Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/46/01. The contractual start date was in September 2014. The draft report began editorial review in October 2017 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
John Strang reports grants and others from Martindale Pharma, grants and others from Mundipharma, and grants and others from Braeburn Pharma, outside the submitted work. In addition, John Strang has a Euro-Celtique patent issued, and a King’s College London patent pending. Michael Kelleher reports grants from the National Institute for Health Research (NIHR) during the conduct of the study, others from South London and Maudsley NHS Foundation Trust, Public Health England, Braeburn Pharma and Reckitt Benckiser, personal fees from Mundipharma and non-financial support from Cephaid, outside the submitted work. Sarah Byford reports grants from NIHR Health Technology Assessment programme during the conduct of the study. John Marsden reports investigator-led, educational grant funding from Indivior (administered by Action-on-Addiction) for a study of personalised psychosocial intervention for non-response to opioid agonist treatment. He declares consultancy for the US National Institute on Drug Abuse Centre for Clinical Trials Network. In the past 3 years, he received honoraria from Merck Serono (2015; clinical oncology training), Martindale (2017; expert meeting on opioid use disorder), and Indivior (via PCM Scientific) as co-chairperson (2015, 2016) and chairperson (2017) for the conference on Improving Outcomes in Treatment of Opioid Dependence.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Strang et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Trial summary
What is the clinical effectiveness and cost-effectiveness of enhanced naltrexone in the treatment of opioid use disorder (OUD)? The Naltrexone Enhanced Addiction Treatment for Opioid Use Disorder (NEAT) trial was the first Phase III UK study to coalesce antagonist medication and behavioural interventions for the treatment of patients with heroin addiction problems.
The study was implemented in two specialist NHS outpatient addiction clinics in London and Birmingham (recruitment centres), each with formal links for research trials with a local university.
A total of 300 recently detoxified, formerly dependent heroin users were to be randomised to one of three treatments, which were to be received on site under supervision:
-
thrice-weekly oral active naltrexone tablets plus placebo extended-release naltrexone at the start of treatment
-
oral placebo naltrexone plus active extended-release naltrexone
-
oral placebo naltrexone plus placebo extended-release naltrexone.
Each condition was delivered over 12 weeks. All participants received standard NHS psychological interventions (weekly individual counselling) and a behavioural protocol incentivising clinic attendance to receive trial medication and complete research assessments.
The primary outcome measure was the number of heroin-negative urine drug screens (UDSs) in treatment (taken thrice weekly during the 12-week treatment phase of the trial; 36 UDSs in total). In addition to societal-focused health-related cost-effectiveness, secondary objectives related to treatment retention/adherence, craving for heroin and cocaine, and monitoring of naltrexone and 6-β-naltrexol (the primary metabolite of naltrexone). Research worker-administered follow-up assessments were at 16, 24 and 36 weeks after the active 12-week treatment phase.
Background and rationale
The term ‘opioids’ refers to semisynthetic and synthetic analgesic compounds with similar properties to the group of psychoactive analgesic substances derived from the poppy plant, including opium, morphine and codeine. In England, ‘street’ heroin is the most harmful illegal opioid in the UK. 1 Illicit heroin makes the user feel intense euphoria. It has an aggressive dependence liability, the predominant symptom being compulsive drug use despite significant health and social harms. 2 Physiologically dependent users need to take heroin every day to avoid the onset of acutely unpleasant flu-like withdrawal symptoms. Users report experiencing intense feelings of wanting and needing to take heroin and find it very difficult to stop. Craving is considered a core symptom of addiction and a prime cause of relapse. 3 Untreated, opioid dependence is a persistent and debilitating condition, associated with high social costs arising from drug misuse. The lives of most heroin addicts are disadvantaged in many ways and there is a strong link between heroin use and acquisitive crime. There is also an associated major public health burden owing to the acquisition and transmission of blood-borne viral infections in this population. Consequently, tackling the problem of opioid dependence is a high priority for the government and the NHS.
Most individuals presenting to specialist NHS community treatment clinics have established harmful illicit OUDs, almost all of which are related to street heroin use. However, the addition of cocaine dependence adds considerable severity to individual cases, and this patient subgroup has a relatively poorer outcome than primary heroin users. 4 Prior to the study’s commission, there were 321,229 individuals in England with problems relating to heroin and/or crack cocaine use (corresponding to 9.4 per 1000 of the population aged 15–64 years in 2008/95). In 2008/9, the combined use of heroin and cocaine was reported in 29% of patients admitted for treatment. 6 Intravenous injection is the preferred route of administration for approximately one-third of heroin users, with associated risks of infection and overdose. There are also substantially elevated rates of mood disorders among heroin users compared with the general population,7 and after opioid detoxification, in addition to craving for heroin, patients often report a syndrome of anhedonia, including affective disorders (depression, dysphoria and anxiety). These symptoms may trigger recurrence in heroin use. 8
The chronic relapsing nature of drug dependence means that helping a patient to achieve stable abstinence is often difficult. In the NHS, the frontline clinical response to heroin dependence is the prescription of a substitute (full or partial) μ-opioid agonist (oral methadone, oral buprenorphine hydrochloride or oral buprenorphine–naloxone medication) taken once daily in the context of case management and general counselling support. If the patient receives an appropriate prescribed dose of opioid agonist maintenance therapy, physiological tolerance to opioids is medically managed and there are usually no breakthrough withdrawal symptoms before the next dosing. Opioid substitution therapy (OST) medication is administered directly under clinical supervision or by the patient at home.
Properly delivered OST creates a platform for patients to receive structured psychosocial interventions. All patients are supported by a keyworker and may also receive structured psychosocial treatment if needed. A specified keyworker (a physician, psychologist or, more commonly, a nurse, social worker or trained non-medical drugs worker) takes the lead role in co-ordinating a patient’s care. Through regular clinic appointments, the keyworker gives practical advice, applies psychological techniques to build motivation to reduce drug-related harms and organises access to community services as required. In around two-thirds of patients receiving substitution treatment, the prescribing physician maintains the patient on a stable daily dose for as long as is clinically indicated, and then supervises a gradual withdrawal to achieve opioid abstinence. In the remainder of cases, prescribing is of shorter duration, usually involving a gradual withdrawal of medication immediately following stabilisation.
However, not all patients derive a clinical benefit from OST. Some respond initially, then lapse to resumed heroin use during treatment; a minority deteriorate progressively during treatment. Some patients and clinicians prefer abstinence, rather than a maintenance approach from the outset, and some patients prefer to continue their personal recovery journey by withdrawing early from agonist therapy and receiving support for abstinence. Overall, reduced therapeutic engagement, ongoing or resumed street heroin and cocaine or amphetamine use, and variations in satisfaction with medication vary widely between programmes. Furthermore, some patients do not wish to receive OST. There are substantially elevated rates of mood disorders among heroin users compared with the general population,7 and, after opioid detoxification, in addition to craving for heroin, patients often report a syndrome of anhedonia, including affective disorders (depression, dysphoria and anxiety). These symptoms may trigger a recurrence in heroin use. 8 Unfortunately, psychological supports have been shown not to be particularly effective at helping patients to maintain abstinence, and the NHS currently has no significant alternative treatment options.
The NEAT trial addressed this need and evaluated an μ-opioid antagonist using naltrexone as part of a relapse prevention maintenance programme for formerly opioid-dependent individuals who were seeking abstinence treatment. Naltrexone blocks the effects of any subsequently ingested heroin and prevents physical dependence. Naltrexone is used as a treatment for alcohol dependence by reducing craving for alcohol and the subjective reinforcement effects of drinking. 9 For opioid dependence, naltrexone does not directly reduce the craving for heroin, but, in the absence of the physical effects of heroin, clinical studies of maintenance therapy indicate that the craving gradually attenuates. 10 This highlights the importance of combining naltrexone with behavioural therapies to maintain abstinence.
Naltrexone is rapidly absorbed, metabolised by the liver and excreted in the urine with an elimination half-life of 4 hours (13 hours for the principal metabolite 6-β-naltrexol). Behaviourally, naltrexone blocks the euphoric effects of opioids. It has no psychoactive effect of its own and tolerance and dependence do not develop. 11 Clinical studies indicate that 50 mg of oral tablet naltrexone hydrochloride will block the pharmacological effects of 25 mg of intravenously administered heroin for a period of at least 24 hours. Doubling this dose provides a blockade for around 48 hours, and tripling the dose provides a pharmacological opioid blockade for approximately 72 hours. Depending on whether 1, 2 or 3 days elapse before a patient’s next clinic visit to receive medication, a dose of 50 mg, 100 mg or 150 mg of oral naltrexone is prescribed. An open-ended and flexible approach to the dosing regimen and the duration of treatment is usually used in routine NHS practice with this medication. Patients may receive 50 mg of oral naltrexone each weekday, with a 100 mg dose on Saturday, or patients may receive 100 mg every other day, or 150 mg every third day. Several clinical trials have used the following dosing regimen: 100 mg on Monday, 100 mg on Wednesday and 150 mg on Friday. This schedule is acceptable to patients, balances the level of attendance at the clinic required to collect research assessments, and was, therefore, used in this study.
Oral naltrexone has an excellent pharmacological profile as an opioid blocker. However, as a relapse prevention pharmacotherapy it has produced disappointing results. The main reason for this is that patients who succumb to cravings (or are otherwise motivated to use heroin) can relatively easily discontinue their medication and then return to heroin use. Consequently, retention has been shown to be poor in all but the most motivated or socially supported patients. There have been several meta-analyses. In 2006, Berglund et al. 12 reported on 10 studies of oral naltrexone versus control (seven placebo) and six studies of psychosocial/psychopharmacological interventions involving 1071 patients randomised to oral naltrexone maintenance therapy for OUD or a control. This review pointed to retention as the key variable in explaining the effectiveness of naltrexone. The studies with the highest retention in the experimental group had better results than the control group for differences in retention, opioid-positive UDSs, psychiatric symptoms and craving for heroin during the experimental period. Among these were those studies that incentivised clinic attendance for each dose by offering vouchers that could be exchanged for recovery-appropriate goods or services. In these trials, there was increased retention and a greater reduction in the number of opioid-positive UDSs. 13
In 2007, Adi et al. 14 reported on 26 studies with 940 participants. They concluded that the methodological quality of the reviewed trials was poor to moderate. The results suggested that oral naltrexone may be better than placebo in terms of retention in treatment, but overall this was not statistically significant. Among the trials including a contingency management element, the mean length of time patients stayed on naltrexone was 7.4 weeks, compared with 2.3–5.6 weeks on naltrexone treatment alone. Nevertheless, based on evidence and clinical experience, according to the National Institute for Health and Care Excellence (NICE) ‘naltrexone is recommended as a treatment option in detoxified formerly opioid-dependent people who are highly motivated to remain in an abstinence programme’. 15
Against the background of clinical evidence for oral naltrexone, there is a clear logic for a sustained-release formulation of naltrexone: it removes the need for the patient to remember to take the medication (usually either daily or thrice weekly). Medical products variously described by prefix as controlled, modified, slow, extended, sustained or prolonged-release (extended-release is the term used herein) are designed to reduce the frequency of dosing by modifying the rate of release and absorption of an active substance. Such products have been available for some time and have been used effectively to treat a wide range of clinical indications. First-generation products achieved modified release through intramuscular or subcutaneous injections of suspensions of insoluble drug complexes.
Chapter 2 Methods
Trial design
The NEAT trial was initiated as a 3-year, definitive, two-centre, three-arm, parallel-group, placebo-controlled, double-blind, double-dummy, Phase III randomised controlled trial. Its objective was to evaluate and compare the clinical effectiveness of oral naltrexone with implanted extended-release naltrexone as relapse prevention therapy for participants with OUD. After a literature review and discussion with experts, 12 weeks was selected as the optimum duration over which to deliver medication, the psychological intervention and the incentivised clinical attendance protocol. Primary and secondary outcomes were assessed after 12 weeks, with follow-up interviews after 16, 24 and 36 weeks.
The trial was designed as double blind. Active and placebo oral medication were produced and encapsulated identically. Active and placebo implant devices were produced and packaged identically. Clinicians and research workers completing baseline assessments, clinic attendance assessments and all follow-ups were blind to group allocation, as were patients and pharmacists. This design ensured that the study had a high level of both treatment integrity (delivery of the treatment as intended) and treatment differentiation (treatment conditions differed from one another in the intended manner). The trial had three groups (Figure 1):
-
group A – active extended-release naltrexone and placebo oral naltrexone
-
group B – placebo extended-release naltrexone and active oral extended-release naltrexone
-
group C – placebo extended-release naltrexone and placebo oral naltrexone.
FIGURE 1.
Participants: patients. ADAPT, Addiction Dimensions for Assessment and Personalised Treatment; ADSUS, Alcohol & Drig adapted Adult Service Use Schedule; BIS, Barratt Impulsiveness Scale; BPAQ-SF, Buss–Perry Aggression Questionnaire-Short Form; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ECG, electrocardiography; EQ-5D, EuroQol-5 Dimensions; EQ-5D-5L, EuroQol-5 Dimensions, five-level version; GAD-7, Generalised Anxiety Disorder-7; HRBS, HIV Risk-taking Behaviour Scale; MCCS, Minnesota Cocaine Craving Scale; MoCA, Montreal Cognitive Assessment; O-NTX, oral naltrexone; PHQ-9, Patient Health Questionnaire-9 items; PIL, patient information leaflet; SCID-V, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; TOP, treatment outcomes profile; WSAS, Work and Social Adjustment Scale; XR-NTX, extended-release naltrexone.

Research governance
The trial was co-sponsored by King’s College London (KCL) and South London and Maudsley (SLaM) NHS Foundation Trust, with regulatory compliance oversight by the King’s Health Partners Clinical Trial Office (KHP-CTO).
An ethics application was submitted to the Dulwich National Research Ethics Service (NRES) Committee London on 21 August 2014 and the NEAT trial received a favourable opinion on 22 September 2014 (reference number: 14/LO/1615). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/104601/#/.
A submission was filed by the KHP-CTO with the Medicines & Healthcare products Regulatory Agency (MHRA) on 8 August 2014. The NEAT trial gained MHRA approval on 2 October 2014 and was given the reference number 28482/0014/001–0001.
To ensure transparency, the trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) Registry on 12 January 2015 (ISRCTN95809946).
The National Institute for Health Research (NIHR) Clinical Research Network (CRN) Portfolio details high-quality clinical research studies that are eligible for support from the NIHR CRN in England. The trial was adopted on to the NIHR CRN Portfolio on 21 September 2015 and was issued the NIHR CRN Portfolio number 17950.
Global NHS permissions were obtained through the Integrated Research Application System on 10 November 2014 and local NHS permissions were obtained from each participating NHS trust. A clinical trial site agreement, based on the model agreement for non-commercial research in the health service, was signed by each participating NHS trust and the sponsor (KCL and SLaM).
Following guidelines from the NIHR, a Trial Steering Committee (TSC), with a majority of independent members, was convened to oversee the trial on behalf of the funder (NIHR) and the sponsor (KCL and SLaM).
The TSC met at least annually during the trial and comprised an independent chairperson, an independent lay member (representing patient perspectives), independent clinicians (specialising in addictions), the chief investigator (JS) and the lead investigator (JM) representing the Trial Management Group (TMG).
Management of the trial
The trial manager was responsible for day-to-day management of the trial with support from the data manager and trial statistician. The TMG was responsible for overseeing day-to-day management of the trial and comprised the chief investigator (JS), the lead investigator (JM) and statisticians (JH and ER). The TMG met regularly throughout the trial to ensure adherence to the trial protocol and monitor the conduct and progress of the trial.
Design and development of the protocol
Clinicians (including doctors, nurses and key workers from the addictions field across the UK), as well as people who previously used drugs, were invited to discuss the trial protocol. This feedback was utilised by the applicants when designing and developing the protocol.
Amendments to the trial protocol
Following receipt of a favourable opinion of the trial protocol from the NRES on 22 September 2014, four substantial amendments were submitted and received favourable opinion.
In summary, these were as follows.
-
Amendment 1 (23 July 2015):
-
When reviewing the patient visit timeline the TMG realised that certain measures were not needed as often or at all. Electrocardiograms (ECGs) were deemed necessary only at baseline and the treatment outcomes profile and both the outcome rating scale and the session rating scale were not needed.
-
The TMG decided that comparing the patterns of heroin relapse between treatment arms among the extended-release naltrexone and the extended-release naltrexone and placebo conditions was needed, and this was added as a secondary end point.
-
It was noted in the investigator brochure that severe renal impairment was included in the list of contraindications but not in the protocol, so this was added as an exclusion criterion.
-
A new formula of placebo from the manufacturer was released so changes from the new investigational medicinal product dossier were added to the protocol. They included an amended version of the undesirable effects, which was added to the protocol and Patient Information Leaflet. The new description of the drug was added to the protocol too.
-
The telephone number of the emergency unblinding service was added to the protocol.
-
It was noted that the dosing level for the ‘take away’ period was incorrect and was deleted from the protocol.
-
It was no longer the case that, if a patient missed six consecutive appointments, the 14-day rule would apply, and this rule was deleted from the protocol.
-
-
Amendment 2 (13 August 2015):
-
The pharmacy indicated to us that a dosing leaflet was needed to instruct patients on the correct dosing instructions for the oral tablets taken in the trial.
-
-
Amendment 3 (8 December 2015):
-
Additional text was added to the ‘Economic’ section of the ‘Primary Endpoints’ section to reflect the clinical end points.
-
The ‘Investigational Medicinal Product (IMP), Dosage and Route Of Administration’ section had the words ‘immediately following randomisation’ deleted as there was no clinical reason for the implant to be given immediately after randomisation.
-
‘Plasma naltrexone’ was added to the secondary end points as it was mentioned in the text of the ‘plasma monitoring’ section but not in the secondary objectives.
-
Reference to ‘antipsychotics, anticonvulsants, antidepressants and anxiolytics’ was removed in the ‘Concomitant Medication’ section as there was no evidence to support those types of drugs being prohibited medication.
-
The 48-hour minimum time period was removed from the consenting period to ensure that patients – particularly those at risk of drug overdose – were able to access the study more quickly.
-
The text in inclusion criterion 5 – ‘a Morphone 2000 [opioid]’ (Alere, Loughton, UK) was removed, as that type of UDS cup was no longer being used in the study.
-
The note about failing the naloxone challenge in criterion 6 of the inclusion criteria was changed from having a 1-month minimum time period during which a patient can return to a patient being able to return when it was clinically indicated. This was because there was no evidence that a 1-month minimum time period was required.
-
Exclusion criterion 2 was amended to make it clearer that patients were not to be excluded from the study if they fail the breathalyser test.
-
The text in exclusion criterion 4 – ‘a Morphone 2000 [opioid]’ was removed, as that type of UDS cup was no longer being used in the study.
-
In exclusion criterion 5, the word ‘opiate’ was replaced with the word ‘opioid’ as it was the more correct word to use in this instance.
-
Exclusion criterion 18 was amended as a result of concern about potentially vulnerable patients not having naltrexone to carry them through the screening period. Some potential patients needed naltrexone during the screening period as they would have recently been released from prison and the normal clinical practice was to give these patients naltrexone to prevent overdose. If they were not given naltrexone, the risk of overdose increases greatly.
-
Exclusion criterion 20 was amended to make the wording was more in line with clinical practice.
-
There was the addition of new exclusion criterion, number 22. This exclusion criterion was to stop patients entering this study if they were on other studies.
-
A ‘Patient Identification Centres’ section was added as to better reflect recruitment practices.
-
In the ‘Primary Effectiveness Parameters’ the analysis model was changed from an analysis of covariance model to a regression model.
-
-
Amendment 4 (11 March 2016):
-
The primary care sites [general practitioner (GP) practices] in the West Midlands trust were added as non-recruiting sites as they covered the GP practices that were administering the insertion of the naltrexone implant.
-
There was a change in the language of the exclusion criteria to allow for a holding dose of naltrexone if there was a length of time between randomisation and the start of treatment.
-
The recruitment flyer was added to help increase recruitment to the study.
-
Support costs
Trials with participants with addictions are challenging. Experienced staff were needed to be able to deal with the complicated issues of the population group. To this end, resource equivalent to 1.0 whole-time equivalent (WTE) band 7 research nurse, a 1.0 WTE band 8 research nurse and a 0.05 WTE clinician were budgeted into the cost of the trial.
Patient and public involvement
The original study proposal was reviewed and endorsed by a member of the public through INVOLVE-related activities. The former service user was a member of the Mental Health Research Network and became an independent member of the TSC. He provided input into the conduct of the trial, for example by reviewing literature to be given to patients and their families (e.g. patient information sheets and patient newsletters).
Site initiation
Site teams from all participating sites attended a site initiation meeting prior to the commencement of patient screening. The purpose of these meetings was to present the background and rationale for the NEAT trial and to discuss delivery of the protocol, including screening and recruiting patients, delivery of the treatment, data collection and validation, and safety monitoring. The operational challenges of conducting the trial at sites were discussed in detail, including strategies for ensuring effective communication within the unit. The principal investigator (PI) from each participating site was required to attend the meeting. If key research staff were unable to attend, or new staff came into post, additional site initiation meetings were conducted as required, either at sites or via teleconference. A standardised slide set from the site initiation meetings was circulated to facilitate internal training within a participating site.
Investigator site file
An investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the trial and included the approved trial protocol; all relevant approvals (e.g. local NHS permissions); a signed copy of the clinical trial site agreement; the delegation of trial duties log; copies of the approved patient information sheets, patient consent form and personal/professional consultee agreement forms; and all standard operating procedures, for example for screening participants, for obtaining informed consent or consultee agreement, for randomising patients, for delivery of the intervention and for collecting and entering data on to the secure, dedicated, electronic case report form (eCRF). The site PI was responsible for maintaining the investigator site file. Responsible staff at sites were authorised to carry out trial duties (e.g. consenting, delivering the intervention) by the site PI on the delegation of trial duties log. This included a confirmation that the individual had been adequately training to carry out the specific duty.
Site management
Site monitoring visits
At least one routine monitoring visit was conducted by the KHP-CTO at all participating sites that recruited a patient during the trial. During the site visit, the investigator site file was checked for completeness, that is, that all essential documents were present, that the patient consent forms and personal/professional consultee agreement forms were checked to ensure that the relevant correctly completed form was present for every patient recruited into the trial and that patient case report forms were checked against the source data for accuracy and completeness. After the visit, the PI and site team were provided with a report summarising the documents that had been reviewed and the actions that were required by the site team. The site PI was responsible for addressing the actions and reporting back to the KHP-CTO. Additional visits were conducted on a risk-based approach, using recruitment rates, data quality and adherence to the protocol as central monitoring triggers.
Inclusion criteria
Inclusion criteria for the study were intended to be as close to clinical practice as possible. Each participant in the trial had to meet all the following criteria:
-
be aged ≥ 18 years
-
be able to demonstrate a verbal understanding of the study patient information material, able to provide written consent and able to understand and confirm willingness to comply with the protocol
-
have a diagnosis of OUD based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) in the past 12 months, as assessed at baseline
-
be completing or have recently completed an inpatient or outpatient treatment for opioid detoxification or has been completely and continuously abstinent from all opioids for at least 7 days
-
have no tolerance to opioids, as verified by a negative urine toxicology screening test prior to randomisation (using an instant result immunoassay device)
-
pass a naloxone challenge test (to confirm zero opioid tolerance by demonstrating no clinical sign or subjective report of opioid withdrawal before randomisation and prior to implant procedure) (individuals failing screening were allowed to enter screening as clinically indicated)
-
be voluntarily seeking opioid antagonist treatment for OUD
-
live in stable/secure accommodation in the community
-
have a personal (mobile/cellular) phone and are able to nominate at least one locator individual (e.g. a family member, friend or recovery mentor) with a verifiable address and a telephone number to assist with the arrangement of follow-up appointments as required
-
if female, not be pregnant or breastfeeding and agree to use a birth control method [oral hormonal contraceptives, barrier (condom or diaphragm) or Nexplanon implant (Merck, Kenilworth, NJ, USA)] for the duration of the study.
Exclusion criteria
Otherwise eligible individuals who met any of the following criteria were excluded from the study:
-
have a clinically significant medical condition or observed abnormalities on physical examination or laboratory investigation, including but not limited to:
-
uncontrolled hypertension
-
significant heart disease (including angina and myocardial infarction in past 12 months)
-
any ECG/cardiovascular abnormality that, in the investigator’s judgement, is clinically significant
-
-
suffer from severe alcohol dependence and/or alcohol withdrawal (by clinical assessment)
-
currently undergoing opioid withdrawal syndrome
-
test positive for presence of opioids in urine (i.e. indicating current opioid use) prior to randomisation (using an instant result immunoassay device)
-
have a clinical diagnosis of opioid dependence syndrome (F11.2 on the DSM-5) with current physical dependence such that an antagonist medication (e.g. naloxone, naltrexone) could precipitate a withdrawal syndrome
-
receive a positive naloxone challenge test at randomisation (confirming opioid use) or having an absence of a recorded result from a naloxone provocation test
-
have acute hepatitis taken as clinical jaundice on examination and/or blood bilirubin level above the normal range for local reference criteria or aspartate aminotransferase or alanine aminotransferase levels (more than three times the upper limit of the normal range)
-
have hepatic insufficiency (taken as more than three times the upper limit of the normal range of aspartate aminotransferase or alanine aminotransferase levels)
-
have severe renal impairment evaluated by clinical decision
-
have known Icenko-Cushing syndrome or require investigation if suspected Cushingoid features/symptoms
-
have systemic mycoses
-
have a clinical history of glaucoma
-
have a clinical history of osteoporosis
-
be pregnant, have a positive or unclear pregnancy test result, or intend to try to become pregnant during the study period or be sexually active without using a birth control method [oral hormonal contraceptives, barrier method (condom or diaphragm) or Nexplanon implant] for the duration of the trial
-
be currently breastfeeding
-
have a history of hypersensitivity to opioid receptor blockers (naloxone and naltrexone formulations) and other components of the formulation
-
have a history of hypersensitivity to triamcinolone or related compounds
-
be currently taking oral or depot naltrexone therapy or have been enrolled in any form of naltrexone therapy within 90 days prior to study screening, apart from treatment given by trial team between screening and the start of treatment
-
be undergoing current criminal justice involvement with legal proceedings (not including current probation supervision) and, in the opinion of the clinical worker, an expectation that the participant would fail to complete the study protocol owing to reincarceration or relocation from the centre’s catchment area
-
be currently (past 30 day) suicidal planning or have recently (past 6 months) attempted suicide
-
have an active, uncontrolled severe mental illness (e.g. psychosis, bipolar I disorder, schizoaffective disorder) and/or a history or evidence of organic brain disease or dementia that would compromise the participant’s ability to comply with the study protocol
-
be currently participating in any interventional trial or completed participation in any interventional trial (which in the view of the chief investigator might interfere with the NEAT trial) within the past 3 months.
Screening and recruitment
Following attendance at a site initiation meeting, screening and recruitment were commenced at participating units once the clinical trial site agreement had been signed and all necessary approvals were in place.
Potentially eligible patients were identified and approached by authorised members of staff about taking part in the trial. Information about the trial was provided to the patient, which included the purpose of the trial, the consequences of taking part or not, data security and funding of the trial. This information was also provided in a patient information sheet, along with the name and contact details of the local PI, which was given to the patient to read before making their decision to take part, or not, in the trial.
Informed consent
Potential participants were approached by a member of the clinical team. Each screening procedure was overseen by the centre PI or a medical officer reporting to the PI. Individuals failing screening were allowed to enter screening again as clinically indicated.
A study doctor or trial nurse implemented the enrolment procedure and obtained informed consent. If the taking of consent had been appropriately delegated to a non-physician, patients were offered the opportunity to speak with the study doctor, who documented that they had confirmed the patient’s eligibility in the medical notes before the patient was randomised. The study information sheet was read to the potential participant and discussed to ensure that he/she fully understood the purpose and key conditions of the trial, what is required and the risks and benefits arising from taking part. Each interested participant received an informed consent document with participant information and was asked to read the information and ask questions.
If the patient wanted to participate, he or she was required to sign the informed consent document prior to the conduct of any study-specific clinical procedures. This document was witnessed and signed by the clinical worker.
In addition (and not a requirement of participation in the trial), participants were asked if they were also willing to participate in the collection of venous blood samples at intervals over the course of their treatment to enable the study of blood levels of naltrexone and its metabolites. If participants agreed to participate, a written record of their consent was additionally collected (as earlier). This was considered separately.
Randomisation and allocation procedure
Randomisation was requested by study sites online using a bespoke web-based randomisation system hosted at the King’s Clinical Trials Unit (KCTU).
Once baseline assessments were complete, the individuals were randomised to one of the three treatment arms. Randomisation was at the patient level and was performed using an online randomisation system set up by the KCTU at the Institute of Psychiatry, Psychology & Neuroscience at KCL in London. Recruiting centre research staff randomised participants to one of the three arms of the study (ratio 1 : 1 : 1), stratifying by clinical centre, prison or community referral and recent cocaine use (yes or no), using randomly varying block sizes via the online randomisation system based at the KCTU.
Only study site staff authorised by the trial manager were given login details to the randomisation system. Authorised staff were allocated a username and password for the randomisation system. Once a patient was consented, all baseline data were collected and eligibility was confirmed, the staff member would log in to the randomisation system and enter the patient’s details. Once randomised, the system automatically generated confirmation e-mails to key staff, with or without treatment allocation information depending on their role in the study.
Screening log
To enable full and transparent reporting for the trial, brief details of all patients who met the eligibility criteria or who met all of the inclusion criteria plus one or more of the exclusion criteria were recorded in the screening log. The reasons for eligible patients not being recruited were recorded, which included the patient declining the invitation to take part, the patient being excluded by the treating clinician and logistical reasons. No patient identifiers were recorded in the screening log.
Treatment groups
Three hundred recently detoxified, formerly dependent heroin users were to be randomised to one of three conditions to receive (on site and supervised) one of the following:
-
thrice-weekly oral active naltrexone tablets plus placebo extended-release naltrexone at the start of treatment; or
-
oral placebo plus active extended-release naltrexone; or
-
oral placebo naltrexone plus placebo extended-release naltrexone.
Each condition was delivered over 12 weeks. All participants received standard NHS psychological interventions (weekly individual counselling) and a behavioural protocol incentivising clinic attendance to receive trial medication and complete research assessments.
Dosing regimen
Oral medication was administered under direct supervision in the outpatient clinics on Mondays (100 mg), Wednesdays (100 mg) and Fridays (150 mg, a higher dose to last until Monday) for the first 4 weeks. Oral medication during weeks 1–4 was directly observed. Small doses were given as takeaway medication if clinic attendance was impossible (e.g. owing to court appearances or urgent hospital appointments). Contingent on good adherence during the first month, patients were able to self-administer oral medication in weeks 5–12, dispensed on a week-by-week basis and contingent on attendance at the clinic three times a week to complete research measures and return packaging and report dosing. If there were any adherence problems, the patient was supervised for 2 weeks and would return to self-administration if adherence improved.
The single iGen/Atral-Cipan device (iGen/Atral-Cipan, Castanheira do Ribatejo, Portugal) was administered on a day-patient basis by a centre doctor (a local GP or hospital physician) appropriately experienced in general practice minor surgical procedures. A clinical consultant with extensive experience in these procedures was secured to guide the training programme. The implant procedure took approximately 30 minutes in an appropriate clinical facility attached to each centre with one of the two trial nurses assisting. A single-use minor surgical pack was used for each procedure.
Each participant was scheduled to receive the following study interventions:
-
1 × iGen/Atral-Cipan (extended-release naltrexone) implant (765 mg) or matching placebo at day 0 of study week 1
-
3 × oral naltrexone tablets (2 × 50 mg, Monday and Wednesday; 3 × 50 mg, Friday) or matching placebo at day 0 of study week 1 (for 12 weeks), directly observed for the first 4 weeks and then patient reporting self-consumption for next 8 weeks when attending clinic to complete research measures (the higher dose given on Fridays was to cover the weekend period).
The oral placebo tablet had the same excipients as the active medication. The tablet core contains lactose anhydrous, lactose monohydrate, microcrystalline cellulose and magnesium stearate. Each tablet was film-coated with Opadry® II Yellow (Colorcon Ltd, Dartford, UK) and purified water phEUR.
Case management
-
1 × weekly standard clinical case management sessions (for 12 weeks) using mapping-based task- and goal-setting tools and a general relapse-prevention skills training and craving coping approach.
Participants received a package of best supportive care with 12 weekly sessions of practical, manual-guided, personal goal-setting and relapse-prevention oriented counselling with a clinic keyworker. Each patient also had appointments with their prescribing physician (the clinic centre PI) monthly or more frequently if required.
In NHS outpatient addiction clinics, each patient was assigned a keyworker to provide case management and to support the patient through their intervention pathway across regular clinic appointments. The keyworker gave practical advice and applied psychological techniques, building motivation to reduce drug-related harms and prevent relapse, and organised required access to community services. A practical goal-setting and relapse-prevention protocol was delivered based on node–link mapping techniques to provide an effective method of helping the patient identify personal goals and monitor tasks. Mapping is a counselling tool that has been adapted in the UK and reflects four key elements of the counselling process:
-
Communication – using maps can provide a clear visual representation to help the communication skills of the patient.
-
Focus – maps provide a way to cluster and summarise information to guide and focus a discussion and maintain attention. Evidence suggests that maps help counsellors and patients maintain their focus.
-
Producing ideas – node–link maps can provide a strategy for idea generation and may also facilitate causal thinking by making patients examine what influences their behaviour or what may happen next. This process may be most useful when therapists and patients are struggling to remember details, or need a fresh approach.
-
Memory – memory for session information is related to the effectiveness of counselling. Node–link maps have been shown to enhance the recall of information in both educational and clinical settings.
Reinforcement protocol
-
Three weekly behavioural reinforcements to attend the clinic for oral naltrexone doses and to complete assessments in each of the 12 weeks, with an ascending voucher-based schedule (contingent on attendance and ingestion of medication).
Given the well-recognised problem of oral naltrexone adherence, a clinic attendance reinforcement protocol was used to maximise adherence to trial medication. This was as recommended by NICE. 15 The theoretical model underpinning this approach is contingency management (CM), a form of behaviour therapy in which a tangible reinforcement, contingent on a sought behaviour, is elicited from a participant. This, in turn, increases the probability of a subsequent desired response. Research in the target populations indicated that one of the most effective protocols links each successive behaviour elicited with an increase in the level of reinforcement, thereby increasing motivation.
Effective CM interventions have the following features. First, the clinician arranges the environment so that target behaviours (e.g. drug abstinence, clinic attendance, medication compliance) are readily detected. Second, incentives are provided when the target behaviour is demonstrated. Third, incentives are withheld when the target behaviour does not occur. In addition to three randomised controlled trials of oral naltrexone compliance using CM techniques for opioid dependence, the meta-analysis by NICE indicates a medium to large effect. 15
In the NEAT trial, an incentive was offered to each participant for attending the clinic. This was a trial nurse-administered, voucher-based reinforcement protocol, contingent on attendance to screening visits and then thrice-weekly during weeks 1–12 to provide urine samples and complete research measures. Participants received non-cash high-street store vouchers that could be exchanged for recovery-appropriate goods and services. Starting at a low level (£5 in value), the reinforcement value increased at a set rate for each attendance. If a participant attended for each of their 37 clinic appointments, they received vouchers worth a total of £400.
Outcome measures
Primary outcome measure
The primary outcome was the proportion of the thrice-weekly urine toxicology samples that were negative for heroin during the 12 weeks of treatment. The number of negative samples collected in the 12-week treatment period was used to calculate the proportion for each participant. The denominator, the maximum number of samples, was 36. A negative urine result was indicated by a negative result on a temperature-sensitive, instant-result immune assay urine drug test; this was a qualitative result.
Secondary outcome measures (for primary outcome paper)
-
Treatment retention.
-
Adherence to the oral study medication and psychological interventions, which can be ascertained via the dosing schedule and clinic visits for research measures.
-
Heroin and cocaine craving scores [measured by the adapted Minnesota Cocaine Craving Scale (MCCS) and adapted MCCS for heroin] at weekly intervals from baseline to 12 weeks post randomisation, and at 12, 16, 24 and 36 weeks.
-
Self-reported heroin, cocaine and benzodiazepine use (and their active class metabolites via urine drug screening). This was assessed during weeks 1–12 post randomisation using the 7-day drug and alcohol use self-report form. In the follow-up stage, data were to be collected at weeks 16, 24 and 36 for the 28 days prior to the visit using the 28-day drug and alcohol use self-report form.
-
Alcohol use (measured same as above).
-
Injection health risk behaviour [as measured by the HIV (human immunodeficiency virus) Risk-taking Behaviour Scale questionnaire at 12 and 36 weeks post randomisation; a total score is obtained for this measure] and psychological health (depression and anxiety symptoms) (as measured by Patient Health Questionnaire-9 items and Generalised Anxiety Disorder-7 questionnaire at baseline, 12 and 36 weeks post randomisation).
-
Health-related quality of life (measured by the EuroQol-5 Dimensions, five-level version, and the Work and Social Adjustment Scale), collected at baseline and at 12 and 36 weeks post randomisation.
-
Safety data and outcomes. Serious adverse events (SAEs) were to be defined in accordance with the EU Clinical Trials Directive. 16
Safety monitoring
In each clinical recruitment site, patients were asked to give blood samples for liver function (hepatotoxicity) testing during screening and monthly over the course of active treatment (weeks 4, 8 and 12). Samples were analysed at the local hospital pathology service, with the following clinical biochemistry assays conducted: albumin, bilirubin, liver transaminases (aspartate aminotransferase/alanine aminotransferase/serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase) and transaminases.
Safety reporting followed the requirements described in The Medicines for Human Use (Clinical Trials) Regulation 2004: SI 2004/103117 and the EU Directive 2001/20/EC. 16 Each participant was given a study identification (ID) card, which described the trial and provided the following information: some cough and cold medicines containing opiates may not work as well as they should and alternatives are recommended, emergency pain relief following an accident may not be achieved using opiates, taking an extremely large dose of heroin to overcome naltrexone blockade could result in serious overdose, and there may be sensitivity to small doses of opiates after discontinuing naltrexone. The patient ID card listed telephone contact information to enable emergency unblinding. A 24-hour emergency code break was available through Emergency Scientific and Medical Services, London, UK.
It is possible that the naltrexone implant procedure led to local site infection or other complications. Prophylactic antibiotic medication was able to be used post surgery and participants were monitored and checked by the trial nurse on each clinic visit and by each centre PI (physician) each month. Site inflammation was man-managed on a case-by-case basis and potentially involved steroidal anti-inflammatory treatment. All SAEs and non-SAEs were identified.
All adverse events (AEs) were recorded in the eCRF and reported, as part of routine reporting throughout the trial, to the Data Monitoring and Ethics Committee and Research Ethics Committee. AEs that were assessed to be serious (i.e. prolonging hospitalisation or resulting in persistent or significant disability/incapacity), life-threatening or fatal – collectively termed SAEs – were reported to the KHP-CTO and reviewed by the PI. SAEs that were unspecified and considered to be possibly, probably or definitely related to the trial treatment were reported to the Research Ethics Committee within 15 calendar days of the event being reported.
The chief investigator acted as custodian for the trial data. The following guidelines were strictly adhered to:
Baseline data were collected and entered by researchers in each study site prior to randomisation. Each participant was assigned a unique trial ID number via the InferMed MACRO (Elsevier, Amsterdam, the Netherlands) eCRF system hosted at the KCTU at the start of the assessment process. This number was written on all clinical assessment forms, datasheets and databases used to record participant data. Trial data were first entered on to paper source datasheets provided to each centre during the preparation phase. The research team endeavoured to minimise the use of paper. A hard copy of a record sheet linking patient identity, contact details and trial ID number (including medication pack number) for all participants was kept at each site. This was placed securely in a locked filing cabinet separate from datasheets. All data were kept secure and maintained in accordance with the requirements of the Data Protection Act and archived locally according to the KHP-CTO Archiving SOP and the host institution’s additional procedures.
Randomisation
Data were collected to enable patients to be randomised and included confirmation that the patient met all of the inclusion criteria and none of the exclusion criteria.
Baseline
The following data were collected at baseline to enable follow-up and to describe the patient population.
Patient demographics:
-
age
-
sex
-
ethnicity.
Stratification factors:
-
clinical centre
-
prison or community referral
-
cocaine use (yes or no).
Intervention period
Data were collected thrice weekly throughout the 12-week intervention period to monitor adherence to treatment allocation (active extended-release naltrexone and placebo oral naltrexone vs. placebo extended-release naltrexone and active oral naltrexone vs. placebo extended-release naltrexone and placebo oral naltrexone) and to describe and cost delivery of the intervention.
Follow-up
Participants completed their primary end point, the proportion of opioid-negative urine samples, at the end of the 12-week post-randomisation period (denominator 36). Urine samples were taken three times a week for 12 weeks.
Secondary outcome results were measured at 12 weeks from randomisation and at 16, 24 and 36 weeks’ follow-up.
Data management
Data management was an ongoing process. Data entered by sites on to the eCRF were monitored and checked throughout the recruitment period to ensure that data were as complete and accurate as possible.
Two levels of data validation were incorporated into the eCRF. The first was to prevent obviously erroneous data from being entered, for example entering a date of birth that occurred after the date of randomisation. The second level involved checks for data completeness and any unusual data entered, for example a physiological variable, such as blood pressure, which was outside the predefined range. The KCTU could generate data validation reports, listing all outstanding data queries, at any time via the eCRF. The site PI was responsible for ensuring that all the data queries were resolved. Ongoing data entry and validation at sites was closely monitored by the trial manager and any concerns were raised with the site PI.
Sample size
Power calculation
Estimated treatment effect size and retention to guide the required number of participants for NEAT was based on best available trial evidence and meta-analysis. The trial was designed to compare the effectiveness of extended-release naltrexone and oral naltrexone on an intention-to-treat basis at 12 weeks post randomisation. There were two comparisons: extended-release naltrexone versus placebo and extended-release naltrexone versus oral naltrexone. Based on a 2007 Health Technology Assessment systematic review20 and the naltrexone depot trial by Comer et al. ,21 the following assumptions were made:
-
The mean percentage of heroin-free UDSs at 12 weeks post randomisation would be approximately 30% (0.30) in the placebo and oral naltrexone treatment arms, respectively, and 55% (0.55) in the extended-release naltrexone treatment arm. This would be measured with a denominator of 36, assuming missed screens are positive.
-
The standard deviations of the treatment groups would be of a similar magnitude. 3 The common standard deviation was estimated to be 30.
-
The minimal clinically significant difference between the extended-release naltrexone and oral naltrexone/placebo groups would be a 25-point difference in percentage points observable at 12 weeks post randomisation, equating to an effect size of 0.8.
-
There would be an expected 40% attrition rate based on previous trial data. 3
-
To control for multiple comparisons in the analyses for the main report, the Bonferroni correction was applied to the significance level, reducing it by a factor 2. Thus, the significance level for primary analysis was to be considered at 2.5%.
Estimated required sample size
With an anticipated 0.8 effect size, a common standard deviation of 30, expected attrition at 40% and testing significance at 2.5%, a sample size of 300 participants randomised on a 1 : 1 : 1 basis to the three arms (100 participants in each arm) had 98% power to detect a 25-point difference in the percentage of heroin-negative urine samples for the planned comparisons of active treatment arm extended-release naltrexone versus placebo and active treatment arm extended-release naltrexone versus standard oral treatment oral naltrexone.
Data analysis plan
Analysis principles
Analyses were to be carried out by the trial statistician (ER) who, was to remain blind whenever possible until the main analyses had been completed. In the first instance, data were to be analysed under intention-to-treat assumptions (i.e. analyse all those with data in groups as randomised irrespective of treatment received). Per-protocol analyses were then to be conducted to determine the influence of non-compliers. The significance level was to be 2.5% (two-sided) for the primary outcome analysis and 5% (two-sided) for secondary outcome analyses. Group difference estimates and associated confidence intervals were to be reported.
Data description
For progress reports to the Data Monitoring and Ethics Committee and the TSC, the Consolidating Standards of Reporting Trials (CONSORT) flow diagram was constructed (Figure 2 and see Figure 27). 2 This included the number of eligible patients, the number of patients agreeing to enter the trial, the number of patients refusing, and, by treatment arm, the number of patients not/inadequately/adequately treated or compliant/non-compliant, the number of patients continuing through the trial, the number of patients withdrawing, the number of patients lost to follow-up and the numbers of patients excluded/analysed.
FIGURE 2.
Template CONSORT flow diagram for NEAT trial. O-NTX, oral naltrexone; XR-NTX, extended-release naltrexone.

Planned analysis of primary outcome
The proportions of opioid-negative urine samples at the end of the study period (denominator 36) were to be analysed using general(ised) linear models, adjusted for treatment arm and stratification factors (centre, referral and recent cocaine use) modelled as fixed effects, with summary estimates for extended-release naltrexone versus placebo, and extended-release naltrexone versus oral naltrexone. The binomial distribution is the natural choice for analysing proportion data, but can be approximated only by a normal distribution for a large sample size. The assumptions for a linear regression may also not be met, and thus a general linear model may not be suitable. A logit transform may instead be required (using the logistic function) to model the proportion of negative urine samples. A small adjustment to the data (adding 0.5) may be required as there may be counts of 0 in the data.
It was proposed that urine samples be recorded as positive if patients did not attend (DNA) (or refused) clinic visits or refused to give urine samples. For other missing post-randomisation assessments, it was planned to implement multiple imputation based on chained equations [Stata® (StataCorp LP, College Station, TX, USA) command ice] to impute missing values for the urine samples, provided there was a reasonable assumption that the missing data mechanism was ignorable (i.e. at least ‘missing at random’).
Planned analysis of secondary outcomes
Treatment retention (time to withdrawal from trial treatment) for each arm was to be analysed as a time to event outcome. To begin with, the data were to be declared to be time to event using the ‘stset’ command in Stata. This entails specifying the failure variable and the dates when coming under and leaving observation. Cox regression (proportional hazards models) was to be used with right-censored time to event as the dependent variable and trial arm and prognostic variables (referral, cocaine use and study site) as explanatory variables. Censoring was to take place when a participant reached the end of the follow-up period. The assumptions of the Cox model were to be checked.
Adherence to treatment was to be described by the median and range of the number of clinic visits attended. A binary measure of adherence was to be constructed, in which adherence to treatment was to be classified as having taken at least half of their oral medication. Adherence rates were to be compared across the different treatment arms using a chi-squared analysis.
To assess the opioid, cocaine, amphetamine, benzodiazepine and alcohol use, the self-report and urine screening results had to be merged. Any report/evidence of drug use was taken (either on the self-report or the urine screen) as a positive result to form a binary variable. Each drug (heroin, cocaine, amphetamine and benzodiazepines and alcohol) was modelled as a separate outcome.
Treatment effects on all secondary outcomes that were measured repeatedly over the follow-up period (namely heroin and cocaine craving; self-reported heroin, cocaine, amphetamine, benzodiazepine and alcohol use; injection risk behaviours; and psychological health) were to be modelled using mixed-effects models, using generalisations of the linear mixed model to allow for non-normal data when necessary. These models would also be adjusted for the stratification factors. The mixed-effects models were to be fitted using maximum likelihood methods that are valid under the missing-at-random assumption. Exploratory moderator analyses were not to be considered for the primary publication.
Safety analyses were to be performed using chi-squared (Fisher’s exact) tests to describe any differences between treatment arms. The potential AEs that were anticipated were to be summarised separately, such as overdose and infection of the implant site. The most frequent AEs were to be summarised by proportions in each treatment arm, along with corresponding risk differences and confidence intervals.
A generalised linear model was to be used to analyse treatment differences in specific AEs, such as opioid poisoning (heroin overdose) or hepatotoxicity. The statistical modelling would feature the outcome measure(s) as the dependent variable with stratification factors, clinically relevant variables and treatment group featuring as covariates.
Data analysis performed
Owing to the small number of patients recruited into the NEAT trial (six patients), the statistical analyses performed were exploratory/descriptive rather than inferential, and no statistical tests were performed. A CONSORT flow diagram was constructed (see Figures 2 and 27). This included the number of:
-
patients screened
-
eligible patients
-
patients agreeing to enter the trial
-
patients refusing to give samples by treatment arm
-
patients not/inadequately/adequately treated or compliant/non-compliant
-
patients continuing through the trial
-
patients withdrawing
-
patients lost to follow-up
-
patients excluded/analysed.
Owing to the small numbers, baseline variables were described overall, rather than by treatment arm. The following baseline variables were described: age, sex, ethnicity, duration of addiction history, previous therapies and duration of treatment. The stratification variables, clinical centre and whether or not patients had used cocaine in the past 30 days were described. The following clinical measures were also described: DSM-IV criteria for major depressive disorder, post-traumatic stress disorder (PTSD), panic disorder or generalised anxiety disorder (GAD), medical history and concomitant medications.
The number of negative urine samples collected in the 12-week treatment period was used to calculate the proportion of negative urine samples for each participant, using a denominator of 36. For patients who DNA or refused clinic visits or to give urine samples, it was assumed that these samples were positive.
The lapse and relapse patterns of each patient (based on urine samples and self-report) were summarised numerically and presented graphically over the 36 urine samples taken during the study period. Owing to the small number of patients, Kaplan–Meier curves could not be used here. Self-reported heroin use was also examined over the 12-week treatment period. Adherence and lapse–relapse were summarised by trial arm.
The first heroin lapse was summarised for each patient who had a heroin lapse–relapse, in terms of the main reason that they used heroin and whether or not they used other opioids, cocaine, amphetamine-type stimulants, alcohol or benzodiazepines on that day. Heroin use post treatment was also summarised when available.
Adherence to the oral study medication was ascertained via the dosing schedule and clinic visits for research measures. A patient is classified as being ‘compliant’ if they take at least 50% of their oral study medication. Adherence to treatment was described by the median and range of the number of clinic visits attended. The number of therapy sessions attended by each patient was summarised.
Adverse events were summarised by intervention arm and time point, in particular implant site infections.
Patient treatment guess and the clinician’s guess of the treatment for each patient (for both the implant and the tablets) were also summarised.
Economic evaluation
Given that implanted extended-release naltrexone is currently an unlicensed medication in Europe, and is more expensive than oral naltrexone, the relative cost-effectiveness of extended-release naltrexone and oral naltrexone treatments was to be assessed by including opportunity costs for stakeholders and comparing ratios of incremental opportunity costs (for all stakeholders) and incremental outcome (health-related quality of life). Costs of the study interventions and external health services and expenditure by the social and criminal justice sectors were to be combined with the primary clinical outcome measure and quality-adjusted life-years to produce incremental ratios that will determine relative cost and cost-effectiveness.
Economic outcome assessments were to be carried out after the 12-week treatment period and at the 36-week follow-up. The a priori primary economic outcome measure was to be quality-adjusted life-years using the EuroQol-5 Dimensions. The economic evaluation was to take a broad policy perspective, including costs borne by hospital and community health and social services and the criminal justice sector, plus the costs of criminal activity. Detailed information on the resources associated with the treatments, including study medications, equipment, dispensing services, urine tests, nurse time, and contact with key workers, medical, nurse and psychology staff, was to be collected from clinical records. Resources external to the clinics, including staffed/supported accommodation, hospital contacts, community health and social services, criminal justice sector resources and crimes committed, were to be collected in interviews with study participants at baseline, after the 12-week treatment period and at the 36-week follow-up.
Chapter 3 Problems
Acquisition of the ultra-long-acting naltrexone implant/depot injection and naltrexone oral tablet
Difficulty of sourcing naltrexone implant as two established companies already producing licensed product could not accommodate study requirements
The original contract date to start the NEAT study was 1 September 2012. Owing to protracted difficulties with sourcing IMP for the study, a contract start date variation was instituted and the contract start date was revised to 1 September 2014.
The original study was proposed using a Russian naltrexone implant called Prodetoxon (NPK ECHO, Moscow, Russia), which had the merit of being the only naltrexone implant for which any national licence had been granted. We had been assured by the company (Fidelity International, London, UK) that supplies of both the active implant and the placebo implant would be provided. However, as we moved forward with plans for actual implementation of the trial, we experienced repeated failure to provide information and a failure to deliver the products when requested. Communication difficulties came to a head and we were unable to elicit a response from the company via e-mail, telephone or by writing. As a result, the situation was reviewed with NIHR and it was agreed this was not a feasible basis on which to proceed.
The next option was to consider switching to the use of a depot injection version of naltrexone (Vivitrol), which had been licensed in the US for alcohol treatment and for which a licence for treatment of opiate addiction had also subsequently been obtained. Negotiations with the US company (Waltham, MA, USA) continued, with a view to securing donations of a supply of both the active depot injection and the placebo. However, the company was not willing to provide the requested trial supplies unless the planned trial design was changed. As discussions continued, the company made it clear that unless we were willing to change the study design, should we choose to purchase the active depot injection and manufacture a matched placebo for the trial independently, they would obstruct any efforts to purchase the necessary stock of depot injection. This was considered unacceptable and, in discussion with NIHR, the decision was made to seek an alternative product.
Less satisfactory options were explored with NIHR, such as removal of the double-blind, double-dummy design so that the study could proceed without any stock of placebo implant, but this was clearly a less satisfactory pathway and was not pursued.
Difficulties finding a manufacturer
We then set out to find a manufacturer of the specially produced naltrexone implants and placebo implants. This process was time-consuming, but we eventually found a manufacturer in the EU that met the required standards (iGen in Portugal). There was then further delay as the drug had to be manufactured rather than simply purchased off the shelf.
Difficulties with the scheduled supply of the oral naltrexone tablets
Unexpectedly, some difficulties were then experienced with the scheduled supply of the oral naltrexone tablets (which are licensed medicinal products in the UK and many other countries) when we were informed by the pharmaceutical company that it was uncertain whether or not it was going to continue to produce and supply the medication, as the quantities being prescribed were very low and hence the profit margins were not sufficient to maintain the business basis. This caused significant concern as the arrangements for placebo naltrexone tablets were based on the product from this particular manufacturer. An acceptable alternative arrangement for the supply of the oral naltrexone tablets from a different manufacturer was able to be organised quickly, and we were able to alter the arrangements for the oral placebo tablets so as to match the new supplier, but this took time and pushed back the recruitment start date.
Change in environment owing to retendering of trusts to third-sector providers
Retendering led to the loss of the Darlington site
A significant problem was the major distraction and destabilisation for all addiction treatment services in England caused by the extensive retendering and competitive tendering, and the associated contract negotiation and potential staff redundancy or staff transfer processes. Over the past few years, many community addiction services have been required to go through this retendering process, some on several occasions, whereby they are considered for possible continuity or, alternatively, for placement with another provider, such as a third-sector provider. The extent of the change is such that, as of 2015, more than half of all addiction services in England have been moved out of the NHS to a non-NHS third-sector provider organisation.
This has two profound implications for health-care research in England. First, it creates a major distraction and destabilisation of the clinical teams with whom we are endeavouring to negotiate secure commitment to participation in a challenging research trial for the next few years. This includes the possibility/reality of the original team failing to secure funding, plus the uncertainty of whether or not the new provider will have interest in continuing participation in the trial. This leads on to the second profound implication, which is that there is no/minimal history within these third-sector organisations of the expectations of participation in randomised trials, and no funding stream to support this. Moreover, in a competitive tendering environment, the expectation of additional commitment is generally considered unacceptable.
This retendering led to a major problem with the Darlington site, led by Dr Soraya Mayet. As the County Durham and Darlington NHS Foundation Trust was retendered to a third-sector provider, Dr Mayet had to move trust. She moved to the Humber NHS Foundation Trust, where she had a consultant post at her new site of Humber. This move appeared straightforward as she was still able to access referrals from local drug treatment services. However, it became clear to both Dr Mayet and us that the Humber cluster was not going to be able to recruit the expected 100 study participants over the assigned period. Dr Mayet and her research and development (R&D) office examined the local situation and they decided that they would not be able to secure even half of this number; accordingly, Dr Mayet was advised by her R&D office to withdraw from being a site for the NEAT trial. This loss of the third site was a large blow for the NEAT trial and a third site was never found to take the place of the Darlington/Humber site.
Retendering led to the Birmingham site having to move to a smaller catchment area
In August 2014 it was announced that Birmingham Drug Action Team, the largest such body in the UK, had decided to award the treatment contract to a new provider, starting in March 2015. There then followed a 6-month period in which the existing NHS provider had to organise the transfer of patients and a new provider had to start effectively from scratch in the city. This period was problematic, as many staff left their posts and were not replaced, and much of the clinical effort was devoted purely to keeping existing treatment provision running. The new treatment provider had an enormous task to take on 3500 OST prescriptions across the city while trying to recruit new staff, and struggled to find suitable buildings from which to deliver services.
In addition, the new provider was not a NHS provider, making governance arrangements for a CTIMP (Clinical Trial of an Investigational Madicinal Product) study extremely difficult. For example, there were no easily identifiable doctors to conduct the implant procedure, and no suitable and accredited premises in which to deliver it. All this meant that a large population of potential candidates were not available to the study.
Dr Ed Day moved to the neighbouring borough of Solihull, where the local NHS provider continued to run treatment services. Birmingham has a catchment area of 1 million people, whereas the catchment area of Solihull is 250,000 people, and so potential study participants were fewer. He also recruited in Wolverhampton, another neighbouring area with clinical services run by the NHS, which has a catchment area of about 350,000 people. However, there were some delays in getting the site open, most importantly surrounding the implant procedure. Finding a doctor qualified in minor operations/surgical procedures was difficult but achieved for both sites, but the main delay came in getting them trained to conduct the implant procedure. Both GPs wanted to see the procedure performed, and this meant waiting for an implant to be fitted in London on a day that one or both could attend.
There was a demand for implants in Solihull, but the number of people exiting OST drug free was very low. The throughput was sufficiently low that co-ordinating all the elements of the research assessment, medical review and implant procedure was very difficult with low staffing levels and high case-loads.
New third-sector providers were unable to engage in research
New third-sector providers are nervous and not especially motivated about allowing or engaging in research and appear to have some fundamental misunderstandings of its role. It was hoped that the third-sector providers would identify patients, provide information about the study, advertise the opportunity to participate in the study (e.g. via posters in waiting rooms), or put potential participants in touch with the NEAT researchers. After much negotiation, the study was granted approval for the actions above by many third-sector providers but in practice we received no engagement.
Further to this, there seems to be a hurdle that cannot be overcome when dealing with third-sector providers in that they cannot provide indemnity cover for the site. This seems to be a death knell for research at sites run by third-sector providers. This is because the sponsors of a trial put together the protocol for everyone to follow. If everyone follows the protocol but there is still a SAE, then the sponsor’s policy will cover the individuals regardless of whether they are NHS or private. If an individual was not to follow the protocol and caused bodily injury then it would be dealt with initially by the sponsor’s insurance as a claim but then would be given back to the NHS, private sector or the individual. NHS employees have CNST (Clinical Negligence Scheme for Trusts) cover and non-NHS employees would have to have their own indemnity cover, but the third-sector providers we were dealing with did not have this cover.
General practitioner implant insertion training, contract negotiation and approval
Protracted contract negotiation with both general practitioner surgery’s delayed recruitment start date at the Birmingham site
Owing to the nature of the extended-release naltrexone implant, a surgeon was required for the implant procedure. As the Birmingham site did not have a surgeon, GPs surgeries that had staff that could perform the implant procedure were found. As the GP surgeries were not part of the same trust as the Birmingham site, a subcontract between the sponsor, the site’s trust and the GP was needed. This seemed simple but in practice took much longer, much longer than one would expect, and delayed the site from opening to recruitment.
Miscommunication with the local Birmingham Research and Development office led to the necessary approvals being late
Adding the GP surgeries to the R&D application led to a lengthy delay in R&D approval for the Birmingham site. There was a miscommunication with the R&D department of the Birmingham site whereby the study team was instructed to add the GPs as separate sites in the R&D application. These new GP sites needed to be added to the protocol, and, thus, a substantial amendment needed to be submitted both the head ethics board as well as the MHRA. The information given to the team was incorrect and, in fact, the GP surgeries did not need to be added as separate sites and simply could have fallen under the umbrella of both the Birmingham site and the Birmingham R&D submission. The time taken to create a separate R&D submission, to add the sites to the protocol, and to obtain the subsequent ethics and MHRA submissions was very considerable and led to the Birmingham site being very late to open.
Delays in the time it took to train the general practitioners also led to delayed recruitment start date at the Birmingham site
The training of the GPs who were to be performing the naltrexone implant procedure at the Birmingham site proved to be difficult. To perform the implant procedure, the GPs wanted to observe an implant procedure being performed. Without this observation of an implant procedure, the Birmingham site could not recruit. Low recruitment at the London site meant that the GPs had to wait a significantly long time to witness an implant procedure.
A private clinic that performs naltrexone implant procedures was approached but a bid to have the GPs attend the clinic was unsuccessful.
Lack of referrals led to decreased recruitment
The loss of NHS inpatient clinics led to decreased referrals to the study
With the change of environment in the addictions field leading to the close of NHS inpatient clinics, the NEAT trial lost its major referral pathway. This pathway was expected to bring most of the patients to the trial; we were unable to recover from its loss and this is one of the main reasons the trial failed. There was discussion at the time of the loss whether to continue with the trial or not, but it was decided by the TMG to push on.
No pre-existing referral pathway for the patient group hurt the study
The fact that in a community setting there was no referral pathway for people who wanted abstinence treatment led to fewer patients being referred to the study at the treatment centres. This was remedied in time, and at Lorraine Hewitt House (London, UK) this pathway was created during the study. But this lack of a pathway and then lack of a culture of referral led to many fewer referrals than we expected.
The expected referrals from the prison system never eventuated
After the loss of the NHS inpatient clinics it was expected that the prison system would be able to pick up the slack of these lost referrals. It was expected that any abstinent drug user in a prison local to the study sites, when released, would be sent directly to that site. The vulnerability of these patients because of their increased likelihood of overdose certainly led the study team to believe that these patients would need to attend a clinic straight after release. This referral pathway never eventuated and was another major reason why the trial failed.
There was organisational inability to refer patients across trusts (Birmingham to Solihull)
After Dr Ed Day had to move to the smaller Solihull Trust, the research team hoped that the larger, and now tendered by a third-sector provider, Birmingham Trust could refer patients to the Solihull Trust. This was not achievable because there was no way for the Solihull Trust to pay for the treatment of a Birmingham patient; thus, the third-sector provider would be out of pocket.
Lack of referrals from third-sector providers
After making good advances with the third-sector providers working in the addictions fields, the Research Oversight Group at one of the largest third-sector providers in the addictions field approved the study and the field workers for that third-sector provider could refer patients directly to the London site. This approval took many high-level talks between members of the TMG, including the chief investigator, and their upper management. After receiving this approval, it was expected that many patients would be referred to the London site, and potentially in the future the Solihull site. However, after this extensive work, no referrals were received from this third-sector provider.
We also achieved geographic expansion for referrals from colleagues in third-sector providers in other London boroughs. We had agreements in place with third-sector providers of community addictions services to refer patients to the study, but again, after extensive work with these organisations, no referrals came the way of the site.
Patients
There was a substantial group of patients on opioid substitution treatment who wanted to join the study but could not lower their dose in time to become eligible
As no referrals came from the community or prison populations, the clinics had to rely on abstinent patients in their own clinics. An analysis of the London site’s screening log showed many patients who were still on methadone or buprenorphine and were not eligible for the study. A large number of these patients wanted to be abstinent, so the team had to wait for these patients to lower their doses to a point at which they could be eligible. These patients were known to the recruiting teams and were working their way down the doses needed to become abstinent. Owing to short staffing among the key workers at the site, patients did not always meet their own key workers and instead met covering key workers who were reluctant to reduce the dose of methadone and buprenorphine for a patient whom they had just met. It was difficult for these patients to reduce their dose to a point at which they were eligible for the study and no patients were recruited from this population group. If there was more time we would have captured the vast majority of this large patient group.
There was discussion within the team about using low-dose naltrexone alongside buprenorphine. It was proposed that patients are given a large dose of buprenorphine and a very low dose (i.e. 1 mg) naltrexone on the first day and no further buprenorphine, as the depot effect would last through at least the next 5 days, during which time the naltrexone dose would be steadily increased. Owing to time constraints, this treatment was not implemented, although it is food for thought for further research.
Problems in the prison setting
Protracted negotiations with the multiple levels of the Ministry of Justice, Her Majesty’s Prison Service and a local prison led to lengthy delays
As prisons are not part of the NHS, the in-reach was limited. Dealing with the myriad levels of bureaucracy to get approval for the study led to time delays. It was only when the chief investigator, Professor John Strang, met with the governor of a local prison that permission was given for the study to be discussed with potential patients within the prison.
Ideological differences concerning naltrexone use led to a complete stop of recruitment in a local prison
The governor of a local prison further endorsed the trial and allowed NEAT posters to be placed around the prison. This allowed patients who were not aware of the trial to approach members of the prison staff to enquire about the trial. Further to that, permission was granted for research nurses to enter the prison to discuss the trial with prisoners. They themselves were able to personally give the prisoners a NEAT flyer, discuss the trial with them and then either book an appointment for the prisoner or even meet them at the gate to escort them to the clinic to begin the screening process. Following this news, the Lorraine Hewitt House team were to hire a nurse with the specific idea of going to the prison to meet potential patients, explain the trial to them and book appointments for them when they are released from prison. We thought this was a significant achievement for the study and we believed this would unlock many potential patients to be recruited. Unfortunately, a third-sector provider based in the prison believed that abstinence therapy was the best treatment and effectively blocked entry into the prison.
Chapter 4 Results
Explanatory statement
Following the Health Technology Assessment meeting held on 2 December 2016, in which the difficulties of recruiting participants to the trial were discussed, a decision was made that the NEAT trial should be closed with immediate effect, apart from the continuation of provision of trial treatment and data collection for study participants already recruited into the trial plus analysis and write-up. A later decision was made to conduct a qualitative investigation of the perspectives of potential and recruited study participants (patients) and other key stakeholders, including clinical staff, at the study sites, either as a separate report or as a publication of these findings, with conclusions being made available later.
A brief final report is shown below. No statistical tests were performed owing to the small number of patients recruited into the trial. A brief description of the participants and their outcomes is provided below.
Description of the participants in the Naltrexone Enhanced Addiction Treatment trial
Six patients were recruited into the NEAT trial. All patients were recruited from the London site by community referral. The first patient was recruited on 2 February 2016 and the last patient was recruited on 26 October 2016. The patients were recruited in February 2016, April 2016, August 2016 and October 2016 (three patients were recruited in October 2016). Patients received their implants and first dose of oral medication 1–5 days after they were randomised. The last date of patient follow-up was 27 February 2017 (a 16-week visit).
Four males and two females were enrolled in the study. The patient ages were 28, 30, 32, 38, 44 and 55 years. One patient was white and five patients were mixed race.
The duration of addiction history ranged from 2 to 18 years. Two patients had previously had methadone maintenance therapy, five patients had previously had buprenorphine maintenance therapy and one patient had previously used Suboxone (Reckitt Benckiser, Slough, UK; sublingual buprenorphine–naloxone combination tablet) (Table 1). Two patients had used cocaine in the past 30 days at the time of randomisation but were not taking cocaine in a significantly problematic or dependent manner such as to constitute criteria for exclusion. No patients had previously had naltrexone relapse prevention medication.
| Patient | Treatment history | |||||
|---|---|---|---|---|---|---|
| Duration of addiction history (years) | Duration of treatment with methadone (years) | Methadone maintenance in past 12 months | Duration of treatment with buprenorphine (years)a | Buprenorphine maintenance in past 12 months | Other maintenance treatment for opiate dependence | |
| 1 | 9 | 7 | Yes | 7 | No | Suboxone for 7 years – not used in past 12 months |
| 2 | 9 | – | – | 0 | Yes | |
| 3 | 18 | 11 | Yes | 5 | Yes | |
| 4 | 2 | – | – | 0 | Yes | |
| 5 | 2 | – | – | – | – | |
| 6 | 9 | – | – | 1 | Yes | |
None of the patients met the Stuctured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders-IV criteria for major depressive episode, PTSD, panic disorder or GAD. Medical history and concomitant medications of the patients are summarised in Appendix 1.
Treatment allocation
Three patients (patients 1, 3 and 6) were allocated to extended-release naltrexone placebo and oral naltrexone placebo (double placebo). Two patients (patients 2 and 5) were allocated to active extended-release naltrexone and oral naltrexone placebo (oral placebo). One patient (patient 4) was allocated to extended-release naltrexone placebo (implant placebo) and active oral naltrexone.
Summary of patient behaviour during trial
Patient 1
Patient 1 was on the double placebo and had 28 out of 36 negative UDSs (for heroin), no positive UDSs (for heroin) and eight DNAs (Figures 3 and 4; see also Figures 15 and 16). This gives a proportion of 0.78. This patient did not disclose using heroin in the 12-week treatment period (or any other substance). This patient did not have any positive urine samples for any other substances during the 12-week treatment period. However, this patient disclosed using heroin for 6 days in weeks 12–16, 28 days in weeks 17–20, 23 days in weeks 20–24 and 28 days in weeks 25–32 (as well as other substances in the follow-up period). They did not disclose using heroin in weeks 32–36. Patient 1 reported having their first lapse during week 16 and used heroin ‘to feel less sad or bored’. They also took cocaine at this time (after heroin).
FIGURE 3.
Patient 1’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.
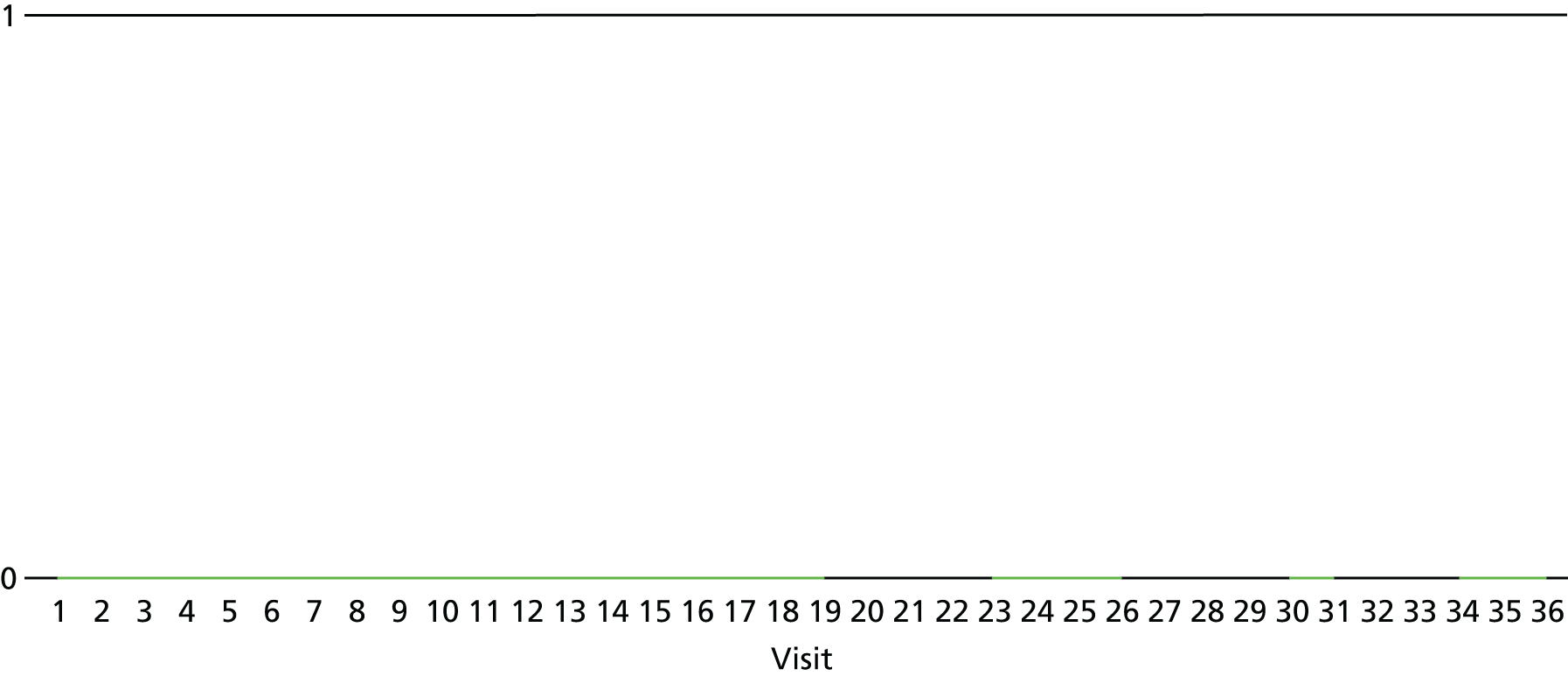
FIGURE 4.
Patient 1’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.

Patient 1 took all their study medication up to the end of week 8, missed two doses in week 9 and did not take their tablets in weeks 10–12.
At week 16, patient 1 had a positive urine sample for benzodiazepine. At week 24 patient 1 had a positive urine sample for heroin, cocaine and methadone. At week 36 patient 1 had a positive urine sample for methadone.
Patient 1 overdosed on heroin 17 weeks after randomisation. This patient also experienced redness, swelling and pain at the implant site, reported 1 week after the date of implant. Patient 1 also had pus reported at the implant site 2 weeks after implantation.
Neither the patient nor the clinician correctly guessed the study participant’s treatment allocation.
Patient 2
Patient 2 was on the active implant and oral placebo and had 34 out of 36 negative urine (for heroin), no positive urine (for heroin) and two DNAs (Figures 5 and 6; see also Figures 17 and 18). This gives a proportion of 0.94 for the negative urine. This patient did not disclose using heroin in the 12-week period (or any other substance) and did not fill out a ‘first heroin lapse’ form. This patient did not have any positive urine samples for any other substances. This patient did not disclose using heroin in the follow-up period and did not have any positive urine samples in the follow-up period. Patient 2 took of all their study medication for all 12 weeks.
FIGURE 5.
Patient 2’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.
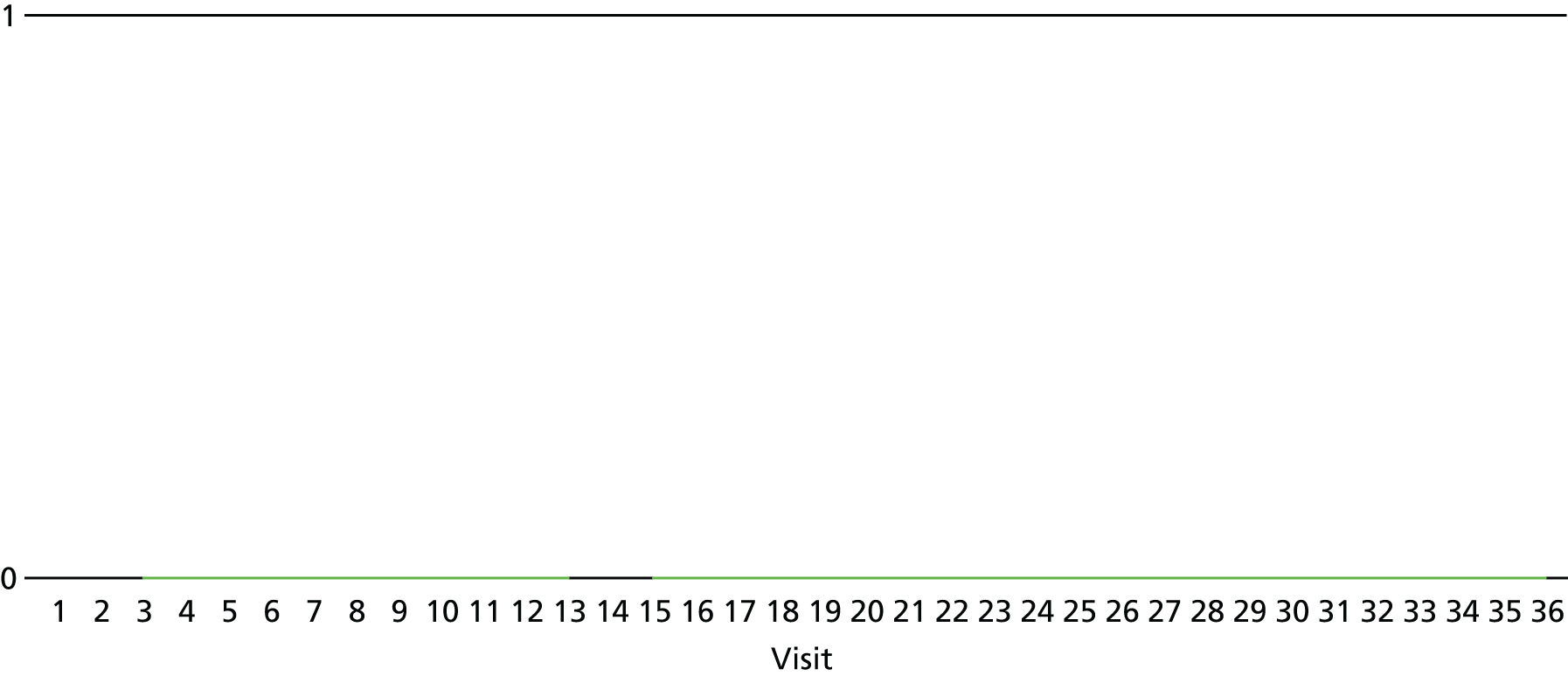
FIGURE 6.
Patient 2’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.
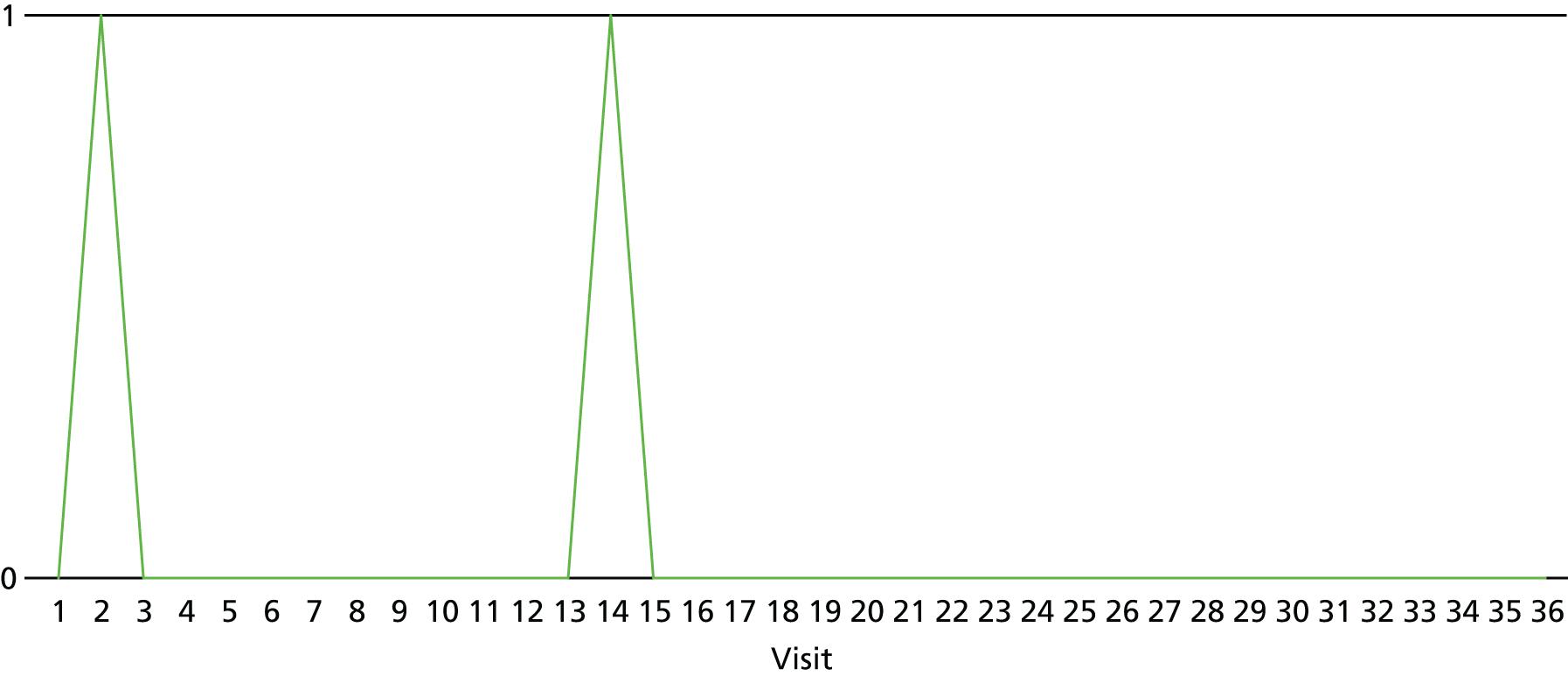
Patient 2 experienced intermittent burning and pain at the site of the implant, reported at 2 weeks, as well as at 26 days after the date of implant. The implant was reported to be unchanged at 5 weeks after implant date.
Neither the patient nor the clinician correctly guessed the patient’s treatment allocation.
Patient 3
Patient 3 was on the double placebo and had 14 out of 36 negative urine samples (for heroin), 8 out of 36 positive urine samples for heroin and 14 DNAs (Figures 7 and 8; see also Figures 19 and 20). This gives a proportion of 0.39 negative urine samples. Patient 3 reported their first heroin lapse to have occurred during week 4 (30 days after randomisation) but did not have any positive urine samples or self-report of substance use. They stated that they used heroin at this time ‘because they had already used something else’ and reported consuming alcohol that day.
FIGURE 7.
Patient 3’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.
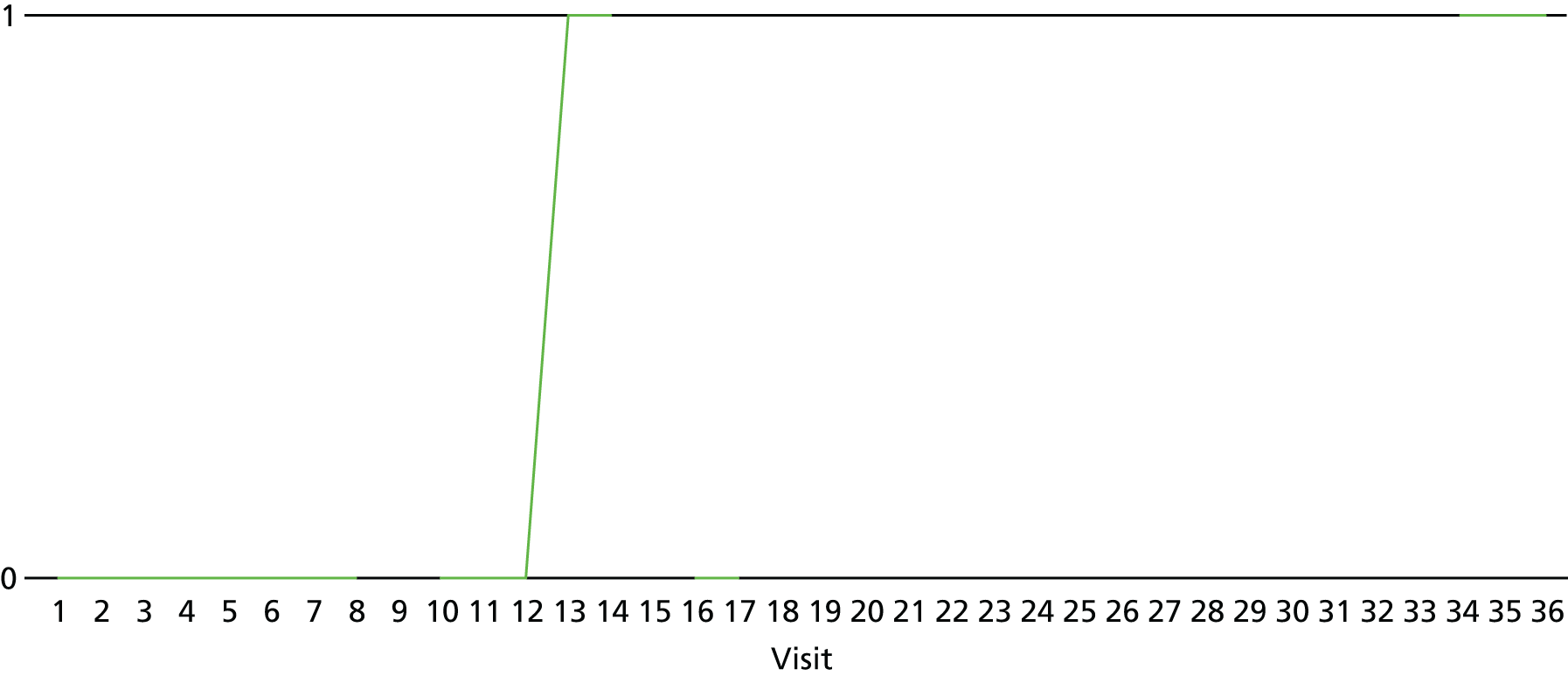
FIGURE 8.
Patient 3’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.
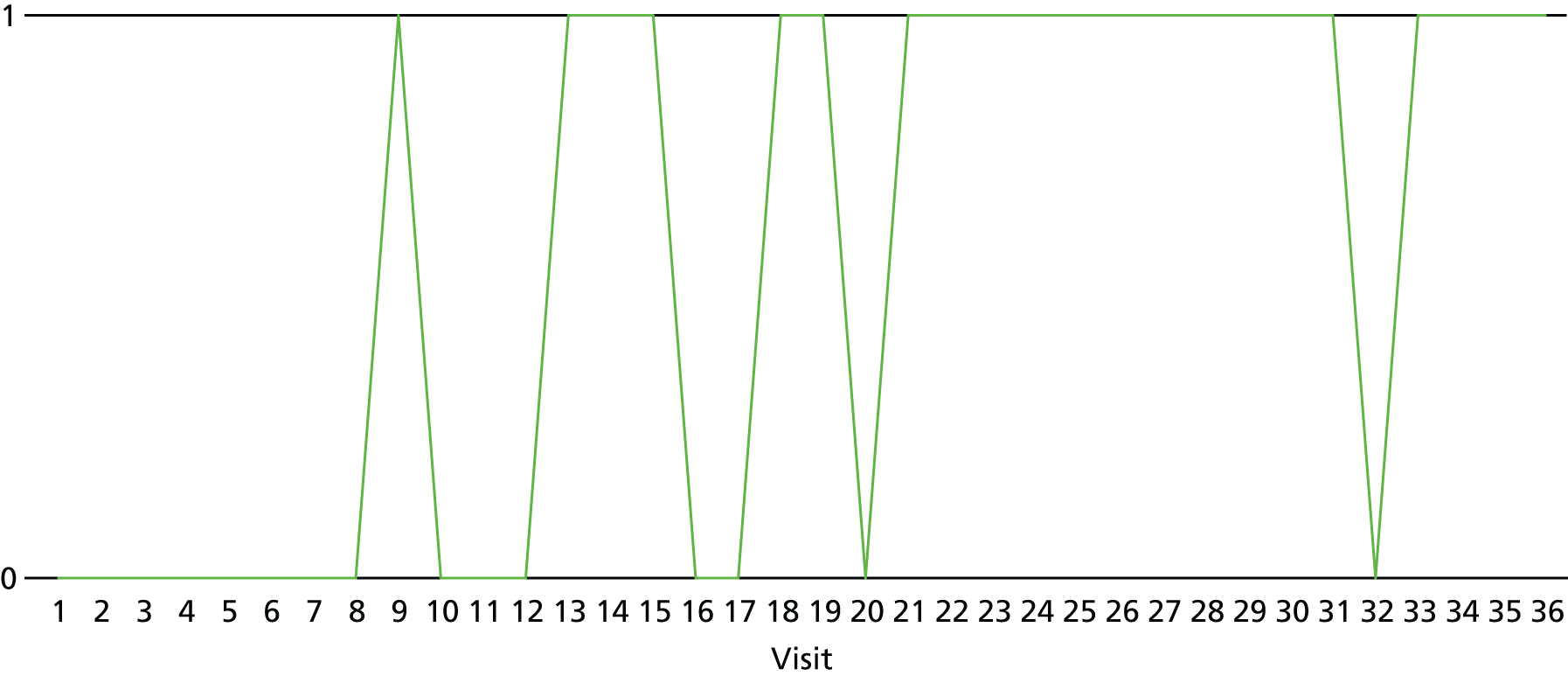
In week 5, patient 3 tested positive for heroin in the first two visits but did not self-report heroin use, and had a positive cocaine sample (they did not disclose cocaine use). They did disclose that they had used amphetamines later in the week (after the positive samples had occurred). In week 8 the patient did not attend the first two visits and had a positive urine sample for heroin (and cocaine) at the third visit, and disclosed using heroin, other opioids and cocaine on days 4–7 of that week. In week 9 the patient did not attend twice for the visits, had one positive urine sample for heroin and cocaine, and self-reported using heroin every day that week and using cocaine on day 7. In week 12 all three visits had positive urine samples for heroin and the patient self-reported using heroin every day. The second and third visits had positive methadone urine samples and the patient self-reported using other opioids on days 5 and 6. Patient 3 used heroin, cocaine, crack cocaine, cannabis, benzodiazepine, methadone and alcohol in the follow-up period (only data up to week 24 available). Patient 3 took all their medication in weeks 1–4, missed two doses in week 5, missed four doses in week 6 and did not take any study medication for the remainder of the trial.
Both the patient and the clinician correctly guessed the patient’s treatment allocation.
Patient 4
Patient 4 was on placebo implant and active oral medication. They had 31 out of 36 negative urine samples (for heroin), one positive urine sample (for heroin, in week 7) and four DNAs (Figures 9 and 10; see also Figures 21 and 22). This gave a proportion of 0.86 negative urine samples. This patient did not disclose using heroin in the 12-week period (or any other substance) and did not fill out a ‘first heroin lapse’ form. At screening, patient 4 had a positive urine screen for buprenorphine (sample 1). The remainder of their urine samples at screening were negative.
FIGURE 9.
Patient 4’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.

FIGURE 10.
Patient 4’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.

This patient did not have any other positive urine samples for any other substances. This patient did not disclose using heroin in the follow-up period and did not have any positive urine samples in the follow-up period (data only up to week 16 were available). Patient 4 took all of their study medication for all 12 weeks.
Neither the patient nor the clinician correctly guessed the patient’s treatment allocation.
Patient 5
Patient 5 was on active implant and placebo oral medication. This patient had 20 out of 36 negative urine samples for heroin, two positive urine samples for heroin and 14 DNAs (Figures 11 and 12; see also Figures 23 and 24). This gave a proportion of 0.56 negative urine samples. At screening, patient 5 had a positive urine screen for heroin and cocaine.
FIGURE 11.
Patient 5’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.
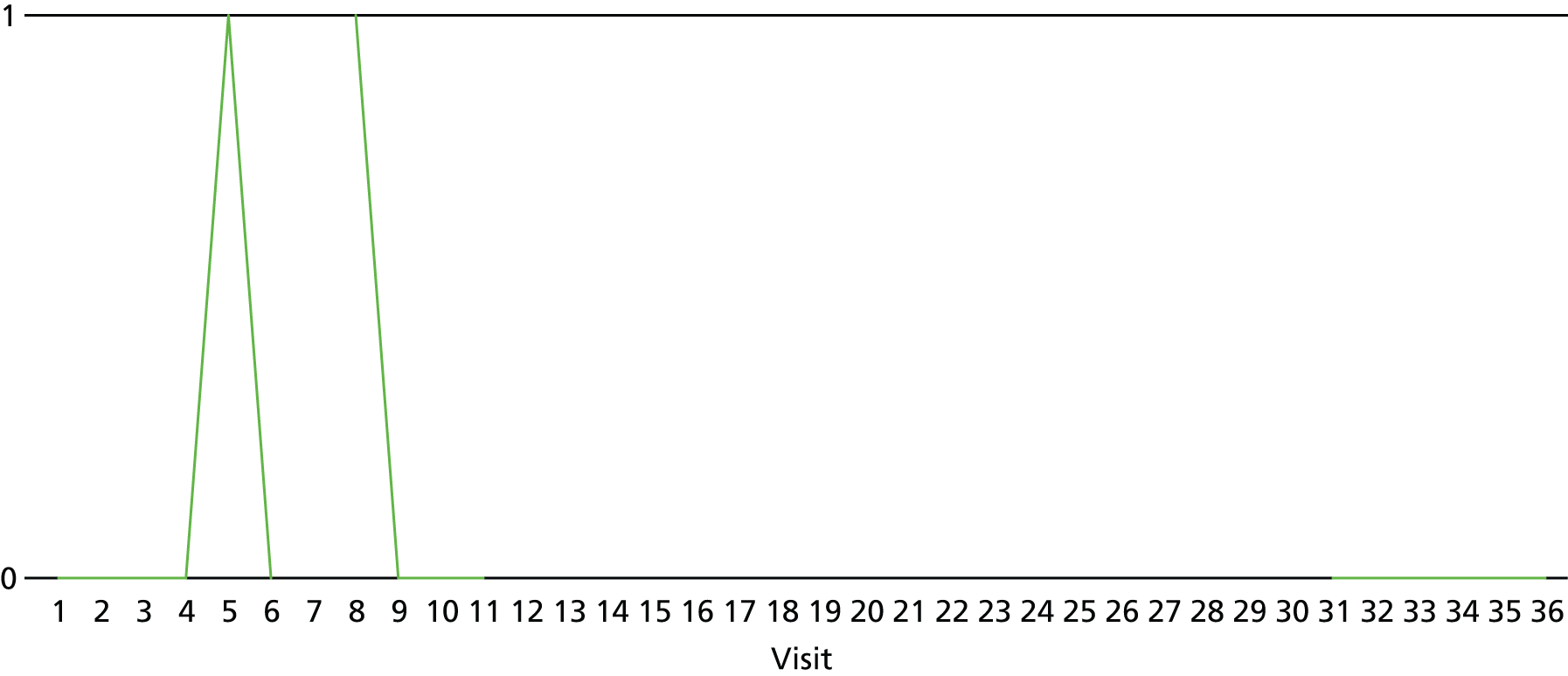
FIGURE 12.
Patient 5’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.
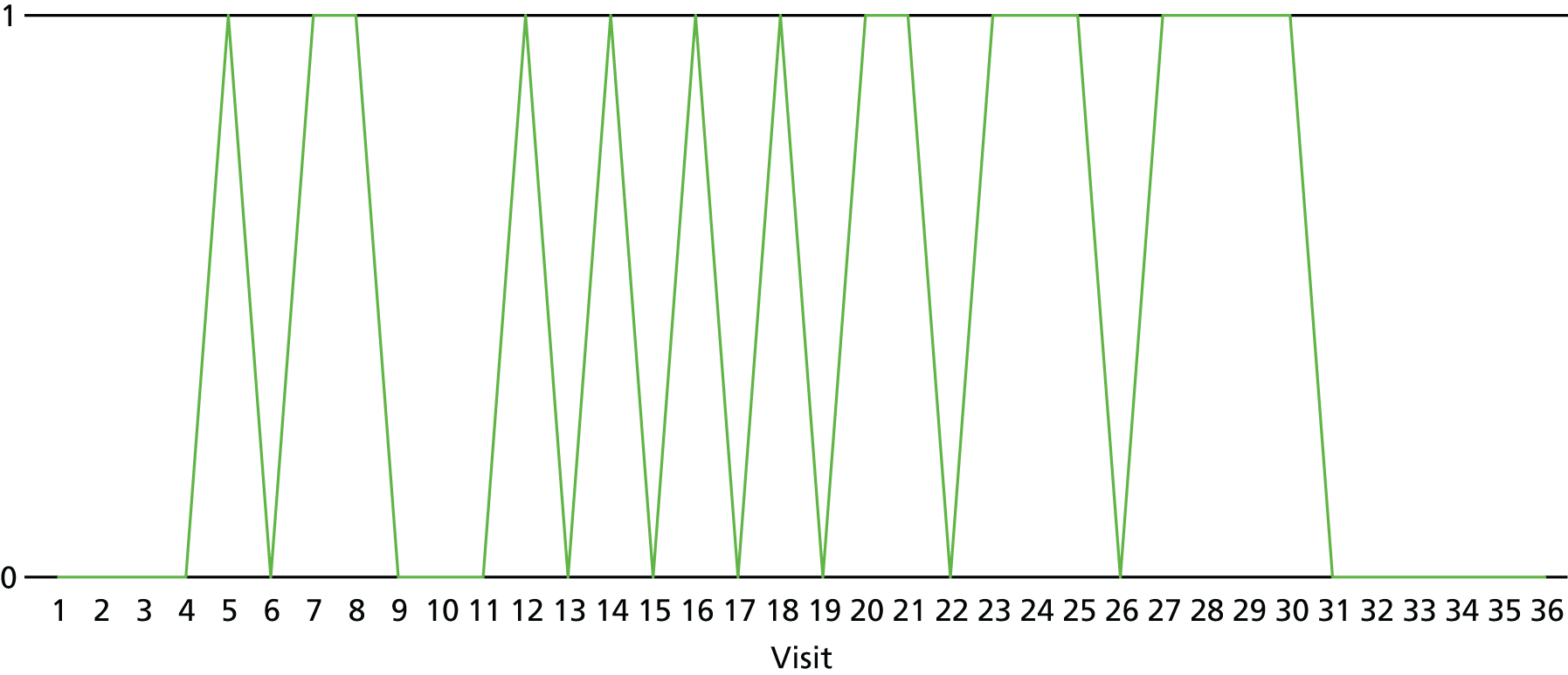
Patient 5 did not self-report using heroin during the 12-week treatment period (according to the self-report form for substance use in past 7 days), but did fill out a first heroin lapse form, which indicated a first lapse at the start of week 5. The reason the patient gave for this first lapse was ‘to manage the effects of other drugs’, and they used cocaine and alcohol before taking heroin on that occasion.
This patient did disclose using cocaine, amphetamines, other opioids and benzodiazepine during the 12-week treatment period. Patient 5 had 15 positive urine samples for cocaine (not including DNAs) and one positive sample for amphetamines. Patient 5 had a positive urine sample for cocaine at the 16-week follow-up visit, and disclosed using alcohol, crack, cocaine, amphetamines, other opioids and diazepam at the 16-week follow-up visit (no additional follow-up data were available). Patient 5 took all the required doses of study medication in the first 2 weeks, missed three doses in week 3, missed two doses in week 4, took all doses in week 5, missed two doses in week 6, took all doses in weeks 7–9, did not take any doses in week 10 and took complete medication in weeks 11 and 12.
Patient 5 reported swelling at the implant site 3 weeks after the date of implant.
Neither the patient nor the clinician correctly guessed the patient’s treatment allocation.
Patient 6
Patient 6 was on double placebo, had 29 out of 36 negative urine samples for heroin, 6 out of 36 positive urine samples and one DNA (Figures 13 and 14; see also Figure 24). This gave a proportion of 0.81 negative urine samples. Patient 6 had a positive urine for heroin and cocaine at the first visit in week 1 but did not self-report substance use at this time. Their first heroin lapse form indicated that they first lapsed at the beginning of week 5. They did not have any positive urine samples in week 5, but self-reported using heroin on day 2 of this week. The reason they provided for this first lapse was to determine their treatment allocation. They did not take any other substances on that occasion.
FIGURE 13.
Patient 6’s urine screen results for heroin, where 0 = negative for heroin, 1 = positive urine sample for heroin. DNAs are shown as blanks.

FIGURE 14.
Patient 6’s urine screen results for heroin, where 0 = negative for heroin. DNAs are shown as positive urine samples.

In week 9 patient 6 had two positive urine samples for heroin – they did not report using heroin at the first visit but reported using heroin on day 6, which coincided with the second positive sample of that week. In week 10 the patient self-reported using heroin on days 3 and 7, but had a positive urine sample only at the third visit. Patient 6 continued using heroin until the end of the treatment period.
Patient 6 had 19 positive urine samples for cocaine during the 12-week treatment period. Patient 6 reported using crack, cannabis and alcohol in the follow-up period (week 16 and 24 data; week 36 data were not available) and had a positive urine sample for cocaine at week 16 (no data were available for weeks 24 and 36). Patient 6 took all their study medication for all 12 weeks.
Neither the patient nor the clinician correctly guessed the patient’s treatment allocation.
Tables displaying the urine screen results for heroin at each visit are given in Appendix 2. Urine screen results for substances other than heroin are given in Appendix 3.
Self-reported heroin use for weeks 1–12
Self-report of other substance use is displayed in Appendix 3.
| Days | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1: days used | None | None | None | None | None | None |
| 2: days used | None | None | None | None | None | None |
| 3: days used | None | None | None | None | None | None |
| 4: days used | None | None | None | None | Missing | None |
| 5: days used | None | None | Day 2 | None | None | Day 2 |
| 6: days used | None | None | Missing | None | None | None |
| 7: days used | None | None | Missing | None | None | None |
| 8: days used | None | None | Days 4–7 | None | Missing | None |
| 9: days used | None | None | Days 1–7 | None | None | Day 6 |
| 10: days used | None | None | Missing | None | None | Days 3, 7 |
| 11: days used | None | None | Missing | None | None | None |
| 12: days used | None | None | Days 1–7 | None | None | Days 3, 7 |
Combining urine screen log results with self-report for heroin and other substance use
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 2 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 3 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 4 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 5 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 6 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 7 | Negative heroin urine | DNA: no self-reported heroin use or other substance use | DNA: no self-reported heroin use or other substance use |
| 8 | DNA: no self-reported heroin use or other substance use | Negative heroin urine | Negative heroin urine |
| 9 | Negative heroin urine | Negative heroin urine | DNA: no self-reported heroin use or other substance use |
| 10 | DNA: no self-reported heroin use or other substance use | DNA: no self-reported heroin use or other substance use | Negative heroin urine |
| 11 | Negative heroin urine | DNA: no self-reported heroin use or other substance use | DNA: no self-reported heroin use or other substance use |
| 12 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
FIGURE 15.
Patient 1’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits (provided patient did not already have evidence of using heroin) are shown as blanks.

FIGURE 16.
Patient 1’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits are shown as positive for using heroin.
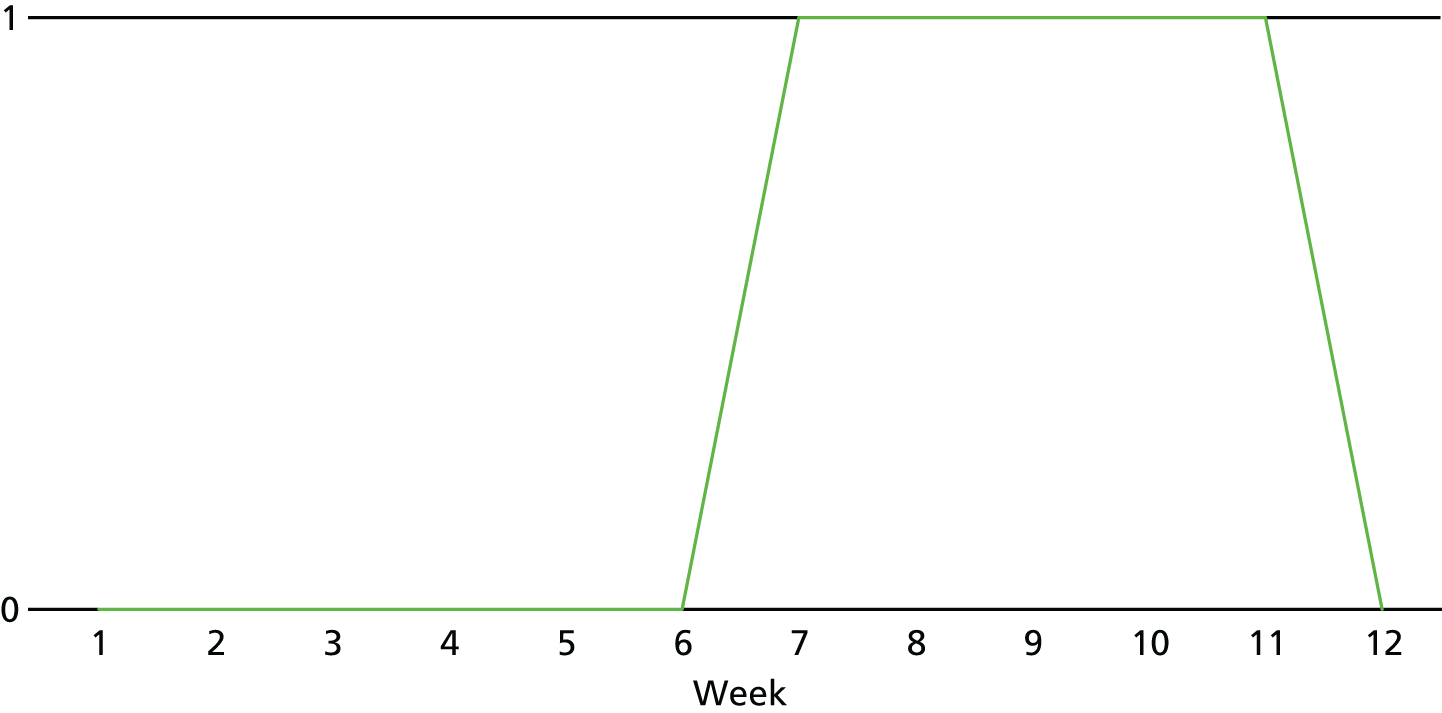
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative heroin urine | DNA: no self-reported heroin or other substance use | Negative heroin urine |
| 2 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 3 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 4 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 5 | Negative heroin urine | DNA: no self-reported heroin or other substance use | Negative urine |
| 6 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 7 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 8 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 9 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 10 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 11 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 12 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
FIGURE 17.
Patient 2’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits (provided patient did not already have evidence of using heroin) are shown as blanks.
FIGURE 18.
Patient 2’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits are shown as positive for using heroin.

| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 2 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 3 | Negative heroin urine | Negative heroin urine | DNA No self-reported heroin use |
| 4 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 5 |
|
|
DNA: self-reported amphetamine use on day 6 |
| 6 | Negative heroin urine | Negative heroin urine | DNA: missing data on substance use |
| 7 | DNA: missing data on substance use | Negative heroin urine | DNA: missing data on substance use |
| 8 |
|
|
|
| 9 | DNA: self-reported heroin use every day |
|
DNA: self-reported heroin use every day; self-reported cocaine use day 7 |
| 10 | DNA: missing data on substance use | DNA: missing data on substance use | DNA: missing data on substance use |
| 11 | DNA: missing data on substance use | Negative heroin urine | DNA: missing data on substance use |
| 12 |
|
|
|
FIGURE 19.
Patient 3’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits (provided patient did not already have evidence of using heroin) are shown as blanks.
FIGURE 20.
Patient 3’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits are shown as positive for using heroin.
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 2 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 3 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 4 | Negative heroin urine | Negative heroin urine | DNA: no self-reported heroin use |
| 5 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 6 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 7 | Negative heroin urine |
|
Negative heroin urine |
| 8 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 9 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 10 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
| 11 | Negative heroin urine | DNA: no self-reported heroin use | DNA: no self-reported heroin use |
| 12 | Negative heroin urine | Negative heroin urine | DNA: no self-reported heroin use |
FIGURE 21.
Patient 4’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits (provided patient did not already have evidence of using heroin) are shown as blanks.

FIGURE 22.
Patient 4’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits are shown as positive for using heroin.

| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative heroin urine | Negative heroin urine |
|
| 2 |
|
|
|
| 3 | DNA: no self-report of heroin use |
|
|
| 4 |
|
|
DNA: missing data on substance use |
| 5 |
|
|
|
| 6 |
|
|
|
| 7 |
|
|
|
| 8 |
|
DNA: missing data on substance use | DNA: missing data on substance use |
| 9 | DNA: no self-report of heroin use |
|
|
| 10 | DNA: no self-report of heroin use |
|
|
| 11 |
|
Negative heroin urine | Negative heroin urine |
| 12 | Negative heroin urine | Negative heroin urine | Negative heroin urine |
FIGURE 23.
Patient 5’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits (provided patient did not already have evidence of using heroin) are shown as blanks.

FIGURE 24.
Patient 5’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of each of the week’s three visits are shown as positive for using heroin.

| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 |
|
|
|
| 2 |
|
|
Negative urine heroin |
| 3 |
|
|
Negative urine heroin |
| 4 |
|
|
Negative urine heroin |
| 5 |
|
|
Negative urine heroin |
| 6 |
|
|
Negative urine heroin |
| 7 | Negative urine heroin | Negative urine heroin | Negative urine heroin |
| 8 |
|
|
Negative urine heroin |
| 9 |
|
DNA |
|
| 10 |
|
|
|
| 11 | Negative urine heroin | Negative urine heroin |
|
| 12 |
|
|
|
FIGURE 25.
Patient 6’s heroin use results over the 12-week treatment period (combining urine screen and self-report), where 0 = did not use heroin, 1 = used heroin. DNAs in any of the week’s three visits are shown as positive for using heroin.
Substance use in follow-up
At each follow-up point, the questionnaire asked about substance use in the past 4 weeks.
| Patient | Follow-up (number of days used in 28 day period) | ||
|---|---|---|---|
| Week 16 | Week 24 | Week 36 | |
| 1 (received double placebo) | Heroin: 6 days; crack: 6 days; cannabis: 10 days | Alcohol: 5 days; heroin: 23 days; crack: 24 days; cannabis: 1 day; methadone: 28 days | Alcohol: 4 days |
| 2 (received active implant, placebo oral) | Alcohol: 7 days; cannabis: 19 days | Alcohol: 6 days; cannabis: 7 days | Alcohol: 28 days; cannabis: 28 days |
| 3 (received double placebo) | Heroin: 28 days; crack: 12 days; cannabis: 28 days; methadone: 10 days | Alcohol: 2 days; cannabis: 7 days | Missing |
| 4 (received placebo implant, active oral) | Alcohol: 2 days | Missing | Missing |
| 5 (received active implant, placebo oral) | Alcohol: 20 days; crack: 3 days; cocaine: 2 days; amphetamines: 1 day; other opioids: 2 days; diazepam: 1 day | Missing | Missing |
| 6 (received double placebo) | Crack: 3 days; cannabis: 16 days | Alcohol: 1 day; crack: 4 days; cannabis: 20 days | Missing |
First heroin lapse
| Study participant | Reason | Other substance use that day | Before or after heroin | Days since randomisation that first lapse occurred |
|---|---|---|---|---|
| 1 | To feel less sad or bored | Cocaine | After heroin | 113 |
| 2 | NA | |||
| 3 | Because I had already used something else | Alcohol | Before heroin | 30 |
| 4 | NA | |||
| 5 | To manage the effects of other drugs | Cocaine and alcohol | Before heroin | 35 |
| 6 | I wanted to see what treatment I was receiving | No | NA | 35 |
Adherence to oral tablet
Full details of the oral dosing record are provided in Appendix 1.
Three of the participants took all of their oral study medication over the 12 weeks. All participants took all of their study medication for the first 2 weeks. One participant dropped to 250 mg at week 9 (takeaway dose) and did not take any of their study medication for weeks 10–12 (takeaway week 10 and DNA in weeks 11 and 12). One participant dropped to 250 mg at week 5, then 150 mg at week 6 and then did not take any study medication from week 7 onwards (takeaway week 7 and DNA in remaining weeks). One participant dropped to 200 mg at week 3 and 250 mg week 4, took all doses in week 5, dropped to 250 mg week 6, took all doses in weeks 7–9, missed week 10 and took all doses in weeks 11 and 12 (Figure 26).
FIGURE 26.
Total weekly dose received by each patient (mg).
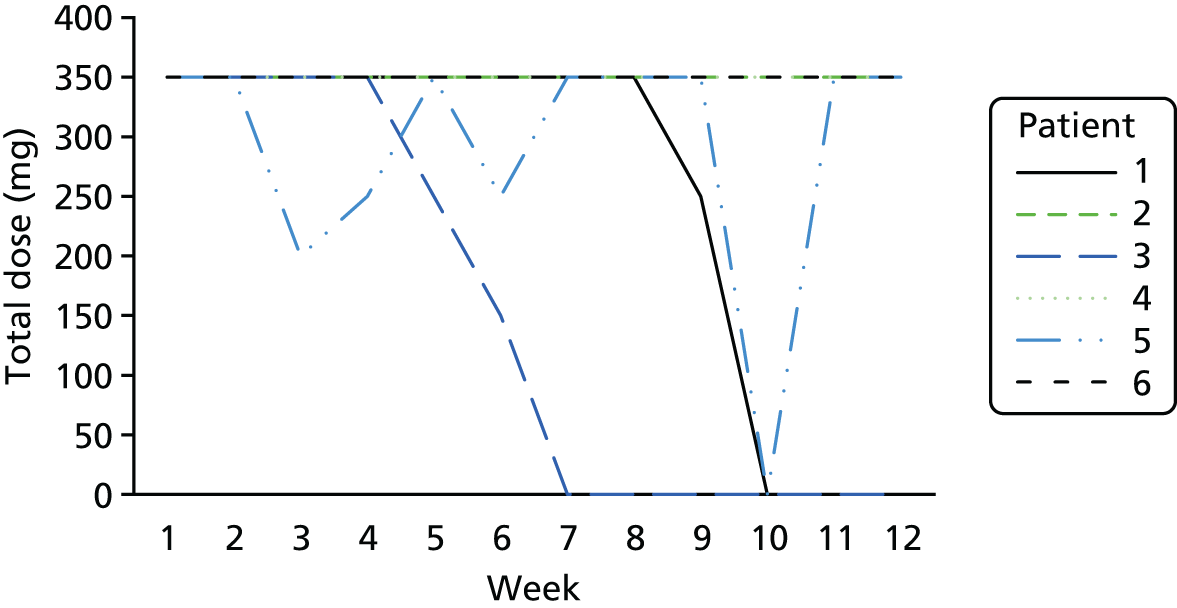
Withdrawal
No patients withdrew from the trial.
Adverse events
All AEs are reported in Appendix 1. Here, only implant-related AEs are reported.
| Patient | AE | Ongoing | Intensity | Related to oral study drug? | Related to implant? | Is this a SAE? |
|---|---|---|---|---|---|---|
| 1 (double placebo) | Redness and swelling at implant site | No | Mild | No | Definitely | No |
| Pain at implant site | No | Mild | No | Definitely | No | |
| Pus at implant site | No | Mild | No | Definitely | No | |
| Pruritus | No | Mild | Possibly | Possibly | No | |
| Acute deterioration in renal function | No | Moderate | Possibly | Possibly | No | |
| 2 (active implant, oral placebo) | Intermittent burning at site of implant | No | Mild | No | Possibly | No |
| Implant remains unchanged | Yes | Moderate | No | Probably | No | |
| Intermittent burning/pain around implant site | No | Mild | No | Possibly | No | |
| 5 (active implant, oral placebo) | Swelling at implant site | Yes | Mild | No | Possibly | No |
Patient treatment guess
| Patient | Guess | Actual treatment | Correct | |
|---|---|---|---|---|
| Implant | Tablet | |||
| 1 | Definitely placebo naltrexone | Definitely active naltrexone |
|
No (wrong tablet guess) |
| 2 | Definitely placebo naltrexone | Definitely placebo naltrexone |
|
No (wrong implant guess) |
| 3 | Definitely placebo naltrexone | Definitely placebo naltrexone |
|
Yes |
| 4 | Probably active naltrexone | Probably active naltrexone |
|
No (wrong implant) |
| 5 | Probably active naltrexone | Probably active naltrexone |
|
No (wrong tablet) |
| 6 | Probably active naltrexone | Probably active naltrexone |
|
No (both wrong) |
Only one patient correctly guessed their treatment.
Clinician treatment guess
| Patient | Guess | Actual treatment | Correct | |
|---|---|---|---|---|
| Implant | Tablet | |||
| 1 | Probably active naltrexone | Probably active naltrexone |
|
No (both wrong) |
| 2 | I cannot say | I cannot say |
|
NA |
| 3 | Definitely placebo naltrexone | Definitely placebo naltrexone |
|
Yes |
| 4 | Probably active naltrexone | Probably active naltrexone |
|
No (implant wrong) |
| 5 | Probably active naltrexone | Probably active naltrexone |
|
No (tablet wrong) |
| 6 | Probably active naltrexone | Probably active naltrexone |
|
No (both wrong) |
The clinician(s) correctly guessed only one patient’s allocation.
The CONSORT flow diagram
FIGURE 27.
The CONSORT flow diagram for the NEAT study. a, Breakdown of exclusions (prior to consent): not abstinent from all opioids for 7 days/not completing treatment for opioid detoxification (n = 1081); not seeking opioid antagonist treatment for opioid use disorder (n = 10); opiate dependence syndrome (n = 33); currently taking naltrexone therapy (n = 3); severe alcohol dependence/withdrawal (n = 4); current/recent suicidal ideation/plan/attempt (n = 2); does not live in stable/secure accommodation in the community (n = 1); current criminal justice involvement (n = 1); declined participation (n = 13); unable to consent (n = 1); other (n = 19). O-NTX, oral naltrexone; XR-TX, extended-release naltrexone.

Chapter 5 Findings
The NEAT trial was terminated at an early stage because of the major problems that were encountered with recruitment of study participants. Despite the early termination, it is possible to make observations that may help understanding of research and clinical processes and procedures and may also guide research and clinical colleagues in possible future investigations of this patient population and of this specific treatment. Some of the observations and conclusions that are considered of relevance, and potentially valuable as guidance for future planned research, are now listed.
Major upheaval of the commissioning, organisation and delivery of clinical services for this patient population occurred over the period during which the trial was planned and delivered. Regular cycles of retendering and recommissioning of services led to insecurity among both staff and treatment-providing agencies, as well as a need to identify ways of reducing operational costs so as to succeed in competitive tendering exercises (for which cost often appeared to be the dominant consideration). In such a context, considerations of any variation to standard clinical practice were often regarded as unwelcome and/or distractions and/or unrealistic. As a result, it was possible to consider clinical sites only if there was already a lead clinician with a commitment to the academic research endeavour. Even with such support from academically sympathetic senior clinical colleagues, the retendering process often led to major disruptions to the clinical services in which they worked. As illustrative examples, the senior coinvestigator in Darlington found that service was due to be closed and consequently moved to a new service in Humber, only then to discover the following year that this service too was reassigned to a different provider (this time a non-NHS service provider) that did not have the appropriate understanding or willingness to give financial and strategic commitment to participation in a research study of this sort.
Several research and clinical process loops became substantial obstacles. These included the requirement that the patient had been successfully detoxified prior to consideration of naltrexone administration. In the procedures, this became a necessary ‘detoxified’ status, which required verification and the establishment of several days of drug-free status. However, this then caused a considerable clinical problem because it might be regarded as the point of greatest vulnerability for relapse, over which period the naltrexone (or placebo) was not being provided. In our opinion, it may be necessary to develop research and clinical procedures that permit the prompt initiation onto naltrexone as soon as the patient has reached this status. Indeed, if the opportunity existed, we would look to investigate some of the more recently proposed methods of incremental low-dose naltrexone induction during the later stages of detoxification, so as to improve clinical procedures and also to enable prompt recruitment into a naltrexone trial.
The typical cooling-off period after consent that had been secured was also a potentially problematic area, because this might be the very period during which naltrexone treatment might be considered most appropriate. We were able to secure agreement that the normal cooling-off period could be shortened, and also that, if clinically considered important, an interim prescribed dose of oral naltrexone (outside the active trial period) could be provided if absolutely necessary in the period running up to the start of study medication.
The need for a separate clinician to do the actual surgical implantation contributed additional delays to the process of reaching the first day of active medication. In one of the sites (Lambeth), we were able to keep this delay to a minimum because we built on the existing Gwent joint working relationship with clinicians familiar with the procedure of surgical implantation of implants/contraceptive rods. However, with the other sites (i.e. Birmingham/Solihull), this was an ongoing problem that was not resolved by the time the decision was made to close the trial, with local interested GPs requiring special training in the implant procedure (which itself involved bringing them down to London to the Lambeth site on a day when the surgical implant was due to be inserted – this was particularly problematic as it would be at short notice, which then did not suit the diaries of the Birmingham GPs). If the study were to be conducted again, we would develop better methods for this element of the clinical procedures (perhaps developing the clinical skills within the addiction clinical team, or, alternatively, having much smoother clinical training processes for the sessional medical staff who might deliver the procedure).
An important finding was that, for a sufficiently large proportion of the patients, the robust trial design (double-blind, double-dummy, placebo-controlled) was broadly acceptable once the reason and process had been properly explained. Patients expressed anxieties about the uncertainty of whether they were on active or placebo, but a sufficient number of patients considered it acceptable to consider entry to the trial. The problems with recruitment were more markedly concentrated around the clinical process of completion of detoxification and the initiation of the antagonist, etc., not around the acceptability or otherwise of the trial design. A similar level of acceptability was observed with clinical staff also. These aspects will be explored more fully in the qualitative interviews that are being conducted (after the completion of the clinical aspects of the trial) and that will be reported separately.
The specially produced implant products (both active and placebo naltrexone) were produced for us by a company in Portugal and seemed broadly acceptable, at least for the purposes of the trial, although the opportunity did not exist to collect blood samples to be sure of pharmacokinetics and the adequacy of plasma levels through the study period. The reader will see that a number of patients reported pain and discomfort at the implant site, but that these were mostly considered mild and did not require any corrective clinical intervention. There were reports of a degree of infection at the insert site but this was not considered to be to an extent that required antibiotic treatment. This observation is of interest, even with only a very small number of study participants, because common practice in independent private practice of the unlicensed forms of depot naltrexone has typically been accompanied by use of types of implant to which triamcinalone has been added, or alternatively prophylactic provision of oral steroids and antibiotics. This practice was considered at the planning stage of the trial but it was decided that, for trial purposes, we did not wish steroids to be included in the implant (triamcinalone is often included in the actual implants themselves with other non-licensed naltrexone implant products) and we did not wish to initiate antibiotic treatment prophylactically. Our wish was to be able to observe the extent to which this added medication was, or was not, required.
An important further finding was that the double-blind procedure appeared to work very well. We had wondered if, with the drug effect as powerful as an opiate antagonist, the blinding might be poor, with patients quickly detecting whether or not they were on active placebo after instances of lapse to heroin/opiate use. Observations from the six study participants is that, across both clinicians as well as the patients themselves, there was a remarkable failure to identify correctly random allocation, and this included not only erroneous allocation guess when active implant or active oral naltrexone was being given, but also a failure to identify the double-placebo random allocation for two of the three patients in this condition. The process of double-blinding thus appears to be feasible and effective.
Chapter 6 Discussion and conclusions
In this final chapter, we give consideration first to the implications of the findings reported here (as reported in Chapter 4 and summarised in Chapter 5) and, second, to the implications for possible future research study of this area. However, before commencing these considerations, it is appropriate to remind ourselves of the limitations of the findings reported.
Limitations
Firm conclusions cannot be reached from the observations on the small number of study participants who entered the study, but it is still possible to reach conclusions on some elements of the work planned and undertaken. Thus, although this does need to be acknowledged as a major limitation within this report, we are nevertheless able to reach tentative conclusions about some aspects and gain an understanding of the obstacles encountered. We are also currently conducting qualitative interviews with study participants recruited, patients who considered participation, clinical staff involved in the trial and key informants in the overall trial conduct, and so it will also be possible to report on the lived experience of participation in the conduct of the trial from the perspective of potential patients approached and interested in participation. This qualitative study comprises an investigation of the knowledge, attitudes and behaviour of the six study participants, or potential study participants, all involved clinical staff and other key individuals. These interviews will explore participants’ perspectives of the trial design and trial validity.
The double-blind, double-dummy trial design was recognised from the outset as new to the particular clinical field (the addictions treatment field) because it involved introducing this trial design to a treatment field that had not previously encountered it. It was also challenging in terms of the necessary pre-trial arrangements to produce the supply of active and placebo treatments. However, once established, it was broadly understood and acceptable to many potential study participants as well as to staff.
One area of particular challenge warrants more detailed description. Considerable problems were encountered with the stipulated requirement of a validated ‘detoxified’ status prior to the initiation of the study naltrexone, and also with the requirement for a ‘cooling-off’ period for the consent that had been provided by the participant for participation in the trial. A further area of challenge concerned the additional delay awaiting these surgical implant procedure. Ways around these obstacles were developed during the preparation and early stages of conduct of the trial, at least to some extent, which were able to mitigate the problem.
The possibility of direct recruitment of study participants on release from prison (because they represent a population with a particular likelihood of having achieved a state of general abstinence which, in view of their imminent release, might at least be considered fragile) was also explored. However, the operational aspects of securing organisational commitment to participation, alongside the lack of previous significant experience in trial participation, led to problems that could not be resolved in the time available to add within-prison participation to the NEAT trial processes. For future work, we recommend consideration of this potentially important patient population.
Consideration of the findings
From the findings described above, we select the following as warranting particular consideration.
We note the success of the double-blind procedure, with the finding of a remarkable failure on the part of participating patients, and also on the part of clinical staff, to identify correctly the random allocation. It was particularly noteworthy that this observation was applicable not only for the active implant and active oral, but also for the two study participants in the double-placebo condition.
It is noted that at least one patient for whom trial participation, and the provision of an antagonist blocking medication with unknown status as either active or placebo, appears to have triggered the first instance of heroin use in order to test the blockade and thereby learn whether they were in the placebo or active condition. This unintended consequence needs to be borne in mind when considering trial designs and their potential advantages and disadvantages.
We also note that, even though an opiate antagonist medication was being taken, there was self-report and urine analysis evidence of a considerable extent of other drug use (e.g. amphetamine, alcohol). Furthermore, there seemed to be evidence from self-report that these instances of use of other drugs often directly preceded instances of use of heroin/opiates.
It is also interesting to look at the clinical course of the six study participants on the basis of whether they received the active implant, active oral or placebo. There is evidence of observed benefit and good continuing recovery with patient 2 (active implant), whose the good progress continued into the follow-up period, and also of the continuing recovery with patient 4 (active oral), whose the good progress similarly continued into the follow-up period. In contrast, for two of the three patients who received double-placebo there was evidence of considerable drug use, including use of heroin.
Implications for future research
A potentially important observation is the difference between lapse and relapse – we suggest that this needs to be explored more carefully. For several study participants, instances of ‘lapse’ (i.e. instances of heroin use) occurred but were not necessarily followed by immediate clinical ‘relapse’. This is potentially a particularly interesting area when we consider the likely beneficial effect of naltrexone. Let us give this some consideration. Instances of ‘lapse’ might occur as harbingers of major relapse, or they may alternatively be isolated instances that have little bearing on longer-term outcomes – the difference is clearly important. Indeed, at least one of the study participants who participated in the NEAT trial specifically attributed their first episode of use to a wish to test their double-blind assignment and their presumed opiate blockade.
Following on from this consideration, it also needs to be borne in mind that it could reasonably be presumed that it is precisely this lapse-to-relapse likelihood that is the area in which naltrexone might be expected to deliver benefit (i.e. that naltrexone might reduce the likelihood that an instance of lapse might occur). If this is correct, then it has profound implications for the selection of study design and choice of outcome measures that should be deployed in future trials.
Overall conclusion
Lessons need to be learnt from the difficulties encountered by the NEAT trial team so as to ensure that these do not occur again (whether for the NEAT team members or for another group of investigators). Some of the problems encountered relate to the choice of trial elements and their conduct, whereas others relate to the severely disrupted and disturbed nature of the addictions treatment field at this time, particularly in the light of the introduction of aggressive competitive tendering processes, with their focus not only on value for money but also on baseline cost specifically, and also the move of commissioning out of health care into local authorities. We consider that, given wider variations in practice and operational uncertainties in the addictions treatment field, it is particularly important that attention is paid to reduce the potential for practitioner and observer bias. We also conclude that, on the basis of our modest experiences in the NEAT trial, a mixture of self-report, urine analysis and subsequent independent description of lapse episodes has blended value in the understanding of extent of resumption of heroin/opioid and other drug use. It is noted that it has been possible to produce placebo medications and to use these in a trial setting with good resulting blinding, and that specially produced medications can effectively be used in such a trial. There remains a need to assess the value of the new ultra-long-acting formulation of naltrexone (depot naltrexone or naltrexone implant) in a properly constructed randomised trial informed by a external pilot.
Acknowledgements
We wish to thank the NIHR Health Technology Assessment programme for funding this trial. We also wish to thank all of the patients and staff from all of the sites that participated in the trial.
A thank you also to all of the staff at the KCTU, as well as Martin Heasman and Glynis Ivin from the pharmacy department at the SLaM NHS Foundation Trust, Rob Van Der Waal and Alison Peachey at the SLaM NHS Foundation Trust, Shabana Akhtar at the Birmingham and Solihull Mental Health NHS Trust and Oliver Gupta at Modepharma.
Contributions of authors
Professor Sir John Strang (Professor of Addiction Psychiatry) conceived and designed the trial, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Michael Kelleher (Consultant Psychiatrist) contributed to the design of the trial, the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Soraya Mayet (Consultant Psychiatrist) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Ed Day (Senior Clinical Lecturer in Addiction Psychiatry) contributed to the design of the trial, the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Ms Jennifer Hellier (Biostatistician) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor Sarah Byford (Professor of Health Economics) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Ms Caroline Murphy (Director, KCTU) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Mr Blair McLennan (Trial Manager) managed the trial, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr James Shearer (Lecturer in Health Economics) contributed to the design of the trial, the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Dr Elizabeth Ryan (Biostatistician) contributed to the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Professor John Marsden (Professor of Addiction Psychology) conceived and designed the trial, contributed to the analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Nutt DJ, King LA, Phillips LD. Independent Scientific Committee on Drugs . Drug harms in the UK: a multicriteria decision analysis. Lancet 2010;376:1558-65. https://doi.org/10.1016/S0140-6736(10)61462-6.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Tiffany ST. New perspectives on the measurement, manipulation and meaning of drug craving. Hum Psychopharmacol: Clin Exp 1997;12:103-13. https://doi.org/10.1002/(SICI)1099-1077(199706)12:2+<S103::AID-HUP907>3.0.CO;2-8.
- Marsden J, Eastwood B, Bradbury C, Dale-Perera A, Farrell M, Hammond P, et al. Effectiveness of community treatments for heroin and crack cocaine addiction in England: a prospective, in-treatment cohort study. Lancet 2009;374:1262-70. https://doi.org/10.1016/S0140-6736(09)61420-3.
- Hay G, Gannon M, Casey J, Millar T. National and Regional Estimates of the Prevalence of Opiate and/or Crack Cocaine Use 2008–09: A Summary of Key Findings. Glasgow: National Treatment Agency for Substance Use; 2010.
- National Treatment Agency Annual Accounts 2010/2011: Presented to Parliament Pursuant to Schedule 15 of the National Health Service Act 2006. London: The Stationery Office; 2011.
- Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry 1997;54:71-80. https://doi.org/10.1001/archpsyc.1997.01830130077015.
- McLellan AT, Luborsky L, Woody GE, O’Brien CP, Druley KA. Predicting response to alcohol and drug abuse treatments. Role of psychiatric severity. Arch Gen Psychiatry 1983;40:620-5. https://doi.org/10.1001/archpsyc.1983.04390010030004.
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006;295:2003-17. https://doi.org/10.1001/jama.295.17.2003.
- Sideroff SI, Charuvastra VC, Jarvik ME. Craving in heroin addicts maintained on the opiate antagonist naltrexone. Am J Drug Alcohol Abuse 1978;5:415-23. https://doi.org/10.3109/00952997809007017.
- Rawson RA, McCann MJ, Hasson AJ, Ling W. Addiction pharmacotherapy 2000: new options, new challenges. J Psychoactive Drugs 2000;32:371-8. https://doi.org/10.1080/02791072.2000.10400238.
- Axel B. Addiction 1996;101:491-503.
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster C. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend 1999;54:127-35. https://doi.org/10.1016/S0376-8716(98)00152-5.
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11060.
- Drug Misuse: Psychosocial Interventions. London: NICE; 2007.
- Directive 2001/20/EC of 4 April 2001, of the European Parliament and of the Council on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Brussels: European Commission; 2001.
- The Medicines for Human Use (Clinical Trials) Regulation 2004: SI 2004/1031. London: The Stationery Office; 2004.
- The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006. London: The Stationary Office; 2006.
- Data Protection Act 2018. London: The Stationary Office; 2018.
- Hanney S, Buxton M, Green C, Coulson D, Raftery J. An assessment of the impact of NHS Health Technology Assessment Programme. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11530.
- Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: novel treatment for opioid dependence. Expert Opin Investig Drugs 2007;16:1285-94. https://doi.org/10.1517/13543784.16.8.1285.
Appendix 1 Medical history
| Patient | Medical history | Ongoing at start of trial | BMI (kg/m2) |
|---|---|---|---|
| 1 | Gastrointestinal (gastritis), psychiatric (depression) | Gastrointestinal: unknown; depression: no | 35 |
| 2 | Hepatic (hepatitis C), dermatological | Yes for both | 22 |
| 3 | Genitourinary (urgency nocturia), psychiatric (low mood in the context of childhood difficulties, drug dependence), other (termination of pregnancy – twice) | Genitourinary: yes; psychiatric: yes; terminations of pregnancy: no | 25 |
| 4 | Musculoskeletal (sciatica), psychiatric (depression) | Both ongoing | 39 |
| 5 | Respiratory (childhood asthma), psychiatric (depression) | No for both | 24 |
| 6 | Musculoskeletal (osteoporosis), psychiatric (psychosis – drug induced?), dermatological (chronic abscesses on buttocks), allergies (penicillin – gastrointestinal tract upset) | Osteoporosis: yes; chronic abscesses: yes; allergies: yes; psychosis: no | 24 |
| Patient | Medications | Dose | Continuing at end of study |
|---|---|---|---|
| 1 | Mirtazapine | 15 mg | No |
| Lansoprazole | 30 mg | No | |
| Flucloxacillin | 500 mg | No | |
| Lidocaine | 8 ml × 1% (5mg/5 ml) | No | |
| Chlorphenamine | 4 mg | No | |
| Loratadine | 10 mg | No | |
| Methadone | 55 mg | Yes | |
| 2 | Viekirax | Two tablets | Yes |
| 3 | Mirtazapine | 30 mg | Missing |
| 4 | Fluoxetine | 20 mg | Not at study end |
| Naproxen | 500 mg | No | |
| Nexplanon implant | 68 mg | Not at study end | |
| Azithromycin | 1 g | No | |
| Metronidazole | 1 g/800 mg | No | |
| Ibuprofen gel | 10% w/w tds PRN | No | |
| 6 | Olanzapine | 20 mg | Missing |
| Lymecycline | 408 mg | Missing | |
| Allendronic acid | 280 mg once weekly | Missing | |
| Colecalciferol 400 IU + calcium carbonate 1.5 g | Two tablets | Missing | |
| Sertraline | 100 mg | Missing | |
| Omeprazole | 20 mg | Missing | |
| Sertraline | 50 mg | Missing | |
| Sildenafil | 100 mg PRN | Missing | |
| Testosterone undecanoate | 1 g/4 ml per 3 months | Missing |
Appendix 2 Urine screen results (based on urine screen log): heroin
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative | Negative | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | Negative |
| 4 | Negative | Negative | Negative |
| 5 | Negative | Negative | Negative |
| 6 | Negative | Negative | Negative |
| 7 | Negative | DNA | DNA |
| 8 | DNA | Negative | Negative |
| 9 | Negative | Negative | DNA |
| 10 | DNA | DNA | Negative |
| 11 | Negative | DNA | DNA |
| 12 | Negative | Negative | Negative |
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative | DNA | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | Negative |
| 4 | Negative | Negative | Negative |
| 5 | Negative | DNA | Negative |
| 6 | Negative | Negative | Negative |
| 7 | Negative | Negative | Negative |
| 8 | Negative | Negative | Negative |
| 9 | Negative | Negative | Negative |
| 10 | Negative | Negative | Negative |
| 11 | Negative | Negative | Negative |
| 12 | Negative | Negative | Negative |
| Week | Sample | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative | Negative | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | DNA |
| 4 | Negative | Negative | Negative |
| 5 | Positive | Positive | DNA |
| 6 | Negative | Negative | DNA |
| 7 | DNA | Negative | DNA |
| 8 | DNA | DNA | Positive |
| 9 | DNA | Positive | DNA |
| 10 | DNA | DNA | DNA |
| 11 | DNA | Negative | DNA |
| 12 | Positive | Positive | Positive |
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative | Negative | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | Negative |
| 4 | Negative | Negative | DNA |
| 5 | Negative | Negative | Negative |
| 6 | Negative | Negative | Negative |
| 7 | Negative | Positive | Negative |
| 8 | Negative | Negative | Negative |
| 9 | Negative | Negative | Negative |
| 10 | Negative | Negative | Negative |
| 11 | Negative | DNA | DNA |
| 12 | Negative | Negative | DNA |
| Week | Sample | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Negative | Negative | Negative |
| 2 | Negative | Positive | Negative |
| 3 | DNA | Positive | Negative |
| 4 | Negative | Negative | DNA |
| 5 | Negative | DNA | Negative |
| 6 | DNA | Negative | DNA |
| 7 | Negative | DNA | DNA |
| 8 | Negative | DNA | DNA |
| 9 | DNA | Negative | DNA |
| 10 | DNA | DNA | DNA |
| 11 | Negative | Negative | Negative |
| 12 | Negative | Negative | Negative |
| Week | Sample number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | Positive | Negative | Negative |
| 2 | Negative | Negative | Negative |
| 3 | Negative | Negative | Negative |
| 4 | Negative | Negative | Negative |
| 5 | Negative | Negative | Negative |
| 6 | Negative | Negative | Negative |
| 7 | Negative | Negative | Negative |
| 8 | Negative | Negative | Negative |
| 9 | Positive | DNA | Positive |
| 10 | Negative | Negative | Positive |
| 11 | Negative | Negative | Negative |
| 12 | Negative | Positive | Positive |
Appendix 3 Urine screen results (based on urine screen log): other substances
Patient 1
Patient 1 had positive urine samples for other substances only in follow-up (after treatment period):
-
At week 16, patient 1 was positive for benzodiazepine.
-
At week 24, patient 1 was positive for heroin, cocaine and methadone.
-
At week 36, patient 1 was positive for methadone.
Patient 2
No positive urine samples for other substances.
Patient 3
-
Patient 3 had a positive urine sample for cocaine at the week-5 (sample 1) visit, week-8 (sample 3) visit and week-9 (sample 2) visit.
-
They had a positive methadone urine sample at week 12, samples 2 and 3.
-
At week 16, patient 3 was positive for heroin, cocaine, benzodiazepine and methadone.
-
At week 24, patient 3 was positive for heroin, cocaine and benzodiazepine.
Patient 4
No positive urine samples for other substances.
Patient 5
Patient 5 had positive cocaine samples at the following visits:
-
week 1, sample 3
-
week 2, sample 1
-
week 2, sample 2
-
week 2, sample 3
-
week 3, sample 2
-
week 3, sample 3
-
week 4, sample 1
-
week 4, sample 2
-
week 5, sample 1
-
week 5, sample 3
-
week 6, sample 2
-
week 7, sample 1
-
week 8, sample 1
-
week 9, sample 2
-
week 11, sample 1.
They were also positive for amphetamines at the week 5 (sample 3) visit.
Patient 5 was positive for cocaine at the week 16 visit; there were no data available for the week 24 and week 36 visits.
Patient 6
Patient 6 had positive samples for cocaine at the following visits:
-
week 1, sample 1
-
week 1, sample 2
-
week 1, sample 3
-
week 2, sample 2
-
week 3, sample 1
-
week 4, sample 1
-
week 4, sample 2
-
week 6, sample 1
-
week 6, sample 2
-
week 8, sample 2
-
week 9, sample 1
-
week 9, sample 3
-
week 10, sample 1
-
week 10, sample 2
-
week 10, sample 3
-
week 11, sample 3
-
week 12, sample 1
-
week 12, sample 2
-
week 12, sample 3.
Patient 6 also had a positive sample for cocaine at week 16; there were no data available for weeks 24 and 36.
Appendix 4 Self-report of other substance use for weeks 1–12 (based on ‘substance use for last 7 days form’)
| Week (days used) | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 | None | None | None | None | Cocaine day 6 | Cocaine day 4 |
| 2 | None | None | None | None | Cocaine days 2 and 4; amphetamines day 7 | Cocaine day 3 |
| 3 | None | None | None | None | Other opioids day 3; cocaine days 4 and 5 | Cocaine day 3 |
| 4 | None | None | None | None | Missing | Cocaine day 3 |
| 5 | None | None | Amphetamines day 6 | None | Cocaine days 4 and 5; amphetamines day 5 | None |
| 6 | None | None | Missing | None | Cocaine days 4 and 6; amphetamines day 3 | Cocaine day 3 |
| 7 | None | None | Missing | None | Cocaine day 4; benzodiazepine day 5 | None |
| 8 | None | None | Other opioids days 4–7; cocaine days 4–7 | None | Missing | Cocaine day 3 |
| 9 | None | None | Cocaine day 7 | None | Cocaine day 6 | Cocaine day 6 |
| 10 | None | None | Missing | None | Cocaine days 5 and 7 | Cocaine days 3 and 7 |
| 11 | None | None | Missing | None | None | None |
| 12 | None | None | Other opioids days 5 and 6 | None | None | Cocaine days 3 and 7 |
Appendix 5 Oral dosing record
| Week dose (mg) | Patient | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 | 350 | 350 | 350 | 350 | 350 | 350 |
| 2 | 350 | 350 | 350 | 350 | 350 | 350 |
| 3 | 350 | 350 | 350 | 350 | 200 (missed 3 doses) | 350 |
| 4 | 350 | 350 | 350 | 350 | 250 (missed 2 doses) | 350 |
| 5 | 350 supervised | 350 takeaway | 250 (missed 2 doses) supervised | 350 takeaway | 350 takeaway | 350 takeaway |
| 6 | 350 supervised | 350 takeaway | 150 (missed 4 doses) takeaway | 350 takeaway | 250 (missed 2 doses) takeaway | 350 takeaway |
| 7 | 350 takeaway | 350 takeaway | 0 (missed all) takeaway | 350 takeaway | 350 takeaway | 350 takeaway |
| 8 | 350 takeaway | 350 takeaway | 0 (missed all) DNA | 350 takeaway | 350 takeaway | 350 takeaway |
| 9 | 250 (missed 2 doses) takeaway | 350 takeaway | 0 (missed all) DNA | 350 takeaway | 350 takeaway | 350 takeaway |
| 10 | 0 (missed all) takeaway | 350 takeaway | 0 (missed all) DNA | 350 takeaway | 0 (missed all) takeaway | 350 takeaway |
| 11 | 0 (missed all) DNA | 350 takeaway | 0 (missed all) DNA | 350 takeaway | 350 takeaway | 350 takeaway |
| 12 | 0 (missed all) DNA | 350 takeaway | 0 (missed all) DNA | 350 takeaway | 350 takeaway | 350 takeaway |
Appendix 6 Adverse events
| Patient | AE | Ongoing | Intensity | Related to oral study drug? | Related to implant? | Is this a SAE? |
|---|---|---|---|---|---|---|
| 1 (double placebo) | Lower back pain | No | Mild | Remote | Remote | No |
| Redness and swelling at implant site | No | Mild | None | Definite | No | |
| Pain at implant site | No | Mild | None | Definite | No | |
| Pus at implant site | No | Mild | None | Definite | No | |
| Pruritus | No | Mild | Possible | Possible | No | |
| Raised potassium | No | Mild | Remote | Remote | No | |
| Raised urea | Yes | Mild | Remote | Remote | No | |
| Acute deterioration in renal function | No | Moderate | Possible | Possible | No | |
| Heroin overdose | No | Severe | None | None | Yes | |
| 2 (active implant, oral placebo) | Intermittent burning at site of implant | No | Mild | None | Possible | No |
| Nausea/loss of appetite | Yes | Mild | Possible | Possible | No | |
| Implant remains unchanged | Yes | Moderate | None | Probable | No | |
| Diarrhoea | Yes | Moderate | Remote | Remote | No | |
| Retching | Yes | Mild | Remote | Remote | No | |
| Intermittent burning/pain around implant site | No | Mild | None | Possible | No | |
| 4 (placebo implant, active oral) | Pregnancy | No | NA | None | None | Yes |
| 5 (active implant, oral placebo) | Cold | No | Mild | None | None | No |
| Swelling at implant site | Yes | Mild | None | Possible | No | |
| Alapecia | Yes | Mild | None | None | No |
List of abbreviations
- AE
- adverse event
- CM
- contingency management
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- DNA
- did not attend
- DSM-V
- Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- ECG
- electrocardiogram
- eCRF
- electronic case report form
- GAD
- generalised anxiety disorder
- GP
- general practitioner
- ID
- identification
- IMP
- Investigational Medicinal Product
- ISRCTN
- International Standardised Randomised Controlled Trial Number
- KCL
- King’s College London
- KCTU
- King’s Clinical Trials Unit
- KHP-CTO
- King’s Health Partners Clinical Trials Office
- MCCS
- Minnesota Cocaine Craving Scale
- MHRA
- Medicines & Healthcare products Regulatory Agency
- NEAT
- Naltrexone Enhanced Addiction Treatment
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- OST
- opioid substitution therapy
- OUD
- opioid use disorder
- PI
- principal investigator
- PTSD
- post-traumatic stress disorder
- R&D
- Research and Development
- SAE
- serious adverse event
- SLaM
- South London and Maudsely
- SOP
- standard operating procedure
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UDS
- urine drug screen
- WTE
- whole-time equivalent
