Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/15/04. The contractual start date was in October 2012. The draft report began editorial review in September 2017 and was accepted for publication in January 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Martin Knapp received funding from Lundbeck Limited (St Albans, UK) in relation to work on depression in younger adults and workplace mental health and from Takeda UK Limited (High Wycombe, UK) for advice on measures of the impact on carers of caring for people with dementia. Zoe Hoare is a member of the Health Services and Delivery Research Associate Board. Bob Woods received a grant from the Welsh Government via Health and Care Research Wales (Cardiff, UK).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Clare et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
There is a greater need than ever before to identify effective and beneficial interventions for people with early-stage dementia. Timely diagnosis of dementia creates an opportunity to equip people with dementia and their carers to manage and live well with the condition. Psychological and social interventions can help to reduce or delay the development or progression of functional disability, depression or behavioural difficulties, maintain independence, support management of comorbid health conditions and, hence, avoid or reduce hospitalisation, maintain quality of life and ultimately delay institutionalisation. 1 At present, however, the chances of accessing psychological or social interventions following a diagnosis of dementia are limited. 2 There is a need to develop relevant and helpful interventions and to provide research evidence regarding the efficacy of these interventions. Research priorities set out by the Ministerial Advisory Group on Dementia Research in 2011 emphasised the need to identify ways of enabling people with dementia and their family members or other supporters (here referred to as ‘carers’) to enjoy a better quality of life and to evaluate the effects of psychological and social interventions for people with dementia living in the community. Nevertheless, there is still a significant ‘psychosocial intervention gap’ that remains to be addressed. 2
What is needed is a range of accessible psychological and social interventions that are effective in supporting or enabling people to live well with dementia and tackling the specific challenges that people face in managing everyday life with the condition. In the early stages of dementia, this includes approaches that can enable people to function as well as possible and remain as independent as possible. 3 Neuropsychological and behavioural studies show that people with early-stage dementia have many retained cognitive and behavioural capacities and are capable of behaviour change and new learning, although this is likely to require extra support. 4–7 It should be possible to harness these retained capabilities to enable people to manage daily activities better and support engagement and participation. Models of disability8–10 make an important distinction between underlying impairment, resulting from pathology, and disability, resulting from limitations on activity and restrictions on social participation. Furthermore, the possibilities for engaging in activity and participating in society are not solely determined by the extent of impairment, but are influenced by a range of other personal, relational, social and environmental factors. Unhelpful, unsupportive or negative influences can contribute to the development and maintenance of excess disability,11 when functional disability is greater than would be predicted by the degree of impairment; an example would be when an unsupportive environment leads to a loss of confidence. This is similar to Kitwood’s12 account of the way in which a negative social context can undermine well-being for people with dementia. In contrast, facilitative and positive influences can enable a person to function optimally. A focus on support and overcoming barriers to activity and participation should therefore produce benefits for people with dementia and their family members.
Traditionally, however, and despite the expressed concerns of clinicians,13 considerable effort has been devoted to using non-pharmacological approaches to attempt to address the underlying impairments in memory and other cognitive functions that are a defining feature of mild dementia, rather than focusing directly on enabling people to function well in everyday life. An example is the use of cognitive (or ‘brain’) training, which involves repeated, structured practice of tasks targeting specific cognitive domains, such as working memory or attention. A Cochrane systematic review14,15 found no evidence for significant benefits in early-stage dementia, and expert consensus endorses this finding. 16 A general issue with cognitive training (CT) that is a concern also in work with healthy older people or those with mild cognitive impairment is the lack of generalisation of benefits. Even in people in whom improvements are observed in trained domains, there is no evidence that these generalise to other areas, improve the ability to undertake everyday activities or have any beneficial impact in real life. 17 There is a need for more directly relevant approaches that can enable better functioning or reduce functional disability for people with dementia.
Interventions that aim to enable functional ability by targeting activity and participation, drawing on retained strengths to support adaptive behaviour, are typically described as forms of rehabilitation. The aim of rehabilitation is to enable people to function at their optimal level in the context of their intrinsic capacity and current health state. 18 The rehabilitation of people with cognitive impairments is termed cognitive (or neuropsychological) rehabilitation. The work described here has applied this approach in the care and support of people with early-stage dementia.
Principles of cognitive rehabilitation
Cognitive rehabilitation (CR) is an individualised behavioural therapy based on a problem-solving approach. 3,19,20 It represents the application of rehabilitation principles to address the effects of cognitive impairment. CR aims to address the impact of cognitive disability by enabling people with cognitive impairments to function at the highest possible level, given the nature and extent of these impairments. Supporting optimal functioning means enabling people to manage their daily lives, engage in worthwhile and meaningful activities and sustain as much independence as possible. This, in turn, allows people to feel more in control of their lives and supports the continuing experience of a coherent sense of identity. CR is person centred, acknowledging that each person’s combination of life experience, motivations, values, preferences, skills and needs is unique, and views the person holistically, taking account of the person’s relationships and environment.
Cognitive rehabilitation does not aim to train cognition or directly improve performance on cognitive tasks. Its goal is the functional rehabilitation of people with cognitive impairment. The focus is on better management of the functional disability that results from cognitive impairment and on reducing any excess or unnecessary disability resulting from secondary consequences, such as a loss of confidence. This is achieved by working with people on the goals that are important to them and that will make a difference in their daily lives.
Concept and terminology
Most people are familiar with the concept of rehabilitation following injury or illness, aiming to return the person to a former state of functioning or, if this is not possible, to enable the person to adjust to altered capacity and function at the best possible level given the residual impairments. 18 In the acute phase during recovery, intensive rehabilitation in specialist settings may be indicated, whereas at later stages, a less intensive community-based approach may be appropriate. Rehabilitation may target physical or cognitive functioning. The concept of rehabilitation is equally relevant for people with progressive impairments, who may benefit from episodes of community-based rehabilitation at various stages or as circumstances change.
In community settings, the term ‘rehabilitation’ is now sometimes replaced by ‘reablement’, which is derived from the same root and essentially shares the same meaning, but is perhaps viewed as a more readily understandable label. Rehabilitation can also be considered as being related to the concept of ‘tertiary prevention’, which is used in public health. We will use the term ‘rehabilitation’ here. The key point is that rehabilitation (or reablement) is grounded in a philosophy of enablement, which reflects a positive approach to finding solutions and encouraging optimal functioning. This philosophy emphasises a collaborative approach in service delivery, which can be summarised as ‘doing with’ rather than ‘doing for’ or ‘doing to’21 and which translates into specific individualised interventions aimed at optimising functioning.
Application to dementia
Living with dementia means living with disability resulting from cognitive impairment. The United Nations Convention on the Rights of Persons with Disabilities sets out a range of rights, including the right to be able to attain and maintain as much independence as possible through the assistance of comprehensive rehabilitation services [Article 26 (1)]. 22 For people with dementia, rehabilitation has been proposed both as an overarching principle of care and service provision, reflecting the aim of enabling optimal functioning,3,20,23,24 and as a specific intervention approach that aims to support the attainment of practical functional goals.
The principles of rehabilitation can be applied flexibly to address different types of need at various stages of dementia. These might include needs resulting from the impact on functioning of cognitive, behavioural, emotional, communication-related, relational, social or physical changes or difficulties. CR for people with dementia focuses primarily on the effects on functioning of the cognitive, behavioural and social communication impairments that form the core symptoms of dementia and the emotional and relational impact of these. A person might have several episodes of rehabilitation over time as needs change or in response to particular circumstances, such as being discharged after a period of hospitalisation. CR is distinct from physical rehabilitation, but it is important to note that people with dementia can benefit from exercise-based interventions and should of course have access to intensive physical rehabilitation when needed following injury or illness. 25
Rehabilitation, with its focus on optimising functioning, provides a highly relevant framework for supporting people with dementia and their carers, and for designing interventions to meet their needs. However, the term ‘cognitive rehabilitation’ (or ‘neuropsychological rehabilitation’), although familiar in areas, such as brain injury research, needs to be better understood in the dementia field. ‘Rehabilitation’ signifies that the intervention aims to enable people to function optimally given any impairments they may have and ‘cognitive’ signifies that the intervention specifically addresses the impact of cognitive impairment on functional ability. This impact may be the direct result of the cognitive impairment (e.g. difficulty remembering) or may reflect secondary effects, such as loss of confidence. CR has a different focus and takes a different approach to other interventions that include the term ‘cognitive’ in their titles. 15 CT and cognitive stimulation focus on cognitive function and target specific domains or global functioning, respectively; the term CR is sometimes incorrectly used to describe these types of interventions, or as an umbrella term for them. Cognitive or cognitive–behavioural therapy targets unhelpful or self-defeating thought patterns that may underlie mental health difficulties or adjustment issues. CR is distinct from all of these other approaches, which include the term ‘cognitive’, and should not be confused with them.
Cognitive rehabilitation in practice
Cognitive rehabilitation is focused on the attainment of realistic personal goals26 that are meaningful to the individual and address relevant needs. Goal-setting is a powerful behavioural strategy,27 and goal-oriented approaches are widely used in rehabilitation interventions, including rehabilitation for people with brain injury,28,29 stroke,30 neurological illness,31 memory difficulties,32 physical disability,33 chronic pain34,35 and age-related frailty. 36 Goals for rehabilitation are expressed in a form that meets the description captured in the acronym SMART (specific, measurable, achievable, realistic, time-bound). The goal-oriented approach has hitherto rarely been used in dementia care, but it is consistent with person-centred principles.
Goals are identified collaboratively and realistic targets are established, leading to the generation and implementation of strategies to support goal attainment. This process is based on a formulation, or understanding, of the individual’s intrinsic capacity, current functioning, strengths and needs, which considers cognitive, behavioural, emotional, relational and environmental factors.
To arrive at a formulation reflecting this global level of understanding, the CR therapist assesses the person’s intrinsic cognitive and functional capacity and current level of functioning. This makes it possible to understand the person’s potential and to pinpoint any areas in which the person is functioning below capacity. Understanding the reasons for this can indicate avenues that need to be addressed before specific rehabilitation goals are tackled. For example, depression or a loss of confidence may lead to reluctance to engage in activities, with a consequent loss of skills, creating an unnecessary burden of excess disability. An early stage of therapy may therefore involve addressing issues of this kind.
The overall formulation provides a framework for identifying specific areas of daily life that the person would like to manage better and establishing which of these may be amenable to change. Through a collaborative process, which can be facilitated by using a structured interview schedule, individual personally meaningful and achievable goals are identified. These relate to particular activities or situations that give rise to concern for the individual. For each of these, the CR therapist assesses the demands of the activity or situation that the person wishes to engage in or manage better, identifies any areas of mismatch between these demands and what the person is able to do, and pinpoints areas in which difficulties are likely to arise and why. This is an important precursor to devising strategies for goal attainment. For example, a person could encounter difficulty with engaging in an activity as a result of not remembering what to do or being unable to concentrate (cognitive), lacking some of the skills needed (behavioural), feeling anxious or fearful (emotional), being in surroundings that are not conducive to carrying out the activity (environmental) or lacking someone to do the activity with (social), or some combination of these. Understanding where the difficulties arise provides a focus for the problem-solving process and for starting to work together to generate possible solutions that can support goal attainment. For example, if the difficulty arises from a lack of necessary skills, the solution may be to teach these skills or to modify the activity; if the difficulty is attributable to memory problems, the solution may be to provide support for remembering; and if the difficultly is due to anxiety, the solution may be to find ways of regulating emotions. The CR therapist can select from a range of methods and strategies, which could involve new learning, relearning, use of compensatory strategies, task modification, environmental modification, application of assistive technology, or some combination of these.
Once a possible solution is chosen, a plan for goal attainment is devised. Specific strategies that can help with implementing the solution are identified collaboratively and tested out in practice. Evidence-based rehabilitative strategies include techniques (such as spaced retrieval) that support new learning or relearning of information or skills, techniques to support the introduction and use of compensatory aids and the introduction of environmental adaptations. Assistive technology may be used to augment the person’s capacity.
Progress towards attaining therapy goals is reviewed continually and strategies are adjusted as needed. Throughout this process, the therapist provides important psychological support and models a positive, problem-solving orientation. Alongside the focus on problem-solving, goal-setting and strategy application, CR incorporates other behavioural therapy methods. First, many people with dementia experience low mood and apathy, and this may need to be addressed at the outset of therapy. Behavioural activation is used to increase engagement in activities that would usually be enjoyable, with the experience of engagement and pleasure providing a source of motivation to make changes and improvements. Secondly, tackling rehabilitation goals can trigger distress, including fear, despondency or frustration, and therapists provide important psychological support in acknowledging these emotions and helping people to develop ways of dealing with them and overcoming the barrier they can present.
Rehabilitation interventions for people with dementia need to offer practical benefits in daily life. When providing behavioural interventions, it is essential to consider first whether or not benefits will transfer from the specific situation to application in real life and second whether or not these benefits generalise, for example to other similar activities. The potential for transfer and generalisation is often limited in the absence of specific efforts, and this is a particular concern in the context of cognitive impairment. For this reason, CR interventions for people with dementia are designed to circumvent the issue by being conducted in the person’s everyday setting in which the skills and strategies learned need to be applied. Whenever possible, carers and other family members are involved to help to implement and maintain changes in daily life.
Supporting carers is an essential part of CR for people with dementia. For family carers, this includes both explaining and demonstrating the strategies and skills employed to promote goal attainment and attending to the carer’s own needs and well-being by providing psychological support, discussing needs and signposting to appropriate sources of help. When the needs and wishes of the person with dementia and those of the family carer differ, resulting in tensions, the therapist has to negotiate a balance between the two perspectives, and this can be one of the most challenging aspects in delivering CR interventions, requiring sensitivity and skill.
Evaluating the outcomes of cognitive rehabilitation
For a behavioural intervention, the first requirement is to demonstrate change in the behaviour or behaviours targeted, and hence progress with therapy goals must be the primary outcome for CR. 19 As CR interventions are based on individual formulations and address personally relevant goals, this has important implications for the assessment of primary outcomes at a group level, for example in clinical trials. In a trial, the overall therapeutic approach and the structure of the intervention (e.g. number and duration of sessions) will be consistent across all participants receiving the intervention, but the content and focus of the intervention and the specific strategies applied will be different for each individual. This is typical for psychological interventions based on individual formulations, for example cognitive–behavioural therapy for depression. However, CR does not address a single defined clinical problem, such as depression, which can be clearly targeted as a common outcome across all participants. Instead, it aims to enable each individual to manage aspects of his or her daily life more effectively and with greater satisfaction. Therefore, the appropriate proximal outcome is the individual’s performance in relation to these selected aspects of daily life.
In single-case designs, the outcome can readily be assessed directly in relation to the therapy goal – for example, whether or not a given activity is completed successfully or a desired behaviour is demonstrated. For effective outcome evaluation in large trials, however, there is a need for a standardised means of capturing individual functioning and changes in functioning. Observational methods can be used in single-case or small-group studies, but are unlikely to be feasible for large trials. Patient-reported outcomes are increasingly understood to be not only valuable but indeed an essential component in evaluating the effectiveness of psychological and social interventions. This is particularly the case in rehabilitative interventions in which the approach is one of collaboration in identifying and solving practical problems, and patient-reported outcome measures are central to researching rehabilitation outcomes. Although goal attainment scaling37 was developed as a means of evaluating the overall effectiveness of multicomponent rehabilitation programmes,26,28,36 client-centred performance measures have been developed that aim to identify outcomes for individuals. The most widely used example of such a measure is the Canadian Occupational Performance Measure (COPM),38 which provides a structured format for identifying individual goals and rating current performance in relation to these. Research using this measure has provided evidence for the reliability, validity and sensitivity to change of the rating method. 30,39–42
Patient-reported outcomes may raise questions about the accuracy with which people rate specific aspects of their own experience. However, there is increasing recognition that people in the mild to moderate stages of dementia can provide meaningful accounts of their own experience. 43 This issue of awareness and accuracy in reporting rehabilitation outcomes stimulated our extensive investigations of awareness in people with early-stage dementia. 44–46 Evidence from these studies shows that, although people with early-stage dementia are likely to overestimate their cognitive abilities relative to their objective test score,47 they appear to be relatively accurate in estimating their functional ability in everyday tasks relative to objective test scores based on observation, and indeed may be more accurate than carers. 48 Therefore, patient-reported outcomes in relation to performance of the activities that are the subject of rehabilitation goals can be considered to be an appropriate means of evaluating intervention effectiveness.
Development work undertaken prior to GREAT
Experience with CR for people with cognitive disability resulting from non-progressive acquired brain injury led to the formulation of the research question: ‘Can cognitive rehabilitation be adapted to enable people with dementia and their carers to better manage the effects of cognitive disability?’ Literature searches identified a few examples of interventions for people with dementia consistent with the principles of CR,49–51 and some descriptions of the application of specific learning strategies, mainly using single-case designs. 52 We carried out a Cochrane systematic review,14 which confirmed that there were no relevant randomised controlled trials (RCTs).
A series of feasibility studies conducted by our group demonstrated that it was possible for people with early-stage dementia to identify personal rehabilitation goals and to apply rehabilitation strategies to change behaviour and improve functioning in relation to these goals. These were either single-case experimental designs53–56 or small-group pre/post comparisons. 57 Behavioural change was observed in relation to the identified goals, and sometimes this generalised to other situations. Secondary benefits included maintained social engagement and reduction in carer burden. Gains were maintained for several months, and this was also the case for one participant with long-term follow-up over several years. 58 Additional work extended the evidence for efficacy, relevance and acceptability of specific rehabilitation methods, such as spaced retrieval or errorless learning. 59,60 These findings were supported by reports from other research groups. 61,62
We next conducted a single-site pilot trial of individual, goal-oriented CR in North Wales from 2005 to 2009, funded by the Alzheimer’s Society. 63 This was the first RCT of CR for people with early-stage dementia. We anticipated that the CR intervention would result in improvements in participants’ functioning in the areas targeted in the intervention, but not in cognitive test scores. We included measures of mood and quality of life to explore whether or not the intervention had any effects in these domains, and to allow us to check that the intervention did not have any adverse effects, given the concerns expressed by clinicians that CT interventions could adversely affect mood and well-being. 13
The participants in the pilot trial were 69 people with dementia recruited from NHS memory clinics, of whom 44 had a family carer who also contributed. Participants had an International Classification of Diseases, Tenth Edition(ICD-10), diagnosis of Alzheimer’s disease or mixed Alzheimer’s disease and vascular dementia, were in the early stages as indicated by a Mini Mental State Examination (MMSE) score of ≥ 18 points, and were receiving a stable dose of either donepezil (Aricept®, Eisai Co., Ltd, Tokyo, Japan; Pfizer, New York, NY, USA), galantamine (Reminyl®, Shire Plc, Dublin, Republic of Ireland) or rivastigmine (Exelon®, Novartis, Basel, Switzerland). All participants identified personal rehabilitation goals during the baseline assessment, using the structured interview format of the COPM. 38 Participants were then randomised to one of three trial arms: CR, relaxation therapy or treatment as usual (TAU). The CR intervention involved weekly 1-hour home visits by the therapist for 8 weeks. The main focus of the intervention was addressing the identified personal rehabilitation goals, and this was supported by improving strategies for emotion regulation, retaining information and enhancing concentration and managing everyday activities. Carers were included in part of each session when they were available and willing. Selected goals related primarily to managing the impact of memory, communication or organisational difficulties, improving the performance of practical skills and activities, learning new skills, regaining confidence and motivation to engage in activities and increasing social interaction. 64 The relaxation therapy intervention, delivered by the same therapist, involved eight weekly 1-hour home visits in which participants were taught progressive muscle relaxation and breathing exercises. Participants allocated to receive TAU had no contact with the therapist.
The primary outcome was participant-reported goal performance using the COPM rating system. At the post-intervention follow-up, ratings of goal performance and satisfaction with functioning in relation to goals improved significantly for the CR group and did not change for the other two groups; effect sizes in favour of CR were large. Behavioural changes in the CR group were corroborated by therapist ratings of performance and of the extent to which goals were attained. The average performance ratings made by participants and therapists improved by a magnitude greater than the 2-point change required to indicate clinical significance. 38
For the secondary outcomes, CR produced benefits in quality of life, mood and cognition for the person with dementia and in stress, well-being and quality of life for the carer, relative to relaxation therapy and TAU. Some of these secondary benefits were maintained 6 months later. There were no differences between the relaxation therapy and TAU groups. A subset of participants underwent functional magnetic resonance imaging scanning using a recognition memory task;65 at the post-intervention follow-up, participants from the CR group showed higher, and those from the control groups showed lower, brain activation in relevant areas, although neither group improved performance on the task. This was interpreted as suggesting that CR may have promoted a partial restoration of function in frontal brain areas. 66
The intervention was acceptable to participants and carers. Attrition was low, with 64 out of the 69 randomised participants (93%) completing the post-intervention assessment and 56 participants (81%) completing the 6-month follow-up (19% attrition overall). Reasons for loss to follow-up included death (n = 3), illness (n = 1), moving out of the area (n = 3) and change of diagnosis (n = 1), with elective withdrawal accounting for only five cases.
In summary, the pilot trial provided evidence to show that people with early-stage dementia can identify realistic goals and make significant improvements in functioning with regard to their chosen goals during a brief CR intervention.
Lessons learned from the pilot trial
We used the experience gained during the pilot trial to develop plans for a large, definitive trial. We updated our Cochrane review during the course of the pilot trial in 200767 and continued to monitor the emerging literature, but found no other RCTs to inform our development work.
A key area of learning from the pilot trial related to outcome measurement. In the pilot trial, we used the COPM, which provides a pragmatic rating system based on a simple 0–10 scale. This is accessible for people with cognitive impairments and can be presented visually as well as verbally. There was consistency in the ratings over time for the non-treated groups, and for the CR group, the measure was sensitive to change, corroborated by therapist observation. This reflects similar findings from other clinical groups30,40–42,68,69 and suggests that goal performance ratings made by people with early-stage dementia can be considered to be reliable and valid64 and that changes in ratings are a valid indicator of treatment effectiveness, with improvements of 2 points being considered to be clinically significant.
Although the COPM rating system proved to be suitable in the pilot trial, the semistructured interview format specified domains of self-care, leisure and productivity, reflecting its generic nature. These domains do not necessarily cover everything that might be relevant for specific groups, such as people with early-stage dementia, or that might be addressed in individual research projects. We therefore developed a semistructured goal-setting interview that used the same rating method, but placed this within the context of a more directly relevant and targeted discussion and goal-setting process, which could be adapted to the needs of specific groups or projects. The measure we developed, the Bangor Goal-Setting Interview (BGSI), has been used to elicit goals and evaluate progress towards goal attainment in trials with cognitively healthy older people70 and people with mild cognitive impairment,71 and is used in GREAT (Goal-oriented cognitive Rehabilitation in Early-stage Alzheimer’s and related dementias: multicentre single-blind randomised controlled Trial), as described here.
In the pilot trial, we included people who did not have a carer available to participate, as people living alone with dementia may be in particular need of support to manage everyday activities, and therefore carer ratings were not obtained for these participants. However, when conducting CR with people who have cognitive impairments, it is good practice, if possible, to obtain a collateral perspective from a family carer,30,42 and such a perspective is particularly valuable in research trials. For this reason, we concluded that an inclusion criterion for participating in GREAT should be the availability of a carer who is willing to provide collateral information. The BGSI provides for the inclusion of parallel informant ratings. A further limitation in the pilot trial was that ratings were obtained post intervention only and not at the 6-month follow-up. In GREAT, we assessed goal attainment at each follow-up.
In the pilot trial, 46% of goals were rated as being fully achieved, 50% were rated as being partially achieved and 4% were rated as being not achieved within the 8-week time frame of the intervention. Reviewing the therapy logs kept by the therapist indicated that, in the case most of the ‘partially achieved’ goals, the therapist considered that further improvements could have been achieved with a little more time. The therapist’s view was that a slightly longer intervention was needed in order to optimise and consolidate benefits. For GREAT, we therefore decided on a 10-session intervention with four additional maintenance sessions.
Finally, the lack of observed differences between the relaxation therapy and TAU groups suggested that in a further trial, a two-arm design comparing CR with TAU should be acceptable.
Aims of GREAT
Building on our extensive development work, we aimed to provide definitive evidence about whether or not goal-oriented CR is a clinically effective and cost-effective intervention for people with early-stage Alzheimer’s disease or vascular or mixed dementia and their carers.
We hypothesised that:
-
This personalised intervention would improve functioning in areas directly targeted in the therapy sessions, and this would be reflected in self-ratings and carer ratings.
-
The intervention might have an impact on perceived self-efficacy, reflecting a possible psychological mechanism of action.
-
Carers of participants receiving the intervention, having learned new ways of supporting and enabling their relatives, might report feeling less stressed following the intervention.
In line with our theoretical model of CR, we did not anticipate changes in performance on cognitive tests, as CR does not directly target or train specific underlying cognitive processes. We did not plan to select participants on the basis of having clinical levels of depression or anxiety, poor scores on quality-of-life measures or carers who reported that their quality of life was poor, although we expected that some participants would show these features. This would necessarily limit the potential for demonstrating improvements in these domains. Nevertheless, there were some improvements in these domains in the pilot trial,63 and we therefore planned to include relevant measures in our assessment of secondary outcomes. This would also make it possible to identify any harms arising if the intervention had a negative impact on well-being.
We set the following specific objectives:
-
To compare the effectiveness of goal-oriented CR with that of TAU, with regard to (1) improving self-reported and carer-rated functional performance in areas identified as causing concern by people with early-stage dementia, (2) improving the quality of life, self-efficacy, mood and cognition of people with early-stage dementia and (3) reducing stress levels and ameliorating the quality of life of carers of participants with early-stage dementia.
-
To estimate the incremental cost-effectiveness of goal-oriented CR compared with TAU.
-
To examine how the goal-oriented CR approach could most effectively be integrated into routine NHS provision, to develop a pragmatic approach that could be directly applied within standard NHS services and to develop materials to support the implementation of this approach within the NHS following trial completion.
A short journal article presenting the results of the GREAT trial has been published in the International Journal of Geriatric Psychiatry. 72 The following chapters provide a detailed account of all aspects of the trial.
Chapter 2 Methods
Design
This was a multicentre, two-arm, single-blind randomised (on a 1 : 1 basis) controlled trial comparing CR added to usual treatment with TAU alone. The design and planned flow of participants through the trial are summarised in Figure 1.
FIGURE 1.
Overview of trial design and planned flow of participants through the trial. CLRN, Comprehensive Local Research Network; MHRN, Mental Health Research Network; NISCHR CRC, National Institute of Social Care and Health Research Clinical Research Collaboration; PwD, person/people living with dementia.

Ethics
The study was reviewed by Wales Research Ethics Committee (REC) 5, which issued a favourable opinion on 25 June 2012 (reference number 12/WA/0185), and was approved by the Bangor University School of Psychology REC. Based on findings from the pilot trial, it was expected that participants and carers who were allocated to receive the CR intervention would derive some benefits, whereas people who were allocated to receive TAU would not be harmed by this allocation. As there was no existing large-scale evidence about the effects of CR, it was not considered to be unethical to withhold the treatment from those allocated to receive TAU. In addition, based on existing evidence, there were no known risks associated with CR. However, trial researchers and therapists were trained to be alert to any concerns about participants’ well-being and to refer any serious concerns to the clinician responsible for the person’s care whenever possible, with the knowledge and permission of the person and their carer.
Governance
The trial was sponsored first by Bangor University (from its start on 1 October 2012 to 28 February 2015) and then, following transfer of the co-ordinating centre, by the University of Exeter (from 1 March 2015 until its completion on 31 December 2016). The governance was overseen by a Trial Steering Committee (TSC), which included two Alzheimer’s Society research volunteers who were former carers and a sponsor’s representative, and by a Data Monitoring and Ethics Committee.
Trial registration
The trial was registered with Current Controlled Trials under reference ISRCTN21027481.
Trial protocol
The trial protocol was published in 2013. 73
Participants
Participants were individuals of any age who were diagnosed with Alzheimer’s disease or vascular or mixed dementia, and who were in the mild stages of the condition. Each participant was recruited together with a carer.
Eligibility
Inclusion criteria
-
Participants had to have been assigned an ICD-10 diagnosis of Alzheimer’s disease, vascular dementia or mixed Alzheimer’s disease and vascular dementia. These conditions are estimated to account for 89% of all dementia diagnoses. 74 Although people with rarer subtypes of dementia could potentially benefit from CR, they might require an intervention that is tailored to take account of the specific profile of their condition, and we considered that this would best be assessed in separate studies.
-
Participants had to be in the relatively early stages of dementia, with mild to moderate cognitive impairment as indicated by a MMSE75 score of ≥ 18 points. Although people with more advanced dementia could potentially benefit from CR, the focus and specific approach would differ, and using a cut-off score in this way provided a basic means of ensuring that the approach was appropriately targeted.
-
It was acknowledged that some, but not all, participants would be receiving dementia-specific medication in accordance with standard practice guidelines. To ensure that the results were not affected by changes in medication use, participants taking dementia-specific medication, such as acetylcholinesterase inhibitors, must have been receiving a stable dose for at least 1 month before entering the trial, with no expectation that the dose would be changed during the course of the trial unless a specific clinical need emerged.
-
Participants had to have a carer who was willing to take part. Having a carer involved is not essential, although it is helpful, for CR; however, for the purposes of the trial, it was important to have collateral information and informant ratings of progress, and it was valuable to be able to determine whether or not CR provided any benefits for carers. By ‘carer’, we mean a family member or close friend who provides unpaid care and support; we acknowledge that some people undertaking this role may not use the term ‘carer’ to describe their role.
-
Participants had to be able to give informed consent to participation. This CR intervention was aimed at people in the earlier stages of dementia and involved engaging the person with dementia in a collaborative process of identifying and addressing meaningful and personally relevant goals. It was therefore essential for participants to understand the process and make a positive choice to engage with it.
Exclusion criteria
-
Potential participants were excluded if they had a prior history of stroke, brain injury or other significant neurological condition. Such conditions would be expected to affect cognitive, behavioural and emotional functioning, and people who have one of these conditions prior to developing dementia could have additional rehabilitation needs. Although such individuals might benefit from CR, their inclusion would have represented a potential confounding factor.
-
Participants were excluded if they were unable to speak English. This criterion was applied for practical reasons, because of the time and costs that would be involved in translating standardised measures and providing interpreters for assessment and therapy sessions. However, when setting this criterion, we expected that no, or only very few, individuals would be excluded from participation owing to an inability to communicate in English.
Any cases of participants for whom eligibility was unclear were referred to an eligibility panel consisting of four clinically qualified co-investigators (two old age psychiatrists, one neuropsychiatrist and one clinical psychologist) for a decision.
Recruitment
Participants were recruited through NHS services, such as memory clinics and old age psychiatry teams, carer and patient support groups, led by NHS staff, support groups and networks run by the Alzheimer’s Society and Join Dementia Research. Recruitment to the trial covered a 36-month period from 1 April 2013 to 31 March 2016.
Potentially eligible individuals were initially identified by either GREAT researchers or National Institute for Health Research (NIHR) Clinical Research Network staff in England and Health and Care Research Wales staff in Wales (previously the National Institute of Social Care and Health Research Clinical Research Collaboration). GREAT researchers and research network staff visited clinics to provide information and ascertain interest in participating. Research network staff also identified possible participants through note-screening and wrote to them on behalf of the responsible clinician; they were invited to indicate their interest by sending a reply slip directly to a GREAT researcher, who then made contact by telephone, sent written information and made a further telephone call to ascertain the participant’s willingness to continue.
When a possible interest in participating was identified, a GREAT researcher visited the potential participant and carer to explain the study in detail, answer any questions, recheck eligibility and ensure that the person with dementia had the capacity to consent. Informed consent from both the person with dementia and the carer was taken at this visit or, if either person required more time to decide, at a subsequent visit. As participants were in the early stages of dementia, we expected that they would continue to have the capacity to consent throughout the period of participation. However, on entry to the trial, participants were asked whether or not, in the event that they did lose capacity, they would wish to continue to be included in the trial and to have their data used in the analysis.
Obtaining informed consent at the start of the trial was only the beginning of an ongoing process. This is particularly crucial in an intervention of this kind, which requires the participant’s active engagement. The trial researchers and therapists were trained to monitor ongoing consent and identify and respond to any indication of a possible withdrawal of consent.
Locations
The trial was conducted in eight NHS sites throughout England and Wales. These were:
-
North Wales – Betsi Cadwaladr University Health Board (Bangor site)
-
South Wales – Cardiff and Vale University Health Board (Cardiff site)
-
London – South London and Maudsley NHS Foundation Trust, with recruitment supported by King’s College Hospital NHS Foundation Trust, Guy’s and St Thomas’ NHS Foundation Trust, St George’s Healthcare NHS Trust, and Oxleas NHS Foundation Trust (London site)
-
South East England – Kent and Medway NHS and Social Care Partnership Trust (Kent site)
-
South West England – RICE (Research Institute for the Care of Older People), Bath, with recruitment supported by Royal United Hospitals Bath NHS Foundation Trust and by general practitioner (GP) practices within the Wiltshire NHS Clinical Commissioning Group [(CCG) Bath site]
-
West Midlands – Birmingham and Solihull Mental Health NHS Foundation Trust, with recruitment supported by Black Country Partnership NHS Foundation Trust and Heart of England NHS Foundation Trust (Birmingham site)
-
North West England – Manchester Mental Health and Social Care Trust, with recruitment supported by Pennine Care NHS Foundation Trust (Manchester site)
-
North East England – Northumberland, Tyne and Wear NHS Foundation Trust (Newcastle site).
Setting
All assessments and intervention sessions were conducted in participants’ own homes.
Sample size
Power calculations were based on findings from the pilot trial. Improvement in goal performance was assessed in the pilot trial, with the rating scale of the COPM,38 which is equivalent to the rating of goal attainment used as the primary outcome measure in GREAT. The effect size for improvement in goal performance was large, with a standardised effect of > 1 at the post-intervention assessment. However, it was important to be able to detect at least medium effect sizes of 0.3 for both the primary and secondary outcomes. To achieve 80% power to detect a medium effect size of 0.3, with alpha 0.05, in primary and secondary outcomes, 175 people with dementia, together with their carers, were needed to complete the trial in each treatment arm. Attrition in the pilot trial was 19% overall, but as the rate could be higher in a longer multicentre trial, we adopted a more conservative estimate of 27%. Allowing for the potential attrition of 27%, it was necessary to randomise 480 people with dementia, each with a carer.
To meet this target, we calculated that each centre would need to recruit three participants per month over 27 months, a total of 80 participants per site. Experience suggested that one in three of the people with dementia who were identified as eligible and invited to participate would be successfully recruited; thus, each month, nine potentially eligible participants would need to be approached in each centre.
Randomisation
Participants were individually randomised following consent and a baseline assessment. Randomisation was triggered by the trial researchers on completion of the baseline assessment through secure web access to the remote randomisation centre, the North Wales Organisation for Randomised Trials in Health (NWORTH) Clinical Trials Unit (CTU) at Bangor University. In this system, which was maintained and monitored independently of the trial statistician or other trial staff, the randomisation was performed by dynamic allocation76 to protect against subversion while ensuring that the trial maintained good balance to the allocation ratio of 1 : 1, both within each stratification variable and across the trial. Participants were stratified by centre, sex, age (< 75 years vs. ≥ 75 years) and MMSE score (< 24 points vs. ≥ 24 points). For validation purposes, additional information was recorded, including the participant’s trial number, initials and date of birth and the details of the person requesting the randomisation. Group allocation was notified to the trial therapists.
Blinding
The trial researchers were blind to participants’ group allocation. The importance of maintaining blinding was emphasised in the training of both the researchers and the therapists. The potential for unblinding could arise through the researchers’ contact with participants at the 3- and 9-month assessments and through day-to-day contact between the researchers and the therapists at each site.
To address the potential for unblinding through day-to-day contact between researchers and therapists at each site, we ensured that they were based in different offices and did not share telephones or printers. Arrangements for the follow-up assessment visits by the researcher were made by the therapist for all participants. As the participants and carers could not be blinded to their group allocation, they were specifically asked not to comment at post-intervention and follow-up assessments on the nature of their involvement in the study and not to reveal to the researcher whether or not they had been visited by the therapist. This was explained by the researcher during baseline visits and included in the written information given to participants, and it was reiterated by the therapists when they contacted participants to confirm the dates of the 3- and 9-month assessments.
Following each assessment at the 3- and 9-month points, the blinded researcher noted to which condition s/he thought the participant had been allocated and how certain s/he was of the allocation.
Owing to the nature of the intervention, it was not possible to blind participants and carers to group allocation.
Intervention
Participants allocated to the intervention group received CR in addition to usual treatment. CR is an individualised, goal-oriented, problem-solving approach aimed at managing or reducing functional disability and maximising engagement and social participation, in which people with dementia and their carers work together with a health professional over a number of sessions to identify personally relevant goals and devise and implement strategies for achieving these. In this trial, CR was delivered by appropriately qualified therapists with experience of rehabilitative interventions. We set out to recruit therapists with psychology, occupational therapy or nursing backgrounds. In the event, our therapists were from occupational therapy and nursing backgrounds. Ten therapists worked on the trial [nine occupational therapists (OTs) and one nurse]; two sites (West Midlands and North West England) had a change of therapist during the trial.
Cognitive rehabilitation was delivered in 10 individual sessions over 3 months, followed by four maintenance sessions over 6 months. Carers were involved in part of each session whenever possible, and were kept informed when direct involvement was not possible (e.g. because the carer was at work). The involvement of a carer helps to ensure that skills are maintained and applied to novel situations, and it facilitates communication about how current or possible future difficulties might be managed.
Over the course of the 10 weekly sessions, participants with dementia worked collaboratively with the therapist to address personal rehabilitation goals. Alongside the information from the initial assessment with the BGSI, the therapists used the Pool Activity Level (PAL) instrument77 to facilitate their understanding of participants’ current level of functioning and potential for goal attainment and to support the development of a comprehensive formulation. The PAL instrument provides a framework for care-planning with people who have cognitive impairments caused by conditions related to dementia, stroke and intellectual disability. 77,78 The PAL instrument contains a valid and reliable tool for assessing functional ability in nine domains, with ability being graded at one of four levels for each domain. It was recommended in the national clinical practice guideline for dementia79 for activity of daily living skill training and for activity planning. The instrument also contains profiling tools for interpreting the assessment in order to plan and deliver effective enabling care and support. As part of the assessment for each participant, the therapist completed the PAL checklist with the carer, and used the resulting profile in planning and implementing the intervention.
Drawing on the goals identified at the baseline assessment, up to three behavioural goals were operationalised for each participant and strategies for addressing these were devised and implemented. These strategies could include environmental adaptations and prompts, use of compensatory memory aids, procedural learning of relevant skills, supported learning of important new information and restorative learning methods to reactivate prior knowledge. For each goal, a set of strategies was formulated into an individual plan, following discussion of the possible options and selection of the most promising solutions. Following the introduction and modelling of strategies and skills during the therapy sessions, the participant and carer worked on the selected goal between sessions following an agreed schedule of activities. Progress was reviewed and the strategies adopted were adjusted as necessary on a weekly basis. Goals were introduced one at a time, in a flexible manner depending on the rate of progress. Performance for each goal was independently rated at the outset and in week 10 by the participant, carer and therapist.
Work on the identified goals was supplemented by five key therapy components, which were considered at appropriate stages across the 10 sessions:
-
Developing a problem-solving orientation. Introduction of, and practice in applying, a solution-focused problem-solving approach by following a short sequence of steps to specify and test possible solutions. This was emphasised at the start of therapy and provided a continued focus throughout.
-
Addressing motivational and affective issues. Strategies to tackle motivational and affective responses that could affect the progress of therapy were considered at an early stage:
-
Emotion regulation strategies. Encountering problems with functioning in daily life can result in emotional reactions, such as anxiety, distress or frustration. Tackling therapy goals and finding these challenging could potentially trigger similar responses. Therefore, it was important to assess participants’ strengths and needs in this area and, when appropriate, introduce, or enhance, emotion regulation strategies for managing anxiety and other affective reactions, and provide practice in strategy use and application.
-
Behavioural activation strategies. Lack of interest, anhedonia, apathy and withdrawal are common and can lead to further loss of skills and confidence. Therefore, it was important to assess participants’ activity levels and, when appropriate, to identify plans for increasing engagement in meaningful and enjoyable activities and to support the implementation of these plans.
-
-
Addressing cognitive disability. A comprehensive set of skills and strategies was developed to help to manage the effects of cognitive disability, complementing the goal-specific problem-solving work:
-
The participant’s use of compensatory strategies (e.g. calendars, diaries, reminder systems) was reviewed and a plan for improving strategy use was developed and implemented, which might include both increasing the efficiency of existing strategies and introducing new strategies.
-
The participant’s knowledge and use of strategies for retaining new information or improving recall was reviewed, and practice in applying key strategies (mnemonics, semantic association and spaced retrieval) was provided, enabling the participant to identify a preferred strategy that could be used in everyday situations.
-
– Difficulties with attention and concentration can interfere with strategy application. Methods for maintaining or improving attention and concentration were taught and practised.
-
-
Carer support. Specific support for the carer included discussion of the carer’s well-being and sources of stress, and identification of strategies the carer could use, or enhance, to manage stress more effectively.
-
Signposting to other sources of support. For both the carer and the participant, the therapist explored options for further sources of help and support, and encouraged them to take advantage of these.
The four maintenance sessions were focused on supporting the maintenance of gains and encouraging continued goal performance and strategy use.
It was acknowledged that participants’ progress with goals would be variable, and although it was suggested that participants work on three goals, the number of goals tackled was likely to vary. Similarly, participants’ needs with regard to the other therapy components were expected to vary, and hence both the amount of time spent on these and the stage at which they were introduced could also vary for different individuals. The therapists needed to be flexible in structuring the sessions in order to take account of individual differences. An example of a session-by-session protocol for the CR intervention, which assumes that three goals are addressed, is shown in Table 1.
| Session | Participant with dementia | Carer | Between sessions |
|---|---|---|---|
| 1 | Orientation to the intervention and explanation of between-session tasks; goal 1 selection and rating; emotion regulation strategies; activity monitoring exercise | Orientation and explanation; goal 1 rating; emotion regulation; activity monitoring | Monitor current activities using diary sheet; practise emotion regulation strategies |
| 2 | Review of activity monitoring and plans for increasing activities; introduction of solution-focused problem-solving approach; intervention plan for goal 1; emotion regulation | Problem-solving; goal 1 intervention; plans for increasing activities | Agreed tasks for goal 1; practise emotion regulation strategies; develop plans for increasing activities; practise solution-focused approach |
| 3 | Progress review for goal 1; progress review for increasing activities; review of adaptations and compensatory strategy use; emotion regulation | Progress review; review of adaptations and compensatory strategy use; increasing activities | Agreed tasks for goal 1; practise emotion regulation strategies; implement plans for increasing activities |
| 4 | Progress review for goal 1; progress review for increasing activities; goal selection and rating – goal 2; plan to improve compensatory strategy use | Progress review; goal 2a rating; plan to improve compensatory strategy use | Agreed tasks for goal 1; implement changes to compensatory strategies |
| 5 | Progress review for goal 1; progress review for compensatory strategy use; intervention plan for goal 2; strategies for improving attention and concentration | Progress review; goal 2 intervention; strategies for improving attention and concentration | Agreed tasks for goals 1 and 2; changes to compensatory strategies; practise maintaining attention and concentration |
| 6 | Progress review for goals 1 and 2; progress review for compensatory strategy use; goal selection and rating – goal 3; improving attention and concentration | Progress review; goal 3a rating; carer well-being | Agreed tasks for goals 1 and 2; practise in maintaining attention and concentration |
| 7 | Progress review for goals 1 and 2; intervention plan for goal 3; restorative strategies for taking in new information | Progress review; restorative strategies; carer well-being | Agreed tasks for goals 1–3; practise restorative strategies |
| 8 | Progress review for goals 1–3; practise restorative strategies | Progress review; application of restorative strategies | Agreed tasks for goals 1–3; practise restorative strategies |
| 9 | Progress review for goals 1–3; practise restorative strategies; preparation for ending weekly sessions | Progress review; discuss other sources of help and support | Agreed tasks for goals 1–3; practise restorative strategies; investigate other sources of support |
| 10 | Progress review for goals 1–3; review of strategy use for emotion regulation, attention and concentration strategies, compensatory strategies and restorative strategies; re-rating of goal performance | Progress review; re-rating of goal performance; review other sources of help and support | Review written information provided about strategies; monitor progress; when appropriate, access other sources of support |
| M1 | Reorientation to problem-solving approach; review of progress with goals; review of strategy use | Problem-solving approach; progress review | Review information given; monitor progress |
| M2 | Problem-solving; review of progress with goals; review of strategy use | Problem-solving; progress review | Review information given; monitor progress |
| M3 | Problem-solving; review of progress with goals; review of strategy use | Problem-solving; progress review | Review information given; monitor progress |
| M4 | Review of progress; goal ratings; reminder of problem-solving approach and strategies; goodbyes | Progress review; goal ratings; future orientation; goodbyes | N/A |
Intervention fidelity
In line with practice recommendations,80 intervention fidelity was promoted through the provision of initial training, a therapy manual, regular centralised supervision and the recording of information about each session in therapy logs:
-
Training – therapists participated in a 2-day training course to prepare them for delivering the intervention at the start of the trial, and subsequently attended a refresher training day annually. The co-investigator Jackie Pool, an OT, specialist consultant and experienced trainer with expertise in applying rehabilitation in dementia care, delivered initial training to all of the trial therapists and guided them throughout the trial in the effective and consistent application of the therapy protocol.
-
Therapy manual – therapists were provided with a detailed therapists’ manual that included information about the principles and key elements of CR, as well as session-by-session overviews and references to the relevant literature.
-
Supervision – therapists had monthly individual supervision via video-conferencing and 3-monthly face-to-face group supervision meetings with Jackie Pool, with ad hoc advice available between meetings if needed. Supervision meetings offered detailed guidance on the delivery of CR and enabled ongoing monitoring of fidelity to the protocol, with potential concerns discussed and resolved as they were raised. Each supervision session was documented and notes were reviewed annually to ensure the appropriate involvement of all therapists in the supervisory process. The trial manager attended the quarterly supervision meetings to review progress with therapy provision and regular updates were given to the chief investigator and the trial management group. The supervision meetings were focused on reviewing therapists’ plans for achieving individual therapy goals, resolving any specific difficulties relating to individual participants and reviewing overall progress with implementing the therapy protocol for current participants in the CR group. Advice was also given about achieving a positive therapeutic relationship with participants and managing caseloads. Group meetings provided a platform for sharing best practice and ensuring consistency across sites.
-
Therapy logs – supervision was facilitated by the use of therapy logs summarising session content (with participant details anonymised). A therapy log was maintained for each participant receiving CR, with notes on session content added by the therapist after each session. The logs were submitted to the supervisor for review prior to the supervision sessions and formed a basis for discussion during the sessions.
Treatment fidelity was considered in relation to form and function. 81 While the therapy protocol was prescriptive in relation to the number and length of sessions and provided guidelines on the typical content of each session (form), a degree of flexibility was required in order to facilitate individual goal attainment, as this was a key aspect of the intervention (function). Therapists could therefore make adjustments to the content of therapy sessions in order to take account of participants’ preferences, levels of cognitive and functional ability, and social and family context.
Comparator
The comparator was TAU. Participants allocated to the control group received usual treatment only, and had no contact with the research team between assessments. TAU consisted of dementia-specific medication when prescribed and any other services normally provided, apart from specific programmes of CR or other cognition-focused interventions. TAU could include, for example, routine monitoring by the Memory Clinic, information provision and attendance at drop-in groups or support groups, or carer participation in support groups, as well as the receipt of any services provided by voluntary organisations.
Outcomes
The assessment measures are summarised in Table 2, which indicates which measures were administered at each time point by the trial researchers. The assessments were completed by 15 trial researchers, all with backgrounds in psychology, nursing or clinical research. Some sites employed more than one researcher. There were changes of researchers during the trial at three sites (West Midlands, South West England and London).
| Domain | Measure | Time point | ||
|---|---|---|---|---|
| Baseline | 3 months post baseline | 9 months post baseline | ||
| Person with dementia | ||||
| Goal attainment | BGSI | ✗ | ✗ | ✗ |
| Satisfaction with goal attainment | BGSI | ✗ | ✗ | ✗ |
| Quality of life | DEMQOL | ✗ | ✗ | ✗ |
| Self-efficacy | GSES | ✗ | ✗ | ✗ |
| Depression | HADS | ✗ | ✗ | ✗ |
| Anxiety | HADS | ✗ | ✗ | ✗ |
| Memory | RBMT story recall | ✗ | ✗ | ✗ |
| Attention | TEA elevator counting | ✗ | ✗ | ✗ |
| Executive function | D-KEFS letter fluency | ✗ | ✗ | ✗ |
| Comorbid conditions | Charlson Index | ✗ | ||
| Service utilisation | CSRI | ✗ | ✗ | ✗ |
| Carer | ||||
| Participant’s goal attainment | BGSI | ✗ | ✗ | ✗ |
| Stress | RSS | ✗ | ✗ | ✗ |
| Quality of life | WHOQOL-BREF | ✗ | ✗ | ✗ |
| Health status | EQ-5D | ✗ | ✗ | ✗ |
Demographic details
At the baseline assessment, we collected demographic and background information for the person with dementia and their carer, including sex, age, relationship between the person with dementia and the carer and whether they live together, age at onset of dementia, educational level, social class and comorbid health conditions assessed using the Charlson Comorbidity Index,82 to provide both the number of conditions and the weighted comorbidity score. A weighted score of ≥ 5 is used to indicate people with a particularly high level of comorbidity translating into a high risk of mortality. This was intended to provide a profile of the sample and to allow us to examine the effects of demographic and social variables on treatment efficacy.
Primary outcome measure
The primary outcome for GREAT was participant-reported goal attainment at 3 months post randomisation (the 3-month follow-up). Participant-reported goal attainment was also assessed at 9 months post randomisation (the 9-month follow-up). Parallel carer ratings of goal attainment were obtained at both the 3-month and 9-month assessments. These ratings were obtained using the structured interview protocol of the BGSI. The trial researchers participated in a 2-day initial training course to prepare them for conducting goal-setting using the BGSI, attended annual refresher training days and participated in monthly telephone supervision with two of the investigators (from a rotating panel of four), which was focused specifically on optimising the goal-setting process.
During the initial assessment using the BGSI, participants were asked how memory and other cognitive difficulties affect (1) everyday tasks, activities and routines, (2) the possibility of engaging in pleasurable and meaningful activities and (3) social contacts and relationships. For each of these domains, participants rated how important it was to them and how ready they were to try to make changes, in each case using a scale of 1–10. This provided a basis for identifying areas in which participants would like to make changes or improvements and for setting specific goals. Participants could select up to three goals. Goals were expressed in behavioural terms using SMART principles: specific, measurable, achievable, realistic and attainable within a defined period of time. Participants described what they want to be able to do and what they are currently doing, and made ratings of their current level of goal attainment on a scale of 1–10, whereby 1 was unable to do or not currently doing and 10 was able to do well with no difficulty. Participants also rated their satisfaction with this level of attainment on the same scale of 1–10, where 1 was extremely dissatisfied and 10 was extremely satisfied. The scale was presented in a visual format as well as through verbal explanation. The mean levels of attainment and satisfaction were calculated by summing the ratings across all of the identified goals and dividing by the number of goals identified.
Carers provided their own descriptions of the person’s current functioning and made parallel ratings of attainment on the same scale of 1–10. The mean ratings for attainment were calculated by summing the ratings across all of the identified goals and dividing by the number of goals.
At the 3-month and 9-month follow-ups, participants and carers were shown the goal descriptions and baseline ratings for the originally identified goals and asked to rate the current attainment and satisfaction levels for each goal. The mean attainment and satisfaction ratings were calculated as before.
The key information that the performance and satisfaction ratings provide is an indication of the extent and direction of change. In clinical practice, it is usual for people to remember and consider previous ratings or previously obtained information when making such ratings, and this can make current ratings more informative and the process of completing the ratings more transparent. 83 However, people with dementia may find it difficult to remember their previous ratings as a result of their memory difficulties. Various approaches to obtaining follow-up ratings are used in clinical trials, and one question that arises is whether or not participants should have access to their previous ratings when completing a new set of ratings at follow-up. Evidence shows that participants prefer to be reminded of previous scores and that, in the case of healthy adults, being reminded of previous scores produces no significant differences in ratings compared with not being reminded. 84 People with dementia, because of their difficulties with memory, may benefit more than other groups from being reminded about the rating process and being given access to their earlier ratings. Furthermore, in GREAT, participants in the CR group made in-session ratings of goal attainment in the sessions prior to the 3- and 9-month assessments, and providing a reminder of previous scores to all study participants removed this source of inequity.
Secondary outcome measures for participants with dementia
The secondary outcomes for the person with dementia at the 3- and 9-month follow-ups were self-rated quality of life, self-efficacy and mood, cognitive test scores (memory, attention and executive function) and extent of service utilisation. The following measures were taken at baseline and follow-up.
DEMentia Quality Of Life questionnaire85
The DEMentia Quality Of Life (DEMQOL) questionnaire is a measure of the health-related quality of life (HRQoL) of people with dementia, with good internal consistency (Cronbach’s alpha 0.87) and test–retest reliability (intraclass correlation coefficient 0.76) in people with mild to moderate dementia. We used the 28-item interviewer-administered questionnaire for the person with dementia to obtain participants’ ratings of their own quality of life. Items are rated on a 4-point scale, with potential scores ranging from 28 to 112. This measure was also used in the economic evaluation, drawing on the algorithm that has been developed to generate quality-adjusted life-year (QALY) scores from DEMQOL questionnaire scores. 86
Generalized Self-Efficacy Scale87
The Generalized Self-Efficacy Scale (GSES) is a 10-item scale that assesses a general sense of perceived self-efficacy, which is the potential to influence one’s situation through one’s own actions. Responses are made on a 4-point scale. Responses to all 10 items are summed to yield the final composite score with a range from 10 to 40. Cronbach’s alphas range from 0.76 to 0.90. 88
Hospital Anxiety and Depression Scale89
The Hospital Anxiety and Depression Scale (HADS) contains 14 items that form subscales for anxiety and depression. Each item is rated on a 4-point scale, giving maximum scores of 21 for anxiety and for depression. Scores of ≥ 11 on either subscale are considered to be a significant ‘case’ of psychological morbidity, with scores of 8–10 being classified as ‘borderline’ and scores of 0–7 being classified as ‘normal’. The HADS has been employed and validated in studies of people with dementia and carers. 90,91
Brief cognitive assessment battery
The brief cognitive assessment battery consisted of brief tests of memory, attention and executive function, suitable for people with early-stage dementia, each taking < 5 minutes to administer:
-
Memory – Rivermead Behavioural Memory Test (RBMT),92 a story recall subtest. The RBMT is a well-established, ecologically valid test of everyday memory. In the story recall task, the researcher reads out a short story, similar to a brief report of a newsworthy event in a daily newspaper, and the participant is asked for immediate and, after 20 minutes, delayed recall of the content. Recall is scored following a standard protocol (inter-rater reliability of > 0.9) with a maximum possible score of 21 for the immediate and for the delayed component. Four parallel versions of equivalent difficulty are available to permit reassessment without the risk of practice effects; practice effects are not anticipated with test–retest intervals of 3 and 6 months, but as a precaution we used a different version at each time point. The raw scores were used in the analysis, as they provide a greater range than the condensed standardised profile score that is used in the calculation of the overall RBMT score.
-
Attention – Test of Everyday Attention (TEA),93 elevator counting and elevator counting with distraction subtests. The TEA is a well-established, ecologically valid test of everyday attention, with subtests assessing different components of attention. The elevator counting subtest assesses sustained attention. Participants are required to count a short string of monotonous tones and give the total number. Seven strings are presented, and the total score is the number of strings correctly counted. The elevator counting with distraction subtest assesses auditory selective attention. Ten strings of tones are presented, this time also including distractor (high-pitched) tones that are not to be counted. The total score is the number of strings correctly counted. Three equivalent versions of each subtest are available to permit reassessment without the risk of practice effects; as above, practice effects were not anticipated, but as a precaution we used a different version at each time point.
-
Executive function – letter fluency subtest of the Delis–Kaplan Executive Function System (D-KEFS). 94 D-KEFS consists of a set of standardised tests of executive function. The verbal letter fluency task evaluates the executive subdomains of initiation, response generation and inhibition,95 as well as drawing on semantic memory and language ability. In this task, the participant is asked to list as many words as possible beginning with a specific letter of the alphabet in a 1-minute period, excluding proper nouns and repetitions. Three letters, F, A and S, are used. The total number of correct responses to the three letters is the unit of analysis. This task has been extensively examined in people with early-stage dementia. 96 Evidence suggests that even in healthy participants there are no practice effects for most components of this task, even at test–retest intervals of < 2 weeks; there are minimal practice effects for the switching component with test–retest intervals of < 2 weeks, but not with longer intervals.
-
The Client Service Receipt Inventory (CSRI),97 completed by the person with dementia and their carer together. The CSRI provides a template that can be adapted to the needs of each specific study. In GREAT, the CSRI focused on both the person with dementia and the carer. Respondents were asked about the use of health and social care services by the person with dementia in the 3-month period preceding each assessment. The questions cover contact with a range of health and social care professionals, prescription of medications, hospital appointments and stays, participation in local authority-funded activities (such as day centres), participation in activities run by voluntary organisations and the contribution of informal carers. Questions to examine the nature and extent of any dementia-specific treatment received from the Memory Clinic were included. Carers were asked about their own accommodation, the impact of caring on employment (when applicable), the involvement of others in the person’s care and the costs involved in accompanying the person with dementia to appointments.
Secondary outcome measures for the carer
The outcomes of interest for carers were stress, quality of life and health status, assessed with the following self-rated measures at baseline and at the 3- and 9-month follow-ups:
-
The Relatives’ Stress Scale (RSS). 98 The RSS is a 15-item dementia-specific measure of caregiver stress with items rated on a 5-point Likert scale and summed (score range 0–60). A higher overall score indicates higher levels of caregiving-specific stress.
-
World Health Organization’s Quality of Life Instrument – brief version (WHOQOL-BREF). 99 The WHOQOL-BREF is a 26-item scale assessing perceived quality of life, with each item scored on a 5-point Likert scale (with the lowest score being 1 and the highest score being 5) giving scores in four domains: environment (raw score range 8–40), social relationships (raw score range 3–15), psychological health (raw score range 6–30) and physical health (raw score range 7–35). Scores within each domain are summed and the mean domain score is calculated (range 1–5); this is multiplied by 4, giving a score in the range of 4–20.
-
EuroQol-5 Dimensions (EQ-5D). 100 The EQ-5D is a standardised measure of health status and health outcome, applicable to a wide range of health conditions. In the first section, the respondent is asked to select one of three options for each of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For each dimension, the three response options are coded on a 3-point scale from 1 (no problems) to 3 (unable to perform/extreme problems). This yields a descriptive profile (e.g. 11232) across the five dimensions. Based on the EQ-5D descriptive categories, a summary index can be calculated. 101 Each level in each dimension has a weight attached, and the summary index is calculated by deducting the appropriate weights from 1. The EQ-5D, three-level version (EQ-5D-3L) has an index score from –0.594 to 1.0, with a higher index score indicating a higher quality of life and a negative index score indicating a quality of life that is considered by some respondents to be ‘worse than death’. The second part of the measure is a visual analogue scale (VAS) for the self-rating of HRQoL (‘your health state today’) on a scale of 0–100. This measure was included so that the EQ-5D score could be used to generate QALY scores using societal weights.
Process evaluation measures
The process evaluation first addressed the process of goal-setting, and second, the process of therapy. A summary of the process evaluation measures is provided in Table 3.
| Source | Measure | Time point (when applicable) |
|---|---|---|
| Whole sample | ||
| BGSI | Goals set | Baseline |
| Goal ratings – importance | Baseline | |
| Goal ratings – readiness to change | Baseline | |
| CR group | ||
| Therapy logs | Goals addressed | |
| Adjustments made to BGSI goal statements | ||
| Goal attainment ratings by person with dementia | When work on goal is introduced and in session 10 | |
| Goal attainment ratings by carer | When work on goal is introduced and in session 10 | |
| Goal attainment ratings by therapist | When work on goal is introduced and in session 10 | |
| Therapist selection of goal attainment scaling indicators | In sessions 10 and 14 | |
| Therapist rating of carer involvement in therapy | Following session 14 | |
| Therapist rating of confidence in addressing the participants’ goals | Following session 14 | |
| Compliance – number of sessions the participant received | Following session 14 | |
| Therapy logs and therapist supervision | Therapist adherence to protocol | |
| Focus group with therapists | Therapist experience of delivering therapy | |
| Interviews with participants and carers | Participant and carer experience of CR | After the 9-month follow-up |
The goal-setting process
All participants, irrespective of allocation, identified personal goals in the baseline assessment. Participants rated the importance to them of each goal and their readiness to change. We extracted details of the goals that participants identified at baseline, together with the corresponding ratings.
The process of therapy
For participants allocated to receive the CR intervention, we evaluated the process of therapy through several means, from the perspectives of the therapists and of the participants and carers.
Goals addressed in therapy
For every goal originally set at baseline by participants subsequently randomised to receive CR, we noted whether or not the goal was addressed in therapy, any adjustments to the original phrasing or modifications made by the therapist in order to operationalise the goal and details of the corresponding ratings by the participant and the carer.
Therapist perceptions of goal attainment
Therapist perceptions of goal attainment were captured in two ways.
First, the therapist made in-session parallel ratings of goal attainment alongside the participant and the carer when each goal was introduced, when the work on therapy goals concluded (usually in session 10) and at the final maintenance session (usually session 14).
Second, the therapist applied a simplified goal attainment scaling procedure26 for each goal addressed in the intervention. When starting work on the goal, clearly specified behavioural indicators of full and partial goal achievement were established. The therapist subsequently rated progress in accordance with these criteria following session 10 and again following session 14 (maintenance session 4).
Effectiveness of supervision
The supervision process was reviewed through annual evaluation of the supervision session attendance rates and rates of submission and review of the therapy logs to ensure the appropriate involvement of all therapists in the supervisory process.
Therapist perspectives on the process of therapy
Following each session, therapists noted relevant information in the therapy log. Therapy logs contained the therapists’ notes from each session, with comments on participants’ progress in relation to goals and other prespecified topics relating to the delivery of the intervention. The logs were not intended to provide a comprehensive summary of every session. Each therapist completed a separate record for each session. See Appendix 1 for full details of the topics recorded in the therapy logs.
Therapist experience of providing the intervention
A focus group was held with six therapists in June 2014, during an annual training event for the trial team held at the end of the therapists’ first year. The aim was to explore their experience of delivering the intervention.
Participant treatment compliance
Treatment compliance was indexed by the number of sessions completed for each participant, up to the maximum of 14 sessions (10 therapy sessions and four maintenance sessions).
Participant and carer experience of the intervention
In three sites where an independent researcher not involved in the trial was available to contribute, a consecutive series of participants and carers completing the trial in the CR arm were approached after the 9-month follow-up and invited to participate in an interview to reflect on their experience of the intervention. The sites were Bangor, Cardiff and Manchester. The interviews addressed the following questions:
-
How did participants and carers experience the intervention?
-
What were their overall perceptions, how useful did they find it, and what did they feel about the degree of effort required?
-
What impact, if any, did the participants and carers feel the intervention had on their everyday life?
The interview schedule is presented in Appendix 2.
Data management
A detailed data management plan covering all of the quantitative data gathered in the trial was developed in collaboration with the CTU and was followed throughout the trial. Quality-assurance procedures included an audit of sites, random checks of the accuracy of data entry and ongoing monitoring of scoring accuracy.
We used the online MACRO Electronic Data Capture (InferMed, London, UK) data management system, which was managed by the NWORTH CTU. A range of built-in checks prevent the entering of invalid information into MACRO. The accuracy of data entry was examined during initial site visits, and 5% of the data at each time point were cross-checked against hard-copy case report forms. Additional checks were conducted centrally, including missing data verification, searches for unusual patterns and identification of unexpected data ranges. These checks indicated a high level of accuracy in data entry.
The GREAT researchers received training in administering all outcome measures and in following data management procedures at the start of the trial, and thereafter through annual refresher training days, and were provided with a detailed researcher’s handbook together with a number of other resources: a guide to using the BGSI, an administration and scoring guide for all other measures, a MACRO user guide, randomisation instructions, details of the safety monitoring and reporting procedure and template spreadsheets for recording and managing contact with participants. The researchers recorded assessment data manually during the participant visits and entered anonymised data item by item into MACRO.
The GREAT therapists received training in data management procedures and the use of the MACRO system at the start of the trial and this was updated during their annual refresher training. In addition to the therapists’ manual, they were provided with a MACRO user guide, randomisation instructions, details of the safety monitoring and reporting procedure and template spreadsheets for recording and managing contact with participants. The therapists entered into MACRO basic information about the participant, details of the PAL assessment, the date and length of each therapy session and details of any missed sessions, details of the goals worked on, ratings of goal attainment, goal attainment scaling indicators and ratings of participant awareness and response to therapy.
Information about adverse events was recorded in the MACRO system by both researchers and therapists, or it was provided on paper-based forms to the co-ordinating centre, where it was entered into the MACRO system.
A separate plan covered management of the data gathered by the therapists and of the qualitative interview data, and this was overseen by the trial manager. Therapy Record Sheets for each participant (a therapy log, a goal-rating sheet and a goal intervention sheet) were stored within a secure shared drive through which the documents could be updated by the therapists following each therapy session and accessed by the supervisor to inform the monthly supervisory sessions. A similar data-sharing strategy was used to manage interview substudy data. In Bangor, we used a shared drive accessed with WinSCP software (developed by Martin Přikryl: https://sourceforge.net/projects/winscp) or the Oracle Secure Global Desktop platform (Oracle Corporation, Redwood Shores, CA, USA), and in Exeter we used an Alfresco web-based platform (Alfresco Software Inc., San Mateo, CA, USA).
Outcome analyses
The primary statistical analysis was based on the treatment-as-allocated principle and was conducted as an intention-to-treat analysis. The trial statistician conducted analyses while being blind to allocation, not knowing which of the two groups had received the intervention. The statistician was unblinded only after the primary analysis had been completed and approved by the TSC. The analyses were conducted in R version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA).
Primary outcome
The main analysis was an analysis of covariance (ANCOVA) adjusted for baseline score, allocation group and the stratification variables (age, sex, MMSE score and centre), which were treated as random effects. The analysis used a mixed-effects model. However, the standardised effect size estimates (Cohen’s d) and the confidence intervals (CIs) were based on a fixed-effects size model, as these parameters cannot be derived from a mixed-effects model. Effect sizes were calculated by converting eta-squared to r and then converting r to Cohen’s d, using the formula provided by McGrath and Meyer. 102
Additional regression modelling was undertaken to identify factors that could be important in attaining and maximising the observed effects. This was done separately for people with dementia and carers. For the participants with dementia, in addition to the stratification variables (age, sex, MMSE score and centre), the following factors were retained in the final model on a priori theoretical grounds: diagnosis, medication, education and comorbidity. The remaining factors [marital status, living situation, first language, ethnicity, socioeconomic status (SES) and self-rated health] were each subjected to a simple regression analysis to determine whether or not the given factor was significantly associated with outcome; if so, that factor was added to the regression model. For the carers, the following factors relating to the carer were included in the final model on a priori theoretical grounds: relationship with the person with dementia, age, sex and education. The remaining factors (first language, ethnicity, SES and self-rated health) were each subjected to a regression analysis to determine whether or not the given factor was significantly associated with outcome; if so, that factor was added to the regression model.
Secondary outcomes
The main analyses for the secondary outcomes used an ANCOVA adjusted for baseline score, allocation group and stratification variables.
We conducted exploratory analyses to examine the influence of demographic variables on key secondary outcomes at baseline and follow-up. For the person with dementia, we examined the influence of the following factors on BGSI performance ratings at 9 months and BGSI satisfaction ratings: centre, age, sex, educational level, social class, ethnicity, living situation, diagnosis, baseline MMSE score, whether or not the person was taking medication for dementia, comorbidity and effectiveness of blinding. For the carer, we examined the influence of the following factors on carer stress: age, sex, education, social class, ethnicity, relationship to the participant, whether or not the carer was co-resident with the person with dementia and self-rated health status.
Sensitivity analyses
Sensitivity analyses for the primary and main secondary outcomes were planned based on:
-
Treatment received irrespective of group allocation. This analysis was planned in order to compare the outcomes of statistical analyses in which the group allocation was determined by random assignment with the outcomes of statistical analyses in which group allocation was based on the treatment received. Because the group allocation and treatment received was 100% consistent, a treatment-received analysis was not necessary.
-
Complete case with no data imputation. We compared the outcomes of statistical analyses that included both imputed and complete-case data with the outcomes of statistical analyses that included complete cases only. This proposed a comparison involved appraising whether or not the results from the complete-case data analysis and the imputed data analysis are substantially different.
Adherence
For the CR group, we examined the impact of the ‘dose’ of treatment received on primary and secondary outcomes at the 3- and 9-month follow-ups. Adherence was calculated as the number of therapy sessions completed. The analysis was intended to be conducted on the basis of treatment received rather than group allocation.
Effectiveness of blinding
We examined whether or not the researcher’s ability to correctly surmise the participant’s group allocation was associated with the primary and secondary outcomes at the 3- and 9-month follow-ups, and whether this ability varied by centre or depending on any participant characteristics.
Process evaluation analyses
We undertook a range of process evaluation analyses. These are briefly detailed below. A fuller account of the methods is presented along with the findings in Chapter 4.
Nature of goals identified
To better understand the concerns of participants and the areas addressed by therapists in the intervention, we examined the goals set by participants in the trial and used descriptive content analysis to classify them into groups based on the content and focus of the goal.
Goal-setting and goal attainment
We compared the goal attainment ratings made by the participant, the carer and the therapist at each stage and the changes in these ratings between the point at which work on the goal was introduced and sessions 10 and 14, in order to examine the similarities and differences in perspective regarding level of functioning and change in functioning. We also compared the goal attainment ratings with the therapists’ goal attainment scaling process (which classified goals as being fully or partially achieved, with a percentage score) to determine whether or not these two procedures yielded consistent information.
Intervention fidelity
Fidelity of form was evaluated in relation to the provision of core elements of the intervention. This was indexed by the number and length of therapy sessions completed and the number of goals operationalised and addressed.
Fidelity of function was considered in relation to the therapists’ ability to deliver the intervention in a flexible, person-centred manner adapted to the needs of each individual participant. In order to evaluate the level of flexibility applied by the therapists, we reviewed the times at which additional goals were introduced and the nature and extent of modifications to goals formulated in the baseline assessment. As fidelity of function was promoted through regular supervision, with the aid of the detailed therapy logs produced by therapists, we also assessed attendance at supervision sessions and the rates of completion of therapy logs.
Therapist perspectives on the intervention and factors associated with positive outcomes
We explored what factors were thought to influence treatment outcome and the characteristics of participants who seemed most and least likely to benefit from the intervention, and why, with two sets of data: (1) data from a focus group conducted with the therapists and (2) a selection of therapy logs.
We analysed data from the focus group conducted with the therapists to identify their views about delivering the intervention and about what factors influence treatment outcome. From the existing literature, we identified a number of factors that we expected the therapists to consider; these were the level of cognitive impairment, health status, awareness of difficulties, motivation, the degree of carer involvement and family circumstances. We used a deductive approach, coding the data in relation to these factors, but also adopted an inductive perspective, which allowed new factors to emerge.
The therapy logs provided a rich source of data about the process of therapy for each individual participant and were analysed to identify significant features and processes of the intervention. We analysed therapy logs for the 25 participants with the best outcomes on the BGSI and the 25 participants with the worst outcomes on the BGSI using a matrix analysis to examine which factors identified from the focus group data were noted as being relevant in the therapy logs. This analysis was also inductive, allowing additional factors to emerge from the therapy logs. The aim was to understand more about which participants were or were not likely to benefit from the intervention.
Participant and carer experience of the intervention
We analysed the transcribed recordings of the interviews with participants and carers using a thematic analysis. The aim was to identify the level of satisfaction with the intervention and any features that contributed to greater or lesser degrees of satisfaction.
Feasibility of implementation
Towards the end of the trial, once recruitment had finished and therapists had completed the majority of the intervention work, sites were invited to consider examining the feasibility of future implementation of CR within their routine services. This was achieved by therapists offering training in the approach to groups of clinical staff and supporting these staff to use the approach. This initiative was evaluated informally through reports from the local principal investigators (PIs). The findings are described in Appendix 3.
Economic analyses
The main economic evaluation was a cost-effectiveness analysis, conducted first from a health and social care perspective and second from a societal perspective. The methods and results for the economic analyses are described in Chapter 5.
Patient and public involvement
Two Alzheimer’s Society Research Network volunteers were involved as advisors to the pilot trial, and they contributed to the development of the GREAT protocol and served as experts by experience on the TSC, in which they were joined by a third Alzheimer’s Society Research Network volunteer.
Changes to protocol
There were two changes to the protocol.
The trial was initially set up in six sites. To meet our recruitment target, each site was requested to randomise 80 participants, representing approximately three participants per month over a 27-month recruitment period. All centres regarded this recruitment target as feasible. However, it proved to be challenging for some sites, and although other sites did meet and in some cases exceed targets (Bangor and Bath agreed to increase their target to 90 participants each), under-recruitment was a concern. Attrition was lower than expected, suggesting that randomisation targets could be reduced, but the funder advised that the original estimate should be retained and no alteration should be made to the target sample size. To address the likely shortfall in recruitment, two additional sites were identified, with a target of 25 randomisations each: Kent and Medway NHS and Social Care Partnership Trust and Northumberland, Tyne and Wear NHS Foundation Trust. The inclusion of these sites was agreed with the funder and approved as an amendment by Wales REC 5. The two sites achieved their first randomisations in June 2015, allowing for a 10-month recruitment period in each site.
The process evaluation component of the data analysis plan was initially envisaged to include (1) convergent evidence about changes in goal performance in the CR group derived from in-session ratings and therapist assessment of goal attainment, (2) intervention fidelity on the part of the therapists and (3) treatment compliance in terms of the number of sessions received by the participant. This was expanded following discussion within the trial team and with the TSC. Qualitative interviews were added in order to capture the experience and perspectives of participants, carers, therapists and researchers; this was approved as an amendment by Wales REC 5. A subset of participants and carers was interviewed following completion of the trial after the 9-month follow-up by an independent researcher who was not part of the trial team in order to elicit the participants’ and carers’ experience of the intervention, and a focus group was conducted with the therapists to capture their experience of delivering the therapy sessions.
Chapter 3 Results
Recruitment and participant flow
The flow of participants through GREAT is shown in the Consolidated Standards of Reporting Trials diagram in Figure 2.
FIGURE 2.
Consolidated Standards of Reporting Trials chart showing participant flow through the trial. Reproduced with permission from Clare et al. 72

Participants were recruited in eight NHS sites in England and Wales: Bangor, Cardiff, London, Kent, Bath, Birmingham, Manchester and Newcastle. Recruitment began on 1 April 2013 and was completed by 31 March 2016. Follow-up assessments were completed by 31 December 2016. The cumulative recruitment figures are provided in Appendix 4.
Overall, 1731 participants were identified as being potentially eligible, of whom 583 (34%) were screened for eligibility and 538 (31%) were seen for the baseline assessment. Following the baseline assessment, 475 participants (88% of those assessed) were randomised to either the CR (n = 239) arm or the TAU (n = 236) arm. Of the participants who did not proceed, a small proportion had difficulty identifying any goals to work on, as they felt content with their current situation and did not think that they had any particular needs. One participant who did not meet the diagnostic criteria was incorrectly randomised (to the CR arm) and was withdrawn from the analysis; this participant had a diagnosis of Parkinson’s disease dementia. All other participants received their allocated condition. Six participants in the CR group requested withdrawal from the intervention after at least two sessions, but remained in the trial to complete the follow-up assessments. One person withdrew after two sessions, three people withdrew after four sessions, one person withdrew after 10 sessions and one person withdrew after 11 sessions. The reasons for withdrawal were stressful life circumstances, lack of motivation to engage in therapy, comorbid health problems and rapid cognitive decline.
Retention in the trial was 94% at 3 months and 90% at 9 months. The overall figure of 10% attrition was considerably lower than the conservative 27% estimate used in the sample size calculation. The trial was adequately powered, and would have been adequately powered even with a smaller sample size consisting of 385 participants randomised.
Sample characteristics
The demographic characteristics of participants and carers are summarised in Table 4. Details are provided for the sample as a whole and separately for the CR and TAU groups. The demographic characteristics are presented in more detail in Appendix 5.
| Measure | Whole sample (N = 474) | Treatment group | |
|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||
| Participants with dementia | |||
| Age (years), mean (SD); range | 78.56 (7.07); 53–95 | 78.25 (7.13); 53–95 | 78.87 (7.01); 55–95 |
| Sex (male), n (%) | 248 (52.3) | 124 (52.1) | 124 (52.5) |
| Ethnicity, n (%) | |||
| White | 457 (96.4) | 226 (95.0) | 231 (97.9) |
| Mixed/multiple ethnic group | 2 (0.42) | 2 (0.84) | 0 (0) |
| Asian/Asian British | 6 (1.27) | 3 (1.26) | 3 (1.27) |
| Black/African/Caribbean/black British | 7 (1.48) | 5 (2.10) | 2 (0.85) |
| Other ethnic group | 2 (0.42) | 2 (0.84) | 0 (0) |
| First language (English), n (%) | 445 (93.9) | 222 (93.3) | 223 (94.5) |
| Marital status (married), n (%) |
330 (69.6) n = 474 |
167 (70.2) n = 238 |
163 (69.1) n = 236 |
| Years of education, mean (SD); range |
12.57 (3.37); 5–33 n = 471 |
12.57 (3.33); 6–24 n = 236 |
12.58 (3.42); 5–33 n = 235 |
| Occupational status, n (%) | |||
| I: professional | 52 (11) | 23 (9.7) | 29 (12.3) |
| II: managerial/technical | 157 (33.1) | 81 (34) | 76 (32.2) |
| III N: skilled, non-manual | 103 (21.7) | 54 (22.7) | 49 (20.8) |
| III M: skilled, manual | 80 (16.9) | 41 (17.2) | 39 (16.5) |
| IV: partly skilled | 50 (10.5) | 24 (10.1) | 26 (11) |
| V: unskilled | 32 (6.8) | 15 (6.3) | 17 (7.2) |
| Carers | |||
| Relationship to participant with dementia, n (%) | |||
| Spouse/partner | 331 (69.8) | 167 (70.2) | 164 (69.5) |
| Adult child (including in-law) | 118 (24.9) | 58 (24.3) | 60 (25.4) |
| Other | 25 (5.3) | 13 (5.5) | 12 (5.1) |
| Age, mean (SD); range | 68.74 (13.01); 17–92 | 68.45 (13.76); 17–92 | 69.04 (12.24); 23–92 |
| Sex (male), n (%) | 142 (30) | 75 (31.5) | 67 (28.4) |
| Ethnicity, n (%) | |||
| White | 449 (94.7) | 224 (94.1) | 225 (95.3) |
| Mixed/multiple ethnic group | 5 (1.1) | 4 (1.7) | 1 (0.42) |
| Asian/Asian British | 10 (2.1) | 4 (1.7) | 6 (2.5) |
| Black/African/Caribbean/black British | 8 (1.7) | 6 (2.5) | 2 (0.85) |
| Other ethnic group | 2 (0.42) | 0 (0) | 2 (0.85) |
| First language (English), n (%) | 443 (93.5) | 222 (93.3) | 221 (93.6) |
| Marital status (married), n (%) | 393 (82.9) | 187 (78.6) | 206 (87.3) |
| Years of education, mean (SD); range |
13.49 (3.52); 4–26 n = 472 |
13.67 (3.45); 5–25 n = 237 |
13.32 (3.58); 4–26 n = 235 |
| Occupational status, n (%) | |||
| I: professional | 49 (10.3) | 30 (12.6) | 19 (8.1) |
| II: managerial/technical | 158 (33.3) | 74 (31.1) | 84 (35.6) |
| III N: skilled, non-manual | 137 (28.9) | 64 (26.9) | 73 (30.9) |
| III M: skilled, manual | 47 (9.9) | 24 (10.1) | 23 (9.7) |
| IV: partly skilled | 55 (11.6) | 27 (11.3) | 28 (11.9) |
| V: unskilled | 20 (4.2) | 14 (5.9) | 6 (2.5) |
| NA | 8 (1.7) | 5 (2.1) | 3 (1.3) |
We examined whether or not participants who were randomised but dropped out before the 3-month follow-up had different demographic characteristics and substantially different scores on the primary outcome from the participants who completed the 3-month follow-up. None of the comparisons between non-completers and completers was statistically significant (p < 0.05) after correcting for multiple comparisons (see Appendix 6).
The participants’ mean age was 78.56 years, with the age of individual participants ranging from 53 to 92 years. Only 20 (4.2%) were aged < 65 years on entry to the trial and four participants (0.8%) were aged < 60 years. Most participants (69.6%) were currently married and 21.3% were widowed. Just over half were male (52.1%) and the overwhelming majority of participants were of white ethnicity (95%) and had English as their first language (93.3%). On average, they had 12.57 years of education, but nearly half (42.2%) had left education by the age of 16 years and only 19% had completed higher education. Two-thirds had a previous occupational background that was professional, managerial/technical or skilled non-manual.
The majority of carers were spouses or partners of the participants (70%) and another 25% were adult children (or sons- or daughters-in-law). The carers’ mean age was 68.74 years. They were predominantly female (70%), currently married (82.9%) and of white ethnicity (94.8%), with English as their first language (93.5%). They were a relatively well-educated group, with a mean of 13.49 years of education, although one-third (32.9%) had left education by the age of 16 years and only one-quarter (25.3%) had engaged in higher education, and 72.5% had a current or previous occupational background that was classed as professional, managerial/technical or skilled non-manual. Counter to protocol, there were four paid carers in the sample. One was a long-term, live-in housekeeper with a longstanding knowledge of the participant, who was not specifically employed to provide dementia care and was considered to be appropriate for inclusion. Three were paid dementia care workers, of whom two (a support worker and an activities co-ordinator) provided both baseline and follow-up data; these data were retained in the analyses on the advice of the trial statistician.
The clinical characteristics are shown in Table 5. Over half of the sample (59.5%) had a diagnosis of Alzheimer’s disease, around one-quarter had a diagnosis of mixed Alzheimer’s disease and vascular dementia (24.5%), and the remainder (15.6%) had a diagnosis of vascular dementia.
| Measure | Whole sample (N = 474) | Treatment group | |
|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||
| Participants with dementia | |||
| Diagnosis, n (%) | |||
| Alzheimer’s disease | 284 (59.9) | 139 (58.4) | 145 (61.4) |
| Vascular dementia | 74 (15.6) | 43 (18.1) | 31 (13.1) |
| Mixed | 116 (24.5) | 56 (23.5) | 60 (25.4) |
| MMSE, mean (SD); range | 23.82 (3.02); 18–30 | 23.89 (3.04); 18–30 | 23.75 (3.02); 18–30 |
| Charlson Comorbidity Index weighted score, mean (SD); range | 2.52 (1.47); 1–11 | 2.49 (1.47); 1–11 | 2.55 (1.48); 1–10 |
| Subjective rating of health, n (%) | |||
| Excellent | 39 (8.2) | 20 (8.4) | 19 (8.1) |
| Very good | 125 (26.4) | 65 (27.3) | 60 (25.4) |
| Good | 159 (33.5) | 77 (32.4) | 82 (34.7) |
| Fair | 121 (25.5) | 61 (25.6) | 60 (25.4) |
| Poor | 30 (6.3) | 15 (6.3) | 15 (6.4) |
| DEMQOL, mean (SD); range |
92.3 (12.33); 39–112 n = 472 |
92 (12.9); 39–112 n = 237 |
92.61 (11.75); 39–112 n = 235 |
| GSES, mean (SD); range | 30.94 (5.09); 11–40; n = 469 | 30.75 (4.81); 13–40; n = 237 | 31.13 (5.35); 11–40; n = 232 |
| HADS, mean (SD); range | N = 472 | N = 238 | N = 234 |
| Depression | 3.77 (2.79); 0–14 | 3.87 (2.83); 0–12 | 3.67 (2.75); 0–14 |
| Anxiety | 5.14 (3.64); 0–16 | 5.29 (3.67); 0–16 | 4.98 (3.62); 0–16 |
| RBMT, mean (SD); range | N = 473 | N = 237 | N = 236 |
| Immediate recall | 2.66 (2.11); 0–11.5 | 2.58 (2.1); 0–9.5 | 2.73 (2.12); 0–11.5 |
| Delayed recall | 0.38 (1.96); –1 to 9 | 0.39 (1.94); –1 to 8 | 0.37 (1.97); –1 to 9 |
| TEA, mean (SD); range | |||
| Elevator counting |
6.39 (1.16); 0–7 n = 463 |
6.35 (1.27); 0–7 n = 232 |
6.42 (1.05); 1–7 n = 231 |
| Elevator counting with distraction |
4.55 (2.72); 0–9 n = 448 |
4.39 (2.68); 0–9 n = 223 |
4.72 (2.75); 0–9 n = 225 |
| D-KEFS verbal fluency, mean (SD); range |
26.27 (11.82); 2–64 n = 470 |
25.78 (11.61); 2–64 n = 235 |
26.77 (12.03); 3–58 n = 235 |
| Carers | |||
| Stress (RSS), mean (SD); range |
18.96 (9.44); 0–52 n = 471 |
18.85 (9.04); 2–46 n = 236 |
19.08 (9.83); 0–52 n = 235 |
| Subjective rating of health, n (%) | |||
| Excellent | 68 (14.3) | 30 (12.6) | 38 (16.1) |
| Very good | 113 (23.8) | 59 (24.8) | 54 (22.9) |
| Good | 179 (37.8) | 89 (37.4) | 90 (38.1) |
| Fair | 83 (17.5) | 42 (17.6) | 41 (17.4) |
| Poor | 31 (6.5) | 18 (7.6) | 13 (5.5) |
| WHOQOL-BREF domains, mean (SD); range | |||
| Physical |
15.34 (2.95); 5–20 n = 470 |
15.3 (3.0); 5–20 n = 237 |
15.37 (2.9); 7–20 n = 233 |
| Psychological |
15.14 (2.15); 8–20 n = 470 |
15.13 (2.19); 8–20 n = 237 |
15.15 (2.1); 8–20 n = 233 |
| Social |
15.13 (2.66); 5–20 n = 468 |
15.19 (2.67); 5–20 n = 235 |
15.07 (2.66); 7–20 n = 233 |
| Environmental |
16.43 (2.15); 10–20 n = 470 |
16.35 (2.3); 10–20 n = 237 |
16.52 (1.99); 10–20 n = 233 |
| EQ-5D-3L, mean (SD); range | |||
| Index |
0.78 (0.25); –0.18 to 1 n = 468 |
0.77 (0.25); –0.18 to 1 n = 235 |
0.79 (0.24); –0.07 to 1 n = 233 |
| VAS |
74.48 (19.95); 0–100 n = 467 |
73.52 (20.95); 1–100 n = 234 |
75.44 (18.9); 0–100 n = 233 |
Participants’ mean MMSE score was 23.82 points, with a range from 18 to 30. Over two-thirds of participants (68%) rated their health as good, very good or excellent and only 6.3% rated their health as poor. However, levels of comorbidity were high, with 312 participants (65.8%) having at least one other condition in addition to dementia. The mean Charlson Comorbidity Index weighted score was 2.52, with 44 participants (9.28%) identified as having a high risk of mortality, which is a score of ≥ 5. 82 Full details of comorbidity are presented in Appendix 5. Three-quarters of the carers (75.9%) rated their health as good, very good or excellent and only 6.5% rated their health as poor. On average, the carers recorded low levels of stress and good scores for quality of life, although there was individual variability.
Eight participants with dementia (1.7%) had a HADS depression score of ≥ 11, indicating clinical levels of depression [six participants in the CR group (2.5%) and two participants in the TAU group (0.9%)]. Forty-seven participants with dementia (10%) had a HADS depression score of 8–10, indicating possible depression [22 participants in the CR group (9.2%) and 25 participants in the TAU group (10.7%)]. Forty-one participants with dementia (8.7%) had a HADS anxiety score of ≥ 11, indicating clinical levels of anxiety [23 participants in the CR group (9.7%) and 18 participants in the TAU group (7.7%)]. Sixty-six participants with dementia (14%) had a HADS anxiety score of 8–10, indicating raised anxiety levels [33 participants in the CR group (13.9%) and 33 participants in the TAU group (14.1%)]. Full details of the HADS depression and anxiety scores are shown in Appendix 5.
Intervention adherence in the cognitive rehabilitation group
The CR intervention consisted of 10 weekly sessions, followed by four maintenance sessions given at 6-week intervals; all sessions were conducted in participants’ own homes. The details of the number of sessions completed are summarised in Table 6.
| Number of sessions completed | Participants, n (%) |
|---|---|
| 1 | 2 (0.9) |
| 2 | 2 (0.9) |
| 3 | 5 (2.1) |
| 4 | 4 (1.7) |
| 5 | 4 (1.7) |
| 6 | 2 (0.9) |
| 7 | 0 (0) |
| 8 | 1 (0.4) |
| 9 | 2 (0.9) |
| 10 | 7 (3) |
| 11 | 8 (3.4) |
| 12 | 10 (4.3) |
| 13 | 21 (9.0) |
| 14 | 166 (70.9) |
Ninety per cent of participants randomised to receive CR completed at least 10 sessions, with 166 participants (70%) completing all 14 sessions. Another 46 participants (19.32%) completed between 10 and 13 sessions; sessions were missed as a result of difficulty in scheduling therapy sessions in the available time frame (n = 40), for example owing to sickness of the participant, carer and/or therapist or therapist annual leave, or because the participant withdrew from therapy (n = 2) or from the trial entirely (n = 4). Only 26 participants (10.92%) completed fewer than 10 sessions; 20 of these participants withdrew from the trial (three of these before the first session) and six remained in the trial to complete the follow-up assessments (four participants discontinued therapy, one participant completed only eight sessions because of the delayed start of a new therapist following a staff change at the site and one participant did not receive any sessions, as the therapist was on sick leave for an extended period). One ineligible participant with a diagnosis of Parkinson’s disease dementia, who was excluded from the analysis because they did not meet the inclusion criteria, completed all sessions but is not included in Table 6.
Data collection
The 3-month follow-up assessments were scheduled within a 4-week window from weeks 14 to 17 post randomisation. The assessments were completed within this time frame for 420 participants (94%); five participants (1%) completed the assessment over 1 month late. The 9-month follow-up assessments were scheduled within a 4-week window in weeks 39–42 post randomisation. The assessments were completed within this time frame for 367 participants (86%), with two participants (0.5%) completing the assessment over 1 month late.
Adverse events
Details of serious adverse events (SAEs) recorded for participants and carers at each site are summarised in Tables 7 and 8. The majority involved hospitalisation. There were 111 SAEs reported at eight sites. Sixty-eight participants and 26 carers were involved in 83 and 28 SAEs, respectively.
| Site | SAEs by treatment group (n) | Total | |||||
|---|---|---|---|---|---|---|---|
| Participant | Carer | ||||||
| CR | TAU | Before randomisation | CR | TAU | Before randomisation | ||
| Bath | 7 | 3 | 0 | 2 | 0 | 0 | 12 |
| Birmingham | 6 | 2 | 0 | 3 | 2 | 0 | 13 |
| Cardiff | 9 | 5 | 3 | 4 | 1 | 1 | 23 |
| Kent | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| London | 9 | 0 | 1 | 0 | 0 | 0 | 10 |
| Manchester | 8 | 10 | 3 | 5 | 3 | 0 | 29 |
| Newcastle | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| North Wales | 5 | 6 | 0 | 3 | 3 | 1 | 18 |
| Total | 49 | 27 | 7 | 17 | 9 | 2 | 111 |
| Classification | Individual classification reported (n) | Reported within a multiple classification (n) | Total number per classification | ||||
|---|---|---|---|---|---|---|---|
| Participant | Carer | Total | Participant | Carer | Total | ||
| Death | 7 | 1 | 8 | 0 | 0 | 0 | 8 |
| Life-threatening | 0 | 2 | 2 | 5 | 0 | 5 | 7 |
| Hospitalisation or prolongation of existing hospitalisation | 62 | 19 | 81 | 8 | 2 | 10 | 91 |
| Persistent or significant disability or incapacity | 1 | 1 | 2 | 9 | 2 | 11 | 13 |
| Otherwise considered to be clinically significant by the investigator | 1 | 3 | 4 | 5 | 0 | 5 | 9 |
| Alleged/suspected abuse or neglect | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Total | 71 | 26 | 97 | 28 | 4 | 32 | 129 |
Of the 111 events, 66 were reported in the CR group (54 different individuals) and 36 were reported in the TAU group (32 different individuals); nine SAEs occurred prior to randomisation.
SAEs were reported in one of six categories: death, a life-threatening event, hospitalisation or prolongation of existing hospitalisation, persistent or significant disability or incapacity, an event otherwise considered to be clinically significant by the PI, and alleged or suspected abuse or neglect. Of the 111 SAEs, 98 were recorded as a single-classification SAE and 13 SAEs had multiple classifications. Hospitalisation or prolongation of existing hospitalisation was the most frequently reported type of SAE (82%). Eight deaths were notified to the trial team.
None of these events was considered to be related to participation in the trial. The SAE reports were reviewed biannually by the Data Monitoring and Ethics Committee, which concluded that the nature of the SAEs appeared to reflect health problems commonly seen in an older population. An increased number of SAEs in the CR group was seen as an artefact of the reporting procedure, as the CR group received 14 additional visits from the therapist over a 9-month period.
Numbers analysed
The analysis, by assigned group, included data from 474 participants at baseline (CR, n = 238; TAU, n = 236), 445 participants at 3 months (CR, n = 218; TAU, n = 227) and 426 participants at 9 months (CR, n = 208; TAU, n = 218).
Missing data
Details of missing data for the primary outcome at the 3-month and 9-month follow-ups are shown in Table 9; there were no missing data on this measure at baseline. Details of missing data for the secondary outcomes are provided in Appendix 7.
| Measure at each follow-up point | Whole sample | Treatment group | ||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Missing, n (%) | Total | Missing, n (%) | Total | Missing, n (%) | Total | |
| 3 months | ||||||
| Participant rating of attainment | 0 (0.00) | 445 | 0 (0.00) | 218 | 0 (0.00) | 227 |
| Participant rating of satisfaction | 0 (0.00) | 445 | 0 (0.00) | 218 | 0 (0.00) | 227 |
| Carer rating of attainment | 6 (1.27) | 439 | 2 (0.84) | 216 | 4 (1.69) | 223 |
| 9 months | ||||||
| Participant rating of attainment | 10 (2.11) | 416 | 3 (1.26) | 205 | 7 (2.97) | 211 |
| Participant rating of satisfaction | 14 (2.95) | 412 | 5 (2.09) | 203 | 9 (3.81) | 209 |
| Carer rating of attainment | 11 (2.32) | 415 | 4 (1.67) | 204 | 7 (2.97) | 211 |
Multiple imputation of missing values was conducted using the mice R package. 103 For all of the measures, a predictive mean-matching algorithm was used. The missing outcome measures at baseline were imputed using the centre-level factors and the participant’s sex, age and baseline MMSE scores. The missing outcome measure scores at the 3-month and 9-month assessments were estimated based on centre-level factors, baseline characteristics and scores for the same outcome at the earlier time point(s). In line with Grund et al. 104 simulation-based observations of the D2 statistics performance for pooling p-values, 25 sets of imputations were generated using the method described above.
Results for the primary outcome measure
The primary outcome was participants’ goal attainment ratings on the BGSI at the 3-month follow-up. Participants’ goal attainment ratings were also obtained at the 9-month follow-up, and carers provided informant ratings of attainment at the 3- and 9-month points. Participants gave ratings of satisfaction with their goal attainment at both time points. For convenience, all of these BGSI measures will be considered together here. Participant attainment and satisfaction ratings and carer attainment ratings across all time points are summarised in Tables 10 and 11 and shown graphically in Figure 3. Tables 10 and 11 also summarise the statistical analyses of changes in the BGSI ratings at 3 and 9 months.
| Measure | Treatment group | |||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Baseline (N = 238) | 3 months (N = 218) | 9 months (N = 205) | Baseline (N = 236) | 3 months (N = 227) | 9 months (N = 211) | |
| Participant rating of attainment | 3.53 (1.74) | 6.10 (1.99) | 6.05 (2.21) | 3.55 (1.59) | 4.41 (1.84) | 4.22 (2.00) |
| Participant rating of satisfaction | 3.76 (1.76) | 6.47 (1.88) | 6.75 (1.97) | 3.86 (1.49) | 5.05 (1.94) | 5.26 (2.05) |
| Carer rating of attainment | 2.76 (1.43) | 5.46 (1.94) | 5.21 (2.33) | 2.72 (1.32) | 3.55 (1.73) | 3.31 (1.96) |
| Measure at each follow-up point | p-value | Bonferroni-adjusted p-value | Mean difference (95% CI) | Cohen’s d (95% CI) |
|---|---|---|---|---|
| 3 months | ||||
| Participant rating of attainment | < 0.001 | NA | 1.58 (1.27 to 1.9) | 0.81 (0.62 to 1) |
| Participant rating of satisfaction | < 0.001 | < 0.001 | 1.34 (1.01 to 1.66) | 0.7 (0.51 to 0.88) |
| Carer rating of attainment | < 0.001 | < 0.001 | 1.75 (1.42 to 2.07) | 0.93 (0.74 to 1.12) |
| 9 months | ||||
| Participant rating of attainment | < 0.001 | < 0.001 | 1.71 (1.35 to 2.08) | 0.8 (0.61 to 0.99) |
| Participant rating of satisfaction | < 0.001 | < 0.001 | 1.36 (1 to 1.73) | 0.67 (0.49 to 0.86) |
| Carer rating of attainment | < 0.001 | < 0.001 | 1.7 (1.32 to 2.09) | 0.79 (0.6 to 0.97) |
FIGURE 3.
Goal attainment ratings by participants and carers in the CR and TAU groups at baseline and 3-month and 9-month follow-ups. Data are mean scores and the error bars show the standard errors.
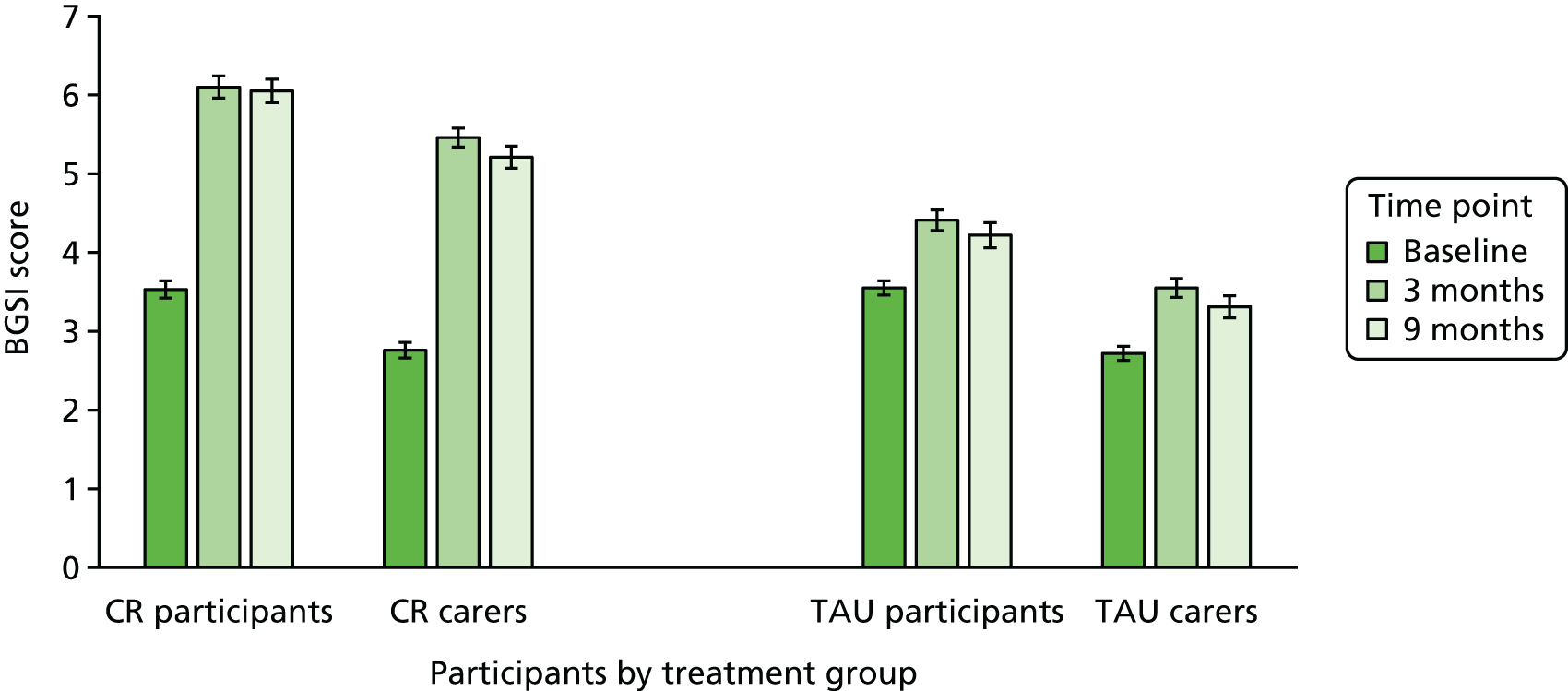
For the CR group, participant attainment ratings improved at the 3-month follow-up by 2.57 points on average, and this improvement was maintained at 9 months. The average ratings in the TAU group showed a negligible improvement of < 1 point at 3 months. The ANCOVA indicated that the differences between the CR and TAU groups were significant at both 3 and 9 months, with large effect sizes of 0.81 and 0.8, respectively.
The same pattern was observed for informant attainment ratings, with the CR group improving by an average of 2.7 points and maintaining the improvement at 9 months, whereas the TAU group ratings showed a negligible improvement of < 1 point. The ANCOVA indicated that the differences between the CR and TAU groups were significant at both 3 and 9 months, with large effect sizes of 0.93 and 0.79, respectively. The carers’ informant ratings of attainment were slightly lower on average than the participants’ attainment ratings, but followed similar trajectories. The participant and carer attainment ratings were highly correlated at all time points, with Pearson’s product–moment correlation coefficients of 0.68 at baseline, 0.77 at the 3-month follow-up and 0.81 at the 9-month follow-up.
In the CR group, the average satisfaction ratings improved by 2.7 points at 3 months and increased further to give a 3-point improvement over baseline at 9 months. The average satisfaction ratings in the TAU group improved by 1.2 points at 3 months, with a further slight increase at 9 months. The ANCOVA indicated that the differences between the CR and TAU groups were significant at both 3 and 9 months, with large effect sizes of 0.7 and 0.67, respectively.
These results demonstrate that, according to the patient- and informant-reported outcomes assessed by the BGSI, the CR intervention was effective in improving functioning in the targeted areas. Furthermore, participants in the CR group were more satisfied with their ability to carry out the everyday activities targeted in the intervention.
Exploratory analyses for the primary outcome measure
Linear mixed-effects models were fitted to identify factors predicting change in the BGSI ratings from baseline to follow-up for participants in the CR group. There were no significant group-by-centre interactions in any of the models, indicating that the results did not differ across centres. Details of these analyses are provided in Appendix 8.
Participants’ goal attainment ratings
A linear mixed-effects model was fitted to identify whether or not any factors predicted the difference in participants’ goal attainment ratings at baseline and the 3-month follow-up. Centre was treated as a random effect and the following factors were treated as fixed effects: sex, age (stratified), MMSE score (stratified), diagnosis, medication use, education, comorbidity, SES and blinding efficiency. At the 3-month follow-up, the model was not statistically significant [χ2(18) = 41.74, R2 = 0.19; p = 0.001]. Two factors within the model had a statistically significant effect in predicting a change in BGSI attainment ratings: blinding efficiency [b = 1.24, standard error (SE) = 0.30, t(216.87) = 4.12, 95% CI 0.54 to 1.76; p < 0.001] and participant SES [χ2(5) = 16.66; p = 0.005]. Greater improvement was seen when blinding was ineffective. For SES, there was a statistically significant difference in the change in BGSI goal attainment rating between professionals and non-manual skilled workers [b = –1.66, SE = 0.65, t(214.71) = –2.55, 95% CI –3.39 to –0.56; p = 0.012], professionals and manual skilled workers [b = –2.11, SE = 0.65, t(216.57) = –3.28, 95% CI –4.17 to –1.05; p = 0.001] and professionals and partly skilled workers [b = –1.93, SE = 0.75, t(216.88) = –2.58, 95% CI –3.83 to –0.67; p = 0.011]. These observed differences show that CR was more effective at improving BGSI performance ratings at the 3-month follow-up for professionals than for non-manual skilled workers, manual skilled workers or non-skilled workers.
A linear mixed-effects model was fitted to identify whether or not any factors predicted the difference between the BGSI attainment ratings at baseline and the 9-month follow-up for participants in the CR group. Centre was treated as a random effect and the following factors were treated as fixed effects: blinding effectiveness, participant’s age (stratified), sex, education, social status, ethnicity, living situation (alone or with others), diagnosis, MMSE score (stratified), medication use and comorbidity. The model was statistically significant [χ2(28) = 68.61, R2 = 0.32; p < 0.001]. Factors within the model that were individually statistically significant were participant’s age, MMSE score and blinding efficiency. Greater improvement was seen in participants who were younger [b = –0.05, SE = 0.02, t(200.60) = –2.08, 95% CI –0.09 to –0.00; p = 0.038] and had higher MMSE scores [b = 0.16, SE = 0.05, t(199.31) = 3.06, 95% CI 0.06 to 0.26; p = 0.002], and when the researcher was able to correctly identify the participant’s group allocation [b = 1.35, SE = 0.32, t(199.86) = 4.21, 95% CI 0.71 to 1.99; p < 0.001].
Linear mixed-effects models were fitted to identify whether or not any carer factors predicted the difference between the participants’ BGSI attainment ratings at baseline and the 3- and 9-month follow-ups for participants in the CR group. Centre was treated as a random effect and the following factors were treated as fixed effects: carer’s sex, carer’s age, carer’s education, hours spent helping the person with dementia per day and the relationship between the carer and the participant. The models were not statistically significant and no factors within the models were statistically significant.
Carers’ ratings of participant goal attainment
Linear mixed-effects models were fitted to identify whether or not any carer factors predicted the difference between carers’ BGSI attainment ratings at baseline and at the 3- and 9-month follow-ups. The models were not statistically significant and no factors within the models were statistically significant.
Linear mixed-effects models were fitted to identify whether or not any participant characteristics predicted the difference between carers’ BGSI attainment ratings at baseline and the 3-month follow-up. The overall model included centre as a random effect and the following factors as fixed effects: MMSE score (stratified), diagnosis, medication use, education, comorbidity, SES. The model was not statistically significant [χ2(17) = 27.24, R2 = 0.12; p = 0.055]. Within the model, SES was statistically significant [χ2(5) = 14.54; p = 0.013]. There was a statistically significant difference in the change in carer BGSI attainment rating between professionals and non-manual skilled workers [b = –1.83, SE = 0.68, t(214.14) = –2.70, 95% CI –3.15 to –0.50; p = 0.007], professionals and manual skilled workers [b = –1.63, SE = 0.67, t(214.99) = –2.46, 95% CI –2.94 to –0.33; p = 0.015]. These results mirror the findings for participant goal attainment ratings at the 3-month follow-up.
Linear mixed-effects models were fitted to identify whether or not any participant characteristics predicted the difference between the carers’ BGSI attainment ratings at baseline and the 9-month follow-up. The overall model included centre as a random effect and the following factors as fixed effects: MMSE score (stratified), diagnosis, medication use, education, comorbidity, SES. The model was not statistically significant [χ2(17) = 31.18, R2 = 0.15; p = 0.019]. Within the model, MMSE (stratified) was a statistically significant factor [χ2(1) = 7.91; p = 0.005]. This shows that there was a statistically significant difference in carer attainment ratings for participants with MMSE scores of ≥ 24 points compared with participants with MMSE scores of < 24 points [b = 0.96, SE = 0.34, t(202.98) = 2.81, 95% CI 0.29 to 1.63; p = 0.005]. The improvement in carer attainment ratings was greater when participants had higher MMSE scores.
Participants’ ratings of satisfaction with goal attainment
A linear mixed-effects model was fitted to identify whether or not any participant characteristics predicted the difference between the BGSI satisfaction ratings at baseline and the 3-month follow-up for participants in the CR group. Centre was treated as a random effect and the following factors were treated as fixed effects: sex, ethnicity, age (stratified), MMSE score (stratified), diagnosis, medication use, education, comorbidity and SES. The model was statistically significant [χ2(28) = 49.89, R2 = 0.21; p = 0.007]. Factors within the model that were individually statistically significant were participant social status [χ2(5) = 16.82; p = 0.005] and blinding efficiency [chi-squared test (1) = 10.30; p = 0.001]. There was a statistically significant difference in the change in BGSI satisfaction rating, showing that satisfaction ratings improved more in professionals than in non-manual skilled workers [b = –1.73, SE = 0.65, t(217) = –2.67, 95% CI –3.01 to –0.46; p = 0.008], manual skilled workers [b = –2.10, SE = 0.63, t(217) = –3.34, 95% CI –3.33 to –0.86; p = 0.001] and partly skilled workers [b = –1.92, SE = 0.75, t(217) = –2.57, 95% CI –2.93 to –0.26; p = 0.011]. There was greater improvement in the BGSI satisfaction ratings from baseline to the 3-month follow-up when blinding was ineffective and the researcher was able to correctly identify that the participant belonged to the CR group than when blinding was effective [b = 0.96, SE = 0.30, t(217) = 3.21, 95% CI 1.04 to 9.49; p = 0.002].
A linear mixed-effects model was fitted to predict the difference between the BGSI satisfaction ratings at baseline and the 9-month follow-up for participants in the CR group. Centre was treated as a random effect and the following factors were treated as fixed effects: blinding efficiency, participant’s age, sex, education, social status, ethnicity, living situation (living alone or with others), diagnosis, MMSE score, medication use and comorbidity. The model was statistically significant [χ2(27) = 54.26, R2 = 0.25; p = 0.001]. The only effects that were statistically significant were MMSE score [χ2(1) = 15.79; p < 0.001] and blinding efficiency [χ2(1) = 14.35; p < 0.001]. Participants with higher MMSE scores at baseline showed greater improvement in BGSI satisfaction ratings from baseline to the 9-month follow-up than participants with lower MMSE scores [b = 0.20, SE = 0.05, t(199.45) = 3.97, 95% CI 0.22 to 0.27; p < 0.001]. There was greater improvement in the BGSI satisfaction ratings from baseline to the 9-month follow-up when blinding was ineffective and the researcher was able to correctly identify that the participant belonged to the CR group than when blinding was effective [b = 1.23, SE = 0.33, t(200.33) = 3.79, 95% CI 0.58 to 1.88; p < 0.001].
Ratings of importance and readiness to change
We examined whether or not participants’ BGSI ratings of importance of the functional domain addressed by each goal and ratings of readiness to change made at baseline were associated with the attainment ratings at the 3-month follow-up. Participants’ baseline ratings of the importance of the functional domain addressed by each goal did not predict improvement. Participants’ ratings of readiness to change at baseline, however, were significantly associated with improvement in attainment ratings at the 3-month follow-up [t(403) = 2.66, r = 0.13; p = 0.008].
Participants’ ratings of readiness to change remained significantly associated with improvement in their BGSI attainment ratings at the 9-month follow-up [t(379) = 2.79, r = 0.14; p = 0.005].
Results for the secondary outcomes
The scores on secondary outcome measures at all time points and the ANCOVA results are summarised in Tables 12 and 13. Following Bonferroni correction of the p-values for the secondary outcomes, there were no significant between-group differences in any secondary outcome measures at the 3- or 9-month follow-ups. The effect sizes were small to negligible, although in some cases these had wide CIs.
| Measure | Treatment group | |||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Baseline | 3 months | 9 months | Baseline | 3 months | 9 months | |
| Participants with dementia | ||||||
| DEMQOL |
n = 237 92 (12.9); 39–112 |
n = 218 92.79 (11.95); 51–112 |
n = 204 92.36 (12.00); 54–112 |
n = 235 92.61 (11.75); 47–111 |
n = 227 93.198 (12.00); 51–111 |
n = 213 92.25 (12.82); 45–112 |
| GSES |
n = 237 30.75 (4.81); 13–40 |
n = 215 30.98 (4.62); 18–40 |
n = 194 30.76 (4.91); 15–40 |
n = 232 31.13 (5.35); 11–40 |
n = 224 30.59 (5.61); 11–40 |
n = 207 30.62 (5.60); 10–40 |
| HADS depression |
n = 238 3.87 (2.83); 0–12 |
n = 218 3.90 (2.86); 0–15 |
n = 194 4.19 (3.23); 0–17 |
n = 234 3.67 (2.75); 0–14 |
n = 226 3.74 (2.69); 0–12 |
n = 210 3.83 (2.82); 0–17 |
| HADS anxiety |
n = 238 5.29 (3.67); 0–16 |
n = 216 5.13 (3.66); 0–17 |
n = 193 5.63 (3.83); 0–18 |
n = 234 4.98 (3.62); 0–16 |
n = 226 4.61 (3.41); 0–15 |
n = 210 4.88 (3.37); 0–20 |
| RBMT immediate recall |
n = 237 2.58 (2.1); 0–9.5 |
n = 218 2.88 (2.16); 0–10.0 |
n = 200 2.34 (2.09); 0–10.0 |
n = 236 2.73 (2.12); 0–11.5 |
n = 226 2.79 (2.12); 0–11.0 |
n = 211 2.37 (1.96); 0–10.0 |
| RBMT delayed recall |
n = 237 0.39 (1.94); –1 to 8 |
n = 217 0.94 (2.31); –1.0 to 8.5 |
n = 200 0.23 (1.97); –1.0 to 8.5 |
n = 236 0.37 (1.97); –1 to 9 |
n = 225 0.66 (2.16); –1.0 to 11.0 |
n = 210 0.36 (1.97); –1.0 to 9.5 |
| TEA elevator counting |
n = 232 6.35 (1.27); 0–7 |
n = 210 6.31 (1.23); 0–7 |
n = 191 6.21 (1.41); 0–7 |
n = 231 6.42 (1.05); 1–7 |
n = 219 6.36 (1.22); 0–7 |
n = 206 6.24 (1.32); 1–7 |
| TEA elevator counting with distraction |
n = 223 4.39 (2.68); 0–9 |
n = 198 4.62 (3.08); 0–10 |
n = 177 4.66 (3.11); 0–10 |
n = 225 4.72 (2.75); 0–9 |
n = 208 4.90 (3.15); 0–10 |
n = 193 4.52 (3.07); 0–10 |
| D-KEFS verbal fluency |
n = 235 25.78 (11.61); 2–64 |
n = 217 26.29 (12.56); 0–58 |
n = 198 26.30 (13.32); 0–62 |
n = 235 26.77 (12.03); 3–58 |
n = 227 26.80 (12.38); 3–68 |
n = 211 25.9 (12.36); 1–67 |
| Carers | ||||||
| RSS |
n = 236 18.85 (9.04); 2–46 |
n = 212 19.42 (9.62); 2–46 |
n = 200 21.23 (9.92); 2–51 |
n = 235 19.08 (9.83); 0–52 |
n = 221 20.42 (10.33); 1–54 |
n = 211 21.65 (10.74); 2–50 |
| WHOQOL-BREF physical |
n = 237 15.3 (3.00); 5–20 |
n = 212 15.20 (2.93); 5–20 |
n = 199 14.95 (3.14); 6–20 |
n = 233 15.37 (2.9); 7–20 |
n = 220 15.07 (2.86); 6–20 |
n = 210 14.78 (2.97); 6–20 |
| WHOQOL-BREF psychological |
n = 237 15.13 (2.19); 8–20 |
n = 212 14.98 (2.21); 7–20 |
n = 199 14.74 (2.41); 7–20 |
n = 233 15.15 (2.1); 8–20 |
n = 220 14.74 (2.20); 7–20 |
n = 210 14.53 (2.38); 7–20 |
| WHOQOL-BREF social |
n = 235 15.19 (2.67); 5–20 |
n = 211 15.03 (2.47); 7–20 |
n = 197 15.04 (2.72); 8–20 |
n = 233 15.07 (2.66); 7–20 |
n = 219 14.80 (2.58); 7–20 |
n = 210 14.51 (2.83); 5–20 |
| WHOQOL-BREF environmental |
n = 237 16.35 (2.3); 10–20 |
n = 212 16.33 (2.26); 9–20 |
n = 199 16.00 (2.40); 9–20 |
n = 233 16.52 (1.99); 10–20 |
n = 220 16.18 (2.04); 10–20 |
n = 210 16.04 (2.05); 11–20 |
| EQ-5D-3L index |
n = 235 0.77 (0.25); –0.18 to 1 |
n = 209 0.75 (0.24); –0.18 to 1 |
n = 196 0.73 (0.27); –0.18 to 1 |
n = 233 0.79 (0.24); –0.07 to 1 |
n = 217 0.74 (0.25); –0.24 to 1 |
n = 211 0.75 (0.23); –0.07 to 1 |
| EQ-5D-3L VAS |
n = 234 73.52 (20.95); 1–100 |
n = 208 74.13 (18.92); 0–100 |
n = 198 74.14 (19.16); 10–100 |
n = 233 75.44 (18.9); 0–100 |
n = 217 73.14 (18.95); 0–100 |
n = 211 72.42 (19.13); 0–100 |
| Measure | p-value | Adjusted p-value | Mean difference (95% CI) | Cohen’s d (95% CI) |
|---|---|---|---|---|
| 3-month follow-up | ||||
| Participants with dementia | ||||
| DEMQOL | 0.738 | 1 | 0.24 (–1.27 to 1.75) | 0.02 (–0.16 to 0.2) |
| GSES | 0.126 | 1 | 0.58 (–0.16 to 1.32) | 0.11 (–0.07 to 0.29) |
| HADS depression | 0.861 | 1 | 0 (–0.42 to 0.41) | 0.02 (–0.16 to 0.2) |
| HADS anxiety | 0.478 | 1 | 0.17 (–0.3 to 0.65) | 0.06 (–0.12 to 0.24) |
| RBMT immediate recall | 0.189 | 1 | 0.19 (–0.1 to 0.48) | 0.1 (–0.08 to 0.28) |
| RBMT delayed recall | 0.096 | 1 | 0.24 (–0.04 to 0.52) | 0.12 (–0.06 to 0.3) |
| TEA elevator counting | 0.799 | 1 | 0.01 (–0.19 to 0.21) | 0.02 (–0.16 to 0.2) |
| TEA elevator counting with distraction | 0.784 | 1 | 0.01 (–0.45 to 0.47) | 0.03 (–0.15 to 0.21) |
| D-KEFS verbal fluency | 0.794 | 1 | 0.15 (–1.12 to 1.41) | 0.02 (–0.16 to 0.2) |
| Carers | ||||
| RSS | 0.382 | 1 | –0.5 (–1.61 to 0.62) | 0.05 (–0.13 to 0.23) |
| WHOQOL-BREF physical | 0.431 | 1 | 0.12 (–0.18 to 0.42) | 0.04 (–0.14 to 0.22) |
| WHOQOL-BREF psychological | 0.214 | 1 | 0.18 (–0.1 to 0.47) | 0.08 (–0.1 to 0.26) |
| WHOQOL-BREF social | 0.572 | 1 | 0.1 (–0.25 to 0.45) | 0.05 (–0.13 to 0.23) |
| WHOQOL-BREF environmental | 0.05 | 0.947 | 0.26 (0 to 0.51) | 0.13 (–0.06 to 0.31) |
| EQ-5D-3L index | 0.295 | 1 | 0.02 (–0.01 to 0.05) | 0.07 (–0.11 to 0.25) |
| EQ-5D VAS | 0.286 | 1 | 1.58 (–1.31 to 4.47) | 0.09 (–0.09 to 0.27) |
| 9-month follow-up | ||||
| Participants with dementia | ||||
| DEMQOL | 0.215 | 1 | 1.08 (–0.62 to 2.78) | 0.09 (–0.09 to 0.27) |
| GSES | 0.38 | 1 | 0.37 (–0.45 to 1.18) | 0.07 (–0.11 to 0.25) |
| HADS depression | 0.614 | 1 | 0.12 (–0.35 to 0.6) | 0.05 (–0.13 to 0.23) |
| HADS anxiety | 0.334 | 1 | 0.26 (–0.26 to 0.77) | 0.08 (–0.1 to 0.26) |
| RBMT immediate recall | 0.496 | 1 | 0.1 (–0.19 to 0.4) | 0.06 (–0.12 to 0.24) |
| RBMT delayed recall | 0.466 | 1 | –0.1 (–0.37 to 0.17) | 0.06 (–0.12 to 0.24) |
| TEA elevator counting | 0.718 | 1 | –0.01 (–0.27 to 0.25) | 0.04 (–0.14 to 0.22) |
| TEA elevator counting with distraction | 0.334 | 1 | 0.23 (–0.23 to 0.69) | 0.09 (–0.09 to 0.27) |
| D-KEFS verbal fluency | 0.342 | 1 | 0.71 (–0.75 to 2.16) | 0.06 (–0.12 to 0.24) |
| Carers | ||||
| RSS | 0.808 | 1 | 0.08 (–1.09 to 1.25) | 0.02 (–0.16 to 0.2) |
| WHOQOL-BREF physical | 0.399 | 1 | 0.14 (–0.19 to 0.47) | 0.05 (–0.13 to 0.23) |
| WHOQOL-BREF psychological | 0.346 | 1 | 0.15 (–0.16 to 0.45) | 0.06 (–0.12 to 0.24) |
| WHOQOL-BREF social | 0.049 | 0.93 | 0.41 (0 to 0.81) | 0.15 (–0.03 to 0.33) |
| WHOQOL-BREF environmental | 0.371 | 1 | 0.13 (–0.15 to 0.4) | 0.06 (–0.12 to 0.24) |
| EQ-5D-3L index | 0.547 | 1 | –0.01 (–0.04 to 0.02) | 0.04 (–0.14 to 0.22) |
| EQ-5D-3L VAS | 0.071 | 1 | 2.6 (–0.22 to 5.42) | 0.14 (–0.04 to 0.32) |
Exploratory analyses for the secondary outcomes
As there were no overall effects on secondary outcomes, we examined whether or not benefits were seen for particular subgroups on key outcome measures. The subgroups reflected centre, age (< 75 years; ≥ 75 years), sex, SES, diagnosis, and MMSE score (< 24 points; ≥ 24 points). Details of these analyses are provided in Appendix 9.
For the person with dementia, the measures examined were DEMQOL, HADS anxiety and depression scores and GSES at the 3- and 9-month follow-ups. No statistically significant models or individual factors were observed for DEMQOL or the HADS at either time point. For the GSES, the overall models were also not significant, but at 3 months sex was significant (p = 0.044), and at 9 months diagnosis was significant (p = 0.021).
For the carer, the measures examined were the RSS and the WHOQOL-BREF. No statistically significant models or individual factors were observed for the RSS. For the WHOQOL-BREF, there were no statistically significant models. The only significant individual factor was in the WHOQOL-BREF physical scale, in which centre was significant at the 3-month follow-up [χ2(1) = 4.48; p = 0.034]. The model containing this factor was not itself significant, [χ2(12) = 8.12, R2 = 0.07; p = 0.775] and the centre factor was not statistically significant at the 9-month follow-up.
Sensitivity analyses
As all participants received the allocated condition, it was not necessary to conduct the planned analysis based on treatment received irrespective of group allocation.
The results from the analysis of complete-case data were very similar to those of the multiple imputation analysis and did not alter the overall picture in any way. A summary of the statistical analyses for the primary and secondary outcome measures using the full data set with no imputations is shown in Appendix 10.
An examination of the change from baseline at the 3- and 9-month follow-ups in the CR group alone using an analysis of variance demonstrated the same pattern as the main analysis, with significant changes in participant and carer BGSI attainment ratings and participant BGSI satisfaction ratings, all with large effect sizes, and no significant changes in other outcomes. Details are shown in Appendix 11.
Effectiveness of blinding
At the 3- and 9-month follow-ups, researchers recorded their estimations of group allocation for each participant. In the majority of cases, researchers were able to correctly guess the participant’s allocation (see Appendix 12).
At the 3-month follow-up, researchers were very certain about the accuracy of their estimations in 16.6% of cases, quite certain in 31.7% of cases, uncertain in 33.3% of cases and very uncertain in 18.2% of cases. Participants explicitly disclosed their group allocation to the researchers in 14.8% of cases, whereas in 8.3% of cases, researchers noticed some indirect clues, such as the presence of memory aids and adaptions. In 48.4% of cases, researchers acknowledged that their guesses were influenced by the presence or absence of change in the participant’s goal performance rating. Researchers were more often very certain about their estimations of participants in the CR group (20.6% of CR participants) than their estimations of participants in the TAU group (12.8% of TAU participants), and they were more likely to be directly unblinded by participants in the CR group (20.2% of CR participants) than by those in the TAU group (9.7% of TAU participants). The picture at the 9-month follow-up was similar.
Binomial tests to assess the difference in proportions between group allocation identified by the researcher and group allocation not identified by the researcher showed a statistically significant difference for all sites and follow-up combinations (p < 0.001). As noted above under the exploratory analyses for the primary outcome measure, the researcher’s ability to correctly surmise the participant’s group allocation was associated with participant attainment and satisfaction scores at the 3- and 9-month follow-ups.
Associations between adherence and outcomes
For the CR group, we examined the association of the number of sessions completed with all primary and secondary outcomes at 3 and 9 months. These analyses are summarised in Appendix 13.
At the 3-month follow-up, adherence was significantly associated with BGSI participant attainment ratings [b = 0.17, SE = 0.09, t(215) = 2.01, 95% CI 0 to 0.34; p = 0.046] and carer attainment ratings [b = 0.21, SE = 0.08, t(213) = 2.46, 95% CI 0.04 to 0.37; p = 0.015]. At the 9-month follow-up, adherence was significantly associated with BGSI participant attainment ratings [b = 0.24, SE = 0.10, t(202) = 2.36, 95% CI 0.04 to 0.44; p = 0.019] and carer attainment ratings [b = 0.28, SE = 0.10, t(201) = 2.68, 95% CI 0.07 to 0.48; p = 0.008], with participant BGSI satisfaction ratings [b = 0.25, SE = 0.11, t(200) = 2.33, 95% CI 0.04 to 0.47; p = 0.021] and with carer EQ-5D-3L VAS scores [b = –1.55, SE = 0.78, t(193) = –1.98, 95% CI –3.09 to –0.01; p = 0.049].
These adherence analyses indicate that attending more sessions was associated with more positive ratings of goal attainment. For each therapy session attended, the participant’s BGSI attainment rating increased on average by 0.24 at the 9-month follow-up.
Chapter 4 Process evaluation
We undertook a range of process evaluation analyses to gain a better understanding of how the intervention was delivered, the influences on treatment outcome and the mechanisms of action through which the intervention operated. We also undertook work to assess the feasibility of implementation in NHS services. As outlined in Chapter 2, we explored the following areas:
-
goal-setting and goal attainment
-
intervention fidelity
-
factors associated with positive outcomes and therapist perspectives on delivering the intervention
-
participant and carer experience of the intervention
-
feasibility of implementation.
Goal-setting and goal attainment
The use of individual goals was central to the intervention and a range of process evaluation analyses were undertaken to explore aspects of goal-setting and the goals chosen.
Goal-setting
All participants were invited to identify up to three therapy goals. Researchers were encouraged to ensure that at least two goals were identified, to ensure that the participant understood the aims of the therapy and was motivated to participate. In total, 1358 therapy goals were identified by 474 randomised participants. The majority of participants (411; 86.7%) identified three therapy goals, 62 participants (13.1%) identified two therapy goals and one participant (0.2%) identified one therapy goal. Further details are provided in Table 14.
| Number of goals set by participants | Whole sample (N = 474) | Treatment group | |
|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||
| One goal identified, n (%) | 1 (0.2) | 0 | 1 (0.4) |
| Two goals identified, n (%) | 62 (13.1) | 35 (14.7) | 27 (11.4) |
| Three goals identified, n (%) | 411 (86.7) | 203 (85.3) | 208 (88.1) |
| Total number of goals in the group | 1358 | 679 | 679 |
Goal selection
The 1358 goals identified by participants were listed and the content and focus were analysed to identify the areas that were of concern to participants. This built on a preliminary analysis of 591 goals set by the first 209 participants enrolled into the trial by December 2014. 105 In the current analysis, the first 300 goals were reviewed in detail and grouped thematically to form the basis of a detailed coding system. This system was then applied to the remaining goals.
The goals were grouped into the following categories:
-
Engaging in activities and personal projects (21%). This included planning and engaging in enjoyable activities, taking up new activities, restarting previously enjoyed activities, doing activities regularly or more often, doing activities independently, practising skills, keeping occupied, undertaking personal projects and doing things for others.
-
Using appliances, devices and the internet (17%). This category focused on the use of household appliances, such as washing machines or microwave ovens, devices such as mobile phones, smartphones, tablets, TVs, computers, laptops, cameras, CD or DVD players, satnavs and personal safety aids, such as wristbands or pull cords. Participants wanted to learn to use, or be able to use, appliances and devices, to feel confident in using them or to use them independently. This was for a range of purposes, including completing household tasks, communication, entertainment and occupation, obtaining information and staying safe.
-
Managing everyday activities, tasks and situations (16%). This included managing money, shopping, cooking and baking, correspondence and telephone calls, carrying out household tasks, going out, wayfinding, using transportation, telling time and staying safe. Participants wanted to feel confident in doing their everyday activities or to be able to do them independently or safely.
-
Knowing what is happening (9%). Participants wanted to know the day and the date, the schedule for the day or the week ahead and the timing of any appointments. They were keen to know without asking others or having to check repeatedly, and sometimes mentioned particular strategies that they wished to use, such as a whiteboard, calendar or diary.
-
Retaining or keeping track of information and events (8%). Participants wanted to find ways of remembering important information, information that they were recently given, information that they had told others, events and activities and messages to pass on. They wanted to be able to retain key elements of the plot of a novel or TV programme or to keep the score when watching or playing sports or games.
-
Locating belongings (7%). This category was about finding personal items and items around the house, knowing where things had been left and putting things back in the right place.
-
Recognising, identifying and naming (6%). Participants wanted to remember the names of family members, friends, people they met and prominent people involved in current affairs, as well as information about these people. They wanted to be able to recognise and identify people and objects.
-
Engaging in conversation (5%). This category was about being able to engage in and participate in conversation, follow the thread of a conversation and retain key points, find words, keep the conversation going and speak without repetition. Participants wanted to be able to contribute confidently, in a range of settings, for example at family mealtimes or when walking with a group.
-
Organising, improving and finishing (4%). Some participants emphasised a wish to organise aspects of their lives or their environment, to complete tasks they had set themselves and to improve their performance in areas like handwriting, spelling or vocabulary.
-
Caring for self (3%). Participants wanted to be able to do some basic life tasks regularly or independently. These included shaving, bathing, changing dirty clothes and eating and drinking regularly.
-
Keeping in contact and staying engaged with family and friends (2%). In this category, participants specifically mentioned wanting to keep in contact with family and friends through various means, to take an interest in and remember what is going on in their lives and to attend family gatherings.
-
Managing emotions (2%). Participants wanted to manage anxiety or frustration, be more patient and worry less, or to cope better with changes in routine.
Goal attainment in the cognitive rehabilitation group
Participants randomised to the CR group had previously identified 679 goals at baseline. Of these, 591 goals were introduced in therapy sessions by the therapist, initial in-session goal attainment ratings were made for 590 goals and follow-up ratings were made for 563 goals. In-session goal attainment ratings showed a similar pattern to the ratings made at the baseline and follow-up assessments (details are shown in Tables 15 and 16). Correlations between the goal attainment ratings made by the participant, the carer and the therapist at each stage were large, ranging from 0.665 to 0.934, and were significant at a p-value of < 0.0001 (two-tailed), indicating a high degree of consistency among the three raters. All ratings improved, on average, by over 2 points, reflecting a clinically significant change. The change scores are shown in Table 16; correlations among the change scores for participants, carers and therapists were large, ranging from 0.679 to 0.861, and were significant at a p-value of < 0.0001 (two-tailed), again reflecting a high degree of consistency.
| In-session attainment ratings | On introduction of goal | Session | |
|---|---|---|---|
| 10 | 14 | ||
| Participant rating of attainment (N = 232) | 4.09 (1.79), 590 | 6.75 (1.68), 554 | 6.96 (1.91), 494 |
| Carer rating of attainment (N = 232) | 3.21 (1.58), 584 | 6.24 (1.75), 550 | 6.29 (2.14), 484 |
| Therapist rating of attainment (N = 232) | 3.03 (1.50), 590 | 6.36 (1.69), 557 | 6.55 (2.06), 496 |
| Change from initial in-session attainment ratings | Session | |
|---|---|---|
| 10 | Session 14 | |
| Participant attainment rating | 2.66 (1.95) | 2.87 (2.08) |
| Carer attainment rating | 3.03 (1.74) | 3.07 (2.14) |
| Therapist attainment rating | 3.33 (1.66) | 3.52 (2.02) |
On introducing goals, therapists also independently established indicators to classify the extent of goal attainment in percentage terms. On completion of the therapy sessions, they rated the extent of attainment for each goal. In total, 54.8% of goals were rated as being at least 75% attained and 79.8% were rated as being at least 50% attained. Only 5% of goals showed no progress towards attainment. Figure 4 provides a summary of the therapists’ goal attainment ratings made after session 10 and Table 17 breaks this down by the order of introduction of the goals.
FIGURE 4.
Attainment of therapy goals by session 10. Proportion of therapy goals rated as being fully achieved (100%), partially achieved (25%, 50%, or 75%) and not achieved (0%) after session 10.
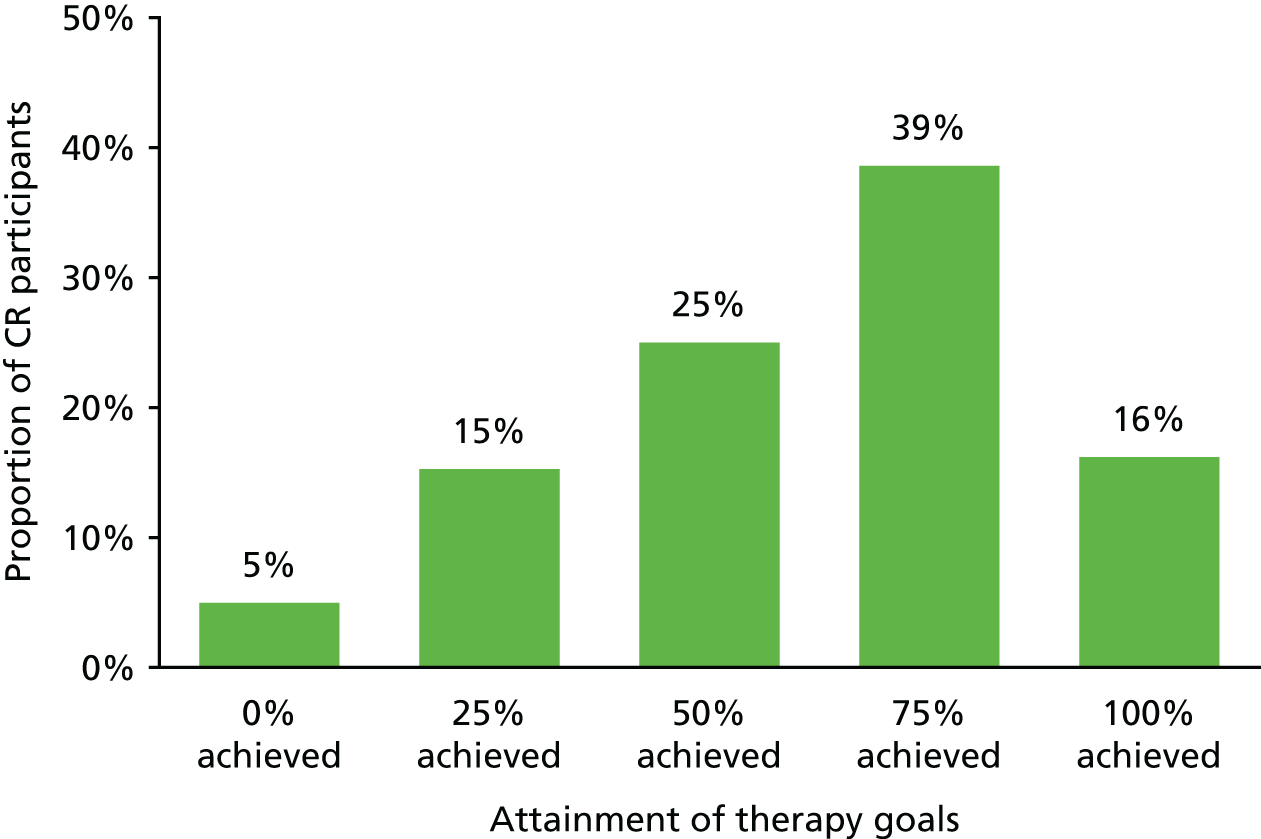
| Proportion achieved | All goals | Goal | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 0% achieved | 27 (5.0) | 7 (2.9) | 10 (4.2) | 10 (4.2) |
| 25% achieved | 83 (15.3) | 27 (11.3) | 33 (13.9) | 23 (9.7) |
| 50% achieved | 136 (25.0) | 51 (21.4) | 53 (22.3) | 32 (13.4) |
| 75% achieved | 210 (38.6) | 72 (30.3) | 86 (36.1) | 52 (21.8) |
| 100% achieved | 88 (16.2) | 49 (20.6) | 25 (10.5) | 14 (5.9) |
We compared the therapists’ goal attainment scaling with the in-session goal attainment ratings made by participants, carers and therapists in or following both sessions 10 and 14 to determine whether or not these two procedures yielded consistent information. For the session 10 goal attainment scaling, the correlations were 0.557 with participant ratings, 0.786 with carer ratings and 0.862 with therapist ratings, and for the session 14 goal attainment scaling, the correlations were 0.652 with participant ratings, 0.881 with carer ratings and 0.932 with therapist ratings. All correlations were significant at a p-value of < 0.0001, two-tailed. This indicates generally good consistency.
Intervention fidelity
We evaluated fidelity of form81 in relation to the provision of core elements of the intervention, completion of the therapy sessions (in terms of number and length of sessions) and the number of therapy goals identified at the baseline assessment that were introduced and addressed in the therapy sessions.
As presented in Chapter 3, Table 6, the majority of participants (70%) received all 14 sessions and only 26 participants (11%) completed < 10 sessions. Therapy sessions lasted between 43 and 120 minutes, with an average of 75.5 minutes [standard deviation (SD) = 12.4 minutes] per session.
Out of the 679 goals identified at the baseline assessment, as noted above, the therapists discussed and confirmed with the participants 591 of the therapy goals. Initial in-session ratings were completed for 590 goals and 563 goals were subsequently re-rated at a later stage of therapy (details are shown in Tables 18–20). Some goals were not introduced because the participants withdrew from the study, because the goals were no longer seen as being suitable or relevant by the participant and no replacement was identified or because there was not enough time to work on them.
| Therapy goals at baseline | All goals | Goal | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Goal confirmed by therapist | 591 (100) | 232 (100) | 217 (100) | 142 (100) |
| Initial goal rating completed | 590 (99.8) | 232 (100) | 217 (100) | 141 (99.3) |
| Goal re-rated during therapy | 563 (95.3) | 214 (0.9) | 213 (98.2) | 136 (95.8) |
| Session number | Goal | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | 207 (89.2) | 33 (15.2) | 29 (20.6) |
| 2 | 20 (8.6) | 6 (2.8) | 4 (2.8) |
| 3 | 4 (1.7) | 15 (6.9) | 4 (2.8) |
| 4 | 1 (0.4) | 100 (46.1) | 12 (8.5) |
| 5 | 43 (19.8) | 9 (6.4) | |
| 6 | 10 (4.6) | 42 (29.8) | |
| 7 | 8 (3.7) | 28 (19.9) | |
| 8 | 1 (0.5) | 10 (7.1) | |
| 10 | 1 (0.5) | 2 (1.4) | |
| 11 | 1 (0.7) | ||
| Total introduced | 232 (100) | 217 (100) | 141 (100) |
| Approach taken | All goals | Goal | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Goal was addressed exactly as set at baseline or very slightly modified | 525 (88.8) | 213 (89.5) | 193 (81.1) | 119 (50.0) |
| Goal set at baseline was used, but significantly modified | 21 (3.6) | 8 (3.4) | 7 (2.9) | 6 (2.5) |
| New goal was developed with the therapist | 36 (6.1) | 8 (3.4) | 13 (5.5) | 15 (6.3) |
| Other | 9 (1.5) | 3 (1.3) | 4 (1.7) | 2 (0.8) |
In order to evaluate fidelity of function,81 we assessed the level of flexibility applied by the therapists in relation to the timing of introducing additional therapy goals (as goals identified at baseline were introduced one at a time during the course of therapy) and the extent to which goal statements formulated at baseline were modified, for example to make them more specific, realistic or achievable (see Tables 18–20). In the majority of cases, the first goal was introduced in the first session, with other goals being introduced gradually in the subsequent sessions. Only a small proportion of participants had more than one goal introduced at the first session.
As summarised in Tables 18–20, the majority (89%) of the goals introduced in the therapy sessions were used exactly as formulated during the baseline assessment or were very slightly modified. This attests to the skill the researchers developed in supporting participants to identify goals that were both meaningful and realistic – a process that in routine practice would be undertaken by the therapist. However, therapists showed flexibility in cases in which goal modification was required and a small proportion of goals were significantly modified (4%) or replaced (6%). In 36 cases in which no third goal was identified at baseline or when a goal set at baseline was no longer felt to be relevant, therapists were able to agree and introduce a new goal. In two instances, two goals were amalgamated into one, in six instances participants decided during the initial discussion that they did not want to work on a particular goal and no other goal was introduced and in one case it was not possible to address the goal as a result of a change in circumstances.
As fidelity of function was promoted through regular supervision with the aid of the detailed therapy logs produced by therapists, we also reviewed attendance at supervision sessions, rates of completion of therapy logs and therapists’ confidence in their ability to address participants’ goals. Therapists had 16 hours of face-to-face group supervision per year, with an average attendance rate of 90%. In addition, therapists had, on average, 7 hours of individual supervision each year, conducted via telephone or Skype™ (Microsoft Corporation, Redmond, WA, USA). Therapy logs were compiled for every participant in the CR group.
Therapists’ evaluation of their confidence in addressing participants’ therapy goals indicated that overall they felt fairly confident, with a mean rating of 7 (SD 2.31) on a scale of 1 to 10, in which 1 is not at all confident and 10 is completely confident.
Therapist views on factors associated with positive outcomes
We explored the therapists’ views about what factors influence treatment outcome and which groups of participants were most and least likely to benefit from the intervention and why. Two sets of data were analysed: data from a focus group conducted with the therapists and a selection of therapy logs. The focus group was analysed using thematic analysis,106 with NVivo 11 (QSR International, Warrington, UK). The focus group analysis and stage 1 of the therapy log analysis informed the development of a list of significant features of the therapy experience. Stages 2 and 3 of the therapy log analysis involved an exploration of the factors influencing good and poor therapy outcomes, and the potential causal relationships contributing to outcomes, in more depth.
Focus group conducted with the therapists
Methods
A focus group was conducted with the trial therapists to examine how the intervention was delivered, the nature of participant and carer engagement and the mechanisms of action through which the intervention operated. The focus group was conducted in June 2014 with six therapists from the original research sites and it lasted for 1 hour. The focus group was digitally recorded and transcribed verbatim.
The focus group data were analysed by a qualitative researcher who had not been otherwise involved in the trial and who was independent of the trial team. The analysis was conducted by first developing a codebook based on two initial readings of the focus group transcript, using a combined deductive and inductive approach. In the deductive approach, aspects of the intervention that were already thought to be critical to success at the intervention development stage – participant motivation, participant acknowledgement of difficulties, cognitive impairment, health status and carer involvement – were included as initial codes in the analysis. Data were also coded inductively, whereby codes were identified in a ‘bottom-up’ way from the data rather than being predetermined. Fourteen codes were identified, including the four deductive codes. One deductive code (‘overall health status’) was dropped, as no data were associated with this code. Data were then coded in NVivo 11 using the resulting 14 deductive and inductive codes, which were subsequently grouped into five descriptive themes: trajectory of the intervention; characteristics and role of the participant; characteristics and role of the carer; role of the therapist; and contextual factors affecting the intervention. The five themes and 14 codes were summarised in a report and translated into two graphics to further explore the relationships between them. Data for each code were reread at each stage of the analysis, to ensure that the groupings and interpretation of the data retained validity.
The two graphics and the summary of the themes and codes were then reviewed and discussed in a meeting between the researcher and the trial manager to reflect on the findings, including consideration of any surprising results, how the findings related to the intervention design and intervention manual and any hypotheses about the implications of the findings. This formed the basis for reporting the findings.
Findings
There were several different aspects to the work that therapists conducted, including engaging carers, tailoring the intervention in accordance with participant capacity and developing relationships and providing support to both carers and participants. The participation of carers and participants’ cognitive and functional ability and readiness to acknowledge difficulties emerged as important factors affecting the success of the intervention. The development of relationships and working with goals flexibly over time was a feature of how the intervention was implemented and it was clear that achievements could create a positive cycle over time, although not all ‘small’ achievements were necessarily formally noted. These key findings are presented below.
Therapists commented on several ways in which carers were important for the success of the intervention and also described how they worked with carers to facilitate their involvement. The role of carers in the intervention was regarded as an important one by therapists, because their facilitation of the intervention (such as prompting the participant between sessions) was thought to have an impact on participant motivation and engagement. Therapists thought that carers were more likely to engage with goals if the goal affected the carer as well as the participant, and that carers’ engagement was negatively affected by carer beliefs in a participant’s lack of functional ability, although this could change if participants made progress. Therapists would adapt visit times to ensure that they met with carers who could be limited by other commitments (such as work) and also made efforts to identify and meet the carers who were most involved in the participant’s life, who were not always the same person as the nominated carer for the intervention. Therapists also provided support for carers, which included education, socioemotional support (which, one therapist commented, could be the primary benefit for the carer) and referral to other services.
Therapists commented on different participant profiles. Some had busy and active lives. Of concern, however, were those participants who were functioning less well. Therapists felt that goal-centred therapy was more difficult for this group, because they could be less likely to engage with goals in the first place as a result of limited acknowledgement of difficulties, they could have difficulty setting relevant goals or remembering them and they could find it hard to complete goal ratings. A lack of awareness of particular difficulties resulted in lower motivation to engage in the intervention. Therapists also commented that it was difficult for some participants to absorb all of the information that they wanted to deliver in the time allocated for the session and that it was important to be able to present concepts in an accessible manner. Therapists responded to differences in participant ability levels by tailoring the timing of material and highlighting the most relevant sections in the session handouts provided.
The therapists described various ways in which they tried to engage participants and carers in the intervention and to help them to progress to achieving goals. In some cases, they explained the evidence-based nature of the intervention in order to engage carers. They thought that participants who had a relatively recent diagnosis, and their carers, were easier to engage because they were still adapting to the diagnosis and were more open to change. The relationships created and the support provided by therapists seemed to be an element that supported their work with participants and carers and promoted effective working towards goals. Therapists worked with goals flexibly, as sometimes the goals set at baseline were not optimal and therapists needed to get to know carers and participants in order to adapt and operationalise them or, if necessary, identify a more relevant goal. Goals could also change in response to an event in a participant’s life. Therapists reflected on the way in which goals could sometimes appear to reflect small issues, but these could make a significant difference to the participant’s life. Achieving goals could be motivating for participants and carers, although sometimes this could make them too ambitious, and achieving goals could also have beneficial effects in other areas of their lives, for example through promoting social engagement.
Therapists described elements of the intervention they were delivering that were, or were perceived to be, additional to the intervention protocol. These included social support, relationship-building, managing relationship conflict between carers and participants, contacting social services to enable carers to have a break and extending the length of their visits. For example, a few therapists mentioned evening visits or additional hours, or made referrals to social services for specific needs.
In talking about the participants, therapists appeared to focus on several dichotomies. They compared participants with lower and higher cognitive and functional ability, and felt that the approach was more relevant for, or easier to apply with, those who were functioning better. They made a distinction between those participants who had received a diagnosis relatively recently and those who had had a diagnosis for longer, and felt that those currently adapting to a recent dementia diagnosis were more likely to engage well with the intervention. These two categories, higher levels of functioning and recency of diagnosis, naturally tended to overlap. They also differentiated between those needing longer and shorter maintenance visits, whereby participants who were doing well needed shorter visits.
A limitation of these findings is that they are based on data from a single conducted focus group with six therapists, and some points were made by only one or two individuals.
To summarise the findings, a number of features were perceived by the therapists as influencing the outcomes of therapy:
-
Participants’ level of functioning. Participants who were functioning better were more likely to engage well in setting goals and working on goals, and were more motivated to make changes.
-
Proximity of diagnosis. Participants who had been diagnosed more recently, and their carers, tended to be more motivated to engage in the intervention.
-
Individual tailoring. Therapists tailored the intervention to individual goals and needs, and greater individual tailoring was thought to be related to better outcomes.
-
Carer engagement. Low levels of carer engagement were thought to be linked to outcomes, but could be mitigated by the therapists working to engage carers more fully.
-
Therapeutic relationship. The relationships that the therapists developed with participants and carers were very important influences on outcome, and these relationships changed over time as therapy progressed.
-
Positive cycles. The achievement of goals led to greater carer engagement and other benefits.
Analysis of the therapy logs
Following the analysis of the focus groups, a set of therapy logs was analysed. The findings from the focus group analysis informed the analysis of the therapy log data, as described below.
Methods
Two subsets of CR group participants were identified, representing the 25 participants with the best goal attainment outcomes and the 25 participants with the poorest outcomes. The therapy outcome was operationalised for this analysis as a change in the BGSI participant goal attainment ratings between baseline and the 3-month follow-up. Scrutiny of the demographic and clinical characteristics of the two groups showed no evident differences. Tables 21–25 summarise the demographic and clinical characteristics and mean BGSI ratings for the two groups.
| Measure | Participants with a | |
|---|---|---|
| Poor outcome (N = 25) | Good outcome (N = 25) | |
| Age (years), mean (SD); range | 79 (5.5); 66–90 | 77.36 (6.8); 62–91 |
| Sex (male), n (%) | 11 (44.0) | 16 (64.0) |
| Ethnicity, n (%) | ||
| White | 23 (92.0) | 24 (96.0) |
| Mixed/multiple ethnic group | 1 (4.0) | 0 |
| Black/African/Caribbean/black British | 1 (4.0) | 0 |
| Other ethnic group | 0 | 1 (4.0) |
| First language (English), n (%) | 25 (100) | 23 (92.0) |
| Marital status (married), n (%) | 19 (76.0) | 18 (72.0) |
| Years of education, mean (SD); range | 12.1 (3.1); 8–20 | 12.3 (3.3); 8–21.5 |
| Occupational status, n (%) | ||
| I: professional | 1 (4.0) | 4 (16.0) |
| II: managerial/technical | 7 (28.0) | 13 (52.0) |
| III N: skilled, non-manual | 8 (32.0) | 3 (12.0) |
| III M: skilled, manual | 5 (20.0) | 1 (4.0) |
| IV: partly skilled | 4 (16.0) | 1 (4.0) |
| V: unskilled | 0 | 3 (12.0) |
| Measure | Participants with a | |
|---|---|---|
| Poor outcome (N = 25) | Good outcome (N = 25) | |
| Relationship to participant with dementia, n (%) | ||
| Spouse/partner | 18 (72.0) | 19 (76.0) |
| Adult child (including in-law) | 5 (20.0) | 6 (24.0) |
| Other | 2 (8.0) | 0 |
| Age, mean (SD); range | 70.1 (10.5); 50–89 | 68.4 (13.9); 29–82 |
| Sex (male), n (%) | 10 (40.0) | 6 (24.0) |
| Ethnicity, n (%) | ||
| White | 25 (100.0) | 23 (92.0) |
| Mixed/multiple ethnic group | 0 | 1 (4.0) |
| Other ethnic group | 0 | 1 (4.0) |
| First language (English), n (%) | 24 (96.0) | 25 (100.0) |
| Marital status (married), n (%) | 18 (72.0) | 24 (96.0) |
| Years of education, mean (SD); range | 12.0 (3.1); 5–20 | 13.8 (3.6); 8–22 |
| Occupational status, n (%) | ||
| I: professional | 3 (12.0) | (24.0) |
| II: managerial/technical | 9 (36.0) | (24.0) |
| III N: skilled, non-manual | 7 (28.0) | 10 (40.0) |
| III M: skilled, manual | 3 (12.0) | 0 |
| IV: partly skilled | 2 (8.0) | 1 (4.0) |
| V: unskilled | 1 (4.0) | 2 (8.0) |
| Measure | Participants with a | |
|---|---|---|
| Poor outcome (N = 25) | Good outcome (N = 25) | |
| Diagnosis, n (%) | ||
| Alzheimer’s disease | 16 (64) | 16 (64) |
| Vascular dementia | 4 (16) | 2 (8) |
| Mixed Alzheimer’s disease and vascular dementia | 5 (20) | 7 (28) |
| MMSE, mean (SD); range | 23.08 (2.27); 18–26 | 24.92 (3.0); 20–29 |
| Charlson Comorbidity Index weighted score, mean (SD); range | 2.7 (2.1); 1–10 | 2.8 (2.2); 1–11 |
| Subjective rating of health, n (%) | ||
| Excellent | 1 (4.0) | 0 |
| Very good | 8 (32.0) | 5 (20.0) |
| Good | 7 (28.0) | 7 (28.0) |
| Fair | 8 (32.0) | 11 (44.0) |
| Poor | 1 (4.0) | 2 (8.0) |
| DEMQOL, mean (SD); range | 90.5 (18.0); 39–109 | 92.28 (10.8); 68–112 |
| GSES, mean (SD); range | 29.7 (4.5); 14–36; N = 24 | 30.20 (4.1); 18–36; N = 25 |
| HADS, mean (SD); range | ||
| Depression | 4.2 (2.9); 1–10 | 3.75 (2.3); 0–8 |
| Anxiety | 5.6 (3.7); 0–15 | 5.76 (3.5); 0–14 |
| RBMT, mean (SD); range | ||
| Immediate recall | 2.3 (2.0); 0–9.5 | 3.08 (2.1); 0–6.5 |
| Delayed recall | –0.4 (1.0); –1 to 2.5 | 1.16 (2.1); –1 to 5 |
| TEA, mean (SD); range | ||
| Elevator counting | 6.2 (1.1); 3–7; N = 22 | 6.36 (0.9); 4–7; N = 25 |
| Elevator counting with distraction | 4.8 (2.8); 1–9; N = 21 | 5.26 (2.9); 0–9; N = 23 |
| D-KEFS verbal fluency, mean (SD); range | 27.6 (11.6); 2–51; N = 24 | 22.24 (8.5); 7–43; N = 25 |
| Measure | Participants with a | |
|---|---|---|
| Poor outcome (N = 25) | Good outcome (N = 25) | |
| Subjective rating of health, n (%) | ||
| Excellent | 0 | 1 (4.0) |
| Very good | 3 (12.0) | 8 (32.0) |
| Good | 12 (48.0) | 10 (40.0) |
| Fair | 8 (32.0) | 5 (20.0) |
| Poor | 2 (8.0) | 1 (4.0) |
| RSS, mean (SD); range | 19.69 (8.0); 6–44; N = 24 | 19.06 (7.2); 6–35 |
| WHOQOL-BREF domains, mean (SD); range | ||
| Physical | 14.58 (3.1); 9–20 | 14.56 (3.3); 6–19 |
| Psychological | 15.13 (2.1); 11–20 | 15. 20 (2.0); 11–18 |
| Social | 14.42 (2.4); 11–20 | 15.32 (2.2); 11–20 |
| Environmental | 15.75 (2.2); 12–20 | 16.24 (2.5); 10–20 |
| EQ-5D-3L, mean (SD); range | ||
| Index | 0.69 (0.29); –0.02 to 1; N = 24 | 0.79 (0.1); 0.5–1 |
| VAS | 72.48 (22.3); 8–99; N = 23 | 73.52 (19.5); 30–100 |
| Measure | Participants with a | |||||
|---|---|---|---|---|---|---|
| Poor outcome (N = 25) | Good outcome (N = 25) | |||||
| Baseline | 3 months | 9 months | Baseline | 3 months | 9 months | |
| Participant rating of attainment | 4.55 (2.30) | 3.94 (2.08) | 4.94 (2.55); N = 24 | 1.91 (0.83) | 8.13 (0.94) | 7.86 (1.64) |
| Participant rating of satisfaction | 4.09 (1.86) | 4.91 (1.99) | 6.24 (2.05); N = 23 | 2.71 (1.34) | 8.01 (0.71) | 8.12 (1.39) |
| Carer rating of attainment | 3.37 (1.85) | 3.35 (1.63) | 3.79 (2.27) | 1.96 (1.18) | 7.09 (1.62) | 6.99 (1.90) |
Therapy logs were maintained by the therapists in Microsoft Word (Microsoft Corporation, Redmond, WA, USA) document format, and for the analysis, these documents were used to create two parallel summary Excel spreadsheets, one for the ‘best outcome’ group and one for the ‘poor outcome’ group. Each file contained 15 tabs; the first tab contained information about the participant’s current functional ability and therapy goals, and each of the remaining 14 tabs referred to one therapy session. Within each tab, each row contained data for one participant and each column contained comments about one topic relating to treatment delivery (e.g. goal progress, use of restorative and compensatory strategies, anxiety management strategies applied), with 6–7 columns in each tab. The trajectories of participants’ progress through the intervention were analysed to identify factors that could help to explain good or poor outcomes.
The analysis was conducted through several stages, using an adapted framework analysis method,107 by a researcher who was not otherwise involved in the trial and was independent of the trial team:
-
In stage 1, the therapy logs were analysed by session, on the basis of the pre-existing topics. This was to provide a perspective on the intervention in relation to how the intervention was structured and the progress recorded after each session.
-
In stage 2, the therapy logs were analysed by the participant, in order to identify critical factors affecting involvement and progress. This stage was informed by findings from the stage 1 analysis and findings from the therapists’ focus group.
-
In stage 3, a ‘negative case analysis’ was conducted in order to explore in more depth the secondary factors influencing therapy outcomes for those participants who did not fit with the general patterns emerging from the analysis.
In the stage 1 analysis, the therapy logs for each participant were summarised using an adapted framework analysis approach, with the framework categories representing pre-existing categories in the therapy logs rather than being identified as themes in the data by the researcher. Summaries were produced for each component of the intervention delivery (i.e. each column in the matrix), for each of the 14 therapy sessions. The summaries produced were subdivided into different themes depending on the content, for example ‘carer stress.’ The summaries included details, such as frequencies, for example numbers mentioning particular types of goals at this stage, to avoid impressionistic bias in the analysis. These summaries also used the voice of the therapist, such as terms employed, as much as possible, to maintain the validity of the analysis. The summaries were then further summarised into short versions in accordance with the key content and themes in order to identify patterns in how participants engaged with and benefited (or not) from different aspects of the intervention. The findings for the two participant groups were then compared, to identify any differences in treatment and treatment experience between the two groups, and to identify and refine explanatory themes emerging from the analysis. Memos reflecting other features of the intervention and participants’ experiences were also recorded, to help in identifying factors that appeared to be important but were not captured by the therapy log categories; the factors noted were:
-
progression or stage of dementia
-
physical health of participants
-
participants’ anxiety
-
carer engagement – commitment to the intervention and participation in the sessions
-
carer difficulties (e.g. stress, health problems)
-
adaptation of goals, for example when participants rejected previously chosen goals, or extension of goals or strategies when original aims were surpassed
-
life events, holidays or family visits
-
type of goal – for example, whether the goal related to household chores, personal projects or social activities.
Factors emerging from the focus group analysis and from the stage 1 therapy log analysis were combined into the following final list of nine significant features of therapy experience that was used for stage 2 of the therapy log analysis:
-
stage or severity of dementia
-
participants’ physical health
-
low mood or depression
-
anxiety
-
carers’ difficulties (e.g. stress, health problems)
-
carer engagement
-
type of goal
-
changes in goals (either rejecting, changing or surpassing original goals)
-
life events, holidays or family visits.
In stage 2, a summary of each of these categories was produced for each participant, identifying the therapist comments made for each domain (if any). The summaries for both groups were then compared, to identify any differences in the experiences between the two groups of participants. The initial analysis examined frequencies to identify broad differences between the groups and then explored potential explanations of why groups were different. This included the consideration of differences with regard to specific factors (e.g. the type of problematic carer engagement) and the exploration of links between the initial findings (e.g. whether or not participants whose dementia had progressed further tended to select different types of goals to work towards).
The summaries of the key findings from the analysis of the therapy logs included a comparison of the findings from each of the three main stages of analysis and further refinement of themes. The findings were discussed by the researcher and trial manager to confirm the basis for, and to discuss the implications of, the findings.
The stage 3 analysis built on stages 1 and 2, to investigate negative cases in which participants did not fit the general pattern for their group, for example when participants who had lower functional ability achieved good BGSI outcomes. The analysis focused on therapy log entries from three milestone sessions in which therapists summarised goal progress and the factors affecting progress: session 10 (the end of the main phase of the intervention), session 11 (the beginning of the maintenance phase) and session 14 (the end of the maintenance phase). The similarities between negative cases in the ‘poor outcome’ and ‘good outcome’ groups were investigated to try to identify factors contributing to participant outcomes.
A limitation of the analysis is that the therapy logs were notes made by the therapists about significant events or issues, rather than a systematic summary of each session. There were also aspects of the log entries that could be unclear:
-
Some entries were ambiguous; for example, some noted strategies without being clear about whether these were being suggested by the therapist or if they were actually being applied by the participant/carer.
-
Some entries implied, rather than explicitly stated, the presence of features, such as anxiety (e.g. references were made to ‘frustration’ or ‘stress’) or dementia symptoms (such as lack of motivation, which could be caused by several quite different types of difficulties).
-
Some entries noted ‘nil’/none and it was not clear whether this referred to lack of progress or if it indicated that this topic was not covered in the session.
When the data were unclear, they were not included in the data reduction summaries for the analysis.
Findings
The analysis identified differences between the two groups in relation to two areas: level of functioning and anxiety, and there were also secondary factors influencing outcomes.
Level of functioning
The initial stage of the therapy logs analysis found that participants in the poor outcome group were more likely to be described as experiencing greater cognitive difficulties, as being less likely to acknowledge difficulties and as having limited motivation to engage in therapy. Cognitive decline over the study period was noted for participants in both groups to an equal extent, but therapists were more likely to comment on a lack of acknowledgement of difficulties in the group with poorer outcomes. This overarching difference between the groups was reflected in several important themes that were evident in the therapy logs.
Although there was some similarity in the type of goals identified by participants in both groups (e.g. similar numbers of participants in both groups wanted to improve their use of mobile phones), there were also marked differences. Participants in the poor outcome group tended to select more basic goals, such as knowing the date, whereas participants in the best outcome group tended to identify more recreational goals, such as engaging in social activities outside the house. In the poor outcome group, notes from the initial therapy sessions contained more concerns about the person’s ability to carry out basic daily activities, such as dressing.
The frequency with which therapists amended the goals identified during the researchers’ visit was the same for both groups. Participants in the best outcome group were more likely to extend their goals and aim for more ambitious targets, whereas those in the poor outcome group were more likely to give up on a goal entirely. This could be linked to lower motivation or to difficulties in identifying suitable objectives or it could possibly be seen as a way to avoid confronting difficulties.
Differences in the rates of progression appeared following the first three sessions. In the best outcome group, the first three sessions were often enough for the participants to start making noticeable progress and there was an increase in positive comments about progress towards achieving therapy goals from the third session onwards. In contrast, for participants in the poor outcome group, there were comments about low levels of engagement with working on goals and insufficient between-session practice. The poor outcome group were also less likely to engage with a third goal, if they had one, or else they started working on it later. As expected, participants in the best outcome group gave more positive in-session ratings for goal attainment, and both participants and therapists made more positive comments about progress towards achieving goals than those in the poor outcome group. Therapists made more comments on wholly positive progress for the best outcome group at the end of the maintenance phase. The lack of progress in the poor outcome group was attributed by therapists to some participants not acknowledging difficulties.
Participants in the best outcome group had slightly higher levels of functioning, as indicated by the PAL77 score, and were more likely to report interest in increasing their activity levels and to make plans to do so than those in the poor outcome group, who were more likely to be described as inactive.
The types of strategies adopted were similar for both groups, although participants in the best outcome group were often already using some strategies and were more likely to make positive progress with new ones.
Participants in the best outcome group were also more likely to be already using some restorative strategies to remedy their cognitive difficulties and were more likely to use new ones successfully and to apply them to a wider range of areas. Participants in the poor outcome group were less likely to engage with these strategies or to even have a discussion about using them.
Although there were similar types of strategies adopted in both groups to support attention and concentration, participants in the best outcome group appeared to have adopted strategies earlier in the course of therapy, and made more positive comments about using the strategies.
Participants in the poor outcome group tended to engage less with therapy, expressed more reluctance, appeared to be withdrawn during sessions and had more difficulty recalling previous sessions. At the end of the intervention, they were more likely to refer to the relational or social aspect of the therapist visiting them as a positive element of the intervention or to make very general positive comments. Participants in the best outcome group were more likely to give examples of specific elements of the intervention that had benefited them.
In the poor outcome group, the relationships between the participant and the carer appeared to be more unbalanced, with dominant carers and/or more passive or dependent participants, although it was not clear to what extent this reflected participants’ cognitive or functional ability and to what extent it was a more intrinsic characteristic of the relationship. Relationship strains were mentioned for both groups, but therapists were slightly more likely to report wholly positive relationships for the participants in the best outcome group.
Towards the end of therapy, the plans for ongoing support differed between the good outcome group and the poor outcome group. Participants in the poor outcome group tended to consider whether or not additional external support, such as day care or respite, would be helpful, whereas those in the best outcome group were more likely to discuss getting involved in social activities, such as support groups or dementia choirs.
Anxiety
In both groups, the therapists noted a range of psychological difficulties experienced by participants, including low levels of confidence, agitation, frustration and low mood. However, anxiety problems were more often noted for participants in the best outcome group, whereas participants in the poor outcome group and their carers were more likely to report that anxiety was not a problem.
Secondary factors influencing therapy outcomes
Stages 2 and 3 of the analysis explored in more detail the secondary factors influencing therapy outcomes. In the second stage, therapy sessions were examined with regard to the final list of nine significant features of the therapy experience that were perceived as being likely to influence outcome. The list included cognitive difficulties as discussed above, as well as factors that did not appear to differentiate the groups clearly, such as participants’ health, carer engagement in therapy and support provided by the carer. To further investigate the impact of these factors on therapy, a negative case analysis explored potential relationships in more depth. Specifically, therapy logs for participants who had poor outcomes, but for whom no particular dementia-related problems were noted (n = 11), were reviewed as a group and therapy logs for participants who had good outcomes, but for whom specific dementia-related problems were noted (n = 6), were reviewed as a group.
The negative case analysis of the six participants who were in the ‘good outcome’ group despite particular dementia-related problems being noted, indicated that their symptoms were milder than those in the ‘poor outcome’ group or that their symptoms fluctuated, creating opportunities for more effective therapy work. The analysis of this group of participants therefore supports the view that the extent of cognitive difficulties or dementia-related problems is associated with outcome.
For the 11 participants in the ‘poor outcome’ group for whom no particular dementia-related problems were noted, progress with therapy appeared to be affected by several secondary factors. The most significant of these factors were either not engaging with goals or setting an inappropriate goal, lack of carer support for between-session practice and significant health problems or additional disability, such as visual impairment. Several participants in this subgroup had a combination of two or three of these factors hindering therapy progress. It is important to note, however, that these secondary factors appear to have less impact on the therapy outcome than their level of functional ability, as discussed above.
There were a number of factors that did not appear from the therapists’ records to have any consistent relationship with therapy outcomes, such as therapeutic alliance, number of sessions attended, carer health and well-being, and therapist support in the areas not related to goals.
Summary
The main findings indicate that the intervention appears to be more effective for participants with less severe cognitive difficulties and with better functional ability. This concurs with findings from the therapist focus group. Although other factors, such as carer support and participant health, influence participants’ behaviour, such as levels of between-session practice, these factors do not seem to be linked to overall intervention outcomes.
Participant and carer experience of the intervention
A subset of participants and carers from the CR group were interviewed about their experience of the intervention to gain insight into the way in which they experienced the therapy and what aspects of the therapy were found to be particularly challenging or helpful. An in-depth understanding of the participants’ subjective experience of the intervention is important for understanding the mechanisms of therapy. It also enables participants to formally contribute their views and experiences to the therapy evaluation.
Method
Three sites were able to contribute to this component of the evaluation, as they each identified an independent researcher not involved in the trial who could conduct detailed interviews. Interviews were conducted in the Bangor (21 March 2014 to 20 January 2015), Cardiff (31 July 2015 to 9 December 2015) and Manchester (1 April 2015 to 28 May 2015) sites. In each site, a consecutive series of participants and carers was approached following the 9-month follow-up assessment and invited to participate in the interview to discuss their experiences of the therapy sessions. The trial manager issued the researchers with site-specific lists of all participants in the CR group who were due to complete their final follow-up assessment in the designated recruitment period. In total, 36 couples were approached and 26 agreed to be interviewed, although, in the case of one couple, only the carer participated in the interview. We interviewed 12 carers and 11 participants at Bangor (100% of those approached), 10 carers and 10 participants at Cardiff (50% of those approached) and four carers and four participants at Manchester (100% of those approached). The demographic and clinical characteristics of these participants, and their BGSI ratings, are shown in Tables 26–30. Of note, six of the participants were also included in the therapy logs analysis, two in the poor-outcome group and four in the good-outcome group.
| Measure | Participants interviewed (N = 25) |
|---|---|
| Age (years), mean (SD); range | 76.64 (5.7); 66–87 |
| Sex (male), n (%) | 13 (52.0) |
| Ethnicity, n (%) | |
| White | 24 (96.0) |
| Mixed/multiple ethnic group | 0 |
| Asian/Asian British | 0 |
| Black/African/Caribbean/black British | 1 (4.0) |
| Other ethnic group | 0 |
| First language (English), n (%) | 23 (92.0) |
| Marital status (married), n (%) | 21 (84.0) |
| Years of education, mean (SD); range | 12.74 (2.7); 10–21.5 |
| Occupational status, n (%) | |
| I: professional | 3 (12.0) |
| II: managerial/technical | 6 (24.0) |
| III N: skilled, non-manual | 6 (24.0) |
| III M: skilled, manual | 5 (20.0) |
| IV: partly skilled | 4 (16.0) |
| V: unskilled | 1 (4.0) |
| Measure | Participants interviewed (N = 26) |
|---|---|
| Relationship to participant with dementia, n (%) | |
| Spouse/partner | 21 (80.8) |
| Adult child (including in-law) | 5 (19.2) |
| Other | 0 |
| Age, mean (SD); range | 70.38 (11.2); 46–85 |
| Sex (male), n (%) | 8 (30.8) |
| Ethnicity, n (%) | |
| White | 26 (100) |
| Mixed/multiple ethnic group | 0 |
| Asian/Asian British | 0 |
| Black/African/Caribbean/black British | 0 |
| Other ethnic group | 0 |
| First language (English), n (%) | 24 (92.3) |
| Marital status (married), n (%) | 25 (96.2) |
| Years of education, mean (SD); range | 14.40 (3.1); 10–20.5 |
| Occupational status, n (%) | |
| I: professional | 6 (23.1) |
| II: managerial/technical | 7 (26.9) |
| III N: skilled, non-manual | 6 (23.1) |
| III M: skilled, manual | 2 (7.7) |
| IV: partly skilled | 4 (15.4) |
| V: unskilled | 1 (3.8) |
| Measure | Participants interviewed (N = 25) |
|---|---|
| Diagnosis, n (%) | |
| Alzheimer’s disease | 19 (76.0) |
| Vascular dementia | 3 (12.0) |
| Mixed Alzheimer’s and vascular dementia | 3 (12.0) |
| MMSE, mean (SD); range | 23.40 (2.3); 19–28 |
| Charlson Comorbidity Index weighted score, mean (SD); range | 2.1 (1.2); 1–6 |
| Subjective rating of health, n (%) | |
| Excellent | 2 (8.0) |
| Very good | 8 (32.0) |
| Good | 7 (28.0) |
| Fair | 8 (32.0) |
| Poor | 0 |
| DEMQOL, mean (SD); range | 91.84 (12.3); 53–106 |
| GSES, mean (SD); range | 31.84 (3.7); 24–39 (n = 25) |
| HADS, mean (SD); range | |
| Depression | 3.28 (2.6); 0–9 |
| Anxiety | 4.88 (3.2); 1–14 |
| RBMT, mean (SD); range | |
| Immediate recall | 2.84 (2.3); 0–7.5 |
| Delayed recall | 0.34 (1.9); –1 to 5 |
| TEA, mean (SD); range | |
| Elevator counting | 6.6 (0.8); 4–7 |
| Elevator counting with distraction | 5.36 (2.7); 1–9 |
| D-KEFS verbal fluency, mean (SD); range | 25.04 (13.2); 2–55 |
| Measure | Participants interviewed (N = 26) |
|---|---|
| Subjective rating of health, n (%) | |
| Excellent | 1 (3.8) |
| Very good | 9 (34.6) |
| Good | 13 (50.0) |
| Fair | 3 (11.5) |
| Poor | 0 |
| RSS, mean (SD); range | 17.02 (7.7); 5–36 |
| WHOQOL-BREF domains, mean (SD); range | |
| Physical | 16.00 (2.4); 10–19 |
| Psychological | 15.58 (1.4); 12–18 |
| Social | 15.19 (2.3); 11–20 |
| Environmental | 17.15 (1.7); 13–20 |
| EQ-5D-3L, mean (SD); range | |
| Index | 0.82 (0.2); 0.09–1 |
| VAS | 80.42 (11.0); 60–100 |
| Rating | Time point | ||
|---|---|---|---|
| Baseline | 3 months | 9 months | |
| Participant rating of attainment (N = 25) | 3.37 (1.74) | 6.10 (1.67) | 6.66 (1.94) |
| Participant rating of satisfaction (N = 25) | 3.57 (1.82) | 6.54 (1.31) | 7.06 (1.70); n = 24 |
| Carer rating of attainment (N = 26) | 2.59 (1.21) | 5.49 (1.76) | 5.72 (2.33) |
The interviews followed a semistructured schedule, and interviewers encouraged the participants and carers to talk freely about their experience of the intervention (see Appendix 2 for the interview schedules). The interviews covered the following topics:
-
How did participants and carers experience the intervention?
-
What were their overall perceptions, how useful did they find it, and what did they feel about the degree of effort required?
-
What impact, if any, did the participants and carers feel that the intervention had on their everyday life?
The interviewers had an overall understanding of what the intervention involved, but no specific knowledge of the individual participants’ therapy goals or the therapy process, in order to avoid bias. Participants and carers were interviewed separately whenever possible, starting with the person with dementia. Interviewers took a photograph of the therapist on the visit to prompt the participant’s memory of the therapy sessions. If the participant was struggling to recall the therapy sessions, the interview was completed jointly with the carer. The first interview conducted by each interviewer was reviewed at the co-ordinating centre to ensure adherence to the interview schedule. Interviews were found to have been conducted satisfactorily in each case. All interviews were audio-recorded, transcribed verbatim and anonymised.
Thematic analysis started from a critical realist position and was based on an inductive approach to identifying and exploring patterns of meaning in relation to the research question. 106,108 Four researchers who were not involved in conducting the interviews analysed the interviews; three of these researchers were independent from the trial and one was the trial manager. The process was overseen by the chief investigator. Initially, two researchers read and reread the first five transcripts to familiarise themselves with the data and then identified and coded (briefly summarised and characterised) units of meaning within each transcript. Codes were listed separately, reviewed and organised into meaningful groups, representing the initial themes for each interview. The resulting lists of themes were compared and discussed by the two researchers until a consensus was reached about content and organisation, after which each researcher recoded the transcripts. Related themes were clustered together and the clusters were ordered into group-level themes and subthemes, and the two researchers worked together to integrate these into an overall thematic map. The remaining transcripts within the set were then coded by a single researcher, using the identified list of themes.
Findings
Overall, the therapy was received positively by both carers and people with dementia and, generally, there were very few criticisms of the therapy. Participants mostly said that they had nothing negative to report about the experience. Several key themes emerged, reflecting factors that influenced the experience of the intervention and whether or not it was considered to be beneficial.
Therapeutic relationship
The relationship with the therapist played an important role in participants’ perceptions of the intervention, especially as the participants with dementia were often unable to recall the specific goals that they had been working towards in the therapy sessions. The therapeutic relationship was what both the carers and the people with dementia enjoyed about their experience of the intervention. They looked forward to the therapist’s visits, and they said that they would miss the visits now that the therapy had ended.
A positive interaction with the therapist was believed to be very important for the therapy by the people with dementia and their carers. The therapists were described as being pleasant, nice, responsive, knowledgeable and professional. The people with dementia described feeling comfortable, relaxed and at ease when talking to their therapists. Moreover, they also said that they did not feel distressed or disturbed during these interactions. The carers and the people with dementia believed that the therapeutic relationship was the foundation for several aspects for the intervention. The three most commonly reported aspects were education for carers and people with dementia about the dementia experience, provision of social support and provision of information or resources that could help with daily functioning in the future.
The information and explanations that therapists gave about dementia were considered to be very beneficial for both the carers and the people with dementia, who found it helpful to have written information and educational materials about living with and caring for someone with the condition. Both the carers and the people with dementia valued the chance to have any questions answered by the therapist. In particular, the relationship with the therapist made asking questions and communication easy and less frightening, as one person with dementia described:
Oh fine, yeah fine, got on well . . . Easy, yeah she explained everything and, you know, it was no hardship [laughs] . . . That’s right, yeah, well sometimes when people come to see you, . . . you’re afraid to talk, you know, afraid to say anything when it’s a little bit dumb. But she made me feel so, er, comfortable and within a couple of minutes we were just like as though we’d been friends for a long time.
Person with dementia 1
The educational component was related to several perceived positive outcomes for people with dementia and their carers:
Yeah, she was very good explaining things and, you know . . . I did become very positive . . . after she’d been . . . ’Cos she, she did, she made me feel good.
Person with dementia 1
Particularly, it increased participants’ understanding and awareness of dementia, to which they attributed their resulting better psychological adjustment. Some participants reported a new and more positive perspective on the diagnosis, which resulted in less worry. One person with dementia described how the therapist increased awareness and reduced worry:
She explored areas, you know that I hadn’t thought about, and . . . I found a great help. I think it, uh . . . I don’t think I’ve been sort of . . . on edge about the Alzheimer’s . . . But . . . it showed me to be less worried about it . . .
Person with dementia 2
People with dementia described being wary of or worrying about performing tasks or coping in some situations prior to the therapy. Participants discussed how the therapist empowered them to make their own decisions about what they wanted to do and about working towards their goals. One person with dementia described how the therapist improved their self-view and made them feel better about themselves:
I’m not as soft as I think I am.
Person with dementia 1
They went on to describe how the therapist had changed their self-view by enabling them to make more decisions for themselves.
Carers also related this increased awareness and understanding to having more patience with the person with dementia and to other improvements in their relationship (e.g. less conflict, more affection and thoughtfulness). An increase in patience and a reduction in frustration with the person with dementia was mentioned by several carers. For example, they reported ‘yelling’ less at the person with dementia and doing tasks more slowly, to help the person with dementia to understand. This increase in patience was seen as reducing conflicts and misunderstandings. In addition, a few people with dementia commented that they had greater self-awareness and better social awareness. They said that they would now think before saying something in social situations, and they would consider how others (particularly, their carer) would perceive what they wanted to say before saying it. This change also seemed to benefit the relationship.
Social support and contact seemed to be important elements provided by the therapist. Carers reflected on how the participants with dementia ‘enjoyed the company’, suggesting that they do not have visitors who engage with them regularly:
I think my mum just enjoyed it more that somebody was, the social aspects of it, that somebody was coming.
Carer 1
They described the visits as helpful because they offered opportunities to talk and provided support, which was something that they described as lacking in their lives. These discussions were often conducted with humour and included shared interests. The visits also became part of the routine for the person with dementia, which was viewed positively.
Several carers described how the therapist gave them a recognition that their experience was shared, as described below:
And she made you feel that . . . this was a problem that other people have and in a way it sort of normalises what is not a normal problem and she sort of made you feel it’s . . . something that other people experience, that there are ways through it . . .
Carer 2
Other people who were also caring for a person with dementia were talked about as being ‘in the same boat’. This knowledge helped the carers to feel that they were not alone or ‘neglected’ and ‘ignored’, which was what they mostly experienced in relation to others or society as a whole. In a few cases, the carer described how the therapist acted as a mediator, or a neutral person, for conflicts between the carer and the person with dementia. Therapists could provide suggestions without seeming confrontational or prompting other negative feelings (e.g. embarrassment). Sometimes, the carer reported that the person with dementia would respond better to the therapist than to the carer or other people.
Strategies to improve functioning
Through the therapeutic relationship, carers and people with dementia learned about strategies to improve functioning and how to implement these. One carer described how the input from the therapist helped to develop ideas that were used to achieve goals:
It was a great help really to talk to somebody other than [person with dementia], obviously, and [the therapist] gave us some ideas about goals which [person with dementia] couldn’t remember but they were [about] your calendar and your medication. And we found that the calendar has worked very well.
Carer 3
Participants discussed the effectiveness of a variety of different strategies, such as simple instructions, memory prompts or reminders. For carers, these strategies were deemed to be especially helpful for functioning if they eased the burden of caregiving (e.g. by supporting dressing or cooking). Trying out these strategies and practising them was key to improvements for the person with dementia. Carers expressed how the intervention needed the investment of time and effort from the participants to gain any benefit. If the strategies were too challenging, effortful or repetitive, the carers believed that the people with dementia would get bored, ‘worn down’ or frustrated, and would no longer continue to use them.
The carers and the people with dementia both mentioned improvements as a result of the strategies. These improvements related to general, daily functioning, and the strategies that were put into place were deemed to be helpful for the person with dementia. For example, the new strategies helped with remembering to take medication (as illustrated in the quote above) or knowing what activities were planned for the day without prompting. Often, the improvements were in small tasks, but some were deemed to be ‘vital’ for relieving some of the burden for the carer or they were things that brought the person with dementia enjoyment, which seemed to enhance their well-being.
Many carers described how they developed a more problem-focused and practical viewpoint as a result of the therapy sessions, which enabled them to create new goals and strategies:
[Therapist] made you think about things that you thought you perhaps knew, but think about them in a different way . . . And approach them in a different way . . . That made it in a very practical way.
Carer 4
This perspective was considered to be valuable, as it would help in coping with future decline or challenges. There was also a shift in thinking that emphasised the ‘value [of] little things’ and enjoyment. Both the carers and the people with dementia got great pleasure from small and simple tasks (e.g. trips into town, a walk and a meal in a pub or a restaurant), and these tasks were thought to improve well-being.
Participants also discussed how the strategies had positive psychological outcomes – that is, they led to greater confidence, autonomy and empowerment. The people with dementia found a sense of achievement when they were able to remember or do something as a result of the intervention. One carer expressed how the act of achieving the goal was beneficial:
I should think sometimes just reaching his goal, being able to do it after a few days. Something like that, you know, seeing that he can do it and just putting whatever [therapist] said into place . . . Just little things like that, they help I think.
Carer 3
Both the carers and the people with dementia described how the therapy had improved the confidence of the person with dementia. Carers noted that the strategies and aids were helpful for improving or supporting independence. Carers remarked how the person with dementia was more willing to try different things and initiate conservations as a result of the enhanced confidence. For carers, perceiving any improvements or achievements was a source of motivation to continue with the intervention.
Although the changes and strategies encouraged in the therapy were generally felt to be useful, a very small number of carers commented that these were a ‘hindrance’ to an established routine.
Person-centred approach
Carers and people with dementia both appreciated the person-centred approach of the intervention. The individual tailoring and flexibility that this approach provided was crucial for developing and implementing strategies and gaining confidence with problem-solving and finding solutions. As one carer described, there was flexibility to ensure that what was covered was relevant to the person and that this was done at the right pace:
. . . they were always relevant to . . . obviously relevant to the issues that [therapist] wanted to raise . . . And also relevant to, the issues that were important for [person with dementia] . . . And, the issues that were – she worked at a pace that was good for him as well.
Carer 5
For people with dementia, this meant that time was taken to understand their needs and preferences, as well as personalising intervention components to suit their interests, abilities and needs. That is, the specific goal or task preferences of the people with dementia were considered and acknowledged. By individualising goals and strategies, the people with dementia gained more enjoyment, empowerment and a sense of achievement from completing these, and therefore, this potentially produced more positive outcomes for both the person with dementia and the carer.
The flexibility of this approach also allowed for the adaption and modification of tasks or goals over time, which was deemed to be important by the people with dementia and their carers. These changes made the tasks ‘fit in’ to their lives, and alterations could be made to manage changes in cognitive or physical health. This flexibility was closely related to the therapist’s responsiveness and the ability to address certain challenges in order to accomplish the goals.
Dementia-related beliefs
A few carers and people with dementia were not sure whether or not the therapy had been truly beneficial, and they noted that it did not improve memory as such. This uncertainty about the impact of the therapy was attributable to the progressive nature and inevitable decline associated with the condition. This was illustrated by the response of one carer when asked about the impact of the therapy:
. . . now, we come onto the issue of . . . the problem of Alzheimer’s itself, so that, to be honest, is very, very difficult to answer . . . Certain things have slipped away, but is that the fault of the programme or the fault of the condition? And so it’s really difficult to equate what the programme has done and what the condition has not allowed it to do . . .
Carer 6
A few carers questioned to what extent the intervention was worthwhile, given that normal functioning could not be restored and that decline was inevitable. Furthermore, there was a concern about the lasting benefit of the therapy. A couple of carers and people with dementia believed that the future deterioration would possibly undo any improvements from the therapy. With this thought, a few carers suggested that the intervention may be most relevant and beneficial in the earlier stages of dementia.
Summary
The main findings can be summarised as follows:
-
The relationship with the therapist played a major role in the therapy. It was the vehicle for providing information, education and support and was the means by which rehabilitative strategies were developed, accepted and personalised.
-
The most frequently reported impact of the therapy was improved psychological adjustment to dementia and a more positive perspective, reflected in greater confidence, less anxiety, better coping, empowerment and improved well-being.
-
Participants and carers found that the intervention was effective in supporting activities of daily living and in improving psychosocial well-being and quality of life.
-
The perceived effect of the therapy on cognition and memory was mixed. Some carers and people with dementia were not sure whether or not the therapy was beneficial for cognition as a result of the believed progressive nature and the inevitable decline associated with the condition. It should be noted that the therapy was not expected to benefit cognition as such and was not presented to participants as a treatment to improve cognition.
-
People with dementia and carers expressed an overarching need for social contact and support.
Case studies
People living with early-stage dementia face many challenges in everyday life as cognitive impairments and other changes affect functioning. The exact nature of these challenges is different for each individual, depending on personal characteristics, circumstances, interests and preferences. Appendix 14 presents four illustrative case studies from GREAT, showing the kinds of needs and concerns that prompted participants and carers to choose particular goals and demonstrating how the therapists worked with participants and carers to address their goals during the CR intervention.
Feasibility of implementation
In the later stages of the trial, the GREAT team undertook to explore the feasibility of implementing the CR approach within NHS services. This was an opportunity to examine the challenges that could arise when translating the intervention to a real-world setting, and to consider how these might be overcome to facilitate successful implementation. The results are presented in Appendix 3.
Chapter 5 Economic evaluation
Research question
Using a multicentre, pragmatic randomised controlled design, the study compared goal-oriented CR with TAU. The aim of the economic evaluation was to examine whether or not CR is a cost-effective intervention, compared with TAU, for people with early-stage Alzheimer’s disease, or vascular or mixed dementia, and their carers, over a 9-month period post randomisation.
Methods
Form of evaluation
The economic evaluation included cost-effectiveness and cost–utility analyses.
Effectiveness
The economic evaluation examined four outcome measures, three for participants with dementia and one for carers.
Cost-effectiveness measures:
-
incremental cost of achieving a standardised mean difference (SMD) of 1.32 points in the participant-reported goal attainment measure (the BGSI), the primary outcome for the trial
-
incremental cost of achieving a difference in effect of 0.30 in the GSES, which assesses a participant’s general sense of perceived self-efficacy.
Cost–utility measures:
-
incremental cost per QALY assessed by the participant-rated DEMQOL
-
Incremental cost per QALY for carers’ self-rated HRQoL, using the EQ-5D-3L.
A SMD for the GSES was calculated by multiplying the effect size of 0.3 by the (non-imputed) SD of the mean across sample participants at baseline following Samsa et al. 109 The second outcome in the list above thus equates to an incremental cost of achieving a SMD of 1.53 points in the GSES. These outcome measures are described in more detail in Chapter 2.
We calculated utility scores for participants with dementia from the DEMQOL instrument (the DEMQOL-U index) using published societal weights. 110 We derived QALYs from these scores using the area-under-the-curve method, with linear interpolation between the three assessment points. We calculated carers’ utility scores from the EQ-5D-3L with published societal weights. 111
Perspective
The economic evaluation first took a health and social care perspective, and second a societal perspective. Broadly speaking, the health and social care perspective took into account those costs falling to the NHS and to local authority social services departments (SSDs). Most service costs (e.g. hospital, community health care, community day care and home-based care) were considered to fall entirely to these agencies. However, only adaptations and equipment reported as being provided by the NHS or SSDs were considered within this perspective. The societal perspective was considered to encompass not only health and social care costs, but also the costs to the participant–carer dyad: lost production in terms of carer’s wages forgone because of providing care; costs of providing care in terms of hours of care provided; out-of-pocket payments (privately purchased equipment and travel costs of attending appointments related to dementia treatment).
Time horizon
The outcomes and costs considered in the economic evaluation were measured at baseline and at the 3-month and 9-month assessment points. The costs collected using the CSRI covered the 3-month period prior to each assessment point. The 9-month costs were calculated from these three data collections. The costs of services between 3 months post baseline and 6 months post baseline were assumed to be the same as the costs in the 3 months prior to the 9-month follow-up. In other words, to calculate the 9-month costs, we estimated the 3-month costs based on data from the second follow-up, multiplied this estimate by two and added this to the costs in the 3 months prior to the first follow-up. It was not necessary to apply discount rates to either costs or outcomes, because the time horizon was shorter than 1 year.
Costs
The analysis considered the comprehensive costs of care and support to the person with dementia. The costs were calculated drawing on the following collections:
-
data on services used by the person with dementia, as observed and reported by carers using the CSRI112
-
data on carer time spent on care and support activities and lost employment, using the CSRI
-
data on time spent by professionals in delivering the intervention, using the therapy logs
-
data on professionals’ labour costs, using a pro forma distributed to therapists
-
costs of training (fees, materials) supplied by the project management team.
The costs of health and social care services were calculated from service use data by applying relevant, nationally generalisable unit costs taken from NHS Reference Costs 2012 to 2013113 and the Personal Social Services Research Unit (PSSRU)’s costs compendium. 114 We calculated the costs of carers’ inputs using opportunity costs (base case) and replacement costs (sensitivity) methods. 115–117 The opportunity costs approach involved attaching a value to each hour of unpaid carer time in providing care and support equal to the minimum wage. In the replacement cost approach, the cost of an hour of home care was used to value unpaid time spent providing care. The unit costs used in valuing resource use118–143 are summarised in Table 31 and reported in full in Appendix 15.
| Service use item | Unit cost, £ (2013–14) |
|---|---|
| Inpatient bed-day, per specialty | Range: 324–896 |
| Inpatient bed-day, weighted average across adult specialties | 495 |
| Day attendances, per specialty | Range: 374–1333 |
| Day case, weighted average across specialties | 698 |
| Outpatient attendances | Range: 42–271 |
| A&E attendances, admitted and non-admitted | 124 |
| Outpatient, weighted average of follow-up attendances across adult specialties | 102 |
| Primary, community and community mental health services: contacts with services | Range: 0.5–221 |
| Primary and community health services: minutes | Range: 0.5–4.43 |
| Residential care per day | Range: 79–157 |
| Nursing home care per day | 104 |
| Community-based social care: minutes | Range: 0.33–0.50 |
| Day services per session per day | Range: 3–146 |
| Medications: standard quantity units | Range: 0.029–8.45 |
| Equipment and adaptations, cost over 3 months per item | Range: 0.22–106 |
| Carer hour, valued at replacement cost: home care worker, per hour | 19.64 |
| Carer hour, valued at opportunity cost: minimum wage, per hour | 6.31 |
Costs are reported in the following categories: hospital services, primary and community health, mental health services, overnight respite care, community social care, day services, equipment and adaptations, mental health medication, costs of the CR intervention and unpaid care. Costs are also reported as aggregated total costs from the health and social care perspective and from the societal perspective.
The costs of the CR intervention were calculated by drawing on a number of sources. We gathered comprehensive information on the time spent by therapists delivering the CR intervention. The intervention time consisted of three elements:
-
Direct (face-to-face) contact time with participants – therapists entered their contact time per visit in ‘therapy logs’ in the MACRO database system.
-
Indirect contact time (planning, travel) with participants – the set-up time was estimated by the GREAT project team as 10–15 minutes. Therefore, 12.5 minutes of indirect time were allocated per visit. Therapists estimated the time taken to make a one-way journey by the participant as an average and entered this into the MACRO database system.
-
Non-contact time (general training and individual training/supervision) – a pro forma collected non-contact costs (supervision and training of therapists) from the project team. These comprised the number of hours spent in general training sessions, the costs of providing general training sessions (trainers’ fees, costs of venues and materials), the travel costs of attending general training sessions, the number of hours spent in individual training sessions, the costs of providing individual training sessions (trainers’ fees, costs of venues and materials) and the travel costs of attending individual training sessions.
Therapists also completed a pro forma providing information on Agenda for Change (AfC) band, the proportion of full-time equivalent (FTE) hours worked, the start and end dates of the project, the usual mode of transport, the average number of miles travelled by car, the average parking charges and the average cost of a public transport fare.
To value the time spent by therapists in direct, indirect and travel activities, we calculated comprehensive therapy staff costs, including salary costs (by median FTE earnings per AfC band, including oncosts: superannuation at 14% of salary and national insurance contributions), administrative overheads (management and non-staff costs) and capital overheads calculated as a percentage of salary cost. Staffing costs were weighted by salary band and time contributions (FTEs at each band) to estimate a weighted cost per hour of therapy time. To value the costs of therapists’ journeys made to participants, we estimated the costs of public transport or car travel per centre using the data from each therapist-completed pro forma.
The study ran over 4 years: pro formas were issued every financial year. The costs of general training and supervision in years following the base year (2013–14) were deflated to 2013–14 prices using the Hospital and Community Health Service (HCHS) index. 144 The total across all years was divided by the number of CR participants (n = 238) multiplied by 14 sessions, to give a per-session overhead to be attached to each session attended by participants.
Missing data
Resource use data collected using the CSRI may be missing for any use of a service, for the frequency of using the service or for the duration of a service (e.g. length of a home care visit). When service use was indicated but frequency was missing, a suitable nationally applicable unit cost was used if available (e.g. cost per visit). For each case, items in each cost category were added together to give the total cost for the category. The category-level costs were then summed to give a total overall cost per case. If all costs in the category were missing, the category total (per case) was calculated as missing; if some items were missing, these were treated as zeros and the case was assigned the cost of the sum of available costs in the category.
Missing category-level costs were multiply imputed by predictive mean matching (k = 5 nearest neighbours) in a regression model that included demographic variables of the dyad, centre and stratified MMSE score and the outcome measures to be used in the cost-effectiveness analysis, using the Stata® programme MI impute (StataCorp LP, College Station, TX, USA). 145 Whether people with dementia who had been lost to follow-up or withdrew prior to the 9-month follow-up had died during this time was unknown. The model therefore also included the survival of the person with dementia to the end of the 9-month assessment as an imputation variable. Carers who were part of a dyad that had been lost to follow-up or withdrawn were assumed to have survived. Costs and outcomes were imputed separately by allocation and depending on survival. 146 The number of imputations was guided by White et al.’s147 rule of thumb that the number of imputations should be set at the percentage of incomplete cases for variables to be used in the analyses.
Analyses
The use of services and the mean use of services within allocation groups were compared descriptively, with no tests of between-group differences, given the large numbers of potential comparisons. The descriptive statistics of costs and outcomes are presented in terms of the mean and SE in each group, and the between-group difference and the SE of the difference. All service use, cost and outcome data are summarised in terms of the sample of dyads for which unpaid carers were available for the completion of the CSRI section of the assessment at each time point. All analyses were conducted using Stata version 14. Cost outliers were identified by following the adjusted box plot technique described by Vanderviere and Huber148 and recommended for skewed data. This involved calculating the medcouple, a measure of skewness (using a user-written programme medcouple in Stata),149 establishing the upper fence of a box plot interval and defining the observations falling above the upper fence as high-cost outliers.
Cost-effectiveness
Cognitive rehabilitation was to be defined as cost-effective if it was:
-
less costly and more effective than TAU or
-
more costly and more effective than TAU, and society is willing to pay the additional cost in order to achieve the gain in outcome, or
-
less costly and less effective than TAU, and society is willing to sacrifice some of the outcome difference in order to make a saving.
The intervention was to be defined as not cost-effective if it was both significantly more costly and less effective than TAU or if society was not willing to pay the cost of a gain in outcome. The criteria for this decision were based on the following rule:
Here ΔC represents the additional cost, ΔE is the gain in outcome associated with the treatment, and λ is the willingness to pay (WTP) for that outcome gain. 150 The incremental cost-effectiveness ratio [(ICER) ΔC/ΔE] must be below the decision-maker’s WTP (λ) to be considered cost-effective.
The ICER was calculated as the difference in the mean costs of the CR and TAU groups over the period of follow-up (9 months) divided by the difference in the mean end-point outcome measure (the BGSI and the GSES for participants with dementia) between groups. In the case of the ratio of incremental costs and QALYs (based on the DEMQOL-U for participants with dementia and the EQ-5D for carers), the denominator was the difference in mean QALYs. The ICER point estimates presented in the tables are based on the ratio of the cost results rounded to one decimal place and the outcome results rounded to two decimal places.
The cost-effectiveness decision rule can be rearranged in terms of the net monetary benefit, the monetary value of a gain in effect associated with the treatment at a given WTP and the net of the additional cost of the treatment:150
The net monetary benefit must be greater than zero if the costs associated with the intervention are not to outweigh the benefits of the intervention.
Cost-effectiveness analyses
The incremental costs and outcomes were estimated by seemingly unrelated regressions (SUR). 151 This approach was combined with non-parametric bootstrapping. The estimates were adjusted by centre, baseline outcome measures and baseline costs, as well as demographic variables (sex, age and stratified MMSE score). The regression coefficients on the allocation term were used to calculate the net monetary benefit over a range of societal WTP levels for incremental differences in the primary outcome measures and for QALY gains.
The number of bootstrap samples used in the analyses was determined by a method suggested by Gould and Pitblado. 152 This involves examining the bootstrap variance estimates for the variable of interest plotted against the number of replications. The bootstrapped SE for the allocation term in the cost regression was examined in this way. At the point when consecutive SEs produced by the bootstrap samples (increasing from a base of 1000 replications by 1000 additional replications) differed by < 1%, this was considered to be an adequate number of replications. The complete-case analyses used 60,000 replications; analyses with multiply imputed data used 3000 replications.
The proportion of bootstrap replicates in which the net benefit was greater than zero was plotted over a range of WTP values to produce cost-effectiveness acceptability curves (CEACs). These illustrate the probability of making a correct decision to fund the intervention,150 and also the sampling uncertainty around the point estimate of the ICER. 153 The cost–outcome difference pairs were plotted as points on the cost-effectiveness plane as a further means to illustrate sampling uncertainty, while providing graphical information on the joint distribution of costs and outcomes across the quadrants of the plane. For instance, cost–outcome differences that fall into the north-east quadrant of the plane indicate that CR is associated with higher costs and better outcomes than TAU. 154 Points falling into the south-east quadrant indicate that lower costs and better outcomes are produced by the new intervention relative to the old intervention (in which case the new intervention is said to ‘dominate’ the old intervention). Points falling into the north-west and south-west quadrants represent situations in which the new intervention respectively costs more (in which case, the new intervention is ‘dominated’) and in which the intervention costs less than the alternative; in either case, the new intervention produces worse outcomes.
The analyses took into account the data of those participants and carers with sufficient information to calculate both service costs and carer costs. Thus, the analyses did not consider data for which only one member of the dyad had contributed information. In addition, given the need to reflect the societal perspective, data from participants with only paid carers were not analysed. This was for two reasons: first, paid carers were not providing unpaid care; and, second, the reason that the informant was a paid carer was likely to reflect the absence of an unpaid carer who would incur any unpaid care costs.
Results
Sample numbers
The numbers of people who formally participated in assessments were:
-
at baseline, 474 people with dementia and their carers (238 in the CR arm; 236 in the TAU arm)
-
at 3 months, 445 people with dementia (218 in the CR arm; 227 in the TAU arm) and 442 carers (217 in the CR arm and 225 in the TAU arm)
-
at 9 months, 426 people with dementia (208 in the CR arm; 218 in the TAU arm) and 422 carers (207 in the CR arm; 215 in the TAU arm).
In terms of complete dyads who formally participated in the assessment:
-
at baseline, 474 participated (238 in the CR arm; 236 in the TAU arm)
-
at 3 months, 442 participated (217 in the CR arm; 225 in the TAU arm)
-
at 9 months, 422 participated (207 in the CR arm; 215 in the TAU arm).
With regard to CSRI questionnaires that were partially or wholly completed by dyads consisting of a person with dementia and an unpaid carer, the numbers were:
-
at baseline, 469 CSRI questionnaires (236 in the CR arm; 233 in the TAU arm)
-
at 3 months, 437 CSRI questionnaires (215 in the CR arm; 222 in the TAU arm)
-
at 9 months, 415 CSRI questionnaires (205 in the CR arm; 210 in the TAU arm).
Information from complete dyads (who had not withdrawn or been lost to follow-up and had an unpaid carer participating) that was sufficiently complete to calculate health and social care and societal costs was available:
-
at baseline, for 469 dyads (236 in the CR arm; 233 in the TAU arm)
-
at 3 months, for 435 dyads (213 in the CR arm; 222 in the TAU arm)
-
at 9 months, from 414 dyads (204 in the CR arm; 210 in the TAU arm).
The outcome data available at each assessment point are described in Chapter 3. The numbers of dyads included in the cost-effectiveness analyses varied depending on the measures; the relevant valid numbers of observations associated with each measure are presented with the results of the analyses. There were four paid carers who completed the CSRI (see Chapter 3). At the end of the study, seven participants with dementia had died and one carer had died.
The complete-case sample of economic data available for analysis at 9 months (those with data from complete dyads across the three assessment points who had not withdrawn or been lost to follow-up and had an unpaid carer participating) was 412 (203 in the CR arm; 209 in the TAU arm). The numbers of cases available for the cost-effectiveness analyses varied depending on the outcome:
-
For QALYs calculated using the DEMQOL-U, there were 401 cases available (196 in the CR arm; 205 in the TAU arm).
-
For BGSI scores, 407 cases were available (201 in the CR arm; 206 in the TAU arm).
-
For GSES scores, 389 cases were available (190 in the CR arm; 199 in the TAU arm).
-
For carers’ QALYs calculated using the EQ-5D-3L, there were 390 cases (192 in the CR arm; 198 in the TAU arm).
The sample of cases available in the analysis of complete data sets as a product of the multiple imputation process was 462.
Use and costs of care and support services at each assessment point
At baseline, both the CR group and the TAU group used a wide variety of services (see Appendix 16, Tables 125–127). The groups exhibited a high use of services, such as outpatient appointments and GP and practice nursing contacts. In both groups, 11% of participants received some form of home help or home care, and roughly the same number attended a day centre. One-fifth of the dyads had cleaning services. The proportions using these services remained relatively stable over the course of the study. The proportion of participants who reported taking any mental health medications was stable over the three assessment points; approximately three-quarters of both groups took antidementia medications.
The mean number of home care contacts and hours demonstrates a problematic feature of the data. There were small numbers receiving very high levels of home care at each assessment point and these were concentrated in the TAU group. At baseline and 9 months, the average number of contacts and hours of home care in the TAU group were twice those in the CR group. However, the variation in contacts and hours was higher in the TAU group, as evidenced by larger SEs relative to the means. This complicates an assessment of the size of these differences: a unit-free measure is useful in these circumstances. The SMD (the mean CR – TAU difference divided by the SD across the groups) in home-care hours between CR and TAU was –16% at baseline, 14% at 3 months and –33% at 9 months (not presented in the table). This suggests very substantial variability in receipt, or reporting of receipt, of home care over the study period and also a large difference in home care received at 9 months in the TAU versus CR comparison. The same pattern occurred in unpaid care time of the principal carer: the number of unpaid care hours at baseline and 9 months was higher in the CR group than in the TAU group but at 3 months was higher in the TAU group.
The types of care and support provided by carers are described in Table 128 in Appendix 16. A majority of carers reported providing social support (keeping the person with dementia company) and taking the person to appointments, helping with medications, providing practical help and supervision. Fewer than one-third of carers in either group reported assisting with personal care at baseline or 3-month assessments. At the 9-month assessment point, however, the proportion of carers providing personal care was 38% in the CR group and 43% in the TAU group.
The raw costs of health and social care services, unpaid care and out-of-pocket expenses in the 3 months prior to assessment are presented in Table 32. The total health and social care and societal costs are also given. The mean differences in costs between the groups exhibited wide CIs. The pattern of apparently higher costs in the CR group at baseline and 9 months echoed that seen in the utilisation of home care and unpaid care hours; however, the unadjusted differences were not significantly different from zero at any point, as evidenced by CIs crossing zero.
| Category of cost | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | CR – TAU | ||||||
| n | Mean | SE | n | Mean | SE | Mean difference | 95% CI | |
| Baseline | ||||||||
| Hospital | 236 | 426 | 91 | 233 | 276 | 44 | 151 | –50 to 351 |
| Primary and community health | 236 | 153 | 11 | 233 | 164 | 14 | –11 | –46 to 25 |
| Respite residential/nursing home | 236 | 3 | 3 | 233 | 0 | 0 | 3 | –3 to 9 |
| Community care | 236 | 203 | 52 | 233 | 458 | 177 | –255 | –615 to 105 |
| Community mental health | 236 | 58 | 11 | 233 | 51 | 10 | 8 | –21 to 36 |
| Day care (any provider) | 236 | 107 | 25 | 233 | 85 | 19 | 22 | –40 to 83 |
| Medicationsb | 236 | 182 | 10 | 233 | 181 | 9 | 1 | –26 to 27 |
| Equipment and adaptationsc | 236 | 10 | 2 | 233 | 10 | 2 | 0 | –6 to 6 |
| Health and social cared | 236 | 1142 | 116 | 233 | 1224 | 194 | –82 | –524 to 360 |
| Unpaid caree | 236 | 5899 | 401 | 233 | 5632 | 369 | 267 | –806 to 1340 |
| Out of pocketf | 236 | 53 | 5 | 233 | 71 | 6 | –18 | –33 to –3 |
| Societalg | 236 | 7041 | 422 | 233 | 6857 | 418 | 185 | –983 to 1352 |
| Sensitivity: unpaid careh | 236 | 15,236 | 1128 | 233 | 13,497 | 982 | 1739 | –1202 to 4681 |
| Sensitivity: societali | 236 | 16,378 | 1143 | 233 | 14,721 | 1007 | 1657 | –1339 to 4653 |
| 3 months | ||||||||
| Hospital | 213 | 292 | 53 | 222 | 310 | 77 | –18 | –203 to 168 |
| Primary and community health | 213 | 131 | 10 | 222 | 144 | 18 | –14 | –54 to 27 |
| Respite residential/nursing home | 213 | 62 | 55 | 222 | 0 | 0 | 62 | –45 to 168 |
| Community care | 213 | 423 | 157 | 222 | 387 | 182 | 36 | –439 to 510 |
| Community mental health | 213 | 43 | 15 | 222 | 24 | 5 | 18 | –12 to 48 |
| Day care (any provider) | 213 | 112 | 23 | 222 | 107 | 22 | 5 | –59 to 68 |
| Medicationsb | 213 | 174 | 11 | 222 | 192 | 10 | –18 | –48 to 12 |
| Equipment and adaptationsc | 213 | 13 | 3 | 222 | 11 | 2 | 2 | –5 to 9 |
| Health and social cared | 213 | 1250 | 186 | 222 | 1177 | 234 | 73 | –518 to 665 |
| Unpaid caree | 213 | 5985 | 385 | 222 | 6199 | 397 | –214 | –1303 to 875 |
| Out of pocketf | 213 | 56 | 5 | 222 | 66 | 6 | –9 | –25 to 6 |
| Societalg | 213 | 7235 | 462 | 222 | 7376 | 452 | –141 | –1411 to 1130 |
| Sensitivity: unpaid careh | 213 | 14,846 | 1099 | 222 | 15,026 | 1061 | –181 | –3182 to 2820 |
| Sensitivity: societali | 213 | 16,096 | 1162 | 222 | 16,203 | 1080 | –107 | –3221 to 3007 |
| 9 months | ||||||||
| Hospital | 204 | 424 | 114 | 210 | 308 | 69 | 116 | –144 to 376 |
| Primary and community health | 204 | 129 | 9 | 210 | 168 | 14 | –39 | –72 to –5 |
| Respite residential/nursing home | 204 | 69 | 48 | 210 | 154 | 63 | –86 | –243 to 71 |
| Community care | 204 | 317 | 88 | 210 | 622 | 243 | –305 | –819 to 209 |
| Community mental health | 204 | 34 | 11 | 210 | 63 | 20 | –29 | –75 to 16 |
| Day care (any provider) | 204 | 123 | 24 | 210 | 133 | 29 | –10 | –84 to 63 |
| Medicationsb | 204 | 172 | 11 | 210 | 184 | 10 | –12 | –41 to 17 |
| Equipment and adaptationsc | 204 | 15 | 3 | 210 | 14 | 3 | 0 | –8 to 9 |
| Health and social cared | 204 | 1282 | 155 | 210 | 1647 | 299 | –365 | –1033 to 303 |
| Unpaid caree | 204 | 6317 | 428 | 210 | 6276 | 410 | 41 | –1123 to 1205 |
| Out of pocketf | 204 | 61 | 5 | 210 | 73 | 6 | –12 | –27 to 4 |
| Societalg | 204 | 7599 | 490 | 210 | 7923 | 494 | –324 | –1691 to 1043 |
| Sensitivity: unpaid careh | 204 | 16,110 | 1198 | 210 | 15,695 | 1131 | 415 | –2821 to 3650 |
| Sensitivity: societali | 204 | 17,391 | 1249 | 210 | 17,342 | 1160 | 50 | –3299 to 3398 |
The health and social care costs were, on average, relatively modest, being substantially lower than societal costs at each assessment point. These averages mask some extremely high-cost cases, particularly in the TAU group. At the 9-month follow-up, the maximum total health and social care costs over the prior 3 months reached £18,063 (vs. the mean of £1282) in the CR group and £42,504 (vs. the mean of £1647) in the TAU group. The data were highly skewed: on a test for normality, the hypothesis that the data were distributed normally was rejected (p = 0.000) for both health and social care costs and societal costs.
Unit costs and per-participant costs of the cognitive rehabilitation intervention
The cost components used to value the CR intervention are given in Table 33. CR-specific training and supervision costs per session were substantial at £33, and may not reflect the level of supervision and support that would be available in routine clinical practice. The mean number of visits in the 3 months after baseline in the economic evaluation sample (n = 215) was 9.61 (SE 0.09), as shown in Table 34; the costs in the first 3 months were £1259 (SE £18). In the period between the 3-month and the 9-month assessments in the economic evaluation sample (n = 204), the mean number of visits was 3.74 (SE 0.07) and the costs were £474 (SE £10).
| Training and supervision costs for professionals delivering CR | Costs (£) | |
|---|---|---|
| Total | Unit | |
| Spend in year 1 | 23,347 | |
| Spend in year 2a | 26,153 | |
| Spend in year 3a | 39,549 | |
| Spend in year 4a | 9702 | |
| 4-year total | 98,751.01 | |
| 4-year total divided by 2970 (total sessions provided to participants)b | 33.25 | |
| Weighted cost per hour of professionals delivering CR | 42.70 | |
| Mileage cost per centre of a one-way journeyc | Range: 3–19 | |
| Health professional visits | Valid (n) | Mean (CR, N = 238) | SE |
|---|---|---|---|
| 3 months | |||
| Number of visitsb | 213 | 9.61 | 0.09 |
| Total hours of visitsb | 213 | 20.17 | 0.33 |
| Mean duration per completed visit (hours)c | 213 | 2.10 | 0.03 |
| Costs (£) | |||
| (a) Face-to-face visits | 213 | 523 | 7 |
| (b) Preparation | 213 | 85 | 1 |
| (c) CR training and individual supervision | 213 | 320 | 3 |
| (d) Travel (time and mileage) | 213 | 331 | 12 |
| Mean cost per person (includes a–d) | 213 | 1259 | 18 |
| 9 months | |||
| Number of visitsb | 204 | 3.74 | 0.07 |
| Total hours of visitsb | 204 | 7.46 | 0.17 |
| Mean duration per completed visit (hours)c | 197 | 2.00 | 0.03 |
| Costs (£) | |||
| (a) Face-to-face visits | 204 | 188 | 4 |
| (b) Preparation | 204 | 33 | 1 |
| (c) CR training and individual supervision | 204 | 124 | 2 |
| (d) Travel (time and mileage) | 204 | 128 | 5 |
| Mean cost per person (includes a–d) | 204 | 474 | 10 |
Use and costs of care and support services over the period of the study
Over the 9-month period of the study, the total cost of the CR intervention (as shown in Table 35) was £1736 (SE £25) per participant. The average total estimated health and social care costs, including the costs of CR over 9 months, were £3998 (SE £539) in the CR group and £4556 (SE £815) in the TAU group. Societal costs were of the order of five times higher. Again, although the mean health and social care costs over 9 months were quite modest, there was a small number of high-cost cases in the TAU group, with the costs exceeding £130,000. A total of eight outliers were identified following the adjusted box-plot method: three in the TAU group, ranging from approximately £28,000 to £133,000, and five in the CR group, ranging from approximately £31,000 to £63,000. In contrast, no outliers were identified by this method in the societal costs.
Cost-effectiveness analyses
Outcomes and costs for the person with dementia
The raw mean outcome scores in the economic evaluation sample (Table 35) show that the CR and TAU groups had similar scores in the BGSI attainment ratings outcomes at baseline. At 3 months, the CR group mean score was 1.63 points (95% CI 1.27 to 1.99 points) higher than that of the TAU group. The CR group mean score at 9 months was also higher than that in the TAU group, by 1.79 points (95% CI 1.38 to 2.20 points). The groups did not differ on any other measure.
| Total and CR costs | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | CR – TAU | ||||||
| n | Mean | SE | n | Mean | SE | Mean difference | 95% CI | |
| Health and social carea | 203 | 3787 | 455 | 209 | 4485 | 796 | –698 | –2514 to 1119 |
| CRb | 203 | 1736 | 25 | 209 | 0 | 0 | 1736 | 1687 to 1784 |
| Health and social care plus CRa,b | 203 | 5523 | 453 | 209 | 4485 | 796 | 1038 | –777 to 2853 |
| Societalc | 203 | 22,417 | 1356 | 209 | 23,290 | 1335 | –873 | –4614 to 2868 |
| Societal and CRb,c | 203 | 24,153 | 1355 | 209 | 23,290 | 1335 | 863 | –2877 to 4602 |
Adjusted differences in outcomes (derived from SUR models) between the groups are given in Table 36. The BGSI attainment ratings were significantly higher in the CR group, by 1.35 points (95% CI 1.09 to 1.64 points). The adjusted differences in other outcomes were not significantly different from zero.
| Outcome | Treatment group | |||||||
|---|---|---|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | CR – TAU | ||||||
| n | Mean | SE | n | Mean | SE | Mean difference | 95% CI | |
| Baseline | ||||||||
| Person with dementia | ||||||||
| BGSI attainment rating | 236 | 3.51 | 0.11 | 233 | 3.56 | 0.1 | –0.05 | –0.35 to 0.25 |
| GSES | 235 | 30.74 | 0.31 | 229 | 31.07 | 0.35 | –0.33 | –1.25 to 0.60 |
| DEMQOL-U | 235 | 0.61 | 0.01 | 232 | 0.59 | 0.01 | 0.02 | –0.00 to 0.03 |
| Carer | ||||||||
| EQ-5D-3L | 233 | 0.76 | 0.02 | 230 | 0.78 | 0.02 | –0.02 | –0.07 to 0.02 |
| 3 months | ||||||||
| Person with dementia | ||||||||
| BGSI attainment rating | 213 | 6.06 | 0.14 | 222 | 4.43 | 0.12 | 1.63 | 1.27 to 1.99 |
| GSES | 211 | 30.94 | 0.32 | 219 | 30.5 | 0.38 | 0.45 | –0.53 to 1.42 |
| DEMQOL-U | 212 | 0.6 | 0.01 | 222 | 0.59 | 0.01 | 0.01 | –0.01 to 0.03 |
| Carer | ||||||||
| EQ-5D-3L | 207 | 0.75 | 0.02 | 215 | 0.74 | 0.02 | 0.01 | –0.04 to 0.06 |
| 9 months | ||||||||
| Person with dementia | ||||||||
| BGSI attainment rating | 202 | 6.04 | 0.16 | 207 | 4.25 | 0.14 | 1.79 | 1.38 to 2.20 |
| GSES | 191 | 30.72 | 0.36 | 202 | 30.62 | 0.4 | 0.1 | –0.95 to 1.15 |
| DEMQOL-U | 199 | 0.59 | 0.01 | 207 | 0.59 | 0.01 | 0 | –0.02 to 0.02 |
| QALY (DEMQOL-U) | 197 | 0.45 | 0 | 206 | 0.44 | 0 | 0.01 | –0.01 to 0.02 |
| Carer | ||||||||
| EQ-5D-3L | 194 | 0.72 | 0.02 | 207 | 0.75 | 0.02 | –0.03 | –0.08 to 0.02 |
| 9-month QALY (EQ-5D-3L) | 192 | 0.56 | 0.01 | 198 | 0.56 | 0.01 | 0 | –0.04 to 0.03 |
The adjusted health and social care cost differences varied substantially depending on the number of complete cases available. In the case of the BGSI attainment ratings, the costs were significantly higher in the CR group than in the TAU group (£1474, 95% CI £59 to £2646). In the slightly smaller samples available in the case of the GSES and QALYs (DEMQOL-U), the adjusted costs did not differ between groups. In contrast, the between-group difference in societal costs was not significantly different from zero in the case of any of the outcome measures; the sign on the difference was negative.
Outcomes and costs for carers
There was no difference in carer QALYs (EQ-5D-3L) between groups, as shown in Table 37. The groups did not differ in terms of health and social care costs or societal costs, with wide CIs around the differences.
| Outcome | CRa | 95% CIb | TAUa | 95% CIb | CR – TAU mean difference | 95% CIb | p-value |
|---|---|---|---|---|---|---|---|
| Person with dementia | N = 201 | N = 206 | |||||
| BGSIc | 4.57 | 4.36 to 4.79 | 3.21 | 3.02 to 3.41 | 1.37 | 1.09 to 1.64 | 0.000 |
| Health and social cared costs | 5502 | 4683 to 6587 | 4027 | 3126 to 5355 | 1474 | 59 to 2646 | 0.024 |
| Societald costs | 23,366 | 21,229 to 25,665 | 23,379 | 21,186 to 25,774 | –13 | –2661 to 2628 | 0.896 |
| Person with dementia | N = 190 | N = 199 | |||||
| GSESc | 20.14 | 19.71 to 20.56 | 19.92 | 19.45 to 20.37 | 0.23 | –0.32 to 0.78 | 0.427 |
| Health and social cared costs | 5197 | 4415 to 6151 | 4169 | 3219 to 5576 | 1028 | –454 to 2067 | 0.109 |
| Societald costs | 22,703 | 20,603 to 24,976 | 23,384 | 21,249 to 25,765 | –681 | –3259 to 1796 | 0.626 |
| Person with dementia | N = 196 | N = 205 | |||||
| QALYc,e (DEMQOL-U) | 0.45 | 0.44 to 0.46 | 0.45 | 0.44 to 0.46 | 0.00 | –0.01 to 0.01 | 0.906 |
| Health and social cared costs | 5397 | 4563 to 6388 | 4286 | 3353 to 5672 | 1110 | –382 to 2187 | 0.091 |
| Societald costs | 23,271 | 21,104 to 25,538 | 23,798 | 21,662 to 26,179 | –526 | –3108 to 1927 | 0.684 |
| Carer | N = 192 | N = 198 | |||||
| QALYe,f (EQ-5D-3L) | 0.56 | 0.54 to 0.58 | 0.56 | 0.54 to 0.58 | 0.00 | –0.02 to 0.02 | 0.893 |
| Health and social careg costs | 5146 | 4504 to 6006 | 4514 | 3316 to 6210 | 632 | –1058 to 1880 | 0.389 |
| Societalg costs | 22,896 | 20,912 to 24,943 | 23,798 | 21,503 to 26,361 | –902 | –3616 to 1705 | 0.592 |
Incremental costs and outcomes: person with dementia
Cost-effectiveness
The ICER point estimates are given in Table 38. The cost of an increase of 1.32 points in the BGSI attainment rating was £1296 from the health and social care perspective and –£9 from the societal perspective. The probability of cost-effectiveness (Figure 5) was over 99% at a WTP of £2500 from the health and social care perspective and from the societal perspective, and over 50% at a WTP of £1300 or more. The distribution of costs and BGSI attainment rating differences on the cost-effectiveness plane is illustrated in Figure 6. Because the difference in health and social care costs was significantly greater than zero and the difference in the outcome was also significantly greater, the cloud of cost–outcome pairs lies mostly in the north-east quadrant.
| Perspective | BGSIa (n = 407) | GSESb (n = 389) | QALY | |
|---|---|---|---|---|
| DEMQOL-Uc (n = 401) | EQ-5D-3Lc (n = 390) | |||
| Person with dementia: 9 months | ||||
| Health and social care | 1474/1.37 = 1296 | 1028/0.23 = 4470 | 1110/0.001 = 1,110,000 | N/A |
| Societal | –13/1.37 = –9 | –681/0.23 = –2961 | –526/0.0005 = –1,052,000 | N/A |
| Carer: 9 months | ||||
| Health and social care | NA | NA | NA | 632/0.001 = 632,000 |
| Societal | NA | NA | NA | –902/0.001 = –902,000 |
FIGURE 5.
Cost-effectiveness acceptability curve: BGSI score (person with dementia).

FIGURE 6.
Cost-effectiveness plane: incremental costs and end-point difference for the BGSI score at 9 months (person with dementia).
On the GSES, the cost of attaining an increase of 1.53 points (ICER point estimate) was £4470 from the health and social care perspective and –£2961 from the societal perspective. The probability of cost-effectiveness (shown in Figure 7) was 76% at a WTP of £50,000 from the health and social care perspective and 79% at the same WTP value from the societal perspective. However, as can be seen in Figure 8, the position of the cloud of societal cost–outcome difference pairs covers all four quadrants of the cost-effectiveness plane such that in any quadrant, no line drawn from the origin could exclude 2.5% of the joint cost–outcome distribution. There is no WTP at which it would be possible to be confident that CR would be more cost-effective than TAU (or vice versa) on this measure. 153
FIGURE 7.
Cost-effectiveness acceptability curve: GSES score (person with dementia).
FIGURE 8.
Cost-effectiveness plane: incremental costs and end-point difference for the GSES score at 9 months (person with dementia).
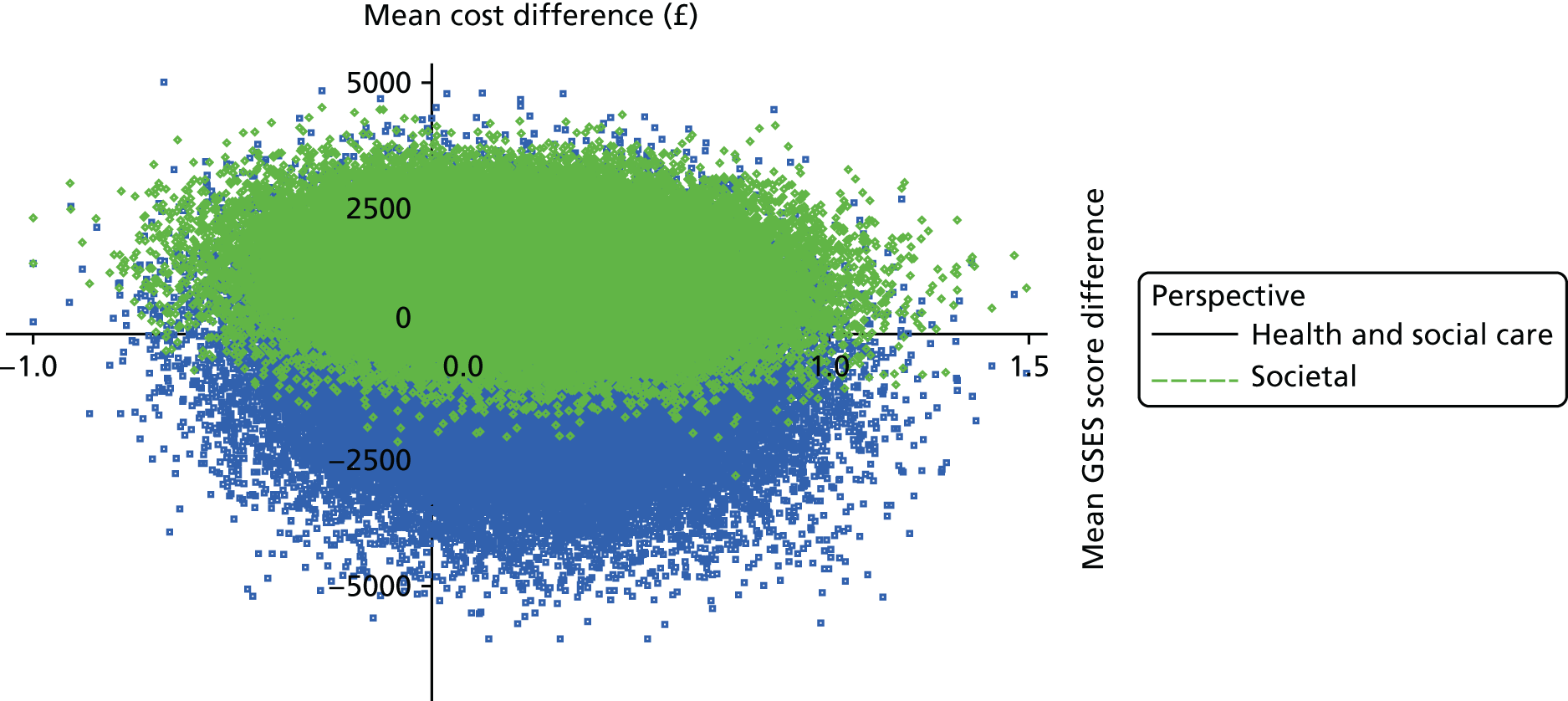
Cost–utility
The cost per QALY derived from the DEMQOL-U (ICER point estimate) (see Table 38) was £1,110,000 from the health and social care perspective. The ICER was negative (–£1,052,000) from the societal perspective, with the cost being somewhat lower (difference of £526, 95% CI –£3108 to £1927) in the intervention group from this perspective. There were no differences between the groups in terms of QALY gain. The probability of cost-effectiveness, shown in Figure 9, was very low at all WTP values per DEMQOL-U QALY (from £0 to £50,000) from the health and social care perspective; the probability of cost-effectiveness was just at or under 65% for all values of WTP over the same range. As illustrated in Figure 10, the cloud of societal cost–outcome difference pairs covers all four quadrants of the plane in approximately equal proportions, indicating that it is not possible to be certain that either strategy is cost-effective by reference to QALY gains at any level of WTP.
FIGURE 9.
Cost-effectiveness acceptability curve: QALY [DEMQOL-U (person with dementia)].

FIGURE 10.
Cost-effectiveness plane: incremental costs and QALYs (DEMQOL-U) at 9 months (person with dementia).
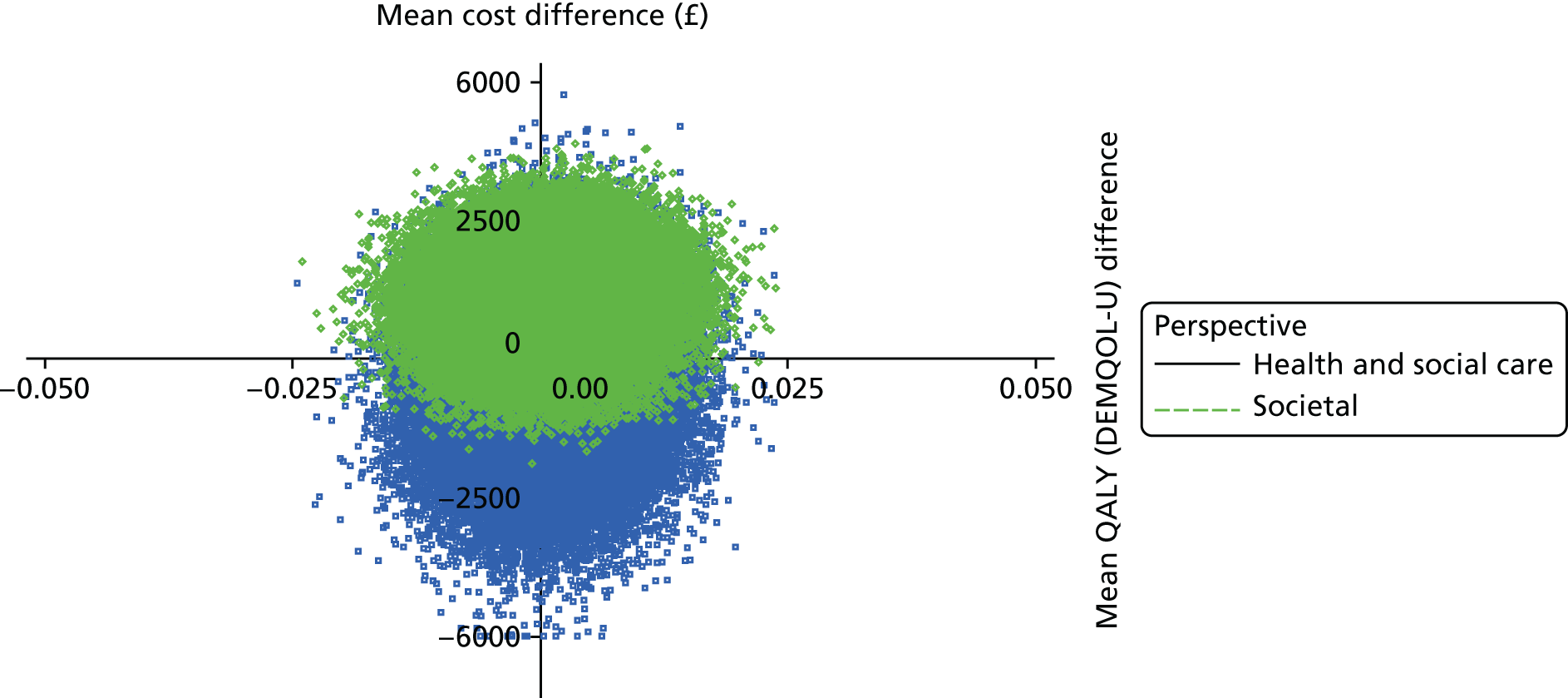
Incremental costs and outcomes: carers
Cost–utility
The cost per QALY for the carer, derived from the EQ-5D-3L (see Table 38), was £632,000 from the health and social care perspective; the ICER was negative (–£902,000) from the societal perspective, with costs being somewhat lower in the CR group than in the TAU group (by £902, 95% CI –£3616 to £1705). There were no differences in terms of QALYs between the CR and TAU groups. The probability of cost-effectiveness, shown in Figure 11, was between 17% and 22% at a range of WTP per EQ-5D-3L QALY values between £0 and £50,000 from the health and social care perspective, and approximately 74% across this range from the societal perspective. The cloud of cost–outcome pairs (Figure 12) is fairly evenly distributed across all four quadrants of the plane, suggesting no certainty in the cost-effectiveness of the intervention versus TAU (or vice versa) at any WTP from this perspective.
FIGURE 11.
Cost-effectiveness acceptability curve: QALY [EQ-5D-3L (carer)].

FIGURE 12.
Cost-effectiveness plane: incremental costs and QALYs (EQ-5D-3L) at 9 months (carer).

Sensitivity analyses
We investigated the robustness of results to several key assumptions made in the base-case analyses.
Replacement costs of unpaid care
We took a replacement-costs approach to calculating societal costs, valuing unpaid carer time at the hourly cost of a home-care worker (the raw mean costs are given in Table 32; the regression results are given in Appendix 16, Tables 129–131). The groups did not significantly differ in societal costs, with wide CIs of the mean difference, across samples associated with all outcome measures. However, the size of the differences (across samples associated with the outcome measures) was much greater than in the base case; the sign on the differences remained negative. Point ICERs were much larger for each outcome measure. In terms of the uncertainty around the point estimates (see Appendix 17, Figures 15–18) for the GSES score and QALYs (DEMQOL-U and EQ-5D-3L), although the probability of cost-effectiveness over the £0 to £50,000 WTP range was higher than in the base-case estimates, we could not be confident that the intervention was more cost-effective, given that the CEAC cuts the y-axis at the 80% or 90% level and remains relatively flat (or declines, in the case of the GSES) over the whole range. This point is illustrated in the cost-effectiveness plane (see Appendix 17, Figures 19–22), in which the cloud of cost–outcome differences is distributed widely on either side of the x-axis (crossing all quadrants of the plane).
Outliers
We examined the influence of cost outliers on health and social care costs and cost-effectiveness (see Appendix 16, Tables 132–134). The exclusion of outliers had a large impact on costs so that, although the size of the differences associated with all outcome measures was little different from the base-case results, these differences were significantly different from zero. The point ICER associated with the BGSI attainment rating was similar to that of the base case; the point ICER associated with the GSES was approximately £3000 greater. The cost per DEMQOL-U QALY (the ICER) was approximately half the size of that in the base case; the cost per EQ-5D-3L QALY (the ICER) was one-third the size of that in the base case.
The CEACs (see Appendix 17, Figures 23–25) and cost-effectiveness plane plots (see Appendix 17, Figures 26–29) were similar to those produced by the base-case results for the BGSI and the GSES. On the person with dementia and carer QALY measures, the probability of cost-effectiveness over the £0 to £50,000 range was low, such that the probability of cost-effectiveness on the person with dementia QALY measure (DEMQOL-U) was close to zero (the CEAC for this outcome is not presented for this reason) and the probability of cost-effectiveness on the carer QALY measure (EQ-5D-3L) was low, not exceeding 5% over this range. Nonetheless, the inferences to be drawn from these results remain the same as those drawn from the base case.
Multiple imputations
The results drawing on the 25 completed data sets produced by the multiple imputation process (see Appendix 16, Tables 135–137) produced a larger sample (231 participants in the CR group, 231 participants in the TAU group). The magnitude of the difference between the groups in BGSI score was very similar to the main analysis (1.35 vs. 1.37 points, respectively). As in the base case, the groups did not differ in GSES scores; however, the coefficient for the between-group difference was smaller than in the base case. On the person with dementia QALY measure (DEMQOL-U), the coefficient was considerably lower than in the base-case result (0.0003 vs. 0.001); on the carer QALY measure (EQ-5D-3L), the coefficient was slightly larger (0.001 vs. 0.003). The (non-significant) difference between groups in health and social care costs was similar to that of the complete-case analyses of GSES score and QALYs (DEMQOL and EQ-5D-3L). The result of the imputed data analyses was in contrast to the complete-case BGSI attainment rating analyses results, whereby the between-group difference in health and social care costs was significant. Societal costs did not differ between the groups, as in the base case. From the health and social care perspective, the point ICER for the BGSI was somewhat lower than in the complete-case analyses (812 vs. 1296); the point ICER for the GSES was slightly higher (5224 vs. 4470). The cost per QALY for participants with dementia was more than three times higher than in the base case; the cost per QALY for carers was twice as high as in the base case. The CEACs and cost-effectiveness plane plots were similar to the base case; however, the probability of cost-effectiveness across the £0–50,000 range was lower across all measures (see Appendix 17, Figures 30–37). Thus, although most of the results were similar to the complete-case analyses, the results for the BGSI attainment rating suggest that the intervention was more effective and no more costly.
Conclusions
There was no evidence of QALY gains for participants or carers, or of cost-effectiveness by reference to QALY gains over a range of WTP values. As assessed in the BGSI attainment rating, the CR intervention was cost-effective at WTP values of ≥ £2500 from the health and social care and societal perspectives. On the GSES, there was relatively little additional evidence of cost-effectiveness over a range of WTP values. The average cost of the CR intervention for participants over 9 months was £1736 (SE £25).
Strengths and weaknesses
The data on the care and support received by participants who finished the trial were well completed, with few items missing in most cases. The evaluation was able to collect detailed information on both the number and the duration of CR visits made to participants, and thereby the per-participant costs of the CR intervention could be estimated. Cost-effectiveness analytic methods have taken account of the correlation between the cost and outcome error terms and presented information on the cost of achieving improvements in outcomes in light of sampling uncertainty.
The analyses encountered some issues. Cost data in the complete-case sample were skewed and there were a number of extreme outliers in the case of health and social care costs. The analyses therefore combined a parametric approach (SUR models, which assume normality) with non-parametric bootstrap sampling, which makes no distributional assumptions. A large number of replications were necessary to produce more efficient bias-corrected SEs for the cost difference in the complete-case analyses. Other analytic models that permit a mixture of distributions for costs and effects could be investigated, for instance, gamma-normal or gamma-beta bivariate models, estimated using Bayesian techniques. 155 The 3-month costs in the interval between 3 and 6 months post baseline were assumed to be the same as the costs between 6 and 9 months post baseline, and the total costs could be overestimated if these were in fact more similar to costs over the initial 3 months post baseline or were rising over this interval.
Concerns with the validity of the EQ-5D86,156,157 to reflect the HRQoL of people with dementia led us to choose DEMQOL, a condition-specific measure, as an outcome for the economic evaluation. The authors of the instrument recommended using the EQ-5D alongside DEMQOL,86 as they considered that the instrument required further testing to better understand its psychometric properties. However, the EQ-5D was not included in the battery of questionnaires, and so whether or not using that instrument would have changed the assessment of the cost-effectiveness of the CR intervention cannot be assessed. Emerging evidence suggests that the EQ-5D and DEMQOL capture different aspects of changes in HRQoL over time. 158 In this study, it is possible that the DEMQOL domains (social relationships, loneliness, negative emotion, positive emotion) were better suited to the types of goals identified by participants (engaging in conversation, knowing what is happening, keeping track of information).
We did not examine the budget impact of introducing the intervention at a national level as part of the economic evaluation. An implementation study following on from the trial will combine the data from the original GREAT with observational data from at least 15 participating sites to model the impact of scaling up the intervention at local and national levels.
The BGSI is a relatively new instrument; no societal WTP threshold has been established for attaining an improvement in the BGSI attainment rating, such as that associated with a QALY gain in the NHS in the deliberations of the National Institute for Health and Care Excellence. 159
Implications
The results indicate that in terms of achieving an improvement in goal attainment as rated by the participant, the intervention is cost-effective from the health and social care and societal perspectives at WTP values of ≥ £2500. This achievement does not appear to have affected parallel improvements in participants’ sense of self-efficacy, or participants’ or carers’ QALYs (assessed by HRQoL and by quality of life in dementia, respectively) or to have reduced costs from the health and social care or societal perspectives. Considering the QALY results, there are a few possible reasons for the apparent lack of effect. The intervention was not expected to affect survival, so there was no reason to expect a difference between groups in the quantity of months lived. In addition, the goals that were chosen by each individual participant would vary depending on that individual’s circumstances and interests. The goals that were set might therefore be unrelated to the domains covered by DEMQOL; furthermore, goals that were set by the participant would not necessarily impinge upon domains covered by the EQ-5D, which was chosen to measure carer quality of life (e.g. the carer’s self-care and usual activities).
Funding decisions regarding CR programmes may not be limited to cost-effectiveness in terms of the cost per QALY gained by the participant or carer (by which measures the intervention was not cost-effective). There is also a need to consider the value of goal attainment – the primary focus of this trial – in the context of other information provided in this report (see the evidence on clinical effectiveness in Chapter 3). The costs of implementing CR in the trial included the costs of substantial supervision for the therapists. In routine clinical practice, these levels of supervision and support would be lower, thus reducing the costs of CR. In turn, the different level of supervision and support could affect the outcomes achieved, although we cannot examine that possibility with the data from the present trial. A follow-on implementation study of CR delivered at scale in routine practice will provide an opportunity to examine these possibilities.
Chapter 6 Discussion
GREAT72 has provided definitive evidence about whether individualised, goal-oriented CR is a clinically effective and cost-effective intervention for people with early-stage Alzheimer’s disease or vascular or mixed dementia and their carers. Based on both participant-reported and carer-reported outcome measures, the CR intervention was effective in improving functioning in the areas targeted in the therapy at the 3-month follow-up, and this improvement was maintained at the 9-month follow-up. Improvements met the criteria for clinical significance. Furthermore, participants in the CR group were more satisfied with their ability to carry out the everyday activities targeted in the intervention. There were no significant effects on secondary outcome measures for either participants with dementia or their carers. However, participants and carers who were interviewed in depth about their experience of the intervention described improved psychological adjustment to living with dementia and a more positive perspective, reflected in greater confidence, less anxiety, better coping, a sense of empowerment and better well-being and quality of life. CR was relatively inexpensive, given that it was individual, home-delivered, provided by skilled therapists and comprised up to 14 sessions, and costs would be lower without the trial-specific centralised supervision of therapists. CR was cost-effective from both the health and social care and societal perspectives at WTP values of ≥ £2500, in terms of achieving an improvement in participant-rated goal attainment.
We first consider these findings in relation to our objectives and hypotheses regarding clinical and cost-effectiveness, and in relation to other relevant literature.
Evidence on clinical effectiveness
Our main objective was to compare the clinical effectiveness of goal-oriented CR with that of TAU. Our first hypothesis was that this personalised intervention would improve functioning in areas directly targeted in the therapy sessions, and that this would be reflected in self and informant ratings. This hypothesis was supported by the quantitative data, with large effect sizes. The perceived improvements were also reflected in the interview responses. These clinically significant improvements indicate that the therapy was perceived as having enabled participants to manage their daily lives better, participate in meaningful activities and address personally relevant needs and goals.
Our second hypothesis was that the intervention might work through improving self-efficacy. This was not borne out by the quantitative data, as there were no differences in self-efficacy scores for the CR and TAU groups. However, qualitative interview data obtained from consecutive series of people completing the trial indicated that following intervention, participants and carers experienced greater confidence and felt that they coped better with the challenges of life with dementia. These descriptions reflect the essence of the self-efficacy construct. This suggests that the hypothesis, although not borne out by our quantitative data, remains worthy of further exploration.
Our third hypothesis was that carers of participants receiving the intervention, having learned new ways of supporting and enabling their relatives, might report feeling less stressed following the intervention. This again was not borne out by quantitative data, as there were no differences in carer scores for stress between the CR and TAU groups. However, as noted above, in the qualitative interviews, carers reported positive outcomes of the intervention and commented that it had helped them to be more understanding and patient in their interactions with the participant. CR does require some effort from carers, first to engage when they may feel that they have already tried various strategies without success and second to support the implementation of strategies through the therapy sessions, but most carers found the effort worthwhile. Therefore, although the specific hypothesis was not supported, it appears that carers did experience some benefits that were not captured by questionnaire measures.
Examination of the clinical effectiveness covered several additional secondary outcomes, which showed changes in the pilot trial. 63 The finding of no differences in cognitive test scores was unsurprising, as the intervention does not directly seek to improve cognitive function. The finding of no differences in scores for depression or anxiety is understandable, as only a small proportion of participants reported clinical levels of depression or anxiety at baseline, and therefore there was little scope for the intervention to demonstrate improvements in these domains. At the same time, it provides confidence that the intervention did no harm. There were also no differences between the groups in quality-of-life scores for either participants with dementia or carers.
Relating the findings on clinical effectiveness to other literature
We updated the Cochrane review covering CR during the course of the trial. 15 At this time, there were no further RCTs of CR, correctly defined, that could be included, and our 2010 pilot63 trial remained the only available trial. At present, we are preparing to update the review again, and to this end a new systematic literature search using the search terms and methods outlined in the published review was conducted by the Cochrane Dementia and Cognitive Improvement Group co-ordinating centre on 9 May 2017. We used the results of this search to check that the three potentially relevant trials published since 2013 of which we were already aware appeared in the output, and to identify any additional potentially relevant trials, of which we found one, published in 2017. Of these four trials, one described what appeared to be a CR intervention, although it is also described as ‘training’,160,161 one described a mixed CR and CT intervention162 and two described the structured training of activities of daily living; one of these variously described the intervention as ‘cognitive rehabilitation’ or ‘cognitive training’,163 whereas the other described the intervention as ‘structured relearning’ or ‘training’. 164
The most important of these trials for present purposes is the ETNA3 (Évaluation de 3 Thérapies Non médicamenteuses dans la maladie d’Alzheimer trial,160,161 a large trial (n = 653 participants) conducted in France, which compared individual CR with group CT, group reminiscence and usual treatment for people with mild to moderate Alzheimer’s disease (MMSE score of 16–26 points). Participants in the CR group received individual 90-minute sessions weekly for 3 months and then 6-weekly for the next 21 months, and their carers received telephone support weekly for 3 months and 6-weekly for the next 21 months. This equates to approximately 28 90-minute sessions of CR for participants and 28 sessions of telephone support for carers. The CT and reminiscence conditions received group sessions weekly for 3 months and 6-weekly for 21 months, whereas their carers participated in parallel-group psychoeducation sessions.
The CR intervention in ETNA3, which was delivered by psychologists, was not well described. Furthermore, an unspecified proportion of participants in this group did not receive CR, but instead followed an individualised reminiscence programme; the reason for this is unclear. For those who did receive CR, the goals involved either improving an activity of daily living or maintaining a leisure activity and had to be personally meaningful, although it is not clear how this was defined or in what way participants and carers were involved in choosing goals. The goals to be addressed were defined in the first two sessions and could be changed by the treating psychologist at any time. The approach used to address goals is described as ‘training’ of particular ‘activities’, without any further explanation, so it is possible that the intervention was closer to the structured training approaches described below than to the approach used in GREAT.
The design of the CR intervention in ETNA3 appears to have drawn on some of our early feasibility studies, which are cited, but the authors do not acknowledge our pilot trial, even though they cite the updated Cochrane review,15 in which the pilot trial is discussed. In their 2013 paper,160 they incorrectly stated that there were no published RCTs of CR, and in their 2015 paper they modified this to state that there is ‘no large RCT’. Some of our early feasibility studies focused on the possible benefits of errorless learning techniques, building on work on this topic in the brain injury field, and these techniques were used in ETNA3,161 in which ‘the psychologist could rely on “errorless learning procedure” to train a particular activity’. However, by 2008 we had accumulated evidence ourselves and synthesised other available evidence, showing, first, that people with early-stage Alzheimer’s disease appear to learn equally well with errorless and trial-and-error methods and, second, that even for groups for which it does convey benefits, errorless learning is more suited to some types of task than others. 59,60 Interestingly, the most recent of the four trials identified164 also failed to take note of this evidence; the REDALI-DEM (RElearning methods on DAily LIving task performance of persons with DEMentia) trial164 assigned participants to structured relearning of activities of daily living using either errorless or trial-and-error instructional methods, and found no differences between the two groups, indicating, as we would expect, that the two types of learning strategy were equally effective.
The primary outcome in ETNA3 was survival without moderately severe to severe dementia at 2 years. This appears to suggest that the three psychological therapies were being evaluated as disease-modifying treatments expected to alter the trajectory of decline. This is an unusual and probably unrealistic expectation for interventions of this kind. Not surprisingly, in ETNA3 none of the three treatments was effective in relation to this outcome. In relation to the kinds of outcomes more typically evaluated in trials of these kinds of interventions, evidence was already available in 2003 to show that CT is not effective in short- to medium-term outcomes for people with dementia,14 and a recent definitive trial of group reminiscence failed to show any benefits in primary or secondary outcomes compared with usual treatment. 165 These findings were supported by ETNA3, as neither CT nor reminiscence yielded any benefits over usual care. With regard to CR, there was no direct assessment of functional outcomes for the CR group, and no information is provided about whether or not goals were addressed successfully or if any changes in behaviour or perceptions of behaviour were seen as a result of the intervention. However, in secondary outcomes, CR was the only one of the three interventions to show any benefits relative to usual care. Participants in the CR group had lower functional decline at 24 months, measured on the Disability Assessment for Dementia,166 a 6-month delay in institutionalisation compared with the usual-treatment group and lower rates of institutionalisation than all other conditions. There were no other significant differences in secondary outcomes, but the authors noted trends in neuropsychiatric symptoms, caregiver burden and service utilisation. The authors concluded that cognition-focused group interventions are not effective and that individualised CR interventions should be used to delay institutionalisation for people with mild to moderate Alzheimer’s disease.
Another trial combined CR with CT exercises and cognitive strategy training. Kim162 reported a small trial (n = 43 participants) conducted in South Korea, in which participants with early-stage Alzheimer’s disease (MMSE score of ≥ 18 points) were randomised to receive eight weekly sessions of either CR (n = 22) or an active control condition (n = 21) consisting of group meetings with structured conversation and health-related videos. The CR sessions consisted of 30-minute group CT exercises and cognitive strategy training and 30-minute individual CR, focused on addressing a personally meaningful goal. The primary outcome was goal performance and satisfaction rated on the COPM. 38 The CR group improved significantly in goal performance and satisfaction, and quality-of-life scores also improved, whereas participants in the control group did not show any changes. However, this was a small trial yielding evidence of limited quality; analyses were restricted to separate pre–post comparisons for each group using t-tests, so the results should be interpreted with caution.
In the other two trials identified, by Voigt-Radloff et al. 164 and Thivierge et al. ,163 the intervention involved structured training of activities of daily living.
Voigt-Radloff et al. 164 report an adequately powered trial (n = 161 participants) conducted in Germany, in which participants were randomly assigned to receive nine 1-hour sessions in which everyday tasks were trained through either errorless (n = 81) or trial-and-error (n = 80) instructional methods. Up to three sessions were devoted to choosing the tasks to work on, which were selected from a list of 43, covering household tasks, leisure activities and cognitively challenging tasks. Task performance was assessed with ratings based on observation. Performance improved in both groups with no differences between the two instructional methods. There were no changes in secondary outcomes for either group. As noted above, this trial failed to take note of available evidence, indicating that both types of learning strategy would be expected to be equally effective. 60
Thivierge et al. 163 report a small trial (n = 20 participants) conducted in Canada, using a block-randomisation wait-list control design. This tested an intervention that was variously described as ‘cognitive rehabilitation’ and ‘cognitive training’, but appeared to involve structured training of instrumental activities of daily living (IADLs). In this study, following assessment to identify problematic IADLs, participants and carers selected one to be the focus of training. Participants were trained in the chosen activity twice a week for 4 weeks, using errorless learning and expanding rehearsal methods, and they practised between sessions. Observational assessment indicated that task performance in the trained group improved and these gains were maintained at 3 months, but the groups did not differ on any other outcome measures on completion of the training or 3 months later.
These four trials taken together all focus on functional ability. However, they reflect two distinct approaches: one is goal-oriented CR addressing functioning in real-life contexts and the other is a form of CT involving structured training in and practice of everyday tasks. The appearance of a form of CT applied to everyday functional activities suggests that the concept of CT, which describes practice on abstract cognitive tasks completed with pencil and paper or via a computer, is being adapted for people with dementia to address the kinds of functional activities undertaken in everyday life. However, structured training does not address issues of transfer and generalisation of learning to the real-life setting,163,164 and the learning may never be integrated into or used in daily life. The focus on relevant everyday tasks in these studies is very positive, but the potential difficulty with generalisation and integration into daily life precisely illustrates the reason why we took a different approach in GREAT and focused on improving functioning in the real-life setting with the activities participants were actually undertaking.
In terms of effectiveness, the results from these trials confirm that the interventions produce improvements in the specific areas targeted and clearly demonstrate that it is possible to improve functional ability for people with mild to moderate dementia. However, with the exception of the functional disability measure in ETNA3, the interventions have no effect on scores on secondary outcome measures, such as quality of life or carer burden. This is consistent with our findings in GREAT. One possibility is that changes in everyday functioning or the ability to carry out specific activities, although very important in their own right and potentially very beneficial, simply are not associated with appraisals of quality of life or carer stress. The quality of life of people with dementia, for example, is associated to a small degree with many different factors, and changes in one area may have little impact overall. 167 Another possibility is that changes in functional ability do have effects in other areas of life, but we do not have outcome measures that are sensitive to these changes. This is suggested by the responses identified from in-depth interviews with GREAT participants and carers. These interviews were undertaken and analysed by researchers otherwise independent of the trial, to reduce the risk of positive response bias, and it seems unlikely that participants and carers would make strong statements about feeling more confident and better able to cope if they did not genuinely feel some benefit in these areas alongside their observations of improved functional ability.
Evidence on cost-effectiveness
Our second objective was to evaluate the cost–utility and cost-effectiveness of goal-oriented CR compared with TAU.
There was no evidence for cost–utility in terms of QALY gains (using DEMQOL-U for people with dementia or the EQ-5D-3L for carers) from either the health and social care system or the societal cost perspective. By reference to the improvement of functioning in areas directly targeted in therapy, which was the primary clinical outcome for the trial, CR could be cost-effective from the health and social care and societal perspectives, depending on decision-makers’ WTP for these gains in participant-rated goal attainment. These improvements associated with CR were perceived by participants as enabling them to achieve a number of goals that were personally relevant to them. These included better management of their daily lives and participation in activities that were meaningful to them. Assessed against this effectiveness measure, CR intervention is cost-effective at WTP values of ≥ £2500 for a SMD of 1.32 on the BGSI scale.
The economic evaluation had some strengths and weaknesses, over and above those discussed for the overall trial below. Data completion was good in relation to care and support, and there was detailed information on the number and duration of CR visits per participant, in contrast to some other studies (e.g. Amieva et al. 161). The cost-effectiveness analyses took account of the correlation between cost and outcome error terms, and sampling uncertainty. The cost data were highly skewed, which is very common in dementia studies, and statistical methods were used to address the issue, although there were a number of extreme outliers in health and social care costs, requiring a large number of replications in the non-parametric bootstrap analyses. Because the BGSI is a new instrument, there are no established WTP thresholds to guide the economic evaluation.
No previous studies of CR for people with dementia have looked at cost-effectiveness. There are some studies of cognitive remediation for other groups, such as people with schizophrenia,168 that have included cost-effectiveness evaluations, but, although they might offer some methodological pointers, their findings are not especially relevant to the interpretation of the findings from GREAT.
From an economics standpoint, the most relevant previous study of CR for people with dementia is the ETNA3 trial. 161 Despite a number of design differences from GREAT, there are some similarities in outcome findings between the two studies. The ETNA3 study found that CR was associated with a significant delay in institutionalisation over a 24-month follow-up period. We did not follow up participants for the same duration of time in GREAT, and, although the health and social care systems differ between France (where the ETNA3 trial was conducted) and the UK, this finding of delayed institutionalisation could suggest that some similar longer-term economic gains might be achieved with CR in the UK, even though no differences between the CR and TAU groups were observed in health care, social care or other costs over the 9-month study period in GREAT.
It is important to consider what outcome domains are important for different decision-makers. For people with dementia, and indeed also their carers, improvements in personally defined goal attainment is fundamentally important, and it is therefore clearly relevant that CR is found to be cost-effective by reference to that outcome. For commissioning purposes, however, we did not find that CR is cost-effective when gauged against QALY gains for either participants with dementia or their carers. It would appear that the attainment of personally set goals did not bring about changes in those domains that are measured in the dementia-specific HRQoL measure (DEMQOL), and it did not improve carer HRQoL (measured using the EQ-5D).
The average cost per participant for the CR intervention was £1736. In routine clinical practice, the levels of supervision and support would be lower than in the trial, which would reduce the cost of CR below that observed in an experimental context. It might, however, also alter the outcomes achieved from CR. The next steps will be to examine the effectiveness and cost of CR implementation in routine practice.
Implications for future implementation
Our final objective in GREAT was to examine how the goal-oriented CR approach could most effectively be integrated into routine NHS provision, to develop a pragmatic approach that could be directly applied within standard NHS services and to develop materials to support the implementation of this approach within the NHS following trial completion.
The feasibility pilot work undertaken in the later stages of GREAT demonstrated the potential for CR to be integrated into NHS provision and showed that improvements in goal attainment comparable to those seen in the main trial can be achieved, even using a pragmatic approach involving fewer sessions delivered by less qualified staff under local supervision. The experience gained, together with the results of supplementary and process evaluation analyses, highlights a number of issues relevant for future implementation efforts.
Goal-setting
Goals identified by participants and addressed in therapy reflected the multiple ways in which cognitive impairment has an impact on everyday life for people with mild to moderate dementia. Some participants used goal-setting as a means to promote engagement in activities. Other aspects of the need for engagement were reflected in goals focusing on keeping in contact with family and friends and engaging in conversation. Some participants focused on managing everyday tasks and being able to use household appliances or devices, such as mobile phones, to help conduct their daily lives and to occupy and entertain themselves. A small number of goals focused on basic aspects of self-care, such as washing and dressing. Many goals reflected the challenges of living with memory difficulties; participants wanted to be well oriented, organised and better able to retain or keep track of information and events, locate belongings and recognise, identify and name people and objects. Managing emotions was an issue for a handful of participants.
Furthermore, a more detailed analysis of the goals participants chose will help to further develop the therapy, especially when goals can be cross-referenced with therapy logs to illustrate the different ways in which cognitive impairment affected functional ability, the problems that therapists identified and the strategies therapists used to overcome or offer solutions for these problems and help participants to achieve their goals. This will provide valuable information to support wider implementation.
It is important to note that some participants who initially expressed interest in GREAT and were assessed at baseline did not proceed to randomisation, because they felt content with their current situation and were unable to identify any areas of need in which they could formulate goals. CR does require active engagement and hence will not be appropriate for everyone. However, it is possible that carers in these cases had a different perspective and may have benefited from support in solving problems and developing strategies. In clinical practice, there may be opportunities to work directly with carers under these circumstances, even if the person with dementia does not wish to engage.
Perceptions of participants and carers
The low attrition rates and good adherence rates, together with the positive evaluations recorded in the qualitative interview data, suggest that the therapy was very acceptable to those participants and carers who opted to take part. In addition to the specific focus on improving everyday functioning and the opportunity to develop new or more effective strategies, they valued the person-centred approach, the relationship they developed with the therapist and the support this provided. The strong emphasis on the therapeutic relationship as the vehicle for change is instructive in terms of future implementation. Some people with dementia and their carers may be able to make use of information about the approach and strategies they could use to engage in self-management, but most are likely to require input from a therapist who can build trust and provide support in finding solutions to everyday challenges.
The qualitative data revealed some areas in which participant and carer perceptions might have been managed better. First, a few people were disappointed that, although the intervention improved functioning, it did not improve memory. CR was not presented as an intervention to improve memory or cognition per se, and indeed this is something that would have been made explicit from the start. Possibly this was not sufficiently explained in some cases, or perhaps it may have needed to be emphasised more throughout the therapy. Second, a few carers wondered if it was worthwhile intervening, given that dementia would progress anyway. This is a completely understandable reaction from carers who are facing, and perhaps grieving over, the gradual decline they observe in their relative, but also suggests that the aims of rehabilitation and what it can realistically achieve may have needed more explanation in some cases in order to convince carers of the value of optimising functioning and reducing excess disability at any stage of dementia. It will be important to ensure that these messages are conveyed effectively in any future implementation, so that people with dementia and their carers have realistic expectations about what CR can help them to achieve.
Challenges in delivering the intervention
Therapists were able to deliver the intervention in line with the protocol. They could successfully engage participants and carers, explain the rationale for the CR approach and conduct individually tailored interventions, addressing goals that participants and carers identified as relevant and meaningful. Therapists in GREAT faced the challenge of working with goals that had been set prior to their first meeting with the participant and carer as part of the baseline assessment. In clinical practice, and hence also in any future implementation, goals would typically be negotiated during the initial sessions with the therapist. Therapists noted the importance of being able to explain the therapy approach and the kinds of strategies used in a way that was accessible to participants and carers.
Therapists were expected to, and did, draw on their wider clinical skills and experience by developing good therapeutic relationships, managing relationship conflict between participants and carers when this emerged during sessions, providing information and, when necessary, making onward referrals, for example to social services. These non-specific elements of the therapy need consideration in preparing therapists to work in this way in any future implementation, especially when CR is delivered by less qualified staff, and need to be taken into account in training and in providing appropriate supervision arrangements.
Identifying who is most likely to benefit
Rehabilitation focuses on ‘doing with’ rather than ‘doing for’ or ‘doing to’. 21 For people in the early stages of dementia, CR requires some degree of active engagement. This implies that participants need to be able to identify something that they would like to change, improve or manage better. It is not necessary for them to acknowledge a specific dementia diagnosis or even the full range of difficulties they may be experiencing, but there needs to be something that they want to work on. This is not the case for everyone. We have conducted detailed investigations of the awareness of difficulties among people with dementia; these show that a small proportion of people with dementia are unwilling or unable to acknowledge any difficulties169 and a larger proportion underestimate the impact of memory problems,47 although there is some evidence that people with early-stage dementia are more accurate in estimating their own functional ability than their carers. 48 Even when difficulties are acknowledged, people may have reached an acceptance of these or may not wish to make the effort required to bring about changes. Approximately 5% of the people with dementia assessed at baseline said that they were content with their situation and could not identify any areas in which they felt changes were needed, and hence did not proceed to randomisation. Participants joining GREAT were able to identify areas for improvement and to evidence some motivation for change. Nevertheless, goal-setting could be challenging for some individuals, who required more time and support to identify suitable goals. As noted above, although in the trial goals were set as part of the initial assessment by the researcher, in usual practice, goals would be negotiated by the therapist and could evolve over a number of therapy sessions, making this process more accessible.
Exploratory statistical analyses revealed few predictors to indicate those participants who were likely to show the greatest gains in goal attainment. Ratings of readiness to change at baseline and the number of sessions completed were associated with greater gains. At 3 months, participants from professional occupational backgrounds had better outcomes than those from other occupational groups according to both participant ratings and carer ratings. At 9 months, the MMSE score was predictive, and participants with MMSE scores of ≥ 24 points had better outcomes according to both participant ratings and carer ratings than those with MMSE scores of ≤ 23 points, whereas participants aged < 75 years did better than those aged ≥ 75 years according to participant ratings only. Centre, sex, diagnosis, medication use and the presence of comorbid conditions were not linked to outcomes.
Data from the therapy logs analysis supplement these findings and suggest that participants with the best outcomes tended to have relatively good cognitive and functional ability, to be socially and physically active and to be highly motivated. These participants were likely to acknowledge their difficulties and express anxiety about the impact of these, and to focus on challenging goals relating to IADLs or increasing engagement in social or leisure activities, and progress was evident early in the course of therapy. Participants who made the least progress were likely to have more extensive cognitive and functional difficulties and lower motivation, and were less likely to acknowledge difficulties or express anxiety about them. For these participants, goals were more likely to relate to basic activities of daily living, and progress followed a slower trajectory.
In GREAT, participants had to have a carer who was willing to contribute, so that we could obtain collateral information, such as carer ratings of goal attainment. However, in naturalistic settings, people with dementia may not have a carer or may have a carer who is not able to support the intervention. This would not preclude them from engaging in CR, but it would be an important factor for the therapist to consider when planning the intervention.
Differing levels and types of need
In discussing their work in the focus group, the therapists seemed to distinguish different groups of participants based on levels of cognitive and functional ability and on time since diagnosis. These different groups were perceived to have different needs and to respond differently to the intervention. Participants who had received a dementia diagnosis in the previous few months, and their carers, were in the process of adjusting to dementia and were actively seeking strategies and solutions to help to manage their everyday lives. Those who had been living with dementia for longer tended to have adapted to living with the condition and to be less motivated to make changes, and in some cases carers felt that they had already tried various strategies without much success. Although participants in this latter group also benefited, working with these participants presented more of a challenge for the therapists.
This provides valuable guidance on how to target the CR intervention in future implementation. Participants in GREAT had mild to moderate dementia, but this broad grouping encompasses a wide range of cognitive and functional ability. In terms of UK NHS care pathways, it spans both the cluster 18 cognitive impairment (low need) and cluster 19 cognitive impairment (moderate need) care clusters. The principles of CR can be applied in different ways to optimise functioning, depending on need, and somewhat different approaches are likely to be required for different groups. The protocol used in GREAT, although applied effectively with both groups, appears to have been seen by therapists as more suitable for ‘cluster 18’ participants.
Cognitive rehabilitation may be a particularly valuable approach for people in the months following a dementia diagnosis and could represent an important component of post-diagnostic support within the first year of being diagnosed. For this group, the aim would be to support people in remaining active and engaged, maintaining confidence and developing a range of strategies to support practical and emotional coping. An additional element for some, although not attempted in GREAT, might be vocational rehabilitation to support continuation in employment or transition to less demanding employment or voluntary roles.
For people who have been living with dementia for longer, the aims may be more about maintaining basic skills of daily living, limiting the effects of excess disability, encouraging people to remain socially engaged and ensuring opportunities to participate in pleasurable activities. As these individuals may find it harder to identify goals, carers are likely to be more involved in the goal selection process. An additional element for some might be the use of CR to support people with dementia who are returning home after a period of hospitalisation caused by illness or injury, or who have additional physical or mental health conditions.
This highlights the need for flexibility in applying the principles of CR to support optimal functioning for people with dementia. Although GREAT followed a structured protocol, CR itself is not a fixed intervention and can be adapted to different contexts to meet a variety of needs. 3 Although the number of sessions was fixed for the trial, in practice the duration of the intervention could vary and could be tailored to participants’ needs. Some individuals may need only one or a few sessions to address specific issues, whereas others may need longer periods of support to develop and implement strategies, or perhaps to regain function after a period of illness or hospitalisation. Some may benefit from several short episodes of CR as their needs change over time. In the pilot work on implementation that we conducted in NHS sites, we trialled shorter, 6- and 8-session protocols as a starting point. However, experience with GREAT suggests that the group of people defined as having early-stage Alzheimer’s or vascular or mixed dementia could be further subdivided into two broad groups reflecting differing degrees of dementia progression. In future implementation, it will be important to prepare CR intervention protocols that meet the needs of each of these groups, to identify optimal timing and duration, and to explore outcomes accordingly.
Limitations
Some limitations relating to trial design, outcome measures and participant inclusion criteria must be acknowledged.
Trial design
This was a pragmatic trial comparing CR with TAU. This design did not provide a means of controlling for the time and attention provided by the therapist, raising questions about whether treatment gains are specific to the therapy or non-specific. In this instance, however, the gains observed related specifically to the effects of CR and were demonstrated in improvements in goal attainment for goals directly targeted in the therapy. It is unlikely that these could be attributed to non-specific effects of the intervention. Furthermore, in the pilot trial, CR demonstrated effectiveness when compared with an active control condition, relaxation therapy, with an equivalent number of therapist visits as well as when compared with TAU.
In trials of behavioural interventions that involve active participation and engagement, it is not possible for participants to remain blind to their group allocation, especially when, as in this case, the intervention is compared with TAU, and this creates the potential for bias in responding on self-reported outcomes. The inclusion of parallel carer ratings may go some way towards mitigating this concern. Furthermore, if bias were present, we would expect to see it on all self-reported measures, and not just the ratings of goal attainment.
All possible precautions were taken to ensure that researchers collecting follow-up data remained blind to participants’ group allocation. However, effective blinding is extremely difficult to achieve in trials of psychosocial interventions that do not include an active control condition. Despite all precautions taken, researchers conducting assessments are likely to surmise whether or not the participant received the intervention, as was the case in GREAT. It could possibly be argued that the statistically significant association of blinding inefficiency with greater improvement in BGSI ratings at follow-up could reflect researcher bias. However, it is more plausible to consider that the ability of the researchers to correctly identify which participants had received the intervention reflects the close relationship between the CR therapy and the outcome measure. The BGSI provides a proximal measure of treatment outcome that directly assesses whether or not the intervention addressed its intended targets. Participants who received CR would be expected to be more engaged with the personal goals identified and to remember them better. These participants would also have made ratings of progress with these goals in sessions with the therapist. Therefore, it is likely that if a participant had engaged well with the CR intervention and derived benefit from working on the identified goals, this would have been evident when making the BGSI ratings in the follow-up assessment, and consequently the researcher would have been able to correctly identify the participant’s group allocation. Conversely, if it was not evident to the researcher that a participant who received CR was allocated in this way, it is likely that the participant was less engaged with the goals identified in the BGSI and that the treatment was less effective.
Outcome measures
Primary and secondary outcomes in GREAT were evaluated mainly through participant-reported outcome measures, with collateral information from carers. This was a positive choice, aiming to foreground the perspective of people with dementia and carers. It was also a practical choice, because participant numbers and the focus on behavioural change in real-life situations precluded observational assessment as a means of evaluating therapy outcomes, and because, as CR was not expected to be a disease-modifying treatment, there was no particular justification for the use of physiological markers. The pilot trial63 included a neuroimaging component, which examined whether or not participation in therapy was associated with changes in particular brain areas seen on functional MRI; the findings were difficult to interpret, especially as only 28% of participants were able or willing to undergo MRI scanning, but some differences in activation patterns between CR and control participants were found following intervention, suggesting that the intervention produced a degree of restoration of function in frontal brain areas. 66 Physiological markers, therefore, may have some relevance, but the crucial question is a behavioural one: whether or not we can improve functioning in everyday life to enable people with dementia and carers to manage everyday life more effectively and with greater satisfaction. For the present trial, therefore, the focus was on behavioural change and on the perceptions of participants and carers.
The primary outcome measure, the BGSI, proved sensitive to changes resulting from the intervention. This used a valid and reliable rating method and participant responses were corroborated by the independent carer ratings. Ratings were collected by a blinded researcher not involved in the therapy, and given that scores on secondary outcome measures showed no changes, it is unlikely that changes in BGSI ratings in the CR group reflected a positive response bias. As discussed above, it is possible that secondary outcome measures were not sensitive to other changes resulting from the therapy. One limitation in outcome measurement is the lack of inclusion of a measure of functional ability. We did not have scope to include a long-term follow-up to assess whether or not the intervention had any impact on rates of institutionalisation. In future trials, this is more likely to be feasible through the use of routine data and data linkage, but this option was not available to us when designing GREAT.
Participant selection
Because of the need for collateral information, we excluded any potential participants who did not have a carer available to contribute. This will have encompassed those who had a carer who was unwilling to contribute and those who had no carer involved and were perhaps living alone. People with dementia in either of these groups may be most in need of psychosocial support, and in clinical practice it would be important to be able to offer CR in these circumstances. Consideration would need to be given to the best way of providing CR when there is no carer involved; this could include options, such as working with volunteers or incorporating between-session contact with the therapist, as a means of supporting practice with targeted activities.
Participation was restricted to people with Alzheimer’s disease or vascular or mixed dementia. Although this accounts for the majority of dementia diagnoses,74 people with other forms of dementia also have needs that could be addressed through CR. Although GREAT was in progress, we undertook a pilot trial with people who have either Parkinson’s disease dementia or dementia with Lewy bodies. 170,171 This pilot trial replicated the methods used in our pilot trial of CR63 and yielded similar results. This indicates that CR, in a similar form to that used in GREAT, is feasible and potentially effective for people with dementias associated with Parkinson’s disease, and provides justification for a larger trial. 172 There is scope for future exploration of the adaptation of CR for people with other rare dementias.
Strengths
Alongside these limitations, it is important to bear in mind the strengths of the trial. It included a diverse group of 474 people living with early-stage dementia and their carers, and attrition was low. The trial was conducted in eight regions of England and Wales, encompassing both urban and rural contexts, with consistent results across the eight centres. The intervention targeted real-life situations and aimed to improve participants’ functioning in areas that were meaningful to them and that would make a difference in their daily lives, avoiding any problems relating to lack of transfer or generalisation of effects. The primary outcome measure was a proximal measure of outcome, directly evaluating perceptions of change in the areas targeted in the intervention, and demonstrated that the intervention led to improvements in ratings of goal attainment, with large effect sizes.
Next steps
Building on the findings from GREAT, we will explore further the potential for implementation in health and social care services offering support to people living with dementia in the early stages, with similar profiles to those involved in the trial. We have secured implementation grant funding, which will enable us to begin this process, starting in 2018. To support the implementation, we will develop materials, resources and training programmes. We will work with a number of partner organisations, including NHS Trusts, local authority and non-profit social-care providers to identify implementation plans, facilitate adoption of the approach into routine practice and evaluate clinical and economic outcomes. We also aim to explore further the effectiveness of CR for people with dementias associated with Parkinson’s disease and the possible adaptation of this approach for people with other rare forms of dementia.
Conclusions
GREAT has demonstrated that individual, goal-oriented CR is clinically effective in enabling people with early-stage Alzheimer’s disease or vascular or mixed dementia to improve their everyday functioning in relation to individual goals targeted in the therapy. This approach can facilitate the process of adjustment to living with dementia and increase confidence in managing the challenges dementia brings. The trial adds further evidence to support the view that individualised interventions that can be tailored to the particular current needs of people with dementia and their carers, and address these in a real-life context, offer important benefits. CR represents an important contribution to improving the choice of interventions available to support people living with early-stage dementia and addressing the ‘psychosocial intervention gap’. 1,2
Acknowledgements
We thank all the participants with dementia and their family supporters who generously contributed their time to be part of this study. We also thank the Alzheimer’s Society for supporting the study, and in particular we are grateful to Dr James Pickett and Malayka Rahman-Amin for their helpful advice.
We would like to acknowledge the key roles in the research played by the following people:
-
Trial Therapists – Jenny Aindow, Eleanor Besso, Sue Evans, Jane Green, Myles Howard, Jane Hughes, Ruth Johns, Gayl Keddie, Pippa Page and Alexis Tranah
-
Trial Researchers – Stephanie Barningham, Jasmine Blane, Claire Carter, Helen Cunnane, Dr Clare Freestone, Sophia Gerbase, India Hart, Dr Catherine Lawrence, James Middleton, Dr Jadwiga Nazimek, Emily Shah, Zoe Simkin, Nadezda Starkova, Olana Tansley-Hancock and Joanna Waring
-
Local Team Leaders – Andrew Hamilton, Amy Hammond and Angela Parker
-
NWORTH CTU Team Members – Statisticians Yvonne Sylvestre and Dr Lou Zhou, IT Specialists Dr David Hunnisett and Lexi Bastable, Quality Assurance and Compliance Officer Debbie Skelhorn and Trials Unit Managers Dr Huw Roberts and Jean Ryan
-
Qualitative Interviewers – Ho Yin Chan, Matthew Lewis and Julie Nixon
-
Qualitative Data Analysts – Dr Gill Toms and Dr Krystal Warmoth
-
Support for Data Analysis and Reporting – Dr Rachel Collins.
We are grateful for the support of the trial development and governance from the following people:
-
TSC members – Professor Ian Robertson (Chairperson), Professor Ian Anderson, Hefin Francis (sponsor’s representative, Bangor University), Dave Hanbury (expert by experience), Professor Narinder Kapur, Sue Lawrence (expert by experience), Victoria Morgan (expert by experience) and Gail Seymour (sponsor’s representative, University of Exeter)
-
Data Monitoring and Ethics Committee members – Professor Stephen Walters (Chairperson), Dr Thomas Chadwick, Dr Lesley Collier
-
NIHR Health Technology Assessment research managers – Simon Bevan and Zoe Hurworth.
We acknowledge the support of the participating universities: University of Exeter (co-ordinating centre from 1 March 2015), Bangor University (co-ordinating centre to 28 February 2015), University of Bradford, Cardiff University, King’s College London, London School of Economics and Political Science and University of Manchester. We also acknowledge the support of our partner organisation Dementia Pal Ltd (Southampton, UK).
We acknowledge the support of the NHS organisations that hosted the trial and funded the work of the therapists by contributing NHS treatment costs: Betsi Cadwaladr University Health Board, Cardiff and Vale University Health Board and the National Institute for Social Care and Health Research Wales (now Health and Care Research Wales), Birmingham and Solihull Mental Health Foundation Trust, Kent and Medway NHS and Social Care Partnership Trust, Manchester Mental Health and Social Care Trust, Northumberland, Tyne and Wear NHS Foundation Trust, RICE, NHS Bath and North East Somerset CCG and the South London and Maudsley NHS Foundation Trust.
We thank the following NHS organisations that supported recruitment by serving as participant identification centres for the trial: Black Country Partnership NHS Foundation Trust; Dudley and Walsall Mental Health Partnership NHS Trust; Guy’s and St Thomas’ NHS Foundation Trust; Heart of England NHS Foundation Trust; King’s College Hospital NHS Foundation Trust; Oxleas NHS Foundation Trust; Pennine Care NHS Foundation Trust; Royal United Hospitals Bath NHS Foundation Trust and general practices in Wiltshire; and St George’s Healthcare NHS Trust.
We are grateful for the support with participant recruitment from clinical research networks in England and Wales: NIHR Clinical Research Network and National Institute for Social Care and Health Research Wales (now Health and Care Research Wales).
Contributions of authors
Professor Linda Clare (Professor of Clinical Psychology of Ageing and Dementia, University of Exeter, Exeter, UK) was the Chief Investigator. Professor Clare developed the intervention, provided overall leadership for the trial, contributed to researcher supervision and drafted the report.
Dr Aleksandra Kudlicka (Trial Manager, University of Exeter, Exeter, UK) provided operational management for the trial.
Professor Jan R Oyebode (Professor of Dementia Care, University of Bradford, Bradford, UK) was the PI for the West Midlands site and provided project leadership at the site, supervised the day-to-day work of the researchers and therapists and contributed to researcher supervision and trial management.
Professor Roy W Jones (Consultant Geriatrician and Director of RICE, Bath, UK) was the PI for the South West site and provided project leadership at the site, supervised the day-to-day work of the researchers and therapists and contributed to trial management.
Professor Antony Bayer (Professor of Geriatric Medicine, Cardiff University, Cardiff, UK) was the PI for the South Wales site and provided project leadership at the site, supervised the day-to-day work of the researchers and the therapist and contributed to trial management.
Dr Iracema Leroi (Clinical Senior Lecturer and Honorary Consultant Psychiatrist, University of Manchester, Manchester, UK) was the PI for the North West site and provided project leadership at the site, supervised the day-to-day work of the researcher and therapists and contributed to trial management.
Professor Michael Kopelman (Professor of Neuropsychiatry, King’s College London, London, UK) was the PI for the London site and provided project leadership at the site, supervised the day-to-day work of the researchers and therapist and contributed to trial management.
Dr Ian A James (Consultant Clinical Psychologist and Head of Newcastle Psychology and Challenging Behaviour Teams, Northumberland Tyne and Wear NHS Foundation Trust, Newcastle upon Tyne, UK) was the PI for the North East site and provided project leadership at the site, supervised the day-to-day work of the researchers and therapists and contributed to trial management.
Dr Alison Culverwell (Consultant Clinical Psychologist and Research Lead for Older Peoples Service Line Research Lead, Kent and Medway NHS and Social Care Partnership Trust, Maidstone, UK) was the PI for the South East site and provided project leadership at the site, supervised the day-to-day work of the researchers and therapist and contributed to trial management.
Mrs Jackie Pool (Occupational Therapist and Director of Dementia Pal Ltd, Southampton, UK) provided expertise in applying CR in dementia care, led on therapist training, supervised the trial therapists and contributed to researcher supervision and trial management.
Dr Andrew Brand (Trial Statistician, NWORTH, Bangor University, Bangor, UK) conducted the analysis of quantitative data.
Dr Catherine Henderson (Assistant Professorial Research Fellow, London School of Economics and Political Science, London, UK) conducted the health economic analyses.
Dr Zoe Hoare (Principal Trial Statistician, NWORTH, Bangor University, Bangor, UK) oversaw the analysis of quantitative data.
Professor Martin Knapp (Professor of Social Policy and Director of London School of Economics Health and Social Care, London School of Economics, London, UK) led on the health economic evaluation and contributed to trial management.
Dr Sarah Morgan-Trimmer (Research Fellow, University of Exeter, Exeter, UK) contributed to the process evaluation analyses and oversaw the analysis of the qualitative data.
Professor Alistair Burns (Professor of Old Age Psychiatry and Honorary Consultant Old Age Psychiatrist, University of Manchester, Manchester, UK) contributed expertise in dementia research.
Dr Anne Corbett (Senior Lecturer in Dementia Research, University of Exeter, Exeter, UK) contributed expertise on patient and public involvement.
Mrs Rhiannon Whitaker (Statistical and Methodological Consultant, Whitaker Research Ltd, Bangor, Bangor, UK) contributed to the study design and set-up while acting as Associate Scientific Director, NWORTH CTU (until 30 September 2014).
Professor Bob Woods [Professor of the Clinical Psychology of Ageing and Director of the Dementia Services Development Centre (DSDC), Bangor University, Bangor, UK] was the PI for the Bangor site and provided project leadership at the site, supervised the day-to-day work of the researcher and the therapist and contributed to researcher supervision and trial management.
Publications
Clare L, Bayer A, Burns A, Corbett A, Jones R, Knapp M, et al. Goal-oriented cognitive rehabilitation in early-stage dementia: study protocol for a multi-centre single-blind randomised controlled trial (GREAT). Trials 2013;14:152.
Bahar-Fuchs A, Kudlicka A, Clare L. Cognitive rehabilitation for people with dementia: what is it and does it work? Aust J Dement Care 2016;5:37–40.
Clare L. Rehabilitation for people living with dementia: a practical framework of positive support. PLOS Med 2017;14:e1002245.
Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: a multi-centre randomised controlled trial (the GREAT trial) [published online ahead of print February 6 2019]. Int J Geriatr Psychiatry 2019.
Clare L, Teale J, Toms G, Kudlicka A, Evans I, Abrahams S, et al. Cognitive rehabilitation, self-management, psychotherapeutic and caregiver support interventions in progressive neurodegenerative conditions: a scoping review. NeuroRehabilitation 2018;43:443–71.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Psychological Dimensions of Care: Putting the Person at the Centre of Care. Leicester: British Psychological Society; 2016.
- Clinical Psychology in the Early-Stage Dementia Pathway. Leicester: British Psychological Society; 2014.
- Clare L. Rehabilitation for people living with dementia: a practical framework of positive support. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002245.
- Squire LR, Knowlton BJ, Gazzaniga M. The Cognitive Neurosciences. Boston, MA: MIT Press; 1995.
- Fernández-Ballesteros R, Zamarrón MD, Tàrraga L, Moya R, Iniguez J. Cognitive plasticity in healthy, mild cognitive impairment (MCI) subjects and Alzheimer’s disease patients: a research project in Spain. Eur Psychol 2003;8:148-59. https://doi.org/10.1027//1016-9040.8.3.148.
- Little AG, Volans PJ, Hemsley DR, Levy R. The retention of new information in senile dementia. Br J Clin Psychol 1986;25:71-2. https://doi.org/10.1111/j.2044-8260.1986.tb00673.x.
- Bäckman L. Memory training and memory improvement in Alzheimer’s disease: rules and exceptions. Acta Neurol Scand 1992;139:84-9. https://doi.org/10.1111/j.1600-0404.1992.tb04461.x.
- International Classification of Impairments, Disabilities, and Handicaps. Geneva: World Health Organization; 1980.
- International Classification of Impairments, Disabilities and Handicaps. Geneva: World Health Organization; 1998.
- International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2017.
- Reifler BV, Larson E, Light E, Lebowitz BD. Alzheimer’s Disease Treatment and Family Stress. New York, NY: Hemisphere; 1990.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997;278:1363-71. https://doi.org/10.1001/jama.1997.03550160083043.
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2003;4. https://doi.org/10.1002/14651858.CD003260.
- Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD003260.pub2.
- Global Council on Brain Health . Engage Your Brain: GCBH Recommendations on Cognitively Stimulating Activities 2017. www.globalcouncilonbrainhealth.org (accessed 21 August 2018).
- Sitzer DI, Twamley EW, Jeste DV. Cognitive training in Alzheimer’s disease: a meta-analysis of the literature. Acta Psychiatr Scand 2006;114:75-90. https://doi.org/10.1111/j.1600-0447.2006.00789.x.
- McLellan DL. Functional Recovery and the Principles of Disability Medicine. London: Churchill Livingstone; 1991.
- Wilson BA. Towards a comprehensive model of cognitive rehabilitation. Neuropsychol Rehabil 2002;12:97-110. https://doi.org/10.1080/09602010244000020.
- Clare L. Neuropsychological Rehabilitation and People with Dementia. Hove: Psychology Press; 2008.
- Aspinal F, Glasby J, Rostgaard T, Tuntland H, Westendorp RG. New horizons: reablement – supporting older people towards independence. Age Ageing 2016;45:572-6. https://doi.org/10.1093/ageing/afw094.
- Convention on the Rights of Persons with Disabilities. New York, NY: United Nations; 2006.
- Cohen D, Eisdorfer C. The Loss of Self: A Family Resource for the Care of Alzheimer’s Disease and Related Disorders. New York, NY: W. W. Norton & Company; 1986.
- Marshall M. Perspectives on Rehabilitation and Dementia. London: Jessica Kingsley; 2005.
- Allen J, Koziak A, Buddingh S, Liang J, Buckingham J, Beaupre LA. Rehabilitation in patients with dementia following hip fracture: a systematic review. Physiother Can 2012;64:190-201. https://doi.org/10.3138/ptc.2011-06BH.
- Malec JF. Goal attainment scaling in rehabilitation. Neuropsychol Rehabil 1999;9:253-75. https://doi.org/10.1080/096020199389365.
- Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. A 35-year odyssey. Am Psychol 2002;57:705-17. https://doi.org/10.1037/0003-066X.57.9.705.
- Rockwood K, Joyce B, Stolee P. Use of goal attainment scaling in measuring clinically important change in cognitive rehabilitation patients. J Clin Epidemiol 1997;50:581-8. https://doi.org/10.1016/S0895-4356(97)00014-0.
- Trombly CA, Radomski MV, Trexel C, Burnet-Smith SE. Occupational therapy and achievement of self-identified goals by adults with acquired brain injury: phase II. Am J Occup Ther 2002;56:489-98. https://doi.org/10.5014/ajot.56.5.489.
- Cup EH, Scholte op Reimer WJ, Thijssen MC, van Kuyk-Minis MA. Reliability and validity of the Canadian Occupational Performance Measure in stroke patients. Clin Rehabil 2003;17:402-9. https://doi.org/10.1191/0269215503cr635oa.
- Khan F, Pallant JF, Turner-Stokes L. Use of goal attainment scaling in inpatient rehabilitation for persons with multiple sclerosis. Arch Phys Med Rehabil 2008;89:652-9. https://doi.org/10.1016/j.apmr.2007.09.049.
- Dewar BK, Kapur N, Kopelman M. Do memory aids help everyday memory? A controlled trial of a Memory Aids Service. Neuropsychol Rehabil 2018;28:614-32. https://doi.org/10.1080/09602011.2016.1189342.
- Rushton PW, Miller WC. Goal attainment scaling in the rehabilitation of patients with lower-extremity amputations: a pilot study. Arch Phys Med Rehabil 2002;83:771-5. https://doi.org/10.1053/apmr.2002.32636.
- Fisher K, Hardie RJ. Goal attainment scaling in evaluating a multidisciplinary pain management programme. Clin Rehabil 2002;16:871-7. https://doi.org/10.1191/0269215502cr554oa.
- Fisher K. Assessing clinically meaningful change following a programme for managing chronic pain. Clin Rehabil 2008;22:252-9. https://doi.org/10.1177/0269215507081928.
- Rockwood K, Howlett S, Stadnyk K, Carver D, Powell C, Stolee P. Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. J Clin Epidemiol 2003;56:736-43. https://doi.org/10.1016/S0895-4356(03)00132-X.
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Community Ment Health J 1968;4:443-53. https://doi.org/10.1007/BF01530764.
- Law M, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure. Ottawa, ON: Canadian Association of Occupational Therapists Publications; 2005.
- Carswell A, McColl MA, Baptiste S, Law M, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: a research and clinical literature review. Can J Occup Ther 2004;71:210-22. https://doi.org/10.1177/000841740407100406.
- Donnelly C, Carswell A. Individualized outcome measures: a review of the literature. Can J Occup Ther 2002;69:84-9. https://doi.org/10.1177/000841740206900204.
- Eyssen IC, Beelen A, Dedding C, Cardol M, Dekker J. The reproducibility of the Canadian Occupational Performance Measure. Clin Rehabil 2005;19:888-94. https://doi.org/10.1191/0269215505cr883oa.
- Jenkinson N, Ownsworth T, Shum D. Utility of the Canadian Occupational Performance Measure in community-based brain injury rehabilitation. Brain Inj 2007;21:1283-94. https://doi.org/10.1080/02699050701739531.
- Steeman E, de Casterlé BD, Godderis J, Grypdonck M. Living with early-stage dementia: a review of qualitative studies. J Adv Nurs 2006;54:722-38. https://doi.org/10.1111/j.1365-2648.2006.03874.x.
- Clare L. The construction of awareness in early-stage Alzheimer’s disease: a review of concepts and models. Br J Clin Psychol 2004;43:155-75. https://doi.org/10.1348/014466504323088033.
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Awareness in early-stage Alzheimer’s disease: relationship to outcome of cognitive rehabilitation. J Clin Exp Neuropsychol 2004;26:215-26. https://doi.org/10.1076/jcen.26.2.215.28088.
- Clare L, Marková IS, Roth I, Morris RG. Awareness in Alzheimer’s disease and associated dementias: theoretical framework and clinical implications. Aging Ment Health 2011;15:936-44. https://doi.org/10.1080/13607863.2011.583630.
- Clare L, Whitaker CJ, Roberts JL, Nelis SM, Martyr A, Marková IS, et al. Memory awareness profiles differentiate mild cognitive impairment from early-stage dementia: evidence from assessments of performance monitoring and evaluative judgement. Dement Geriatr Cogn Disord 2013;35:266-79. https://doi.org/10.1159/000346735.
- Martyr A, Clare L. Awareness of functional ability in people with early-stage dementia. Int J Geriatr Psychiatry 2018;33:31-8. https://doi.org/10.1002/gps.4664.
- Kurlychek RT. Use of a digital alarm chronograph as a memory aid in early dementia. Clin Gerontol 1983;1:93-4.
- Bourgeois MS. Enhancing conversation skills in patients with Alzheimer’s disease using a prosthetic memory aid. J Appl Behav Anal 1990;23:29-42. https://doi.org/10.1901/jaba.1990.23-29.
- Josephsson S, Backman L, Borell L, Bernspang B, Nygard L, Ronnberg L. Supporting everyday activities in dementia: an intervention study. Int J Geriatr Psychiatry 1993;8:395-400. https://doi.org/10.1002/gps.930080505.
- Camp CJ. Facilitation of New Learning in Alzheimer’s Disease. New York, NY: Springer; 1989.
- Clare L, Wilson BA, Breen K, Hodges JR. Errorless learning of face-name associations in early Alzheimer’s disease. Neurocase 1999;5:37-46. https://doi.org/10.1080/13554799908404063.
- Clare L, Wilson BA, Carter G, Breen K, Gosses A, Hodges JR. Intervening with everyday memory problems in dementia of Alzheimer type: an errorless learning approach. J Clin Exp Neuropsychol 2000;22:132-46. https://doi.org/10.1076/1380-3395(200002)22:1;1-8;FT132.
- Clare L, Wilson BA, Carter G, Hodges JR. Cognitive rehabilitation as a component of early intervention in Alzheimer’s disease: a single case study. Aging Ment Health 2003;7:15-21. https://doi.org/10.1080/1360786021000045854.
- Clare L, Wilson BA. Memory rehabilitation for people with early-stage dementia: a single case comparison of four errorless learning methods. Z Gerontol Psychol Psychiat 2004;17:109-17.
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning face-name associations in early Alzheimer’s disease. Neuropsychology 2002;16:538-47. https://doi.org/10.1037/0894-4105.16.4.538.
- Clare L, Wilson BA, Carter G, Hodges JR, Adams M. Long-term maintenance of treatment gains following a cognitive rehabilitation intervention in early dementia. Neuropsychol Rehabil 2001;11:477-94. https://doi.org/10.1080/09602010042000213.
- Dunn J, Clare L. Learning face-name associations in early-stage dementia: comparing the effects of errorless learning and effortful processing. Neuropsychol Rehabil 2007;17:735-54. https://doi.org/10.1080/09602010701218317.
- Clare L, Jones RS. Errorless learning in the rehabilitation of memory impairment: a critical review. Neuropsychol Rev 2008;18:1-23. https://doi.org/10.1007/s11065-008-9051-4.
- Bird M. Behavioural difficulties and cued recall of adaptive behaviour in dementia: experimental and clinical evidence. Neuropsychol Rehabil 2001;11:357-75. https://doi.org/10.1080/09602010042000042.
- Lekeu F, Wojtasik V, Van der Linden M, Salmon E. Training early Alzheimer patients to use a mobile phone. Acta Neurol Belg 2002;102:114-21.
- Clare L, Linden DE, Woods RT, Whitaker R, Evans SJ, Parkinson CH, et al. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am J Geriatr Psychiatry 2010;18:928-39. https://doi.org/10.1097/JGP.0b013e3181d5792a.
- Clare L, Evans S, Parkinson C, Woods RT, Linden D. Goal-setting in cognitive rehabilitation for people with early-stage Alzheimer’s disease. Clin Gerontol 2011;34:220-36. https://doi.org/10.1080/07317115.2011.555937.
- van Paasschen J, Clare L, Woods RT, Linden DE. Can we change brain functioning with cognition-focused interventions in Alzheimer’s disease? The role of functional neuroimaging. Restor Neurol Neurosci 2009;27:473-91. https://doi.org/10.3233/RNN-2009-0494.
- van Paasschen J, Clare L, Yuen KS, Woods RT, Evans SJ, Parkinson CH, et al. Cognitive rehabilitation changes memory-related brain activity in people with Alzheimer disease. Neurorehabil Neural Repair 2013;27:448-59. https://doi.org/10.1177/1545968312471902.
- Clare L, Woods RT. Cognitive rehabilitation and cognitive training in early-stage Alzheimer’s disease and vascular dementia (updated version). Cochrane Database Syst Rev 2007;4.
- Wressle E, Marcusson J, Henriksson C. Clinical utility of the Canadian Occupational Performance Measure – Swedish version. Can J Occup Ther 2002;69:40-8. https://doi.org/10.1177/000841740206900104.
- Cusick A, McIntyre S, Novak I, Lannin N, Lowe K. A comparison of goal attainment scaling and the Canadian Occupational Performance Measure for paediatric rehabilitation research. Pediatr Rehabil 2006;9:149-57. https://doi.org/10.1080/13638490500235581.
- Clare L, Nelis SM, Jones IR, Hindle JV, Thom JM, Nixon JA, et al. The Agewell trial: a pilot randomised controlled trial of a behaviour change intervention to promote healthy ageing and reduce risk of dementia in later life. BMC Psychiatry 2015;15. https://doi.org/10.1186/s12888-015-0402-4.
- Lautenschlager NT, Goh A, Etherton-Beer C, Liew D, Ames D, LoGiudice D, et al. The INDIGO Study: a randomized controlled trial of physical activity with individual goal-setting and volunteer mentors to overcome sedentary lifestyle in older adults at risk of cognitive decline. Alzheimers Dement 2014;10. https://doi.org/10.1016/j.jalz.2014.04.044.
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: a multi-centre randomised controlled trial (the GREAT trial). Int J Geriatr Psychiatry 2019.
- Clare L, Bayer A, Burns A, Corbett A, Jones R, Knapp M, et al. Goal-oriented cognitive rehabilitation in early-stage dementia: study protocol for a multi-centre single-blind randomised controlled trial (GREAT). Trials 2013;14. https://doi.org/10.1186/1745-6215-14-152.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK. London: The Alzheimer’s Society; 2014.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Pool J. The Pool Activity Level (PAL) Instrument for Occupational Profiling: A Practical Resource for Carers of People with Cognitive Impairment. London: Jessica Kingsley; 2012.
- Wenborn J, Challis D, Pool J, Burgess J, Elliott N, Orrell M. Assessing the validity and reliability of the Pool Activity Level (PAL) Checklist for use with older people with dementia. Aging Ment Health 2008;12:202-11. https://doi.org/10.1080/13607860801984375.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: NICE; 2006.
- Moncher FJ, Prinz RJ. Treatment fidelity in outcome studies. Clin Psychol Rev 1991;11:247-66. https://doi.org/10.1016/0272-7358(91)90103-2.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. https://doi.org/10.1136/bmj.328.7455.1561.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
- Cusick A, Lannin NA, Lowe K. Adapting the Canadian Occupational Performance Measure for use in a paediatric clinical trial. Disabil Rehabil 2007;29:761-6. https://doi.org/10.1080/09638280600929201.
- Wallen MA, Ziviani JM. Canadian Occupational Performance Measure: impact of blinded parent-proxy ratings on outcome. Can J Occup Ther 2012;79:7-14. https://doi.org/10.2182/cjot.2012.79.1.2.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- Schwarzer R, Jerusalem M, Weinman J, Wright S, Johnston M. Measures in Health Psychology: A User’s Portfolio. Windsor: NFER-Nelson; 1995.
- Luszczynska A, Gutierrez-Dona B, Schwarzer R. General self-efficacy in various domains of human functioning: evidence from five countries. Int J Psychol 2005;40:80-9. https://doi.org/10.1080/00207590444000041.
- Snaith RP, Zigmond AS. The Hospital Anxiety and Depression Scale. Windsor: NFER-Nelson; 1994.
- Ablitt A, Jones G, Muers J. Awareness of carer distress in people with dementia. Int J Geriatr Psychiatry 2010;25:1246-52. https://doi.org/10.1002/gps.2461.
- Cooper C, Katona C, Orrell M, Livingston G. Coping strategies and anxiety in caregivers of people with Alzheimer’s disease: the LASER-AD study. J Affect Disord 2006;90:15-20. https://doi.org/10.1016/j.jad.2005.08.017.
- Wilson BA, Baddeley AD, Cockburn J. Rivermead Behavioural Memory Test II. Bury St Edmunds: Thames Valley Test Company; 2003.
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The Test of Everyday Attention. Bury St Edmunds: Thames Valley Test Company; 1994.
- Delis DC, Kaplan E, Kramer JH. Delis–Kaplan Executive Function System (D-KEFS). London: Pearson; 2001.
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 2007;17:213-33. https://doi.org/10.1007/s11065-007-9040-z.
- Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 2004;42:1212-22. https://doi.org/10.1016/j.neuropsychologia.2004.02.001.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskill; 1992.
- Greene JG, Smith R, Gardiner M, Timbury GC. Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: a factor analytic study. Age Ageing 1982;11:121-6. https://doi.org/10.1093/ageing/11.2.121.
- Skevington SM, Lotfy M, O’Connell KA. WHOQOL Group . The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res 2004;13:299-310. https://doi.org/10.1023/B:QURE.0000018486.91360.00.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- McGrath RE, Meyer GJ. When effect sizes disagree: the case of r and d. Psychol Methods 2006;11:386-401. https://doi.org/10.1037/1082-989X.11.4.386.
- van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Grund S, Lüdtke O, Robitzsch A. Pooling ANOVA results from multiply imputed datasets. Methodology 2016;12:75-88. https://doi.org/10.1027/1614-2241/a000111.
- Evans S, Pool J, Besso E, Cunnane H, Freestone C, Gerbase S, et al. What do people with early-stage dementia identify as meaningful therapy goals?. Br J Occup Ther 2015;78:78-9.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Ritchie J, Spencer L, O’Connor W, Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage Publications; 2003.
- Smith JA, Osborn M, Jarman J, Chamberlain MMJ. Qualitative Health Psychology: Theories and Methods. London: Sage Publications; 1999.
- Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. PharmacoEconomics 1999;15:141-55. https://doi.org/10.2165/00019053-199915020-00003.
- Rowen D, Mulhern B, Banerjee S, Hout Bv, Young TA, Knapp M, et al. Estimating preference-based single index measures for dementia using DEMQOL and DEMQOL-Proxy. Value Health 2012;15:346-56. https://doi.org/10.1016/j.jval.2011.10.016.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey (discussion paper 138). York: University of York; 1995.
- Beecham JK, Knapp MRJ, Thornicroft G, Brewin C, Wing JK. Measuring Mental Health Needs. London: Gaskell; 2001.
- NHS Reference Costs 2012 to 2013. London: Department of Health and Social Care; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. PharmacoEconomics 2008;26:269-80. https://doi.org/10.2165/00019053-200826040-00001.
- Pritchard C, Sculpher MJ. Productivity Costs. London: Office of Health Economics; 2000.
- NHS Reference Costs 2013 to 2014. London: Department of Health and Social Care; 2014.
- Greenleaf Cleaning . Our Prices: Regular Domestic Cleaning n.d. www.greenleaf-cleaning.co.uk/our-services/ (accessed 28 August 2018).
- Hassle.com . Pricing n.d. www.hassle.com/uk/pricing (accessed 15 November 2016).
- Homeclean . What We Do n.d. www.homeclean.co.uk/ (accessed 15 November 2016).
- Wandsworth Council . Laundry Service 2008 n.d. www.wandsworth.gov.uk/moderngov/mgConvert2PDF.aspx?ID=6962 (accessed 20 June 2016).
- Banks L, Barnes M. Evaluation of the East Sussex Carers’ Breaks Demonstrator Site. Brighton: University of Brighton, Social Science Policy and Research Centre; 2011.
- Optics at a Glance 2014. London: Optical Confederation; 2014.
- General Ophthalmic Services: Increases to NHS Sight Test Fee, Pre-Registration Supervisors Allowance and Continuing Education and Training Allowance and Arrangements for Universal Credit. London: Department of Health and Social Care; 2014.
- Romeo R, Knapp M, Banerjee S, Morris J, Baldwin R, Tarrier N, et al. Treatment and prevention of depression after surgery for hip fracture in older people: cost-effectiveness analysis. J Affect Disord 2011;128:211-19. https://doi.org/10.1016/j.jad.2010.07.026.
- NHS West Norfolk CCG . Freedom of Information Request: WN-2015-00077(IR)–Structured Diabetes 2015 2015.
- D’Amico F, Rehill A, Knapp M, Aguirre E, Donovan H, Hoare Z, et al. Maintenance cognitive stimulation therapy: an economic evaluation within a randomized controlled trial. J Am Med Dir Assoc 2015;16:63-70. https://doi.org/10.1016/j.jamda.2014.10.020.
- Orgeta V, Leung P, Yates L, Kang S, Hoare Z, Henderson C, et al. Individual cognitive stimulation therapy for dementia: a clinical effectiveness and cost-effectiveness pragmatic, multicentre, randomised controlled trial. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19640.
- Quinn C, Toms G, Jones C, Brand A, Edwards RT, Sanders F, et al. A pilot randomized controlled trial of a self-management group intervention for people with early-stage dementia (The SMART study). Int Psychogeriatr 2016;28:787-800. https://doi.org/10.1017/S1041610215002094.
- Dementia Partnerships . Peer Support Projects 2014 n.d. www.dementiapartnerships.org.uk (accessed 14 April 2014).
- Alzheimer’s Society (Hull and East Riding) . Singing For The Brain: Alzheimer’s Society n.d. www.dementiaeastriding.org.uk (accessed 31 May 2017).
- Alzheimer’s Society (Surrey) . New ‘Singing for the Brain’® Group for People With Dementia in Oxted 2016. www.woldingham.com/wp-content/uploads/2016/10/Singing-for-the-brain-PressRelease.pdf (accessed 31 May 2017).
- Alzheimer’s Society (Surrey) . Singing With Us 2017. www.surreymusichub.com/assets/documents/singing-for-the-brain (accessed 31 May 2017).
- Alzheimer’s Society – Singing for the Brain. Sheffield: Sheffield Directory; 2017.
- Witham MD, Fulton RL, Greig CA, Johnston DW, Lang CC, van der Pol M, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2012;5:209-16. https://doi.org/10.1161/CIRCHEARTFAILURE.111.963132.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- National Catalogue and Tariff for Simple Aids to Daily Living 2010. London: Department of Health and Social Care; 2010.
- Building Telecare in England. London: Department of Health and Social Care; 2005.
- Prescription Cost Analysis, England 2013. Leeds: NHS Digital; 2014.
- GOV.UK . National Minimum Wage and National Living Wage Rates 2017. www.gov.uk/national-minimum-wage-rates (accessed 1 February 2017).
- Motoring Costs 2013. Basingstoke: Automobile Association; 2013.
- NHS Employers . Pay Circular (AforC) 3 2013 n.d. www.nhsemployers.org/∼/media/Employers/Publications/Pay%20circulars/pay-circular-afc-3-2013.pdf (accessed 24 November 2014).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Stata Multiple-Imputation Reference Manual: Release 14. College Station, TX: StataCorp LP; 2015.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Vanderviere E, Huber M, Antoch J. COMPSTAT 2004 Symposium: Proceedings in Computational Statistics. Heidelberg: Physica-Verlag; 2004.
- Gelade W, Verardi V, Vermandele C. MEDCOUPLE: Stata Module to Compute Medcouple Measure of Asymmetry and Heaviness of the Tails. Boston, MA: Statistical Software Components, Boston College Department of Economics; 2013.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Gould W, Pitblado J. How Large Should the Bootstrapped Samples Be Relative to the Total Number of Cases in the Dataset? 2017. www.stata.com/support/faqs/statistics/bootstrapped-samples-guidelines/ (accessed 16 March 2017).
- Glick H. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-52.
- Mantopoulos T, Mitchell PM, Welton NJ, McManus R, Andronis L. Choice of statistical model for cost-effectiveness analysis and covariate adjustment: empirical application of prominent models and assessment of their results. Eur J Health Econ 2016;17:927-38. https://doi.org/10.1007/s10198-015-0731-8.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. https://doi.org/10.1016/j.jval.2010.08.002.
- Aguirre E, Kang S, Hoare Z, Edwards RT, Orrell M. How does the EQ-5D perform when measuring quality of life in dementia against two other dementia-specific outcome measures?. Qual Life Res 2016;25:45-9. https://doi.org/10.1007/s11136-015-1065-9.
- Ratcliffe J, Flint T, Easton T, Killington M, Cameron I, Davies O, et al. An empirical comparison of the EQ-5D-5L, DEMQOL-U and DEMQOL-Proxy-U in a post-hospitalisation population of frail older people living in residential aged care. Appl Health Econ Health Policy 2017;15:399-412. https://doi.org/10.1007/s40258-016-0293-7.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Amieva H, Dartigues JF. ETNA3, a clinical randomized study assessing three cognitive-oriented therapies in dementia: rationale and general design. Rev Neurol 2013;169:752-6. https://doi.org/10.1016/j.neurol.2013.07.011.
- Amieva H, Robert PH, Grandoulier AS, Meillon C, De Rotrou J, Andrieu S, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr 2016;28:707-17. https://doi.org/10.1017/S1041610215001830.
- Kim S. Cognitive rehabilitation for elderly people with early-stage Alzheimer’s disease. J Phys Ther Sci 2015;27:543-6. https://doi.org/10.1589/jpts.27.543.
- Thivierge S, Jean L, Simard M. A randomized cross-over controlled study on cognitive rehabilitation of instrumental activities of daily living in Alzheimer disease. Am J Geriatr Psychiatry 2014;22:1188-99. https://doi.org/10.1016/j.jagp.2013.03.008.
- Voigt-Radloff S, de Werd MM, Leonhart R, Boelen DH, Olde Rikkert MG, Fliessbach K, et al. Structured relearning of activities of daily living in dementia: the randomized controlled REDALI-DEM trial on errorless learning. Alzheimers Res Ther 2017;9. https://doi.org/10.1186/s13195-017-0247-9.
- Woods RT, Orrell M, Bruce E, Edwards RT, Hoare Z, Hounsome B, et al. REMCARE: pragmatic multi-centre randomised trial of reminiscence groups for people with dementia and their family carers: effectiveness and economic analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0152843.
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 1999;53:471-81. https://doi.org/10.5014/ajot.53.5.471.
- Clare L, Wu YT, Teale JC, MacLeod C, Matthews F, Brayne C, et al. CFAS-Wales study team . Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: a cross-sectional study. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002259.
- Patel A, Knapp M, Romeo R, Reeder C, Matthiasson P, Everitt B, et al. Cognitive remediation therapy in schizophrenia: cost-effectiveness analysis. Schizophr Res 2010;120:217-24. https://doi.org/10.1016/j.schres.2009.12.003.
- Clare L, Quinn C, Jones IR, Woods RT. ‘I don’t think of it as an illness’: illness representations in mild to moderate dementia. J Alzheimers Dis 2016;51:139-50. https://doi.org/10.3233/JAD-150794.
- Hindle JV, Watermeyer T, Roberts J, Martyr A, Lloyd-Williams H, Brand A, et al. Cognitive rehabilitation for Parkinson’s disease dementia: study protocol for a randomised controlled trial (CORD-PD). Trials 2016;17. https://doi.org/10.1186/s13063-016-1253-0.
- Watermeyer TJ, Hindle JV, Roberts J, Lawrence CL, Martyr A, Lloyd-Williams H, et al. Goal-setting for cognitive rehabilitation in mild to moderate Parkinson’s disease dementia and dementia with Lewy bodies. Parkinsons Dis 2016;2016. https://doi.org/10.1155/2016/8285041.
- Watermeyer T, Hindle JV, Clare L. Cognitive Rehabilitation for People With Parkinson’s Disease Dementia and Dementia With Lewy Bodies: The CORD-PD Pilot Trial n.d.
Appendix 1 Topics recorded by therapists in the therapy logs
| Therapy log topic | Therapist ratings or comments recorded under relevant sessions |
|---|---|
| Compliance | Whether or not given session was completed |
| Relationships | The relationship the therapist developed with the participant and the carer, and the relationship between the participant and the carer |
| Goals |
Participant and carer responsiveness to the solution-focused problem-solving approach Goal 1: agreed strategies, between-session practice and progress Goal 2: agreed strategies, between-session practice and progress Goal 3: agreed strategies, between-session practice and progress Any adjustments or modifications to goal statements provided at baseline In-session goal attainment ratings by participant, carer and therapist (sessions 10 and 14) and comments on these Selection of goal attainment scaling indicators for each goal (sessions 10 and 14) and comments on these |
| Activity levels | Review of activity levels, plan for behavioural activation to increase activity engagement and comments on progress |
| Compensatory strategy use | Review of the current use of compensatory strategies and environmental adaptations, plan to develop strategy use and comments on progress |
| Restorative strategy use | Response to information about restorative strategies, plan for developing restorative strategy use and comments on progress |
| Attention and concentration | Strategies introduced to help maintain attention and concentration, and progress with applying these strategies |
| Anxiety management | Current use of anxiety-management strategies, carer’s perspective on the participant’s use of these strategies, introduction or refinement of anxiety management technique(s) and progress with use of anxiety-management strategies |
| Carer well-being | Review with carer, and plan for enhancing carer well-being |
| Carer involvement | The extent to which the carer was engaged in supporting the process of therapy |
| Ending therapy | Plans for maintaining progress after the end of the intervention, and review of other sources of help and support |
| Experience of therapy | Review with the participant and with the carer, and therapist reflection on the process of therapy; therapist confidence in addressing participants’ goals (following the 9-month follow-up) |
Appendix 2 Interview schedule for exploring participant and carer experience of the GREAT intervention
Interview schedule for the person with dementia
The interview will take form of a conversation and the interviewer will encourage the participant to talk freely about the experience of the CR intervention.
The researcher will begin by re-establishing consent for the interview and for audio-recording.
The researcher may begin with some general conversation to build rapport as appropriate.
The researcher will introduce the main part of the interview by saying:
You’ve been taking part in the GREAT study and having visits from the therapist, and I’d like to know your views on what it was like. I’m interested in what it was like to take part in the study and how you found the visits from the therapist.
Experiences
The researcher will explore the participant’s experiences and feelings starting with general questions:
How did you find the therapist’s visits over the past few weeks?
What was it like to work with the therapist on your goals?
More specific information will then be elicited using prompts, such as the following:
What were the more enjoyable things about your work with the therapist?
What were the less enjoyable things about your work with the therapist?
What aspects of your work with the therapist were more/less helpful?
Did you find it hard work to take part in the therapy?
What aspects of your work with the therapist were most challenging?
The interviewer will encourage the person to give specific examples, when possible.
Outcomes
The researcher will explore the impact of taking part in CR on the person’s everyday life and self-perceptions.
What difference (if any) has your work with the therapist made to your daily life?
Has the experience changed anything in the way you think about your dementia/about yourself/about the future?
Has the experience changed anything in the way you relate to your carer/family?
The researcher will draw on positive comments from the participant to end the conversation on a positive note.
Interview schedule for the carer
The interview will take the form of a conversation and the interviewer will encourage the carer to talk freely about the experience of the CR intervention.
The researcher will begin by re-establishing consent for the interview and for audio-recording.
The researcher may begin with some general conversation to build rapport as appropriate.
The researcher will introduce the main part of the interview by saying:
[Your relative] has been taking part in the GREAT study and having visits from the therapist, and I’d like to know your views on what it was like. I’m interested in what it was like to take part in the study and how you found the visits from the therapist.
Experiences
The researcher will explore the carer’s experiences and feelings starting with general questions:
How did you find the therapist’s visits over the past few weeks?
How do you think [your relative] felt about working with the therapist on his/her goals?
More specific information will then be elicited using prompts, such as the following:
What were the more enjoyable things about working with the therapist on [your relative’s] goals?
What were the less enjoyable things about working with the therapist on [your relative’s] goals?
What aspects of working with the therapist were more/less helpful?
Do you think [your relative] found it hard work to take part in the therapy?
What aspects of working with the therapist were most challenging?
The interviewer will encourage the person to give specific examples, when possible.
Outcomes
The researcher will explore the impact of taking part in CR on the person’s everyday life, and the perception of the person with memory difficulties.
What difference (if any) has the therapy made to your daily life?
What difference (if any) has the therapy made to [your relative’s] daily life?
Has the experience changed anything in the way you think about [your relative’s] memory difficulties/about [him/her]/about the future?
Has the experience changed anything in the way you relate to [your relative]?
The researcher will draw on positive comments from the carer to end the conversation on a positive note.
Appendix 3 Feasibility of implementation
In the later stages of the trial, the GREAT team undertook to explore the feasibility of implementing the CR approach within NHS services. This was an opportunity to examine the challenges that could arise when translating the intervention to a real-world setting and to consider how these might be overcome to facilitate successful implementation. The aims of this feasibility project were to:
-
explore how best to approach the training of staff to enable them to offer CR
-
explore how goal-oriented CR can best be incorporated into practice
-
evaluate the effectiveness of the intervention for people with dementia and their carers under these circumstances
-
understand the views of the therapists delivering the intervention
-
identify lessons learned to inform future implementation work.
Three sites were involved: Bangor, Birmingham and Kent.
Implementation methods
At the Bangor site, the Occupational Therapy Service within the Betsi Cadwaladr University Health Board Older People’s Mental Health Directorate expressed interest and was identified as a suitable service to provide the intervention. OTs and technical instructors (TIs) from the service attended a 1-day training event in May 2016, facilitated by the GREAT therapist and researcher. Following training, 10 OTs and their linked TIs from different memory clinics across Betsi Cadwaladr University Health Board offered the intervention to a total of eight service users. Based on early feedback from the OTs, the intervention was adapted from the 10 sessions used in the trial so that it could be delivered in either six sessions or eight sessions. Typically, the OT undertook initial assessment and goal-setting, supervised the TI in conducting the intervention and evaluated the outcome. Specialist supervision and support were provided to all OTs and TIs involved by the GREAT therapist with the support of the GREAT local PI.
At the Kent site, staff from Kent and Medway NHS Partnership Trust were offered a 1½-day training course. Six staff members completed the course: one OT, one OT assistant, two community psychiatric nurses (CPNs) and two support workers. Following training, four staff members each worked with one service user and one worked with two; one did not implement CR as a result of workload pressures. The intervention was offered in the form of six weekly sessions followed by two fortnightly sessions, with two fortnightly follow-up telephone calls. Small group supervision was provided and specialist advice was available from the GREAT therapist on request. It should be noted that this site, having joined GREAT at a late stage, overcame particular time constraints to join the feasibility study.
At the Birmingham site, eight OTs from Birmingham and Solihull Mental Health NHS Foundation Trust participated in training workshops and were keen to try out the approach in practice. However, around the time the workshops were delivered, the trust unexpectedly introduced a reorganisation of work roles and proposed to downgrade all OTs, leading to uncertainty and loss of morale. As a result, the trained OTs were not in a position to proceed with the implementation pilot.
Format of the intervention
The intervention delivered during the feasibility pilot in Bangor and in Kent followed the key principles of the GREAT intervention: individualised, evidence-based rehabilitation, addressing needs through identification of personally meaningful goals that were SMART. The core element involved work on up to two personally meaningful therapy goals, based on a problem-solving approach and using compensatory and restorative strategies as appropriate. Additional content was provided in modular form and could be selected or omitted depending on the needs of the person with dementia. These additional modules covered behavioural activation to increase activity levels, emotion regulation and anxiety management, improving attention and concentration, work on a further personal goal, identification of local sources of support and a focus on carer well-being. In general, work focused mainly on personal goals, augmented by other approaches when relevant.
The service users receiving CR ranged in age from 58 to 90 years and were diagnosed with either Alzheimer’s disease or vascular or mixed dementia. Although the trial required the involvement of a carer who could provide collateral information on progress and outcomes, in this clinical implementation we included people with dementia who did not have support available from a carer or a friend.
Outcomes for participants
The CR therapists used the BGSI as a means of enabling participants to identify goals and eliciting ratings of progress toward goal attainment. Ratings for each goal were made independently by participants, carers (when available) and therapists at the start and end of therapy. To support the therapists in evaluating the intervention, at the initial assessment, participants completed the Addenbrooke’s Cognitive Examination – Third Revision (ACE-III) and DEMQOL, and carers completed the RSS. The DEMQOL and the RSS were completed again on completion of the intervention.
Results
The results provided by the Bangor and Kent sites are presented below.
Bangor
Eight participants with dementia, five of whom were female, received the intervention. Five had a diagnosis of Alzheimer’s disease, one had a diagnosis of vascular dementia and two, although known to have dementia, were still awaiting a specific diagnosis from the memory service. Those with a diagnosis had received this within the previous 12 months. The mean ACE-III score was 71 points (range 62–78 points). Six participants had a carer involved; the carers were five spouses and an adult child. Five participants received a six-session intervention and three received an eight-session intervention.
Participants identified a total of 16 goals: two identified one goal, four identified two goals and two identified three goals. The goals included learning to use new technology, remembering names and activities undertaken or planned, locating items around the house, orientation to the current day and date, planning activities and engaging in social contact and community activities.
Participant and therapist pre- and post-intervention ratings of goal attainment and satisfaction with attainment were available for 13 goals. Carer ratings were available for nine goals. Mean scores improved significantly post intervention, as shown in Table 39. Scrutiny of individual goals indicated that a clinically meaningful improvement of at least 2 points was obtained for attainment of 12 out of 13 goals as rated by participants.
| Rating | Intervention | Statistical comparison | |||
|---|---|---|---|---|---|
| Pre | Post | ||||
| n | Mean (SD), range | n | Mean (SD), range | ||
| Service user goal attainment | 13 | 3.23 (1.83), 1–6 | 13 | 7.54 (1.98), 3–10 | t(12) = –7.018; p < 0.005 |
| Service user satisfaction | 13 | 3.38 (1.98), 1–7 | 13 | 8.15 (1.51), 5–10 | t(12) = –7.315; p < 0.005 |
| Carer goal attainment | 9 | 3.33 (1.73), 1–6 | 9 | 7.44 (1.74), 5–10 | t(8) = –5.094; p < 0.005 |
| Therapist goal attainment | 13 | 2.38 (1.26), 1–4 | 13 | 7.85 (2.12), 4–10 | t(12) = –8.309; p < 0.005 |
Occupational therapists and TIs also rated the extent of goal attainment post intervention by matching current functioning to the goal statements and goal attainment descriptors identified at the initial assessment. Nine out of 16 goals (56%) were rated as fully achieved, six were rated as partially achieved and only one was rated as not achieved.
The mean DEMQOL scores, when available (n = 7), increased from 90.29 (SD 12.55) at initial assessment to 96.14 (SD 6.47) at the end of the intervention, reflecting more positive ratings of quality of life, and the mean RSS scores (n = 6) decreased from 16.5 (SD 3.51) at initial assessment to 13.5 (SD 5.68) at the end of the intervention, reflecting lower levels of stress. However, the small numbers precluded statistical analysis.
Kent
Outcome information was available for five of the six service users included. Between them, these five service users worked on eight goals. BGSI attainment ratings were made by service users for all eight goals, by carers for seven goals and by therapists for four goals (two participants). The mean initial attainment ratings by service users were 3.13 (SD 1.46) and the mean post-intervention ratings were 7.75 (SD 0.89), reflecting a significant improvement [t(7) = –6.56; p < 0.001]. Carers’ mean initial attainment ratings were 3.43 (SD 1.27) and their mean post-intervention ratings were 7.86 (SD 1.07), which was a significant improvement [t(6) = –7.75; p < 0.001]. Therapists’ mean initial ratings were 2.75 (SD 0.96) and their post-intervention ratings were 8.25 (SD 0.96), again reflecting a significant improvement [t(3) = –8.52; p < 0.05]. Service user satisfaction ratings improved from a mean of 2.88 (SD 0.99) at initial assessment to 8.13 (SD 0.83) post intervention, also reflecting a significant change [t(7) = –9.98; p < 0.01]. Carers’ scores for stress decreased only minimally from 36.67 (SD 17.01) at initial assessment to 36.33 (SD 11.93) post intervention, and this was non-significant.
Service users’ experience of the intervention
At the Bangor site, at the end of therapy, participants and carers were asked to complete a questionnaire about their experiences and return it to the GREAT therapist in a prepaid envelope. Four responses were received. Comments were sought on both helpful and less helpful aspects of the intervention. No unhelpful aspects were identified. The responses suggested that the intervention had a positive impact on participants’ lives in various ways:
Feedback from the Kent site indicated that the most important benefit that service users mentioned was an increase in confidence.
Staff members’ experience of delivering the intervention
At the Bangor site, views of the OTs and TIs were sought at two time points. Following the training day, they were asked to give their views on the training and orientation to the intervention via an anonymous questionnaire. All found that the training was informative, easy to follow and pitched at the right level, with useful materials provided. All felt that they would be able to apply what they had learned. Most felt that adequate time was provided, but one would have liked more time for discussion. They felt that completing the 1-day training in a single session was quite intensive and preferred the idea of training being split over two half-days.
On completing their work with the feasibility pilot, the OTs and TIs were invited to complete anonymised questionnaires reporting on their experiences of learning about and delivering the intervention, and to share their experiences in a group discussion with the supervisor.
The therapists initially found it challenging at the start to get to grips with something new, and most had taken the therapy resource materials home to study in their own time. However, once they had begun to use the approach, they quickly became keen on working in this way and saw it as something that they wanted to be involved in:
It’s what we joined the profession to do, actually do interventions with people.
They thought that the goal-setting process was useful, finding that it gave focus to the intervention, facilitated the process of change for participants and family members and was a valuable source of feedback. The intervention could make participants feel valued in that someone was taking the time to help, and could create opportunities for carers to respond more positively to the person with dementia. Positive responses from participants and carers stimulated the therapists’ enthusiasm:
It’s so rewarding, each time I went they were full of praise.
They felt that the intervention was of an appropriate length, although they could sometimes have continued working for longer as additional needs emerged. Some found the modular approach confusing. The therapists uniformly disliked paperwork and preferred not to use questionnaires to evaluate outcomes. All were convinced that there was a service need for this kind of intervention to be more widely available:
We’re going out and seeing a lot more people that we could be doing it with, but with time restraints and waiting lists and other work commitments, there’s just not the time to be able to do it with them all is there. It’s such a shame.
They indicated that time constraints and existing workloads would make it difficult to continue using the intervention, especially given current staff shortages, and that they were looking into ways of being able to sustain their work; for example, one TI was introducing goal-setting into an existing group, one OT and TI pair were putting together a business case for more TI hours and one TI was actively seeking to have CR included in her role.
At the Kent site, the views of staff were obtained, first on the training and support provided and second on delivering the therapy. Staff found the training helpful and the ratings at the end of training indicated that they felt reasonably confident to start delivering CR. However, they found the training very intensive and would have preferred it to be split over several days. They also felt that additional, or ‘refresher’, sessions would have been useful once they were providing CR. They valued the small group supervision and access to specialist advice. Reflecting on the experience of delivering CR, staff found the process of developing goals that were genuinely important to the service user very helpful in focusing their work, and felt that this added to their skills and transferred to other areas of work. They noted how service users increased in confidence, and found it very motivating to see this positive change and receive positive feedback from service users and carers. Some commented that doing CR made them feel like a ‘proper OT’ or a ‘proper CPN’ again. They were unsure about the value of adding follow-up telephone contacts and felt that this was helpful for some service users but less so for others who struggled to use the telephone or were difficult to reach by telephone. Some staff found the dual role of delivering therapy to service users for whom they acted as care co-ordinator challenging.
Lessons for future implementation
This feasibility pilot has demonstrated that delivery on the ground can achieve improvements in goal attainment comparable to those observed in the main trial, even with a considerably shortened intervention period. The approach clearly appeals to staff and offers them the opportunity to work in a way that they feel meets their professional aspirations, and the positive benefits they observe in service users enhance motivation and morale. The pilot has also provided valuable insights into what will be required to implement CR in routine clinical practice. These are discussed below.
Training
Sufficient time is needed for initial training to enable staff to assimilate new information and engage with the approach. Staff prefer training spread over several days in shorter sessions rather than intensive 1- or 2-day workshops. Staff also need some study time to review training materials and consolidate their learning. Training needs to be flexible to take account of different staff backgrounds and levels of experience, and some types of staff will need more input than others. It may be helpful to provide follow-up training sessions once staff are engaged in delivering CR. With regard to the content of training, it is important for staff to understand how CR is different from their current everyday practice. Some staff may feel that they already set treatment goals and suggest strategies, and need to understand how this differs from the goal-setting and strategy application approaches used in CR, which is a more detailed and collaborative process based on careful assessment of intrinsic capacity, current ability and task demands, and which uses a problem-solving approach to identify goals and possible solutions and expresses goals in clear behavioural terms. Providing staff with a checklist to use following training, to remind them of what they should be considering in their work, may help to enhance and maintain adherence to the therapy model.
Supervision
Staff find supervision and access to advice extremely helpful and it is important that time is allocated for staff to participate in supervision, especially in the early stages of familiarisation with the approach.
Intervention
The adaptation to a six- or eight-session format worked well, and increasing the length of time between later sessions, for example by moving from weekly to fortnightly sessions, may be helpful. Telephone follow-up was helpful in some cases but not others, and should be used flexibly. The modular protocol was intended to allow for individual tailoring, but requires revision to enable staff to utilise the available guidance to tailor the intervention optimally for each person. It will clearly be important to limit ‘paperwork’ and recording requirements, and to ensure that any evaluation is not burdensome.
Service users
As with participants in the main trial, service users differed in the extent to which they were able to identify goals and in their motivation to make changes. It is important to be able to identify those service users who are likely to benefit from CR and, equally, to be able to offer other, more appropriate, kinds of support to those who are not likely to engage with the approach. It is also important that the intervention approach is sufficiently flexible to accommodate service users with different degrees of cognitive difficulty and that therapists are trained and supported to apply CR in ways that meet the needs of a range of service users.
Staff
It may be preferable to avoid staff being in the dual roles of care co-ordinator and CR therapist.
Service constraints
Unsurprisingly, time constraints, heavy workloads and staff shortages presented challenges for the implementation. The unexpected reorganisation at the Birmingham site was a classic example of the way in which wider issues can hinder implementation efforts. Future implementation work should be considered only in services for which reorganisation is not envisaged within the time frame of the work. A fuller implementation programme will need to address this through a top-down approach, by engaging service managers and key decision-makers. The consistent support of management at all levels, both senior managers and local line managers, is essential to achieve effective implementation and sustainability.
Appendix 4 Participant recruitment
Here we present cumulative recruitment figures in relation to targets (Figure 13), month-by-month recruitment figures (Figure 14) and a breakdown of recruitment by site (Table 40).
FIGURE 13.
Cumulative recruitment figures in relation to targets.

FIGURE 14.
Recruitment figures by month. Number of recruiting sites: months 1–3, three sites; months 4–25, six sites; months 26–30, eight sites; months 31–36, seven sites.

| Site | Recruitment started | First randomisation | Recruitment ended | Months of recruitment | Original (and adjusted)a targets, n | Actual randomisations, n (%) |
|---|---|---|---|---|---|---|
| Bangor | 24 April 2013 | 4 June 2013 | 31 March 2016 | 36 | 80 (90) | 90 (19) |
| Bath | 24 April 2013 | 30 May 2013 | 31 March 2016 | 36 | 80 (90) | 85 (17.9) |
| Birmingham | 24 April 2013 | 9 July 2013 | 30 September 2015 | 30 | 80 | 52 (11) |
| Cardiff | 28 June 2013 | 9 January 2014 | 31 March 2016 | 34 | 80 | 82 (17.3) |
| Kent | 16 May 2015 | 21 August 2015 | 31 March 2016 | 11 | 25 | 25 (5.3) |
| London | 28 June 2013 | 25 September 2013 | 31 March 2016 | 34 | 80 | 51 (10.8) |
| Manchester | 28 June 2013 | 20 August 2013 | 31 March 2016 | 34 | 80 | 62 (13.1) |
| Newcastle | 16 May 2015 | 17 July 2015 | 31 March 2016 | 11 | 25 | 27 (5.7) |
Appendix 5 Demographic and clinical characteristics of the sample: additional details
| Measure | Whole sample (N = 474) | Treatment group | |
|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||
| Participants with dementia | |||
| Age (years), mean (SD); range | 78.56 (7.07); 53–95 | 78.25 (7.13); 53–95 | 78.87 (7.01); 55–95 |
| Sex, n (%) | |||
| Male | 248 (52.3) | 124 (52.1) | 124 (52.5) |
| Female | 226 (47.7) | 114 (47.9) | 112 (47.5) |
| Ethnicity, n (%) | |||
| White | 457 (96.4) | 226 (95.0) | 231 (97.9) |
| Mixed/multiple ethnic group | 2 (0.42) | 2 (0.84) | 0 (0) |
| Asian/Asian British | 6 (1.27) | 3 (1.26) | 3 (1.27) |
| Black/African/Caribbean/black British | 7 (1.48) | 5 (2.10) | 2 (0.85) |
| Other ethnic group | 2 (0.42) | 2 (0.84) | 0 (0) |
| First language, n (%) | |||
| English | 445 (93.9) | 222 (93.3) | 223 (94.5) |
| Welsh | 10 (2.1) | 5 (2.1) | 5 (2.1) |
| Other | 19 (4) | 11 (4.6) | 8 (3.4) |
| Marital status, n (%) | |||
| Single | 5 (1.1) | 1 (0.4) | 4 (1.7) |
| Married/remarried | 330 (69.6) | 167 (70.2) | 163 (69.1) |
| Civil partnership | 2 (0.4) | 1 (0.4) | 1 (0.4) |
| Separated/divorced | 25 (5.3) | 14 (5.9) | 11 (4.7) |
| Widowed | 101 (21.3) | 48 (20.2) | 53 (22.5) |
| Other | 10 (2.1) | 7 (2.9) | 3 (1.3) |
| Missing | 1 (0.2) | 0 (0) | 1 (0.4) |
| Length of marriage (years), mean (SD); range |
N = 437 39.86 (19.22); 0–70 |
N = 217 40.24 (19.79); 0–70 |
N = 220 39.5 (18.67); 0–69 |
| Age (years) at start of education, mean (SD); range |
N = 473 4.86 (0.92); 2–15 |
N = 237 4.86 (0.79); 2–8 |
N = 236 4.85 (1.04); 3–15 |
| Age (years) when left education, mean (SD); range | 16.03 (2.32); 11–29 | 15.98 (2.24); 11–27 | 16.09 (2.41); 11–29 |
| Years of education, mean (SD); range |
N = 471 12.57 (3.37); 5–33 |
N = 236 12.57 (3.33); 6–24 |
N = 235 12.58 (3.42); 5–33 |
| Further study, n (%) | |||
| Yes | 243 (51.3) | 124 (52.1) | 119 (50.4) |
| No | 231 (48.7) | 114 (47.9) | 117 (49.6) |
| Years in further study, mean (SD); range |
N = 472 1.38 (1.94); 0–10 |
N = 237 1.43 (1.89); 0–9.5 |
N = 235 1.34 (1.98); 0–10 |
| Education type, n (%) | |||
| Left school at age 14–16 years and did not go back to education | 200 (42.2) | 103 (43.3) | 97 (41.1) |
| Left school at age 17–18 years and did not go back to education | 21 (4.4) | 7 (2.9) | 14 (5.9) |
| Further education (e.g. vocational qualifications: GNVQ/NVQ/HND) | 161 (34) | 82 (34.5) | 79 (33.5) |
| Higher education (BSc/BA or equivalent) | 52 (11) | 25 (10.5) | 27 (11.4) |
| Postgraduate education (MSc/MA/PhD or equivalent) | 38 (8) | 20 (8.4) | 18 (7.6) |
| Missing | 2 (0.4) | 1 (0.4) | 1 (0.4) |
| Occupational status, n (%) | |||
| I: professional | 52 (11) | 23 (9.7) | 29 (12.3) |
| II: managerial/technical | 157 (33.1) | 81 (34) | 76 (32.2) |
| III N: skilled, non-manual | 103 (21.7) | 54 (22.7) | 49 (20.8) |
| III M: skilled, manual | 80 (16.9) | 41 (17.2) | 39 (16.5) |
| IV: partly skilled | 50 (10.5) | 24 (10.1) | 26 (11) |
| V: unskilled | 32 (6.8) | 15 (6.3) | 17 (7.2) |
| Carers | |||
| Relationship to person with dementia, n (%) | |||
| Spouse | 296 (62.4) | 149 (62.6) | 147 (62.3) |
| Partner | 35 (7.4) | 18 (7.6) | 17 (7.2) |
| Son/daughter | 108 (22.8) | 52 (21.8) | 56 (23.7) |
| Step-child | 0 (0) | 0 (0) | 0 (0) |
| Son/daughter-in-law | 10 (2.1) | 6 (2.5) | 4 (1.7) |
| Grandchild | 2 (0.4) | 2 (0.8) | 0 (0) |
| Brother/sister | 6 (1.3) | 3 (1.3) | 3 (1.3) |
| Nephew/niece | 1 (0.2) | 0 (0) | 1 (0.4) |
| Friend | 7 (1.5) | 5 (2.1) | 2 (0.8) |
| Neighbour | 3 (0.6) | 0 (0) | 3 (1.3) |
| Other | 6 (1.3) | 3 (1.3) | 3 (1.3) |
| Age (years), mean (SD); range | 68.74 (13.01); 17–92 | 68.45 (13.76); 17–92 | 69.04 (12.24); 23–92 |
| Sex, n (%) | |||
| Male | 142 (30) | 75 (31.5) | 67 (28.4) |
| Female | 332 (70) | 16 (68.5) | 169 (71.6) |
| Ethnicity, n (%) | |||
| White | 449 (94.7) | 224 (94.1) | 225 (95.3) |
| Mixed/multiple ethnic group | 5 (1.1) | 4 (1.7) | 1 (0.42) |
| Asian/Asian British | 10 (2.1) | 4 (1.7) | 6 (2.5) |
| Black/African/Caribbean/black British | 8 (1.7) | 6 (2.5) | 2 (0.85) |
| Other ethnic group | 2 (0.42) | 0 (0) | 2 (0.85) |
| First language, n (%) | |||
| English | 443 (93.5) | 222 (93.3) | 221 (93.6) |
| Welsh | 12 (2.5) | 6 (2.5) | 6 (2.5) |
| Other | 19 (4) | 10 (4.2) | 9 (3.8) |
| Marital status, n (%) | |||
| Single | 31 (6.5) | 23 (9.7) | 8 (3.4) |
| Married/remarried | 393 (82.9) | 187 (78.6) | 206 (87.3) |
| Civil partnership | 5 (1.1) | 3 (1.3) | 2 (0.8) |
| Separated/divorced | 20 (4.2) | 10 (4.2) | 10 (4.2) |
| Widowed | 12 (2.5) | 8 (3.4) | 4 (1.7) |
| Other | 13 (2.7) | 7 (2.9) | 6 (2.5) |
| Marital status length (years), mean (SD); range |
N = 410 41.21 (17.7); 0–69 |
N = 196 41.9 (17.96); 1–68 |
N = 214 40.57 (17.4); 0–69 |
| Age (years) at start of education, mean (SD); range | 4.66 (0.74); 2.5–8 | 4.64 (0.69); 2.5–7 | 4.69 (0.78); 3–8 |
| Age (years) when left education, mean (SD); range |
N = 473 16.57 (2.35); 7.5–27 |
N = 237 16.7 (2.29); 11–25 |
N = 236 16.44 (2.41); 7.5–27 |
| Years in education, mean (SD); range |
N = 472 13.49 (3.52); 4–26 |
N = 237 13.67 (3.45); 5–25 |
N = 235 13.32 (3.58); 4–26 |
| Carer further study, n (%) | |||
| Yes | 266 (56.1) | 135 (56.7) | 131 (55.5) |
| No | 207 (43.7) | 102 (42.9) | 105 (44.5) |
| Not answered | 1 (0.2) | 1 (0.4) | 0 (0) |
| Years in further study, mean (SD); range |
N = 472 1.59 (2.12); 0–15 |
N = 237 1.61 (2.09); 0–12 |
N = 235 1.56 (2.15); 0–15 |
| Education type, n (%) | |||
| Left school at age 14–16, and did not go back to education | 156 (32.9) | 77 (32.4) | 79 (33.5) |
| Left school at age 17–18, and did not go back to education | 31 (6.5) | 16 (6.7) | 15 (6.4) |
| Further education (e.g. vocational qualifications: GNVQ/NVQ/HND) | 163 (34.4) | 79 (33.2) | 84 (35.6) |
| Higher education (BSc/BA or equivalent) | 72 (15.2) | 35 (14.7) | 37 (15.7) |
| Postgraduate education (MSc/MA/PhD or equivalent) | 48 (10.1) | 29 (12.2) | 19 (8.1) |
| Missing | 4 (0.8) | 2 (0.8) | 2 (0.8) |
| Occupational status, n (%) | |||
| I: professional | 49 (10.3) | 30 (12.6) | 19 (8.1) |
| II: managerial/technical | 158 (33.3) | 74 (31.1) | 84 (35.6) |
| III N: skilled, non-manual | 137 (28.9) | 64 (26.9) | 73 (30.9) |
| III M: skilled, manual | 47 (9.9) | 24 (10.1) | 23 (9.7) |
| IV: partly skilled | 55 (11.6) | 27 (11.3) | 28 (11.9) |
| V: unskilled | 20 (4.2) | 14 (5.9) | 6 (2.5) |
| Missing | 8 (1.7) | 5 (2.1) | 3 (1.3) |
| Carer health, n (%) | |||
| Excellent | 68 (14.3) | 30 (12.6) | 38 (16.1) |
| Very good | 113 (23.8) | 59 (24.8) | 54 (22.9) |
| Good | 179 (37.8) | 89 (37.4) | 90 (38.1) |
| Fair | 83 (17.5) | 42 (17.6) | 41 (17.4) |
| Poor | 31 (6.5) | 18 (7.6) | 13 (5.5) |
| Condition | Yes, n (%) | No, n (%) | Unknown, n (%) |
|---|---|---|---|
| Connective tissue disease | 163 (34.4) | 307 (64.8) | 4 (0.8) |
| Peripheral vascular disease | 76 (16.0) | 389 (82.1) | 9 (1.9) |
| Diabetes | 71 (15.0) | 402 (84.8) | 1 (0.2) |
| Any tumour | 52 (11.0) | 421 (88.8) | 1 (0.2) |
| Chronic pulmonary disease | 49 (10.3) | 423 (89.2) | 2 (0.4) |
| Myocardial infarction | 40 (8.4) | 430 (90.7) | 4 (0.8) |
| Congestive heart failure | 39 (8.2) | 428 (90.3) | 7 (1.5) |
| Ulcer disease | 15 (3.2) | 456 (96.2) | 3 (0.6) |
| Moderate or severe renal disease | 8 (1.7) | 464 (97.9) | 2 (0.4) |
| Diabetes with end organ damage | 3 (0.6) | 471 (99.4) | 0 (0) |
| Lymphoma | 3 (0.6) | 470 (99.2) | 1 (0.2) |
| Metastatic solid tumour | 3 (0.6) | 470 (99.2) | 1 (0.2) |
| Mild liver disease | 2 (0.4) | 468 (98.7) | 4 (0.8) |
| Moderate or severe liver disease | 2 (0.4) | 469 (98.9) | 3 (0.6) |
| Leukaemia | 1 (0.2) | 472 (99.6) | 1 (0.2) |
| Hemiplegia | 0 (0) | 474 (100.0) | 0 (0) |
| AIDS | 0 (0) | 474 (100.0) | 0 (0) |
| Total number of comorbid conditions | Whole sample, n (%) (N = 474) | Participants in each treatment group, n (%) | |
|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||
| 0 | 162 (34.2) | 83 (34.9) | 79 (33.5) |
| 1 | 177 (37.3) | 86 (36.1) | 91 (38.6) |
| 2 | 78 (16.5) | 38 (16.0) | 40 (16.9) |
| 3 | 38 (8.0) | 21 (8.8) | 17 (7.2) |
| 4 | 16 (3.4) | 9 (3.8) | 7 (3.0) |
| 5 | 2 (0.4) | 1 (0.4) | 1 (0.4) |
| 6 | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Charlson Comorbidity Weighted Index | Participants in each treatment group, n (%) | ||
|---|---|---|---|
| Whole sample (N = 474) | CR (N = 238) | TAU (N = 236) | |
| 1 | 125 (26.4) | 63 (26.5) | 62 (26.3) |
| 2 | 151 (31.9) | 78 (32.8) | 73 (30.9) |
| 3 | 103 (21.7) | 53 (22.3) | 50 (21.2) |
| 4 | 51 (10.8) | 22 (9.2) | 29 (12.3) |
| 5 | 27 (5.7) | 14 (5.9) | 13 (5.5) |
| 6 | 10 (2.1) | 6 (2.5) | 4 (1.7) |
| 7 | 3 (0.6) | 0 (0.0) | 3 (1.3) |
| 8 | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| 10 | 2 (0.4) | 1 (0.4) | 1 (0.4) |
| 11 | 1 (0.2) | 1 (0.4) | 62 (26.3) |
| Charlson Comorbidity Weighted Index | Participants in each treatment group, n (%) | ||
|---|---|---|---|
| Whole sample (N = 474) | CR (N = 238) | TAU (N = 236) | |
| 0–4 | 430 (90.72) | 216 (90.76) | 214 (90.68) |
| 5–11 | 44 (9.28) | 22 (9.24) | 22 (9.32) |
| Age-adjusted Charlson Comorbidity Index | Participants in each treatment group, n (%) | ||
|---|---|---|---|
| Whole sample (N = 474) | CR (N = 238) | TAU (N = 236) | |
| 3 | 4 (0.8) | 4 (1.7) | 0.0 (0.0) |
| 4 | 24 (5.1) | 9 (3.8) | 15 (6.4) |
| 5 | 70 (14.8) | 40 (16.8) | 30 (12.7) |
| 6 | 117 (24.7) | 60 (25.2) | 57 (24.2) |
| 7 | 113 (23.8) | 51 (21.4) | 62 (26.3) |
| 8 | 77 (16.2) | 39 (16.4) | 38 (16.1) |
| 9 | 40 (8.4) | 19 (8.0) | 21 (8.9) |
| 10 | 20 (4.2) | 13 (5.5) | 7 (3.0) |
| 11 | 3 (0.6) | 1 (0.4) | 2 (0.8) |
| 12 | 3 (0.6) | 0 (0.0) | 3 (1.3) |
| 14 | 1 (0.2) | 1 (0.4) | 0 (0.0) |
| 15 | 2 (0.4) | 1 (0.4) | 1 (0.4) |
| HADS score | Participants at each time point, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 9 months | |||||||
| Total (N = 472) | CR group (N = 238) | TAU group (N = 234) | Total (N = 444) | CR group (N = 218) | TAU group (N = 226) | Total (N = 404) | CR group (N = 194) | TAU group (N = 210) | |
| ≥ 11 | 8 (1.7) | 6 (2.5) | 2 (0.9) | 12 (2.7) | 8 (0.9) | 4 (1.8) | 10 (2.5) | 7 (3.6) | 3 (1.4) |
| 8–10 | 47 (10.0) | 22 (9.2) | 25 (10.7) | 35 (7.9) | 17 (10.7) | 18 (8.0) | 38 (9.4) | 22 (11.3) | 16 (7.6) |
| 0–7 | 417 (88.3) | 210 (88.2) | 207 (88.5) | 397 (89.4) | 193 (88.5) | 204 (90.3) | 356 (88.1) | 165 (85.1) | 191 (91.0) |
| HADS score | Participants at each time point, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 9 months | |||||||
| Total (N = 472) | CR group (N = 238) | TAU group (N = 234) | Total (N = 442) | CR group (N = 216) | TAU group (N = 193) | Total (N = 403) | CR group (N = 226) | TAU group (N = 210) | |
| ≥ 11 | 41 (8.7) | 23 (9.7) | 18 (7.7) | 35 (7.9) | 18 (8.3) | 21 (10.9) | 37 (9.2) | 17 (7.5) | 16 (7.6) |
| 8–10 | 66 (14.0) | 33 (13.9) | 33 (14.1) | 61 (13.8) | 31 (14.4) | 38 (19.7) | 66 (16.4) | 30 (13.3) | 28 (13.3) |
| 0–7 | 365 (77.3) | 182 (76.5) | 183 (78.2) | 346 (78.3) | 167 (77.3) | 134 (69.4) | 300 (74.4) | 179 (79.2) | 166 (79.0) |
Appendix 6 Comparison of participants who did and did not complete the trial
Comparison of the demographic characteristics of completers and non-completers who withdrew before the 3-month follow-up
| Measure | Completers, n, mean (SD) | Non-completers, n, mean (SD) | Effect size (completers – non-completers) | 95% CI for mean difference | Test statistic | Unadjusted p-value |
|---|---|---|---|---|---|---|
| Age (years) | 474, 78.56 (7.07) | 29, 78.69 (6.90) | –0.13 | –2.83 to 2.56 | t(31.7) = –0.1 | 0.92 |
| Years of education | 471, 12.57 (3.37) | 29, 12.05 (3.09) | 0.52 | –0.69 to 1.73 | t(32.23) = 0.88 | 0.39 |
| Length of marriage (years) | 437, 39.86 (19.22) | 29, 35.21 (19.09) | 4.66 | –2.80 to 12.12 | t(31.88) = 1.27 | 0.21 |
| Charlson Comorbidity Index age-adjusted score | 474, 6.84 (1.71) | 29, 7.17 (2.14) | –0.34 | –1.16 to 0.49 | t(30.23) = –0.83 | 0.41 |
| Participant characteristics | Completers, n (%) | Non-completers, n (%) | Fisher’s exact count test: p-value |
|---|---|---|---|
| Group | |||
| CR | 238 (50) | 20 (69) | 0.06 |
| TAU | 236 (50) | 9 (31) | |
| Sex | |||
| Male | 248 (52) | 15 (52) | 1 |
| Female | 226 (48) | 14 (48) | |
| Ethnicity | |||
| White | 457 (97) | 28 (97) | 0.62 |
| Mixed/multiple ethnic group | 2 (0) | 0 (0) | |
| Asian/Asian British | 6 (1) | 1 (0) | |
| Black/African/Caribbean/black British | 7 (1) | 0 (0) | |
| Other ethnic group | 2 (0) | 0 (0) | |
| First language | |||
| English | 445 (94) | 28 (97) | 1 |
| Welsh | 10 (2) | 0 (0) | |
| Other | 19 (4) | 1 (3) | |
| Occupational status | |||
| I: professional | 52 (11) | 4 (14) | 0.55 |
| II: managerial/technical | 157 (33) | 7 (24) | |
| III N: skilled, non-manual | 103 (22) | 4 (14) | |
| III M: skilled, manual | 80 (17) | 7 (24) | |
| IV: partly skilled | 50 (11) | 4 (14) | |
| V: unskilled | 32 (7) | 3 (10) | |
| Health | |||
| Excellent | 39 (8) | 2 (7) | 0.19 |
| Very good | 125 (26) | 7 (24) | |
| Good | 159 (34) | 11 (38) | |
| Fair | 121 (26) | 4 (14) | |
| Poor | 30 (6) | 5 (17) | |
| Marital status | |||
| Single | 5 (1) | 0 (0) | 0.81 |
| Married/remarried | 330 (70) | 19 (66) | |
| Civil partnership | 2 (0) | 0 (0) | |
| Separated/divorced | 25 (5) | 1 (3) | |
| Widowed | 101 (21) | 9 (31) | |
| Other | 10 (2) | 0 (0) | |
| Centre | |||
| Bangor | 90 (19) | 6 (21) | 0.63 |
| Cardiff | 82 (17) | 5 (17) | |
| Manchester | 62 (13) | 6 (21) | |
| Bath | 85 (18) | 3 (10) | |
| Birmingham | 52 (11) | 1 (3) | |
| London | 51 (11) | 4 (14) | |
| Kent | 25 (5) | 1 (3) | |
| Newcastle | 27 (6) | 3 (10) | |
| Measure | Completers, n, mean (SD) | Non-completers, n, mean (SD) | Effect size (completers–dropouts) | Lower CI limit | Test statistic | Unadjusted p-value |
|---|---|---|---|---|---|---|
| Carer age (years) | 474, 68.84 (13.17) | 11, 59.45 (18.6) | 9.38 | –3.15 to 21.91 | t(10.23) = 1.66 | 0.13 |
| Years of education | 472, 13.49 (3.52) | 11, 13.09 (3.22) | 0.4 | –1.78 to 2.58 | t(10.56) = 0.41 | 0.69 |
| Duration of marriage for spouse carers (years) | 410, 41.21 (17.7) | 9, 32.56 (21.35) | 8.65 | –7.8 to 25.11 | t(8.24) = 1.21 | 0.26 |
| Carer characteristics | Completers, n (%) | Non-completers, n (%) | Fisher’s exact count test: p-value |
|---|---|---|---|
| Carer relationship | |||
| Spouse/partner | 331 (69) | 18 (62) | 0.69 |
| Adult child (including in-law) | 64 (14) | 5 (17) | |
| Other | 79 (16) | 6 (21) | |
| Sex | |||
| Male | 142 (30) | 4 (36) | 0.74 |
| Female | 332 (70) | 7 (64) | |
| Ethnicity | |||
| White | 449 (94) | 10 (91) | 0.24 |
| Mixed/multiple ethnic group | 5 (1) | 0 (0) | |
| Asian/Asian British | 10 (2) | 0 (0) | |
| Black/African/Caribbean/black British | 8 (2) | 1 (9) | |
| Other ethnic group | 2 (0) | 0 (0) | |
| First language | |||
| English | 443 (93) | 8 (73) | 0.04 |
| Welsh | 12 (3) | 1 (9) | |
| Other | 19 (4) | 2 (18) | |
| Marital status | |||
| Single | 31 (7) | 1 (9) | 0.5 |
| Married/remarried | 393 (83) | 9 (82) | |
| Civil partnership | 5 (1) | 0 (0) | |
| Separated/divorced | 20 (4) | 0 (0) | |
| Widowed | 12 (3) | 1 (9) | |
| Other | 13 (3) | 0 (0) | |
| Occupational status | |||
| I: professional | 49 (11) | 1 (9) | 0.16 |
| II: managerial/technical | 158 (34) | 2 (18) | |
| III N: skilled, non-manual | 137 (29) | 2 (18) | |
| III M: skilled, manual | 47 (10) | 1 (9) | |
| IV: partly skilled | 55 (12) | 4 (36) | |
| V: unskilled | 20 (4) | 1 (9) | |
| Measure | Completers, n, mean (SD) | Non-completers, n, mean (SD) | Effect size (completers – non-completers) | 95% CI for mean difference | Test statistic | Unadjusted p-value |
|---|---|---|---|---|---|---|
| Primary outcome measure | ||||||
| Participant rating of goal attainment | 474, 3.54 (1.67) | 29, 3.20 (1.56) | 0.33 | –0.28 to 0.94 | t(32.06) = 1.12 | 0.27 |
| Participant rating of satisfaction | 474, 3.81 (1.63) | 29, 4.25 (1.55) | –0.44 | –1.05 to 0.16 | t(31.89) = –1.49 | 0.15 |
| Carer rating of goal attainment | 474, 2.74 (1.38) | 29, 2.36 (1.35) | 0.39 | –0.14 to 0.92 | t(31.66) = 1.49 | 0.15 |
| Secondary outcome measures: participants with dementia | ||||||
| DEMQOL | 472, 92.30 (12.33) | 29, 94.62 (11.28) | –2.32 | –6.73 to 2.10 | t(32.25) = –1.07 | 0.29 |
| GSES score | 469, 30.94 (5.09) | 28, 31.86 (4.36) | –0.92 | –2.67 to 0.83 | t(31.55) = –1.07 | 0.29 |
| HADS score | 472, 8.91 (5.54) | 29, 8.62 (5.21) | 0.29 | –1.75 to 2.33 | t(32.02) = 0.29 | 0.77 |
| HADS anxiety score | 472, 5.14 (3.64) | 29, 4.48 (3.29) | 0.65 | –0.64 to 1.95 | t(32.36) = 1.03 | 0.30 |
| HADS depression score | 472, 3.77 (2.79) | 29, 4.14 (3.31) | –0.37 | –1.65 to 0.92 | t(30.49) = –0.58 | 0.56 |
| RBMT immediate recall | 473, 2.66 (2.11) | 29, 2.52 (2.43) | 0.14 | –0.80 to 1.08 | t(30.63) = 0.31 | 0.76 |
| RBMT delayed recall | 473, 0.38 (1.96) | 29, 0.12 (1.54) | 0.26 | –0.35 to 0.87 | t(33.79) = 0.86 | 0.40 |
| TEA elevator counting | 463, 6.39 (1.16) | 28, 6.25 (1.40) | 0.14 | –0.42 to 0.69 | t(29.29) = 0.5 | 0.62 |
| TEA elevator counting with distraction | 448, 4.55 (2.72) | 27, 4.07 (2.91) | 0.48 | –0.70 to 1.65 | t(28.8) = 0.83 | 0.41 |
| D-KEFS verbal fluency | 470, 26.27 (11.82) | 29, 21.76 (11.6) | 4.52 | –0.01 to 9.04 | t(31.69) = 2.03 | 0.05 |
| Secondary outcome measures: carers | ||||||
| RSS | 471, 18.96 (9.44) | 29, 21.14 (8.77) | –2.17 | –5.61 to 1.26 | t(32.12) = –1.29 | 0.21 |
| WHOQOL-BREF physical | 470, 15.34 (2.95) | 28, 15.86 (3.04) | –0.52 | –1.73 to 0.68 | t(30.1) = –0.88 | 0.38 |
| WHOQOL-BREF psychological | 470, 15.14 (2.15) | 28, 14.57 (2.70) | 0.57 | –0.49 to 1.63 | t(29.07) = 1.09 | 0.28 |
| WHOQOL-BREF social | 468, 15.13 (2.66) | 28, 15.18 (2.88) | –0.05 | –1.19 to 1.09 | t(29.83) = –0.09 | 0.93 |
| WHOQOL-BREF environmental | 470, 16.43 (2.15) | 28, 16.39 (2.20) | 0.04 | –0.83 to 0.91 | t(30.15) = 0.09 | 0.93 |
| EQ-5D-3L index | 468, 0.78 (0.25) | 29, 0.80 (0.24) | –0.03 | –0.12 to 0.07 | t(31.71) = –0.6 | 0.55 |
| EQ-5D-3L VAS | 467, 74.48 (19.95) | 28, 74.79 (23.64) | –0.31 | –9.64 to 9.02 | t(29.35) = –0.07 | 0.95 |
Appendix 7 Summary of missing data for secondary outcomes
Missing data for the secondary outcome measures
| Measure | Whole sample | Treatment group | ||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Missing, n (%) | Total, N | Missing, n (%) | Total, N | Missing, n (%) | Total, N | |
| Participants with dementia | ||||||
| DEMQOL score | 2 (0.42) | 472 | 1 (0) | 237 | 1 (0.42) | 235 |
| GSES score | 5 (1.05) | 469 | 1 (0) | 237 | 4 (1.69) | 232 |
| HADS depression score | 2 (0.42) | 472 | 0 (0) | 238 | 2 (0.85) | 234 |
| HADS anxiety score | 2 (0.42) | 472 | 0 (0) | 238 | 2 (0.85) | 234 |
| RBMT immediate recall | 1 (0.21) | 473 | 1 (0) | 237 | 0 (0.00) | 236 |
| RBMT delayed recall | 1 (0.21) | 473 | 1 (0) | 237 | 0 (0.00) | 236 |
| TEA elevator counting | 11 (2.32) | 463 | 6 (3) | 232 | 5 (2.12) | 231 |
| TEA elevator counting with distraction | 26 (5.47) | 448 | 15 (6) | 223 | 11 (4.66) | 225 |
| D-KEFS verbal fluency | 4 (0.84) | 470 | 3 (1) | 235 | 1 (0.42) | 235 |
| Carers | ||||||
| RSS | 3 (0.63) | 471 | 2 (1) | 236 | 1 (0.42) | 235 |
| WHOQOL-BREF physical | 4 (0.84) | 470 | 1 (0) | 237 | 3 (1.27) | 233 |
| WHOQOL-BREF psychological | 4 (0.84) | 470 | 1 (0) | 237 | 3 (1.27) | 233 |
| WHOQOL-BREF social | 6 (1.26) | 468 | 3 (1) | 235 | 3 (1.27) | 233 |
| WHOQOL-BREF environmental | 4 (0.84) | 470 | 1 (0) | 237 | 3 (1.27) | 233 |
| EQ-5D-3L index | 6 (1.26) | 468 | 3 (1) | 235 | 3 (1.27) | 233 |
| EQ-5D-3L VAS | 7 (1.47) | 467 | 4 (2) | 234 | 3 (1.27) | 233 |
| Measure | Whole sample | Treatment group | ||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Missing, n (%) | Total, N | Missing, n (%) | Total, N | Missing, n (%) | Total, N | |
| Participants with dementia | ||||||
| DEMQOL score | 0 (0.00) | 445 | 0 (0.00) | 218 | 0 (0.00) | 227 |
| GSES score | 6 (1.35) | 439 | 3 (1.38) | 215 | 3 (1.32) | 224 |
| HADS depression score | 1 (0.22) | 444 | 0 (0.00) | 218 | 1 (0.44) | 226 |
| HADS anxiety score | 3 (0.67) | 442 | 2 (0.92) | 216 | 1 (0.44) | 226 |
| RBMT immediate recall | 1 (0.22) | 444 | 0 (0.00) | 218 | 1 (0.44) | 226 |
| RBMT delayed recall | 3 (0.67) | 442 | 1 (0.46) | 217 | 2 (0.88) | 225 |
| TEA elevator counting | 16 (3.60) | 429 | 8 (3.67) | 210 | 8 (3.52) | 219 |
| TEA elevator counting with distraction | 39 (8.76) | 406 | 20 (9.17) | 198 | 19 (8.37) | 208 |
| D-KEFS verbal fluency | 1 (0.22) | 444 | 1 (0.46) | 217 | 0 (0.00) | 227 |
| Carers | ||||||
| RSS | 12 (2.70) | 433 | 6 (2.75) | 212 | 6 (2.64) | 221 |
| WHOQOL-BREF physical | 13 (2.92) | 432 | 6 (2.75) | 212 | 7 (3.08) | 220 |
| WHOQOL-BREF psychological | 13 (2.92) | 432 | 6 (2.75) | 212 | 7 (3.08) | 220 |
| WHOQOL-BREF social | 15 (3.37) | 430 | 7 (3.21) | 211 | 8 (3.52) | 219 |
| WHOQOL-BREF environmental | 13 (2.92) | 432 | 6 (2.75) | 212 | 7 (3.08) | 220 |
| EQ-5D-3L index | 19 (4.27) | 426 | 9 (4.13) | 209 | 10 (4.41) | 217 |
| EQ-5D-3L VAS | 20 (4.49) | 425 | 10 (4.59) | 208 | 10 (4.41) | 217 |
| Measure | Whole sample | Treatment group | ||||
|---|---|---|---|---|---|---|
| CR | TAU | |||||
| Missing, n (%) | Total, N | Missing, n (%) | Total, N | Missing, n (%) | Total, N | |
| Participants with dementia | ||||||
| DEMQOL score | 9 (2.11) | 417 | 4 (1.92) | 204 | 5 (2.29) | 213 |
| GSES score | 25 (5.87) | 401 | 14 (6.73) | 194 | 11 (5.05) | 207 |
| HADS depression score | 22 (5.16) | 404 | 14 (6.73) | 194 | 8 (3.67) | 210 |
| HADS anxiety score | 23 (5.40) | 403 | 15 (7.21) | 193 | 8 (3.67) | 210 |
| RBMT immediate recall | 15 (3.52) | 411 | 8 (3.85) | 200 | 7 (3.21) | 211 |
| RBMT delayed recall | 16 (3.76) | 410 | 8 (3.85) | 200 | 8 (3.67) | 210 |
| TEA elevator counting | 29 (6.81) | 397 | 17 (8.17) | 191 | 12 (5.50) | 206 |
| TEA elevator counting with distraction | 56 (13.15) | 370 | 31 (14.90) | 177 | 25 (11.47) | 193 |
| D-KEFS verbal fluency | 17 (3.99) | 409 | 10 (4.81) | 198 | 7 (3.21) | 211 |
| Carers | ||||||
| RSS | 15 (3.52) | 411 | 8 (3.85) | 200 | 7 (3.21) | 211 |
| WHOQOL-BREF physical | 17 (3.99) | 409 | 9 (4.33) | 199 | 8 (3.67) | 210 |
| WHOQOL-BREF psychological | 17 (3.99) | 409 | 9 (4.33) | 199 | 8 (3.67) | 210 |
| WHOQOL-BREF social | 19 (4.46) | 407 | 11 (5.29) | 197 | 8 (3.67) | 210 |
| WHOQOL-BREF environmental | 17 (3.99) | 409 | 9 (4.33) | 199 | 8 (3.67) | 210 |
| EQ-5D-3L index | 19 (4.46) | 407 | 12 (5.77) | 196 | 7 (3.21) | 211 |
| EQ-5D-3L VAS | 17 (3.99) | 409 | 10 (4.81) | 198 | 7 (3.21) | 211 |
| Measure | Missing, n (%) | Total, N |
|---|---|---|
| TEA distractor task at 9 months | 104 (21.90) | 370 |
| TEA no distractor task at 9 months | 77 (16.20) | 397 |
| GSES score at 9 months | 73 (15.40) | 401 |
| HADS score at 9 months | 71 (15.00) | 403 |
| TEA ECD at 3 months | 68 (14.30) | 406 |
| WHOQOL-BREF social scale at 9 months | 67 (14.10) | 407 |
| EQ-5D-3L index at 9 months | 67 (14.10) | 407 |
| WHOQOL-BREF environmental scale at 9 months | 65 (13.70) | 409 |
| WHOQOL-BREF psychological scale at 9 months | 65 (13.70) | 409 |
| WHOQOL-BREF physical scale at 9 months | 65 (13.70) | 409 |
| EQ-5D-3L VAS at 9 months | 65 (13.70) | 409 |
| D-KEFS verbal fluency at 9 months | 65 (13.70) | 409 |
| RBMT delayed recall score at 9 months | 64 (13.50) | 410 |
| RSS at 9 months | 63 (13.30) | 411 |
| RBMT immediate recall score at 9 months | 63 (13.30) | 411 |
| BGSI participant satisfaction rating at 9 months | 62 (13.10) | 412 |
| BGSI carer goal attainment rating at 9 months | 59 (12.40) | 415 |
| BGSI participant goal attainment rating at 9 months | 58 (12.20) | 416 |
| DEMQOL at 9 months | 57 (12.00) | 417 |
| EQ-5D-3L VAS at 3 months | 49 (10.30) | 425 |
| EQ-5D-3L index at 3 months | 48 (10.10) | 426 |
| TEA elevator counting at 3 months | 45 (9.50) | 429 |
| WHOQOL-BREF social scale at 3 months | 44 (9.30) | 430 |
| WHOQOL-BREF environmental scale at 3 months | 42 (8.90) | 432 |
| WHOQOL-BREF psychological scale at 3 months | 42 (8.90) | 432 |
| WHOQOL-BREF physical scale at 3 months | 42 (8.90) | 432 |
| RSS at 3 months | 41 (8.60) | 433 |
| GSES at 3 months | 35 (7.40) | 439 |
| BGSI carer goal attainment rating at 3 months | 35 (7.40) | 439 |
| RBMT delayed recall score at 3 months | 32 (6.80) | 442 |
| HADS at 3 months | 32 (6.80) | 442 |
| D-KEFS FAS test at 3 months | 30 (6.30) | 444 |
| RBMT immediate recall score at 3 months | 30 (6.30) | 444 |
| DEMQOL at 3 months | 29 (6.10) | 445 |
| BGSI participant satisfaction rating at 3 months | 29 (6.10) | 445 |
| BGSI participant goal attainment rating at 3 months | 29 (6.10) | 445 |
| TEA ECD at baseline | 26 (5.50) | 448 |
| TEA elevator counting at baseline | 11 (2.30) | 463 |
| EQ-5D-3L VAS at baseline | 7 (1.50) | 467 |
| WHOQOL-BREF social scale at baseline | 6 (1.30) | 468 |
| EQ-5D-3L index at baseline | 6 (1.30) | 468 |
| GSES at baseline | 5 (1.10) | 469 |
| WHOQOL-BREF environmental scale at baseline | 4 (0.80) | 470 |
| WHOQOL-BREF psychological scale at baseline | 4 (0.80) | 470 |
| WHOQOL-BREF physical scale at baseline | 4 (0.80) | 470 |
| D-KEFS verbal fluency at baseline | 4 (0.80) | 470 |
| RSS at baseline | 3 (0.60) | 471 |
| HADS at baseline | 2 (0.40) | 472 |
| DEMQOL at baseline | 2 (0.40) | 472 |
| RBMT delayed recall score at baseline | 1 (0.20) | 473 |
| RBMT immediate recall score at baseline | 1 (0.20) | 473 |
| BGSI carer goal attainment score at baseline | 0 (0.00) | 474 |
| BGSI participant satisfaction score at baseline | 0 (0.00) | 474 |
| BGSI participant goal attainment score at baseline | 0 (0.00) | 474 |
Appendix 8 Exploratory analyses for the primary outcome measure
Participants’ goal attainment ratings
Participant characteristics as predictors of differences in participants’ own Bangor Goal-Setting Interview goal attainment ratings
Linear mixed-effects model fitted to identify potential participant characteristics as predictors of differences between the Bangor Goal-Setting Interview attainment ratings at baseline and 3 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 15.52 | 1 | 0.000 |
| Sex | 0.21 | 1 | 0.646 |
| Age (stratified) | 1.91 | 1 | 0.167 |
| MMSE score | 0.51 | 1 | 0.476 |
| Diagnosis | 2.05 | 2 | 0.358 |
| Medication | 0.18 | 1 | 0.674 |
| Education | 0.71 | 4 | 0.950 |
| Comorbidity | 0.02 | 1 | 0.897 |
| Social status | 16.66 | 5 | 0.005 |
| Blinding inefficient | 16.95 | 1 | 0.000 |
| Centre | 1.58 | 1 | 0.209 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 3.49 | 0.89 | 212.87 | 3.94 | 0.000 | 1.73 to 5.25 |
| Aged ≥ 75 years vs. < 75 years | –0.44 | 0.32 | 216.78 | –1.38 | 0.168 | –1.06 to 0.19 |
| Blinding inefficient vs. maintained | 1.24 | 0.30 | 216.87 | 4.12 | 0.000 | 0.54 to 1.76 |
| Sex: male vs. female | –0.14 | 0.31 | 213.81 | –0.46 | 0.647 | –0.77 to 0.48 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.20 | 0.28 | 216.70 | 0.71 | 0.476 | –0.35 to 0.75 |
| Comorbidity | 0.01 | 0.09 | 216.93 | 0.13 | 0.897 | –0.18 to 0.20 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | –0.49 | 0.53 | 214.37 | –0.92 | 0.359 | –1.55 to 0.57 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.26 | 0.35 | 216.99 | 0.74 | 0.462 | –0.44 to 0.96 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –0.56 | 0.80 | 216.26 | –0.70 | 0.484 | –2.15 to 1.02 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.15 | 0.33 | 216.77 | –0.46 | 0.648 | –0.79 to 0.49 |
| Person with dementia: education – higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.22 | 0.52 | 212.44 | –0.42 | 0.673 | –1.25 to 0.81 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.28 | 0.61 | 210.50 | –0.47 | 0.640 | –1.48 to 0.91 |
| Medication: yes vs. no | –0.18 | 0.44 | 216.02 | –0.42 | 0.674 | –1.04 to 0.68 |
| Social status: II managerial/technical vs. I professional | –0.86 | 0.56 | 211.15 | –1.53 | 0.127 | –2.48 to 0.08 |
| Social status: III N skilled, non-manual vs. I professional | –1.66 | 0.65 | 214.71 | –2.55 | 0.012 | –3.39 to –0.56 |
| Social status: III M skilled, manual vs. I professional | –2.11 | 0.65 | 216.57 | –3.28 | 0.001 | –4.17 to –1.05 |
| Social status: IV partly skilled vs. I professional | –1.93 | 0.75 | 216.88 | –2.58 | 0.011 | –3.83 to –0.67 |
| Social status: V unskilled vs. I professional | –0.84 | 0.81 | 216.41 | –1.03 | 0.302 | –2.73 to 0.56 |
Linear mixed-effects model fitted to identify potential participant characteristics as predictors of differences between the Bangor Goal-Setting Interview attainment ratings at baseline and 9 months’ follow-up
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 2.38 | 1 | 0.123 |
| Blinding inefficient | 17.75 | 1 | 0.000 |
| Age | 4.35 | 1 | 0.037 |
| Sex | 0.14 | 1 | 0.712 |
| Education | 1.82 | 4 | 0.769 |
| Social status | 8.96 | 5 | 0.111 |
| Ethnicity | 8.68 | 9 | 0.468 |
| Living situation | 0.00 | 1 | 0.976 |
| Diagnosis | 5.10 | 2 | 0.078 |
| MMSE score | 9.38 | 1 | 0.002 |
| Medication | 1.96 | 1 | 0.161 |
| Comorbidity | 0.03 | 1 | 0.855 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 3.71 | 2.40 | 202.66 | 1.54 | 0.124 | –1.03 to 8.45 |
| Blinding inefficient vs. maintained | 1.35 | 0.32 | 199.86 | 4.21 | 0.000 | 0.71 to 1.99 |
| Aged ≥ 75 years vs. < 75 years | –0.05 | 0.02 | 200.60 | –2.08 | 0.038 | –0.09 to 0.00 |
| Sex: female vs. male | 0.13 | 0.35 | 198.44 | 0.37 | 0.713 | –0.56 to 0.82 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.16 | 0.05 | 199.31 | 3.06 | 0.002 | 0.06 to 0.26 |
| Comorbidity | –0.02 | 0.11 | 199.72 | –0.18 | 0.855 | –0.24 to 0.20 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | –1.25 | 0.58 | 201.57 | –2.14 | 0.034 | –2.40 to –0.10 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.58 | 0.39 | 200.38 | –1.47 | 0.144 | –1.35 to 0.20 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.32 | 0.90 | 200.09 | 0.36 | 0.719 | –1.47 to 2.11 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.40 | 0.37 | 200.34 | –1.08 | 0.283 | –1.13 to 0.33 |
| Education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.46 | 0.56 | 197.23 | –0.82 | 0.415 | –1.56 to 0.64 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.43 | 0.65 | 196.81 | –0.65 | 0.514 | –1.72 to 0.86 |
| Ethnicity: any other Asian background vs. Welsh/English/Scottish/Northern Irish/British | 0.85 | 1.55 | 201.37 | 0.55 | 0.583 | –2.22 to 3.92 |
| Ethnicity: African vs. Welsh/English/Scottish/Northern Irish/British | –0.11 | 2.12 | 199.11 | –0.05 | 0.959 | –4.30 to 4.07 |
| Ethnicity: Caribbean vs. Welsh/English/Scottish/Northern Irish/British | –0.79 | 1.48 | 198.49 | –0.53 | 0.594 | –3.72 to 2.13 |
| Ethnicity: any other ethnic group vs. Welsh/English/Scottish/Northern Irish/British | 0.35 | 1.49 | 198.44 | 0.24 | 0.813 | –2.58 to 3.29 |
| Ethnicity: Irish vs. Welsh/English/Scottish/Northern Irish/British | –1.40 | 0.98 | 200.21 | –1.43 | 0.156 | –3.34 to 0.54 |
| Ethnicity: any other white background vs. Welsh/English/Scottish/Northern Irish/British | 1.19 | 1.07 | 198.49 | 1.11 | 0.268 | –0.92 to 3.30 |
| Ethnicity: white and black Caribbean vs. Welsh/English/Scottish/Northern Irish/British | 0.91 | 2.10 | 198.39 | 0.43 | 0.665 | –3.23 to 5.05 |
| Ethnicity: any other mixed/multiple ethnic background vs. Welsh/English/Scottish/Northern Irish/British | –2.26 | 2.12 | 200.30 | –1.07 | 0.287 | –6.46 to 1.93 |
| Ethnicity: Indian vs. Welsh/English/Scottish/Northern Irish/British | 4.45 | 2.36 | 201.54 | 1.88 | 0.061 | –0.24 to 9.12 |
| Living situation: not alone vs. alone | 0.01 | 0.41 | 197.96 | 0.03 | 0.977 | –0.79 to 0.82 |
| Medication: yes vs. no | –0.68 | 0.48 | 198.58 | –1.40 | 0.163 | –1.63 to 0.27 |
| Social status: II managerial/technical vs. I professional | –1.02 | 0.61 | 196.38 | –1.67 | 0.096 | –2.21 to 0.18 |
| Social status: III N skilled, non-manual vs. I professional | –1.42 | 0.71 | 198.56 | –2.01 | 0.045 | –2.82 to –0.03 |
| Social status: III M skilled, manual vs. I professional | –1.77 | 0.69 | 200.80 | –2.55 | 0.011 | –3.13 to –0.40 |
| Social status: IV partly skilled vs. I professional | –2.02 | 0.82 | 199.67 | –2.47 | 0.014 | –3.63 to –0.41 |
| Social status: V unskilled vs. I professional | –0.98 | 0.90 | 201.09 | –1.09 | 0.276 | –2.76 to 0.79 |
Carer characteristics as predictors of differences in participant’s own rating Bangor Goal-Setting Interview goal attainment
Linear mixed-effects model to identify potential carer factors as predictors of differences between the participants’ Bangor Goal-Setting Interview attainment ratings at baseline and 3 months’ follow-up for participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 0.02 | 1 | 0.00 | 0.946 |
| Carer age | 0.18 | 1 | 0.04 | 0.845 |
| Carer sex | 3.95 | 1 | 0.85 | 0.358 |
| Carer education | 36.00 | 4 | 1.93 | 0.107 |
| Person with dementia and carer relationship | 27.38 | 7 | 0.84 | 0.556 |
| Carer hours | 29.16 | 8 | 0.78 | 0.619 |
| Residuals | 866.40 | 186 | NA | NA |
| Carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –0.13 | 1.97 | 186 | –0.07 | 0.946 | –4.02 to 3.76 |
| Carer age | 0.00 | 0.02 | 186 | 0.20 | 0.845 | –0.03 to 0.04 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 1.51 | 0.66 | 186 | 2.28 | 0.024 | 0.21 to 2.82 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 0.36 | 0.39 | 186 | 0.93 | 0.355 | –0.41 to 1.14 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.41 | 0.51 | 186 | 0.81 | 0.420 | –0.59 to 1.41 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | 1.06 | 0.52 | 186 | 2.05 | 0.042 | 0.04 to 2.08 |
| Carer sex: male vs. female | –0.32 | 0.35 | 186 | –0.92 | 0.358 | –1.00 to 0.36 |
| Hours providing care in a typical day: < 1 hour vs. none | 0.51 | 0.72 | 186 | 0.71 | 0.476 | –0.91 to 1.93 |
| Hours providing care in a typical day: > 1 hour and up to 2 hours vs. none | –0.02 | 0.76 | 186 | –0.03 | 0.975 | –1.52 to 1.47 |
| Hours providing care in a typical day: > 2 hours and up to 3 hours vs. none | 0.52 | 0.79 | 186 | 0.65 | 0.515 | –1.04 to 2.07 |
| Hours providing care in a typical day: > 3 hours and up to 5 hours vs. none | 1.12 | 0.82 | 186 | 1.38 | 0.171 | –0.49 to 2.73 |
| Hours providing care in a typical day: > 5 hours and up to 10 hours vs. none | 1.01 | 0.80 | 186 | 1.26 | 0.209 | –0.57 to 2.60 |
| Hours providing care in a typical day: > 10 hours, but not overnight vs. none | 0.85 | 0.95 | 186 | 0.89 | 0.373 | –1.02 to 2.72 |
| Hours providing care in a typical day: > 10 hours and/including overnight vs. none | 0.57 | 0.74 | 186 | 0.77 | 0.445 | –0.89 to 2.02 |
| Hours providing care in a typical day: other, describe vs. none | 2.08 | 1.67 | 186 | 1.24 | 0.215 | –1.22 to 5.38 |
| Person with dementia and carer relationship: friend vs. brother/sister | 0.35 | 1.79 | 186 | 0.20 | 0.845 | –3.18 to 3.88 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 1.48 | 2.65 | 186 | 0.56 | 0.577 | –3.74 to 6.70 |
| Person with dementia and carer relationship: other vs. brother/sister | 2.77 | 1.79 | 186 | 1.55 | 0.124 | –0.76 to 6.30 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.00 | 1.39 | 186 | 0.72 | 0.470 | –1.73 to 3.74 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 1.48 | 1.37 | 186 | 1.08 | 0.283 | –1.23 to 4.19 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 0.62 | 1.72 | 186 | 0.36 | 0.720 | –2.78 to 4.01 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.72 | 1.28 | 186 | 1.34 | 0.182 | –0.81 to 4.26 |
Linear mixed-effects model to identify potential carer factors as predictors of differences between the participants’ Bangor Goal-Setting Interview attainment ratings at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 0.07 | 1 | 0.01 | 0.913 |
| Carer age | 2.31 | 1 | 0.39 | 0.532 |
| Carer sex | 1.39 | 1 | 0.24 | 0.627 |
| Carer education | 24.30 | 4 | 1.03 | 0.391 |
| Person with dementia and carer relationship | 34.18 | 7 | 0.83 | 0.563 |
| Carer hours | 84.75 | 8 | 1.80 | 0.080 |
| Residuals | 1017.09 | 173 | NA | NA |
| Carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 0.25 | 2.25 | 173 | 0.11 | 0.913 | –4.19 to 4.68 |
| Carer age | –0.01 | 0.02 | 173 | –0.63 | 0.532 | –0.06 to 0.03 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.80 | 0.75 | 173 | 1.07 | 0.287 | –0.68 to 2.28 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 0.75 | 0.46 | 173 | 1.62 | 0.107 | –0.16 to 1.65 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.38 | 0.59 | 173 | 0.64 | 0.523 | –0.78 to 1.54 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | 1.01 | 0.60 | 173 | 1.67 | 0.097 | –0.18 to 2.20 |
| Carer sex: male vs. female | –0.20 | 0.41 | 173 | –0.49 | 0.627 | –1.00 to 0.61 |
| Hours providing care in a typical day: < 1 hour vs. none | 2.42 | 0.83 | 173 | 2.93 | 0.004 | 0.79 to 4.05 |
| Hours providing care in a typical day: > 1 hour and up to 2 hours vs. none | 1.55 | 0.85 | 173 | 1.81 | 0.072 | –0.14 to 3.24 |
| Hours providing care in a typical day: > 2 hours and up to 3 hours vs. none | 2.34 | 0.89 | 173 | 2.63 | 0.009 | 0.58 to 4.09 |
| Hours providing care in a typical day: > 3 hours and up to 5 hours vs. none | 2.82 | 0.95 | 173 | 2.97 | 0.003 | 0.94 to 4.69 |
| Hours providing care in a typical day: > 5 hours and up to 10 hours vs. none | 2.06 | 0.91 | 173 | 2.27 | 0.024 | 0.27 to 3.85 |
| Hours providing care in a typical day: > 10 hours, but not overnight vs. none | 2.23 | 1.10 | 173 | 2.03 | 0.044 | 0.06 to 4.40 |
| Hours providing care in a typical day: > 10 hours and/including overnight vs. none | 1.64 | 0.84 | 173 | 1.97 | 0.051 | –0.01 to 3.29 |
| Hours providing care in a typical day: other, describe vs. none | 4.07 | 1.88 | 173 | 2.16 | 0.032 | 0.35 to 7.78 |
| Person with dementia and carer relationship: friend vs. brother/sister | –1.81 | 2.25 | 173 | –0.81 | 0.421 | –6.25 to 2.62 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | –1.33 | 2.98 | 173 | –0.44 | 0.657 | –7.22 to 4.56 |
| Person with dementia and carer relationship: other vs. brother/sister | –0.71 | 2.27 | 173 | –0.32 | 0.753 | –5.19 to 3.76 |
| Person with dementia and carer relationship: partner vs. brother/sister | 0.71 | 1.57 | 173 | 0.45 | 0.653 | –2.39 to 3.80 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.25 | 1.55 | 173 | 0.16 | 0.873 | –2.81 to 3.31 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | –0.32 | 1.94 | 173 | –0.17 | 0.867 | –4.15 to 3.50 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.16 | 1.45 | 173 | 0.80 | 0.423 | –1.69 to 4.01 |
Carers’ ratings of participant goal attainment
Carer characteristics as predictors of differences in carer’s rating of Bangor Goal-Setting Interview goal attainment
Linear mixed-effects model fitted to identify potential carer factors as predictors of differences between the carers’ Bangor Goal-Setting Interview attainment ratings at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 0.31 | 1 | 0.07 | 0.793 |
| Carer’s age | 0.26 | 1 | 0.06 | 0.809 |
| Carer’s sex | 7.92 | 1 | 1.76 | 0.186 |
| Carer’s education | 38.27 | 4 | 2.13 | 0.078 |
| Person with dementia and carer relationship | 32.22 | 7 | 1.03 | 0.415 |
| Residuals | 897.85 | 200 | NA | NA |
| Carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| Carer’s age | 0.88 | 1.83 | 202 | 0.48 | 0.631 | –2.73 to 4.49 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –0.00 | 0.02 | 202 | –0.12 | 0.908 | –0.04 to 0.03 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 1.38 | 0.66 | 202 | 2.09 | 0.038 | 0.08 to 2.68 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.19 | 0.37 | 202 | 0.51 | 0.607 | –0.54 to 0.93 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.20 | 0.49 | 202 | 0.41 | 0.685 | –0.76 to 1.15 |
| Carer sex: male vs. female | 0.73 | 0.49 | 202 | 1.49 | 0.138 | –0.24 to 1.70 |
| Person with dementia and carer relationship: friend vs. brother/sister | –0.22 | 0.33 | 202 | –0.67 | 0.507 | –0.88 to 0.43 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 1.22 | 1.58 | 202 | 0.77 | 0.444 | –1.91 to 4.34 |
| Person with dementia and carer relationship: other vs. brother/sister | 1.32 | 2.62 | 202 | 0.51 | 0.614 | –3.85 to 6.49 |
| Person with dementia and carer relationship: partner vs. brother/sister | 2.90 | 1.78 | 202 | 1.63 | 0.106 | –0.62 to 6.41 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 1.18 | 1.36 | 202 | 0.87 | 0.388 | –1.51 to 3.86 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 1.43 | 1.36 | 202 | 1.05 | 0.294 | –1.25 to 4.11 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 0.57 | 1.71 | 202 | 0.33 | 0.740 | –2.80 to 3.94 |
Linear mixed-effects model to identify carer characteristics predicting differences between carers’ Bangor Goal-Setting Interview attainment ratings at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 6.55 | 1 | 1.05 | 0.306 |
| Carer’s age | 4.15 | 1 | 0.67 | 0.415 |
| Carer’s sex | 4.56 | 1 | 0.73 | 0.393 |
| Carer’s education | 19.24 | 4 | 0.77 | 0.544 |
| Person with dementia and carer relationship | 65.52 | 7 | 1.51 | 0.168 |
| Residuals | 1169.09 | 188 | NA | NA |
| Carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| Carer’s age | 2.67 | 2.15 | 189 | 1.24 | 0.217 | –1.58 to 6.91 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –0.02 | 0.02 | 189 | –0.78 | 0.439 | –0.06 to 0.03 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 0.62 | 0.77 | 189 | 0.80 | 0.422 | –0.90 to 2.13 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.41 | 0.45 | 189 | 0.91 | 0.364 | –0.48 to 1.29 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.19 | 0.58 | 189 | 0.32 | 0.749 | –0.96 to 1.33 |
| Carer sex: male vs. female | 0.61 | 0.59 | 189 | 1.04 | 0.301 | –0.55 to 1.76 |
| Person with dementia and carer relationship: friend vs. brother/sister | –0.01 | 0.40 | 189 | –0.02 | 0.982 | –0.79 to 0.77 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 0.29 | 1.92 | 189 | 0.15 | 0.878 | –3.50 to 4.08 |
| Person with dementia and carer relationship: other vs. brother/sister | –0.89 | 3.04 | 189 | –0.29 | 0.771 | –6.89 to 5.11 |
| Person with dementia and carer relationship: partner vs. brother/sister | –0.16 | 2.30 | 189 | –0.07 | 0.943 | –4.70 to 4.37 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.58 | 1.58 | 189 | 0.37 | 0.713 | –2.54 to 3.70 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 0.35 | 1.58 | 189 | 0.22 | 0.824 | –2.76 to 3.46 |
| Person with dementia and carer relationship: spouse vs. brother/sister | –0.62 | 1.98 | 189 | –0.31 | 0.755 | –4.52 to 3.28 |
Participant characteristics as predictors of differences in carers’ Bangor Goal-Setting Interview goal attainment ratings
Linear mixed-effects model to identify participant characteristics predicting the difference between carer Bangor Goal-Setting Interview goal attainment ratings at baseline and at 3 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 16.18 | 1 | 0.000 |
| Sex | 0.07 | 1 | 0.797 |
| Age | 0.28 | 1 | 0.597 |
| MMSE | 2.41 | 1 | 0.121 |
| Diagnosis | 0.17 | 2 | 0.920 |
| Medication | 0.43 | 1 | 0.511 |
| Education | 3.74 | 4 | 0.442 |
| Comorbidity | 0.09 | 1 | 0.762 |
| Social status | 14.54 | 5 | 0.013 |
| Centre | 0.10 | 1 | 0.752 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 3.52 | 0.88 | 209.17 | 4.02 | 0.000 | 1.82 to 5.26 |
| Aged ≥ 75 years vs. < 75 years | –0.17 | 0.32 | 213.40 | –0.53 | 0.598 | –0.81 to 0.47 |
| Sex: female vs. male | –0.08 | 0.33 | 212.67 | –0.26 | 0.797 | –0.73 to 0.56 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.45 | 0.29 | 214.41 | 1.55 | 0.122 | –0.11 to 1.02 |
| Comorbidity | –0.03 | 0.10 | 212.65 | –0.30 | 0.762 | –0.22 to 0.16 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | 0.18 | 0.55 | 182.80 | 0.33 | 0.741 | –0.90 to 1.27 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.12 | 0.36 | 213.42 | 0.33 | 0.743 | –0.60 to 0.83 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –1.24 | 0.82 | 214.98 | –1.51 | 0.133 | –2.86 to 0.37 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 0.09 | 0.34 | 214.77 | 0.26 | 0.798 | –0.58 to 0.75 |
| Education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.38 | 0.54 | 211.43 | 0.70 | 0.482 | –0.68 to 1.42 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.29 | 0.62 | 207.88 | –0.47 | 0.636 | –1.53 to 0.92 |
| Medication: yes vs. no | 0.30 | 0.46 | 214.45 | 0.66 | 0.512 | –0.60 to 1.19 |
| Social status: II managerial/technical vs. I professional | –0.85 | 0.58 | 209.24 | –1.46 | 0.146 | –2.00 to 0.29 |
| Person with dementia social status: III N skilled, non-manual vs. I professional | –1.83 | 0.68 | 214.14 | –2.70 | 0.007 | –3.15 to – 0.50 |
| Social status: III M skilled, manual vs. I professional | –1.63 | 0.67 | 214.99 | –2.46 | 0.015 | –2.94 to –0.33 |
| Social status: IV partly skilled vs. I professional | –1.30 | 0.77 | 214.36 | –1.70 | 0.091 | –2.81 to 0.21 |
| Social status: V unskilled vs. I professional | –0.21 | 0.84 | 214.47 | –0.25 | 0.803 | –1.85 to 1.44 |
Linear mixed-effects model to identify participant characteristics predicting the difference between the carer Bangor Goal-Setting Interview attainment ratings at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 11.81 | 1 | 0.001 |
| Sex | 0.03 | 1 | 0.873 |
| Age | 1.46 | 1 | 0.227 |
| MMSE | 7.91 | 1 | 0.005 |
| Diagnosis | 1.28 | 2 | 0.528 |
| Medication | 0.56 | 1 | 0.454 |
| Education | 2.03 | 4 | 0.731 |
| Comorbidity | 0.27 | 1 | 0.603 |
| Social status | 9.97 | 5 | 0.076 |
| Centre | 2.48 | 1 | 0.115 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 3.59 | 1.04 | 199.06 | 3.44 | 0.001 | 1.52 to 5.65 |
| Aged ≥ 75 years vs. < 75 years | –0.46 | 0.38 | 203.22 | –1.21 | 0.228 | –1.22 to 0.29 |
| Sex: female vs. male | 0.06 | 0.39 | 200.54 | 0.16 | 0.873 | –0.71 to 0.83 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.96 | 0.34 | 202.98 | 2.81 | 0.005 | 0.29 to 1.63 |
| Comorbidity | 0.06 | 0.11 | 203.31 | 0.52 | 0.604 | –0.17 to 0.28 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | –0.75 | 0.66 | 203.73 | –1.13 | 0.260 | –2.06 to 0.56 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.16 | 0.43 | 203.66 | –0.37 | 0.712 | –1.00 to 0.68 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –1.04 | 1.01 | 203.00 | –1.03 | 0.305 | –3.07 to 0.98 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.19 | 0.40 | 201.80 | –0.47 | 0.640 | –0.97 to 0.50 |
| Education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.69 | 0.62 | 199.50 | –1.12 | 0.263 | –1.92 to 0.53 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.20 | 0.72 | 197.40 | –0.27 | 0.784 | –1.62 to 1.22 |
| Medication: yes vs. no | –0.42 | 0.55 | 200.68 | –0.75 | 0.454 | –1.51 to 0.68 |
| Social status: II managerial/technical vs. I professional | –0.50 | 0.68 | 197.44 | –0.74 | 0.463 | –0.94 to 0.42 |
| Social status: III N skilled, non-manual vs. I professional | –1.51 | 0.79 | 201.73 | –1.92 | 0.056 | –2.09 to –0.48 |
| Social status: III M skilled, manual vs. I professional | –1.06 | 0.78 | 203.06 | –1.36 | 0.175 | –1.73 to –0.09 |
| Social status: IV partly skilled vs. I professional | –1.87 | 0.90 | 203.39 | –2.08 | 0.039 | –2.71 to –0.78 |
| Social status: V unskilled vs. I professional | –0.13 | 1.00 | 203.32 | –0.14 | 0.893 | –0.54 to 1.30 |
Participants’ ratings of satisfaction with goal attainment
Participant characteristics as predictors of differences in participants Bangor Goal-Setting Interview satisfaction ratings
Linear mixed-effects model to identify participant characteristics predicting the difference between Bangor Goal-Setting Interview satisfaction ratings at baseline and at 3 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 6.02 | 1 | 0.014 |
| Blinding inefficient | 10.30 | 1 | 0.001 |
| Age (stratified) | 3.08 | 1 | 0.079 |
| Sex | 0.50 | 1 | 0.478 |
| Education | 6.06 | 4 | 0.194 |
| Social status | 16.82 | 5 | 0.005 |
| Ethnicity | 4.41 | 9 | 0.882 |
| Living situation | 1.46 | 1 | 0.227 |
| Diagnosis | 2.36 | 2 | 0.307 |
| MMSE score (stratified) | 2.61 | 1 | 0.106 |
| Medication | 0.31 | 1 | 0.576 |
| Comorbidity | 0.00 | 1 | 0.973 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 5.26 | 2.15 | 217 | 2.45 | 0.015 | 1.04 to 9.49 |
| Blinding inefficient vs. maintained | 0.96 | 0.30 | 217 | 3.21 | 0.002 | 0.37 to 1.54 |
| Aged ≥ 75 years vs. < 75 years | –0.03 | 0.02 | 217 | –1.75 | 0.081 | –0.07 to 0.00 |
| Sex: female vs. male | –0.23 | 0.32 | 217 | –0.71 | 0.479 | –0.86 to 0.41 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.07 | 0.05 | 217 | 1.62 | 0.107 | –0.02 to 0.17 |
| Comorbidity | 0.00 | 0.10 | 217 | 0.03 | 0.973 | –0.19 to 0.20 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | –0.57 | 0.52 | 217 | –1.10 | 0.274 | –1.58 to 0.45 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.23 | 0.35 | 217 | 0.66 | 0.512 | –0.46 to 0.92 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.12 | 0.79 | 217 | 0.15 | 0.878 | –1.44 to 1.69 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.63 | 0.33 | 217 | –1.91 | 0.058 | –1.29 to 0.02 |
| Education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.06 | 0.52 | 217 | 0.11 | 0.915 | –0.97 to 1.09 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.98 | 0.61 | 217 | –1.60 | 0.112 | –2.18 to 0.23 |
| Ethnicity: any other Asian background vs. Welsh/English/Scottish/Northern Irish/British | –0.85 | 1.42 | 217 | –0.60 | 0.550 | –3.64 to 1.94 |
| Ethnicity: African vs. Welsh/English/Scottish/Northern Irish/British | –0.82 | 1.98 | 217 | –0.42 | 0.678 | –4.71 to 3.07 |
| Ethnicity: Caribbean vs. Welsh/English/Scottish/Northern Irish/British | –0.72 | 1.02 | 217 | –0.71 | 0.481 | –2.73 to 1.29 |
| Ethnicity: any other ethnic group vs. Welsh/English/Scottish/Northern Irish/British | 0.47 | 1.40 | 217 | 0.33 | 0.739 | –2.28 to 3.21 |
| Ethnicity: Irish vs. Welsh/English/Scottish/Northern Irish/British | –1.06 | 0.91 | 217 | –1.16 | 0.248 | –2.85 to 0.74 |
| Ethnicity: any other white background vs. Welsh/English/Scottish/Northern Irish/British | 0.67 | 1.00 | 217 | 0.67 | 0.504 | –1.30 to 2.65 |
| Ethnicity: white and black Caribbean vs. Welsh/English/Scottish/Northern Irish/British | –2.09 | 1.97 | 217 | –1.06 | 0.290 | –5.97 to 1.79 |
| Ethnicity: any other mixed/multiple ethnic background vs. Welsh/English/Scottish/Northern Irish/British | –0.41 | 1.97 | 217 | –0.21 | 0.837 | –4.30 to 3.48 |
| Ethnicity: Indian vs. Welsh/English/Scottish/Northern Irish/British | 1.93 | 2.15 | 217 | 0.90 | 0.369 | –2.29 to 6.15 |
| Living situation: not alone vs. alone | –0.45 | 0.37 | 217 | –1.21 | 0.229 | –1.18 to 0.28 |
| Medication: yes vs. no | –0.24 | 0.43 | 217 | –0.56 | 0.577 | –1.09 to 0.61 |
| Social status: II managerial/technical vs. I professional | –0.82 | 0.56 | 217 | –1.46 | 0.145 | –1.92 to 0.28 |
| Social status: III N skilled, non-manual vs. I professional | –1.73 | 0.65 | 217 | –2.67 | 0.008 | –3.01 to –0.46 |
| Social status: III M skilled, manual vs. I professional | –2.10 | 0.63 | 217 | –3.34 | 0.001 | –3.33 to –0.86 |
| Social status: IV partly skilled | –1.92 | 0.75 | 217 | –2.57 | 0.011 | –3.39 to –0.45 |
| Social status: V unskilled vs. I professional | –1.33 | 0.81 | 217 | –1.64 | 0.102 | –2.93 to 0.26 |
Linear mixed-effects model fitted to predict the difference between the participants’ Bangor Goal-Setting Interview satisfaction ratings at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.36 | 1 | 0.549 |
| Blinding inefficient | 14.35 | 1 | 0.000 |
| Age | 0.99 | 1 | 0.320 |
| Sex | 0.04 | 1 | 0.846 |
| Education | 5.16 | 4 | 0.271 |
| Social status | 7.45 | 5 | 0.189 |
| Ethnicity | 6.55 | 8 | 0.586 |
| Living situation | 0.30 | 1 | 0.582 |
| Diagnosis | 4.07 | 2 | 0.131 |
| MMSE score | 15.79 | 1 | 0.000 |
| Medication | 0.71 | 1 | 0.399 |
| Comorbidity | 0.04 | 1 | 0.848 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 1.46 | 2.44 | 200.31 | 0.60 | 0.550 | –3.35 to 6.27 |
| Blinding inefficient vs. maintained | 1.23 | 0.33 | 200.33 | 3.79 | 0.000 | 0.58 to 1.88 |
| Aged ≥ 75 years vs. < 75 years | –0.02 | 0.02 | 201.19 | –0.99 | 0.321 | –0.00 to 0.01 |
| Sex: female vs. male | 0.07 | 0.36 | 197.40 | 0.19 | 0.846 | 0.10 to 0.52 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.20 | 0.05 | 199.45 | 3.97 | 0.000 | 0.22 to 0.27 |
| Comorbidity | –0.02 | 0.11 | 200.71 | –0.19 | 0.848 | –0.05 to 0.10 |
| Diagnosis: vascular dementia vs. Alzheimer’s disease | –1.18 | 0.59 | 201.99 | –2.01 | 0.046 | –2.34 to –0.02 |
| Diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.21 | 0.40 | 201.19 | –0.54 | 0.592 | –1.00 to 0.57 |
| Education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.61 | 0.91 | 200.35 | 0.67 | 0.501 | 0.04 to 1.42 |
| Education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.73 | 0.38 | 200.91 | –1.96 | 0.052 | –0.53 to –0.21 |
| Education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.17 | 0.57 | 196.08 | –0.29 | 0.771 | –0.25 to 0.48 |
| Education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.62 | 0.67 | 194.91 | –0.93 | 0.355 | –0.66 to 0.17 |
| Ethnicity: any other Asian background vs. Welsh/English/Scottish/Northern Irish/British | 0.27 | 1.56 | 201.79 | 0.17 | 0.864 | –0.49 to 1.74 |
| Ethnicity: Caribbean vs. Welsh/English/Scottish/Northern Irish/British | –2.70 | 1.50 | 198.31 | –1.80 | 0.073 | –3.29 to –1.19 |
| Ethnicity: any other ethnic group vs. Welsh/English/Scottish/Northern Irish/British | 0.29 | 1.51 | 198.61 | 0.19 | 0.848 | 0.27 to 2.10 |
| Ethnicity: Irish vs. Welsh/English/Scottish/Northern Irish/British | –0.37 | 0.99 | 200.81 | –0.37 | 0.711 | –0.85 to 0.57 |
| Ethnicity: any other white background vs. Welsh/English/Scottish/Northern Irish/British | 1.34 | 1.09 | 198.63 | 1.24 | 0.218 | 1.14 to 2.55 |
| Ethnicity: white and black Caribbean vs. Welsh/English/Scottish/Northern Irish/British | –0.74 | 2.13 | 198.56 | –0.35 | 0.729 | –0.44 to 1.96 |
| Ethnicity: any other mixed/multiple ethnic background vs. Welsh/English/Scottish/Northern Irish/British | –1.80 | 2.14 | 200.71 | –0.84 | 0.399 | –6.07 to 0.15 |
| Ethnicity: Indian vs. Welsh/English/Scottish/Northern Irish/British | 2.47 | 2.38 | 201.86 | 1.04 | 0.300 | –2.27 to 7.20 |
| Living situation: not alone vs. alone | –0.23 | 0.41 | 197.11 | –0.55 | 0.582 | –1.05 to 0.59 |
| Medication: yes vs. no | –0.41 | 0.49 | 198.71 | –0.84 | 0.400 | –0.46 to 0.16 |
| Social status: II managerial/technical vs. I professional | –1.14 | 0.62 | 194.38 | –1.85 | 0.066 | –1.20 to –0.41 |
| Social status: III N skilled, non-manual vs. I professional | –1.60 | 0.72 | 198.09 | –2.23 | 0.027 | –1.79 to –0.83 |
| Social status: III M skilled, manual vs. I professional | –1.61 | 0.70 | 201.23 | –2.30 | 0.022 | –1.85 to –0.90 |
| Social status: IV partly skilled vs. I professional | –2.05 | 0.83 | 199.62 | –2.48 | 0.014 | –2.31 to –1.18 |
| Social status: V unskilled vs. I professional | –1.38 | 0.91 | 200.01 | –1.51 | 0.132 | –1.21 to –0.20 |
Appendix 9 Exploratory analyses for the secondary outcomes
Participant outcomes
Linear mixed-effects model fitted to identify participant characteristics predicting differences between participant DEMentia Quality Of Life scores at baseline and 3 months’ follow-up
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.22 | 1 | 0.635 |
| Sex | 0.13 | 1 | 0.720 |
| Age | 0.01 | 1 | 0.943 |
| MMSE | 0.08 | 1 | 0.774 |
| Diagnosis | 0.34 | 2 | 0.844 |
| Social status | 3.03 | 5 | 0.695 |
| Centre | 0.21 | 1 | 0.646 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 1.16 | 2.44 | 191.98 | 0.47 | 0.636 | –3.67 to 5.97 |
| Aged ≥ 75 years vs. < 75 years | 0.10 | 1.43 | 215.87 | 0.07 | 0.943 | –2.64 to 2.94 |
| Sex: female vs. male | –0.52 | 1.45 | 214.82 | –0.36 | 0.720 | –3.36 to 2.33 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.36 | 1.26 | 216.76 | –0.29 | 0.775 | –2.76 to 2.15 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.63 | 1.75 | 199.73 | –0.36 | 0.719 | –4.13 to 2.67 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.52 | 1.55 | 214.58 | 0.34 | 0.737 | –2.61 to 3.42 |
| Social status: II managerial/technical vs. I professional | 0.07 | 2.32 | 214.08 | 0.03 | 0.975 | –4.52 to 4.55 |
| Social status: III N skilled, non-manual vs. I professional | 0.48 | 2.60 | 216.95 | 0.18 | 0.855 | –4.66 to 5.47 |
| Social status: III M skilled, manual vs. I professional | –1.01 | 2.53 | 214.51 | –0.40 | 0.691 | –6.03 to 3.84 |
| Social status: IV partly skilled vs. I professional | 1.76 | 2.91 | 209.83 | 0.60 | 0.547 | –4.00 to 7.34 |
| Social status: V unskilled vs. I professional | 3.71 | 3.31 | 216.86 | 1.12 | 0.263 | –2.74 to 10.16 |
Linear mixed-effects model fitted to identify participant characteristics as predicting differences between participant DEMentia Quality Of Life scores at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.31 | 1 | 0.577 |
| Sex | 0.53 | 1 | 0.466 |
| Age | 0.00 | 1 | 1.000 |
| MMSE | 1.94 | 1 | 0.164 |
| Diagnosis | 1.13 | 2 | 0.567 |
| Social status | 1.78 | 5 | 0.879 |
| Centre | 0.00 | 1 | 1.000 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 1.45 | 2.60 | 203.00 | 0.56 | 0.577 | –3.67 to 6.58 |
| Aged ≥ 75 years vs. < 75 years | 0.00 | 1.55 | 203.00 | 0.00 | 1.000 | –3.05 to 3.05 |
| Sex: female vs. male | –1.13 | 1.55 | 203.00 | –0.73 | 0.467 | –4.18 to 1.92 |
| MMSE score: ≥ 24 points vs. < 24 points | –1.87 | 1.34 | 203.00 | –1.39 | 0.165 | –4.52 to 0.78 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 1.68 | 1.85 | 203.00 | 0.91 | 0.365 | –1.96 to 5.32 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 1.33 | 1.66 | 203.00 | 0.80 | 0.424 | –1.94 to 4.61 |
| Social status: II managerial/technical vs. I professional | –0.60 | 2.53 | 203.00 | –0.24 | 0.813 | –5.59 to 4.39 |
| Social status: III N skilled, non-manual vs. I professional | 0.66 | 2.83 | 203.00 | 0.24 | 0.814 | –4.90 to 6.23 |
| Social status: III M skilled, manual vs. I professional | 0.29 | 2.76 | 203.00 | 0.11 | 0.916 | –5.15 to 5.73 |
| Social status: IV partly skilled vs. I professional | 2.16 | 3.11 | 203.00 | 0.69 | 0.488 | –3.96 to 8.29 |
| Social status: V unskilled vs. I professional | 1.78 | 3.62 | 203.00 | 0.49 | 0.624 | –5.35 to 8.91 |
Linear mixed-effects model fitted to identify participant characteristics predicting differences between participant Hospital Anxiety and Depression Scale anxiety scores at baseline and 3 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.25 | 1 | 0.619 |
| Sex | 0.09 | 1 | 0.761 |
| Age category | 0.15 | 1 | 0.696 |
| MMSE score level | 1.70 | 1 | 0.192 |
| Person with dementia diagnosis | 3.16 | 2 | 0.206 |
| Person with dementia social status | 1.52 | 5 | 0.911 |
| Centre | 0.11 | 1 | 0.735 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –0.38 | 0.77 | 188.86 | –0.50 | 0.619 | –1.88 to 1.14 |
| Aged ≥ 75 years vs. < 75 years | –0.18 | 0.45 | 212.99 | –0.39 | 0.696 | –1.07 to 0.71 |
| Sex: female vs. male | –0.14 | 0.46 | 214.31 | –0.30 | 0.761 | –1.04 to 0.76 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.52 | 0.40 | 214.79 | –1.30 | 0.193 | –1.30 to 0.26 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.94 | 0.55 | 189.06 | 1.69 | 0.092 | –0.15 to 2.02 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.02 | 0.49 | 212.76 | –0.04 | 0.970 | –0.98 to 0.94 |
| Social status: II managerial/technical vs. I professional | 0.30 | 0.73 | 212.86 | 0.41 | 0.680 | –1.14 to 1.75 |
| Social status: III N skilled, non-manual vs. I professional | 0.60 | 0.82 | 215.85 | 0.74 | 0.462 | –1.01 to 2.21 |
| Social status: III M skilled, manual vs. I professional | –0.00 | 0.80 | 212.50 | –0.00 | 0.999 | –1.58 to 1.56 |
| Social status: IV partly skilled vs. I professional | 0.80 | 0.93 | 205.55 | 0.86 | 0.391 | –1.03 to 2.61 |
| Social status: V unskilled vs. I professional | 0.16 | 1.04 | 215.78 | 0.16 | 0.875 | –1.90 to 2.23 |
Linear mixed-effects model fitted to identify participant characteristics predicting differences between participant Hospital Anxiety and Depression Scale anxiety scores at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.01 | 1 | 0.932 |
| Sex | 0.11 | 1 | 0.740 |
| Age | 1.94 | 1 | 0.163 |
| MMSE | 0.14 | 1 | 0.707 |
| Diagnosis | 0.94 | 2 | 0.625 |
| Social status | 2.09 | 5 | 0.836 |
| Centre | 0.00 | 1 | 1.000 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 0.08 | 0.88 | 193.00 | 0.09 | 0.932 | –1.66 to 1.81 |
| Aged ≥ 75 years vs. < 75 years | –0.72 | 0.52 | 193.00 | –1.39 | 0.165 | –1.75 to 0.30 |
| Sex: female vs. male | 0.18 | 0.53 | 193.00 | 0.33 | 0.741 | –0.87 to 1.22 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.17 | 0.46 | 193.00 | –0.38 | 0.707 | –1.07 to 0.73 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.12 | 0.63 | 193.00 | –0.19 | 0.848 | –1.37 to 1.12 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.55 | 0.57 | 193.00 | –0.96 | 0.337 | –1.66 to 0.57 |
| Social status: II managerial/technical vs. I professional | 0.99 | 0.87 | 193.00 | 1.14 | 0.258 | –0.73 to 2.70 |
| Social status: III N skilled, non-manual vs. I professional | 0.52 | 0.97 | 193.00 | 0.53 | 0.593 | –1.40 to 2.44 |
| Social status: III M skilled, manual vs. I professional | 1.03 | 0.94 | 193.00 | 1.10 | 0.273 | –0.82 to 2.89 |
| Social status: IV partly skilled vs. I professional | 0.78 | 1.05 | 193.00 | 0.74 | 0.458 | –1.28 to 2.84 |
| Social status: V unskilled vs. I professional | 0.35 | 1.22 | 193.00 | 0.29 | 0.772 | –2.0 to 2.75 |
Linear mixed-effects model fitted to identify participant characteristics predicting differences between participant Hospital Anxiety and Depression Scale depression scores at baseline and 3 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.08 | 1 | 0.779 |
| Sex | 0.05 | 1 | 0.822 |
| Age | 0.17 | 1 | 0.681 |
| MMSE | 1.81 | 1 | 0.179 |
| Diagnosis | 0.71 | 2 | 0.702 |
| Social status | 3.07 | 5 | 0.689 |
| Centre | 0.00 | 1 | 1.000 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 0.19 | 0.69 | 218.00 | 0.28 | 0.779 | –1.16 to 1.54 |
| Aged ≥ 75 years vs. < 75 years | –0.17 | 0.40 | 218.00 | –0.41 | 0.681 | –0.96 to 0.63 |
| Sex: female vs. male | 0.09 | 0.41 | 218.00 | 0.22 | 0.823 | –0.71 to 0.89 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.48 | 0.35 | 218.00 | –1.34 | 0.180 | –1.17 to 0.22 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.23 | 0.49 | 218.00 | 0.47 | 0.636 | –0.73 to 1.20 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.23 | 0.44 | 218.00 | –0.53 | 0.594 | –1.09 to 0.62 |
| Social status: II managerial/technical vs. I professional | 0.16 | 0.65 | 218.00 | 0.24 | 0.807 | –1.13 to 1.45 |
| Social status: III N skilled, non-manual vs. I professional | –0.15 | 0.73 | 218.00 | –0.21 | 0.836 | –1.59 to 1.29 |
| Social status: III M skilled, manual vs. I professional | 0.75 | 0.71 | 218.00 | 1.05 | 0.294 | –0.65 to 2.16 |
| Social status: IV partly skilled vs. I professional | –0.00 | 0.82 | 218.00 | –0.00 | 0.998 | –1.61 to 1.61 |
| Social status: V unskilled vs. I professional | 0.64 | 0.93 | 218.00 | 0.68 | 0.494 | –1.20 to 2.47 |
Linear mixed-effects model fitted to identify participant characteristics predicting for differences between participant Hospital Anxiety and Depression Scale depression scores at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Participant characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.07 | 1 | 0.794 |
| Sex | 0.69 | 1 | 0.407 |
| Age | 0.90 | 1 | 0.343 |
| MMSE | 0.00 | 1 | 0.979 |
| Diagnosis | 0.20 | 2 | 0.907 |
| Social status | 2.00 | 5 | 0.849 |
| Centre | –0.00 | 1 | 1.000 |
| Participant characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | 0.19 | 0.74 | 194.00 | 0.26 | 0.795 | –1.27 to 1.65 |
| Aged ≥ 75 years vs. < 75 years | 0.41 | 0.43 | 194.00 | 0.95 | 0.344 | –0.44 to 1.27 |
| Sex: female vs. male | 0.37 | 0.44 | 194.00 | 0.83 | 0.408 | –0.51 to 1.24 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.01 | 0.38 | 194.00 | –0.03 | 0.979 | –0.77 to 0.75 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.13 | 0.53 | 194.00 | 0.24 | 0.810 | –0.92 to 1.17 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.13 | 0.48 | 194.00 | –0.28 | 0.779 | –1.07 to 0.80 |
| Social status: II managerial/technical vs. I professional | –0.01 | 0.73 | 194.00 | –0.02 | 0.987 | –1.45 to 1.42 |
| Social status: III N skilled, non-manual vs. I professional | –0.65 | 0.82 | 194.00 | –0.80 | 0.425 | –2.26 to 0.96 |
| Social status: III M skilled, manual vs. I professional | –0.09 | 0.79 | 194.00 | –0.12 | 0.905 | –1.65 to 1.46 |
| Social status: IV partly skilled vs. I professional | –0.60 | 0.88 | 194.00 | –0.69 | 0.493 | –2.34 to 1.13 |
| Social status: V unskilled vs. I professional | –0.11 | 1.02 | 194.00 | –0.11 | 0.914 | –2.13 to 1.91 |
Linear mixed-effects model fitted to identify carer characteristics predicting differences between participant total Generalized Self-Efficacy Scale scores at baseline and 3 months’ follow-up for participants in the cognitive rehabilitation group
| Carer characteristics | χ2 | df | p value |
|---|---|---|---|
| (Intercept) | 0.04 | 1 | 0.847 |
| Sex | 4.04 | 1 | 0.044 |
| Age | 0.17 | 1 | 0.681 |
| MMSE | 0.14 | 1 | 0.710 |
| Diagnosis | 5.20 | 2 | 0.074 |
| Social status | 4.92 | 5 | 0.425 |
| Centre | 0.00 | 1 | 1.000 |
| Carer characteristics | B (estimate) | SE | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| (Intercept) | 0.22 | 1.12 | 214.00 | 0.19 | 0.847 |
| Aged ≥ 75 years vs. < 75 years | –0.27 | 0.66 | 214.00 | –0.41 | 0.682 |
| Sex: female vs. male | 1.35 | 0.67 | 214.00 | 2.01 | 0.046 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.22 | 0.58 | 214.00 | –0.37 | 0.711 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.95 | 0.81 | 214.00 | 1.17 | 0.241 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –1.10 | 0.71 | 214.00 | –1.54 | 0.125 |
| Social status: II managerial/technical vs. I professional | 0.01 | 1.06 | 214.00 | 0.01 | 0.995 |
| Social status: III N skilled, non-manual vs. I professional | –0.92 | 1.20 | 214.00 | –0.77 | 0.444 |
| Social status: III M skilled, manual vs. I professional | 0.34 | 1.16 | 214.00 | 0.29 | 0.770 |
| Social status: IV partly skilled vs. I professional | –1.20 | 1.33 | 214.00 | –0.90 | 0.368 |
| Social status: V unskilled vs. I professional | 1.31 | 1.52 | 214.00 | 0.86 | 0.390 |
Linear mixed-effects model fitted to identify carer characteristics predicting differences between participant Generalized Self-Efficacy Scale scores at baseline and 9 months’ follow-up for participants in the cognitive rehabilitation group
| Carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.80 | 1 | 0.371 |
| Sex | 1.09 | 1 | 0.296 |
| Age | 0.70 | 1 | 0.402 |
| MMSE | 1.22 | 1 | 0.269 |
| Diagnosis | 7.71 | 2 | 0.021 |
| Social status | 2.07 | 5 | 0.839 |
| Centre | 0.00 | 1 | 1.000 |
| Carer characteristics | B (estimate) | SE | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| (Intercept) | –1.19 | 1.33 | 193.00 | –0.90 | 0.372 to –3.81 |
| Aged ≥ 75 years vs. < 75 years | –0.66 | 0.79 | 193.00 | –0.84 | 0.403 to –2.21 |
| Sex: female vs. male | 0.83 | 0.80 | 193.00 | 1.04 | 0.297 to –0.74 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.76 | 0.69 | 193.00 | 1.11 | 0.270 to –0.60 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 2.67 | 0.96 | 193.00 | 2.77 | 0.006 to 0.77 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.82 | 0.86 | 193.00 | 0.96 | 0.337 to –0.86 |
| Social status: II managerial/technical vs. I professional | 0.42 | 1.30 | 193.00 | 0.32 | 0.748 to –2.15 |
| Social status: III N skilled, non-manual vs. I professional | 0.35 | 1.46 | 193.00 | 0.24 | 0.809 to –2.53 |
| Social status: III M skilled, manual vs. I professional | 0.24 | 1.42 | 193.00 | 0.17 | 0.868 to –2.55 |
| Social status: IV partly skilled vs. I professional | –0.64 | 1.60 | 193.00 | –0.40 | 0.688 to –3.79 |
| Social status: V unskilled vs. I professional | 1.77 | 1.83 | 193.00 | 0.97 | 0.335 to –1.84 |
Carer outcomes
Linear mixed-effects model fitted to identify carer characteristics predicting differences between carer Relatives’ Stress Scale scores at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 21.38 | 1 | 0.62 | 0.433 |
| Carer age | 9.20 | 1 | 0.27 | 0.607 |
| Carer sex | 121.97 | 1 | 3.52 | 0.062 |
| Carer education | 192.63 | 4 | 1.39 | 0.240 |
| Carer social status | 243.56 | 5 | 1.41 | 0.225 |
| Carer ethnicity | 282.88 | 6 | 1.36 | 0.234 |
| Person with dementia diagnosis and carer relationship | 327.08 | 7 | 1.35 | 0.231 |
| Carer health | 54.27 | 4 | 0.39 | 0.815 |
| Carer hours | 194.42 | 8 | 0.70 | 0.690 |
| Residuals | 5581.21 | 161 | NA | NA |
| Carer characteristics | B (estimate) | SE | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| (Intercept) | –5.32 | 6.77 | –0.79 | 0.433 | –18.69 to 8.05 |
| Carer age | 0.03 | 0.06 | 0.52 | 0.607 | –0.09 to 0.15 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | 0.73 | 2.01 | 0.36 | 0.716 | –3.24 to 4.70 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | 0.63 | 1.20 | 0.53 | 0.597 | –1.73 to 2.99 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | 0.06 | 1.74 | 0.03 | 0.975 | –3.38 to 3.49 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –3.25 | 1.88 | –1.72 | 0.086 | –6.96 to 0.47 |
| Carer ethnicity: any other Asian background vs. Welsh/English/Scottish/Northern Irish/British | –5.50 | 4.56 | –1.21 | 0.229 | –14.50 to 3.50 |
| Carer ethnicity: Caribbean vs. Welsh/English/Scottish/Northern Irish/British | 0.64 | 2.72 | 0.23 | 0.815 | –4.73 to 6.01 |
| Carer ethnicity: Irish vs. Welsh/English/Scottish/Northern Irish/British | 6.74 | 3.36 | 2.00 | 0.047 | 0.10 to 13.39 |
| Carer ethnicity: any other white background vs. Welsh/English/Scottish/Northern Irish/British | 0.57 | 4.65 | 0.12 | 0.902 | –8.62 to 9.76 |
| Carer ethnicity: any other mixed/multiple ethnic background | 6.16 | 3.75 | 1.64 | 0.102 | –1.24 to 13.56 |
| Carer ethnicity: Indian vs. Welsh/English/Scottish/Northern Irish/British | –2.21 | 6.30 | –0.35 | 0.727 | –14.66 to 10.24 |
| Carer sex: male vs. female | –2.11 | 1.13 | –1.88 | 0.062 | –4.34 to 0.11 |
| Carer health: fair vs. excellent | –0.27 | 1.70 | –0.16 | 0.874 | –3.63 to 3.09 |
| Carer health: good vs. excellent | –0.71 | 1.51 | –0.47 | 0.641 | –3.69 to 2.28 |
| Carer health: poor vs. excellent | –1.96 | 2.14 | –0.91 | 0.362 | –6.18 to 2.27 |
| Carer health: very good vs. excellent | –1.37 | 1.54 | –0.89 | 0.374 | –4.42 to 1.67 |
| Hours providing care in a typical day: < 1 hour vs. none | –0.57 | 2.13 | –0.27 | 0.789 | –4.79 to 3.64 |
| Hours providing care in a typical day: > 1 hour and up to 2 hours vs. none | –0.12 | 2.22 | –0.05 | 0.958 | –4.50 to 4.26 |
| Hours providing care in a typical day: > 1 hour and up to 3 hours vs. none | –0.77 | 2.37 | –0.33 | 0.744 | –5.45 to 3.90 |
| Hours providing care in a typical day: > 3 hours and up to 5 hours vs. none | –0.74 | 2.41 | –0.31 | 0.758 | –5.50 to 4.01 |
| Hours providing care in a typical day: > 5 hours and up to 10 hours vs. none | 1.12 | 2.44 | 0.46 | 0.648 | –3.71 to 5.94 |
| Hours providing care in a typical day: > 10 hours, but not overnight vs. none | –2.75 | 2.76 | –1.00 | 0.321 | –8.21 to 2.71 |
| Hours providing care in a typical day: > 10 hours and/including overnight vs. none | 1.34 | 2.19 | 0.61 | 0.540 | –2.98 to 5.66 |
| Hours providing care in a typical day: other vs. none | 1.06 | 4.64 | 0.23 | 0.820 | –8.11 to 10.23 |
| Carer social status: II managerial/technical vs. I professional | –1.42 | 1.66 | –0.86 | 0.392 | –4.70 to 1.85 |
| Carer social status: III N skilled, non-manual vs. I professional | –2.63 | 1.95 | –1.35 | 0.180 | –6.49 to 1.22 |
| Carer social status: III M skilled, manual vs. I professional | 1.50 | 2.29 | 0.65 | 0.514 | –3.03 to 6.02 |
| Carer social status: IV partly skilled vs. I professional | –0.73 | 2.24 | –0.33 | 0.745 | –5.16 to 3.70 |
| Carer social status: V unskilled vs. I professional | –3.85 | 2.77 | –1.39 | 0.168 | –9.33 to 1.63 |
| Person with dementia and carer relationship: friend vs. brother/sister | 1.33 | 5.79 | 0.23 | 0.819 | –10.10 to 12.76 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 12.90 | 7.73 | 1.67 | 0.097 | –2.37 to 28.16 |
| Person with dementia and carer relationship: other vs. brother/sister | 0.95 | 5.33 | 0.18 | 0.858 | –9.57 to 11.48 |
| Person with dementia and carer relationship: partner vs. brother/sister | 7.21 | 4.16 | 1.73 | 0.085 | –1.00 to 15.42 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 6.52 | 4.12 | 1.58 | 0.115 | –1.61 to 14.65 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 10.45 | 5.34 | 1.96 | 0.052 | –0.10 to 20.99 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 7.24 | 3.91 | 1.85 | 0.066 | –0.47 to 14.95 |
Linear mixed-effects model fitted to identify carer characteristics predicting differences between carer Relatives’ Stress Scale scores at baseline and 9 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Carer characteristics | Sum of squares | df | F-value | p-value |
|---|---|---|---|---|
| (Intercept) | 27.77 | 1 | 0.76 | 0.385 |
| Carer age | 24.16 | 1 | 0.66 | 0.417 |
| Carer sex | 124.16 | 1 | 3.40 | 0.067 |
| Carer education | 44.90 | 4 | 0.31 | 0.873 |
| Carer social status | 83.90 | 5 | 0.46 | 0.806 |
| Carer ethnicity | 313.05 | 6 | 1.43 | 0.207 |
| Person with dementia and carer relationship | 229.18 | 7 | 0.90 | 0.511 |
| Carer health | 148.86 | 4 | 1.02 | 0.400 |
| Carer hours | 150.49 | 8 | 0.51 | 0.844 |
| Residuals | 5516.54 | 151 | NA | NA |
| Carer characteristics | B (estimate) | SE | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| (Intercept) | –6.23 | 7.14 | –0.87 | 0.385 | –20.35 to 7.89 |
| Carer age | 0.05 | 0.06 | 0.81 | 0.417 | –0.07 to 0.18 |
| Carer education: left school at age 17–18 years and did not go back to education vs. left school at age 14–16 years and did not go back to education | –2.21 | 2.09 | –1.06 | 0.292 | –6.35 to 1.92 |
| Carer education: further education (e.g. vocational qualifications: GNVQ/NVQ/HND) vs. left school at age 14–16 years and did not go back to education | –0.16 | 1.25 | –0.13 | 0.896 | –2.64 to 2.31 |
| Carer education: higher education (BSc/BA or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.70 | 1.87 | –0.38 | 0.708 | –4.39 to 2.99 |
| Carer education: postgraduate education (MSc/MA/PhD or equivalent) vs. left school at age 14–16 years and did not go back to education | –0.82 | 1.98 | –0.41 | 0.680 | –4.73 to 3.09 |
| Carer ethnicity: any other Asian background vs. Welsh/English/Scottish/Northern Irish/British | –8.93 | 4.72 | –1.89 | 0.060 | –18.26 to 0.40 |
| Carer ethnicity: Caribbean vs. Welsh/English/Scottish/Northern Irish/British | 4.18 | 3.58 | 1.17 | 0.246 | –2.90 to 11.25 |
| Carer ethnicity: Irish vs. Welsh/English/Scottish/Northern Irish/British | 5.80 | 3.47 | 1.67 | 0.097 | –1.06 to 12.65 |
| Carer ethnicity: any other white background vs. Welsh/English/Scottish/Northern Irish/British | 1.35 | 6.51 | 0.21 | 0.836 | –11.51 to 14.21 |
| Carer ethnicity: any other mixed/multiple ethnic background | 2.59 | 3.87 | 0.67 | 0.504 | –5.06 to 10.25 |
| Carer ethnicity: Indian vs. Welsh/English/Scottish/Northern Irish/British | –4.24 | 6.52 | –0.65 | 0.516 | –17.14 to 8.65 |
| Carer sex: male vs. female | –2.32 | 1.26 | –1.84 | 0.067 | –4.82 to 0.17 |
| Carer health: fair vs. excellent | 2.18 | 1.86 | 1.17 | 0.243 | –1.50 to 5.86 |
| Carer health: good vs. excellent | –0.49 | 1.61 | –0.30 | 0.761 | –3.66 to 2.68 |
| Carer health: poor vs. excellent | –0.44 | 2.27 | –0.19 | 0.846 | –4.92 to 4.04 |
| Carer health: very good vs. excellent | –0.52 | 1.66 | –0.31 | 0.754 | –3.79 to 2.75 |
| Hours providing help in a typical day: < 1 hour vs. none | –0.36 | 2.22 | –0.16 | 0.870 | –4.74 to 4.02 |
| Hours providing help in a typical day: > 1 hour and up to 2 hours vs. none | 0.69 | 2.31 | 0.30 | 0.764 | –3.87 to 5.26 |
| Hours providing help in a typical day: > 2 hours and up to 3 hours vs. none | 1.38 | 2.42 | 0.57 | 0.570 | –3.40 to 6.15 |
| Hours providing help in a typical day: > 3 hours and up to 5 hours vs. none | 1.63 | 2.62 | 0.62 | 0.535 | –3.54 to 6.80 |
| Hours providing help in a typical day: > 5 hours and up to 10 hours vs. none | 2.68 | 2.47 | 1.09 | 0.280 | –2.20 to 7.57 |
| Hours providing help in a typical day: > 10 hours but not overnight vs. none | 1.03 | 2.91 | 0.35 | 0.724 | –4.72 to 6.78 |
| Hours providing help in a typical day: > 10 hours and/including overnight vs. none | 1.72 | 2.26 | 0.76 | 0.447 | –2.73 to 6.18 |
| Hours providing help in a typical day: other, describe vs. none | –0.98 | 4.76 | –0.21 | 0.837 | –10.39 to 8.43 |
| Carer social status: II managerial/technical vs. I professional | 0.30 | 1.77 | 0.17 | 0.864 | –3.20 to 3.81 |
| Carer social status: III N skilled, non-manual vs. I professional | 1.07 | 2.09 | 0.51 | 0.608 | –3.05 to 5.20 |
| Carer social status: III M skilled, manual vs. I professional | 1.92 | 2.51 | 0.76 | 0.445 | –3.04 to 6.88 |
| Carer social status: IV partly skilled vs. I professional | –0.41 | 2.38 | –0.17 | 0.863 | –5.11 to 4.29 |
| Carer social status: V unskilled vs. I professional | –1.53 | 3.12 | –0.49 | 0.625 | –7.70 to 4.64 |
| Person with dementia and carer relationship: friend vs. brother/sister | –0.20 | 6.13 | –0.03 | 0.974 | –12.31 to 11.91 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 11.23 | 8.09 | 1.39 | 0.167 | –4.76 to 27.22 |
| Person with dementia and carer relationship: other vs. brother/sister | 0.66 | 5.54 | 0.12 | 0.906 | –10.29 to 11.60 |
| Person with dementia and carer relationship: partner vs. brother/sister | 4.53 | 4.38 | 1.03 | 0.303 | –4.13 to 13.18 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 5.29 | 4.37 | 1.21 | 0.228 | –3.34 to 13.91 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 8.51 | 5.28 | 1.61 | 0.109 | –1.93 to 18.96 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 5.16 | 4.12 | 1.25 | 0.213 | –2.99 to 13.30 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version physical scores at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 1.17 | 1 | 0.280 |
| Person with dementia and carer relationship | 2.37 | 7 | 0.937 |
| Carer sex | 1.02 | 1 | 0.311 |
| Person with dementia diagnosis | 0.71 | 2 | 0.702 |
| Person with dementia MMSE score | 0.22 | 1 | 0.641 |
| Centre | 4.48 | 1 | 0.034 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –1.12 | 1.04 | 211.30 | –1.08 | 0.281 | –3.16 to 0.92 |
| Carer’s sex: male vs. female | –0.27 | 0.27 | 205.29 | –1.01 | 0.313 | –0.79 to 0.25 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.12 | 0.25 | 210.15 | –0.47 | 0.641 | –0.62 to 0.38 |
| Person with dementia and carer relationship: friend vs. brother/sister | 1.49 | 1.29 | 207.44 | 1.15 | 0.250 | –1.05 to 4.04 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 0.54 | 1.61 | 206.90 | 0.33 | 0.739 | –2.64 to 3.73 |
| Person with dementia and carer relationship: other vs. brother/sister | 1.28 | 1.44 | 207.82 | 0.89 | 0.375 | –1.56 to 4.12 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.22 | 1.10 | 206.57 | 1.10 | 0.271 | –0.96 to 3.39 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 1.00 | 1.05 | 206.69 | 0.95 | 0.344 | –1.08 to 3.08 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 0.51 | 1.44 | 208.43 | 0.36 | 0.722 | –2.33 to 3.36 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.17 | 1.04 | 209.03 | 1.13 | 0.260 | –0.88 to 3.22 |
| Person with dementia and carer relationship diagnosis: vascular dementia vs. Alzheimer’s disease | 0.20 | 0.35 | 211.87 | 0.57 | 0.566 | –0.49 to 0.89 |
| Person with dementia and carer relationship diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.14 | 0.31 | 211.86 | –0.44 | 0.661 | –0.74 to 0.47 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version physical scores at baseline and 9 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 1.59 | 1 | 0.207 |
| Person with dementia and carer relationship | 4.25 | 7 | 0.751 |
| Carer sex | 0.15 | 1 | 0.698 |
| Person with dementia diagnosis | 0.79 | 2 | 0.672 |
| Person with dementia MMSE score | 0.33 | 1 | 0.563 |
| Centre | 1.88 | 1 | 0.171 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –1.25 | 0.99 | 198.87 | –1.26 | 0.209 | –3.20 to 0.70 |
| Carer’s sex: male vs. female | 0.10 | 0.26 | 193.28 | 0.39 | 0.699 | –0.42 to 0.62 |
| MMSE score: ≥ 24 points vs. < 24 points | –0.15 | 0.25 | 198.65 | –0.58 | 0.564 | –0.64 to 0.35 |
| Person with dementia and carer relationship: friend vs. brother/sister | 2.07 | 1.30 | 197.15 | 1.59 | 0.114 | –0.50 to 4.65 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | –0.13 | 1.55 | 195.57 | –0.08 | 0.934 | –3.19 to 2.94 |
| Person with dementia and carer relationship: other vs. brother/sister | 1.25 | 1.39 | 196.15 | 0.90 | 0.369 | –1.48 to 0.98 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.14 | 1.07 | 194.98 | 1.06 | 0.290 | –0.97 to 3.25 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.81 | 1.02 | 195.49 | 0.80 | 0.423 | –1.19 to 2.82 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 0.99 | 1.39 | 197.33 | 0.71 | 0.476 | –1.74 to 3.73 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.05 | 1.00 | 197.92 | 1.05 | 0.296 | –0.93 to 3.02 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.25 | 0.34 | 197.18 | –0.73 | 0.469 | –0.93 to 0.43 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.09 | 0.30 | 198.68 | 0.30 | 0.765 | –0.50 to 0.68 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version psychological scores at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 5.37 | 1 | 0.020 |
| Person with dementia and carer relationship | 8.62 | 7 | 0.281 |
| Carer sex | 0.01 | 1 | 0.907 |
| Person with dementia diagnosis | 0.50 | 2 | 0.781 |
| Person with dementia MMSE score | 0.79 | 1 | 0.375 |
| Centre | 1.04 | 1 | 0.307 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –2.04 | 0.88 | 211.89 | –2.32 | 0.021 | –3.78 to –0.31 |
| Carer’s sex: male vs. female | 0.03 | 0.23 | 206.53 | 0.12 | 0.907 | –0.42 to 0.48 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.19 | 0.22 | 211.67 | 0.89 | 0.376 | –0.24 to 0.62 |
| Person with dementia and carer relationship: friend vs. brother/sister | 3.06 | 1.11 | 209.23 | 2.76 | 0.006 | 0.88 to 5.25 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 1.75 | 1.39 | 208.87 | 1.26 | 0.209 | –0.99 to 4.49 |
| Person with dementia and carer relationship: other vs. brother/sister | 2.00 | 1.24 | 209.05 | 1.62 | 0.108 | –0.44 to 4.44 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.82 | 0.95 | 208.40 | 1.92 | 0.056 | –0.05 to 3.69 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 1.58 | 0.90 | 208.48 | 1.75 | 0.081 | –0.20 to 3.37 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 1.94 | 1.24 | 210.50 | 1.57 | 0.118 | –0.50 to 4.38 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.60 | 0.89 | 211.08 | 1.80 | 0.074 | –0.16 to 3.35 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.07 | 0.29 | 207.32 | 0.24 | 0.810 | –0.52 to 0.66 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.18 | 0.26 | 211.10 | 0.70 | 0.484 | –0.33 to 0.70 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version psychological scores at baseline and 9 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 1.23 | 1 | 0.268 |
| Person with dementia and carer relationship | 1.74 | 7 | 0.973 |
| Carer sex | 0.37 | 1 | 0.545 |
| Person with dementia diagnosis | 2.13 | 2 | 0.344 |
| Person with dementia MMSE score | 0.01 | 1 | 0.917 |
| Centre | 0.92 | 1 | 0.338 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –1.01 | 0.91 | 198.86 | –1.11 | 0.269 | –2.81 to 0.79 |
| Carer’s sex: male vs. female | 0.15 | 0.24 | 190.77 | 0.61 | 0.546 | –0.30 to 0.60 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.02 | 0.23 | 198.76 | 0.10 | 0.917 | –0.38 to 0.47 |
| Person with dementia and carer relationship: friend vs. brother/sister | 0.43 | 1.20 | 196.79 | 0.35 | 0.724 | –1.67 to 2.82 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | –0.06 | 1.43 | 194.44 | –0.04 | 0.967 | –2.62 to 2.66 |
| Person with dementia and carer relationship: other vs. brother/sister | 0.25 | 1.28 | 195.05 | 0.20 | 0.843 | –2.03 to 2.68 |
| Person with dementia and carer relationship: partner vs. brother/sister | 0.86 | 0.99 | 193.47 | 0.87 | 0.385 | –0.96 to 2.68 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.59 | 0.94 | 194.27 | 0.63 | 0.528 | –1.08 to 2.37 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | 0.30 | 1.28 | 197.04 | 0.23 | 0.815 | –2.04 to 2.66 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 0.44 | 0.92 | 197.90 | 0.48 | 0.630 | –1.17 to 2.21 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.30 | 0.31 | 194.16 | –0.95 | 0.346 | –0.91 to 0.24 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.22 | 0.27 | 197.85 | 0.82 | 0.414 | –0.27 to 0.73 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version social scores at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.79 | 1 | 0.374 |
| Person with dementia and carer relationship | 2.90 | 7 | 0.894 |
| Carer sex | 0.06 | 1 | 0.811 |
| Person with dementia diagnosis | 2.40 | 2 | 0.301 |
| Person with dementia MMSE score | 0.21 | 1 | 0.647 |
| Centre | 0.00 | 1 | 1.000 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –1.07 | 1.20 | 211.00 | –0.89 | 0.375 | –3.44 to 1.30 |
| Carer’s sex: male vs. female | 0.07 | 0.31 | 211.00 | 0.24 | 0.811 | –0.54 to 0.69 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.14 | 0.30 | 211.00 | 0.46 | 0.648 | –0.45 to 0.72 |
| Person with dementia and carer relationship: friend vs. brother/sister | 1.03 | 1.52 | 211.00 | 0.68 | 0.498 | –1.96 to 4.02 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | –0.07 | 1.90 | 211.00 | –0.03 | 0.972 | –3.81 to 3.68 |
| Person with dementia and carer relationship: other vs. brother/sister | 1.01 | 1.70 | 211.00 | 0.60 | 0.551 | –2.33 to 4.36 |
| Person with dementia and carer relationship: partner vs. brother/sister | 0.26 | 1.30 | 211.00 | 0.20 | 0.839 | –2.29 to 2.82 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.92 | 1.24 | 211.00 | 0.74 | 0.459 | –1.52 to 3.36 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | –0.36 | 1.69 | 211.00 | –0.21 | 0.830 | –3.70 to 2.97 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 0.61 | 1.22 | 211.00 | 0.50 | 0.616 | –1.78 to 3.00 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.62 | 0.40 | 211.00 | 1.55 | 0.123 | –0.17 to 1.40 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.17 | 0.36 | 211.00 | 0.46 | 0.643 | –0.54 to 0.87 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version social scores at baseline and 9 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 3.10 | 1 | 0.078 |
| Person with dementia and carer relationship | 7.08 | 7 | 0.420 |
| Carer sex | 3.34 | 1 | 0.068 |
| Person with dementia diagnosis | 0.63 | 2 | 0.730 |
| Person with dementia MMSE score | 0.12 | 1 | 0.728 |
| Centre | 0.00 | 1 | 1.000 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –2.25 | 1.28 | 196.00 | –1.76 | 0.080 | –4.76 to 0.27 |
| Carer’s sex: male vs. female | 0.63 | 0.34 | 196.00 | 1.83 | 0.069 | –0.05 to 1.31 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.11 | 0.33 | 196.00 | 0.35 | 0.728 | –0.53 to 0.76 |
| Person with dementia and carer relationship: friend vs. brother/sister | 3.31 | 1.69 | 196.00 | 1.96 | 0.052 | –0.02 to 6.64 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 1.63 | 2.02 | 196.00 | 0.81 | 0.419 | –2.34 to 5.61 |
| Person with dementia and carer relationship: other vs. brother/sister | 2.81 | 1.80 | 196.00 | 1.56 | 0.120 | –0.74 to 6.36 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.82 | 1.39 | 196.00 | 1.30 | 0.194 | –0.93 to 4.56 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 1.69 | 1.32 | 196.00 | 1.28 | 0.201 | –0.91 to 4.29 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | –0.23 | 1.80 | 196.00 | –0.13 | 0.900 | –3.77 to 3.32 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.78 | 1.29 | 196.00 | 1.38 | 0.170 | –0.76 to 4.33 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | 0.11 | 0.44 | 196.00 | 0.24 | 0.808 | –0.76 to 0.98 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.31 | 0.39 | 196.00 | 0.79 | 0.429 | –0.46 to 1.07 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version environmental scores at baseline and 3 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 0.61 | 1 | 0.434 |
| Person with dementia and carer relationship | 6.59 | 7 | 0.473 |
| Carer sex | 1.54 | 1 | 0.215 |
| Person with dementia diagnosis | 1.84 | 2 | 0.399 |
| Person with dementia MMSE score | 0.23 | 1 | 0.629 |
| Centre | 0.00 | 1 | 1.000 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –0.61 | 0.78 | 212.00 | –0.78 | 0.435 | –2.16 to 0.93 |
| Carer’s sex: male vs. female | –0.25 | 0.20 | 212.00 | –1.24 | 0.216 | –0.66 to 0.15 |
| MMSE score: ≥ 24 points vs. < 24 points | 0.09 | 0.19 | 212.00 | 0.48 | 0.629 | –0.29 to 0.47 |
| Person with dementia and carer relationship: friend vs. brother/sister | 0.03 | 0.99 | 212.00 | 0.03 | 0.976 | –1.92 to 1.98 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 0.02 | 1.24 | 212.00 | 0.02 | 0.987 | –2.42 to 2.46 |
| Person with dementia and carer relationship: other vs. brother/sister | 1.38 | 1.11 | 212.00 | 1.25 | 0.212 | –0.79 to 3.56 |
| Person with dementia and carer relationship: partner vs. brother/sister | 0.40 | 0.85 | 212.00 | 0.47 | 0.641 | –1.27 to 2.06 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.83 | 0.81 | 212.00 | 1.02 | 0.307 | –0.76 to 2.42 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | –0.31 | 1.10 | 212.00 | –0.28 | 0.777 | –2.49 to 1.86 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 0.77 | 0.79 | 212.00 | 0.98 | 0.331 | –0.79 to 2.33 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.05 | 0.26 | 212.00 | –0.19 | 0.853 | –0.56 to 0.46 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | –0.31 | 0.23 | 212.00 | –1.35 | 0.180 | –0.77 to 0.14 |
Linear mixed-effects model fitted to identify characteristics predicting differences between carer World Health Organization’s Quality of Life Instrument – brief version environmental scores at baseline and 9 months’ follow-up for carers of participants in the cognitive rehabilitation group
| Person with dementia and carer characteristics | χ2 | df | p-value |
|---|---|---|---|
| (Intercept) | 2.57 | 1 | 0.109 |
| Person with dementia and carer relationship | 11.65 | 7 | 0.113 |
| Carer sex | 0.01 | 1 | 0.944 |
| Person with dementia diagnosis | 0.21 | 2 | 0.901 |
| Person with dementia MMSE score | 0.19 | 1 | 0.662 |
| Centre | –0.00 | 1 | 1.000 |
| Person with dementia and carer characteristics | B (estimate) | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| (Intercept) | –1.37 | 0.85 | 199.00 | –1.60 | 0.110 | –3.05 to 0.31 |
| Carer’s sex: male vs. female | 0.02 | 0.23 | 199.00 | 0.07 | 0.944 | –0.44 to 0.47 |
| MMSE: ≥ 24 points vs. < 24 points | 0.10 | 0.22 | 199.00 | 0.44 | 0.662 | –0.33 to 0.52 |
| Person with dementia and carer relationship: friend vs. brother/sister | 0.79 | 1.13 | 199.00 | 0.70 | 0.484 | –1.43 to 3.02 |
| Person with dementia and carer relationship: grandchild vs. brother/sister | 0.28 | 1.35 | 199.00 | 0.20 | 0.839 | –2.38 to 2.93 |
| Person with dementia and carer relationship: other vs. brother/sister | 3.34 | 1.21 | 199.00 | 2.77 | 0.006 | 0.97 to 5.72 |
| Person with dementia and carer relationship: partner vs. brother/sister | 1.32 | 0.93 | 199.00 | 1.42 | 0.157 | –0.51 to 3.16 |
| Person with dementia and carer relationship: son/daughter vs. brother/sister | 0.94 | 0.88 | 199.00 | 1.06 | 0.289 | –0.80 to 2.68 |
| Person with dementia and carer relationship: son/daughter-in-law vs. brother/sister | –0.02 | 1.20 | 199.00 | –0.02 | 0.985 | –2.39 to 2.35 |
| Person with dementia and carer relationship: spouse vs. brother/sister | 1.02 | 0.86 | 199.00 | 1.18 | 0.239 | –0.68 to 2.72 |
| Person with dementia diagnosis: vascular dementia vs. Alzheimer’s disease | –0.06 | 0.29 | 199.00 | –0.21 | 0.831 | –0.64 to 0.51 |
| Person with dementia diagnosis: mixed Alzheimer’s disease and vascular dementia vs. Alzheimer’s disease | 0.09 | 0.26 | 199.00 | 0.33 | 0.739 | –0.42 to 0.59 |
Appendix 10 Complete-case analyses with no imputation
| Measure | p-value | Adjusted p-value | Mean difference | 95% CI for mean difference | Cohen’s d | 95% CI for Cohen’s d |
|---|---|---|---|---|---|---|
| 3-month follow-up | ||||||
| Participant rating of attainment | < 0.001 | < 0.001 | 1.71 | 1.32 to 2.1 | 0.91 | 0.7 to 1.13 |
| Participant rating of satisfaction | < 0.001 | < 0.001 | 1.56 | 1.14 to 1.98 | 0.78 | 0.56 to 0.99 |
| Carer rating of attainment | < 0.001 | < 0.001 | 1.89 | 1.51 to 2.28 | 1.03 | 0.81 to 1.25 |
| 9-month follow-up | ||||||
| Participant rating of attainment | < 0.001 | < 0.001 | 1.81 | 1.34 to 2.28 | 0.86 | 0.63 to 1.09 |
| Participant rating of satisfaction | < 0.001 | < 0.001 | 1.69 | 1.21 to 2.17 | 0.78 | 0.55 to 1.01 |
| Carer rating of attainment | < 0.001 | < 0.001 | 1.85 | 1.38 to 2.31 | 0.88 | 0.65 to 1.11 |
| Measure | p-value | Adjusted p-value | Mean difference | 95% CI for mean difference | Cohen’s d | 95% CI for Cohen’s d |
|---|---|---|---|---|---|---|
| 3-month follow-up | ||||||
| Participants with dementia | ||||||
| DEMQOL score | 0.505 | 1 | 0.59 | –1.14 to 2.31 | 0.07 | –0.14 to 0.28 |
| GSES score | 0.157 | 1 | 0.65 | –0.25 to 1.56 | 0.15 | –0.06 to 0.36 |
| HADS score | 0.659 | 1 | –0.2 | –1.07 to 0.68 | –0.05 | –0.25 to 0.16 |
| RBMT immediate recall | 0.259 | 1 | 0.22 | –0.16 to 0.6 | 0.12 | –0.09 to 0.33 |
| RBMT delayed recall | 0.668 | 1 | 0.07 | –0.27 to 0.41 | 0.05 | –0.16 to 0.25 |
| TEA elevator counting | 0.391 | 1 | 0.1 | –0.13 to 0.32 | 0.09 | –0.12 to 0.3 |
| TEA elevator counting with distraction | 0.427 | 1 | 0.21 | –0.31 to 0.73 | 0.08 | –0.12 to 0.29 |
| D-KEFS verbal fluency | 0.924 | 1 | 0.07 | –1.35 to 1.49 | 0.01 | –0.2 to 0.22 |
| Carers | ||||||
| RSS | 0.362 | 1 | –0.59 | –1.87 to 0.68 | –0.1 | –0.3 to 0.11 |
| WHOQOL-BREF physical | 0.171 | 1 | 0.23 | –0.1 to 0.55 | 0.15 | –0.06 to 0.35 |
| WHOQOL-BREF psychological | 0.133 | 1 | 0.25 | –0.08 to 0.57 | 0.16 | –0.05 to 0.37 |
| WHOQOL-BREF social | 0.930 | 1 | –0.02 | –0.46 to 0.42 | –0.01 | –0.22 to 0.2 |
| WHOQOL-BREF environmental | 0.029 | 0.488 | 0.33 | 0.03 to 0.63 | 0.23 | 0.03 to 0.44 |
| EQ-5D-3L index | 0.189 | 1 | 0.02 | –0.01 to 0.06 | 0.14 | –0.07 to 0.35 |
| EQ-5D-3L VAS | 0.153 | 1 | 2.72 | –1.02 to 6.47 | 0.15 | –0.06 to 0.36 |
| 9-month follow-up | ||||||
| Participants with dementia | ||||||
| DEMQOL score | 0.254 | 1 | 1.23 | –0.89 to 3.36 | 0.13 | –0.09 to 0.35 |
| GSES score | 0.357 | 1 | 0.48 | –0.54 to 1.5 | 0.1 | –0.12 to 0.32 |
| HADS score | 0.121 | 1 | 0.76 | –0.2 to 1.72 | 0.18 | –0.05 to 0.4 |
| RBMT immediate recall | 0.704 | 1 | 0.08 | –0.32 to 0.47 | 0.04 | –0.18 to 0.26 |
| RBMT delayed recall | 0.335 | 1 | –0.18 | –0.54 to 0.19 | –0.11 | –0.33 to 0.11 |
| TEA elevator counting | 0.764 | 1 | –0.04 | –0.33 to 0.24 | –0.03 | –0.25 to 0.19 |
| TEA elevator counting with distraction | 0.176 | 1 | 0.38 | –0.17 to 0.93 | 0.15 | –0.07 to 0.37 |
| D-KEFS verbal fluency | 0.679 | 1 | 0.36 | –1.34 to 2.05 | 0.05 | –0.17 to 0.27 |
| Carers | ||||||
| RSS | 0.529 | 1 | 0.44 | –0.93 to 1.81 | 0.07 | –0.15 to 0.29 |
| WHOQOL-BREF physical | 0.126 | 1 | 0.31 | –0.09 to 0.72 | 0.17 | –0.05 to 0.39 |
| WHOQOL-BREF psychological | 0.544 | 1 | 0.11 | –0.25 to 0.46 | 0.07 | –0.15 to 0.29 |
| WHOQOL-BREF social | 0.165 | 1 | 0.36 | –0.15 to 0.87 | 0.16 | –0.06 to 0.38 |
| WHOQOL-BREF environmental | 0.607 | 1 | 0.09 | –0.24 to 0.41 | 0.06 | –0.16 to 0.28 |
| EQ-5D-3L index | 0.858 | 1 | 0 | –0.05 to 0.04 | –0.02 | –0.24 to 0.2 |
| EQ-5D-3L VAS | 0.009 | 0.147 | 4.72 | 1.21 to 8.24 | 0.3 | 0.08 to 0.52 |
Appendix 11 Analyses of the change in scores between baseline and follow-ups
| Measure | F-value | df | p-value | Adjusted p-value | Cohen’s d | 95% CI for Cohen’s d | Mean difference | 95% CI for Mean difference |
|---|---|---|---|---|---|---|---|---|
| BGSI performance rating by participant with dementia | 73.82 | 1344 | < 0.001 | < 0.001 | 0.91 | 0.7 to 1.13 | 1.71 | 1.32 to 2.1 |
| BGSI satisfaction rating by participant with dementia | 53.61 | 1344 | < 0.001 | < 0.001 | 0.78 | 0.56 to 0.99 | 1.56 | 1.14 to 1.98 |
| BGSI performance rating by carer | 93.3 | 1344 | < 0.001 | < 0.001 | 1.03 | 0.81 to 1.25 | 1.89 | 1.51 to 2.28 |
| DEMQOL score | 0.45 | 1344 | 0.505 | 1 | 0.07 | –0.14 to 0.28 | 0.59 | –1.14 to 2.31 |
| GSES score | 2.01 | 1344 | 0.157 | 1 | 0.15 | –0.06 to 0.36 | 0.65 | –0.25 to 1.56 |
| HADS score | 0.19 | 1344 | 0.659 | 1 | –0.05 | –0.25 to 0.16 | –0.2 | –1.07 to 0.68 |
| RBMT immediate recall | 1.28 | 1344 | 0.259 | 1 | 0.12 | –0.09 to 0.33 | 0.22 | –0.16 to 0.6 |
| RBMT delayed recall | 0.18 | 1344 | 0.668 | 1 | 0.05 | –0.16 to 0.25 | 0.07 | –0.27 to 0.41 |
| TEA elevator counting | 0.74 | 1344 | 0.391 | 1 | 0.09 | –0.12 to 0.3 | 0.1 | –0.13 to 0.32 |
| TEA elevator counting with distraction | 0.63 | 1344 | 0.427 | 1 | 0.08 | –0.12 to 0.29 | 0.21 | –0.31 to 0.73 |
| D-KEFS verbal fluency | 0.01 | 1344 | 0.924 | 1 | 0.01 | –0.2 to 0.22 | 0.07 | –1.35 to 1.49 |
| RSS | 0.83 | 1344 | 0.362 | 1 | –0.10 | –0.3 to 0.11 | –0.59 | –1.87 to 0.68 |
| EQ-5D-3L index | 1.73 | 1344 | 0.189 | 1 | 0.14 | –0.07 to 0.35 | 0.02 | –0.01 to 0.06 |
| EQ-5D-3L VAS | 2.05 | 1344 | 0.153 | 1 | 0.15 | –0.06 to 0.36 | 2.72 | –1.02 to 6.47 |
| WHOQOL-BREF physical | 1.89 | 1344 | 0.171 | 1 | 0.15 | –0.06 to 0.35 | 0.23 | –0.1 to 0.55 |
| WHOQOL-BREF physchological | 2.26 | 1344 | 0.133 | 1 | 0.16 | –0.05 to 0.37 | 0.25 | –0.08 to 0.57 |
| WHOQOL-BREF social | 0.01 | 1344 | 0.930 | 1 | –0.01 | –0.22 to 0.2 | –0.02 | –0.46 to 0.42 |
| WHOQOL-BREF environmental | 4.83 | 1344 | 0.029 | 0.488 | 0.23 | 0.03 to 0.44 | 0.33 | 0.03 to 0.63 |
| Measure | F-value | df | p-value | Adjusted p-value | Cohen’s d | 95% CI for Cohen’s d | Mean difference | 95% CI for Mean difference |
|---|---|---|---|---|---|---|---|---|
| BGSI performance rating by participant with dementia | 58.01 | 1305 | < 0.001 | < 0.001 | 0.86 | 0.63 to 1.09 | 1.81 | 1.34 to 2.28 |
| BGSI satisfaction rating by participant with dementia | 47.34 | 1305 | < 0.001 | < 0.001 | 0.78 | 0.55 to 1.01 | 1.69 | 1.21 to 2.17 |
| BGSI performance Rating by carer | 60.97 | 1305 | < 0.001 | < 0.001 | 0.88 | 0.65 to 1.11 | 1.85 | 1.38 to 2.31 |
| DEMQOL score | 1.31 | 1305 | 0.254 | 1 | 0.13 | –0.09 to 0.35 | 1.23 | –0.89 to 3.36 |
| GSES score | 0.85 | 1305 | 0.357 | 1 | 0.10 | –0.12 to 0.32 | 0.48 | –0.54 to 1.5 |
| HADS score | 2.42 | 1305 | 0.121 | 1 | 0.18 | –0.05 to 0.4 | 0.76 | –0.2 to 1.72 |
| RBMT immediate recall | 0.14 | 1305 | 0.704 | 1 | 0.04 | –0.18 to 0.26 | 0.08 | –0.32 to 0.47 |
| RBMT delayed recall | 0.93 | 1305 | 0.335 | 1 | –0.11 | –0.33 to 0.11 | –0.18 | –0.54 to 0.19 |
| TEA elevator counting | 0.09 | 1305 | 0.764 | 1 | –0.03 | –0.25 to 0.19 | –0.04 | –0.33 to 0.24 |
| TEA elevator counting with distraction | 1.84 | 1305 | 0.176 | 1 | 0.15 | –0.07 to 0.37 | 0.38 | –0.17 to 0.93 |
| D-KEFS verbal fluency | 0.17 | 1305 | 0.679 | 1 | 0.05 | –0.17 to 0.27 | 0.36 | –1.34 to 2.05 |
| RSS | 0.4 | 1305 | 0.529 | 1 | 0.07 | –0.15 to 0.29 | 0.44 | –0.93 to 1.81 |
| EQ-5D-3L index | 0.03 | 1305 | 0.858 | 1 | –0.02 | –0.24 to 0.2 | 0 | –0.05 to 0.04 |
| EQ-5D-3L VAS | 6.98 | 1305 | 0.009 | 0.147 | 0.30 | 0.08 to 0.52 | 4.72 | 1.21 to 8.24 |
| WHOQOL-BREF physical | 2.35 | 1305 | 0.126 | 1 | 0.17 | –0.05 to 0.39 | 0.31 | –0.09 to 0.72 |
| WHOQOL-BREF physchological | 0.37 | 1305 | 0.544 | 1 | 0.07 | –0.15 to 0.29 | 0.11 | –0.25 to 0.46 |
| WHOQOL-BREF social | 1.94 | 1305 | 0.165 | 1 | 0.16 | –0.06 to 0.38 | 0.36 | –0.15 to 0.87 |
| WHOQOL-BREF environmental | 0.26 | 1305 | 0.607 | 1 | 0.06 | –0.16 to 0.28 | 0.09 | –0.24 to 0.41 |
Appendix 12 Effectiveness of blinding
| Researcher estimates | Whole sample, n (%) | Treatment group, n (%) | |
|---|---|---|---|
| CR | TAU | ||
| 3-month follow-up (N = 444) | |||
| Incorrect estimation | 86 (19.3) | 64 (29.4) | 22 (9.7) |
| Correct estimation | 358 (80.4) | 154 (70.6) | 204 (89.9) |
| 9-month follow-up (N = 426) | |||
| Incorrect estimation | 93 (21.8) | 66 (31.7) | 27 (12.4) |
| Correct estimation | 333 (78.2) | 142 (68.3) | 191 (87.6) |
| Questions asked of researchers | Whole sample (N = 444), n (%) | Treatment group, n (%) | |
|---|---|---|---|
| CR (N = 218) | TAU (N = 226) | ||
| Which condition the researcher thought the person with dementia had been allocated to | |||
| Indicated CR | 176 (39.6) | 154 (70.6) | 22 (9.7) |
| Indicated TAU | 268 (60.2) | 64 (29.4) | 204 (89.9) |
| How confident/certain was the researcher of their judgement about group allocation? | |||
| Very uncertain (complete guess) | 81 (18.2) | 43 (19.7) | 38 (16.7) |
| Uncertain | 148 (33.3) | 73 (33.5) | 75 (33.0) |
| Quite certain | 141 (31.7) | 57 (26.1) | 84 (37.0) |
| Very certain | 74 (16.6) | 45 (20.6) | 29 (12.8) |
| Did the participant make the group allocation explicit to the researcher? | |||
| Yes | 66 (14.8) | 44 (20.2) | 22 (9.7) |
| No | 378 (84.9) | 174 (79.8) | 204 (89.9) |
| Were there any indirect clues about group allocation? (e.g. new adaptations at home)? | |||
| Yes | 37 (8.3) | 33 (15.1) | 4 (1.8) |
| No | 407 (91.5) | 185 (84.9) | 222 (97.8) |
| Were the responses to these questions influenced by change/no change in the participant’s goal performance rating? | |||
| Yes | 215 (48.3) | 93 (42.7) | 122 (53.7) |
| No | 229 (51.5) | 125 (57.3) | 104 (45.8) |
| Questions asked of researchers | Whole sample (N = 426), n (%) | Treatment group, n (%) | |
|---|---|---|---|
| CR (N = 208) | TAU (N = 218) | ||
| Which condition the researcher thought the person with dementia had been allocated to | |||
| Indicated CR | 169 (39.7) | 142 (68.3) | 27 (12.4) |
| Indicated TAU | 257 (60.3) | 66 (31.7) | 191 (87.6) |
| How confident/certain was the researcher of their judgement about group allocation? | |||
| Very uncertain (complete guess) | 63 (14.8) | 31 (14.9) | 32 (14.7) |
| Uncertain | 149 (35.0) | 66 (31.7) | 83 (38.1) |
| Quite certain | 130 (30.5) | 54 (26.0) | 76 (34.9) |
| Very certain | 84 (19.7) | 57 (27.4) | 27 (12.4) |
| Did the participant make the group allocation explicit to the researcher: | |||
| Yes | 71 (16.7) | 54 (26.0) | 17 (7.8) |
| No | 355 (83.3) | 154 (74.0) | 201 (92.2) |
| Were there any indirect clues about group allocation? (e.g. new adaptations at home)? | |||
| Yes | 38 (8.9) | 32 (15.4) | 6 (2.8) |
| No | 388 (91.1) | 176 (84.6) | 212 (97.2) |
| Were the responses to these questions influenced by change/no change in the participant’s goal performance rating? | N = 425 | N = 207 | |
| Yes | 188 (44.2) | 84 (40.6) | 104 (47.7) |
| No | 237 (55.8) | 123 (59.4) | 114 (52.3) |
| Question | Blinding, n (%) | |
|---|---|---|
| Ineffective (N = 358) | Effective (N = 86) | |
| Which condition the researcher thought the person with dementia had been allocated to | ||
| Indicated CR | 154 (43.0) | 22 (25.6) |
| Indicated TAU | 204 (57.0) | 64 (74.4) |
| How confident/certain was the researcher of their judgement about group allocation? | ||
| Very uncertain (complete guess) | 52 (14.5) | 29 (33.7) |
| Uncertain | 112 (31.3) | 36 (41.9) |
| Quite certain | 121 (33.8) | 20 (23.3) |
| Very certain | 73 (20.4) | 1 (1.2) |
| Did the participant make the group allocation explicit to the researcher? | ||
| Yes | 66 (18.4) | 0 (0) |
| No | 292 (81.6) | 86 (100.0) |
| Were there any indirect clues about group allocation (e.g. new adaptations at home)? | ||
| Yes | 35 (9.8) | 2 (2.3) |
| No | 323 (90.2) | 84 (97.7) |
| Were the responses to these questions influenced by change/no change in the participant’s goal performance rating? | ||
| Yes | 183 (51.1) | 32 (37.2) |
| No | 175 (48.9) | 54 (62.8) |
| Question | Blinding, n (%) | |
|---|---|---|
| Ineffective (N = 333) | Effective (N = 93) | |
| Which condition the researcher thought the person with dementia had been allocated to | ||
| Indicated CR | 142 (42.6) | 27 (29.0) |
| Indicated TAU | 191 (57.4) | 66 (71.0) |
| How confident/certain was the researcher of their judgement about group allocation? | ||
| Very uncertain (complete guess) | 44 (13.2) | 19 (20.4) |
| Uncertain | 105 (31.5) | 44 (47.3) |
| Quite certain | 107 (32.1) | 23 (24.7) |
| Very certain | 77 (23.1) | 7 (7.5) |
| Have the participants made the group allocation explicit to the researcher? | ||
| Yes | 70 (21.0) | 1 (1.1) |
| No | 263 (79.0) | 92 (98.9) |
| Were there any indirect clues about group allocation (e.g. new adaptations at home)? | ||
| Yes | 34 (10.2) | 4 (4.3) |
| No | 299 (89.8) | 89 (95.7) |
| Were the responses to these questions influenced by change/no change in the participant’s goal performance rating? | ||
| Yes | 154 (46.4) | 34 (36.6) |
| No | 178 (53.6) | 59 (63.4) |
Appendix 13 Relationship of adherence to outcome
| Outcome | Estimate | SE | df | t-value | p-value | 95% CI |
|---|---|---|---|---|---|---|
| 3-month follow-up | ||||||
| Primary outcomes | ||||||
| Participant rating of attainment | 0.17 | 0.09 | 215 | 2.01 | 0.046 | 0 to 0.34 |
| Participant rating of satisfaction | 0.15 | 0.09 | 215 | 1.8 | 0.073 | –0.01 to 0.32 |
| Carer rating of attainment | 0.21 | 0.08 | 213 | 2.46 | 0.015 | 0.04 to 0.37 |
| Secondary outcomes: participants with dementia | ||||||
| DEMQOL score | –0.3 | 0.37 | 214 | –0.81 | 0.421 | –1.02 to 0.43 |
| GSES score | –0.33 | 0.18 | 211 | –1.8 | 0.073 | –0.69 to 0.03 |
| HADS depression score | 0.14 | 0.12 | 213 | 1.18 | 0.241 | –0.09 to 0.37 |
| HADS anxiety score | 0.02 | 0.1 | 215 | 0.23 | 0.816 | –0.18 to 0.23 |
| RBMT immediate recall | –0.03 | 0.07 | 214 | –0.36 | 0.722 | –0.16 to 0.11 |
| RBMT delayed recall | 0 | 0.06 | 213 | –0.01 | 0.995 | –0.12 to 0.12 |
| TEA elevator counting | 0.07 | 0.05 | 207 | 1.44 | 0.151 | –0.03 to 0.17 |
| TEA elevator counting with distraction | 0 | 0.11 | 190 | –0.04 | 0.971 | –0.22 to 0.21 |
| D-KEFS verbal fluency | 0.2 | 0.28 | 211 | 0.72 | 0.472 | –0.35 to 0.74 |
| Secondary outcomes: carers | ||||||
| RSS | –0.1 | 0.25 | 208 | –0.39 | 0.698 | –0.6 to 0.40 |
| WHOQOL-BREF physical | 0.03 | 0.08 | 209 | 0.35 | 0.729 | –0.13 to 0.18 |
| WHOQOL-BREF psychological | 0.04 | 0.07 | 209 | 0.65 | 0.516 | –0.09 to 0.18 |
| WHOQOL-BREF social | 0.1 | 0.09 | 208 | 1.09 | 0.278 | –0.08 to 0.27 |
| WHOQOL-BREF environmental | –0.04 | 0.06 | 209 | –0.65 | 0.514 | –0.16 to 0.08 |
| EQ-5D-3L index | 0 | 0.01 | 204 | 0.62 | 0.535 | –0.01 to 0.02 |
| EQ-5D-3L VAS | –0.73 | 0.79 | 205 | –0.92 | 0.361 | –2.29 to 0.84 |
| 9-month follow-up | ||||||
| Primary outcomes | ||||||
| Participant rating of attainment | 0.24 | 0.1 | 202 | 2.36 | 0.019 | 0.04 to 0.44 |
| Participant rating of satisfaction | 0.25 | 0.11 | 200 | 2.33 | 0.021 | 0.04 to 0.47 |
| Carer rating of attainment | 0.28 | 0.1 | 201 | 2.68 | 0.008 | 0.07 to 0.48 |
| Secondary outcomes: participants with dementia | ||||||
| DEMQOL score | –0.43 | 0.44 | 200 | –0.98 | 0.329 | –1.3 to 0.44 |
| GSES score | –0.38 | 0.25 | 190 | –1.54 | 0.126 | –0.86 to 0.11 |
| HADS depression score | –0.06 | 0.15 | 190 | –0.43 | 0.671 | –0.36 to 0.23 |
| HADS anxiety score | –0.04 | 0.12 | 191 | –0.3 | 0.763 | –0.28 to 0.21 |
| RBMT immediate recall | –0.07 | 0.08 | 197 | –0.89 | 0.376 | –0.24 to 0.09 |
| RBMT delayed recall | –0.01 | 0.06 | 197 | –0.16 | 0.874 | –0.14 to 0.12 |
| TEA elevator counting | –0.1 | 0.07 | 186 | –1.4 | 0.162 | –0.23 to 0.04 |
| TEA elevator counting with distraction | 0.1 | 0.13 | 168 | 0.72 | 0.472 | –0.17 to 0.36 |
| D-KEFS verbal fluency | 0.25 | 0.36 | 193 | 0.71 | 0.481 | –0.46 to 0.96 |
| Secondary outcomes: carers | ||||||
| RSS | 0.09 | 0.28 | 197 | 0.32 | 0.749 | –0.47 to 0.65 |
| WHOQOL-BREF physical | 0 | 0.01 | 194 | –0.44 | 0.657 | –0.02 to 0.01 |
| WHOQOL-BREF psychological | –1.55 | 0.78 | 193 | –1.98 | 0.049 | –3.09 to 0.01 |
| WHOQOL-BREF social | –0.06 | 0.08 | 196 | –0.79 | 0.432 | –0.22 to 0.09 |
| WHOQOL-BREF environmental | –0.06 | 0.07 | 196 | –0.81 | 0.419 | –0.2 to 0.08 |
| EQ-5D-3L index | –0.14 | 0.1 | 194 | –1.4 | 0.164 | –0.34 to 0.06 |
| EQ-5D-3L VAS | –0.12 | 0.07 | 196 | –1.81 | 0.071 | –0.25 to 0.01 |
Appendix 14 Four case studies from GREAT
Four illustrative case studies from GREAT show the kinds of needs and concerns that prompted participants and carers to choose particular goals and demonstrate how the therapists worked with participants and carers to address their goals during the CR intervention. Names and identifying details have been changed.
David: overcoming anxiety to maintain independence
David, a retired factory worker aged 70 years, lived with his wife Julie on the outskirts of a small town. Both were involved in numerous community activities. David had been diagnosed with Alzheimer’s disease a few months prior to joining the trial.
Although quite capable in everyday activities and household tasks, David was afraid to use appliances of any kind for fear of making a mistake and getting things wrong. He found this tremendously frustrating. It was severely compromising his independence, and his increasing reliance on Julie was causing friction between them. Julie felt frustrated when she tried unsuccessfully to explain how things worked, and she regretted that at times she could be very impatient with David. At the same time, she felt that David was capable of managing better and that she should be pushing him to do more for himself. David and Julie wanted to work on this area of difficulty.
The therapist’s assessment showed that David had the capacity to manage daily activities with only a small amount of guidance or support but was functioning considerably below this level. David’s anxiety needed to be understood in the context of his previous experience of episodes of anxiety and depression, and the therapist noted that he was currently taking antidepressants to try to stabilise his mood. The results of his cognitive tests indicated that he would be able to direct his attention to a task or activity but would need extra support with taking in information or remembering instructions, as it would be difficult for him to take in and retain information or instructions given verbally, especially if the surroundings were distracting. Although David was worried about his memory, the therapist found that he had some good strategies for managing memory difficulties; for example, when he needed to learn new songs for the choir he belonged to, he would break down the lyrics and learn a couple of lines at a time, building up to the whole song. This suggested that David had good potential to develop new ways of coping and should be able to learn to overcome his anxiety and manage to use various appliances. Both David and Julie were keen to try out any strategies that might help. The therapist’s work with David and Julie therefore focused mainly on enabling David to achieve his aim of being able to use various appliances without experiencing crippling levels of anxiety, with the wider aim of allowing him to function more independently.
We illustrate this here in relation to one of the goals: for David to be able to use his mobile phone whenever he wanted or needed to. Being able to use the mobile phone would give David the confidence to be out and about on his own, either to do shopping or errands or to participate in his chosen activities, knowing that he could contact Julie if he needed to. At the start of therapy, David could ‘wake up’ the phone and display the contacts list on the screen, but could not get beyond this step as he developed feelings of panic at the thought that he might do the wrong thing and then the phone would not work at all. David, Julie and the therapist all independently rated his current use of the phone at 2 out of 10. Julie was sceptical that any progress could be made as she had already obtained a simple ‘Doro’ phone for David and tried to teach him to use it, without success, but the therapist convinced her that it was worth trying to apply more specific learning techniques.
The first priority was to find a way to reduce David’s extreme anxiety. This was done by identifying a single key-press that would always take David back to the main menu if necessary. The phone had two smart keys. One of these was for cancelling choices and returning to the main menu; David and the therapist called this the ‘No, go back’ key. The other was for confirming choices on the display; David and the therapist labelled this the ‘Yes, go ahead’ key. The therapist initially taught David, using action-based learning with spaced retrieval, the functions of two smart keys on the phone. The left-hand key was designated ‘Yes’ and the right-hand key was designated ‘No, go back’. David was encouraged to use the ‘No, go back’ key to return to the main menu at any time, so that he did not need to fear that he would make a mistake.
Once the use of the ‘No, go back’ key was well established, the therapist and David identified the different ways in which David needed to use the phone. Initially, the focus was on receiving and making calls, and this was later extended to receiving and sending texts. Each activity was taught in sequence, with an appropriate set of learning strategies applied. The therapist worked with David to list the steps involved in the activity and develop step-by-step instructions that made sense to David. David had to engage in thinking about each step and write down the instructions for himself, reflecting effortful processing of the information. He kept these instructions together in a folder that he could refer to at any time.
Using an action-learning approach, each step was taught in turn, with the therapist demonstrating the actions needed and David repeating them, using an expanding rehearsal approach, with practice spaced at gradually increasing intervals. David was encouraged to practise in a quiet environment without distractions and to allow plenty of time in order to help him stay focused. Simple steps were taught first and more complex ones were taught later, following the principle of graded activity. For example, David first learned to access the text screen, and then when he was confident in doing this, he learned to write a text. This was followed, in sequence, by learning to sending the text, then receiving a text and reading it. David then practised the full sequence of steps, including sending texts to the therapist between sessions and receiving texts from the therapist. As David became more confident, Julie demonstrated how to add punctuation to his text messages and taught him how to delete old messages. She did this using expanding rehearsal strategies, focusing on one instruction at a time.
Having learned how to carry out a task, such as making a call, the next stage involved gaining confidence in using the phone through graded exposure to increasingly demanding situations. David began to practise using the phone in the house. First he used it in staged situations, such as making a test call to Julie or to the therapist, and then moved on to using it for real-life purposes, such as making a call about one of his activities or to a company representative. David then practised using the phone while out in the garden, with Julie on standby in the house in case he needed help. Finally, he practised using the phone while he was out and about, initially contacting the therapist and then using it for real-life purposes. Julie helped by identifying situations in which David could use the phone and encouraging him to do so. Julie gave verbal prompts to ensure that David took his phone with him and had switched it on before leaving the house; these prompts were gradually faded out as the routine became established.
At this stage, one last issue emerged. Now that David had largely mastered the skills of using his phone, he needed to remember to always take it with him when he went out, so that he could contact Julie if needed. A solution-focused problem-solving approach was used to develop a strategy to help David remember to take the phone with him. The method David and the therapist selected involved creating a cue card as a reminder. When going out, he usually remembered to take his bus pass and his wallet, both of which he kept in the same specific place, so the cue card was placed in the same location. The card contained the mnemonic BMW, standing for Bus pass, Mobile phone and Wallet and had ‘I’m taking the BMW’ written on it. David first had to learn the mnemonic and this was achieved by Julie prompting him twice daily, with the prompts gradually faded out once David was reliably able to respond.
By the end of the intervention period, David, Julie and the therapist all found that David’s ability to use the mobile phone had improved considerably. David and Julie both rated his ability to use the phone as 7 out of 10, and the therapist as 8 out of 10. David’s ability and confidence continued to improve throughout the 6-month maintenance period, as he regularly practised his new skills. He still experienced occasional anxiety, but was much better able to manage it. In session 14, David and Julie both gave attainment ratings of 8 out of 10 and the therapist rated his goal attainment as 9 out of 10. Using predefined goal attainment descriptors, the therapist rated the goal as 100% achieved in both session 10 and session 14, as David was able to use the phone routinely to make and receive calls and return to the home screen when any difficulties arose, and in addition he was using the phone to send and reply to texts.
During the therapy, David and Julie also worked on David’s ability to use other appliances independently, and similar improvements were seen in these other areas. Julie gained some valuable new skills to help with learning and relearning, and during the course of the therapy she applied these to help David to learn to use the cooker and the washing machine, using compensatory strategies, such as colour-coding controls.
At the end of therapy, David said that his ‘fear has gone’. The anxiety around using appliances had considerably abated and he was much less afraid of making mistakes. David felt more confident to try things out and gain new skills, knowing that he could determine how to learn at his own pace. Julie felt that she had become more willing to allow him to complete activities at his own pace and was much more patient with him, which meant that there were fewer tensions between them.
Doris: staying safe and in control
Doris, aged 63 years, lived independently in an inner-city area. She had a large extended family, many of whom would call in during the course of each day. Doris said she had been experiencing memory problems for around 4 years, and these had worsened considerably over the past 2 years. She had been diagnosed with vascular dementia within the previous month. Her eldest daughter, Dawn, was the main carer and was very protective of Doris, being justifiably concerned about her safety. Dawn frequently expressed anger about other family members who she felt were not doing enough to support her. Doris also frequently dealt with her feelings of stress by expressing anger at Dawn and other family members.
Doris valued her independence and it was important to her to feel in control. She was worried about her difficulties with memory and decision-making, found that her thoughts were muddled and felt that she had trouble making herself understood. She often experienced feelings of fear, even panic, as if something awful was about to happen, and was especially anxious in new situations or situations in which something was expected of her. She used to be very sociable and outgoing, and enjoyed going into town or to the pub but was now uncomfortable in crowds and had almost completely stopped going out alone. Even going along the road to the local post office could produce feelings of panic, which Doris could not account for.
The therapist’s work with Doris focused largely on enabling her to safely remain in control and be as independent as possible, both in and out of the house. Doris readily adopted the problem-solving approach; she considered solving problems one of her particular strengths. One important consideration for the therapist was the discovery that Doris had struggled at school as a child and had never learned to read or write, other than her name.
Doris usually forgot to lock her door when she went out or went to bed, creating a security risk, and Doris and Dawn agreed that this was an important goal to work on. They, and the therapist, all scored current attainment at 1 out of 10. The therapist worked with Doris to rehearse the procedure of locking the doors, followed by telephoning Dawn to confirm that she had done it, using an action-learning approach. To stimulate this behaviour, visual prompts were created, consisting of a photograph of the door keys, and these were placed next to the front door, in the living room and at the top of the stairs where Doris transferred off the stair lift. Family members were asked to prompt Doris to lock the door whenever they were leaving at the end of a visit, and to telephone Doris at night to remind her to lock the door before going to bed, although this did not happen consistently. In session 10, Doris and her daughter both rated attainment as 6 out of 10 and the therapist rated it as 7 out of 10. All of these ratings increased to 8 out of 10 at session 14, and at this stage the therapist rated this goal as 75% achieved, as Doris was mostly locking the door independently but still required some prompting on occasion.
Doris used a cash machine at the local post office to withdraw money but found that this was anxiety-provoking and was unable to remember the PIN (personal identification number) she had to enter into the cash machine to retrieve her money, describing herself as ‘stupid’. She had written the number on a piece of paper and placed it in her purse under a clear plastic window visible on opening the purse. This was very unsafe, especially as she tended to misplace her purse. She was increasingly anxious about using her card and inclined to avoid going altogether. Instead, family members had started to withdraw money on her behalf. Current attainment was rated by Doris, Dawn and the therapist as 1 out of 10. Doris could recognise numbers and indeed was considered to be ‘good with numbers’, and the therapist judged that she was capable of learning the PIN to enable her to use her bank card independently, while removing the risk of financial exploitation. She herself was very motivated to work on this, as she found it important to be in control of her finances and saw this as a marker of independence. This was a sensitive area, however, for the therapist to work with, and one that required extra safeguards. The approach to be taken was discussed in depth with Doris, with her family and with the trial team. Doris was deemed to have the capacity both to choose the goal and to give an opinion about the proposed strategy; had there been concerns about capacity for these specific decisions, a best-interests decision would have been needed. It might have been possible to work indirectly through the carer, but Doris’s daughter preferred the therapist to work on this goal directly with Doris rather than providing her with strategies to assist Doris and guidance on implementing them. In weighing up all of these factors, everyone involved agreed that given the risks Doris was exposed to currently, sharing the PIN with the therapist represented a safer option and, in this instance, was the best way to proceed. Full details of the circumstances and the team’s discussions were also recorded in Doris’s clinical notes held by the NHS memory service.
To help Doris learn the PIN, the number was first changed to something that would be relatively easy to remember. Chunking the information meant that initially the first two numbers were learned using expanding rehearsal, followed by the second two numbers, and finally all four digits. Visual mapping of the numbers on the key pad was attempted using action-based learning to set up a habitual pattern of movement. By session 10, Doris and Dawn rated attainment as 5 out of 10, with the therapist selecting 6 out of 10.
The therapist introduced controlled breathing techniques that Doris thought she could put into practice quickly and effectively. Doris understood the principles of controlled breathing and was able to demonstrate the technique when relaxed, but found it hard to put the technique into practice when she was anxious. Dawn and Doris used solution-focused problem-solving to identify ways of reducing anxiety about going to the post office and fear of experiencing a panic attack; this included identifying the days and times when the post office was quiet and planning to go at these specific times, so that Doris could use her card independently without feeling rushed. A plan for graded exposure was followed, whereby Doris gradually increased the frequency of visits to the post office to practise using her card. By session 14, Doris could reliably remember her PIN, and Doris, Dawn and the therapist rated attainment as 10 out of 10, with the goal rated as 100% achieved. Doris continued to work on managing her anxiety about going to the post office.
Other work focused on ensuring that Doris remembered to have her mobile phone with her at all times so that she could be in contact with her family, while retaining her independence. Everyone was concerned that she might fall or otherwise need help and wanted to make sure she could summon help if needed. This was achieved by using visual prompts to remind Doris to take her phone with her when going out and return it to a designated place in the house when indoors. Doris made good use of these strategies and her family felt reassured about her safety.
The therapist spent time with Dawn to help her to understand and deal with Doris’s behaviour and to point her towards local resources for carers. As a consequence, Dawn established an extensive network to support Doris’s needs, identified some sources of support for herself and started to allocate time for her own needs. Dawn was very engaged in the intervention and provided considerable support with grading activity and prompting the use of anxiety-management strategies. Following the intervention, she felt that she had a better understanding of Doris’s abilities, and began to apply similar principles to other situations, such as helping Doris to create and use her own shopping lists using visual prompts, such as collecting and storing product labels. Dawn was very positive about Doris’s progress with goals, development of compensatory strategies and general increase in motivation, and felt that participation had been beneficial in terms of helping Doris to maximise goal attainment and maintain independence and well-being.
Doris and Dawn both felt that they had always been ‘problem solvers’ but they found the framework for solution-focused problem-solving used in the intervention particularly useful in that it gave consideration to what had worked in the past. This enabled them to identify and develop strategies to maximise Doris’s independence, self-efficacy and self-esteem and apply these to a range of situations, as well as bringing the family together to support Doris’s goal attainment.
Shahid: re-engaging with people and activities
Shahid, aged 77 years, had worked in marketing prior to retirement. He lived with his wife Sylvia near their daughter and grandchildren. He had previously been actively involved in his local community and an accomplished public speaker. He was a keen photographer but had not done any photography for over 1 year. Shahid had been diagnosed with Alzheimer’s disease around 2 months before joining the trial.
Shahid had lost confidence and had become anxious about engaging with people and activities. One reason for this was his difficulty with word-finding, which made it hard to engage in conversation. He sometimes had trouble finding the correct words to use, which interrupted his flow of speech, and this led to him feeling embarrassed and getting quite frustrated and annoyed with himself. Often Sylvia would supply the word for him, but sometimes she was unsure of the word he was searching for, leading to more frustration. He wanted to be able to speak fluently again, and in particular he wanted to find the right word during a conversation to enable him to participate. Shahid also wanted to take up photography again and meet up with other photographers. However, he was confused and unsure about how to manage the camera settings and lacked the confidence to try.
The therapist’s assessment showed that Shahid was able to carry out most activities independently but he had difficulty motivating himself or initiating activity, and occasionally needed reminding about self-care. He was worried and anxious about his poor memory and lack of concentration and had become quite withdrawn and reluctant to participate in social interactions.
The therapist’s work with Shahid focused on helping him to feel more confident about engaging with people and activities. The first priority was to help Shahid feel better able to find his words during a conversation and hence less anxious about engaging with people. Initially, Shahid and Sylvia rated his current attainment as 5 out of 10, whereas the therapist opted for 4 out of 10. This indicated that his ability in conversation was fair, but reflected his desire for improvement.
The therapist, Shahid and Sylvia developed a plan to tackle word-finding problems. This had several elements. The first involved effortful processing and errorless learning. Instead of supplying the missing word, Sylvia instead gave either a cue, such as the first letter, or a clue to help Shahid find the word, so that he was more likely to retrieve the correct word himself. The cues or clues were intended to be precise enough to prompt the desired word, increasing the probability that this would also be recalled in future; this provided a natural opportunity for errorless learning.
The second element involved providing support for naming everyday items and objects. Items around the room were labelled to encourage Shahid to associate each object with its name, and he practised naming both labelled and unlabelled items when requested by Sylvia. The therapist prepared a set of picture cards and Shahid practised naming the items depicted, with Sylvia’s help. As Shahid gained confidence with naming the objects, these activities were graded by gradually increasing the number of items shown in any one session and by presenting them at greater speed.
The third element was the use of word exercises. These were practised during the session and further examples were left for Shahid and Sylvia to practise between sessions. Several different types of exercises were used, including supplying missing words in a sentence, providing antonyms or synonyms, listing items under a given category (e.g. modes of transport), identifying similarities and differences between pairs of words and answering comprehension questions about short stories.
The fourth element involved devising specific strategies for particular words or types of words. Shahid developed mnemonics and used expanding rehearsal within an errorless learning framework to remember specific words that often eluded him.
Shahid seemed to enjoy focusing on word-finding and doing the various exercises, and he made good progress. Sylvia became adept at providing cues or clues whenever he was unable to retrieve a word. By session 10, Shahid felt much better able to engage in conversation, and he no longer saw word-finding or engaging in conversation as a problem. Shahid and Sylvia both rated his attainment as 8 out of 10. The therapist observed that his conversation was much more fluent and rated his attainment as 9 out of 10. Shahid kept up his progress and the ratings made in session 14 were identical. The therapist rated the goal as 100% achieved in both session 10 and session 14, noting that Shahid was more confident about his word-finding ability and was usually able to find the necessary word and able to continue a conversation.
During the therapy, Shahid also worked on re-engaging with his interest in photography. There were technical issues with managing camera settings and using digital cameras, and Shahid preferred to discuss these with his son rather than with the therapist. The therapist’s role was to encourage Shahid to persist with solving the problems. The eventual solution was to provide Shahid with a phone that had a good-quality camera and was easy to link to his computer and TV screen to download and show images. This did enable Shahid to take photographs, and the good results that ensued gave him confidence and motivated him to continue. Holidays and visits to family provided interesting photographic opportunities, and he was able to produce some good photographs. Ratings of attainment improved from 2 at the start of therapy to 8 in session 10 and 9 (Shahid and Sylvia) or 10 (the therapist) in session 14. The therapist identified this goal as 75% achieved by session 10 and 100% achieved by session 14.
Shahid also developed his use of compensatory strategies, such as using a calendar to remember appointments, and began to carry a small notebook in his pocket containing a daily ‘to do’ list. This increased self-determination was mirrored by Sylvia offering prompts rather than doing things for him. He learned a strategy of intentional chanting for times when he might get distracted or interrupted, for example when going upstairs to fetch something, and practised various anxiety-management strategies before settling on using music to calm himself. He managed to motivate himself to clear his computer room and make space for working on his photographs, and this increased motivation also extended to getting other tasks done around the house. Shahid and Sylvia both became more active, developing a routine of playing golf once a week and going for a walk together once a week.
Gareth: managing everyday challenges
Gareth, a 71-year-old widower, had retired from a skilled technical job a few years previously. He lived independently and kept in contact with his daughters and grandchildren, mainly by telephone, although they lived nearby. Gareth had been diagnosed with mixed Alzheimer’s disease and vascular dementia 3 months before joining the trial. He also had some other health problems, which meant that he needed to eat regularly and keep to a healthy diet, and which limited his physical ability. His main source of support was his eldest daughter, Ginny.
Gareth was troubled by difficulties with concentrating, planning and organising his activities, remembering appointments, remembering things he needed to do, such as taking his medication, and finding key items, such as his keys, wallet or phone. His strategy of using a Dictaphone to record notes and messages was only partially successful. These difficulties were making it hard for him to complete everyday tasks independently and safely, manage his health problems and participate in social events. Gareth was particularly frustrated by his difficulties with planning and the impact these were having on his everyday life. He and Ginny identified some basic everyday skills in which improvements would help him to maintain independence and reduce the need to rely on Ginny. The therapist’s assessment showed that although Gareth had the potential to manage many activities and tasks independently, he needed some practical guidance and support to enable him to function optimally.
The therapist’s work with Gareth focused on improving everyday skills to support his independence. The first priority was cooking. Gareth prepared his own meals, but tended to lose track of what he was doing and forget that food was in the oven. This meant that food was often burnt and inedible. To make matters worse, Gareth had lost his sense of smell, so that he could not detect the olfactory cues associated with food overcooking. Gareth often felt tired and would leave the kitchen to sit down comfortably while food was cooking, but he was hard of hearing, so that if he was not in the kitchen, he did not hear the oven timer. In addition, he often fell asleep while waiting for food to be ready and then woke up feeling confused about what he had been doing or what he needed to do. When Gareth was in the kitchen, his tendency to lose track meant that he often picked up trays or plates from the oven without realising that they were hot and burnt himself, and there was a risk of the gas hob being turned on but unlit. Because of these difficulties, Gareth was limiting the extent to which he cooked for himself, either eating out, which was proving to be too expensive, or just having snacks. Gareth was keen to manage his cooking better and Ginny was very concerned about his safety, wanting to make sure that he was able to eat a healthy diet without hurting himself or setting fire to the kitchen. Gareth, Ginny and the therapist rated Gareth’s current ability in cooking his own meals as 4 out of 10.
The strategy that Gareth and the therapist devised involved two main components. First, Gareth was encouraged to focus his attention on the process of planning the meal and to work through a series of steps, reading the food packaging, writing down the cooking instructions, listing what preparation was needed and then recording what time the food went into the oven and when it was due to be ready. For this, Gareth used a whiteboard in the kitchen. Second, a portable timer was introduced to provide an auditory cue to check the oven at the appropriate time. Gareth learned to take the whiteboard and timer with him when leaving the kitchen, so that he would be able to hear it if he was in another room or if he fell asleep. Gareth opted to set the alarm to go off shortly before the food was due to be ready as this gave him time to get to the kitchen.
One additional practical change that the therapist recommended and Gareth and Ginny followed up was to purchase a halogen worktop cooker that turned off automatically, to remove safety concerns about leaving the gas on. This was intended to replace using the oven as it had a built-in timer and so turned itself off and ‘beeped’ when done. Gareth had no difficulty adjusting to using this and was able to demonstrate to the therapist how it worked. The therapist also involved telecare for an assessment of gas safety and provision of additional sensors, over and above Gareth’s two functioning smoke detectors.
Gareth could see an immediate improvement as a result of using the whiteboard and timer and readily adopted the use of these aids. By week 10, he was cooking meals safely without burning the food. He now cooked for himself at home most days. Having got used to the halogen cooker, Gareth gained confidence in using the hob to boil vegetables and rediscovered how to use the microwave. At both session 10 and session 14, Gareth rated his current ability as 7 out of 10, Ginny rated it as 6 out of 10 and the therapist rated it as 8 out of 10. The therapist rated this goal as 100% achieved in week 10.
A second area of concern for which similar strategies were adopted was remembering to take essential medication. Gareth had to take medication both in the morning and in the evening and, although he usually remembered his morning regime, he often forgot his evening medication, especially when he had been out during the day, potentially putting his health at risk. Some tablets were in a blister pack, but others were not, which made it harder to determine whether or not a dose had been missed. Usually one of his daughters would telephone to remind him, but this was not always possible and was proving stressful for the family. Gareth rated his current functioning as 5 out of 10, Ginny rated it as 6 out of 10 and the therapist rated it as 3 out of 10.
The strategy adopted had several components. First, a specific ‘workstation’ was set up on a table in the living room as the special place where medication would be taken. This was clearly visible from Gareth’s favourite chair, as this was where he usually went to take his tablets. The medication was placed permanently on the table along with a bottle of water, so that Gareth would always be able to see his medication and would not be distracted by leaving the room to get a drink of water. An attempt to link evening medication with an established routine, such as watching the 6 p.m. news on television was trialled, but this was not effective, as Gareth often went out in the evening, especially in the summer. As an alternative, an alarm clock was set for 7.30 a.m. and 7.30 p.m. as an auditory cue to remind Gareth to take his morning and evening doses. Gareth chose the timing of the alarm himself as the one that best fitted with his routine, and the therapist taught Gareth to respond to the alarm clock cue by taking medication at the appropriate time. To adapt the strategy for use on days out, Gareth’s mobile phone was also set to give an alarm at 7.30 p.m., and if going out, Gareth took his medication with him in a small container. Gareth found the strategy very useful, and when the alarm went off he would only cancel it after he had taken his tablets, to ensure that he did not get distracted. The therapist rated this goal as 75% achieved in week 10 and 100% achieved in week 14. Gareth rated his current ability as 8 in week 10 and 9 in week 14; Ginny’s ratings were 7 and 6, respectively, and the therapist rated his ability as 8 and 10, respectively.
During the intervention, Gareth also worked on other areas with the therapist, including remembering names of family and friends, staying engaged in conversation and improving attention. He became anxious when confronted with tasks, events or activities for which he was not prepared; to manage this better, the therapist introduced the idea of using a wall calendar to write down appointments and messages and a notebook for details. The therapist modelled the use of these aids and enabled Gareth to incorporate them in his daily routine. Gareth himself used the problem-solving approach to tackle other challenges, such as keeping his paperwork in order, organising telecare documents and managing his financial information. He used filing boxes and made lists, and he reviewed things weekly with Ginny to make sure everything was as it should be.
Gareth tended to get bored on his own at home and this was another area that the therapist focused on. Gareth’s usual strategy for dealing with boredom was to go out for a drive but he did not like to drive in the dark or when the weather was bad. The therapist worked with Gareth to identify activities he could do at home to occupy himself in the evenings or during bad weather. Gareth was also encouraged to practise using public transport, to prepare for a time when driving may no longer be feasible, and he began to try using the bus instead of driving. As Gareth often felt lonely, the therapist introduced him to the local Alzheimer’s Society branch and he started to attend their groups and activities, which he engaged with enthusiastically, feeling that his social life had been greatly improved.
Gareth embraced the CR intervention, was enthusiastic about adopting a range of compensatory strategies and integrated these very effectively into his daily life. He enjoyed working with the therapist and thought that he would miss the regular visits. Ginny and his other daughters were equally enthusiastic and willing to try new ideas. They all gained skills in problem-solving and developing new strategies and felt able to manage daily challenges better.
Concluding comments
These four typical case studies taken from the therapy logs compiled by GREAT therapists illustrate the types of goals participants chose and the way in which participants, carers and therapists worked together to apply a problem-solving approach and to develop strategies to enable participants to improve their functioning and attain their goals. They are consistent with the findings from the qualitative analysis of interviews with participants and carers. These emphasised the key importance of the relationship with the therapist as the vehicle for change and the time taken to understand needs and develop personalised strategies.
Appendix 15 Full unit costs
| Variable name | Unit cost, £ (2013–14) | Unit | Source | Notes/assumptions |
|---|---|---|---|---|
| Respite and care home use | ||||
| Private-sector residential care for older people, cost of stay | 79 | Per day | PSSRU 2014, table 1.2114 | Excludes personal living expenses |
| Private- and other independent-sector residential home for people with dementia, cost of stay | 91 | Per day | PSSRU 2014, table 1.3114 | Excludes personal living expenses |
| Local authority residential care for older people, cost of stay | 157 | Per day | PSSRU 2014, table 1.3114 | Excludes personal living expenses |
| Private-sector nursing home for older people, cost of stay | 104 | Per day | PSSRU 2014, table 1.1114 | Excludes personal living expenses |
| Social care schemes, cost of stay | 84 | Per day | PSSRU 2014, table 1.2114 | Average across the four schemes |
| Community health and social care services | ||||
| GP time, home visit | 3.60 | Per minute | PSSRU 2013, table 10.8b115 | No information about home visits in the 2014 volume. Assumed that the ratio of clinic-to-home cost per minute remained the same |
| GP time, home visit average visit cost (23.4 minutes) | 85 | Per visit | PSSRU 2013, table 10.8b115 | No information about home visits in the 2014 volume. Assumed that the ratio of clinic-to-home cost per minute remained the same. Assumed that the average duration of the visit remained the same |
| GP time, clinic visit | 2.90 | Per minute | PSSRU 2014, table 10.8b114 | No direct care staff and no qualification costs |
| GP time, clinic visit | 50 | Per visit | PSSRU 2014, table 10.8b114 | No direct care staff and no qualification costs |
| Practice nurse, face-to-face time | 0.73 | Per minute | PSSRU 2014, table 10.6114 | Excludes qualification costs |
| Practice nurse, face-to-face time | 11.37 | Per consultation | PSSRU 2014, table 10.6114 | Excludes qualification costs |
| District nursing time, face-to-face contact | 37 | Per contact | NHS Reference Costs 2013 to 2014 118 | |
| District nursing time, direct contact time | 1.23 | Per minute | NHS Reference Costs 2013 to 2014 118 | Assumes 30-minute contact |
| District nursing time, home visit | 1 | Per minute | PSSRU 2013, table 10.1115 | Excludes qualification cost |
| District nursing visit, per home visit | 56 | Per visit | PSSRU 2013, table 10.1115 | Excludes qualification cost |
| Nurse (mental health), face-to-face contact | 1.1 | Per minute | PSSRU 2014, table 10.2114 | Excludes qualification costs |
| Nurse (mental health), face-to-face contact | 33 | Per contact | PSSRU 2014, table 10.2114 | Excludes qualification costs |
| Consultant: psychiatrist, face-to-face session | 4.43 | Per minute | PSSRU 2014, table 15.7114 | Excludes qualification costs |
| Consultant: psychiatrist, face-to-face session | 221.45 | Per contact | PSSRU 2014, table 15.7114 | Excludes qualification costs |
| Clinical psychologist | 2.23 | Per minute | PSSRU 2014, table 9.5114 | Excludes qualification costs (no information on them) |
| Social worker, face-to-face time | 2.65 | Per minute | PSSRU 2014, table 11.2114 | Excludes qualification costs |
| Community physiotherapist, home visit | 52 | Per contact | NHS Reference Costs 2013 to 2014 118 | Home/clinic not specified |
| Community physiotherapist, home visit | 0.5 | Per minute | PSSRU 2014, table 9.1114 | Excludes qualification costs |
| Community physiotherapist, per clinic visit | 12.43 | Per contact | PSSRU 2014, table 13.1114 | Home/clinic not specified. Excludes qualification costs |
| Community physiotherapist, per clinic visit | 0.53 | Per minute | PSSRU 2014, table 13.1114 | Home/clinic not specified. Excludes qualification costs |
| NHS OT, cost per hour | 0.53 | Per minute | PSSRU 2014, table 9.2114 | Excludes qualification costs |
| NHS clinical support worker, cost per hour | 0.33 | Per minute | PSSRU 2014, table 10.5114 | Excludes qualification costs |
| Community pharmacist, patient-related activities | 1.07 | Per minute | PSSRU 2014, table 10.5114 | Excludes qualification costs |
| Community OT (social services), cost per hour | 0.68 | Per minute | PSSRU 2014, table 11.5114 | Excludes qualification costs |
| Dietitian, cost per hour | 0.55 | Per minute | PSSRU 2014, table 13.4114 | Excludes qualification costs |
| Dietitian, cost per visit | 80 | Per contact | NHS Reference Costs 2013 to 2014 118 | |
| Counselling services in primary care | 0.83 | Per minute | PSSRU 2014, table 2.7114 | |
| Counselling services in primary care | 45.83 | Per visit | PSSRU 2014, table 2.7114 | |
| NHS community mental health team (CMHT) worker for older people (OP) with mental health problems, per team member | 0.68 | Per minute | PSSRU 2014, table 12.1114 | |
| NHS Community Mental Health Team worker for older people with mental health problems, per team member | 41 | Per visit | PSSRU 2014, table 12.1114 | |
| Home care: average of independent and social services (average cost per hour: £19.64) | 0.33 | Per minute | PSSRU 2014, table 11.6114 | Average cost of private and social services costs; weighted average of weekday and weekend costs |
| Cleaner | 0.17 | Per minute | Greenleaf Cleaning,119 Hassle.com,120 Homeclean121 |
Internet search of three cleaning companies; average across prices Deflated using the HCHS index |
| Meals on Wheels | 5.8 | Per meal | PSSRU compendium 2014, table 8.1.1114 | Uprated using the HCHS Pay & Price Index |
| Laundry service, cost per week per service user | 26.43 | Per service user per week | Wandsworth Council122 | Uprated from 2008 to 2014 prices using HCHS Pay & Price inflator |
| Sitting service (i.e. Crossroads) | 0.29 | Per minute | Evaluation of the East Sussex Carers’ Breaks Demonstrator Site123 | Cost of a short break for carers |
| Carer support worker | 0.5 | Per minute | Evaluation of the East Sussex Carers’ Breaks Demonstrator Site123 | |
| Chiropodist | 0.53 | Per minute | PSSRU 2014, table 9.4114 | |
| Chiropodist | 39 | Per visit | NHS Reference Costs 2013 to 2014 118 | |
| Optician | 21.1 | Per visit | Optics at a Glance 2014; Optical Confederation124 | Cost of sight test |
| Optician | 57.92 | Per visit | Department of Health and Social Care125 | Adds the fee payable for the first patient seen at one domiciliary visit, NHS |
| Dentist, weighted average cost per hour of patient contact for a performer only and providing performer | 1.71 | Per minute | PSSRU 2014114 | Tables 10.9 and 10.10 |
| Dentist, community dental service | 125 | Per visit | NHS Reference Costs 2013 to 2014 118 | |
| Dentist, general dental service | 85 | Per visit | NHS Reference Costs 2013 to 2014 118 | |
| Audiologist | 51.94 | Per contact | NHS Reference Costs 2013 to 2014 118 | |
| Health visitor, patient-related work | 1.08 | Per minute | PSSRU 2014, schema 10.3114 | |
| Health visitor, per contact | 45.07 | Per contact | NHS Reference Costs 2013 to 2014 118 | CHS tab, N03F – health visitor, other clinical intervention |
| Speech and language therapist | 84 | Per contact | NHS Reference Costs 2013 to 2014 118 | A13A1 – speech and language therapist, adult, one to one |
| Speech and language therapist | 0.53 | Per minute | PSSRU 2014, table 13.3114 | Excludes qualifications |
| Day care for older people, per session | 56 | Per session | PSSRU 2014, table 1.6114 | |
| Day care in NHS facilities, per attendance | 146 | Attendance | NHS Reference Costs 2013 to 2014 118 | CHS tab |
| Day care for people with mental health problems, per session | 40 | Per session | PSSRU 2014, table 2.5114 | |
| Lunch club | 7.71 | Per session | Romeo et al.126 | Uprated using the HCHS Pay & Price inflator |
| Patient education classes |
Range: 9–36 11–9 |
Per session | NHS West Norfolk CCG,127 D’Amico et al.,128 Orgeta et al.,129 Quinn et al.130 | 2015 prices, downrated using the HCHS inflator; Uprated to 2013–14 prices using the HCHS Pay & Price inflator; uprated to 2013–14 using the HCHS Pay & Price inflator |
| Respiratory support group | 5.50 | Per session | Romeo et al.126 | Assumed social club; uprated using the HCHS Pay & Price inflator |
| Community memory café run by voluntary sector | 3 | Per session | Dementia Partnerships131 | |
| Befriending of older adults | 7.33 | Per session | PSSRU 2014, schema 2.11114 | |
| Choral singing, people with dementia | 3.13 | Per session | Alzheimer’s Society132–135 | Charges across four choirs |
| Exercise classes | 38.66 | Per person per class | Witham et al.136 | Uprated to 2013–14 using the HCHS Pay & Price inflator |
| Paramedic visit, see and treat and refer | 180 | Per attendance | NHS Reference Costs 2013 to 2014 118 | ASS01 – see and treat or refer |
| Memory clinic | 422 | Per attendance | PSSRU 2014, table 1.10114 | Assumes 1-hour contact |
| Minor injuries unit, weighted average of all attendances (admission and non-admission) | 55.16 | Per attendance | PSSRU 2010, table 7.1137 | Uprated to 2013–14 Pay & Price inflator |
| Consultant contact: community-based | 1.68 | Per minute | PSSRU 2014, table 15.5114 | Assumed the same cost as a medical consultant in hospital |
| Non-emergency ambulance use, average cost of a non-emergency patient transfer | 43.26 | Per transfer | PSSRU 2010 (p. 60)137 | Uprated using HCHS Pay & Price inflator. The 2013 compendium does not record |
| Equipment and adaptations | ||||
| Wheelchair (non-powered average of active user and self-/attendant propelled), mean annual equipment cost | 34.25 | Per item | PSSRU 2014, table 7.2114 | Annuitised over 10 years; cost over 3 months |
| Wheelchair (powered), mean annual equipment cost | 106 | Per item | PSSRU 2014, table 7.2114 | Annuitised over 10 years; cost over 3 months |
| Outdoor rail | 0.85 | Per item | PSSRU 2014, table 7.3.2114 | Annuitised over 10 years; cost over 3 months |
| Stair/grab rail | 0.6 | Per item | PSSRU 2014, table 7.3.2114 | Annuitised over 10 years; cost over 3 months |
| Over-bath shower | 36 | Per item | PSSRU 2014, table 7.3.2114 | Annuitised over 10 years; cost over 3 months |
| Walk-in shower/shower cubicle replacing bath | 119.75 | Per item | PSSRU 2014, table 7.3.1114 | Annuitised over 10 years; cost over 3 months |
| Outdoor ramp | 3.5 | Per item | PSSRU 2014, table 7.3.2114 | Annuitised over 10 years; cost over 3 months |
| Perching stool | 0.68 | Per item | PSSRU 2013, table 7.3.1115 | Uprated using the HCHS Pay & Price inflator – annuitised over 10 years;* cost over 3 months |
| Commode | 1.73 | Per item | PSSRU 2013, table 7.3.1115 | Uprated using the HCHS Pay & Price inflator – annuitised over 10 years;* cost over 3 months |
| Kitchen trolley | 1.04 | Per item | PSSRU 2013, table 7.3.1115 | Uprated using the HCHS Pay & Price inflator – annuitised over 10 years;* cost over 3 months |
| Toilet frame/raised toilet seat | 0.91 | Per item | PSSRU 2013, table 7.3.1115 | Annuitised over 10 years; cost over 3 months. Uprated to 2013–14 prices using the HCHS Pay & Price inflator |
| Walking stick | 0.22 | Per item | Transforming Community Equipment Services National Catalogue and Tariff for Simple Aids to Daily Living 138 | Annuitised over 10 years; cost over 3 months. Uprated to 2013–14 prices using the HCHS Pay & Price inflator |
| All four-wheeled and four-footed walking frames | 0.93 | Per item | Transforming Community Equipment Services National Catalogue and Tariff for Simple Aids to Daily Living 138 | Annuitised over 10 years; cost over 3 months. Uprated to 2013–14 prices using the HCHS Pay & Price inflator |
| Bath seat | 0.51 | Per item | Transforming Community Equipment Services National Catalogue and Tariff for Simple Aids to Daily Living 138 | Annuitised over 10 years; cost over 3 months. Uprated to 2013–14 prices using the HCHS Pay & Price inflator |
| Bed lever/rail | 0.91 | Per item | Transforming Community Equipment Services National Catalogue and Tariff for Simple Aids to Daily Living 138 | Annuitised over 10 years; cost over 3 months. Uprated to 2013–14 prices using the HCHS Pay & Price inflator |
| Individual alarm system | 98.53 | Per item | Building Telecare in England (pp. 1–21)139 | Uprated using the HCHS Pay & Price inflator – annuitised over 10 years;* cost over 3 months |
| Medications | ||||
| Various | Range: 0.029–8.45 | Standard quantity units | Prescription Cost Analysis, England 2013 140 | |
| Unpaid carer costs | ||||
| National minimum wage | 6.31 | Per hour | National Minimum Wage and National Living Wage Rates 141 | |
| Travel costs | ||||
| Cost per mile of travel for carer (car running costs), per mile | 0.25 | Per mile | Automobile Association car running costs 2013142 | Price when new between £22,000 to £26,000 |
| Professional travel for delivery of CR: NHS reimbursement rate | 0.43 | Per mile | NHS mileage rates 2013143 | |
| Hospital services | ||||
| A&E attendances, weighted average of admitted and non-admitted | 124 | Attendance | NHS Reference Costs 2013 to 2014 118 | |
| Minor injury unit | 55 | Attendance | PSSRU 2010, table 7.1137 | Uprated using HCHS Pay & Price inflator |
| Inpatients | ||||
| Subchapter AA: nervous system procedures and disorders | 420 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter BZ: eyes and periorbital procedures and disorders | 565 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter CA: ear, nose, mouth, throat and neck procedures | 841 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter DZ: thoracic procedures and disorders | 370 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter EA: cardiac procedures | 896 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter EB: cardiac disorders | 412 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter FZ: digestive system procedures and disorders | 482 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter HA: orthopaedic trauma procedures | 1661 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter HB: orthopaedic non-trauma procedures | 567 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter HC: spinal surgery and disorders | 651 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter HD: musculoskeletal disorders | 374 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter LA: renal procedures and disorders | 522 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter LB: urological and male reproductive system procedures and disorders | 598 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter MB: urological and male reproductive system procedures and disorders | 452 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Subchapter VC: rehabilitation | 298 | Day | NHS Reference Costs 2013 to 2014 118 | REHAB tab |
| Subchapter WA: multiple trauma | 356 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter WD: treatment of mental health patients by non-mental health service providers | 474 | Day | NHS Reference Costs 2013 to 2014 118 | NEL and NEL _ XS tabs |
| Subchapter YR: vascular imaging interventions | 646 | Day | NHS Reference Costs 2013 to 2014 118 | NEL tab |
| Day cases | ||||
| Subchapter CA: ear, nose, mouth, throat and neck procedures | 938 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Subchapter EA: cardiac procedures | 1333 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Subchapter BZ: eyes and periorbital procedures and disorders | 784 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Subchapter FZ: digestive system procedures and disorders | 566 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Subchapter HD: musculoskeletal disorders | 374 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Subchapter JD: skin disorders | 513 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Day cases, weighted average across specialties | 698 | Day | NHS Reference Costs 2013 to 2014 118 | DC tab |
| Outpatients | ||||
| Service code 100: general surgery | 112 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 101: urology | 92 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 103: breast surgery | 118 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 104: colorectal surgery | 110 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 105: hepatobiliary and pancreatic surgery | 156 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 107: vascular surgery | 138 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 108: spinal surgery service | 133 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 110: trauma and orthopaedics | 104 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 120: ear, nose and throat | 83 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 130: ophthalmology | 80 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 141: restorative dentistry | 117 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 144: maxillofacial surgery | 104 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 150: neurosurgery | 172 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 160: plastic surgery | 85 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 170: cardiothoracic surgery | 271 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 191: pain management | 121 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 301: gastroenterology | 118 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 302: endocrinology | 131 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 303: clinical haematology | 156 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 304: clinical physiology | 60 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 307: diabetic medicine | 142 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 320: cardiology | 118 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 324: anticoagulant service | 27 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 330: dermatology | 93 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 340: respiratory medicine | 138 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 341: respiratory physiology | 128 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 350: infectious diseases | 210 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 361: nephrology | 141 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 370: medical oncology | 138 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 400: neurology | 156 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 410: rheumatology | 122 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 430: geriatric medicine | 175 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 502: gynaecology | 120 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 650: physiotherapy | 44 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 652: speech and language therapy | 84 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 653: podiatry | 42 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 654: dietetics | 61 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 656: clinical psychology | 184 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 657: prosthetics | 53 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 658: orthotics | 111 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 662: optometry | 95 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 715: old age psychiatry | 107 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 812: diagnostic imaging | 44 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Service code 840: audiology | 122 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
| Weighted average of follow-up attendances across service codes | 102 | Attendance | NHS Reference Costs 2013 to 2014 118 | Consultant and non-consultant-led follow-up face-to-face attendances, CL tab |
Appendix 16 Participant resource use and replacement costs
| Resources | Units | Treatment group | |||
|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||||
| Users, N/n valid | Sample mean (SE) | Users, N/n valid | Sample mean (SE) | ||
| Hospital services | |||||
| A&E | Attendances | 28/236 | 0.19 (0.05) | 15/233 | 0.07 (0.02) |
| Inpatient | Days | 13/236 | 0.66 (0.28) | 12/233 | 0.17 (0.06) |
| Outpatient | Attendances | 123/236 | 1.10 (0.11) | 107/233 | 0.98 (0.10) |
| Day hospital | Days | 2/236 | 0.01 (0.01) | 4/233 | 0.04 (0.02) |
| Primary and community health | |||||
| GP | Contactsa | 161/236 | 1.60 (0.13) | 172/233 | 1.69 (0.15) |
| Practice nurse | Contactsa | 115/236 | 0.85 (0.09) | 131/233 | 1.16 (0.12) |
| Community nurse | Contactsa | 21/236 | 0.36 (0.14) | 24/233 | 0.48 (0.23) |
| Physiotherapist | Contactsa | 24/236 | 0.25 (0.07) | 22/233 | 0.38 (0.10) |
| OT | Contactsa | 15/236 | 0.18 (0.06) | 11/233 | 0.15 (0.07) |
| Specialist nurse | Contactsa | 40/236 | 0.25 (0.04) | 36/233 | 0.38 (0.17) |
| Dietitian | Contactsa | 4/236 | 0.02 (0.01) | 5/233 | 0.03 (0.01) |
| Counsellor | Contactsa | 1/236 | 0.01 (0.01) | 0/233 | 0 |
| Optician | Contactsa | 75/236 | 0.40 (0.04) | 62/233 | 0.33 (0.04) |
| Chiropodist | Contactsa | 71/236 | 0.39 (0.04) | 83/233 | 0.54 (0.06) |
| Dentist | Contactsa | 90/236 | 0.53 (0.06) | 84/233 | 0.49 (0.05) |
| Mental health | |||||
| Mental health nurse | Contactsa | 23/236 | 0.19 (0.05) | 18/233 | 0.12 (0.04) |
| Psychiatrist | Contactsa | 38/236 | 0.17 (0.03) | 32/233 | 0.16 (0.03) |
| Psychologist | Contactsa | 24/236 | 0.17 (0.06) | 20/233 | 0.17 (0.05) |
| Mental health team worker | Contactsa | 4/236 | 0.03 (0.01) | 5/233 | 0.06 (0.04) |
| Community care | |||||
| Social worker/care manager | Contactsa | 18/236 | 0.11 (0.03) | 12/233 | 0.07 (0.02) |
| Home care/home help | Contacts | 25/236 | 6.32 (1.65) | 26/233 | 12.07 (3.18) |
| Home care/home help | Hours | 25/236 | 73.43 (6.68) | 26/233 | 105.01 (13.80) |
| Cleaner | Contacts | 50/236 | 2.20 (0.41) | 53/233 | 2.53 (0.36) |
| Meals on Wheels | Contacts | 1/236 | 0.05 (0.05) | 3/233 | 0.48 (0.37) |
| Laundry service | Contacts | 3/236 | 0.13 (0.08) | 9/233 | 0.24 (0.10) |
| Sitting service | Contacts | 1/236 | 0.05 (0.05) | 7/233 | 0.22 (0.10) |
| Carer support worker | Contacts | 9/236 | 0.09 (0.04) | 9/233 | 0.07 (0.03) |
| Other health and social care services | |||||
| SALTb | Contacts | 1/230 | 0.01 (0.01) | 1/230 | 0 |
| Health visitorb | Contacts | 1/230 | 0.01 (0.01) | 0/230 | 0 |
| Medical consultantb | Contacts | 1/230 | 0 | 0/230 | 0 |
| Paramedicb | Contacts | 0/230 | 0 | 0/230 | 0 |
| Audiologyb | Contacts | 2/229 | 0.02 (0.01) | 5/230 | 0.02 (0.01) |
| Community pharmacistb | Contacts | 0/229 | – | 0/230 | – |
| Health-care support workerb | Contacts | 6/233 | 0.09 (0.05) | 4/231 | 0.03 (0.01) |
| Day care | |||||
| Day centre | Attendances | 23/236 | 1.51 (0.44) | 25/233 | 1.26 (0.31) |
| Lunch club | Attendances | 23/236 | 0.83 (0.24) | 12/233 | 0.47 (0.15) |
| Patient education course | Attendances | 8/236 | 0.29 (0.12) | 10/233 | 0.24 (0.09) |
| Other day services | |||||
| Memory café/dementia supportc | Attendances | 16/236 | 0.33 (0.11) | 12/233 | 0.25 (0.09) |
| Befrienderc | Attendances | 2/236 | 0.06 (0.06) | 2/233 | 0.11 (0.11) |
| Singing for the brain/dementia choirsc | Attendances | 3/236 | 0.08 (0.06) | 5/233 | 0.10 (0.05) |
| Exercise classes for older peoplec | Attendances | 3/236 | 0.14 (0.08) | 1/233 | 0.06 (0.06) |
| Respiratory support groupsc | Attendances | 1/236 | 0.04 (0.04) | 0/233 | 0 |
| Other | |||||
| Equipment adaptations (NHS/SSD) | Items | 76/236 | 0.86 (0.11) | 76/233 | 0.80 (0.10) |
| Equipment privately purchased | Items | 130/236 | 1.27 (0.11) | 140/233 | 1.45 (0.11) |
| Medications | Units | 190/236 | 1.15 (0.06) | 193/233 | 1.11 (0.05) |
| Hypnotics and anxiolytics | Units | 3/236 | 0.02 (0.01) | 2/233 | 0.01 (0.01) |
| Antipsychotics | Units | 2/236 | 0.01 (0.01) | 2/233 | 0.01 (0.01) |
| Antidepressants | Units | 59/236 | 0.26 (0.03) | 42/233 | 0.21 (0.03) |
| Antiepileptics | Units | 1/236 | 0.00 (0.00) | 1/233 | 0.00 (0.00) |
| Dementia medications | Units | 170/236 | 0.82 (0.04) | 182/233 | 0.83 (0.03) |
| Principal carer care | Hours | 207/235 | 609.43 (52.25) | 200/232 | 534.91 (47.67) |
| Other carer care | Hours | 81/236 | 92.94 (19.80) | 75/232 | 57.50 (10.87) |
| Resources | Units | Treatment group | |||
|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||||
| Users, N/n valid | Sample mean use (SE) | Users, N/n valid | Sample mean use (SE) | ||
| Hospital services | |||||
| A&E | Attendances | 21/215 | 0.13 (0.03) | 22/223 | 0.11 (0.02) |
| Inpatient | Days | 8/215 | 0.35 (0.15) | 15/223 | 0.52 (0.23) |
| Outpatient | Attendances | 114/215 | 1.23 (0.21) | 96/223 | 0.93 (0.19) |
| Day hospital | Days | 3/215 | 0.05 (0.03) | 0/223 | 0 |
| Primary and community health | |||||
| GP | Contactsa | 143/215 | 1.37 (0.10) | 147/223 | 1.41 (0.11) |
| Practice nurse | Contactsa | 104/215 | 0.89 (0.09) | 113/223 | 1.07 (0.13) |
| Community nurse | Contactsa | 12/215 | 0.38 (0.17) | 14/223 | 0.74 (0.40) |
| Physiotherapist | Contactsa | 19/215 | 0.42 (0.15) | 15/223 | 0.27 (0.09) |
| OT | Contactsa | 14/215 | 0.16 (0.06) | 9/223 | 0.08 (0.03) |
| Specialist nurse | Contactsa | 23/215 | 0.15 (0.04) | 23/223 | 0.15 (0.03) |
| Dietitian | Contactsa | 5/215 | 0.03 (0.01) | 6/223 | 0.03 (0.01) |
| Counsellor | Contactsa | 1/215 | 0.02 (0.02) | 0/223 | 0 |
| Optician | Contactsa | 56/215 | 0.30 (0.04) | 62/223 | 0.35 (0.04) |
| Chiropodist | Contactsa | 66/215 | 0.46 (0.06) | 75/223 | 0.51 (0.06) |
| Dentist | Contactsa | 78/215 | 0.55 (0.06) | 79/223 | 0.52 (0.06) |
| Mental health | |||||
| Mental health nurse | Contactsa | 10/215 | 0.05 (0.02) | 8/223 | 0.09 (0.06) |
| Psychiatrist | Contactsa | 24/215 | 0.11 (0.02) | 20/223 | 0.09 (0.02) |
| Psychologist | Contactsa | 7/215 | 0.07 (0.04) | 6/223 | 0.04 (0.02) |
| Mental health team worker | Contactsa | 5/215 | 0.09 (0.06) | 8/223 | 0.11 (0.05) |
| Community care | |||||
| Social worker | Contacts | 16/215 | 0.17 (0.05) | 15/223 | 0.14 (0.05) |
| Home care/home help | Contacts | 27/215 | 8.43 (2.22) | 30/223 | 11.02 (2.72) |
| Home care/home help | Hours | 27/215 | 95.98 (8.60) | 30/223 | 67.61 (7.40) |
| Cleaner | Contacts | 48/215 | 2.84 (0.59) | 52/223 | 2.71 (0.38) |
| Meals on Wheels | Contacts | 1/215 | 0.01 (0.01) | 3/223 | 0.33 (0.27) |
| Laundry service | Contacts | 5/215 | 0.20 (0.09) | 7/223 | 0.23 (0.12) |
| Sitting service | Contacts | 2/215 | 0.05 (0.04) | 4/223 | 0.11 (0.06) |
| Carer support worker | Contacts | 9/215 | 0.11 (0.06) | 8/223 | 0.05 (0.02) |
| Other health and social care services | |||||
| SALTb | Contacts | 1/215 | 0 | 0/223 | 0 |
| Health visitorb | Contacts | 0/215 | 0 | 0/223 | 0 |
| Medical consultantb | Contacts | 1/215 | 0 | 0/223 | 0 |
| Paramedicb | Contacts | 3/212 | 0.01 (0.01) | 1/218 | 0 |
| Audiologyb | Contacts | 1/209 | 0 | 2/216 | 0.01 (0.01) |
| Community pharmacistb | Contacts | 0/209 | – | 0/216 | – |
| Health-care support workerb | Contacts | 2/211 | 0.03 (0.02) | 3/217 | 0.06 (0.05) |
| Day care | |||||
| Day centre | Attendances | 30/215 | 1.72 (0.40) | 28/223 | 1.63 (0.38) |
| Lunch club | Attendances | 17/215 | 0.64 (0.22) | 21/223 | 0.91 (0.23) |
| Patient education course | Attendances | 8/215 | 0.31 (0.12) | 3/223 | 0.04 (0.04) |
| Other day services | |||||
| Memory café/dementia supportc | Attendances | 21/215 | 0.48 (0.13) | 14/223 | 0.39 (0.13) |
| Befrienderc | Attendances | 2/215 | 0.07 (0.06) | 5/223 | 0.04 (0.03) |
| Singing for the brain/dementia choirsc | Attendances | 6/215 | 0.19 (0.11) | 5/223 | 0.13 (0.07) |
| Exercise classes for older peoplec | Attendances | 0/215 | 0 | 2/223 | 0.17 (0.13) |
| Respiratory support groupsc | Attendances | 1/215 | 0.06 (0.06) | 0/223 | 0 |
| Other | |||||
| Equipment adaptations (NHS/SSD) | Items | 86/215 | 1.04 (0.12) | 82/223 | 0.83 (0.10) |
| Equipment privately purchased | Items | 130/215 | 1.36 (0.12) | 131/223 | 1.43 (0.11) |
| Medications | Items | 170/215 | 1.16 (0.06) | 186/223 | 1.13 (0.06) |
| Hypnotics and anxiolytics | Units | 1/215 | 0.00 (0.00) | 2/223 | 0.01 (0.01) |
| Medications used in psychoses | Units | 2/215 | 0.01 (0.01) | 4/223 | 0.02 (0.01) |
| Antidepressants | Units | 57/215 | 0.31 (0.04) | 41/223 | 0.21 (0.03) |
| Antiepileptics | Units | 1/215 | 0.00 (0.00) | 1/223 | 0.00 (0.00) |
| Dementia medications | Units | 157/215 | 0.80 (0.04) | 175/223 | 0.86 (0.04) |
| Principal carer care | Hours | 189/213 | 578.13 (53.01) | 191/221 | 613.98 (52.21) |
| Other carer care | Hours | 76/214 | 95.75 (20.21) | 73/222 | 50.14 (8.78) |
| Resources | Units | Treatment group | |||
|---|---|---|---|---|---|
| CR (N = 238) | TAU (N = 236) | ||||
| Users, N/n valid | Sample mean use (SE) | Users, N/n valid | Sample mean use (SE) | ||
| Hospital services | |||||
| A&E | Attendances | 24/205 | 0.14 (0.03) | 25/210 | 0.18 (0.05) |
| Inpatient | Days | 12/205 | 0.72 (0.36) | 7/210 | 0.35 (0.17) |
| Outpatient | Attendances | 96/205 | 1.40 (0.24) | 103/210 | 1.19 (0.21) |
| Day hospital | Days | 3/205 | 0.04 (0.02) | 2/210 | 0.02 (0.01) |
| Primary and community health | |||||
| GP | Contactsa | 139/205 | 1.42 (0.12) | 145/210 | 1.56 (0.11) |
| Practice nurse | Contactsa | 108/205 | 1.17 (0.16) | 104/210 | 0.91 (0.10) |
| Community nurse | Contactsa | 12/205 | 0.39 (0.17) | 12/210 | 0.60 (0.23) |
| Physiotherapist | Contactsa | 21/205 | 0.22 (0.06) | 28/210 | 0.60 (0.16) |
| OT | Contactsa | 22/205 | 0.24 (0.08) | 19/210 | 0.13 (0.03) |
| Specialist nurse | Contactsa | 22/205 | 0.16 (0.04) | 18/210 | 0.15 (0.05) |
| Dietitian | Contactsa | 4/205 | 0.02 (0.01) | 1/210 | 0 |
| Counsellor | Contactsa | 1/205 | 0.05 (0.05) | 0/210 | 0 |
| Optician | Contactsa | 48/205 | 0.27 (0.04) | 52/210 | 0.28 (0.04) |
| Chiropodist | Contactsa | 67/205 | 0.46 (0.05) | 84/210 | 0.65 (0.07) |
| Dentist | Contactsa | 75/205 | 0.48 (0.05) | 83/210 | 0.57 (0.07) |
| Mental health | |||||
| Mental health nurse | Contactsa | 8/205 | 0.05 (0.02) | 12/210 | 0.08 (0.03) |
| Psychiatrist | Contactsa | 18/205 | 0.12 (0.03) | 29/210 | 0.16 (0.03) |
| Psychologist | Contactsa | 8/205 | 0.10 (0.06) | 12/210 | 0.06 (0.02) |
| Mental health team worker | Contactsa | 3/205 | 0.02 (0.01) | 3/210 | 0.03 (0.02) |
| Community care | |||||
| Social worker/care manager | Contacts | 14/205 | 0.12 (0.04) | 22/210 | 0.16 (0.04) |
| Home care/home help | Contacts | 29/205 | 9.16 (2.16) | 28/209 | 13.73 (3.49) |
| Home care/home help | Hours | 29/205 | 77.51 (8.18) | 28/210 | 141.89 (17.13) |
| Cleaner | Contacts | 47/205 | 2.66 (0.52) | 49/209 | 2.68 (0.42) |
| Meals on Wheels | Contacts | 3/205 | 0.48 (0.45) | 6/209 | 0.31 (0.22) |
| Laundry service | Contacts | 5/205 | 0.29 (0.16) | 7/209 | 0.29 (0.12) |
| Sitting service | Contacts | 3/205 | 0.26 (0.17) | 5/209 | 0.11 (0.06) |
| Carer support worker | Contacts | 8/205 | 0.06 (0.02) | 13/209 | 0.22 (0.10) |
| Other health and social care services | |||||
| SALTb | Contacts | 1/202 | 0.01 (0.01) | 5/203 | 0.03 (0.02) |
| Health visitorb | Contacts | 0/202 | 0 | 0/203 | 0 |
| Medical consultantb | Contacts | 0/202 | 0 | 1/203 | 0.01 (0.01) |
| Paramedicb | Contacts | 1/202 | 0 | 3/204 | 0.02 (0.02) |
| Audiologyb | Contacts | 0/202 | 0 | 2/204 | 0.01 (0.01) |
| Community pharmacistb | Contacts | 1/202 | – | 0/204 | – |
| Health-care support workerb | Contacts | 2/202 | 0.07 (0.07) | 2/203 | 0 |
| Day care | |||||
| Day centre | Attendances | 30/205 | 1.82 (0.39) | 25/210 | 2.01 (0.48) |
| Lunch club | Attendances | 19/205 | 0.92 (0.26) | 16/210 | 0.48 (0.14) |
| Patient education course | Attendances | 6/205 | 0.18 (0.12) | 1/210 | 0.06 (0.06) |
| Other day services | |||||
| Memory café/dementia supportc | Attendances | 26/205 | 0.84 (0.24) | 17/210 | 0.40 (0.13) |
| Befrienderc | Attendances | 3/205 | 0.08 (0.07) | 6/210 | 0.32 (0.16) |
| Singing for the brain/dementia choirsc | Attendances | 8/205 | 0.31 (0.12) | 9/210 | 0.34 (0.13) |
| Exercise classes for older peoplec | Attendances | 1/205 | 0.05 (0.05) | 1/210 | 0.19 (0.19) |
| Respiratory support groupsc | Attendances | 1/205 | 0.06 (0.06) | 1/210 | 0.06 (0.06) |
| Other | |||||
| Equipment (NHS/SSD) | Items | 92/205 | 1.26 (0.15) | 79/210 | 1.07 (0.14) |
| Equipment privately purchased | Items | 129/205 | 1.47 (0.12) | 141/210 | 1.67 (0.12) |
| Medications | Items | 162/205 | 1.20 (0.07) | 175/210 | 1.16 (0.06) |
| Hypnotics and anxiolytics | Units | 4/205 | 0.02 (0.01) | 2/210 | 0.01 (0.01) |
| Medications used in psychoses | Units | 5/205 | 0.02 (0.01) | 5/210 | 0.02 (0.01) |
| Antidepressants | Units | 58/205 | 0.31 (0.04) | 46/210 | 0.25 (0.03) |
| Antiepileptics | Units | 1/205 | 0.00 (0.00) | 2/210 | 0.01 (0.01) |
| Dementia medications | Units | 150/205 | 0.80 (0.04) | 163/210 | 0.81 (0.03) |
| Principal carer care | Hours | 181/203 | 660.67 (58.16) | 209/209 | 647.52 (54.78) |
| Other carer care | Hours | 84/204 | 71.73 (14.28) | 82/209 | 59.39 (12.40) |
| Type of care or support | Treatment group, n (%) | |
|---|---|---|
| CR (N = 236) | TAU (N = 233) | |
| Baseline | ||
| Personal care | 74 (31) | 66 (28) |
| Helping with finances | 179 (76) | 175 (75) |
| Practical help | 191 (81) | 176 (76) |
| Taking the person to appointments | 205 (87) | 201 (87) |
| Medications | 178 (75) | 172 (74) |
| Keeping the person company | 202 (86) | 200 (86) |
| Making sure the person is safe (supervision) | 146 (62) | 148 (64) |
| Helping the person to organise their scheduleb | 6 (3) | 3 (2) |
| Helping the person’s mental state – moraleb | 0 (0) | 5 (3) |
| Driving or navigating for the personb | 4 (2) | 3 (2) |
| 3-month follow-up | ||
| Personal care | 64 (30) | 80 (36) |
| Helping with finances | 161 (75) | 182 (83) |
| Practical help | 171 (80) | 179 (81) |
| Taking the person to appointments | 185 (86) | 199 (90) |
| Medications | 163 (76) | 168 (76) |
| Keeping the person company | 183 (86) | 195 (88) |
| Making sure the person is safe (supervision) | 135 (63) | 144 (65) |
| Helping person to organise scheduleb | 2 (1) | 3 (2) |
| Helping person’s mental state – moraleb | 1 (1) | 2 (1) |
| Driving or navigating for personb | 3 (2) | 5 (3) |
| 9-month follow-up | ||
| Personal care | 78 (38) | 90 (43) |
| Helping with finances | 162 (80) | 171 (82) |
| Practical help | 160 (79) | 176 (84) |
| Taking the person to appointments | 175 (86) | 187 (89) |
| Medications | 158 (78) | 160 (77) |
| Keeping the person company | 186 (92) | 185 (89) |
| Making sure the person is safe (supervision) | 135 (67) | 146 (70) |
| Helping the person to organise their scheduleb | 4 (3) | 3 (2) |
| Helping the person’s mental state – moraleb | 1 (1) | 0 (0) |
| Driving or navigating for the personb | 4 (3) | 7 (5) |
| Outcomes/costs | CRa (N = 201) | 95% CIb | TAUa (N = 206) | 95% CIb | CR – TAU mean difference | 95% CIb | p-value |
|---|---|---|---|---|---|---|---|
| BGSI scorec | 4.57 | 4.36 to 4.79 | 3.21 | 3.02 to 3.41 | 1.37 | 1.09 to 1.64 | 0.000 |
| Societal costs (£)e | 49,394 | 44,237 to 54,811 | 52,588 | 47,256 to 58,260 | –3195 | –9329 to 2760 | 0.350 |
| (N = 190) | (N = 199) | ||||||
| GSES scorec | 20.14 | 19.70 to 20.56 | 19.92 | 19.45 to 20.37 | 0.23 | –0.32 to 0.78 | 0.435 |
| Societal costs (£)e | 48,042 | 42,909 to 53,544 | 52,327 | 46,999 to 58,073 | –4285 | –10,480 to 1693 | 0.172 |
| (N = 196) | (N = 205) | ||||||
| QALYsc,d (DEMQOL-U) | 0.45 | 0.44 to 0.46 | 0.45 | 0.44 to 0.46 | 0.00 | –0.01 to 0.01 | 0.897 |
| Societal costs (£)e | 49,561 | 44,276 to 55,039 | 53,276 | 47,988 to 58,970 | –3716 | –9795 to 2230 | 0.278 |
| Outcomes/costs | CRa (N = 192) | 95% CIb | TAUa (N = 198) | 95% CIb | CR – TAUa mean difference | 95% CIb | p-value |
|---|---|---|---|---|---|---|---|
| QALYsc,d (EQ-5D-3L) | 0.56 | 0.54 to 0.58 | 0.56 | 0.54 to 0.58 | 0.00 | –0.02 to 0.02 | 0.926 |
| Societal costs (£)e | 48,709 | 43,702 to 54,009 | 52,989 | 47,560 to 58,777 | –4280 | –10,423 to 1758 | 0.223 |
| Outcomes/costs | BGSIa (N = 407) | GSESb (N = 389) | QALYs [DEMQOL-Uc (N = 401)] | QALY [EQ-5D-3Lc (N = 390)] |
|---|---|---|---|---|
| Person with dementia, 9 months | ||||
| Societal costs (£) | –3195/1.37 = –2332 | –4285/0.23 = –18,630 | –3716/0.0005 = –7,432,000 | NA |
| Carer, 9 months | ||||
| Societal costs (£) | NA | NA | NA | –4280/0.001 = –4,280,000 |
| Outcomes/costs | CRa (N = 196) | 95% CIb | TAUa (N = 204) | 95% CIb | CR – TAU mean difference | 95% CIa | p-value |
|---|---|---|---|---|---|---|---|
| BGSIc | 4.60 | 4.38 to 4.82 | 3.22 | 3.03 to 3.42 | 1.38 | 1.09 to 1.65 | 0.000 |
| Health and social care costs (£)d | 4595 | 4253 to 5026 | 3295 | 2807 to 3826 | 1300 | 696 to 1953 | 0.000 |
| (N = 186) | (N = 197) | ||||||
| GSESc | 20.16 | 19.72 to 20.60 | 20.00 | 19.54 to 20.44 | 0.17 | –0.395 to 0.732 | 0.564 |
| Health and social care costs (£)d | 4543 | 4174 to 4951 | 3288 | 2833 to 3822 | 1255 | 628 to 1831 | 0.000 |
| (N = 191) | (N = 203) | ||||||
| QALYc,e (DEMQOL-U) | 0.45 | 0.44 to 0.46 | 0.45 | 0.44 to 0.46 | 0.00 | –0.01 to 0.01 | 0.721 |
| Health and social care costs (£)d | 4592 | 4226 to 4986 | 3432 | 2960 to 3981 | 1160 | 520 to 1742 | 0.000 |
| Outcomes/costs | CR (N = 189)a | 95% CIb | TAU (N = 195)a | 95% CIb | CR – TAUa mean difference | 95% CIa | p-value |
|---|---|---|---|---|---|---|---|
| QALYsc,d (EQ-5D-3L) | 0.56 | 0.54 to 0.58 | 0.56 | 0.54 to 0.58 | 0.01 | –0.01 to 0.02 | 0.553 |
| Health and social care costs (£)e | 4647 | 4294 to 5090 | 3342 | 2819 to 3900 | 1305 | 669 to 2008 | 0.000 |
| Outcomes/costs | BGSI (N = 400)a | GSES (N = 383)b | QALY | |
|---|---|---|---|---|
| DEMQOL-Uc (N = 394) | EQ-5D-3Lc (N = 384) | |||
| Person with dementia, 9 months | ||||
| Health and social care costs (£) | 1300/1.38 = 942 | 1255/0.17 = 7382 | 1160/0.002 = 580,000 | |
| Carer, 9 months | ||||
| Health and social care costs (£) | NA | NA | NA | 1305/0.01 = 130,500 |
| Outcomes/costs | CRa (N = 231) | 95% CIb | TAUa (N = 231) | 95% CIb | CR – TAU mean difference | 95% CIb | p-value |
|---|---|---|---|---|---|---|---|
| BGSIc | 4.56 | 4.37 to 4.76 | 3.209 | 3.03 to 3.39 | 1.35 | 1.10 to 1.62 | 0.000 |
| GSESc | 20.160 | 19.791 to 20.537 | 19.946 | 19.559 to 20.337 | 0.21 | –0.27 to 0.71 | 0.388 |
| QALYc,d (DEMQOL-U) | 0.45 | 0.44 to 0.45 | 0.45 | 0.44 to 0.46 | 0.00 | –0.01 to 0.01 | 0.938 |
| Health and social care costs (£)e | 5426 | 4692 to 6452 | 4329 | 3357 to 5745 | 1097 | –326 to 2258 | 0.102 |
| Societal costs (£)e | 23,359 | 21,404 to 25,659 | 23,343 | 21,407 to 25,468 | –15 | –2545 to 2279 | 0.990 |
| Outcomes/costs | CRa (N = 231) | 95% CIb | TAUa (N = 231) | 95% CIb | CR – TAUa mean difference | 95% CIb | p-value |
|---|---|---|---|---|---|---|---|
| QALYc,d (EQ-5D-3L) | 0.56 | 0.54 to 0.58 | 0.56 | 0.54 to 0.58 | 0.00 | –0.01 to 0.01 | 0.715 |
| Health and social care costs (£)e | 5423 | 4689 to 6435 | 4332 | 3363 to 5771 | 1091 | –337 to 2236 | 0.102 |
| Societal costs (£)e | 23,298 | 21,351 to 25,446 | 23,404 | 21,434 to 25,700 | –106 | –2560 to 2163 | 0.930 |
| Outcomes/costs | BGSIa (N = 462) | GSESb (N = 462) | QALY | |
|---|---|---|---|---|
| DEMQOL-Uc (N = 462) | EQ-5D-3Lc (N = 462) | |||
| Person with dementia, 9 months | ||||
| Health and social care costs (£) | 1097/1.35 = 813 | 1097/0.21 = 5224 | 1097/0.0003 = 3,656,667 | NA |
| Societal costs (£) | –15/1.35 = –11 | –15/0.21 = –71 | –15/0.0003 = –50,000 | NA |
| Carer, 9 months | ||||
| Health and social care costs (£) | NA | NA | NA | 1091/0.003 = 363,667 |
| Societal costs (£) | NA | NA | NA | –106/0.003 = –35,333 |
Appendix 17 Cost-effectiveness acceptability curves and cost-effectiveness planes
FIGURE 15.
Cost-effectiveness acceptability curve: BGSI, person with dementia; replacement costs of unpaid care.
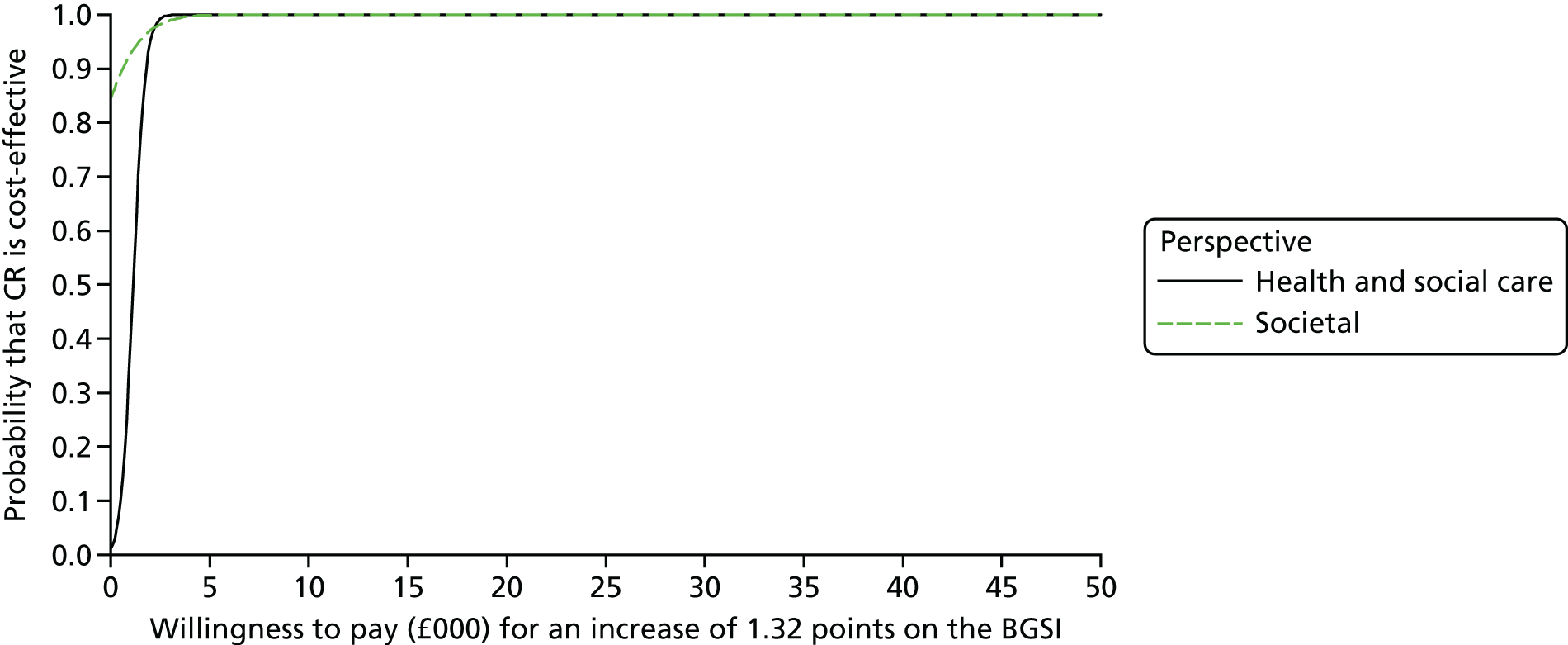
FIGURE 16.
Cost-effectiveness acceptability curve: GSES, person with dementia; replacement costs of unpaid care.
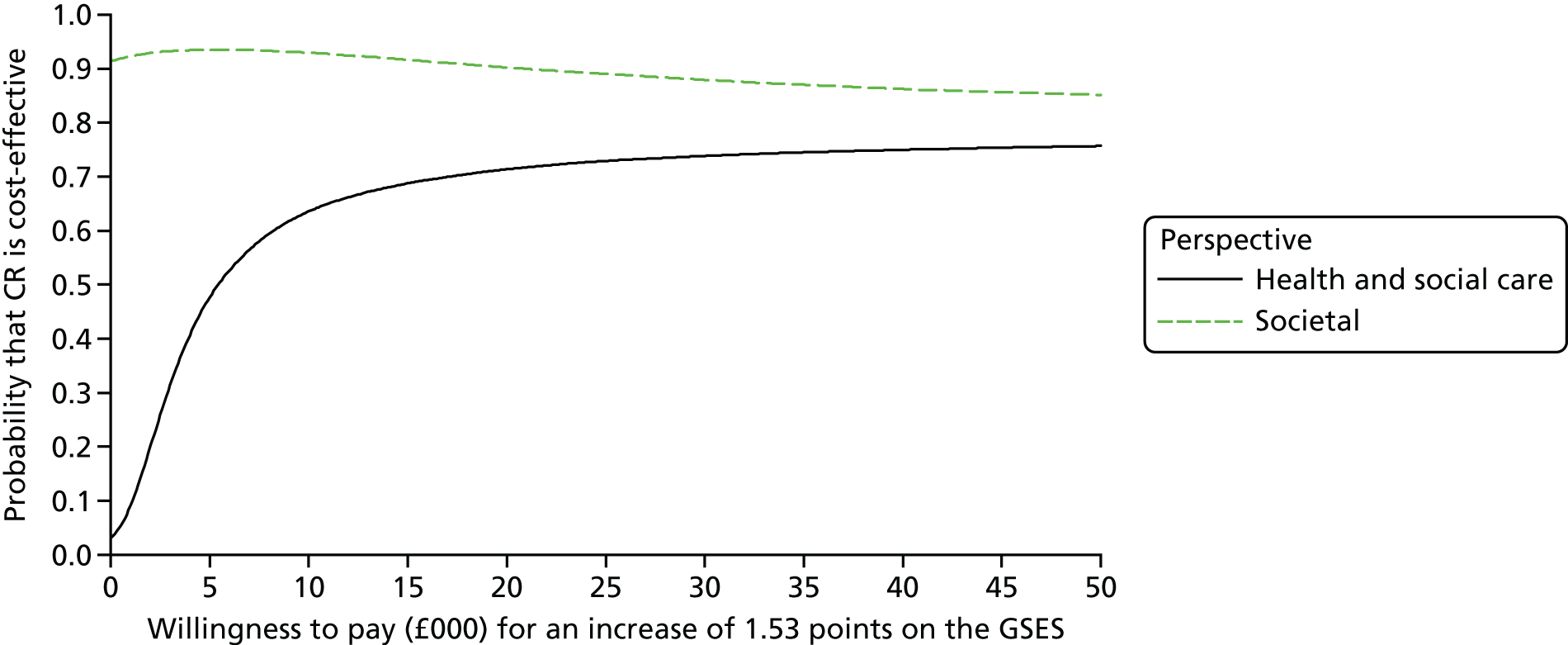
FIGURE 17.
Cost-effectiveness acceptability curve: QALYs (DEMQOL-U), person with dementia; replacement costs of unpaid care.

FIGURE 18.
Cost-effectiveness acceptability curve: QALYs (EQ-5D-3L), carer; replacement costs of unpaid care.

FIGURE 19.
Cost-effectiveness plane: incremental costs and end-point difference in BGSI score at 9 months, person with dementia; replacement costs of unpaid care.
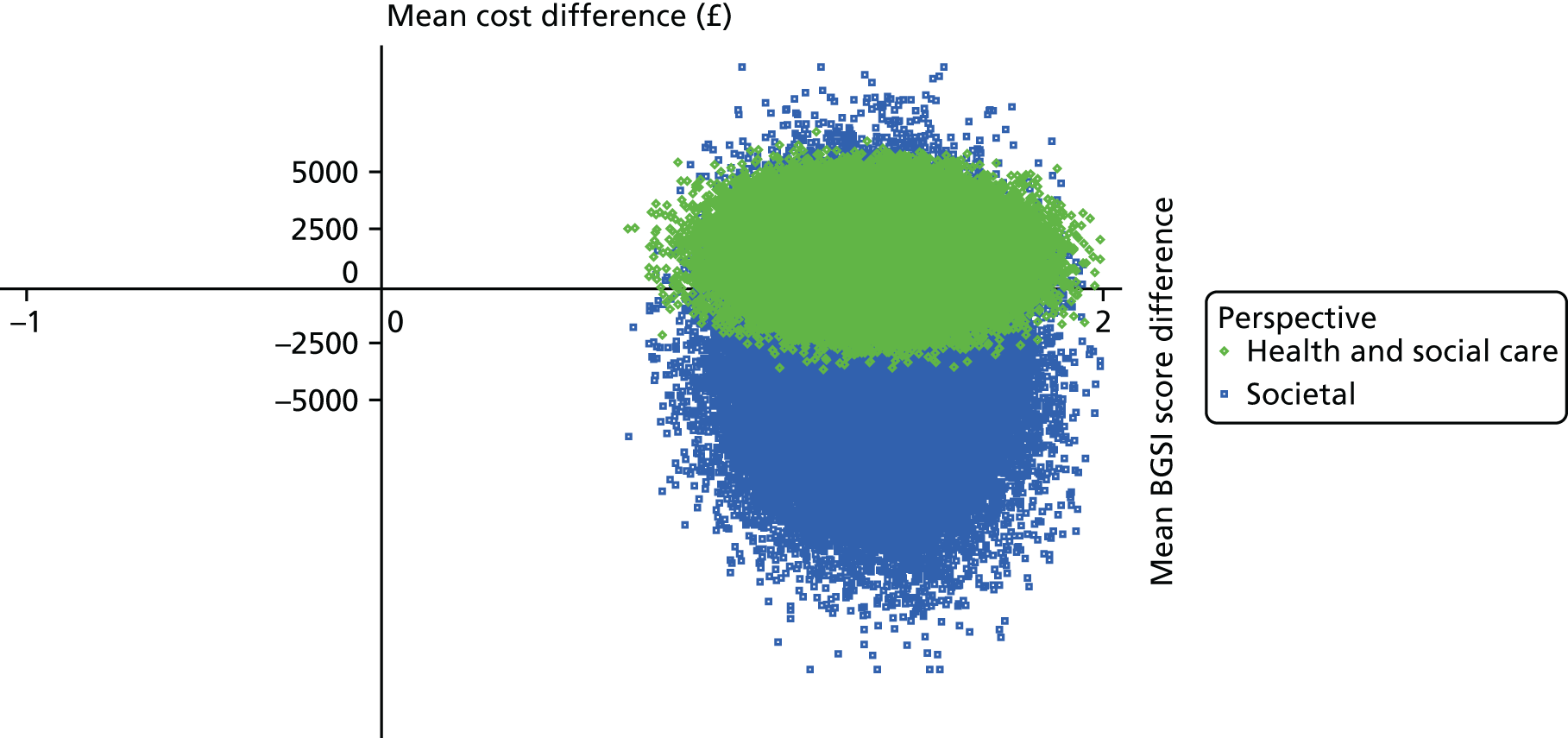
FIGURE 20.
Cost-effectiveness plane: incremental costs and end-point difference in GSES score at 9 months, person with dementia; replacement costs of unpaid care.

FIGURE 21.
Cost-effectiveness plane: incremental costs and QALYs (DEMQOL-U) at 9 months, person with dementia; replacement costs of unpaid care.
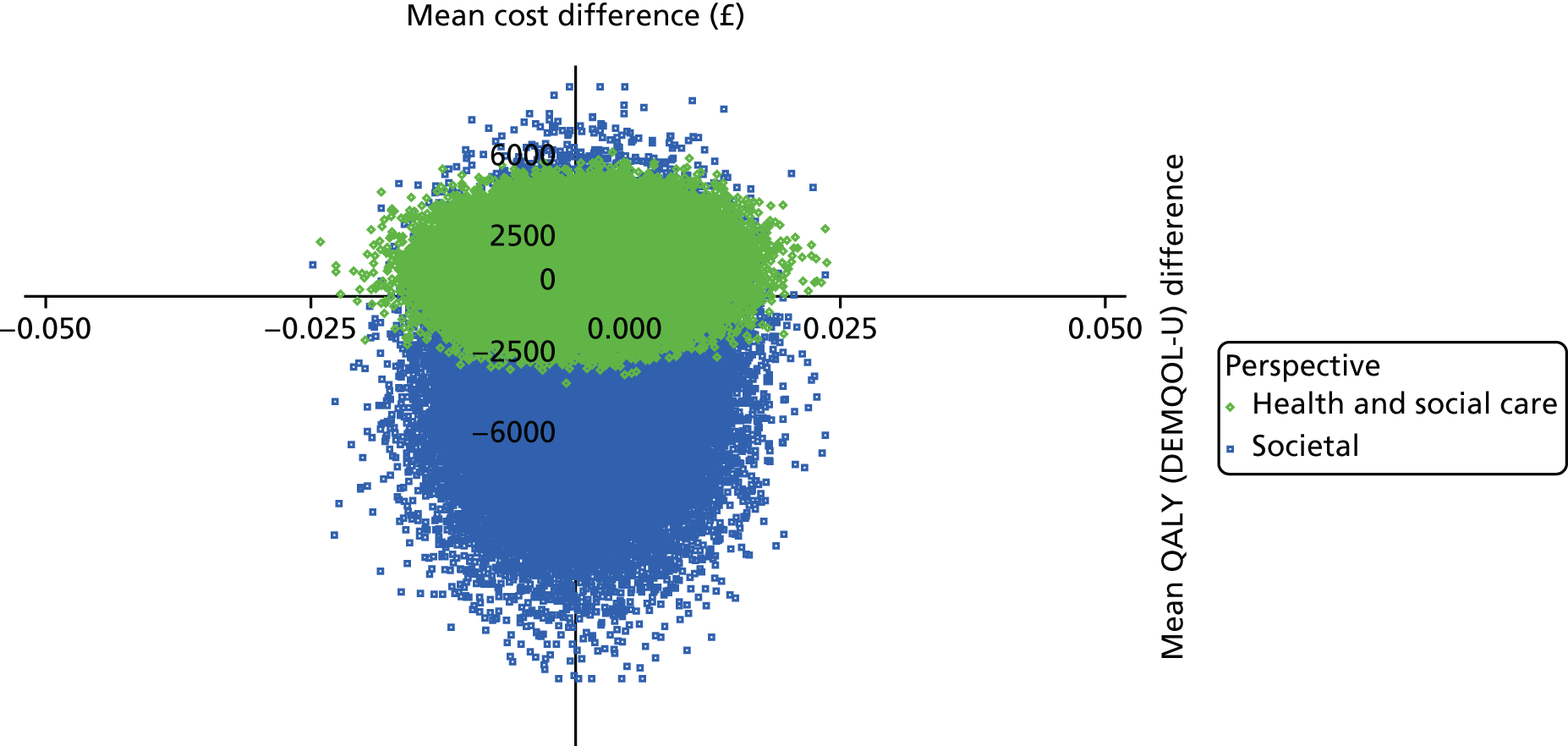
FIGURE 22.
Cost-effectiveness plane: incremental costs and QALYs (EQ-5D-3L) at 9 months, carer; replacement costs of unpaid care.
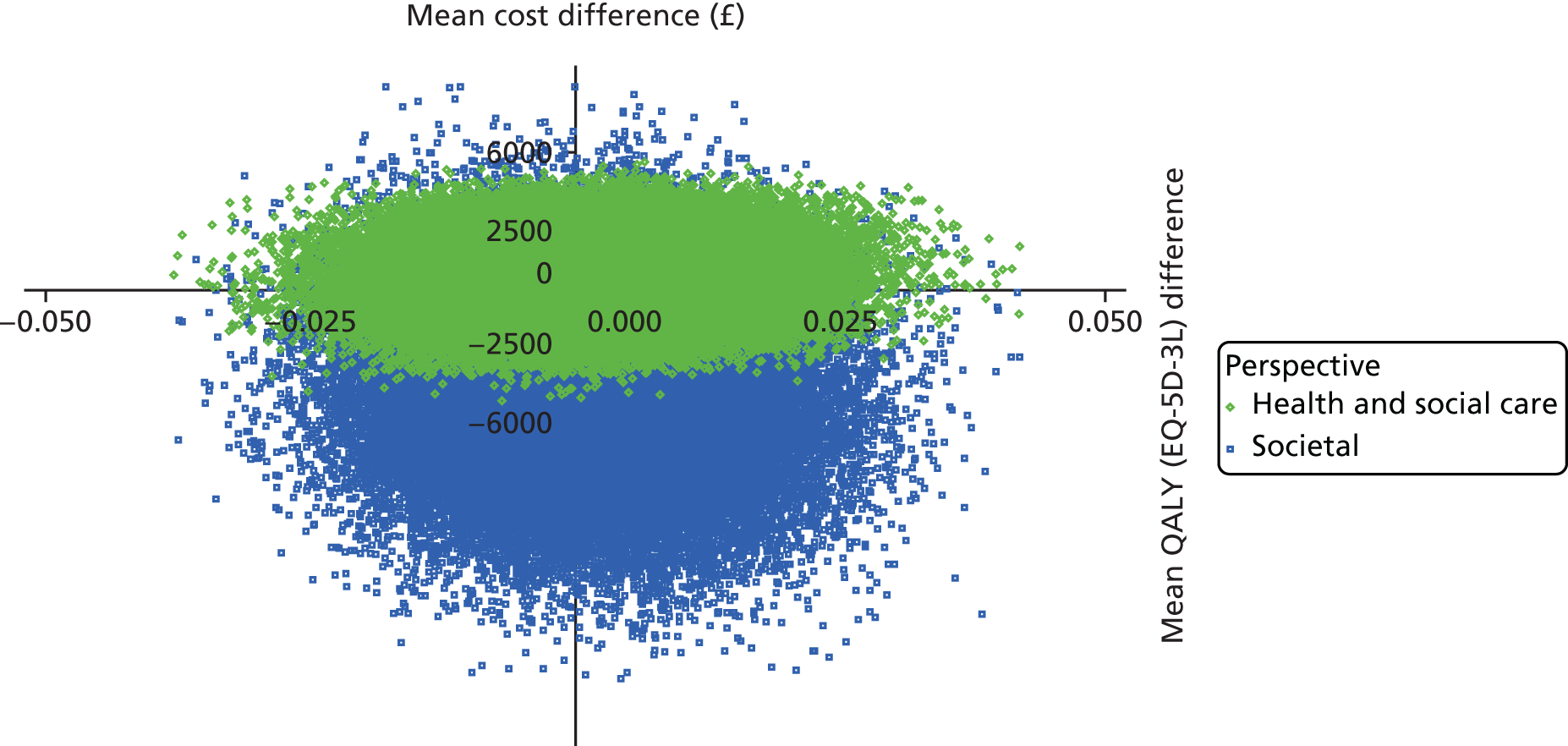
FIGURE 23.
Cost-effectiveness acceptability curve: BGSI, person with dementia; excluding high-cost outliers.

FIGURE 24.
Cost-effectiveness acceptability curve: GSES, person with dementia; excluding high-cost outliers.
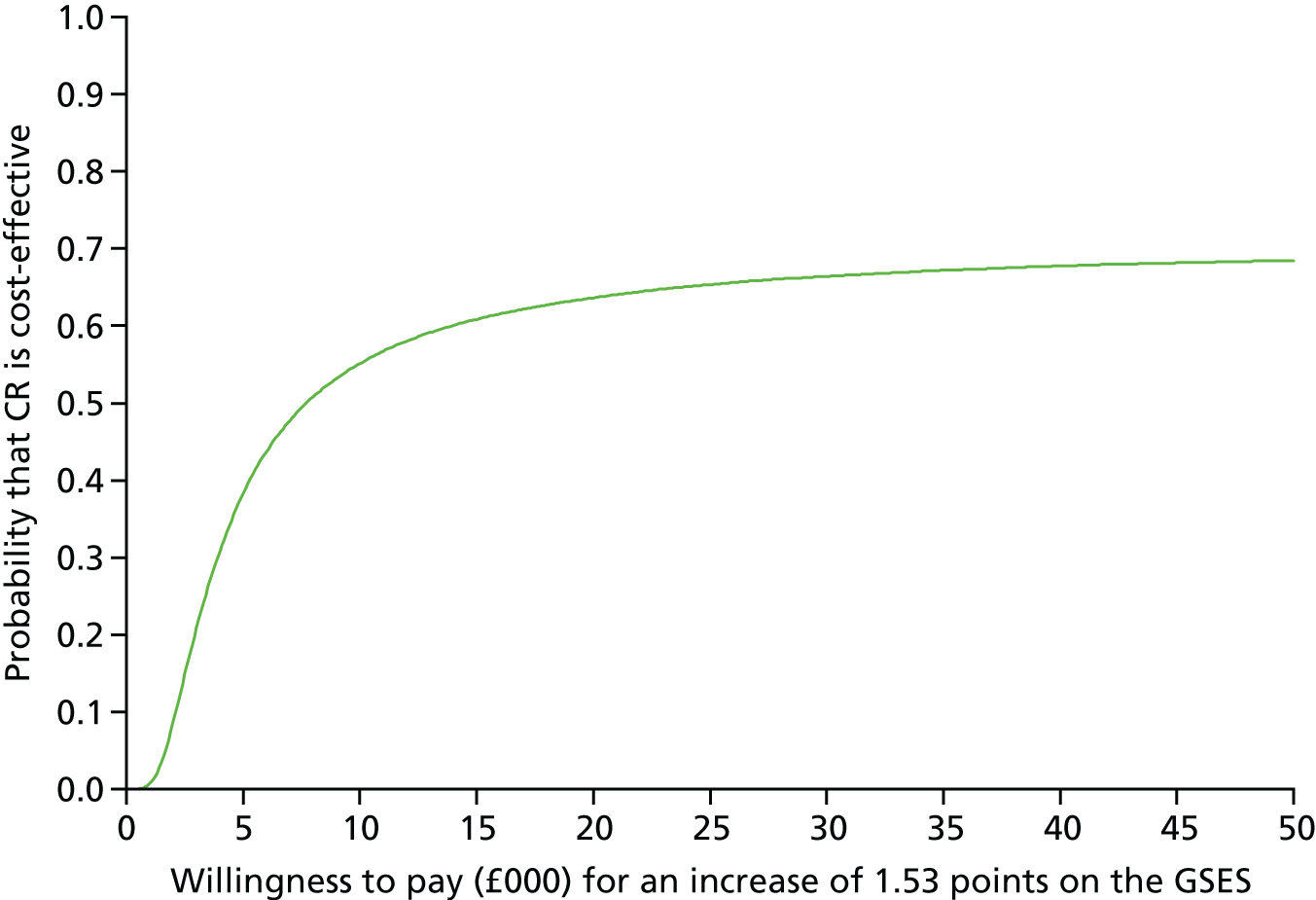
FIGURE 25.
Cost-effectiveness acceptability curve: QALYs (EQ-5D-3L), carer; excluding high-cost outliers.

FIGURE 26.
Cost-effectiveness plane: incremental costs (excluding high-cost outliers) and end-point difference in BGSI score at 9 months, person with dementia.

FIGURE 27.
Cost-effectiveness plane: incremental costs (excluding high-cost outliers) and end-point difference in GSES at 9 months, person with dementia.

FIGURE 28.
Cost-effectiveness plane: incremental costs (excluding high-cost outliers) and QALYs (DEMQOL-U) at 9 months, person with dementia.
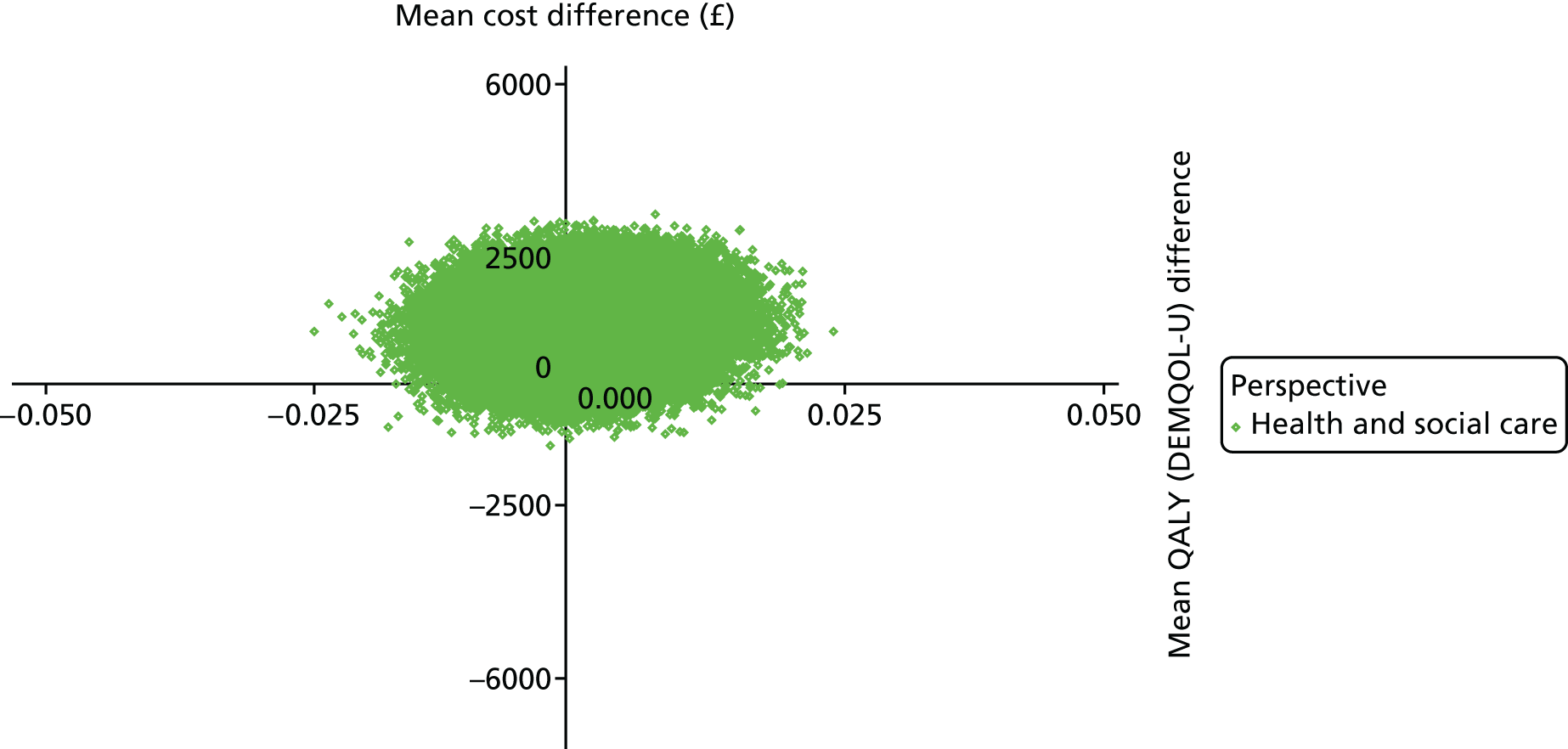
FIGURE 29.
Cost-effectiveness plane: incremental costs (excluding high-cost outliers) and QALYs (EQ-5D-3L) at 9 months, carer.
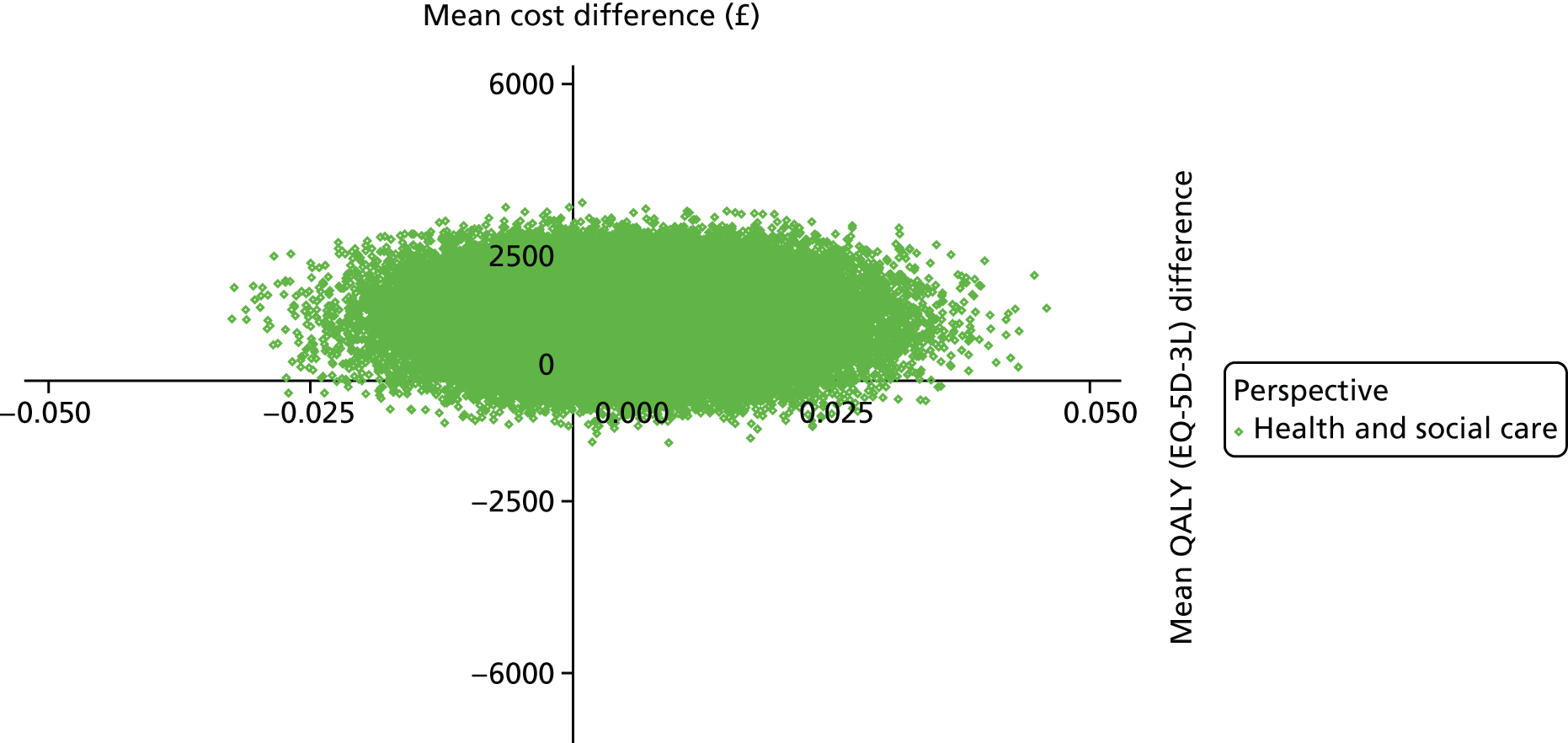
FIGURE 30.
Cost-effectiveness acceptability curve: BGSI, person with dementia; imputed data.

FIGURE 31.
Cost-effectiveness acceptability curve: GSES, person with dementia; imputed data.
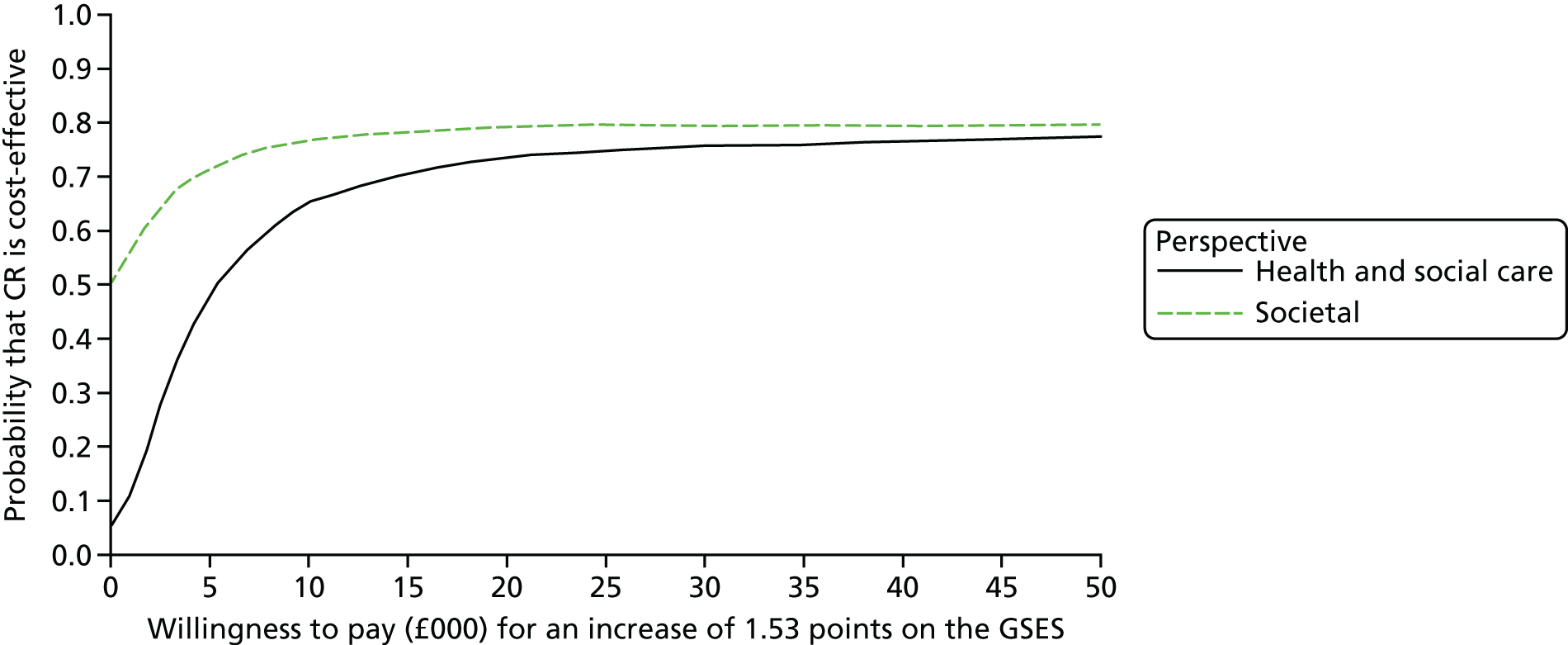
FIGURE 32.
Cost-effectiveness acceptability curve: QALYs (DEMQOL-U), person with dementia; imputed data.
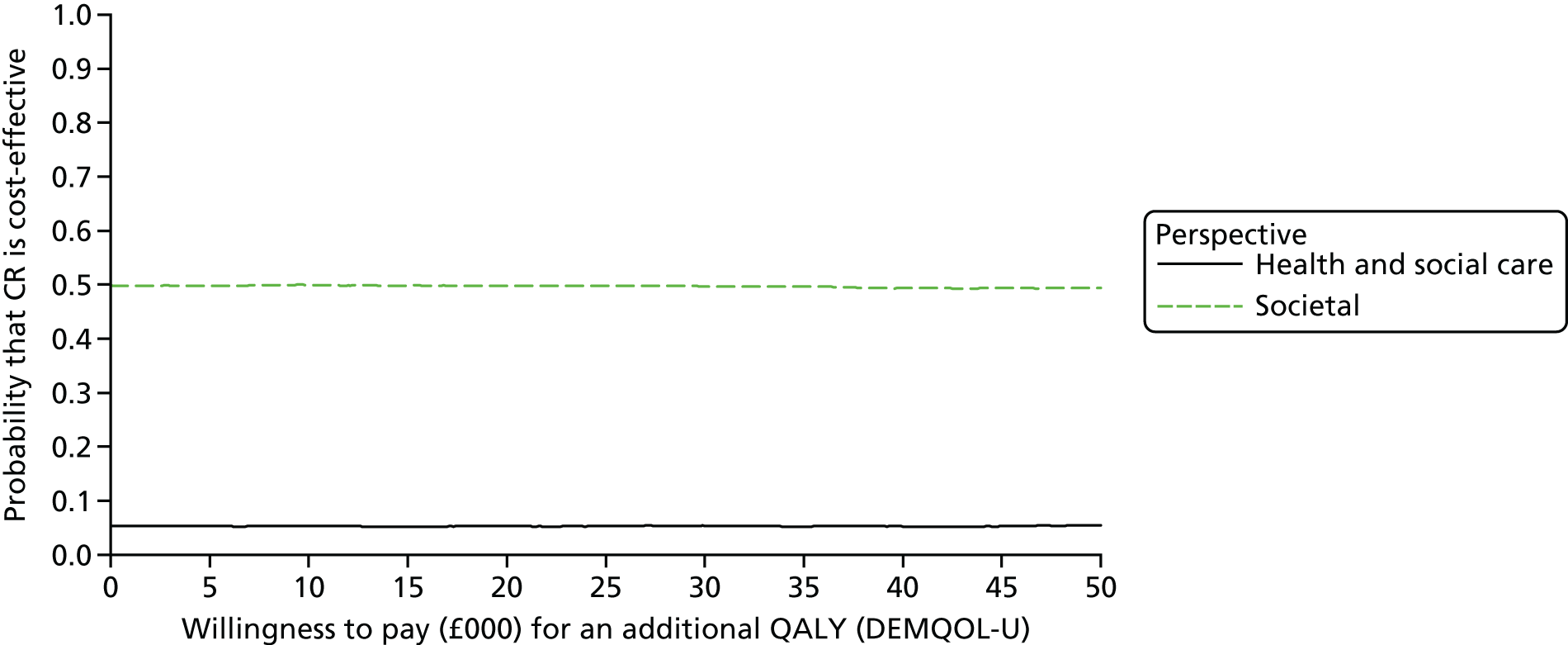
FIGURE 33.
Cost-effectiveness acceptability curve: QALYs (EQ-5D-3L), carer; imputed data.
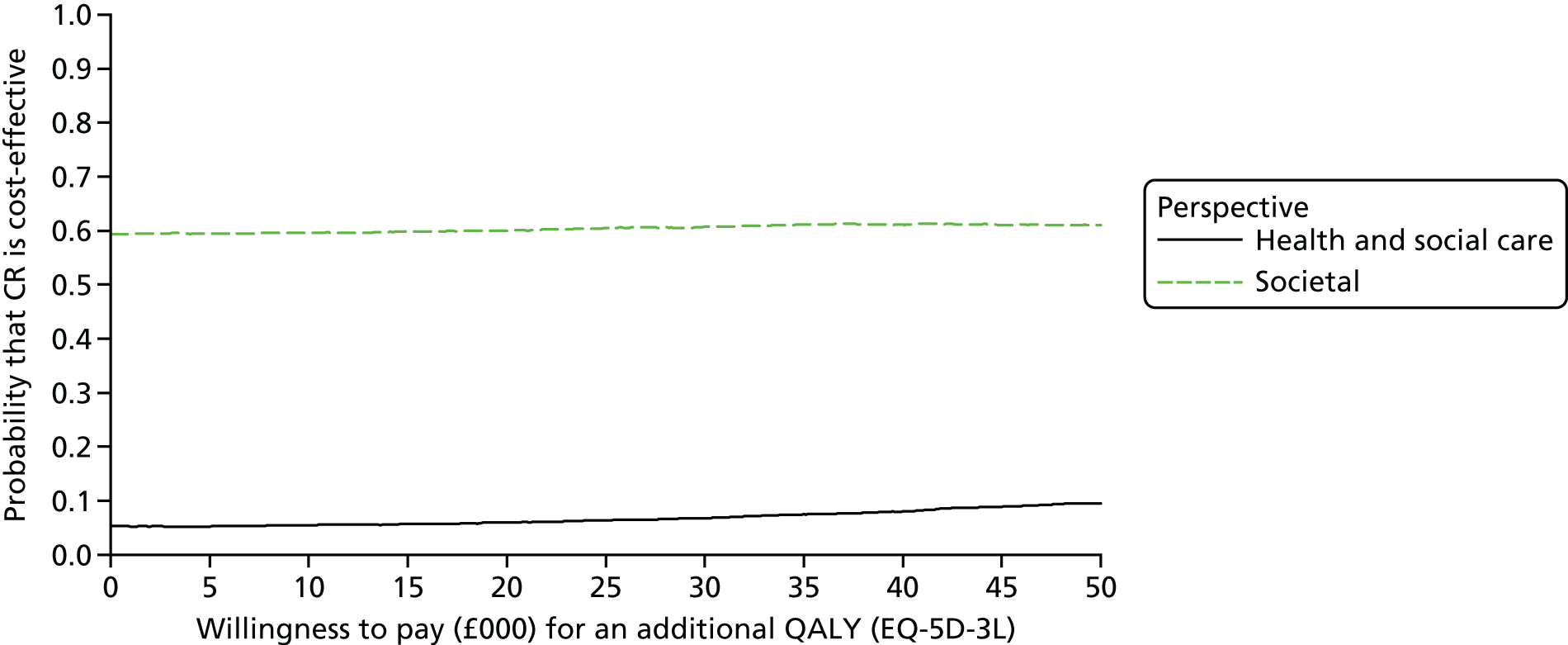
FIGURE 34.
Cost-effectiveness plane: incremental costs and end-point difference in BGSI score at 9 months, person with dementia; imputed data.

FIGURE 35.
Cost-effectiveness plane: incremental costs and end-point difference in GSES score at 9 months, person with dementia; imputed data.

FIGURE 36.
Cost-effectiveness plane: incremental costs and QALYs (DEMQOL-U) at 9 months, person with dementia; imputed data.
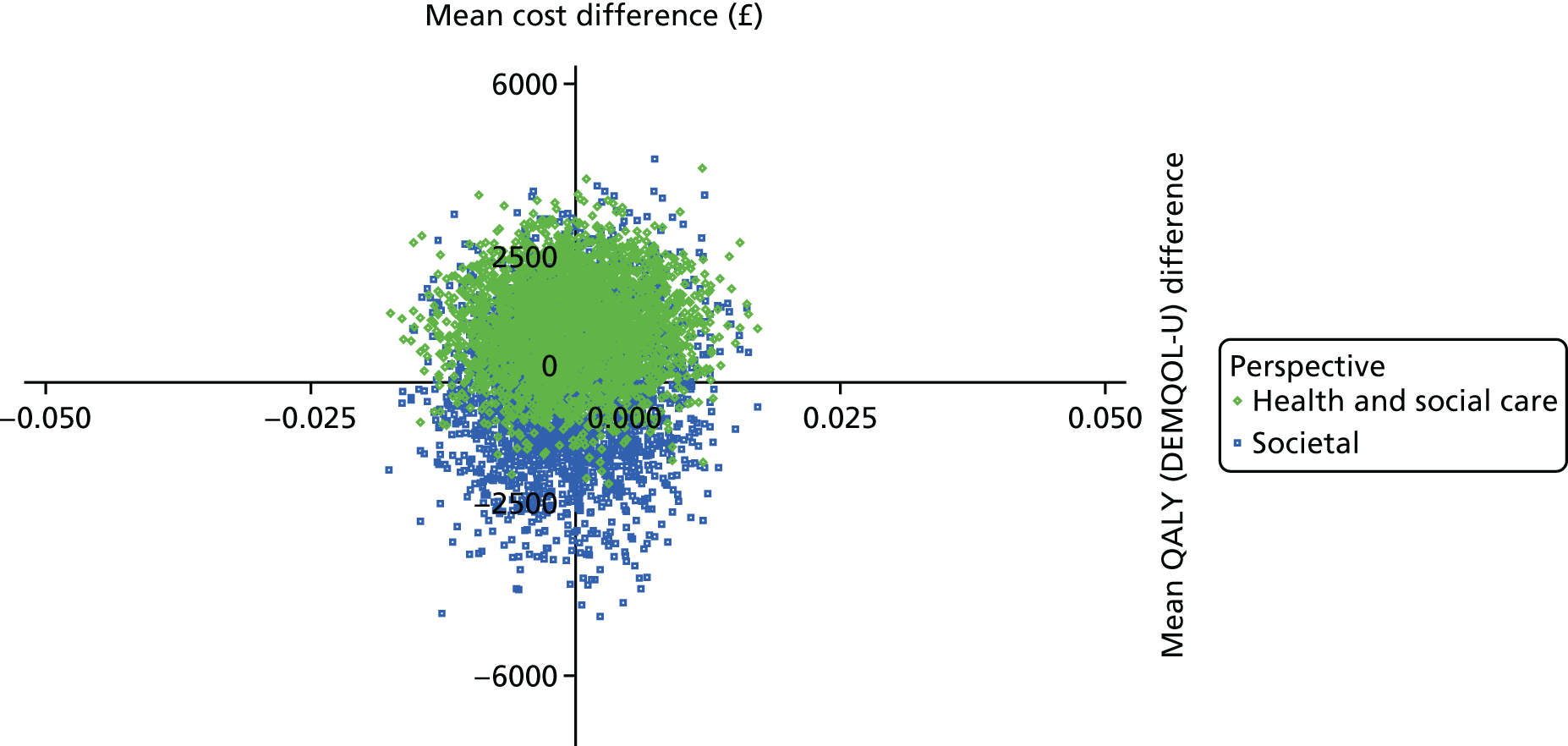
FIGURE 37.
Cost-effectiveness plane: incremental costs and QALYs (EQ-5D-3L) at 9 months, carer; imputed data.
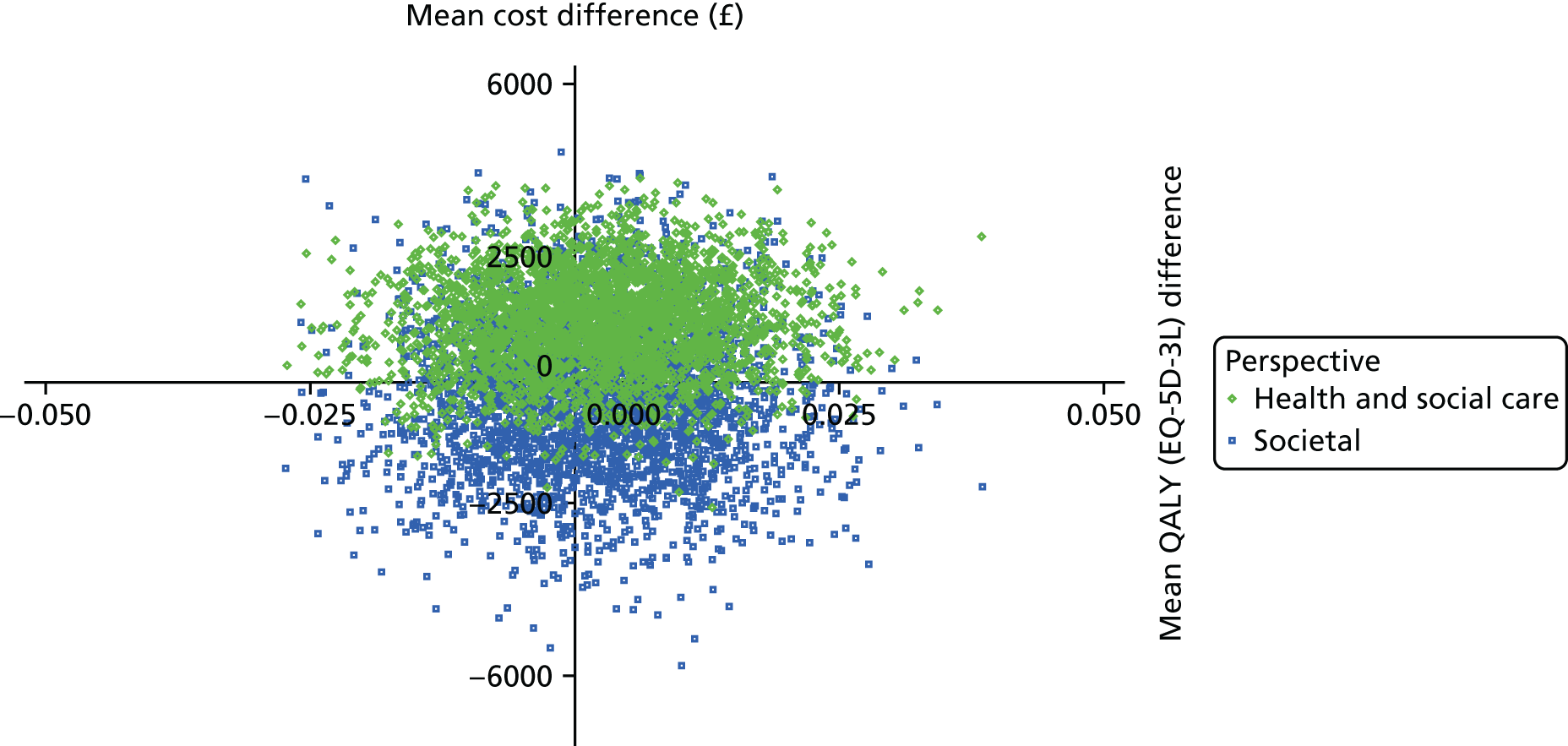
List of abbreviations
- ACE-III
- Addenbrooke’s Cognitive Examination – Third Revision
- AfC
- Agenda for Change
- ANCOVA
- analysis of covariance
- BGSI
- Bangor Goal-Setting Interview
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COPM
- Canadian Occupational Performance Measure
- CPN
- community psychiatric nurse
- CR
- cognitive rehabilitation
- CSRI
- Client Service Receipt Inventory
- CT
- cognitive training
- CTU
- Clinical Trials Unit
- DEMQOL
- DEMentia Quality Of Life
- DEMQOL-U
- DEMentia Quality Of Life – utility score
- df
- degrees of freedom
- D-KEFS
- Delis–Kaplan Executive Function System
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ETNA3
- Évaluation de 3 Thérapies Non médicamenteuses dans la maladie d’Alzheimer
- FTE
- full-time equivalent
- GP
- general practitioner
- GREAT
- Goal-oriented cognitive Rehabilitation in Early-stage Alzheimer’s and related dementias: multicentre single-blind randomised controlled Trial
- GSES
- Generalized Self-Efficacy Scale
- HADS
- Hospital Anxiety and Depression Scale
- HCHS
- Hospital and Community Health Service
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IADL
- instrumental activity of daily living
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- MAGDR
- Ministerial Advisory Group on Dementia Research
- MMSE
- Mini Mental State Examination
- NIHR
- National Institute for Health Research
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- OT
- occupational therapist
- PAL
- Pool Activity Level
- PI
- principal investigator
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RBMT
- Rivermead Behavioural Memory Test
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RICE
- Research Institute for the Care of Older People
- RSS
- Relatives’ Stress Scale
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SES
- socioeconomic status
- SMART
- specific, measurable, achievable, realistic, time-bound
- SMD
- standardised mean difference
- SSD
- social services department
- SUR
- seemingly unrelated regressions
- TAU
- treatment as usual
- TEA
- Test of Everyday Attention
- TI
- technical instructor
- TSC
- Trial Steering Committee
- UN
- United Nations
- VAS
- visual analogue scale
- WHOQOL-BREF
- World Health Organization’s Quality of Life Instrument – brief version
- WTP
- willingness to pay
