Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/88/10. The contractual start date was in January 2015. The draft report began editorial review in January 2018 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Martin C Gulliford is a member of the Health Services and Delivery Research Board. A Toby Prevost is an employee of Imperial College London and is a member of the Public Health Research (PHR) Funding Board. Alastair D Hay is a member of the Health Technology Assessment (HTA) Clinical Trials Board. Paul Little is a member of the HTA Pandemic Influenza Board, the National Institute for Health Research Journals Library Board and the Programme Grants for Applied Research Expression of Interests – HTA Projects remit. Lucy Yardley is a member of the HTA Antimicrobial Resistance Themed Call Board, the HTA Efficient Study Designs Board and the PHR Funding Board. Michael Moore is a member of the government’s Advisory Committee on Antimicrobial Prescribing, Resistance and Healthcare Associated Infection.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gulliford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and rationale
This research was proposed in response to the National Institute for Health Research (NIHR)’s call for proposals on antimicrobial drug resistance (AMR). There is growing concern that the widespread, and sometimes unnecessary, use of antibiotics is leading to the development of AMR. 1,2 In economic terms, the antimicrobial effect of drugs may be viewed as a natural resource. Antimicrobial resistance represents a negative externality of antibiotic use. If resistant organisms persist over time, then the resource of antibiotic drugs will be exhaustible. 3,4 Reducing inappropriate use of antibiotics, as well as ensuring that antibiotics can be used when they are needed, represents an important component of a strategy to control infectious diseases. 5
Governments of all countries need to adopt a stewardship role so as to ensure that effective antimicrobial drugs are available to future generations. 1 This should include responding to the requirement to improve governance and standards of clinical practice with respect to antimicrobial drug utilisation. The present research addresses a subject of great public health importance because overutilisation of antibiotics contributes to the emergence of AMR and, consequently, infections that may be very difficult to treat. The review on antimicrobial resistance,2 chaired by Jim O’Neill, identified education and training to reduce inappropriate and unnecessary antibiotic use as key measures to fight AMR. This research specifically aims to address the problem of inappropriate and unnecessary prescribing of antibiotics to patients with self-limiting respiratory tract infections (RTIs) in primary care.
Self-limiting respiratory tract infections in primary care
Self-limiting RTIs, including colds, sore throats, cough, bronchitis, rhinosinusitis and otitis media, represent common reasons for consultation with a general practitioner (GP). 6 Each year there are about 200 consultations for RTIs per 1000 registered patients in primary care. 7 Antibiotics are prescribed at about 50% of all RTI consultations,8 with RTIs commonly stated to account for about 60% of all antibiotics prescribed in primary care. There are substantial age-related differences in consultations for RTIs, with children < 5 years of age having extremely high consultation rates. 9 However, on a per-consultation basis, antibiotic prescribing at RTI consultations generally increases with age. 9,10
Most respiratory infections are self-limiting without specific treatment. 11 Antibiotic treatment generally offers minimal benefit in terms of duration and severity of symptoms, but may be associated with side effects such as diarrhoea or rashes. 12,13 Patients prescribed antibiotics are more likely to believe that this is an effective treatment and are more likely to consult in future. 11 The small minority of individuals who may benefit from antibiotics can be positively identified through indicators of severity of illness or comorbidity. 6 Patterns of microbial colonisation begin to change soon after antibiotics are started, leading to the emergence of drug-resistant organisms. 14
Databases of primary care electronic health records (EHRs), such as the Clinical Practice Research Datalink (CPRD), provide an important resource for understanding the epidemiology and public health impact of respiratory infections and antibiotic prescribing in primary care. Previous research using the CPRD showed that there has been a long-term decline in consultation for RTIs. 7 During the 1990s, following the publication of the Standing Medical Advisory Committee’s report, The Path of Least Resistance,15 there was some reduction in the proportion of consultations at which antibiotics were prescribed, but there was little change in antibiotic prescribing for RTIs after 2000. 7
Recent CPRD analyses for 2012 from the eCRT (a cluster randomised trial using electronic records)8,16 showed that antibiotics are prescribed for about one-third of consultations with patients presenting with common colds, more than half of consultations with patients presenting with sore throat or otitis media and about 90% of consultations with patients presenting with sinusitis. In the context of treatment recommendations that advise that most acute respiratory infections can be managed without antibiotics, these data clearly indicate an opportunity to make a major impact on unnecessary antibiotic prescribing.
There are striking variations between general practices in the rates of consultation and antibiotic prescribing for RTIs. 8 The rate of antibiotic prescribing per 1000 registered patients is always less than the consultation rate for RTIs; this is consistent with an overall prescribing proportion of between 50% and 60%. In the CPRD, in 2012, < 1% of general practices prescribed antibiotics at < 20% of RTI consultations, other general practices prescribed antibiotics at > 80% of RTI consultations, with 89% of general practices prescribing antibiotics at > 40% of RTI consultations. Most general practices are unaware of their pattern of antibiotic prescribing for particular indications, and its standing in relation to their peers, with only aggregated data for all antibiotic prescriptions dispensed being generally available for performance management. 8 Some antibiotic-prescribing indicators based on aggregated data for prescriptions dispensed for general practices in England can be viewed through the OpenPrescribing website (https://openprescribing.net; accessed October 2018).
Linder17 observed that nearly all general practices are currently prescribing antibiotics at rates that are ‘way off the mark’ in the context of good practice recommendations, which advise that most RTIs can be managed without the prescription of antibiotics. 6 Based on this guidance, most practices might optimally be prescribing antibiotics at considerably fewer RTI consultations. For example, in the Netherlands, antibiotics are prescribed at a little over 20% of RTI consultations. 18 These CPRD data suggest that considerable reductions in antibiotic utilisation for RTIs are necessary in UK primary care. The then prime minister, David Cameron, pledged to half inappropriate antibiotic prescribing by 2020. This raises a question concerning how reductions in antibiotic prescribing can be achieved.
Evidence from previous trials and systematic reviews
Strategies to reduce unnecessary antibiotic prescribing have been tested in a number of previously published randomised controlled trials (RCTs). Ranji et al. 19,20 performed a systematic review up to 2007. In 30 trials contributing to a quantitative analysis, Ranji et al. found a median reduction of 9.7% [interquartile range (IQR) 6.6–13.7%] in the proportion of participants receiving antibiotics. Most studies employed educational activities aimed at clinicians or patients, an audit of antibiotic prescribing with feedback of results or a combination of these interventions. More recent trials have demonstrated similar reductions in antibiotic utilisation (Table 1), with a reduction in antibiotic prescribing of up to 15%, as reported by Little et al. 21 These recent trials have used similar intervention strategies, but have more frequently used electronic media to deliver advice on appropriate prescribing. 21,26
| Trial (authors and year of publication) | Setting | Intervention | Effect |
|---|---|---|---|
| Little et al. (2013)21 | EU |
Training in communication skills CRP level testing |
9–15% reduction in antibiotic prescriptions |
| Gerber et al. (2013)22 | USA | Education, audit and feedback | 6.7% net reduction in antibiotic prescribing |
| Gonzales et al. (2013)23 | USA |
Education, audit and feedback Electronic decision support |
≈12% net reduction in antibiotic prescribing |
| Gjelstad et al. (2013)24 | Norway | Education, audit and feedback | 1.3% reduction in antibiotic prescribing |
| Butler et al. (2012)25 | UK | Education, audit and feedback | 4.2% net reduction |
Some authors recommend a delayed antibiotic-prescribing strategy, in which a prescription is issued but used only if symptoms fail to improve. This approach is sometimes recommended as a method for reducing antibiotic utilisation in the management of RTIs. 27
Systematic reviews28,29 of the wider implementation science literature are also informative in identifying features of audit and feedback or decision support that are associated with greater intervention effects. Ivers et al. 28 found that feedback was more effective when performance is suboptimal, when feedback is given in written and verbal formats, and when explicit targets and actions are recommended. Roshanov et al. 29 found that clinical decision support systems were more likely to be effective when these required active measures before they could be over-ridden or if patient information was provided in addition to clinician information.
The eCRT
The systematic review and recent trials are important in identifying strategies that may be effective at changing prescribers’ behaviour. However, previous trials required resource-intensive interventions and these intervention techniques have not yet been translated on a wide and sustainable scale into the NHS. For example, the trial by Gonzales et al. 23 required clinicians to participate in a half-day training session, triage nurses to provide patients with education leaflets to read before their consultation, a specially designed structured template to be programmed into the practice system to provide an algorithm-based probability of the patient having pneumonia, and ‘order sets’ to be created for group diagnosis and treatment options for different types of RTIs. The challenge now is to take the components of intervention that have been shown to be effective and to find methods to deploy these efficiently into routine practice settings.
The methods of deploying the components of the intervention into routine practice settings have been investigated in the recently completed eCRT, which involved cluster randomisation of general practices that contribute EHRs to a national primary care database, the CPRD. 16 The study included 104 general practices in England and Scotland. Decision support tools (DSTs) were delivered remotely to general practices. The effectiveness of the intervention was evaluated by analysing EHRs that are routinely collected into the database. Data were analysed for > 600,000 individual participants, with a financial cost of about 27 pence per participant. Even with a very simple intervention, the trial showed a near 2% reduction in antibiotic prescribing. This eCRT showed that it was feasible to use the CPRD to evaluate interventions that may be readily scaled up to the population level. Feedback received in the eCRT process evaluation,30 together with evidence from other trials cited above, identifies ways to increase engagement in the intervention and increase effect sizes.
Safety outcomes
Material in this section is reproduced from Gulliford et al. 31 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
The present research also addresses the safety of reducing antibiotic prescribing in primary care. There may be well-grounded clinical concern that reducing antibiotic use might increase the risk of complications following RTIs. Clinical trial evidence suggests that antibiotics may reduce the risk of suppurative complications of RTIs,13 but the more serious complications are generally too rare to evaluate precisely in randomised studies. Petersen et al. 32 conducted a cohort study in 162 general practices in the General Practice Research Database (the predecessor of the CPRD) from 1991 to 2001 to evaluate the effect of antibiotic treatment on the incidence of pneumonia after ‘chest infection’, peritonsillar abscess after sore throat, and mastoiditis after otitis media. The results suggested that antibiotic treatment was associated with lower odds of each these complications, but the overall risk of complications was generally small and the number of patients who would have to be treated to avoid one complication was estimated to be in excess of 4000. However, pneumonia following chest infection was more frequent. In people aged < 65 years, it was estimated that there might be one case for every 100 antibiotic prescriptions for chest infection avoided, and one case for every 39 prescriptions avoided in people aged > 65 years. Infections of the middle ear or sinuses may rarely be complicated by intracranial abscess. 33 Lemierre syndrome,34 from thrombophlebitis of the internal jugular vein associated with Fusobacterium necrophorum infection, is a rare complication of sore throat,34 but F. necrophorum infections may be frequently detectable in patients with symptoms of sore throat. 35,36 The annual number of cases of Lemierre syndrome in England was reported to have increased from 19 in 1997 to 34 in 1999, prompting a reminder from the Chief Medical Officer that some sore throat symptoms may require antibiotic treatment. 37 In addition to concerns about complications, medical practitioners may be concerned about the potential consequences of diagnostic misclassification. The initial symptoms of meningitis may sometimes resemble an influenza-like illness. 38 Awareness of the possibility of a more serious diagnosis might prompt GPs to issue an antibiotic prescription for conditions in which this is not usually indicated.
These observations raise important questions for a policy to reduce antibiotic prescribing for RTIs in primary care: is there a safe level of antibiotic prescribing for RTIs? What target can general practices safely adopt in reducing the proportion of RTI consultations with antibiotics prescribed? Is there a threshold for antibiotic prescribing below which complications may increase? An additional aim was added to the present research to evaluate the safety of a policy to reduce antibiotic prescribing for RTIs in primary care. The incidence of pneumonia, peritonsillar abscess, mastoiditis, empyema, meningitis, intracranial abscess and Lemierre syndrome was evaluated. The study aimed to determine whether or not these complications were more frequent at general practices that prescribe fewer antibiotics for self-limiting RTIs than at higher-prescribing practices. The use of this information was aimed to quantify the potential clinical and public health impact of changes in antibiotic-prescribing practices.
Evidence explaining why this research is needed now
The recent systematic review, together with the additional, more recent, trials, show that interventions to modify prescribing behaviour in primary care can be effective. However, there is a block in the translational pathway because it has not been possible to roll-out this evidence into routine practice; antibiotic prescribing for RTIs remains high outside trial settings. There is a lack of effective interventions that can easily be translated, in a sustained way, into routine practice settings. This research aimed to use the strengths of EHRs to inform, deliver and evaluate an intervention. This will be achieved with a high degree of efficiency by employing at the research environment a database of EHRs accessed through the CPRD.
This research is at a later stage of translation than previous trials. In order to overcome the block in the translational pathway, the study aimed to develop and evaluate more effective complex multicomponent interventions that can be implemented and delivered remotely. Development of the interventions was informed by evidence from recent trials, as well the process evaluation of the eCRT study. 30 The research focused on deployment of interventions with potential to be readily scaled up, through remote delivery using electronic media, to large samples of unselected practices. The present research built on previous experience of implementing the eCRT within the CPRD. In the eCRT, the intervention was an educational and DST39 that aimed to support evidence-based antibiotic prescribing for respiratory illness in primary care. The intervention was installed remotely at practices and utilisation of the intervention was monitored.
The approach of utilising the EHRs of the CPRD to provide the environment for delivering and testing the interventions has several advantages:
-
both the interventions and a cluster randomised trial of the interventions can be implemented at a very low cost
-
the sample available for the study is nationally representative for the UK and large sample sizes are expected
-
the sustainability of the effect of the intervention may be evaluated after the end of the trial, because data continue to be collected from trial practices
-
utilisation of the intervention can be routinely monitored through electronic information routinely collected into EHRs
-
a cost-effectiveness analysis may be implemented using data on health-care utilisation, which are collected for all patients in CPRD40
-
translation of the trial results is readily feasible because the interventions are delivered using the practice systems that are employed in delivering routine care within the NHS.
Aim and objectives
Aim
This main aim of this research was to test the effectiveness, in a cluster RCT, of electronically delivered, multicomponent interventions to reduce unnecessary antibiotic prescribing when patients consult for RTIs in primary care.
Objectives
Specific objectives were to:
-
Develop, refine and implement complex multicomponent interventions, but low-cost interventions, to influence GPs’ prescribing of antibiotics when patients consult with RTIs. The intervention comprised:
-
feedback of monthly updated antibiotic-prescribing information from the CPRD as a major novel component
-
educational and DSTs, which include a summary of antibiotic-prescribing recommendations, a summary of research evidence concerning no antibiotic-prescribing strategies, information on the definite indications for antibiotic prescription, information and evidence on the risks from non-prescribing and patient information
-
brief web-based training (a webinar) to introduce, provide training on and promote effective utilisation of the intervention materials.
-
-
Conduct a parallel-group cluster RCT, with general practices as the unit of allocation, over 12 months to estimate the difference between intervention and control practices in primary outcome (i.e. antibiotic prescription rate per 1000 patients) and secondary outcomes (i.e. proportion of RTI consultations with antibiotics prescribed, RTI consultations, subgroups of age, gender, comorbidity and infection type and intervention utilisation, safety outcomes and costs of health-care utilisation), in an intention-to-treat analysis.
-
Conduct a population-based cohort study in general practices not exposed to trial interventions to determine whether or not the incidence of pneumonia, peritonsillar abscess, mastoiditis, empyema, meningitis, intracranial abscess or Lemierre syndrome is higher at general practices that prescribe fewer antibiotics for self-limiting RTIs.
Chapter 2 Methods
Intervention development and delivery
Theoretical framework
The research for intervention development drew on the framework that was used previously in the eCRT. 16,39 McDermott et al. 39 identified theoretical components that relate directly to effective implementation in health-care settings, identifying aspects of social cognitive theory41 and self-determination theory42 as possible influences on GP prescribing behaviour. The feedback received from the process evaluation for the eCRT is also incorporated. 30
Social cognitive theory proposes that the environment plays a key role in influencing an individual’s behaviour. 41 An individual’s belief in their ability to exercise control over their environment is one of the most important mechanisms involved in successful behaviour change. If an individual perceives their environment to be controllable and supportive, they will be more likely to succeed in performing the desired behaviour. 41 In the present research, this suggests that interventions that are embedded into the consultation environment and become active during the flow of care are more likely to be successful. Social cognitive theory also proposes that the strength of an individual’s belief in his/her own ability to reach goals (i.e. their self-efficacy) functions as a key determinant of motivation for a specific behaviour. GPs’ self-efficacy has also been implicated as a predictor of intended adherence to recommendations for prescribing. 43,44 Social cognitive theory also suggests that anticipated outcomes or ‘outcome expectancies’ of a behaviour influence the likelihood that it will be performed. Outcome expectancies relevant to prescribing decisions might include anticipated patient pressure45 or beliefs about risks and benefits associated with characteristics of a disease. 46
Qualitative interviews in the previous study39 identified views that were consistent with self-determination theory. 42 The theory proposes that behaviour change will occur and persist if it is autonomously motivated, in contrast to behaviour change that is brought about by perceived enforcement. GPs reported, for example, that they would be unlikely to engage with an intervention that they were forced to view or that they considered was attempting to control their behaviour, but in contrast they would be more inclined to engage with an intervention that they considered was there to support and aid them.
This study’s approach to developing the intervention aimed to create a controllable and supportive environment, increase self-efficacy, provide a meaningful rationale for engagement in the intervention, and promote expectations of positive outcomes, while reducing perceived negative risks, in order to support better GP adherence to prescribing recommendations. 39
The description of the intervention was also guided by the TIDieR (Template for Intervention Description and Replication) checklist. 47 This ensured that the intervention was sufficiently described to enable replication, future development and implementation into practice.
Design of intervention development study
A qualitative semistructured interview study was conducted to obtain empirical feedback on prototype interventions and to provide contextual information. Follow-up interviews were conducted with selected participants to further refine the study tools. Interviews were conducted by three experienced researchers, who met on a regular basis during data collection phase to compare and discuss their field notes.
Participant selection
Participants were GPs and nurse prescribers (NPs) employed by the NHS and working in non-trial general practices across England (London, Oxfordshire and Yorkshire). The minimum sample of 30 participants was set, aiming for saturation. GPs and NPs included a convenience sample and were recruited via clinical research networks, snowballing, mail-outs and social media [i.e. the young GP Facebook (Facebook, Inc., Menlo Park, CA, USA) community]. Those GPs and NPs interested in taking part were sent the participant information sheet and consent form. Participants were reimbursed for their time.
Procedure
Each interview followed an interview guide, which contained a list of questions and prompts. A semistructured interview was designed to identify factors likely to influence successful implementation of the intervention tools and discover likely responses to the proposed messages, in order to further inform development and aid refinement of the messages. During the interview participants were encouraged to interact with the study tools using a tablet as they would during their routine clinical practice. The initial section of the interview explored participants’ views on the webinar. Then the discussion moved onto the demonstration of the DSTs, followed by the discussion of practice-prescribing feedback. Field notes were made after each interview summarising the main points raised. All interviews were digitally recorded and then transcribed using professional services. Data collection and tools refinement were iterative processes and data collection continued until saturation was achieved. The study was progressively recruiting and interviewing GPs and introducing changes based on their comments until the last interviewed GPs considered no improvements could be made.
Analysis
The full analysis of transcripts was performed by two coders. A thematic analysis was conducted. 48 An inductive approach to data analysis was used and themes were derived from the data. NVivo software (version 11; QSR International, Warrington, UK) was used to facilitate data analysis. Participants did not provide feedback on the findings; transcripts were not returned to participants for comments or corrections.
Patient and public involvement
We engaged with a primary care patient participation group (in Lewisham, south London). The trial procedure and the proposed intervention were presented to the group, and feedback and views were obtained on all aspects of the intervention, including the way in which messages would appear on GP screens, and information that would be presented to patients (such as patient information sheets). A member of this patient participation group continued in an advisory role throughout the intervention development, commenting on the intervention materials and giving feedback on the clarity, style and wording.
Cluster randomised trial
The trial was conducted in general practices contributing to the UK’s CPRD. The CPRD is one of the world’s largest databases of primary care EHRs; it includes data from general practices throughout the UK. CPRD data have been extensively evaluated and employed for epidemiological research. 49 Recently, the CPRD has begun to be used as a resource for interventional research. 16,50–52
Research ethics
The protocol for the study was approved by the NHS Health Research Authority National Research Ethics Service (NRES) Committee London–Dulwich (reference number 14/LO/1730). The protocol was also reviewed and approved by the CPRD Independent Scientific Advisory Committee (ISAC; reference number 14_130). An Independent Trial Steering Committee, and a Data Monitoring Committee, had oversight of the trial. The responsible partner at each practice gave written informed consent for the participation of the practice in the trial. The intervention was implemented at the general practice level and there was no requirement for individual patient consent because all individual treatment decisions were at the discretion of GPs and NPs. The study protocol has been reported previously,53 and the updated protocol can be accessed from the NIHR Health Technology Assessment (HTA) programme website (https://njl-admin.nihr.ac.uk/document/download/2010718; accessed on 1 November 2018).
Target population
The target population for this trial was the general population registered with general practices in the UK, including England, Scotland, Wales and Northern Ireland. The immediate participants in the research were health professionals who may issue prescriptions for antibiotics at UK general practices. Outcomes were evaluated using the anonymised EHRs for individual patients registered with UK general practices who presented with RTIs and were eligible to be prescribed antibiotics.
Inclusion/exclusion criteria
General practices were included in the trial if they were contributing up-to-standard data to the CPRD and consented to participation in the trial, and research governance approval for the study was in place. Data for individual participants were included if they were currently registered with the CPRD general practices. There were no exclusion criteria.
Recruitment and allocation
All practices contributing to the CPRD were invited to participate in the study from September 2015. Cluster randomisation was employed because intervention was at cluster level. Allocation was performed at King’s College London by Martin Gulliford and Toby Prevost, and CPRD staff responsible for recruiting practices to the trial, and communicating allocations (JS and KS), had no access to the allocation procedure. Practices were allocated to intervention and control trial arms using minimisation according to the MINIM program (www-users.york.ac.uk/∼mb55/guide/minim.htm; accessed October 2018),54 stratifying for CPRD region/country and pre-trial antibiotic-prescribing quartile. Practices were allocated in six waves: November 2015 (19 practices), January 2016 (12 practices), February 2016 (19 practices), June 2016 (18 practices), July 2016 (8 practices), and August 2016 (4 practices). For analysis, the six waves were combined into three periods: period 1, practices randomised in November 2015; period 2, January and February 2016; and period 3, June to August 2016. One practice allocated to the intervention trial arm withdrew from the CPRD before the intervention started and was excluded from further analysis because no data were available. The trial was stopped when the last general practices completed 12 months of follow-up.
Sample size calculations
Key measures included the consultation rate for RTIs, the antibiotic-prescribing rate for RTIs (both per 1000 registered patient-years) and the proportion of RTI consultations with antibiotics prescribed (%). As the primary aim of the study was to reduce antibiotic prescribing, antibiotic prescriptions for RTIs per 1000 registered patients was the primary outcome for the trial. Design parameters for the eCRT, which included participants aged 18–59 years, are shown in Table 2.
| Measure | Mean number (SD) | Coefficient of variation | Correlation before–after |
|---|---|---|---|
| Antibiotic-prescribing rate (per 1000 patient-years) | 111.9 (39.8) | 0.36 | 0.82 |
| RTI consultation rate (per 1000 patient-years) | 214.7 (56.5) | 0.26 | 0.83 |
| Percentage of consultations with antibiotics prescribed | 52.0 (10.5) | 0.20 | 0.91 |
The estimation of sample size requirements was informed by the analysis of the CPRD data for the eCRT. 16 The mean general practice-specific antibiotic-prescribing rate in the CPRD was 111.9 per 1000 patient-years [standard deviation (SD) 39.8 per 1000 patient-years]. The study employed analysis of covariance, with measures over 12 months before and after the intervention, giving a correlation coefficient of 0.82. Initially, it was aimed to recruit 120 CPRD general practices, which would have enabled detection of a 12 per 1000 reduction in antibiotic-prescribing rate. It was considered that a recruitment target of 120 practices was achievable because of the two previous cluster trials in the CPRD each recruiting more than 100 CPRD general practices. 16,50
The REDUCE study depended on data from Vision (In Practice Systems Ltd, London, UK) practices contributing to the CPRD. Of the 385 Vision practices invited to take part, 21% agreed, 26% declined, 17% were undecided and 36% did not respond. During the recruitment phase, 26% (99 practices) changed their GP software system from Vision, precluding their involvement in the current study. The original target of 120 practices for this study was not therefore achievable, so the sample size calculation was revised. It was estimated that if there were 40 practices in each of two trial arms then, with an alpha of 0.05, there would be 80% power to detect an absolute reduction in antibiotic-prescribing rate of 15 per 1000 registered patient-years, and > 90% power to detect an absolute reduction of 3.5% in the proportion of RTI consultations at which antibiotics are prescribed. This revised sample size calculation was discussed and agreed with the HTA programme representatives at a HTA monitoring visit on 11 July 2016. The revised sample size calculation was also submitted to the CPRD ISAC and the Research Ethics Committee as an amendment.
Main measures
Outcomes were evaluated from the EHRs of registered patients at participating CPRD general practices. For each participant, the person-time at risk during the 12-month intervention period of the trial was evaluated. In addition, whether or not each patient had consultations for RTIs was evaluated. Self-limiting RTIs, including cough and bronchitis, otitis media, rhinosinusitis, sore throat and common cold, were evaluated using Read medical codes. Repeat consultations during the same episode were excluded using a 14-day time window. It was determined whether or not antibiotics were prescribed on the same date as the RTI consultation. Antibiotic prescribing was evaluated using product codes for antibiotics included in the British National Formulary (BNF),55 section 5.1, excluding antituberculous and antilepromatous drugs. Comorbidity was classified as present or absent using the ‘seasonal flu at-risk Read codes’ as employed in the NHS. 56 Seasonal flu at-risk Read codes include diagnoses of significant heart, lung, renal, liver or neuromuscular disease, as well as cystic fibrosis, diabetes and immunosuppression or immunosuppressive treatment. Criteria based on age and pregnancy were not included.
Outcome measures
The proposed outcome measures for the trial are outlined in Table 3. The primary outcome measure was the rate of antibiotic prescribing for RTI per 1000 participant-years over the 12-month intervention period. Secondary outcome measures were the proportion of RTI consultations with antibiotics prescribed; the consultation rate for RTI per 1000 participant-years, and estimates for each of cough and bronchitis, colds, otitis media, rhinosinusitis and sore throat. In addition, total antibiotic prescribing for all indications was evaluated. Deferred prescriptions using the Read code for ‘deferred antibiotic prescription’ (8BP0) was evaluated. Health-care utilisation and costs, using methods reported previously, were also evaluated,57,58 obtaining utilisation estimates from the CPRD and costs of care from reference sources. 59
| Measure | Definition | Details |
|---|---|---|
| Primary | ||
| Antibiotic-prescribing rate | Number of antibiotic prescriptions for RTI per 1000 registered patient-years | Antibiotics included in the BNF,55 section 5.1, excluding sections 5.1.9 (TB) and 5.1.10 (leprosy) |
| Secondary | ||
| RTI consultation rate | Number of consultations for RTI per 1000 registered patient-years | Read codes for RTIs. Repeat consultations within 10 days excluded |
| Proportion of RTI consultations with antibiotics prescribed | Number of consultations for RTIs with antibiotics prescribed/total RTI consultations (%) | |
| Total antibiotic-prescribing ratea | All antibiotic prescriptions per 1000 registered patient-years | |
| Subgroups of RTIa | Broad categories including colds, sore throat, cough and bronchitis, otitis media and rhinosinusitis (see NICE6) | Subgroups of Read codes |
| Health-care costsa | Estimated costs of all health-care utilisation per 1000 registered patient-years | Health-care utilisation from the CPRD clinical, referral and consultation records.57,58 Costs from reference sources59 |
| Safety outcomes | ||
| Pneumonia and lower RTIs, peritonsillar abscess, mastoiditis, intracranial abscess, empyema, scarlet fever, pyelonephritis, septic arthritis, osteomyelitis, meningitis, toxic shock syndrome and septicaemia, and Lemierre syndrome | Number of events (by category) per 1000 registered patient-years | Lists of Read codes |
Analysis plan
The trial protocol envisaged that a general practice-level analysis would be performed, with data aggregated to practice level. 53 Subsequently, two considerations favoured an individual level for analysis of the primary, secondary and safety outcomes. First, there was attrition of trial general practices, with six practices withdrawing from the intervention trial arm and five from the control trial arm during the 12-month intervention period. This attrition was accounted for by practices leaving the Vision practice system that is employed by CPRD general practices. Data from practices that withdraw from the study were included in the intention-to-treat analysis but, because the conditions of interest have a pronounced seasonal distribution, bias might be introduced if comparable periods of time are not included for each practice. Second, a preliminary publication from the study group,31 as well as analyses for the trial DMC, drew greater attention to safety outcomes of the study. Analysis of safety outcomes requires consideration of individual-level covariates (including age and comorbidity), and these are also relevant for decisions to prescribe antibiotics. Thus, inferences are intended both at general practice level and at individual patient level. Consequently, an analysis was conducted of individual participant-level data as the primary analysis. The statistical analysis plan included prespecified subgroup analyses by age group, gender, comorbidity, region, type of infection and baseline antibiotic-prescribing quartile. Age group was categorised from 0 to 14 years, then in 10-year bands until ≥ 85 years. The subgroup effect was assessed statistically on this basis. The effect was summarised, more simply, in those aged 0–14 years being classed as children, and those aged 15–84 and ≥ 85 years being classed as adults.
The trial data set comprised full EHRs data for all participants who consulted with an RTI on one or more occasion during the trial baseline and intervention periods, together with denominator data for all patients registered at trial practices. In the primary intention-to-treat analysis, data for antibiotic prescriptions issued at consultations for RTIs and person-years at each practice were aggregated by age group, gender, comorbidity status and study quarter (as aggregation by month led to non-convergence) following the intervention start date. A random-effects Poisson model was fitted using the ‘hglm’ package in the R program (The R Foundation for Statistical Computing, Vienna, Austria). 60,61 The dependent variable was a count of antibiotic prescriptions. Explanatory variables were trial arm, gender, age group, comorbidity status, region, study quarter and baseline antibiotic-prescribing rate. The baseline antibiotic-prescribing rate was included as an age-standardised rate for each practice, using the European standard population62 for reference. For practices that withdrew during the intervention period, baseline time was included pro rata. The period of randomisation was included, as well as the interaction of period with the baseline antibiotic-prescribing rate. A random effect was included for general practice. The offset was the log of person-years. The intervention effect was tested by considering the statistical significance of the effect of trial arm. Analyses were conducted using the same analytical framework for secondary outcomes. The log of respiratory consultations was employed as offset in the analysis of the antibiotic-prescribing proportion. Interaction terms were tested and prespecified subgroup analyses were conducted in the same framework. A forest plot was constructed. Results for the primary outcome are expressed as the number of antibiotic prescriptions for RTIs per 1000 registered patient-years, referred to as the antibiotic-prescribing rate; and, as secondary outcomes, the number of consultations for RTIs per 1000 registered patient-years (the RTI consultation rate) and the number of consultations for RTIs with antibiotics prescribed/total RTI consultations (%) (the antibiotic-prescribing proportion). Data for all antibiotic prescriptions for any indication were also analysed as a secondary outcome.
Cluster-level analyses were conducted, using general practice-specific aged-standardised rates as observations, as a secondary analysis. Scatterplots were presented in order to visualise the data, and rates were compared using analysis of covariance weighted for the number of person-years at the practice.
Analyses for safety outcomes
The incidence of safety outcomes was estimated as rates per 100,000 person-years. Safety outcomes were analysed by fitting a Poisson model and adjusting for age group, gender and comorbidity. A random effect for general practice was included for the most common outcome of pneumonia. The random effect for general practice was omitted for the remaining, more sparsely distributed outcomes, because of non-convergence.
Sensitivity analyses
Several sensitivity analyses were conducted for the primary outcome. The impact on estimates of a better-fitting model was evaluated, which included respiratory consultation rates as covariates as well as interactions of age group with covariates; the effect of fitting baseline age-specific rather than age-standardised rates as covariates was explored. Extended quasi-likelihood (EQL) and EQL1 estimation methods for ‘hglm’ were compared. The goodness of fit of the ‘hglm’ mode was evaluated and, as there was evidence of overdispersion, double hierarchical generalised linear models (DHGLMs) were evaluated using the ‘dhglm’ package in R. 61 DHGLMs allow for additional random effects in the dispersion part of the model, as well as in the mean model for fixed effects, which can be used to model heavy-tailed distributions and may provide more robust analysis when outliers are present. 61 An overdispersed Poisson model was fitted, incorporating an additional random effect in the dispersion part of the model.
Process evaluation
A process evaluation of the trial was conducted, including a questionnaire to participating GPs and analysis of data on intervention utilisation collected into DXS Point-of-Care™ (DXS International plc, Farnham, UK). The design of the process evaluation questionnaire was informed by criteria suggested by Linnan and Steckler63 for the process evaluation of public health interventions and research. These authors suggest that rigorous process evaluation should encompass five dimensions: fidelity (the extent to which the intervention was delivered as planned), reach (how many intended recipients took part in the intervention), dose (how much of the intended intervention was delivered), recruitment and context. The questionnaire included items concerning respondents’ experience with each component of the intervention. In addition, data on the utilisation of DSTs were also collected. Data were analysed for each practice on the number of times DSTs were viewed and for the number of information leaflets printed. As these two measures were highly correlated, the number of DST views was analysed. For each general practice the proportion (%) of RTI consultations at which DSTs were viewed was estimated. The intervention trial arm practices were divided into quartiles and a linear trend was evaluated across levels of DST utilisation for the primary outcome, adjusting for the same covariates as in the primary analysis.
Analysis of costs of health-care utilisation
There were some reasons why a full cost-effectiveness analysis was not appropriate for this study: reducing antibiotic prescribing may result in benefits to non-trial participants and the intervention may contribute to a reduction or delayed development of AMR. The latter has the properties of a public good whose benefits may be widespread in geography and time. A cost-effectiveness analysis would require a model that would relate trial outcomes to the wider impact of reducing AMR. This trial has the potential to generate information that could be used in a full cost-effectiveness analysis, but such an analysis will require wider research.
For these reasons, a cost analysis that focused on analysis of health-care utilisation was conducted. Previously developed and reported methods were employed to evaluate the costs of health-care utilisation,57,58,64 obtaining utilisation estimates from the CPRD and unit costs of care from reference sources. 59 Health-care utilisation was evaluated from participants’ EHRs in the CPRD. Hospital Episode Statistics data were not used because linked data for the relevant time period were not yet available at the time of analysis. Analyses evaluated primary care utilisation, including consultations at the general practice, emergency consultations, home visits, out-of-hours visits and telephone consultations, and hospital utilisation (as noted in primary care records) including inpatient admissions, outpatient episodes, day cases and emergency episodes, as reported previously. 57,58,64 The number of events was calculated for each patient. The costs of health-care utilisation were estimated using unit costs for 2017 from standard reference sources. 59 The cost of a general practice consultation was £38, a telephone consultation was £27, an inpatient stay was £634, a home visit was £96.40, an outpatient visit was £137, an out-of-hours and emergency consultation was £38, a day-case procedure was £727, and an emergency referral was £137. 59 Drug prescriptions were enumerated and prescription costs calculated by linking the product code for each prescription to the drug price from a dictionary obtained from RESIP Drug Database UK Ltd (Chertsey, UK).
The cost analysis focused on the question of whether or not the total costs of health-care utilisation for patients who consulted at least once for RTIs during the trial intervention period might be increased through intervention. This may be a concern if patients are more likely to re-consult if they are not given a prescription. The distribution of total costs was compared for participants in the control and intervention trial arms from the time of their first RTI consultation to the end of their general practice’s participation in the trial. As the analyses included participants who had consulted at least once, there were no cost zero values. As expected, the distribution of total costs was skewed. A hierarchical general linear model (HGLM) was fitted with a log-link and gamma error distribution, and a random effect was included to allow for correlation by general practice. Analyses were also adjusted for the fixed effects of gender, age group, comorbidity, region and trial period. The coefficient for trial arm was used to evaluate whether or not there was evidence that costs of health-care utilisation might be increased through interventions that aim to reduce antibiotic prescribing.
Changes to original trial protocol
This section outlines changes to the original trial protocol. Each modification was submitted to the CPRD ISAC as an amendment for approval; the Research Ethics Committee was also notified, when appropriate.
Definition of safety outcomes
Initially, safety outcomes were listed as pneumonia and lower RTIs, peritonsillar abscess, mastoiditis, and skin and bacterial infections. Following a review of the recent National Institute for Health and Care Excellence (NICE) guidance,65 intracranial abscess and empyema were included as safety outcomes, whereas bacterial infections and skin infections were omitted as lacking specificity. Following a meeting of the trial Data Monitoring Committee (DMC), held on 20 October 2015, it was recommended to include the following conditions as safety outcomes to be monitored during the trial and reported to the DMC: scarlet fever, pyelonephritis, septic arthritis, osteomyelitis, meningitis, toxic shock syndrome and septicaemia. Amendments to the protocol to evaluate these safety outcomes were submitted to the ISAC and these were approved.
Sample size calculation
As noted in Sample size calculations, an amendment was submitted for approval of the reduced recruitment target of 80 general practices, rather than the initially envisaged 120.
Statistical analysis plan
As noted in Analysis, the trial protocol envisaged that a general practice-level analysis would be performed, with data aggregated to practice level. However, two considerations favoured an individual level for the analysis of primary, secondary and safety outcomes. (1) There was significant attrition of CPRD trial practices, with six practices withdrawing from the intervention trial arm and five from the control trial arm. Although data from practices that withdraw from the study can be included in the analysis, bias may be introduced if comparable periods of time are not included for each practice, when the condition of interest has a seasonal distribution. (2) The cohort study,7 as well as analyses for the trial DMC, drew greater attention to safety outcomes of the study. Analysis of safety outcomes requires consideration of individual-level covariates (e.g. age and comorbidity), and these are also relevant for decisions to prescribe antibiotics. Consequently, it was decided to conduct an individual-level analysis as the primary analysis with a cluster-level analysis being considered as secondary.
The statistical analysis plan (SAP) was written by the trial statistician (AP) and the principal investigator (MG). The SAP was reviewed and approved by the independent DMC (meeting on 20 April 2017) and the Trial Steering Committee (meeting on 8 June 2017).
Population-based cohort study of safety outcomes
Material in this section is reproduced from Gulliford et al. 31 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
The data source for the cohort study was the UK’s CPRD. 49 For the present study, data were included for the 10-year period, from 2005 to 2014, before the start of the cluster trial. The CPRD included data for an open cohort of about 4.5 million registered patients during this period.
Definition of infective complications of respiratory infections
The number of first episodes of infective complications were evaluated in the entire registered population of patients in the CPRD from 2005 to 2014. Infective complications of RTIs were defined using Read medical codes recorded in participants’ EHRs. EHR data include diagnoses recorded at primary care consultations and home visits. In addition, the CPRD referral file includes coded data for hospital referrals and hospital discharges. Analyses evaluated pneumonia (57 codes), empyema (14 codes), peritonsillar abscess (five codes), mastoiditis (13 codes), bacterial meningitis (19 codes) and intracranial abscess (14 codes). Codes for pneumonia were drawn from section H2 of the Read code classification, which includes codes for pneumonia and influenza. Codes were included if they indicated the presence of pneumonia without a viral aetiology. Bacterial meningitis included codes for meningococcal meningitis, meningococcal septicaemia, pneumococcal meningitis and Haemophilus meningitis, as well as unspecified bacterial meningitis. Data were extracted for all participants with records of infective complications from 2005 to 2014. Incident events were defined as the first record of an event in a participant that was recorded > 12 months after the start of the participant’s CPRD record. Gender, year and age group were included as individual-level covariates. Nine 10-year age groups were employed, with categories of 0–14 years and ≥ 85 years. Incident events were aggregated by year, age group, gender and general practice. Person-time for the registered CPRD population was estimated by year, age group, gender and general practice in order to estimate rates of infective complications. Cluster-level covariates included CPRD region, with 10 regions in England, as well as regions in Wales, Scotland and Northern Ireland. Deprivation quintile was included, based on general practice-level data for indices of multiple deprivation score66 for England and equivalent scores in Wales, Scotland and Northern Ireland. Linked data were provided by the CPRD. 67 The distribution of deprivation quintiles is not expected to be equivalent between countries.
Definition of respiratory consultation and antibiotic-prescribing rates
Age-standardised measures were estimated for RTI consultations and antibiotic prescribing, as reported previously. 7 For each CPRD general practice, the rate of RTI consultations per 1000 registered patients was estimated; the antibiotic-prescribing rate for RTI per 1000 registered patients, and the proportion (%) of RTI consultations with antibiotics prescribed. These prescribing measures were estimated on a sample of CPRD data because it was not feasible, and the licence did not allow us, to perform the analysis on the entire CPRD database. Participants were sampled from all acceptable patients included in the CPRD. A random sample of 75 currently registered patients was drawn without replacement from each year from 2005 to 2014. This gave a maximum sample of 750 participants, with up to 7500 person-years of observation per practice. The study aimed to achieve a total sample of < 0.5 million and the total sample for analysis was 411,226 participants from 643 general practices. This allowed the estimation of practice-specific proportions with a margin of error of 1%. For participants in the sample, person-years were estimated as the denominator from the start of CPRD registration, or 1 January 2005, to the end of the participant’s CPRD record, or 31 December 2014. Self-limiting RTIs were identified using medical codes recorded during general practice consultations. These were classified into five groups following the recommendations of NICE:6 colds and upper respiratory infections; sore throat, including pharyngitis and laryngitis; cough and acute bronchitis; otitis media; and rhinosinusitis. Acute bronchitis was included because current recommendations are to avoid antibiotic treatment. 6 Consultations for RTIs were identified and first consultations within a 14-day time window were selected. Data for participants aged ≥ 100 years were excluded. Antibiotic prescriptions issued on the same day as respiratory consultations were identified. For each general practice rates of consultations for respiratory infections per 1000 person-years (i.e. the RTI consultation rate), rates of antibiotic prescribing for RTIs per 1000 person-years (i.e. the antibiotic-prescribing rate) and proportions (per cent) of RTI consultations with antibiotics prescribed (i.e. the antibiotic-prescribing proportion) were estimated. Rates and proportions were standardised for age and gender using the 2013 European standard population. 62 After excluding practices with insufficient data, because of short periods of CPRD contribution rates were estimated for 610 CPRD general practices.
Analysis
Age-standardised incidence rates (per 100,000) were estimated by year for each safety outcome, and trends over time were estimated using a linear model. In the final stage of the analysis, the number of infective complications, with person-years at risk, were evaluated in relation to general practice-specific rates of RTI consultations and antibiotic prescribing. Mixed-effects Poisson models were fitted using the ‘hglm’ package60 in the R program. 68 General practice was fitted as a random effect. The log of person-years was included as offset. Fixed effects included gender, year, age group, region and deprivation quintile. The association of age-standardised RTI consultation rate with rates of infective complications was evaluated. The association of the antibiotic-prescribing rate, and the antibiotic-prescribing proportion, with infective complication rates were evaluated after adjusting for the RTI consultation rate. Incident rate ratios (IRRs) [along with 95% confidence intervals (CIs)] were estimated for each quartile of RTI consultation rate, antibiotic-prescribing rate or antibiotic-prescribing proportion using the lowest quartile for reference. The RTI consultation rate, antibiotic-prescribing rate or antibiotic-prescribing proportion were also fitted as continuous predictors and IRRs were estimated per 10-unit change in the predictor. Whether or not the addition of quadratic terms improved goodness of fit was evaluated. As there were small numbers of events for intracranial abscess and mixed-effects models did not converge, the random effect for general practice was omitted for this outcome. Regression models were not fitted for Lemierre syndrome because this was extremely rare. The ‘ggplot2’69 and ‘forestplot’70 packages in R were used to present the results.
In order to present the clinical implications of these findings, the number of events expected in a general practice with 7000 patients, the general practice mean list size for England during 10 years of follow-up was calculated. The median (95% range) was used for the RTI consultation rate to estimate the expected number of RTI consultations. The disease incidence and distribution of antibiotic-prescribing proportion for the highest-prescribing quartile was used to estimate expected numbers of complications and antibiotic prescriptions. The relative risk increase for a 10% change in antibiotic prescribing was used, from the Poisson model, to estimate the expected change in number of infective complications.
Research ethics
The protocol for the study was approved by the CPRD ISAC (reference 14_130A2). The CPRD has broad NRES ethics approval for observational research studies.
Chapter 3 Results: intervention development and delivery
Parts of this chapter have been reproduced with permission from Gulliford et al. 71 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
The initial sections of this chapter present empirical data for contextual themes and participants’ views on the prototype interventions that emerged from qualitative analysis of interview data. The final section of this chapter describes the main features of the REDUCE trial intervention.
Participants
Data collection for the intervention study was completed between March 2015 and September 2015. There were 28 GPs and three NPs who took part in the interviews and 35 interviews were conducted in total (three GPs and one nurse were interviewed on two occasions). There were 31 interviews conducted face to face in general practices and four were conducted over the telephone. Participants were recruited from 21 non-trial general practices covering several areas of England (London, Oxfordshire and Yorkshire) and from general practices of different sizes – ranging in the number of registered patients from 5891 to 17,797 (on average, 10,968 registered patients). Interviews lasted between 20 and 76 minutes.
Intervention development: emerging contextual themes
The interviews for intervention development provided contextual information, which contributed to the understanding of influences on practitioners’ interaction with the intervention materials. Table 4 summarises the main themes relevant to the implementation of the trial intervention.
| Theme | Subtheme |
|---|---|
| Researchers may not understand the general practice context |
|
| The problem of antibiotic overutilisation may not be perceived as personally applicable |
|
Theme 1: researchers may not understand the general practice context
Participants considered that researchers did not have a good understanding of the way general practice operates, suggesting a number of reasons why the research might be difficult to sustain within the general practice environment.
Respiratory tract infection consultations are time-consuming
Clinicians were agreed that they see a high number of patients with RTIs during their daily medical practice:
I’ve seen loads [of patients with RTIs] this morning. And I’m sure I’ve got some in the afternoon. I can predict that I’ll have at least one or two in my 15 patients in the afternoon.
GP21
Many felt frustrated that patients would visit the practice several times during the same episode of RTI until they were given a prescription for antibiotics:
When we see patients, if I look at a consultation and the patient has been in two or three times for the same problem, you’ll end up giving them antibiotics because you know they’re going to keep coming back . . . And we probably know the patient doesn’t need antibiotics, but the problem is, they’re now taking up three consultations and they’ll probably come back a fourth time if we don’t give them.
GP21
Routine daily task
Participants were in agreement that seeing patients with RTIs is a routine daily task, and they felt confident in diagnosing respiratory infections. Some participants were surprised that the intervention is targeting such a well-established behaviour:
We wouldn’t click [on the intervention tools], because this is bread and butter stuff we will already be doing these things . . . And I don’t think anybody needs to really remind us [of] these things.
GP18
A majority of interviewed clinicians indicated that they are fixed in their approaches to dealing with patients presenting with RTIs as a time-saving strategy; a change of approach might increase their workload:
Although it’s there [i.e. the REDUCE trial notification] as a reminder, it won’t trigger in the brain as a reminder, because it’s like when you know you’re on the computer and you click ‘next’, you know what screen is coming next and you’re already ready to type . . . So it can become quite routine . . . So it may not have the effect that you want it to have.
GP17
It eats into our time. I personally will just tell them face to face. I wouldn’t like to go through this [study tools]. It just adds on to my consultation time.
GP10
Loss of clinical autonomy and judgment
Some participants perceived the intervention as a source of external pressure to withhold antibiotics for patients for whom clinicians considered antibiotics were recommended and felt this external pressure to not prescribe undermined their clinical judgment and autonomy:
Unless you’re the clinician sitting behind that desk, then don’t come and tell me what I can and can’t prescribe and in what condition and when I can’t, because you haven’t seen the patient . . . because my rate in this practice might be a 100% because every single patient who comes in might clinically need it.
GP7
Some participants were concerned that involvement in a trial, such as the REDUCE trial, might undermine their relationship and rapport with their patients or change the focus of the consultations from the patient to the study tools:
Then my concern is that if you just give them a leaflet, I feel they may just think I’m patronising them, because they feel like ‘Well actually I wanted antibiotics, I didn’t want a piece of paper.’ People can get annoyed very easily and they can kind of affect that doctor–patient relationship which isn’t ideal.
GP25
More consumerist patients
Many participants considered there was a shift in patient expectations and demands towards a more ‘consumerist’ approach:
People just don’t care, they want to be better by 1 day when they have a terrible cold. That’s what I think is one of the biggest barriers.
GP6
Adults are harder . . . because parents hear about antibiotics being bad and giving them diarrhoea and are quite keen to avoid them . . . it’s more when it’s for themselves. Because they want to get back to work and they think it will hurry it up.
GP13
As a result of this consumerist attitude, combined with a feeling of constantly being audited by the NHS, some GPs felt uncertain about potential bacterial complications of the infection:
It’s the fear of litigation or things going wrong, and if you have arbitrary targets like this, which is way beyond the usual targets, it puts unnecessary pressure on GPs not to prescribe . . . And I don’t want to prescribe, but if it’s needed, then pressure of some sort of appraisal and maybe told off is not really needed.
GP18
Research involvement by clinicians
Participants emphasised a change within general practice towards more target-driven culture. As work began to shift towards this pattern, participants considered that the study should be aligned with the changes at the system level (incentives for meeting certain clinical targets). For many participants this would encourage them to take part:
If you want to make it work, incentivise the GPs to make use of it. Otherwise we’ve got cancer tools, we’ve got all these other tools coming through our system . . . and literally go straight into my dustbin. There’s no funding or extra incentive attached to it. There’s more paperwork there, bits to fill in, you have go from one screen to another screen, go to a website. You haven’t got time for all of that. So I pretend to use it when they come, ‘yes, yes, I’m using it, it’s great’. I’m not using it at all.
GP7
Others participants commented that, despite possible incentives for getting involved, there would be no recognition for the efforts of individual GPs:
Could you have this information [feedback on antibiotic prescribing] done on individuals rather than practices? . . . it’s a bit of a ‘name and shame’ situation, isn’t it if this data comes back and doctor so and so is prescribing 80% antibiotics and – you know, that would certainly probably make you think, ‘Blimey I need to do something about this’.
GP1
Theme 2: the problem may not be perceived as personally applicable
The problem does not belong to me
A majority of interviewed participants considered that they were familiar with current recommendations for antibiotic prescribing but were confident that own their prescribing was appropriate:
Personally I don’t think we would prescribe unnecessarily . . . I will only prescribe it when I think it’s needed rather when the patient is thinking they want it . . .
GP18
In several cases, participants reported that they do not overprescribe because high levels of prescribing is justified for their patient population:
Well if it [feedback] is population-based, then, you know, if your sick population who are poor, they are going to be sicker aren’t they? So you can’t compare us to [an affluent area] can you?
GP4
Distrust of the evidence
Some participants questioned the outline proposals for reduced antibiotic-prescribing rates for RTIs:
You can say it’s safe to half this amount of antibiotics. You don’t know what they’ve seen, you know. Who says it’s safe? What’s this? ‘You can safely aim to halve antibiotic prescribing.’ That means that half our patients didn’t need it. How do you know?
NP3
Other participants acknowledged the importance of following the NICE recommendations,6 but considered these recommendations may be unachievable in GP daily practice:
I just think it looks like the target is completely unachievable . . . It just looks, yes, it just looks like no one is ever going to get near it.
GP13
Out-of-hours prescribing
Participants identified groups of individuals or institutions which, in their view, were responsible for high antibiotic-prescribing rates:
I think 50% of our doctors at the moment are locum doctors and, you see, I don’t know if I’m being judgemental. But it just seems to be ‘chest infection, amoxicillin’ – when I did my audit, none of the regular doctors did it.
GP6
There was a visible tension in GPs accounts between general practices and out-of-hours services, which in the views of GPs, were not only responsible for high antibiotic-prescribing rates, but also for creating more workload for GPs:
I think GPs would also want to know what percentage of like A&E [accident and emergency] and walk-in centres are doing as well . . . because that’s another problem – we tell [patients] ‘no’, then they go to A&E and they get antibiotics. And the next time they come, they go, ‘Last time I had to go to A&E to get my antibiotics.’ We do get that quite a lot.
GP13
Many participants considered that the intervention should be directly targeting ‘high-prescribing’ practices or individuals:
It seems to me that it would make sense that before you go to all this trouble . . . you can see which practices are perhaps being excessive and then target those with all this material . . .
NP2
And I think much better would be this to be targeted to those doctors that are already prescribing high. Because what is it about them, you know, what – are they not doing what the others are doing.
GP4
Intervention development: feedback from interviews
Table 5 provides a summary of themes relevant to intervention design.
| Intervention component | Key themes |
|---|---|
| Webinar |
Watch with other practitioners during practice meeting Intervention not going to increase workload |
| Support tools |
Use condition-specific leaflet Emphasise symptom duration Support tools available when issuing a prescription Add prescription indication criteria |
| Monthly feedback |
Add comparison with other local practices/CCGs Feedback reports could be included in appraisals |
Webinar
There was a consensus among interviewed GPs and nurses that the webinar was positive and communicated messages that were relevant to health-care professionals, such as safety of reduced prescribing. Some GPs suggested that the webinar should be watched together during the practice meeting:
If it’s a clinical meeting, everyone is sitting there, you’re playing it, difficult to avoid it . . . It covers the whole, covers the whole practice. So in the meeting we have everyone, senior partners, salaried, nurses, health-care assistants, it would encompass everyone, even if say the health-care assistants are not directly involved, clinical staff would watch the video and would be aware that we were part of the study.
P16
It was important to emphasise in the webinar that the intervention would not increase workload, as this might be a major barrier to involvement. In contrast, participation in the intervention might lower GPs’ consultation burden associated with RTIs (as patients would be less likely to re-consult with future RTIs):
What it’s saying, it’s addressing our concerns about spending more time talking to the patients . . . I guess probably, I guess that feeds back to the anxiety everyone has about their workload at the moment . . . guess if you market it to GPs by saying, ‘reduce your consultation rates,’ or something like that, you know, if the trial is going to show that if, you know, when you don’t prescribe, they don’t come back the next time.
P32
Decision support tools: patient leaflets
A majority of GPs preferred a condition-specific patient leaflet, rather than the generic one, as the condition-specific leaflets appear more tailored to patient needs:
Once you’ve told someone, ‘You have a middle ear infection, this is some information about it,’ if it’s more specific to their condition, they might prefer it or it might give them more incentive to read it, although the information is pretty much the same as the other one.
P26
Handing out a leaflet could help draw a consultation to a close and patients would not leave the GP office empty handed. Some GPs suggested adding condition-specific ways of easing and managing symptoms associated with upper respiratory infections:
Maybe suggest other options like, for example, sinusitis – consider steam inhalation, [nasal] spray, just give the option to the doctor what else they can do.
P28
Participants particularly liked the idea of including expected illness duration and stressing that antibiotics will not reduce the duration of symptoms:
That’s really helpful. Well the infection is likely to last 21 days, that’s helpful because people come back every week.
P26
However, others felt that there was a shift in patient expectations and demands towards a more ‘consumerist’ approach and that patients would request antibiotics irrespective of information presented on the patient leaflet:
People just don’t care, they want to be better by 1 day when they have a terrible cold. That’s what I think is one of the biggest barriers.
GP6
Intervention activation
Some GPs expressed a view that they would not type in a Read code (diagnosis) during the consultation, but would start with issuing a prescription. They considered that the study box should appear when a GP writes a prescription for a common RTI antibiotic (e.g. amoxicillin), not just in response to a Read code. However, other GPs considered this approach might not be appropriate as common antibiotics are commonly used to treat non-RTI infections, such as infected tooth:
The problem is there will be no Read code by the time it’s prescribed sometimes. So often the last thing that’s sometimes put in is a Read code . . . So if this was to pop up, then the best time for it to pop up would be as an antibiotic is prescribed. But obviously software wise you’d have to link it in to a diagnosis as well. And give some sort of warning there going, whatever, you know, ‘reduce antibiotics,’ or, ‘are you certain this is indicated?’ or something.
P21
Some GPs felt that the addition of prescription indication criteria for each condition would be beneficial. This would help to reassure both the GP (that they are making a correct diagnosis) and patients (that the diagnosis and possible antibiotic prescription are based on official criteria):
I know there isn’t a scoring system for all of them [RTIs], but on, I mean, without a scoring system or something like that – I’m not sure how this is going to reduce the prescribing . . . if somebody pushes back, so if someone is insistent, you know, going back to the original script, if someone is insistent . . . you can come back and say, ‘Look I’ve put it, we’ve done – this is a recognised validated scoring system, and you don’t warrant antibiotics because your score is . . . whatever’.
P30
Patient information screen
Some GPs were not keen on the use of a patient information screen, as they felt that this would lengthen consultation times:
If I say, ‘read this [patient information screen]’, then I have to sit there and wait for them to actually read it and then that would mean they’re not going with anything to take away with and they may forget it as well.
P16
Prescribing reports
A majority of GPs considered that they did not prescribe antibiotics unnecessarily and some were surprised that their practice would be part of the intervention:
Personally I don’t think we would prescribe unnecessarily . . . I will only prescribe it when I think it’s needed rather when the patient is thinking they want it . . .
GP18
It seems to me that it would make sense that before you go to all this trouble . . . you can see which practices are perhaps being excessive and then target those with all this material . . .
NP2
A majority of GPs did not favour the use of prescribing target rates or of optimal prescribing rates. Some perceived the target prescribing rate as unachievable, as it was much lower than current rates:
Yes, because otherwise you’ll get this and think, ‘where on earth are they getting 22% from?’
P26
Whereas I think, with this, people are going to be aiming to be better than other people (other CCGs [Clinical Commissioning Groups]). But I just think it looks like the target is completely unachievable. It just looks, yes, it just looks like no one is ever going to get near it.
P23
A majority of GPs considered that a comparison with local practices/local CCGs should be presented in the reports, as then the results would feel relevant. Many GPs acknowledged though that they would not aim to lower their prescribing if they were at the same level as or a lower level than other local practices:
The national rate is useful, but we’d probably be comparing ourselves within our CCG . . . similar population, similar ways of practising, probably meeting each other in our CCG meetings, similar budgets, similar pressures.
P16
There was no agreement among interviewees about whether feedback reports should present data for different RTI types separately or combined:
If we are looking at individual conditions, this is easier to interpret, it’s easier to understand and it’s a summary, it’s an overall summary. Whereas if you have 1, 2, 3, 4, 5, five graphs to look at, five graphs to interpret and the people will say, ‘We are better than the CCG in cough, we are not so good in rhinosinusitis and we are average in sore throat, so where are we overall?’
P16
All participants welcomed the idea that feedback reports would count as an audit towards practice or individual GP appraisals:
I was looking to see well what are practices going to get out of it, but I think if the practice did it, they’d all feel they could write it on their appraisal and that they participated.
P20
Trial interventions
A summary of the main features of the final versions of trial interventions is given in Table 6. There were no modifications to the interventions following the start of the trial.
| Intervention component | Content | Delivery |
|---|---|---|
| Webinar | Professionally produced video narrated by a practising GP in a general practice setting, summarising:
|
Webinar delivered through an electronic link embedded in trial start letter |
| Webinar also delivered into practice system using DXS Point-of-Care, with active alerting | ||
| GPs encouraged to present and discuss webinar in practice meetings | ||
| Antibiotic-prescribing reports | Monthly updated reports on antibiotic-prescribing rates for RTIs, including:
|
Delivered by e-mail to the GP identified as the ‘champion’ for the trial at the practice |
| GP requested to circulate prescribing reports to all prescribers at the practice | ||
| GPs encouraged to discuss prescribing reports in practice meetings | ||
| Decision support tools | Professionally designed DSTs, including:
|
Decision support tools delivered into general practice systems through DXS Point-of-Care |
| Activated during consultations when medical codes for RTIs were entered into patient electronic records |
Webinar
Purpose
A webinar was developed to fulfil several functions:
-
making practice staff aware of their practice’s participation in the trial
-
raising awareness of and concern at the problem of AMR
-
introducing the REDUCE trial DSTs and providing training by demonstrating how to use the support tools
-
introducing and outlining the value of the monthly antibiotic-prescribing reports that were produced for the trial
-
reassuring GPs about the safety of reduced antibiotic prescribing
-
identifying potential benefits of this approach to the practice, including a reduced number of RTI consultations in the future.
The webinar was narrated by an academic GP member of the study team and was recorded in a general practice setting. Initial versions of the webinar were recorded and presented to interview respondents on a tablet computer. The final version of the webinar, which incorporated suggestions from the qualitative interviews and patient advisory group feedback, was produced professionally.
The main elements of the webinar narrative were as follows, with the narrator:
-
drawing attention to the increasing problem of AMR, referencing the Chief Medical Officer’s report1 and highlighting AMR as a potentially ‘catastrophic threat’
-
discussing general practice antibiotic prescribing, emphasising that RTIs represent the most frequent indication for antibiotic prescribing in primary care, observing that RTI consultations can be time-consuming and recognising that GPs may find it difficult to withhold antibiotics
-
introducing the REDUCE trial DSTs, explaining that these were developed by leading GPs, explaining that the tools are easy to access and may help to manage RTI consultations
-
demonstrating the use of the DSTs, showing how the ‘banner’ is triggered by entering Read codes for RTIs
-
introducing the condition-specific patient information leaflets, noting that these are patient friendly and have been based on good evidence and developed with input from practising GPs and patients
-
explaining that the REDUCE trial tools are designed to safely help antibiotic prescribing, demonstrating that the ‘prescription indication’ page can be used to decide whether or not patients are at risk of complications
-
introducing the REDUCE trial antibiotic-prescribing reports, explaining that these reports can be used as evidence of audit and can be included in appraisals
-
commenting that reducing antibiotic prescribing is expected to be associated with reducing RTI consultations and that investing time in improved management may be associated with future benefits
-
explaining that reducing the rates of antibiotic prescribing is not associated with reduced patient satisfaction when patients are explained the reasons for the no antibiotic strategy.
Each intervention trial arm practice was encouraged to identify a GP ‘champion’ for the study, who was asked to ensure that all prescribers at the practice were aware of the trial and had seen the webinar, ideally through the presentation of the webinar at practice meetings.
Antibiotic-prescribing reports
Antibiotic-prescribing reports were presented on professionally designed templates, including the logos of the NIHR and King’s College London. The main features of the antibiotic-prescribing reports were as follows:
-
The intervention trial arm practices were sent antibiotic-prescribing reports at the start of the trial, and after each completed month of the 12-month intervention period.
-
At the end of the trial, practices received a final report accompanied by a certificate of completion of the trial.
-
Prescribing reports were portable document format (PDF) files each presented as a single side of A4 paper.
-
The reports presented data in a table, including a count of the number of RTI consultations and number of antibiotic prescriptions, together with the proportion of RTI consultations with antibiotics prescribed for each month of the intervention period.
-
Data were compared with the preceding 12 months at the same practice for reference.
-
A brief accompanying narrative explained the numerical data included in the table and summarised any change in antibiotic utilisation based on the proportion of RTI consultations with antibiotics prescribed.
-
The narrative also included a reminder on how to access the REDUCE trial DSTs.
-
A bar chart illustrated changes in the proportion of RTI consultations with antibiotics prescribed in comparison with the preceding 12 months at the same practice.
-
Based on interview feedback, it was decided not to include an external comparator (such as antibiotic-prescribing data for the whole of the CPRD) because this might offer little incentive for those practices already prescribing fewer antibiotics than average.
-
Data reported were aggregated across all ages and for men and women.
-
No changes were made to the overall design of the prescribing reports during the course of the trial.
-
Data analysis and report writing were performed using bespoke code written in the R program.
Decision support tools
The REDUCE trial DSTs (Figure 1) were delivered into trial practices using proprietary software known as DXS Point-of-Care, which is integrated with the Vision practice system. The DSTs were triggered when a Read code for a RTI was entered into the practice system. A ‘banner’ then appeared in the DXS Point-of-Care tool bar. Practitioners gained access to the DSTs by clicking on the toolbar. The process was explained in the introductory webinar.
FIGURE 1.
Map of DSTs.
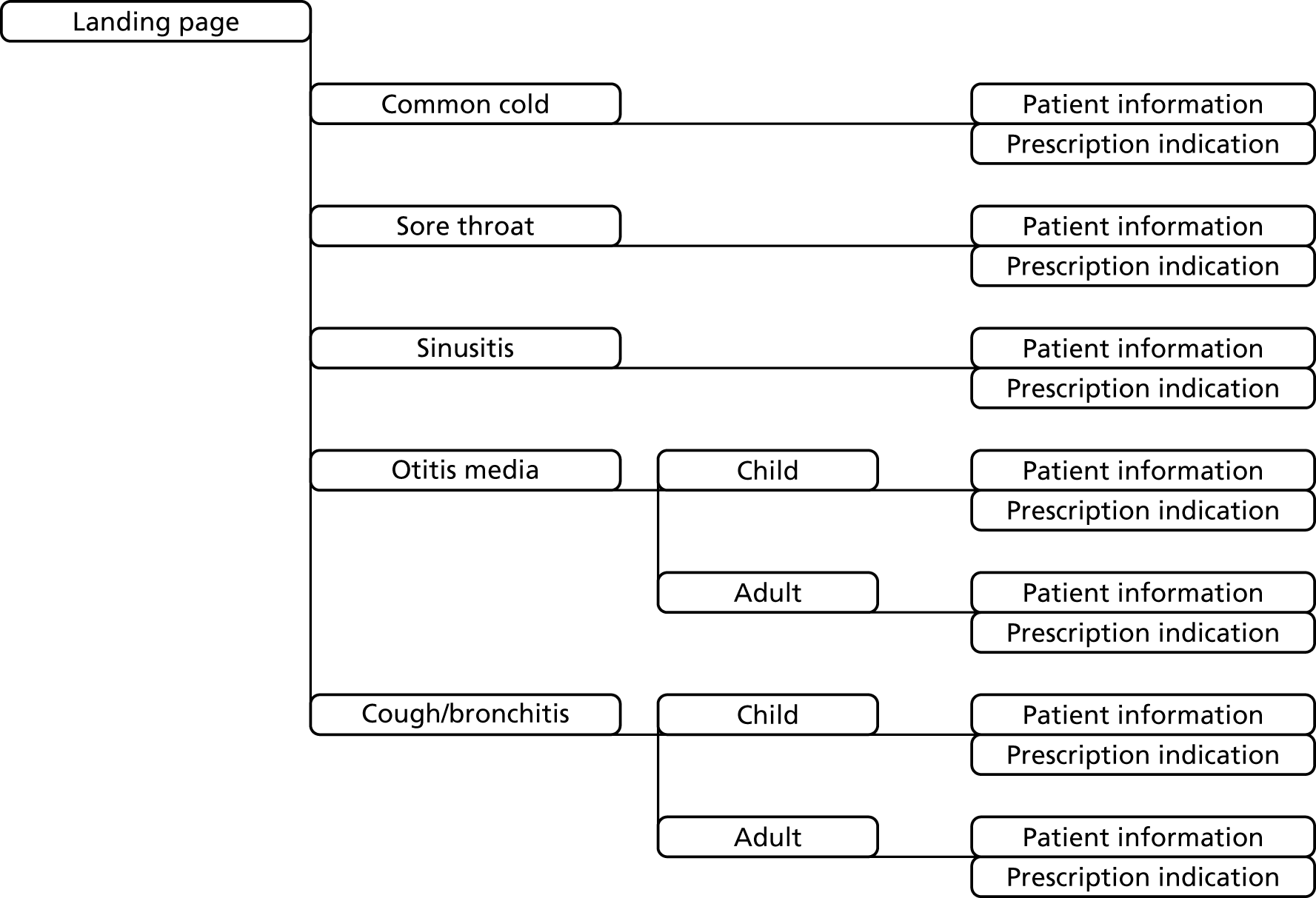
Figure 1 provides a map of the DSTs. After clicking on the DXS Point-of-Care toolbar, practitioners were presented with a ‘landing page’ that offered advice for the five groups of self-limiting RTI, including common cold, sore throat, sinusitis, otitis media and cough/bronchitis. Advice for the last two conditions was specifically tailored for either children or adults, based on clinical advice from GP members of the study team. For each group of conditions, practitioners were offered the alternative of accessing a patient information leaflet (Figure 2) or a prescription indication page (Figure 3).
FIGURE 2.
Part of one of the REDUCE trial patient information leaflets.
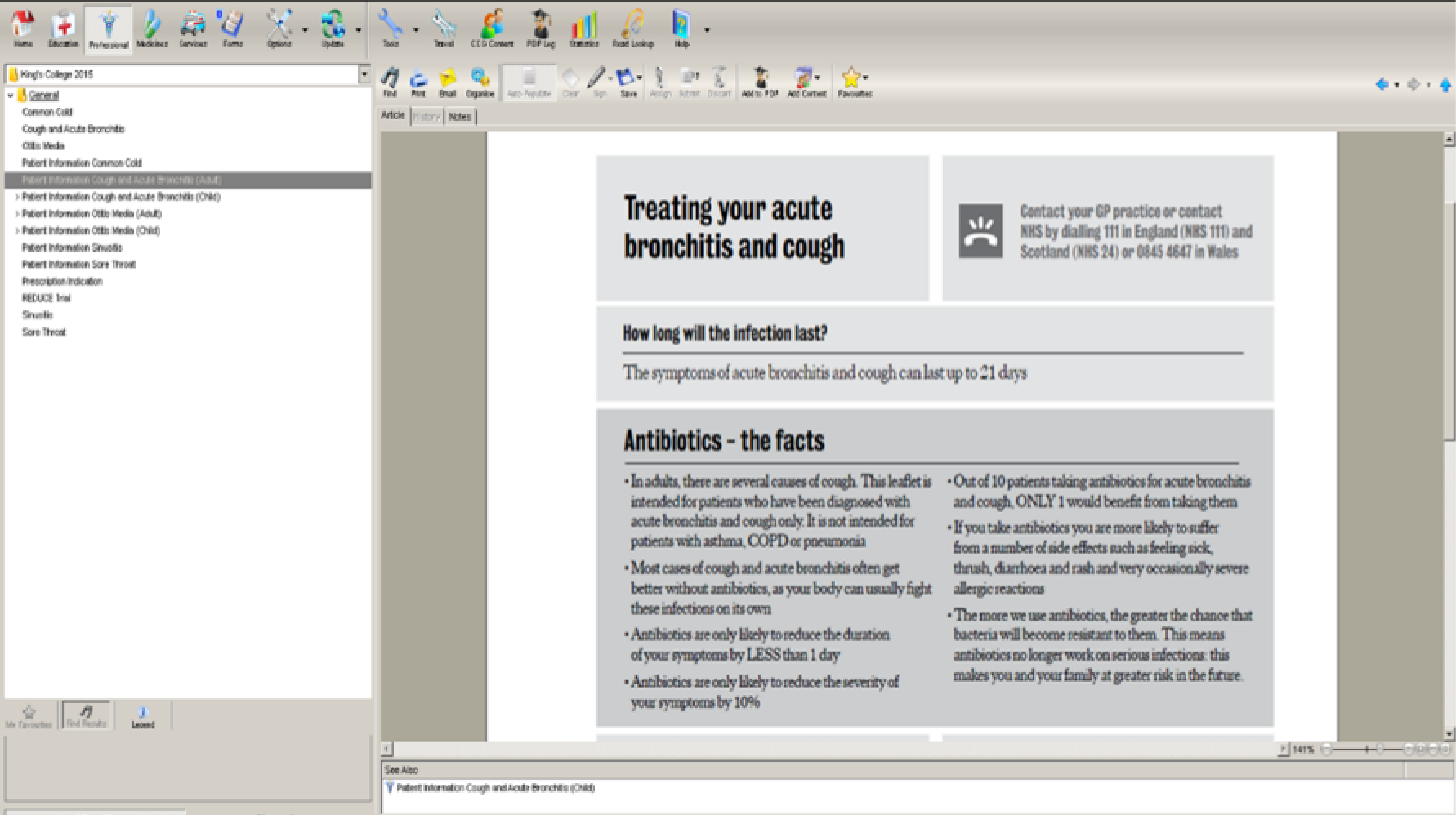
FIGURE 3.
Screenshot showing the REDUCE trial prescription indication page.
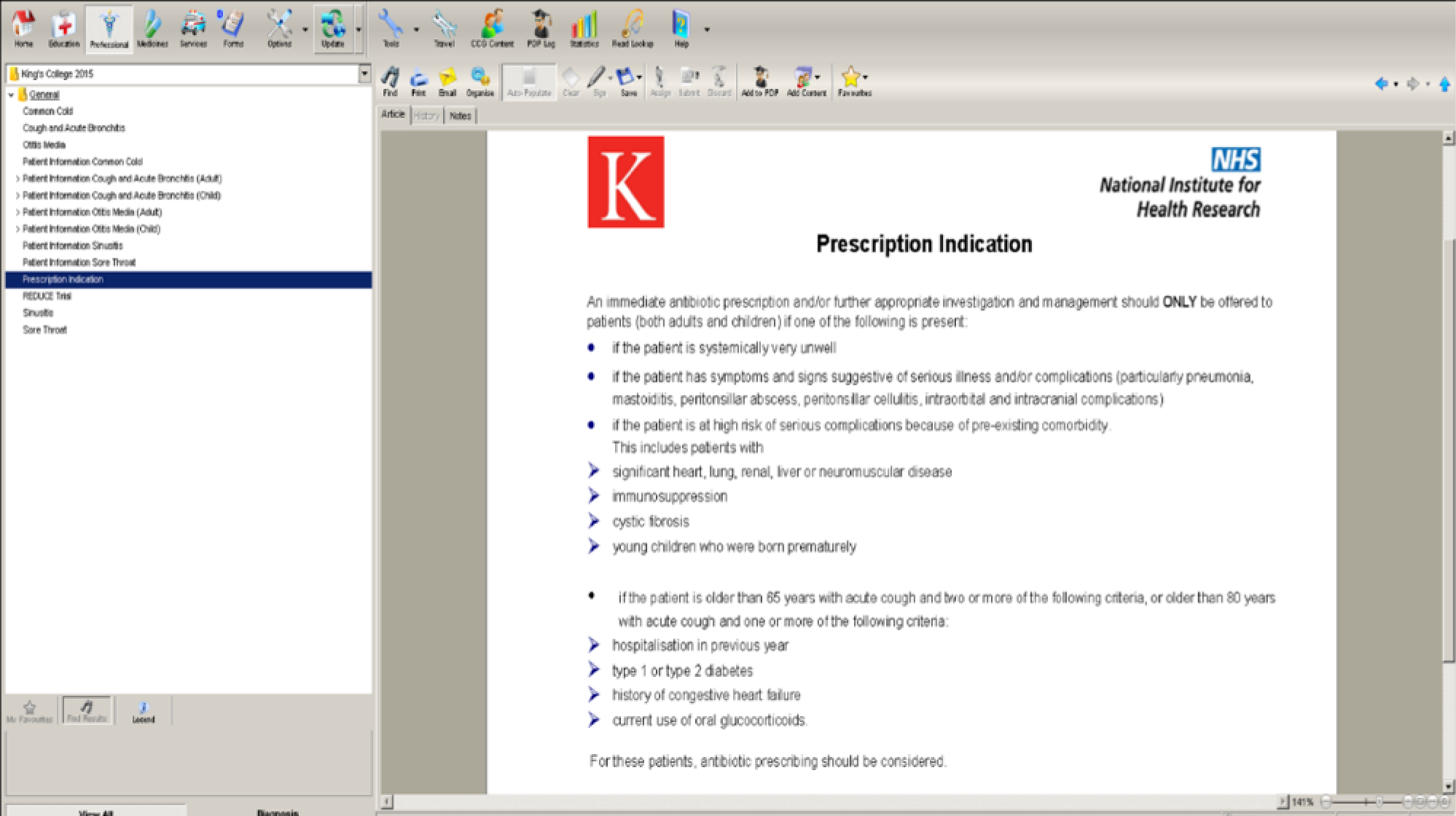
The REDUCE trial patient information leaflets included the following items of information:
-
the expected duration of symptoms from the specified class of self-limiting RTI
-
evidence concerning antibiotic utilisation including:
-
lack of clinical effectiveness of antibiotics at reducing the duration or severity of symptoms
-
increased risk of common drug side effects
-
risks to individuals and families from AMR
-
-
advice on self-management of symptoms including:
-
need for rest
-
fluid intake
-
management of fever with paracetamol or ibuprofen
-
obtaining advice on symptomatic treatments from a pharmacist
-
-
advice on seeking help when there are possible warning signs including:
-
possible signs of meningitis (headache or vomiting)
-
respiratory difficulties (rapid breathing, cyanosis)
-
possible signs of septicaemia (cold skin, discoloration, rash)
-
local complications (difficulty swallowing, drooling)
-
‘feeling a lot worse’.
-
The patient information leaflets were professionally designed and included the NIHR and King’s College London logos. The leaflets were presented as a single side of A4 in a PDF, which could be viewed on a computer screen or printed and handed to patients.
The REDUCE trial prescription indication page drew on the criteria for antibiotic prescribing in the NICE guidelines (see paragraph 1.7);6 these guidelines identify patients at risk of complications using criteria that include how unwell the patient is, whether or not there are clinical features suggestive of complications if comorbidity is present, or if the patient is of an older age and having features that suggest a higher level of risk. The prescription-indication page enabled practitioners to evaluate with patients whether or not an indication for an immediate antibiotic prescription was present.
Chapter 4 Results: cluster randomised trial
Recruitment and baseline characteristics
The trial recruited 80 (25%) general practices to the trial, of which one withdrew from the CPRD before the start of the intervention and the remaining 79 were included in the intention-to-treat analysis. Figure 4 provides a flow chart showing the progress of practices and participants through the trial.
FIGURE 4.
A flow chart showing trial practices and registered populations.

The trial included general practices from throughout the UK, including 19 practices from Scotland, 15 from Wales, nine from Northern Ireland and the remaining 36 from regions in England (Table 7). There were 18 practices allocated in November 2015, 31 in January and February 2016 and 30 in June–August 2016. The registered population included participants of all ages, including approximately 17% aged under 15 years and approximately 8% aged ≥ 75 years. General practice list sizes were generally slightly higher in intervention trial arm practices, but the range of list sizes was similar across trial arms. There were 17.1% of participants with comorbidity in intervention trial arm practices and 14.5% at control trial arm practices. In the baseline period, the point estimates for RTI consultation rate and antibiotic-prescribing rate were slightly higher at control trial arm practices, but in the context of the wide range of variation among practices (Table 7).
| Characteristic | Trial arm | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Number | % | Number | % | |
| Region | ||||
| London | 4 | 9.8 | 3 | 7.9 |
| Midlands and North | 4 | 9.8 | 4 | 10.5 |
| Northern Ireland | 4 | 9.8 | 5 | 13.2 |
| Scotland | 10 | 24.4 | 9 | 23.7 |
| South and East | 8 | 19.5 | 6 | 15.8 |
| South West | 3 | 7.3 | 4 | 10.5 |
| Wales | 8 | 19.5 | 7 | 18.4 |
| Period of randomisation | ||||
| November 2015 | 7 | 17.1 | 11 | 28.9 |
| January–February 2016 | 18 | 43.9 | 13 | 34.2 |
| June–August 2016 | 16 | 39.0 | 14 | 36.8 |
| Practice list sizea | 8936 | Range 1086–18,425 | 6777 | Range 2530–18,557 |
| Age (years) group | ||||
| < 15 | 55,577 | 16.0 | 47,509 | 17.1 |
| 15–24 | 40,544 | 11.6 | 30,610 | 11.0 |
| 25–34 | 45,545 | 13.1 | 37,444 | 13.4 |
| 35–44 | 46,288 | 13.3 | 38,766 | 13.9 |
| 45–54 | 52,447 | 15.1 | 41,507 | 14.9 |
| 55–64 | 42,275 | 12.1 | 33,769 | 12.1 |
| 65–74 | 35,746 | 10.3 | 26,760 | 9.6 |
| 75–84 | 20,919 | 6.0 | 15,264 | 5.5 |
| ≥ 85 | 8817 | 2.5 | 6838 | 2.5 |
| Gender | ||||
| Male | 173,383 | 49.8 | 138,588 | 49.8 |
| Female | 174,775 | 50.2 | 139,879 | 50.2 |
| Comorbidity | ||||
| No | 288,594 | 82.9 | 238,106 | 85.5 |
| Yes | 59,564 | 17.1 | 40,361 | 14.5 |
| Antibiotic-prescribing rate (per 1000 patient-years)a,b | 108 (range 4–244) | 114 (range 20–266) | ||
| RTI consultation rate (per 1000 patient-years)a,b | 261 (range 11–454) | 261 (range 76–526) | ||
| Antibiotic-prescribing proportion (%)a,b | 43 (range 12–64) | 43 (range 24–78) | ||
Main results
Initial analyses were conducted using cluster-level statistics. Figure 5 presents a plot of the age-standardised estimates for each general practice for the intervention phase of the trial. The baseline antibiotic-prescribing rate is plotted on the x-axis and the change in antibiotic-prescribing rate (trial period – baseline) on the y-axis. Linear models, weighted for practice size, are plotted by trial arm, together with 95% CIs. There was a wide range of antibiotic-prescribing rates. Both intervention and control trial arm practices showed lower mean antibiotic-prescribing rates during the trial than with baseline, with no clear difference between trial arms. However, changes in antibiotic-prescribing rates showed wide variation at any level of baseline antibiotic prescribing. The data appear to be overdispersed, with several extreme results possibly having undue influence in this representation of the data. In an analysis of covariance, weighted for practice size, the adjusted mean difference between the intervention and control trial arms for the antibiotic-prescribing rate for RTI was –0.5 per 1000 patient-years (95% CI –8.2 to 7.2 per 1000 patient-years).
FIGURE 5.
Scatterplot showing change (trial period – baseline) in age-standardised antibiotic-prescribing rate for each trial practice plotted against the baseline antibiotic-prescribing rate.
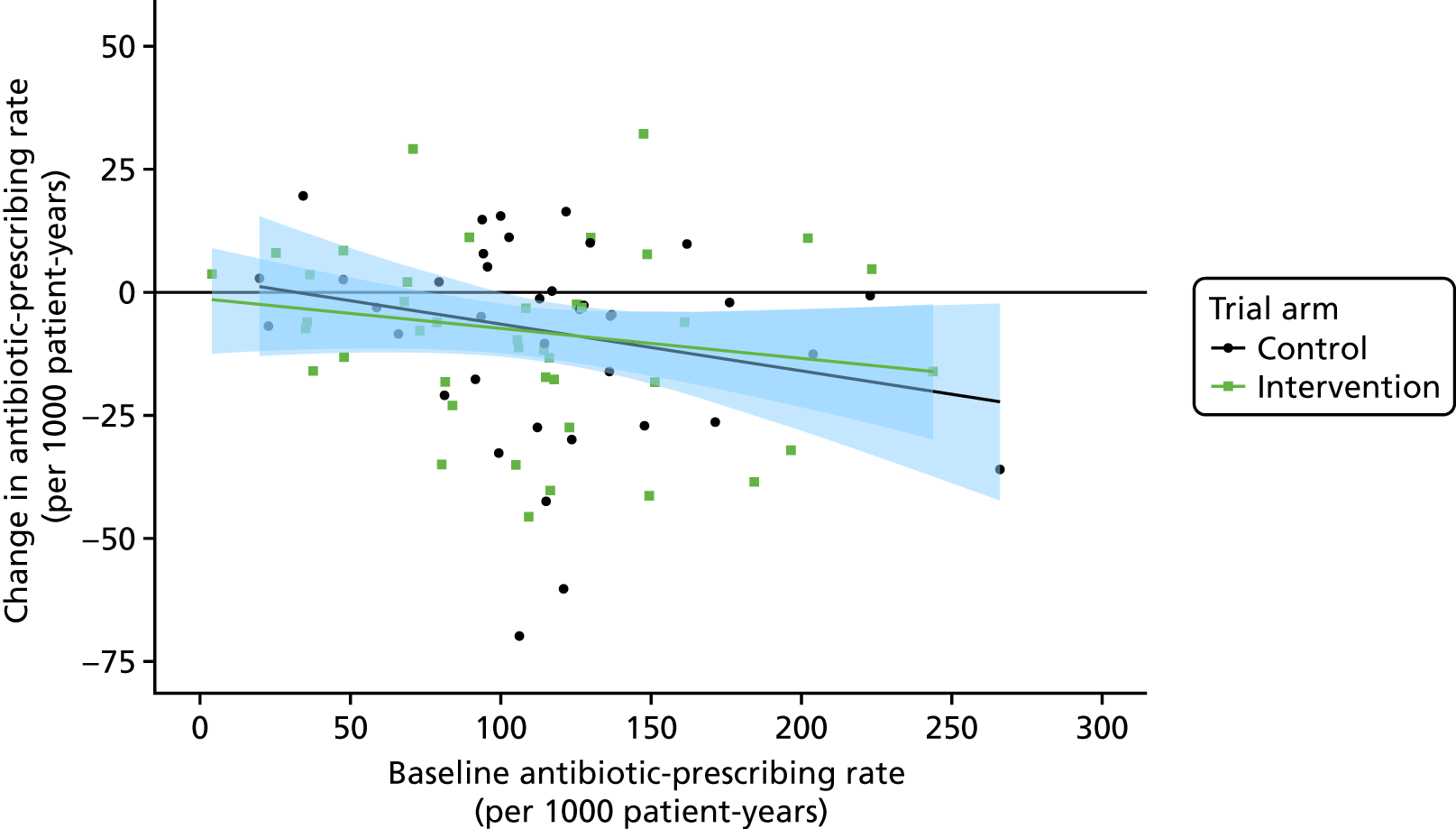
Figure 6 presents a density plot for the distribution of changes in antibiotic-prescribing rate from baseline for the intervention and control trial arms separately. Although the trial analysis was not based on changes from baseline, in a cautious interpretation of Figure 6 there appears to be substantial overlap in the distributions for the two trial arms, although the curve for intervention trial arm practices in green is generally shifted to the left in comparison with control trial arm practices in black. Both Figures 5 and 6 illustrate wide variation among trial general practices, which might make it difficult to draw clear conclusions from the trial.
FIGURE 6.
Density plot showing distribution of change from baseline in antibiotic prescribing at ages for intervention (green) and control (black) trial arms.
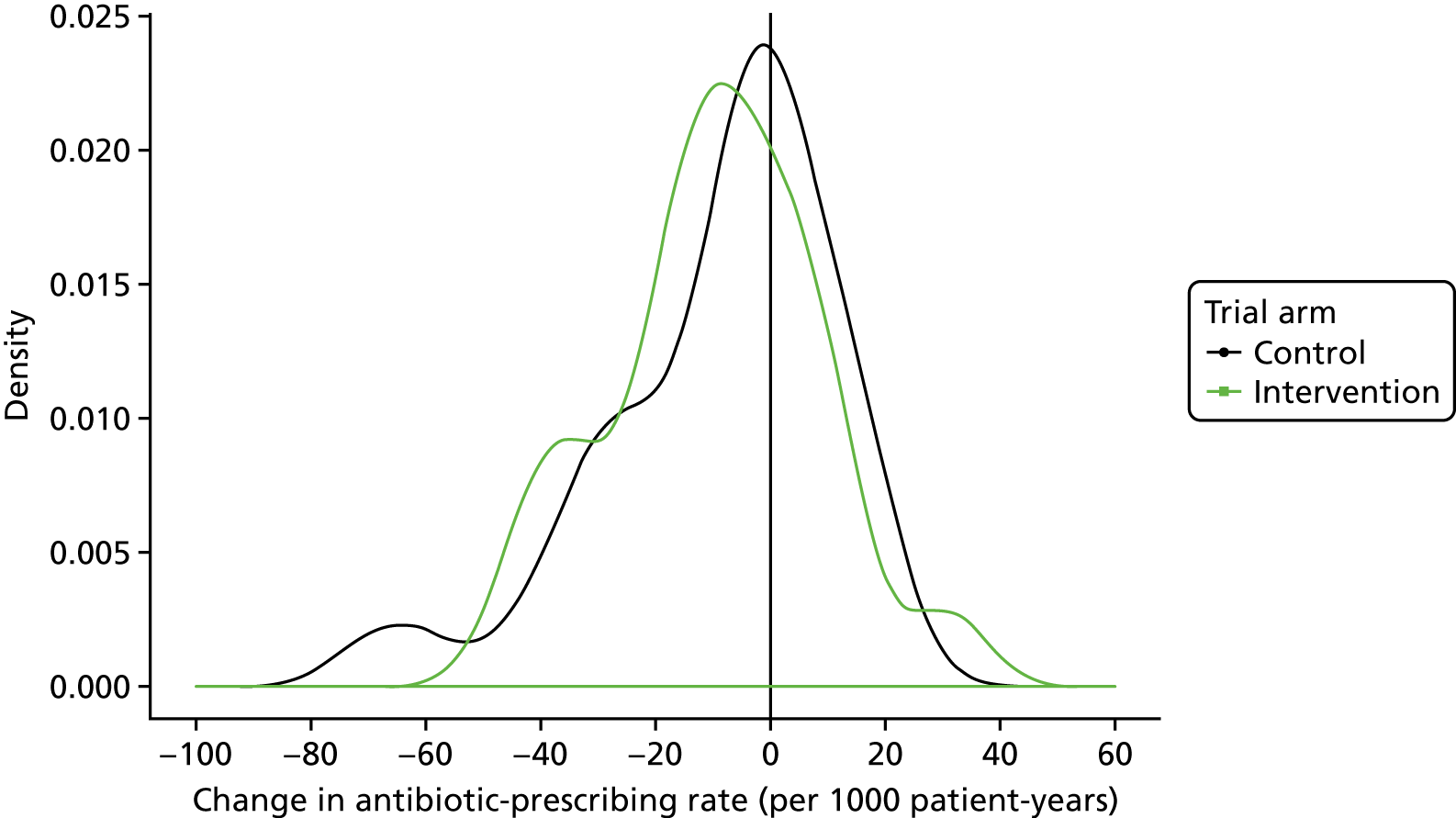
Table 8 presents the results of the primary analysis. There were 31,907 antibiotic prescriptions for RTIs during 323,155 patient-years at 41 intervention trial arm practices and 27,923 antibiotic prescriptions during 259,520 person-years at 38 control trial arm practices. There were 98.7 antibiotic prescriptions per 1000 patient-years in the intervention trial arm and 107.6 per 1000 in the control arm, giving an unadjusted RR of 0.92. The RR for antibiotic prescribing for RTIs was 0.88 (95% CI 0.78 to 0.99; p = 0.040). Point estimates for the RR for RTI consultations (adjusted RR 0.94, 0.86 to 1.03; p = 0.186) and the proportion of antibiotic consultations with antibiotics prescribed (adjusted RR 0.96, 0.89 to 1.03; p = 0.253) were both lower than unity. However, the CIs were wide and the p-values were large, which was consistent with an insufficiently precise estimate being obtained given the size of the trial and the substantial level of variation in these measures.
| Measure | Trial arm | Adjusted RR (95% CI)a | p-value | |
|---|---|---|---|---|
| Intervention | Control | |||
| Antibiotic-prescribing rate | ||||
| Antibiotic prescriptions | 31,907 | 27,923 | 0.88 (0.78 to 0.99) | 0.040 |
| Person-years | 323,155.4 | 259,519.7 | ||
| Crude rate (per 1000 patient-years) | 98.7 | 107.6 | ||
| RTI consultation rate | ||||
| RTI consultations | 78,324 | 66,114 | 0.94 (0.86 to 1.03) | 0.186 |
| Person-years | 323,155.4 | 259,519.7 | ||
| Crude rate (per 1000 patient-years) | 242.4 | 254.8 | ||
| Antibiotic-prescribing proportion | ||||
| Antibiotic prescriptions | 31,907 | 27,923 | 0.96 (0.89 to 1.03) | 0.253 |
| RTI consultations | 78,324 | 66,114 | ||
| Proportion (%) | 40.7 | 42.2 | ||
Intervention effect by the subgroup of age
Respiratory tract infection consultations and antibiotic prescribing were strongly associated with age. In the control trial arm, children aged 0–14 years accounted for 18% of all person-time, but 31% of all RTI consultations and 23% of antibiotic prescriptions for RTIs. People aged 45–54 years accounted for 15% of all person-time, but 11% of all RTI consultations and 12% of all antibiotic prescriptions. People aged 65–74 years accounted for 9% of all person-time, but 12% of antibiotic prescriptions. These figures are presented as per cent of the total person-time in each trial arm and an effect of intervention is not intended to be demonstrated.
There was strong evidence that the effect of the intervention varied by age group. A Wald test of the trial arm by age–group interaction terms gave a p-value of < 0.001. The results of a prespecified subgroup analysis, by age, are shown in Figure 7. There was no evidence of an effect of intervention in children aged < 15 years (adjusted RR 0.96, 0.82 to 1.12) or in people aged ≥ 85 years (adjusted RR 0.97, 95% CI 0.79 to 1.18). At intermediate ages, antibiotic prescribing was lower in the intervention trial arm. Adjusted RRs were 0.84 (95% CI 0.73 to 0.96) for people aged 45–54 years; 0.79 (95% CI 0.69 to 0.91) for 55- to 64-year-olds; 0.80 (95% CI 0.70 to 0.91) for 65- to 74-year-olds; and 0.79 (95% CI 0.69 to 0.91) for 75- to 84-year-olds. Antibiotic prescribing was generally similar across categories of gender and comorbidity.
FIGURE 7.
Effect of intervention for primary outcome, by age group. (a) All ages; and (b) 15- to 84-year-olds. RRs were adjusted for the random effect of general practice and fixed effects of gender, comorbidity, region, quarter in study, practice-specific baseline rate and interaction with period of randomisation. LL, lower limit of CI; UL, upper limit of CI.

Effect modification was summarised by age by comparing effect measures in children, adults aged 15–84 years and senior elderly aged ≥ 85 years (Table 9). The intervention was associated with lower antibiotic prescribing for RTIs in adults aged 15–84 years (adjusted RR 0.84, 95% CI 0.75 to 0.95). During the intervention period the mean rate of antibiotic prescribing for RTIs in adults aged 15–84 years at control trial arm practices was 100.2 per 1000 patient-years. The relative risk reduction was used to estimate an absolute risk reduction of 16.0 (95% CI 5.0 to 25.1) antibiotic prescriptions per 1000 patient-years. In adults, one antibiotic prescription was avoided for every 62 (95% CI 40 to 200) registered patients aged 15–84 years per year. Figure 8 presents a density plot of changes in antibiotic prescribing for adults aged 15–84 years. This reveals a higher proportion of general practices showing reductions in antibiotic prescribing in the intervention trial arm, providing visual confirmation of the estimates provided from statistical modelling.
| Measure | Trial arm | Adjusted RR (95% CI)a | |
|---|---|---|---|
| Intervention | Control | ||
| Antibiotic-prescribing rate (antibiotic/person-years) | |||
| Children aged 0–14 years | 7497/53,826.3 | 6432/46,019.6 | 0.96 (0.82 to 1.12) |
| Adults aged 15–84 years | 23,551/261,841.3 | 20,811/207,611.4 | 0.84 (0.75 to 0.95) |
| Adults aged ≥ 85 years | 859/7487.8 | 680/5888.7 | 0.97 (0.79 to 1.18) |
| RTI consultation rate (RTI/person-years) | |||
| Children aged 0–14 years | 24,365/53,826.3 | 20,820/46,019.6 | 1.02 (0.93 to 1.13) |
| Adults aged 15–84 years | 52,166/261,841.3 | 43,853/207,611.4 | 0.90 (0.82 to 0.98) |
| Adults aged ≥ 85 years | 1793/7487.8 | 1441/5888.7 | 0.93 (0.80 to 1.09) |
| Antibiotic-prescribing proportion (antibiotic/RTI) | |||
| Children aged 0–14 years | 7497/24,365 | 6432/20,820 | 0.96 (0.85 to 1.07) |
| Adults aged 15–84 years | 23,551/52,166 | 20,811/43,853 | 0.95 (0.89 to 1.03) |
| Adults aged ≥ 85 years | 859/1793 | 680/1441 | 1.04 (0.94 to 1.15) |
FIGURE 8.
Density plot showing distribution of change from baseline in antibiotic prescribing at ages 15–84 years for the intervention (green) and control (black) trial arms.
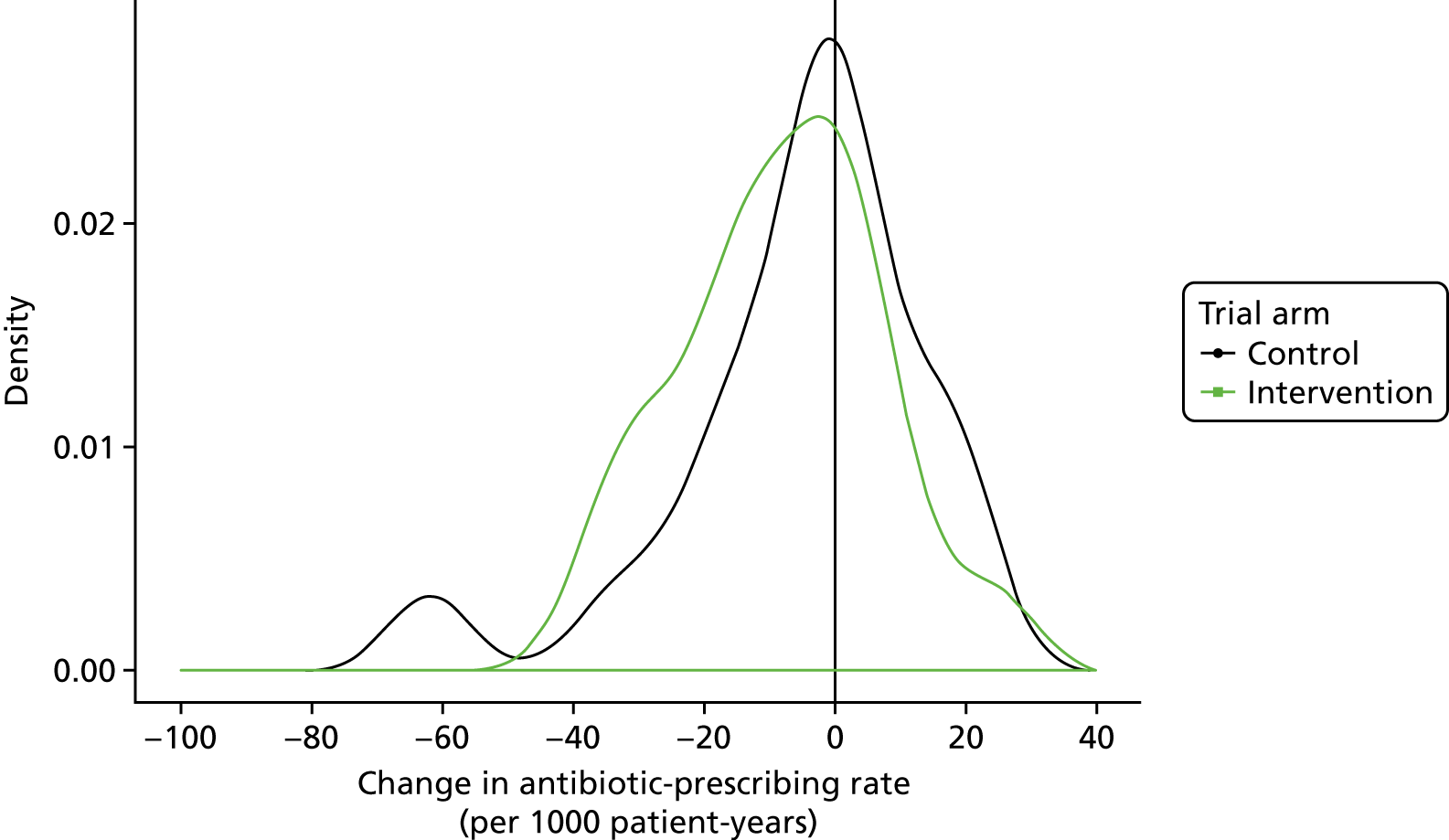
For adults aged 15–84 years, the relative risk estimate was lower than unity for the proportion of RTI consultations with antibiotics prescribed (adjusted RR 0.95, 95% CI 0.89 to 1.03; p = 0.209), but the estimate was imprecise and the p-value was large, which is consistent with a lack of evidence of an effect on this measure (Table 9). There was evidence that, for adults aged 15–84 years, the rate of RTI consultations was lower in the intervention trial arm (adjusted RR 0.90, 95% CI 0.82 to 0.98). These findings suggest that the effect observed for the primary outcome might be more strongly associated with differences in respiratory consultation rates rather than changes in the proportion of RTI consultations with antibiotics prescribed. Figure 9 shows a series of density plots comparing changes in age-standardised general practice-specific values for RTI consultation rates, antibiotic-prescribing rates and proportion of consultations with antibiotics prescribed, for all ages and for the age range 15–84 years. This series shows that RTI consultation rates were generally lower during the trial period than in the baseline period, with similar reductions being observed in both trial arms. Antibiotic-prescribing rates, and the proportion of RTI consultations with antibiotics prescribed, showed substantial overlap, but any reductions tended to be slightly greater in the intervention trial arm. There were several outlying values. This provided visual confirmation of the statistical modelling results.
FIGURE 9.
Density plots showing distribution of change from baseline for RTI consultation rates, antibiotic-prescribing rates and proportion of RTI consultations with antibiotics prescribed at all ages and for ages 15–84 years for the intervention (green) and control (black) trial arms.


Additional subgroup analyses
The intervention effects for the primary outcome (Figure 10) were generally similar for men and women and for comorbidity categories. Estimated effects varied widely among regions, but caution must be observed when drawing firm conclusions because of the small numbers of general practices included in each region. When the primary outcome was analysed by subgroups of baseline antibiotic-prescribing rate quartile, there was no evidence for any consistent pattern of effect. Adjusted RRs were 0.94 (95% CI 0.66 to 1.34) for the lowest quartile, 0.79 (95% CI 0.57 to 1.09) for the second quartile, 0.99 (95% CI 0.81 to 1.20) for the third quartile and 0.99 (95% CI 0.89 to 1.10) for the highest quartile.
FIGURE 10.
Forest plot showing RRs for antibiotic prescribing, by the subgroups of gender, age, comorbidity and region. Estimates were adjusted for the random effect of general practice and the fixed effects of quarter in study, practice-specific baseline rate and interaction with period, as well as each of the variables shown. LL, lower limit of CI; UL, upper limit of CI.

Estimated effects for the proportion of RTI consultations with antibiotics prescribed (Figure 11) showed consistent results for men and women and for comorbidity categories. Estimates by age group were generally consistent with the overall effect, but there was no evidence of a clear pattern of variation by age, though the estimate for people aged ≥ 85 years was consistent with absence of effect. It is relevant to note that the analyses for the antibiotic-prescribing proportion uses a different denominator, that is, the number of RTI consultations, as compared with the analyses for antibiotic-prescribing rate and RTI consultation rate, which both incorporate person-time as denominator.
FIGURE 11.
Forest plot showing RRs for the antibiotic-prescribing proportion, by the subgroups of gender, age, comorbidity and region. Estimates were adjusted for the random effect of general practice and the fixed effects of quarter in study, practice-specific baseline rate and interaction with period, as well as each of the variables shown. LL, lower limit of CI; UL, upper limit of CI.

Sensitivity analyses
Sensitivity analyses were conducted to evaluate whether or not the use of potentially better-fitting models might influence conclusions (Table 10). The rate of respiratory consultations was adjusted for in the trial period. In addition, interactions of covariates with age group was allowed for. Estimates and p-values from these models were generally consistent with the findings from the base-case model. DHGLMs were fitted to address possible overdispersion of the general practice-derived data. In DHGLMs, random effects may be fitted in the dispersion part of the model, as well as in the mean model for the fixed effects. In the DHGLM framework, an overdispersed Poisson model yielded a similar estimate to the base case. In addition, results were compared using EQL and EQL1 estimation, as the former is known to give biased estimates in some data configurations, but the same estimates were obtained with either option.
| Analysis | Intervention effect, adjusted RR (95% CI) | p-value |
|---|---|---|
| Base case | ||
| Model 1 | 0.88 (0.78 to 0.99) | 0.040 |
| Model 1 + | ||
| RTI consultation rate | 0.89 (0.79 to 0.99) | 0.040 |
| Age group × covariate interactions | 0.88 (0.78 to 0.99) | 0.030 |
| DHGLM | ||
| Poisson model 1 | 0.85 (0.75 to 0.97) | 0.014 |
| Overdispersed Poisson model 1 | 0.86 (0.75 to 0.97) | 0.018 |
Total antibiotic prescribing
Table 11 presents data for antibiotic prescribing for all indications during the trial. There were 185,924 antibiotic prescriptions in intervention trial arm practices and 150,539 in control trial arm practices. Crude rates were 575.3 and 581.0 per 1000 patient-years, respectively. The adjusted RR was 0.93 (95% CI 0.83 to 1.04; p = 0.184). There was no evidence that total antibiotic prescribing was reduced by this intervention.
| Measure | Trial arm | |
|---|---|---|
| Intervention | Control | |
| Person-years | 323,155 | 259,520 |
| Antibiotic prescriptions | 185,924 | 150,539 |
| Rate per 1000 patient-years | 575.3 | 581.0 |
| Adjusted RR (95% CI)a | 0.93 (0.83 to 1.04) | |
| p-value | 0.184 | |
Antibiotic prescribing by type of respiratory tract infection
Table 12 presents data disaggregated by type of RTI. Although the proportion of consultations with antibiotics prescribed varied considerably by type of RTI, there was no evidence that the intervention might selectively impact on prescribing for any single subgroup of RTI. Adjusted RRs were all lower than unity, but there was no evidence that the intervention could be judged to be effective in any subgroup of RTI considered separately.
| Type of RTI | Trial arm | RR (95% CI)a | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Consultations (n) | Antibiotic prescriptions (n) | Per cent | Consultations (n) | Antibiotic prescriptions (n) | Per cent | ||
| Colds and URTI | 15,571 | 3304 | 21.2 | 12,892 | 3072 | 23.8 | 1.00 (0.69 to 1.44) |
| Cough and bronchitis | 38,337 | 15,152 | 39.5 | 32,743 | 13,109 | 40.0 | 0.85 (0.71 to 1.03) |
| Otitis media | 5932 | 3282 | 55.3 | 4486 | 2647 | 59.0 | 0.93 (0.75 to 1.14) |
| Rhinosinusitis | 3214 | 2552 | 79.4 | 2921 | 2391 | 81.9 | 0.90 (0.69 to 1.18) |
| Sore throat | 15,270 | 7617 | 49.9 | 13,072 | 6704 | 51.3 | 0.92 (0.79 to 1.08) |
Deferred prescriptions
Table 13 shows the number of deferred prescriptions by trial arm and period. During the intervention period of the trial, 20 control practices and 22 intervention trial arm practices recorded no deferred antibiotic prescriptions. There were three control practices and six intervention trial arm practices that recorded > 50 deferred antibiotic prescriptions. Across all practices, the rate of deferred antibiotic prescriptions was similar in the intervention and control trial arms and did not increase after the introduction of the intervention.
| Trial arm | Trial arm | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Number of deferred antibiotic prescriptions | Rate per 1000 patient-years | Number of deferred antibiotic prescriptions | Rate per 1000 patient-years | |
| Baseline | 874 | 2.84 | 627 | 2.53 |
| Intervention | 878 | 2.72 | 503 | 1.94 |
Safety outcomes
The safety outcomes for the trial were those agreed by the Data Monitoring Committee and the study team. Table 14 presents the number of safety outcomes and rates per 100,000 patient-years by trial arm. Figure 12 presents a forest plot showing relative incidence rates for each safety outcome. There was no evidence to suggest that any of these outcomes were more frequent in the intervention trial arm. Estimates were imprecise for rare outcome events. Figure 13 shows the distribution of counts of safety events by age group and trial arm. Scarlet fever was the most frequently recorded outcome in children; pyelonephritis and peritonsillar abscess were most frequent at intermediate ages, whereas pneumonia was the most frequent outcome at older ages. It may be noted that the denominator population is slightly larger for the intervention trial arm. There was weak evidence that scarlet fever might have been more frequent in the control trial arm, but this might have been a chance finding.
| Condition | Trial arm | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Number | Rate (per 100,000 patient-year) | Number | Rate (per 100,000 patient-year) | |
| Pneumonia | 367 | 113.6 | 299 | 115.2 |
| Pyelonephritis | 115 | 35.6 | 84 | 32.4 |
| Scarlet fever | 60 | 18.6 | 72 | 27.7 |
| Peritonsillar abscess | 49 | 15.2 | 42 | 16.2 |
| Septic arthritis | 13 | 4.0 | 14 | 5.4 |
| Osteomyelitis | 21 | 6.5 | 11 | 4.2 |
| Mastoiditis | 7 | 2.2 | 7 | 2.7 |
| Meningitis | 9 | 2.8 | 6 | 2.3 |
| Empyema | 13 | 4.0 | 4 | 1.5 |
| Intracranial abscess | 1 | 0.3 | 3 | 1.2 |
| Toxic shock/septicaemia | 7 | 2.2 | 4 | 1.5 |
| Lemierre syndrome | 0 | 0 | 1 | 0.4 |
FIGURE 12.
Forest plot showing RRs (95% CI) of safety outcomes in the intervention trial arm compared with the control trial arm as reference. Estimates were from a Poisson model adjusted for age group, gender and comorbidity. Analyses for pneumonia were adjusted for the random effect of general practice. LL, lower limit of CI; UL, upper limit of CI.
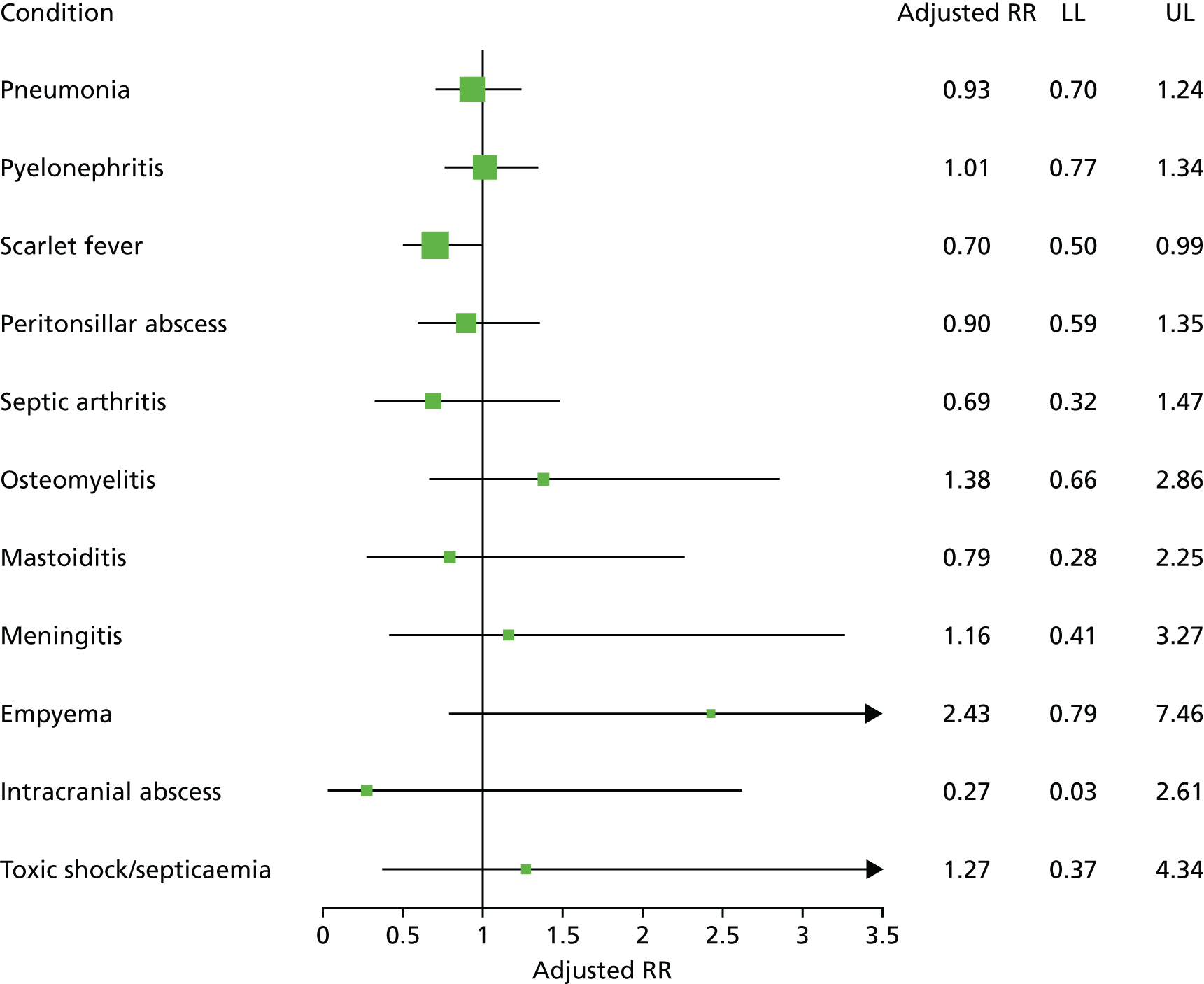
FIGURE 13.
The number of safety outcomes, by trial arm and age group.

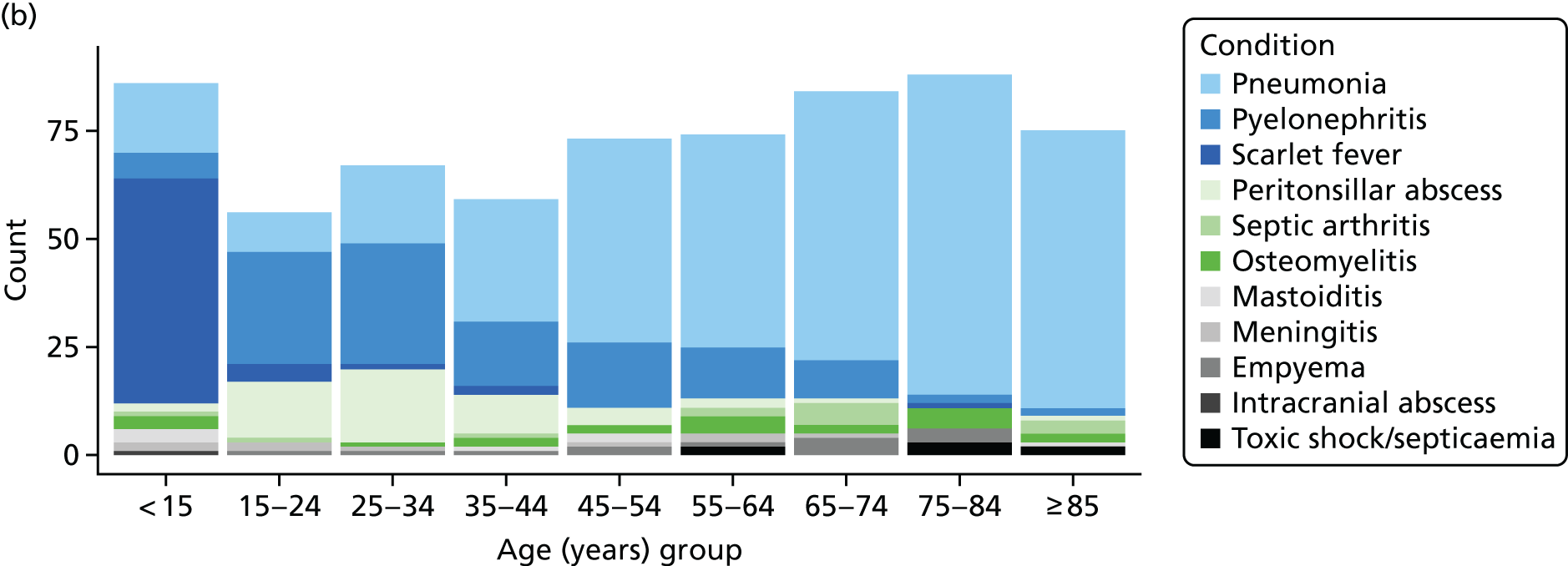
Process evaluation
The trial outcome measures were evaluated in relation to the level of utilisation of DSTs at the intervention trial arm practices. In the lowest quartile of utilisation, DSTs were viewed at < 1% of RTI consultations. In the highest quartile, up to 28% of RTI consultations were associated with DST viewing (Table 15). In adults aged 15–84 years, there was evidence of a linear trend across quartiles of DST utilisation (adjusted RR 0.96, 95% CI 0.93 to 0.99). This association was not evident for children (adjusted RR 0.98, 95% CI 0.94 to 1.03), the senior elderly (adjusted RR 0.99, 95% CI 0.94 to 1.05) and, only weakly, for the sample as a whole (adjusted RR 0.97, 95% CI 0.93 to 1.00; p = 0.043).
| Questionnaire item | Agree or strongly agree, frequency (%) |
|---|---|
| The REDUCE trial prescribing reports of antibiotics for RTIs . . . | |
| were easy to understand | 93.5 |
| I was pleased to receive the prescribing report data | 93.5 |
| were discussed among clinicians at my practice | 89.1 |
| presented credible data on antibiotic-prescribing rates | 84.8 |
| were easy to reada | 82.6 |
| were useful for my practice | 82.6 |
| encouraged me to reduce antibiotic prescribing for RTIs | 78.3 |
| had impact on the prescribing of antibiotics for RTIs at my practicea | 76.1 |
| have helped to reduce the prescribing of antibiotics for RTIs at my practice | 69.6 |
| The REDUCE trial webinar . . . | |
| there no were problems with the webinar streaminga | 100.0 |
| was not too longa | 96.8 |
| was relevant to my clinical practicea | 93.5 |
| was easy to access | 87.1 |
| encouraged me to try out the computer-delivered support tools | 87.1 |
| encouraged me to read the monthly prescribing reports for the REDUCE trial | 77.4 |
| The REDUCE trial DSTs . . . | |
| prescription indication pages were easy to read during a consultation for RTIs | 80.8 |
| there were no problems accessing the tools during a consultation for RTIsa | 76.9 |
| appeared at an appropriate time during consultations for RTIs | 73.1 |
| were not too slow to use during RTI consultationsa | 73.1 |
| patient information leaflet pages were easy to select during a consultation | 73.1 |
| patient information leaflet pages supported me in reducing antibiotic prescribing | 65.4 |
| prescription indication pages supported me in reducing the prescribing of antibiotics | 61.5 |
Table 16 shows the responses of GPs to the process evaluation questionnaire items. There were 51 respondents from 31 out of 41 (76%) intervention trial arm practices. Respondents generally gave positive responses to items concerning the monthly antibiotic-prescribing reports. Respondents were pleased to receive the reports and found that they provided credible data, which were was easy to read and understand. Respondents discussed these with colleagues and found that these data were useful for their practice. However, items concerning the possible impact on antibiotic prescribing were generally slightly less favourably addressed, with < 80% of respondents agreeing that the reports encouraged them to reduce antibiotic prescribing or had impact on the prescribing of antibiotics at the practice. The webinar was generally favourably received. The DSTs tended to be slightly less favourably received than the prescribing reports, with nearly one-third of respondents not affirming that the tools might support their reduction in antibiotic prescribing.
| Group | RTI consultations with DSTs viewed (%) | Age group, antibiotic/person-years | |||
|---|---|---|---|---|---|
| All | Children aged 0–14 years | Adults aged | |||
| 15–84 years | ≥ 85 years | ||||
| Control trial arm | – | 27,923/259,519.7 | 6432/46,019.6 | 20,811/207,611.4 | 680/5888.7 |
| Lowest quartile | 0 to 0.6 | 7190/85,805.1 | 1932/15,699.9 | 5089/68,220.1 | 169/1885.1 |
| Second quartile | 0.6 to 2.9 | 7765/74,868.3 | 1706/12,009.4 | 5837/60,825.5 | 222/2033.4 |
| Third quartile | 2.9 to 6.1 | 10,647/91,986.9 | 2339/15,233.4 | 7957/74,735.5 | 351/2018.0 |
| Highest quartile | 6.1 to 27.6 | 6305/70,495.1 | 1520/10,883.6 | 4668/58,060.1 | 117/1551.3 |
| Test for linear trend, adjusted RR (95% CI)a,b | 0.97 (0.93 to 1.00) | 0.98 (0.94 to 1.03) | 0.96 (0.93 to 0.99) | 0.99 (0.94 to 1.05) | |
| p-value | 0.043 | ||||
Cost analysis
The total costs of health-care utilisation were estimated for patients who made one or more consultations for RTIs during the trial intervention period. Costs were evaluated from the date of the index consultation until the end of the trial and were scaled to annual costs using the person-time available for analysis. During the trial there were 144,438 RTI consultations by 106,219 patients. Costs of health-care utilisation were estimated for 105,389 (99%) patients. The overall distribution of health-care costs per patient-year are shown by trial arm in the Table 17.
| Trial arm | Number | 25% | 50% | Mean | 75% |
|---|---|---|---|---|---|
| Intervention | 57,616 | 233 | 508 | 1001 | 1107 |
| Control | 47,773 | 236 | 535 | 1052 | 1178 |
The coefficient associated with intervention trial arm was –0.081 (standard error 0.078); after exponentiation this may be interpreted as a multiplicative factor, showing that costs in the intervention trial arm were 92% of those observed in the control trial arm. The 95% CI was from 79% to 107%, with a p-value of 0.298. Therefore, the trial provided no evidence that the total costs of health-care utilisation might differ as a result of intervention, at least during the time horizon of the trial.
Chapter 5 Results: cohort study of safety outcomes
Material in this section is reproduced from Gulliford et al. 31 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
Data were analysed for 610 CPRD general practices, with 45,465,200 registered person-years of observation over the period from 2005 to 2014. The RTI consultation rate continued a long-term decline7 during the period from 2005 to 2014, reducing it from 256 to 220 per 1000 patient-years in men and from 351 to 307 per 1000 patient-years in women. The antibiotic-prescribing rate for RTI declined from 128 to 106 per 1000 patient-years in men and from 184 to 155 per 1000 patient-years in women. The proportion of RTI consultations with antibiotics prescribed declined from 53.9% to 50.5% in men, and from 54.5% to 51.5% in women.
General practices were divided into quartiles on the basis of the proportion of RTI consultations with antibiotics prescribed (Table 18). General practices in the highest quartile prescribed antibiotics at a median of 65% (range 58–79%) of RTI consultations, whereas general practices in the lowest quartile prescribed antibiotics at a median of 38% (range 29–44%) of RTI consultations. Age-standardised incidence rates for each of the infective complications are shown in Table 18. The incidence of pneumonia was 157 (95% CI 154 to 159) per 100,000 patient-years at low-prescribing practices, but 119 (95% CI 117 to 121) per 100,000 patient-years at high prescribing practices. The incidence of peritonsillar abscess was 15.6 (95% CI 15.5 to 15.8) per 100,000 patient-years at low-prescribing practices, but 12.9 (95% CI 12.8 to 13.0) per 100,000 patient-years at high prescribing practices. Mastoiditis, empyema and bacterial meningitis and intracranial abscess showed lower rates of incidence rates, which did not appear to be associated with antibiotic-prescribing category. There were 14 cases of Lemierre syndrome, evenly distributed between prescribing categories, with an overall incidence rate of 0.31 per one million patient-years.
| Variables | Quartiles of proportion of RTI consultations with antibiotics prescribed | |||
|---|---|---|---|---|
| High ≥ 58% | 51–58% | 44–51% | Low < 44% | |
| Number of general practices | 152 | 153 | 152 | 153 |
| Number of person-years from registered patients | 10,573,885 | 12,135,183 | 12,109,005 | 10,647,128 |
| Proportion of RTI consultations with antibiotics prescribed (median, 95% range) | 65 (58–79) | 54 (51–57) | 48 (45–51) | 38 (29–44) |
| Age- and gender-standardised incidence rate per 100,000 (95% CI) | ||||
| Pneumonia | 119.2 (117.0 to 121.3) | 129.1 (126.9 to 131.2) | 156.4 (154.0 to 158.7) | 156.6 (154.0 to 159.1) |
| Peritonsillar abscess | 12.9 (12.8 to 13.0) | 13.2 (13.1 to 13.3) | 14.1 (13.9 to 14.2) | 15.6 (15.5 to 15.8) |
| Mastoiditis | 3.48 (3.37 to 3.60) | 3.31 (3.21 to 3.42) | 3.32 (3.19 to 3.46) | 3.38 (3.25 to 3.51) |
| Empyema | 3.64 (3.27 to 4.01) | 4.00 (3.63 to 4.37) | 3.66 (3.31 to 4.01) | 4.00 (3.61 to 4.40) |
| Bacterial meningitis | 2.19 (1.90 to 2.47) | 2.16 (1.90 to 2.42) | 2.24 (1.97 to 2.51) | 2.45 (2.15 to 2.75) |
| Intracranial abscess | 0.37 (0.25 to 0.48) | 0.35 (0.24 to 0.46) | 0.55 (0.42 to 0.69) | 0.42 (0.29 to 0.55) |
| Lemierre syndrome | 4 cases | 3 cases | 2 cases | 5 cases |
A general practice with the mean list size for England of 7000 registered patients is expected to have 20,300 (95% range 11,340–30,380) consultations for RTIs over a period of 10 years (Table 19). A general practice of this size, with an average RTI consultation rate, may issue 13,195 (95% range 11,744–16,037) antibiotic prescriptions during this period if it is in the highest prescribing quartile. If the practice reduces the proportion of RTI consultations with antibiotics prescribed by 10%, the general practice will issue 20,300 (95% range 11,340–30,380) fewer antibiotic prescriptions for RTIs. This reduction in antibiotic prescribing is expected to be associated with 11 (95% range 6–15) more cases of pneumonia per decade, and 0.9 (95% range 0.5–1.3) more cases of peritonsillar abscess per decade (Table 19). Mastoiditis, empyema, bacterial meningitis, intracranial abscess and Lemierre syndrome are not expected to increase.
| Measure | Number expected in a general practice with 7000 patients over 10 years | Change over 10 years after a 10% absolute decrease in the proportion of RTI consultations with antibiotics prescribed |
|---|---|---|
| RTI consultations | 20,300 (11,340 to 30,380) | 0 |
| Antibiotic prescriptions for RTI | 13,195 (11,744 to 16,037)a | –2030 (–3038 to –1134)b |
| Number of first episodes | ||
| Pneumonia | 83 (82 to 85) | 11 (6 to 15) |
| Peritonsillar abscess | 9 (9 to 9) | 0.9 (0.5 to 1.3) |
| Mastoiditis | 2 (2 to 3) | 0 |
| Empyema | 3 (2 to 3) | 0 |
| Bacterial meningitis | 2 (1 to 2) | 0 |
| Intracranial abscess | < 1 | 0 |
Figure 14 shows the association between the antibiotic-prescribing rate and infective complications. Pneumonia showed an association with the antibiotic-prescribing rate [incidence rate ratio (IRR) 0.74, 95% range 0.58–0.95; p = 0.02], peritonsillar abscess showed a weak association (IRR 0.84, 95% range 0.68–1.03; p = 0.09), but the other bacterial infections did not.
FIGURE 14.
Association of incidence of infective complications with quartile of antibiotic-prescribing rate. IRRs were adjusted for RTI consultation rate, gender, age group, region, deprivation quintile and clustering by general practice. AB rate, rate of antibiotic prescriptions for RTIs per 1000 registered patients; Ref., reference category.
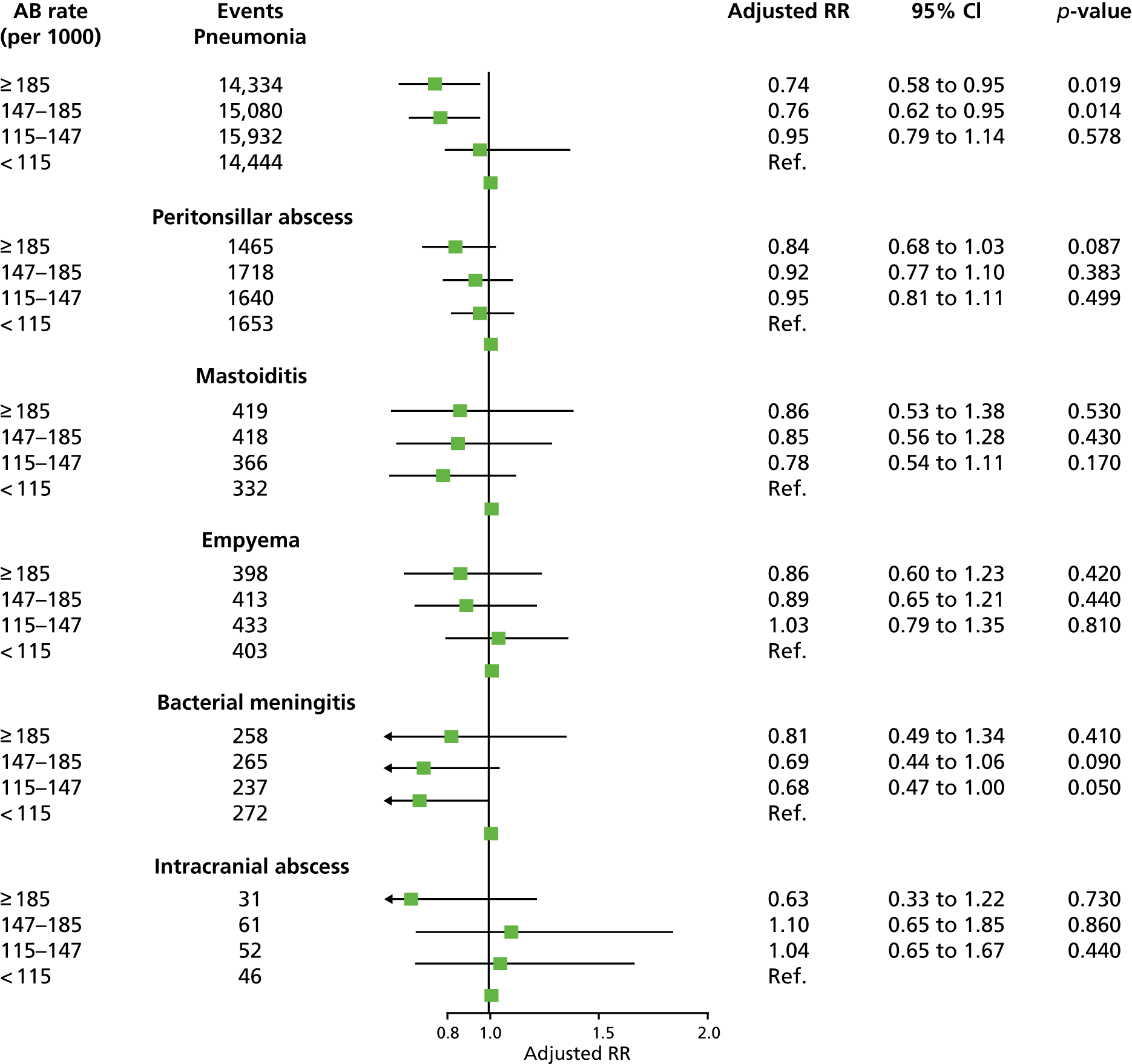
Table 20 shows IRRs estimated after fitting the predictors as continuous variables. These estimates are consistent with linear associations; adding quadratic terms did not improve the goodness of fit. Increasing the RTI consultation rate was associated with increasing incidence of pneumonia and mastoiditis. Increasing antibiotic-prescribing proportion was associated with a declining incidence of pneumonia and peritonsillar abscess. Each 10% increase in antibiotic-prescribing proportion was associated with a 12.8% (95% range 7.8–17.5%) relative decrease in pneumonia, and a 9.9% (95% range 5.6–14.0%) decrease in peritonsillar abscess. Associations with the antibiotic-prescribing rate fitted as a linear predictor were consistent with those for the antibiotic-prescribing proportion.
| Bacterial infection | RTI consultation rate (10 consultations per 1000 patient-years) | Antibiotic-prescribing rate for RTIs (10 antibiotic prescriptions for RTIs per 1000 patient-years) | Proportion of RTI consultations with antibiotics prescribed (for 10% increase) | |||
|---|---|---|---|---|---|---|
| RRa (95% CI) | p-value | RRb (95% CI) | p-value | RRb (95% CI) | p-value | |
| Pneumonia | 1.015 (1.008 to 1.023) | < 0.001 | 0.959 (0.941 to 0.976) | < 0.001 | 0.87 (0.83 to 0.92) | < 0.001 |
| Peritonsillar abscess | 1.004 (0.998 to 1.010) | 0.18 | 0.968 (0.953 to 0.982) | < 0.001 | 0.90 (0.86 to 0.94) | < 0.001 |
| Mastoiditis | 1.020 (1.005 to 1.034) | 0.007 | 1.008 (0.973 to 1.044) | 0.67 | 1.00 (0.90 to 1.12) | 0.95 |
| Empyema | 1.005 (0.995 to 1.016) | 0.35 | 0.979 (0.953 to 1.005) | 0.11 | 0.93 (0.86 to 1.01) | 0.10 |
| Bacterial meningitis | 1.001 (0.987 to 1.016) | 0.86 | 0.986 (0.949 to 1.023) | 0.45 | 0.94 (0.84 to 1.06) | 0.30 |
| Intracranial abscessc | 1.003 (0.984 to 1.022) | 0.75 | 0.986 (0.938 to 1.035) | 0.57 | 0.94 (0.81 to 1.09) | 0.40 |
A general practice with the mean list size for England of 7000 registered patients is expected to have 20,300 (95% range 11,340–30,380) consultations for RTIs over a period of 10 years (Table 20). A general practice of this size, with an average RTI consultation rate, may issue 13,195 (95% range 11,744–16,037) antibiotic prescriptions during this period if it is in the highest prescribing quartile. If the practice reduces the proportion of RTI consultations with antibiotics prescribed by 10% it will issue 2030 (95% range 11,340–30,380) fewer antibiotic prescriptions for RTIs. This reduction in antibiotic prescribing is expected to be associated with 11 (95% range 6–15) more cases of pneumonia per decade, and 0.9 (95% range 0.5–1.3) more cases of peritonsillar abscess per decade (Table 20). The number of cases of mastoiditis, empyema, bacterial meningitis, intracranial abscess and Lemierre syndrome is not expected to increase.
Chapter 6 Discussion
Main findings of the intervention development study
The intervention development study led to the refinement of the intervention materials for the trial, including electronically delivered antibiotic-prescribing reports and DSTs, supported by a webinar. The development study also provided insights into the preparedness of health professionals to engage in this antimicrobial stewardship intervention. The analysis revealed two main themes: the perception that the problem of overprescribing is not individually applicable and the perception that the researchers responsible for planning interventions in primary care do not understand how general practices operate.
Participants considered that they were not personally responsible for high levels of antibiotic prescribing, sometimes taking the view that the problem of overprescribing did not apply to them. This is inconsistent with analysis of data from 568 UK general practices contributing to the CPRD, which showed an overall antibiotic-prescribing proportion of between 50% and 60% for RTIs. 8 Such rates of prescribing suggest that most UK GPs and NPs prescribe at rates in excess of the current recommendations. 17 This perception may result from the ‘better than average effect’72 – a phenomenon in which people perceive themselves as special and feel that a special set of psychological rules apply to them. McKay and Dennett73 have argued that the illusion of superiority is an evolved human trait, serving an adaptive function by motivating adaptive behaviours. This illusion of superiority might be resistant to modification and should be recognised as part of normal human functioning.
A second overarching theme was the perception that researchers do not understand the demands of general practice. Most of the clinicians interviewed considered that clinical uncertainty concerning RTIs was low and were confident in diagnosing RTIs. However, convincing patients that antibiotics are not needed for a RTI episode was time-consuming and frustrating, and this perception was strengthened by the high-pressure working environment. Many participating GPs expressed the view that they have been de-professionalised in the process of achieving targets and pursing financial incentives, to the detriment of patient-centred care. Some GPs perceived certain elements of the intervention as being potentially unhelpful, with frequent audits being inconsistent with patient demands. The small risk of adverse complications representing an important concern in the context of performance review. These factors combined undermine GPs’ sense of professional competency and create anxiety. Therefore, it is possible that the intervention was not perceived as a possible source of support for GPs, resulting in less likelihood of using these tools. Similar barriers have been reported in the implementation literature,74,75 literature on GP morale76,77 and studies exploring the reasons why GPs leave practice early. 78
Although participants expressed concerns about the target-driven NHS culture and de-professionalised role of a GP, at the same time a majority of interviewed clinicians considered that funding to incentivise antibiotic reduction initiatives should be provided, as this would demonstrate the importance of reducing antibiotic prescribing to the NHS and would promote engagement. 79,80 Although the introduction of the pay-for-performance scheme in the NHS in 2004/2005 was associated with some improvements in quality of care for chronic diseases, evidence also suggest that practices divert their attention to activities associated with incentives. 81 It is possible that the scheme has contributed to the paradox reported by clinicians in this study, when they recognise the negative aspects of the target paradigm (i.e. improvements are achieved at the expense of those aspects of care that are not incentivised) and when clinicians are focused on ‘ticking the boxes’ rather than demonstrating empathy towards the patient.
Previous reviews of qualitative data suggest that GPs would welcome interventions that would bring benefits once implemented into general practice. 82 One such benefit in the REDUCE trial intervention is decreased workload, as patients who are not prescribed antibiotics for RTIs are less likely to consult in the future. 83 However, interviewed clinicians were concerned that involvement would have negative consequences in terms of workload and time. It is possible, therefore, that the tools developed for this study were not successful at conveying the message that the intervention would not disrupt usual daily practice and might bring future benefits in terms of a reduced number of consultations for RTIs.
The results demonstrate the potential limitations of implementing individual-level interventions, even when informed by a substantial understanding of user perspective and grounded in well-established behaviour change theory. A review of interventions to reduce unnecessary antibiotic prescribing found only a modest reduction of 9.7% (interquartile range 6.6–13.7%) in the proportion of patients receiving antibiotics. 20 More recent studies have achieved similar reductions, although the GRACE [Genomics to combat Resistance against Antibiotics for Community-acquired lower respiratory tract infection (LRTI) in Europe] trial reported a reduction of 15%. 21 Factors identified in this study, such as lack of perception of personal responsibility for changing antibiotic prescribing, might be less amenable to individual-level interventions. Attempts to curtail the clinical autonomy of GPs are likely to be counterproductive and the findings suggest that approaches aligned to the professional values of GPs are likely to be more acceptable.
Limitations of the intervention development study
The intervention development study included a convenience sample of GPs and NPs from non-trial practices. It is possible that those who volunteered for the study could be more motivated by, for example, vocational interest or a sense of professional duty. It is also not known whether the GPs recruited were comparable either with the national GP profile (e.g. representing group vs. single handed practices) or with those who later took part in the trial. The study cannot be sure whether or not intervention development respondents represented a mix of low and high antibiotic prescribers. The results of the interview study might overestimate the importance of individual-level factors and underestimate the importance of patient-level factors, organisational factors and the cultural and social context. Each interview was conducted using the same interview guide, but the differences in interviewing styles might have elicited different responses. Finally, this study asked clinicians about hypothetical, rather than the actual, use of the intervention, reducing the generalisability of our findings. Previous research suggests that initial scepticism and belief that the intervention would not be beneficial diminished over time and with experience in using the intervention that may not have been captured in our study. 84
Main findings of the cluster randomised trial
The cluster randomised trial evaluated the effectiveness of a complex electronically delivered intervention including antibiotic-prescribing reports and DSTs, supported by a webinar, at reducing antibiotic prescribing for self-limiting RTIs to people of all ages in primary care. The study provided evidence that these interventions might be associated with lower overall antibiotic prescribing for RTIs in primary care, but, given the size of the trial, this evidence may not be viewed as strong. This might be accounted for, in part, by the wide variations in practice that exist. It was observed that antibiotic utilisation for RTIs is patterned by age, with children accounting for a high proportion of all RTI consultations and antibiotic prescriptions. 9 There was no evidence that the intervention was effective at reducing antibiotic prescribing to children or the very old. In the adult population aged 15–84 years, there was evidence that antibiotic utilisation for RTIs was 16% lower in the intervention trial arm. The reduction in antibiotic utilisation was greater at practices that used the trial DSTs more frequently, which suggests a causal association. The study did not find evidence that the secondary outcome of proportion of RTI consultations with antibiotics prescribed was reduced. There was no evidence that the costs of health-care utilisation differed between trial arms over the course of the trial.
The agreed aim of the study, following discussion between the study team and the funders, was to address antibiotic prescribing in people of all ages. The trial general practice prescribing reports presented data aggregated across age groups. This was justified because any effective strategy must be shown to reduce antibiotic utilisation across the whole population. The results of the trial suggest that future interventions should stratify feedback and advice by age group. The trial DSTs specifically addressed common diagnostic concerns in children, including otitis media and cough and bronchitis. Prescribing to the youngest and oldest age groups may be more difficult to modify because safety concerns may be more salient at these ages. More research is needed to evaluate safety outcomes for more vulnerable subgroups of the population.
Strengths and limitations of the cluster randomised trial
The trial was conducted in the context of system-wide efforts to reduce unnecessary antibiotic prescribing in primary care. The NHS introduced an incentive known as the ‘Quality Premium’ to encourage reduced antibiotic utilisation. Antimicrobial stewardship interventions became available from a number of national and local sources. Perhaps as a result of some of these interventions, the English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) found that there was a 13% decrease in the number of antibiotic prescriptions dispensed from GP settings between 2012 and 2016. These system-wide interventions will have ‘contaminated’ both trial arms of the study. It is possible that a greater intervention effect might have been observed in their absence.
The intervention materials were successfully delivered into intervention trial arm practices. The DSTs were triggered when a Read code was entered into the general practice system. Thus, there was no control over the exact point during the consultation at which the intervention would be triggered. Practitioners had discretion over whether or not to view the intervention materials; viewing of the materials was not ‘forced’ through active-alerting or ‘pop-ups’ because these have been associated with negative feedback in previous studies. The study included a large sample of general practices. These were drawn from all parts of the UK including England, Scotland, Wales and Northern Ireland, including all registered patients in trial analyses. These features of the study enhanced the external validity of the study. However, it is possible that general practices that agreed to take part in the study might be more motivated to reduce antibiotic prescribing. At the time of the study, data could only be used from CPRD general practices employing the Vision practice system, many of which, during the course of the study, migrated to other practice systems and thus could not provide data to the REDUCE trial. There was a smaller pool of eligible practices than anticipated, the final number of practices included was smaller than originally intended and several practices were unable to continue with the trial before the end of the intervention period because they transferred to a different practice system. It should be noted that since this study was completed, the CPRD has increased its coverage to almost 1000 practices [the majority of which use the EMIS GP software system (EMIS Health, London, UK)] and the CPRD general practice coverage continues to grow. Data from EMIS practices could not be used in this study, but data are planned to be made available for future studies with the CPRD. Additionally, there was very wide variation in antibiotic prescribing, with different general practices prescribing antibiotics at as few as 12% or as many as 78% of RTI consultations. The sample size calculation did not allow for this overdispersion. Interventions might offer little benefit to those practices that had low prescribing rates. Consequently, the effect of intervention was not estimated precisely and there may be a risk of overlooking small effects that could be of clinical or public importance. Based on prespecified subgroup analysis, the study identified effects in adults aged 15–84 years. Interpretation of effects for subgroups carries a risk of false-positive interpretation. However, it was found that utilisation of intervention DSTs was associated with effect size, which points to a causal association. The study analysed data for antibiotic prescriptions issued by trial general practices. Patients may have received antibiotic prescriptions at consultations with walk-in centres and out-of-hours or emergency services; some antibiotic prescriptions may have been issued as delayed or deferred prescriptions but not recorded as such. It is possible that these alternative patterns of antibiotic utilisation differed between the trial arms, potentially contributing to biased assessment of intervention effects. It is also possible that altered diagnostic code selection may have occurred in order to justify antibiotic prescriptions. 85 There was necessarily no blinding of general practice staff to the intervention, which included feedback of counts of antibiotic prescriptions that were the outcome for the study. The study employed a hierarchical random-effects model for analysis. This provided estimates that can be interpreted as being conditional on cluster/general practice membership. Marginal population-averaged estimates are sometimes recommended in randomised trials, but Lee and Nelder86 showed that marginal predictions may also be obtained from a conditional model and this will usually have advantages compared with a marginal model. The data obtained from general practices showed evidence of overdispersion, but models were fitted that allow for overdispersion and these provided similar estimates. There was some evidence for regional variation in intervention effects and these could be investigated further, perhaps through qualitative research.
At the design stage of the project, it was concluded, through discussion with a health economist, that attempting a cost-effectiveness analysis would not be appropriate for this project. The study focused on the costs of health-care utilisation on the grounds that decision-makers need to be aware of whether or not an intervention leads to overall increased costs. The study did not identify any difference in health-care utilisation between general practices in the intervention and control trial arms. However, the study did not have access to linked Hospital Episode Statistics data for all trial practices, which would have strengthened the assessment of hospital utilisation. The research was conducted within the set of general practices contributing to the CPRD, which represents an existing research infrastructure. The CPRD draws on a single general practice information system; practices were further selected for this study that used, or were prepared to use, the DXS system. The immediate costs of delivering the intervention comprised the fees paid to the CPRD and DXS for work on the trial and for EHR data, as well as costs of staff time and materials for developing the intervention materials. The resources required to roll out a similar intervention into routine practice in the NHS might differ. On the one hand, economies of scale might reduce the marginal cost of delivering the intervention to a single general practice. On the other hand, there will be significant logistical challenges associated with working across general practices that employ different practice systems, sometimes with no established systems for data collection and analysis. Consequently, further feasibility work to evaluate resource requirements and logistical issues will be required before any possible scaling up of these interventions. Caution is also needed, as the intervention effects estimated in this 12-month trial might not be sustained in the longer term.
Comparison of trial results with other studies
Previous studies of audit and feedback interventions show that these often have only small effects. 29 Roshanov et al. 29 found that feedback interventions that provide advice to patients as well as physicians are associated with a greater chance of success. This was exemplified in the REDUCE trial DSTs, which offered patient information leaflets that could be viewed online or printed, as well as offering advice to physicians on the recognised indications for giving an antibiotic prescription. A recent trial reported on the outcome of quarterly feedback on antibiotic utilisation over 2 years among 2900 Swiss physicians. 87 Over the first and second years of the trial, there was no difference in antibiotic prescribing to all patients. The study found that there was nearly a 5% lower antibiotic utilisation to adults aged 19–65 years in the second year of the study, but not in the first year. The study appeared to identify age-related changes in prescribing that were not consistent over time. However, there are important differences from the present study because the feedback employed by Hemkens et al. 87 was less immediate (this study provided monthly rather than quarterly feedback) and because Switzerland already has very low antibiotic prescribing rates. 88 A study of dental practices in Scotland, which might account for 10% of community antibiotic prescribing, found that feedback of past antibiotic prescription data were associated with a 5.7% relative reduction in antibiotic prescribing over 12 months. 89 Audit and feedback have also been employed successfully to reduce other forms of high-risk prescribing in primary care. 90 Antibiotic prescribing to children was investigated by Cabral et al. ;91 the authors reported an analysis of four qualitative studies. Their analysis suggested that both parents and clinicians view children as being more vulnerable to the risks associated with RTIs. For parents and for doctors, giving an antibiotic prescription are viewed as safe courses of action. ‘Failing’ to intervene appropriately was anticipated to incur future disapproval from peers. 91 Similar considerations may influence the management of very old people. The development of clearer criteria for the prescribing of antibiotics to these more vulnerable groups is desirable. 92
Main findings of the cohort study of safety outcomes
Material in this section is reproduced from Gulliford et al. 31 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
The study used a large data set of EHRs to investigate the safety of reducing unnecessary antibiotic prescribing for RTIs in primary care. The results show that non-trial general practices that prescribe fewer antibiotics for respiratory infections may expect to have a higher incidence of pneumonia and peritonsillar abscess than higher-prescribing general practices. If a general practice, with an average list size of 7000 patients, reduced the proportion of RTI consultations with antibiotics prescribed by 10%, it might encounter about one additional case of pneumonia each year and one additional case of peritonsillar abscess each decade. Changes will be proportionately greater for larger reductions in antibiotic prescribing. These estimates represent averages across general practice populations, but complications might be fewer than expected if GPs are able to effectively stratify antibiotic prescribing on the basis of the level of risk. There was no evidence that diagnoses of mastoiditis, empyema, bacterial meningitis or intracranial abscess might increase. Lemierre syndrome was rare, with about one case per 2 million person-years, but there was no evidence that this was more frequent at low-prescribing practices. This is reassuring in view of recent suggestions that F. necrophorum may often be present in patients with sore throat. 36 These estimates must be viewed in the context of quantitatively important declining secular trends in incidence for several infective complications of RTIs, including peritonsillar abscess, mastoiditis and meningitis. Bacterial meningitis from pneumococcal, meningococcal or Haemophilus infection has declined following the introduction of vaccination programmes. 93 However, the incidence of pneumonia showed a slight increase over time, consistent with previous studies based on hospital admissions. 94,95
Reducing the proportion of RTI consultations with antibiotics prescribed by 10% is expected to be accompanied by some 2000 fewer antibiotic prescriptions per practice over a 10-year period. Benefits to individual patients from avoiding antibiotics include reductions in common adverse reactions to antibiotics (including rashes, vomiting and diarrhoea), which may affect 10% of patients,12 as well as less common side-effects, such as anaphylaxis. Benefits to general practices may include a demedicalisation of RTIs followed by a decline in the consultation rate, as previous observational studies show higher-prescribing general practices attract more consultations for RTIs. 83 Trial evidence shows that even one antibiotic prescription increases the likelihood of re-consultation with a new episode of RTI. 11 Most of the ‘complications’ identified do not require hospital admission and, currently, respond well to antibiotics, so simply the occurrence of an uncommon complication rates is not in itself a reason to justify more widespread prescribing of antibiotics for initially uncomplicated presentations. There was no evidence for a threshold between ‘safe’ or ‘unsafe’ prescribing levels. Inspection of forest plots suggested some departure from linearity, but the addition of non-linear terms did not improve the goodness of fit of regression models.
Strengths and weaknesses of the cohort study in relation to other studies
Previous studies have consistently demonstrated high levels of unnecessary prescribing of antibiotics for RTIs in primary care. 96 Although there has been a declining trend in the consultation rate for RTIs,7 there has been little change in the proportion of consultations with antibiotics prescribed in the UK7 in spite of the efforts of researchers, clinicians and policy-makers to bring about changes. This is in contrast to Sweden, where there has been a long-term decline in antibiotic prescribing for respiratory indications without evidence of increased risk of bacterial complications. 97 General practitioners may often be concerned to meet patients’ expectations for antibiotic prescriptions,98 but both patients and prescribers may also have concerns about the safety of non-prescribing strategies. 98 Petersen et al. 32 provided evidence that antibiotics reduced the risk of pneumonia, mastoiditis and peritonsillar abscess (quinsy), but did not quantify the potential population impact of these complications.
The present results represent averages across general practice populations. There is considerable diversity among the population of patients at risk of RTIs. Current management guidelines for RTIs recommend that specific groups of patients should be considered to have positive indications for antibiotic treatment. 12 An immediate antibiotic prescription is recommended if patients are very unwell, or have clinical features suggestive of serious illness or complications,99 or if comorbidity is present, or the patient is very young or very old. Further research is needed to evaluate whether or not the present results will be confirmed when subgroups that may be at higher risk, including older adults, are analysed separately. It is possible that general practices with the same overall level of antibiotic prescribing may differ in the appropriateness of their management of patients with defined markers of vulnerability and this could influence the rate of complications. However, the clinical features of a RTI episode may have only limited predictive value for the future occurrence of complications, and a high proportion of complications may occur in patients who appeared to be at low risk. 100 A delayed antibiotic-prescribing strategy, in which a prescription is issued but used only if symptoms fail to improve, is sometimes recommended as a method for reducing antibiotic utilisation in the management of RTIs. 27 Delayed antibiotic prescribing may be as effective as immediate use of antibiotics in the prevention of complications of sore throat. 101 The development and application of point-of-care testing to guide antibiotic prescribing may have a future role in identifying individuals who may benefit from antibiotic treatment. 102,103
Strengths and weaknesses of the cohort study
This study comprised > 600 general practices, with a registered population of > 4 million patients and 45 million person-years of observation. Consequently, the study provided very precise estimates for the more frequent outcomes evaluated. It is acknowledged that there was lower power to evaluate potential changes in less frequent outcomes. It can be concluded that the absolute risks of mastoiditis, empyema, intracranial abscess or Lemierre syndrome remain small even in low-prescribing practices. This study adopted a population perspective, aiming to quantify the outcomes of either high- or low-prescribing strategies in the management of RTIs. Consequently, the study evaluated changes in infective complications at the level of the general practice population. The research did not address variation in prescribing at the level of the individual physician. The research did not show whether or not individual patients who experienced complications received antibiotics. Conclusions might differ if individual-level analyses showed that complications arise in patients who were treated with antibiotics. This study did not evaluate the outcomes of individual patients who were diagnosed with complications. Further research is required to evaluate the severity of complications, such as pneumonia, and their outcomes, including mortality. Future studies might also make use of linked hospital episode data, which are available for selected CPRD practices in England in more recent years, in order to evaluate hospitalised cases in more detail. The risk of complications associated with different classes of antibiotics also merits study. It is acknowledged that there may be other complications, such as a proportion of all cases of septicaemia diagnosed in primary care, which might follow from RTIs. The study acknowledges several sources of misclassification: the study used a sample of the CPRD to estimate consultation and prescribing rates; there is variation among general practices in the use of diagnostic categories;85 general practice populations may vary in their use of out-of-hours and emergency services, whose generally higher antibiotic prescribing may not be captured in the CPRD; and some general practices may use delayed antibiotic-prescribing strategies,101 but these were not distinguished in the analysis of prescriptions issued. It is also possible that use of near-patient testing may have contributed to better diagnosis during the period. These forms of misclassification will generally tend to diminish estimated associations, but might cause bias if effects are differentially distributed across prescribing categories. Diagnostic coding may have a subjective element104 and bias might arise if low-prescribing practices are more likely than high-prescribing practices to code ‘pneumonia’ in order to justify the prescription of an antibiotic. The research utilised non-randomised data, and the data were adjusted for age, gender, region, deprivation category and general practice, but it is possible that unmeasured confounders might have biased the reported associations. Caution is required in the analysis of large data sets; ‘significant’ results must be judged in relation to their clinical importance. Antibiotic prescribing in the UK is high compared with some international comparators, and the study cannot be sure that the associations reported here would also hold at very low antibiotic-prescribing levels.
Conclusions
The study identified a range of views that may influence GPs’ acceptance of an intervention addressing the problem of inappropriate antibiotic prescribing to patients with RTIs. Primary care prescribers may tend to deny personal responsibility for overprescribing and may not engage with the intervention as having relevance for them. Such individual attitudinal barriers might suggest that solutions need to be developed and implemented at an organisational level as well as, or instead of, at the individual practitioner level. It appears likely that barriers to active engagement in research interventions identified in this study might go beyond antibiotic prescribing and this might suggest a need to shift the focus of research towards less individual-based approaches, as well as addressing the possible negative attitudes of individual health professional. These findings might be useful for researchers, professional organisations and guideline developers who engage in designing interventions for primary care.
This research successfully conducted an efficient trial to evaluate the effectiveness of electronically delivered antimicrobial stewardship interventions in a large population. The study included 79 general practices, with a registered population of > 600,000 people. The study found evidence that, overall, general practice antibiotic prescribing for RTI is reduced by these electronically delivered interventions. The imprecise result reflected very wide variations in clinical practice. The study found evidence that antibiotic prescribing might be reduced by 16% in adults aged 15–84 years, but not in children or very old people. This finding is consistent with previous research that shows that parents and doctors may be cautious in their management of children. It can be concluded that future intervention strategies for antimicrobial stewardship should employ stratified interventions that are tailored to the needs and concerns of specific age groups, with reporting of antibiotic utilisation disaggregated by age.
There was no evidence from trial data that any safety outcomes might be increased through interaction with the intervention. The larger cohort study in non-trial practices provides evidence that general practices that prescribe antibiotics less frequently at consultations for RTIs may experience a slight increase in the incidence of pneumonia and peritonsillar abscess, which would be expected to respond to treatment while bacterial pathogens remain sensitive to antibiotics. No increase in mastoiditis, empyema, meningitis, intracranial abscess or Lemierre syndrome is likely. Even a large reduction in antibiotic prescribing was predicted to be associated with only a small increase in numbers of cases observed over a 10-year period, and this would be expected to reduce the risks of antibiotic resistance, the side effects of antibiotics and, the medicalisation of largely self-limiting illnesses. The safety outcomes of no antibiotic-prescribing strategies for RTIs are an important aspect for communication to patients and the public in the context of wider communication strategies to support antimicrobial stewardship. 105
Research recommendations
This research provides some evidence to support scaling up the interventions employed in this trial. The research illustrates the value of using EHR data to provide practitioners with detailed feedback on antibiotic prescribing for particular prescribing indications. Any decision to scale up should be made in the context of the changing policy environment, current antibiotic stewardship policies, advances in computerised decision support design, the need for increased patient involvement in design and awareness of the possible waning of effect over time among other considerations. The contributions of pharmacists, microbiologists and health economists with expertise in antimicrobial stewardship will also be essential.
Interventions need to be developed and implemented at an organisational level as well, or instead of, at the individual practitioner level. It appears likely that barriers to active engagement in research interventions identified in this study might go beyond antibiotic prescribing and this might suggest a need to shift the focus of research towards less individual-based approaches.
It is recommended that future research should develop and test age-stratified interventions specifically focusing on antibiotic prescribing for more vulnerable age groups.
Research should further investigate the safety of no prescribing strategies using individual patient data, a more systematic selection of safety outcomes and analyses that specifically address vulnerable groups.
Acknowledgements
The authors thank Tim Foster and DXS-UK Ltd for assistance with intervention design, delivery and monitoring. The authors thank Lars Rönnegård, Dalarna University, Sweden, for statistical advice and additional R programming. The authors also thank all of the general practices and patients who contributed to the study.
Data sources
The study is based, in part, on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
Funding
The trial is funded by the NIHR Health Technology Assessment Programme (reference number 13/88/10). Martin C Gulliford was supported by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ Hospitals.
Trial registration
The trial was registered as ISRCTN95232781. The trial was registered on 18 November 2014.
Trial Steering Committee
Jackie Cassell (chairperson), Susan Hopkins (Public Health England), Tim Chadborn (Public Health England) and Nanik Pursani (Lay member).
Data Monitoring Committee
Christine A’Court (chairperson), Helen Strongman (CPRD/MHRA), Derek Cook (St George’s, University of London) and Jason Oke (University of Oxford).
Contributions of authors
Martin C Gulliford (Professor of Public Health) contributed to trial design, conduct and analysis and drafting of the report.
Dorota Juszczyk (Research Associate) led the intervention study and the design of the intervention. She analysed the qualitative data and reported on the intervention development study.
A Toby Prevost (Professor of Medical Statistics and Clinical Trials) contributed statistical advice on the design and conduct of the study. He drafted the statistical analysis plan and advised on the conduct and reporting of the statistical analyses.
Jamie Soames (Senior Clinical Project Manager) was responsible for recruiting general practices to the study and liaising with practices for intervention delivery and process evaluation.
Lisa McDermott (Research Associate) contributed to the design of the trial, the development of the intervention and the design of the process evaluation.
Kirin Sultana (Senior Clinical Project Manager) was responsible for recruiting general practices to the study and liaising with practices for intervention delivery and process evaluation.
Mark Wright (Director of Interventional Research) was responsible for overseeing the design and conduct of the CPRD contribution to the study.
Robin Fox (General Practitioner) contributed to the design of the study and provided clinical advice on intervention development and interpretation of results.
Alastair D Hay (Professor of Primary Care) contributed clinical advice to the study, including intervention design and development and interpretation of trial and safety outcomes.
Paul Little (Professor of Primary Care Research) contributed clinical advice to the design of the study, including intervention design and development and analysis of trial and safety outcomes.
Michael Moore (Professor of Primary Care) contributed clinical advice to the study, including intervention design and development and analysis of trial and safety outcomes.
Lucy Yardley (Professor of Health Psychology) contributed advice on the intervention development study, the design of the intervention, and the design and analysis of the process evaluation.
Mark Ashworth (Reader in General Practice) contributed clinical advice to the study, including intervention design and development and interpretation of results.
Judith Charlton (Research Associate) analysed all the CPRD data for the prescribing reports for the study, wrote a program to prepare the reports on an automated basis, and programmed the main trial analyses from the statistical analysis plan.
All authors contributed to, and approved, the final manuscript.
Publications
Juszczyk D, Charlton J, McDermott L, Soames J, Sultana K, Ashworth M, et al. Electronically delivered, multicomponent intervention to reduce unnecessary antibiotic prescribing for respiratory infections in primary care: a cluster randomised trial using electronic health records-REDUCE Trial study original protocol. BMJ Open 2016;6:e010892.
Gulliford MC, Moore MV, Little P, Hay AD, Fox R, Prevost AT, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016;354:i3410.
Gulliford MC, Prevost AT, Charlton J, Juszczyk D, Soames J, McDermott L, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 2019;364:l236.
Data-sharing statement
The data analysed in this study were obtained from the CPRD (www.cprd.com) under licence. The agreements in place for these data do not permit further distribution or sharing. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Annual Report of the Chief Medical Officer. Volume 2, 2011. Infections and the Rise of Antimicrobial Resistance. London: Department of Health and Social Care; 2013.
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: Review on Antimicrobial Resistance; 2016.
- Kaier K. Economic modeling of the persistence of antimicrobial resistance. Nat Res Model 2012;25:388-402. https://doi.org/10.1111/j.1939-7445.2011.00114.x.
- Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know?. Clin Microbiol Infect 2014;20:973-80. https://doi.org/10.1111/1469-0691.12798.
- Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016;387:168-75. https://doi.org/10.1016/S0140-6736(15)00474-2.
- Prescribing of Antibiotics for Self-limiting Respiratory Tract Infections in Adults and Children in Primary Care. London: NICE; 2008.
- Gulliford M, Latinovic R, Charlton J, Little P, van Staa T, Ashworth M. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health 2009;31:512-20. https://doi.org/10.1093/pubmed/fdp081.
- Gulliford MC, Dregan A, Moore MV, Ashworth M, Staa Tv, McCann G, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006245.
- Ashworth M, Charlton J, Latinovic R, Gulliford M. Age-related changes in consultations and antibiotic prescribing for acute respiratory infections, 1995–2000. Data from the UK General Practice Research Database. J Clin Pharm Ther 2006;31:461-7. https://doi.org/10.1111/j.1365-2710.2006.00765.x.
- Fossum GH, Lindbæk M, Gjelstad S, Dalen I, Kværner KJ. Are children carrying the burden of broad-spectrum antibiotics in general practice? Prescription pattern for paediatric outpatients with respiratory tract infections in Norway. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002285.
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315:350-2. https://doi.org/10.1136/bmj.315.7104.350.
- Venekamp RP, Sanders S, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2013;1. https://doi.org/10.1002/14651858.CD000219.pub3.
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD000023.pub4.
- Dagan R, Leibovitz E, Greenberg D, Yagupsky P, Fliss DM, Leiberman A. Dynamics of pneumococcal nasopharyngeal colonization during the first days of antibiotic treatment in pediatric patients. Pediatr Infect Dis J 1998;17:880-5. https://doi.org/10.1097/00006454-199810000-00006.
- The Path of Least Resistance. London: Department of Health and Social Care; 2009.
- Gulliford MC, van Staa T, Dregan A, McDermott L, McCann G, Ashworth M, et al. Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study). Ann Fam Med 2014;12:344-51. https://doi.org/10.1370/afm.1659.
- Linder JA. Antibiotic prescribing for acute respiratory infections – success that’s way off the mark: comment on ‘A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis’. JAMA Intern Med 2013;173:273-5. https://doi.org/10.1001/jamainternmed.2013.1984.
- van den Broek d’Obrenan J, Verheij TJ, Numans ME, van der Velden AW. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014;69:1701-7. https://doi.org/10.1093/jac/dku005.
- Ranji SR, Steinman MA, Shojania KG, Sundaram V, Lewis R, Arnold S, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Volume 4: Antibiotic Prescribing Behaviour. Rockville, MD: Agency for Healthcare Research and Quality; 2006.
- Ranji SR, Steinman MA, Shojania KG, Gonzales R. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care 2008;46:847-62. https://doi.org/10.1097/MLR.0b013e318178eabd.
- Little P, Stuart B, Francis N, Douglas E, Tonkin-Crine S, Anthierens S, et al. Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet 2013;382:1175-82. https://doi.org/10.1016/S0140-6736(13)60994-0.
- Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013;309:2345-52. https://doi.org/10.1001/jama.2013.6287.
- Gonzales R, Anderer T, McCulloch CE, Maselli JH, Bloom FJ, Graf TR, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med 2013;173:267-73. https://doi.org/10.1001/jamainternmed.2013.1589.
- Gjelstad S, Høye S, Straand J, Brekke M, Dalen I, Lindbæk M. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing (Rx-PAD) study). BMJ 2013;347. https://doi.org/10.1136/bmj.f4403.
- Butler CC, Simpson SA, Dunstan F, Rollnick S, Cohen D, Gillespie D, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 2012;344. https://doi.org/10.1136/bmj.d8173.
- Høye S, Gjelstad S, Lindbæk M. Effects on antibiotic dispensing rates of interventions to promote delayed prescribing for respiratory tract infections in primary care. Br J Gen Pract 2013;63:e777-86. https://doi.org/10.3399/bjgp13X674468.
- Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4. https://doi.org/10.1002/14651858.CD004417.pub4.
- Ivers NM, Grimshaw JM, Jamtvedt G, Flottorp S, O’Brien MA, French SD, et al. Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med 2014;29:1534-41. https://doi.org/10.1007/s11606-014-2913-y.
- Roshanov PS, Fernandes N, Wilczynski JM, Hemens BJ, You JJ, Handler SM, et al. Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013;346. https://doi.org/10.1136/bmj.f657.
- McDermott L, Yardley L, Little P, van Staa T, Dregan A, McCann G, et al. Process evaluation of a point-of-care cluster randomised trial using a computer-delivered intervention to reduce antibiotic prescribing in primary care. BMC Health Serv Res 2014;14. https://doi.org/10.1186/s12913-014-0594-1.
- Gulliford MC, Moore MV, Little P, Hay AD, Fox R, Prevost AT, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016;354. https://doi.org/10.1136/bmj.i3410.
- Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007;335. https://doi.org/10.1136/bmj.39345.405243.BE.
- Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 2014;82:806-13. https://doi.org/10.1212/WNL.0000000000000172.
- Sinave CP, Hardy GJ, Fardy PW. The Lemierre syndrome: suppurative thrombophlebitis of the internal jugular vein secondary to oropharyngeal infection. Medicine 1989;68:85-94. https://doi.org/10.1097/00005792-198903000-00002.
- Björk H, Bieber L, Hedin K, Sundqvist M. Tonsillar colonisation of Fusobacterium necrophorum in patients subjected to tonsillectomy. BMC Infect Dis 2015;15. https://doi.org/10.1186/s12879-015-0975-z.
- Centor RM, Atkinson TP, Ratliff AE, Xiao L, Crabb DM, Estrada CA, et al. The clinical presentation of fusobacterium-positive and streptococcal-positive pharyngitis in a university health clinic: a cross-sectional study. Ann Int Med 2015;162:241-7. https://doi.org/10.7326/M14-1305.
- Unusual Disease Diagnosis. Lemierre’s Syndrome. CMO’s Update 29th February 2001. London: Department of Health and Social Care; 2001.
- Maes N. I thought my awful headache was flu – but it was meningitis. Daily Mail 2015. https://www.dailymail.co.uk/health/article-3047737/I-thought-awful-headache-flu-meningitis-s-not-just-children-risk-terrifying-illness-s-easy-adults-mistake-symptoms.html (accessed 5 January 2018).
- McDermott L, Yardley L, Little P, Ashworth M, Gulliford M. eCRT Research Team . Developing a computer delivered, theory based intervention for guideline implementation in general practice. BMC Fam Pract 2010;11. https://doi.org/10.1186/1471-2296-11-90.
- Gulliford MC, Charlton J, Prevost T, Booth H, Fildes A, Ashworth M, et al. Costs and outcomes of increasing access to bariatric surgery: cohort study and cost-effectiveness analysis using electronic health records. Value Health 2017;20:85-92. https://doi.org/10.1016/j.jval.2016.08.734.
- Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Proc 1991;50:248-87. https://doi.org/10.1016/0749-5978(91)90022-L.
- Deci EL, Ryan RM. The empirical exploration of intrinsic motivational processes. Adv Exp Soc Psychol 1980;13:39-80. https://doi.org/10.1016/S0065-2601(08)60130-6.
- Eccles MP, Grimshaw JM, Johnston M, Steen N, Pitts NB, Thomas R, et al. Applying psychological theories to evidence-based clinical practice: identifying factors predictive of managing upper respiratory tract infections without antibiotics. Implement Sci 2007;2. https://doi.org/10.1186/1748-5908-2-26.
- Hrisos S, Eccles M, Johnston M, Francis J, Kaner EF, Steen N, et al. An intervention modelling experiment to change GPs’ intentions to implement evidence-based practice: using theory-based interventions to promote GP management of upper respiratory tract infection without prescribing antibiotics. BMC Health Serv Res 2008;8. https://doi.org/10.1186/1472-6963-8-10.
- Little P. Advice to parents has limited effect – where next?. BMJ 2004;329. https://doi.org/10.1136/bmj.329.7460.269.
- Rashidian A, Eccles MP, Russell I. Falling on stony ground? A qualitative study of implementation of clinical guidelines’ prescribing recommendations in primary care. Health Policy 2008;85:148-61. https://doi.org/10.1016/j.healthpol.2007.07.011.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Braun V, Clarke V, Cooper H. The Handbook of Research Methods in Psychology. Washington, DC: American Psychological Association; 2012.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Dregan A, van Staa TP, McDermott L, McCann G, Ashworth M, Charlton J, et al. Point-of-care cluster randomized trial in stroke secondary prevention using electronic health records. Stroke 2014;45:2066-71. https://doi.org/10.1161/STROKEAHA.114.005713.
- Herrett E, Williamson E, van Staa T, Ranopa M, Free C, Chadborn T, et al. Text messaging reminders for influenza vaccine in primary care: a cluster randomised controlled trial (TXT4FLUJAB). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010069.
- Julious SA, Horspool MJ, Davis S, Bradburn M, Norman P, Shephard N, et al. PLEASANT: Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term – a cluster randomised controlled trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20930.
- Juszczyk D, Charlton J, McDermott L, Soames J, Sultana K, Ashworth M, et al. Electronically delivered, multicomponent intervention to reduce unnecessary antibiotic prescribing for respiratory infections in primary care: a cluster randomised trial using electronic health records – REDUCE trial study original protocol. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010892.
- Altman DG, Bland JM. Treatment allocation by minimisation. BMJ 2005;330. https://doi.org/10.1136/bmj.330.7495.843.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Seasonal Flu At Risk Read Codes 2015–2016. Leeds: NHSE; 2016.
- Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Prevalence of depression and utilization of health care in single and multiple morbidity: a population-based cohort study. Psychol Med 2013;43:1423-31. https://doi.org/10.1017/S0033291712002498.
- Charlton J, Rudisill C, Bhattarai N, Gulliford M. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy 2013;18:215-23. https://doi.org/10.1177/1355819613493772.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Ronnegard L, Shen X, Alam M. HGLM: a package for fitting hierarchical generalized linear models. R J 2010;2:20-8.
- Lee Y, Ronnegard L, Noh M. Data Analysis Using Hierarchical Generalized Linear Models with R. Boca Raton, FL: CRC Press; 2017.
- Office for National Statistics (ONS) . Revision of the European Standard Population (ESP) – Effect on UK Statistics n.d. www.ons.gov.uk/ons/guide-method/user-guidance/health-and-life-events/revised-european-standard-population-2013-2013-esp-/esp-effect-on-uk-official-statistics–a-summary-forusers-of-statistics.doc (accessed October 2018).
- Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Hazra NC, Rudisill C, Gulliford MC. Determinants of health care costs in the senior elderly: age, comorbidity, impairment, or proximity to death?. Eur J Health Econ 2018;19:831-42. https://doi.org/10.1007/s10198-017-0926-2.
- National Institute for Health and Care Excellence . Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use n.d. www.nice.org.uk/guidance/ng15 (accessed January 2019).
- English Indices of Deprivation 2010. London: Ministry of Housing, Communities and Local Government; 2015.
- Clinical Practice Research Datalink. London: Medicines and Healthcare products Regulatory Agency; 2018.
- R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010.
- Wickham H. GGPLOT2: Elegant Graphics for Data Analysis. Heidelberg: Springer; 2009.
- Gordon M, Lumley T. Package ‘Forestplot’. Advanced Forest Plot Using ‘Grid’ Graphics. Vienna: The Comprehensive R Archive Network; 2016.
- Gulliford MC, Prevost AT, Charlton J, Juszczyk D, Soames J, McDermott L, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 2019;364. https://doi.org/10.1136/bmj.l236.
- Guenther CL, Alicke MD. Deconstructing the better-than-average effect. J Pers Soc Psychol 2010;99:755-70. https://doi.org/10.1037/a0020959.
- McKay RT, Dennett DC. The evolution of misbelief. Behav Brain Sci 2009;32:493-510. https://doi.org/10.1017/S0140525X09990975.
- Cranney M, Warren E, Barton S, Gardner K, Walley T. Why do GPs not implement evidence-based guidelines? A descriptive study. Family Practice 2001;18:359-63. https://doi.org/10.1093/fampra/18.4.359.
- Freeman AC, Sweeney K. Why general practitioners do not implement evidence: qualitative study. BMJ 2001;323. https://doi.org/10.1136/bmj.323.7321.1100.
- Ham C, Alberti KG. The medical profession, the public, and the government. BMJ 2002;324:838-42. https://doi.org/10.1136/bmj.324.7341.838.
- Iacobucci G. GP confronts health minister over pressures of the job. BMJ 2013;347. https://doi.org/10.1136/bmj.f6291.
- Doran N, Fox F, Rodham K, Taylor G, Harris M. Qualitative Study Exploring the Reasons Why GP’s Leave Practice Early in Their Careers and the Barriers to Their Return. Bath: University of Bath; 2014.
- Quality Premium: 2016/17 Guidance for CCGs. Leeds: NHS England; 2016.
- Mc Hugh S, O’Mullane M, Perry IJ, Bradley C. Barriers to, and facilitators in, introducing integrated diabetes care in Ireland: a qualitative study of views in general practice. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-003217.
- Doran T, Kontopantelis E, Valderas JM, Campbell S, Roland M, Salisbury C, et al. Effect of financial incentives on incentivised and non-incentivised clinical activities: longitudinal analysis of data from the UK Quality and Outcomes Framework. BMJ 2011;342. https://doi.org/10.1136/bmj.d3590.
- Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother 2011;66:2215-23. https://doi.org/10.1093/jac/dkr279.
- Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 2005;55:603-8.
- Leydon GM, McDermott L, Moore M, Williamson I, Hobbs FD, Lambton T, et al. A qualitative study of GP, NP and patient views about the use of rapid streptococcal antigen detection tests (RADTs) in primary care: ‘swamped with sore throats?’. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002460.
- Stocks N, Fahey T. Labelling of acute respiratory illness: evidence of between-practitioner variation in the UK. Fam Pract 2002;19:375-7. https://doi.org/10.1093/fampra/19.4.375.
- Lee Y, Nelder J. Conditional and marginal models: another view. Stat Sci 2004;19:219-38. https://doi.org/10.1214/088342304000000305.
- Hemkens LG, Saccilotto R, Reyes SL, Glinz D, Zumbrunn T, Grolimund O, et al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care: a randomized clinical trial. JAMA Intern Med 2017;177:176-83. https://doi.org/10.1001/jamainternmed.2016.8040.
- Achermann R, Suter K, Kronenberg A, Gyger P, Mühlemann K, Zimmerli W, et al. Antibiotic use in adult outpatients in Switzerland in relation to regions, seasonality and point of care tests. Clin Microbiol Infect 2011;17:855-61. https://doi.org/10.1111/j.1469-0691.2010.03348.x.
- Elouafkaoui P, Young L, Newlands R, Duncan EM, Elders A, Clarkson JE, et al. Translation Research in a Dental Setting (TRiaDS) Research Methodology Group. An audit and feedback intervention for Reducing Antibiotic Prescribing in general Dental practice: the RAPiD cluster randomised controlled trial. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002115.
- Guthrie B, Kavanagh K, Robertson C, Barnett K, Treweek S, Petrie D, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ 2016;354. https://doi.org/10.1136/bmj.i4079.
- Cabral C, Lucas PJ, Ingram J, Hay AD, Horwood J. ‘It’s safer to . . .’ parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: An analysis across four qualitative studies. Soc Sci Med 2015;136–7:156-64. https://doi.org/10.1016/j.socscimed.2015.05.027.
- Hay AD, Redmond NM, Turnbull S, Christensen H, Thornton H, Little P, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902-10. https://doi.org/10.1016/S2213-2600(16)30223-5.
- Okike IO, Ribeiro S, Ramsay ME, Heath PT, Sharland M, Ladhani SN. Trends in bacterial, mycobacterial, and fungal meningitis in England and Wales 2004–11: an observational study. Lancet Infect Dis 2014;14:301-7. https://doi.org/10.1016/S1473-3099(13)70332-3.
- Quan TP, Fawcett NJ, Wrightson JM, Finney J, Wyllie D, Jeffery K, et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998–2014. Thorax 2016;71:535-42. https://doi.org/10.1136/thoraxjnl-2015-207688.
- Trotter CL, Stuart JM, George R, Miller E. Increasing hospital admissions for pneumonia, England. Emerging Infect Dis 2008;14:727-33. https://doi.org/10.3201/eid1405.071011.
- Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health 2004;26:268-74. https://doi.org/10.1093/pubmed/fdh160.
- Cars T, Eriksson I, Granath A, Wettermark B, Hellman J, Norman C, et al. Antibiotic use and bacterial complications following upper respiratory tract infections: a population-based study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016221.
- Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015;33:11-20. https://doi.org/10.3109/02813432.2015.1001942.
- Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239-46. https://doi.org/10.1177/0272989X8100100304.
- Little P, Stuart B, Hobbs FD, Butler CC, Hay AD, Campbell J, et al. Predictors of suppurative complications for acute sore throat in primary care: prospective clinical cohort study. BMJ 2013;347. https://doi.org/10.1136/bmj.f6867.
- Little P, Stuart B, Hobbs FD, Butler CC, Hay AD, Delaney B, et al. Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis 2014;14:213-19. https://doi.org/10.1016/S1473-3099(13)70294-9.
- Van den Bruel A, Jones C, Thompson M, Mant D. C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Arch Dis Child 2016;101:382-5. https://doi.org/10.1136/archdischild-2015-309228.
- Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013;347. https://doi.org/10.1136/bmj.f5806.
- Howie JG, Richardson IM, Gill G, Durno D. Respiratory illness and antibiotic use in general practice. J R Coll Gen Pract 1971;21:657-63.
- Huttner B, Goossens H, Verheij T, Harbarth S. CHAMP consortium. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis 2010;10:17-31. https://doi.org/10.1016/S1473-3099(09)70305-6.
Glossary
- Antibiotic resistance
- A condition in which bacteria that cause infections are no longer susceptible to antibiotics.
- Bacterial meningitis
- An infection of the space between the brain and the skull.
- Empyema
- An infection of the space between the lung and the chest wall, leading to pus formation.
- Intracranial abscess
- An infection in the brain or adjacent tissues, leading to the accumulation of pus.
- Mastoiditis
- An infection of the bony cavities adjacent to the ear.
- Peritonsillar abscess
- A severe infection in the tonsils leading to pus formation, also known as quinsy.
- Pneumonia
- An infection of the lung tissue causing consolidation with infected secretions.
- R
- A program for statistical computing.
List of abbreviations
- ABPP
- antibiotic-prescribing proportion
- AMR
- antimicrobial drug resistance
- BNF
- British National Formulary
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- DHGLM
- double hierarchical generalised linear model
- DMC
- Data Monitoring Committee
- DST
- decision support tool
- eCRT
- cluster randomised trial using electronic media
- EHR
- electronic health record
- EQL
- extended quasi-likelihood
- GP
- general practitioner
- HGLM
- hierarchical generalised linear model
- HTA
- Health Technology Assessment
- IQR
- interquartile range
- IRR
- incident rate ratio
- ISAC
- Independent Scientific Advisory Committee
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NP
- nurse prescriber
- NRES
- National Research Ethics Service
- portable document format
- RCT
- randomised controlled trial
- RR
- rate ratio
- RTI
- respiratory tract infection
- SAP
- statistical analysis plan
- SD
- standard deviation
- TIDieR
- Template for Intervention Description and Replication
