Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/192. The contractual start date was in January 2011. The draft report began editorial review in August 2017 and was accepted for publication in March 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Wendy Atkin and Amanda J Cross report grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, grants from Cancer Research UK (Population Research Committee – Programme Award C8171/A16894) and non-financial support from Eiken Chemical Co. Ltd (Tokyo, Japan) (MAST is UK distributor) during the conduct of the study. Stephen Morris is a member of the NIHR Health Services and Delivery Research funding board. Sheena Pearson, Carolyn Piggott and Julia Snowball all report grants from the NIHR HTA programme during the conduct of the study.
Disclaimer
This is a summary of independent research funded by the NIHR HTA programme and the Bobby Moore Fund for Cancer Research UK. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, the Department of Health and Social Care or Cancer Research UK. Infractructure support for this work was provided by the NIHR Imperial Biomedical Research Centre.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Atkin et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Colorectal cancer (CRC) is a UK health-care priority. It is estimated that 1 in 14 men and 1 in 19 women will develop the disease in their lifetime. 1 CRC is the second most frequent cause of cancer death in the UK, with 41,000 new diagnoses and 16,000 deaths in 2014. The estimated annual cost of CRC to the NHS is more than £1B. 1,2
Most CRCs develop from adenomas. 3–6 Adenomas are common and their prevalence is around 30–40% at 60 years. 7 Most adenomas do not, however, become cancerous. 8
Colorectal cancer screening
Screening enables the early detection of CRC and endoscopic removal of adenomas and is highly effective at reducing CRC mortality rates. 9–15 In England, the NHS Bowel Cancer Screening Programme (BCSP) offers CRC screening to men and women aged between 60 and 74 years. Every 2 years, patients are invited to complete a stool test, which is currently the guaiac faecal occult blood test (gFOBT). Among the first million people tested by the BCSP, 2% (21,106/1,079,293) had an abnormal gFOBT result using a three-test-kit algorithm, of whom 83% (17,518/21,106) had a follow-up colonoscopy or other investigative examination. 16 Adoption of an additional screening modality, flexible sigmoidoscopy at age 55 years, began in 2013. 17
Adenoma surveillance
Adenomas detected through screening, surveillance or among patients presenting symptomatically, are typically removed by polypectomy during colonoscopy, flexible sigmoidoscopy or surgery. Following adenoma removal, a proportion of people remain at increased risk of developing advanced adenomas (AAs) (i.e. sized ≥ 10 mm, tubulovillous or villous histology or high-grade dysplasia) or CRC, and are recommended to undergo a surveillance colonoscopy at a later date. 18–20 The risk of developing AAs or CRC following polypectomy is dependent on the characteristics of the baseline colonoscopy, such as completeness and quality of bowel preparation, and on the characteristics of adenomas found at baseline, including number of adenomas, size and pathology. 18–20 Based on the characteristics of adenomas found at baseline, the UK adenoma surveillance guideline (UK-ASG) defines three risk groups with consequent recommendations for surveillance colonoscopy following polypectomy (Figure 1). 21
FIGURE 1.
The UK-ASG. 21–23 a, Other considerations: age, comorbidity, family history, accuracy and completeness of examination. Reproduced from surveillance guidelines after removal of colorectal adenomatous polyps, Atkins and Saunders, vol. 51, pp. v6–9, 2002,21 with permission from BMJ Publishing Group Ltd.
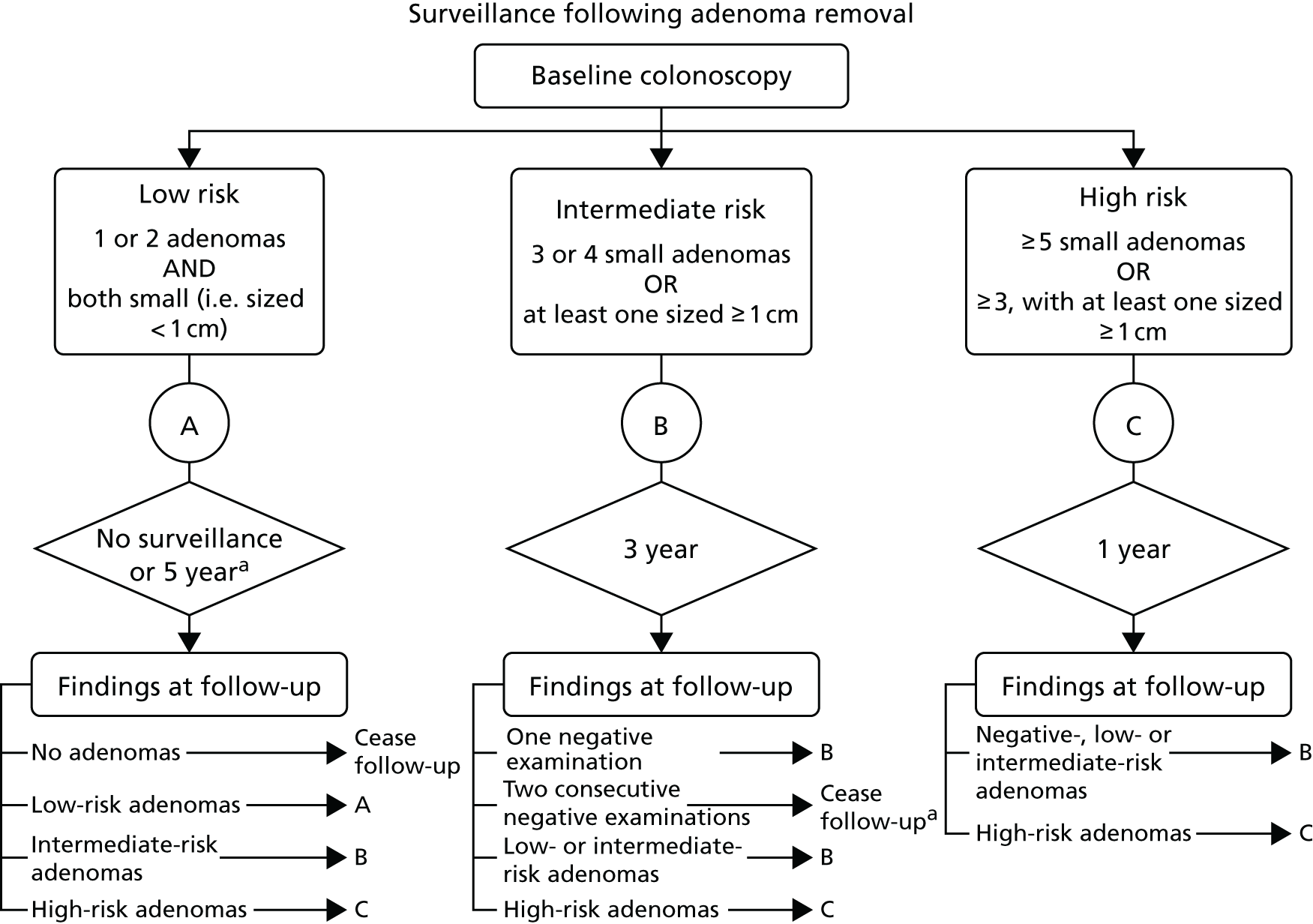
Low risk: patients with only one or two small adenomas (i.e. sized < 10 mm)
Low-risk patients identified after referral to hospital, because of symptoms or diagnostic test results, are commonly offered surveillance at 5 years. 24,25 In contrast, low-risk patients identified by the BCSP are not offered surveillance but return to routine biennial gFOBT screening for as long as they remain within the eligible age range. 26
Among the first million individuals tested by the BCSP, 7514 out of the 17,518 (42.9%) individuals attending colonic examination following positive gFOBT were found to have adenomas. Around 37% (2743/7514) of patients with adenomas identified by the BCSP were considered as low risk. 16
Intermediate risk: patients with three or four small adenomas (i.e. sized < 10 mm) or one or two large adenomas (i.e. sized ≥ 10 mm)
This group constituted 41% (3050/7514) of adenoma patients identified by the BCSP among the first million tested. 16 Based on the UK-ASG, the BCSP recommends colonoscopy surveillance for the intermediate-risk group at 3 years.
High risk: patients with five or more small adenomas (i.e. sized < 10 mm) or three or more adenomas, at least one of which is large (i.e. sized ≥ 10 mm)
This group constituted 23% (1721/7514) of adenoma patients identified by the BCSP among the first million tested. 16 Based on the UK-ASG, the BCSP recommends a clearing colonoscopy 1 year after first diagnosis for high-risk patients. This recommendation is based on the high detection rate of AAs after 1 year and on the risk of missed or incompletely removed lesions when large or multiple adenomas are removed. 19,27,28
Colonoscopy in post-polypectomy surveillance
Colonoscopy is the most sensitive examination for CRC and adenomas. However, there are a number of problems with its use as a surveillance method.
Colonoscopy is an expensive procedure because of the requirement for skilled endoscopists and the use of sedation. The increased detection of adenomas requiring colonoscopy surveillance from the BCSP is reaching the point at which demand is overwhelming the available endoscopy workforce. 29 Currently, post-polypectomy surveillance accounts for approximately 20% of colonoscopies in the UK, and this figure will inevitably rise as more people enter surveillance. 30
There are disadvantages to colonoscopy for the patient undergoing the examination. Bowel preparation is typically unpleasant and the colonoscopy itself can be uncomfortable. 31,32 There is a small risk of serious complications (e.g. severe bleeding and colon perforation) and that risk increases with age. 33 Attendance for colonoscopy after a positive gFOBT is poor (around 80%), possibly because of procedural anxiety, in addition to other patient-reported barriers such as fear of finding cancer and anticipated pain. 16,34–36
Colonoscopy is not 100% sensitive and sometimes lesions are missed. Studies suggest that > 50% of post-colonoscopy CRCs result from missed lesions. 37 Delayed diagnosis of CRC occurring as a result of the surveillance interval could be detrimental to patient outcomes. 38
The yield of CRC and AAs at each surveillance colonoscopy is low. Even in higher-risk groups, the yield is < 20%, meaning that > 80% of colonoscopies will either be negative or detect only small adenomas of low malignant potential. 18,19,39,40 Negative colonoscopies provide no therapeutic benefit other than reassurance while contributing to cost, discomfort and risk.
For these reasons, a different, more cost-effective, method of surveillance following adenoma removal is urgently required. The faecal immunochemical test (FIT) for occult blood in stool may be an effective alternative.
Faecal immunochemical tests and guaiac faecal occult blood tests
Many screening programmes use gFOBT. In randomised controlled trials (RCTs), CRC mortality rates were reduced by 15%, on average, in people offered the gFOBT, and by 25% in those accepting the gFOBT. 13,41,42 However, an alternative stool test, FIT, which is due to replace gFOBT in the BCSP, has many advantages over gFOBT. 43,44
The FIT uses antibodies raised against the globin component of haemoglobin, whereas gFOBT is based on a chemical reaction involving haem. Unlike gFOBT, FIT is not subject to interference from dietary haemoglobin in red meat or peroxidases in vegetables. 45 Reduced dietary interference with FIT enables its increased analytical sensitivity to be exploited without diminution of analytical specificity. Furthermore, FIT is less susceptible than gFOBT to false positives from upper gastrointestinal (GI) tract bleeding as the globin component of haemoglobin is degraded by gastric proteases in the upper GI tract.
Quantitative FIT enables the selection of a preferred faecal haemoglobin threshold level for positivity, allowing the adjustment of clinical sensitivity and specificity so that the test is clinically acceptable while not overwhelming endoscopy resources. 46–48 At a low haemoglobin threshold, FIT can detect lower levels of bleeding than gFOBT and, therefore, has a higher sensitivity for CRC and AAs. 49–51 Quantitative FIT provides the opportunity to incorporate haemoglobin concentration into a multivariable CRC risk score, rather than the binary risk (positive or negative) afforded by gFOBT. 52
Test uptake is reported to be higher with FIT than gFOBT. 53,54 Individuals may find FIT more acceptable than gFOBT because the kit is typically simpler to use, less messy and usually requires only one stool sample (unlike the widely used three-sample, six-window gFOBT). 53
Given the advantages of FIT over gFOBT, many national screening programmes have started to use FIT to screen average-risk populations. 42 A systematic review examined the performance of FIT in the screening context and found that at a threshold of 20 µg haemoglobin per gram of faeces (hereafter referred to as µg/g), sensitivity for CRC approximated 90%. 55 Sensitivity for AAs is reported to be lower, typically < 50%. 56
Faecal immunochemical tests in post-polypectomy surveillance
Given the high sensitivity of low-threshold FIT for CRC in the screening setting, FIT could be useful for post-polypectomy surveillance. However, few published studies have looked at the use of FIT for this purpose and those few that have included patients undergoing surveillance for reasons other than adenomas (e.g. family or personal history of CRC). 46,57–62
A number of studies have examined the performance of interval FIT in addition to colonoscopy surveillance. 58,60,61 In the first of these studies,58 1641 individuals enrolled in colonoscopy surveillance (538 of whom were undergoing surveillance because of a personal history of neoplasia) were invited to complete a qualitative FIT [Inform, Enterix Pty Ltd, Sydney, NSW, Australia; Inform is known as InSure (Enterix Inc., Edison, NJ) in the USA] in the interval prior to colonoscopy. Among the 792 who responded and completed a FIT, 57 (7.2%) tested positive. Of the 57 who tested positive, six (10.5%) were diagnosed with CRC and eight (14.0%) had a significant adenoma. The study authors suggested that using FIT in surveillance could speed detection of interval CRCs. However, the study did not report the results of subsequent colonoscopy in those testing FIT negative and, therefore, sensitivity and specificity of FIT could not be calculated.
A second study, by Cole et al. ,60 is, to our knowledge, the only published study besides ours to have examined the performance of annual FIT in surveillance. A total of 1736 individuals enrolled in colonoscopy surveillance (984 of whom had prior adenomas) were invited to complete annual qualitative FIT (InSure) in the interval prior to surveillance colonoscopy. Of these 1736 individuals, 1071 (61.7%) completed at least one FIT (the median number completed was two) in the interval prior to surveillance colonoscopy, of whom 379 (35.4%) tested positive at least once. Colonoscopy was performed either following positive FIT or, in those testing FIT negative, after the designated surveillance interval. Sensitivity of repeated FIT was 85.7% (12/14) for CRC and 62.5% (60/96) for AAs. In cases in which CRC and AAs were diagnosed following a positive test, it is estimated that FIT brought forward colonoscopy by a median of 25 months and 24 months, respectively.
Most recently, Cole et al. 61 examined the performance of two-sample interval quantitative FIT (OC-Sensor, Eiken Chemical Co. Ltd, Tokyo, Japan; 20 µg/g threshold) in addition to colonoscopy surveillance. In total, 804 colonoscopies were performed early bacause of a positive FIT. As a result, nine (1.1%) patients were diagnosed with CRC and 162 (20.1%) patients were diagnosed with AAs. The results of subsequent surveillance colonoscopy in those patients testing negative were not reported and, therefore, sensitivity could not be calculated. However, the findings indicate that FITs could bring forward detection of significant neoplasia if used in combination with colonoscopy surveillance.
In addition to these studies, there have been a number of others that have tested the performance of one round of FIT directly prior to surveillance colonoscopy. Robinson et al. 57 invited 919 individuals (420 of whom had prior adenomas) to complete a qualitative FIT (HemeSelect, SmithKline Diagnostics, San Jose, CA, USA) before surveillance colonoscopy. In the 808 who complied, sensitivity for CRC was 70.0% (7/10) and sensitivity for large adenomas (i.e. sized ≥ 10 mm) was 44.4% (16/36). Hazazi et al. 59 invited 1469 individuals to complete three FITs (OC-Micro, Eiken Chemical Co. Ltd) prior to colonoscopy. Of 1469 invited, 1000 completed a FIT (337 of whom had prior polyps). Sensitivity of the first of three FITs at 10 µg/g was 100.0% (8/8) for CRC and 44.4% (32/72) for advanced colorectal neoplasia (ACN) (CRC and/or AAs). Using the highest haemoglobin concentration of all three FITs, sensitivity for ACN increased to 65.3% (47/72).
Although the study by Hazazi et al. 59 indicated that low-threshold quantitative FITs had high accuracy for ACN, a more recent study by Terhaar sive Droste et al. 46 of quantitative FIT (OC-Sensor) reported lower sensitivity. At a threshold of 10 µg/g, sensitivity was 80.0% (4/5) for CRC and 30.2% (32/106) for ACN. Both studies, however, had limited sample sizes and did not test the performance of multiple rounds of FIT.
These studies provide some evidence to suggest that FIT could be a useful tool for surveillance following adenoma removal. However, further research is needed on the accuracy, acceptability and cost-effectiveness of repeated FIT for detecting CRC and AAs in a large cohort of individuals. For this reason we developed the FIT for Follow-Up study.
Study aim
The aim of the FIT for Follow-Up study was to determine whether or not annual FIT is a feasible, safe, acceptable and cost-saving alternative to colonoscopy surveillance for the detection of CRC and AAs following adenoma removal. We proposed to offer annual FIT to people deemed at intermediate risk because of adenomas (see Figure 1) detected following a positive gFOBT completed as part of the BCSP in England. As is standard UK practice,21 these individuals were scheduled to have a surveillance colonoscopy at 3 years. In the BCSP, intermediate-risk patients account for approximately 40% of all patients diagnosed with adenomas, and approximately 5000 intermediate-risk patients a year are identified. 16 We hypothesised that, by using FIT as an alternative to colonoscopy surveillance, the number of colonoscopies could be greatly reduced, with minimal loss of sensitivity.
Primary objective
To determine the 3-year programme sensitivity of annual FITs compared with colonoscopy surveillance at 3 years, for the detection of CRC or AAs in patients categorised as at intermediate risk because of adenomas detected at colonoscopy following a positive gFOBT completed as part of the BCSP.
Secondary objectives
-
To estimate the diagnostic accuracy of FIT at first, second and third tests and over two or three tests at various thresholds.
-
To examine the acceptability of FIT, compared with colonoscopy, as a method of surveillance for people at increased risk of CRC.
-
To calculate the incremental costs and cost-effectiveness of FIT versus colonoscopy surveillance.
Chapter 2 Methods
The FIT for Follow-Up study compared the accuracy, acceptability and cost-effectiveness of three annual FITs compared with colonoscopy at 3 years for surveillance of intermediate-risk patients following the removal of adenomas. The reporting of this study is in accordance with Standards of Reporting of Diagnostic Accuracy (STARD) guidelines. 63
Literature search
We searched MEDLINE and EMBASE, using text words and medical subject heading (MeSH)/EMTREE terms, to identify studies using FIT in a surveillance setting published on or before 6 April 2017. Reference lists of relevant articles were also reviewed to identify further pertinent studies.
Research governance and ethics arrangements
The FIT for Follow-Up study was prospectively registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry (ISRCTN18040196). Imperial College London was the nominated sponsor of the study. A trial steering committee provided independent oversight and advice.
In line with Imperial College London research governance procedures, relevant approvals were obtained before the study commenced, and appropriate regulations and guidelines were followed. The BCSP Research Committee granted support for the study on 7 October 2009. The London – City and East Research Ethics Committee approved the study on 17 May 2011 (reference number 11/LO/0326). Further approval was granted for substantial amendments to (1) invite a subset of participants to an end-of-study interview, (2) add a ‘health and lifestyle’ questionnaire to round 3 of testing and increase the FIT positivity threshold from 20 µg/g to 40 µg/g and (3) include a reminder letter to be sent to participants who had not yet attended their routine 3-year surveillance colonoscopy. The Ethics and Confidentiality Committee (ECC) of the National Information Governance Board (now known as the Health Research Authority Confidentiality Advisory Group) granted approval [reference number ECC 3-04(p)/2011] to access patient identifiers and contact details on 10 June 2011, allowing the identification of eligible participants and invitation by the BCSP.
Participant recruitment
Individuals who had been identified as being at intermediate risk (one or two adenomas of ≥ 10 mm in size, or three or four adenomas of < 10 mm in size, no CRC) within the previous 12 months were identified from those who had attended colonoscopy after positive gFOBT in the English BCSP.
Individuals meeting the eligibility criteria were identified by NHS Digital (formerly known as the Health and Social Care Information Centre) using the BCSP’s database, the Bowel Cancer Screening System (BCSS). NHS Digital sent encrypted information on potentially eligible individuals to the BCSP Southern Hub. In the BCSP, there are five regional hubs (Southern Hub, Eastern Hub, Midlands and North West Hub, London Hub and North East Hub) that receive and analyse gFOBT kits. 16 However, for the purpose of this study, analysis and distribution of kits were centralised; kits were sent and analysed from one hub, the BCSP Southern Hub, regardless of the invitee’s location. The BCSP Southern Hub sent an invitation letter, a detailed participant information sheet, a consent form, a short baseline questionnaire and a FIT kit to eligible individuals between January 2012 and December 2013 (see Report Supplementary Material 1 for study documentation). Individuals who returned a completed consent form and an analysable FIT constituted the study cohort.
Eligibility criteria
Criteria for inclusion were:
-
aged 60–72 years at recruitment
-
diagnosed < 1 year previously with intermediate-risk adenomas at colonoscopy following positive gFOBT in the BCSP
-
scheduled for surveillance colonoscopy 3 years after initial colonoscopy, in line with BCSP guidelines for post-polypectomy surveillance of intermediate-risk patients.
Although individuals aged 72–74 years are invited to gFOBT screening in the BCSP, this group was excluded from this study. Given that they would be ≥ 75 years by the end of the study, they would not be eligible for a surveillance colonoscopy according to BCSP guidelines.
After study initiation, it was found that some potentially eligible individuals had received multiple baseline colonoscopies. These individuals were excluded from the study to prevent patient overinvestigation from a further colonoscopy at years 1 or 2 because of a positive FIT result.
Faecal immunochemical test kit processing
The FIT kit comprised a step-by-step instruction wallet, the FIT sampling device (OC-Sensor sampling device, Eiken Chemical Co. Ltd), a plastic zip-lock bag with absorbent material in case of leakage and a foil-lined pre-paid envelope in which to return the completed kit. The distribution of FIT kits and analysis of returned tests was conducted by the BCSP Southern Hub through the patient management system (PMS) (see Data processing and information governance).
Laboratory analysis of the FIT was carried out at the BCSP Southern Hub, using the OC-Sensor DIANA analyser (Eiken Chemical Co. Ltd) in accordance with the manufacturer’s instructions. Samples were refrigerated on receipt and analysis took place within 7 days. Results were uploaded onto the PMS as comma-separated values (CSV) files. Any samples that were > 10 days old on receipt were not analysed. If the sample was spoilt in any other way [damaged packaging, technical fail, non-technical fail (e.g. insufficient sample to analyse)], then a replacement kit was sent to the participant. The coefficient of variation for FIT was 4.5% at a concentration of 20 µg/g and 3.3% at a concentration of 90 µg/g. 64
In the pilot study, the threshold for a FIT positive result was set at 20 µg/g. However, as the percentage of participants testing positive in this pilot was higher than expected, the threshold was subsequently increased to 40 µg/g. This change of threshold was made to ensure that the range of predicted positivity rates would not lead to unsustainable numbers of colonoscopies that would swamp screening centres. The threshold was not changed retrospectively for participants screened within the pilot study (see Pilot study).
Study design
The study was divided into three annual FIT rounds at 1, 2 and 3 years after baseline colonoscopy (i.e. colonoscopy at which intermediate-risk adenomas were detected), as shown in the study flow diagram (Figure 2).
FIGURE 2.
The FIT for Follow-Up study flow diagram. PEQ, participant experience questionnaire; R1, round 1; R2, round 2; R3, round 3 (intervention rounds).
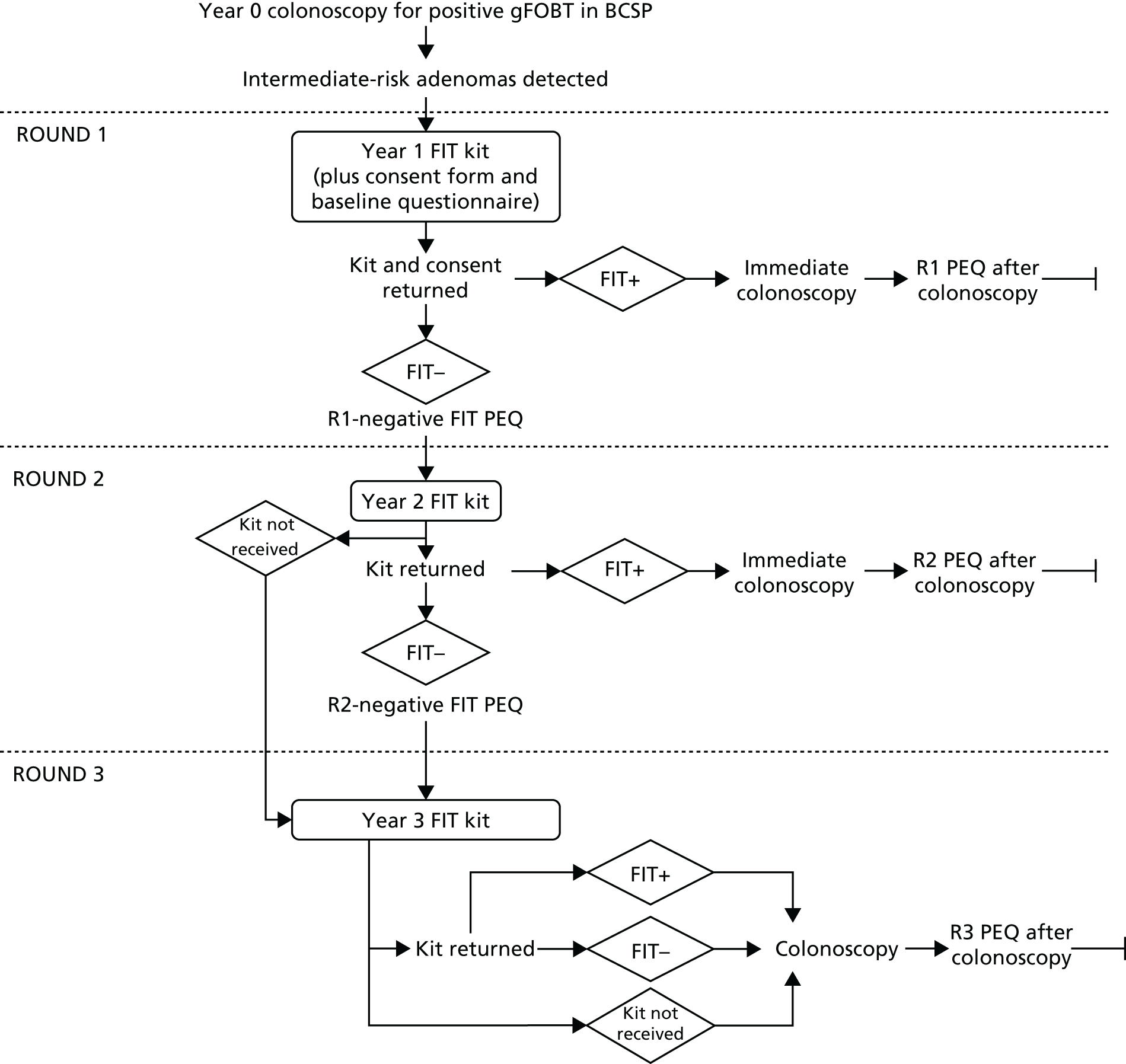
Participants who tested FIT positive at round 1 or 2 were offered an early surveillance colonic examination (typically colonoscopy) and were not invited to further FIT rounds. For participants who accepted the offer, the early colonic examination was the reference standard. If the participant declined the offer in round 1 or 2, they were still invited to an end-of-study colonic examination at 3 years after baseline colonoscopy, which served as the reference standard.
Participants who tested FIT negative in rounds 1 and 2 were eligible for further FIT rounds. All participants offered a round 3 FIT were invited to the routine 3-year surveillance colonic examination, which again served as the reference standard. Those who attended a colonic examination following positive FIT, because of symptoms or at the end of the study, returned to the appropriate surveillance pathway according to the outcomes of the examination (see Figure 1).
Intervention round 1 (year 1)
Eligible individuals were invited to take part in the study approximately 10 weeks before the first anniversary of their baseline colonoscopy. Individuals who did not respond within 3 weeks were sent a reminder to participate.
We decided that the interval between FIT rounds should be at least 9 months. If the participant returned a round 1 negative FIT late, and a round 2 FIT invite would have meant an interval between rounds of < 9 months, then the participant was classed as a round 1 late responder. These participants were not invited to complete a FIT in round 2 but were invited in round 3.
Intervention round 2 (year 2)
Participants who tested FIT negative in round 1 and who were not round 1 late responders were sent a FIT kit in round 2. As in round 1, participants who did not reply within 3 weeks were sent a reminder letter.
Intervention round 3 (year 3)
Participants who tested FIT negative in round 2, who were round 1 late responders and who did not return an analysable round 2 FIT were sent a round 3 FIT. As in rounds 1 and 2, participants who did not reply within 3 weeks were sent a reminder letter.
Throughout the study, the participants and their general practitioners (GPs) were informed of the results of each completed FIT. A dedicated freephone helpline managed by the BCSP Southern Hub was available to participants, GPs and screening centre staff. A study website was also available. 65
Questionnaires and interviews
Participant experience questionnaires were sent by post (1) with the consent form in round 1 (baseline questionnaire), (2) with FIT-negative results letters in rounds 1 and 2 (round 1 negative questionnaire, round 2 negative questionnaire), (3) after attending/declining an early surveillance colonoscopy after a FIT positive result in rounds 1 and 2 (round 1 positive questionnaire, round 2 positive questionnaire) and (4) after the participant had attended their year 3 end-of-study colonic examination (round 3 end-of-study questionnaire) (see Report Supplementary Material 2 for study questionnaires). To avoid unnecessary distress, participants diagnosed with cancer were not sent questionnaires.
End-of-study phone interviews were conducted with a subsample of participants in order to better understand attitudes towards the use of FIT in surveillance (see Chapter 4).
Colonic examinations
The default colonic examination was colonoscopy. However, as is current practice in the BCSP, computed tomographic (CT) colonography or flexible sigmoidoscopy were alternative options when colonoscopy was not deemed appropriate. In the BCSP, only a minority of patients (typically < 3%) are deemed unfit for colonoscopy. 16
If participants tested FIT positive in round 1 or 2, the participant’s affiliated BCSP screening centre was informed that the participant had consented to take part in the study, had tested FIT positive and should be offered an early colonic examination.
Before commencement of the study, all screening centre directors and nominated specialist screening practitioners at the 64 English screening centres were encouraged to support the study, and to contact FIT-positive participants to discuss arranging an earlier colonic examination in a timely manner to reduce participant anxiety. Collaborators at NHS Digital modified the BCSS to allow specialist screening practitioners to amend a participant’s surveillance due date, bring forward the date of the colonic examination and indicate that this was as a result of them taking part in the study and testing FIT positive. The Imperial College London study team contacted the appropriate screening centre on a case-by-case basis for all those who tested FIT positive, retrieved the relevant colonic examination and histology reports for those who attended and updated the PMS to show that the participant had declined an earlier examination.
All participants who did not attend (DNA) an early colonic examination as a result of a positive FIT were still invited for their year 3 surveillance examination. To manage the timely collection of the year 3 surveillance examination reports for participants who tested FIT negative, monthly data downloads from the BCSS with information on participants’ scheduled and attended colonic examinations were sent by NHS Digital to the Imperial College London study team. The team were responsible for contacting the screening centres to determine whether colonic examinations had been arranged or declined and, once colonic examinations had occurred, to retrieve copies of the relevant colonic examination and histology reports. Colonic examination and histology reports were sent directly to data clerks at the Imperial College London central study office by encrypted NHS.net e-mail or secure fax, and were immediately logged on receipt.
Data clerks entered data according to a standard operating procedure (see Report Supplementary Material 3). All data entered were audited by a second data clerk.
Colonic examination data entered included:
-
date of examination
-
type of examination
-
pre-medications used (if any)
-
name of the endoscopist
-
bowel preparation used
-
segment of the bowel reached
-
whether or not the examination was completed
-
the final results of the examination
-
details of polyps discovered in the bowel
-
whether or not a polyp was removed and successfully retrieved
-
polyp pathology (if known)
-
evidence of CRC or AA.
Data processing and information governance
The study was co-ordinated through a purpose-built PMS. The PMS was hosted in an ISO 27001 high-security data centre by ioko365 (Piksel Ltd, York, UK). The Clinical Informatics Research Unit at the University of Southampton processed the data, and was responsible for the development, maintenance and security of the PMS and had policies in place for information governance (IG) that were compliant with the Data Protection Act and the European Clinical Trials Directive.
Every aspect of the study was conducted through the PMS, creating a complete record of each individual’s passage through the study. The PMS was used by the following organisations that collaborated on this study:
-
The BCSP Southern Hub (based at Royal Surrey County Hospital NHS Trust).
-
Nominated members of the BCSP Southern Hub had access to the PMS in order to upload information about potentially eligible individuals and for patient management purposes.
-
The IG toolkit organisation code was RA2.
-
-
Cancer Screening and Prevention Research Group (CSPRG), Imperial College London.
-
Nominated members of the CSPRG could receive paper records from study participants and NHS sites involved with participants’ medical care. All records were stored in secure locked cabinets.
-
Nominated members of the CSPRG received pseudonymised electronic BCSS data, which were stored in a managed and secure area on the server.
-
Nominated members of the CSPRG had access to the PMS in order to carry out trial management duties, record the information received and extract information on consented participants for analysis.
-
The CSPRG held a level 3 IG certificate that was subsequently superseded by the IG toolkit version 14 (CSPRG organisational code: 8HL46-FOM-CSPRG).
-
The PMS was hosted and developed outside Imperial College London but complied with Imperial College London’s policy on data handling and data storage. ioko365 (Piksel Ltd, York, UK), the company that hosted the PMS, held an IG toolkit (IG Toolkit Organisation Code 8GX09). The Head of the CSPRG was the data controller.
-
-
Department of Behavioural Science and Health, University College London.
-
Selected members had limited access to the PMS in order to record the questionnaires. They could not access participant-identifiable information. Access to the system was from the CSPRG office with a guest user account valid for a limited time period.
-
Statistical methods
Primary outcome
To determine the 3-year sensitivity of annual FIT for the detection of CRC or AAs compared with colonoscopy undertaken at 3 years.
Secondary outcomes
-
Uptake and positivity of FIT in rounds 1, 2 and 3.
-
Positive predictive value (PPV) of FIT for ACN, CRC or AAs in rounds 1, 2 and 3 in participants attending colonic examination following positive FIT.
-
Diagnostic yield of ACN, CRC or AAs in participants attending colonic examination following negative FIT in rounds 1, 2 and 3.
-
Positivity, sensitivity, specificity, PPV and negative predictive value (NPV) of FIT for ACN, CRC or AAs at first, second and third tests and over two or three tests at various thresholds.
-
Subjective physical and mental well-being following each FIT round (see Chapter 4).
-
Participant preference for annual FIT versus 3-yearly colonoscopy for surveillance, and participant satisfaction with FIT at the 3-year assessment (see Chapter 4).
-
Incremental costs and cost-effectiveness of annual FIT versus 3-yearly colonoscopy surveillance (see Chapter 5).
Sample size
The sample size calculation was based on the estimation of the relative sensitivity of three annual FITs compared with 3-yearly colonoscopy to detect ACN. Under conservative assumptions of a prevalence of ACN of 2.5%, and a relative FIT sensitivity of 75%, we required 72 cases and 2881 adherent participants in order to provide an estimate of the sensitivity with a 95% confidence interval (CI) within ± 10% among adherent participants. Allowing for a conservative estimate of compliance with all tests of 40%, we calculated that we would need 7203 invitees. Given a ± 10% margin of error, this led to the calculated required sample size of 8000.
Outcome definitions
Outcomes of CRC and AA were ascertained from colonic examination and histology reports. Participants may have had more than one colonic examination (colonoscopy, flexible sigmoidoscopy or CT colonography) performed and may have also had surgery. All colonic procedures performed were considered when defining outcomes.
The CRC sites were defined by the International Classification of Diseases, Tenth Edition, and we included codes C18-C20. CRC morphology was coded with ICD-O2 codes and we included cancers with codes of 8140/3, 8211/3, 8246/3, and 8263/3.
Polyp size was determined by the maximum of the microscopic size at pathology, endoscopy or surgery for each polyp. An AA was defined as an adenoma meeting one of the following criteria: ≥ 10 mm, tubulovillous or villous histology, or high grade dysplasia. Adenocarcinomas were not classed as adenomas; therefore, participants with CRC were included as having an AA detected only if they had a separate lesion which met the criteria of an AA. ACN was defined as CRC and/or AAs.
Data analyses
In order to estimate the diagnostic accuracy of FIT, we made the important assumption that any colorectal neoplasia detected at year 1, 2 or 3 was present at year 1 and remained present and unmodified, in the absence of colonic examination, to year 3. We also assumed that the same colorectal neoplasia would be detected in each participant regardless of the year in which colonic examination was performed. Under these assumptions, AAs or CRCs detected at year 2 or 3 were considered missed by previous FITs and it was assumed that any colorectal neoplasia found at early colonic examination (year 1 or 2) would have been found at year 3. Furthermore, these assumptions allowed us to estimate the sensitivity and specificity of FIT at multiple FIT thresholds (10 µg/g, 20 µg/g, 30 µg/g and 40 µg/g). Under these assumptions, if a participant would have tested positive at a lower threshold (e.g.10 µg/g) than that used (40 µg/g), the resulting early colonic examination would have found what was actually found at a later examination.
For the outcomes of ACN, CRC and AA, we calculated positivity, sensitivity, specificity, PPV and NPV at each round and for different FIT thresholds. In order to perform calculations at each round for the thresholds lower than 40 µg/g used in the study, any of a participant’s FIT results from later rounds were ignored in the analysis once the participant had a FIT result above the threshold being analysed, analogous to participants being excluded from further FIT rounds once testing positive during the trial. For example, in the analysis considering a threshold of 20 µg/g, a participant who had a FIT result of 22 µg/g at year 1 would be considered positive at year 1, and any FIT results from year 2 or 3 would be ignored, despite the fact that they may have completed additional rounds of FIT. For calculations of sensitivity, specificity, PPV and NPV, we included only participants who had at least one colonic examination.
As well as estimating positivity, sensitivity, specificity, PPV and NPV for each round, we also calculated these figures for a combination of two tests and for a combination of three tests.
In ‘cumulative test analyses’, we included only those participants who either completed the specified number of rounds of FIT or tested positive at a previous round. The two-test analysis included participants who completed at least two rounds of FIT or who tested positive at round 1. The three-test analysis included participants who completed all three rounds of FIT or who tested positive at any round.
‘Programme analyses’ included all participants who completed FIT at round 1, regardless of whether or not they participated at any further round. A participant was classed as positive if their FIT was positive at any of the first two rounds at which they completed FIT (two-test analysis), or if their FIT was positive at any round at which they completed FIT (three-test analysis).
Results were presented by sex and by age group (≤ 65 years and > 65 years). We compared sex and age in individuals who did and did not participate using chi-squared tests. A number of methods for computing CIs for binomial proportions were considered, including asymptotic CI calculated assuming a normal approximation of the sampling distribution. However, accuracy suffers when these methods are used for proportions very close to zero or 1. For the asymptotic method to be appropriate, Bland66 anticipated that both the number of tests giving a negative result and the number giving a positive result should exceed five. Given the low number of CRC cases identified in this study, and given the capability to make use of more accurate methods [using the statistical software package Stata® (StataCorp LP, College Station, TX, USA)], exact Clopper–Pearson CIs were used.
All data analyses were performed in Stata/IC 13.
Patient and public involvement
Patient and public representatives were involved throughout this study by means of workshops, individual consultations (e.g. to co-participate in designing patient materials and questionnaires) and as members of the independent steering committee. We consulted a range of representatives, some of whom had no history of colorectal investigations, some of whom had tested negative in the BCSP and had no experience of colonoscopy, some of whom had tested positive in the BCSP and had experienced an investigative colonoscopy, and some of whom were in an active adenoma surveillance programme.
While planning the study, we took advice from a panel of patient experts on contacting individuals prior to consent. The panel felt that, given the importance of the research, this was acceptable, as long as the details of individuals who had not given explicit consent to take part in the study were not shared outside the BCSP Southern Hub. Personnel at the BCSP Southern Hub were the only members of the study team who could view unconsented individuals’ personal details, in order to produce the initial invitation letters.
Representatives were consulted in the pre-pilot phase (see Preparation for the study), during which the PMS and study materials (e.g. flyer, letter, consent form, baseline questionnaire, FIT kit instructions, participant information sheet) were being developed. Representatives’ feedback strongly influenced the type of study materials that were used and their layout and content.
Service users were also interviewed to assess the likely acceptability of FIT as a CRC surveillance tool. A discussion group was convened in November 2011 to discuss the practicalities of using FIT, the implications of the results and the possible risks associated with FIT. As a result of users’ feedback about the psychological implications of receiving a false-positive test result, patient materials were modified to clearly demonstrate the possibility of testing false positive (see Chapter 4 and Bowyer et al. 67 for full details and results).
Users were also involved in assessment of the pilot study (see Pilot study), the aim of which was to test the processes for invitation, analysis and transmission of results, and storage of information on the PMS. A number of modifications to the PMS and to the study materials were made as a result of the pilot study.
Health psychology assessment
A secondary objective of the FIT for Follow-Up study was to examine the acceptability of FIT compared with colonoscopy as a surveillance mechanism for people at increased risk of developing CRC. Participant experience questionnaires after each round of FIT allowed us to assess various aspects of the study, including the FIT kit instructions, how easy participants found it to use the FIT kit, how they felt when receiving the results, how any subsequent colonoscopy affected them, and their screening preferences (see Chapter 4).
We assessed the psychological consequences of annual surveillance over time using an itinerary of questions, including a short version of the Spielberger State–Trait Anxiety Inventory (STAI)68 and more specific measures of CRC-related worry. 69 We also investigated the emotional impact of FIT outcomes (e.g. false-negative and false-positive results) through telephone interviews. Participants suspected of having cancer were not sent questionnaires to avoid causing unnecessary distress. Chapter 4 fully documents the methods and analyses pertaining to the health psychology assessment.
Economic evaluation
We assessed the cost and cost-effectiveness of annual FIT with colonoscopy only for a positive result versus 3-yearly colonoscopy surveillance. Further details are in Chapter 5.
Preparation for the study
In July 2011, we conducted a pre-pilot study to review study materials and to assess PMS functionality. Volunteers were asked to review and comment on the flyer introducing the study, invitation letter, participant information sheet, consent form, baseline questionnaire, FIT kit instruction wallet and foil-lined sample return envelope.
Feedback on the study materials was received from 27 age-appropriate volunteers (60–71 years), six other volunteers (< 60 years) and 13 members of the research teams.
It was decided that a flyer, designed to introduce potential participants to the study, should not be used in the main study, as feedback indicated that it did not contain enough information about the study and would be likely to confuse potential participants. Other feedback warranted changes to the invitation letter and the participant information sheet.
A qualitative study with discussion groups was conducted (November 2011) to gain an understanding of public attitudes towards FIT, identify potential issues for the main study and facilitate the design of study materials. Details of this study are further described in Chapter 4, Study 1: patient attitudes towards the faecal immunochemical test as an alternative to colonoscopy surveillance of groups at increased risk of colorectal cancer – a qualitative discussion group study.
Pilot study
The aim of the pilot study was to test the processes for invitation, analysis, transmission of results and storage of information on the PMS. It also enabled us to examine uptake, requirements for reminders, attendance for colonic examination of those with a positive FIT and the frequency and nature of calls to the freephone helpline. Following the pilot study, a number of modifications to the PMS and to the information and study materials were made. A major change was to the haemoglobin threshold set to define a positive FIT result. Initially, the threshold was set at 20 µg/g, as this is commonly used in screening programmes and has been used frequently by other studies. 70–76 It was subsequently changed to 40 µg/g as the proportion positive at a threshold of 20 µg/g was 6.5%, which was higher than we expected given the literature. 77 We were concerned that this level of positivity had the potential to cause an excess of colonoscopies, with consequences on patient risk and endoscopist workload.
Chapter 3 Results
Invitation to participate in the study
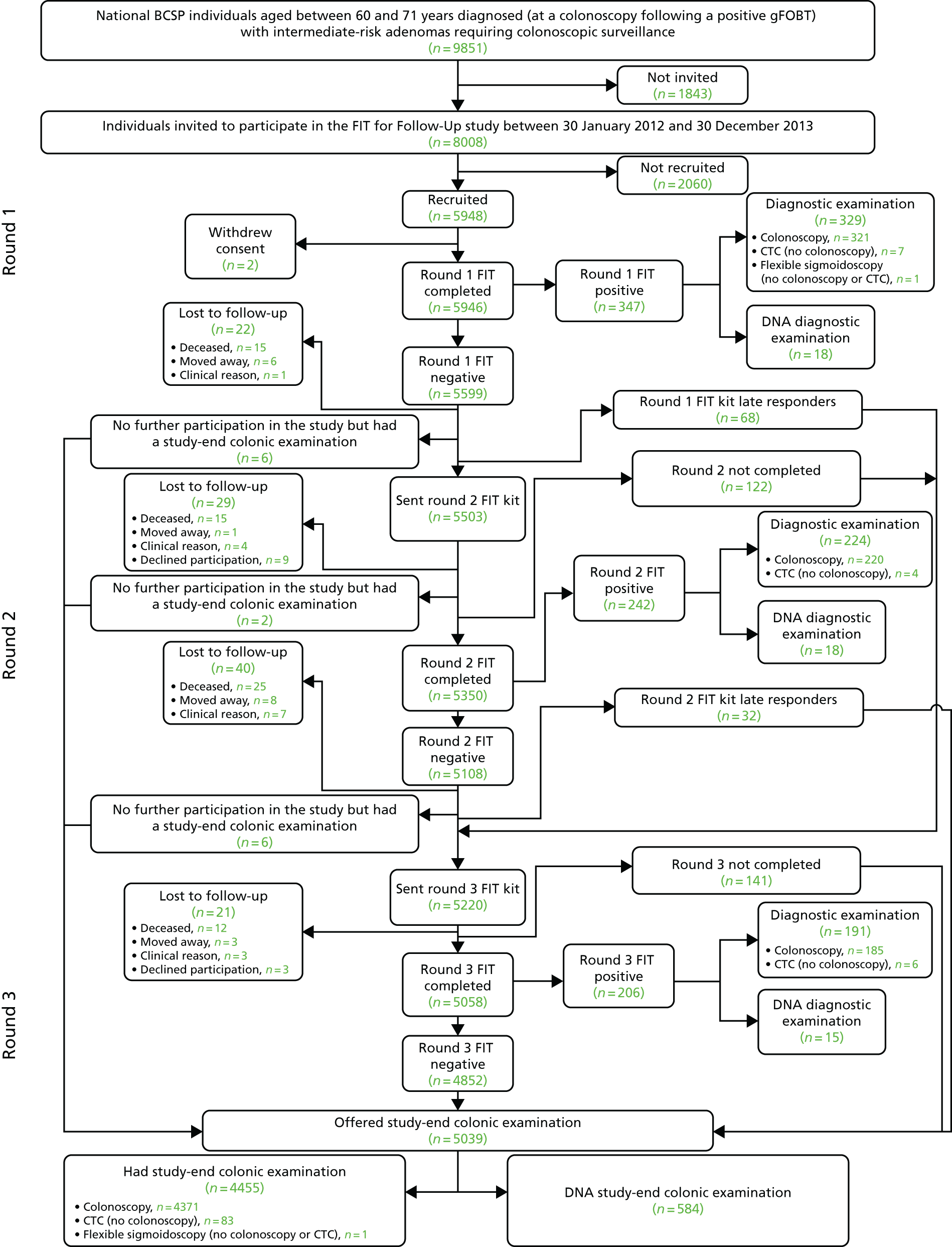
In total, 9851 individuals were identified as potentially eligible for the study. Of these, 296 were excluded after eligibility assessment for the following reasons: 187 individuals had received more than one colonoscopy prior to invitation and were therefore excluded to prevent patient overinvestigation, and 109 individuals were excluded by the BCSP for clinical reasons, or because of informed dissent, death or emigration. A further 1547 were not invited as the recruitment target of 8000 participants had been met. The remaining 8008 individuals were invited to participate in the study between 30 January 2012 and 30 December 2013 (Figure 3). The mean age of these invited individuals was 65.7 years (standard deviation 3.4 years; 49.3% were aged ≤ 65 years and 50.7% were aged > 65 years), and 65.3% were men (Table 1).
FIGURE 3.
Participant flow diagram from invitation through to end-of-study colonic examination. Invited: 9851 individuals were identified as potentially eligible for the study and were ordered by surveillance due date; 8008 individuals were invited to participate. A total of 1843 of the potentially eligible individuals were not invited to participate in the study; 296 were not invited as they were excluded after the eligibility assessment and a further 1547 individuals were not invited as the recruitment target of 8000 had already been met. Not recruited: 2055 were because of lack of consent, one consented but had not returned a FIT kit and four consented but returned a FIT kit that could not be analysed. DNA: includes participants who (using data collected on 6 October 2016) were offered, but DNA, a diagnostic examination after a positive kit, or DNA a study-end colonic examination after being offered such.
| Sex and age (years) at invite | Participation, n (%) | p-valuec | ||
|---|---|---|---|---|
| Inviteda | Participatedb | Did not participateb | ||
| All | ||||
| All ages | 8008 (100) | 5946 (74.3) | 2062 (25.7) | |
| ≤ 65 | 3950 (49.3) | 2880 (72.9) | 1070 (27.1) | 0.007 |
| > 65 | 4058 (50.7) | 3066 (75.6) | 992 (24.4) | |
| Men | ||||
| All ages | 5227 (65.3) | 3898 (74.6) | 1329 (25.4) | |
| ≤ 65 | 2634 (32.9) | 1903 (72.2) | 731 (27.8) | < 0.001 |
| > 65 | 2593 (32.4) | 1995 (76.9) | 598 (23.1) | |
| Women | ||||
| All ages | 2781 (34.7) | 2048 (73.6) | 733 (26.4) | |
| ≤ 65 | 1316 (16.4) | 977 (74.2) | 339 (25.8) | 0.498 |
| > 65 | 1465 (18.3) | 1071 (73.1) | 394 (26.9) | |
Participation in surveillance by round
Round 1
Following the invitation to participate, 5948 out of 8008 invitees returned a completed consent form and an analysable FIT. Of the 2060 invitees not recruited, 2055 did not provide consent and five consented but did not return an analysable FIT. Of the 5948 invitees who returned a completed consent form and an analysable FIT, two individuals subsequently withdrew consent. The remaining 5946 participants (74.3% of the 8008 invited) formed the study cohort.
Participation was slightly higher in male (74.6%, 3898/5227) than in female (73.6%, 2048/2781) invitees, although the difference was not significant (p = 0.364) (see Table 1). Older men were more likely to participate than younger men (76.9% of men aged > 65 years participated vs. 72.2% of men aged ≤ 65 years; p < 0.001). However, among women, the proportion who participated was similar across age groups (73.1% of women aged > 65 years participated vs. 74.2% of women aged ≤ 65 years; p = 0.498).
In the study cohort of 5946 participants, 347 (5.8%) tested FIT positive and 5599 (94.2%) tested FIT negative in round 1 (Table 2 and see Figure 3). Most FIT-positive participants (94.8%, 329/347) attended a colonic examination (colonoscopy, CT colonography or flexible sigmoidoscopy). Reasons for non-attendance were that the participant declined colonic examination (n = 12), the participant could not be contacted (n = 3) or the participant was lost to follow-up and not contacted again (n = 3).
| Findings | Round | End-of-study colonic examination | Entire study findings | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Cumulative (across all rounds) | ||||||||||||||
| n | % of round 1 | % of entire study findings | n | % of round 2 | % of entire study findings | n | % of round 3 | % of entire study findings | n | % of all rounds | % of entire study findings | n | % of end-of-study colonic exam | % of entire study findings | n | % of entire study findings | |
| Invited | 8008 | 5503 | 5220 | 8008 | |||||||||||||
| Completed FITa | 5946 | 74.3 | 5350 | 97.2 | 5058 | 96.9 | 5946 | 74.3 | |||||||||
| Tested positive | 347b | 5.8c | 242 | 4.5c | 206 | 4.1c | 795d | 13.4c | |||||||||
| Invited for colonic examination | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Colonic examination performed | 329 | 94.8e | 6.3f | 224 | 92.6e | 4.3f | 191 | 92.7e | 3.7f | 744 | 93.6e | 14.3f | 4455 | – | 85.7f | 5199 | |
| Colonoscopy | 321 | 97.6g | 6.3f | 220 | 98.2g | 4.3f | 185 | 96.9g | 3.6f | 726 | 97.6g | 14.2f | 4371 | 98.1g | 85.8f | 5097 | 98.0g |
| CTC (no colonoscopy) | 7 | 2.1g | 7.0f | 4 | 1.8g | 4.0f | 6 | 3.1g | 6.0f | 17 | 2.3g | 17.0f | 83 | 1.9g | 83.0f | 100 | 1.9g |
| FS (no colonoscopy or CTC) | 1 | 0.3g | 50.0f | 0 | 0g | 0f | 0 | 0g | 0f | 1 | 0.1g | 50.0f | 1 | 0.02g | 50.0f | 2 | 0.04g |
| Diagnostic yield | |||||||||||||||||
| ACNh | 83 | 25.2i | 17.9f | 43 | 19.2i | 9.3f | 39 | 20.4i | 8.4f | 165 | 22.2i | 35.6f | 298 | 6.7i | 64.4f | 463 | 8.9i |
| CRC | 8 | 2.4i | 30.8f | 8 | 3.6i | 30.8f | 2 | 1.0i | 7.7f | 18 | 2.4i | 69.2f | 8 | 0.2i | 30.8f | 26 | 0.5i |
| AAs | 78 | 23.7i | 17.6f | 37 | 16.5i | 8.4f | 37 | 19.4i | 8.4f | 152 | 20.4i | 34.3f | 291 | 6.5i | 65.7f | 443 | 8.5i |
Round 2
Round 2 FIT kits were sent to 5503 out of 5599 (98.3%) participants who tested FIT negative in round 1. There were 96 round 1 FIT-negative participants who were not sent a round 2 FIT, for the following reasons: 68 were late responders to round 1, 22 were lost to follow-up (deceased, n = 15; moved away, n = 6; clinical reason, n = 1) and were not contacted again, and six missed subsequent FITs because of an administrative error or investigation of symptoms, but did have a colonic examination performed during the study period.
In total, 5350 out of 5503 (97.2%) participants sent a round 2 FIT returned an analysable test. Of the 153 participants who did not return an analysable FIT, 29 were lost to follow-up (deceased, n = 15; moved away, n = 1; clinical reason, n = 4; declined further participation, n = 9) and were not contacted again, 122 did not return an analysable FIT but were sent a FIT in round 3, and two were not sent a FIT in round 3 as a result of an investigation of symptoms.
Overall, 5108 out of 5350 (95.5%) participants completing a round 2 FIT tested negative, and 242 (4.5%) tested positive. Of the 242 participants testing positive, 224 (92.6%) attended a colonic investigation. Eighteen (7.4%) DNA the colonic examination (12 participants declined, one could not be contacted and five were lost to follow-up and not contacted again).
Round 3
In round 3, 5220 participants were sent a FIT. This included 5030 participants who tested FIT negative in round 2 and who were receiving their third consecutive FIT, 68 participants who were round 1 late responders and had therefore not been invited to round 2, and 122 participants who had been invited in round 2 but had not returned an analysable FIT. A number of round 2 FIT-negative participants were not sent a round 3 FIT (n = 78), for the following reasons: 40 were lost to follow-up (deceased, n = 25; moved away, n = 8; clinical reason, n = 7) and were not contacted again, 32 participants were late responders to round 2, and six missed round 3 because of an administrative error. The 38 participants who were not lost to follow-up were invited to an end-of-study colonic examination.
In total, 5058 out of 5220 (96.9%) participants who were sent a round 3 FIT returned an analysable test. Of the 162 participants who did not return an analysable FIT, 21 were lost to follow-up (deceased, n = 12; moved away, n = 3; clinical reason, n = 3; declined participation, n = 3) and were not contacted again; 141 participants did not return an analysable FIT but were invited to an end-of-study colonic examination.
For 60 out of 5058 (1.2%) participants completing a round 3 FIT, it was their second completed FIT, and for 4998 (98.8%) participants it was their third. Of these 5058 round 3 participants, 4852 (95.9%) tested negative and 206 (4.1%) tested positive. Not all participants testing positive attended colonic investigation: 15 out of 206 (7.3%) DNA, either because they declined (n = 3) or for reasons unknown (n = 12).
End-of-study colonic examination
Of the 5946 study participants who completed at least the round 1 FIT, 5039 (84.7%) were offered an end-of-study colonic examination. There were 907 (15.3%) participants who were not offered an end-of-study colonic examination: 795 (13.4%) because of a prior positive FIT and 112 (1.9%) because the participant had been lost to follow-up.
The 5039 participants offered an end-of-study colonic examination were composed of 4852 (96.3%) round 3 FIT-negative participants, 32 (0.6%) round 2 late responders, 141 (2.8%) participants who did not return a completed round 3 FIT and 14 participants who ceased participation in FIT prior to round 3 (because of an investigation of symptoms or an administrative error) but who did have an end-of-study colonic examination. Among the 5039 invited to an end-of-study colonic examination, 584 (11.6%) DNA. Participants DNA for the following reasons: they declined (n = 298), they could not be contacted (n = 172), they postponed (n = 107) or for clinical reasons (n = 7).
Uptake and test positivity
Uptake of faecal immunochemical test overall and by round
In round 1, 5946 out of 8008 (74.3%) invited individuals returned a completed consent form and an analysable FIT (see Table 2). Only individuals who returned a completed consent form and negative FIT in round 1 were eligible for round 2. Uptake of FIT was 97.2% (5350/5503) in round 2 and 96.9% (5058/5220) in round 3. Uptake was marginally higher in men than in women in round 1 (74.6% vs. 73.6%), round 2 (97.3% vs. 97.0%) and round 3 (97.3% vs. 96.1%) (see Report Supplementary Material 4, Table 1).
Although participation in the study in round 1 was greater in participants aged > 65 years than in those aged ≤ 65 years (75.6% vs. 72.9%), subsequent uptake of FIT in rounds 2 and 3 did not differ considerably by age category (see Report Supplementary Material 5, Table 2). Uptake in round 2 was 97.3% in participants aged > 65 years and 97.1% in those aged ≤ 65 years. In round 3, uptake was 96.8% in participants aged > 65 years and 97.0% in those aged ≤ 65 years.
Faecal immunochemical test positivity overall and by round
Faecal immunochemical test positivity decreased from 5.8% (347/5946) in round 1 to 4.5% (242/5350) in round 2, and 4.1% (206/5058) in round 3 (see Table 2). The cumulative test positivity over all three rounds was 13.4% (795/5946).
Positivity at each round was greater in men than in women (see Report Supplementary Material 4, Table 1). At round 1, positivity was 6.6% in men and 4.3% in women. At round 2, positivity was 4.9% in men and 3.8% in women. At round 3, positivity was 4.5% in men and 3.2% in women. The cumulative positivity over all three rounds was 14.8% in men and 10.6% in women.
Positivity was greater, in every round, in older (> 65 years) participants than in younger participants (see Report Supplementary Material 5, Table 2). Cumulative positivity over all three rounds was 12.2% in participants aged ≤ 65 years and 14.4% in participants aged > 65 years.
Attendance for colonic examination and yield of colorectal cancer, advanced adenomas and advanced colorectal neoplasia
Attendance for colonic examination
Attendance for colonic examination following a positive FIT was 94.8% (329/347) in round 1, 92.6% (224/242) in round 2 and 92.7% (191/206) in round 3 (see Table 2). Over all three rounds, cumulative attendance for colonic examination following a positive FIT was 93.6% (744/795). The majority of participants attending colonic examination following a positive FIT received a colonoscopy (97.6%, 726/744). Of the 18 who did not receive a colonoscopy, 17 received CT colonography and one received a flexible sigmoidoscopy.
Among all FIT-positive participants, a greater proportion of men (95.0%, 549/578) than in women (89.9%, 195/217) attended a colonic examination (see Report Supplementary Material 4, Table 1). There was little difference in attendance by age category (93.5% of participants aged ≤ 65 years attended vs. 93.7% of those aged > 65 years) (see Report Supplementary Material 5, Table 2).
Attendance for an end-of-study colonic examination following prior negative FITs was 88.4% (4455/5039). Most participants attending an end-of-study colonic examination received a colonoscopy (98.1%, 4371/4455).
Diagnostic yield of colorectal cancer, advanced adenomas and advanced colorectal neoplasia
In total, 5199 out of 5946 (87.4%) participants had a colonic examination at some point during the study (see Table 2). Among these 5199 participants, CRC was found in 26 (0.5%), AAs in 443 (8.5%) and ACN in 463 (8.9%); there were six individuals with an AA and a CRC.
Among the 744 participants who, in any round, attended colonic examination following a positive FIT, the diagnostic yields of CRC, AA and ACN were 2.4% (18/744), 20.4% (152/744) and 22.2% (165/744), respectively (see Table 2). The diagnostic yield of CRC was greater in rounds 1 (2.4%, 8/329) and 2 (3.6%, 8/224) than in round 3 (1.0%, 2/191). The diagnostic yield of AA was greatest in round 1 (23.7%, 78/329) and lower in rounds 2 (16.5%, 37/224) and 3 (19.4%, 37/191).
The diagnostic yield of ACN from colonic examination was slightly higher in men (9.3%, 321/3454) than in women (8.1%, 142/1745), and in participants aged > 65 years (9.4%, 252/2667) than in those aged ≤ 65 years (8.3%, 211/2532) (see Report Supplementary Material 4, Table 1 and Report Supplementary Material 5, Table 2). Furthermore, the mean number of adenomas per patient with ACN was slightly greater in men than women (2.20 vs. 1.92 – data not presented). The mean number of adenomas in participants aged > 65 years with ACN was similar to that in those aged ≤ 65 years (2.13 vs. 2.09 – data not presented).
The diagnostic yield of CRC from colonic examination following a positive FIT, over all three rounds, was greater in female (4.6%, 9/195) than in male (1.6%, 9/549) participants (see Report Supplementary Material 4, Table 1). There was little difference between men and women in the diagnostic yield of AA (20.4% vs. 20.5%). The diagnostic yield of CRC and AA was greater in participants aged > 65 years (2.7% and 21.0%, respectively) than in those aged ≤ 65 years (2.1% and 19.8%, respectively) (see Report Supplementary Material 5, Table 2).
Among the 4455 participants attending an end-of-study colonic examination, eight (0.2%) were found to have CRC, 291 (6.5%) had AAs and 298 participants (6.7%) had ACN (see Table 2).
Performance of the faecal immunochemical test at different faecal haemoglobin thresholds
Faecal immunochemical test positivity at different thresholds
We calculated FIT positivity, by number of completed FITs, at different faecal haemoglobin thresholds among the 5946 study participants (Table 3). FIT positivity rates with thresholds of < 40 µg/g were estimated on the assumption that following a positive test at a given threshold, the participant would not have been offered further FITs. With higher positivity rates expected at lower thresholds, the number available for subsequent rounds of FIT would be lower.
| FIT threshold (µg/g) and test | All | Sex | ||||
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Completed a test,a n | Tested +ve, n (%) | Completed a test,a n | Tested +ve, n (%) | Completed a test,a n | Tested +ve, n (%) | |
| 40 | ||||||
| First | 5946 | 344b (5.8) | 3898 | 256 (6.6) | 2048 | 88 (4.3) |
| Secondc | 5481 | 251 (4.6) | 3571 | 178 (5.0) | 1910 | 73 (3.8) |
| Thirdd | 4927 | 197 (4.0) | 3194 | 142 (4.4) | 1733 | 55 (3.2) |
| Cumulative test analysis | ||||||
| Two testse | 5825 | 595 (10.2) | 3827 | 434 (11.3) | 1998 | 161 (8.1) |
| Three testsf | 5522 | 792 (14.3) | 3628 | 576 (15.9) | 1894 | 216 (11.4) |
| Programme analysis | ||||||
| Two testsg | 5946 | 595 (10.0) | 3898 | 434 (11.1) | 2048 | 161 (7.9) |
| Three testsh | 5946 | 792 (13.3) | 3898 | 576 (14.8) | 2048 | 216 (10.5) |
| 30 | ||||||
| First | 5946 | 416 (7.0) | 3898 | 309 (7.9) | 2048 | 107 (5.2) |
| Secondc | 5415 | 299 (5.5) | 3522 | 217 (6.2) | 1893 | 82 (4.3) |
| Third | 4820 | 241 (5.0) | 3111 | 171 (5.5) | 1709 | 70 (4.1) |
| Cumulative test analysis | ||||||
| Two testse | 5831 | 715 (12.3) | 3831 | 526 (13.7) | 2000 | 189 (9.5) |
| Three testsf | 5535 | 956 (17.3) | 3637 | 697 (19.2) | 1898 | 259 (13.6) |
| Programme analysis | ||||||
| Two testsg | 5946 | 715 (12.0) | 3898 | 526 (13.5) | 2048 | 189 (9.2) |
| Three testsh | 5946 | 956 (16.1) | 3898 | 697 (17.9) | 2048 | 259 (12.6) |
| 20 | ||||||
| First | 5946 | 546 (9.2) | 3898 | 399 (10.2) | 2048 | 147 (7.2) |
| Secondc | 5294 | 362 (6.8) | 3438 | 259 (7.5) | 1856 | 103 (5.5) |
| Third | 4649 | 295 (6.3) | 2994 | 212 (7.1) | 1655 | 83 (5.0) |
| Cumulative test analysis | ||||||
| Two testse | 5840 | 908 (15.5) | 3837 | 658 (17.1) | 2003 | 250 (12.5) |
| Three testsf | 5557 | 1203 (21.6) | 3652 | 870 (23.8) | 1905 | 333 (17.5) |
| Programme analysis | ||||||
| Two testsg | 5946 | 908 (15.3) | 3898 | 658 (16.9) | 2048 | 250 (12.2) |
| Three testsh | 5946 | 1203 (20.2) | 3898 | 870 (22.3) | 2048 | 333 (16.3) |
| 10 | ||||||
| First | 5946 | 844 (14.2) | 3898 | 598 (15.3) | 2048 | 246 (12.0) |
| Secondc | 5004 | 491 (9.8) | 3243 | 343 (10.6) | 1761 | 148 (8.4) |
| Third | 4254 | 388 (9.1) | 2729 | 272 (10.0) | 1525 | 116 (7.6) |
| Cumulative test analysis | ||||||
| Two testse | 5848 | 1335 (22.8) | 3841 | 941 (24.5) | 2007 | 394 (19.6) |
| Three testsf | 5589 | 1723 (30.8) | 3670 | 1213 (33.1) | 1919 | 510 (26.6) |
| Programme analysis | ||||||
| Two testsg | 5946 | 1335 (22.5) | 3898 | 941 (24.1) | 2048 | 394 (19.2) |
| Three testsh | 5946 | 1723 (29.0) | 3898 | 1213 (31.1) | 2048 | 510 (24.9) |
Faecal immunochemical test positivity was higher with lower faecal haemoglobin thresholds. At 40 µg/g, positivity was 5.8% (344/5946), 4.6% (251/5481) and 4.0% (197/4927) for the first, second and third completed FIT, respectively. At a lower threshold of 30 µg/g, positivity was greater, at 7.0% (416/5946), 5.5% (299/5415) and 5.0% (241/4820). Positivity increased further with a threshold of 20 µg/g to 9.2% (546/5946), 6.8% (362/5294) and 6.3% (295/4649). At the lowest studied threshold of 10 µg/g, the highest positivity was observed: 14.2% (844/5946), 9.8% (491/5004) and 9.1% (388/4254) for the first, second and third completed FIT, respectively.
Higher positivity at lower thresholds was reflected in cumulative test analysis and programme analysis. In cumulative test analysis, examining only individuals who completed all three tests or who tested positive prior to the third test, positivity was 14.3% (792/5522) at a threshold of 40 µg/g, 17.3% (956/5535) at a threshold of 30 µg/g, 21.6% (1203/5557) at a threshold of 20 µg/g and 30.8% (1723/5589) at a threshold of 10 µg/g. Cumulative test positivity of two, rather than three, tests was lower: 10.2% (595/5825) at a threshold of 40 µg/g, 12.3% (715/5831) at a threshold of 30 µg/g, 15.5% (908/5840) at a threshold of 20 µg/g and 22.8% (1335/5848) at a threshold of 10 µg/g.
Similarly, in programme analysis, examining all 5946 participants completing a test at round 1 regardless of subsequent participation in further rounds, positivity was higher at lower thresholds. Over three rounds, 13.3% (792/5946) tested positive at a threshold of 40 µg/g, 16.1% (956/5946) at a threshold of 30 µg/g, 20.2% (1203/5946) at a threshold of 20 µg/g and 29.0% (1723/5946) at a threshold of 10 µg/g. Positivity was lower after up to two, rather than up to three, tests: 10.0% (595/5946) at a threshold of 40 µg/g, 12.0% (715/5946) at a threshold of 30 µg/g, 15.3% (908/5946) at a threshold of 20 µg/g and 22.5% (1335/5946) at a threshold of 10 µg/g.
Regardless of threshold and number of tests performed, positivity was higher in men than in women. In programme analysis, after three rounds of testing using a threshold of 40 µg/g, 14.8% (576/3898) of men and 10.5% (216/2048) women were FIT positive; and at a threshold of 10 µg/g, 31.1% (1213/3898) of men and 24.9% (510/2048) of women tested positive (see Table 3). Positivity was in general, although not in all cases, higher in participants aged > 65 years than in those aged ≤ 65 years (see Report Supplementary Material 6, Table 3). At a threshold of 40 µg/g, in programme analysis after up to three tests, 12.2% (350/2880) of participants aged ≤ 65 years and 14.4% (442/3066) of those aged > 65 tested positive; at a threshold of 10 µg/g, 27.6% (795/2880) of participants aged ≤ 65 years and 30.3% (928/3066) of those aged > 65 years tested positive.
Accuracy of the faecal immunochemical test at different faecal haemoglobin thresholds
We estimated the sensitivity, specificity, PPV and NPV of FIT at different faecal haemoglobin thresholds among the 5199 participants who underwent a colonic examination (see Tables 4–6).
Sensitivity, specificity, positive predictive value and negative predictive value of the faecal immunochemical test for colorectal cancer
Of the 5199 participants who underwent a colonic examination, 26 (0.5%) were diagnosed with CRC during the study (Table 4). For the first completed FIT, at a threshold of 40 µg/g, sensitivity for CRC was 30.8%, specificity was 93.9%, the PPV was 2.5% and the NPV was 99.6%.
| FIT threshold (µg/g) | Completed a FIT,a n | Tested +ve, n (%) | CRCs | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Total cases, n | FIT +ve cases, n | |||||||
| 40 | ||||||||
| First | 5199 | 326 (6.3) | 26 | 8 | 30.8 (14.3 to 51.8) | 93.9 (93.2 to 94.5) | 2.5 (1.1 to 4.8) | 99.6 (99.4 to 99.8) |
| Secondb | 4837 | 233 (4.8) | 16 | 8 | 50.0 (24.7 to 75.3) | 95.3 (94.7 to 95.9) | 3.4 (1.5 to 6.7) | 99.8 (99.7 to 99.9) |
| Thirdc | 4429 | 182 (4.1) | 6 | 2 | 33.3 (4.3 to 77.7) | 95.9 (95.3 to 96.5) | 1.1 (0.1 to 3.9) | 99.9 (99.8 to 100.0) |
| Cumulative test analysis | ||||||||
| Two testsd | 5163 | 559 (10.8) | 24 | 16 | 66.7 (44.7 to 84.4) | 89.4 (88.6 to 90.3) | 2.9 (1.6 to 4.6) | 99.8 (99.7 to 99.9) |
| Three testse | 4988 | 741 (14.9) | 22 | 18 | 81.8 (59.7 to 94.8) | 85.4 (84.4 to 86.4) | 2.4 (1.4 to 3.8) | 99.9 (99.8 to 100.0) |
| Programme analysis | ||||||||
| Two testsf | 5199 | 559 (10.8) | 26 | 16 | 61.5 (40.6 to 79.8) | 89.5 (88.6 to 90.3) | 2.9 (1.6 to 4.6) | 99.8 (99.6 to 99.9) |
| Three testsg | 5199 | 741 (14.3) | 26 | 18 | 69.2 (48.2 to 85.7) | 86.0 (85.0 to 87.0) | 2.4 (1.4 to 3.8) | 99.8 (99.6 to 99.9) |
| 30 | ||||||||
| First | 5199 | 386 (7.4) | 26 | 10 | 38.5 (20.2 to 59.4) | 92.7 (92.0 to 93.4) | 2.6 (1.2 to 4.7) | 99.7 (99.5 to 99.8) |
| Secondb | 4778 | 276 (5.8) | 14 | 6 | 42.9 (17.7 to 71.1) | 94.3 (93.6 to 95.0) | 2.2 (0.8 to 4.7) | 99.8 (99.7 to 99.9) |
| Thirdc | 4330 | 220 (5.1) | 6 | 3 | 50.0 (11.8 to 88.2) | 95.0 (94.3 to 95.6) | 1.4 (0.3 to 3.9) | 99.9 (99.8 to 100.0) |
| Cumulative test analysis | ||||||||
| Two testsd | 5164 | 662 (12.8) | 24 | 16 | 66.7 (44.7 to 84.4) | 87.4 (86.5 to 88.3) | 2.4 (1.4 to 3.9) | 99.8 (99.7 to 99.9) |
| Three testse | 4992 | 882 (17.7) | 22 | 19 | 86.4 (65.1 to 97.1) | 82.6 (81.6 to 83.7) | 2.2 (1.3 to 3.3) | 99.9 (99.8 to 100.0) |
| Programme analysis | ||||||||
| Two testsf | 5199 | 662 (12.7) | 26 | 16 | 61.5 (40.6 to 79.8) | 87.5 (86.6 to 88.4) | 2.4 (1.4 to 3.9) | 99.8 (99.6 to 99.9) |
| Three testsg | 5199 | 882 (17.0) | 26 | 19 | 73.1 (52.2 to 88.4) | 83.3 (82.3 to 84.3) | 2.2 (1.3 to 3.3) | 99.8 (99.7 to 99.9) |
| 20 | ||||||||
| First | 5199 | 492 (9.5) | 26 | 12 | 46.2 (26.6 to 66.6) | 90.7 (89.9 to 91.5) | 2.4 (1.3 to 4.2) | 99.7 (99.5 to 99.8) |
| Secondb | 4677 | 331 (7.1) | 13 | 6 | 46.2 (19.2 to 74.9) | 93.0 (92.3 to 93.7) | 1.8 (0.7 to 3.9) | 99.8 (99.7 to 99.9) |
| Thirdc | 4182 | 267 (6.4) | 5 | 3 | 60.0 (14.7 to 94.7) | 93.7 (92.9 to 94.4) | 1.1 (0.2 to 3.2) | 99.9 (99.8 to 100.0) |
| Cumulative test analysis | ||||||||
| Two testsd | 5169 | 823 (15.9) | 25 | 18 | 72.0 (50.6 to 87.9) | 84.4 (83.3 to 85.3) | 2.2 (1.3 to 3.4) | 99.8 (99.7 to 99.9) |
| Three testse | 5005 | 1090 (21.8) | 23 | 21 | 91.3 (72.0 to 98.9) | 78.5 (77.4 to 79.7) | 1.9 (1.2 to 2.9) | 99.9 (99.8 to 100.0) |
| Programme analysis | ||||||||
| Two testsf | 5199 | 823 (15.8) | 26 | 18 | 69.2 (48.2 to 85.7) | 84.4 (83.4 to 85.4) | 2.2 (1.3 to 3.4) | 99.8 (99.6 to 99.9) |
| Three testsg | 5199 | 1090 (21.0) | 26 | 21 | 80.8 (60.6 to 93.4) | 79.3 (78.2 to 80.4) | 1.9 (1.2 to 2.9) | 99.9 (99.7 to 100.0) |
| 10 | ||||||||
| First | 5199 | 742 (14.3) | 26 | 16 | 61.5 (40.6 to 79.8) | 86.0 (85.0 to 86.9) | 2.2 (1.2 to 3.5) | 99.8 (99.6 to 99.9) |
| Secondb | 4429 | 441 (10.0) | 10 | 5 | 50.0 (18.7 to 81.3) | 90.1 (89.2 to 91.0) | 1.1 (0.4 to 2.6) | 99.9 (99.7 to 100.0) |
| Thirdc | 3837 | 347 (9.0) | 3 | 1 | 33.3 (0.8 to 90.6) | 91.0 (90.0 to 91.9) | 0.3 (0.0 to 1.6) | 99.9 (99.8 to 100.0) |
| Cumulative test analysis | ||||||||
| Two testsd | 5171 | 1183 (22.9) | 26 | 21 | 80.8 (60.6 to 93.4) | 77.4 (76.2 to 78.6) | 1.8 (1.1 to 2.7) | 99.9 (99.7 to 100.0) |
| Three testse | 5020 | 1530 (30.5) | 24 | 22 | 91.7 (73.0 to 99.0) | 69.8 (68.5 to 71.1) | 1.4 (0.9 to 2.2) | 99.9 (99.8 to 100.0) |
| Programme analysis | ||||||||
| Two testsf | 5199 | 1183 (22.8) | 26 | 21 | 80.8 (60.6 to 93.4) | 77.5 (76.4 to 78.7) | 1.8 (1.1 to 2.7) | 99.9 (99.7 to 100.0) |
| Three testsg | 5199 | 1530 (29.4) | 26 | 22 | 84.6 (65.1 to 95.6) | 70.8 (69.6 to 72.1) | 1.4 (0.9 to 2.2) | 99.9 (99.7 to 100.0) |
Sensitivity increased and specificity decreased with lower faecal haemoglobin thresholds. For the first completed FIT, sensitivity and specificity for CRC were, respectively, 38.5% and 92.7% at a threshold of 30 µg/g, 46.2% and 90.7% at a threshold of 20 µg/g, and 61.5% and 86.0% at a threshold of 10 µg/g.
Sensitivity also increased, and specificity decreased, when taking into account multiple FITs. In cumulative test analysis, examining only individuals who completed all three tests or who tested positive prior to the third test, sensitivity and specificity for CRC were, respectively, 81.8% and 85.4% at a threshold of 40 µg/g, 86.4% and 82.6% at a threshold of 30 µg/g, 91.3% and 78.5% at a threshold of 20 µg/g, and 91.7% and 69.8% at a threshold of 10 µg/g (see Table 4). The highest observed sensitivity for CRC in the study was obtained using the 10 µg/g threshold (91.7%).
Sensitivity of two FITs was lower, and specificity higher, than that of three FITs. In cumulative test analysis, sensitivity and specificity for CRC after two FITs were, respectively, 66.7% and 89.4% at a threshold of 40 µg/g, 66.7% and 87.4% at a threshold of 30 µg/g, 72.0% and 84.4% at a threshold of 20 µg/g, and 80.8% and 77.4% at a threshold of 10 µg/g. In programme analysis, sensitivity and specificity for CRC after two FITs were, respectively, 61.5% and 89.5% at a threshold of 40 µg/g, 61.5% and 87.5% at a threshold of 30 µg/g, 69.2% and 84.4% at a threshold of 20 µg/g, and 80.8% and 77.5% at a threshold of 10 µg/g (see Table 4).
In both cumulative test analysis and programme analysis, the PPV decreased at lower faecal haemoglobin thresholds and with multiple tests, whereas the NPV remained the same. For instance, in cumulative analysis after three tests, the PPVs and NPVs were, respectively, 2.4% and 99.9% at a threshold of 40 µg/g, 2.2% and 99.9% at a threshold of 30 µg/g, 1.9% and 99.9% at a threshold of 20 µg/g, and 1.4% and 99.9% at a threshold of 10 µg/g (see Table 4).
Sensitivity, specificity, positive predictive value and negative predictive value of the faecal immunochemical test for advanced adenomas
Of the 5199 participants who underwent a colonic examination, 443 (8.5%) participants were diagnosed with AAs (Table 5).
| FIT threshold (µg/g) | Completed a FIT,a n | Tested +ve, n (%) | AAsb | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Total cases, n | FIT +ve cases, n | |||||||
| 40 | ||||||||
| First | 5199 | 326 (6.3) | 443 | 78 | 17.6 (14.2 to 21.5) | 94.8 (94.1 to 95.4) | 23.9 (19.4 to 28.9) | 92.5 (91.7 to 93.2) |
| Secondc | 4837 | 233 (4.8) | 363 | 38 | 10.5 (7.5 to 14.1) | 95.6 (95.0 to 96.2) | 16.3 (11.8 to 21.7) | 92.9 (92.2 to 93.7) |
| Thirdd | 4429 | 182 (4.1) | 308 | 36 | 11.7 (8.3 to 15.8) | 96.5 (95.8 to 97.0) | 19.8 (14.3 to 26.3) | 93.6 (92.8 to 94.3) |
| Cumulative test analysis | ||||||||
| Two testse | 5163 | 559 (10.8) | 441 | 116 | 26.3 (22.3 to 30.7) | 90.6 (89.8 to 91.4) | 20.8 (17.5 to 24.4) | 92.9 (92.2 to 93.7) |
| Three testsf | 4988 | 741 (14.9) | 424 | 152 | 35.8 (31.3 to 40.6) | 87.1 (86.1 to 88.1) | 20.5 (17.7 to 23.6) | 93.6 (92.8 to 94.3) |
| Programme analysis | ||||||||
| Two testsg | 5199 | 559 (10.8) | 443 | 116 | 26.2 (22.1 to 30.5) | 90.7 (89.8 to 91.5) | 20.8 (17.5 to 24.4) | 93.0 (92.2 to 93.7) |
| Three testsh | 5199 | 741 (14.3) | 443 | 152 | 34.3 (29.9 to 38.9) | 87.6 (86.6 to 88.5) | 20.5 (17.7 to 23.6) | 93.5 (92.7 to 94.2) |
| 30 | ||||||||
| First | 5199 | 386 (7.4) | 443 | 88 | 19.9 (16.2 to 23.9) | 93.7 (93.0 to 94.4) | 22.8 (18.7 to 27.3) | 92.6 (91.8 to 93.3) |
| Secondc | 4778 | 276 (5.8) | 353 | 50 | 14.2 (10.7 to 18.2) | 94.9 (94.2 to 95.5) | 18.1 (13.8 to 23.2) | 93.3 (92.5 to 94.0) |
| Thirdd | 4330 | 220 (5.1) | 287 | 44 | 15.3 (11.4 to 20.0) | 95.6 (95.0 to 96.3) | 20.0 (14.9 to 25.9) | 94.1 (93.3 to 94.8) |
| Cumulative test analysis | ||||||||
| Two testse | 5164 | 662 (12.8) | 441 | 138 | 31.3 (27.0 to 35.8) | 88.9 (88.0 to 89.8) | 20.8 (17.8 to 24.1) | 93.3 (92.5 to 94.0) |
| Three testsf | 4992 | 882 (17.7) | 425 | 182 | 42.8 (38.1 to 47.7) | 84.7 (83.6 to 85.7) | 20.6 (18.0 to 23.5) | 94.1 (93.3 to 94.8) |
| Programme analysis | ||||||||
| Two testsg | 5199 | 662 (12.7) | 443 | 138 | 31.2 (26.9 to 35.7) | 89.0 (88.1 to 89.9) | 20.8 (17.8 to 24.1) | 93.3 (92.5 to 94.0) |
| Three testsh | 5199 | 882 (17.0) | 443 | 182 | 41.1 (36.5 to 45.8) | 85.3 (84.2 to 86.3) | 20.6 (18.0 to 23.5) | 94.0 (93.2 to 94.6) |
| 20 | ||||||||
| First | 5199 | 492 (9.5) | 443 | 111 | 25.1 (21.1 to 29.4) | 92.0 (91.2 to 92.7) | 22.6 (18.9 to 26.5) | 92.9 (92.2 to 93.7) |
| Secondc | 4677 | 331 (7.1) | 330 | 54 | 16.4 (12.5 to 20.8) | 93.6 (92.9 to 94.3) | 16.3 (12.5 to 20.7) | 93.6 (92.9 to 94.4) |
| Thirdd | 4182 | 267 (6.4) | 260 | 40 | 15.4 (11.2 to 20.4) | 94.2 (93.4 to 94.9) | 15.0 (10.9 to 19.8) | 94.4 (93.6 to 95.1) |
| Cumulative test analysis | ||||||||
| Two testse | 5169 | 823 (15.9) | 441 | 165 | 37.4 (32.9 to 42.1) | 86.1 (85.1 to 87.1) | 20.0 (17.4 to 22.9) | 93.6 (92.9 to 94.4) |
| Three testsf | 5005 | 1090 (21.8) | 425 | 205 | 48.2 (43.4 to 53.1) | 80.7 (79.5 to 81.8) | 18.8 (16.5 to 21.3) | 94.4 (93.6 to 95.1) |
| Programme analysis | ||||||||
| Two testsg | 5199 | 823 (15.8) | 443 | 165 | 37.2 (32.7 to 41.9) | 86.2 (85.2 to 87.1) | 20.0 (17.4 to 22.9) | 93.6 (92.9 to 94.4) |
| Three testsh | 5199 | 1090 (21.0) | 443 | 205 | 46.3 (41.6 to 51.0) | 81.4 (80.3 to 82.5) | 18.8 (16.5 to 21.3) | 94.2 (93.4 to 94.9) |
| 10 | ||||||||
| First | 5199 | 742 (14.3) | 443 | 147 | 33.2 (28.8 to 37.8) | 87.5 (86.5 to 88.4) | 19.8 (17.0 to 22.9) | 93.4 (92.6 to 94.1) |
| Secondc | 4429 | 441 (10.0) | 294 | 65 | 22.1 (17.5 to 27.3) | 90.9 (90.0 to 91.8) | 14.7 (11.6 to 18.4) | 94.3 (93.5 to 95.0) |
| Thirdd | 3837 | 347 (9.0) | 216 | 42 | 19.4 (14.4 to 25.4) | 91.6 (90.6 to 92.5) | 12.1 (8.9 to 16.0) | 95.0 (94.2 to 95.7) |
| Cumulative test analysis | ||||||||
| Two testse | 5171 | 1183 (22.9) | 441 | 212 | 48.1 (43.3 to 52.8) | 79.5 (78.3 to 80.6) | 17.9 (15.8 to 20.2) | 94.3 (93.5 to 95.0) |
| Three testsf | 5020 | 1530 (30.5) | 428 | 254 | 59.3 (54.5 to 64.0) | 72.2 (70.9 to 73.5) | 16.6 (14.8 to 18.6) | 95.0 (94.2 to 95.7) |
| Programme analysis | ||||||||
| Two testsg | 5199 | 1183 (22.8) | 443 | 212 | 47.9 (43.1 to 52.6) | 79.6 (78.4 to 80.7) | 17.9 (15.8 to 20.2) | 94.2 (93.5 to 94.9) |
| Three testsh | 5199 | 1530 (29.4) | 443 | 254 | 57.3 (52.6 to 62.0) | 73.2 (71.9 to 74.4) | 16.6 (14.8 to 18.6) | 94.8 (94.1 to 95.5) |
Sensitivity of FIT for AAs was lower, and specificity higher, than for CRC. The sensitivity of the first completed FIT for AAs was 17.6% at a threshold of 40 µg/g, 19.9% at a threshold of 30 µg/g, 25.1% at a threshold of 20 µg/g and 33.2% at a threshold of 10 µg/g. Specificity of the first completed FIT for AAs was 94.8% at a threshold of 40 µg/g, 93.7% at a threshold of 30 µg/g, 92.0% at a threshold of 20 µg/g and 87.5% at a threshold of 10 µg/g. Comparable sensitivities for CRC were 30.8%, 38.5%, 46.2% and 61.5% at each of the thresholds and comparable specificities for CRC were 93.9%, 92.7%, 90.7% and 86.0% (see Table 4).
As with CRC, sensitivities for AAs were higher, and specificities lower, with multiple FITs and lower faecal haemoglobin thresholds. In cumulative test analysis after three tests, sensitivity and specificity for AAs were, respectively, 35.8% and 87.1% at a threshold of 40 µg/g, 42.8% and 84.7% at a threshold of 30 µg/g, 48.2% and 80.7% at a threshold of 20 µg/g, and 59.3% and 72.2% at a threshold of 10 µg/g. In programme analysis after three tests, sensitivity and specificity were, respectively, 34.3% and 87.6% at a threshold of 40 µg/g, 41.1% and 85.3% at a threshold of 30 µg/g, 46.3% and 81.4% at a threshold of 20 µg/g, and 57.3% and 73.2% at a threshold of 10 µg/g (see Table 5).
The PPVs of FIT were higher and NPVs lower for AAs than for CRC. For instance, at a threshold of 40 µg/g, the PPV of the first completed FIT for AAs was 23.9% and the NPV was 92.5%. At the same threshold, the PPV and NPV for CRC were 2.5% and 99.6%, respectively.
Sensitivity, specificity, positive predictive value and negative predictive value of the faecal immunochemical test for advanced colorectal neoplasia
Of the 5199 participants who underwent a colonic examination, 463 were diagnosed with ACN (Table 6); 437 (94.4%) with AAs but not CRC, 20 (4.3%) with CRC but not AAs and 6 (1.3%) with both AAs and CRC.
| FIT threshold (µg/g) | Completed a FIT,a n | Tested +ve, n (%) | ACNb | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Total cases | FIT +ve cases | |||||||
| 40 | ||||||||
| First | 5199 | 326 (6.3) | 463c | 83 | 17.9 (14.5 to 21.7) | 94.9 (94.2 to 95.5) | 25.5 (20.8 to 30.6) | 92.2 (91.4 to 92.9) |
| Secondd | 4837 | 233 (4.8) | 376 | 44 | 11.7 (8.6 to 15.4) | 95.8 (95.1 to 96.3) | 18.9 (14.1 to 24.5) | 92.8 (92.0 to 93.5) |
| Thirde | 4429 | 182 (4.1) | 313 | 38 | 12.1 (8.7 to 16.3) | 96.5 (95.9 to 97.0) | 20.9 (15.2 to 27.5) | 93.5 (92.7 to 94.2) |
| Cumulative test analysis | ||||||||
| Two testsf | 5163 | 559 (10.8) | 459 | 127 | 27.7 (23.6 to 32.0) | 90.8 (90.0 to 91.6) | 22.7 (19.3 to 26.4) | 92.8 (92.0 to 93.5) |
| Three testsg | 4988 | 741 (14.9) | 440 | 165 | 37.5 (33.0 to 42.2) | 87.3 (86.3 to 88.3) | 22.3 (19.3 to 25.4) | 93.5 (92.7 to 94.2) |
| Programme analysis | ||||||||
| Two testsh | 5199 | 559 (10.8) | 463 | 127 | 27.4 (23.4 to 31.7) | 90.9 (90.0 to 91.7) | 22.7 (19.3 to 26.4) | 92.8 (92.0 to 93.5) |
| Three testsi | 5199 | 741 (14.3) | 463 | 165 | 35.6 (31.3 to 40.2) | 87.8 (86.9 to 88.8) | 22.3 (19.3 to 25.4) | 93.3 (92.5 to 94.0) |
| 30 | ||||||||
| First | 5199 | 386 (7.4) | 463 | 95 | 20.5 (16.9 to 24.5) | 93.9 (93.1 to 94.5) | 24.6 (20.4 to 29.2) | 92.4 (91.6 to 93.1) |
| Secondd | 4778 | 276 (5.8) | 364 | 54 | 14.8 (11.3 to 18.9) | 95.0 (94.3 to 95.6) | 19.6 (15.1 to 24.7) | 93.1 (92.3 to 93.8) |
| Thirde | 4330 | 220 (5.1) | 292 | 46 | 15.8 (11.8 to 20.4) | 95.7 (95.0 to 96.3) | 20.9 (15.7 to 26.9) | 94.0 (93.2 to 94.7) |
| Cumulative test analysis | ||||||||
| Two testsf | 5164 | 662 (12.8) | 459 | 149 | 32.5 (28.2 to 37.0) | 89.1 (88.2 to 90.0) | 22.5 (19.4 to 25.9) | 93.1 (92.3 to 93.8) |
| Three testsg | 4992 | 882 (17.7) | 441 | 195 | 44.2 (39.5 to 49.0) | 84.9 (83.8 to 85.9) | 22.1 (19.4 to 25.0) | 94.0 (93.2 to 94.7) |
| Programme analysis | ||||||||
| Two testsh | 5199 | 662 (12.7) | 463 | 149 | 32.2 (27.9 to 36.6) | 89.2 (88.2 to 90.0) | 22.5 (19.4 to 25.9) | 93.1 (92.3 to 93.8) |
| Three testsi | 5199 | 882 (17.0) | 463 | 195 | 42.1 (37.6 to 46.8) | 85.5 (84.5 to 86.5) | 22.1 (19.4 to 25.0) | 93.8 (93.0 to 94.5) |
| 20 | ||||||||
| First | 5199 | 492 (9.5) | 463 | 119 | 25.7 (21.8 to 29.9) | 92.1 (91.3 to 92.9) | 24.2 (20.5 to 28.2) | 92.7 (91.9 to 93.4) |
| Secondd | 4677 | 331 (7.1) | 341 | 59 | 17.3 (13.4 to 21.7) | 93.7 (93.0 to 94.4) | 17.8 (13.9 to 22.4) | 93.5 (92.7 to 94.2) |
| Thirde | 4182 | 267 (6.4) | 264 | 42 | 15.9 (11.7 to 20.9) | 94.3 (93.5 to 95.0) | 15.7 (11.6 to 20.7) | 94.3 (93.6 to 95.0) |
| Cumulative test analysis | ||||||||
| Two testsf | 5169 | 823 (15.9) | 460 | 178 | 38.7 (34.2 to 43.3) | 86.3 (85.3 to 87.3) | 21.6 (18.9 to 24.6) | 93.5 (92.7 to 94.2) |
| Three testsg | 5005 | 1090 (21.8) | 442 | 220 | 49.8 (45.0 to 54.5) | 80.9 (79.8 to 82.1) | 20.2 (17.8 to 22.7) | 94.3 (93.6 to 95.0) |
| Programme analysis | ||||||||
| Two testsh | 5199 | 823 (15.8) | 463 | 178 | 38.4 (34.0 to 43.0) | 86.4 (85.4 to 87.3) | 21.6 (18.9 to 24.6) | 93.5 (92.7 to 94.2) |
| Three testsi | 5199 | 1090 (21.0) | 463 | 220 | 47.5 (42.9 to 52.2) | 81.6 (80.5 to 82.7) | 20.2 (17.8 to 22.7) | 94.1 (93.3 to 94.8) |
| 10 | ||||||||
| First | 5199 | 742 (14.3) | 463 | 159 | 34.3 (30.0 to 38.9) | 87.7 (86.7 to 88.6) | 21.4 (18.5 to 24.6) | 93.2 (92.4 to 93.9) |
| Secondd | 4429 | 441 (10.0) | 302 | 68 | 22.5 (17.9 to 27.7) | 91.0 (90.0 to 91.8) | 15.4 (12.2 to 19.1) | 94.1 (93.4 to 94.8) |
| Thirde | 3837 | 347 (9.0) | 219 | 43 | 19.6 (14.6 to 25.5) | 91.6 (90.6 to 92.5) | 12.4 (9.1 to 16.3) | 95.0 (94.2 to 95.7) |
| Cumulative test analysis | ||||||||
| Two testsf | 5171 | 1183 (22.9) | 461 | 227 | 49.2 (44.6 to 53.9) | 79.7 (78.5 to 80.8) | 19.2 (17.0 to 21.6) | 94.1 (93.4 to 94.8) |
| Three testsg | 5020 | 1530 (30.5) | 446 | 270 | 60.5 (55.8 to 65.1) | 72.5 (71.1 to 73.7) | 17.6 (15.8 to 19.7) | 95.0 (94.2 to 95.7) |
| Programme analysis | ||||||||
| Two testsh | 5199 | 1183 (22.8) | 463 | 227 | 49.0 (44.4 to 53.7) | 79.8 (78.6 to 80.9) | 19.2 (17.0 to 21.6) | 94.1 (93.4 to 94.8) |
| Three testsi | 5199 | 1530 (29.4) | 463 | 270 | 58.3 (53.7 to 62.8) | 73.4 (72.1 to 74.6) | 17.6 (15.8 to 19.7) | 94.7 (94.0 to 95.4) |
Sensitivities and specificities of FIT for ACN were similar to the values observed for AAs. For instance, for the first completed FIT, sensitivity and specificity for ACN were, respectively, 17.9% and 94.9% at a threshold of 40 µg/g, 20.5% and 93.9% at a threshold of 30 µg/g, 25.7% and 92.1% at a threshold of 20 µg/g, and 34.3% and 87.7% at a threshold of 10 µg/g (see Table 6).
As with CRC and AAs, sensitivity for ACN increased and specificity decreased with multiple FITs and lower faecal haemoglobin thresholds. In cumulative test analysis after three tests, sensitivity and specificity for ACN were, respectively, 37.5% and 87.3% at a threshold of 40 µg/g, 44.2% and 84.9% at a threshold of 30 µg/g, 49.8% and 80.9% at a threshold of 20 µg/g, and 60.5% and 72.5% at a threshold of 10 µg/g. In programme analysis after three tests, sensitivity and specificity were, respectively, 35.6% and 87.8% at a threshold of 40 µg/g, 42.1% and 85.5% at a threshold of 30 µg/g, 47.5% and 81.6% at a threshold of 20 µg/g, and 58.3% and 73.4% at a threshold of 10 µg/g.
The PPVs were higher and NPVs lower for ACN than for either AAs or CRC. For instance, for the first completed FIT, the PPVs and NPVs for ACN were, respectively, 25.5% and 92.2% at a threshold of 40 µg/g, 24.6% and 92.4% at a threshold of 30 µg/g, 24.2% and 92.7% at a threshold of 20 µg/g, and 21.4% and 93.2% at a threshold of 10 µg/g. In comparison, PPVs and NPVs of the first completed FIT for AAs were, respectively, 23.9% and 92.5% at a threshold of 40 µg/g, 22.8% and 92.6% at a threshold of 30 µg/g, 22.6% and 92.9% at a threshold of 20 µg/g, and 19.8% and 93.4% at a threshold of 10 µg/g. For CRC, these values were, respectively, 2.5% and 99.6% at a threshold of 40 µg/g, 2.6% and 99.7% at a threshold of 30 µg/g, 2.4% and 99.7% at a threshold of 20 µg/g, and 2.2% and 99.8% at a threshold of 10 µg/g.
Performance of the faecal immunochemical test by sex and age at invite
Of the 26 participants diagnosed with CRC, 13 were men (see Report Supplementary Material 7, Table 4). Furthermore, 12 out of 26 (33.3%) participants with CRC were aged ≤ 65 years (see Report Supplementary Material 8, Table 5). The number of CRCs when stratified by subgroup was small and, therefore, the subgroup analyses lacked statistical power.
The sensitivity, specificity, PPV and NPV for CRC of the first completed FIT at a threshold of 40 µg/g were, respectively, 38.5%, 93.0%, 2.0% and 99.8% in men, and 23.1%, 95.6%, 3.8% and 99.4% in women (see Report Supplementary Material 7, Table 4). PPVs for CRC were consistently higher in women than in men across all thresholds (e.g. in cumulative test analysis after three tests, PPVs in women compared with men were, respectively, 4.6% vs. 1.6% at a threshold of 40 µg/g, 3.9% vs. 1.5% at a threshold of 30 µg/g, 3.8% vs. 1.3% at a threshold of 20 µg/g, and 2.5% vs. 1.0% at a threshold of 10 µg/g).
The sensitivity, specificity, PPV and NPV for CRC of the first completed FIT at a threshold of 40 µg/g were, respectively, 16.7%, 94.7%, 1.5%, and 99.6% in participants aged ≤ 65 years, and 42.9%, 93.1%, 3.2%, and 99.7% in participants aged > 65 years (see Report Supplementary Material 8, Table 5).
Of the 443 participants with AAs, 311 (70.2%) were men (see Report Supplementary Material 9, Table 6) and 202 (45.6%) were aged ≤ 65 years (see Report Supplementary Material 10, Table 7).
The sensitivity, specificity, PPV and NPV for AAs of the first completed FIT at a threshold of 40 µg/g were, respectively, 19.0%, 94.1%, 24.0% and 92.1% in men, 14.4%, 96.2%, 23.8% and 93.2% in women, 17.8%, 95.7%, 26.5% and 93.1% in participants aged ≤ 65 years, and 17.4%, 93.9%, 22.1% and 92.0% in participants aged > 65 years.
Sensitivities for AAs were generally higher, and specificities lower, in men and older participants. For instance, in cumulative test analysis after three tests, the sensitivity and specificity for AAs in men were, respectively, 37.8% and 85.5% at a threshold of 40 µg/g, 45.6% and 82.8% at a threshold of 30 µg/g, 51.0% and 78.5% at a threshold of 20 µg/g, and 61.5% and 70.0% at a threshold of 10 µg/g. In women, these values were 31.3% and 90.1% at a threshold of 40 µg/g, 36.4% and 88.2% at a threshold of 30 µg/g, 41.9%% and 84.8% at a threshold of 20 µg/g, and 54.3% and 76.5% at a threshold of 10 µg/g (see Report Supplementary Material 9, Table 6).
Among participants aged ≤ 65 years, the sensitivity and specificity for AAs in cumulative test analysis after three tests were, respectively, 33.5% and 88.2% at a threshold of 40 µg/g, 39.2% and 86.2% at a threshold of 30 µg/g, 43.3% and 81.9% at a threshold of 20 µg/g, and 52.6% and 72.9% at a threshold of 10 µg/g. In those aged > 65 years, these values were 37.8% and 86.0% at 40 µg/g, 45.9% and 83.3% at a threshold of 30 µg/g, 52.4% and 79.5% at a threshold of 20 µg/g, and 65.1% and 71.5% at a threshold of 10 µg/g (see Report Supplementary Material 10, Table 7).
Of the 463 participants with ACN, 321 were men (69.3%) (see Report Supplementary Material 11, Table 8) and 211 (45.6%) were aged ≤ 65 years (see Report Supplementary Material 12, Table 9).
The sensitivity, specificity, PPV and NPV for ACN of the first completed FIT at a threshold of 40 µg/g were, respectively, 19.6%, 94.2%, 25.6% and 92.0% in men, 14.1%, 96.3%, 25.0% and 92.7% in women (see Report Supplementary Material 11, Table 8), 17.1%, 95.7%, 26.5% and 92.7% in participants aged ≤ 65 years, and 18.7%, 94.1%, 24.7% and 91.7% in participants aged > 65 years (see Report Supplementary Material 12, Table 9).
As with AAs, sensitivity for ACN was generally higher and specificity lower in men and older participants. For instance, in cumulative test analysis after three tests, at a threshold of 10 µg/g, sensitivity and specificity for ACN were, respectively, 62.3% and 70.2% in men, 56.5% and 76.9% in women, 53.7% and 73.1% in participants aged ≤ 65 years, and 66.3% and 71.8% in participants aged > 65 years.
Characteristics of detected colorectal cancers and advanced adenomas
Information on CRC stage and site was available for 23 out of 26 participants with CRC (Table 7). Of the 23 CRCs with stage and site information, 11 were primary tumours (pT) 3 or pT4, 12 were proximal to the descending colon and 11 were distal to the splenic flexure.
| Age at invite (years) | Sex | Cancer | TNM stage | Number of FITs completed | Faecal haemoglobin count (µg/g) | ||
|---|---|---|---|---|---|---|---|
| Site | Type | First completed FIT | Highest observed | ||||
| Proximal to the descending colon | |||||||
| 67 | F | Caecum | Adenocarcinoma | pT4, N0, Mx | 1 | 786 | 786 |
| 61 | M | Caecum | Adenocarcinoma | pT3, N0, Mx | 3 | 0 | 0.4 |
| 69 | M | Appendix | Carcinoid exgoblet cell | pT3, N1, Mx | 1 | 61.2 | 61.2 |
| 70 | F | Ascending colon | Adenocarcinoma | pT1, N1, Mx | 2 | 6 | 502.4 |
| 68 | F | Ascending colon | Adenocarcinoma | pT1, N0, Mx | 2 | 2.2 | 157.8 |
| 66 | M | Ascending colon | Adenocarcinoma | pT2, N1, Mx | 2 | 38.4 | 42.6 |
| 70 | F | Ascending colon | Adenocarcinoma | pT3, N0, Mx | 3 | 12.8 | 23.6 |
| 61 | F | Ascending colon | Adenocarcinoma | pT4, N1, Mx | 1 | 184.8 | 184.8 |
| 71 | F | Transverse colon | Adenocarcinoma | pT1, Nx, Mx | 3 | 1.2 | 2.6 |
| 64 | M | Transverse colon | Adenocarcinoma | pT4, N0, Mx | 3 | 7.6 | 35 |
| 61 | M | Transverse colon | Adenocarcinoma | pT3, N0, Mx | 2 | 0 | 112.8 |
| 63 | F | Transverse colon | Adenocarcinoma | pT1, N0, Mx | 3 | 1.8 | 52 |
| Distal to the splenic flexure | |||||||
| 72 | M | Descending colon | Adenocarcinoma | pT1, N0, Mx | 2 | 20.8 | 71 |
| 68 | M | Sigmoid colon | Adenocarcinoma | pT3, N0, Mx | 1 | 271.4 | 271.4 |
| 65 | M | Sigmoid colon | Adenocarcinoma | pT4, N2, M1 | 1 | 1937.4 | 1937.4 |
| 68 | F | Sigmoid colon | Adenocarcinoma | pT1, Nx, Mx | 1 | 51 | 51 |
| 63 | M | Sigmoid colon | Adenocarcinoma | pT3, N0, Mx | 2 | 37.8 | 1522.6 |
| 65 | F | Sigmoid colon | Adenocarcinoma | pT3, N1, Mx | 3 | 5.4 | 5409.6 |
| 67 | M | Rectosigmoid | Adenocarcinoma | pT1, N0, Mx | 1 | 97.2 | 97.2 |
| 63 | F | Rectum | Adenocarcinoma | pT2, N1, Mx | 2 | 11.2 | 95.4 |
| 61 | F | Rectum | Neuroendocrine tumour | pT1 (from biopsy) | 2 | 0.4 | 1.2 |
| 68 | F | Rectum | Adenocarcinoma | pT1, Nx, Mx | 2 | 16.4 | 752 |
| 69 | M | Rectum | Adenocarcinoma | pT1, Nx, Mx | 1 | 54.8 | 54.8 |
| Unknown | |||||||
| 67 | F | Unknown | Adenocarcinoma | Unknown | 1 | 21.4 | 21.4 |
| 63 | M | Unknown | Adenocarcinoma | Unknown | 2 | 0 | 0 |
| 65 | M | Unknown | Unknown | Unknown | 1 | 14.4 | 14.4 |
Characteristics of the 524 AAs diagnosed in 443 participants are detailed in Report Supplementary Material 13, Table 10, including information on site, size, dysplasia and histology. AAs were most frequently located in the sigmoid colon (28.6%) and the vast majority (93.7%) had low-grade dysplasia. Approximately half of the AAs were ≥ 10 mm in size (n = 269; 51.3%). Histology was known for 510 (97.3%) AAs, and 357 (68.1%) were tubulovillous adenomas. Out of the 443 participants with at least one AA diagnosed, 383 (86.5%) had a single AA and 49 (11.1%) had two AAs, whereas only 11 (2.4%) participants had three or more AAs (see Report Supplementary Material 14, Table 11).
Chapter 4 Health psychology assessment
This chapter is partly reproduced from Bowyer et al. 67 Patient attitudes towards faecal immunochemical testing for haemoglobin as an alternative to colonoscopic surveillance of groups at increased risk of colorectal cancer. Journal of Medical Screening vol. 20, iss. 3, pp. 149–56. Copyright © 2013 by the Authors. Reprinted by permission of SAGE Publications, Ltd. This property is the exclusive property of the SAGE Publishing and is protected by copyright and other intellectual property laws. User may not modify, publish, transmit, participate in the transfer or sale of, reproduce, create derivative works (including course packs) from, distribute, perform, display, or in any way exploit any of the content of the file(s) in whole or in part. Permission may be sought for further use from Publications Ltd., Rights & Permissions Department, 1, Oliver’s Yar, 55, City Road, London EC1Y 1SP, Email: permissions@sagepub.co.uk. By accessing the file(s), the User acknowledges and agrees to these terms. URL: www.sagepub.co.uk.
Introduction
In this chapter, we present studies evaluating perceptions and preferences for FIT surveillance and summarise the experience of participants in the main FIT for Follow-Up study. Colonoscopy surveillance is currently considered the optimal test for individuals at intermediate or high risk of CRC. 21 However, since the introduction of the BCSP, there have been concerns about the implications for endoscopy departments and the risk associated with repeat colonoscopies at regular intervals. These concerns are further compounded by the modest diagnostic yield of surveillance colonoscopy. 19,39 Another issue with surveillance colonoscopy is that one in five individuals at intermediate or high risk of CRC do not attend the surveillance examinations, which is comparable to the uptake of colonoscopy following an abnormal gFOBT in the BCSP. 16,35,36 Factors associated with non-attendance at colonoscopy include anxiety about having to prepare for the test with a laxative, anxiety about the procedure itself (such as the risk of bowel perforation) and expectations of pain and embarrassment. 34
FIT might be a viable alternative as the primary surveillance test. FIT is administered at home, is non-invasive and requires only a single stool sample, thereby significantly reducing the unpleasantness associated with other home-based stool tests. 67 As such, FIT is aligned with people’s preferences for CRC tests that are non-invasive, avoid pain as much as possible and do not require bowel preparation, while offering high levels of sensitivity and specificity. 78 One study found that average-risk participants who were concerned about procedural discomfort were more likely to choose FIT over colonoscopy as a screening modality. 79 In these patients, FIT completion rates were higher than colonoscopy attendance, a finding supported in a recent RCT. 80 However, there is a lack of research into the perceptions of and preferences for different forms of post-polypectomy surveillance among higher-risk individuals.
Here, we present the findings of the health psychology work stream nested within the FIT for Follow-Up study. Its aim was to better understand what people think about FIT in the context of surveillance, to monitor the actual participant-reported experience associated with an annual FIT and begin to investigate what preferences people at intermediate risk have for their adenoma surveillance.
Study 1: patient attitudes towards the faecal immunochemical test as an alternative to colonoscopy surveillance of groups at increased risk of colorectal cancer – a qualitative discussion group study
The following is an executive summary of a study into public attitudes towards FIT. Its main aim was to gain an early understanding of potential issues for the main study and to facilitate the design of participant materials. A full summary has been published by Bowyer et al. 67
Methods
Recruitment
A total of 198 individuals aged 60–74 years who resided in the London Boroughs of Brent and Harrow were identified by the BCSP London Hub and St Mark’s Hospital Endoscopy Unit. Participants varied in CRC risk level and experience of gFOBT, baseline colonoscopy and colonoscopy surveillance. Previous research has demonstrated that patient preferences differ according to perceived risk and prior experience of colonoscopy surveillance. 69,81–84
Average-risk groups included individuals with and without prior experience of colonoscopy, all of whom had received a negative gFOBT result from the BCSP and were awaiting their next routine BCSP invitation. Intermediate-risk surveillance-naive groups included individuals who were awaiting their first surveillance colonoscopy, having been referred via the BCSP.
Intermediate-risk surveillance-experienced groups consisted of individuals who had been referred for colonoscopy via their GP or as part of the UK Flexible Sigmoidoscopy Screening Trial. 9 The study was approved by the North London Research Ethics Committee (reference 10/H0717/82).
Discussion guide
The discussion groups were conducted in November 2011 and used a comprehensive stepwise discussion guide (see Report Supplementary Material 15). After each information segment on CRC surveillance, participants responded to the following question: ‘Consider the information you have just seen about FIT replacing a routine colonoscopy. How would you feel about the offer of a FIT every year instead of a three-yearly colonoscopy?’. They responded using an electronic response device, selecting an option on a six-point scale from ‘very positive’ to ‘very negative’, before discussing each section as a group. Discussions were transcribed and coded using thematic analysis. 85
Results
Of the 198 people who were sent invitation letters, 45 (22.7%) agreed to participate. Of these, 28 people eventually took part in one of five discussion groups. The type of risk profile had an important impact on how information about the FIT was interpreted. There was agreement in all groups that a FIT would be easier to complete and safer than a colonoscopy. However, there was concern among intermediate-risk participants with a history of previous colonoscopy whether or not a FIT would be reliable. One female from the surveillance-experienced group voiced her reservations:
Once you’ve had a colonoscopy you feel very reassured yourself because you’ve actually seen the whole procedure . . . whereas with this FIT, does it work, does it not . . . you don’t know.
Female, surveillance-experience group
Interestingly, many of the concerns about FIT related to the test relying on a single sample:
I’m very comfortable with the FIT but it also has a down-side that it . . . wouldn’t detect as much as providing three samples, possibly.
Female, average-risk group
This was further compounded by the belief that polyps might bleed only intermittently.
Discussion
Overall, FIT was preferred over colonoscopy by people who were at average risk or surveillance naive, who cited the importance of being tested more frequently. By contrast, the surveillance-experienced group did not endorse the idea of annual FIT replacing 3-yearly colonoscopy surveillance because of concerns about the sensitivity of FIT. They would endorse FIT only in addition to, rather than instead of, 3-yearly colonoscopy surveillance. The study had several limitations. It is possible that, among individuals who attend a focus group, levels of anxiety about CRC are different from those in the general population. Unfortunately, we were unable to test this possibility and how this would have affected preferences stated in the groups.
Some patients had also previously undergone a colonoscopy with a high satisfaction rate so they might have been more likely to prefer to have another in the future. This group may also have been told that they need a colonoscopy for follow-up and so may have been biased. As discussed in General discussion in more detail, future research should focus on individuals who have not yet undergone colonoscopy.
Study 2: acceptability of annual faecal immunochemical tests for post-polypectomy surveillance – findings from the main study
Introduction
As part of the main study, we monitored experiences associated with FIT-based annual surveillance. The key areas were (1) the psychological impact of annual surveillance, (2) acceptability of completing the test kit and (3) preferences for future surveillance.
Methods
Baseline questionnaire
The baseline questionnaire was sent to people alongside the consent form and round 1 FIT kit (see Report Supplementary Material 1). Participants were asked to complete the questionnaire and return it with the consent form and FIT kit. Those who did not respond within 3 weeks of the invitation were sent a reminder letter.
Participants were asked to rate their experience of their baseline colonoscopy (‘Was your overall experience of your most recent colonoscopy?’) on a four-point scale ranging from ‘not at all acceptable’ (1) to ‘very acceptable’ (4).
The baseline questionnaire assessed specific aspects of CRC-related worry [‘Over the last two weeks, how often have you worried about having bowel polyps (how often have you thought about your own chance of developing bowel cancer?’)] using a four-point scale ranging from ‘not at all’ (1) to ‘every day’ (4).
The questionnaire also assessed participants’ current emotional state using the STAI,86 which contains six items (‘calm’, ‘tense’, ‘upset’, ‘relaxed’, ‘content’ and ‘worried’), using a scale ranging from ‘not at all’ (1) to ‘very much so’ (4). The scale met the standard threshold for internal reliability in each round (Cronbach’s alpha = 0.80, 0.81, 0.81 and 0.79, respectively). Positive emotional items were reverse coded so that higher scores reflected greater degrees of state anxiety.
Round 1 questionnaire
Questionnaires were administered at the same time as the test outcomes (i.e. a negative FIT result letter or a covering letter once the participant had attended or declined the offer of an early surveillance colonic examination). See Report Supplementary Material 2 for study questionnaires. Questionnaires were not posted to participants who had been diagnosed with cancer to minimise distress.
The round 1 questionnaire for participants with a negative FIT result (round 1 FIT negative) repeated the measurement of current emotional well-being and specific CRC-related beliefs and worry (as per the baseline questionnaire). In addition, the questionnaire assessed motivation to take part in the study, acceptability of the FIT invitation materials, clarity of instructions and ease of completing the FIT kit.
In the section on completing the test, participants rated the ease of ‘catching the bowel motion’, ‘removing the stick from the sampling bottle’, ‘collecting the sample with the stick’, ‘reinserting the stick into the sample bottle’ and ‘closing the sample bottle after use’, on a four-point scale ranging from ‘very difficult’ (1) to ‘very easy’ (4). Participants were also asked to respond to the following negative effects of completing the FIT (‘Doing the FIT made me feel anxious’, ‘The thought of an abnormal result from the FIT scared me’, ‘After seeing the FIT, I was concerned about its ability to detect new polyps’) on a four-point scale ranging from ‘strongly disagree’ (1) to ‘strongly agree’ (4). This question was administered only at round 1.
Participants were asked in all three rounds how they felt when waiting for the FIT results and when receiving the FIT results using a four-point scale ranging from ‘not worried’ (1) to ‘very worried’ (4).
The questionnaires for all three rounds also contained three items adapted from the emotional subscale of the Positive Psychological Consequences of Screening questionnaire87 to ascertain the degree of reassurance gained after each round of FIT surveillance: did the FIT . . . ‘reassure you that you did not have bowel cancer?’, ‘make you feel more hopeful about the future?’, ‘make you feel less anxious about bowel cancer?’. Participants responded to each item on a four-point scale ranging from ‘not at all’ (1) to ‘a great deal’ (4). For participants with a positive FIT result, the item was modified to ‘Did your last colonoscopy. . .’ followed by the same response options. The scale had acceptable to good inter-reliability in the three rounds with Cronbach’s alpha of 0.84, 0.80 and 0.76 in rounds 1–3, respectively, and will be referred to as the Positive Consequences of Surveillance Scale (PCSS).
Round 2 questionnaire
The round 2 questionnaire repeated the assessment of emotional well-being and perceived CRC worry (see Baseline questionnaire).
Participant questionnaires for faecal immunochemical test-positive participants in rounds 1 and 2
Participants with a FIT positive result were given the same questionnaire for rounds 1 and 2 but with a few additional items for round 2, including a question about how they felt about being invited to have a follow-up test (in the vast majority of cases a colonoscopy) earlier than they had expected; for this they could answer on a four-point scale ranging from ‘very dissatisfied’ (1) to ‘very satisfied’ (4). Participants with a FIT positive result were also asked to state a preference between four potential options for future surveillance: ‘Routine colonoscopy every 3 years’, ‘Routine colonoscopy every 3 years plus a FIT every year’, ‘FIT every year only’ or ‘No further surveillance’.
Round 3 questionnaire
End-of-study questionnaires (identical to those used in rounds 1 and 2) were sent to all participants (irrespective of their round 3 FIT result) after they had attended their end-of-study colonic examination.
End-of-study interviews
A subsample of participants were invited to participate in an end-of-study interview with a research assistant (see Report Supplementary Material 16 for the interview guide). Participants were invited for an interview at the end of round 1 or 2 if they had received a positive FIT during these two rounds, or at the end of the study if they had completed a round 3 FIT and attended their end-of-study colonic examination. The aim of the interviews was to enrich the quantitative evaluation of participant-reported experience.
Surveillance outcomes
At the end of the study, participants fell into one of four groups. Respondents who had received three negative FIT results were divided into two subgroups according to the outcome of the end-of-study colonic examination. Respondents who had no CRC-related abnormality detected at their end-of-study examination were classified as ‘true negative.’ In contrast, those who had some form of therapeutic intervention to remove a CRC-related abnormality (e.g. polyps or adenoma) were classified as ‘false negative’. In the group of respondents who had received a positive FIT result in rounds 1–3, the outcome of the diagnostic colonoscopy determined whether a participant was classified as ‘true positive’ (when the diagnostic colonoscopy detected an AA or CRC-related abnormality) or ‘false positive’ (when the diagnostic colonoscopy did not detect an AA or CRC-related abnormality). Our criteria were based on previous research, which suggested that participants would consider any form of CRC-related abnormality a significant clinical finding. 67,88 These categories excluded people who did not complete their end-of-study questionnaire and participants who had CRC detected by whatever means. As a result, our definition of an abnormal result was different from the one used to categorise clinical findings in the main study. For example, we classified any colonoscopy which detected a CRC-related abnormality as abnormal, whereas in the main study the definition of abnormal was restricted to colonoscopies which had detected AAs or CRC.
Statistical analysis
Basic information on demographics (i.e. age, sex) and participant outcomes for FIT and diagnostic/surveillance examinations were obtained from data recorded in the PMS. Initial analysis revealed that most responses were skewed towards the upper end (in the case of acceptability) or the lower end (in the case of CRC-related worry and risk) of the distribution. As a result, we used non-parametric tests of differences to compare between different test outcomes and within individuals responding in repeated rounds. The Mann–Whitney U-test was used for between-group comparisons and Wilcoxon signed-rank test for comparison across different rounds. Chi-squared tests were used for individual items that had been collapsed into binary outcomes.
Results
Table 8 gives a demographic breakdown of responders in all three rounds. Of 5946 participants, 5879 (98.9%) completed a baseline questionnaire with 5020 participants (84.4%) completing a round 1 questionnaire. Of 5350 participants, 4491 (83.9%) completed a round 2 questionnaire, and of 4646 participants, 3881 (83.5%) completed a round 3 questionnaire.
| Variables | Time point | |||
|---|---|---|---|---|
| Baseline, n (%) | Round, n (%) | |||
| 1 | 2 | 3 | ||
| All participants | 5879 | 5020 | 4491 | 3881 |
| Gender | ||||
| Female | 2030 (34.5) | 1751 (34.9) | 1576 (35.1) | 1319 (34.4) |
| Male | 3849 (65.5) | 3269 (65.1) | 2915 (64.9) | 2562 (65.6) |
| Age | ||||
| ≤ 65 years | 2847 (48.4) | 2364 (47.1) | 2130 (47.4) | 1866 (48.1) |
| > 65 years | 3032 (51.6) | 2656 (52.9) | 2361 (52.6) | 2015 (51.9) |
Satisfaction with previous colonoscopy at baseline was extremely high, with 95.8% (5370/5604) of participants responding to this question rating their baseline colonoscopy as acceptable (Table 9). However, female participants were significantly more likely than male participants to report that their colonoscopy was not acceptable (6.7% vs. 2.8% respectively; χ2 = 47.94; p < 0.001). There was no significant difference by age (χ2 = 0.02; p = 0.89).
| Experience with previous colonoscopy at baseline | |||||
|---|---|---|---|---|---|
| Variables | n a | Not acceptable, n (%) | Acceptable, n (%) | χ2 | p-value |
| All participants | 5604 | 234 (4.2) | 5370 (95.8) | ||
| Gender | 47.94 | < 0.001 | |||
| Female | 1933 | 130 (6.7) | 1603 (93.3) | ||
| Male | 3671 | 104 (2.8) | 3567 (97.2) | ||
| Age (years) | 0.02 | 0.894 | |||
| ≤ 65 | 2707 | 112 (4.1) | 2595 (95.9) | ||
| > 65 | 2897 | 122 (4.2) | 2775 (95.8) | ||
| How did you feel about being invited to have a colonoscopy earlier than you had expected? | |||||
| Variables | n | Dissatisfied, n (%) | Satisfied, n (%) | χ2 | p-value |
| All participants | 450 | 34 (7.6) | 416 (92.4) | ||
| Round | 9.199 | 0.002 | |||
| 1 | 176 | 5 (2.8) | 171 (97.2) | ||
| 2 | 274 | 29 (10.6) | 245 (89.4) | ||
| Gender | 0.001 | 0.978 | |||
| Female | 105 | 8 (7.6) | 97 (92.4) | ||
| Male | 345 | 26 (7.5) | 319 (92.5) | ||
| Age (years) | 0.137 | 0.720 | |||
| ≤ 65 | 185 | 15 (8.1) | 170 (91.9) | ||
| > 65 | 265 | 19 (7.2) | 246 (92.8) | ||
| How did you find catching the bowel motion? | |||||
| Variables | n | Difficult, n (%) | Easy, n (%) | χ2 | p-value |
| All participants | 4990 | 255 (5.1) | 4735 (94.9) | ||
| Gender | 1.084 | 0.312 | |||
| Female | 1736 | 81 (4.7) | 1655 (95.4) | ||
| Male | 3254 | 174 (5.3) | 3080 (94.7) | ||
| Age (years) | 0.186 | 0.699 | |||
| ≤ 65 | 2355 | 117 (5.0) | 2497 (95.0) | ||
| > 65 | 2635 | 138 (5.1) | 2497 (94.8) | ||
| How did you find removing the stick from the sampling bottle? | |||||
| Variables | n | Difficult, n (%) | Easy, n (%) | χ2 | p-value |
| All participants | 4798 | 47 (1.0) | 4751 (99.0) | ||
| Gender | 5.529 | 0.016 | |||
| Female | 1670 | 24 (1.4) | 1646 (98.6) | ||
| Male | 3128 | 23 (0.7) | 3105 (99.3) | ||
| Age (years) | 4.562 | 0.039 | |||
| ≤ 65 | 2274 | 15 (0.7) | 2259 (99.3) | ||
| > 65 | 2524 | 32 (1.3) | 2492 (98.7) | ||
| How did you find collecting the sample with the stick? | |||||
| Variables | n | Difficult, n (%) | Easy, n (%) | χ2 | p-value |
| All participants | 4790 | 137 (2.9) | 4653 (97.1) | ||
| Gender | 7.806 | 0.006 | |||
| Female | 1666 | 63 (3.8) | 1603 (96.2) | ||
| Male | 3124 | 74 (2.4) | 3050 (97.6) | ||
| Age (years) | 0.290 | 0.603 | |||
| ≤ 65 | 2269 | 68 (3.0) | 2201 (97.0) | ||
| > 65 | 2521 | 69 (2.7) | 2452 (97.3) | ||
| How did you find reinserting the stick into the sampling bottle? | |||||
| Variables | n | Difficult, n (%) | Easy, n (%) | χ2 | p-value |
| All participants | 4801 | 246 (5.1) | 4555 (94.9) | ||
| Gender | 25.067 | < 0.001 | |||
| Female | 1670 | 122 (7.3) | 1548 (92.7) | ||
| Male | 3131 | 124 (4.0) | 3007 (96.0) | ||
| Age (years) | 2.220 | 0.077 | |||
| ≤ 65 | 2271 | 105 (4.6) | 2166 (95.4) | ||
| > 65 | 2530 | 141 (5.6) | 2389 (94.4) | ||
| How did you find closing the sampling bottle? | |||||
| Variables | n | Difficult, n (%) | Easy, n (%) | χ2 | p-value |
| All participants | 4801 | 36 (0.7) | 4765 (99.3) | ||
| Gender | 2.447 | 0.159 | |||
| Female | 1673 | 17 (1.0) | 1656 (99.0) | ||
| Male | 3128 | 19 (0.6) | 3109 (99.4) | ||
| Age (years) | 0.099 | 0.753 | |||
| ≤ 65 | 2275 | 18 (0.8) | 2257 (99.2) | ||
| > 65 | 2526 | 18 (0.7) | 2508 (99.3) | ||
| Doing the FIT made me anxious | |||||
| Variables | n | Disagree, n (%) | Agree, n (%) | χ2 | p-value |
| All participants | 4877 | 3570 (73.2) | 1307 (26.8) | ||
| Gender | 31.253 | 0.001 | |||
| Female | 1675 | 1144 (68.3) | 531 (31.7) | ||
| Male | 3202 | 2426 (75.8) | 776 (24.2) | ||
| Age (years) | 1.010 | 0.316 | |||
| ≤ 65 | 2319 | 1682 (72.5) | 637 (27.5) | ||
| > 65 | 2558 | 1888 (73.8) | 670 (26.2) | ||
| The thought of an abnormal result from the FIT scared me | |||||
| Variables | n | Disagree, n (%) | Agree, n (%) | χ2 | p-value |
| All participants | 4840 | 2500 (51.7) | 2340 (48.3) | ||
| Gender | 92.093 | < 0.001 | |||
| Female | 1670 | 704 (42.2) | 966 (57.8) | ||
| Male | 3170 | 1796 (56.7) | 1374 (43.3) | ||
| Age (years) | 1.399 | 0.238 | |||
| ≤ 65 | 2301 | 1168 (50.8) | 1133 (49.2) | ||
| > 65 | 2539 | 1332 (52.5) | 2500 (52.5) | ||
| After seeing the FIT, I was concerned about its ability to detect new polyps | |||||
| Variables | n | Disagree, n (%) | Agree, n (%) | χ2 | p-value |
| All participants | 4856 | 3440 (70.8) | 1416 (29.2) | ||
| Gender | 30.436 | < 0.001 | |||
| Female | 1680 | 1107 (65.9) | 573 (34.1) | ||
| Male | 3176 | 2333 (73.5) | 843 (26.5) | ||
| Age (years) | 2.67 | 0.107 | |||
| ≤ 65 | 2304 | 1658 (72.0) | 646 (28.0) | ||
| > 65 | 2552 | 1782 (69.8) | 770 (30.2) | ||
Participants unanimously reported that catching the bowel motion, removing the stick, collecting the sample with the stick, reinserting the stick into the sampling bottle and closing the sampling bottle was easy (94.9%, 99.0%, 97.1%, 95.4% and 99.3%, respectively; see Table 9). Participants aged > 65 years were slightly more likely than those aged ≤ 65 years to find removing the stick from the sampling bottle difficult (1.3% vs. 0.7%; χ2 = 4.56, p = 0.04). There were no other significant differences by age.
There were a number of small but significant differences by sex (see Table 9). Females were slightly more likely than males to report difficulties with removing the stick from the bottle (1.4% vs. 0.7%, χ2 = 5.5; p = 0.02), collecting the sample with the stick (3.8% vs. 2.4%, χ2 = 7.8; p = 0.006) and reinserting the stick into the sampling bottle (7.3% vs. 4.0%; χ2 = 25.1; p < 0.001).
In terms of the psychological consequences of screening, 26.8% of participants in round 1 reported that completing the FIT kit made them feel anxious (see Table 9), 48.3% reported that the thought of an abnormal test result had scared them (see Table 9) and 7.3% reported being worried when waiting for the round 1 FIT result (Table 10). A total of 29.2% reported being concerned about the ability of FIT to detect new polyps (see Table 9).
| Variables | Time point, how did you feel when waiting for the FIT results? | |||||
|---|---|---|---|---|---|---|
| Round 1 | Round 2 | Round 3 | ||||
| Not worried, n (%) | Worried, n (%) | Not worried, n (%) | Worried, n (%) | Not worried, n (%) | Worried, n (%) | |
| All participants | 4628 (92.7) | 362 (7.3) | 4125 (92.4) | 337 (7.6) | 4125 (92.4) | 337 (7.6) |
| Age (years) | ||||||
| ≤ 65 | 2175 (92.4) | 179 (7.6) | 1962 (92.7) | 155 (7.3) | 1744 (94.2) | 108 (5.8) |
| > 65 | 2453 (93.1) | 183 (6.9) | 2163 (92.2) | 182 (7.8) | 1889 (94.5) | 111 (5.6) |
| p-value | 0.368 | 0.579 | 0.579 | |||
| Sex | ||||||
| Female | 1554 (85.9) | 182 (10.5) | 1391 (88.9) | 173 (11.1) | 1191 (91.0) | 118 (9.0) |
| Male | 3074 (95.4) | 180 (5.5) | 2734 (94.3) | 164 (5.7) | 2442 (96.0) | 101 (4.0) |
| p-value | < 0.001 | < 0.001 | < 0.706 | |||
| FIT result | ||||||
| Negative | 4392 (93.2) | 321 (6.8) | 3975 (92.8) | 310 (7.2) | 3456 (5.6) | 205 (5.6) |
| Positive | 236 (85.2) | 41 (14.8) | 150 (84.7) | 27 (15.3) | 142 (92.2) | 12 (7.8) |
| p-value | < 0.001 | < 0.001 | 0.283 | |||
There were significant differences by sex but not by age in the responses to these items. Female responders were significantly more likely to report being anxious when completing the test kit than male responders (31.7% vs. 24.2%, χ2 = 31.25; p = 0.001; see Table 9). Female responders were also more likely to report that the thought of an abnormal test result had scared them (57.8% vs. 43.3%, χ2 = 92.10; p < 0.001; see Table 9) and were more likely than male responders to report being worried while waiting for the round 1 FIT results (10.5% vs. 5.5%, χ2 = 41.63; p < 0.001; see Table 10). Female responders were also more likely than male responders to be concerned about the ability of FIT to detect new polyps (34.1% vs. 29.2%, χ2 = 30.44; p< 0.001; see Table 9).
Spielberger State–Trait Anxiety Inventory scores across different rounds
Anxiety was highest at baseline (baseline = 9, round 1 = 7, round 2 = 7, round 3 = 7; Table 11). Comparison across the four assessments of anxiety confirmed that anxiety at baseline was significantly higher than at round 1 (p < 0.001), round 2 (p < 0.001) and round 3 (p < 0.001). There were, however, no significant differences between rounds 1 and 2 (p = 0.36) or between rounds 2 and 3 (p = 0.18) (see Table 11).
| Psychological scale | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Round 1 | Round 2 | Round 3 | ||||
| Median | FIT–ve median | FIT+ve median | FIT–ve median | FIT+ve median | FIT–ve median | FIT+ve median | |
| STAI | |||||||
| I feel calm | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| I am tense | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| I am upset | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| I am relaxed | 2.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| I feel content | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| I am worried | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Overall | 9.00 | 7.00 | 7.00 | 7.00 | 6.00 | 7.00 | 6.00 |
| PCSS | |||||||
| Reassurance | – | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| More hope | – | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Less anxious | – | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Overall | – | 11.00 | 10.00 | 10.00 | 11.00 | 11.00 | 11.00 |
There was no significant difference in anxiety between people who had received FIT-positive versus FIT-negative results in round 1 (FIT–ve = 7 vs. FIT+ve = 7; p = 0.37), round 2 (FIT–ve = 7 vs. FIT+ve = 6; p = 0.26) or round 3 (FIT–ve = 7 vs. FIT+ve = 6; p = 0.10; see Table 11). There was a small but statistically significant trend of males reporting slightly higher levels of anxiety than females across the three rounds (Table 12).
| Variables | Time point, median STAI score | |||
|---|---|---|---|---|
| Baseline | Round 1 | Round 2 | Round 3 | |
| All participants | 9.00 | 7.00 | 7.00 | 7.00 |
| Age (years) | ||||
| ≤ 65 | 9.00 | 7.00 | 7.00 | 7.00 |
| > 65 | 9.00 | 7.00 | 7.00 | 7.00 |
| p-value | 0.905 | 0.374 | 0.725 | 0.342 |
| Sex | ||||
| Female | 9.00 | 6.00 | 7.00 | 7.00 |
| Male | 10.00 | 8.00 | 7.00 | 8.00 |
| p-value | 0.001 | < 0.001 | < 0.01 | < 0.001 |
We observed a very similar pattern for CRC-related worry by round. The proportion of respondents worrying more than once a week was 7.2%, 8.7% and 9.2% at baseline, round 1 and round 2, respectively, with a decline at round 3 (3.0%) (p = 0.001; Table 13). There were no between-group differences. Furthermore, the vast majority of participants who had a positive result in round 1 or 2 felt satisfied about being called early for their colonoscopy as a result of a positive FIT (97.2% and 89.4%, respectively; see Table 9). However, participants were significantly more likely to be dissatisfied about being called early for colonoscopy in round 2 than in round 1 (10.6% vs. 2.8%, χ2 = 9.20; p = 0.002; see Table 9).
| Variables | Time point, doing the FIT kit | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Round 1 | Round 2 | Round 3 | |||||
| Not worried, n (%) | Worried, n (%) | Not worried, n (%) | Worried, n (%) | Not worried, n (%) | Worried, n (%) | Not worried, n (%) | Worried, n (%) | |
| All participants | 5213 (92.8) | 404 (7.2) | 4512 (91.3) | 428 (8.7) | 4007 (90.8) | 407 (9.2) | 3672 (97.0) | 114 (3.0) |
| Age (years) | ||||||||
| ≤ 65 | 2499(92.2) | 212 (7.8) | 2109 (90.3) | 226 (9.7) | 1898 (90.6) | 197 (9.4) | 1764 (96.7) | 61 (3.3) |
| > 65 | 2714(93.4) | 192 (6.6) | 2403 (92.2) | 202 (7.8) | 2109 (90.9) | 210 (9.1) | 1908 (97.3) | 53 (2.7) |
| p-value | 0.079 | < 0.05 | 0.715 | 0.255 | ||||
| Sex | ||||||||
| Female | 1760(90.9) | 176 (176) | 1518 (88.7) | 194 (11.3) | 1332 (86.1) | 215 (13.9) | 1214 (95.0) | 64 (5.0) |
| Male | 3453(93.8) | 228 (6.2) | 2994 (92.8) | 234 (7.2) | 2675 (93.3) | 192 (6.7) | 2458 (98.0) | 50 (2.0) |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| FIT result | ||||||||
| Negative | NA | 4275 (91.5) | 398 (8.5) | 3845 (90.7) | 395 (9.3) | 3495 (97.1) | 301 (2.9) | |
| Positive | NA | 237 (88.8) | 30 (11.2) | 162 (93.1) | 12 (6.9) | 143 (96.0) | 6 (4.0) | |
| p-value | 0.145 | 0.348 | 0.172 | |||||
Positive consequences of faecal immunochemical test surveillance
Participants reported very high levels of reassurance (median = 4 out of 4), feeling more hopeful (median = 4 out of 4) and being less anxious (median = 3 out of 4) as a result of taking part in the study (see Table 10). There were no meaningful differences by round or FIT results (see Table 11).
Preferences for different types of adenoma surveillance
Table 14 offers a breakdown of surveillance preferences. As can be seen by the grand total, a majority of participants (57.9%, n = 2478) preferred ‘Routine colonoscopy every three years plus a FIT every year (RC + FIT)’, followed by ‘FIT every year with colonoscopy only for a positive result (FIT only)’ (31.5%, n = 1347), ‘Routine colonoscopy every three years and no FIT (RC only)’ (8.9%, n = 379) and ‘No further colonoscopies or FIT (No surveillance)’ (1.8%, n = 75).
| Variables | Surveillance preference, n (%) | |||
|---|---|---|---|---|
| Routine colonoscopy every 3 years and no FIT (‘RC only’)a | Routine colonoscopy every 3 years plus a FIT every year (‘RC + FIT’) | FIT every year with colonoscopy only for a positive result (‘FIT only’) | No further colonoscopies or FIT (‘no surveillance’) | |
| All participants | 379 (8.9) | 2478 (57.9) | 1347 (31.5) | 75 (1.8) |
| Sexb | ||||
| Female | 81 (5.8) | 801 (57.1) | 492 (35.0) | 30 (2.1) |
| Male | 298 (10.4) | 1677 (58.3) | 855 (29.7) | 45 (1.6) |
| Age (years) | ||||
| ≤ 65 | 165 (8.1) | 1175 (58.0) | 655 (32.3) | 31 (1.5) |
| > 65 | 214 (9.5) | 1303 (57.8) | 692 (30.7) | 44 (2.0) |
| Roundb | ||||
| 1 | 41 (15.2) | 105 (39.0) | 118 (43.9) | 5 (1.9) |
| 2 | 22 (12.2) | 82 (45.6) | 73 (40.6) | 3 (1.7) |
| 3 | 15 (10.1) | 81 (54.4) | 52 (34.9) | 1 (0.7) |
| Year 3 (FIT–ve) | 300 (8.2) | 2206 (60.0) | 1104 (30.0) | 66 (1.8) |
| Previous experience with baseline colonoscopyb | ||||
| Not acceptable | 12 (8.6) | 56 (40.0) | 68 (48.6) | 4 (2.9) |
| Acceptable | 347 (8.9) | 2301 (58.7) | 1201 (30.7) | 68 (1.7) |
| Outcomesb | ||||
| True negative (FIT–ve RC–ve) | 122 (7.3) | 908 (54.5) | 602 (36.1) | 66 (1.7) |
| False negative (FIT–ve RC+ve) | 167 (8.5) | 1275 (65.1) | 485 (24.8) | 34 (2.0) |
| False positive (FIT+ve RC–ve) | 6 (11.8) | 30 (58.8) | 15 (29.4) | 0 (0.0) |
| True positive (FIT+ve RC+ve) | 10 (9.7) | 54 (52.4) | 38 (36.9) | 1 (1.0) |
Table 14 shows that there were some notable differences between female and male participants. For example, females were more likely than males to prefer ‘FIT only’ (35.0% vs. 29.7%). Males were more likely than females to opt for ‘RC only’ (10.4% vs. 5.8%). There were no significant differences in preference by age.
Table 14 shows the pattern of preferences by screening round among FIT-positive participants. There were notable differences to this pattern among respondents who received a FIT positive result in rounds 1–3. In round 1, the largest proportion of respondents (43.9%, n = 118) opted for ‘FIT only’, followed closely by ‘RC + FIT’ (39.0%, n = 105). One out of six respondents (15.2%, n = 41) opted for receiving ‘RC only’, whereas a very small minority (1.9%, n = 5) preferred ‘No surveillance’. Interestingly, the proportion of people choosing ‘FIT only’ as their preferred option reduced to 40.6%, (n = 73) in round 2 and to 34.9% (n = 52) in round 3. By comparison, the proportion of respondents choosing ‘RC + FIT’ increased to 45.6% (n = 82) in round 2 and to 54.4% (n = 81) in round 3. In contrast, participants who received consistent FIT-negative results across the study and completed the end-of-study questionnaire in round 3 showed a strong preference for ‘RC + FIT’ (60.0%, n = 2206) compared with ‘FIT only’ (30.0%, n = 1104), ‘RC only’ (8.2%, n = 300) and ‘No surveillance’ (1.8%, n = 66).
Preferences by satisfaction with baseline colonoscopy
Among participants with a negative experience at baseline colonoscopy, 48.6% (n = 68) preferred ‘FIT only’ compared with 30.7% (n = 1201) of respondents who had rated their baseline colonoscopy as acceptable (χ2 = 23.01; p < 0.001; see Table 14).
Preferences by final diagnostic/surveillance outcome
Preference for ‘FIT-only’ surveillance was highest in the true-positive group (36.9%, n = 38) and true-negative group (36.1%, n = 602), and was lowest among respondents who had a false-negative FIT result (24.8%, n = 485) (see Table 14). In contrast, the option of ‘RC + FIT’ was most frequently chosen among those in the false-negative group (65.1%, n = 1275).
End-of-study interviews
We invited 31 participants who had received a positive FIT result in round 1 to interview; 22 (71.0%) attended (male, n = 17; female, n = 5). In round 2, 16 individuals with FIT-positive results were invited, of whom 13 (81.0%) agreed to be interviewed (male, n = 6; female, n = 7). In round 3, we conducted a further 24 interviews (60.0% response rate) with participants after their end-of-study colonic examination.
Acceptability of the faecal immunochemical test
Overall, participants were very satisfied with their experience of taking part in the study and particularly remarked how much easier it was for them to complete the FIT than the gFOBT they completed in the BCSP (Box 1).
I mean if you’re prone to have these little polyps and things appear then you should be looking out for them and if this is a way of finding that out fairly easily, simply and cost-effectively then I think it should be done.
Participant 3: male, round 1, true positive
I’d say it comes, it’s very simple to do, it’s very easily . . . I mean, I’m quite a layperson. It is very simple to do and the instructions are very clear. . . .
Participant 59: female, round 3, true negative
The FIT was easier and more hygienic to administer than the gFOBT
Well, it was a lot easier to do, it was not as messy as the other, err, you just have to take one sample, erm, as I say, a hundred times better
Participant 6: male, round 1, true positive
Once it’s clean, far more hygienic . . . if you are hygienic yourself it doesn’t really matter, I suppose, but far more pleasant than the previous way of testing and the fact that it had to be done three times.
Participant 28: female, round 2, false positive
Opinions about sensitivity of testing a single stool sample
Minor concerns, yes, for the reasons I mentioned. Three tests in a series might be better to highlight a rogue reading, whereas the one test might be the rogue reading.
Participant 41: male, round 3, true negative
I thought well if they can pick something up from such a small sample as that, I think it’s a much more . . . to me, it’s a much more accurate fine-tune test.
Participant 33: female, round 3, true positive
An annual FIT test provided more frequent reassurance
You think, well, although I tried not to think . . . oh well 3 years is quite a long time, actually, I suppose a lot of something could happen in 3 years, whereas yearly it’s caught earlier, isn’t it?
Participant 30: female, round 2, true positive
Three years is quite a long time, and I understand that the polyps can actually turn cancerous, certain ones can. So it’s [the FIT test] worth doing because if you have anything and anything shows up then you can get someone to take a look or have another colonoscopy.
Participant 40: female, round 3, true negative
The colonoscopy was tolerable
The colonoscopy was fine, the staff were absolutely brilliant, the clinician that carried it out was exceptionally good. The worst part was the . . . err, what’s the word I’m looking for . . . the substances I had to take beforehand.
Participant 21: male, round 1, false positive
Yes. I mean, it’s not comfortable, it’s not something you want to go through, like anything like that, but, I mean, it wasn’t bad and they [medical staff] are all very nice and very supportive on both times I’ve had it done. Very, very good. I couldn’t fault them.
Participant 32: female, round 2, true positive
Negative colonoscopy experiences
. . . and I felt really unpleasant for a good week. I had a lot of back trouble, and he said it was because what they were having to do, and I believe what they removed this time was a little bit bigger.
Participant 2: female, round 1, true positive
. . . you have that horrendous solution to drink the night prior to the colonoscopy to flush your system out. I found that really hard to get through, however many litres of water solution it was, to get it through the system. That was quite gagging in some way, to drink it.
Participant 46: female, round 2, true positive
Reassurance
Another frequently endorsed advantage was that the annual FIT provided more frequent reassurance and helped to bridge the gap between 3-yearly colonoscopies. Interviewees frequently commented on the fact that FIT required only a single stool sample. Some participants described this as a ‘mark of quality’, whereas others thought that it might limit the ability of FIT to detect intermittently bleeding polyps and cancers.
Test preferences by faecal immunochemical test outcomes
Participants who received a positive FIT result in round 1 or 2, and then had CRC-related abnormalities detected at their colonic examination, often expressed surprise (Boxes 2 and 3 show quotations from ‘end-of-study interviews’ in rounds 1 and 2). Importantly, some participants mentioned that any abnormalities detected in round 1 would probably have been missed during the baseline colonoscopy (see Box 2). Participants who received false-positive FIT results commonly mentioned feeling relieved by the negative result of their colonic examination and did not express any concern or regret associated with having had a ‘false alarm’. Some of these participants preferred to be offered an annual FIT and a 3-yearly colonoscopy, to ensure that any abnormalities not detected by the FIT could be picked up by a more thorough examination (see Box 3).
. . . well it did surprise me in a sense that, because they told me I wasn’t to have another check for 3 years, you see what I mean?
Participant 5: male, true positive
Yeah, well with only being, you know, being told that 3 years . . . erm . . . before they want to see me again, and then it’s within just over 12 months that, something has developed.
Participant 6: male, true positive
Relief that polyps were found and removed
No, not really, it’s just that, I mean, in a way it’s when they do find something, right, then it’s like a relief when they take it away because you know they took it away.
Participant 5: male, true positive
Well, again, disbelief I suppose. But I suppose relieved that they’d removed whatever it was and again, just get on with life, I mean what can you do, sit and worry and there’s no point in it.
Participant 2: female, true positive
Understood that polyps may have been missed during the first colonoscopy
. . . no problem, they just found a small, another small polyp, which they probably missed the first time.
Participant 5: male, true positive
I asked him why, err, err they had appeared again so sudden. He said most probably they might have just left a bit of root in or something like that.
Participant 6: male, true positive
Relief that no polyps were found during colonoscopy
I was very pleased, well relieved err and obviously you know you forget about it err once it’s been done and you’re given the all clear you’re at ease.
Participant 14: male, false positive
Relieved. I would have been surprised because it was so quickly . . . so soon after the anterior resection, err polyps growing . . . would not have grown that rapidly so I suspect any blood in the test was a result of possible haemorrhoids or whatever.
Participant 21: male, false positive
Concerns about possible harms of colonoscopy
I just don’t think it’s a procedure that should be undertaken lightly, you know there are dangers attached to it.
Participant 2: female, true positive
. . . if the experts in their wisdom have decided that this is the way to proceed and go ahead, with the least amount of fuss but with the maximum amount of result it’s err, it’s got to be the way forward.
Participant 1: male, false positive
It was a bit of a surprise, yes it was. But, as I say, I tried to stay positive thinking well, alright, something has been picked up and it will be sort of dealt with further.
Participant 30: female, true positive
It was like here we go again, because obviously I’ve had the feeling twice now and it got, right, OK, they’ve found something else, let’s hope it’s the same as the previous time and just go on from there.
Participant 28: female, false positive
Glad to be monitored annually
. . . well, because if I hadn’t have had that done and missed it for 3 years, if you hadn’t sent me that follow-up one, I wouldn’t have had a normal bowel screening test possibly for 3 years and there might have been a problem, which I will be eternally grateful for, that you did send me it again.
Participant 33: female, true positive
I feel grateful in a way that I’m being monitored at a close level sort of thing because if it had been left, my dad didn’t know . . . we didn’t know my dad had it, we were told 3 days before he died.
Participant 34: female, true positive
Relief that no polyps were found during their colonoscopy
Oh yes, I was pleased there was nothing there, yes.
Participant 25: male, false positive
Well obviously one was pleased, yes.
Participant 27: male, false positive
Concerns about harms of colonoscopy
I can’t say I’d queue up to have a colonoscopy. The FIT is the easier option, if it is accurate.
Participant 31: female, true positive
Yes, because there’s no point in having a colonoscopy if you don’t need to have one. Because it’s not something you’d recommend to anybody but, having said that, that probably is only because of my experience.
Participant 30: female, true positive
Reducing the risk of missing cancer
So yes, it probably would be best to run it in conjunction, when I think about it, just in case there is a slip-up where it’s not detected in the yearly test. Yes, when I think about it, it probably would be nice for the two things to run together.
Participant 28: female, false positive
On the safe side of things, if you can catch it early, at least you can do something about it.
Participant 26: female, false positive
In round 3, all remaining participants received a colonoscopy regardless of their FIT outcome. Participants who received true-positive FIT results at round 3 commonly mentioned being reassured by the accuracy of the FIT in detecting CRC-related abnormalities, and feeling relieved when abnormalities were removed during the colonoscopy. In contrast, participants who received false-negative FIT results mostly mentioned feeling disappointed by having abnormalities found during the colonoscopy. Some participants mentioned that they did not believe that abnormalities would bleed all the time, rendering a test relying on a single stool sample unreliable (Box 4).
It made me feel good because it had been picked up from there, and if I hadn’t have done the FIT, that wouldn’t have happened. I had no reason or other symptoms to have gone to the doctor and ask. So very positive.
Participant 39: female, true positive
Well, I’d probably just . . . It made me believe the FIT was actually a reasonably accurate test.
Participant 46: female, true positive
Reassured by the FIT that there was nothing sinister
I was very pleased that I’d done the FIT as well because I didn’t expect that there would be anything seeing as I had done the test, so my mind was at ease before I went in because I’d done that one and obviously she said it was all clear and there was no polyps or anything. So yes, I was really pleased.
Participant 40: female, true negative
I guess that the FIT, if it showed anything amiss, you would have . . . well you wrote to me and said, on each occasion, that the test was satisfactory. I think that it was fine.
Participant 36: male, true negative
Understood bleeding could have been caused by other conditions
The explanation I got was that why I had a positive test and then nothing showed on the colonoscopy was because something briefly had released blood into the faecal matter and then healed up.
Participant 50: male, false positive
Well I just put that down to an error in the system. As I said, I’ve got haemorrhoids. Not that they are that bad, I’ve just had a bit of a bleed and some of the blood has got into the sample. I wasn’t particularly concerned about it.
Participant 38: male, false positive
Awareness of intermittent bleeding
It must be necessary to have several tests because there are times when I was having blood in my stool and there were times when I wasn’t. So I’m not entirely surprised that there are times when it shows and there are times when it doesn’t show.
Participant 37: male, false negative
There was no blood. So the polyps were . . . They’re very, very small and they obviously weren’t bleeding. Yes. That was fine.
Participant 53: male, false negative
Preference for only being offered a colonoscopy if an abnormal FIT result is found
I mean, the colonoscopy, it’s a very good thing, but it’s not very comfortable [laughs]. If you could get away without having one of them I think I’d be quite happy.
Participant 40: female, true negative
Avoiding the risk of false negatives
I know there’s no guarantees. There could have been blood and there could be nothing at all. Even if I had the procedure and they found nothing, I’d rather be safe than sorry.
Participant 46: female, true positive
Not to have the colonoscopy, I think I would start to worry with the fact that polyps could be there but we are not looking for them. Unless the polyps are bleeding then it’s not going to show up on the FIT, is it?
Participant 53: male, false negative
I think I’d have more faith in the colonoscopy than in the actual test. I think the test may just be, as I said, an indicator but I feel the colonoscopy is more of an examination rather than just a test.
Participant 38: male, false positive
General discussion
Participant-reported satisfaction with the FIT for Follow-Up study was very high. This was probably in part bolstered by the participants’ previous experience with gFOBT. Completing the FIT kit was unanimously perceived as easier and more convenient, especially as it required only one stool sample. However, many participants remained sceptical that a single sample stool kit would detect CRC reliably and preferred to have the test in addition to, rather than as a replacement for, colonoscopy.
Although the act of completing the test kit and waiting for the results was associated with some anxiety, this did not seem to have lasting effects as CRC-related worry or general anxiety was reported to be very low, even for those who had received positive FIT results.
Overall, the main message from these studies seems to be that participants valued colonoscopy for its ability to visually inspect the bowel and were concerned that a single stool sample test might miss intermittently bleeding polyps and cancers. From a participant perspective, therefore, the FIT was appreciated as offering a quick and easy way to gain additional reassurance rather than as a stand-alone test.
There are limitations to bear in mind when interpreting these results. Despite the high level of recruitment, our findings might not represent the entire surveillance population. Importantly, our end-of-study preference data are restricted to participants who had undergone an end-of-study colonic examination, excluding participants who withdrew during the study, failed to attend their end-of-study colonic examination or did not return their final questionnaire. As a result, our findings might over-represent people who are concerned about CRC and have positive attitudes towards colonoscopy.
Across all of our studies we were unable to record the ethnic mix of participants. If FIT for surveillance were to be implemented, it would be vital to ensure active participation from patients from ethnically diverse backgrounds. Future work should also investigate the views of patients who have not previously been exposed to colonoscopy surveillance. In a recent hypothetical survey of 491 screening-naive individuals, the majority (61%) stated a preference for a surveillance test resembling a home-based FIT, whereas only 31% reported a preference for colonoscopy. Increased frequency of testing was a commonly cited reason for the preference for FIT. Unfortunately, the response rate of the survey was only 16%, limiting its generalisability. Future work should investigate whether or not the preference for FIT in screening-naive individuals translates to greater adherence in the surveillance setting. 89
Future research should try to identify the extent to which FIT might enable the small but substantial proportion of people not attending surveillance colonoscopy to have adenoma surveillance. This study also revealed that the key concerns about annual FIT surveillance are the lack of visual inspection and the reliance on a single stool sample, which might miss intermittently bleeding polyps and cancers. These concerns are based on complex beliefs, which need to be explored further.
Another important question for future research will be to determine what precise additional benefit people expect to gain from annual FIT. In this study, it was interesting to observe the response from people who had CRC-related abnormalities detected following a positive FIT result in round 1 or 2. Here, our end-of-study interviews revealed ‘surprise’ or even ‘shock’, often combined with a realisation that abnormalities detected so soon after the baseline colonoscopy would probably have been missed at that examination. The extent to which people value FIT for its ability to detect disease that has been missed previously should be investigated further.
To conclude, these investigations have shown that, although there is enthusiasm for the use of FIT in CRC surveillance, it is often restricted to the use of FIT as an additional rather than a stand-alone test. Future research is needed to better understand the concerns of participants with regard to FIT missing polyps and cancers.
Chapter 5 Economic evaluation of faecal immunochemical tests versus colonoscopy surveillance
Introduction
We investigated the incremental cost and cost-effectiveness of annual FIT versus colonoscopy at 3 years post baseline colonoscopy for post-polypectomy surveillance of intermediate-risk patients.
As defined in the protocol, under the assumption that FIT was as accurate as colonoscopy surveillance, the aim of the economic evaluation was to undertake a cost-minimisation analysis of annual FIT surveillance versus colonoscopy surveillance at 3 years. In the event that the marginal effectiveness of FIT surveillance was significantly less than that of colonoscopy surveillance, the analysis would instead comprise a cost and cost-effectiveness analysis, balancing test costs against test outcomes.
Method
Overview of economic evaluation
Given the results of the main clinical analysis, we undertook both a cost analysis and cost-effectiveness analysis comparing annual FIT surveillance with colonoscopy surveillance at 3 years. For the cost analysis, we calculated the cost of each surveillance alternative. In the cost-effectiveness analysis, the outcomes were the number of AAs detected and the number of CRCs detected. Cost-effectiveness was expressed in terms of incremental saving per AA that was not detected by FIT versus colonoscopy surveillance, and per CRC that was not detected by FIT versus colonoscopy surveillance (see Economic analysis).
The analysis took a UK NHS perspective. 90 Costs were calculated in 2015 Great British pounds and inflated when necessary. 91 The time horizon was 3 years, reflecting the cycle time for colonoscopy surveillance, and all costs were discounted at 3.5% for each year after the first year.
As stated in the protocol, a full lifetime cost-effectiveness model using quality-adjusted life-years (QALYs) as the outcome measure was not conducted because this would have required separate data describing the outcomes and treatment pathways associated with the two options.
There were no missing data for the analyses.
Generating a control group
The FIT for Follow-Up study was a single-arm trial, which meant that for the economic analysis we had to generate a control arm (a pseudo-control group) to consider the cost and outcomes of people who would have had routine 3-yearly colonoscopy surveillance in the absence of FIT.
In order to create this pseudo-control group, we assumed that all DNA participants for colonic examinations scheduled because of positive FIT would also be DNA participants for routine colonoscopy surveillance, and that those participants lost to follow-up during the study would also be lost to routine colonoscopy surveillance. As a starting point, we defined the size of the intervention and control groups as the 5946 participants who completed round 1 of the FIT regimen. To estimate the number of participants receiving colonoscopy surveillance at 3 years in the absence of FIT, we used the sum of all participants involved in the trial who had a colonic examination (4455 participants who had the routine 3-year colonic examination, plus 744 participants who had an examination after having a FIT positive result, which resulted in 5199 participants in total).
The appropriateness of the size of the pseudo-control arm receiving colonoscopy surveillance at 3 years can be confirmed by subtracting the participants who DNA their colonic examination and those who were lost to follow-up from the original 5946 participants who completed round 1 of the FIT regimen (5946 minus 584 DNA participants for the 3-year colonic examination, minus 51 DNA participants for early colonic examination after a FIT positive result, minus 112 participants who were lost to follow-up during the course of the 3-year study period, which resulted in 5199 participants in total).
Figure 4 shows the actual number of study participants completing each round of FIT and the estimated number undergoing colonoscopy surveillance at 3 years in the absence of FIT. Figure 5 shows the resulting costs, number of colonoscopies, and AAs and CRCs detected in each group.
FIGURE 4.
Participants included in the economic analysis. FIT round completed data were drawn from the FIT for Follow-Up study Consolidated Standards of Reporting Trials (CONSORT) diagram. The pseudo-control arm estimates the number of surveillance colonoscopies that would have taken place in the absence of the FIT for Follow-Up study.
FIGURE 5.
Estimated costs and outcomes associated with annual FIT surveillance and 3-yearly colonoscopy surveillance.
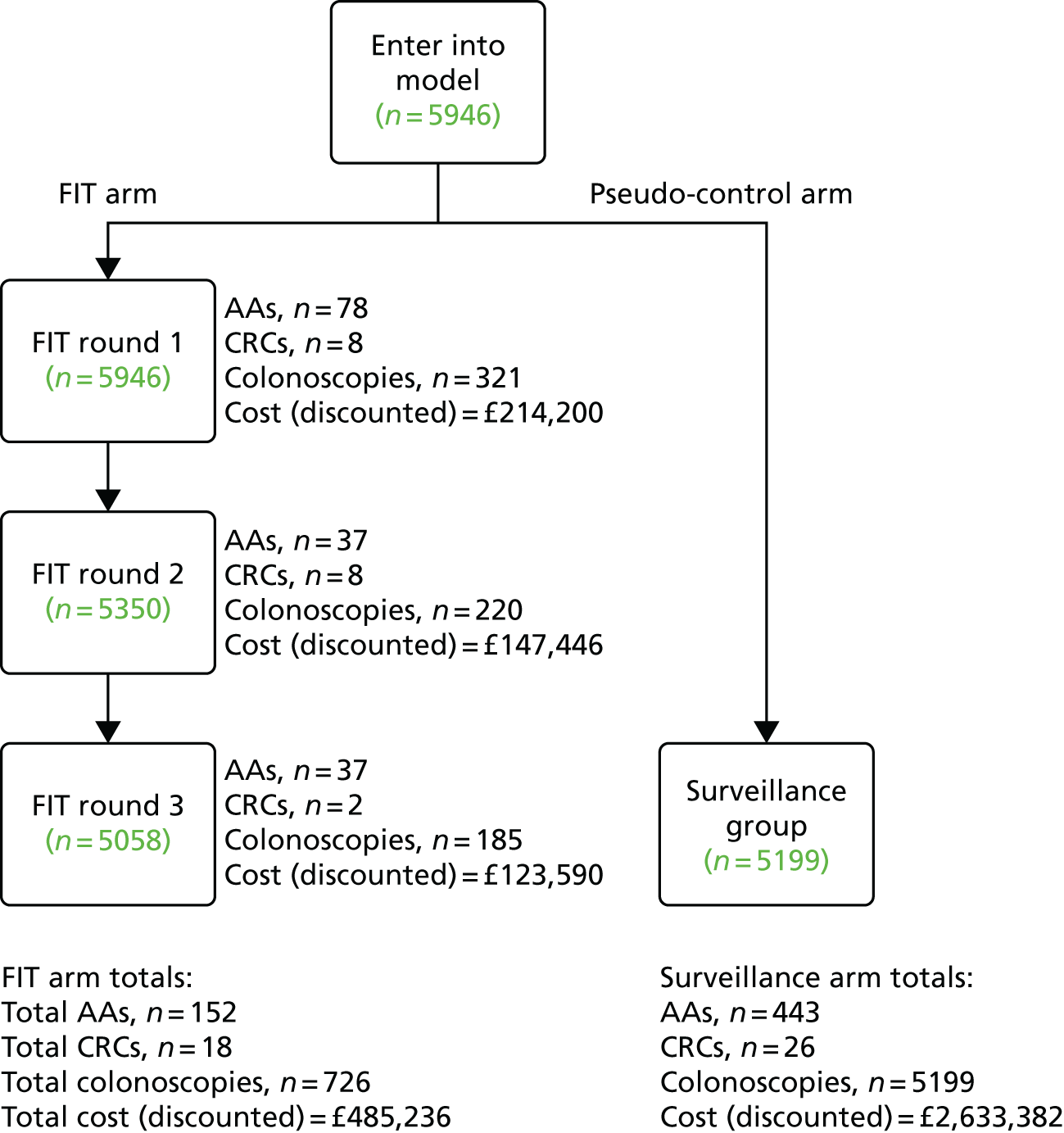
Resource use and costs
As specified in the study protocol, the costs used in the analyses were restricted to surveillance costs. We did not include the cost of treating observed cases of disease, other than polypectomy during a surveillance procedure, but we did include the costs of treating complications of colonoscopy. For every participant, we calculated the cost of FIT kits92 and of all diagnostic testing that took place as a result of a positive FIT (Table 15). Unit costs for diagnostic procedures were sourced from the NHS Reference Costs 2014 to 2015,93 and the average cost for each procedure was used. For the unit cost of CT colonography, we took the weighted average of a CT scan of one area with pre contrast, post contrast and both. For colonoscopy complications, we included the costs of treating bowel perforations and GI bleeding. The probabilities of bowel perforation during colonoscopy without and with polypectomy were assumed to be 0.0008 and 0.0017,94 respectively, and the probability of GI bleeding following colonoscopy was assumed to be 0.00439. 94 Unit costs for dealing with these complications were taken from NHS Reference Costs 2014 to 201593 as above (see Table 15). In cases in which an AA was detected, the procedures were costed with polypectomy, assuming that the adenoma would be excised during the procedure. For the base-case analysis, participants who DNA their diagnostic procedures were assumed to incur no cost.
| Parameters | Value | Source |
|---|---|---|
| Colonoscopy | £519.42 | NHS Reference Costs 2014 to 2015,93 diagnostic, 19 years and over |
| Colonoscopy with polypectomy | £601.86 | NHS Reference Costs 2014 to 2015,93 therapeutic, 19 years and over |
| Diagnostic flexible sigmoidoscopy | £381.61 | NHS Reference Costs 2014 to 2015,93 diagnostic, 19 years and over |
| Flexible sigmoidoscopy with polypectomy | £480.76 | NHS Reference Costs 2014 to 2015,93 therapeutic, 19 years and over |
| CT colonography | £87.92 | NHS Reference Costs 2014 to 2015,93 Diagnostic Imaging |
| Cost of treating bowel perforation | £5911.08 | NHS Reference Costs 2014 to 2015,93 Major Large Intestine Procedure, 19 years and over |
| Cost of treating GI bleed | £2498.14 | NHS Reference Costs 2014 to 2015,93 gastrointestinal bleed with single intervention |
| FIT kit returned | £5.14 | Murphy and Gray,92 inflated to 2014/15 |
| FIT kit not returned | £1.66 | Murphy and Gray,92 inflated to 2014/15 |
| Probability of bowel perforation during colonoscopy (without polypectomy) | 0.0008 | Atkin et al.94 |
| Probability of bowel perforation during colonoscopy (with polypectomy) | 0.0017 | Atkin et al.94 |
| Probability of GI bleeding after colonoscopy | 0.00439 | Atkin et al.94 |
Outcome
The effectiveness of FIT surveillance was expressed in terms of the number of AAs and number of CRCs that were detected. AAs are of relevance to CRC screening as they pose a high risk of malignancy.
Economic analysis
Mean costs per participant and the difference between the costs of FIT surveillance versus colonoscopy surveillance were calculated. Incremental cost-effectiveness ratios (ICERs) were calculated in terms of the incremental cost per additional AA detected and the incremental cost per additional CRC detected. These were calculated as the costs of FIT surveillance minus the costs of colonoscopy surveillance divided by the AAs (or CRCs) detected by FIT surveillance minus the AAs (or CRCs) detected by colonoscopy surveillance. As FIT surveillance was both cheaper and less effective than colonoscopy surveillance, incremental cost-effectiveness was presented in terms of incremental saving per AA and per CRC not detected. Note that in this scenario, the numerator of the ICER (incremental costs) and the denominator (differences in AAs and CRCs detected) are both negative so the ICER will be positive. In this case, higher positive values of the ICER reflect better value for money as they represent a larger cost saving for each AA or CRC missed. Calculations of net monetary benefit were not possible because there are no cost-effectiveness thresholds for these outcomes.
Sensitivity analyses
We undertook a probabilistic sensitivity analysis. 90 We varied the following parameters: number of FIT kits returned at each round, number of colonoscopies required at each round, number of participants who DNA their diagnostic procedure at each round, number of AAs and CRCs detected by FIT at each round, and unit costs of colonoscopy, treating bowel perforations and treating GI bleeding following colonoscopy.
Distributions were assigned to the parameters to reflect the uncertainty associated with each parameter value. We used probabilities to characterise the number of FIT kits returned, colonoscopies, DNAs for diagnostic testing, and AAs and CRCs, and used beta distributions to model uncertainty in these probabilities. 95 We used uniform distributions to model uncertainty in unit costs, allowing the values to vary randomly between ± 25% of the base-case value.
A random value from the corresponding distribution for each parameter was selected. This generated an estimate of the mean cost and mean number of detected AAs and CRCs associated with FIT surveillance for each of the 5000 simulations, and these were used to calculate the incremental costs, differences in AAs and CRCs detected and ICERs for FIT versus colonoscopy surveillance. This was repeated 5000 times and the results for each simulation were noted. The 95% uncertainty intervals were calculated as the 2.5th and 97.5th percentiles of the simulated values.
We also undertook a deterministic sensitivity analysis. The effect on cost and cost-effectiveness of using different faecal haemoglobin thresholds to denote FIT positivity was explored using data on the performance of FIT at different thresholds, reported in Chapter 3. The study used a threshold of 40 µg/g, although lower thresholds were considered in the analyses. The number of participants classed as positive was higher with lower thresholds. Therefore, lower thresholds would increase costs associated with FIT surveillance as more patients would be referred for colonic examinations. To explore the effect of lower thresholds on the analysis, we assumed a linear relationship between the number of positive FIT results and overall costs of FIT surveillance.
The impact of the diagnostic procedure unit costs was also explored using univariate sensitivity analysis. We first assumed that all procedures were undertaken as elective inpatient procedures. We then assumed that all procedures were undertaken on an outpatient basis. Weighted averages of the upper and lower quartiles were used for the unit cost of CT colonography (for a procedure with pre contrast, post contrast and both). The effect of the unit cost of the FIT kits was analysed by increasing and decreasing the cost by 25%. We similarly varied the unit costs of treating bowel perforations and GI bleeding associated with colonoscopy by ± 25%. The cost of managing DNA participants was also explored, valuing them at the full cost of a colonoscopy without polypectomy, instead of at zero cost as in the base-case analysis.
Budget impact
We estimated the budget impact if FIT surveillance were to replace colonoscopy surveillance nationally over a screening cycle by multiplying the incremental costs per patient as calculated above by the total estimated eligible patients. We assumed that approximately 4.5 million people between 60 and 74 years would be screened adequately for CRC in England over a 2.5-year cycle;96 2% would have an abnormal screening result and be offered a colonic examination,97 uptake of which would be 88%,98 and 16% of those attending a colonic examination would be diagnosed with intermediate-risk adenomas. 99
Results
Costs and outcomes
For the cost analysis, we calculated the total cost of FIT surveillance over 3 years to be £485,236, using a threshold of 40 µg/g, and the cost of colonoscopy surveillance over 3 years to be £2,633,382 (Table 16). Hence, FIT surveillance produced a cost saving of £2,148,146 compared with colonoscopy surveillance.
| Absolute costs and outcomes of regimen | Surveillance | ||||
|---|---|---|---|---|---|
| Colonoscopy | FIT threshold (µg/g) | ||||
| 40 | 30 | 20 | 10 | ||
| Cost | £2,633,382 | £485,236 | £568,601 | £693,148 | £956,602 |
| AAs detected | 443 | 152 | 182 | 205 | 254 |
| CRCs detected | 26 | 18 | 19 | 21 | 22 |
The mean total cost per participant was £81.61 for FIT surveillance and £442.88 for colonoscopy surveillance. For FIT surveillance, approximately 80% of the total cost was the cost of colonoscopies undertaken for a positive FIT result, with the remainder split between the cost of FIT kits, complications of colonoscopy and the cost of alternative diagnostic tests (flexible sigmoidoscopy and CT colonography). For colonoscopy surveillance, 100% of the cost was accounted for by colonoscopies undertaken.
The FIT surveillance detected fewer AAs and CRCs (n = 152 and n = 18, respectively) than colonoscopy surveillance (n = 443 and n = 26, respectively).
Cost-effectiveness analysis
The mean incremental cost per participant for FIT versus colonoscopy surveillance was –£361 (95% uncertainty interval –£386 to –£321) over the 3-year period. FIT surveillance detected 291 fewer (i.e. –291) AAs than colonoscopy surveillance (95% uncertainty interval –312 to –269) and eight fewer CRCs (95% uncertainty interval –15 to 1) using a threshold of 40 µg/g (Table 17). These findings suggest that FIT surveillance was less costly than colonoscopy surveillance and was also less effective at detecting AAs. The point estimate for the difference in number of CRCs detected suggests that FIT surveillance was less effective at detecting CRCs than colonoscopy surveillance; however, the size of the 95% uncertainty interval indicates that there is a chance that FIT surveillance was not less effective. The incremental cost-effectiveness of FIT surveillance versus colonoscopy surveillance was £7382 (95% uncertainty interval £6475 to £8191) per AA not detected and £268,518 (95% uncertainty interval –£868,248 to £1,718,956) per CRC not detected.
| Output parameter | FIT positivity threshold | |||
|---|---|---|---|---|
| Base case (40 µg/g) | 30 µg/g | 20 µg/g | 10 µg/g | |
| Absolute difference in cost compared with surveillance colonoscopy | –£2,148,146 (–£2,292,629 to –£1,911,004) | –£2,064,780 (–£2,230,445 to –£1,817,489) | –£1,940,234 (–2,127,787 to –£1,687,479) | –£1,676,779 (–£2,494,145 to £415,940) |
| Difference in number of AAs detected | –291 (–312 to –269) | –261 (–284 to –238) | –238 (–263 to –213) | –189 (–213 to –166) |
| Difference in number of CRCs detected | –8 (–15 to 1) | –7 (–14 to 3) | –5 (–13 to 5) | –4 (–9 to 2) |
| Incremental cost per participant | –£361 (–£386 to –£321) | –£347 (–£375 to –£306) | –£326 (–£358 to –£284) | –£282 (–£419 to £70) |
| Incremental cost-effectiveness per AA not detected | £7382 (£6475 to £8191) | £7911 (£6823 to £8983) | £8152 (£6884 to £9478) | £8872 (–£2341 to £13,884) |
| Incremental cost-effectiveness per CRC not detected | £268,518 (–£868,248 to £1,718,956) | £294,969 (–£1,786,046 to £2,546,573) | £388,047 (–£2,766,964 to –£3,844,425) | £419,195 (–£3,495,732 to £3,822,391) |
Faecal immunochemical test positivity threshold sensitivity analysis
The effect of varying the FIT positivity threshold on cost and cost-effectiveness was explored, relative to the base case threshold of 40 µg/g. The numbers of AAs and CRCs that would have been detected using different thresholds were estimated based on the data reported in Chapter 3. Tables 16 and 17 show the results of this sensitivity analysis. Qualitatively, the findings were similar to the base case threshold of 40 µg/g, indicating that FIT surveillance was less costly than colonoscopy surveillance and less effective at detecting AAs and CRCs, with the 95% uncertainty interval indicating that there is a chance that FIT was not less effective at detecting CRCs. However, the absolute cost difference between FIT surveillance and colonoscopy surveillance fell as the threshold was lowered, and the incremental cost-effectiveness increased (i.e. improved) as fewer AAs and CRCs were missed. At a threshold of 10 µg/g, the incremental cost per participant for FIT versus colonoscopy surveillance was –£282 (95% uncertainty interval –£419 to £70); the 95% uncertainty interval indicates that there is a chance that FIT was not less costly than colonoscopy surveillance.
Sensitivity analysis if did-not-attend participants were valued at full cost
When valuing the diagnostic procedures of DNA participants at the full cost of a colonoscopy without polypectomy (instead of at zero cost as in the base-case analysis), the total difference in cost of FIT versus colonoscopy surveillance was –£2,429,331 over the 3-year period. This corresponds to an incremental cost per participant for FIT versus colonoscopy surveillance of –£409. The incremental cost-effectiveness of FIT versus colonoscopy surveillance was £8348 per AA not detected and £303,666 per CRC not detected (Table 18).
| Input parameters | Incremental cost per participant | Incremental cost-effectiveness | |
|---|---|---|---|
| Per AA not detected | Per CRC not detected | ||
| Base case | –£361 | £7382 | £268,518 |
| DNAs valued at full cost | –£409 | £8348 | £303,666 |
| High-cost colonoscopy | –£572 | £11,692 | £425,280 |
| High-cost colonoscopy with polypectomy | –£378 | £7719 | £280,777 |
| High-cost flexible sigmoidoscopy | –£359 | £7341 | £267,045 |
| High-cost computed tomographic colonography | –£361 | £7380 | £268,448 |
| High cost of treating bowel perforation | –£362 | £7400 | £269,175 |
| High cost of treating GI bleed | –£363 | £7421 | £269,939 |
| FIT kit cost +25% | –£358 | £7312 | £265,961 |
| Low-cost colonoscopy | –£263 | £5375 | £195,531 |
| Low-cost colonoscopy with polypectomy | –£342 | £6983 | £254,000 |
| Low-cost flexible sigmoidoscopy | –£362 | £7395 | £268,983 |
| Low-cost computed topographic colonography | –£361 | £7384 | £268,604 |
| Low cost of treating bowel perforation | –£358 | £7315 | £266,088 |
| Low cost of treating GI bleed | –£354 | £7237 | £263,260 |
| FIT kit cost –25% | –£365 | £7452 | £271,075 |
Diagnostic procedure cost sensitivity analysis
The effect of unit costs on the incremental cost and cost-effectiveness was explored in a sensitivity analysis. Table 18 shows the results of this sensitivity analysis. Incremental cost and cost-effectiveness was largely insensitive to changes in unit costs, with the exception of the cost of colonoscopies. The cost of FIT kits did not have an appreciable effect on the analysis.
Budget impact analysis
Based on the assumptions and data described above, we estimated that up to 12,777 individuals would be eligible for FIT surveillance. Given a cost saving per participant for FIT versus colonoscopy surveillance of –£361 (–£386 to –£321) for the base-case FIT threshold of 40 µg/g, the budget impact from replacing colonoscopy surveillance with FIT surveillance would be –£4.6M (95% uncertainty interval –£4.9M to –£4.1M). If the FIT threshold was amended to 30 µg/g, 20 µg/g or 10 µg/g, the resulting cost savings per participant for FIT versus colonoscopy surveillance would be –£347 (95% uncertainty interval –£375 to –£306), –£326 (95% uncertainty interval –£358 to –£284) or –£282 (95% uncertainty interval –£419 to £70), respectively, while the budget impact would be –£4.4M (95% uncertainty interval –£4.8M to –£3.9M), –£4.2M (95% uncertainty interval –£4.6M to –£3.6M) or –£3.6M (95% uncertainty interval –£5.4M to £0.9M), respectively. As noted, these figures do not include the costs of treating missed AAs and CRCs.
Discussion
Our economic analysis to evaluate the cost-effectiveness of annual FIT surveillance versus 3-yearly colonoscopy surveillance showed that FIT surveillance was cheaper than colonoscopy surveillance, but also less effective at detecting AAs and CRCs. Sensitivity analysis suggested that the cost of DNAs did have an effect on the analysis, as did the cost of colonoscopies.
The total cost associated with FIT surveillance was sensitive to the faecal haemoglobin threshold selected to define FIT positivity. This is not surprising, as more people would be classed as positive and referred for colonic examination with lower thresholds. In all cases, annual FIT surveillance was less costly than 3-yearly colonoscopy surveillance; however, at the lowest threshold (10 µg/g), there is a chance that FIT surveillance was not less costly than colonoscopy surveillance. The incremental cost-effectiveness per AA and CRC not detected increased significantly (i.e. reflecting better value for money) at lower thresholds. This is because, despite higher diagnostic costs associated with higher numbers of colonic examinations at lower thresholds, FIT surveillance would miss fewer participants with AAs and CRC at these lower thresholds. This is despite the increase in diagnostic costs associated with the higher numbers of colonic examinations that would be performed. The findings suggest that using FIT as an alternative to colonoscopy for surveillance could result in significant financial savings, although this would mean missing a number of AAs and CRCs.
Limitations of the economic analysis and further research
Our analysis had two main limitations. The first was that the study was based on a single-arm trial and, therefore, to undertake the economic analysis, we had to create a pseudo-control group for colonoscopy surveillance. This is a suboptimal alternative to using a separate control group in a RCT. However, we did test the impact of the assumptions made in generating the pseudo-control group and showed that the conclusions of the economic analysis did not change.
The second main limitation, also stemming from the study design, was that our analysis included only the short-term costs and outcomes associated with surveillance. In particular, we were not able to estimate lifetime costs and QALYs associated with FIT and colonoscopy surveillance, including the long-term costs and outcomes associated with undiagnosed CRCs. As stated in the study protocol, such an analysis was not planned because it would require separate data describing the outcomes and treatment pathways associated with FIT and colonoscopy surveillance. However, this meant that we could not include the treatment costs for CRCs that were detected, meaning we are underestimating the total cost of both surveillance regimens. This underestimation might be particularly pronounced for FIT surveillance given the potential cost implications of undiagnosed CRCs.
In the light of these limitations, further research would be beneficial, in particular a cost–utility analysis of annual FIT versus 3-yearly colonoscopy surveillance in terms of the lifetime incremental costs per QALYs gained. Ideally, such an analysis would be undertaken within a full RCT, enabling a direct comparison between a treatment and control group and thereby removing the need to generate control group data, as was done in the present study. Such an analysis would account for costs of treatment associated with FIT and colonoscopy surveillance, and would also include the costs of treating diagnosed and undiagnosed CRCs. It would also account for the impact of both surveillance regimens on survival and health-related quality of life.
Chapter 6 Discussion
In the UK, individuals deemed to be at intermediate-risk of developing CRC or AAs after polypectomy are currently recommended 3-yearly surveillance colonoscopy. 22 Although colonoscopy is the most sensitive examination for colorectal neoplasia, there are disadvantages to its use in this context. Most intermediate-risk individuals undergoing surveillance colonoscopy will not have CRC or AAs. 100–102 Colonoscopy is an uncomfortable procedure that carries a small risk of serious adverse events. 33,103 Furthermore, colonoscopy is expensive and surveillance colonoscopy is putting a growing strain on already overburdened endoscopy services. 29 In the light of these issues, we developed the FIT for Follow-Up study to examine the diagnostic accuracy, acceptability and cost-effectiveness of annual FIT as an alternative to colonoscopy for surveillance of patients at intermediate risk of CRC or AAs following polypectomy.
Main findings
In our study, the sensitivity of FIT was greater for CRC than for AAs and depended strongly on the faecal haemoglobin threshold applied and the number of completed FIT rounds. The sensitivity of the first completed FIT at a threshold of 40 µg/g was only half that estimated at a threshold of 10 µg/g (30.8% vs. 61.5% for CRC and 17.6% vs. 33.2% for AAs). Sensitivity was even higher at 10 µg/g in those who completed all FITs offered (91.7% and 59.3% for CRC and AAs, respectively, in cumulative test analysis).
Although using a low threshold and multiple rounds of FITs reduced the number of CRCs and AAs missed, this came at a cost of reduced specificity and an increase in cumulative positivity and requirement for colonoscopy. However, even when the lowest threshold of 10 µg/g was applied, 71% of participants did not test positive at any round. Therefore, using three rounds of annual FIT as an alternative to the first surveillance colonoscopy at year 3 would considerably reduce the number of colonoscopies performed.
The FIT positivity declined by round of testing, reducing from approximately 6% at the first round to 4% at the third round with a threshold of 40 µg/g, and from 14% to 9% with a threshold of 10 µg/g. The decrease in positivity is not surprising given that participants who would have tested positive at a given threshold would have been offered an early colonoscopy and, therefore, would not have been invited to subsequent FIT rounds.
Sensitivity also declined by round of testing; for example, at a threshold of 40 µg/g, sensitivity for ACN was estimated to be 17.9% for the first completed FIT, 11.7% for the second FIT and 12.1% for the third FIT. At 10 µg/g, sensitivity for ACN was 34.3% for the first FIT, 22.5% for the second FIT and 19.6% for the third FIT. A possible explanation for this is that participants with an AA or CRC that caused high faecal haemoglobin levels were likely to test positive at an early round.
There were differences in the performance of FIT by age and sex. FIT positivity was higher in older and male participants. This reflects the prevalence of ACN, which, in both this study and more generally, increases with age and is higher in men. 104,105 However, sensitivity for AAs and CRC was also generally higher, and specificity lower, in older participants and men. The reasons for these differences in FIT positivity and sensitivity between older and younger patients, as well as between men and women, are not yet fully understood.
Looking at the stage distribution of the CRCs detected during the study, 11 were pT stage three or four. Considering that these were detected within 3 years following colonoscopy, it is likely that they were present but missed during the baseline examination. Although advanced-stage CRCs are more likely to be detected by FIT than early-stage CRCs,75 our reported sensitivities for FIT are not likely to be significantly inflated compared with what would be achieved in clinical practice; colonoscopy is not perfectly sensitive and a small proportion of CRCs, including those at advanced stages, are routinely missed. 106–108 A surveillance programme employing annual FIT would therefore help to speed detection of missed CRCs compared with a programme adopting surveillance colonoscopy at 3 years. Indeed, in our study, FIT detected 8 of the 11 pT3/4 CRCs at 40 µg/g and 10 out of the 11 at 10 µg/g, with seven detectable in round 1 or 2 at either threshold.
Comparison with other studies of faecal immunochemical tests in surveillance
The FIT for Follow-Up study is, to our knowledge, the largest study to date to have examined the performance of FIT in post-polypectomy surveillance. In our study, 5199 participants completed at least one FIT and attended colonoscopy in the study period, during which time 26 CRCs and 443 AAs were diagnosed.
Estimated sensitivity for CRC of a single FIT at the lowest studied threshold (10 µg/g) was lower in our study (61.5%) than in previous studies of both qualitative and quantitative FIT in a surveillance setting.
Robinson et al. 57 reported the sensitivity for CRC of a qualitative FIT (Hemeselect, SmithKline Diagnostics, San Jose, CA, USA) before surveillance colonoscopy to be 70.0% (7/10). Terhaar sive Droste et al. 46 reported the sensitivity for CRC of a single quantitative FIT (OC-Sensor) to be 80.0% (4/5) at 10 µg/g. Hazazi et al. 59 found the sensitivity of three consecutive quantitative FITs (OC-Micro at 10 µg/g) for CRC before surveillance colonoscopy to be 100.0% (8/8). Low numbers of CRCs in each study limited the precision of these estimates. One potential reason for lower sensitivity in our study might be that the first FIT was completed 2 years prior to, rather than immediately before, the year 3 planned surveillance colonoscopy. Faecal haemoglobin concentration may have increased over time as neoplasia progressed.
The sensitivity for AAs or ACN of a single FIT at 10 µg/g was not markedly different in our study (33.2% for AAs and 34.3% for ACN) to figures reported in previous studies of FIT in surveillance. Robinson et al. 57 noted a sensitivity of 44.4% (16/36) of a qualitative FIT for large adenomas (i.e. sized ≥ 10 mm). Hazazi et al. 59 found the sensitivity for ACN of three consecutive quantitative FITs at 10 µg/g to be 44.4% (32/72). Terhaar sive Droste et al. 46 reported the sensitivity for AAs of a single FIT at 10 µg/g to be 27.7% (28/101).
The study conducted by Lane et al. 60 is the only other study to have examined the sensitivity of annual FIT in a surveillance setting. Annual qualitative FIT (median of two rounds) identified 12 out of 14 patients with CRC (sensitivity of 85.7%) and 60 of 96 patients with AAs (sensitivity of 64.5%). Comparable, but slightly lower, sensitivity was observed in our study with three annual FIT rounds and a threshold of 10 µg/g (programme analysis: 84.6% for CRC and 57.3% for AAs).
Comparison with other studies of faecal immunochemical tests in screening
More extensive research has been conducted into the use of FIT for CRC screening. A systematic review commissioned by the US Preventive Services Task Force,56 published in 2016, reported the sensitivity of a single FIT for CRC, from studies using a FDA-approved qualitative or quantitative FIT and colonoscopy as reference standard, to be in the range 73–88% and specificity to be in the range 90–98%. Sensitivity of a single FIT for AAs was reported to be in the range 22–40% and specificity to be in the range 91–97%. An earlier systematic review with meta-analysis of FIT for CRC screening calculated a pooled sensitivity and specificity for CRC of FIT at 20 µg/g of 86% (95% CI 75% to 92%) and 91% (95% CI 89% to 93%), respectively. 55
The sensitivity of FIT for CRC reported by these systematic reviews is higher than was observed in our study. One potential explanation for this may be differences in the size, site and stage of CRCs detected through surveillance relative to screening. A systematic review with meta-analysis by Hirai et al. 109 found lower sensitivity of FIT for proximal than distal CRC. Furthermore, faecal haemoglobin levels appear to be higher in patients with larger AAs and in those with a greater number of AAs. 110
Health psychology assessment
Participants generally reported high levels of satisfaction with the FIT for Follow-Up study. Although completing the test was associated with some increased anxiety, this did not seem to have a long-lasting effect. Participants also reported that FIT was easier and more convenient than the three stool sample gFOBT (hema-screen™; Immunostics, Eatontown, NJ, USA) used at the time in the BCSP.
There was some scepticism about whether or not FIT alone would detect cancer reliably, particularly with only one stool sample. Although participants were generally in favour of the use of FIT in surveillance, most preferred annual FIT in addition to colonoscopy every 3 years (57.9%, 2478/4279), with a lower proportion (31.5%, 1347/4279) preferring annual FIT alone. Many participants valued annual FIT in addition to colonoscopy for the more frequent reassurance this provided them.
Health economic assessment
In incremental cost and cost-effectiveness analyses, annual FIT surveillance was cheaper than 3-yearly colonoscopy surveillance. At a threshold of 40 µg/g, the cost of FIT surveillance for the 5946 study participants was estimated to be £485,236, compared with £2,633,382 for colonoscopy surveillance. However, compared with colonoscopy surveillance, FIT surveillance resulted in eight missed CRCs (8/26) and 291 missed AAs (291/443).
Reducing the FIT threshold increased the cost of FIT surveillance and reduced the number of colorectal lesions missed. With a threshold of 10 µg/g, the cost of FIT surveillance was estimated to be £956,602, with 4 out of 26 CRCs missed and 189 out of 443 AAs missed. Incremental cost-effectiveness per AA and per CRC not detected increased (i.e. improved) with lower thresholds. These findings demonstrate that low-threshold annual FIT could be a cheaper alternative strategy for surveillance of intermediate-risk patients following the removal of adenomas. However, further economic analysis would be beneficial to understand the cost implications of AAs and CRCs missed by FIT, and the impact on expected QALYs lost.
Strengths and limitations
Our study had a number of strengths, including a relatively large sample size, a well-defined population comprised solely of individuals undergoing surveillance for intermediate-risk adenomas, and results from multiple rounds, rather than a single round, of FIT.
The study also had limitations. One possible limitation is that we assumed that the population with ACN was static, such that any ACN detected was present and would remain present and unchanged in the absence of colonic examination at years 1, 2 and 3. This is a reasonable assumption as it is widely thought that annual polyp progression rates are low. A systematic review of the natural history of small polyps (i.e. sized < 10 mm) found that, in the few studies that have been conducted, the majority of untreated polyps did not progress to become an AA over 2–3 years. 111 Furthermore, in a study of 226 patients with large untreated polyps (i.e. sized ≥ 10 mm), the risk of CRC at 5 years post-baseline assessment was only 2.5%. 112
Under the assumption of a static ACN population and with a constant FIT sensitivity, we would expect the number of neoplasms detected to decrease with each round. The detection of eight CRC cases at round 1, eight CRC cases at round 2 and two CRC cases at round 3 is consistent with a static population assumption for CRC. However, the detection of 78 AAs at the first round and 37 at each of the second and third rounds is less consistent with a static population of AAs, and suggests that even within the study period of 3 years, there is some movement in and out of this population. However, if anything, the static population assumption is likely to lead to conservative results with respect to the effect of FIT at the various thresholds, as it takes no account of the improved outcome associated with lesions detected earlier.
A further limitation of the study is that we assumed that the colonic surveillance examination had perfect sensitivity for AAs and CRCs. Most participants attending colonic examination received a colonoscopy(98.0%, 5097/5199). Although considered the ‘gold-standard’ colonic examination, colonoscopy occasionally misses polyps and, on rare occasions, CRCs. 113,114 A small proportion of participants attending colonic examination did not receive colonoscopy but did receive CT colonography. A randomised trial of CT colonography and colonoscopy in 1610 symptomatic patients found little difference in the detection rate of CRC and large polyps between the examinations. 115 In a study of 2531 asymptomatic participants undergoing CT colonography followed by colonoscopy, sensitivity of CT colonography for adenomas or CRCs ≥ 10 mm in size was 90% relative to colonoscopy. 116 Flexible sigmoidoscopy has lower sensitivity than CT colonography or colonoscopy as the proximal colon is not examined. However, in our study, only two participants received flexible sigmoidoscopy without additional colonoscopy or CT colonography (0.04%, 2/5199).
The generalisability of our findings was limited to an extent by the inclusion criteria, which stipulated that all participants had to return a first round FIT to be included. Uptake of FIT at rounds 2 and 3 is likely to be higher in our study than if participants who did not respond to the first FIT invitation had also been reinvited. Participation was slightly higher in older invitees (75.6% among invitees aged > 65 years and 72.9% among invitees aged ≤ 65 years) and in men (74.6% of male invitees participated and 73.6% of female invitees participated). Given that we stratified our findings by age and sex, the negative consequences of this are limited.
The generalisability of the findings from the health psychology assessment are limited in a similar way, as questionnaires and interviews were administered only to individuals participating in the main study. The attitudes of these individuals towards FIT and surveillance are likely to have been more favourable than those of individuals who did not agree to participate.
A further potential issue of generalisability is that we evaluated just one FIT brand (OC-Sensor). Evidence from a recent study of nine different quantitative FITs does, however, suggest that this is not a significant limitation. 117 When directly compared in a single screening cohort, different FIT brands achieved near-equal sensitivities when thresholds were set to yield defined rates of test positivity.
As noted, the economic evaluation was limited as it was based on a single-arm trial and did not consider the cost implications of missed AAs and CRCs and impact on QALYs.
In addition, although we hoped to model the potential of FIT screening to replace colonoscopy surveillance for groups at intermediate risk of developing CRC, including those with a personal or family history of CRC, we unfortunately were not able to realise this aim in the study period. A cost-effectiveness analysis of different CRC screening and surveillance strategies has recently been conducted, using data from the Dutch general population. 118 The analysis used the Adenoma and Serrated pathway to Colorectal Cancer (ASCCA) model, populated with Dutch data for colorectal lesion prevalence rates and CRC incidence and mortality rates, to compare no screening or surveillance, FIT screening alone and FIT screening plus surveillance colonoscopy. It was revealed that both FIT screening alone and FIT screening with surveillance colonoscopy were more effective and cost saving than no screening or surveillance. FIT screening with surveillance colonoscopy was not, however, cost-effective compared with FIT screening alone (using the Dutch cost-effectiveness threshold).
Implications for clinical practice
Our findings suggest that a programme of low-threshold annual FIT could perform a useful role in the surveillance of intermediate-risk patients. However, in a recent study examining heterogeneity of CRC incidence in intermediate-risk patients, we identified a lower-risk subgroup, for whom the value of colonoscopy surveillance was unclear, and a higher-risk subgroup for whom colonoscopy surveillance was clearly beneficial. 102 The utility of FIT surveillance may differ dependent on risk subgroup.
Currently, most patients deemed at intermediate risk following polypectomy cease surveillance after two negative surveillance colonoscopies. 22 We have examined the performance of FIT relative to the initial surveillance colonoscopy but we do not have sufficient data on the performance of FIT in the longer term. FIT might also perform a useful role in patients diagnosed with high-risk adenomas instead of the first 12-month surveillance examination or after the first surveillance colonoscopy.
Recommendations for research
-
Further research on FIT-based surveillance is needed to examine the implications of missed ACN, considering effects on QALYs.
-
The identification of subgroups among intermediate-risk patients102 requires further research into the performance and value of FIT by subgroup.
-
The efficacy, safety and cost-effectiveness of FIT in longer-term surveillance, as well as in high-risk patients, should be evaluated in RCTs.
-
Future studies should aim to determine whether molecular stool testing in addition to FIT could improve sensitivity further. This is important as the diagnostic accuracy of FIT is to some extent limited given that, as it is a test for occult blood in stool, non-bleeding lesions will not be detected. Although van Lanschot et al. 119 are currently evaluating FIT and a stool DNA test performed separately in a surveillance setting, the sensitivity of these tests in combination requires investigation.
-
Finally, effort should be placed on building a natural history model of CRC, similar to the ASCCA model,118 which incorporates data from the British population and from intermediate-risk patients. This would enable evaluation of the potential to replace 3-yearly colonoscopy surveillance with annual FIT surveillance.
Conclusion
This study has demonstrated the potential value of FIT for the surveillance of patients deemed to be at intermediate risk following polypectomy. Annual rounds of FIT, using a low faecal haemoglobin threshold and with colonoscopy in positive cases, achieved high cumulative sensitivity for CRC, was well accepted by patients, and would be cost saving compared with 3-yearly colonoscopy surveillance. However, with higher thresholds, FIT surveillance could miss 15–30% of CRCs and 40–70% of AAs, and most participants preferred annual FIT in combination with 3-yearly colonoscopy. Further research is needed to define a clear role for FIT in post-polypectomy surveillance. A full RCT with cost–utility analysis that addresses the cost and health implications of AAs and CRCs that were not detected is recommended.
Acknowledgements
The investigators are grateful to the people listed here for their involvement in this study. In addition to those named, we would like to thank the personnel within the 64 bowel cancer screening centres we liaised with to follow the investigations and subsequent clinician management for the participants who tested FIT positive, and for all their invaluable support with retrieving the relevant colonic examination and histology reports for all participants in the study.
Thank you also to all of the participants who engaged with the study.
Trial Steering Committee
Dr Andrew Veitch (chairperson), Ms Helen Watson and Ms Lynn Faulds-Wood (patient representatives), Professor Allan Hackshaw, Professor Sue Moss, Professor Marco Novelli, Professor Matt Rutter and Professor Christopher Todd.
Patient and Public Involvement
Throughout the study, our patient and public involvement representatives sat on the Trial Steering Committee and provided relevant insight into the views of patients with regard to the study, and wider issues relating to bowel cancer diagnosis, screening and surveillance.
Trial office staff, Cancer Screening and Prevention Research Group
Ms Amy Brenner, Ms Elizabeth Coles, Dr Paula Kirby, Ms Jessica Martin, Miss Emma Robbins, Mrs Urvi Shah, Ms Irene Simmonds, Mr Iain Stenson and Dr Laura Turner.
Collaborators
Faecal immunochemical test kit provision
Mr Iain McElarney, MAST Group Ltd FIT kits were generously provided by Eiken Chemical Co. Ltd (through MAST Group Ltd).
Bowel Cancer Screening Programme Southern Hub
Mrs Sally Benton, Mrs Helen Bruce, Ms Carole Burtonwood, Dr Magdalen Carroll, Ms Cerin John, Ms Kate Randall, Mrs Katy Allen, Dr Helen Seaman and Mr Neil Stubbs.
Clinical Informatics Research Unit at the University of Southampton
Professor James Batchelor and Mr Wez Morris.
Health Behaviour Research Centre
Ms Bernardette Bonello, Ms Harriet Bowyer, Dr Gemma Vart and Professor Jane Wardle (deceased).
Public Health England
Mr Tariq Malik, Ms Claire Nickerson and Dr Suzanne Wright.
Clinical advice
Professor Brian Saunders, Professor Robert Steele and Dr Roland Valori.
Questionnaire design
Mr Phillip Dove.
Contributions of authors
Wendy Atkin was the principal investigator for the study.
Wendy Atkin, Amanda J Cross, Kate Wooldrage, Aaron Prendergast and Stephen W Duffy were responsible for data analysis and interpretation.
Wendy Atkin, Ines Kralj-Hans, Stephen W Duffy, Stephen Morris, Christian von Wagner and Stephen Halloran were responsible for obtaining funding and research approvals and study design.
Eilidh MacRae, Bhavita Patel, Kevin Pack and Rosemary Howe were responsible for trial management, including collecting, entering and cleaning the data.
Wendy Atkin, Amanda J Cross, Ines Kralj-Hans, Eilidh MacRae, Carolyn Piggott, Sheena Pearson, Kate Wooldrage, Jeremy Brown, Fiona Lucas, Aaron Prendergast, Natalie Marchevsky, Bhavita Patel, Kevin Pack, Rosemary Howe, Hanna Skrobanski, Robert Kerrison, Nicholas Swart, Julia Snowball, Stephen W Duffy, Stephen Morris, Christian von Wagner and Stephen Halloran reviewed and edited the report.
Jeremy Brown and Fiona Lucas drafted the final report.
Hanna Skrobanski, Robert Kerrison and Christian von Wagner were responsible for the psychological analyses.
Nicholas Swart and Stephen Morris were responsible for the economic analysis.
Publications
Bowyer HL, Vart G, Kralj-Hans I, Atkin W, Halloran SP, Seaman H, et al. Patient attitudes towards faecal immunochemical testing for haemoglobin as an alternative to colonoscopic surveillance of groups at increased risk of colorectal cancer. J Med Screen 2013;20:149–56.
Bonello B, Ghanouni A, Bowyer HL, MacRae E, Atkin W, Halloran SP, et al. Using a hypothetical scenario to assess public preferences for colorectal surveillance following screening-detected, intermediate-risk adenomas: annual home-based stool test vs. triennial colonoscopy. BMC Gastroenterol 2016;16:113.
Cross AJ, Wooldrage K, Robbins EC, Kralj-Hans I, MacRae E, Piggott C, et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: a diagnostic accuracy and cost-effectiveness study [published online ahead of print December 11 2018]. Gut 2018.
Data-sharing statement
Data sharing requests should be directed to the corresponding author. Access to available anonymised, aggregated data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Bowel Cancer Survival Statistics. London: Cancer Research UK; 2014.
- Bending MW, Trueman P, Lowson KV, Pilgrim H, Tappenden P, Chilcott J, et al. Estimating the direct costs of bowel cancer services provided by the National Health Service in England. Int J Technol Assess Health Care 2010;26:362-9. https://doi.org/10.1017/S0266462310001078.
- Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251-70. https://doi.org/10.1002/cncr.2820360944.
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525-32. https://doi.org/10.1056/NEJM198809013190901.
- Morson BC, Bussey HJ. Magnitude of risk for cancer in patients with colorectal adenomas. Br J Surg 1985;72:S23-5. https://doi.org/10.1002/bjs.1800721315.
- Bussey HJ, Wallace MH, Morson BC. Metachronous carcinoma of the large intestine and intestinal polyps. Proc R Soc Med 1967;60:208-10.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343:162-8. https://doi.org/10.1056/NEJM200007203430301.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490-502. https://doi.org/10.1016/S0140-6736(13)61649-9.
- Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624-33. https://doi.org/10.1016/S0140-6736(10)60551-X.
- Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345-57. https://doi.org/10.1056/NEJMoa1114635.
- Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial – SCORE. J Natl Cancer Inst 2011;103:1310-22. https://doi.org/10.1093/jnci/djr284.
- Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606-15. https://doi.org/10.1001/jama.2014.8266.
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541-9. https://doi.org/10.1111/j.1572-0241.2008.01875.x.
- Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-96. https://doi.org/10.1056/NEJMoa1100370.
- Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095-105. https://doi.org/10.1056/NEJMoa1301969.
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. English Bowel Cancer Screening Evaluation Committee . Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. https://doi.org/10.1136/gutjnl-2011-300843.
- Geurts SM, Massat NJ, Duffy SW. Likely effect of adding flexible sigmoidoscopy to the English NHS Bowel Cancer Screening Programme: impact on colorectal cancer cases and deaths. Br J Cancer 2015;113:142-9. https://doi.org/10.1038/bjc.2015.76.
- Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136:832-41. https://doi.org/10.1053/j.gastro.2008.12.007.
- Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993;328:901-6. https://doi.org/10.1056/NEJM199304013281301.
- Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992;326:658-62. https://doi.org/10.1056/NEJM199203053261002.
- Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002;51:V6-9. https://doi.org/10.1136/gut.51.suppl_5.v6.
- Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. https://doi.org/10.1136/gut.2009.179804.
- Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn’s Disease or Adenomas. NICE Clinical Guidelines. London: NICE; 2011.
- Hornung TA, Bevan R, Mumtaz S, Hornung BR, Rutter MD. Surveillance colonoscopy in low-risk postpolypectomy patients: is it necessary?. Frontline Gastroenterol 2015;6:77-84. https://doi.org/10.1136/flgastro-2014-100524.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. https://doi.org/10.1053/j.gastro.2012.06.001.
- BCSP Guidance Note No 1. Adenoma Surveillance Version 1 September 2009. Sheffield: NHS Cancer Screening Programmes; 2009.
- Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24-8. https://doi.org/10.1016/S0016-5085(97)70214-2.
- Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769-72. https://doi.org/10.1093/jnci/82.22.1769.
- Brown H, Wyatt S, Croft S, Gale N, Turner A, Mulla A. Scoping the Future: An Evaluation of Endoscopy Capacity Across the NHS in England. London: Cancer Research; 2015.
- Gavin DR, Valori RM, Anderson JT, Donnelly MT, Williams JG, Swarbrick ET. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013;62:242-9. https://doi.org/10.1136/gutjnl-2011-301848.
- von Wagner C, Ghanouni A, Halligan S, Smith S, Dadswell E, Lilford RJ, et al. Patient acceptability and psychologic consequences of CT colonography compared with those of colonoscopy: results from a multicenter randomized controlled trial of symptomatic patients. Radiology 2012;263:723-31. https://doi.org/10.1148/radiol.12111523.
- Ghanouni A, Plumb A, Hewitson P, Nickerson C, Rees CJ, von Wagner C. Patients’ experience of colonoscopy in the English Bowel Cancer Screening Programme. Endoscopy 2016;48:232-40. https://doi.org/10.1055/s-0042-100613.
- Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol 2016;111:1092-101. https://doi.org/10.1038/ajg.2016.234.
- McLachlan SA, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context – a systematic review of the literature. Patient Educ Couns 2012;86:137-46. https://doi.org/10.1016/j.pec.2011.04.010.
- Lund JN, Scholefield JH, Grainge MJ, Smith SJ, Mangham C, Armitage NC, et al. Risks, costs, and compliance limit colorectal adenoma surveillance: lessons from a randomised trial. Gut 2001;49:91-6. https://doi.org/10.1136/gut.49.1.91.
- Steele RJ, McClements PL, Libby G, Black R, Morton C, Birrell J, et al. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009;58:530-5. https://doi.org/10.1136/gut.2008.162883.
- Adler J, Robertson DJ. Interval colorectal cancer after colonoscopy: exploring explanations and solutions. Am J Gastroenterol 2015;110:1657-64. https://doi.org/10.1038/ajg.2015.365.
- Richards MA. The size of the prize for earlier diagnosis of cancer in England. Br J Cancer 2009;101:125-9. https://doi.org/10.1038/sj.bjc.6605402.
- van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. Gastroenterology 1998;115:13-8. https://doi.org/10.1016/S0016-5085(98)70359-2.
- Lee TJ, Nickerson C, Goddard AF, Rees CJ, McNally RJ, Rutter MD. Outcome of 12-month surveillance colonoscopy in high-risk patients in the National Health Service Bowel Cancer Screening Programme. Colorectal Dis 2013;15:e435-42. https://doi.org/10.1111/codi.12278.
- Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 1998;317:559-65. https://doi.org/10.1136/bmj.317.7158.559.
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637-49. https://doi.org/10.1136/gutjnl-2014-309086.
- Allison JE, Fraser CG, Halloran SP, Young GP. Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver 2014;8:117-30. https://doi.org/10.5009/gnl.2014.8.2.117.
- Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, et al. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci 2015;60:609-22. https://doi.org/10.1007/s10620-014-3445-3.
- Burch JA, Soares-Weiser K, St John DJ, Duffy S, Smith S, Kleijnen J, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. J Med Screen 2007;14:132-7. https://doi.org/10.1258/096914107782066220.
- Terhaar sive Droste JS, van Turenhout ST, Oort FA, van der Hulst RW, Steeman VA, Coblijn U, et al. Faecal immunochemical test accuracy in patients referred for surveillance colonoscopy: a multi-centre cohort study. BMC Gastroenterol 2012;12. https://doi.org/10.1186/1471-230X-12-94.
- Steele RJ, McDonald PJ, Digby J, Brownlee L, Strachan JA, Libby G, et al. Clinical outcomes using a faecal immunochemical test for haemoglobin as a first-line test in a national programme constrained by colonoscopy capacity. United European Gastroenterol J 2013;1:198-205. https://doi.org/10.1177/2050640613489281.
- Grazzini G, Visioli CB, Zorzi M, Ciatto S, Banovich F, Bonanomi AG, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening?. Br J Cancer 2009;100:259-65. https://doi.org/10.1038/sj.bjc.6604864.
- Dancourt V, Lejeune C, Lepage C, Gailliard MC, Meny B, Faivre J. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. Eur J Cancer 2008;44:2254-8. https://doi.org/10.1016/j.ejca.2008.06.041.
- Guittet L, Bouvier V, Mariotte N, Vallee JP, Levillain R, Tichet J, et al. Comparison of a guaiac and an immunochemical faecal occult blood test for the detection of colonic lesions according to lesion type and location. Br J Cancer 2009;100:1230-5. https://doi.org/10.1038/sj.bjc.6604996.
- van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135:82-90. https://doi.org/10.1053/j.gastro.2008.03.040.
- Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam ME, Dekker E, van Ballegooijen M, et al. Combining risk factors with faecal immunochemical test outcome for selecting CRC screenees for colonoscopy. Gut 2014;63:466-71. https://doi.org/10.1136/gutjnl-2013-305013.
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012;55:87-92. https://doi.org/10.1016/j.ypmed.2012.05.006.
- Moss S, Mathews C, Day TJ, Smith S, Seaman HE, Snowball J, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2017;66:1631-44. https://doi.org/10.1136/gutjnl-2015-310691.
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160. https://doi.org/10.7326/M13-1484.
- Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315:2576-94. https://doi.org/10.1001/jama.2016.3332.
- Robinson MH, Kronborg O, Williams CB, Bostock K, Rooney PS, Hunt LM, et al. Faecal occult blood testing and colonoscopy in the surveillance of subjects at high risk of colorectal neoplasia. Br J Surg 1995;82:318-20. https://doi.org/10.1002/bjs.1800820310.
- Bampton PA, Sandford JJ, Cole SR, Smith A, Morcom J, Cadd B, et al. Interval faecal occult blood testing in a colonoscopy based screening programme detects additional pathology. Gut 2005;54:803-6. https://doi.org/10.1136/gut.2004.043786.
- Hazazi R, Rozen P, Leshno M, Levi Z, Samuel Z, Waked A, et al. Can patients at high risk for significant colorectal neoplasms and having normal quantitative faecal occult blood test postpone elective colonoscopy?. Aliment Pharmacol Ther 2010;31:523-33. https://doi.org/10.1111/j.1365-2036.2009.04202.x.
- Lane JM, Chow E, Young GP, Good N, Smith A, Bull J, et al. Interval fecal immunochemical testing in a colonoscopic surveillance program speeds detection of colorectal neoplasia. Gastroenterology 2010;139:1918-26. https://doi.org/10.1053/j.gastro.2010.08.005.
- Symonds EL, Osborne J, Young GP, Bampton PA, Fraser RJ, Cole SR. Significance of a positive faecal immunochemical test in a colonoscopic surveillance program. J Gastroenterol Hepatol 2013;28.
- Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 2007;146:244-55. https://doi.org/10.7326/0003-4819-146-4-200702200-00003.
- Bossuyt PM, Cohen JF, Gatsonis CA, Korevaar DA. STARD group . STARD 2015: updated reporting guidelines for all diagnostic accuracy studies. Ann Transl Med 2016;4. https://doi.org/10.3978/j.issn.2305-5839.2016.02.06.
- Carroll M, Piggott C, Pearson S, Seaman H, Halloran S. Evaluation of Quantitative Faecal Immunochemical Tests for Haemoglobin. Guildford: Guildford Medical Device Evaluation Centre (GMEC); 2013.
- Imperial College London . FIT for Follow-Up Study n.d. www.fit4followup.org.uk/ (accessed 31 July 2017).
- Bland M. An Introduction to Medical Statistics 1995.
- Bowyer HL, Vart G, Kralj-Hans I, Atkin W, Halloran SP, Seaman H, et al. Patient attitudes towards faecal immunochemical testing for haemoglobin as an alternative to colonoscopic surveillance of groups at increased risk of colorectal cancer. J Med Screen 2013;20:149-56. https://doi.org/10.1177/0969141313503953.
- Marteau TM, Bekker H. The development of a 6-item short-form of the state scale of the Spielberger State Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. https://doi.org/10.1111/j.2044-8260.1992.tb00997.x.
- Miles A, Atkin WS, Kralj-Hans I, Wardle J. The psychological impact of being offered surveillance colonoscopy following attendance at colorectal screening using flexible sigmoidoscopy. J Med Screen 2009;16:124-30. https://doi.org/10.1258/jms.2009.009041.
- Castiglione G, Grazzini G, Miccinesi G, Rubeca T, Sani C, Turco P, et al. Basic variables at different positivity thresholds of a quantitative immunochemical test for faecal occult blood. J Med Screen 2002;9:99-103. https://doi.org/10.1136/jms.9.3.99.
- Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007;132:2304-12. https://doi.org/10.1053/j.gastro.2007.03.030.
- Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 2007;99:1462-70. https://doi.org/10.1093/jnci/djm150.
- Nakama H, Zhang B, Fukazawa K, Zhang X. Comparisons of cancer detection rate and costs of one cancer detected among different age-cohorts in immunochemical occult blood screening. J Cancer Res Clin Oncol 2001;127:439-43. https://doi.org/10.1007/s004320000230.
- Nakama H, Zhang B, Zhang X. Evaluation of the optimum cut-off point in immunochemical occult blood testing in screening for colorectal cancer. Eur J Cancer 2001;37:398-401. https://doi.org/10.1016/S0959-8049(00)00387-7.
- Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005;129:422-8. https://doi.org/10.1016/j.gastro.2005.05.056.
- Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010;59:62-8. https://doi.org/10.1136/gut.2009.177089.
- Zorzi M, Grazzini G, Senore C, Vettorazzi M. Screening for colorectal cancer in Italy: 2004 survey. Epidemiol Prev 2006;30:41-50.
- Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health 2007;10:415-30. https://doi.org/10.1111/j.1524-4733.2007.00196.x.
- Wong MC, Tsoi KK, Ng SS, Lou VW, Choi SY, Ling KW, et al. A comparison of the acceptance of immunochemical faecal occult blood test and colonoscopy in colorectal cancer screening: a prospective study among Chinese. Aliment Pharmacol Ther 2010;32:74-82. https://doi.org/10.1111/j.1365-2036.2010.04312.x.
- Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697-706. https://doi.org/10.1056/NEJMoa1108895.
- Schroy PC, Glick JT, Robinson PA, Heeren T. Screening preferences of patients at familial risk of colorectal cancer. Dig Dis Sci 2007;52:2788-95. https://doi.org/10.1007/s10620-006-9670-7.
- Wolf RL, Basch CE, Brouse CH, Shmukler C, Shea S. Patient preferences and adherence to colorectal cancer screening in an urban population. Am J Public Health 2006;96:809-11. https://doi.org/10.2105/AJPH.2004.049684.
- Michie S, McDonald V, Marteau T. Understanding responses to predictive genetic testing: a grounded theory approach. Psychol Health 1996;11:455-70. https://doi.org/10.1080/08870449608401982.
- Liljegren A, Lindgren G, Brandberg Y, Rotstein S, Nilsson B, Hatschek T, et al. Individuals with an increased risk of colorectal cancer: perceived benefits and psychological aspects of surveillance by means of regular colonoscopies. J Clin Oncol 2004;22:1736-42. https://doi.org/10.1200/JCO.2004.04.138.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Spielberger CD. State–Trait Anxiety Inventory: Bibliography. Palo Alto, CA: Consulting Psychologists Press; 1989.
- Cockburn J, De Luise T, Hurley S, Clover K. Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. Soc Sci Med 1992;34:1129-34. https://doi.org/10.1016/0277-9536(92)90286-Y.
- Banks J, Hollinghurst S, Bigwood L, Peters TJ, Walter FM, Hamilton W. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol 2014;15:232-40. https://doi.org/10.1016/S1470-2045(13)70588-6.
- Bonello B, Ghanouni A, Bowyer HL, MacRae E, Atkin W, Halloran SP, et al. Using a hypothetical scenario to assess public preferences for colorectal surveillance following screening-detected, intermediate-risk adenomas: annual home-based stool test vs. triennial colonoscopy. BMC Gastroenterol 2016;16. https://doi.org/10.1186/s12876-016-0517-1.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care. University of Kent: Personal Social Services Research Unit; 2015.
- Murphy J, Gray A. The Cost-effectiveness of Immunochemical Faecal Occult Blood Testing vs. Guaiac Faecal Occult Blood Testing for Colorectal Cancer Screening in the NHS Bowel Cancer Screening Programme: Report to the UK National Screening Committee. Oxford: University of Oxford, Health Economics Research Centre; 2015.
- NHS Reference Costs 2014 to 2015. London: DHSC; 2015.
- Atkin WS, Cook CF, Cuzick J, Edwards R, Northover JM, Wardle J. UK flexible sigmoidoscopy screening trial investigators . Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet 2002;359:1291-300. https://doi.org/10.1016/S0140-6736(02)08268-5.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- NHS England Data Catalogue . Public Health England. Cancer Screening Coverage – Bowel Cancer n.d. https://data.england.nhs.uk/dataset/phe-indicator-91720 (accessed 31 July 2017).
- Bowel Cancer Screening: The Facts. London: Cancer Research UK; 2016.
- Morris S, Baio G, Kendall E, von Wagner C, Wardle J, Atkin W, et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer 2012;107:765-71. https://doi.org/10.1038/bjc.2012.303.
- Seaman H. NHS Bowel Cancer Screening Programme Southern Programme Hub. Annual Report, 2014 15 n.d. www.royalsurrey.nhs.uk/wp-content/uploads/2015/12/BCSP-Southern-Hub-Annual-Report-2014-2015.pdf (accessed 31 July 2017).
- Majumdar D, Lee TJ, Nickerson C, Patnick J, Rutter MD. Outcome of 3 year surveillance colonoscopy in patients with intermediate risk adenomas: Analysis of the NHS bowel cancer screening programme national database. Gut 2011;60. https://doi.org/10.1136/gut.2011.239301.11.
- Atkin W, Brenner A, Martin J, Wooldrage K, Shah U, Lucas F, et al. The clinical effectiveness of different surveillance strategies to prevent colorectal cancer in people with intermediate-grade colorectal adenomas: a retrospective cohort analysis, and psychological and economic evaluations. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21250.
- Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017;18:823-34. https://doi.org/10.1016/S1470-2045(17)30187-0.
- Ball AJ, Rees CJ, Corfe BM, Riley SA. Sedation practice and comfort during colonoscopy: lessons learnt from a national screening programme. Eur J Gastroenterol Hepatol 2015;27:741-6. https://doi.org/10.1097/MEG.0000000000000360.
- Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011;306:1352-8. https://doi.org/10.1001/jama.2011.1362.
- Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol 2013;11:172-80. https://doi.org/10.1016/j.cgh.2012.09.010.
- Cha JM. Colonoscopy quality is the answer for the emerging issue of interval cancer. Intest Res 2014;12:110-16. https://doi.org/10.5217/ir.2014.12.2.110.
- Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol 2010;105:2588-96. https://doi.org/10.1038/ajg.2010.390.
- Erichsen R, Baron JA, Stoffel EM, Laurberg S, Sandler RS, Sørensen HT. Characteristics and survival of interval and sporadic colorectal cancer patients: a nationwide population-based cohort study. Am J Gastroenterol 2013;108:1332-40. https://doi.org/10.1038/ajg.2013.175.
- Hirai HW, Tsoi KK, Chan JY, Wong SH, Ching JY, Wong MC, et al. Systematic review with meta-analysis: faecal occult blood tests show lower colorectal cancer detection rates in the proximal colon in colonoscopy-verified diagnostic studies. Aliment Pharmacol Ther 2016;43:755-64. https://doi.org/10.1111/apt.13556.
- Digby J, Fraser CG, Carey FA, McDonald PJ, Strachan JA, Diament RH, et al. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol 2013;66:415-19. https://doi.org/10.1136/jclinpath-2013-201445.
- Vleugels JLA, Hazewinkel Y, Fockens P, Dekker E. Natural history of diminutive and small colorectal polyps: a systematic literature review. Gastrointest Endosc 2017;85:1169-76. https://doi.org/10.1016/j.gie.2016.12.014.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 1987;93:1009-13. https://doi.org/10.1016/0016-5085(87)90563-4.
- Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 2014;63:949-56. https://doi.org/10.1136/gutjnl-2012-303796.
- van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343-50. https://doi.org/10.1111/j.1572-0241.2006.00390.x.
- Halligan S, Dadswell E, Wooldrage K, Wardle J, von Wagner C, Lilford R, et al. Computed tomographic colonography compared with colonoscopy or barium enema for diagnosis of colorectal cancer in older symptomatic patients: two multicentre randomised trials with economic evaluation (the SIGGAR trials). Health Technol Assess 2015;19. https://doi.org/10.3310/hta19540.
- Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359:1207-17. https://doi.org/10.1056/NEJMoa0800996.
- Gies A, Cuk K, Schrotz-King P, Brenner H. Direct comparison of diagnostic performance of 9 quantitative fecal immunochemical tests for colorectal cancer screening. Gastroenterology 2018;154:93-104. https://doi.org/10.1053/j.gastro.2017.09.018.
- Greuter MJE, de Klerk CM, Meijer GA, Dekker E, Coupé VMH. Screening for colorectal cancer with fecal immunochemical testing with and without postpolypectomy surveillance colonoscopy: a cost-effectiveness analysis. Ann Intern Med 2017;167:544-54. https://doi.org/10.7326/M16-2891.
- van Lanschot MC, Carvalho B, Coupé VM, van Engeland M, Dekker E, Meijer GA. Molecular stool testing as an alternative for surveillance colonoscopy: a cross-sectional cohort study. BMC Cancer 2017;17. https://doi.org/10.1186/s12885-017-3078-y.
Glossary
- Cumulative test analyses
- Included only those participants who either completed the specified number of rounds of faecal immunochemical tests or tested positive at a previous round. The two-test analysis included participants who completed at least two rounds of faecal immunochemical tests or who tested positive at round 1. The three-test analysis included participants who completed all three rounds of faecal immunochemical tests or who tested positive at any round.
- Programme analyses
- Included all participants who completed a faecal immunochemical test at round 1, regardless of whether or not they participated at any further round. A participant was classed as positive if their faecal immunochemical test was positive at any of the first two rounds at which they completed a faecal immunochemical test (two-test analysis), or if their faecal immunochemical test was positive at any round at which they completed a faecal immunochemical test (three-test analysis).
List of abbreviations
- AA
- advanced adenoma
- ACN
- advanced colorectal neoplasia
- ASCCA
- Adenoma and Serrated pathway to Colorectal Cancer
- BCSP
- Bowel Cancer Screening Programme
- BCSS
- Bowel Cancer Screening System
- CI
- confidence interval
- CRC
- colorectal cancer
- CSPRG
- Cancer Screening and Prevention Research Group
- CT
- computed tomography
- DNA
- did not attend
- ECC
- Ethics and Confidentiality Committee
- FIT
- faecal immunochemical test
- gFOBT
- guaiac faecal occult blood test
- GI
- gastrointestinal
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- IG
- information governance
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- PCSS
- Positive Consequences Of Surveillance Scale
- PMS
- patient management system
- PPV
- positive predictive value
- pT
- primary tumour
- QALY
- quality-adjusted life-year
- RC
- routine colonoscopy
- RCT
- randomised controlled trial
- STAI
- Spielberger State–Trait Anxiety Inventory
- UK-ASG
- UK adenoma surveillance guideline
