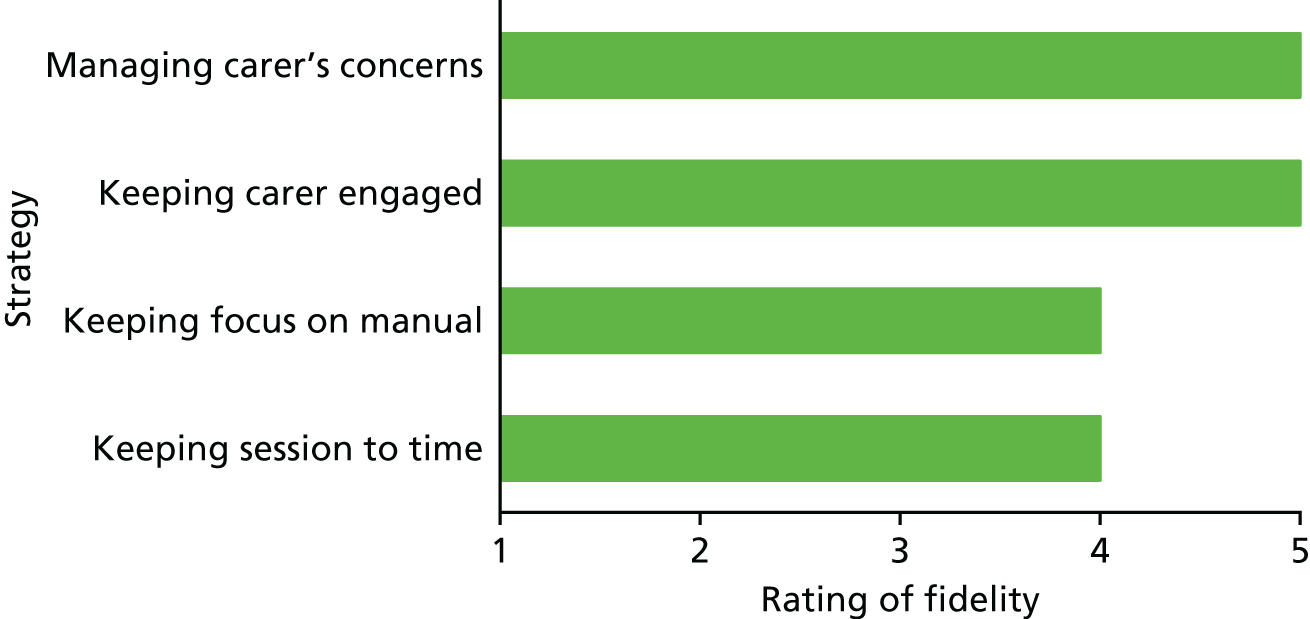
Table 7 shows the strategies that the 37 carers who attended session 6 wrote down as their plan to use in future. Most of them intended to use several strategies.
| Strategies that the carers intended to keep using | n (%) (N = 37) |
|---|---|
| Increasing natural light or using light box | 37 (100) |
| Increasing daytime activity or physical exercise | 37 (100) |
| Making a new routine or strengthening the bed–sleep link | 32 (86) |
| Bedroom and lifestyle changes to improve sleep | 28 (76) |
| Managing night-time behaviours | 26 (70) |
| Carer looking after her/himself and challenging unhelpful thoughts | 32 (86) |
| Relaxation | 28 (75) |
Potential outcomes for the main trial
Completion rate and results of validated interviews at baseline and 3 months’ follow-up
Table 8 summarises completion rates and scores of patient- and carer-validated questionnaire scores at baseline. The NPI, PSQI, SCI and HSQ-12 were completed for 61 out of 62 (98.4%) participants. All other measures were completed for everyone. Summary scores are similar between the two groups, although carers in the intervention group had consistently better scores than carers in the TAU group.
| Interview measures at baseline | Trial group | |
|---|---|---|
| Intervention (N = 42) | TAU (N = 20) | |
| Person with dementia | ||
| SDI global score | ||
| Median (IQR) | 2.57 (1.35 to 3.67) | 2.98 (1.85 to 3.96) |
| ESS total score | ||
| Mean (SD) | 9.95 (6.02) | 8.85 (6.31) |
| NPI total score | ||
| Mean (SD) | 42.02 (23.17) (n = 41) | 46.90 (23.48) |
| DEMQoL-Proxy | ||
| Mean (SD) | 87.57 (10.73) | 88.51 (10.14) |
| Carer | ||
| PSQI global score | ||
| Mean (SD) | 9.22 (4.08) (n = 41) | 10.40 (4.52) |
| SCI total score | ||
| Mean (SD) | 15.32 (8.22) (n = 41) | 13.50 (5.92) |
| HADS scores, mean (SD) | ||
| Anxiety | 8.17 (4.66) | 9.30 (3.80) |
| Depression | 5.24 (4.33) | 7.65 (4.60) |
| ZBI score | ||
| Mean (SD) | 37.69 (18.39) | 38.30 (19.27) |
| HSQ scores, mean (SD) | ||
| Physical health | 67.89 (32.79) (n = 41) | 52.50 (39.47) |
| Mental health | 60.95 (23.17) | 52.33 (24.02) |
Table 9 summarises the patient- and carer-validated questionnaire scores at 3 months. More than 90% of those who were randomised at baseline completed the SDI, HADS, ZBI, SCI and HSQ-12. Over 85% completed the other questionnaires. Summary data indicate generally better scores for the intervention group for both patient and carer measures. After adjusting for site and baseline score, significant improvements due to the intervention are evident for ESS total score, DEMQoL-Proxy and ZBI score.
| Interview measures at 3 months | Trial group, mean (SD) [n] | Adjusted treatment effect (I – TAU)a (95% CI)b [n] | |
|---|---|---|---|
| Intervention (N = 42) | TAU (N = 20) | ||
| Person with dementia | |||
| SDI global score, median (IQR) | 0.92 (0.49–2.94) | 2.43 (0.82–3.88) | –0.30 (–1.42 to 0.82)c |
| ESS total score | 7.17 (5.87) [36] | 9.00 (7.55) [18] | –2.86 (–5.54 to –0.17) [54] |
| DEMQoL-Proxy | 93.52 (10.12) [37] | 87.07 (10.22) [18] | 7.08 (2.25 to 11.91) [55] |
| NPI total score | 38.69 (23.57) [36] | 44.72 (23.22) [18] | –1.99 (–11.66 to 7.68) [54] |
| Carer | |||
| PSQI global score | 9.37 (4.16) [38] | 9.5 (4.49) [18] | 1.03 (–1.05 to 3.11) [55] |
| SCI total score | 15.45 (7.45) [38] | 14.53 (8.54) [19] | –0.41 (–3.75 to 2.93) [56] |
| HADS scores | |||
| Anxiety | 8.76 (5.57) | 9.05 (4.22) | 1.13 (–0.31 to 2.56) |
| Depression | 5.71 (4.43) | 8.79 (4.88) | –1.05 (–3.01 to 0.91) |
| Total | 14.47 (9.20) [38] | 17.84 (8.43) [19] | 0.51 (–2.39 to 3.42) [57] |
| ZBI score | 36.5 (17.07) [38] | 42.16 (16.45) [19] | –5.32 (–9.83 to –0.82) [57] |
| HSQ scores | |||
| Physical health | 68.42 (32.60) [38] | 54.39 (35.94) [19] | 3.12 (–12.27 to 18.52) [57] |
| Mental health | 55.96 (26.19) [38] | 48.07 (21.15) [19] | 1.25 (–9.52 to 12.02) [57] |
Analysis of actigraphy data
Table 10 shows the baseline sleep data for those who wore the Actiwatches for seven or more nights and had complete NPCRA data, that is not including 24-hour periods with ≥ 3 hours of missing data. Only one of the 62 randomised participants with dementia did not wear the watch for seven or more nights at baseline. The sleep diary or event markers were used by 50 out of 62 (81%) randomised participants to record the person with dementia’s bedtimes and rise times for actigraphy. Of the remaining 12 participants, eight (13%) gave a verbal report of sleep pattern, including any atypical bed or rise times (e.g. due to medical appointments), whereas four (6%) did not.
| Baseline actigraphy measures | Trial group | |
|---|---|---|
| Intervention | TAU | |
| Sleep measures | (n = 41) | (n = 20) |
| Sleep efficiency (%), median (IQR) | 76.90 (66.60–83.00) | 80.68 (70.75–86.30) |
| Average duration of sleep time (minutes), median (IQR) | 448.00 (376–503) | 442.00 (388–568.50) |
| Average duration of wake time (minutes), median (IQR) | 111 (77–152) | 114 (58.5–147.5) |
| Average time of lights out/bedtime,a median (IQR) | 22:26 (22:07–23:08) | 22:25 (21:37–23:25) |
| Average time of falling asleep,a median (IQR) | 22:43 (22:07–23:40) | 22:45 (22:04–00:16) |
| Average time of waking up, median (IQR) | 08:10 (07:41–08:54) | 08:20 (07:48–08:46) |
| Average time of getting up, median (IQR) | 08:14 (07:52–09:00) | 08:23 (07:56–08:56) |
| Average duration of time in bed (hours), mean (SD) | 9.91 (1.35) | 10.01 (1.80) |
| NPCRA measures | (n = 42) | (n = 20) |
| Relative amplitude, median (IQR) | 0.71 (0.48–0.82) | 0.78 (0.56–0.90) |
| Interdaily stability, mean (SD) | 0.38 (0.16) | 0.43 (0.17) |
| Intradaily variability, mean (SD) | 1.11 (0.40) | 1.03 (0.32) |
| L5 – average activity count for 5 most restful hours, median (IQR) | 1381 (572–1919) | 761 (358.5–1616) |
| Start hour of 5 most restful hours,a median (IQR) | 01:00 (00:00–03:00) | 01:00 (00:00–02:00) |
| M10 – average activity count for 10 most active hours, median (IQR) | 8155 (5127–12,281) | 8212.0 (3730.5–14,545.5) |
| Start hour of 10 most active hours, median (IQR) | 10:00 (8:00–12:00) | 8:30 (8:00–11:00) |
| Core night-time sleep measures | (n = 41) | (n = 20) |
| Sleep efficiency (%), median (IQR) | 76.4 (68.1–83.9) | 81.3 (67.15–91.05) |
| Average duration of sleep time (minutes), median (IQR) [n] | 278 (245–303) [39] | 300 (246–331) [19] |
| Average duration of wake time (minutes), median (IQR) [n] | 57 (47–75) [39] | 52 (26–76) [19] |
Table 11 shows the sleep data at follow-up for those who wore the watch for seven or more nights and NPCRA data with all 24-hour periods with ≥ 3 hours of missing data excluded. Of the 57 people with dementia who were randomised and still in the trial at follow-up, six refused to wear the watch again; 49 out of 51 (96%) of those who did wear it had actigraphy data for seven or more nights. Carers of 42 (82%) of these participants provided their relative’s bedtimes and rise times via the sleep diary or event markers. Of the remaining 11, eight (16%) provided a verbal report of their relative’s sleep pattern; three (6%) did not.
| Actigraphy measures at 3 months | Trial arm | Adjusted treatment effect (I – TAU)a (95% CI)b | |
|---|---|---|---|
| Intervention | TAU | ||
| Sleep measures | (n = 32) | (n = 17) | (n = 49) |
| Sleep efficiency (%), median (IQR) | 76.05 (65.0–81.9) | 78.6 (72.1–82.3) | 0.39 (–4.89 to 5.68)c |
| Average duration of sleep time (minutes), median (IQR) | 418.5 (383.5–506) | 475 (396–534) | |
| Average duration of wake time (minutes), median (IQR) | 112.5 (94.5–157.5) | 105 (88–133) | |
| Average time of lights out/bedtime,d median (IQR) | 22:15 (21:36–22:50) | 22:03 (21:21–23:28) | |
| Average time of falling asleep,d median (IQR) | 22:49 (21:59–23:23) | 22:22 (21:39–23:55) | |
| Average time of waking up, median (IQR) | 08:04 (07:16–08:38) | 08:09 (07:38–08:41) | |
| Average time of getting up, median (IQR) | 08:06 (07:20–08:44) | 08:17 (07:48–08:43) | |
| Average duration of time in bed (hours), mean (SD) | 9.89 (1.83) | 10.05 (1.83) | |
| NPCRA measures | (n = 34) | (n = 17) | (n = 51) |
| Relative amplitude, median (IQR) | 0.71 (0.49–0.87) | 0.76 (0.51–0.90) | –0.02 (–0.10 to 0.06)c |
| Interdaily stability, mean (SD) | 0.38 (0.18) | 0.47 (0.19) | |
| Intradaily variability, mean (SD) | 1.04 (0.38) | 0.98 (0.40) | |
| L5 – average activity count for 5 most restful hours, median (IQR) | 1067 (621–2003) | 981 (493–1940) | 76.88 (–521.40 to 675.16)c |
| Start hour of 5 most restful hours,d median (IQR) | 01:00 (00:00–03:00) | 01:00 (01:00–02:00) | |
| M10 – average activity count for 10 most active hours, median (IQR) | 8247 (4258–12,155) | 8132 (5960–17,158) | –198.29 (–1717.83 to 1321.25)c |
| Start hour of 10 most active hours, median (IQR) | 10:00 (8:00–11:00) | 9:00 (8:00–10:00) | |
| Core night-time sleep measures | (n = 32) | (n = 17) | (n = 49) |
| Sleep efficiency (%), median (IQR) | 79.0 (63.75–82.6) | 80.9 (69.8–86.5) | |
| Average duration of sleep time (minutes), median (IQR) [n] | 285 (243–303) [30] | 291 (251–311) [17] | |
| Average duration of wake time (minutes), median (IQR) [n] | 62 (43–80) [30] | 49 (39–70) [17] | |
Sensitivity analysis of actigraphy estimates including only those co-resident with a carer
Table 12 shows the follow-up actigraphy data for the people living with a family carer.
| Actigraphy measures at 3 months | Trial group | Adjusted treatment effect (I – TAU)a (95% CI)b (N = 40) | |
|---|---|---|---|
| Intervention (N = 25) | TAU (N = 15) | ||
| Sleep measures | |||
| Sleep efficiency (%), median (IQR) | 77.10 (67.6–85) | 78.6 (71.8–85.6) | 2.32 (–3.48 to 8.13)b |
| Average sleep duration (minutes), median (IQR) | 439 (393–504) | 488 (396–578) | |
| Average wake duration (minutes), median (IQR) | 111 (96–149) | 105 (88–138) | |
| Average time of lights out/bedtime,b median (IQR) | 22:17 (21:36–23:02) | 21:53 (21:16–22:25) | |
| Average time of falling asleep,b median (IQR) | 22:50 (21:54–23:25) | 22:08 (21:32–23:30) | |
| Average time of waking up, median (IQR) | 08:03 (07:12–08:35) | 08:07 (07:30–08:41) | |
| Average time of getting up, median (IQR) | 08:06 (07:16–08:44) | 08:17 (07:37–08:43) | |
| Average duration of time in bed (hours), mean (SD) | 9.80 (1.98) | 10.22 (1.89) | |
| Fragmentation index, median (IQR) | 46.4 (35.4–61.1) | 41.9 (34.3–45.9) | 0.77 (–9.91 to 8.37)b |
| NPCRA measures | |||
| Relative amplitude, median (IQR) | 0.69 (0.49–0.83) | 0.76 (0.49–0.90) | –0.02 (–0.14 to 0.11)b |
| Interdaily stability, mean (SD) | 0.37 (0.18) | 0.46 (0.19) | |
| Intradaily variability, mean (SD) | 1.07 (0.33) | 1.05 (0.40) | |
| L5 – average activity count for 5 most restful hours, median (IQR) | 1176 (544–1814) | 981 (474–2314) | 81.58 (–723.94 to 887.10)b |
| Start hour of 5 most restful hours (24 hour),a median (IQR) | 01:00 (11:00–02:00) | 01:00 (01:00–03:00) | |
| M10 – average activity count for 10 most active hours, median (IQR) | 7632 (4048–11,251) | 8132 (4392–17,886) | –869.46 (–2189.40 to 450.48)b |
| Start hour of 10 most active hours (24 hour), median (IQR) | 10:00 (8:00–12:00) | 9:00 (8:00–11:00) | |
| Core night-time sleep measures | |||
| Sleep efficiency (%), median (IQR) | 78.8 (65.3–89.0) | 80.9 (69.8–87) | |
| Average sleep duration (minutes), median (IQR) | 284 (240.5–308.5) (n = 24) | 291 (251–313) | |
| Average wake duration (minutes), median (IQR) | 64.5 (42–83.5) (n = 24) | 49 (36–73) | |
Table 13 shows the correlation between scores on the baseline and follow-up questionnaire and actigraphy measures to contribute to power analysis for a full trial.
| Measure | Correlation |
|---|---|
| Person with dementia | |
| NPI total score | 0.73 |
| ESS total score | 0.66 |
| DEMQoL-Proxy | 0.55 |
| SDI total score | 0.57 |
| Sleep measures | |
| Sleep efficiency (%) | 0.85 |
| Average sleep time (minutes) | 0.65 |
| NPCRA measures | |
| Fragmentation index | 0.82 |
| Relative amplitude | 0.78 |
| L5 activity count – least active 5 hours | 0.86 |
| M10 activity count – most active 10 hours | 0.90 |
| Carer | |
| PSQI global score | 0.58 |
| SCI total score | 0.69 |
| HADS | |
| Anxiety | 0.87 |
| Depression | 0.73 |
| Total | 0.84 |
| ZBI score, mean (SD) | 0.87 |
| HSQ | |
| Physical health | 0.67 |
| Mental health | 0.68 |
Assumptions and sample size calculation
It is envisaged that the main DREAMS trial will retain the same basic characteristics as the feasibility study. It will be a parallel, multicentre trial with two arms and 2 : 1 randomisation to the DREAMS intervention versus TAU and randomisation at the individual level. The primary outcome will be SDI score at 6 months (to allow more flexibility in intervention delivery time). As the intervention is delivered by therapists (with each therapist likely to see approximately 15 people), it is expected that there could be therapist clustering in the intervention group. Analysis will compare groups allowing for this clustering and making adjustment for baseline SDI score. Table 14 shows calculated estimates of the required sample size based on variance and correlation of SDI scores observed from the feasibility work, and plausible ranges for other unknown parameters.
| ICC assumed | 0 | 0.03 | 0.05 | 0.08 | ||||
|---|---|---|---|---|---|---|---|---|
| Design effect | 1 | 1.33 | 1.55 | 1.88 | ||||
| Estimates of sample size | I | TAU | I | TAU | I | TAU | I | TAU |
| Difference in mean SDI score = 0.8 (effect size 0.4) | ||||||||
| N | 168 | 84 | 188 | 94 | 200 | 100 | 218 | 109 |
| N with dropout | 198 | 991 | 220 | 110 | 233 | 118 | 255 | 128 |
| N with inflation if non-normal | 230 | 115 | 254 | 127 | 272 | 136 | 296 | 148 |
| Difference in mean SDI score = 0.6 (effect size 0.3) | ||||||||
| N | 297 | 149 | 330 | 165 | 352 | 176 | 386 | 193 |
| N with dropout | 349 | 175 | 388 | 194 | 414 | 207 | 454 | 228 |
| N with inflation if non-normal | 406 | 203 | 450 | 225 | 480 | 240 | 528 | 264 |
The analysis would compare groups allowing for clustering with adjustment for baseline SDI. We wanted to detect a difference in mean SDI (on the SDI 0–12 scale) scores of 0.6 (effect size 0.3) or 0.8 (effect size 0.4) because a mean difference of 0.8 was observed in the feasibility study, although there was a much larger difference in medians as data are skewed. SD estimated from baseline feasibility data was 2.24. Correlation between baseline and follow-up measurements was assumed to be 0.57 from feasibility study estimates.
Intracluster correlation (ICC) could be between 0 and 0.08 (0.08 indicated by upper confidence limit from START trial. This was used as the feasibility study was too small to provide a useful estimate.
We will aim for 2 : 1 randomisation (achieved by calculating unequal sample sizes before adjusting for clustering and inflated by the design effect to achieve a 2 : 1 ratio), with 90% power and significance of 0.05. Inflation for a non-parametric analysis is included for the case of data being non-normal (feasibility study data indicate that this may be the case for SDI). This calculation should provide a conservative estimate of the sample size needed in the case when analyses are based on transformed data (e.g. log or square-root transformation, which may be appropriate). 79
Chapter 4 Results: qualitative study
Post-intervention qualitative interviews: intervention refinement
Demographics
Sixteen carers who received the DREAMS START intervention were interviewed, 15 of whom had completed all six sessions and one who had completed only two. The demographic characteristics of the carers are displayed in Table 15. The 16 carers included two paid carers and 14 family carers. The majority of carers were women and they were from a diverse range of ethnicities. Most spoke English as a first language (n = 11). Their ages ranged from 22 to 87 years (median 53.5 years, IQR 48.5–64.0 years). Thirteen carers lived with the person with dementia, one of whom was a paid live-in carer Monday–Friday; the other three carers comprised two family carers who were non-resident and a paid carer who worked shifts as part of a team of carers. The qualitative findings are presented in three main headings: (1) the acceptability of the assessment tools and intervention, (2) aspects of the intervention that the carers found helpful and (3) the acceptability and practicality of the intervention.
| Characteristics | n (%) |
|---|---|
| Sex | |
| Female | 12 (75) |
| Age (years) | |
| < 25 | 1 (6.25) |
| 40–49 | 4 (25) |
| 50–59 | 5 (31.25) |
| 60–69 | 5 (31.25) |
| ≥ 80 | 1 (6.25) |
| Ethnicity | |
| White British | 5 (31.25) |
| White Irish | 1 (6.25) |
| White Other | 6 (37.5) |
| Spanish | 2 (12.5) |
| Polish or German | 1 (6.25) |
| Greek Cypriot | 2 (12.5) |
| Turkish/Greek Cypriot | 1 (6.25) |
| Black British Caribbean | 1 (6.25) |
| Black British African | 1 (6.25) |
| Asian Indian British | 1 (6.25) |
| South American (Brazilian) | 1 (6.25) |
| Relationship to person with dementia | |
| Husband | 2 (12.5) |
| Wife | 2 (12.5) |
| Son | 2 (12.5) |
| Daughter | 6 (37.5) |
| Daughter-in-law | 1 (6.25) |
| Granddaughter | 1 (6.25) |
| Paid carer | 2 (12.5) |
Acceptability of the assessment tools and intervention
Questionnaires
Carers highlighted the lengthy and repetitive nature of the assessment questionnaires but recognised that this was important for the research:
But whether you, you know, prior to starting the questionnaire you say, look . . . they will seem really repetitive but it’s just we really need this information because it’s going to be really helpful in our, putting together the study.
Carer (C)1; co-resident daughter
One carer spoke of the difficulty in completing the SDI for the previous 2 weeks, as these may not be typical weeks in terms of the sleep disturbance:
I have a, you know, a slight question about that because things keep changing, so the last 2 weeks may not actually be typical.
C2; co-resident wife
Two participants spoke about their dislike of the PSQI measure; they did not see how measuring their sleep was relevant, had difficulty in averaging times or how long it takes to fall asleep and thought that it was long:
I think Pittsburgh is a bit long winded.
C3; co-resident husband
Both participants preferred the SCI over the PSQI, although one of them found it difficult to come up with an average time of how long it takes them to fall asleep and how long they are awake for during the night for the SCI.
One carer thought that there should be more options in possible answers to the HADS, as they felt that their answer did not reflect how they felt, and another carer did not think that it was relevant to their situation:
I think I found this one quite difficult . . . there seems to be a big jump from time to time, occasionally, to a lot of the time. I think it needs a bit more subtlety . . . Because, I mean, for instance, you know, I still enjoy the things I used to enjoy, yes, definitely as much, but there are times if I’m feeling very stressed that I don’t, you know.
C2; co-resident wife
Three participants disliked the ZBI, considering it to be invasive, repetitive and subjective:
I remember thinking this is incredibly subjective . . . I think the day I filled it in I was feeling quite stressed, so I was emphasising the, you know, the more, you know, the stronger responses.
C2; co-resident wife
Two carers discussed how they had recently had operations, which affected their health-related quality-of-life answers.
One individual with dementia, who was present during the interview, suggested that there should be a question about whether or not the person with dementia and carer sleep separately, as this can affect how the sleep questions about the person with dementia are answered:
[Speaking to the person with dementia] So, you’re saying there should be a question about do you sleep with your relative or your person you’re caring for?
C2; co-resident wife
Actigraphy
Carers discussed both the person with dementia’s experience of wearing the watch and the feedback they received about the person with dementia’s sleep during the intervention sessions and after completion of the trial.
Wearing the Actiwatch
Many of the carers reported no problems with the person they care for wearing the watch, with the person with dementia either enjoying the experience or seeming to be unaware of it once it was on. The absence of problems wearing the watch was surprising to some of the carers:
Even after her shower if the carer forgot to put it back on her she used to tell her ‘the watch, the watch’.
C4; co-resident daughter-in-law
And she kept it on for 2 weeks, which I was really impressed . . . Which I wasn’t expecting, I have to be honest.
C5; co-resident daughter
There were some issues with the watch being taken off by either the person with dementia or the carers, whether paid or family, owing to either the person with dementia forgetting what the watch was or practical reasons, such as having a wash. Only one carer was unable to find the watch for an extended period of time:
It did come off one day, didn’t it, we didn’t find it for a day . . . but we found it. I think the carer had taken it off.
C2; co-resident wife
Ensuring that the watch was worn and kept safe was a cause for concern for some carers, as this carer described:
She’d taken it off once and had left it, they found it; another day someone else was wearing it during the course of the day, so quite how mum had managed that I don’t know.
C6; co-resident son
Other minor problems with wearing the Actiwatch were reported by one carer each and included not liking its appearance, skin irritation and clothing covering the light sensor on the watch. Two participants commented on the event marker button on the face of the watch, which can be pressed to signal bedtimes or wake-up times. Both found that the person they cared for liked pressing the button themselves. In one case it was pressed repeatedly by the person with dementia, frequently at the wrong times:
As I say, I used to press it and she used to press it.
C4; co-resident daughter-in-law
Actiwatch feedback
Many of the carers liked receiving the baseline Actiwatch data in session 2 of the intervention, especially when it confirmed their own impression of their relative’s sleep pattern:
It sort of correlated with my own diary, and it correlated with my own experience, of the amount of activity that mum has, and the amount of light.
C1; co-resident daughter
Carers also discussed how the watch gave them new information, like how often the person with dementia woke in the night, especially if they lived separately, and how active they were during the day if the carer was absent:
It’s good to know what happens in the day with her. Because I’m not here and it’s interesting to see the sleeping, the movement and just generally what’s going on and I think that’s a really clever little watch.
C11; co-resident daughter
The watch where, you know, making a note of how many times she woke up in the night and that sort of thing. Because I’m sure she wakes up and gets up in the night many times without me even knowing.
C8; co-resident husband
Carers discussed the feedback participants received at study completion from the analysis of the Actiwatch worn at the 3-month follow-up. This feedback often did not accord with the carer’s judgement of changes that had occurred, and one carer described disappointment at the lack of change on actigraph, but also thought this was because the follow-up Actiwatch recording was too soon to notice any changes:
I was quite disappointed that there was not much of a change, it was exactly the same as last time, and I was saying to myself; how come? . . . There wasn’t much change to her routine and structure. It’s only now that it’s starting to pick up, so it could be that time it was early stages to ask for a good review.
C12; co-resident granddaughter
Intervention overall
Almost all the carers interviewed spoke positively about the intervention overall:
I found it really quite empowering. And with someone who suffers with dementia, or a family member of someone [who suffers with dementia], everything is just so bleak and, sort of, grey. And you’re dealing with so much that actually having some idea of clarity and putting things into perspective and knowing that you can, [that] there are some things that you can do rather than all being completely unmanageable.
C7; live-out daughter
Only one participant, who dropped out of the intervention, reported that it was not helpful for her relative:
I have been on a course [about] how to deal with dementia in the past . . . So in a way I didn’t find that very beneficial to be honest with you.
C5; co-resident daughter
The positive feedback mentioned how the intervention was enlightening, interesting, well-designed and provided a new perspective. Six of the interviewees planned to use the manual in the future:
It was really enlightening; it was, on a personal level, it was very, very . . . I found it to be really useful because there were things that I would overlook that I didn’t realise were that important.
C7; live-out daughter
No, I think, really, it was just the total thing . . . was just interesting for us because we’d never have even thought about any of these things before.
C8; co-resident husband
Look, I got nearly 25 or 30 certificates. And I think this was the most complete. The most useful, the easiest. And compared to the others, I think there’s nothing to add to this one . . . very well designed.
C9; paid carer
The relevance of the intervention was discussed, and participants highlighted how the relevance varied according to the individual presentation of their relative. Even when participants did not find some parts of the intervention relevant, they found other parts useful and judged the overall intervention to be interesting and useful:
But anyway, we were slightly difficult in our case because we’re both quite active, you know, and we were already doing a lot of the things . . . so some of it didn’t mean an awful lot to us. But, I mean, overall, somebody’s done an awful lot of work, putting all this together and, you know, this package is quite useful, I think.
C8; co-resident husband
I think the DREAMS programme was quite interesting. Even though some of it didn’t apply to my mum. A lot of the things were for the ones that move about, you know, are more mobile and everything. But some of the points were quite useful, you know, for me to use on my mum.
C10; co-resident daughter
Manual design
Participants found the manual layout user-friendly and easy to read, considered the balance of pictures, vignettes and text appropriate and felt the direct quotations from carers to be important. Two people wanted more examples and quotations:
It was well explained, it was in plain English, it was all understandable.
C13; live-out daughter
Pictures are always good . . . but I think more importantly is the case studies, you know, and anecdotes, yes, that’s more important.
C1; co-resident daughter
One carer would have liked an electronic version of the manual and two would have liked to be able to use it on their mobile telephone:
It would’ve been better if the paperwork was more electronic, that way it doesn’t get lost.
C12; live-in granddaughter
It might’ve been easier for me to just talk into my phone at the end of the day or, you know, to keep an audio diary.
C2; co-resident wife
Some application could be developed that would send you a message.
C5; co-resident daughter
Another carer suggested organising the manuals with tabs for reference to make it easier to find things.
Content of the manuals
Participants found the content of the manual relevant and useful:
In my mind, there isn’t anything that I think you’ve missed or anything different or that could be improved on. I think you covered an extensive range [of] matters in terms of relaxation, breathing, sleep, and understanding, all of those things.
C11; co-resident daughter
I found it very good, I didn’t find anything boring about it; it was well calculated, and well put down on paper. I found every part of it very imaginative.
C3; co-resident husband
Participants also liked receiving information about caring for someone with dementia, the biological processes of sleep and how dementia can affect an individual’s sleep. The diagrams used in the manual to describe circadian rhythms and sleep pressure were described as easy to understand for carers, but not for people with dementia. Having this specific information clearly explained helped the carers to make sense of sleep difficulties in dementia and justified making behavioural changes, such as increasing natural light:
The sleep cycle, REM [rapid eye movement] and all of this, and that it’s in cycles, it isn’t just one cycle that lasts the entire night . . . I think it’s useful to know that, actually, if I, you know, if I do this it affects this and that’s why then it has a knock-on effect.
C1; co-resident daughter
I really liked the fact that how sleep and dementia are correlating with each other, I found it very helpful. Because with people that have dementia, as their mind is slowly becoming less integrated, they’re very more sensitive as to how it can impact their sleeping. Because if you have dementia . . . you don’t have a tough shell against having a routine which can change dramatically, like myself.
C12; co-resident granddaughter
Now I’m really aware that being out in the conservatory is really good for her because of the light. Whereas before I thought, ‘oh, it’s better because it’s a bit oppressive in the front of the house, a bit dark’ . . . But now I know that there is a physical reason for it, it’s not just, you know, a hunch.
C7; live-out daughter
Sleep diary and record forms
One aspect of the manual that received mixed feedback was the sleep diary, designed for participants to use between sessions to record sleep patterns and strategies tried. Completing a sleep diary was described as useful both for understanding the sleep patterns of the person with dementia and in enabling carers to provide further details to others, such as health professionals:
The diary, the timesheet, everything you know because it helps me to learn, it helped me to be able to see exactly what’s going on. And I can give exact times to people now as to when she’s awake whereas before I might’ve missed it.
C11; co-resident daughter
Although some carers found the sleep diary easy to complete, a number of carers spoke about the difficulties of completing it. They attributed this to the design of the diary and the added stress of record-keeping, especially when the person with dementia awoke in the night:
But it was a nightmare because of the ways, yes, because it goes from noon . . . sometimes if you forget also to do it you can think, ‘oh, you know what we didn’t . . . let’s leave yesterday, let’s just do today’ . . . but by the time the next day came it just got really, yes, really confusing so we just left it.
C7; live-out daughter
But it was crazy because when you try to sleep and she’s woken up you have to memorise the time, or otherwise you have to leave the bed and go and write it. Next day you think, what time was she awake? It was 3? It was 2?
C9; paid carer
One suggestion was to change the timings of the sleep diary to start recording at an earlier time of day, instead of noon, and to use day and night symbols:
I don’t know what time would be good, maybe 6 a.m., you know, so you definitely knew that that was Tuesday, all that line.
C2; live-in wife
Other record forms within the manuals were described as easy to use and useful.
Process of intervention delivery
Carers valued a number of aspects of the process of intervention delivery. These included, first, having the space to talk; second, developing individualised strategies; and, third, being flexibly guided through the intervention.
Having the space to talk
Many carers simply appreciated the chance to talk through challenges with the therapist, something that had not always been available to them:
It was good that someone else had some ideas; it’s always good to talk to people who have any vague idea of what you’re going on about . . . It’s rare to come up against someone with some understanding, even professionals.
C6; co-resident son
And actually it was nice knowing that I had an hour . . . to just speak about problems and try to find out answers, work it through because nobody in the . . . national health system, you know, has that time.
C7; live-out daughter
Often carers related this to the specific qualities of the therapists, who were seen to both listen and give space while gently guiding carers through the sessions, facilitating the process:
I used to look forward to my sessions with [T1]. He’s very professional and also a gentle man . . . He’s softly spoken but he puts the point across.
C11; co-resident daughter
[T2] was very understanding, she was engaged and patient and she was dealing with my quirkiness every time I randomly asked her a random question out of the blue.
C12; co-resident granddaughter
Developing individualised strategies
Carers valued how the therapists and the manual supported them to both build on existing strategies and develop new ones. In particular, they valued that they were encouraged to make specific plans and try new strategies out:
Quite often I was doing something similar, or I was just on the edge of it or doing parallel but not quite achieving, and he, sort of, fine-tuned it.
C6; co-resident son
She knew my tendency of being slightly forgetful and being all over the place. So she tried to give me a plan that was realistic and not overambitious.
C12; co-resident granddaughter
Realising that they already had strategies to build on and being encouraged to try things out and notice changes was validating and reinforcing:
There was a lot of positive reinforcement, which I think was very helpful, actually, because I think it’s very easy in this sort of situation to become depressed and, you know, kind of overwhelmed by a kind of inertia.
C2; co-resident wife
It just made me aware that I was already doing stuff, whereas before I was, like, ‘oh, I’m not doing enough’ and, you know . . . reaffirming, reassurance and also reminding.
C1; co-resident daughter
One carer discussed the manual using real-life examples as validating their own experiences of caring:
Well, I think what made it easier was looking at examples of people with dementia and how their family and carers dealt with it. And it made it easier for me knowing that I’m not the only one who finds it hard, you know. Because sometimes you can question yourself and say, ‘well, I’m just not doing this right.’
C11; co-resident daughter
Being guided through the intervention
Participants found it useful to have the therapist guide them through the manual, rather than a more self-help style intervention. This process increased motivation to try to practise new strategies:
She talked through the manual, rather than just letting me, on my own time, read it, which really helped . . . it gave a lot of insight into what the manual was about, because her talking through it gave a lot more understanding.
C12; co-resident granddaughter
In the beginning, I said, ‘no I’ll do that [relaxation and exercise] when I’m on my own’, but she made us do it. Well, not forcefully, but, you know, she had a nice way to get around you. So we did it with her . . . I realised it was easy to do it so we did it when she wasn’t here, yes.
C14; co-resident wife
Carers valued the flexible pacing of the sessions, including being given the manuals one at a time and each session having a different focus:
Yes it was because each week T1 would give me, like, a new session of paperwork to look through and I found that easy . . . Because it wasn’t given to you all in one big bulk . . . And each session covered a different thing.
C11; co-resident daughter
In general, carers felt that the number of sessions was appropriate. Some highlighted that fitting in weekly sessions could be challenging. Some suggested more flexibility around both the number (e.g. having four longer sessions instead of six) and spacing of the sessions (e.g. having up to 2 weeks between them). Having time between the sessions was also valued as an opportunity to try out new strategies, without losing momentum:
They did vary sometimes, they weren’t as . . . slightly as long. So it was, yes, it met the needs of that particular subject.
C13; live-out daughter
At the time I was thinking, ‘oh, that’s come round quite quickly’ . . . However, the times where we did wait for 2 weeks, yes, I wouldn’t want to go over longer than that because then you forget about stuff . . . Yes, I think between 1 and 2 weeks is ideal, yes, absolutely.
C7; live-out daughter
Which aspects of the intervention did carers find useful?
Strategies from the intervention included using the light box; increasing natural light, exercise and daytime activity; practising the relaxation exercises; carers looking after themselves; changes to the bedroom environment; changes to the bedtime routine; managing night-time behaviours; lifestyle changes; and reducing daytime naps. Carers spoke about what strategies they did and did not use and what effects they noticed on themselves and their relatives. How relevant certain strategies were to carers varied in relation to the specific circumstances or presentation of their relative; for example, if a person with dementia went out every day, increasing natural light or activity was not as relevant.
Using the light box
Of the 16 participants interviewed, 14 had tried to use the light box to increase their relative’s light during the intervention. Only three of them were using the light box every day at the time of the interview:
Every morning he asks me to switch on the light, you know . . . he totally seems to count on the light.
C14; co-resident wife
However, five of those not using it every day spoke about using the light box again, particularly in the autumn or/winter when there is less light:
Well, we didn’t use it after, sort of end of March really, mid-March because she was out so much then and the nights were getting lighter. But certainly I think it was February time for a period of about 3 weeks or 4 weeks that we did we just put it on while she was in the room . . . It probably did help; it did because she wasn’t going to sleep so much during the day.
C7; live out daughter
The others spoke of their relative disliking the light because of its brightness or because it did not seem to be working:
She didn’t want it on a lot of the time; she said it was too bright.
C13; live-out daughter
My son said, you know, it’s making their nan sleep more.
C10; co-resident daughter
Increasing natural light
Five people spoke about trying to increase natural light, often indoors, by sitting nearer to windows or opening curtains, or spending more time outdoors:
We changed the furniture, so she was near the window. So she has more light.
C9; paid carer
What we still try and do is take my mother out daily. So still trying to give her some exercise. Still to get her out into the sunlight for at least half an hour.
C15; co-resident son
Carers had mixed opinions on the effects of increasing natural light; some felt that it aided sleep, whereas others felt that it had no effect:
Yes, she’s more settled when she goes out and she got more light. And then she becomes more tired and she sleeps better.
C9; paid carer
Getting my mom up in the morning, lots of daylight, it didn’t really seem to have that much effect.
C6; co-resident son
Increasing exercise
Increasing walking
Four carers spoke about increasing exercise by walking outdoors, which also increased exposure to natural light, and had continued using this strategy beyond the intervention. One carer increased their relative’s exercise by parking farther away from places they were visiting:
Well, also now she goes back out walking the dog with [other family carer] because she’s got now the walker . . . I know it’s, you know, getting dark by then.
C4; co-resident daughter-in-law
What we still try and do is take my mother out daily. So still trying to give her some exercise.
C15; co-resident son
Using seated exercises
The seated exercise routine was meant for people who could not go out with ease or exercise standing. It was discussed by five of the interviewees. Four people enjoyed the exercises and were still continuing to use them beyond the intervention (three people with dementia and one carer). Another carer was trying the exercises with their relative, but was considering stopping, because they were too tired as they were sleeping very badly:
And he joins in which is brilliant. Because it’s very hard to get him to do his exercises for his joints but he loves this.
C14; co-resident wife
Yes, there was the one I thought was quite good because my mum doesn’t do a lot of activity, walking around. There was one with your feet, moving your feet up and down as an exercise. She’d be sitting down but still moving her feet and her arms.
C13; live-out daughter
I was doing exercise with her before but I sort of tailed off because then, you know, her sleep’s been so bad, and she’s so tired.
C1; co-resident daughter
Increasing daytime activity
The majority of the interviewees discussed small things they had tried in order to increase daytime activity in the person with dementia, including listening to audiobooks, music and the radio, looking through photographs, watching television programmes and videos, playing bingo, reading newspapers and playing with sensory objects. Carers appreciated the focus on daytime activities and on the things that they could try, especially when it built on what they were already doing:
It just made me aware that I was already doing stuff, whereas before I was, like, ‘oh, I’m not doing enough’ and, you know.
C1; co-resident daughter
I did like the reminders of the importance of going out every day. I mean, I sort of do, but there were lots of reminders of sensible things to do . . . but I was thinking about it, you know, trying to do fun things together.
C2; co-resident wife
Some valued how the sessions had guided them to adapt existing activities or the environment where the activities took place to make it easier for the person with dementia to participate:
We worked out headphones for the TV for her because she has to have it so, so loud, so she can enjoy TV more without trying to read the subtitles. So she’s enjoying TV a lot more and programmes that she used to like to watch.
C4; co-resident daughter-in-law
For carers whose relatives were already very active, the focus on activities was less relevant.
Practising the relaxation exercises
Although many of the interviewees enjoyed the relaxation exercises within the sessions, some felt that the exercises were not for them and struggled to practise between sessions. Carers were more likely to use elements of the exercises that did not require the CD, such as the breathing exercises, or used it as a reminder to take a moment to relax and notice when they were feeling stressed:
I try and incorporate the breathing when I’m feeling a bit, like today I thought, ‘ugh, God, I just haven’t got the patience today to deal with mum’s agitation’ . . . but it’s, like, you do it, for that moment of time you’re relaxed and then it’s, like, you have to go back into it.
C1; co-resident daughter
One carer also described using the imagery relaxation exercises with their relative, adapting it to make it more relevant and briefer for use over the telephone:
I did try and get my mum using that kind of technique. Imaging herself in a particular environment. I changed it sometimes to the Caribbean. To somewhere that she is familiar with . . . Sometimes I’d speak to her over the phone and try to describe . . . an imagery to think of that she’d like that made her feel happy.
C13; live-out daughter
Carer looking after themselves
Carers spoke about how the session around looking after themselves was important, but that they needed more in-depth opportunity to address more long-standing patterns and habits:
What it did do was it stopped you and it said, ‘hang on a minute, you know, this is a management programme it’s not just about the person, it’s about the family member and looking after . . .’ but actually the fundamentals of looking after yourself, you know, when it comes to practice it’s difficult.
C7; live-out daughter
I think it’s absolutely key, you know, it’s probably the most important aspect of the whole thing, but also very, very difficult because if one’s spent, you know, nearly 70 years of one’s life practising, you know, all the buzzing.
C2; co-resident wife
Changes to the bedroom environment
Changes to the bedroom environment covered a range of topics, from making the room more comfortable to adding more personal objects so that, when the person living with dementia woke up, they recognised that they were at home. Mostly carers felt the bedroom was too warm, which some confirmed using the thermometer provided:
I think that is part of this really just to try and keep things in order in her bedroom, so when she goes to bed she doesn’t have to go to bed in chaos.
C7; live-out daughter
And I did put the heating, central heating, on the timer. So it came off a bit earlier, so that it was slightly cooler. And yes, she was saying she was freezing . . . You know, and it was just mild.
C13; live-out daughter
Some participants had, before the intervention, thought about changes that they could make to improve the bedroom environment, such as blackout curtains, either themselves or with help from their memory service. For others, changes to the bedroom environment were not possible: for example, one person with dementia had never liked having their bedroom curtains closed at night, and their carer felt that this was impossible to change:
Just before we started this I actually got blackout curtains, which had already helped a bit to make her sleep a bit.
C6; co-resident son
Changes to bedtime routine
The changes made to the bedtime routine encompassed making the wind-down period start earlier, trying to decrease stimulating activities before bedtime and changing the time the person with dementia went to bed, which seemed to improve sleep:
I would phone my mum sometimes about 10 o’clock just to say goodnight and I’ve just put that a bit more forward now so I phone her at 9 o’clock. And then that 9 to 10 o’clock is a sort of period of winding down.
C7; live-out daughter
Trying to keep her up as long as possible so she’ll sleep better through the night. All those sort of things, they did help, because obviously when she goes to bed early she will get up early.
C15; co-resident son
Some discussed how the bedtime routine was fixed because their relative lived alone and depended on paid carers, or because they thought changing the routine may be too confusing for their relative.
Managing night-time behaviours
Strategies for managing night-time behaviours included thinking more about the causes of behaviours and carers reacting differently to being disturbed in the night:
I’ve now figured out, look, she’s not going back to bed because it’s not right, she doesn’t know what to say and what to do. And she’s actually started coming into my room and at first I couldn’t understand why. Now I know she’s trying to tell me something’s wrong . . . So I know now to just go in and, I have a spare, and I can have mom’s bed changed so that she’s back in bed and asleep in 15 minutes flat.
C6; co-resident son
One carer also adapted the home environment by putting signs on bathroom and bedroom doors, which helped their relative find their way to and from the bathroom in the night, and prevented them wandering around the house in the night.
Lifestyle changes
The main lifestyle change mentioned was reducing the consumption of caffeine from the late afternoon onwards, and especially before bed, which most people did. However, this was not always successful. Another carer discussed how her relative drank caffeine at night but did not think that had any impact on his sleep:
We talked about having a snack before bed and cutting out caffeine, which I tried, again, for a little while, but we seem to have slid back to . . . Well, P does have, does actually drink decaffeinated tea now, which he didn’t before . . . But he likes a particular form of coffee, which is very sweet and has caffeine, and I haven’t been able to wean him off that.
C2; co-resident wife
Reducing daytime naps
Carers used a number of the strategies, such as going out and increased activity, to try to reduce daytime napping in the person with dementia. Some of the carers had some success with these strategies, but other carers discussed finding it an ongoing struggle to reduce naps:
But a couple of, like, past photos, family photos and everything, you know, like we recorded and had on a CD . . . A few other videos, places that she was familiar with, like her village, where she comes from, that kept her awake a few times.
C10; co-resident daughter
If you wake her up she’ll get really angry, so my dad phones me and he says, ‘she’s been asleep for about 45 minutes, can you phone?’. So, he’ll put the phone right next to her to wake her. So I’ll phone up, she’ll pick it, I’ll say, ‘hi Mum, how are you? What you doing?’ you know, and I’ll try.
C7; live-out daughter
And it’s impossible if she wants to go to sleep, she will go to sleep, no matter what you do. Napping, you know.
C4; co-resident daughter-in-law
Overall benefits for carer and person with dementia
Overall, nine of the carers interviewed spoke about their relative sleeping better after taking part in the intervention, but five of them were unsure whether or not it was due to the intervention or other reasons, such as medication or progression of dementia over 3 months:
You’ve created a miracle, because it really did work.
C11; co-resident daughter
For years, she’s been very bad at sleeping and it seems to have improved quite a bit, but whether that’s anything to do with this course, I don’t know.
C8; co-resident husband
Mum was put on mirtazapine and we started the research. So, it is very difficult to say what . . . where the benefit came from . . . And again I don’t know whether it’s the mirtazapine. She’s actually gone down on her mirtazapine from 30 to 15 [mg] and her sleep is still OK.
C7; live out daughter
Some attributed the improvements to a changed routine:
She definitely stays upstairs in her room during the night for longer. She came less times downstairs to eat or just listen to the radio, as she used to . . . Yes, it’s a better routine.
C9; paid carer
One carer described how the intervention improved not only the sleep but also the mood of the person she cared for; this person with dementia had taken part in the sessions with the carer:
But I have to say he enjoyed it every week, he looked forward to it. And he seemed to think it was making him better. And his mood, everything changed and, touch wood, he’s still the same.
C14; co-resident wife
In terms of benefits for carers, two described how the intervention had also improved their own sleep and two spoke about feeling calmer from the skills that they had learnt from the intervention:
I really was and even people at work noticed a difference with me. It was like, you seem full of life. I’d say it’s because I’m sleeping at night now . . . The programme as I saw it for us, for my mum and for me, worked really well.
C11; co-resident daughter
I’ve learnt to be calmer though and more patient by sort of . . . To stop thinking about it and think of something else.
C10; co-resident daughter
One carer also mentioned the feeling of altruism she felt from taking part in the research:
And I think there’s something, it sounds a bit weird, but there is something comforting about having done this, having been involved in something that is hopefully going to lead to some helpful outcomes for a lot of people, so I think that in itself is good.
C2; live-in wife
One carer of a relative with very severe dementia felt that the intervention had a limited effect:
I’m not saying don’t offer it to them [people with severe dementia] . . . but if you find that, actually, it’s more helpful in people with mild to moderate dementia, you might feel that it’ll be of more benefit and you’ll be able to reach more people within those categories, and help more people as a result.
C1; co-resident daughter
Acceptability and practicality of the intervention
What made it easier to put strategies into practice?
Carers described various factors that made it easier to overcome challenges and put strategies into practice.
Support from others
Having additional care and support from others, including other family members and paid carers who could try out strategies and encourage their relatives to make changes, was described as helpful:
When my cousin came, it did help make sense of ways we could give a change to my nan’s well-being. Because he’s the only one who convinced my nan, I could not convince her.
C12; co-resident granddaughter
I made the point of asking, when the carer was at home with her all day, get her out as well. So it just made me think of it, that, to do, rather than leave everything for me to do when I come in from work, so, good ideas.
C6; co-resident son
Routine and organisation
A number of carers discussed how building activities into an existing routine and planning ahead made it easier to increase daytime activities, particularly if their relative was a person who liked to go out anyway:
And I had suggested about taking out her clothes in advance for . . . To encourage her to go to church on the Sunday. So on the Saturday I would come, and I have done that a few times . . . And probably half of the time she has taken it up. So that’s kind of encouraged her because her clothes are all ready, she knows what she’s going to wear.
C13; live-out daughter
My mother is the sort of person who likes to go out, so that was quite easy.
C15; co-resident son
Indirect changes rather than confrontation
The carers described how coming up with creative solutions, which could involve distracting relatives, rather than trying to directly get them to change a behaviour, worked well:
We’ll park a little bit further away from where we’re going for a destination, she’s got to walk to get there . . . She has done a bit more walking herself, so I think it’s helped her. That does tire her . . . Helps her sleep.
C15; co-resident son
Support and suggestions from the therapist
The manner and approach of the therapists was seen to facilitate and motivate the carers to make changes:
[Regarding the relaxation exercises] In the beginning I said, ‘no I’ll do that when I’m on my own’ . . . but you know, she had a nice way to get around you. So we did it with her. No, I realised it was easy to do it so we did it when she wasn’t here, yes.
C14; co-resident wife
What made it harder to put strategies into practice?
The barriers to making use of the intervention have been organised into three subthemes: (1) factors related to the person with dementia, (2) factors related to the carer and (3) practical issues.
Difficulties relating to dementia and comorbid physical illness
Most carers described the impact of the dementia itself on their ability to make changes or try out new strategies. They often attributed this to the unpredictability of the condition and fluctuation in their relative’s wishes, for example the person with dementia not understanding something, or it not being possible to leave things lying around as the person with dementia would destroy them:
The only difficulty I found was if I made arrangements, or I wanted to make arrangements, I’ll ask [her] first, if she says yes, then we go . . . Once we got there, I am afraid it was no, I don’t want to go in, I don’t like it, don’t like this place.
C3; co-resident husband
I can’t leave stuff like this out and about, I have to lock everything away, so you then get a situation of it’s all locked away, I’ve finally got mum to bed, I’m doing this, I’m doing that and I don’t remember to go back and do it . . . if I want to keep anything safe, complete, stop it being ripped apart . . . everything’s got be hidden. That’s the big thing for me personally.
C6; co-resident son
Carers found inflexibility in their relatives particularly challenging, especially when strategies involved making changes to long-standing routines, such as having the room a certain temperature or having the curtains closed during the day:
Every suggestion that I gave her, she threw it out of the window. So, I knew that even if I did put my 100% into it, I was not going to get anything out of it, because my nan is reluctant.
C12; co-resident granddaughter
We discussed the room temperature, how she likes the room so hot and . . . [paid carer] keeps trying cooling it down and my mum would then go mad having a go at her, ‘why are you doing that? Are you trying to get me to catch a cold?’.
C7; live-out daughter
One man caring for his mother with dementia described how having the therapist in their home affected his mother, as she did not understand who the therapist was and why they were talking to him:
She didn’t quite know who [T2] was and she got . . . She started getting this jealous rage thinking that [T2]’s going to take me away.
C15; co-resident son
One carer spoke about being unable to stop her relative from daytime napping, including the difficulties arising from the hangover effects of sleep medication taken the night before, which prevented her from being able to practise strategies with her relative:
If she does sleep, she’s completely out of it, I think I’ve said this before, and she doesn’t rouse at all for an entire day, so it’s like she’s accumulated all these hours of not sleeping and just making up for it, and that can last 2 days, even.
C1; co-resident daughter
The impact of comorbid physical health problems were frequently highlighted, for example infections increasing confusion, continence issues at night waking a relative or mobility issues making it harder to increase activity:
When she had the urine infection in between so that can affect her sleep. You know, like going into a deeper sleep.
C10; co-resident daughter
I think with Parkinson’s [disease], which means that he needs to get up and go to the loo several times during the night, that kind of thing is not able to be influenced by taking part in this kind of project.
C2; co-resident wife
There’s not a lot he can do . . . It’s the mobility that’s holding him back.
C14; co-resident wife
Carers’ tiredness, competing demands and relationship with the care recipient
Carers spoke about their own stress, health problems and tiredness affecting their motivation. The carer we interviewed who did not complete the intervention spoke about the added stress of the intervention being part of why they dropped out:
That made it harder? . . . it’s just a vicious circle, isn’t it, because if you’re tired, you’re less hopeful and you don’t have the patience and then you start thinking about yourself and how unfair things are.
C7; live-out daughter
It would’ve put more of a stress for myself and I didn’t want any more.
C5; co-resident daughter
The relationship between either family carers or paid carers with the person with dementia was also discussed as something that made it harder to try strategies:
I mean, some of the things, I think what may make it a bit harder for me is that she’s not my mother, she’s my mother-in-law, you know.
C4; co-resident daughter-in-law
One carer also discussed having additional caring responsibility for another parent who also had dementia and how that made it harder to try strategies with their parent who was taking part in DREAMS:
Yes, because it’s not just my mother. I look after my dad as well, so when you’re not looking after one, you’re looking after the other one.
C15; co-resident son
Sometimes, carers spoke in more general terms about multiple competing demands on their time, stressful life events and feeling overwhelmed or hopeless. This affected their ability to engage with the intervention and make changes:
When the sessions were going to start with T2, I was very much disorganised and scattered and I had a lot of things that I was thinking, and that was going over my mind . . . It was not the right time for me to focus on it.
C12; co-resident granddaughter
I don’t know. You read a section and think, ‘yes, yes, yes, I’ve got it, I can do that’, we’ll do that and it doesn’t work out. It just doesn’t work out. So you leave it, and if you try it again another day and it will, but most of the times it doesn’t.
C4; co-resident daughter-in-law
Well, I actually, I had a bit of a breakdown when she was in a state . . . all night she stayed awake one night, she was awake every 10 to 20 minutes, she’s knocking on my door, calling me, you know, quite angry and it carried on until the next afternoon, into the evening. I just had enough, I couldn’t take it any more. I just slept for like 36 hours. I had to get my sister to come here to look after them for about 2 or 3 days.
C15; co-resident son
But I have so many things to think about, what with finding things for her to do, doing the cooking, the housework and all. I don’t want any more things to think about.
C8; co-resident husband
Practical issues
There were various practical issues that made it harder for carers to make use of the intervention. This included being at work during the day or at night, which resulted in them being unable to try out strategies with their relative:
If I was here to increase daytime activity, it would be a lot easier . . . But the fact that I’m not here and I’m at work, it’s just hard.
C11; co-resident daughter
In addition, when the carer did not live with their relative or if they slept separately, it was difficult to try new strategies or to see any effects:
Sometimes I’d . . . Yes, that was one thing, sometimes I didn’t think there was enough time to try things out, because by the following week, you know, you either haven’t had time, or it didn’t work so well . . . and based on the fact as well that I’m not living with my mum.
C13; live-out daughter
And the other thing, it was suggested to try to keep her up a bit later. And that wasn’t quite working at all, me not living with her.
C13; live-out daughter
Because P sleeps down here and I sleep upstairs, so I wasn’t checking whether the temperature was optimal for sleep.
C2; live-in wife
Carers also described the lack of flexibility available in paid care, especially for working carers who were not with their relative during the day:
Very hard. I said to T1 that, for me, that’s almost going to be impossible unless we change the whole package for mummy with the carers, created more time per session with them and getting them to do other things with her, other than just feed her and take her to the toilet.
C11; live-in daughter
Another barrier raised by carers related to the time of year the interventions took place. Participants spoke about how it was harder to get their relative to leave the house when the weather was bad in the winter months, which affected both activity and light levels:
But the middle of winter, you come home at 4 in the afternoon and it’s pitch black, cold, damp and horrible, and I don’t particularly want to start dragging her out.
C6; live-in son
Post-intervention patient and public involvement focus group’s views about further improvements to the manual
The post-intervention patient and public involvement (PPI) focus group was facilitated by Penny Rapaport and Lucy Webster and was hosted by James A Pickett of the Alzheimer’s Society. Five carers who had not had the intervention attended the focus group, all of whom had contributed to the earlier intervention development process.
The key themes elicited from the focus group related to what the carers liked about the intervention, what they felt should be added or enhanced in future versions, what they felt were barriers to delivery and effectiveness of the intervention and general suggestions for a future trial of DREAMS START.
The focus group participants highlighted aspects of both the content and process of intervention delivery that they thought were useful and important. In terms of content, they felt that a focus on behavioural activation and physical activity was important for both carers and people with dementia, and could lead to a reduction in daytime napping and night-time wakening. In terms of process, the carers highlighted how combining a methodical, manualised and structured approach with support from a therapist to put strategies into practice and troubleshoot difficulties was beneficial. They also appreciated the flexible and personalised delivery of the intervention and felt that the manual was easy to read and follow.
One of the main challenges that they perceived was that increasing activity or making daytime changes, especially if the person with dementia lives alone, relies on adequate local services being available, which in their experiences was rarely the case. They also perceived the intervention to be harder both to deliver and put into practice if the person with dementia did not recognise that they had a sleep problem or had a different understanding of how they slept than their relative.
The main suggestions made by the focus group participants related to building technology into any future iterations of the intervention. Carers suggested having application-based technology both to remind carers to try a particular strategy at a set time and to facilitate real-time recording. Some acknowledged the importance of having simple paper-recording available as an alternative. They felt that using telecare and other assistive technology (which was often part of plans) could be further explored in a future trial, as this would be useful for those people with dementia who are alone during the day and could help manage difficult or risky behaviours at night. The carers also suggested that future versions of the manual should be even more personalised, with more detailed instructions for therapists on how to deliver the intervention flexibly based on the particular presentation of the person with dementia and the specific impact on family carers. They suggested including more detailed vignettes of the sleep difficulties people with dementia may experience, based on the data collected in this feasibility study. Focus group participants also felt that having a peer support component in which participants could either directly or virtually connect with others participating in the intervention would be beneficial to carers.
Interestingly, all of the carers asked questions or expressed frustration that the manual would not be widely available until a full RCT had been completed. What they felt was the most important outcome for a future trial was discussed and the participants felt that there needs to be a continued focus on improving carers’ sleep as well as the sleep of people with dementia. One carer suggested testing the manual in a self-help format for carers alongside testing in individual, facilitated sessions.
Chapter 5 Discussion
Main findings
The study fulfilled the primary outcomes for feasibility and acceptability to continue to a full-scale trial. The expected recruitment rate to indicate feasibility stipulated in the protocol of potentially eligible participants was 50%, but the actual percentage of eligible referrals recruited exceeded this at 65%. Those who were recruited were, in the main, demographically similar to those who were not. However, family carers who cared for men with dementia were more likely to refuse to participate than those who cared for women with dementia. The research team are unsure why this should be. There was no difference in the sex of the carer who consented to the study or refused to participate, or whether carers were the care recipient’s spouse or child. The proportion of men recruited also reflects the proportion of men with dementia in the older population.
In terms of acceptability, the percentage of participants randomised to the intervention group attending four or more of six sessions was 88%, exceeding the expected value of 75%. The median number of sessions attended was six, with two people dropping out before they began the sessions (one because of lack of time and one because the person with dementia refused), but only three of those who started did not adhere to the intervention. Thus, most of those in the intervention group appeared to find it acceptable to attend all sessions once they had started.
Recruitment and follow-up
The referral rates were four potential participants per week from two memory clinics, which informs the number of trusts required for a full trial. We used JDR and recruited two people over the recruitment period. There was clearly more potential, but 19 out of 25 (76%) of those registered on the list did not respond to contact and only two of those we contacted consented. Thus, it seems that JDR is most useful as a supplementary method to recruit people rather than as the sole or main method. Generally, ≥ 80% follow-up is regarded as satisfactory, and this trial achieved 92%.
Completion of outcome measures
Very high completion rates of the validated questionnaire measures were achieved, using carers as informants. At baseline, all but one person with dementia wore the watch and 50 (81%) carers completed the sleep diary or event markers to record the person with dementia’s bedtimes and rise times. However, at follow-up 49 (79%) of the 62 people with dementia in the randomised group had ≥ 7 days of actigraphy data and 42 (82%) of their carers provided the bedtimes and rise times on the sleep diary or by using event markers to aid interpretation.
Primary and secondary outcomes for a full trial
Actigraphy as outcome and tool
It was originally envisaged that actigraphy data would be the primary outcome in a full trial. However, in the feasibility study, it was available for only 79% of randomised participants at follow-up (for ≥ 7 days; the usual ‘gold’ standard). In addition, the sleep diary or event markers, which give times of going to bed or getting up, are required to interpret the data. Only 68% of people randomised provided this, so data interpretation was difficult for some of those with records, as it relied solely on verbal reports or the researchers’ supposition. Therefore, our feasibility study indicates that we are unlikely to have the level of reliable data required to use actigraphy as a primary outcome. The research team suggest that it would be helpful to validate the use of Actiwatches in this population before considering actigraphy measures as a primary outcome in any efficacy trial.
There is, in addition, a paucity of validation data about actigraphy in this population. Although sleep efficiency is often taken as a good summary sleep measure from actigraphy in younger people, it appears not to be as well measured in older people possibly because sleep in actigraphy is inferred from time in bed and movement. Movement is, in contrast, directly measured. According to the actigraphy data, both groups were still spending a long time in bed after the intervention and it did not look as if the intervention changed this sleep behaviour in any substantial way. There was an indication from our actigraphy results that the intervention participants were more active during the day and less active during the night. These findings accord with the qualitative feedback from the carers in the study as well as the results of the validated instruments. The carers in the study and the PPI group judged the important outcomes to be that the person with dementia was less restless during the night, more awake during the day, disturbed them less during the night-time and seemed happier (which are measured in the SDI). In these circumstances, they were unsure that these measurements of sleep added additional outcome information, or were accurate.
In contrast to their disappointment with actigraphy as feedback, the carers and therapists found the information from actigraphy valuable to use at baseline to consider the rest–activity pattern and help make a plan and this would continue to be incorporated in the manual as part of the intervention in a full trial.
Overall, the place of actigraphy in a full study would, therefore, be to measure changes in daytime and night-time activity as a secondary outcome and as part of the intervention.
Validated interview measures
In the qualitative assessment participants commented on the combined length of the questionnaires, and felt that this was acceptable if participants need to be reassured that this information is being collected for the purposes of the research and will be useful. The completion rate for all validated instruments at baseline was very high, ranging from 98% to 100%.
Instruments for the person with dementia
The validated instruments to measure sleep disorder had a high rate of completion at follow-up, and the SDI was completed at follow-up by 90% of those initially recruited to the trial. This appeared to be the most practical way to measure sleep. The qualitative feedback from carers participating in the trial and PPI indicated that they felt that it reflected their experience of sleep as well and had relevance. Therefore, it is proposed as the outcome in a main trial.
Summary data for the carer-reported instruments indicated generally better scores for the intervention group and, after adjusting for site and baseline problems, there was significant improvement in daytime sleepiness and in quality of life of people with dementia, despite the small numbers in the study. There was also no increase and a possible reduction in the numbers of people who were prescribed at least one medication for sleep. These may be related to each other. Although power for these results had not been considered, the consistency in the direction of all the results suggests that they may be real. It is important that the intervention may reduce daytime sleepiness, whereas sedative medication sometimes increases it. Similarly, it is always important to consider quality of life for someone with dementia, as an intervention may improve a specific domain while reducing overall quality of life. We would therefore conclude that the DEMQoL-Proxy and ESS should be measured in a full trial. We also administered the CSRI, to assess the feasibility of its use, and suggest that it too should be included in a full trial, as this would enable the cost-effectiveness of an intervention to be calculated.
Harms
There was no clear increase or difference between groups in the use of psychotropic medication and melatonin, with the intervention group possibly being prescribed slightly fewer medications afterwards, taking into account baseline measures. This suggests that any effects were not due to psychotropics being used as rescue medication in the intervention group. There was no indication of important harms in terms of side effects in either group.
Carer outcomes
The carers in the intervention group reported significant improvements in ZBI-measured burden, and the direction of results suggested that there may be an improvement in depressive symptoms (HADS). It is important to consider the effect of sleeplessness on carers’ stress levels and mental health, and we would conclude that these measurements should be retained for a full trial. A few of the carers disliked the questionnaires used to measure carer sleep as they found it difficult to report averages. It is possible that they might be willing to record their own sleep using a sleep diary, attributing any sleep disturbance to disturbance of their relative’s sleep or any other cause. However, this was not tested and the carers were not very positive about sleep diaries for their relatives.
As carer sleep is often disturbed by the person with dementia, it seems worthwhile to measure it. In order to select one measure for future trials, two instruments were used: the PSQI and the SCI. The rate of carers who filled in these questionnaires was satisfactory, with 61 (98%) of the randomised carers completing both at baseline. Fifty-six (90%) completed the PSQI at follow-up and 57 (92%) completed the SCI. The SCI gives the information to consider whether or not criteria for insomnia have been fulfilled, whereas the PSQI does not. Overall, more than half of the carers fulfilled the SCI criteria for poor sleep at baseline. In the qualitative interviews, two carers were of the opinion that the PSQI did not measure relevant features of sleep and that it was too long. They preferred the SCI to the PSQI, and we will go with their preferences.
Additional data
One individual with dementia present during the interview also suggested that it may be important to record whether or not carers and the person they care for share a bed or bedroom as part of the assessments, as this can affect how the questionnaire is answered. This would be added to an assessment for a full trial.
Power for a full trial
To calculate the sample size required for the main study, feasibility estimates were used by calculating the SD of baseline SDI scores (2.24) and the correlation between baseline and 3-month measurements (0.57). As there is no estimate of clinically significant difference, a SD of 0.4 was used, different sizes were calculated considering the CI for ICC from the earlier START study. A full study with 2 : 1 randomisation (which is feasible from the results above) will require 230–296 participants in the intervention arm and 115–148 participants in the TAU arm (assuming an average of 15 participants per therapist, 2 : 1 randomisation and a drop-out rate of ≤ 15%, and including an inflation for the case of non-normality).
Where to recruit participants for a full trial
It was found that memory clinics (four referrals per week from two trusts) were a better source of referrals than JDR (two consented in total). JDR would be used as an adjunct rather than as the main source of referrals for a trial.
Changes to those recruited for a full trial
Participants from a range of dementia diagnoses and living situations were recruited. It had been expected that the intervention would be delivered mainly to family carers, but consenting participants chose a complex variety of methods of delivery. Slightly fewer than half were to family members by themselves. The intervention was thus delivered to family carers, paid carers and sometimes to both. Frequently the person with dementia was also included and, in one case, half of the sessions were delivered to a person with dementia alone. Delivering the intervention with the person with dementia present, although not always problematic, did present challenges for the therapists that were addressed in clinical supervision. In a few cases, the person with dementia and the family member presented conflicting views of their sleep difficulties and the potential solutions and strategies. This was especially the case when the person with dementia denied that they had any difficulties with sleep and, therefore, did not see the need for making any changes. It became clear during and after the first session for the one person with dementia who had some sessions alone that they could not retain or recall the information being discussed and, therefore, were unable to make use of the sessions. In a future trial, people with dementia would not be excluded from jointly participating in the intervention sessions; however, additional training would be built in for the therapists on delivering sessions with people with dementia present and how to manage any conflict and interpersonal challenges that arose.
It was envisaged that someone with sleep disturbance and dementia would have a paid carer or family member with them at night to ensure safety. This was not always the case. When people lived alone, the carers (whether family or paid) were unable to implement strategies, for example a scheduled bedtime or wind-down routine. It was also difficult to gain reliable information about the sleep patterns of people with dementia living alone. Therefore, people without a night-time carer would be excluded in a full trial. In addition, the intervention would be delivered only to people with dementia who have a carer also participating.
Families also found that making changes during the day was difficult if the person with dementia was alone during the day. This led to them being offered time switches for the light boxes. They found increasing activity if there was no one at home depended on the availability of services, such as going to a centre or having someone to take the person with dementia out. It is important to discuss this explicitly and consider the options available.
When paid carers attended the intervention, they were also able to implement strategies. Working with them may be important to allow people to remain living at home, as care agencies insist on full-day rates and two carers if the carer is disturbed frequently during the night. This may become financially non-viable and make it harder for someone with dementia to stay at home. Since it appeared feasible and is potentially useful, paid carers would be included in a full trial if people with dementia and their families wished.
People with a diagnosis of alcohol-related dementia or who were currently drinking were not excluded from the study. Two participants drank alcohol during the intervention sessions. These people were unable to work with a plan to change their sleep, which involved reducing alcohol, and there was also concern for the safety of our therapists visiting the homes by themselves, if the family were often absent. The participants then dropped out. Therefore, in future, anyone with current heavy drinking habits would be excluded.
It was not considered that people who were leaving the country for months or forever would be referred to the study. However, one person was leaving the UK and, although they did not fufil inclusion criteria in other ways, being in the UK for the trial and follow-up would be specified as a criterion. This would not exclude those going on holiday, only those who would be away for a period that prevented them from receiving the intervention or being assessed after the intervention.
The intervention content and delivery
Manual design and delivery
Generally carers felt that the number of sessions was appropriate and liked the balance of pictures, vignettes and text. They appreciated the direct quotations from carers. Some thought that they would continue to use the manual itself as well as strategies within it. To make it easier for participants to use the manuals in between the sessions and after the intervention, one participant’s suggestion of tabs will be used.
Topics
Carers liked the information about caring for someone with dementia, the biological processes of sleep and how dementia can affect an individual’s sleep, and found the diagrams helped them to understand why they should make changes, such as increasing natural light and activity, including exercise. They tried different components depending on individual needs. Many of them asked for specific help from relatives, which would continue after the study. They often made adaptations to the environment, for example putting signs on the bathroom door. Some realised that their relative got up during the night for a reason that they were able to address, which helped improve their relative’s comfort and reduce their own sleep disturbance.
At session 6, all carers made a plan for the future and clearly appreciated a multimodal intervention as they wanted to continue using a range of strategies. All intended to persevere with the light box (although not necessarily in the summer) and continue to increase activity or physical exercise. Many intended to carry on with a later bedtime routine, usually going to bed around half-an-hour later than before, and continued to be aware that a comfortable bedroom is important. Most carers became more aware of looking after themselves by giving themselves time, challenging negative thoughts or using relaxation. They felt that this had improved their own quality of life and made them a better carer, and so they would continue with these self-care strategies.
The carers interviewed wanted to ensure that, in future, therapists did not go through all the content of the manual on topics that were not relevant to them, for example sections about increasing activity if the person with dementia was very active. For a full trial, it will be stated at the beginning of the sessions that not all of the topics will be relevant, and, if they are irrelevant, they will be omitted, but participants will still have the information within their manual should it later become relevant (e.g. if their relative’s sleep disturbance or physical health changes, or as their dementia progresses).
The carers felt that using telecare and other assistive technology (which was often part of plans) could be further emphasised in a future trial, as this would be useful for those people with dementia alone during the day and could help manage difficult or risky behaviours.
Some carers mentioned in the qualitative interviews that they would have preferred electronic versions of the sleep diaries that were used for the actigraphy and in the intervention, and found that it was difficult to complete the next day when they had to remember when someone had been awake in the night. They suggested that the timings of the sleep diary be changed to start recording from 06:00 instead of 12:00, and day and night symbols be used to make it a lot clearer; this will be done. The research team believe that it is worth investigating the possibility of, for example, sending an automated message to prompt a carer to report information, such as bedtimes or rise times. This could be done by simply replying to the text or by writing the information in the diary.
Delivery by therapists
The therapists were clinically supervised psychology graduates who were delivering the intervention to varying combinations of carers and people with dementia. Both the participants and the PPI group felt that the therapists were taking a facilitative and supportive approach. This was essential to the therapeutic process, as it was complex for participants to overcome the barriers to helping relatives who were often unable to formulate and remember plans themselves. They valued face-to-face contact and that the strategies were delivered to them and tailored to their individual needs.
Complexity
The intervention was complex and could be individually tailored, but took only six sessions. As sleep problems are complex and diverse, this allowed it to be appropriately individualised.
Fidelity
Fidelity ratings were high, suggesting that different therapists were able to deliver the intervention consistently.
Flexibility
The intervention was offered weekly; the median time taken to deliver the intervention was 7 weeks. Most participants thought it was ideal to have 1–2 weeks between sessions, rather than necessarily aiming for weekly sessions. Therefore, a degree of flexibility in timing would be offered for an intervention in future, allowing for the intervention to take up to 3 months.
Strengths and limitations
The trial recruited people from urban and suburban environments in London only, which is a limitation to the external validity. Although there were both male and female care recipients, men were more likely to refuse. However, people from a range of age groups and with varying types and severity of dementia, relationships to the care recipient, marital status and educational backgrounds were successfully recruited. In particular, it is often the case that ethnic minorities are under-represented in studies; this study recruited ≈35% of people of ethnic minority status. In general, the findings should have good external validity.
Although one carer commented that sleep patterns in the last 2 weeks may be atypical, the analysis of such data at a group level should eliminate any systematic bias.
A very high proportion of carers remained in the study. Although the outcome assessors were blinded to outcome, the participants were not. It is possible that there was some bias in the ratings, as some carers might have felt that they had to report a positive result to please the researcher interviewing them, who was not the therapist, but in most cases had met them for screening and baseline assessment. However, the instruments are validated, and in other studies carers have frequently reported no beneficial effect in quantitative interviews. 80 In addition, those in the intervention group were prescribed slightly less medication for sleep, suggesting that independent doctors, using family reports, may have felt that sleep had improved.
All participants who were interviewed and had completed the intervention liked it. Of the three carers who had started the intervention but not completed at least four sessions, only one could be recruited for a post-intervention qualitative interview. The researchers were trained in qualitative interview techniques, including how to make the interviewee feel comfortable and avoid leading questions. During the interviews, they emphasised to the carer that they wanted to hear suggestions for changes and what had not worked, as that would be very useful to inform further development of the intervention and the research programme. The slight reduction in the prescription of psychotropic medication also suggests that the participants were reporting some improvement to their doctors.
If the person with dementia lived alone, the sleep pattern was reported by the person with dementia, who may have had difficulties remembering it, although their ability to live alone may indicate that they were less impaired. The carer would, therefore, have been likely to report their relative’s assessment and this assessment may be less reliable.
Data were gathered to enable the design of a full trial, in terms of both a power calculation and primary and secondary outcomes. The primary outcome is SDI rather than actigraphy, as had been envisaged. The results were used to calculate the numbers needed for a full trial. The clinically relevant difference in SDI score is not known, but an effect size > 0.2 is usually accepted as such. 81
Participants were asked to wear the Actiwatch on the same wrist (most often on the non-dominant side) at baseline and follow-up, to help consistency of interpretation of results. Actigraphy interprets sleep from movement rather than measures sleep. Detection of ‘sleep’ is made by inference from lack of activity. The accuracy of this may vary with the population, device and circumstances. The validated sleep estimation algorithms all assume the intention to sleep, so are for people in bed around their habitual bedtime. 82 There is little validation in people with dementia, who may frequently be quite still while awake, move around during sleep or may sleep during the day. The families thought that the person whom they looked after was sleeping better, but the actigraphy results did not support this. They also felt that the person with dementia was less sleepy during the day, but the Actiwatch may not pick this up, because the algorithms are not expected to do so.
Interpretation
A manual was coproduced in this study that shows acceptability and feasibility. Minor changes to the manual content and delivery would be made in a full trial. These comprise adding more flexibility in timing; stating at the beginning of the sessions that not all of the topics will be relevant and omitting those that are not; discussing how to get help to increase activity early on for those who are alone during the day; emphasising interventions for safety, including telecare; and adding tabs to the manual to make it easier to use. The diary design would also be improved and the possibility of sending an automated message would also be investigated, to prompt a carer to report information, such as bedtimes or rise times, by replying to the text or by writing the information in the diary.
Changes can be made to the inclusion and exclusion criteria, such as excluding those without a family or paid carer at night, those who are not staying in the UK for the period of the study or those who are currently drinking. The primary outcome (SDI) can be stated, a full trial can be powered and appropriate secondary outcomes can be chosen.
Conclusions
This acceptability and feasibility study fulfilled its primary outcomes and indicated that the SDI, which is a questionnaire measuring sleep and completed using carer information, would be a satisfactory primary outcome.
Implications for health care
There is now a feasible and acceptable manual that can be used in a future trial, and the information to design such a trial.
Recommendations for future research
The study was not powered for efficacy, but the evidence from the validated questionnaires suggested that there was potential for efficacy. However, as it cannot be certain from this indication, this is something that could be tested in a full trial. It may be appropriate to evaluate a comprehensive intervention first, with options to see how it might be delivered more cheaply after the efficacy trial. This encompasses both improving sleep disturbance and increasing the quality of life of the person with dementia, and reducing the stress on their family carer. The recruitment and retention reflect the salience and potential benefits of the intervention, which augurs well for the next step, that is, a full trial.
Acknowledgements
We would like to thank the participating people with dementia and carers, and the clinical practitioners from these trusts for referring the patients. We would also like to thank the members of the Trial Steering Committee: Esme Moniz-Cook (Chairperson), Kate Maxmin (Member), Judy Leibowitz (Member), Sue Boex (Public Member), Rosemary Copsey (Public Member), John Cape (Observer), Tabitha Kavoi (Observer), Aryana Chopra and Amal Qureshi (Observers). Rossana Horsley, Sue Boex and Rosemary Copsey gave advice throughout as expert family carers. We would like to thank Rebecca Turner and Adam Kadri for delivering the intervention. Many thanks to the Alzheimer’s Society for providing support for the project through James A Pickett’s involvement and by connecting us with the Research Network. The DREAMS START research team further acknowledges the support of the NIHR through the North Thames Clinical Research Network.
Funding acknowledgements
Camden and Islington NHS Foundation Trust and Barnet, Enfield and Haringey Mental Health NHS Trust provided funding to pay for excess treatment costs from therapist training and supervision and intervention delivery.
The research was designed, conducted, analysed and interpreted by the authors, entirely independently of the funding sources.
Contributions of authors
Kirsi M Kinnunen (DREAMS START Study Manager and Postdoctoral Researcher specialising in dementia) assisted with the editing of the manual, trained the staff for the interviews and use of the manuals, finalised the document changes made for ethics approval, developed the Trial Master File and Case Report Form, submitted the study for Health Research Authority approval and trial registration. Kirsi M Kinnunen also prepared site information packs, wrote the standard operating procedures and policies, set up databases, liaised with research and development, Local Clinical Research Networks and the sponsor for site setup, was responsible for the day-to-day management of the trial and screened the participants for eligibility and consented them. She also gathered baseline and follow-up quantitative data, analysed the actigraphy data, contributed to the quantitative analytic plan, carried out the qualitative interviews, identified themes for the initial coding framework and wrote initial parts of the report.
Penny Rapaport (Principal Clinical Psychologist in Older People) contributed to the conception and design of the study, designed and revised the manual, trained the staff for the interviews and use of the manuals, supervised the intervention delivery, devised the qualitative interview, identified themes for the initial coding framework, supervised the qualitative analysis and wrote initial parts of the report.
Lucy Webster (DREAMS START and MARQUE Research Assistant in the Division of Psychiatry) assisted with the editing of the manual, screened the participants for eligibility and consented them, gathered baseline and follow-up quantitative data, analysed the actigraphy data, delivered the intervention, carried out the qualitative interviews, identified themes for the initial coding framework, coded the majority of the qualitative interviews and wrote initial parts of the report.
Julie Barber (Senior Lecturer in Medical Statistics) contributed to the conception and design of the study, drafted the quantitative analytic plan, analysed the quantitative data and wrote initial parts of the report.
Simon D Kyle (Senior Research Fellow in Sleep Disorders) contributed to the conception and design of the study, designed and revised the manual and contributed to the quantitative analytic plan.
Brendan Hallam (DREAMS START Research Assistant in the Division of Psychiatry) assisted with the editing of the manual, screened the participants for eligibility and consented them, gathered baseline and follow-up quantitative data, analysed the actigraphy data, delivered the intervention, carried out the qualitative interviews and identified themes for the initial coding framework.
Claudia Cooper (Clinical Reader in Psychiatry) contributed to the conception and design of the study, designed and revised the manual, trained the staff for the interviews and use of the manuals and contributed to the quantitative analytic plan.
Rossana Horsley (Alzheimer’s Society Research Network Member) contributed to the conception and design of the study, commented on the manual and led the PPI contribution.
James A Pickett (Head of Research at Alzheimer’s Society) commented on the manual and co-ordinated the PPI contribution.
Anastasia Vikhanova (DREAMS START Research Intern) coded the majority of the qualitative interviews.
Colin A Espie (Professor of Sleep Medicine) contributed to the conception and design of the study.
Gill Livingston (Professor of Psychiatry of Older People) wrote the application and protocol, acted as chief investigator, contributed to the conception and design of the study, designed and revised the manual, trained the staff for the interviews and use of the manuals, wrote the ethics application and submitted it, contributed to the quantitative analytic plan, devised the qualitative interview, identified themes for the initial coding framework, wrote initial parts of the report and acts as guarantor.
All authors revised the report critically for important intellectual content and gave final approval of the version to be published.
Publications
Kinnunen KM, Vikhanova A, Livingston G. The management of sleep disorders in dementia: an update. Curr Opin Psychiatry 2017;30:491–7.
Livingston G, Barber JA, Kinnunen KM, Webster L, Kyle SD, Cooper C, et al. DREAMS-START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people with dementia and sleep disturbances: a single-blind feasibility and acceptability randomised controlled trial. Int Psychogeriatric 2018;17:1–15.
Rapaport P, Webster L, Horsley R, Kyle S, Kinnunen K, Hallam B, et al. An intervention to improve sleep for people living with dementia: reflections on the development and co-production of DREAMS:START. (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives). Dementia 2018;17:976–89.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015 – The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2015.
- Prince M, Comas-Herrera MA, Knapp M, Guerchet M, Karagiannidou MM. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage Quality and Costs Now and in the Future. London: Alzheimer’s Disease International; 2016.
- Dementia UK: Update. 2nd edn. London: Alzheimer’s Society; 2014.
- Lewis F, Schaffer SK, Sussex J, O’Neill P, Cockcroft L. The Trajectory of Dementia in the UK – Making a Difference. London: Office of Health Economics Consulting; 2014.
- Ooms S, Ju YE. Treatment of sleep disorders in dementia. Curr Treat Options Neurol 2016;18. https://doi.org/10.1007/s11940-016-0424-3.
- de Oliveira AM, Radanovic M, de Mello PC, Buchain PC, Vizzotto AD, Celestino DL, et al. Nonpharmacological Interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/218980.
- Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord 2016;190:264-71. https://doi.org/10.1016/j.jad.2015.09.069.
- Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med 2005;6:347-52. https://doi.org/10.1016/j.sleep.2004.12.005.
- Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med 2007;8:27-34. https://doi.org/10.1016/S1389-9457(08)70006-6.
- Miu DKY, Szeto SSL. Sleep disturbances among a group of dementia participants. J Clin Gerontol Geriatr 2012;3:105-9. https://doi.org/10.1016/j.jcgg.2012.04.008.
- Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging 2016;41:107-14. https://doi.org/10.1016/j.neurobiolaging.2016.02.009.
- Pavlova M. Circadian rhythm sleep-wake disorders. Continuum (Minneap Minn) 2017;23:1051-63. https://doi.org/10.1212/CON.0000000000000499.
- Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology – a bidirectional relationship. Nat Rev Neurol 2014;10:115-19. https://doi.org/10.1038/nrneurol.2013.269.
- Figueiro MG. Light, sleep and circadian rhythms in older adults with Alzheimer’s disease and related dementias. Neurodegener Dis Manag 2017;7:119-45. https://doi.org/10.2217/nmt-2016-0060.
- Cagnin A, Fragiacomo F, Camporese G, Turco M, Bussè C, Ermani M, et al. Sleep–wake profile in dementia with Lewy bodies, Alzheimer’s disease, and normal aging. J Alzheimers Dis 2017;55:1529-36. https://doi.org/10.3233/JAD-160385.
- Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics 2015;15:65-74. https://doi.org/10.1111/psyg.12069.
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev 2007;11:143-53. https://doi.org/10.1016/j.smrv.2006.09.002.
- Gehrman P, Gooneratne NS, Brewster GS, Richards KC, Karlawish J. Impact of Alzheimer disease patients’ sleep disturbances on their caregivers. Geriatr Nurs 2018;39:60-5. https://doi.org/10.1016/j.gerinurse.2017.06.003.
- McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD009178.pub3.
- Scoralick FM, Louzada LL, Quintas JL, Naves JOS, Camargos EF, Nóbrega OT. Mirtazapine does not improve sleep disorders in Alzheimer’s disease: results from a double-blind, placebo-controlled pilot study. Psychogeriatrics 2017;17:89-96. https://doi.org/10.1111/psyg.12191.
- Leonpacher AK, Peters ME, Drye LT, Makino KM, Newell JA, Devanand DP, et al. Effects of citalopram on neuropsychiatric symptoms in Alzheimer’s dementia: evidence from the CitAD study. Am J Psychiatry 2016;173:473-80. https://doi.org/10.1176/appi.ajp.2016.15020248.
- Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, Xiang YT. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. Int J Geriatr Psychiatry 2017;32:50-7. https://doi.org/10.1002/gps.4571.
- Altınyazar V, Kiylioglu N. Insomnia and dementia: is agomelatine treatment helpful? Case report and review of the literature. Ther Adv Psychopharmacol 2016;6:263-8. https://doi.org/10.1177/2045125316646064.
- Ishikawa I, Shinno H, Ando N, Mori T, Nakamura Y. The effect of memantine on sleep architecture and psychiatric symptoms in patients with Alzheimer’s disease. Acta Neuropsychiatr 2016;28:157-64. https://doi.org/10.1017/neu.2015.61.
- Kazui H, Adachi H, Kanemoto H, Yoshiyama K, Wada T, Tokumasu Nomura K, et al. Effects of donepezil on sleep disturbances in patients with dementia with Lewy bodies: an open-label study with actigraphy. Psychiatry Res 2017;251:312-18. https://doi.org/10.1016/j.psychres.2017.02.039.
- Brown CA, Berry R, Tan MC, Khoshia A, Turlapati L, Swedlove F. A critique of the evidence base for non-pharmacological sleep interventions for persons with dementia. Dementia 2013;12:210-37. https://doi.org/10.1177/1471301211426909.
- Living Well with Dementia: A National Dementia Strategy for England. London: Department of Health and Social Care; 2009.
- Final Report from the MAGDR Subgroup 1 on Priority Topics in Dementia Research, February 2011. London: The Stationery Office; 2011.
- Dzierzewski JM, O’Brien EM, Kay D, McCrae CS. Tackling sleeplessness: psychological treatment options for insomnia in older adults. Nat Sci Sleep 2010;2:47-61. https://doi.org/10.2147/NSS.S7064.
- van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev 2016;29:52-6. https://doi.org/10.1016/j.smrv.2015.08.009.
- Sekiguchi H, Iritani S, Fujita K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer’s type dementia: a case series. Psychogeriatrics 2017;17:275-81. https://doi.org/10.1111/psyg.12233.
- Tewary S, Cook N, Pandya N, McCurry SM. Pilot test of a six-week group delivery caregiver training program to reduce sleep disturbances among older adults with dementia (innovative practice). Dementia 2018;17:234-43. https://doi.org/10.1177/1471301216643191.
- Gibson RH, Gander PH, Dowell AC, Jones LM. Non-pharmacological interventions for managing dementia-related sleep problems within community dwelling pairs: a mixed-method approach. Dementia 2017;16:967-84. https://doi.org/10.1177/1471301215625821.
- van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry 1996;40:259-70. https://doi.org/10.1016/0006-3223(95)00370-3.
- Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, King D, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f6276.
- Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, King D, et al. Long-term clinical and cost-effectiveness of psychological intervention for family carers of people with dementia: a single-blind, randomised, controlled trial. Lancet Psychiatry 2014;1:539-48. https://doi.org/10.1016/S2215-0366(14)00073-X.
- Montgomery P, Dennis JA. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev 2002;2.
- Sivertsen B, Nordhus IH. Management of insomnia in older adults. Br J Psychiatry 2007;190:285-6. https://doi.org/10.1192/bjp.bp.106.031278.
- Tractenberg RE, Singer CM, Cummings JL, Thal LJ. The Sleep Disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. J Sleep Res 2003;12:331-7. https://doi.org/10.1046/j.0962-1105.2003.00374.x.
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. https://doi.org/10.1192/bjp.140.6.566.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. https://doi.org/10.1212/WNL.43.11.2412-a.
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9:173-6. https://doi.org/10.1017/S1041610297004870.
- Beecham J, Knapp M, Thornicroft G, Brewin CR, Wing J. Measuring Mental Health Needs. London: Gaskell/Royal College of Psychiatrists; 1992.
- Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology 1997;48:10-6. https://doi.org/10.1212/WNL.48.5_Suppl_6.10S.
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 1994;17:703-10. https://doi.org/10.1093/sleep/17.8.703.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Mulhern B, Smith SC, Rowen D, Brazier JE, Knapp M, Lamping DL, et al. Improving the measurement of QALYs in dementia: developing patient- and carer-reported health state classification systems using Rasch analysis. Value Health 2012;15:323-33. https://doi.org/10.1016/j.jval.2011.09.006.
- Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 2013;36:1747-55. https://doi.org/10.5665/sleep.3142.
- Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. J Clin Sleep Med 2013;9:217-25. https://doi.org/10.5664/jcsm.2482.
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 1997;20:24-7. https://doi.org/10.1093/sleep/20.1.24.
- McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc 2005;53:793-802. https://doi.org/10.1111/j.1532-5415.2005.53252.x.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-21. https://doi.org/10.1016/0165-1781(89)90047-4.
- Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004183.
- Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 1997;27:363-70. https://doi.org/10.1017/S0033291796004382.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Barry TL, Kaiser KL, Atwood JR. Reliability, validity, and scoring of the Health Status Questionnaire-12 version 2.0. J Nurs Meas 2007;15:24-35. https://doi.org/10.1891/106137407780851778.
- Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev 2014;26.
- Espie CA, Perlis M, Aloia M, Kuhn B. Behavioral Treatments for Sleep Disorders. London: Academic Press; 2011.
- START Manuals. London: University College London; n.d.
- Livingston G, Barber J, Rapaport P, Knapp M, Griffin M, Romeo R, et al. START (STrAtegies for RelaTives) study: a pragmatic randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of a manual-based coping strategy programme in promoting the mental health of carers of people with dementia. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18610.
- Bothelius K, Kyhle K, Espie CA, Broman JE. Manual-guided cognitive–behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res 2013;22:688-96. https://doi.org/10.1111/jsr.12067.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage Publications; 1994.
- Flesch R. A new readability yardstick. J Appl Psychol 1948;32:221-33. https://doi.org/10.1037/h0057532.
- NHS . Sitting Exercises n.d. www.nhs.uk/live-well/exercise/sitting-exercises/ (accessed 7 September 2018).
- Dementia Overview. London: NICE; n.d.
- Bryman A. Social Research Methods. Oxford: Oxford University Press; 2012.
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int 1999;16:505-18. https://doi.org/10.3109/07420529908998724.
- Rock P, Goodwin G, Harmer C, Wulff K. Daily rest–activity patterns in the bipolar phenotype: a controlled actigraphy study. Chronobiol Int 2014;31:290-6. https://doi.org/10.3109/07420528.2013.843542.
- Livingston G, Leavey G, Manela M, Livingston D, Rait G, Sampson E, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2010;341. https://doi.org/10.1136/bmj.c4184.
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91. https://doi.org/10.1002/pst.185.
- Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. Thousand Oaks, CA: Sage Publications, Inc.; 2014.
- ICH Topic E9 Statistical Principles for Clinical Trials. London: European Medicines Agency; 1998.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Moher D, Schulz KF, Altman DG. CONSORT Group (Consolidated Standards of Reporting Trials) . The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. J Am Podiatr Med Assoc 2001;91:437-42. https://doi.org/10.7547/87507315-91-8-437.
- Moher D, Schulz KF, Altman D. CONSORT Group (Consolidated Standards of Reporting Trials) . The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987-91. https://doi.org/10.1001/jama.285.15.1987.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- Randles RH, Wolfe DA. Introduction to the Theory of Nonparametric Statistics. Malabar: Krieger Publishing Company; 1991.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. https://doi.org/10.1016/S0140-6736(17)31363-6.
- Long PW. When Is A Difference Between Two Groups Significant? 2011. www.mentalhealth.com/dis-rs/rs-effect_size.html (accessed 15 September 2017).
- Kazlausky T. Actigraphy – Sleep Review 2013. www.sleepreviewmag.com/2013/06/actigraphy-q-a/ (accessed 2 October 2017).
Appendix 1 Ethics approval
Appendix 2 Safety and tolerability assessment
Appendix 3 Actiwatch instructions and sleep diary
The image of the Actigraph watch (MotionWatch8) has been reproduced with permission from CamNtech Ltd, Cambridge, UK.
The image of the Actigraph watch (MotionWatch8) has been reproduced with permission from CamNtech Ltd, Cambridge, UK.
Appendix 4 Focus group interview schedules
DREAMS START (Dementia Related Manual for Sleep; Strategies for Relatives) feasibility and pilot study: focus group feedback on intervention manual for development
Introductions
Thank you for agreeing to take part in this group. My name is . . . . As you know, I am a researcher from UCL and I will conduct and record this focus group. Everything you say is confidential but I would like you to introduce yourselves so that the typist can identify you.
Description of the research
We are in the process of developing a manual, which, over the next year, we are going to be testing out with people with dementia and their family carers as part of a trial. It will be delivered to individuals at home, and we hope that it will help with sleep and lead to improvement in quality of life for people with dementia and their families. The intervention will be based on what we know works from research and practice with people who do not have dementia and on our specialist research and clinical knowledge of people with dementia and their families.
Before we finalise the intervention, we want to hear your views and opinions about what we have developed, and then make further changes to the intervention manual. We are asking you because we know that you have responded to the Alzheimer’s Society invitation as this is something you are interested in and probably have experience of. We want to make use of your expertise.
Sleep difficulties/current understandings
Question (Q). Could you tell us about any sleep difficulties that your relative experiences?
-
What do you notice happening at night and during the day?
-
What effect do these problems have on you and your relative?
-
How do you understand what may be causing these sleep difficulties for your relative?
-
Which sleep difficulties do you find most difficult to manage?
-
What has worked well in managing sleep difficulties?
-
What have you tried?
-
What has not worked well?
We are particularly interested in how to make the manual practical and to fit in with people’s lives.
Introduce the manual
Show them the draft manual/structure and present an overall summary.
-
People with dementia and their families will receive their own copy of the manual to write in and keep.
-
They will wear an actigraph (special watch) to see how their sleep rhythms are.
-
There will be practical suggestions to try out between sessions.
-
There will be one member of staff working with the person with dementia and their family.
-
The manual contains information about sleep and dementia.
-
Each session will include a combination of information giving, discussion and making an individualised plan with practical exercises, including increasing light at particular times and increasing activity.
-
There will be a relaxation exercise and people will be given a relaxation CD or MP3 files.
-
Each session will be around 1 hour.
-
The staff member delivering the intervention will be supervised by a clinical psychologist and their general practitioner (GP) and memory service team will know that they are in the study.
The sessions of the DREAMS intervention comprise the following:
-
Learning about sleep and dementia.
-
Relaxation and bedroom comfort/pre-bed and mealtime routine.
-
Using natural light, morning wake-up light.
-
Pleasant activities.
-
Management of daytime naps.
-
Relaxation, especially at night.
-
What works? Using skills and strategies in the future.
Give them a few minutes to look through and make any general comments. These prompts are only if not already covered. (Explain that this is only a draft/outline).
Q. What are your initial thoughts on the manual?
Q. What do you think about the design of the manual?
Prompts:
-
How do you find the layout?
-
Is it easy to read and follow, for example not too much text on each page/fonts/colours?
-
What do you think of the pictures and images – more, less, different?
-
How do you find the balance of information-giving and discussion?
Q. What do you think about the outline structure and content of the sessions?
Prompts:
-
Do the topics and examples fit with what your experience is or has been?
-
Is there anything important that you feel is missing?
-
Does the order of the sessions make sense?
-
Is it pitched at the right level for a range of people?
-
Is it easy to understand?
-
Do the key points stand out?
Q. Do you have any other feedback regarding layout, design, content, etc.?
Q. What do you think might make it harder for people to participate in the sessions and use the manual? (Prompt about wearing actigraph, using light box, increasing activities.)
Q. What do you think might make it easier for people to participate in the sessions and use the manual? (Prompt about wearing actigraph, using light box, increasing activities.)
Q. Before we finish, is there anything else you would like to mention that we have not already covered?
Thank you for taking part today.
DREAMS START virtual/e-mail reference group (July 2016)
-
Seven participants.
-
Each sent different sections (two) of the manual; two participants sent the full manual (Trial Steering Committee members).
-
Four questions via e-mail:
-
What do you think about the design? For example, how do you find the layout? Is it easy to read and follow, for example not too much text on each page/fonts/colours? What do you think of the pictures and images – more, less, different?
-
What do you think about the content? For example, is it easy to understand? Do the key points stand out? Do the topics and examples fit with what your experience is or has been? Is there anything important that you feel is missing? Is it pitched at the right level for a range of people?
-
How do you find the balance of information-giving and discussion?
-
Any other feedback (layout, design, content, acceptability of intervention, etc.)?
-
DREAMS START feasibility and pilot study: focus group with family carers post intervention
Introductions
Thank you for agreeing to take part in this group. My name is . . . . As you know, I am a researcher from UCL and I will conduct and record this interview. Everything you say is confidential but I would like you to introduce yourselves so that the typist can identify you.
Description of the research
We have now completed the feasibility and pilot study of the DREAMS START intervention and wanted to meet with you all today to discuss our findings and any potential changes to the manual in the future. Before we begin talking, we will briefly tell you about the final version of the manual we used in the study and how we delivered it and then XXX will tell you about how the study has gone and what the people who received the intervention told us about how they found it.
Following our discussion today and based on the findings of the study so far, we will probably make further changes to the manual and how we deliver it, which we will hopefully test in a randomised controlled trial in the future. Therefore, we want to hear your views and opinions about what we have developed and tested and how it could be improved before further testing.
Revisiting the manual
Show them the manual used in the study and present an overall summary (present summary slides of content and process).
-
People with dementia and their families received their own copy of the manual to write in and keep.
-
They wore an actigraph (special watch) to see how their sleep rhythms were.
-
There were practical suggestions to try out between sessions.
-
There was one member of staff working with the person with dementia and their family.
-
The manual contained some information about sleep in dementia.
-
Each session included a combination of information-giving, discussion, looking at the results of the sleep actigraph from the week before and practical exercises, including increasing light at particular times and increasing activity.
-
There were relaxation exercises and people were given relaxation CD or MP3 files.
-
Each session was around 1 hour.
-
The staff members delivering the intervention were supervised by a clinical psychologist, and the participant’s GP and memory service team will know that they are in the study.
The sessions of the DREAM intervention include the following:
-
Learning about sleep and dementia.
-
The importance of routine pre bed and at mealtimes.
-
Relaxation and bedroom comfort.
-
Using natural light, morning wake-up light.
-
Pleasant activities.
-
Management of daytime naps.
-
Relaxation, especially at night.
-
What works? Using skills and strategies in the future.
Give them a few minutes to look through and make any general comments. These prompts are only if not already covered.
Q. What are your initial thoughts on the manual we used?
What do you think about the design of the manual?
Prompts:
-
How do you find the layout?
-
Is it easy to read and follow, for example not too much text on each page/fonts/colours?
-
How do you find the quotes? Prompts relevance, length.
-
What do you think of the pictures and images – more, less, different?
-
How do you find the balance of information-giving and discussion?
Q. What do you think about the content of the sessions?
Prompts:
-
Do the topics and examples fit with what your experience is or has been?
-
Is there anything important that you feel is missing?
-
Is it pitched at the right level for a range of people?
-
Is it easy to understand?
-
Do the key points stand out?
Q. What do you think about the structure of the sessions?
Prompts:
-
Does the order of sessions make sense?
-
What do you think about the length of each session?
-
Is there anything else we could do to make it easier to understand?
-
What do you think about having relaxation and homework in each session?
-
Do you think it is better to give out the manual session by session or all at once?
Brief presentation of findings to date:
-
What are your first thoughts on the findings?
-
Did anything surprise you that you heard?
-
What do you think we could do to address some of the barriers raised? (Put up slide about what made it harder?)
-
Do you have any suggestions about how to make it easier for people to make use of DREAMS START? (Put up slide about what made it easier?)
-
Based on what you have heard today, do you have any other suggestions about changes we could make to the design/content/structure and process the sessions?
Q. Before we finish, is there anything else you would like to mention that we have not already covered?
Thank you for taking part today.
Appendix 5 Final version of the DREAMS START manual: carer and facilitator versions
Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport.
Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport. Parts of this appendix have been reproduced with permission from CamNtech Ltd, Cambridge, UK; Dolores Gallagher-Thompson; Moïse Roche; iStockPhoto LP (Getty Images International, Dublin, Ireland); Openclipart (San Francisco, CA, USA; URL: https://openclipart.org) (This is an Open access article distributed under the terms of the Creative Commons Attribution (CC0 1.0) license, which permits others to copy, modify, distribute and perform the work, even for commercial purposes. See: https://creativecommons.org/publicdomain/zero/1.0/.); NHS Photo Library (URL: http://capture.co.uk/nhs/); and Gill Livingston and Penny Rapaport.
Appendix 6 Treatment-as-usual baseline data and information pack
Appendix 7 End-of-involvement letter template
Appendix 8 Fidelity checklists for intervention sessions
Appendix 9 Schedule for individual qualitative interviews
DREAMS START individual interview post-intervention guide
Before starting the recording
Give the carer a case report form to look at, a copy of the manual and the DREAMS START session card. Let the participant look at these for a few minutes.
Introduction of researchers
Thanks . . . . My name is . . . (As you know) I am a researcher from University College London and will be recording this interview. I am interested in your opinion about and experience of the DREAMS sessions. Everything you say is confidential, but I would like you to introduce yourselves for the recording so that the typist can identify you.
Description of research topic
We are asking you because you have tried out the sessions. We want to know about your experience and what was good and what was less good and how it has made a difference to you and your relative.
As a reminder, here’s a card listing the six sessions:
-
Session 1: Understanding sleep and dementia.
-
Session 2: Making a plan.
-
Session 3: Daytime activity and routine.
-
Session 4: Difficult night-time behaviours.
-
Session 5: Taking care of your own sleep.
-
Session 6: What works? Using strategies in the future.
You can use the manual too, if having your copy on hand is helpful.
Q. Can we start by hearing about how you found the intervention in general?
Prompts:
-
What did you like best about the sessions?
-
Was there anything that you did not like about the sessions?
-
Is there anything important that you feel was missing/not covered?
-
What were the key points that stood out for you?
-
How did you find the length of the sessions? How about the number of the sessions? Were the sessions spaced apart enough?
-
What aspects of the sessions/manual have you continued to use? Have you gone back to the manuals since the intervention stopped?
-
What difference has the intervention made to you and/or your relative during the day? How about during the night?
Q. What did you think about the content of each sessions?
Prompts:
How did you find . . .
-
Increasing natural light.
-
Using the light box (and are you still using the light box now?).
-
Increasing daytime activity/physical exercise.
-
Making a new routine/strengthening the link between bed and sleep.
-
Bedroom and lifestyle changes to improve sleep.
-
Managing night-time behaviours.
-
Looking after yourself/challenging unhelpful thoughts.
-
Relaxation.
Is there anything else you would like to mention regarding content?
Q. How did you find the manual itself?
Prompts:
-
How did you find the layout?
-
Was it easy to read and follow, for example not too much text on each page/fonts/colours?
-
What did you think of the diagrams and pictures – more, less, different?
-
Were the sleep diary/record forms easy to use? How could we improve them?
Q. How did you find the plans and trying things out between the sessions?
Prompts:
-
What made it easier for you to try things out between the sessions?
-
What made it harder for you to do the tasks between the sessions?
-
Did it make a difference trying things out together?
Q. How did the intervention fit with the sorts of sleep difficulties that your relative has been experiencing?
Q. How did your relative find wearing the watch?
Q. What did you think about the feedback from the letter comparing the difference in sleep from baseline to 3-month follow-up? What other information would you have liked to know? For example, about naps, if time to bed or rise has changed.
Q. What did you think of the initial and follow-up assessments, particularly the measures (questionnaires) we used?
Q. Before we finish, is there anything else you would like to mention that we have not already covered?
Thank you for taking part in the study and meeting me today.
Appendix 10 Standard operating procedure: processing Actiwatch data
DREAMS START
Standard operating procedure: processing Actiwatch data
Downloading Actiwatch data
-
Clean the watch with an antiseptic wipe (including the insides).
-
Download the data (Read Data) using MotionWare software 1.1.25. Save the data in [Actiwatch participant data folder].
-
Rename the file as, for example, 0101-bl-orig OR -fu-orig for follow-up.
-
IMPORTANT: download the data (Read Data) again, and save the data in [Actiwatch participant data folder].
-
Rename the file as, for example, 0101-bl OR -fu for follow-up. THIS IS THE FILE USED FOR THE REST.
-
Choose Utilities and Shutdown Watch, then disconnect.
-
Choose the file used for the analysis, and in the Actogram window, choose File > Properties, and edit any recording properties [date of birth, UserID (user identification), UserName] before proceeding.
-
– The UserID should be like this: 0101.
-
– The UserName should contain the UserID and watch serial number: 0101_S004324.
-
Preparing the data set
-
Select Sleep Analysis.
-
Highlight the whole recording period.
-
Choose Edit to remove missing data from the analysis; anything from 17:01 on day 14 AND all periods when the watch was off the wrist (based on the carer’s report and/or judging from the recording).
-
IMPORTANT: make sure that the remaining period is exactly 14 days long; should be from 17:00 of the first day to 17:00 of the last day.
-
IMPORTANT (needed for the manual – see below): find Average Lux by selecting the whole 14-day period. In the Sleep Analysis window, Average Light (in lux) is under the Summary option. Make a note of the lux value, to later record it in the light document (instructions below).
-
IMPORTANT: While the window is still open, click on NPCRA and choose Save Period, to view the edited 14-day actigraphy (otherwise the report will not include NPCRA).
Producing graphs for the manual
-
Each graph will need to be produced separately. To produce:
-
– Light graph – click the downwards arrow on the left-hand scale until the black data (activity) disappears. The range for light should always be 0–2000. Then move on to step b.
-
– Activity graph – Click the downwards arrow on the right-hand scale until the yellow data (light) disappears. The range for activity will depend on the participant, but 0–5000 appears to work quite well. In some cases, you cannot choose 5000 so either go 4000 or 6000, whichever is better for the participant’s activity based on what you know. Move on to step b.
-
-
Select Tools, then Report.
-
Under report options, tick only:
-
– Include recording properties.
-
– Include actogram.
-
– Push actogram onto separate page.
-
-
Keep the default values for all other settings (i.e. do not change anything under Change Actogram Options).
-
Click copy to Word Processor.
-
In Word, create a new document, and paste the clipboard contents.
-
For the:
-
– Light graph – use cropping to delete all extra (edited out) periods from the end of the report. Also crop the scale on the left-hand side, leaving only the scale on the right-hand side, as long as this does not cut out the dates.
-
– IMPORTANT (needed for the manual – see below): add the Average Lux from above to the light document, just underneath the participant data table.
-
– Activity graph – crop the scale on the right-hand side, leaving only the scale on the left-hand side; also delete all extra (edited out) periods from the end of the report.
-
-
Name the file as, for example, 0101_light-bl OR 0101_activity-bl (-fu for followup) and save in: [intervention delivery actigraph data for session 2 folder].
Obtaining outcome measures
-
Next, using the edited actigraphy, highlight the first sleep period, choosing as the starting and ending points the event button presses (or one of those, preferably the first, if any), then editing and re-editing the period in the Sleep Analysis window as necessary.
-
Once happy with a sleep period, choose Save Sleep Period. Do the same for each night that the watch was worn.
-
N.B. If the carer has been filling in the Actiwatch sleep diary, use Tools > Sleep Summary Table and enter the Lights out (Bed time) and Got up times from the diary to use those as initial start/end points. Then edit the sleep period by editing these times, using the button presses (when available).
-
N.B. If the carer has NOT filled in the Actiwatch sleep diary, highlight the area believed to be the sleep period based on the carer’s verbal report and the activity data. If it becomes too difficult to create a sleep window based on activity, then attempt the second strategy: using the light and activity data. When unsure, ask one of the other two researchers to help and reach a consensus on each problematic sleep window.
-
With all Sleep Periods defined, choose Tools and Report. IMPORTANT: make sure both the Sleep Analysis and NPCRA are ticked, then Print Preview. Save the Report in [Actiwatch participant data folder] as, for example, 0101_report-bl (OR -fu).
For those delivering the intervention:
-
Open Session 2 manual and paste the light and activity data into the designated place (light actograms will be placed on p. 8 and activity actograms will be placed on p. 12).
-
Verify that the correct data have been selected for the participant: check the table (i.e. UserID, Sex) and then delete these, leaving only the actograms.
-
Add Average Lux (from the light document) to p. 8 of Session 2.
List of abbreviations
- A
- assessor
- AD
- Alzheimer’s disease
- ASRN
- Alzheimer’s Society Research Network
- C
- carer
- CD
- compact disc
- CDR
- Clinical Dementia Rating
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- DEMQoL-Proxy
- Dementia Quality of Life – Proxy
- DREAMS START
- Dementia RElAted Manual for Sleep; STrAtegies for RelaTives
- ESS
- Epworth Sleepiness Scale
- HADS
- Hospital Anxiety and Depression Scale
- HSQ-12
- Health Status Questionnaire-12
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation coefficient
- ID
- identification
- IQR
- interquartile range
- JDR
- Join Dementia Research
- MARQUE
- Managing Agitation and Raising Quality of Life
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPCRA
- non-parametric circadian rhythm analysis
- NPI
- Neuropsychiatric Inventory
- PPI
- patient and public involvement
- PSQI
- Pittsburgh Sleep Quality Index
- Q
- question
- RCT
- randomised controlled trial
- SCI
- Sleep Condition Indicator
- SCN
- suprachiasmatic nucleus
- SD
- standard deviation
- SDI
- Sleep Disorders Inventory
- START
- STrAtegies for RelaTives
- T
- Therapist
- TAU
- treatment as usual
- UCL
- University College London
- ZBI
- Zarit Burden Interview
