Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/69/02. The contractual start date was in September 2013. The draft report began editorial review in January 2018 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Little was Programme Director of the Programme Grants for Applied Research (PGfAR) programme, Editor-in-Chief for the PGfAR journal and a member of the National Institute for Health Research (NIHR) Journals Library Editorial Group and the NIHR PGfAR expressions of interest – Health Technology Assessment Projects Remit Meeting. Trudie Chalder reports grants from Guy’s and St Thomas’ Charity. She was a faculty member at the Third International Conference on Functional (Psychogenic) Neurological Disorders, September 2017, Edinburgh, UK; a member of the Improving Access to Psychological Therapies (IAPT) Education and Training Evidence Review Group (2016); a member of the IAPT Outcomes and Informatics Meeting (2016–present); and the president of the British Association for Behavioural and Cognitive Psychotherapies (2012–15), for which she did not receive payment. She delivered workshops on medically unexplained symptoms during the conduct of the study (money paid into King’s College London for future research). Trudie Chalder has a patent for the background intellectual property (IP) of the manuals that were developed prior to the trial starting. The Trial Steering Committee Chairperson, Peter White, was a colleague of Trudie Chalder in the past but he has recently retired. Rona Moss-Morris reports personal fees from training in irritable bowel syndrome interventions for Central and North West London NHS Foundation Trust and the University of East Anglia outside the submitted work. The patient manual is background IP developed by Rona Moss-Morris and Trudie Chalder in previous work. The therapist manual was developed for the Assessing Cognitive–behavioural Therapy in Irritable Bowel (ACTIB) trial. These manuals were made available only once the 12-month ACTIB follow-up was complete. Sabine Landau reports support via the Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Everitt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Randomised controlled trial
Patient and public involvement
People with irritable bowel syndrome (IBS) have been involved in the Assessing Cognitive–behavioural Therapy in Irritable Bowel (ACTIB) trial throughout, from the time of application for the Health Technology Assessment (HTA)-commissioned call through to discussion of the results and dissemination of the findings, including the development of this patient and public involvement (PPI) section of the report.
We were very fortunate to identify two PPI representatives, Jill Durnell and Kate Riley, who had participated in the Management of Irritable Bowel Syndrome (MIBS) feasibility study1 and who were willing to act as PPI representatives for ACTIB. They helped to develop the grant application to HTA for the ACTIB trial. They were positive about the idea of taking the website from MIBS forward to a full trial and commented on drafts of the outline application and full applications for feedback. In particular, our PPI representatives assisted with highlighting areas important to patients to include, with consideration of outcome measures and by helping to develop the Plain English summary and ensuring that it was clear and accessible for a non-specialist audience.
During the early development phase of the ACTIB study materials, our PPI representatives were involved in commenting on paperwork, questionnaires and the updated website to ensure that these were as accessible and user friendly as possible.
Our PPI representatives were part of our trial management team and received communications and updates on all trial management issues. One of our PPI representatives was happy to focus on participation in the Trial Management Group and our other PPI representative on the TSC.
Our PPI representatives have also been involved in the development of this HTA report (in particular the Plain English summary and this PPI section) and have agreed to continue in their PPI roles during our funded extension to complete a 24-month follow-up and process evaluation.
Some specific examples of our PPI representative activities and input are as follows:
-
Jill Durnell has presented to the local Clinical Research Network on being a PPI representative and participating in research.
-
Our PPI provided input and views on potential ways to improve follow-up rates. We instituted multiple methods of contacting participants to remind them to complete their questionnaires and offered them a Love2shop voucher (www.love2shop.co.uk; accessed 4 December 2018) for taking part in the study.
-
Our PPI representatives recently provided quotations for the Southampton primary care website to highlight the benefits/realities of PPI involvement:
Having benefited from participating in the ACTIB pilot study, I felt that I wanted to play a part in ensuring other IBS sufferers had the same opportunity. Being a PPI [representative] provides the opportunity to contribute to expressing the science into what it means to patients. Recruitment and retention of participants in studies is often a challenge. PPI input can enhance any literature provided to patients, by ensuring the information is not bewildering. Hopefully, this helps to keep them involved and the research to achieve its objectives.
Introduction
Irritable bowel syndrome is a common chronic gastrointestinal (GI) disorder, affecting 10–22% of the UK population, with NHS costs of > £200M per year. 2,3 Abdominal pain, bloating and altered bowel habits affect quality of life (QoL) and social functioning and can lead to time off work. 4,5 Treatment relies on a positive diagnosis, reassurance, lifestyle advice and drug therapies. However, many patients suffer ongoing symptoms.
Face-to-face cognitive–behavioural therapy (CBT) has been shown to help IBS, reducing symptom scores and improving QoL measures,6–8 but NHS availability is poor and cost-effectiveness is uncertain. 8 However, National Institute for Health and Care Excellence (NICE) guidance9 recommends CBT for patients with refractory IBS symptoms (i.e. ongoing symptoms after 12 months despite being offered appropriate medications and lifestyle advice).
Web-based CBT has shown promise in other long-term conditions, for example depression,10 tinnitus11 and fatigue in multiple sclerosis,12 and is recommended in guidelines13 for the management of depression. It has advantages, for example being accessible at a time, place and rate of completion convenient to the participant, without extra travel time and costs, but some research studies14 have found low levels of uptake and limited benefits. Small pilot trials have shown promise for web-based CBT in IBS15–17 but indicate that some therapist input is needed.
We previously developed a CBT self-management website to support patients with IBS (Regul8) and trialled it among 135 patients in the National Institute for Health Research (NIHR) Research for Patient Benefit-funded MIBS feasibility study. 1,15
This NIHR HTA ACTIB trial was in response to a commissioned call to assess the clinical effectiveness and cost-effectiveness of psychological interventions for patients with refractory IBS.
Aims and objectives
The primary aim of ACTIB was to determine the clinical effectiveness of therapist telephone-delivered CBT (TCBT) and web-based CBT (WCBT) compared with treatment as usual (TAU) for reducing the severity and impact of symptoms in IBS at 12 months from randomisation.
The secondary aims were to:
-
determine the cost-effectiveness of TCBT and WCBT compared with TAU for reducing severity and impact of symptoms in IBS at 12 months
-
determine the clinical effectiveness and cost-effectiveness of TCBT and WCBT compared with TAU for reducing severity and impact of symptoms in IBS at 3 and 6 months
-
assess the effect of TCBT and WCBT on relief of IBS symptoms, QoL, enablement, anxiety and depression compared with TAU at 3, 6 and 12 months’ follow-up, and acceptability of the treatment.
Methods
The trial protocol for this trial has been published in Everitt et al. 18
Study design
We conducted an open, pragmatic randomised controlled trial (RCT) in primary and secondary care to determine the clinical effectiveness of TCBT and WCBT in patients with refractory IBS.
Setting
Patients were recruited from general practices from Wessex and South London Clinical Commissioning Groups (CCGs), and from one Southampton and two London secondary care sites [Southampton University Hospital Trust (SUHT), King’s College Hospital (KCH) and Guy’s and St Thomas’ NHS Foundation Trust (GSTT)] between May 2014 and March 2016. Research was co-ordinated by two academic centres: the University of Southampton and the Institute of Psychiatry, King’s College London. Treatment took place at participants’ homes via telephone and the internet. Therapists were based at the South London and Maudsley NHS Foundation Trust (SLAM).
Ethics approval and research governance
Ethics approval was awarded by the National Research Ethics Service (NRES) Committee South Central – Berkshire on 11 June 2013 (reference number 13/SC/0206) and local research governance approval was obtained from all participating CCGs.
The trial was registered with the International Standard Randomised Controlled Trial Number (ISRCTN) under the reference number 44427879.
Summary of any changes to the protocol
A total of four substantial amendments were approved by the ethics committee and included a change to the primary outcome, the addition of a 24-month outcome time point and an increase in the recruitment target. Two minor amendments were submitted and acknowledged (Table 1).
| Amendment | Date | Description |
|---|---|---|
| Initial application conditions | Approved 11 June 2013 | |
| Modified amendment V1.0 | Approved 4 February 2014 | Primary outcome changed from SGA of Relief to WSAS |
| Added SAE and withdrawal forms | ||
| Minor changes to study documentation | ||
| Minor amendment 1 | Acknowledged 27 March 2014 | Amended questionnaire instruction |
| Substantial amendment V2.0 | Unfavourable opinion 30 April 2014 | SAE form to include self-harm |
| Substantial amendment V3.0 | Approved 4 April 2014 | Reimbursement for participant travel |
| Substantial amendment V4.0 | Approved 23 May 2015 | Increase recruitment from 495 to 570 participants |
| Substantial amendment V5.0 | Approved 3 December 2015 | Add 24-month follow-up measure |
| Therapist qualitative study documents added | ||
| Vouchers to patients to incentivise completion of questionnaires | ||
| Minor amendment 2 | Acknowledged 7 March 2016 | Comorbidities added to note review form |
| Substantial amendment V6.0 | Approved 26 June 2017 | Protocol title changed to funder’s contractual title |
| Timetable updated to reflect extra recruitment and follow-up time |
Training of co-ordinating centres
Research teams from the co-ordinating centres in Southampton and London were trained in January 2014 at the University of Southampton. The processes covered included telephone screening of patients accepting the invitation and the information required for fully informed consent, therapist procedures, setting up sites (site files, logs and packs), completing the research team database and record-keeping, communication with sites and patients (texts for e-mails and letters) and monitoring LifeGuide (https://lifeguidehealth.org/; accessed 27 January 2019) queries and patient e-mails.
Recruitment of general practices
Expressions of interest from general practices were gathered by Wessex and South London Clinical Research Networks (CRNs) and passed on to the research teams, who then contacted the practices to provide further information on the trial. The number of general practice sites required was estimated based on information from the MIBS feasibility study. 1,15 An assumption of 30–80 patients with a computer diagnosis of IBS per general practitioner (GP) was made (2–5% prevalence and 1600 registered patients per GP). In addition, it was estimated that 5–10% of those would fulfil the inclusion criteria and be willing to participate in the trial (three to eight per GP). Thus, we initially anticipated recruiting approximately 30 general practices.
Recruitment of secondary care sites
Secondary care sites were identified for participation in ACTIB by expert contacts of the research team and comprised SUHT, KCH and GSTT. It was estimated that over 500 patients with IBS attend Southampton hospital GI clinics each year and over 1000 attend the London GI clinics, providing a large population of patients attending secondary care to invite to the study.
Training of sites
Training was undertaken by providing a written training manual and a training log was signed by all site personnel with a delegated role. The training manual included a list of study materials to be supplied by the co-ordinating centre. It covered procedures for identifying patients from the practice records using Read codes to identify adult patients with an IBS diagnosis and preparation of the invitation letters using a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet pre-populated with identification (ID) numbers, recruitment of patients from an opportunistic consultation using a pre-numbered pack, recruitment of patients using a poster (see Report Supplementary Material 1) in receptions, instructions for the blood test including postage of the blood samples and completing paperwork for the co-ordinators and pathology and instructions for completing a serious adverse event (SAE) form if needed. Prior to starting the search, all personnel were added to a delegation log and signed off by the principal investigator at the site. All sites received a site file and a copy of the NICE guidelines for IBS. Sites were kept up to date with trial progress and their individual performance by monthly e-mail updates from the co-ordinating centres.
Patient recruitment
Participating sites were asked to recruit patients by sending invitation letters, by opportunistically recruiting during consultations and by displaying posters in receptions. Because of the limited availability of therapy sessions, site initiation was staggered and the response rate monitored to ensure adequate recruitment and a steady workload for the therapists.
Primary care patients were identified by searching GPs’ lists for those with a diagnosis of IBS, by opportunistically recruiting patients presenting with symptoms consistent with IBS and by displaying posters in receptions. General practice administration staff ran searches for eligible patients using appropriate clinical diagnosis and symptom codes. GPs checked the lists of patients to be invited prior to the invitation letters being sent out to ensure that it was appropriate to contact them.
Secondary care patients were identified from gastroenterology clinics at SUHT, KCH and GSTT. When possible, clinic administration staff searched clinic lists for patients with a diagnosis of IBS. Potential participants were contacted by letter (sent from the clinic), which informed them about the trial and invited them to take part. When clinics needed more support, researcher administration staff were available to hand out packs and answer patient queries. The consultants checked the lists of patients to be contacted prior to the invitation letters being sent out to ensure that it was appropriate to contact them. Invitation letters were sent in batches.
Patients received an invitation letter (see Report Supplementary Material 2) and a patient information sheet (PIS) (see Report Supplementary Material 3) by post from the site, or from the GP/consultant, or from the research team following interest from the poster. Patients were asked to return the reply slip in a pre-paid envelope with contact details to the researchers either indicating that they were interested in being contacted or stating that they were not interested by ticking a list of potential reasons (and with an option for free-text responses). The researchers attempted to contact the patients who indicated that they were interested in participating for screening, either by telephone (including attempts during the evening and at weekends) or via text/e-mail, to arrange a convenient day and time to telephone.
Patient screening
Screening was conducted to ensure that potential participants met the inclusion criteria (Box 1), including being aged ≥ 18 years with refractory IBS [clinically significant symptoms defined by an Irritable Bowel Syndrome Symptom Severity Score (IBS SSS) of > 75], fulfilling Rome III criteria19 and having already been offered first-line therapies (e.g. antispasmodics, antidepressants or fibre-based medications) but still had continuing IBS symptoms for ≥ 12 months. Potential participants aged > 60 years were included only if they had undertaken a consultant review in the previous 2 years to confirm that their symptoms were related to IBS and that other serious bowel conditions had been excluded. This was because NICE guidelines9 advise that a new change in bowel habit in those aged > 60 years should be investigated further, as there is an increased risk of bowel cancer in this age group.
-
Patient is aged ≥ 18 years.
-
Patient has refractory IBS (clinically significant symptoms defined by an IBS SSS of > 75).
-
Patient fulfils Rome III criteria.
-
Patient has been offered first-line therapies (e.g. antispasmodics, antidepressants or fibre-based medications) but still has continuing IBS symptoms for ≥ 12 months.
-
If > 60 years old, patient has had a consultant review in the previous 2 years to confirm that their symptoms are related to IBS and that other serious bowel conditions have been excluded.
During the telephone screening, researchers followed a script and completed a paper screening questionnaire (see Appendix 1). Screening was conducted to assess the patient’s full eligibility for the study, check their understanding of the study procedures (including the need for a blood test), answer any questions to ensure that informed consent could be undertaken online and discuss the time commitment and the constraints around availability for therapy; it included the validating measures IBS SSS and Rome III. Screening also checked participants’ access to the internet.
Potential participants were excluded if they had unexplained rectal bleeding or weight loss; had a diagnosis of inflammatory bowel disease (IBD), coeliac disease, peptic ulcer disease or colorectal carcinoma; were unable to participate in CBT because of speech or language difficulties; had no access to an internet-enabled computer to be able to undertake the WCBT; had received CBT for IBS in the past 2 years; had had previous access to the MIBS website; or were currently participating in an IBS/intervention trial (Box 2).
-
Patient has unexplained rectal bleeding or weight loss.
-
Patient has a diagnosis of IBD.
-
Patient has coeliac disease.
-
Patient has peptic ulcer disease.
-
Patient has colorectal carcinoma.
-
Patient is unable to participate in CBT because of speech or language difficulties.
-
Patient has no access to an internet-enabled computer to be able to undertake the WCBT.
-
Patient has received CBT for IBS in the past 2 years.
-
Patient has had previous access to the MIBS website.
-
Patient is currently participating in an IBS/intervention trial.
General practitioners were notified of any of their patients who failed screening for unexplained weight loss or rectal bleeding so that the symptoms could be followed up.
Patient consent
Patients meeting the screening criteria were e-mailed instructions for the website address/uniform resource locator (URL) to log in to the LifeGuide online consent form (see Report Supplementary Material 4). The research team received an automated e-mail once the form was completed, which prompted the researchers to send another e-mail to the patient, customised for each general practice or secondary care site, with an instruction to make a blood test appointment for the next stage of screening.
Blood test
Patients telephoned their general practice surgery or gastrointestinal (GI) clinic to make an appointment for the blood test. When patients already had recent (within the previous 3 months) blood test results, these were requested from the site so that patients were not required to undergo another set of tests. The function of the blood tests was to exclude alternative diagnoses to IBS, as recommended for IBS diagnosis in the NICE guidelines. 9 The blood tests undertaken were full blood count (FBC) for anaemia, tissue transglutaminase antibodies (TTG) as screening for coeliac disease and C-reactive protein (CRP) for inflammation (marker for IBD). The blood tests were undertaken by practice nurses, by GPs within the surgeries or by phlebotomists or research nurses at the secondary care sites. Sites were pre-supplied with vacutainers, a form to fax back to the research team and a form to post with the sample. Patients were instructed to arrive with their ID number so that all forms could be identified by ID number only. Samples were sent to the SUHT pathology laboratory for testing and were then destroyed. The results were posted to the research team and the GP and were checked by the chief investigator. Blood sample results were stored in a locked filing cabinet.
Abnormal blood test results
A patient with abnormal blood test results (e.g. indicating anaemia or a positive TTG) was excluded from the study and referred back to his or her GP for further assessment and the GP was informed of the abnormal results by post. Patients with a CRP level above the normal laboratory range were phoned by the team and given the option to have a second test after 4 weeks (as CRP can be raised temporarily because of a minor intercurrent illness). A second high CRP result excluded them from the trial and their GP was informed of the test result.
Baseline questionnaire
Patients with acceptable blood test results were e-mailed instructions to log on to LifeGuide to complete their baseline questionnaire (see Appendix 3) and, on completion, the team received an automated e-mail that prompted them to initiate patient randomisation.
Randomisation
Randomisation was provided by the randomisation service at the UK Clinical Research Collaboration-registered King’s College London Clinical Trials Unit (CTU) and accessed by the research team via a web-based system. Randomisation was at the level of the individual, using block randomisation with randomly varying block sizes stratified by centre (Southampton general practices, Southampton secondary care, London general practices, London secondary care). The CTU procedure was as follows: on completion of the baseline questionnaire, the trial manager or research assistant electronically submitted details of each participant to the CTU. This included participant ID number, site, initials and date of birth. The system immediately notified the unblinded researchers and recorded the randomisation outcome. Research staff were allocating patients to therapists so could not be kept blinded.
Once a patient was randomised to the study, a letter was sent to their GP (see Report Supplementary Material 5) to inform them of the patient’s participation, their allocated group and their blood test results. Patients were e-mailed instructions to receive their group by logging on to LifeGuide as promptly as possible to enter one of three codes. This directed them to one of three alternative web pages within LifeGuide, allowing them access to the relevant intervention. Patients were asked to register, which triggered future automated reminder e-mails to them at each of the follow-up collection points and directed them to further instructions for each arm of the trial. Having separate sites ensured non-contamination of treatments. Patients in the TCBT arm were asked not to share their therapy manual with others to avoid cross-contamination.
Interventions
Two active interventions were assessed in the study: TCBT with a detailed patient manual; and low-intensity WCBT (the Regul8 programme developed in the MIBS trial20), with some therapist support.
Those in the control arm received TAU. Patients in the two active intervention arms also received TAU.
The CBT content of the two treatments was the same. The CBT was based on an empirical cognitive–behavioural model of IBS,20,21 which specifies that factors such as stress and gastric infection trigger the symptoms of IBS, which are then maintained by patients’ cognitive, behavioural and emotional responses to the symptoms. For instance, if a patient becomes anxious (emotion) about the symptoms, believes that he or she has no control over them (cognitions) and responds by avoiding social situations (behaviour), this can increase anxiety and maintain symptoms through the link between a heightened autonomic nervous system and the enteric nervous system. This model was used to structure the content of the therapy sessions.
The therapy consisted of education, behavioural and cognitive techniques, aimed at improving bowel habits, developing stable healthy eating patterns, addressing unhelpful thoughts, managing stress, reducing symptom focusing and preventing relapse (Table 2).
| Session | Summary |
|---|---|
| 1. Understanding your IBS | Rationale for self-management, which includes the following explanations:
|
| 2. Assessing your symptoms | Self-assessment of the interaction between thoughts, feeling and behaviours and how these can impact on stress levels and gut symptoms |
| Development of a personal model of IBS that incorporates these elements | |
| Homework: daily diaries of the severity and experience of IBS symptoms in conjunction with stress levels and eating routines/behaviours | |
| 3. Managing symptoms and eating | Review of the symptom diary |
| Behavioural management of the symptoms of diarrhoea and constipation, and common myths in this area are discussed. Goal-setting is explained | |
| The importance of healthy, regular eating and not being overly focused on elimination is covered | |
| Homework: goal-setting for managing symptoms and regular/healthy eating. Goal-setting, monitoring and evaluation continue weekly throughout the programme | |
| 4. Exercise and activity | The importance of exercise in symptom management is covered |
| Identifying activity patterns such as resting too much in response to symptoms or an all-or-nothing style of activity is addressed | |
| Homework: goal-setting for regular exercise and managing unhelpful activity patterns, if relevant | |
| 5. Identifying your thought patterns | Identifying unhelpful thoughts (negative automatic thoughts) in relation to high personal expectations and IBS symptoms is introduced |
| The link between these thoughts, feelings, behaviours and symptoms is reinforced | |
| Homework: goal-setting plus daily thought records of unhelpful thoughts related to personal expectations and patterns of overactivity | |
| 6. Alternative thoughts | The steps for coming up with alternatives to unhelpful thoughts are covered together with personal examples |
| Homework: goal-setting plus daily thought records including coming up with realistic alternative thoughts | |
| 7. Learning to relax, improving sleep, managing stress and emotions | Basic stress management and sleep hygiene are discussed |
| Diaphragmatic breathing, progressive muscle relaxation and guided imagery relaxation are presented in video and audio formats | |
| Identifying common positive and negative emotions and the participant’s current ways of dealing with these | |
| New strategies to facilitate expression of emotion as well as coping with negative or difficult emotions are discussed | |
| Homework: goal-setting for stress management, good sleep habits and emotional processing | |
| 8. Managing flare-ups and the future | The probability of flare-ups is discussed and patients are encouraged to develop achievable long-term goals and to continue to employ the skills they have learned throughout the manual to manage flare-ups and ongoing symptoms |
The two main differences between the therapy trial arms were:
-
The amount of therapy contact was predetermined (TCBT participants received 8 hours and WCBT participants received 2.5 hours of therapy). Those in the TCBT arm had six 1-hour telephone sessions with a CBT therapist at weeks 1, 2, 3, 5, 7 and 9 and homework tasks. They also received two 1-hour booster sessions at 4 and 8 months. WCBT participants received three 30-minute telephone therapy support calls at weeks 1, 3 and 5 and two 30-minute booster sessions at 4 and 8 months.
-
The TCBT patients used a self-management CBT manual and the WCBT patients had access to an interactive website.
Development of therapy manuals
Telephone-delivered cognitive–behavioural therapy patient manual
The patient manual was an updated version of the manual used in a pilot RCT of CBT-based self-management for IBS,20 which drew on some content from a nurse-delivered CBT trial. 7 Minor updates were made to ensure that the content of the manual closely mirrored the content of the Regul8 website, including incorporating the most recent NICE guidance regarding issues such as diet. 9 Content was written to be user friendly, with minimal technical jargon and easy-to-read text broken up by imagery and diagrams. Chapters included example scenarios and educational diagrams regarding the digestive system, the fight-or-flight stress response and links between the enteric and autonomic nervous system. Homework tasks were linked to the content in each chapter and included sheets to allow participants to record activities (see Table 2).
Telephone-delivered cognitive–behavioural therapy therapist manual
The therapist manual was developed by drawing on the IBS patient manual and the therapist manual used in the PACE (Pacing, graded Activity, and Cognitive behaviour therapy; a randomised Evaluation) trial for chronic fatigue,22 adapting the content to IBS rather than fatigue. For TCBT, the manual was designed to be used flexibly by therapists for formulation-driven sessions. The manual also provided guidelines for therapists to use alongside the more structured WCBT-guided self-help sessions. This included instructions for the optimum setting for the telephone calls (i.e. ensuring that patients were in a quiet environment without interruptions). Sessions were less formulation driven than in the TCBT arm and related more closely to the sequence of the Regul8 sessions. However, therapists were encouraged to be responsive to the issues patients raised. The first two support sessions focused on clarifying and reviewing material such as the personal model, eliciting updates on clients’ progress with the programme, helping to set realistic behavioural goals and monitoring progress on goals. Later sessions focused more on eliciting and challenging unhelpful thoughts, managing stress and sleep, and possible setbacks.
Standard operating procedures for adverse event (AE) and SAE reporting, assessing low mood and suicide risk, scheduling of sessions and the non-attendance of sessions, were detailed in the manual, with additional forms provided to therapists to fill out when necessary.
Allocation to therapists
Once participants were randomised to a treatment condition, they were allocated to therapists based on therapist availability in terms of client caseload and ability to arrange sessions at times that suited the participant.
Participants randomised to TCBT were immediately sent a CBT manual, including homework sessions, in the post. A digital copy was also sent via e-mail alongside three additional documents: (1) a standard information sheet on lifestyle and diet in IBS based on the NICE guidance (see Report Supplementary Material 6), (2) a participant information sheet about the allocated treatment and (3) a brief profile of their therapist including a picture. The allocated therapist contacted participants within 1 week of allocation to arrange the first session. Participants randomised to the WCBT arm were e-mailed a login with access to the Regul8 website. 15 E-mails included the same attachments as for TCBT (excluding the CBT manual).
Therapist scheduling
Ten CBT-trained therapists undertook the therapy sessions for both the TCBT arm and the WCBT arm of the study (see Therapy summary for more details of therapist characteristics). The therapists had different work schedules (some worked full time and some part time) and there was some changeover in therapists as a result of scheduled maternity leave and therapists leaving the service. Prior to therapist allocation, participants were asked by the research assistants to detail any times that they would be unavailable for sessions. Two therapists could take calls in the evenings with participants who were unavailable during the day. A few patients were unable to participate in the trial because of the low availability of evening sessions.
For both TCBT and WCBT, therapists sought to schedule all non-booster sessions within the 9-week period following randomisation. When possible, booster sessions were arranged around the 4- and 8-month post-randomisation points. However, ability to do this was variable dependent on the availability of the patient, finding mutually agreed times for sessions and session non-attendance without prior notice. Trial research assistants provided some limited practical support to the therapists during the process of scheduling sessions for some participants (approximately 20% of the participants who were allocated to the CBT arms). This was because of repeated non-attendance or lack of reciprocal contact from participants.
Intervention fidelity and supervision
The therapists received a 1-day training session in the two CBT interventions before recruitment started, which was delivered by co-applicants (RMM and TC). During the first 3 hours of the training, the research assistants delivered oral presentations focused on the aetiology and pathophysiology of IBS, the diagnostic criteria, the financial and humanistic burden of IBS, the treatment evidence, the cognitive–behavioural interventions for managing symptoms and impact in IBS and the main processes involved with the trial. Rona Moss-Morris and Trudie Chalder contributed to these presentations by sharing their clinical experience and knowledge. The second half of the training was led by Rona Moss-Morris and Trudie Chalder. It focused on the model of understanding IBS and interventions that therapists could potentially discuss with patients using guided discovery.
Telephone therapy sessions were audio-recorded for the purpose of providing regular supervision, assessing treatment fidelity and recording the length and number of telephone sessions. Therapists completed a protocol deviation form when they were unable to record a session.
The therapists attended one 90-minute group supervision session every fortnight in the first half of the trial, which reduced to monthly in the second half of the trial. Supervision was led by Rona Moss-Morris and Trudie Chalder. Regular supervision is part of good clinical practice in CBT. Rona Moss-Morris focused on supervising the WCBT and Trudie Chalder on the TCBT. Rona Moss-Morris and Trudie Chalder listened to an audio-recording of one session from each therapist chosen by one of the research assistants. The selection of sessions sent was aimed to provide a variety of sessions across the progression of therapy for the different therapists. Therapists were also encouraged to ask supervisors to listen to recordings of sessions for which they felt that feedback might be particularly helpful. Feedback was provided on the session by the supervisors in a collaborative manner, with all therapists providing suggestions and input.
Treatment integrity and competence were assessed at the end of the trial by two independent clinical psychologists who were experienced in using CBT for medically unexplained syndromes. A random selection of 20% of session 2 for WCBT and session 3 for TCBT was rated. Randomisation was carried out by the trial statistician and stratified so that at least two sessions for every therapist and for each therapy type were available. These were rated in terms of adherence to the TCBT manual or WCBT approach. A scale used in a large RCT of treatments for chronic fatigue syndrome23 was modified and simplified for these ratings in the ACTIB trial. There were three key areas to rate on a seven-point Likert scale: (1) overall therapeutic alliance (single item), (2) CBT skills (five items) and (3) overall therapist adherence to the manual (single item). Therapy manuals covered each session in both arms.
The independent clinical psychology raters received an initial training in the fidelity ratings delivered by Rona Moss-Morris and Trudie Chalder. The training was conducted face to face and lasted approximately 2.5 hours. Tapes were rated using the scale and ratings were cross-checked and discussed. Approximately 10% of the tapes were double rated to check for inter-rater reliability and cross-checked for consistency by Rona Moss-Morris and Trudie Chalder.
Three further telephone calls were scheduled to be checked for consistency after double ratings were completed. This training ensured that the clinical psychologists worked in a similar way and that there was adequate inter-rater reliability. Raters were blind to the identity of the patient. Ratings were made independently after listening to an entire session of therapy.
Treatment as usual
Patients in all three arms received TAU, with the control arm being TAU alone. TAU was defined as continuation of current medications and usual GP or consultant follow-up with no psychological therapy. All general practice sites or secondary care sites involved in the study received a copy of the NICE guidance for IBS9 at the start to ensure that all clinicians had the standard best practice information on IBS management. They also received a deskside reminder (see Report Supplementary Material 7) to remind them of the guidelines, protocol guidance on prescribing psychological therapies and inclusion criteria. All participants received a standard information sheet on lifestyle and diet in IBS based on the NICE guidance (see Report Supplementary Material 6). Information was collected on any changes in IBS treatment/management during the study, and numbers of GP and consultant consultations were recorded for all three arms. The TAU-alone participants had access to the WCBT website (but with no therapy support) at the end of the 12-month follow-up period.
Treatment adherence for intervention arms
Treatment adherence was defined separately for the two active treatment arms. Participants allocated to TCBT who completed at least four of the initial telephone CBT sessions were deemed as having adhered to treatment. Those who were offered WCBT and completed four or more website sessions and at least one telephone support session were considered treatment adherent.
Withdrawal from treatment and/or follow-up
In accordance with good clinical practice, patients were free to withdraw from the treatment and/or follow-up at any time without this affecting their medical care. Therapists made two attempts to contact non-attenders of the therapy sessions before withdrawing patients. All information on the event was collected in the drop-out report form (see Report Supplementary Material 8) and added to the CTU commercial data entry system [InferMed MACRO (InferMed, London, UK)].
Blinding
As with any therapy trial, blinding was not possible for participants or therapists. It was also impractical for the research assistants, who liaised with the participants and therapists regarding administration tasks. However, blinding was pre-planned for the outcome assessors and the trial statisticians, as described below.
After the database was locked, a decision was taken to use multiple imputation (MI) to deal with missing data in the formal statistical analysis (see Statistical methods), which meant that the trial statistician would become aware of treatment allocation when carrying out these analyses. However, these analyses were undertaken at the end so that any that could be carried out without revealing treatment allocations were carried out by a blinded statistician.
Outcome measures were collected via the web (when possible) and were patient reported. The researcher who contacted patients by telephone to capture primary outcome data in a short questionnaire (see Appendix 4), for those patients who did not complete follow-up questionnaires after reminders, was kept blinded to the participant’s treatment group to avoid bias. Statisticians, the principal investigators and all oversight committee members were also kept blinded to treatment group.
Baseline data
The baseline data included sociodemographic details, current medication, past medical history and medications, duration of IBS symptoms, previous or current psychiatric diagnoses, Rome III, IBS SSS, Work and Social Adjustment Scale (WSAS) , EuroQol-5 Dimensions (EQ-5D), Client Service Receipt Inventory (CSRI), Hospital Anxiety and Depression Scale (HADS), Cognitive scale for Functional Bowel Disorders (CG-FBD), Brief Illness Perception Questionnaire for IBS (IPQ), Irritable Bowel Syndrome Behavioural Responses Questionnaire, Beliefs about Emotions Scale (BES), impoverished emotional experience (IEE) factor of the Emotional Processing Scale-25 and Positive and Negative Affect Schedule (PANAS).
Primary outcome measures
The clinical effectiveness of the intervention was assessed by two co-primary measures: IBS SSS24 and WSAS. 25
The IBS SSS is widely used in IBS studies (a 50-point within-participant change from baseline is regarded as clinically significant). 24 We powered this trial to detect a 35-point difference between groups at 12 months for the sample size calculations. This was to account for a 15-point placebo response in the TAU arm (the placebo response is known to be important in IBS, and the MIBS trial1,15 showed a 24-point difference in the no-website group from baseline to 12-week follow-up; thus, allowing for a 15-point placebo response at 12 months was prudent). The IBS SSS24 is a five-item, self-administered questionnaire measuring severity of abdominal pain, duration of abdominal pain, abdominal distension/tightness, bowel habit and QoL. It has a maximum score of 500: a score of < 75 indicates normal bowel function, 75–174 indicates mild IBS, 175–299 indicates moderate IBS and 300–500 indicates severe IBS.
The WSAS measures the effect of the IBS on people’s ability to work and manage at home and to participate in social and private leisure activities and relationships. 25 WSAS has been shown to be sensitive to change in IBS trials. 7,20 It has five aspects, each scored from 0 (not affected) to 8 (severely affected), with a possible total score of 40.
Secondary outcome measures
The Subject’s Global Assessment (SGA) of Relief26 is frequently used in treatment trials to identify IBS responders for therapy. 26 Participants rate their relief from IBS symptoms on a scale of 1 to 5, ranging from ‘completely relieved’ to ‘worse’. Scores are dichotomised so that patients scoring 1–3 are considered responders and those scoring 4–5 are considered non-responders.
The HADS27 is a well-validated, commonly used self-report instrument for detecting depression and anxiety in patients with medical illnesses.
The Patient Enablement Questionnaire (PEQ)21 assesses participants’ ability to cope with their illness and life.
The CSRI28 and EQ-5D29 were used to gather information on use of health services and QoL.
Patients’ GP notes were reviewed at 12 months to assess GP and other consultations in the year prior to entering the study and in the 12 months since entry into the study. Other studies20,30 have shown an impact on GP contacts from patient self-management programmes.
Patients’ adherence to the CBT treatments was measured by recording the number of telephone sessions and an automated count of web sessions accessed. A patient completing four or more sessions of the website and one or more of the telephone support calls was deemed compliant with the WCBT arms. In the TCBT arm, a patient completing four or more of the initial telephone CBT sessions was deemed compliant. Patients kept a simple log of homework tasks to complete.
Process/mediator variables
Process and mediator variables were collected in this trial and will be used to inform a process evaluation that will be undertaken in a funded extension agreed by the HTA programme, and will be reported separately from this HTA report.
The measures collected were:
-
The CG-FBD,31 a 31-item scale assessing unhelpful cognitions related to IBS.
-
The IPQ,32 an 8-point scale to assess participants’ perceptions of their illness.
-
The Irritable Bowel Syndrome-Behavioural Responses Questionnaire,33 a 26-item scale that measures changes in behaviour specific to managing IBS symptoms.
-
The BES,34 a 12-item questionnaire that measures beliefs about the unacceptability of experiencing and expressing negative emotions. These beliefs are likely to have implications for emotion regulation and processing. Principal components analysis identified one factor and the scale had high internal consistency (0.91). 34
-
The IEE factor of the Emotional Processing Scale,35 composed of five items and related to the labelling and awareness of emotional events, which influence the way people process their emotions. The subscale has high internal consistency (0.82). 35
-
The PANAS36 measures both positive and negative affect. The reliabilities of the PANAS, as measured by Cronbach’s alpha, were 0.89 for positive affect and 0.85 for negative affect. 37 Participants completed only the positive affect subscale because the HADS measures negative affect.
Adverse event reporting
An AE was defined as any clinical change, disease or disorder experienced by the participant during their participation in the trial, whether or not it was considered related to the intervention. A SAE was defined as an AE that was life-threatening or that resulted in inpatient hospitalisation, a disability/incapacity, a congenital anomaly/birth defect in the offspring of a subject, or another medical event requiring intervention to prevent one of these outcomes. All sites and therapists were supplied with SAE forms (see Report Supplementary Material 9) to complete and a SAE standard operating procedure (SOP).
In addition, patients were asked to self-report any medical events at each assessment.
Patients were asked the following online questions at the 3-, 6- and 12-month follow-up points:
-
Since you started the study have you had any of the following events – a life-threatening event, admission to hospital where you had to stay overnight, permanent disability/incapacity, a congenital anomaly/birth defect in a child of yours, or other medical events requiring medical attention to prevent one of the above? If yes, please give details.
-
Has your health been adversely affected since the start of the study? If yes, please give details.
The chief investigator, on behalf of the sponsor, assessed each completed SAE form for relatedness to the intervention and expectedness, to identify serious adverse reactions (SARs) and suspected unexpected serious adverse reactions. The co-ordinating centres’ SOPs were followed with respect to reporting to the sponsor, Research Ethics Committee (REC), Data Monitoring and Ethics Committee (DMEC), Trial Steering Committee (TSC) and local governance offices. Annual safety reports were submitted to the REC. All AEs and SAEs were entered onto MACRO.
Economic evaluation measures
These are discussed in Chapter 3.
Follow-up procedures
Participants completed follow-up measures online at 3, 6 and 12 months after baseline using the LifeGuide website (see Appendix 5). Those who were unable to complete the outcome measures online received a paper copy of the questionnaires. If this was not completed, then they received a telephone call from a blinded researcher, who took the participant through a limited selection of the main outcome measures. These were the IBS SSS, WSAS, SGA, PEQ and HADS.
The follow-up procedure was as follows: the baseline assessment was conducted within 90 days of screening (to allow time for the blood tests) and randomisation was conducted within 3 days of baseline (to allow for weekends). Patients received two automated reminders from LifeGuide to complete their follow-up assessments at 3, 6 and 12 months. In addition, the researchers received an automated message from LifeGuide after 3 weeks if the questionnaires had not been completed, which prompted the team to send a personalised e-mail to the patient. This was followed by a further text, a paper copy of the full questionnaire sent to the patient’s home address and then, if still not complete, a telephone call from a blinded member of the research team to complete a short questionnaire.
All baseline and most 3-, 6- and 12-month outcome data were self-completed by the patient on a data collection section of the Regul8 programme website (LifeGuide). LifeGuide was maintained and hosted by the University of Southampton. The research team was notified when LifeGuide data were missing, and actions were taken to collect these data via paper questionnaires in the post and by telephone. Primary outcome data and some secondary outcome data that were collected by paper questionnaires or short telephone questionnaires were entered by the research team onto MACRO; these included PEQ, IBS SSS, WSAS, SGA and HADS. Other paper-collected outcome measures, including CSRI, IPQ, Behavioural Responses Questionnaire, IEE-EPS (Emotional Processing Scale), BES, PANAS and EQ-5D, were entered onto a spreadsheet. In addition, all questionnaire completion dates and sources of data were entered onto MACRO, as were economic data, AEs and SAEs and withdrawals.
Error checking was carried out in the full questionnaire database of the ACTIB trial. This was to check that the error rates for the primary outcomes were < 1% and the error rates for the secondary outcomes were < 5%; 20% checks were carried out on all variables for the 3-, 6- and 12-month questionnaires. Data checking was carried out by the London data entry research assistants, with Stephanie Hughes, Alice Sibelli and Sula Windgassen ensuring that the checks were carried out by a different person from the original data entry:
-
3 months – 89 questionnaires, every fifth ID selected = 18 patients
-
6 months – 103 questionnaires, every fifth ID selected = 21 patients
-
12 months – 139 questionnaires, every fifth ID selected = 28 patients.
A spreadsheet of any errors and changes needed was kept, listing variable name, original data entry, change, date, initial and reason.
Patient retention
In late 2014, follow-up monitoring revealed that follow-up rates were not as high as the 80% anticipated in the original sample size calculation. Follow-up procedures were very carefully scrutinised and every effort was made to increase rates using the follow-up methods described in the previous section. In December 2015, the REC approved an increase in the number of participants to be recruited, with a new target of up to 570 participants [as per an updated sample size calculation to allow for the lower follow-up rates (see Sample size)]. The REC also approved sending vouchers to participants, as evidence from the literature38 suggested that non-conditional vouchers could help to incentivise patients to complete follow-up questionnaires. Cards with an enclosed £10 Love2shop voucher were posted to all participants before their 3-month questionnaire date (and immediately for any patients who had passed this date).
Sample size
A 35-point difference between therapy groups and TAU on IBS SSS at 12 months was regarded as the minimal clinically important difference (MCID) (assuming a 15-point placebo response in the TAU arm in the trial). 15,24 Assuming a within-group IBS SSS standard deviation (SD) of 76 (taken from the MIBS pilot study1), this equated to an effect size of 0.46. To achieve 90% power to detect such an effect or larger using a two-sided independent-samples t-test at the 2.5% significance level (adjusting for two primary outcomes), it was estimated that the trial would require 119 subjects per group. Based on each of 10 therapists delivering therapy to 17 patients in the WCBT and TCBT groups, and an intraclass correlation of 0.02, taken from Baldwin et al.,39 this sample size was increased by an inflation factor of 1.32 to take account of therapist effects. We measured IBS SSS at baseline and assumed that baseline values were predictive of post-treatment values (correlation 0.4). Accounting for this in our statistical analysis model allowed us to decrease the sample size by a deflation factor of 0.84. Finally, we applied a further inflation factor of 1.25 on the assumption that attrition would be < 20%. The final sample size requirement was thus calculated as 165 patients per group or 495 patients in total.
In terms of our second primary outcome (WSAS), the initial 495 sample size was calculated to be sufficient to detect a difference between the WCBT (or TCBT) and TAU groups of ≥ 3.7. Specifically, we assumed inflation factors of 1.32 for correlation of outcomes within therapists and of 1.25 for attrition and a deflation factor of 0.84 for correlation between baseline and follow-up measures. Based on this, a moderate effect size of 0.46 could be found with 90% power at the 2.5% significance level, given 119 participants per group. Assuming a SD of 8.0 (as estimated in a study of CBT for IBS7), this would equate to a treatment difference of 3.7 on this scale. This is less than the difference of 5.4 in change of means in WSAS that was found in a trial of a CBT-based self-management intervention for IBS. 20
As the trial progressed, we found that the attrition rate was closer to 30% (November 2014 estimate). The sample size was recalculated using the same group size of 119 subjects, with inflation and deflation factors of 1.32 and 0.84 kept constant. The updated attrition rate of 30% gave a sample size of 189 patients per group and a total of 567 patients. We gained ethics approval to increase recruitment to the trial within the same recruitment time frame to aim for this larger calculated sample size.
Statistical methods
A statistical analysis plan (SAP) (see Appendix 6) was developed by the statisticians (KG, RH and SL), discussed by the trial management team and the DMEC and approved by the chief investigator (HE) and chairperson of the TSC before database lock. The following approach was used to formally compare the primary and secondary outcomes between a CBT arm and TAU: an intention-to-treat (ITT) approach was used for all primary and secondary outcomes, that is, participants were analysed in the groups to which they were randomised. For each outcome and assessment time point (3, 6 or 12 months), we estimated the effect of treatment (TCBT or WCBT) compared with TAU to assess treatment effectiveness. The TCBT and WCBT arms were not formally compared.
As we had two primary outcomes (IBS SSS and WSAS at 12 months), significance testing for these two variables was conducted at the Bonferroni-adjusted significance level of alpha = 0.025 to account for two outcome comparisons. Furthermore, because we carried out two planned comparisons per outcome measure, further adjustments were not necessary.
The primary outcome measures and the secondary outcome measure, HADS score, were continuous variables. Their modelling relied on normal assumptions for error terms and treatment effects were quantified by trial arm differences (and standardised differences). We had planned to analyse PEQ under a normal assumption but found inflated floor and ceiling effects for this variable. To facilitate modelling, the original PEQ was reclassified as a binary responder measure (0 = non-response, 1 = response). SGA measures were also reclassified as binary responders, as originally described in the SAP. A ‘PEQ responder’ was defined as a participant achieving a score of ≥ 6 at the post-randomisation time point. A ‘SGA responder’ was defined as a participant achieving a score of between 1 and 3, as planned in the SAP. Binary outcome variables were analysed within a logistic regression framework and treatment effects quantified by odds ratios (ORs).
Need for multiple imputation
Formal trial arm comparisons were carried out by MI, more specifically by using the flexible multivariate imputation by chained equations (MICE) approach. 40 This was necessary because non-adherence to treatment, defined as completing four of the telephone calls (not including booster sessions) for the TCBT arm and as completing four or more of the website sessions and at least one telephone call (not including booster sessions) for the WCBT arm, was found to be predictive of missing primary outcomes at 12 months in each of the CBT arms. To avoid unblinding the trial statisticians, testing for whether or not adherence to treatment was associated with missing data at the final time point was carried out by an independent statistician. The association between treatment adherence and missing data at the final time point in the TCBT or WCBT arms was tested using Fisher’s exact tests. Adherence was found to be predictive in both CBT arms. Thus, a MI approach was pursued to allow for a missing data-generating mechanism that was missing at random (MAR), with the observed variables allowed to drive missingness including adherence with TCBT or WCBT.
We empirically assessed whether or not baseline variables were predictive of missing data using logistic regression. The following baseline variables were considered: IBS SSS, HADS, WSAS, age at randomisation, Index of Multiple Deprivation (IMD), duration of IBS symptoms, duration of IBS symptoms before diagnosis, whether or not the participant was registered with an IBS specialist, who they lived with, their marital status, the type of residence in which they lived, their choice of group at baseline, their highest level of education and their gender. Variables were considered to be potentially important, and later considered for inclusion in the imputation model, if an unadjusted logistic regression of missingness at 12 months on the baseline variable was statistically significant at a liberal 20% test level. The variables found to be potentially important predictors of missingness were then included in a multivariate logistic regression, starting with baseline IBS SSS and IMD. At each stage, the more complex model was tested against the simpler model using a likelihood ratio test, again at the 20% level. The preferred model of baseline predictors of missingness was found to be baseline IBS SSS and IMD only.
The MICE approach was used with regression models for imputation of missing values in continuous variables and logistic regression models for imputation of missing values in binary variables. In addition, predictive mean matching to a randomly chosen value from one of the 10 nearest neighbours was used for continuous outcome variables (IBS SSS, WSAS and HADS) to ensure that all imputed values lay within the observed data range.
Analysis model
The analysis models used to estimate treatment effectiveness included the outcome variable as the dependent variable and the trial arm (two dummy variables indicating the TCBT and WCBT arms), baseline values of the outcome (if available) and randomisation stratifier (dummy variables for four levels) as explanatory variables. As both TCBT and WCBT were delivered by therapists, possible therapist effects were investigated. For this purpose, two therapists who saw only a small number of participants, all of whom were compliers, were merged into a single group that also included those participants in the TCBT and WCBT arms who were not assigned a therapist. This was to avoid the computational instability and perfect prediction issues that were seen when the two therapists with few participants were considered as single therapists. To select appropriate therapist effects, a series of models were fitted for the participants who completed follow-up (completers) and compared using likelihood ratio tests. Model A allowed for therapist-varying random intercepts in each of the CBT arms with the variances of these random effects allowed to differ between TCBT and WCBT. Model B included therapist-varying random intercepts, but only in the TCBT arm. Model C did not include any therapist effects. Model A fitting significantly better than model B at a liberal 10% level was interpreted as evidence for therapist effects in both CBT arms; model B fitting better than model C was evidence for therapist effects in the TCBT arm only. We detected therapist effects in the TCBT arm for some variables (see Table 31) in both continuous and binary outcomes. Hence, the analysis models were extended to also include therapist-varying random intercepts in the TCBT arm. For binary outcomes affected by therapist effects, this meant that estimated ORs were conditioned on the therapist. In such cases, the OR was marginalised across therapists within a stratifier level to ensure that all quoted ORs estimated the same effect size measure (OR of treatment within stratifier level).
Imputation model
For each outcome variable, the imputation model included (1) all of the variables of the analysis model, (2) measures of the outcome variable at other assessment time points including baseline and (3) known predictors of missingness (binary adherence variables for each of TCBT and WCBT, IBS SSS and IMD). (1) is stipulated by MI theory, (2) was carried out to improve the precision of the imputed values and also allow outcome measures at earlier time points to drive dropout at later time points, and (3) accommodates identified predictors of missingness and allowed us to make a more realistic MAR assumption. For analysis models that contained (random) therapist effects, (4) fixed effects for therapists in the TCBT arm were also added to ensure that the imputation model remained more general than the analysis model.
Relevant assumptions were checked. Normality and homogeneity assumptions were checked for modelling of IBS SSS, WSAS and HADS using residual diagnostics. All of these checks were satisfactory. This was also carried out for PEQ and highlighted that PEQ scores could not be treated as normally distributed.
The validity of the imputations was checked using the Stata® version 14.2 (StataCorp LP, College Station, TX, USA) command midiagplots,41 comparing the cumulative distribution of the imputed data against the fully observed data and the merged completed and imputed data. The differences in distribution across different iterations of the imputation were also investigated.
Sensitivity analyses
Four sets of sensitivity analyses were conducted. The first sensitivity analysis assessed the impact of excluding participants who had IBS SSS values at baseline below the inclusion threshold of 75 from the analysis set. (The IBS SSS eligibility criterion was determined at screening.) For the primary outcomes, non-eligible participants were dropped from the analysis set, the reduced data were reanalysed and the finding was compared with the original results to evaluate sensitivity. The second sensitivity analysis looked at the impact of using only observations that were recorded within the prespecified assessment time windows. Again, those outside the window were dropped from the analyses of the primary outcomes. The third sensitivity analysis evaluated the impact of defining PEQ responders according to an alternative threshold. ‘PEQ responders’ were defined according to another possible threshold and PEQ response was reanalysed at the primary outcome time point (12 months). Finally, the complier-average causal effect (CACE) was estimated in order to estimate efficacy without bias. The original ITT analyses estimate the clinical effectiveness of the interventions, but these estimates are biased for efficacy in the presence of non-compliance. To understand the extent to which the ITT estimates were affected by non-compliance with randomised treatment, we carried out a simple CACE analysis using complete cases. To this end we modelled the effect of the binary endogenous variables ‘receipt of TCBT’ and ‘receipt of WCBT’ on the co-primaries using randomisation to TCBT or WCBT, respectively, as instrumental variables. Further covariates in the models were baseline values of the outcome and dummy variables reflecting the randomisation stratifier. We had also considered a sensitivity analysis to investigate the impact on results of the missingness process being not MAR. However, follow-up rates were reasonably good and there was no information to inform such sensitivity analyses, so this was not carried out.
All analyses were carried out in Stata version 14.2.
Software
Data management
Two online data collection systems were used: LifeGuide and MACRO. The senior research assistant (SH) in charge of LifeGuide extracted the data from the main database and removed unblinding data or any disclosive information if required. MACRO was hosted on a dedicated server at King’s College London and managed by the King’s College London CTU. Its data manager extracted data periodically as needed.
Results
Recruitment
Sites were entered into the study in stages to ensure a continuous and steady feed of patients into the available therapy slots. The first sites to participate were given the instruction to search databases in February 2014 and the first invitation letters were sent in March 2014. The patient recruitment rate was monitored and subsequent sites were started each month over the following 23 months.
A total of 15,065 invitations were given out by 77 sites (74 general practice surgeries and three secondary care centres). There were 14,908 invitation letters (98.96%) delivered by mail-outs following a note search of potential participants. Forty-four invitation letters (0.29%) were sent after potential participants saw displayed posters and contacted the research teams, and 113 invitation letters (0.75%) were handed out by consultants or GPs during GI consultations as opportunistic recruitment (Table 3).
| Centre | Recruitment method (n) | Total (n) | ||
|---|---|---|---|---|
| Mail-out | Poster | Opportunistic | ||
| GSTT | 203 | 80 | 283 | |
| KCH | 208 | 208 | ||
| LPC sites | 5482 | 2 | 7 | 5491 |
| SPC sites | 8841 | 40 | 25 | 8906 |
| UHS | 174 | 2 | 1 | 177 |
| Total | 14,908 | 44 | 113 | 15,065 |
Characteristics of general practices
Of the 103 (38 South London and 65 Wessex) general practices that initially agreed to participate, 74 (28 South London and 46 Wessex) progressed to carrying out note searches and mail-outs (some general practice sites dropped out, citing that they were too busy, or did not reply to prompts). At each practice, a GP or practice manager acting as site principal investigator was assigned to the study. Practices were reimbursed for activity time by Department of Health and Social Care service support costs. Sites were recruited from both urban and rural settings and had a range of sociodemographic characteristics (Tables 4 and 5).
| CCG | Number of general practices |
|---|---|
| South London | |
| Bexley | 2 |
| Bromley | 4 |
| Croydon | 1 |
| Greenwich | 1 |
| Lambeth | 10 |
| Lewisham | 1 |
| Merton | 2 |
| Southwark | 6 |
| Wandsworth | 1 |
| Total | 28 |
| Wessex | |
| Dorset | 8 |
| Fareham and Gosport | 3 |
| Hampshire | 1 |
| Isle of Wight | 2 |
| North East Hampshire and Farnham | 3 |
| North Hampshire | 2 |
| Portsmouth | 3 |
| South Eastern Hampshire | 9 |
| Southampton City | 4 |
| West Hampshire | 5 |
| Wiltshire | 6 |
| Total | 46 |
| Characteristic | General practice (n) | Total (n) | |
|---|---|---|---|
| London | Wessex | ||
| Practice list size | |||
| 1–4999 | 1 | 5 | 6 |
| 5000–14,999 | 23 | 31 | 54 |
| ≥ 15,000 | 4 | 10 | 14 |
| Number of GP partners | |||
| 0–5 | 11 | 12 | 23 |
| 6–10 | 15 | 28 | 43 |
| 11–15 | 2 | 5 | 7 |
| ≥ 16 | 0 | 1 | 1 |
| Deprivation score (IMD decile): 1 (most deprived) to 10 (least deprived) | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 |
| 2 | 4 | 0 | 4 |
| 3 | 6 | 0 | 6 |
| 4 | 5 | 10 | 15 |
| 5 | 5 | 2 | 7 |
| 6 | 3 | 5 | 8 |
| 7 | 1 | 5 | 6 |
| 8 | 1 | 6 | 7 |
| 9 | 1 | 5 | 6 |
| 10 | 2 | 13 | 15 |
| Total | 28 | 46 | 74 |
An indication of the level of deprivation of the practice setting was calculated using the IMD score 2016 obtained from the Public Health England website (www.gov.uk/government/statistics/english-indices-of-deprivation-2015; accessed 27 January 2019), using practice postcode (8). This website was recommended by the National Institute for Health Research (NIHR) CRN Wessex for its reliability. The IMD measures the deprivation of small areas using 38 separate indicators in each of the seven domains: (1) income deprivation, (2) employment deprivation, (3) health deprivation and disability, (4) education, skills and training deprivation, (5) barriers to housing and services, (6) living environment deprivation and (7) crime. These indicators are then combined using appropriate weightage to calculate the IMD. IMD scores in deciles are presented as numbers ranging from 1 (most deprived) to 10 (least deprived).
Characteristics of invited participants
Data on the gender and date of birth of invitees were collected by the sites and reported to the research team anonymously. A total of 71.4% of those invited were female and the average age was 47.7 years (Tables 6 and 7).
| Centre | Gender (n) | Total (n) | ||
|---|---|---|---|---|
| Female | Male | Missing | ||
| GSTT | 201 | 82 | 0 | 283 |
| KCH | 140 | 63 | 5 | 208 |
| LPC sites | 3618 | 1791 | 82 | 5491 |
| SPC sites | 6451 | 2228 | 227 | 8906 |
| UHS | 134 | 42 | 1 | 177 |
| Total | 10,544 | 4206 | 315 | 15,065 |
| Variable | Mean | SD | Number of participants | Minimum | Maximum | Median | IQR | Possible range |
|---|---|---|---|---|---|---|---|---|
| Age at invitation (years) | 47.7 | 16.8 | 15,065 | 15.9 | 102.2 | 47 | 34–62 | 16–98 |
Reasons for declining the ACTIB invitation
When sending out the ACTIB invitations, we included an option to return a ‘reason for decline’ slip so that we could gather information on why people chose not to participate in the ACTIB trial.
The main reasons that those invited gave for declining participation were that their IBS symptoms had improved and they did not need further input, that they did not have the time and that they did not want to take part in CBT or an online programme (Table 8). The number who declined was 2423 (multiple answers were allowed).
| Centre | Reason (n) | ||||||
|---|---|---|---|---|---|---|---|
| MIBS accessa | MIBS studyb | No timec | Declined TCBTd | Declined onlinee | IBS OKf | Other reasong | |
| GSTT | 0 | 1 | 11 | 5 | 4 | 4 | 9 |
| KCH | 0 | 1 | 13 | 5 | 7 | 6 | 11 |
| LPC sites | 10 | 12 | 235 | 172 | 158 | 314 | 225 |
| SPC sites | 23 | 13 | 521 | 551 | 504 | 893 | 616 |
| UHS | 0 | 0 | 12 | 7 | 6 | 10 | 8 |
| Total | 33 | 27 | 792 | 740 | 679 | 1227 | 869 |
Telephone screen and consent
Screening commenced 2 months ahead of the first scheduled randomisation to allow time for the screening blood tests. A total of 1525 replies that indicated an interest in participating were returned and 1452 patients were screened, with 558 patients (38.4%) randomised into the study over 23 months. Tables 9 and 10 show the gender and age of those interested in participating. The main reasons for ineligibility at screening were not having been offered first-line therapies (e.g. antispasmodics, antidepressants or fibre-based medications) and/or not having continuing IBS symptoms for ≥ 12 months, being > 60 years of age with no consultant review, failing to meet IBS SSS criteria, failing to meet Rome III criteria, or having no access to the internet (see Table 13).
| Centre | Gender (n) | Total (n) | ||
|---|---|---|---|---|
| Female | Male | Missing | ||
| GSTT | 71 | 29 | 0 | 100 |
| KCH | 0 | 0 | 44 | 44 |
| LPC sites | 99 | 21 | 294 | 414 |
| SPC sites | 733 | 205 | 0 | 938 |
| UHS | 21 | 8 | 0 | 29 |
| Total | 924 | 263 | 338 | 1525 |
| Variable | Mean | SD | Number of participants | Minimum | Maximum | Median | IQR | Possible range |
|---|---|---|---|---|---|---|---|---|
| Age at invitation (years) | 47.87 | 15.77 | 47.87 | 16 | 94 | 48 | 34–60 | 16–94 |
The mean age of potential participants interested in participating was 47.8 years, the mean age of those invited was 47.7 years and the mean age of those randomised was 43.1 years (some potential participants aged > 60 years were excluded on safety grounds at screening because they had not had a consultant review).
The proportion of females was 77.8% among those interested, 71.5% among those invited and 75.8% among those randomised.
Screening blood test results
The reasons for failing the blood test are given in Table 11.
| Blood test failure reason | n |
|---|---|
| Abnormal CRP | 33 |
| Abnormal FBC | 13 |
| Abnormal FBC and CRP | 5 |
| Abnormal TTG | 2 |
| Missing sample/unlabelled blood/haemolysed | 6 |
| Other medical reason | 1 |
| No blood from veins | 1 |
| Total | 61 |
Summary of recruitment
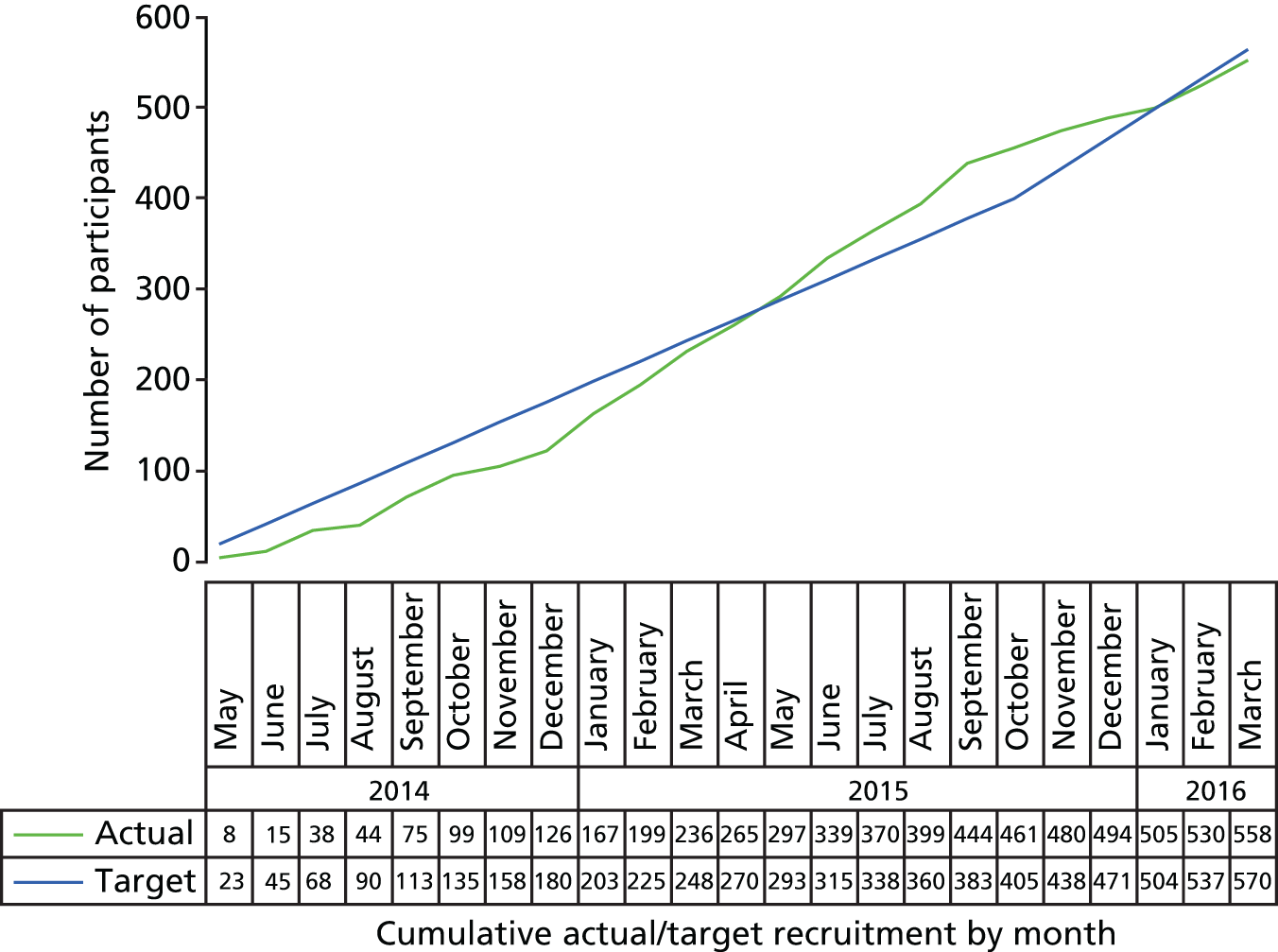
Table 12 and Figure 1 show recruitment by centre and over time.
| Pathway | Site (n) | All centres (n) | ||||
|---|---|---|---|---|---|---|
| GSTT | KCH | LPC sites | SPC sites | UHS | ||
| Sites | 1 | 1 | 28 | 46 | 1 | 77 |
| Mailout | 203 | 208 | 5482 | 8841 | 174 | 14,908 |
| Poster | 0 | 0 | 2 | 40 | 2 | 44 |
| Opportunistic | 80 | 0 | 7 | 25 | 1 | 113 |
| Patient invitations | 283 | 208 | 5491 | 8906 | 177 | 15,065 |
| Invitations | ||||||
| ‘Yes’ | 100 | 44 | 414 | 938 | 29 | 1525 |
| ‘Declined’ | 18 | 28 | 633 | 1719 | 25 | 2423 |
| Screened | 96 | 41 | 370 | 916 | 29 | 1452 |
| Randomised | 59 | 19 | 141 | 324 | 15 | 558 |
FIGURE 1.
Participant recruitment against target.
Consolidating Standards of Reporting Trials
A CONSORT flow chart has been constructed. This includes the number of potential patients contacted and screened, the number of eligible patients, the number of patients agreeing to enter the trial and the number of patients refusing to enter the trial, and then, by treatment arm, the number of patients adherent to treatment, the number of patients continuing through the trial, the number of patients withdrawing, the number of patients lost to follow-up and the number of patients excluded/analysed.
Treatment adherence was defined separately for the two active treatment arms. Participants allocated to TCBT who completed at least four of the initial telephone calls were deemed adherent to treatment. Those who were offered WCBT and completed four or more website sessions and at least one telephone support call were considered as treatment adherent.
Details of the patient pathway through the recruitment process are included in the CONSORT flow diagram (Figure 2), with details of the inclusion and exclusion criteria given in Table 13.
| Ineligibility criteria | Number of participants |
|---|---|
| Failed inclusion criteria | 547 |
| Not aged ≥ 18 years | 2 |
| Does not have refractory IBS (clinically significant symptoms defined by a IBS SSS of > 75) | 54 |
| Does not fulfil Rome III criteria | 43 |
| Has not been offered first-line therapies (e.g. antispasmodics, antidepressants or fibre-based medications) and does not have continuing IBS symptoms for ≥ 12 months | 297 |
| If > 60 years old, patient has not had a consultant review in the in the previous 2 years | 151 |
| Exclusion criteria | 76 |
| Unexplained rectal bleeding or weight loss | 5 |
| Diagnosis of IBD | 8 |
| Diagnosis of coeliac disease | 2 |
| Diagnosis of peptic ulcer disease | 0 |
| Diagnosis of colorectal carcinoma | 0 |
| Unable to participate in CBT because of speech or language difficulties | 4 |
| No access to an internet computer to be able to undertake the lower-intensity WCBT | 53 |
| Received CBT for IBS in the past 2 years | 3 |
| Had previous access to the MIBS website | 1 |
| Currently participating in an IBS/intervention trial | 0 |
Baseline data summary
A total of 558 (38.4%) out of 1452 patients screened for eligibility were randomised, with 186 randomly allocated to TCBT, 185 to WCBT and 187 to TAU.
Table 14 shows that, as expected from randomisation, the three trial arms were well balanced with regard to clinical and demographic variables. In particular, the distribution of recruitment centre (stratification factor) was almost identical across trial arms.
| Participant variables | Trial arm | All (N = 558) | ||
|---|---|---|---|---|
| TCBT (N = 186) | WCBT (N = 185) | TAU (N = 187) | ||
| Number from recruitment centre,a n (%) | ||||
| Southampton general practices | 108 (58.1) | 108 (58.4) | 108 (57.8) | 324 (58.1) |
| Southampton secondary care | 5 (2.7) | 5 (2.7) | 5 (2.7) | 15 (2.7) |
| London general practices | 46 (24.7) | 47 (25.4) | 48 (25.7) | 141 (25.3) |
| London secondary care | 27 (14.5) | 25 (13.5) | 26 (13.9) | 78 (14.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Age in years | ||||
| Mean (SD) | 43.4 (12.5) | 43.8 (13.6) | 42.0 (13.5) | 43.1 (13.2) |
| N (median) | 186 (43.9) | 185 (42.8) | 187 (40.8) | 558 (42.5) |
| Minimum (maximum) | 20.0 (75.6) | 19.4 (74.2) | 18.8 (81.0) | 18.8 (81.0) |
| Missing | 0 | 0 | 0 | 0 |
| Duration of symptoms before diagnosis in years | ||||
| Mean (SD) | 6.4 (8.0) | 6.3 (7.6) | 5.6 (6.8) | 6.1 (7.5) |
| N (median) | 186 (3.29) | 185 (3.0) | 187 (3.0) | 558 (3.0) |
| Minimum (maximum) | 0.0 (47.0) | 0.0 (40.0) | 0.0 (40.0) | 0.0 (47.0) |
| Missing | 0 | 0 | 0 | 0 |
| Duration of IBS in years | ||||
| Mean (SD) | 9.9 (9.8) | 10.8 (9.3) | 10.4 (10.2) | 10.4 (9.7) |
| N (median) | 185 (6.5) | 185 (8.6) | 187 (6.3) | 557 (7.4) |
| Minimum (maximum) | 1.0 (65) | 0.7 (45.4) | 0.3 (49.9) | 0.3 (64.6) |
| Missing | 1 | 0 | 0 | 1 |
| Deprivation score: IMD 2010 | ||||
| Mean (SD) | 16.7 (12.0) | 17.3 (12.3) | 17.2 (11.8) | 17.1 (12.0) |
| N (median) | 186 (13.5) | 185 (13.1) | 187 (14.1) | 557 (13.7) |
| Minimum (maximum) | 0.8 (47.9) | 1.1 (53.6) | 1.8 (54.0) | 0.8 (54.0) |
| Missing | 0 | 0 | 1 | 1 |
| Type of residence, n (%) | ||||
| Owner-occupied flat or house | 138 (74.2) | 124 (67.0) | 121 (64.7) | 383 (68.6) |
| Privately rented flat or house | 30 (16.1) | 34 (18.4) | 54 (28.9) | 118 (21.2) |
| Flat or house rented from local authority | 12 (6.5) | 15 (8.1) | 8 (4.3) | 35 (6.3) |
| Other | 6 (3.2) | 12 (6.5) | 4 (2.1) | 22 (3.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Gender, n (%) | ||||
| Male | 47 (25.3) | 40 (21.6) | 48 (25.7) | 135 (24.2) |
| Female | 139 (73.7) | 145 (78.4) | 139 (74.3) | 423 (75.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Marital status, n (%) | ||||
| Single | 46 (24.7) | 49 (26.5) | 54 (28.9) | 149 (26.7) |
| Married | 92 (49.5) | 91 (49.2) | 80 (42.8) | 263 (47.1) |
| Living with partner | 31 (16.7) | 31 (16.8) | 36 (19.2) | 98 (17.6) |
| Separated | 6 (3.2) | 3 (1.6) | 2 (1.1) | 11 (2.0) |
| Divorced | 7 (3.8) | 8 (4.3) | 12 (6.4) | 27 (4.8) |
| Widowed | 4 (2.2) | 3 (1.6) | 3 (1.6) | 10 (1.8) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Living with, n (%) | ||||
| Spouse/partner | 68 (36.6) | 79 (42.7) | 84 (44.9) | 231 (41.4) |
| Spouse/partner and children | 55 (29.6) | 43 (23.2) | 32 (17.1) | 130 (23.3) |
| Children (without spouse) | 5 (2.7) | 7 (3.8) | 11 (5.9) | 23 (4.1) |
| Parents | 14 (7.5) | 8 (4.3) | 13 (7.0) | 35 (6.3) |
| Alone | 25 (13.4) | 28 (15.1) | 30 (16.0) | 83 (14.9) |
| Other | 19 (10.2) | 20 (10.8) | 17 (9.1) | 56 (10.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Had a consultant at time of baseline assessment, n (%) | ||||
| No | 166 (89.3) | 170 (91.9) | 165 (88.2) | 501 (89.8) |
| Yes | 20 (10.7) | 15 (8.1) | 22 (11.8) | 57 (10.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Tried specific IBS diets previously, n (%) | ||||
| Yes | 83 (44.6) | 93 (50.3) | 98 (52.4) | 274 (49.1) |
| No | 103 (55.4) | 92 (49.7) | 89 (47.6) | 284 (50.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Taking part in self-help group, n (%) | ||||
| Yes | 4 (2.2) | 1 (0.5) | 1 (0.5) | 6 (1.1) |
| No | 182 (97.9) | 184 (99.5) | 186 (99.5) | 552 (98.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Previous anxiety treatment, n (%) | ||||
| Yes | 49 (26.3) | 61 (33.0) | 59 (31.6) | 169 (30.3) |
| No | 137 (73.7) | 124 (67.0) | 128 (68.4) | 389 (69.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Previous depression treatment, n (%) | ||||
| Yes | 67 (36.0) | 78 (42.2) | 75 (40.1) | 220 (39.4) |
| No | 119 (64.0) | 107 (57.8) | 112 (59.9) | 338 (60.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Treatment preference, n (%) | ||||
| TCBT (paper manual and eight 1-hour CBT sessions) | 73 (39.3) | 67 (36.2) | 72 (38.5) | 212 (38.0) |
| WCBT (eight online modules and five CBT sessions) | 72 (38.7) | 68 (36.8) | 62 (33.2) | 202 (36.2) |
| TAU (usual treatment) | 8 (4.3) | 10 (5.4) | 14 (7.5) | 32 (5.7) |
| No preference | 33 (17.7) | 40 (21.6) | 39 (20.9) | 112 (20.1) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Highest education level, n (%) | ||||
| No formal education | 5 (2.7) | 7 (3.8) | 10 (5.4) | 22 (3.9) |
| GCSE/O level or equivalent | 32 (17.2) | 42 (22.7) | 38 (20.3) | 112 (20.1) |
| A level or equivalent | 28 (15.0) | 24 (13.0) | 31 (16.6) | 83 (14.9) |
| Degree | 71 (38.2) | 61 (33.0) | 58 (31.0) | 190 (34.1) |
| Postgraduate | 39 (21.0) | 33 (17.8) | 37 (19.8) | 109 (19.5) |
| Other | 11 (5.9) | 18 (9.7) | 13 (7.0) | 42 (7.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Ethnicity, n (%) | ||||
| White | 162 (87.1) | 171 (92.4) | 174 (93.0) | 507 (90.9) |
| Mixed/multiple ethnic groups | 6 (3.2) | 2 (1.1) | 3 (1.6) | 11 (2.0) |
| Asian/Asian British | 1 (0.5) | 1 (0.5) | 0 (0.0) | 2 (0.4) |
| Other | 0 (0.0) | 3 (1.6) | 2 (1.1) | 5 (0.9) |
| Missing | 17 (9.1) | 8 (4.3) | 8 (4.3) | 33 (5.9) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Children aged ≥ 5 years, n (%) | ||||
| 0 | 77 (41.4) | 73 (39.5) | 84 (44.9) | 234 (41.9) |
| 1 | 25 (13.4) | 25 (13.5) | 16 (8.6) | 66 (11.8) |
| 2 | 23 (12.4) | 17 (9.2) | 17 (9.1) | 57 (10.2) |
| 3 | 5 (2.7) | 4 (2.2) | 5 (2.7) | 14 (2.5) |
| 4 | 2 (1.1) | 1 (0.5) | 3 (1.6) | 6 (1.1) |
| 5 | 1 (0.5) | 0 (0.0) | 1 (0.5) | 2 (0.4) |
| 6 | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Missing | 52 (28.0) | 65 (35.1) | 61 (32.6) | 178 (31.9) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Children aged < 5 years, n (%) | ||||
| 0 | 107 (57.5) | 103 (55.7) | 111 (59.4) | 321 (57.5) |
| 1 | 18 (9.7) | 11 (5.9) | 11 (5.9) | 40 (7.2) |
| 2 | 5 (2.7) | 3 (1.6) | 0 (0.0) | 8 (1.4) |
| Missing | 56 (30.1) | 68 (36.8) | 65 (34.7) | 189 (33.9) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Number of elderly people living with participant, n (%) | ||||
| 0 | 117 (62.9) | 107 (57.8) | 112 (59.9) | 336 (60.2) |
| 1 | 12 (6.5) | 6 (3.2) | 10 (5.4) | 28 (5.0) |
| 2 | 1 (0.5) | 0 (0.0) | 4 (2.1) | 5 (0.9) |
| 3 | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Missing | 55 (29.6) | 72 (38.9) | 61 (32.6) | 188 (33.7) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| HADS anxiety score at baseline | ||||
| Mean (SD) | 10.6 (4.3) | 11.1 (4.3) | 10.5 (4.0) | 10.7 (4.2) |
| N (median) | 186 (10.0) | 185 (11.0) | 187 (11.0) | 558 (11.0) |
| Minimum (maximum) | 1 (21) | 1 (20) | 2 (20) | 1 (21) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HADS depression score at baseline | ||||
| Mean (SD) | 5.5 (3.6) | 5.9 (3.8) | 5.6 (3.5) | 5.7 (3.7) |
| N (median) | 186 (5.0) | 185 (5.0) | 187 (5.0) | 558 (5.0) |
| Minimum (maximum) | 0 (18) | 0 (18) | 0 (19) | 0 (19) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HADS anxiety score at baseline, n (%) | ||||
| No | 97 (52.2) | 87 (47.0) | 91 (48.7) | 275 (49.3) |
| Yes | 89 (47.9) | 98 (53.0) | 96 (51.3) | 283 (50.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| HADS depression score at baseline, n (%) | ||||
| No | 139 (74.7) | 125 (67.6) | 137 (73.3) | 401 (71.9) |
| Yes | 47 (25.3) | 60 (32.4) | 50 (26.7) | 157 (28.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| HADS distress at baseline, n (%) | ||||
| No | 116 (62.4) | 102 (55.1) | 118 (63.1) | 336 (60.2) |
| Yes | 70 (37.6) | 83 (44.9) | 69 (36.9) | 222 (39.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
| Screening IBS SSSb | ||||
| Mean (SD) | 294.9 (82.1) | 294.0 (84.5) | 288.0 (86.2) | 292.3 (84.2) |
| N (median) | 185 (295.0) | 185 (300.0) | 187 (290.0) | 557 (295.0) |
| Minimum (maximum) | 95 (500) | 75 (480) | 75 (470) | 75 (500) |
| Missing | 1 | 0 | 0 | 1 |
| Number with IBS subtype, n (%) | ||||
| IBS_D (diarrhoea) | 60 (32.3) | 60 (32.4) | 58 (31.0) | 178 (31.9) |
| IBS_C (constipation) | 26 (14.0) | 23 (12.4) | 27 (14.5) | 76 (13.6) |
| IBS_A (alternating)c | 93 (50.3) | 98 (53.0) | 96 (51.3) | 287 (51.5) |
| IBS_U (unclassified) | 6 (3.2) | 4 (2.2) | 6 (3.2) | 16 (2.9) |
| Unknown | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Total | 186 (100) | 185 (100) | 187 (100) | 558 (100) |
Participants had a mean age of 43.1 years at baseline and the majority were female (75.8%). Over 90% of participants were white, 64.7% of participants were married or living with a partner and 35.3% were single, separated, divorced or widowed. The median duration since diagnosis was 7.4 years and the median duration of symptoms prior to diagnosis was 3.0 years. At baseline, only 10.2% of participants had seen a consultant. Approximately 30% of participants had received treatment for anxiety and nearly 40% had received treatment for depression. In accordance with the HADS, approximately 60% of participants were not distressed at baseline.
A very small proportion, approximately 1%, of participants had joined a self-help organisation. Most of those who had done so had joined an online group.
Participants were asked their highest level of formal education. The largest single group (54.6% of participants) were those with at least a degree. Approximately 20% of participants had only General Certificate of Secondary Education (GCSE) or equivalent-level qualifications and a similar proportion had postgraduate qualifications. The majority of participants, 68.6%, owned their own property, and 27.5% lived in rented accommodation, of whom only 6.3% rented their accommodation from a local authority. In addition, 14.9% of our sample lived alone, 41.4% lived with their spouse only and a further 23.3% lived with their spouse and children. The mean deprivation (IMD) score was 17.1 (range 0.8–54.0).
At screening, the mean IBS SSS was 292.3 (range 75–500) but at baseline the mean IBS SSS was 265.0 [range 14–500 (see Table 26)].
At baseline, participants had a mean HADS anxiety score of 10.7 (range 1–21), a mean HADS distress score of 16.4 (range 1–39), a mean HADS depression score of 5.7 (range 0–19) and a mean WSAS score of 12.5 (range 0–40) (see Table 26).
The majority of participants, 74.2%, would have preferred one of the CBT groups, with 36.2% preferring the online CBT. Only 25.8% expressed no preference or preferred the standard care.
Therapy summary
All therapists were available to work in both therapy arms (TCBT and WCBT) and with any participant regardless of recruitment centre. There were 13 therapists, of whom 10 (77%) were female. Six therapists were clinical psychologists (46%) and seven were CBT psychotherapists (54%). They had worked for a median of 7 years in their professions (minimum 4 years, maximum 24 years), with clinical psychologists having worked for a median of 7.5 years in their profession (minimum 4 years, maximum 12 years) and CBT psychotherapists for a median of 5 years (minimum 4 years, maximum 24 years). They had worked within medically unexplained symptoms for a median of 4 years (minimum 1 year, maximum 20 years). Their mean age was 42 years (SD 5.9 years), with a minimum age of 34 years and a maximum age of 52 years. Table 15 shows the number of participants allocated to each therapist.
| Therapist ID | Participants (n) | |
|---|---|---|
| TCBT | WCBT | |
| T1 | 48 | 54 |
| T2 | 9 | 10 |
| T3 | 26 | 19 |
| T4 | 22 | 21 |
| T5 | 11 | 11 |
| T6 | 13 | 16 |
| T7 | 10 | 13 |
| T8 | 38 | 36 |
| Mixeda | 9 | 5 |
| Total | 186 | 185 |
Therapy protocol deviations
Twenty-five therapy protocol deviations were recorded. The majority of these concerned treatment scheduling falling outside the allocated treatment windows (n = 15), of which 33% (n = 5) were recorded in London participants. The predominant reason for deviations was issues with participant availability for sessions. Other protocol deviations concerned not recording sessions (n = 7). These were not always logged by therapists. When a session was not recorded, this was usually due to technical issues with recorders (battery failure, not working with the telephone, failure to switch on) rather than participants requesting that sessions not be recorded. There were seven logged instances of sessions not being recorded; however, it was estimated that non-recording of sessions happened more frequently than this. There was one reported instance of a participant refusing the recording of sessions.
Therapy fidelity
Six recorded therapy sessions (three TCBT and three WCBT) were double rated by the external clinical psychologists. The results of the first round of double ratings showed adequate inter-rater reliability (i.e. k > 0.5, approximately 90% of agreement) for items 1A and 5B (Table 16).
| Item | Weighted kappa | Percentage of agreement |
|---|---|---|
| 1A (therapeutic alliance) | 0.818 | 96.30 |
| 1B (CBT skills subscale) | 0.160 | 70.83 |
| 2B (CBT skills subscale) | 0.307 | 83.33 |
| 3B (CBT skills subscale) | 0.222 | 70.83 |
| 4B (CBT skills subscale) | 0.483 | 79.17 |
| 5B (CBT skills subscale) | 0.870 | 97.92 |
| 1C (overall therapist adherence) | 0. 250 | 88.90 |
A second meeting between Rona Moss-Morris and the external clinical psychologists took place to discuss the main reasons underlying the disagreements. It was decided to assess two further therapy sessions (one TCBT and one WCBT) (total n = 8). The second round of double ratings showed adequate inter-rater reliability for the following items: 1A, 3B, 4B, 5B and 1C (Table 17).
| Item | Weighted kappa | Percentage of agreement |
|---|---|---|
| 1A (therapeutic alliance) | 0.74 | 95.8 |
| 1B (CBT skills subscale) | –0.13 | 81.9 |
| 2B (CBT skills subscale) | 0.05 | 83.5 |
| 3B (CBT skills subscale) | 0.79 | 95.31 |
| 4B (CBT skills subscale) | 0.55 | 84.38 |
| 5B (CBT skills subscale) | 0.76 | 96.5 |
| 1C (overall therapist adherence) | 0.71 | 94.44 |
A second telephone call with Rona Moss-Morris took place to clarify the discrepancies concerning the two new recordings and it was agreed to rate a further telephone session (total n = 9). The third round of double ratings showed adequate inter-rater reliability for all items except 1B (Table 18). A third meeting with Rona Moss-Morris and Trudie Chalder took place to discuss the ratings of the last recorded session and any further queries concerning fidelity checks.
| Item | Weighted kappa | Percentage of agreement |
|---|---|---|
| 1A (therapeutic alliance) | 0.76 | 96.30 |
| 1B (CBT skills subscale) | 0.11 | 83.95 |
| 2B (CBT skills subscale) | 0.50 | 95.14 |
| 3B (CBT skills subscale) | 0.81 | 95.06 |
| 4B (CBT skills subscale) | 0.97 | 99.31 |
| 5B (CBT skills subscale) | 0.82 | 97.53 |
| 1C (overall therapist adherence) | 0.77 | 96.53 |
Table 19 shows the treatment fidelity ratings for therapeutic alliance. All three fidelity score means were high, with similar results for TCBT and WCBT in suggesting good treatment fidelity.
| Item | Fidelity ratings, mean (SD) | |
|---|---|---|
| TCBT | WCBT | |
| Therapeutic alliance | 93.2 (10.4) | 94.4 (10.3) |
| CBT skills | 78.5 (13.1) | 82.8 (12.1) |
| Therapy adherence | 90.1 (10.0) | 89.4 (15.1) |
Therapy process variables (measured during treatment period, not at baseline)
A number of variables, including number of telephone sessions and number of times patients accessed the website, were assessed during treatment. These will be analysed as part of the mediator and moderator analysis in the process evaluation that will be reported separately.
Withdrawal from treatment
Forty-five participants withdrew from treatment at some point during the trial (Table 20). More than half of those withdrawing from treatment also withdrew from follow-up and did not supply any further outcome data after ceasing therapy sessions.
| Time of withdrawal | Trial arm (n) | Total (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| TCBT | WCBT | TAU | ||||||
| Treatment only | Trial | Treatment only | Trial | Treatment only | Trial | Treatment only | Trial | |
| By 3 months | 7 | 4 | 8 | 6 | N/A | 4 | 15 | 14 |
| Between 3 and 6 months | 6 | 2 | 8 | 5 | N/A | 10 | 14 | 17 |
| Between 6 and 12 months | 2 | 3 | 5 | 4 | N/A | 7 | 7 | 14 |
| Total | 15 | 9 | 21 | 15 | N/A | 21 | 36 | 45 |
Compliance with treatment
For those in the TCBT arm, treatment compliance was defined as taking part in four telephone sessions out of the six initial sessions offered. Approximately 84% of participants completed at least four telephone calls; 16% did not comply. The take-up of therapy and booster sessions offered is described in Table 21.
| Number of sessions received | Participants, n (%) |
|---|---|
| Telephone | |
| 0 | 9 (4.8) |
| 1 | 5 (2.7) |
| 2 | 10 (5.4) |
| 3 | 5 (2.7) |
| 4 | 5 (2.7) |
| 5 | 11 (5.9) |
| 6 | 141 (75.8) |
| Booster | |
| 0 | 50 (26.9) |
| 1 | 9 (4.8) |
| 2 | 127 (68.3) |
For those in the WCBT arm, treatment compliance was defined as taking part in one telephone session out of three sessions offered and accessing at least four web sessions. Approximately 88.1% of participants allocated to the WCBT arm completed at least one telephone call, and 69.8% completed four web sessions (Table 22).
| Number of sessions received | Participants, n (%) |
|---|---|
| Telephone | |
| 0 | 22 (11.9) |
| 1 | 19 (10.3) |
| 2 | 17 (9.2) |
| 3 | 120 (64.9) |
| 4 | 6 (3.2) |
| 5 | 1 (0.5) |
| Booster | |
| 0 | 76 (41.8) |
| 1 | 18 (9.7) |
| 2 | 91 (49.2) |
| Web sessions accessed | |
| 0 | 15 (8.1) |
| 1 | 13 (7.0) |
| 2 | 18 (9.7) |
| 3 | 11 (6.0) |
| 4 | 12 (6.5) |
| 5 | 16 (8.7) |
| 6 | 33 (17.8) |
| 7 | 27 (14.6) |
| 8 | 40 (21.6) |
A larger percentage of participants failed to comply with WCBT (30.8%) than with TCBT (16%).
Follow-up summary
Follow-up assessments were scheduled at 3, 6 and 12 months after randomisation. Follow-up rates in terms of primary outcomes were good, with 136, 124 and 131 participants completing both final outcome measures in the TCBT, WCBT and TAU arms, respectively (see Figure 2). These numbers constitute follow-up rates at 12 months of 73% for TCBT, 67% for WCBT and 70% for TAU. Table 23 shows how many of the measures were recorded within the prespecified time windows (ranging from 1 week before the intended time point until 4 weeks after). At 12 months, approximately 75% of recorded outcomes were obtained within the prescribed window.
| 12-month scores | Trial arm | All (N = 558) | ||||||
|---|---|---|---|---|---|---|---|---|
| TCBT (n = 186) | WCBT (n = 185) | TAU (n = 187) | ||||||
| Mean (SD) | Number recorded | Mean (SD) | Number recorded | Mean (SD) | Number recorded | Mean (SD) | Number recorded | |
| All IBS SSS records | 139.0 (94.8) | 136 | 163.0 (108.8) | 124 | 205.6 (100.5) | 131 | 168.9 (104.8) | 391 |
| IBS SSS recorded within time window | 134.0 (98.4) | 99 | 148.4 (102.7) | 92 | 199.4 (103.3) | 102 | 161.3 (105.1) | 293 |
| All WSAS records | 6.0 (7.5) | 138 | 7.4 (7.7) | 124 | 10.8 (9.3) | 132 | 8.1 (8.5) | 394 |
| WSASs recorded within time window | 6.3 (7.4) | 101 | 6.8 (7.1) | 92 | 10.8 (9.7) | 103 | 8.0 (8.4) | 296 |
The overall follow-up rates were 76.5% at 3 months (427/558), 72.9% at 6 months (407/558) and 70.3% at 12 months (392/558) (see Figure 2 for a summary of patient follow-up).
We tested whether or not compliance with treatment offered was predictive of missing primary outcomes at 12 months using Fisher’s exact test. The test found that non-compliance with treatment offered predicted missing outcomes in both the TCBT (p < 0.001) and the WCBT (p < 0.001) arms. Logistic regression tests found that higher baseline IBS SSS (p < 0.001) and higher IMD scores (p < 0.001) were associated with more missing values in the primary outcomes at 12 months. Thus, all of these variables were included as predictors of missing values in the imputation step of our MI approach (for details see Methods). All results reported here are based on MI with k = 100 imputations to avoid missing data biases.
Outcome measures
Outcome measures are summarised by assessment time point and trial arm in Tables 24–26, and the results of the formal trial arm comparisons are provided in Tables 27 and 28.
| Outcome variable | Trial arm | All (N = 558) | ||||||
|---|---|---|---|---|---|---|---|---|
| TCBT (n = 186) | WCBT (n = 185) | TAU (n = 187) | ||||||
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | |
| IBS SSS | ||||||||
| Baseline | 272.3 (95.5) | 186 | 264.2 (99.3) | 185 | 258.5 (91.6) | 187 | 265.0 (95.5) | 558 |
| 3 months | 174.0 (98.6) | 148 | 179.1 (104.2) | 138 | 236.5 (97.1) | 141 | 196.3 (103.7) | 427 |
| 6 months | 154.9 (92.0) | 145 | 170.3 (104.9) | 128 | 212.7 (104.8) | 135 | 178.8 (103.2) | 408 |
| 12 months | 139.0 (94.8) | 136 | 163.0 (108.8) | 124 | 205.6 (100.5) | 131 | 168.9 (104.8) | 391 |
| WSAS | ||||||||
| Baseline | 12.3 (8.8) | 186 | 13.0 (9.3) | 185 | 12.4 (7.4) | 187 | 12.5 (8.5) | 558 |
| 3 months | 8.4 (7.8) | 149 | 9.4 (8.2) | 138 | 12.1 (8.2) | 140 | 9.9 (8.2) | 427 |
| 6 months | 7.0 (7.8) | 146 | 7.6 (7.5) | 127 | 10.5 (8.1) | 135 | 8.3 (7.9) | 408 |
| 12 months | 6.0 (7.5) | 138 | 7.4 (7.7) | 124 | 10.8 (9.3) | 132 | 8.1 (8.5) | 394 |
| HADS distress | ||||||||
| Baseline | 16.1 (6.9) | 186 | 17.0 (7.3) | 185 | 16.0 (6.4) | 187 | 16.4 (6.9) | 558 |
| 3 months | 13.5 (6.6) | 147 | 13.4 (6.9) | 136 | 15.6 (7.4) | 139 | 14.1 (7.0) | 422 |
| 6 months | 12.4 (6.7) | 123 | 12.7 (6.9) | 115 | 15.2 (7.3) | 120 | 13.4 (7.1) | 358 |
| 12 months | 12.2 (6.5) | 120 | 12.7 (7.4) | 117 | 15.0 (7.2) | 113 | 13.3 (7.1) | 350 |
| Binary outcome | Trial arm | All (N = 558) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCBT (n = 186) | WCBT (n = 185) | TAU (n = 187) | ||||||||||
| Non-responder (%) | Responder (%) | n | Non-responder (%) | Responder (%) | n | Non-responder (%) | Responder (%) | n | Non-responder (%) | Responder (%) | n | |
| PEQ respondersa | ||||||||||||
| 3 months | 55 (37.2) | 93 (62.8) | 148 | 71 (51.4) | 67 (48.6) | 138 | 128 (90.8) | 13 (9.2) | 141 | 254 (59.5) | 173 (40.5) | 427 |
| 6 months | 35 (24.0) | 111 (76.0) | 146 | 57 (44.9) | 70 (55.1) | 127 | 113 (83.1) | 23 (16.9) | 136 | 205 (50.1) | 204 (49.9) | 409 |
| 12 months | 30 (21.7) | 108 (78.3) | 138 | 56 (45.2) | 68 (54.8) | 124 | 101 (76.5) | 31 (23.5) | 132 | 187 (47.5) | 207 (52.5) | 394 |
| SGA respondersb | ||||||||||||
| 3 months | 36 (24.2) | 113 (75.8) | 149 | 44 (31.9) | 94 (68.1) | 138 | 111 (79.3) | 29 (20.7) | 140 | 191 (44.7) | 236 (55.3) | 427 |
| 6 months | 28 (19.2) | 118 (80.8) | 146 | 32 (25.2) | 95 (74.8) | 127 | 95 (69.9) | 41 (30.1) | 136 | 155 (37.9) | 254 (62.1) | 409 |
| 12 months | 21 (15.2) | 117 (84.8) | 138 | 31 (25.0) | 93 (75.0) | 124 | 77 (58.3) | 55 (41.7) | 132 | 129 (32.7) | 265 (67.3) | 394 |
| IBS SSS respondersc | ||||||||||||
| 3 months | 48 (32.4) | 100 (67.6) | 148 | 51 (37.0) | 87 (63.0) | 138 | 101 (71.6) | 40 (28.4) | 141 | 200 (46.8) | 227 (53.2) | 427 |
| 6 months | 39 (26.9) | 106 (73.1) | 145 | 40 (31.3) | 88 (68.8) | 128 | 78 (57.8) | 57 (42.2) | 135 | 157 (38.5) | 251 (61.5) | 408 |
| 12 months | 37 (27.2) | 99 (72.8) | 136 | 42 (33.9) | 82 (66.1) | 124 | 73 (55.7) | 58 (44.3) | 131 | 152 (38.9) | 239 (61.1) | 391 |
| WSAS respondersd | ||||||||||||
| 3 months | 99 (66.4) | 50 (33.6) | 149 | 95 (68.8) | 43 (31.2) | 138 | 120 (85.7) | 20 (14.3) | 140 | 314 (73.5) | 113 (26.5) | 427 |
| 6 months | 90 (61.6) | 56 (38.4) | 146 | 72 (56.7) | 55 (43.3) | 127 | 103 (76.3) | 32 (23.7) | 135 | 265 (65.0) | 143 (35.0) | 408 |
| 12 months | 78 (56.5) | 60 (43.5) | 138 | 70 (56.4) | 54 (43.6) | 124 | 101 (76.5) | 31 (23.5) | 132 | 249 (63.2) | 145 (36.8) | 394 |
| Observations | IBS SSS severity,a n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial arm | All (N = 558) | |||||||||||||||
| TCBT (n = 186) | WCBT (n = 185) | TAU (n = 187) | ||||||||||||||
| None | Mild | Moderate | Severe | None | Mild | Moderate | Severe | None | Mild | Moderate | Severe | None | Mild | Moderate | Severe | |
| Baseline (n = 558) | 3 (1.6) | 25 (13.4) | 84 (45.2) | 74 (39.8) | 8 (4.3) | 25 (13.5) | 80 (43.2) | 72 (38.9) | 5 (2.7) | 33 (17.6) | 89 (47.6) | 60 (32.1) | 16 (2.9) | 83 (14.9) | 253 (45.3) | 206 (36.9) |
| 3 months (n = 427) | 22 (14.9) | 58 (39.2) | 52 (35.1) | 16 (10.8) | 20 (14.5) | 53 (38.4) | 48 (34.8) | 17 (12.3) | 9 (6.4) | 26 (18.4) | 71 (50.4) | 35 (24.8) | 51 (11.9) | 137 (32.1) | 171 (40.0) | 68 (15.9) |
| 6 months (n = 408) | 31 (21.4) | 54 (37.2) | 52 (35.9) | 8 (5.5) | 28 (21.9) | 40 (31.3) | 43 (33.6) | 17 (13.3) | 13 (9.6) | 38 (28.2) | 56 (41.5) | 28 (20.7) | 72 (17.6) | 132 (32.4) | 151 (37.0) | 53 (13.0) |
| 12 months (n = 391) | 39 (28.7) | 52 (38.2) | 37 (27.2) | 8 (5.9) | 31 (25.0) | 42 (33.9) | 35 (28.2) | 16 (12.9) | 11 (8.4) | 39 (29.8) | 57 (43.5) | 24 (18.3) | 81 (20.7) | 133 (34.0) | 129 (33.0) | 48 (12.3) |
| Observations | TCBT vs. TAU | WCBT vs. TAU | ||||
|---|---|---|---|---|---|---|
| Estimated difference (95% CI) | Test (degrees of freedom); p-value | Standardised differencea | Estimated difference (95% CI) | Test (degrees of freedom); p-value | Standardised differencea | |
| IBS SSS | ||||||
| 3 months | –69.2 (–88.7 to –49.7) | t = –7.0 (367); p < 0.001 | 0.73 | –53.0 (–74.9 to –31.1) | t = –4.8 (237); p < 0.001 | 0.56 |
| 6 months | –58.3 (–80.3 to –36.3) | t = –5.2 (295); p < 0.001 | 0.61 | –35.7 (–58.5 to –12.9) | t = –3.1 (257); p = 0.002 | 0.37 |
| 12 months | –61.6 (–89.5 to –33.8) | t = –4.3 (1581); p < 0.001 | 0.65 | –35.5 (–57.8 to –12.6) | t = –3.1 (275); p = 0.002 | 0.37 |
| WSAS | ||||||
| 3 months | –3.4 (–4.8 to –2.0) | t = –4.8 (355); p < 0.001 | 0.40 | –3.0 (–4.4 to –1.5) | t = –3.9 (288); p < 0.001 | 0.35 |
| 6 months | –2.7 (–4.2 to –1.2) | t = –3.6 (280); p < 0.001 | 0.32 | –2.5 (–4.0 to –1.0) | t = –3.3 (257); p = 0.001 | 0.30 |
| 12 months | –3.5 (–5.1 to –1.9) | t = –4.2 (268); p < 0.001 | 0.41 | –3.0 (–4.6 to –1.3) | t = –3.5 (258); p = 0.001 | 0.35 |
| HADS | ||||||
| 3 months | –2.1 (–3.2 to –0.9) | t = –3.6 (306); p < 0.001 | 0.30 | –2.5 (–3.7 to –1.3) | t = –4.0 (261); p < 0.001 | 0.36 |
| 6 months | –2.2 (–3.5 to –0.8) | t = –3.2 (227); p = 0.002 | 0.32 | –2.9 (–4.2 to –1.6) | t = –4.2 (230); p < 0.001 | 0.42 |
| 12 months | –2.8 (–4.1 to –1.5) | t = –4.3 (248); p < 0.001 | 0.41 | –2.3 (–3.7 to –1.0) | t = –3.4 (217); p = 0.001 | 0.34 |
Table 24 shows that improvements over time were observed for both primary outcomes (IBS SSS and WSAS) and in all trial arms. Table 26 illustrates IBS SSS changes in terms of severity categories and also shows that the percentage of participants with no or only mild symptoms increases after CBT treatment in both the TCBT and the WCBT arms, and also somewhat in the TAU arm.
Primary outcome measures
Table 27 shows the results of the formal statistical analyses of the continuous outcomes.
Irritable Bowel Syndrome Symptom Severity Score
The estimated IBS SSS differences for TCBT versus TAU were –69.2, –58.3 and –61.6 at 3, 6 and 12 months, respectively. For WCBT versus TAU, the differences were lower, at –53.0, –35.7 and –35.2, respectively (Figure 3). The comparison of the CBT arms with the TAU arm at all times showed statistically significant benefits in terms of IBS SSS. At 12 months, moderate-sized effects were found for TCBT (p < 0.001, standardised effect = 0.65) and WCBT (p = 0.002, standardised effect = 0.37) (see Table 29). The estimated differences between the TCBT arm (a reduction of 61.6) or the WCBT arm (a reduction of 35.2) and the TAU arm were larger than our stated minimum clinically significant difference (MCID = 35, as per our sample size calculation; see Sample size).
FIGURE 3.
The IBS SSS means by trial arm. IBS SSS possible range is 0–500.
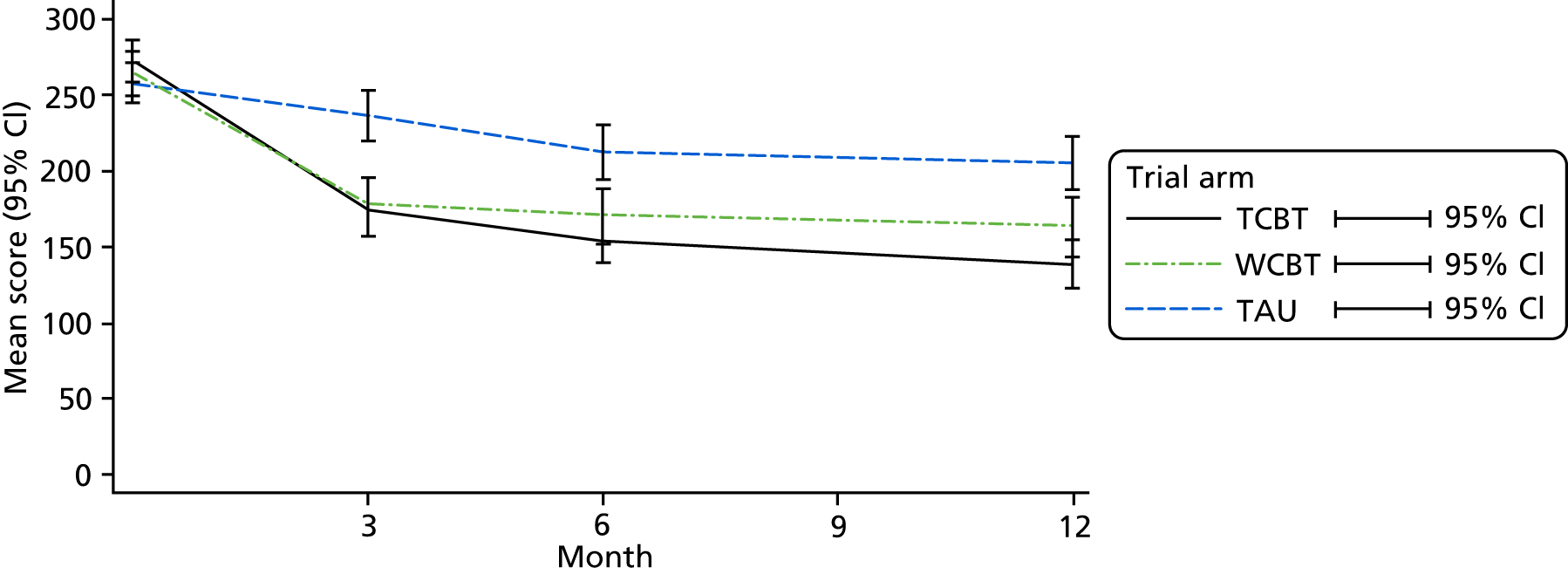
Therapist effects were only detected and modelled in the TCBT arm at 12 months.
The number needed to treat (NNT) to improve the IBS SSS for one case by 50 at 12 months was 3.5 for TCBT and 4.6 for WCBT.
Work and Social Adjustment Scale
The estimated WSAS score differences for TCBT versus TAU were –3.4, –2.7 and –3.5 at 3, 6 and 12 months, respectively. For WCBT versus TAU, the differences were –3.0, –2.5 and –3.0, respectively. The comparison of the CBT arms with the TAU arm at all time points showed statistically significant benefits for both TCBT and WCBT. At 12 months, small to moderate effects were found for TCBT (p < 0.001, standardised effect = 0.4) and WCBT (p < 0.001, standardised effect = 0.3) (Figure 4). Therapist effects were not detected for any time point or CBT treatment arm.
FIGURE 4.
The WSAS means by trial arm. WSAS possible range is 0–40.
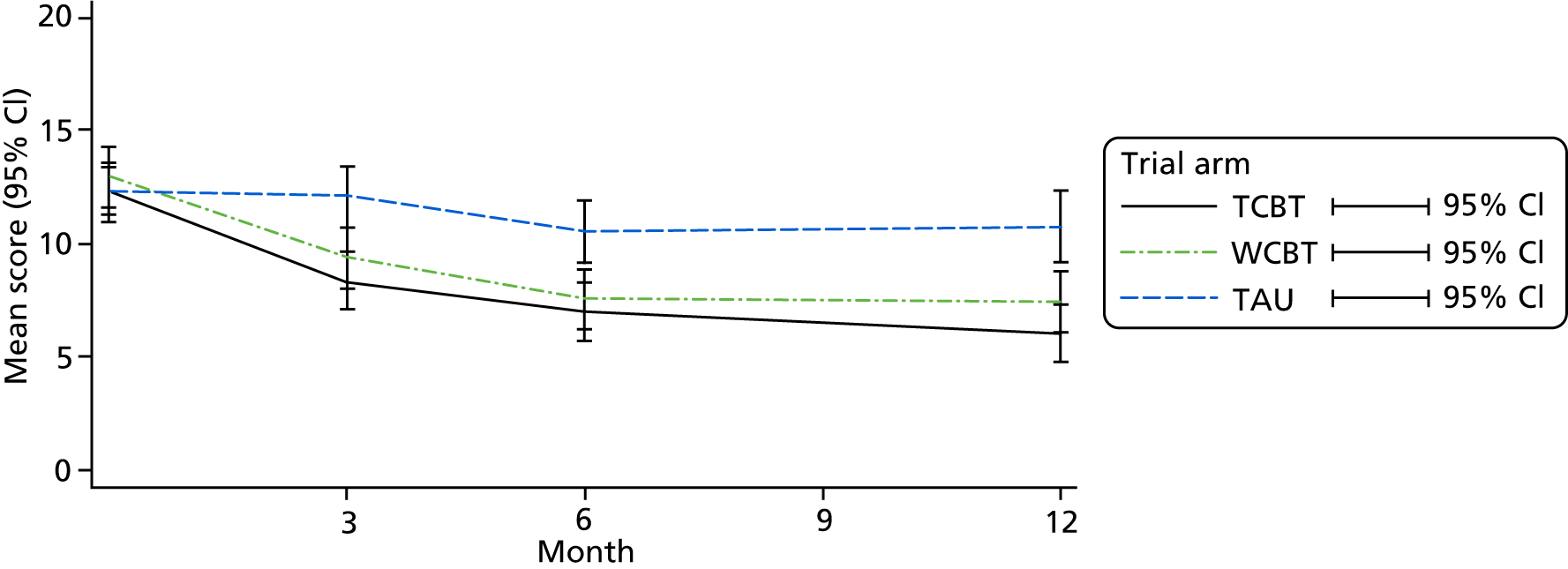
The NNT to improve the WSAS score for one case by 5.9 at 12 months was 5 for both TCBT and WCBT.
Secondary outcome measures
Descriptive summaries for the two binary secondary outcomes, PEQ responder and SGA responder, are provided in Table 26. The responder percentage increased over time for both responder types and in all trial arms. The results of the formal trial arm comparisons are shown in Table 28.
| Responder | TCBT vs. TAU | WCBT vs. TAU | ||
|---|---|---|---|---|
| Estimated OR (95% CI) | Test (degrees of freedom); p-value | Estimated OR (95% CI) | Test (degrees of freedom); p-value | |
| PEQ responders | ||||
| 3 months | 13.3 (6.9 to 25.8) | t = 7.7 (1347); p < 0.001 | 6.6 (3.3 to 13.2) | t = 5.4 (918); p < 0.001 |
| 6 months | 11.6 (6.4 to 20.9) | t = 8.1 (1134); p < 0.001 | 4.8 (2.7 to 8.7) | t = 5.2 (889); p < 0.001 |
| 12 monthsa | 9.3b (4.5 to 19.3) | t = 6.0 (2369); p < 0.001 | 3.5 (2.0 to 5.9) | t = 4.5 (1079); p < 0.001 |
| SGA responders | ||||
| 3 months | 10.2 (5.8 to 18.1) | t = 8.1 (1289); p < 0.001 | 6.4 (3.6 to 11.3) | t = 6.3 (824); p < 0.001 |
| 6 months | 6.2 (3.7 to 10.4) | t = 6.9 (1637); p < 0.001 | 5.0 (2.8 to 8.8) | t = 5.6 (630); p < 0.001 |
| 12 monthsa | 6.1c (2.5 to 15.0) | t = 3.9 (2957); p < 0.001 | 3.6 (2.0 to 6.3) | t = 4.4 (600); p < 0.001 |
Patient enablement
For the TCBT arm, the odds of being a responder on the PEQ (defined as a score of ≥ 6) were 13.3, 11.6 and 9.3 (3, 6 and 12 months, respectively) times those in the TAU arm. The ORs for WCBT compared with TAU were lower, at 6.6, 4.8 and 3.5 at 3, 6 and 12 months, respectively, but still indicated large improvements in responder rates (Figure 5). These results were statistically significant for both CBT arms and at all time points, with p-values of < 0.001 at all times. Therapist effects were detected only in the TCBT arm at 12 months.
FIGURE 5.
The PEQ responders by trial arm.
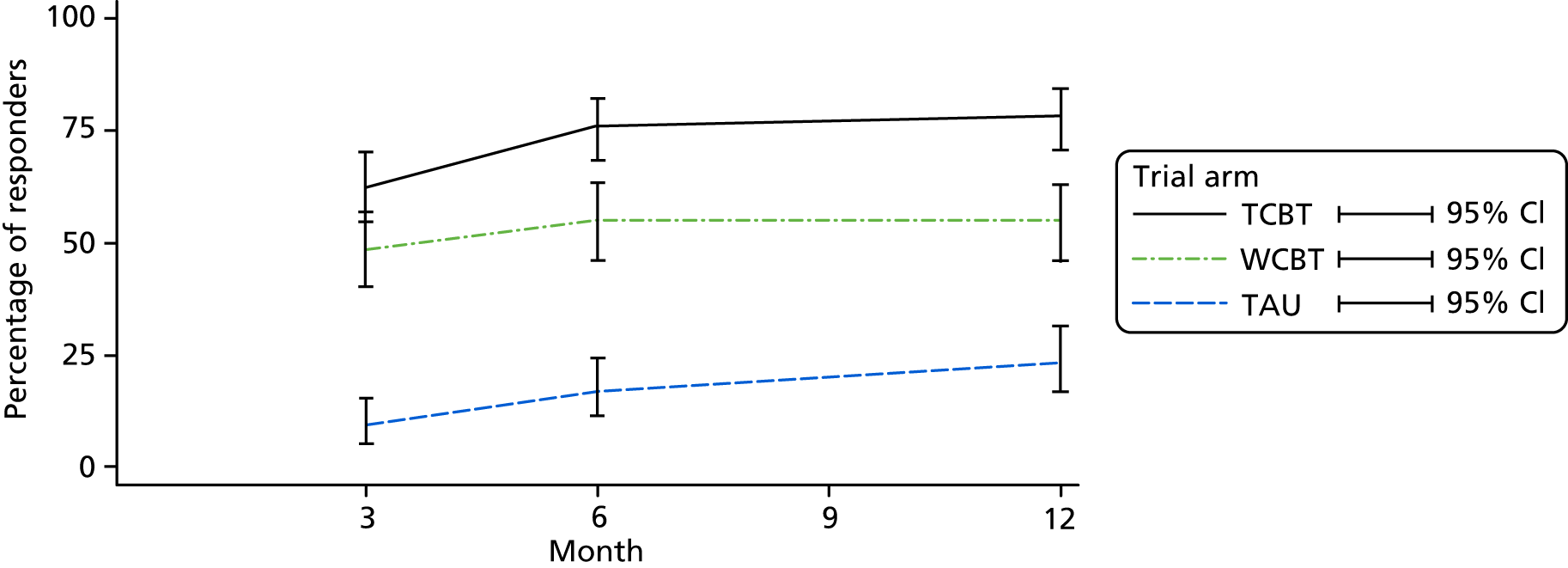
Subject’s Global Assessment of Relief
For SGA responders, the ORs were 10.2, 6.2 and 6.1 for TCBT and 6.4, 5.0 and 3.6 for WCBT (3, 6 and 12 months, respectively) (Figure 6). These results were also statistically significant for both CBT arms and at all time points. Therapist effects were again detected only in the TCBT arm at 12 months.
FIGURE 6.
The SGA responders by trial arm.

As mentioned above, therapist effects were detected at 12 months for the primary outcome, IBS SSS, and the secondary outcomes, SGA and PEQ. Such effects were seen only in the TCBT arm. To understand these effects, we tabulated the outcome improvements by 12 months by therapist in the TCBT arm. Table 29 indicates that this therapist variability was largely due to lower responses on all three measures in the patient groups that were seen by therapist T2 or tended not have a therapist assigned at all. Participants were randomised only to treatment group, not to therapist. It cannot be assumed that the groups seen by an individual therapist were equivalent.
| Therapist | Number assigned | Number of participants completing outcomes | Mean baseline IBS SSS (SD) | IBS SSS mean difference (SD) | Responders, n (%) | |
|---|---|---|---|---|---|---|
| PEQ | SGA | |||||
| T1 | 46 | 38 | 288.3 (13.3) | –156.8 (15.2) | 35 (92.1) | 36 (94.7) |
| T2 | 9 | 9 | 268.3 (48.7) | –37.8 (31.9) | 6 (66.7) | 6 (66.7) |
| T3 | 24 | 15 | 279.3 (23.8) | –133.1 (36.1) | 13 (86.7) | 13 (86.7) |
| T4 | 22 | 17 | 284.3 (14.7) | –132.6 (20.1) | 15 (88.2) | 14 (82.4) |
| T5 | 11 | 9 | 266.5 (19.6) | –140.2 (23.6) | 7 (77.8) | 9 (100.0) |
| T6 | 13 | 9 | 278.5 (30.7) | –116.4 (30.1) | 6 (66.7) | 9 (100.0) |
| T7 | 8 | 5 | 247.0 (39.6) | –108.6 (42.0) | 3 (60.0) | 4 (80.0) |
| T8 | 36 | 29 | 253.3 (15.1) | –84.6 (18.5) | 19 (65.5) | 21 (72.4) |
| Mixeda | 17 | 7 | 256.9 (22.1) | –69.9 (48.5) | 4 (57.1) | 5 (71.4) |
| Total | 186 | 138 | 272.3 (7.0) | –119.2 (8.8) | 108 (78.3) | 117 (84.8) |
Hospital Anxiety and Depression Scale
For HADS distress, the estimated differences for TCBT or WCBT compared with TAU were found to be –2.1 for TCBT to TAU at 3 months, –2.2 for TCBT to TAU at 6 months and –2.8 for TCBT to TAU at 12 months, and –2.5 for WCBT to TAU at 3 months, –2.9 for WCBT to TAU at 6 months and –2.3 for WCBT to TAU at 12 months (Figure 7 and see Table 27). These results were statistically significant at all time points and in both CBT arms (Table 29). The effect sizes for HADS, once standardised, were small to moderate. Therapist effects were not detected for any time point or CBT treatment arm.
FIGURE 7.
The HADS means by trial arm. HADS possible range is 1–39.

General practitioner visits
The number of GP visits recorded in the GP records in the year before and after randomisation were recorded on a notes review form (see Appendix 7). The numbers of visits did not noticeably change and were similar across the three trial arms. The mean number of visits for all arms was between four and five per year both pre and post randomisation (see Appendix 8, Table 41).
Sensitivity analysis
Four sets of sensitivity analyses were conducted. The first sensitivity analysis assessed the impact of excluding participants who had an IBS SSS at baseline below the inclusion threshold of 75 from the analysis set. (The IBS SSS eligibility criterion was determined at screening.) The second sensitivity analysis looked at the impact of using only observations that were recorded within the prespecified assessment time windows. The third sensitivity analysis evaluated the impact of defining PEQ responders according to an alternative threshold. Finally, the fourth sensitivity analysis explicitly targeted the efficacy rather than the effectiveness of the CBT treatments and assessed the impact of this on effect size estimates for the two co-primaries.
Sixteen participants had scores that were lower than the eligibility threshold when this was reassessed at baseline. In the TCBT arm, three participants were below 75 on the IBS SSS at baseline; in the WCBT arm, eight participants were below this threshold; in the TAU group, five participants were below this threshold. These participants were dropped from the analysis and the co-primaries were analysed in the same way as before. For IBS SSS at 12 months, the estimated trial arm differences were –63.0 (95% CI –91.9 to –34.2; p < 0.001) for TCBT compared with TAU and –35.5 (95% CI –58.2 to –12.7; p = 0.002) for WCBT compared with TAU. For WSAS at 12 months, the estimated trial arm differences were –3.5 (95% CI –6.1 to –0.9; p = 0.02) for TCBT compared with TAU and –3.4 (95% CI –4.7 to –2.1; p < 0.001) for WCBT compared with TAU. Comparisons of these findings with the original analysis results shown in Table 29 demonstrate that the analyses were robust regarding the timing of the IBS SSS eligibility assessment.
Participants were asked to record outcomes at 3, 6 and 12 months after randomisation within the prespecified time window, ranging from 7 days before the intended assessment date to 4 weeks afterwards. Treatment effects were expected to be reasonably constant over the 5-week period. For primary outcomes at the 12-month assessment time point, 98 out of 391 (25.0%) IBS SSS values and 98 out of 394 (24.9%) WSAS values were recorded outside this assessment window (see Table 25). These participants were dropped from the analysis and the co-primaries were analysed in the same way as before. For IBS SSS at 12 months, the estimated trial arm differences were –64.3 (95% CI –93.2 to –35.5; p < 0.001) for TCBT compared with TAU and –37.2 (95% CI –64.1 to –10.4; p = 0.007) for WCBT compared with TAU. For WSAS at 12 months, the estimated trial arm differences were –3.3 (95% CI –4.9 to –1.8; p < 0.001) for TCBT compared with TAU and –3.3 (95% CI –3.8 to 10.4; p = 0.20) for WCBT compared with TAU. The results demonstrate that, apart from some loss of power (wider CIs), the IBS SSS analysis was not sensitive to outcomes being recorded within the specified time period. However, for the WSAS, power loss was associated with dropping one-quarter of the sample, leading to the WCBT effect becoming non-significant (see Table 29).
As we had not anticipated analysing PEQ scores on a binary scale, we confirmed that the substantive analysis results were not sensitive to the choice of threshold for defining a participant to be a ‘PEQ responder’. In the main analysis, we considered those reporting a PEQ score of ≥ 6 to be responders. For this sensitivity analysis, we considered responders to be those reporting a PEQ score of ≥ 4. The ORs of being a responder reported at the primary outcome time of 12 months using these criteria were estimated to be 12.7 (conditioned on therapist and stratification site, 95% CI 4.9 to 33.0; p < 0.001) for TCBT versus TAU. For the comparison of WCBT with TAU, which was not affected by therapist effects, the OR was estimated to be 4.0 (95% CI 2.3 to 6.9; p < 0.001). Comparing these findings with those reported in Table 30 shows that the definition of a ‘PEQ responder’ does not affect the substantive conclusions.
An instrumental variables analysis was carried out to assess the efficacy of TCBT and WCBT, based on complete cases. The 12-month efficacy (quantified by CACE) for IBS SSS was estimated to be 71.8 (95% CI 93.7 to 59.9; p < 0.001) for TCBT compared with TAU and 50.4 (95% CI 75.2 to 25.5; p < 0.001) for WCBT compared with TAU. For WSAS, the estimated efficacy was –4.5 (95% CI –6.1 to –3.0; p < 0.001) for TCBT compared with TAU and –4.2 (95% CI –5.9 to –2.4 points; p < 0.001) for WCBT compared with TAU. This suggests that the intervention might be more efficacious (the effects of actually receiving the intervention stronger) than the effects suggested by the ITT analyses.
Adverse events
There were 77 recorded AEs in the TCBT arm, 61 in the WCBT arm and 55 in the TAU arm (Table 30). We would expect to see an increased rate of reported events in the CBT arms, particularly in the TCBT arm, as the therapists completed AE forms for any AEs that were mentioned during the therapy sessions.
| Body system code | Trial arm, n (%) | Overall, n (%) | ||
|---|---|---|---|---|
| TCBT | WCBT | TAU | ||
| 1. Cardiovascular | 5 (6.5) | 1 (1.6) | 5 (9.1) | 11 (5.7) |
| 2. Respiratory | 5 (6.5) | 3 (4.9) | 6 (10.9) | 14 (7.3) |
| 3. Hepatic | 2 (2.6) | 2 (3.3) | 0 (0.0) | 4 (2.1) |
| 4. Gastrointestinal | 14 (18.2) | 10 (16.4) | 11 (20.0) | 35 (18.1) |
| 5. Genitourinary | 5 (6.5) | 2 (3.3) | 8 (14.5) | 15 (7.8) |
| 6. Endocrine | 0 (0.0) | 2 (3.3) | 0 (0.0) | 2 (1.0) |
| 7. Haematological | 1 (1.3) | 0 (0.0) | 1 (1.8) | 2 (1.0) |
| 8. Musculoskeletal | 15 (19.5) | 8 (13.1) | 4 (7.3) | 27 (14.0) |
| 10. Neurological | 4 (5.2) | 6 (9.8) | 0 (0.0) | 10 (5.2) |
| 11. Psychological | 18 (23.4) | 17 (20.9) | 10 (18.2) | 45 (23.3) |
| 12. Immunological | 0 (0.0) | 2 (3.3) | 0 (0.0) | 2 (1.0) |
| 13. Dermatological | 2 (2.6) | 2 (3.3) | 1 (1.8) | 5 (2.6) |
| 14. Allergies | 0 (0.0) | 0 (0.0) | 1 (1.8) | 1 (0.5) |
| 15. Ear, nose, throat | 0 (0.0) | 2 (3.3) | 4 (7.3) | 6 (3.1) |
| 16. Other | 6 (7.8) | 2 (3.3) | 4 (7.3) | 12 (6.2) |
| Missing code | 0 (0.0) | 2 (3.3) | 0 (0.0) | 2 (1.0) |
| Total | 77 (100.0) | 61 (100.0) | 55 (100.0) | 193 (100.0) |
Of these, 18.1% were gastrointestinal related (see Table 11). Fourteen of these events were seen in the TCBT arm, 10 in the WCBT arm and 11 in the TAU arm. Of the AEs, 23.3% were psychological events, of which 18 were seen in the TCBT arm, with 17 and 10 events seen in the WCBT and TAU arms, respectively. The other 14 body system codes made up 58.6% of AEs, most individual system codes accounting for < 10% of events.
There were four GI AEs in the TAU arm, one psychological event in the TCBT arm and no events related to the treatment in the WCBT arm that were recorded prior to unblinding as possibly related to the intervention (Table 31). There were two events in each of the TAU, WCBT and TCBT arms for which, prior to unblinding, it was recorded as unknown whether or not they were related to the intervention.
| Body system code | Trial arm (n) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCBT | WCBT | TAU | |||||||||||||
| Possible | Remote | None | Unknown | Total | Possible | Remote | None | Unknown | Total | Possible | Remote | None | Unknown | Total | |
| 1. Cardiovascular | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 5 | 0 | 5 |
| 2. Respiratory | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 6 | 0 | 6 |
| 3. Hepatic | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 4. Gastrointestinal | 0 | 0 | 13 | 1 | 14 | 0 | 0 | 10 | 0 | 10 | 4 | 0 | 4 | 3 | 11 |
| 5. Genitourinary | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 8 | 0 | 8 |
| 6. Endocrine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7. Haematological | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 8. Musculoskeletal | 0 | 0 | 15 | 0 | 15 | 0 | 0 | 8 | 0 | 8 | 0 | 0 | 4 | 0 | 4 |
| 10. Neurological | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 6 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
| 11. Psychological | 0 | 1 | 17 | 0 | 18 | 0 | 0 | 14 | 3 | 17 | 0 | 0 | 10 | 0 | 10 |
| 12. Immunological | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 13. Dermatological | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 | 3 | 0 | 0 | 1 | 0 | 1 |
| 14. Allergies | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 15. Ear, nose, throat | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | 0 | 4 |
| 16. Other | 0 | 0 | 5 | 1 | 6 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 4 | 0 | 4 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 4 | 0 | 0 | 0 | 0 |
| Total | 0 | 1 | 74 | 2 | 77 | 0 | 0 | 58 | 3 | 61 | 4 | 0 | 48 | 3 | 55 |
Protocol deviations
Therapist-related protocol deviations are outlined in Appendix 9.
A full list of protocol deviations can be found in Appendix 9.
Discussion
Summary of results
The ACTIB trial was a RCT of TCBT with a patient manual and web-delivered CBT with minimal therapist input (WCBT), both compared with TAU in adults with refractory IBS with a 12-month follow-up. Both therapy arms received TAU as well as CBT. A total of 558 out of 1452 (38.4%) of patients screened for eligibility were recruited: 186 were randomised to TCBT, 185 were randomised to WCBT and 186 were randomised to TAU. The mean IBS SSS was 265.0 at baseline, indicating moderately severe IBS symptoms. The trial arms were well balanced at baseline.
Follow-up rates were 76.5% at 3 months (427/558), 72.9% at 6 months (407/558) and 70.3% at 12 months (392/558). ITT analysis was undertaken with MI.
Compared with the TAU arm, both the TCBT and WCBT intervention arms showed a significant improvement in primary (IBS SSS and WSAS) and secondary outcomes over the trial period. In terms of the two primary outcomes, at 12 months, compared with the TAU arm (IBS SSS of 205.6), IBS SSS were 61.6 lower for the TCBT arm (95% CI 89.5 to 33.8; p < 0.001) and 35.2 lower for the WCBT arm (95% CI 57.8 to 12.6; p = 0.002). The WSAS score in the TAU arm was 10.8 (SD 9.3) at 12 months, 3.5 lower with TCBT (95% CI 5.1 to 1.9; p < 0.001) and 3.0 lower with WCBT (95% CI 4.6 to 1.3; p = 0.001). Thus, both primary outcomes were clinically and statistically significant at 12 months.
In terms of secondary outcomes, SGA of relief of symptoms had a 84.8% response rate in the TCBT arm at 12 months compared with 41.7% in the TAU arm (OR 6.1, 95% CI 2.5 to 15.0; p < 0.001) and 75.0% in the WCBT arm (OR 3.6, 95% CI 2.0 to 6.3; p < 0.001). PEQ had a 78.3% response rate in the TCBT arm compared with 23.5% in the TAU arm (OR 9.3, 95% CI 4.5 to 19.3; p < 0.001) and 54.8% in the WCBT arm (OR 3.5, 95% CI 2.0 to 5.9; p < 0.001). For HADS, compared with the TAU arm [mean HADS score of 16.4 (SD 6.9) at 12 months], scores were 2.8 (95% CI 4.1 to 1.5) lower (p < 0.001) in the TCBT arm and 2.3 (95% CI 3.7 to 1.0) lower (p < 0.001) in the WCBT arm at 12 months.
Strengths and limitations
Strengths
To our knowledge, this is the largest trial of CBT for IBS worldwide to date and it has the advantage of a 12-month follow-up and assessment of both a higher-intensity therapist-led CBT (TCBT) and a lower-intensity CBT self-management intervention (WCBT) with a nested qualitative study and health economic evaluation.
The trial was rigorously conducted with high levels of research and clinical governance, a careful trial design, an explicit theoretical model, detailed patient and therapy manuals, a rigorously developed CBT website (Regul8) and highly trained therapists with experience in delivering CBT. The CBT interventions were specifically tailored to IBS rather than more generic CBT, and there was regular clinical supervision and good treatment fidelity.
The baseline tables indicated that the three trial arms had very similar characteristics and, thus, that randomisation appeared to work well.
The results were highly significant in favour of the intervention arms for all primary and secondary outcomes despite positive improvements in outcome in the TAU group; thus, one can be fairly confident that the results are robust.
Limitations
As with most RCTs, there is a potential for selection bias in the participants entering a trial compared with those with a diagnosis of IBS in the general population. We attempted to reduce this as much as possible by broad inclusive recruitment methods from both primary and secondary care. GPs invited all patients on their lists with a computer record of an IBS diagnosis; patients were also recruited through opportunistic recruitment and posters. However, only approximately 10% responded to the invitations. Among those who responded with a reason for declining participation in ACTIB, the main reasons were that their IBS symptoms did not need further management, they lacked time or they did not want to undertake CBT. Thus, the low response rate to invitation is probably partly a result of a proportion of people invited who no longer had troublesome IBS symptoms. This group would not have fulfilled screening for refractory IBS and would have appropriately self-selected to not participate. However, people with IBS who were unwilling to consider undertaking CBT for IBS are also likely to have declined to enter the trial and so this could affect generalisability of the findings to the wider population of those with refractory IBS. Encouragingly, the age and gender profiles of those invited were very similar to the age and gender profiles of those indicating that they would like to participate and those randomised, which suggests that, in terms of these basic demographics, the sample was representative of the broader population. We did not have data on the ethnicities of those invited so cannot be sure of the representativeness of the sample in terms of ethnicity. A total of 90.9% of those randomised were white, so people from ethnic minorities may have been under-represented.
Follow-up rates were lower than the 80% we had anticipated in the original sample size calculation despite extensive efforts to follow up participants. The 3-month follow-up rate was 76.5% and the 12-month follow-up rate was 70.3%. To mitigate this lower than anticipated follow-up, and to maintain power, we recruited more participants than initially planned (558 were randomised, compared with an initial estimated sample size of 495). In addition, an ITT analysis with MI was used to ensure that the results remained internally valid in the presence of missing data.
Interpretation of ACTIB results
Clinical relevance and magnitude of effect
Our results show a statistically significant improvement in both therapy arms over TAU at all trial time points (3, 6 and 12 months) in both primary outcomes and most secondary outcomes, suggesting that both forms of CBT delivery are effective treatments for IBS when compared with TAU. It is also important to consider the clinical significance of the changes observed. We also found a clinically important improvement at all time points for both therapy arms compared with TAU for IBS SSS. The MCID between groups for IBS SSS was defined a priori in our sample size calculation as 35 (a within-patient change of 50 is deemed a significant clinical change and for group differences we assumed a 15-point placebo response in the TAU arm in the trial). 15,24 We saw a change in IBS SSS greater than the MCID even though the TAU arm had a much greater improvement in IBS SSS than we had anticipated (at 12 months the TAU arm had improved by 52.9 on average compared with baseline). This large change in the TAU arm also highlights the importance of the placebo or Hawthorne effect in trials in IBS and reinforces the importance of having a TAU arm.
The size of the difference in IBS SSS between therapy arm and the TAU arm was greater at all time points for TCBT than for WCBT, which is what would be expected given the significantly greater therapist time input for the TCBT arm (8 hours) than for the WCBT arm (2.5 hours). Previous trials of web-based or lower-intensity CBT for IBS have commonly shown smaller effect sizes than TCBT for IBS. 43 TCBT also had greater adherence rates, with 84% of patients meeting our threshold for adherence, compared with 70% in the WCBT arm. This is consistent with a systematic review of CBT for depression, which reports greater adherence to individual face-to-face CBT than to guided WCBT. 44 However, the comparisons between face-to-face CBT and WCBT in this review are drawn from different trials. 44 The current study is one of the first studies to include TCBT and WCBT in the same trial.
Although adherence was lower in the WCBT arm, the changes were moderate, clinically significant and, most importantly, sustained at 12 months. Most other minimal-contact IBS interventions have relied on self-referral and shown larger dropout rates among patients referred to treatment. 45 As all IBS patients in participating centres were invited to the ACTIB trial, we can conclude that Regul8 with minimal contact is largely acceptable to a broad range of patients.
Comparison with results of other studies
Previous research7,8,20 has shown face-to-face CBT to be helpful for IBS, particularly immediately after completing treatment. However, a Cochrane review8 concluded that it was unclear whether or not the effects were maintained in the longer term.
In this ACTIB trial, we found that the beneficial results of the TCBT arm were maintained at 12 months. This may be related to the comprehensive CBT manual supplied to participants and the two booster sessions at 4 and 8 months, which were aimed at maintaining improvement. It may also be because the interventions were based on a clear theoretical model that targeted specific cognitive and behavioural responses to IBS symptoms, which gave it good face validity, and participants perceived it to be high quality in the nested ACTIB qualitative study.
In addition, previous research8 into the use of CBT for IBS found that adherence to therapy was limited, especially with face-to-face therapy, although also with web-based therapy. 43 For instance, in the trial by Kennedy et al. ,7 which assessed therapist-delivered CBT, fewer than half of the participants were considered to have completed therapy by the end of the intervention and 41% were recorded as declining therapy or dropping out, often because of time issues such as work and childcare commitments. However, we found good adherence to therapy, with 84% of participants compliant in the TCBT arm and 70% compliant in the WCBT arm. The improved concordance rates may have been because therapy was delivered over the telephone but also because participants were offered flexibility regarding appointment times. A key component of ensuring engagement in the intervention is likely to be the provision of a physiological explanation of IBS symptoms and how these physiological changes may link to factors such as the lack of an eating routine and an autonomic nervous system response to stress.
Some recent small pilot trials1,12,16,17 have shown promise for web-based CBT for IBS but suggested that some therapist input is needed. Our results indicate that significant improvements in IBS symptoms can be achieved and maintained at 12 months with quite low levels of therapist input (2.5 hours in total). However, our qualitative work reinforces the importance of some therapist input, rather than a standalone website. In the qualitative study, it was found that participants felt that therapists had an important role to play in supporting patients to engage with CBT and to make sense of the therapy and their IBS. Patients valued having therapist support available alongside Regul8 and this may have helped enhance their engagement and outcomes.
Generalisability of findings
As described in Strengths and limitations, we made every effort to ensure that a broad range of patients were included in this trial with recruitment from primary and secondary care from different regions of the UK. This will enhance generalisability of the findings. However, all of those who entered the trial were willing to participate in a trial of CBT for IBS and this may not be the case for all people with refractory IBS. Interestingly, the qualitative findings showed that even those with some initial scepticism about CBT were able to engage with the CBT interventions and make improvements. Other factors that may affect the generalisability of the results were our sample had limited ethnic diversity and participants were relatively highly educated. We found in the MI statistical model that a high baseline deprivation score was predictive of missing outcomes.
Conclusion
Both CBT intervention arms showed clinically and statistically significant improvements in IBS outcome measures, compared with TAU, that were sustained at 12 months.
Implications for health care
Currently, clinicians have few options to offer people with refractory IBS. This study shows that providing CBT has the potential to provide significant improvement in symptoms and is achievable within a NHS setting (NHS therapists delivered the interventions). Both the higher-intensity TCBT and the web-based self-management (Regul8) with minimal therapist support showed significant clinically important improvements in symptom severity. Therapists who currently work in the Improving Access to Psychological Therapies (IAPT) services have suitable skills, are well placed to provide CBT for IBS and could be trained using the manuals carefully developed for this study. A potential option could be a ‘matched’ approach in which people with IBS are initially offered the most appropriate type of CBT for them, or a ‘stepped care’ approach in which all patients are offered lower-intensity WCBT and further therapist input is considered for those with particularly troublesome or resistant symptoms. We are currently undertaking a process evaluation that is likely to provide further useful information on which form of CBT is most appropriate for which patients with IBS.
Recommendations for research
Further research is needed to clarify the best ways to implement CBT for IBS in clinical settings and whether or not CBT therapy effects can be maintained in the longer term.
Chapter 2 Qualitative study
Summary
Objectives of the qualitative work
The objectives were to identify the factors that facilitate or impede adherence to web-delivered and TCBT in patients with refractory IBS, to provide insight into the quantitative results of the ACTIB trial and to identify social and psychological processes of change that took place during the trial.
Background
Face-to-face CBT has been shown to improve IBS symptom severity and QoL. However, its availability in primary care is limited and its cost-effectiveness is uncertain. Furthermore, it has been associated with high dropout rates. Both telephone- and web-based CBT are becoming widely used psychological interventions to treat a diverse range of medical and mental health conditions. Web-based CBT affords individuals the opportunity to engage in a therapy by overcoming some of the known barriers to traditional face-to-face CBT.
Methods
Semistructured interviews were undertaken with 52 ACTIB trial participants at 3 months and 42 interviews were undertaken at 12 months post baseline (see Appendix 10 for interview schedules). Inductive thematic analysis was used.
Findings
Key themes related to the perceived benefits of CBT, the role of the therapist and the processes by which CBT may have elicited benefits. Perceived benefits of CBT include reduced symptoms and, arguably more importantly, an increased capacity to cope with symptoms, negative emotions and other challenges of daily life. Therapists have an important role to play in supporting patients to engage with CBT and to make sense of the therapy and their IBS. Patients valued having therapist support available alongside Regul8 and this may have helped enhance their engagement, adherence and outcomes. The perceived high quality of the intervention itself also facilitated adherence. CBT appeared to help patients make changes to their ways of thinking and behaving in relation to their IBS, broadly consistent with the theoretical mechanisms of change underpinning this intervention.
Conclusion
Telephone- and web-based CBT for IBS appears to help patients change how they think and feel about IBS, and can produce benefits that go beyond symptom reduction to more fundamental changes in coping and self-management strategies. If rolled out in practice, such interventions would benefit from offering therapist support alongside any web-based intervention, could be offered to patients despite initial scepticism regarding psychological interventions and could be augmented with longer-term support for maintenance.
Introduction
Web-based CBT is becoming a widely used psychological intervention to treat a diverse range of medical and mental health conditions, with continued success. 46 Furthermore, a growing body of evidence has shown reliable improvements in mental health conditions if CBT is delivered in a web-based format and accompanied by a text-based manual with therapist support. 47,48 WCBT provides individuals with an opportunity to access a psychological therapy that they would otherwise not have access to. Irrespective of the documented success rates for CBT in IBS,6 it is not without its significant barriers to access and implementation. For example, there are shortages of adequately trained individuals to run sessions; getting to a session can be a burden because of issues around travel, family and cost; individuals do not always have the time to attend sessions for various personal and professional reasons; individuals are known to experience various psychosocial issues such as embarrassment and stigmatisation; and not all people who are offered help take it up because of a limited perceived need for treatment and a preference for managing their condition independently. 49–55 In addition, findings from RCTs of WCBT have found it to be comparable to face-to-face CBT56 in the development of a therapeutic relationship. 57
In a study by Hadjistavropoulos et al. 58 exploring participants’ views on therapist-assisted WCBT, participants completed, on average, 8.57 of the 12 modules over 18 weeks, with approximately 51% completing all 12 modules. 58 Acceptability of this form of therapy was rated as high (mean 5.58, SD 1.52) on a rating scale of 0 to 7. Furthermore, participants reported valuing the information they were given (e.g. ‘knowing I am not alone’ and ‘finally having access to good information’) and the skills they learned (e.g. ‘challenging negative thoughts’). Participants also acknowledged the role of the therapist in their experiences of WCBT, noting ‘really a good therapist is what has kept me here’, and how they benefited from the therapist’s support (e.g. ‘the best part were the email exchanges’). More importantly, participants noted a preference for web-based CBT over face-to-face CBT because of ‘more frequent treatment than seeking a counsellor’. Another such study59 found that participants like the anonymity that this mode of therapy provides. There appears to be a variation in how people respond to and engage with the programmes and participants need a differing amount of support to keep them engaged. However, the qualitative research on the use of non-face-to-face CBT for health conditions is limited.
Moreover, Hadjistavropoulos et al. 58 noted that most participants reported negative aspects of CBT rather than WCBT, for instance with regard to completing the recommended homework (e.g. ‘the exercises after each module’) and not liking certain aspects of CBT that involved confronting their fears (e.g. ‘worry exposure’). 58 Regarding the WCBT, it was found that motivation was a particularly hard aspect of completing the web-based modules, and participants found it difficult to open up to a therapist about their problems (e.g. ‘having to put everything into words’). Conversely, it was also found that some of the participants felt that they needed more contact with the therapist (e.g. ‘communication only being weekly’) or that the information was too generalised (e.g. ‘the fact that it was generalised’). Furthermore, when asked about limitations of WCBT, participants expressed that they ‘can’t really think of a worst part!’.
Specific to IBS, the MIBS feasibility study60 is the only known trial to date that has explored whether or not an internet-delivered CBT programme is acceptable to IBS patients. Using qualitative methods, this study found that, on the whole, participants perceived web-based CBT positively. In reporting their findings, Tonkin-Crine et al. 60 noted that ‘the website was well designed and easy to understand and use’, although ‘a user had to be self-motivated to work through the material’. Participants engaged with the website to varying degrees, with some having limited or no engagement because ‘they did not find the website relevant to them’ or ‘the website was too impersonal’. Participants differed in terms of the aspect they engaged with most; for example, some found cognitive aspects, such as challenging negative thoughts, most helpful, whereas others found the website more helpful for encouraging lifestyle changes. 60
Exploring participants’ experiences of complex interventions and other health interventions has many benefits that quantitative measures cannot capture,61,62 for example to examine whether or not the intervention was delivered as it was intended, to explore the deliverers’ and participants’ responses to the intervention, to explore reasons for the findings and to explain variations in effectiveness within the sample. 62 Similarly, others63,64 have found that interviewees find the interview an opportunity for self-reflection, appraisal, catharsis, their conditions being validated and a feeling of being listened to. Furthermore, inductive qualitative methods allow an in-depth insight into a topic area and can add valuable information to quantitative analysis to enrich the data as a whole, for example by explaining how an intervention may succeed or fail, how the intervention can be optimised and how acceptable the intervention was to participants. 63
The current qualitative study was nested within the ACTIB RCT. As previously mentioned, the ACTIB trial aims to determine the clinical effectiveness and cost-effectiveness of TCBT and WCBT in IBS. At present, there is a dearth of literature exploring the use of telephone- and web-based CBT for IBS, so this qualitative study will add to what is already known while supplementing the quantitative findings.
Aims and objectives
The overarching aim was to explore patients’ experiences of the CBT for IBS. The objectives were to identify factors that facilitate or impede adherence to web-delivered and therapist telephone-delivered CBT in this patient group, to provide insight into the quantitative results of this complex trial and to identify social and psychological processes of change that took place during the trial. The purpose was to use qualitative methods to add scientific value concerning understanding of change processes and practical value concerning the relative merits of each type of CBT and delivery issues to attend to in any future widespread implementation.
Methods
Design
This qualitative study was nested within the main ACTIB trial. Semistructured interviews were subjected to thematic analysis, incorporating techniques from grounded theory and framework analysis. Data collection and initial analysis proceeded iteratively, with coding beginning after completing the first few interviews and informing subsequent interviews. The analysis was data driven (i.e. inductive) in that the researchers sought to identify themes in the data rather than imposing any pre-existing interpretive framework. Subsequently, inductively identified themes were compared across subgroups, mapping the qualitative findings against prespecified quantitative data. This approach enabled the strengths of qualitative data and inductive analysis to be realised while also relating these findings to their context, that is, the main trial.
Ethics consideration
At the beginning of the trial, all trial participants completed an online consent form (see Appendix 2) and agreed to be contacted to discuss their involvement in a qualitative interview at 3 months post baseline (i.e. post treatment) and at 12 months post baseline. On subsequently being invited to take part in interviews, the purpose and scope of the interviews were reiterated. Before commencing each interview, interviewers obtained and recorded oral consent. All interviews were anonymised on transcription; pseudonyms and/or participant numbers have been used throughout the reporting of this study, and to minimise the risk of participant identification, combinations of multiple participant characteristics are not reported at an individual participant level.
The qualitative study was included in the main ethics application approved by the NRES Committee South Central – Berkshire (REC reference 13/SC/0206) on 11 June 2013. The interview topic guides were approved by the same committee on 4 February 2014.
Sampling and recruitment
The aim was to interview a diverse sample of approximately 17–20 interviewees per arm at baseline (i.e. 10–12%). Interviewing participants from each active arm allows factors to be identified that relate to adherence and change processes; including participants from the TAU arm provides insight into the quantitative results. Interviewing the same individuals at 3 and 12 months allows greater depth to explore change processes over time and the potential to better understand any differences in the quantitative results between 3 and 12 months. Interviewees were sampled purposively to encompass variety in gender, age, ethnic background, baseline symptom severity scores, recruiting site (London/Southampton) and setting (primary/secondary care). Sampling for variety on such key characteristics helps ensure that the qualitative findings capture the breadth of participants’ experiences and are not dominated by any one subgroup.
Participants were invited in batches to permit iterative sampling, interviewing and analysis. The researchers attempted to contact participants via two e-mails, one text message and two telephone calls, after which no further attempts were made. In total, 100 trial participants were contacted in this way. Recruitment to interviews ended when no new themes emerged and existing themes were well developed within a diverse sample. The ACTIB trial recruited a total of 558 participants, 52 of whom were interviewed.
Interviews
Semistructured interviews were used to elicit interviewees’ experiences of taking part in the trial and IBS more broadly. Separate topic guides for the 3- and 12-month interviews (see Appendix 10) were developed collaboratively by the research team and refined after being piloted with two people with IBS. The topic guides comprised a series of open-ended questions and prompts used by the interviewer to elicit participants’ experiences of, reflections on and thoughts and feelings about the trial within the broader context of managing IBS. The questions were open ended and designed to elicit concrete descriptions of interviewees’ experiences and reflections thereon. The questions were grouped into four sections: taking part in the trial and trial treatments, other treatments tried for IBS, experiencing and managing feelings and thoughts about the future. Topic guides were used flexibly to ensure that interviewers covered all required topics while allowing for interviewees to introduce unanticipated issues and to have some control over the flow of the interviews. Interviewees were offered the choice of being interviewed by telephone or face to face. All interviews were audio-recorded using a digital audio-recorder.
The 3-month interviews
The 3-month interviews explored interviewees’ experiences of taking part in the trial and the treatment they were allocated to, their experiences of other treatments for IBS and their thoughts about the future. Most of the interviews were conducted by a female research assistant (n = 42) and the remainder (n = 10) were conducted by a male postgraduate student. Most 3-month interviews were conducted over the telephone (n = 42); the remainder were conducted face to face at the request of participants (n = 10). The 3-month interviews lasted from 23 to 116 minutes (mean 56 minutes). They took place over a 19-month period (between September 2014 and July 2016).
The 12-month interviews
The 12-month interviews explored interviewees’ reflections on taking part in the trial and the treatment they were allocated to, any other treatments they had tried since the 3-month interviews and their thoughts about the future. The 12-month interviews were all conducted by a female research assistant. Forty were conducted over the telephone and two face to face. These interviews lasted from 11 to 107 minutes (mean 45.52 minutes). They took place over a 23-month period (from July 2015 to June 2017).
Analysis methods
Interviews were transcribed verbatim with identifying details (e.g. names) removed. Analysis began on completion of the first few interviews and proceeded iteratively; this allowed early insights or puzzling findings to be explored more fully in later interviews and, if necessary, for improvements to be made to the topic guide and interviewing technique. An inductive thematic analysis employing supplementary techniques from grounded theory65,66 was used to code the data and to identify themes that captured key concepts and processes. The thematic analysis procedure outlined by Braun and Clarke67 was used, moving (as recommended) backwards and forwards through the phases, rather than approaching the analysis in a linear fashion. These phases were supplemented with techniques from grounded theory, as shown in Table 32.
| Phase | Thematic analysis | Supplementary techniques |
|---|---|---|
| 1 | Familiarisation with the data through reading and re-reading transcripts | Listen to audio-recordings |
| 2 | Generate initial codes | Line-by-line open coding on a portion of the data; constant comparison |
| 3 | Searching for themes | Constant comparison; identifying key concepts in the data; write memos |
| 4 | Reviewing themes for fit with coded extracts and entire data set; generate a thematic ‘map’ | Constant comparison; search for negative/deviant cases; generate case summaries for individual interviewees to capture whole stories and changes across the 3- and 12-month interviews |
| 5 | Defining and naming themes and their inter-relations | Constant comparison |
| 6 | Reporting: select compelling examples, final analysis and contextualisation with the literature and research objectives | Identify the limits of the analysis |
NVivo version 11 (QSR International, Warrington, UK) was used to facilitate data management and coding and to undertake thematic comparisons across subgroups of participants. In addition to the analytic procedures described above, the following procedures were used to enhance the trustworthiness of the analysis: multiple researchers contributed to the analysis to avoid producing idiosyncratic interpretations, a ‘member check’ was conducted whereby interviewees were invited to comment on summaries of their interviews, an audit trail was produced to enhance transparency, including memos and a coding manual, and field notes were written after each interview to capture initial impressions and non-verbal/contextual observations.
Findings
Participants
Fifty-two trial participants took part in 3-month interviews; their characteristics are shown in Table 33. Ten individuals declined to take part in follow-up interviews: five from each of the CBT arms. Forty-two interviewees were interviewed again at 12 months; their characteristics are shown in Table 34. The reasons participants did not take part in the second interview were that they had no time available in their schedule (n = 3), they had childcare responsibilities (n = 2) or they were not contactable (n = 5).
| Characteristic | Trial arm | Total (N = 52) | ||
|---|---|---|---|---|
| TCBT (N = 17) | WCBT (N = 17) | TAU (N = 18) | ||
| Gender (n) | ||||
| Female | 13 | 14 | 13 | 40 |
| Male | 4 | 3 | 5 | 12 |
| Ethnicity (n) | ||||
| African | 1 | 1 | ||
| Indian | 1 | 1 | ||
| Irish | 1 | 1 | ||
| White | ||||
| Asian | 1 | 1 | ||
| British | 11 | 12 | 15 | 38 |
| Other | 4 | 4 | 1 | 9 |
| Other | 1 | 1 | ||
| Age (years), mean (SD) | 39.94 (11.71) | 42.41 (17.31) | 39.72 (13.23) | 40.67 (14.06) |
| IBS SSS, mean (SD) | 283.37 (117.11) | 259.65 (124.39) | 236.83 (86.36) | 259.54 (109.61) |
| Recruitment site (n) | ||||
| Primary care | 11 | 13 | 11 | 35 |
| Secondary care | 6 | 4 | 7 | 17 |
| Characteristic | Trial arm | Total (N = 52) | ||
|---|---|---|---|---|
| TCBT (N = 17) | WCBT (N = 17) | TAU (N = 18) | ||
| Gender (n) | ||||
| Female | 10 | 9 | 13 | 31 |
| Male | 3 | 3 | 5 | 11 |
| Ethnicity (n) | ||||
| African | 1 | 1 | ||
| Indian | 1 | 1 | ||
| Irish | 1 | 1 | ||
| White | ||||
| Asian | 1 | 1 | ||
| British | 8 | 9 | 15 | 31 |
| Other | 4 | 3 | 1 | 8 |
| Other | 1 | 1 | ||
| Age (years), mean (SD) | 40.38 (12.26) | 45.00 (18.63) | 40.82 (12.76) | 41.88 (14.31) |
| IBS SSS, mean (SD) | 290.62 (128.26) | 219.58 (123.01) | 232.53 (87.54) | 246.81 (113.03) |
| Recruitment site (n) | ||||
| Primary care | 6 | 9 | 10 | 25 |
| Secondary care | 7 | 3 | 7 | 17 |
Facilitators to and barriers of web-delivered and therapist telephone-delivered cognitive–behavioural therapy for irritable bowel syndrome
The facilitators to and barriers of engaging with CBT for IBS can be best understood within the broader context of interviewees’ previous experiences of IBS treatments and their reasons for entering the ACTIB trial. A focused analysis of 3-month interviews was conducted to explore interviewees’ experiences of seeking and appraising treatments for IBS before entering the trial. 68 In summary, this analysis demonstrated that interviewees entered the trial having previously tried a diverse range of treatments for IBS without satisfactory effects on their symptoms or QoL. Accounts of treatment seeking were characterised by a sense of being trapped within a ‘vicious cycle’ of alternating hope (for new treatments) and despair (on finding them ineffective). A desperation and willingness drove interviewees to try any treatment modality available if it offered potential relief. Interviewees derived hope that a new treatment would resolve their symptoms (at the extreme, provide a cure) from various sources, including word-of-mouth recommendations, internet sources, marketing claims and advice from health-care professionals. Treatments that had been tried included various medications (prescribed and over the counter), special diets and complementary or alternative therapies. Interviewees appraised treatments for their effects on symptoms and QoL while also considering, but rarely prioritising, other aspects, including convenience of the regimen itself in the broader context of one’s personal social and working life, whether or not it addressed the perceived root causes of IBS, perceived side effects and adverse impacts on QoL, and cost. On finding a treatment to be ineffective, disappointment often ensued before the search resumed for a more effective remedy for ongoing symptom flare-ups. Repeated disappointing experiences contributed to some interviewees feeling exploited by marketing companies, having reduced confidence in health-care professionals and feeling negatively about themselves and their QoL. Escaping the vicious cycle was helped when individuals found some symptom relief and began to accept that their IBS might need ongoing self-management rather than continuing to hope for a cure.
The invitation to take part in the ACTIB trial thus came to people within a complex context of treatment-seeking behaviours and disappointing experiences. Some of the reasons people gave for enrolling in ACTIB strongly reflected this context. For example, interviewees talked about wanting help with their IBS, being willing to try anything that might help or provide new insight into their IBS, wanting to try a non-medical approach in general or CBT in particular (having tried many medications previously), feeling that their GP had nothing else left to offer them and feeling frustrated or down about their ongoing symptoms and reduced QoL. Less commonly described reasons for participating were altruistic (e.g. to help research and other patients in the future) or passive (e.g. because they received an invitation from their GP):
I was interested when I first heard about it because – of it being about CBT as a treatment because I’ve tried all the medications that my GP had suggested.
ID40370, TAU, 3 months
I was just feeling really miserable about my symptoms and – and anything would help, really.
ID28570, WCBT, 3 months
Typically, interviewees were commencing CBT with some hope that it would provide either a cure or effective relief, but this hope was often tempered by a long history of disappointing experiences of treatments. At the most extreme, some interviewees described fearing that CBT would become another in a long line of disappointing treatments, whereas others were simply desperate for some relief:
I did the programme at a time when I was desperate to find a solution, so I was really willing to – give it a try; so I was motivated, but same time I really didn’t believe it would.
ID40015, TCBT, 12 months
Facilitators
Two key factors appeared to facilitate engagement with both forms of CBT in the trial: the perceived high quality of the intervention itself and the relationship that patients developed with their therapist. Interviewees reported finding the materials and sessions informative and well structured, and liked particular features, such as having some flexibility to self-pace, the follow-up sessions and reminders, the online/telephone format and the ability to refer back to materials at a later date. The relationship that patients developed with their therapist encouraged interviewees to continue engaging with both therapist telephone-delivered and web-delivered CBT. Especially (but not only) in the TCBT arm, this relationship helped interviewees to overcome challenges and to make sense of their experiences over the course of therapy; interviewees particularly valued working with the same therapist across all sessions and praised their support, compassion and professionalism:
Well I liked the fact that – I was not left on my own, I was just – I had someone to talk to and actually follow the progress and then – it was really good because there were some really – there were some times when it was quite hard, like emotionally – I mean things happened last year and at some point I think – I think the symptoms got – they actually began to get worse again and then the fact that I could speak to [therapist] about this, she explained and we went through everything, I think it just really helped, because I think – a month into the programme I would have given [up] because of what happened. And because [therapist] was there, she really gave me the support I needed at that point, so it was really good. I mean otherwise I think I would have stopped the programme before I finished it.
ID40015, WCBT, 3 months
Interviewees from the WCBT arm appeared to value the therapist interactions (which took the form of telephone support calls) predominantly in practical terms (as reminders and/or opportunities to clarify and ask questions about the materials) or in terms of feeling supported and listened to (i.e. having someone to talk to about their IBS):
Except that it made me feel a bit better, to talk to somebody who understood, that I had the freedom to talk about these things that people don’t normally talk about and other people don’t really want to hear. So it was nice to be able to talk to someone about that and be listened to with some understanding; that was great and that was helpful in that sense.
ID39958, WCBT, 12 months
A sense of progression during the trial also appeared to encourage some participants to continue engaging with CBT. This could take the form of experiencing early improvements in symptoms and/or revising and then introducing new topics in each session:
Literally after the second week of doing it, sort of reading through the books and then – talking to [therapist] for the hour and going through everything, it was brilliant and the fact that it did really help, you know, week by week we were talking about different behaviours and – and I think, literally, I sort of saw improvement quite quickly really.
ID24527, TCBT, 3 months
Barriers
An initial scepticism about having CBT for IBS was a potential barrier to engagement mentioned by interviewees from both CBT arms. This scepticism appeared to be driven by having a physical model of IBS, in which there was no logical place for mental processes such as thoughts and feelings. However, many of the interviewees who described initial scepticism also typically reported overcoming this during therapy, suggesting that the intervention materials successfully addressed this issue for most people:
To be honest, when I started I was very sceptical, I couldn’t see how thought processes and things would actually affect your tummy, but when it’s explained through the literature and when you speak to a therapist, you can really see the connection between how you think and how your tummy reacts and – I think it just takes somebody to tell you . . .
ID25119, TCBT, 3 months
The demands that the interventions made on the interviewees and their time presented an ongoing barrier or challenge to engagement with both web-delivered and therapist telephone-delivered CBT. Participants in both therapy arms referred to the need for self-discipline to complete the homework tasks in the CBT programme. A few interviewees felt that the web-delivered sessions were repetitive, which exacerbated the sense of the intervention making excessive demands on their time:
I found it hard um . . . I’m not very good at doing homework and never have been and I don’t suppose I was, um . . . so where it’s given my homework to do, I’ve not – I’ve not been, um let’s say a grade A student.
ID21339, WCBT, 3 months
What did you dislike about being in this group?
I think probably the discipline of having to do the homework, but then I wanted to do the – it’s kind of a bit of a paradox; I wanted to do the homework because I’m keen to participate and kind of make the best of it, but it’s kind of remembering to do it and – having something else to do during the week.
ID25044, TCBT, 3 months
Some interviewees from the WCBT arm reported disengaging with therapy when they felt that the materials were not right for them. This manifested in three main ways:
-
Some interviewees felt that the materials did not provide for their particular level of symptom severity (typically, they felt that their symptoms were more severe than those described in the materials).
-
Some felt that they were not learning anything new about IBS, often describing how they had had it for many years and had already tried the strategies recommended by Regul8.
-
Some felt that the topics were covered in the wrong order for them; for example, they wanted to address stress earlier on so that they would then feel better able to engage with other topics:ID20066, WCBT, 3 months
I’ve followed all the little sections on the trial, looking at your diet, looking at your stress, looking at your activity and I’ve kind of gone through all of those on my own in the last few, you know, over the years. So from – from my point of view, I didn’t get an awful lot out of it because it was already telling what I already knew.
A sense of reluctance about, or discomfort with, talking about IBS symptoms was an initial deterrent for some interviewees, particularly, but not only, those allocated to the TCBT arm. It may have been that the increased frequency and intensity of telephone conversations in the therapist-delivered group helped these individuals to become more accustomed to speaking about their symptoms and to be more at ease with their specific therapist than those in the web-delivered group, who received fewer telephone calls:
I did think it is odd to do counselling over the phone, but now I think – actually – it doesn’t matter, it doesn’t really matter at all as long as the counselling is good.
ID40210, TCBT, 3 months
The phone calls were OK but I wasn’t as comfortable discussing stuff over the phone.
ID40567, WCBT, 12 months
Insight into the quantitative results of ACTIB
The quantitative results showed that, compared with the TAU arm, both the TCBT and WCBT intervention arms showed significant improvement in primary (IBS SSS and WSAS) and secondary (PEQ and SGA of relief of symptoms) outcomes. Expanding on these results, interviewees described, in their own words, the benefits that they perceived as resulting from their participation in ACTIB and, specifically, from the CBT that they received. The majority of participants in the therapy arms reported improvements that they attributed to CBT. Perceived benefits included symptom-related improvements (e.g. reduced symptoms and less severe symptoms), reduced use of medications/other health services and broader improvements in coping (e.g. with symptoms, emotions, stress, life events, interpersonal relationships) and QoL (e.g. confidence in daily life, re-engaging with social activities):
I think it is much improved really; I’ve not had as much sort of constipation as what I used to have, so – so yes – for me, it has been really, really good.
ID24547, TCBT, 3 months
Previously I would maybe see the doctor every 6 months maybe or when I was about to have a flight or a journey or some stressful situation and I’d end up with some omeprazole or something, to stop acid and IBS-type symptoms. And I haven’t had to do that for all of the time since the course finished, the ACTIB course finished.
ID20822, TCBT, 12 months
I think, in a way, it has helped me cope with some of the emotional side of that better than if I hadn’t had any therapy. I mean actually I think probably if I hadn’t had the CBT at all and I’d gone through the same problems, I would be even worse than it is at the moment.
ID40210, TCBT, 12 months
I’ve learned some new things about the condition and things that you can do to – help any symptoms when they come on and they were things that were different, from things I’ve been told by the doctor.
ID20068, WCBT, 12 months
But I mean overall I’ve seen a huge difference in who I am, how I am, what I say, think, how I behave and my outlook on life; there has been a lot of changes in that sense.
ID10074, WCBT, 12 months
. . . [it has changed] the way that I feel about my IBS, it’s just something there and if it happens it happens and I can deal with it, whereas it’s not kind of overtaking my life any more.
ID28570, WCBT, 12 months
Yes, I think it’s really helped me to – not ignore my symptoms, but to see them for what they are and so knowing that, OK, I know what’s happening now, I know why it’s happening, but still [carry on with] my day-to-day life much more confidently. I think I’ve become more confident and will just go out anyway and stuff like that.
ID25044, TCBT, 12 months
It is possible that some of the subjective perceived effects of CBT had not been fully captured by the quantitative outcome measures. A small number of interviewees described struggling to express their experiences within the confines of the questionnaires:
Some questions I felt you could elaborate on, um [Interviewer: Uh huh], for instance, if it said – ‘does it stop you going out?’. I mean, I could put yes, but I – I said no because I force myself to go out, but just to answer no, it makes you feel like you’re not worrying about it.
ID21140, TAU, 3 months
So it’s been very frustrating and it’s frustrating because – my experience of IBS – does not seem to fit the questions.
ID39958, WCBT, 3 months
A few interviewees reported not experiencing benefits from CBT, particularly in the longer term, when interviewees talked about forgetting or neglecting newly learned practices and finding old habits returning:
I would like to say that – things changed and it was helpful . . . but . . . but I don’t think so.
ID39958, WCBT, 12 months
I found it very helpful at the beginning, and obviously as time [fades], you forget about the practices.
ID40192, TCBT, 12 months
Potential social and psychological change processes
In addition to the changes in symptoms, QoL and other ‘outcomes’ described above, interviewees talked about changes that might have contributed to these outcomes. Notably, many of these changes mapped well onto the theoretical model underpinning CBT. For example, interviewees described changes in the attitudes, beliefs and behaviours that are broadly consistent with the CBT model of IBS:
So I think – there isn’t enough – promotion of anxiety and stress and things that can make your IBS worse; so I think understanding that more and [learning] techniques to help manage those situations has helped.
ID40567, WCBT, 12 months
I have now come to understand that the physical is just the manifestation of what is going on in the head.
ID10074, WCBT, 12 months
I don’t know what your name for them was but there are reminders of things to say or do just to remind yourself of – of some of the thought processes. And I had three or four of those as tools to use, so if I did feel the anxiety coming on then a little bit of deep breathing, diaphragm-type breathing, I felt that that was the solution and if it was a solution or not, it worked. So that gives you confidence and that works. Also looking – looking at things from a much wider perspective, so – look at the evidence, that was another one, a real little trick. You’re sitting in an aeroplane, everyone around you is just chatting, reading their books, having drinks, nobody’s fretting and getting unwell or anything; it’s just a journey. So the evidence all around you is overwhelming and positive, compared to the tiny little bit that your brain is trying to manoeuvre you into, to feeling negative or anxious. So that’s another little tool I’ve remembered and set aside to use if I need to. All those things like eating well, going to bed earlier, exercise. Another one: if I feel that I’m going to have a situation where stress has built up to a point, I can sense, if you like, the triggers of maybe being a bit forgetful or whatever and your mind being too much on stressful things, to get some exercise and get the cortisol levels down and do something physical. So I’ve maintained physical exercise that I hadn’t had before the CBT, which I think is probably favourable, as well.
ID20822, TCBT, 12 months
I started thinking to myself, you know, I don’t need to stress and worry about it, there is a toilet here, it’s there if I can use it, which has helped, because I used to go home early from parties and things like that with a tummy ache. But now I can just think, you know, I’m working myself up about it and then, more often than not, the feeling goes away and then I’m absolutely fine.
ID25119, TCBT, 3 months
I think like with the thoughts, just being aware of the sort of things that can kind of perpetuate the cycle of stress. I think catastrophising or black-and-white thinking and things like that, I think I can see them in myself and I think just being aware of that, you can kind of try and take a back step and see it in another way and re-evaluate the situation.
ID26417, TCBT, 3 months
Interviewees also described gaining greater insight into the nature of IBS. Crucially for some, this entailed the realisation that they should no longer be seeking a single curative treatment. In other words, it helped them to break out of the vicious cycle of treatment seeking that had characterised their approach before enrolling in ACTIB. This was made easier by having gained effective coping strategies from the CBT:
Well managing stress and obviously helping to reduce or minimise the flare-ups of the IBS; that’s the benefits that I got out of it; understanding what’s happening, I suppose is a benefit, because when you understand something a lot better, you’re better prepared to deal with it, so that’s obviously very important, very useful.
ID20850, TCBT, 12 months
I think that I understand a bit more about my IBS and stop thinking that there is going to be one solution to this, because before I used to think that.
ID16045, TCBT, 12 months
I just think that I can cope much better with my symptoms, which is maybe not that – has helped me with not having so many bad stomach aches, because I sort of do know how to manage my symptoms now. Before, I think my mindset was – I’m never going to get over this, it’s not going to go away; whereas now I can understand – it doesn’t stop me – my symptoms don’t stop me from doing stuff.
ID24547, TCBT, 12 months
For some participants, the CBT offered during the trial seemed to improve their understanding of emotional states and triggers:
I do think that – the therapy [received during the trial] I guess, because I think sometimes you – you don’t – know what triggers your emotions and I think, you know, going on the sort of different courses has helped understand how to control and trigger.
ID24547, TCBT, 3 months
The CBT has helped untangle what upsets me sometimes [Interviewer: OK] or what – like – how I feel about things and writing it down. Previously it’s been quite difficult to figure out how I feel about things. [Interviewer: OK] I just can’t figure it out – but now I can figure it out a bit better, because I think – if it’s manually written down, I felt like this – this made me feel this – and then I’ve looked down the sheet and then I’m like, OK, so I feel anxious a lot of the time.
ID33746, WCBT, 3 months
Furthermore, only participants from the CBT groups tended to describe their personalities as strong or resilient when talking about problem-solving emotions, suggesting that they felt more empowered than the TAU group. A sense of empowerment to manage negative emotions was a common pattern reported by the CBT participants when asked about their experiences of the main trial. The way CBT participants talked about a perceived increased control over their IBS symptoms, gained through new self-management techniques learned during the study, may have had an impact on the way they subsequently identified and described active coping strategies related specifically to managing their negative emotions. Furthermore, emotional regulation may have been addressed with some individuals if it arose as a pertinent issue in the therapy sessions:
I think it’s just when I feel very negatively about something, I actually do something to correct it. So I’m not someone who just accepts the situation; I’ve got quite like a strong fighting personality, so – so when things get too negative, then I just – I just change them.
ID40015, TCBT, 3 months
Negative feelings? [. . .] so I’m not a negative person and when I deal with negative things, I try and turn it around to positive.
ID40102, TCBT, 3 months
Participants receiving CBT also explained in more detail the analytical processes that allowed them to reframe a situation. Some explicitly acknowledged the positive role CBT played in changing their thought processes:
Yes. I think I am getting better because – so yes – they’re going down because I’m more aware of – that the feelings that I’m getting, I can recognise a bit more when I’m feeling anxious or when I’m feeling stressed. And I am trying to put into use the things I’ve learned from my online sessions and my sessions with the therapist [. . .] Yes, so the sessions have helped with that, the way you have to think about what’s causing you to feel like that and what’s the worst that can happen; kind of breaking it down to try and think better or more helpful thoughts. So that’s what I’m trying to do now.
ID40567, WCBT, 3 months
I think, in a way, it has helped me cope with some of the emotional side of that better than if I hadn’t had any therapy. I mean actually I think probably if I hadn’t had the CBT at all and I’d gone through the same problems, I would be even worse than it is at the moment.
ID40210, TC, 12 months
Conclusions
Summary
-
The benefits of CBT included reduced symptoms and, arguably more importantly, an increased capacity to cope with symptoms, negative emotions and other challenges of daily life.
-
Therapists had an important role to play in supporting patients to engage with CBT and to make sense of the therapy and their IBS. Patients valued having therapist support available alongside Regul8 and this may have helped enhance their engagement and outcomes.
-
The CBT appeared to help patients make changes to their ways of thinking and behaving in relation to their IBS, which was broadly consistent with the underpinning theoretical mechanisms of change underpinning this intervention.
Strengths and limitations
Strengths
Using purposive sampling meant that a diverse sample of participants from each trial arm were included in the qualitative study. This ensures that our findings do not relate to a narrow subset of the people who took part in the trial.
The rigorous analysis of interviews has produced novel insights into the subjective experience of having CBT for IBS. The findings capture participants’ more immediate perceptions as well as their longer-term reflections on the process and outcomes of CBT.
Conducting interviews with the same participants repeatedly over time permitted a detailed analysis of potential mechanisms of change that might underpin the effects of CBT that were described by participants and captured by the quantitative outcomes.
Limitations
Although the breadth of topics addressed in the interviews enabled a wide-ranging analysis of participants’ experiences of different aspects of the trial and the perceived effects of CBT, it meant that some issues were not addressed in depth (e.g. the perceived limitations of the outcome measures).
Although we used purposive sampling to gain a diverse range of views, participants willing to undertake the qualitative interviews may not hold the same views as of all of the participants in the study.
Given the inclusion criteria for the main trial, the qualitative findings may not transfer to people earlier in their IBS journey.
Implications for practice
If the interventions were rolled out in practice, the qualitative data suggest that it would be important to:
-
offer therapist support alongside Regul8
-
consider augmenting the intervention to provide additional support for sustaining behaviour changes over time
-
help GPs/consultants to encourage even those patients who are initially sceptical about a talking therapy to try CBT for IBS.
Chapter 3 Economic analysis
Heath economic objectives
-
To investigate the differences in health service costs and societal costs between participants allocated to TCBT or WCBT and those allocated to TAU at all outcome time points (3, 6 and 12 months after randomisation) and over the entire follow-up period.
-
To compare the cost-effectiveness of both TCBT and WCBT with TAU over the follow-up period using quality-adjusted life-years (QALYs) and the primary outcome measures.
Methods
We measured costs and assessed the cost-effectiveness from both a health service and a societal perspective over a 1-year period after baseline. To calculate the cost of TCBT, the number of sessions with therapists was recorded and combined with the unit cost of therapist time. The latter was calculated using therapy costs reported in the Personal Social Services Research Unit’s annual compendium,69 which gave a figure of £98 per session. Data were available on the number and duration of sessions attended. For TCBT, the median length of sessions was 55 minutes, and this was used with the £98 to derive a cost per minute of therapist time that was combined with both TCBT and WCBT therapist time. WCBT also incurred a cost of the system. The WCBT development costs were estimated and apportioned over those using the intervention and this was estimated at £13.51 per participant. Other service use was measured with the CSRI28 at baseline (going back 6 months) and each follow-up (with measurements covering the whole period since the previous interview). The schedule was based on other versions of the CSRI used in similar research. 28 Services included primary and secondary health care (including inpatient stays), investigations and medication. Service costs were generated by combining these data with appropriate unit cost information (e.g. NHS Reference Costs69 and the British National Formulary70) for the year 2015/16, and these costs were added to the intervention costs to generate total health costs per person. A table of unit costs is provided in Appendix 11.
Societal costs were calculated by including family care costs and lost work. Family care costs were recorded by asking patients to state how much time per week family members (and friends) spent providing support in specific areas because of IBS. This time was combined with an average hourly wage rate of £15.73. Lost days from work were recorded on the schedule and combined with an average daily wage rate of £105 to generate lost work costs. Cost comparisons between the three groups were made at 3, 6 and 12 months and over the entire follow-up period, in both cases controlling for baseline costs. Intervention costs were added to the costs for only the whole follow-up period rather than for each individual time period. Although medication costs were calculated, the data were not of high quality and these costs were excluded from the analysis of total costs.
Analysis
The study was not statistically powered to detect differences between TCBT and WCBT. In these economic analyses, we have made this comparison given the focus on non-frequentist approaches in many economic studies. However, caution is necessary in interpreting these differences. Non-intervention cost comparisons between the three groups were made at 3, 6 and 12 months, controlling for baseline costs. Costs over the follow-up were then combined with the intervention costs and again compared between the three groups. Cost data are usually skewed and cost comparisons used bootstrapped regression models to generate appropriate 95% CIs around the cost differences.
Cost-effectiveness was assessed from the health-care perspective by combining the cost data with the follow-up scores on the IBS SSS and WSAS and with QALYs. The latter were generated from the EQ-5D combined with UK-specific tariffs. Area under the curve methods were used to calculate the number of QALYs accrued over the follow-up period and comparisons between groups were made using a regression model controlling for baseline EQ-5D scores. Similar models were used for the IBS SSS and WSAS but the coefficient for group differences was subsequently multiplied by –1 so that a positive score represented an improvement.
In deterministic analyses, mean incremental costs and incremental QALYs were calculated for both intervention arms, TCBT and WCBT, compared with TAU. The main analyses were conducted on cases in which data on both costs and QALYs were available. If outcomes were better for one intervention group than another, and costs were lower, then that intervention was defined as being ‘dominant’. If outcomes were better and costs were higher, then an incremental cost-effectiveness ratio (ICER) was generated to indicate the extra cost incurred to achieve an extra QALY. Cost-effectiveness planes were produced using 1000 cost and outcome differences (from bootstrapped regression models) for TCBT compared with TAU and for WCBT compared with TAU. For completeness, we also produced a cost-effectiveness plane comparing TCBT with WCBT. Cost-effectiveness acceptability curves (CEACs) were generated from the incremental cost and QALY data and also the differences between groups on the IBS SSS and WSAS. Net benefit values were produced by multiplying incremental effects by a range of assumed values placed on a 1-unit difference and subtracting the incremental cost. The assumed values are straightforward for QALYs because NICE commonly approves interventions that have a cost per QALY below around £20,000. Therefore, we used a range of £0–60,000 in these analyses. For the IBS SSS and WSAS, there is no accepted threshold, so a range was chosen based on the ICER using the mean differences. We also tried to demonstrate situations in which one intervention had a 60%, 70%, 80% and 90% likelihood of being the most cost-effective option.
Sensitivity analyses were conducted by changing the intervention costs upwards and downwards by 25% and 50%. We also imputed for missing cost and EuroQol-5 Dimensions, five-level version (EQ-5D-5L), data using the impute procedure in Stata. Available cost data were used for the cost imputations, and for EQ-5D-5L scores we used available EQ-5D-5L data and the WSAS scores and IBS SSS. Further sensitivity analyses were conducted by using the minimum wage of £7.50 per hour to value informal care and lost work days (assuming 7.5 hours per day) and by using the unit cost of a home care worker (£20 per hour) to value informal care. 71
Modelling beyond the trial period and making comparisons with other interventions was not in the scope of this project.
Health economic results
Service use and lost employment data were available for 186 TCBT, 185 WCBT and 187 TAU participants at baseline; 142 (76% of baseline number) TCBT, 132 (71%) WCBT and 134 (72%) TAU participants at the 3-month follow-up; 135 (73%) TCBT, 115 (62%) WCBT and 128 (68%) TAU participants at the 6-month follow-up; and 130 (70%) TCBT, 120 (65%) WCBT and 130 (70%) TAU participants at the 12-month follow-up.
Service use and costs by time period
In the 6 months prior to baseline, > 80% of participants had contact with GPs (Table 35). Relative to other services, there were high rates of contact with other doctors, pharmacists and practice nurses. Relatively few had inpatient stays. Medication was received by just over half of each group and investigations (usually blood tests) were carried out in around three-quarters of participants. The mean number of contacts in Table 37 (and similar tables for other time periods) is only for those with at least one contact (i.e. excluding those not using the service). If a service was used, then there were usually < 10 contacts during the period. Health service costs were highest for inpatient care (despite its low use), GP contacts and other doctor contacts. There were few notable differences between the three groups prior to baseline. The mean total health-care costs were £681 for TCBT, £620 for WCBT and £802 for TAU. Although the TAU costs are relatively high, the SDs around the mean costs are substantial, as is common with cost data, at £122 more than for TCBT (bootstrapped 95% CI –£93 to £351) and £182 more than for WCBT (bootstrapped 95% CI –£27 to £393). During the baseline period, about one-fifth of each group received care from family or friends because of health problems. For those in receipt of this informal care, the number of hours was, on average, between 9 and 13 per week. This time, valued using the average hourly wage of £15, resulted in costs that were relatively high compared with the health-care costs. Lost work days were experienced for about half of the TCBT and TAU groups and 40% of the WCBT group. An average of about 10 days were lost from work for those for whom this was the case. Combining the informal care and lost employment costs with the health-care costs results in mean societal costs at baseline of £1995 for TCBT, £1965 for WCBT and £2352 for TAU. TCBT had societal costs that were £357 lower than those for TAU (bootstrapped 95% CI –£607 to £1214) and WCBT had costs that were £387 lower than those for TAU (bootstrapped 95% CI –£683 to £1349).
| Health-care contacts/days | Trial arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TCBT (N = 186) | WCBT (N = 185) | TAU (N = 187) | |||||||
| n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | |
| Gastroenterologist | 29 (16) | 1.5 (0.7) | 32 (85) | 28 (15) | 2.8 (5.5) | 57 (317) | 37 (20) | 2.6 (4.8) | 69 (320) |
| GP | 149 (80) | 3.9 (3.2) | 106 (106) | 160 (86) | 3.8 (3.2) | 110 (106) | 162 (87) | 4.2 (4.2) | 122 (136) |
| Other doctor | 37 (20) | 3.6 (4.8) | 97 (346) | 38 (21) | 2.1 (1.4) | 57 (140) | 51 (27) | 3.4 (5.4) | 124 (432) |
| Pharmacist | 53 (28) | 3.6 (2.3) | 26 (51) | 60 (32) | 4.4 (5.2) | 36 (90) | 67 (36) | 4.4 (5.3) | 40 (95) |
| Physiotherapist | 18 (10) | 3.2 (2.7) | 15 (62) | 19 (10) | 6.5 (7.7) | 33 (153) | 24 (13) | 6.0 (4.2) | 38 (122) |
| Practice nurse | 67 (36) | 2.1 (2.3) | 11 (25) | 61 (33) | 2.2 (1.8) | 11 (22) | 80 (43) | 1.7 (0.8) | 11 (15) |
| Other nurse at home | 1 (1) | 2.0 (0.0) | < 1 (4) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) |
| Other nurse in hospital | 11 (6) | 2.7 (2.1) | 7 (35) | 14 (8) | 2.0 (1.2) | 7 (26) | 21 (11) | 1.4 (0.7) | 7 (21) |
| Psychiatrist | 5 (3) | 3.0 (2.3) | 11 (81) | 6 (3) | 2.0 (1.5) | 9 (59) | 0 (0) | – | 0 (0) |
| Social worker | 4 (2) | 3.5 (3.0) | 3 (25) | 2 (1) | 6.0 (2.8) | 3 (26) | 2 (1) | 1.0 (0.0) | < 1 (4) |
| Other therapist | 15 (8) | 5.7 (4.7) | 36 (160) | 21 (11) | 7.3 (7.1) | 66 (261) | 16 (9) | 5.9 (4.4) | 40 (163) |
| Acupuncturist | 10 (6) | 4.4 (3.0) | 12 (59) | 5 (3) | 4.8 (3.2) | 6 (46) | 11 (6) | 5.4 (2.5) | 16 (70) |
| Dietitian | 27 (15) | 1.7 (0.7) | 20 (53) | 23 (12) | 1.6 (0.8) | 16 (49) | 29 (16) | 1.8 (1.1) | 22 (62) |
| Homeopath | 8 (4) | 2.5 (1.2) | 5 (28) | 5 (3) | 2.4 (1.5) | 3 (22) | 4 (2) | 11.0 (12.7) | 12 (114) |
| Occupational therapist | 3 (2) | 4.3 (4.9) | 6 (59) | 3 (2) | 3.3 (4.0) | 4 (47) | 1 (1) | 1.0 (0.0) | < 1 (6) |
| Osteopath | 12 (6) | 3.1 (2.5) | 10 (48) | 15 (8) | 4.0 (4.0) | 16 (78) | 12 (6) | 3.3 (2.1) | 10 (47) |
| Inpatient | 13 (7) | – | 163 (745) | 11 (6) | – | 71 (335) | 18 (10) | – | 156 (335) |
| A&E | 24 (13) | 1.3 (0.9) | 23 (73) | 19 (10) | 1.2 (0.5) | 16 (53) | 27 (14) | 1.3 (0.9) | 27 (80) |
| Medication | 99 (53) | – | 23 (35) | 109 (59) | – | 28 (41) | 108 (58) | – | 36 (92) |
| Investigations | 147 (79) | 2.6 (1.8) | 98 (182) | 138 (75) | 2.7 (2.9) | 99 (202) | 151 (81) | 2.6 (1.8) | 108 (196) |
| Total health cost | 681 (948) | 620 (881) | 802 (1258) | ||||||
| Informal care | 36 (19) | 9.6 (14.8) | 759 (3058) | 36 (19) | 11.5 (22.7) | 915 (4456) | 37 (20) | 12.9 (20.2) | 1048 (4198) |
| Lost work days | 96 (52) | 10.3 (20.4) | 556 (1627) | 74 (40) | 10.2 (23.1) | 430 (1618) | 91 (49) | 9.8 (15.8) | 502 (1266) |
| Total cost | 1995 (4201) | 1965 (5176) | 2352 (5006) | ||||||
In the period prior to the 3-month follow-up, around half of participants in each group had GP contacts (Table 36). The next most used service was pharmacist contacts. No clear differences in service use were observed between the groups. Inpatient use was low in each group, but in the WCBT group the cost of inpatient care was very high because of one participant having an extended period of time in hospital. Again, costs tended to be high for GP and other doctor contacts. Inpatient costs for the WCBT group were substantially higher than for the other two groups. Medication was used by around one-third of the TCBT group and slightly more of the other two groups. Investigations were received by around one-third of each group. Overall, the groups were similar in terms of service use. The mean total health-care costs excluding the intervention were £271 for TCBT, £346 for WCBT and £227 for TAU. Controlling for baseline, the TCBT group had costs that were £52 higher than those for TAU (bootstrapped 95% CI –£60 to £170) and WCBT had costs that were £152 higher than those for TAU (bootstrapped 95% CI –£66 to £508). Informal care was received by similar proportions in each group, but TAU participants received more hours per week, which resulted in higher informal care costs for this group. Lost work days were similar in each group. The mean societal costs were £682 for TCBT, £723 for WCBT and £836 for TAU. Controlling for baseline, TCBT had mean costs that were £28 higher than those for TAU (bootstrapped 95% CI –£309 to £361) and WCBT had costs that were £218 higher than those for TAU (bootstrapped 95% CI –£226 to £649).
| Health-care contacts/days | Trial arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TCBT (N = 142) | WCBT (N = 132) | TAU (N = 134) | |||||||
| n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | |
| Gastroenterologist | 6 (4) | 1.2 (0.4) | 7 (34) | 5 (4) | 1.4 (0.5) | 7 (39) | 12 (9) | 1.2 (0.4) | 14 (48) |
| GP | 71 (50) | 2.4 (3.9) | 39 (98) | 67 (51) | 2.1 (1.8) | 36 (55) | 69 (51) | 3.0 (3.7) | 50 (100) |
| Other doctor | 18 (13) | 2.3 (2.1) | 39 (143) | 20 (15) | 1.8 (0.9) | 36 (96) | 21 (16) | 1.8 (1.4) | 37 (113) |
| Pharmacist | 41 (29) | 3.0 (6.9) | 22 (98) | 37 (28) | 2.7 (4.1) | 19 (62) | 32 (24) | 2.8 (5.2) | 17 (69) |
| Physiotherapist | 14 (10) | 3.4 (3.8) | 16 (75) | 9 (7) | 3.0 (2.8) | 10 (50) | 15 (11) | 1.9 (1.0) | 11 (34) |
| Practice nurse | 23 (16) | 1.7 (2.1) | 4 (16) | 27 (20) | 1.6 (1.8) | 5 (15) | 29 (22) | 1.3 (0.6) | 4 (9) |
| Other nurse at home | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) |
| Other nurse in hospital | 3 (2) | 1.7 (1.2) | 2 (12) | 14 (11) | 1.7 (1.6) | 8 (32) | 6 (4) | 1.2 (0.4) | 2 (11) |
| Psychiatrist | 2 (1) | 2.5 (2.1) | 5 (47) | 0 (0) | – | 0 (0) | 1 (1) | 1.0 (0.0) | 1 (12) |
| Social worker | 1 (1) | 3.0 (0.0) | 1 (10) | 2 (2) | 6.0 (5.7) | 4 (35) | 1 (1) | 1.0 (0.0) | < 1 (3) |
| Other therapist | 7 (5) | 5.6 (3.3) | 22 (110) | 11 (8) | 3.8 (3.5) | 25 (113) | 7 (5) | 3.9 (2.0) | 16 (76) |
| Acupuncturist | 6 (4) | 1.8 (0.8) | 4 (20) | 1 (1) | 5.0 (0.0) | 2 (22) | 3 (2) | 2.0 (0.0) | 2 (15) |
| Dietitian | 4 (3) | 1.0 (0.0) | 2 (13) | 3 (2) | 3.0 (3.5) | 6 (50) | 7 (5) | 1.4 (0.5) | 6 (27) |
| Homeopath | 3 (2) | 3.3 (1.5) | 4 (26) | 0 (0) | – | 0 (0) | 3 (2) | 1.0 (0.0) | 1 (7) |
| Occupational therapist | 1 (1) | 6.0 (0.0) | 3 (40) | 1 (1) | 3.0 (0.0) | 2 (21) | 1 (1) | 1.0 (0.0) | 1 (7) |
| Osteopath | 7 (5) | 1.7 (0.8) | 4 (20) | 6 (5) | 2.3 (1.9) | 5 (30) | 8 (6) | 2.4 (1.2) | 7 (31) |
| Inpatient | 3 (2) | – | 56 (424) | 2 (2) | – | 121 (1349) | 2 (1) | – | 13 (117) |
| A&E | 7 (5) | 1.0 (0.0) | 7 (30) | 8 (6) | 1.1 (0.4) | 9 (39) | 9 (7) | 1.6 (1.1) | 14 (66) |
| Medication | 50 (35) | – | 6 (11) | 56 (42) | – | 12 (47) | 64 (48) | – | 15 (48) |
| Investigations | 45 (32) | 1.8 (1.2) | 35 (126) | 42 (32) | 2.5 (5.4) | 52 (231) | 42 (31) | 2.0 (1.3) | 32 (101) |
| Total health cost | 271 (623) | 346 (1691) | 227 (326) | ||||||
| Informal care | 21 (15) | 6.7 (9.0) | 203 (846) | 20 (15) | 7.5 (10.1) | 232 (961) | 26 (19) | 10.7 (17.7) | 423 (1795) |
| Lost work days | 33 (23) | 8.5 (16.5) | 208 (907) | 36 (27) | 5.1 (11.7) | 145 (679) | 45 (34) | 5.3 (6.2) | 186 (456) |
| Total cost | 682 (1571) | 723 (2707) | 836 (1950) | ||||||
During the 3 months prior to the 6-month follow-up, similar patterns of service use as before were observed, with around half of all participants receiving GP care, around one-quarter seeing a pharmacist and small numbers having inpatient care (Table 37). Costs tended to be highest for GPs and other doctors. Mean non-intervention health-care costs were £281 for TCBT, £224 for WCBT and £206 for TAU. After controlling for baseline costs, it was shown that TCBT had £84 higher costs than TAU (bootstrapped 95% CI –£41 to £230) and WCBT had costs that were £41 higher than those for TAU (bootstrapped 95% CI –£70 to £165). Informal care costs were very different during this period. Although the proportions in receipt were similar, the mean number of hours per week was far greater for the TAU group. The number of lost work days was lowest for the WCBT group. The mean societal costs were £840 for TCBT, £503 for WCBT and £1344 for TAU. Controlling for baseline resulted in TCBT having costs that were, on average, £350 lower than those for TAU (bootstrapped 95% CI –£332 to £996) and WCBT having costs that were £407 lower than those for TAU (bootstrapped 95% CI –£74 to £923). It is of interest that none of these differences was statistically significant. This is due to the large SDs around the costs and the pre-existing baseline differences.
| Health-care contacts/days | Trial arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TCBT (N = 135) | WCBT (N = 115) | TAU (N = 128) | |||||||
| n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost (£) (SD) | |
| Gastroenterologist | 4 (3) | 2.3 (2.5) | 9 (73) | 2 (2) | 11.0 (14.1) | 26 (268) | 10 (8) | 1.3 (0.5) | 14 (51) |
| GP | 71 (53) | 2.0 (1.5) | 34 (49) | 52 (45) | 2.3 (3.3) | 35 (82) | 68 (53) | 2.7 (2.5) | 47 (75) |
| Other doctor | 19 (14) | 4.7 (11.2) | 90 (597) | 16 (14) | 2.1 (1.2) | 39 (113) | 20 (16) | 1.5 (0.8) | 32 (86) |
| Pharmacist | 35 (26) | 2.0 (1.7) | 13 (31) | 25 (22) | 2.2 (1.7) | 12 (30) | 32 (25) | 2.0 (1.3) | 13 (27) |
| Physiotherapist | 19 (14) | 3.6 (2.2) | 25 (74) | 8 (7) | 2.9 (2.4) | 10 (46) | 14 (11) | 3.7 (7.7) | 20 (133) |
| Practice nurse | 23 (17) | 1.9 (2.3) | 5 (18) | 27 (23) | 1.3 (0.6) | 5 (10) | 27 (21) | 1.5 (0.8) | 5 (10) |
| Other nurse at home | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) |
| Other nurse in hospital | 6 (4) | 2.2 (2.9) | 4 (31) | 5 (4) | 1.6 (0.9) | 3 (16) | 7 (5) | 1.3 (0.8) | 3 (14) |
| Psychiatrist | 0 (0) | – | 0 (0) | 1 (1) | 3.0 (0.0) | 4 (38) | 2 (2) | 2.0 (1.4) | 4 (38) |
| Social worker | 2 (1) | 3.0 (0.0) | 2 (15) | 2 (2) | 1.0 (0.0) | 1 (5) | 1 (1) | 1.0 (0.0) | < 1 (4) |
| Other therapist | 5 (4) | 4.4 (3.4) | 13 (80) | 9 (8) | 4.8 (4.6) | 30 (140) | 6 (5) | 2.0 (2.0) | 7 (46) |
| Acupuncturist | 5 (4) | 3.6 (2.2) | 7 (39) | 2 (2) | 2.0 (0.0) | 2 (13) | 4 (3) | 1.5 (1.0) | 2 (15) |
| Dietitian | 0 (0) | – | 0 (0) | 3 (3) | 1.0 (0.0) | 2 (13) | 9 (7) | 1.7 (0.7) | 9 (38) |
| Homeopath | 3 (2) | 1.7 (1.2) | 2 (14) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) |
| Occupational therapist | 3 (2) | 1.7 (1.2) | 3 (20) | 0 (0) | – | 0 (0) | 0 (0) | – | 0 (0) |
| Osteopath | 9 (7) | 2.3 (1.6) | 8 (35) | 8 (7) | 1.3 (0.7) | 4 (18) | 7 (5) | 1.7 (1.0) | 5 (22) |
| Inpatient | 3 (2) | – | 24 (175) | 1 (1) | – | 17 (184) | 3 (2) | – | 17 (116) |
| A&E | 8 (6) | 1.0 (0.0) | 8 (33) | 9 (8) | 1.3 (0.7) | 14 (56) | 7 (5) | 1.0 (0.0) | 8 (32) |
| Medication | 45 (33) | – | 5 (10) | 39 (34) | – | 6 (17) | 58 (45) | – | 12 (45) |
| Investigations | 44 (33) | 1.9 (1.6) | 36 (117) | 24 (21) | 1.6 (1.0) | 22 (102) | 35 (27) | 1.9 (1.5) | 21 (79) |
| Total health cost | 280 (728) | 224 (532) | 206 (308) | ||||||
| Informal care | 24 (18) | 7.2 (15.8) | 261 (1449) | 19 (17) | 4.8 (4.1) | 161 (495) | 21 (16) | 26.0 (35.0) | 873 (3459) |
| Lost work days | 39 (29) | 9.9 (18.3) | 299 (1128) | 42 (37) | 3.1 (6.4) | 118 (435) | 47 (37) | 6.9 (11.1) | 265 (784) |
| Total cost | 840 (2090) | 503 (858) | 1344 (3850) | ||||||
Finally, in the 6 months prior to the 12-month follow-up, we see highest use for GPs, other doctors, pharmacists and practice nurses (Table 38). Inpatient use was slightly higher than before and this resulted in relatively high inpatient costs, especially in the TCBT and TAU arms. The mean total health-care costs were £519 for TCBT, £325 for WCBT and £393 for TAU. After controlling for baseline, TCBT was shown to have non-intervention health-care costs that were, on average, £141 higher than those for TAU (bootstrapped 95% CI –£118 to £474) and WCBT had costs that were £43 lower than those for TAU (bootstrapped 95% CI –£115 to £205). The higher level of informal care for TAU was maintained during this period. TAU also had higher lost employment costs than the other two arms. The mean societal costs were £1055 for TCBT, £1029 for WCBT and £2103 for TAU. Controlling for baseline, TCBT had costs that were £850 lower than those for TAU (bootstrapped 95% CI –£262 to £2181) and WCBT had costs that were £748 lower than those for TAU (bootstrapped 95% CI –£175 to £1953).
| Health-care contacts/days | Trial arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TCBT (N = 130) | WCBT (N = 120) | TAU (N = 130) | |||||||
| n (%) | Mean number of contacts/days (SD) | Mean cost(£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost(£) (SD) | n (%) | Mean number of contacts/days (SD) | Mean cost(£) (SD) | |
| Gastroenterologist | 7 (5) | 1.3 (0.5) | 9 (42) | 8 (7) | 1.6 (1.1) | 15 (66) | 15 (12) | 3.7 (10.0) | 59 (480) |
| GP | 82 (63) | 2.0 (1.7) | 42 (56) | 71 (59) | 2.4 (2.2) | 47 (68) | 73 (56) | 2.6 (1.9) | 48 (63) |
| Other doctor | 42 (32) | 1.6 (1.0) | 70 (127) | 27 (23) | 2.1 (1.4) | 65 (152) | 28 (22) | 2.6 (3.7) | 77 (273) |
| Pharmacist | 44 (34) | 2.0 (1.5) | 17 (32) | 34 (28) | 2.1 (1.7) | 15 (33) | 32 (25) | 2.6 (1.8) | 16 (36) |
| Physiotherapist | 16 (12) | 3.9 (7.2) | 24 (135) | 12 (10) | 4.3 (2.5) | 21 (74) | 12 (9) | 3.2 (2.9) | 14 (62) |
| Practice nurse | 27 (21) | 1.6 (1.3) | 5 (13) | 26 (22) | 1.5 (0.9) | 5 (11) | 25 (19) | 1.6 (0.9) | 5 (11) |
| Other nurse at home | 1 (1) | 20.0 (0.0) | 4 (49) | 0 (0) | – | 0 (0) | 2 (2) | 2.0 (1.4) | 1 (8) |
| Other nurse in hospital | 13 (10) | 1.8 (1.8) | 8 (34) | 11 (9) | 1.4 (1.2) | 5 (23) | 11 (8) | 1.8 (1.3) | 7 (26) |
| Psychiatrist | 2 (2) | 2.5 (2.1) | 5 (49) | 1 (1) | 2.0 (0.0) | 2 (25) | 0 (0) | – | 0 (0) |
| Social worker | 3 (2) | 2.3 (1.5) | 2 (16) | 1 (1) | 1.0 (0.0) | < 1 (4) | 1 (1) | 4.0 (0.0) | 1 (14) |
| Other therapist | 7 (5) | 2.9 (1.8) | 12 (59) | 7 (6) | 5.0 (8.4) | 23 (176) | 6 (5) | 2.7 (1.9) | 10 (53) |
| Acupuncturist | 8 (6) | 4.1 (2.5) | 13 (58) | 1 (1) | 1.0 (0.0) | < 1 (5) | 6 (5) | 3.3 (4.3) | 8 (55) |
| Dietitian | 2 (2) | 1.0 (0.0) | 1 (10) | 6 (5) | 2.2 (1.5) | 9 (46) | 5 (4) | 1.4 (0.9) | 4 (25) |
| Homeopath | 4 (3) | 1.0 (0.0) | 2 (9) | 2 (2) | 1.0 (0.0) | 1 (6) | 2 (2) | 2.5 (0.7) | 2 (16) |
| Occupational therapist | 0 (0) | – | 0 (0) | 2 (2) | 4.0 (4.2) | 5 (51) | 1 (1) | 1.0 (0.0) | 1 (7) |
| Osteopath | 7 (5) | 1.9 (0.9) | 5 (23) | 5 (4) | 5.2 (8.3) | 11 (92) | 8 (6) | 2.1 (1.4) | 7 (30) |
| Inpatient | 8 (6) | – | 202 (1381) | 5 (4) | – | 36 (191) | 7 (5) | – | 103 (504) |
| A&E | 14 (11) | 1.1 (0.4) | 17 (52) | 7 (6) | 1.0 (0.0) | 8 (32) | 15 (12) | 1.3 (0.5) | 20 (60) |
| Medication | 48 (37) | – | 11 (18) | 46 (38) | – | 15 (38) | 60 (46) | – | 20 (47) |
| Investigations | 62 (48) | 3.1 (4.0) | 81 (273) | 42 (35) | 2.0 (1.7) | 55 (195) | 51 (39) | 2.0 (1.2) | 43 (153) |
| Total health cost | 519 (1635) | 325 (597) | 393 (791) | ||||||
| Informal care | 13 (10) | 5.6 (6.7) | 230 (1082) | 15 (13) | 10.0 (14.6) | 510 (2454) | 22 (17) | 16.5 (36.4) | 1144 (6532) |
| Lost work days | 48 (37) | 7.9 (21.3) | 307 (1409) | 52 (43) | 4.3 (5.3) | 195 (430) | 61 (47) | 11.5 (23.6) | 566 (1798) |
| Total cost | 1055 (3496) | 1029 (2665) | 2103 (6959) | ||||||
Costs over follow-up
The mean total health-care costs, including the intervention, over the 1-year follow-up period were £1650 (SD £1931) for TCBT, £943 (SD £955) for WCBT and £715 (SD £884) for TAU. Adjusting for baseline differences, TCBT was on average, £943 more costly than TAU, a difference that was statistically significant (bootstrapped 95% CI £572 to £1363), and WCBT was £278 more costly than TAU, which was also a statistically significant difference (bootstrapped 95% CI £11 to £514). The cost differences for the participants for whom EQ-5D-5L data were available (which is relevant for the complete-case analysis) were £956 (bootstrapped 95% CI £601 to £1435) for TCBT and £224 (bootstrapped 95% CI –£11 to £448) for WCBT.
The mean societal costs over the follow-up period, including therapy costs, were £3065 (SD £5179) for TCBT, £2094 (SD £3069) for WCBT and £4374 (SD £11,843) for TAU. After controlling for baseline, it was found that TCBT cost £858 less than TAU (bootstrapped 95% CI –£1063 to £2812) and WCBT costs £1028 less than TAU (bootstrapped 95% CI –£404 to £2626).
As stated above, medication costs were not included in the totals because of the quality of the data. We did know what medications were taken but the data on frequency and dose were not complete. What is evident from Tables 35–38 is that there was a slight reduction in medication use after baseline and participants in the TAU group were slightly more likely to be on medication. The majority of prescriptions were for very inexpensive oral drugs and so any cost saving would be very small.
Quality-adjusted life-years
Table 39 shows that EQ-5D-5L scores were relatively high for each group at each time point. It is evident that improvements were greater for the two therapy groups than for TAU. Controlling for baseline EQ-5D-5L utility scores, TCBT resulted in 0.0414 more QALYs than TAU (95% CI 0.0194 to 0.0635 QALYs) and WCBT resulted in 0.0269 more QALYs than TAU (95% CI 0.0041 to 0.0497 QALYs). The QALY differences for those with follow-up cost data are 0.0429 QALYs (95% CI 0.0205 to 0.0653 QALYs) and 0.0290 QALYs (95% CI 0.0063 to 0.0518 QALYs), respectively.
| Time period and QALYs | Trial arm | |||||
|---|---|---|---|---|---|---|
| TCBT (N = 186) | WCBT (N = 185) | TAU (N = 187) | ||||
| n (%) | Mean utility score (SD) | n (%) | Mean utility score (SD) | n (%) | Mean utility score (SD) | |
| Baseline | 185 (99) | 0.8191 (0.1283) | 185 (100) | 0.8016 (0.1651) | 187 (100) | 0.8101 (0.1468) |
| 3-month follow-up | 147 (79) | 0.8499 (0.1253) | 133 (72) | 0.8392 (0.1657) | 132 (71) | 0.8083 (0.1547) |
| 6-month follow-up | 134 (72) | 0.8761 (0.1128) | 112 (60) | 0.8563 (0.1404) | 128 (68) | 0.8251 (0.1438) |
| 12-month follow-up | 120 (65) | 0.8799 (0.1425) | 113 (61) | 0.8459 (0.1513) | 123 (66) | 0.8265 (0.1497) |
| QALYs | 106 (57) | 0.8786 (0.0786) | 92 (50) | 0.8525 (0.1244) | 102 (55) | 0.8254 (0.1313) |
Cost-effectiveness results: complete-case analyses
Dividing the health-care cost differences by the QALY differences for those with both sets of data results in the following ICERs: TCBT versus TAU, £22,280; and WCBT versus TAU, £7724. The ICER for TCBT versus WCBT was £52,662.
Figures 8–10 show the uncertainty around the ICERs. The points on these cost-effectiveness planes represent a pair of incremental costs and QALYs from 1000 bootstrapped samples. Figure 8 shows that TCBT is certain to result in higher costs than TAU and to produce more QALYs. From Figure 9, which compares WCBT with TAU, we see that there is a 96.2% likelihood that WCBT is more expensive than TAU and produces more QALYs, a 3.2% likelihood of lower costs and more QALYs, a 0% likelihood of lower costs and fewer QALYs and a 0.6% likelihood of higher costs and fewer QALYs. Figure 10 reveals that, compared with WCBT, there is a 91.4% likelihood that TCBT is more expensive and produces more QALYs, a 0.0% likelihood that it is less expensive and produces more QALYs, a 0% likelihood it is less expensive and produces fewer QALYs and a 8.6% likelihood it is more expensive and produces fewer QALYs. Finally, the CEACs in Figure 11 reveal that, at very low values placed on a QALY gain, TAU is most likely to be cost-effective. With higher values placed on a QALY, WCBT becomes the most likely treatment to be cost-effective. The probability that TCBT is most cost-effective does increase but only exceeds the probability for WCBT at £55,000 per QALY. At the £20,000 threshold commonly used in evaluations in the UK, WCBT is most likely to be cost-effective, followed by TAU and then TCBT.
FIGURE 8.
Cost-effectiveness plane for TCBT vs. TAU (health-care perspective, complete-case analysis).

FIGURE 9.
Cost-effectiveness plane for WCBT vs. TAU using QALYs.
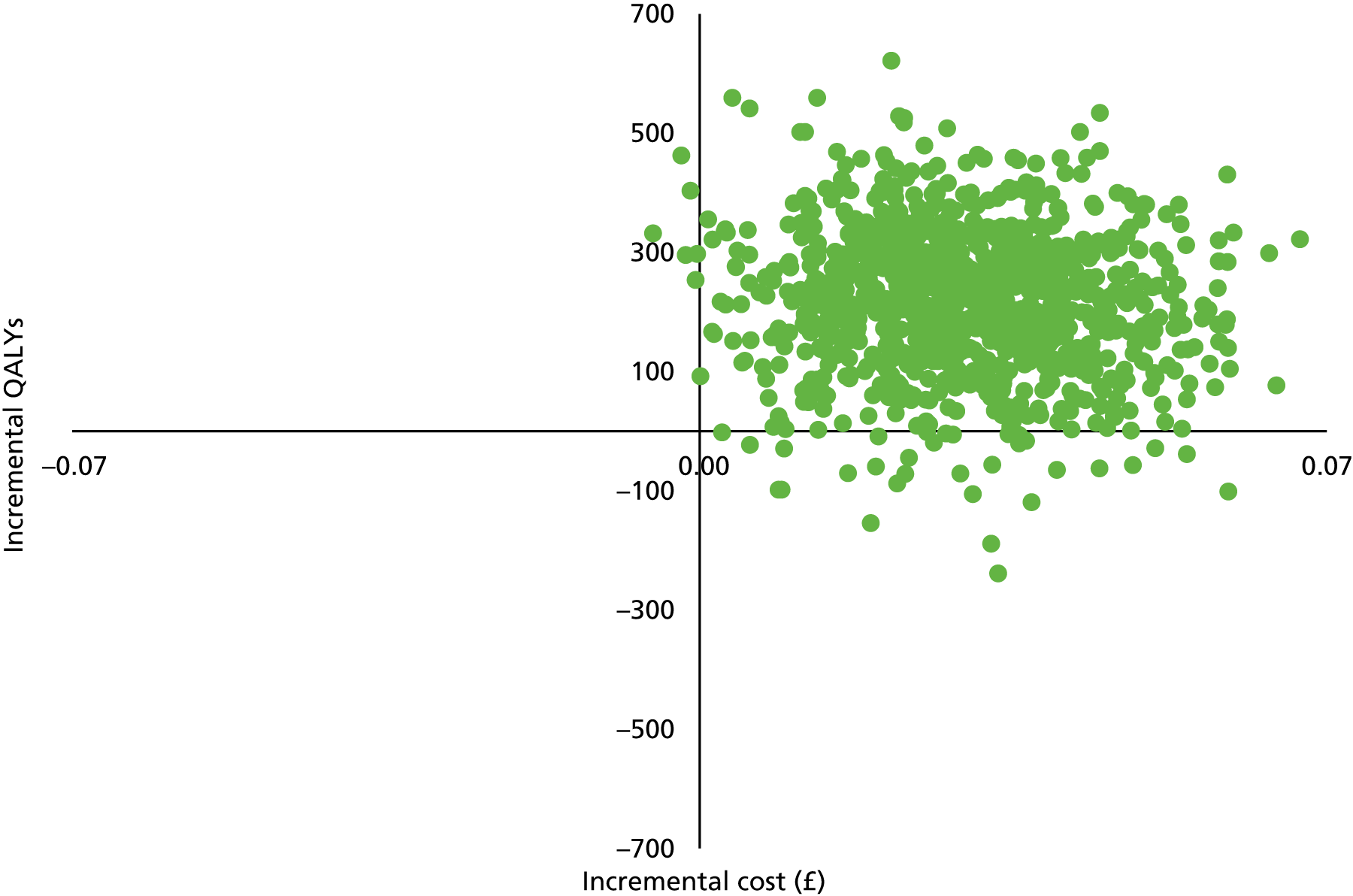
FIGURE 10.
Cost-effectiveness plane for TCBT vs. WCBT.
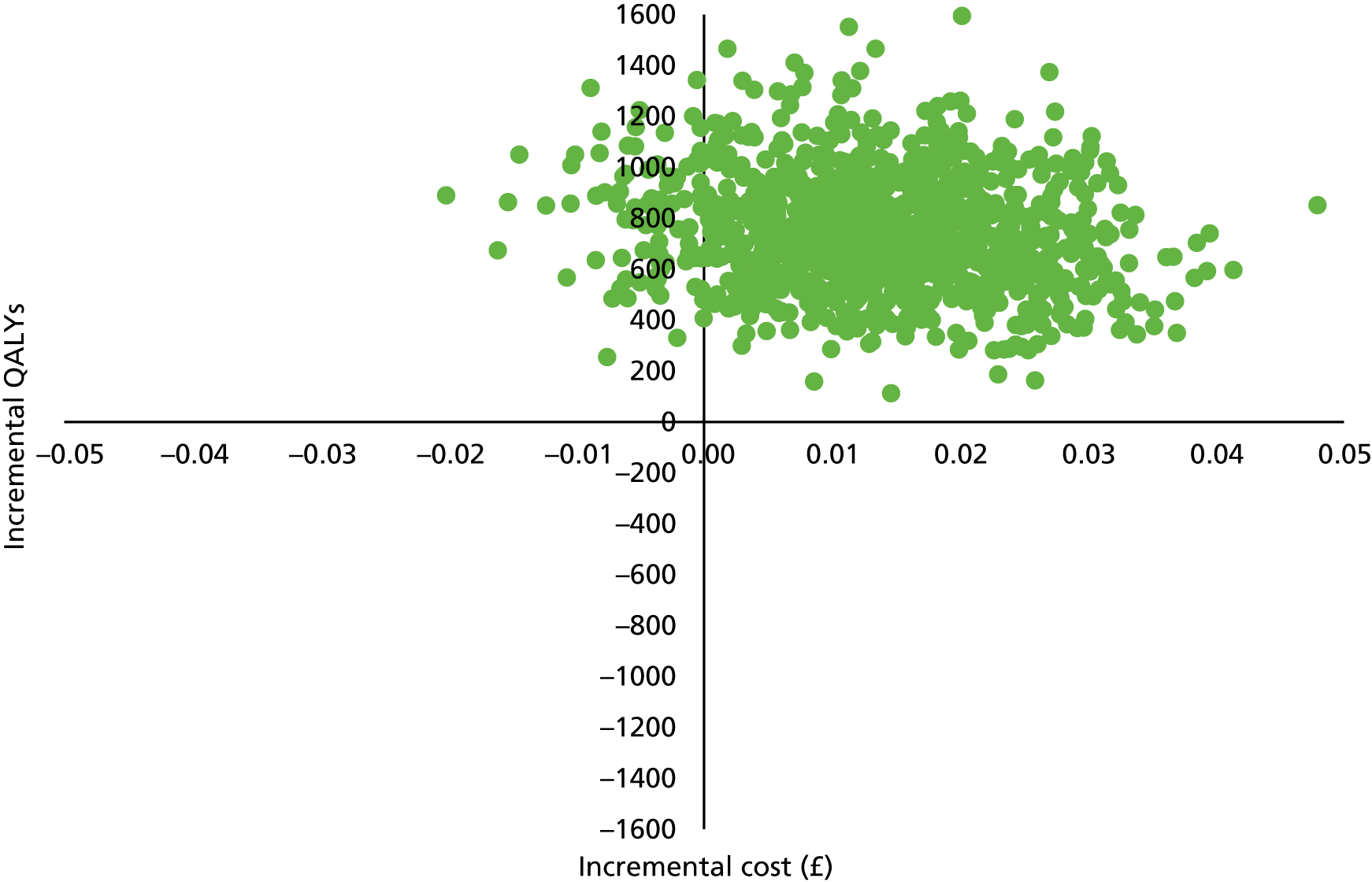
FIGURE 11.
Cost-effectiveness acceptability curves based on QALYs.
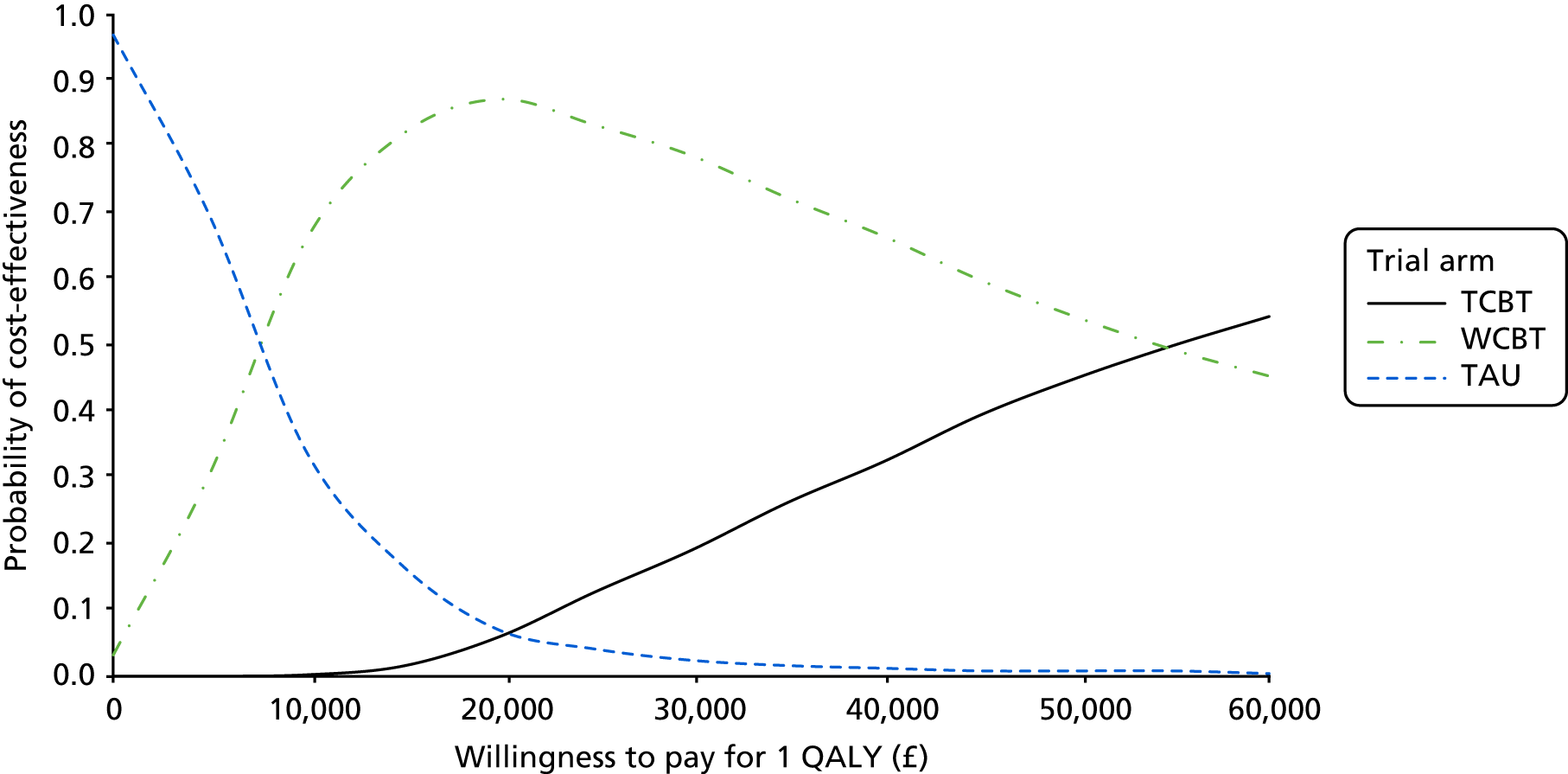
Using complete data, the difference in IBS SSS at 12 months compared with TAU, controlling for baseline, was 84 for TCBT and 56 for WCBT. The corresponding cost differences are £962 and £281, respectively. This leads to an ICER for TCBT compared with TAU of £11 per unit improvement on the IBS SSS, and an ICER for WCBT compared with TAU of £5. CEACs based on the IBS SSS outcome measure are shown in Figure 12. As with the QALYs, TAU is most likely to be cost-effective for very small values placed on a 1-point improvement on this measure. However, if the value is around £5 per 1-point improvement, then WCBT becomes the option that is most likely to be cost-effective. TCBT becomes most likely to be cost-effective if the value placed on a 1-unit improvement exceeds £24.
FIGURE 12.
Cost-effectiveness acceptability curves based on IBS SSS.

The difference at 12 months on the WSAS between TCBT and TAU was 5.1 and between WCBT and TAU was 4.0. The corresponding cost differences are £943 and £278, respectively. The ICERs based on these figures are £185 for TCBT and £70 for WCBT. In Figure 13, it can be seen that, when the WSAS is used, the probability that WCBT is the most cost-effective option occurs for values placed on a 1-unit improvement on this measure in excess of £60. TCBT is unlikely to be the most cost-effective option for values placed on a 1-unit improvement on the WSAS within the range £0–200.
FIGURE 13.
Cost-effectiveness acceptability curves based on WSAS scores.

Cost-effectiveness results: sensitivity analyses
When therapy costs were reduced by 25% and 50%, the ICERs (health-care perspective) compared with TAU were reduced for both groups and brought TCBT well below the £20,000 threshold (Table 40). Increasing therapy costs had the opposite effect, but even with a 50% increase the WCBT option was still well below the £20,000 threshold.
| Incremental costs and ICERs (£) following reductions/increases in therapy costs | Therapy 50% less | Therapy 25% less | Base case | Therapy 25% more | Therapy 50% more |
|---|---|---|---|---|---|
| TCBT | |||||
| Incremental cost | 614 | 785 | 956 | 1126 | 1297 |
| ICER | 14,312 | 18,298 | 22,284 | 26,247 | 30,233 |
| WCBT | |||||
| Incremental cost | 97 | 160 | 224 | 288 | 352 |
| ICER | 3345 | 5517 | 7724 | 9931 | 12,138 |
As stated previously, there were many cases with missing EQ-5D-5L data and, consequently, with missing QALYs. After imputation, the number of QALYs over the follow-up was 0.8519 for TCBT, 0.8300 for WCBT and 0.8184 for TAU. After controlling for baseline, the incremental QALY gain for TCBT compared with TAU was 0.0280 and for WCBT compared with TAU was 0.0172. The mean service costs after imputation were £1650 for TCBT, £1151 for WCBT and £886 for TAU. The incremental costs compared with TAU were £812 for TCBT and £337 for WCBT. This results in an ICER for TCBT of £29,000 per QALY and an ICER for WCBT of £19,593 per QALY.
When the minimum wage was used to value informal care and lost work days, the mean societal costs were reduced to £2362 for TCBT, £1520 for WCBT and £2518 for TAU. After controlling for baseline, TCBT cost £45 more than TAU (bootstrapped 95% CI –£1145 to £1124), whereas WCBT cost £389 less than TAU (bootstrapped 95% CI –£391 to £1283). When informal care was valued using the cost of a home-care worker, the mean societal costs were £3281 for TCBT, £2275 for WCBT and £5098 for TAU. After controlling for baseline, we found that TCBT cost £1228 less than TAU (bootstrapped 95% CI –£1061 to £3917) and WCBT cost £1241 less than TAU (bootstrapped 95% CI –£498 to £3351).
Health economic discussion
This cost-effectiveness analysis found that TCBT, and to a lesser extent WCBT, increased health-care costs over the follow-up period. This finding is not surprising because any health-care cost savings would have required a high use to start with and most patients receive relatively low-cost care. Therefore, any active therapy would be likely to increase costs. Cost-effectiveness does not mean cost saving, and higher costs can be justified if outcomes are sufficiently improved. In terms of the main clinical outcomes, WCBT produced improvements for lower costs than TCBT did. Interpretation of clinically specific outcomes is problematic for decision-makers and so QALYs were also used. The complete-case analysis showed that the cost per QALY for WCBT was very much lower than the threshold often assumed to guide NICE decisions in England (£20,000). TCBT had a cost-effectiveness ratio slightly above this threshold. On this basis, WCBT would be the preferred option. An analysis of uncertainty around the estimates suggests that these are robust findings. When imputation for the missing data was carried out, the ICERs both increased substantially.
From a societal perspective, we found that both interventions resulted in reduced costs. This was particularly because of reduced informal care from family and friends compared with TAU. From this perspective, then, we may deduce that TCBT and WCBT both dominate TAU (i.e. they are more effective and less expensive). However, the CIs are wide and do not exclude zero cost differences. Furthermore, NICE does not usually consider carer costs in its decision-making process.
Limitations
There were limitations to this economic evaluation. First, and most importantly, the number of missing EQ-5D-5L data was a concern. Those with missing data had worse IBS SSS and WSAS scores at each time point and so imputation from these resulted in smaller QALY gains for TCBT and WCBT relative to TAU. Second, service use data were provided by participants themselves and there may have been recall accuracy problems. However, this would not be likely to affect one group more than another and it was the only option for collecting comprehensive data. Third, medication data were not of high quality. We did know what medications were taken but quantities and durations were not complete. However, these costs would be a small proportion of the total and their inclusion would have only a marginal effect. Finally, this was a trial and the implementation and delivery of interventions outside the trial setting may be less than optimal.
Health economic conclusion
In conclusion, the complete-case analysis suggests that the therapies are cost-effective from a health-care perspective but this is reduced when using imputation methods. The interventions appear to produce important savings in terms of carer time and, if this is valued, then the interventions do cover their cost, although the societal costs are not significantly statistically different. However, when imputation for missing data was carried out, the ICERs increased to levels at which NICE would not usually recommend a treatment.
Chapter 4 Drawing all workstreams together
The HTA ACTIB trial was a rigorously conducted three-arm RCT of TCBT and WCBT compared with TAU in adults with refractory IBS with 12-month follow-up. It consisted of a RCT with a nested qualitative study and health economic evaluation.
Recruitment from both primary and secondary care sites in London and the south of England was undertaken to maximise the range of patients included and the generalisability of the findings. Recruitment was achieved on time and over target to allow for lower than initially predicted follow-up rates. A total of 558 participants were recruited, with 70.3% followed up at 12 months. We believe that this is the largest trial of CBT for IBS to date.
Clinical effectiveness outcomes showed that, compared with the TAU arm, both the TCBT and WCBT intervention arms showed clinically and statistically significant improvements in primary (IBS SSS and WSAS) and secondary outcomes over the trial period.
A large nested qualitative study consisted of interviews with 52 participants from the three trial arms to identify the factors that facilitate or impede adherence to web-delivered and therapist telephone-delivered CBT in patients with refractory IBS, to provide insight into the quantitative results of the ACTIB trial and to identify social and psychological processes of change that took place during the trial. The qualitative findings highlighted an increased capacity to cope with symptoms, negative emotions and other challenges of daily life in those in the CBT trial arms. It also indicated that therapists have an important role to play in supporting patients to engage with CBT and to make sense of the therapy and their IBS. Patients reported valuing the therapist support available alongside the website (Regul8), and this may have helped enhance their engagement and outcomes. The results suggest that, if rolled out in practice, such interventions would benefit from offering therapist support alongside any web-based intervention, could be offered to patients despite initial scepticism regarding psychological interventions and could be augmented with longer-term support for maintenance.
The health economic analysis aimed to investigate the treatment differences in cost-effectiveness of health service use (as measured using the CSRI and EQ-5D) between participants allocated to TCBT or WCBT and those allocated to TAU at all outcome time points (3, 6 and 12 months after randomisation). It showed that at 12 months the ICER (QALYs) for TCBT versus TAU was £20,125 and for WCBT versus TAU was £9905 in a complete-case analysis. However, cost-effectiveness was reduced after imputation for missing values on the EQ-5D. From a societal perspective, both interventions resulted in reduced costs (but not significantly) because of reduced informal care from family and friends in the CBT arms compared with TAU.
Overall conclusion
In this large, rigorously conducted RCT, both CBT arms showed significant improvements in IBS outcomes compared with TAU. WCBT had a lower cost per QALY than TCBT. Sustained improvements in IBS symptoms are possible at an acceptable cost.
Acknowledgements
The authors would like to acknowledge the key role played by the following people/groups:
-
all patients who volunteered for the study
-
all patient and public involvement (PPI) input, particularly from Jill Durnell and Kate Riley
-
the local CRNs who assisted with recruitment to the study
-
all general practices and gastroenterology clinics that hosted the study.
Trial Steering Committee and Data Monitoring and Ethics Committee members
Trial Steering Committee
Professor Peter White, Professor of Psychological Medicine (Chairperson); Professor Else Guthrie, Professor of Psychological Medicine and Medical Psychotherapy; Professor Qasim Aziz, Professor of Neurogastroenterology; Professor Tom Sensky, Emeritus Professor in Psychological Medicine; and Ms Jill Durnell, Owner and Managing Director at JayDee Solutions (PPI member). Observers: Dr Hazel Everitt, Professor Trudie Chalder, Professor Rona Moss-Morris, Dr Gilly O’Reilly, Dr Martina Prude (Sponsor Representative) and Dr Jenny Baverstock (Primary Care Research Network Representative).
Data Monitoring and Ethics Committee members
Professor Astrid Fletcher, Professor of Epidemiology of Ageing (Chairperson); Dr Charlotte Feinman, Consultant Psychiatrist; Professor Ronan O’Carroll, Professor of Psychology. Observers: Dr Hazel Everitt, Professor Trudie Chalder, Professor Rona Moss-Morris and Dr Gilly O’Reilly.
Contributions of authors
Hazel Everitt (Associate Professor in General Practice) was the chief investigator and led the ACTIB trial.
Sabine Landau (Professor of Statistics) was a co-applicant and led the ACTIB statistics.
Paul Little (Professor of Primary Care) was a co-applicant who provided expert trials advice and oversight.
Felicity L Bishop (Senior Researcher) led the qualitative study.
Gillian O’Reilly (Trial Manager of ACTIB) assisted with trial management.
Alice Sibelli (Research Assistant for ACTIB) assisted with trial recruitment and management.
Rachel Holland (Statistician) provided statistical support.
Stephanie Hughes (Research Assistant for ACTIB) assisted with trial recruitment and management.
Sula Windgassen (Research Assistant for ACTIB) assisted with trial recruitment and management.
Paul McCrone (Professor of Health Economics) was a co-applicant and led the health economics study.
Kim Goldsmith (Statistician, ACTIB Senior Researcher in Statistics) assisted with statistics.
Nicholas Coleman (Consultant Gastroenterologist) was a co-applicant and assisted with recruitment in Southampton secondary care.
Robert Logan (Consultant Gastroenterologist) was a co-applicant and assisted with recruitment in London secondary care.
Trudie Chalder (Professor of Health Psychology) was a co-applicant, was co-principal investigator and co-led the therapist supervision.
Rona Moss-Morris (Professor of Health Psychology) was a co-applicant, was co-principal investigator and co-led the therapist supervision.
Publications
Everitt H, Landau S, Little P, Bishop FL, McCrone P, O’Reilly G, et al. Assessing Cognitive behavioural Therapy in Irritable Bowel (ACTIB): protocol for a randomised controlled trial of clinical-effectiveness and cost-effectiveness of therapist delivered cognitive behavioural therapy and web-based self-management in irritable bowel syndrome in adults. BMJ Open 2015;5:e008622.
Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. Assessing telephone-delivered cognitive–behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial [published online ahead of print April 11 2019]. Gut 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. The CBT patient and therapist manuals used in the telephone CBT arm are freely available on the IAPT for the Long Term Conditions/Medically Unexplained Symptoms website as part of evidenced-based resources for IAPT.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Everitt H, Moss-Morris R, Sibelli A, Tapp L, Coleman N, Yardley L, et al. Management of irritable bowel syndrome in primary care: the results of an exploratory randomised controlled trial of mebeverine, methylcellulose, placebo and a self-management website. BMC Gastroenterol 2013;13. https://doi.org/10.1186/1471-230X-13-68.
- Hellier M, Sanderson J, Morris A, Elias E, De Caestecker J. Care Of Patients with Gastrointestinal Disorders in the United Kingdom: A Strategy for the Future. London: British Society of Gastroenterology; 2006.
- Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut 2001;48:272-82. https://doi.org/10.1136/gut.48.2.272.
- Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770-98. https://doi.org/10.1136/gut.2007.119446.
- Prior A, Whorwell PJ. Double blind study of ispaghula in irritable bowel syndrome. Gut 1987;28:1510-13. https://doi.org/10.1136/gut.28.11.1510.
- Hayee B, Forgacs I. Psychological approach to managing irritable bowel syndrome. BMJ 2007;334:1105-9. https://doi.org/10.1136/bmj.39199.679236.AE.
- Kennedy T, Jones R, Darnley S, Seed P, Wessely S, Chalder T. Cognitive behaviour therapy in addition to antispasmodic treatment for irritable bowel syndrome in primary care: randomised controlled trial. BMJ 2005;331. https://doi.org/10.1136/bmj.38545.505764.06.
- Zijdenbos IL, de Wit NJ, van der Heijden GJ, Rubin G, Quartero AO. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD006442.pub2.
- Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care. Manchester: NICE; 2015.
- Andersson G, Bergström J, Holländare F, Carlbring P, Kaldo V, Ekselius L. Internet-based self-help for depression: randomised controlled trial. Br J Psychiatry 2005;187:456-61. https://doi.org/10.1192/bjp.187.5.456.
- Andersson G, Strömgren T, Ström L, Lyttkens L. Randomized controlled trial of internet-based cognitive behavioural therapy for distress associated with tinnitus. Psychosom Med 2002;64:810-6.
- Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther 2012;50:415-21. https://doi.org/10.1016/j.brat.2012.03.001.
- Depression and Anxiety – Computerised Cognitive Behavioural Therapy (CCBT). Manchester: NICE; 2006.
- Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, Araya R, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ 2015;351. https://doi.org/10.1136/bmj.h5627.
- Everitt HA, Moss-Morris RE, Sibelli A, Tapp L, Coleman NS, Yardley L, et al. Management of irritable bowel syndrome in primary care: feasibility randomised controlled trial of mebeverine, methylcellulose, placebo and a patient self-management cognitive behavioural therapy website. (MIBS trial). BMC Gastroenterol 2010;10. https://doi.org/10.1186/1471-230X-10-136.
- Ljótsson B, Falk L, Vesterlund AW, Hedman E, Lindfors P, Rück C, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome – a randomized controlled trial. Behav Res Ther 2010;48:531-9. https://doi.org/10.1016/j.brat.2010.03.003.
- Hunt MG, Moshier S, Milonova M. Brief cognitive-behavioral internet therapy for irritable bowel syndrome. Behav Res Ther 2009;47:797-802. https://doi.org/10.1016/j.brat.2009.05.002.
- Everitt H, Landau S, Little P, Bishop F, McCrone P, O’Reilly G, et al. Assessing Cognitive behavioural Therapy in Irritable Bowel (ACTIB): protocol for a randomised controlled trial of clinical-effectiveness and cost-effectiveness of therapist delivered cognitive behavioural therapy and web-based self-management in irritable bowel syndrome in adults. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008622.
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 2006;130:1377-90.
- Moss-Morris R, McAlpine L, Didsbury LP, Spence MJ. A randomized controlled trial of a cognitive behavioural therapy-based self-management intervention for irritable bowel syndrome in primary care. Psychol Med 2010;40:85-94. https://doi.org/10.1017/S0033291709990195.
- Howie JG, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract 1998;15:165-71. https://doi.org/10.1093/fampra/15.2.165.
- Burgess M, Chalder T. PACE Manual for Therapists: Cognitive Behaviour Therapy for CFS/ME. London: King’s College London, PACE Trial Management Group; 2004.
- White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet 2011;377:823-36. https://doi.org/10.1016/S0140-6736(11)60096-2.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395-402. https://doi.org/10.1046/j.1365-2036.1997.142318000.x.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. https://doi.org/10.1192/bjp.180.5.461.
- Müller-Lissner S, Koch G, Talley NJ, Drossman D, Rueegg P, Dunger-Baldauf C, et al. Subject’s Global Assessment of Relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epid 2003;56:310-16. https://doi.org/10.1016/S0895-4356(03)00027-1.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2011.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, et al. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut 2006;55:643-8. https://doi.org/10.1136/gut.2004.062901.
- Toner BB, Stuckless N, Ali A, Downie F, Emmott S, Akman D. The development of a cognitive scale for functional bowel disorders. Psychosom Med 1998;60:492-7. https://doi.org/10.1097/00006842-199807000-00017.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. https://doi.org/10.1016/j.jpsychores.2005.10.020.
- Reme SE, Darnley S, Kennedy T, Chalder T. The development of the irritable bowel syndrome-behavioral responses questionnaire. J Psychosom Res 2010;69:319-25. https://doi.org/10.1016/j.jpsychores.2010.01.025.
- Rimes KA, Chalder T. The Beliefs about Emotions Scale: validity, reliability and sensitivity to change. J Psychosom Res 2010;68:285-92. https://doi.org/10.1016/j.jpsychores.2009.09.014.
- Baker R, Thomas S, Thomas PW, Gower P, Santonastaso M, Whittlesea A. The Emotional Processing Scale: scale refinement and abridgement (EPS-25). J Psychosom Res 2010;68:83-8. https://doi.org/10.1016/j.jpsychores.2009.07.007.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063-70. https://doi.org/10.1037/0022-3514.54.6.1063.
- Crawford J, Henry J. The Positive And Negative Affect Schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol 2004;43:245-65. https://doi.org/10.1348/0144665031752934.
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003821.
- Baldwin SA, Murray DM, Shadish WR, Pals SL, Holland JM, Abramowitz JS, et al. Intraclass correlation associated with therapists: estimates and applications in planning psychotherapy research. Cogn Behav Ther 2011;40:15-33. https://doi.org/10.1080/16506073.2010.520731.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2010;30:377-99. https://doi.org/10.1002/sim.4067.
- Eddings WM, Marchenko Y. Diagnostics for multiple imputation in Stata. Stata J 2012;12:353-67. https://doi.org/10.1177/1536867X1201200301.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Ljótsson B, Andersson G, Andersson E, Hedman E, Lindfors P, Andréewitch S, et al. Acceptability, effectiveness, and cost-effectiveness of internet-based exposure treatment for irritable bowel syndrome in a clinical sample: a randomized controlled trial. BMC Gastroenterol 2011;11. https://doi.org/10.1186/1471-230X-11-110.
- van Ballegooijen W, Cuijpers P, van Straten A, Karyotaki E, Andersson G, Smit JH, et al. Adherence to internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0100674.
- Pajak R, Lackner J, Kamboj SK. A systematic review of minimal-contact psychological treatments for symptom management in irritable bowel syndrome. J Psychosom Res 2013;75:103-12. https://doi.org/10.1016/j.jpsychores.2013.05.007.
- McCombie A, Gearry R, Andrews J, Mikocka-Walus A, Mulder R. Computerised cognitive behavioural therapy for psychological distress in patients with physical illnesses: a systematic review. J Clin Psychol Med Settings 2015;22:20-44. https://doi.org/10.1007/s10880-015-9420-0.
- Andersson G, Bergstrom J, Buhrman M, Caribring P, Hollandare F, Kaldo V, et al. Development of a new approach to guided self-help via the internet: the Swedish experience. J Tech Hum Serv 2008;26:161-81. https://doi.org/10.1080/15228830802094627.
- Spek V, Cuijpers P, Nyklícek I, Riper H, Keyzer J, Pop V. Internet-based cognitive behaviour therapy for symptoms of depression and anxiety: a meta-analysis. Psychol Med 2007;37:319-28. https://doi.org/10.1017/S0033291706008944.
- Andrade LH, Alonso J, Mneimneh Z, Wells JE, Al-Hamzawi A, Borges G, et al. Barriers to mental health treatment: results from the WHO World Mental Health surveys. Psychol Med 2014;44:1303-17. https://doi.org/10.1017/S0033291713001943.
- Castelein S, Bruggeman R, Davidson L, van der Gaag M. Creating a supportive environment: peer support groups for psychotic disorders. Schizophr Bull 2015;41:1211-13. https://doi.org/10.1093/schbul/sbv113.
- Bennett-Levy J, Perry H. The promise of online cognitive behavioural therapy training for rural and remote mental health professionals. Australas Psychiatry 2009;17:121-4. https://doi.org/10.1080/10398560902948126.
- Cartreine JA, Ahern DK, Locke SE. A roadmap to computer-based psychotherapy in the United States. Harv Rev Psychiatry 2010;18:80-95. https://doi.org/10.3109/10673221003707702.
- Coulson NS. Receiving social support online: an analysis of a computer-mediated support group for individuals living with irritable bowel syndrome. Cyberpsychol Behav 2005;8:580-4. https://doi.org/10.1089/cpb.2005.8.580.
- Prasko J, Jelenova D, Mihal V. Psychological aspects and psychotherapy of inflammatory bowel diseases and irritable bowel syndrome in children. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:307-14. https://doi.org/10.5507/bp.2010.046.
- van den Berg S, Shapiro DA, Bickerstaffe D, Cavanagh K. Computerized cognitive-behaviour therapy for anxiety and depression: a practical solution to the shortage of trained therapists. J Psychiatr Ment Health Nurs 2004;11:508-13. https://doi.org/10.1111/j.1365-2850.2004.00745.x.
- Cuijpers P, Donker T, van Straten A, Li J, Andersson G. Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol Med 2010;40:1943-57. https://doi.org/10.1017/S0033291710000772.
- Klein B, Mitchell J, Gilson K, Shandley K, Austin D, Kiropoulos L, et al. A therapist-assisted Internet-based CBT intervention for posttraumatic stress disorder: preliminary results. Cogn Behav Ther 2009;38:121-31. https://doi.org/10.1080/16506070902803483.
- Hadjistavropoulos HD, Alberts NM, Nugent M, Marchildon G. Improving access to psychological services through therapist-assisted, internet-delivered cognitive behaviour therapy. Can Psychol 2014;55:303-11. https://doi.org/10.1037/a0037716.
- Beattie A, Shaw A, Kaur S, Kessler D. Primary-care patients’ expectations and experiences of online cognitive behavioural therapy for depression: a qualitative study. Health Expect 2009;12:45-59. https://doi.org/10.1111/j.1369-7625.2008.00531.x.
- Tonkin-Crine S, Bishop FL, Ellis M, Moss-Morris R, Everitt H. Exploring patients’ views of a cognitive behavioral therapy-based website for the self-management of irritable bowel syndrome symptoms. J Med Internet Res 2013;15. https://doi.org/10.2196/jmir.2672.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. https://doi.org/10.1136/bmj.b3496.
- Clark T. On ‘being researched’: why do people engage with qualitative research?. Qual Res 2010;10:399-41. https://doi.org/10.1177/1468794110366796.
- Campbell R, Adams AE, Wasco SM, Ahrens CE, Sefl T. ‘What has it been like for you to talk with me today?’: the impact of participating in interview research on rape survivors. Violence Against Women 2010;16:60-83. https://doi.org/10.1177/1077801209353576.
- Strauss AL, Corbin JM. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage Publications Ltd; 1990.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publisher Company; 1967.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psych 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Harvey JM, Sibelli A, Chalder T, Everitt H, Moss-Morris R, Bishop FL. Desperately seeking a cure: Treatment seeking and appraisal in irritable bowel syndrome [published online ahead of print March 5 2018]. Br J Health Psychol 2018. https://doi.org/10.1111/bjhp.12304.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2018.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- NHS Reference Costs 2015 to 2016. n.d.
- Annual Survey of Hours and Earnings: 2016 Provisional Results. n.d.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444-55. https://doi.org/10.2307/2291629.
Appendix 1 Screening questionnaire
Questionnaire not reproduced owing to copyright restrictions. Please contact the corresponding author for more information.
Appendix 2 Consent form
Appendix 3 Baseline questionnaire
Questionnaire not reproduced owing to copyright restrictions. Please contact the corresponding author for more information.
Appendix 4 Short questionnaire
Questionnaire not reproduced owing to copyright restrictions. Please contact the corresponding author for more information.
Appendix 5 The 3-, 6- and 12-month questionnaire
Questionnaires not reproduced owing to copyright restrictions. Please contact the corresponding author for more information.
Appendix 6 Statistical analysis plan
Assessing Cognitive–behavioural Therapy in Irritable Bowel (ACTIB) Trial.
A randomised controlled trial of clinical and cost effectiveness of therapist-delivered CBT and web-based self-management in irritable bowel syndrome.
Version 1.1 9 March 2017
Appendix 7 Note review form
Appendix 8 General practitioner visits
The numbers of GP visits in the year before and the year after randomisation were recorded when the GP made this information available. The number of visits did not noticeably change and was similar across the three trial arms.
| GP visits | Trial arm | All | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCBT | WCBT | TAU | ||||||||||
| Mean (SD) | Minimum/maximum | Median/observed | Mean (SD) | Minimum/maximum | Median/observed | Mean (SD) | Minimum/maximum | Median/observed | Mean (SD) | Minimum/maximum | Median/observed | |
| In the year before randomisation | 4.5 (4.4) | 0/32 | 4/147 | 4.6 (3.5) | 0/20 | 4/144 | 4.1 (3.2) | 0/16 | 3/147 | 4.4 (3.7) | 0/32 | 4/438 |
| In the year after randomisation | 4.1 (3.7) | 0/17 | 4/139 | 4.2 (4.1) | 0/28 | 3/134 | 4.1 (3.4) | 0/20 | 3/138 | 4.1 (3.6) | 0/28 | 3/411 |
Appendix 9 Protocol deviations
Therapy protocol deviations are recorded in Chapter 1, Therapy protocol deviations.
All other protocol deviations are listed below.
| ID | Date | Description |
|---|---|---|
| 20140; 20022; 20130 | 3 April 2014 | LifeGuide message to do baseline set up after 1 week of consent, had to reconsent these participants |
| 10045 | 30 June 2014 | Randomised before participant completed baseline questionnaire |
| 21678 | 15 July 2014 | Participant was sent blood instruction prematurely before consent instruction and therefore had blood test without consent |
| 40002 | 21 July 2014 | Time frame between the FBC and the baseline questionnaire date was a bit more than 3.5 months |
| 20630 | 29 July 2014 | An incorrect ID (20630) was used to request a randomisation for participant 21630 |
| All participants | 30 July 2014 | Automated e-mails sent to participants asking them to do their questionnaires had the wrong link to the website |
| 10074; 40004 | 5 August 2014 | Automated e-mail failed to arrive from LifeGuide notifying randomisation complete step; LifeGuide support resolved |
| 30100 | 14 October 2014 | Baseline released before receiving the TTG result (she had the other two blood results). The participant completed baseline straight away |
| 23255 | 8 October 2014 | Blood sample came to Aldermoor Health Centre and was forwarded on to pathology by a member of staff in van downstairs |
| 29891 | 23 June 2015 | The participant had blood taken prior to consent and screening |
| 37858 | 12 March 2015 | The screening questionnaire was missing |
| 40192 | 19 March 2015 | Participant with ID 40192 was randomised as a general practice surgery (primary care London) patient. However, they were recruited through London secondary care |
| 16176 | 23 February 2016 | Participant was sent blood instruction prematurely before consent instruction and therefore had blood test without consent |
| 33272 | 24 February 2016 | Randomised before baseline but on the same day |
| 30959 | 15 March 2016 | The participant has been allocated to the LICBT group. However, he is unable to access the online intervention regularly because of constant problems with their laptops. He has requested a paper version of the website. As we do not have a paper version of each webpage from the Regul8 intervention, the participant will receive the manual developed for the HICBT group |
Appendix 10 Qualitative interview schedules
Three-month interview schedule
Introduction
-
Thank you for agreeing to take part in this interview [short hello].
-
I would like to record the conversation we have today so that I can refer back to it at a later date. It enables me to listen to you better. Is that OK?
-
Before we start there are a few things I would just like to mention.
-
What we talk about will be used as part of the study, but anything said will remain anonymous. We are going to ensure this by not using your real name when we type up the interview. Is that OK?
-
If I ask a question that you do not want to answer, that is absolutely fine. Just say so and I will ask you a different question. If at any point you would like to stop participating then please just tell me and we will stop the interview.
-
I am currently contacting a number of people who are taking part in the ACTIB study to find out about their experience of taking part in the trial, the treatments used in the trial, other IBS treatments they have tried in the past and the way they feel.
-
Anything you can tell me about your experiences including good and bad points would be useful.
-
Do you have any questions before we start? Are you happy to continue?
Section A
First I would like to ask you about the trial you are taking part in.
-
Could you tell me all about your experience of taking part in the trial so far?
Prompt: I am interested in why you agreed to take part in the trial and what you were initially expecting from it.
I am very interested to hear about the treatment that you have had as part of being in the trial (website delivered, therapist delivered, treatment as usual).
-
Could you tell me all about the treatment that you had for your IBS as part of the trial?
Additional prompts to be used flexibly if necessary:
-
What were your expectations of this group before initially trying it?
-
How did you feel about being allocated to this group in the beginning?
-
How did you feel about the website-delivered group after a while?
-
-
What did you like about being in this group?
-
What did you dislike about being in this group?
-
How has your IBS been since you have been using this treatment?
Can you tell me about anything that you feel changed while being in this group? (Symptoms, thoughts, feelings, lifestyle, social/relationships.)
-
Are there any other things that have changed?
Looking back on this treatment now . . .
-
What do you think about this treatment for IBS?
-
Do you have any particular feelings about the use of this treatment for IBS?
Section B
In this section of the interview, I would like to find out about other treatments that you have tried for your IBS.
-
Could you tell me about the most helpful treatments you have tried for your IBS?
-
What did you like/dislike about these treatments?
-
-
Could you tell me about the least helpful treatments you have tried for your IBS?
-
What did you like/dislike about these treatments?
-
Prompt: I’m interested in the treatments you have tried before taking part in the trial or any that you have tried since.
For the treatment:
-
What led you to try out this treatment?
-
How would you compare these treatments to each other?
-
How do the past treatments compare with the treatment received during the trial?
Section C
Until now, we have talked about your experience of taking part in the trial and the different treatments for your IBS. In the following section of the interview, I would like to find out more about how you feel in general and the way you manage your feelings. There are no right or wrong answers; I am just interested in your own personal experience.
-
Could you talk me through how you feel on a typical day?
-
Has it always been like this for you? Prompts: have there been any changes recently? If yes, which ones?
-
-
How do you express your emotions or feelings to other people? Prompt: why do you think this is? What about other people like colleagues of friends?
-
Has it always been like this for you? Prompts: have there been any changes recently? If yes, which ones?
-
-
Could you describe a time that you have experienced negative feelings?
-
Can you tell me what you do/did when you experience negative feelings?
-
Is there anything else that you do to cope with negative feelings? If yes, please describe.
-
-
Could you now describe a time that you have experienced positive feelings? Prompt: can you tell me what you do when you experience positive feelings?
Additional prompts to be used flexibly if necessary:
-
How easy/difficult is to work out how you feel?
-
Do you feel in control of your emotions? Why do you think this is?
Section D
In the final section of the interview, I am interested in your thoughts about what happens after the trial.
-
How have you been doing since you finished the first set of telephone support sessions? (For active arms only.)
-
What do you think will happen next with your IBS?
-
Is there anything that we could do differently to improve our treatments for people with IBS?
Closing and ending
-
Thank you very much for sharing your thoughts and experiences with me today.
-
What you have told me will really help us to understand patients’ experiences and hopefully to improve our treatments for IBS.
-
Before we finish, is there anything else you want to tell me? Is there anything you want to ask me?
-
Offer the participant a copy of the transcript and/or a summary of the findings.
Twelve-month interview schedule
Introduction
-
Thank you for agreeing to take part in this interview [short hello].
-
I would like to record the conversation we have today so that I can refer back to it at a later date. It enables me to listen to you better, is that OK?
-
Before we start there are a few things I would just like to mention.
-
What we talk about will be used as part of the study, but anything said will remain anonymous. We are going to ensure this by not using your real name when we type up the interview. Is that OK?
-
If I ask a question that you do not want to answer that is absolutely fine, just say so and I’ll ask you a different question. If at any point you would like to stop participating then please just tell me and we will stop the interview.
-
I’m currently contacting a number of people who are taking part in the ACTIB study to find out about their experience of taking part in the trial, the treatments used in the trial, other IBS treatments they have tried in the past and the way they feel.
-
Anything you can tell me about your experiences including good and bad points would be useful.
-
Do you have any questions before we start? Are you happy to continue?
Section A
First I would like to ask you about the trial you are taking part in.
-
Now that it has been a year since you started in the trial, can you tell me what you think about your experiences with the study?
As part of the trial, you received [treatment as usual/website + telephone support/telephone CBT]. Now that it has been a little while since your treatment finished, could you tell me what you think about it?
Additional prompts to be used flexibly if necessary:
-
Can you tell me about anything that you feel changed because of this treatment? (Symptoms, thoughts, feelings, lifestyle, social/relationships.)
-
Are there any other things that have changed?
-
-
How has your IBS been since we last spoke?
-
Do you think the treatments had any long-term effects on you?
-
Looking back on this treatment now . . .
-
What do you think about this treatment for IBS?
-
How do you feel about the use of this treatment for IBS?
-
What do you like about this treatment?
-
What do you dislike about this treatment?
-
Section B
In this section of the interview, I would like to find out about other treatments that you have tried for your IBS.
-
Could you tell me about the most helpful treatments you have tried for your IBS since we last spoke?
-
What did you like/dislike about these treatments?
-
-
Could you tell me about the least helpful treatments you have tried for your IBS since we last spoke?
-
What did you like/dislike about these treatments?
-
For the treatment:
-
What led you to try out this treatment?
-
How would you compare these treatments to each other?
-
How do the past treatments compare with the treatment received during the trial?
Section C
Until now, we have talked about your experience of taking part in the trial and the different treatments for your IBS. In the following section of the interview, I would like to find out more about how you feel in general and the way you manage your feelings. There are no right or wrong answers; I am just interested in your own personal experience.
-
Could you talk me through how you feel on a typical day?
-
Has it always been like this for you? Prompts: have there been any changes recently? If yes, which ones?
-
-
How do you express your emotions or feelings to other people? Prompt: why do you think this is? What about other people like colleagues of friends?
-
Has it always been like this for you? Prompts: have there been any changes recently? If yes, which ones?
-
-
Could you describe a time that you have experienced negative feelings?
-
Can you tell me what you do/did when you experience negative feelings?
-
-
Is there anything else that you do to cope with negative feelings? If yes, please describe.
-
Could you now describe a time that you have experienced positive feelings? Prompt: can you tell me what you do when you experience positive feelings?
Additional prompts to be used flexibly if necessary:
-
How easy/difficult is to work out how you feel?
-
Do you feel in control of your emotions? Why do you think this is?
Section D
In the final section of the interview, I’m interested in your thoughts about what happens after the trial.
-
How have you been doing since you finished the first set of telephone support sessions? (For active arms only.)
-
What do you think will happen next with your IBS?
-
Is there anything that we could do differently to improve our treatments for people with IBS?
Closing and ending
-
Thank you very much for sharing your thoughts and experiences with me today.
-
What you have told me will really help us to understand patients’ experiences and hopefully to improve our treatments for IBS.
-
Before we finish, is there anything else you want to tell me? Is there anything you want to ask me?
-
Offer the participant a copy of the transcript and/or a summary of the findings.
Appendix 11 Unit costs used in economic analyses
| Service | Unit cost (2016/17 £) |
|---|---|
| TCBT/WCBT per hour | 98 |
| Gastroenterologist | 137 |
| GP | 33 |
| Other doctor | 135 |
| Pharmacist | 25 |
| Physiotherapist | 49 |
| Practice nurse | 15 |
| Psychiatrist | 135 |
| Social worker | 40 |
| Other therapist | 79 |
| Acupuncturist | 50 |
| Dietitian | 81 |
| Homeopath | 50 |
| Occupational therapist | 79 |
| Osteopath | 50 |
| Nurse at home | 28 |
| Nurse in hospital | 43 |
| Inpatient day | Depends on specialty |
| Accident and emergency | 138 |
| Allergy test | 51 |
| Blood test | 8 |
| CT scan | 99 |
| EEG | 72 |
| Endoscopy | 416 |
| MRI | 146 |
| Ultrasound | 51 |
| X-ray | 26 |
| Informal care (per hour) | 16 |
| Lost work (per day) | 105 |
| Medication | Depends on drug |
List of abbreviations
- ACTIB
- Assessing Cognitive–behavioural Therapy in Irritable Bowel
- AE
- adverse event
- BES
- Beliefs about Emotions Scale
- CACE
- complier-average causal effect
- CBT
- cognitive–behavioural therapy
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CG-FBD
- Cognitive Scale for Functional Bowel Disorders
- CI
- confidence interval
- CONSORT
- Consolidated Standards Of Reporting Trials
- CRN
- Clinical Research Network
- CRP
- C-reactive protein
- CSRI
- Client Service Receipt Inventory
- CTU
- clinical trials unit
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FBC
- full blood count
- GI
- gastrointestinal
- GP
- general practitioner
- GSTT
- Guy’s and St Thomas’ NHS Foundation Trust
- HADS
- Hospital Anxiety and Depression Scale
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- IBD
- inflammatory bowel disease
- IBS
- irritable bowel syndrome
- IBS SSS
- Irritable Bowel Syndrome Symptom Severity Score
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IEE
- impoverished emotional experience
- IMD
- Index of Multiple Deprivation
- IPQ
- Brief Illness Perception Questionnaire for irritable bowel syndrome
- ISRCTN
- International Standard Randomised Controlled Trial Number
- ITT
- intention to treat
- KCH
- King’s College Hospital
- MAR
- missing at random
- MCID
- minimal clinically important difference
- MI
- multiple imputation
- MIBS
- Management of Irritable Bowel Syndrome
- MICE
- multivariate imputation by chained equations
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NNT
- number needed to treat
- NRES
- National Research Ethics Service
- OR
- odds ratio
- PANAS
- Positive and Negative Affect Schedule
- PEQ
- Patient Enablement Questionnaire
- PIS
- patient information sheet
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SD
- standard deviation
- SGA
- Subject’s Global Assessment
- SLAM
- South London and Maudsley NHS Foundation Trust
- SOP
- standard operating procedure
- SUHT
- Southampton University Hospital Trust
- TAU
- treatment as usual
- TCBT
- therapist telephone-delivered cognitive–behavioural therapy
- TSC
- Trial Steering Committee
- TTG
- tissue transglutaminase antibodies
- URL
- uniform resource locator
- WCBT
- web-based cognitive–behavioural therapy
- WSAS
- Work and Social Adjustment Scale

