Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/20. The contractual start date was in April 2012. The draft report began editorial review in April 2017 and was accepted for publication in July 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Matthew L Costa is a member of the general board for the Health Technology Funding stream. Keith Willett received royalty payments from Zimmer for implant design outside the submitted work. Sarah E Lamb is a member of the Health Technology Assessment (HTA) Additional Capacity Funding Board, HTA End of Life Care and Add-on Studies, HTA Prioritisation Group and HTA Trauma Board.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Costa et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Fractures of the lower limb are extremely common injuries in both the civilian and military populations. The majority of these injuries are ‘closed’, that is, the skin around the fracture is intact. In such cases, the risk of infection is low; however, if the fracture is ‘open’, such that the barrier provided by the skin is breached, then the broken bone is exposed to contamination from the environment. In open fractures, the risk of infection is greatly increased. 1
Wounds associated with open fractures of the lower limb are graded by severity, as part of routine clinical practice, using the classification of Gustilo and Anderson2 (G&A). Grade 1 injuries are small clean wounds (< 1 cm in length), grade 2 injuries involve larger wounds (> 1 cm in length) but without extensive soft-tissue damage, and grade 3 injuries are wounds of > 1 cm in length with extensive soft-tissue damage (Table 1). In addition, Gustilo and Anderson2 described a special type of grade 3 injury that involved damage to a major blood vessel that required surgical repair. The greater the extent of the injury to the soft tissues around the broken bone, the greater the risk of infection. In severe, high-energy fractures of the lower limb, infection rates of 27% have been reported,3 even in specialist trauma centres.
| Fracture type | Description |
|---|---|
| Grade 1 | An open fracture with a wound of < 1 cm in length and clean |
| Grade 2 | An open fracture with a laceration of > 1 cm in length without extensive soft-tissue damage, flaps or avulsions |
| Grade 3 | An open segmental fracture, an open fracture with extensive soft-tissue damage or a traumatic amputation. Special categories in grade 3 are gunshot injuries, any open fracture caused by a farm injury and any open fracture with accompanying vascular injury requiring repair |
If complications, such as deep surgical site infection (SSI) occur, treatment frequently continues for years after the open fracture. There is a huge health-care cost associated with such injuries (US study:4 US$163,000 if the limb can be salvaged and > US$500,000 if amputation is required) and this is a fraction of the subsequent personal and societal cost. In the UK civilian population, the risk of an open long-bone fracture is approximately 11.5 per 100,000 per year,5 but this is much higher in the military population and the severity of the injuries is frequently greater. 6
The initial management of open fractures of the lower limb in an emergency department involves the removal of gross contamination, the application of a sealed dressing and the administration of antibiotics, as described in the joint British Orthopaedic Association and British Association of Plastic, Reconstructive and Aesthetic Surgeons ‘BOAST’ publication The Management of Severe Open Lower Limb Fractures (www.boa.ac.uk/wp-content/uploads/2014/12/BOAST-4.pdf; accessed 9 October 2017)7 and the National Institute for Health and Care Excellence (NICE) complex fracture guidelines 2016 (www.nice.org.uk/guidance/ng37; accessed 9 October 2017). 8 Many patients admitted to local hospitals are transferred immediately to specialist facilities, such as a major trauma centre (MTC). However, the key component of the management pathway is the surgical ‘debridement’ (removal of all contaminated tissue and washout of the open fracture under anaesthetic). Once the wound is clean, the fracture is usually immobilised with some form of internal or external fixation and a dressing is applied at the end of surgery.
Traditionally, if the wound cannot be closed primarily, a sealed, non-adhesive layer is applied to the surface of the wound to protect the open fracture from further contamination. The wound is covered in this way until a reassessment and further debridement is performed in the operating theatre, usually within 48–72 hours after the initial injury. This method has been used throughout the NHS and in military practice for many years.
This study concerns the type of dressing that is applied to the wound at the end of the operation.
Negative-pressure wound therapy (NPWT) is an alternative form of dressing that may be applied to open fractures. In this treatment, an ‘open-cell’ foam or gauze is cut to size and laid onto the wound, followed by a sealed dressing. A hole is made in the dressing overlying the foam or gauze and a sealed tube is used to connect the dressing to a pump, which creates a partial vacuum over the wound. This NPWT removes blood and exudate from the area of the wound, and may also remove any residual bacteria, encouraging ‘granulation’ (healing) tissue. 9 Recent laboratory studies have also suggested that NPWT may stimulate the release of ’cytokines’ that encourage new blood vessel formation. 10 These NPWT dressings are widely used for other surgical wounds after elective surgery and are increasingly used throughout the NHS. However, NPWT is considerably more expensive than traditional wound dressings for the dressing and the associated machinery that generates the partial vacuum.
Negative-pressure wound therapy has shown encouraging results in clinical trials related to diabetic foot wounds11 and abdominal wounds. 12 A systematic review13 of the literature before the Wound management of Open Lower Limb Fractures (WOLLF) study showed only one randomised controlled trial14 (RCT) comparing standard wound dressing with NPWT for patients with open fractures of the lower limb. This trial14 demonstrated a reduction in the rate of wound infection in the group of patients treated with NPWT [5.4% vs. 20%; relative risk (RR) 0.199, 95% CI 0.05 to 0.87]. However, this was a small trial (59 patients, 63 fractures) at a single centre and was funded by a commercial company which produces a NPWT system. There were no similar trials registered on the international trials database.
Relevance of project
Despite the limited supporting evidence, the 2009 UK BOAST7 and 2016 NICE guidelines8 for the management of open fractures of the lower limb included a recommendation for the use of NPWT. A consensus paper, published by the International Expert Panel on NPWT,13 also recommended that, when the use of primary closure is not possible, NPWT needs to be considered in the management of wounds associated with open fractures, but acknowledged that the evidence base to support this statement was very limited.
There was a pressing need to evaluate this relatively expensive technology given the increasing use in clinical practice without a strong supporting evidence base. This multicentre, pragmatic RCT was conducted to compare NPWT with standard dressings for patients with wounds associated with open fractures of the lower limb.
Objectives
The study was conducted in two phases with objectives for each.
Feasibility study
-
To conduct a qualitative assessment of (a) patients’ experience of sustaining fracture of the lower limb; (b) patients’ experience of being enrolled into a clinical trial, giving or declining consent for the trial; and (c) the acceptability of the trial procedures to patients and staff.
-
To determine the number of eligible, recruited and withdrawn patients in five trauma centres over the course of 6 months. In addition, to determine whether or not any trial participants lacked capacity to give consent 6 weeks post injury.
Main study
The primary objective for the main RCT was to:
-
estimate differences between the treatment groups in the Disability Rating Index (DRI) at 12 months after open fracture.
The secondary objectives were to:
-
estimate the rate of ‘deep infection’ (deep SSI) of the limb at 30 days after open fracture
-
estimate differences in health-related quality of life (HRQoL) [Short Form questionnaire-12 items (SF-12) and EuroQol-5 Dimensions, three-level version (EQ-5D-3L)] up to 12 months after open fracture
-
determine the number and nature of complications and further surgical interventions related to the injury during the first 12 months after open fracture
-
investigate, using appropriate statistical and economic analysis methods, health-care resource use and thereby the cost-effectiveness of NPWT versus standard dressing for wounds associated with open fractures of the lower limb.
Chapter 2 Methods
Trial design
This was a multicentre pragmatic RCT, recruiting patients with an open lower limb fracture. Patients were randomly assigned to receive either a standard wound dressing or NPWT after lower limb surgery.
Setting
The trial was conducted in 24 NHS hospitals across the UK. Eighteen were designated MTCs and six were large trauma units.
Participants
Inclusion criteria
Patients were eligible for the trial if they:
-
were aged ≥ 16 years
-
presented (or were transferred) to a trial hospital within 72 hours of injury
-
had sustained an open fracture of the lower limb assessed as G&A grade 2 or 3. The treating surgeon determined the G&A grade at the end of surgical debridement as per routine operative practice.
We anticipated that only a very small number of patients would present after 72 hours, but that, in such cases, it was likely that open-fracture wounds would already be infected.
Exclusion criteria
Patients were excluded from the trial if:
-
there were any contraindications to anaesthesia such that the patient was unfit for surgery
-
there was evidence that the patient was unable to adhere to trial procedures or complete questionnaires, such as permanent cognitive impairment. In a small proportion of patients, this exclusion criterion could be determined only after randomisation and emergency surgery had taken place. These patients were withdrawn from the study and no patient-identifiable data were retained.
Screening procedures
Patients with an open fracture of the lower limb are admitted to the hospital from the emergency department. All patients were screened in the emergency department or the trauma wards for eligibility by a trained research associate. Screening logs from each centre were used to determine the number of eligible patients and reasons for exclusions. Patients who declined to take part were offered the opportunity to discuss reasons for this with a member of the research team.
Recruitment challenges
The key challenge to recruitment was the procedure for obtaining the patients’ informed consent. The nature of these injuries meant that the majority of patients were operated on immediately or were allocated to the next available trauma operating list. Some patients were unconscious or had reduced levels of consciousness, and the great majority were given strong opiate-based analgesia. Therefore, many of the patients lacked capacity to provide informed consent before their surgery. The feasibility phase of the WOLLF study was designed to address this issue, as well as to estimate the rate of recruitment.
Consent
Conducting research in the emergency setting is regulated by the Mental Capacity Act (MCA) 2005. 15 As patients were likely to lack capacity, as described above, and because of the urgent nature of the treatment limiting access to, and appropriate discussion with, personal consultees, we acted in accordance with section 32, subsection 9b of the MCA for following a process approved by the Research Ethics Committee (REC).
Patients consented preoperatively
Patients who were able to give informed consent preoperatively were approached and recruited by a member of the research team.
Patients consented postoperatively
Patients who were unconscious or lacking ability to process information were recruited to the study under consultee agreement. Patient consent was not obtained prior to surgery, but a consultee was approached to provide agreement for entry to the trial. The consultee was a next of kin, when available, or a medically trained clinician independent of the trial. At the first appropriate time when the patient had regained capacity, the research associate provided all the study information. The patients were given the opportunity to ask questions and discuss the study with their family and friends. They were then asked to provide written consent for continuation in the study. Participants recruited and randomised under consultee agreement were withdrawn from the trial if they were unable to give informed consent within 6 weeks of randomisation. Any other participant randomised to treatment could withdraw at any time.
Randomisation
A computer-generated random allocation sequence was generated and controlled by York Clinical Trials Unit. The unit of randomisation was the individual patient on a 1 : 1 basis, stratified by trial centre and G&A grade. When a patient entered the trial, non-identifiable details were logged on the secure, encrypted, web-based randomisation system and then the allocation was generated. Information included patient initials, date of birth, gender and eligibility checks. The trial and clinical teams were informed of the unique trial number (TNO) for each participant; this TNO was used on all subsequent trial documentation.
Allocation of treatment
Trial participants were assigned to their treatment allocation intraoperatively at the end of initial surgery, but before a wound dressing was applied. All operating theatres included a computer with internet access. Therefore, a secure, 24-hour, web-based randomisation system was used to generate treatment allocation.
Blinding
As the wound dressings were clearly identifiable, it was not possible to blind trial participants or clinical teams to treatment allocation. However, outcome assessment was undertaken by trained research associates (nurses or research physiotherapists) independent of the clinical care team. For patient-reported outcomes [disability, pain, quality of life (QoL), resource use, other complications], trial participants completed follow-up questionnaires themselves and these were returned directly to the central trial office.
Interventions
Usual-care group
Participants allocated to usual care had a standard dressing applied to the open wound. This comprised a non-adhesive layer applied directly to the wound covered by a sealed dressing or bandage. The standard dressing did not use ‘negative pressure’. The exact details of the materials used were left to the discretion of the treating surgeon as per routine care. Details of each dressing applied in the trial were recorded and classified according to British National Formulary (BNF) classification.
Negative-pressure wound therapy
The NPWT dressing used an ‘open-cell’ solid foam or gauze which was laid onto the wound followed by an adherent dressing. A sealed tube was connected from the dressing to a pump which created a partial vacuum over the wound. The basic features of the NPWT are universal, but the exact details of the dressing and pressure (mmHg) were left to the discretion of the treating health-care team as per routine care. Details of dressings used were recorded in trial documentation.
Post-randomisation withdrawals
Lack of consent
Participants recruited and randomised under consultee agreement were withdrawn from the trial within 6 weeks of randomisation if they were unable to give informed consent by that time.
Participant withdrawal
Participants could decline to take part in the trial at any time. There were different levels of withdrawal:
-
withdrawal from the trial with approval for use of all trial data
-
withdrawal from the trial with approval for use of partial trial data (e.g. clinical records, radiographs only or questionnaires only)
-
withdrawal from the trial rescinding approval for access to trial and clinical data.
Participant care pathway
All of the participants followed routine clinical pathways, other than the allocation of the wound dressing at the end of the initial surgical debridement of the open-fracture wound.
All patients received a general or regional anaesthetic. Antibiotic prophylaxis and prophylaxis for venous thromboembolism was used as per routine protocol at each centre. The wound associated with the fracture was ‘debrided’ (surgically decontaminated and cleaned) in the operating theatre and the fracture was treated with either internal or external fixation. At the end of the initial operation, if the open-fracture wound could not be closed primarily, patients were randomised and allocated to either standard dressing or NPWT.
Both groups of patients then followed the normal postoperative management of patients with an open fracture of the lower limb. This usually involved a ‘second-look’ operation between 48 and 72 hours, at which time a further debridement was performed and the wound closed with sutures or a soft-tissue reconstruction as necessary. Depending on the specific injury and depending on the treating surgeon’s normal practice, the wound may have been redressed again pending further surgery. Any further wound dressing followed the allocated treatment until definitive closure/cover of the wound was achieved.
Postoperative rehabilitation was left to the discretion of the treating surgeon and clinical team, depending on the patient’s injuries and usual clinical practice at that centre.
Primary outcome
The primary outcome for the trial was the DRI at 12 months after randomisation. The DRI is a self-administered, 12-item visual analogue disability scale questionnaire that is transformed to a 100-point score, where 0 represents normal function and 100 represents complete disability. 16
If more than six items were missing, the DRI was considered to be invalid and was marked as missing; 3 out of the 377 participants (0.8%) with a DRI at 12 months did not provide responses to sufficient items to enable a valid score to be calculated. This outcome measure was chosen because it addressed gross function in the lower limbs, rather than specific joints or body segments. Therefore, it allowed for the different fractures and different injury patterns sustained by the trial participants.
The default method of data capture at baseline (pre injury) was a face-to-face meeting. On later occasions, the default method of data capture was via postal correspondence.
Secondary outcomes
The secondary outcomes were HRQoL, deep SSI, other postoperative complications and resource use. Patient-reported outcomes, including the DRI, HRQoL measures, criteria for SSI and other complications, and health-care resource use, were collected by questionnaire at baseline, 3, 6, 9 and 12 months after randomisation. Baseline assessments were made primarily to allow study participants to retrospectively assess their pre-injury status. Definitions for outcomes and procedures for data collection are described below.
Health-related quality of life
Health-related QoL was captured using the following measures.
-
EuroQoL-5 Dimensions (EQ-5D): a validated measure of HRQoL, consisting of a five-dimension health status classification system and a separate visual analogue scale (VAS). 17 Responses to the health status classification system were converted into multiattribute utility (MAU) scores using a published utility algorithm, anchored at 1 (perfect health) and 0 (death). 18 These MAU scores were combined with survival data to generate quality-adjusted life-year (QALY) profiles for the economic evaluation. In addition, health status was also assessed using the EQ-5D VAS, which required participants to assess their own health from the worst imaginable (0) to the best imaginable (100). These assessments were made by study participants pre injury (retrospectively),19 immediately post injury and at 3, 6, 9 and 12 months post injury.
-
SF-12: a validated and widely used health-related QoL measure. 17 The UK factor score coefficients20 were used to give physical component scores (PCSs) and mental component scores (MCSs). Each permutation of response to the SF-12 was converted into a Short Form questionnaire 6-Dimensions (SF-6D) health utility score using a published utility algorithm. 21 These data were also combined with survival data to generate QALY profiles for a sensitivity analysis within the economic evaluation. HRQoL using the SF-12 was assessed at pre-injury baseline (retrospectively recalled) and at 3, 6, 9 and 12 months post injury.
Surgical site infection and wound healing
The Centers for Disease Control and Prevention (CDC) definitions of SSIs were used (Box 1 and see Appendices 10 and 11). The CDC definition for superficial and deep SSIs is at 30 days following surgery (randomisation). Wounds were assessed and medical records reviewed at discharge, or at the first outpatient appointment after discharge from hospital if the patient was discharged before 30 days. Patients discharged before 30 days had their first follow-up appointment between 30 days and 6 weeks after surgery as part of normal clinical practice in the UK. Wounds were directly observed and infection characteristics were recorded by research staff. The CDC criteria23 for deep SSI also include any deep infection occurring within 1 year if an implant has been left in place. Therefore, we also recorded deep infection presenting within 12 months of the injury: any wound infection that required continuing medical or surgical intervention after 30 days, including infections leading to amputation, was considered a deep SSI.
Must meet the following criteria:
Infection occurs within 30 days after the operative procedure if no implant is left in place or within 1 year if an implant is in place and the infection appears to be related to the operative procedure.
AND
Involves deep soft tissues of the incision (e.g. fascial and muscle layers).
AND
Patient has at least one of the following:
-
Purulent drainage from the deep incision but not organ/space component of surgical site.
-
A deep incision that spontaneously dehisces or is deliberately opened by a surgeon and is culture-positive or not cultured when the patient has at least one of the following signs or symptoms: fever (> 38 °C), or localised pain or tenderness. A culture-negative finding does not meet this criterion.
-
An abscess or other evidence of infection involving the deep incision is found on direct examination, during reoperation or by histopathologic or radiological examination.
-
Diagnosis of a deep incisional SSI by a surgeon or attending physician.
Wound photographs
Wound photographs were taken at 6 weeks. A Samsung ES9 digital camera with flash (Samsung Electronics Limited, Surrey, UK) was given to each centre. Staff were trained to adhere to a standard wound protocol to ensure that images were of adequate quality (e.g. instructions for lighting). Nurses were instructed to remove wound dressings from the open-fracture wound and place a 15-cm paper ruler next to the wound for scaling. This paper ruler included the participant TNO. All images were password protected and returned to the trial co-ordinating centre. Photographs were reviewed blind to treatment allocation by two experienced wound healing specialists. Disagreement was resolved by a third reviewer.
Other postoperative complications
All complications and further surgical interventions related to the open-fracture wound or treatment of the wound were recorded using multiple approaches. Complications were documented at routine follow-up appointments, were self-reported by patients or were notified as adverse events (AEs) or serious adverse events (SAEs) (see Approval for main trial).
All participants were invited for clinical review and a radiograph at 12 months, as per routine practice after this type of injury. If a participant had not returned a 12-month postal questionnaire, this was completed in clinic.
Radiographic images (radiographs)
Radiographic images were taken at 6 weeks and 12 months post surgery as part of routine follow-up for this group of patients. Standard anteroposterior and lateral radiographs were taken at each centre. Copies of original radiographs were stored on a secure compact disc and returned to the central trial office. The radiographs were reviewed by an independent surgeon who was blinded to the treatment allocation. As part of the assessment of complications, each set of radiographic images was assessed for failure of fixation (yes or no). For long-bone fractures, the sagittal and coronal angulation were measured for the index fixation; sagittal angulation > 10° and coronal angulation > 5° were considered to be clinically important. At 12 months, each set of radiographs was also assessed for bony union (bridging cortical bone across three cortices).
Health-care and social care resource use
Resource use was measured for the purposes of the health economic evaluation. Unit cost data were obtained from national databases such as the BNF24 and Personal Social Services Research Unit (PSSRU)’s Unit Costs of Health and Social Care 2012. 25 When these were not available, the unit cost was estimated in consultation with the University Hospitals Coventry and Warwickshire (UHCW) NHS Trust finance department. The cost–consequences following hospital discharge, including NHS costs and patients’ out-of-pocket expenses, were estimated using questions included within a questionnaire sent to participants at 3, 6, 9 and 12 months post randomisation. Patient self-reported information on service use has previously been shown to be accurate in terms of the intensity of use of different services. 26
Data management: postal questionnaires
All questionnaires were sent from and returned to the Warwick Clinical Trials Unit (WCTU), managed by data clerks. If no questionnaire was received within 2 weeks, a reminder questionnaire was sent. When there was no response to reminders, participants were contacted by telephone and invited to answer questions on core outcomes (DRI and EQ-5D). A small proportion of participants were invited to complete the 12-month questionnaire during their routine clinic follow-up appointment 1 year post surgery.
We used techniques common in long-term cohort studies to ensure minimum loss to follow-up such as collection of multiple contact addresses, telephone numbers, mobile telephone numbers and e-mail addresses. Considerable efforts were made by the trial team to maintain contact with participants throughout the trial, including regular participant newsletters.
Approval for main trial
On completion of the 6-month feasibility study, results were reviewed by the Trial Steering Committee (TSC). The target recruitment rate was achieved across five trauma centres (one patient per month per centre) indicating that it was feasible to proceed. In brief, findings from the qualitative interviews suggested that patients were willing to consent and they understood the rationale for the study. Therefore, no changes to the protocol or recruitment targets were made. Following the TSC report, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme granted approval for progression to the main phase of the trial.
Adverse event management
An AE is defined as any untoward medical occurrence in a clinical trial participant that does not necessarily have a causal relationship with the treatment. All AEs were listed on the appropriate case report form for routine return to the central trial office.
A SAE is defined as any untoward and unexpected medical occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing inpatient hospitalisation
-
results in persistent or significant disability or incapacity
-
is a congenital anomaly or birth defect
or any other important medical condition that, although not included in the above, may require medical or surgical intervention to prevent one of the outcomes listed.
All SAEs were entered onto a SAE reporting form and faxed to the dedicated fax system at WCTU within 24 hours of the investigator becoming aware of them. Once received, causality and expectedness were confirmed by the chief investigator. SAEs that were deemed to be unexpected and related to the trial were notified to the REC within 15 days. All such events were reported to the TSC and Data Monitoring Committee (DMC) at regular meetings.
Serious adverse events that were expected as part of the surgical interventions and that did not require reporting to the main REC were complications of anaesthesia or surgery (wound infection, bleeding or damage to adjacent structures such as nerves, tendons and blood vessels; delayed unions/non-unions; delayed wound healing; further surgery to remove/replace metal work; and thromboembolic events). All participants experiencing SAEs were followed up as per protocol (PP) until the end of the trial.
Risks and benefits
The risks associated with this trial were predominantly those related to the injury and surgery, for example postoperative infection and bleeding and damage to adjacent structures such as nerves, blood vessels and tendons. Participants in both groups underwent surgery and were potentially at risk from any/all of these complications. Allocation of the trial intervention took place at the end of the initial surgery so that there was no difference between the groups in terms of surgical or anaesthetic risk. Both standard dressings and NPWT have been used widely in the civilian and military settings, and there were no specific risks anticipated with the use of either type of wound management, which was the focus of this trial.
Statistical analysis
Sample size
The minimal clinically important difference (MCID) for the primary outcome measure (DRI) is 8 points. At an individual patient level, a difference of 8 points represented the ability to climb stairs or run with ‘some difficulty’ versus with ‘great difficulty’. At a population level, 8 points represented the difference between a ‘healthy patient’ and a ‘patient with a minor disability’.
In Table 2, the figure of 412 participants represents a conservative scenario, based on a standard deviation (SD) of 25 participants and 90% power to detect the selected MCID. Allowing a margin of 10% loss during follow-up, including the small number of patients who die in the first year following their injury, gave a total sample size of 460 patients. Therefore, 230 patients consented to each intervention arm would provide 90% power to detect a difference of 8 points in DRI at 12 months at the 5% significance level.
| SD | Power | |
|---|---|---|
| 80% | 90% | |
| 15 | 112 | 150 participants |
| 20 | 198 | 264 participants |
| 25 | 308 | 412 participants |
Analysis plan
Feasibility study
At the end of the feasibility phase, the overall mean recruitment at the five selected centres for this phase of the study was estimated (with a 95% CI) and compared with the target rate of one patient per month per centre. The estimated recruitment rate and the overall rate of withdrawn patients in the feasibility phase informed the design and the decision to proceed to the main RCT.
Main randomised controlled trial
Standard statistical summaries (e.g. medians and ranges or means and variances dependent on the distribution of the outcome) and graphical plots showing correlations were presented for the primary outcome measure and all secondary outcome measures. Baseline data were summarised to check comparability between treatment arms and to highlight any characteristic differences between those individuals in the study, those ineligible and those eligible but withholding consent.
The main analysis investigated differences in the primary outcome measure, the DRI score at 1 year after injury, between the two treatment groups (standard dressings and NPWT) on an intention-to-treat (ITT) basis. In addition, early functional status was assessed and reported at 3, 6 and 9 months. Differences between groups were assessed based on a normal approximation for the DRI score at 12 months post injury and at interim occasions. Tests were two-sided and considered to provide evidence for a significant difference if p-values were < 0.05 (5% significance level).
Although generally we had no reason to expect that clustering effects would be important for this study, in reality the data were hierarchical in nature, with patients naturally clustered into groups by the recruiting centre. Therefore, we accounted for this by generalising the conventional linear (fixed-effects) regression approach to a mixed-effects modelling approach, in which patients were naturally grouped by recruiting centres (random effects). This model formally incorporated terms that allowed for possible heterogeneity in responses for patients owing to the recruiting centre, in addition to the fixed effects of the treatment groups, G&A grade and other patient characteristics that proved to be important moderators of the treatment effect, such as age and gender.
It seemed likely that some data would not be available owing to voluntary withdrawal of patients, lack of completion of individual data items or general loss to follow-up. When possible, the reasons for data ‘missingness’ were ascertained and reported. Although missing data were not expected to be a problem for this study, the nature and pattern of the missingness were carefully considered including, in particular, whether or not data were treated as missing completely at random. If judged appropriate, missing data were imputed, using the multiple imputation facilities available in R (The R Foundation for Statistical Computing, Vienna, Austria). The resulting imputed data sets were analysed and reported, together with appropriate sensitivity analyses. Any imputation methods used for scores and other derived variables were carefully considered and justified. Reasons for ineligibility, non-compliance, withdrawal or other protocol violations were stated and any pattern observed was summarised. More formal analysis, for example using logistic regression with ’protocol violation’ as a response, was also considered, when appropriate, to aid interpretation. About 1–2% of patients were expected to die during follow-up; therefore, this is unlikely to be a serious cause of bias. However, we conducted a secondary analysis taking account of the competing risk of death, using methods described by Varadhan et al. 27
A detailed statistical analysis plan (SAP) was agreed with the DMC. Any subsequent amendments to this initial SAP were clearly stated and justified. Interim analyses were performed only when directed by the DMC. The routine statistical analysis was carried out using R.
Secondary analyses were undertaken using the above strategy for approximately normally distributed outcome measures SF-12 and EQ-5D. For dichotomous outcome variables, such as indicators of deep infection and other complications related to the trial interventions, mixed-effects logistic regression analysis was undertaken with results presented as odds ratios (ORs) [and 95% confidence intervals (CIs)] between the trial groups. In addition, temporal patterns of any complications were presented graphically and, if appropriate, a time-to-event analysis (Kaplan–Meier survival analysis) was used to assess the overall risk and risk within individual classes of complications.
Health economic analysis plan
An economic evaluation was integrated into the trial design. The economic evaluation was conducted from the recommended NHS and Personal Social Services (PSS) perspective. 28 Data were collected on the health and social service resources used in the treatment of each trial participant during the period between randomisation and 12 months post randomisation. Trial data collection forms recorded the duration of each form of hospital care, surgical procedures, adjunctive interventions, medication profiles, and tests and procedures. Observational research was required to detail additional staff and material inputs associated with clinical complications. At 3, 6, 9 and 12 months post randomisation, trial participants were asked to complete economic questionnaires profiling hospital (inpatient and outpatient) and community health and social care resource use and, for the purposes of sensitivity analysis, out-of-pocket expenditures and costs associated with lost productivity. Current UK unit costs were applied to each resource item to value total resource use in each arm of the trial. Per diem costs for hospital care, delineated by level or intensity of care, were largely derived from national reference cost schedules. The unit costs of clinical events that were unique to this trial were derived from the hospital accounts of the trial participating centres, although primary research that used established accounting methods was also required. 29 The unit costs of community health and social services were largely derived from national sources. Trial participants were asked to complete the EQ-5D-3L17 and SF-1230 measures at baseline and at 3, 6, 9 and 12 months post randomisation. Responses to the EQ-5D-3L and SF-12 were converted into health utility scores using established algorithms. 18,21
An incremental cost-effectiveness analysis, expressed in terms of incremental cost per QALY gained, was performed. Results were presented using incremental cost-effectiveness ratios (ICERs) and cost-effectiveness acceptability curves (CEACs) generated via non-parametric bootstrapping. This accommodated sampling (or stochastic) uncertainty and varying levels of willingness to pay for an additional QALY. A series of sensitivity analyses were undertaken to explore the implications of parameter uncertainty on the ICERs. In addition, CEACs were constructed using the net benefits approach.
Ethics approval and monitoring
Standard NHS cover for negligent harm was in place. There was no cover for non-negligent harm.
Ethics committee approval
The WOLLF study was approved by the Coventry REC on 6 February 2012 (REC reference 10/57/20) and by the research and development department of each participating centre. The trial protocol31 was published in the BMJ Open.
Trial Management Group
The day-to-day trial management was the responsibility of the trial co-ordinator, based at WCTU, and supported by administrative staff. The Trial Management Group (TMG) met monthly to assess overall trial progress. It was also the responsibility of the trial co-ordinator to train the research associates at each of the trial centres.
Trial Steering Committee
A TSC was appointed by the NIHR HTA programme and was responsible for oversight, monitoring and supervising trial progress. The TSC consisted of four independent experts, a lay member and the chief investigator. Members of the TSC are listed in Acknowledgements.
Data Monitoring Committee
The DMC was also appointed by the NIHR HTA programme and was tasked with monitoring ethics, safety and data integrity. The trial statistician provided data and analyses requested by the DMC at each meeting. Members of the DMC are listed in Acknowledgements.
Patient and public involvement
Prior to the submission of the grant application for the WOLLF study, an informal survey was conducted at a large university hospital trust to establish the opinion of patients and their carers with regard to research in orthopaedic trauma. We established that patients place great importance on research comparing different types of interventions in the area of trauma surgery. Furthermore, they have demonstrated that they are willing to take part in such trials.
Throughout the trial, a patient with direct experience of sustaining an open lower limb fracture and the subsequent recovery path reviewed all patient documents prior to submission to the sponsor and the ethics committee. Furthermore, advice was sought from this lay collaborator during management meetings when issues were discussed directly related to patient engagement and commitment.
Independent lay representation was present on the TSC. Members of the Trauma Patient and Public Involvement Group also reviewed the progress of the WOLLF study at the annual NIHR Trauma Trials Meetings.
Chapter 3 Qualitative study
Background
This study explores the experiences of patients in acute care who have an open fracture of the lower limb and their experience of being in a trial. Research that examines the experience of having an open fracture of the lower limb is limited, particularly in the acute phase of recovery. Some studies explore recovery at a variety of time points post hospitalisation. For example, in order to develop an outcome tool, Trickett et al. 32 interviewed nine participants 1.2–2.8 years post injury. They identified the serious impact of injury on participants’ daily life as they struggled to recover in terms of a range of different types of pain including stiffness and discomfort, reduced mobility and flexibility, the impact of temperature on their body, frustration and fear, anxiety around their appearance, concerns about getting back to work, a fear of falling, reduced finances and the impact of injury on family and friends. Using a grounded theory approach, Shauver et al. 33 sought to explore the relationship between high satisfaction levels and poor outcomes. Semistructured interviews (n = 20) were undertaken 2.3–12 years post injury. Satisfaction despite poor outcomes was explained by approaches to coping, problem-solving practical difficulties and using cognitive restructuring to identify positive aspects of experience. Personal growth was present as participants developed alternative careers to accommodate their injury.
Other studies that include lower limb injury provide useful insights into patient experience. Griffiths and Jordan34 used diaries and semistructured interviews (n = 9) 5–8 weeks post surgery. Three themes of (1) dealing with uncertainty, (2) seeking control and (3) returning to normality were identified. The participants experienced high levels of pain, had difficulty controlling pain and found dependency on others problematic in their attempt to return to normality. Participants studied by Forsberg et al. 35 were interviewed (n = 9) from 1 to 12 months after injury and identified feelings of frustration, helplessness and vulnerability as they struggled to feel in control and safe as they regained their autonomy. Like the participants in the Griffiths and Jordan study,34 they sought to control their pain but also felt vulnerable while waiting for, and going to, the operating theatre for surgery; security was gained from supportive staff. Studies of ankle fracture support these aspects of experience. McPhail et al. 36 interviewed 12 participants aged < 60 years at 6 weeks to > 2 years post injury and six staff members. They identified ongoing swelling and pain, frustration and depression, an inability to return to normal activities and to wear usual footwear, and a reduction in social life and reduced finances. The emotional vulnerability of older people (aged > 60 years) with an unstable ankle fracture was identified by Keene et al. 37 Unstructured interviews (n = 36) at 6–10 weeks post treatment identified the emotion work participants undertook as they processed being injured and feeling older and renegotiated interdependency with their partners as they coped with non-weight bearing. Rethinking taken-for-granted activities and finding ways of keeping busy was key for their physical and mental well-being. Struggling to move was hampered by comorbidities, lack of skill in using walking aids, pain, swelling and lack of confidence. Support from family and friends was crucial to the maintenance of well-being during this period.
Understanding how participants make sense of trauma trials in an emergency situation when their ability to make decisions may be impaired by pain, medication and emergency treatments is part of the feasibility phase of the trial. In other research studies,37–39 trust in the clinical team and altruism have been identified as reasons to take part in a trial. Often, understanding of trial methodology, such as randomisation, can be limited, which has led to a belief that staff have provided the best treatment for them, often termed ‘therapeutic misconception’. 38,39 The acceptability of randomisation or equipoise when clinical treatment is required is called into question by trial and lay participants40,41 and can threaten participants’ feelings of trust in the clinician. 40 Patients may also make decisions based on a limited understanding of the risks and disadvantages of being in a trial42 and how the trial benefits them personally. 39,43
Our current understanding of patient experience of taking part in orthopaedic trauma trials is limited. In a study of ankle injury management,37 in which participants often had at least 24 hours to consider entering the study, they developed a strong preference for surgery or the non-surgical intervention and were disappointed when they did not get the treatment they desired. Sometimes the experience of family and friends supported their preference, also found by Canvin and Jacoby. 38 However, when interviewed at 6 weeks, they felt that they had received the right treatment for them. 37 This suggests that a process of acceptance developed over time as participants made sense of their treatment as the best course of action for them. This may be an extension of therapeutic misconception, a term used to describe the inability of patients to distinguish between trial participation and normal clinical care or a way of living with their allocated treatment. This study also identified the importance of experiential knowing at the time of consenting and how participants felt they could not know the interventions as they had no prior experience of them. The timing of consent and assessing individuals’ capacity to make an informed decision is problematic in studies of emergency orthopaedic trauma. From a systematic review of a range of studies, Gobat et al. 44 concluded that researchers face a conflict between providing an opportunity for patients to take part in a trial and being viewed as immoral by causing high levels of patient distress, related to patients’ ability to absorb sufficient information, and the additional distress of family members. Third-party consent was generally considered acceptable but there were concerns in high-risk studies and a suggestion that the burden of consent should be shared between several people. Further evidence is required in relation to this study and in particular the use of presumed consent, using personal and nominated consultees, with informed written consent provided for continuation in the study when the participants were well enough to make a decision.
As outlined above, it is not easy for patients to make a full recovery from open fracture of the lower limb, and psychological, physical and social consequences limit many from returning to a pre-injury state. Research involving patients with a range of lower limb injuries adds depth in relation to the impact of such injuries on patients’ emotions, the need for control over pain management and the effects on daily life and relationships. Studies are variable in the quality of their reporting of methodology and rigour, and in some studies samples are small. The theoretical perspectives and timing of the interviews are also variable, and it is difficult to determine how experiences change over time and what may influence change. There is currently a gap in the literature regarding patient experience in the early phase of injury while in acute care. There is also limited evidence regarding the experience of taking part in an orthopaedic trauma trial during the early phase of treatment and recovery. An understanding of this phase will help lay the foundation for improving care for this group of patients and lead to better outcomes of care in the future. This study intends to add to the body of knowledge on patient experience of injury and being in a trial while in hospital.
Methods
The study is underpinned by phenomenology drawing on the work of Heidegger. 45 This is a philosophical approach that focuses on ‘being in the world’ and what it is like to be in the world or ‘dasein’. Research focuses on the meanings inherent in everyday life, including aspects that may be taken for granted. Madjar and Walton46 suggest using a ‘listening gaze’ to focus on the unknown in order to gain a deeper understanding of the person within his or her life world. The central tenets of phenomenology convey ‘being’ within the social, historical context of the person, and include temporality and a sense of space, both spatially but also in relation to aspects of ‘concern for’ the other. 45 The research process therefore is framed by a focus on what life is like for an individual and the taken for granted meanings inherent in their everyday world. Interviews are often the method of choice; through descriptions of what it is like, notions of being, context, time and space can be examined.
In this study, unstructured interviews were undertaken while participants were in acute care. The interview focused on their experience of being injured. This was followed by prompts such as ‘tell me more about that’, ‘how did you feel?’, ‘what did you think at that time?’ and ‘how did that differ from?’. The intention was to give participants the opportunity to describe what it was like to be injured from their perspective, and to explore the issues, concerns and taken-for-granted aspects that were part of their everyday experience. In addition, one focus group with five clinical and research staff, including doctors and nurses, focused on what it was like to recruit patients to the trial.
A purposive sample of 20 patients of different ages, genders and breadth of experience was recruited; participants had been hospitalised for treatment for open fracture of the lower limb and had consented to be part of the WOLLF study. 31 All patients had fractures, G&A grade 2 (n = 4) or grade 3 (n = 16), often alongside other injuries; two participants had an extended period in critical care. Injuries had been sustained through motorbike/car collisions and activities at work or in the home. They were aged 20–82 years (mean 40 years, median 38 years). Time since first surgical intervention (within 72 hours of injury) ranged from 5 to 35 days (mean 12 days, median 11 days). Interview length ranged from 25 to 86 minutes (mean 54 minutes, median 58 minutes). In addition, one focus group was undertaken (46 minutes) with five interdisciplinary staff to ascertain their experience of undertaking the study.
Interviews took place between July 2012 and July 2013. The researcher approached those who gave permission and discussed the study with them. They received an information sheet and had at least 24 hours to consider participation before signing a consent form. NHS research ethics approval was granted. Interviews took place in a ward environment because the nature of participants’ injuries necessitated them being confined to the ward area. Interviews in busy clinical environments are problematic as maintaining privacy and dignity can be challenging. This was discussed with participants, who were given control over when to stop the interview; visiting times were avoided and the researcher left the area during clinical visits and mealtimes. The participants were comfortable with the interview environment and not concerned when interruptions occurred.
Interviews were recorded (digital–audio) and transcribed verbatim. Analysis was undertaken by drawing together codes that conveyed inherent meaning within the descriptions to form categories and themes while being aware of similarities and differences within the meanings. 47 For example, descriptions underpinning codes reflecting death, saviours, miracles and being lucky were drawn together within the category ‘being alive’, which best reflected the meaning underlying the codes. This was then drawn into the theme ‘being emotionally fragile,’ as emotions reflected the broader theme underlying each of these categories. Reflection on the process drew on notions of the hermeneutic circle,48 considering each meaning in relation to each other and the emerging whole. NVivo 10 (QSR International, Warrington, UK) was used to help manage the data. The same process was undertaken for the focus group.
Rigour was demonstrated through the notions of trustworthiness. 49 The researcher was immersed in the data for a prolonged period of time and used verbatim quotes. A range of experiences were presented, reflecting the breadth of data to enhance transferability and resonance with the reader. Auditability was demonstrated through identification of the research process. Intersubjectivity of the researcher with the data was examined throughout by reflection on data collection and the process of analysis with peers and in field notes. The previous experience of the researcher (ET) of researching concepts such as comfort, hope and other areas of injury was part of this reflective process.
The findings
The findings identify the overall theme of embodied vulnerability, in which the impact of the injury goes beyond the physical body to have an impact on all aspects of the individual’s self and their life.
Embodied vulnerability in this study was defined as a response to injury in which participants experienced a new way of being in the world expressed through their emotionally fragile, visibly wounded, constrained and painful body. They suffered and endured their recovery in hospital in the context of uncertainty and, when ready, they reimagined how their life would be at home and at work.
In this group of previously generally healthy, independent people with busy lives, there were high levels of emotional and physical distress as a result of sudden injury. The experience was extremely challenging, requiring dependency on others and a high degree of personal resilience. The participants vulnerability was expressed through four themes depicted in Figure 1: (1) being emotionally fragile, with categories of being alive, being close to losing a leg, being a person with strong emotions and being aware of others; (2) being injured, with categories of being a person with wounds, being constrained and being in pain; (3) living with injury, with categories of being at home and being at work; and (4) being compromised, with categories of being dependent on others, being trusting, being grateful, and being without prior experience.
FIGURE 1.
Conceptual framework: embodied vulnerability – relationships between themes.

Theme 1: being emotionally fragile
Being emotionally fragile reflects the feelings required to make sense of the event, live with continued uncertainty and process their own strong emotions and those of others. It was expressed through being alive, being close to losing a leg, being a person with strong emotions and being aware of others.
Category: being alive
Being alive conveyed the dramatic, life-threatening nature of this injury, often caused by high-impact road traffic events, many involving motorbikes, or industrial incidents. Many of the participants felt that they could have died and the last their families would see of them was in a ‘box’ (participant 10). The shocking nature of the near-death event created a sense of being saved and, for some, a sense of spirituality, a ‘sort of a miracle’ (participant 3), with professionals regarded as saviours. Being grateful that they had received such good care went alongside being lucky, which was linked to what could have happened and how the situation could have been so much worse:
I’ve just got to go with what happens really but at the same time I’ve still got to harp back to the fact that in the first place I was lucky. I could easily have died in that incident so you’ve got to think about relative situations really haven’t you and the injury that I eventually sustained . . .
Participant 5
Being alive, being saved and being lucky were notions that participants reflected on when talking about their recovery and planning their life in the future.
Category: being close to losing a leg
Participants were horrified and shocked, when, at some point in their recovery, they felt, or were told, that losing their leg had been, or remained, a possibility. The ease with which this could happen, and the inherent uncertainty about whether or not it would, created a high degree of anxiety:
Emotionally it’s been a real shock and quite a rollercoaster . . . and to be told that if they didn’t get it right I could lose my leg which was a bit of a shock and just to realise how easy it is to do something so serious.
Participant 16
Interviewees felt a mixture of relief and hope in relation to the potential loss of a leg. It was not something they had envisaged before the accident and they hoped it would never happen. Relief that they had kept their leg so far was mixed with apprehension regarding the uncertain progression of their recovery and the potential threat of its loss in the future.
Category: being a person with strong emotions
The participants’ stories conveyed an emotional fragility that pervaded every aspect of their lives. Some had not felt this degree of fragility before; others had, but only in relation to extreme events, such as the death of a loved one. All interviewees expressed strong emotions, and sometimes these spilled out as ‘meltdowns’, when they cried and felt they were unable to cope. A few participants in this study preferred not to talk about their injury as the feelings engendered were too strong. Some struggled with these more than others and, at times, it felt like a rollercoaster as the slow realisation of the seriousness of the impact of injury on themselves, their families and their lives unfolded. They were unprepared for the emotional hit of the accident; in addition, the repeated surgical interventions, despite knowing they were in safe hands, made participants feel vulnerable, a feeling they were not used to in their busy successful lives:
. . . initially I think I found it quite difficult and to be emotional. It’s just not something for me at all. It would take a lot for me to bring my emotion out, it would take a significant thing in my life and I’ve probably only experienced it twice, so yes quite a lot to deal with, I found it quite strange but I think we’re getting there.
Participant 9
The injury event was shocking and overwhelming; many participants relived the event through talking; for others, it was too upsetting. Some processed the fear and anxiety through regular dreams and nightmares:
I have nightmares about not being able to walk ever again or my children will walk in with my leg in their hands and stupid stuff like that.
Participant 19
The long, slow process of repeatedly requiring further surgery and coping with the impact of injury in a hospital setting reduced the participants’ capacity to emotionally cope with their recovery:
. . . it wasn’t until I got right down to the anaesthetics room that the penny dropped and then I was like a big girl’s blouse because I didn’t have the wife there or anybody there just two strangers and I felt lonely and vulnerable and basically my life is in their hands.
Participant 3
Strong emotions, such as anxiety, fear and low mood, were expressed in relation to sleep patterns, the next surgical step, the anaesthetic and being in hospital. As the extent of care required and the impact on their lives came to the fore, emotional resilience was required to endure the interventions, but participants often lacked emotional capacity and felt ‘like flipping, and going absolutely off my head . . . going absolutely schizo’ (participant 14). Psychologically, it was hard work with frustration setting in after the initial relief at being in the right place with the right care. The strength of emotions was a surprise for participants, but something they learnt to manage in a public place. However, on occasions, it was directed at staff or spilled out when insurance personnel or police asked about the accident. They also suppressed the emotional consequences of their injury for the sake of family and friends.
Category: being aware of others
The participants were not only processing their own emotions but were also facilitating family and friends’ responses who were also experiencing strong emotions. For example, a group of friends came in singing with cards and balloons but were stunned when they saw the degree of injury sustained:
I realised they were shocked and that was quite shocking to me, and they realised the grief and looked back and just joked about it.
Participant 2
There was some degree of suppression of their own emotions with the intention of maintaining normality and instilling a sense of hope that recovery was progressing:
Inside I really am as tough as old boots. I was more upset when I saw my wife upset and the effect it had had on her. I don’t think I really realised just how close I did come but as I said, I am still here and it didn’t happen, it’s just part of life . . . I put on a brave face so the wife can start to relax and start being normal again, that’s the most important thing to me for the kids to stop worrying, Dad’s on the mend and it’s all going to be good. There is light at the end of the tunnel.
Participant 3
The younger participants found Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) and the social contact with a wide range of friends incredibly supportive and a good way of maintaining contact with everyday life, family and friends; however, the nature of the interactions and type of support was not explored in this study.
Theme 2: being injured
Being injured was an emotional and physical shock for all participants and they struggled to manage their bodies through the categories of being a person with wounds, being constrained and being in pain.
Category: being a person with wounds
The wound itself and the state of the injured leg created a real sense of panic and shock. Interviewees were reluctant to see the actual wound and had to be ready to do so. They had to prepare: ‘giving it time and psych myself up’ (participant 1). The visual look of the wounds often left participants feeling shocked and sick:
It was off and I looked down and thought oh my God they’ve obviously got to cut my leg off because it just looked horrendous . . .
Participant 2
Participants were concerned about how they would live with the resulting wound. They were surprised by staff responses when they described their wound as brilliant, beautiful or healing well. Preparation of participants for seeing their wound was varied; good preparation was beneficial:
I saw it [the wound] for the first time yesterday, which was a bit weird, but if I hadn’t seen the pictures in the book I wouldn’t have known what to expect at all and it would have been horrible and it probably would have made me feel a bit sick . . . I’m glad again that I was shown the pictures so I knew what to expect . . .
Participant 11
There was a sense of being damaged and concern about what others would think:
I was worried that she wouldn’t find me attractive and she’s so pretty and so perfect and I thought ‘what is she going to think of me now?’.
Participant 2
Some became accustomed to their wounds and, on some level, accepted them and took a cognitive interest in their healing and progression:
Yes, last couple of days, side by side (both legs undressed and visible), a bit overwhelming but also a big sigh of relief because they are on the mend, well definitely very far into the mend. Compared to what they were like. Everything seems to be alright with them, the plastics are happy with them . . . I was just intrigued to be honest. I took photos to send to the family. It’s not pretty to look at but to me looking at it, it makes me feel happy to be honest because my legs are still there. It is not pretty to look at but it is what my legs are like now.
Participant 19
For certain participants, at some point in their recovery, there was a feeling of detachment from their body; they needed time to assimilate what was happening or were unable to cope with the implications and so temporarily disconnected from the situation:
The only time I actually felt detachment was when Jim first mentioned the possibility, the extreme possibility, of amputation. When another surgeon came in and mentioned it again I almost felt like I was in heaven and just detached slightly. I was listening to him and thought blimey I’ve completely disconnected from this, that’s when I feel detachment when that gets raised, I’m not consciously, it’s not a decision to detach, but it just seems to happen because it’s something that even though I’m aware of it I don’t really want to have to consider it right now.
Participant 15
Readiness to see a wound and preparedness are key elements in being a person with wounds; inclusion of family and friends is important in this process where they can offer support for the emotional impact of having a wound with a focus on the natural bodily responses and how they change over time.
Category: being constrained
The participants felt they were constrained by their body’s inability to perform in its normal way. The majority were outdoor, active people who had physically busy lifestyles. Being constrained by their body was boring and frustrating. Instead of being taken for granted, their body now required surveillance and they observed their body, noting the swelling and changes in bruising and watched for signs of healing. They expressed their shock at how limited they were in terms of what they could do. Their bodies were lacking in strength and constrained in their ability to function which affected what they did and how they felt:
Yes, the strength in my legs is so reduced it’s quite incredible and so you can imagine a few more weeks like this and it’s going to take a while to get my strength back, it’s your core strength. If I transfer from this to a wheelchair I’m absolutely exhausted and you’ve just got no trunk strength or virtually none.
Participant 2
Many participants had lost the use of both legs and had other injuries that left them constrained to the bed, resulting in frustration, boredom and stress as they tried to cope with the daily discomforts and waited for their body to heal:
The largest frustration has been the scaphoid fracture in my right wrist which is just another thing to go with the left leg.
Participant 13
Hospital life was frustrating, particularly being on bed rest. Once they could physically move, there was a sense of surprise that they were unable to move naturally, had to plan everything and required help. Participants had to learn how to cope with prolonged periods of bed rest and immobility, deal with the frustrations of limited mobility, accept the pace of recovery dictated by the healing process and move their bodies within the limits of their injuries. There was a heightened sense of surveillance in relation to monitoring their body for signs of recovery but also a mix of frustration and acceptance combined with relief when they could finally move about.
Category: being in pain
Participants experienced extreme pain at some point that they found difficult to control and expressed strong emotions that they would not normally express. Many had support from the pain relief team with some success. Others struggled to find pain relief that suited them, to get timely access to pain relief, to balance activities around pain relief and to control their emotions as a result of pain. Pain was a constant source of concern and was worse early on in the recovery process. Interviewees who had patient-controlled analgesia were fairly happy with their pain control once they had worked out how to use it, but timely access to oral medication was difficult. For some, the pain varied in nature but was persistent, wearing down their ability to cope:
Yes there are days that the pain is bad and there have been days where I can’t bear the pain. I’ve been asking for painkillers and I’ve curled up . . . to try and deal with the pain. It does have its days of coming and going, the pain. I had pain when I woke up just now, and now it’s gone into an ache, which is sometimes worse because obviously an ache you can’t do anything about. It’s aching now and that’s why I keep fidgeting . . . It’s not always just pain, it’s like itching where it’s healing and I can’t itch it, which is annoying. There’s aching, itching, pain, throbbing, there’s a burning pain like when you’ve got sunburn, it feels like that on my legs where they took the skin grafts from.
Participant 19
Trying to be ‘big and strong’ was a difficult facade to maintain in the face of persistent acute pain; resilience was lowered by pain to a point at which participants expressed distress:
Yes, it has made me cry a few times and I’m not really the sort of person to cry but obviously where I’m so stressed out in here and in so much pain it’s bound to come to it.
Participant 14
Overall, pain was a source of concern to all participants at some point in their recovery. This was complicated by the variety of sources of pain, access to medication and a general reluctance of patients to take medication. The group appeared to suffer considerably, which reduced their energy to cope and often led to expression of strong emotions in public that were not normal for them. Thus, being a person in pain was an inherent part of the injury experience and affected their ability to process their emotions and actively manage their recovery.
Theme 3: living with injury
Living with injury evolved as they found mental space to rethink or reimagine how they might live their life at home and at work in the future and was conveyed through the categories of being at home and being at work. This thinking was done within the context of a high level of uncertainty.
Category: being at home
The participants felt that the future was unknown and uncertain but that they were lucky to have a future. The need to get home was overwhelming, but as they progressed through the recovery process it was something they felt was more tangible and they could ‘visualise’ (participant 19) what it would be like to go home. Emotionally, they were anxious and daunted about going home which was sometimes expressed in nightmares. Cognitively working through how it would be at home and how they would manage physically was mixed with concern about how their families would cope. Managing false hope and having realistic expectations of what being at home would be like were of concern. Returning to their hobbies was also a preoccupation, with some planning ways to continue them and others processing the loss of their ability to undertake their hobby:
It’s really hard and it sickens me the thought of losing my bikes but it’s a small sacrifice. If I want to live another 30 years on this planet and I want to walk these beautiful girls down the aisle, then it’s a small price to pay.
Participant 3
Planning a return to home was largely about thinking it through, supporting activities at home to make living at home doable for the family and planning how they might cope. There was sadness at the loss of the life they had before and a degree of uncertainty about how things would work out but also a determination to get home and cope with the changes in their lives.
Category: being at work
Part of getting back to normal was getting back to work. This proved to be a slippery concept that was difficult to visualise owing to the uncertainty of recovery from trauma and degree of functional recovery expected. Any information on this aspect was gratefully received, but participants felt that clarity about timescales was unlikely owing to the complex nature of their injury and individual recovery paths.
Participants with physical jobs had difficulty imagining what they would do. Some had casual contracts with no other skills and were thinking about what they could do to earn a living, some had plans and others were unsure how they would manage. Uncertainty over their ability to function as before was a source of concern:
Yes but I’m not too worried about getting back on a horse or anything like that or the nerves and it’s whether I can physically do it. Mentally I will be fine, there’s no question about that but physically I don’t know . . . we’ll see whether it’s time to pack up and get a new trade. I don’t know but it’s a possibility.
Participant 8
For some interviewees, the accident had provided an opportunity to re-evaluate their lives. Getting back to work was seen as part of a return to normality and necessary for financial security but they were uncertain about their ability to function and make it happen. Those with physical jobs were aware that their ability to work as before was unlikely but could not see an alternative at this stage.
Theme 4: being compromised
The decision to go into the trial was based on being compromised, defined as being dependent on others owing to the acute/emergency nature of the injury and a high degree of trust invested in the clinical team. Being grateful included feelings of altruism and the need to give something back, supported by prior research knowledge and the minimal burden of the research process. Being without prior experience of the two treatments led to a state of not knowing about the interventions; however, experience of the two treatments or information from others clearly identifed a technological preference for NPWT.
Category: being dependent on others
There were challenges in relation to the acute circumstances of the injury, the high degree of trust in the clinical team and the lack of prior experience of the two treatments. The decision to go into the trial was made at a time of great stress, ‘I don’t think I was in any fit state to make any decisions’ (participant 6), and there was acceptance that ‘somebody had to make a decision on your behalf’ (participant 2); however, some were able to make sense of the trial and, based on the fact that both interventions were commonly used and not invasive, were happy to go into the trial:
I was informed about it on the first day and I can half remember being informed about it because I was on gas and air and morphine, tramadol, I was on all sorts of stuff the first day, but yes I can remember being asked about it. I can remember agreeing to it then but not quite with it and then they mentioned it afterwards and yes I can’t really see it having that much effect on me.
Participant 11
Verbal consent before going to the operating theatre sometimes helped them make sense of the trial when they were later asked to provide written consent:
They asked me beforehand and I can remember giving verbal consent so the written consent afterwards didn’t bother me at all.
Participant 11
The nature of the trial was unproblematic for participants but the principle of being asked to take part was important for some participants. Some preferred, if at all possible, to be asked about the trial before the surgery – ‘I would rather that they woke me up and asked me given the choice’ (participant 15) – but, in light of their poor state and lack of concern about the nature of the dressings, were not concerned about the study itself. There was a general acceptance that, in some areas of life, there is no choice. Some participants, despite knowing they were not in a fit state to be asked, considered being a ‘guinea pig’ as a way of making sense of their participation, while others did not mind and still others felt rather ‘shocked’ (participant 8) at being asked to consent after surgical intervention, but balanced this against any potential harm and their ability to get better in the longer term. There was some confusion or lack of memory in relation to the trial, but generally doctors were seen to ‘know best’ (participant 20):
To be honest I was hurt, but they’re doctors and they know what they’re doing so I didn’t really mind because they know best. I can’t really tell them that that’s not right, you can’t let the computer choose for me you should have asked me first, it was right for me. I’m not really angry, I’m quite happy about it.
Participant 20
For others, consenting after surgery was not a problem and they would have made the same decision (participant 19). They found it helpful that they could withdraw at any time and that the study was low impact in relation to their time and the effect on their life:
It genuinely didn’t bother me about that and I felt free at any point to say no.
Participant 16
One person chose not to take part in the trial, but this was on the basis of paperwork only. In principle, there were no concerns about the trial itself:
To be honest the only thing I said to her about doing paperwork was that I would never get round to doing it, it’s just like homework at school, I never did do it and I had it in my head that I never would do it. Paperwork is not one of my strong points, that’s why I didn’t do it.
Participant 14
The staff focus group supported the above findings, with staff having experience of both those who wished to be informed in the emergency department even though they were not able to formally consent and those who deferred to the doctors owing to their condition:
I think they probably think of the broken bone and that being fixed is probably the important thing, they don’t appreciate fully the open nature of the fracture is more likely to affect their outcome. I think it’s hard and invariably these patients have had a good dose of morphine plus or minus ketamine or whatever else.
Focus group, staff participant 5
In addition, they identified those who felt that the surgeon should decide on the dressing, rejecting randomisation as described by Robinson et al. 41 They noted that patients thought the burden was minimal and liked having extra contact with known staff. There was some discomfort approaching patients who had experienced major injury or death of a loved one, as the study felt insignificant in the light of the patient’s circumstances. They noted that patients who gave consent to continue in the trial often indicated that they felt the paperwork was not really necessary:
I think some of them think what’s the fuss, it’s happened so why are you wanting me to sign a piece of paper, what’s the big deal and then especially if they’re seen after they’ve had their flap for them to try and remember what they had prior to that, it’s moved on in their pathway of what’s happened.
Focus group, staff participant 2
In general, the staff felt they were in equipoise and had no concerns about entering patients into the trial but had a heightened awareness of patients’ dependence on them in the early phase of treatment and recovery.
Category: being trusting
Owing to the severe nature of the injury and intervention required there was an enormous degree of trust in the clinical team: ‘they thought I would be OK for it, so I didn’t have any qualms about it’ (participant 16). Many felt that they were not in a position to make a decision because of their general state and that the medical team knew more and were in a better position to know what was best for them:
I’m stood here to tell the tale and I’m just glad of that and taking part in any trials or vacuum pumps [NPWT] as such, the decisions were made for me as I wasn’t in a fit state of mind to do that at the time. To be honest, even if I was I would have agreed to it anyway. If they had said to me they had got this and that I would have said to them straight, what do you think? I would have passed the buck back, which was the best one . . .
Participant 3
The degree of trust on occasions worried the staff, who felt:
I think there’s too much going on and, like Donald said, they’re just willing to do anything.
Focus group, staff participant 3
Knowing what is best for them suggests a degree of therapeutic misconception on behalf of the patient in relation to understanding randomisation, but this was not necessarily the case. There was a need to trust in the team and know that they can ‘bring me out the other side, as long as that happens I’m happy regardless of how they do it’ (participant 5). A need to believe in the team and see a future in which they would recover was of greater importance than the type of dressing they received. Within this was acknowledgement of the knowledge differential between themselves and the team in relation to wound healing: ‘they know what they’re doing and it’s obviously better that they’re doing it and I’m not’ (participant 14); ‘I know nothing about these dressings’ (participant 6). However, those who received advice from medical family members showed a distinct preference for the NPWT: ‘I have to say I would probably have been happier if they had randomised me to the vac [NPWT] system’ (participant 6). The staff focus group noted that having a full description of the NPWT in the information sheet might lead to an expectation that it was a new and better treatment.
Category: being grateful
The decision to go into the trial was facilitated by a degree of altrusim, prior research knowledge and the low burden of the research process. There was a sense of privilege at being asked to be in a trial and a need to give something back as a way of being grateful for the care they received: ‘I have done my bit’ (partcipant 9). It would also be ‘selfish’ (partcipant 10) not to help when they could ‘make it easier for others’ (partcipant 19), particularly to the biker community (partcipant 11):
I am here, I’m a captive audience and what else have I got to do.
Partcipant 9
Having an appreciation of research and being a ‘quite keen supporter of science’ (participant 15), ‘the fact that I appreciate research and I would always sign up for something like that if I was asked to, so it didn’t bother me’ (participant 16), ‘I’m aware of the necessity for trials’ (participant 6) facilitated a decision to join the trial. The trial processes were also of minimal burden and participants recognised this as no cost to themselves and of limited effort:
At the end of the day I know it’s just information that you need to gather to get what you need. It’s free, it doesn’t cost anything does it.
Participant 5
Category: being without prior experience
In making the decision to participate in the trial, participants felt that they did not really understand or know the two interventions because they had not experienced them before. For some, a visual image of what the other intervention was like would help:
I don’t know because I don’t know what the sucking thing is or what it’s like. I probably wouldn’t have minded. I probably would have just accepted that this is what they’re doing for the best. Because I’ve never had one of the suction dressings before I don’t know what it’s like so I can’t really compare it. I can’t really comment on it. I’m just happy that they’ve done what they’ve done.
Participant 17
There was a technological preference for the NPWT gained from those with family in medical positions, interactions with the clinical team and from personal experience. The staff focus group indicated that the majority of staff were in equipoise regarding the use of NPWT; however, if, as occurred occasionally, a preference was stated, the patient was entered in a trial. Patients considered the NPWT to be ‘another piece of clever technology’ (participant 3) that provided security and was visible [‘you can see something there, less pressure on the foot’ (participant 7)] and ‘reduced the smell’ (participant 19). It was felt to be a good option and had ‘helped’ them. They wanted other people to know about the benefits of the NPWT (participant 19). The NPWT dressing was good because it sucked up the ‘goo’ (participant 2), removed ‘nasty fluid’ (participant 20), took the ‘bad stuff’ (participant 19) away that would otherwise be sitting there ‘festering’ (participant 2) and was felt to be particularly important if the wound was dirty [‘loads of dirt and building stuff went in my legs’ (participant 20)]. Based on personal experience there was a desire to have NPWT should they ever require one again:
I would say I would have that again. I would ask them to use that one instead of the normal one.
Participant 20
The dressing was considered to have an active element ‘propelling your recovery’ (participant 19) and ‘increasing blood flow’ (participant 15). It was also considered a ‘clean space’, ‘sealed’, ‘impervious’ (participant 15) and a protective advantage as dirt could not enter the wound:
. . . I’d be happy if, say, the kids came in and got a bit of mud over it, I feel that it’s protective, it is because there’s plastic over it there isn’t much that could get into it unless it’s cut but with a normal bandage dressing it means having to change the whole bandage but the vac dressing [NPWT] you just wipe down.
Participant 19
The combination of actively taking fluid away and being enclosed enabled participants to experience a psychological advantage and a feeling of reassurance:
Rather than bandages soaking up all the goo that was coming off to see it being mechanically extracted for me was as engineer more reassuring than just stood there. I wouldn’t like to think that my leg could lay in a puddle of goo when it could be freshly vacuumed, so in a way I found that reassuring. That was good enough for me so there were no issues there.
Participant 2
The decision to go into the trial was made against a background of a high degree of technological intervention required at this stage of their care so ‘having another tube wouldn’t have really bothered me at all’ (participant 11). But there were challenges around the NPWT, particularly in relation to movement. Tubes could get caught up when patients were sleeping, and carrying the dressing pack when walking with a frame or crutches created issues with balance and concern about falling. Some were concerned about moving in case they damaged anything: ‘you are very scared to move’ (participant 15). Some would have preferred not to see the fluid flowing though the tube (participant 3). Dressing changes could cause excruciating pain as a result of removal of hairs along with the dressing and extremely sensitive skin which stung after removal:
The actual time came when they were removing the dressing that was definitely the most painful moment . . . it really was quite painful . . . they got it off eventually, it made me sweat a little bit and I was pulling on the covers of the bed and squeezing it that tight that they’re still probably trying to get the creases out now with the iron.
Participant 2
Participants with the standard dressing were accepting of it. There was a feeling that the dressing was ‘just there’ and that, as long as they were not ‘in pain’ (participant 10) and the nurses kept the wound clean [‘as long as I see it being cleaned properly’ (participant 1)], they were happy with it. Reflections on whether or not the dressings actually worked reflected the lack of knowing until the wounds healed or an infection occurred:
I suppose having fractured it in the house there’s not going to be an awful lot of bad contamination, it’s probably a lower risk than if I had fractured it at football but I don’t know until it heals up perfectly well and I don’t get infected do I?
Participant 6
Being compromised by the nature of the injury meant that participants were dependent on the clinical team to get them through acute recovery towards their lives outside hospital. They trusted the team and invested their hopes in the ability of the team. Being grateful for the care they received and feelings of altruism facilitated their decision to go into the trial. The type of dressing they received was considered a small aspect of their care and they were generally happy for the team to put them in the trial with presumed consent. However, some preferred to be informed and remembered the conversation later on when approached for their written consent to continue in the trial. Participants also felt that their decision-making was compromised as they did not have prior experience of the two treatments; however, those with experience of the NPWT clearly demonstrated a preference for this.
Overall summary
The core concept of embodied vulnerability conveyed the enormity of the impact of injury for this group; the experience of hospitalisation, while feeling grateful for being saved and lucky that it was not worse, was an emotional rollercoaster. They experienced strong emotions, often to a degree they had not felt before, severe pain, shock and fear in relation to their injured body. There was a physical and emotional fragility which, despite inner strength and determination, meant that they had few reserves to fall back on when they encountered a new threat to their body. Repeated surgery, severe pain, anxiety and fear lowered their ability to contain their emotions and led to what they described as meltdowns. Injury had changed their lives and they reframed their future in the context of uncertainty in relation to their return to normal function, their home lives, their hobbies and their work. Taking part in the trial was within the context of being compromised by the acute event, placing their trust in the clinical team and a feeling of being grateful for their care. A lack of experience of the two interventions limited their decision-making ability but, once they had experience of the interventions, a technological preference was identified.
Discussion
The discussion explores embodied vulnerability through the four themes: (1) being emotionally fragile, (2) being injured, (3) living with injury and (4) being compromised.
Being emotionally fragile
Being emotionally fragile can be explained through notions of an ‘emotionally fragile body’: making sense of the event and injury, learning to live with uncertainty and processing strong emotions were part of recovery.
Making sense of the event
Feeling close to death after a traumatic event is common in trauma patients50 and evident in people who have experienced a vehicle collision,51 many of whom repeatedly relive the event. In this study, interviewees talked about the event in great detail. Some had met the crew who transported them to hospital, which helped them to make sense of what had happened and the resulting nature of the injury. Making sense of their experience, filling missing gaps in their memory, sorting out what is real/unreal and finding familiar things is evident in others who have been critically ill. 52 Ogilvie et al. 53 note how survivors of a life-threatening injury are helped to make sense of the injury by their families, who make connections between their past, present and future life. In situations when loss leads to prolonged grief, Maccallum and Bryant54 suggest that memories need to become integrated with autobiographical information to enable contextualisation in order to facilitate recovery. Feeling lucky to be saved provides a certain degree of comfort from the harsh reality of the consequences of the event: an outlook that is viewed by others as cognitive restructuring. 33 Talking about the event, and the feelings engendered, asking questions and listening to others’ experiences may help them make sense of what has happened and incorporate it into their life story.
Living with uncertainty
Living with uncertainty was part of the emotional work inherent in being emotionally fragile. The future for participants felt largely unknown owing to the unfolding nature of clinical interventions combined with anxiety regarding possible loss of a limb. Learning to live with uncertainty is a core concept evident in many conditions. 55,56 Morse and Penrod57 suggest that uncertainty is present when the outcome is clear but the route to achieve it is not. In this study, there was uncertainty regarding healing, further treatment and return of function; the hoped-for outcome of a return to normal was not necessarily achievable. Aspirational hope was expressed in participants’ desire to ride their motorbikes again or to walk their daughters down the aisle: hopes which can be sustaining during recovery. 58 Although some took control to limit uncertainty such as smoking cessation, the participants had to learn to live with continued uncertainty about the future. Living in the present, a ‘wait and see’ approach and focusing on small obtainable goals has been noted as helpful in early recovery from trauma. 59 Later on in recovery, the sense of uncertainty through closeness to injury and death can be experienced as heightened awareness of potential danger60 and changing activities to minimise danger. 51 Strategies to help participants to process anxiety and fear created by injury and continued treatment such as counselling or coaching may help them make sense of uncertainty within the context of their life.
Processing strong emotions
The injury event, hospitalisation and treatment resulted in an emotional rollercoaster, as noted in other research. 35,50 Participants experienced strong emotions, often a new experience for them, and their ability to contain their emotions was impaired. Seeing their family/friends demonstrably upset was also particularly hard. Morse et al. 61 suggests that emotions spill out when people move from a state of enduring or emotional containment towards a more emotionally expressive state of suffering. Containment of emotions is identified by Lawlor62 as ‘the ways in which emotion is experienced or avoided, managed or denied, kept in or passed on, so that its effects are either mitigated or amplified’. In this study, participants demonstrated a determination to mitigate the enormity of their emotional response in order to protect their family and friends, to maintain a sense of normality for them. Parallels can be drawn with work in a burns unit, in which ‘the trauma bubble’ describes containment of emotions that threaten the self; acting as a form of insulation that can lead to isolation from others. 63 Clifton,64 from his auto ethnography of spinal cord injury, argues that expressing grief is an important part of processing loss and that feelings of ‘sadness, anger and melancholy’ are part of the process towards acceptance of injury. Interviewees with high levels of stress and anxiety up to 23 months after injury identified ongoing strong emotions of frustration, fear, despair and thoughts of death. They also felt that emotional recovery was not highly valued within trauma services. 60 The experience of overwhelming strong emotions as a result of injury suggests that emotional suffering needs a higher profile alongside physical suffering and an exploration of interventions that might be helpful for this group.
Being injured
Being injured highlighted the difficulty of being a person with wounds and of the visibly wounded body on the sense of self, living in a constrained way with the need for full intimate bodily help, and enduring and managing being in pain.
Sense of self
Interviewees experienced their bodies as changed beyond recognition and they had no immediate resources of comfort from which to draw. From experiences of having a stoma, Thorpe et al. 65 presents this as a ‘loss of embodied wholeness’, when a part of the body becomes separated from the subjective self. This was evident when legs were referred to in the third person or they felt detached from their body. Morse66 and Morse and Mitcham67 note how patients with burns disembody damaged parts of their body to maintain control of their sense of self. Often staff used a similar approach referring to removal of ‘the leg’, presenting it as separate from the person. In our study, the participants referred to their wounds as revolting, disrupting their ability to walk and care for themselves, similar to the ‘awareness of a disrupted lived body’ in Thorpe et al. 65 Using the model of altered body image presented by Price,68 there was a gap between the body reality and the body ideal, how individuals would like their bodies to be, and a disruption in body presentation to others. This exposed them to notions of disfigurement and stigma, and there was concern about how the world would react to them as damaged people and as potential wheelchair users. In a study of burns, by Johnson et al. ,69 participants developed a sense of self-acceptance in relation to their changed ‘physical otherness’. In our study, family members’ and friends’ responses to patients’ wounds was important, and interviewees did not want loved ones to feel as horrified as they did on seeing their wounds. Gullick et al. 63 highlight the vicarious suffering of family and friends, also termed ‘compathy’ by Morse and Mitcham. 67 Preparation through instruction and feelings of readiness to see their wounds were important. In time, and with exposure to the wound, participants began to assimilate the wound as part of themselves, but this was not easy. Support was required to help them and their families process their emotions and enable them to understand their wounds and the likely process of healing.
Living in a constrained way
Living in a constrained way was a form of embodied endurance as identified by Keene et al. 37 in patients with an unstable ankle fracture who were non-weight bearing. Participants’ geographical space had shrunk to the bed/chair and room in a hospital ward. The resulting frustrations of living with a ‘constrained body’ had to be endured, a state recognised by Morse and Penrod57 alongside suffering, uncertainty and hope. Their injured body had become a source of constraint, disrupting their way of being in the world and they had to rethink the simplest of activities as in the non-spontaneous body identified by Kvigne and Kirkevold. 70 If they were able to move, they were reliant on crutches, walking frames and support from others, evident in the extended body. 70
Dependence on others for their comfort, privacy and dignity was frustrating and participants struggled with bedpans, plastic mattresses and a variety of hospital appliances. While finding technology reassuring, they were also frustrated by it, a dissonance noted by Stayt et al. 71 Dependence on others for intimate bodily activities, termed the dependent body by Kvigne and Kirkevold,70 was also frustrating, and there was relief when they could get to the toilet. The indignity created by hospital life is noted by other patients. 72 Dependency in early recovery can put a strain on relationships and can continue in longer-term recovery. 73 The pace of life for participants was slower than normal, and there was an increased watchfulness for bodily changes that may or may not indicate healing. Increased bodily surveillance is evident in other injuries37 and the importance of being watchful in self-management of musculoskeletal disorders has been recognised. 74 Frustrations with living in a constrained way were expressed by participants but largely endured, and emotions contained as a means of getting on with daily hospital life.
Being in pain
Being in pain was something that all participants struggled with at some point during their hospitalisation. The energy required to endure ongoing pain reduced their ability to handle their daily life and contributed to their emotional fragility. Pain is recognised as part of the experience of injury and surgery75 and is difficult to manage. Other studies of lower limb injury identify pain as a key problem. 32,34,35 Studies specifically including open fracture of the lower limb34 identify getting through the pain and seeking control as two key experiences. Pain management was an overriding concern, and patients struggled to live with the pain and manage the side effects of medication. The resilience required to manage a ‘painful body’ can be considerable; Gullick et al. 63 note the all-consuming nature of embodied pain whereby it is difficult to carry out other functions. The emotional impact is also evident in pelvic pain, the experience of which can be an ‘overwhelming emotional rollercoaster’. 76 For participants in this study, being in pain was an inherent part of being injured, and considerable energy was required to manage and learn to live with the pain. The embodied nature of pain was expressed through the interrelationship of pain with emotions, their ability to function, uncertainty and hopes for the future. Opportunities for better clinical management of pain may ease the burden of being in pain and facilitate their ability to manage their recovery.
Living with injury
Living with injury incorporated a way of envisaging the future: both being at home and being at work.
Being at home
Interviewees expressed a high level of optimism and determination to go home alongside the ideal of returning to normal, as found in other studies in trauma. 59 A degree of readiness, based on feelings of wellness/mental energy, was required before they could think of home. There was sadness at what they had lost but also a feeling of being lucky that they had a future. Planning for the future was an embodied process that required emotion work and involved reimagining how life would be for them and their family. This was undertaken with a high degree of uncertainty about their recovery and what the future would be like. Rethinking and reimagining may provide some form of control over the future. Ogilvie et al. 53 suggest that this may be helpful when the future is unknown in terms of recovery. There was an awareness that they might have false hope about what they may achieve, particularly in relation to their hobbies such as riding a motorbike. But these hopes conveyed a sense of aspiration and could be a source of motivation for recovery, as in other specialties. 58 Richmond et al. 73 identified the long-lasting impact of injury that required energy and resilience in order to move on in daily life. Continued disability and dependency in their study could both reduce or increase opportunities in life. In this study, the participants were developing a sense of preparedness for the transition to home but could not fully imagine what it would be like until they had experienced it.
Being at work
Rethinking and reimagining continued in relation to returning to work, which felt like a much longer-term goal. There was a strong sense of uncertainty, linked to ability to recover and return to normal function. Many of those in manual jobs felt that it was unlikely that they could return to the same job; others imagined how they could reconfigure their work to facilitate their return. Johnson et al. 69 note how patients with burns rethink how they will manage at work and struggled when medical staff did not understand their fear or anxiety. They experienced a slow return to normal, with a gradual increase in the number of good days; however, normal was tangibly different from their pre-injury normal. Some injured participants questioned their role in society and needed to find meaning in their very existence. 53 In this study, there was evidence for some of a re-evaluation of life plans and opportunity to make a new path in their working life. At this stage of recovery, what it was like to be at work was unknown and hard to imagine.
Being compromised
The findings identify that making a decision to take part in a trauma trial takes place within the context of participants’ broader experience of recovery. Participants experienced being in the trial within the context of being compromised and being dependent on others to facilitate their recovery. The type of wound dressing was a small part of a much larger life-changing experience. This may be similar to the finding that women chose to take part in oncology drug trials because of their cancer treatment goals rather than because of information they received about the trial. 77 Therapeutic misconception37,38,40 was present on occasions, with some participants trusting the clinicians to provide the right treatment for them. However, the nature of their trust was also based on their belief that the team had saved them and that they were lucky to be alive; they focused on a longer-term hope that the clinical team would be able to lead them to recovery, not noted in other studies. There was some evidence that information about the study before surgery, despite being compromised, was useful for participants and could facilitate a feeling of being involved and having a say in their care. The principles of inclusionary consent in which individuals can actively take part despite their current limitations as identified in dementia care78 may, therefore, have utility in emergency orthopaedic trauma studies.
Participants’ rationale for taking part or continuing with the study was gratitude for the care they received, which included some element of altruism also found in other studies. 38,39,43,79 Some subgroups of patients felt that they were helping their peer-group as well as society more generally, for example the motorcycle community, whose fractures are often open. The limited burden of follow-up activities on participants was also important. 38,39 In relation to understanding the trial, not having experience of the two dressings added to participants’ belief that they were not really in a position to judge the utility of the dressings and, hence, they trusted that staff would not support a trial that would cause them harm. Experiential knowledge was also important to participants in the ankle injury management study. 37 However, once the NPWT had been experienced, there was a strong technological preference for the dressing as it was considered cleaner and had a visible, dynamic component of suction, which removed exudate away from the wound. Participants reported that they wanted this intervention if they ever had a wound again, suggesting that experience was important to how they make sense of an intervention.
Limitations
The sample was purposive in relation to gender, age, severity of injury and a range of experience; however, it was not ethnically diverse. The study was undertaken in hospital where privacy and dignity were negotiated but could not be ensured. A more private setting may have facilitated deeper explorations of the concepts; however, the participants were keen to talk and often asked if another meeting was possible. A longitudinal study would help to highlight the change in concepts over time.
Conclusions
This study, the first exploring lower limb injury in acute care, adds to current evidence through the identification of the concept of embodied vulnerability as a result of lower limb injury, which conveys the vast amount of emotional and physical work undertaken by participants while in hospital. It demonstrated that patients, although comfortable with others leading on informed consent, wished to be involved to the extent of their abilities at the time. The focus of the study in this case was of minimal concern but they did have a strong preference for NPWT once they had experience of this dressing. Lack of experience of the interventions was an important factor and influenced how they understood the study. Trust in the team went beyond therapeutic misconception but was linked to longer-term hopes of recovery. The study highlights how previous taken-for-granted ways of being in the world were changed and new ways of being, incorporating their injury, were sought. Participants were actively processing the impact of injury and treatment and staff have a vital role to play in supporting this activity through the early recovery phase. Supportive activities may include emotion-focused activities related to emotional fragility enabling them to make sense of the event, live with uncertainty, process strong emotions, integrate their injury with their sense of self, develop resilience for managing pain and envisage the future. Potential for further support at this stage will require exploration in light of patient-important outcomes along the recovery continuum. Implications for practice are to create a heightened awareness of emotional work and a requirement for skills to support this work, resources to provide time for specific supportive work such as coaching, innovative ways of facilitating better active management of pain, and creative ways of helping participants envisage the future to ease their transition to a home/work environment.
Summary
-
Both treatment groups, those receiving standard dressings and those receiving NPWT, require support, when possible, to ease their experience of vulnerability caused by traumatic injury. That support should focus on (1) participants’ emotional fragility, closeness to death and/or loss of a limb and helping them to process strong emotions while containing their emotions for the benefit of other people; (2) the state of being injured and participants’ changed sense of self as they became a person with wounds, are constrained by their broken body and live with pain; and (3) living with injury and reimagining how it will be at home and at work in the future.
-
Both treatment groups wanted to be involved within the limits of their ability in this emergency orthopaedic trauma trial. They were comfortable with other people providing informed consent because of their own physical/emotional state and the treatment of the wound being of comparative low importance at that time.
-
Both groups had an overall faith in the team regarding their longer-term recovery and hopes for the future and trusted that they would not put them in a study that caused them harm.
-
Both groups felt that they did not really know the interventions as they had no experience of them but those who had NPWT developed a strong technological preference for this treatment.
Chapter 4 Results
Screening
Patient screening for potential study participants started in July 2012, with n = 2434 patients screened. For 1809 patients, the local research associate provided the reason for ineligibility (Table 3).
| Reason | Year | Total | % | |||
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | |||
| Aged < 16 years | 2 | 14 | 12 | 12 | 40 | 2.2 |
| Presented/transferred to trial hospital > 72 hours after injury | 7 | 25 | 35 | 26 | 93 | 5.1 |
| Fracture G&A grade 1 | 11 | 65 | 87 | 73 | 236 | 13.0 |
| Patient unable to adhere to trial procedures | 4 | 23 | 80 | 50 | 157 | 8.7 |
| Amputation | 3 | 3 | 18 | 13 | 37 | 2.0 |
| Primary closure | 19 | 139 | 316 | 401 | 875 | 48.4 |
| Other/unknown | 8 | 52 | 79 | 71 | 210 | 11.6 |
| Missed | 0 | 16 | 36 | 34 | 86 | 4.8 |
| Randomisation temporarily suspended | 0 | 12 | 2 | 0 | 14 | 0.8 |
| Surgeon decision | 1 | 5 | 22 | 21 | 49 | 2.7 |
| Surgeon preference: NPWT | 0 | 0 | 1 | 0 | 1 | 0.1 |
| Surgeon preference: standard dressing | 0 | 0 | 3 | 0 | 3 | 0.2 |
| Polytrauma or unlikely to survive | 0 | 1 | 0 | 5 | 6 | 0.3 |
| No pump available | 0 | 0 | 0 | 2 | 2 | 0.1 |
| Total | 55 | 355 | 691 | 708 | 1809 | 100 |
Rates of primary closure increased during the course of the WOLLF study, from 34.5% in 2012 to 56.6% in 2015. Figure 2 shows changes in primary closure during study recruitment. The increase in the rate of primary closure was statistically significant; a Poisson regression model for counts provided a regression coefficient for the recruitment year term of 1.2 (95% CI 1.1 to 1.3; p < 0.001). The relative rate of increase in primary closures was approximately 20% per year.
FIGURE 2.
Rates of primary closure from 2012 to 2015 in screened population. Observed rates (circles) with 95% CIs, fitted line (solid line) and 95% CI on fit (dashed line).

Recruitment
Overall recruitment and recruitment by centre
A total of 625 patients were recruited and randomised into the study. Of these, 460 were consented. Recruitment started in July 2012 and was completed in December 2015. Recruitment took place at 24 centres:
-
Royal London Hospital
-
Poole Hospital
-
Aintree University Hospital
-
Leeds General Infirmary
-
Addenbrookes Hospital, Cambridge
-
University Hospital Southampton
-
Northern General Hospital Sheffield
-
Queen Alexandra Hospital
-
Royal Berkshire Hospital
-
Kings College Hospital
-
University Hospital of North Staffordshire
-
Plymouth Hospitals
-
University Hospital Coventry and Warwickshire
-
Norfolk and Norwich University Hospital
-
Queen Elizabeth Hospital, Birmingham
-
Royal Victoria Infirmary
-
Royal Derby Hospital
-
John Radcliffe Hospital
-
Frenchay Hospital
-
Hull Royal Infirmary
-
University Hospital Leicester
-
Nottingham University Hospital
-
Royal Sussex County Hospital, Brighton
-
Morriston Hospital Swansea.
For those patients who lacked capacity to consent prospectively, consent for continuation in the trial was made at the first appropriate time point in the postoperative period. Therefore, a proportion of randomised patients did not consent to be in the study. Table 4 shows that a total of 625 patients were recruited and randomised, with 460 consenting to take part in the study and 165 not consenting.
| Treatment group | Consented | Total | |
|---|---|---|---|
| No | Yes | ||
| NPWT | 85 | 226 | 311 |
| Standard | 80 | 234 | 314 |
| Total | 165 | 460 | 625 |
The planned overall required recruitment rate for the WOLLF study was approximately 0.75 patients per centre per month, based on 460 patients recruited and consented over 36 months at 24 centres.
Overall recruitment across centres was 0.7 patients per month. This was lower than the planned rate, based on the original recruitment period, therefore the trial recruitment period was extended by 3 months to the end of 2015 to reach the target of 460. Full details of recruitment by centre are shown in Appendix 1 (see Table 34).
Population characteristics
The breakdown of the recruited population (n = 625) by age group (< 40 and ≥ 40 years), gender and treatment group is shown in Table 5.
| Gender | Age group (years) | Treatment group, n (%) | Total | |
|---|---|---|---|---|
| NPWT | Standard | |||
| Male | < 40 | 123 (55) | 102 (45) | 225 |
| ≥ 40 | 115 (53) | 104 (47) | 219 | |
| Female | < 40 | 16 (47) | 18 (53) | 34 |
| ≥ 40 | 57 (39) | 90 (61) | 147 | |
| Total | 311 | 314 | 625 | |
The majority (444/625; 71.0%) of patients recruited to the trial were male. There was also a clear difference in age structure between genders. Figure 3 shows age distributions by gender.
FIGURE 3.
Age distribution by 10-year age bands for (a) males and (b) females. Solid lines show smoothed distributions.
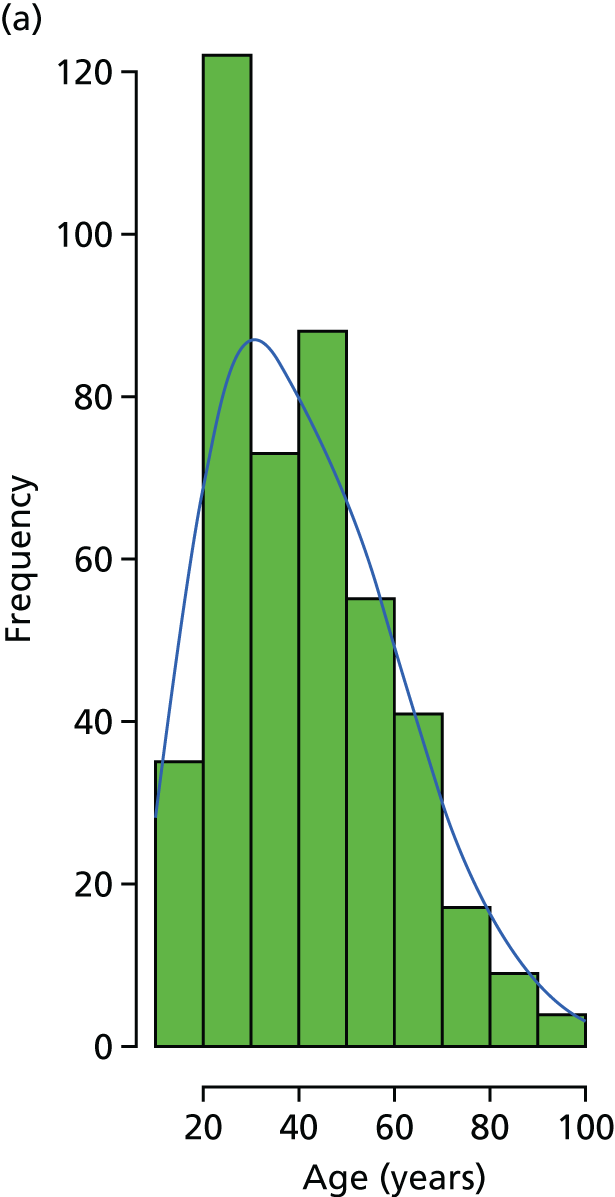

The figures show the relative size of male and female population recruited to the study and also the differing age structure between genders. Males were predominantly younger [median age 39 years; interquartile range (IQR) 26–52 years] and females were older (median age 61 years; IQR 44–79 years). This is probably due to differences in the mechanism of injury between genders (see Table 10).
Consented participants and interventions
Consented and non-consented participants
Figure 4 shows the study CONSORT (Consolidated Standards of Reporting Trials) plot. Of the 625 participants recruited into the study, 460 consented to take part. The pattern and number of pre-consent withdrawals were well balanced between intervention groups (NPWT group, n = 85; standard dressing group, n = 80). Only a small proportion of those randomised decided subsequently not to consent to take part in the study [NPWT group, n = 14 (4.5%); standard dressing group, n = 15 (4.8%)]. The main reason for post-randomisation (pre-consent) withdrawal was primary closure of the index wound during surgery (NPWT group, n = 20; standard dressing group, n = 18). This made the patients ineligible for entry into the study.
FIGURE 4.
The WOLLF study CONSORT plot. FU, follow-up.
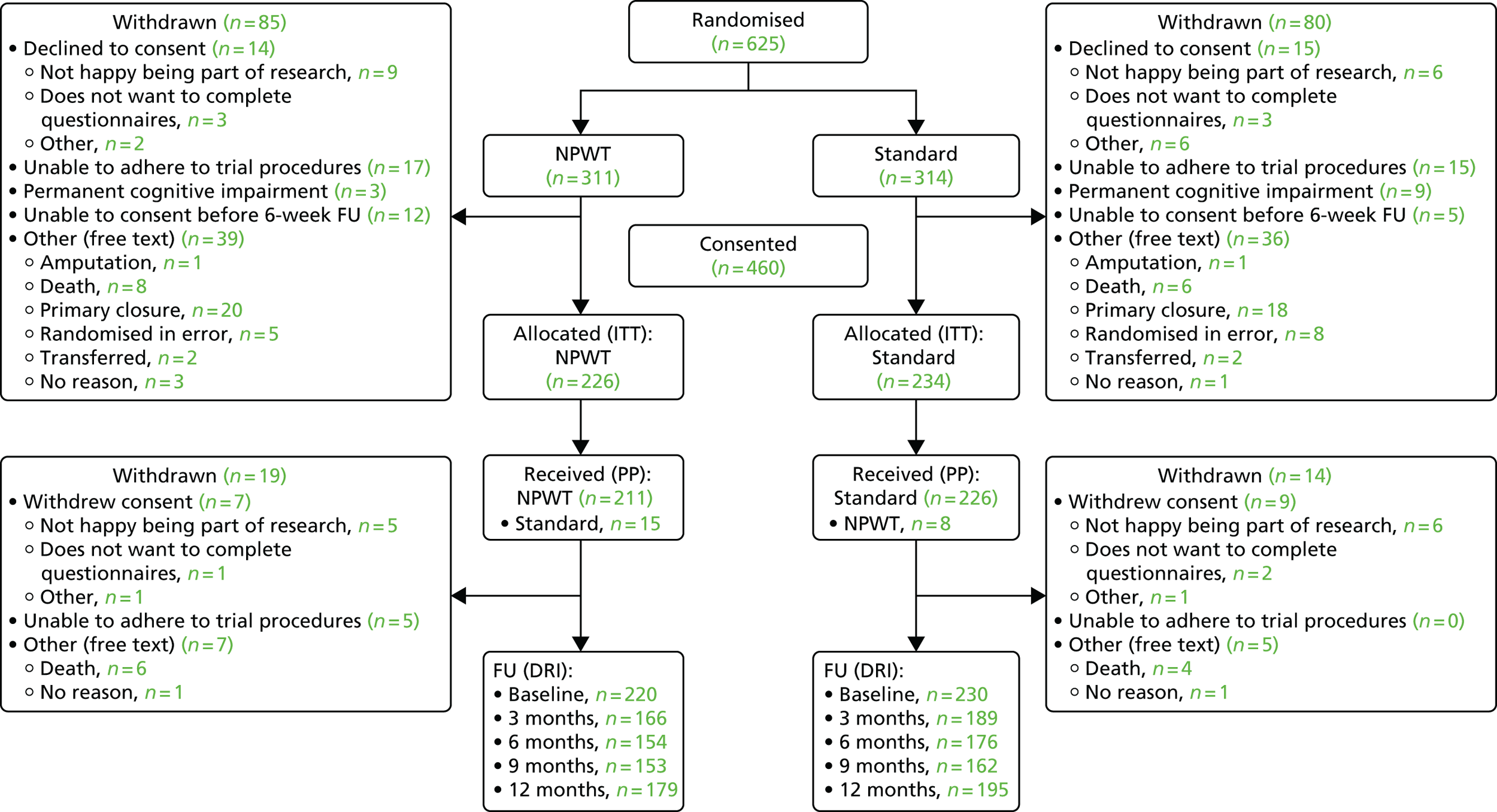
Table 6 presents the characteristics of those consented and those not consented.
| Characteristic | Treatment group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Consented (N = 460) | Non-consented (N = 165) | |||||||||
| NPWT (n = 226) | Standard (n = 234) | Total | NPWT (n = 83) | Standard (n = 82) | Total | |||||
| Consent type, n (%) | ||||||||||
| Hospital data | 1 | (0.4) | 2 | (0.9) | 3 | – | – | – | – | – |
| Prospective | 19 | (8.4) | 24 | (10.3) | 43 | – | – | – | – | – |
| Retrospective | 206 | (91.2) | 208 | (88.9) | 414 | – | – | – | – | – |
| Gender, n (%) | ||||||||||
| Female | 48 | (21.2) | 70 | (29.9) | 118 | 27 | (32.5) | 30 | (36.6) | 57 |
| Male | 178 | (78.8) | 164 | (70.1) | 342 | 56 | (67.5) | 52 | (63.4) | 108 |
| G&A grade,a n (%) | ||||||||||
| 2 | 34 | (15.0) | 30 | (12.8) | 64 | 12 | (14.5) | 17 | (20.7) | 29 |
| 3 | 171 | (75.7) | 180 | (76.9) | 351 | 62 | (74.7) | 59 | (72.0) | 121 |
| 3C | 21 | (9.3) | 24 | (10.3) | 45 | 9 | (10.8) | 6 | (7.3) | 15 |
| Age group (years), n (%) | ||||||||||
| < 40 | 102 | (45.1) | 99 | (42.3) | 201 | 36 | (43.4) | 29 | (35.4) | 65 |
| ≥ 40 | 124 | (54.9) | 135 | (57.7) | 259 | 47 | (56.6) | 53 | (64.6) | 100 |
| Age (years), median (IQR) | 42 (29–61) | 43 (26–57) | 50 (28–65) | 48 (29–68) | ||||||
A chi-squared test indicated that the proportion of females was significantly (p = 0.037) higher in the non-consented population than in the consented population: 34.5% (57/165) versus 25.7% (118/460). The difference in median age between the consented and non-consented population was 5 years (43 years for consented; 48 years for non-consented). Analysis indicated that this difference did not quite reach statistical significance (Mann–Whitney U-test; p = 0.056). Therefore, we conclude that patients who declined to consent, who were not able to consent or who were ineligible for inclusion in the study after initial recruitment were generally older and more likely to be female than those who consented to take part (see Figure 3, distribution of patient age by gender).
Interventions
Table 7 shows the details of operative procedures by study intervention arm. There were no important differences in the length of the operation, surgeon experience, intraoperative problems or additional injuries between the intervention groups.
| Characteristic | Treatment group | Total | |||
|---|---|---|---|---|---|
| NPWT (N = 226) | Standard (N = 234) | ||||
| First operative procedures | |||||
| Lead surgeon grade, n (%) | |||||
| Consultant | 148 | (65.5) | 165 | (70.5) | 313 |
| Associate specialist | 10 | (4.4) | 11 | (4.7) | 21 |
| Specialist trainee | 56 | (24.8) | 49 | (20.9) | 105 |
| Other | 9 | (4.0) | 7 | (3.0) | 16 |
| Number of surgeons, median (IQR) | 2 (1–2) | 2 (1–2) | |||
| Operation time in minutes, median (IQR) | 126 (90–182) | 120 (86–183) | |||
| Side, n (%) | |||||
| Left | 118 | (52.2) | 111 | (47.4) | 229 |
| Right | 108 | (47.8) | 123 | (52.6) | 231 |
| Bone, n (%) | |||||
| Femur | 16 | (7.1) | 26 | (11.1) | 42 |
| Patella | 3 | (1.3) | 2 | (0.9) | 5 |
| Fibula/tibia | 187 | (82.7) | 192 | (82.1) | 379 |
| Foot | 20 | (8.8) | 13 | (5.6) | 33 |
| Type of fixation, n (%) | |||||
| Nail | 49 | (21.7) | 56 | (23.9) | 105 |
| Plate and screws | 38 | (16.8) | 32 | (13.7) | 70 |
| Wires/tension band wires | 7 | (3.1) | 3 | (1.3) | 10 |
| External fixator-half-pin | 107 | (47.3) | 111 | (47.4) | 218 |
| External fixator-fine-wire | 3 | (1.3) | 11 | (4.7) | 14 |
| Other | 21 | (9.3) | 21 | (9.0) | 42 |
| Intraoperative problems, n (%) | |||||
| No | 212 | (93.8) | 213 | (91) | 425 |
| Yes | 14 | (6.2) | 21 | (9) | 35 |
| Nerve injury | |||||
| No | 222 | (98.2) | 234 | (100) | 456 |
| Yes | 4 | (1.8) | 0 | (0) | 4 |
| Vascular injury | |||||
| No | 222 | (98.2) | 228 | (97.4) | 450 |
| Yes | 4 | (1.8) | 6 | (2.6) | 10 |
| Tendon injury | |||||
| No | 221 | (97.8) | 228 | (97.4) | 449 |
| Yes | 5 | (2.2) | 6 | (2.6) | 11 |
| Extension of fracture | |||||
| No | 225 | (99.6) | 231 | (98.7) | 456 |
| Yes | 1 | (0.4) | 3 | (1.3) | 4 |
| Other problems | |||||
| No | 220 | (97.3) | 227 | (97) | 447 |
| Yes | 6 | (2.7) | 7 | (3) | 13 |
| Surgeon satisfaction with debridement, n (%) | |||||
| No | 1 | (0.4) | 3 | (1.3) | 4 |
| Yes | 201 | (88.9) | 210 | (89.7) | 411 |
| Surgeon satisfaction with dressing, n (%) | |||||
| No | 6 | (2.7) | 4 | (1.7) | 10 |
| Yes | 195 | (86.3) | 208 | (88.9) | 403 |
| Other surgery at time of wound debridement, n (%) | |||||
| No | 148 | (65.5) | 149 | (63.7) | 297 |
| Yes | 78 | (34.5) | 85 | (36.3) | 163 |
| Other operative procedures | |||||
| Head, n (%) | |||||
| No | 215 | (95.1) | 231 | (98.7) | 446 |
| Yes | 11 | (4.9) | 3 | (1.3) | 14 |
| Chest, n (%) | |||||
| No | 223 | (98.7) | 231 | (98.7) | 454 |
| Yes | 3 | (1.3) | 3 | (1.3) | 6 |
| Abdomen, n (%) | |||||
| No | 219 | (96.9) | 230 | (98.3) | 449 |
| Yes | 7 | (3.1) | 4 | (1.7) | 11 |
| Pelvis, n (%) | |||||
| No | 217 | (96) | 226 | (96.6) | 443 |
| Yes | 9 | (4) | 8 | (3.4) | 17 |
| Spine, n (%) | |||||
| No | 222 | (98.2) | 231 | (98.7) | 453 |
| Yes | 4 | (1.8) | 3 | (1.3) | 7 |
| Upper limb, n (%) | |||||
| No | 203 | (89.8) | 209 | (89.3) | 412 |
| Yes | 23 | (10.2) | 25 | (10.7) | 48 |
| Ipsilateral limb, n (%) | |||||
| No | 193 | (85.4) | 189 | (80.8) | 382 |
| Yes | 33 | (14.6) | 45 | (19.2) | 78 |
| Ipsilateral limb fracture, n (%) | |||||
| Open | 13 | (5.8) | 18 | (7.7) | 31 |
| Closed | 13 | (5.8) | 21 | (9.0) | 34 |
| Contralateral limb, n (%) | |||||
| No | 201 | (88.9) | 203 | (86.8) | 404 |
| Yes | 25 | (11.1) | 31 | (13.2) | 56 |
| Contralateral limb fracture, n (%) | |||||
| Open | 6 | (2.7) | 10 | (4.3) | 16 |
| Closed | 13 | (5.8) | 15 | (6.4) | 28 |
| Treatment of other open injuries, n (%) | |||||
| Standard | 13 | (5.8) | 18 | (7.7) | 31 |
| NPWT | 7 | (3.1) | 3 | (1.3) | 10 |
| Primary closure | 10 | (4.4) | 12 | (5.1) | 22 |
| Flap graft | 1 | (0.4) | 1 | (0.4) | 2 |
| Other | 0 | (0.0) | 1 | (0.4) | 1 |
| No other open injuries | 195 | (86.3) | 199 | (85.0) | 394 |
| Prophylactic antibiotics, n (%) | |||||
| No | 5 | (2.2) | 3 | (1.3) | 8 |
| Yes | 200 | (88.5) | 209 | (89.3) | 409 |
| Lead surgeon experience: number of open fractures, n (%) | |||||
| < 5 | 2 | (0.9) | 3 | (1.3) | 5 |
| 5–10 | 7 | (3.1) | 11 | (4.7) | 18 |
| 10–20 | 16 | (7.1) | 13 | (5.6) | 29 |
| > 20 | 195 | (86.3) | 197 | (84.2) | 392 |
| Lead surgeon experience: number of NPWT dressings, n (%) | |||||
| < 5 | 10 | (4.4) | 9 | (3.8) | 19 |
| 5–10 | 7 | (3.1) | 16 | (6.8) | 23 |
| 10–20 | 29 | (12.8) | 28 | (12) | 57 |
| > 20 | 171 | (75.7) | 167 | (71.4) | 338 |
Treatment allocation
There were 23 deviations (crossovers) from the allocated treatment (NPWT group, n = 15; standard dressing group, n = 8). Unavailability of equipment accounted for two crossovers in the NPWT group and one in the standard dressing group. In nine cases in the NPWT group, the surgeon chose to use a different dressing, and, in three cases in the standard dressing group, the surgeon chose to use NPWT. There were four cases in each group in which the allocated dressing was not communicated to the operating team.
The details of the types of dressing used for the standard and NPWT interventions are shown in Table 8. There were 241 standard dressing procedures in total (226 in the standard dressing arm of the study and 15 in the NPWT arm). The median number of dressing changes was 1 for the standard dressing arm (IQR 0–2). There were 219 NPWT procedures in total (211 in the NPWT arm of the study and eight in the standard dressing arm). The median number of dressing changes for the NPWT arm was 1 (IQR 0–2).
| Procedure | Treatment group | Total | |||
|---|---|---|---|---|---|
| NPWT (N = 211) | Standard (N = 8) | ||||
| Number of dressing packs used, median (IQR) | 1 (1–1) | 1 (1–1) | – | ||
| Pump pressure 125 mmHg, n (%) | |||||
| No | 35 | (16.6) | 3 | (37.5) | 38 |
| Yes | 156 | (73.9) | 3 | (37.5) | 159 |
| Unknown | 20 | (9.5) | 2 | (25.0) | 22 |
| Pressure in mmHg, median (IQR) | 125 (125–125) | 100 (100–110) | – | ||
| Number of days pump on, median (IQR) | 3 (2–5) | 5 (3–5) | – | ||
| NPWT operation, n (%) | |||||
| Continuous | 163 | (77.3) | 2 | (25.0) | 165 |
| Intermittent | 13 | (6.2) | 1 | (12.5) | 14 |
| Unknown | 35 | (16.6) | 5 | (62.5) | 40 |
| Irrigation, n (%) | |||||
| No | 181 | (85.8) | 4 | (50.0) | 185 |
| Yes | 7 | (3.3) | 1 | (12.5) | 8 |
| Unknown | 23 | (10.9) | 3 | (37.5) | 26 |
| Irrigation and antibiotics, n (%) | |||||
| No | 2 | (28.6) | 1 | (100) | 3 |
| Yes | 3 | (42.9) | 0 | (0.0) | 3 |
| Unknown | 2 | (28.6) | 0 | (0.0) | 2 |
| NPWT (N = 15) | Standard (N = 226) | Total | |||
| Antiseptic gauze, wool crepe bandage, n (%) | 7 | (46.7) | 99 | (43.8) | 106 |
| Bead pouch, n (%) | 0 | (0) | 62 | (27.4) | 62 |
| Other antibiotic-impregnated dressing, n (%) | 0 | (0) | 5 | (2.2) | 5 |
| Gauze, wool or crepe bandage, n (%) | 2 | (13.3) | 57 | (25.2) | 59 |
| Other, n (%) | 0 | (0) | 1 | (0.4) | 1 |
| Unknown, n (%) | 6 | (40) | 2 | (0.9) | 8 |
Baseline characteristics
Unless stated otherwise, all analyses reported here are on an ITT basis, that is, by allocated treatment.
Baseline participant characteristics
Table 9 shows the baseline patient characteristics for the 460 participants recruited and consented into the WOLLF study.
| Characteristic | Treatment group | Total | |||
|---|---|---|---|---|---|
| NPWT (N = 226) | Standard (N = 234) | ||||
| Gender, n (%) | |||||
| Female | 48 | (21.2) | 70 | (29.9) | 118 |
| Male | 178 | (78.8) | 164 | (70.1) | 342 |
| Diabetes mellitus, n (%) | |||||
| No | 208 | (92.0) | 218 | (93.2) | 426 |
| Yes | 14 | (6.2) | 13 | (5.6) | 27 |
| Age (years), median (IQR) | 42 (29–61) | 43 (26–57) | |||
| Height (cm), median (IQR) | 175 (170–182) | 175 (165–180) | |||
| Weight (kg), median (IQR) | 79.4 (69.9–90.0) | 78.5 (68.5–90.0) | |||
| Smoker, n (%) | |||||
| No | 149 | (65.9) | 151 | (64.5) | 300 |
| Yes | 70 | (31) | 79 | (33.8) | 149 |
| If yes, how many smoked per day, median (IQR) | 11 (9–20) | 15 (10–20) | |||
| If yes, how many years, median (IQR) | 15 (10–21) | 15 (7–25) | |||
| Training, n (%) | |||||
| None | 76 | (33.6) | 84 | (35.9) | 160 |
| Work-based training | 51 | (22.6) | 44 | (18.8) | 95 |
| Non-degree qualification | 62 | (27.4) | 65 | (27.8) | 127 |
| College/university degree | 26 | (11.5) | 36 | (15.4) | 62 |
| Employment, n (%) | |||||
| Full-time employed | 105 | (46.5) | 106 | (45.3) | 211 |
| Part-time employed | 9 | (4.0) | 14 | (6.0) | 23 |
| Self-employed | 25 | (11.1) | 31 | (13.2) | 56 |
| Retired/inactive | 44 | (19.5) | 44 | (18.8) | 88 |
| Unpaid work | 2 | (0.9) | 0 | (0.0) | 2 |
| Unemployed | 28 | (12.4) | 25 | (10.7) | 53 |
| Full-time student | 6 | (2.7) | 9 | (3.8) | 15 |
| Alcohol per normal week (units), n (%) | |||||
| 0–7 | 130 | (57.5) | 141 | (60.3) | 271 |
| 8–14 | 33 | (14.6) | 46 | (19.7) | 79 |
| 15–21 | 28 | (12.4) | 17 | (7.3) | 45 |
| > 21 | 27 | (11.9) | 24 | (10.3) | 51 |
| Marital status, n (%) | |||||
| Single | 84 | (37.2) | 80 | (34.2) | 164 |
| Separated | 5 | (2.2) | 8 | (3.4) | 13 |
| Married/civil partner | 63 | (27.9) | 87 | (37.2) | 150 |
| Living with a partner | 42 | (18.6) | 38 | (16.2) | 80 |
| Divorced | 13 | (5.8) | 10 | (4.3) | 23 |
| Widowed | 15 | (6.6) | 9 | (3.8) | 24 |
| Ethnic group, n (%) | |||||
| White | 207 | (91.6) | 215 | (91.9) | 422 |
| Black Caribbean | 2 | (0.9) | 2 | (0.9) | 4 |
| Black African | 3 | (1.3) | 1 | (0.4) | 4 |
| Black other | 0 | (0) | 0 | (0) | 0 |
| Indian | 4 | (1.8) | 2 | (0.9) | 6 |
| Pakistani | 1 | (0.4) | 2 | (0.9) | 3 |
| Bangladeshi | 1 | (0.4) | 2 | (0.9) | 3 |
| Chinese | 0 | (0) | 0 | (0) | 0 |
| Other | 3 | (1.3) | 7 | (3) | 10 |
Table 10 shows details of the mechanism of injury and the participants’ previous medical history.
| Characteristic | Treatment group, n (%) | Total | |||
|---|---|---|---|---|---|
| NPWT (N = 226) | Standard (N = 234) | ||||
| Mechanism of injury | |||||
| Low-energy fall | 34 | (15) | 39 | (16.7) | 73 |
| High-energy fall | 34 | (15) | 25 | (10.7) | 59 |
| Road traffic accident | 125 | (55.3) | 139 | (59.4) | 264 |
| Crush injury | 17 | (7.5) | 19 | (8.1) | 36 |
| Contact sports injury | 3 | (1.3) | 1 | (0.4) | 4 |
| Other | 13 | (5.8) | 9 | (3.8) | 22 |
| Hospital transfer | |||||
| No | 164 | (72.6) | 166 | (70.9) | 330 |
| Yes | 58 | (25.7) | 65 | (27.8) | 123 |
| Other injury | |||||
| No | 168 | (74.3) | 158 | (67.5) | 326 |
| Yes | 58 | (25.7) | 76 | (32.5) | 134 |
| Head | |||||
| Yes | 14 | (6.2) | 11 | (4.7) | 25 |
| Chest | |||||
| Yes | 24 | (10.6) | 22 | (9.4) | 46 |
| Abdomen | |||||
| Yes | 3 | (1.3) | 12 | (5.1) | 15 |
| Pelvis | |||||
| Yes | 8 | (3.5) | 15 | (6.4) | 23 |
| Spine | |||||
| Yes | 21 | (9.3) | 22 | (9.4) | 43 |
| Upper limb | |||||
| Yes | 17 | (7.5) | 32 | (13.7) | 49 |
| Ipsilateral limb | |||||
| Yes | 6 | (2.7) | 16 | (6.8) | 22 |
| Ipsilateral limb fracture | |||||
| Open | 3 | (1.3) | 4 | (1.7) | 7 |
| Closed | 3 | (1.3) | 8 | (3.4) | 11 |
| Contralateral limb | |||||
| Yes | 4 | (1.8) | 14 | (6) | 18 |
| Contralateral limb fracture | |||||
| Open | 0 | (0) | 1 | (0.4) | 1 |
| Closed | 3 | (1.3) | 8 | (3.4) | 11 |
| Previous problem with injured limb | |||||
| No | 163 | (72.1) | 175 | (74.8) | 338 |
| Yes | 63 | (27.9) | 59 | (25.2) | 122 |
| Fracture | |||||
| Yes | 16 | (7.1) | 25 | (10.7) | 41 |
| Ligament/tendon/nerve | |||||
| Yes | 12 | (5.3) | 9 | (3.8) | 21 |
| Arthritis | |||||
| Yes | 13 | (5.8) | 8 | (3.4) | 21 |
| Other | |||||
| Yes | 25 | (11.1) | 23 | (9.8) | 48 |
| Regular analgesia before injury | |||||
| No | 193 | (85.4) | 201 | (85.9) | 394 |
| Yes | 28 | (12.4) | 27 | (11.5) | 55 |
| Other medication before injury | |||||
| No | 126 | (55.8) | 133 | (56.8) | 259 |
| Yes | 84 | (37.2) | 94 | (40.2) | 178 |
Operative procedures
Table 11 reports the methods used for fixation (temporary and definitive) and wound closure in planned operations.
| Method | Treatment group | Total | p-valuea | |||
|---|---|---|---|---|---|---|
| NPWT (n = 226) | % | Standard (n = 234) | % | |||
| Fixation | ||||||
| Temporary fixation: external | 81 | 35.8 | 93 | 39.7 | 174 | 0.443 |
| Temporary fixation: POP | 1 | 0.4 | 1 | 0.4 | 2 | 1.000a |
| Definitive fixation: ORIF | 88 | 38.9 | 92 | 39.3 | 180 | 1.000 |
| Definitive fixation: IM nail | 88 | 38.9 | 103 | 44.0 | 191 | 0.312 |
| Definitive fixation: K-wire TBW | 8 | 3.5 | 3 | 1.3 | 11 | 0.201 |
| Definitive fixation: external circular frame | 19 | 8.4 | 33 | 14.1 | 52 | 0.075 |
| Definitive fixation: external half pin | 17 | 7.5 | 12 | 5.1 | 29 | 0.387 |
| Definitive fixation: external NOS | 8 | 3.5 | 3 | 1.3 | 11 | 0.201 |
| Definitive fixation: other | 10 | 4.4 | 5 | 2.1 | 15 | 0.263 |
| Segmental defect Masquelet first stage | 4 | 1.8 | 6 | 2.6 | 10 | 0.752a |
| Segmental defect bone transport first stage | 0 | 0.0 | 3 | 1.3 | 3 | 0.249a |
| Amputation | 2 | 0.9 | 1 | 0.4 | 3 | 0.618a |
| Wound closure | ||||||
| Primary closure | 37 | 16.4 | 47 | 20.1 | 84 | 0.363 |
| Local flap | 22 | 9.7 | 23 | 9.8 | 45 | 1.000 |
| Free flap | 90 | 39.8 | 82 | 35.0 | 172 | 0.336 |
| Local flap and SSG | 11 | 4.9 | 10 | 4.3 | 21 | 0.935 |
| Skin graft | 45 | 19.9 | 49 | 20.9 | 94 | 0.875 |
There was no evidence to suggest that methods of closure or fixation differed between treatment groups (see p-values in Table 11).
Primary outcome
The primary outcome measure for the WOLLF study was the DRI at 12 months post injury. Baseline DRI, based on retrospective recall of pre-injury health, was also assessed, as was early disability at 3, 6 and 9 months. Table 12 shows the observed means and SDs for DRI and Figure 5 shows the full data distributions and trends in the mean scores.
| Treatment group | Time point, DRI scores | |||||
|---|---|---|---|---|---|---|
| Pre injury | Post injury | 3 months | 6 months | 9 months | 12 months | |
| NPWT | ||||||
| n | 220 | – | 166 | 154 | 153 | 179 |
| Mean (points) | 12.1 | – | 64.3 | 53.2 | 49.2 | 45.5 |
| SD (points) | 22.6 | _ | 22.3 | 23.8 | 25.9 | 28.0 |
| Standard | ||||||
| n | 231 | – | 188 | 175 | 161 | 195 |
| Mean (points) | 12.6 | – | 65.6 | 50.3 | 45.4 | 42.4 |
| SD (points) | 24.3 | – | 20.1 | 24.1 | 25.2 | 24.2 |
FIGURE 5.
Pre-injury baseline, 3, 6, 9 and 12 months. (a) Box plots of DRI scores and (b) trends in means (with 95% CIs).

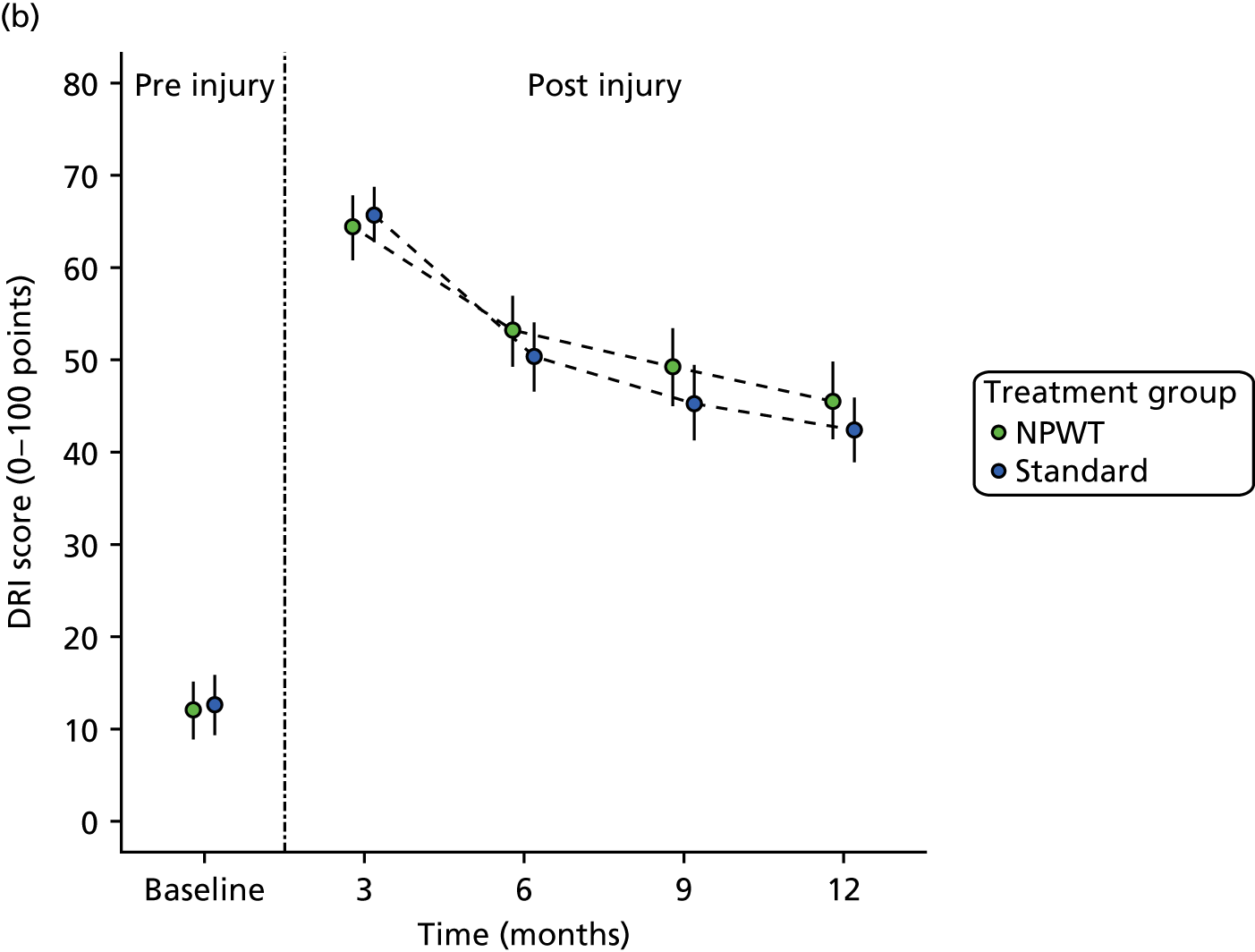
The rates and method of data collection (face to face, postal, telephone or e-mail) are described in Appendix 2 (see Table 35). The method of data collection was very similar between the groups.
Analysis of primary outcome
The primary outcome for the WOLLF study is DRI at 12 months post injury. DRI scores recover in the postoperative period in both groups, but function is still worse than before the injury.
Table 12 shows the means and SDs by treatment group. A mixed-effects linear regression model is used to estimate treatment differences (and 95% CIs). The full (planned) model incorporated terms that allowed for possible heterogeneity in responses for patients owing to the recruiting centre (random effect), in addition to the fixed effects of the wound grade (G&A grade), gender (male or female), patient age (< 40 years and ≥ 40 years), pre-injury DRI and the intervention groups (NPWT and standard dressing) on an ITT basis.
Table 13 shows the raw and adjusted estimates of treatment effects for DRI at 12 months and earlier occasions; a negative value is in favour of the standard treatment, as lower DRI scores indicate less disability. The covariates used to adjust the treatment effect estimates generally showed strong statistical significance, indicating that the inclusion of these terms improved the overall model fit. For the 12-month DRI model, a higher pre-injury DRI was associated with a higher 12-month DRI (p < 0.001); 12-month DRI was higher (i.e. they were more disabled) for participants aged ≥ 40 years than those aged < 40 years (p < 0.001); and 12-month DRI was higher for those participants with G&A grade of 3, or 3 with vascular involvement, than for those participants with G&A grade of 2 (p = 0.047).
| Outcome | Treatment group | Difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|
| NPWT | Standard | ||||||
| Mean (SD) | n | Mean (SD) | n | Rawa | Adjustedb | ||
| Primary outcome | |||||||
| DRI, 12 months (points) | 45.5 (28.0) | 179 | 42.4 (24.2) | 195 | –3.1 | –3.9 (–8.9 to 1.2) | 0.132 |
| DRI scores (points) over time | |||||||
| 3 months | 64.3 (22.3) | 166 | 65.6 (20.1) | 188 | 1.3 | 0.7 (–3.7 to 5.0) | 0.761 |
| 6 months | 53.2 (23.8) | 154 | 50.3 (24.1) | 175 | –2.8 | –3.5 (–8.4 to 1.5) | 0.172 |
| 9 months | 49.2 (25.9) | 153 | 45.4 (25.2) | 161 | –3.8 | –4.4 (–10.0 to 1.3) | 0.128 |
The adjusted estimate of the treatment effect for the 12-month DRI is –3.9 (95% CI –8.9 to 1.2) in favour of the standard dressing group; a lower DRI score indicates less disability. The p-value of 0.132 (see Table 13) indicates that there is no evidence for a statistically significant difference in DRI scores between the treatment groups at 12 months post injury. The MCID for the DRI is 8 points. Therefore, we must conclude, based on our estimated CIs that show evidence in favour of the standard dressing group, that it is extremely unlikely that there is a clinically important difference in DRI scores in favour of the NPWT dressing.
Disability Rating Index at 12 months was positively correlated with earlier DRI scores; the Pearson correlation coefficient with pre-injury DRI is 0.23, 3-month DRI is 0.53, 6-month DRI is 0.71 and 9-month DRI is 0.70. The strong associations between 12-month DRI and earlier (3, 6 and 9 months) DRI can be seen in the similar adjusted treatment effect estimates for these outcomes in Table 13 to those for 12-month DRI. There was no evidence of statistically significant differences in early DRI outcomes between the NPWT and standard dressing treatment groups.
Secondary analyses of the Disability Rating Index data
There were 23 deviations (crossovers) from the allocated treatment (NPWT group, n = 15; standard, n = 8). The PP treatment means for the groups, as defined by the actual treatment participants received rather than the treatment to which they were allocated, were as follows: for NPWT, mean DRI at 12 months was 45.4 points (SD 27.7 points) and for the standard dressing mean DRI at 12 months was 42.5 points (SD 24.5 points), giving a raw mean difference of –2.9. A mixed-effects regression analysis, adjusting exactly as for the ITT model (see Table 13), gave an adjusted treatment effect estimate of –4.0 (95% CI –9.1 to 1.0) in favour of standard dressings with a p-value of 0.119.
Missing data analysis
There were 86 study participants with missing DRI data at the 12-month study end point; 33 participants withdrew from the study after consenting but prior to the 12-month study end point and so, based on the available participants, the study end point is 88% (374/427) towards being complete. Three participants returned 12-month DRI assessments that were classed as missing because more than six of the items were missing. A summary of missing values, by age group and sex, for DRI at 12 months is shown in Appendix 2 (see Table 36).
Secondary outcomes
Health-related quality of life
Health-related QoL was assessed using the EQ-5D-3L and the SF-12. Table 14 shows the observed means and SDs and Figures 6–9 show the full data distributions and trends in the mean scores.
| Outcome | Time point | |||||
|---|---|---|---|---|---|---|
| Pre injury | Post injury | 3 months | 6 months | 9 months | 12 months | |
| NPWT | ||||||
| EQ-5D utility | ||||||
| n | 220 | 210 | 152 | 146 | 144 | 172 |
| Mean | 0.88 | 0.00 | 0.34 | 0.47 | 0.51 | 0.55 |
| SD | 0.22 | 0.29 | 0.33 | 0.33 | 0.33 | 0.33 |
| EQ-5D VAS | ||||||
| n | 219 | 210 | 151 | 144 | 144 | 174 |
| Mean | 82.22 | 43.47 | 56.87 | 62.79 | 65.56 | 67.73 |
| SD | 18.07 | 23.53 | 22.61 | 23.11 | 23.41 | 24.12 |
| SF-12 PCS | ||||||
| n | 214 | 138 | 132 | 130 | 154 | |
| Mean | 54 | 21.1 | 26.6 | 29.5 | 32.2 | |
| SD | 13 | 11.8 | 14.4 | 16.3 | 17.4 | |
| SF-12 MCS | ||||||
| n | 214 | 138 | 132 | 130 | 154 | |
| Mean | 47.8 | 43 | 45.5 | 43.8 | 44.7 | |
| SD | 7.5 | 8.9 | 9.0 | 9.0 | 8.4 | |
| Standard | ||||||
| EQ-5D utility | ||||||
| n | 231 | 226 | 175 | 166 | 154 | 192 |
| Mean | 0.90 | 0.00 | 0.34 | 0.47 | 0.54 | 0.56 |
| SD | 0.21 | 0.30 | 0.32 | 0.32 | 0.29 | 0.32 |
| EQ-5D VAS | ||||||
| n | 231 | 223 | 175 | 165 | 151 | 190 |
| Mean | 82.64 | 42.56 | 60.15 | 63.27 | 67.32 | 68.34 |
| SD | 17.98 | 23.7 | 21.66 | 22.67 | 22.68 | 22.75 |
| SF-12 PCS | ||||||
| n | 227 | 164 | 156 | 137 | 175 | |
| Mean | 55.3 | 21.6 | 26.7 | 29.7 | 32.7 | |
| SD | 11.8 | 12.4 | 15.3 | 16.6 | 15.5 | |
| SF-12 MCS | ||||||
| n | 227 | 164 | 156 | 137 | 175 | |
| Mean | 47.6 | 43.4 | 45.1 | 45.2 | 44.3 | |
| SD | 7.7 | 9.1 | 9.8 | 8.1 | 8.2 | |
FIGURE 6.
(a) Box plots of EQ-5D-3L utility scores and (b) trends in means (with 95% CIs) pre injury, at post-injury baseline and at 3, 6, 9 and 12 months post injury.


FIGURE 7.
(a) Box plots of EQ-5D VAS scores and (b) trends in means (with 95% CIs) pre injury, at post-injury baseline and at 3, 6, 9 and 12 months post injury.


FIGURE 8.
(a) Box plots of SF-12 PCSs and (b) trends in means (with 95% CIs) pre injury, at post-injury baseline and at 3, 6, 9 and 12 months post injury.
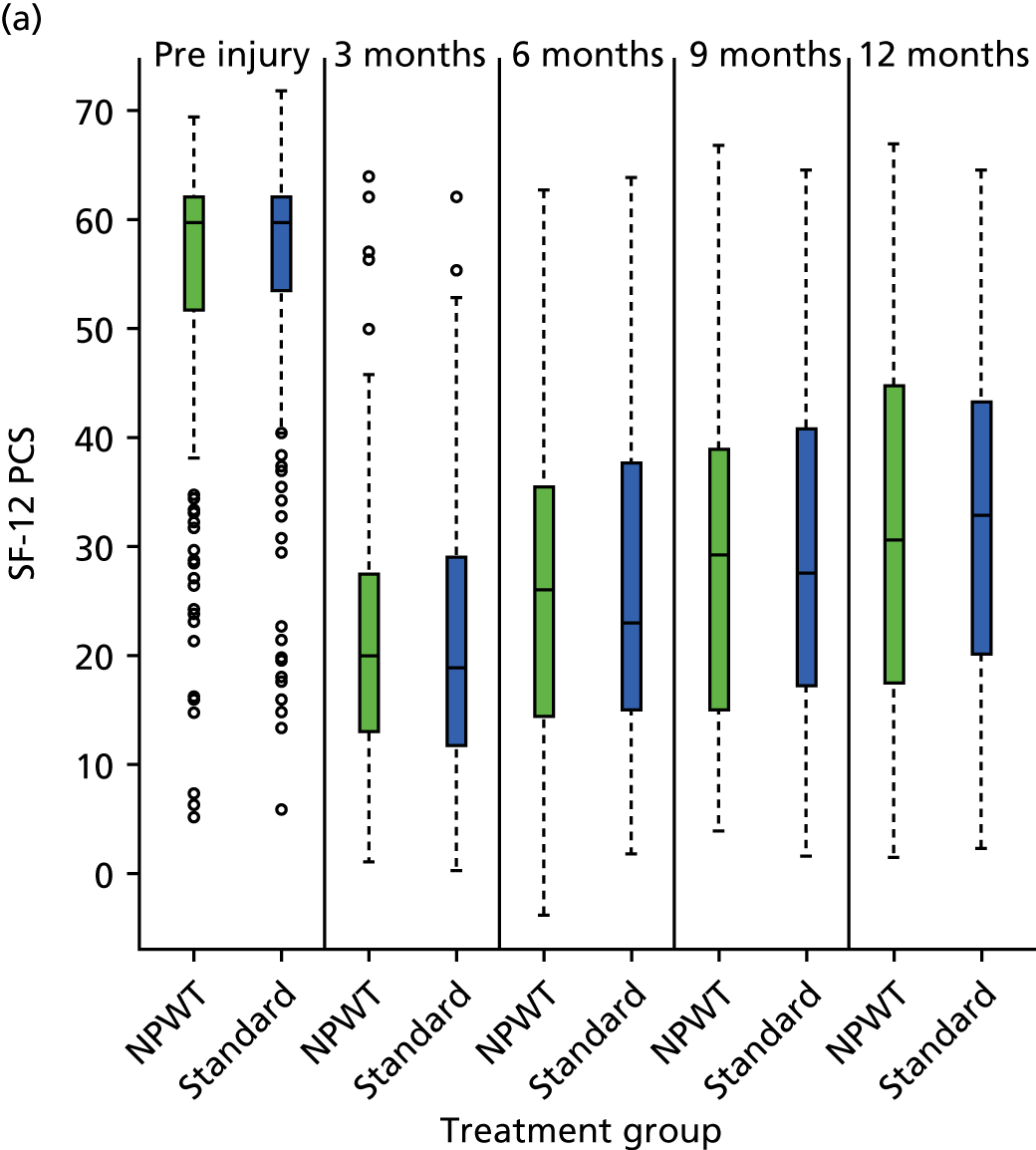

FIGURE 9.
(a) Box plots of SF-12 MCSs and (b) trends in means (with 95% CIs) pre injury, at post-injury baseline and at 3, 6, 9 and 12 months post injury.
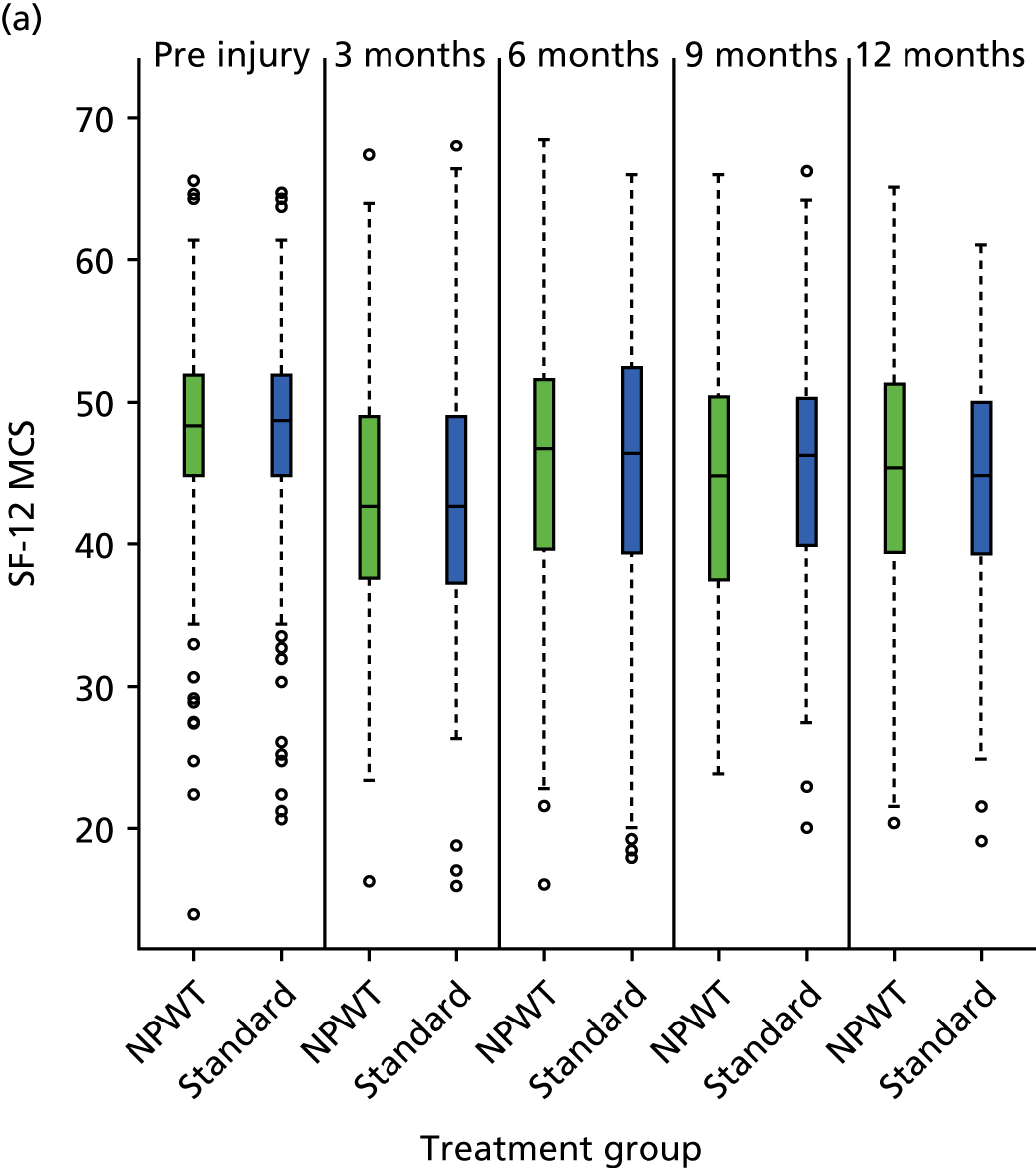
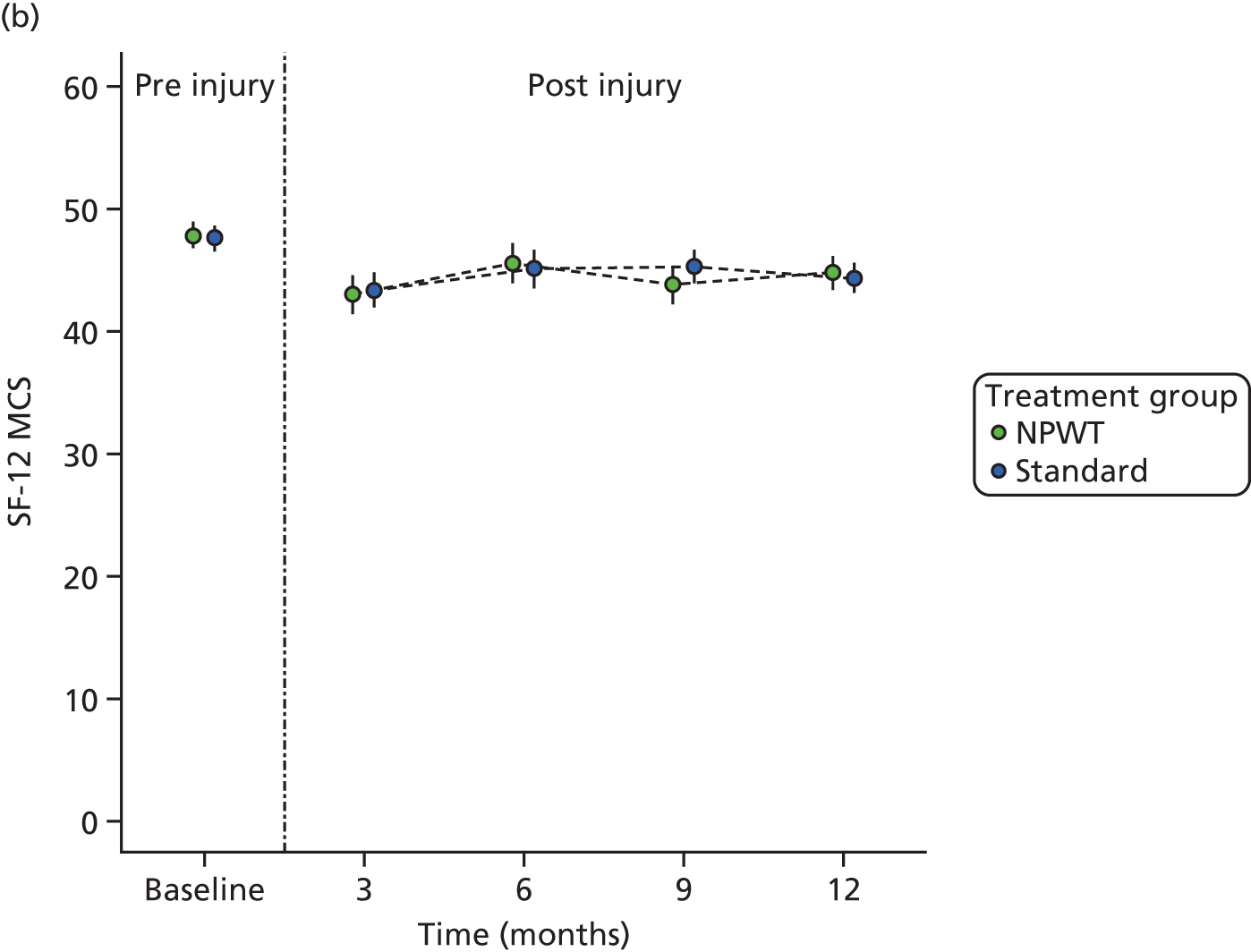
Analysis of health-related quality of life
Health-related QoL was measured using the EQ-5D-3L utility score, EQ-5D VAS score, physical health score (PCS from SF-12) and mental health score (MCS from SF-12) at 12 months post randomisation. There appears to be, from a visual inspection of Figures 6–9, very little evidence of a treatment group difference in these outcomes. Scores for all outcome measures other than MCS improve significantly in the postoperative period in both groups, indicating improved physical health, but participants never recover to pre-injury levels. Mental health score, as measured by MCS, does not vary much in the 12 months following injury, but is always marginally lower than pre-injury levels.
Table 15 shows raw and adjusted estimates of treatment effects for QoL measures across all time points. There is no evidence to support statistically significant differences between treatment groups for any of these measures, at any time point in the first year after injury.
| Outcome | Treatment group | Difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|
| NPWT | Standard | ||||||
| Mean (SD) | n | Mean (SD) | n | Rawa | Adjustedb | ||
| QoL at 12 months | |||||||
| EQ-5D utility | 0.55 (0.33) | 172 | 0.56 (0.32) | 192 | 0.02 | 0.01 (–0.06 to 0.07) | 0.823 |
| EQ-5D VAS | 67.7 (24.1) | 174 | 68.3 (22.7) | 190 | 0.6 | 1.0 (–3.6 to 5.7) | 0.660 |
| SF-12 PCS | 32.2 (17.4) | 154 | 32.7 (15.5) | 175 | 0.5 | 0.4 (–3.0 to 3.8) | 0.817 |
| SF-12 MCS | 44.7 (8.4) | 154 | 44.3 (8.2) | 175 | –0.4 | –0.2 (–2.1 to 1.6) | 0.797 |
| QoL over time | |||||||
| 3 months | |||||||
| EQ-5D utility | 0.34 (0.33) | 152 | 0.34 (0.32) | 175 | 0.00 | 0.00 (–0.07 to 0.07) | 0.948 |
| EQ-5D VAS | 56.9 (22.6) | 151 | 60.1 (21.7) | 175 | 3.3 | 3.6 (–1.2 to 8.4) | 0.140 |
| SF-12 PCS | 21.1 (11.8) | 138 | 21.6 (12.4) | 164 | 0.5 | 0.4 (–2.3 to 3.2) | 0.758 |
| SF-12 MCS | 43.0 (8.9) | 138 | 43.4 (9.1) | 164 | 0.4 | 0.8 (–1.3 to 2.8) | 0.465 |
| 6 months | |||||||
| EQ-5D utility | 0.47 (0.33) | 146 | 0.47 (0.32) | 166 | 0.01 | 0.01 (–0.06 to 0.08) | 0.793 |
| EQ-5D VAS | 62.8 (23.1) | 144 | 63.3 (22.7) | 165 | 0.5 | 1.2 (–3.7 to 6.2) | 0.632 |
| SF-12 PCS | 26.6 (14.4) | 132 | 26.7 (15.3) | 156 | 0.1 | 0.5 (–2.8 to 3.9) | 0.761 |
| SF-12 MCS | 45.5 (9.0) | 132 | 45.1 (9.8) | 156 | –0.5 | –0.4 (–2.6 to 1.8) | 0.730 |
| 9 months | |||||||
| EQ-5D utility | 0.51 (0.33) | 144 | 0.54 (0.29) | 154 | 0.03 | 0.04 (–0.03 to 0.10) | 0.301 |
| EQ-5D VAS | 65.6 (23.4) | 144 | 67.3 (22.7) | 151 | 1.8 | 2.9 (–2.2 to 8.0) | 0.267 |
| SF-12 PCS | 29.5 (16.3) | 130 | 29.7 (16.6) | 137 | 0.2 | 1.0 (–2.8 to 4.8) | 0.604 |
| SF-12 MCS | 43.8 (9.0) | 130 | 45.3 (8.1) | 137 | 1.5 | 1.8 (–0.3 to 3.9) | 0.094 |
There is no evidence that missingness patterns differed between treatment groups. Of the 86 study participants who did not provide DRI score data at 12 months, 47 were in the NPWT group and 39 were in the standard dressing group. Only nine study participants who provided consent had no post-baseline DRI data: six in the NPWT group and three in the standard dressing group.
Logistic regression, with missing data coded as 1 and complete data as 0, indicated that none of age group, gender, wound grade or treatment allocation was predictive of DRI missingness at 12 months; p-values from chi-squared tests for age group, gender, wound grade and treatment group in the logistic regression model were 0.723, 0.306, 0.787 and 0.318, respectively Given that these variables are the most likely to affect missingness patterns and they show no statistically significant association with missingness, it seems reasonable to assume that data are missing at random (MAR).
Imputing the missing data and rerunning the analysis on fully complete data provides a useful sensitivity analysis. Missing data were imputed using the MICE (multiple imputation by chained equations) package in R and pooled estimates of model parameters based on 50 data imputations, and were obtained for the mixed-effects regression models (see Table 13). The pooled estimate of the treatment group effect for DRI at 12 months was –4.5 (95% CI –4.2 to 1.9), with the percentage of the variability attributable to the uncertainty caused by the missing data estimated at 12.8%. Equivalent analyses for the early outcomes at 3, 6 and 9 months were 0.2 (95% CI –4.0 to 4.5), –4.0 (95% CI –8.6 to 0.7) and –5.3 (95% CI –10.5 to –0.1), respectively, with percentages for the variability attributable to the uncertainty caused by the missing data estimated at 19.8%, 18.9% and 24.7%, respectively.
In summary, the inferences based on the complete data, after imputation, are not markedly different from those reported from the complete-case analysis in Appendix 2 (see Table 37).
Complications
Complications were recorded from several sources as part of the routine clinical follow-up for all study participants, through SAEs and directly from participants to the trial team. The routinely reported complications are summarised in Appendix 12 (see Tables 40–45). However, the complete pattern and extent of complications was considerably more complex than these routinely reported assessments.
In some cases, it was unclear whether complications were related or unrelated to the index WOLLF study wound. Therefore, to provide a more comprehensive and comprehensible summary of the full extent of complications, the totality of data was assessed by a surgeon independent from the trial team and categorised into the groupings that are reported in Local complications related to the open fracture or its treatment and Systemic complications potentially related to the open fracture or its treatment. We present summaries of trial-related and unrelated complications, and related and unrelated reoperations.
Local complications related to the open fracture or its treatment
Surgical site infections
Deep surgical site infection at 30 days
In total, 35 out of the 460 participants (7.6%) had a deep SSI: 16 (7.1%) in the NPWT group and 19 (8.1%) in the standard dressing group. Mixed-effects logistic regression with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect showed no evidence that deep SSI rates differed between treatment groups; estimated OR 1.18 (95% CI 0.59 to 2.37), with p-values from analysis of variance (ANOVA) F-test given by 0.638.
Superficial surgical site infection within 30 days
In total, 68 out of the 460 participants (14.8%) had a superficial SSI: 35 (15.5%) in the NPWT group and 33 (14.1%) in the standard dressing group. Mixed-effects logistic regression with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect showed no evidence that superficial SSI rates differed between treatment groups; estimated OR 0.89 (95% CI 0.53 to 1.51), with p-values from ANOVA F-test given by 0.675.
Deep surgical site infection diagnosed after 30 days but before 12 months
In total, 28 out of the 460 participants (6.1%) had persistent or new symptoms of deep SSI after 30 days (but before 12 months): 12 (5.3%) in the NPWT group and 16 (6.8%) in the standard dressing group. Mixed-effects logistic regression with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect showed no evidence that late deep SSI rates differed between treatment groups; estimated OR 1.39 (95% CI 0.53 to 3.02), with p-values from ANOVA F-test given by 0.407.
Specific signs/symptoms
Table 16 shows the number of participants with each of the identified signs/symptoms by treatment group at 30 days. There was no evidence of a difference in individual signs or symptoms.
| Response | Within 30 days | ||||
|---|---|---|---|---|---|
| Treatment group | p-valuea | ||||
| NPWT (N = 226) | Standard (N = 234) | ||||
| n | % | n | % | ||
| Red and inflamed | 34 | 15.0 | 28 | 12.0 | 0.494 |
| Swollen | 26 | 11.5 | 33 | 14.1 | 0.325 |
| Painful/tender | 26 | 11.5 | 35 | 15.0 | 0.165 |
| Fluid leaking | 40 | 17.7 | 42 | 18.0 | 0.712 |
| Fluid (pus) cloudy | 16 | 7.1 | 15 | 6.4 | 0.837 |
| Gaping open | 4 | 1.8 | 8 | 3.4 | 0.255 |
| Surgeon opened | 13 | 5.8 | 10 | 4.3 | 0.670 |
| Fever > 38 °C | 22 | 9.7 | 16 | 6.8 | 0.396 |
| Abscess/infection | 7 | 3.1 | 8 | 3.4 | 0.796 |
| Culture swab taken | 40 | 17.7 | 28 | 12.0 | 0.184 |
| Antibiotics | 34 | 15.0 | 37 | 16.4 | 0.603 |
Assessment of photographic images taken at 6 weeks
Photographic images of the index wound were assessed as ‘healed’ or ‘unhealed’ and ‘infected’ or ‘uninfected’ by two independent clinical assessors blinded to the treatment allocation. Agreement between the two assessors was quantified using Cohen’s kappa statistic, which measures inter-rater agreement for categorical items. For ‘wound healed’, there was substantial agreement between assessors (Cohen’s kappa 0.80 with 95% bootstrapped CI 0.73 to 0.86). There was also substantial, but less, agreement between assessors for ‘wound infected’ (Cohen’s kappa 0.63 with 95% bootstrapped CI 0.52 to 0.72).
Table 17 shows percentage wound healing and signs of infection, based on photographic image data only, by treatment group.
| Healed/infected | Treatment group | OR (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|
| NPWT | Standard | ||||||
| % (n) | N | % (n) | N | Rawa | Adjustedb | ||
| Wound healed | 52.0 (91) | 175 | 51.7 (93) | 180 | 0.99 | 1.0 (0.6 to 1.6) | 0.994 |
| Wound infected | 15.4 (27) | 175 | 17.2 (31) | 180 | 1.14 | 1.1 (0.6 to 1.9) | 0.798 |
Adjusted ORs (see Table 17), based on a mixed-effects logistic regression with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect, show no evidence from photographic images to support differences in wound healing or infection rates between treatment groups.
Other complications
Table 18 shows other complications related to the open fracture, by treatment group.
| Complication | Treatment group | Total | p-valuea | |||
|---|---|---|---|---|---|---|
| NPWT (n = 226) | % | Standard (n = 234) | % | |||
| Soft tissue (other) | 20 | 8.8 | 17 | 7.3 | 37 | 0.650 |
| Neurovascular | 5 | 2.2 | 8 | 3.4 | 13 | 0.618 |
| Pain | 8 | 3.5 | 11 | 4.7 | 19 | 0.696 |
| DVT/PE | 6 | 2.7 | 4 | 1.7 | 10 | 0.538a |
| Other | 0 | 0.0 | 3 | 1.3 | 3 | 0.249a |
There was no evidence to suggest that numbers of trial-related complications differed between treatment groups (see p-values in Table 18).
Further surgery related to the open fracture
Table 19 shows details of the further surgery related to the open fracture in the 12 months after the injury, by treatment group.
| Further surgery | Treatment group | Total | p-valuea | |||
|---|---|---|---|---|---|---|
| NPWT (n = 226) | % | Standard (n = 234) | % | |||
| Removal external fixation | 4 | 1.8 | 8 | 3.4 | 12 | 0.414 |
| Removal internal fixation | 14 | 6.2 | 18 | 7.7 | 32 | 0.654 |
| Revision coverage free flap | 4 | 1.8 | 5 | 2.1 | 9 | 1.000a |
| Revision coverage not specified | 2 | 0.9 | 0 | 0.0 | 2 | 0.241a |
| Revision coverage local flap + SSG | 0 | 0.0 | 5 | 2.1 | 5 | 0.061a |
| Revision coverage primary | 1 | 0.4 | 0 | 0.0 | 1 | 0.491a |
| Revision coverage free flap + SSG | 2 | 0.9 | 1 | 0.4 | 3 | 0.618a |
| Revision coverage skin graft | 9 | 4.0 | 4 | 1.7 | 13 | 0.234 |
| Amputation | 4 | 1.8 | 6 | 2.6 | 10 | 0.752a |
| Other | 0 | 0.0 | 3 | 1.3 | 3 | 0.249a |
| Wound management | 19 | 8.4 | 21 | 9.0 | 40 | 0.960 |
| Revision fixation: ORIF | 9 | 4.0 | 4 | 1.7 | 13 | 0.234 |
| Revision fixation: IM nail | 6 | 2.7 | 4 | 1.7 | 10 | 0.538a |
| Revision fixation: external fixation circular frame | 5 | 2.2 | 6 | 2.6 | 11 | 1.000 |
| Revision fixation: POP | 0 | 0.0 | 2 | 0.9 | 2 | 0.499a |
| Cement nail | 1 | 0.4 | 2 | 0.9 | 3 | 1.000a |
| Reoperation for non-fixation/non-coverage failure | 7 | 3.1 | 7 | 3.0 | 14 | 1.000 |
| Segmental defect Masquelet | 4 | 1.8 | 6 | 2.6 | 10 | 0.752a |
| Local antibiotic therapy | 1 | 0.4 | 3 | 1.3 | 4 | 0.624a |
| Bone biopsy | 1 | 0.4 | 3 | 1.3 | 4 | 0.624a |
| Segmental defect bone transport | 0 | 0.0 | 1 | 0.4 | 1 | 1.000a |
| Other operation | 2 | 0.9 | 2 | 0.9 | 4 | 1.000a |
| Bone graft | 10 | 4.4 | 18 | 7.7 | 28 | 0.204 |
There was no evidence to suggest that the number of related reoperations differed between treatment groups (see p-values in Table 19). Mixed-effects logistic regression with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect showed no evidence that overall revision fixation rates differed between treatment groups (8.0% in the NPWT group and 6.4% in the standard dressing group; see Table 19); estimated OR 0.78 (95% CI 0.38 to 1.60), with p-values from ANOVA F-test given by 0.494.
Radiographic assessment of complications
Radiographs were assessed at 6 weeks for failure of fixation (yes or no) and the sagittal and coronal angulation measured for the index fixation. Union (yes or no) was assessed using 12-month radiographs. Table 20 shows percentages by treatment group.
| Assessment | Treatment group | OR (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|
| NPWT | Standard | ||||||
| % (n) | N | % (n) | N | Rawa | Adjustedb | ||
| 6 weeks | |||||||
| Fixation intact | 96.2 (177) | 184 | 96.8 (179) | 185 | 1.18 | 1.2 (0.4 to 3.6) | 0.800 |
| Sagittal angle (> 5°) | 23.7 (23) | 97 | 19.6 (18) | 92 | 0.78 | 0.8 (0.4 to 1.7) | 0.580 |
| Coronal angle (> 10°) | 7.5 (7) | 93 | 4.4 (4) | 91 | 0.57 | 0.5 (0.1 to 2.4) | 0.397 |
| 12 months (discharge) | |||||||
| Union | 69.6 (112) | 161 | 71.9 (110) | 153 | 1.12 | 1.1 (0.7 to 1.9) | 0.682 |
Adjusted ORs, based on a mixed-effects logistic regression model with treatment group, age group, gender and wound grade as covariates (fixed effects) and recruiting centre as a random effect, show no evidence from radiographs to support differences between treatment groups.
Systemic complications potentially related to the open fracture or its treatment
There were 10 participant deaths reported during the study: six in the NPWT group and four in the standard dressing group. Table 21 shows details of the events.
| Treatment group | Cause of death | Time (days) |
|---|---|---|
| NPWT | Complications after below-knee amputationa | 29 |
| Unknownb | 46 | |
| Respiratory failureb | 84 | |
| House firec | 192 | |
| Complications after below-knee amputationa | 265 | |
| Chronic pulmonary embolismb | 332 | |
| Standard | Acute renal failure and dehydration due to gastrointestinal infectionb | 25 |
| Septic shockb | 37 | |
| Septicaemia and bronchopneumoniab | 45 | |
| Acute kidney injury and urosepsisb | 96 |
Kaplan–Meier curves based on cause-specific survival (censoring the unrelated house fire death) are shown in Figure 10. The number of deaths was small, so a cause-specific analysis was not attempted.
FIGURE 10.
Kaplan–Meier survivor curves: plus symbols show censored events in which participants withdrew from the study before the end of 12 months’ follow-up.
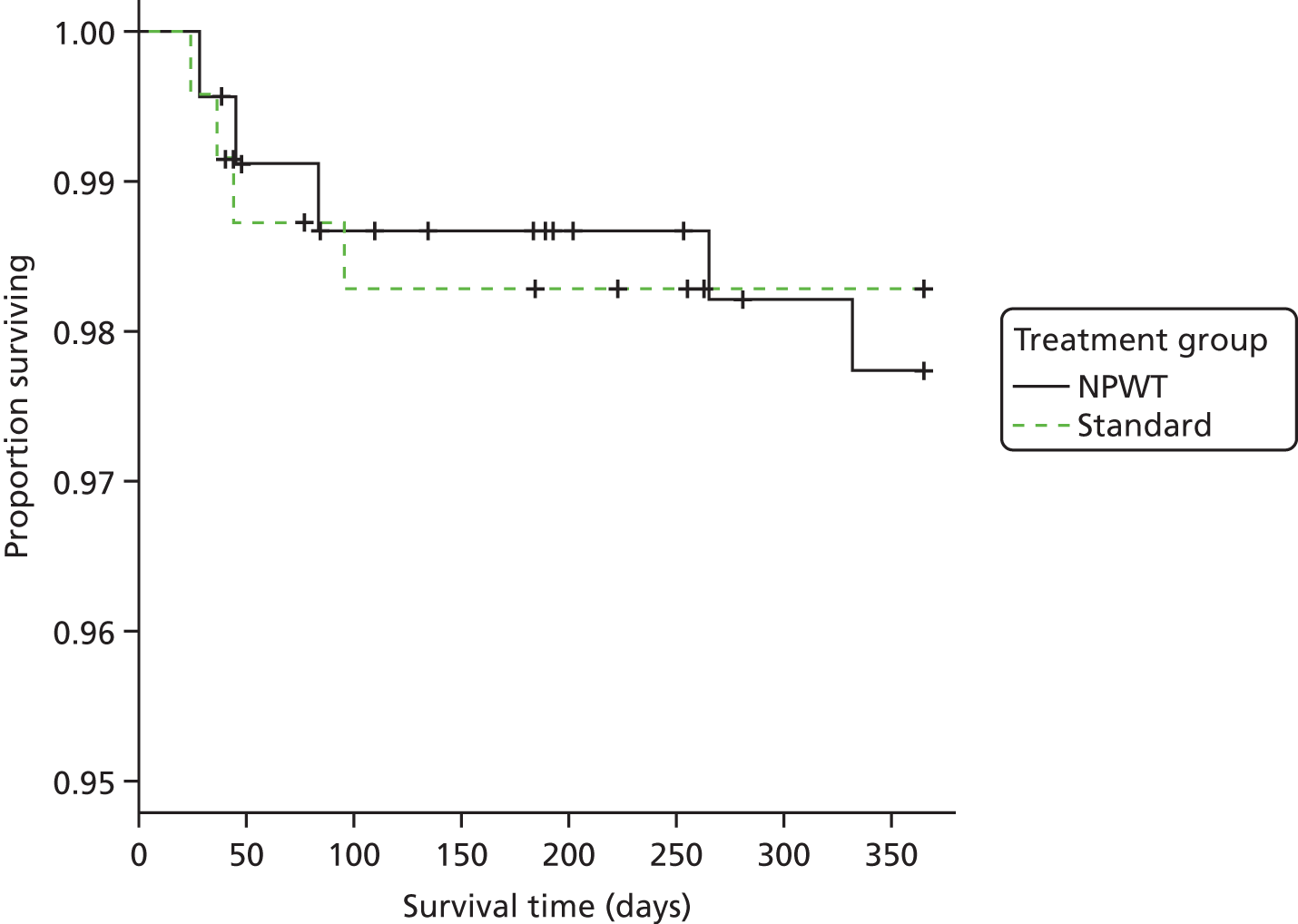
A log-rank test provided no evidence to support a difference in survival between treatment groups; the chi-squared statistic is 0.1 on 1 degree of freedom and p-value is 0.699.
Unrelated serious adverse events
There was no evidence to suggest that numbers of SAEs differed between treatment groups (see p-values in Table 22).
| Specialty area | Treatment group | Total | p-valuea | |||
|---|---|---|---|---|---|---|
| NPWT (n = 226) | % | Standard (n = 234) | % | |||
| Surgical | 7 | 3.1 | 7 | 3.0 | 14 | 1.000 |
| Medical | 19 | 8.4 | 21 | 9.0 | 40 | 0.960 |
| Trauma | 5 | 2.2 | 8 | 3.4 | 13 | 0.618 |
| Psychiatric | 3 | 1.3 | 0 | 0.0 | 3 | 0.118a |
| Anaesthetic | 3 | 1.3 | 4 | 1.7 | 7 | 1.000a |
In addition, there was no evidence to suggest that numbers of unrelated reoperations differed between treatment groups (see p-value in Table 23).
| Operation | Treatment group | Total | p-valuea | |||
|---|---|---|---|---|---|---|
| NPWT (n = 226) | % | Standard (n = 234) | % | |||
| Any operation | 68 | 30.1 | 75 | 32.1 | 143 | 0.723 |
Chapter 5 Health economic evaluation
Overview
In order to estimate the cost-effectiveness of NPWT compared with standard dressings, a prospective economic evaluation was conducted alongside the RCT. The primary analysis adopted a NHS and PSS perspective, as recommended by NICE. 28 This analysis excluded costs that fell outside the NHS and PSS sectors. A secondary analysis conducted from a societal perspective, which included broader societal costs, was also carried out as part of the sensitivity analyses. The economic evaluation took the form of a cost–utility analysis, expressed in terms of incremental cost attributable to the NPWT dressing per QALY gained.
There were three main components to the strategy used to estimate incremental costs associated with NPWT dressings. These were estimation of the costs associated with:
-
the NPWT and standard dressings, as well as costs associated with dressing changes during the period of initial hospitalisation
-
each participant’s initial hospitalisation as well as any further readmissions related to their WOLLF study wound, taking account of all procedures carried out during each inpatient admission
-
broader resource use recorded in patient questionnaires completed at 3, 6, 9 and 12 months post randomisation.
All costs were expressed in Great British pounds and valued at 2014/15 prices. When necessary, costs were inflated or deflated to 2014/15 prices using the NHS Hospital and Community Health Services Pay and Prices Index,80 or the October 2016 annual consumer prices index81 to deflate from 2016/17 prices.
Costings of negative-pressure wound therapy and standard dressings
The components of both types of dressing and their standard NHS costs were obtained from the finance department within the UHCW NHS Trust. The costs of dressing changes were determined from a sample of trial participants for whom additional details were recorded on dressing changes during the initial hospital admission. This data were used to calculate the average number and cost of dressing changes within each trial arm for 38 WOLLF study participants (randomised to NPWT, n = 20; randomised to standard, n = 18). The NPWT dressing comprised the dressing pack (an average cost for the available sizes was used), the canister and the pump (a daily rental cost was applied to the period of initial hospitalisation relevant to the open-fracture wound). The standard dressing comprised Mepitel® (Mölnlycke Health Care, Gothenburg, Sweden) (a non-adhesive dressing), dressing gauze and a wool and crepe bandage.
Costing of initial hospitalisation and readmissions
Inpatient resource use for many participants within the WOLLF study was complex and featured multiple procedures and complications as well as multiple readmissions. Given the importance of capturing the costs associated with complications and further surgeries, all of the trial data related to the initial admission were collated from the background information, operation notes, 6-week follow-up and SAE forms. This information was then assessed for each participant individually to determine the procedures carried out. Procedures were then grouped manually for all participants to determine Healthcare Resource Groups (HRGs) for each initial admission, using the HRG4+ 2015/16 Reference Costs82 code to group guidance, which could then be related to costs using the NHS Reference Costs 2014 to 2015 schedule. 83 When insufficient information was available to reliably assess the procedure(s) carried out during the initial inpatient admission, operation notes and/or discharge letters were requested from trial sites for further clarification.
In order to assign costs to readmissions, it was necessary first to determine which admissions were related to the WOLLF study wound, the process of which was based on clinical judgement, using data from the 3-, 6-, 9- and 12-month follow-up forms as well as the SAE forms. This process was analogous to the process that determined whether AEs were related or unrelated to the open fracture or its treatment. Operation notes were then requested from sites for all relevant admissions, allowing the procedures carried out during each readmission to be determined. HRG codes could then be determined for each readmission and related to NHS Reference Costs. For cases in which there was evidence of a related readmission but the specific procedure carried out was unknown, a weighted average of the base costs, average length of stay and excess bed-day cost of the five most common procedures carried out during readmissions were assumed.
Measurement of broader resource use
As well as inpatient resource use related to the initial admission and readmissions, data were also collected about broader NHS and PSS resource use as well as broader societal resource use for the period between randomisation and 12 months post randomisation. Trial participants self-completed resource use questionnaires at 3, 6, 9 and 12 months post randomisation, covering their use of outpatient and day-case hospital services and community health care, as well as their use of medications, PSSs, and aids and adaptations (e.g. crutches, walking sticks, grab rails, etc.). The 3-month questionnaire covered the period following initial hospitalisation to 6 months post randomisation, with subsequent questionnaires covering the 3 months since the previous questionnaire. Further questionnaires captured wider societal resource use, with data collected on time off work, over-the-counter medications, aids and adaptations purchased privately, as well as any additional costs (e.g. travel costs, lost earnings, child care costs and help with housework) borne by themselves, their partners or their friends and relatives. A copy of the follow-up questionnaire can be found in Appendix 8.
Value of broader resource use
Resource inputs were valued using a combination of primary research, based on established accounting methods and data from secondary national tariff sets. Costs were applied to inpatient hospital care resource use as described in Costing of initial hospitalisation and readmissions. For outpatient hospital care, costs per contact from the NHS Reference Costs 2014 to 201583 were multiplied by the numbers of contacts in each department. For community health care and PSS, unit costs were primarily extracted from the PSSRU Unit Costs of Health and Social Care 201580 compendium. When trial participants provided data on the average duration of each community health or social care contact, the per-minute unit cost was applied to these inputs. When unavailable, the mean cost per contact was used. Medication costs were derived from NHS Prescription Costs Analysis84 data. Data on dose, dose frequency (number of times daily) and dose duration (number of days used) were used when available. When this information was unavailable, a combination of clinical input and the mean values of other trial participants using the same medication was used. Aids and adaptations were valued using a combination of data from the NHS Supply Chain Catalogue85 as well as other sources. Aids and adaptations that could reasonably be assumed to be returned to the NHS following use were assumed to last for 5 years with no resale value, and a discount rate of 3.5% was used to calculate an annuitised cost which was then applied to the period of use during the trial. 86 Time off work was valued using income data from the Office for National Statistics 2014 New Earnings Survey,87 categorised by age and gender. Unit costs were inflated/deflated to 2014/15 prices when necessary using the NHS Hospital and Community Health Services Pay and Prices Index80 for health service resource inputs and the consumer prices index81 for broader resource inputs. No discounting of costs was applied because cost-effectiveness was determined over a 1-year time horizon.
Calculation of utilities and quality-adjusted life-years
The HRQoL of WOLLF study participants was measured using the EQ-5D-3L collected at baseline (which provided an immediate assessment of post-injury HRQoL, as well as separate retrospectively recalled pre-injury values) and at 3, 6, 9 and 12 months post randomisation. 88 The EQ-5D-3L consists of both a descriptive system and a VAS ranging from 100 (best imaginable health state) to 0 (worst imaginable health state). The descriptive system defines HRQoL across five dimensions: mobility, self-care, usual activities, pain, and anxiety or depression. Responses in each dimension take the form of ‘no problems’, ‘some or moderate problems’ or ‘serious or extreme problems’. Responses to each of these five dimensions can then be valued on a health utility scale from –0.59 to 1, with negative values relating to health states considered worse than death, 0 equivalent to being dead, and 1 being a state of full health. For the purposes of this study, the UK time trade-off tariff was applied to each set of responses to generate a EQ-5D utility score (preference weight) for each participant. 18 QALYs were calculated as the area under the baseline-adjusted utility curve of EQ-5D-3L utility scores at (immediate post injury) baseline, 3, 6, 9 and 12 months, using the trapezoidal rule. 86 No discounting of QALYs was applied because cost-effectiveness was determined over a 1-year time horizon.
Within the WOLLF study, the SF-12 score was also collected at baseline (when participants were asked to retrospectively recall their pre-injury health state) as well at 3, 6, 9 and 12 months post randomisation. The SF-12 is a generic health measure with 12 questions covering aspects of physical and mental health across eight dimensions. The UK standard gamble tariff was applied to the responses to the SF-12 in order to generate SF-6D utility scores, from which QALYs could be recalculated using the trapezoidal rule for the purposes of a sensitivity analysis. 21,89,90
Missing data
Missing data were anticipated to be a problem; 69% of data inputs were incomplete when assessing all study time points for the base-case analysis. Multiple imputation was therefore used for all analyses except the complete-case analysis. Multiple imputation produces unbiased estimates of treatment effect when data are MAR. This assumption was explored using logistic regression on missingness of costs and QALYs against baseline covariates. Multiple imputation using chained equations with predictive mean matching was carried out on total QALYs over the entire 1-year follow-up period, total costs in each follow-up period (baseline to 3 months, 3–6 months, 6–9 months and 9–12 months) and on pre- and post-injury baseline EQ-5D utility scores. 91 Included also within the imputation models were predictive covariates; these included costs associated with initial hospitalisation and dressing changes, trial site, G&A grade, gender and age. A total of 69 imputed data sets were generated for the base-case analysis, following the ‘rule of thumb’ suggested in recent methodological guidance. 91
Analyses of costs and outcome data
The mean and standard error (SE) of cost values for each cost category at each time point and within each trial allocation were calculated. Differences between these means were calculated and tested for statistically significant differences from zero using t-tests. For total costs and for each cost category, non-parametric bootstrap estimates based on 10,000 replications were calculated for differences in mean costs and their respective 95% bootstrap CIs also calculated.
The proportion of participants in each arm of the trial providing a suboptimal response at each time point of follow-up for each EQ-5D-3L dimension were tested for equality using the chi-squared test. The mean EQ-5D VAS scores and EQ-5D-3L utility scores for each arm of the trial were also tested for equality at each time point of follow-up using t-tests.
Cost-effectiveness analysis
Bivariate regression using seemingly unrelated regression was used to model total costs and total QALYs over the 1-year follow-up period. This approach allows for correlation between costs and outcomes and estimates the two regression equations jointly, potentially improving the precision of the estimates. By specifying the treatment group as an indicator within each equation, the incremental costs and QALYs attributable to NPWT was estimated, while controlling for baseline covariates (age, gender, trial site and G&A grade). Within the equation for QALYs, baseline EQ-5D utility scores (both pre- and post-injury) were included to adjust for potential baseline imbalances between the trial allocation groups. 92 The cost-effectiveness result was expressed as an ICER, defined as the incremental cost of NPWT divided by the incremental QALYs produced by NPWT. The ICER was then compared with cost-effectiveness threshold values for an additional QALY. The NICE cost-effectiveness threshold for British studies93 ranges between £20,000 and £30,000 per QALY. In addition, a £15,000 cost-effectiveness threshold was included to reflect more recent trends in health-care decision-making. 94
By bootstrapping the data with replacement and recalculating these incremental costs and QALYs 1000 times, a cost-effectiveness plane was populated with 1000 simulated ICER values. Net monetary benefits were estimated from the incremental costs and QALYs at each given cost-effectiveness threshold value and describes the resource gain or loss due to investing in NPWT, given that resources can be used elsewhere within the NHS at the same cost-effectiveness threshold level. By calculating net monetary benefits for each of these 1000 simulated ICER values at levels of the cost-effectiveness threshold varying from £0 to £50,000 per QALY gained, the probability of cost-effectiveness of NPWT (defined as the proportion of positive net monetary benefits at a given threshold level) was calculated, and plotted as a CEAC.
Sensitivity and subgroup analyses
Several sensitivity analyses were undertaken to explore the effects of alternative perspectives or scenarios on the cost-effectiveness results. The cost-effectiveness analysis was therefore repeated under the following assumptions: (1) adopting a wider societal costing perspective (described under Value of broader resource use), (2) calculating QALYs using the SF-6D instead of the EQ-5D21,89,90 and (3) restricting the analysis only to complete cases (i.e. those with complete cost and outcome data).
A single prespecified subgroup analysis was also conducted to explore potential heterogeneity in the incremental cost-effectiveness of NPWT related to whether or not there was evidence of deep infection, assessed using the CDC SSI algorithm.
Long-term cost-effectiveness model
The trial-based economic evaluation focused on the short- and medium-term costs and consequences of NPWT dressing in the treatment of adult patients with an open fracture of the lower limb. The study protocol31 allowed for extrapolation of costs and consequences over a longer time horizon if the trial demonstrated statistically significant differences in medium-term outcomes. This would have required the development of a de novo decision-analytic model. Accepted guidelines for good practice in decision-analytic modelling and the general principles outlined in the NICE ‘reference case’ were to be followed. 95,96 Long-term extrapolations of outcomes were to be expressed in terms of QALYs in the event of differences in medium-term outcomes. Both costs and outcomes accruing beyond the first year post randomisation were to be discounted using a 3.5% annual discount rate in line with current guidance. 28
Results
Study population
A total of 460 participants were consented into the WOLLF study, of whom 226 were randomised to the NPWT dressing and 234 were randomised to the standard dressing. These participants formed the baseline study population for the trial-based health economic evaluation. A complete profile of resource use, from a NHS and PSS perspective, was collected for 285 participants (62%) at 3 months post randomisation, 277 participants (60%) at 6 months post randomisation, 262 participants (57%) at 9 months post randomisation and 322 participants (70%) at 12 months post randomisation. A complete profile of EQ-5D data was collected for 218 participants (47%) and a complete profile of both EQ-5D and resource use values (from a NHS and PSS perspective), across all time points, was collected for 144 participants (31%). Results of the primary clinical and health economic outcomes collected are detailed in Table 24. The completeness of the relevant health economic data items, by follow-up point and by resource category, is detailed in Table 25.
| Follow-up point | All (n) | Treatment group | Mean EQ-5D (SE) | p-value difference in mean EQ-5D | Mean DRI score, points (SE) | p-value difference in mean DRI | |||
|---|---|---|---|---|---|---|---|---|---|
| NPWT (n) | Standard (n) | NPWT | Standard | NPWT | Standard | ||||
| Baseline: pre injury | 451 | 220 | 231 | 0.881 (0.015) | 0.897 (0.014) | 0.427 | – | – | – |
| Baseline: post injury | 436 | 210 | 226 | 0.002 (0.02) | –0.003 (0.02) | 0.833 | – | – | – |
| 3 months | 325 | 151 | 174 | 0.342 (0.027) | 0.346 (0.024) | 0.900 | 64.2 (1.8) | 64.9 (1.5) | 0.799 |
| 6 months | 308 | 143 | 165 | 0.468 (0.027) | 0.471 (0.025) | 0.934 | 52.5 (2) | 50.2 (1.9) | 0.414 |
| 9 months | 294 | 142 | 152 | 0.51 (0.027) | 0.533 (0.024) | 0.527 | 48.8 (2.1) | 45.4 (2) | 0.251 |
| 12 months | 363 | 171 | 192 | 0.545 (0.025) | 0.564 (0.023) | 0.582 | 45.4 (2.1) | 42.7 (1.7) | 0.338 |
| Time point | Treatment group, n (%) | Total (N = 460) | |
|---|---|---|---|
| NPWT (N = 226) | Standard (N = 234) | ||
| Health status | |||
| Pre-injury baseline EQ-5D | 220 (97) | 231 (99) | 451 (98) |
| Post-injury baseline EQ-5D | 210 (93) | 226 (97) | 436 (95) |
| 3-month EQ-5D | 152 (67) | 175 (75) | 327 (71) |
| 6-month EQ-5D | 146 (65) | 166 (71) | 312 (68) |
| 9-month EQ-5D | 144 (64) | 154 (66) | 298 (65) |
| 12-month EQ-5D | 172 (76) | 192 (82) | 364 (79) |
| QALYs complete cases | 94 (42) | 124 (53) | 218 (47) |
| Resource use | |||
| 3 months | |||
| Inpatient care | 157 (69) | 174 (74) | 331 (72) |
| Outpatient care | 148 (65) | 164 (70) | 312 (68) |
| Community care | 148 (65) | 162 (69) | 310 (67) |
| Medications | 142 (63) | 159 (68) | 301 (65) |
| PSSs | 144 (64) | 158 (68) | 302 (66) |
| Aids and adaptations | 144 (64) | 162 (69) | 306 (67) |
| Total costs | 136 (60) | 149 (64) | 285 (62) |
| 6 months | |||
| Inpatient care | 145 (64) | 164 (70) | 309 (67) |
| Outpatient care | 142 (63) | 157 (67) | 299 (65) |
| Community care | 138 (61) | 156 (67) | 294 (64) |
| Medications | 136 (60) | 155 (66) | 291 (63) |
| PSSs | 141 (62) | 157 (67) | 298 (65) |
| Aids and adaptations | 138 (61) | 156 (67) | 294 (64) |
| Total costs | 128 (57) | 149 (64) | 277 (60) |
| 9 months | |||
| Inpatient care | 145 (64) | 150 (64) | 295 (64) |
| Outpatient care | 140 (62) | 143 (61) | 283 (62) |
| Community care | 138 (61) | 138 (59) | 276 (60) |
| Medications | 139 (62) | 143 (61) | 282 (61) |
| PSSs | 141 (62) | 142 (61) | 283 (62) |
| Aids and adaptations | 139 (62) | 140 (60) | 279 (61) |
| Total costs | 129 (57) | 133 (57) | 262 (57) |
| 12 months | |||
| Inpatient care | 168 (74) | 186 (79) | 354 (77) |
| Outpatient care | 162 (72) | 180 (77) | 342 (74) |
| Community care | 161 (71) | 180 (77) | 341 (74) |
| Medications | 159 (70) | 179 (76) | 338 (73) |
| PSSs | 163 (72) | 182 (78) | 345 (75) |
| Aids and adaptations | 163 (72) | 180 (77) | 343 (75) |
| Total costs | 152 (67) | 170 (73) | 322 (70) |
| Complete cases | |||
| EQ-5D and resource use | 65 (29) | 79 (34) | 144 (31) |
Resource use and costs
Initial hospitalisation
The costs associated with each participant’s initial hospitalisation were based on the primary procedure, which was the most resource-intensive procedure during their hospitalisation. The number of primary procedures, and their associate lengths of hospital stay, are broken down by trial arm and detailed in Table 26. The most common primary procedure was microvascular free tissue transfer of flap of muscle of shoulder (latissimus dorsi), with 130 participants having this as their primary procedure. There was a statistically significant difference between the two trial arms in the number of participants receiving primary open reduction of fracture of long bone and extramedullary fixation using plate of hip as their primary procedure (p = 0.005), with none of the participants receiving NPWT having this as their primary procedure, compared with eight participants in the standard dressing arm. There were no other differences in the type of fixation. There were no statistically significant differences in length of stay for each primary procedure between the two trial arms.
| Primary initial procedure name | Number of procedures: NPWT | Mean length of stay: NPWT (days) | Number of procedures: standard | Mean lengthof stay: standard (days) | p-value difference in number of procedures | p-value difference in length of stay |
|---|---|---|---|---|---|---|
| Microvascular free tissue transfer of flap of muscle of shoulder (latissimus dorsi) | 70 | 18.5 | 60 | 20 | 0.204 | 0.512 |
| Primary open reduction of fracture of bone and external fixation HFQ of knee | 53 | 22.7 | 60 | 18.3 | 0.585 | 0.12 |
| Primary open reduction of fracture of long bone and fixation using rigid nail NEC of knee | 28 | 17 | 33 | 15.2 | 0.588 | 0.654 |
| Primary open reduction of fracture of long bone and extramedullary fixation using plate NEC of knee | 17 | 15.2 | 17 | 15.2 | 0.916 | 0.988 |
| Microvascular free tissue transfer of flap of muscle of hip | 18 | 16.9 | 14 | 28.2 | 0.404 | 0.148 |
| Primary open reduction of fracture of long bone and fixation using rigid nail NEC of hip | 5 | 7.4 | 9 | 14.1 | 0.308 | 0.094 |
| Primary open reduction of fracture of bone and external fixation HFQ of hip | 9 | 18.2 | 4 | 23.8 | 0.141 | 0.652 |
| Primary open reduction of fracture of long bone and extramedullary fixation using plate NEC of hip | 0 | – | 8 | 31.5 | 0.005 | – |
| Primary open reduction of fracture dislocation of joint and internal fixation NEC of foot | 4 | 7.5 | 2 | 12.5 | 0.387 | 0.691 |
| Amputation of leg below knee | 4 | 33 | 2 | 21 | 0.387 | 0.441 |
| Primary open reduction of fracture of bone and external fixation HFQ of foot | 3 | 19 | 2 | 15.5 | 0.625 | 0.442 |
| Primary open reduction of fragment of bone and fixation using wire system of foot | 3 | 5.7 | 2 | 20.5 | 0.625 | 0.279 |
| Unspecified split autograft of skin | 3 | 17 | 1 | 8 | 0.299 | – |
| Application of external ring fixation to bone NEC of knee | 0 | – | 3 | 30 | 0.088 | – |
| Revision of microvascular vessel anastomosis of blood vessel of lower limb | 0 | – | 3 | 33 | 0.088 | – |
| Primary open reduction of fragment of bone and fixation using screw of knee | 0 | – | 2 | 7 | 0.164 | – |
| Amputation of leg above knee | 0 | – | 2 | 40.5 | 0.164 | – |
| Primary open reduction of fracture of ankle and extramedullary fixation NEC | 1 | 0 | 1 | 9 | 0.98 | – |
| Amputation of leg through knee | 0 | – | 2 | 37 | 0.164 | – |
| Primary open reduction of fragment of bone and fixation using wire system of knee | 1 | 14 | 1 | 18 | 0.98 | – |
| Application of skeletal traction to bone NEC of hip | 0 | – | 1 | 62 | 0.325 | – |
| Debridement of soft tissue NEC of knee | 1 | 15 | 0 | – | 0.308 | – |
| Application of external fixation to bone NEC of knee | 0 | – | 1 | 1 | 0.325 | – |
| Unspecified local flap of skin and muscle | 1 | 12 | 0 | – | 0.308 | – |
| Application of external fixation to bone NEC of foot | 0 | – | 1 | 4 | 0.325 | – |
| Debridement of soft tissue NEC of foot | 1 | 1 | 0 | – | 0.308 | – |
| Other specified transplantation of muscle of hip | 1 | 50 | 0 | – | 0.308 | – |
| Primary open reduction of fragment of bone and fixation using screw of foot | 1 | 7 | 0 | – | 0.308 | – |
| Microvascular free-tissue transfer of flap of muscle of knee | 0 | – | 1 | 18 | 0.325 | – |
| Remanipulation of fracture of bone and external fixation HFQ of knee | 0 | – | 1 | 25 | 0.325 | – |
| Remanipulation of fragment of bone and fixation using screw of knee | 0 | – | 1 | 2 | 0.325 | – |
| Primary open reduction of fracture of long bone and extramedullary fixation using plate NEC of foot | 1 | 8 | 0 | – | 0.308 | – |
| Primary simple repair of tendon of foot | 1 | 41 | 0 | – | 0.308 | – |
| Total | 226 | 18.5 | 234 | 19.5 | NA | 0.437 |
Costs of negative-pressure wound therapy and standard dressings
The resource components associated with NPWT and standard dressings and their associated costs are summarised in Table 27. A further component of dressing costs is those costs associated with dressing changes. In order to estimate these costs, data on dressing changes were collected for 38 participants (randomised to NPWT, n = 20; randomised to standard dressing, n = 18) and the mean costs associated with dressing changes as well as the mean number of dressing changes in each group is also summarised in Table 27.
| Dressing component | Cost (£) | Source |
|---|---|---|
| NPWT dressing elements | ||
| VAC dressing pack | 31.67 | Sue Wetton, UHCW NHS Trust, 2016, personal communication |
| VAC canister | 34.87 | Sue Wetton, UHCW NHS Trust, 2016, personal communication |
| VAC pump (daily rental cost) | 17.56 | Janet Sensicle, UHCW NHS Trust, 2016, personal communication |
| Standard dressing elements | ||
| Mepitel 8 cm × 10 cm | 3.46 | NHS Supply Chain Catalogue 2015/16 85 |
| Dressing gauze | 0.21 | NHS Supply Chain Catalogue 2015/16 85 |
| Formflex Natural Sterile Padding Bandage 10 cm × 2.7 m (Lantor, Bridgewater, UK) | 0.39 | NHS Supply Chain Catalogue 2015/16 85 |
| Premier Band Light Support Bandage 10 cm × 4.5 cm (Shermond, Coalville, UK) | 0.60 | NHS Supply Chain Catalogue 2015/16 85 |
| Mean cost per dressing change (NPWT) | 22.40 | |
| Mean cost per dressing change (standard dressing) | 6.12 | |
| Mean additional cost of NPWT | 16.28 | |
| Mean number of dressing changes (NPWT) | 2.05 | |
| Total additional cost of NPWT dressing changes | 33.37 | |
Broader resource use
Table 28 presents resource use for trial participants with complete data (for each resource category and follow-up point) by trial allocation and study period. The resource use values are presented for each resource category (inpatient care, outpatient care, community care, medication, PSSs, and aids and adaptations) and by individual items within each category. Resource use values were combined with unit costs for each resource item (Table 29) to estimate economic costs for each resource category.
| Resource use type | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months | 6 months | 9 months | 12 months | |||||||||
| NPWT mean (SE) | Standard mean (SE) | p-value difference between means | NPWT mean (SE) | Standard mean (SE) | p-value difference between means | NPWT mean (SE) | Standard mean (SE) | p-value difference between means | NPWT mean (SE) | Standard mean (SE) | p-value difference between means | |
| Inpatient resource use | n = 157 | n = 174 | n = 145 | n = 164 | n = 145 | n = 150 | n = 168 | n = 186 | ||||
| Readmissions related to WOLLF study wound | 0.22 (0.03) | 0.22 (0.03) | 0.869 | 0.22 (0.03) | 0.26 (0.03) | 0.467 | 0.21 (0.03) | 0.17 (0.03) | 0.305 | 0.11 (0.02) | 0.11 (0.02) | 0.991 |
| Outpatient resource use | n = 148 | n = 164 | n = 142 | n = 157 | n = 140 | n = 143 | n = 162 | n = 180 | ||||
| Orthopaedics | 2.36 (0.27) | 2.55 (0.21) | 0.576 | 1.97 (0.18) | 2.12 (0.22) | 0.593 | 1.16 (0.14) | 1.34 (0.15) | 0.388 | 1.05 (0.13) | 0.98 (0.12) | 0.715 |
| Pathology | 0.16 (0.09) | 0.20 (0.06) | 0.766 | 0.32 (0.11) | 0.21 (0.07) | 0.383 | 0.17 (0.05) | 0.15 (0.04) | 0.803 | 0.15 (0.05) | 0.14 (0.06) | 0.894 |
| Radiology | 1.16 (0.16) | 1.28 (0.11) | 0.552 | 1.27 (0.16) | 1.29 (0.12) | 0.898 | 0.62 (0.10) | 0.76 (0.11) | 0.368 | 0.66 (0.11) | 0.75 (0.14) | 0.608 |
| Physiotherapy (NHS) | 1.85 (0.25) | 2.07 (0.32) | 0.588 | 3.72 (0.45) | 3.99 (0.62) | 0.726 | 1.64 (0.26) | 3.21 (0.72) | 0.040 | 1.05 (0.20) | 1.24 (0.23) | 0.539 |
| Physiotherapy (private) | 0.33 (0.16) | 0.59 (0.24) | 0.358 | 0.84 (0.38) | 0.60 (0.20) | 0.576 | 0.41 (0.17) | 0.78 (0.28) | 0.265 | 0.34 (0.15) | 0.28 (0.11) | 0.768 |
| Emergency department (injury related) | 0.04 (0.02) | 0.06 (0.03) | 0.596 | 0.07 (0.06) | 0.04 (0.02) | 0.668 | 0.07 (0.03) | 0.04 (0.02) | 0.364 | 0.02 (0.02) | 0.03 (0.01) | 0.874 |
| Other | 0.94 (0.33) | 1.46 (0.45) | 0.347 | 0.41 (0.17) | 0.38 (0.12) | 0.900 | 0.10 (0.04) | 0.20 (0.07) | 0.226 | 0.19 (0.09) | 0.11 (0.05) | 0.393 |
| Community care resource use | n = 148 | n = 162 | n = 138 | n = 156 | n = 138 | n = 138 | n = 161 | n = 180 | ||||
| GP surgery | 0.48 (0.09) | 0.72 (0.16) | 0.185 | 1.37 (0.36) | 0.65 (0.13) | 0.063 | 0.72 (0.14) | 0.56 (0.13) | 0.422 | 0.42 (0.13) | 0.34 (0.06) | 0.599 |
| GP home | 0.23 (0.06) | 0.18 (0.05) | 0.531 | 0.14 (0.05) | 0.10 (0.03) | 0.579 | 0.05 (0.03) | 0.08 (0.04) | 0.572 | 0.04 (0.03) | 0.02 (0.02) | 0.525 |
| GP phone | 0.43 (0.15) | 0.51 (0.09) | 0.633 | 0.28 (0.09) | 0.44 (0.11) | 0.265 | 0.25 (0.09) | 0.30 (0.08) | 0.664 | 0.11 (0.05) | 0.17 (0.05) | 0.427 |
| Practice nurse | 2.07 (0.49) | 1.12 (0.31) | 0.099 | 0.86 (0.28) | 0.75 (0.25) | 0.758 | 0.40 (0.18) | 0.51 (0.28) | 0.727 | 0.43 (0.26) | 0.09 (0.04) | 0.190 |
| District nurse | 4.74 (0.93) | 4.24 (0.63) | 0.66 | 2.75 (1.00) | 1.50 (0.39) | 0.243 | 0.54 (0.27) | 1.25 (0.45) | 0.173 | 0.38 (0.18) | 0.47 (0.22) | 0.740 |
| Community physiotherapist | 0.67 (0.21) | 0.59 (0.13) | 0.736 | 1.93 (0.75) | 1.72 (0.43) | 0.808 | 0.33 (0.12) | 0.60 (0.21) | 0.260 | 0.18 (0.10) | 0.64 (0.28) | 0.125 |
| NHS direct call | 0.10 (0.07) | 0.07 (0.03) | 0.696 | 0.04 (0.03) | 0.03 (0.01) | 0.715 | 0.10 (0.07) | 0.02 (0.02) | 0.238 | 0.01 (0.01) | 0.07 (0.06) | 0.314 |
| Ambulance or paramedic | 0.04 (0.02) | 0.06 (0.03) | 0.659 | 0.15 (0.08) | 0.04 (0.02) | 0.149 | 0.03 (0.02) | 0.00 (0.00) | 0.207 | 0.00 (0.00) | 0.00 (0.00) | – |
| Occupational therapy | 0.26 (0.10) | 0.27 (0.07) | 0.945 | 0.12 (0.08) | 0.17 (0.06) | 0.665 | 0.12 (0.09) | 0.05 (0.03) | 0.499 | 0.01 (0.01) | 0.02 (0.02) | 0.838 |
| Other | 0.25 (0.16) | 0.32 (0.17) | 0.759 | 0.16 (0.10) | 0.16 (0.09) | 0.995 | 0.12 (0.09) | 0.02 (0.02) | 0.266 | 0.13 (0.11) | 0.01 (0.01) | 0.296 |
| PSS resource use | n = 144 | n = 158 | n = 141 | n = 157 | n = 141 | n = 142 | n = 163 | n = 182 | ||||
| Meals on wheels (hot) | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.46 (0.46) | 0.319 |
| Laundry | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.10 (0.07) | 0.180 |
| Social worker | 0.13 (0.09) | 0.01 (0.01) | 0.178 | 0.04 (0.04) | 0.00 (0.00) | 0.319 | 0.06 (0.06) | 0.03 (0.03) | 0.652 | 0.02 (0.02) | 0.00 (0.00) | 0.207 |
| Care worker | 1.52 (0.78) | 4.26 (1.74) | 0.151 | 2.44 (1.88) | 2.39 (1.34) | 0.985 | 0.71 (0.46) | 1.37 (0.85) | 0.493 | 1.41 (0.81) | 1.39 (0.97) | 0.988 |
| Other | 0.08 (0.05) | 1.19 (1.07) | 0.300 | 0.02 (0.01) | 0.11 (0.11) | 0.426 | 0.00 (0.00) | 0.00 (0.00) | – | 0.00 (0.00) | 0.02 (0.02) | 0.319 |
| Aids and adaptations resource use | n = 144 | n = 162 | n = 138 | n = 156 | n = 139 | n = 140 | n = 163 | n = 180 | ||||
| Crutches | 0.83 (0.08) | 0.80 (0.08) | 0.743 | 0.38 (0.07) | 0.40 (0.06) | 0.824 | 0.23 (0.05) | 0.13 (0.04) | 0.121 | 0.16 (0.04) | 0.09 (0.03) | 0.201 |
| Sticks | 0.03 (0.02) | 0.08 (0.02) | 0.102 | 0.13 (0.04) | 0.21 (0.05) | 0.201 | 0.09 (0.03) | 0.14 (0.04) | 0.399 | 0.08 (0.02) | 0.12 (0.03) | 0.253 |
| Zimmer frames | 0.31 (0.05) | 0.40 (0.05) | 0.217 | 0.09 (0.03) | 0.15 (0.03) | 0.172 | 0.03 (0.01) | 0.01 (0.01) | 0.407 | 0.02 (0.01) | 0.03 (0.01) | 0.563 |
| Grab rails | 0.06 (0.04) | 0.14 (0.03) | 0.088 | 0.01 (0.01) | 0.12 (0.04) | 0.008 | 0.06 (0.03) | 0.06 (0.03) | 0.856 | 0.01 (0.01) | 0.02 (0.01) | 0.280 |
| Dressing aids | 0.25 (0.13) | 0.20 (0.10) | 0.749 | 0.01 (0.01) | 0.04 (0.02) | 0.195 | 0.79 (0.72) | 0.14 (0.14) | 0.375 | 0.00 (0.00) | 0.03 (0.03) | 0.319 |
| Long-handle shoe horns | 0.04 (0.02) | 0.04 (0.01) | 0.836 | 0.02 (0.01) | 0.03 (0.02) | 0.845 | 0.02 (0.01) | 0.01 (0.01) | 0.313 | 0.01 (0.01) | 0.00 (0.00) | 0.158 |
| Other | 0.47 (0.05) | 0.52 (0.04) | 0.463 | 0.16 (0.03) | 0.22 (0.04) | 0.215 | 0.05 (0.02) | 0.10 (0.05) | 0.335 | 0.10 (0.03) | 0.04 (0.01) | 0.027 |
| NPWT n (%) | Standard n (%) | All n (%) | NPWT n (%) | Standard n (%) | NPWT n (%) | Standard n (%) | NPWT n (%) | Standard n (%) | ||||
| Medications resource use | n = 142 | n = 159 | n = 136 | n = 155 | n = 139 | n = 143 | n = 159 | n = 179 | ||||
| Prescription medication | 76 (54) | 85 (53) | 0.991 | 49 (36) | 74 (48) | 0.044 | 41 (29) | 47 (33) | 0.541 | 44 (28) | 67 (37) | 0.057 |
| Resource item | Unit cost (£) | Source | |
|---|---|---|---|
| Per contact | Per minute | ||
| Outpatient care | |||
| Orthopaedics | 112.50 | NHS Reference Costs 2014 to 2015 83 | |
| Pathology | 77.70 | NHS Reference Costs 2014 to 2015 83 | |
| Radiology | 82.37 | NHS Reference Costs 2014 to 2015 83 | |
| Physiotherapy (NHS) | 46.00 | NHS Reference Costs 2014 to 2015 83 | |
| Physiotherapy (private) | 45.54 | NHS Reference Costs 2014 to 2015 83 | |
| Emergency department | 140.59 | NHS Reference Costs 2014 to 2015 83 | |
| Community care | |||
| GP visits in surgery | 44.46 | 3.80 | Unit Costs of Health and Social Care 2015 80 |
| GP home visits | 43.32 | 3.80 | Unit Costs of Health and Social Care 2015 80 |
| GP telephone contacts | 26.98 | 3.80 | Unit Costs of Health and Social Care 2015 80 |
| Practice nurse contacts | 14.47 | 0.93 | Unit Costs of Health and Social Care 2015 80 |
| District nurse contacts | 38.00 | 1.12 | Unit Costs of Health and Social Care 2015 80 |
| Community physiotherapy contacts | 34.05 | 0.61 | Unit Costs of Health and Social Care 2014 97 |
| Calls to NHS Direct | 20.18 | BBC98 | |
| Calls for an ambulance or paramedic | 99.00 | Unit Costs of Health and Social Care 2015 80 | |
| Occupational therapy contacts | 77.69 | 0.61 | Unit Costs of Health and Social Care 2014 97 |
| PSSs | |||
| Meals on wheels | 6.55 | Unit Costs of Health and Social Care 2010 99 | |
| Laundry services | 9.78 | Elderly Accommodation Counsel100 | |
| Social worker contacts | 49.28 | 1.32 | Unit Costs of Health and Social Care 2015 80 |
| Care worker contacts | 18.50 | 0.62 | Unit Costs of Health and Social Care 2015 80 |
| Aids and adaptations | |||
| Crutches | 5.06 | NHS Supply Chain Catalogue 2015/16 85 | |
| Stick | 3.94 | NHS Supply Chain Catalogue 2015/16 85 | |
| Zimmer frame | 21.54 | Complete Care Shop101 | |
| Grab rail | 1.61 | NHS Supply Chain Catalogue 2015/16 85 | |
| Dressing aids | 5.34 | NHS Supply Chain Catalogue 2015/16 85 | |
| Long-handle shoe horn | 1.66 | NHS Supply Chain Catalogue 2015/16 85 | |
In terms of inpatient resource use, readmissions related to the WOLLF study wound were broadly similar in both trial arms, varying between 0.11 readmissions per participant during the 9- to 12-month follow-up period (in both trial arms) and 0.26 readmissions per participant during the 3- to 6-month follow-up period, for participants receiving standard dressings. Within outpatient resource use, the department with the highest average visits per participant was NHS physiotherapy, which peaked at 3.99 visits per participant during the 3- to 6-month follow-up period for participants receiving standard dressings. In the 6- to 9-month follow-up period, participants receiving standard dressings made statistically significantly (p = 0.040) more NHS physiotherapy visits (3.21 compared with 1.64), an effect mirrored for private physiotherapy visits (0.78 compared with 0.41 visits), although the latter difference was not statistically significant. This was followed by orthopaedics, with 2.55 visits per patient in the 0- to 3-month follow-up period for participants receiving standard dressings. This fell to 0.98 visits per participant during the 9- to 12-month follow-up period. Within community care, the district nurse category received the greatest average number of visits per participant, with 4.74 visits per participant during the 0- to 3-month follow-up period, for participants randomised to NPWT. Within the aids and adaptations category, statistically significantly (p = 0.008) more grab rails were utilised on average by the participants in the standard dressing arm during the 3- to 6-month follow-up period (0.12 grab rails on average, compared with 0.01 for those receiving NPWT).
Prescription medication usage was highest during the first 3 months of the post-randomisation period, with 53% of all participants using some form of prescription medication. In the 3- to 6-month follow-up period, this fell to 42% of participants, with statistically significantly (p = 0.044) higher usage for those participants randomised to standard dressings (48% compared with 36%).
Economic costs
Economic costs for trial participants with complete data at each time point are presented in Table 30 by trial arm, study period and cost category. With the exception of the cost of the initial inpatient stay (including costs associated with dressings and dressing changes), there were no statistically significant differences in costs between the trial arms in any cost category. For the initial patient stay, mean costs were £1223 higher in the NPWT arm (p = 0.030). Over the entire follow-up period, mean (SE) total NHS and PSS costs, inclusive of the additional cost of the intervention and associated dressing changes, was £14,079 (£1109) in the NPWT group, compared with £14,002 (£654) in the standard dressing group, generating a mean cost difference of £77 (bootstrap 95% CI –£2114 to £2925).
| Cost category | Treatment group, mean cost (£) (SE) | Mean difference | p-value | Bootstrap 95% CI | |
|---|---|---|---|---|---|
| NPWT | Standard | ||||
| Baseline to 3 months (n = 285 complete cases out of 460 total) | |||||
| Initial inpatient stay | 10,324.10 (460.00) | 9101.00 (319.10) | 1223.10 | 0.030 | 210.60 to 2363.80 |
| Inpatient care | 717.90 (190.40) | 814.50 (170.20) | –96.60 | 0.705 | –568.60 to 434.60 |
| Outpatient care | 545.90 (54.30) | 625.50 (47.30) | –79.60 | 0.271 | –216.20 to 70.70 |
| Community care | 263.90 (45.00) | 280.70 (33.90) | –16.80 | 0.766 | –121.00 to 101.00 |
| Medications | 25.90 (7.80) | 19.30 (3.30) | 6.60 | 0.440 | –6.30 to 28.50 |
| PSSs | 32.00 (14.80) | 85.30 (34.10) | –53.30 | 0.153 | –154.80 to 1.80 |
| Aids and adaptations | 10.20 (1.60) | 13.60 (2.70) | –3.40 | 0.277 | –10.80 to 1.80 |
| Total cost | 11,919.90 (504.70) | 10,939.90 (358.20) | 980.00 | 0.115 | –162.50 to 2255.00 |
| 3–6 months (n = 277 complete cases out of 460 total) | |||||
| Inpatient care | 724.70 (204.70) | 842.60 (167.70) | –117.90 | 0.656 | –582.30 to 464.90 |
| Outpatient care | 542.80 (51.90) | 591.70 (49.70) | –48.90 | 0.496 | –193.70 to 96.50 |
| Community care | 349.20 (94.10) | 174.70 (25.90) | 174.50 | 0.076 | 36.30 to 459.10 |
| Medications | 46.20 (28.10) | 23.40 (10.30) | 22.80 | 0.449 | –13.90 to 134.80 |
| PSSs | 200.60 (164.40) | 49.90 (24.90) | 150.70 | 0.367 | –34.60 to 857.50 |
| Aids and adaptations | 7.70 (2.90) | 7.90 (2.60) | –0.20 | 0.965 | –7.40 to 7.90 |
| Total cost | 1871.10 (311.10) | 1690.10 (193.00) | 181.00 | 0.622 | –464.70 to 996.00 |
| 6–9 months (n = 262 complete cases out of 460 total) | |||||
| Inpatient care | 651.90 (148.00) | 461.30 (153.00) | 190.60 | 0.371 | –263.50 to 572.70 |
| Outpatient care | 290.20 (29.30) | 386.80 (47.80) | –96.60 | 0.086 | –225.30 to –1.10 |
| Community care | 101.30 (21.80) | 101.50 (20.80) | –0.20 | 0.994 | –58.60 to 58.00 |
| Medications | 27.10 (9.40) | 12.10 (3.50) | 15.00 | 0.135 | 0.20 to 42.40 |
| PSSs | 37.30 (20.90) | 31.70 (19.50) | 5.60 | 0.843 | –49.40 to 63.90 |
| Aids and adaptations | 1.50 (0.60) | 1.30 (0.60) | 0.20 | 0.751 | –1.80 to 1.90 |
| Total cost | 1109.50 (176.40) | 994.60 (182.80) | 114.90 | 0.652 | –403.60 to 598.80 |
| 9–12 months (n = 322 complete cases out of 460 total) | |||||
| Inpatient care | 440.40 (183.50) | 275.10 (79.30) | 165.30 | 0.409 | –111.50 to 765.90 |
| Outpatient care | 261.70 (33.00) | 254.30 (32.90) | 7.40 | 0.873 | –87.70 to 95.70 |
| Community care | 50.30 (14.20) | 66.80 (16.20) | –16.50 | 0.444 | –61.70 to 22.50 |
| Medications | 16.40 (5.90) | 24.60 (10.80) | –8.20 | 0.504 | –44.60 to 9.70 |
| PSSs | 38.20 (26.50) | 35.70 (23.00) | 2.50 | 0.943 | –65.80 to 71.60 |
| Aids and adaptations | 4.10 (2.10) | 4.60 (3.70) | –0.50 | 0.918 | –14.70 to 5.10 |
| Total cost | 811.20 (204.30) | 661.10 (97.20) | 150.10 | 0.508 | –176.10 to 815.40 |
| Total NHS and PSS costs, including intervention costs (n = 152 complete cases out of 460 total) | |||||
| 14,078.90 (1108.60) | 14,002.10 (653.60) | 76.80 | 0.953 | –2114.30 to 2925.40 | |
Health-related quality-of-life outcomes
Table 31 presents the numbers and proportions of responses to each level of the EQ-5D-3L for each of the five EQ-5D dimensions, across all follow-up time points, as well as the means and SEs for the EQ-5D VAS and EQ-5D-3L utility scores for all follow-up time points. Presented also are p-values for the test of equality of proportions of participants with suboptimal function between the two trial arms, for each EQ-5D dimension, as well as p-values for the test of equality for the mean EQ-5D VAS and utility scores between the two trial arms, at each follow-up time point. Participants in the two trial arms were strikingly similar, with no statistically significant differences in EQ-5D outcomes across any of the tests carried out.
| Time/allocation | Domain, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | ||||||||||
| Level 1 | Level 2 | Level 3 | Suboptimal | Level 1 | Level 2 | Level 3 | Suboptimal | Level 1 | Level 2 | Level 3 | Suboptimal | |
| Pre-injury baseline (n = 450) | ||||||||||||
| Intervention (n = 219) | 184 (84) | 34 (16) | 1 (0.46) | 35 (16) | 202 (92) | 15 (7) | 2 (0.91) | 17 (8) | 195 (89) | 22 (10) | 2 (0.91) | 24 (11) |
| Control (n = 231) | 202 (87) | 29 (13) | 0 (0) | 29 (13) 0.298a | 218 (94) | 13 (6) | 0 (0) | 13 (6) 0.364a | 210 (91) | 16 (7) | 5 (2) | 21 (9) 0.509a |
| Post-injury baseline (n = 432) | ||||||||||||
| Intervention (n = 209) | 3 (1) | 50 (24) | 156 (75) | 206 (99) | 27 (13) | 117 (56) | 65 (31) | 182 (87) | 3 (1) | 34 (16) | 172 (82) | 206 (99) |
| Control (n = 223) | 3 (1) | 45 (20) | 175 (79) | 220 (99) 0.936a | 22 (10) | 139 (62) | 62 (28) | 201 (90) 0.317a | 2 (1) | 32 (14) | 189 (85) | 221 (99) 0.601a |
| 3 months (n = 322) | ||||||||||||
| Intervention (n = 149) | 6 (4) | 125 (84) | 18 (12) | 143 (96) | 65 (44) | 75 (50) | 9 (6) | 84 (56) | 9 (6) | 63 (42) | 77 (52) | 140 (94) |
| Control (n = 173) | 6 (3) | 146 (84) | 21 (12) | 167 (97) 0.792a | 66 (38) | 105 (61) | 2 (1) | 107 (62) 0.319a | 5 (3) | 84 (49) | 84 (49) | 168 (97) 0.167a |
| 6 months (n = 308) | ||||||||||||
| Intervention (n = 143) | 19 (13) | 121 (85) | 3 (2) | 124 (87) | 87 (61) | 54 (38) | 2 (1) | 56 (39) | 12 (8) | 84 (59) | 47 (33) | 131 (92) |
| Control (n = 165) | 27 (16) | 134 (81) | 4 (2) | 138 (84) 0.450a | 102 (62) | 60 (36) | 3 (2) | 63 (38) 0.860a | 21 (13) | 94 (57) | 50 (30) | 144 (87) 0.220a |
| 9 months (n = 294) | ||||||||||||
| Intervention (n = 143) | 30 (21) | 108 (76) | 5 (4) | 113 (79) | 90 (63) | 46 (32) | 7 (5) | 53 (37) | 22 (15) | 87 (61) | 34 (24) | 121 (85) |
| Control (n = 151) | 31 (21) | 116 (77) | 4 (3) | 120 (80) 0.924a | 95 (63) | 54 (36) | 2 (1) | 56 (37) 0.997a | 25 (17) | 91 (60) | 35 (23) | 126 (83) 0.784a |
| 12 months (n = 362) | ||||||||||||
| Intervention (n = 172) | 47 (27) | 123 (72) | 2 (1) | 125 (73) | 121 (70) | 48 (28) | 3 (2) | 51 (30) | 43 (25) | 92 (54) | 37 (22) | 129 (75) |
| Control (n = 190) | 50 (26) | 136 (72) | 4 (2) | 140 (74) 0.82a | 132 (70) | 56 (30) | 2 (1) | 58 (31) 0.856a | 39 (21) | 120 (63) | 31 (16) | 151 (80) 0.310a |
| Time/allocation | Domain, n (%) | EQ-5D VAS score | EQ-5D-3L utility score | |||||||||
| Pain | Anxiety/depression | |||||||||||
| Level 1 | Level 2 | Level 3 | Suboptimal | Level 1 | Level 2 | Level 3 | Suboptimal | Mean (SE) | Mean (SE) | |||
| Pre-injury baseline (n = 450) | ||||||||||||
| Intervention (n = 219) | 169 (77) | 46 (21) | 4 (2) | 50 (23) | 177 (81) | 36 (16) | 6 (3) | 42 (19) | 82.2 (1.2) | 0.880 (0.015) | ||
| Control (n = 231) | 192 (83) | 34 (15) | 5 (2) | 39 (17) 0.113a | 193 (84) | 29 (13) | 9 (4) | 38 (17) 0.449a | 82.6 (1.2) 0.804b | 0.897 (0.014) 0.412b | ||
| Post-injury baseline (n = 432) | ||||||||||||
| Intervention (n = 209) | 25 (12) | 139 (67) | 45 (22) | 184 (88) | 93 (45) | 101 (48) | 15 (7) | 116 (56) | 43.4 (1.6) | 0.002 (0.020) | ||
| Control (n = 223) | 25 (11) | 155 (70) | 43 (19) | 198 (89) 0.807a | 105 (47) | 98 (44) | 20 (9) | 118 (53) 0.590a | 42.6 (1.6) 0.702b | –0.001 (0.021) 0.918b | ||
| 3 months (n = 322) | ||||||||||||
| Intervention (n = 149) | 22 (15) | 103 (69) | 24 (16) | 127 (85) | 69 (46) | 73 (49) | 7 (5) | 80 (54) | 57.2 (1.9) | 0.345 (0.027) | ||
| Control (n = 173) | 18 (10) | 132 (76) | 23 (13) | 155 (90) 0.237a | 70 (41) | 91 (53) | 12 (7) | 103 (60) 0.291a | 60.0 (1.8) 0.253b | 0.348 (0.026) 0.936b | ||
| 6 months (n = 308) | ||||||||||||
| Intervention (n = 143) | 19 (13) | 107 (75) | 17 (12) | 124 (87) | 61 (43) | 69 (48) | 13 (9) | 82 (57) | 62.8 (1.9) | 0.468 (0.027) | ||
| Control (n = 165) | 21 (13) | 121 (73) | 23 (14) | 144 (87) 0.884a | 80 (49) | 72 (44) | 13 (8) | 85 (52) 0.306a | 63.3 (1.9) 0.850b | 0.477 (0.027) 0.809b | ||
| 9 months (n = 294) | ||||||||||||
| Intervention (n = 143) | 23 (16) | 102 (71) | 18 (13) | 120 (84) | 69 (48) | 65 (46) | 9 (6) | 74 (52) | 65.5 (2.0) | 0.505 (0.028) | ||
| Control (n = 151) | 22 (15) | 119 (79) | 10 (7) | 129 (85) 0.719a | 78 (52) | 62 (41) | 11 (7) | 73 (48) 0.560a | 67.3 (1.9) 0.498b | 0.531 (0.025) 0.478b | ||
| 12 months (n = 362) | ||||||||||||
| Intervention (n = 172) | 30 (17) | 122 (71) | 20 (12) | 142 (83) | 85 (49) | 73 (42) | 14 (8) | 87 (51) | 67.7 (1.9) | 0.547 (0.025) | ||
| Control (n = 190) | 36 (19) | 134 (71) | 20 (11) | 154 (81) 0.711a | 105 (55) | 72 (38) | 13 (7) | 85 (45) 0.266a | 68.3 (1.7) 0.789b | 0.563 (0.024) 0.640b | ||
Cost-effectiveness results
Table 32 presents the incremental cost-effectiveness results for the NPWT dressing in the base-case analysis as well as in each of the sensitivity analyses. The probability that NPWT is cost-effective is also presented, at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY gained, as well as the 95% CIs for net monetary benefit at each of these cost-effectiveness thresholds. All analyses were adjusted for potential imbalances in baseline covariates of age, gender, G&A grade and trial site. The analysis of QALYs was adjusted for both pre-injury and post-injury baseline EQ-5D-3L utility scores. 92
| Base-case and sensitivity analyses | Incremental cost (£) (95% CI) | Incremental QALYs (95% CI) | ICER (£) | Probability cost-effectivea | Probability cost-effectiveb | Probability cost-effectivec | Net monetary benefita (£) (95% CI) | Net monetary benefitb (£) (95% CI) | Net monetary benefitc (£) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Base-case NHS and PSS perspective – imputed costs and QALYs, covariate adjusted | 678 (–1082 to 2438) | 0.002 (–0.054 to 0.059) | 267,910 | 0.233 | 0.244 | 0.271 | –615 (–2163 to 848) | –606 (–2210 to 938) | –588 (–2320 to 1169) |
| Societal perspective – imputed costs and QALYs, covariate adjusted | 2264 (–1271 to 5800) | 0.008 (–0.043 to 0.059) | 282,858 | 0.076 | 0.081 | 0.104 | –2156 (–5177 to 826) | –2121 (–5197 to 922) | –2051 (–5324 to 1163) |
| QALYs calculated using SF-6D – imputed costs and QALYs, covariate adjusted | 796 (–925 to 2518) | –0.002 (–0.030 to 0.027) | Dominated | 0.119 | 0.120 | 0.127 | –823 (–2216 to 585) | –833 (–2268 to 595) | –853 (–2347 to 674) |
| Complete-case analysis – covariate adjusted | –452 (–2926 to 2022) | 0.022 (–0.041 to 0.086) | Dominant | 0.709 | 0.721 | 0.736 | 760 (–1820 to 3401) | 862 (–1919 to 3745) | 1068 (–2195 to 4694) |
| Subgroup analysis – deep infection | 3295 (–3680 to 10,269) | –0.036 (–0.243 to 0.171) | Dominated | 0.139 | 0.137 | 0.142 | –3821 (–11,083 to 3414) | –3982 (–11,557 to 3777) | –4304 (–12,455 to 4155) |
Base-case analysis
The base-case analysis used multiply imputed data and produced an ICER of £267,910 per QALY gained, reflecting higher costs on average and marginally higher QALYs on average in the NPWT arm. The probability that NPWT was cost-effective was also computed at the cost-effectiveness thresholds referred to previously but never exceeded 27% (24.4% at the widely used £20,000 per QALY cost-effectiveness threshold). The base-case analysis indicates that NPWT is highly unlikely to be cost-effective in this patient population. The cost-effectiveness plane and CEAC for the base-case analysis are displayed in Figure 11.
FIGURE 11.
Base-case analysis. (a) Cost-effectiveness plane; and (b) CEAC.

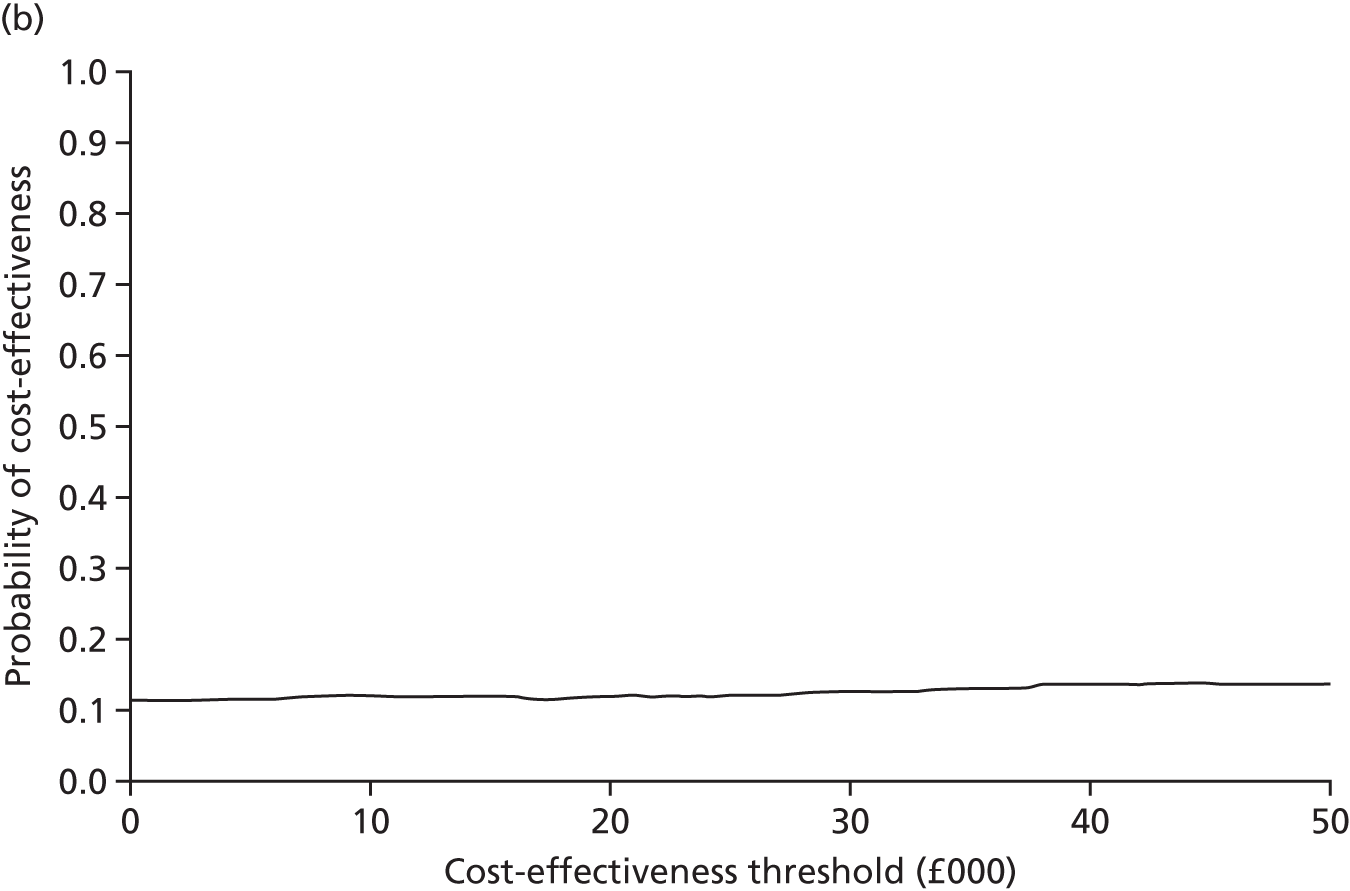
Sensitivity analyses
Societal perspective
When taking a broader costing perspective that additionally included costs that fell outside the NHS and PSS sectors, the ICER increased to £282,858 per QALY gained, largely driven by an increased incremental cost attributable to NPWT; the probability that NPWT is cost-effective did not exceed 11% (8.1% at a £20,000 cost-effectiveness threshold). The cost-effectiveness plane and CEAC for the analysis carried out taking a societal perspective is shown in Figure 12.
FIGURE 12.
Sensitivity analysis adopting a societal perspective. (a) Cost-effectiveness plane; and (b) CEAC.
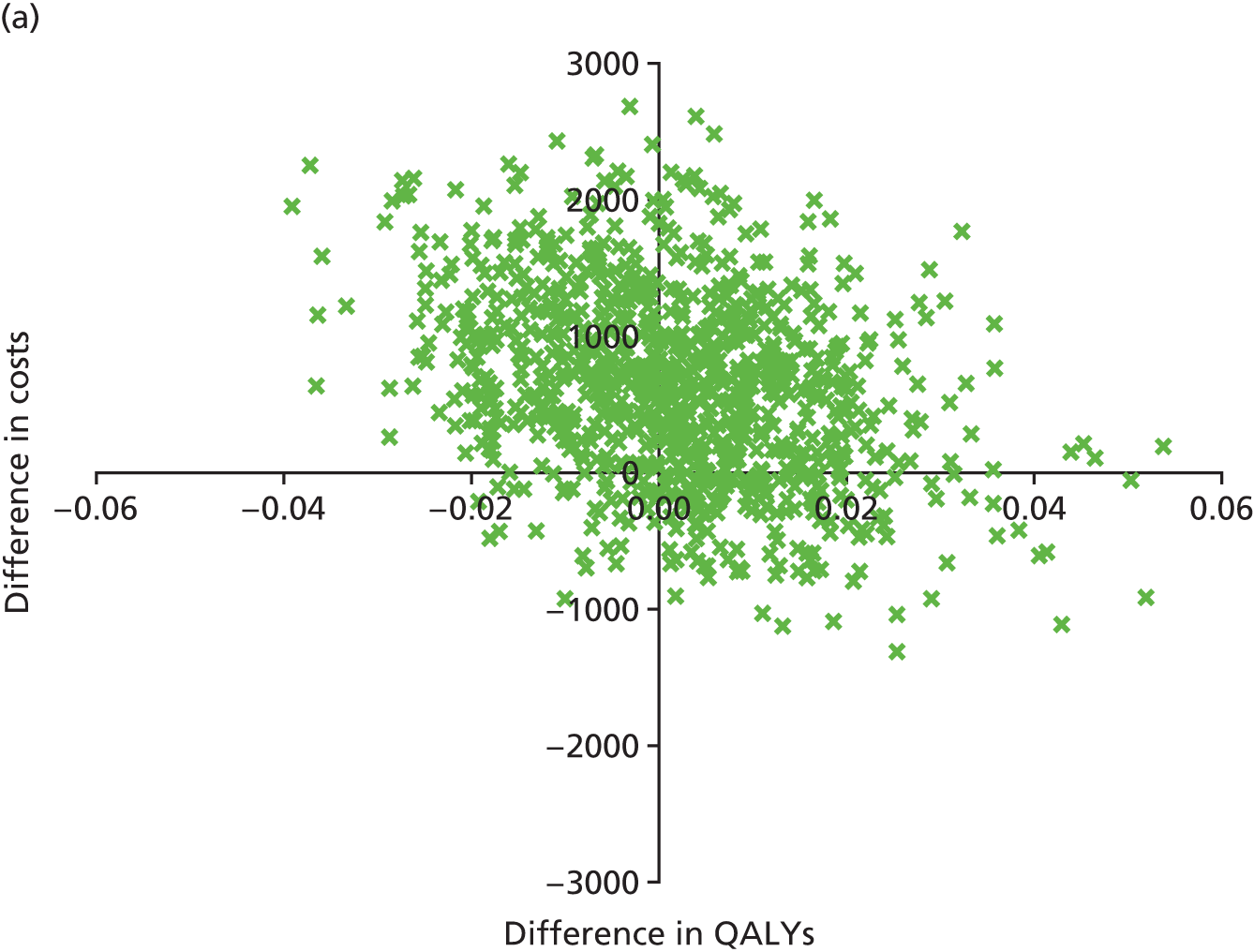
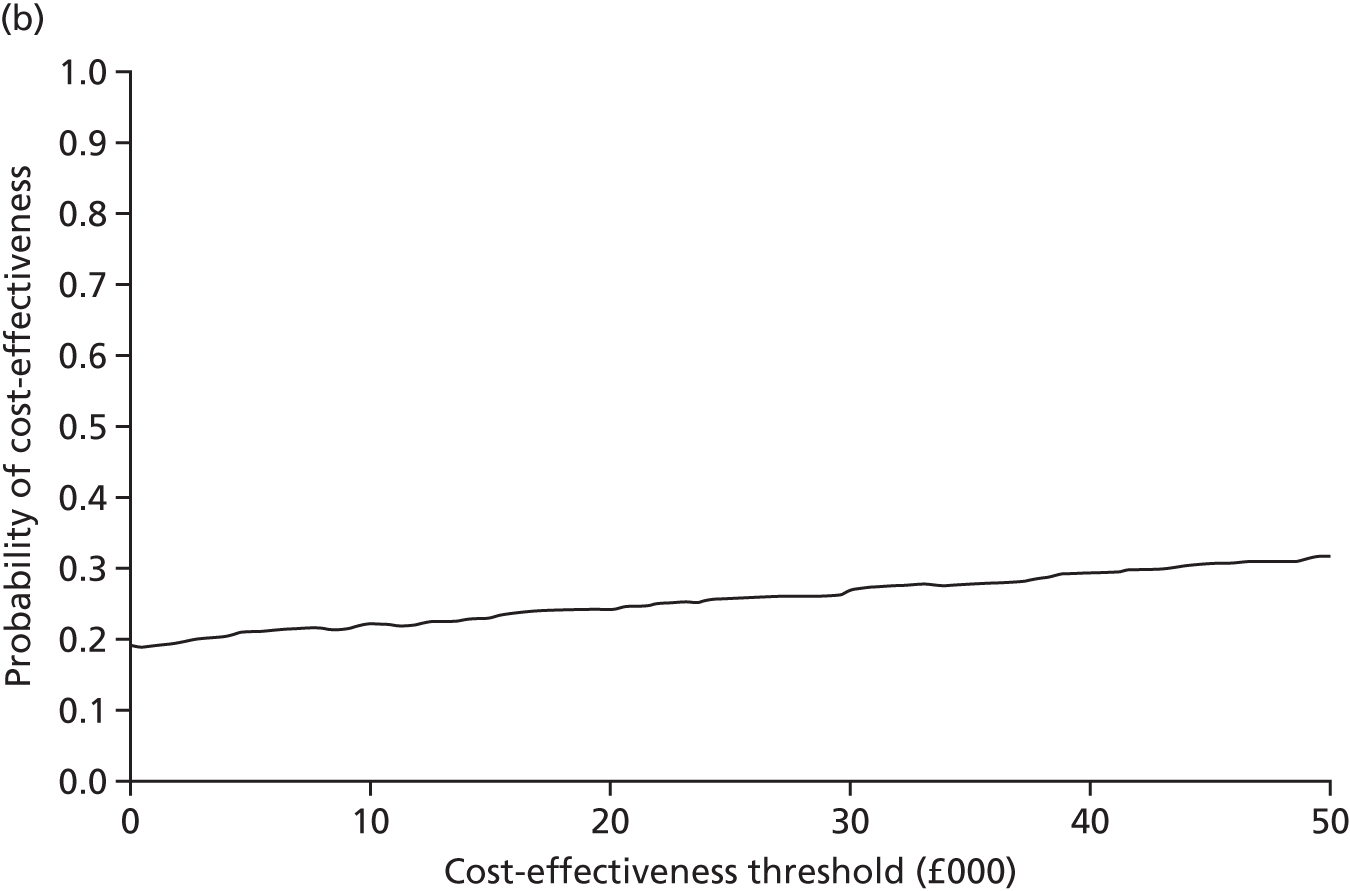
Quality-adjusted life-years calculated using the Short Form questionnaire-6 Dimensions
With QALYs calculated using the SF-6D utility measure rather than the EQ-5D-3L, NPWT was strictly dominated by standard dressings, meaning that NPWT resulted in both higher costs and worse outcomes, on average. The probability that NPWT is cost-effective did not exceed 13% (12.0% at a £20,000 per QALY cost-effectiveness threshold). The cost-effectiveness plane and CEAC for the analysis with QALYs calculated using the SF-6D is shown in Figure 13.
FIGURE 13.
Sensitivity analysis calculating QALYs using SF-6D rather than EQ-5D. (a) Cost-effectiveness plane; and (b) CEAC.
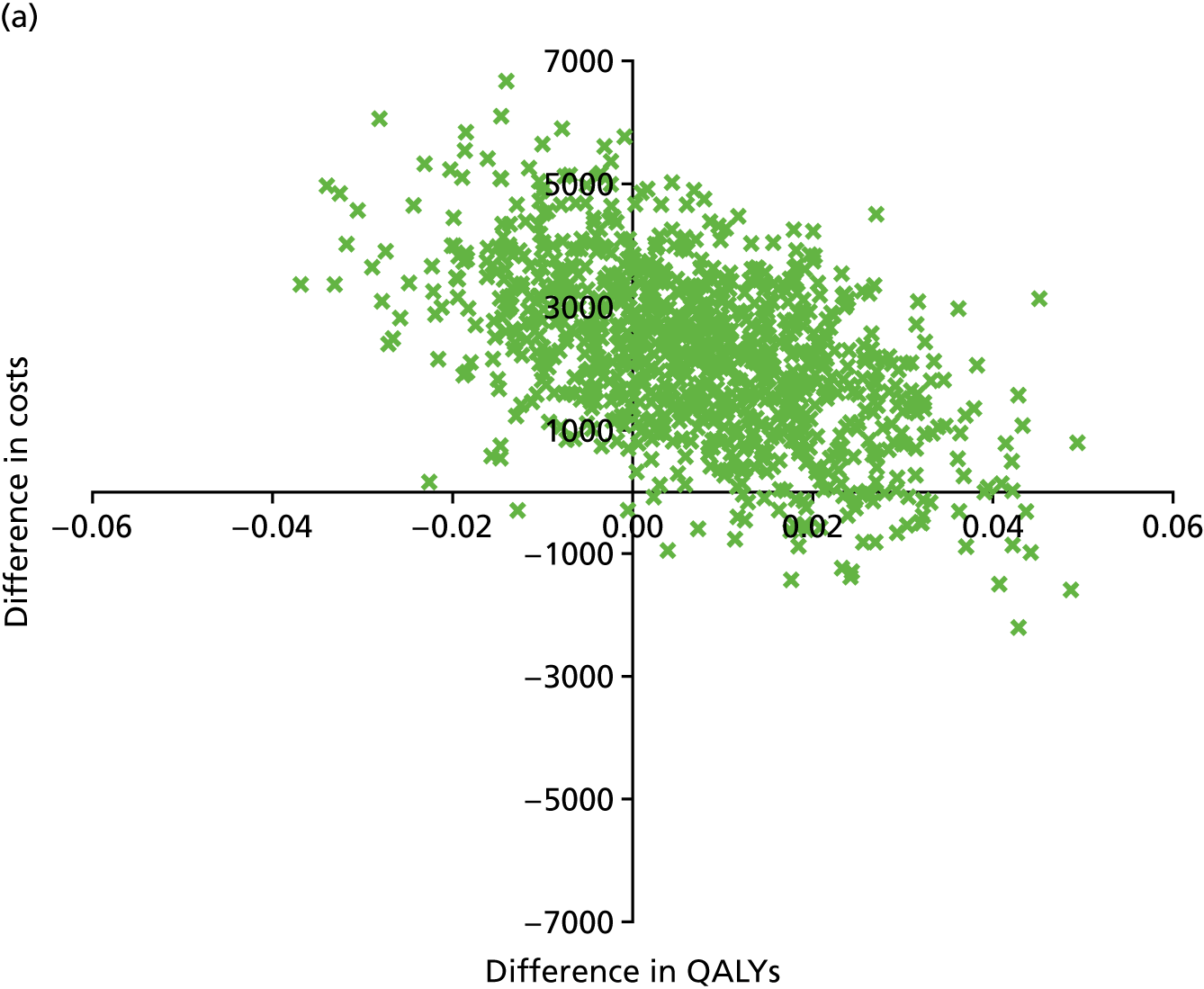
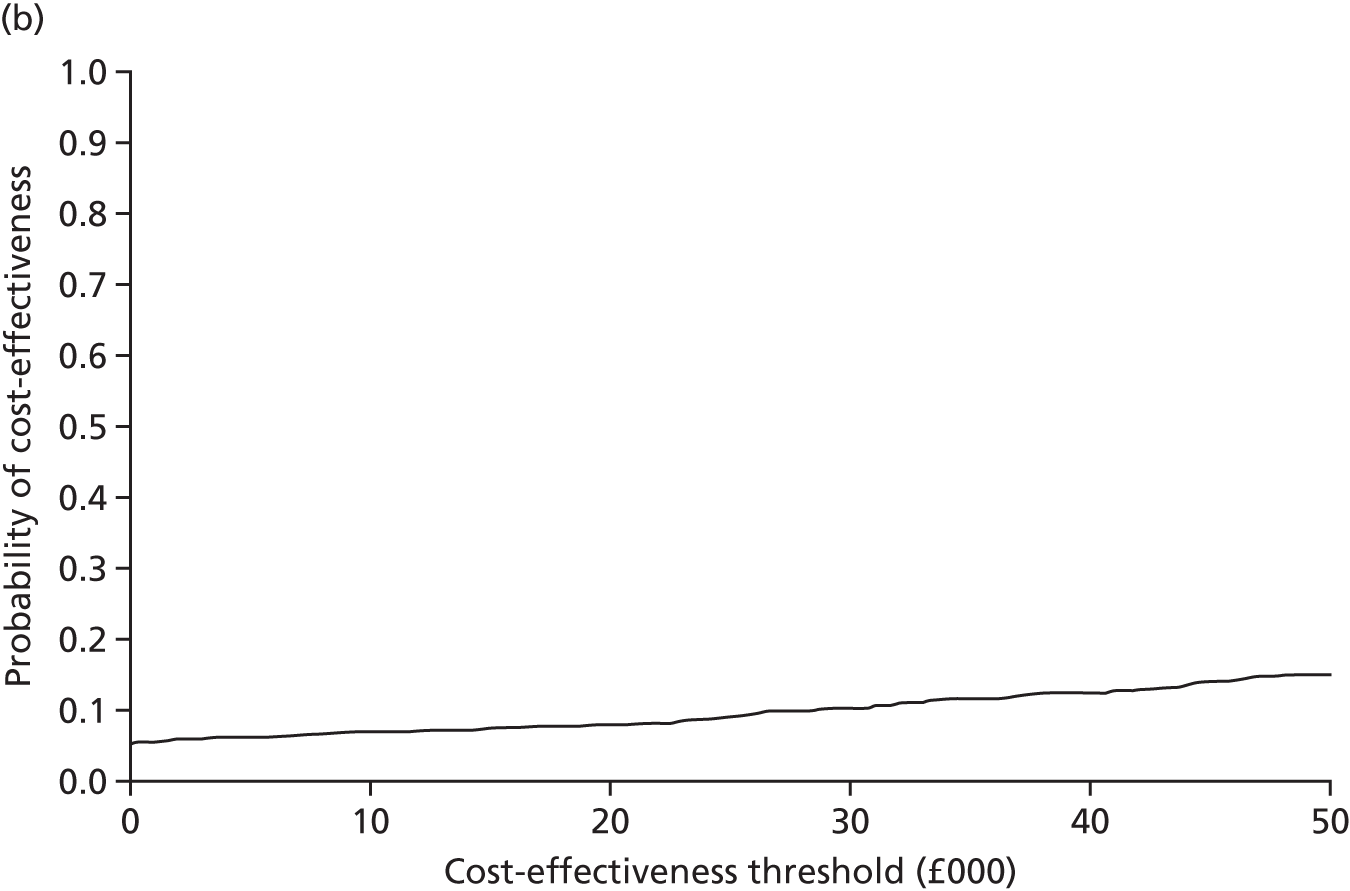
Complete-case analysis
The complete-case analysis restricted the analysis to individuals with complete data and resulted in a qualitative change in the direction of the results, with NPWT becoming dominant in health economic terms; that is, it generated both lower costs and higher QALYs, on average. The probability that NPWT is cost-effective was estimated as 71%, 72% and 74% at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY, respectively. The cost-effectiveness plane and CEAC for the complete-case analysis are shown in Figure 14. Although there was a clear difference in the results of the base-case analysis using multiply imputed data and the results of the complete-case analysis, this difference is plausible given the large number of missing data. For the base-case analysis, complete cases were available for only 31% of the total cases and logistic regressions on missingness of total QALYs and total costs support the MAR assumption.
FIGURE 14.
Sensitivity analysis restricted to complete case. (a) Cost-effectiveness plane; and (b) CEAC.
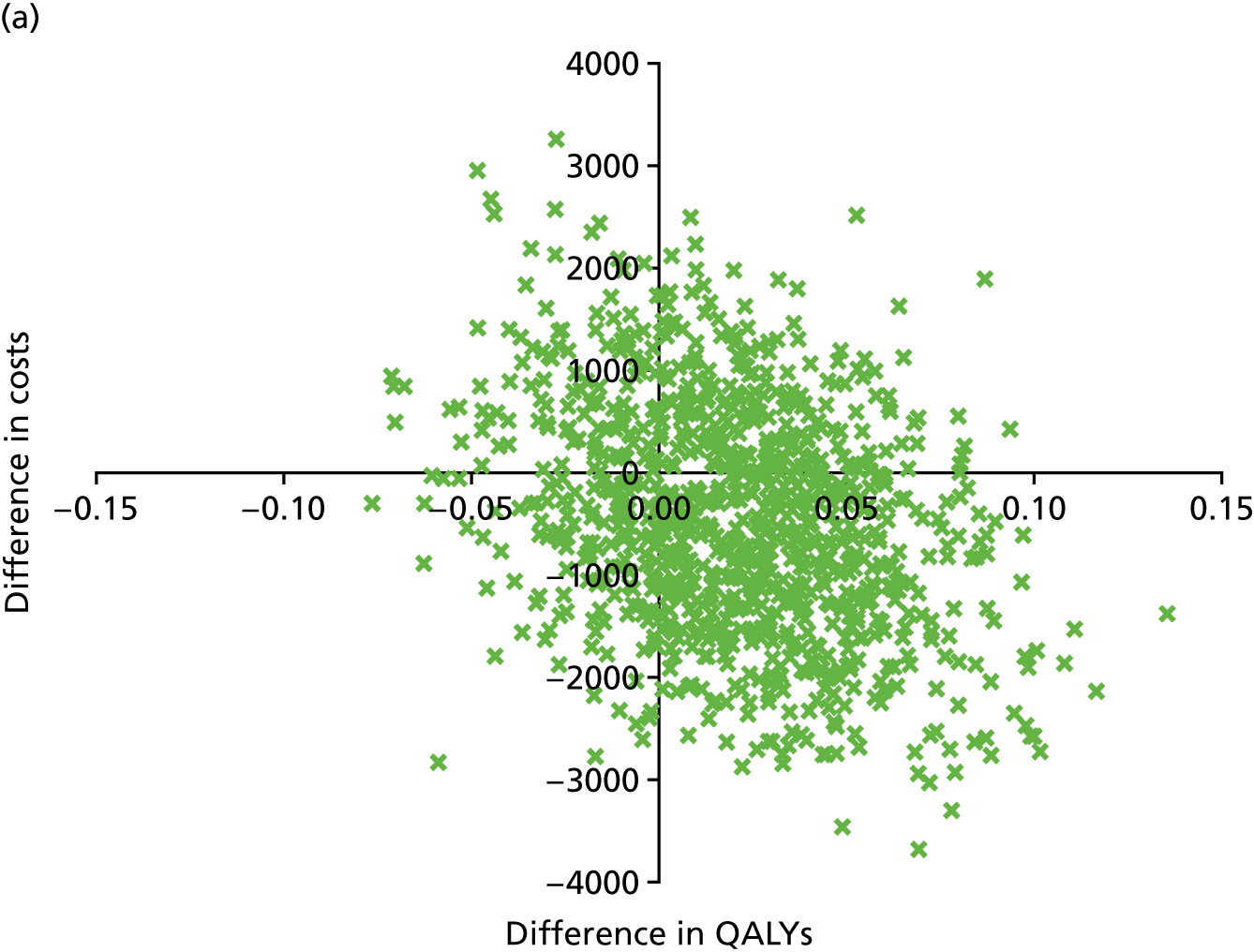

Subgroup analyses
A post hoc subgroup analysis was also carried out to explore the cost-effectiveness of NPWT in those participants with a deep infection. NPWT was dominated in this group of participants, generating increased costs and lower QALYs, on average. The probability of cost-effectiveness did not exceed 15% and, as such, the results suggest that NPWT is highly unlikely to be cost-effective in this subgroup. The cost-effectiveness plane and CEAC for the deep infection subgroup is shown in Figure 15.
FIGURE 15.
Deep infection subgroup. (a) Cost-effectiveness plane; and (b) CEAC.
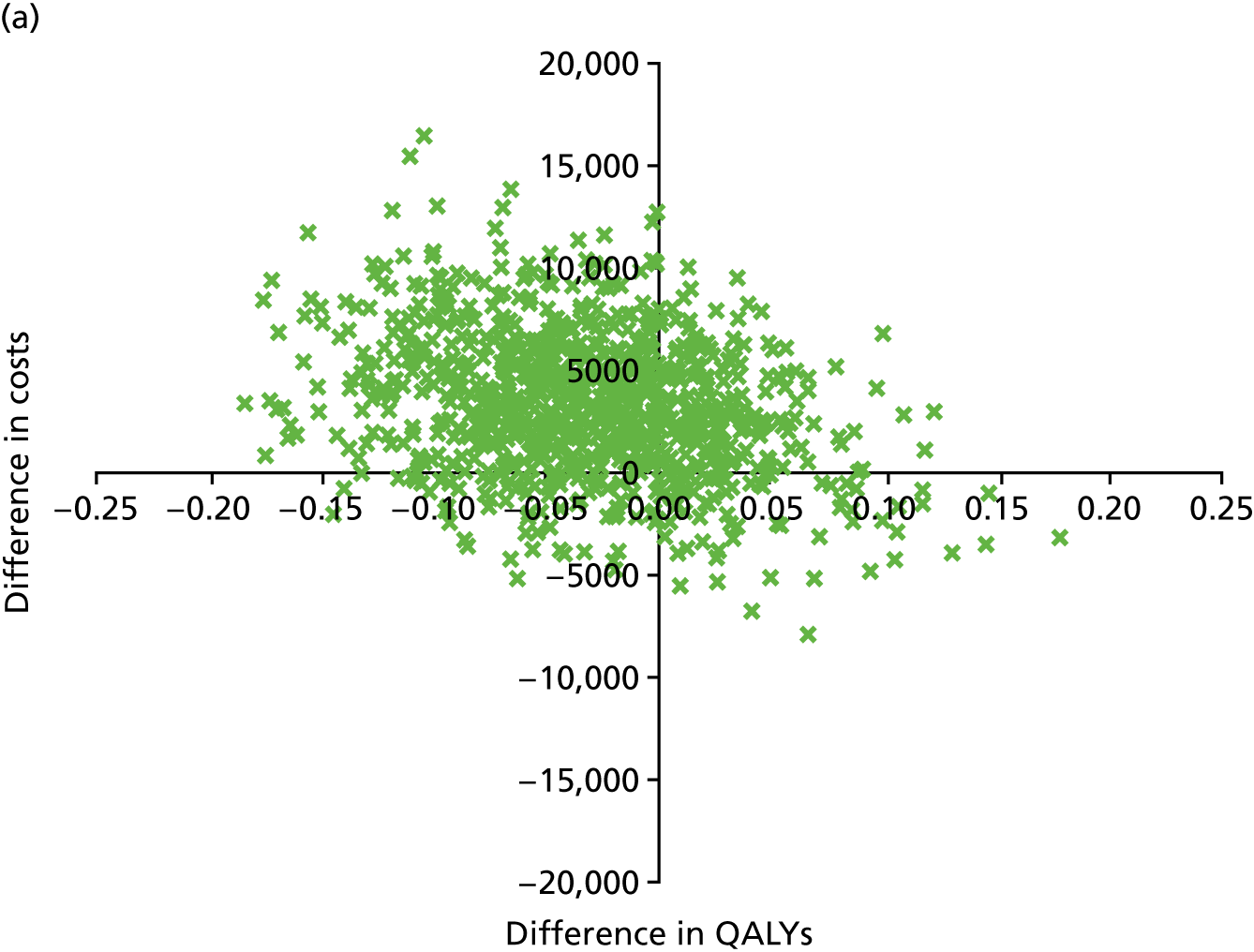

Chapter 6 Summary and discussion
Qualitative study
The integrated qualitative study (Chapter 3) was very important in the progression of the trial from the feasibility to the main phase of recruitment. In particular, it informed the process for consent into the trial in the context of acute trauma, emergency surgery and a potentially limb-threatening injury. There were two related components to the qualitative work: first, the patient’s experience of having an open fracture of the lower limb and its subsequent treatment and, second, the patient’s experience of taking part in the trial under these difficult circumstances.
All of the patients involved in the interviews experienced a strong sense of vulnerability caused by the traumatic injury. The extent of this emotional as well as physical vulnerability, although recognised in previous studies, is brought home by the first-hand accounts described in the WOLLF study. There were several clear themes: (1) emotional fragility, closeness to death and the loss of a limb and the processing of strong emotions while containing emotions for the benefit of other people; (2) the state of being injured and experiencing a changed sense of self as participants became a person with wounds, were constrained by their broken body and lived with pain; and (3) living with injury and reimagining how home and work life will be in the future. These themes underline the need for support that focuses on these areas: support in both the acute phase of treatment in the hospital, but also on-going support following discharge into the community.
Despite the very serious nature of the injuries and the requirement for repeat operations and prolonged periods in hospital, both treatment groups expressed a clear desire to be involved in this clinical trial of emergency orthopaedic trauma.
With particular regard to the process of consent, the patients were comfortable with other people providing consultee agreement in lieu of their personal consent, owing to their physical/emotional state in the immediate period after the injury. Both groups had an overall faith in the team regarding their longer-term recovery and hopes for the future and trusted that they would not put them in a study that caused them harm. They expressed a clear view that they would like to be involved, to the limit of their capacity, in the decision-making process before surgery. However, they were comfortable with the formal written consent process taking place after surgery, when they had recovered full capacity and could consider all aspects of the trial. Although the great majority of the patients gave their consent to continue in the study, it was important to them that they had the option to withdraw at any time.
These findings were important in the trial-specific training that was provided during the main phase of the WOLLF study. Patients should be included in the decision-making process as much as possible, but formal written consent could, when appropriate, wait until after the initial surgery. This approach was also supported by the staff who took part in the associated focus group.
Another important theme, and one which has implications for other trials in emergency surgery, is the importance of the nature of the intervention under investigation. Both groups of patients felt that they did not really know the interventions as they had no prior experience of them. This was particularly the case for the group allocated to the NPWT intervention, although participants in this group generally felt that the ‘new technology’ was likely to be beneficial. This lack of prior knowledge is, of course, the case in most emergency trials. In terms of this particular intervention, the patients also felt that the type of dressing applied to the wound was of comparatively low importance at that time, in the context of their injury/injuries more broadly. This emphasises the importance of tailoring the trial process to the nature of the intervention. The involvement of patients and the public in the development of trial design, with particular reference to the consent process, is therefore of great importance.
Screening
Over 2400 patients were screened during the course of the trial, which is testament to the hard work of the clinical and research associate teams in the trial centres. As expected, primary closure of the open-fracture wound – that is, closure of the wound after the first surgical debridement of the open fracture – was by far the most common reason, accounting for just under half of all patients excluded. Other exclusion criteria were used much less commonly: injury sustained > 72 hours before presentation at a trial site (5%), G&A grade 1 injury (13%), patient unable to adhere to trial procedures (8.7%) and primary amputation of the limb (2%). Reassuringly, in terms of the external validity of the trial, only 3% of patients were excluded because of ‘surgeon decision’, and only 0.3% of these because of surgical preference for one dressing or another. Therefore, it is very unlikely that lack of surgical equipoise created a selection bias within the trial.
Interestingly, the rate of primary closure increased during the course of the WOLLF study, from 34.5% in 2012 to 56.6% in 2015 (see Figure 2), and this change of clinical practice was statistically significant (p < 0.001). Although the WOLLF study was not designed to investigate such a change in clinical practice, the study does provide an opportunity to quantify this increase in the rate of primary wound closure following an open fracture of the lower limb; most MTCs in the UK taking part in the trial. The trial coincided with the most significant change to the system for looking after patients with ‘major trauma’ that the NHS has known, namely the creation of the Major Trauma Networks in England in 2012. In the Major Trauma Network system, and for the first time in the UK, patients with serious traumatic injuries, including those with open fractures of the lower limb, bypassed the nearest hospital to be taken to a designated MTC. The MTC specification included the colocation of orthopaedic trauma surgeons and plastic surgeons, creating ’orthoplastic’ centres. These centres facilitated joint care between the two surgical specialties and greatly increased the volume of patients with open fractures seen in each centre, and hence the experience of the surgical and support teams. Traditional teaching in orthopaedic practice was that it is ‘dangerous’ to primarily close any wound associated with an open fracture of the lower limb, because of the high risk of infection. 2 A ’second-look’ wound debridement was advocated, usually at 48–72 hours, when any non-viable or contaminated tissue would have ‘declared’ itself and could be removed. This teaching was, at least in part, based on the fact that each individual surgeon in the 200 acute hospitals in England would see only one or two severe open fractures of the lower limb each year and, therefore, few surgeons would gain sufficient experience to be entirely confident in their primary wound debridement to the point at which they would close the open-fracture wound at the first visit to the operating theatre. With the development of the Major Trauma Network, the surgeons in each of these 22 adult MTCs would individually become much more experienced. This greater experience added to the fact that joint orthoplastic working enabled shared decision-making and therefore even more confidence in early radical wound debridement. While it is, to some degree, speculation, it seems likely that this great experience/confidence led to the increased use of primary wound closure noted during the course of the trial.
Declined to participate
In total, 625 patients were randomised into the WOLLF study. Of these, 460 were eligible and consented to participate in the trial. A total of 165 patients did not consent to enter the trial: 85 in the NPWT group and 80 in the standard dressing group.
The patients who declined to consent, were not able to consent or were ineligible for inclusion in the study after initial recruitment (see Table 6) were generally older and more likely to be female. One of the exclusion criteria was that patients were unable to adhere to trial procedures, including filling out questionnaires, and this was most commonly because of permanent cognitive impairment. This may explain why the patients who did not consent were older than those who did consent. However, the number of patients in this category was small, so it seems more likely that older female patients were either less likely to survive their injuries, or simply less likely to agree to participate.
There were two main reasons for patients being unable to take part in the trial post randomisation.
First, owing to the emergency nature of the surgery, many patients went straight to the operating theatre before their eligibility for the trial could be definitively confirmed. For example, some patients were unconscious or had been anaesthetised before transfer to the hospital, so that, at the time of surgery, it was not clear whether or not they would be able to ‘adhere to trial procedures’. Therefore, it was anticipated that a number of patients randomised would subsequently be deemed ineligible to participate in the trial.
Second, and in keeping with the findings of the integrated qualitative study, we anticipated that a small number of patients who were not able to consent to enter the trial prospectively, owing to lack of capacity, would subsequently choose not to take part in the trial.
Regarding the first group, 140 out of the 165 patients who did not consent for the trial were found to be ineligible in the postoperative period and were therefore not consented to take part. Of these, 75 were in the NPWT group and 65 in the standard dressing group (see Figure 6). Some of the reasons for ineligibility were anticipated. These included 28 patients in each group who were deemed ‘unable to adhere to trial procedures’ or ‘unable to consent before 6 weeks’, most of whom had significant head injuries in association with their open fracture of the lower limb or were found to have permanent cognitive impairment which could not be identified preoperatively. In keeping with the major trauma sustained by patients in this trial, 14 patients died in the postoperative period and were never able to consent. However, of the ‘other’ reasons (see Figure 6), the great majority were actually related to failure of the surgical team to follow the trial procedures. Several sites, particularly at the beginning of the trial, chose to randomise the patients during surgery, rather than waiting until the end of the operation. This was understandable, as it allowed the operating theatre team to prepare the dressings in advance of the wound closure. However, the result was that several patients were randomised before a final decision about the open-fracture wound was taken. A total of 20 patients in the NPWT group and 18 patients in the standard dressing group went on to have a primary wound closure and were therefore no longer eligible for the trial. One patient in each group went on to have an amputation as the extent of the injury became clear during surgery. As this issue was recognised, the sites received further training and the number of patients randomised before the end of surgery reduced as the trial continued. When possible, all patients who were randomised into the trial but who were subsequently found to be ineligible were told about the trial but no other data were collected on these patients.
There is no reason to suspect that these patients, who were randomised but later found to be ineligible, created a selection bias within the trial.
Of more potential concern were the second group of patients who were eligible and were randomised, but who later declined to participate. If this group of patients was large, it could draw into question the external validity of the trial, that is, the ability to generalise the results of the trial to the broader population of patients with an open fracture of the lower limb. However, in keeping with the findings of the integrated qualitative study, the great majority of patients were happy to take part. In fact, only 25 potentially eligible patients declined to participate in the WOLLF study: 10 in the NPWT group and 15 in the standard dressing group. The most common reason for declining was that the patient ‘did not want to be part of a research project’ or ‘did not want to complete questionnaires’. Overall, 460 out of the 485 (95%) patients who were randomised and eligible for the trial agreed to participate in the WOLLF study. Therefore, we can be very confident that the patients who took part were representative of the population with open fractures of the lower limb.
Treatment according to allocation
There were 23 deviations (crossovers) from the allocated treatment: 15 in the NPWT group and eight in the standard dressing group. Eight of these (four in each group) were because of miscommunication between the surgical team and the operating theatre staff and three were caused by lack of equipment. However, nine cases in the NPWT group and three in the standard dressing group were because of interoperative surgeon preference, which may reflect a lack of equipoise. However, as this number was a very small proportion of the total number of patients we did not expect it to affect the results of the trial (i.e. we did not anticipate a difference between the ITT and secondary per-treatment analysis).
Recruitment by centre
There was considerable variation in the rate of recruitment by centre. The fastest rate of recruitment was in Bristol MTC, at just under two patients per month. Other units struggled to recruit to the trial. In some cases, this was caused by the increasing effects of the Major Trauma Network service reconfiguration. For example, the only centre to withdraw from the trial was Reading after a Network decision that all patients with a suspected open fracture of the lower limb were to bypass the Royal Berkshire Hospital and go directly to the regional MTC in Oxford. In general, recruitment in the ‘Trauma Units’ reduced over time as more patients with an open fracture of the lower limb were diverted to the regional MTCs as they matured. In some centres, the slow rate of recruitment was largely caused by staffing issues in the local research team.
However, more pertinently, the breakdown of recruited participants by centre and G&A grade showed good balance between treatments both across and within centres (see Table 6).
Baseline characteristics of the two groups
Overall, the baseline characteristics of the two groups of participants were very well balanced by the randomisation process.
Patients
The largest group of patients in the WOLLF study were young men, with the most common age group being in their 20s. This fits with the idea that younger men are more likely to suffer the sort of high-energy trauma associated with open fractures of the lower limb, the sort of trauma that can cause severe injuries even in the presence of normal bone quality. Over half of all the open fractures were caused by road traffic accidents, many of which involved motorcycles.
Only 30% of the open fractures occurred in women. However, in this group, the most common age group was in their 70s. This suggests that the mechanism of injury is different in women, being more likely to be low-energy trauma in association with osteoporosis (brittle bones).
Fractures
Over 80% of all of the open fractures in the trial occurred in the tibia. The tibia is subcutaneous for its entire length from the knee down to the ankle (i.e. there is very little soft tissue covering the tibia so the bone is more likely to penetrate the overlying skin when it is broken). The next most commonly broken bone was the femur, closely followed by the bones in the foot.
Just over one-third of the patients in the trial required concomitant surgery for other injuries sustained at the time of the open fracture. This highlights the fact that the majority of patients sustained high-energy trauma, during which other areas of the body were also likely to be injured. The most common other areas requiring surgery were the contralateral lower limb (≈17%) and another part of the ipsilateral limb (≈12%), followed by upper limb injuries in around 10% of patients. Head (≈3%), spine (≈2%), chest (≈1%), abdominal (≈2%) and pelvic (≈4%) surgery were much less common.
Surgeons and surgery
Two-thirds of operations were performed by consultant surgeons, with the majority of the other procedures performed by trainees under the supervision of consultant surgeons. Associate specialist surgeons performed < 5% of operations in each treatment group. In keeping with the principle of joint orthoplastic surgical operating, there was a median of two surgeons involved in each case. On average, the primary wound debridement and associated surgery took 2 hours. However, as expected given the wide range of injuries and variable requirement for fixation of the broken bones, there was considerable variation around this 2-hour average operating time.
Current clinical guidelines8 suggest that definitive fixation of the bone involved in an open fracture should be performed at the same time as definitive closure of the wound. Therefore, it is no surprise that the most common form of initial fixation was external fixation, this being the common ‘temporary’ method to hold the position of the broken bone pending definitive internal fixation at a second sitting when the wound was closed.
A total of 47% of patients in both groups had ‘half-pin’ external fixation at their initial surgery. Half-pins were generally used for temporary fixation rather than ‘fine-wire’ external fixators (see Table 7), with the latter being more commonly used for definitive fixation. Table 11 shows that in the great majority of patients treated with a half-pin external fixator at the initial surgery this was later converted to definitive internal fixation at the second procedure when the wound was closed. In some cases, external fixation may be the definitive form of fixation. This most commonly involved ‘fine-wire’ fixation. However, in only 1.3% of the NPWT group and 4.7% of the standard dressing group was fine-wire fixation the first operation. These figrures rose to 8.1% and 14.7%, respectively, at the second operation. This probably reflects the fact that application of circular frames using fine-wires is technically demanding (not all surgeons perform this procedure) and can be time-consuming, so surgeons are less likely to perform fine-wire external fixation in the emergency setting of the first wound debridement. Interestingly, despite the current NICE guidelines,8 half of the patients in both groups had definitive internal fixation at their first operation. This may reflect the fact that the WOLLF study started before the guidelines were developed, so surgeons may have been unaware of the evidence behind this particular NICE guideline. In keeping with the fact that the most commonly injured bone was the tibia, the most common type of internal fixation was intramedullary nail (around 22% in both groups), followed by plate and screw fixation (around 15%). It is important to note that the choice of initial fixation took place before the patient was randomised, so it did not influence the choice of dressing at the end of the procedure.
As anticipated, there was also considerable variation in the type of procedure used to close the wound definitively (see Table 11). The most commonly used option was a free flap – this option was used in 40% of patients in the NPWT group and 35% of the standard dressing group. Free flaps are a technique whereby tissue is transported from a site away from the zone of injury into the open-fracture wound together with its own blood vessels. These blood vessels are then ‘anastamosed’ (sewn into) the local blood vessels to restore blood supply. The formation of free flaps is a technically demanding procedure and may take several hours to perform but, providing that the local blood vessels are intact, a free flap can be used to cover essentially any type of wound. Local flaps were used in 15% of NPWT patients and 14% of the patients in the standard dressing group. Local flaps, as the name would suggest, involve moving tissue locally using that tissue’s existing blood supply. Although generally less time-consuming, the surgeon’s ability to use a local flap depends on how much damage there has been to the tissues around the open-fracture wound. The amount of damage may not always be obvious, so local flaps are considered less reliable than free flaps in many cases. Approximately 20% of both groups had a simple split skin graft. A split skin graft involves taking a partial thickness area of skin from a part of the body outside the zone of injury and transporting it into the area of the open fracture. A split skin graft does not need its own blood vessels so it is a relatively simple and quick procedure. However, it can be used only if there is healthy muscle covering the broken bone, as split skin grafts do not heal when applied directly to the surface of bone. Therefore, split skin grafts are generally used only in less severe open fractures. The majority of the remaining patients underwent delayed primary closure of their wound (i.e. the surgeons were able to sew together the edges of the open-fracture wound without the need for a graft). In the context of the WOLLF study, there was no statistically significant difference in the proportion of patients having skin grafting or primary closure of the wound.
In some areas of the world, NPWT is used for prolonged periods of time following an open fracture of the lower limb, the theory being that NPWT may reduce the size of the wound over time and, therefore, reduce the need for complex free-tissue transfer surgery. This is particularly the case in the developing world, where access to the resources and skills needed for flap reconstruction is limited. Since the start of the WOLLF study, there have been two other reports of the use of NPWT in association with open fractures. The first trial compared two types of fixation for one particular fracture (heel fracture) rather than comparing NPWT with another type of dressing and, therefore, is not relevant to this research question. 102 The second trial103 did, however, compare NPWT with standard dressings. This single-centre trial randomised 90 patients into two groups and followed them for 1 month only. The ‘rate’ of wound healing (defined by the ‘wound surface area’) was noted to be quicker in the group treated with NPWT, but there was no difference in the incidence of infection. This trial describes the use of NPWT over a prolonged period of time, as described in the following text:
Wounds were examined weekly and following, measurements were recorded presence of granulation tissue, wound bed becomes redder, decrease in wound drainage, and decrease in dimensions of wound.
Arti et al. 103
This trial also describes when the use of NPWT was stopped:
V.A.C Therapy [a trade name for NPWT] was terminated when adequate granulation base was achieved allowing for change to conventional dressing, split-thickness skin graft, or flap closure.
Arti et al. 103
This is a very different use of NPWT from that advocated by the BOAST7 and NICE8 guidelines for the management of open fractures, in which early definitive wound closure (within 72 hours) is recommended. It is unlikely that NPWT applied for < 72 hours would have led to a reduction in the size of the open-fracture wound and hence an increase in the use of simple split skin grafting or primary closure. This is borne out in the trial results; if anything, fewer patients in the NPWT group (16%) had primary closure of their open-fracture wound than in the standard dressing group (20%). The length of follow-up in the Arti et al. 103 trial was short and no patient-reported outcomes were described. Therefore, although that trial ostensibly addresses the same question as the WOLLF study, it is not clear whether or not the results are pertinent to the UK NHS or indeed other health-care systems.
Follow-up rate
Inevitably, some patients did not complete the study. The largest group of participants were men aged < 40 years, who are not normally considered to be the most reliable demographic for returning study questionnaires. However, thanks to the efforts of the trial administration team, the number of patients lost to follow-up in the WOLLF study was actually small. A total of 86 study participants did not provide DRI data at the 12-month study end point and 33 participants withdrew from the study after consenting but before the study end point; therefore, based on the available participants, the study is 88% (374/427) complete.
Outcomes
Primary outcome
The primary outcome measure for the WOLLF study was the DRI. The DRI allowed the study participants to make their own assessment of their disability between 0 and 100 points, where 0 represents no disability and 100 represents complete disability. The primary end point for the trial was 12 months. Baseline (retrospective, pre-injury) DRI was also assessed, as was earlier outcome at 3, 6 and 9 months.
Table 12 shows observed means and SDs for the DRI and Figure 6 shows the full data distributions and trends in the mean scores. At baseline, pre injury, the majority of patients reported that they had no disability or only minor disability. However, a few patients had quite significant pre-existing disability and so the mean DRI before the open fracture was 12 points. This correlates with the fact that, although the open fractures occurred most commonly in young fit patients, older patients and those with significant pre-injury disability were also affected. The DRI scores improved in the 12 months following the open fracture. However, in keeping with the existing literature,1–4 patients still had considerable disability at 1 year after this very significant injury. At 3 months, the mean DRI score was 65 points, improving to 52 points at 6 months, 47 points at 9 months and finally 44 points at 12 months.
The covariates used to adjust the treatment effect estimates generally showed strong statistical significance, indicating that the inclusion of these terms improved the overall model fit. For example, a higher pre-injury DRI was associated with a higher 12-month DRI (p < 0.001). Participants aged ≥ 40 years had a higher 12-month DRI than those aged < 40 years (p < 0.001). As per the existing literature, the 12-month DRI was higher for those participants with a G&A grade of 3, or 3 with vascular involvement, than for those participants with a G&A grade of 2 (p = 0.047). 2
The main results of the WOLLF study is that there was no evidence of a difference in the DRI at 12 months between those patients treated with NPWT and those treated with standard wound dressings. The mean DRI in the NPWT group was 45.5 points (SD 28.0 points), compared with 42.4 points (SD 24.2 points) in the standard dressing group, giving a difference of –3.9 points (95% CI –8.9 to 1.2 points) in favour of standard dressings (p = 0.132). As the MCID for the DRI is 8 points, we conclude that it is extremely unlikely that NPWT dressings confer a clinically important difference in DRI scores for patients with an open fracture of the lower limb. Similarly, there was no evidence of a difference in DRI scores at 3, 6 or 9 months.
A systematic review of the literature before this trial showed only one RCT comparing standard wound dressing with NPWT for patients with open fractures of the lower limb. Stannard et al. 14 did not report a Disability Rating so there is no existing evidence with which to compare this result.
Pre-planned subgroup and secondary analyses of the Disability Rating Index
The secondary PP (per treatment) analysis did not differ from the primary ITT analysis, and the difference between groups was –4.0 points (95% CI –9.1 to 1.0 points) in favour of the standard dressings with p-value of 0.119. This was as expected because the number of patients who crossed over (i.e. did not receive the treatment allocated) within the trial was small.
As noted above, the number of participants who did not complete the trial was small. However, we performed a secondary analysis using imputation to account for missing data (see Table 37, Appendix 2). As expected, the results were very similar to those from the complete-case analysis, indicating that missing data do not affect the result of the trial.
Secondary outcome measures
Health-related quality of life
Health-related QoL was measured using the EQ-5D-3L utility score, EQ-5D VAS score, physical health score (PCS from SF-12) and mental health score (MCS from SF-12) in the 12 months post randomisation. Scores for all outcome measures, other than mental health score, improve significantly in both groups, indicating improved physical health but participants never recover to pre-injury levels. This is in keeping with the results of the primary outcome measure of Disability Rating. Mental health score, as measured by MCS, does not vary much in the 12 months following injury, but is always marginally lower than pre-injury levels. As per the results of the integrated qualitative research presented in Chapter 3, it may be that physical recovery following an open fracture of the lower limb proceeds more rapidly than recovery in mental health.
The analysis of the HRQoL data by treatment group corroborates the analysis of the primary Disability Rating outcome. There is no evidence to support statistically significant differences between treatment groups in any of these measures, at any time point in the first year after injury.
A systematic review of the literature identified only one RCT14 comparing standard wound dressing with NPWT for patients with open fractures of the lower limb. Stannard et al. 14 reported HRQoL using the Short Form questionnaire-36 items at 3-, 6-, 9- and 12-month follow-ups and final follow-up. They found:
There were no significant differences in the mental component score between the 2 groups at any time point, and there was no significant difference between the groups in PCS for patients who did not develop an infection.
The authors also found that:
However, there was a significant difference at 3 months (P = 0.013), 6 months (P = 0.049), and 9 months (0.005) after injury in favor of a higher PCS in patients randomized to receive NPWT.
Clearly, the rate of deep infection noted in that trial had a large influence on the physical component of HRQoL. This is discussed further in Complications and Complications local to the open fracture.
Complications
When interpreting the primary outcome of any trial, it is important to take account of the complication profile of the two interventions. This is particularly important in the WOLLF study as the interventions under investigation are specifically designed to improve wound healing and thereby reduce the risk of deep infection at the site of the open fracture. We have broken these AEs down into ‘complications local to the open fracture’, most notably infection, ‘systemic complications of the open fracture or its treatment’ and ‘unrelated adverse events’, during the 12 months after the injury.
Complications local to the open fracture
Deep surgical site infection
In total, 35 out of the 460 (7.6%) participants in the WOLLF study had a deep SSI. From any perspective, this rate of deep infection is high, but it is lower than in most other published series of severe open fractures of the lower limb. 2–4 Although comparisons with other series in the literature should be made with caution, given the different mechanisms of injury and health-care systems, this relatively low rate of deep infection could be considered ‘encouraging’. It is tempting to attribute this to the development of multidisciplinary orthoplastic units and the Major Trauma Network in England. However, longitudinal investigations would be required to investigate this relationship more thoroughly.
With regard to the rate of deep SSI by treatment group, 16 (7.1%) participants in the NPWT treatment group and 19 (8.1%) participants in the standard dressing group had a deep infection. Mixed-effects logistic regression showed no evidence that the rate of deep SSI differed between treatment groups [estimated OR 1.18 (95% CI 0.59 to 2.37), with p-value from ANOVA F-test 0.638].
As noted earlier, a systematic review of the literature identified only one other RCT14 comparing standard wound dressing with NPWT for patients with open fractures of the lower limb. Stannard et al. 14 demonstrated a reduction in the rate of deep wound infection in the group of patients treated with NPWT compared with control [5.4% vs. 20%; RR 0.199 (95% CI 0.05 to 0.87)]. However, this was a small trial (59 patients, 63 fractures) and there were only seven deep infections in the control group and two in the NPWT group. It is possible that the different rate of deep SSI found in that trial was due to systematic differences in the patients and/or the treatment pathway in a single centre in the USA; the WOLLF study, in contrast, took place in the much broader setting of 24 centres in the UK. However, given the relatively small number of cases in the Stannard et al. trial,14 it is perhaps more likely that the result represents a lack of precision in the estimate of the incidence of deep infection.
Other complications local to the open fracture
In keeping with the data for deep SSI, the WOLLF study found no evidence to suggest that numbers of other local related complications differed between treatment groups (see p-values in Tables 16 and 18).
There was no difference in the numbers of superficial SSIs. As expected, superficial infections were more common than deep infections. In total, 68 out of the 460 participants (14.8%) had a superficial SSI according to the CDC criteria: 35 (15.5%) in the NPWT treatment group and 33 (14.1%) in the standard dressing group. In the WOLLF study, we used photographs of the wound as a supplementary assessment of infection. The correlation between photographs and other criteria for the diagnosis of infection requires further investigation. However, it is reassuring that the estimate of the rate of superficial infection on the photographs was similar to that estimated using the more usual clinical criteria: 27 (15.4%) in the NPWT group and 31 (17.2%) in the standard dressing group.
In the context of severely injured patients, the number of deep-vein thromboses/pulmonary emboli was low: six in the NPWT group and four in the standard dressing group. As the wound debridement and fixation (albeit temporary in some cases) took place before the allocation of treatment, there was no reason to suspect that the incidence of soft-tissue complications [e.g. nerve or tendon damage, or early failure of fixation (within 6 weeks)], malunion or non-union would differ between groups and, indeed, no difference was observed in the trial. Thirty-nine participants had radiographic evidence of non-union at 12 months, 21 in the NPWT group (9.3%) and 18 in the standard dressing group (7.7%).
Patients with an open fracture of the lower limb often require more than one further operation, either as part of the primary debridement and fixation of the wound and open fracture or in relation to local related complications such as non-union or infection. This was also the case in the WOLLF study, with some patients requiring repeat surgery. The most common reasons for reoperation were as expected. Removal of metalwork (either internal fixation or removal of external fixation under anaesthetic) was required in 44 participants: 18 in the NPWT group and 26 in the standard dressing group. Revision of the wound coverage was relatively rare, occurring in 33 patients, but only six in each group required a free flap. This may also reflect increased experience and improved decision-making in the new orthoplastic centres discussed earlier. Ten patients required amputation of the injured limb: four in the NPWT group and six in the standard dressing group. This is lower than in other published series,104 but it should be remembered that patients who underwent primary amputation at the first wound debridement (37 patients in the screening log) were not eligible for the trial (i.e. patients with unreconstructable leg trauma were excluded from participation). However, two of the patients who underwent amputation later died of complications that may have been related to the amputation.
Overall, there was no evidence to suggest that the number of related reoperations differed between treatment groups (see p-values in Table 19). Mixed-effects logistic regression showed no evidence that overall revision fixation rates differed between treatment groups [8.0% in the NPWT group and 6.4% in the standard dressing group (see Table 19); estimated OR 0.78 (95% CI 0.38 to 1.60), with p-value from ANOVA F-test 0.494].
Systematic complications
As noted previously, open fractures frequently occur in the context of patients suffering multiple injuries. It may be difficult to separate the systemic effects of an open fracture of the lower limb from the effects of trauma to other areas of the body. For the purposes of the trial, we took a conservative view and presumed that systemic complications, including death, were related to the open fracture and its treatment, unless demonstrably otherwise. Seven participants died of systemic complications during the 12 months after the injury.
Unrelated adverse events
Medical complications included the treatment of chest and urinary infections, etc. These were the most common systemic complications occurring in 40 patients: 19 in the NPWT group and 21 in the standard dressing group. There were relatively few psychiatric referrals, which is somewhat surprising given the patient experiences documented in Chapter 3. This may reflect the lack of psychiatric/psychological support services available to trauma patients, rather than a lack of need.
Surgical complications included any return to the operating theatre for treatment other than to the open fracture. These were common, occurring in 143 participants: 68 in the NPWT group and 75 in the standard dressing group. Some of these were for the treatment of injuries sustained at the same time as the open fracture, but most were entirely unrelated, for example elective removal of gallstones. There was no evidence to suggest that the number of unrelated reoperations differed between treatment groups.
In total, 10 participant deaths were reported during the study: six in the NPWT group and four in the standard dressing group. As noted previously, two deaths were related to complications following amputation of the limb with the open fracture and so were deemed related and local and seven were possibly related but systemic (chest infection, pulmonary embolus, etc.). Only one death was considered completely unrelated. That patient died in a house fire.
Health economic evaluation
The health economic evaluation shows that costs for individual participants varied greatly across the WOLLF study. Patients who underwent a successful primary delayed wound closure and definitive fixation of their fracture with no complications incurred low costs, but patients requiring free-flap surgery or repeat surgery for complications incurred dramatically increased costs. This is in keeping with previous studies that reported that complex soft-tissue reconstruction procedures and complications, including amputation, greatly increased morbidity and cost in the context of open fractures. 105
The index hospital admission costs were, to a large degree, determined by the primary operative procedure associated with that admission, that is, the most resource-intensive procedure. The most common primary procedure was free flap, which is also the most expensive. In terms of the choice of fixation, the only statistically significant difference was in the number of participants receiving ‘primary open reduction of fracture of long bone and extramedullary fixation using plate of hip’ as their primary procedure (p = 0.005). However, as temporary external fixation is hardly every used in the region of the hip joint, the decision to use a plate fixation was almost certainly made before the patient was randomised (i.e. any difference is by chance). With the exception of the cost of the initial inpatient stay (including costs associated with dressings and dressing changes), there were no statistically significant differences in costs between the trial arms in any cost category. For the initial patient stay, mean costs were £1223 higher in the NPWT arm (p = 0.030).
However, over the entire follow-up period, there was no evidence of a difference in costs; mean total NHS and PSS costs were £14,079 in the NPWT group, compared with £14,002 in the standard dressing arm, generating a mean cost difference of just £77 (bootstrap 95% CI –£2114 to £2925).
Cost-effectiveness
Given that there was very little difference in HRQoL outcomes measured during the 12 months after the injury and little difference in cost, the cost-effectiveness analysis is very clear. The base-case analysis used multiply imputed data and produced an ICER of £267,910 per QALY gained, reflecting higher costs on average and marginally higher QALYs, on average, in the NPWT arm. The probability that NPWT was cost-effective was low.
The conclusion from the base-case analysis is not altered by the various sensitivity analyses. When broader social costs are included (e.g. time off work), the ICER increases to £282,858 per QALY gained. With QALYs calculated using the SF-6D rather than the EQ-5D-3L, NPWT was strictly dominated by standard dressings, meaning that NPWT resulted in both higher costs and worse outcomes. The only doubt is raised by the complete-case analysis, for which NPWT generated slightly lower costs and slightly higher QALYs, on average. The probability that NPWT is cost-effective was estimated as 72% at cost-effectiveness thresholds of £20,000 per QALY in the complete-case analysis. However, under the MAR assumption, it is multiple imputation that produces unbiased estimates of treatment effect. 106 Given that complete health economic data were available in only 31% of cases, it is thus reasonable and unsurprising that the complete-case analysis produced different results, as this analysis is based only on a subset of the data and a subset for which missingness could be predicted from the other covariates, rather than being missing completely at random. Therefore, it is the base-case analysis using multiple imputation that makes use of all the relevant health economic data and that, under the MAR assumption, provides an unbiased estimate of the cost-effectiveness of NPWT dressings.
Limitations
There are, of course, some limitations to the trial. A total of 625 potentially eligible participants were randomised into the trial, but only 460 were eligible and able to provide informed consent. This could pose a risk to the external validity (generalisability) of the trial. However, the great majority of these patients were found to be ineligible after randomisation owing to, for example, primary closure of the wound or permanent cognitive impairment that could not be recognised before surgery/randomisation. In fact, only 25 potentially eligible patients actually declined to participate in the WOLLF study: 10 in the NPWT group and 15 in the standard dressing group. The most common reasons for declining were that the patient ‘did not want to be part of a research project’ or ‘did not want to complete questionnaires’. Overall, 460 out of the 485 (95%) patients who were randomised and eligible for the trial agreed to participate in the WOLLF study. Therefore, we can be confident that the patients who took part were representative of the population with open fractures of the lower limb.
Another possible limitation was post-randomisation crossover of patients from one group to the other. However, only 23 patients crossed over; therefore, 95% of patients received the treatment to which they had been allocated. A bigger concern in the early phases of the study was loss to follow-up. The largest number of participants were men < 40 years, who are not normally considered to be the most reliable demographic for returning study questionnaires. However, following the pilot stage of the trial we included e-mail and text message reminders to this group of patients and contacted those who preferred to do so by telephone. Thanks to the efforts of the trial administration team, the number of patients lost to follow-up in the WOLLF study was actually small. A total of 86 study participants did not provide DRI data at the 12-month study end point and 33 participants withdrew from the study after consenting but before the study end point. Therefore, based on the available participants, the study is 88% (374/427) complete. This is slightly less than the 90% predicted in the protocol but the 2% difference is highly unlikely to alter the result.
Chapter 7 Conclusion
Contrary to the existing literature and current clinical guidelines,7,8,13 NPWT dressings do not provide a clinical or economic benefit for patients with an open fracture of the lower limb. Future work should investigate alternative strategies to reduce the incidence of infection and improve outcomes for patients with open fractures of the lower limb. Two specific areas of potentially great benefit are (1) the use of topical antibiotic preparations in the open-fracture wound and (2) the role of orthopaedic implants with antimicrobial coatings when fixing the associated fracture.
Acknowledgements
Trial team
Trial management team
Professor Matthew Costa, chief investigator; Dr Nick R Parsons, trial statistician; Dr Juul Achten, research manager; Dr Julie Bruce, principal research fellow; Mrs Sonia Davis, trial co-ordinator; Professor Stavros Petrou, Professor of Health Economics; Ben Parker, health economist; Professor Sarah Lamb, Professor of Rehabilitation; Mrs Susie Hennings, senior project manager; James Masters, specialist registrar; Rishpal Rai, trial administrator; and Liz Weaver, data clerk.
Trial applicants
Professor Matthew Costa, chief investigator; Dr Juul Achten, coapplicant; Professor Sarah Lamb, coapplicant; Professor Keith Willett, coapplicant; Professor Stavros Petrou, coapplicant; Lt.Col. Steven Jeffery, coapplicant; Professor Damian Griffin, coapplicant; Dr Elizabeth Tutton, coapplicant; and Dr Julie Bruce, coapplicant.
Trial Steering Committee
Professor Matthew Costa, chief investigator; Professor James Mason, Professor of Health Economics; Professor Amar Rangan, Professor of Orthopaedic Surgery (independent member); Professor Mike Reed, consultant orthopaedic surgeon (independent member); Mrs Carole Beamish, lay member, quality assurance (independent member); Mrs Ceri Jones, manager, research and development, and UHCW (sponsor representative).
Data Management Committee
Professor Lee Shepstone, Professor of Medical Statistics (chairperson); Professor Simon Donell, consultant orthopaedic surgeon (independent member); and Mr Phillip Johnston, consultant orthopaedic surgeon (independent member).
Statistician
Dr Nick Parsons.
Health economists
Professor Stavros Petrou and Mr Ben Parker.
Radiological evaluation
Mr James Masters.
Programming team
Henry Adjei (Programmer, University of Warwick), Chockalingam Muthiah (Programmer, University of Warwick) and Adrian Willis (Senior Programmer, University of Warwick).
Research team
Principal investigators
Jill Arrowsmith, Gorav Datta, Mick Dennison, Mark Farrar, Peter Giannoudis, Andrew Gray, Philip Henman, Peter Hull, Umraz Khan, Charlotte Lewis, David Loveday, Jitendra Mangwani, Andrew McAndrew, Damian McClelland, Mick McNicholas, David Noyes, Ben Ollivere, Ian Pallister, Keith Porter, Manoj Ramachandran, Rory Rickard, Benedict Rogers, Hemant Sharma, Adel Tavakkolizadeh and Jonathan Young.
Research associates
Gokturk Abdulkerim, Shanaz Ahmad, Naheed Akhtar, Manjit Attwal, Sheeba Babu, Steven Barnfield, Conor Bentley, Jackie Berry, Natalie Blytt-Jordens, Racquel Carpio, Katherine Coates, Louise Clarkson, Carolyn Colvin, Bernadette Cook, Lauren Cooper, Shirley Cooper, Maria Dibua, Alisen Dube, Julie Foxton, Christina Haines, Ruth Halliday, Simone Hargreaves, Elizabeth Hawes, Jamila Kassam, Laura Latter, Maria Letts, Kathryn Lewis, Candice Matthews, Jess Nightingale, Ismail Patel, Sandra Owdziej, Tracey Potter, Carrie Ridley, Elizabeth Saunders, Karen Smith, Louise Spoors, Rosalyn Squire, Sylvia Turner, Ylenia Vigo, Maria Vincent and Lisa Wilson.
Contributions of authors
Matthew L Costa was the chief investigator and was responsible for study conception and design, clinical responsibility and writing and reviewing the report.
Juul Achten was responsible for study conception and design, developing the protocol, writing and reviewing the report, and was a member of the TMG.
Julie Bruce was responsible for developing the protocol, quality assurance of photographic review, writing and reviewing the report, and was a member of the TMG.
Sonia Davis was responsible for study management, writing and reviewing the report, and was a member of the TMG.
Susie Hennings was responsible for study management and was a member of the TMG.
Keith Willett was responsible for developing the protocol and writing and reviewing the report.
Stavros Petrou was responsible for developing the protocol, the health economic analysis, writing and reviewing the report, and was a member of the TMG.
Steven Jeffery was responsible for developing the protocol and writing and reviewing the report.
Damian Griffin was responsible for developing the protocol and writing and reviewing the report.
Ben Parker was responsible for performing the health economic analysis, writing and reviewing the report, and was a member of the TMG.
James Masters was responsible for independent clinical review of the outcome data and writing and reviewing the report.
Sarah E Lamb was responsible for developing the protocol, writing and reviewing the report, and was a member of the TMG.
Elizabeth Tutton was responsible for developing the protocol, developing the qualitative study design and conduct and writing and reviewing the report.
Nick Parsons was responsible for developing the protocol, developing the statistical analysis of the trial, writing and reviewing the report, and was a member of the TMG.
Publication
Costa ML, Achten J, Bruce J, Tutton E, Petrou S, Lamb SE, et al. Effect of negative pressure wound therapy vs standard wound management on 12-month disability among adults with severe open fracture of the lower limb: the WOLLF randomized clinical trial. JAMA 2018;319:2280–8.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Louie KW. Management of open fractures of the lower limb. BMJ 2009;339. https://doi.org/10.1136/bmj.b5092.
- Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 1976;58:453-8. https://doi.org/10.2106/00004623-197658040-00004.
- Pollak AN, Jones AL, Castillo RC, Bosse MJ, MacKenzie EJ. LEAP Study Group . The relationship between time to surgical debridement and incidence of infection after open high-energy lower extremity trauma. J Bone Joint Surg Am 2010;92:7-15. https://doi.org/10.2106/JBJS.H.00984.
- MacKenzie EJ, Jones AS, Bosse MJ, Castillo RC, Pollak AN, Webb LX, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am 2007;89:1685-92.
- Court-Brown CM, Rimmer S, Prakash U, McQueen MM. The epidemiology of open long bone fractures. Injury 1998;29:529-34. https://doi.org/10.1016/S0020-1383(98)00125-9.
- Mody RM, Zapor M, Hartzell JD, Robben PM, Waterman P, Wood-Morris R, et al. Infectious complications of damage control orthopedics in war trauma. J Trauma 2009;67:758-61. https://doi.org/10.1097/TA.0b013e3181af6aa6.
- British Orthopaedic Association and British Association of Plastic, Reconstructive and Aesthetic Surgeons . BOAST 4: The Management of Severe Open Lower Limb Fractures 2009. www.boa.ac.uk/wp-content/uploads/2014/12/BOAST-4.pdf (accessed 9 October 2017).
- Fractures (Complex): Assessment and Management. London: NICE; 2016.
- Pollak AN, Powell ET, Fang R, Cooper EO, Ficke JR, Flaherty SF. Use of negative pressure wound therapy during aeromedical evacuation of patients with combat-related blast injuries. J Surg Orthop Adv 2010;19:44-8.
- Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M. Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 2009;66:749-57. https://doi.org/10.1097/TA.0b013e318171971a.
- Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008;195:782-8. https://doi.org/10.1016/j.amjsurg.2007.06.023.
- Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, Minard G, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma 2008;65:337-42. https://doi.org/10.1097/TA.0b013e31817fa451.
- Krug E, Berg L, Lee C, Hudson D, Birke-Sorensen H, Depoorter M, et al. Evidence-based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury 2011;42:S1-12. https://doi.org/10.1016/S0020-1383(11)00041-6.
- Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 2009;23:552-7. https://doi.org/10.1097/BOT.0b013e3181a2e2b6.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Salén BA, Spangfort EV, Nygren AL, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol 1994;47:1423-35. https://doi.org/10.1016/0895-4356(94)90086-8.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Parsons N, Griffin XL, Achten J, Costa ML. Outcome assessment after hip fracture: is EQ-5D the answer?. Bone Joint Res 2014;3:69-75. https://doi.org/10.1302/2046-3758.33.2000250.
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C. Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health 1999;53:46-50. https://doi.org/10.1136/jech.53.1.46.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. https://doi.org/10.1016/j.ajic.2008.03.002.
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606-8. https://doi.org/10.1017/S0195941700015241.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- van den Brink M, van den Hout WB, Stiggelbout AM, . Self Reports of Health Care Utilisation: Can a Questionnaire Replace a Diary? n.d.
- Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care 2010;48:96-105. https://doi.org/10.1097/MLR.0b013e3181d99107.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Allen C, Beecham J, Netten A, Beecham J. Costing Community Care: Theory and Practice. Aldershot: Ashgate Publishing Ltd; 1993.
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993.
- Achten J, Parsons NR, Bruce J, Petrou S, Tutton E, Willett K, et al. Protocol for a randomised controlled trial of standard wound management versus negative pressure wound therapy in the treatment of adult patients with an open fracture of the lower limb: UK Wound management of Open Lower Limb Fractures (UK WOLFF). BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009087.
- Trickett RW, Mudge E, Price P, Pallister I. A qualitative approach to recovery after open tibial fracture: the road to a novel, patient-derived recovery scale. Injury 2012;43:1071-8. https://doi.org/10.1016/j.injury.2012.01.027.
- Shauver MS, Aravind MS, Chung KC. A qualitative study of recovery from type III-B and III-C tibial fractures. Ann Plast Surg 2011;66:73-9. https://doi.org/10.1097/SAP.0b013e3181d50eba.
- Griffiths H, Jordan S. Thinking of the future and walking back to normal: an exploratory study of patients’ experiences during recovery from lower limb fracture. J Adv Nurs 1998;28:1276-88. https://doi.org/10.1046/j.1365-2648.1998.00847.x.
- Forsberg A, Söderberg S, Engström Å. People’s experiences of suffering a lower limb fracture and undergoing surgery. J Clin Nurs 2014;23:191-200. https://doi.org/10.1111/jocn.12292.
- McPhail SM, Dunstan J, Canning J, Haines TP. Life impact of ankle fractures: qualitative analysis of patient and clinician experiences. BMC Musculoskelet Disord 2012;13. https://doi.org/10.1186/1471-2474-13-224.
- Keene DJ, Mistry D, Nam J, Tutton E, Handley R, Morgan L, et al. The Ankle Injury Management (AIM) trial: a pragmatic, multicentre, equivalence randomised controlled trial and economic evaluation comparing close contact casting with open surgical reduction and internal fixation in the treatment of unstable ankle fractures in patients aged over 60 years. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20750.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-32.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. https://doi.org/10.1177/1740774510392257.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19. https://doi.org/10.1016/S0277-9536(01)00197-6.
- Robinson EJ, Kerr CE, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al. Lay public’s understanding of equipoise and randomisation in randomised controlled trials. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9080.
- Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med 2004;58:1689-97. https://doi.org/10.1016/S0277-9536(03)00338-1.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Gobat NH, Gal M, Francis NA, Hood K, Watkins A, Turner J, et al. Key stakeholder perceptions about consent to participate in acute illness research: a rapid, systematic review to inform epi/pandemic research preparedness. Trials 2015;16. https://doi.org/10.1186/s13063-015-1110-6.
- Heidegger M. Being and Time. Oxford: Blackwell Publishing Ltd; 1962.
- Madjar I, Walton JA. Nursing and the Experience of Illness: Phenomenology in Practice. London: Routledge; 1999.
- van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. Albany, NY: State University of New York Press; 1990.
- Gadamer H. Truth and Method. London: Bloomsbury Publications Plc; 1975.
- Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park, CA: Sage Publications Inc.; 1985.
- Tutton E, Seers K, Langstaff D. Professional nursing culture on a trauma unit: experiences of patients and staff. J Adv Nurs 2008;61:145-53. https://doi.org/10.1111/j.1365-2648.2007.04471.x.
- Doohan I, Saveman BI. Impact on life after a major bus crash – a qualitative study of survivors’ experiences. Scand J Caring Sci 2014;28:155-63. https://doi.org/10.1111/scs.12040.
- Stayt LC, Seers K, Tutton L. Making sense of it: intensive care patients’ phenomenological accounts of story construction. Nurs Crit Care 2016;21:225-32. https://doi.org/10.1111/nicc.12224.
- Ogilvie R, McCloughen A, Curtis K, Foster K. The experience of surviving life-threatening injury: a qualitative synthesis. Int Nurs Rev 2012;59:312-20. https://doi.org/10.1111/j.1466-7657.2012.00993.x.
- Maccallum F, Bryant RA. A Cognitive Attachment Model of prolonged grief: integrating attachments, memory, and identity. Clin Psychol Rev 2013;33:713-27. https://doi.org/10.1016/j.cpr.2013.05.001.
- Penrod J. Refinement of the concept of uncertainty. J Adv Nurs 2001;34:238-45. https://doi.org/10.1046/j.1365-2648.2001.01750.x.
- Penrod J. Living with uncertainty: concept advancement. J Adv Nurs 2007;57:658-67. https://doi.org/10.1111/j.1365-2648.2006.04008.x.
- Morse JM, Penrod J. Linking concepts of enduring, uncertainty, suffering, and hope. Image J Nurs Sch 1999;31:145-50. https://doi.org/10.1111/j.1547-5069.1999.tb00455.x.
- Tutton E, Seers K, Langstaff D, Westwood M. Staff and patient views of the concept of hope on a stroke unit: a qualitative study. J Adv Nurs 2012;68:2061-9. https://doi.org/10.1111/j.1365-2648.2011.05899.x.
- Tutton E, Seers K, Langstaff D. Hope in orthopaedic trauma: a qualitative study. Int J Nurs Stud 2012;49:872-9. https://doi.org/10.1016/j.ijnurstu.2012.01.013.
- Wiseman T, Foster K, Curtis K. The experience of emotional wellbeing for patients with physical injury: a qualitative follow-up study. Injury 2016;47:1983-9. https://doi.org/10.1016/j.injury.2016.03.021.
- Morse JM, Beres MA, Spiers JA, Mayan M, Olson K. Identifying signals of suffering by linking verbal and facial cues. Qual Health Res 2003;13:1063-77. https://doi.org/10.1177/1049732303256401.
- Lawlor D. Test of time: a case study in the functioning of social systems as a defence against anxiety: rereading 50 years on. Clin Child Psychol Psychiatry 2009;14:523-30. https://doi.org/10.1177/1359104509339545.
- Gullick JG, Taggart SB, Johnston RA, Ko N. The trauma bubble: patient and family experience of serious burn injury. J Burn Care Res 2014;35:e413-27. https://doi.org/10.1097/BCR.0000000000000030.
- Clifton S. Grieving my broken body: an autoethnographic account of spinal cord injury as an experience of grief. Disabil Rehabil 2014;36:1823-9. https://doi.org/10.3109/09638288.2013.872202.
- Thorpe G, McArthur M, Richardson B. Bodily change following faecal stoma formation: qualitative interpretive synthesis. J Adv Nurs 2009;65:1778-89. https://doi.org/10.1111/j.1365-2648.2009.05059.x.
- Morse JM. Using qualitative methods to access the pain experience. Br J Pain 2015;9:26-31. https://doi.org/10.1177/2049463714550507.
- Morse JM, Mitcham C. The experience of agonizing pain and signals of disembodiment. J Psychosom Res 1998;44:667-80. https://doi.org/10.1016/S0022-3999(97)00301-2.
- Price B. A model for body-image care. J Adv Nurs 1990;15:585-93. https://doi.org/10.1111/j.1365-2648.1990.tb01858.x.
- Johnson RA, Taggart SB, Gullick JG. Emerging from the trauma bubble: Redefining ‘normal’ after burn injury. Burns 2016;42:1223-32. https://doi.org/10.1016/j.burns.2016.03.016.
- Kvigne K, Kirkevold M. Living with bodily strangeness: women’s experiences of their changing and unpredictable body following a stroke. Qual Health Res 2003;13:1291-310. https://doi.org/10.1177/1049732303257224.
- Stayt LC, Seers K, Tutton E. Patients’ experiences of technology and care in adult intensive care. J Adv Nurs 2015;71:2051-61. https://doi.org/10.1111/jan.12664.
- Baillie L. Patient dignity in an acute hospital setting: a case study. Int J Nurs Stud 2009;46:23-36. https://doi.org/10.1016/j.ijnurstu.2008.08.003.
- Richmond TS, Thompson HJ, Deatrick JA, Kauder DR. Journey towards recovery following physical trauma. J Adv Nurs 2000;32:1341-7. https://doi.org/10.1046/j.1365-2648.2000.01629.x.
- Smith-Young J, Solberg S, Gaudine A. Constant negotiating: managing work-related musculoskeletal disorders while remaining at the workplace. Qual Health Res 2014;24:217-31. https://doi.org/10.1177/1049732313519868.
- Closs SJ, Briggs M. Patients’ verbal descriptions of pain and discomfort following orthopaedic surgery. Int J Nurs Stud 2002;39:563-72. https://doi.org/10.1016/S0020-7489(01)00067-0.
- Toye F, Seers K, Barker K. A meta-ethnography of patients’ experiences of chronic pelvic pain: struggling to construct chronic pelvic pain as ‘real’. J Adv Nurs 2014;70:2713-27. https://doi.org/10.1111/jan.12485.
- Abhyankar P, Velikova G, Summers B, Bekker HL. Identifying components in consent information needed to support informed decision making about trial participation: an interview study with women managing cancer. Soc Sci Med 2016;161:83-91. https://doi.org/10.1016/j.socscimed.2016.05.040.
- Dewing J. Process consent and research with older persons living with dementia . Research Ethics 2008;4:59-64. https://doi.org/10.1177/174701610800400205.
- Harrop E, Noble S, Edwards M, Sivell S, Moore B, Nelson A. behalf of the FRAGMATIC Trial Management Group (TMG) . ‘I didn’t really understand it, I just thought it’d help’: exploring the motivations, understandings and experiences of patients with advanced lung cancer participating in a non-placebo clinical IMP trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1460-8.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Consumer Price Inflation Time Series Dataset (MM23). London: ONS; 2016.
- HRG4+ 2015/16 Reference Costs Code to Group User Manual. Leeds: Health and Social Care Information Centre; 2016.
- NHS Reference Costs 2014 to 2015. London: Department of Health; 2015.
- Prescription Costs Analysis, England – 2014. Leeds: NHS Digital; 2014.
- NHS Business Services Authority . NHS Supply Chain Catalogue 2015 16 2015.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- New Earnings Survey Panel Dataset, 1975–2016: Secure Access. [data collection]. UK Data Service; 2017.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Kharroubi SA, Brazier JE, Roberts J, O’Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ 2007;26:597-612. https://doi.org/10.1016/j.jhealeco.2006.09.002.
- McCabe C, Brazier J, Gilks P, Tsuchiya A, Roberts J, O’Hagan A, et al. Using rank data to estimate health state utility models. J Health Econ 2006;25:418-31. https://doi.org/10.1016/j.jhealeco.2005.07.008.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- The E-Vita Open Plus for Treating Complex Aneurysms and Dissections of the Thoracic Aorta. London: NICE; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- British Broadcasting Corporation . Q&Amp;A: NHS 111 n.d. www.bbc.co.uk/news/health-223706212013 (accessed 1 October 2016).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Elderly Accommodation Counsel . Laundry Service n.d. www.housingcare.org/service/ser-info-11938-laundry-servic.aspx (accessed 1 October 2016).
- Complete Care Shop n.d. www.completecareshop.co.uk (accessed 1 October 2016).
- Zhang T, Yan Y, Xie X, Mu W. Minimally invasive sinus tarsi approach with cannulated screw fixation combined with vacuum-assisted closure for treatment of severe open calcaneal fractures with medial wounds. J Foot Ankle Surg 2016;55:112-16. https://doi.org/10.1053/j.jfas.2015.07.023.
- Arti H, Khorami M, Ebrahimi-Nejad V. Comparison of negative pressure wound therapy (NPWT) & conventional wound dressings in the open fracture wounds. Pak J Med Sci 2016;32:65-9. https://doi.org/10.12669/pjms.321.8568.
- Saddawi-Konefka D, Kim HM, Chung KC. A systematic review of outcomes and complications of reconstruction and amputation for type IIIB and IIIC fractures of the tibia. Plast Reconstr Surg 2008;122:1796-805.
- Chung KC, Saddawi-Konefka D, Haase SC, Kaul G. A cost-utility analysis of amputation versus salvage for Gustilo type IIIB and IIIC open tibial fractures. Plast Reconstr Surg 2009;124:1965-73. https://doi.org/10.1097/PRS.0b013e3181bcf156.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
Appendix 1 Study recruitment
Table 33 shows the breakdown of recruited participants (n = 625) by centre and G&A grade. There is a good balance between treatments across and within centres, and between G&A grades.
| Centre | G&A gradea | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 3C | |||||||
| NPWT | Standard | NPWT | Standard | NPWT | Standard | NPWT | Standard | Total | |
| RLH | 1 | 3 | 16 | 15 | 4 | 4 | 21 | 22 | 43 |
| POH | 5 | 5 | 5 | 4 | 0 | 0 | 10 | 9 | 19 |
| AUH | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 |
| LGI | 0 | 1 | 4 | 5 | 0 | 0 | 4 | 6 | 10 |
| ADH | 3 | 4 | 17 | 16 | 0 | 0 | 20 | 20 | 40 |
| UHS | 3 | 2 | 5 | 5 | 1 | 0 | 9 | 7 | 16 |
| STH | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 4 |
| QAH | 2 | 2 | 0 | 0 | 1 | 1 | 3 | 3 | 6 |
| RBH | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 3 | 4 |
| KCH | 1 | 0 | 12 | 13 | 1 | 0 | 14 | 13 | 27 |
| UNS | 2 | 2 | 4 | 5 | 1 | 0 | 7 | 7 | 14 |
| PLY | 5 | 5 | 9 | 10 | 1 | 0 | 15 | 15 | 30 |
| UHC | 8 | 8 | 26 | 27 | 5 | 4 | 39 | 39 | 78 |
| NNH | 1 | 2 | 2 | 2 | 0 | 0 | 3 | 4 | 7 |
| UHB | 4 | 2 | 6 | 6 | 8 | 10 | 18 | 18 | 36 |
| RVI | 2 | 1 | 10 | 9 | 0 | 0 | 12 | 10 | 22 |
| RDH | 2 | 2 | 4 | 4 | 0 | 1 | 6 | 7 | 13 |
| JRH | 0 | 3 | 24 | 21 | 2 | 2 | 26 | 26 | 52 |
| FRH | 2 | 1 | 48 | 47 | 7 | 10 | 57 | 58 | 115 |
| HRI | 0 | 1 | 6 | 6 | 0 | 0 | 6 | 7 | 13 |
| UHL | 1 | 2 | 2 | 2 | 0 | 0 | 3 | 4 | 7 |
| NUH | 2 | 1 | 13 | 14 | 1 | 2 | 16 | 17 | 33 |
| RSC | 2 | 2 | 11 | 10 | 0 | 0 | 13 | 12 | 25 |
| MHS | 0 | 0 | 5 | 4 | 0 | 0 | 5 | 4 | 9 |
| Total | 47 | 50 | 232 | 230 | 32 | 34 | 311 | 314 | 625 |
Table 34 shows the recruitment rate for consented participants (n = 460) by centre (hospital), and the estimated monthly recruitment rate.
| Centre | Date opened | Months open | Recruited | Rate per month | ||
|---|---|---|---|---|---|---|
| 1 | FRH | Frenchay Hospital | 10 September 2012 | 40 | 77 | 1.93 |
| 2 | UHC | UHCW | 9 July 2012 | 42 | 62 | 1.48 |
| 3 | JRH | John Radcliffe Hospital | 1 December 2012 | 37 | 44 | 1.19 |
| 4 | RLH | Royal London Hospital | 13 November 2013 | 25 | 28 | 1.12 |
| 5 | KCH | Kings College Hospital | 21 March 2014 | 21 | 22 | 1.05 |
| 6 | ADH | Addenbrookes Hospital Cambridge | 13 July 2013 | 30 | 29 | 0.97 |
| 7 | NUH | Nottingham University Hospital | 12 June 2013 | 31 | 25 | 0.81 |
| 8 | HRI | Hull Royal Infirmary | 25 August 2014 | 16 | 12 | 0.75 |
| 9 | UHB | Queen Elizabeth Hospital Birmingham | 4 February 2013 | 35 | 25 | 0.71 |
| 10 | PLY | Plymouth Hospital | 13 June 2013 | 31 | 21 | 0.68 |
| 11 | RSC | Royal Sussex County hospital Brighton | 12 August 2013 | 29 | 18 | 0.62 |
| 12 | LGI | Leeds General Infirmary | 23 September 2014 | 15 | 9 | 0.60 |
| 13 | POH | Poole Hospital | 6 August 2013 | 29 | 16 | 0.55 |
| 14 | RDH | Royal Derby Hospital | 18 November 2013 | 25 | 11 | 0.44 |
| 15 | MHS | Morriston Hospital Swansea | 11 June 2014 | 18 | 7 | 0.39 |
| 16 | RVI | Royal Victoria Infirmary | 4 October 2012 | 39 | 15 | 0.38 |
| 17 | UNS | University Hospital of North Staffordshire | 28 November 2013 | 25 | 8 | 0.32 |
| 18 | UHS | University Hospital Southampton | 5 September 2013 | 28 | 8 | 0.29 |
| 19 | UHL | University Hospital Leicester | 23 October 2013 | 26 | 7 | 0.27 |
| 20 | QAH | Queen Alexandra Hospital | 5 April 2014 | 21 | 5 | 0.24 |
| 21 | NNH | Norfolk and Norwich University Hospital | 17 September 2013 | 27 | 5 | 0.19 |
| 22 | STH | North General Hospital Sheffield | 11 August 2014 | 16 | 2 | 0.12 |
| 23 | RBH | Royal Berkshire Hospital | 8 May 2013 | 32 | 3 | 0.09 |
| 24 | AUH | Aintree University Hospital | 5 August 2014 | 17 | 1 | 0.06 |
| Total | 655 | 460 | 0.70 | |||
Figure 16 shows the overall progress of study recruitment for consented participants towards the required target sample of 460. Shown also is the required recruitment, based on a crude model of centre opening rates for the 24 recruiting centres to reach the target of 460 participants by September 2015.
FIGURE 16.
Rate of overall recruitment (solid line) and required recruitment (dashed line).

Figure 17 shows the recruitment rates for consented participants (n = 460) for individual centres in addition to the overall data. This is presented on a log-transformed scale to aid visualisation.
FIGURE 17.
Rate of overall recruitment (solid line), recruitment by individual centre and required recruitment (dashed line). A log-transformation is used to aid visualisation. Centres are numbered as in Table 34.

Appendix 2 Disability Rating Index
Table 35 shows the method of data capture (percentage rates in each category by treatment group and assessment occasion) for DRI.
| Occasion | Treatment group | Total, n | Method, n (%) | |||
|---|---|---|---|---|---|---|
| Face to face | Postal | Telephone | ||||
| Pre injury | NPWT | 220 | 217 (99) | 2 (1) | 1 (0) | 0 (0) |
| Standard | 231 | 230 (100) | 1 (0) | 0 (0) | 0 (0) | |
| 3 months | NPWT | 166 | 1 (1) | 148 (89) | 13 (8) | 4 (2) |
| Standard | 188 | 3 (2) | 165 (88) | 19 (10) | 1 (1) | |
| 6 months | NPWT | 154 | 1 (1) | 137 (89) | 15 (10) | 1 (1) |
| Standard | 175 | 3 (2) | 156 (89) | 12 (7) | 4 (2) | |
| 9 months | NPWT | 153 | 1 (1) | 127 (83) | 20 (13) | 5 (3) |
| Standard | 161 | 1 (1) | 139 (86) | 20 (12) | 1 (1) | |
| 12 months | NPWT | 179 | 26 (15) | 131 (73) | 19 (11) | 3 (2) |
| Standard | 195 | 29 (15) | 149 (76) | 15 (8) | 2 (1) | |
A summary of missing values, by age group and sex for DRI at 12 months is shown in Table 36. The full pattern of missing values is shown in Table 37 for NPWT and standard dressing intervention arms.
| Age (years) | Gender | Data available | Data missing | Total | % missing |
|---|---|---|---|---|---|
| < 40 | Female | 21 | 3 | 24 | 12.5 |
| Male | 138 | 39 | 177 | 22.0 | |
| ≥ 40 | Female | 79 | 15 | 94 | 16.0 |
| Male | 136 | 29 | 165 | 17.6 | |
| Total | 374 | 86 | 460 | 18.7 |
| Treatment | Numbera | Cumulative (%)b | DRIc | Missing | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | ||||
| NPWT | 111 | 49 | 1 | 1 | 1 | 1 | 1 | 0 |
| 1 | 50 | 0 | 1 | 1 | 1 | 1 | 1 | |
| 12 | 55 | 1 | 0 | 1 | 1 | 1 | 1 | |
| 13 | 61 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 13 | 66 | 1 | 1 | 1 | 0 | 1 | 1 | |
| 6 | 69 | 1 | 1 | 1 | 1 | 0 | 1 | |
| 5 | 71 | 1 | 0 | 0 | 1 | 1 | 2 | |
| 6 | 74 | 1 | 0 | 1 | 0 | 1 | 2 | |
| 8 | 77 | 1 | 1 | 0 | 0 | 1 | 2 | |
| 1 | 78 | 1 | 0 | 1 | 1 | 0 | 2 | |
| 2 | 79 | 1 | 1 | 0 | 1 | 0 | 2 | |
| 2 | 80 | 1 | 1 | 1 | 0 | 0 | 2 | |
| 1 | 80 | 0 | 0 | 0 | 1 | 1 | 3 | |
| 9 | 84 | 1 | 0 | 0 | 0 | 1 | 3 | |
| 1 | 85 | 1 | 0 | 0 | 1 | 0 | 3 | |
| 2 | 85 | 1 | 0 | 1 | 0 | 0 | 3 | |
| 10 | 90 | 1 | 1 | 0 | 0 | 0 | 3 | |
| 19 | 98 | 1 | 0 | 0 | 0 | 0 | 4 | |
| 4 | 100 | 0 | 0 | 0 | 0 | 0 | 5 | |
| Total | 226 | – | 6 | 60 | 72 | 73 | 47 | 258 |
| Standard | 138 | 59 | 1 | 1 | 1 | 1 | 1 | 0 |
| 4 | 61 | 1 | 0 | 1 | 1 | 1 | 1 | |
| 9 | 65 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 16 | 71 | 1 | 1 | 1 | 0 | 1 | 1 | |
| 5 | 74 | 1 | 1 | 1 | 1 | 0 | 1 | |
| 4 | 75 | 1 | 0 | 0 | 1 | 1 | 2 | |
| 5 | 77 | 1 | 0 | 1 | 0 | 1 | 2 | |
| 10 | 82 | 1 | 1 | 0 | 0 | 1 | 2 | |
| 5 | 84 | 1 | 1 | 1 | 0 | 0 | 2 | |
| 9 | 88 | 1 | 0 | 0 | 0 | 1 | 3 | |
| 1 | 88 | 1 | 0 | 0 | 1 | 0 | 3 | |
| 2 | 89 | 1 | 0 | 1 | 0 | 0 | 3 | |
| 5 | 91 | 1 | 1 | 0 | 0 | 0 | 3 | |
| 18 | 99 | 1 | 0 | 0 | 0 | 0 | 4 | |
| 3 | 100 | 0 | 0 | 0 | 0 | 0 | 5 | |
| Total | 234 | – | 3 | 46 | 59 | 73 | 39 | 220 |
Appendix 3 Background information
Appendix 5 Patient entry form
Appendix 6 Operation note
Appendix 7 Six-week follow-up form
Appendix 8 Three-month questionnaire
EQ-5D redacted for copyright reasons.
Appendix 9 Serious adverse event form
Appendix 10 Surgical site infection diagnosis algorithm
| Purulent drainage from the deep incision (purulent drainage) | (d) Is there any fluid leaking from the wound? |
| (e) If yes, is the fluid pus or cloudy yellow? | |
| Accept if (d) AND (e) = present today OR | |
| Accept if (e) = present today AND (d) = No or missing | |
| A deep incision that spontaneously dehisces and fever > 38 °C (dehiscence and fever) | (f) Is the wound gaping open (dehisced)? |
| (h) Any fever of > 38 °C since surgery? | |
| Accept if (f) AND (h) = present today OR | |
| Accept if (f) = present today AND (h) = no or missing | |
| A deep incision that spontaneously dehisces and localised pain or tenderness (dehiscence and pain) | (f) Is the wound gaping open (dehisced)? |
| (c) Is the area around the wound painful or tender? | |
| Accept if (f) AND (c) = present today OR | |
| Accept if (f) = present today AND (c) = no or missing | |
| A deep incision that is deliberately opened by surgeon or attending physician or other designee and fever > 38 °C (surgery and fever) | (g) Has a surgeon deliberately opened the wound? |
| (h) Any fever of > 38 °C since surgery? | |
| Accept if (g) AND (h) = present today OR | |
| Accept if (g) = present today AND (h) = no or missing | |
| A deep incision that is deliberately opened by surgeon or attending physician or other designee and localised pain or tenderness (surgery and pain) | (g) Has a surgeon deliberately opened the wound? |
| (c) Is the area around the wound painful or tender? | |
| Accept if (g) AND (c) = present today OR | |
| Accept if (g) = present today AND (c) = no or missing | |
| An abscess or other evidence of infection involving the deep incision that is detected on gross examination (abscess) | (i) Is there any sign of abscess or infection on direct examination? |
| Accept if (i) = present today | |
| Culture swabs and antibiotics (antibiotics) | (j) Has a culture swab been obtained? |
| (k) Have antibiotics been prescribed for a wound infection? | |
| Accept if (j) = present today AND (k) = yes |
Appendix 11 Superficial surgical site infection
| Purulent drainage from the superficial incision (purulent drainage) | (d) Is there any fluid leaking from the wound? |
| (e) If yes, is the fluid pus or cloudy yellow? | |
| Accept if (d) AND (e) = previous time OR | |
| Accept if (e) = previous time AND (d) = no or missing | |
| Organisms isolated from an aseptically obtained specimen from the superficial incision or subcutaneous tissue by a culture or non-culture based microbiological testing method performed for the purposes of clinical diagnosis or treatment (antibiotics) | (j) Has a culture swab been obtained? |
| (k) Have antibiotics been prescribed for a wound infection? | |
| Accept if (j) = previous time AND (k) = yes | |
| Superficial incision that is deliberately opened by a surgeon, attending physician or other designee and has pain or tenderness (surgery and pain) | (g) Has a surgeon deliberately opened the wound? |
| (c) Is the area around the wound painful or tender? | |
| Accept if (g) AND (c) = previous time OR | |
| Accept if (g) = previous time AND (c) = no or missing | |
| Superficial incision that is deliberately opened by a surgeon, attending physician or other designee and has localised swelling (surgery and swelling) | (g) Has a surgeon deliberately opened the wound? |
| (b) Is the area around the wound swollen? | |
| Accept if (g) AND (b) = previous time OR | |
| Accept if (g) = previous time AND (b) = no or missing | |
| Superficial incision that is deliberately opened by a surgeon, attending physician or other designee and has erythema or heat (surgery and inflammation) | (g) Has a surgeon deliberately opened the wound? |
| (a) Is the wound red or inflamed? | |
| Accept if (g) AND (a) = previous time OR | |
| Accept if (g) = previous time AND (a) = no or missing | |
| An abscess or other evidence of infection involving the deep incision that is detected on gross examination (abscess) | (i) Is there any sign of abscess or infection on direct examination? |
| Accept if (i) = previous time |
Appendix 12 Routinely reported complications
| Wound complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Red and inflamed | |||||
| Any time since surgery | 34 | 28 | 62 | 15.0 | 12.0 |
| No | 159 | 153 | 312 | 70.4 | 65.4 |
| Symptoms present today | 13 | 19 | 32 | 5.8 | 8.1 |
| Missing | 20 | 34 | 54 | 8.8 | 14.5 |
| Chi-squared test: p-value = 0.420 (statistic = 1.733, df = 2). Fisher’s exact test: p-value = 0.439 | |||||
| Swollen | |||||
| Any time since surgery | 26 | 33 | 59 | 11.5 | 14.1 |
| No | 142 | 119 | 261 | 62.8 | 50.9 |
| Symptoms present today | 38 | 49 | 87 | 16.8 | 20.9 |
| Missing | 20 | 33 | 53 | 8.8 | 14.1 |
| Chi-squared test: p-value = 0.123 (statistic = 4.187, df = 2). Fisher’s exact test: p-value = 0.125 | |||||
| Painful or tender | |||||
| Any time since surgery | 26 | 35 | 61 | 11.5 | 15 |
| No | 147 | 130 | 277 | 65.0 | 55.6 |
| Symptoms present today | 35 | 33 | 68 | 15.5 | 14.1 |
| Missing | 18 | 36 | 54 | 8.0 | 15.4 |
| Chi-squared test: p-value = 0.335 (statistic = 2.185, df = 2). Fisher’s exact test: p-value = 0.346 | |||||
| Fluid leakage | |||||
| Any time since surgery | 40 | 42 | 82 | 17.7 | 17.9 |
| No | 138 | 131 | 269 | 61.1 | 56.0 |
| Symptoms present today | 28 | 27 | 55 | 12.4 | 11.5 |
| Missing | 20 | 34 | 54 | 8.8 | 14.5 |
| Chi-squared test: p-value = 0.923 (statistic = 0.160, df = 2). Fisher’s exact test: p-value = 0.912 | |||||
| If yes, fluid pus or cloudy yellow | |||||
| Any time since surgery | 16 | 15 | 31 | 7.1 | 6.4 |
| No | 35 | 41 | 76 | 15.5 | 17.5 |
| Symptoms present today | 11 | 10 | 21 | 4.9 | 4.3 |
| Missing | 164 | 168 | 332 | 72.6 | 71.8 |
| Chi-squared test: p-value = 0.807 (statistic = 0.429, df = 2). Fisher’s exact test: p-value = 0.800 | |||||
| Gaping open | |||||
| Any time since surgery | 4 | 8 | 12 | 1.8 | 3.4 |
| No | 195 | 188 | 383 | 86.3 | 80.3 |
| Symptoms present today | 6 | 4 | 10 | 2.7 | 1.7 |
| Missing | 21 | 34 | 55 | 9.3 | 14.5 |
| Chi-squared test: p-value = 0.407 (statistic = 1.8, df = 2). Fisher’s exact test: p-value = 0.425 | |||||
| Surgeon deliberately opened | |||||
| Any time since surgery | 13 | 10 | 23 | 5.8 | 4.3 |
| No | 194 | 190 | 384 | 85.8 | 81.2 |
| Symptoms present today | 2 | 0 | 2 | 0.9 | 0.0 |
| Missing | 17 | 34 | 51 | 7.5 | 14.5 |
| Chi-squared test: p-value = 0.327 (statistic = 2.236, df = 2). Fisher’s exact test: p-value = 0.435 | |||||
| Fever | |||||
| Any time since surgery | 22 | 16 | 38 | 9.7 | 6.8 |
| No | 184 | 186 | 370 | 81.4 | 79.5 |
| Missing | 20 | 32 | 52 | 8.8 | 13.7 |
| Chi-squared test: p-value = 0.431 (statistic = 0.621, df = 1). Fisher’s exact test: p-value = 0.396 | |||||
| Abscess or infection | |||||
| Any time since surgery | 7 | 8 | 15 | 3.1 | 3.4 |
| No | 198 | 185 | 383 | 87.6 | 79.1 |
| Symptoms present today | 3 | 5 | 8 | 1.3 | 2.1 |
| Missing | 18 | 36 | 54 | 8.0 | 15.4 |
| Chi-squared test: p-value = 0.683 (statistic = 0.762, df = 2). Fisher’s exact test: p-value = 0.710 | |||||
| Culture swab taken | |||||
| Any time since surgery | 40 | 28 | 68 | 17.7 | 12.0 |
| No | 164 | 164 | 328 | 72.6 | 70.1 |
| Symptoms present today | 5 | 10 | 15 | 2.2 | 4.3 |
| Missing | 17 | 32 | 49 | 7.5 | 13.7 |
| Chi-squared test: p-value = 0.160 (statistic = 3.666, df = 2). Fisher’s exact test: p-value = 0.170 | |||||
| Organism | |||||
| Bacillus spp. | 1 | 0 | 1 | 0.4 | 0.0 |
| Clostridium | 0 | 1 | 1 | 0.0 | 0.4 |
| Coliform | 2 | 0 | 2 | 0.9 | 0.0 |
| Enterobacter | 3 | 0 | 3 | 1.3 | 0.0 |
| Enterobacter and Pseudomonas spp. | 1 | 0 | 1 | 0.4 | 0.0 |
| Enterococcus spp. | 1 | 1 | 2 | 0.4 | 0.4 |
| Escherichia coli ESB2 | 1 | 0 | 1 | 0.4 | 0.0 |
| Faecal flora and Staphylococcus aureus (unknown sensitivity) | 1 | 0 | 1 | 0.4 | 0.0 |
| Gram-negative rods | 0 | 1 | 1 | 0.0 | 0.4 |
| Mixed growth | 1 | 3 | 4 | 0.4 | 1.3 |
| Nil observed | 13 | 9 | 22 | 5.8 | 3.8 |
| Pseudomonas spp. | 2 | 1 | 3 | 0.9 | 0.4 |
| Skin flora | 0 | 2 | 2 | 0.0 | 0.9 |
| Skin flora and faecal flora | 1 | 0 | 1 | 0.4 | 0.0 |
| Staphylococcus aureus (meticillin resistant) | 1 | 3 | 4 | 0.4 | 1.3 |
| Staphylococcus aureus (unknown sensitivity) | 4 | 4 | 8 | 1.8 | 1.7 |
| Staphylococcus aureus (unknown sensitivity) and enterococci | 2 | 0 | 2 | 0.9 | 0.0 |
| Staphylococcus epidermidis (unknown sensitivity) | 1 | 1 | 2 | 0.4 | 0.4 |
| Staphylococcal spp. (mixed) | 1 | 0 | 1 | 0.4 | 0.0 |
| Streptococcal spp. | 1 | 0 | 1 | 0.4 | 0.0 |
| Missing | 189 | 208 | 397 | 83.6 | 88.9 |
| Chi-squared test: p-value = 0.410 (statistic = 19.742, df = 19). Fisher’s exact test: p-value = 0.472 | |||||
| Antibiotics prescribed for trial wound | |||||
| No | 174 | 163 | 337 | 77.0 | 69.7 |
| Yes | 34 | 37 | 71 | 15.0 | 15.8 |
| Missing | 18 | 34 | 52 | 8.0 | 14.5 |
| Chi-squared test: p-value = 0.658 (statistic = 0.196, df = 1). Fisher’s exact test: p-value = 0.603 | |||||
| Other antibiotics | |||||
| No | 140 | 135 | 275 | 61.9 | 57.7 |
| Yes | 44 | 38 | 82 | 19.5 | 16.2 |
| Missing | 42 | 61 | 103 | 18.6 | 26.1 |
| Chi-squared test: p-value = 0.756 (statistic = 0.097, df = 1). Fisher’s exact test: p-value = 0.706 | |||||
| Infection diagnosed | |||||
| No | 182 | 187 | 369 | 80.5 | 79.9 |
| Yes | 26 | 21 | 47 | 11.5 | 9.0 |
| Missing | 18 | 26 | 44 | 8 | 11.1 |
| Chi-squared test: p-value = 0.536 (statistic = 0.384, df = 1). Fisher’s exact test: p-value = 0.536 | |||||
| Fully healed | |||||
| No | 100 | 101 | 201 | 44.2 | 43.2 |
| Yes | 85 | 80 | 165 | 37.6 | 34.2 |
| Missing | 41 | 53 | 94 | 18.1 | 22.6 |
| Chi-squared test: p-value = 0.817 (statistic = 0.053, df = 1). Fisher’s exact test: p-value = 0.754 | |||||
| Patient thinks fully healed | |||||
| No | 86 | 109 | 195 | 38.1 | 46.6 |
| Yes | 115 | 89 | 204 | 50.9 | 38.0 |
| Missing | 25 | 36 | 61 | 11.1 | 15.4 |
| Chi-squared test: p-value = 0.019 (statistic = 5.524, df = 1). Fisher’s exact test: p-value = 0.016 | |||||
| Complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Anaesthesia | |||||
| No | 221 | 230 | 451 | 97.8 | 98.3 |
| Yes | 3 | 2 | 5 | 1.3 | 0.9 |
| Chi-squared test: p-value = 0.969 (statistic = 0.002, df = 1). Fisher’s exact test: p-value = 0.681 | |||||
| Postoperative bleeding | |||||
| No | 222 | 230 | 452 | 98.2 | 98.3 |
| Yes | 2 | 2 | 4 | 0.9 | 0.9 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| DVT | |||||
| No | 218 | 229 | 447 | 96.5 | 97.9 |
| Yes | 6 | 3 | 9 | 2.7 | 1.3 |
| Chi-squared test: p-value = 0.467 (statistic = 0.528, df = 1). Fisher’s exact test: p-value = 0.331 | |||||
| PE | |||||
| No | 222 | 228 | 450 | 98.2 | 97.4 |
| Yes | 2 | 4 | 6 | 0.9 | 1.7 |
| Chi-squared test: p-value = 0.713 (statistic = 0.135, df = 1). Fisher’s exact test: p-value = 0.686 | |||||
| Nerve damage | |||||
| No | 217 | 221 | 438 | 96.0 | 94.4 |
| Yes | 7 | 11 | 18 | 3.1 | 4.7 |
| Chi-squared test: p-value = 0.519 (statistic = 0.417, df = 1). Fisher’s exact test: p-value = 0.473 | |||||
| Tendon damage | |||||
| No | 218 | 230 | 448 | 96.5 | 98.3 |
| Yes | 6 | 2 | 8 | 2.7 | 0.9 |
| Chi-squared test: p-value = 0.263 (statistic = 1.255, df = 1). Fisher’s exact test: p-value = 0.169 | |||||
| Blood vessel damage | |||||
| No | 218 | 228 | 446 | 96.5 | 97.4 |
| Yes | 6 | 4 | 10 | 2.7 | 1.7 |
| Chi-squared test: p-value = 0.707 (statistic = 0.141, df = 1). Fisher’s exact test: p-value = 0.538 | |||||
| Delayed (non-) union | |||||
| No | 216 | 229 | 445 | 95.6 | 97.9 |
| Yes | 8 | 3 | 11 | 3.5 | 1.3 |
| Chi-squared test: p-value = 0.201 (statistic = 1.638, df = 1). Fisher’s exact test: p-value = 0.135 | |||||
| Surgery to remove metalwork | |||||
| No | 203 | 209 | 412 | 89.8 | 89.3 |
| Yes | 21 | 23 | 44 | 9.3 | 9.8 |
| Chi-squared test: p-value = 0.971 (statistic = 0.001, df = 1). Fisher’s exact test: p-value = 0.875 | |||||
| Regional pain syndrome | |||||
| No | 224 | 231 | 455 | 99.1 | 98.7 |
| Yes | 0 | 1 | 1 | 0.0 | 0.4 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Other | |||||
| No | 185 | 187 | 372 | 81.9 | 79.9 |
| Yes | 39 | 45 | 84 | 17.3 | 19.2 |
| Chi-squared test: p-value = 0.670 (statistic = 0.182, df = 1). Fisher’s exact test: p-value = 0.630 | |||||
| Final wound closure method | |||||
| Free flap | 91 | 81 | 172 | 40.3 | 34.6 |
| Local flap | 22 | 20 | 42 | 9.7 | 8.5 |
| Other | 10 | 13 | 23 | 4.4 | 5.6 |
| Primarily | 37 | 44 | 81 | 16.4 | 18.8 |
| Skin graft | 48 | 51 | 99 | 21.2 | 21.8 |
| Chi-squared test: p-value = 0.780 (statistic = 1.761, df = 4). Fisher’s exact test: p-value = 0.782 | |||||
| Complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Red and inflamed | |||||
| No | 117 | 123 | 240 | 51.8 | 52.6 |
| Yes | 30 | 48 | 78 | 13.3 | 20.5 |
| Chi-squared test: p-value = 0.146 (statistic = 2.11, df = 1). Fisher’s exact test: p-value = 0.119 | |||||
| Swollen | |||||
| No | 118 | 133 | 251 | 52.2 | 56.8 |
| Yes | 29 | 38 | 67 | 12.8 | 16.2 |
| Chi-squared test: p-value = 0.685 (statistic = 0.165, df = 1). Fisher’s exact test: p-value = 0.679 | |||||
| Fluid leakage | |||||
| No | 93 | 102 | 195 | 41.2 | 43.6 |
| Yes | 54 | 69 | 123 | 23.9 | 29.5 |
| Chi-squared test: p-value = 0.586 (statistic = 0.297, df = 1). Fisher’s exact test: p-value = 0.564 | |||||
| Fluid clear or blood stained | |||||
| No | 108 | 115 | 223 | 47.8 | 49.1 |
| Yes | 39 | 56 | 95 | 17.3 | 23.9 |
| Chi-squared test: p-value = 0.278 (statistic = 1.177, df = 1). Fisher’s exact test: p-value = 0.269 | |||||
| Fluid yellow or green pus | |||||
| No | 115 | 142 | 257 | 50.9 | 60.7 |
| Yes | 32 | 29 | 61 | 14.2 | 12.4 |
| Chi-squared test: p-value = 0.346 (statistic = 0.89, df = 1). Fisher’s exact test: p-value = 0.318 | |||||
| Increased pain around wound | |||||
| No | 108 | 125 | 233 | 47.8 | 53.4 |
| Yes | 39 | 46 | 85 | 17.3 | 19.7 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Edge of wound open | |||||
| No | 121 | 142 | 263 | 53.5 | 60.7 |
| Yes | 26 | 29 | 55 | 11.5 | 12.4 |
| Chi-squared test: p-value = 0.982 (statistic = 0.001, df = 1). Fisher’s exact test: p-value = 0.883 | |||||
| Sample for laboratory | |||||
| No | 120 | 136 | 256 | 53.1 | 58.1 |
| Yes | 27 | 35 | 62 | 11.9 | 15.0 |
| Chi-squared test: p-value = 0.742 (statistic = 0.109, df = 1). Fisher’s exact test: p-value = 0.672 | |||||
| Further surgery for fracture | |||||
| No | 132 | 152 | 284 | 58.4 | 65.0 |
| Yes | 15 | 19 | 34 | 6.6 | 8.1 |
| Chi-squared test: p-value = 0.937 (statistic = 0.006, df = 1). Fisher’s exact test: p-value = 0.857 | |||||
| DVT | |||||
| No | 145 | 165 | 310 | 64.2 | 70.5 |
| Yes | 2 | 6 | 8 | 0.9 | 2.6 |
| Chi-squared test: p-value = 0.390 (statistic = 0.74, df = 1). Fisher’s exact test: p-value = 0.294 | |||||
| If yes, did you see DVT nurse | |||||
| No | 145 | 168 | 313 | 64.2 | 71.8 |
| Yes | 2 | 3 | 5 | 0.9 | 1.3 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| DVT medication | |||||
| No | 145 | 166 | 311 | 64.2 | 70.9 |
| Yes | 2 | 5 | 7 | 0.9 | 2.1 |
| Chi-squared test: p-value = 0.573 (statistic = 0.318, df = 1). Fisher’s exact test: p-value = 0.457 | |||||
| Other complication | |||||
| No | 117 | 135 | 252 | 51.8 | 57.7 |
| Yes | 30 | 36 | 66 | 13.3 | 15.4 |
| Chi-squared test: p-value = 0.998 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Unscheduled hospital appointment | |||||
| No | 130 | 146 | 276 | 57.5 | 62.4 |
| Yes | 17 | 25 | 42 | 7.5 | 10.7 |
| Chi-squared test: p-value = 0.525 (statistic = 0.405, df = 1). Fisher’s exact test: p-value = 0.507 | |||||
| Any other problems | |||||
| No | 80 | 85 | 165 | 35.4 | 36.3 |
| Yes | 67 | 86 | 153 | 29.6 | 36.8 |
| Chi-squared test: p-value = 0.468 (statistic = 0.528, df = 1). Fisher’s exact test: p-value = 0.432 | |||||
| Complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Red and inflamed | |||||
| No | 115 | 120 | 235 | 50.9 | 51.3 |
| Yes | 24 | 35 | 59 | 10.6 | 15.0 |
| Chi-squared test. p-value = 0.322 (statistic = 0.98, df = 1). Fisher’s exact test: p-value = 0.308 | |||||
| Swollen | |||||
| No | 108 | 123 | 231 | 47.8 | 52.6 |
| Yes | 31 | 32 | 63 | 13.7 | 13.7 |
| Chi-squared test: p-value = 0.839 (statistic = 0.041, df = 1). Fisher’s exact test: p-value = 0.777 | |||||
| Fluid leakage | |||||
| No | 103 | 109 | 212 | 45.6 | 46.6 |
| Yes | 36 | 46 | 82 | 15.9 | 19.7 |
| Chi-squared test: p-value = 0.555 (statistic = 0.349, df = 1). Fisher’s exact test: p-value = 0.516 | |||||
| Fluid clear or blood stained | |||||
| No | 116 | 127 | 243 | 51.3 | 54.3 |
| Yes | 23 | 28 | 51 | 10.2 | 12.0 |
| Chi-squared test: p-value = 0.850 (statistic = 0.036, df = 1). Fisher’s exact test: p-value = 0.760 | |||||
| Fluid yellow or green pus | |||||
| No | 118 | 130 | 248 | 52.2 | 55.6 |
| Yes | 21 | 25 | 46 | 9.3 | 10.7 |
| Chi-squared test: p-value = 0.936 (statistic = 0.006, df = 1). Fisher’s exact test: p-value = 0.873 | |||||
| Increased pain around wound | |||||
| No | 109 | 118 | 227 | 48.2 | 50.4 |
| Yes | 30 | 37 | 67 | 13.3 | 15.8 |
| Chi-squared test: p-value = 0.743 (statistic = 0.107, df = 1). Fisher’s exact test: p-value = 0.678 | |||||
| Edge of wound open | |||||
| No | 125 | 138 | 263 | 55.3 | 59.0 |
| Yes | 14 | 17 | 31 | 6.2 | 7.3 |
| Chi-squared test: p-value = 0.953 (statistic = 0.004, df = 1). Fisher’s exact test: p-value = 0.851 | |||||
| Sample for laboratory | |||||
| No | 120 | 128 | 248 | 53.1 | 54.7 |
| Yes | 19 | 27 | 46 | 8.4 | 11.5 |
| Chi-squared test: p-value = 0.470 (statistic = 0.523, df = 1). Fisher’s exact test: p-value = 0.423 | |||||
| Further surgery for fracture | |||||
| No | 118 | 128 | 246 | 52.2 | 54.7 |
| Yes | 21 | 27 | 48 | 9.3 | 11.5 |
| Chi-squared test: p-value = 0.706 (statistic = 0.142, df = 1). Fisher’s exact test: p-value = 0.638 | |||||
| DVT | |||||
| No | 135 | 152 | 287 | 59.7 | 65.0 |
| Yes | 4 | 3 | 7 | 1.8 | 1.3 |
| Chi-squared test: p-value = 0.884 (statistic = 0.021, df = 1). Fisher’s exact test: p-value = 0.711 | |||||
| If yes, did you see DVT nurse | |||||
| No | 136 | 152 | 288 | 60.2 | 65.0 |
| Yes | 3 | 3 | 6 | 1.3 | 1.3 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| DVT medication | |||||
| No | 135 | 152 | 287 | 59.7 | 65.0 |
| Yes | 4 | 3 | 7 | 1.8 | 1.3 |
| Chi-squared test: p-value = 0.884 (statistic = 0.021, df = 1). Fisher’s exact test: p-value = 0.711 | |||||
| Other complication | |||||
| No | 114 | 124 | 238 | 50.4 | 53.0 |
| Yes | 25 | 31 | 56 | 11.1 | 13.2 |
| Chi-squared test: p-value = 0.772 (statistic = 0.084, df = 1). Fisher’s exact test: p-value = 0.766 | |||||
| Unscheduled hospital appointment | |||||
| No | 128 | 140 | 268 | 56.6 | 59.8 |
| Yes | 11 | 15 | 26 | 4.9 | 6.4 |
| Chi-squared test: p-value = 0.744 (statistic = 0.106, df = 1). Fisher’s exact test: p-value = 0.683 | |||||
| Any other problems | |||||
| No | 79 | 88 | 167 | 35.0 | 37.6 |
| Yes | 60 | 67 | 127 | 26.5 | 28.6 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Red and inflamed | |||||
| No | 106 | 119 | 225 | 46.9 | 50.9 |
| Yes | 25 | 23 | 48 | 11.1 | 9.8 |
| Chi-squared test: p-value = 0.641 (statistic = 0.218, df = 1). Fisher’s exact test: p-value = 0.633 | |||||
| Swollen | |||||
| No | 112 | 118 | 230 | 49.6 | 50.4 |
| Yes | 19 | 24 | 43 | 8.4 | 10.3 |
| Chi-squared test: p-value = 0.706 (statistic = 0.142, df = 1). Fisher’s exact test: p-value = 0.621 | |||||
| Fluid leakage | |||||
| No | 107 | 118 | 225 | 47.3 | 50.4 |
| Yes | 24 | 24 | 48 | 10.6 | 10.3 |
| Chi-squared test: p-value = 0.882 (statistic = 0.022, df = 1). Fisher’s exact test: p-value = 0.874 | |||||
| Fluid clear or blood stained | |||||
| No | 115 | 122 | 237 | 50.9 | 52.1 |
| Yes | 16 | 20 | 36 | 7.1 | 8.5 |
| Chi-squared test: p-value = 0.781 (statistic = 0.077, df = 1). Fisher’s exact test: p-value = 0.722 | |||||
| Fluid yellow or green pus | |||||
| No | 122 | 128 | 250 | 54.0 | 54.7 |
| Yes | 9 | 14 | 23 | 4.0 | 6.0 |
| Chi-squared test: p-value = 0.503 (statistic = 0.449, df = 1). Fisher’s exact test: p-value = 0.394 | |||||
| Increased pain around wound | |||||
| No | 107 | 120 | 227 | 47.3 | 51.3 |
| Yes | 24 | 22 | 46 | 10.6 | 9.4 |
| Chi-squared test: p-value = 0.644 (statistic = 0.213, df = 1). Fisher’s exact test: p-value = 0.628 | |||||
| Edge of wound open | |||||
| No | 124 | 136 | 260 | 54.9 | 58.1 |
| Yes | 7 | 6 | 13 | 3.1 | 2.6 |
| Chi-squared test: p-value = 0.882 (statistic = 0.022, df = 1). Fisher’s exact test: p-value = 0.779 | |||||
| Sample for laboratory | |||||
| No | 122 | 132 | 254 | 54.0 | 56.4 |
| Yes | 9 | 10 | 19 | 4.0 | 4.3 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Further surgery for fracture | |||||
| No | 111 | 120 | 231 | 49.1 | 51.3 |
| Yes | 20 | 22 | 42 | 8.8 | 9.4 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| DVT | |||||
| No | 129 | 140 | 269 | 57.1 | 59.8 |
| Yes | 2 | 2 | 4 | 0.9 | 0.9 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| If yes, did you see DVT nurse | |||||
| No | 130 | 140 | 270 | 57.5 | 59.8 |
| Yes | 1 | 2 | 3 | 0.4 | 0.9 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| DVT medication | |||||
| No | 130 | 141 | 271 | 57.5 | 60.3 |
| Yes | 1 | 1 | 2 | 0.4 | 0.4 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Other complication | |||||
| No | 109 | 113 | 222 | 48.2 | 48.3 |
| Yes | 22 | 29 | 51 | 9.7 | 12.4 |
| Chi-squared test: p-value = 0.540 (statistic = 0.376, df = 1). Fisher’s exact test: p-value = 0.535 | |||||
| Unscheduled hospital appointment | |||||
| No | 119 | 135 | 254 | 52.7 | 57.7 |
| Yes | 12 | 7 | 19 | 5.3 | 3.0 |
| Chi-squared test: p-value = 0.257 (statistic = 1.287, df = 1). Fisher’s exact test: p-value = 0.234 | |||||
| Any other problems | |||||
| No | 86 | 103 | 189 | 38.1 | 44.0 |
| Yes | 45 | 39 | 84 | 19.9 | 16.7 |
| Chi-squared test: p-value = 0.271 (statistic = 1.211, df = 1). Fisher’s exact test: p-value = 0.239 | |||||
| Complications | Treatment group | Total | Treatment group (%) | ||
|---|---|---|---|---|---|
| NPWT (n = 226) | Standard (n = 234) | NPWT | Standard | ||
| Red and inflamed | |||||
| No | 139 | 155 | 294 | 61.5 | 66.2 |
| Yes | 18 | 25 | 43 | 8.0 | 10.7 |
| Chi-squared test: p-value = 0.616 (statistic = 0.252, df = 1). Fisher’s exact test: p-value = 0.518 | |||||
| Swollen | |||||
| No | 137 | 156 | 293 | 60.6 | 66.7 |
| Yes | 20 | 24 | 44 | 8.8 | 10.3 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Fluid leakage | |||||
| No | 142 | 150 | 292 | 62.8 | 64.1 |
| Yes | 15 | 30 | 45 | 6.6 | 12.8 |
| Chi-squared test: p-value = 0.079 (statistic = 3.078, df = 1). Fisher’s exact test: p-value = 0.077 | |||||
| Fluid clear or blood stained | |||||
| No | 143 | 155 | 298 | 63.3 | 66.2 |
| Yes | 14 | 25 | 39 | 6.2 | 10.7 |
| Chi-squared test: p-value = 0.210 (statistic = 1.569, df = 1). Fisher’s exact test: p-value = 0.174 | |||||
| Fluid yellow or green pus | |||||
| No | 153 | 169 | 322 | 67.7 | 72.2 |
| Yes | 4 | 11 | 15 | 1.8 | 4.7 |
| Chi-squared test: p-value = 0.188 (statistic = 1.736, df = 1). Fisher’s exact test: p-value = 0.184 | |||||
| Increased pain around wound | |||||
| No | 136 | 152 | 288 | 60.2 | 65.0 |
| Yes | 21 | 28 | 49 | 9.3 | 12.0 |
| Chi-squared test: p-value = 0.681 (statistic = 0.169, df = 1). Fisher’s exact test: p-value = 0.643 | |||||
| Edge of wound open | |||||
| No | 152 | 170 | 322 | 67.3 | 72.6 |
| Yes | 5 | 10 | 15 | 2.2 | 4.3 |
| Chi-squared test: p-value = 0.431 (statistic = 0.621, df = 1). Fisher’s exact test: p-value = 0.428 | |||||
| Sample for laboratory | |||||
| No | 146 | 166 | 312 | 64.6 | 70.9 |
| Yes | 11 | 14 | 25 | 4.9 | 6.0 |
| Chi-squared test: p-value = 0.951 (statistic = 0.004, df = 1). Fisher’s exact test: p-value = 0.837 | |||||
| Further surgery for fracture | |||||
| No | 142 | 157 | 299 | 62.8 | 67.1 |
| Yes | 15 | 23 | 38 | 6.6 | 9.8 |
| Chi-squared test: p-value = 0.447 (statistic = 0.579, df = 1). Fisher’s exact test: p-value = 0.391 | |||||
| DVT | |||||
| No | 154 | 177 | 331 | 68.1 | 75.6 |
| Yes | 3 | 3 | 6 | 1.3 | 1.3 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| If yes, did you see DVT nurse | |||||
| No | 156 | 177 | 333 | 69.0 | 75.6 |
| Yes | 1 | 3 | 4 | 0.4 | 1.3 |
| Chi-squared test: p-value = 0.714 (statistic = 0.134, df = 1). Fisher’s exact test: p-value = 0.626 | |||||
| DVT medication | |||||
| No | 155 | 178 | 333 | 68.6 | 76.1 |
| Yes | 2 | 2 | 4 | 0.9 | 0.9 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Other complication | |||||
| No | 134 | 147 | 281 | 59.3 | 62.8 |
| Yes | 23 | 33 | 56 | 10.2 | 14.1 |
| Chi-squared test: p-value = 0.448 (statistic = 0.577, df = 1). Fisher’s exact test: p-value = 0.383 | |||||
| Unscheduled hospital appointment | |||||
| No | 146 | 168 | 314 | 64.6 | 71.8 |
| Yes | 11 | 12 | 23 | 4.9 | 5.1 |
| Chi-squared test: p-value = 1 (statistic = 0, df = 1). Fisher’s exact test: p-value = 1 | |||||
| Any other problems | |||||
| No | 121 | 135 | 256 | 53.5 | 57.7 |
| Yes | 36 | 45 | 81 | 15.9 | 19.2 |
| Chi-squared test: p-value = 0.752 (statistic = 0.1, df = 1). Fisher’s exact test: p-value = 0.702 | |||||
List of abbreviations
- AE
- adverse event
- ANOVA
- analysis of variance
- BNF
- British National Formulary
- CDC
- Centers for Disease Control and Prevention
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- DRI
- Disability Rating Index
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- G&A
- Gustilo and Anderson
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MAR
- missing at random
- MAU
- multiattribute utility
- MCA
- Mental Capacity Act
- MCID
- minimal clinically important difference
- MCS
- mental component score
- MTC
- major trauma centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPWT
- negative-pressure wound therapy
- OR
- odds ratio
- PCS
- physical component score
- PP
- per protocol
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- SF-6D
- Short Form questionnaire 6-Dimensions
- SF-12
- Short Form questionnaire-12 items
- SSI
- surgical site infection
- TMG
- Trial Management Group
- TNO
- trial number
- TSC
- Trial Steering Committee
- UHCW
- University Hospitals Coventry and Warwickshire
- VAS
- visual analogue scale
- WCTU
- Warwick Clinical Trials Unit
- WOLLF
- Wound management of Open Lower Limb Fractures
