Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/139/17. The contractual start date was in May 2015. The draft report began editorial review in November 2016 and was accepted for publication in May 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ioannis Gallos, Metin Gülmezoglu, Justus Hofmeyr and Arri Coomarasamy have been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of postpartum haemorrhage that were considered for inclusion in this review. Ferring Pharmaceuticals (Saint-Prex, Switzerland) and Novartis Pharmaceuticals UK Ltd (Surrey, UK) have supplied carbetocin and oxytocin to these studies. Ioannis Gallos, Metin Gülmezoglu, Justus Hofmeyr and Arri Coomarasamy have not participated in decisions regarding inclusion of these trials in this review or any tasks related to them such as data extraction or quality assessment. Arri Coomarasamy is involved in a World Health Organization-sponsored randomised controlled trial of carbetocin versus oxytocin, supported by Merck for Mothers (Merck & Co., Inc., Kenilworth, NJ, USA). Metin Gülmezoglu was involved in a large multicentre trial included in the review as part of the central co-ordination unit. As part of the central co-ordination unit, he is also involved in an ongoing World Health Organization-sponsored randomised controlled trial of carbetocin versus oxytocin supported by Merck for Mothers. Abi Merriel is part-funded by Ammalife (a UK-registered charity 1120236) and the Birmingham Women’s NHS Foundation Trust. Harry Gee and Arri Coomarasamy are trustees of Ammalife. Jonathan Deeks is a member of the Health Technology Assessment (HTA) Commissioning Board and the HTA Efficient Study and Designs Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gallos et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Existing knowledge
Postpartum haemorrhage
An estimated 289,000 women worldwide died during childbirth in 2013. 1 Postpartum haemorrhage (PPH) is the leading direct cause of maternal death worldwide, accounting for up to one-third of all maternal deaths. 2 PPH is very common, affecting 1 in 10 women at childbirth in Europe and 67,000 women in England alone every year. 3,4 In the UK, death from PPH is usually averted, but it remains an important cause of severe morbidity (e.g. when receiving a blood transfusion) and surgery, including hysterectomy. 5
The third stage of labour, defined as the period of time from birth until the birth of the placenta, and the immediate postpartum period are the most hazardous phases of childbirth because of the risk of PPH. The World Health Organization (WHO) defines PPH as blood loss exceeding 500 ml in the first 24 hours after birth. 6 Though healthy women can physiologically adapt to this amount of blood loss, for women with a coexisting disease, such as anaemia, it can cause considerable morbidity and mortality. The primary cause of PPH, as defined by WHO, is uterine atony, which accounts for 75% of cases. 7 Even though risk factors for adverse maternal outcomes from severe haemorrhage have been identified,8 PPH is often unpredictable because it occurs in the absence of identifiable clinical or historical risk factors. 9 Therefore, effective prevention of PPH is advocated for all women during childbirth. 6 The routine administration of uterotonic drugs during the third stage of labour is a key intervention that prevents PPH, although there is uncertainty about which drug may be the most effective.
Uterotonic drugs
The active management of the third stage of labour refers to a package of interventions. The administration of uterotonic drugs to prevent PPH is the main intervention within this package and can prevent two-thirds of PPH. 6,10 Uterotonics are also essential for the treatment of PPH, but treatment is not the focus of this review.
Several different uterotonic drugs have been used for preventing PPH. These drugs include ergometrine, misoprostol (Cytotec®; Pfizer Inc., New York, NY, USA), misoprostol plus oxytocin (Syntocinon®; Novartis International AG, Basel, Switzerland), carbetocin (Pabal®; Ferring Pharmaceuticals, Saint-Prex, Switzerland), ergometrine plus oxytocin and oxytocin when used alone.
Oxytocin
Oxytocin is the most widely used uterotonic drug. At low doses, it produces rhythmic uterine contractions that are indistinguishable in frequency, force and duration from those observed during spontaneous labour; however, at higher dosages, it causes sustained tetanic uterine contractions. 11 It has a short half-life, approximately 3–5 minutes, and can be used as an infusion to maintain uterine contraction. When used intramuscularly, the latent phase lasts 2–5 minutes, but the uterine activity can last 2–3 hours. 11 However, oxytocin cannot be used orally. Oxytocin is unstable at room temperature and it requires cold storage and transport. It cannot be given intravenously as a large bolus, because it can cause severe hypotension. 12 Owing to its antidiuretic effect, water intoxication can occur with prolonged infusion of oxytocin. 11 Oxytocin has a favourable side-effect profile for common side effects, such as nausea and vomiting, but the evidence is scarce. 13
Ergometrine
Ergometrine and methylergometrine are ergot alkaloids that increase the uterine muscle tone by causing continuous tetanic contractions. It takes 2–5 minutes after intramuscular injection for the drug to become effective and the plasma half-life is 30–120 minutes. 14 However, ergometrine and methylergometrine are unstable in heat and cannot be used orally. 15 They are vasoconstrictive and increase the risk of hypertension post partum. 16 Other side effects with ergot alkaloids are pain after birth, nausea and vomiting. 16
Misoprostol
Misoprostol is a prostaglandin E1 analogue that is licensed for the prevention and treatment of gastric ulcers. It is widely used off-label as a uterotonic agent. 17 It is water soluble and heat stable. 18 It takes 9–15 minutes after sublingual, oral, vaginal and rectal use for the drug to be effective. The half-life is about 20–40 minutes. Oral and sublingual routes have the advantage of rapid onset of action, whereas the vaginal and rectal routes result in prolonged activity and greater bioavailability. 19 However, misoprostol is associated with side effects, such as diarrhoea, abdominal pain, nausea and vomiting, shivering and pyrexia. 17
Carbetocin
Carbetocin is a newer long-acting synthetic analogue of oxytocin with agonist properties. After intravenous injection, it produces tetanic uterine contractions within 2 minutes, lasting for approximately 6 minutes followed by rhythmic contractions for 60 minutes. 20 When carbetocin is administered by an intramuscular injection the tetanic contractions last for approximately 11 minutes and the rhythmic contractions for 120 minutes. 20 Carbetocin is heat stable and the side-effect profile appears to be similar to oxytocin. 21
Combinations of uterotonic drugs
The use of combinations of uterotonic drugs is also popular and the most commonly used preparation is oxytocin plus ergometrine. This combination is suggested to be associated with a statistically significant reduction of PPH blood loss of ≥ 500 ml when compared with oxytocin alone, attributable to the additive ergometrine effect. 22 Another combination is oxytocin plus misoprostol, which is also found to be associated with a small reduction in PPH blood loss of ≥ 500 ml. 17 However, both these combinations are associated with significant side effects and, despite the small difference in PPH, there is no difference found for severe PPH when compared with oxytocin. This has led the WHO to recommend oxytocin over these combinations. 6
The WHO recommends that all women giving birth should be offered uterotonics during the third stage of labour for the prevention of PPH; oxytocin [given intramuscularly/intravenously at a dose of 10 international units (IU)] is the uterotonic drug of choice. 6 Other injectable uterotonics and misoprostol are recommended as alternatives for the prevention of PPH in settings where oxytocin is not available.
Costs to the National Health Service
Treatment of PPH costs the NHS £32–180M per year. The National Institute for Health and Care Excellence (NICE) recently estimated the costs of treating PPH to be between £488 and £2700 for each woman, depending on the severity of PPH. 23 Treating PPH also has societal implications, as it can reduce economic productivity by causing physical disability or a psychological burden to parents and families. A relative risk reduction of 34% in PPH occurrence can represent a saving of £10–60M per year for the NHS, with important benefits for public health.
Existing research
Before conducting the search through Cochrane, a scoping literature search was conducted for trials and reviews of the use of uterotonics for preventing PPH. The databases MEDLINE, EMBASE, Cochrane Controlled Trials Register, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, ISI Proceedings, ISRCTN Register and metaRegister of Current Controlled Trials were searched from the respective database inception to July 2014. The search terms aimed to capture trials assessing the effectiveness of uterotonic drugs to prevent PPH include ‘post-partum period of haemorrhage’, ‘third stage of labour’, ‘caesarean section’ and ‘obstetric delivery’ AND (‘Oxytocin’, ‘misoprostol’, ‘ergometrine’, ‘syntometrine’, ‘carbetocin’ and ‘prostaglandins’). The scoping literature search had identified 445 randomised trials that could be eligible for inclusion in the network meta-analysis (NMA). There were five separate Cochrane reviews,13,16,17,21,22 including an aggregate total of 115 trials and 77,447 participants, that have compared a uterotonic drug against another or with a placebo or no treatment. These meta-analyses were suggesting that oxytocin plus ergometrine [odds ratio (OR) 0.82, 95% confidence interval (CI) 0.71 to 0.95], oxytocin plus misoprostol [risk ratio (RR) 0.71, 95% CI 0.53 to 0.95] and carbetocin (RR 0.66, 95% CI 0.42 to 1.06) may be more effective than oxytocin in preventing PPH. The Cochrane reviews were pairwise meta-analyses and, therefore, could only compare two drugs that have been compared directly in head-to-head trials (direct evidence), did not make use of the large amount of indirect evidence available and could not always be used for drawing inferences across all the possible comparisons. In the absence of a single randomised controlled trial comparing all uterotonic drugs, uncertainty remained over their relative effectiveness and ranking.
The existing Cochrane reviews were also becoming out of date. In total, 58 new trials (n = 22,071 participants) were identified that could be eligible for inclusion in these reviews and 43 active randomised trials (n = 63,326 participants) due for completion before the end of 2015 (Table 1). These were assessed for inclusion in the NMA in addition to the existing evidence (see Figure 1).
| Cochrane review (first author and date of publication) | Included trials (number of participants) | Latest search update | Available comparisons | Trials awaiting classification (number of participants) | Active trials to be completed by December 2015 (number of participants) |
|---|---|---|---|---|---|
| Liabsuetrakul et al.,16 2007 | 6 (n = 1996) | 30 April 2011 | Ergometrine vs. placebo or no treatment | 2 (n = 340) | 0 |
| McDonald et al.,22 2004 | 6 (n = 9332) | 30 April 2007 | Oxytocin plus ergometrine vs. oxytocin | 4 (n = 946) | 3 (n = 6860) |
| Su et al.,21 2012 | 11 (n = 2635) | 1 March 2011 | Carbetocin vs. oxytocin | 20 (n = 5898) | 17 (n = 41,583) |
| Carbetocin vs. oxytocin plus ergometrine | |||||
| Tunçalp et al.,17 2012 | 72 (n = 52,678) | 7 January 2011 | Misoprostol vs. oxytocin | 24 (n = 10,666) | 15 (n = 8067) |
| Misoprostol vs. ergometrine | |||||
| Misoprostol vs. placebo or no treatment | |||||
| Misoprostol vs. oxytocin plus ergometrine | |||||
| Misoprostol vs. oxytocin plus misoprostol | |||||
| Westhoff et al.,13 2013 | 20 (n = 10,806) | 21 May 2013 | Oxytocin vs. placebo or no treatment | 8 (n = 4221) | 8 (n = 6816) |
| Oxytocin vs. ergometrine | |||||
| Oxytocin plus ergometrine vs. ergometrine | |||||
| Total | 115 (n = 77,447) | 58 (n = 22,071) | 43 (n = 63,326) |
A systematic review and a NMA were performed synthesising all available, up-to-date direct and indirect evidence of relative treatment effects in a single coherent analysis for all uterotonic drugs. Indirect evidence is obtained when the relative effectiveness of two competing drugs is inferred through a common comparator, even though this pair may not have been compared directly. 24 The NMA aimed to provide robust estimates or relative effectiveness, side-effect profile and the relative ranking for each uterotonic drug with a model-based economic evaluation.
Objectives
Primary
To identify the most effective and cost-effective uterotonic drug(s) to prevent PPH, and to generate a clinically useful ranking of available uterotonics according to their effectiveness.
Secondary
-
To provide the relative effectiveness and side-effect profile of each drug for the primary outcomes within (1) treatment subgroups (different dosages and regimens and routes of administration of each uterotonic drug), and (2) population subgroups (prior risk of PPH, mode of birth and health-care setting).
-
To produce effectiveness and side-effect hierarchies of all uterotonic drugs considered, and to estimate the probability that each drug is the best for each outcome.
-
To evaluate the cost-effectiveness for each drug for preventing PPH overall and in the subgroups defined earlier in the UK.
Chapter 2 Review methods
Criteria for considering studies for this review
Types of studies
All randomised controlled comparisons or cluster trials of effectiveness or side-effects of uterotonic drugs for preventing PPH were included. Quasi-randomised trials and crossover trials were excluded.
Types of participants
The review included studies of pregnant women following a vaginal birth or caesarean section conducted in hospital and community settings.
Types of interventions
The study considered trials of uterotonic drugs, described by the WHO (ergometrine, misoprostol, misoprostol plus oxytocin, carbetocin, ergometrine plus oxytocin, oxytocin and a placebo or no treatment), administered prophylactically by health-care professionals for preventing PPH via any systemic route (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion) compared with another uterotonic drug or with a placebo or no treatment. Trials were included in which non-pharmacological co-interventions, such as controlled cord traction, cord clamping or uterine massage, were performed as a randomised intervention in all arms of the trial and the effects of such co-interventions were tested through a sensitivity analysis. All drugs were stratified according to mode of birth, prior risk of PPH, health-care setting and specific dosage, regimen and route of drug administration to detect inequalities in subgroups that could affect comparative effectiveness.
Multiarm trials that compared different dosages, regimens or routes of one uterotonic drug, but also compared any of these drugs versus another uterotonic drug, were included. Intervention arms of different dosages, regimens or routes of administration of the same uterotonic drug were merged together for the global analysis of all outcomes and treated as separate independent comparisons for only the relevant subgroup analysis according to dosage, regimen and route of drug administration, while considering the correlation between the comparisons. Trials comparing exclusively different dosages, regimens or routes of drug administration of the same uterotonic drug were excluded. The review was restricted to studies evaluating uterotonic drugs administered systemically at the birth of the baby to prevent PPH. Studies considering non-uterotonic drugs, uterotonic drugs administered locally (e.g. via intraumbilical or intrauterine routes) or at a later stage of birth (e.g. for the treatment of PPH or for retained placenta) were excluded.
For this review, it was assumed that any woman that meets the inclusion criteria is, in principle, equally likely to be randomised to any of the eligible uterotonic drugs.
Types of outcome measures
The study estimated the relative effects and ranking of the competing interventions according to the following outcomes.
Primary outcomes
The primary outcomes of the review were:
-
PPH blood loss of ≥ 500 ml
-
PPH blood loss of ≥ 1000 ml.
Secondary outcomes
The secondary outcomes of the review were:
-
maternal deaths
-
maternal deaths or severe morbidity events adapted from the WHO’s ‘near-miss’ criteria25 to include major surgery [laparotomy, uterine artery ligation, internal iliac artery ligation, B-Lynch suture, hysterectomy, extensive vaginal repair, admission to the intensive care unit or vital organ failure (temporary or permanent)]
-
additional uterotonics requirement
-
transfusion requirement
-
manual removal of the placenta
-
mean volume of blood loss (ml)
-
mean duration of the third stage of labour (minutes)
-
change in haemoglobin (Hb) measurements before and after birth (g/l)
-
clinical signs of excessive blood loss (as defined by the triallists)
-
neonatal unit admission requirement
-
breastfeeding at discharge
-
side effects, such as nausea, vomiting, hypertension, headache, tachycardia, hypotension, abdominal pain, fever and shivering, in the first 24 hours post partum.
There are two primary outcomes for this NMA: a PPH blood loss of ≥ 500 ml and ≥ 1000 ml. The former is the WHO’s definition6 of PPH, but the latter was considered as one of the three critical outcomes (together with blood transfusion and maternal death) for the WHO’s recommendations6 for PPH prevention in which outcomes were rated by an independent panel.
Data sources
Electronic searches
The trials search co-ordinator for the pregnancy and childbirth group performed the search (September 2015) using their trials register, which contained trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) weekly searches of MEDLINE (via Ovid)
-
weekly searches of EMBASE (via Ovid)
-
monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost)
-
hand-searches of 30 journals and the proceedings of major conferences
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central e-mail alerts.
Details of the search strategies for CENTRAL, MEDLINE, EMBASE and CINAHL, the list of hand-searched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialised Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group (CPCG). 26 Trials identified through the searching activities described above are each assigned to a review topic (or topics). The trials search co-ordinator searched the register for each review using the topic list rather than keywords (see Appendix 1).
In addition, ClinicalTrials.gov and the WHO’s International Clinical Trials Registry Platform (ICTRP) were searched for unpublished trial reports. The search terms we used are given in Appendix 1.
Searching other resources
Additional relevant references cited in papers, identified through the above search strategy, were retrieved and the full texts of trials initially identified as abstracts were searched. Information was sought from primary authors to investigate whether or not these studies met the study’s eligibility criteria, and to obtain outcome and study data. Trials that compared at least two of the drugs were eligible and all possible comparisons formed by the drugs of interest were searched for. No language or date restrictions were applied.
Study selection
Three review authors retrieved and independently assessed for inclusion all the potential studies that were identified (IDG, AM and HW). Any disagreements were resolved through discussion or, if required, in consultation with a fourth person (AC). A study flow diagram was created to map out the number of records identified, included and excluded (Figure 1).
FIGURE 1.
Network plot of eligible drug comparisons for the prevention of PPH.

Data extraction
An electronic form was designed on Microsoft Access® 2010 (Microsoft Corporation, Redmond, WA, USA) to extract data. For eligible studies, at least three review authors independently extracted the data using a blank electronic form (IDG, HW, AM, DL, HG or OT). Discrepancies were resolved through discussion or, if required, another person (AC) was consulted. Data were entered into Stata® version 14 (StataCorp LP, College Station, TX, USA) and Review Manager software 5.2 [2014 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark)] and checked for accuracy. When information was unclear, an attempt was made to contact authors of the original reports to provide further details.
Data extracted
Outcome data
From each included study the number of participants, the gestational age and parity of participants, and any exclusion criteria were extracted. In addition, the interventions being compared and their respective primary and secondary outcomes were extracted. All relevant arm-level data were extracted (e.g. number of events and number of patients for binary outcomes).
Data on potential effect modifiers
From each included study the following data were extracted that may have acted as effect modifiers:
-
mode of birth (vaginal birth or caesarean section)
-
prior risk of PPH (as defined by triallists and categorised as low, high, mixed or not stated)
-
dosage, regimen and route of drug administration (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion)
-
setting of the study (community or hospital).
Other data
From each included study the following additional information was extracted:
-
country or countries in which the study was performed
-
date of publication
-
type of publication (full text publication, abstract publication, unpublished data)
-
trial registration reference.
Critical appraisal
At least three (IDG, HW, AM, DL, HG or OT) review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. 27 Any disagreements were resolved by discussion or by involving a third assessor (AC).
(1) Random sequence generation (checking for possible selection bias)
For each included study, the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not the study should produce comparable groups were described. Trials rated as being at a high risk of bias for allocation sequence generation were excluded from the review (any non-random process, e.g. odd or even date of birth, hospital or clinic record number).
The methods were assessed as being at:
-
a low risk of bias (any truly random process, e.g. random number table, computer random number generator)
-
an unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, the methods used to conceal allocation to interventions prior to assignment and to assess whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment were described.
The methods were assessed as being at:
-
a low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes)
-
a high risk of bias (e.g. open random allocation, unsealed or non-opaque envelopes, alternation, date of birth)
-
an unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received were described. Studies were considered as being at a low risk of bias if they were blinded or, if judged, that the lack of blinding would be unlikely to have affected the results.
The methods were assessed as being at a:
-
low, high or unclear risk of bias for participants
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received were described.
The methods used to blind outcome assessment were assessed as being at a:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias caused by the amount, nature and handling of incomplete outcome data)
For each included study, and for each primary outcome, the completeness of data, including attrition and exclusions from the analysis, was described. The reasons were stated for attrition and exclusions and the numbers included in the analysis at each stage (compared with the total randomised participants), and a judgement was made on whether missing data were balanced across groups or were related to outcomes.
The methods to handle incomplete outcome data were assessed as being at a:
-
low risk of bias (e.g. no missing outcome data or missing outcome data balanced across groups and < 10% of missing outcome data)
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation or > 10% of missing outcome data)
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study how the possibility of selective outcome reporting bias was investigated and what was found were described.
The methods were assessed as being at a:
-
low risk of bias (in which it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported)
-
high risk of bias (in which not all the study’s prespecified outcomes had been reported, one or more reported primary outcomes were not prespecified, outcomes of interest were reported incompletely and so could not be used, or the study failed to include results of a key outcome that would have been expected to have been reported)
-
unclear risk of bias.
(6) Other bias [checking for bias caused by problems not covered by (1) to (5)]
For each included study any important concerns about other possible sources of bias, such as the source of funding and potential conflicts of interest, were described.
The interests were assessed as being at a:
-
low risk of other bias (e.g. public funding or no funding and no significant conflicts of interest identified)
-
high risk of other bias (e.g. industry funding or significant conflicts of interest identified)
-
unclear risk of other bias.
Another source of bias could be generated by the method of measuring blood loss. An assessment was made of the method described in each study and it was classified as being at a:
-
low risk of other bias (e.g. objective measurements, such as weighing sponges, measurements in drapes, volumetric assessment and tagged red cells)
-
high risk of other bias (subjective measurement, such as clinical or visual estimates)
-
unclear risk of other bias (unspecified methods of measurement).
(7) Overall risk of bias
Explicit judgements were made about whether or not studies were rated as being at a high risk of bias, according to the criteria given in the Cochrane handbook. 27 With reference to (1)–(6), the likely magnitude and direction of the bias, and whether or not the magnitude and direction of the bias was considered to have an impact on the findings were assessed. For the primary outcomes, quality items and judged trials were rated as being at a ‘low risk of bias’ if they were double-blinded and had allocation concealment, with little loss to follow-up (< 10%). Trials were judged as being at an ‘intermediate risk of bias’ if they demonstrated adequate allocation concealment, with assessor blinding and little loss to follow-up (< 10%). Alternatively, trials were considered to be at a ‘high risk of bias’. See Sensitivity analysis for information about how this risk of bias has impacted the results.
Measures of treatment effect
Relative treatment effects
Relative treatment effects were summarised for dichotomous outcomes as the RR and 95% CIs. For continuous scales of measurement, the mean difference with 95% CIs was used. 28
Relative treatment ranking
The ranking probabilities were estimated for all treatments of being at each possible rank for each intervention, then a treatment hierarchy was obtained using the surface under the cumulative ranking curve (SUCRA). 29 The SUCRA index can also be expressed as a percentage interpreted as the percentage of effectiveness or side effects of a treatment that would be ranked first without uncertainty.
Unit of analysis
Cluster randomised trials
Cluster randomised trials were included in the analyses along with individually randomised trials. The standard errors of the trials were adjusted using the methods described in the Cochrane handbook using an estimate of the intracluster correlation coefficient derived from the trial. 27 It was considered reasonable to combine the results from cluster randomised and individually randomised trials, as there is little heterogeneity between the study designs and any interaction between the relative effects of interventions and the choice of randomisation unit is considered to be unlikely. However, performed sensitivity analyses were performed to assess the validity of this assumption for the primary outcomes.
Crossover trials
This type of trial was not deemed appropriate for this intervention.
Multiarm trials
Multiarm trials were included and the correlation between the effect sizes were accounted for in the NMA. Multiarm studies were treated as multiple independent comparisons in pairwise meta-analyses.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data was explored in the overall assessment of treatment effect by using sensitivity analyses. For all outcomes, analyses were carried out, as far as possible, on a modified intention-to-treat basis, that is, all participants randomised to each group were included in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The number of participants randomised minus any participants whose outcomes are known to be missing was used as the denominator for each outcome in each trial. No assumptions or imputations were made for the missing outcomes. If any participants were inappropriately excluded by the triallists from the analysis, and the data were available, these participants were reincluded in the analyses.
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, descriptive statistics were generated for each trial and study population characteristics across all included trials that compare each pair of interventions. The presence of clinical heterogeneity was assessed within each pairwise comparison by comparing these characteristics.
Assessment of transitivity across treatment comparisons
The assumption of transitivity was assessed by comparing the distribution of potential effect modifiers across the different pairwise comparisons. In this context it was expected that the transitivity assumption holds assuming the following: (1) the common treatment used to compare different uterotonics indirectly is similar when it appears in different trials (e.g. oxytocin is administered in a similar way in oxytocin vs. misoprostol trials and in oxytocin vs. oxytocin plus ergometrine trials); and (2) all pairwise comparisons do not differ with respect to the distribution of effect modifiers (e.g. the design and study characteristics of oxytocin vs. misoprostol trials are similar to oxytocin vs. oxytocin plus ergometrine trials). The assumption of transitivity is evaluated epidemiologically by comparing the clinical and methodological characteristics of sets of studies grouped by treatment comparisons.
Assessment of reporting biases
Potential reporting bias was evaluated for the primary outcomes by assessing the sensitivity of results to exclusion of studies with < 400 participants.
Data synthesis
Methods for direct treatment comparisons
Initially, standard pairwise meta-analyses were performed using a random-effects model,30 in Stata, for every treatment comparison with at least two studies.
Methods for indirect and mixed comparisons
The NMA was performed within a frequentist framework using multivariate meta-analysis models. 31 All analyses were carried out using Stata statistical software, version 14. The network suite of Stata commands designed for this purpose was used. 32 The a priori belief was that a random-effects model is more appropriate because a degree of clinical heterogeneity between trials was expected.
Assessment of statistical heterogeneity
Assumptions when estimating the heterogeneity
In pairwise meta-analyses different heterogeneity variances were estimated for each pairwise comparison. In the NMA, a common estimate was assumed for the heterogeneity variance across the different comparisons, by defining a proportional between-studies variance–covariance matrix. 31
Measures and tests for heterogeneity
The presence of heterogeneity was statistically assessed within each pairwise comparison for the primary outcomes using the I2-statistic, which measures the percentage of variability that cannot be attributed to random error. 33 The assessment of statistical heterogeneity in the entire network is based on the magnitude of the heterogeneity variance parameter estimated from the multivariate meta-analysis model.
Assessment of statistical inconsistency
To check the assumption of consistency in the entire network, the ‘design-by-treatment’ interaction model, as described by Higgins et al.,34 was used. This model accounts for a different source of inconsistency that can occur when studies with different designs (i.e. two-arm trials vs. three-arm trials) give different results as well as disagreement between direct and indirect evidence. Using this approach, the presence of inconsistency was inferred from any source in the entire network based on a chi-squared test.
Investigation of heterogeneity and inconsistency
When important heterogeneity and/or inconsistency was found, the possible sources for primary outcomes were explored. Databases were rechecked for mistakes and inconsistencies in data extraction and entry. When sufficient studies were available, multivariate meta-analyses or subgroup analyses were performed by using the following potential effect modifiers as possible sources of inconsistency and/or heterogeneity:
-
Population – prior risk of PPH (high vs. low), mode of birth (vaginal birth vs. caesarean section) and setting (hospital vs. community).
-
Intervention – dose, regimen and route.
-
Quality of the studies – studies are rated as being at a ‘low risk of bias’ if they are double-blinded and have allocation concealment with little loss to follow-up (< 10%). The concealed studies with assessor blinding and little loss to follow-up (< 10%) are rated as being at an ‘intermediate risk of bias’ and the rest are rated as being at a ‘high risk of bias’. Assessor blinding was considered to be very important, in order to eliminate any risk of bias in subjective measurements or estimates of blood loss (not all studies measure this outcome objectively). Protocol publication was considered in advance of the results to be an unsuitable criterion for sensitivity analyses, because protocol publication has only became widespread in recent years.
-
Funding source – high versus low risk of bias.
-
Whether or not an objective method of outcome assessment was employed (objective vs. subjective) Objective methods of blood loss measurement were considered to be all methods that employed a measurement of the blood loss. This is in contrast to subjective methods, in which a health-care professional is estimating the blood loss, usually visually.
-
Trial size – excluding small studies, in recognition of the greater likelihood for small studies than large or multicentre studies to suffer publication bias. In terms of trial size, there is evidence that smaller studies can exaggerate estimated benefits. 35 However, the cut-off point for deciding the definition of a small study can vary between research topics. For this topic, it appears that trials with > 400 participants were more likely to be rated as being of higher quality, prospectively registered and, overall, being rated as at a low risk of bias.
-
Randomisation unit – cluster versus individual.
Subgroup analysis
For the primary outcomes, the following subgroup analyses were carried out:
-
population – prior risk of PPH (high vs. low), mode of birth (vaginal birth vs. caesarean section) and setting (hospital vs. community)
-
intervention – dose, regimen and route.
Subgroup differences were assessed by evaluating the relative effects and assessing model fit.
Sensitivity analysis
For the primary outcomes, sensitivity analysis was performed for the following:
-
the quality of the studies (as described previously)
-
funding source (as described previously)
-
whether or not an objective method of outcome assessment was employed
-
trial size (as described previously)
-
trials that randomised participants to co-interventions, such as uterine massage or controlled cord traction
-
trials with > 10% missing data
-
trials published before 1990
-
randomisation unit (cluster vs. individual)
-
choice of relative effect measure (RR vs. OR)
-
use of fixed-effects versus random-effects model.
Differences were assessed by evaluating the relative effects and assessing model fit.
Changes to the protocol
Preliminary protocol development
-
26 February 2014: meta-analytic title registration (not including cost-effectiveness analysis) with the Cochrane Collaboration.
-
5 September 2014: submission of the initial study proposal, including cost-effectiveness analysis, to the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme.
-
10 January 2015: submission of a more detailed study proposal, including cost-effectiveness analysis to the NIHR HTA programme (recommendation for funding 5 February 2015).
Publication of protocol
-
22 April 2015: finalisation of the comprehensive study protocol, including cost-effectiveness analysis, for the NIHR HTA programme, version 1.0
-
30 April 2015: typographic corrections only to the comprehensive study protocol, including cost-effectiveness analysis for the NIHR HTA programme, version 1.1
-
18 May 2015: publication of the meta-analytic protocol (not including the cost-effectiveness analysis) by the Cochrane Collaboration [contents in accordance with (4) Incomplete outcome data (checking for possible attrition bias caused by the amount, nature and handling of incomplete outcome data) and (5) Selective reporting (checking for reporting bias) above; available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011689/pdf (accessed 25 April 2018)].
Changes post publication
-
November 2016: submission of the NMA and cost-effectiveness analysis to the NIHR HTA programme, with meta-analysis performed in Stata rather than WinBUGS (MRC Biostatistics Unit, Cambridge, UK) for reasons of future reproducibility.
Patient and public involvement
The study team undertook patient and public involvement (PPI) primarily as consultation and collaboration to ensure that the study objectives and outcomes appropriately reflected the priorities of maternity service users. This was also undertaken to disseminate any findings of relevance to women of reproductive age and a wider public. The study team sought, and drew on, the contributions of lay stakeholders to conceive and develop the project, with facilitation from Gillian Gyte, who is the consumer editor of the CPCG and is a long-standing member of the National Childbirth Trust (NCT). Comments and suggestions were collected from the CPCG consumer panel via editorial feedback to the systematic review protocol prior to publication of this document and, subsequently, from the CPCG consumer panel and NCT representatives. Gillian Gyte established a study-specific PPI group (a group of women with experience of childbirth and willing to comment on provisional drafts of this report and the Cochrane review). The group comprised 10 women, six of whom had experienced PPH. These women also contributed to the Plain English summary of this report and the plain language summary of the Cochrane review. 36 Comments and suggestions were also collected from the Public and Researcher Involvement in Maternity and Early pregnancy (PRIME) research group. The comments and suggestions were collected, in April 2016, from 19 members, at a face-to-face meeting of the PRIME research group.
Overall, the women and parents who contributed to the study articulated the belief that reducing the occurrence of PPH is a top priority for preserving maternal well-being and endorsed the study objectives to identify the most effective uterotonic agent with minimal side effects. The women and parents encouraged the research team to evaluate additional outcomes, including women’s views regarding the drugs used, clinical signs of excessive blood loss, abdominal pain after birth, neonatal unit admissions and breastfeeding.
Chapter 3 Results
Study selection
The results of the search strategy are summarised in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram (Figure 2). Included in this systematic review are 137 randomised trials for a total of 87,466 women (see Appendix 2 for details). 37–173 Excluded, with reasons, are 133 randomised trials; the specific references and reasons for exclusion are given in Appendices 3–6.
FIGURE 2.
The PRISMA study flow diagram.
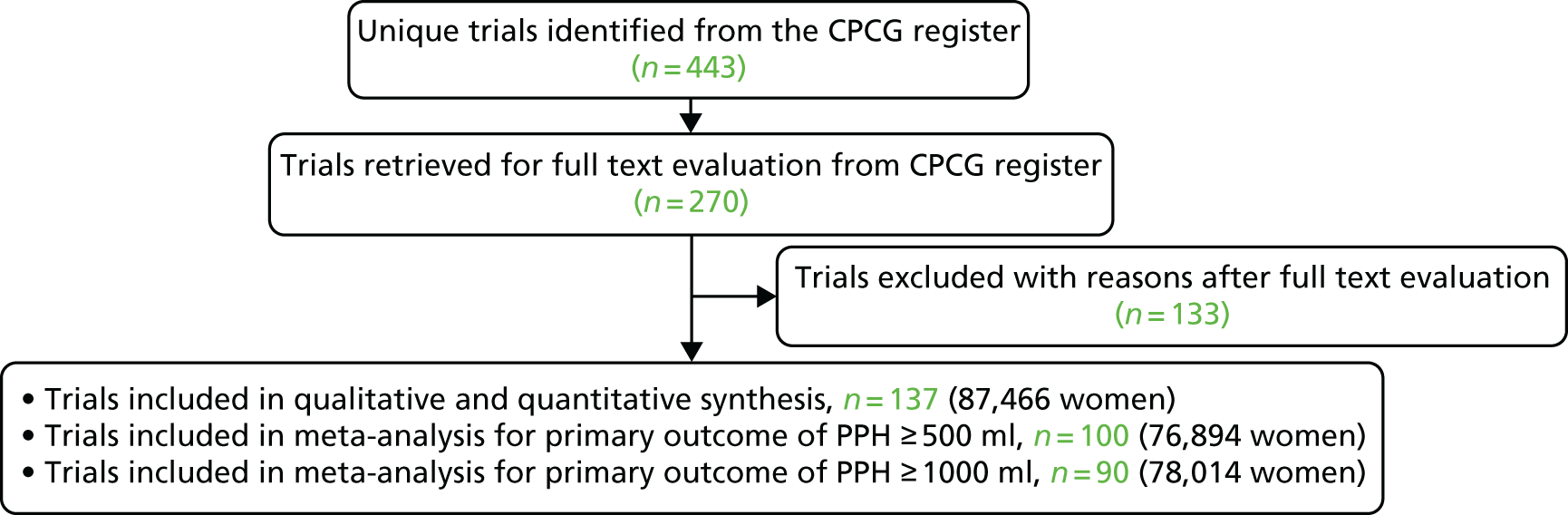
The authors were contacted from 93 primary randomised trials for additional data or clarifications and, for 38 randomised trials, they were able to add to this review data not reported in the published reports (see Appendix 7 for unpublished data from triallists). In October 2017, an updated CPCG Register search was carried out that retrieved an additional 85 trial reports listed under studies awaiting classification.
Study characteristics
Study characteristics of participants and interventions for the 137 included studies are reported in Appendix 2. Most studies were reported in English and seven translations were obtained (four Spanish, two French, two Turkish and one Chinese). The studies were conducted in various countries and often involved more than one country. The UK was the country where most studies were conducted (i.e. 11 studies). A number of multiarm trials were identified: two five-arm trials, five four-arm trials and 14 three-arm trials (see Appendix 3). The median size of the trials was 250 participants (interquartile range 140–602 participants).
Included trials involved women undergoing a vaginal birth in 102 out of 137 trials (74.5%) and 35 trials (25.5%) involved women undergoing elective or emergency caesareans. Women included in the trials were judged to be at high risk for PPH in 42 out of 137 trials (30.7%), at low risk in 42 out of 137 trials (30.7%) and at either high or low risk in 48 out of 137 trials (35%). The risk for PPH was not specified in five trials (3.6%). There were 132 trials conducted in the hospital setting (96.4%), with only four community trials (2.9%) and one (0.7%) with a mixed setting.
The gestational ages included in the trials were not specified in 67 out of 137 trials (48.9%) and, when it was specified, 32 trials (23.4%) included term pregnancies with the remaining 38 trials (27.7%) including women with both preterm and term pregnancies. There were 81 trials (59.1%) that included women with a singleton pregnancy, 21 trials (15.3%) that included women with either singleton or multiple pregnancies and 35 trials (25.6%) did not specify this criterion. Three trials (2.2%) included only nulliparous or primigravida women, 34 trials (24.8%) included women of varying parity and 100 trials (73%) did not specify the parity of the women included in the trials. Exclusion criteria varied significantly and often encompassed women with significant medical comorbidities.
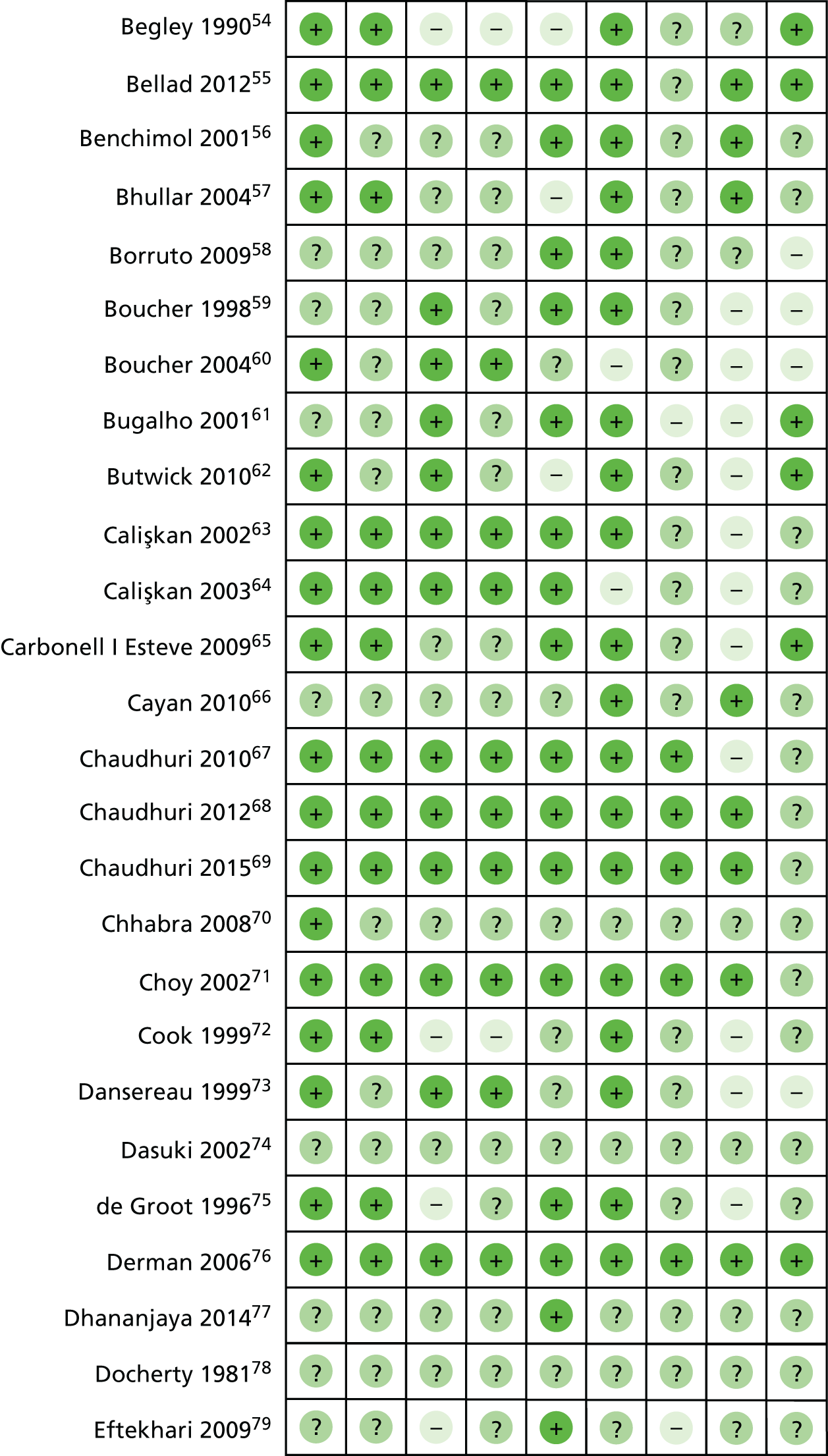
Risk of bias in included studies
Summaries of the methodological quality of the included studies are presented for each of the domains that were assessed across all studies (Figure 3) and for each included study (Figure 4).
FIGURE 3.
Risk-of-bias graph: review authors’ judgements about each risk-of-bias item presented as percentages across all included studies.
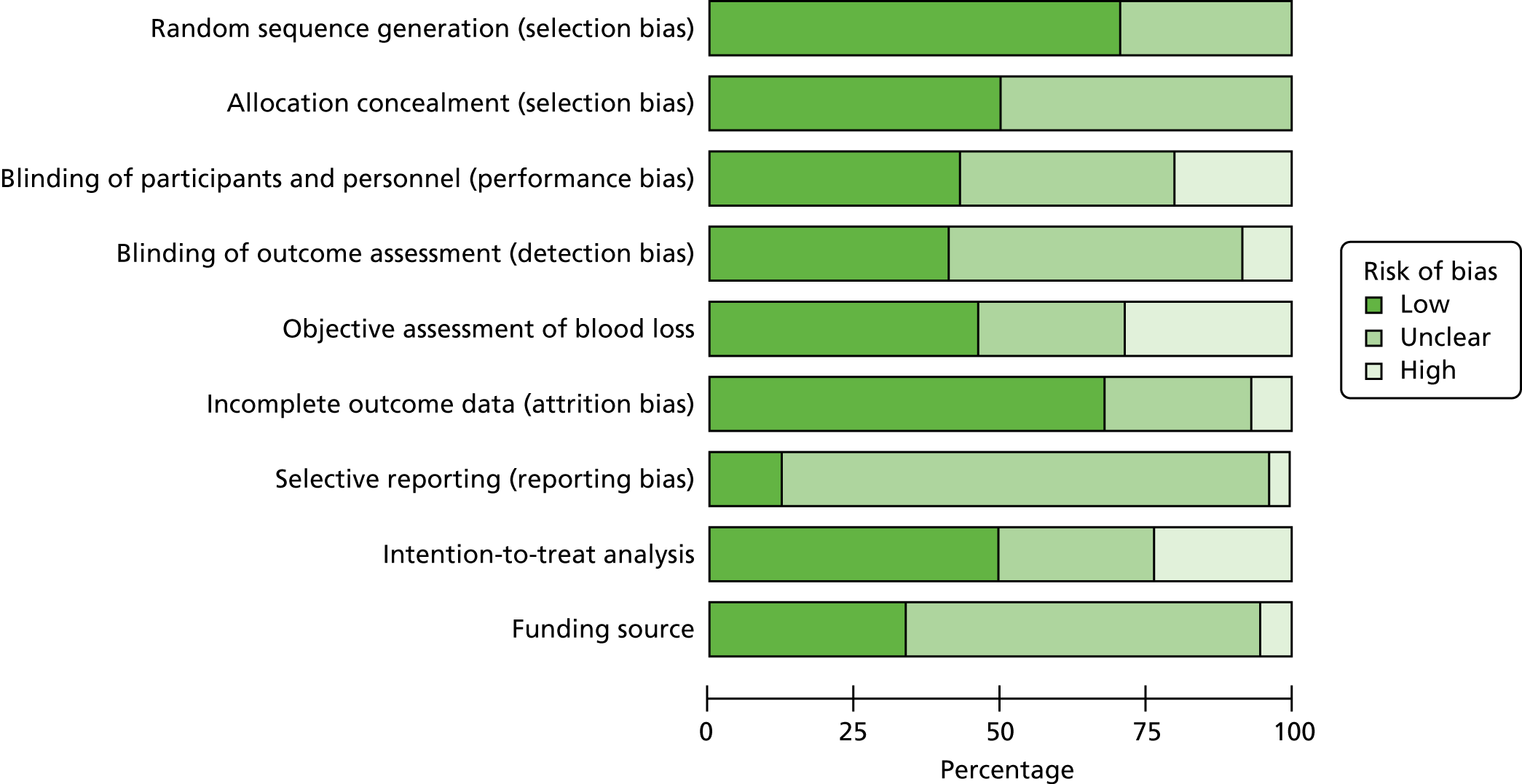
FIGURE 4.
Risk-of-bias summary: review authors’ judgements about each risk-of-bias item for each included study. +, low risk of bias; ?, uncertain risk of bias; – high risk of bias.





Random sequence generation
Trials with evidence of inadequate random sequence generation were excluded from this review. As a result, 99 out of 137 included trials (72.3%) were found to have used an adequate method of generating the random sequence and were rated as being at a low risk of bias. However, 38 trials (27.7%) did not report the method used in sufficient detail and the risk of bias was rated as being unclear.
Allocation concealment
Out of 137 trials, 70 (51.1%) reported adequate methods for allocation concealment and were rated as being at a low risk of bias, and 67 trials (48.9%) did not provide enough information to assess allocation concealment and the risk of bias was rated as being unclear.
Blinding of participants and personnel
There were 59 out of 137 trials (43.1%) reporting adequate methods for blinding both participants and personnel to treatment allocation, and 29 trials (21.2%) were rated as being at a high risk of bias for blinding of participants and personnel. A further 49 trials (35.8%) did not provide enough information to assess the blinding of participants and personnel and the risk of bias was rated as being unclear.
Blinding of outcome assessment
For blinding the assessment of the primary outcomes, 56 out of 137 trials (40.9%) reported adequate methods, and 11 (8%) were rated as being at a high risk of bias for blinding the assessment of the primary outcomes. Seventy trials (51.1%) did not provide enough information for blinding the assessment of the primary outcomes and the risk of bias was rated as being unclear.
Incomplete outcome data
There were 94 out of 137 trials (68.6%) that were rated as being at a low risk of bias. In these trials, missing outcome data were < 10% and balanced in numbers across intervention groups, with similar reasons for missing data across groups. In 11 trials (8%), > 10% of patients dropped out or were not analysed as per the intention-to-treat principles following randomisation, indicating as being at a high risk of bias. Moreover, 32 trials (23.4%) did not provide enough information to be assessed, so it was uncertain whether or not the handling of incomplete data was appropriate, and the risk of bias was rated as being unclear in these trials.
Selective reporting
Only 14 out of 137 trials (10.2%) prespecified all outcomes in publicly available study protocols and were rated as being at a low risk of bias. Ten trials (7.3%) did not report all prespecified outcomes as reported in their published protocols or methodology within the main report and were rated as being at a high risk of bias for selective reporting. For most trials [i.e. 113 trials (82.5%)], it was not possible to trace a published protocol and the risk of bias was rated as being unclear.
Other bias (source of funding and conflicts of interest)
Several trials [i.e. 47 out of 137 (34.3%)] were conducted with either public or no funding and did not declare potential conflicts of interest. Eight trials (5.8%) were rated as being at a high risk of bias, as they were funded directly by the pharmaceutical industry. Eighty-two trials (59.9%) did not provide enough information to assess the source of funding or potential conflicts of interest and the risk of bias was rated as being unclear.
Method of measuring blood loss
Only 14 out of 137 trials (10.2%) did not report blood loss outcomes or it was not possible to extract data for these outcomes from the published reports. From the studies that reported blood loss outcomes, 65 out of 123 trials (52.8%) reported relatively objective methods for measuring blood loss, such as weighing sponges, measurements in drapes or volumetric assessment, and were rated as being at a low risk of bias. In addition, 38 trials (30.9%) were rated as being at a high risk of bias for measuring blood loss, as the studies used subjective measurements, such as clinical or visual estimates, and 20 trials (16.3%) did not provide enough information to assess the method for measuring blood loss and the risk of bias was rated as being unclear.
Overall risk of bias
For the purpose of the sensitivity analysis, the number trials rated at a low, intermediate or high overall risk of bias have been assessed. For PPH blood loss of ≥ 500 ml, 29 out of 100 trials (29%) were rated as being at a low overall risk of bias, and 71 trials (71%) were rated as being at a high risk of bias as they were to be at either high risk or unclear risk of bias for at least one of the domains mentioned above. There were no trials that were rated as being at an intermediate risk of bias – see Sensitivity analysis for information about how this risk of bias impacted the results.
Effects of interventions
Primary postpartum haemorrhage blood loss of ≥ 500 ml
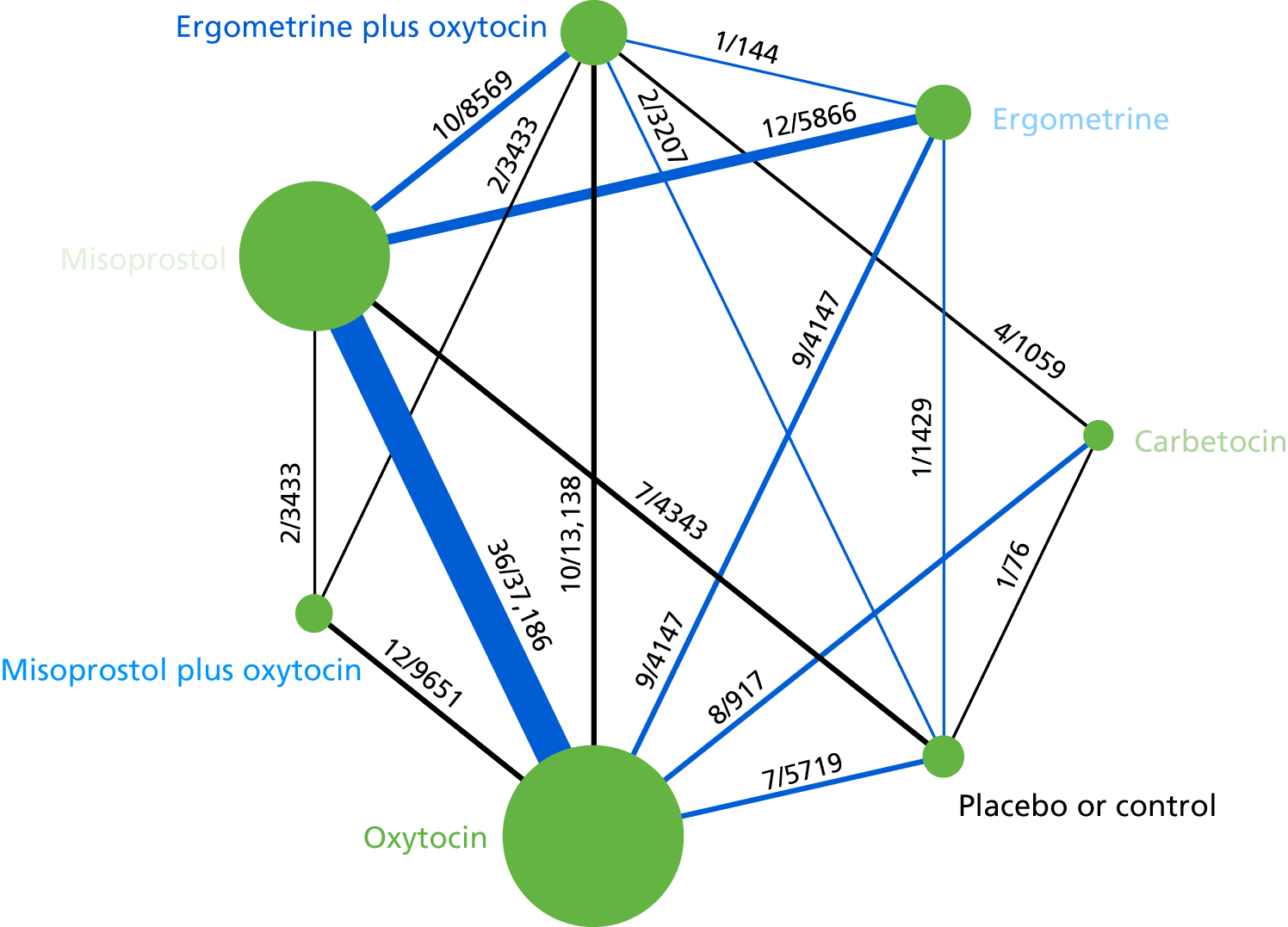
The network diagram for PPH blood loss of ≥ 500 ml is presented in Figure 5. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention with any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. The numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is black when > 50% of the trials involved in the specific direct comparison are rated as being at a low risk of bias if they were double-blinded and had allocation concealment with little loss to follow-up (i.e. < 10%). The colour is blue when < 50% of the trials are rated as being at a low risk of bias. Multiarm trials contribute to more than one comparison. Oxytocin was the most frequently investigated intervention (i.e. in 82 trials), whereas carbetocin was investigated in only 13 trials (see Figure 5).
FIGURE 5.
Network diagram for PPH blood loss of ≥ 500 ml.
Pooled effect sizes from the NMA of 100 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo (Figure 6). There is good statistical evidence that ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin are more effective than the standard intervention, oxytocin (ergometrine plus oxytocin vs. oxytocin: RR 0.69, 95% CI 0.57 to 0.83; carbetocin vs. oxytocin: RR 0.72, 95% CI 0.52 to 1.00; misoprostol plus oxytocin vs. oxytocin: RR 0.73, 95% CI 0.60 to 0.90; see Figure 6]. Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was evidence of global inconsistency, in which the direct and indirect randomised evidence are not in agreement, in this analysis (p = 0.046). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results, except for ergometrine versus placebo or the control based on a single study.
FIGURE 6.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml. NA, not applicable.
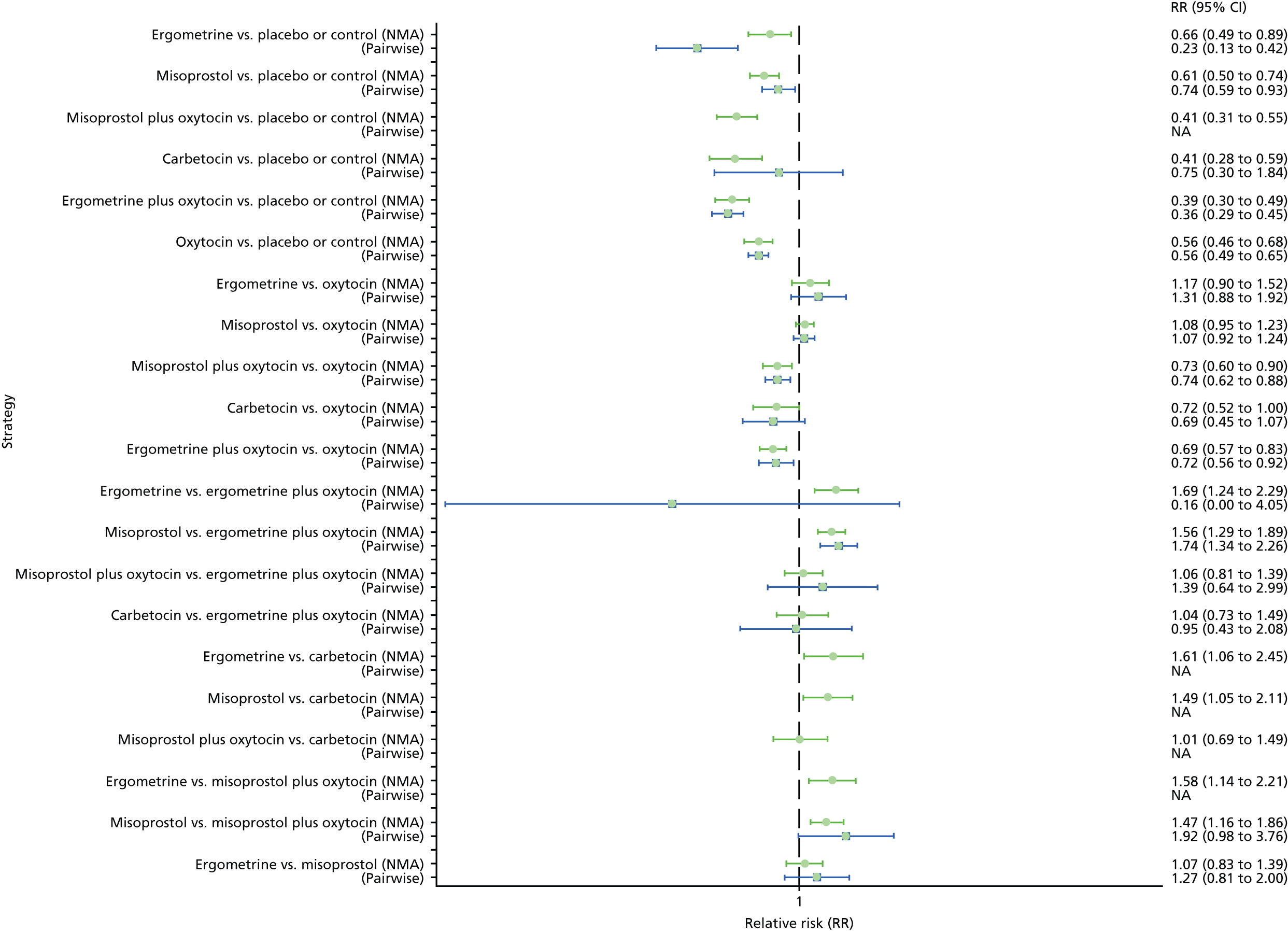
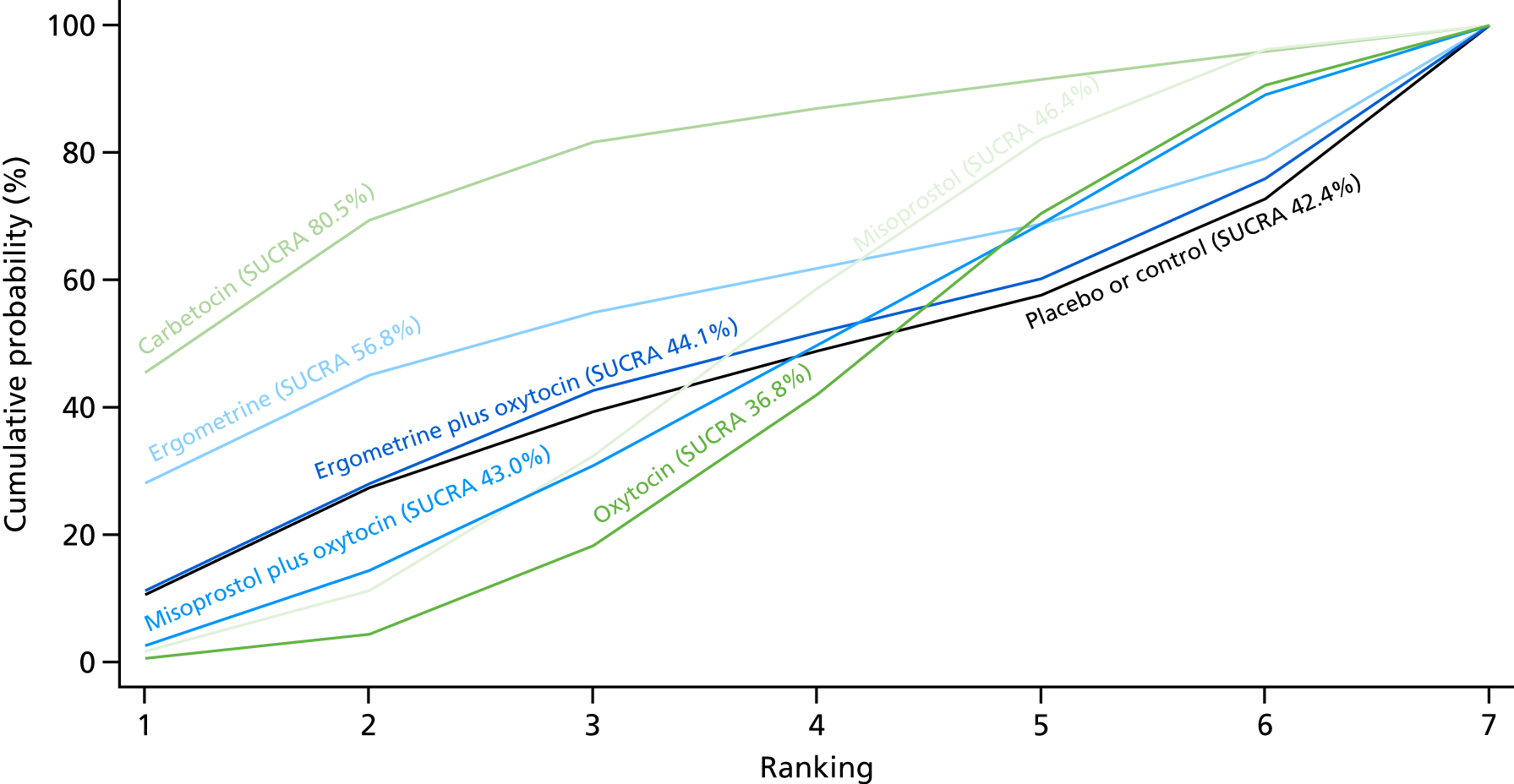
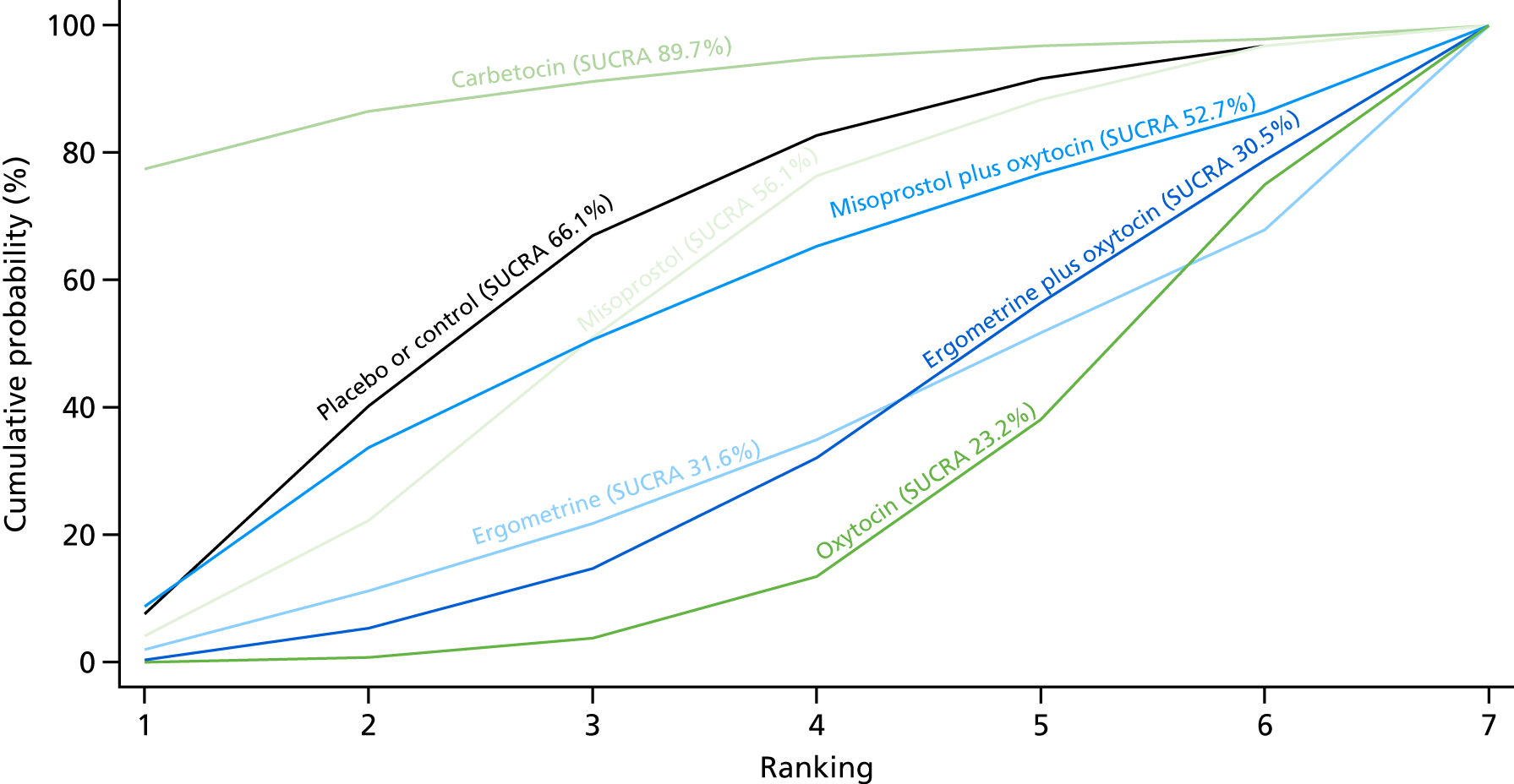
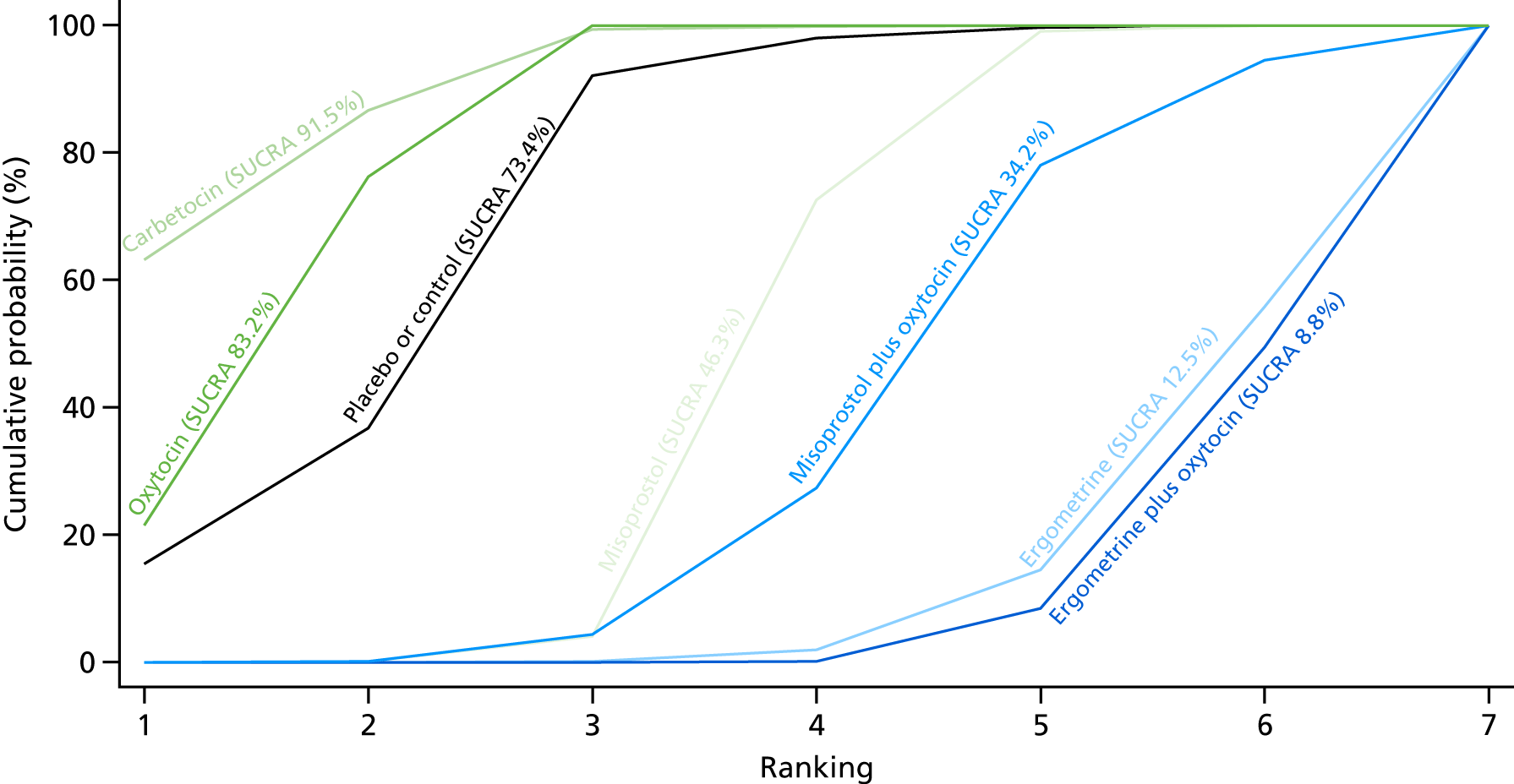
Figure 7 shows the cumulative probabilities, in the absence of bias, for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml. The x-axis reports each of the possible ranks, for which position 1 means that the intervention is ranked the highest and position 7 the lowest. The y-axis shows the cumulative probability with which each intervention has been ranked at each of the seven possible positions. To compare interventions the SUCRA was used. SUCRA can also be interpreted as the percentage of effectiveness or side effects of a treatment that would be ranked first without uncertainty. For example, ergometrine plus oxytocin has the highest probability (around 45%) of being the best drug. The probability of this intervention being either the best or the second-best drug is around 80% and being the best, the second best or the third best is 100%. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin, with an almost 100% probability of these three interventions being ranked first, second and third. Oxytocin is ranked fourth and its probability in being ranked in the top three interventions was close to 0%.
FIGURE 7.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml.

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml is presented in Figure 8. Oxytocin was the most frequently investigated intervention (i.e. in 77 trials), whereas carbetocin was investigated in only 11 trials (see Figure 8).
FIGURE 8.
Network diagram for PPH blood loss of ≥ 1000 ml.

Pooled effect sizes from the NMA of 90 trials suggested that all interventions, except ergometrine, are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo (Figure 9). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin and misoprostol plus oxytocin demonstrated a trend towards reduction of this outcome (see Figure 9). There was no evidence of global inconsistency in this analysis (p = 0.345).
FIGURE 9.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml. NA, not applicable.
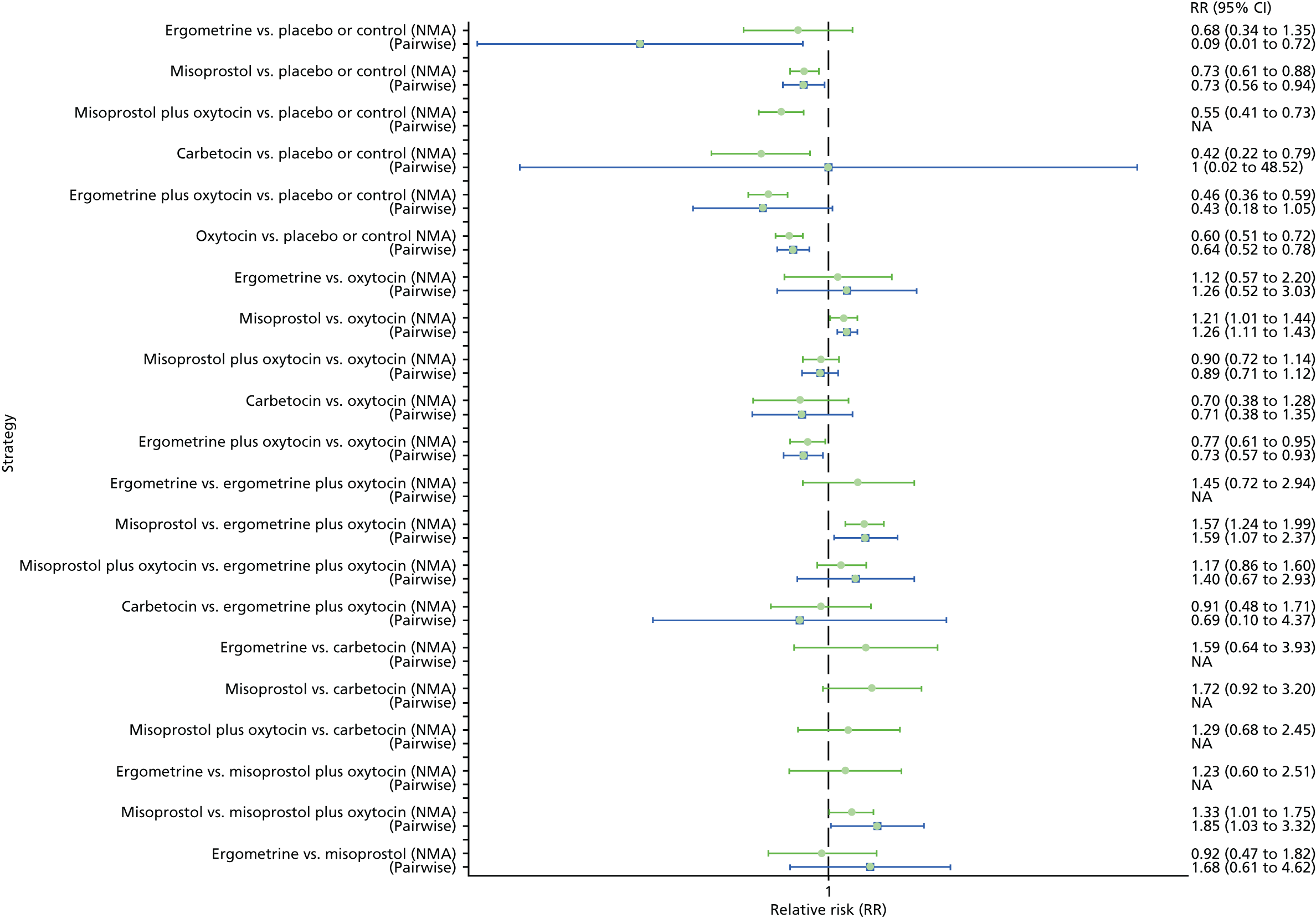
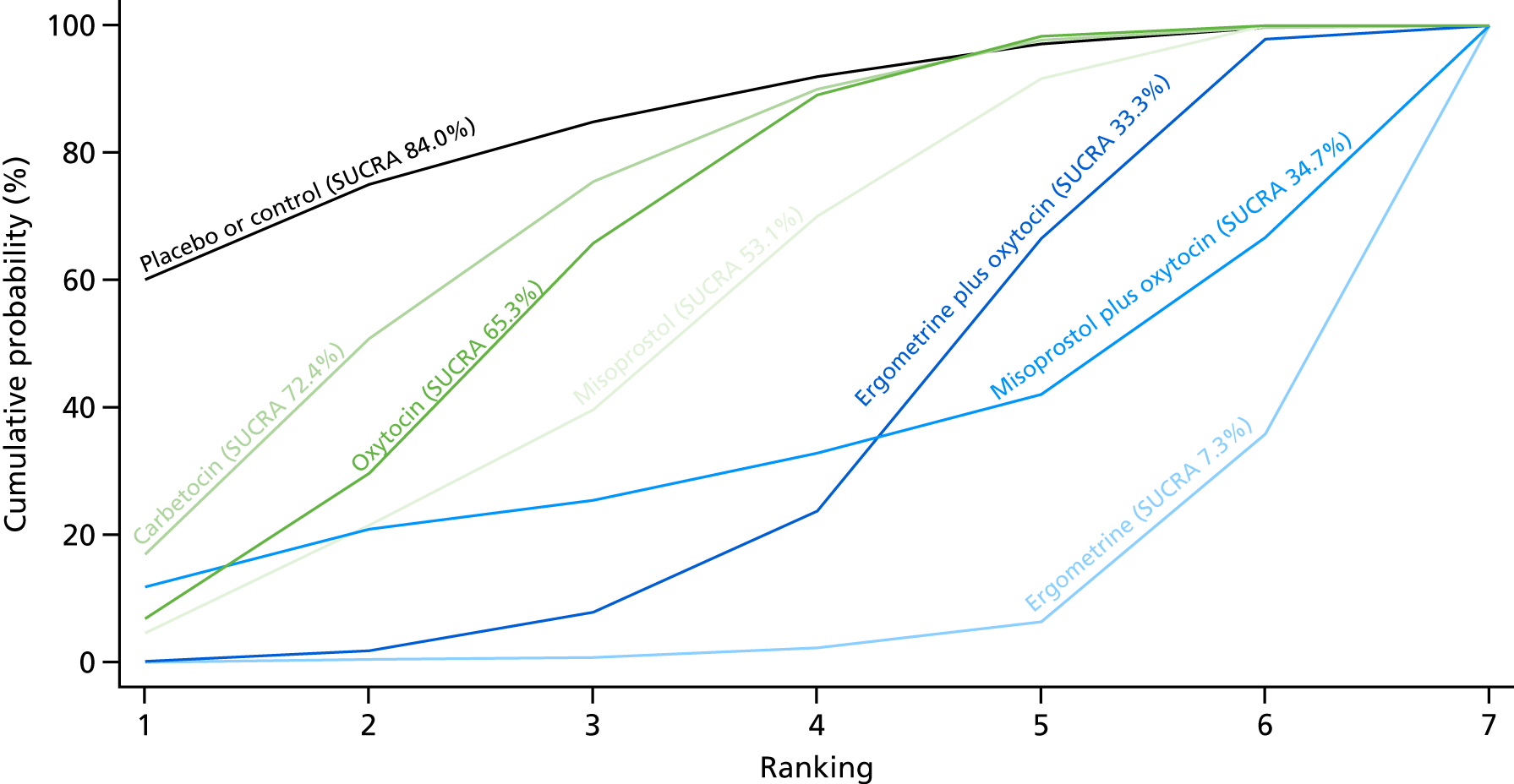
Figure 10 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin. Oxytocin is still ranked fourth and its probability in being ranked in the top three interventions was close to 20%.
FIGURE 10.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml.

Maternal death
The network diagram for maternal death is presented in Appendix 8. Pooled effect sizes from the NMA of 50 trials suggested that there are no meaningful differences between all interventions for maternal death, as this outcome was so rare (Figure 11). There was no evidence of global inconsistency in this analysis (p = 0.999).
FIGURE 11.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for maternal death. NA, not applicable.
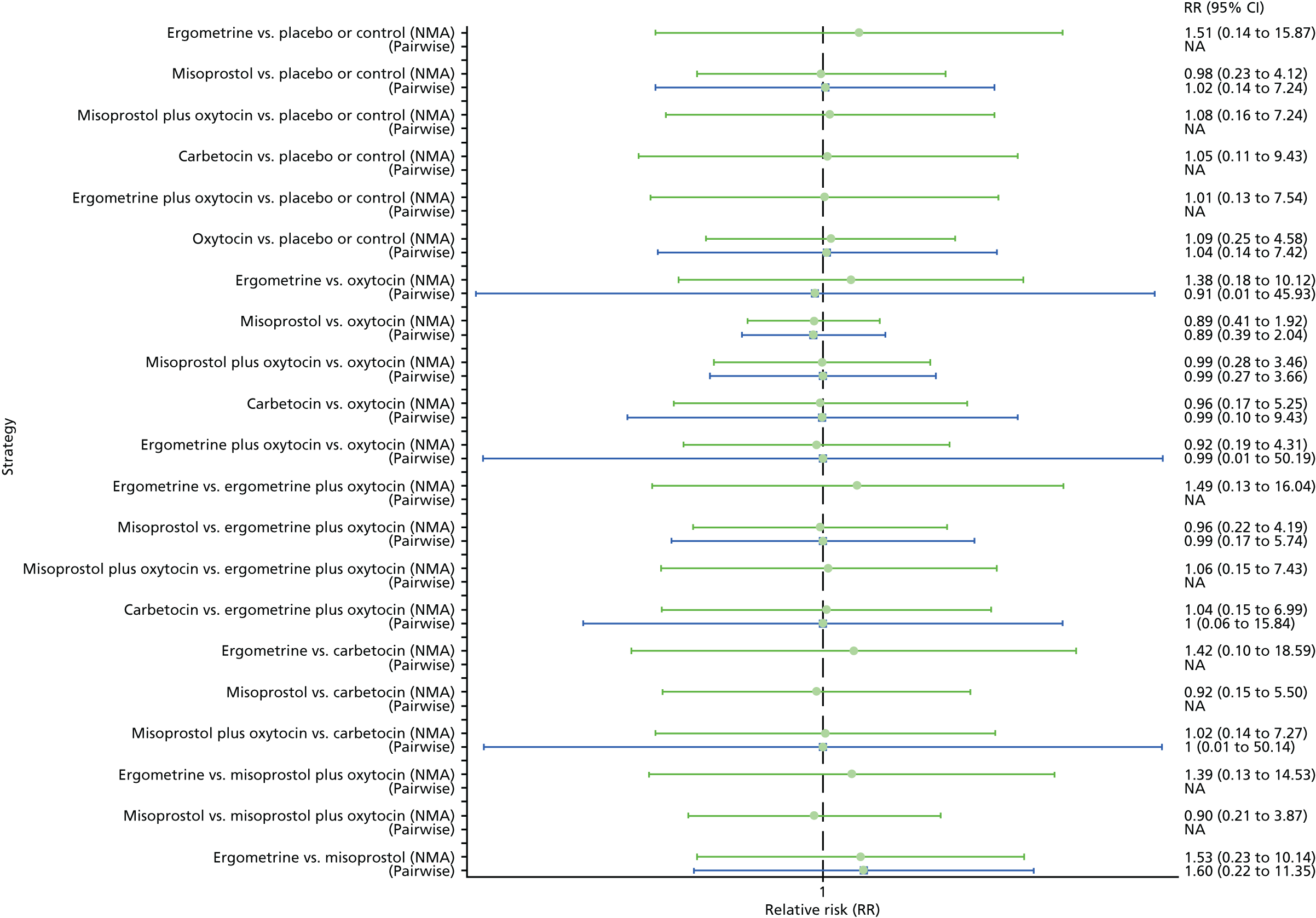
Figure 12 shows the cumulative probabilities for each intervention being at each possible rank for maternal death. No reliable ranking can be derived for this outcome.
FIGURE 12.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the prevention of maternal death.

Maternal deaths or severe morbidity
The network diagram for maternal death or severe morbidity is presented in Appendix 8. Pooled effect sizes from the NMA of 37 trials suggested that there are no detectable differences between all interventions for maternal deaths or severe morbidity, as this outcome was still so rare (Figure 13). There was no evidence of global inconsistency in this analysis (p = 0.884).
FIGURE 13.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for maternal death or severe morbidity. NA, not applicable.

Figure 14 shows the cumulative probabilities for each intervention being at each possible rank for maternal death or severe morbidity. No sensible ranking can be derived for this outcome because of limited data.
FIGURE 14.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the prevention of maternal death or severe morbidity events.
Additional uterotonics
The network diagram for the requirement of additional uterotonics is presented in Appendix 8. Pooled effect sizes from the NMA of 107 trials suggested that all interventions are effective at reducing the requirement of additional uterotonics when compared with placebo (Figure 15). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin (see Figure 15). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (p = 0.275).
FIGURE 15.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the requirement of additional uterotonics. NA, not applicable.

Figure 16 shows the cumulative probabilities for each intervention being at each possible rank for the requirement of additional uterotonics. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin, with an almost 100% probability of these three interventions being ranked in the top three. Oxytocin is ranked fourth and its probability in being ranked in the top three interventions was close to 0%. The lowest-ranked interventions were misoprostol, ergometrine and placebo or the control.
FIGURE 16.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the requirement of additional uterotonics.

Transfusion
The network diagram for blood transfusion is presented in Appendix 8. Pooled effect sizes from the NMA of 92 trials suggests that all interventions, except ergometrine, are effective for preventing blood transfusion when compared with placebo (Figure 17). Misoprostol plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin and ergometrine plus oxytocin demonstrated a trend towards reduction of this outcome (see Figure 17). There was no evidence of global inconsistency in this analysis (p = 0.061).
FIGURE 17.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the requirement of blood transfusion. NA, not applicable.

Figure 18 shows the cumulative probabilities for each intervention being at each possible rank for preventing blood transfusion. The highest-ranked interventions are misoprostol plus oxytocin, carbetocin and ergometrine plus oxytocin. Oxytocin is ranked fifth behind misoprostol and its probability of being ranked in the top three interventions was < 10%.
FIGURE 18.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the requirement of blood transfusion.

Manual removal of the placenta
The network diagram for the requirement of manual removal of placenta is presented in Appendix 8. Pooled effect sizes from the NMA of 67 trials suggest that there are no significant differences between all interventions for this outcome (Figure 19). There was evidence of global inconsistency in this analysis (p = 0.025). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results except for ergometrine versus placebo or the control and carbetocin versus oxytocin based on single studies.
FIGURE 19.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the requirement of manual removal of placenta. NA, not applicable.

Figure 20 shows the cumulative probabilities for each intervention being at each possible rank for the prevention of blood transfusion. No clear ranking can be derived for this outcome, with all interventions being comparable except for carbetocin, as that drug appeared to have the highest probability being of the top-ranked intervention, with a probability close to 80%.
FIGURE 20.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for the requirement of manual removal of placenta.
Mean volumes of blood loss
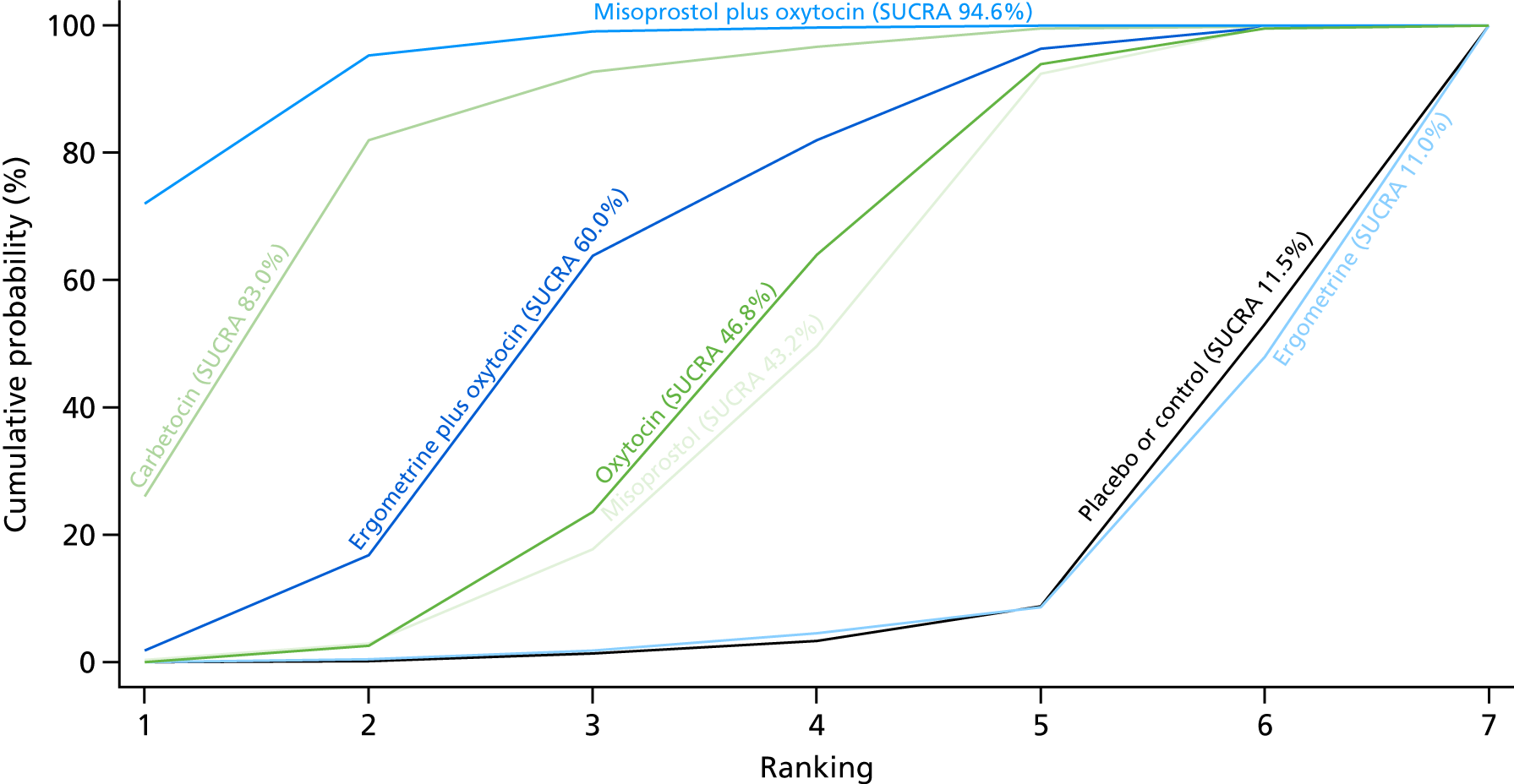
The network diagram for blood loss (as reported in ml), as a continuous outcome, is presented in Appendix 8. Pooled effect sizes from the NMA of 102 trials suggested that all interventions are effective for reducing blood loss as a continuous outcome when compared with placebo (Figure 21). Carbetocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin, even though ergometrine plus oxytocin also demonstrated a trend towards reduction of this outcome (see Figure 21). Carbetocin and misoprostol plus oxytocin were more effective than ergometrine plus oxytocin in reducing blood loss. Carbetocin and misoprostol plus oxytocin were also found to be more effective in reducing blood loss than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (p = 0.111).
FIGURE 21.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for blood loss (ml). NA, not applicable.
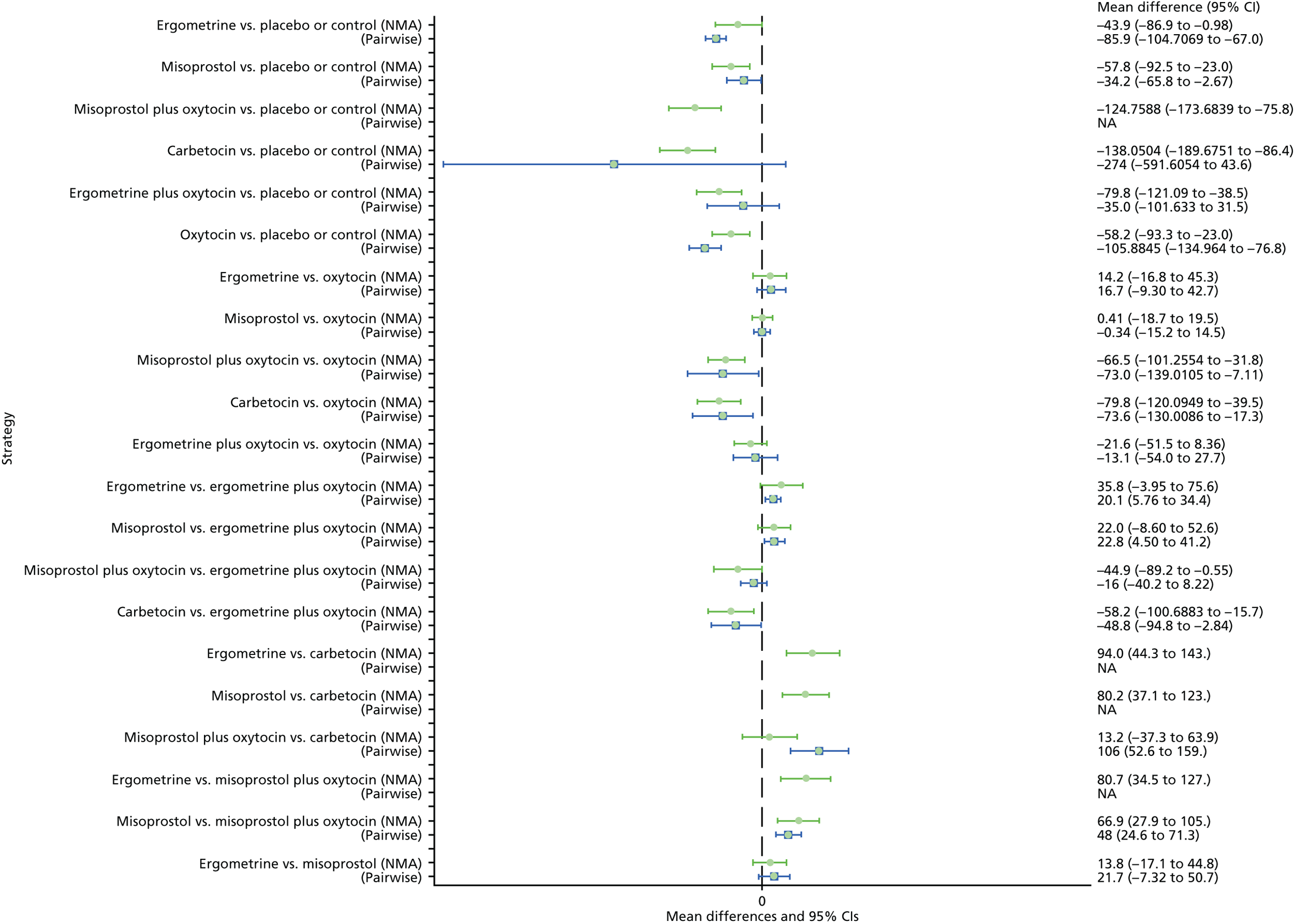
Figure 22 shows the cumulative probabilities for each intervention being at each possible rank for preventing blood loss (as reported in ml) as a continuous outcome. The highest-ranked interventions are carbetocin, misoprostol plus oxytocin and ergometrine plus oxytocin. Oxytocin is ranked fourth and its probability in being ranked in the top three interventions was > 10%. The lowest-ranked interventions were misoprostol, ergometrine and placebo or the control.
FIGURE 22.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for blood loss (ml).

Mean duration of the third stage of labour
The network diagram for the duration of the third stage (as reported in minutes), as a continuous outcome, is presented in Appendix 8. Pooled effect sizes from the NMA of 58 trials suggested that all interventions are effective for reducing the duration of the third stage as a continuous outcome when compared with placebo, except for carbetocin and misoprostol plus oxytocin, even though they demonstrated a similar trend towards reduction of this outcome (Figure 23). There were no significant differences between all active interventions for this outcome (see Figure 23). There was evidence of global inconsistency in this analysis (p = 0.011) and these results need to be interpreted with caution.
FIGURE 23.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for duration of third stage (minutes). NA, not applicable.

Figure 24 shows the cumulative probabilities for each intervention being at each possible rank for the reduction of the third stage as a continuous outcome. No sensible ranking can be derived for this outcome, with all interventions being comparable. The exception is ergometrine plus oxytocin as this intervention appeared to have the highest probability in being the top-ranked intervention, with a probability close to 60%, and the placebo or the control, which appeared to have the lowest ranking, with a probability of > 80%.
FIGURE 24.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for duration of third stage (minutes).

Change in haemoglobin levels
The network diagram for the change in Hb measurements before and after birth (as measured in g/l) is presented in Appendix 8. Pooled effect sizes from the NMA of 74 trials suggested that misoprostol plus oxytocin and carbetocin are effective for reducing the change in Hb measurements than placebo (Figure 25). Misoprostol plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin (see Figure 25). Misoprostol plus oxytocin were also more effective than misoprostol and ergometrine when used alone. Carbetocin was more effective than ergometrine when used alone. However, there was evidence of substantial global inconsistency in this analysis (p = 0.001).
FIGURE 25.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for change in Hb measurements before and after birth (g/l). NA, not applicable.

Figure 26 shows the cumulative probabilities for each intervention being at each possible rank for change in Hb measurements before and after birth. The highest-ranked interventions are misoprostol plus oxytocin, carbetocin and ergometrine plus oxytocin. Oxytocin is ranked fourth and its probability in being ranked in the top three interventions was just over 20%. The lowest-ranked interventions were misoprostol, ergometrine and placebo or the control.
FIGURE 26.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for change in Hb measurements before and after birth (g/l).
Clinical signs of blood loss
There were no trials reporting clinical signs of acute blood loss.
Neonatal unit admission
The network diagram for neonatal unit admissions is presented in Appendix 8. Pooled effect sizes from the NMA of only six trials did not point towards any meaningful differences between all interventions for this outcome (Figure 27). There was no evidence of global inconsistency in this analysis (p = 0.989).
FIGURE 27.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for neonatal unit admissions. NA, not applicable.

Figure 28 shows the cumulative probabilities for each intervention being at each possible rank for neonatal unit admissions. No sensible ranking can be derived for this outcome because of too few studies.
FIGURE 28.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for neonatal unit admissions.

Breastfeeding at discharge
The network diagram for breastfeeding at discharge is presented in Appendix 8. Pooled effect sizes from the NMA of only five trials did not point towards any meaningful differences between interventions for this outcome (Figure 29). There was no evidence of global inconsistency in this analysis (p = 0.167).
FIGURE 29.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for breastfeeding at discharge. NA, not applicable.
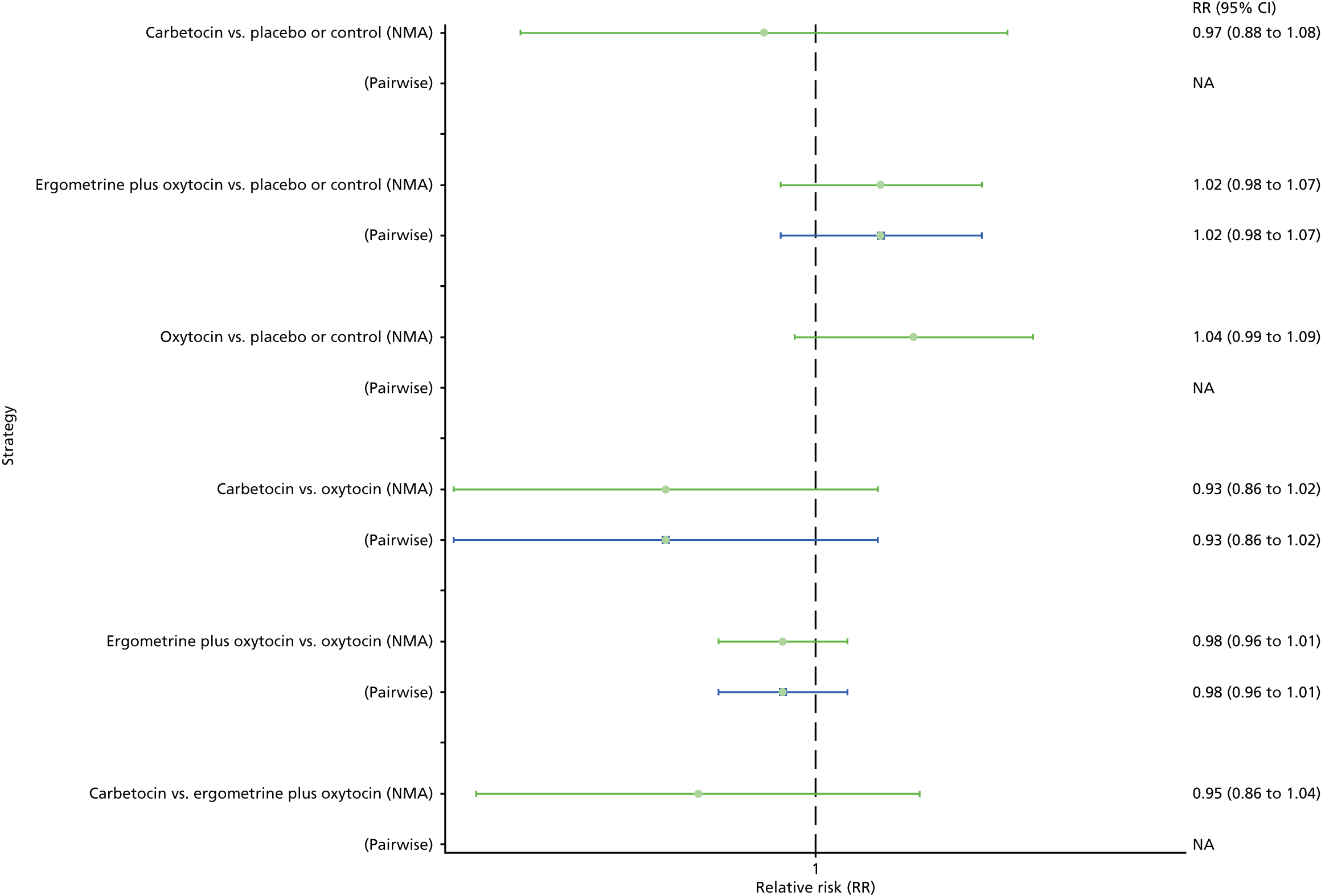
Figure 30 shows the cumulative probabilities for each intervention being at each possible rank for breastfeeding at discharge. No clear ranking can be derived for this outcome, with all interventions being comparable again because of too few studies.
FIGURE 30.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for breastfeeding at discharge.

Side effects
Nausea
The network diagram for nausea is presented in Appendix 8. Pooled effect sizes from the NMA of 74 trials suggested that ergometrine and ergometrine plus oxytocin are worse than the placebo or the control in causing nausea (Figure 31). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus oxytocin were found to be worse in causing nausea than the standard intervention, oxytocin (see Figure 31). Ergometrine, ergometrine plus oxytocin and misoprostol plus oxytocin were significantly worse in causing nausea than carbetocin. There was evidence of global inconsistency in this analysis (p = 0.005). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results except for ergometrine versus placebo or the control based on a single study.
FIGURE 31.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for nausea. NA, not applicable.

Figure 32 shows the cumulative probabilities for each intervention being at each possible rank for causing nausea. The highest-ranked and least likely interventions to cause nausea are carbetocin, oxytocin and placebo or the control. The lowest-ranked and most likely interventions to cause nausea are ergometrine plus oxytocin and ergometrine.
FIGURE 32.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for nausea.

Vomiting
The network diagram for vomiting is presented in Appendix 8. Pooled effect sizes from the NMA of 83 trials suggested that ergometrine and ergometrine plus oxytocin are worse than the placebo or the control in causing vomiting (Figure 33). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus oxytocin were found to be worse in causing vomiting than the standard intervention, oxytocin (see Figure 33). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus oxytocin were significantly worse in causing vomiting than carbetocin. There was no evidence of global inconsistency in this analysis (p = 0.06).
FIGURE 33.
Forest plot with relative RRs and 95% CIs from the NMAs and pairwise analyses for vomiting. NA, not applicable.

Figure 34 shows the cumulative probabilities for each intervention being at each possible rank for causing vomiting. The highest-ranked interventions are carbetocin, oxytocin and placebo or the control, with an almost 100% probability of these three interventions being ranked in the top three. The lowest-ranked interventions were ergometrine plus oxytocin and ergometrine.
FIGURE 34.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for vomiting.
Hypertension
The network diagram for hypertension is presented in Appendix 8. Pooled effect sizes from the NMA of 15 trials suggested that ergometrine is worse than the placebo or the control in causing hypertension (Figure 35). Ergometrine was found to be worse in causing hypertension than the standard intervention, oxytocin (see Figure 35). Ergometrine is also significantly worse in causing hypertension than carbetocin and misoprostol. There was no evidence of global inconsistency in this analysis (p = 0.481).
FIGURE 35.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for hypertension. NA, not applicable.
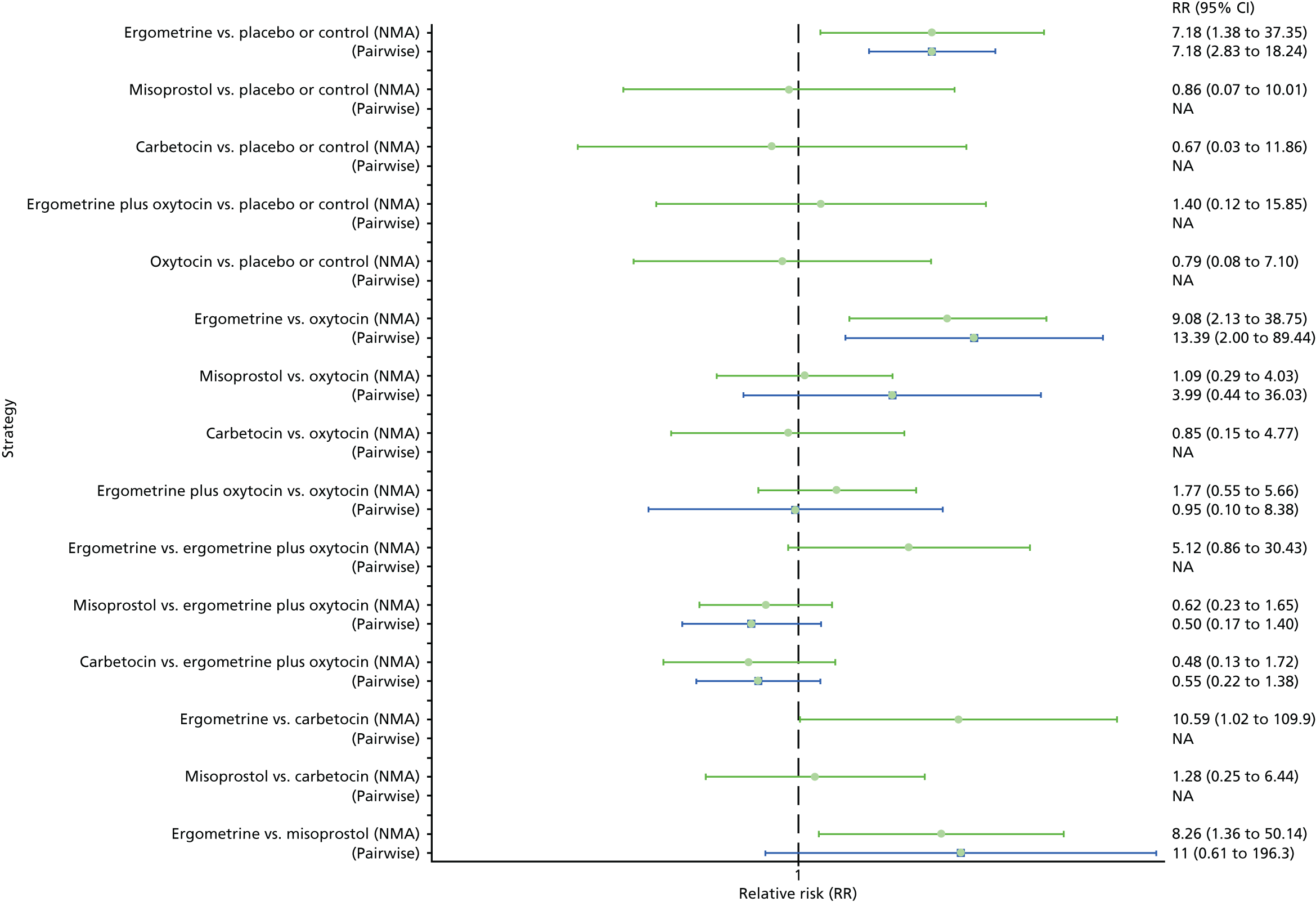
Figure 36 shows the cumulative probabilities for each intervention being at each possible rank for causing hypertension. The lowest-ranked interventions were ergometrine and ergometrine plus oxytocin. However, not all interventions could be ranked because of the lack of studies in this analysis.
FIGURE 36.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for hypertension.

Headache
The network diagram for headache is presented in Appendix 8. Pooled effect sizes from the NMA of 45 trials suggested that ergometrine is worse than the placebo or the control in causing headaches (Figure 37). Ergometrine was found to be worse in causing headache than the standard intervention, oxytocin (see Figure 37). Ergometrine is also significantly worse in causing headaches than carbetocin and misoprostol. There was no evidence of global inconsistency in this analysis (p = 0.826).
FIGURE 37.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for headaches. NA, not applicable.
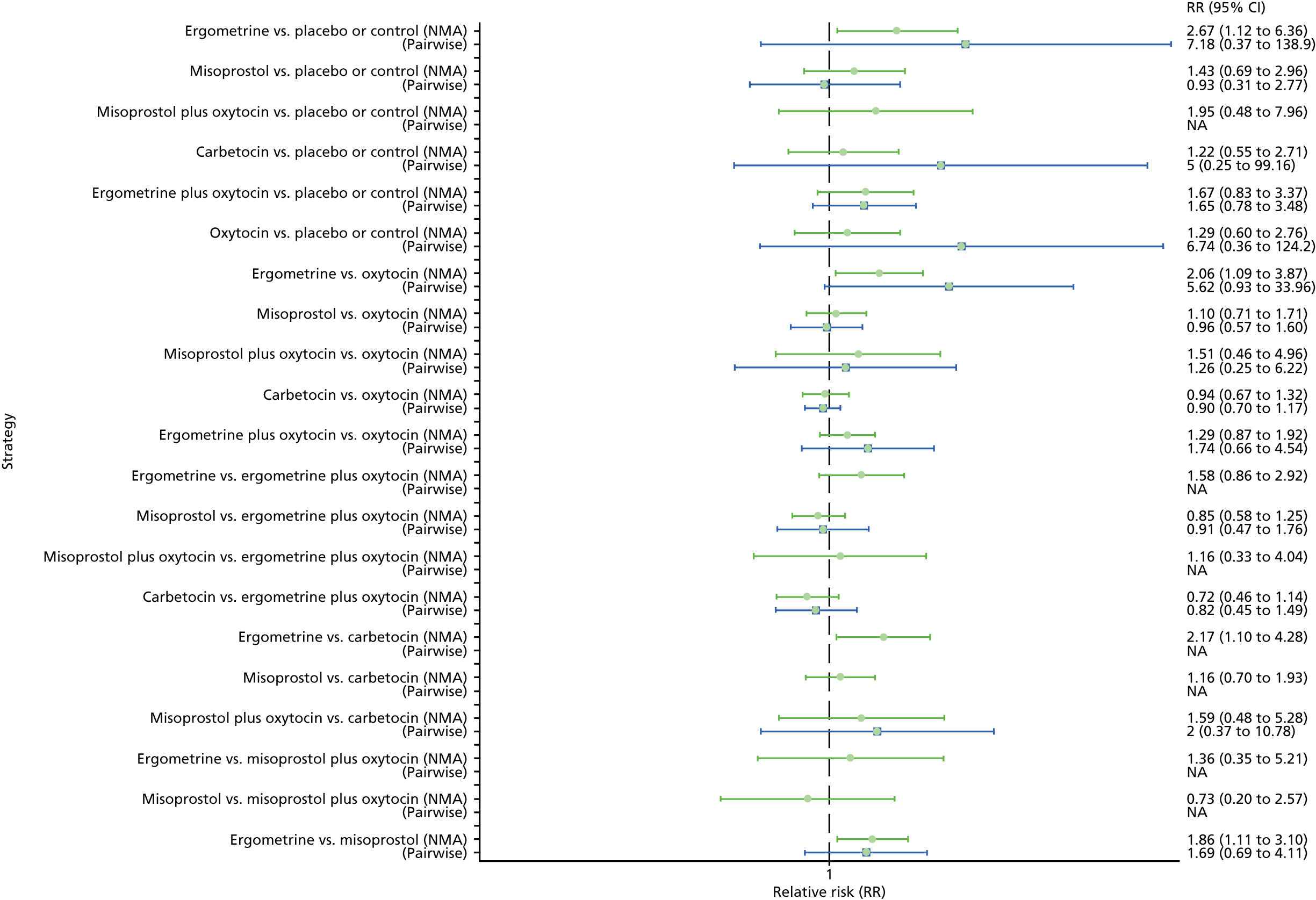
Figure 38 shows the cumulative probabilities for each intervention being at each possible rank for causing headache. The lowest-ranked interventions were ergometrine, misoprostol plus oxytocin and ergometrine plus oxytocin. The highest-ranked interventions are placebo or the control, carbetocin and oxytocin.
FIGURE 38.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for headaches.
Fever
The network diagram for fever is presented in Appendix 8. Pooled effect sizes from the NMA of 64 trials suggested that misoprostol and misoprostol plus oxytocin are worse than the placebo or the control in causing fever (Figure 39). Misoprostol and misoprostol plus oxytocin were found to be worse in causing fever than the standard intervention, oxytocin (see Figure 39). Misoprostol and misoprostol plus oxytocin were also significantly worse in causing fever than carbetocin, ergometrine and ergometrine plus oxytocin, with the exception of the comparison carbetocin versus misoprostol plus oxytocin, which fell just short of being statistically significant. There was no evidence of global inconsistency in this analysis (p = 0.352).
FIGURE 39.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for fever. NA, not applicable.
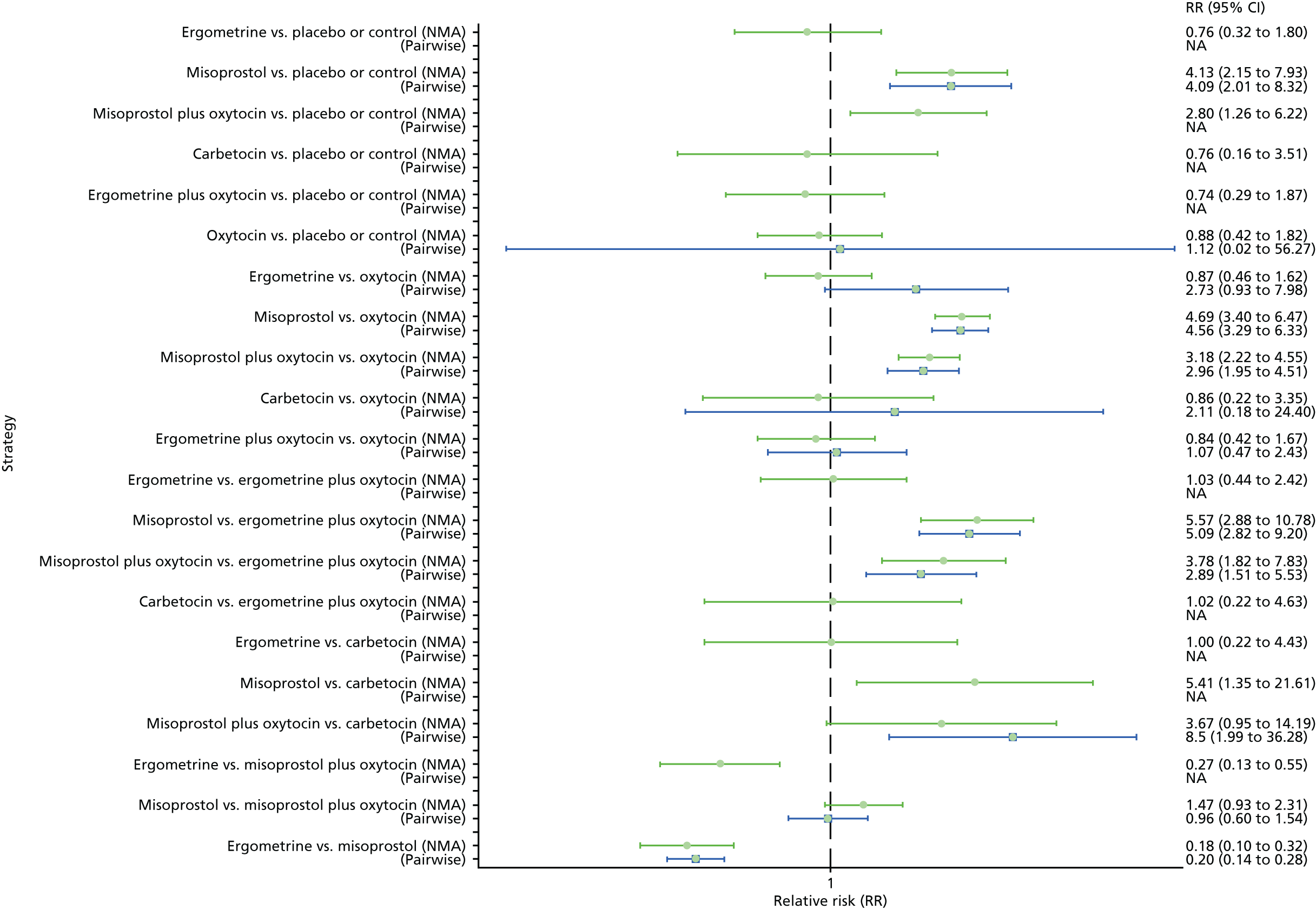
Figure 40 shows the cumulative probabilities for each intervention being at each possible rank for causing fever. The highest-ranked interventions are carbetocin, oxytocin and placebo or the control. The lowest-ranked interventions were misoprostol and misoprostol plus oxytocin. The rest of the interventions were similar in ranking to the placebo or the control group.
FIGURE 40.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof and combinations thereof for fever.
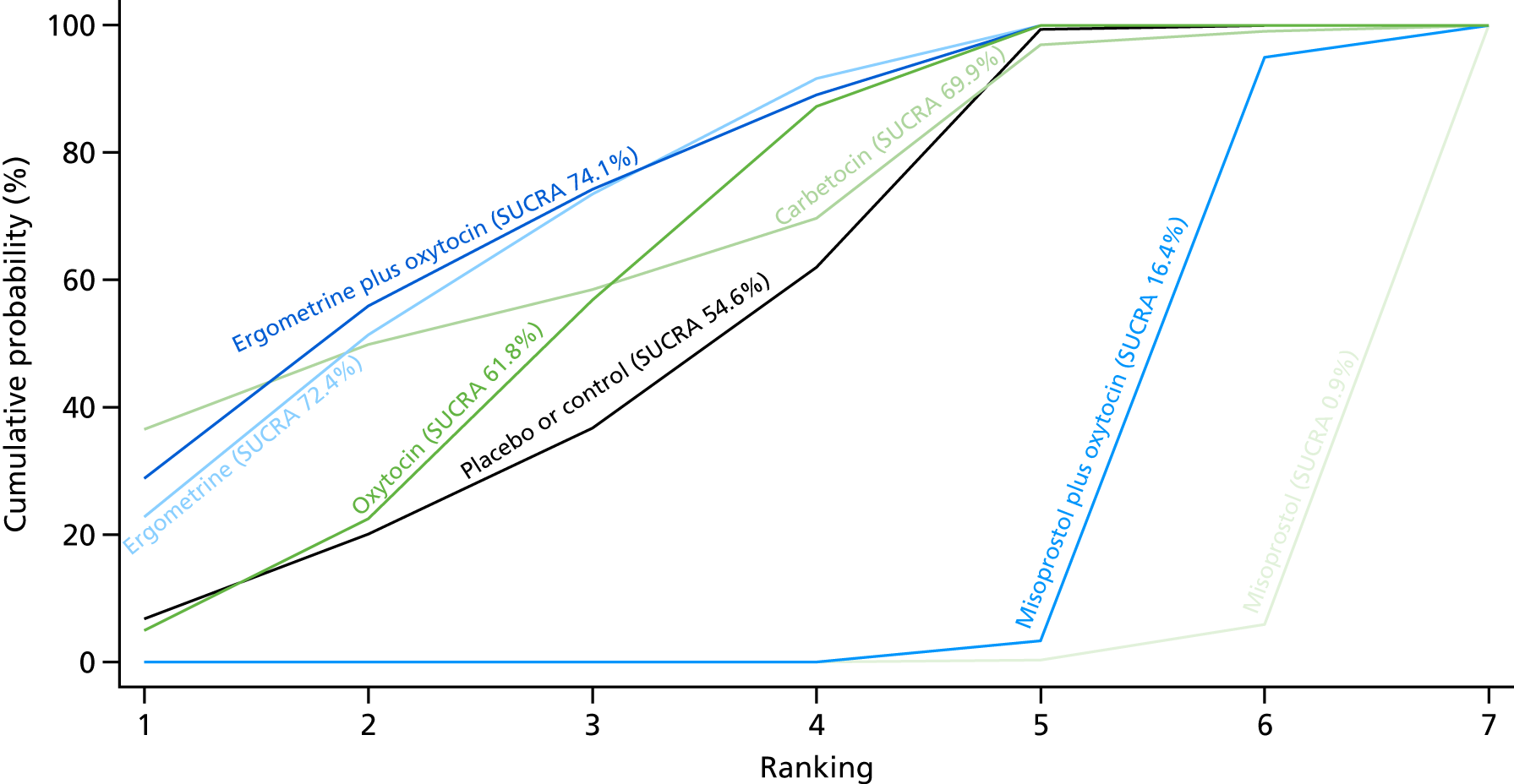
Shivering
The network diagram for shivering is presented in Appendix 8. Pooled effect sizes from the NMA of 87 trials suggested that misoprostol and misoprostol plus oxytocin are worse than the placebo or the control in causing shivering (Figure 41). Misoprostol and misoprostol plus oxytocin were found to be worse in causing shivering than the standard intervention, oxytocin (see Figure 41). Misoprostol and misoprostol plus oxytocin were also significantly worse in causing shivering than carbetocin, ergometrine and ergometrine plus oxytocin. There was no evidence of global inconsistency in this analysis (p = 0.923).
FIGURE 41.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for shivering. NA, not applicable.
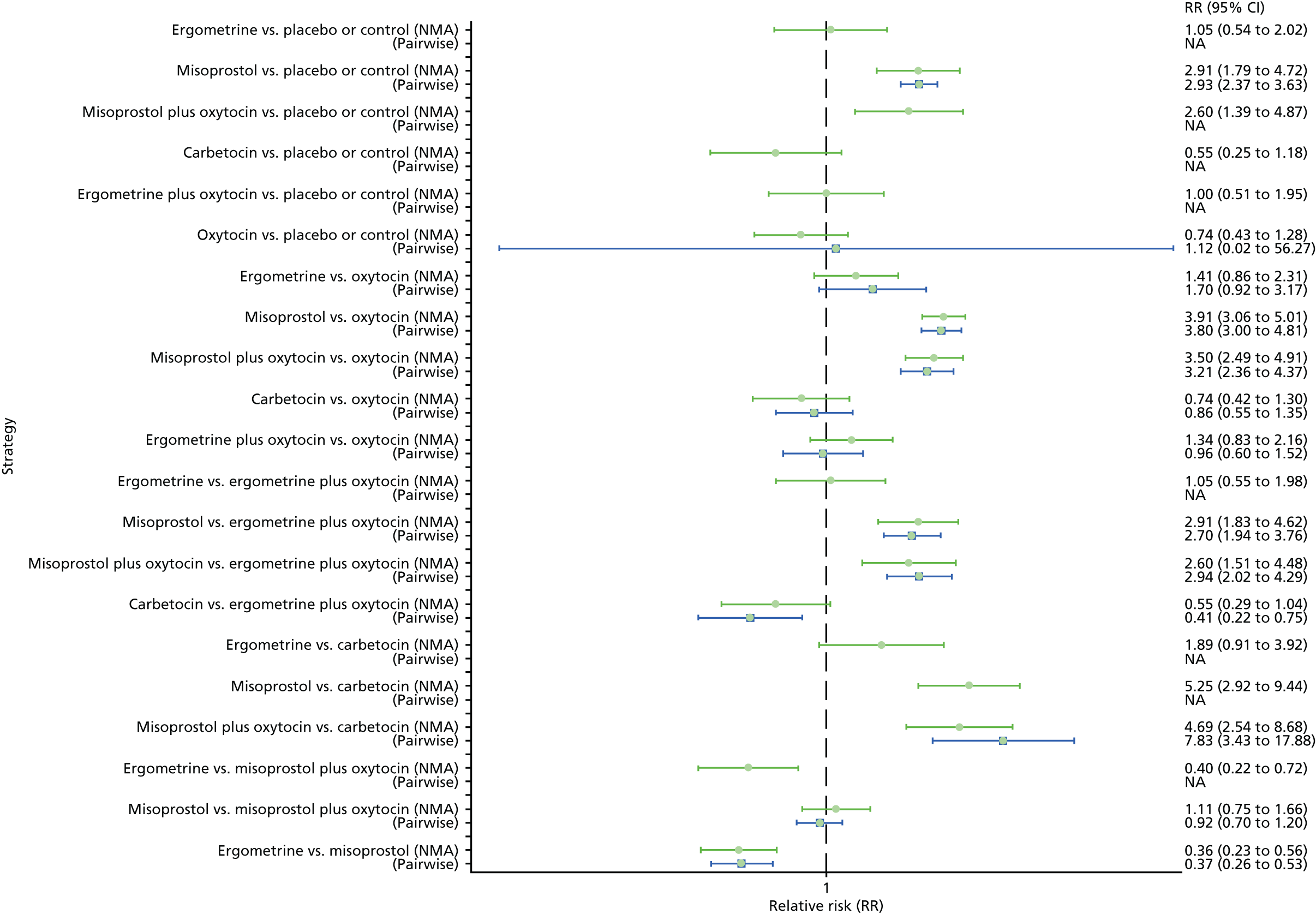
Figure 42 shows the cumulative probabilities for each intervention being at each possible rank for causing shivering. The highest-ranked interventions are carbetocin and oxytocin. The lowest-ranked interventions were misoprostol and misoprostol plus oxytocin. Ergometrine and ergometrine plus oxytocin were similar in ranking to the placebo or the control group.
FIGURE 42.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for shivering.

Tachycardia
The network diagram for tachycardia is presented in Appendix 8. Pooled effect sizes from the NMA of seven trials suggested that only carbetocin is worse than oxytocin and ergometrine plus oxytocin in causing tachycardia, but most of the comparisons were based on single studies (Figure 43). There was no evidence of global inconsistency in this analysis (p = 0.361).
FIGURE 43.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for tachycardia. NA, not applicable.

Figure 44 shows the cumulative probabilities for each intervention being at each possible rank for causing tachycardia. No clear ranking emerges and not all interventions could be ranked because of the lack of studies in this analysis.
FIGURE 44.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for tachycardia.
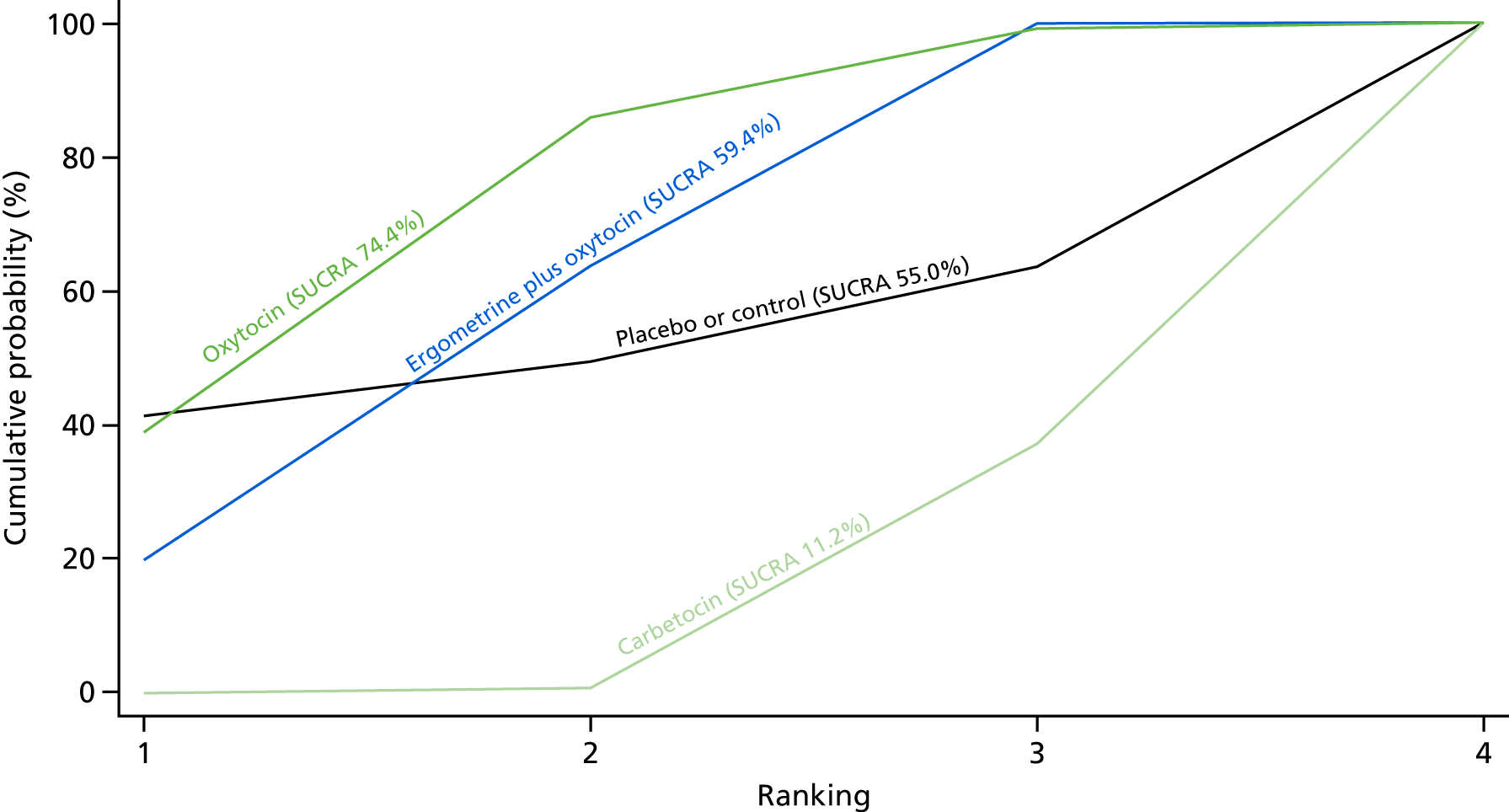
Hypotension
The network diagram for hypotension is presented in Appendix 8. Pooled effect sizes from the NMA of eight trials suggested a lack of evidence that any intervention is worse or better than any other, but most of the comparisons were based on single studies (Figure 45). There was no evidence of global inconsistency in this analysis (p = 0.304).
FIGURE 45.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for hypotension. NA, not applicable.
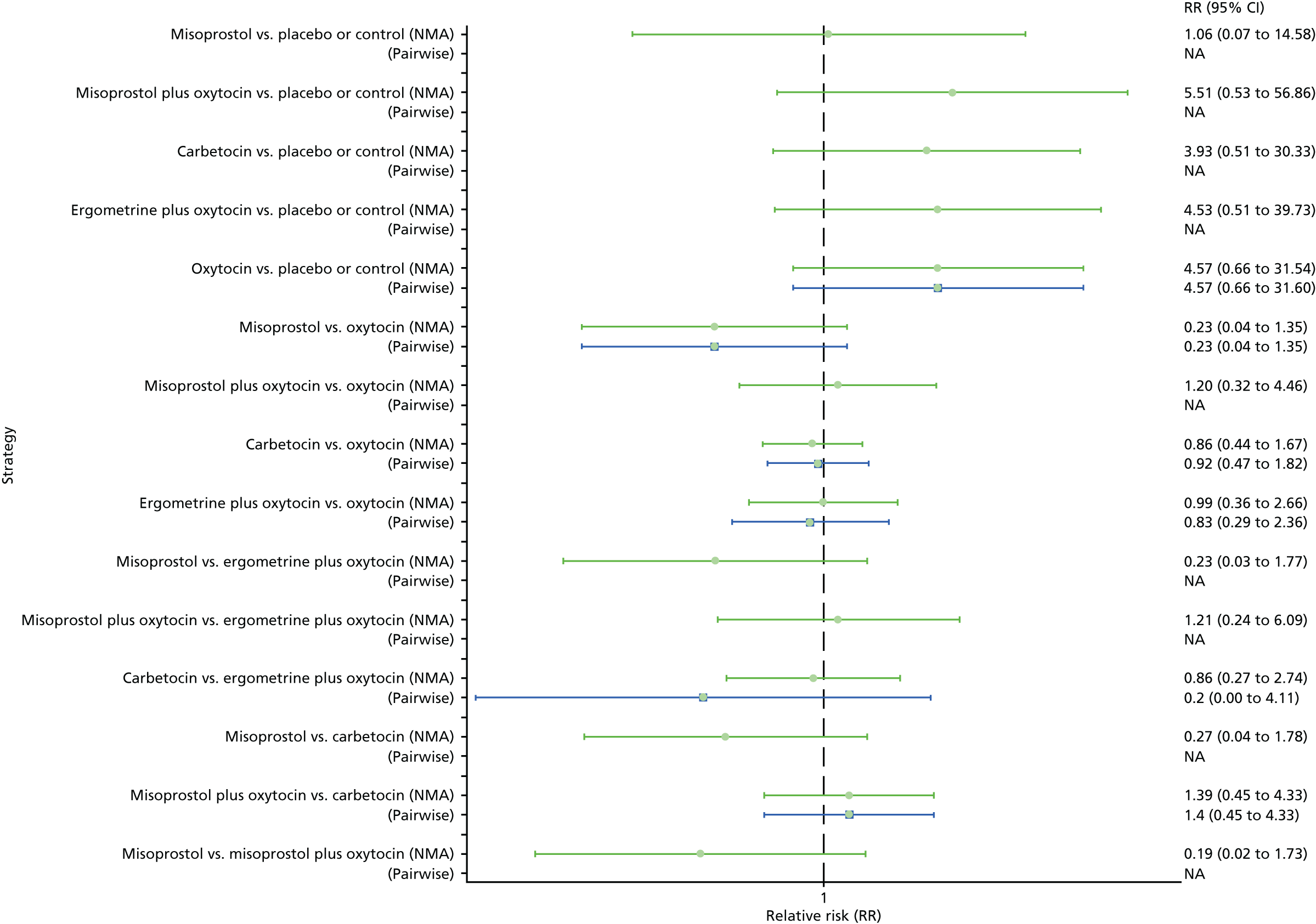
Figure 46 shows the cumulative probabilities for each intervention being at each possible rank for causing hypotension. The highest-ranked interventions were misoprostol and placebo or the control. For the rest of the interventions no clear ranking emerges and not all interventions could be ranked because of the lack of studies in this analysis.
FIGURE 46.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for hypotension.

Abdominal pain
The network diagram for abdominal pain is presented in Appendix 8. Pooled effect sizes from the NMA of 25 trials suggested that misoprostol plus oxytocin is worse than the placebo or the control in causing abdominal pain (Figure 47). No active intervention was found to be worse or better than any other. There was evidence of global inconsistency in this analysis (p = 0.035). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally fairly consistent results.
FIGURE 47.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for abdominal pain. NA, not applicable.
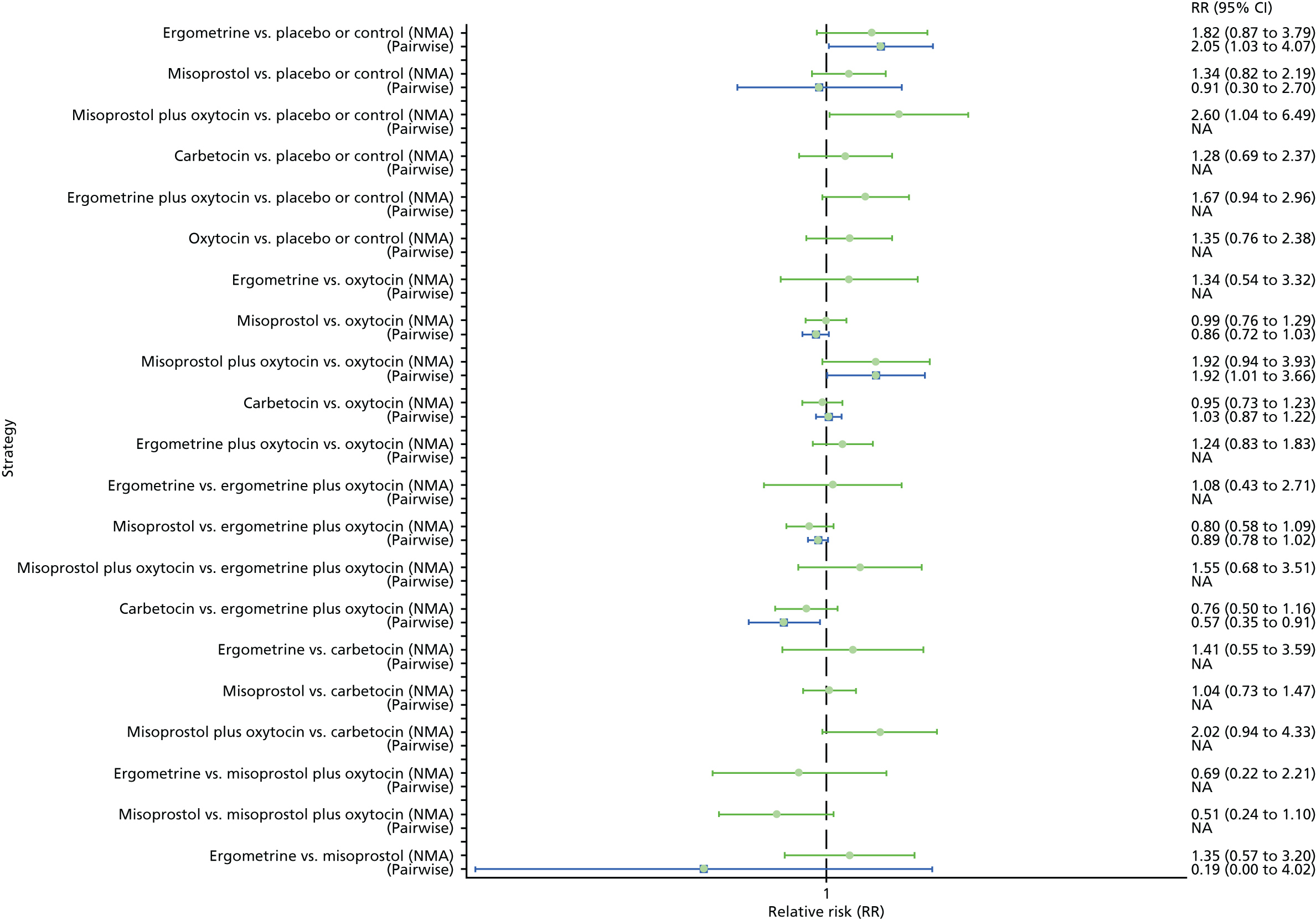
Figure 48 shows the cumulative probabilities for each intervention being at each possible rank for causing abdominal pain. The highest-ranked intervention was placebo or the control. For the rest of the interventions no clear ranking emerges because of the lack of studies in this analysis.
FIGURE 48.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for abdominal pain.

Subgroup analyses
Mode of birth
Vaginal birth
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the subgroup, including only vaginal births, is presented in Appendix 8. Pooled effect sizes from the NMA of 85 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with the placebo (Figure 49). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin, even though carbetocin also demonstrated a trend towards reduction of this outcome (see Figure 49). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (p = 0.06).
FIGURE 49.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, by mode of birth (vaginal birth). NA, not applicable.
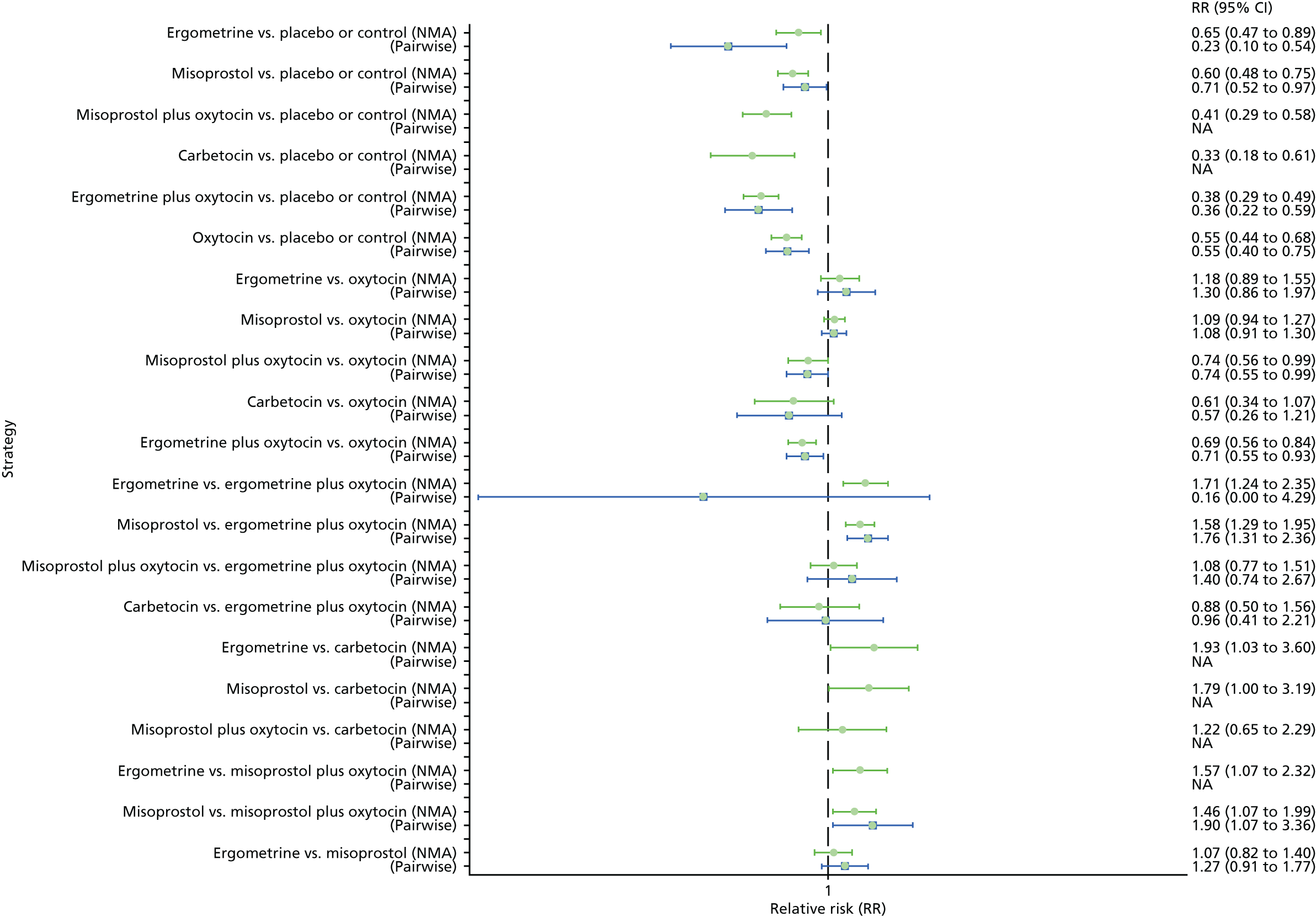
Figure 50 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including only vaginal births. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin, with an almost 100% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was close to 0%.
FIGURE 50.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by mode of birth (vaginal birth).
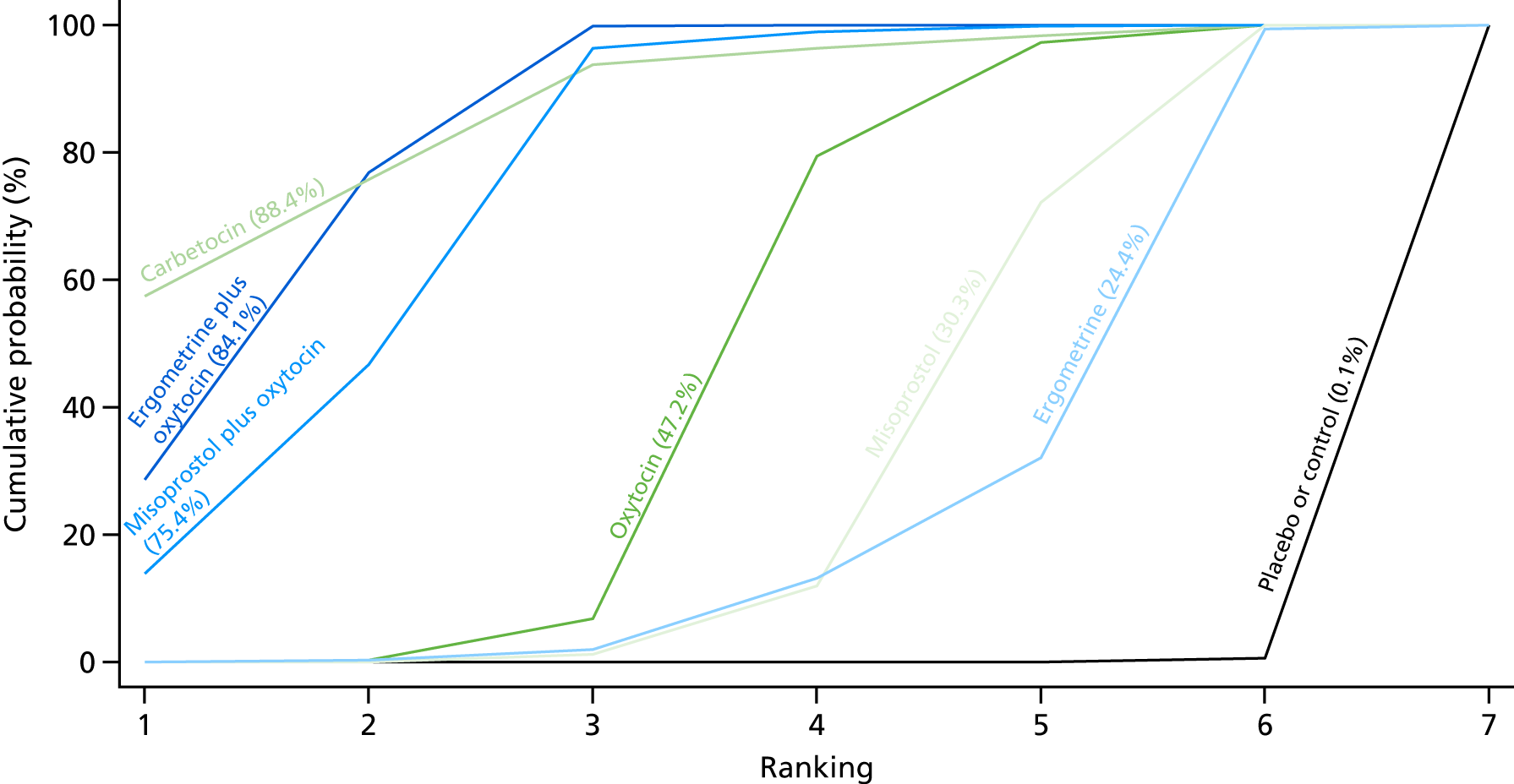
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 71 trials suggested that all interventions except carbetocin and ergometrine are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo (Figure 51). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin and misoprostol plus oxytocin demonstrated a trend towards reduction of this outcome (see Figure 51). There was no evidence of global inconsistency in this analysis (p = 0.206).
FIGURE 51.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by mode of birth (vaginal birth). NA, not applicable.
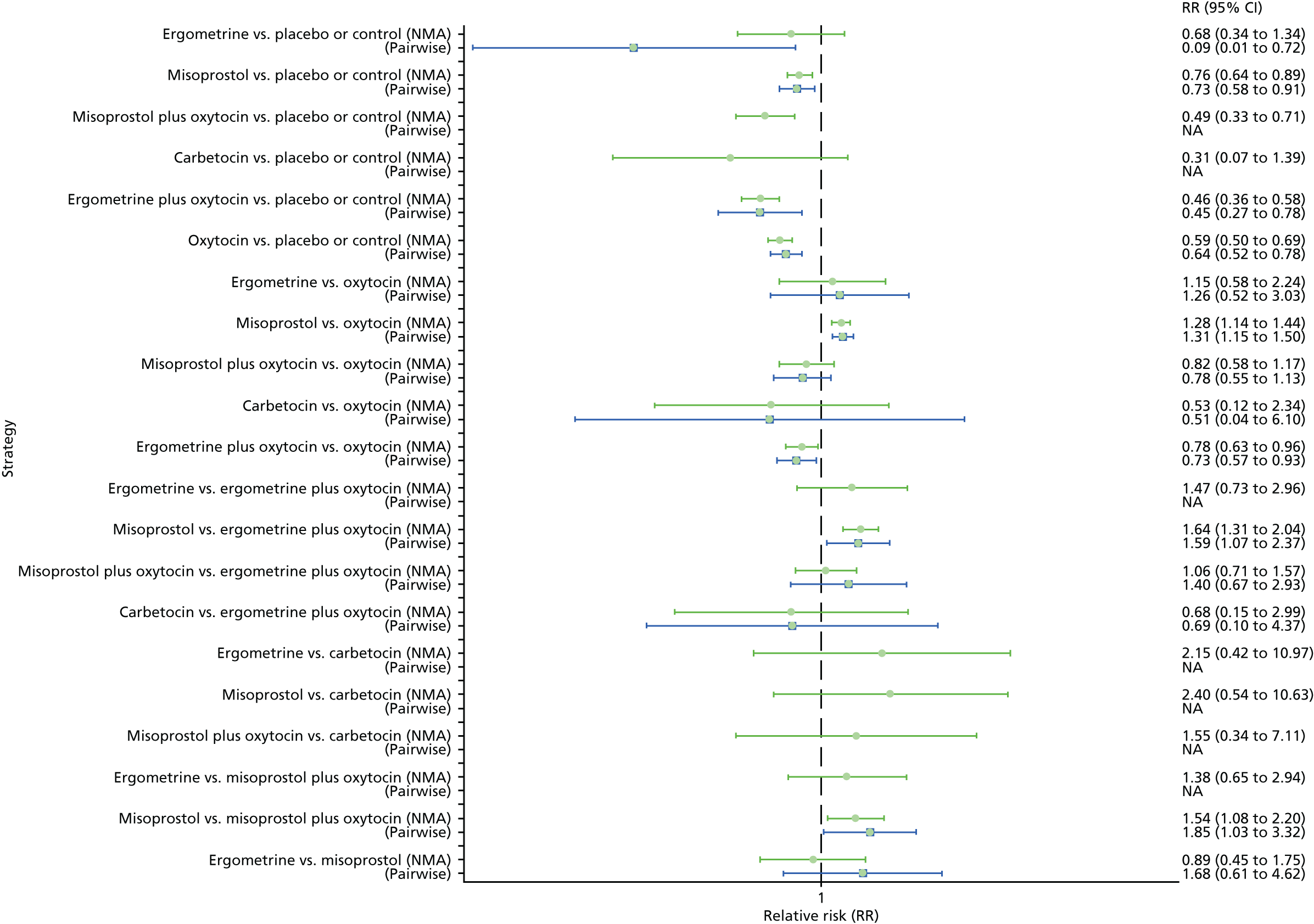
Figure 52 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including only vaginal births. The highest-ranked interventions are carbetocin, ergometrine plus oxytocin, and misoprostol plus oxytocin. Oxytocin is ranked fourth and its probability of being ranked in the top two interventions was close to 0%.
FIGURE 52.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml by mode of birth (vaginal birth).
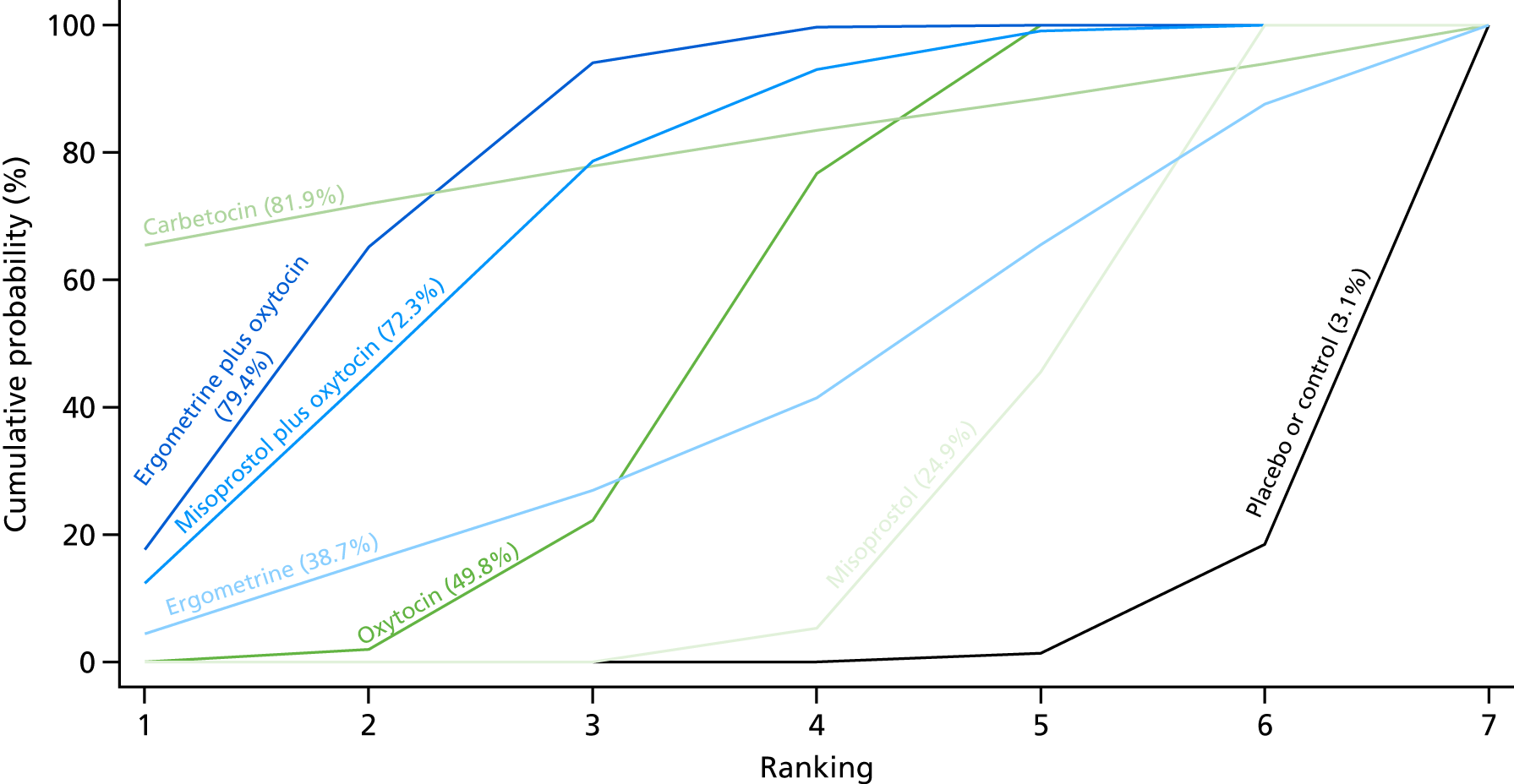
Caesarean section
Primary postpartum haemorrhage blood loss of ≥ 500 ml
Pooled effect sizes from the NMA of 15 trials suggested that only misoprostol plus oxytocin is better than oxytocin alone in preventing PPH blood loss of ≥ 500 ml in women undergoing caesareans, but most of the comparisons were based on single studies (Figure 53). There was no evidence of global inconsistency in this analysis (p = 0.249).
FIGURE 53.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml by mode of birth (caesarean). NA, not applicable.
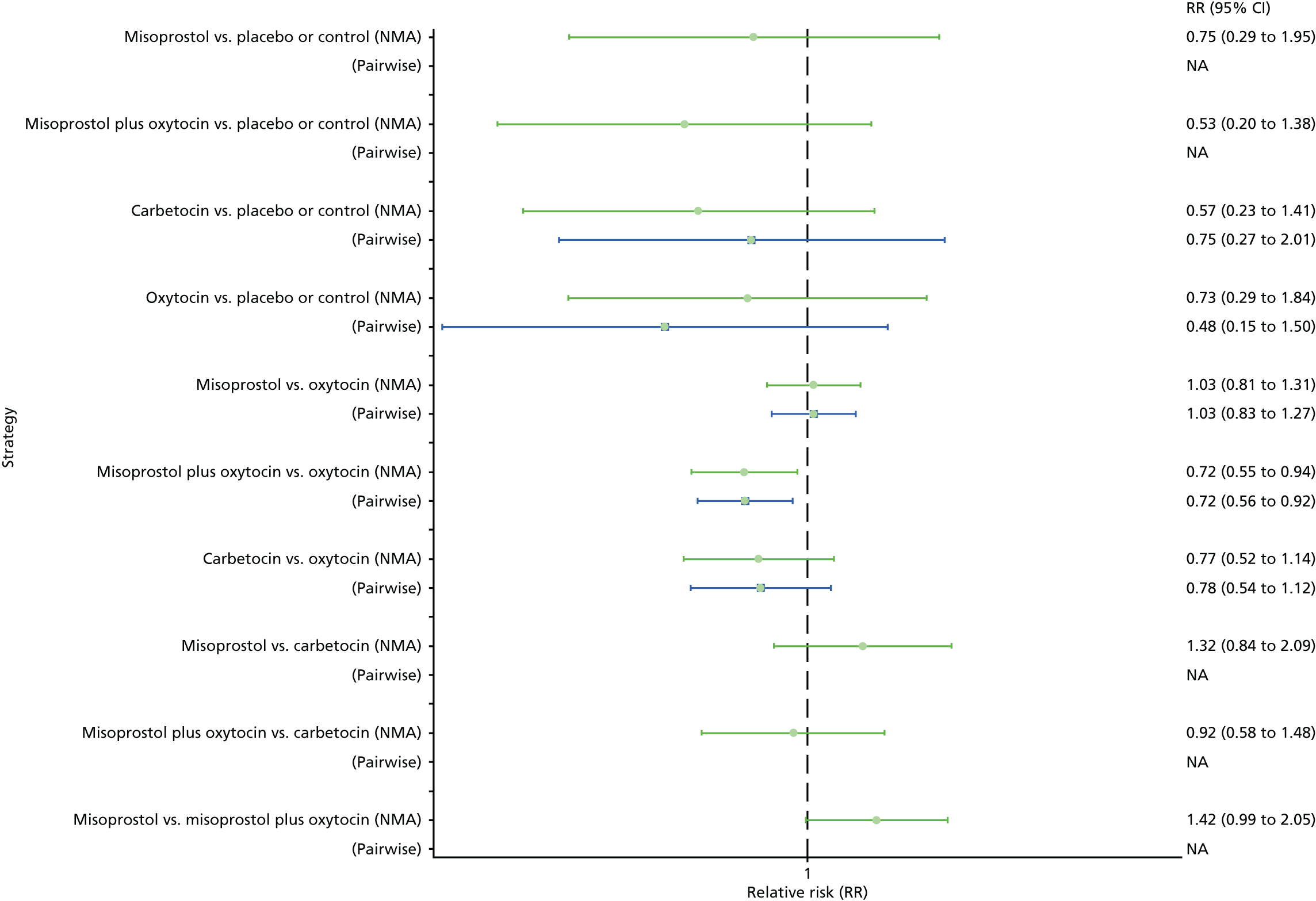
Figure 54 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including only caesareans. The highest-ranked interventions are misoprostol plus oxytocin and carbetocin. Oxytocin is ranked third and its probability in being ranked in the top two interventions was close to 5%. Ergometrine and ergometrine plus oxytocin could not be ranked, as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 54.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by mode of birth (caesarean).
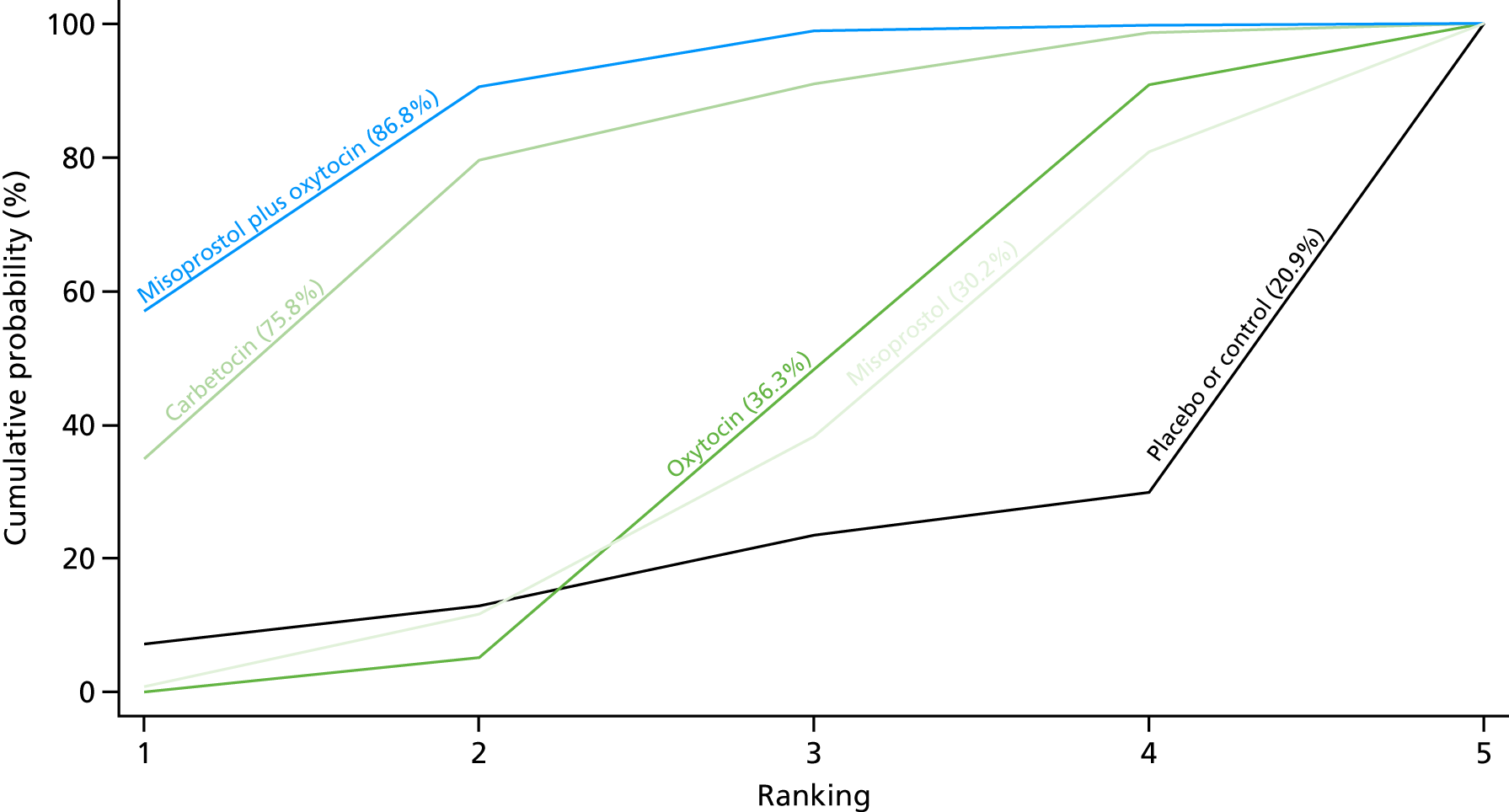
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 19 trials suggested a lack of evidence that any intervention is worse or better than any other in preventing PPH blood loss of ≥ 1000 ml in women undergoing caesareans, but many of the comparisons were based on single studies (Figure 55). There was no evidence of global inconsistency in this analysis (p = 0.86).
FIGURE 55.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by mode of birth (caesarean). NA, not applicable.
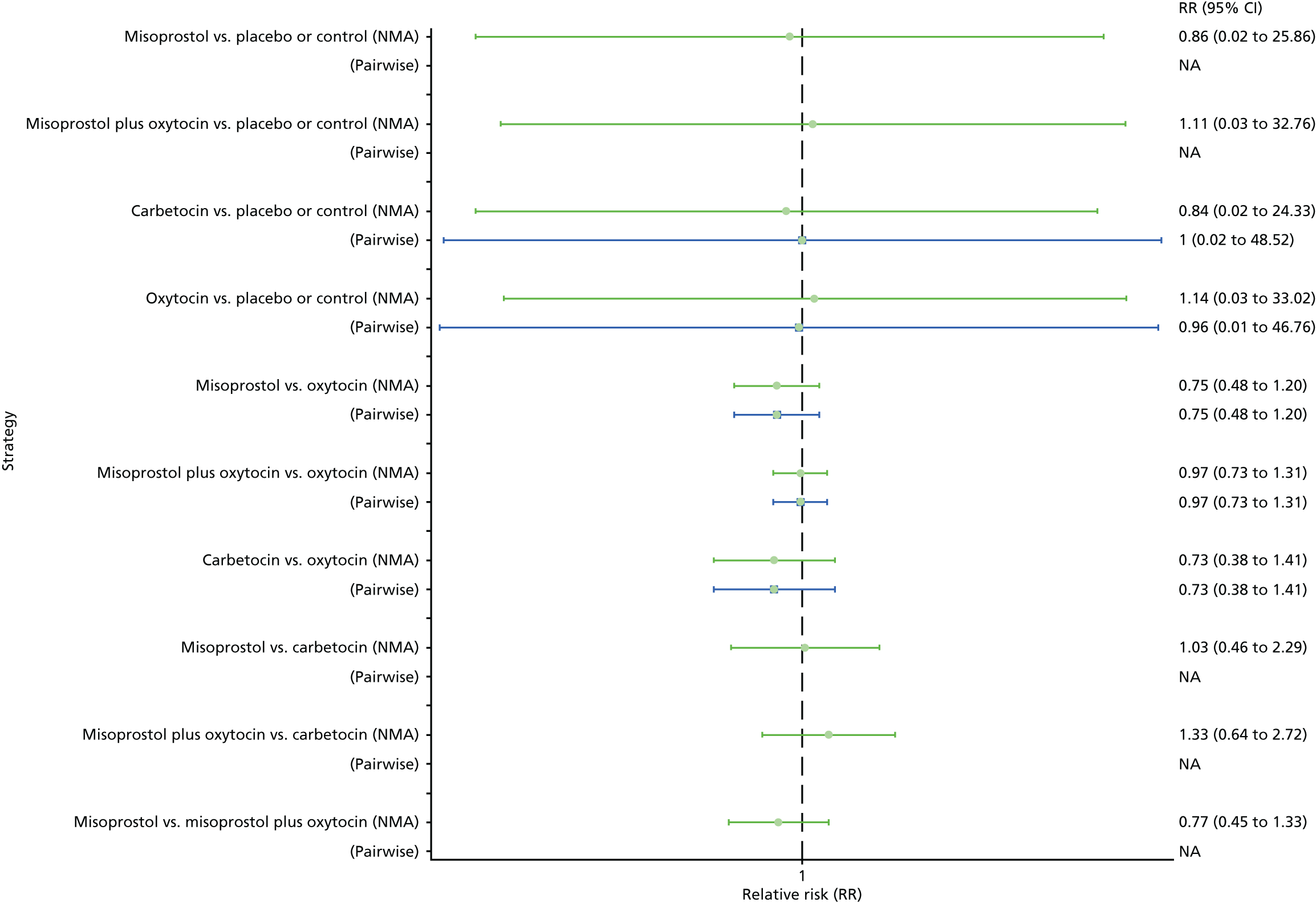
Figure 56 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including only caesareans. No clear ranking emerges in this analysis. Ergometrine and ergometrine plus oxytocin could not be ranked, as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 56.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, by mode of birth (caesarean).

Prior risk of postpartum haemorrhage
Low risk for postpartum haemorrhage
Primary postpartum haemorrhage blood loss of ≥ 500 ml
Pooled effect sizes from the NMA of 35 trials suggested that only ergometrine plus oxytocin and misoprostol are better than the placebo in preventing PPH blood loss of ≥ 500 ml in women at low risk for PPH, but most of the comparisons were based on single studies (Figure 57). There was no evidence of global inconsistency in this analysis (p = 0.236).
FIGURE 57.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, by prior risk for PPH (low risk). NA, not applicable.
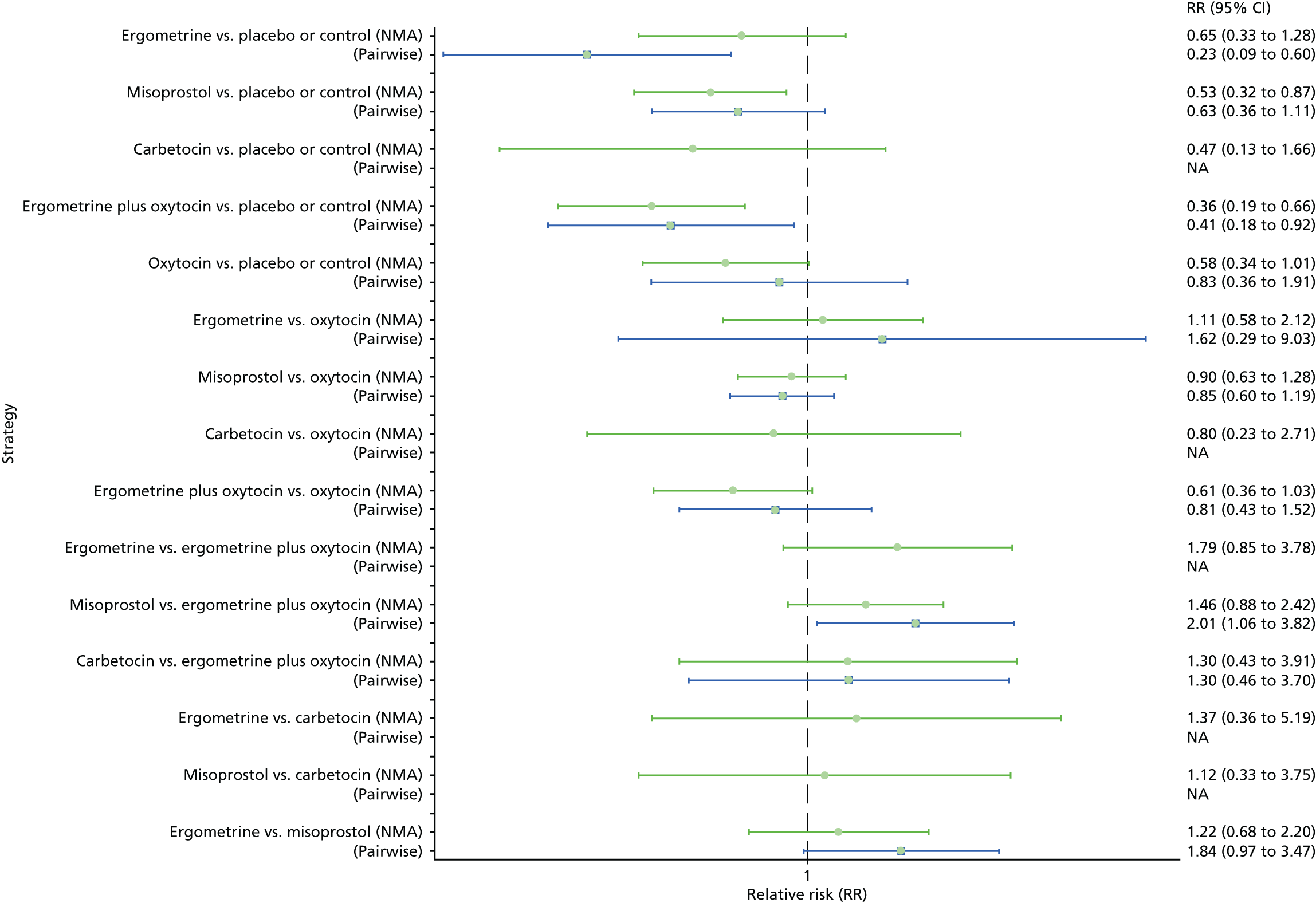
Figure 58 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including trials with only women at low risk for PPH. The highest-ranked interventions are ergometrine plus oxytocin and carbetocin. Oxytocin is ranked fourth behind misoprostol and its probability in being ranked in the top two interventions was close to 10%. Misoprostol plus oxytocin could not be ranked, as there were no studies found comparing this intervention with any other interventions in the network.
FIGURE 58.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by prior risk for PPH (low risk).

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 32 trials suggested that ergometrine plus oxytocin, oxytocin, ergometrine and misoprostol are better than placebo in preventing PPH blood loss of ≥ 1000 ml in women at low risk for PPH (Figure 59). The comparisons between active interventions appeared to be underpowered to detect differences between them. There was no evidence of global inconsistency in this analysis (p = 0.477).
FIGURE 59.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by prior risk for PPH (low risk). NA, not applicable.

Figure 60 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including trials with only women at low risk for PPH. No clear ranking emerges in this analysis. Ergometrine could not be ranked, as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 60.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, by prior risk for PPH (low risk).

High risk for postpartum haemorrhage
Primary postpartum haemorrhage blood loss of ≥ 500 ml
Pooled effect sizes from the NMA of 21 trials suggested that only misoprostol plus oxytocin is better than oxytocin in preventing PPH blood loss of ≥ 500 ml and carbetocin showed a similar trend towards prevention of this outcome for women at high risk for PPH, but most of the comparisons were based on single studies (Figure 61). There was no evidence of global inconsistency in this analysis (p = 0.211).
FIGURE 61.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml by prior risk for PPH (high risk). NA, not applicable.

Figure 62 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including trials with only women at high risk for PPH. The highest-ranked interventions are misoprostol plus oxytocin and carbetocin. Oxytocin is ranked third closely followed by misoprostol and its probability in being ranked in the top two interventions was close to 0%.
FIGURE 62.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by prior risk for PPH (high risk).
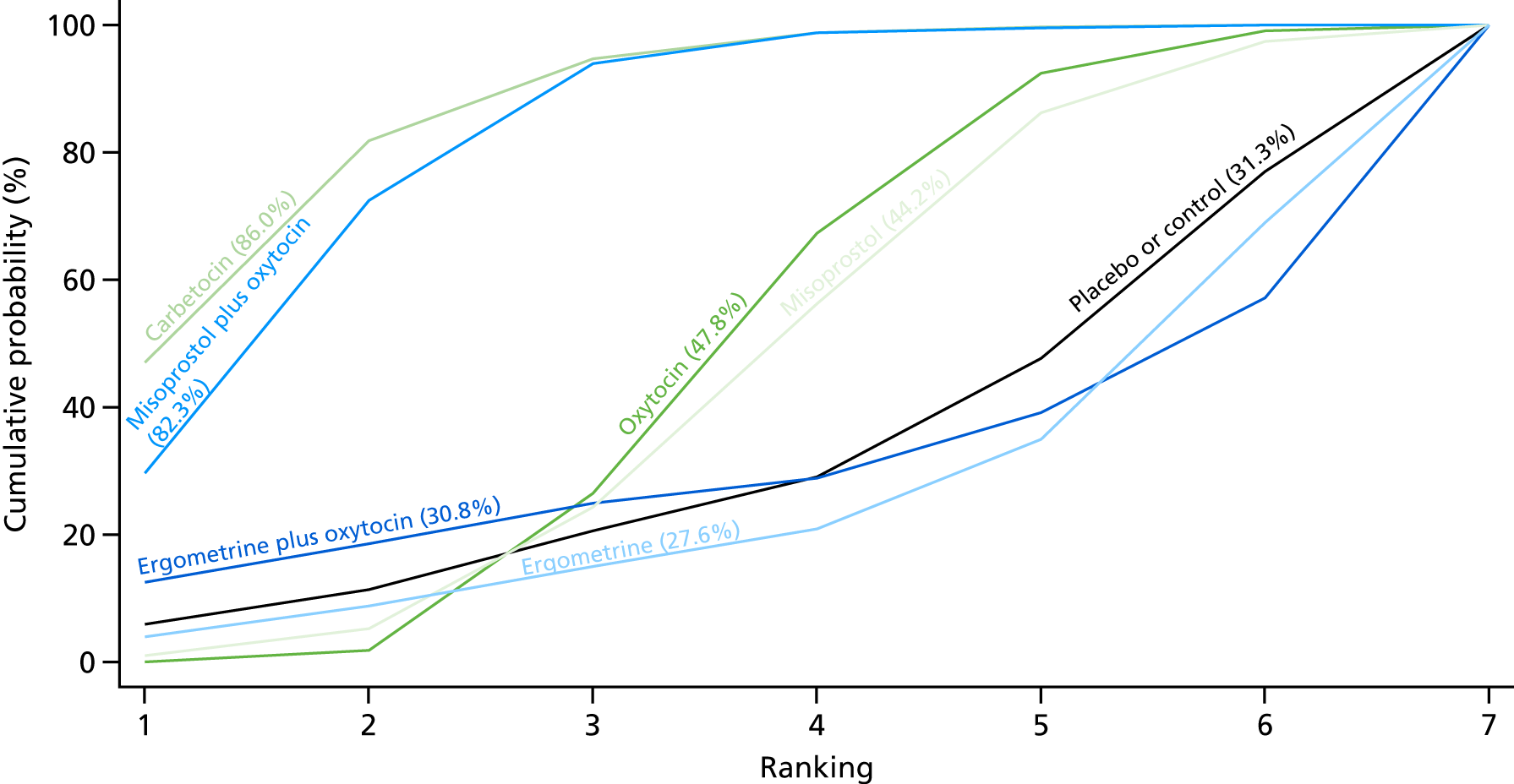
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 22 trials suggested a lack of evidence that any intervention is worse or better than any other in preventing PPH blood loss of ≥ 1000 ml in women at high risk for PPH, and many of the comparisons were based on single studies (Figure 63). There was no evidence of global inconsistency in this analysis (p = 0.851).
FIGURE 63.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by prior risk for PPH (high risk). NA, not applicable.
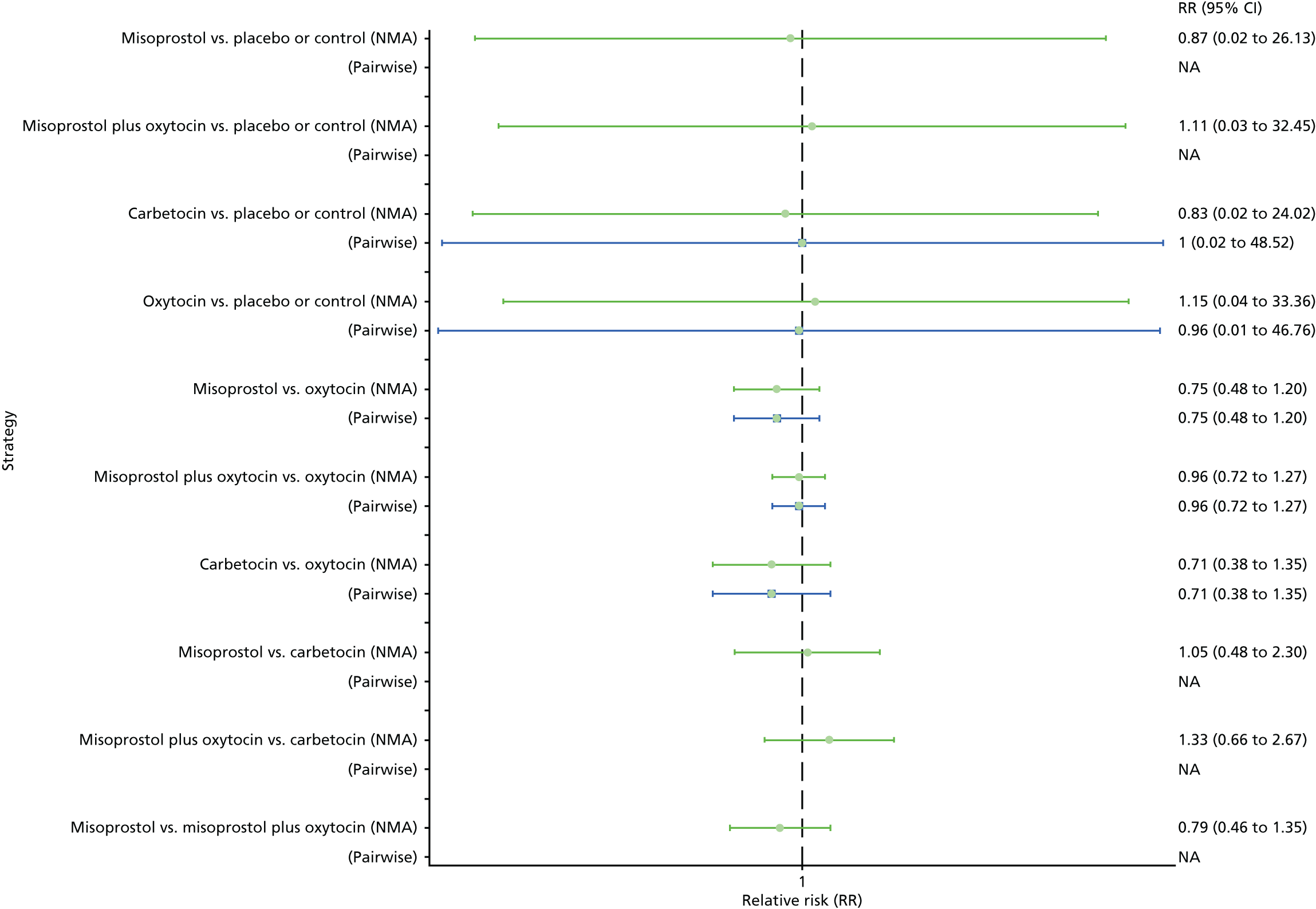
Figure 64 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including trials with only women at high risk for PPH. No clear ranking emerges in this analysis. Ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 64.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, by prior risk for PPH (high risk).

Health-care setting
Hospital setting
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the subgroup including trials carried out in the hospital setting is presented in Appendix 8. Pooled effect sizes from the NMA of 95 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo (Figure 65). Ergometrine plus oxytocin, and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin, even though carbetocin also demonstrated a trend towards reduction of this outcome (Figure 65). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (p = 0.0448). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results except for ergometrine versus placebo or the control based on a single study.
FIGURE 65.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, by health-care setting (hospital setting). NA, not applicable.
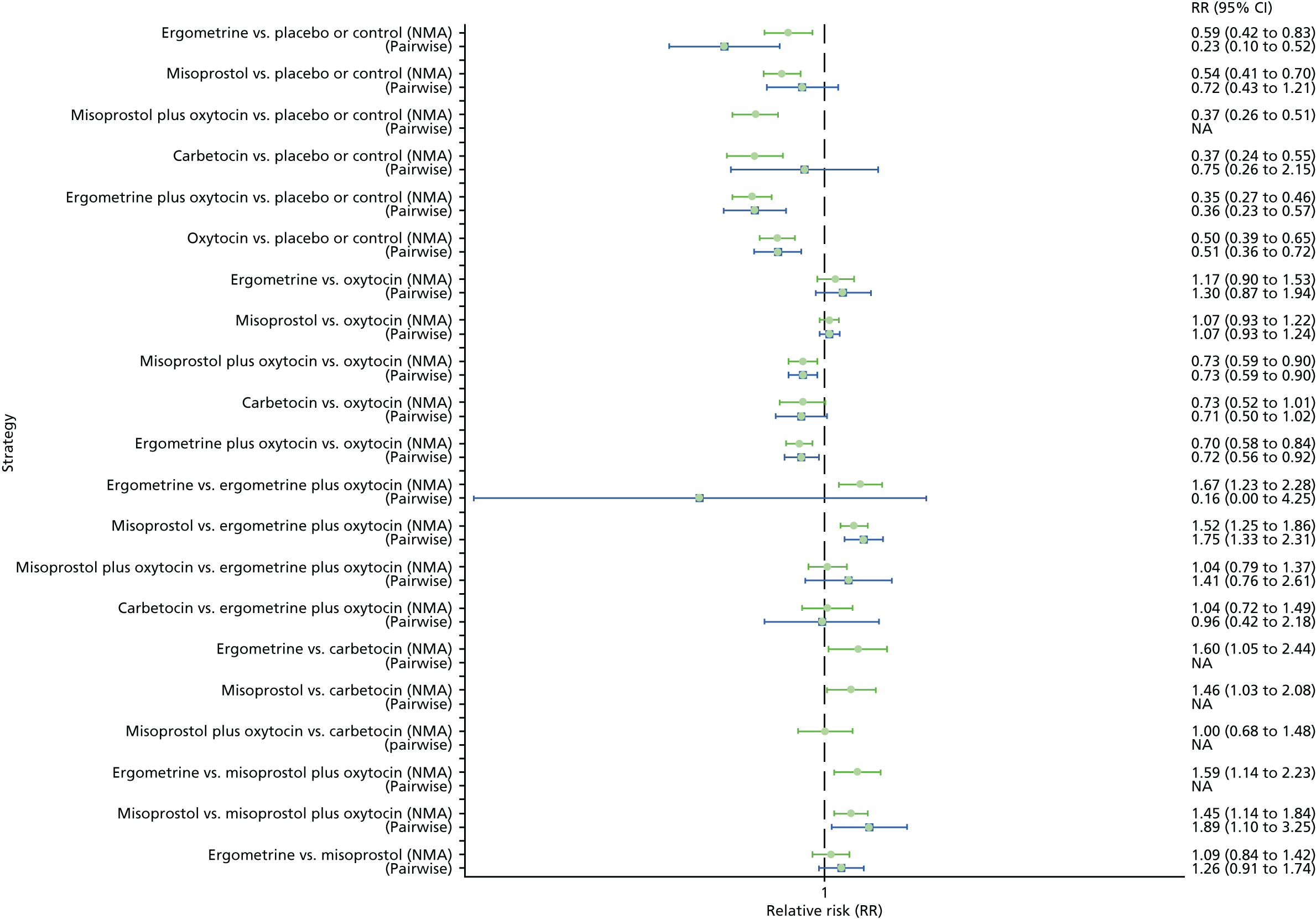
Figure 66 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including trials carried out in the hospital setting. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin, with an almost 100% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was close to 0%.
FIGURE 66.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by health-care setting (hospital setting).

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 85 trials suggested that all interventions, except ergometrine, are effective for preventing PPH blood loss of ≥ 1000 ml when compared with the placebo for the subgroup including trials carried out in the hospital setting (Figure 67). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin and misoprostol plus oxytocin demonstrated a trend towards reduction of this outcome (Figure 67). There was no evidence of global inconsistency in this analysis (p = 0.389).
FIGURE 67.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by health-care setting (hospital setting). NA, not applicable.

Figure 68 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including trials carried out in the hospital setting. The highest-ranked interventions are carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin is still ranked fourth and its probability of being ranked in the top three interventions was close to 20%.
FIGURE 68.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, by health-care setting (hospital setting).

Community setting
Primary postpartum haemorrhage blood loss of ≥ 500 ml
Pooled effect sizes from the NMA of four trials suggested that only oxytocin and misoprostol are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup including trials carried out in the community setting (Figure 69). There was evidence of global inconsistency in this analysis (p = 0.03), but most of the comparisons are based on a small number of studies.
FIGURE 69.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, by health-care setting (community setting).
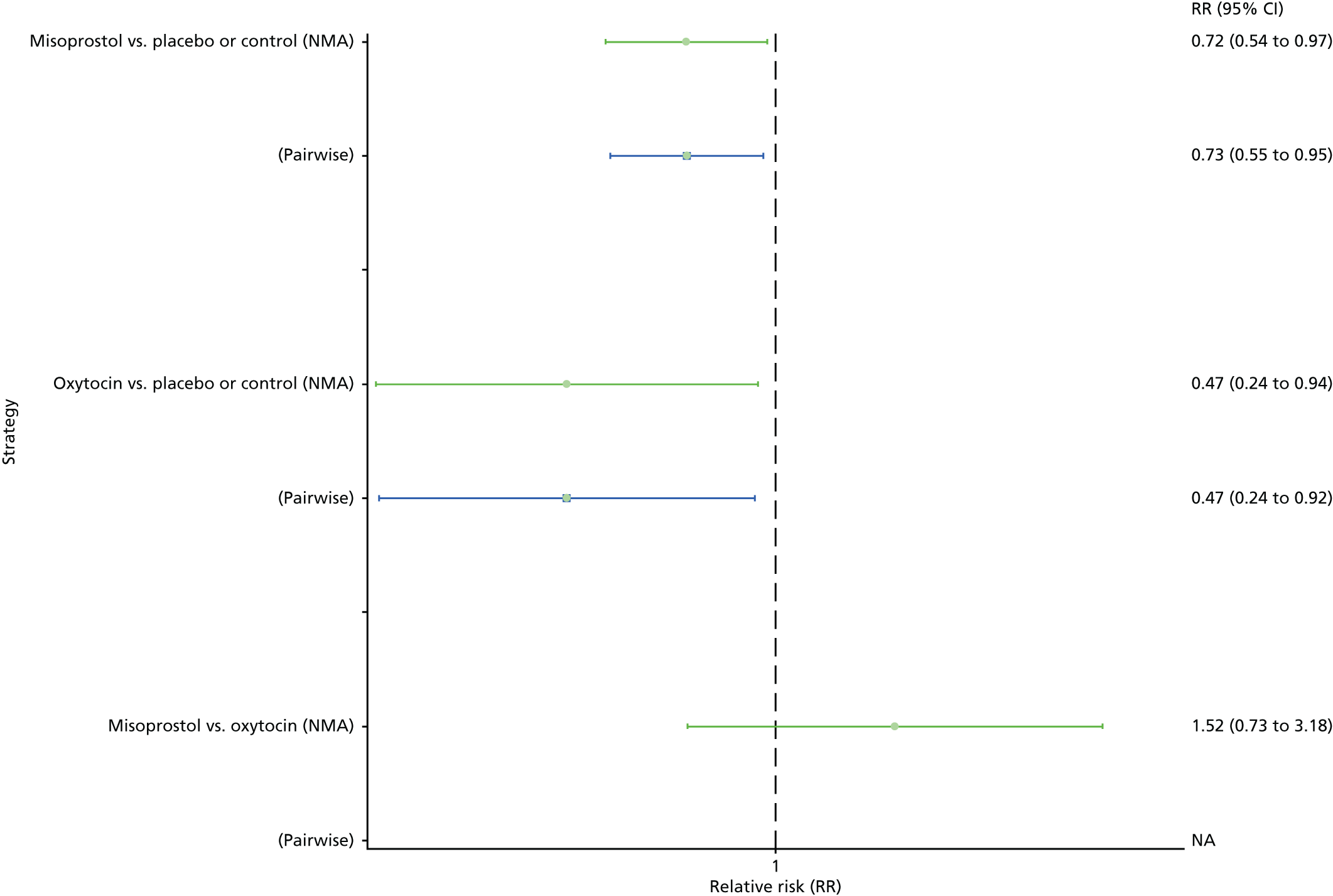
Figure 70 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including trials carried out in the community setting. No clear ranking emerges in this analysis. Carbetocin, misoprostol plus oxytocin, ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 70.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, by health-care setting (community setting).

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of four trials suggested that only misoprostol is more effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo, even though oxytocin also demonstrated a trend towards reduction of this outcome for the subgroup including trials carried out in the community setting (Figure 71). There was evidence of global inconsistency in this analysis (p = 0.004), but most of the comparisons are based on a small number of studies.
FIGURE 71.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, by health-care setting (community setting). NA, not applicable.
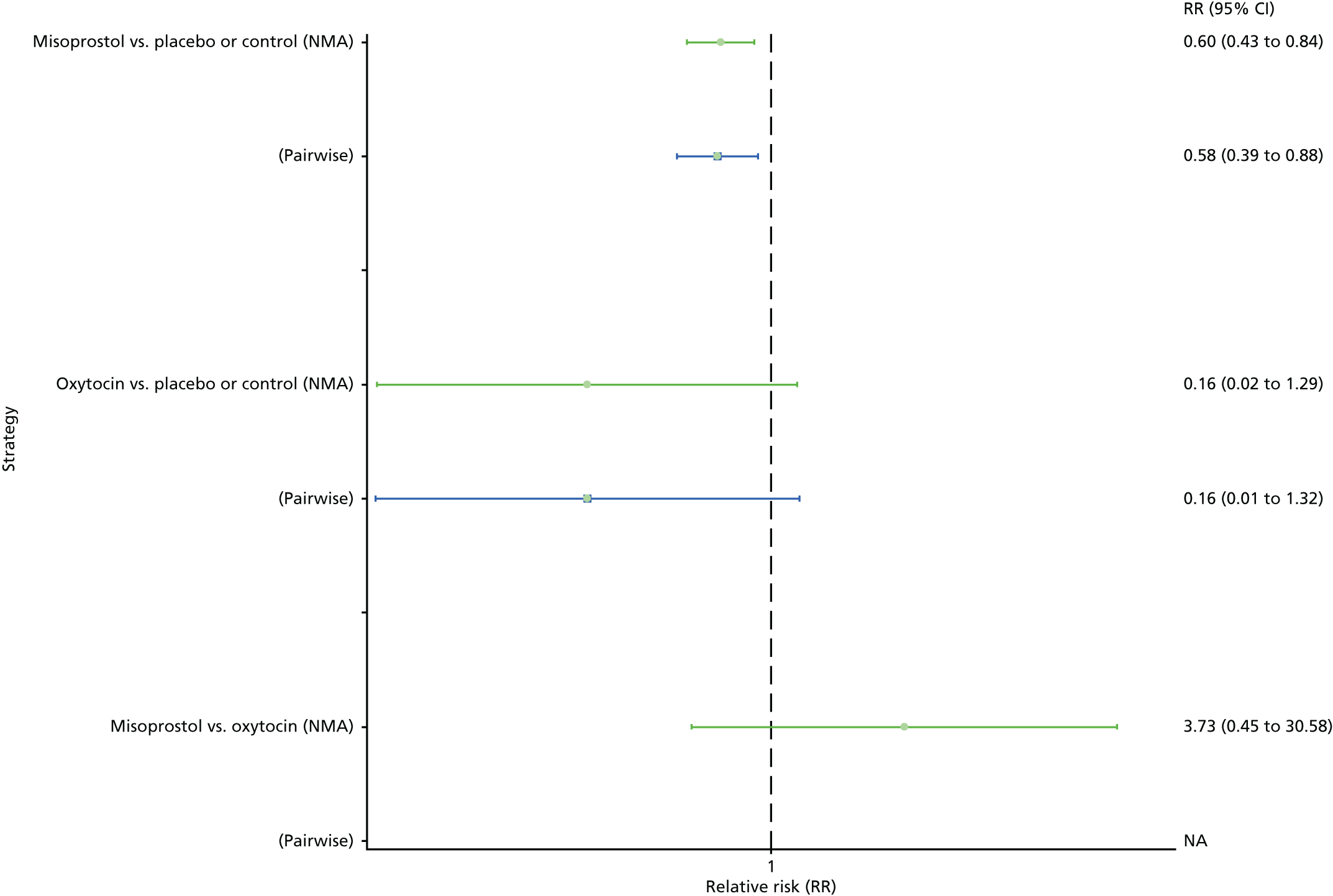
Figure 72 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including trials carried out in the community setting. No clear ranking emerges in this analysis. Carbetocin, misoprostol plus oxytocin, ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those drugs with any other interventions in the network.
FIGURE 72.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, by health-care setting (community setting).
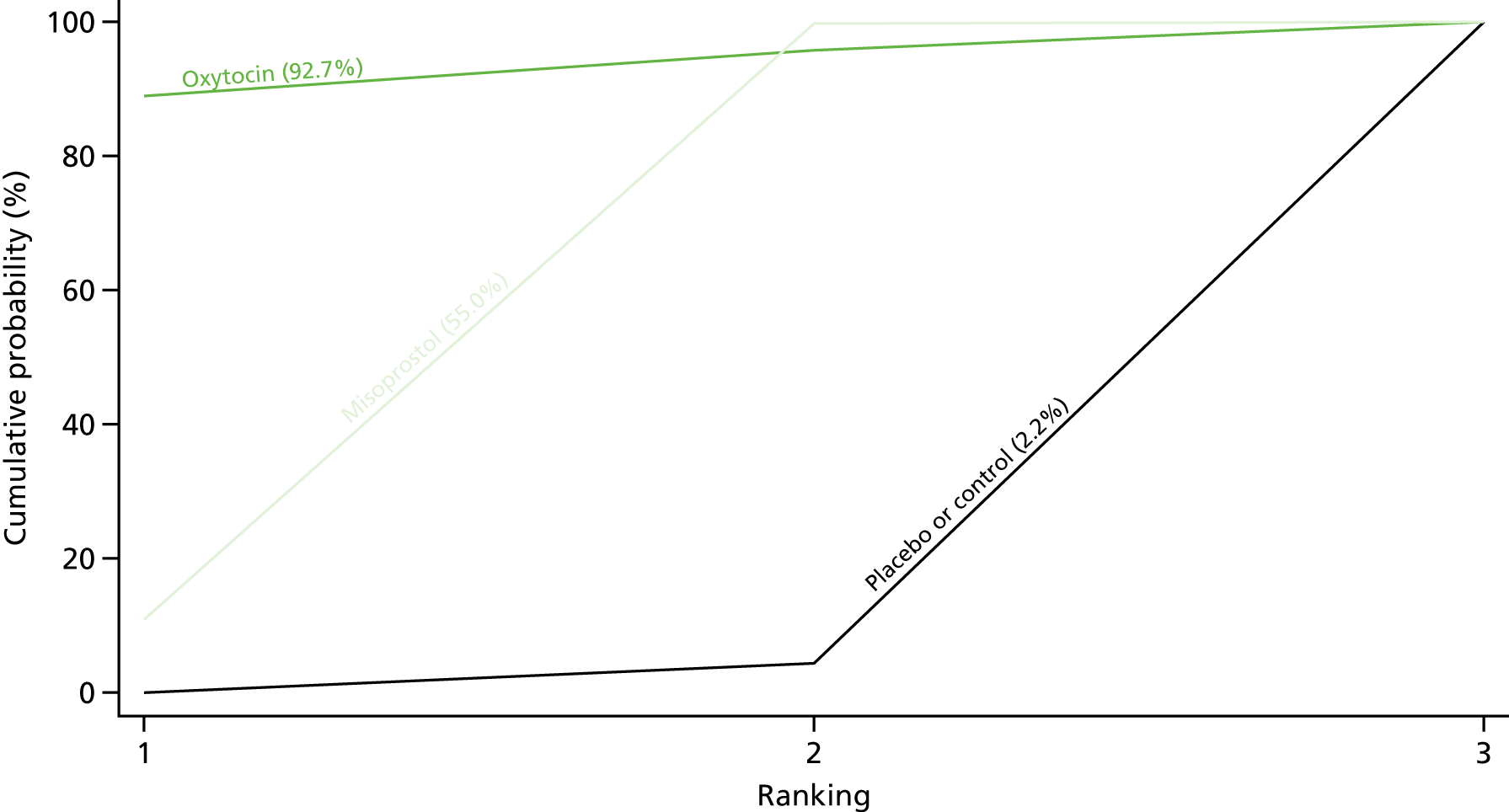
Intervention dose, regimen or route
Low-dose misoprostol
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the subgroup including only misoprostol studies, which used a low dose (i.e. < 500 µg) of misoprostol is presented in Appendix 8. Pooled effect sizes from the NMA of 72 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with the placebo (Figure 73). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin (Figure 73). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (p = 0.016). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results except for ergometrine versus placebo or the control based on a single study.
FIGURE 73.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg). NA, not applicable.
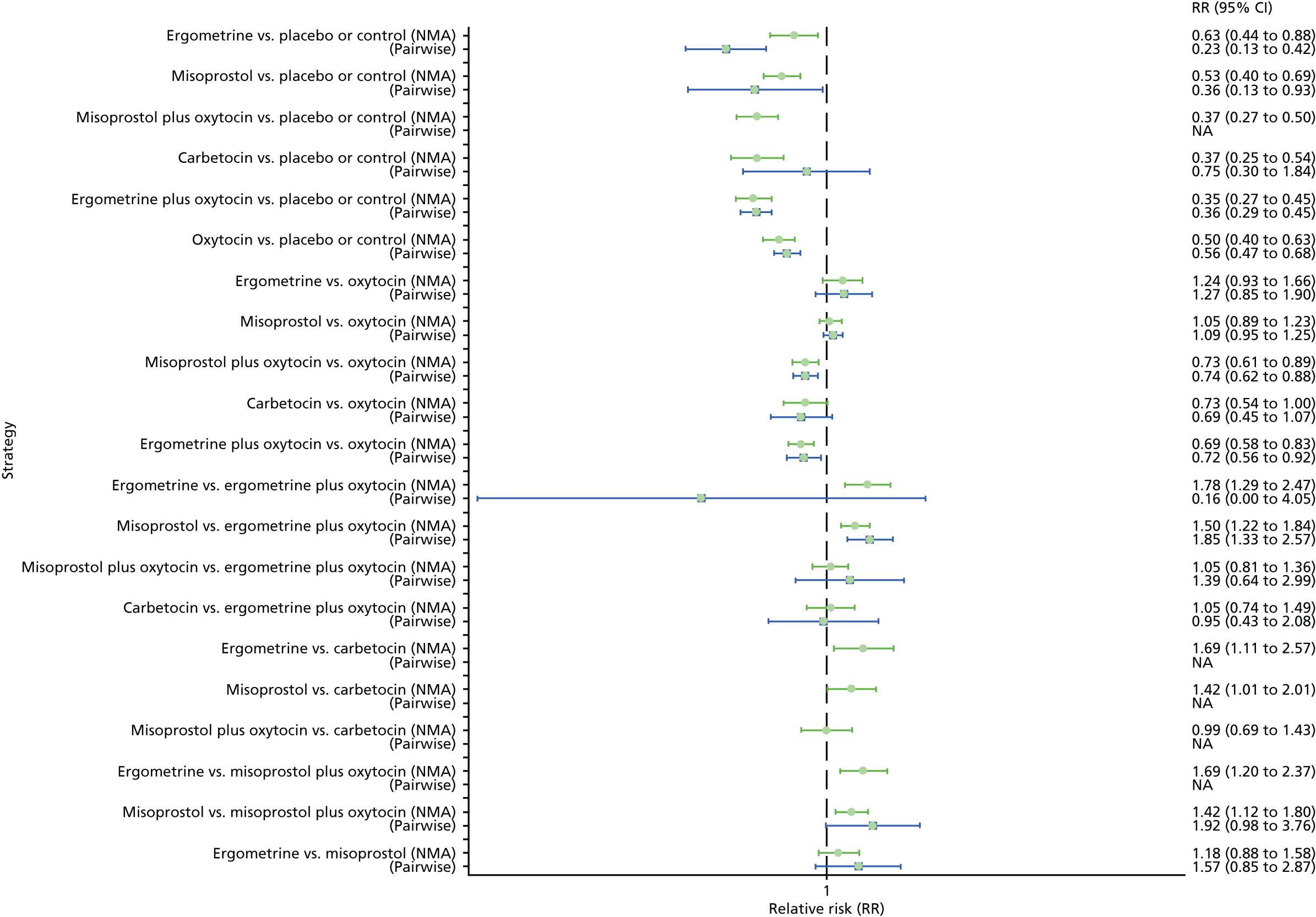
Figure 74 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including misoprostol trials that used a low dose. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin, with almost 100% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was close to 0%.
FIGURE 74.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg).

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 69 trials suggested that all interventions except ergometrine are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo for the subgroup, including misoprostol trials that used a low dose (Figure 75). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin also demonstrated a trend towards reduction of this outcome (Figure 75). There was no evidence of global inconsistency in this analysis (p = 0.401).
FIGURE 75.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg). NA, not applicable.

Figure 76 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including misoprostol trials that used a low dose. The highest-ranked interventions are carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin is still ranked fourth and its probability of being ranked in the top three interventions was close to 20%.
FIGURE 76.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg).
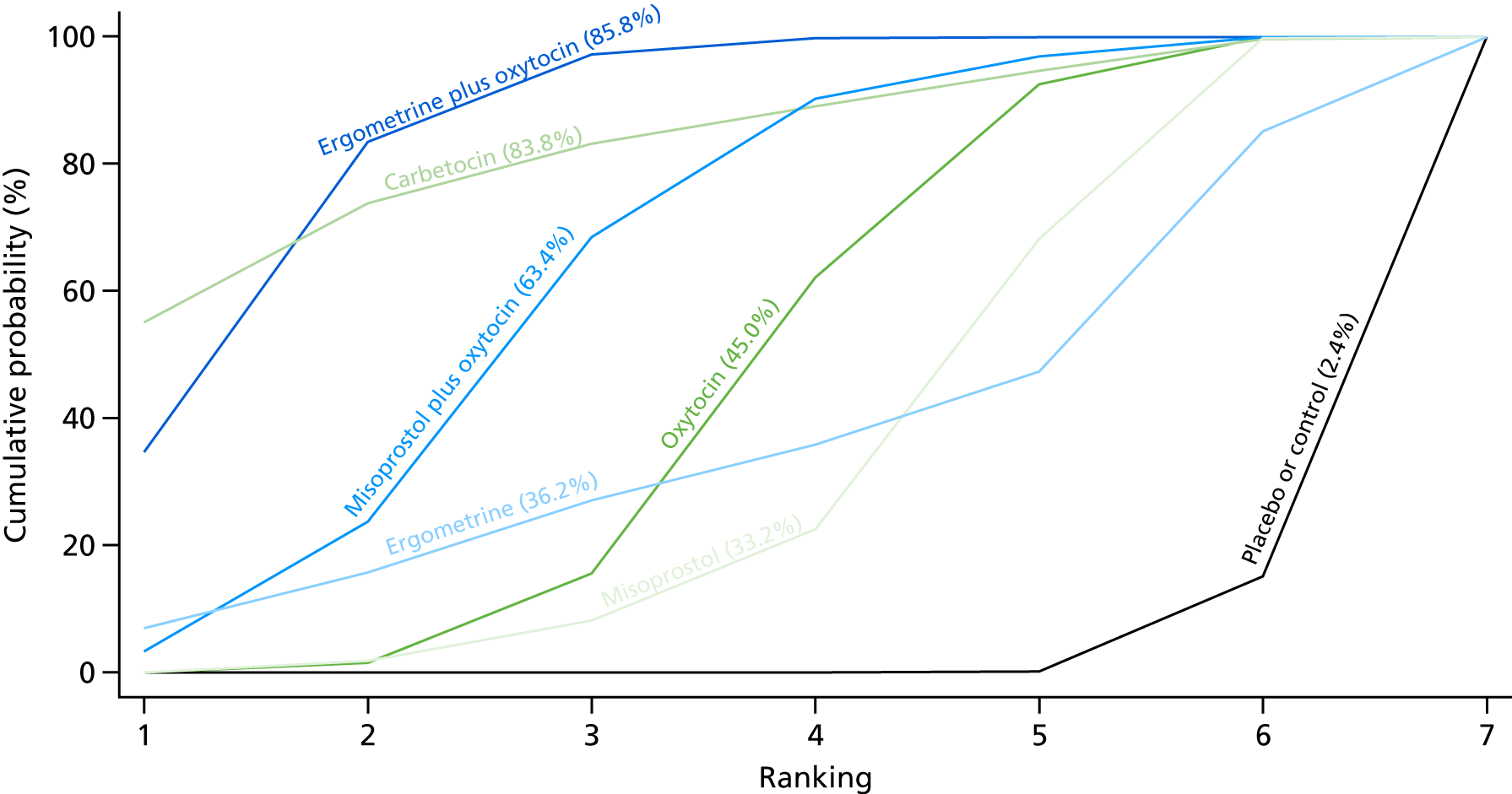
High-dose misoprostol
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the subgroup only misoprostol studies, which used a high dose (i.e. ≥ 600 µg) of misoprostol is presented in Appendix 8. Pooled effect sizes from the NMA of 83 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup, including only misoprostol trials that used a high dose (Figure 77). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin, even though carbetocin also showed a trend towards reduction of this outcome (see Figure 77). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (p = 0.322).
FIGURE 77.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to misoprostol studies that used a high dose (i.e. ≥ 600 µg). NA, not applicable.
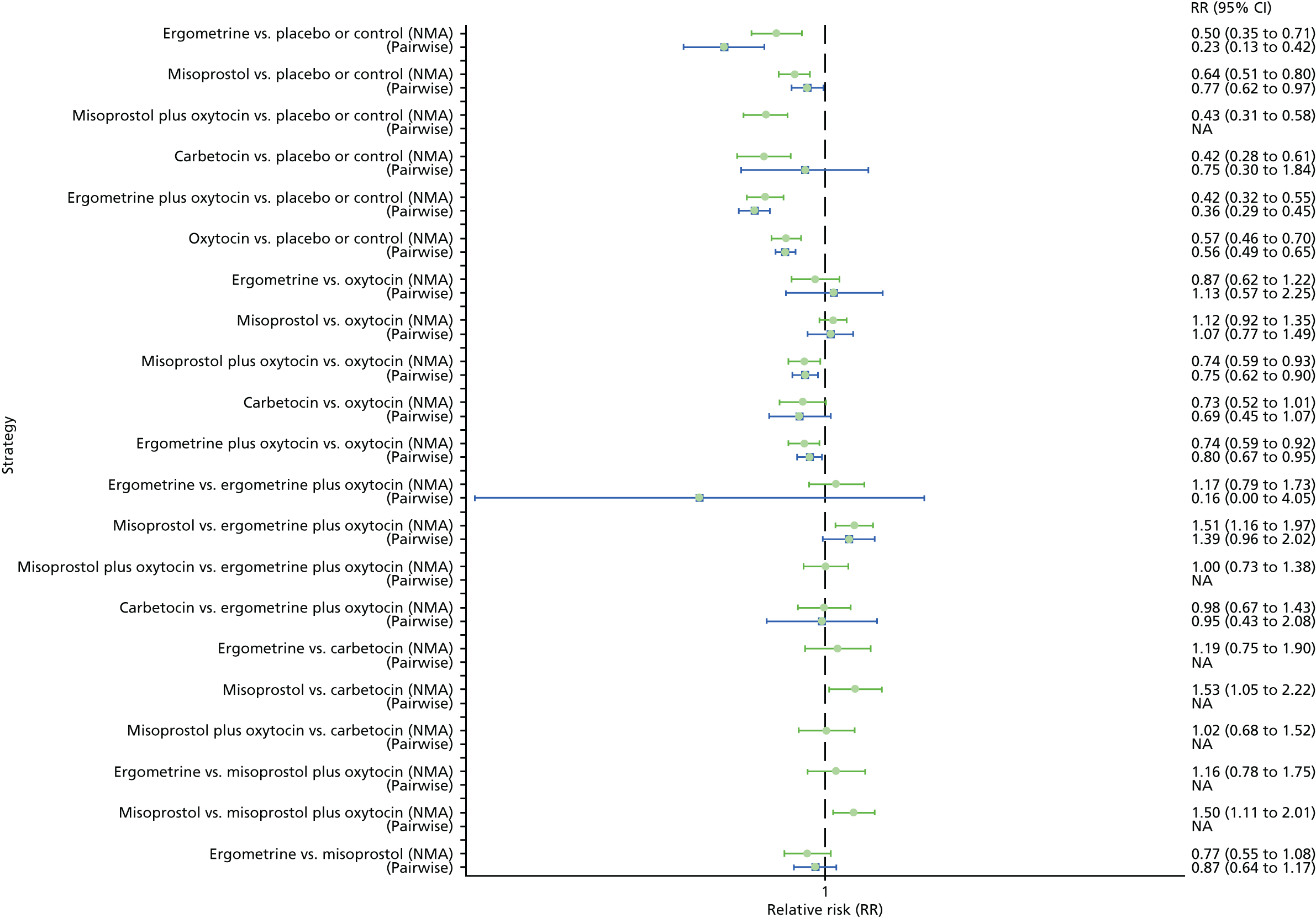
Figure 78 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup, including misoprostol trials that used a low dose. The highest-ranked interventions are ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin, with > 80% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fifth behind ergometrine and its probability of being ranked in the top three interventions was close to 0%.
FIGURE 78.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to misoprostol studies that used a high dose (i.e. ≥ 600 µg).
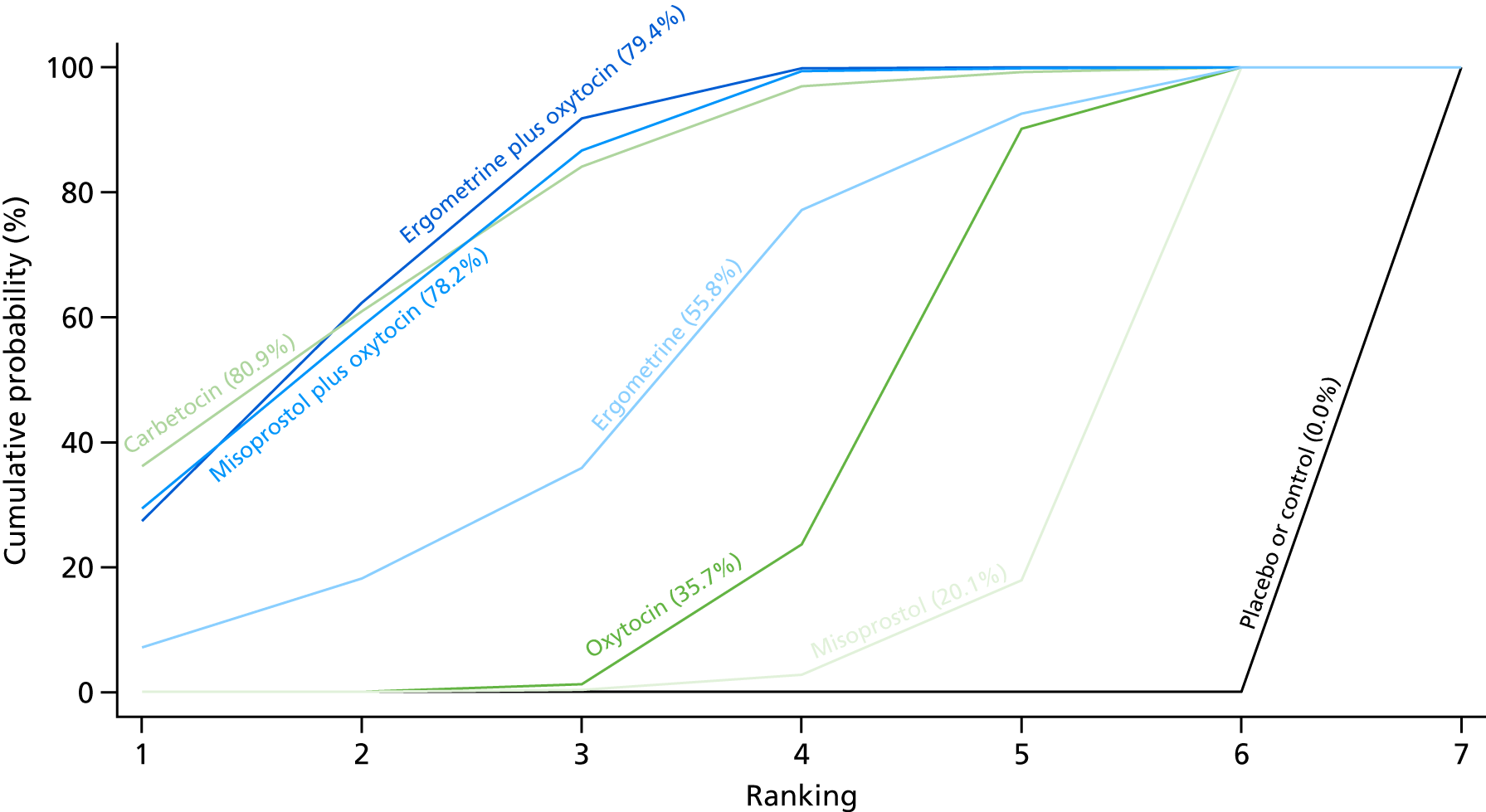
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
Pooled effect sizes from the NMA of 62 trials suggested that all interventions except ergometrine are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo for the subgroup including misoprostol trials that used a low dose (Figure 79). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin also demonstrated a trend towards reduction of this outcome (see Figure 79). Ergometrine plus oxytocin, carbetocin, misoprostol plus oxytocin and oxytocin when used alone were found to be more effective than misoprostol, despite misoprostol being used at a higher dose. There was no evidence of global inconsistency in this analysis (p = 0.625).
FIGURE 79.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to misoprostol studies that used a high dose (i.e. ≥ 600 µg). NA, not applicable.

Figure 80 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including misoprostol trials that used a high dose. The highest-ranked interventions are carbetocin, ergometrine plus oxytocin, ergometrine and misoprostol plus oxytocin. Oxytocin is still ranked fifth and its probability of being ranked in the top three interventions was < 20%.
FIGURE 80.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to misoprostol studies that used a high dose (i.e. ≥ 600 µg).
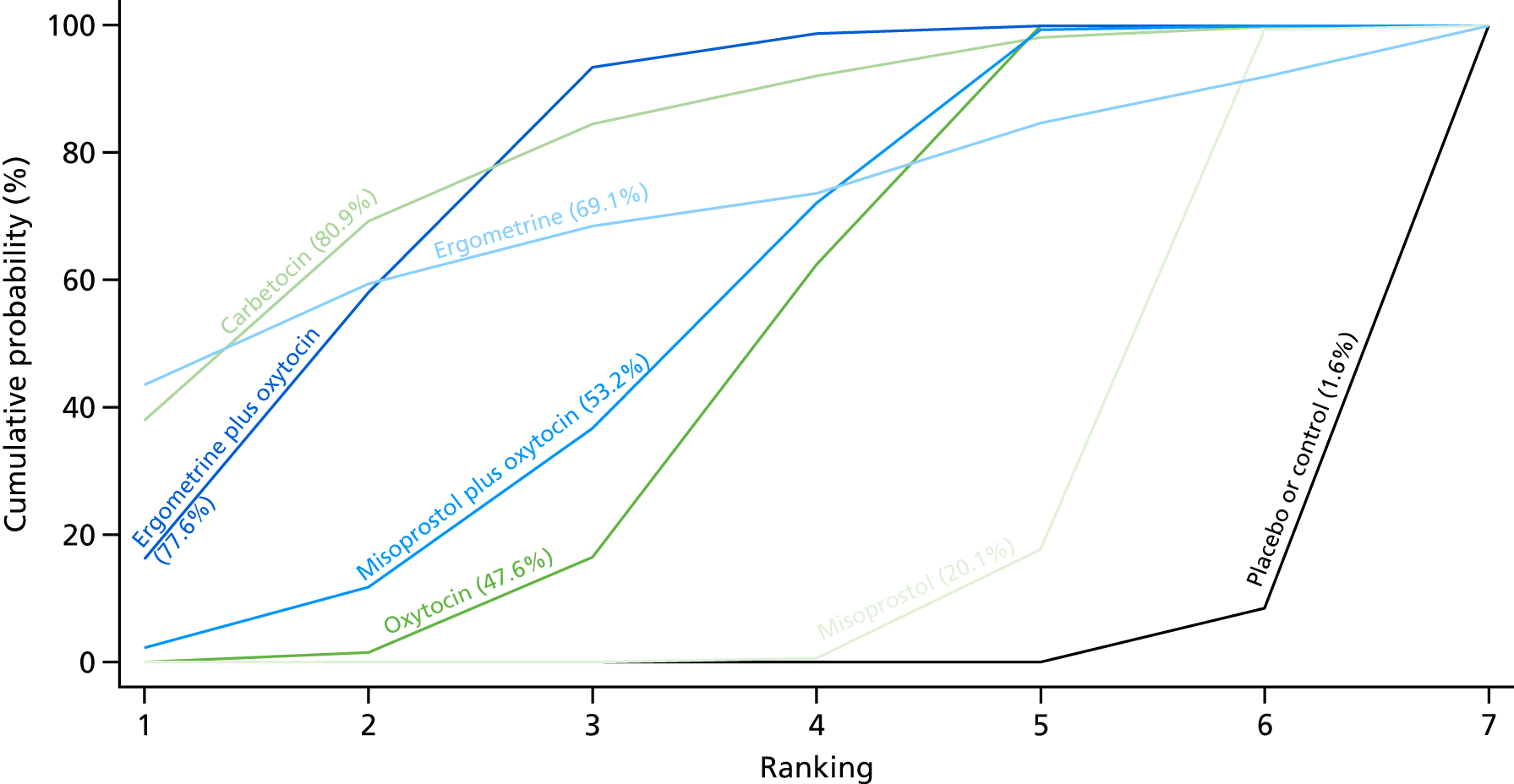
Oxytocin bolus only
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the subgroup is presented in Appendix 8. This subgroup includes all trials, but when oxytocin was used as an arm in the trial this analysis is restricted to oxytocin studies that used an intravenous or intramuscular bolus of any dose and excluded studies that used a bolus plus infusion or infusion only of oxytocin. Pooled effect sizes from the NMA of 84 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup, including oxytocin trials that used an intramuscular or intravenous bolus of any dose (Figure 81). Ergometrine plus oxytocin was the only intervention found to be more effective than the standard intervention, oxytocin, even though carbetocin and misoprostol plus oxytocin also demonstrated a trend towards reduction of this outcome (see Figure 81). Ergometrine plus oxytocin was also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (p = 0.134).
FIGURE 81.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose. NA, not applicable.

Figure 82 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup including oxytocin trials that used an intramuscular or intravenous bolus of any dose. The highest-ranked interventions are ergometrine plus oxytocin, misoprostol plus oxytocin and carbetocin, with > 80% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was > 20%.
FIGURE 82.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.
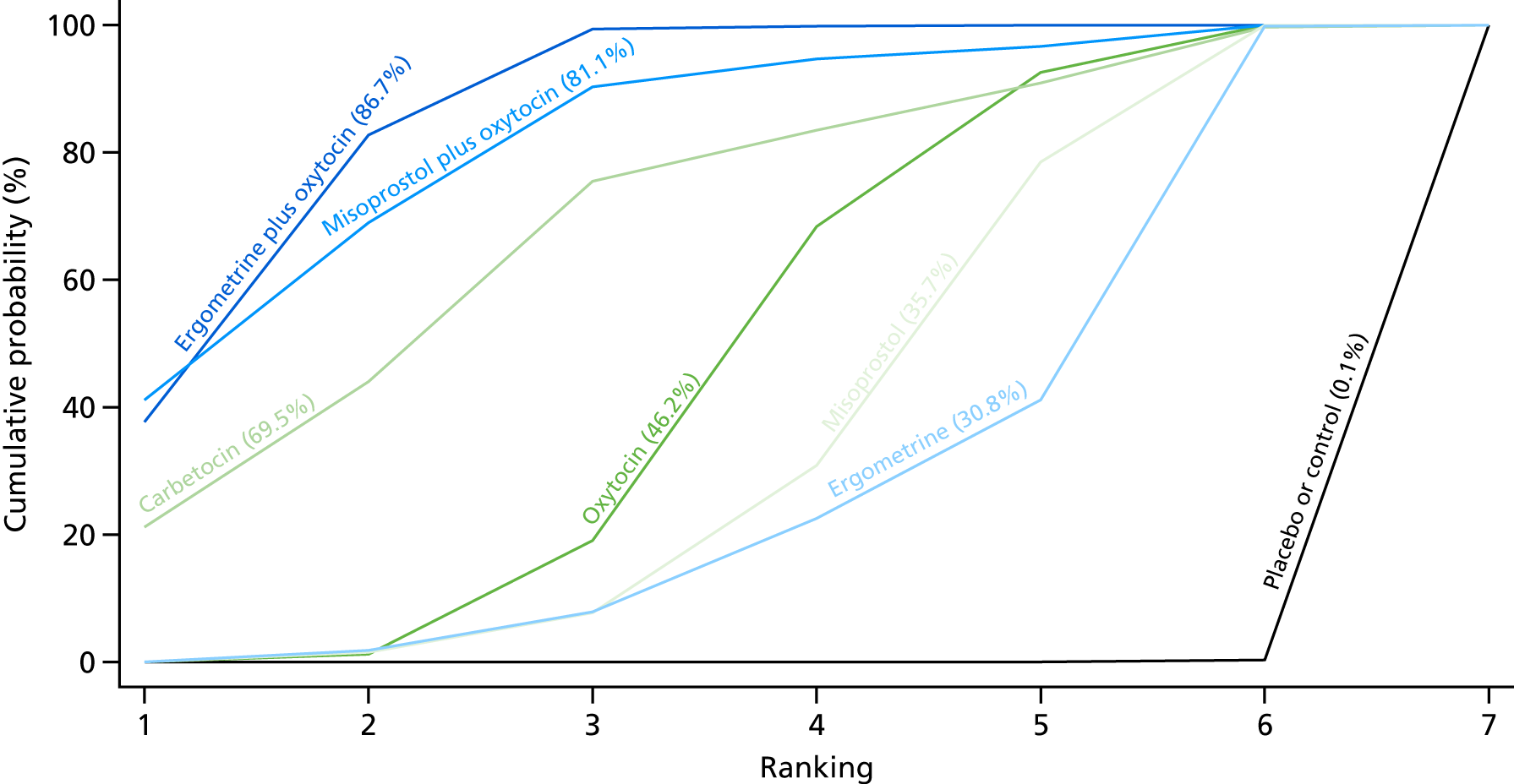
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for the subgroup including all trials, but restricting to oxytocin trials that used an intravenous or intramuscular bolus of any dose, is presented in Appendix 8. Pooled effect sizes from the NMA of 68 trials suggested that all interventions, except carbetocin and ergometrine, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup including only oxytocin trials that used an intramuscular or intravenous bolus of any dose (Figure 83). None of the interventions was found to be more effective than the standard intervention, oxytocin (see Figure 83). Ergometrine plus oxytocin and oxytocin when used alone were found to be more effective than misoprostol. There was no evidence of global inconsistency in this analysis (p = 0.468).
FIGURE 83.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose. NA, not applicable.
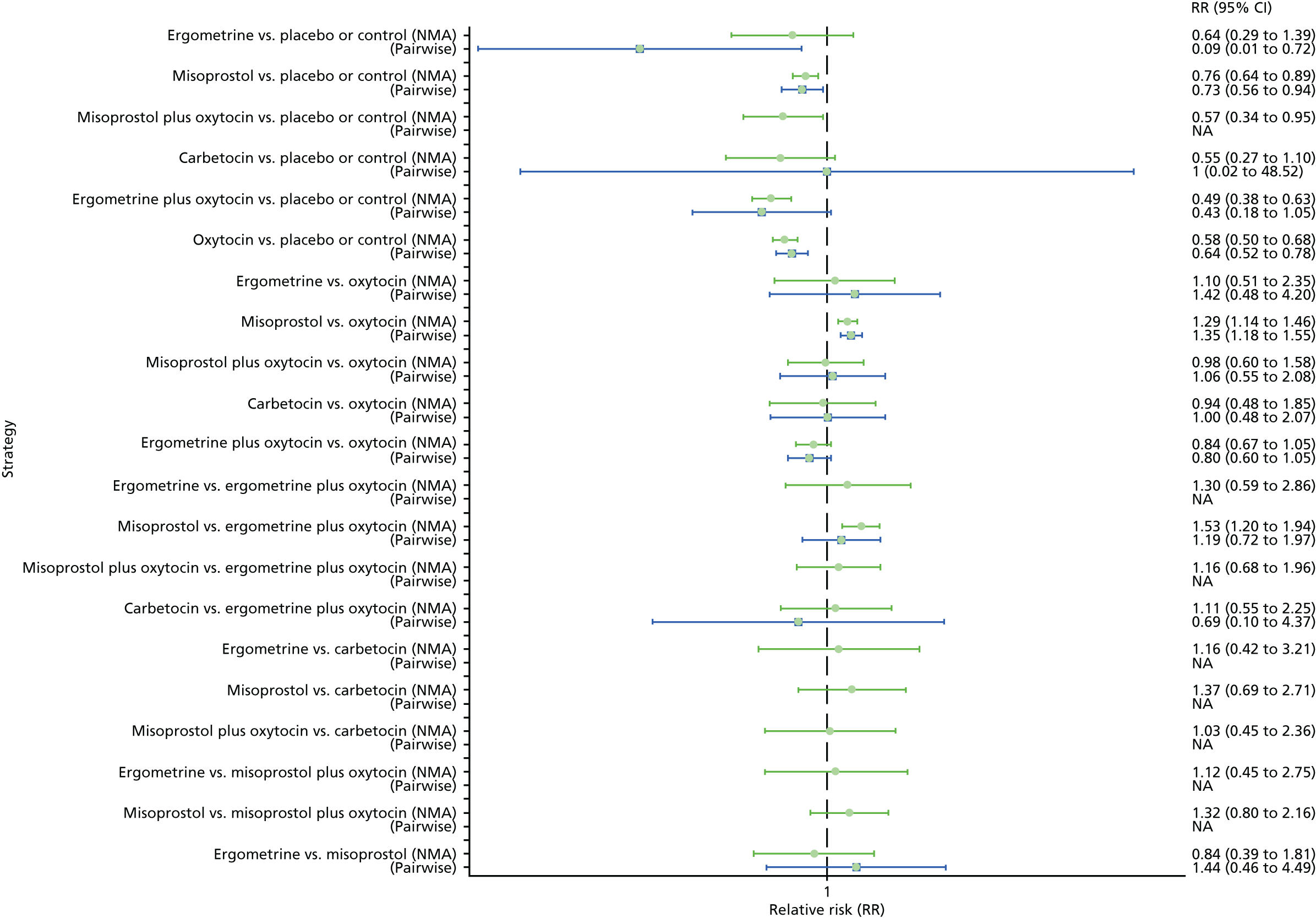
Figure 84 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose. The highest-ranked intervention is ergometrine plus oxytocin, with a less clear ranking among the other interventions.
FIGURE 84.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.

Oxytocin bolus plus infusion
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for this subgroup is presented in Appendix 8. This subgroup includes all trials, but when oxytocin was used as an arm in the trial this analysis is restricted to oxytocin studies that used an intravenous bolus with an intravenous infusion of any dose and excluded studies that used an intravenous or intramuscular bolus or an intravenous infusion only of oxytocin. Pooled effect sizes from the NMA of 31 trials suggested that all interventions, except oxytocin and misoprostol plus oxytocin, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup including oxytocin trials that used an intravenous bolus plus an infusion of any dose (Figure 85). The active interventions were comparable between them, but most of the comparisons were too underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (p = 0.081).
FIGURE 85.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose. NA, not applicable.

Figure 86 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup, including trials only of oxytocin that used an intravenous bolus plus an infusion of any dose. No clear ranking emerges in this analysis.
FIGURE 86.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for this subgroup is presented in Appendix 8. This subgroup includes all trials, but it is restricted to oxytocin studies that used an intravenous bolus with an intravenous infusion of any dose. Pooled effect sizes from the NMA of 29 trials suggested that all interventions demonstrated a similar trend for reducing occurrence of this outcome, but only ergometrine, misoprostol and ergometrine plus oxytocin reached statistical significance when compared with the placebo for this subgroup (Figure 87). The active interventions were comparable between them, but most of the comparisons were too underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (p = 0.315).
FIGURE 87.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose. NA, not applicable.

Figure 88 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including oxytocin studies that used an intravenous bolus plus an infusion of any dose. No clear ranking emerges in this analysis.
FIGURE 88.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.

Oxytocin infusion only
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for this subgroup is presented in Appendix 8. This subgroup includes all trials, but when oxytocin was used as an arm in the trial this analysis is restricted to oxytocin studies that used an intravenous infusion only of any dose, and excluded studies that used an intravenous or intramuscular bolus or an intravenous bolus plus an intravenous infusion of oxytocin. Pooled effect sizes from the NMA of 48 trials suggested that all interventions are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup, including oxytocin trials that used an intravenous infusion only of any dose (Figure 89). The active interventions were comparable between them, but most of the comparisons were too underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (p = 0.135).
FIGURE 89.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intravenous infusion only of any dose. NA, not applicable.

Figure 90 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the subgroup, including oxytocin trials trials that used an intravenous infusion only of any dose. The highest-ranked interventions are carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin, with almost 100% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability in being ranked in the top three interventions was almost 0%.
FIGURE 90.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to oxytocin studies that used an intravenous infusion only of any dose.

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for the subgroup including all trials, but restricting to oxytocin trials that used an intravenous infusion only of any dose is presented in Appendix 8. Pooled effect sizes from the NMA of 41 trials suggested that all interventions except oxytocin and ergometrine are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo for the subgroup, including only oxytocin trials that used an intravenous infusion only of any dose (see Figure 83). Ergometrine plus oxytocin and carbetocin were found to be more effective than the standard intervention, oxytocin (Figure 91). Ergometrine plus oxytocin and carbetocin were also found to be more effective than misoprostol. There was no evidence of global inconsistency in this analysis (p = 0.232).
FIGURE 91.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intravenous infusion only of any dose. NA, not applicable.

Figure 92 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the subgroup including oxytocin studies that used an intravenous infusion only of any dose. The highest-ranked intervention is carbetocin. There is less clear ranking for the rest of the interventions, but on this analysis, oxytocin is ranked sixth, lower than ergometrine and misoprostol, with 0% probability of it being ranked in the top three.
FIGURE 92.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to oxytocin studies that used an intravenous infusion only of any dose.

Sensitivity analyses
High-quality studies
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for high-quality trials (double-blinded, adequately concealed, with < 10% loss to follow-up) is presented in Appendix 8. Pooled effect sizes from the NMA of 29 high-quality trials suggested that all interventions, except carbetocin, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo, even though carbetocin demonstrated a similar trend towards reduction of this outcome (Figure 93). Ergometrine plus oxytocin, and ergometrine were found to be more effective than the standard intervention, oxytocin (see Figure 93). Ergometrine plus oxytocin and ergometrine were also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (p = 0.844).
FIGURE 93.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to only high-quality studies. NA, not applicable.

Figure 94 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for the high-quality trials. The highest-ranked interventions are ergometrine, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was < 10%. Carbetocin dropped its ranking from second in the global analysis for PPH blood loss of ≥ 500 ml to fifth behind oxytocin in this analysis including only high-quality trials. The ranking of ergometrine is an outlier in this analysis and is based on a single study.
FIGURE 94.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to only high-quality studies.

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for high-quality trials is presented in Appendix 8. Pooled effect sizes from the NMA of 30 high-quality trials suggested that all interventions, except carbetocin, are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo, even though carbetocin demonstrated a similar trend towards reduction of this outcome (Figure 95). Oxytocin was found to be better than misoprostol when used alone (see Figure 95). Ergometrine plus oxytocin was also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (p = 0.802).
FIGURE 95.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to only high-quality studies. NA, not applicable.
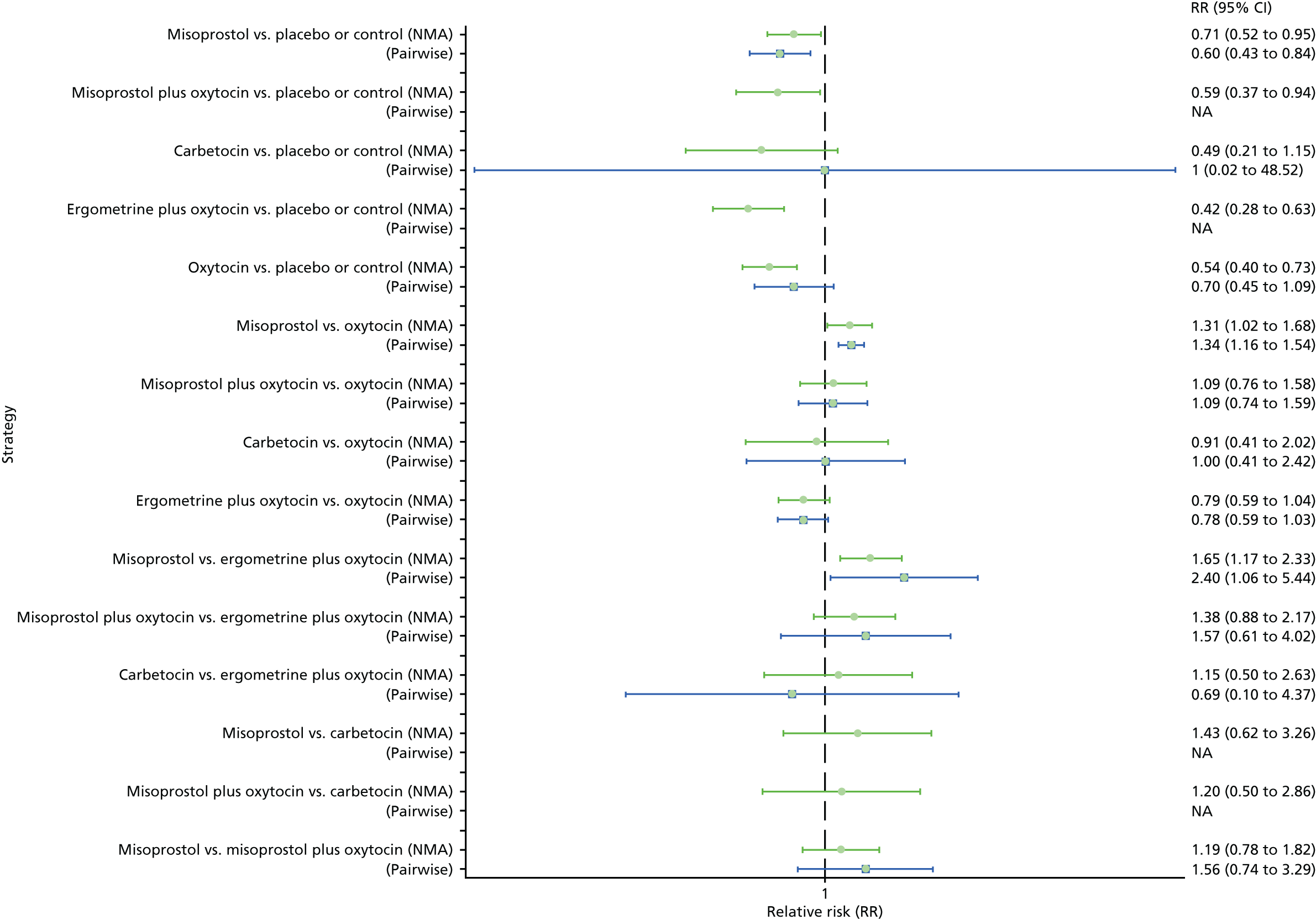
Figure 96 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the high-quality trials. The highest-ranked intervention is ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin is very close, without a clear hierarchy.
FIGURE 96.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to only high-quality studies.
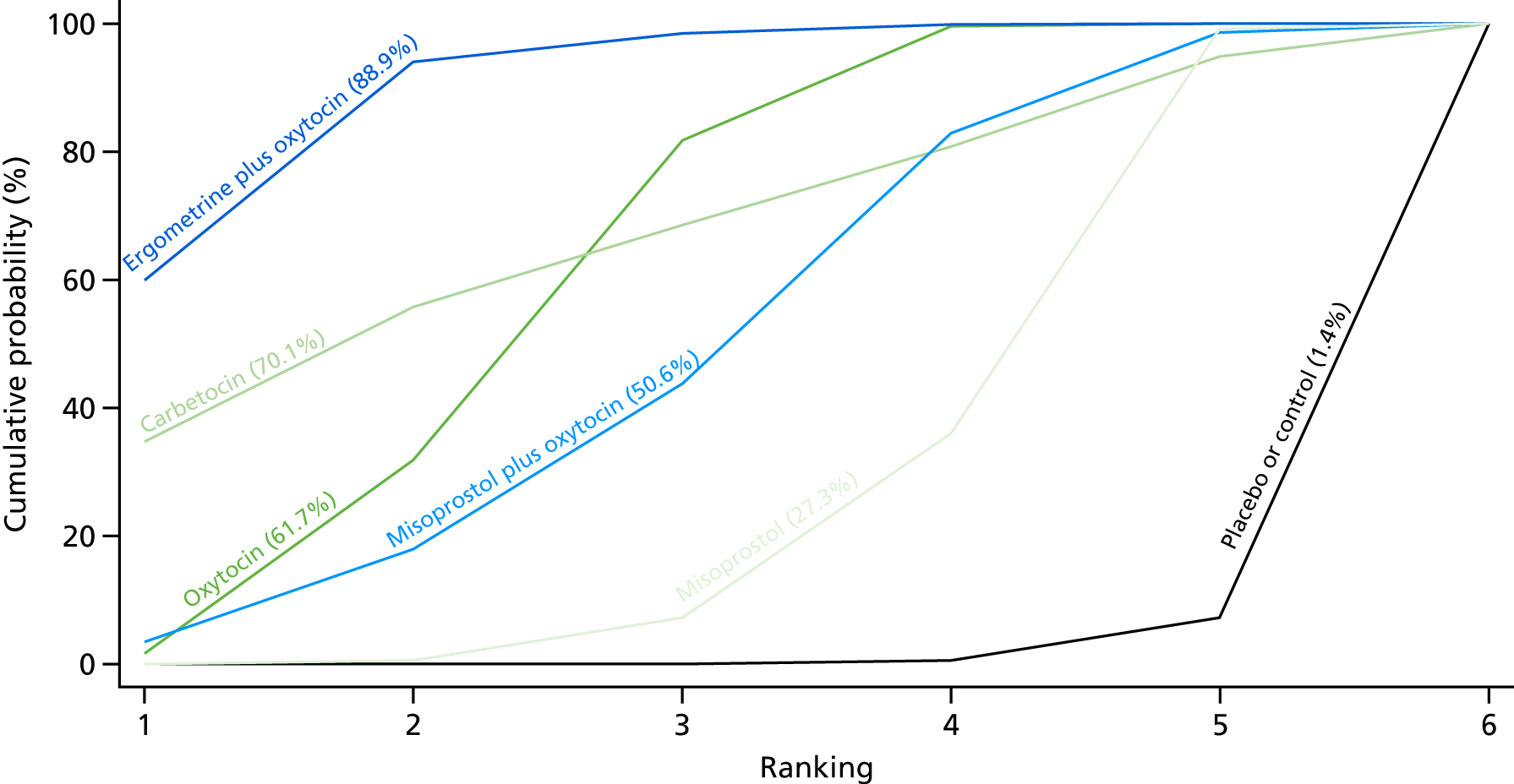
Studies with funding source rated as being at low risk of bias (public or no funding)
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for studies with public or no funding is presented in Appendix 8. Pooled effect sizes from the NMA of 32 trials suggested that all interventions, except carbetocin and ergometrine, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo, even though they all demonstrated a similar trend towards reduction of this outcome (Figure 97). There were no significant differences between the active interventions. There was evidence of global inconsistency in this analysis (p = 0.0003). However, it is noted that the CIs for both the NMA and direct evidence were overlapping across all comparisons, suggesting locally consistent results except for ergometrine versus misoprostol based on a single study.
FIGURE 97.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to studies with funding source rated as being at a low risk of bias (public or no funding). NA, not applicable.
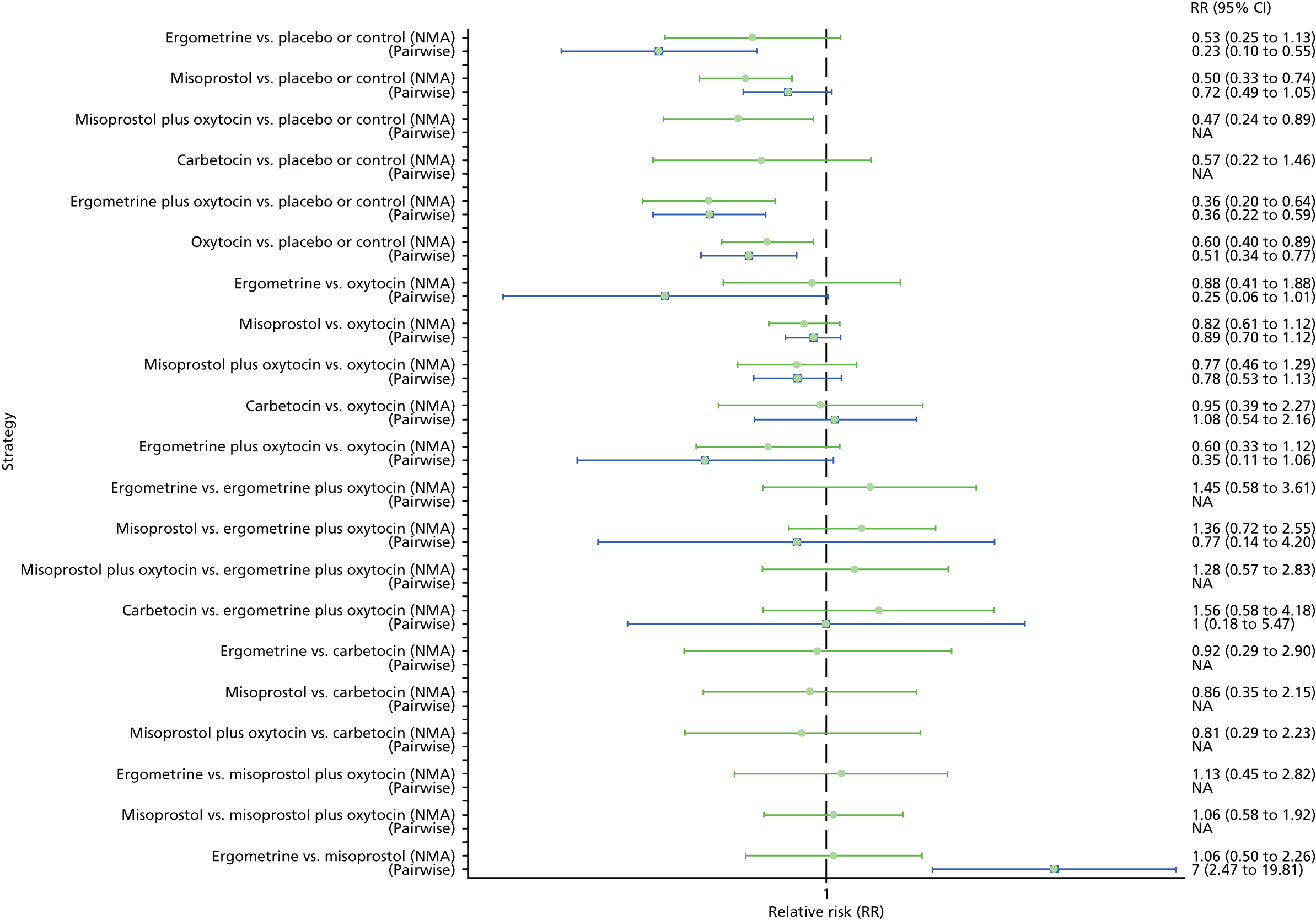
Figure 98 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for trials with public or no funding. The highest-ranked intervention is ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin is very close without a clear hierarchy.
FIGURE 98.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to studies with funding source rated as being at a low risk of bias (public or no funding).
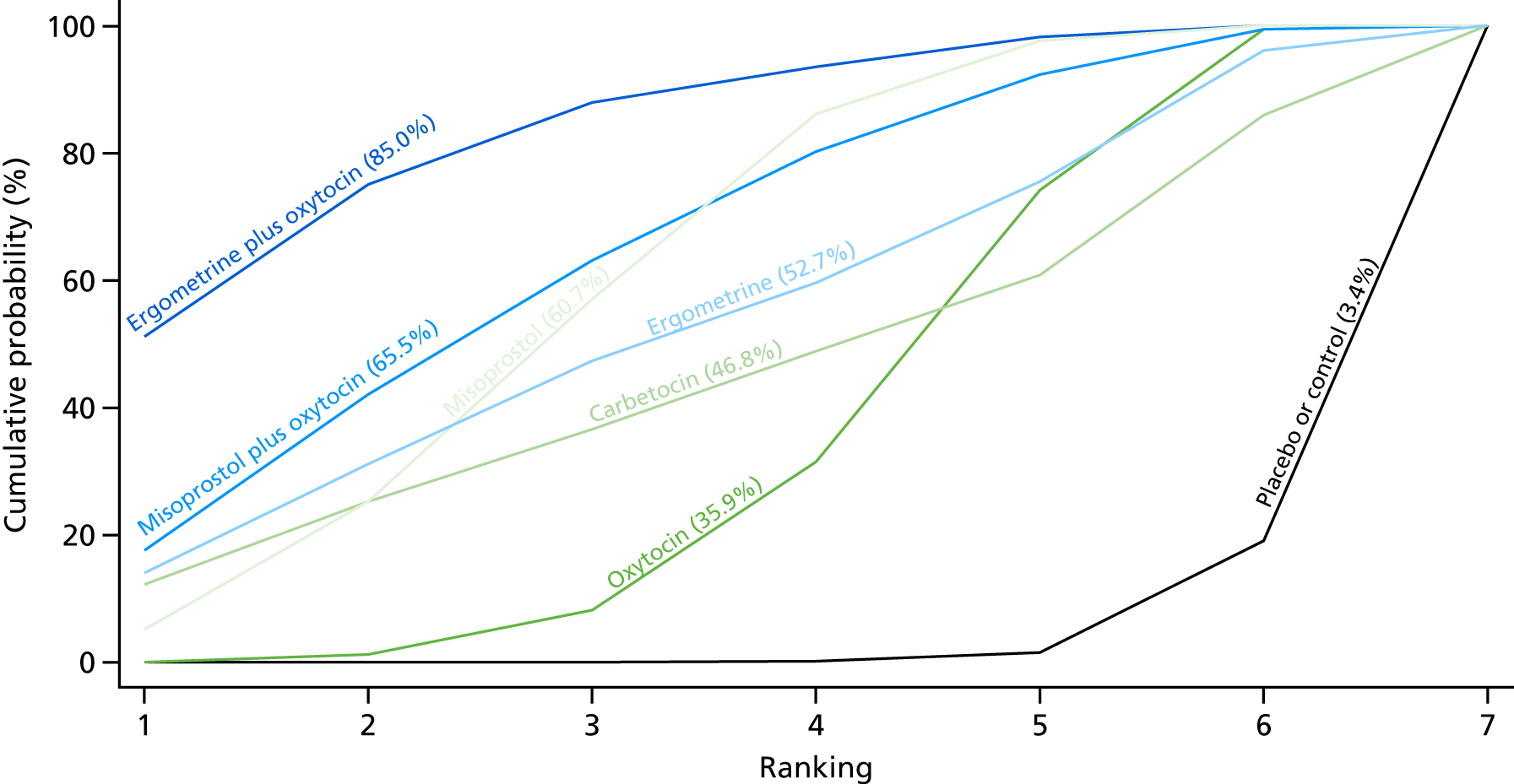
Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for trials with public or no funding is presented in Appendix 8. Pooled effect sizes from the NMA of 35 trials suggested that all interventions, except carbetocin, are effective for preventing PPH blood loss of ≥ 1000 ml when compared with placebo, even though carbetocin demonstrated a similar trend towards reduction of this outcome (Figure 99). No intervention was found to be significantly better or worse than oxytocin (see Figure 99). Ergometrine was found to be more effective than misoprostol. There was no evidence of global inconsistency in this analysis (p = 0.739).
FIGURE 99.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to studies with funding source rated as being at a low risk of bias (public or no funding). NA, not applicable.

Figure 100 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the trials with public or no funding. The highest-ranked interventions are ergometrine and ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin is very close without a clear hierarchy. The ranking of ergometrine is an outlier in this analysis and is based on a single study.
FIGURE 100.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to studies with funding source rated as being at a low risk of bias (public or no funding).

Studies with an objective method of measuring blood loss
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the trials with an objective method for measuring blood loss is presented in Appendix 8. Pooled effect sizes from the NMA of 56 trials suggested that all interventions, except ergometrine, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo (Figure 101). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective than the standard intervention, oxytocin, with carbetocin also demonstrating a similar trend (see Figure 101). Ergometrine plus oxytocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (p = 0.455).
FIGURE 101.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to studies with an objective method of measuring blood loss. NA, not applicable.
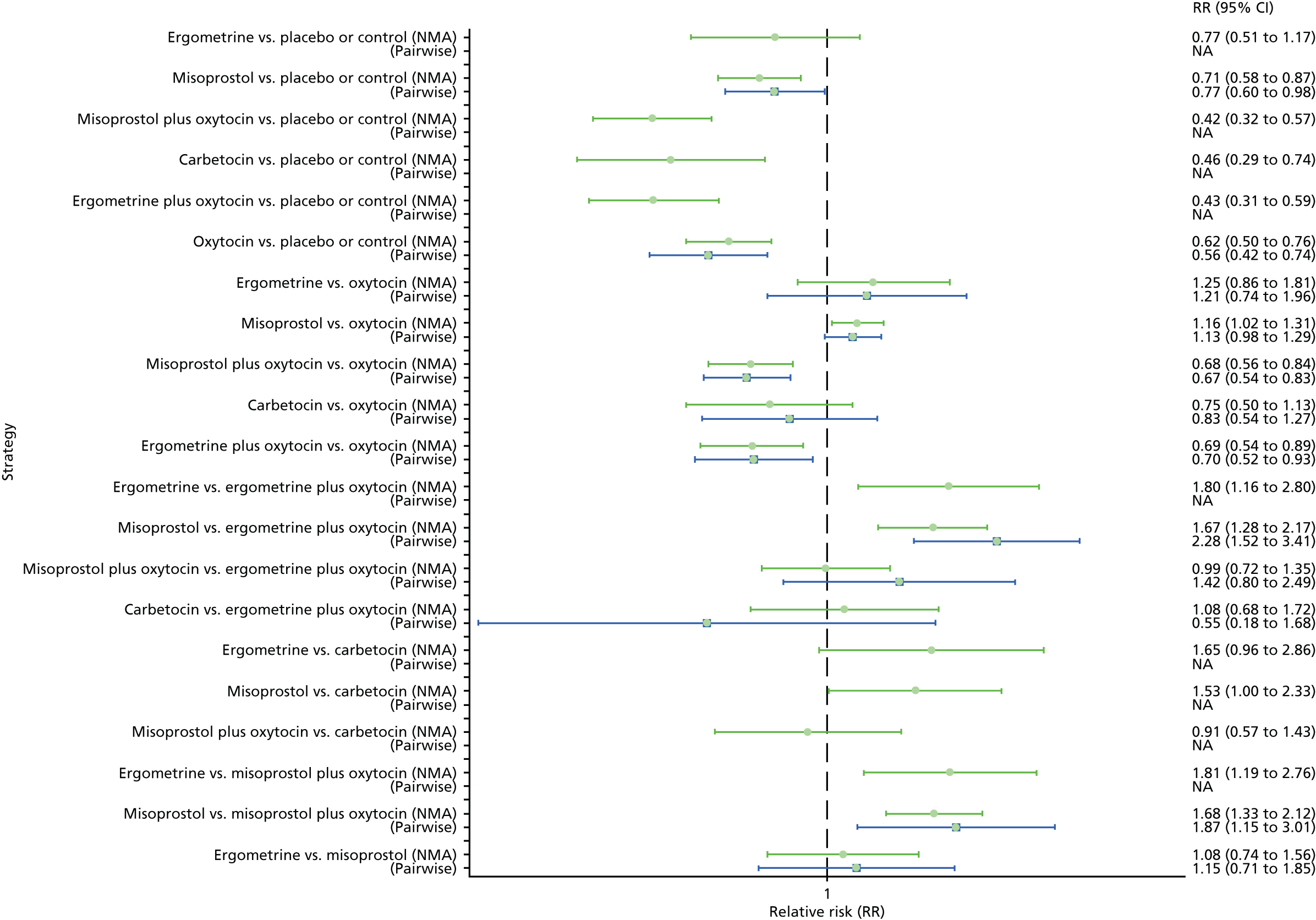
Figure 102 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for trials with an objective method of measuring blood loss. The highest-ranked interventions are ergometrine plus oxytocin and misoprostol plus oxytocin followed closely by carbetocin, with almost 100% probability of these three interventions being ranked first, second or third. Oxytocin is ranked fourth and its probability of being ranked in the top three interventions was < 0%.
FIGURE 102.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to studies with an objective method of measuring blood loss.

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for studies with an objective method of measuring blood loss is presented in Appendix 8. Pooled effect sizes from the NMA of 49 high-quality trials suggested that all interventions, except carbetocin and ergometrine, are effective for preventing PPH blood loss of ≥ 1000 ml when compared with the placebo, even though carbetocin demonstrated a similar trend towards reduction of this outcome (Figure 103). Ergometrine plus oxytocin was found to be more effective than the standard intervention, oxytocin. Ergometrine plus oxytocin was also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (p = 0.606).
FIGURE 103.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to studies with an objective method of measuring blood loss. NA, not applicable.

Figure 104 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the studies that used an objective method of measuring blood loss. The highest-ranked intervention is ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin is very close, without a clear hierarchy.
FIGURE 104.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to studies with an objective method of measuring blood loss.

Large studies only (i.e. > 400 participants)
Primary postpartum haemorrhage blood loss of ≥ 500 ml
The network diagram for PPH blood loss of ≥ 500 ml for the large trials with > 400 participants is presented in Appendix 8. Pooled effect sizes from the NMA of 46 trials suggested that all interventions, except ergometrine, are effective for preventing PPH blood loss of ≥ 500 ml when compared with the placebo (Figure 105). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective than with the standard intervention, oxytocin, with carbetocin not being included in this analysis as there were no large studies comparing carbetocin with any of the other interventions (see Figure 105). Ergometrine plus oxytocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (p = 0.011). However, it is noted that the CIs for both the NMA and the direct evidence were overlapping across all comparisons, suggesting locally consistent results, except for ergometrine versus placebo or the control based on a single study.
FIGURE 105.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 500 ml, restricted to large studies (i.e. > 400 participants). NA, not applicable.

Figure 106 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 500 ml for large trials with > 400 participants. The highest-ranked interventions are ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin is ranked third and its probability of being ranked in the top two interventions was close to 0%. Carbetocin could not be ranked as there were no studies found comparing it with any other interventions in the network.
FIGURE 106.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 500 ml, restricted to large studies (i.e. > 400 participants).

Primary postpartum haemorrhage blood loss of ≥ 1000 ml
The network diagram for PPH blood loss of ≥ 1000 ml for the large trials with > 400 participants is presented in Appendix 8. Pooled effect sizes from the NMA of 46 trials suggested that all interventions, except ergometrine, are effective for preventing PPH blood loss of ≥ 500 ml when compared with placebo (Figure 107). Ergometrine plus oxytocin was found to be more effective than the standard intervention, oxytocin, with carbetocin not being included in this analysis as there were no large studies comparing carbetocin with any of the other interventions (Figure 107). Ergometrine plus oxytocin and oxytocin alone were also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (p = 0.122).
FIGURE 107.
Forest plot with relative RRs and 95% CIs from the NMA and pairwise analyses for the prevention of PPH blood loss of ≥ 1000 ml, restricted to large studies (i.e. > 400 participants). NA, not applicable.
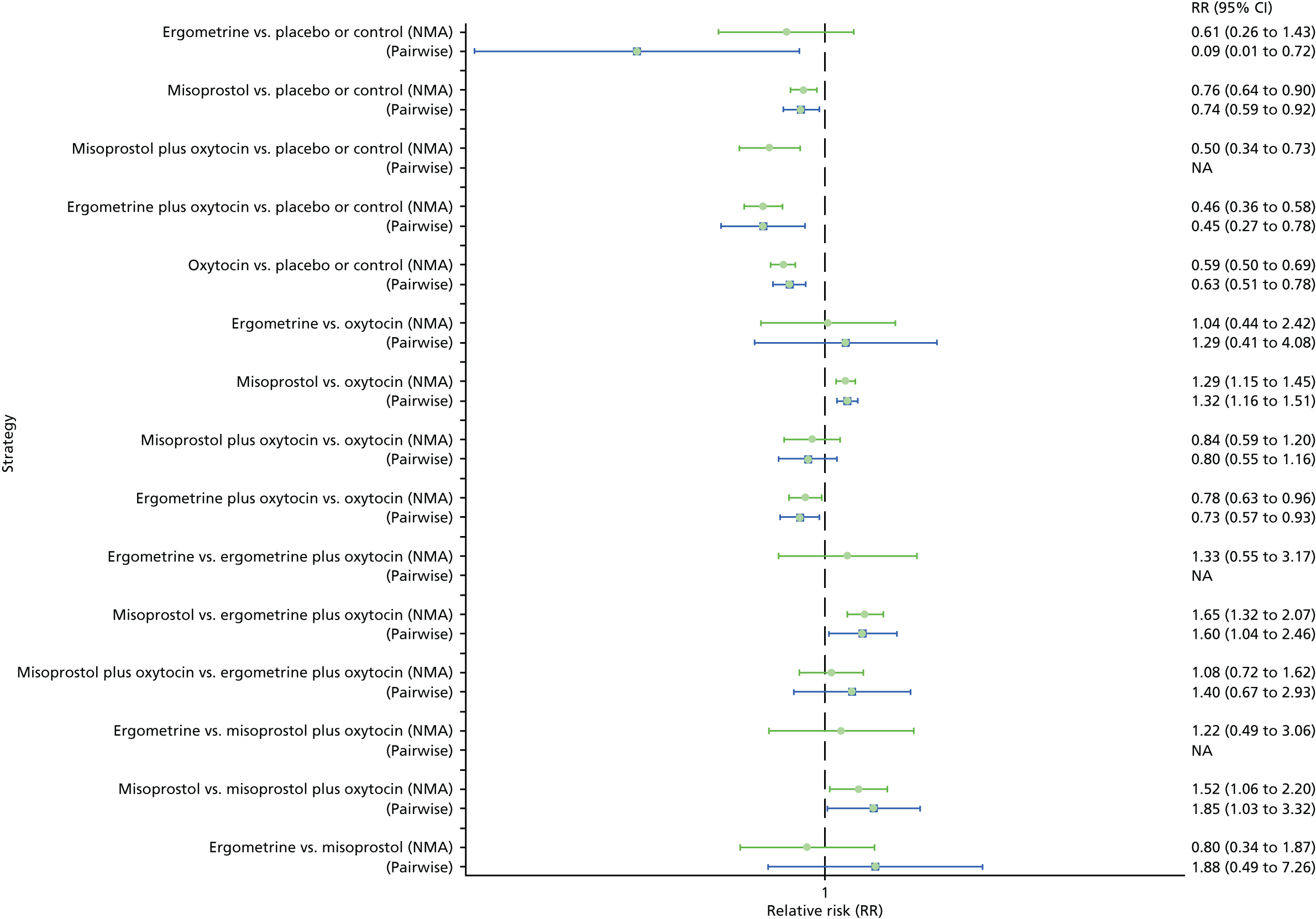
Figure 108 shows the cumulative probabilities for each intervention being at each possible rank for PPH blood loss of ≥ 1000 ml for the large trials. The highest-ranked interventions are ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin is ranked third and its probability of being ranked in the top two interventions was close to 10%. Carbetocin could not be ranked as there were no studies found comparing it with any other interventions in the network.
FIGURE 108.
Cumulative rankograms comparing each of the uterotonic drugs and combinations thereof for the prevention of PPH blood loss of ≥ 1000 ml, restricted to large studies (i.e. > 400 participants).
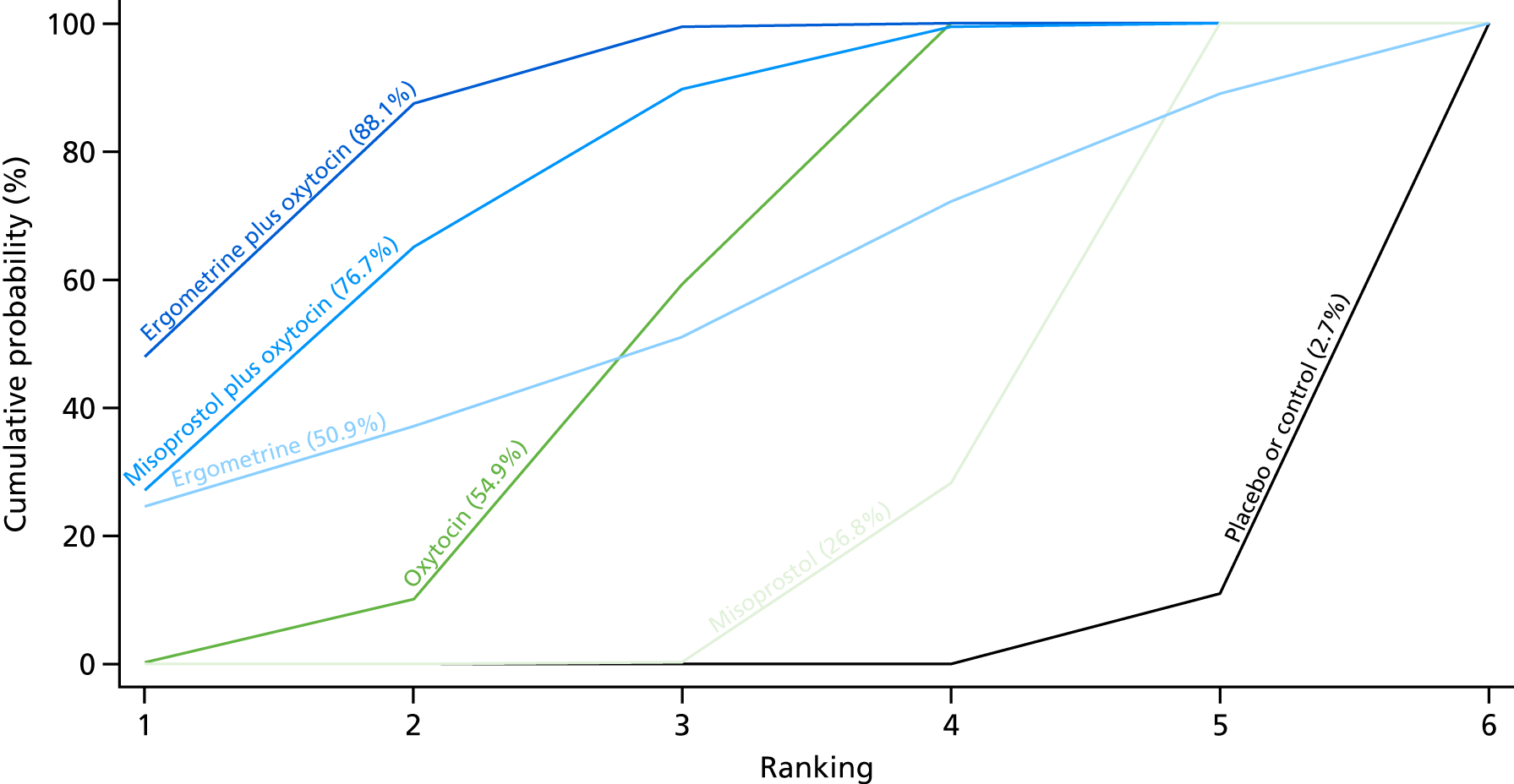
Further sensitivity analyses for our primary outcomes were performed by removing trials published earlier than 1990 (three trials), a cluster trial (one trial), removing trials with a high number of missing data (10 trials) and removing trials in which participants were also randomised to co-interventions such as uterine massage and/or early controlled cord traction (three trials). Sensitivity analyses were also performed according to the choice of relative effect measure (RR vs. OR) and the statistical model (fixed-effects vs. random-effects model). It was found that the overall ranking did not vary, and the CIs of the relative effects did not substantially change. Of note is that the global inconsistency was less when the trials randomising to co-interventions were removed (p = 0.218).
Chapter 4 Health economics
Background
Uterotonic drugs administered at the birth of the baby are routinely recommended for the prevention of PPH, but there is lack of clarity over which uterotonic drug is best. Oxytocin is currently recommended in the UK for preventing PPH23,174 because of its relatively low price and incidence of side effects. Few previous attempts have been made to compare the cost-effectiveness of one uterotonic drug with standard care for the prevention of PPH. 175–179 The literature is lacking any comparison of more than two uterotonic drugs or any ranking of cost-effectiveness for multiple uterotonics.
A model-based economic evaluation was carried out to compare the relative cost-effectiveness of the full range of uterotonic drugs available for the prevention of PPH. The model follows women from the point of administration of the uterotonic drug for the purpose of prevention through a pathway where, in some cases, the same drug or alternatives are given for the treatment of PPH.
In the economic evaluation reported here, the modes of birth (vaginal birth and birth by caesarean section) were separated for the analyses, and vaginal birth in a community health-care setting was also analysed. When possible, the results from the NMA are used in the health economics model. Costs and resource-use data were collected from appropriate sources as described in the Methods section.
Methods
The model was constructed to facilitate all the relevant comparisons in order to determine the most cost-effective uterotonic drug for the prevention of PPH. The analyses were carried out from the perspective of the UK NHS, as this cost-effectiveness analysis is targeted at a UK audience. The primary outcome measure was cost per case of haemorrhage avoided (i.e. ≥ 500 ml of blood lost). Secondary outcome measures of cost per case of haemorrhage avoided (i.e. ≥ 1000 ml of blood lost) and cost per major outcome averted were also analysed. It was not possible to present results in terms of quality-adjusted life-years (QALYs) because of the lack of appropriate utility data in the literature. The results are presented in terms of the incremental cost-effectiveness ratio (ICER), namely the additional cost per case of PPH blood loss of ≥ 500 ml avoided, additional cost per case of PPH blood loss of ≥ 1000 ml avoided, and additional cost per major outcome averted.
Model structure
A decision tree model was developed in TreeAge Pro 2016 (TreeAge Software, Inc., Williamstown, MA, USA) to represent the alternative strategies. A decision tree was chosen as the most appropriate model for evaluating the cost-effectiveness of uterotonic drugs for the prevention of PPH, because of the relatively short-term impact of the intervention and treatment of PPH. 180 The pathways of the model represent, as far as possible, the clinical steps carried out in a UK hospital in the event of PPH. NICE and the Royal College of Obstetricians and Gynaecologists (RCOG) guidelines for the management and treatment of PPH were followed to establish the model pathways. 23,174 Additional information about the steps taken by clinicians to treat PPH were identified via expert opinion, which consisted of a team of five obstetricians. The obstetricians were part of the research team and helped finalise the model pathways.
The decision tree structure is presented in Figure 109. The start of the model is assumed to be when women are approaching what is referred to as the third stage of labour. This is defined as the point when women have given birth to their baby, but the placenta is yet to be delivered.
FIGURE 109.
Summarised version of the clinical pathways in the model.

At the prevention stage (stage 0) of the model, women are given one of six active-prevention strategies:
-
carbetocin
-
ergometrine
-
ergometrine plus oxytocin
-
misoprostol plus oxytocin
-
misoprostol
-
oxytocin.
After a uterotonic drug has been administered as prevention for PPH, a woman may require treatment for PPH. The pathways are defined as the uterotonic drug that is given for prevention, acknowledging that after a uterotonic drug is given for prevention, treatment for PPH may be required. It is assumed that women receiving each strategy in the model have a possibility of either continuing to bleed (with a PPH blood loss of ≥ 500 ml) or experiencing no PPH. The pathways combine the probability of a woman following a particular path and the associated cost.
If, after the prevention stage, a woman continues to bleed, she is assumed to follow a consecutive series of four treatments in an attempt to stop the bleeding:
-
prevention stage
-
treatment stage 1 – if bleeding continues, the woman will be treated with a combination of two drugs: an oxytocin infusion and ergometrine plus oxytocin
-
treatment stage 2 – if bleeding continues, the woman will be treated with two alternative drugs: carboprost and misoprostol
-
treatment stage 3 – if bleeding continues, the woman will receive a non-invasive balloon (balloon tamponade)
-
treatment stage 4 – if bleeding continues, a surgical procedure, such as a hysterectomy, will be carried out on the woman.
In the model, the woman is then assumed to either survive or die.
Vaginal birth versus caesarean section
Expert opinion expressed that the third stage of labour can differ greatly depending on mode of birth. Therefore, vaginal birth and birth by caesarean section were analysed separately using different decision tree models. Both models follow the same structure. Women at high risk and low risk of PPH do not require a separate model analysis, as they would follow the same pathways depending on mode of birth.
In the base case, all births are assumed to take place in an obstetric unit, where appropriate treatment for PPH is readily available should the woman require it. This is true of 87% of births in the UK. 181 Vaginal birth in a community health-care setting, such as at home or in a midwife-led unit, is analysed in the scenario analysis.
Adverse events
It was assumed that after receiving a drug for either prevention or treatment of PPH, a woman has a chance of suffering an adverse event. Adverse events suffered in the model included:
-
nausea
-
vomiting
-
hypertension
-
headache
-
tachycardia
-
hypotension
-
fever
-
shivering
-
abdominal pain.
Adverse events were not given separate branches in the model. The probability of a woman suffering adverse events and the associated costs were included as a weighted average after the woman has been given a drug or combination of drugs to prevent or treat PPH. The model runs for a short time period and is for the immediate postpartum period only.
Data
Effectiveness data
The effectiveness data required for the model were, as far as possible, based on the results of the trials sourced from the NMA. When necessary, data were supplemented by the literature. Owing to limited information on carboprost treatment, the effectiveness of balloon tamponades and surgical procedures reported by the NMA were based on literature estimates.
The absolute probabilities used in the model were defined as relative probabilities, relative to oxytocin. Oxytocin was deemed most suitable as the main comparator in the base case because it is the uterotonic agent currently recommended as prevention for PPH in the UK. The NMA revealed a large number of studies comparing oxytocin with an alternative strategy, so data around the oxytocin strategy were considered to be the most robust.
The main effectiveness data from the NMA were defined by blood loss of ≥ 500 ml and of ≥ 1000 ml. It was assumed that the preventing PPH blood loss of ≥ 500 ml was parallel to reaching the prevention stage of the model (stage 0) and not requiring any treatment. Preventing PPH blood loss of ≥ 1000 ml was assumed to mean that the woman had received treatment stage 1, but no further treatment was required.
No data were available in the NMA for PPH blood loss of ≥ 500 ml and of ≥ 1000 ml for the interventions ergometrine and ergometrine plus oxytocin, for caesarean section. In this case, the relative probabilities used for ergometrine and ergometrine plus oxytocin in vaginal birth were also used for caesarean section.
For the effectiveness of treatment stage 2 (carboprost and misoprostol), the effectiveness of carboprost was used, which was sourced from Butwick et al. ,182 who compared the risk of haemorrhage-related morbidity, for birth by caesarean section only, in those women exposed to methylergonovine versus carboprost. Given that this estimate is limited to only one study and, additionally, that it is only for birth by caesarean section, this probability was explored in the sensitivity analysis.
The effectiveness of a balloon tamponade was based on a literature estimate. Doumouchtsis et al. ,183 in a systematic review looking at studies that discuss the management of PPH, found nine studies evaluating the success rate of a balloon tamponade.
The effectiveness of a ‘surgical procedure’ was also based on a literature estimate for hysterectomy. Knight184 performed a study across all UK hospitals with consultant-led maternity units looking at women undergoing peripartum hysterectomy. Different surgical procedures can be carried out to treat PPH [e.g. laparotomy, B-Lynch suturing technique (brace suture)], but as a hysterectomy is the procedure usually used as a life-saving measure for PPH, the source was considered appropriate. 184,185
The probability of haemorrhage (i.e. of ≥ 500 ml and ≥ 1000 ml) and the effectiveness of treatment strategies are presented in Table 2 (vaginal birth) and Table 3 (caesarean section). The tables provide absolute probabilities with standard errors and 95% CIs. Where no standard errors for probabilities were provided in the literature estimates, they were calculated as one-tenth of one minus its value. 186
| Item | Strategy | Probability of successa | Standard errorb | 95% CI (%) | Sources |
|---|---|---|---|---|---|
| Prevention | Oxytocin | 0.908 | 0.009 | 0.891 to 0.925 | NMA |
| Prevention | Carbetocin | 0.944 | 0.288 | 0.883 to 0.974 | NMA |
| Prevention | Ergometrine plus oxytocin | 0.936 | 0.101 | 0.908 to 0.958 | NMA |
| Prevention | Ergometrine | 0.891 | 0.140 | 0.830 to 0.933 | NMA |
| Prevention | Misoprostol plus oxytocin | 0.931 | 0.144 | 0.892 to 0.958 | NMA |
| Prevention | Misoprostol | 0.899 | 0.078 | 0.861 to 0.929 | NMA |
| Treatment stage 1 | Oxytocin | 0.977 | 0.003 | 0.971 to 0.997 | NMA |
| Treatment stage 1 | Carbetocin | 0.988 | 0.756 | 0.932 to 0.244 | NMA |
| Treatment stage 1 | Ergometrine plus oxytocin | 0.982 | 0.105 | 0.972 to 0.895 | NMA |
| Treatment stage 1 | Ergometrine | 0.973 | 0.342 | 0.935 to 0.658 | NMA |
| Treatment stage 1 | Misoprostol plus oxytocin | 0.981 | 0.176 | 0.966 to 0.824 | NMA |
| Treatment stage 1 | Misoprostol | 0.970 | 0.060 | 0.958 to 0.940 | NMA |
| Treatment stage 2 | Carboprost | 0.840 | 0.016 | 0.755 to 0.887 | Butwick et al.62 |
| Treatment stage 3 | Balloon tamponade | 0.840 | 0.016 | 0.775 to 0.888 | Doumouchtsis et al.183 |
| Treatment stage 4 | Surgery | 0.994 | 0.0006 | 0.85 to 1.00 | Knight184 |
| Item | Strategy | Probability of successa | Standard errorb | 95% CI (%) | Sources |
|---|---|---|---|---|---|
| Prevention | Oxytocin | 0.401 | 0.074 | 0.256 to 0.547 | NMA |
| Prevention | Carbetocin | 0.534 | 0.197 | 0.147 to 0.761 | NMA |
| Prevention | Ergometrine plus oxytocin | 0.586 | 0.101 | 0.372 to 0.743 | NMA |
| Prevention | Ergometrine | 0.291 | 0.140 | –0.160 to 0.593 | NMA |
| Prevention | Misoprostol plus oxytocin | 0.567 | 0.139 | 0.293 to 0.751 | NMA |
| Prevention | Misoprostol | 0.382 | 0.122 | 0.024 to 0.632 | NMA |
| Treatment stage 1 | Oxytocin | 0.895 | 0.019 | 0.858 to 0.932 | NMA |
| Treatment stage 1 | Carbetocin | 0.923 | 0.334 | 0.799 to 0.974 | NMA |
| Treatment stage 1 | Ergometrine plus oxytocin | 0.082 | 0.105 | 0.864 to 0.956 | NMA |
| Treatment stage 1 | Ergometrine | 0.121 | 0.342 | 0.681 to 0.960 | NMA |
| Treatment stage 1 | Misoprostol plus oxytocin | 0.897 | 0.149 | 0.814 to 0.950 | NMA |
| Treatment stage 1 | Misoprostol | 0.920 | 0.234 | 0.830 to 0.967 | NMA |
| Treatment stage 2 | Carboprost | 0.840 | 0.084 | 0.755 to 0.887 | Butwick et al.62 |
| Treatment stage 3 | Balloon tamponade | 0.840 | 0.084 | 0.775 to 0.888 | Doumouchtsis et al.183 |
| Treatment stage 4 | Surgery | 0.994 | 0.099 | 0.85 to 1.00 | Knight184 |
The probability of experiencing an adverse event as a result of a uterotonic drug or combination of drugs is presented in Appendix 9. The absolute probabilities used for adverse events were defined via relative probabilities, relative to oxytocin. Owing to the lack of complete data in the NMA, the likelihood of experiencing some adverse events was not recorded for all prevention strategies. Several assumptions were made to complete the data set. Based on the evidence from the NMA, it is reasonable to assume that the adverse event profile of carbetocin and oxytocin is similar. Similarly, the adverse event profile of ergometrine plus oxytocin could be assumed to be identical to ergometrine, and the adverse event profile of misoprostol plus oxytocin to be identical to misoprostol. If data were missing for carbetocin but available for oxytocin, then the probability for the adverse event was based on oxytocin. This reasoning was applied to other similar uterotonic drugs. If data were missing for both uterotonic drugs with the same adverse events profile (e.g. data were missing for both carbetocin and oxytocin), then an average of the probabilities available for that side effect was used. Ergometrine is commonly known to be associated with a high level of all side effects, with the exceptions of fever and shivering, so uterotonic drugs containing ergometrine were removed from the averaging process, apart from when considering fever and shivering. Misoprostol is commonly known to be associated with fever and shivering, so uterotonic drugs containing misoprostol were removed from the averaging process for these adverse events.
Resource use and costs
Owing to the large variety of countries included in the NMA, there was no clear justification for any particular choice of country on which to base costs for the whole analysis apart from the UK, where the current study is hosted and funded by the UK research money. All costs sourced are reported in 2016 UK prices, having been appropriately inflated if necessary. Key costs are presented in Table 4.
| Item | Drug/treatment | Unit cost (£) | Other information | Sources |
|---|---|---|---|---|
| Birth costs | Birth costs associated with vaginal birth | 1826.04 | Per birth. See Appendix 10 for a breakdown of the calculation | NHS Reference Costs 2014–15 187 |
| Birth costs | Birth costs associated with birth by caesarean section | 3801.70 | Per birth. See Appendix 10 for a breakdown of the calculation | NHS Reference Costs 2014–15 187 |
| Birth costs | Birth costs in a community health-care setting | 1282.93 | Per birth. See Appendix 11 for a breakdown of the calculation | NHS Reference Costs 2013–14 188 |
| Uterotonic drug | Oxytocin | 0.91 | Per 10 IU, intramuscularly | British National Formulary 189 |
| Uterotonic drug | Misoprostol | 0.17 | Per 200-mcg tablet | NHS Electronic Drug Tariff 190 |
| Uterotonic drug | Ergometrine | 1.50 | Per 500 mcg, intramuscularly | British National Formulary 189 |
| Uterotonic drug | Ergometrine plus oxytocin | 1.57 | Per 500 mcg (ergometrine) plus 5 IU, intramuscularly (oxytocin) | British National Formulary 189 |
| Uterotonic drug | Misoprostol plus oxytocin | 1.08 | Per person (cost of misoprostol plus cost of oxytocin) | British National Formulary 189 |
| Uterotonic drug | Carbetocin | 17.64 | Per 100 mcg, intramuscular | British National Formulary 189 |
| Treatment for PPH | Oxytocin infusion | 0.91 | Per 10 IU, infusion | British National Formulary 189 |
| Treatment for PPH | Carboprost | 18.2 | Per 250 mcg, intramuscular | British National Formulary 189 |
| Treatment for PPH | Balloon tamponade | 1280.42 | Per procedure | NHS Reference Costs 2014–15 187 |
| Treatment for PPH | Postpartum surgery | 3780.40 | Per procedure | NHS Reference Costs 2014–15 187 |
| Treatment for PPH | Blood transfusion | 171.84–163.63 | Per unit. £171.84 (first unit), £163.63 (subsequent units) | Putting NICE Guidance into Practice: Costing Statement Blood Transfusion. Implementing the NICE Guideline on Blood Transfusion 191 |
| Hospital stay | Excess bed-days (vaginal birth) | 440.49 | Per excess day in hospital. The figure is a weighted average of all excess bed-day costs for a vaginal birth (normal or assisted) within an inpatient setting (see Appendix 12) | NHS Reference Costs 2014–15 187 |
| Hospital stay | Excess bed-days (caesarean section) | 444.39 | Per excess day in hospital. The figure is a weighted average of all excess bed-day costs for birth by caesarean section within an inpatient setting (see Appendix 13) | NHS Reference Costs 2014–15 187 |
| Transport | Ambulance call out and transfer to hospital | 239.99 | Per person. Cost includes the cost to see, treat and transfer patient to hospital | NHS Reference Costs 2014–15 187 |
NHS reference costs include information on birth costs. The average birth cost was calculated separately for a vaginal birth in an inpatient setting, a vaginal birth in a community health-care setting and for a caesarean section. A full breakdown of how birth costs were calculated is supplied in Appendices 11–13.
Standard-practice dosage and route of administration were identified for each uterotonic drug, via the study team. The costs attached to each uterotonic drug were sourced from the British National Formulary189 and NHS Electronic Drug Tariff. 190
In line with UK practice, it was assumed that women reaching treatment stage 3 (i.e. balloon tamponade) of the model would require admission to theatre. The cost for the balloon tamponade procedure was assumed to be equivalent to the NHS reference costs for a minor upper genital tract procedure at £1280.42. 187
Being consistent with the effectiveness data for surgery, costs applied to a surgical procedure were based on the cost of a hysterectomy. The cost of this is assumed to be equivalent to the NHS reference cost for a major open upper genital tract procedure with a comorbidities and complications score of 0–5, in an inpatient setting that is £3780.40. 187,192 It was acknowledged that a surgical procedure carried out to treat PPH is performed when the woman is in a life-threatening condition, so there will probably be more serious complications, hence the allowance for a higher comorbidities and complications score (0–5). It was also acknowledged that the cost for a peripartum hysterectomy may be in excess of the assumed standard hysterectomy costs for reasons such as more senior surgeons being required to carry out the procedure. This was tested in the sensitivity analysis to allow for these potential extra costs.
It was assumed that a woman requiring treatment at stage 4 (surgery, having already had a failed attempt at balloon tamponade) will remain in theatre throughout stages 3 and 4. In order to avoid duplication of some costs by summing these procedures, it was assumed that women who ultimately required the more serious intervention of hysterectomy additionally incurred half of the cost for a balloon tamponade. This assumption was explored in the sensitivity analysis.
The assumed lengths of hospital stay were based on blood loss and are based on real data collected for 2000 patients from the Birmingham Women’s Hospital over a 3-month period (March–May 2016) (see Appendix 14). The data were retrieved through K2 Medical Systems™: Athena™ Maternity Information System (Plymouth, UK). The length of hospital stay data for women reaching treatment stage 4 were unable to be sourced from the Birmingham Women’s Hospital because of a lack of patient numbers. Data on length of hospital stay for this stage of the model were based on literature estimates. Women surviving surgery were assumed to stay in hospital for 6 days following both a vaginal birth or a caesarean section. 193
The associated costs attached to an extra day in hospital were calculated using a weighted average of all excess bed-day costs, identified in the NHS Reference Costs 2014–15. 187 The weighted average daily cost of hospitalisation associated with birth is £440.49 (vaginal) and £444.39 (caesarean section). A full breakdown of how excess bed-day costs were calculated is presented in Appendices 15 and 16.
Treatment for adverse events in their worst case, and their associated costs are presented in Appendix 17. Adverse events were assumed to be treated with drugs, intravenous fluids and monitoring in hospital overnight. Worst-case treatment of adverse events was sourced via expert opinion, which consisted of a team of five obstetricians. The obstetricians were part of the research team. It was acknowledged that the severity of adverse events can differ greatly from person to person. In mild cases, adverse events would not be treated with any drug or intervention, and so the costs attached would be zero. The costs assigned to adverse events were explored in the sensitivity analysis. Other resource use includes costs associated with blood transfusion. Costs associated with blood transfusion were sourced from NICE. Two units of blood were assumed to be given to women reaching treatment stage 3 and an additional two units of blood were assumed to be given to women reaching treatment stage 4 of the model.
Subgroup analysis compares an inpatient setting and community health-care setting for birth. Transportation costs were sourced for those needing to be transferred to hospital. It was assumed that women requiring treatment (PPH blood loss of ≥ 500 ml) would be required to be transferred to hospital.
Assumptions
Several assumptions were required in order to develop a workable model. These are summarised and described below and divided into three categories: birth, model pathways and model inputs.
Birth
Women are assumed to enter the model when approaching the third stage of labour.
In the principal analyses, all births are assumed to take place in an obstetric unit, where appropriate treatment for PPH is readily available should the woman require it. This is true of 87% of births in the UK. 181
Women giving birth in a community health-care setting, such as at home or in a midwife-led unit, are assumed to only give birth via vaginal birth and not caesarean section. All deliveries by caesarean section are assumed to take place in hospital.
Birth costs are calculated for all levels of comorbidities and complications. It is assumed, therefore, that the costs for any other complications other than PPH are included in the birth costs.
Model pathways
It is assumed that no routine drug for PPH has been administered to women prior to them entering the model.
All prevention strategies follow the same stages of treatment, apart from where misoprostol has been given for prevention of PPH. In this case the same drug may not be repeated for treatment and the patient will forgo this stage of treatment, that is, misoprostol is not to be replaced by another drug or form of treatment.
After ‘no PPH’, ‘bleeding stops’ and ‘survive’ pathways, women are assumed to return to full health.
It is assumed that women who reached PPH blood loss of ≥ 500 ml would have completed the first stage of treatment, women reaching PPH blood loss of ≥ 1000 ml have completed the second stage of treatment, and women reaching PPH blood loss of ≥ 1500 ml have completed the third stage of treatment.
It is assumed that a probability of death can only occur after treatment stage 4.
Model inputs
The relative probability used for the probability of PPH blood loss of ≥ 500 ml from using ergometrine in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 500 ml when using ergometrine in birth by caesarean section. Similarly, the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine in birth by caesarean section.
The relative probability used for the probability of PPH blood loss of ≥ 500 ml from using ergometrine plus oxytocin in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 500 ml when using ergometrine plus oxytocin in birth by caesarean section. Similarly, the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine plus oxytocin in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine plus oxytocin in birth by caesarean section.
Effectiveness of carboprost, balloon tamponade and surgery were assumed to be standard across modes of birth.
Costs for uterotonic drugs were assumed to be standard across the model. That is, drug costs are assumed to carry the same cost, regardless of whether they are given for prevention or treatment.
Costs for administration of treatment, that is, staff time, were assumed to be included in birth costs and excess bed-day costs. Therefore, no extra staff costs were attached to treatment costs of PPH.
Nausea, vomiting, hypertension, headache, tachycardia, hypotension, fever, shivering and abdominal pain were assumed to be the only adverse events that can occur as a side effect of taking a uterotonic drug.
The effectiveness of treatments used for adverse events was assumed to be 100% successful.
The cost of treatment for adverse events, as a weighted average, was attached to every outcome of the model, except death.
An outcome of death assumed no excess bed-day costs.
Analysis
Various alternative analyses were carried out. Because of the multiple missing data for adverse events, analyses were carried out including and excluding adverse events. Additionally, because of the lack of data for ergometrine and ergometrine plus oxytocin PPH blood loss of ≥ 500 ml and PPH blood loss of ≥ 1000 ml for caesarean section, analysis was carried out including and excluding these uterotonic drugs. Each analysis was carried out for three outcome measures:
-
cost per case of PPH blood loss of ≥ 500 ml avoided
-
cost per case of PPH blood loss of ≥ 1000 ml avoided
-
cost per major outcome averted, in which a major outcome refers to treatment stage 4 of the model (surgery).
Principal analyses
-
Analysis 1. A deterministic analysis analysing the relative cost-effectiveness of a range of uterotonic drugs for the prevention of PPH for vaginal birth. The results are presented in terms of the ICER, namely the additional cost per case of PPH blood loss of ≥ 500ml avoided. In this analysis, no adverse events are included in the model.
-
Analysis 2. A deterministic analysis similar to analysis 1, but adverse events are included in this analysis.
-
Analysis 3. A deterministic analysis similar to analysis 1, but birth is by caesarean section. Ergometrine plus oxytocin and ergometrine are excluded from this analysis because of a lack of any data on these interventions related to caesarean sections.
-
Analysis 4. A deterministic analysis for caesarean section (similar to analysis 3), but adverse events are included in this analysis. Ergometrine and ergometrine plus oxytocin are excluded from this analysis.
-
Analysis 5. A deterministic analysis for caesarean section including ergometrine plus oxytocin and ergometrine in the analysis. Adverse events are excluded from this analysis. There were no data available in the NMA for the probability of PPH blood loss of ≥ 500 ml and PPH blood loss of ≥ 1000 ml when using ergometrine or ergometrine plus oxytocin as prevention for PPH in the case of birth by caesarean section. Probabilities for PPH blood loss of ≥ 500 ml and ≥ 1000 ml when using ergometrine or ergometrine plus oxytocin for prevention were included in this analysis by making the following assumptions:
-
The relative probability used for the probability of PPH blood loss of ≥ 500 ml from using ergometrine in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 500 ml when using ergometrine in birth by caesarean section. Similarly, the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine in birth by caesarean section.
-
The relative probability used for the probability of PPH blood loss of ≥ 500 ml from using ergometrine plus oxytocin in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 500 ml when using ergometrine plus oxytocin in birth by caesarean section. Similarly, the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine plus oxytocin in vaginal birth was assumed to be equal to the relative probability used for the probability of PPH blood loss of ≥ 1000 ml when using ergometrine plus oxytocin in birth by caesarean section.
-
-
Analysis 6. A deterministic analysis similar to analysis 5, but adverse events are included in this analysis.
Scenario analysis
In addition to the six principal analyses, scenario analyses were carried out to explore the results of the cost-effectiveness of the uterotonic drugs in a different setting, namely a community health-care setting. All scenario analyses were for vaginal birth only.
-
Scenario analysis. A deterministic analysis, similar to analysis 2, but for birth in a community health-care setting. Birth costs for a community health-care setting are included, as well as transport costs to transfer the woman to hospital in the event of PPH blood loss of ≥ 500 ml. The probability of PPH blood loss of ≥ 1000 ml was also doubled to account for the potential delay in the woman receiving these drugs, because she is being transferred to hospital.
Sensitivity analyses
Deterministic and probabilistic sensitivity analyses (PSAs) were carried out to explore the uncertainty of the model input data. In deterministic analysis, there is no randomness and individual parameters are explored using their specified point value. In PSA, distributions are assigned to uncertain model parameters, and by drawing randomly from these distributions, a large number (i.e. 10,000) of mean cost and effectiveness estimates are generated. These estimates are used jointly, to form an empirical distribution of the differences in cost and effectiveness of the interventions.
Probabilistic sensitivity analyses
Sensitivity analysis 1
A PSA of analyses 2 and 6.
One-way sensitivity analyses
Deterministic one-way sensitivity analyses were carried out to further explore the robustness of costs of surgery (treatment stage 4) and the effectiveness of carboprost (treatment stage 3).
Sensitivity analysis 2
Similar to analyses 2 and 6, but increasing the cost of treatment stage 4 to allow the full cost of the balloon tamponade on top of surgery.
Sensitivity analysis 3
Similar to analyses 2 and 6, but decreasing the cost of treatment stage 4 to discount the cost of the balloon tamponade completely.
Sensitivity analysis 4
Similar to analyses 2 and 6, but increasing the cost of a hysterectomy by 50% to allow for an increase in costs caused by complications or extra/more senior staff being required to be present for the procedure.
Sensitivity analysis 5
Similar to analyses 2 and 6, but changing the effectiveness of carboprost (treatment stage 3). The range of probabilities explored was from 0 to 1 in 10 intervals.
Results
In the majority of cases effectiveness results are given to three decimal places. When rounding resulted in identical effectiveness ratios, effectiveness ratios are given to six decimal places. The results of the analyses are presented in Table 5.
| Analysis | Cost (£) per average woman | PPH | Major outcome averted | ||||
|---|---|---|---|---|---|---|---|
| ≥ 500ml avoided | ≥ 1000ml avoided | ||||||
| Effectiveness | ICERa (£) | Effectiveness | ICERa (£) | Effectiveness | ICERa (£) | ||
| 1: vaginal birth with no adverse events | |||||||
| Ergometrine plus oxytocin | 2537.67 | 0.936 | – | 0.998843 | – | 0.999970 | – |
| Carbetocin | 2551.43 | 0.944 | 1888.75 | 0.999301 | 30,012.87 | 0.999982 | 1,172,377.79 |
| Misoprostol plus oxytocin | 2538.78 | 0.931 | Dominated | 0.998843 | Dominated | 0.999966 | Dominated |
| Oxytocin | 2545.02 | 0.908 | Dominated | 0.998668 | Dominated | 0.999946 | Dominated |
| Misoprostol | 2547.85 | 0.899 | Dominated | 0.997859 | Dominated | 0.999924 | Dominated |
| Ergometrine | 2551.32 | 0.891 | Dominated | 0.996982 | Dominated | 0.999926 | Dominated |
| 2: vaginal birth with adverse events | |||||||
| Oxytocin | 2617.78 | 0.908 | – | 0.997859 | – | 0.999945 | – |
| Carbetocin | 2650.79 | 0.944 | 927.65 | 0.999301 | 22,899.57 | 0.999982 | 894,514.46 |
| Ergometrine plus oxytocin | 2662.87 | 0.936 | Dominated | 0.998843 | Dominated | 0.999970 | Dominated |
| Ergometrine | 2752.04 | 0.891 | Dominated | 0.997082 | Dominated | 0.999925 | Dominated |
| Misoprostol plus oxytocin | 2762.39 | 0.931 | Dominated | 0.998668 | Dominated | 0.999966 | Dominated |
| Misoprostol | 2771.66 | 0.899 | Dominated | 0.996982 | Dominated | 0.999923 | Dominated |
| 3: caesarean section excluding ergometrine and ergometrine plus oxytocin, and with no adverse events | |||||||
| Misoprostol plus oxytocin | 5170.13 | 0.567 | – | 0.955 | – | 0.998858 | – |
| Carbetocin | 5189.25 | 0.534 | Dominated | 0.964 | 2251.77 | 0.999076 | 87,959.83 |
| Misoprostol | 5213.50 | 0.382 | Dominated | 0.951 | Dominated | 0.998737 | Dominated |
| Oxytocin | 5217.92 | 0.401 | Dominated | 0.937 | Dominated | 0.998387 | Dominated |
| 4: caesarean section excluding ergometrine and ergometrine plus oxytocin, and with adverse events | |||||||
| Carbetocin | 5469.57 | 0.534 | – | 0.964 | – | 0.999076 | – |
| Misoprostol plus oxytocin | 5552.12 | 0.567 | 2480.19 | 0.955 | Dominated | 0.998858 | Dominated |
| Oxytocin | 5474.38 | 0.401 | Dominated | 0.937 | Dominated | 0.998387 | Dominated |
| Misoprostol | 5519.16 | 0.382 | Dominated | 0.951 | Dominated | 0.998737 | Dominated |
| 5: caesarean section including ergometrine and ergometrine plus oxytocin, and with no adverse events | |||||||
| Ergometrine plus oxytocin | 5160.36 | 0.586 | – | 0.966 | – | 0.999128 | – |
| Misoprostol plus oxytocin | 5170.13 | 0.567 | Dominated | 0.955 | Dominated | 0.998858 | Dominated |
| Carbetocin | 5189.25 | 0.534 | Dominated | 0.964 | Dominated | 0.999076 | Dominated |
| Misoprostol | 5213.50 | 0.382 | Dominated | 0.951 | Dominated | 0.998737 | Dominated |
| Oxytocin | 5217.92 | 0.401 | Dominated | 0.937 | Dominated | 0.998387 | Dominated |
| Ergometrine | 5256.46 | 0.291 | Dominated | 0.914 | Dominated | 0.997802 | Dominated |
| 6: caesarean section including ergometrine and ergometrine plus oxytocin, and with adverse events | |||||||
| Ergometrine plus oxytocin | 5452.77 | 0.586 | – | 0.966 | – | 0.999128 | – |
| Carbetocin | 5469.57 | 0.534 | Dominated | 0.964 | Dominated | 0.999076 | Dominated |
| Oxytocin | 5474.38 | 0.401 | Dominated | 0.937 | Dominated | 0.998387 | Dominated |
| Misoprostol | 5519.16 | 0.382 | Dominated | 0.951 | Dominated | 0.998737 | Dominated |
| Ergometrine | 5548.87 | 0.291 | Dominated | 0.914 | Dominated | 0.997802 | Dominated |
| Misoprostol plus oxytocin | 5552.12 | 0.567 | Dominated | 0.955 | Dominated | 0.998858 | Dominated |
Analysis 1: vaginal birth with no adverse events
Table 5 shows that ergometrine plus oxytocin is the least costly prevention strategy, with an average cost of £2537.67 per woman. The strategy in which carbetocin is given as the uterotonic drug for prevention, is the most effective strategy, and ergometrine plus oxytocin is the second most effective strategy. All other prevention strategies are dominated by ergometrine plus oxytocin, as they are both more costly and less effective than ergometrine plus oxytocin. However, carbetocin is more effective than ergometrine plus oxytocin. Therefore, compared with ergometrine plus oxytocin, carbetocin is both more costly but more effective. The estimated ICER for prevention with carbetocin compared with ergometrine plus oxytocin is £1888.75 per case of PPH blood loss of ≥ 500 ml avoided. This means that every additional case of PPH blood loss of ≥ 500 ml avoided by using carbetocin over oxytocin costs an extra £1888.75 (see Table 5).
Similarly, an outcome measure of PPH blood loss of ≥ 1000 ml avoided results in an ICER of £30,012.87 per case of PPH blood loss of ≥ 1000 ml avoided for prevention with carbetocin compared with ergometrine plus oxytocin (see Table 5).
An outcome measure of major outcome averted results in an ICER for prevention with carbetocin compared with ergometrine plus oxytocin is £1,172,377.79 per major outcome averted (see Table 5).
Analysis 2: vaginal birth with adverse events
Table 5 shows that oxytocin is the least costly prevention strategy, with an average cost of £2617.78 per woman. Carbetocin is the most effective strategy, and oxytocin is the fourth most effective strategy. All other prevention strategies are dominated by carbetocin, as they are both more costly and less effective than carbetocin. However, oxytocin is less costly than carbetocin. Therefore, compared with oxytocin, carbetocin is both more costly but more effective. The estimated ICER for prevention with carbetocin compared with oxytocin is £927.65 per case of PPH blood loss of ≥ 500 ml avoided. This means that every additional case of PPH blood loss of ≥ 500 ml avoided by using carbetocin over oxytocin costs an extra £927.65.
Analysis 3: birth by caesarean section excluding ergometrine and ergometrine plus oxytocin, and with no adverse events
Table 5 shows that the strategy of misoprostol plus oxytocin dominates all other strategies. The strategy of misoprostol plus oxytocin is both less costly and more effective than all other strategies.
For an outcome measure of PPH blood loss of ≥ 1000 ml avoided, oxytocin is the least costly prevention strategy, with an average cost of £5170.13 per woman. Carbetocin is shown to be the most effective strategy and misoprostol plus oxytocin is shown to be the second most effective strategy. All other strategies are dominated by misoprostol plus oxytocin, as they are both more costly and less effective than misoprostol plus oxytocin. Therefore, compared with misoprostol plus oxytocin, carbetocin is both more costly and more effective. The estimated ICER for prevention with carbetocin compared with misoprostol plus oxytocin is £2251.77 per case of PPH blood loss of ≥ 1000 ml avoided. This means that every additional case of PPH blood loss of ≥ 1000 ml avoided by using carbetocin over oxytocin costs an extra £2251.77.
Similarly, an outcome measure of major outcome averted results in an ICER for prevention with carbetocin compared with misoprostol plus oxytocin is £87,959.83 per major outcome averted.
Analysis 4: birth by caesarean section excluding ergometrine and ergometrine plus oxytocin, and with adverse events
Table 5 shows that carbetocin is the least costly prevention strategy, with an average cost of £5469.57 per woman. Misoprostol plus oxytocin is the most effective strategy, and carbetocin is the second-most effective strategy. All other prevention strategies are dominated by carbetocin, as they are both more costly and less effective than carbetocin. However, misoprostol plus oxytocin is more effective than carbetocin. Therefore, compared with carbetocin, misoprostol plus oxytocin is both more costly and more effective. The estimated ICER for prevention with misoprostol plus oxytocin compared with carbetocin is £2480.19 per case of PPH blood loss of ≥ 500 ml avoided. This means that every additional case of PPH blood loss of ≥ 500 ml avoided by using misoprostol plus oxytocin over carbetocin costs an extra £2480.19.
The results in Table 5 show that the strategy of carbetocin dominates all other strategies. The strategy of carbetocin is both less costly and more effective than all other strategies.
Analysis 5: birth by caesarean section including ergometrine and ergometrine plus oxytocin, and with no adverse events
The results in Table 5 show that the strategy of ergometrine plus oxytocin dominates all other strategies. The strategy of ergometrine plus oxytocin is both less costly and more effective than all other strategies.
Analysis 6: birth by caesarean section including ergometrine and ergometrine plus oxytocin, and with adverse events
Table 5 shows that the strategy of ergometrine plus oxytocin dominates all other strategies. The strategy of ergometrine plus oxytocin is both less costly and more effective than all other strategies.
Scenario analyses: vaginal birth in a community health-care setting
The results in the table show that the addition of transport costs and doubling the probability of PPH blood loss of ≥ 1000 ml (treatment stage 1) to account for a delay in effectiveness do not change the decisions from analysis 2. Full results are presented in Appendix 18.
Sensitivity analysis 1: probabilistic sensitivity analysis
(a) Vaginal birth
The results of the PSA for analysis 2 show moderate uncertainty in the results.
The cost-effectiveness acceptability curve (CEAC) is presented in Figure 110. The CEAC shows the probability that each intervention is cost-effective, compared with the alternative, for a range of values of the maximum acceptable ceiling ratio. 194
FIGURE 110.
Cost-effectiveness acceptability curve between prevention strategies oxytocin and carbetocin, for vaginal birth, using distributions around the accuracy data.

Figure 110 shows the CEAC for the leading strategies, carbetocin and oxytocin. For a maximum willingness-to-pay (WTP) threshold of £863 per PPH blood loss of ≥ 500 ml avoided, oxytocin is considered the optimal strategy. Given a maximum WTP threshold of £864 per PPH blood loss of ≥ 500 ml avoided, there is an equal probability that carbetocin and oxytocin are cost-effective compared with the other strategy. At a WTP threshold of £865 per PPH blood loss of ≥ 500 ml avoided, carbetocin is the optimal strategy. As the WTP per PPH blood loss of ≥ 500 ml avoided tends to infinity, the probability that carbetocin is cost-effective compared with oxytocin tends to 95%. The difference in probabilities over WTP thresholds reflects uncertainty in the model.
(b) Caesarean section
Figure 111 shows the CEAC for the dominant strategy, ergometrine plus oxytocin, and UK current practice, oxytocin. The CEAC shows that at any WTP threshold greater than zero, ergometrine plus oxytocin is shown to be the optimal strategy compared with oxytocin. As the WTP per PPH blood loss of ≥ 500ml avoided tends to infinity, the probability that ergometrine plus oxytocin is cost-effective compared with oxytocin tends to 99%.
FIGURE 111.
Cost-effectiveness acceptability curve between prevention strategies ergometrine plus oxytocin, and oxytocin, for caesarean section birth, using distributions around the accuracy data.
Figure 112 shows the CEAC for the dominant strategy ergometrine plus oxytocin, and second-place prevention strategy, carbetocin. The CEAC shows that at any WTP threshold below £1105 per PPH blood loss of ≥ 500 ml avoided, carbetocin is the optimal strategy compared with oxytocin. Given a maximum WTP threshold of £1106 per PPH blood loss of ≥ 500 ml avoided, there is an equal probability that ergometrine and carbetocin are cost-effective compared with the other strategy. At a WTP threshold of £1107 per PPH blood loss of ≥ 500 ml avoided, ergometrine plus oxytocin is the optimal strategy. As the WTP per PPH blood loss of ≥ 500 ml avoided tends to infinity, the probability that ergometrine plus oxytocin is the optimal strategy compared with carbetocin tends to 70%.
FIGURE 112.
Cost-effectiveness acceptability curve between prevention strategies carbetocin and ergometrine plus oxytocin, for caesarean section birth, using distributions around the accuracy data.

Sensitivity analyses 2–4: changing the cost of treatment stage 4 (surgery)
Allowing for an increase or decrease in treatment stage 4 made no substantial difference to the results. Full results are presented in Appendix 18.
Sensitivity analysis 5: changing the effectiveness of treatment stage 3 (carboprost)
Allowing for a change in the effectiveness of treatment stage 3 made no substantial difference to the results. Full results are presented in Appendix 18.
Discussion
Principal findings and interpretation of the results
(a) Vaginal birth
Analysis 1: vaginal birth with no adverse events
The results of the full range of model-based analyses on the range of different outcomes show that for vaginal birth, all but one of the prevention strategies are dominated by ergometrine plus oxytocin, as they are all more costly and less effective than ergometrine plus oxytocin. The only exception is prevention with carbetocin. Carbetocin is the most effective strategy, but it is also the most costly. Carbetocin is both more costly and more effective than prevention with ergometrine plus oxytocin in the prevention of PPH for the three main outcomes of PPH blood loss of ≥ 500 ml, PPH blood loss of ≥ 1000 ml and avoiding a major outcome (surgery).
Analysis 2: vaginal birth with adverse events
When including adverse events into the model for vaginal birth, the results show that all but one of the prevention strategies are dominated by carbetocin, as the prevention strategies are all more costly and less effective than carbetocin. The only exception is prevention with the UK’s current practice drug, oxytocin. Oxytocin is the least costly strategy, but it is ranked fourth in terms of effectiveness. Carbetocin is both more costly and more effective than prevention with oxytocin in the prevention of PPH for the three main outcomes of PPH blood loss of ≥ 500 ml, PPH blood loss of ≥ 1000 ml and avoiding a major outcome (surgery). These results are also valid in women giving birth in a community health-care setting.
Information or data on the impact of the outcome on quality of life were not available in this analysis. Therefore, presenting results in terms of outcomes in natural units, such as extent of haemorrhage avoided, is necessary but such results are difficult to interpret for the purpose of determining the most cost-effective uterotonic drug. To inform considerations about the relative cost-effectiveness of the different interventions, the resulting ICERs can be considered in the light of the accepted thresholds used by NICE even though such thresholds are presented in QALYs. 195 For example, to convert the ICER for analysis 2(a) of £927.65 into cost per QALY, the ICER is divided by the upper limit of NICE’s cost-effectiveness threshold (£30,000 per QALY). This gives a quality-of-life value of 0.031 (= £927.65/£30,000). If 1 QALY is equal to 1 year in full health, then 0.031 is roughly equal to 11 days in full health [= 0.031/(1/365)]. The result can therefore be interpreted as follows: for carbetocin to be considered cost-effective compared with oxytocin for preventing PPH blood loss of ≥ 500 ml, having an outcome of PPH blood loss of ≥ 500 ml must be equivalent to losing 11 days of full health.
Given that a typical blood donation is typically 470 ml196 with no loss to health, it can be argued the state of losing 500 ml is probably not equivalent to losing 11 days at full health. Although being in labour is very different from a person donating blood, this reasoning suggests that carbetocin is not likely to be considered a cost-effective strategy compared with oxytocin.
By similar reasoning:
-
For carbetocin to be considered cost-effective compared with oxytocin for preventing PPH blood loss of ≥ 1000 ml, having an outcome of PPH blood loss of ≥ 1000 ml must be equivalent to losing over 9 months of full health (referring to analysis 2b).
-
For carbetocin to be considered cost-effective compared with oxytocin for preventing a major outcome (i.e. surgery) having an outcome of major surgery must be equivalent to losing almost 30 years of full health (referring to analysis 2c).
Thus, the prevention strategy of carbetocin is not likely to be considered cost-effective for preventing PPH blood loss of ≥ 1000 ml and surgery.
The ICERs were lower in the scenario analysis for a community health-care setting. This may be more transferable to developing countries.
The results of the sensitivity analysis show moderate uncertainty in the input parameters. The one-way sensitivity analysis demonstrates robustness in the surgery costs, but the PSA shows that a small change in input parameters can change the decision ICER. The CEAC (see Figure 110) shows a WTP threshold of £865 per PPH blood loss of ≥ 500 ml avoided changes the decision as to whether carbetocin or oxytocin is the preferred prevention strategy. Being in natural units makes this result difficult to interpret. At a WTP threshold of £927.65 (the ICER value, analysis 2a), the probability that carbetocin is the optimal strategy compared with oxytocin is 53%. This probability is not much higher than that probability that oxytocin is the optimal strategy at the same WTP threshold (47%), which further reflects uncertainty in interpreting the results.
(b) Caesarean section
For women delivering by caesarean section, the results are mixed.
Analysis 3: birth by caesarean section excluding ergometrine and ergometrine plus oxytocin, and with no adverse events
For analysis 3, misoprostol plus oxytocin is the dominant strategy for an outcome of PPH blood loss of ≥ 500 ml. For an outcome measure of PPH blood loss of ≥ 1000 ml avoided carbetocin is shown to be the most effective strategy and misoprostol plus oxytocin is shown to be the second most effective strategy. The estimated ICER for prevention with carbetocin compared with misoprostol plus oxytocin is £2251.77 per case of PPH blood loss of ≥ 1000 ml avoided. Similarly, an outcome measure of major outcome averted results in an ICER for prevention with carbetocin compared with misoprostol plus oxytocin is £87,959.83 per major outcome averted.
Analysis 4: birth by caesarean section excluding ergometrine and ergometrine plus oxytocin, and with adverse events
When adverse events are included in analysis 4, carbetocin is the least costly prevention strategy. For an outcome of PPH blood loss of ≥ 500 ml, carbetocin dominates all strategies except misoprostol plus oxytocin. The estimated ICER for prevention with misoprostol plus oxytocin compared with carbetocin is £2480.19 per case of PPH blood loss of ≥ 500 ml avoided. This means that every additional case of PPH blood loss of ≥ 500 ml avoided by using misoprostol plus oxytocin over carbetocin costs an extra £2480.19. Following the intuition described Analysis 2: vaginal birth with adverse events, for misoprostol plus oxytocin to be considered cost-effective compared with carbetocin for preventing PPH blood loss of ≥ 500 ml, having an outcome of PPH blood loss of ≥ 500 ml must be equivalent to losing 30 days of full health. Therefore, it is doubtful that misoprostol plus oxytocin can be considered cost-effective.
For outcome measures of cost per case of PPH blood loss of ≥ 1000 ml avoided, and cost per major outcome averted, carbetocin is the dominant strategy, being less costly and more effective than all other prevention strategies.
Analysis 5: birth by caesarean section including ergometrine and ergometrine plus oxytocin, and with no adverse events
Including ergometrine and ergometrine plus oxytocin in this analysis changes the dominant strategy. In this case, the results show the prevention strategy of ergometrine plus oxytocin to be the only dominant strategy, being less costly and more effective than all other strategies.
Analysis 6: birth by caesarean section including ergometrine and ergometrine plus oxytocin, and with adverse events
Similar to analysis 5, the results of analysis 6 show that the prevention strategy of ergometrine plus oxytocin to be the only dominant strategy. The UK current practice of oxytocin is dominated by ergometrine plus oxytocin and carbetocin, as both ergometrine plus oxytocin and carbetocin are less costly and more effective than oxytocin for all outcome measures. The CEAC (see Figure 111) shows that for any given WTP threshold greater than zero, ergometrine plus oxytocin is the optimal strategy compared with the UK’s current practice oxytocin for preventing PPH blood loss of ≥ 500 ml. The CEAC for ergometrine plus oxytocin compared with carbetocin shows less certainty in ergometrine plus oxytocin being the optimal strategy compared with carbetocin in preventing PPH blood loss of ≥ 500 ml for all WTP thresholds.
Strengths and limitations
Strengths
The strength of this model-based economic evaluation is that it is the first model-based economic evaluation to compare the cost-effectiveness of six different active strategies for preventing PPH. Previous cost-effectiveness studies have compared only two interventions. Being able to directly compare six active interventions and to rank them in terms of cost and effectiveness is especially helpful for policy-makers.
Second, by using effectiveness data from the NMA, it ensures that the pooled effectiveness data are a good reflection of the effectiveness of the prevention strategies. As opposed to a randomised control trial, which may have several biases attached and may be limited to specific countries or health-care settings, pooling the effectiveness data over so many trials from all over the world is intended to create more accurate data on the effectiveness of the uterotonic drugs.
Limitations
The main limitation in this economic evaluation was accurately accounting for missing data in the model. In the NMA, no studies had analysed the effect of ergometrine plus oxytocin or ergometrine for prevention of PPH for caesarean section, and so assumptions had to be made around these estimates in order to analyse their cost-effectiveness (analyses 5 and 6). Similarly, there were multiple data missing for adverse events from the uterotonic drugs in the model. This meant that several assumptions had to be made about the probability of certain adverse events resulting from different prevention strategies. Attempts were made to make missing probabilities as accurate as possible, and the probability of adverse events was explored in the PSA in an attempt to rectify this limitation. However, another method of including adverse events in the model, such as quality of life immediately post partum, may be more appropriate to capture the effect adverse events have on the women.
For analyses 5 and 6, ergometrine plus oxytocin results as the only dominant strategy for caesarean section for all outcome measures. However, the widely known risk factors associated with ergometrine were not addressed specifically in the model. For example, in the UK, under current guidelines,197 ergometrine plus oxytocin is not to be given to hypertensive women as this can worsen hypertension and put the women at risk of more serious adverse events, such as stroke. The model does not address any further risks attached to the women other than the nine adverse events discussed in Adverse events.
The model-based economic evaluation makes no comparisons for different dosages of uterotonic or different routes of administration. Comparing the effects of different dosages and routes of administration for the dominant strategies may be useful for future research.
The model-based economic evaluation also includes only UK guidelines for model pathways, and attaches UK costs to resource use. It therefore does not consider different model pathways taken to treat PPH in developing countries where resources may be unavailable. It also fails to consider the costs and resources needed to store the uterotonic drugs. For example, oxytocin is required to be refrigerated, which may not be possible in some health-care settings.
Recommendations for practice
The findings of the health economic evaluation are insufficient on their own to dictate changes in practice, because of the varied results and uncertainty caused by missing data. However, the results do suggest that uterotonic drugs for the prevention of PPH, other than current UK practice, may be more effective and cost-effective for preventing PPH blood loss of ≥ 500 ml and PPH blood loss of ≥ 1000 ml.
Chapter 5 Discussion
Key findings
Key findings of the effectiveness network meta-analysis
A systematic review and NMA, using Cochrane methods, were performed to identify the most effective uterotonic drug for the prevention of PPH. The study included 137 randomised trials involving 87,466 women and compared six active drugs between them and with placebo or the control for prevention of PPH. Most trials were performed in the hospital setting and included women undergoing a vaginal birth, who were at either high or low risk for PPH. The study found that 29% of included trials were rated at being at a low overall risk of bias, but for most trials bias was uncertain because of insufficient reporting.
The strategies that were most effective for prevention of PPH blood loss of ≥ 500 ml were ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin, and all three strategies were found to reduce the risk of PPH blood loss of ≥ 500 ml compared with the current WHO-recommended drug, oxytocin (ergometrine plus oxytocin: RR 0.69, 95% CI 0.57 to 0.83; carbetocin: RR 0.72, 95% CI 0.52 to 1.00; misoprostol plus oxytocin: RR 0.73, 95% CI 0.6 to 0.9). These three strategies had an almost 100% probability of being ranked the first, second or third most effective strategy. Oxytocin was ranked fourth, with an almost 0% probability of being ranked in the top three. A similar performance of these three strategies was noted for the reduction of PPH blood loss of ≥ 1000 ml [ergometrine plus oxytocin: RR 0.77, 95% CI 0.61 to 0.95; carbetocin: RR 0.70, 95% CI 0.38 to 1.28; misoprostol plus oxytocin: RR 0.90, 95% CI 0.72 to 1.14), but the CIs were wider as this outcome is more rare. However, these three strategies had an almost 80% probability of being ranked the first, second or third most effective strategy. Oxytocin was ranked fourth, with an approximately 20% probability of being ranked in the top three strategies for this outcome.
For our secondary outcomes, including requirement for additional uterotonics, transfusion, change in Hb level and blood loss as a continuous outcome, again ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were the three most effective strategies. Oxytocin was consistently ranked fourth behind these three strategies. For some outcomes, such as maternal death, the composite outcome of maternal death or severe morbidity and manual removal of placenta, the study found that there were too few events to make analysis useful. For the duration of the third stage there was no clear ranking that emerged from this analysis. For the outcome of clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge, there were too few studies to make the analysis useful.
In terms of side effects, ergometrine and ergometrine plus oxytocin are the lowest-ranked drugs for nausea, vomiting, hypertension and headache. Misoprostol and misoprostol plus oxytocin are the lowest-ranked drugs for fever and shivering. Misoprostol plus oxytocin and ergometrine plus oxytocin are the lowest-ranked drugs for causing abdominal pain. For hypotension and tachycardia, there were too few studies to make the analysis useful. Carbetocin, oxytocin and placebo or the control had a similar side-effect profile and were the highest-ranked drugs for all side effects. There were no serious adverse effects noted with any of the drugs in the included trials.
Subgroup analyses were carried out according to mode of birth, prior risk of PPH, health-care setting and dose and route of administration of the drugs. The study found that the results were, as expected, less powered and more unstable, but generally in agreement with the overall results. Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were the highest-ranked drug strategies, with oxytocin being consistently fourth. However, no studies have used ergometrine plus oxytocin or ergometrine alone for women undergoing caesareans and effectiveness estimates could not be provided for these drug strategies. Interestingly, in the subgroup including only oxytocin trials in which the drug was administered intramuscularly or intravenously via a bolus as currently recommended, the ranking did not change. Similarly, restricting the analysis to high- or low-dose misoprostol trials did not alter the ranking of this drug.
In our sensitivity analyses, when we restricted the analysis to high-quality studies or studies rated as being at low risk of bias in terms of their funding, carbetocin lost its ranking and was comparable to oxytocin, but ergometrine plus oxytocin and misoprostol plus oxytocin were still ranked higher than oxytocin. When the analysis was restricted to studies that assessed the blood loss objectively, it was found that ergometrine plus oxytocin was ranked the highest, but there was no clear ranking hierarchy for the rest of the drugs or drug combinations. When the analysis was restricted to large studies we found that there were no studies investigating carbetocin and, again, ergometrine plus oxytocin and misoprostol plus oxytocin were ranked higher than oxytocin.
Key findings of the cost-effectiveness analysis
Alongside the NMA, the study aimed to discover the most cost-effective uterotonic drug for the prevention of PPH. The analyses took the perspective of the NHS, and costs were presented in Great British pounds. The results were presented as ICERs, with a primary outcome measure of cost per case of PPH blood loss of ≥ 500 ml avoided. Secondary outcome measures were cost per case of PPH blood loss of ≥ 1000 ml avoided and cost per major adverse outcome averted. The analysis was carried out separately for vaginal birth and caesarean section birth.
The results of the cost-effectiveness for vaginal birth, excluding adverse events, show ergometrine plus oxytocin and carbetocin to be the leading strategies. Ergometrine plus oxytocin is the least costly strategy, and carbetocin is the most-effective strategy. The estimated ICER for prevention with carbetocin compared with ergometrine plus oxytocin is £1888.75 per case of PPH blood loss of ≥ 500 ml avoided. This means that every additional case of PPH blood loss of ≥ 500 ml avoided by using carbetocin over oxytocin costs an extra £1888.75 (see Table 4). When adverse events were included in the analysis, the dominant strategies were carbetocin and oxytocin. Oxytocin is the least costly strategy, and carbetocin is the most effective. The estimated ICER for prevention with carbetocin compared with oxytocin is £927.65 per case of PPH blood loss of ≥ 500 ml avoided. This means that taking into account side effects, every additional case of PPH blood loss of ≥ 500 ml avoided by using carbetocin over oxytocin costs an extra £927.65. There is a case for carbetocin being considered cost-effective compared with oxytocin, particularly in a community setting, where treatment for PPH may not be as easily accessible.
The results for birth by caesarean section were mixed because of a large number of missing data. The probabilities of PPH blood loss of ≥ 500 ml and of ≥ 1000 ml for ergometrine and ergometrine plus oxytocin were unavailable from the NMA, so the strategies were initially excluded from the analysis. These results showed misoprostol plus oxytocin and carbetocin to be the leading strategies. Including ergometrine and ergometrine plus oxytocin in the analysis, by making assumptions about the effectiveness of these strategies, shows ergometrine plus oxytocin to dominate all other strategies.
The results of the PSA show moderate uncertainty in the input parameters. This reflects the differing results shown in the principal analysis.
Strengths and limitations
Strengths and limitations of the effectiveness network meta-analysis
Strengths
The systematic review answers a defined question through a comprehensive literature search using Cochrane methods. The study excluded quasi-randomised trials to improve the quality of the included evidence. Study selection and extraction of relevant quantitative and quality assessment data were performed by three reviewers (IG, HW, AM, OT, HG or DL) for all randomised trials. The NMA provides the relative effectiveness of all drugs used for the prevention of PPH in a coherent and methodologically robust way across important clinical outcomes by combining both direct and indirect evidence increasing the power and confidence in the results.
The study found that most of the included trials reported the primary outcomes, most of the secondary outcomes and often side effects. This increased the power across most of the analyses and underpins the consistency in the ranking across all blood loss outcomes, which also increases the confidence in the results.
The NMA is valid only assuming that all drugs in the network were suitable for all included women. We were thorough in the evaluation of the six important potential treatment effect modifiers (mode of birth, prior risk of PPH, health-care setting, dose, route and regimen of the drugs) and found no clinically important differences in the distribution of these potential effect modifiers across the interventions with a ranking in each of the subgroups comparable to the overall ranking. The results of the NMAs were mostly consistent, and when there was significant inconsistency this was normally because of unstable estimates from single studies. Through the sensitivity analyses, it was possible to identify that the research underpinning the carbetocin effectiveness is based on small studies of low quality.
Limitations
Included studies were rated as being at a low risk of bias when the quality domains were reported, but around half of the quality domains were not reported. The most common reason for concerns regarding selective reporting was insufficient information regarding protocol publication to confidently judge if the trial has selectively reported results. This is affected by the fact that protocol publication only became common practice recently. Often studies did not report their sources of funding or their methods for measuring the blood loss. This latter outcome was particularly inconsistent because some trials measured blood loss objectively by weighing swabs and drapes and others subjectively by visual estimation. Around half of the studies used adequate concealment and blinded professionals and participants.
Patients identified the clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge as important. These were not reported often enough in the trials to make conclusions based on their analyses.
Heterogeneity in the analyses may have been caused by the fact that trials were carried out over a long time period, during which the clinical response to PPH may have improved. These temporal changes could have contributed to heterogeneity and increased uncertainty of findings. As objective methods of measuring blood loss become increasingly available this could perhaps have also led to apparent increases in reported blood loss. However, a sensitivity analysis was carried out removing trials published before 1990, and this did not vary the ranking of the drugs.
The trials included in the review recruited women with varied clinical characteristics, and it is important to bear this in mind when interpreting results. The inclusion criteria were not always reported in detail and, when they were, these varied across trials. Many trials excluded women with significant comorbidities and at very high risk of PPH. Predominantly, women recruited to trials were > 37 weeks of gestation. Most of the trials were carried out in hospital settings and for women having a vaginal birth.
Clinical heterogeneity was encountered in settings and inclusion criteria, as described in Chapter 3, Study characteristics. However, some heterogeneity may also be present in the overall analysis related to the dose, route of drug administration or regimen of the drugs. Even though subgroup effects were not observed when the dose of misoprostol or regimen of oxytocin administration were varied, it was felt that they were most relevant. Subgroup analyses was not performed for every single increment in dosage or change in route or regimen of drug administration. Studies comparing exclusively different doses, routes or regimens of the drugs were excluded, as this was not the aim of this analysis.
Limitations in the cost-effectiveness analysis were mostly because of the missing data needed for the model. Several assumptions had to be made to impute probabilities for missing data, but there were uncertainties around these estimates. Attempts were made to make missing probabilities as accurate as possible, and the probability of adverse events was explored in the PSA in an attempt to rectify this limitation. Furthermore, the model does not address any further risks attached to the women other than the nine adverse events discussed in the methods. This includes serious adverse events, such as strokes, that can be more likely to occur when ergometrine plus oxytocin is given to hypertensive women.
The model-based economic evaluation includes only UK guidelines for model pathways, and attaches UK costs to resource use. It therefore does not consider different model pathways taken to treat PPH in developing countries where resources may be unavailable. It also fails to consider the costs and resources needed to store the uterotonic drugs.
Clinical implications of findings
This NMA found that ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin are more effective uterotonic drugs for preventing PPH than the standard drug recommendation of oxytocin. However, ergometrine plus oxytocin and misoprostol plus oxytocin cause significant side effects. Carbetocin has a favourable side-effect profile similar to oxytocin and placebo or the control. However, carbetocin trials are small and of poor quality, and when the analysis is restricted to high-quality trials, carbetocin loses its top ranking and does not appear to be more effective than oxytocin, but there is significant uncertainty around the effect estimate.
The ranking of the available drugs was similar in the subgroups including trials only of women having a vaginal birth or undergoing a caesarean. However, there are no trials that have used ergometrine plus oxytocin or ergometrine alone for prevention of PPH at caesarean section and these strategies cannot be recommended in this circumstance. However, these strategies are often used for treatment of PPH at the time of a caesarean section and should also be effective for prevention. The ranking is relevant to women at high or low risk of PPH in the hospital setting. There were not enough trials to be able to recommend a ranking in the community setting, even though a similar ranking in terms of effectiveness can be expected.
The advantages of carbetocin over existing practice using oxytocin as the agent of choice are evident. Carbetocin is always found to be more effective than oxytocin. Overall, carbetocin is also less costly than oxytocin, being the least costly in all but one of the analyses, despite the unit cost for carbetocin being relatively more expensive. Carbetocin, like oxytocin, has a relatively favourable side-effect profile, making it more appealing than uterotonic drugs, such as ergometrine plus oxytocin and misoprostol plus oxytocin, in which adverse events are more likely and the risks are less clear.
The current recommendation from NICE,23 RCOG174 and WHO6 is for 10 IU of intramuscular or intravenous oxytocin for the prevention of PPH. However, several studies have demonstrated that oxytocin loses potency if not stored at room temperature (i.e. ≤ 25 °C) for a restricted amount of time or refrigerated (at 2–8 °C), making its use difficult in low-income countries. 198 The manufacturer of carbetocin, Ferring Pharmaceuticals (Saint-Prex, Switzerland), has recently developed a room temperature-stable (RTS) formulation (i.e. carbetocin RTS), which makes it an attractive option for countries where maintaining cold storage is problematic. 199 As oxytocin is ranked fourth in terms of effectiveness and carbetocin is more cost-effective with a similar side-effect profile, our results can have important implications for clinical practice. However, when the analysis is restricted to high-quality trials it changes the ranking of carbetocin and it does not appear to be more effective than oxytocin in this analysis. The conclusion from this is that there is an urgent need for a high-quality large trial, comparing carbetocin with the current standard of oxytocin, to confirm or reject the findings of the current small and poor-quality trials that involve carbetocin.
There are two key studies that will inform a future update of this review. The first one is a WHO-led multicentre Phase III clinical study200 comparing the effectiveness of carbetocin RTS and oxytocin (administered intramuscularly) in the prevention of PPH for women having a vaginal birth. This study was recently published201 and included approximately 30,000 women from 10 countries: Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda and the UK. Carbetocin RTS was found to be non-inferior to oxytocin and the aim of the collaborating organisations is to now provide access to heat-stable carbetocin to public sector providers in low-income countries, with a high burden of maternal mortality, at an affordable and sustainable price. This is particularly important for low-resource countries where cold storage is difficult to achieve and maintain. Another trial,202 based in the UK, is recruiting > 6000 women to a three-arm trial comparing carbetocin, ergometrine plus oxytocin and oxytocin. This trial is also expected to report in due course. These trials aim to provide the high-quality evidence to support a change in practice, if the effectiveness of carbetocin is confirmed.
Recommendations for research
This NMA and cost-effectiveness analysis will require further updates in the future, especially as new evidence from randomised trials becomes available. An updated search in October 2017 identified a further 85 trial reports listed under studies awaiting classification. The priority is to update this analysis once the WHO-led trial mentioned in Clinical implications of findings is complete. If such a large and high-quality trial confirms the effectiveness of carbetocin, this updated report is likely to support a change in clinical practice. If such a recommendation is issued, then future research should focus on the implementation of such a policy in different settings.
More research into patient-reported outcomes, such as women’s views about the drugs, is important. After our consultation with the PPI group of this study, it was clear that preventing PPH is a top priority for preserving maternal well-being, and the group considered it important to evaluate additional outcomes, including women’s views regarding the drugs used, clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge. However, existing trials rarely investigated these outcomes. Side effects of the drugs are also considered equally important and these were often not reported. All triallists should consider reporting these outcomes and side effects for each drug in all future randomised trials.
Additionally, future evidence synthesis research should compare the effects of different dosages and routes of administration for the dominant strategies. Attaching other developed, and also developing, country costs and model pathways should also be explored, as this may change the ranking order of cost-effective uterotonics.
Chapter 6 Other information
Trial registration
HTA reference number: 14/139/17.
PROSPERO reference number: CRD42015020005.
Cochrane Pregnancy and Childbirth Group (substudy) reference number: 0871.
PROSPERO Cochrane (substudy) reference number: CRD42015026568.
Sponsor’s reference number: ERN_13–1414.
Protocol versions
Preliminary protocol development
26 February 2014
Meta-analytic title registration (not including cost-effectiveness analysis) with the Cochrane Collaboration.
5 September 2014
Submission of our initial study proposal including cost-effectiveness analysis to the NIHR HTA programme.
10 January 2015
Submission of a more-detailed study proposal including cost-effectiveness analysis to the NIHR HTA programme (recommendation for funding 5 February 2015).
Publication of protocol
22 April 2015
Finalisation of our comprehensive study protocol including cost-effectiveness analysis for the NIHR Journals Library version 1.0.
30 April 2015
Typographic corrections only to the comprehensive study protocol, including cost-effectiveness analysis for the NIHR Journals Library version 1.1.
18 May 2015
Publication of meta-analytic protocol (not including the cost-effectiveness analysis) by the Cochrane Collaboration.
Changes post publication
November 2016
Submission of the NMA and cost-effectiveness analysis to the NIHR HTA programme, with meta-analysis performed in Stata rather than WinBUGS for reasons of future reproducibility.
Acknowledgements
Contributions of authors
All of the following named authors contributed substantially to the development of the research question and study design, implementation, analysis and/or interpretation of data and submission of the final report.
Particular contributions are denoted below.
Ioannis Gallos (Clinician Scientist and Honorary Consultant in Obstetrics and Gynaecology) conceived the idea for the project and contributed to protocol development, management of the project, design of electronic data collection forms, data collection and quality assessment for the systematic review, clinical interpretation of findings and co-ordination of the initial draft of the final report.
Helen Williams (Research Associate, Women’s Health) was responsible for the completion of data gathering, providing data quality assurance, co-ordination of the analysis and the writing groups, and co-ordination of the initial draft of the final report.
Malcolm Price (Clinical Lecturer in Statistics) contributed to protocol development, conducted statistical analyses and drafted and edited the report.
Karen Pickering (Research Associate) conducted the economic analysis and modelling, and drafted and edited the report.
Abi Merriel (Research Fellow) contributed to data collection, data set management and quality assessment, and commented on drafts of the report.
Aurelio Tobias (Statistician) conducted statistical analyses and drafted and edited the report.
David Lissauer (Clinical Lecturer in Obstetrics and Gynaecology) contributed to data collection, data set management and quality assessment, and commented on drafts of the report.
Harry Gee (Retired Consultant in Obstetrics) contributed to data collection, data set management and quality assessment, and commented on drafts of the report.
Ôzge Tunçalp (Research Fellow) contributed to data collection, data set management and quality assessment, and commented on drafts of the report.
Gillian Gyte (Consumer Representative) co-ordinated consumer groups, contributed to protocol development, commented on drafts of all project documentation and commented on drafts of the report.
Vidhya Moorthy (Obstetrician) contributed to data collection, data set management and quality assessment and commented on drafts of the report.
Tracy Roberts (Professor of Health Economics) contributed to protocol development, supervised the economic analysis and modelling and drafted and edited the report.
Jonathan Deeks (Professor of Statistics) contributed to protocol development and commented on drafts of the report.
Justus Hofmeyr (Professor of Obstetrics) contributed to protocol development, drafted and edited the report.
Metin Gülmezoglu (WHO Co-ordinator for Maternal and Perinatal Health) contributed to protocol development, drafted and edited the report.
Arri Coomarasamy (Professor of Gynaecology) conceived the project and contributed to protocol development, and drafted and edited the report.
The Cochrane Collaboration
As part of the pre-publication editorial process, the review protocol was commented on by five peers (an editor and four referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group’s international panel of consumers, and the Group’s Statistical Advisers.
Other researchers
We are grateful to the investigators of the Postpartum Haemorrhage Core Outcome Sets Project, and particularly to Shireen Meher, Anna Cuthbert, Zarko Alfirevic, Jamie Kirkham and Paula Williamson, for discussing the progress of their work with us.
Publications
Coomarasamy A, Gallos ID, Williams H, Price M, Gee H, Merriel A, et al. Effects of uterotonic drugs for preventing postpartum haemorrhage: a network meta-analysis. Int J Gynaecol Obstet 2015;131(Suppl. 5):083.4.
Gallos ID, Williams H, Price M, Merriel A, Gee HY, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev 2018;4:CD011689.
Pickering K, Gallos ID, Williams H, Price MJ, Merriel A, Lissauer D, et al. Uterotonic drugs for the prevention of postpartum haemorrhage: a cost-effectiveness analysis. PharmacoEconomics – Open 2018:1–14.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Trends in Maternal Mortality: 1990 to 2013 – Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva: WHO; 2014.
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323-33. https://doi.org/10.1016/S2214-109X(14)70227-X.
- Carroli G, Cuesta C, Abalos E, Gülmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol 2008;22:999-1012. https://doi.org/10.1016/j.bpobgyn.2008.08.004.
- Health and Social Care Information Centre . Hospital Episode Statistics Analysis: NHS Maternity Statistics – England, 2012–13 2013. www.hscic.gov.uk/catalogue/PUB12744 (accessed 20 August 2014).
- Penney G, Brace V. Near miss audit in obstetrics. Curr Opin Obstet Gynecol 2007;19:145-50. https://doi.org/10.1097/GCO.0b013e328014a860.
- WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: WHO; 2012.
- Weekes LR, O’Toole DM. Postpartum hemorrhage; a five-year study at Queen of Angels Hospital. Am J Obstet Gynecol 1956;71:45-50. https://doi.org/10.1016/0002-9378(56)90676-7.
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747-55. https://doi.org/10.1016/S0140-6736(13)60686-8.
- Combs CA, Murphy EL, Laros RK. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol 1991;77:69-76.
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD007412.pub3.
- eMC . Syntocinon Ampoules 5 IU Ml – Summary of Product Characteristics (SPC) 2014. www.medicines.org.uk/emc/medicine/16423/SPC (accessed 10 October 2014).
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth 2007;98:116-19. https://doi.org/10.1093/bja/ael302.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD001808.pub2.
- de Groot AN, van Dongen PW, Vree TB, Hekster YA, van Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56:523-35.
- de Groot AN. The role of oral (methyl)ergometrine in the prevention of postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol 1996;69:31-6. https://doi.org/10.1016/0301-2115(95)02531-6.
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database Syst Rev 2007;2. https://doi.org/10.1002/14651858.CD005456.pub2.
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD000494.pub4.
- Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy 2001;21:60-73. https://doi.org/10.1592/phco.21.1.60.34442.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 2005;71:22-5. https://doi.org/10.1016/j.contraception.2004.06.014.
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther 1992;52:60-7. https://doi.org/10.1038/clpt.1992.103.
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2012;2. https://doi.org/10.1002/14651858.CD005457.pub3.
- McDonald S, Abbott JM, Higgins SP. Prophylactic ergometrine-oxytocin versus oxytocin for the third stage of labour. Cochrane Database Syst Rev 2004;1. https://doi.org/10.1002/14651858.CD000201.pub2.
- Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth. London: NICE; 2014.
- Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009;181:488-93. https://doi.org/10.1503/cmaj.081086.
- Evaluating the Quality of Care for Severe Pregnancy Complications: Who Near-Miss Approach for Maternal Health. Geneva: WHO; 2011.
- Detailed search methods used to maintain and update the Specialised Register. London: The Cochrane Collaboration; 2017.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. https://doi.org/10.1177/0272989X12458724.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. https://doi.org/10.1016/j.jclinepi.2010.03.016.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111-25. https://doi.org/10.1002/jrsm.1045.
- White IR. Network meta-analysis. Stata J 2015;15:951-85. https://doi.org/10.1177/1536867X1501500403.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. https://doi.org/10.1002/sim.1186.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. https://doi.org/10.1002/jrsm.1044.
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341. https://doi.org/10.1136/bmj.c3515.
- Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev 2018;4.
- Abdel-Aleem H, Singata M, Abdel-Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. Int J Gynaecol Obstet 2010;111:32-6. https://doi.org/10.1016/j.ijgo.2010.04.036.
- Acharya G, Al-Sammarai MT, Patel N, Al-Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous Syntocinon on intra-operative blood loss during cesarean section. Acta Obstet Gynecol Scand 2001;80:245-50. https://doi.org/10.1034/j.1600-0412.2001.080003245.x.
- Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post-caesarean section. Int J Gynaecol Obstet 2012;119. https://doi.org/10.1016/S0020-7292(12)62094-3.
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Med J 2010;51:207-11.
- Ahmed WAS, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. J Matern Fetal Neonatal Med 2014;27.
- Al-Sawaf A, El-Mazny A, Shohayeb A. A randomised controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. J Obstet Gynaecol 2013;33:277-9. https://doi.org/10.3109/01443615.2012.755503.
- Amant F, Spitz B, Timmerman D, Corremans A, Van Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double-blind randomised trial. Br J Obstet Gynaecol 1999;106:1066-70. https://doi.org/10.1111/j.1471-0528.1999.tb08115.x.
- Amin N. Prophylactic use of misoprostol in management of third stage of labour and prevention of atonic uterus. J Postgrad Med Inst 2014;28:196-200.
- Askar AA, Ismail MT, El-Ezz AA, Rabie NH. Carbetocin versus Syntometrine in the management of third stage of labor following vaginal delivery. Arch Gynecol Obstet 2011;284:1359-65. https://doi.org/10.1007/s00404-011-1851-8.
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. BJOG 2010;117:929-36. https://doi.org/10.1111/j.1471-0528.2010.02585.x.
- Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001752.
- Badejoko OO, Ijarotimi AO, Awowole IO, Loto OM, Badejoko BO, Olaiya DS, et al. Adjunctive rectal misoprostol versus oxytocin infusion for prevention of postpartum hemorrhage in women at risk: a randomized controlled trial. J Obstet Gynaecol Res 2012;38:1294-301. https://doi.org/10.1111/j.1447-0756.2012.01869.x.
- Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin-ergometrine co-administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG 2008;115:579-84. https://doi.org/10.1111/j.1471-0528.2007.01658.x.
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. Am J Obstet Gynecol 1998;179:1043-6. https://doi.org/10.1016/S0002-9378(98)70212-1.
- Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with Syntometrine for management of third stage of labor. Acta Obstet Gynecol Scand 1998;77:178-81. https://doi.org/10.1080/j.1600-0412.1998.770209.x.
- Barton SR, Jackson A. The safety and efficiency of carbetocin to control uterine bleeding following caesarean section. Prenatal Neonatal Med 1996;1.
- Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. Int J Gynaecol Obstet 2007;97:2-5. https://doi.org/10.1016/j.ijgo.2006.12.016.
- Begley CM. A comparison of ‘active’ and ‘physiological’ management of the third stage of labour. Midwifery 1990;6:3-17. https://doi.org/10.1016/S0266-6138(05)80091-9.
- Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, et al. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double-blind randomised controlled trial. BJOG 2012;119:975-82. https://doi.org/10.1111/j.1471-0528.2012.03341.x.
- Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Role of misoprostol in the delivery outcome. J Gynecol Obstet Biol Reprod 2001;30:576-83.
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol 2004;104:1282-8. https://doi.org/10.1097/01.AOG.0000144119.94565.18.
- Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Arch Gynecol Obstet 2009;280:707-12. https://doi.org/10.1007/s00404-009-0973-8.
- Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol 1998;18:202-7.
- Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery: a double-blind randomized trial. J Obstet Gynaecol Can 2004;26:481-8. https://doi.org/10.1016/S1701-2163(16)30659-4.
- Bugalho A, Daniel A, Faúndes A, Cunha M. Misoprostol for prevention of postpartum hemorrhage. Int J Gynaecol Obstet 2001;73:1-6. https://doi.org/10.1016/S0020-7292(01)00346-0.
- Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective Caesarean delivery. Br J Anaesth 2010;104:338-43. https://doi.org/10.1093/bja/aeq004.
- Calişkan E, Meydanli MM, Dilbaz B, Aykan B, Sönmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. Am J Obstet Gynecol 2002;187:1038-45. https://doi.org/10.1067/mob.2002.126293.
- Calişkan E, Dilbaz B, Meydanli MM, Ozturk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstet Gynecol 2003;101:921-8. https://doi.org/10.1097/00006250-200305000-00017.
- Carbonell I Esteve JL, Hernandez JMR, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 µg of sublingual misoprostol and 200 µg of rectal misoprostol versus active management only in the prevention of post-partum haemorrhage. A randomised clinical trial. Progres Obstet Ginecol 2009;52:543-51. https://doi.org/10.1016/S0304-5013(09)72619-6.
- Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on intestinal motility and pain score in high risk cesarean delivery. Turk Klinik J Med Sci 2010;30:1154-9. https://doi.org/10.5336/medsci.2008-10206.
- Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. Int J Gynaecol Obstet 2010;109:25-9. https://doi.org/10.1016/j.ijgo.2009.11.009.
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low-risk women. Int J Gynaecol Obstet 2012;116:138-42. https://doi.org/10.1016/j.ijgo.2011.09.016.
- Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum hemorrhage. Int J Gynaecol Obstet 2015;128:48-52. https://doi.org/10.1016/j.ijgo.2014.07.029.
- Chhabra S, Tickoo C. Low-dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. J Obstet Gynaecol Res 2008;34:820-3. https://doi.org/10.1111/j.1447-0756.2008.00843.x.
- Choy CM, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular Syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG 2002;109:173-7. https://doi.org/10.1111/j.1471-0528.2002.01204.x.
- Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or Syntometrine in the third stage of labour. Aust N Z J Obstet Gynaecol 1999;39:414-19. https://doi.org/10.1111/j.1479-828X.1999.tb03124.x.
- Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. Am J Obstet Gynecol 1999;18:670-6. https://doi.org/10.1016/S0002-9378(99)70271-1.
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage. J Obstet Gynaecol Res 2002;28.
- de Groot AN, van Roosmalen J, van Dongen PW, Borm GF. A placebo-controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstet Gynecol Scand 1996;75:464-8. https://doi.org/10.3109/00016349609033355.
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial. Lancet 2006;368:1248-53. https://doi.org/10.1016/S0140-6736(06)69522-6.
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Res J Pharm Biol Chem Sci 2014;5:734-9.
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. J Obstet Gynaecol 1981;2.
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. J Obstet Gynaecol 2009;29:633-6. https://doi.org/10.1080/01443610903061744.
- El Behery MM, El Sayed GA, El Hameed AA, Soliman BS, Abdelsalam WA, Bahaa A. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in obese nulliparous women undergoing emergency cesarean delivery. J Matern Fetal Neonatal Med 2016;29:1257-60. https://doi.org/10.3109/14767058.2015.1043882.
- El Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al. Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Rev Bras Anestesiol 2012;62:625-35. https://doi.org/10.1016/S0034-7094(12)70162-9.
- El-Refaey H, Nooh R, O’Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG 2000;107:1104-10. https://doi.org/10.1111/j.1471-0528.2000.tb11108.x.
- Elgafor el Sharkwy IA. Carbetocin versus sublingual misoprostol plus oxytocin infusion for prevention of postpartum hemorrhage at cesarean section in patients with risk factors: a randomized, open trail study. Arch Gynecol Obstet 2013;288:1231-6. https://doi.org/10.1007/s00404-013-2896-7.
- Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. Int J Gynaecol Obstet 2012;118:149-52. https://doi.org/10.1016/j.ijgo.2012.03.038.
- Enakpene CA, Morhason-Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post-partum hemorrhage during third stage of labor. J Obstet Gynaecol Res 2007;33:810-17. https://doi.org/10.1111/j.1447-0756.2007.00661.x.
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. Int J Gynaecol Obstet 2014;124:67-71. https://doi.org/10.1016/j.ijgo.2013.07.020.
- Fararjeh C, Gezer A, Cepni I, Benian A, Ocal P, Kosebay D. The efficacy of misoprostol in preventing postpartum bleeding. Jinekoloji Ve Obstetrik Dergisi 2003;17:218-23.
- Fazel MR, Mansoure S, Esmaeil F. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East J Anaesthesiol 2013;22:41-6.
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. J Gynecol Obstet Biol Reprod 2009;38:588-93. https://doi.org/10.1016/j.jgyn.2009.09.006.
- Fenix AM. Double-blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum hemorrhage among high risk women following vaginal delivery. Int J Gynaecol Obstet 2012;119:S347-8. https://doi.org/10.1016/S0020-7292(12)60677-8.
- Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. J Nurs Sci 2003;18:910-1.
- Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methylergometrine in management of the third stage of labor. Int J Gynaecol Obstet 2005;91:160-1. https://doi.org/10.1016/j.ijgo.2005.07.005.
- Gavilanes P, Morales MF, Velasco S, Teran E. Sublingual misoprostol is as effective as intravenous oxytocin to reduce intra-operative blood loss during cesarean delivery in women living at high altitude. J Matern Fetal Neonatal Med 2015;29:559-61. https://doi.org/10.3109/14767058.2015.1011115.
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. Am J Obstet Gynecol 2001;185:878-82. https://doi.org/10.1067/mob.2001.117360.
- Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689-95. https://doi.org/10.1016/S0140-6736(01)05835-4.
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage – a pilot study. Int J Gynaecol Obstet 2006;94:139-40. https://doi.org/10.1016/S0020-7292(06)60014-3.
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. Am J Obstet Gynecol 2005;192:1404-6. https://doi.org/10.1016/j.ajog.2004.12.033.
- Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with Syntometrine on blood loss in the third stage of labour. West Indian Med J 2009;58:201-6.
- Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol 1998;105:971-5. https://doi.org/10.1111/j.1471-0528.1998.tb10259.x.
- Hofmeyr GJ, Nikodem VC, de Jager M, Drakely A. Side-effects of oral misoprostol in the third stage of labour – a randomised placebo-controlled trial. S Afr Med J 2001;91:432-5.
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400 µg of misoprostol to augment routine active management of the third stage of labor. Int J Gynaecol Obstet 2011;112:98-102. https://doi.org/10.1016/j.ijgo.2010.08.019.
- Høj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea-Bissau: randomised double blind clinical trial. BMJ 2005;331. https://doi.org/10.1136/bmj.331.7519.723.
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. Am J Obstet Gynecol 2007;197. https://doi.org/10.1016/j.ajog.2007.10.336.
- Is S, Gr V, Keranahalli S. Comparison of intramuscular ergometrine and per rectal misoprostol for prophylaxis against atonic post partum haemorrhage. Int J Gynaecol Obstet 2012;119:S797-8. https://doi.org/10.1016/S0020-7292(12)62007-4.
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. J Matern Fetal Neonatal Med 2007;20:703-5. https://doi.org/10.1080/14767050701500406.
- Jangsten E, Mattsson LÅ, Lyckestam I, Hellström AL, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG 2011;118:362-9. https://doi.org/10.1111/j.1471-0528.2010.02800.x.
- Jerbi M, Hidar S, Elmoueddeb S, Chaieb A, Khairi H. Oxytocin in the third stage of labor. Int J Gynaecol Obstet 2007;96:198-9. https://doi.org/10.1016/j.ijgo.2006.11.019.
- Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai J Obstet Gynaecol 2000;12.
- Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. J Obstet Gynaecol Can 2002;24:149-54. https://doi.org/10.1016/S1701-2163(16)30296-1.
- Kerekes L, Domokos N. The effect of prostaglandin F2 alpha on third stage labor. Prostaglandins 1979;18:161-6. https://doi.org/10.1016/S0090-6980(79)80034-9.
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus Syntometrine in the active management of the third stage of labour. Eur J Obstet Gynecol Reprod Biol 1995;58:147-51. https://doi.org/10.1016/0028-2243(95)80014-J.
- Kumru S, Gurates B, Parmaksiz C. Investigation of the usefulness of methyl ergonovine application in cesarean section cases. J Turk Ger Gynecol Asso 2005;6:42-5.
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. Int J Gynaecol Obstet 2001;75:235-41. https://doi.org/10.1016/S0020-7292(01)00498-2.
- Lam H, Tang OS, Lee CP, Ho PC. A pilot-randomized comparison of sublingual misoprostol with Syntometrine on the blood loss in third stage of labor. Acta Obstet Gynecol Scand 2004;83:647-50. https://doi.org/10.1111/j.0001-6349.2004.00572.x.
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. Int J Gynaecol Obstet 2006;95:2-7. https://doi.org/10.1016/j.ijgo.2006.05.031.
- Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus Syntometrine in the management of the third stage of labour. BJOG 2006;113:1459-64. https://doi.org/10.1111/j.1471-0528.2006.01105.x.
- Lokugamage AU, Paine M, Bassaw-Balroop K, Sullivan KR, Refaey HE, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus Syntocinon. Aust N Z J Obstet Gynaecol 2001;41:411-14. https://doi.org/10.1111/j.1479-828X.2001.tb01319.x.
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose-related shivering and pyrexia in the third stage of labour. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labour. Br J Obstet Gynaecol 1999;106:304-8. https://doi.org/10.1111/j.1471-0528.1999.tb08266.x.
- Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery in high risk women. J Matern Fetal Neonatal Med 2016;29:532-6. https://doi.org/10.3109/14767058.2015.1011121.
- McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone versus oxytocin and ergometrine in active management of third stage of labour. BMJ 1993;307:1167-71. https://doi.org/10.1136/bmj.307.6913.1167.
- Mitchell GG, Elbourne DR. The Salford Third Stage Trial: oxytocin plus ergometrine vs oxytocin alone in the active management of the third stage of labor. Online J Curr Clin Trial 1993;2.
- Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG 2011;118:353-61. https://doi.org/10.1111/j.1471-0528.2010.02807.x.
- Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG 2011;118:1349-56. https://doi.org/10.1111/j.1471-0528.2011.03022.x.
- Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side-effects at spontaneous vertex delivery. Br J Anaesth 1979;51:113-17. https://doi.org/10.1093/bja/51.2.113.
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. Br J Anaesth 1976;48:571-4. https://doi.org/10.1093/bja/48.6.571.
- Mukta M, Sahay PB. Role of misoprostol 600 mcg oral in active management of third stage of labor: a comparative study with oxytocin 10 IU i.m. J Obstet Gynaecol India 2013;63:325-7. https://doi.org/10.1007/s13224-012-0330-x.
- Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, et al. Double-blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. Int J Gynaecol Obstet 2015;129:227-30. https://doi.org/10.1016/j.ijgo.2015.01.008.
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. Int J Gynaecol Obstet 2009;105:244-7. https://doi.org/10.1016/j.ijgo.2009.01.018.
- Ng PS, Chan AS, Sin WK, Tang LC, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and i.m. Syntometrine in the management of the third stage of labour. Hum Reprod 2001;16:31-5. https://doi.org/10.1093/humrep/16.1.31.
- Ng PS, Lai CY, Sahota DS, Yuen PM. A double-blind randomized controlled trial of oral misoprostol and intramuscular Syntometrine in the management of the third stage of labor. Gynecol Obstet Invest 2007;63:55-60. https://doi.org/10.1159/000095498.
- Nirmala K, Zainuddin AA, Ghani NA, Zulkifli S, Jamil MA. Carbetocin versus syntometrine in prevention of post-partum hemorrhage following vaginal delivery. J Obstet Gynaecol Res 2009;35:48-54. https://doi.org/10.1111/j.1447-0756.2008.00829.x.
- Nordström L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. Br J Obstet Gynaecol 1997;104:781-6. https://doi.org/10.1111/j.1471-0528.1997.tb12020.x.
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. J Obstet Gynaecol 2003;23:13-6. https://doi.org/10.1080/0144361021000043146.
- Ogunbode O, Obisesan K, Ayeni O. Methergin in the management of the third stage of labor: a comparative clinical trial with Syntometrine and ergometrine. Curr Ther Res Clin Exp 1979;26:460-5.
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. Int J Gynaecol Obstet 2008;101:129-32. https://doi.org/10.1016/j.ijgo.2007.11.009.
- Ortiz-Gómez JR, Morillas-Ramírez F, Fornet-Ruiz I, Palacio-Abizanda FJ, Bermejo-Albares L. Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin. Rev Esp Anestesiol Reanim 2013;60:7-15. https://doi.org/10.1016/j.redar.2012.06.013.
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. J Obstet Gynaecol Res 2011;37:715-21. https://doi.org/10.1111/j.1447-0756.2010.01399.x.
- Parsons SM, Walley RL, Crane JMG, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. J Obstet Gynaecol Can 2006;28:20-6. https://doi.org/10.1016/S1701-2163(16)32029-1.
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. J Obstet Gynaecol Can 2007;29:711-18. https://doi.org/10.1016/S1701-2163(16)32594-4.
- Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the hospital de maternidad Rafael Calvo. Rev Colomb Obstet Ginecol 2002;53:87-92.
- Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297:1295-300. https://doi.org/10.1136/bmj.297.6659.1295.
- Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for the prevention of postpartum hemorrhage. J Pregnancy 2014;2014. https://doi.org/10.1155/2014/713879.
- Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular Syntometrine and intravenous Syntocinon, in preventing postpartum haemorrhage. J Obstet Gynaecol 2009;29:396-401. https://doi.org/10.1080/01443610902946929.
- Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. J Obstet Gynaecol India 2001;51:53-4.
- Reyes OA. Carbetocin vs. oxytocin for the prevention of postpartum hemorrhage grand multipara patients: randomized controlled trial. Clin Invest Ginecol Obstet 2011;38:2-7. https://doi.org/10.1016/j.gine.2010.01.001.
- Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in patients with severe preeclampsia: a double-blind randomized controlled trial. J Obstet Gynaecol Can 2011;33:1099-104. https://doi.org/10.1016/S1701-2163(16)35077-0.
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351:693-9. https://doi.org/10.1016/S0140-6736(97)09409-9.
- Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesæter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013;119:541-51. https://doi.org/10.1097/ALN.0b013e31829416dd.
- Rozenberg P, Quibel T, Ghout I, Salomon L, Bussiere L, Goffinet F. Active management of the third stage of labor with routine oxytocin and misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. Am J Obstet Gynecol 2015;212. https://doi.org/10.1016/j.ajog.2014.10.071.
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. Int Res J Pharm 2011;2:76-81.
- Samimi M, Imani-Harsini A, Abedzadeh-Kalahroudi M. Carbetocin vs. syntometrine in prevention of postpartum hemorrhage: a double blind randomized control trial. Iran Red Crescent Med J 2013;15:817-22. https://doi.org/10.5812/ircmj.7881.
- Shrestha A, Dongol A, Chawla CD, Adhikari RK. Rectal misoprostol versus intramuscular oxytocin for prevention of post partum hemorrhage. Kathmandu Univ Med J 2011;9:8-12. https://doi.org/10.3126/kumj.v9i1.6254.
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. Int J Gynaecol Obstet 2009;107:130-4. https://doi.org/10.1016/j.ijgo.2009.06.007.
- Soltan MH, El-Gendi E, Imam HH, Fathi O. Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. Int J Health Sci 2007;1:229-36.
- Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. J Obstet Gynaecol India 2012;62:162-7. https://doi.org/10.1007/s13224-012-0168-2.
- Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community-based, cluster-randomized trial. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001524.
- Su LL, Rauff M, Chan YH, Mohamad Suphan N, Lau TP, Biswas A, et al. Carbetocin versus Syntometrine for the third stage of labour following vaginal delivery – a double-blind randomised controlled trial. BJOG 2009;116:1461-6. https://doi.org/10.1111/j.1471-0528.2009.02226.x.
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. J Bangladesh Coll Phys Surg 2007;25:73-6. https://doi.org/10.3329/jbcps.v25i2.373.
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labour. Gynakol Geburtshilfliche Rundsch 1999;39:255-8.
- Tewatia R, Rani S, Srivastav U, Makhija B. Sublingual misoprostol versus intravenous oxytocin in prevention of post-partum hemorrhage. Arch Gynecol Obstet 2014;289:739-42. https://doi.org/10.1007/s00404-013-3026-2.
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol 1993;48:19-22. https://doi.org/10.1016/0028-2243(93)90048-H.
- Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. J Obstet Gynaecol 2014;34:407-11. https://doi.org/10.3109/01443615.2014.899329.
- Un Nisa S, Un Nisa S, Usmani SY. Role of intravenous Syntocinon in prevention of primary postpartum haemorrhage. Pak J Med Health Sci 2012;6:1020-4.
- Uncu Y, Karahasan M, Uyaniklar Ö, Uncu G. Prophylactic misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. Eur Rev Med Pharmacol Sci 2015;19:15-22.
- Vagge DS, Mamatha KR, Shivamurthy G, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. J Chem Pharm Res 2014;6:1134-40.
- Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl-ergometrine versus intramuscular 15-methyl PGF2alpha in active management of third stage of labor. Arch Gynecol Obstet 2009;280:893-7. https://doi.org/10.1007/s00404-009-1019-y.
- Verma P, Aggarwal N, Jain V, Suri V. A double-blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. Int J Gynaecol Obstet 2006;94:137-8. https://doi.org/10.1016/S0020-7292(06)60013-1.
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Int J Gynaecol Obstet 2004;87:1-5. https://doi.org/10.1016/j.ijgo.2004.05.016.
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynaecol Obstet 2006;92:106-10. https://doi.org/10.1016/j.ijgo.2005.10.008.
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double-blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG 2000;107:1111-15. https://doi.org/10.1111/j.1471-0528.2000.tb11109.x.
- Whigham CA, Gorelik A, Loughnan T, Trivedi A. Carbetocin versus oxytocin in active labour. BJOG 2014;121.
- Yuen PM, Chan NS, Yim SF, Chang AM. A randomised double blind comparison of Syntometrine and Syntocinon in the management of the third stage of labour. Br J Obstet Gynaecol 1995;102:377-80. https://doi.org/10.1111/j.1471-0528.1995.tb11288.x.
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. Int J Gynaecol Obstet 2006;92:23-6. https://doi.org/10.1016/j.ijgo.2005.08.026.
- Postpartum Haemorrhage, Prevention and Management. London: RCOG; 2009.
- Pichon-Riviere A, Glujovsky D, Garay OU, Augustovski F, Ciapponi A, Serpa M, et al. Oxytocin in Uniject disposable auto-disable injection system versus standard use for the prevention of postpartum hemorrhage in Latin America and the Caribbean: a cost-effectiveness analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0129044.
- Sutherland T, Bishai DM. Cost-effectiveness of misoprostol and prenatal iron supplementation as maternal mortality interventions in home births in rural India. Int J Gynaecol Obstet 2009;104:189-93. https://doi.org/10.1016/j.ijgo.2008.10.011.
- Sutherland T, Meyer C, Bishai DM, Geller S, Miller S. Community-based distribution of misoprostol for treatment or prevention of postpartum hemorrhage: cost-effectiveness, mortality, and morbidity reduction analysis. Int J Gynaecol Obstet 2010;108:289-94. https://doi.org/10.1016/j.ijgo.2009.11.007.
- Tsu VD, Levin C, Tran MP, Hoang MV, Luu HT. Cost-effectiveness analysis of active management of third-stage labour in Vietnam. Health Policy Plan 2009;24:438-44. https://doi.org/10.1093/heapol/czp020.
- Bradley SE, Prata N, Young-Lin N, Bishai DM. Cost-effectiveness of misoprostol to control postpartum hemorrhage in low-resource settings. Int J Gynaecol Obstet 2007;97:52-6. https://doi.org/10.1016/j.ijgo.2006.12.005.
- Barton P, Bryan S, Robinson S. Modelling in the economic evaluation of health care: selecting the appropriate approach. J Health Serv Res Policy 2004;9:110-18. https://doi.org/10.1258/135581904322987535.
- Morse A. Maternity Services in England. London: The Stationery Office; 2013.
- Butwick AJ, Carvalho B, Blumenfeld YJ, El-Sayed YY, Nelson LM, Bateman BT. Second-line uterotonics and the risk of hemorrhage-related morbidity. Am J Obstet Gynecol 2015;212:642.e1-7. https://doi.org/10.1016/j.ajog.2015.01.008.
- Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv 2007;62:540-7. https://doi.org/10.1097/01.ogx.0000271137.81361.93.
- Knight M. UKOSS . Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG 2007;114:1380-7. https://doi.org/10.1111/j.1471-0528.2007.01507.x.
- Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartum bleeding: a systematic review. Obstet Gynecol 2010;115:637-44. https://doi.org/10.1097/AOG.0b013e3181cfc007.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- NHS Reference Costs 2014–15. London: DHSC; 2015.
- NHS Reference Costs 2013–14. London: DHSC; 2014.
- BNF 71 (British National Formulary March–September 2016). London: Pharmaceutical Press; 2016.
- NHS Electronic Drug Tariff. London: Department of Health and Social Care; 2016.
- Putting NICE Guidance into Practice: Costing Statement Blood Transfusion. Implementing the NICE Guideline on Blood Transfusion. London: NICE; 2015.
- Khan KS, Tryposkiadis K, Tirlapur SA, Middleton LJ, Sutton AJ, Priest L, et al. MRI versus laparoscopy to diagnose the main causes of chronic pelvic pain in women: a test-accuracy study and economic evaluation. Health Technol Assess 2018;9.
- Glaze S, Ekwalanga P, Roberts G, Lange I, Birch C, Rosengarten A, et al. Peripartum hysterectomy: 1999 to 2006. Obstet Gynecol 2008;111:732-8. https://doi.org/10.1097/AOG.0b013e31816569f2.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-52.
- NICE. London: NICE; 2018.
- The Donation Process: What Happens on the Day. Watford: NHS Blood and Transplant; 2016.
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–8. The eighth report on confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118:1-203.
- Hogerzeil HV, Walker GJA, de Goeje MJ. Stability of Injectable Oxytocics in Tropical Climates. Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. Geneva: WHO; 1993.
- Heat-Stable Oxytocin. Technology Opportunity Assessment: Prepared for the Merck for Mothers Program. Seattle, WA: PATH; 2013.
- Gülmezoglu M. The WHO champion trial. Int J Gynecol Obstet 2015;131:E29-30.
- Widmer M, Piaggio G, Nguyen TMH, Osoti A, Olorunfemi O, Sujata MD, et al. Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth. N Engl J Med 2018;379:743-52. https://doi.org/10.1056/NEJMoa1805489.
- Draycott T, van der Nelson HA. Intramuscular Oxytocics: A Comparison Study of Intramuscular Carbetocin, Syntocinon and Syntometrine for the Third Stage of Labour Following Vaginal Birth (IMox). 2014. clinicaltrials.gov/ct2/show/NCT02216383 (accessed 28 September 2016).
- Fleiss JL. Statistical Methods for Rates and Proportions. London: John Wiley & Sons; 1981.
- Uterotonic Agents for Preventing Postpartum Haemorrhage: A Network Meta-Analysis n.d. https://edata.bham.ac.uk/284/ (accessed 21 February 2019).
Appendix 1 Search strategy: Cochrane Pregnancy and Childbirth Group
ClinicalTrials.gov and the WHO’S International Clinical Trials Registry Platform (ICTRP).
Search strategy
Third stage AND labo(u)r AND oxytocin.
Third stage AND labo(u)r AND misoprostol.
Third stage AND labo(u)r AND carbetocin.
Third stage AND labo(u)r AND ergometrine.
uterotonic* AND oxytocin.
uterotonic* AND misoprostol.
uterotonic* AND carbetocin.
uterotonic* AND ergometrine.
uterotonic* AND labo(u)r.
uterotonic* AND h(a)emorrhage.
h(a)emorrhage AND postpartum AND ergometrine.
h(a)emorrhage AND postpartum AND oxytocin.
h(a)emorrhage AND postpartum AND carbetocin.
h(a)emorrhage AND postpartum AND misoprostol.
Appendix 2 Description of included studies
| Study (author and year of publication) | Methods | Participants | Interventions | Outcomes | Quality rating |
|---|---|---|---|---|---|
| Abdel-Aleem et al., 201037 | Three-arm controlled randomised trial |
There were 1964 parturients randomised in a hospital setting in Egypt and South Africa The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with medical complications, such as hypertension and diabetes mellitus, previous caesarean section, or an abdominal wall that was not thin enough to allow easy palpation of the uterus after delivery |
10 IU of i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Acharya et al., 200138 | Two-arm active-controlled randomised trial |
There were 60 parturients randomised in a hospital setting in the UK The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria were not specified |
10 IU of i.v. oxytocin (bolus) vs. 400 µg of p.o. misoprostol |
|
High risk of bias |
| Adanikin et al., 201239 | Two-arm active-controlled double-dummy randomised trial |
There were 218 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with altered serum electrolyte levels, peritonitis, sepsis, previous bowel surgery, thyroid disease, inflammatory bowel disease or chronic constipation |
25 IU of i.v. oxytocin (bolus plus infusion) vs. 600 µg of p.r. misoprostol plus 5 IU of i.v. oxytocin (bolus) |
|
Low risk of bias |
| Afolabi et al., 201040 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in Nigeria The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction of labour or caesarean section, or those with haematocrit of < 30%, pre-eclampsia/eclampsia, grand multiparity (five or more), multiple pregnancy, coagulopathy or medical disorders |
10 IU of oxytocin i.m. vs. 400 µg of p.o. misoprostol |
|
High risk of bias |
| Ahmed et al., 201441 | Two-arm active-controlled randomised trial |
There were 80 parturients randomised in a hospital setting in Egypt The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients with risk factors for excessive blood loss, for example those women with placenta praevia or placental abruption |
100 µg of i.v. carbetocin (bolus) vs. 10 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Al-Sawaf et al., 201342 | Three-arm controlled randomised trial |
There were 120 parturients randomised in a hospital setting in Egypt The population comprised women of parity ≤ 4, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction of labour or instrumental delivery, or those with previous caesarean section, extensive perineal, vaginal or cervical lacerations, bleeding disorders, a Hb level of < 100 g/l, uterine malformations, grand multiparity, multiple pregnancy, polyhydramnios, intrauterine fetal death, medical problems such as pre-eclampsia, diabetes mellitus, cardiopulmonary problems, bowel disease or allergy to prostaglandins |
200 µg of s.l. misoprostol vs. 5 IU of i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Amant et al., 199943 | Two-arm active-controlled double-dummy randomised trial |
There were 213 parturients randomised in a hospital setting in Belgium The population comprised women of unspecified parity, either singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertensive disorders, gestational age of < 32 weeks, intrauterine fetal death, uterine malformations, inflammatory bowel disease, obliterative vascular or coronary disease, sepsis or allergy to prostaglandins or alkaloids |
600 µg of p.o. misoprostol vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Amin, 201444 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in Pakistan The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, bleeding disorders, prolonged labour, placenta praevia, placental abruption, multiple pregnancy, a BMI of > 30 kg/m2 or previous PPH |
5 IU of i.v. oxytocin (bolus) vs. 800 µg of p.r. misoprostol |
|
High risk of bias |
| Askar et al., 201145 | Two-arm active-controlled double-blind randomised trial |
There were 240 parturients randomised in a hospital setting in Kuwait The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients < 18 years old and those with known or suspected coagulopathy, grand multiparity (≥ 5), uterine fibroids, polyhydramnios, multiple pregnancy, fetal macrosomia, severe anaemia, cervical tears or who required prophylactic oxytocin infusion The presence of contraindications to the use of either Syntometrine or carbetocin that include pre-existing hypertension, pre-eclampsia, asthma, cardiac, renal or liver diseases, epilepsy, or history of hypersensitivity to Syntometrine or carbetocin |
100 µg of i.m. carbetocin vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
Low risk of bias |
| Attilakos et al., 201046 | Two-arm active-controlled double-blind randomised trial |
There were 377 parturients randomised in a hospital setting in the UK The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, gestational age of < 37 weeks performed for fetal or maternal distress where, because of time constraints, it was not possible to recruit or randomise, or those with multiple pregnancy, placenta praevia or placental abruption |
100 µg of i.v. carbetocin (bolus) vs. 5 IU of i.v. oxytocin (bolus) |
|
Low risk of bias |
| Atukunda et al., 201447 | Two-arm active-controlled double-dummy randomised trial |
There were 1140 parturients randomised in a hospital setting in Uganda The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or elective caesarean section, or those with intrauterine fetal death, heart disease, severe malaria or acute bacterial infection, multiple pregnancy, antepartum haemorrhage, altered cognitive status or reported hypersensitivity to prostaglandins |
10 IU of oxytocin i.m. vs. 600 µg of s.l. misoprostol |
|
Low risk of bias |
| Badejoko et al., 201248 | Two-arm active-controlled double-dummy randomised trial |
There were 264 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients in the second or third stage of labour, or those women with cervical lacerations or coagulopathy |
30 IU of i.v. oxytocin (bolus and infusion) vs. 600 µg of p.r. misoprostol plus 20 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Balki et al., 200849 | Two-arm active-controlled double-blind randomised trial |
There were 48 parturients randomised in a hospital setting in Canada The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section Exclusion criteria comprised parturients requiring general anaesthesia, or those with cardiac disease, hypertension or any condition predisposing to uterine atony and PPH, such as placenta praevia, multiple pregnancy, pre-eclampsia, macrosomia, polyhydramnios, uterine fibroids, bleeding disorders, chorioamnionitis, previous uterine atony, previous PPH or allergy/hypersensitivity to oxytocin or ergot derivatives |
250 µg of ergometrine plus 20 IU of i.v. oxytocin (bolus and infusion) vs. 20 IU of i.v. oxytocin (bolus and infusion) |
|
Low risk of bias |
| Bamigboye et al., 199850 | Two-arm placebo-controlled randomised trial |
There were 550 parturients randomised in a hospital setting in South Africa The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
400 µg of p.r. misoprostol vs. placebo |
|
High risk of bias |
| Bamigboye et al., 199851 | Two-arm active-controlled randomised trial |
There were 491 parturients randomised in a hospital setting in South Africa The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
400 µg of p.r. misoprostol vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Barton and Jackson, 199652 | Two-arm placebo-controlled randomised trial |
There were 119 parturients randomised in a hospital setting in the USA The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria were not specified |
100 µg of i.v. carbetocin (bolus) vs. placebo |
|
High risk of bias |
| Baskett et al., 200753 | Two-arm active-controlled double-dummy randomised trial |
There were 622 parturients randomised in a hospital setting in Canada The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta previa, placental abruption, coagulopathy or unstable asthma |
5 IU of i.v. oxytocin (bolus) vs. 400 µg of p.o. misoprostol |
|
Low risk of bias |
| Begley, 199054 | Two-arm controlled randomised trial |
There were 1429 parturients randomised in a hospital setting in Ireland The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, vaginal breech or instrumental delivery, or those with hypertension, epidural anaesthesia, antepartum haemorrhage, placenta praevia, placental abruption, first stage of labour > 15 hours, ‘quick’ delivery or needing resuscitation |
500 µg of i.v. ergometrine (bolus) vs. no treatment |
|
High risk of bias |
| Bellad et al., 201255 | Two-arm active-controlled double-dummy randomised trial |
There were 652 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with medical disorders, in active labour with > 4-cm dilatation or stillbirths |
400 µg of s.l. misoprostol vs. 10 IU of i.m. oxytocin |
|
Low risk of bias |
| Benchimol et al., 200156 | Three-arm controlled randomised trial |
There were 602 parturients randomised in a hospital setting in France The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age of < 32 weeks, previous PPH, intrauterine fetal death, previous uterine scar, multiple pregnancy or pre-eclampsia |
2.5 IU of i.m. oxytocin vs. 600 µg of p.o. misoprostol vs. no treatment |
|
High risk of bias |
| Bhullar et al., 200457 | Two-arm placebo-controlled randomised trial |
There were 756 parturients randomised in a hospital setting in the USA The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with a bleeding disorder |
200 µg of s.l. misoprostol plus 20 IU of i.v. oxytocin (infusion) vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Borruto et al., 200958 | Two-arm active-controlled randomised trial |
There were 104 parturients randomised in a hospital setting in France and Italy The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients with toxaemia, eclampsia or epilepsy |
100 µg of i.v. carbetocin (bolus) vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Boucher et al., 199859 | Two-arm active-controlled double-dummy randomised trial |
There were 60 parturients randomised in a hospital setting in Canada The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with heart disease or cardiac arrhythmia, hypertension or liver/renal/endocrine disease |
100 µg of i.v. carbetocin (bolus) vs. 32.5 IU of i.v. oxytocin (bolus and infusion) |
|
High risk of bias |
| Boucher et al., 200460 | Two-arm active-controlled double-dummy randomised trial |
There were 164 parturients randomised in a hospital setting in Canada The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients < 18 years old, or those without known PPH risk, known or suspected coagulopathy, heart disease or cardiac arrhythmia, chronic liver/renal/endocrine disease or hypersensitivity to study drugs |
100 µg of i.m. carbetocin vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Bugalho et al., 200161 | Two-arm active-controlled double-dummy randomised trial |
There were 700 parturients randomised in a hospital setting in Mozambique The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour |
400 µg of p.r. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Butwick et al., 201062 | Five-arm placebo-controlled randomised trial |
There were 75 parturients randomised in a hospital setting in the USA The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with active labour, ruptured membranes, drug allergy, multiple pregnancy, significant obstetric disease, risk factors for PPH (abnormal placentation, fibroids, previous PPH, previous classical uterine incision), coagulopathy or thrombocytopenia |
5, 3, 1 or 0.5 IU of i.v. oxytocin (bolus) vs. placebo |
|
High risk of bias |
| Calişkan et al., 200364 | Four-arm active-controlled double-dummy randomised trial |
There were 1800 parturients randomised in a hospital setting in Turkey The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age of < 32 weeks or hypersensitivity to prostaglandins |
400 µg of p.o. misoprostol plus 10 IU of i.v. oxytocin (infusion) vs. 400 µg of p.o. misoprostol vs. 10 IU of i.v. oxytocin (infusion) vs. 200 µg of i.m. ergometrine plus 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Calişkan et al., 200263 | Four-arm active-controlled double-dummy randomised trial |
There were 1633 parturients randomised in a hospital setting in Turkey The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with a gestational age of < 32 weeks or hypersensitivity to prostaglandins |
400 µg of p.r. misoprostol plus 10 IU of i.v. oxytocin (infusion) vs. 400 µg of p.r. misoprostol vs. 10 IU of i.v. oxytocin (infusion) vs. 200 µg of i.m. ergometrine plus 10 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Carbonell I Esteve et al., 200965 | Two-arm active-controlled randomised trial |
There were 1410 parturients randomised in a hospital setting in Spain The population comprised women of parity ≤ 4, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with a gestational age of < 32 weeks, coagulopathy, a Hb level < 80 g/l, liver or kidney disorder, grand multiparity (five or more), hypersensitivity or any contraindication for use of prostaglandins |
400 µg of s.l. misoprostol plus 200 µg of p.r. misoprostol plus 10 IU of i.m. oxytocin vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Cayan et al., 201066 | Four-arm controlled randomised trial |
There were 160 parturients randomised in a hospital setting in Turkey The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean section Exclusion criteria comprised parturients with thyroid disorder, inflammatory bowel disease or other bowel diseases, previous bariatric surgery or hypersensitivity to prostaglandins |
200, 400 or 600 µg of p.r. misoprostol plus 10 IU of i.v. oxytocin (infusion) vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Chaudhuri et al., 201067 | Two-arm active-controlled double-dummy randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients undergoing caesarean section for cord prolapse or bradycardia, or those with cardiovascular, respiratory, liver or haematological disorders or known hypersensitivity to prostaglandins |
800 µg of p.r. misoprostol vs. 40 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Chaudhuri et al., 201268 | Two-arm active-controlled double-dummy randomised trial |
There were 530 parturients randomised in a hospital setting in India The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with risk factors for PPH, including a BMI of > 30 kg/m2, grand multiparity (five or more), polyhydramnios, fetal macrosomia, antepartum haemorrhage, prolonged labour, previous PPH, a Hb level of < 80 g/l, severe pre-eclampsia, asthma or coagulopathy |
400 µg of s.l. misoprostol vs. 10 IU of i.m. oxytocin |
|
Low risk of bias |
| Chaudhuri and Majumdar, 201569 | Two-arm active-controlled double-dummy randomised trial |
There were 396 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section Exclusion criteria comprised parturients requiring conversion to general anaesthesia, or those with cardiovascular, hepatic, or haematological disorders or any contraindication for the use of misoprostol or oxytocin |
400 µg of s.l. misoprostol plus 20 IU of i.v. oxytocin (bolus and infusion) vs. 20 IU of i.v. oxytocin (bolus and infusion) |
|
Low risk of bias |
| Chhabra and Tickoo, 200870 | Three-arm active-controlled randomised trial |
There were 300 parturients were randomised in a hospital setting in India The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with grand multiparity (more than five), multiple pregnancy, pregnancy-induced hypertension, antepartum haemorrhage, previous caesarean, a Hb level of < 80 g/l, other obstetric problems or known hypersensitivity to prostaglandins |
100 or 200 µg of s.l. misoprostol vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Choy et al., 200271 | Two-arm active-controlled randomised trial |
There were 991 parturients randomised in a hospital setting in Hong Kong The population comprised women of parity ≤ 3, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with medical conditions that precluded the use of ergometrine, such as pre-eclampsia, cardiac disease or conditions that required prophylactic oxytocin infusion after delivery such as grand multiparity (four or more) or presence of uterine fibroids |
500 µg ergometrine plus 5 IU of i.m. oxytocin vs. 10 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Cook et al., 199972 | Three-arm active-controlled randomised trial |
There were 930 parturients randomised in a hospital setting in Australia, Papua New Guinea and China The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section, or those with coagulopathy, asthma, heart disease, severe renal disease, epilepsy or hypertension |
400 µg of p.o. misoprostol vs. 500 µg plus 5 IU of ergometrine plus i.m. oxytocin vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Dansereau et al., 199973 | Two-arm active-controlled double-blind randomised trial |
There were 694 parturients randomised in a hospital setting in Canada The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients undergoing general anaesthesia or requiring a classical uterine incision, or those with heart disease, chronic hypertension requiring treatment, liver/renal/endocrine disorders, coagulopathy, placenta praevia or placental abruption |
100 µg of i.v. carbetocin (bolus) vs. 25 IU of i.v. oxytocin (bolus and infusion) |
|
High risk of bias |
| Dasuki et al., 200274 | Two-arm active-controlled randomised trial |
There were 196 parturients randomised in a hospital setting in Indonesia The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| de Groot et al., 199675 | Three-arm placebo-controlled randomised trial |
There were 371 parturients randomised in a hospital and community setting in the Netherlands The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, requiring tocolysis or those who refuse to take part or with cardiac disease, multiple pregnancies, non-cephalic presentation, polyhydramnios, coagulopathy, stillbirth, antepartum haemorrhage, a Hb level of < 4.8 mmol/l or previous complication in third stage |
5 IU of i.m. oxytocin vs. placebo |
|
High risk of bias |
| Derman et al., 200676 | Two-arm placebo-controlled randomised trial |
There were 1620 parturients randomised in a community setting in India The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients at high risk and inappropriate for home or community births according to India’s Ministry of Health guidelines including those women undergoing elective caesarean section or breech vaginal delivery, or those women who have had a caesarean section previously, a Hb level of < 80 g/l, antepartum haemorrhage, hypertension, multiple pregnancy, history of previous antepartum or PPH, retained placenta, uterine inversion, diabetes mellitus, heart disease, seizures, placenta praevia, asthma or contraindications to misoprostol |
600 µg of p.o. misoprostol vs. placebo |
|
Low risk of bias |
| Dhananjaya and Charishma, 201477 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in India The population comprised women of parity ≤ 4, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with grand multiparity (not defined), rhesus-negative blood group, cardiac disease, diabetes mellitus, bleeding disorder, precipitated labour, overdistended uterus, traumatic PPH, PROM/chorioamnionitis, intrauterine death, previous caesarean section/scar on uterus or inability to obtain the informed consent |
10 IU of i.m. oxytocin vs. 200 µg of i.m. ergometrine |
|
High risk of bias |
| Docherty et al., 198178 | Two-arm active-controlled randomised trial |
There were 50 parturients randomised in a hospital setting in UK The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
10 IU of i.m. oxytocin vs. 500 µg ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Eftekhari et al., 200979 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in Iran The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with multiple pregnancy, prolonged labour > 12 hours, two or more previous caesarean sections, previous uterine rupture, a Hb level of < 80 g/l, who had a history of heart/renal/liver disorders or had a coagulopathy did not enter the study |
400 µg of s.l. misoprostol vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| El Behery et al., 201680 | Two-arm active-controlled double-dummy randomised trial |
There were 180 parturients randomised in a hospital setting in Egypt The population comprised women of nulliparous, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section Exclusion criteria comprised parturients undergoing elective caesarean section, vaginal delivery or general anaesthesia, those women who were multigravida, or with malpresentation, fetal anomalies, placenta praevia, diabetes mellitus, hypertension, pre-eclampsia or cardiac disease |
100 µg of i.v. carbetocin (bolus) vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| El Tahan et al., 201281 | Two-arm placebo-controlled randomised trial |
There were 382 parturients randomised in a hospital setting in Egypt The population comprised women of parity ≤ 3, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with asthma, anaemia, bleeding disorders, cardiac disease, inflammatory disease, bowel disease, multiple pregnancy, pre-eclampsia, placenta praevia, placental abruption, previous APH, previous PPH, grand multiparity (not defined), fibroids, growth restriction, fetal malformations or allergy to prostaglandins |
400 µg of s.l. misoprostol plus 10 IU of i.v. oxytocin (bolus) vs. 10 IU of i.v. oxytocin (bolus) |
|
Low risk of bias |
| Elgafor el Sharkwy, 201383 | Two-arm active-controlled double-dummy randomised trial |
There were 380 parturients randomised in a hospital setting in Egypt The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients undergoing general anaesthesia, or those with coagulopathy, coronary artery disease, hypertension, PPH due to causes other than uterine atony or hypersensitivity to carbetocin |
400 µg s.l. misoprostol plus 20 IU of s.l. i.v. oxytocin (infusion) vs. 100 µg of i.v. carbetocin (bolus) |
|
Low risk of bias |
| El-Refaey et al., 200082 | Two-arm active-controlled randomised trial |
There were 1000 parturients randomised in a hospital setting in UK The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section or water birth, or those women with severe asthma |
500 µg of p.o. misoprostol vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Elsedeek et al., 201284 | Two-arm placebo-controlled randomised trial |
There were 400 parturients randomised in a hospital setting in Egypt The population comprised women of parity ≤ 4, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients undergoing their first elective caesarean section, those unsure of gestation or with hypertension, diabetes mellitus, oligohydramnios, abnormal placenta or abnormal laboratory investigations |
400 µg p.r. misoprostol plus 10 IU of i.v. oxytocin (infusion) vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Enakpene et al., 200785 | Two-arm active-controlled randomised trial |
There were 864 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with pre-eclampsia, hypertension, cardiac disease, severe anaemia, asthma, renal/hepatic disorders, grand multiparity (not defined), multiple pregnancy, polyhydramnios, previous PPH, fibroids or contraindications to misoprostol or ergometrine |
400 µg of p.o. misoprostol vs. 500 µg of i.m. ergometrine |
|
High risk of bias |
| Ezeama et al., 201486 | Two-arm active-controlled double-dummy randomised trial |
There were 300 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with premature labour (i.e. < 28 weeks’ gestation), multiple pregnancy, antepartum haemorrhage, hypertension in pregnancy, severe anaemia or haemoglobinopathy |
10 IU of i.m. oxytocin vs. 500 µg of i.m. ergometrine |
|
Low risk of bias |
| Fararjeh et al., 200387 | Two-arm active-controlled randomised trial |
There were 97 parturients randomised in a hospital setting in Turkey The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section or instrumental delivery, or those with premature labour (i.e. < 37 weeks’ gestation), post maturity (i.e. > 43 weeks’ gestation), grand multiparity (more than four), twin pregnancy, growth restriction, macrosomia, a Hb level of < 100 g/l, systemic disorder, prolonged third stage, manual removal of placenta or additional lacerations due to episiotomy or where it took > 30 minutes to repair lacerations after episiotomy |
400 µg of p.r. misoprostol vs. 200 µg of ergometrine plus 10 IU of i.m. oxytocin |
|
High risk of bias |
| Fazel et al., 201388 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in Iran The population comprised women of parity ≤ 3, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with twin pregnancy, fetal distress, pregnancy-induced hypertension, oligohydramnios, polyhydramnios, macrosomia, grand multiparity (≥ 4), HELLP syndrome, coagulopathy, asthma, heart/lung/liver disease, previous more than one caesarean section, previous myomectomy, previous other abdominal operations, febrile diseases or sensitivity to prostaglandins |
400 µg of p.r. misoprostol vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Fekih et al., 200989 | Two-arm active-controlled randomised trial |
There were 250 parturients randomised in a hospital setting in Tunisia The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean section Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, or those with placenta praevia, retroplacental clot, multiple pregnancy, premature labour (i.e. < 32 weeks’ gestation), intrauterine death, a Hb level of < 80 g/l, coagulopathy, HELLP syndrome, antepartum haemorrhage, ruptured uterus, previous more than two caesareans or other uterine scar, prolonged labour (i.e. > 12 hours) or pyrexia |
200 µg s.l. misoprostol plus 20 IU of i.v. oxytocin (bolus plus infusion) vs. 20 IU of i.v. oxytocin (bolus plus infusion) |
|
High risk of bias |
| Fenix, 201290 | Two-arm active-controlled double-dummy randomised trial |
There were 75 parturients randomised in a hospital setting in the Philippines The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with pre-existing hypertension, pre-eclampsia, diabetes mellitus, asthma, cardiac/renal diseases, coagulopathy, abnormal laboratory tests or allergy to the study medication |
100 µg of i.v. carbetocin (bolus) vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Fu et al., 200391 | Two-arm controlled randomised trial |
There were 156 parturients randomised in a hospital setting in China The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
400 µg of p.o. misoprostol vs. no treatment |
|
High risk of bias |
| Garg et al., 200592 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women who were primigravid, of a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of p.o. misoprostol vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Gavilanes et al., 201593 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in Ecuador The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with a Hb level of < 80 g/l, multiple pregnancy, polyhydramnios, previous uterine rupture, bleeding disorders, intrauterine death or hyperthermia (i.e. > 38.5 °C) |
400 µg of s.l. misoprostol vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Gerstenfeld and Wing, 200194 | Two-arm placebo-controlled randomised trial |
There were 400 parturients randomised in a hospital setting in USA The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with multiple pregnancy, coagulopathy, a Hb level of < 70 g/l, indication for caesarean section or contraindication to prostaglandin or oxytocin use |
400 µg of p.r. misoprostol vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Gülmezoglu et al., 200195 | Two-arm active-controlled double-blind randomised trial |
There were 18,530 parturients randomised in hospital settings in Argentina, China, Egypt, Ireland, Nigeria, South Africa, Switzerland, Thailand and Vietnam The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective or emergency caesarean section after randomisation, or those with asthma, severe chronic allergic conditions, abortion, pyrexia (i.e. > 38 °C) or inability to give consent |
600 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin or i.v. (bolus) |
|
Low risk of bias |
| Gupta et al., 200696 | Two-arm active-controlled double-blind randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of p.r. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Hamm et al., 200597 | Two-arm placebo-controlled randomised trial |
There were 352 parturients randomised in a hospital setting in the USA The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria were not specified |
200 µg of s.l. misoprostol plus 20 IU of i.v. oxytocin (infusion) vs. 20 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Harriott et al., 200998 | Two-arm active-controlled randomised trial |
There were 140 parturients randomised in a hospital setting in the West Indies The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with previous PPH, hypertension, previous caesarean, intrauterine death, sepsis/pyrexia (i.e. > 38 °C), antepartum haemorrhage or a Hb level of < 80 g/l |
500 µg of ergometrine plus 10 IU of i.m. oxytocin vs. 400 µg of p.r. misoprostol |
|
High risk of bias |
| Hofmeyr et al., 199899 | Two-arm placebo-controlled randomised trial |
There were 500 parturients randomised in a hospital setting in South Africa The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing augmentation of labour, or those with hypertension, diabetes mellitus or previous caesarean |
400 µg of p.o. misoprostol vs. placebo |
|
High risk of bias |
| Hofmeyr et al., 2001100 | Two-arm placebo-controlled randomised trial |
There were 600 parturients randomised in a hospital setting in South Africa The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of p.o. misoprostol vs. placebo |
|
High risk of bias |
| Hofmeyr et al., 2011101 | Two-arm placebo-controlled randomised trial |
There were 1103 parturients randomised in a hospital setting in South Africa, Uganda and Nigeria The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those who declined participation or were unable to consent, were too ill or distressed to participate or with an unviable pregnancy |
400 µg of s.l. misoprostol plus 10 IU of i.m. oxytocin vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Høj et al., 2005102 | Two-arm placebo-controlled randomised trial |
There were 661 parturients randomised in a community setting in Guinea-Bissau The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of s.l. misoprostol vs. placebo |
|
Low risk of bias |
| Hong et al., 2007103 | Two-arm placebo-controlled randomised trial |
There were 214 parturients randomised in a hospital setting in Korea The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by caesarean (unspecified whether elective or emergency) Exclusion criteria were not specified |
20 IU of i.v. oxytocin (infusion) vs. 400 µg of p.r. misoprostol plus 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Is et al., 2012104 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
400 µg of p.r. misoprostol vs. an unspecified dose of i.m. ergometrine |
|
High risk of bias |
| Jago et al., 2007105 | Two-arm active-controlled randomised trial |
There were 510 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those requiring epidural analgesia or with hypertension in pregnancy, existing hypertension, chronic renal disease, diabetes mellitus, vascular diseases, cardiac disease, anticoagulation therapy or allergy to ergometrine or oxytocin |
500 µg of i.m. ergometrine vs. 10 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Jangsten et al., 2011106 | Two-arm controlled randomised trial |
There were 1802 parturients randomised in a hospital setting in Sweden The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section, or those who were non-Swedish speaking or with previous PPH, pre-eclampsia, grand multiparity (> four) or intrauterine death |
10 IU of i.v. oxytocin (bolus) vs. no treatment |
|
High risk of bias |
| Jerbi et al., 2007107 | Two-arm controlled randomised trial |
There were 130 parturients randomised in a hospital setting in Tunisia The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with placenta praevia, antepartum haemorrhage, non-cephalic presentation, intrauterine death, grand multiparity, (more than five), fibroids, anticoagulation therapy, previous PPH or previous caesarean section |
5 IU of i.v. oxytocin (bolus) vs. no treatment |
|
High risk of bias |
| Jirakulsawas and Khooarmompattana, 2000108 | Two-arm active-controlled randomised trial |
There were 140 parturients randomised in a hospital setting in Thailand The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
600 µg of p.o. misoprostol vs. 200 µg of i.m. ergometrine |
|
High risk of bias |
| Karkanis et al., 2002109 | Two-arm active-controlled randomised trial |
There were 238 parturients randomised in a hospital setting in Canada The population comprised women of parity ≤ 5, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with coagulopathy, anticoagulation therapy, previous PPH or previous caesarean section |
400 µg of p.r. misoprostol vs. 5 IU of i.v. oxytocin (bolus) or i.m. |
|
High risk of bias |
| Kerekes and Domokos, 1979110 | Three-arm controlled randomised trial |
There were 140 parturients randomised in a hospital setting in Hungary The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
200 µg of i.v. ergometrine (bolus) vs. no treatment |
|
High risk of bias |
| Khan et al., 1995111 | Two-arm active-controlled double-blind randomised trial |
There were 2040 parturients randomised in a hospital setting in the United Arab Emirates The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour, caesarean section or instrumental delivery, or requiring general anaesthesia, epidural or diazepam, or those with antenatal hypertension (≥ 160/100 mmHg), hypertension on antihypertensive drugs, multiple pregnancy, cardiac disease or a Hb level of ≤ 90 g/l |
10 IU of i.m. oxytocin vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Kumru et al., 2005112 | Two-arm active-controlled randomised trial |
There were 55 parturients randomised in a hospital setting in Turkey The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean section Exclusion criteria comprised parturients with multiple pregnancy, hypertension or vascular diseases |
10 IU of i.v. oxytocin (bolus plus infusion) vs. 200 µg of ergometrine plus 10 IU of i.v. oxytocin (bolus plus infusion) |
|
High risk of bias |
| Kundodyiwa et al., 2001113 | Two-arm placebo-controlled randomised trial |
There were 500 parturients randomised in a hospital setting in Zimbabwe The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, antepartum haemorrhage, coagulopathy, multiple pregnancy, asthma or allergies to prostaglandins or oxytocin |
400 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Lam et al., 2004114 | Two-arm active-controlled randomised trial |
There were 60 parturients randomised in a hospital setting in Hong Kong The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with antepartum haemorrhage, anaemia, two or more surgical terminations, previous manual removal of placenta, previous PPH or previous third-stage complications |
500 µg of ergometrine plus 5 IU of i.v. oxytocin (bolus) vs. 600 µg of s.l. misoprostol |
|
High risk of bias |
| Lapaire et al., 2006115 | Two-arm active-controlled double-blind randomised trial |
There were 56 parturients randomised in a hospital setting in Switzerland The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with fetal distress, fetal malformations, pre-eclampsia, HELLP syndrome, coagulopathy, severe systemic disorders, an American Society of Anaesthetists physical status of ≥ III, severe asthma, previous myomectomy, pyrexia (i.e. > 38.5 °C) or hypersensitivity to prostaglandins |
25 IU of i.v. oxytocin (bolus plus infusion) vs. 800 µg of p.o. misoprostol plus 5 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Leung et al., 2006116 | Two-arm active-controlled double-dummy randomised trial |
There were 329 parturients randomised in a hospital setting in Hong Kong The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients requiring prophylactic oxytocin infusion, or those with pre-existing hypertension, pre-eclampsia, asthma, cardiac/renal/liver diseases, grand multiparity or fibroids |
100 µg of i.m. carbetocin vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
Low risk of bias |
| Lokugamage et al., 2001117 | Two-arm active-controlled randomised trial |
There were 40 parturients randomised in a hospital setting in UK The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean section Exclusion criteria comprised parturients with two or more previous caesarean sections or previous uterine rupture |
10 IU of i.v. oxytocin (bolus) vs. 500 µg of p.o. misoprostol |
|
High risk of bias |
| Lumbiganon et al., 1999118 | Three-arm active-controlled double-dummy randomised trial |
There were 597 parturients randomised in a hospital setting in South Africa and Thailand The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section or abortion, or those with asthma, other severe chronic allergic conditions a contraindication to the use of misoprostol or if they were not willing or able to give informed consent |
600 µg or 400 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
Low risk of bias |
| Maged et al., 2015119 | Two-arm active-controlled double-dummy randomised trial |
There were 200 parturients randomised in a hospital setting in Egypt The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with placenta praevia, coagulopathy, pre-eclampsia, cardiac/renal/liver disorders, epilepsy or known hypersensitivity to oxytocin or carbetocin |
100 µg of i.m. carbetocin vs. 5 IU of i.m. oxytocin |
|
High risk of bias |
| McDonald et al., 1993120 | Two-arm active-controlled double-blind randomised trial |
There were 3497 parturients randomised in a hospital setting in Australia The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or requiring general anaesthetic for instrumental delivery, or those with hypertension in labour (i.e. >150/100 mmHg), antenatal hypertension, maternal distress, advanced stage in labour, language barrier, fetal abnormality, intrauterine death or medical disorder |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. 10 IU of i.m. oxytocin |
|
Low risk of bias |
| Mitchell et al., 1993121 | Two-arm active-controlled double-blind randomised trial |
There were 461 parturients randomised in a hospital setting in the UK The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section, or those with significant hypertension or cardiac disease |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. 5 IU of i.m. oxytocin |
|
High risk of bias |
| Mobeen et al., 2011122 | Two-arm placebo-controlled randomised trial |
There were 1119 parturients randomised in a community setting in Pakistan The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with hypertension, non-cephalic presentation, polyhydramnios, previous caesarean section, multiple pregnancy, intrauterine death, antepartum haemorrhage or a Hb level of > 80 g/l |
600 µg of p.o. misoprostol vs. placebo |
|
Low risk of bias |
| Moertl et al., 2011123 | Two-arm active-controlled double-blind randomised trial |
There were 84 parturients randomised in a hospital setting in Austria The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients requiring general anaesthesia, or those with placenta praevia, placental abruption, multiple pregnancy, pre-eclampsia, gestational diabetes mellitus, pre-existing insulin-dependent diabetes mellitus, cardiovascular/renal disorders, hypo/hyperthyroidism or women on cardiovascular system medications |
100 µg of i.v. carbetocin (bolus) vs. 5 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Moir and Amoa, 1979124 | Two-arm active-controlled randomised trial |
There were 88 parturients randomised in a hospital setting in the UK The population comprised women who were primigravid, of a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
500 µg of i.v. ergometrine (bolus) vs. 10 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Moodie and Moir, 1976125 | Two-arm active-controlled randomised trial |
There were 148 parturients randomised in a hospital setting in the UK The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
500 µg of i.v. ergometrine (bolus) vs. 5 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Mukta and Sahay, 2013126 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or those with eclampsia, asthma, epilepsy, cardiac/kidney disorder or coagulopathy |
600 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Musa et al., 2015127 | Two-arm active-controlled double-dummy randomised trial |
There were 235 parturients randomised in a hospital setting in Nigeria The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing planned instrumental birth, or those who received oxytocin and/or misoprostol other than in the third stage of labour, or those with grand multiparity (more than four), multiple pregnancy, fibroids, polyhydramnios, pre-eclampsia, eclampsia, hypertension, cardiac disorder, asthma, antepartum haemorrhage previous PPH, prolonged rupture of membranes or a Hb level of < 100 g/l |
600 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Nasr et al., 2009128 | Two-arm active-controlled double-dummy randomised trial |
There were 514 parturients randomised in a hospital setting in Egypt The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with antepartum haemorrhage, coagulopathy, hypertension in pregnancy or the need for anticoagulants |
800 µg of p.r. misoprostol vs. 5 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Ng et al., 2001129 | Two-arm active-controlled randomised trial |
There were 2058 parturients randomised in a hospital setting in Hong Kong The population comprised women of parity ≤ 3, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage of labour, or those with pre-eclampsia, cardiac disorder, asthma, grand multiparity (> 3), fibroids or contraindications to the use of either misoprostol or Syntometrine |
600 µg of p.o. misoprostol vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Ng et al., 2007130 | Two-arm active-controlled double-dummy randomised trial |
There were 360 parturients randomised in a hospital setting in Hong Kong The population comprised women of parity ≤ 3, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre-eclampsia, cardiac disorder, asthma, grand multiparity (> 3), fibroids or contraindications to the use of either misoprostol or Syntometrine |
400 µg of p.o. misoprostol vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
Low risk of bias |
| Nirmala et al., 2009131 | Two-arm active-controlled randomised trial |
There were 120 parturients randomised in a hospital setting in Malaysia The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients aged < 18 years, or those with cardiac disorder, hypertension requiring treatment, liver/renal/vascular/endocrine disorder (excluding gestational diabetes mellitus) or hypersensitivity to oxytocin or carbetocin |
100 µg of i.m. carbetocin vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Nordström et al., 1997132 | Two-arm placebo-controlled randomised trial |
There were 1000 parturients randomised in a hospital setting in Sweden The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
10 IU of i.v. oxytocin (bolus) vs. placebo |
|
Low risk of bias |
| Oboro and Tabowei, 2003133 | Two-arm active-controlled double-dummy randomised trial |
There were 496 parturients randomised in a hospital setting in Nigeria The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with previous caesarean, a Hb level of < 80 g/l, previous PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, fibroids or precipitate labour |
10 IU of i.m. oxytocin vs. 600 µg of p.o. misoprostol |
|
Low risk of bias |
| Ogunbode et al., 1979134 | Three-arm active-controlled randomised trial |
There were 144 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, multiple pregnancy, polyhydramnios or vaginal lacerations |
200 µg or 500 µg of i.m. ergometrine vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Orji et al., 2008135 | Two-arm active-controlled randomised trial |
There were 600 parturients randomised in a hospital setting in Nigeria The population comprised women of parity ≤ 6, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertension in pregnancy, a packed cell volume of < 30%, previous PPH, haemoglobinopathy or cardiac disorder |
10 IU of i.v. oxytocin (bolus) vs. 250 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Ortiz-Gómez et al., 2013136 | Three-arm active-controlled randomised trial |
There were 156 parturients randomised in a hospital setting in Spain The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with comorbidities, refractory hypotension caused by neuraxial blockage, vasoactive drugs needed to control haemodynamic issues or multiple pregnancy |
100 µg of i.v. carbetocin (bolus) vs. 61 IU of i.v. oxytocin (bolus plus infusion) |
|
High risk of bias |
| Owonikoko et al., 2011137 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, antepartum haemorrhage, cardiac/renal/liver disorders, coagulopathy, asthma, glaucoma, pre-eclampsia, eclampsia, prolonged labour or contraindications to administration of prostaglandins |
20 IU of i.v. oxytocin (infusion) vs. 400 µg of s.l. misoprostol |
|
High risk of bias |
| Parsons et al., 2006138 | Two-arm active-controlled randomised trial |
There were 450 parturients randomised in a hospital setting in Ghana The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins |
10 IU of i.m. oxytocin vs. 800 µg of p.o. misoprostol |
|
High risk of bias |
| Parsons et al., 2007139 | Two-arm active-controlled randomised trial |
There were 450 parturients randomised in a hospital setting in Ghana The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins |
10 IU of i.m. oxytocin vs. 800 µg of p.r. misoprostol |
|
High risk of bias |
| Penaranda et al., 2002140 | Three-arm active-controlled randomised trial |
There were 78 parturients randomised in a hospital setting in Colombia The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with asthma, multiple pregnancy, intrauterine death, coagulopathy, cervical tear or water in the blood collector |
50 µg of s.l. misoprostol vs. 16 mIU/minute of i.v. oxytocin (infusion) vs. 200 µg of i.m. ergometrine |
|
High risk of bias |
| Prendiville et al., 1988141 | Two-arm controlled randomised trial |
There were 1695 parturients randomised in a hospital setting in UK The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with cardiac disorder, antepartum haemorrhage, non-cephalic presentation, multiple pregnancy and intrauterine death, but after change in the protocol multiple other exclusion criteria were introduced |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Rajaei et al., 2014142 | Two-arm active-controlled double-dummy randomised trial |
There were 400 parturients randomised in a hospital setting in Iran The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with placenta praevia, placental abruption, coagulopathy, previous caesarean section, macrosomia (i.e. > 4 kg), polyhydramnios or uncontrolled asthma |
20 IU of i.v. oxytocin (infusion) vs. 400 µg of p.o. misoprostol |
|
High risk of bias |
| Rashid et al., 2009143 | Two-arm active-controlled randomised trial |
There were 686 parturients randomised in a hospital setting in Saudi Arabia The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section or requiring oxytocin infusion in the third stage, or those with pre-eclampsia, cardiac disorder, hypertension on treatment, antepartum haemorrhage, pre-term labour (i.e. < 37 weeks’ gestation), post maturity (i.e. > 42 weeks’ gestation) or a Hb level of ≤ 90 g/l |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. 10 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Ray et al., 2001144 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section, or those with pre-term labour (i.e. > 32 weeks’ gestation), prolonged labour, antepartum haemorrhage, pre-eclampsia, intrauterine death, multiple pregnancy, epilepsy, asthma, cardiac/kidney disorder, coagulopathy or anaemia |
400 µg of p.o. misoprostol vs. an unspecified dose and route of ergometrine |
|
High risk of bias |
| Reyes, 2011145 | Two-arm active-controlled randomised trial |
There were 144 parturients randomised in a hospital setting in Panama The population comprised women of parity ≥ 5, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with coagulopathy, unknown parity or known allergy to carbetocin |
100 µg of i.v. carbetocin (bolus) vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Reyes and Gonzalez, 2011146 | Two-arm active-controlled double-dummy randomised trial |
There were 57 parturients randomised in a hospital setting in Panama The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both caesarean section and vaginal delivery Exclusion criteria comprised parturients with HELLP syndrome, blood dyscrasia or multiple pregnancy |
100 µg of i.v. carbetocin (bolus) vs. 10 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Rogers et al., 1998147 | Two-arm controlled randomised trial |
There were 1512 parturients randomised in a hospital setting in UK The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing augmentation of labour or instrumental delivery or requiring epidural analgesia, or those with placenta praevia, previous PPH, antepartum haemorrhage, a Hb level of < 100 g/l or mean corpuscular volume of < 75 fl, non-cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than five), fibroids, anticoagulation therapy, pre-term labour (i.e. < 32 weeks’ gestation) or contraindications to any of the drugs |
Unspecified dose of ergometrine plus i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Rosseland et al., 2013148 | hree-arm placebo-controlled randomised trial |
There were 76 parturients randomised in a hospital setting in Norway The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section Exclusion criteria comprised parturients with pre-eclampsia, placenta praevia, placenta accreta, von Willebrand disease or other bleeding disorder or a preoperative systolic arterial pressure of < 90 mmHg |
5 IU of i.v. oxytocin (bolus) vs. 100 µg of i.v. carbetocin (bolus) vs. placebo |
|
Low risk of bias |
| Rozenberg et al., 2015149 | Two-arm placebo-controlled randomised trial |
There were 1721 parturients randomised in a hospital setting in France The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with known hypersensitivity to prostaglandins |
400 µg of p.o. misoprostol plus 10 IU of i.v. oxytocin (bolus) vs. 10 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Sadiq et al., 2011150 | Two-arm active-controlled randomised trial |
There were 1865 parturients randomised in a hospital setting in Nigeria The population comprised women of parity ≤ 6, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing instrumental delivery, or those with diabetes mellitus, non-cephalic presentation, anaemia, antepartum haemorrhage, multiple pregnancy, grand multiparity (> six) or known allergy |
10 IU of i.v. oxytocin (bolus) vs. 600 µg of p.o. misoprostol |
|
High risk of bias |
| Samimi et al., 2013151 | Two-arm active-controlled double-blind randomised trial |
There were 216 parturients randomised in a hospital setting in Iran The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with hypertension, pre-eclampsia, uterine rupture, cervical tear, asthma, cardiovascular/renal/liver disorders, grand multiparity (not defined), fibroids or previous PPH |
100 µg of i.m. carbetocin vs. 200 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Shrestha et al., 2011152 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in Nepal The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with polyhydramnios, chorioamnionitis, preterm labour, previous caesarean, asthma, cardiac disorder or contraindication/hypersensitivity to the use of prostaglandin and uterotonics |
1000 µg of p.r. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Singh et al., 2009153 | Four-arm active-controlled double-dummy randomised trial |
There were 300 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing augmentation of labour, or those with intrauterine death, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disorder, Rhesus-negative mother, hypertension, a Hb level of < 70 g/l or hypersensitivity/contraindication to prostaglandins |
400 µg or 600 µg of s.l. misoprostol vs. 5 IU of i.v. oxytocin (bolus) vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Soltan et al., 2007154 | Four-arm active-controlled randomised trial |
There were 1228 parturients randomised in a hospital setting in Egypt The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, blood disorders, chorioamnionitis, placenta praevia or placental abruption |
200 µg of i.m. ergometrine vs. 600–1000 µg of s.l. misoprostol |
|
High risk of bias |
| Sood and Singh, 2012155 | Two-arm placebo-controlled randomised trial |
There were 174 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria were not specified |
400 µg of s.l. misoprostol plus 20 IU of i.v. oxytocin (infusion) vs. 20 IU of i.v. oxytocin (infusion) |
|
Low risk of bias |
| Stanton et al., 2013156 | Two-arm cluster-controlled randomised trial |
There were 1586 parturients randomised in a community setting in Ghana The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
10 IU of i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Su et al., 2009157 | Two-arm active-controlled double-blind randomised trial |
There were 370 parturients randomised in a hospital setting in Singapore The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing elective caesarean section, or those with multiple pregnancy, previous PPH, coagulopathy, coronary artery disease, hypertension or hypersensitivity/contraindications to the use of Syntometrine or carbetocin |
100 µg of i.m. carbetocin vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
Low risk of bias |
| Sultana and Khatun, 2007158 | Two-arm active-controlled randomised trial |
There were 400 parturients randomised in a hospital setting in Bangladesh The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with previous caesarean |
400 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Surbek et al., 1999159 | Two-arm placebo-controlled randomised trial |
There were 65 parturients randomised in a hospital setting in Switzerland The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with multiple pregnancy, pre-eclampsia, previous PPH or antepartum haemorrhage |
600 µg of p.o. misoprostol vs. placebo |
|
Low risk of bias |
| Tewatia et al., 2014160 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in India The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with grand multiparity (> 4), anaemia, malpresentation, polyhydramnios, antepartum haemorrhage, liver/renal disorder, previous caesarean, previous PPH, uterine anomaly, traumatic PPH or contraindications to the use of misoprostol or oxytocin |
10 IU of i.v. oxytocin (infusion) vs. 600 µg of s.l. misoprostol |
|
High risk of bias |
| Thilaganathan et al., 1993161 | Two-arm controlled randomised trial |
There were 193 parturients randomised in a hospital setting in the UK The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those with grand multiparity (not defined), malpresentation, multiple pregnancy, previous caesarean, previous PPH, antepartum haemorrhage, hypertension in pregnancy, intrauterine death, preterm rupture of membranes, cervical lacerations or third-degree perineal tears |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. no treatment |
|
High risk of bias |
| Ugwu et al., 2014162 | Two-arm active-controlled randomised trial |
There were 120 parturients randomised in a hospital setting in Nigeria The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, pre-eclampsia, eclampsia, undiagnosed vaginal bleeding, prolonged labour, prolonged obstructed labour, cardiac/renal/liver disorders or fever |
400 µg of s.l. misoprostol plus 20 IU of oxytocin (infusion) vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Un Nisa et al., 2012163 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in India The population comprised women of parity 2–4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with previous PPH, multiple pregnancy, previous caesarean section, macrosomia, pre-eclampsia, diabetes mellitus, cardiac/lung/bleeding/clotting disorders or taking anticoagulants |
10 IU of i.v. oxytocin (bolus) vs. 500 µg of ergometrine plus 5 IU of i.m. oxytocin |
|
High risk of bias |
| Uncu et al., 2015164 | Five-arm controlled randomised trial |
There were 248 parturients randomised in a hospital setting in Turkey The population comprised women of parity ≤ 5, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta praevia, previous PPH, antepartum haemorrhage, non-cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than five), fibroids, pre-eclampsia or anticoagulation therapy |
400–800 µg of p.o. misoprostol, p.v. or p.r. vs. no treatment |
|
High risk of bias |
| Vagge et al., 2014165 | Two-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those women with cardiac disorder in pregnancy, uterine tumour in pregnancy, secondary PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, anaemia, coagulopathy, antepartum haemorrhage, previous PPH, prolonged labour, precipitate labour or known allergic or hypersensitivity reaction to prostaglandins |
10 IU of i.v. oxytocin (infusion) vs. 800 µg of p.r. misoprostol |
|
High risk of bias |
| Vaid et al., 2009166 | Three-arm active-controlled randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of parity ≤ 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients with grand multiparity (> 4), multiple pregnancy, preterm labour (i.e. < 32 weeks’ gestation), HELLP syndrome, polyhydramnios, coagulopathy, asthma, cardiac/renal disorder, epilepsy, hypertension, a Hb level of < 80 g/l or known drug allergy |
400 µg of s.l. misoprostol vs. 200 µg of i.m. ergometrine |
|
High risk of bias |
| Verma et al., 2006167 | Two-arm active-controlled double-dummy randomised trial |
There were 200 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria were not specified |
400 µg of s.l. misoprostol vs. 200 µg of i.m. ergometrine |
|
High risk of bias |
| Vimala et al., 2004168 | Two-arm active-controlled randomised trial |
There were 120 parturients randomised in a hospital setting in India The population comprised women of parity < 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with preterm labour (i.e. < 37 weeks’ gestation), grand multiparity (> 5), multiple pregnancy, hypertension in pregnancy, a Hb level of < 80 g/l or known hypersensitivity to prostaglandins |
400 µg of s.l. misoprostol vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
| Vimala et al., 2006169 | Two-arm active-controlled randomised trial |
There were 100 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective or emergency caesarean Exclusion criteria comprised parturients with multiple pregnancy, antepartum haemorrhage, polyhydramnios, prolonged labour (i.e. > 12 hours), more than one previous caesarean section, previous uterine rupture, cardiac/liver/renal disorder, coagulopathy or a Hb level of < 80 g/l |
400 µg of s.l. misoprostol vs. 20 IU of i.v. oxytocin (infusion) |
|
High risk of bias |
| Walley et al., 2000170 | Two-arm active-controlled double-dummy randomised trial |
There were 401 parturients randomised in a hospital setting in Ghana The population comprised women of parity ≤ 5, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with grand multiparity (> 5), multiple pregnancy, preterm labour (i.e. < 32 weeks’ gestation), hypertension in pregnancy, HELLP syndrome, polyhydramnios, previous PPH, coagulopathy, precipitate labour, chorioamnionitis, a Hb level of < 80 g/l or a known hypersensitivity to prostaglandins |
400 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin |
|
Low risk of bias |
| Whigham et al., 2014171 | Two-arm active-controlled double-blind randomised trial |
There were 58 parturients randomised in a hospital setting in Australia The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section Exclusion criteria comprised parturients undergoing elective caesarean section or requiring general anaesthesia, or those with vascular/liver/renal disorders, preterm labour (i.e. < 37 weeks’ gestation), placenta praevia, placental abruption, previously more than two caesarean sections or an adverse reaction to carbetocin or oxytocin |
100 µg of i.v. carbetocin (bolus) vs. 5 IU of i.v. oxytocin (bolus) |
|
High risk of bias |
| Yuen et al., 1995172 | Two-arm active-controlled double-blind randomised trial |
There were 1000 parturients randomised in a hospital setting in Hong Kong The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage of labour or those with pre-eclampsia or cardiac disorder |
500 µg of ergometrine plus 5 IU of i.m. oxytocin vs. 10 IU of i.m. oxytocin |
|
High risk of bias |
| Zachariah et al., 2006173 | Three-arm active-controlled randomised trial |
There were 2023 parturients randomised in a hospital setting in India The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery Exclusion criteria comprised parturients undergoing caesarean section, or those women with asthma, cardiac disorder, rhesus factor incompatibility or hypertension |
400 µg of p.o. misoprostol vs. 10 IU of i.m. oxytocin vs. 200 µg of i.v. ergometrine (bolus) |
|
High risk of bias |
Appendix 3 Reference list for excluded studies
Abdel-Aleem 1993
Abdel-Aleem H, Abol-Oyoun EM, Moustafa SA, Kamel HS, Abdel-Wahab HA. Carboprost trometamol in the management of the third stage of labor. Int J Gynaecol Obstet 1993;42:247–50.
Abdel-Aleem 1997
Abdel-Aleem H, Mostafa SAM, Makarem MH, Abol-Oyoun EM, Makhlouf A, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum haemorrhage. In Research Activities on Reproductive Health: Annual Report of Assiut University Department of Obstetrics and Gynaecology November 1997. Assiut: Faculty of Medicine, Assiut University; 1997.
Abdel-Aleem 2013
Abdel-Aleem H, Alhusaini TK, Abdel-Aleem MA, Menoufy M, Gülmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. J Matern Fetal Neonatal Med 2013;26:1705–9.
Abdollahy 2000
Abdollahy F. Comparison effect of oxytocin and normal salin injection intra umbelical venuse. Gynaecol Endocrinol 2000;14(Suppl. 2):49.
Al-Harazi 2009
Al-Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum haemorrhage. Saudi Med J 2009;30:912–6.
Anandakrishnan 2013
Anandakrishnan S, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective Cesarean delivery: a randomized controlled trial to determine the effective dose, part 2. Can J Anaesth 2013;60:1054–60.
Anjaneyulu 1988
Anjaneyulu R, Pk D, Jain S, Cr K, Vijaya R, Ks R. Prophylactic use of 15(S) 15-methyl-PGF2α, by intramuscular route – a controlled clinical trial. Acta Obstet Gynecol Scand 1988;67:9–11.
Anvaripour 2013
Anvaripour A, Shahryari H, Ahmadi S, Ghasemi S, Mirzaei K. Comparison the effects of oxytocin and methylergonovine in elective caesarean section under spinal anesthesia. Arch Gynecol Obstet 2013;287:979–83. https://doi.org/10.1007/s00404-012-2671-1
Athavale 1991
Athavale RD, Nerurkar NM, Dalvi SA, Bhattacharya MS. Umbilical vein oxytocin in the management of third stage of labour. J Postgrad Med 1991;37:219–20.
Ayedi 2011
Ayedi M, Jarraya A, Smaoui M, Zouari J, Smaoui L, Kolsi K. Effect of tranexamic acid on post partum haemorrhage by uterine atony: a preliminary result of a randomised, placebo controlled trial. Eur J Anaesthesiol 2011;28:165.
Ayedi 2011a
Ayedi M, Zouche I, Smaoui L, Bouaziz I, Smaoui M, Kolsi K. Comparison of 2 versus 5 units of oxytocin in caesarean section: 11AP2-6. Eur J Anaesthesiol 2011;28:159–60.
Aziz 2014
Aziz S, Kazi S, Haq G, Soomro N. Oral misoprostol versus oxytocin in the management of third stage of labour. J Pak Med Assoc 2014;64:428–32.
Bader 2000
Bader W, Ast S, Hatzmann W. [The significance of acupuncture in the third stage of labour.] Dtsch Z Akupunkt 2000;43:264–8.
Bader 2000a
Bader W, Ast S, Reinehr J, Hackmann J, Hatzmann W. [Oxytocin versus acupuncture in the third stage of labour – a prospective randomised study.] Geburtshilfe Frauenheilkd 2000;60(Suppl. 1):73.
Badhwar 1991
Badhwar L, Singh K, Sethi N, Gupta I, Aggarwal N. The value of nipple stimulation in the management of third stage of labour. Int J Gynaecol Obstet 1991;36:16.
Bai 2014
Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high-risk patients undergoing cesarean delivery. Exp Ther Med 2014;7:46–50. https://doi.org/10.3892/etm.2013.1379
Balki 2005
Balki M, Ronayne M, Davies S, Kingdom J, Windrim R, Carvalho J. Oxytocin requirements at cesarean section for failure to progress in labour: a dose-finding study. Anaesthesiology 2005;102(Suppl. 1):10.
Balki 2006
Balki M, Ronayne M, Davies S, Fallah S, Kingdom J, Windrim R, Carvalho JC. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol 2006;107:45–50.
Banovska 2013
Banovska J, Goffard P, Suball M, Origer P, Delatte P, Kapessidou P. Efficiency of temporary balloon occlusion of iliac arteries in patients at high haemorrhagic risk undergoing cesarean section: 11AP4-5. Eur J Anaesthesiol 2013;30:176.
Barbaro 1961
Barbaro CA, Smith GO. Clinical trial of SE505 – a new oxytocic mixture. Aust NZ J Obstet Gynaecol 1961;1:147–50.
Baumgarten 1983
Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post-partum application of sulprostone and other oxytocics. Eur J Obstet Gynecol Reprod Biol 1983;16:181–92.
Bhattacharya 1988
Bhattacharya P, Pk D, Jain S, Cr K, Ks R. Prophylactic use of 15(s)15 methyl pgf2α by intramuscular route for control of postpartum bleeding – a comparative trial with methylergometrine. Acta Obstet Gynecol Scand 1988;67:13–15. https://doi.org/10.1111/aogs.1988.67.s145.13
Bhavana 2013
Bhavana G, Mittal S. Evaluation of efficacy of prophylactic injection tranexamic acid in decreasing blood loss before and after caesarean section. BJOG 2013;120(Suppl. 1):32.
Bider 1991
Bider D, Zolti M, Menashe Y, Dulitzky M, Mashiach S, Ben-Rafael Z. Oxytocin or saline injected intra-umbilically did not influence the third stage of labor. Acta Obstet Gynecol Scand 1991;70:321–3.
Bider 1992
Bider D, Ben-Rafael Z, Dulitzky M, Menashe Y, Mashiach S, Barkai G. Effect of intraumbilical prostaglandin F2 alpha injection on the third stage of labor. J Reprod Med 1992;37:317–19.
Bisri 2011
Bisri Y, Redjeki IS, Himendra A. The comparative of effect of bolus-infusion oxytocine with infusion oxytocine on blood pressure, heart rate, and uterine contraction of women undergoing elective caesarean section with general anaesthesia N2O-sevoflurane: 11AP2-3. Eur J Anaesthesiol 2011;28:159.
Biswas 2007
Biswas A, Bal R, Kundu MK, Kyal A, Halder M. A study of prophylactic use of 15-methyl prostalglandin F2alpha in the active management of third stage of labour. J Indian Med Assoc 2007;105:506, 508–9.
Bivins 1993
Bivins Jr HA, Cope DA, Newman RB, Eller DP. Randomised trial of intraumbilical vein oxytocin. Am J Obstet Gynaecol 1993;168:435.
Bivins 1993a
Bivins HA, Cope DA, Newman RB, Eller DP. Randomized trial of intraumbilical vein oxytocin in midtrimester pregnancy losses. Am J Obstet Gynecol 1993;169:1070–3.
Blum 2010
Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet 2010;375:217–23. https://doi.org/10.1016/S0140-6736(09)61923-1
Bonham 1963
Bonham DG. Intramuscular oxytocics and cord traction in third state of labour. Br Med J 1963;2:1620–3.
Bonis 2012
De Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. J Matern Fetal Neonatal Med 2012;25:732–5. https://doi.org/10.3109/14767058.2011.587920
Cappiello 2006
Cappiello E, Lugo L, Kodali B, Hepner D, Harnett M, Tsen LC. A double-blinded, randomised, placebo-controlled trial of calcium chloride for the augmentation of uterine tone following cesarean delivery. Anaesthesiology 2006;104(Suppl. 1):32.
Carvalho 2004
Carvalho JCA, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obst Gynaecol 2004;104:1005–10.
Catanzarite 1990
Catanzarite VA. Prophylactic intramyometrial carboprost tromethamine does not substantially reduce blood loss relative to intramyometrial oxytocin at routine cesarean section. Am J Perinatol 1990;7:39–42. https://doi.org/10.1055/s-2007-999443
Chaplin 2009
Chaplin AC, George RB, McKeen D, McLeod LC. Up-down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing an elective caesarean delivery. Canadian J Anaesth 2009;56(Suppl. 1):S62.
Chaudhuri 2014
Chaudhuri P, Mandi S, Mazumdar A. Rectally administrated misoprostol as an alternative to intravenous oxytocin infusion for preventing post-partum hemorrhage after cesarean delivery. J Obstet Gynaecol Res 2014;40:2023–30. https://doi.org/10.1111/jog.12464
Chestnut 1987
Chestnut DH, Wilcox LL. Influence of Umbilical Vein Administration of Oxytocin on the Third Stage of Labour. Society for Obstetric Anaesthesia and Perinatology, 19th Annual Meeting, Halifax, NS, Canada; 20–3 May 1987.
Chestnut 1987a
Chestnut DH, Wilcox LL. Influence of umbilical vein administration of oxytocin on the third stage of labour: a randomized, double-blind, placebo-controlled study. Am J Obstet Gynaecol 1987;157:160–2.
Chou 1994
Chou MM, MacKenzie IZ. A prospective, double-blind, randomized comparison of prophylactic intramyometrial 15-methyl prostaglandin F2, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. Am J Obstet Gynaecol 1994;171:1356–60.
Chua 1995
Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15-methyl F2 alpha compared with syntometrine for prophylactic use in the third stage of labour. Aust N Z J Obstet Gynaecol 1995;35:413–16.
Chukudebelu 1963
Chukudebelu WO, Marshall AT, Chalmers JA. Use of ‘Syntometrine’ in the third stage of labour. Br Med J 1963;1:1390–1.
Cooper 2004
Cooper GM. A Study to Determine the Cardiovascular Effects of Different Methods of Administering the Oxytocic Drug Syntocinon. Current Controlled Trials. 2004. URL: www.isrctn.com/ISRCTN07452238 (accessed 15 September 2004).
Cordovani 2012
Cordovani D, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose. Can J Anaesth 2012;59:751–7. https://doi.org/10.1007/s12630-012-9728-2
Dagdeviren 2014
Dagdeviren H. Intramuscular Versus Intravenous Prophylactic Oxytocin for Haemorrhage After Vaginal Delivery (oxytocin). ClinicalTrials.gov. 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02080104 (accessed 24 March 2014).
Dahiya 1995
Dahiya P, Puri M, Rathee S. Influence of intraumbilical oxytocin on the third stage of labour. Indian J Med Sci 1995;49:23–7.
Daley 1951
Daley D. The use of intramuscular ergometrine at the end of the second stage of normal labour. J Obstet Gynaecol Br Emp 1951;58:388–97.
Daly 1999
Daly S, Andolina K, Tolosa JE, Roberts N, Wapner R. A randomized controlled trial of misoprostol versus oxytocin in preventing postpartum blood loss. Am J Obstet Gynaecol 1999;180:S68.
Dao 2009
Dao B, Blum J, Barrera G, Cherine Ramadan M, Dabash R, Darwish E, et al. Side effect profiles for misoprostol and oxytocin in the treatment of postpartum haemorrhage. Int J Gynaecol Obstet 2009;107(Suppl. 2):150.
Davies 2005
Davies GAL, Tessier JL, Woodman MC, Lipson A, Hahn PM. Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labour: a randomised controlled trial. Obstet Gynaecol 2005;105:294–9.
De Bonis 2012
De Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. J Matern Fetal Neonatal Med 2012;25:732–5. https://doi.org/10.3109/14767058.2011.587920
Dennehy 1998
Dennehy KC, Rosaeg OP, Cicutti NJ, Krepski B, Sylvain JP. Oxytocin injection after caesarean delivery: intravenous or intramyometrial? Can J Anaesth 1998;45:635–9.
Devi 1988
Devi PK, Sutaria UD, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha for control of postpartum bleeding. Acta Obstet Gynecol Scand 1988;67:7–8.
Diab 1999
Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. J Obstet Gynaecol Res 1999;25:327–32.
Dickinson 2009
Dickinson JE, Doherty DA. Optimization of third-stage management after second-trimester medical pregnancy termination. Am J Obstet Gynaecol 2009;201:303.e1–7.
Dong 2011
Dong Y. Effects of carboprost on prevention of haemorrhage after induced labour with scarred uterus. J Shanghai Jiaotong Univ (Med Sci) 2011;31:1212–5.
Durocher 2012
Durocher J, Blum J, Sheldon WR, Trussell J, Winikoff B. Does the effect of oxytocin prophylaxis on post-partum blood loss depend on route of administration? Int J Gynaecol Obstet 2012;119(Suppl. 3):S332.
Dutta 2000
Dutta DK, Saha KK. Comparative Study on Role of Syntometrine and Prostaglandin in the Prevention of PPH. XVI FIGO World Congress of Obstetrics and Gynaecology, Washington, DC, USA, 3–8 September 2000.
Dweck 2000
Dweck MF, Lynch CM, Spellacy WN. Use of methergine for the prevention of postoperative endometritis in non-elective cesarean section patients. Infect Dis Obstet Gynecol 2000;8:151–4.
Dzuba 2012
Dzuba I, Durocher J, Dilbaz B, Gelisen O, Ngoc NTN, Montesinos R, et al. Route of administration of oxytocin in prevention of postpartum haemorrhage. Int J Gynaecol Obstet 2012;119(Suppl. 3):333.
Elati 2011
Elati A, Elmahaishi MS, Elmahaishi MO, Elsraiti OA, Weeks AD. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG 2011;118:466–73. https://doi.org/10.1111/j.1471-0528.2010.02821.x
Erkkola 1984
Erkkola R, Kero P, Kanto J, Korvenranta H, Nanto V, Peltonen T. Delayed cord clamping in cesarean section with general anesthesia. Am J Perinatol 1984;1:165–9.
Farber 2013
Farber MK. Tranexamic Acid and Thromboelastography During Cesarean Delivery (TA TEG). ClinicalTrials.gov. 2013. URL: https://clinicaltrials.gov/ct2/show/NCT02026297 (accessed 21 January 2014).
Farber 2015
Farber MK, Schultz R, Lugo L, Liu X, Huang C, Tsen LC. The effect of co-administration of intravenous calcium chloride and oxytocin on maternal hemodynamics and uterine tone following cesarean delivery: a double-blinded, randomized, placebo-controlled trial. Int J Obstet Anaesth 2015;24:217–24.
Fatemeh 2011
Foroonzhad F, Sadat Z, Mousavi GA, Hatami L. Maternal haemodynamic effects of oxytocin bolus or infusion in the third stage of labour. Pak J Med Sci 2011;27:656–9.
Fawole 2011
Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double-blind, randomized, placebo-controlled trial of misoprostol and routine uterotonics for the prevention of postpartum haemorrhage. Int J Gynaecol Obstet 2011;112:107–11.
Fawzy 2012
Fawzy AEMA, Swelem M, Abdelrehim AI, Titeli S, Elghazal ZS, El-Gahwagi MM, et al. Active management of third stage of labour by intravenous ergometrine and rectal versus sublingual misoprostol (a double-center study). Alex J Med 2012;48:381–5.
Forster 1957
Forster FMC. A comparative study of ergometrine and ‘methergin’ used in the management of the third stage of labour. M J Austr 1957;2:155–6.
Francis 1965
Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin-ergometrine mixture. Aust N Z J Obstet Gynaecol 1965;5:47–51.
Friedman 1957
Friedman EA. Comparative clinical evaluation of postpartum oxytocics. Am J Obstet Gynecol 1957;73:1306–13.
Fugo 1958
Fugo NW, Dieckmann WJ. A comparison of oxytocic drugs in the management of the placental stage. Am J Obstet Gynecol 1958;76:141–6.
Gai 2004
Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after Caesarean section: a multi-centre, randomized trial. Eur J Obstet Gynaecol Reprod Biol 2004;112:154–7.
Gambling 1994
Gambling D, Dansereau J, Schulz M, Horbay GLA, Waasenaar W. Double-blind, randomised comparison of a single dose of carbetocin vs 8 hours oxytocin infusion after cesarean delivery: safety data. A Canadian multi-center trial. Int J Obstet Anaesth 1994;3:113–4.
Gambling 1994a
Gambling DR, Dansereau J, Wassenaar W, Schulz M, Horbay GLA. Double-blind randomised comparison of a single dose of carbetocin versus 8 hours oxytocin infusion after cesarean delivery: safety data. Anesth Analg 1994;78(Suppl. ):S127.
Gawecka 2014
Gawecka E, Rosseland LA. A secondary analysis of a randomized placebo-controlled trial comparing the analgesic effects of oxytocin with carbetocin: postcesarean delivery morphine equivalents. Anesth Analg 2014;119:1004.
Geller 2004
Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, Derman RJ. Conducting international collaborative research in developing nations. Int J Gynaecol Obstet 2004;87:267–71.
Geller 2008
Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low-risk women delivering in rural India. Int J Gynaecol Obstet 2008;101:94–9. https://doi.org/10.1016/j.ijgo.2007.08.025
George 2010
George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Can J Anaesth 2010;57:578–82. https://doi.org/10.1007/s12630-010-9297-1
Ghulmiyyah 2005
Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleban C, Sibai BM. Effects of intraumbilical vein injection of saline versus oxytocin plus saline on duration of the third stage of labour: a randomized double-blind placebo trial. Am J Obstet Gynecol 2005;193(Suppl.):18.
Ghulmiyyah 2007
Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleben C, Sibai BM. Intraumbilical vein injection of oxytocin and the third stage of labour: randomized double-blind placebo trial. Am J Perinatol 2007;24:347–52.
Gobbur 2011
Gobbur VR, Reddy SV, Bijapur UJ. Efficacy of Tranexamic Acid in Reducing Blood Loss During Lower Segment Caesarean Section. The 54th All India Congress of Obstetrics and Gynaecology, Hyderabad, Andhra Pradesh, India, 5–9 January 2011.
Gohel 2007
Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomised case controlled prospective study. J Obstet Gynaecol India 2007;57:228–30.
Goswami 2013
Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anaemic parturients for lower segment cesarean section: a double-blind randomized case control prospective trial. Saudi J Anaesth 2013;7:427–31.
Groeber 1960
Groeber WR, Bishop EH. Methergine and ergonovine in the third stage of labor. Obstet Gynecol 1960;15:85–8.
Güngördük 2010
Güngördük K, Asicioglu O, Besimoglu B, Güngördük OC, Yildirm G, Ark C, Tekirdağ AI. Using intraumbilical vein injection of oxytocin in routine practice with active management of the third stage of labor: a randomized controlled trial. Obstet Gynecol 2010;116:619–24. https://doi.org/10.1097/AOG.0b013e3181edac6b
Güngördük 2010a
Güngördük K, Asicioglu O, Celikkol O, Olgac Y, Ark C. Use of additional oxytocin to reduce blood loss at elective caesarean section: a randomised control trial. Aust N Z J Obstet Gynaecol 2010;50:36–9. https://doi.org/10.1111/j.1479-828X.2009.01106.x
Güngördük 2011
Güngördük K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol 2011;28:233–40.
Güngördük 2013
Güngördük K, Asicioglu O, Yildirim G, Ark C, Tekirdag AI, Besimoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labour in vaginal delivery? A randomised controlled study. Am J Perinatol 2013;30:407–13.
Gupta 2014
Gupta M, Bhosale U. Comparative study of methylergometrine and low dose carboprost (PGF2-x) in active management of 3rd stage labour. BJOG 2014;121(Suppl. 2):139.
Habek 2007
Habek D, Franicević D. Intraumbilical injection of uterotonics for retained placenta. Int J Gynaecol Obstet 2007;99:105–9.
Hacker 1979
Hacker NF, Biggs JS. Blood pressure changes when uterine stimulants are used after normal delivery. Br J Obstet Gynaecol 1979;86:633–6.
Häivä 1994
Häivä L, Hartikainen A. [Pharmacological management of third stage of labour in primiparae and multiparae.] Suomen Lääkärilehti 1994;49:3442–4.
Halder 2013
Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre- and postoperative haemoglobin level: a case-control study. J Indian Med Assoc 2013;111:184–6.
Hoffman 2004
Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labour. Am J Obstet Gynaecol 2004;191:82.
Hoffman 2006
Hoffman M, Castagnola D, Naqvi F. A randomised trial of active versus expectant management of the third stage of labour. Am J Obstet Gynaecol 2006;195:107.
Hofmeyr 1997
Hofmeyr GJ, de Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for Third Stage of Labour Management: A Double Blind, Placebo Controlled Clinical Trial, Proceedings of the 16th Conference on Priorities in Perinatal Care, South Africa, 1997.
Hofmeyr 1998a
Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol 1998;105:971–5.
Hofmeyr 2000
Hofmeyr GJ, Nikodem VC, De Jager M, Drakely AJ. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. J Obstet Gynaecol 2000;20(Suppl. 1):40–1.
Hofmeyr 2004
Hofmeyr GJ, Ferreira S, Nikodem VC, Mangesi L, Singata M, Jafta Z, et al. Misoprostol for treating postpartum haemorrhage: a randomized controlled trial [ISRCTN72263357.] BMC Pregnancy Childbirth 2004;4:16.
Hofmeyr 2008
Hofmeyr GJ. Misoprostol for preventing postpartum haemorrhage. ClinicalTrials.gov. 2008. URL: https://clinicaltrials.gov/ct2/show/NCT00124540?term=Misoprostol+for+preventing+postpartum+haemorrhage.&rank=1 (accessed 20 February 2008).
Howard 1964
Howard WF, McFadden PR, Keettel WC. Oxytocic drugs in fourth stage of labor. JAMA 1964;189:411–13.
Huh 2000
Huh W, Chelmow D, Malone FD. A randomised, double-blinded, placebo controlled trial of oxytocin at the beginning versus the end of the third stage of labour for prevention of postpartum haemorrhage. Am J Obstet Gynaecol 2000;182:S130.
Huh 2004
Huh WK, Chelmow D, Malone FD. A double-blinded, randomised, controlled trial of oxytocin at the beginning versus the end of the third stage of labour for prevention of postpartum haemorrhage. Gynecol Obstet Invest 2004;58:72–6.
Hunt 2013
Hunt BJ. Tranexamic acid for the treatment of postpartum haemorrhage – preliminary results of the woman trial. Transfus Med 2013;23(Suppl. 1):7.
Ilancheran 1990
Ilancheran A, Ratnam SS. Effect of oxytocics on prostaglandin levels in the third stage of labour. Gynecol Obstet Invest 1990;29:177–80. https://doi.org/10.1159/000293371
Irons 1994
Irons DW, Sriskandabalan P, Bullough CH. A simple alternative to parenteral oxytocics for the third stage of labor. Int J Gynaecol Obstet 1994;46:15–18.
Jackson 2001
Jackson KW Jr, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum haemorrhage. Am J Obstet Gynaecol 2001;185:873–7.
Jiang 2001
Jiang Q, Wang P, Cao W. Effect on different doses of misoprostol to prevent postpartum haemorrhage. Chin Nurs Res 2001;15:313–14.
Jin 2000
Jin LJ, Zhou L. Application of anus misoprostol to decrease the volume of post partum haemorrhage. J Pract Nurs 2000;16:9–10.
Jolivet 1978
Jolivet A, Robyn C, Huraux-Rendu C, Gautray JP. [Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period.] J Gynecol Obstet Biol Reprod 1978;7:129–34.
Jonsson 2009
Jonsson M, Norden Lindeberg S, Hanson U. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. Int J Gynaecol Obstet 2009;107(Suppl. 2):214.
Jonsson 2010
Jonsson M, Hanson U, Lidell C, Nordén-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG 2010;117:76–83.
Kashanian 2010
Kashanian M, Fekrat M, Masoomi Z, Sheikh Ansari N. Comparison of active and expectant management on the duration of the third stage of labour and the amount of blood loss during the third and fourth stages of labour: a randomised controlled trial. Midwifery 2010;26:241–5.
Kemp 1963
Kemp J. Clinical trial of ‘Syntometrine’ in the third stage of labour. Br Med J 1963;1:1391–2.
Khan 1997
Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. Am J Obstet Gynecol 1997;177:770–4.
Khan 2003
Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynaecol 2003;101:968–74.
Khan 2012
Khan MS, Sinha SK, Sultana T, Singhal S. Comparison of two oxytocin infusions in patients undergoing emergency cesarean sections: a double blind study. Int J Gynaecol Obstet 2012;119(Suppl. 3):389.
Khanun 2011
Khanun A, Khanum S. Oral versus rectal misoprostol in the prevention of primary postpartum hemorrhage. Pak J Medical Health Sci 2011;5:587–8.
Khurshid 2010
Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 Mg and intravenous methyl ergometrine 0.2 Mg in the active management of third stage labor. Internet J Gynaecol Obstet 2009;14.
Kikutani 2003
Kikutani T, Oshima M, Sugimoto K, Shimada Y. [Effects of intravenous infusion rate of oxytocin on thoracic epidural pressure in parturients undergoing elective cesarean section.] Nihon Ika Daigahu Zasshi 2003;70:475–9.
Kikutani 2003a
Kikutani T, Shimada Y. Effects of methylergometrine and oxytocin on thoracic epidural pressure during cesarean section. J Obstet Gynaecol Res 2003;29:180–5.
Kikutani 2006
Kikutani T, Kikutani M, Oshima M, Sugimoto K, Shimada Y. [Effects of methylergometrine and oxytocin on blood loss and uterine contraction during cesarean section.] Masui 2006;55:590–4.
King 2006
King KJ, Douglas J, Unger W, Wong AB. A randomized double-blind comparison of a 5 unit intravenous oxytocin bolus versus placebo as a strategy to prevent uterine atony at cesarean section in women who are at increased risk of post-partum haemorrhage. Anaesthesiology 2006;104(Suppl. 1):41.
King 2007
King KJ, Douglas J, Unger W, Wong AB, Espinosa V, King RA. 5U bolus oxytocin at cesarean section in women at risk of atony. Anaesthesiology 2007;106(Suppl. 1):14.
King 2010
King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women at risk of atony: a randomized, double-blind, controlled trial. Anesth Analg 2010;111:1460–6.
Kintu 2012
Kintu A, Nakubulwa S, Mijumbi C, Kwizera A, Tindimwebwa J. Uterotonic efficacy of oxytocin 2.5 versus 10 units during caesarean section at Mulago hospital: a double blinded placebo controlled randomised clinical trial. Br J Anaesth 2012;108:ii197–8.
Kiran 2012
Kiran S, Anand A, Singh T, Gupta N. Effective dose of oxytocin in caesarean delivery. B J Anaesth 2012;108:ii195.
Kore 2000
Kore S, Srikrishna S, Hegde A, Ambiye VR, Vaidya PR. Active management of third stage of labour with intraumbilical oxytocin injection. J Obstet GynaecolIndia 2000;50:54–5.
Kovacheva 2015
Kovacheva VP, Soens MA, Tsen LC. A randomized, double-blinded trial of a ‘rule of threes’ algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anaesthesiology 2015;123:92–100.
Kovavisarach 1996
Kovavisarach E, Rojsangruang S. Effect of Umbilical Vein Oxytocin Injection on the Third Stage of Labour: A Randomised Controlled Study. Proceedings of the 9th Congress of the Federation of the Asia and Oceania Perinatal Societies, Singapore, 10–14 November 1996.
Kovavisarach 1998
Kovavisarach E, Rojsangruang S. Effect of umbilical vein oxytocin injection on the third stage of labor: a randomized controlled study. J Med Assoc Thai 1998;81:693–7.
Kumar 2011
Kumar S. A Study to Determine the Efficacy of 600 mcg Misoprostol in Prevention of Post Partum Haemorrhage. Proceedings of the 54th All India Congress of Obstetrics and Gynaecology, Hyderabad, Andhra Pradesh, India, 5–9 January 2011.
Kushtagi 2006
Kushtagi P, Verghese LM. Evaluation of two uterotonic medications for the management of the third stage of labor. Int J Gynaecol Obstet 2006;94:47–8.
Lamont 2001
Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and Syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins Other Lipid Mediat 2001;66:203–10.
Le 2000
Le J. [Prevention of postpartum haemorrhage by carboprost and oxytocin in 90 cases analysis.] Acta Med Sin 2000;13:140–1.
Leader 2002
Leader J, Bujnovsky M, Carlan SJ, Triana T, Richichi K. Effect of oral misoprostol after second-trimester delivery: a randomized, blinded study. Obstet Gynaecol 2002;100:689–94.
Li 2002
Li X, Wang H, Wang J, Cao X L, Ma Y. Prophylactic and therapeutic effect of misoprofil plus oxytocin on postpartum haemorrhage in patients with pregnancy-induced hypertension syndrome. J Postgrad Med 2002;25:34–5.
Li 2003
Li DP, Bei HZ. [Clinical study on reduction of postpartum bleeding in the risk factors by misoprostol.] Hainan Med J 2003;14:11–2.
Li 2011
Li H, Afzal A, Lian Q, Kramer GC, Svenson C, Prough D. Restricted fluid therapy decreases surgical blood loss – a clinical study of two fluid regimens during cesarean section under spinal anaesthesia. Anaesth Analg 2011;112:S291.
Li 2011a
Li H, Simon M, Lian Q, Afzal A, Christer Svenson C, Prough D. Restricted Fluid Therapy Decreases Surgical Blood Loss – A Clinical Study of Two Fluid Regimens During Cesarean Section Under Spinal Anaesthesia. 2011. URL: www.asaabstracts.com/strands/asaabstracts/abstract.htm?year=2011&index=13&absnum=4566 (accessed 19 March 2012).
Lin 2009
Lin JH, Lin QD, Liu XH, Yan JY, He J, Li L, et al. [Multi-center study of motherwort injection to prevent postpartum haemorrhage after caesarean section.] Zhonghua Fu Chan Ke Za Zhi 2009;44:175–8.
Liu 1997
Liu C, Wang D, Li X. [Clinical study on reduction of postpartum bleeding by methyl carprost suppository.] Zhonghua Fu Chan Ke Za Zhi 1997;32:22–4.
Liu 2002
Liu DY, Fan L, Huang XH. [Clinical observation on treatment of postpartum haemorrhage by xuesaitong soft capsule.] Zhongguo Zhong Xi Yi Jie He Za Zhi 2002;22:182–4.
Luamprapas 1994
Luamprapas A. A study of umbilical vein administration of oxytocin to shorten the third stage of labor. Chon Buri Hosp J 1994;19:14–25.
Mangla 2012
Mangla D, Goel JK, Goel R. Prophylactic intramyometrial oxytocin before placenta delivery during cesarean section prevents postpartum haemorrhage: a prospective randomised study of 150 women. J SAFOG 2012;4:93–6.
Mankuta 2006
Mankuta D. Double Blind Placebo Controlled Bellis Perenis and Arnica Montana as a Drug for PPH. ClinicalTrials.gov. 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00405626 (accessed 6 May 2015).
Mansouri 2011
Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Arch Gynecol Obstet 2011;283:935–9.
Martinez 2006
Martínez MM, López Farfán JA, Ramos Alvarez G, López Colombo A. [Oxytocin trough umbilical vein to shorten the third stage of labor.] Ginecol Obstet Mex 2006;74:89–94.
McGinty 1956
McGinity LB. A study of the vasopressor effects of oxytocics when used intravenously in the third stage of labor. West J Surg Obstet Gynecol 1956;64:22–8.
Miller 2009
Miller S, Tudor C, Thorsten V, Nyima, Kalyang, Sonam, et al. Randomized double masked trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum haemorrhage in Lhasa, Tibet. J Midwifery Womens Health 2009;54:133–41.e1.
Mirghafourvand 2013
Mirghafourvand M, Alizadeh SM, Abasalizadeh F, Shirdel M. The effect of intravenous tranexamic acid on haemoglobin and haematocrit levels after vaginal delivery: a randomized controlled trial. Iran J Obstet Gynaecol Infertil 2013;16:1–8.
Mirghafourvand 2015
Mirghafourvand M, Mohammad-Alizadeh S, Abbasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a double-blind randomised controlled trial. Aust N Z J Obstet Gynaecol 2015;55:53–8.
Mobeen 2006
Mobeen N, Walraven G. Misoprostol for the Prevention of Postpartum Haemorrhage in Rural Pakistan. ClinicalTrials.gov. 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00120237 (accessed 21 March 2006).
Mobeen 2009
Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo-controlled trial. Int J Gynaecol Obstet 2009;107(Suppl. 2):92.
Moertl 2008
Moertl M, Kraschl J, Friedrich S, Pickel K, Ulrich D, Eder M, et al. Hemodynamic changes of carbetocin and oxytocin in women undergoing cesarean section. Am J Obstet Gynecol 2008;199(Suppl. 1):86.
Mollitt 2009
Mollitt C, Ssenoga A, Grassman C, Barclay PM. Randomized controlled trial comparing the effects of oxytocin i.v bolus vs oxytocin i.v. infusion on cardiac output during caesarean section. Int J Obstet Anaesth 2009;18(Suppl. 1):11.
Moore 1956
Moore JH. Is methylergonovine tartrate superior to ergonovine maleate? Am J Obstet Gynecol 1956;71:908–11.
Mortl 2008
Mortl M, Pickel K, Friedrich S, Ulrich D, Lang U, Schlembach D. [Hemodynamic changes of carbetocin and oxytocin given as i.v. bolus on women undergoing cesarean section. ] Geburtshilfe Frauenheilkd 2008;68:S01.
Movafegh 2011
Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. Int J Gynaecol Obstet 2011;115:224–6.
Muller 1996
Muller R, Beck G. Active Management of the Third Stage of Labour. Paper presented at the 19th Congress of the Swiss Society of Gynaecology and Obstetrics, Interlaken, Switzerland 1996.
Munishankarappa 2009
Munishankarappa B, McLeod GA, MacGregor H, Murphy D. Maternal haemodynamic at elective caesarean section following oxytocin 5-unit bolus and placebo infusion compared to oxytocin 5-unit bolus and 30-unit infusion. Int J Obstet Anaesth 2009;18(Suppl. 1):48.
Munn 2001
Munn MB, Owen J, Hauth J. Oxytocin regimens for the prevention of uterine atony at cesarean delivery. Am J Obstet Gynaecol 2001;184:S14.
Munn 2001a
Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol 2001;98:386–90.
Murphy 2008
Murphy DJ. A Randomised Controlled Trial of Oxytocin Bolus versus Oxytocin Bolus and Infusion for the Control of Blood Loss at Elective Caesarean Section. Current Controlled Trials. 2008. URL: www.isrctn.com/ISRCTN17813715 (accessed 9 April 2008).
Murphy 2009
Murphy DJ, Carey M, Montgomery AA, Sheehan SR, ECSSIT Study Group. Study protocol. ECSSIT – Elective Caesarean Section Syntocinon Infusion Trial. A multi-centre randomised controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. BMC Pregnancy Childbirth 2009;9:36.
Murphy 2009a
Murphy DJ, MacGregor H, Munishankar B, McLeod G. A randomised controlled trial of oxytocin 5IU and placebo infusion versus oxytocin 5IU and 30IU infusion for the control of blood loss at elective caesarean section – pilot study. Eur J Obstet Gynecol Reprod Biol 2009;142:30–3.
Nankali 2013
Nankali A, Keshavarzi F, Fakheri T, Zare S, Rezaei M, Daeichin S. Effect of intraumbilical vein oxytocin injection on third stage of labor. Taiwan J Obstet Gynecol 2013;52:57–60. https://doi.org/10.1016/j.tjog.2013.01.010
NCT01710566 2012
NCT01710566. Misoprostol and Oxytocin in Uniject® for Postpartum Haemorrhage Prevention in Communities. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01710566 (accessed 9 October 2013).
Nellore 2006
Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs 15-methyl prostaglandin F2alpha for the prevention of postpartum hemorrhage. Int J Gynaecol Obstet 2006;94:45–6.
Nelson 1983
Nelson GH. Use of 15-methyl prostaglandin F2 alpha postpartum to contract the uterus in normal pregnant women. J Med Assoc Ga 1983;72:703–6.
Newton 1961
Newton M, Mosey LM, Egli GE, Gifford WB, Hull CT. Blood loss during and immediately after delivery. Obstet Gynecol 1961;17:9–18.
Nguyen-Lu 2013
Nguyen-Lu N, Carvalho JC, Farine D, Seaward G, Downey K, Balki M. Carbetocin at cesarean delivery for labour arrest: a randomised controlled trial to determine ED90. Can J Anaesth 2013;60(Suppl. 1):S112.
Nieminen 1964
Nieminen U, Jaervinen PA. A comparative study of different medical treatments of the third stage of labour. Ann Chir Gynaecol Fenn 1964;53:424–9.
Norchi 1988
Norchi S, Beretta E, Zanini A, Bottino S. Prevention of Primary Post-Partum Haemorrhage (PPH). Controlled Clinical Trial: Sulprostone vs Metilergometrina, Proceedings of the 12th FIGO World Congress of Gynaecology and Obstetrics, Brazil, 23–8 October 1988.
Oberbaum 2005
Oberbaum M, Galoyan N, Lerner-Geva L, Singer SR, Grisaru S, Shashar D, Samueloff A. The effect of the homeopathic remedies Arnica montana and Bellis perennis on mild postpartum bleeding – a randomized, double-blind, placebo-controlled study – preliminary results. Complement Ther Med 2005;13:87–90.
Oberbaum 2010
Oberbaum M. Effect of a Homeopathic Remedy on the Third Stage of Delivery: A Prospective, Randomised, Double-blind Study. ClinicalTrials.gov. 2010. URL: https://clinicaltrials.gov/ct2/show/NCT01156194 (accessed 6 May 2015).
Oguz 2014
Oguz OE, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. Int J Gynaecol Obstet 2014;127:175–9.
Ozalp 2010
Ozalp E, Tanir HM, Sener T. Dinoprostone vaginal insert versus intravenous oxytocin to reduce postpartum blood loss following vaginal or cesarean delivery. Clin Exp Obstet Gynecol 2010;37:53–5.
Ozcan 1996
Ozcan T, Sahin G, Senöz S. The effect of intraumbilical oxytocin on the third stage of labour. Aust N Z J Obstet Gynaecol 1996;36:9–11.
Ozkaya 2005
Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo-controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum haemorrhage. J Obstet Gynaecol Res 2005;31:389–93.
Padhy 2006
Padhy AK, Panigrahi R, Mohapatra KR. Alternative Method of Active Management of 3rd Stage of Labour with 10 Units of Intraumbilical Oxytocin Injection. Proceedings of the 49th All India Congress of Obstetrics and Gynaecology, Cochin, Kerala State, India, 6–9 January 2006.
Palacio 2011
Palacio FJ, Morillas F, Ortiz-Gómez JR, Fornet I, Bermejo L, Cantalejo F. [Efficacy of low-dose oxytocin during elective cesarean section.] Rev Esp Anestesiol Reanim 2011;58:6–10.
Paull 1977
Paull JD, Ratten GJ. Ergometrine and third stage blood loss. Med J Aust 1977;1:178–9.
Pei 1996
Pei JL, Zhao DF. [Study of the effects of using uterine stimulants on milk secretion during delivery.] Zhonghua Hu Li Za Zhi 1996;31:384–5.
Perdiou 2009
Perdiou A. The Effect of 3rd Generation Colloids on Primary Haemostasis in Pregnant Women. European Haematology Association, 14th Annual Congress, Berlin, Germany, 4–7 June 2009.
Perdiou 2009a
Perdiou A, Kousoulakou A, Papadopoulou G, Leveta G, Trigka A, Andromida M, et al. The effect of 3rd generation colloids on primary haemostasis in pregnant women. Haematologica 2009;94(Suppl. 2):527.
Phromboot 2012
Phromboot T, Chittacharoen A, Pitakkijronnakorn S. Efficacy of intraumbilical vein methylergonovine maleate on duration of third stage of labour. Thai J Obstet Gynaecol 2012;20:29–33.
Pierre 1992
Pierre F, Mesnard L, Body G. For a systematic policy of i.v. oxytocin inducted placenta deliveries in a unit where a fairly active management of third stage of labour is yet applied: results of a controlled trial. Eur J Obstet Gynaecol Reprod Biol 1992;43:131–5.
Pinder 2002
Pinder AJ, Dresner M, Calow C, Shorten GD, O’Riordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth 2002;11:156–9.
Pisani 2012
Pisani I, Tiralongo GM, Gagliardi G, Scala RL, Todde C, Frigo MG, Valensise H. The maternal cardiovascular effect of carbetocin compared to oxytocin in women undergoing caesarean section. Pregnancy Hypertens 2012;2:139–42.
Poeschmann 1988
Poeschmann RP, Eskes TKAB, Doesburg WH, Lemmens WAJG, Benneker JCLH. Oxytocin and Sulprostone Reduce Postpartum Blood Loss in Low Risk Term Women Compared to Saline. Proceedings of the 1st European Congress on Prostaglandins in Reproduction, Vienna, Austria, 6–9 July 1988.
Poeschmann 1991
Poeschmann RP, Doesburg WH, Eskes TK. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. Br J Obstet Gynaecol 1991;98:528–30.
Poeschmann 1991a
Poeschmann RP, Eskes TKAB, Doesburg WH. Oxytocin and sulprostone reduce post partum blood loss and shorten the third stage in low risk term women. Int J Gynaecol Obstet 1991;36(Suppl.):312.
Porter 1991
Porter KB, O’Brien WF, Bruskivage L, Collins MK, Knuppel RA, Givens P. Prospective randomized study on the effects of umbilical vein oxytocin on puerperal blood loss, length of the third stage of labor and on alpha-fetoprotein levels. Am J Obstet Gynaecol 1991;164:326.
Porter 1991a
Porter KB, O’Brien WF, Collins MK, Givens P, Knuppel R, Bruskivage L. A randomized comparison of umbilical vein and intravenous oxytocin during the puerperium. Obstet Gynaecol 1991;78:254–6.
Priya 2015
Priya GP, Veena P, Chaturvedula L, Subitha L. A randomized controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. Arch Gynaecol Obstet 2015;292:1231–7.
Puri 2012
Puri M, Taneja P, Gami N, Rehan HS. Effects of different doses of intraumbilical oxytocin on the third stage of labor. Int J Gynaecol Obstet 2012;118:210–12. https://doi.org/10.1016/j.ijgo.2012.04.010
Qiu 1998
Qiu H, Zhu H, Ouyang W. [Clinical study on chanlibao in accelerating second stage of labor.] Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18:214–16.
Qiu 1999
Qiu H, Zhu H, Ouyang W, Wang Z, Sun H. Clinical effects and mechanism of chanlibao in accelerating second stage of labor. J Tongji Med Univ 1999;19:141–4.
Quiroga 2009
Quiroga Díaz R, Cantú Mata R, Tello Gutiérrez HE, Puente Villalobos M, Montemayor Garza R, Martínez Mendoza A. [Intrauterine misoprostol for the prevention of bleeding cesarean.] Ginecol Obstet Mex 2009;77:469–74.
Rajwani 2000
Rajwani J, Survana K. Active Management of Third Stage of Labour – A Comparative Study. Proceedings of the 16th FIGO World Congress of Obstetrics and Gynaecology, Washington, DC, USA, 3–8 September 2000.
Ramirez 2001
Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? Preliminary results. J Perinat Med 2001;29(Suppl. 1):364.
Reddy 1989
Reddy VV, Carey JC. Effect of umbilical vein oxytocin on puerperal blood loss and length of the third stage of labor. Am J Obstet Gynecol 1989;160:206–8.
Reddy 2001
Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. J Obstet Gynaecol India 2001;51:44–7.
Rooney 1985
Rooney I, Hughes P, Calder AA. Is routine administration of Syntometrine still justified in the management of the third stage of labour? Health Bull 1985;43:99–101.
Rosales-Ortiz 2013
Rosales-Ortiz S. Prophylaxis of obstetric haemorrhage: Experience using carbetocin vs Oxytocin in patients with risk factors for postpartum haemorrhage. J Perinat Med 2013;41(Suppl. 1):140.
Rouse 2011
Rouse D, Abramovici A, Szychowski J, Seals S, Andrews W, Hauth J, et al. Oxytocin dose-regimens to prevent uterine atony after vaginal delivery: does treatment efficacy vary by risk status? Am J Obstet Gynaecol 2011;204(Suppl. 1):50–1.
Sadeghipour 2013
Sadeghipour Z. The Role of Tranexamic Acid in Management of Uterine Atony During Delivery. IRCT Iranian Registry of Clinical Trials. 2013. URL: www.irct.ir/trial/13336 (accessed 2 December 2013).
Saito 2007
Saito K, Haruki A, Ishikawa H, Takahashi T, Nagase H, Koyama M, et al. Prospective study of intramuscular ergometrine compared with intramuscular oxytocin for prevention of postpartum hemorrhage. J Obstet Gynaecol Res 2007;33:254–8.
Samuels 2005
Samuels N, Oberbaum M. The effect of the homoeopathic remedies Arnica montana and Bellis perennis on postpartum bleeding – a randomized, double-blind, placebo-controlled study. Foc Altern Complement Ther 2005;10(Suppl. 1):47.
Sariganont 1999
Sariganont J. Comparative study between Syntocinon and methergin in prevention of postpartum haemorrhage. Thai J Obstet Gynaecol 1999;11:248.
Sarna 1997
Sarna MC, Soni AK, Gomez M, Oriol NE. Intravenous oxytocin in patients undergoing elective cesarean section. Anesth Analg 1997;84:753–6.
Sartain 2008
Sartain JB, Barry JJ, Howat PW, McCormack DI, Bryant M. Intravenous oxytocin bolus of 2 units is superior to 5 units during elective caesarean section. Br J Anaesth 2008;101:822–6.
Schaefer 2004
Schaefer A, Klein L, Wolfe P, Heindricks G, Downs L, Guinn D. Double blind RCT of early versus traditional oxytocin management in the third stage to prevent blood loss. Am J Obstet Gynaecol 2004;191(Suppl. 1):69.
Schemmer 2001
Schemmer G. A randomised controlled trial comparing prophylactic administration of oxytocin before and after placental delivery in the prevention of postpartum haemorrhage. Am J Obstet Gynaecol 2001;184:S20.
Sekhavat 2009
Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. J Matern Fetal Neonatal Med 2009;22:72–5.
Sentilhes 2014
Sentilhes L. Tranexamic Acid for Preventing Postpartum Haemorrhage Following a Vaginal Delivery (TRAAP). ClinicalTrials.gov. 2014. (https://clinicaltrials.gov/ct2/show/NCT02302456) (accessed 10 January 2015).
Sentürk 2013
Sentürk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double-blind, placebo-controlled, randomized clinical trial. Arch Gynecol Obstet 2013;287:641–5.
Shahid 2013
Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. J Coll Physicians Surg Pak 2013;23:459–62.
Sheehan 2009
Sheehan S, Carey M, Murphy D. A cohort study of 500 patients recruited to ECSSIT – Elective Caesarean Section Syntocinon Infusion Trial. Int J Gynaecol Obstet 2009;107:492.
Sheehan 2011
Sheehan S, Montgomery AA, Carey M, McAuliffe F, Eogan M, Gleeson R, et al. ECSSIT-elective caesarean section Syntocinon infusion trial a multicentre randomised controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. Iri J Med Sci 2011;180:119.
Sheehan 2011a
Sheehan SR, Montgomery AA, Carey M, McAuliffe FM, Eogan M, Gleeson R, et al. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: double blind, placebo controlled, randomised trial. BMJ 2011;343:d4661.
Shirazi 2013
Shirazi FH. A Placebo-controlled Clinical Trial to Assess Efficacy of Tranexamic Acid in Reducing Haemorrhage after Vaginal Delivery. IRCT Iranian Registry of Clinical Trials. 2013. URL: www.irct.ir/trial/9921 (accessed 4 December 2013).
Shrestha 2007
Shrestha P, Babu CS. Influence of umbilical vein oxytocin on blood loss and length of third stage of labour. Nepal Med Coll J 2007;9:176–8.
Singh 2005
Singh N, Singh U. Methylergometrine and carboprost tromethamine prophylaxis for postpartum haemorrhage. J Obstet Gynaecol India 2005;55:325–8.
Siriwarakul 1991
Siriwarakul W. A study of umbilical vein administration of oxytocin to shorten the third stage of labour. Chon Buri Hosp J 1991;16:40–51.
Soiva 1964
Soiva K, Koistinen O. Clinical experience with simultaneous intramuscular injection of oxytocin and methylermetrine. Ann Chir Gynaecol Fenn 1964;53:173–8.
Sorbe 1978
Sorbe B. Active pharmacologic management of the third stage of labor. A comparison of oxytocin and ergometrine. Obstet Gynecol 1978;52:694–7.
Soriano 1995
Soriano D, Dulitzki M, Schiff E, Barkai G, Seidman DS. A randomised prospective trial of oxytocin plus ergometrin versus oxytocin alone for prevention of postpartum haemorrhage. Am J Obstet Gynaecol 1995;172:361.
Stearn 1963
Stearn RH. Syntometrine in the management of the third stage of labour. J Obstet Gynaecol Br Commonw 1963;70:593–6.
Svanstrom 2008
Svanström MC, Biber B, Hanes M, Johansson G, Naslund U, Bålfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth 2008;100:683–9.
Symes 1984
Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. J Obstet Gynaecol 1984;5:36–8.
Taj 2014
Taj N, Firdous A, Akhtar N, Chaudhary MH, Sarah, Bajwa Z, et al. Efficacy of tranexamic acid in reducing blood loss during and after cesarean section. Rawal Med J 2014;39:311–13.
Takagi 1976
Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post-partum hemorrhage. Prostaglandins 1976;12:565–79.
Tanir 2009
Tanir H, Sener T, Ozalp E. Dinoprostone vaginal insert versus intravenous oxytocin to reduce the postpartum blood loss following vaginal or cesarean delivery. Int J Gynaecol Obstet 2009;107(Suppl. 2):S506–7.
Tarabrin 2012
Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, Gavrychenko D. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Crit Care 2012;16(Suppl. 1):157.
Tariq 2015
Tariq N, Khakwani M, Parveen R. Effectiveness of misoprostol in the prevention of postpartum haemorrhage. Pak J Med Health Sci 2015;9:268–70.
Tehseen 2008
Tehseen F, Anwar A, Arfat Y. Intraumbilical veinous injection oxytocin in the active management of third stage of labour. J Coll Physicians Surg Pak 2008;18:551–4.
Terry 1970
Terry MF. A management of the third stage to reduce feto-maternal transfusion. J Obstet Gynaecol Br Commonw 1970;77:129–32.
Tessier 2000
Tessier JL, Davies GAL, Woodman MC, Lipson A. Maternal hemodynamics after oxytocin bolus versus infusion in the third stage of labour. Am J Obstet Gynaecol 2000;182:S128.
Tharakan 2007
Tharakan T, Jha J. Randomized double-blind prospective trial of active management of the third stage of labor. Obstet Gynaecol 2007;109(Suppl. 4):1.
Tharakan 2008
Tharakan T, Jha J. Randomized double blind prospective trial of active management of the third stage of labour. Arch Med Sci 2008;4:79–82.
Thomas 2006
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of intravenous bolus or infusion of oxytocin in women undergoing caesarean section. Int J Obstet Anaesth 2006;15(Suppl. 1):13.
Thomas 2007
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth 2007;98:116–19.
Thornton 1987
Thornton S, Davison JM, Baylis PH. Plasma oxytocin in the third stage of human labour with and without Syntometrine. Clin Sci 1987;73(Suppl. 17):2P.
Thornton 1988
Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 1988;297:167–9.
Tita 2012
Tita ATN, Szychowski JM, Rouse DJ, Bean CM, Chapman V, Nothern A, et al. Higher-dose oxytocin and haemorrhage after vaginal delivery: A randomised controlled trial. Obstet Gynaecol 2012;119(2 Pt 1):293–300.
Tripti 2006
Tripti N, Manju E. Intramuscular PGF2 alpha 125 µg versus intravenous methyl ergometrine 0.2 mg in the active management of third stage of labor. J Obstet Gynaecol India 2006;56:396–8.
Tripti 2009
Tripti N, Balram S. 400 µg oral misoprostol versus 0.2 mg intravenous methyl ergometrine for the active management of third stage of labor. J Obstet Gynaecol India 2009;59:228–34.
Tudor 2006
Tudor C, Miller S, Nyima, Sonam, Droyoung, Varner M. Preliminary progress report: randomized double-blind trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum haemorrhage in Lhasa, Tibet. Int J Gynaecol Obstet 2006;94(Suppl. 2):145–6.
Van den Enden 2009
Van den Enden E, Lahousse J, Devlieger R, Vandermeersch E, Van de Velde M. Haemodynamic effects of a bolus or infusion of oxytocin: a randomized double-blind trial. Int J Obstet Anaesth 2009;18(Suppl. 1):45.
Van Selm 1995
Van Selm M, Kanhai HH, Keirse MJ. Preventing the recurrence of atonic postpartum hemorrhage: a double-blind trial. Acta Obstet Gynecol Scand 1995;74:270–4.
Vasegh 2005
Vasegh Rahimparvar F, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labour. Hayat 2005;10:102.
Vaughan 1974
Vaughan Williams C, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. J Obstet Gynaecol Br Commonw 1974;81:596–9.
Ventoskovskiy 1990
Ventoskovskiy BM, Popov AV. Homoeopathy as a practical alternative to traditional obstetrics methods. Br Homoeopath J 1990;79:201–5.
Verghese 2008
Verghese L, Kushtagi P. Evaluation of carboprost as a prophylactic oxytocic in the management of third stage of labour. BJOG 2008;115(Suppl. 1):74.
Vogel 2004
Vogel D, Burkhardt T, Rentsch K, Schweer H, Watzer B, Zimmermann R, Von Mandach U. Misoprostol versus methylergometrine: pharmacokinetics in human milk. Am J Obstet Gynecol 2004;191:2168–73.
Wallace 2008
Wallace EM. A Double-blind Randomised Controlled Trial of Oxytocin Bolus plus Placebo Infusion versus Oxytocin Bolus plus Oxytocin Infusion at Elective Caesarean Section. Australian New Zealand Clinical Trials Registry. 2008. URL: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82466&isReview=true (accessed 19 February 2008).
Walraven 2005
Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, Sloan N. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG 2005;112:1277–83.
Wang 2000
Wang BI, Du JM. Clinical study on reduction of postpartum bleeding using carprost suppository. Henan Med Res 2000;9:155–6.
Weeks 2013
Weeks A, Ditai J, Ononge S, Faragher B, Mirembe F, Byamugisha J, et al. Self-administered misoprostol to prevent bleeding after homebirths in Uganda: a placebo-controlled randomised trial. BJOG 2013;120:76.
Weihong 1998
Weihong H, Hanrong C, Hong L, Linan C. [Preventing of postpartum haemorrhage by carboprost methylate suppository administered through vagina or sublingually.] Acta Acad Med Shanghai 1998;25:137–9.
Weiss 1975
Weiss G, Klein S, Shenkman L, Kataoka K, Hollander CS. Effect of methylergonovine on puerperal prolactin secretion. Obstet Gynecol 1975;46:209–10.
Wetta 2011
Wetta L, Szychowski J, Seals S, Mancuso M, Hauth J, Tita A. Risk factors for uterine atony at vaginal delivery: a comprehensive evaluation. Am J Obstet Gynaecol 2011;204(Suppl. 1):71–2.
Wetta 2013
Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol 2013;209:51.e1–6.
Winikoff 2012
Winikoff B. IV vs i.m. Oxytocin in the Third Stage of Labor for Prevention of Postpartum Haemorrhage. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01608958 (28 March 2016).
Wong 2006
Wong A. Does the Rapid Intravenous Administration of Oxytocin after Delivery of the Baby Decrease the Bleeding During Cesarean Section in Women at Risk of Bleeding During Cesarean Section? ClinicalTrials.gov. 2006. URL: https://clinicaltrials.gov/ct2/show/NCT00257803 (accessed 21 March 2006).
Wright 2006
Wright L. RCT of Zhi Byed 11(ZB11) Versus Misoprostol in Tibet (ongoing trial). ClinicalTrials.gov. 2006. [http://clinicaltrials.gov/] (accessed 21 March 2006).
Wu 2007
Wu LF, Liu Y, Ruan Y. [Clinical study on prevention of postpartum hemorrhage of cesarean section using hemabat in high risk pregnant women.] Zhonghua Fu Chan Ke Za Zhi 2007;42:577–81.
Xu 2003
Xu H. Misoprostol on preventing postpartum bleeding in cesarean. Hebei Medi 2003;9:806–7.
Xu 2013
Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double-blind randomization trial. Arch Gynecol Obstet 2013;287:463–8.
Yamaguchi 2011
Yamaguchi ET, Cardoso MM, Torres ML, Nascimento RC, Ribeiro MC, Frerichs E, et al. Serum oxytocin concentrations in elective caesarean delivery: a randomised comparison of three infusion regimens. Int J Obstet Anaesth 2011;20:224–8.
Yan 2000
Yan WG, Ling MX, Mao HY. Clinical study on reduction of postpartum bleeding in cesarean operation by misoprostol. J Zhenjiang Med Coll 2000;10:440–1.
Yang 2001
Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomised comparative, multicenter trial.] Zhonghua Fu Chan Ke Tsa Zhi 2001;36:590–2.
Young 1988
Young SB, Martelly PD, Greb L, Considine G, Coustan DR. The effect of intraumbilical oxytocin on the third stage of labour. Obstet Gynaecol 1988;71:736–8.
Zamora 1999
Zamora LAL. A randomized controlled trial of oxytocin administered at the end of the second stage of labor versus oxytocin administered at the end of the third stage of labor in the prevention of postpartum haemorrhage. Philipp J Obstet Gynaecol 1999;23:125–33.
Zaporozhan 2013
Zaporozhan V, Tarabrin O, Gavrychenko D, Mazurenko G, Saleh O, Lyoshenko I. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Crit Care 2013;17(Suppl. 2):135–6.
Zhao 1998
Zhao Y, Li X, Peng Y. [Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol.] Zhonghua Fu Chan Ke Za Zhi 1998;33:403–5.
Zhao 2003
Zhao SF, Sun XF. Clinical study on preventing and curing postpartum haemorrhage in the third stage of labour. J Pract Obstet Gynaecol 2003;19:278–80.
Zhou 1994
Zhou HL, Zhang L. Study on the effect of third stage of labour through different channels of injection of oxytocin. Chin J Nurs 1994;29:453–5.
Studies awaiting classification
Begum 2015
Begum T, Yeasmin S, Chakma S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss in caesarean section. BJOG 2015;122(Suppl. 1).
Beigi 2009
Beigi A, Tabarestani H, Moini A, Zarrinkoub F, Kazempour M, Hadian Amree A. [Sublingual misoprostol versus intravenous oxytocin in the management of postpartum haemorrhage.] Tehran Univ Med J 2009;67:556–61.
Bhatti 2014
Bhatti K, Mahar T, Hafeez R, Shoaib-u-Nisa. A randomized controlled trial on prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin. Med For Mon 2014;25:10–12.
Chandhiok 2006
Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. Int J Gynaecol Obstet 2006;92:170–5.
Chatterjee 2000
Chatterjee A. Misoprostol and the 3rd Stage. Proceedings of the 16th FIGO World Congress of Obstetrics & Gynaecology, Washington, DC, USA, 3–8 September 2000.
Del Angel-Garcia 2006
Del Angel-Garcia G, Garcia-Contreras F, Constantino-Casas P, Nevarez-Sida A, Lopez-Gonzalez N, Garcia-Constantino M, et al. Economic evaluation of carbetocin for the prevention of uterine atony in patients with risk factors in Mexico. Value Health 2006;9:A254.
Dell-Kuster 2016a
Dell-Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of intravenous carbetocin as a bolus compared to a short infusion for caesarean section. J Obstet Anaesth 2016;26(Suppl. 1):7.
Dommisse 1980
Dommisse J. The routine use of oxytocic drugs in the third stage of labour. S Afr Med J 1980;58:549.
Frye 2012a
Frye LJ, Diop AR, Kone Y. Comparing Misoprostol and Oxytocin in UnijectTM for Postpartum Hemorrhage (PPH) Prevention in Mali. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01487278 (accessed 28 March 2016).
Fuks 2014
Fuks AM, Khanna P, Yusaf T, Aslian A, Kowalska D, Salafia CM. Use of prophylactic misoprostol in reduction of blood loss at vaginal delivery. Obstet Gynaecol 2014;123:144–5.
Ng 2004
Ng PS, Yuen PM, Sahota DS. Comparison of Oral Misoprostol and Intravascular Syntocinon in the Management of the Third Stage of Labour – A Double-blind Randomised Controlled Trial, 30th British Congress of Obstetrics and Gynaecology, Glasgow, UK, 7–9 July 2004.
Sharma 2014
Sharma M, Kaur P, Kaur K, Kaur A, Kaur PK, Kaur MM. A comparative study of oxytocin/misoprostol/methylergometrine for active management of the third stage of labor. J Obstet Gynaecol India 2014;64:175–9.
Shrivasatava 2012
Shrivasatava DD, Khamsara D. Critical evaluation of sublingual misoprostol and methyl ergometrine in active management of third stage of labour. Int J Gynaecol Obstet 2012;119(Suppl. 3):484.
Appendix 4 Characteristics of excluded studies
| Author | Year | Reason for exclusion |
|---|---|---|
| Abdel-Aleem | 1993 | Not eligible intervention |
| Abdel-Aleem | 1997 | Not eligible intervention |
| Abdel-Aleem | 1997a | Not eligible intervention |
| Abdel-Aleem | 1997a | Not eligible intervention |
| Abdel-Aleem | 2013 | Not eligible intervention |
| Abdollahy | 2000 | Not eligible intervention |
| Al-Harazi | 2009 | Same drug intervention used in both arms and only different route of misoprostol administration |
| Anandakrishnan | 2013 | Same drug intervention used in both arms and only different dose of carbetocin administration |
| Anjaneyulu | 1988 | Not eligible intervention |
| Anvaripour | 2013 | Intervention given after the third stage of labour |
| Athavale | 1991 | Not eligible intervention |
| Ayedi | 2011 | Not eligible intervention |
| Ayedi | 2011a | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Aziz | 2014 | Quasi-randomised |
| Bader | 2000 | Not eligible intervention |
| Bader | 2000a | Not eligible intervention |
| Badhwar | 1991 | Not eligible intervention |
| Bai | 2014 | Not eligible uterotonic |
| Balki | 2005 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Balki | 2006 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Banovska | 2013 | Not eligible intervention |
| Barbaro | 1961 | Not eligible intervention |
| Baumgarten | 1983 | Not eligible uterotonic |
| Bhattacharya | 1988 | Not eligible uterotonic |
| Bhavana | 2013 | Not eligible intervention |
| Bider | 1991 | Not eligible intervention |
| Bider | 1992 | Not eligible intervention |
| Bisri | 2011 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Biswas | 2007 | Not eligible uterotonic |
| Bivins | 1993 | Not eligible uterotonic |
| Bivins | 1993a | Not eligible uterotonic |
| Blum | 2010 | Intervention for treatment of PPH |
| Bonham | 1963 | Quasi-randomised |
| Bonis | 2012 | Quasi-randomised |
| Cappiello | 2006 | Not eligible intervention |
| Carvalho | 2004 | Same drug intervention both arms and only different dose of oxytocin administration |
| Catanzarite | 1990 | Not eligible intervention |
| Chaplin | 2009 | Not eligible intervention |
| Chaudhuri | 2014 | Inappropriate population (excluded women who had PPH) |
| Chestnut | 1987 | Not eligible intervention |
| Chestnut | 1987a | Not eligible intervention |
| Chou | 1994 | Not eligible intervention |
| Chua | 1995 | Not eligible intervention |
| Chukudebelu | 1963 | Quasi-randomised |
| Cooper | 2004 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Cordovani | 2012 | Same drug intervention used in both arms and only different dose of carbetocin administration |
| Dagdeviren | 2014 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Dahiya | 1995 | Not eligible intervention |
| Daley | 1951 | Quasi-randomised |
| Daly | 1999 | Not able to extract outcomes |
| Dao | 2009 | Intervention for treatment of PPH |
| Davies | 2005 | Same drug intervention used in both arms and only different route of oxytocin administration |
| De bonis | 2012 | Quasi-randomised |
| Dennehy | 1998 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Devi | 1988 | Not eligible intervention |
| Diab | 1999 | Quasi-randomised |
| Dickinson | 2009 | Not eligible population (terminations in second trimester) |
| Dommisse | 1980 | Not randomised |
| Dong | 2011 | Not eligible intervention |
| Durocher | 2012 | Quasi-randomised |
| Dutta | 2000 | Quasi-randomised |
| Dweck | 2000 | Not eligible intervention |
| Dzuba | 2012 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Elati | 2011 | Same drug intervention used in both arms and only different route of misoprostol administration |
| Erkkola | 1984 | Not eligible intervention |
| Farber | 2013 | Not eligible intervention |
| Farber | 2015 | Not eligible intervention |
| Fatemeh | 2011 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Fawole | 2011 | The intervention was oxytocin or ergometrine plus oxytocin and data were not given for each of the drugs separately |
| Fawzy | 2012 | Treatment (not prevention) of PPH |
| Forster | 1957 | Quasi-randomised |
| Francis | 1965 | Quasi-randomised |
| Francis | 1965a | Quasi-randomised |
| Friedman | 1957 | Quasi-randomised |
| Frye | 2012 | Study abandoned |
| Fugo | 1958 | Quasi-randomised |
| Gai | 2004 | Not eligible intervention |
| Gambling | 1994 | Duplicate (abstract of Dansereau 1999) |
| Gambling | 1994a | Duplicate (abstract of Dansereau 1999) |
| Gawecka | 2014 | Duplicate (abstract of Rosseland 2013) |
| Geller | 2004 | Duplicate (abstract of Derman 2006) |
| Geller | 2008 | Duplicate (secondary analysis from Derman 2006) |
| George | 2010 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Ghulmiyyah | 2005 | Not eligible intervention |
| Ghulmiyyah | 2007 | Not eligible intervention |
| Gobbur | 2011 | Not eligible intervention |
| Gohel | 2007 | Not eligible intervention |
| Goswami | 2013 | Not eligible intervention |
| Groeber | 1960 | Not eligible intervention |
| Gungorduk | 2010 | Not eligible intervention |
| Gungorduk | 2010a | Same drug intervention used in both arms and only different route of oxytocin administration |
| Gungorduk | 2011 | Not eligible intervention |
| Gungorduk | 2013 | Not eligible intervention |
| Gupta | 2014 | Not eligible intervention |
| Habek | 2007 | Not eligible intervention |
| Hacker | 1979 | No available outcomes |
| Halder | 2013 | Not eligible intervention |
| Hoffman | 2004 | Not appropriate intervention (comparing timing of oxytocin) |
| Hoffman | 2006 | Not appropriate intervention (comparing timing of oxytocin) |
| Hofmeyr | 1997 | Duplicate (interim analysis from Hofmeyr 1998) |
| Hofmeyr | 1998a | Duplicate (from Hofmeyr 1998 and 2001) |
| Hofmeyr | 2000 | Duplicate (abstract from Hofmeyr 2001) |
| Hofmeyr | 2004 | Intervention for treating PPH |
| Hofmeyr | 2008 | Duplicate (trial registration for Hofmeyr 2011) |
| Howard | 1964 | Not eligible intervention |
| Huh | 2000 | Same drug intervention used in both arms and only different regimen of oxytocin administration |
| Huh | 2004 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Hunt | 2013 | Not eligible intervention |
| Häivä | 1994 | Quasi-randomised |
| Ilancheran | 1990 | No outcome data |
| Irons | 1994 | No outcome data |
| Jackson | 2001 | Not eligible intervention |
| Jiang | 2001 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Jin | 2000 | Not eligible intervention |
| Jolivet | 1978 | Not eligible outcomes |
| Jonsson | 2009 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Jonsson | 2010 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Kashanian | 2010 | Ineligible population (excluded women with PPH) |
| Kemp | 1963 | Quasi-randomised |
| Khan | 1997 | Not eligible intervention |
| Khan | 2003 | Same drug intervention used in both arms and only different route of misoprostol administration |
| Khan | 2012 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Khanun | 2011 | Same drug intervention used in both arms and only different route of misoprostol administration |
| Khurshid | 2010 | Not eligible intervention |
| Kikutani | 2003 | Not eligible outcomes |
| Kikutani | 2003a | Not eligible outcomes |
| Kikutani | 2006 | Data cannot be extracted |
| King | 2006 | Same drug intervention used in both arms and only different route of oxytocin administration |
| King | 2007 | Same drug intervention used in both arms and only different route of oxytocin administration |
| King | 2010 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Kintu | 2012 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Kiran | 2012 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Kore | 2000 | Not eligible intervention |
| Kovacheva | 2015 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Kovavisarach | 1996 | Not eligible intervention |
| Kovavisarach | 1998 | Not eligible intervention |
| Kumar | 2011 | Not available outcomes |
| Kushtagi | 2006 | Not eligible intervention (carboprost) |
| Lamont | 2001 | Not eligible intervention (carboprost) |
| Le | 2000 | Not eligible intervention |
| Leader | 2002 | Not eligible population (second trimester) |
| Li | 2002 | Not eligible intervention |
| Li | 2003 | Not eligible intervention |
| Li | 2011 | Not eligible intervention |
| Li | 2011a | Not eligible intervention |
| Lin | 2009 | Not eligible intervention |
| Liu | 1997 | Not eligible intervention |
| Liu | 2002 | Not eligible intervention |
| Luamprapas | 1994 | Not eligible intervention |
| Mangla | 2012 | Not eligible intervention |
| Mankuta | 2006 | Not eligible intervention |
| Mansouri | 2011 | Same drug intervention used in both arms and only different route of misoprostol administration |
| Martinez | 2006 | Not eligible intervention |
| McGinty | 1956 | Quasi-randomised |
| Miller | 2009 | Not eligible intervention |
| Mirghafourvand | 2013 | Not eligible intervention |
| Mirghafourvand | 2015 | Not eligible intervention |
| Mobeen | 2006 | Duplicate (trial registration for Mobeen 2011) |
| Mobeen | 2009 | Duplicate (abstract for Mobeen 2011) |
| Moertl | 2008 | Duplicate (abstract of Moertl 2011) |
| Mollitt | 2009 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Moore | 1956 | Same drug intervention used in both arms and only different type of the same drug |
| Mortl | 2008 | Duplicate (abstract of Moertl 2011) |
| Movafegh | 2011 | Not eligible intervention |
| Muller | 1996 | Outcome data cannot be extracted |
| Munishankarappa | 2009 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Munn | 2001 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Munn | 2001a | Same drug intervention used in both arms and only different route of oxytocin administration |
| Murphy | 2008 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Murphy | 2009 | Same drug intervention used in both arms and only different route of oxytocin administration |
| Murphy | 2009a | Same drug intervention used in both arms and only different route of oxytocin administration |
| Nankali | 2013 | Not eligible intervention |
| Nellore | 2006 | Not eligible intervention |
| NCT01710566 | 2012 | Study withdrawn |
| Nelson | 1983 | Not eligible intervention |
| Newton | 1961 | Quasi-randomised |
| Nguyen-Lu | 2013 | Same drug intervention used in both arms and only different dose of carbetocin administration |
| Nieminen | 1964 | Not eligible intervention |
| Norchi | 1988 | Not eligible intervention |
| Oberbaum | 2005 | Not eligible intervention |
| Oberbaum | 2010 | Not eligible intervention |
| Oguz | 2014 | Same drug intervention used in both arms and only different route and timing of oxytocin administration |
| Ozalp | 2010 | Not eligible intervention |
| Ozcan | 1996 | Not eligible intervention |
| Ozkaya | 2005 | Inappropriate population (excluded women who had PPH) |
| Padhy | 2006 | Not eligible intervention |
| Palacio | 2011 | Same drug intervention used in both arms and only different dose of oxytocin administration |
| Paull | 1977 | Same drug intervention used in both arms and only different doses of drug administration |
| Pei | 1996 | Not eligible outcomes |
| Perdiou | 2009 | Not eligible intervention |
| Perdiou | 2009a | Not eligible intervention |
| Phromboot | 2010 | Not eligible intervention |
| Pierre | 1992 | Quasi-randomised |
| Pinder | 2002 | Same drug intervention used in both arms and only different doses of drug administration |
| Pisani | 2012 | Quasi-randomised |
| Poeschmann | 1988 | Duplicate (abstract of Poeschmann 1991) |
| Poeschmann | 1991 | Quasi-randomised |
| Poeschmann | 1991a | Duplicate (abstract of Poeschmann 1991) |
| Porter | 1991 | Not eligible intervention |
| Porter | 1991a | Not eligible intervention |
| Priya | 2015 | Ineligible outcomes (not measured blood loss in the third stage) |
| Puri | 2012 | Not eligible intervention |
| Qiu | 1998 | Not eligible population (second stage) |
| Qiu | 1999 | Not eligible population (second stage) |
| Quiroga | 2009 | Not eligible intervention |
| Rajwani | 2000 | Not eligible intervention |
| Ramirez | 2001 | No available data |
| Reddy | 1989 | Not eligible intervention |
| Reddy | 2001 | Not eligible intervention |
| Rooney | 1985 | Quasi-randomised |
| Rosales-Ortiz | 2013 | Quasi-randomised |
| Rouse | 2011 | Same drug intervention both arms and only different doses of drug administration |
| Sadeghipour | 2013 | Not eligible intervention |
| Saito | 2007 | Quasi-randomised |
| Samuels | 2005 | Not eligible intervention |
| Sariganont | 1999 | Cannot extract data |
| Sarna | 1997 | Same drug intervention used in both arms and only different doses of drug administration |
| Sartain | 2008 | Same drug intervention used in both arms and only different doses of drug administration |
| Schaefer | 2004 | Same drug intervention used in both arms and only different timings of drug administration |
| Schemmer | 2001 | Same drug intervention used in both arms and only different timings of drug administration |
| Sekhavat | 2009 | Not eligible intervention |
| Sentilhes | 2014 | Not eligible intervention |
| Sentürk | 2013 | Not eligible intervention |
| Shahid | 2013 | Not eligible intervention |
| Sharma | 2014 | Not randomised |
| Sheehan | 2009 | Same drug intervention used in both arms and only different doses of drug administration |
| Sheehan | 2011 | Same drug intervention used in both arms and only different doses of drug administration |
| Sheehan | 2011a | Same drug intervention used in both arms and only different doses of drug administration |
| Shirazi | 2013 | Not eligible intervention |
| Shrestha | 2007 | Not eligible intervention |
| Singh | 2005 | Not eligible intervention |
| Siriwarakul | 1991 | Not eligible intervention |
| Soiva | 1964 | Quasi-randomised |
| Sorbe | 1978 | Quasi-randomised |
| Soriano | 1995 | Quasi-randomised |
| Stearn | 1963 | Quasi-randomised |
| Svanstrom | 2008 | No eligible outcomes |
| Symes | 1984 | No eligible outcomes |
| Taj | 2014 | Not eligible intervention |
| Takagi | 1976 | Not eligible intervention |
| Tanir | 2009 | Not eligible intervention |
| Tarabrin | 2012 | Not eligible intervention |
| Tariq | 2015 | Not eligible intervention |
| Tariq | 2015a | Administered for treatment of PPH |
| Tehseen | 2008 | Administered for treatment of PPH |
| Terry | 1970 | Not eligible intervention |
| Tessier | 2000 | Same drug intervention used in both arms and only different doses of drug administration |
| Tharakan | 2007 | Same drug intervention used in both arms and only different doses of drug administration |
| Tharakan | 2008 | Same drug intervention used in both arms and only different doses of drug administration |
| Thomas | 2006 | Same drug intervention used in both arms and only different doses of drug administration |
| Thomas | 2007 | Same drug intervention used in both arms and only different doses of drug administration |
| Thornton | 1987 | Quasi-randomised |
| Thornton | 1988 | Quasi-randomised |
| Tita | 2012 | Same drug intervention used in both arms and only different doses of drug administration |
| Tripti | 2006 | Not eligible intervention |
| Tripti | 2009 | Not eligible intervention |
| Tudor | 2006 | Same drug intervention used in both arms and only different doses of drug administration |
| Van den | 2009 | Not eligible uterotonic |
| Van Selm | 1995 | Not randomised |
| Vasegh | 2005 | Quasi-randomised |
| Vaughan | 1974 | No effectiveness outcomes reported |
| Ventoskovskiy | 1990 | Not eligible intervention |
| Verghese | 2008 | Not eligible intervention |
| Vogel | 2004 | Not eligible outcomes |
| Wallace | 2008 | Same drug intervention used in both arms and only different regimen of oxytocin administration |
| Walraven | 2005 | Not eligible uterotonic (oral ergometrine) |
| Wang | 2000 | Not eligible intervention |
| Weeks | 2013 | Self-administered drug |
| Weihong | 1998 | Not eligible intervention |
| Weiss | 1975 | Not eligible outcomes |
| Wetta | 2011 | Same drug intervention used in both arms and only different doses of drug administration |
| Wetta | 2013 | Same drug intervention used in both arms and only different doses of drug administration |
| Winikoff | 2012 | Same drug intervention used in both arms and only different doses of drug administration |
| Wong | 2006 | Same drug intervention used in both arms and only different doses of drug administration |
| Wright | 2006 | Not eligible intervention |
| Wu | 2007 | Not eligible intervention |
| Xu | 2003 | Not eligible intervention |
| Xu | 2013 | Not eligible intervention |
| Yamaguchi | 2011 | Same drug used in intervention both arms and only different doses of drug administration |
| Yan | 2000 | Not eligible intervention |
| Yang | 2001 | Not eligible intervention |
| Young | 1988 | Not eligible intervention |
| Zamora | 1999 | Not eligible intervention |
| Zaporozhan | 2013 | Not eligible intervention |
| Zhao | 1998 | Not eligible intervention |
| Zhao | 2003 | Not eligible intervention or able to extract outcomes |
| Zhou | 1994 | Same drug intervention used in both arms and only different doses of drug administration |
Appendix 5 Reference list for studies awaiting classification
Adanikin 2013
Adanikin AI, Orji E, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage after caesarean section. Nepal J Obstet Gynaecol 2013;8:34–7.
Adhikari 2007
Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal J Obstet Gynaecol 2007;2:24–8.
Ahmed 2015
Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. J Matern Fetal Neonatal Med 2015;28:1014–18.
Akinaga 2016
Akinaga C, Uchizaki S, Kurita T, Taniguchi M, Makino H, Suzuki A, et al. Randomized double-blind comparison of the effects of intramyometrial and intravenous oxytocin during elective cesarean section. J Obstet Gynaecol Res 2016;42:404–9.
Ali 2012
Ali R, Hina F. Postpartum haemorrhage; comparison of efficacy of ergometrine with misoprostol in prophylaxis in cesarean section. Prof Med J 2012;19:360–4.
Alli 2013
Alli QO. Comparing effectiveness of sublingual misoprostol with oxytocin infusion to reduce blood loss at caesarean section: double blind, randomized study. BJOG 2013;120:77–8.
Alwani 2014
Alwani M, Singh S, Thakur R, Mishra S. A randomised study comparing rectally administered misoprostol after spinal anaesthesia versus intramuscular oxytocin for prevention of postpartum haemorrhage in caesarean section. Int J Reprod Contracept Obstet Gynaecol 2014;3:512–5.
Ashwal 2016
Ashwal E, Hiersch L, Wertheimer A, Krispin E, Aviram A, Dayan DB, et al. The effect of post-partum oxytocin regimen on haemoglobin decline – a randomized controlled trial. Am J Obstet Gynaecol 2016;214(Suppl.):197–8.
Asmat 2017
Asmat R, Ashraf T, Asmat F, Asmat S, Asmat N. Effectiveness of Per Rectal Misoprostol Versus Intramuscular Oxytocin for Prevention of Primary Postpartum Haemorrhage. J Coll Physicians Surg Pak 2017;27:13–17.
Ayedi 2012
Ayedi M. Effects of Tranexamic Acid on Post Partum Haemorrhage by Uterine Atony After Cesarean Section Delivery: A Randomised, Placebo Controlled Trial. ClinicalTrials.gov. 2012. URL: clinicaltrials.gov/ct2/show/NCT01599468 (accessed 28 March 2016).
Baig 2015
Baig FS, Shahzad N, Khurshid HN, Malik A. Postpartum haemorrhage; comparison of intra umbilical and intra venous injection of oxytocin on blood loss in third stage of labour. Prof M J 2015;22:793–7.
Begum 2015
Begum T, Yeasmin S, Chakma S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss in caesarean section. BJOG 2015;122(Suppl. 1):258.
Beigi 2009
Beigi A, Tabarestani H, Moini A, Zarrinkoub F, Kazempour M, Hadian Amree A. [Sublingual misoprostol versus intravenous oxytocin in the management of postpartum haemorrhage.] Tehran Univ Med J 2009;67:556–61.
Bhatti 2014
Bhatti K, Mahar T, Hafeez R, Shoaib-u-Nisa. A randomised controlled trial on prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin. Med For Mon 2014;25:10–2.
Boopathi 2014
Boopathi A, Nayak SR, Rao A, Rao B. Oxytocin versus methylergometrine in the active management of third stage of labour. Open J Obstet Gynaecol 2014;4:666–71.
Carrillo-Gaucín 2016
Carrillo-Gaucín S, Torres-Gómez LG. [Carbetocin and oxytocin: Prevention of postpartum hemorrhage in patients with risk factors for uterine atony.] Rev Med Inst Mex Seguro Soc 2016;54(Suppl. 3):284–90.
Chalermpolprapa 2010
Chalermpolprapa V. Efficacy of sublingual misoprostol in prevention of postpartum haemorrhage in cesarean section: a randomized double-blinded, placebo-controlled trial. Regi 4–5 Med J 2010;29:325–35.
Chandhiok 2006
Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. Int J Gynaecol Obstet 2006;92:170–5.
Chatterjee 2016
Chatterjee S, Sarkar A, Rao KD. Using misoprostol for primary versus secondary prevention of postpartum haemorrhage – do costs matter? PLOS ONE 2016;11:e0164718.
Chaudhuri 2016
Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third-stage management among women at risk of postpartum haemorrhage. Int J Gynaecol Obstet 2016;132:191–5.
Chou 2015
Chou LT, Da AW, Murizah MZ1, Rushdan M, Rashid Z. A randomised controlled trial on low dose versus high dose oxytocin infusion in prevention of uterine atony at caesarean delivery. J Obstet Gynaecol Res 2015;41(Suppl. 1):44–5.
Cordovani 2011
Cordovani D, Farine D, Balki M, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: A dose-finding study. Can J Anaesth 2011;58(Suppl. 1):90.
Dabbaghi 2012
Dabbaghi Gale T, Elmizadeh KH, Moradi SD, Rashvand Melli E. [Comparison of intravenous oxytocin and oral misoprostol in reduction of postpartum haemorrhage.] J Zanjan Univ Med Sci 2012;20:1–8.
Dagdeviren 2016
Dagdeviren H, Cengiz H, Heydarova U, Caypinar SS, Kanawati A, Guven E, Ekin M. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Arch Gynecol Obstet 2016;294:911–16.
Dell-Kuster 2016
Dell-Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, Girard T. Efficacy and safety of carbetocin applied as an intravenous bolus compared to as a short-infusion for caesarean section: study protocol for a randomised controlled trial. Trials 2016;17:155.
Dell-Kuster 2016a
Dell-Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of intravenous carbetocin as a bolus compared to a short infusion for caesarean section. J Obstet Anaesth 2016;26(Suppl. 1):7.
Dell-Kuster 2017
Dell-Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin given as an intravenous bolus compared with short infusion for caesarean section – double-blind, double-dummy, randomised controlled non-inferiority trial. Brit J Anaesth 2017;118:772–80.
Deshpande 2016
Deshpande HG, Madkar CS, Patel KK. Comparative study between intravenous and intraumbilical oxytocin as active management of third stage in elective and emergency caesarean section. Indian J Obstet Gynaecol Res 2016;3:55–8.
Diop 2016
Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, et al. Oxytocin via Uniject (a prefilled single-use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster-randomised controlled trial. Lancet Glob Health 2016;4:e37–44.
Dutta 2016
Dutta BK, Gupta KR. A comparative study on rectal misoprostol versus intramuscular oxytocin to prevent postpartum haemorrhage. New Indian J Obgyn 2016;2:98–103.
Elbohoty 2016
Elbohoty AEH, Mohammed WE, Sweed M, Eldin AMB, Nabhan A, Abd-El-Maeboud KHI. Randomized controlled trial comparing carbetocin, misoprostol, and oxytocin for the prevention of postpartum haemorrhage following an elective cesarean delivery. Int J Gynaecol Obstet 2016;134:324–8.
Fahmy 2015
Fahmy AA, Fawzy M. Oxytocin infusion after oxytocin bolus and carbetocin bolus to reduce blood loss during and after cesarean section – a randomised clinical trial. Med J Cairo Univ 2015;83:79–83.
Fahmy 2016
Fahmy NG, Yousef HM, Zaki HV. Comparative study between effect of carbetocin and oxytocin on isoflurane-induced uterine hypotonia in twin pregnancy patients undergoing cesarean section. Egypt J Anaesth 2016;32:117–21.
Fakour 2013
Fakour F, Mirzayi M, Reza Naghipour M, Ebrahimi H, Mahdavi M. Comparison between sublingual misoprostol and intravenous oxytocin in management of third stage of labor. Iran J Obstet Gynaecol Infert 2013;15:7–14.
Frye 2012
Frye LJ, Diop AR, Kone Y. Comparing Misoprostol and Oxytocin in UnijectTM for Postpartum Hemorrhage (PPH) Prevention in Mali. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01487278 (accessed 28 March 2016).
Frye 2015
Frye L, Durocher J, Weeks A, Ditai J, Ononge S, Faragher B, et al. On the trail of misoprostol in the community: A secondary analysis of self-administered misoprostol for the prevention of postpartum haemorrhage in Uganda. Int J Gynaecol Obstet 2015;131(Suppl. 5):e354–5.
Fuks 2014
Fuks AM, Khanna P, Yusaf T, Aslian A, Kowalska D, Salafia CM. Use of prophylactic misoprostol in reduction of blood loss at vaginal delivery. Obstet Gynaecol 2014;123(Suppl.):144–5.
Ghulmiyyah 2017
Ghulmiyyah LM, Usta i.m., Ghazeeri G, Taher N, Abu-Ghannam G, Tamim H, Nassar AH. Intravenous oxytocin use to decrease blood loss during scheduled cesarean delivery: a randomized double-blinded controlled trial (OXYTRIAL). Am J Perinatol 2017;34:379–87.
Gülmezoglu 2015
Gülmezoglu M. The WHO champion trial. Int J Gynaecol Obstet 2015;131(Suppl. 5):E29–30.
Hernandez-Castro 2016
Hernandez-Castro F, Lopez-Serna N, Trevino-Salinas EM, Soria-Lopez JA, Sordia-Hernandez LH, Cardenas-Estrada E. Randomized double-blind placebo-controlled trial of buccal misoprostol to reduce the need for additional uterotonic drugs during cesarean delivery. Int J Gynaecol Obstet 2016;132:184–7.
Islam 2008
Islam A, Siraj A, Arif N. Post partum haemorrhage prophylaxis; comparison of the efficacy of misoprostol and ergometrine in cesarean delivery. Prof Med J 2008;15:323–7.
Jagielska 2015
Jagielska I, Kazdepka-Ziemińska A, Kaczorowska A, Madej A, Kolossa T, Grabiec M. [Evaluation of carbetocin and oxytocin efficacy in prevention of postpartum hemorrhage in women after cesarean section.] Ginekol Pol 2015;86:689–93.
Jans 2017
Jans S, Herschderfer KC, van Diem MT, Aitink M, Rijnders M, van der Pal-Bruin K, et al. LENTE Study: Effectiveness of Prophylactic Intramuscular Oxytocin During Third Stage of Labour Among Low Risk Women. A Randomised Controlled Trial. Midwives – Making a Difference in the World. Proceedings of the 31st International Confederation of Midwives Triennial Congress, Toronto, ON, Canada, 18–22 June 2017.
Javadi 2015
Javadi EHS, Sadeghipour Z, Barikani A, Javadi M. Tranexamic acid in the control of uterine atony during labor. Biotech Health Sci 2015;2:e26898.
Kabir 2015
Kabir N, Akter D, Daisy TA, Jesmin S, Razzak M, Tasnim S, et al. Efficacy and safety of carbetocin in comparison to oxytocin in the active management of third stage of labour following vaginal delivery: an open label randomized control trial. Bangladesh J Obstet Gynaecol 2015;30:3–9.
Khan 2013
Khan M, Balki M, Ahmed I, Farine D, Searward G, Carvalho JCA. Carbetocin at Elective Cesarean Delivery: A Randomised Controlled Trial to Determine the Effective Dose, Part 3 Final. Society for Obstetric Anaesthesia and Perinatology, 45th Annual Meeting, San Juan, Puerto Rico, 24–8 April 2013.
Koen 2016
Koen S, Snyman LC, Pattinson RC, Makin JA. A randomised controlled trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. S Afr Med J 2016;106:55–6.
Liu 2015
Liu Y, Chen HX, Kang DL, Kuang XH, Liu WX, Ni J. Influence of dexmedetomidine on incidence of adverse reactions introduced by hemabate in postpartum hemorrhage during cesarean section. Int J Clin Exp Med 2015;8:13776–82.
Liu 2016
Liu W, Ma S, Pan W, Tan W. Combination of motherwort injection and oxytocin for the prevention of postpartum hemorrhage after cesarean section. J Matern Fetal Neonatal Med 2016;29:2490–3.
Maged 2015
Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, et al. A randomized placebo-controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. Int J Gynaecol Obstet 2015;131:265–8.
Maged 2017
Maged AM, Ragab AS, Elnassery N, Al Mostafa W, Dahab S, Kotb A. Carbetocin versus Syntometrine for prevention of postpartum haemorrhage after cesarean section. J Matern Fetal Neonatal Med 2017;30:962–6.
Makvandi 2013
Makvandi S, Shoushtari SZ, Hosseini VZ. Management of third stage of labour: A comparison of intraumbilical oxytocin and placental cord drainage. Shiraz E Med J 2013;14:83–90.
Mirteimouri 2013
Mirteimouri M, Tara F, Teimouri B, Sakhavar N, Vaezi A. Efficacy of rectal misoprostol for prevention of postpartum hemorrhage. Iran J Pharm Res 2013;12:469–74.
Mockler 2015
Mockler JC, Malkoutzis V, Davis-Tuck M, Wallace EM. Oxytocin infusion at elective caesarean section: a double blind, randomised controlled trial. J Paed Child Heal 2015;51(Suppl. 1):54.
Modi 2014
Modi V, Goel JK, Kashyap A, Arya SB, Kar J, Goel R. Active management of third stage of labour: A comparison of various uterotonic. J South Asian Fed Obstet Gynaecol 2014;6:151–5.
Mohamadian 2013
Mohamadian S, Shorab NJ, Mirzakhani K. The effect of the timing of intramuscular oxytocin injection on maternal bleeding during the third stage of labour. J Midwif Reprod Heal 2013;1:66–70.
Mohamed 2015
Mohamed HF, Mustafa GF, Ibrahim MA, Stefanos GE. Comparative study between intravenous bolus dose of carbetocin versus oxytocin during cesarean delivery in healthy parturients on blood loss: a randomized control trial. Med J Cairo Uni 2015;83:167–72.
Murphy 2015
Murphy D. A Study to Compare the Effectiveness of Intravenous Oxytocin with Intramuscular Oxytocin Given at the Third Stage of Labour at Preventing Bleeding at Vaginal Birth. BMC. 2015. URL: isrctn.com/ISRCTN14718882 (accessed 28 March 2016).
Nankaly 2016
Nankaly A, Jalilian N, Eshghiali S, Rezaei M. [The effects of sublingual misoprostol and intravenous oxytocin in reducing bleeding among cesarean deliveries.] Acta Med Mediterr 2016;32:953–7.
Narenji 2012
Narenji F. Comparison the Effect of Intramuscular Injection of Oxytocin and Nipple Stimulation on the Third Stage of Delivery Length and Bleeding. IRCT Iranian Registry of Clinical Trials. 2012. URL: www.irct.ir/trial/10487 (accessed 16 July 2015).
Neri-Mejia 2016
Neri-Mejía M, Pedraza-Avilés AG. [Active management of the third stage of labor: Three schemes of oxytocin: randomised clinical trial.] Ginecol Obstet Mex 2016;84:306–13.
Ng 2004
Ng PS, Yuen PM, Sahota DS. Comparison of Oral Misoprostol and Intravascular Syntocinon in the Management of the Third Stage of Labour – A Double-blind Randomised Controlled Trial. Proceedings of the 30th British Congress of Obstetrics and Gynaecology, Glasgow, UK, 7–9 July 2004.
Nguyen-Lu 2015
Nguyen-Lu N, Carvalho JC, Farine D, Seaward G, Ye XY, Balki M. Carbetocin at Cesarean delivery for labour arrest: a sequential allocation trial to determine the effective dose. Can J Anaesth 2015;62:866–74.
Ononge 2015
Ononge S, Campbell OM, Kaharuza F, Lewis JJ, Fielding K, Mirembe F. Effectiveness and safety of misoprostol distributed to antenatal women to prevent postpartum haemorrhage after child-births: a stepped–wedge cluster-randomized trial. BMC Pregnancy Childbirth 2015;15:315.
Othman 2016
Othman ER, Fayez MF, El Aal DE, El-Dine Mohamed HS, Abbas AM, Ali MK. Sublingual misoprostol versus intravenous oxytocin in reducing bleeding during and after cesarean delivery: A randomized clinical trial. Taiwan J Obstet Gynecol 2016;55:791–5.
Pakniat 2015
Pakniat H, Khezri MB. The effect of combined oxytocin-misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomised double-blind study. J Obstet Gynaecol India 2015;65:376–81.
Patil 2013
Patil NB, Patted SS. A randomised controlled trial of oral misoprostol vs injection methylergometrine for prevention of post partum haemorrhage. Int J Reprod Contracept Obstet Gynaecol 2013;2:296–303.
Quibel 2016
Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, et al. Active Management of the Third Stage of Labor With a Combination of Oxytocin and Misoprostol to Prevent Postpartum Hemorrhage: A Randomized Controlled Trial. Obstet Gynecol 2016;128:805–11.
Rabow 2017
Rabow S, Jonsson E, Jonsson H, Olofsson P. [Cardiovascular effects of oxytocin and carbetocin at caesarean section, a prospective double-blind randomised study using non-invasive pulse wave analysis.] Acta Anaesthesiol Scand 2017;61:1053.
Ragab 2016
Ragab A, Barakat R, Alsammani MA. A randomized clinical trial of preoperative versus postoperative misoprostol in elective cesarean delivery. Int J Gynaecol Obstet 2016;132:82–4.
Raghavan 2016
Raghavan S, Geller S, Miller S, Goudar SS, Anger H, Yadavannavar MC, et al. Misoprostol for primary versus secondary prevention of postpartum haemorrhage: a cluster-randomised non-inferiority community trial. BJOG 2016;123:120–7.
Ray 2012
Ray D, Ghosh S, Bhattacharya S, Mandal RD, Basak A. Oxytocin Administration During Caesarean Delivery: Comparison Between Bolus versus Infusion. Society for Obstetric Anaesthesia and Perinatology, 44th Annual Meeting, Monterey, CA, USA, 2–5 May 2012.
Razali 2016
Razali N, Md Latar IL, Chan YK, Omar SZ, Tan PC. Carbetocin compared to oxytocin in emergency cesarean section: a randomized trial. Eur J Obstet Gynecol Reprod Biol 2016;198:35–9.
Reyes 2011
Reyes OA. [Carbetocin vs oxytocin for the prevention of postpartum haemorrhage in grand multiparous patients: a randomized controlled trial.] Clin Invest Ginecol Obstet 2011;38:2–7.
Rosales-Ortiz 2014
Rosales-Ortiz S, Aguado RP, Hernandez RS, Castorena M, Cristobal FL, Gonzalez MC, et al. Carbetocin versus oxytocin for prevention of postpartum haemorrhage: a randomised controlled trial. Lancet 2014;383:S51.
Sangkhomkhamhang 2012
Sangkhomkhamhang U. A Randomised Controlled Trial of Intravenous versus Intramuscular Oxytocin in the Management of Third Stage of Labour. Australian New Zealand Clinical Trials Registry. 2012. URL: anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN = 12612000624886 (first received 12 June 2012) (accessed 28 March 2016).
Sentilhes 2015
Sentilhes L, Daniel V, Darsonval A, Deruelle P, Vardon D, Perrotin F, et al. Study protocol. TRAAP – TRAnexamic Acid for Preventing postpartum haemorrhage after vaginal delivery: a multicenter randomised, double-blind, placebo-controlled trial. BMC Pregnancy Childbirth 2015;15:135.
Sentürk 2016
Sentürk Ş, Kağıtçı M, Balık G, Arslan H, Kır Şahin F. The effect of the combined use of methylergonovine and oxytocin during caesarean section in the prevention of post-partum haemorrhage. Basic Clin Pharmacol Toxicol 2016;118:338–43.
Shrestha 2008
Shrestha A, Urala MS, Upreti D, Niraula S. Comparison of intramyometrial and intramuscular 15 methyl PGF2x against traditional prophylactic intramuscular methergin for the active management of third stage of labor. Nepal J Obstet Gynaecol 2008;3:35–9.
Shrivasatava 2012
Shrivasatava DD, Khamsara D. Critical evaluation of sublingual misoprostol and methyl ergometrine in active management of third stage of labour. Int J Gynaecol Obstet 2012;119(Suppl. 3):484.
Soleimani 2014
Soleimani Z, Naini AA. [The effectiveness of sublingual misoprostol in prevention of bleeding during cesarean delivery.] Iran J Obstet Gynaecol Infert 2014;17:1–7.
Sunil 2016
Sunil Kumar KS, Shyam S, Batakurki P. Carboprost versus oxytocin for active management of third stage of labor: a prospective randomized control study. J Obstet Gynaecol India 2016;66(Suppl. 1):229–34.
Taheripanah 2017
Taheripanah R, Shoman A, Ali Karimzadeh M, Zamaniyan M, Malih N. Efficacy of oxytocin vs carbetocin in prevention of postpartum haemorrhage after cesarean section under general anaesthesia: a prospective randomised clinical trial. J Matern Fetal Neonatal Med 2018;31:2807–12.
Tali 2016
Tali K, Ignacio Alensuela A. The effect of prophylactic intravenous tranexamic acid in reducing blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a prospective, randomised, double-blind, placebo-controlled study. Aust N Z J Obstet Gynaecol 2016;56(Suppl. 1):61.
Ugwu 2016
Ugwu IA, Oluwasola TA, Enabor OO, Anayochukwu-Ugwu NN, Adeyemi AB, Olayemi OO. Randomized controlled trial comparing 200µg and 400µg sublingual misoprostol for prevention of primary postpartum hemorrhage. Int J Gynaecol Obstet 2016;133:173–7.
Un Nisa 2012
Un Nisa S, Usmani SY. Role of intravenous Syntocinon in prevention of primary postpartum haemorrhage. Pak J Med Health Sci 2012;6:1020–3.
Vlassoff 2016
Vlassoff M, Diallo A, Philbin J, Kost K, Bankole A. Cost-effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. Int J Gynaecol Obstet 2016;133:307–11.
Voltolini 2012
Voltolini C, De Bonis M, Vellucci F, Regini C, Orlandini C, Vannuccini S, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. Int J Gynaecol Obstet 2012;119:S806–7.
Weeks 2015
Weeks AD, Ditai J, Ononge S, Faragher B, Frye LJ, Durocher J, et al. The MamaMiso study of self-administered misoprostol to prevent bleeding after childbirth in rural Uganda: a community-based, placebo-controlled randomised trial. BMC Pregnancy Childbirth 2015;15:219.
Whigham 2016
Whigham CA, Gorelik A, Loughnan TE, Trivedi A. Carbetocin versus oxytocin to reduce additional uterotonic use at non-elective caesarean section: a double-blind, randomised trial (.). J Matern Fetal Neonatal Med 2016;29:3866–9.
Widmer 2016a
Widmer M, Piaggio G, Abdel-Aleem H, Carroli G, Chong YS, Coomarasamy A, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trials 2016;17:143.
Winikoff 2016
Winikoff B, Dzuba I, Carroli G. i.v Versus i.m. Oxytocin for Postpartum Bleeding. ClinicalTrials.gov. 2016. URL: clinicaltrials.gov/show/NCT02954068 (accessed 28 March 2016).
Appendix 6 Reference list for ongoing studies
Castro 2012
Castro FH. Buccal Misoprostol During Cesarean Section for Preventing Postpartum Haemorrhage. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01733329 (accessed 21 May 2013).
Frye 2012
Frye LJ, Diop AR, Kone Y. Comparing Misoprostol and Oxytocin in UnijectTM for Postpartum Haemorrhage (PPH) Prevention in Mali. ClinicalTrials.gov. 2011. URL: https://clinicaltrials.gov/ct2/show/NCT01487278 (accessed 28 March 2016).
Gomez 2011
Gomez MCG. Comparison of the Effectiveness of Carbetocin vs Oxytocin in Managing the Third Stage of Labour in a Group of Women with Risk Factors for Postpartum Haemorrhage. Australian New Zealand Clinical Trials Registry. 2011. URL: www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335628&isReview=true (accessed 16 February 2011).
Kalahroudi 2010
Kalahroudi MA. Comparison Effect of Carbetocin and Syntometrin in Prevention of Post Partum Haemorrhage. IRCT Iranian Registry of Clinical Trials. 2010. URL: www.irct.ir/trial/2719 (accessed 6 December 2010).
Kalahroudi 2010a
Kalahroudi MA. Comparison of the Effect of Rectal Misoprostol and Syntometrin in Prevention of Post Partum Haemorrhage. IRCT Iranian Registry of Clinical Trials. 2010. URL: www.irct.ir/trial/2720 (accessed 6 December 2010).
Moradi 2010
Moradi S. Comparison of Misoprostol and Oxytocin in Reduction of Postpartum Haemorrhage. IRCT Iranian Registry of Clinical Trials. 2010. URL: www.irct.ir (accessed 6 December 2010).
Moss 2006
Moss NM. Oral Misoprostol for Prevention of Postpartum Haemorrhage (Ongoing Trial). ClinicalTrials.gov. 2006. [http://clinicaltrials.gov/] (accessed 21 March 2006).
NCT01487278 2011
NCT01487278. Comparing Misoprostol and Oxytocin in UnijectTM postpartum haemorrhage (PPH) prevention in Mali. ClinicalTrials.gov. 2011. URL: https://clinicaltrials.gov/ct2/show/study/NCT01487278 (accessed 1 February 2015).
NCT01713153 2012
NCT01713153. Comparing Misoprostol and Oxytocin in Uniject for Postpartum Haemorrhage (PPH) Prevention in Senegal. ClinicalTrials.gov. 2012. URL: https://clinicaltrials.gov/ct2/show/NCT01713153 (accessed 9 October 2013).
Shahboodaghi 2013
Shahboodaghi Z. Misoprostol versus Oxytocin for Prevention of Post Partum Haemorrhage. ClinicalTrials.gov. 2013. URL: https://clinicaltrials.gov/ct2/show/NCT01863706 (accessed 5 February 2014).
Sweed 2014
Sweed MS. Comparison Between Rectal and Sublingual Misoprostol Before Caesarean Section to Reduce Intra & Post-Operative Blood Loss. ClinicalTrials.gov. 2014. URL: https://clinicaltrials.gov/ct2/show/NCT02083107 (accessed 24 March 2014).
Appendix 7 Additional data from triallists
| First author and publication year | Additional data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adanikin, 201239 |
Additional data retrieved from: Adanikin A, Orji E, Adanikin P, Olaniyan O. Comparative study of rectal misoprostol to oxytocin in preventing postpartum haemorrhage post caesarean section. Int J Gynaecol Obstet 2012:119(Suppl. 3):S825 |
|||||||||
| Al-Sawaf, 201342 | Response to e-mail queries | |||||||||
| Trial arm | Number of events | Number of participants in trial arm | ||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Control | 8 | 39 | ||||||||
| Misoprostol | 3 | 28 | ||||||||
| Oxytocin | 2 | 37 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Control | 6 | 39 | ||||||||
| Misoprostol | 2 | 28 | ||||||||
| Oxytocin | 1 | 37 | ||||||||
| Change in Hb levels after delivery (g/dl) | ||||||||||
| Trial arm | Mean Hb level (g/dl) change (SD) | Number of participants in trial arm | ||||||||
| Control | 1.3 (0.6) | 39 | ||||||||
| Misoprostol | 1.3 (0.9) | 28 | ||||||||
| Oxytocin | 1.2 (0.9) | 37 | ||||||||
| Amin, 201444 | Response to e-mail queries | |||||||||
|
All patients included in the study were admitted through emergency and operations After a complete history and examination, women who had undergone a previous caesarean section or experienced a traumatic PPQ, bleeding disorders, prolonged difficult labour, placenta previa, placental abruption, PPH or multiple gestations, and women having a BMI of > 30 kg/m2 were excluded However, all other women with a full-term pregnancy and who came to a labour room in spontaneous onset of labour resulting in spontaneous vaginal delivery without episiotomy were included in the study |
||||||||||
| High-risk patients were not included in the study, so no death or major morbidity were noted | ||||||||||
| Askar, 201145 | Response to e-mail queries | |||||||||
| Hypertension | ||||||||||
| Trial arm | Duration (minutes) of third stage of labour (number of women) | Number of participants in trial arm | ||||||||
| 30–60 | 60–120 | |||||||||
| Intervention (carbetocin) | 0 | 0 | 0 | |||||||
| Control (Syntometrine) (these are the same patients) | 7 | 7 | 7 | |||||||
| Attilakos, 201046 | Response to e-mail queries | |||||||||
| Trial arm | ||||||||||
| Carbetocin ( n = 22) | Oxytocin ( n = 26) | |||||||||
| Nausea, n = 1 | Nausea, n = 2 | |||||||||
| Nausea and flushed, n = 2 | Vomiting, n = 3 | |||||||||
| Nausea and headache, n = 1 | Vomiting and trigeminy, n = 1 | |||||||||
| Nausea and abdominal pain, n = 1 | Nausea and headache, n = 1 | |||||||||
| Nausea and vomiting, n = 2 | Nausea and vomiting, n = 2 | |||||||||
| Nausea, vomiting and sweating, n = 1 | Nausea, vomiting and shortness of breath, n = 1 | |||||||||
| Nausea, vomiting and tremors, n = 1 | Nausea, vomiting and tremors, n = 1 | |||||||||
| Nausea, vomiting, flushed and hypotension, n = 1 | Nausea, vomiting, flushed and tremors, n = 1 | |||||||||
| Tight throat, n = 1 | Dizziness, n = 2 | |||||||||
| Dizziness, n = 2 | Dizziness, flushed and sweating, n = 1 | |||||||||
| Flushed, n = 1 | Hypotension, n = 2 | |||||||||
| Hypotension, n = 2 | Tremors, n = 2 | |||||||||
| Hypotension and shortness of breath, n = 1 | Shortness of breath, n = 1 | |||||||||
| ST segment depression, n = 1 | Blurred vision, n = 1 | |||||||||
| Tachycardia, n = 1 | Metallic taste in mouth, n = 1 | |||||||||
| Tremors and tachycardia, n = 1 | Pain in arm, n = 1 | |||||||||
| Metallic taste in mouth, shortness of breath and wheezing, n = 1 | Abdominal pain and shortness of breath, n = 1 | |||||||||
| Metallic taste in mouth and pressure over forehead, n = 1 | Backache, n = 1 | |||||||||
| Headache, n = 1 | Headache, n = 1 | |||||||||
| Atukunda, 201447 | Attachment to response to e-mail queries | |||||||||
| Trial arm | Events, n (%) | Number of participants in trial arm | ||||||||
| Vomiting (generally) | ||||||||||
| Misoprostol | 35 (6.1) | 569 | ||||||||
| Oxytocin | 19 (3.3) | 570 | ||||||||
| Vomiting (severe) | ||||||||||
| Misoprostol | 8 (1.4) | 569 | ||||||||
| Oxytocin | 3 (0.5) | 570 | ||||||||
| Morbidity (extensive vaginal repair) | ||||||||||
| Misoprostol | 11 (1.9) | 570 | ||||||||
| Oxytocin | 8 (1.4) | 570 | ||||||||
| Bamigboye, 199850 | Response to e-mail queries | |||||||||
|
The authors did not document the routine drugs used in the active management of labour in each case At two sites in South Africa (East London and Dora Nginza, Port Elizabeth), and in Uganda the routine was 10 units of i.m. oxytocin At the third site in South Africa (Rob Ferreira) 5 units of i.m. oxytocin was used in 60 out of 155 cases and oxytocin–ergometrine (5 units/0.5 mg) was used in 85 out of 155 cases In Nigeria, either oxytocin or ergometrine was used, but the authors did not have the details As this was a randomised trial, it was expected that the routine management be evenly distributed between the randomised groups |
||||||||||
| Begley, 199054 | Response to e-mail queries | |||||||||
|
Random number tables were used from the statistical textbook by Fleiss203 The first number was selected from the table by a disinterested observer and the numbers were then allocated in blocks of 100, following in sequence |
||||||||||
| Trial arm | Duration (minutes) of third stage of labour (number of women) | Mean number of women (SD/CI) | Total number of women | |||||||
| 0–20 | 21–40 | 41–60 | 61–80 | 81–100 | 101–120 | > 120 | ||||
| Intervention (active) | 674 | 11 | 4 | 0 | 2 | 8 | 6 | 11.26 (19.62) | 705 | |
| Control (physiological) | 670 | 41 | 7 | 1 | 4 | 1 | 0 | 11.56 (8.41) | 724 | |
| Trial arm | Change in Hb levels (g/dl) | Total number of patients | ||||||||
| Mean change in Hb level (g/dl) | SD/CI (g/dl) | |||||||||
| Intervention (active) | + 0.91 | 1.19 | 618 | |||||||
| Control (physiological) | + 0.47 | 1.27 | 645 | |||||||
| Bellad, 201255 | Response to e-mail queries | |||||||||
|
Four women in the misoprostol group and none in the oxytocin group experienced fever (defined as a temperature of > 38 °C); this was entered onto the form as a dichotomous variable and the authors have no information regarding the actual temperature (or whether or not any woman experienced a temperature of > 40 °C) One woman in the oxytocin group had retained placenta and had a blood transfusion; this was the only case of transfusion and required intensive care unit admission for monitoring There were no other complications (e.g. organ failure) and no maternal deaths |
||||||||||
| 58/329 women receiving oxytocin (17.6%) had a second stage of labour of ≥ 30 minutes | ||||||||||
| 53/323 women receiving sublingual misoprostol (16.4%) had a second stage of labour of ≥ 30 minutes | ||||||||||
| One woman receiving oxytocin and no women receiving sublingual misoprostol had a third stage of labour of ≥ 30 minutes | ||||||||||
| Bhullar, 200457 | Response to e-mail queries | |||||||||
| I do not have the raw data anymore, but I am certain we did not have any maternal deaths | ||||||||||
| Bugalho, 200161 | Additional data extracted from published Cochrane review(s)17 | |||||||||
| Chaudhuri, 201268 | Additional data extracted from published Cochrane review(s)17 | |||||||||
| Chhabra, 200870 | Response to e-mail queries | |||||||||
| This was a low-dose study in low-risk cases for prophylaxis | ||||||||||
| The number of women (n/N) in each study group (if any) who needed major surgery: 0 | ||||||||||
| The number of women (n/N) in each study group (if any) who needed ICU admission: 0 | ||||||||||
| The number of women (n/N) in each study group (if any) who had hyperpyrexia (i.e. a temperature of > 40 °C): 0 | ||||||||||
| The number of women (n/N) in each study group (if any) who had vital organ failure: 0 | ||||||||||
| The number of women (n/N) in each study group (if any) who had an estimated blood loss of > 1000 ml: 0 | ||||||||||
| The number of women (n/N) in each study group (if any) who died: 0 | ||||||||||
| Dansereau, 199973 | Response to e-mail queries | |||||||||
|
The paper should have stated that:Because of the difficulty in assessing estimated blood loss, the authors had decided – before the beginning of the study – to not use that variable but to use the judgment of the surgeon (blinded to the study drug), as to whether or not the patient needed additional oxytocic drugs (required in all cases of PPH) Clearly, more than two patients per group had a PPH blood loss of > 500 or even 1000 ml The exact number is not available though, as it was decided not to use this outcome of PPH in the study |
||||||||||
| El Behery, 201580 | Response to e-mail queries: | |||||||||
| Trial arm | Number of events | |||||||||
| PPH > 500 ml | ||||||||||
| Carbetocin | 6 | |||||||||
| Oxytocin | 19 | |||||||||
| Major morbidity or death | ||||||||||
| Carbetocin | 0 | |||||||||
| Oxytocin | 3 | |||||||||
| The authors excluded the following cases from their study: congenital fetal anomalies, placenta previa, diabetes mellitus, hypertension, preeclampsia, cardiac disorders and general anaesthesia | ||||||||||
| El Tahan, 201281 | Response to e-mail queries | |||||||||
Any of the following:
|
||||||||||
| Enakpene, 200785 | Response to e-mail queries | |||||||||
| There were no deaths recorded in either group | ||||||||||
| None of the study participants required additional surgery, such as hysterectomy or arterial ligation, to treat massive postpartum haemorrhage | ||||||||||
| There was only one ICU admission in the misoprostol group for a non-haemorrhage-related condition but caused by postpartum eclampsia | ||||||||||
| No participants in each study group developed hyperpyrexia with a temperature of > 40 °C | ||||||||||
| No participants developed major organ failure | ||||||||||
|
Three participants from the oral misoprostol group had a massive PPH blood loss of > 1000 ml, whereas only one participant in the methylergometrine group developed a massive PPH However, all four women who developed a massive haemorrhage responded very well with additional oxytocic drugs and did not require surgical interventions |
||||||||||
| Fenix, 201290 | Additional data retrieved from an unpublished text entitled ‘Double-blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum haemorrhage among high risk women following vaginal delivery’204 | |||||||||
|
Results The study was conducted over a 4-month period (from May 2011 to August 2011) There was a total of 272 deliveries in our hospital during the study period, of which 111 delivered vaginally Seventy-five women were finally recruited into the study Nine women in the carbetocin group and six women in the oxytocin group failed to have a paired Hb test to measure the change in Hb level 24 hours after delivery because they refused further blood extraction These 15 women were excluded and, therefore, the study had 30 women each in the carbetocin and oxytocin arm in the analysis who were randomly assigned to receive either of the two different interventions There was no significant difference between the two groups in their demographic characteristics (Table 1) Most of the participants were college degree holders, with an average age of 30 years The average age of gestation was 38 weeks for the carbetocin group, whereas it was almost 39 weeks’ gestation in the oxytocin group It was also observed that about two-thirds were multigravid women for both groups The average Hb count 24 hours after delivery of the participants for the oxytocin group (–1.1g/dl) seems to have a greater drop than those in the carbetocin group (–0.6g/dl)204 Participants in the carbetocin group exhibited a relatively lower average estimated blood loss than those in the oxytocin group (296 cc and 493 cc, respectively) There was no case of PPH between the two trial groups The distribution of exposure to additional agents revealed that 9 out of 10 patients in the oxytocin group needed additional uterotonic agents In contrast, 90% of the participants in the carbetocin group did not need any additional agent after drug administration In addition, it was noted that almost all of the patients in the oxytocin group needed a uterine massage compared with a negligible number of those in the carbetocin group Meanwhile, none of the patients needed a blood transfusion204 Carbetocin immediately (1 minute) took effect in the patients of the carbetocin group while those patients in the oxytocin group waited for some time (i.e. > 30 minutes) for oxytocin to take effect204 Adverse effects are presented204 The incidences of headache and hypogastric pain were similar in between trial groups There were no nausea, vomiting, facial flushing or pain in the injection site noted Twenty per cent or 6 out of 30 women in the carbetocin group had tachycardia (defined as a maternal pulse rate of ‡ 100 b.p.m.) within 60 minutes post delivery and were significantly higher than the 10% (3 out of 30) recorded in the oxytocin group; however, the difference was statistically insignificant The mean blood pressure values at different intervals after delivery of each group are also shown,204 although no statistical difference was observed between the two trial interventions To determine if there is a significant difference between the two drugs, the authors will need to perform independent sample t-tests Prior to performing the test, we need to satisfy its assumptions which are as follows:Based on the results, the authors can conclude that there was a significant difference between the carbetocin and oxytocin groups, as the p-values for the estimated mean blood loss and mean difference of the Hb count were approximately zero (i.e. a < LOS = 0.05)204 Looking at the mean difference of the Hb count, having a value of 0.57 implies that carbetocin garnered a significantly lower change in the Hb count after 24 hours204 The mean difference of the estimated blood loss, with value of –197.33 ml, denotes a statistically lower blood loss for women exposed to carbetocin than those who were exposed to oxytocin204 |
||||||||||
| Baseline characteristics of patients – see reference number 204 | ||||||||||
| Primary outcome (peripartum Hb concentration) – see reference number 204 | ||||||||||
| Secondary outcomes – see reference number 204 | ||||||||||
| Adverse reactions – see reference number 204 | ||||||||||
| t-test for independent samples means – see reference number 204 | ||||||||||
| Q–Q plot of estimated blood loss – see reference number 204 | ||||||||||
| Q–Q plot of difference of preoperative Hb count and 24-hour Hb count – see reference number 204 | ||||||||||
| Response to e-mail queries | ||||||||||
|
Thirty parturients received oxytocin with a mean blood loss of 493 ml, but there were no cases of blood loss of > 500 ml because the estimated blood loss during delivery was measured only through eyeballing of the gauzes used In the estimation, the authors did not include the bleeding coming from repair of the laceration One of the recommendations for future studies is to measure the actual blood loss using a more accurate device of measurement In addition, because the estimation of blood loss is often inaccurate during delivery, it was agreed that a fall in Hb level be used as a primary outcome assessing the efficacy of the uterotonic agents in reducing postpartum haemorrhage |
||||||||||
| Gavilanes, 201593 | Response to e-mail queries | |||||||||
| Trial group | Number of events | Total | ||||||||
| PPH blood loss of 500–1000 ml | ||||||||||
| Misoprostol | 33 | 50 | ||||||||
| Oxytocin | 26 | 50 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Misoprostol | 12 | 50 | ||||||||
| Oxytocin | 13 | 50 | ||||||||
|
None of the women had major morbidity There were no deaths either |
||||||||||
| Gülmezoglu, 200195 | Additional data extracted from published Cochrane review(s)17 | |||||||||
| Hofmeyr, 199899 | Response to e-mail queries | |||||||||
| Dosage | Trial arm, n (%) | Relative risk (95% CI) | p -value | |||||||
| Misoprostol ( N = 36) | Placebo ( N = 37) | |||||||||
| > 500 ml | 8 (22) | 15 (41) | 0.55 (0.27 to 1.13) | 0.15 | ||||||
| > 1000 ml | 2 (5.6) | 5 (14) | 0.41 (0.09 to 1.98) | 0.23 | ||||||
| Additional oxytocic drug | 2 (5.6) | 7 (19) | 0.29 (0.07 to 1.32) | 0.08 | ||||||
| Hofmeyr, 2011100 | Response to e-mail queries | |||||||||
| All nine pyrexias were between 39 and 39.9 °C, none was ≥ 40 °C | ||||||||||
| The only severe morbidity that was recorded were the nine laparotomy patients, of whom one had a hysterectomy | ||||||||||
|
There was no overlap of data The Nigeria site in Hofmeyr 2011 was the University College Hospital, Ibadan Fawole 2011 included two other hospitals in Ibadan and other Nigerian sites The University College Hospital occurs in the title, as that is Bukola’s base, but this was not a site |
||||||||||
|
Additional data also retrieved from: Hofmeyr GJ, Gülmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G. Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ 2009;87:666–77 |
||||||||||
|
Additional data were also retrieved from: Hofmeyr GJ, Ferreira S, Nikodem VC, Mangesi L, Singata M, Jafta Z, et al. Misoprostol for treating postpartum haemorrhage: a randomized controlled trial. BMC Pregnancy Childbirth 2004;4:167 |
||||||||||
| Jerbi, 2007107 | Response to e-mail queries | |||||||||
| No blood loss of > 1000 ml in any trial group | ||||||||||
| No transfusion or maternal death in the two trial groups | ||||||||||
| Lapaire, 2006115 | Response to e-mail queries | |||||||||
| Blood loss | Trial arm (number of women) | |||||||||
| Misoprostol ( N = 24) | Oxytocin ( N = 19) | |||||||||
| Calculated | ||||||||||
| > 500 ml | 18 | 15 | ||||||||
| > 1000 ml | 13 | 11 | ||||||||
| Misoprostol (N = 28) | Oxytocin (N = 28) | |||||||||
| Estimated | ||||||||||
| > 500 ml | 18 | 10 | ||||||||
| > 1000 ml | 1 | 14 | ||||||||
| Musa, 2015127 | Response to e-mail queries | |||||||||
|
There was no postpartum blood loss of > 1000 ml in both trial groups Range of blood loss was 20–790 ml in the misoprostol group and 40–790 ml in the oxytocin group There were no maternal deaths recorded, though participants were followed up only in the early puerperium There was no major morbidity The only morbidity recorded was retained placenta that warranted a manual removal of placenta The two cases occurred in the oxytocin group and none in the misoprostol group |
||||||||||
| Nasr, 2009128 | Response to e-mail queries | |||||||||
| No women in either group needed major surgery or ICU admission, nor did any have hyperpyrexia, massive bleeding of > 1000 ml or major organ failure | ||||||||||
| Ortiz-Gómez, 2013136 | Response to e-mail queries | |||||||||
| The method of randomisation was made by the statistical department, and it was believed that it was a computer-generated sequence | ||||||||||
| Owonikoko, 2011137 | Response to e-mail queries | |||||||||
| Trial arm | Number of women | |||||||||
| PPH > 500 ml | ||||||||||
| s.l. misoprostol | 34 | |||||||||
| i.v. oxytocin | 27 | |||||||||
| PPH > 1000 ml | ||||||||||
| s.l. misoprostol | 4 | |||||||||
| i.v. oxytocin | 5 | |||||||||
| Blood loss (ml), mean (SD/CI) | Total | |||||||||
| s.l. misoprostol | 667.12 (213.38) | 50 | ||||||||
| i.v. oxytocin | 649.90 (251.15) | 50 | ||||||||
| Change in Hb levels (%), mean (SD/CI) | Total | |||||||||
| s.l. misoprostol | 4.5 (3.3) | 50 | ||||||||
| i.v. oxytocin | 4.3 (2.97) | 50 | ||||||||
| Parsons, 2007139 | Response to e-mail queries | |||||||||
| Trial arm | Duration (minutes) of third stage of labour | Total | ||||||||
| > 30 minutes (number of women) | Mean number of women, SD (95% CI) | |||||||||
| Intervention | 3 | 6.95, 6.11 (6.13 to 7.76) | 218 | |||||||
| Control | 2 | 6.18, 4.62 (5.57 to 6.79) | 222 | |||||||
| Rosseland, 2013148 | Response to e-mail queries | |||||||||
|
The data on estimated blood loss were the visually estimated blood loss in the OR The authors believed that these data were of limited value and have based their analyses of blood loss on change in Hb level instead A strict perioperative i.v. fluid protocol was followed |
||||||||||
| Trial arm | Number of events | Total | ||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Oxytocin | 4 | 26 | ||||||||
| Carbetocin | 6 | 25 | ||||||||
| Placebo | 8 | 25 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Oxytocin | 0 | 26 | ||||||||
| Carbetocin | 0 | 25 | ||||||||
| Placebo | 0 | 25 | ||||||||
| Change in Hb levels (g/dl), mean (SD/CI) | ||||||||||
| Oxytocin | –0.82 (0.67) | 26 | ||||||||
| Carbetocin | –0.50 (0.82) | 25 | ||||||||
| Placebo | –0.84 (0.53) | 25 | ||||||||
| Change in Hb levels (%), mean (SD/CI) | ||||||||||
| Oxytocin | 27.9 (14.1) | 26 | ||||||||
| Carbetocin | 25.6 (13.6) | 25 | ||||||||
| Placebo | 15.7 (16.5) | 25 | ||||||||
| Sadiq, 2011150 | Response to e-mail queries | |||||||||
| For fever, there were no patients lost to follow-up because each round of the study lasted only 24 hours, and throughout this period the patients were hospitalised (admitted) | ||||||||||
| For fever, the authors have a full data set, but a number of the data were published elsewhere | ||||||||||
| There were no deaths in the study (despite the global reports on maternal mortality in Nigeria) | ||||||||||
|
There were differences in baseline characteristics like age, parity, etc. However, the authors thought to minimise the effects of these differences through randomisation of treatment, even though what was carried out was not the literary meaning of the term ‘randomisation’ as the authors did not initially consider a specific patient population However, the authors suggested further studies (in my reports) in which baseline characteristics are made uniform between the two trial groups In the case of baseline treatment with oxytocin, there is clear demarcation in that the misoprostol group had no pretreatment with oxytocin |
||||||||||
| Samimi, 2013151 | Response to e-mail queries | |||||||||
|
Because the aim of this study was prevention of PPH not treatment of PPH, the authors had no mortality or morbidity in the study population The authors also used Hb level as an indicator of blood loss instead of a measurement of blood loss volume |
||||||||||
| Shrestha, 2011152 | Response to e-mail queries | |||||||||
| The authors did not find hyperpyrexia (i.e. a temperature > 40 °C), vital organ failure, ICU admission, surgery or death in either the intervention or the control group of this study | ||||||||||
| Tewatia, 2014160 | Response to e-mail queries | |||||||||
| Trial arm | Number of events | |||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Misoprostol | 0 | |||||||||
| Oxytocin | 0 | |||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Misoprostol | 0 | |||||||||
| Oxytocin | 0 | |||||||||
| Death | ||||||||||
| Misoprostol | 0 | |||||||||
| Oxytocin | 0 | |||||||||
| Morbidity | ||||||||||
| Misoprostol | 13 fever, 10 shivering, 1 nausea, 1 vomiting | |||||||||
| Oxytocin | 1 nausea, 1 vomiting | |||||||||
| Ugwu, 2014162 | Response to e-mail queries | |||||||||
| Trial arm | Events | Total | ||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Misoprostol | 15 | 60 | ||||||||
| Oxytocin | 33 | 60 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Misoprostol | 1 | 60 | ||||||||
| Oxytocin | 2 | 60 | ||||||||
| Death | ||||||||||
| Misoprostol | 0 | 60 | ||||||||
| Oxytocin | 0 | 60 | ||||||||
| Morbidity | ||||||||||
| Misoprostol | 0 | 60 | ||||||||
| Oxytocin | 0 | 60 | ||||||||
| Walley, 2000170 | Response to e-mail queries | |||||||||
| Trial arm | Mean, SD (95% CI) | |||||||||
| Duration of third stage of labour > 30 minutes | ||||||||||
| Misoprostol | 6.15, 3.76 (5.62 to 6.69) | |||||||||
| Oxytocin | 7.30, 13.08 (5.40 to 9.19) | |||||||||
| Trial arm | Events | Total | ||||||||
| Duration of third stage of labour > 30 minutes | ||||||||||
| Misoprostol | 0 | 194 | ||||||||
| Oxytocin | 2 | 185 | ||||||||
| Whigham, 2014168 | Response to e-mail queries | |||||||||
| Trial arm | Events | Total | ||||||||
| Carbetocin vs. oxytocin in non-elective caesarean section | ||||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Carbetocin | 42 | 59 | ||||||||
| Oxytocin | 37 | 53 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Carbetocin | 6 | 59 | ||||||||
| Oxytocin | 6 | 53 | ||||||||
| Active labour at time of caesarean section | ||||||||||
| PPH blood loss of > 500 ml | ||||||||||
| Carbetocin | 22 | 30 | ||||||||
| Oxytocin | 19 | 28 | ||||||||
| PPH blood loss of > 1000 ml | ||||||||||
| Carbetocin | 4 | 30 | ||||||||
| Oxytocin | 3 | 28 | ||||||||
| Zachariah, 2006173 | Response to e-mail queries | |||||||||
| The authors did not have any maternal deaths in any of the study groups | ||||||||||
Appendix 8 Network diagrams
Secondary outcomes
FIGURE 113.
Network diagram for maternal deaths.
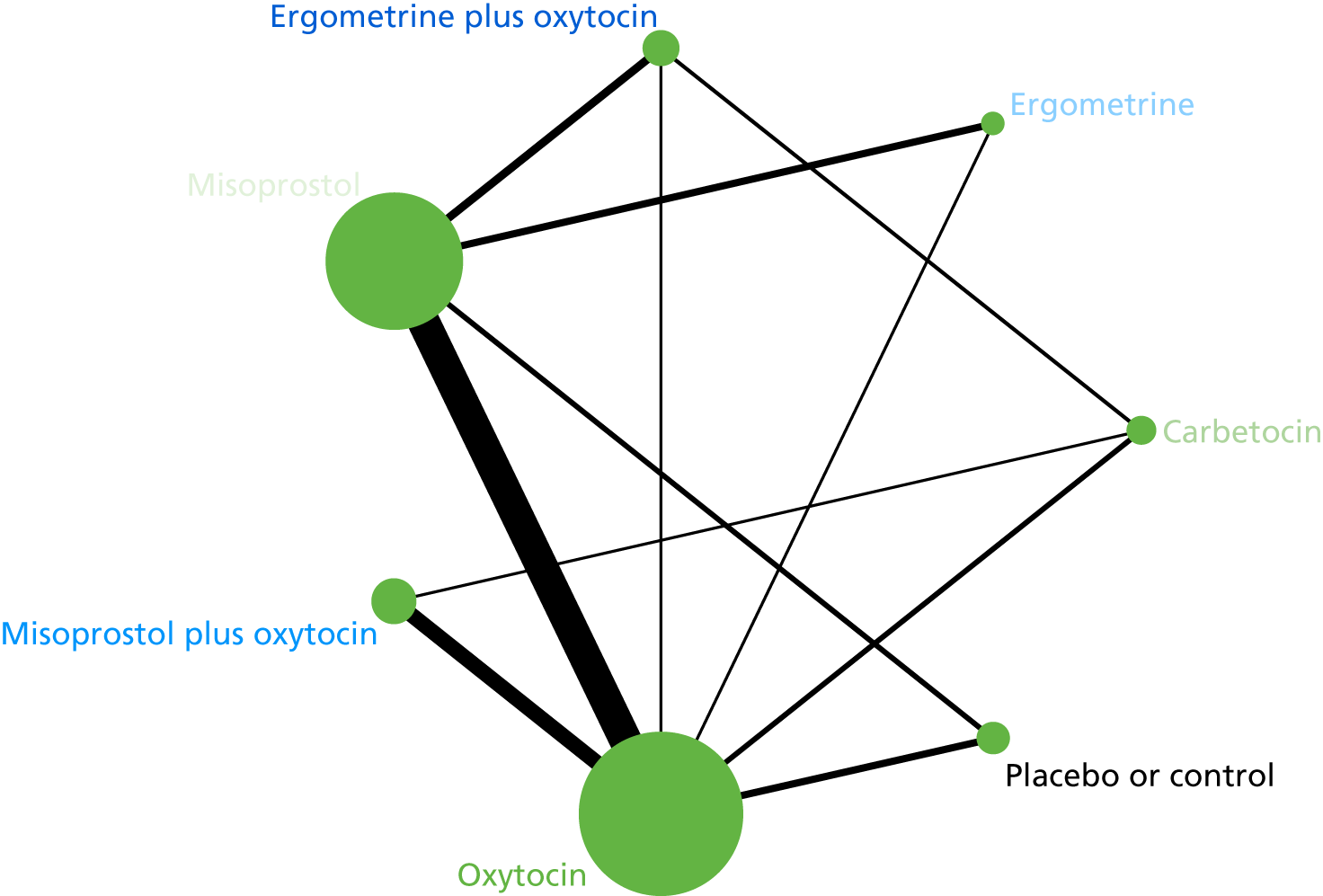
FIGURE 114.
Network diagram for maternal deaths or severe morbidity events adapted from WHO ‘near-miss’ criteria. 25

FIGURE 115.
Network diagram for additional uterotonics requirements.
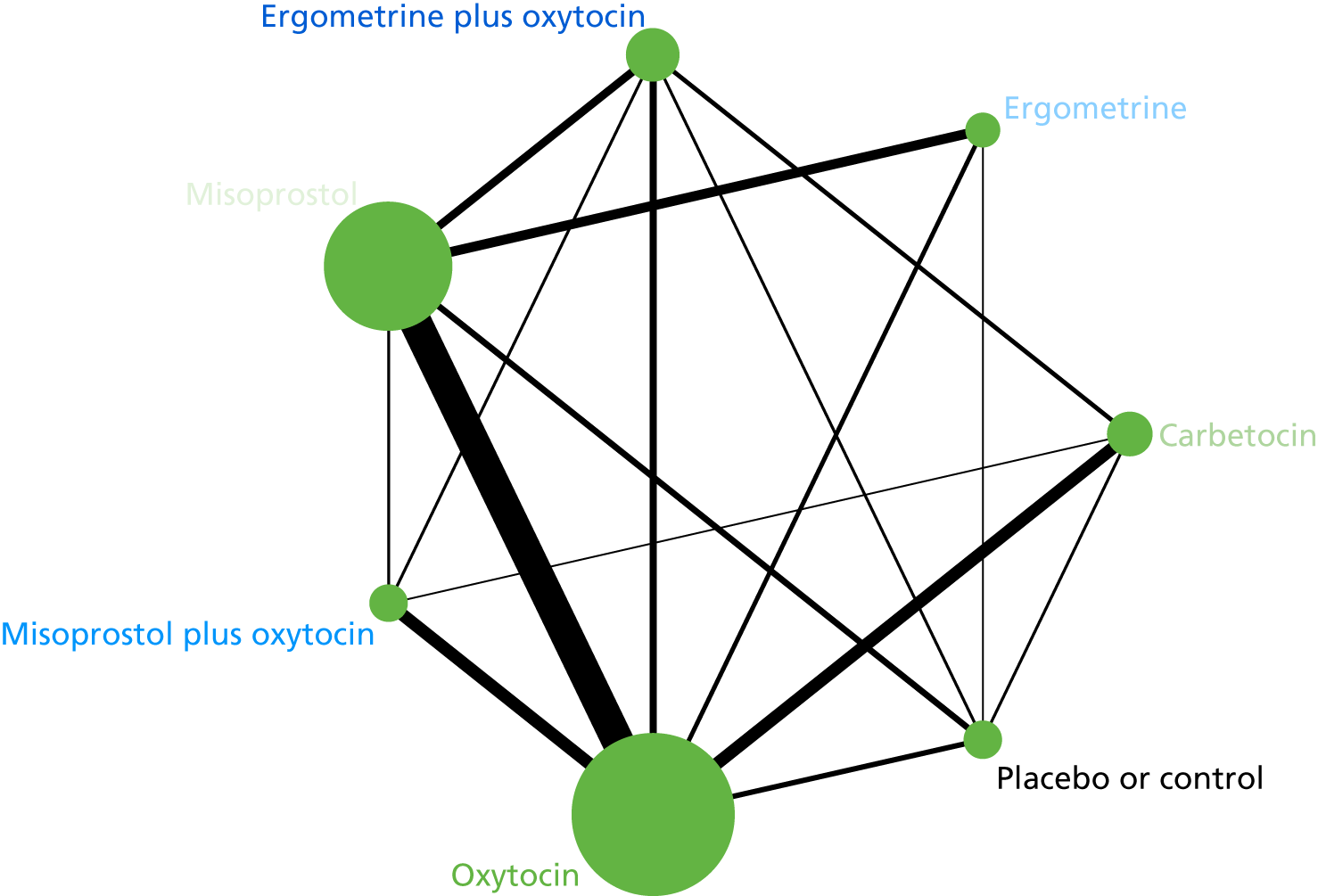
FIGURE 116.
Network diagram for transfusion requirements.
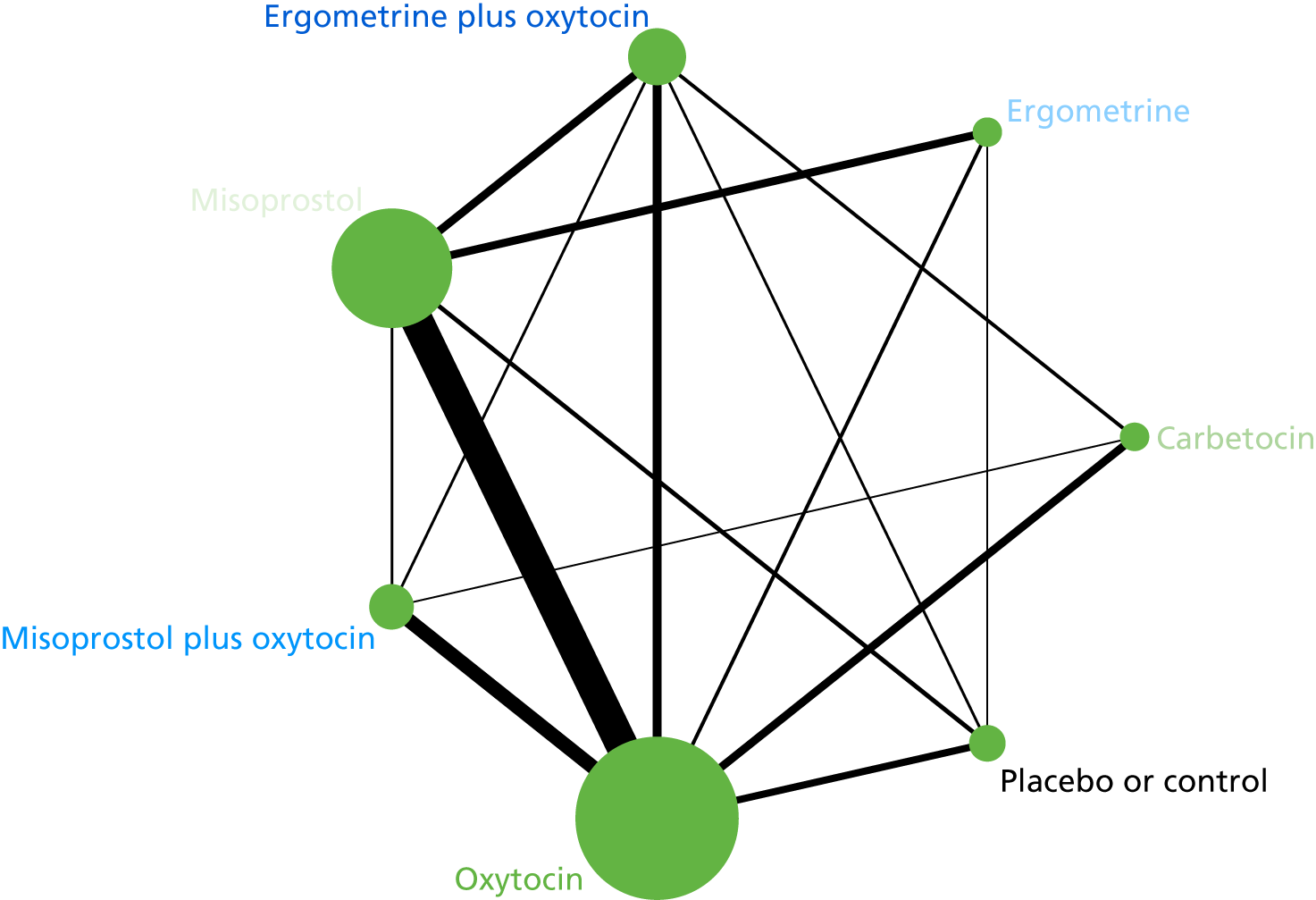
FIGURE 117.
Network diagram for manual removal of the placenta.
FIGURE 118.
Network diagram for mean volumes of blood loss (ml).

FIGURE 119.
Network diagram for mean durations (minutes) of the third stage in labour.
FIGURE 120.
Network diagram for change in Hb (g/l) measurements before and after birth.

FIGURE 121.
Network diagram for neonatal unit admission requirements.

FIGURE 122.
Network diagram for breastfeeding at discharge.
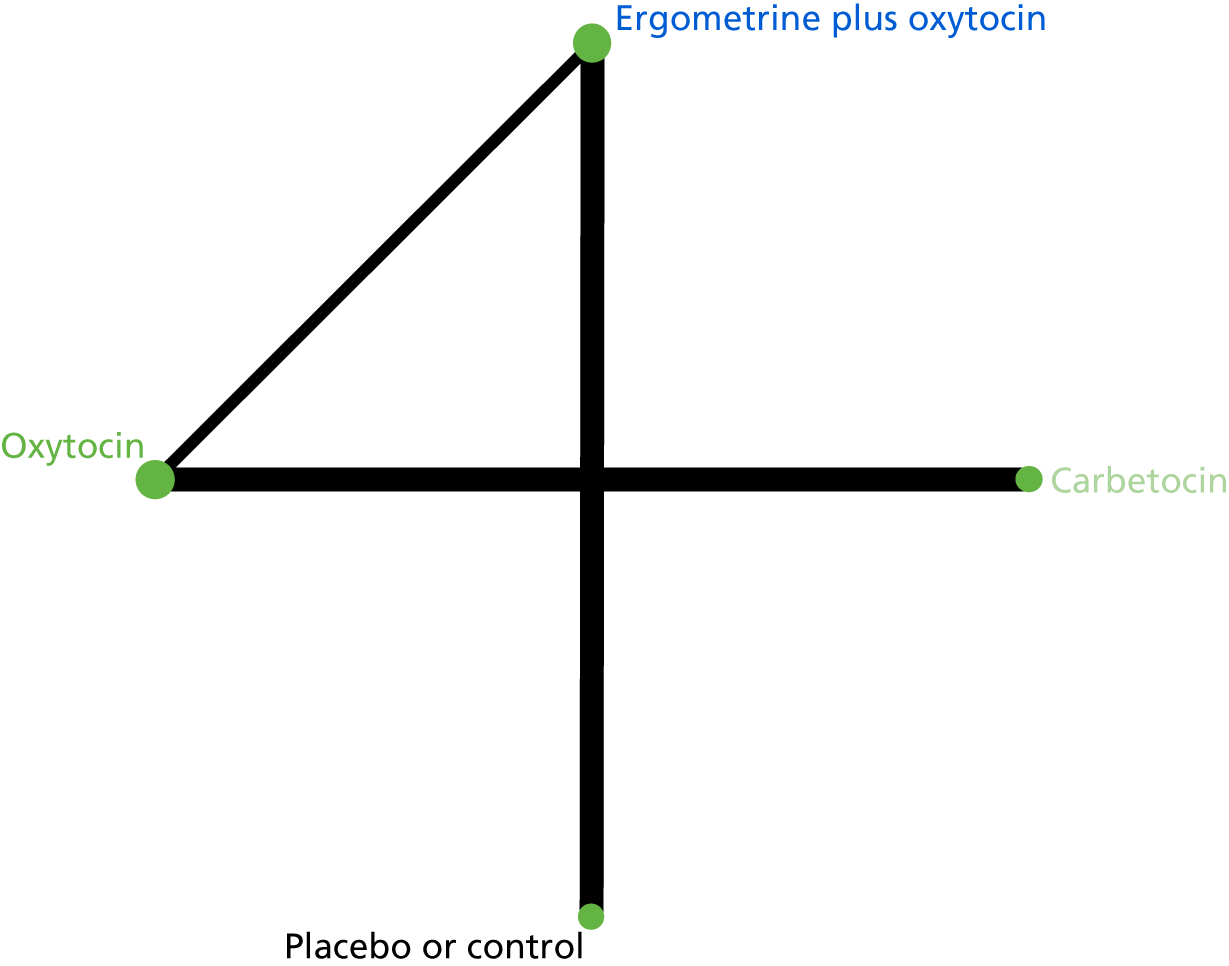
FIGURE 123.
Network diagram for nausea in the first 24 hours post partum.
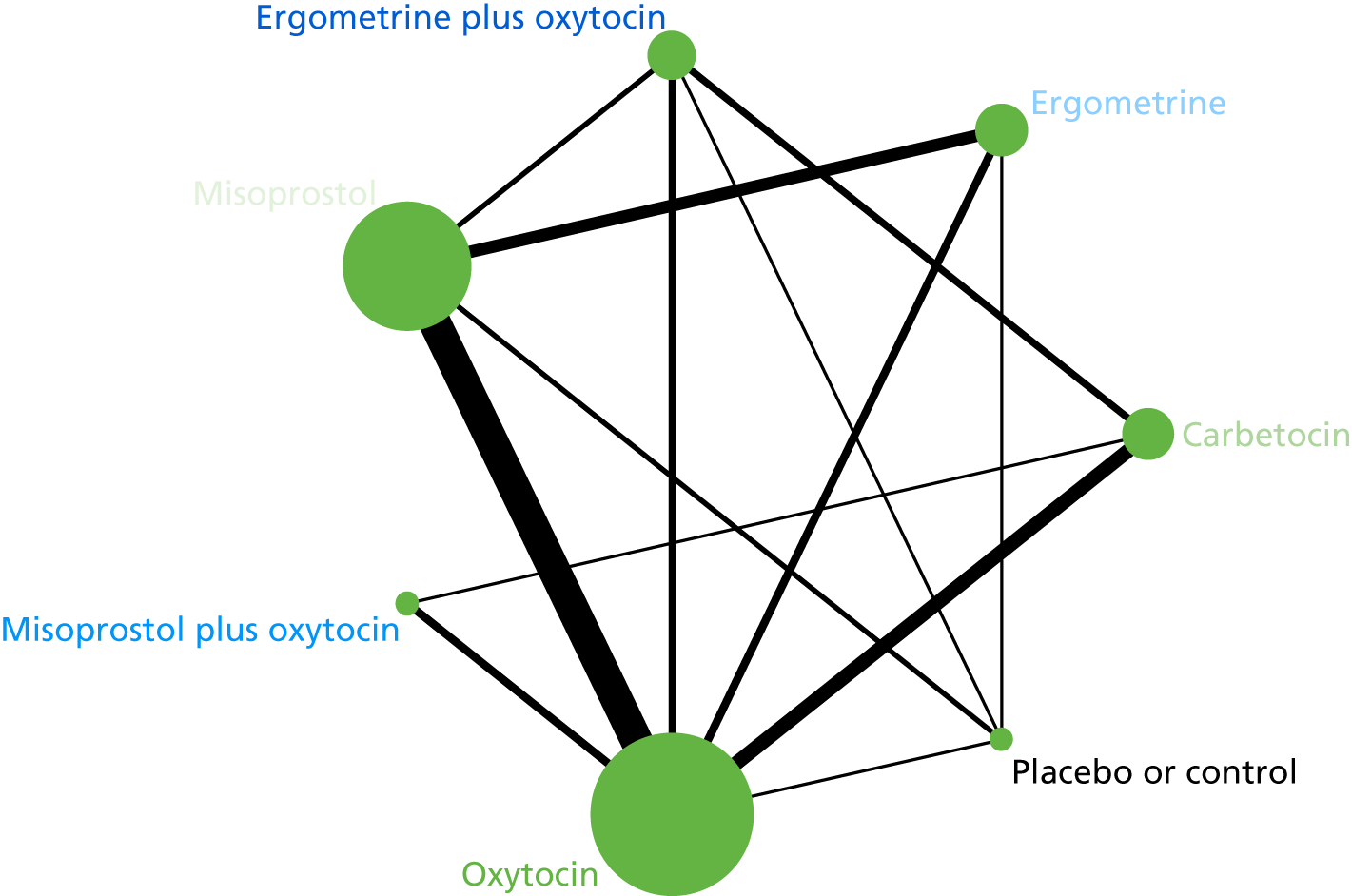
FIGURE 124.
Network diagram for vomiting in the first 24 hours post partum.

FIGURE 125.
Network diagram for hypertension in the first 24 hours post partum.
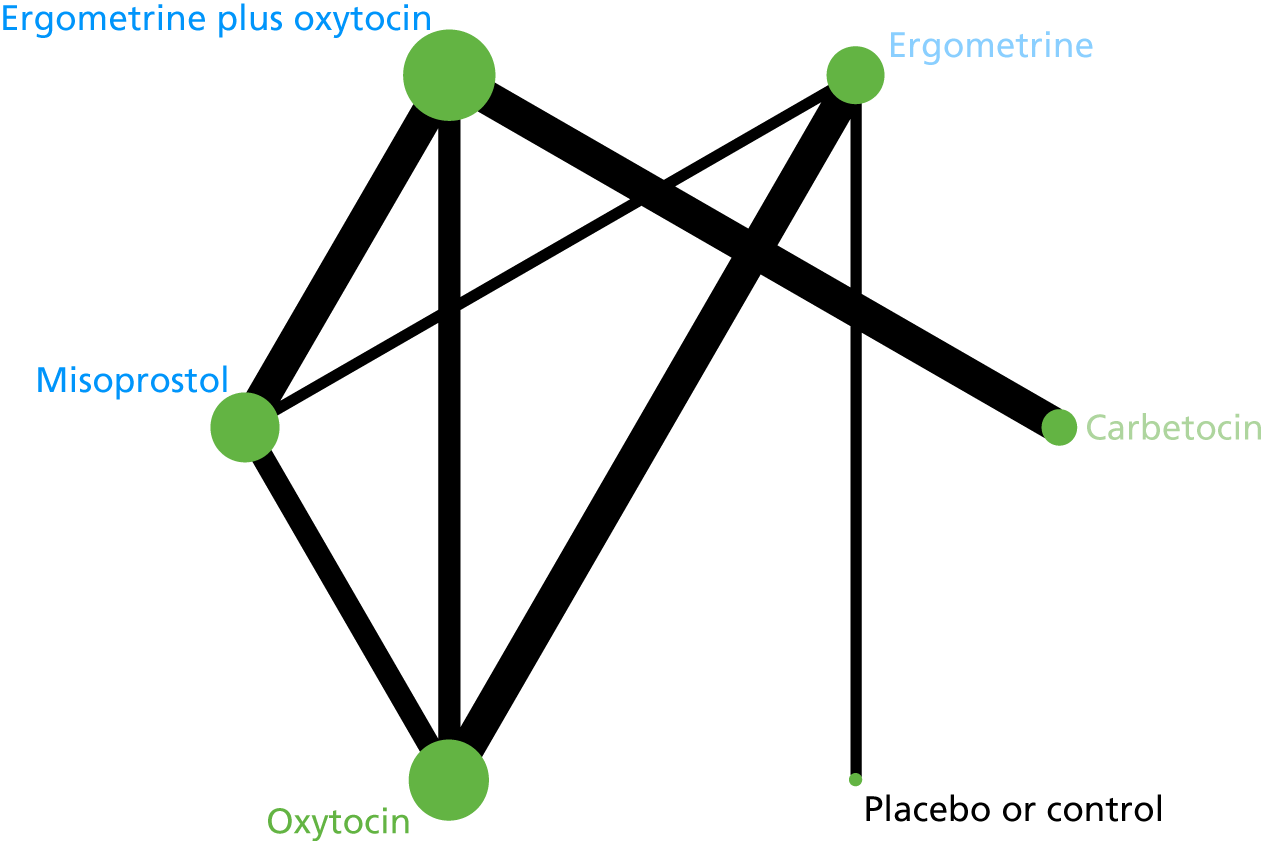
FIGURE 126.
Network diagram for headache in the first 24 hours post partum.
FIGURE 127.
Network diagram for fever in the first 24 hours post partum.
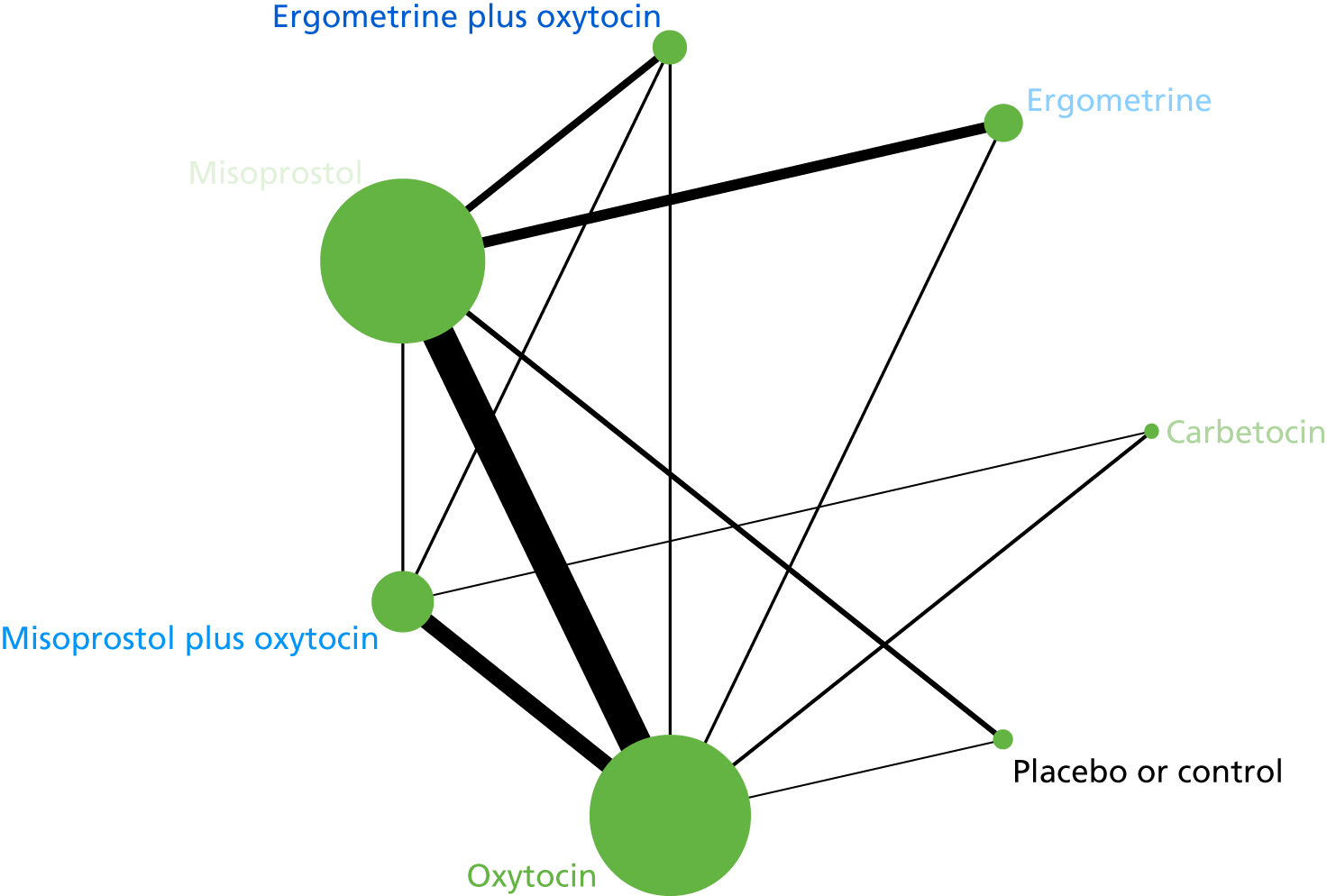
FIGURE 128.
Network diagram for shivering in the first 24 hours post partum.
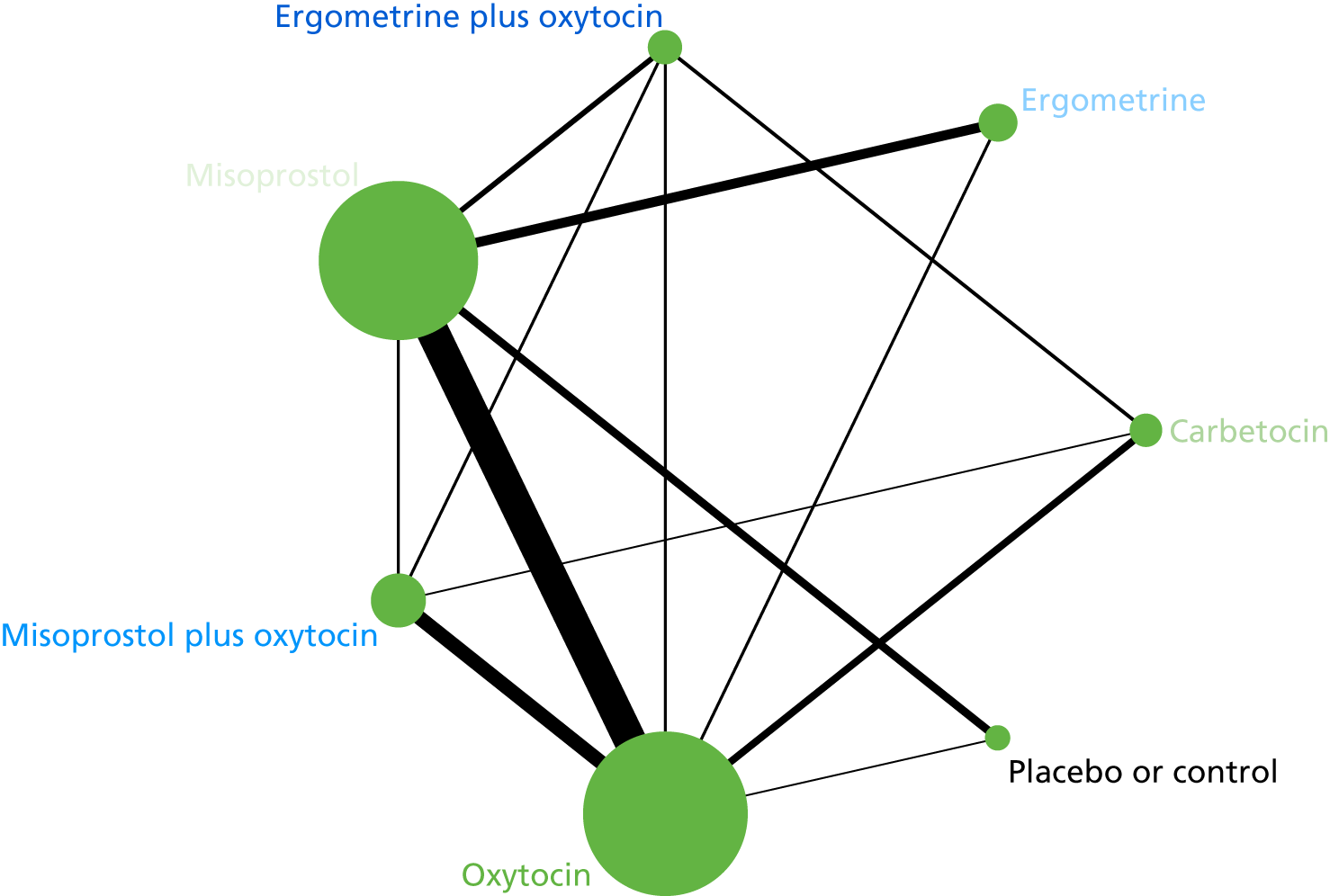
FIGURE 129.
Network diagram for tachycardia in the first 24 hours post partum.

FIGURE 130.
Network diagram for hypotension in the first 24 hours post partum.
FIGURE 131.
Network diagram for abdominal pain in the first 24 hours post partum.

Subgroup analyses
FIGURE 132.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by mode of birth (vaginal birth).
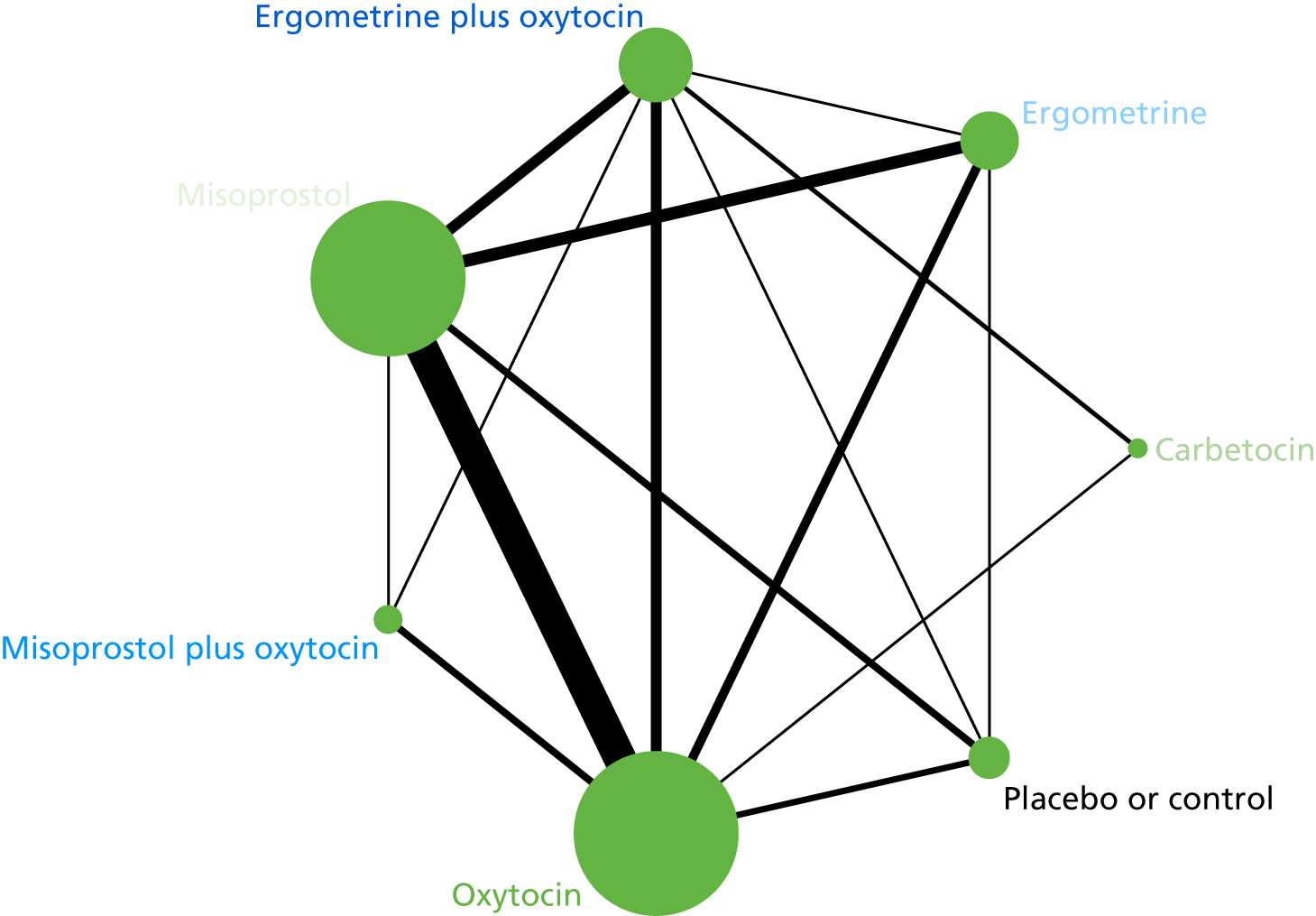
FIGURE 133.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by mode of birth (vaginal birth).

FIGURE 134.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by mode of birth (caesarean).

FIGURE 135.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by mode of birth (caesarean).

FIGURE 136.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by prior risk for PPH (low risk).

FIGURE 137.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by prior risk for PPH (low risk).
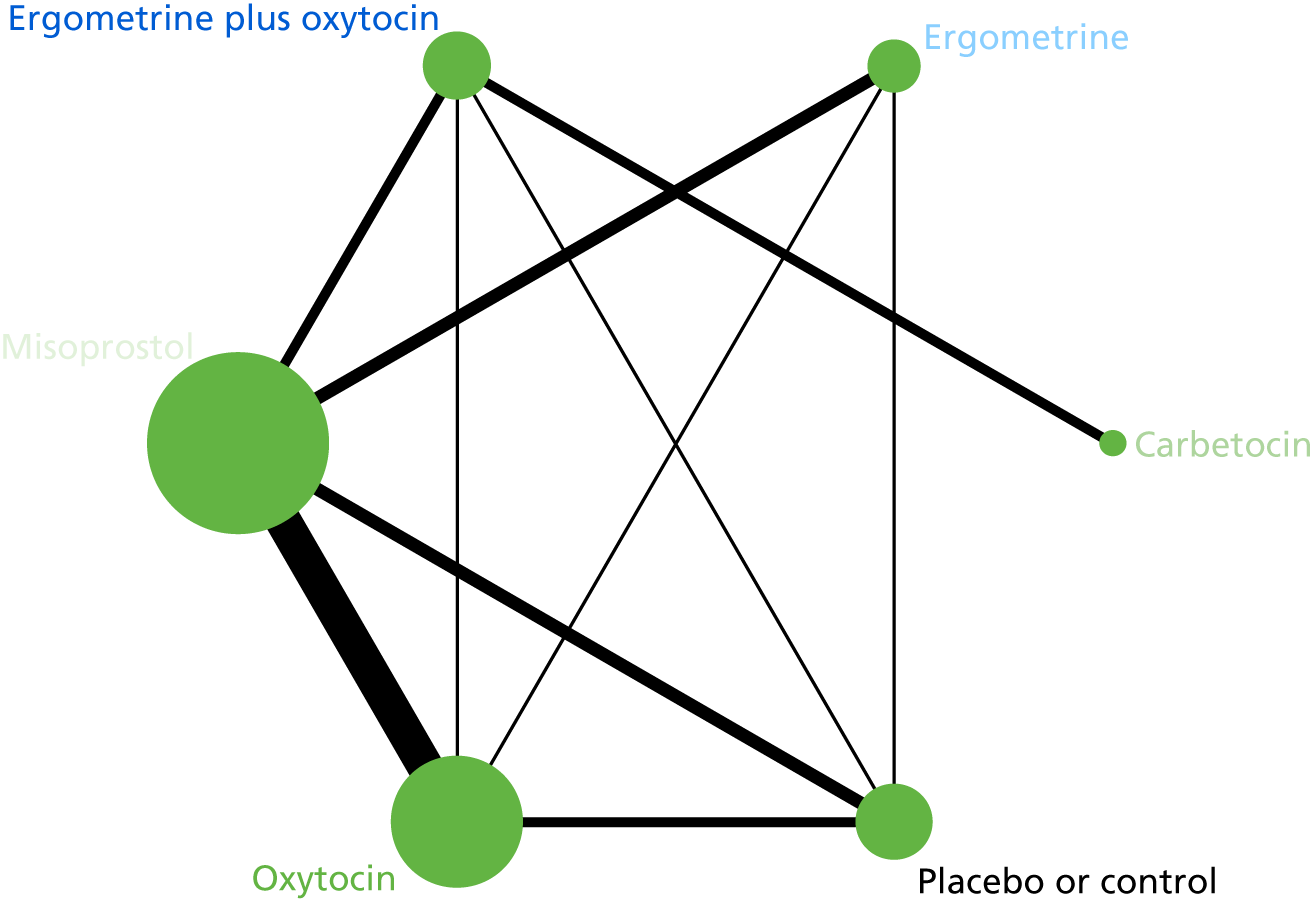
FIGURE 138.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by prior risk for PPH (high risk).

FIGURE 139.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by prior risk for PPH (high risk).
FIGURE 140.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by health-care setting (hospital setting).
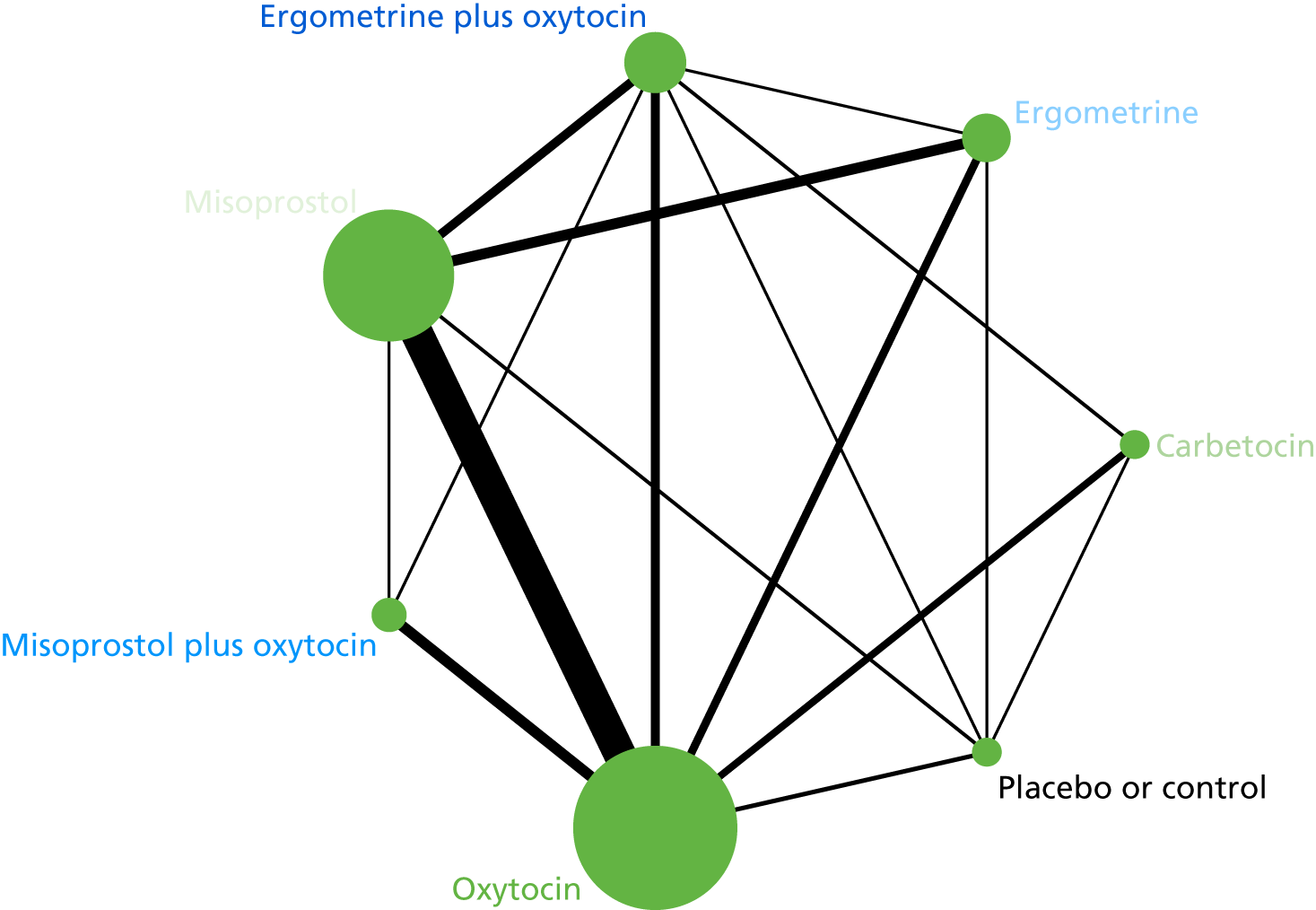
FIGURE 141.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by health-care setting (hospital setting).
FIGURE 142.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml by health-care setting (community setting).

FIGURE 143.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml by health-care setting (community setting).
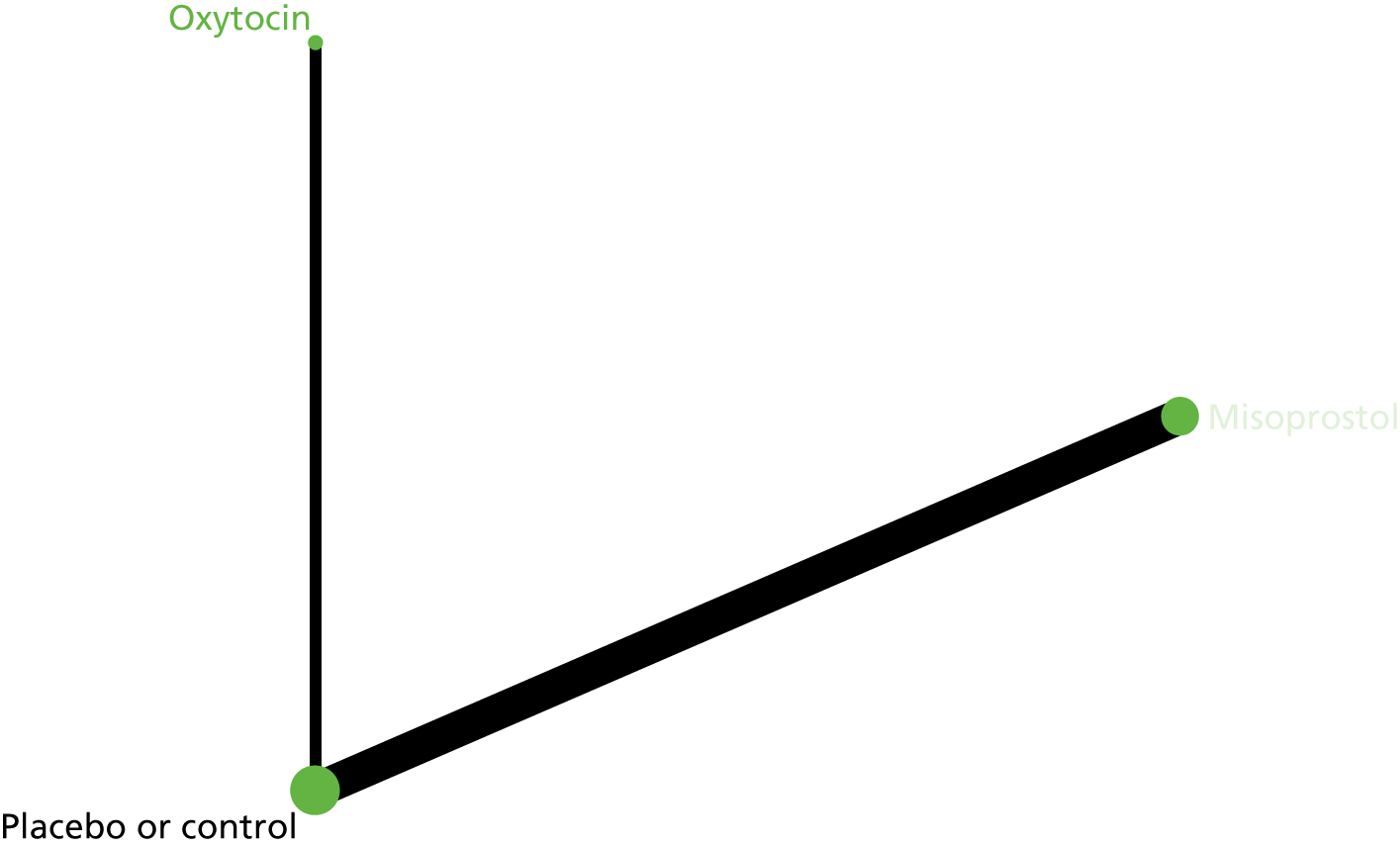
FIGURE 144.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg).

FIGURE 145.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to misoprostol studies that used a low dose (i.e. ≤ 500 µg).

FIGURE 146.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to misoprostol studies that used a high dose (i.e. > 600 µg).

FIGURE 147.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to misoprostol studies that used a high dose (i.e. > 600 µg).

FIGURE 148.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.

FIGURE 149.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.
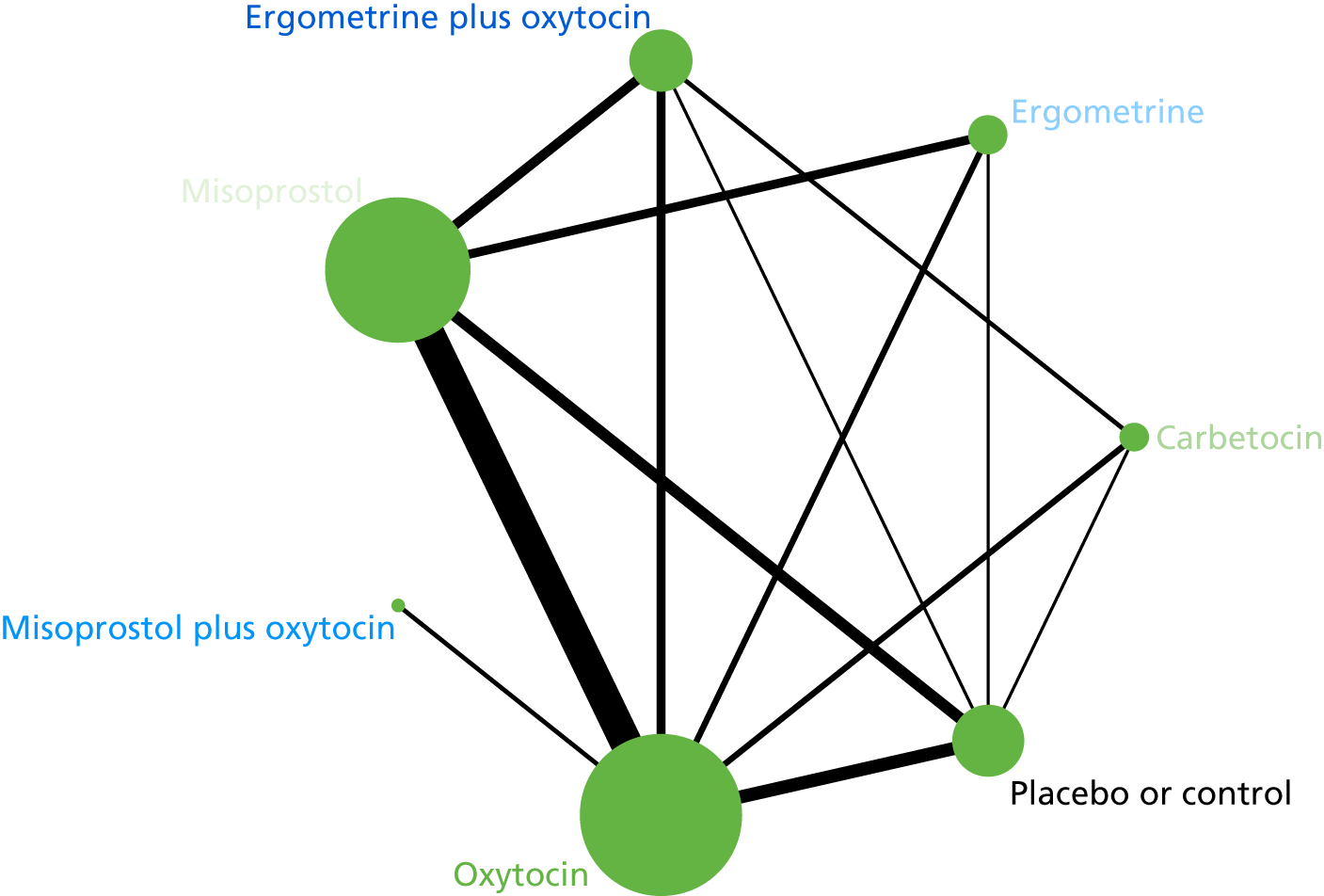
FIGURE 150.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.
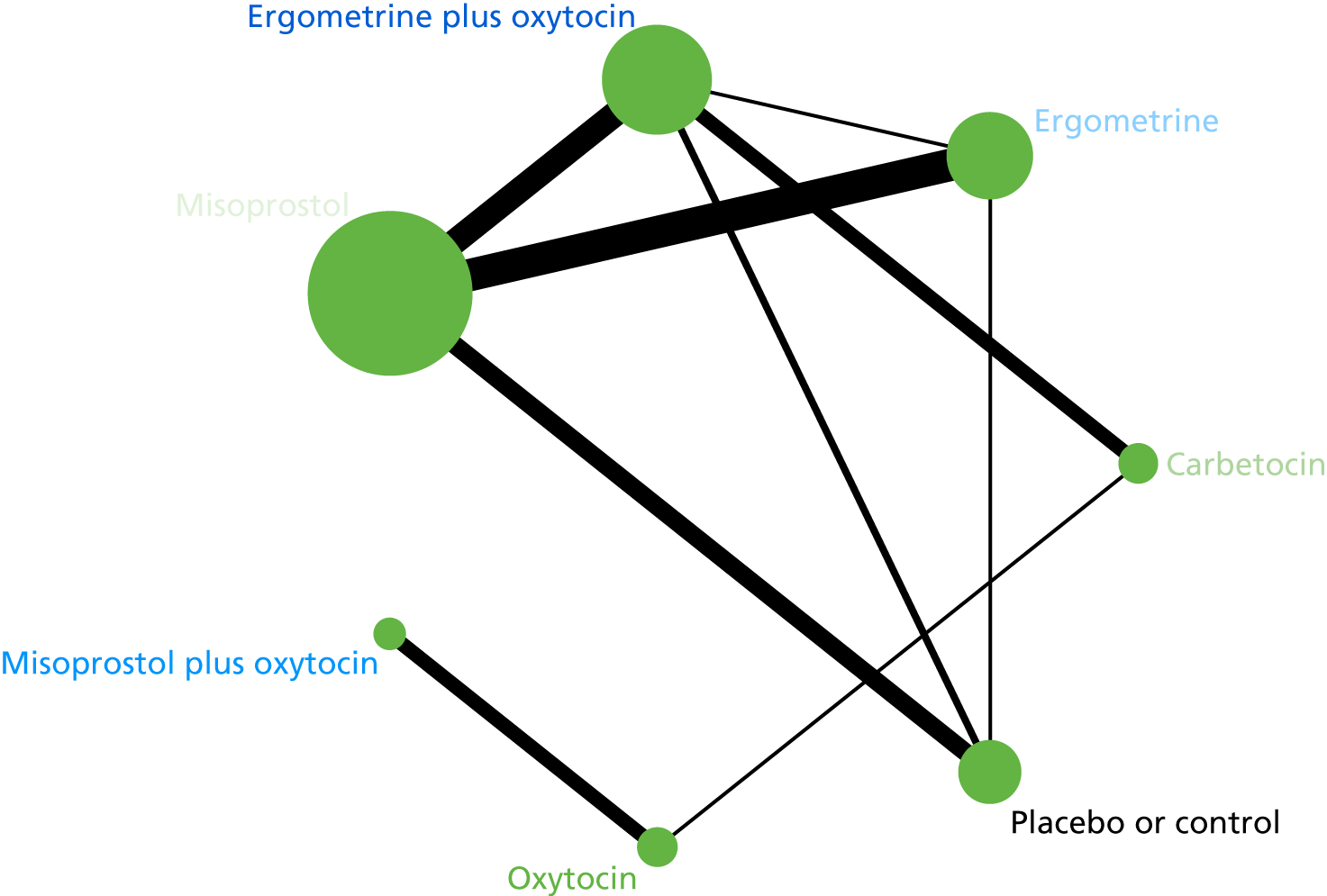
FIGURE 151.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.
FIGURE 152.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to oxytocin studies that used an intravenous infusion only of any dose.

FIGURE 153.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to oxytocin studies that used an intravenous infusion only of any dose.
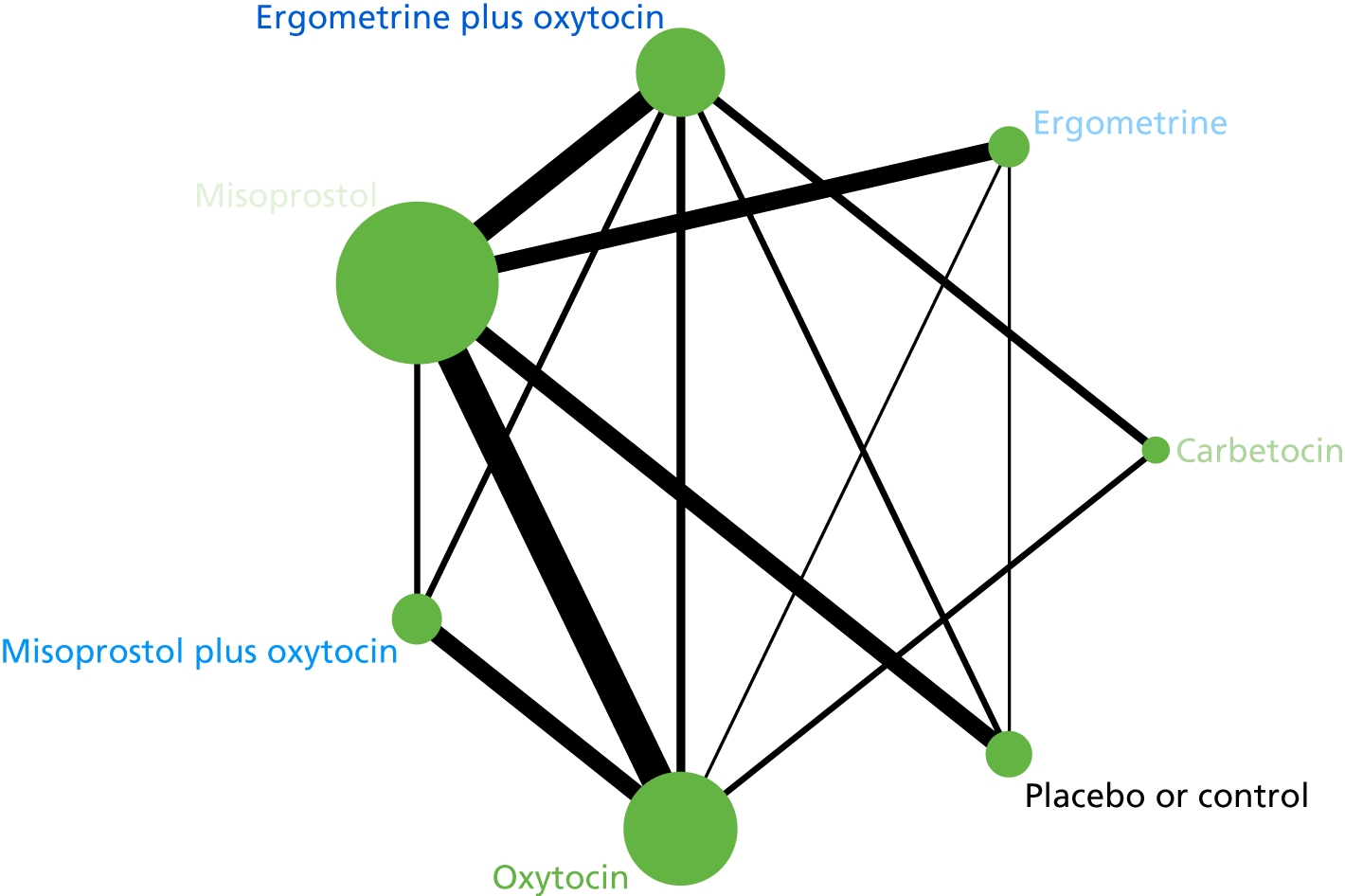
Sensitivity analyses
FIGURE 154.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to high-quality studies only.

FIGURE 155.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to high-quality studies only.
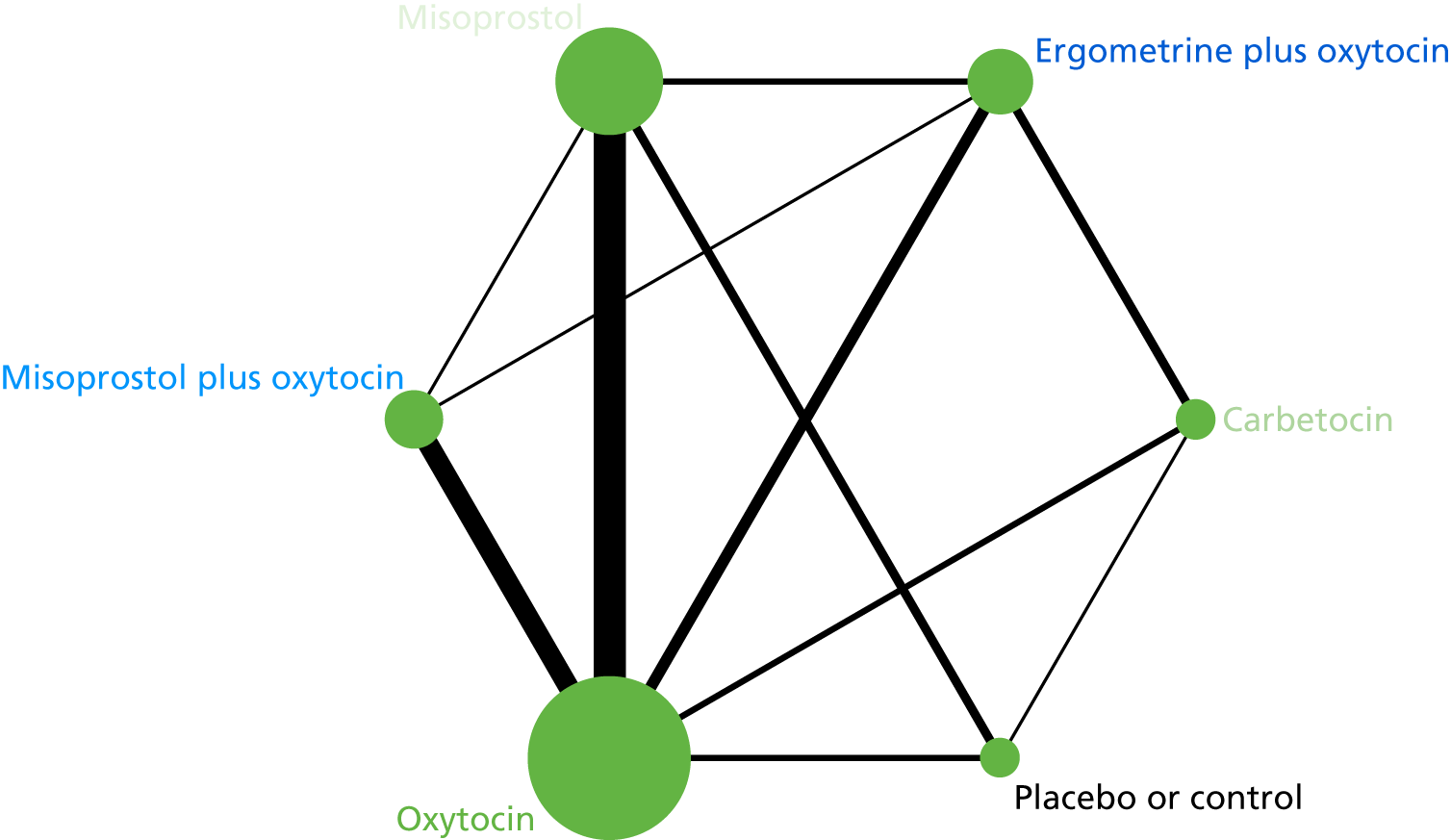
FIGURE 156.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to studies with funding source rated as being at a low risk of bias (public or no funding).
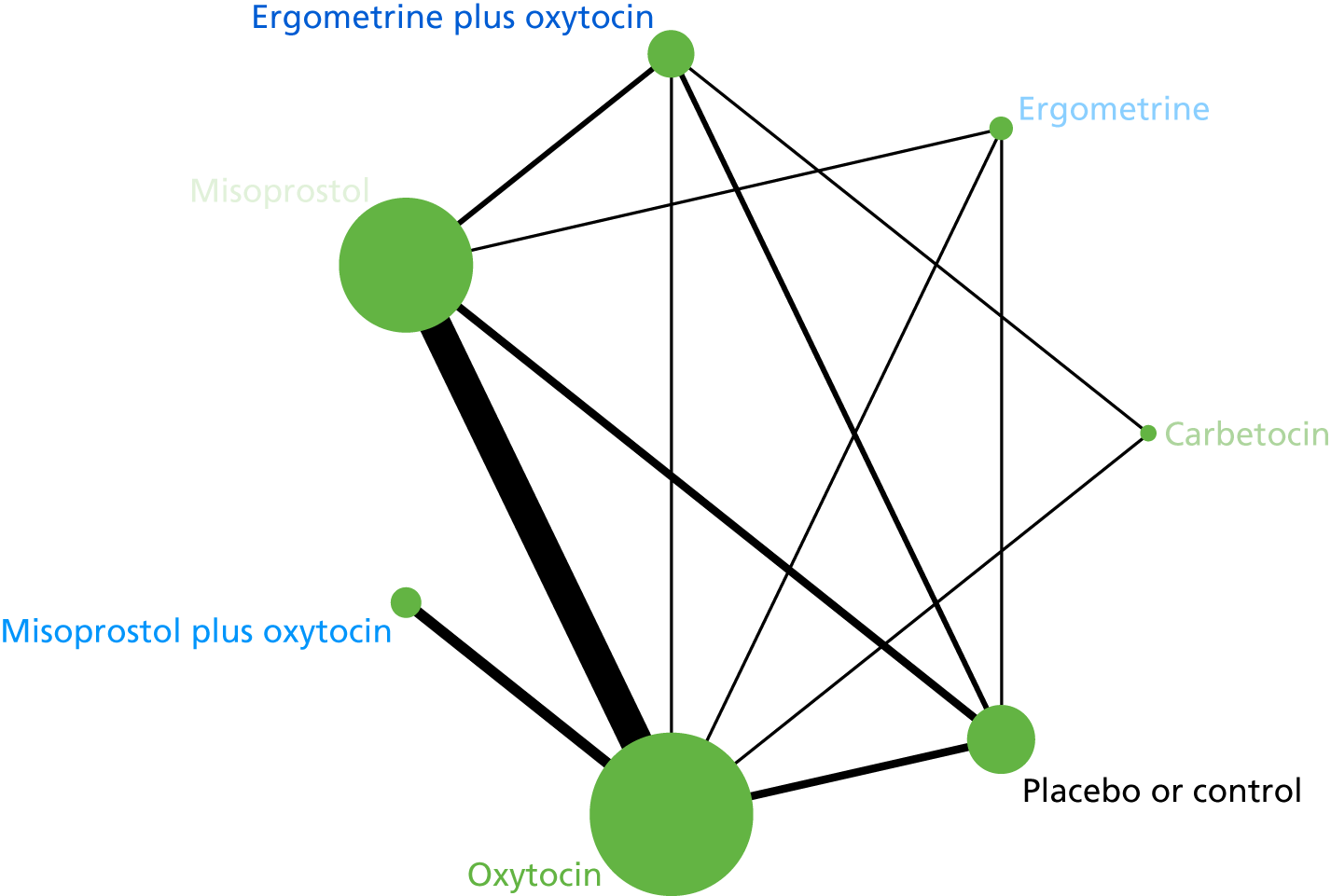
FIGURE 157.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to studies with funding source rated as being at a low risk of bias (public or no funding).

FIGURE 158.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to studies with an objective method of measuring blood loss.
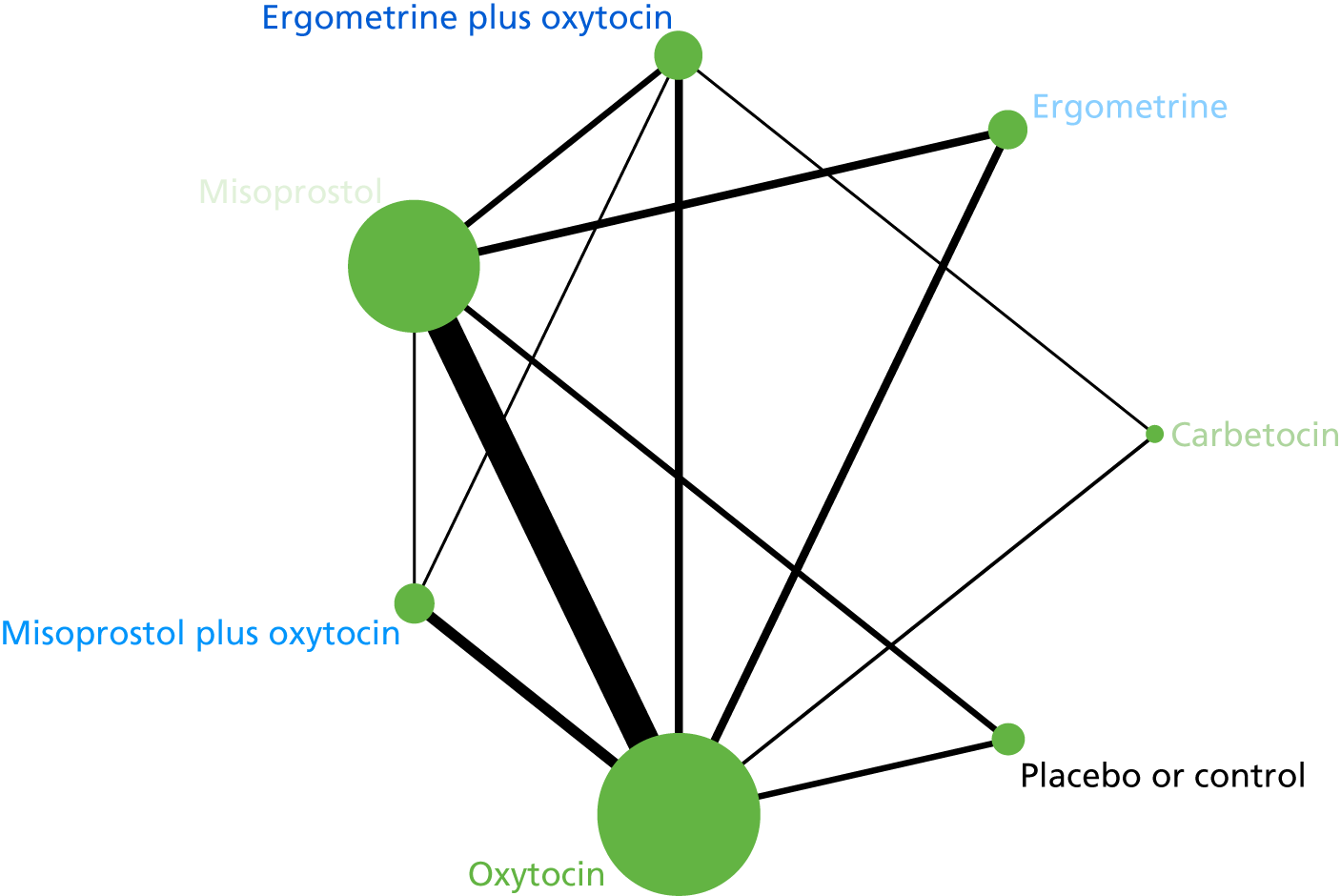
FIGURE 159.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to studies with an objective method of measuring blood loss.
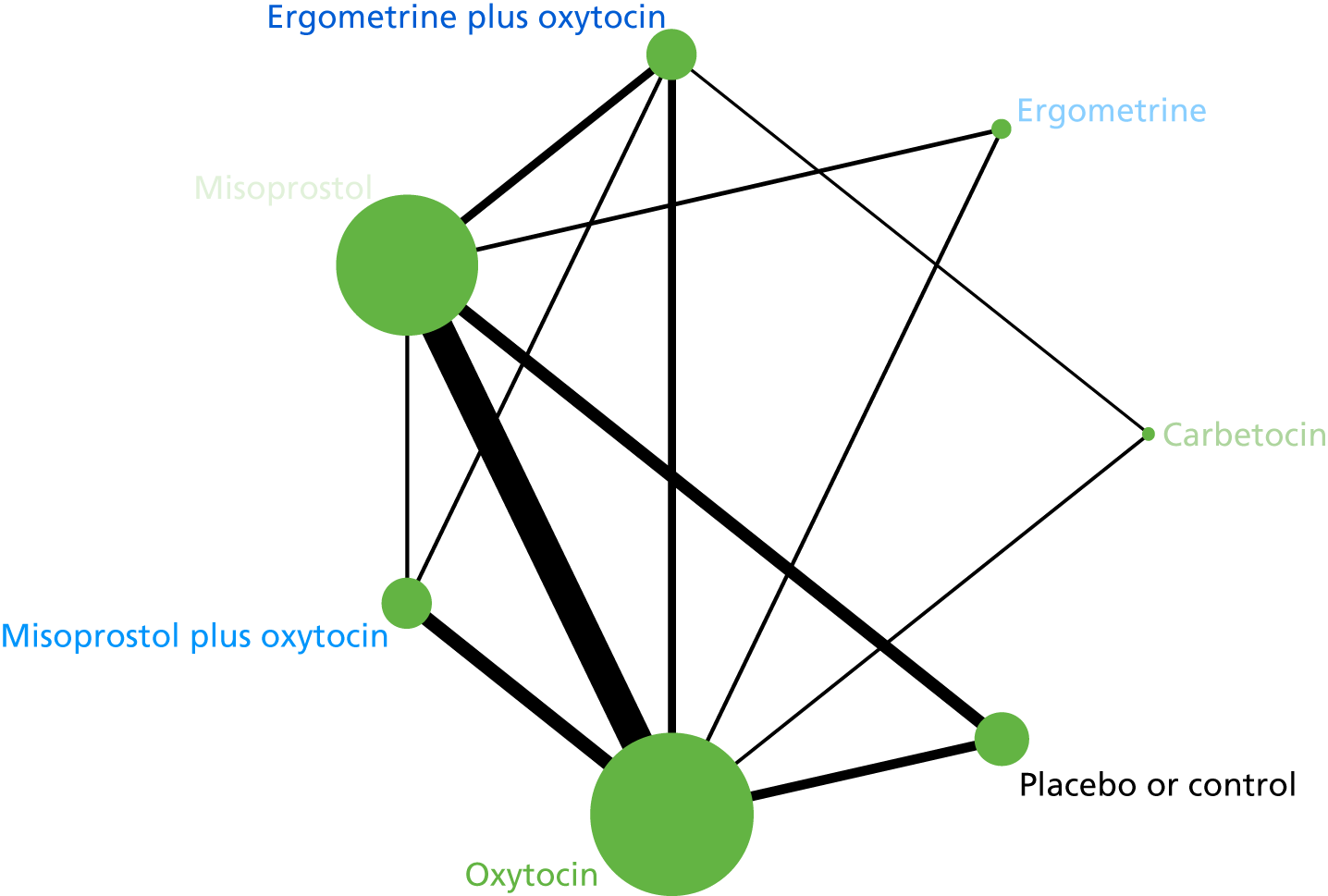
FIGURE 160.
Network diagram for the prevention of PPH blood loss of ≥ 500 ml restricted to large studies (i.e. > 400 participants).
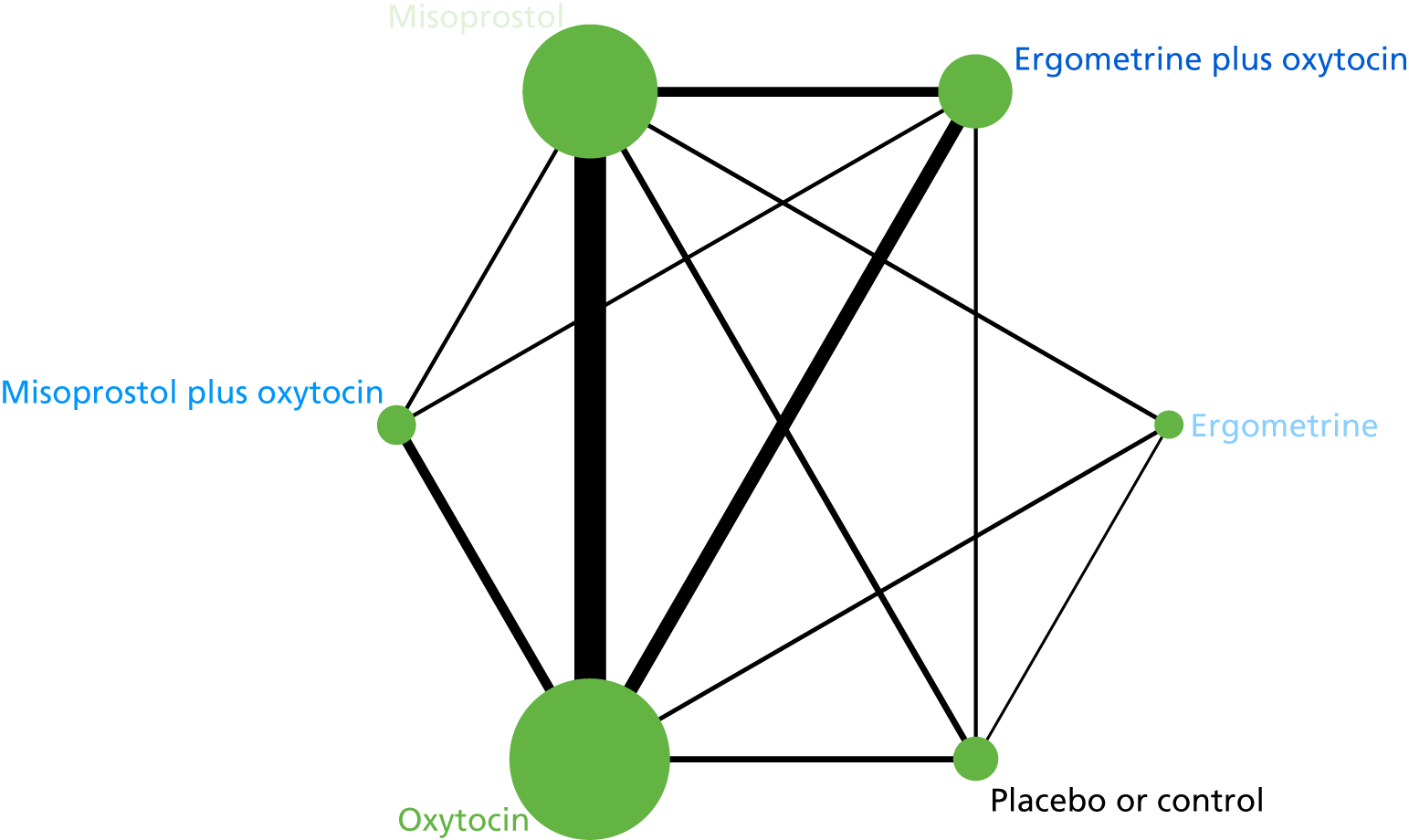
FIGURE 161.
Network diagram for the prevention of PPH blood loss of ≥ 1000 ml restricted to large studies (i.e. > 400 participants).
Appendix 9 Probability of adverse events for each prevention strategy
| Prevention strategy | Probability of adverse events (standard error in parenthesis) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nausea | Vomiting | Hypertension | Headache | Tachycardia | Hypotension | Fever | Shivering | Abdominal pain | |
| Vaginal delivery | |||||||||
| Oxytocin | 0.039 (0.005) | 0.010 (0.002) | 0.021 (0.005) | 0.044 (0.009) | 0.025 (0.014) | 0.005 (0.005) | 0.020 (0.003) | 0.071 (0.007) | 0.134 (0.043) |
| Misoprostol plus oxytocin | 0.270 (0.891) | 0.039 (0.255) | – | – | – | – | 0.090 (0.229) | 0.261 (0.246) | – |
| Misoprostol | 0.058 (0.161) | 0.029 (0.097) | 0.033 (0.655) | 0.068 (0.323) | – | 0.002 (1.630) | 0.105 (0.162) | 0.271 (0.140) | 0.127 (0.158) |
| Ergometrine plus oxytocin | 0.081 (0.202) | 0.043 (0.099) | 0.059 (0.633) | 0.072 (0.294) | 0.040 (0.551) | – | 0.020 (0.336) | 0.087 (0.282) | 0.149 (0.245) |
| Ergometrine | 0.106 (0.226) | 0.042 (0.148) | 0.172 (0.814) | 0.129 (0.412) | – | – | 0.020 (0.303) | 0.097 (0.265) | 0.172 (0.464) |
| Carbetocin | 0.028 (0.341) | 0.010 (0.305) | 0.030 (0.808) | 0.054 (0.382) | 0.074 (0.498) | – | – | – | 0.099 (0.307) |
| Caesarean section | |||||||||
| Oxytocin | 0.091 (0.019) | 0.056 (0.011) | 0.167 (0.076) | 0.094 (0.021) | 0.024 (0.016) | 0.169 (0.065) | 0.033 (0.005) | 0.050 (0.010) | 0.172 (0.071) |
| Misoprostol plus oxytocin | 0.164 (0.393) | 0.085 (0.299) | – | 0.141 (0.576) | – | 0.220 (0.672) | 0.073 (0.274) | 0.160 (0.262) | 0.333 (0.328) |
| Misoprostol | 0.043 (0.687) | 0.048 (0.407) | – | 0.059 (0.451) | – | 0.034 (1.077) | 0.049 (0.639) | 0.244 (0.400) | – |
| Ergometrine plus oxytocin | 0.453 (1.012) | 0.337 (1.127) | 0.042 (1.080) | – | 0.018 (0.707) | 0.141 (0.532) | – | – | – |
| Ergometrine | – | – | – | – | – | – | – | – | – |
| Carbetocin | 0.092 (0.327) | 0.049 (0.282) | – | 0.083 (0.151) | 0.120 (1.546) | 0.157 (0.346) | 0.026 (0.785) | 0.035 (0.392) | 0.178 (0.089) |
Appendix 10 Breakdown of delivery costs: vaginal delivery (normal and assisted)
| Setting | Activitya | National average unit cost (£) | Source |
|---|---|---|---|
| Elective inpatientb | 1362 | 2038.40 | NHS Reference Costs 2014–15 187 |
| Non-elective long stayb | 139,514 | 2634.20 | NHS Reference Costs 2014–15 187 |
| Non-elective short stayb | 223,663 | 1322.60 | NHS Reference Costs 2014–15 187 |
| Day caseb | 77 | 418.51 | NHS Reference Costs 2014–15 187 |
| Totalb | 364,616 | 1826.95 | NHS Reference Costs 2014–15 187 |
| Minus average UK standard practice for preventing and treating PPH (10 IU i.m. injection of oxytocin) | 0.91 | British National Formulary 189 | |
| Total cost of delivery | 1826.04 |
Appendix 11 Breakdown of delivery costs: caesarean section (planned and emergency)
| Setting | Activitya | National average unit cost (£) | Source |
|---|---|---|---|
| Elective inpatientb | 5745 | 3035.09 | NHS Reference Costs 2014–15 187 |
| Non-elective long stayb | 138,750 | 4059.79 | NHS Reference Costs 2014–15 187 |
| Non-elective short stayb | 20,987 | 2312.54 | NHS Reference Costs 2014–15 187 |
| Day caseb | 1 | 1598.44 | NHS Reference Costs 2014–15 187 |
| Totalb | 165,483 | 3802.61 | NHS Reference Costs 2014–15 187 |
| Minus average UK standard practice for preventing and treating PPH (10 IU i.m. injection of oxytocin) | 0.91 | British National Formulary 189 | |
| Total cost of delivery | 3801.70 |
Appendix 12 Breakdown of delivery costs: vaginal delivery (normal and assisted) – community health-care setting
| Setting | Activitya | National average unit cost (£) | Source |
|---|---|---|---|
| Community health-care settingb | 8270 | 1283.84 | NHS Reference Costs 2013–14 188 |
| Minus average UK standard practice for preventing and treating PPH (10 IU i.m. injection of oxytocin) | 0.91 | British National Formulary 189 | |
| Total cost of delivery | 1282.93 |
Appendix 13 Mean length of hospital stay
| Blood loss (ml) | Stage of model | Mean length of hospital stay (days) vaginal delivery | Mean length of hospital stay (days) caesarean section | Source |
|---|---|---|---|---|
| < 500 | No PPH after prevention stage | 1.57 | 2.8 | Birmingham Women’s Hospital real data |
| ≥ 500 | Bleeding stops after treatment stage 1 | 2.2 | 3.3 | Birmingham Women’s Hospital real data |
| ≥ 1000 | Bleeding stops after treatment stage 2 | 2.6 | 3.6 | Birmingham Women’s Hospital real data |
| ≥ 1500 | Bleeding stops after treatment stage 3 | 3 | 4.5 | Birmingham Women’s Hospital real data |
| Bleeding stops after treatment stage 4 | 6 | 6 | Glaze et al.193 |
Appendix 14 Breakdown of excess bed-day costs: vaginal delivery
| Setting | Activitya | National average unit cost (£) | Source |
|---|---|---|---|
| Elective inpatient excess bed-daysb | 173 | 432.56 | NHS Reference Costs 2014–15 187 |
| Non-elective excess bed-daysb | 58,278 | 440.51 | NHS Reference Costs 2014–15 187 |
| Totala | 58,451 | 440.49 |
Appendix 15 Breakdown of excess bed-day costs: caesarean section
| Setting | Activitya | National average unit cost (£) | Source |
|---|---|---|---|
| Elective inpatient excess bed-daysb | 361 | 452.35 | NHS Reference Costs 2014–15 187 |
| Non-elective excess bed-daysb | 34,042 | 444.31 | NHS Reference Costs 2014–15 187 |
| Total | 34,403 | 444.39 |
Appendix 16 Treatment of adverse events with associated costs
| Adverse event | Treatment | Cost (£) | Breakdown of costs | Source |
|---|---|---|---|---|
| Nauseaa | Cyclizine (50 mg, twice, intravenous injection) and ondansatron (4 mg twice, intramuscular) | 28.50 | Cyclizine (£5.42) and ondansetron (£23.08) | NHS Reference Costs 2014–15 187 |
| Vomiting | Prochlorperazine (12.5 mg 3 times daily, intramuscular) with i.v. fluids – 24 hours | 442.05–445.95 | Prochlorperazine (£1.56) and excess bed-day (£440.49, vaginal delivery; £444.39, caesarean section) | NHS Reference Costs 2014–15;187 British National Formulary189 |
| Hypertensiona | Labetalol (200 mg over 24 hours,) and nifedipine (20 mg over 24 hours, orally) | 630.55–634.45 | Labetalol (£189.61) and nifedipine (£0.45) and excess bed-day (£440.49, vaginal delivery; £444.39, caesarean section) | NHS Reference Costs 2014–15;187 British National Formulary189 |
| Headachea | Paracetamol and codeine for 24 hours | 0.66 | Paracetamol (£0.19) and codeine (£0.47) | NHS Reference Costs 2014–15 187 |
| Tachycardia | Observation over 24 hours | 440.49–444.39 | Excess bed-day (£440.49, vaginal delivery; £444.39 caesarean section) | British National Formulary 189 |
| Hypotension | i.v. fluids and observation over 24 hours | 440.49–444.39 | Excess bed-day (£440.49, vaginal delivery; £444.39, caesarean section) | British National Formulary 189 |
| Fevera |
Paracetamol and i.v. antibiotics with fluids Observation over 24 hours, including a blood culture, high vaginal swab, full blood count and C-Reactive Protein (CRP) test |
443.04–446.94 | Paracetamol (£0.19) and amoxicillin (£2.36) and excess bed-day for tests and observation (£440.49, vaginal delivery; £444.39 caesarean section) | NHS Reference Costs 2014–15;187 British National Formulary189 |
| Shivering | Observation over 24 hours | 440.49–444.39 | Excess bed-day (£440.49, vaginal delivery; £444.39 caesarean section) | British National Formulary 189 |
| Abdominal paina | Paracetamol and oral morphine for 24 hours | 0.25 | Paracetamol (£0.06) and ibuprofen (£0.19) | NHS Reference Costs 2014–15 187 |
Appendix 17 Summary of results: scenario analysis (vaginal delivery in a community health-care setting)
| Prevention Strategy | Average cost per woman (£) | Effectiveness | ICERa (£) |
|---|---|---|---|
| PPH blood loss of ≥ 500 ml avoided | |||
| Oxytocin | 2098.01 | 0.908 | – |
| Carbetocin | 2122.46 | 0.944 | 686.92 |
| Ergometrine and oxytocin | 2137.11 | 0.936 | Dominated |
| Misoprostol and oxytocin | 2238.23 | 0.931 | Dominated |
| Ergometrine | 2240.31 | 0.891 | Dominated |
| Misoprostol | 2258.22 | 0.899 | Dominated |
| PPH blood loss of ≥ 1000 ml avoided | |||
| Oxytocin | 2098.01 | 0.995718 | – |
| Carbetocin | 2122.46 | 0.997204 | 16,459.15 |
| Ergometrine and oxytocin | 2137.11 | 0.995370 | Dominated |
| Misoprostol and oxytocin | 2238.23 | 0.994674 | Dominated |
| Ergometrine | 2240.31 | 0.988329 | Dominated |
| Misoprostol | 2258.22 | 0.987929 | Dominated |
| Major outcome averted | |||
| Oxytocin | 2098.01 | 0.999890 | – |
| Carbetocin | 2122.46 | 0.999928 | 642,935.50 |
| Ergometrine and oxytocin | 2137.11 | 0.999881 | Dominated |
| Misoprostol and oxytocin | 2238.23 | 0.999864 | Dominated |
| Ergometrine | 2240.31 | 0.999701 | Dominated |
| Misoprostol | 2258.22 | 0.999691 | Dominated |
Appendix 18 Summary of results: one-way sensitivity analyses
| Prevention strategies not dominated | |||
|---|---|---|---|
| PPH blood loss ≥ 500 ml avoided, ICERa (£) | PPH blood loss ≥ 1000 ml avoided, ICERa (£) | Major outcome averted, ICERa (£) | |
| Sensitivity analysis 2 (increasing the cost of treatment stage 4) | |||
| Vaginal delivery | |||
| Oxytocin | – | – | – |
| Carbetocin | 926.99 | 22,883.10 | 893,874.25 |
| Sensitivity analysis 3 (decreasing the cost of treatment stage 4) | |||
| Vaginal delivery | |||
| Oxytocin | – | – | – |
| Carbetocin | 928.32 | 22,915.96 | 895,154.67 |
| Sensitivity analysis 4 (increasing the cost of treatment stage 4) | |||
| Vaginal delivery | |||
| Oxytocin | – | – | – |
| Carbetocin | 925.69 | 22,851.69 | 892,624.26 |
| Sensitivity analysis 5 (changing the effectiveness of treatment stage 3) | |||
|
1 Treatment stage 3 is 0% effective |
|||
| Vaginal delivery | |||
| Oxytocin | – | – | – |
| Carbetocin | 840.69 | 20,752.77 | 129,704.79 |
|
2 Treatment stage 3 is 100% effective |
|||
| Vaginal delivery | |||
| Oxytocin | – | – | – |
| Carbetocin | 944.22 | 23,308.49 | Dominated |
List of abbreviations
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CPCG
- Cochrane Pregnancy and Childbirth Group
- Hb
- haemoglobin
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- IU
- international units
- NCT
- National Childbirth Trust
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- OR
- odds ratio
- PPH
- postpartum haemorrhage
- PPI
- patient and public involvement
- PRIME
- Public and Researcher Involvement in Maternity and Early pregnancy
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RR
- risk ratio
- RTS
- room temperature stable
- SUCRA
- surface under the cumulative ranking curve
- WHO
- World Health Organization
- WTP
- willingness to pay
