Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/44/01. The contractual start date was in November 2016. The draft report began editorial review in May 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mark J Peters is a member of the National Institute for Health Research (NIHR) Health Technology Assessment General Board. Kathryn M Rowan is a member of the NIHR Health Services and Delivery Research Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Peters et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
Fever is a host response that helps to control infections with a very wide range of pathogens. 1 Fever has been very highly conserved throughout evolution for at least 580 million years,1 and is seen across many species including reptiles, birds and mammals. 2 Recently, even plants have been shown to raise core temperatures to control fungal infections. 3 In humans, fever is known to increase numerous basic immunological processes including neutrophil production, recruitment and killing; monocyte/macrophage/dendritic cell phagocytosis; and antigen presentation, T-cell maturation and lymphocyte recruitment. 1
Studies in non-critically ill patients with chickenpox,4 malaria5 and rhinovirus6 infections have led to a rediscovery of the potential beneficial effects of fever. This is recognised by the National Institute for Health and Care Excellence (NICE) in guidance for the management of feverish illness in children,7 which recommends:
Do not use antipyretic agents with the sole aim of reducing body temperature in children with fever.
However, this advice is not aimed at the management of critically ill children.
Observational studies demonstrate that treatment of fever in critically ill children is inconsistent. 8 In this population, there is a lack of robust data to guide antipyretic intervention. This frequently leaves the decision of if and when to treat fever at the discretion of the bedside nurse. There is genuine uncertainty as to whether or not the immunological advantages of a fever in defending the body against viruses and bacteria during critical illness outweigh the metabolic costs and cardiorespiratory consequences of a high fever. 2 In cases with underlying neurological pathology (e.g. traumatic brain injury, hypoxic–ischaemic encephalopathy and encephalomyelitis), practice is to avoid fever because of consistent associations with worse outcomes, but in the much larger proportion of emergency admissions in whom other organ failures predominate (most commonly respiratory) the optimal approach is unknown. With emerging evidence that fever may be beneficial in critically ill adults, but also cognisant of the physiological differences between adults and children, there is an important need to evaluate whether or not a more permissive approach to fever management in critically ill children improves outcomes.
A recent systematic review9 identified five, small, completed randomised controlled trials (RCTs) of antipyretic interventions in critically ill adults. These trials were small (ranging from 26 to 200 participants) and the results of a meta-analysis on intensive care unit mortality were inconclusive [relative risk for fever control compared with no fever control or a more permissive threshold 0.97, 95% confidence interval (CI) 0.58 to 1.63]. One larger RCT among adults, the HEAT trial10 in Australia and New Zealand, examined the effect of acetaminophen (Perfalgan, Bristol-Myers Squibb) (paracetamol) versus placebo to treat fever in 700 critically ill adults with known or suspected infection. No differences were seen in the primary outcome of the number of paediatric intensive care unit (PICU)-free days to day 28 or in mortality. The CASS trial (NCT01455116)11 found a non-significant increase in 30-day mortality in mechanically ventilated adult patients with septic shock who were actively cooled to hypothermia when compared with normal thermal management. Analysis of the trial’s secondary outcomes revealed that induced hypothermia worsened respiratory failure, circulatory collapse and delayed reduction in serum c-reactive protein. We are not aware of any completed or ongoing RCTs comparing antipyretics or fever thresholds in critically ill children.
A systematic review of observational studies of the association between fever and mortality in critically ill adults found wide variation in the definitions of fever and its association with mortality. 12 Two further observational studies in adults, not included in the systematic review, found different relationships between fever and mortality for patients with and without infection, with fever associated with lower mortality among admissions with infection unless the temperature exceeded 40 °C. 13,14 Similar results have been found in small cohorts of critically ill children with infection. 15
The FEVER feasibility study aimed to establish whether or not it is feasible to conduct a clinical trial to evaluate different temperature thresholds at which clinicians deliver antipyretic intervention in critically ill children with fever owing to infection [i.e. comparing a permissive approach to fever (e.g. treat at ≥ 39.5 °C) with a standard restrictive approach (e.g. treat at ≥ 37.5 °C)].
Clinical trials, such as the proposed FEVER RCT, are expensive and the chances of successful completion are improved if both the feasibility and pilot testing of certain key parameters can be clearly demonstrated. Using a mixed-methods approach comprising three separate studies, the FEVER feasibility study included the FEVER qualitative study, the FEVER observational study and the FEVER pilot RCT with integrated-perspectives study.
Aim
The overarching aim for the FEVER feasibility study was to explore and test important key parameters needed to inform the design and ensure the successful conduct of a definitive FEVER RCT, and to report a clear recommendation for continuation or not to a full trial. Each of the three studies had specific objectives.
Objectives
The FEVER qualitative study
To review, with input from parents/legal representatives:
-
the acceptability of the selection of temperature thresholds and options for analgesia for a definitive FEVER RCT
-
potential barriers to recruitment, the proposed process of decision-making and deferred consenting, and co-develop information and documentation for a definitive FEVER RCT
-
the selection of important, relevant, patient-centred, primary and secondary outcomes for a definitive FEVER RCT.
To review and explore, with input from clinicians:
-
the acceptability of temperature thresholds and options for analgesia for a definitive FEVER RCT
-
potential barriers to recruitment, deferred consenting and associated training needs for a definitive FEVER RCT.
The FEVER observational study
-
Estimate the size of the potentially eligible population for the definitive FEVER RCT.
-
Confirm, using empirical data, the temperature threshold(s) currently employed for a standard approach for antipyretic intervention in NHS PICUs.
-
Estimate the characteristics [e.g. mean and standard deviation (SD)] of selected important, relevant, patient-centred primary outcome measure(s).
The FEVER pilot randomised control trial with integrated-perspectives study
-
Test the willingness of clinicians to screen, recruit and randomise eligible critically ill children.
-
Estimate the recruitment rate of critically ill children.
-
Test the acceptability of the deferred consenting procedure and participant information.
-
Test, following randomisation, the delivery of and adherence to the selected temperature thresholds (intervention and control) for antipyretic intervention and to demonstrate separation between the randomised groups in peak temperature measurement over the first 48 hours following randomisation.
-
Test follow-up for the identified, potential, patient-centred primary and other important secondary outcome measures and for adverse event (AE) reporting.
-
Inform the final selection of a patient-centred primary outcome measure.
The FEVER feasibility study management
The FEVER feasibility study was sponsored and co-ordinated by the Intensive Care National Audit and Research Centre (ICNARC) Clinical Trials Unit (CTU) (UK Clinical Research Collaboration ID number 42). A Study Management Group (SMG) was convened, comprising the chief investigator (MJP) and co-investigators (RA, ESD, BF, DAH, NK, PRM, PR, KMR, ST, LT, JW and KW), and was responsible for overseeing day-to-day management of the entire FEVER feasibility study. The SMG met regularly throughout the duration of the study to monitor its conduct and progress. Two parents (CF and JW) and one young adult (BF) (all co-investigators) with experience of a critical illness caused by a severe infection were members of the SMG and provided valuable input into the design and conduct of the FEVER feasibility study, including reviewing documents for parent interviews (e.g. draft FEVER pilot trial participant documentation) and informing study recruitment approaches (i.e. identification of social media groups and charities), in addition to being involved in reviewing study progress and findings.
Chapter 2 The FEVER qualitative study
Study design
The FEVER qualitative study was a mixed-methods study involving semistructured interviews with parents/legal representatives of children with relevant experience, and focus groups (including quantitative data collected with a voting system) with staff in pilot RCT sites.
Research governance
An ethics application was made to the North West – Liverpool East Research Ethics Committee on 16 October 2015 and received a favourable opinion on 21 December 2016 (reference number 16/NW/0826). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/ (accessed 16 November 2018). Local NHS permissions were obtained for the NHS trusts for recruitment routes 2 and 3 (see Recruitment and sampling procedures).
Study management
The FEVER qualitative study was led by a co-investigator (KW). An experienced research associate (ED) was employed to organise, conduct and analyse the interviews and focus groups. The SMG was responsible for overseeing day-to-day management of the entire FEVER feasibility study, including the FEVER qualitative study.
Network support
To maintain the profile of the FEVER feasibility study, updates were provided at national meetings, such as the biannual Paediatric Intensive Care Society Study Group meetings.
Patient and public involvement
Two parents (CF and JW) and one young adult (BF) with experience of severe infection and admission to hospital were co-investigators and members of the SMG. They provided valuable input into the design and conduct of the study, including reviewing documents for parent interviews (e.g. the draft pilot trial participant information sheets) and informing study recruitment approaches (i.e. identification of social media groups and charities). They were also involved in the review of study progress and findings.
Design and development of the protocol
The design and development of the protocol, including sample estimation, recruitment strategy and interview topic guide, were informed by previous trials conducted in paediatric emergency and critical care in the NHS and earlier research. 16–20 Relevant research was used to develop a parent interview topic guide (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019), two participant information sheets for the pilot trial (bereaved and non-bereaved) (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 16 November 2018),17,18,20,21 a list of potential outcome measures17 to inform discussions with parents/legal representatives during interviews (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019) and a staff focus group topic guide (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019).
The interview and focus group topic guides contained open-ended questions and prompts to help explore parent/legal representative and staff views on the acceptability of a definitive FEVER RCT, including the draft FEVER pilot RCT participant information sheets and approach to consent. A separate section of questions was developed for bereaved parents/legal representatives.
Amendments to the study protocol
Minor amendments to the protocol, patient information sheet, schedule of events and statement of activities were approved by the Health Research Authority (HRA) on 10 January 2017.
A substantial amendment to the qualitative study protocol was submitted to the North West – Liverpool East Research Ethics Committee. The amendment requested the inclusion of an incentive description in the study recruitment advertisements and placement of the study advertisements in newspapers. A favourable opinion was received on 1 March 2017.
Recruitment
Participants
Based on previous studies,17,20 it was anticipated that 15–25 parents/legal representatives would be recruited to reach data saturation (i.e. the point at which no new major themes are discovered in analysis). We aimed to conduct a focus group with site staff (nurses and doctors) working in the four PICUs and associated retrieval services involved in the subsequent pilot RCT. We anticipated that a minimum of 4 and a maximum of 10 clinicians would attend each of the four focus groups (16–40 in total).
Eligibility criteria
Inclusion criteria
Parents/legal representatives who have experienced their child being admitted to an intensive care unit with a fever and suspected infection in the preceding 3 years and clinicians (nurses and doctors) working in the four PICUs/retrieval services planned to be included in the pilot RCT (study 3).
Exclusion criteria
Parents/legal representatives who do not speak English.
Recruitment and sampling procedure
For the focus groups, an e-mail invitation and participant information sheet was sent to lead clinicians (FEVER feasibility study co-investigators) in paediatric emergency and paediatric critical care medicine at the four NHS hospitals taking part in the pilot RCT. Co-investigators disseminated focus group invitations to all relevant staff and arranged a location and time for the focus group.
Parents were recruited through four routes (described in the following sections) to maximise the potential sample within the 6-month active recruitment period and to encourage diversity within the sample.
Recruitment route 1: existing database
Eligible parents were identified from an existing database held by Kerry Woolfall at the University of Liverpool, which contained contact details of parents who participated in the Fluids in Shock (FiSh) feasibility study17 and provided consent to be contacted for future related studies.
Recruitment route 2: postal recruitment
Staff used hospital medical records to identify the 15 most recent parents/legal representatives (including up to five bereaved) of children who met the inclusion criteria. Those identified were sent a postal invitation, including a covering letter and participant information sheet describing how to register interest in taking part.
Recruitment route 3: advertising in paediatric intensive care units
Copies of the FEVER qualitative study participant information leaflet and participant information poster were placed in family/relative waiting rooms and notice boards near the PICUs.
Recruitment route 4: media advertising
An advertisement was posted on Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) and Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com), which invited parents/legal representatives to register interest in participating in the study. Relevant charities and parent support groups were asked to place the advertisement on their website and social media. Data saturation was reached before an advertisement was placed in a newspaper.
Screening and conduct of interviews and focus groups
Interviews
Screening
Parents’ expressions of interest to participate were responded to in sequential order. Once eligibility was confirmed, an interview date and time were scheduled. The draft pilot RCT participant information sheet (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 16 November 2018) and list of potential outcomes (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019) were e-mailed to parents to read prior to the interview. Screening and interview conduct stopped when data saturation and sample variation (recruitment of parents via multiple recruitment routes) were achieved.
Informed consent
Audio-recorded verbal consent was sought over the telephone before the interview. This involved reading each aspect of the consent form to parents, including consent for audio-recording and to receive a copy of the findings when the study is complete. Each box was initialled on the consent form when verbal consent was provided. Informed consent discussions were audio-recorded for auditing purposes.
Conduct of the interviews
Interviews began with a discussion about the aims of the study, an opportunity for questions and a check that the parent had sufficient time to read the draft pilot trial participant information sheet and list of potential outcomes (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019).
The interview then commenced using the interview topic guide to explore:
-
the experience of having a child admitted to the PICU with a fever and suspected infection
-
previous experience of participation in clinical trials
-
length and content of the draft pilot RCT participant information sheet
-
the acceptability of restrictive (e.g. ≥ 37.5 °C) and permissive (e.g. ≥ 40 °C) temperature thresholds
-
the acceptability of research without prior consent (RWPC) in paediatric research and in the proposed FEVER RCT
-
the identification of potential barriers to participation in the trial and how these could be addressed
-
the identification of potential facilitators of trial participation
-
trial design, including the selection of outcome measures
-
whether or not parents would (hypothetically) consent to the use of their child’s data in the proposed FEVER RCT.
Example questions can be found on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019). Respondent validation was used to add unanticipated topics to the topic guide as interviewing and analysis progressed. After the interview, participants were sent a copy of the consent form and a thank you letter, including a £30 Amazon (Amazon.com, Inc., Bellvue, WA, USA) voucher to thank them for their time. A copy of the consent form was retained by the University of Liverpool.
Focus groups
Informed consent
At the start of the focus group, Kerry Woolfall or Elizabeth Deja checked that all participants had read the participant information sheet and understood the purpose of the focus group. The focus group/interview aims and topics to be covered were discussed, followed by an opportunity for questions. Participants were asked to provide written consent using the consent form before the focus group and audio-recording began.
Conduct of the focus groups
A voting system, using TurningPoint software (Turning Technologies, Youngstown, OH, USA), was used alongside verbally administered questions. This involved some of the key questions being presented to the group via a laptop presentation and each participant using a wireless handset to select their answer from those shown on the screen. A test question was used at the beginning of the focus group to help demonstrate how the voting system would work alongside verbally administered questions. This method was used to enable the collection of data from all participants, as well as a means of generating statistical data from all sites alongside qualitative data from group discussions. Once it was established that the handsets were working with the test question, staff were asked to introduce themselves, their role within the PICU and past experience of recruiting to clinical trials.
The focus groups explored site staff views and experiences on:
-
current temperature thresholds for treating pyrexia
-
the acceptability of the proposed FEVER RCT including the selection of temperature thresholds and options for analgesia
-
perceptions of the use of RWPC in the RCT
-
potential barriers to recruitment and consent in the trial and how these might be addressed
-
potential difficulties in adhering to the trial protocol
-
training needs.
Respondent validation22 was used to add unanticipated questions to the topic guide as data collection and analysis progressed; for example, queries raised by staff about the pilot RCT inclusion criteria (e.g. would children need to be on a ventilator to be included in the study?) were added to the topic guide to explore in subsequent focus groups. Once a focus group was completed, staff were thanked for their time and participation in the study.
Transcription
Digital audio-recordings were transcribed verbatim by a professional transcription company (Voicescript Ltd, Bristol, UK) in accordance with the Data Protection Act 1998. 23 Transcripts were anonymised and checked for accuracy. All identifiable information, such as names (e.g. of patients, family members or hospital their child was admitted to), were removed.
Data analysis
Qualitative data analysis of the interviews and focus groups was interpretive and iterative (Table 1). Utilising a thematic analysis approach, the aim was to provide accurate representation of parental views on trial design and acceptability. Thematic analysis is a method for identifying, analysing and reporting patterns (or themes) within data. Analysis was informed by the work of Braun and Clarke24 and their guide to thematic analysis. This approach allows for themes to be identified at a semantic level (i.e. surface meanings or summaries) or at a latent level (i.e. interpretive – theorising the significance of the patterns and their broader meanings and implications). 28 NVivo 10 (QSR International, Warrington, UK) software was used to assist in the organisation and coding of data. Elizabeth Deja (a psychologist) led the analysis with assistance from Kerry Woolfall (a sociologist).
| Phase | Description |
|---|---|
| 1. Familiarising with data | Elizabeth Deja read and re-read transcripts, noting down initial ideas |
| 2. Generating initial codes | Initially, a data-coding framework was developed using a priori codes identified from the project proposal and the interview topic guide. During the familiarisation stage, Elizabeth Deja identified additional data-driven codes and concepts not previously captured in the initial coding frame |
| 3. Developing the coding framework | Kerry Woolfall coded 10% of the transcripts using the initial coding frame and made notes on any new themes identified and how the framework could be refined |
| 4. Defining and naming themes | Following review and reconciliation by Elizabeth Deja and Kerry Woolfall, a revised coding frame was subsequently developed and ordered into themes (nodes) within the NVivo (QSR International, Warrington, UK) database |
| 5. Completing coding of transcripts | Kerry Woolfall and Elizabeth Deja met regularly to discuss developing themes, sample variance and potential data saturation by looking at the data and referring back to the study aims. When saturation was achieved, data collection stopped. Elizabeth Deja completed coding of all transcripts in preparation for write-up |
| 6. Producing the report | Elizabeth Deja and Kerry Woolfall developed the manuscript using themes to relate back to the study aims, ensuring that key findings and recommendations were relevant to the FEVER RCT design and site staff training (i.e. catalytic validity).24–27 Final discussion and development of selected themes took place during the write-up phase |
Results
Participants
Parents/legal representatives
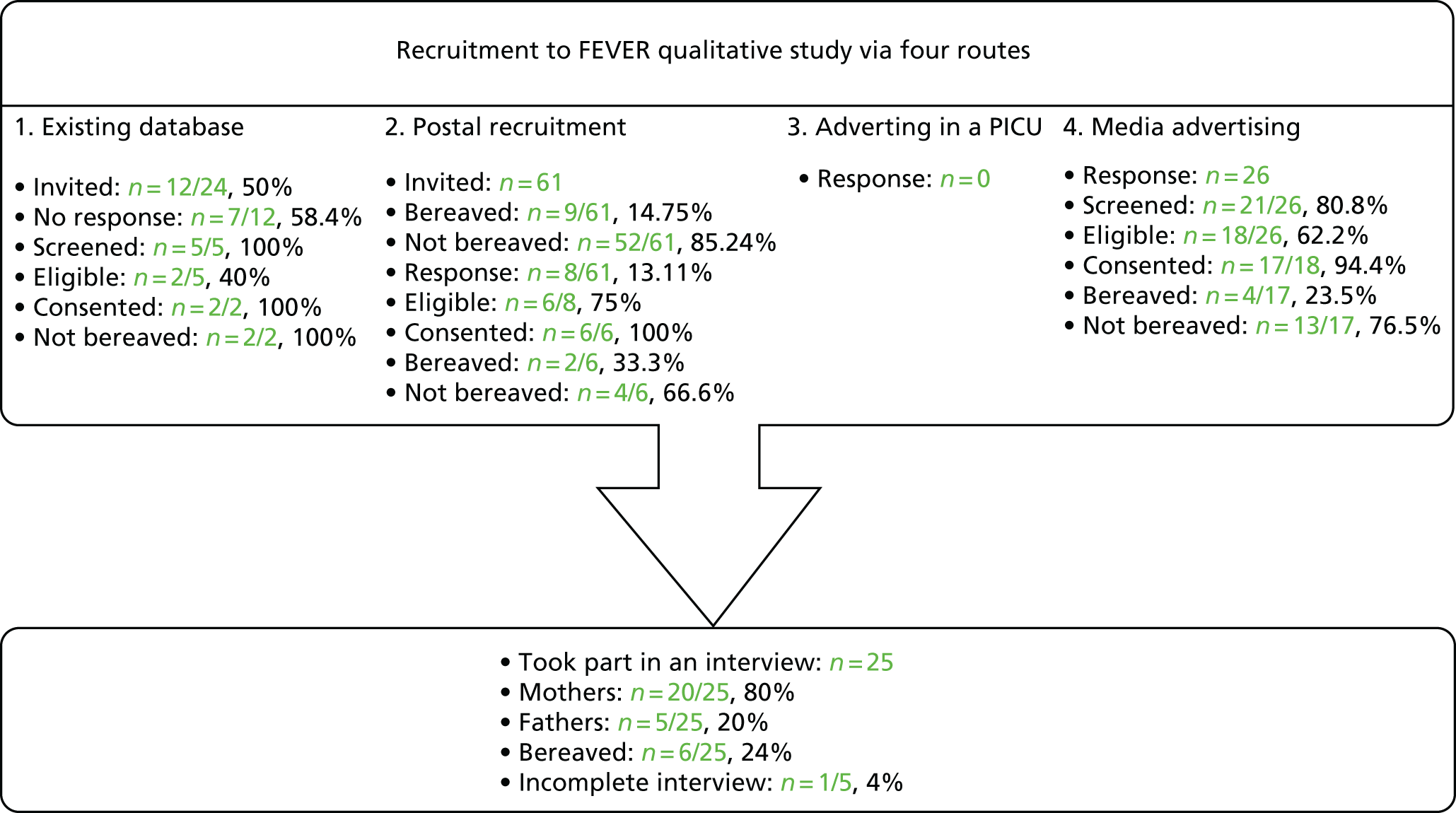
A total of 46 parents registered interest, of whom 34 were screened (Figure 1). Eight were deemed ineligible and one did not confirm a date for interview. No individuals identified themselves as legal representatives; therefore, this term is not used in the remainder of this chapter.
FIGURE 1.
Recruitment to the FEVER qualitative study.
Parent characteristics
The sample included 25 parents: 20 mothers (4 bereaved) and 5 fathers (2 bereaved). Bereaved parents were interviewed at a mean of 19 months (SD 9.8 months) since their child’s admission (range 12–38 months). Children were a median age of 1.96 [interquartile range (IQR) 4.21] years (range 0.0–15.58 years) and had a median hospital length of stay of 12.5 (IQR 33.25) days (range 0.5–140 days) owing to streptococcus (n = 2), meningitis (n = 2), pneumonia (n = 1) and sepsis (n = 2). One bereaved father stopped the interview before diagnosis information was collected. Non-bereaved parents were interviewed a mean of 15.63 months (SD 6.5 months) since admission (range 6–29 months). The children had a median age of 1.5 years (IQR 3.87 years) (range 0.0–15.58 years) and a median hospital stay of 30.67 days (IQR 37.35 days) (range 7–140 days) for streptococcus (n = 2), meningitis (n = 4), sepsis (n = 6), chest infections (pneumonia, n = 5, and bronchitis, n = 2), toxic shock (n = 1), varicella (n = 2) and unknown infection (n = 2).
A total of 9 out of 24 parents (36%) had previous experience of trial participation either directly (themselves) or indirectly (through their child’s participation). Five parents (20%) had previous knowledge of RWPC. Ten out of 24 parents (41.7%) could not recall what temperature their child reached during their admission to hospital with a fever and severe infection. Of those who could recall, 7 out of 24 (29%) stated that their child’s temperature had exceeded 38.0 °C, whereas 5 out of 24 (21%) stated that their child’s temperature had exceeded 40 °C. A total of 15 out of 24 parents (63%) reported giving their child paracetamol at home, whereas the children of 13 out of the 24 parents (54%) were known to have been administered paracetamol by staff in hospital. Interviews took, on average (median), 47.59 minutes (SD 19.26 minutes) (range 15–105 minutes).
Site staff perspectives
A total of 56 staff took part in six focus groups across the four sites, with a median of 49.72 minutes (SD 11.14 minutes) in length (range 30.59–59.48 minutes). Staff mainly self-identified as nurses [20 junior nurses (36%) and 25 senior nurses (45%)]; there were also eight senior doctors (14%) and three junior doctors (5%). All were involved in the clinical care of children.
Parent perspectives
Support for the proposed FEVER randomised controlled trial but some concerns
Prior to the interviews, participants were sent copies of the draft participant information sheet and a list of outcome measures to read through. At the start of the interview, parents were asked questions about their experiences of their child’s hospital admission and the interview then moved on to discussions about the proposed FEVER RCT.
Overall, parents supported the FEVER RCT and all stated that they would give consent for the use of their child’s information in the trial. This acceptability was based on a number of factors, including the child still being treated for the infection; the intervention not being invasive; trust of medical staff; how trial findings might help other children in the future; and the trial question being viewed as important, including whether or not the question being tested ‘makes sense’ in that not treating fever might be beneficial:
Because I think you know from your own kind of anecdotal experience your child’s at home, when they spike a fever, when they’re getting, when they’re ill with something, quite often they feel a lot better afterwards.
P25, mother, non-bereaved
Yeah. Yeah. Because I think erm ‘cause fever is meant to be like part of a fighting off, healing process isn’t it? A natural one. So I can, I can understand exactly why it would be interesting to see what happens.
P07, mother, non-bereaved
No, not if it’s helping other kids. Obviously that’s what they’re there for do you know what I mean, it’s what they’d, I’d expect them to do that, to use it for other kids and stuff.
P09, mother, non-bereaved
Despite parents describing their support for the study, many voiced specific concerns about the acceptability of the higher temperature threshold. Most commonly, parents described their concerns about the negative impact of the higher temperature threshold and if not treating a child’s temperature would increase the likelihood of them having seizures:
I would worry about seizures and I’d worry, that’s what I would worry about, and are we making her more ill by not medicating early?
P03, mother, non-bereaved
Parents were also concerned that children randomised to the higher temperature threshold, may suffer unnecessary discomfort, pain or other detrimental side effects, such as ‘organs shutting down’ (P07, mother, non-bereaved), ‘riggers’ (P06, mother, bereaved) or ‘death’ (P18, father, non-bereaved):
Other problems can arise with a temperature being too high, that, that could have adverse effects on the child or baby regardless of the infection because there’s different problems that it could cause.
P02, mother, bereaved
However, parents stipulated that these concerns were largely addressed by their trust in staff to monitor the children and do what is best for their child:
I think I would trust that my child was being monitored, it’s not like they’re waiting for her condition to get worse before they do something, it’s deciding on a different course of treatment. That wouldn’t concern me, the difference between 39.5 and 40 [°C] . . . I think you’re in that environment where when you are having one-on-one care from, um, a nurse by your bedside at all times, I just had complete trust. And I know that the parents from the beds around us felt the same.
P25, mother, non-bereaved
When a parent spoke of their concern about a high temperature causing seizures, the interviewer explained that that current evidence suggests that fever does not cause a seizure, but rather a seizure is a symptom of an infection. This information was valued by parents, all stating that this explanation needs to be included in trial discussions and information materials, as it would change their views on how acceptable they found the higher temperature threshold in the proposed trial:
Yeah, it’s the infection itself causing the seizure rather than the temperature.
Oh, OK. Could you put that in (trial materials) somewhere?
That’s interesting. OK, OK, so then if that could be explained, I think that’s important to really reiterate to parents.
P03 mother, non-bereaved
Acceptable temperature thresholds
While considering the acceptability of the two proposed temperature thresholds, many parents noted that their opinions on the study were influenced by their understanding of what is ‘normal’ paracetamol use. In their experience, if a child is unwell, has a temperature or is in pain, the first course of action would be to administer an antipyretic such as paracetamol. This was evidenced by parents (15/24, 63%) who said they had given paracetamol to their child prior to taking them to hospital. As shown in the following quotations, parents were concerned that children would be uncomfortable and feel ‘unwell’ if paracetamol was not administered until a high temperature:
It’s normal of a parent if your child, or you know, yourself, if you have a temperature you take paracetamol or Calpol® [Johnson & Johnson Limited], um, and I understand that there’s compelling evidence that, you know having a temperature is the sort of normal response, and therefore actually are we, should we not be treating it. But I suppose at the point where your child has got a temperature, you know generally they’re really not very well, and you just want to make them feel better. And you know what you feel like with a temperature, you know you feel so much better when you’ve had something like paracetamol. So I think I would just feel uncomfortable that my child perhaps was, you know, left to be uncomfortable without having something to cool them down. Um, I think that was what was worrying me about it – I guess it’s just that sort of we’re very used to thinking that you have a temperature and therefore you take something like Calpol or paracetamol.
P01, mother, non-bereaved
When you’re at home with your bottle of paracetamol, as soon as it goes above [laugh] you know, 37 [°C], you start dosing them up don’t you, to try and make them feel better. But these are, these are seriously ill children aren’t they, they’re not going to be feeling great anyway.
P07, mother, non-bereaved
When explicitly asked about the acceptability of waiting until 39.5 °C or 40 °C to treat a child’s fever, many stated that waiting to treat at 40 °C was acceptable. However, parents did state that it is ‘a really frightening temperature, isn’t it? I mean they’re boiling at 40 [°C]’ (P01, mother, non-bereaved). Parents stated that they would give consent for their child’s participation in a trial that did not treat a fever with antipyretics until 40 °C as they trusted staff to act in their child’s best interests. However, owing to concerns about 40 °C being a high and ‘scary number’, the majority suggested that the FEVER RCT would be more acceptable if the higher threshold was set at a lower threshold such as 39.9 °C or 39.5 °C:
I think that, that’s a scary number again when you see you’re little one, err, up at 40 [°C]-odd, you know. It’s, it’s all numbers that you kind of take in, ‘cause they were just staring, that’s all you’re looking at for 24 hours a day, is, is his numbers and hoping they’re taking a turn for the better. So, um, yeah, perhaps I think 39.5 [°C]. But again you guys know best and I think, as I say, you do, do what you need to do, but I’m just saying that is a . . . I think that’s very hot. That would concern me, err, as I say, err, if it’s gone, gone too high before you, before you treat it, if that makes sense?
P17, father, non-bereaved
I would probably go with 39.9 [°C] . . . Yeah. It does sound slightly more agreeable, doesn’t it? 40 [°C] does sound a lot.
P07, mother, non-bereaved
Parents were less concerned about the lower temperature threshold, although a few parents did state that 37.5 °C might be too low a temperature to administer an antipyretic. Some expressed concern that a child may not have a fever at this temperature and may therefore be treated unnecessarily, or the antipyretic might mask how poorly they are:
I remember one night on the ward he, he wasn’t sleeping and one of the nurses said, ‘do you think he wants paracetamol?’. I remember another nurse remember saying, ‘No, I don’t, I think he’s, he’s not in pain. Don’t say yes to paracetamol unless you really do think he needs it because you could be masking something then.’ And I always remember thinking that like, ‘Yeah, what if he like . . . What if his temperature goes up but we’ve given paracetamol and then we don’t realise something’s up with him?’. You know what I mean?
P22, mother, non-bereaved
Research without prior consent
Research without prior consent is acceptable but parents held concerns about the emotive situation.
A definition of RWPC was first read to parents (Box 1). All parents responded positively to the use of RWPC in critical care research and described how, although they would prefer to be asked before their child was entered into a trial, they understood that in time-limited situations this would not be appropriate or possible without delaying emergency treatments:
I think I would feel like I would want to know about it but at the same time I understand why they couldn’t always ask you about it because of how quick, because as we know how quickly things can change and they have to make decisions.
P21, mother, bereaved
I understand why it would have to be done, I understand that it’s important obviously not to delay treatment of the child, I mean I was hysterical at the point that he was admitted to ITU [intensive care unit], so I appreciate it wouldn’t be a time to start having to sit down and decide if you want your child to take part in a study.
P01, mother, non-bereaved
Owing to the need to treat a patient in an emergency without delay, or because parents may not always be present when a child needs treatment, it is not always appropriate or possible to obtain consent before a child is entered into a trial. To enable research to be conducted in the emergency setting, many countries (including the UK) allow consent to be sought as soon as possible afterwards. This is for permission to use the data already collected and to continue in the trial. This is research without prior consent (sometimes called deferred consent). Research without prior consent is a relatively new approach to seeking consent in the UK.
When asked specifically about the acceptability of RWPC in the proposed FEVER RCT, parents made similar comments, such as ‘I understand there’s not really another way you can do it’ (P01, mother, non-bereaved). Many described how they would not be in a position to give informed consent at that point in time as ‘there was panic at that point, so definitely can’t be when it’s all going on. I wouldn’t have been listening’ (P18, father, non-bereaved). One mother commented that she found RWPC reassuring as ‘it’s good because well they’d be treating the child first and prioritising them’ (P16, mother, non-bereaved).
Although the majority supported the use of RWPC, a few were concerned that in such an emotive situation they, or other parents, might be angry or upset at finding out that their child had been placed in the study without their knowledge or consent:
I’d just feel really cross actually, I think, if I found out that this had been done to my child without my consent. Um, even though I understand that you can’t really take consent at the point that the child is that unwell, I think I would just feel that someone else had made that decision. And even though I know that you know it’s not me making the decision about my child’s care anyway, you know if they say that he needs antibiotics, if they say he needs a lumbar puncture, and if actually I don’t like it of course he’s going to have it. I don’t know what it is about this that I would feel uncomfortable about.
P01, mother, non-bereaved
It feels like an experiment. And, as I say, you already feel pretty powerless so I think it’s, it is worth thinking about how people would react to it. ‘Cause I think you would get some bad reactions in some cases.
P12, father, non-bereaved
Parents suggested that the consent process should be carefully managed in order to limit the potential negative impact of RWPC on recruitment and clinician–parent trust. They recommended that clinicians should use their judgement about when it is appropriate to approach families for consent. They also recommended that parents should be informed at the earliest possible time, ‘as long as your child is stable’ (P01, mother, non-bereaved), when the emergency situation has past and ‘people have stopped rushing about and everything’s a bit calmer’ (P03 mother, non-bereaved). All emphasised the importance of a clear explanation of the reasons why their child had been entered into a clinical trial without parental informed consent:
I think you need to communicate why it’s been done in a very clear, very simple way.
P21, mother, bereaved
Interestingly, most parents felt that they would be unlikely to notice that their child’s temperature had not been treated with an antipyretic owing to their emotional distress before their child was stable, as well as all the other medical procedures happening at that point in time. This view is supported by the fact that just under half of parents interviewed (10/24, 41.7%) were unable to recall what temperature their child had reached during their time in the PICU.
Nevertheless, some did suggest that parents of children randomised to the higher threshold ‘would notice’ (P17, father, non-bereaved) that their child’s temperature was not being treated and would therefore broach the subject with the bedside clinician. When it was explained that in this situation clinicians would provide parents with a brief outline of the study, including details of how ‘their child’s treatment was a priority and they would be spoken to in more depth later’, parents felt that this was an acceptable and appropriate response and overall ‘it sounds OK’ (P17, father, non-bereaved).
One father articulated the complexities of information provision in this stressful situation, highlighting the need for site staff to tailor the level of detail provided to meet individual needs:
I think I’d have asked the question, why he’s 38 [°C] and a half, 39 [°C]? Why haven’t you given him any paracetamol? I think I’d have probably asked that question myself, if you weren’t treating him until he was 40 [°C], for example? So I think I would have naturally asked the question anyway. But, err, I don’t suppose everybody else, everybody would. I don’t suppose my wife would’ve because she was just in complete shock . . . You’d want to know more about it then, I think, I don’t think that would sat . . . If you told me that little snippet of information, I’d want to know everything about it. I think. I’d rather, again I think I’d go back to the point just, I think just yeah, just do, do what you think is best at this moment in time. And then if it is a research thereafter, then talk to me about that thereafter.
P17, father, non-bereaved
The FEVER pilot randomised controlled trial participant information sheet
All parents considered the participant information sheet (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 16 November 2018) to be ‘fairly clear’ (P10, mother, non-bereaved), ‘make sense’ (P02, mother, bereaved) and written in comprehensible language:
I think they would understand it. It’s not been put in medical jargon, it’s simple for them to read.
P06, mother, bereaved
Parents identified parts of the participant information sheet that required clarification, including if not treating a temperature could cause a seizure, the importance of explaining RWPC at an early point in the recruitment discussion, that all other treatments would be given and some formatting suggestions (Box 2).
-
Does not treating temperature increase the chance of seizure?
-
How long will they be in the trial for?
-
What if my child has already had paracetamol?
-
It needs to be clear from the start that the child has already been entered in the study.
-
Needs highlighting that the child is receiving all other treatment.
-
Would prefer it if the headings were not in red.
Given the emotive situation in which the participant information sheet was going to be read, many suggested that the draft participant information sheet was too long and would benefit from all key information being summarised on the first page. Parents said that this format would provide them with the essential information needed at the time of the consent discussion with the option to read the rest of the information sheet at a later time. Consequently, during later interviews, we explored views on preferred information for the ‘important things you need to know’ section on the first page of the participant information sheet. As a result, the FEVER pilot RCT participant information sheet summary section was revised using information parents prioritised when considering FEVER information (Figure 2).
FIGURE 2.
Important things that you need to know: revised participant information sheet.

Consenting bereaved parents
The researcher asked bereaved parents to consider a scenario in which their child had been entered into the trial before death and a practitioner approached them after death to discuss the trial. All stated that it was important to approach bereaved parents for consent after a child’s death because they would want their child’s information to be used if it could help other children in the future:
If people feel anything like me then they’d want to do anything to save other children and help other children really. Um, and any data that can be taken from the loss of a child will, could help another.
P19, mother, bereaved
Despite this, as P15 (mother, bereaved) noted, although ‘I think I’d be OK about it’ others ‘might feel angry’ and not give consent for the use of their child’s data. In addition to anger, many anticipated that bereaved parents would question practitioners about whether or not involvement in the study had affected their child’s survival: ‘they’re gonna then ask, aren’t they, could it have been the case that ‘cause he was in the study, that’s why he died?’ (P21, mother, bereaved).
Bereaved parents’ views were then sought on the most appropriate way of contacting families to discuss the trial following the death of a child (Box 3). As shown in other studies,17,20 there was disparity in views about the most appropriate timing and method of approach. Indeed, the mother and father who were jointly interviewed had conflicting views on how and when they should be approached:
. . . but 4 weeks struck me as too soon actually, just thinking that, you know, I just wasn’t really in a space to think about anything really, um.
No. But then, I’d also, my gut instinct was like I’d rather have known kind of right at the time, like almost straight away.
The research associate presented several options to consider:
-
Face-to-face discussion with a nurse or doctor.
-
Telephone call by a nurse or doctor.
-
Personalised letter 4 weeks after randomisation, followed by a second letter 8 weeks after randomisation (i.e. if no response is received after sending the initial letter). Letters would explain how to opt out of the study and that there would be no need to respond if they wanted their child’s data to be used in the trial.
The above quotations suggest that it is difficult to make general recommendations on how best to approach parents in this situation and that an individualised approach to consent may be most appropriate. Bereaved parents described how they trusted hospital staff to make an appropriate case-by-case assessment of the best way to approach bereaved families about the study. They recommended that regardless of how parents would be approached (e.g. face-to-face or postal contact) or timing, the communication needed to be honest, personalised and delivered with compassion. It was also accepted that if a letter was sent, that the proposed time frame of 4 weeks then 8 weeks after death with a ‘opt-out’ option was acceptable:
‘Cause I think parents have a lot on their mind and they could be organising funerals and things and could forget to reply.
P15, mother, bereaved
Outcomes of importance to parents
The list of outcomes and accompanying descriptive text sent to parents prior to the interview can be found on the project web page (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019). In this section of the interview, a definition of an outcome was first read to parents, including an explanation about why it is important to explore parents’ perspectives about important outcomes (Box 4). Parents were then asked:
-
Thinking about your experience of your child being admitted for a fever and suspected infection, what would you hope the treatment (e.g. paracetamol and/or cooling) would do to help your child? (Prompt: what effect would the treatment have to be useful?) What would you be looking for as an indicator that your child was getting better?
As we have discussed, in the FEVER RCT we want to find out whether or not critically ill children with symptoms of severe infection should be given treatments for fever at a higher temperature (up to 40.0 °C) than usual (up to 37.5 °C).
To do this, we will collect information on (read through outcome measures list).
By collecting information on these main things, we hope to find out which treatment for fever should be used in the future. These are called outcome measures.
However, these outcomes have come from research papers and don’t really give us much information on how children or families feel, or what is important to them. It is important that we include outcome measures that matter to children and their families.
Parents found outcomes listed as ‘reasonable’ (P01, mother, non-bereaved) and comprehensive ‘I think everything’s sort of covered really’ (P24, mother, non-bereaved). The majority of outcomes identified coincided with the predefined short-term (measured during a child’s hospital stay) and longer-term (measured at the end of care or following hospital discharge) categories. Many of the outcomes identified and prioritised by parents were included in the list provided. Three additional outcomes were identified:
-
looking and/or behaving like their normal self (examples included improved mood, communication, more like themselves and more alert, sitting up and start to eat and drink)
-
number of visits to general practitioners and other health professionals as a result of illness
-
infection levels coming down.
The following list presents outcomes identified in the analysis of parent descriptions. Outcomes are listed in order of importance (i.e. defined as how many parents mentioned a particular outcome when directly asked which indicators were most important to them or inferred significance from wider interview discussion). Parents prioritised the following outcomes:
-
long-term morbidity (e.g. kidney function, amputation, skin grafts, teeth pushed back, bowel sections removed affect nutrition absorption, difficulties in school, effect on memory, development or learning and post-sepsis syndrome
-
looking and behaving more normally
-
length of time on breathing support
-
time in PICU and hospital
-
how quickly vital statistics are back to normal.
Interestingly, only two parents explicitly stated that survival should be an outcome measure in the proposed FEVER RCT.
Site staff perspectives
Perceived current practice
At the beginning of the focus groups, site staff were asked to use TurningPoint handsets to identify the temperature threshold they use for administering antipyretic interventions in a child with a fever and suspected infection. As shown in Table 2, the majority of site staff indicated that they would administer antipyretic interventions between 38.1 °C and 38.5 °C. Those who indicated that they would administer at a higher threshold (38.6–39.6 °C) were senior doctors or nurses. Only one junior doctor indicated that they administer antipyretic interventions at 39.1–39.5 °C.
| Temperature threshold (°C) | Participants, n (% within threshold) | ||||
|---|---|---|---|---|---|
| All site staff (N = 53a) | Junior doctors (N = 3) | Senior doctors (N = 7) | Junior nurses (N = 19) | Senior nurses (N = 24) | |
| 37.5–38.0 | 4 (7.5) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 1 (25.0) |
| 38.1–38.5 | 34 (64.2) | 1 (2.9) | 1 (2.9) | 15 (44.1) | 17 (50.0) |
| 38.6–39.0 | 14 (26.4) | 1 (7.1) | 6 (42.9) | 1 (7.1) | 6 (42.9) |
| 39.1–39.5 | 1 (1.9) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Although staff in two sites referred to defined thresholds at which they would administer an antipyretic on their unit (38.5 °C in one unit and 38.1–38.5 °C in the other), the majority described how they made individual decisions about temperature thresholds on a case-by-case basis. Many stated that they would lower the threshold at which to administer an antipyretic if they were concerned about a child’s clinical condition and/or the child was showing visible signs that they were in discomfort or were unhappy. Specific conditions or injuries, such as head injuries, tachycardia or febrile seizures or neutropenia, were viewed as requiring a more aggressive temperature management:
Or a child that let’s say is neutropenic, and we don’t get that often . . . but we do get children, then I might be more aggressive and lower my threshold. At least with some kind of consultation with the medical team about actually I’m worried that this child is, um, more compromised than other and therefore is their temperature telling me something that I need to act quicker about.
P01, FG5
Child is miserable, grumpy, and he’s tachycardic of 180 [beats per minute] I would treat that pyrexia.
P04, FG3
Some staff described how they would administer an antipyretic at a lower threshold than they would normally use if parents made a specific request. As the following quotations illustrate, parents’ views on what was a high temperature for their child or a direct request from parents to give their child paracetamol even at a relatively low temperature of 37 °C were acted on, if a child was also showing signs of being in discomfort:
Like I had a patient the other day, the temperature went up as high as 37 [°C], so it’s hardly a temperature but the parent said ‘can we give paracetamol’ and the child was a bit unsettled, so I said ‘yeah’.
P06, FG2
So even families might say to me, oh, but, you know, 37 [°C] is really high for him.
P01, FG5
Acceptable temperature thresholds for the FEVER randomised controlled trial
The researcher sought staff views on acceptable temperature thresholds for the two treatment groups in the proposed FEVER RCT through the use of voting handsets and group discussion.
Site staff views on an acceptable restrictive temperature threshold
The majority (80%, 43/54, two missing) indicated that 38.0 °C should be the lower, restrictive temperature threshold. There were no differences (p = 0.816) in views by staff role, with only 11 members of staff [9/44 (20%) nurses, and 2/10 (20%) doctors] selecting 37.5 °C as the acceptable restrictive threshold for the definitive FEVER RCT.
Concerns about the restrictive threshold in the FEVER randomised controlled trial
Many described how 37.5 °C would be too low a threshold for the trial as it was a ‘normal’ (P01, FG4) temperature and could result in administering an intervention unnecessarily. A few members of staff highlighted the potential impact of the environment and how a temperature of 37.5 °C could be attributable to a hot PICU, which can be controlled without the need for medication:
I’ll tell you what I’ll be more, much more happier with 38 and 39.5 [°C] rather than . . . What I will not be happy is 37.5 and 39.5 [°C] because I don’t want to treat unnecessarily.
P02, FG3
Some children have a temperature 37.5 [°C] is normal.
P01, FG1
We try and give the minimum amount of drugs . . . on this unit, and you would just think why am I giving this . . . at this temperature. Why am I giving this drug for a temperature?
P02, FG5
I think for me it’s about, um, the impact of the, um, environment, and actually as a bedside nurse you’ve got quite a lot of control upon your environment, and bringing the temperature down in that way. And I’ve been in many situations where maybe the baby therm [thermometer] is a bit too warm, or that the unit’s warm and actually when the temperature is 37.5 [°C] and you change it environmentally.
P05, FG5
One focus group participant described how treating a fever at 38 °C was lower than the threshold used in their unit, so they would be personally concerned that the use of an even lower restrictive threshold of 37.5 °C in the FEVER RCT would be changing the standard of clinical care for children presenting with fever and suspected infection:
Well my reasoning was if we don’t normally treat at 38 [°C] why would we then lower the standards of what we’re normally doing in order to do a study?
P05, FG4
There was some discussion about the implications of agreed temperature thresholds on the success of the trial. As the following quotations illustrate, one doctor stated that asking clinicians to treat children differently to their ‘normal’ practice in both the control group and the intervention group may cause concern and have a negative impact on trial success. In contrast, one nurse stated that she had selected 37.5 °C as the acceptable restrictive group threshold for FEVER, as this lower threshold would help ensure sufficient separation between the two trial groups:
I guess I’d have some concerns for a trial if you have a control arm which is not your usual practice, then you might have a problem because then you’re asking people to do something in the control arm and in the intervention arm which is, both of which are different to your current practice.
P03, FG1
I just think, er, I put 37.5 [°C] because I thought would you not get, kind of, a better differentiation of the results. Because, er, if there’s going to be an upper arm as well is it not best to have the lower arm at the lower level so then there might be bigger differences as the results come in.
P07, FG4
Site staff views on an acceptable permissive temperature threshold
Using voting handsets, 82% of staff (45/55, one missing) selected 39.5 °C as an acceptable temperature threshold for the permissive group of the FEVER RCT, whereas only 10 members of staff (18%, five doctors and five nurses) selected 40 °C.
Importantly, the majority of staff were in agreement that that a permissive threshold of 40 °C would not be acceptable:
I think again, if you really want a true answer in your trial you should try for 40 [°C], but realistically I think we will struggle to convince people to let that child wait till 40 [°C].
P08, FG6
A few members of staff described how a permissive threshold of 40 °C may provide a more definitive answer to the trial question. These staff appeared to be more supportive of the study than other participants; they also tended to give examples of personal clinical experience in which it was normal to wait until a higher temperature threshold to treat a fever.
Concerns about the permissive threshold in the FEVER randomised controlled trial
Concerns about not treating a child’s fever until it reached a permissive threshold (e.g. 39–40 °C) included an increased risk of febrile seizures or rigors, discomfort, tachycardia or hallucinations:
Seizures, you’re running the risk I would say of them getting to, er, febrile convulsions.
P08, FG4
I’ve seen kids hallucinate at 39 [°C] so . . .
P01, FG1
An 11-year-old boy who was 39.5 [°C] and he was just on another planet, like he was just so uncomfortable and, you know, you just, as a nurse, to leave that would just, again, that word uncomfortable.
P05, FG5
But also every, every degree above normal you go you increase cardiac workload by, by, by about 15% so, and [inaudible 0:10:08] the child with a temperature of 39 [°C] and a heart rate of 200 [beats per minute], I can’t leave that so I, I have to intervene.
P02, FG1
Some staff described how it would be ‘incredibly difficult to wait and watch’ (P05, FG5) and not administer an antipyretic intervention to a child who was visibly uncomfortable or unsettled with a high temperature. Others discussed the lack of evidence for current practice and the need to conduct the FEVER RCT, yet it seemed that many lacked confidence in waiting to treat in the permissive threshold group as inaction goes against their clinical training or ‘gut instinct’ (P05, FG2):
I think is just a hangover, this is the way, these are the sort of numbers that have triggered your response for decades and there ain’t no science.
P02, FG3
You, [staff name] absolutely right. Well there is, there is a bit of science which suggests we should let the temperature get higher.
P01, FG3
With this it’s more like it’s a gut instinct, isn’t it, ‘cause it’s been drummed into you from the day you start your training and all the pain days, you have to give the paracetamol as well for morphine uptake to improve. And that’s going to be, it’s going to be one of those oh the kid’s temperature 38.5 [°C], it’s going to be an automatic reaction so it’s sort of breaking that as well as . . . it doesn’t matter how much you educate, there’s always going to be that gut reaction first of all.
P05, FG2
One nurse spoke of how it was it was her responsibility to assist children’s recovery by providing treatments that reduce a body’s workload. The need to be proactive and control a child’s temperature was viewed as an important part of a nurse’s role:
That as nurses I think that’s our job, is to maximise their output, I suppose, by reducing the demands. And temperature is one of the things that we can control, we can’t control some of the other stuff, um, but we can control, um, temperature. And I think, and actually it’s probably almost our job, you know, it’s not the medics that come along and administer paracetamol, or strip the child off or cool them, so I think there probably is a very active role that the nurses deliver that intervention. So actually I think even 39.5 [°C] feels quite uncomfortable to me, that, um . . .
P03, FG5
Site staff drew on their own personal experiences, or their child’s experiences, of having a high temperature when describing how they would have concerns about the permissive threshold in FEVER:
I wouldn’t want to have a temperature of 39.5 [°C] and not to give myself some paracetamol, so I just think for the child it will be more comfortable.
P08, FG4
In terms of looking at a ch- in the case of a child, they’d have to get up to 40 [°C] but having personally had um quite regularly get temperatures of 39 [°C] plus, they’re bloody uncomfortable. You’re tachycardic, you, you feel horrible.
P01, FG1
My son rigors at 38.4 [°C] every time . . . He just starts shaking with temperature, so I get it at home, so I’m wary about letting temperatures go up too high.
P02, FG2
As the following quotations illustrate, a few members of staff discussed their concerns about not knowing if the permissive threshold was in the best interests of the child and whether or not waiting to treat the fever could cause harm:
I think it’s just not knowing are you doing the right thing or not. Yeah, I suppose you could be concerned about the other obs [observations], like say tachycardia, high blood pressure, um, and I suppose you’re thinking if I gave paracetamol . . . It’s hard to, to tell whether they’re, I don’t know, whether it’s alright either way or what damage or what good you’re doing.
P02, FG2
Because potentially you could be maybe putting their life at risk and making it worse.
P09, FG4
After considering their own acceptability of the permissive threshold, some considered how acceptable parents would find the permissive threshold group of the trial. A few staff were concerned about how to explain to parents why they were not treating a high temperature:
[H]ow can I justify that to a parent that, yeah, yeah, yeah you can see it rising but we’re not doing anything about it?
P03, FG6
I think they would, you know, feel very uncomfortable about their child being left with a temperature.
P05, FG1
As the following quotations from one focus group illustrate, staff felt that a permissive temperature of 40 °C would seem more frightening and therefore less acceptable than 39 °C to parents:
I think parents would be more concerned as well. If, you know, if you told a parent that their temperature was 40 [°C], I don’t know, maybe it’s just me, that they’d be more con- . . .
. . . Yes, it sounds more scary yeah.
It sounds worse than 39.5 [°C], to them . . .
What if it was 39.9 [°C]? Is it the 40 [°C] that’s scary or that um . . .
For the parents.
Yes, for the parents.
Yeah, their understanding.
Staff concerns about not using paracetamol for analgesia
Seventy-six per cent of staff (42/55, one missing) indicated that they would have concerns about not using paracetamol for analgesia. However, the acceptability of not administering an antipyretic appeared to be influenced by how unwell a child was; for example, staff described how the trial would be more acceptable if it was limited to children who were very poorly and receiving other drugs for pain or discomfort. In contrast, many described how it would not be appropriate to administer alternative analgesic drugs, such as morphine, to treat a discomfort in a spontaneously breathing patient who was not seriously unwell:
I’m more comfortable that they’re sicker because you’ve got the other drugs on board that we could give, and we’re happy we’ve got like back-up vents and . . . whatever we can deal with. Kids who are self-ventilating it feels a bit extreme to jump to an NCA [nurse-controlled analgesia] pump when she’s just a bit grizzly and you could’ve given her paracetamol or something.
P06, FG5
Because when you’re using these drugs, we’re all aware that they have side effects, so if we were going to opioids when we didn’t need to necessarily, then I think that’s what would make me a bit more uncomfortable. But if they already required those then I think that that would be more acceptable.
P04, FG6
When staff views were sought on amending inclusion criteria to include children on ventilators, it was apparent that such a change would help address concerns about not using medication for analgesia. In addition, staff confirmed that limiting the sample to children who were more clinically unwell would also address their concerns about children being uncomfortable or upset, or using alternative painkillers unnecessarily, as ventilated children would already be receiving opioids, such as morphine, for pain:
Maybe if they are intubated and ventilated, they’re having a bit of roc [rocuronium] and a bit more morphine, then actually you wouldn’t feel as guilty or uncomfortable because they’re not crying, and parents aren’t saying ‘what can I do with them ‘cause they’re crying all the time’.
P01, FG6
Approach to consent in the FEVER randomised controlled trial
As shown in Figure 3, 22% (11/49, seven missing) of staff used their handsets to indicate that the use of RWPC was very acceptable or acceptable (25/49, 51%) in the proposed FEVER RCT. Although 14% (7/49) did not have any strong feelings (selected neutral), 12% (6/49) indicated that RWPC was unacceptable in the proposed FEVER RCT.
FIGURE 3.
Staff views on the acceptability of RWPC in the FEVER RCT.

Some members of staff discussed their support for the study and its use of RWPC, saying that there is a need for scientific evidence to address this research question and that they did not view informed consent as feasible owing to parental incapacity in an emergency situation:
. . . by giving paracetamol early, it might be actually prolonging their hospital stay and making them sicker for longer with consequences of actually getting other illnesses in the intensive care . . .
They can’t give consent . . . can they?
. . . they can’t actually give informed consent, they can barely say their name.
Others found it reassuring that although they would not be seeking informed consent, it’s not ‘cloak and dagger stuff’ (R12, FG4), as they anticipated that parents would be informed about trial participation and consent sought fairly shortly after randomisation:
I would anticipate that people do consent quite quickly, I don’t think it would need to be 4 days before you did consent, it would be able to be done within a reasonable . . . I don’t know exactly what time frame but I don’t think the parents would be in the dark about it for very long, so I think that it would be acceptable.
P01, FG6
As the following quotation shows, some staff supported RWPC in this trial, as they stated that the most effective temperature threshold to use in children presenting with a fever and suspected infection is not known, therefore children would not knowingly be denied an effective treatment:
Because we’re not denying a treatment that has a proven benefit, I would find that less acceptable, but because we don’t know whether it helps or not I think it’s acceptable to try and find out.
P02, FG6
Those who used their handsets to indicate a ‘neutral’ response or indicated that RWPC was unacceptable in the FEVER RCT went on to describe a range of reasons for their views. Most commonly, staff were concerned about RWPC in a trial that would require them to not to treat a child’s high temperature, therefore being ‘inactive’ in a potentially life-threatening situation. As the following quotation illustrates, some staff with previous experience of RWPC in paediatric RCTs viewed ‘withholding’ an emergency intervention without prior consent as less acceptable than administering a ‘needed’ intervention:
When you have to do something that’s an emergency, you know, it’s needed, it’s an emergency, you have to intervene and you have to do that without consent, that’s very different to withholding something.
P02, FG4
Staff were also concerned that parents may notice their child’s temperature and respond negatively to finding out that they were not being treated with an antipyretic owing to participation in a trial without their prior consent:
The thing is what won’t it [trial participation] be really obvious to the parents watching.
P03, FG4
I just wonder whether not treating people, which is what this says, is so completely contradictory to parents . . . that it just won’t sit well.
P05, FG3
But if we’re now saying, ‘oh we, we’re not ignoring it but we’re just not treating it,’ I don’t know whether you’d get a good response in an already very highly emotive environment.
P05, FG1
Staff concerns about the impact of research without prior consent on parent–clinician relationships
Many members of staff spoke of how RWPC in the FEVER RCT could negatively impact on rapport, relationship building and communication with parents: ‘You know, potential barrier to communication or, you know, working relationship’ (M3, FG1). One nurse spoke of how the ‘whole ethos of paediatric nursing is you’re meant to do it in partnership with the, you know, child and family’ (P05, FG1), and how RWPC goes against this ethos.
Others were uncomfortable about changing their usual practice of administrating antipyretics for a fever, as they were unsure of the clinical impact that would have on a child. Some stated that they would not give consent for their own child to be in the study, so they were not willing to put someone else’s in the FEVER RCT without prior consent. Nevertheless, they noted their conflicting desire for the study to be conducted to inform future clinical practice:
I think for me it’s a personal thing, I wouldn’t put my daughter into that trial to start off with, so I wouldn’t get, er, uninformed consent from another parent, simple as. There’s no way I would let my daughter’s temperature go that high before giving them paracetamol.
P09, FG4
I suppose the thing is it’s a bit rich isn’t it that, because we’re all happy to benefit from research. But we’re not all . . . that happy to perhaps participate.
P02, FG4
Towards the end of one focus group, provisional findings from the parent interviews were described to staff, including details of how parents viewed the use of RWPC and the higher threshold as acceptable. Although a few queried the representativeness of the interview sample, this information appeared to appease staff concerns, stating that if parents are happy with the study then staff would find taking part in FEVER more acceptable:
If that’s what parents are saying then, how can you dispute what the parents are saying.
P09, FG4
Informing the pilot randomised controlled trial design and training, including inclusion and exclusion criteria
Staff had key questions that were important to consider in the pilot RCT design and site training package development stage. As shown in Box 5, these were often practical questions that required clarification about what would be included the trial protocol, as well as questions related to qualitative study findings about parents’ perceptions of the proposed FEVER RCT. Some queries were prioritised by staff, appearing to influence views on trial acceptability, including concerns about whether a pyretic child would be distressed or combative, the use of environmental cooling methods and the inclusion of children with high heart rates or tachycardia.
-
What if the patient has a high heart rate/tachycardia?
-
What if the patient is combative?
-
What if they have already been given paracetamol or been stripped off?
-
What if they never reach 40 °C?
-
Can we remove blankets if we think the environment has caused the fever?
-
What environmental cooling will we be able to do?
-
How do parents feel about waiting until 40 °C?
-
What do parents think about RWPC in FEVER?
-
How are we measuring temperature?
When specifically asked about what staff thought should be listed as exclusion criteria, suggestions included ‘post op cardiac patient who end up septic’ (P06, FG3), ‘due to cardiovascular instability’ (P01, FG3), ‘oncology patients’ (P01, FG6) and patients admitted with ‘febrile neutropenia’ (P01, FG6). Staff agreed with the researcher’s description of proposed exclusion criteria, including ‘traumatic brain injuries’, ‘seizures’ and ‘children who have a cardiac arrest and need to be kept normothermic’ (researcher descriptions). As described earlier, staff proposed limiting inclusion criteria to only include children who are on ventilators.
Many emphasised the importance of ensuring bedside nurses were supportive of the study, as it is nurses who administer antipyretic interventions, often as a first-line response to an indication of infection:
I think like [name] said, the nurses have to be completely on board with this because they do have their own – I can’t tell you how many times you go into the bed space and they’re like – ‘no, but seriously – oh well temperature was 37.2 [°C] so I’ve given paracetamol’. And you’re like ‘what?’. ‘Well I didn’t want him to get too hot.’ How are you going to know if they’re brewing something anyway?
P06, FG3
Recommendations included the need for further information to help staff feel confident about taking part and explaining the study to families. This included a summary of the clinical evidence, which demonstrates the scientific rationale behind the trial question, as well as previous literature and qualitative research findings on parents’ views on RWPC to help facilitate staff ‘buy-in’ and help staff answer parents’ questions about the study and its approach to consent:
I think arming all staff with the key points from current literature is really helpful . . . we’ll all be in positions where we might have to answer parents’ questions, so it’s really important that everybody’s on the same page with it, because actually it’s not just the people doing the research or the consenting and everything, the bedside nurse is going to be fielding questions and needs to believe in the study. I think that people do really invest and get behind it if they can see what the benefit would be of it, if it has an answer to it . . . I think that needs to be summarised and everybody has to have the same points from it and understand what we’re comparing and why, and why we think it’s safe.
P01, FG6
I had more knowledge and understanding of the benefits to this, as well as the child targets and the thresholds I felt a bit more comfortable with, then I probably would be able to then confidently say to family, yes, actually I know that their temperature is X, and I’m not treating it because there’s this is a study.
P03, FG3
Key findings to inform the FEVER pilot randomised controlled trial
This qualitative study provides insight into the acceptability of the FEVER RCT by exploring the views of parents with relevant experience and site staff at the four pilot RCT sites.
This research found that parents supported the proposed trial and the use of RWPC. All stated that they would (hypothetically) consent to the use of their child’s information in the trial. Parents’ views on trial acceptability appeared to be influenced by a number of factors, including that all other treatments for suspected infection are given, the nature of the intervention (e.g. antipyretics being non-invasive), trust of medical staff to act in the best interests of their child, a desire to help other children in the future and a belief that the trial question is important.
The majority of parents were not concerned about the proposed restrictive temperature threshold of 37.5 °C. In contrast, many voiced specific concerns about the acceptability of the higher temperature threshold, including if not treating a child’s temperature would increase the likelihood of seizures. Parents stated that they would still consent to a trial that did not treat a fever with an antipyretic until 40 °C, as they trusted staff to monitor their child and act in their best interests. However, owing to concerns about 40 °C being a high and ‘scary number’, the majority suggested that the FEVER RCT would be more acceptable if the permissive temperature threshold was slightly lower (e.g. 39.9 °C or 39.5 °C).
Focus groups with site staff revealed concerns about 37.5 °C being too low a temperature threshold at which to administer an antipyretic, and the majority echoed parents’ perspectives in describing a permissive threshold of 40 °C as being too high. Staff were concerned about the acceptability of the permissive threshold to parents and about not using paracetamol for analgesia in the less unwell, spontaneously breathing patients who may be in pain or discomfort.
Many staff viewed RWPC as acceptable in the proposed trial, although for some the acceptability of RWPC was dependent on the permissive temperature threshold selected for the trial. Parents also viewed RWPC as acceptable in FEVER. Many described prospective informed consent as being inappropriate in emotive critical care situations and suggested that the RWPC process should be carefully managed by site staff in order to limit any potential negative impact on parents’ trust in staff. Parents’ views on RWPC were in line with previous research and guidance. 18 They recommended that staff in the proposed FEVER RCT should inform parents that their child has been randomised to the trial at the earliest possible time. Parents also recommended that staff should use their judgement about when is appropriate to approach each family, as well as tailor the level of information provided to meet individual needs. Bereaved parents recommended that communication about a child’s involvement in the proposed FEVER RCT after death should be personalised, honest and compassionate. Parents identified parts of the participant information sheet that required clarification, including if not treating a temperature could cause a seizure, that all other treatments would be given and some formatting suggestions, such as all prioritised information being on the first page.
Suggestions on how staff concerns could be addressed to assist ‘buy-in’ included restricting inclusion criteria to mechanically ventilated children; involving clinical staff as well as research staff in site training; providing information about the scientific evidence underpinning the trial; and clarifying key issues, such as what cooling methods could be used.
Qualitative study findings were used to develop the pilot RCT protocol, including the selection of temperature thresholds, inclusion criteria to require that patients are mechanically ventilated, participant information materials and site staff training package. To help address staff concerns about how parents may react to trial discussions, parent perspectives would be communicated in site training, highlighting parental acceptability of RWPC, temperature thresholds, parents’ questions and concerns about the study and, importantly, suggestions on how staff could address such questions.
Chapter 3 The FEVER observational study
Aim and objectives
The aim of the FEVER observational study was to inform the feasibility of conducting a definitive RCT by addressing the following specific objectives:
-
Estimate the size of the potentially eligible population for the definitive FEVER RCT, both overall and in subgroups defined by confirmed versus suspected infection and by site/type of infection.
-
Confirm, using empirical data, the temperature threshold(s) currently employed for a standard approach for antipyretic intervention in UK PICUs, and whether or not these vary in accordance with confirmed versus suspected infection and site/type of infection.
-
Estimate the characteristics (e.g. mean and SD) of selected important, relevant, patient-centred primary outcome measure(s) to inform both the selection of an ultimate primary outcome and sample size calculation.
Methods
Study design
The FEVER observational study was an epidemiological cohort study of fever attributable to infection in critically ill children following an unplanned admission to a PICU.
Research governance
Approval was granted by the Greater Manchester West Research Ethics Committee on 11 January 2017 (reference number 17/NW/0026) and the HRA on 17 January 2017 (Integrated Research Application System reference number 209929). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/ (accessed 16 November 2018).
The FEVER observational study was registered on the ClinicalTrials.gov database. Registration was confirmed on 20 January 2017 (reference number NCT03028818). The study was adopted in the National Institute for Health Research (NIHR) Clinical Research Network (CRN) Portfolio as a non-consenting study on 15 February 2017 and issued the NIHR CRN Portfolio number 33374.
Local confirmation of capacity and capability was obtained from each participating hospital trust to collect data for the study. For NHS England sites, the statement of activities was used as the study agreement. For sites in Northern Ireland, Wales and Scotland, an adapted clinical trial site agreement, based on the model agreement for non-commercial research in the health service, was signed by each participating hospital trust and the sponsor (ICNARC).
Data collection was embedded in the Paediatric Intensive Care Audit Network (PICANet), which has approval to collect patient-identifiable and personal data without consent. No patient-identifiable data were collected or used for the FEVER observational study. A release of data and a customised data collection request was made to the Healthcare Quality Improvement Partnership for access to unidentifiable routine PICANet data and to collect the additional data required for this study. Healthcare Quality Improvement Partnership approval was received for this purpose on 1 March 2017.
Study management
The study co-ordinator was responsible for day-to-day management of the FEVER observational study, with support from the ICNARC CTU and the SMG.
Amendments to the protocol
After receiving approval of the protocol from the HRA on 17 January 2017, three non-substantial amendments were submitted and categorised:
-
Minor amendment 1 (approved 3 February 2017) consisted of the addition of 16 research sites.
-
Minor amendment 2 (approved 21 February 2017) consisted of the addition of two research sites, and changes in principal investigator (PI) responsibilities in four participating sites.
-
Minor amendment 3 (approved 17 March 2017) consisted of changes in PI responsibilities for a participating site.
NHS support costs
In order to enable screening and data collection for the FEVER observational study, resources equivalent to 0.09 whole-time equivalent of a Band 6 clinical/research nurse per PICU for a period of 6 months were agreed prior to the submission of the research grant for the FEVER feasibility study.
Participants: sites
Twenty-two PICUs (Table 3) were selected from those actively participating in PICANet. These sites represented a variety of UK PICU configurations, including general or combined intensive care units in general academic medical centres or within stand-alone children’s hospitals. In order to be selected to participate in the FEVER observational study, sites had to meet the following inclusion criteria:
-
Meet all responsibilities as stated in the statement of activities (for sites in England) or the adapted clinical trial site agreement (for sites in Scotland, Wales and Northern Ireland).
-
Identify and sign up a local PI.
-
Identify a responsible FEVER observational study research nurse (to be funded or part-funded, centrally).
-
Agree to collect additional FEVER data on all patients eligible for data collection.
-
Agree to enter study data for all eligible patients via data collection screens and send securely to ICNARC.
| Site | PI |
|---|---|
| Addenbrooke’s Hospital (Cambridge) | Dr Nazima Pathan |
| Alder Hey Children’s Hospital (Liverpool) | Dr Lyvonne Tume |
| Birmingham Women’s and Children’s Hospital | Dr Barney Scholefield/Ms Julie Menzies |
| Bristol Royal Hospital for Children | Dr Peter Davis |
| Evelina London Children’s Hospital | Dr Shane Tibby |
| Great North Children’s Hospital (Newcastle upon Tyne) | Dr Rachel Agbeko |
| Great Ormond Street Hospital (London) | Professor Mark Peters |
| James Cook University Hospital (Middlesbrough) | Dr Jonathan Grimbley |
| John Radcliffe Hospital (Oxford) | Dr James Weitz |
| King’s College Hospital (London) | Dr Akash Deep |
| Leicester Children’s Hospital | Dr Raghu Ramaiah |
| Queen’s Medical Centre (Nottingham) | Dr Patrick Davies |
| Royal Brompton Hospital (London) | Dr Angela Aramburro |
| Royal Hospital for Children, Glasgow | Dr Richard Levin |
| Royal London Hospital | Dr Kalaimaran Sadasivam |
| Royal Manchester Children’s Hospital | Dr Peter-Marc Fortune |
| Royal Stoke University Hospital | Dr John Alexander |
| Royal Belfast Hospital for Sick Children | Dr Stewart Reid |
| Southampton General Hospital | Dr John Pappachan |
| St George’s Hospital (London) | Dr Nicholas Prince |
| St Mary’s Hospital (London) | Dr David Inwald |
| University Hospital of Wales (Cardiff) | Dr Siva Oruganti |
Site initiation
Site initiation meetings were held via teleconference with all participating sites prior to the commencement of data collection (between 1 and 8 March 2017). The purpose of these meetings was to present the background and rationale for the study and to discuss data collection and submission. The PI from each participating site attended the meeting.
Following participation in the site initiation meetings, screening and data collection was commenced at participating sites once the statement of activities (sites in England) or clinical trial site agreement (sites in Scotland, Wales and Northern Ireland) had been signed and necessary approvals were in place. The scheduled start of data collection for the FEVER observational study was 1 March 2017. Sites initiated after this date collected data retrospectively on participants admitted from this date.
Investigator site file
An electronic investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the study and included the approved protocol, all relevant approvals (e.g. local confirmation of capacity and capability), a copy of the statement of activities/clinical trial site agreement, copies of the approved participant information leaflet and family poster and all standard operating procedures (e.g. for entering data onto the customised data collection screen).
Communication and motivation
The study co-ordinator, with support from the research assistant, maintained close contact with the research team at participating sites by e-mail and telephone throughout the study. A teleconference was held mid-way through data collection, on 14 June 2017, with research teams at participating sites. The purpose of this was to provide a forum for site teams to ask questions and to discuss interim data submission at 3 months to allow analysis to inform the pilot RCT. PICANet provided regular status reports, which displayed the number of participants eligible for data collection, the data collection completion and the outstanding number of associated validation queries. These reports were used to identify specific sites that were falling behind on data collection.
Study population
Eligibility criteria
All infants and children admitted to a PICU were recorded in routine PICANet data collection. Participants who were recorded as an ‘unplanned PICU admission’ were deemed eligible for data collection for the FEVER observational study. There were no exclusion criteria.
Consent
Informed consent was not required as there was no intervention in the patients’ care and no patient-identifiable data were collected. Patient information sheets (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 16 November 2018) and posters were displayed in participating PICUs to make parents/guardians aware that the unit was taking part in the study. Patients or parents/legal representatives were able to opt out from participation and have their data removed from the study.
Outcomes
-
Potentially eligible population for a definitive FEVER trial.
-
Temperature thresholds employed in usual care.
-
The distribution of potential outcome measures for a definitive FEVER trial.
Data collection
Data entry
The FEVER observational study was nested within routine data collection for PICANet. PICANet is the national clinical audit network of paediatric critical care units in England, Wales, Northern Ireland and Scotland and was established in 2002 by the Universities of Leeds, Leicester and Sheffield with the support of the paediatric intensive care community. Nesting the study in PICANet ensured efficient design (with respect to data collection to address the study objectives) and facilitated effective study management through the ability to monitor data entry. Data were collected for the FEVER observational study through a mixture of routinely collected audit data and additional data collection through an additional screen on PICANet. Paper case report forms (CRFs) were provided to facilitate data collection and entry. Collection of data was delegated by the site PI to qualified members of the research team and recorded on the delegation log. Data entry was monitored via status reports generated by PICANet and was subject to ongoing validation to ensure that data were as complete and accurate as possible.
Data stages
Participants who were recorded as ‘unplanned PICU admissions’ were eligible for the collection of additional data. These data were split into three stages. Stages 1 and 2 aimed to identify potentially eligible participants for the definitive FEVER trial, and stage 3 provided infection data alongside temperature and antipyretic management.
Stage 1
Exclusion criteria:
-
acute encephalopathy, including convulsive status epilepticus
-
postcardiopulmonary bypass
-
known/suspected myocardial disease
-
severe rhabdomyolysis
-
malignant hyperthermia
-
neuroleptic malignant syndrome
-
drug-induced hyperthermia
-
receiving palliative care, or death perceived as imminent.
If the patient met one or more of the stage 1 exclusion criteria, no additional data were collected. If the patient did not meet any of the stage 1 exclusion criteria, data collection moved on to stage 2.
Stage 2
Inclusion criterion:
-
suspected or confirmed infection on admission.
If the patient did not have a suspected or confirmed infection, no additional data were collected. If the patient had a suspected or confirmed infection, the patient was identified as ‘potentially eligible’ for the definitive FEVER trial and data collection moved on to stage 3.
Stage 3
-
Data were collected on site and type of infection.
-
Daily data (for first 5 calendar days in PICU) were collected for the highest daily temperature and any antipyretic and opioid analgesic interventions given.
Paediatric Intensive Care Audit Network data
For all unplanned admissions, routine PICANet data were provided on patient characteristics, daily interventions and status at PICU discharge.
Data submission
Participating sites downloaded a data extract from PICANet, consisting of the data collected specifically for the FEVER observational study and the specified PICANet routine data. The study data set was anonymous and submitted to ICNARC for analysis (Figure 4).
FIGURE 4.
The PICANet processes.

Sample size
Based on PICANet data, a sample size of approximately 1100 children was deemed to be sufficient to permit the calculation of 30-day mortality (anticipated to be 6% based on data from PICANet) with a 95% CI of ±1.4% based on the following assumptions:
-
There were 21,326 emergency/unplanned admissions to the 27 UK PICUs during the period of 1 January 2011 to 31 December 2012. This corresponded to an anticipated overall sample size for the FEVER observational study in a target number of 20 UK PICUs over a 6-month period of approximately 3960 admissions (33 per PICU per month).
-
There were 11,007 (51.6%) emergency/unplanned admissions to the 27 UK PICUs during the same period for patients who were admitted with a primary or secondary diagnosis consistent with a probable infection. Of these, 946 admissions (8.6%) met one or more of the proposed FEVER exclusion criteria, corresponding to approximately 1900 admissions (16 per PICU per month) who required the additional FEVER data collection on temperature and FEVER management.
-
Of admissions with probable infection, 1583 (14.4%) were re-admissions of the same child. Based on data from the UK Paediatric Intensive Care Outcome Study (UK PICOS),29 approximately 70% of children admitted with infection have a peak temperature of ≥ 37.5 °C (estimated to be the likely temperature threshold).
Data analysis
Interim analysis
An interim analysis of data from the FEVER observational study was undertaken in July 2017 to inform the final design of the pilot RCT.
Study populations
All potentially eligible participants for the definitive FEVER trial were included in the analyses. Analyses were carried out based on the following populations:
-
All patients not meeting any stage 1 exclusion criteria and with confirmed or suspected infection (stage 2 criteria).
-
As population 1, restricted to patients receiving any mode of mechanical ventilation (invasive, non-invasive or high-flow oxygen) on days 1–2 of PICU admission.
-
As population 1, restricted to patients receiving invasive ventilation on days 1–2 of PICU admission.
Analysis methods
Potentially eligible populations
The number of potentially eligible participants for a definitive FEVER trial (per site per month) was estimated based on the number of recruited participants divided by the total duration of recruitment in months for each population cohort, considering the temperature thresholds employed on days 1–2 of PICU admission. The potentially eligible population is reported overall and as the median (IQR) across sites, with 95% CIs based on a Poisson distribution for each population.
Subgroup analyses
Subgroup analyses were performed for maximum temperature and current temperature thresholds by confirmed versus suspected infection and site/type of infection.
Temperature thresholds
The temperature thresholds employed in usual care were evaluated in each of the three populations by plotting:
-
the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ X, with X varying from 36.0 °C to 39.5 °C
-
the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of < X, with X varying from 36.0 °C to 39.5 °C
-
the variation across sites in the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ 37.5 °C
-
the variation across sites in the proportion of patients receiving any antipyretic intervention on day 1 among those with a maximum temperature on day 1 of ≥ 38.0 °C
-
for patients not receiving any antipyretic intervention on day 1 and receiving an antipyretic intervention subsequently, the cumulative distribution function of the maximum temperature on the first day on which they received any antipyretic intervention and on the preceding day
-
for patients not receiving any antipyretic intervention on day 1 and receiving an antipyretic intervention subsequently, the variation across sites in the median value of the maximum temperature on the first day on which they received any antipyretic intervention and on the preceding day.
Potential outcome measures
The distribution of potential outcome measures for a definitive FEVER RCT were evaluated for the following potential outcome measures for each of the cohorts of potentially eligible participants for a definitive RCT, as defined in Study populations. Length of stay in PICU is reported as the mean (SD) and median (IQR). Duration of organ support is reported as the number (%) receiving the organ support, the median (IQR) duration of organ support among patients receiving the organ support and the mean (SD) duration of organ support among all patients (with patients not receiving the support assigned a value of 0). PICU mortality is reported as the number and percentage and the 95% CI for the percentage. The number of stays alive and free from a PICU and from mechanical ventilation are reported as the mean (SD).
Statistical analysis
Statistical analyses were conducted in accordance with a prespecified statistical analysis plan written prior to the final data lock. The final analyses were conducted using Stata®/SE version 14.2 (StataCorp LP, College Station, TX, USA) and R version 3.4.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Missing data
All analyses were undertaken in the complete-case population. There was no imputation of missing data.
Results
Participants
From 1 March 2017 to 31 August 2017, a total of 4126 children with unplanned PICU admissions were recorded from 22 hospitals. A total of 4008 patients (97.1%) had completed data entry relating to stage 1 criteria, and, of these, 737 (18.4%) met one or more of the stage 1 exclusions. Of the 3141 patients (78%) meeting stage 1 criteria, 1263 (40%) had no infection and a further 25 (0.8%) had missing data. The remaining 1853 patients (59.3%) meeting stage 2 criteria were identified as the potentially eligible population for a definitive FEVER RCT. We were unable to successfully collect PICANet data from 12 patients (0.6%); therefore, 1841 patients (99.4%) were included in the analysis for population 1, 1532 patients (82.7%) for population 2 and 1156 patients (62.4%) for population 3 (Figure 5). The variation across sites in the number and percentage of patients meeting stage 1 and 2 criteria is presented in Appendix 1.
FIGURE 5.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram for the study.

Baseline characteristics
Participant demographics and case mix are presented in Table 4. Age distribution, temperature at first face-to-face contact and highest temperature recorded on day 1 appear similar across the three populations. The proportions of antipyretic interventions on day 1 also appear consistent across groups; however, the use of intravenous opiates on day 1 is greatest in those receiving invasive mechanical ventilation (population 3) (74% of invasively mechanically ventilated patients received intravenous opiates). Infection was confirmed during the PICU stay for approximately two-thirds of participants in each of the populations, with viral infections and the lungs being the most common type and site of infection, respectively. Again, this was consistent across groups, accounting for > 60% (both type and site) of infections in each of the three populations.
| Variable | Population | ||
|---|---|---|---|
| 1a (N = 1841) | 2b (N = 1532) | 3c (N = 1156) | |
| Age (months) | N = 1749 | N = 1452 | N = 1081 |
| Mean (SD) | 44 (58) | 40 (56) | 39 (55) |
| Median (IQR) | 15 (3–67) | 13 (3–58) | 13 (2–56) |
| Age group (years), n/N (%) | |||
| < 1 | 793/1749 (45.3) | 698/1452 (48.1) | 527/1081 (48.8) |
| 1 | 260/1749 (14.9) | 228/1452 (15.7) | 173/1081 (16.0) |
| 2–4 | 226/1749 (12.9) | 169/1452 (11.6) | 124/1081 (11.5) |
| 5–9 | 227/1749 (13.0) | 179/1452 (12.3) | 126/1081 (11.7) |
| 10–16 | 243/1749 (13.9) | 178/1452 (12.3) | 131/1081 (12.1) |
| Temperature at first face-to-face contact (°C) | N = 1808 | N = 1505 | N = 1136 |
| Mean (SD) | 37.0 (1.1) | 36.9 (1.1) | 36.8 (1.2) |
| Median (IQR) | 36.9 (36.4–37.5) | 36.9 (36.4–37.5) | 36.8 (36.3–37.5) |
| Highest temperature on day 1 (°C) | N = 1808 | N = 1506 | N = 1134 |
| Mean (SD) | 37.6 (1.0) | 37.6 (1.0) | 37.7 (1.0) |
| Median (IQR) | 37.5 (37.0–38.2) | 37.5 (37.0–38.2) | 37.6 (37.0–38.3) |
| Highest level of mechanical ventilation on day 1, n/N (%) | |||
| Invasive | 1098/1841 (59.6) | 1098/1532 (71.7) | 1098/1156 (95.0) |
| Non-invasive | 218/1841 (11.8) | 218/1532 (14.2) | 20/1156 (1.7) |
| High-flow oxygen | 175/1841 (9.5) | 175/1532 (11.4) | 12/1156 (1.0) |
| None | 350/1841 (19.0) | 41/1532 (2.7) | 26/1156 (2.2) |
| Antipyretics received on day 1, n/N (%)d | |||
| Paracetamol | 934/1814 (51.5) | 768/1509 (50.9) | 531/1137 (46.7) |
| NSAID | 67/1814 (3.7) | 49/1509 (3.2) | 23/1137 (2.0) |
| External/other cooling | 176/1814 (9.7) | 160/1509 (10.6) | 131/1137 (11.5) |
| Any antipyretic | 984/1814 (54.2) | 817/1509 (54.1) | 575/1137 (50.6) |
| Received intravenous opiates on day 1, n/N (%) | 935/1821 (51.3) | 882/1513 (58.3) | 847/1143 (74.1) |
| Infection status at admission to PICU, n/N (%) | |||
| Confirmed | 343/1841 (18.6) | 288/1532 (18.8) | 212/1156 (18.3) |
| Suspected | 1498/1841 (81.4) | 1244/1532 (81.2) | 944/1156 (81.7) |
| Site of infection,e n/N (%) | |||
| Lung | 1135/1841 (61.7) | 1028/1532 (67.1) | 734/1156 (63.5) |
| Soft tissue | 60/1841 (3.3) | 42/1532 (2.7) | 39/1156 (3.4) |
| Central nervous system | 93/1841 (5.1) | 71/1532 (4.6) | 67/1156 (5.8) |
| Blood | 300/1841 (16.3) | 227/1532 (14.8) | 187/1156 (16.2) |
| Abdomen | 142/1841 (7.7) | 103/1532 (6.7) | 86/1156 (7.4) |
| Urinary tract | 42/1841 (2.3) | 35/1532 (2.3) | 25/1156 (2.2) |
| Other | 99/1841 (5.4) | 66/1532 (4.3) | 43/1156 (3.7) |
| Unknown | 124/1841 (6.7) | 90/1532 (5.9) | 68/1156 (5.9) |
| Infection confirmed during PICU stay, n/N (%) | 1176/1841 (63.9) | 1008/1532 (65.8) | 753/1156 (65.1) |
| Type of infection,d n/N (% of confirmed infection) | |||
| Virus | 740/1176 (62.9) | 672/1008 (66.7) | 497/753 (66.0) |
| Bacteria | 511/1176 (43.5) | 418/1008 (41.5) | 318/753 (42.2) |
| Fungus | 21/1176 (1.8) | 14/1008 (1.4) | 11/753 (1.5) |
| Parasite | 2/1176 (0.2) | 0/1008 (0.0) | 0/753 (0.0) |
| Unknown | 14/1176 (1.2) | 11/1008 (1.1) | 9/753 (1.2) |
Outcomes
Potentially eligible populations
The rates of potentially eligible participants with temperatures of ≥ 37.5 °C and ≥ 38 °C by site and overall for each analysis population are presented in Table 5. Overall rates of potentially eligible participants in population 1 were 10.7 (95% CI 10.2 to 11.3) participants per site per month with a temperature of ≥ 37.5 °C and 7.0 (95% CI 6.5 to 7.5) participants per site per month with a temperature of ≥ 38 °C. The inclusion of mechanical ventilation (population 2) reduced the overall rates to 9.2 (95% CI 8.6 to 9.7) participants per site per month for a temperature of ≥ 37.5 °C and 6.1 (95% CI 5.6 to 6.5) participants per site per month with a temperature of ≥ 38 °C. Recruitment rates were further reduced in population 3 (invasive mechanical ventilation) to 7.3 (95% CI 6.8 to 7.7) participants per site per month and 5.0 (95% CI 4.6 to 5.4) participants per site per month for temperatures of ≥ 37.5 °C and ≥ 38 °C, respectively.
| Site | Number of months recruiting | Population 1a | Population 2b | Population 3c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | |||||||||||||
| ≥ 37.5 | ≥ 38.0 | ≥ 37.5 | ≥ 38.0 | ≥ 37.5 | ≥ 38.0 | ||||||||
| Number of participants | Rate | Number of participants | Rate | Number of participants | Rate | Number of participants | Rate | Number of participants | Rate | Number of participants | Rate | ||
| Addenbrooke’s Hospital (Cambridge) | 6 | 63 | 10.5 | 44 | 7.3 | 42 | 7.0 | 28 | 4.7 | 35 | 5.8 | 24 | 4.0 |
| Alder Hey Children’s Hospital (Liverpool) | 6 | 71 | 11.8 | 50 | 8.3 | 69 | 11.5 | 49 | 8.2 | 62 | 10.3 | 43 | 7.2 |
| Royal Belfast Hospital for Sick Children | 6 | 54 | 9.0 | 29 | 4.8 | 43 | 7.2 | 26 | 4.3 | 29 | 4.8 | 19 | 3.2 |
| Birmingham Women’s and Children’s Hospital | 6 | 143 | 23.8 | 100 | 16.7 | 130 | 21.7 | 95 | 15.8 | 94 | 15.7 | 68 | 11.3 |
| Bristol Royal Hospital for Children | 6 | 26 | 4.3 | 15 | 2.5 | 26 | 4.3 | 15 | 2.5 | 24 | 4.0 | 13 | 2.2 |
| University Hospital of Wales (Cardiff) | 6 | 36 | 6.0 | 27 | 4.5 | 32 | 5.3 | 23 | 3.8 | 25 | 4.2 | 19 | 3.2 |
| Evelina London Children’s Hospital | 5 | 80 | 16.0 | 51 | 10.2 | 70 | 14.0 | 45 | 9.0 | 65 | 13.0 | 42 | 8.4 |
| Great North Children’s Hospital (Newcastle upon Tyne) | 6 | 62 | 10.3 | 40 | 6.7 | 55 | 9.2 | 36 | 6.0 | 44 | 7.3 | 28 | 4.7 |
| Great Ormond Street Hospital (London) | 6 | 116 | 19.3 | 58 | 9.7 | 100 | 16.7 | 52 | 8.7 | 82 | 13.7 | 41 | 6.8 |
| Royal Hospital for Children, Glasgow | 6 | 63 | 10.5 | 36 | 6.0 | 55 | 9.2 | 33 | 5.5 | 36 | 6.0 | 23 | 3.8 |
| James Cook University Hospital (Middlesbrough) | 6 | 26 | 4.3 | 14 | 2.3 | 13 | 2.2 | 5 | 0.8 | 5 | 0.8 | 3 | 0.5 |
| John Radcliffe Hospital (Oxford) | 6 | 62 | 10.3 | 40 | 6.7 | 52 | 8.7 | 33 | 5.5 | 32 | 5.3 | 23 | 3.8 |
| King’s College Hospital (London) | 6 | 77 | 12.8 | 56 | 9.3 | 63 | 10.5 | 49 | 8.2 | 56 | 9.3 | 45 | 7.5 |
| Leicester Children’s Hospital | 4 | 18 | 4.5 | 12 | 3.0 | 14 | 3.5 | 8 | 2.0 | 10 | 2.5 | 6 | 1.5 |
| Royal Manchester Children’s Hospital | 6 | 83 | 13.8 | 55 | 9.2 | 68 | 11.3 | 45 | 7.5 | 58 | 9.7 | 39 | 6.5 |
| Queen’s Medical Centre (Nottingham) | 2 | 23 | 11.5 | 16 | 8.0 | 18 | 9.0 | 14 | 7.0 | 12 | 6.0 | 11 | 5.5 |
| Royal Brompton Hospital (London) | 6 | 10 | 1.7 | 3 | 0.5 | 7 | 1.2 | 1 | 0.2 | 2 | 0.3 | 0 | 0.0 |
| Royal London Hospital | 6 | 60 | 10.0 | 38 | 6.3 | 49 | 8.2 | 32 | 5.3 | 19 | 3.2 | 15 | 2.5 |
| Royal Stoke University Hospital | 6 | 46 | 7.7 | 31 | 5.2 | 43 | 7.2 | 29 | 4.8 | 38 | 6.3 | 28 | 4.7 |
| Southampton General Hospital | 6 | 87 | 14.5 | 54 | 9.0 | 82 | 13.7 | 52 | 8.7 | 76 | 12.7 | 51 | 8.5 |
| St George’s Hospital (London) | 6 | 77 | 12.8 | 61 | 10.2 | 64 | 10.7 | 51 | 8.5 | 56 | 9.3 | 45 | 7.5 |
| St Mary’s Hospital (London) | 6 | 58 | 9.7 | 43 | 7.2 | 50 | 8.3 | 38 | 6.3 | 47 | 7.8 | 35 | 5.8 |
| Overall (95% CI) | 125 | 1341 | 10.7 (10.2 to 11.3) | 873 | 7.0 (6.5 to 7.5) | 1145 | 9.2 (8.6 to 9.7) | 759 | 6.1 (5.6 to 6.5) | 907 | 7.3 (6.8 to 7.7) | 621 | 5.0 (4.6 to 5.4) |
| Median (IQR) | 10.5 (7.7–12.8) | 7.2 (4.8–9.2) | 9.0 (7.0–11.3) | 6.0 (4.3–8.2) | 6.3 (4.2–9.7) | 4.7 (3.2–7.2) | |||||||
Temperature thresholds
Figure 6 shows the proportion of participants in each population receiving an antipyretic intervention on day 1 with a maximum temperature above or below a given temperature ranging from 36.0 °C to 39.5 °C. In all participants (population 1), 55% of participants with a maximum temperature of > 36 °C received antipyretics, and this increased to 62% of participants at > 37 °C, 82% of participants at > 38 °C and 91% of participants at > 39 °C. The proportion of participants receiving antipyretics at the given temperatures on day 1 remained consistent when narrowing the inclusion criteria across populations 2 and 3 (Figure 6); however, there was variability across sites in temperature thresholds employed for antipyretic interventions on day 1 (Figure 7).
FIGURE 6.
The proportion of participants receiving antipyretic intervention on day 1 with a temperature above or below a given threshold. (a) Population 1; (b) population 2; and (c) population 3.
FIGURE 7.
Variation across sites in the proportion of participants receiving antipyretic intervention on day 1 among participants with a temperature of ≥ 37.5 °C (a, c and e) and ≥ 38 °C (b, d and f). (a and b) Population 1; (c and d) population 2; and (e and f) population 3.






For all participants not receiving an antipyretic intervention on day 1 but receiving subsequent antipyretic intervention, around 15% had a maximum temperature of ≤ 37 °C on the first day they received antipyretics (Figure 8). This increased to 68% of participants with a maximum temperature of ≤ 38 °C and 94% of participants with a temperature of ≤ 39 °C. The proportion of participants treated with antipyretics at these thresholds appeared to remain consistent across populations 2 and 3, with the mean temperature on the first day of receiving antipyretics being 37.8 °C (SD 0.8 °C) in population 1, 37.8 °C (SD 0.8 °C) in population 2 and 37.8 °C (SD 0.7 °C) in population 3. Again, there was considerable variability across sites in terms of temperature thresholds for antipyretic intervention on days 2–5 (Figure 9); however, the majority of participants in populations 1–3 with a temperature of ≥ 37.0 °C received an antipyretic intervention. The subgroup analyses of temperature thresholds employed by confirmed versus suspected and site/type of infection can be found in Appendix 2.
FIGURE 8.
Cumulative distribution of maximum daily temperatures of participants not receiving any antipyretics on day 1 and receiving subsequent antipyretic intervention. (a) Population 1; (b) population 2; and (c) population 3.

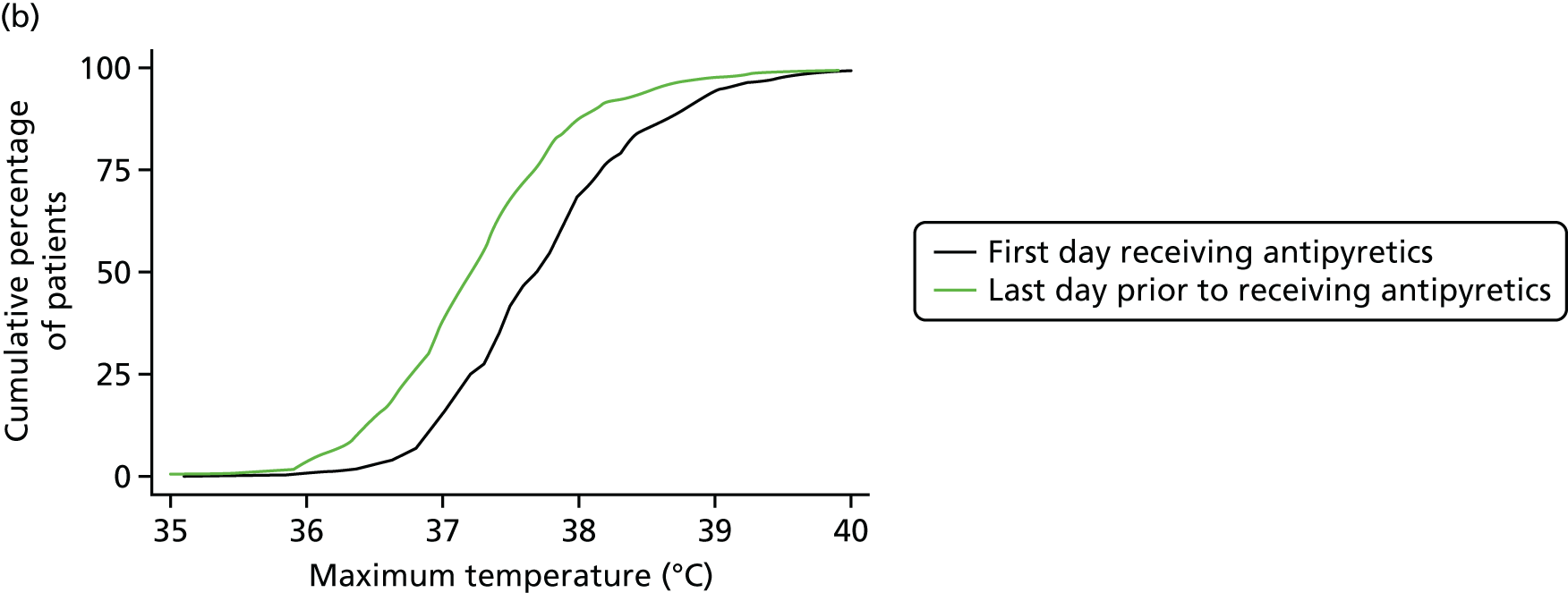

FIGURE 9.
Variation across sites in the median value of maximum temperatures on the first day on which antipyretics were received. (a) Population 1; (b) population 2; and (c) population 3.
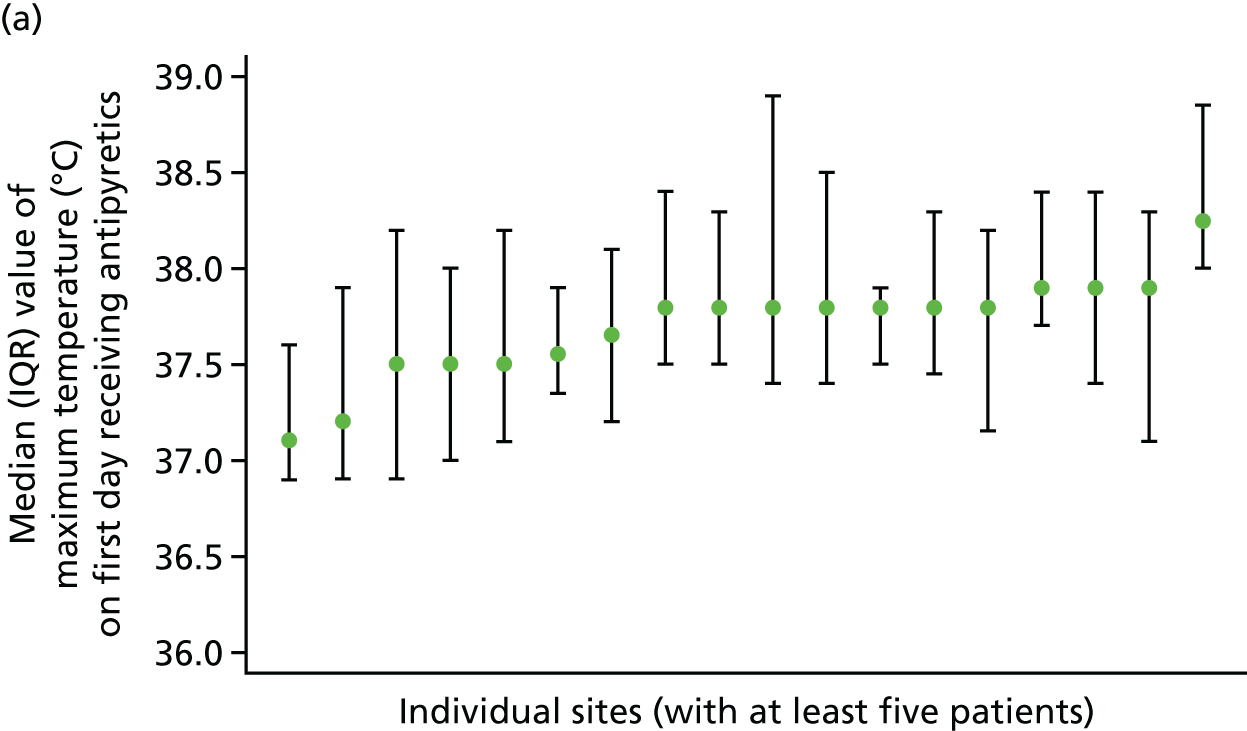

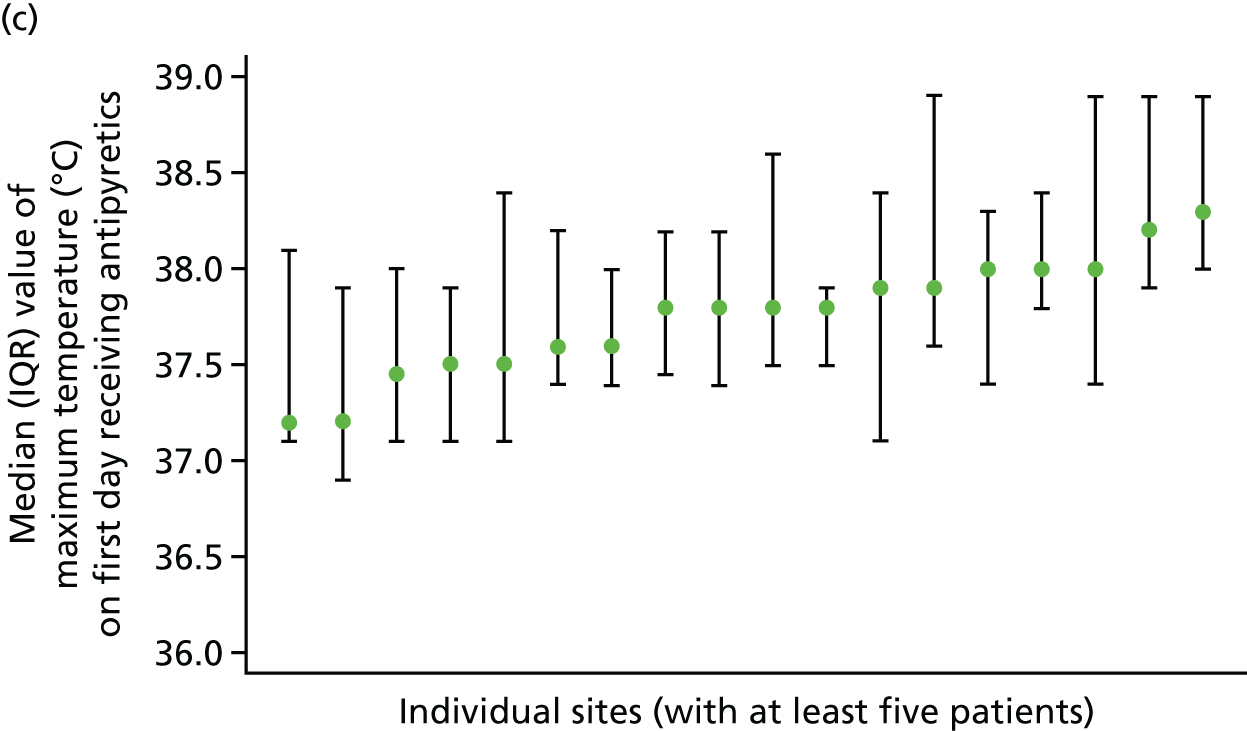
Potential outcome measures
The distributions of potential outcome measures were consistent across the ≥ 37.5 °C and ≥ 38 °C temperature thresholds (Table 6). Overall, PICU mortality (population 1) was around 5%, and this was higher among mechanically ventilated patients, at around 5.5%, and even higher among invasively ventilated patients, at around 6.5%. Similarly, PICU length of stay increased with narrowing inclusion criteria for all participants and those who survived to PICU discharge. Although the median duration of organ support among those receiving the support was similar across all populations, the proportions of patients receiving mechanical ventilation and cardiovascular support and the mean duration across all patients (including those not receiving the support) increased with narrowing inclusion criteria. Use of renal support was low in all populations. Consistent with the results on mortality, length of stay and organ support, the mean number of days alive and free from PICU and mechanical ventilation reduced across populations 1–3 with the narrowing of inclusion criteria.
| Potential outcome measures | Population | N | n (%) | Mean (SD) | Median (IQR) |
|---|---|---|---|---|---|
| PICU mortality | 1: ≥ 37.5 °C | 1341 | 66 (4.9) | – | – |
| 1: ≥ 38 °C | 873 | 42 (4.8) | – | – | |
| 2: ≥ 37.5 °C | 1145 | 65 (5.7) | – | – | |
| 2: ≥ 38 °C | 759 | 42 (5.5) | – | – | |
| 3: ≥ 37.5 °C | 907 | 59 (6.5) | – | – | |
| 3: ≥ 38 °C | 621 | 40 (6.4) | – | – | |
| PICU length of stay (days) | |||||
| All patients | 1: ≥ 37.5 °C | 1341 | – | 8.5 (13.7) | 5 (3–8) |
| 1: ≥ 38 °C | 873 | – | 8.9 (14.3) | 5 (3–9) | |
| 2: ≥ 37.5 °C | 1145 | – | 9.4 (14.6) | 5 (3–9) | |
| 2: ≥ 38 °C | 759 | – | 9.7 (15.1) | 6 (4–10) | |
| 3: ≥ 37.5 °C | 907 | – | 9.7 (14.6) | 6 (4–10) | |
| 3: ≥ 38 °C | 621 | – | 9.9 (14.7) | 6 (4–10) | |
| PICU survivors | 1: ≥ 37.5 °C | 1275 | – | 7.8 (10.9) | 5 (3–8) |
| 1: ≥ 38 °C | 831 | – | 8.2 (11.4) | 5 (3–9) | |
| 2: ≥ 37.5 °C | 1080 | – | 8.7 (11.5) | 5 (3–9) | |
| 2: ≥ 38 °C | 717 | – | 9.0 (12.1) | 6 (4–9) | |
| 3: ≥ 37.5 °C | 848 | – | 8.9 (10.6) | 6 (4–10) | |
| 3: ≥ 38 °C | 581 | – | 9.1 (10.5) | 6 (4–10) | |
| Receipt and duration of organ supporta | |||||
| Mechanical ventilation | 1: ≥ 37.5 °C | 1341 | 1158 (86.4) | 5.9 (6.6) | 5 (2–8) |
| 1: ≥ 38 °C | 873 | 769 (88.1) | 6.2 (6.7) | 5 (3–8) | |
| 2: ≥ 37.5 °C | 1145 | 1145 (100.0) | 6.8 (6.6) | 5 (2–8) | |
| 2: ≥ 38 °C | 759 | 759 (100.0) | 7.1 (6.8) | 5 (3–8) | |
| 3: ≥ 37.5 °C | 907 | 907 (100.0) | 7.2 (6.7) | 5 (3–8) | |
| 3: ≥ 38 °C | 621 | 621 (100.0) | 7.4 (6.8) | 5 (3–9) | |
| Cardiovascular support | 1: ≥ 37.5 °C | 1341 | 393 (29.3) | 1.2 (2.7) | 3 (2–5) |
| 1: ≥ 38 °C | 873 | 293 (33.6) | 1.4 (2.9) | 3 (2–5) | |
| 2: ≥ 37.5 °C | 1145 | 370 (32.3) | 1.3 (2.9) | 3 (2–5) | |
| 2: ≥ 38 °C | 759 | 274 (36.1) | 1.5 (3.0) | 3 (2–5) | |
| 3: ≥ 37.5 °C | 907 | 346 (38.1) | 1.6 (3.0) | 3 (2–5) | |
| 3: ≥ 38 °C | 621 | 258 (41.5) | 1.8 (3.1) | 3 (2–5) | |
| Renal support | 1: ≥ 37.5 °C | 1341 | 44 (3.3) | 0.2 (1.3) | 4 (2–7) |
| 1: ≥ 38 °C | 873 | 33 (3.8) | 0.2 (1.4) | 4 (2–7) | |
| 2: ≥ 37.5 °C | 1145 | 39 (3.4) | 0.2 (1.3) | 4 (2–7) | |
| 2: ≥ 38 °C | 759 | 29 (3.8) | 0.2 (1.4) | 4 (2–7) | |
| 3: ≥ 37.5 °C | 907 | 36 (4.0) | 0.2 (1.4) | 4 (2–7) | |
| 3: ≥ 38 °C | 621 | 26 (4.2) | 0.2 (1.5) | 4 (2–7) | |
| Number of days alive and free (to day 28)b from: | |||||
| PICU | 1: ≥ 37.5 °C | 1341 | – | 20.0 (7.7) | 23 (19–25) |
| 1: ≥ 38 °C | 873 | – | 19.7 (7.8) | 23 (18–25) | |
| 2: ≥ 37.5 °C | 1145 | – | 19.2 (7.9) | 22 (18–25) | |
| 2: ≥ 38 °C | 759 | – | 18.9 (8.0) | 22 (17–24) | |
| 3: ≥ 37.5 °C | 907 | – | 18.6 (8.1) | 22 (17–24) | |
| 3: ≥ 38 °C | 621 | – | 18.4 (8.1) | 22 (16–24) | |
| Mechanical ventilation | 1: ≥ 37.5 °C | 1341 | – | 21.4 (7.7) | 24 (20–26) |
| 1: ≥ 38 °C | 873 | – | 21.1 (7.8) | 24 (20–26) | |
| 2: ≥ 37.5 °C | 1145 | – | 20.3 (7.8) | 23 (19–25) | |
| 2: ≥ 38 °C | 759 | – | 20.1 (7.9) | 23 (19–25) | |
| 3: ≥ 37.5 °C | 907 | – | 19.8 (8.0) | 23 (19–25) | |
| 3: ≥ 38 °C | 621 | – | 19.6 (8.1) | 23 (18–25) | |
Summary of findings informing the FEVER pilot randomised controlled trial
An interim analysis was conducted in July 2017 on data collected from 21 out of 22 sites from between March and May 2017 to inform the design of the pilot RCT. This showed that the proposal to narrow the inclusion criteria to mechanically ventilated patients from the FEVER qualitative study was feasible in terms of recruitment. Data from the interim and full analyses also confirmed the lower temperature threshold for the usual care group of the pilot RCT at ≥ 37.5 °C, as this temperature falls within usual care across UK PICUs.
Chapter 4 Methods for the FEVER pilot randomised controlled trial with integrated-perspectives study
Objectives
-
Test the willingness of clinicians to screen, recruit and randomise eligible critically ill children.
-
Estimate the recruitment rate of critically ill children.
-
Test the acceptability of the deferred consenting procedure and participant information.
-
Test, following randomisation, the delivery of and the adherence to the selected temperature thresholds (intervention and control) for antipyretic intervention and to demonstrate separation between the randomised groups in peak temperature measurement over the first 48 hours following randomisation.
-
Test follow-up for the identified, potential, patient-centred primary and other important secondary outcome measures and for AE reporting.
-
Inform final selection of a patient-centred primary outcome measure.
Study design
The FEVER pilot RCT was a pragmatic, open, multicentre, parallel-group, pilot RCT with an integrated-perspectives study element involving parents/legal representatives and site staff involved in the study. The integrated-perspectives study element comprised questionnaires and interviews with parents/legal representatives and focus groups and questionnaires with site staff towards the end of the FEVER pilot RCT.
Research governance
An application was submitted to the HRA on 20 June 2017 and received full approval on 11 August 2017 (Integrated Research Application System reference number 209931, Research Ethics Committee reference number 17/LO/1139). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/ (accessed 16 November 2018).
The FEVER pilot RCT was registered with the ISRCTN (International Standard Randomised Controlled Trial Number) registry (reference number ISRCTN16022198) on 14 August 2017 and was adopted onto the NIHR CRN Portfolio on 19 July 2017 (portfolio number 34598).
Local confirmation of capacity and capability was obtained from each participating NHS trust. A clinical trial site agreement, based on the model agreement for non-commercial research in the health service, was signed by each participating NHS trust and the sponsor (ICNARC).
In accordance with guidelines, a Trial Steering Committee (TSC), comprising 75% independent members, was convened to oversee the FEVER pilot RCT on behalf of the funder (NIHR). The TSC met twice and comprised an independent chairperson, an independent lay member (representing parent perspectives), independent clinicians and methodologists (specialising in paediatric emergency and critical care medicine), the chief investigator (MJP) and the lead qualitative researcher (KW).
In addition, a fully independent Data Monitoring and Ethics Committee (DMEC) was convened to monitor FEVER pilot RCT data and ensure the safety of participants. The DMEC met twice and comprised two expert clinicians specialising in paediatric emergency and critical care medicine (one acting as chairperson) and an experienced statistician.
Study management
The study co-ordinator at the ICNARC CTU was responsible for the day-to-day management of the FEVER pilot RCT and Kerry Woolfall was responsible for day-to-day management of the integrated-perspectives study. Both aspects were completed with support from the ICNARC CTU and the SMG.
Patient and public involvement
Engagement with patients was vital to the successful conduct of the FEVER pilot RCT with integrated-perspectives study. A parent of a child who was admitted to a PICU with severe infection provided oversight on the FEVER pilot RCT TSC; three members were co-investigators (JW, CF and BF) and members of the SMG. They provided input into the conduct of the FEVER pilot RCT, including reviewing literature to be given to patients and their families (e.g. participant information sheets). In addition to this, patients and members of the public were engaged through the FEVER qualitative study in which the result informed the pilot RCT design and trial materials.
One of the main aspects of the integrated-perspectives study was to obtain input from parents of children who were enrolled in the FEVER pilot RCT.
Design and development of the protocol
The FEVER pilot RCT protocol was designed to inform key parameters and to test the design and possible conduct of the proposed definitive FEVER RCT. The protocol design was informed by the perspectives of parents and clinicians, as described in Chapter 2, and the observational data, as reported in Chapter 3.
Amendments to the FEVER pilot randomised controlled trial protocol
Following receipt of approval from the HRA on 11 August 2017, one substantial and two minor amendments were submitted and categorised.
Substantial amendment 1 (approved on 30 October 2017) consisted of the following:
-
the addition of the inclusion criterion ‘referral requiring PICU admission to a participating unit’, which clarified for transport services that randomisation could only take place during transport for eligible patients being transported to a participating unit
-
the removal of the exclusion criterion ‘sickle cell disease’ as the SMG felt there to be no clinical reason for those with sickle cell disease not to take part in the trial
-
the addition of the exclusion criterion ‘previously recruited into the FEVER pilot RCT’
-
amendment of the existing exclusion criteria ‘severe rhabdomyolysis’ to ‘rhabdomyolysis’ and ‘receiving or requiring mechanical ventilation’ to ‘new requirement for mechanical ventilation’
-
minor administrative changes to the protocol that added clarity to the trial processes.
Minor amendment 1 (approved on 22 November 2017) consisted of the addition of a researcher contact details card to the participant materials given to the parent/legal guardian who agreed to take part in a telephone interview. This was to help assist contact and arrangement of interviews.
Minor amendment 2 (approved on 28 December 2017) consisted of a minor administrative change to the site staff questionnaire to mirror the focus group topic guide.
NHS support costs
The NHS support costs were agreed prior to the submission of the research grant to include screening to identify eligible patients and obtaining informed consent from parents. This equated to 0.15 whole-time equivalent of a Band 6 research nurse for 4 months in each of the four participating PICUs.
The FEVER pilot randomised controlled trial
Sites
Four sites agreed to participate in the FEVER pilot RCT (Table 7). The sites agreed to participate prior to the submission of the grant application, and for study continuity, including participation in the FEVER qualitative study and the FEVER observational study. Each FEVER pilot RCT site was obliged to:
-
meet all responsibilities as stated in the FEVER pilot RCT clinical trial site agreement
-
identify and sign up a local PI
-
identify a responsible research nurse (to be funded, or part-funded, centrally)
-
agree to incorporate the FEVER pilot RCT into routine transport team and PICU activity, particularly highlighting the importance of screening at first contact
-
agree to adhere to randomisation allocation and to ensure adherence to the protocol
-
agree, when possible, to recruit all eligible patients and to maintain a screening log.
| Site | PI |
|---|---|
| Alder Hey Children’s Hospital, Liverpool | Dr Kent Thorburn |
| Great North Children’s Hospital, Newcastle upon Tyne | Dr Rachel Agbeko |
| Great Ormond Street Hospital, London | Professor Mark Peters |
| Evelina London Children’s Hospital | Dr Shane Tibby |
Site initiation
Site teams from participating sites attended a site initiation meeting prior to the commencement of patient screening. Initiation meetings were held at each of the four sites between 22 September 2017 and 31 October 2017. The purpose of these meetings was for the chief investigator to present the background and rationale for the FEVER pilot RCT and integrated-perspectives study. The meetings included an overview of the FEVER qualitative study and the FEVER observational study findings, and a discussion of protocol delivery, including screening and recruiting patients, delivery of the intervention, data collection and validation and safety monitoring. The operational challenges of conducting the FEVER pilot RCT at sites were discussed in detail, including strategies for ensuring effective communication within the PICU and transport teams. The PI from each participating site attended the meeting. A standardised slide set from the site initiation meetings was circulated to facilitate internal training within a participating site.
Investigator site file
An investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the FEVER pilot RCT and included the approved protocol; all relevant approvals (e.g. local confirmation of capacity and capability); a signed copy of the clinical trial site agreement; the delegation of trial duties log; copies of the approved participant information sheets, parent/legal representative consent forms and participant assent form; and all standard operating procedures [e.g. for screening participants, randomising participants, delivery of the intervention, obtaining informed consent or assent and collecting and entering data onto the secure, dedicated, electronic case report form (eCRF)]. The site PI was responsible for maintaining the investigator site file. Responsible staff at sites were authorised to carry out FEVER pilot RCT duties (e.g. consenting and oversight of the delivery of the intervention) by the site PI on the delegation of trial duties log. This included a confirmation that the individual had been adequately trained to carry out the specific duty.
Communication with sites
The ICNARC CTU study team maintained close contact with the PI and research team at participating sites by e-mail and telephone throughout the set-up and delivery periods of the FEVER pilot RCT. Two teleconferences were held with research teams at participating sites during the recruitment period. The purpose of these was to provide updates on progress and to provide a forum for site teams to ask questions, discuss local barriers and challenges to the conduct of the FEVER pilot RCT and delivery of the intervention, and to share successes and best practice. E-mail discussions also took place with individual site teams and the members of the study team to address site-specific issues in the conduct of the FEVER pilot RCT.
Site monitoring visits
A routine monitoring visit was conducted at all participating sites during the FEVER pilot RCT. During the site visit, the investigator site file was checked for completeness (i.e. that all essential documents were present), the parent/legal representative consent forms and participant assent forms (if applicable) were checked to ensure that the relevant correctly completed form was present for every participant recruited into the FEVER pilot RCT (or, if the parents/legal representatives were followed up for consent by post, evidence of the postal consent covering letters for non-responders) and a random sample of patient CRFs were checked against the source data for accuracy and completeness. After the visit, the PI and site team were provided with a report summarising the documents that had been reviewed and actions required by the site team. The site PI was responsible for addressing the actions and reporting back to the ICNARC CTU.
Maintenance and motivation
During the FEVER pilot RCT, frequent e-mails to site teams with updates on patient recruitment and newsletters were sent. These provided an opportunity to clarify any issues related to the conduct of the FEVER pilot RCT, as well as maintaining motivation and involvement through regular updates on progress and next steps. A number of steps were taken to raise and maintain the profile at participating sites, including the inclusion criteria posters being displayed in staff areas and at relevant locations within the PICU, information posters being displayed in family/relative waiting rooms, pocket cards summarising the eligibility being distributed, branded pens being distributed to staff, labels being used for participant notes indicating involvement in the trial and providing bedside posters to aid intervention adherence.
Support
A 24-hour, 7 days per week telephone support service was available, linking site teams to one of the clinical members of the SMG to provide advice on screening and recruitment of participants and on delivery of the intervention. This ensured access to clinicians to answer any queries on the eligibility of patients and delivery of the intervention.
Participants
Recruitment
The trial procedures for recruitment and follow-up of participants are summarised in Figure 10.
FIGURE 10.
Summary of trial procedures for recruitment and follow-up of participants.
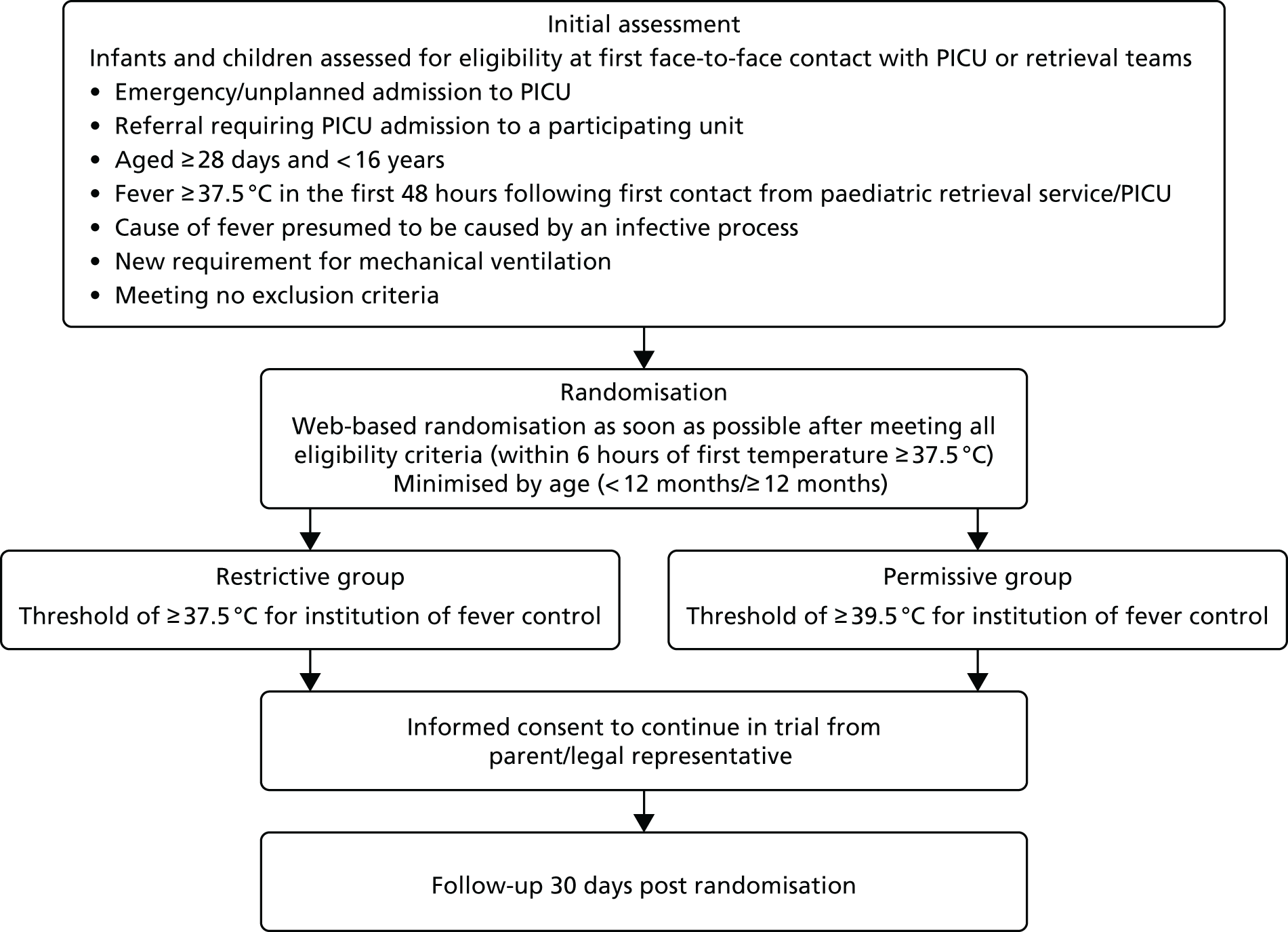
Inclusion criteria
Patients were eligible for inclusion in the FEVER pilot RCT if they met all of the following criteria:
-
unplanned PICU admission
-
aged ≥ 28 days and < 16 years
-
referral requiring PICU admission to a participating unit
-
fever of ≥ 37.5 °C in the first 48 hours following contact with the paediatric retrieval service/PICU
-
new requirement for mechanical ventilation
-
treating clinician presumes the cause of the fever is an infective process.
Exclusion criteria
Patients were excluded if they met any of the following criteria:
-
acute encephalopathy, including convulsive status epilepticus
-
postcardiopulmonary bypass or known/suspected cardiomyopathy/myocarditis
-
rhabdomyolysis (defined as serum creatine kinase concentration at least 10 times the upper limit of normal)
-
malignant hyperthermia, neuroleptic malignant syndrome or drug-induced hyperthermia
-
receiving palliative care, or death perceived as imminent
-
previously recruited to the FEVER pilot RCT.
Initiation of screening and recruitment
Following attendance at a site initiation meeting, screening and recruitment were commenced at participating sites once the clinical trial site agreement had been signed and necessary approvals were in place. Potentially eligible patients were identified, confirmed as eligible by the local research and clinical teams and then randomised prior to obtaining informed consent. RWPC was implemented as fever is typically highest early in the clinical course before either treatment of the cause is attempted or specific treatment of fever is administered, and so any interventions to improve the management of fever will have the greatest impact if they are acted on immediately after a fever is detected. Therefore, any delay to implementation of a temperature-control strategy may be detrimental to patients. In addition, staff priorities are assessment and emergency treatment of the patient, and any delay in commencing treatment could have been detrimental.
Randomisation and allocation procedure
Participants were randomised by authorised staff at participating sites once eligibility was confirmed and within 6 hours of the infant’s or child’s temperature being recorded as ≥ 37.5 °C, using an online web randomisation service. Participants were randomly allocated (1 : 1) to either the permissive group or to the restrictive group by a computer-generated dynamic procedure (minimisation) with a random component. Minimisation was performed on age (< 12 months/≥ 12 months) with a 70 : 30 split in the weighted random element. This meant that each participant was allocated with 70% probability to the group that minimises the between-group differences in those aged < 12 or ≥ 12 months among participants recruited to the trial to date, and 30% probability of allocation to the alternative group.
Staff at participating sites were advised to call either their local FEVER on-call clinicians or the 24 hours, 7 days per week telephone support service if they needed to address any emergency recruitment or randomisation issues.
Screening log
To enable full and transparent reporting, brief details of all patients who met the inclusion criteria were recorded in the screening log. If the patient was deemed ineligible because they met one or more of the exclusion criteria, this was recorded in the screening log. The reasons for eligible patients not being recruited were recorded under two categories: (1) ‘missed’ (e.g. > 6 hours elapsed since becoming eligible) and (2) ‘identified but not randomised’ (e.g. clinical decision made to not include the patient in the study).
Consent
Staff members who had received training on the background, rationale and purpose of FEVER and on the principles good clinical practice (GCP) were authorised by the PI to take informed consent from parents/legal representatives. The method used for the FEVER pilot RCT was RWPC.
Once notified of the recruitment of a participant to the FEVER pilot RCT, a delegated member of the site research team approached the parents/legal representatives as soon as practical and appropriate following randomisation (usually 24–48 hours) to discuss consent. 18,30
Information about the FEVER pilot RCT was provided to the parents/legal representatives, which included the purpose of the trial, the reasons why informed consent prior to randomisation could not be sought from parents/legal representatives, what participation in the trial meant (i.e. permission for the use of data already collected and/or for their child to continue to take part in the trial procedures when applicable18), participant confidentiality, future availability of the results of the trial and funding of the study. It also provided information on completing an optional questionnaire and/or taking part in a telephone interview as part of the integrated-perspectives study. This information was provided in the participant information sheet, along with the name and contact details of the local PI and research nurse(s), which was given to the parents/legal representatives to read before making their decision for their child’s data to be used, or not, and for their child to continue to take part, or not, in the trial. Once the staff member taking informed consent was satisfied that the parents/legal representatives had read and understood the participant information sheet and that all their questions about the trial had been answered, the parents/legal representatives were invited to sign the consent form.
Death prior to consent being sought
The views of bereaved parents who participated in the FEVER qualitative study (see Chapter 2) and guidance on RWPC in emergency and critical care trials were used to inform the approach to consent when a child had died before consent had been sought from parents/legal representatives. 18 In this situation, a researcher would obtain information from colleagues and bereavement counsellors to establish the most appropriate research team member to notify the parents/legal representatives of the trial. Consent could have been sought from parents/legal representatives following the death of their child and prior to their departure from the hospital; however, it was at the discretion of the site staff to determine if this was appropriate for each individual family.
If consent was not sought prior to the parents’/legal representatives’ departure from the hospital, then the parents/legal representatives were sent a covering letter, personalised by the most appropriate clinical team member, and a copy of the participant information sheet (version for bereaved parents/legal representatives) and consent form (postal version for bereaved parents/legal representatives) by post 4 weeks after randomisation. The letter explained how to opt in or out of the trial, directed them to the participant information sheet for detailed information on the trial, and provided telephone contact details if parents/legal representatives wished to discuss the trial with a member of the site research team. If there was no response 4 weeks after sending the initial letter, a follow-up letter along with the participant information sheet and consent form were sent. The second letter provided the same information as the first letter, in addition to confirming that if no consent form was received within 4 weeks of sending the second letter, then the participant’s data would be included in the trial unless the family notified the site research team otherwise.
Discharge prior to consent being sought
In the situation in which a participant was discharged from hospital before consent had been sought, the most appropriate member of the site research team attempted at least one telephone call to the parents/legal representatives within 5 working days of hospital discharge to inform them of the participant’s involvement and provide details of the trial. If the parents/legal representatives gave provisional agreement for their child’s data to be used in the study, this agreement was recorded by the site staff; however, if they refused to provide consent at this stage, this was treated as a consent refusal and their child’s data were withdrawn from the study.
Parents/legal representatives who provisionally agreed during the telephone call, requested further information or could not be reached were sent a covering letter, personalised by the most appropriate clinical team member, and a copy of the participant information sheet and consent form (postal version) by post. The postal procedures then followed those outlined above.
Assent
Because of the severity of illness and its impact on the mental state of the target population, it was not considered possible to involve FEVER pilot RCT participants in the consenting process. Instead, there was a possibility for participants to assent to the trial prior to hospital discharge if the participant was determined old enough to be able to make an informed decision and their condition allowed (e.g. they regained capacity). FEVER pilot RCT participants were provided with an age-appropriate participant information sheet and asked to sign an assent form, if appropriate. Parents/legal representatives would have been involved in this discussion. In all other respects, the assenting procedures followed the consenting procedures as described in Consent. If the participant was likely to regain capacity following hospital discharge, then an age-appropriate participant information sheet was provided to parents/legal representatives to discuss with the participant following recovery. The age-appropriate participant information sheet included a description of the trial and a link to the ‘You took part in research’ animation (https://youtu.be/_Fs1yUxeBFQ; accessed 16 November 2018), which describes what happens when children are included in RWPC. The animation is based on the findings from ‘The VOICES project – Children’s Views’,31 which explored the views of 16 children aged 7–15 years about RWPC.
Interventions
The trial incorporated a pragmatic approach to temperature control in both trial groups. An example treatment algorithm is illustrated in Figure 11. Treatments to reduce temperature were only permitted in response to a temperature threshold of ≥ 39.5 °C in the permissive group and a temperature threshold of ≥ 37.5 °C in the restrictive group. The treatment strategies for the restrictive and permissive group commenced from randomisation and were sustained until PICU discharge or death while receiving mechanical ventilation (defined as invasive, non-invasive or high-flow oxygen). During periods when the participant did not receive mechanical ventilation, the trial processes were not applicable. If the participant subsequently recommenced mechanical ventilation, the trial processes were reapplied.
FIGURE 11.
Example treatment algorithm.
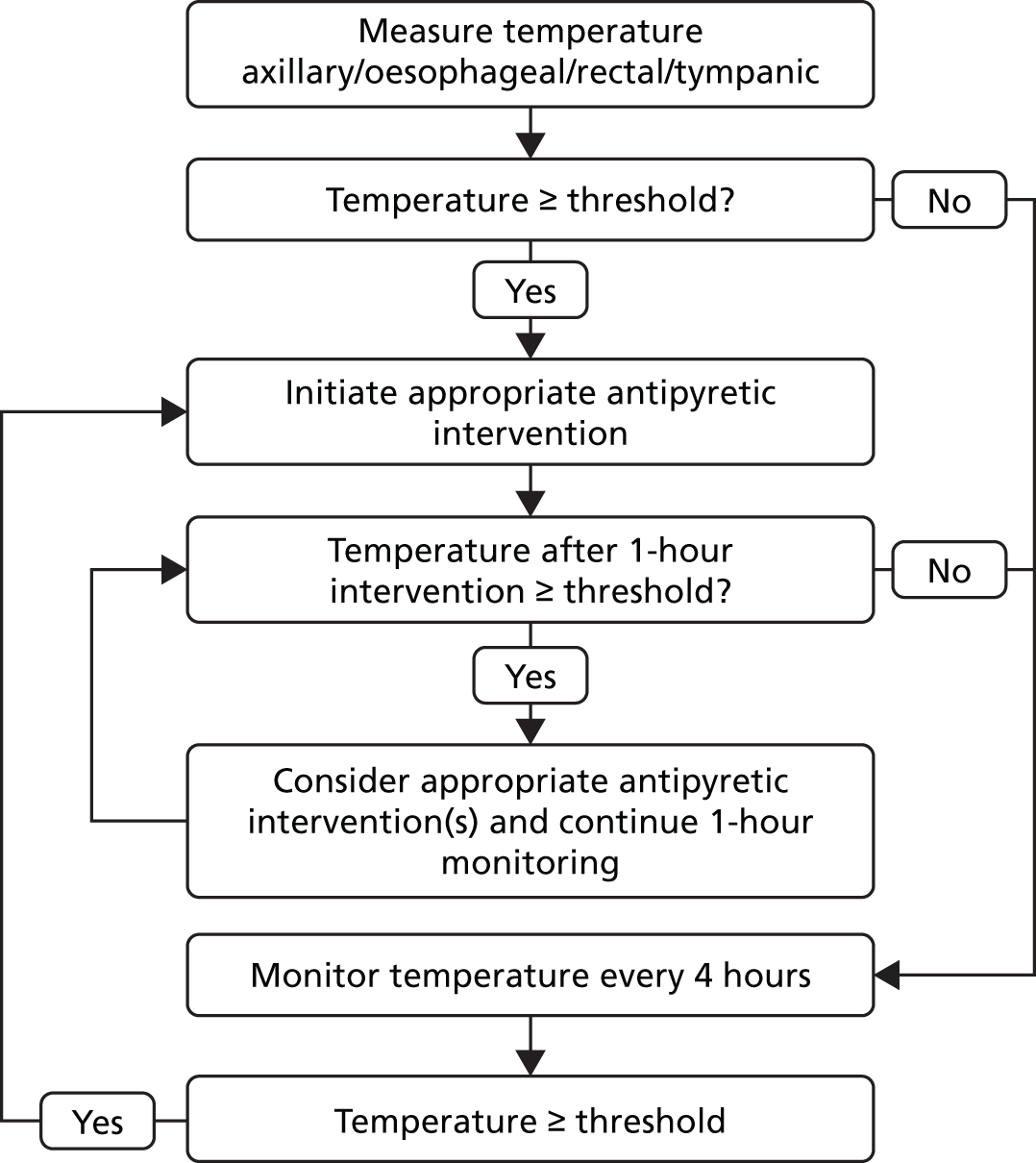
Outcomes
-
The proportion of eligible children recorded in the trial screening logs who were recruited to the FEVER pilot RCT and the reported reasons for non-recruitment.
-
Recruitment rate of eligible, critically ill children.
-
The proportion of recruited children whose parents subsequently declined to give consent or who withdrew their child from the FEVER pilot RCT having initially given consent.
-
Adherence to the temperature thresholds for antipyretic intervention in both the intervention and standard care groups.
-
Separation between the treatment groups in maximum temperature measurement over 48 hours following randomisation.
-
A comparison between treatment groups and assessment of the completeness of follow-up for the following potential outcome measures:
-
Mortality at discharge from a PICU (‘PICU mortality’).
-
Mortality at ultimate discharge from an acute hospital (‘hospital mortality’).
-
Mortality at 30 days post randomisation (‘30-day mortality’).
-
Length of stay in a PICU (calculated in days from the date of randomisation to the date of discharge from or death in a PICU).
-
Duration of organ support (calculated as the number of calendar days on which the organ support was received in a PICU up to day 28) –
-
Number of days of mechanical ventilation.
-
Number of days of cardiovascular support.
-
Number of days of renal support.
-
-
Number of days alive and free from a PICU to day 28.
-
Number of days alive and free from mechanical ventilation to day 28.
-
Safety monitoring
Participants were monitored for AEs occurring between randomisation and PICU discharge. Specified AEs were:
-
seizures
-
rhabdomyolysis (defined as serum creatine kinase concentration at least 10 times the upper limit of normal)
-
cerebral oedema.
Unspecified AEs were defined as an unfavourable symptom or disease temporally associated with the instigation of the FEVER pilot RCT treatments, whether or not it was related to the trial treatment, that was not deemed to be a direct result of the participant’s medical condition and/or standard emergency or critical care treatment. All AEs were recorded in the eCRF and reported as part of routine reporting throughout the FEVER pilot RCT to the DMEC. AEs that were assessed to be serious (i.e. prolonging hospitalisation or resulting in persistent or significant disability/incapacity), life-threatening or fatal – collectively termed serious adverse events (SAEs) – were reported to the ICNARC CTU. SAEs that were unspecified and considered to be possibly, probably or definitely related to the trial treatment were reported to the Research Ethics Committee within 15 calendar days of the event being reported.
Data collection
A secure, dedicated eCRF, hosted by ICNARC, was set up for data to be entered by staff at participating sites. The eCRF was accessible only to authorised users with access being centrally controlled by the study co-ordinator or data manager (access was granted after cross-checking the site delegation of study duties log). Each individual was provided with a unique username and password and had access to data only for participants recruited at their site. The data set for the FEVER pilot RCT included the minimum data required to confirm patient eligibility, describe the patient population, monitor and describe delivery of the intervention, assess outcomes and enable identification of the patient through PICANet to collect applicable routinely collected data.
Baseline data
Baseline data were collected to enable the participant to be randomised and included confirmation that the patient met all of the inclusion criteria and none of the exclusion criteria. The following data were also collected at baseline to describe the patient population:
-
age
-
type of ventilation
-
previous treatment of fever
-
physiology (temperature, lactate, heart rate, mean arterial pressure, systolic blood pressure, diastolic blood pressure and blood pressure measurement type).
Daily data collection during paediatric intensive care unit admission
The following trial-specific data were collected from randomisation until PICU discharge or day 28:
-
whether or not the patient was on mechanical ventilation
-
type(s) of ventilation
-
antipyretic interventions (while receiving mechanical ventilation) and type(s) of antipyretic interventions for days 1–7
-
use of opiates (while receiving mechanical ventilation) on days 1–7 only
-
highest temperature (while receiving mechanical ventilation).
From day 1 (day of randomisation) to day 7, data were collected in 6-hourly intervals (0.00–5.59, 6.00–11.59, 12.00–17.59 and 18.00–23.59); following day 7 (days 8–28), data were recorded daily.
Data collection at discharge from paediatric intensive care unit/hospital
The following data were collected following discharge from a PICU, from the hospital housing the PICU and ultimate discharge from an acute hospital (if relevant):
-
infection site and organism (for patients with confirmed infection at PICU discharge)
-
survival status at discharge
-
date of discharge or death
-
survival status at 30 days following randomisation (and date of death for patients who died within 30 days).
Additional data collection
Additional trial-specific data were also collected on:
-
Adverse events from randomisation to PICU discharge.
-
Consent, assent and withdrawal. When consent for a telephone interview or longer-term contact for future research was obtained, parent/legal representative contact details were collected.
Data management
Data management was an ongoing process. Data entered by sites onto the eCRF were monitored and checked throughout the recruitment period to ensure that they were as complete and accurate as possible. Two levels of data validation were incorporated into the electronic case report. The first was to prevent obviously erroneous data from being entered (e.g. entering observation data before the date of randomisation). The second level involved checks for data completeness and any unusual data entered (e.g. a physiological variable, such as systolic blood pressure) that were outside the predefined range.
Once data were entered onto the eCRF, the data manager generated data clarification checks, listing all outstanding data queries, at regular intervals throughout the recruitment period. The site PI was responsible for ensuring that all data queries were resolved. Ongoing data entry and validation at sites was closely monitored by the data manager and any concerns were raised with the site staff and PI.
Adherence to the FEVER pilot RCT protocol was closely monitored, including adherence to all elements of the trial procedures. Any queries relating to adherence were also generated as data request checks and sent to participating sites at regular intervals via e-mail. For each query, the PI and/or site research team were asked to explain the reason for any non-adherence to the protocol. If deemed necessary, follow-up contact via e-mail or telephone was made to ensure that effective plans were put in place to improve future adherence.
Sample size
The FEVER pilot RCT was set up without a defined primary outcome and, hence, without a usual power calculation to determine sample size. Instead, the sample was determined to be adequate to estimate critical parameters to be tested to a necessary degree of precision.
Data from 1537 children admitted to a PICU with a reason for admission associated with infection and a temperature of ≥ 37.5 °C in UK PICUs indicate a mean peak temperature in the first 24 hours following PICU admission of 38.5 °C with a SD of 0.7 °C (unpublished data from the UK PICOS study;29 Kathryn M Rowan and David A Harrison, ICNARC, 2016, personal communication). Based on this, a sample of 84 children was anticipated to be sufficient to give 90% power to demonstrate a separation of 0.5 °C in mean peak temperature between the higher temperature threshold group and standard care group. The FEVER pilot RCT would therefore need to recruit 100 children to allow for up to an anticipated 16% withdrawal following deferred consent. 32
Allowing for a rate of non-recruitment of 30% of eligible children, recruitment was anticipated to be completed with four PICUs/retrieval teams recruiting for 4 months (anticipated recruitment rate of 6.25 children per PICU per month).
Statistical analysis
Interim analysis
Unblinded, comparative data on recruitment, withdrawal, adherence and AEs were reviewed by an independent DMEC. No formal interim analyses were performed owing to the nature of the FEVER pilot RCT.
Analysis principles
All analyses followed the intention-to-treat principle. Participants were analysed in accordance with the group they were randomised to, irrespective of whether or not the treatment allocated was received. Effect estimates are reported with 95% CIs.
Analyses were performed using Stata®/SE version 14.2 for Microsoft Windows® 64-bit x86-64 (Microsoft Corporation, Redmond, WA, USA) and R version 3.4.3.
Study population
Screening and eligibility
The numbers of participants screened and deemed eligible are reported overall and by site.
Recruitment
The numbers of patients randomised, consented (to continue the study and for the use of their data) and withdrawn are reported by site and treatment group and summarised as a Consolidated Standards of Reporting Trials (CONSORT) flow diagram. The numbers of patients screened and recruited are reported per month and by site (see Table 10 and Figure 14). When reported, the reasons for exclusion and withdrawal are also summarised.
Adherence and protocol deviations
Overall adherence during day 1–7 is summarised by the proportion of time spent below the allocated threshold – calculated for each patient as the sum of the number of 6-hourly time periods during which the maximum temperature was below the allocated threshold divided by the sum of the number of time periods during which the patient was receiving mechanical ventilation – and is reported as the median and IQR in each treatment group.
Potential protocol deviations during day 1–7 were identified as participants who:
-
received antipyretic intervention during time periods when their maximum temperature did not reach the allocated threshold
-
did not receive antipyretic intervention during time periods when their maximum temperature exceeded the allocated threshold.
Potential protocol deviations triggered a query to the participating site, which then had the opportunity to provide a justification/explanation. In some cases (e.g. a highest temperature recorded towards the end of a 6-hour time period with antipyretic interventions not started until early in the next 6-hour time period), the SMG determined that the event was not a protocol deviation.
Separation between groups
Separation between the treatment groups in the maximum temperature measurement over the first 48 hours following randomisation is presented as the difference in the mean with 95% CIs. The maximum temperature over the first eight 6-hourly time periods for each participant was calculated and then the mean of these values for each treatment group was calculated. For participants who spend < 1 hour in their first time period, their first highest temperature measurement as the maximum from the first two time periods was calculated, and this was combined with the values from their seven subsequent 6-hourly time periods.
Highest temperature
The highest temperature measurements are presented graphically (see Figure 15):
-
side-by-side box plots based on individual measurements by time period and treatment group
-
the median and IQR by time period and treatment group
-
the difference between the treatment groups in the mean of the highest temperature with 95% CI by time period and treatment group
-
the distribution of the proportion of time spent at each temperature in each treatment group during (1) the first 48 hours (defined as earlier) and (2) days 1–7.
Antipyretic interventions
The proportion of participants who received any antipyretic intervention while mechanically ventilated is presented by treatment group as a time series plot for days 1–7.
A breakdown of the proportion of participants receiving each type of antipyretic intervention is tabulated by time period and treatment group for days 1–7.
Use of opiates
The use of opiates is presented using a time series plot and table analogous to those for the antipyretic interventions.
Baseline patient characteristics
Baseline demographic and clinical data were summarised for the intention-to-treat population for the two treatment groups and overall. There was no statistical testing for any of the summary measures when comparing the baseline variables between the treatment groups. The following baseline variables were compared between the treatment groups and may be required for risk adjustment and stratification:
-
age (years) – median (IQR) and number (%) by age group (< 1, 1, 2–4, 5–9 or 10–16 years)
-
sex (female or male) – number (%)
-
pre-randomisation antipyretic interventions [paracetamol, non-steroidal anti-inflammatory drug (NSAID), external/other cooling and any antipyretic intervention] – number (%)
-
Paediatric Index of Mortality version 2r (PIM2r) score33,34 (2016 recalibration) – median (IQR)
-
Paediatric Index of Mortality version 3 (PIM3) score35 – median (IQR)
-
source of admission (same hospital, other hospital, clinic or home) – number (%)
-
primary diagnosis (asthma, acute respiratory failure, bronchiolitis, pneumonia/lower respiratory tract infection, sepsis/septic shock, seizures/convulsions and other) – number (%).
Outcome definitions
The following potential outcome measures for the definitive RCT were compared between the treatment groups:
-
PICU mortality
-
hospital mortality
-
30-day mortality
-
length of stay in a PICU (calculated in days from the date of randomisation to the date of discharge from PICU or death in a PICU)
-
duration of organ support (calculated as the number of calendar days on which the organ support was received in a PICU up to day 28) –
-
– number of days of mechanical ventilation
-
– number of days of cardiovascular support
-
– number of days of renal support
-
-
number of days alive and free from PICU to day 28
-
number of days alive and free from mechanical ventilation to day 28.
Participants who died between randomisation and day 28 were assigned a value of zero days alive and free from PICU/mechanical ventilation to day 28. For participants who survived to day 28, the number of days alive and free from PICU (mechanical ventilation) were calculated as the number of calendar days between the day of randomisation and day 28, inclusive, on which the participant was not in a PICU (not receiving mechanical ventilation in a PICU) at any time during the calendar day.
Analysis methods
Mortality outcomes are reported as the number and percentage in each group. The risk ratio and absolute risk reduction were calculated unadjusted, with 95% CIs based on approximate standard errors.
Length of stay in a PICU is reported as the mean (SD) and median (IQR) in each treatment group, overall and stratified by survival to PICU discharge.
Duration of organ support is reported as the number (%) receiving the organ support, the median (IQR) duration of organ support among participants receiving the organ support and the mean (SD) duration of organ support among all participants (with participants not receiving the support assigned a value of zero) in each group. The difference in means was calculated unadjusted, with 95% CIs based on Student’s t-distribution assuming equal variance.
The numbers of days alive and free from a PICU/mechanical ventilation are reported as the mean (SD) in each group and the difference in means with 95% CI, calculated as for the duration of organ support.
In addition, histograms of the continuous outcomes by treatment group are presented.
Missing data
To assess the follow-up procedures, the number (%) of participants with complete follow-up data for each of the potential outcome measures for the definitive RCT, overall and by treatment group, is reported.
All analyses were undertaken in the complete-case population. There was no imputation of missing data.
Harms
The number and percentage of participants experiencing each prespecified AE (plus any other AEs as reported) while in PICU were summarised for each treatment group. Numbers of SAEs, severity and reported relatedness to treatment are also reported.
Additional analyses
If the results of the FEVER observational study suggested a potential narrowing of the inclusion criteria (e.g. a higher temperature threshold or restricting to participants receiving invasive ventilation), then it was planned to repeat the FEVER pilot RCT analyses on the subgroup of patients likely to be eligible for the definitive FEVER RCT.
Integrated-perspectives study
Participants
Based on previous research and the FEVER pilot RCT sample size,17,18 it was estimated that approximately 50 parents/legal representatives (including both mothers and fathers of randomised children) would be recruited to the questionnaire element and 15–25 (depending on data saturation) parents/legal representatives would be recruited to the interview element. In addition, four focus groups with site staff (approximately 6–8 per group) and an online questionnaire for those who could not attend focus groups were planned.
Eligibility
Parents/legal representatives who did and did not consent to the FEVER pilot RCT were eligible to take part in the questionnaire and interview elements, unless they were unable to speak or read English.
Site staff involved in screening, recruiting, randomising and consenting parents/legal representatives during the FEVER pilot RCT were eligible to take part in a focus group or online questionnaire.
Recruitment and consent procedure
Parents/legal representatives
As part of the FEVER pilot RCT participant information sheet and consent discussions, parents/legal representatives were provided with information about the integrated-perspectives study. Researchers explained the different aspects of the FEVER pilot RCT and asked parents/legal representatives if they would like to complete the FEVER consent questionnaire (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019) and/or provide contact details on the consent form if they were willing to take part in a telephone interview. Parents/legal representatives contacted about the FEVER pilot RCT by post (see Death prior to consent being sought and Discharge prior to consent being sought) were provided with information on the telephone interview only.
Site staff
An invitation to participate in a focus group at one of the four participating sites was e-mailed to site PIs and/or lead research nurses. They were also asked to send the invitation to all staff involved in the FEVER pilot RCT at their site. The e-mail included a participant information sheet for staff, which provided information about the purpose of the integrated-perspectives study elements and what the focus group would involve. Written consent was sought from staff before the focus group began. A separate e-mail invitation was sent to PIs and/or lead research nurses a week after each focus group for dissemination to staff who were unable to attend the focus group. The introduction to the questionnaire explained how completion constituted consent for participation.
Participation was entirely voluntary and parents/legal representatives and site staff were able to withdraw at any time without giving a reason.
Procedures
Parent questionnaire administration
Following the consent discussion, one of the members of the FEVER site staff gave a copy of the FEVER consent questionnaire (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019) to each parent/legal representative to complete (one questionnaire per parent). The questionnaire was then placed in a stamped self-addressed envelope, preferably before they left hospital, and returned by post to the University of Liverpool.
Parent telephone interview conduct
A researcher contacted parents/legal representatives to arrange an interview within 1 month of consent. Subsequent consent for audio-recording of interviews was checked verbally at the time of the interview. Parent telephone interviews began with a discussion about their child’s admission to hospital and current well-being, aims of the interview, an outline of the topics to be covered in the interview and an opportunity for questions. The interview then commenced using the interview topic guide to explore views and experiences about their child’s admission to hospital, including diagnosis; the FEVER pilot RCT consent process, including how and when the trial was communicated by staff; information materials; and consent and decision-making. This included questions about how trial processes could be improved, child assent and outcome measures. Example questions can be found on the project web page (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019). Respondent validation was used to add unanticipated topics to the topic guide as interviewing and analysis progressed. 22 After the interview was complete, parents/legal representatives were sent a copy of the consent form, a thank you letter and a £30 Amazon voucher to thank them for their time.
Staff focus group conduct
Focus groups began with asking staff to introduce themselves and their role within the FEVER pilot RCT. The focus group aims and topics to be covered were discussed, followed by an opportunity for questions. In the focus groups, a voting system using TurningPoint software was used alongside verbally administered questions. This involved questions (from the online questionnaire) being presented to the group via a laptop presentation and each participant using a wireless handset to select their answer from those shown on the screen. A test question was used at the beginning of the focus group to help demonstrate how the voting system would work alongside verbally administered questions from the topic guide (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019). This method was used to enable the collection of data from all participants, as well as a means of generating statistical data from all sites alongside qualitative data from group discussions.
Focus groups explored staff views and experiences on site training; acceptability of the proposed trial, including temperature thresholds, trial information and consenting procedures; willingness of staff to screen, recruit and randomise eligible critically ill children; parents’/legal representatives’ responses to RWPC; protocol adherence; and FEVER pilot RCT forms, including CRFs and consent forms. Example questions are shown on the project web page (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019). Respondent validation was used to add unanticipated topics to the topic guide as parent interviewing, focus group conduct and analysis progressed. 22 After focus groups were complete, all participants were sent a copy of the consent form (if applicable) and thanked for their time.
Staff online questionnaire
The invitation e-mail contained a link to the site staff online questionnaire (see www.journalslibrary.nihr.ac.uk/programmes/hta/154401/#/; accessed 23 January 2019) for completion. When the questionnaire was complete, a message thanked participants for their time.
Transcription
Digital audio-recordings were transcribed verbatim by a professional transcription company (Voicescript Ltd, Bristol, UK) in accordance with the Data Protection Act 1998. 23 Transcripts were anonymised and checked for accuracy. All identifiable information, such as names (e.g. of patients, family members or the hospital their child was admitted to), were removed.
Data analysis
Qualitative analysis of interview and focus group data was interpretive and iterative (Table 8). Utilising a thematic analysis approach, the aim was to provide accurate representation of parental views on trial design and acceptability. Thematic analysis is a method of identifying, analysing and reporting patterns (or themes) within data. Analysis was informed by the work of Braun and Clarke24 and their guide to thematic analysis. This approach allows for themes to be identified at a semantic level (i.e. surface meanings or summaries) or at a latent level (i.e. interpretive – theorising the significance of the patterns and their broader meanings and implications). 28 NVivo 10 software was used to assist in the organisation and coding of data. ED (a psychologist) and Kerry Woolfall (a sociologist) analysed and synthesised the data. Quantitative data from the parent and staff questionnaire were cleaned and entered into IBM SPSS Statistics version 20.0. Descriptive statistics are presented with percentages.
| Phase | Description |
|---|---|
| 1. Familiarising with data | ED and KW read and re-read transcripts, noting down initial ideas on themes |
| 2. Generating initial codes | Initially, two complementary data-coding frameworks were developed [one for focus group data (KW) and one for interview data (ED)] using a priori codes identified from the project proposal and topic guides. During the familiarisation stage, ED and KW identified additional data-driven codes and concepts not previously captured in the initial coding frame |
| 3. Developing the coding framework | KW coded 10% of the interview transcripts using the initial coding frame and made notes on any new themes identified and how the framework could be refined. In turn, ED coded 10% of the focus group transcripts following the same procedure |
| 4. Defining and naming themes | Following review and reconciliation by ED and KW, revised coding frames were subsequently developed and ordered into themes (nodes) within the NVivo database |
| 5. Completion of coding of transcripts | ED completed coding interview transcripts and KW completed coding focus group transcripts in preparation for write-up |
| 6. Producing the report | ED and KW developed the manuscript using themes to relate back to the study aims, ensuring that key findings and recommendations were relevant to the FEVER trial design and site staff training (i.e. catalytic validity).24–27 Final discussion and development of selected themes took place during the write-up phase |
Chapter 5 Results of the FEVER pilot randomised controlled trial
Screening and recruitment
Sites
All sites were open to recruitment within 3 months of receiving full HRA approval (Table 9). Initial local document packs were sent in June 2017 and the final packs were sent on 17 August 2017. The median time from receiving the final local document packs to the pilot trial opening at sites (i.e. start of screening) was 51.5 days (range 39–83 days) and to the first participant recruited was 62 days (range 41–84 days). Delays in opening were predominantly attributable to the time taken to obtain local confirmation of capacity and capability.
| Hospital | Date when open to recruitment |
|---|---|
| Great Ormond Street Hospital | 25 September 2017 |
| Alder Hey Children’s Hospital | 2 October 2017 |
| Great North Children’s Hospital | 13 October 2017 |
| Evelina London Children’s Hospital | 8 November 2017 |
Participants
Between 25 September 2017 and 19 December 2017, a total of 154 patients were screened and deemed to meet the inclusion criteria (Figure 12). Of these, 15 patients (9.7%) met one or more of the exclusion criteria. Of those patients deemed to be eligible, 26 (18.7%) were missed and a further 12 (8.6%) were not randomised owing to local clinical decisions. Overall, 101 eligible children (72.7%) were randomised into the pilot RCT. One patient randomised to the permissive group was identified immediately post randomisation as a duplicate randomisation and removed from the study. Therefore, 72.5% (100/138, 95% CI 64.5% to 79.2%) of eligible patients were appropriately randomised into the study.
FIGURE 12.
The CONSORT flow diagram. a, Two patients were under the care of social workers, two had a lack of understanding of study information and one was a site decision. b, Includes 139 patients eligible for randomisation minus one duplicate patient. c, Includes 101 randomised patients minus one duplicate patient.
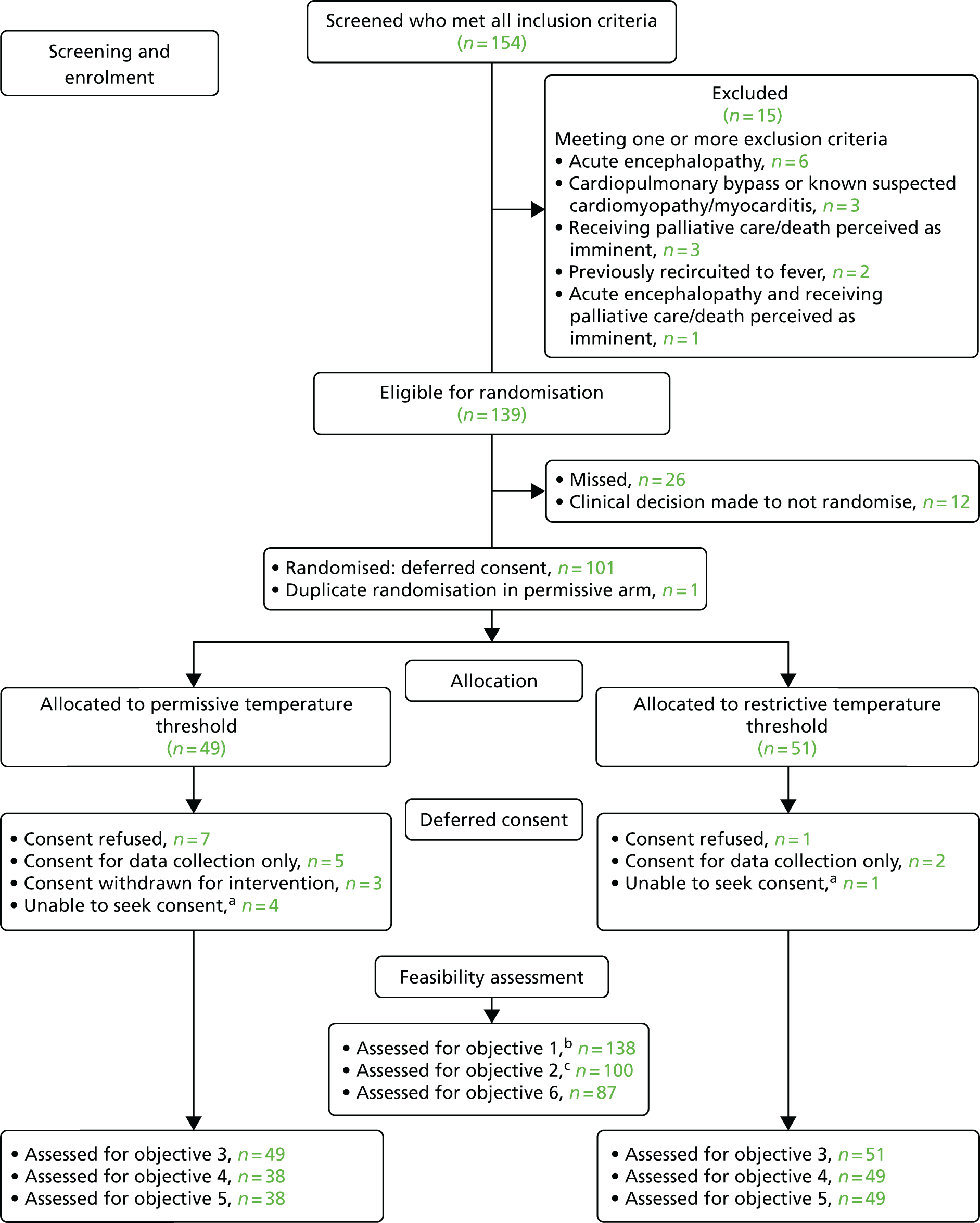
The overall recruitment rate of 11.1 participants per site per month (95% CI 9.0 to 13.5 participants) was greater than the pre-trial estimate of 6.25 (Figure 13); however, recruitment rates varied across sites (Figure 14). The number of patients eligible, screened and recruited per month at each site can be found in Table 10.
FIGURE 13.
Cumulative recruitment over time compared with pre-trial expected recruitment.
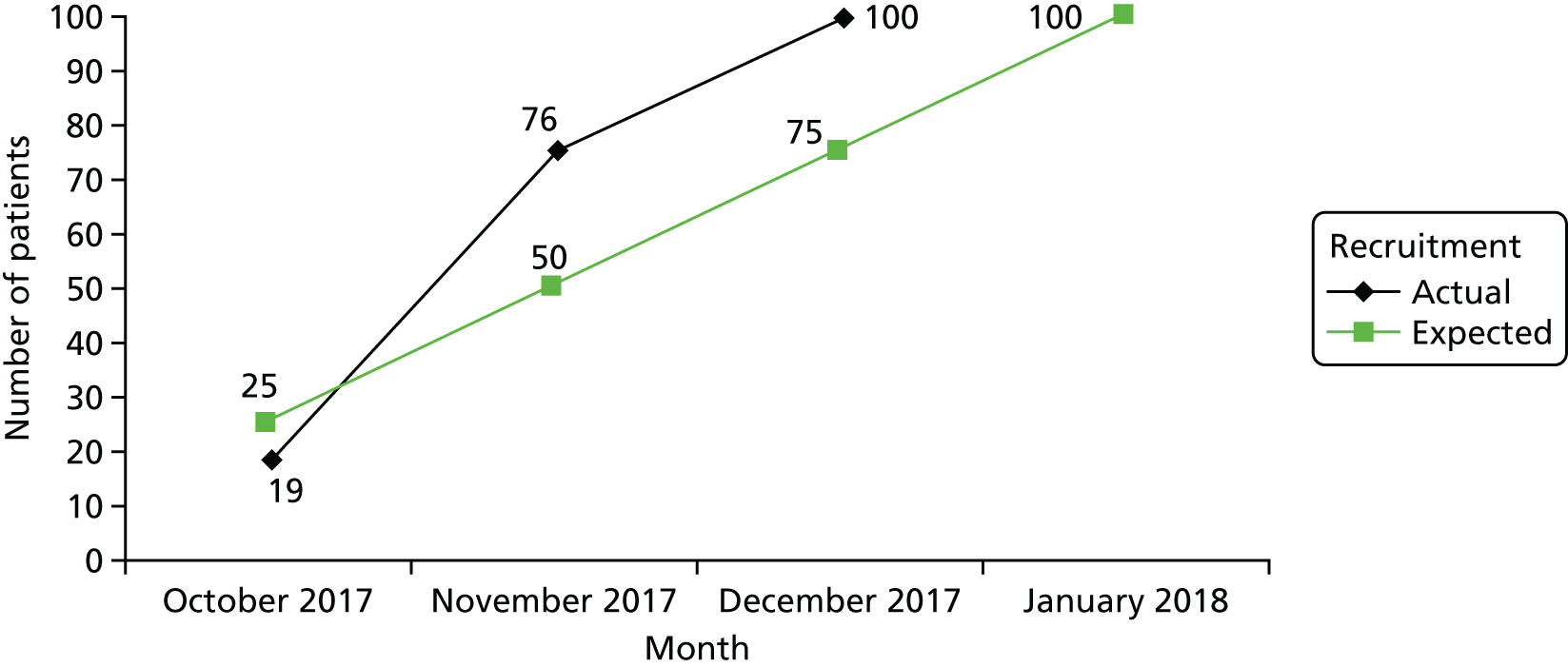
FIGURE 14.
Overall recruitment rate at each site. GOSH, Great Ormond Street Hospital.

| Month | Patients, n (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Total | ||||||||||||||
| Alder Hey Children’s Hospital (open for 2.58 months) | Great North Children’s Hospital (open for 2.23 months) | Great Ormond Street Hospital (open for 2.82 months) | Evelina London Children’s Hospital (open for 1.38 months) | ||||||||||||
| Screened | Eligiblea | Recruitedb | Screened | Eligiblea | Recruitedb | Screened | Eligiblea | Recruitedb | Screened | Eligiblea | Recruitedb | Screened | Eligiblea | Recruitedb | |
| October 2017c | 11 | 7 (63.6) | 4 (57.1) | 4 | 3 (75.0) | 2 (66.7) | 16 | 16 (100) | 13 (81.3) | N/A | N/A | N/A | 31 | 26 (83.9) | 19 (73.1) |
| November 2017 | 17 | 15 (88.2) | 7 (46.7) | 17 | 17 (100) | 16 (94.1) | 17 | 17 (100) | 16 (94.1) | 27 | 25 (92.6) | 17 (68.0) | 78 | 74 (94.9) | 56 (75.7) |
| December 2017 | 10 | 6 (60.0) | 4 (66.7) | 7 | 7 (100) | 7 (100) | 12 | 11 (91.7) | 7 (63.6) | 14 | 14 (100) | 7 (50.0) | 53 | 38 (71.7) | 25 (65.8) |
| Total | 38 | 28 (73.7) | 15 (53.6) | 28 | 27 (96.4) | 25 (92.6) | 46 | 44 (97.8) | 36 (81.8) | 41 | 39 (95.1) | 24 (61.5) | 162 | 138 (85.2) | 100 (72.5) |
Consent
Consent to continue in the study was refused in 8 out of 100 patients (8.0%, 95% CI 4.1% to 15.0%). Of these, seven had been allocated to the permissive treatment group (accounting for 14.3% of patients in this group, 95% CI 7.1% to 26.7%). In addition, seven parents/legal representatives (five in the permissive group and two in the restrictive group) refused consent to be treated in accordance with the study protocol but consented for ongoing data collection, and a further three patients treated in the permissive group had consent withdrawn so that an antipyretic intervention could be given.
In one hospital, 7 out of the 10 patients randomised to the permissive group either refused or withdrew consent to the intervention, which accounted for almost half (46.7%) of the total number across all four sites. The consent procedure was also frequently carried out earlier than stated in the protocol (24–48 hours post randomisation), with the consent procedure being completed on the same day as randomisation for 29 out of the 91 patients (31.9%) to have a face-to-face approach. Eight out of 29 patients (27.6%) refused or withdrew consent if the consent procedure was completed on the day of randomisation, whereas 10 out of 62 (16.1%) refused or withdrew consent on the subsequent days after randomisation.
In addition, five patients were withdrawn from the study centrally as the parents/legal representatives were unable to be approached for consent. Therefore, 87 out of the 100 correctly randomised patients were included in the analysis of objectives 4–6 (87.0%, 95% CI 79.9% to 92.2%).
Baseline patient characteristics
Overall, the two treatment groups appear well matched at baseline (Table 11), with only a small difference between the groups in terms of mean age and a balance across groups in the proportion of participants aged < 1 year. Over half of the participants were male (63.2% in the permissive group and 55.1% in the restrictive group) and bronchiolitis was the primary diagnosis (50% in the permissive group and 49.0% in the restrictive group). Illness severity was balanced across the two groups in terms of PIM2r and PIM3 scores, with approximately 90% of participants invasively ventilated (92.1% in the permissive group and 89.8% in the restrictive group). Baseline temperature was the same in both groups; however, a greater proportion of participants in the restrictive temperature group received pre-randomisation paracetamol (Table 12).
| Variables | Treatment group, n (%) | Total, n (%) (N = 87) | |
|---|---|---|---|
| Permissive (N = 38) | Restrictive (N = 49) | ||
| Age (years) | |||
| Mean (SD) | 1.8 (3.4) | 1.1 (2.1) | 1.4 (2.7) |
| Median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–2) |
| Age group (years), n (%) | |||
| < 1 | 24 (63.2) | 32 (65.3) | 56 (64.4) |
| 1 | 3 (7.9) | 4 (8.2) | 7 (8.0) |
| 2–4 | 5 (13.2) | 9 (18.4) | 14 (16.1) |
| 5–9 | 3 (7.9) | 3 (6.1) | 6 (6.9) |
| 10–15 | 3 (7.9) | 1 (2.0) | 4 (4.6) |
| Sex, n (%) | |||
| Female | 14 (36.8) | 22 (44.9) | 36 (41.4) |
| Male | 24 (63.2) | 27 (55.1) | 51 (58.6) |
| PIM2r scorea | |||
| Mean (SD) | 0.025 (0.030) | 0.025 (0.030) | 0.025 (0.029) |
| Median (IQR) | 0.012 (0.008–0.035) | 0.012 (0.008–0.037) | 0.012 (0.008–0.036) |
| PIM3 score | |||
| Mean (SD) | 0.023 (0.033) | 0.025 (0.036) | 0.024 (0.034) |
| Median (IQR) | 0.007 (0.005–0.033) | 0.007 (0.005–0.038) | 0.007 (0.005–0.037) |
| Source of admission, n (%) | |||
| Same hospital | 6 (15.8) | 13 (26.5) | 19 (21.8) |
| Other hospital | 32 (84.2) | 36 (73.5) | 68 (78.2) |
| Clinic or home | 0 (0) | 0 (0) | 0 (0) |
| Primary diagnosis, n (%) | |||
| Bronchiolitis | 19 (50.0) | 24 (49.0) | 43 (49.4) |
| Pneumonia/LRTI | 9 (23.7) | 8 (16.3) | 17 (19.5) |
| Acute respiratory failure | 4 (10.5) | 7 (14.3) | 11 (12.6) |
| Sepsis/septic shock | 2 (5.3) | 6 (12.2) | 8 (9.2) |
| Asthma | 2 (5.3) | 1 (2.0) | 3 (3.4) |
| Seizures/convulsions | 1 (2.6) | 2 (4.1) | 3 (3.4) |
| Other | 1 (2.6) | 1 (2.0) | 2 (2.3) |
| Temperature prior to randomisation (°C) | |||
| Mean (SD) | 38.1 (0.6) | 38.1 (0.7) | 38.1 (0.6) |
| Median (IQR) | 38.0 (37.7–38.6) | 37.9 (37.6–38.3) | 37.9 (37.7–38.5) |
| Type of ventilation | |||
| High-flow humidified oxygen | 0 (0) | 2 (4.1) | 2 (2.3) |
| Invasive | 35 (92.1) | 44 (89.8) | 79 (90.8) |
| Non-invasive | 3 (7.9) | 3 (6.1) | 6 (6.9) |
| Variables | Treatment group, n (%) | Total, n (%) (N = 87) | |
|---|---|---|---|
| Permissive (N = 38) | Restrictive (N = 49) | ||
| Any antipyretic intervention | 5 (13.2) | 11 (22.4) | 16 (18.4) |
| Paracetamol | 3 (7.9) | 9 (18.4) | 12 (13.8) |
| NSAID | 0 (0) | 1 (2.0) | 1 (1.1) |
| External and other cooling | 3 (7.9) | 3 (6.1) | 6 (6.9) |
Temperatures
Highest temperatures
Figure 15 shows the distribution of the highest temperatures for participants over the first 6 days of the intervention based on individual patient measurements presented as both box plots and medians and IQRs. On average, participants in the restrictive group had reached a temperature of 37.5 °C by 24 hours and participants in the permissive group had a temperature of > 37.5 °C until 42 hours. The permissive group spent a greater proportion of time below the allocated treatment threshold over both the first 48 hours and the first 6 days of the intervention (Figure 16). Overall, the proportion of time spent below the allocated temperature target was a median of 1.0 hours (IQR 0.9–1.0 hours) in the permissive group compared with a median of 0.7 hours (IQR 0.5–0.8 hours) in the restrictive group.
FIGURE 15.
Highest temperature, by 6-hour time period and treatment group. (a) Box plot; and (b) median and IQR.

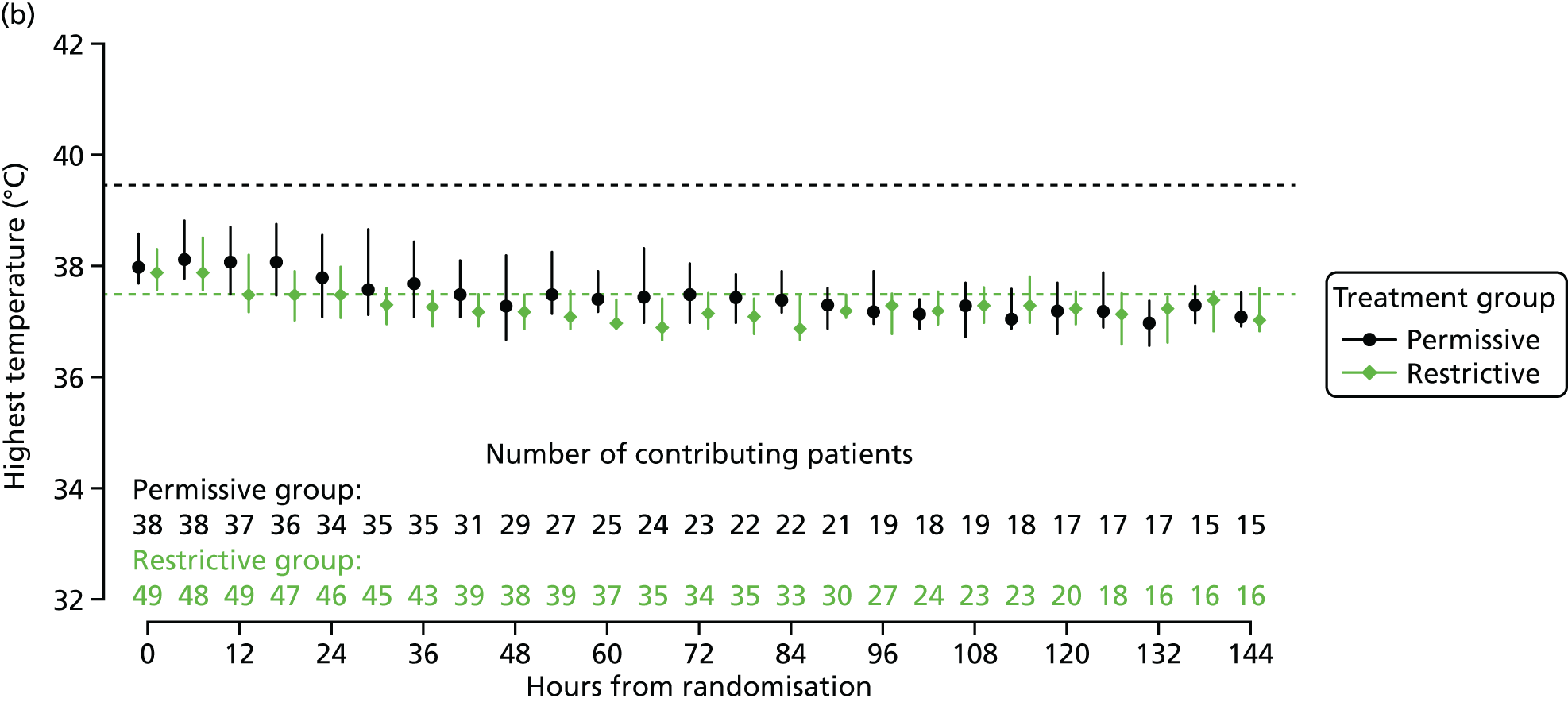
FIGURE 16.
Distribution of time spent at each temperature, by treatment group. (a) During the first 48 hours; and (b) during the first 6 days.


Separation between the groups
Over the first 48 hours, on average, participants in the permissive group had a mean peak temperature of 38.8 °C (95% CI 38.6 to 39.1 °C) compared with 38.4 °C (95% CI 38.2 to 38.6 °C) in the restrictive group. This resulted in a separation in mean peak temperature over the first 48 hours of 0.5 °C (95% CI 0.2 to 0.8 °C) between the groups. The separation of around 0.5 °C between treatment groups was maintained up to around 84 hours following randomisation (Figure 17).
FIGURE 17.
Difference between groups in the mean of the highest temperature at each time point with 95% CI.
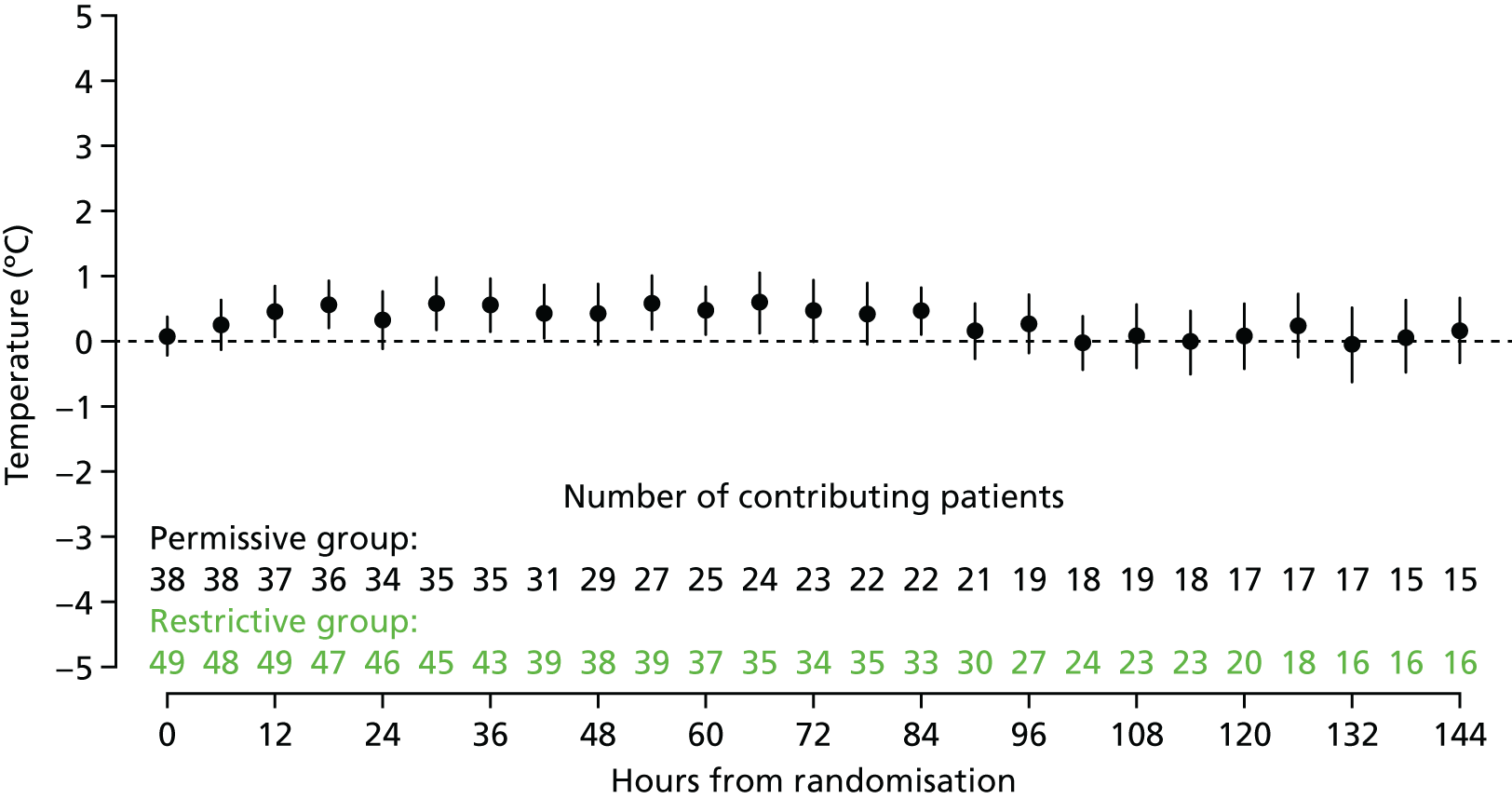
Interventions
Receipt of antipyretic interventions
The proportion of participants receiving antipyretic interventions while mechanically ventilated over the first 6 days of the intervention was greater in the restrictive group (Figure 18) than in the permissive group. This was consistent across both paracetamol and external/other cooling (Table 13).
FIGURE 18.
Proportion of participants who received any antipyretic intervention while mechanically ventilated.

| Hoursa | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| Permissive | Restrictive | |||||||
| n b | Antipyretic intervention (%) | n b | Antipyretic intervention (%) | |||||
| Paracetamol | NSAID | External and other | Paracetamol | NSAID | External and other | |||
| Baseline | 38 | 7.9 | 0.0 | 7.9 | 49 | 18.4 | 2.0 | 6.1 |
| 0–6 | 38 | 13.2 | 0.0 | 13.2 | 49 | 51.0 | 0.0 | 24.5 |
| 6–12 | 37 | 10.8 | 2.7 | 21.6 | 49 | 34.7 | 0.0 | 22.4 |
| 12–18 | 36 | 8.3 | 0.0 | 5.6 | 47 | 29.8 | 0.0 | 23.4 |
| 18–24 | 35 | 11.4 | 0.0 | 2.9 | 46 | 34.8 | 0.0 | 21.7 |
| 24–30 | 35 | 8.6 | 2.9 | 5.7 | 45 | 35.6 | 0.0 | 13.3 |
| 30–36 | 35 | 11.4 | 2.9 | 2.9 | 43 | 18.6 | 0.0 | 11.6 |
| 36–42 | 32 | 9.4 | 3.1 | 6.3 | 40 | 20.0 | 2.5 | 2.5 |
| 42–48 | 29 | 13.8 | 0.0 | 3.4 | 39 | 15.4 | 2.6 | 15.4 |
| 48–54 | 27 | 14.8 | 0.0 | 7.4 | 38 | 15.8 | 0.0 | 21.1 |
| 54–60 | 25 | 8.0 | 0.0 | 0.0 | 37 | 10.8 | 0.0 | 2.7 |
| 60–66 | 24 | 16.7 | 0.0 | 0.0 | 36 | 13.9 | 0.0 | 8.3 |
| 66–72 | 23 | 8.7 | 0.0 | 13.0 | 34 | 29.4 | 2.9 | 11.8 |
| 72–78 | 23 | 4.3 | 0.0 | 4.3 | 36 | 19.4 | 0.0 | 11.1 |
| 78–84 | 22 | 9.1 | 0.0 | 0.0 | 34 | 20.6 | 0.0 | 8.8 |
| 84–90 | 21 | 9.5 | 0.0 | 4.8 | 32 | 21.9 | 0.0 | 9.4 |
| 90–96 | 19 | 15.8 | 0.0 | 5.3 | 28 | 25.0 | 0.0 | 14.3 |
| 96–102 | 18 | 11.1 | 0.0 | 11.1 | 26 | 23.1 | 0.0 | 19.2 |
| 102–108 | 19 | 15.8 | 0.0 | 5.3 | 24 | 29.2 | 0.0 | 12.5 |
| 108–114 | 18 | 0.0 | 0.0 | 0.0 | 23 | 30.4 | 0.0 | 8.7 |
| 114–120 | 17 | 17.6 | 0.0 | 0.0 | 20 | 25.0 | 0.0 | 15.0 |
| 120–126 | 17 | 11.8 | 0.0 | 0.0 | 18 | 27.8 | 0.0 | 11.1 |
| 126–132 | 17 | 17.6 | 0.0 | 0.0 | 16 | 6.2 | 0.0 | 12.5 |
| 132–138 | 15 | 20.0 | 0.0 | 0.0 | 16 | 31.2 | 0.0 | 18.8 |
| 138–144 | 15 | 13.3 | 0.0 | 0.0 | 16 | 12.5 | 0.0 | 12.5 |
Use of opiates
As shown in Figure 19 and Table 14, the vast majority of participants received opiates and the proportions were similar across groups.
FIGURE 19.
Proportion of participants receiving opiates while mechanically ventilated.

| Hoursa | Treatment group | |||||
|---|---|---|---|---|---|---|
| Permissive | Restrictive | |||||
| n b | Opiates (%) | n b | Opiates (%) | |||
| Intravenous opiate infusion | Opiate bolus | Intravenous opiate infusion | Opiate bolus | |||
| 0–6 | 38 | 84.2 | 28.9 | 49 | 85.7 | 28.6 |
| 6–12 | 37 | 83.8 | 18.9 | 49 | 81.6 | 26.5 |
| 12–18 | 36 | 75.0 | 19.4 | 47 | 80.9 | 21.3 |
| 18–24 | 35 | 77.1 | 22.9 | 46 | 82.6 | 10.9 |
| 24–30 | 35 | 74.3 | 8.6 | 45 | 84.4 | 17.8 |
| 30–36 | 35 | 65.7 | 17.1 | 43 | 83.7 | 7.0 |
| 36–42 | 32 | 65.6 | 12.5 | 40 | 80.0 | 12.5 |
| 42–48 | 29 | 69.0 | 13.8 | 39 | 79.5 | 15.4 |
| 48–54 | 27 | 66.7 | 11.1 | 38 | 78.9 | 7.9 |
| 54–60 | 25 | 68.0 | 16.0 | 37 | 73.0 | 10.8 |
| 60–66 | 24 | 66.7 | 16.7 | 36 | 69.4 | 13.9 |
| 66–72 | 23 | 69.6 | 13.0 | 34 | 70.6 | 8.8 |
| 72–78 | 23 | 69.6 | 17.4 | 36 | 63.9 | 13.9 |
| 78–84 | 22 | 68.2 | 13.6 | 34 | 61.8 | 8.8 |
| 84–90 | 21 | 66.7 | 28.6 | 32 | 59.4 | 15.6 |
| 90–96 | 19 | 63.2 | 10.5 | 28 | 64.3 | 10.7 |
| 96–102 | 18 | 66.7 | 5.6 | 26 | 50.0 | 0.0 |
| 102–108 | 19 | 68.4 | 15.8 | 24 | 54.2 | 8.3 |
| 108–114 | 18 | 72.2 | 16.7 | 23 | 56.5 | 13.0 |
| 114–120 | 17 | 58.8 | 17.6 | 20 | 65.0 | 20.0 |
| 120–126 | 17 | 52.9 | 17.6 | 18 | 66.7 | 0.0 |
| 126–132 | 17 | 52.9 | 29.4 | 16 | 62.5 | 6.2 |
| 132–138 | 15 | 46.7 | 13.3 | 16 | 62.5 | 6.2 |
| 138–144 | 15 | 40.0 | 6.7 | 16 | 50.0 | 6.2 |
Protocol adherence
Protocol deviations were reported in 39 out of 628 6-hour time periods (6.2%) in the permissive group and 60 out of 810 time periods (7.4%) in the restrictive temperature group. Overall, 39% of participants in the permissive group and 55% of participants in the restrictive group experienced at least one protocol deviation (Table 15). The number of participants who received antipyretic interventions when below the allocated temperature threshold was higher in the permissive group (14, 36.8%) than in the restrictive group (11, 22.4%). In a total of 37 out of 589 6-hour periods (6.3%), these deviations showed that participants in the permissive group were receiving mechanical ventilation and had a documented temperature measurement below the threshold, compared with 20 out of 504 6-hour periods in the restrictive group (4.0%). In contrast, fewer participants did not receive the recommended antipyretic interventions above the allocated temperature threshold in the permissive group (2, 5.3%) than in the restrictive group (20, 40.8%). In 2 out of 39 6-hour periods (5.1%), these deviations showed that participants in the permissive group were receiving mechanical ventilation and had a documented temperature measurement above the threshold, compared with 40 out of 306 6-hour periods in the restrictive group (13.1%).
| Type of deviation | Treatment group | |
|---|---|---|
| Permissive (N = 38) | Restrictive (N = 49) | |
| Receiving antipyretic when temperature below threshold | ||
| n/N (%) of 6-hour periods | 37/589 (6.3) | 20/504 (4.0) |
| n (%) of participants | 14 (36.8) | 11 (22.4) |
| Not receiving antipyretic when temperature above threshold | ||
| n/N (%) of 6-hour periods | 2/39 (5.1) | 40/306 (13.1) |
| n (%) of participants | 2 (5.3) | 20 (40.8) |
| Any protocol deviation | ||
| n/N (%) of 6-hour periods | 39/628 (6.2) | 60/810 (7.4) |
| n (%) of participants | 15 (39.5) | 27 (55.1) |
For the permissive group, reasons for receiving antipyretic interventions during the 6-hour period when the temperature was below the threshold included paracetamol for pain or discomfort when receiving non-invasive or high-flow oxygen or being weaned from invasive ventilation (16 occurrences); external or other cooling given in error (10 occurrences); lack of trial communication (five occurrences); other staff errors (four occurrences); and withdrawal of consent for the intervention (one occurrence). No clear explanation from the site was provided for the remaining deviation. When restricting the analyses to protocol deviations solely while the participants were receiving invasive ventilation, the proportion of 6-hour time periods when antipyretic interventions were received while the temperature was below the threshold in the permissive group reduces from 6.3% to 3.1% (15 occurrences out of 479 6-hour time periods). This is lower than the corresponding proportion (3.5%, 15 occurrences out of 424 6-hour time periods) in the restrictive group.
For the restrictive group, the circumstances when antipyretic interventions were not received during a 6-hour period with a maximum temperature at or above the threshold were paracetamol having been given in the previous or subsequent time period (19 instances), paracetamol having been given in both the previous and subsequent time periods (six instances), a single-period spike in temperature (six instances), the temperature dropped below the threshold by the subsequent time period (five instances), the participant was no longer mechanically ventilated by the subsequent time period (one instance) and external/other cooling was given in the subsequent time period (one instance). No clear explanation from the site was provided for the remaining deviations. Only two participants had two consecutive 6-hour time periods in which antipyretic interventions were not received when the temperature was at or above the threshold. Of these, one participant received paracetamol in the subsequent time period and the other participant’s temperature subsequently dropped below the threshold.
Potential outcome measures
For participants included in the analyses, the completeness of outcome data was high, with one participant excluded from all mortality outcomes owing to withdrawal of consent and four participants excluded from hospital mortality owing to withdrawal of consent (n = 1) or because they remained in hospital at the time of the data lock (n = 3) (Table 16). As anticipated for a small pilot trial, there were no significant differences between the groups in any of the outcomes (Table 17). Four participants remained in hospital. Histograms plotted for continuous measures are presented in Appendix 3.
| Outcome | Treatment group, n (%) | |
|---|---|---|
| Permissive (N = 38) | Restrictive (N = 49) | |
| PICU mortality | 37 (97.4) | 49 (100.0) |
| Hospital mortality | 36 (94.7) | 46 (93.9) |
| 30-day mortality | 37 (97.4) | 49 (100.0) |
| Length of stay in a PICU | 37 (97.4) | 49 (100.0) |
| Receipt and duration of organ support | 38 (100.0) | 49 (100.0) |
| Number of days alive and free from: | ||
| PICU | 37 (97.0) | 49 (100.0) |
| Mechanical ventilation | 38 (100.0) | 49 (100.0) |
| Potential outcome measures | Treatment group | Effect estimate (95% CI) | |
|---|---|---|---|
| Permissive (N = 38) | Restrictive (N = 49) | ||
| PICU mortality, n/N (%) | 1/37 (2.7) | 1/49 (2.0) | RR 1.3 (0.1 to 20.5); ARR −0.7 (−7.2 to 5.9) |
| Hospital mortality, n/N (%) | 1/36 (2.8) | 2/46 (4.3) | RR 0.6 (0.1 to 6.8); ARR 1.6 (−6.4 to 9.5) |
| 30-day mortality, n/N (%) | 2/37 (5.4) | 1/49 (2.0) | RR 2.6 (0.2 to 28.1); ARR −3.4 (−11.7 to 4.9) |
| Length of stay in PICU (days) | |||
| All participants | N = 37 | N = 49 | |
| Mean (SD) | 7.3 (4.3) | 8.1 (8.6) | |
| Median (IQR) | 6 (4–10) | 6 (4–8) | |
| Survivors to PICU discharge | N = 36 | N = 48 | |
| Mean (SD) | 7.2 (4.3) | 7.9 (8.5) | |
| Median (IQR) | 6 (4–9) | 6 (4–8) | |
| Receipt and duration of organ supporta | |||
| Mechanical ventilation, n (%) | 38 (100) | 49 (100) | |
| Median (IQR) | 5 (3–8) | 5 (3–7) | |
| Mean (SD) | 6.5 (4.6) | 6.2 (5.5) | Mean difference 0.3 (−1.8 to 2.5) |
| Cardiovascular support, n (%) | 8 (21.1) | 13 (26.5) | |
| Median (IQR) | 2 (2–3) | 3 (2–6) | |
| Mean (SD) | 0.5 (1.1) | 1.2 (2.9) | Mean difference −0.7 (−1.6 to 0.2) |
| Renal support, n (%) | 1 (2.6) | 3 (6.1) | |
| Mean (SD) | 0.4 (2.4) | 0.3 (1.6) | Mean difference 0.1 (−0.8 to 1.0) |
| Median (IQR) | N < 5 | N < 5 | |
| Number of days alive and free (to 28 days) from: | |||
| PICU, mean (SD) [n] | 19.8 (6.4) [37] | 20.4 (6.2) [49] | Mean difference −0.7 (−3.4 to 2.1) |
| Mechanical ventilation, mean (SD) [n] | 20.5 (6.7) [38] | 21.6 (6.1) [49] | Mean difference −1.1 (−3.8 to 1.7) |
Safety monitoring
There were no reported SAEs. In total, three AEs were reported. One seizure was reported in the permissive group (an expected AE) that was deemed unlikely to be related to the trial intervention. There were two AEs in the restrictive group: one seizure that was deemed unrelated to the trial intervention and one case of rhabdomyolysis (an expected AE) that was deemed possibly related to the trial intervention (Table 18).
| Treatment group | Event | Severity | Relatedness |
|---|---|---|---|
| Permissive | Seizures | Mild | Unlikely |
| Restrictive | Seizures | Mild | None |
| Restrictive | Rhabdomyolysis | Mild | Possibly |
Additional analyses
As specified in the methods, because the FEVER observational study suggested that it may be beneficial to narrow the inclusion criteria to include invasively ventilated patients only, rather than all those with mechanical ventilation, the FEVER pilot RCT analysis was re-run on this group of patients only (see Appendix 4).
Chapter 6 Results of the integrated-perspectives study
Participants: parents/legal representatives
A total of 60 parents of 57 out of 101 FEVER pilot RCT participants (56%) took part in the integrated-perspective study (Figure 20). Of these, 41 out of 60 (68%) completed a questionnaire, 12 out of 60 (20%) took part in an interview and 7 out of 60 (12%) took part in both. No participants identified themselves as legal representatives. A total of 66 parents consented to be contacted for an interview; 44 out of 66 (67%) were invited by telephone or e-mail. Of these, 19 out of 44 (43%) were interviewed, 15 out of 44 (34%) did not respond, 2 were deemed ineligible owing to an inability to understand English and 8 out of 44 (18%) declined at the point of interview. A total of four parents (4/66, 6%) (all parents of children in the permissive threshold group) who declined some part of the FEVER pilot RCT consented to be approached for interview: two were interviewed, one did not respond to three separate contacts and one declined at the point of interview.
FIGURE 20.
Parent recruitment to the integrated-perspectives study. (a) Parent questionnaire recruitment; and (b) parent interview recruitment. N/A, not applicable.

Data saturation was reached after eight interviews with parents of children who consented to the restrictive temperature threshold and after 11 interviews with parents of children allocated to the permissive temperature threshold. Therefore, 19 out of 66 parents (29%) who consented to interview were not contacted owing to data saturation. One parent was not contacted because of insufficient contact details, one was not contacted because there was too long between the timing of consent and receiving their information and one was not contacted owing to bereavement.
Characteristics
Of the 60 parents (49 mothers and 11 fathers) who took part, eight parents (13%) of seven children reported that they had not provided consent for their child’s continued involvement in the FEVER pilot RCT. One parent interviewed was bereaved at the point of interview. Parents were recruited for interviews and questionnaires from all four sites. Seven of the parents interviewed (7/19, 37%) said that they had previous experience of participating in medical research. No parents reported prior experience of RWPC. Parents were interviewed a median of 31 days after randomisation (range 9–70 days). Their children had received treatment for respiratory illness (e.g. bronchiolitis and respiratory syncytial virus) (18/19, 94%), cancer (1/19, 5%) and septic shock (1/19, 5%). Interviews took between 20 and 50 minutes (mean 32 minutes).
Participants: site staff
The sample included 98 staff at the four FEVER pilot RCT sites. Half (48/98, 50%) completed an online survey and half attended a focus group, which included the administration of survey questions using TurningPoint software voting handsets.
Characteristics
Of the 97 staff who provided information about their role, 75 out of 97 (77%) were nurses, 45 out of 97 (60%) were senior-level staff. Fourteen out of 97 (14%) were doctors, 12 (86%) of whom were consultant-level doctors. The majority of staff (79/98, 81%) were involved in the clinical care of children (44/48, 92% of online questionnaire participants; and 35/48, 73% of focus group participants). Focus groups took an average of 52.5 minutes (range 22.52–106.00 minutes).
Parent perspectives
Research without prior consent is acceptable in the proposed FEVER randomised controlled trial
Questionnaires and interviews to explore parents’ views on RWPC were used in the FEVER pilot RCT and proposed FEVER RCT. As shown in Table 19, 17 parents (35%) who completed a questionnaire indicated that they were initially surprised to find out that their child had already been entered into the FEVER pilot RCT. However, the majority (89%) were satisfied with the RWPC process for this study.
| Statement | Responses, n (%) | ||
|---|---|---|---|
| Agree | Neither agree nor disagree | Disagree | |
| 1. I was initially surprised to find out that my child had already been entered into FEVER | 17 (35.4) | 20 (41.7) | 11 (22.0) |
| 2. I was satisfied with the (RWPC) deferred consent process for FEVER | 42 (89.4) | 1 (2.1) | 4 (8.5)a |
Interview data supported questionnaire findings. Parents described their initial surprise at finding out that their child was involved in a trial without their prior consent. However, many were supportive of RWPC for this trial, as well as other PICU trials, as they understood the reasons why informed consent could not be sought in a critical care situation:
I think it’s not ideal to find out they are in a study, you know, without your prior consent, but I didn’t have any issues with that particular study and I understand the reasons why they’re doing it, and I think it’s a, you know . . . a good reason and for a good, good cause.
P79, interview, mother, permissive
I don’t think there’s any other way better to go about it.
P79, interview, mother, restrictive
Parents described how RWPC was acceptable as the FEVER pilot RCT discussion took place when the situation had calmed down and the emergency situation had passed. Some emphasised the importance of clinicians broaching the trial discussion at the first appropriate opportunity:
No, I think it was better the way they did it because I had calmed down, he was in intensive care, everything was settled down and then they spoke with . . . Yeah, I do actually think it’s better that way around.
P49, interview, mother, permissive
As long as it’s sort of discussed at the first suitable opportunity, erm, then that’s fine . . . Yeah, as soon as it’s appropriate to.
P82, interview, mother, restrictive
Nevertheless, there were some exceptions. Four (4/48, 9%) questionnaire and two interview participants indicated that they were not satisfied with RWPC in the FEVER pilot RCT. Only one out of the eight parents who declined consent and took part in an interview (n = 2) or questionnaire (n = 6) indicated that they were unhappy regarding RWPC; however, the use of RWPC was not the reason they had declined consent.
Interestingly, four parents did not believe that their child had been entered into the trial without their prior consent as they believed that they had provided informed consent prior to randomisation:
Because at that point, um, she hadn’t been assigned to it [a threshold] and it was randomly. I mean ‘cause we made that decision at the time, she may have been in the 39 [°C group].
P53, interview, father, restrictive
They come to me and asked us first . . .
Did they?
. . . before putting him in the trial. Yes, they did, sorry.
Yeah.
They didn’t just put him in. They, they come and asked me first if they could do the trial.
This finding suggests that in a few cases either the consent process was not followed correctly, randomisation to a temperature threshold had not been explained clearly at the point of broaching the trial or the parent did not have the capacity at the time to understand the RWPC process. This finding was subsequently discussed in three staff focus groups. All staff confirmed that RWPC had been used for all randomised participants without exception.
One of the mothers who believed they (the parents) had consented before randomisation explained that although they would have been unhappy that their child had been entered into the FEVER pilot RCT without their consent, they would still have provided consent for the use of their child’s information and to remain in the FEVER pilot RCT as they wished to help others:
I wouldn’t have been ha-, like happy at first that the would go ahead and do it without asking permission but then I would still say, well you can still, you can still do. It . . . it was just one them things if this could help then obviously I am gonna do it, but it’s just not knowing what they were doing that I wouldn’t have been at first.
P85, interview, mother, permissive
Another mother described how her dissatisfaction with RWPC was exacerbated in the FEVER pilot RCT as she had noticed a sticker on her child’s bed indicating trial participation before staff had broached the FEVER pilot RCT:
I walked onto the ward that morning and I saw the, um, little sticker on the side of his bed that said that, the higher temperature and it said FEVER RCT and I had no idea what it meant. I think he came round later and said that, er, and I was a bit shocked that they were asking me for consent for him to be on the trial, even though he was already on the trial.
P84, questionnaire, mother, permissive, declined consent
Others appeared to be unhappy with any alternative to informed consent for research, rather than specific concerns about RWPC in this pilot RCT:
Well I personally don’t agree with, um, somebody else deciding that for me . . . before they come and speak to me about it.
P84, interview, mother, permissive, declined consent
Parents’ experiences of the FEVER pilot randomised controlled trial consent process
The majority of parents, including 96% of those who completed the questionnaire, indicated that site staff had checked that it was a convenient time to discuss the FEVER pilot RCT (Table 20). Most parents (46/48, 96%) also indicated that the information they received about the FEVER pilot RCT was clear and straightforward to understand. This was corroborated during interviews with parents describing how staff had approached them appropriately, with well-timed, clear, comprehensive study information:
It was all, all fine and I understood what they were saying and was happy to sort of go ahead with it, with the trial. If it, if I was, if it wasn’t explained to me too well, I probably wouldn’t have bothered doing it, so I don’t think there was any problems with it being explained to me.
P77, interview, father, restrictive
I feel like it was well-explained to me.
P79, interview, mother, permissive
I didn’t think it [trial information] was missing anything.
P78, interview, mother, restrictive
| Statement | Responses, n (%) | ||
|---|---|---|---|
| Agree | Neither agree nor disagree | Disagree | |
| 1. The practitioner checked that it was a convenient time to discuss research before discussing FEVER | 46 (95.8) | 2 (4.2) | 0 (0.0) |
| 2. The information I received about FEVER was clear and straightforward to understand | 46 (95.8) | 2 (4.2) | 0 (0.0) |
| 3. I had enough opportunity to ask questions about FEVER | 45 (93.8) | 2 (4.2) | 1 (2.1) |
| 4. It was difficult to take in the information I was given about FEVER | 7 (14.6) | 6 (12.5) | 35 (72.9) |
| 5. It was difficult to make a decision about FEVER | 8 (17.0) | 8 (17.0) | 31 (66.0) |
| 6. I made this decision | 41 (89.4) | 2 (4.3) | 3 (6.4) |
| 7. Someone took this decision away from me | 5 (10.4) | 3 (6.3) | 40 (83.3) |
| 8. I was not in control of this decision | 4 (8.3) | 5 (10.4) | 39 (81.3) |
| 9. The decision about the research was inappropriately influenced by others | 4 (8.5) | 2 (4.3) | 41 (87.2) |
Patient information materials were also described as being ‘clear and understandable’ (P80, interview, mother, permissive), ‘jargon free’ (P30, interview, mother, restrictive) and the ‘perfect’ length (P85, interview, mother, permissive):
I mean we were given so much information on various other things and that’s one thing I’ll always say, you know, everything was clear and in plain English. Um, yeah, it was easy to follow all the literature.
P79, interview, father, permissive
One mother suggested that in the proposed FEVER RCT participant information sheet it would be useful to include information about trial outcome measures to help fully understand what the trial aims are:
The outcome measures would be good so people could understand, they understand the reasons why and then understand what it is that is actually being looked at as part of the reasons.
P80, interview, mother, permissive
Parents’ views on trial acceptability
Following discussion of the consent processes, the focus of the interviews shifted to parents’ views on the acceptability of the proposed FEVER RCT, including the temperature thresholds.
All parents interviewed, including those who declined their child’s continued participation, supported the proposed trial:
I think it’s a brilliant idea, so I’m all, I’m all for it.
P80, interview mother, permissive
It sounded quite interesting.
P84, interview, mother, permissive, declined consent
This was underpinned by the perception that ‘it was an interesting trial’ (P30, interview, mother, restrictive) that had been ‘clearly explained’ (P74, interview, mother, permissive), and involved a non-invasive treatment (paracetamol), which parents were familiar with:
It’s just how are they gonna give the paracetamol, when they’re gonna give it. I mean if it was more severe, um, more of an invasive study, um, I might have been a bit, I might have had to query it a little bit more but I was happy with, with everything.
P53, interview, father, restrictive
Many also considered the proposed FEVER RCT to be acceptable and had consented to the FEVER pilot RCT as they did not perceive it as causing harm to their child:
No, I just thought, you know, what’s, what’s the harm? There’s no harm in taking like, letting her into the study.
P78, interview, mother, restrictive
Parents of children allocated to the restrictive temperature threshold found the trial very acceptable as they viewed giving paracetamol at this temperature as being their normal practice at home:
I think it probably helped, the fact that it’s something that I would do myself anyway, so I felt comfortable that they were giving him paracetamol at 37 degrees [°C], which I would do anyway. I think that was the main thing. It wasn’t something that was out of my comfort.
P82, interview, father, restrictive
Parents also viewed the permissive threshold to be acceptable; however, this acceptability was contingent on the perception that their child was not in any discomfort. Interestingly, no parents referred to the temperature their child reached when discussing acceptability:
The only thing would be if she wasn’t on any other kind of pain relief, um, but there’s other things to manage, her discomfort.
P73, interview, father, permissive
Why parents consented to the FEVER pilot randomised controlled trial
The questionnaire included a series of statements to establish why parents had consented for their child’s information to be used and/or provided consent to continue involvement in the FEVER pilot RCT (Table 21). Parents often indicated multiple reasons for providing consent (mode four reasons, mean two reasons). In line with qualitative findings, the main reason why parents provided consent was to help other children in the future:
At the end of the day it will benefit other children in the future so, I wanted to obviously help [. . .] if we can find the information to help other children in the future, that would be good.
P35, interview, mother, restrictive
| Reason for consent | Responses, n (%) | |
|---|---|---|
| Identified as a reason (N = 41) | Identified as the main reason (N = 15) | |
| 1. To help my child | 30 (73.2) | 2 (13.3) |
| 2. To help other children in the future | 32 (78.0) | 8 (53.3) |
| 3. I felt that medical studies like FEVER are important | 32 (78.0) | 2 (13.3) |
| 4. Because I trusted the doctor or nurse who explained FEVER | 25 (61.0) | 0 (0.0) |
| 5. The treatment had already started | 13 (31.7) | 0 (0.0) |
| 6. My child recovered | 5 (12.5) | 1 (6.7) |
| 7. I did not feel comfortable saying no to the nurse or doctor who explained FEVER | 2 (4.9) | 1 (6.7) |
| 8. Other | 5 (12.2) | 1 (6.7) |
A high proportion of parents (32/41, 78%) indicated that they provided consent to help other children in the future and because of a belief that medical research studies like FEVER are important (32/41, 78%). After this desire to help other children and future research, parents were motivated by the belief that trial participation could help their own child (30/41, 73%). ‘Other’ reasons for consent included two parents who felt that their child had benefited from additional monitoring owing to trial participation and two parents who referred to how their child was comfortable or recovering well. Another parent described wanting to ‘give back to medical service as NHS done so much to help my child’ (P59, questionnaire, mother, restrictive threshold).
Although 25 out of 41 parents (60%) reported providing consent because they trusted the doctor or nurse who explained the FEVER pilot RCT, no parents identified this as their main reason for providing consent. During interviews, parents also spoke of their trust in the person who explained the study. However, they also appeared to place their trust in the knowledge, skills and values of clinicians looking after their child. Parents repeatedly emphasised the importance of their trust in doctors and nurses to place the needs of their child above the needs of the study:
She’s in a hospital. I mean them people know better than me, so I understand that they would never put a child in harm’s way.
P81, interview, father, restrictive
Yeah. I mean we were trying to take the view that we were, we’re in their hands and they know what they’re doing so, um, I’ve, I’ve always felt that, you know, it wouldn’t . . . you know, they’re not gonna put any undue risk on the child so yeah, I was happy with that level set. Um, and it’s set at that level for a reason I’m sure.
P73, interview, father, permissive
Parental acceptability of the permissive threshold was underpinned by trust in staff to act in their child’s best interests
During interviews, parents described how they expected staff to act in their child’s best interests, which included not adhering to the protocol by administering an antipyretic if at any point clinical staff felt that it was needed. The parents who were interviewed appeared to understand and value their ability to withdraw or decline consent for their child’s continued involvement in the FEVER pilot RCT:
I know if anything did happen, yous can stop at any time. Stop it if they saw it was getting out of hand and he, and I felt like it, what like it, it wasn’t helping, that I would stop it. Erm, but I knew he was in like capable hands, obviously being in the, intensive care unit that he was going to be absolutely fine, they wouldn’t let him go to the stage of him getting poorly.
P85, interview, father, restrictive
I was happy enough for him to undergo the trial but if at any point the nurses thought he could do with the Calpol, or I thought he could, then I wanted the trial to stop it could do.
P49, interview, mother, permissive
Some parents referred to how the trial was acceptable as ‘my child is comfortable’ (P49, questionnaire, mother, permissive). Indeed, two mothers described how they found the trial acceptable and gave full consent, but later chose to withdraw their child from the study when they were being weaned from the ventilator as they were concerned about their child being in pain or distress:
The only time we eventually pulled him from the trial and gave him paracetamol was when he was awake and he was a lot more distressed, and that was harder for me to watch, especially, especially the way he was, and I said, ‘look, if it’s gonna help, I’d rather you gave him it,’ but when he was sedated and he was ventilated and everything, he did get a temperature and, like I say, he brought it back down himself.
P49, interview, mother, permissive, consented then withdrew
He was withdrawn because, um, he didn’t actually have a temperature at all and he was in a little bit of pain once he’d been extubated. So they wanted to give him some paracetamol but he didn’t have any temperature any way.
P76, interview, mother, permissive, consented then withdrew
Why parents declined consent to the FEVER pilot randomised controlled trial
We gained insight into the reasons why eight parents (8/18, 44% of total decliners) refused consent for their child to continue to take part in the FEVER pilot RCT. Of these, six parents completed a questionnaire and two took part in an interview.
One parent reported declining owing to her child no longer being ventilated and a belief that her child was ‘therefore not part of the research requirements’ (P67, questionnaire, mother, restrictive, decliner). Of the other seven parents, six had children who were allocated to the permissive threshold and one was in the restrictive threshold. Two were parents of the same child: the mother (P84) took part in an interview and the father (P72) completed a questionnaire.
As shown in Table 22, only one parent declined consent to use data already collected, as well as consent for their child to continue in the trial. Parents’ reasons for declining consent included concerns about any negative impact on their child owing to the child’s pre-existing medical condition, concerns about their child being in pain or discomfort, increased workload, risk of epileptic seizures owing to family history, incapacity to make an informed decision and a wish to have the answer to the research question before trial participation.
| Reason for declining (number of parents who provided this reason) | Type of consent declined (e.g. consent to use data already collected and/or consent for child to continue in the FEVER pilot RCT) | |
|---|---|---|
| Concerns about their child being in pain or discomfort (three parents):Given the trauma his little body had entailed, I just don’t want him to be in any more painP70, questionnaire, mother, permissiveI would probably say once they, when they can sort of start telling you how they’re feeling. ‘Cause the trouble is, we just felt because he couldn’t tell us how he was feeling, like whether he was OK, or whether he was in pain, it was very difficultP84, interview, mother, permissiveI feel that my baby had been through so much that if he was to have a temperature I would give him paracetamol straight awayP71 questionnaire, mother, permissive |
P70: full refusal to use data already collected and consent for child to continue P84: consent provided to use data already collected. Declined consent to continue P71: consent provided to use data already collected. Declined consent to continue |
|
| Concern about increased workload (one parent):I think allowing her paracetamol to help her temp [temperature] and heart rate benefits her not tiringP68, questionnaire, mother, permissive | P68: trial identification missing from questionnaire so not possible to identify type of decliner or threshold | |
| Concerns about negative impact owing to child’s pre-existing medical conditions (three parents):Because my child’s brain doesn’t control the body temperature, my child don’t express herself so when she is in pain we have to guess and give her medicationP69, questionnaire, father, restrictiveMy son had too many underlying medical conditions and felt it may hinder his recovery as he was selected to the upper limit before treatmentP73, questionnaire, father, permissiveI think it was, if he had no other underlying medical condition then, and maybe if we hadn’t have been in hospital for 16 weeks previous to that, then possibly, yeahP84, interview, mother, permissive |
P69: consent provided to use data already collected. Declined consent to continue P73 and P84: same child – consent provided to use data already collected. Declined consent to continue P84: consent provided to use data already collected. Declined consent to continue |
|
| Risk of seizures owing to family history (one parent):My nephew has seizures [. . .]. So, we just decided that if there was a possibility that they could stop that from happening by intervening sooner, we decided that we would rather that than cause anything to happen [. . .] the, person that was telling us about the trial said that, you know, there was a very minor chance that it could happen. But, just purely because of the background of the family, we just decided that we just did not want that to even be a possibilityP84, interview, mother, permissive | P84: consent provided to use data already collected. Declined consent to continue | |
| Incapacity and research evidence needed (one parent):At the time he had too much other stuff going on for us to even think about being involved in the study [. . .] it’s seeing whether there is any research and proof that giving paracetamol straight away is the right thing to do or whether it would go away by itselfP83, interview, mother, permissive | P83: consent provided to use data already collected. Declined consent to continue | |
Importantly, interview and questionnaire data suggest that parents who declined some element of their child’s participation often described their support for the proposed FEVER RCT. No parents were unhappy about their child’s involvement in the FEVER pilot RCT:
From being on other wards, as soon as babies or children even spike a little bit of a temperature they seem to be quite quick on the, quick let’s give them paracetamol. We can say that first hand where we’ve always been like a kind of, a hold off [. . .] I think sometimes paracetamol can hide other things going on as well.
P83, interview, mother, permissive, decliner
As described in Chapter 2, Parent perspectives, one mother (P84, interview, mother, decliner) was dissatisfied with RWPC in any trial. However, the parents who declined appeared to be satisfied with the consent process and provided similar questionnaire responses to those who had consented to the FEVER pilot RCT. This was corroborated during the interviews, with both mothers saying that consent discussions were clear and well timed:
And did you think the timing was appropriate?
Yeah, it was absolutely fine. The bloke that I spoke to so lovely about it. But, because we did feel a bit bad afterwards saying no, because he was so nice, but obviously, you know, at the end of the day he respected our decision.
Parent-centred outcomes
As part of an iterative process, findings from the FEVER qualitative study (see Chapter 2) were incorporated into the topic guide as interviewing progressed to further explore parents’ perspectives about important outcome measures. Parents were asked to think about their experience of their child being admitted to hospital with a suspected infection and then prompted to share their views on what effects they hoped the FEVER treatment would do to help their child.
At this point in the interview, the majority of parents’ responses focused on a reduction or reduced fluctuation of their child’s temperature. Parents also prioritised stopping any discomfort and/or pain, getting the child ‘back to themselves’ and reducing time in hospital. Others found this question difficult to understand: ‘I don’t know really’ (P79, interview, mother, permissive) and ‘I have no idea [laughter]’ (P76, interview, mother, permissive). All parents were then asked ‘What would you be looking for as an indicator that your child was getting better?’. The most common responses were:
-
time spent on mechanical ventilation
-
looking and/or behaving like normal self
-
child not in discomfort and/or pain
-
improved mood.
The interviewer then repeated back the outcome measures identified to the individual, as well as any other outcomes that had been referred to and noted by the researcher during interview discussions. Parents were then asked to rank their identified outcomes in order of importance for a proposed FEVER RCT. The top-prioritised outcomes in order of how commonly they were described as being most important were:
-
time spent on mechanical ventilation
-
child not in discomfort and/or pain
-
reduction of, or reduced, fluctuation of child’s temperature
-
looking and/or behaving like normal self
-
time spent in hospital
-
number of re-admissions to hospital.
As no parents had mentioned survival as an outcome measure, the researcher queried the inclusion of survival in the list after parents had prioritised outcomes. All except one parent stipulated that survival is the most important outcome measure for the proposed FEVER RCT and gave a range of reasons why she had not initially mentioned it (Box 6). These reasons included death not being something she wished to consider or that she had not considered owing to her child’s condition (bronchiolitis) and, therefore, she thought that it would not be a salient outcome measure for this trial:
I hadn’t thought about it, but also, yeah, don’t think it’s necessarily the best outcome measure that you could have.
P80, interview, mother, permissive
I suppose with bronchiolitis I never really thought that was a possibility. If he had have had something like that [meningitis/sepsis], I might have mentioned the fact that he had survived, but with bronchiolitis, I didn’t really see that as an option
P49, interview, mother, permissive
Do not want to think of is as a possibility
It’s just something that you don’t, you don’t let enter your mind. So on reflection, when you’re thinking back, you don’t think of it. I can see that is, er, should be number one on the list, definitely
P53, interview, father, restrictive
Because their child survived, they do not think about it
I think because, you know, she has survived it all through, that sort of goes to the back of your mind again, you know. You sort of don’t think that, you think of oh what . . . but now, you know, now you think about it, you just, yeah, you don’t think of that. I don’t know, it’s strange
P78, interview, mother, restrictive
Did not make the link between fever, infection and survival
I wouldn’t have necessarily connected the two, that, um, that fever could be so, um, um, so linked to, to something so, you know, grave but yeah, um, I guess anything can happen, can’t, can’t it?
P73, interview, father, permissive
Not an option – my child was going to survive
There’s no option. I’ve lost a nephew, erm, 5 or 6 years ago, so, obviously, going into hospital and having them on the machines was, er . . . like brought back lots of memories, when they’re in intensive care, there’s only two ways out of it. You either don’t come out or you come out, so like I said to my wife, we’re definitely getting them out, or my son, because he was the only one ill at that time
P55, interview, father, permissive
Told that there was no risk
As soon as we got there, they said, we treat this all the time, she’ll be fine. So I didn’t really ever think at that point that she wasn’t going to survive . . .
P79, interview, mother, permissive
N/A, not applicable.
Therefore, other than survival, six prioritised outcomes were identified from the combined analysis of outcome measure discussions, initial prioritisation and ranking. These were:
-
length of time on breathing support (e.g. ventilator, high-flow oxygen)
-
not in pain and/or discomfort
-
how quickly vital statistics are back to normal (including temperature)
-
looking and/or behaving like normal self
-
length of time in hospital
-
number of re-admissions to hospital.
Interestingly, this list includes only short-term outcomes, in contrast to the outcomes list created from the FEVER qualitative study (see Chapter 2), which included longer-term outcomes. This finding may indicate that parents’ prioritisation of outcomes may be influenced by their experience of their child’s illness, survival and the point at which they are asked about outcomes of importance in the course of their child’s illness.
Combining outcomes-related data from both the qualitative and integrated-perspective studies suggests that, apart from survival, parents would prioritise seven outcomes for measurement in the proposed FEVER RCT (Box 7).
-
Length of time on mechanical ventilation.
-
Long-term effects of illness on child (physical and developmental).
-
Looking and/or behaving like their normal self (examples included improved mood, communication, more like themselves and more alert, sitting up and starting to eat and drink).
-
Not in discomfort and/or pain.
-
Number of days spent in the PICU and hospital.
-
Vital signs back to normal (e.g. heart rate, breathing rate and temperature).
-
Effect on family (e.g. emotional, functional and financial).
Site staff perspectives
The FEVER pilot randomised controlled trial site training
A total of 21 members of staff (21/97, 21%) were trained by the FEVER study team, five of whom also received subsequent training from a colleague at their site. The majority (68/97, 70%, 1 missing) had received protocol training from a member of their site team.
In questionnaires and focus groups (using voting handsets), staff were asked to rate the FEVER pilot RCT training received on a four-point scale (excellent, good, fair and poor). The majority of staff positively rated the training as ‘good’ (47/97, 48%) or ‘excellent’ (24/97, 25%), whereas 14% (14/97) indicated that the training was ‘fair’ and 11% (11/97) indicated that the training was ‘poor’. The 11 staff who provided a poor rating had been trained by a member of their site team. Many staff (67/90, 74%) indicated that the site training prepared them for recruitment and consent in the FEVER pilot RCT; however, 22 out of 90 (24%) indicated that they were not prepared. Eight staff (8/98, 8%) did not respond to this question.
In the focus groups, some staff described how ensuring transfer team and bedside clinical staff availability to attend training sessions during the busy winter period was challenging:
It’s getting the clinical team available. You know, on that, that Thursday everyone was invited but on a busy winter period you’re not going to be able to pull people out, so we did what we could.
P01, FG1
In two sites, staff described how aspects of the protocol remained unclear after site training, including what constituted active cooling of a randomised participant and how long a patient would remain in the FEVER pilot RCT:
So I think we basically got told it on, we had a mandatory study day and we had someone come in and talk to us about it but then I felt I was quite unclear about the, erm, the different parts of what would go on if you had a patient that was randomised and then put on it that it was, it wu-, things like it, not just the giving of the paracetamol but the environmental factors about what constituted coo- cooling them. So taking off blankets or I just felt that, I was quite unclear about that side of things. Erm, and there was a sticker to say if your patient was on the trial but then I didn’t know how long they were supposed to be on it for.
P01, FG2
Although resources were available in a training file, some staff felt that they would have benefited from additional training or ‘simplified resources’ (P01, FG2) to enable a quick refresh when needed:
It would be really useful if you could just have really basic teaching slides available on a quick link for out of hours, um, so that if, even if a nurse in charge knew of it, then they can quickly run through and know really quickly how to randomise and it’s just a really basic overview.
P02, FG5
To help facilitate clinical staff ‘buy-in’, focus group participants suggested that additional scientific information underpinning the trial rationale should be included in any future FEVER RCT training:
I think it would be better to do more in-depth teaching so that more people understood why we were studying it. So that’s probably the only reason that I was really supportive of it, that’s because I was able to read around other studies.
P04, FG5
As the following quotation illustrates, some focus group participants remained unclear about the scientific rationale for the study, which appeared to negatively influence their views on trial acceptability:
A lot more parameters of what the trial involves and the reasons why. Like I get not giving paracetamol but I don’t understand . . . I get the outcome for it, I just don’t agree, I don’t get why parts of it are involved and it . . . that’s what make, to me makes in unacceptable, the, the things about, I don’t get why you wou-, what difference it makes cooling them if it’s paracetamol you’re looking at what the environmental factors come into it. So I think just more training for the rest, the rest of us and I think that’s a unit thing allowing more time for the, us to have specific training instead of just sort of like, you know, ‘cause the research team did a brilliant job but there’s only so many of them and there’s a lot of a, a rest of us.
P03, FG2
Acceptability of the FEVER pilot randomised controlled trial temperature thresholds
Restrictive temperature threshold
In questionnaires and focus groups, staff were asked to rate how acceptable they viewed the restrictive temperature of 37.5 °C. As shown in Table 23, most (95%) indicated that the restrictive temperature was acceptable (66% considered it very acceptable and 29% considered it acceptable). Only five nurses did not view this threshold as acceptable, two of whom described how they were unhappy with the lower threshold as it was not their normal practice to treat a fever at this low temperature:
I understand this was for the trial, however it was mentioned more than once from myself and others that we would not normally treat with paracetamol a temperature of 37.5 [°C].
P49, questionnaire free-text response
So I’ve put not acceptable . . . because actually I wouldn’t normally give it to them at 37.5, so why, all of a sudden, am I overmedicating someone. That’s more uncomfortable to me, than waiting till like 39 [°C].
P01, FG5
| Acceptability | Temperature threshold, n (%) | |
|---|---|---|
| Restrictive | Permissive | |
| Very acceptable | 59 (66) | 5 (6) |
| Acceptable | 26 (29) | 37 (47) |
| Not acceptable | 5 (5) | 23 (29) |
| Very unacceptable | 0 (0) | 14 (18) |
| Total | 90 (100) | 79 (100) |
Overall, these findings are in stark contrast to those reported in the FEVER qualitative study (see Chapter 2), in which the majority of participants had concerns about unnecessarily treating children with an antipyretic. Although this pilot RCT staff sample was larger, the majority of participants had also taken part in the FEVER qualitative study focus groups. As the following quotation shows, experience of conducting the FEVER pilot RCT appeared to change staff views about the acceptability of the restrictive temperature threshold. Parents’ positive responses to their child’s participation in the restrictive group of the FEVER pilot RCT appeared to influence staff views on the acceptability of administering an antipyretic at a lower temperature than their usual practice:
Everybody that was in the lower end of it, I found were like happy to take part.
P01, FG4
Bedside clinical staff acknowledged that they may not have normally given paracetamol at such a low temperature; however, they described how, despite initial reservations, they did follow the FEVER pilot RCT trial protocol and did not find it unacceptable to treat children at 37.5 °C:
I wasn’t like, oh I shouldn’t be doing this. But, erm, but, yeah, I mean I suppose if we, because we were following sort of like a protocol I mean, yeah, it didn’t feel, it didn’t feel wrong if that’s what you’re asking?
P03, FG4
One participant described how it was acceptable to treat at such a low temperature as a child may have a fever or be in pain:
I think it could be acceptable if you’re like looking at different things. Like they might be in pain or there’s a temperature there. If you look at like the whole, you might give them paracetamol if they’re 37.5 [°C].
P02, FG5
Permissive temperature threshold
Staff had mixed views about the acceptability of the permissive temperature threshold. Approximately half (42/79, 53%) indicated that the 39.5 °C threshold was acceptable, whereas just under half (37/79, 47%) did not (Table 24). There were no notable differences between the views of staff who completed the questionnaire and the views of those who took part in the focus group. However, staff trained by their colleagues were more likely to find the permissive threshold not acceptable, or very unacceptable, than staff who received training from the FEVER study team, which included the chief investigator. This may have been linked to the training issues highlighted in The FEVER pilot randomised controlled trial site training, as all staff who rated the site training as poor had been trained by their colleagues. These staff appeared to have remained unclear about the scientific rationale for the study, which negatively influenced their views on the acceptability of the trial.
| Site training provider | Acceptability (number of participants) | Total number of participants | |||
|---|---|---|---|---|---|
| Very acceptable | Acceptable | Not acceptable | Very unacceptable | ||
| Trial team | 3 | 9 | 2 | 0 | 14 |
| Site staff | 1 | 27 | 16 | 14 | 58 |
| Both | 1 | 1 | 2 | 0 | 4 |
| Total | 5 | 37 | 23 | 14 | 79 |
As described in Chapter 2, FEVER qualitative study findings highlighted parent and staff concerns about a permissive threshold of 40 °C. These findings informed the selection of a FEVER pilot RCT permissive temperature threshold of 39.5 °C and limiting inclusion criteria to children already receiving ventilation. In focus groups, many staff who were positive about the permissive threshold in the FEVER pilot RCT described how they valued how staff and parent views had been listened to. Changes made to the protocol positively influenced staff perceptions about the acceptability of the study. Some research nurses responsible for training site staff spoke of how being able to describe how parent and staff views had informed changes to the study had helped engage colleagues in the FEVER pilot RCT:
I know from giving the training, having the feedback that things have been changed according to what parents had said changed the kind of response of the people that we were giving training to. You know, there was an initial kind of barrier up and then once you explained certain parts of it had been changed, staff did relax a little bit into it and go ‘right OK,’ and engage a bit more in the training so whether that helped.
P01, FG1
As the following interview excerpt shows, prior concerns about not being able to use an antipyretic to treat pain had been addressed by the FEVER team limiting the pilot RCT sample to children receiving mechanical ventilation. Staff described how this inclusion criterion had changed their views on trial acceptability, as children would already be receiving other medication, such as an opioid, for pain relief:
It was mainly that they all had an infusion running of kind of something else, an opioid and . . .
And they would have had that anyway?
Because they were ventilated, yeah.
Yeah, OK. Did that make quite a difference [to staff views on trial acceptability] then, that vent [ventilation] and infusion?
Yeah.
You didn’t see the sort of effects and it wasn’t like a self-ventilating patient that you might see sort of crying and being quite distressed.
P02, FG4
Others stated that their previous concerns about high temperatures causing harm or discomfort were not observed. One nurse anticipated that experience of conducting the proposed FEVER RCT would help address staff concerns in the future as ‘seeing is believing’ (P03, FG1):
I think also what’ll happen is as some patients are randomised into the higher temperature and people are, see that they’re actually manageable and it doesn’t cause them any, um, harm, then people generally get more accepting of it can be one or the other category. It’s kind of seeing is believing.
P03, FG1
Some staff were surprised at how positive parents in the FEVER pilot RCT had been about their child’s participation in the permissive threshold group. A few attributed these positive responses to staff explanations about the use of alternative medication for pain. Others described how some parents had an interest in the trial question and the potential to inform the future treatment of fever in children with a suspected infection:
I think a lot of them were happier once they understood there were alternatives we could give for pain.
P04, FG2
I think I definitely found that a lot of families were really interested in it, and like something like temperature, something that they actually understand. And I think, if they understood that we’re doing something because maybe the outcome is, is better, I think then families were a lot more on board.
P04, FG5
In contrast, staff who did not find the permissive threshold acceptable were unhappy about not administering paracetamol when they thought that a child was uncomfortable or in pain. These concerns meant that some staff did not adhere to the protocol and administered paracetamol before a child’s temperature had reached 39.5 °C:
I often looked after patients that I felt were in pain and may benefit [from] paracetamol for comfort. I found it unacceptable that I felt I should not use paracetamol in these circumstances when it is our first line drug for pain. I found doctors often reluctant to prescribe paracetamol for comfort, I am a nurse, it is extremely important to me that my patients are comfortable. I continued to request paracetamol for pain and comfort.
P39, questionnaire response
I’ve never known a trial before where I feel like potentially we’re making our patients more uncomfortable.
P01, FG2
Think the thing I was most uncomfortable with, er, was the, the higher level . . . I thought it was too high because at that stage the patient I was looking after was, was very distressed and very uncomfortable from what I remember I gave paracetamol because I didn’t fi-, I didn’t think it was fair on the child to leave them that hot and that distressed.
P01, FG3
Many were concerned about not giving paracetamol for pain or discomfort when a child was conscious. A few staff said that alternative medications for pain, such as morphine, seemed ‘extreme’ (P01 and P03, FG2). Suggestions were made to revise the proposed FEVER RCT protocol to exclude patients not on mechanical ventilation (e.g. high-flow nasal oxygen) or those close to being extubated when sedation is being weaned:
I think people/nurses have found it difficult to not be able to give there paracetamol when the child is upset.
P22, questionnaire free-text response
We’re much happy to, happier to be compliant, erm, if the child was intubated and ventilated and knocked out . . . most of our problems came in really, I would say, in classifying Optiflow [Fisher & Paykel Healthcare Limited, Panmure, New Zealand] and, you know, high-flow nasal cannular as mechanical ventilation. I think that obviously there are huge problems in . . . because a child is awake.
P05, FG2
Do not include high flow.
P63, questionnaire free-text response
And then at the point that you’re weaning their ventilation . . . you’re weaning . . . their sedation to wake them up. So you’re reducing their level of comfort so you would automatically normally give paracetamol . . . I know it sounds dramatic but a little bit like collateral damage. Like for the greater good that they’re gonna be in more discomfort while they’ve got, they’re in intensive care to find out these results sort of thing. But, yeah, [if] they were on morphine that would be easier.
P03, FG2
Staff in one focus group described how they ‘do not sedate anybody that much unless you are very sick’ (P04, FG3). They discussed the benefits of using paracetamol to ensure that a child is comfortable and how its use can prevent the need for other drugs. As the quotation below suggests, this practice appeared to apply to children on invasive ventilation, which meant that staff had concerns about changing their usual practice in FEVER, leading to protocol deviations when children in the permissive threshold looked uncomfortable or in pain:
As paracetamol is such, erm, a good drug and we use it for lots of situations, it’s not just temperature control . . . You can reduce the amount of other drugs you give, so you actually, you know, you could aim people towards getting them extubated because they’re, they’re breathing better and, you know, ‘cause you can reduce the amount of morphine and use paracetamol as a, as an adjunct with those drugs. And actually taking that away almost, especially with somebody like him because the [FEVER patient] pa-, I remember this case as well, erm, it, it kind of tied your arms behind your back because you’re like that wou-, is what you would use. That’s [paracetamol] we would usually use, you know?
P04, FG3
These members of staff described a clinical decision to withdraw a pilot RCT participant without input from parents owing to staff concerns about how the child looked:
It was easy until that point and, erm, I jus-, I felt, I don’t, you know, I didn’t just make the decision on my own, I think I spoke to other people. The parents weren’t there, erm, I don’t know if I should have got their consent or not, but I just felt, erm, it was difficult to stick to it looking at how the child was . . . So I made that decision, well, along with other people.
P01, FG3
In each focus group, staff provided a few examples of parents who had withdrawn their child from the study because had been unhappy or concerned about their child’s well-being in the permissive temperature threshold group. These examples appeared to be uncommon. In most cases, parents had informed staff that they would have consented for their child to remain in the trial if they had been randomised to the lower threshold:
I knew that one of the parents, erm, I think withdrew because their child was starting to get to like 39 [°C] and was uncomfortable, and they didn’t feel happy keeping their child involved in the study. So, again, if it was slightly lower threshold for giving it you’d probably maintain like a higher participant rate.
P01, FG3
His view was that he would be happy to do the study, but he felt 39.5 [°C] was too high, erm, that the study didn’t need to be done and that we already knew exactly what was going on . . .
P04, FG4
Others described how waiting for a child’s temperature to rise had caused parents stress, leading them to withdraw their child from the FEVER pilot RCT:
Sometimes parents were uneasy about watching the temperature climb even after consenting to the trial. A few families took their child out of the trial because it was causing more stress to them.
P76, questionnaire free-text response
Protocol adherence
In questionnaires and using voting handsets, just under half of staff (44/96, 46%) indicated that they had difficulty adhering to the protocol. Almost all difficulties were attributed to staff concerns about not treating a pyretic child in the permissive group, which, as described, led to the administration of paracetamol before a child’s temperature had reached the stipulated threshold. As the following quotations suggest, others used active cooling therapy, such as fans, and one unit described how they had run out of fans, suggesting that they had misunderstood the protocol and used active cooling:
I was not frontline staff during the trial I was more data input but I found a lot that people gave paracetamol still either for comfort or at parents request and also did not document accurately about other cooling methods like fan therapy etc.
P41, questionnaire free-text response
There was no fan that she could get. So that was only a little, like just a little thing but that was what the protocol said to do and she couldn’t, she did find one in the end but she had to take it off someone else.
P02, FG1
The details like about the cooling measures whether taking a blanket off that had been put on 2 hours previously it’s classed as a cooling measure. Some people would say, yes, some people would say, no.
P01, FG2
In contrast, the other half of participants (52/96, 54%) did not report problems adhering to the protocol, which was described as ‘very easy to follow’ as ‘it’s easy to know what you had to do when, and what you should not do when’ (P02, FG3). There was no significant association (p = 0.416) between who trained staff (the trial team or a site colleague) and reported difficulties in adhering to the protocol.
Acceptability of research without prior consent in FEVER
Just under half of staff (46/97, 47%) had previous involvement in trials that had used a RWPC approach. As the following quotation shows, staff were familiar and supported RWPC, which had become the ‘normal’ approach to consent in paediatric intensive care research:
So many of the trials that we’ve done over the last few years have worked in the same way, without getting consent and things, it’s actually more normal than, hold on a minute, I can’t do that, we need to get consent first. Like, I can’t remember the last time we had to do that, so it’s the nature of what we do, I think.
P01, FG5
A few staff at each site (10/98, 10%) were formally involved in trial discussions and seeking consent from parents for the use of their child’s information and continued involvement in the FEVER pilot RCT. However, 58 out of 93 staff (62%) positively responded to the questionnaire and voting handset question ‘How have parents responded to the FEVER consent discussion?’ and 35 (38%) selected ‘not applicable’. This response and focus group discussions indicate that many clinical staff had informally discussed the FEVER pilot RCT with parents.
Over half indicated that parents had responded positively (39/93, 42%) or very positively (8/93, 9%); however, nine out of 93 (10%) indicated that parents had responded negatively and a few (2/93, 2%) had been very negative:
I think it (RWPC) was dealt with really well.
Yeah, I had no complaints, no-one said anything bad about it at all.
Yeah, the same.
The FEVER posters and leaflets were placed by children’s beds and in communal areas. Nurses said that this was helpful, as trial information was ‘visible before we, the research team even approach them’ (P01, FG1) and meant that some parents had asked whether or not their child had been included in the study before the research discussion had been broached.
Parents were often given brief verbal information about the trial shortly after randomisation and informed that there would be a full trial discussion at a later time. As the following quotations show, this staged approach to information giving appeared to work well in two sites:
We would’ve sort of made them aware as soon as the child went into it that this is what we were doing and that we would consented a or it we would talk to them about it at a later, when it was convenient, you know?
P02, FG2
I think it had been mentioned very briefly, and then before the research team had a chance to come and discuss it properly, they asked me, what’s this all about? So I just explained it and then they were fine about it.
P01, FG4
Nevertheless, staff in one site reflected on how they had approached some parents too soon (e.g. within 1 hour) after randomisation, which had caused them to decline their child’s continued participation. They described how they had adjusted their approach to give parents more time after their child’s PICU admission before broaching the FEVER pilot RCT:
We like to, I think at the very beginning we, we felt as if we stepped in a bit too soon with the consent process and some, we lost a couple . . . erm, I think we learnt to kind of step it back and give them a little bit more time.
P02, FG2
The majority of staff (80%) viewed RWPC as acceptable (28/94, 30%) or very acceptable (47/94, 50%) in the proposed FEVER RCT, and 19 out of 94 staff (20%) did not find RWPC acceptable, one of whom had been involved in RWPC discussions with families. Those who elaborated on why RWPC was not acceptable reiterated their concerns about the permissive temperature threshold and not being able to administer paracetamol to children who were uncomfortable or in pain:
I tick negatively as most parents just wanted their children to be pain free and comfortable, they are not pain free and comfortable with a temperature of 39.5 [°C].
P39, questionnaire free-text response
One parent felt very upset by this and others even if they agreed to consent still felt like they should have been asked first.
P75, questionnaire free-text response
Some staff at one site discussed how consent should be sought prospectively for a trial that was withholding an intervention that could make a child feel better. They were concerned that not seeking consent in the proposed FEVER RCT may have a negative impact on parents’ trust in staff:
Cause you’re withholding what I would consider a, a drug from my child that could potentially make them feel better.
I think I’m the same. Like at the first point that you would feel like you would normally give something, I think that’s when the subject needs to be broached rather than, oh, well we can’t just in case.
It’s an element of trust like I would put a trust in a bedside nurse that they’re actively doing everything they can with the facilities they’ve got around them in a PICU to make my child as comfortable as possible. So then to be told after the event, well no, they, they haven’t had this . . . I feel like there’s an element of trust there that would be broken from my point of view.
Interestingly, 21 out of 97 members of staff (22%) indicated that their views on RWPC had changed owing to conducting the FEVER pilot RCT. These staff were significantly more likely (p = 0.032) to not have previous experience of RWPC before involvement in the FEVER pilot RCT. In focus groups, some staff described how they became ‘more educated I think about research’ (P04, FG1) and realised that informed consent ‘wouldn’t be feasible’ (P03, FG5) in this emergency situation. Therefore, experience of RWPC in the FEVER pilot RCT appeared to address initial concerns that some staff had about the use of RWPC in the proposed FEVER RCT.
Finally, in two sites, staff described the challenge of having sufficient research cover at weekends, which led to a delay in formally discussing the trial and consent-seeking process with parents. They suggested that it would be useful for more staff to be GCP trained so that they could be involved in consent seeking in the proposed FEVER RCT:
There was a bit of anxiety over weekends and stuff as well when, when patients were randomised, and although we did train a number of study members to do, or a number of people to do consent, actually the majority of it was done Monday to Friday and within sort of working, working hours, just because it just happened to be how the rota fell over the study period really. So yeah . . . I’m more than open to doing GCP with the rest of the staff and getting them consenting.
P04, FG4
Screening, randomisation and forms
Approximately half of participants (48/98, 49%) had been involved in screening patients, over one-third (33/93, 35%) of whom suggested that the process could be improved. Limited research support meant that screening was not taking place at evenings and weekends, which meant that eligible patients had been missed:
I think that was across the unit as well, you know, overall we missed 10 patients, the majority, well they were all out of research hours. We haven’t got a full-time research team and the funding wasn’t there to be able to do that.
P01, FG1
If a patient’s first temperature was overnight and then it was a research person come in the next day and said that, ‘oh, this person should’ve been randomised’ . . . ‘Cause there was no one or nobody did it for whichever reason.
P02, FG2
Collaborative working between retrieval teams and clinical and research staff assisted the identification of eligible patients. Although only 30 staff (including 24 nurses) had used the web-based system to randomise patients, in focus groups nurses described having a key role in identifying patients to be screened and randomised:
Although it’s the doctors that do the randomising, actually when a lot of them come in the middle of the night and they need randomising, or they’ve been done by [transfer team], actually it’s usually the nurses saying, you need to randomise this patient. So, although we haven’t formally screened or randomised them, we are actually the ones driving it.
P01, FG5
Most questionnaire and focus group participants (82/98, 84%) stated that they did not experience any difficulties with the randomisation process, and that it could not be improved (81/94, 86%). Suggestions for improvement included allowing more senior nurses to randomise patients and additional research support, particularly at weekends:
Most of the time the senior nurses are chasing medical staff to do this [randomisation] when they could just do it themselves.
P36, questionnaire free-text response
For us, I think having a 7-day cover and not put, not putting the onus on the bedside staff.
P01, FG1
Many (24/27, 89%) of those who had used the CRF found that it was easy to use. A few members of staff suggested that there was a lack of clarity about how to record information, suggesting the need for additional training or changes to the form. This included additional details about what information to record and how to record the final discharge time for children transferred to another hospital:
CRF needs to be more detailed to capture all the information required. Many queries are because it was unclear the depth of information required when completing them the first time round.
P41, questionnaire free-text response
I think the only thing we’ve had more difficulty and it’s not impossible but more difficulty collecting are those children who are transferred to other hospitals is their ultimate discharge from that hospital.
P02, FG2
No suggestions were made to improve the consent form, which 21 out of 22 staff (95%) involved in consent discussions viewed as being easy to use.
Is the proposed FEVER randomised controlled trial practically possible and appropriate to conduct?
At the end of the focus group sessions and in questionnaires, staff views on whether or not the proposed FEVER RCT is practically possible and acceptable to conduct were sought. The majority of staff (80/95, 84%) viewed the trial to be practically possible. A similar proportion of staff (82/95, 86%) were positive about the acceptability of the trial, with 46 out of 95 (47%) stating that the FEVER RCT was acceptable and 36 out of 95 (38%) stating that it was very acceptable. Ten of the 15 staff who did not think that the trial was practically possible had been trained by their site colleagues rather than the trial team, eight of whom also stated that the trial was not acceptable to conduct.
Some questionnaire participants used free-text boxes to state that they would wish to know the FEVER pilot RCT findings, including parents’ views on trial acceptability, before drawing a conclusion about whether or not a future trial should be conducted:
The main issue is whether fever is acceptable to families/if enough families from hot arm don’t withdraw consent then I do think it is a workable large RCT trial.
P72, questionnaire free-text response
Need to see preliminary results before decision to continue.
P62, questionnaire free-text response
At the end of focus groups, many staff confirmed their support for the proposed FEVER RCT, often stating that the trial was needed to provide an answer to an important research question:
I think if you like get some like valuable answers from it, or you then can find out whether it is better and whether it can help children recover more, then it’s a really good, valuable study to see on a larger scale.
P04, FG5
You’re never gonna know the answer if you don’t do a trial.
P04, FG2
Summary of parents’ and clinicians’ perspectives in the FEVER pilot randomised controlled trial
The integrated-perspectives study findings add to the FEVER qualitative study findings presented in Chapter 2 by exploring the views of parents and clinicians involved in the FEVER pilot RCT.
Parents’ perspectives
The findings suggest that the majority of parents who took part in an interview or completed a questionnaire supported the proposed FEVER RCT. Administering an antipyretic for fever was something that parents understood and were familiar with and, as a result, parents were interested in the trial question and felt that the proposed trial was important. The non-invasive nature of the intervention appeared to influence parents’ views on trial acceptability, which they did not perceive to pose a risk to their child’s well-being. As shown in previous studies, parents strongly valued the advancement of medical research and commonly stated that they provided consent as a way to help other families and children in the future. 16,20,36–39
Descriptions of the permissive temperature threshold as a ‘scary number’ by parents in the FEVER qualitative study were not observed in data from parents of children who took part in the FEVER pilot RCT. Interestingly, no parents referred to the temperature their child reached when discussing trial acceptability. Nevertheless, parents only viewed the permissive threshold as being acceptable if their child was not in pain or discomfort. Parents trusted clinicians to act in the best interests of their child and expected them to deviate from the protocol by administering an antipyretic if at any point they felt that it was needed. Both parents interviewed who had withdrawn their child from the trial did so because of concerns about pain or discomfort. Similar concerns were also described by some parents whose children were in the permissive threshold group and had declined their child’s continued participation in the trial. Others had declined because of reasons such as their child’s pre-existing medical condition, risk of epileptic seizures owing to family history or incapacity to make an informed decision owing to the situation.
Importantly, interview and questionnaire data suggest that parents who declined some element of their child’s participation in the FEVER pilot RCT still supported the proposed FEVER RCT. Parents valued clinicians’ willingness to give an antipyretic, as well as the option to withdraw or decline continued participation in the trial when their child was perceived to be uncomfortable or in pain. No parents who took part in this study element expressed anger or dissatisfaction about their child’s involvement in the FEVER pilot RCT.
Using information that parents’ prioritised in the FEVER qualitative study to inform the participant information sheet and staff training appeared to address potential parental concerns about the trial. For example, anticipated concerns from the FEVER qualitative study, such as if not treating a fever could cause a seizure, were not voiced by parents in the FEVER pilot RCT. Parents described the participant information sheet as being clear, comprehensive and an appropriate length to read in a critical care situation.
As shown in previous studies,17,18 some parents were initially surprised to find out that their children had been entered into a trial without their prior consent. However, following a tailored explanation from site staff about why prospective consent was not sought, the majority of parents were satisfied and supported this approach to consent for this trial, as well as other PICU trials. Parents described how RWPC was acceptable, as the FEVER pilot RCT discussion took place when the emergency situation had passed. Some emphasised the importance of clinicians broaching the trial discussion at the first appropriate opportunity, which is consistent with existing guidance. 30 There was a minority of exceptions. A few parents were unhappy that their child had been randomised to FEVER without their prior consent. However, during interviews, parents described how their concerns were not specific to the proposed FEVER RCT, as they would not be happy with any alternative to informed consent for research.
Some parents stated that they had provided informed consent for the FEVER pilot RCT. Although it was found that most did support the use of RWPC, this finding suggests that there were instances in which either the RWPC process was not followed by staff, the trial consent process had not been clearly communicated or parents did not have the capacity to understand information provided in the critical care situation. This finding is perhaps not surprising as at the point of discussing the trial the child’s clinical care may not have been affected for the purposes of the trial. Indeed, if a child’s temperature had not reached a ‘standard care’ threshold (e.g. < 37.5 °C) when staff broached the trial, then parental decision-making was not dissimilar to a prospective informed consent decision as nothing had happened at that point in time, apart from online randomisation. This interpretation, coupled with concerns voiced about children being in pain or discomfort in the permissive threshold group, may help to explain the higher decliner rate in this group.
Outcomes
Combined data on parent-centred outcomes from the qualitative and integrated-perspective studies suggest that, apart from survival, the following outcomes should be prioritised for measurement in the proposed FEVER RCT: length of time on mechanical ventilation, long-term effects of illness on child (physical and developmental), looking and/or behaving like their normal self (examples included improved mood, improved communication, being more like themselves and more alert, sitting up and starting to eat and drink), not being in discomfort and/or pain, number of days spent in the PICU and hospital, vital signs back to normal (e.g. heart rate, breathing rate and temperature) and effect on family (e.g. emotional, functional and financial). Interestingly, as also shown in the FiSh feasibility study,17 parents who were interviewed shortly after their child’s admission in the FEVER pilot RCT prioritised shorter-term outcomes, which were potentially most salient to them at the time of being interviewed. This is in contrast to the prioritisation of longer-term outcomes, such as disabilities, by parents in the FEVER qualitative study, who were interviewed a mean of 19 months after their child’s hospital admission. These findings add further evidence to suggest that parents’ prioritisation of outcomes may be influenced by their experience of their child’s illness, survival of their child and the point at which they are asked about outcomes of importance in the course of their child’s illness. These findings should be considered in any future priority setting or design of core outcome set development work.
Site staff perspectives
Overall, the majority of clinicians viewed the trial and its use of RWPC to be acceptable as well as practically possible to conduct. Many staff confirmed their support for the proposed FEVER RCT, often stating that this trial was needed to provide an answer to an important research question.
Staff viewed the restrictive temperature threshold to be acceptable. This finding was in stark contrast to those reported in the FEVER qualitative study (see Chapter 2), in which the majority of participants had concerns about unnecessarily treating children with an antipyretic. Although the FEVER pilot RCT staff sample was larger, the majority of participants had also taken part in the FEVER qualitative study. As shown in previous critical care research, staff experience of conducting a trial can help address negative preconceptions about how parents may respond to the trial and its use of RWPC. As described by one nurse (P06, FG1), ‘seeing is believing’. Parents’ positive responses to their child’s participation in the restrictive group of the FEVER pilot RCT appeared to influence staff views on the acceptability of administering an antipyretic at a lower temperature than their usual practice. In the permissive group, staff described how parents appeared to value staff explanations about the use of alternative medication for pain. In line with the parent perspective findings, staff described how some parents were interested in the trial question and supported the study as they wished to help inform future treatments for children with a fever and suspected infection.
Those who were positive about both temperature thresholds appeared to value how parent and staff perspectives had been incorporated into the FEVER pilot RCT design. Changes made by the FEVER team, such as the selection of a FEVER pilot RCT permissive temperature threshold of 39.5 °C rather than 40 °C, and amending inclusion criteria to require that patients are mechanically ventilated, had positively influenced staff views on trial acceptability. Some research nurses responsible for the dissemination of training felt that being able to show how FEVER qualitative study findings had informed the protocol had helped to facilitate engagement of colleagues in the FEVER pilot RCT.
Nevertheless, polarised staff views about the acceptability of the permissive temperature threshold were found. Approximately half of the sample indicated that the 39.5 °C threshold was acceptable, whereas just under half did not. Those who raised concerns did not find the permissive threshold acceptable when a child was perceived to be uncomfortable or in pain. This had resulted in staff administering paracetamol or actively cooling a child before their temperature had reached the permissive threshold. As previously described, parents valued staff decisions to administer paracetamol when they felt it was needed. To help address this issue, suggestions were made to exclude patients not on mechanical ventilation (e.g. those on high-flow nasal oxygen) and to end trial participation when sedation is being weaned in preparation for extubation.
The majority of clinicians positively rated site training, which they felt prepared them for recruitment and consent in the FEVER pilot RCT. An important finding was that staff who viewed the FEVER pilot RCT training to be poor were often trained by their colleagues rather than the FEVER team at the site initiation meeting. It was also found that staff trained by their colleagues were more likely to find the permissive threshold unacceptable when compared with staff trained at the site initiation meeting. These staff remained unclear about the scientific rationale for the study, which negatively influenced their views on the acceptability of the trial. Although half of participants did not experience problems adhering to the protocol, others raised uncertainties or misunderstandings about what constituted active cooling, which led to protocol deviations.
As shown in Chapter 5, the FEVER pilot RCT recruitment target was reached ahead of time, yet the findings of this research suggest that in a future trial, recruitment rates could be further increased. Collaborative working between retrieval teams and clinical and research staff assisted the identification of eligible patients at sites. However, the FEVER pilot RCT was set up during a very busy winter period, which led to difficulties in ensuring that all clinical staff and retrieval teams were able to attend training. One retrieval team was unable to engage in training and limited research support meant that screening did not always taking place at evenings and weekends; therefore, eligible patients were missed. Nurses played an important role in identifying patients yet were unable to randomise until a GCP-trained doctor was present. Many nurses were enthusiastic about the study and wanted to play a more active role in randomisation in a future trial.
When combined, these findings suggest the need to focus on the content, timing and dissemination of training in the proposed FEVER RCT. Suggestions to help improve recruitment and trial conduct for the proposed trial include additional research support, GCP training for senior nurses so they can randomise patients and a comprehensive training package that can be disseminated to all staff. This research identified that training content in the proposed FEVER RCT should include clarification on what constitutes active cooling, the scientific rationale and existing evidence that informed the study question, presentation of the findings on parents’ and staff perspectives about trial acceptability, and any changes made to the FEVER RCT protocol based on these findings.
Overall, the findings of this research highlight the value of doing pre-trial research to inform the design of challenging clinical trials. 40 Incorporation of these findings into staff training, and the protocol for the proposed FEVER RCT, should help assist staff buy in to improve recruitment, protocol adherence and, importantly, ensure that the trial is patient centred.
Chapter 7 Discussion and conclusions
Principal findings
The FEVER qualitative study
The FEVER qualitative study provided insight into the acceptability of a definitive FEVER RCT by exploring the views of parents with relevant experience and site staff at the four pilot RCT sites.
Interviews were conducted with 25 parents – 20 mothers (four bereaved) and five fathers (two bereaved) – of children admitted to a UK PICU in the preceding 3 years. Overall, parents supported the conduct of a definitive FEVER RCT. Despite describing support for the study, concerns were raised regarding the acceptability of the permissive temperature threshold, with parents voicing specific concerns regarding pain and discomfort, and if not treating a child’s temperature would increase the likelihood of seizures. Owing to these concerns, the majority of parents suggested that a permissive temperature threshold of 39.5 °C would be more acceptable than 40 °C. In line with previous research and guidance,18 RWPC was considered to be an appropriate methodology by parents with all stating that they would consent for the use of their child’s information in the study.
Parents’ views on which potential outcome measures they perceived to be the most important were sought. Parents prioritised the following outcomes: (1) long-term morbidity, (2) looking and behaving more normally, (3) length of time on breathing support, (4) time in a PICU and hospital and (5) how quickly vital statistics are back to normal. Parents’ views on outcomes were incorporated into potential outcome measures collected in the pilot RCT. Interestingly, parents rarely prioritised survival as an outcome. This finding was also observed in the recent FiSh feasibility study. 17 As it is surprising that all parents would not prioritise survival as the most important outcome, this finding was further explored with parents of children involved in the pilot RCT.
Fifty-six clinical staff from the four sites involved in the pilot RCT took part in six focus groups. Site staff had concerns regarding the temperature thresholds, suggesting that 37.5 °C for the restrictive temperature group was too low for the administration of antipyretic intervention. Staff also echoed parents’ concerns regarding the permissive temperature threshold and were concerned about not using paracetamol for analgesia in the less unwell, spontaneously breathing patients, who may be in pain or discomfort. Many staff found RWPC acceptable for the proposed trial; however, concerns were raised regarding acceptability to parents of participants randomised to the permissive group.
Overall, the clinical trial protocol was considered acceptable by most parents and staff.
Qualitative study findings were used to develop the pilot RCT protocol, including reducing the higher temperature threshold, narrowing inclusion criteria to require that patients were mechanically ventilated, revising participant information materials and developing a site staff training package. To help address staff concerns about how parents may react to trial discussions, parent perspectives were communicated in site training, highlighting parental acceptability of RWPC, temperature thresholds, parents’ questions and concerns about the study and, importantly, suggestions on how staff could address such questions.
The FEVER observational study
The FEVER observational study, conducted in 22 PICUs from March to August 2017, identified a potentially eligible population of > 3000 cases per year or 10.9 (95% CI 10.3 to 11.5) eligible cases per site per month. The high number of potential patients permitted consideration of adjustments of study design by testing the impact of more stringent inclusion criteria. Importantly, mandating invasive mechanical ventilation rather than including non-invasive ventilation and high-flow humidified oxygen reduced the eligible patient population to around 2000 per year or 7.6 (95% CI 7.1 to 8.1) per site per month.
Wide variation in the temperature thresholds associated with antipyretic interventions was observed both across participants and across sites; however, the majority of critically ill children with a maximum temperature of ≥ 37.5 °C received antipyretic interventions on the first 5 days in a PICU. These findings remained consistent when the inclusion criteria were narrowed to include only mechanically ventilated or invasively mechanically ventilated patients.
The PICU mortality in the potentially eligible population was around 5%, increasing to 5.5% and 6.5% in mechanically ventilated and invasively mechanically ventilated patients, respectively. PICU length of stay and duration of mechanical ventilation and cardiovascular support also increased with narrowing inclusion criteria, whereas the number of days alive and free from a PICU/mechanical ventilation reduced.
The FEVER pilot randomised controlled trial
The FEVER pilot RCT was successfully delivered with 100 eligible children randomised from four PICUs between September and December 2017. Consistent with the larger than expected eligible population in the FEVER observational study, recruitment was completed ahead of schedule. The observed recruitment rate of 11.1 participants per site per month was almost double the predicted rate of 6.25 participants per site per month, with > 70% of eligible children included in the study. One-third of the parents/legal representatives of children allocated to the permissive group who were approached for consent either refused or subsequently withdrew consent to the intervention, compared with only 6% in the restrictive group. Refusals and withdrawals were more common among parents/legal representatives who completed the consent procedures early in the PICU stay. The refusals of consent included eight parents/legal representatives who did not give consent for routine data to be used in the study, and for a further five it was not possible to approach the parent/legal representative to obtain consent and so data for these 13 children could not be included in the analysis.
Non-adherence to the temperature thresholds was reported in 6% of the 6-hour time periods when participants were receiving mechanical ventilation in the permissive group and 7% in the restrictive group. Overall, 39% of participants in the permissive group and 55% in the restrictive group were non-adherent at least once during the course of their PICU stay. The most frequent reason given for receiving antipyretic intervention when below the threshold was the administration of paracetamol for pain and discomfort when receiving non-invasive ventilation or high-flow oxygen or when being weaned from invasive mechanical ventilation. When restricting the analyses to non-adherence solely while participants were receiving invasive mechanical ventilation, the proportion of 6-hour time periods when antipyretic interventions were received when below the temperature threshold in the permissive group halved from 6% to 3%. Despite the observed non-adherence, the difference in maximum temperature over the first 48 hours following randomisation achieved the prespecified separation of 0.5 °C between the groups (95% CI 0.2 to 0.8 °C).
There were no SAEs reported in either treatment group. There was a single PICU death in each group and a further one participant in each group died after PICU discharge. PICU length of stay, number of days alive and free from PICU and mechanical ventilation, and the duration of organ support were similar between the groups; however, as a pilot, the trial was not powered to detect differences in clinical outcomes.
Integrated-perspectives study
A total of 60 parents (49 mothers and 11 fathers) of 57 children recruited to the FEVER pilot RCT took part in the integrated-perspectives study, of whom 41 completed a questionnaire, 12 took part in an interview and 7 took part in both parts. Most parents supported the proposed FEVER RCT and thought that the proposed trial was important. No parents referred to the temperature their child reached when discussing trial acceptability. However, parents only viewed the permissive threshold as being acceptable if their child was not in pain or discomfort. Concerns about pain or discomfort were cited as reasons for non-consent and withdrawal among parents of children randomised to the permissive group. Nevertheless, both interview and questionnaire data from parents who declined their child’s participation in different elements of the pilot RCT still supported the conduct of the proposed FEVER RCT. One-third of parents were surprised that their child had been entered into the pilot RCT; however, the vast majority (89%) were satisfied with the RWPC approach and supported its use.
Parents in the integrated-perspectives study prioritised the following outcomes: (1) length of time on breathing support, (2) not in pain and/or discomfort, (3) how quickly vital statistics are back to normal, (4) looking and/or behaving like normal self, (5) length of time in hospital and (6) number of re-admissions to hospital. However, when prompted by the researcher all except one parent stated that survival is the most important outcome measure for the proposed FEVER RCT.
Ninety-eight members of staff at the four FEVER pilot RCT sites took part in the integrated-perspectives study, with half completing an online survey and half attending a focus group. Overall, staff supported the proposed FEVER RCT and thought that the research question was important. Staff found the restrictive threshold of 37.5 °C acceptable; however, only just over half indicated that the permissive threshold of 39.5 °C was acceptable. Staff trained by the study team, which included the chief investigator, were more likely to find the permissive threshold acceptable than staff trained by the trial team or who received ‘secondary’ training by site staff. When questioned, 46% of staff admitted difficulty in adhering to the protocol. The majority of difficulties related to willingness of staff to not treat pain and discomfort, resulting in staff administering paracetamol or actively cooling a child before their temperature had reached the permissive threshold.
Interpretation
Barriers to delivering the definitive FEVER randomised controlled trial
Synthesising the results from the multiple studies identifies a number of barriers to delivering the definitive FEVER RCT, but also informs how these barriers may be overcome (Table 25).
| Barriers identified | Reasons attributed | Suggested modifications to study design and treatment protocol |
|---|---|---|
| Rate of non-consent and withdrawal in permissive group | Refusals of consent and withdrawals as parents wanted their child to be treated with paracetamol for pain/discomfort | Restrict intervention to while the patient is invasively ventilated |
| Lack of understanding of the rationale for the study, particularly among staff receiving secondary training from local site staff | Improved training of site teams (e.g. using data from the feasibility studies to underpin the usual practice and acceptability to parents and providing additional resources to use for secondary training) | |
| Consent procedures completed early in the PICU stay | Ensure that the procedure and rationale for approaching later is clear | |
| Improved training of site teams to ensure that (1) parents are approached at an appropriate time, not too soon after their child’s admission,18 and (2) they can address parents’ questions about the study after seeing study posters | ||
| High proportion at one site (7/10 compared with 8/39) | Improve tailoring of site training to local context | |
| Proportion (13%) of patients whose data could not be used in the analysis | Patients for whom parents/legal representatives could not be approached | Seek approval to use data from the clinical record and routine data sources (e.g. PICANet and death registrations) without explicit consent (while ensuring that parents/legal representatives are informed about the use of their child’s data and retain the ability to opt out) |
| Parents/legal representatives refusing consent to study continuation, which included use of data already collected, despite their primary concern being with the intervention rather than use of data | ||
| Protocol adherence in the permissive group, in which 37% of patients received antipyretic intervention with a temperature of < 39.5 °C | Treatment for discomfort on non-invasive ventilation or high-flow humidified oxygen | Restrict intervention to while the patient is invasively ventilated |
| Misunderstanding of the allowance for external/other cooling measures (e.g. adding fans or removing blankets) | Increased clarity in training package and documentation | |
| Protocol adherence in the restrictive group, in which 41% of patients did not receive antipyretic intervention when their temperature was ≥ 37.5 °C | Possible lack of documentation on external/other cooling in the patient record | Improve training package (e.g. using data from the feasibility studies to underpin the usual practice and acceptability to parents, as well as what constitutes external/other cooling and how to ensure that this is documented) |
| Overstrict definition of non-adherence | Improve site monitoring to focus on important deviations from the study protocol |
A major concern is the acceptability of the temperature threshold in the permissive group. Both parents and clinicians raised concerns regarding the permissive temperature threshold in the qualitative elements of the study, suggesting that it is only acceptable if the child is not in pain or discomfort. This is likely to have resulted in higher rates of both non-consent and withdrawal in this treatment group and a higher proportion of patients being treated with antipyretics when below the temperature threshold. The findings suggest that this could be addressed in the definitive RCT by restricting the inclusion criteria to only those patients who were receiving invasive mechanical ventilation (comprising 80% of ventilated patients with a temperature of ≥ 37.5 °C in the FEVER observational study and 90% of patients recruited to the FEVER pilot RCT) and ceasing the intervention when beginning to wean the patient from invasive ventilation.
The integrated-perspectives study identified a lack of acceptability of the permissive threshold among some site staff, particularly those not trained directly by the FEVER trial team, which may therefore have stemmed from a lack of understanding of the rationale for the study. This is likely to have been transmitted to parents when seeking consent, contributing to the higher rates of non-consent. This may be addressed through improved training of the site teams, making use of data from the feasibility studies to underpin the variation in usual practice, the importance of the research question and acceptability of the trial to parents. In addition, further resources should be made available for use during secondary training by local site staff, such as a video from the chief investigator explaining the rationale for the study, example study and consent explanations and answers to frequently asked questions.
Despite advice to not approach parents about the study until it was considered an appropriate time, many parents were approached on the first day their child was in a PICU, and the rates of non-consent and withdrawal were much higher when the consent procedure was completed on the first day. The site training (including resources for secondary training) must reinforce the rationale for not approaching the parents too early. In addition, the high visibility of the study within the PICU (e.g. stickers within the bed space) prompted some parents to ask questions to the bedside nurses before the site team had the opportunity to properly explain the study at an appropriate time. The need to ensure that the clinical team are aware of the treatment allocation and adhere to the assigned threshold needs to be balanced against the additional concerns that this visibility may create when the child’s condition has not stabilised.
Non-consent and withdrawal were particular issues in one of the four study sites and, although this may just have been bad luck (e.g. the parents would have not provided/withdrawn consent regardless of the study being conducted and approach to consent), the site team considered that this could have been attributable in part to their local approach to sedation. Tailoring of the site training to specific local needs – in this case, any adjustment required to their usual approach to sedation and analgesia to facilitate delivery of the study – may have helped to mitigate these issues.
A notable proportion (13%) of recruited patients could not be included in the analysis either because of not being able to approach parents/legal representatives to obtain consent or as a result of a complete refusal of consent for the study. Although the first of these situations will reduce the power of the study, the second is of greater concern as increased refusals in the permissive group may introduce a bias in the study results, particularly if these refusals relate to the patient’s ongoing clinical trajectory in the time period between randomisation and the approach for consent. As well as damaging the scientific integrity of the study, excluding participants who have been randomised and received the intervention raises ethical concerns as, for example, if consent was refused following an AE caused by the intervention, then potential safety signals would be missed. Therefore, there is a strong rationale to seek approval for the definitive RCT to use data from the clinical record and other routine sources (including PICANet and death registrations) without explicit consent, while ensuring that parents/legal representatives are fully informed of any processing of data whenever possible and retain the option to opt out. We would seek consent for conducting longer term quality-of-life follow-up with the participant.
Although non-adherence to the higher temperature threshold in the permissive group happened in only 6% of the 6-hourly monitoring time periods, these affected over one-third of patients at some point during their stay. Around half of the non-adherence was owing to paracetamol being used for analgesia among patients receiving non-invasive ventilation or high-flow oxygen, or who were being weaned from invasive mechanical ventilation, and, therefore, the proposed change to include only invasively mechanically ventilated patients would address this. The remaining instances of non-adherence were mainly attributable to misunderstandings of what did or did not constitute ‘external cooling’ or ‘other cooling’, which may be improved by closer attention in site training. Cooling was pragmatically defined in the study as ‘any action taken with the express intention to reduce body temperature’. These measures, which may be as simple as removing a blanket, were also not reliably documented in the patient record, making retrospective collection and validation of study data for these items difficult.
Lack of understanding, and lack of documentation, of cooling measures may also have contributed to the apparently high rate of non-adherence in the restrictive group, in which 41% of patients had at least one 6-hour time period during which their temperature was > 37.5 °C and they did not receive any antipyretic intervention. However, this definition of non-adherence was very strict, with over half (65%) of occurrences of non-adherence being for patients who received paracetamol during one 6-hour time period and then did not receive a subsequent dose during the next 6-hour time period, often as their temperature was already dropping and a number of participants (15%) with a brief, transient spike in temperature. Indeed, only 11 participants (four in the permissive group and seven in the restrictive group) had two or more consecutive periods of non-adherence. The fact that, for most participants in the restrictive group, temperatures dropped below the threshold relatively quickly and the prespecified separation in temperature was achieved indicates that adherence was not as bad as the headline figure of 41% would suggest. For a large trial, it would be necessary to refine the adherence monitoring to identify the most important non-adherence events, for example, when antipyretic interventions were not given for an extended period of time.
Primary outcome measure
With regard to the choice of a primary outcome measure for a definitive RCT, opinions of parents differed between those in the FEVER qualitative study whose experience of a PICU had been some time previously (and who favoured outcomes related to longer-term recovery) and those in the integrated-perspectives study whose experience was more recent (and who favoured outcomes related to time on machines). Combined data on parent-centred outcomes from the qualitative and integrated-perspectives studies suggest that, apart from survival, the following outcomes should be prioritised for measurement in the proposed FEVER RCT: length of time on mechanical ventilation, long-term effects of illness on child (physical and developmental), looking and/or behaving like their normal self, not in discomfort and/or pain, number of days spent in the PICU and hospital, vital signs back to normal and effect on family.
Mortality was 5.0–6.5% across the populations considered, and, therefore, there would not be a sufficient number of events to power a trial to detect a feasible reduction in mortality as a primary outcome. Longer-term functional and developmental outcomes are difficult to assess in this population owing to the wide age range and the pre-existing impairments of many patients. Consequently, the recommended primary outcome for the trial (consistent with the top-prioritised outcome from parents across the qualitative and integrated-perspectives studies) would be either number of days alive and free of mechanical ventilation or time to liberation from mechanical ventilation (accounting for a competing risk of death).
Table 26 illustrates alternative scenarios for the sample size of a definitive trial based on the number of days alive and free of mechanical ventilation. The calculations assume 28-day mortality in the restrictive group of 6% and a mean duration of mechanical ventilation of 7 days among survivors, based on data from patients receiving invasive mechanical ventilation with a temperature of ≥ 37.5 °C in the FEVER observational study. This corresponds to a mean of (28 – 7) × (1 − 0.06) = 19.74 days alive and free of mechanical ventilation. A SD of 8 days was assumed for all calculations, based on the FEVER observational study. This results in required sample sizes of between 750 and 3000 patients, depending on the assumptions regarding effect size and power. Based on an average rate of eligible patients of 8.5 per site per month (from the FEVER observational study, excluding the four smallest sites, which would not participate in a trial), with recruitment of 60% of eligible patients (73% achieved in the FEVER pilot RCT) and allowing for 13% withdrawal (as observed in the FEVER pilot RCT), 10 sites would achieve these sample sizes after recruiting for between 17 and 69 months and 15 sites would achieve these sample sizes after recruiting for between 12 and 46 months.
| Absolute reduction in mortality (%) | Absolute reduction in mean duration of MV (days) | Mortality in permissive group (%) | Mean duration of MV in permissive group (days) | Mean number of days alive and free of MV in permissive groupa | Treatment effectb | Total sample size for | |
|---|---|---|---|---|---|---|---|
| 80% power | 90% power | ||||||
| 0.00 | 1.00 | 6.00 | 6.00 | 20.68 | 0.94 | 2276 | 3046 |
| 0.50 | 1.00 | 5.50 | 6.00 | 20.79 | 1.05 | 1824 | 2440 |
| 1.00 | 1.00 | 5.00 | 6.00 | 20.90 | 1.16 | 1494 | 2000 |
| 0.00 | 1.25 | 6.00 | 5.75 | 20.92 | 1.18 | 1456 | 1950 |
| 0.50 | 1.25 | 5.50 | 5.75 | 21.03 | 1.29 | 1216 | 1626 |
| 1.00 | 1.25 | 5.00 | 5.75 | 21.14 | 1.40 | 1030 | 1378 |
| 0.00 | 1.50 | 6.00 | 5.50 | 21.15 | 1.41 | 1012 | 1354 |
| 0.50 | 1.50 | 5.50 | 5.50 | 21.26 | 1.52 | 868 | 1162 |
| 1.00 | 1.50 | 5.00 | 5.50 | 21.38 | 1.64 | 752 | 1008 |
Strengths and limitations
The FEVER qualitative study
The FEVER qualitative study recruited rapidly and achieved saturation with opinions from both parents and clinical staff. Overall, the findings supported the proposed FEVER RCT including the use of RWPC and were used to inform the methods and conduct of the FEVER pilot RCT. However, the majority of parents were recruited via social media advertising, with no parents being recruited directly from a PICU, and, therefore, the findings may be biased towards parents with a pre-existing interest in research and may not be representative of the population recruited into the trial.
The FEVER observational study
The FEVER observational study had the great strength of over-recruiting sites above the original plan, with 22 out of the 27 UK PICUs participating. No previous prospective study of UK PICUs has achieved this level of national coverage. This provides strong support for the importance of the question of fever thresholds to PICU staff. This study also confirmed that data linkage to PICANet is a highly efficient approach to study design. Despite only recruiting in spring and summer months, this research was able to identify high numbers of patients who would be suitable for inclusion in a clinical trial. However, that fact that data collection took place over a short period of time means that it did not capture the full range of seasonal variability that may happen in emergency admissions, and, therefore, the results may not be fully representative of the population of a future RCT. Finally, although the linked observational study design enabled a large number of patients to be recruited, this made it impossible to distinguish the reasons why particular interventions were being delivered, for example, whether paracetamol was given as an antipyretic or for pain relief, limiting the ability to draw definitive conclusions regarding thresholds for antipyretic management in usual practice.
The FEVER pilot randomised controlled trial
The FEVER pilot RCT was the first attempt to compare fever thresholds in critically ill children globally and was delivered successfully. In particular, the FEVER pilot RCT recruited within a little over two-thirds of the planned recruitment period despite some delays in site approvals. The inclusion and exclusion criteria were easily followed by site staff and a high completeness of outcome data was achieved. The between-group separation in maximum temperature in the first 48 hours of 0.5 °C was greater than many clinicians expected and compares favourably with other studies in this field. 10,41 No safety concerns were identified. Although recruitment ahead of schedule is a strength of the pilot RCT, the fact that the recruitment period was conducted over a different period to the FEVER observational study is an important limitation. Seasonal differences in admissions (i.e. ≈50% of admissions for bronchiolitis in the pilot RCT) may have resulted in differences between the potential trial populations included in the observational and pilot studies, in which 49% of mechanically ventilated patients (population 3) were < 1 year old in the observational study, compared with 64% being 1 year old in the pilot RCT. Therefore, this may misrepresent the true population of a definitive RCT that would recruit year round. However, despite this, the prognostic variables of interest appear to be consistent across the study populations. Another important weakness is the proportion of parents who declined consent to continue in the study, especially in the permissive group. If this were to be reproduced in a full clinical trial, this would threaten the validity of any result. Finally, the relatively high level of non-adherence observed in both groups is a weakness of the pilot RCT, although this may be somewhat mitigated through restricting the inclusion criteria and intervention period to both recruit patients and deliver the intervention only when invasively ventilated.
Integrated-perspectives study
The findings are strengthened by the involvement of parents with direct experience of their child being enrolled into the FEVER pilot RCT, as well as staff involved in recruitment and consent procedures and the clinical care of randomised children. The recruitment targets were either reached or exceeded, involving 60 parents of 57 out of 100 patients. Insight was gained into the views of 8 out of 18 parents (44%) who had declined their child’s continued participation in one or more aspect of the pilot RCT. In particular, the interviews with parents who declined consent and nursing staff who found the protocol challenging to follow provided valuable information to assist with refining the study process for a definitive RCT. However, it is unknown whether or not the predominantly positive views of the declining parents who took part in an interview or questionnaire were shared by other parents who declined the FEVER pilot RCT and also declined participation in the integrated-perspectives study. In addition, the insight into the consent process was limited by the subjective recollections of parents and practitioners rather than insight gained through observations or the analysis of audio-recoded consent discussions. Nevertheless, the findings were strengthened by the integrated-perspectives mixed-methods approach, providing a multiperspective understanding of parent and site staff opinions and experiences of RWPC, specifically in the context of the FEVER pilot RCT.
Summaries of key research recommendations
A definitive trial of permissive versus restrictive temperature thresholds for antipyretic intervention using the FEVER protocol tested here should not be conducted
Our studies identified major concerns with respect to the feasibility of a definitive trial using the same protocol as the FEVER pilot RCT, particularly with regard to the acceptability of the intervention among less sick children and the use of paracetamol as analgesia for patients who were not invasively ventilated. As a result, our recommendation is to not attempt a definitive trial with this protocol.
A definitive trial of permissive versus restrictive temperature thresholds for antipyretic intervention using a modified protocol should be conducted
The thorough, mixed-methods approach to evaluating feasibility conducted here has confirmed both the continuing importance of the research question to parents and to clinical staff and the size of the population with the potential to benefit. Approaches to temperature management vary widely but are largely very restrictive. The unmet need of evidence to guide this very common intervention is therefore confirmed.
We recommend that a definitive trial is undertaken to answer this important question with some key protocol amendments, as outlined previously, that will increase the acceptability of the trial protocol to parents and bedside staff and improve the retention of children in the trial.
The definitive trial should include an internal pilot phase to ensure that these amendments have achieved the desired effect.
Further international work is required to agree important outcome measures and/or develop new outcome measures for clinical trials among critically ill children
Although we were able to identify outcomes that were important to parents, the mixed-methods approach that we undertook highlighted the difficulty of selecting a primary outcome that is measurable, free from bias and with which it would be feasible to detect a plausible treatment effect. We recommend further research to achieve international consensus on a core outcome measurement set42 for paediatric intensive care and, if required, to develop new measures and instruments, or further validate existing measures and instruments, to address any gaps identified during this process.
Implications for health care/practice
As a feasibility study, this project has no direct implications for health care or practice.
Acknowledgements
We wish to thank the NIHR Health Technology Assessment programme for funding this trial. We also wish to thank all the patients and staff from the sites that participated in the trial. Thank you to all the staff at PICANet and ICNARC, and special thanks to Caroline Lamming, Lee Norman, Martin Perkins (PICANet) and Joe Collins (ICNARC).
Research staff at participating sites
We acknowledge that there have been many other individuals who made a contribution within the participating sites. It is impossible to thank everyone personally; however, we would like to thank the following research staff:
-
Addenbrooke’s Hospital – Nazima Pathan and Deborah White
-
Alder Hey Children’s Hospital – Kentigan Thorburn, Dawn Jones and Laura Walsh
-
Birmingham Children’s Hospital – Barney Scholefield and Julie Menzies
-
Bristol Royal Hospital for Children – Peter Davies and Anna Laskey
-
Evelina London Children’s Hospital – Shane Tibby and Paul Wellman
-
Great Ormond Street Hospital – Lauran O’Neill, Guilia Scarpa, Holly Bellfield and Samiran Ray
-
Great North Children’s Hospital – Rachel Agbeko, Christine Mackerness and Lindsey Cooper
-
James Cook University Hospital – Jonathan Grimbley and Maxine Stephens
-
King’s College Hospital – Akash Deep
-
Leicester Children’s Hospital – Ramaiah Raghu and Wendy McCabe
-
Nottingham Children’s Hospital – Patrick Davies and Stephanie Larter
-
John Radcliff Hospital – James Weitz and Kirsten Beadon
-
Royal Belfast Hospital for Sick Children – Stewart Reid and Carolyn Green
-
Royal Brompton Hospital – Duncan Macrae and Angela Aramburo
-
Royal Hospital for Children, Glasgow – Richard Levin
-
Royal London Hospital – Kalai Sadasivam and Leanne Reardon
-
Royal Manchester Children’s Hospital – Peter-Marc Fortune and Claire Jennings
-
Southampton General Hospital – John Pappachan and Danny Pratt
-
St George’s Hospital – Nick Prince
-
St Mary’s Hospital – David Inwald and Calandra Feather
-
Royal Stoke Hospital – John Alexander and Rachel Pringle
-
University Hospital of Wales – Siva Oruganti.
Contributions of authors
Professor Mark J Peters (Professor of Paediatric Intensive Care) conceived and designed the studies, contributed to the acquisition and analysis of data and drafted and critically revised the manuscript.
Mr Imran Khan (Project Manager) managed the studies, contributed to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Dr Kerry Woolfall (Senior Lecturer) conceived and designed the qualitative elements of the study, contributed to acquisition, analysis and interpretation of the data and drafted and critically revised the manuscript.
Dr Elizabeth Deja (Research Associate) carried out the qualitative elements of the study, contributed to acquisition, analysis and interpretation of the data and drafted and critically revised the manuscript.
Mr Paul R Mouncey (Head of Research) contributed to the design of the study and the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Dr Jerome Wulff (Statistician) contributed to the analysis and interpretation of the data and critically reviewed the manuscript.
Dr Alexina Mason (Statistician) contributed to the analysis and interpretation of the data and critically reviewed the manuscript.
Dr Rachel Agbeko (Consultant in Paediatric Intensive Care) contributed to the design of the study and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Professor Elizabeth S Draper (Professor of Perinatal and Paediatric Epidemiology) contributed to the design of the trial and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Ms Blaise Fenn (Patient Representative) provided patient input into the study, contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Dr Doug W Gould (Senior Researcher) contributed to the analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Ms Abby Koelewyn (Data Manager) contributed to the analysis and interpretation of the data and critically reviewed the manuscript.
Professor Nigel Klein (Professor of Infectious Disease and Immunology) contributed to the design of the study and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Ms Christine Mackerness (Senior Sister/Research Nurse) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Ms Sian Martin (Study Co-ordinator) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Ms Lauran O’Neill (Critical Care Research Nurse) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Dr Padmanabhan Ramnarayan (Consultant in Paediatric Intensive Care) contributed to the design of the study and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Dr Shane Tibby (Consultant in Paediatric Intensive Care) contributed to the design of the study and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Dr Lyvonne Tume (Associate Professor of Child Health and Intensive Care Nursing) contributed to the design of the trial and to the acquisition, analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Mr Jason Watkins (Parent Representative) provided parental input into the study, contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Dr Kent Thorburn (Consultant in Paediatric Intensive Care) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Mr Paul Wellman (Senior Paediatric Intensive Care Research Nurse) contributed to the acquisition, analysis and interpretation of the data and critically reviewed the manuscript.
Professor David A Harrison (Head Statistician) contributed to the design of the study, the analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Professor Kathryn M Rowan (CTU Director) designed the trial, contributed to acquisition, analysis and interpretation of the data and drafted and critically revised the manuscript.
Study Management Group
Dr Elizabeth Deja (Research Associate), Dr Rachel Agbeko (Co-investigator), Professor Elizabeth S Draper (Co-investigator), Ms Blaise Fenn (Co-investigator), Ms Clara Francis (Co-investigator), Mr Imran Khan (Trial Co-ordinator), Professor Nigel Klein (Co-investigator), Dr David Harrison (Co-investigator), Ms Sian Martin (Research Assistant), Mr Paul Mouncey (Co-investigator), Professor Mark Peters (Chief Investigator), Dr Padmanabhan Ramnarayan (Co-investigator), Professor Kathryn Rowan (Co-investigator), Dr Shane Tibby (Co-investigator), Dr Lyvonne Tume (Co-investigator), Mr Jason Watkins (Co-investigator), Dr Kerry Woolfall (Co-investigator) and Dr Jerome Wulff (Study Statistician).
Trial Steering Committee
Dr Duncan Mcrae (independent, Chairperson), Dr Bronagh Blackwood (independent), Dr Balazs Fule (independent), Dr Joseph Manning (independent), Dr Nazima Pathan (independent), Mr Lewis Veale (independent), Professor Mark Peters (Chief Investigator) and Dr Kerry Woolfall (Co-investigator).
Data Monitoring and Ethics Committee
Dr Nicholas Prince (Chairperson), Ms Laura Pankhurst and Dr Barney Scholefield.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015;15:335-49. https://doi.org/10.1038/nri3843.
- Young PJ, Saxena M. Fever management in intensive care patients with infections. Crit Care 2014;18. https://doi.org/10.1186/cc13773.
- Zhu Y, Lu J, Wang J, Chen F, Leng F, Li H. Regulation of thermogenesis in plants: the interaction of alternative oxidase and plant uncoupling mitochondrial protein. J Integr Plant Biol 2011;53:7-13. https://doi.org/10.1111/j.1744-7909.2010.01004.x.
- Doran TF, De Angelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chickenpox?. J Pediatr 1989;114:1045-8. https://doi.org/10.1016/S0022-3476(89)80461-5.
- Brandts CH, Ndjavé M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in plasmodium falciparum malaria. Lancet 1997;350:704-9. https://doi.org/10.1016/S0140-6736(97)02255-1.
- Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975;231:1248-51. https://doi.org/10.1001/jama.1975.03240240018017.
- Fever in Under 5s: Assessment and Initial Management. London: NICE; 2013.
- Brick T, Agbeko RS, Davies P, Davis PJ, Deep A, Fortune P-M, et al. Too hot to handle: a survey of attitudes towards fever of 462 pediatric intensive care unit staff. Eur J Pediatr 2016;176:423-7. https://doi.org/10.1007/s00431-016-2844-1.
- Niven DJ, Stelfox HT, Laupland KB. Antipyretic therapy in febrile critically ill adults: a systematic review and meta-analysis. J Crit Care 2013;28:303-10. https://doi.org/10.1016/j.jcrc.2012.09.009.
- Young P, Saxena M, Bellomo R, Freebairn R, Hammond N, van Haren F, et al. Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med 2015;373:2215-24. https://doi.org/10.1056/NEJMoa1508375.
- Johansen ME, Jensen JU, Bestle MH, Ostrowski SR, Thormar K, Christensen H, et al. Mild induced hypothermia: effects on sepsis-related coagulopathy – results from a randomized controlled trial. Thromb Res 2015;135:175-82. https://doi.org/10.1016/j.thromres.2014.10.028.
- Egi M, Morita K. Fever in non-neurological critically ill patients: a systematic review of observational studies. J Crit Care 2012;27:428-33. https://doi.org/10.1016/j.jcrc.2011.11.016.
- Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care 2012;16. https://doi.org/10.1186/cc11211.
- Young PJ, Saxena M, Beasley R, Bellomo R, Bailey M, Pilcher D, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 2012;38:437-44. https://doi.org/10.1007/s00134-012-2478-3.
- Brick T. In PICU Patients With Suspected Infection, Fever Is Not Associated With Decreased Mortality n.d.
- Molyneux S, Njue M, Boga M, Akello L, Olupot-Olupot P, Engoru C, et al. ‘The words will pass with the blowing wind’: staff and parent views of the deferred consent process, with prior assent, used in an emergency fluids trial in two African hospitals. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0054894.
- O’Hara CB, Canter RR, Mouncey PR, Carter A, Jones N, Nadel S, et al. A qualitative feasibility study to inform a randomised controlled trial of fluid bolus therapy in septic shock. Arch Dis Child 2017;103:28-32. https://doi.org/10.1136/archdischild-2016-312515.
- Woolfall K, Frith L, Gamble C, Gilbert R, Mok Q, Young B. CONNECT Advisory Group . How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008522.
- Woolfall K, Frith L, Gamble C, Young B. How experience makes a difference: practitioners’ views on the use of deferred consent in paediatric and neonatal emergency care trials. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-45.
- Woolfall K, Young B, Frith L, Appleton R, Iyer A, Messahel S, et al. Doing challenging research studies in a patient-centred way: a qualitative study to inform a randomised controlled trial in the paediatric emergency care setting. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005045.
- Knapp P, Raynor DK, Silcock J, Parkinson B. Can user testing of a clinical trial patient information sheet make it fit-for-purpose? – a randomized controlled trial. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-89.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Braun V, Clarke V. What can ‘thematic analysis’ offer health and wellbeing researchers?. Int J Qual Stud Health Well-Being 2014;9. https://doi.org/10.3402/qhw.v9.26152.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45. https://doi.org/10.2307/798843.
- Strauss A CJ. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks, CA: Sage Publications Ltd; 1998.
- Patton MQ. Qualitative Research and Evaluation Methods. London: Sage Publications Ltd; 2002.
- Brady AR, Harrison D, Black S, Jones S, Rowan K, Pearson G, et al. Assessment and optimization of mortality prediction tools for admissions to pediatric intensive care in the United Kingdom. Pediatrics 2006;117:e733-42. https://doi.org/10.1542/peds.2005-1853.
- Woolfall K, Frith L, Dawson A, Gamble C, Young B. Research Without Prior Consent (Deferred Consent) in Trials Investigating the Emergency Treatmemt of Critically Ill Children: CONNECT Study Guidance. Liverpool: University of Liverpool; 2015.
- Roper L, Sherratt FC, Young B, McNamara P, Dawson A, Appleton R, et al. Children’s views on research without prior consent in emergency situations: a UK qualitative study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022894.
- Harron K, Woolfall K, Dwan K, Gamble C, Mok Q, Ramnarayan P, et al. Deferred consent for randomized controlled trials in emergency care settings. Pediatrics 2015;136:e1316-22. https://doi.org/10.1542/peds.2015-0512.
- Slater A, Shann F, Pearson G. Paediatric Index of Mortality (PIM) Study Group . PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003;29:278-85. https://doi.org/10.1007/s00134-002-1601-2.
- Network PICA. Annual Report of the Paediatric Intensive Care Audit Network: November 2016. University of Leeds and University of Leicester; 2016.
- Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, et al. Paediatric Index of Mortality 3. Pediatr Crit Care Med 2013;14:673-81. https://doi.org/10.1097/PCC.0b013e31829760cf.
- Morris MC, Nadkarni VM, Ward FR, Nelson RM. Exception from informed consent for pediatric resuscitation research: community consultation for a trial of brain cooling after in-hospital cardiac arrest. Pediatrics 2004;114:776-81. https://doi.org/10.1542/peds.2004-0482.
- Jansen TC, Kompanje EJ, Bakker J. Deferred proxy consent in emergency critical care research: ethically valid and practically feasible. Crit Care Med 2009;37:65-8. https://doi.org/10.1097/CCM.0b013e3181920851.
- Brierley J, Larcher V. Emergency research in children: options for ethical recruitment. J Med Ethics 2011;37:429-32. https://doi.org/10.1136/jme.2010.040667.
- Maitland K, Molyneux S, Boga M, Kiguli S, Lang T. Use of deferred consent for severely ill children in a multi-centre phase III trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-90.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18380.
- Ray S, Rogers L, Brown KL, Peters MJ. The effect of acetaminophen on temperature in critically ill children: a retrospective analysis of over 50,000 doses. Pediatr Crit Care Med 2018;19:204-9. https://doi.org/10.1097/PCC.0000000000001426.
- Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a ‘Core Outcome Set’ – a practical guideline. Trials 2016;17. https://doi.org/10.1186/s13063-016-1555-2.
Appendix 1 Proportion of patients meeting stage 1 and stage 2 criteria at each site
FIGURE 21.
Percentage of patients (a) meeting any stage 1 criteria and (b) with confirmed or suspected infection.
| Site | Number of unplanned admissions | Number of patients who completed stage 1 | Criteria, n/N (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Death imminent (yes/stage 1 completed) | Cardiopulmonary bypassa | Cardiomyopathy/myocarditisa | Malignant hyperthermiaa | Drug-induced hyperthermiaa | Acute encephaopathya | Neuroleptic malignant syndromea | Severe rhabdomyolysisa | Any stage 1 failed [yes for one or more of eight questions (% of those completed stage 1)] | Any stage 1 failed [yes for one or more of eight questions (% of those who completed stage 1)] | Stage 1 passed [no to all eight questions (% of stage 1 completed)] | |||
| Addenbrooke’s | 186 | 186 | 3/186 (1.6) | 0/186 (0.0) | 1/186 (0.5) | 0/186 (0.0) | 0/186 (0.0) | 10/186 (5.4) | 0/186 (0.0) | 0/186 (0.0) | 14/186 (7.5) | 1/186 (0.5) | 171/186 (91.9) |
| Alder Hey | 266 | 266 | 6/266 (2.3) | 0/266 (0.0) | 3/266 (1.1) | 0/266 (0.0) | 1/266 (0.4) | 20/266 (7.5) | 0/266 (0.0) | 0/266 (0.0) | 30/266 (11.3) | 21/266 (7.9) | 215/266 (80.8) |
| Belfast | 155 | 155 | 1/155 (0.6) | 0/155 (0.0) | 2/155 (1.3) | 0/155 (0.0) | 0/155 (0.0) | 43/155 (27.7) | 0/155 (0.0) | 0/155 (0.0) | 45/155 (29.0) | 1/155 (0.6) | 109/155 (70.3) |
| Birmingham | 453 | 378 | 1/378 (0.3) | 2/378 (0.5) | 5/378 (1.3) | 0/378 (0.0) | 0/378 (0.0) | 41/378 (10.8) | 0/378 (0.0) | 0/378 (0.0) | 49/378 (13.0) | 11/378 (2.9) | 318/378 (84.1) |
| Bristol | 207 | 207 | 3/207 (1.4) | 1/207 (0.5) | 5/207 (2.4) | 0/207 (0.0) | 1/207 (0.5) | 14/207 (6.8) | 0/207 (0.0) | 1/207 (0.5) | 25/207 (12.1) | 68/207 (32.9) | 114/207 (55.1) |
| Cardiff | 156 | 156 | 0/156 (0.0) | 1/156 (0.6) | 18/156 (11.5) | 0/156 (0.0) | 0/156 (0.0) | 51/156 (32.7) | 0/156 (0.0) | 0/156 (0.0) | 67/156 (42.9) | 1/156 (0.6) | 88/156 (56.4) |
| Evelina | 141 | 141 | 2/141 (1.4) | 2/141 (1.4) | 3/141 (2.1) | 1/141 (0.7) | 2/141 (1.4) | 7/141 (5.0) | 0/141 (0.0) | 0/141 (0.0) | 15/141 (10.6) | 0/141 (0.0) | 126/141 (89.4) |
| GNCH Newcastle | 218 | 218 | 0/218 (0.0) | 0/218 (0.0) | 3/218 (1.4) | 0/218 (0.0) | 0/218 (0.0) | 72/218 (33.0) | 0/218 (0.0) | 0/218 (0.0) | 75/218 (34.4) | 0/218 (0.0) | 143/218 (65.6) |
| GOSH | 273 | 273 | 4/273 (1.5) | 1/273 (0.4) | 7/273 (2.6) | 0/273 (0.0) | 1/273 (0.4) | 69/273 (25.3) | 0/273 (0.0) | 0/273 (0.0) | 78/273 (28.6) | 0/273 (0.0) | 195/273 (71.4) |
| Glasgow | 188 | 188 | 4/188 (2.1) | 0/188 (0.0) | 9/188 (4.8) | 0/188 (0.0) | 3/188 (1.6) | 15/188 (8.0) | 1/188 (0.5) | 0/188 (0.0) | 31/188 (16.5) | 3/188 (1.6) | 154/188 (81.9) |
| James Cook | 116 | 116 | 2/116 (1.7) | 0/116 (0.0) | 3/116 (2.6) | 0/116 (0.0) | 0/116 (0.0) | 0/116 (0.0) | 0/116 (0.0) | 0/116 (0.0) | 5/116 (4.3) | 3/116 (2.6) | 108/116 (93.1) |
| John Radcliffe | 192 | 192 | 4/192 (2.1) | 1/192 (0.5) | 2/192 (1.0) | 0/192 (0.0) | 0/192 (0.0) | 64/192 (33.3) | 0/192 (0.0) | 0/192 (0.0) | 69/192 (35.9) | 6/192 (3.1) | 117/192 (60.9) |
| King’s College | 163 | 139 | 0/139 (0.0) | 0/139 (0.0) | 0/139 (0.0) | 0/139 (0.0) | 0/139 (0.0) | 13/139 (9.4) | 0/139 (0.0) | 0/139 (0.0) | 13/139 (9.4) | 9/139 (6.5) | 117/139 (84.2) |
| Leicester | 89 | 81 | 0/81 (0.0) | 0/81 (0.0) | 1/81 (1.2) | 0/81 (0.0) | 0/81 (0.0) | 3/81 (3.7) | 0/81 (0.0) | 0/81 (0.0) | 4/81 (4.9) | 5/81 (6.2) | 72/81 (88.9) |
| Manchester | 244 | 244 | 3/244 (1.2) | 0/244 (0.0) | 17/244 (7.0) | 0/244 (0.0) | 0/244 (0.0) | 28/244 (11.5) | 0/244 (0.0) | 2/244 (0.8) | 50/244 (20.5) | 0/244 (0.0) | 194/244 (79.5) |
| Nottingham | 73 | 73 | 1/73 (1.4) | 0/73 (0.0) | 1/73 (1.4) | 0/73 (0.0) | 0/73 (0.0) | 4/73 (5.5) | 0/73 (0.0) | 0/73 (0.0) | 6/73 (8.2) | 0/73 (0.0) | 67/73 (91.8) |
| Royal Brompton | 133 | 131 | 0/131 (0.0) | 0/131 (0.0) | 12/131 (9.2) | 0/131 (0.0) | 0/131 (0.0) | 0/131 (0.0) | 0/131 (0.0) | 0/131 (0.0) | 12/131 (9.2) | 0/131 (0.0) | 119/131 (90.8) |
| Royal London | 149 | 140 | 4/140 (2.9) | 0/140 (0.0) | 1/140 (0.7) | 0/140 (0.0) | 0/140 (0.0) | 13/140 (9.3) | 0/140 (0.0) | 0/140 (0.0) | 18/140 (12.9) | 0/140 (0.0) | 122/140 (87.1) |
| Royal Stoke | 118 | 118 | 4/118 (3.4) | 0/118 (0.0) | 6/118 (5.1) | 0/118 (0.0) | 0/118 (0.0) | 32/118 (27.1) | 0/118 (0.0) | 0/118 (0.0) | 41/118 (34.7) | 0/118 (0.0) | 77/118 (65.3) |
| Southampton | 249 | 249 | 4/249 (1.6) | 0/249 (0.0) | 4/249 (1.6) | 1/249 (0.4) | 0/249 (0.0) | 28/249 (11.2) | 0/249 (0.0) | 0/249 (0.0) | 36/249 (14.5) | 0/249 (0.0) | 213/249 (85.5) |
| St George’s | 216 | 216 | 10/216 (4.6) | 0/216 (0.0) | 2/216 (0.9) | 0/216 (0.0) | 0/216 (0.0) | 6/216 (2.8) | 0/216 (0.0) | 0/216 (0.0) | 12/216 (5.6) | 1/216 (0.5) | 203/216 (94.0) |
| St Mary’s | 141 | 141 | 6/141 (4.3) | 0/141 (0.0) | 1/141 (0.7) | 0/141 (0.0) | 0/141 (0.0) | 51/141 (36.2) | 0/141 (0.0) | 0/141 (0.0) | 56/141 (39.7) | 0/141 (0.0) | 85/141 (60.3) |
| Total | 4126 | 4008 | 62/4008 (1.5) | 8/4008 (0.2) | 106/4008 (2.6) | 2/4008 (0.0) | 8/4008 (0.2) | 584/4008 (14.6) | 1/4008 (0.0) | 3/4008 (0.1) | 751/4008 (18.7) | 130/4008 (3.2) | 3127/4008 (78.0) |
| Site | Stage 1 passed (n) | Infection status at PICU admission, n/N (% of stage 1 passed) | Stage 2 passed, n/N (% of stage 1 passed) | |||
|---|---|---|---|---|---|---|
| Confirmed infection | Suspected infection | No infection | Unknown/missing | |||
| Addenbrooke’s | 171 | 11/171 (6.4) | 76/171 (44.4) | 83/171 (48.5) | 1/171 (0.6) | 87/171 (50.9) |
| Alder Hey | 215 | 6/215 (2.8) | 104/215 (48.4) | 105/215 (48.8) | 0/215 (0.0) | 110/215 (51.2) |
| Belfast | 109 | 1/109 (0.9) | 63/109 (57.8) | 45/109 (41.3) | 0/109 (0.0) | 64/109 (58.7) |
| Birmingham | 318 | 34/318 (10.7) | 185/318 (58.2) | 92/318 (28.9) | 7/318 (2.2) | 219/318 (68.9) |
| Bristol | 114 | 5/114 (4.4) | 24/114 (21.1) | 83/114 (72.8) | 2/114 (1.8) | 29/114 (25.4) |
| Cardiff | 102 | 17/102 (16.7) | 43/102 (42.2) | 41/102 (40.2) | 1/102 (1.0) | 60/102 (58.8) |
| Evelina | 126 | 12/126 (9.5) | 82/126 (65.1) | 25/126 (19.8) | 7/126 (5.6) | 94/126 (74.6) |
| GNCH Newcastle | 143 | 1/143 (0.7) | 77/143 (53.8) | 65/143 (45.5) | 0/143 (0.0) | 78/143 (54.5) |
| GOSH | 195 | 9/195 (4.6) | 131/195 (67.2) | 55/195 (28.2) | 0/195 (0.0) | 140/195 (71.8) |
| Glasgow | 154 | 18/154 (11.7) | 60/154 (39.0) | 75/154 (48.7) | 1/154 (0.6) | 78/154 (50.6) |
| James Cook | 108 | 5/108 (4.6) | 51/108 (47.2) | 52/108 (48.1) | 0/108 (0.0) | 56/108 (51.9) |
| John Radcliffe | 117 | 8/117 (6.8) | 71/117 (60.7) | 38/117 (32.5) | 0/117 (0.0) | 79/117 (67.5) |
| King’s College | 117 | 7/117 (6.0) | 94/117 (80.3) | 13/117 (11.1) | 3/117 (2.6) | 101/117 (86.3) |
| Leicester | 72 | 20/72 (27.8) | 20/72 (27.8) | 32/72 (44.4) | 0/72 (0.0) | 40/72 (55.6) |
| Manchester | 194 | 38/194 (19.6) | 68/194 (35.1) | 88/194 (45.4) | 0/194 (0.0) | 106/194 (54.6) |
| Nottingham | 67 | 16/67 (23.9) | 35/67 (52.2) | 15/67 (22.4) | 1/67 (1.5) | 51/67 (76.1) |
| Royal Brompton | 119 | 0/119 (0.0) | 16/119 (13.4) | 102/119 (85.7) | 1/119 (0.8) | 16/119 (13.4) |
| Royal London | 122 | 29/122 (23.8) | 46/122 (37.7) | 47/122 (38.5) | 0/122 (0.0) | 75/122 (61.5) |
| Royal Stoke | 77 | 18/77 (23.4) | 45/77 (58.4) | 14/77 (18.2) | 0/77 (0.0) | 63/77 (81.8) |
| Southampton | 213 | 13/213 (6.1) | 137/213 (64.3) | 62/213 (29.1) | 1/213 (0.5) | 150/213 (70.4) |
| St George’s | 203 | 67/203 (33.0) | 29/203 (14.3) | 107/203 (52.7) | 0/203 (0.0) | 96/203 (47.3) |
| St Mary’s | 85 | 11/85 (12.9) | 50/85 (58.8) | 24/85 (28.2) | 0/85 (0.0) | 61/85 (71.8) |
| Total | 3141 | 346/3141 (11.0) | 1507/3141 (48.0) | 1263/3141 (40.2) | 25/3141 (0.8) | 1853/3141 (59.0) |
Appendix 2 Subgroup analysis of maximum temperature and current temperature thresholds by confirmed versus suspected infection and site/type of infection
FIGURE 22.
The proportion of patients receiving antipyretic intervention on day 1 with a temperature above or below a given threshold for all patients (population 1), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.
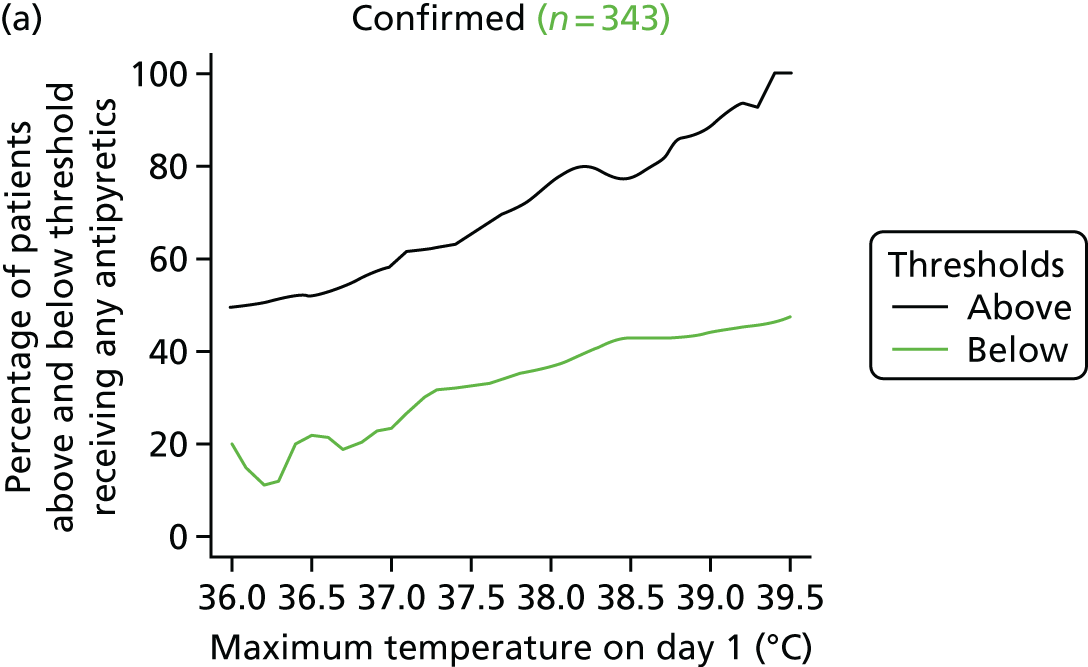
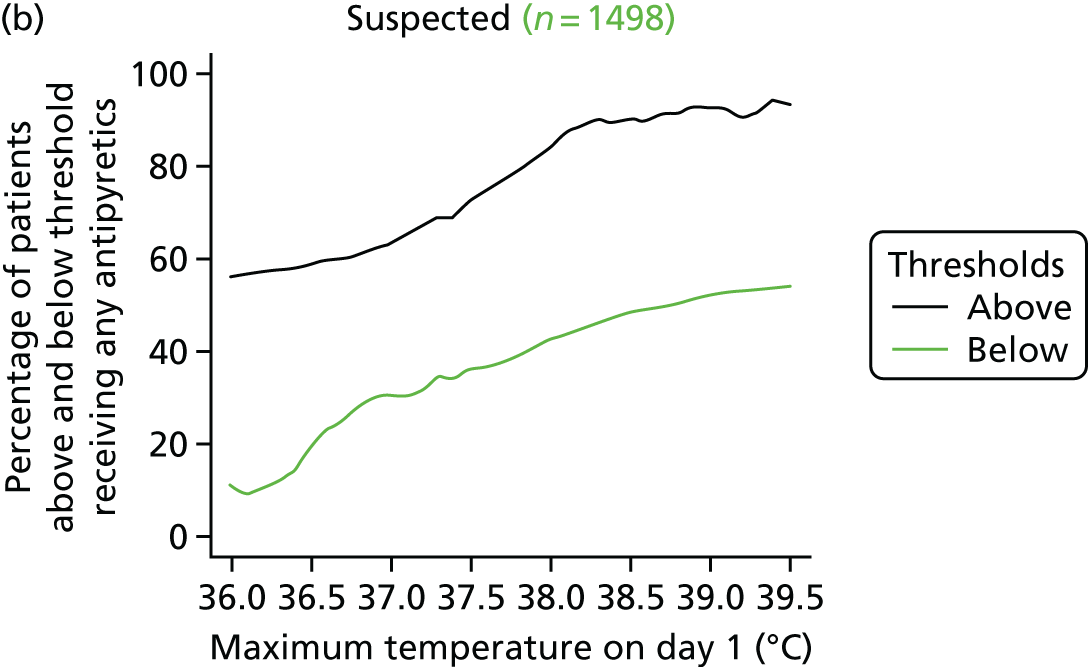
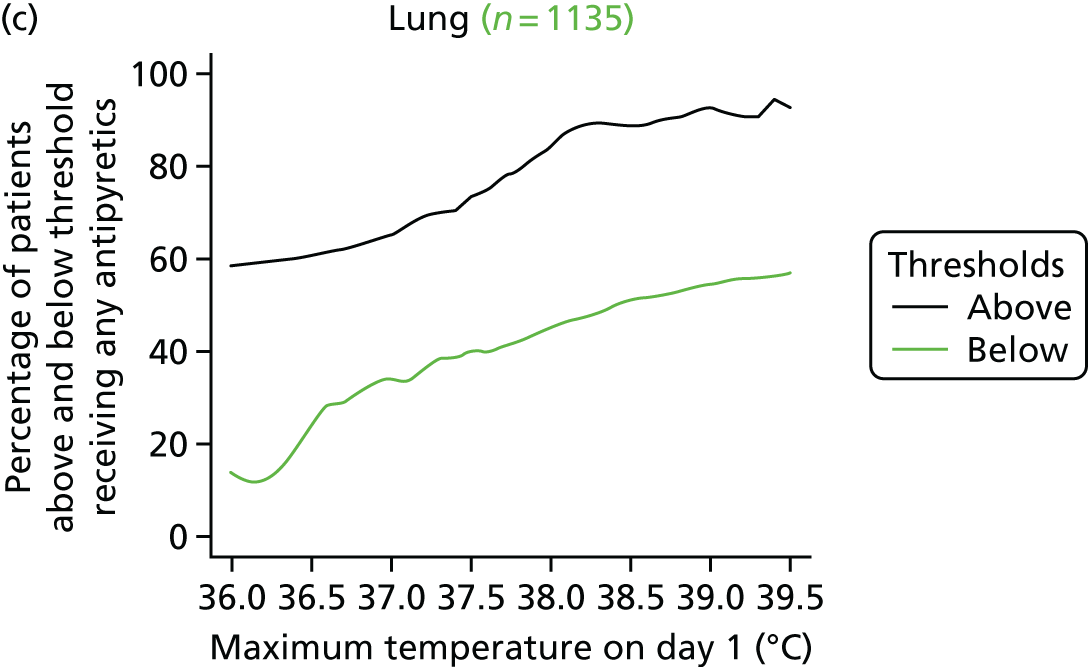



FIGURE 23.
The proportion of patients receiving antipyretic intervention on day 1 with a temperature above or below a given threshold for ventilated patients (population 2), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.
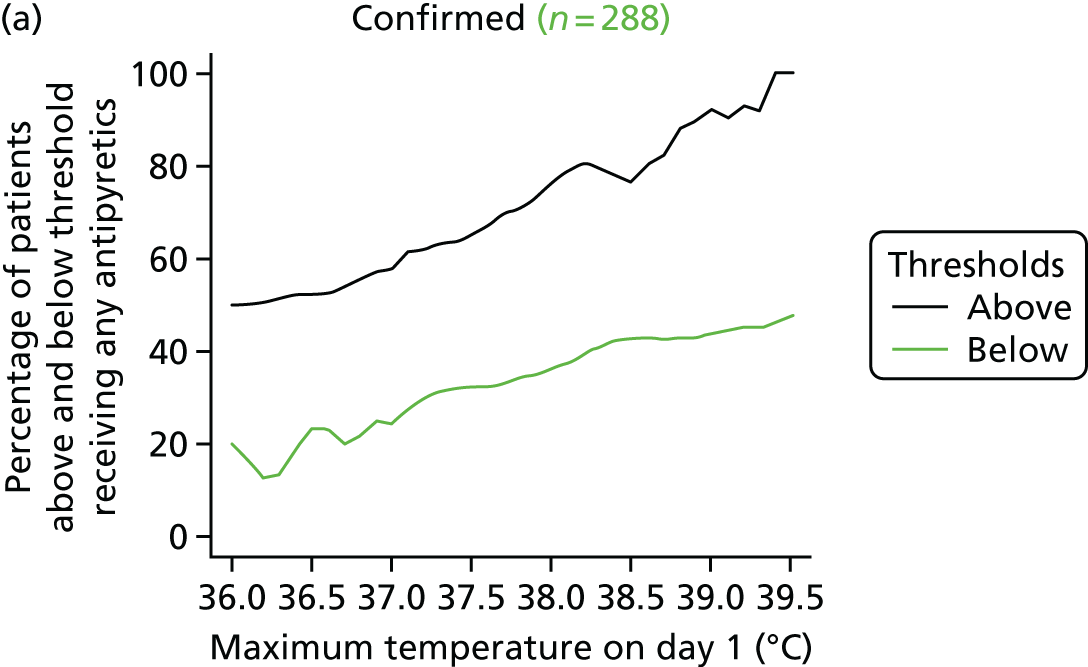

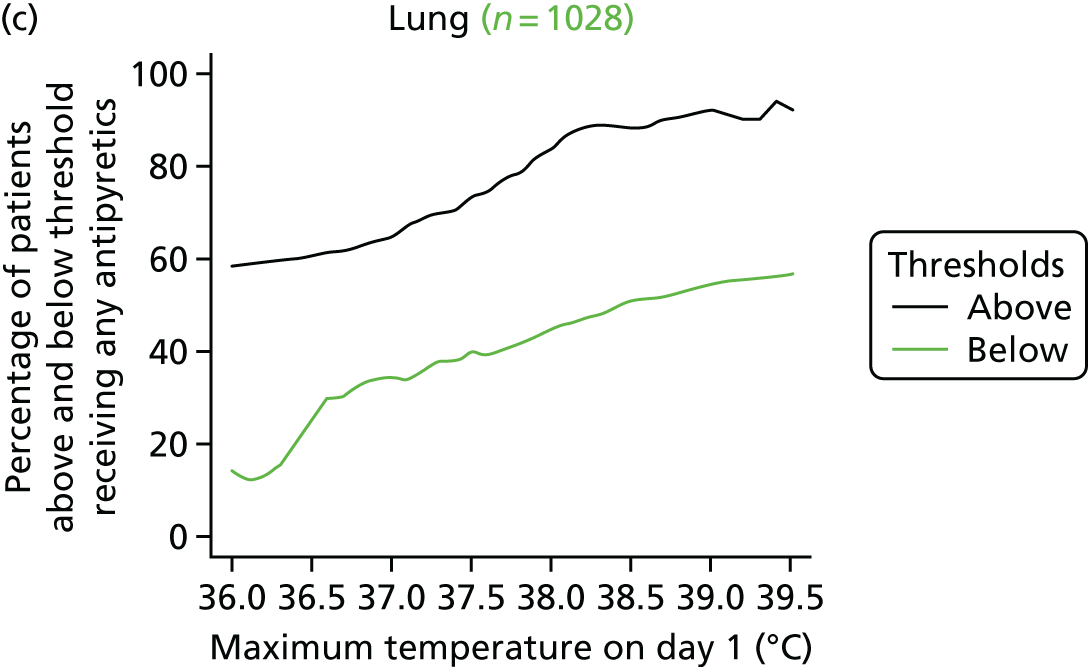
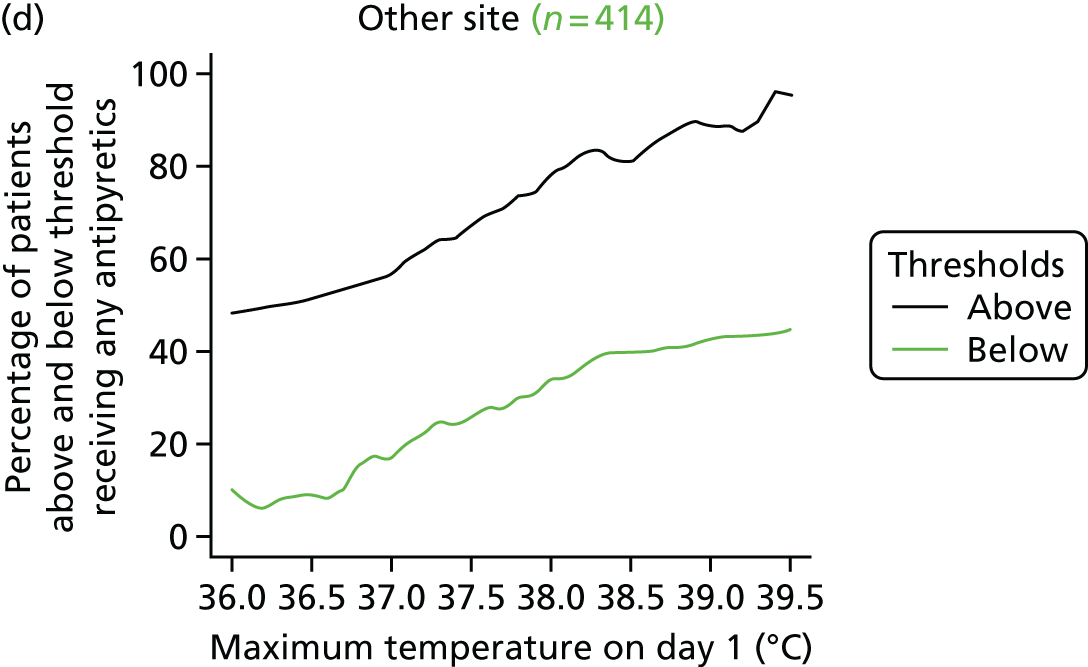


FIGURE 24.
The proportion of patients receiving antipyretic intervention on day 1 with a temperature above or below a given threshold for ventilated patients (population 3), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.
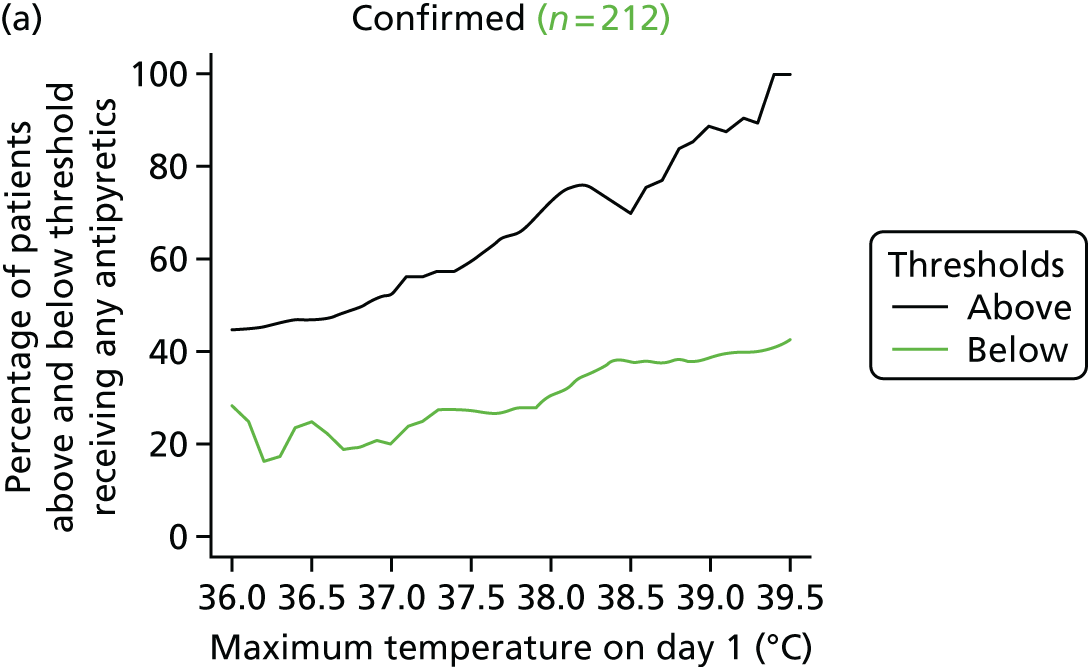
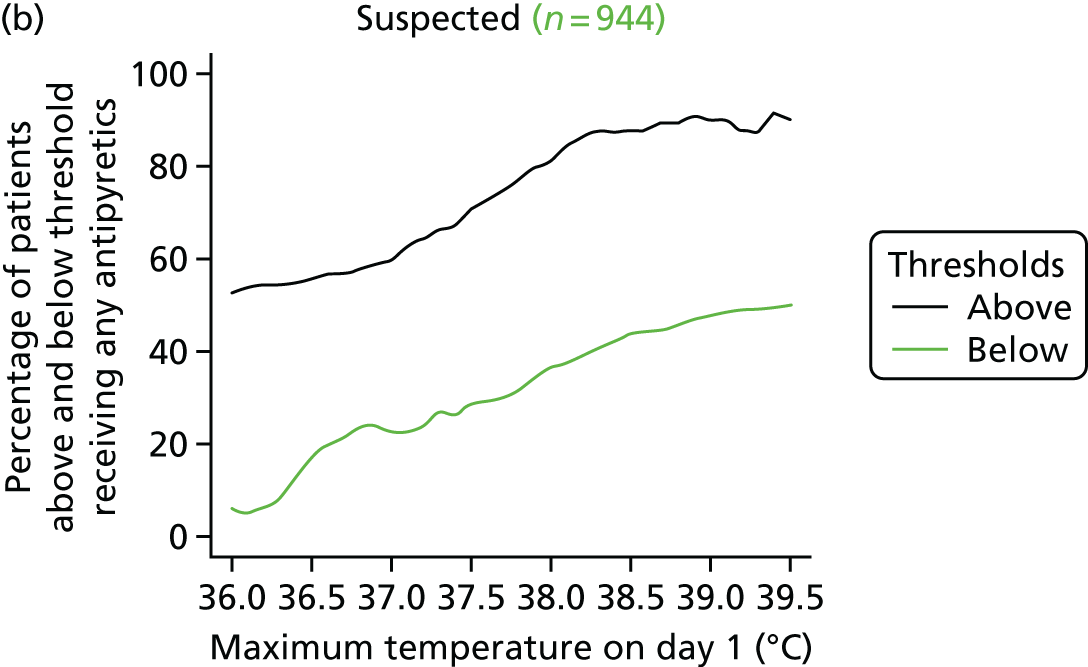



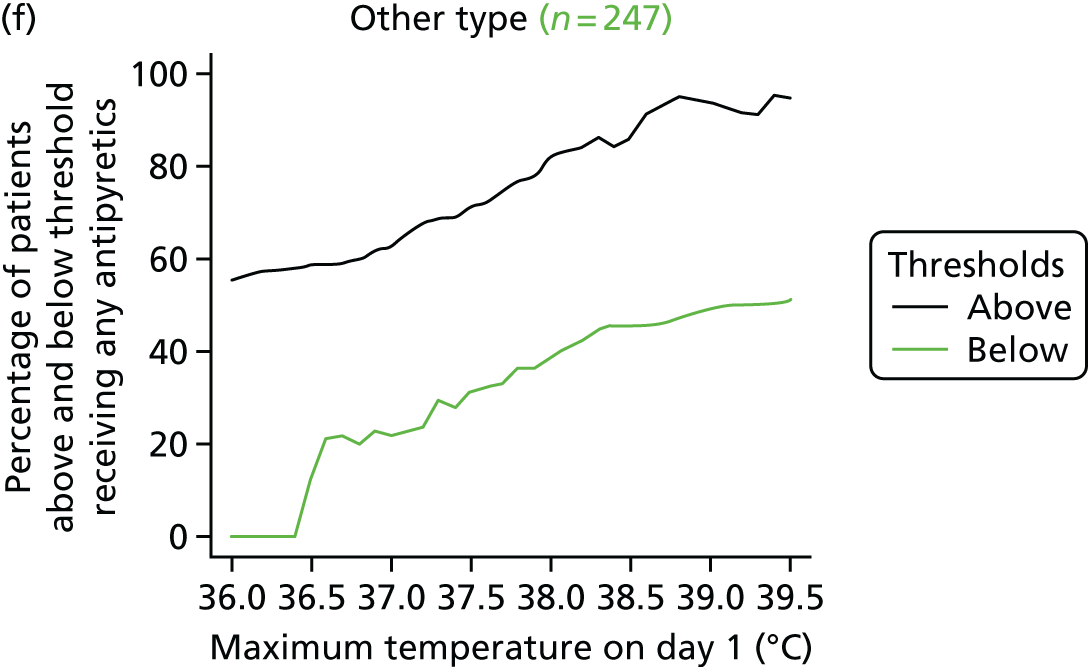
FIGURE 25.
Cumulative distribution of maximum daily temperatures of patients not receiving antipyretics on day 1 and receiving subsequent antipyretic intervention threshold for all patients (population 1), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.



FIGURE 26.
Cumulative distribution of maximum daily temperatures of patients not receiving antipyretics on day 1 and receiving subsequent antipyretic intervention threshold for mechanically ventilated patients (population 2), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.

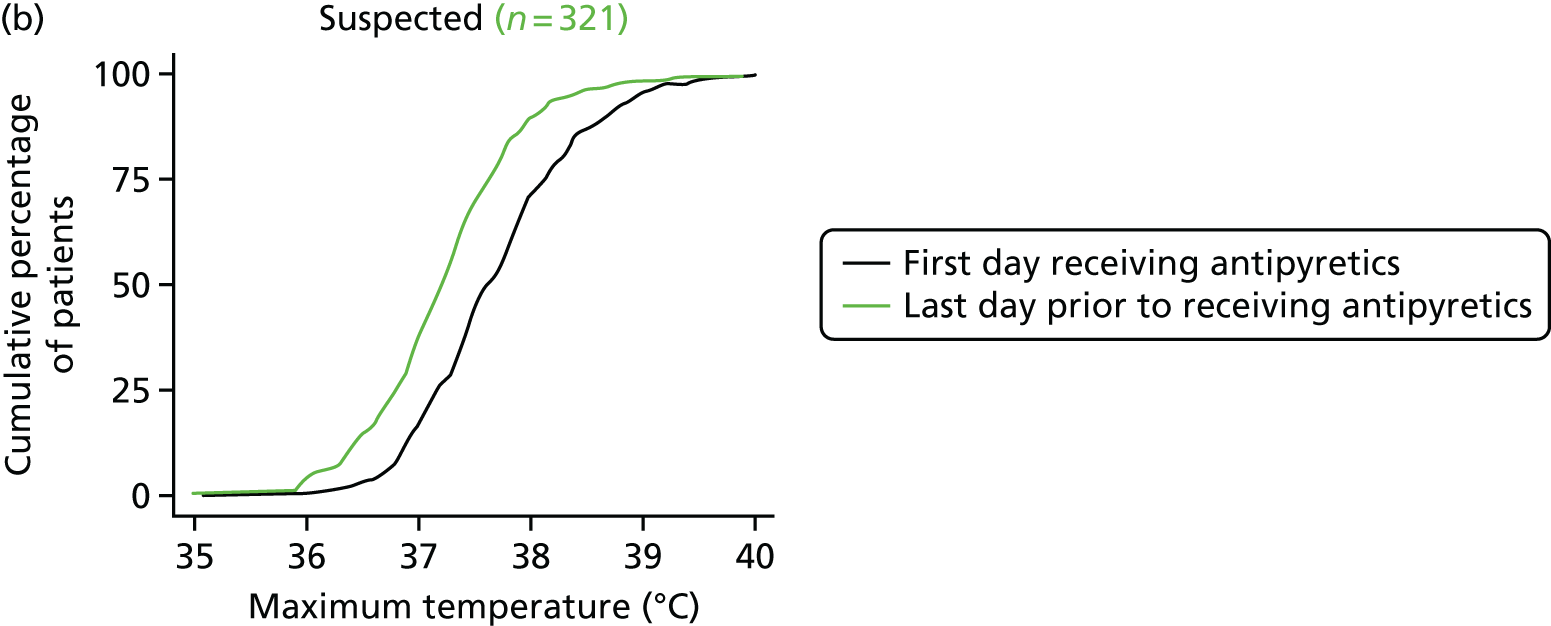

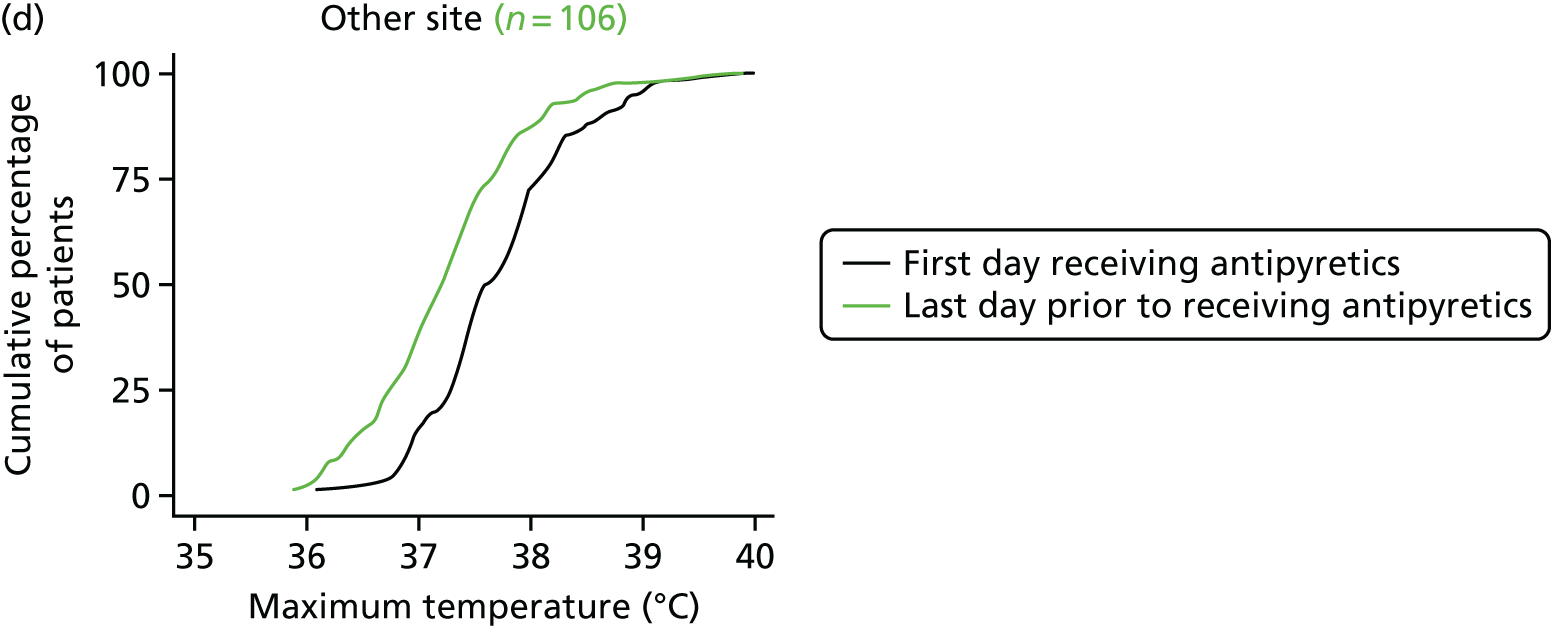
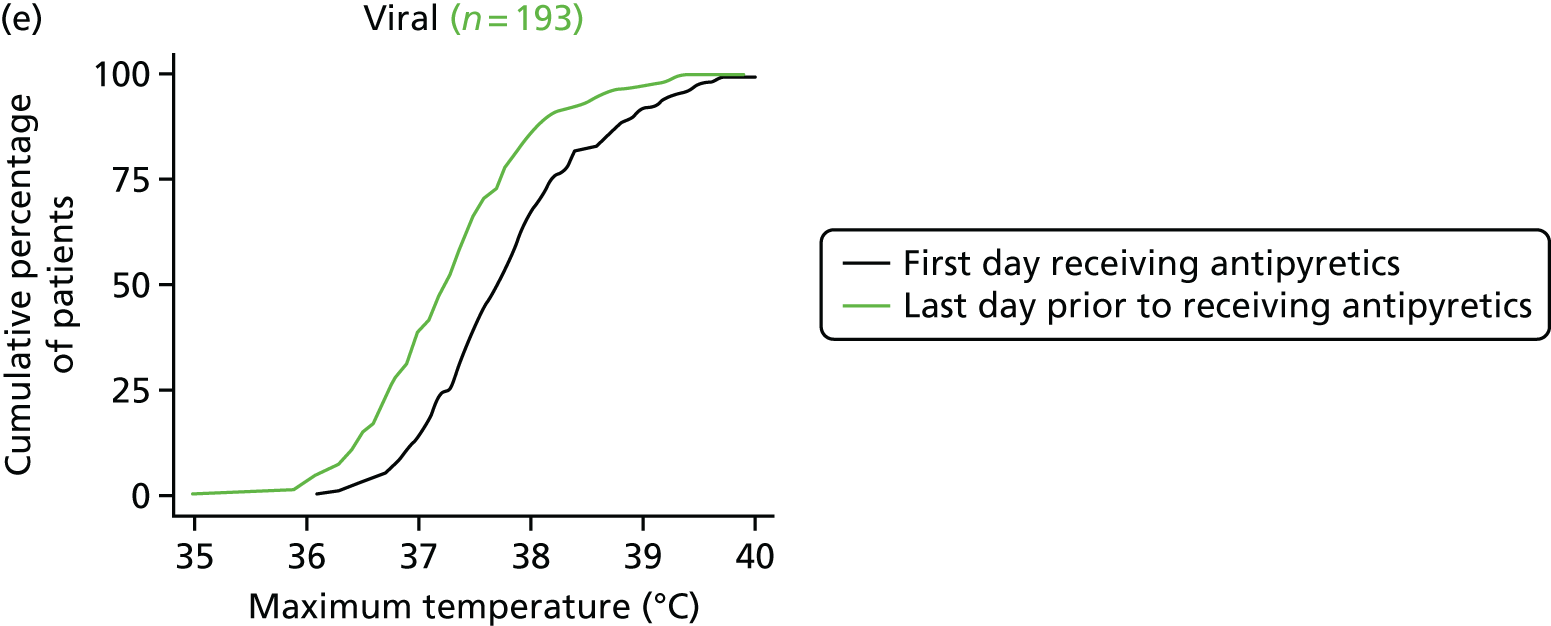
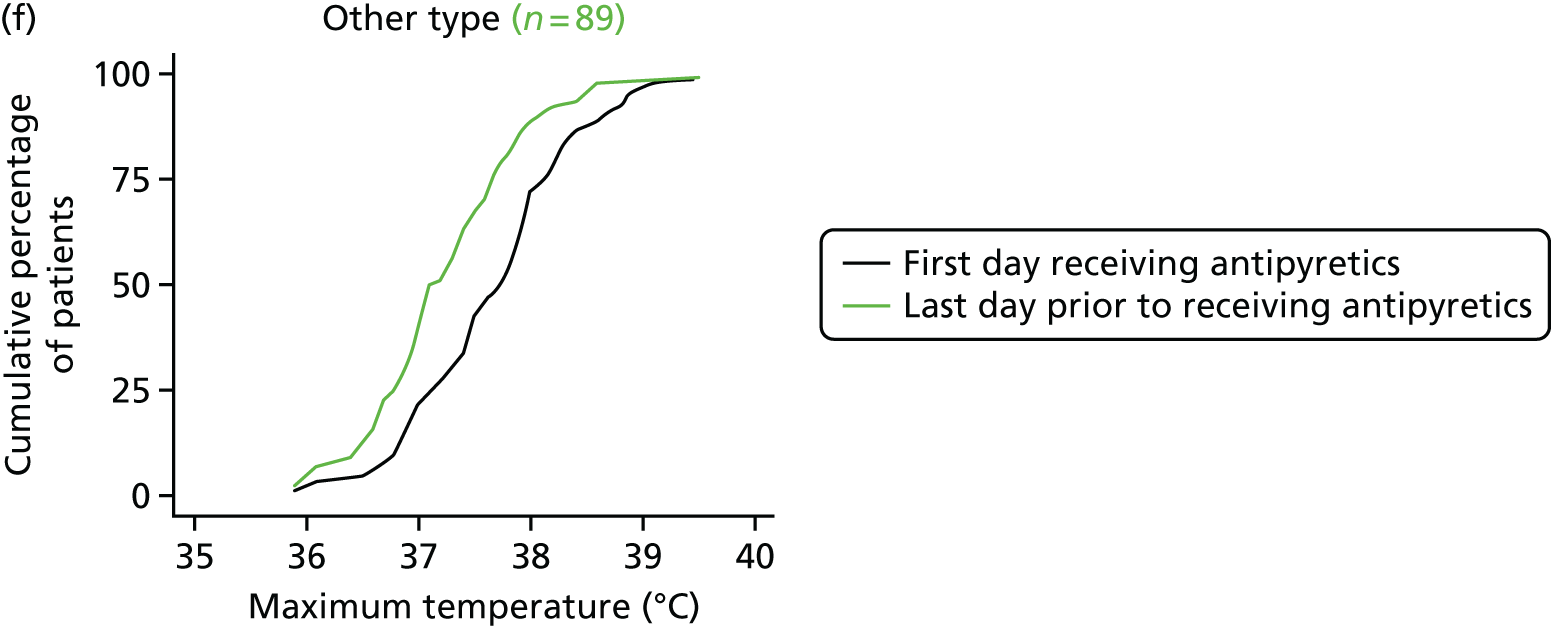
FIGURE 27.
Cumulative distribution of maximum daily temperatures of patients not receiving antipyretics on day 1 and receiving subsequent antipyretic intervention threshold for invasively ventilated patients (population 3), by (a) confirmed or (b) suspected (c and d) site and (e and f) type of infection.


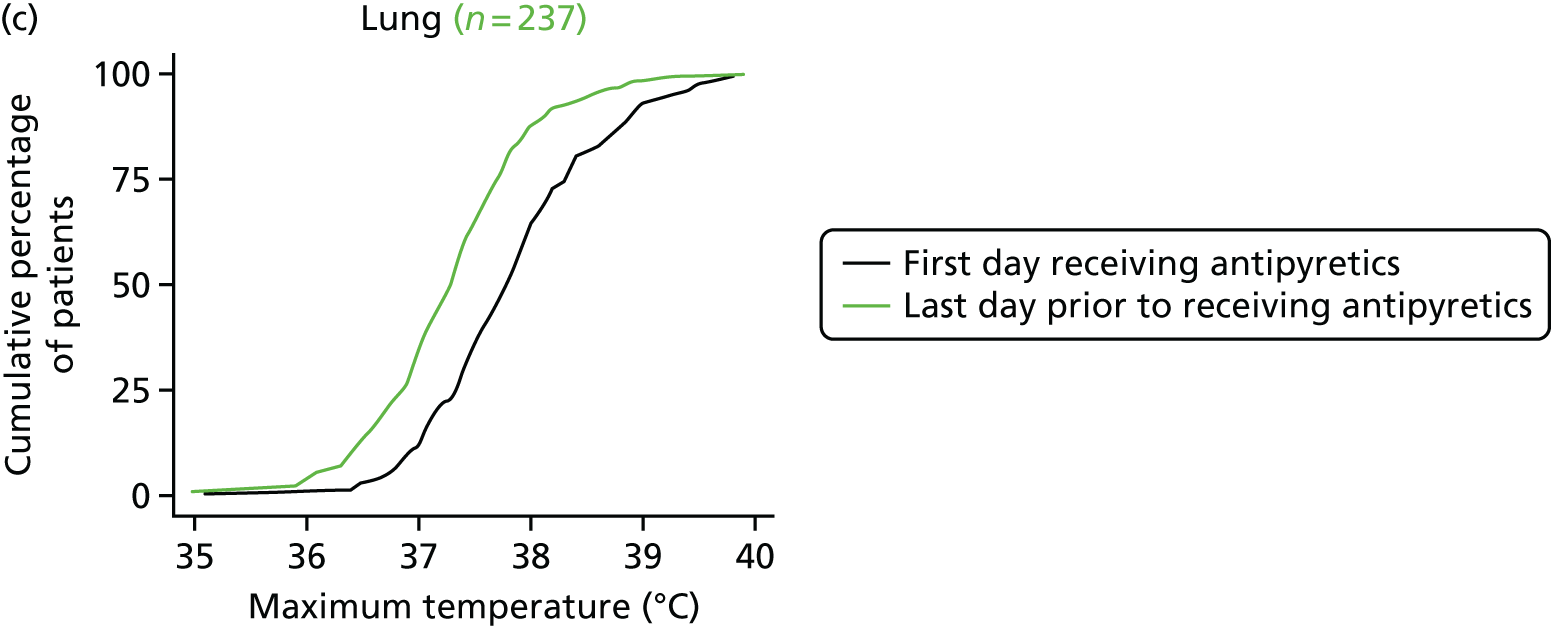


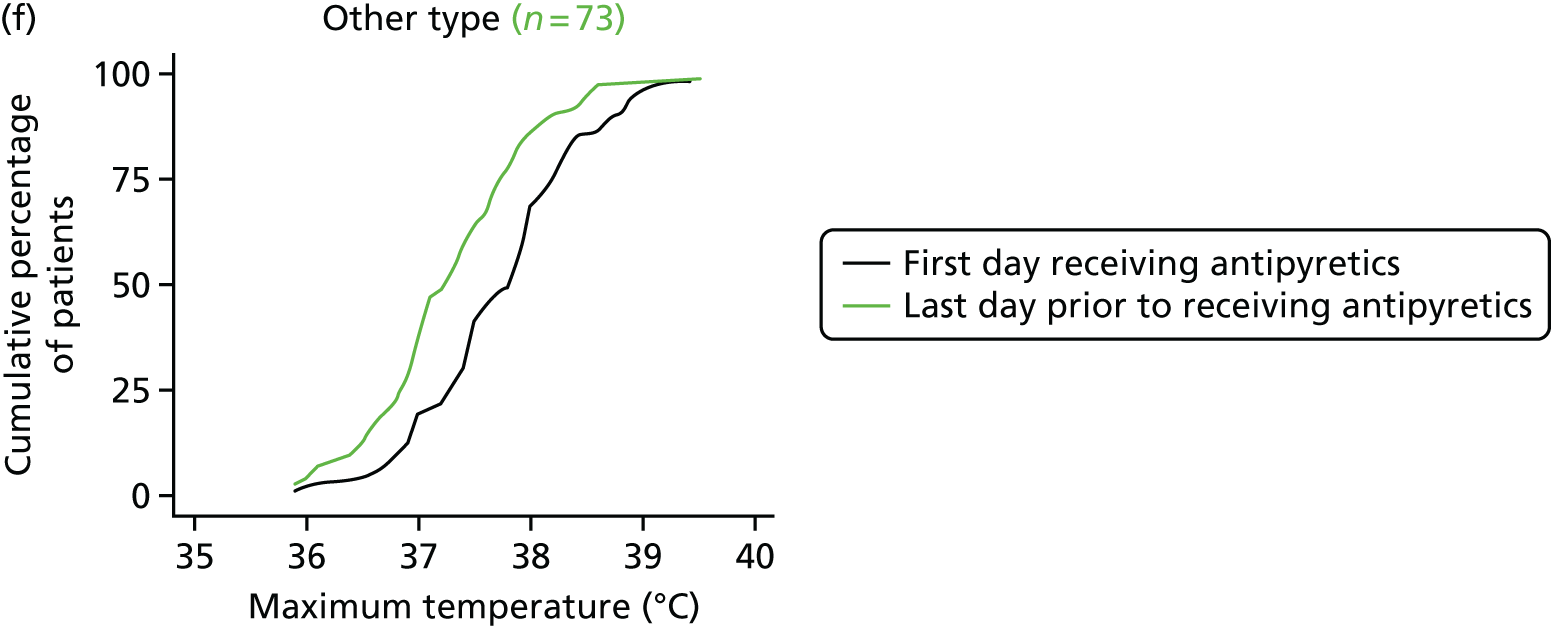
Appendix 3 Histograms of continuous outcomes for the pilot randomised controlled trial
FIGURE 28.
Number of days alive and free from (a and b) a PICU and (c and d) mechanical ventilation to day 28 in each treatment group.
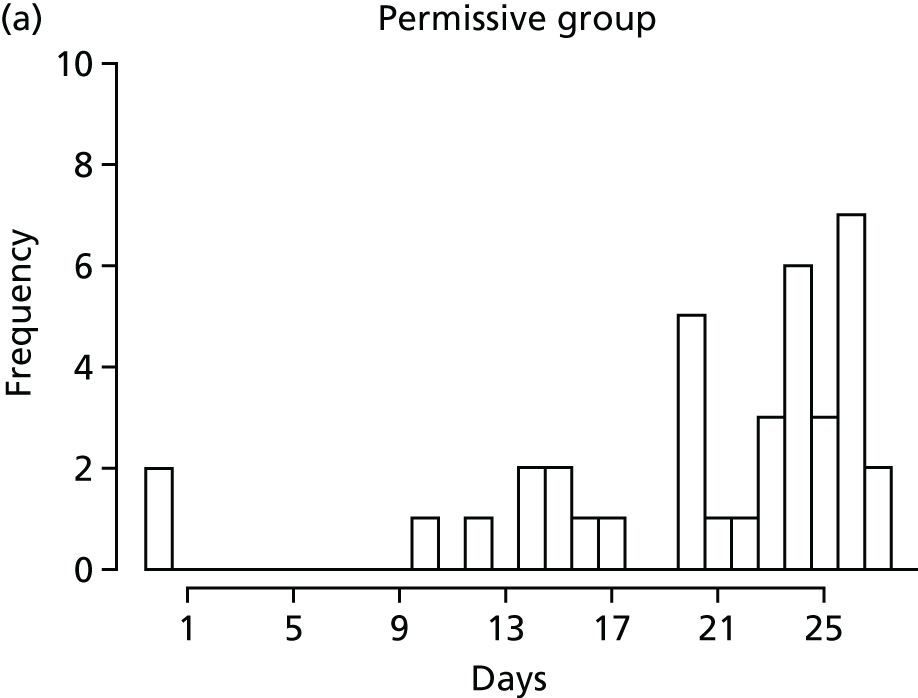
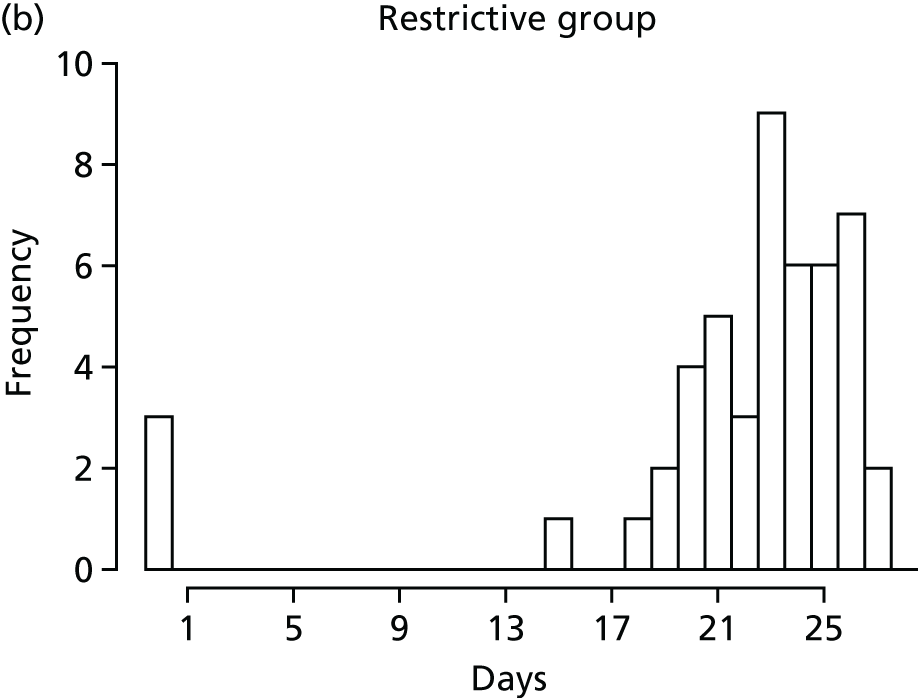


FIGURE 29.
Length of stay in a PICU by treatment group (all patients). (a) Permissive group; and (b) restrictive group.
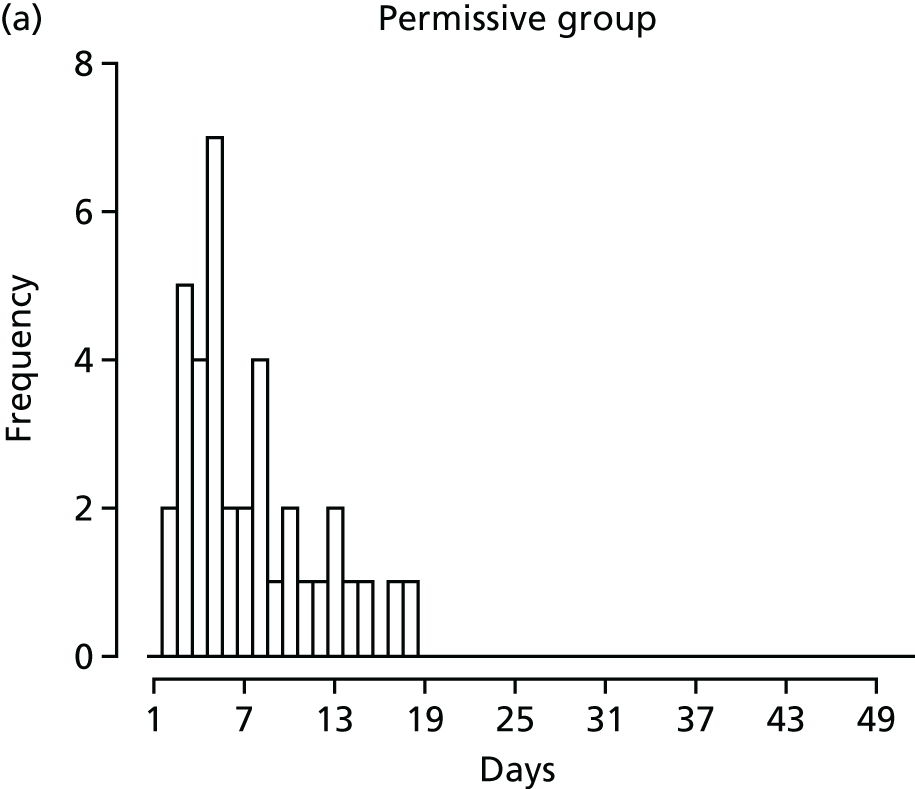
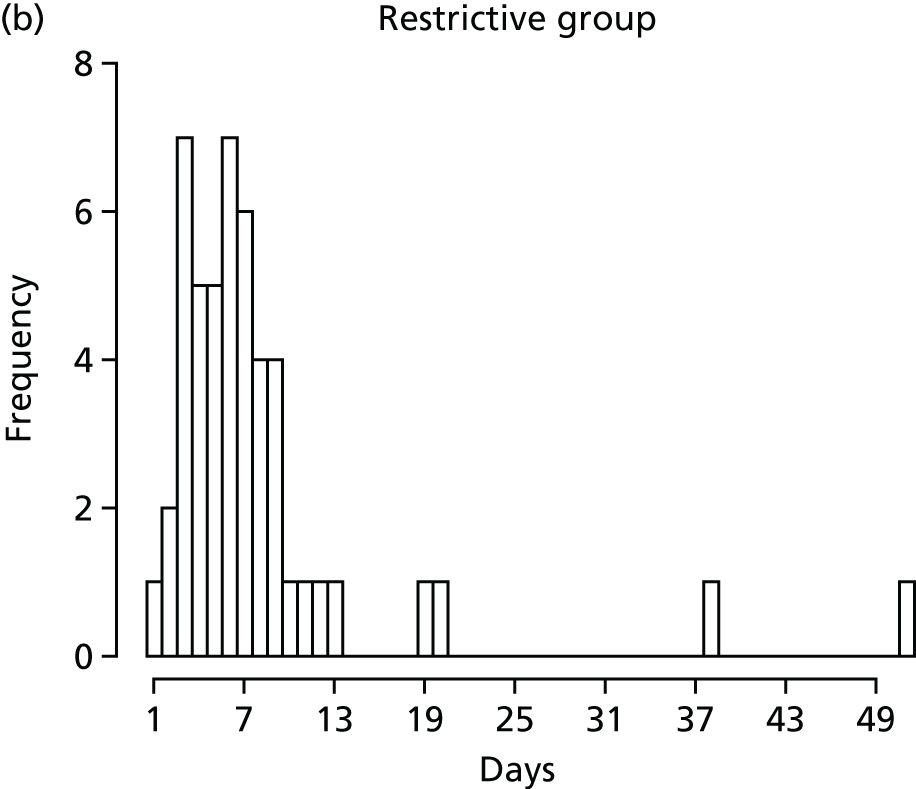
FIGURE 30.
Number of days receiving (a and b) mechanical ventilation, (c and d) cardiovascular support and (e and f) renal support from randomisation to day 28 in each treatment group.
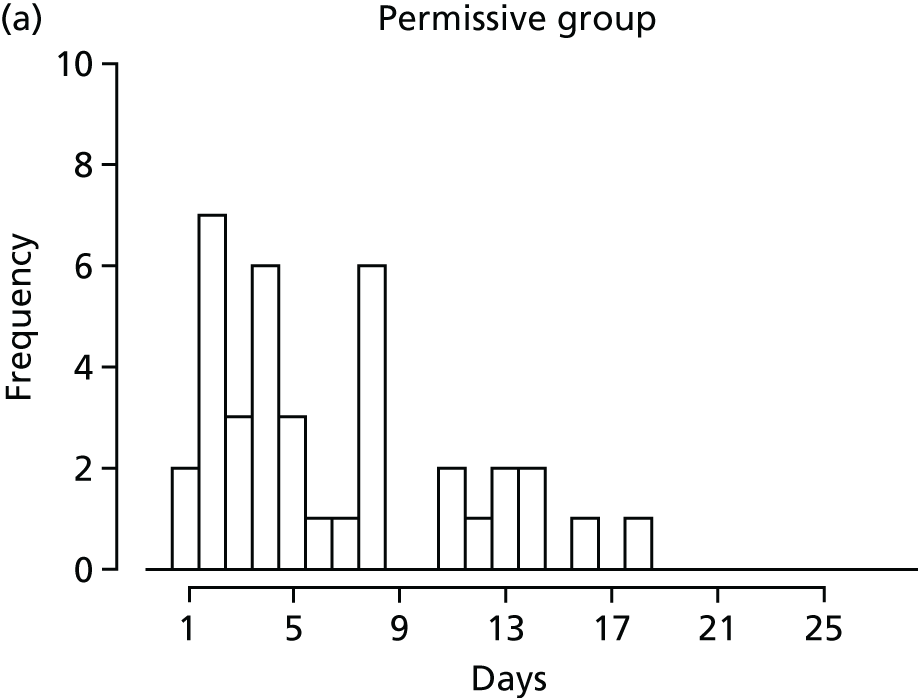


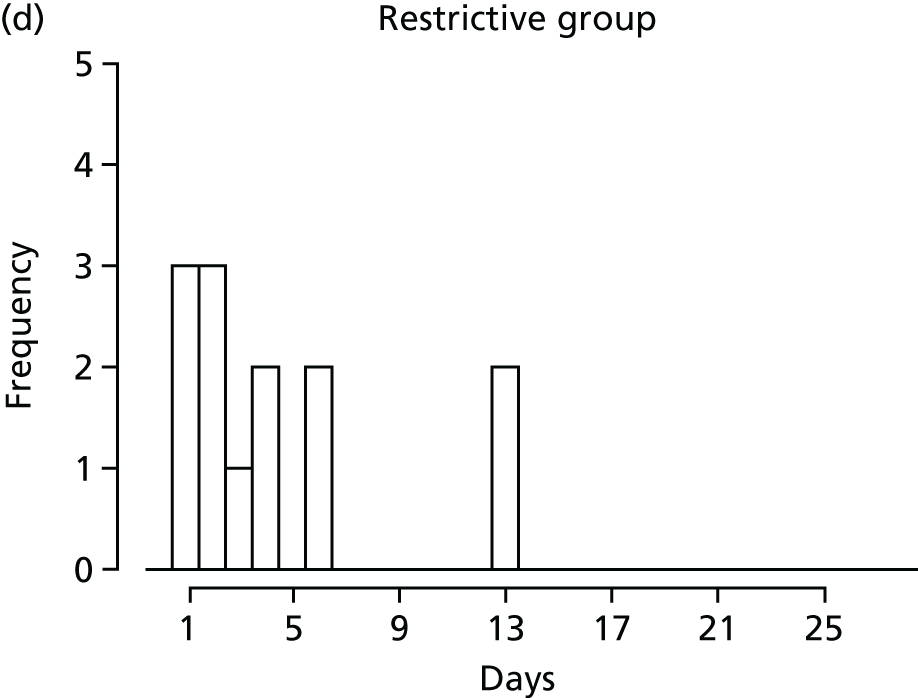

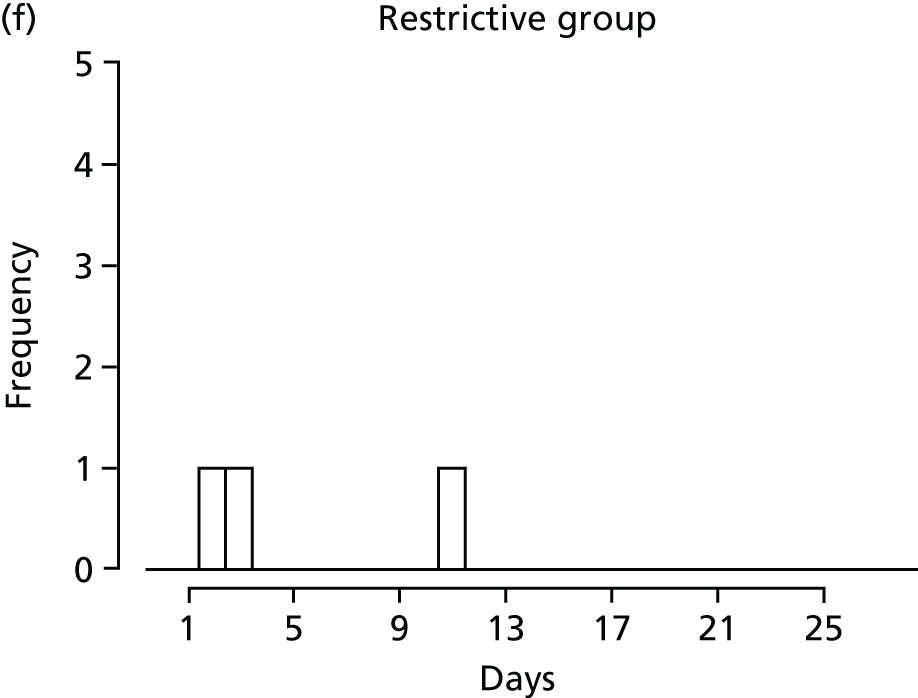
Appendix 4 Subgroup analysis of patients receiving invasive mechanical ventilation
Baseline patient characteristics
| Variables | Treatment group | Total (N = 79) | |
|---|---|---|---|
| Permissive (N = 35) | Restrictive (N = 44) | ||
| Age (years) | |||
| Mean (SD) | 1.4 (2.8) | 1.1 (2.2) | 1.2 (2.5) |
| Median (IQR) | 0 (0–2) | 0 (0–1) | 0 (0–2) |
| Age group (years) | |||
| < 1 | 23 (65.7) | 30 (68.2) | 53 (67.1) |
| 1 | 3 (8.6) | 3 (6.8) | 6 (7.6) |
| 2–4 | 5 (14.3) | 7 (15.9) | 12 (15.2) |
| 5–9 | 2 (5.7) | 3 (6.8) | 5 (6.3) |
| 10–15 | 2 (5.7) | 1 (2.3) | 3 (3.8) |
| Sex, n (%) | |||
| Female | 13 (37.1) | 18 (40.9) | 31 (39.2) |
| Male | 22 (62.9) | 26 (59.1) | 48 (60.8) |
| PIM2r score | |||
| Mean (SD) | 0.025 (0.031) | 0.026 (0.031) | 0.026 (0.031) |
| Median (IQR) | 0.011 (0.007–0.033) | 0.012 (0.008–0.037) | 0.012 (0.008–0.036) |
| PIM3 score | |||
| Mean (SD) | 0.023 (0.034) | 0.026 (0.038) | 0.024 (0.036) |
| Median (IQR) | 0.006 (0.004–0.033) | 0.007 (0.005–0.038) | 0.006 (0.005–0.037) |
| Source of admission | |||
| Same hospital | 5 (14.3) | 8 (18.2) | 13 (16.5) |
| Other hospital | 30 (85.7) | 36 (81.8) | 66 (83.5) |
| Clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Home | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Main reason for PICU admission, n (%) | |||
| Asthma | 2 (5.7) | 1 (2.3) | 3 (3.8) |
| Bronchiolitis | 21 (60.0) | 23 (52.3) | 44 (55.7) |
| Croup | 1 (2.9) | 1 (2.3) | 2 (2.5) |
| Obstructive sleep apnoea | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diabetic ketoacidosis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Recovery from surgery | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Seizure disorder | 1 (2.9) | 1 (2.3) | 2 (2.5) |
| Other | 10 (28.6) | 18 (40.9) | 28 (35.4) |
| Temperature (°C) | |||
| Mean (SD) | 38.1 (0.6) | 38.0 (0.6) | 38.0 (0.6) |
| Median (IQR) | 38.0 (37.7–38.6) | 37.8 (37.7–38.2) | 37.9 (37.7–38.5) |
| Variables | Treatment group, n (%) | Total, n (%) | |
|---|---|---|---|
| Permissive | Restrictive | ||
| Any treatment of fever | 5 (14.3) | 9 (20.5) | 14 (17.7) |
| Paracetamol | 3 (8.6) | 7 (15.9) | 10 (12.7) |
| NSAID | 0 (0.0) | 1 (2.3) | 1 (1.3) |
| External cooling | 2 (5.7) | 3 (6.8) | 5 (6.3) |
| Other cooling | 1 (2.9) | 0 (0.0) | 1 (1.3) |
| Treatment group | n | Time spent below allocated temperature threshold (hours), median (IQR) |
|---|---|---|
| Permissive | 35 | 1.0 (1.0–1.0) |
| Restrictive | 44 | 0.7 (0.4–0.8) |
Temperatures
Highest temperatures
FIGURE 31.
Highest temperature, by 6-hour period and treatment group. (a) Box plot; and (b) median and IQRs.
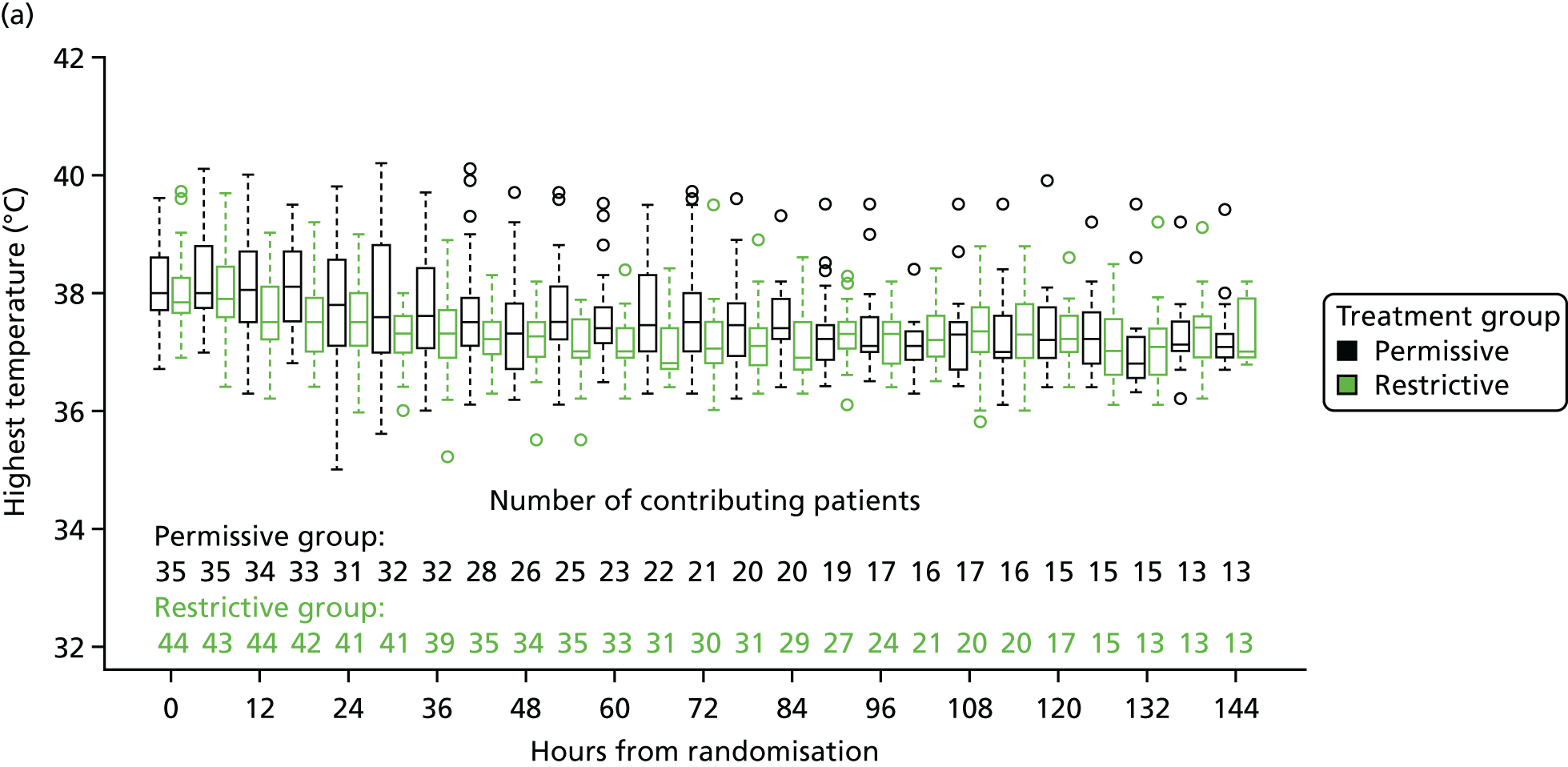
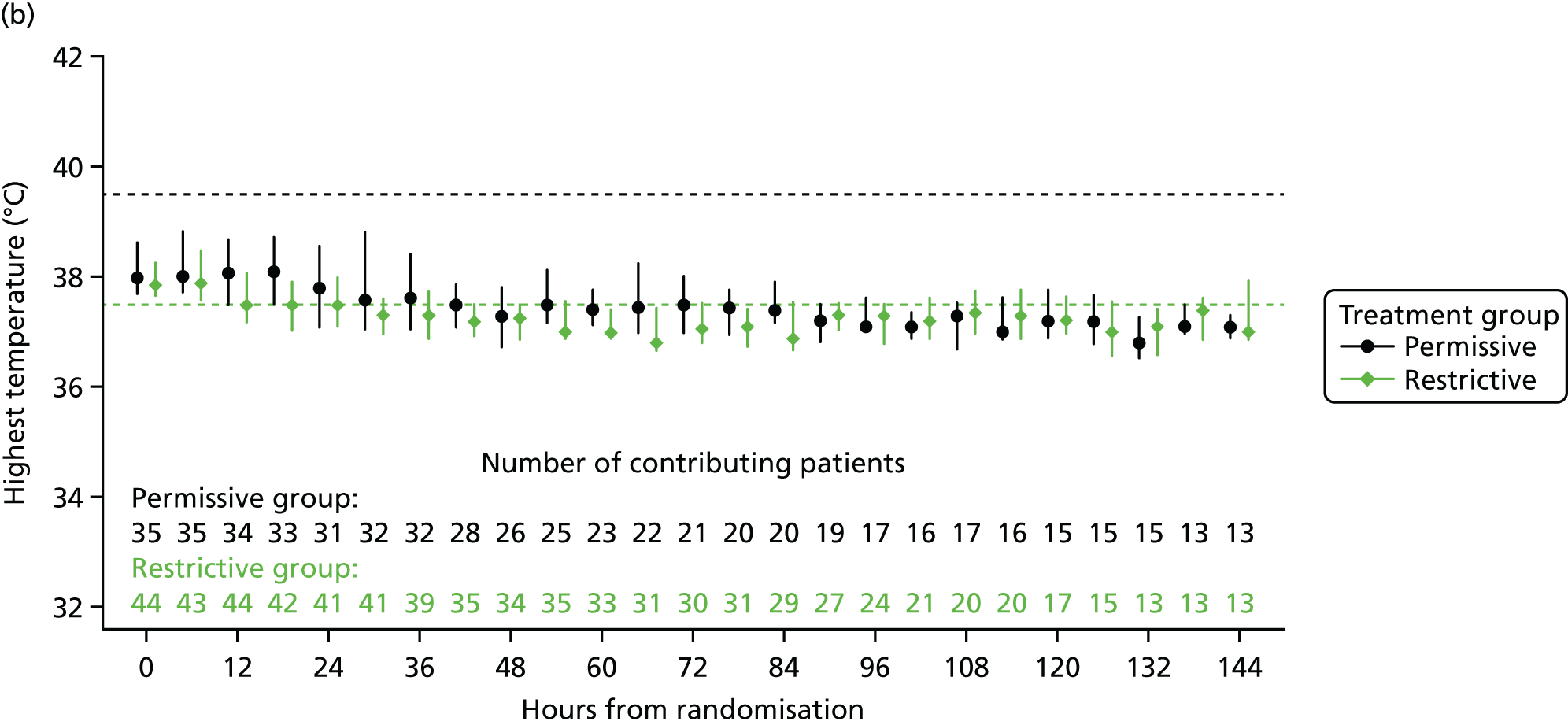
FIGURE 32.
Distribution of time spent at each temperature, by treatment group. (a) During the first 48 hours; and (b) during the first 6 days.
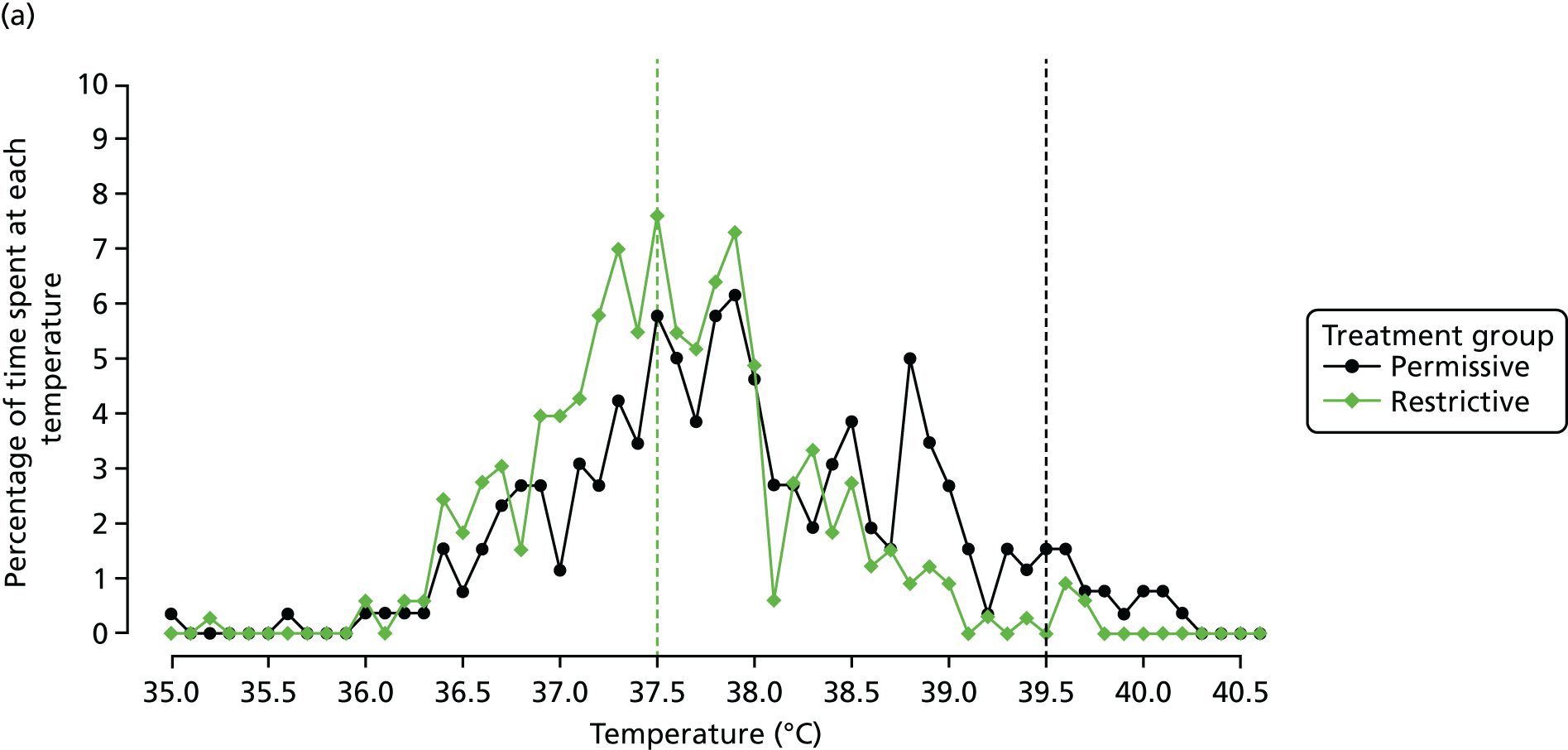
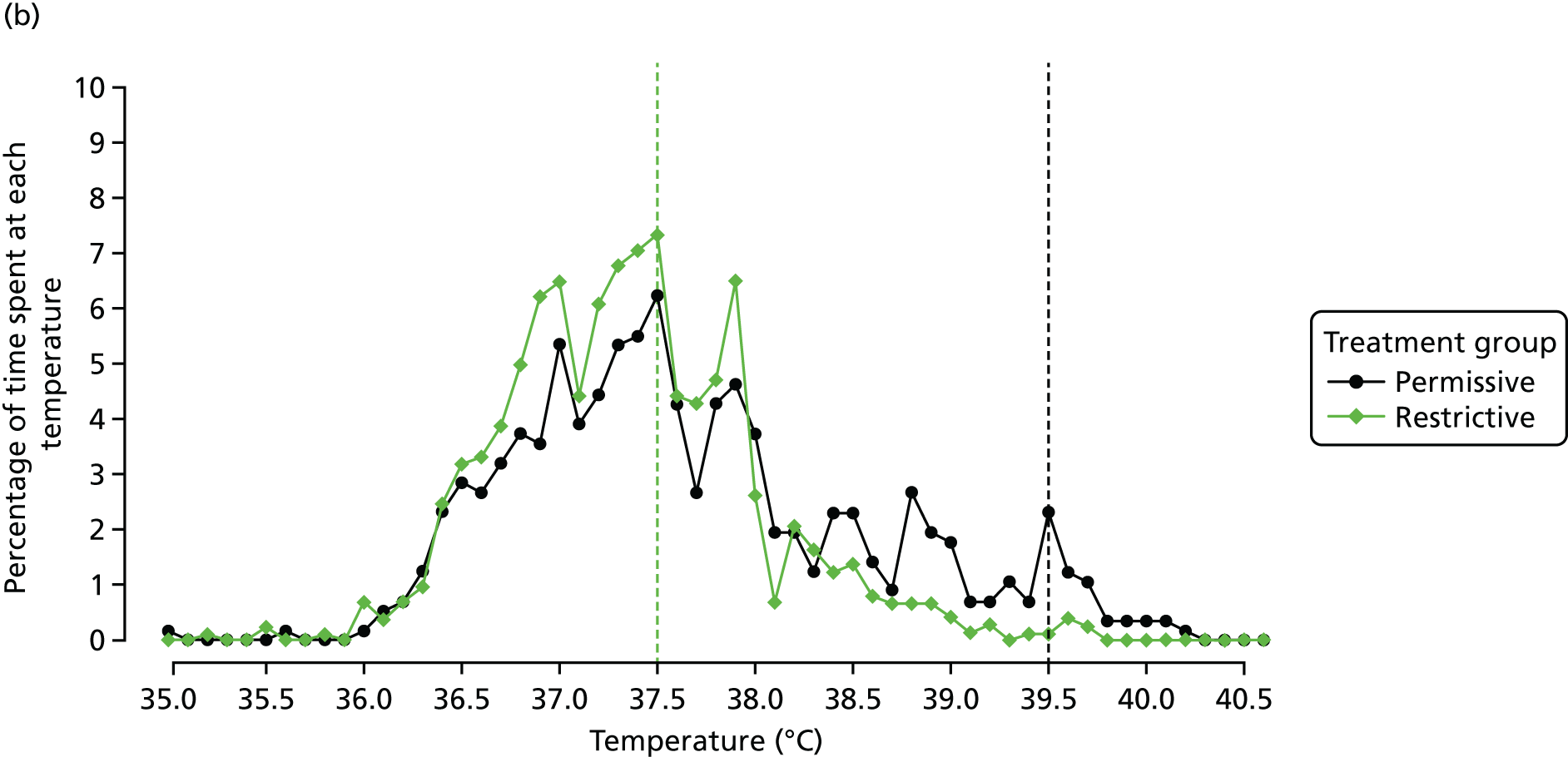
Separation between groups
FIGURE 33.
The difference between groups in the mean of the highest temperature at each time point with 95% CIs.
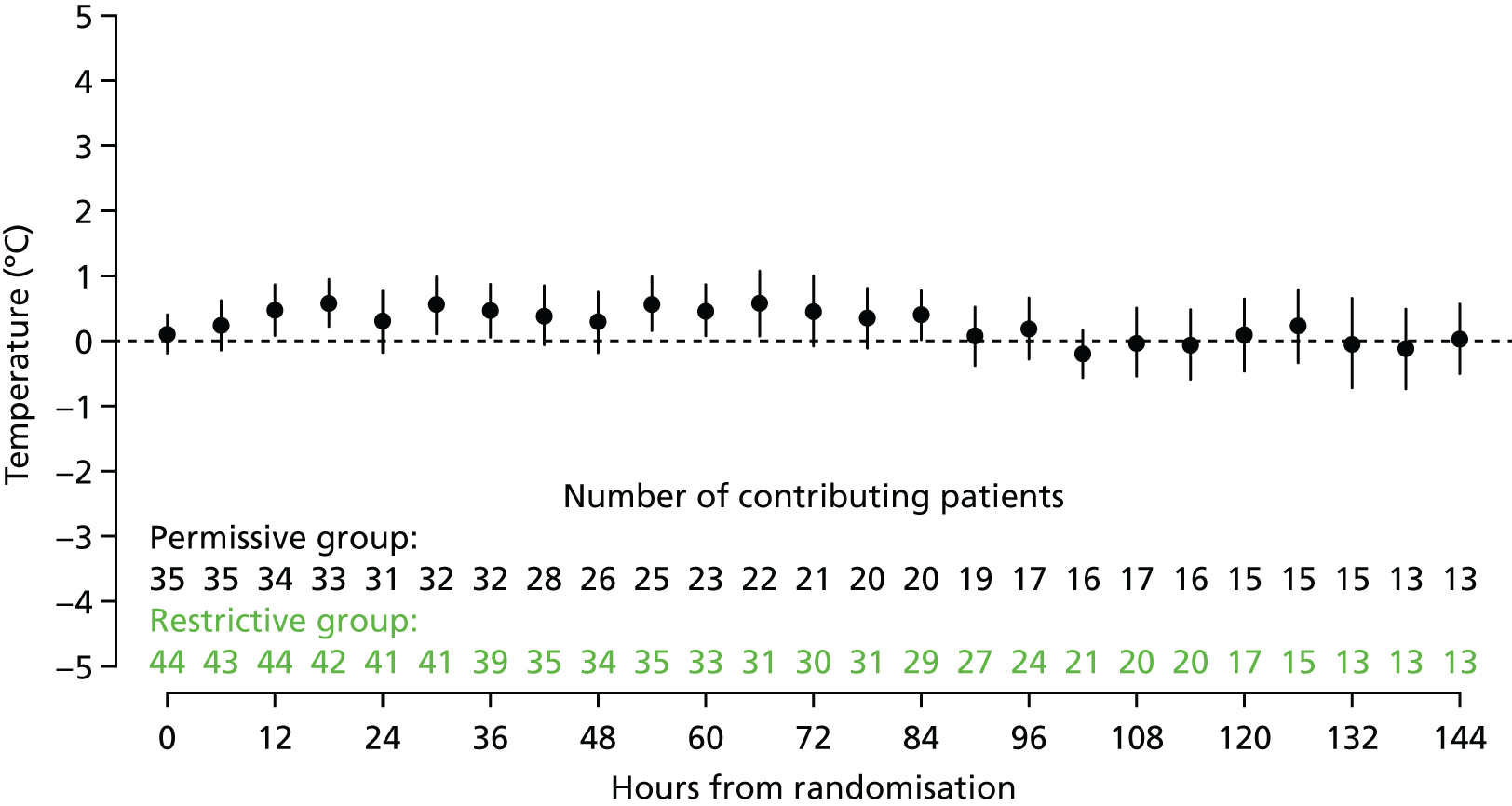
| Treatment group | n | Maximum temperature (°C), mean (95% CI) |
|---|---|---|
| Permissive | 35 | 38.8 (38.5 to 39.1) |
| Restrictive | 44 | 38.3 (38.1 to 38.5) |
| Difference | 0.5 (0.1 to 0.8) |
Interventions
Antipyretics
| Hours | Treatment group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Permissive | Restrictive | |||||||||
| n | Antipyretic intervention (%) | n | Antipyretic intervention (%) | |||||||
| Paracetamol | NSAID | External | Other | Paracetamol | NSAID | External | Other | |||
| Baseline | 35 | 8.6 | 0.0 | 5.7 | 2.9 | 44 | 15.9 | 2.3 | 6.8 | 0.0 |
| 0–6 | 35 | 14.3 | 0.0 | 8.6 | 5.7 | 44 | 52.3 | 0.0 | 20.5 | 6.8 |
| 6–12 | 34 | 8.8 | 2.9 | 14.7 | 5.9 | 44 | 31.8 | 0.0 | 20.5 | 4.5 |
| 12–18 | 33 | 9.1 | 0.0 | 6.1 | 0.0 | 42 | 28.6 | 0.0 | 21.4 | 4.8 |
| 18–24 | 32 | 12.5 | 0.0 | 3.1 | 0.0 | 41 | 31.7 | 0.0 | 19.5 | 2.4 |
| 24–30 | 32 | 9.4 | 3.1 | 6.2 | 0.0 | 41 | 34.1 | 0.0 | 14.6 | 0.0 |
| 30–36 | 32 | 9.4 | 3.1 | 3.1 | 0.0 | 39 | 20.5 | 0.0 | 10.3 | 2.6 |
| 36–42 | 29 | 10.3 | 3.4 | 6.9 | 0.0 | 36 | 22.2 | 2.8 | 2.8 | 0.0 |
| 42–48 | 26 | 11.5 | 0.0 | 3.8 | 0.0 | 35 | 14.3 | 2.9 | 14.3 | 2.9 |
| 48–54 | 25 | 16.0 | 0.0 | 8.0 | 0.0 | 34 | 17.6 | 0.0 | 14.7 | 8.8 |
| 54–60 | 23 | 4.3 | 0.0 | 0.0 | 0.0 | 33 | 9.1 | 0.0 | 3.0 | 0.0 |
| 60–66 | 22 | 18.2 | 0.0 | 0.0 | 0.0 | 32 | 15.6 | 0.0 | 6.2 | 3.1 |
| 66–72 | 21 | 9.5 | 0.0 | 9.5 | 4.8 | 30 | 33.3 | 3.3 | 10.0 | 3.3 |
| 72–78 | 21 | 0.0 | 0.0 | 0.0 | 4.8 | 32 | 15.6 | 0.0 | 9.4 | 3.1 |
| 78–84 | 20 | 10.0 | 0.0 | 0.0 | 0.0 | 30 | 23.3 | 0.0 | 10.0 | 0.0 |
| 84–90 | 19 | 10.5 | 0.0 | 5.3 | 0.0 | 29 | 20.7 | 0.0 | 6.9 | 3.4 |
| 90–96 | 17 | 17.6 | 0.0 | 0.0 | 5.9 | 25 | 20.0 | 0.0 | 12.0 | 4.0 |
| 96–102 | 16 | 6.2 | 0.0 | 6.2 | 6.2 | 23 | 26.1 | 0.0 | 8.7 | 13 |
| 102–108 | 17 | 11.8 | 0.0 | 5.9 | 0.0 | 21 | 28.6 | 0.0 | 9.5 | 0.0 |
| 108–114 | 16 | 0.0 | 0.0 | 0.0 | 0.0 | 20 | 30.0 | 0.0 | 5.0 | 5.0 |
| 114–120 | 15 | 13.3 | 0.0 | 0.0 | 0.0 | 17 | 23.5 | 0.0 | 17.6 | 0.0 |
| 120–126 | 15 | 6.7 | 0.0 | 0.0 | 0.0 | 15 | 33.3 | 0.0 | 13.3 | 0.0 |
| 126–132 | 15 | 20.0 | 0.0 | 0.0 | 0.0 | 13 | 7.7 | 0.0 | 7.7 | 7.7 |
| 132–138 | 13 | 15.4 | 0.0 | 0.0 | 0.0 | 13 | 38.5 | 0.0 | 15.4 | 7.7 |
| 138–144 | 13 | 15.4 | 0.0 | 0.0 | 0.0 | 13 | 15.4 | 0.0 | 7.7 | 0.0 |
FIGURE 34.
Proportion of patients who received antipyretic interventions while receiving invasive mechanical ventilation.
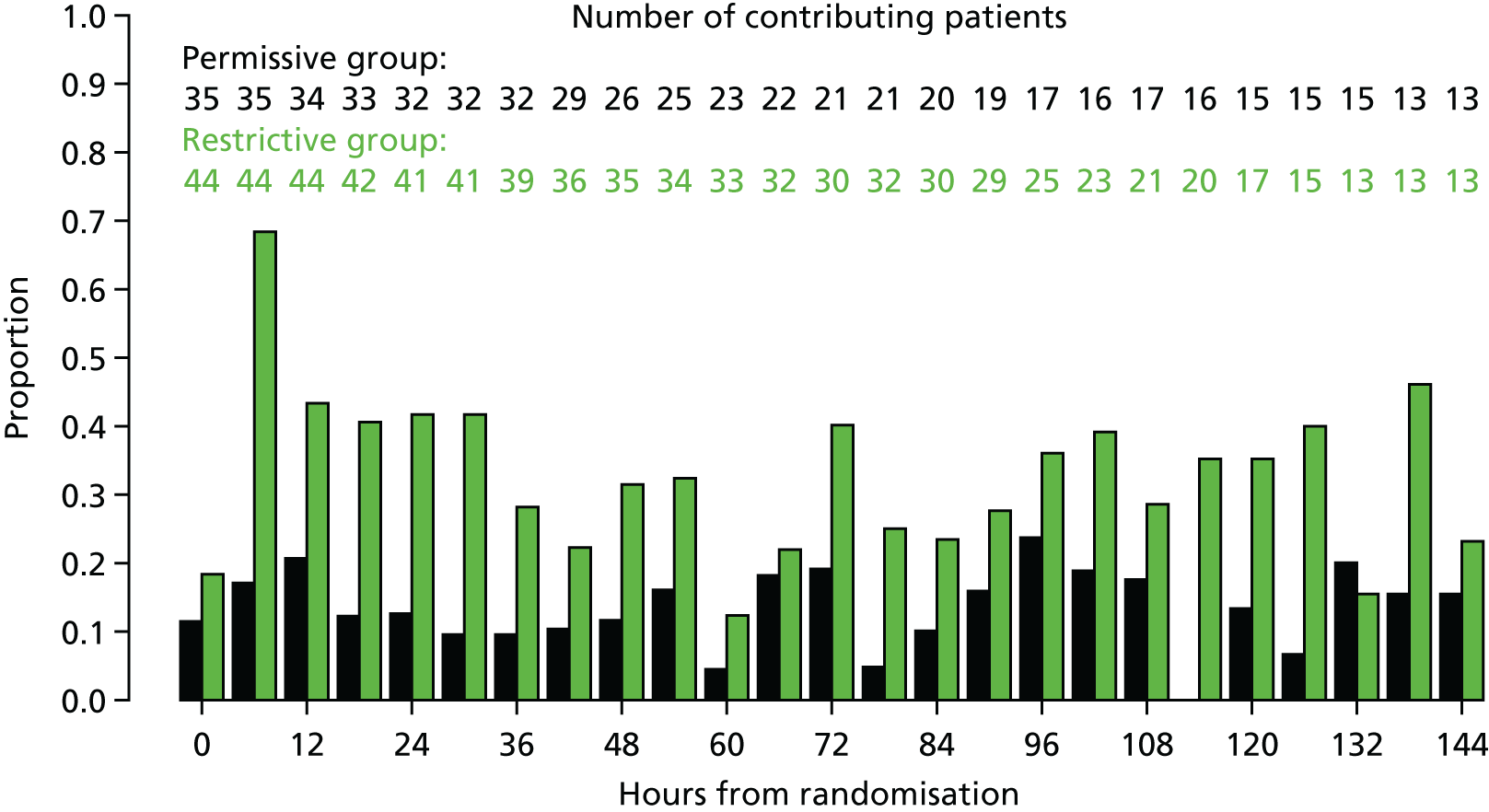
Opiates
| Hours | Treatment group | |||||
|---|---|---|---|---|---|---|
| Permissive | Restrictive | |||||
| n | Opiates (%) | n | Opiates (%) | |||
| Intravenous opiate infusion | Opiate bolus | Intravenous opiate infusion | Opiate bolus | |||
| 0–6 | 35 | 85.7 | 28.6 | 44 | 93.2 | 31.8 |
| 6–12 | 34 | 85.3 | 14.7 | 44 | 88.6 | 29.5 |
| 12–18 | 33 | 75.8 | 18.2 | 42 | 88.1 | 23.8 |
| 18–24 | 32 | 78.1 | 18.8 | 41 | 87.8 | 12.2 |
| 24–30 | 32 | 75.0 | 9.4 | 41 | 87.8 | 17.1 |
| 30–36 | 32 | 65.6 | 15.6 | 39 | 87.2 | 7.7 |
| 36–42 | 29 | 65.5 | 10.3 | 36 | 83.3 | 13.9 |
| 42–48 | 26 | 69.2 | 11.5 | 35 | 82.9 | 17.1 |
| 48–54 | 25 | 68.0 | 12.0 | 34 | 82.4 | 8.8 |
| 54–60 | 23 | 69.6 | 17.4 | 33 | 75.8 | 12.1 |
| 60–66 | 22 | 68.2 | 18.2 | 32 | 71.9 | 15.6 |
| 66–72 | 21 | 71.4 | 14.3 | 30 | 73.3 | 10.0 |
| 72–78 | 21 | 71.4 | 19.0 | 32 | 65.6 | 15.6 |
| 78–84 | 20 | 70.0 | 15.0 | 30 | 63.3 | 6.7 |
| 84–90 | 19 | 68.4 | 26.3 | 29 | 58.6 | 17.2 |
| 90–96 | 17 | 64.7 | 11.8 | 25 | 64.0 | 12.0 |
| 96–102 | 16 | 68.8 | 6.2 | 23 | 47.8 | 0.0 |
| 102–108 | 17 | 70.6 | 17.6 | 21 | 52.4 | 9.5 |
| 108–114 | 16 | 75.0 | 18.8 | 20 | 55.0 | 15.0 |
| 114–120 | 15 | 60.0 | 20.0 | 17 | 64.7 | 17.6 |
| 120–126 | 15 | 53.3 | 20.0 | 15 | 66.7 | 0.0 |
| 126–132 | 15 | 53.3 | 33.3 | 13 | 61.5 | 7.7 |
| 132–138 | 13 | 53.8 | 15.4 | 13 | 61.5 | 7.7 |
| 138–144 | 13 | 46.2 | 7.7 | 13 | 46.2 | 7.7 |
FIGURE 35.
Proportion of patients receiving opiates while mechanically ventilated.
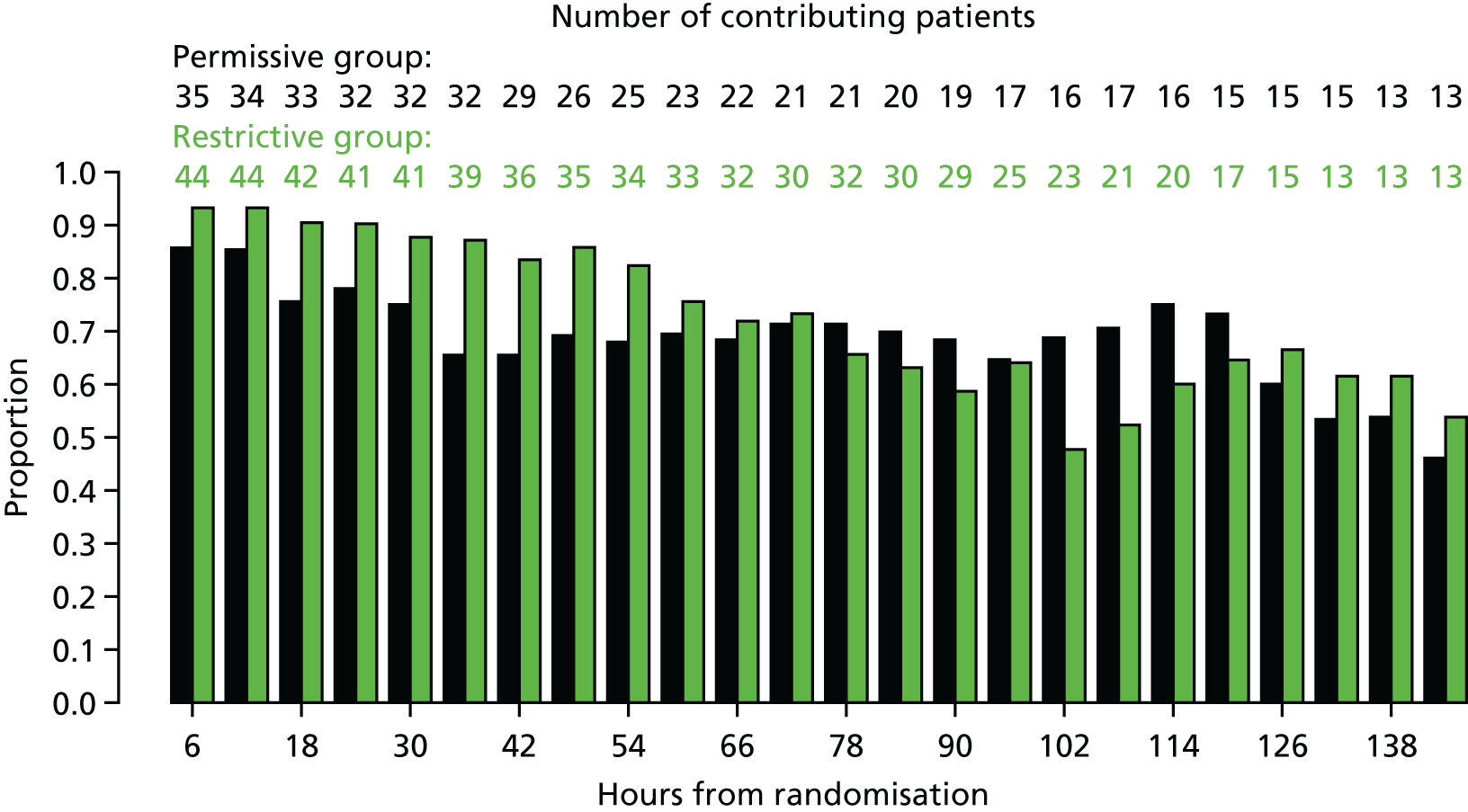
Potential outcome measures in patients receiving invasive mechanical ventilation
| Potential outcome measures | Treatment group | Effect estimate (95% CI) | ||
|---|---|---|---|---|
| Permissive (N = 35) | Restrictive (N = 44) | |||
| PICU mortality, n/N (%) | 0/34 (0) | 1/44 (2.3) | –0.02 (–0.16 to 0.28)a | |
| Ultimate discharge mortality, n/N (%) | 0/33 (0) | 1/42 (2.4) | –0.02 (–0.16 to 0.29)a | |
| Day 30 mortality, n/N (%) | 1/34 (2.9) | 1/44 (2.3) | 0.77 (0.02 to 25.26)b | –0.01 (–0.24 to 0.20)a |
| Length of hospital stay (days) | N = 34 | N = 44 | ||
| Mean (SD) | 7.0 (4.0) | 7.3 (7.6) | ||
| Median (IQR) | 6 (4–9) | 6 (4–8) | ||
| Number of survivors to PICU discharge | N = 34 | N = 43 | ||
| Mean (SD) | 7.0 (4.0) | 7.0 (7.4) | ||
| Median (IQR) | 6 (4–9) | 6 (4–8) | ||
| Days alive and free from, mean (SD) [n] | ||||
| PICU | 20.4 (5.4) [34] | 21.0 (5.4) [44] | –0.6 (–3.0 to 1.9)c | |
| Mechanical ventilation | 21.2 (5.8) [35] | 22.2 (5.4) [44] | –1.0 (–3.5 to 1.5)c | |
| Duration of organ support | ||||
| Mechanical ventilation, n (%) | 35 (100) | 44 (100) | ||
| Mean (SD) | 6.3 (4.5) | 5.6 (4.6) | 0.3 (–1.8 to 2.5)c | |
| Median (IQR) | 5 (2–8) | 5 (3–7) | ||
| Cardiovascular support, n (%) | 7 (20.0) | 12 (27.3) | ||
| Mean (SD) | 0.5 (1.1) | 1.2 (3.0) | –0.7 (–1.6 to 0.2)c | |
| Median (IQR) | 2 (2–2) | 2 (2–6) | ||
| Renal support, n (%) | 0 (0) | 2 (4.5) | ||
| Mean (SD) | 0.3 (1.7) | 0.1 (–0.8 to 1.0)c | ||
| Median (IQR) | 6 (4–9) | |||
FIGURE 36.
Length of stay in PICUs (all patients). (a) Permissive group; and (b) restrictive group.


FIGURE 37.
Days alive and free from (a and b) a PICU and (c and d) mechanical ventilation to day 28.

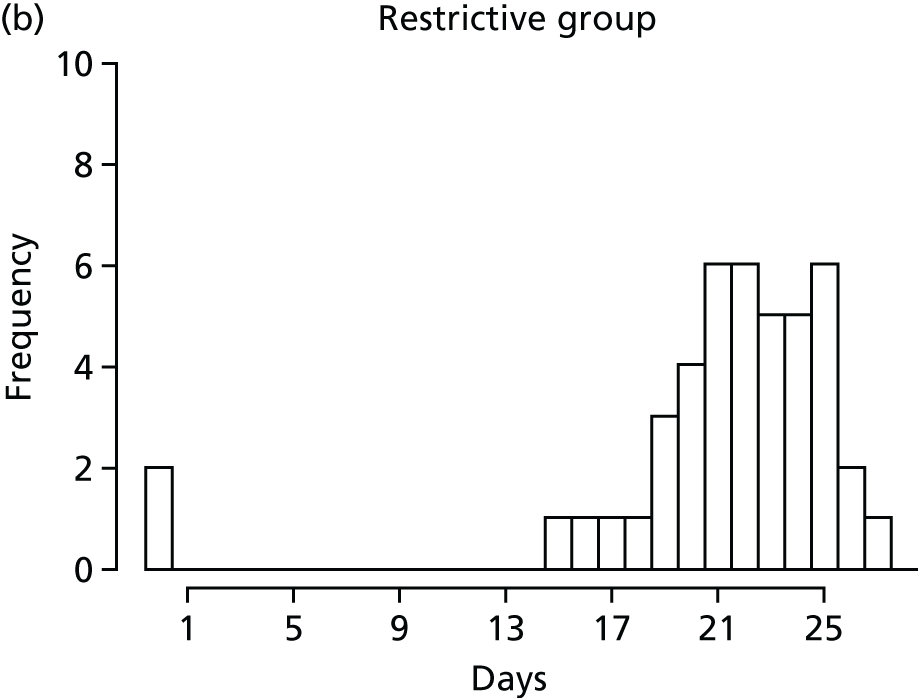

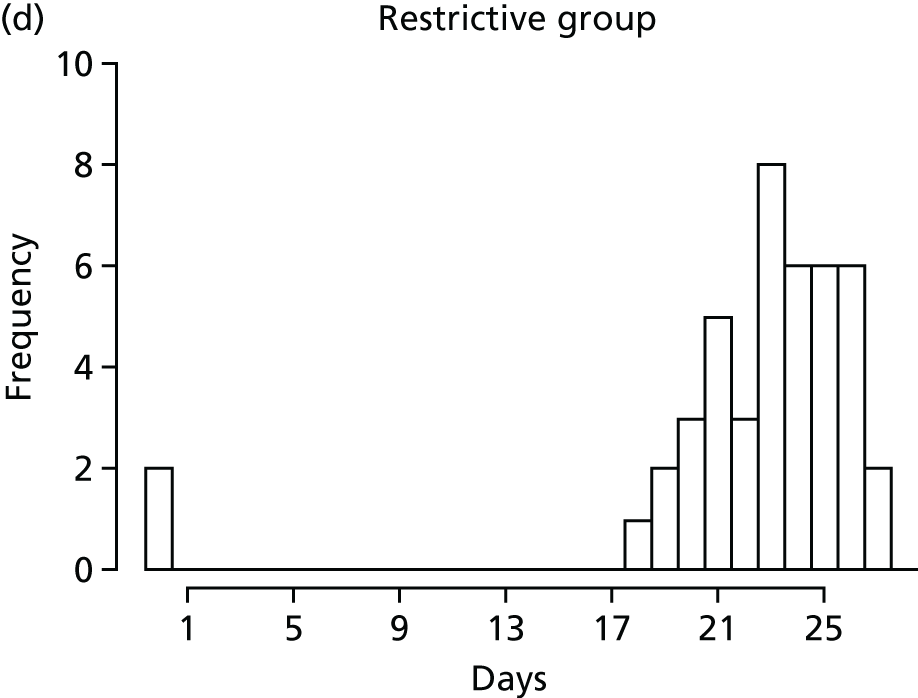
FIGURE 38.
Days receiving (a and b) mechanical ventilation, (c and d) cardiovascular support and (e and f) renal support, by treatment group.

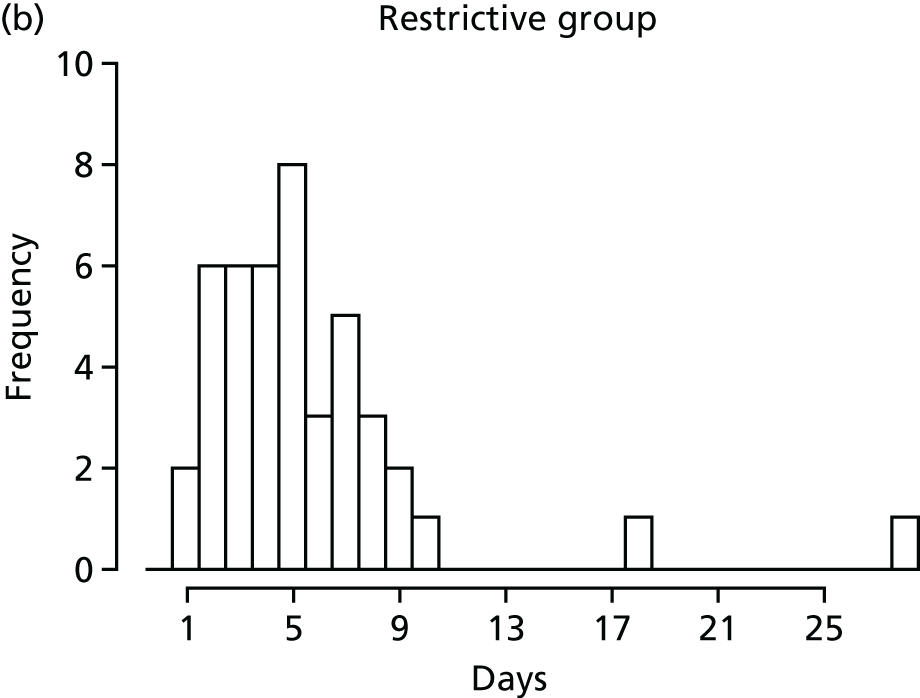




List of abbreviations
- AE
- adverse event
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN
- Clinical Research Network
- CTU
- clinical trials unit
- DMEC
- Data Monitoring and Ethics Committee
- eCRF
- electronic case report form
- FiSh
- Fluids in Shock
- GCP
- good clinical practice
- HRA
- Health Research Authority
- ICNARC
- Intensive Care National Audit and Research Centre
- IQR
- interquartile range
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- PI
- principal investigator
- PICANet
- Paediatric Intensive Care Audit Network
- PICOS
- Paediatric Intensive Care Outcome Study
- PICU
- paediatric intensive care unit
- PIM2r
- Paediatric Index of Mortality version 2r
- PIM3
- Paediatric Index of Mortality version 3
- RCT
- randomised controlled trial
- RWPC
- research without prior consent
- SAE
- serious adverse event
- SD
- standard deviation
- SMG
- Study Management Group
- TSC
- Trial Steering Committee
