Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/160/01. The contractual start date was in January 2016. The draft report began editorial review in June 2018 and was accepted for publication in October 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Nigel Arden has received honoraria from, held advisory board positions (which involved receipt of fees) in and received consortium research grants from Merck & Co. (Kenilworth, NJ, USA) (honorarium), Roche Holding AG (Basel, Switzerland), Novartis (Basel, Switzerland) and Bioiberica S.A. (Barcelona, Spain) (grants), Smith & Nephew plc (London, UK), NicOx S.A. (Valbonne, France), Flexion Bioventus (Bioventus LLC, Durham, NC, USA) and Freshfields Bruckhaus Deringer LLP (London, UK) (personal fees) outside the submitted work. Amar Rangan reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme, Orthopaedic Research UK (London, UK), DePuy Synthes UK (Leeds, UK) and JRI Orthopaedics (Sheffield, UK) outside the submitted work. Andrew Judge is a subpanel member of the NIHR Programme Grants for Applied Research (PGfAR) programme, has received consultancy fees from Freshfields Bruckhaus Deringer LLP and has held advisory board positions (which involved receipt of fees) from Anthera Pharmaceuticals Inc. (Hayward, CA, USA). Daniel Prieto Alhambra has received grants and other support from Amgen Inc. (Thousand Oaks, CA, USA) and UCB Biopharmal Srl (Brussels, Belgium); grants from Laboratories Servier (Neuilly-sur-Seine, France), Novartis International AG (Basel, Switzerland), Astellas Pharma Inc. (Tokyo, Japan), the NIHR HTA programme and from NIHR Research for Patient Benefit (RfPB), outside the submitted work. He is also a member of the NIHR HTA Clinical Evaluation and Trials panel (from November 2017 to present) and the NIHR RfPB South-Central Regional Advisory Committee panel (from 2013 to 2017). Tim Holt is a general practitioner (GP) in London and is a GP advisor for, but not employed by, the Clinical Practice Research Datalink. Gary S Collins is a member of the HTA Commissioning Board and has received grants from the NIHR HTA programme, NIHR RfPB, NIHR Biomedical Research Centre (BRC) and British Heart Foundation outside the submitted work. Sarah E Lamb was on the HTA Additional Capacity Funding Board (2012–15), HTA End of Life Care and Add-on Studies Board (2012–15), HTA Prioritisation Group Board (2010–15), HTA Trauma Board (2013–15), HTA Clinical Trials Board (2010–15) and the HTA Funding Boards Policy Group (2010–15) within 36 months of the start of the study. Andrew Carr has received other grants from the NIHR HTA programme, the Medical Research Council and the Wellcome Trust during the conduct of this study. He is a panel member on the Medical Research Council Developmental Pathway Funding Scheme (2016–present), a theme leader for the NIHR Oxford Biomedical Research Centre (2017–present) and was the Director of the NIHR Oxford Musculoskeletal Biomedical Research Unit (2008–17). Jonathan L Rees has received other grants from the NIHR HTA and NIHR PGfAR programmes. He works within a NIHR BRC and currently holds other grants from the Royal College of Surgeons of England, the Dinwoodie Charitable Company (Macclesfield, UK), McLaren Applied Technologies (Woking, UK) and the National Joint Register (NJR). He sits on committees at the NJR, National Institute for Health and Care Excellence and the Orthopaedic Data Evaluation Panel (ODEP), advises the Medicines and Healthcare Products Regulatory Agency and is a council member of the British Elbow and Shoulder Society.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Rees et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background and study introduction
This study is in response to a research commission from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme and, as such, this avenue of research has already been deemed necessary. Since the commissioned call, no systematic reviews or randomised clinical trials that answer the brief have been published.
The most common joint dislocations seen in hospital accident and emergency departments affect the shoulder (8.2–17 cases per 100,000 people per year). 1 Around 95% of traumatic dislocations of the shoulder occur anteriorly, where the top end of the arm bone (humerus) is forced frontwards out of the shoulder socket. 1 The mobility of the shoulder joint renders it particularly unstable and susceptible to re-dislocation. Traumatic anterior shoulder dislocation (TASD) is particularly common in younger patients and often occurs as a result of injury during contact sports. 2 When it occurs, it is very painful and the shoulder often stays dislocated until it is repositioned. The condition is associated with significant morbidity as, following a first-time dislocation, there will probably be damage to the soft tissue and ligaments that are responsible for stabilising the joint, rendering it susceptible to re-dislocation. The literature reports that recurrent dislocation can occur in 85–92% of cases. 3 However, the most effective treatment for the management of first-time traumatic shoulder dislocation in preventing further dislocations remains uncertain.
Surgery versus conservative treatment
Current options for the management of TASD include surgical or conservative treatment (usually physiotherapy) that aims to restore the stability and function of the shoulder joint. 4 However, there is a lack of consensus and a lack of good-quality evidence in support of a particular treatment regime. 2 Prior to both surgical and non-surgical intervention, closed reduction techniques tend to be used to restore the correct position of the shoulder joint. 4 Subsequent surgical management tends to include either soft-tissue reconstruction (e.g. Bankart labral repair) or bony procedures (e.g. coracoid process transfer). 5 Alternatively, non-surgical treatments involve immobilisation of the arm using slings or splints, followed by physical rehabilitation. 2 It is currently unclear from the literature which treatment approach to use following a first-time TASD to restore the stability and function of the shoulder and to help prevent recurrent dislocations.
The use of traditional conservative management approaches after initial reduction and joint immobilisation has been challenged because of high rates of recurrent dislocation among some population groups. In younger patients, rates of recurrence as high as 92–96% have been reported. 6 An incidence study of shoulder instability among athletes at a US military academy showed that 85% of athletes experienced a recurrent event within a 9-month period. 7 A systematic review showed that there were some limited data to support primary surgery following a first-time TASD among young adults engaged in demanding physical activities (military personnel and athletes). 5 A later systematic review also showed that among younger patients, a significantly lower rate of recurrent instability was identified in a 2-year period following a first-time TASD for those having surgery than for those having no surgery (7% vs. 46%). 8 Consequently, there appears to be some limited evidence for surgical intervention following a first-time TASD among younger and/or highly active patients; however, the literature emphasises that there is no evidence to challenge the use of non-surgical techniques for other patient groups. 5
Concerning non-operative treatment approaches in the management of first-time TASD, not only is there a lack of evidence for non-surgical over surgical treatment, but there are also uncertainties regarding the type of non-operative treatment used. For example, there is debate over the length of time the arm should be immobilised and the position (i.e. internal or external rotation) in which it should be immobilised. 6 Some studies have found a lower recurrence rate in patients treated using external rotation (26% recurrence) than in those treated using internal rotation (42% recurrence) methods, and that this technique was also more effective for the younger, < 30 years age group. 9 However, an earlier systematic review did not identify any statistically significant results in re-dislocation rates among patients treated using internal or external rotation methods. 2 The literature has highlighted the absence of and the usefulness of future trials looking at these aspects of non-operative management for TASD. 2
The use of surgical intervention for the management of TASD goes back to 1923, when Bankart described an anterior labral avulsion of the glenoid during shoulder dislocation. 10 Current approaches involve stabilising the joint using open or arthroscopic (keyhole) surgery; however, the literature is unclear as to which strategy is most effective. No significant differences have been identified between open and arthroscopic approaches in terms of recurrent instability or re-injury. 8,11,12
Incidence studies
Studies of the incidence of traumatic shoulder dislocation have been conducted outside the UK. An early, highly cited study of the incidence of shoulder dislocation was carried out in Sweden in 1982 by Hovelius. 13 In a random sample of 2092 people aged 18–70 years, it was shown that 1.7% of participants had a history of dislocation, with re-dislocation more common in young adults and with a male-to-female ratio of 3 : 1 overall (although varying with age). 13 In a 10-year follow-up study of 247 Swedish patients aged 12–22 years at the time of their dislocation, 66% of patients had one or more re-dislocations but only 24% had a recurrence between 30 and 40 years of age. 14
The incidence of shoulder dislocation was again examined in a later (2010) study based on a US population. 15 This study utilised data from the National Electronic Injury Surveillance System (NEISS) and was based on patients of all ages who experienced a shoulder dislocation from 2002 to 2006. Their findings showed an overall adjusted incidence rate of 23.9 per 100,000 person-years [95% confidence interval (CI) 20.8 to 27.0 per 100,000 person-years], a rate that was more than double that originally thought. The majority of dislocations occurred in men (72%), with the highest incidence observed in those aged 20–29 years (47.8 per 100,000 person-years, 95% CI 41.0 to 54.4 per 100,000 person-years). In males, the overall incidence rate was 34.9 per 100,000 person-years (95% CI 30.1 to 39.7 per 100,000 person-years), whereas in females this was 13.3 per 100,000 person-years (95% CI 11.6 to 15.0 per 100,000 person-years).
A further study, by Leroux et al. 16 in 2014, also looked at the incidence rate of primary anterior shoulder dislocation in a Canadian population of 16- to 70-year-olds who were diagnosed between April 2002 and September 2010. Compared with the US study, the Canadian data showed a similar rate of dislocations in men (74%), with an incidence rate highest for 16- to 20-year-olds (98.3 per 100,000 person-years). Similar figures for the overall adjusted incidence rate were observed, which in males was 34.3 per 100,000 person-years and in females was 11.8 per 100,000 person-years.
It is unclear in the current literature as to the most effective treatment pathway (i.e. surgery vs. no surgery) in the management of first-time TASD. Regarding conservative treatment, the optimum position for arm immobilisation and the duration of time are still in question. Concerning surgery, it is debated what technique (i.e. open or arthroscopic, soft tissue or bony) is more effective, and when or if surgery is needed following a first-time TASD. The main problem is an absence of data on the natural history of shoulder dislocation, including in the UK where age and sex incidence data have not been published. The literature also highlights the lack of good-quality evidence and supports the need for further research and randomised trials to address these issues.
Aims
The commissioned aims were:
-
to study the association between surgical treatment and recurrence rates following a first-time TASD in young adults who had surgery compared with those who had not had surgery
-
to identify clinical predictors of recurrent dislocation in young adults with a TASD for surgical and non-surgical patients.
Objectives
To use routinely collected data from two NHS computerised databases [i.e. the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES)] to study the association between surgical treatment and rates of re-dislocation, compared with no surgery in young adults with a first-time TASD. Potential predictors of re-dislocation in patients from each treatment group were further investigated.
The research questions were addressed by implementing a two-stage approach using two work packages.
Work package 1
Work package 1 consists of an internal and external validation study to test the quality and completeness of coding in the CPRD for identifying patients aged 16–35 years diagnosed with and treated for a first-time TASD. From these data, age and sex prevalence rates for first-time TASD in the UK were produced and these were externally validated against reported rates published in other settings.
Work package 2
A propensity-score-matched cohort analysis was conducted using CPRD and HES data. The cohort of participants used in the analysis comprised young adults (aged 16–35 years) with a TASD, who had at least 2 years of coding in the CPRD prior to a first-time entry Read code for shoulder dislocation (washout period) and at least 2 years of follow-up coding. The association between treatment strategy (i.e. surgery compared with no surgery) and rates of re-dislocation were then studied. Propensity matching ensured that patients undergoing surgery were matched and compared with a non-surgical control patient. Risk factors that may play an important role in re-dislocation in both the surgical and the non-surgical groups were further investigated.
Methods
Work package 1
The first phase of this project involved conducting an internal and external validation study to test the suitability of using the CPRD data set for identifying patients with a first-time TASD, and then externally validating the findings against published results from other settings. Relevant risk factors that were identifiable in the CPRD were recorded and used to inform the formal analysis regarding future predictors.
Internal validation
An internal validation study was conducted to check the quality of the coding for shoulder dislocations and treatments in the CPRD. A cohort of patients aged 16–70 years who were diagnosed with a shoulder dislocation in the UK between 1995 and 2015 were initially identified from the CPRD, to use as UK incidence data for all age groups. The included patients all had at least 2 years of coding in the CPRD prior to a first-time entry Read code for shoulder dislocation and at least another 2 years of subsequent coding. The internal validation exercise then focused on the planned study cohort of patients identified from the CPRD who were aged 16–35 years and had the same washout period.
A general practitioner (GP) questionnaire was designed with the help of GPs to internally validate the coding of shoulder dislocations and treatments in the CPRD. A random sample of 172 patients was then selected from those identified as meeting the above selection criteria. A questionnaire was sent to the general practice of each patient using the CPRD GP questionnaire service. A clinician at the practice completed the questionnaire by comparing the records on the CPRD with the clinical records of the patient. Written reminders to complete the questionnaire were sent to the general practice by the CPRD every 2 weeks (up to a total of four reminders). The data from returned questionnaire responses were double-entered into a database, and an academic orthopaedic shoulder surgeon was consulted to resolve data input queries.
The following criteria were established a priori to ensure that shoulder dislocation coding in the CPRD was of high quality prior to moving forwards with the main analysis:
-
The coding of shoulder dislocation within the CPRD needed to have a positive predictive value of ≥ 75%.
-
The coding of ‘primary’ or ‘first-time’ shoulder dislocation coding in the CPRD also had to have a positive predictive value of ≥ 75%.
External validation
The external validation exercise compared the age and sex incidence rates for TASD produced for the UK with those reported in other settings. For this analysis, the original cohort of patients aged 16–70 years who were identified from the CPRD with a shoulder dislocation between 1995 and 2015 were used. The CPRD has a representative coverage of around 6.9% of the UK and includes 11.3 million patients, making it broadly generalisable in terms of age, sex and ethnicity for the UK population as a whole. The external validation study itself produced population-based age- and sex-specific incidence rates for TASD for the UK. Comparing these with the published rates reported from other settings allows for external validation of the UK data.
Work package 2
Propensity-score-matched cohort analysis
The main study is a population-based propensity-score-matched cohort study comparing the association between surgery (vs. no surgery) and rates of re-dislocation in patients diagnosed with a TASD. The cohort of patients used for this analysis consisted of young adults aged 16–35 years with a TASD, with 2 years of coding in the CPRD prior to a first-time entry Read code for shoulder dislocation, and at least another 2 years of follow-up coding after the initial event. A pre-agreed list of Read codes (CPRD) and HES Office of Population Censuses and Surveys (OPCS) 4.7 codes for shoulder dislocation and treatments was used to collect all related outcomes and events; these were further informed by the earlier validation work (work package 1) (see Appendix 1). A ‘primary’ or ‘first-time’ TASD is defined here as a first-time entry Read code for shoulder dislocation.
Identified patients were allocated to the intervention (surgical) or control (non-surgical) groups. Patients in the intervention group were those who underwent shoulder stabilisation surgery following a first-time episode of TASD (early surgical repair is defined here as a ‘decision to treat surgically after a first-time TASD’). Patients in the control group were those who did not receive a surgical intervention following a first-time episode of TASD.
Propensity score matching methods were used to match each patient receiving surgery to a comparable patient in the non-surgical group. Propensity score methods were used because they provide the best approach to handling observational data sets that may be influenced by confounding (e.g. some patients being more likely to have surgery than others). Propensity score methods allow for bias being introduced into the data set through confounding, as the type of treatment received (i.e. surgery or no surgery) was not randomly allocated in this study.
After the process of matching surgical patients to non-surgical control patients, a Cox regression survival model was used to assess the association between surgery and time to re-dislocation over a 2-year period.
Identify clinical predictors of recurrent dislocations by treatment type
In this component, investigated potential risk factors associated with re-dislocation for both the surgical and the non-surgical groups were investigated. Prediction models were developed using linked CPRD and HES data, including any risk factors defined a priori by consensus that were available and those identified through the earlier validation work.
Conclusion
The relevant background information supporting the need for research into the efficacy of management options for patients with first-time traumatic shoulder dislocation has been described. Each of the four key components of the study has been outlined in this chapter and they are described in more detail in Chapters 2–5.
Chapter 2 Internal validation study of shoulder dislocation coding within the Clinical Practice Research Datalink
Results from the validation study have been published in Shah et al. 17 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Introduction
The first phase of this study was to carry out an internal validation of shoulder dislocation coding in routinely collected data from the CPRD to confirm the feasibility of using the CPRD to study shoulder dislocations for the main analyses. It also sought to identify which risk factors were relevant to shoulder dislocation and were readily available in the CPRD for use in the main analyses.
Objectives of Chapter 2
-
Identify patients in the CPRD aged 16–35 years who were diagnosed with a traumatic shoulder dislocation between 1 April 1997 and 31 March 2015 in England.
-
Develop a GP questionnaire using a validation algorithm and with input from GPs.
-
Take a random sample of patients from those identified in the CPRD and use the CPRD GP questionnaire service to send the questionnaire to the respective patient practices for completion.
Methods
Data source
Population-based primary care data from the CPRD were used to identify a cohort of patients diagnosed with a traumatic shoulder dislocation (aged 16–35 years) in the UK from 1 April 1997 to 31 March 2015. The CPRD covers 11.3 million people from 674 UK general practices and provides a representative coverage of around 6.9% of the UK population, which is broadly representative of the population in terms of age, sex and ethnicity. 18 Patient and practice data are anonymised, but patient-level data are available on age, sex, geographic region, body mass index (BMI), smoking status (i.e. current smoker, ex-smoker, non-smoker) and drinking status (i.e. current drinker, ex-drinker, non-drinker). The CPRD data were linked to data from the Index of Multiple Deprivation (IMD) 200419 for English patients and the Charlson Comorbidity Index (CCI) score was calculated using predefined Read codes.
Participants
The Read codes were used to identify patients from the CPRD with a shoulder dislocation. These codes had been established a priori through consensus by specialist shoulder surgeons with clinical experience and experts in epidemiology research (see Appendix 1). To ensure that ‘primary’ or ‘first-time’ shoulder dislocations were captured, patients were required to have no recorded shoulder dislocations in their CPRD clinical data for 2 years prior to first entry of a shoulder dislocation Read code. The 2-year washout period was defined using the date that the general practice was classified as ‘up to standard’ and the date that the patient was first registered at the general practice. This first entry of a shoulder dislocation Read code was defined as the primary dislocation.
The patients had to be registered at ‘active’ CPRD practices. An ‘active’ practice was defined as a practice that had contributed to the CPRD database in the previous 6 months. No general practices were classified as active in the East Midlands, and so it was not possible to include patients from this region. Following the identification of the shoulder dislocation cohort from within the CPRD data set, a predefined set of patient exclusion criteria was applied to facilitate the validation (Table 1).
| Exclusions | n (%) |
|---|---|
| Total number of CPRD shoulder dislocation patients received | 63,324 (100) |
| Unacceptable patients (i.e. CPRD flags that data quality for a patient is insufficient for medical research) | 806 (1) |
| Unacceptable dates (i.e. impossible to find a shoulder dislocation code between the CPRD minimum and maximum acceptable dates as defined by data management standard operating procedures for clinical research)18 | 34,710 (55) |
| Shoulder dislocation date prior to 1 April 1997 | 2507 (4) |
| Shoulder dislocation date after 31 March 2015 | 823 (1) |
| < 2-year minimum washout period (i.e. washout period defined using the date the general practice was classified as ‘up to standard’ and the date the patient first registered at the practice) | 3694 (6) |
| Aged < 16 years | 825 (1) |
| Aged > 35 years | 12,125 (19) |
| Non-resident of England patients | 1788 (3) |
| Patients remaining in cohort | 6046 (10) |
General practitioner questionnaire design and implementation
The GP questionnaire was designed with the assistance of GPs and based on a developed validation algorithm (see Appendices 2 and 3) and a random sample of 172 patients was selected from a list of the 6046 eligible patients. CPRD personnel then sent the questionnaire to each patient’s general practice for a clinician to complete by comparing the records on their CPRD computer system with the patient’s clinical records. The GPs assessed the use of shoulder dislocation codes for traumatic dislocation, confirmation of first-time shoulder dislocation, subsequent codes used for further events, physiotherapy referral codes and confirmation that physiotherapy took place.
Four written reminders were sent every 2 weeks to the general practices. Data from the returned questionnaires were double-entered into a data set by a statistician and a project manager. Any queries were resolved by an academic orthopaedic shoulder surgeon. In the instance that two questionnaires were received for the same patient with differing answers (on three occasions two questionnaires were sent back for one patient, i.e. three patients and six questionnaires; differing responses were only received for questions 6 and 7), clarification was sought from the general practice via CPRD personnel (clarification was received for one patient).
The following validation criteria were defined a priori to reflect that the coding of shoulder dislocations in the CPRD was of a sufficiently high quality to proceed with the main analyses planned in work package 2:
-
a positive predictive value of ≥ 75% accuracy for shoulder dislocation coding in the CPRD
-
a positive predictive value of ≥ 75% accuracy in coding ‘primary’ or ‘first-time’ traumatic shoulder dislocation within the CPRD.
Results
Cohort
An initial cohort of 63,324 patients with codes for shoulder dislocation was identified from the CPRD database. A database manager and statistician assessed the cohort against clear predefined and important exclusion criteria (see Table 1). Unacceptable patients were defined as those whose records had not met quality standards and had been flagged by the CPRD as ‘unacceptable’. Unacceptable dates were defined as when it was impossible to find a shoulder dislocation code between the CPRD minimum and maximum acceptable dates, as defined by data management standard operating procedures for clinical research. 18 During this process, the majority of patients were excluded, either because they had a shoulder dislocation diagnosis outside the study time period (55%) or because they were outside the study age limits (16–35 years) (20%). The final cohort included 6046 patients aged 16–35 years who were diagnosed with a shoulder dislocation between 1 April 1997 and 31 March 2015 in England.
Internal validation
Of the 172 patients whose GP received a copy of the validation questionnaire, a response for 95 (55%) patients was received. For two patients, their GPs confirmed that they had transferred out of the practice and that no further information was available for them on the CPRD system.
Table 2 presents demographic characteristics for the following patient groups:
-
the cohort of 6046 patients from the CPRD aged 16–35 years and diagnosed with a shoulder dislocation between 1 April 1997 and 31 March 2015 in England
-
the 172 patients randomly selected to have their GPs receive a questionnaire
-
the 97 patients for whom completed questionnaires were returned by their general practice
-
the 75 patients for whom questionnaires were not returned by their general practice.
| Demographic characteristic | Patient group | |||
|---|---|---|---|---|
| Whole cohort | All GP questionnaires | Responders | Non-responders | |
| Cohort size, (n) | 6046 | 172 | 97 | 75 |
| Sex, n (%) | ||||
| Male | 4991 (83) | 137 (80) | 81 (84) | 56 (75) |
| Female | 1055 (17) | 35 (20) | 16 (16) | 19 (25) |
| Median age (years) (IQR) | 24 (20–34) | 24 (20–29) | 24 (20–29) | 24 (19–29) |
| Median BMI (kg/m2) (IQR) | 24 (22–27) | 24 (21–27) | 25 (22–28) | 23 (21–26) |
| Median CCI score (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Region, n (%) | ||||
| East Midlands | 263 (4) | 0 (0) | 0 (0) | 0 (0) |
| East of England | 673 (11) | 21 (12) | 17 (18) | 4 (5) |
| London | 695 (12) | 23 (13) | 11 (11) | 12 (16) |
| North East | 133 (2) | 4 (2) | 3 (3) | 1 (1) |
| North West | 951 (16) | 29 (17) | 13 (13) | 16 (21) |
| South Central | 965 (16) | 32 (19) | 17 (18) | 15 (20) |
| South East Coast | 702 (12) | 25 (15) | 12 (12) | 13 (17) |
| South West | 743 (12) | 17 (10) | 17 (18) | 0 (0) |
| West Midlands | 667 (11) | 15 (9) | 7 (7) | 8 (11) |
| Yorkshire and the Humber | 254 (4) | 6 (3) | 0 (0) | 6 (8) |
| IMD 2004 quintile, n (%) | ||||
| 1 (affluent) | 1279 (21) | 53 (31) | 28 (29) | 26 (35) |
| 2 | 1077 (18) | 35 (20) | 20 (21) | 15 (20) |
| 3 | 958 (16) | 24 (14) | 16 (16) | 8 (11) |
| 4 | 876 (14) | 38 (22) | 19 (20) | 18 (24) |
| 5 (deprived) | 624 (10) | 22 (13) | 14 (14) | 8 (11) |
| Missing | 1232 (20) | 0 (0) | 0 (0) | 0 (0) |
All four groups were similar with respect to demographic characteristics, including age, BMI and CCI score. The highest response rate (100%) was received from general practices in the South West of England, but otherwise the proportion of responses received reflected the regional distribution of patients included in the cohort. Data on the IMD 200419 were obtained and linked after the selection of the 172 records to be validated. There were no missing data on deprivation, as all practices sampled were ‘active practices’. A higher proportion of patients had been sampled from category 1 (affluent) and category 4 (somewhat deprived) than in the initial cohort of 6046 patients, but otherwise response rates were similar from all deprivation groups.
The distribution of CPRD Read codes used by GPs to code shoulder dislocations is given in Table 3. Codes S41..00, S41z.00 and 14G5.00 for dislocation of shoulder accounted for 82% of all shoulder dislocation coding in the data. Recurrent shoulder dislocation codes only identified another 10% of patients, indicating that the 2-year washout period was a successful approach to identifying primary or first-time shoulder dislocations. Of the seven patients who had a recurrent shoulder dislocation code and for whom a GP questionnaire response was obtained, four were confirmed as having had a primary shoulder dislocation and one was confirmed as not having had a shoulder dislocation at all.
| CPRD description (Read code) | Patient group (%) | |||
|---|---|---|---|---|
| Whole cohort | All GP questionnaires | Responders | Non-responders | |
| Total number of patients | 6046 | 172 | 97 | 75 |
| CPRD description (Read code) | ||||
| Dislocation or subluxation of shoulder (S41..00) | 55 | 55 | 52 | 60 |
| Dislocation of shoulder NOSa (S41z.00) | 10 | 10 | 9 | 11 |
| H/O:a dislocated shoulder (14G5.00) | 17 | 19 | 20 | 17 |
| Closed reduction of dislocation of shoulder (7K6G300) | 3 | 3 | 5 | 1 |
| Closed traumatic dislocation of shoulder (S410.00) | 2 | 1 | 1 | 0 |
| Recurrent dislocation of shoulder, anterior (N083A00) | 6 | 6 | 5 | 7 |
| Anterior dislocation of shoulder (S410111) | 2 | 1 | 2 | 0 |
| Recurrent joint dislocation, of shoulder region (N083100) | 2 | 0 | 0 | 0 |
| Recurrent subluxation of shoulder, anterior (N083C00) | 2 | 2 | 2 | 3 |
| Closed traumatic dislocation shoulder joint, anterior (subcoracoid) (S410100) | < 1 | 1 | 0 | 1 |
| Closed traumatic dislocation shoulder joint, unspecified (S410000) | < 1 | 1 | 2 | 0 |
| Closed traumatic subluxation, shoulder (S412.00) | < 1 | 1 | 2 | 0 |
Shoulder dislocation was confirmed as having been coded correctly in 89% (95% CI 83% to 95%) of all patients (Table 4). The remaining 11% (10 patients) had been miscoded and the patient had suffered other shoulder trauma or injuries, such as strains or dislocations of the acromioclavicular joint, as confirmed by their GP. Of all patients, a first-time or primary shoulder dislocation was confirmed in 76% (95% CI 67% to 85%) of cases. Subsequent dislocations occurring up to 2 years after the primary dislocation were recorded in the CPRD for 32% of patients. From the GP responses, an additional 11% of patients experienced a re-dislocation during this time that was not recorded in the CPRD.
| Validation of shoulder dislocations | n (%) |
|---|---|
| GP confirmation of shoulder dislocation | 85 (89) |
| Patients who had a confirmed ‘primary’ shoulder dislocation | 72 (76) |
| Patients who had a further dislocation within 2 years of the primary dislocationa | 27 (32) |
| Confirmation that this was a further dislocation episode and not a review of the problem | 21 (78) |
| Patients with further dislocations that have not been noted in the CPRD | 9 (11) |
| Patients who have CPRD Read codes for physiotherapy in the 2 years following the first dislocation code | 24 (28) |
| It is clear that this physiotherapy code indicates that the patient received physiotherapy for their shoulder | 15 (63) |
| Patients who did not have a CPRD Read code for physiotherapy, but for whom documentation exists confirming that they received physiotherapy for their shoulder | 17 (21) |
Twenty-eight per cent of patients had been coded as having received physiotherapy within the CPRD, and GPs confirmed that physiotherapy had been given to 63% of these. However, a further 17 patients had received physiotherapy for their shoulder dislocation that was not recorded within the CPRD. Thus, 41% of patients known to be receiving physiotherapy were not recorded within the CPRD.
Conclusion
This validation exercise, carried out, to our knowledge, for the first time for this condition in the CPRD, has demonstrated that the CPRD is an acceptable data set to identify and study shoulder dislocation patients. The validity of GP coding of shoulder dislocations within the CPRD in a subset of patients proved very high, at 89%. Of all patients, 76% were confirmed to have primary shoulder dislocations. All of the CPRD Read codes used to identify shoulder dislocation patients were useful for identifying patients who had a primary shoulder dislocation, including the three codes that are specific to re-dislocations (i.e. N083A00, N083100 and N083C00). There was a small amount of under-reporting of subsequent shoulder dislocations. Physiotherapy treatment coding was of a poorer quality given that it is under-reported, at 41%, and, as such, the effectiveness of physiotherapy cannot be evaluated using the CPRD in any subsequent analyses of shoulder dislocations. Although not all general practices responded to the questionnaire, those that did and those that did not respond to the questionnaire survey were similar by deprivation level, geography and other demographic characteristics.
The strength of the CPRD is that it is a large, population-based primary care cohort that is representative of the UK general population. The positive internal validation result achieved on the correct coding of shoulder dislocations in the CPRD now provides the opportunity to use these codes and study definitions in the main study analysis.
Chapter 3 External validation study of shoulder dislocation data within the Clinical Practice Research Datalink
Results from the external validation study have been published in Shah et al. 17 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Introduction
The second phase of this study was to produce, for the first time, the age- and sex-specific incidence rates for shoulder dislocations in the UK. These would then allow the comparison of numbers and incidence rates of shoulder dislocations in the UK with those published from the USA and Canada. The comparison will facilitate an external validation of the data contained within the CPRD on shoulder dislocations.
Objectives of Chapter 3
The objectives of this chapter are to produce age- and sex-specific incidence rates for shoulder dislocation for the UK population and to validate UK data by comparing age- and sex-specific incidence rates of first-time TASD with those of similar studies from the USA and Canada. 15,16
Methods
Data source
The CPRD of population-based primary care data was used to identify a cohort of patients diagnosed with a traumatic shoulder dislocation aged 16–70 years during 1995–2015 in the UK. A description of the CPRD and the potential risk factors available within it was presented in Chapter 2.
Participants
The cohort of 16,763 CPRD patients aged 16–70 years with a TASD during 1995–2015 in the UK was used. The patients were identified using predefined Read codes as described in Chapter 2 (see also Appendix 1).
Statistical analysis
Descriptive statistics were used to summarise the epidemiology of primary shoulder dislocations by demographic factors. The incidence rates by age and sex per 100,000 person-years and incidence rate ratios with 95% CIs and p-values were calculated for all age and sex groups using Stata® software version 14.1 (StataCorp LP, College Station, TX, USA).
Incidence rate denominators were constructed using the patient-level data from the CPRD. Observation time per patient was calculated between 1995 and 2015 as the sum of total year-time contributed by all subjects, wherein person-years start as the latest of first registration date, practice up-to-standard date and 1 January 1995, and end as the earliest from patient transfer out date, practice last collection date, death date and 31 December 2015.
Results
An initial cohort of 63,324 patients with codes for shoulder dislocation was identified. A predefined set of exclusion criteria was applied (Table 5). During this process, many patients were excluded either because they had a shoulder dislocation diagnosis outside the study time period (55%) or because they were outside the study age limits (16–70 years) (8%).
| Exclusion | n (%) |
|---|---|
| Total number of CPRD shoulder dislocation patients received | 63,324 (100) |
| Unacceptable patients (i.e. CPRD flags that data quality for a patient is insufficient for medical research) | 806 (1) |
| Unacceptable dates (i.e. impossible to find a shoulder dislocation code between the CPRD minimum and maximum acceptable dates, as defined by data management standard operating procedures for clinical research) | 34,710 (55) |
| Shoulder dislocation date prior to 1 January 1995 | 1446 (2) |
| Shoulder dislocation date after 31 December 2015 | 75 (< 1) |
| < 2-year minimum washout period (i.e. washout period defined using the date the GP was classified as ‘up to standard’ and the date the patient first registered at the general practice) | 4008 (6) |
| Aged < 16 years | 878 (1) |
| Aged > 70 years | 4638 (7) |
| Patients remaining in cohort | 16,763 (26) |
The final cohort produced included 16,763 patients aged 16–70 years who were diagnosed with a shoulder dislocation between 1995 and 2015 in the UK. The numbers of patients identified by CPRD Read codes are given in Table 6. Table 7 highlights the baseline characteristics of the cohort. Most (72%) of the shoulder dislocations occurred in men and the median age for the whole cohort was 36 years [interquartile range (IQR) 24–52 years]. Most patients had a ‘normal’ BMI (18.5–24.9 kg/m2) and 88% of patients had no comorbidities.
| Description | Read code | Number of patients |
|---|---|---|
| Dislocation or subluxation of shoulder | S41..00 | 9600 |
| Dislocation of shoulder NOSa | S41z.00 | 2066 |
| H/O: dislocated shouldera | 14G5.00 | 2331 |
| Closed reduction of dislocation of shoulder | 7K6G300 | 739 |
| Closed traumatic dislocation of shoulder | S410.00 | 410 |
| Recurrent dislocation of shoulder, anterior | N083A00 | 646 |
| Anterior dislocation of shoulder | S410111 | 424 |
| Recurrent joint dislocation, of shoulder region | N083100 | 176 |
| Recurrent subluxation of shoulder, anterior | N083C00 | 168 |
| Closed traumatic dislocation shoulder joint, anterior (subcoracoid) | S410100 | 64 |
| Closed traumatic dislocation shoulder joint, unspecified | S410000 | 78 |
| Closed traumatic subluxation, shoulder | S412.00 | 61 |
| Total | 16,763 | |
| Characteristic | n (%) |
|---|---|
| Total | 16,763 (100) |
| Sex | |
| Male | 12,148 (72) |
| Female | 4615 (28) |
| Age at shoulder dislocation (years) | |
| 16–20 | 2561 (15) |
| 21–30 | 4266 (25) |
| 31–40 | 3021 (18) |
| 41–70 | 6915 (41) |
| BMI (kg/m2) | |
| < 18.5 | 180 (1) |
| 18.5–24.9 | 3392 (20) |
| 25.0–29.9 | 3020 (18) |
| 30.0–34.9 | 1292 (8) |
| ≥ 35.0 | 768 (5) |
| Missing | 8111 (48) |
| Smoking | |
| Non-smoker | 6674 (40) |
| Current smoker | 3388 (20) |
| Ex-smoker | 2014 (12) |
| Missing | 4687 (28) |
| Drinking | |
| Current drinker | 6854 (41) |
| Non-drinker | 1113 (7) |
| Ex-drinker | 188 (1) |
| Missing | 8608 (51) |
| CCI score | |
| 0 | 14,834 (88) |
| 1 | 950 (6) |
| 2 | 523 (3) |
| ≥ 3 | 456 (3) |
| Region | |
| East Midlands | 600 (4) |
| East of England | 1444 (9) |
| London | 1484 (9) |
| North East | 279 (2) |
| North West | 2071 (12) |
| Northern Ireland | 602 (4) |
| Scotland | 1626 (10) |
| South Central | 2005 (12) |
| South East Coast | 1572 (9) |
| South West | 1462 (9) |
| Wales | 1591 (9) |
| West Midlands | 1470 (9) |
| Yorkshire and the Humber | 557 (3) |
| IMD 2004 (quintile of deprivation) | |
| 1 (affluent) | 2790 (17) |
| 2 | 2345 (14) |
| 3 | 2001 (12) |
| 4 | 1793 (11) |
| 5 (deprived) | 1309 (8) |
| Missing | 6525 (39) |
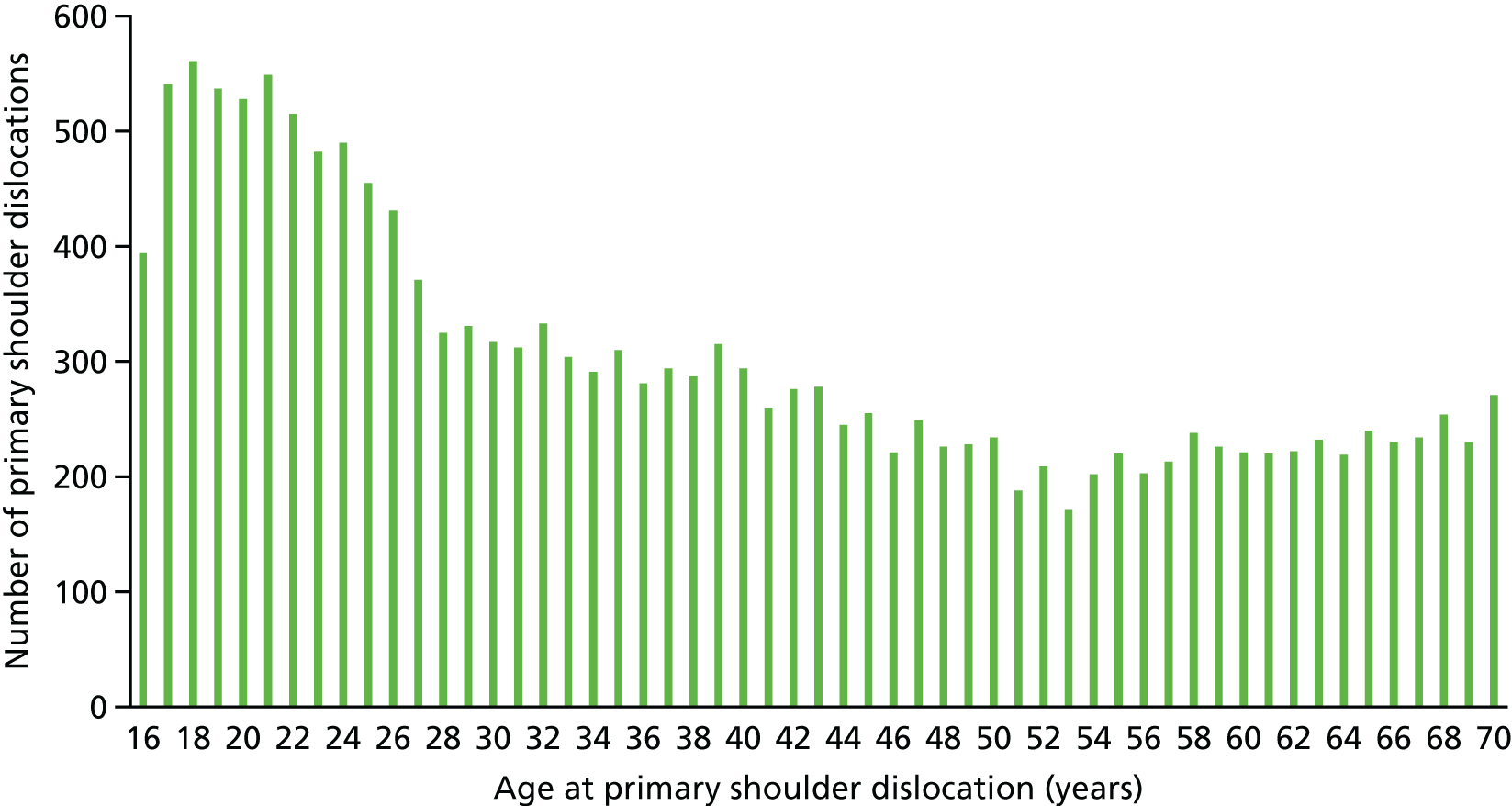
The age distribution of primary shoulder dislocation patients during 1995–2015 in the UK within the CPRD is given in Figure 1. A peak of > 500 patients per year of age occurs in patients aged 17–21 years, which then decreases until the age of 53 years. Between 55 years and 70 years, there is a gradual increase in the number of patients with a primary shoulder dislocation.
FIGURE 1.
Distribution of primary shoulder dislocation patients by age (years), demonstrating the overall distribution within CPRD during 1995–2015 in the UK.
UK incidence rates
The incidence rates and incidence rate ratios by age and sex for primary shoulder dislocation patients in the UK are presented in Table 8. The overall incidence rate in males was seen to be 40.39 per 100,000 person-years (95% CI 40.38 to 40.41 per 100,000 person-years) and in females was 15.52 per 100,000 person-years (95% CI 15.51 to 15.52 per 100,000 person-years). The highest incidence observed was in 16- to 20-year-old males (80.55 per 100,000 person-years, 95% CI 80.45 to 80.65 per 100,000 person-years). The incidence in men decreased with an increase in age. A U-shaped pattern of incidence was observed in women. The incidence was 16.36 per 100,000 person-years in those aged 16–20 years. This decreased in women aged 21–50 years and then increased to 28.64 per 100,000 person-years in women aged 61–70 years. Overall, the incidence was significantly higher in men than in women in almost all age groups, with an overall incidence rate ratio of 2.60 (95% CI 2.52 to 2.69). The exception was found in men and women aged 61–70 years, in whom no significant difference in incidence was observed (p = 0.334).
| Demographic category | Number of patients | Person-yearsa | Incidence rateb | 95% CI | Demographic comparison | Incidence rate ratio | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 12,148 | 30,074,078 | 40.39 | 40.38 to 40.1 | Male vs. female | 2.60 | 2.52 to 2.69 | < 0.001 |
| Female | 4615 | 29,741,559 | 15.52 | 15.51 to 15.52 | ||||
| Age (years) | ||||||||
| 16–20 | 2561 | 5,245,428 | 48.82 | 48.78 to 48.87 | ||||
| 21–30 | 4266 | 11,006,586 | 38.76 | 38.74 to 38.78 | 16–20 vs. 21–30 | 1.26 | 1.20 to 1.32 | < 0.001 |
| 31–40 | 3021 | 12,362,061 | 24.44 | 24.42 to 24.45 | 16–20 vs. 31–40 | 2.00 | 1.90 to 2.11 | < 0.001 |
| 41–50 | 2472 | 12,244,890 | 20.19 | 20.18 to 20.20 | 16–20 vs. 41–50 | 2.42 | 2.29 to 2.56 | < 0.001 |
| 51–60 | 2091 | 10,583,309 | 19.76 | 19.75 to 19.77 | 16–20 vs. 51–60 | 2.47 | 2.33 to 2.62 | < 0.001 |
| 61–70 | 2352 | 8,373,363 | 28.09 | 28.07 to 28.11 | 16–20 vs. 61–70 | 1.74 | 1.64 to 1.84 | < 0.001 |
| Age (years), sex (male) | ||||||||
| 16–20 | 2137 | 2,653,062 | 80.55 | 80.45 to 80.65 | ||||
| 21–30 | 3588 | 5,463,830 | 65.67 | 65.61 to 65.72 | 16–20 vs. 21–30 | 1.23 | 1.16 to 1.29 | < 0.001 |
| 31–40 | 2316 | 6,265,348 | 36.97 | 36.94 to 36.99 | 16–20 vs. 31–40 | 2.18 | 2.05 to 2.31 | < 0.001 |
| 41–50 | 1733 | 6,243,377 | 27.76 | 27.74 to 27.78 | 16–20 vs. 41–50 | 2.90 | 2.72 to 3.094 | < 0.001 |
| 51–60 | 1244 | 5,342,095 | 23.29 | 23.27 to 23.31 | 16–20 vs. 51–60 | 3.46 | 3.22 to 3.71 | < 0.001 |
| 61–70 | 1130 | 4,106,366 | 27.52 | 27.49 to 27.54 | 16–20 vs. 61–70 | 2.93 | 2.72 to 3.15 | < 0.001 |
| Age (years), sex (female) | ||||||||
| 16–20 | 424 | 2,592,366 | 16.36 | 16.34 to 16.38 | ||||
| 21–30 | 678 | 5,542,756 | 12.23 | 12.22 to 12.24 | 16–20 vs. 21–30 | 1.34 | 1.18 to 1.51 | < 0.001 |
| 31–40 | 705 | 6,069,714 | 11.56 | 11.55 to 11.57 | 16–20 vs. 31–40 | 1.41 | 1.25 to 1.60 | < 0.001 |
| 41–50 | 739 | 6,001,514 | 12.31 | 12.30 to 12.32 | 16–20 vs. 41–50 | 1.33 | 1.18 to 1.50 | < 0.001 |
| 51–60 | 847 | 5,241,214 | 16.16 | 16.15 to 16.17 | 16–20 vs. 51–60 | 1.01 | 0.90 to 1.14 | 0.840 |
| 61–70 | 1222 | 4,266,996 | 28.64 | 28.61 to 28.67 | 16–20 vs. 61–70 | 0.57 | 0.51 to 0.64 | < 0.001 |
| Age (years), sex (male vs. female) | ||||||||
| 16–20 | 16–20 vs. 16–20 | 4.92 | 4.44 to 5.47 | < 0.001 | ||||
| 21–30 | 21–30 vs. 21–30 | 5.37 | 4.95 to 5.83 | < 0.001 | ||||
| 31–40 | 31–40 vs. 31–40 | 3.20 | 2.94 to 3.48 | < 0.001 | ||||
| 41–50 | 41–50 vs. 41–50 | 2.25 | 2.07 to 2.46 | < 0.001 | ||||
| 51–60 | 51–60 vs. 51–60 | 1.44 | 1.32 to 1.57 | < 0.001 | ||||
| 61–70 | 61–70 vs. 61–70 | 0.96 | 0.89 to 1.04 | 0.334 | ||||
Comparison of UK incidence data with Canadian incidence data
Incidence rates for TASD in the UK were also compared by age and sex with those reported in Canada. 16 A comparative summary of the characteristics of the UK and Canadian cohorts is shown in Table 9. The UK cohort included in the analysis consisted of 15,666 patients aged 16–70 years with a primary shoulder dislocation in the UK as recorded in the CPRD between 1 April 1997 and 31 March 2015. Denominators for incidence analyses were obtained from the CPRD by individual year, age and sex. Data on urban or rural residence were not available in the CPRD, and thus this comparison could not be made.
| Details | Canadian data (Leroux et al.16) | UK data |
|---|---|---|
| Setting | Hospital records of patients having closed reduction of the shoulder | Primary care records of coded shoulder dislocations |
| Geography | Ontario cohort | UK sample (CPRD) |
| Dates | April 2002–September 2012 | April 1997–March 2015 |
| Patient age (years) | 16–70 | 16–70 |
| Numbers of patients | 20,719 | 15,666 |
The demographic data for the UK cohort (Table 10) were similar in age and sex distribution to those observed in the Canadian cohort. 16 The median age in both cohorts was 35 years, with a similar IQR. In the UK, 72% of primary shoulder dislocations occurred in men; in the Canadian cohort, this was slightly higher, at 74%.
| Demographic variable | UK cohort | Canadian cohort (Leroux et al.16) |
|---|---|---|
| Cohort size (n) | 15,666 | 20,719 |
| Age (years) | ||
| Mean (SD) | 38.29 (16.31) | 37.99 (16.62) |
| Median | 35 | 35 |
| IQR | 24–52 | 22–51 |
| Sex, n (%) | ||
| Male | 11,357 (72) | 15,399 (74) |
| Female | 4309 (28) | 5320 (26) |
| Deprivation quintile,a n (%) | ||
| 5 (most deprived) | 1224 (8) | 3698 (18) |
| 4 | 1686 (11) | 3862 (19) |
| 3 | 1889 (12) | 4071 (20) |
| 2 | 2188 (14) | 4356 (21) |
| 1 (most affluent) | 2625 (17) | 4732 (23) |
| Missing | 6054 (39) | 0 (0) |
The English IMD 200419 is a composite deprivation index at the small-area level, based on seven domains: income, employment, health and disability, education, barriers to housing and services, living environment, and crime. The Canadian measure of deprivation is solely based on income. These two measures are not directly comparable but a similar pattern of deprivation is observed, with increasing numbers of patients linked to increasing affluence. There was a large number of missing data for the UK cohort because the IMD results were available for English patients only, whereas complete data on deprivation were available for the Canadian patients.
Figures 2 and 3 present the percentage of primary shoulder dislocation patients by age and sex in the UK and Canada, respectively. In the UK, the peak in numbers for men is spread over those aged 17–22 years, whereas there is a distinct peak in men aged 17 years in Canada. The pattern in the number of women with shoulder dislocations is similar in both cohorts, with a high percentage in those aged 16 years, which decreases up to the early 30s and then increases until the age of 70 years.
FIGURE 2.
The percentage of primary shoulder dislocation patients by age (16–70 years) and sex recorded within CPRD (UK) between 1 April 1997 and 31 March 2015. Reproduced from Shah et al. 17 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
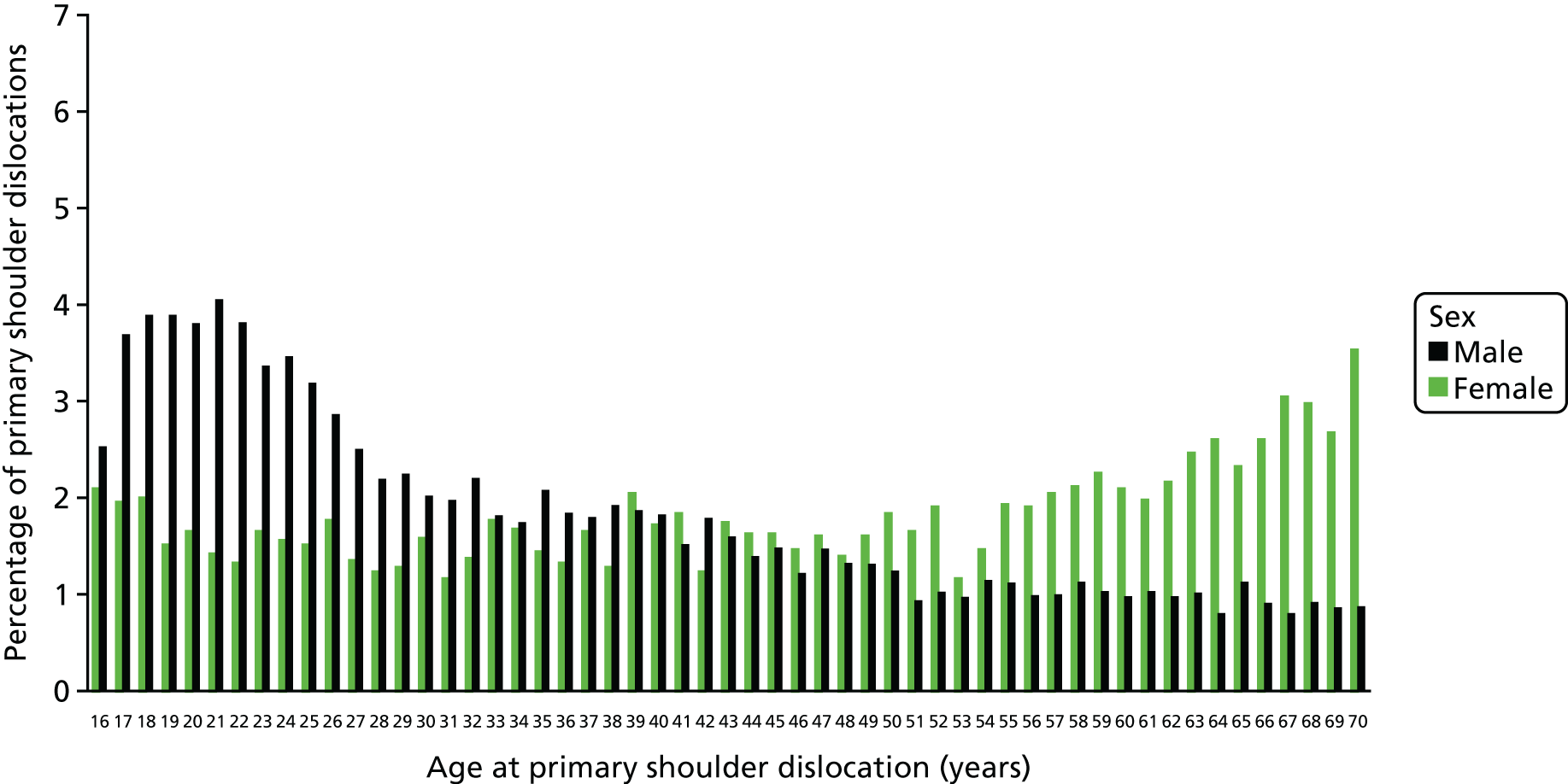
FIGURE 3.
The percentage of primary shoulder dislocation patients by age and sex recorded in Canada. Leroux T, Wasserstein D, Veillette C, Khoshbin A, Henry P, Chahal J, et al. , American Journal of Sports Medicine, vol. 42, issue 2, pp. 442–50, copyright © 2014 by American Orthopaedic Society for Sports Medicine, reprinted by Permission of SAGE Publications, Inc. 16

The incidence rates by age and sex for primary shoulder dislocation patients in the UK and an extract of similar Canadian incidence data are presented in Table 11. The patterns of incidence by age and sex were similar and are also represented in Figures 4 and 5. Incidence rates were higher in the UK for all combinations of age groups and sex, except for men aged 16–20 years [UK men (81.6 per 100,000 person-years) vs. Canadian men (98.3 per 100,000 person-years)].
| Demographic category | UK cohort | Canadian cohort (Leroux et al.16) | |
|---|---|---|---|
| Incidence ratea | 95% CI | Incidence ratea | |
| Sex | |||
| Male | 40.4 | 40.4 to 40.4 | 34.3 |
| Female | 15.5 | 15.5 to 15.5 | 11.8 |
| Age (years) | |||
| 16–20 | 48.8 | 48.8 to 48.9 | 56.6 |
| 21–30 | 38.8 | 38.7 to 38.8 | 27.0 |
| 31–40 | 24.4 | 24.4 to 24.5 | 16.5 |
| 41–70 | 22.2 | 22.2 to 22.2 | 18.8 |
| Age (years), sex (male) | |||
| 16–20 | 80.5 | 80.5 to 80.6 | 98.3 |
| 21–30 | 65.7 | 65.6 to 65.7 | 46.9 |
| 31–40 | 37.0 | 36.9 to 37.0 | 25.9 |
| 41–70 | 26.2 | 26.2 to 26.2 | 22.3 |
| Age (years), sex (female) | |||
| 16–20 | 16.4 | 16.3 to 16.4 | 13.8 |
| 21–30 | 12.2 | 12.2 to 12.2 | 7.5 |
| 31–40 | 11.6 | 11.6 to 11.6 | 7.1 |
| 41–70 | 18.1 | 18.1 to 18.1 | 15.2 |
FIGURE 4.
The incidence rate of primary shoulder dislocation by age and sex among patients aged 16–70 years with a primary shoulder dislocation recorded within CPRD (UK) between 1 April 1997 and 31 March 2015.

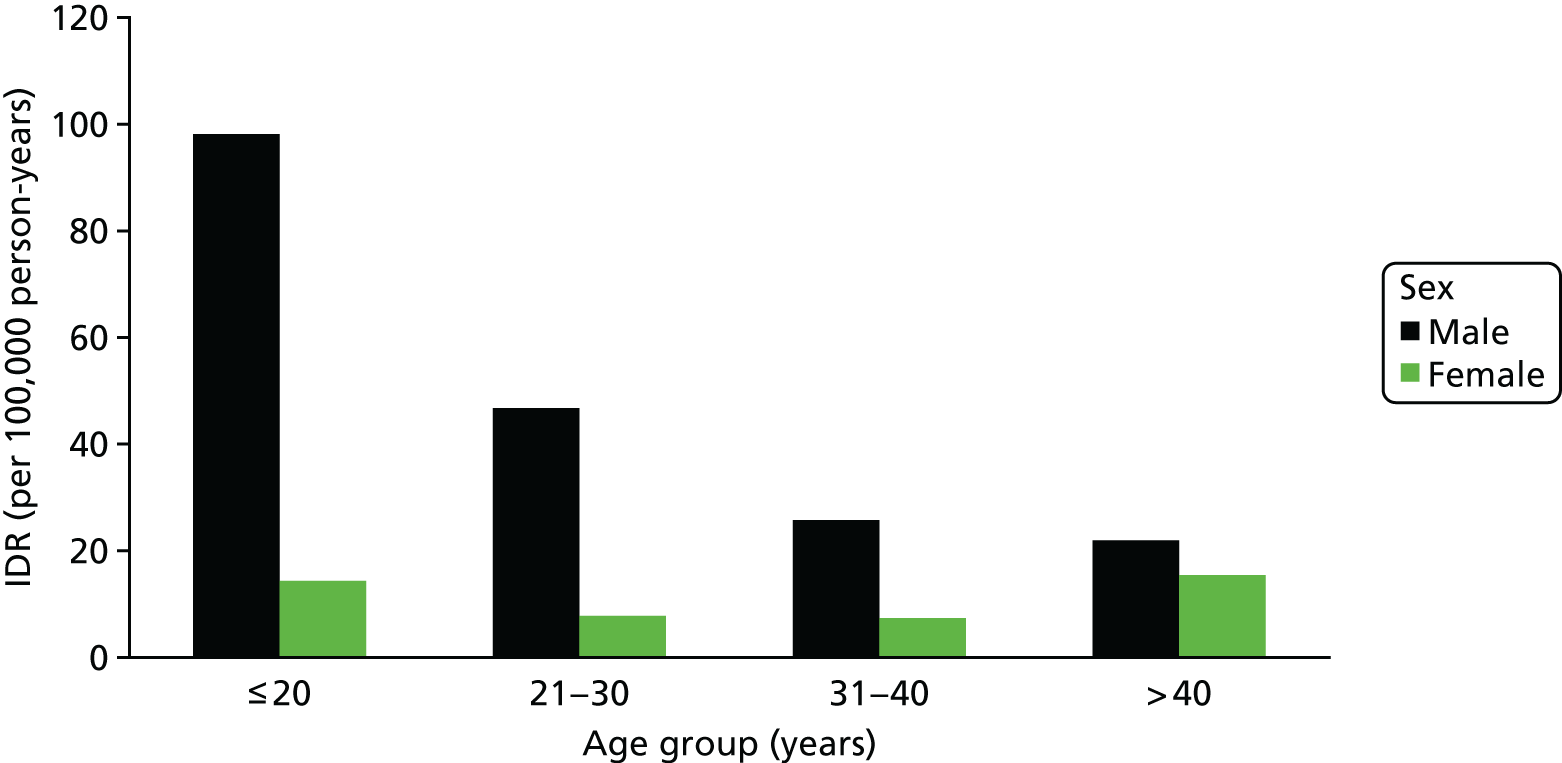
FIGURE 5.
The overall IDR of primary anterior shoulder dislocation in Canada requiring closed reduction in accordance with both age and sex. The y-axis depicts the IDR per 100,000 person-years and the x-axis depicts each age category. IDR, incidence density rate. Leroux T, Wasserstein D, Veillette C, Khoshbin A, Henry P, Chahal J, et al. , American Journal of Sports Medicine, vol. 42, issue 2, pp. 442–50, copyright © 2014 by American Orthopaedic Society for Sports Medicine, reprinted by permission of SAGE Publications, Inc. 16
Comparison of UK incidence data with US incidence data
The age and sex incidence rates for primary shoulder dislocation in the UK were also compared with published data from the USA. 15 A comparison of the characteristics between the UK and US cohorts is shown in Table 12. The UK cohort included in the analysis comprised 20,784 patients of all ages with a primary shoulder dislocation between 1 April 1997 and 31 March 2015. Data on ethnicity were not available within the CPRD, thus this comparison could not be made. Denominators for incidence analyses were obtained from the CPRD by individual year, age and sex.
| Details | USA data (Zacchilli and Owens15) | UK data |
|---|---|---|
| Setting | Hospital records of patients presenting to 100 hospital emergency departments with a shoulder dislocation | Primary care records of coded shoulder dislocations |
| Geography | USA sample (NEISS) | UK sample (CPRD) |
| Dates | 2002–2006 | April 1997–March 2015 |
| Patient age (years) | All ages | All ages |
| Numbers of patients | 8940 | 20,784 |
The overall incidence rate in the UK was 26.6 per 100,000 person-years (95% CI 26.2 to 26.9 per 100,000 person-years), which was similar to the incidence rate reported in the USA (23.9 per 100,000 person-years, 95% CI 20.8 to 27.0 per 100,000 person-years). The peak incidence occurred in patients aged 20–29 years in the UK (41.8 per 100,000 person-years, 95% CI 40.6 to 43.1 per 100,000 person-years) (Table 13), which is similar to the peak in 20- to 29-year-olds observed in the USA (47.8 per 100,000 person-years, 95% CI 41.0 to 54.5 per 100,000 person-years). 15 Significantly higher rates of incidence were observed in the UK among patients aged > 50 years in comparison with those in the USA (see Table 13).
| Demographic category | UK cohort data | USA cohort data (Zacchilli and Owens15) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence ratea | 95% CI | Incidence rate ratio | 95% CI | p-value | Incidence ratea | 95% CI | Incidence rate ratio | 95% CI | p-value | |
| Sex | ||||||||||
| Female | 19.02 | 18.59 to 19.45 | Referenceb | 13.26 | 11.56 to 14.96 | Referenceb | ||||
| Male | 34.29 | 33.71 to 34.88 | 1.80 | 1.75 to 1.86 | < 0.001 | 34.90 | 30.08 to 39.73 | 2.64 | 2.39 to 2.88 | < 0.05 |
| Age (years) | ||||||||||
| 0–9 | 1.23 | 1.01 to 1.49 | 0.06 | 0.05 to 0.08 | < 0.001 | 0.92 | 0.56 to 1.29 | 0.07 | 0.04 to 0.10 | < 0.05 |
| 10–19 | 27.34 | 26.32 to 28.41 | 1.38 | 1.30 to 1.46 | < 0.001 | 39.71 | 34.05 to 45.37 | 3.07 | 2.62 to 3.53 | < 0.05 |
| 20–29 | 41.80 | 40.55 to 43.08 | 2.11 | 2.00 to 2.23 | < 0.001 | 47.76 | 41.02 to 54.50 | 3.70 | 3.15 to 4.25 | < 0.05 |
| 30–39 | 25.05 | 24.14 to 25.99 | 1.26 | 1.19 to 1.34 | < 0.001 | 25.69 | 21.85 to 29.53 | 1.99 | 1.73 to 2.25 | < 0.05 |
| 40–49 | 20.66 | 19.84 to 21.51 | 1.04 | 0.98 to 1.11 | 0.169 | 17.59 | 14.22 to 20.96 | 1.36 | 0.91 to 1.82 | > 0.05 |
| 50–59 | 19.81 | 18.95 to 20.70 | Referenceb | 12.89 | 10.48 to 15.30 | Referenceb | ||||
| 60–69 | 26.71 | 25.60 to 27.87 | 1.35 | 1.27 to 1.43 | < 0.001 | 16.96 | 14.06 to 19.87 | 1.31 | 0.98 to 1.65 | > 0.05 |
| 70–79 | 41.12 | 39.48 to 42.82 | 2.08 | 1.95 to 2.20 | < 0.001 | 22.56 | 17.51 to 27.61 | 1.74 | 1.45 to 2.03 | < 0.05 |
| 80–89 | 58.03 | 55.40 to 60.79 | 2.93 | 2.75 to 3.12 | < 0.001 | 31.34 | 25.05 to 37.63 | 2.43 | 1.93–2.93 | < 0.05 |
| ≥ 90 | 65.55 | 59.65 to 72.03 | 3.31 | 2.98 to 3.67 | < 0.001 | 28.38 | 17.97 to 38.79 | 2.20 | 1.30–3.10 | < 0.05 |
In the UK, the incidence of primary shoulder dislocation was significantly higher in men (34.3 per 100,000 person-years, 95% CI 33.7 to 34.9 per 100,000 person-years) than in women (19.0 per 100,000 person-years, 95% CI 18.6 to 19.5 per 100,000 person-years) (p < 0.001), which is similar to the pattern observed in the USA (see Table 13). Incidence of shoulder dislocation in men was similar between the UK and US cohorts, but incidence in women in the UK was much higher than incidence in women in the USA. In the UK cohort, 36% of shoulder dislocations occurred in women, in contrast to 28% of shoulder dislocations in the US cohort.
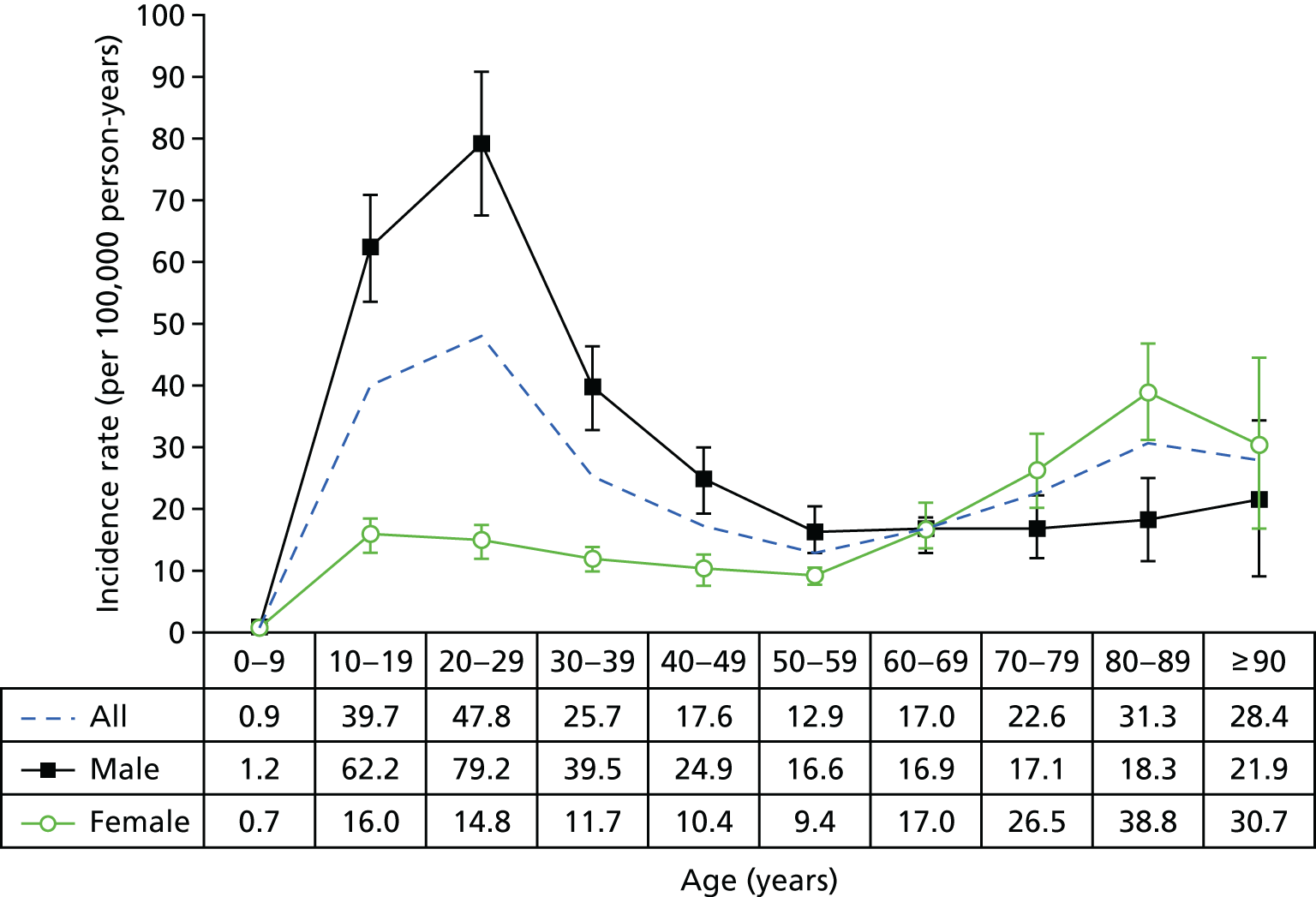
Figure 6 shows the peak of incidence in men aged 20–29 years in the UK (71.5 per 100,000 person-years), which was similar to the peak in men in the same age group in the USA (79.2 per 100,000 person-years) (Figure 7). The peak in women in the UK was observed in those aged > 90 years (71.7 per 100,000 person-years), in contrast with the 38.8 per 100,000 person-years in women aged 80–89 years in the USA. A possible reason for the differences in incidence may be caused by the UK study being based on primary care records and the US study being based on emergency department records.
FIGURE 6.
Shoulder dislocation incidence rates and 95% CIs by age and sex in CPRD (UK) between 1 April 1997 and 31 March 2015.

FIGURE 7.
Total weighted NEISS estimates of all US shoulder dislocations between 2002 and 2006 by age and sex, demonstrating a bimodal distribution with peaks among men (aged 20–29 years) and women (aged 80–89 years). The vertical bars denote the 95% CIs. 15 Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg 1992;3:542–9. https://insights.ovid.com/pubmed?pmid=20194311. Reprinted by permission of Wolters Kluwer.
Conclusion
This chapter describes a large population-based cohort of 16,763 patients aged 16–70 years in the UK during 1995–2015 identified in the CPRD data set in relation to shoulder dislocations. Most shoulder dislocations occurred in males (72%), with an overall incidence rate of 40.4 per 100,000 person-years. In females, the overall incidence rate was 15.5 per 100,000 person-years. The highest incidence was observed in 16- to 20-year-old males (80.5 per 100,000 person-years). An unexpected finding was that incidence in women increased beyond the age of 50 years to 28.1 per 100,000 person-years among those aged 61–70 years; this pattern was not observed in men.
The results from the UK cohort were then compared with other cohorts in other countries. The UK cohort was similar in age, sex distribution and incidence patterns to those observed in the Canadian, US and Norwegian cohorts. 15,16,20 Although the incidence patterns were similar between countries, in the UK the peak in numbers observed for men is spread over those aged 17–22 years, whereas there is a distinct peak in men aged 17–18 years in Canada and the USA. Possible reasons for this difference may be the high numbers of young men playing ice hockey and American football at school, aged 17–18 years, of whom not all continue to play at college. In a smaller study of the causes of shoulder dislocations in Sweden, incidence was high (8%) among ice-hockey players. 13 Other explanations might be the under-reporting of shoulder dislocations among college students or a genuine decrease because of better skeletal maturity and shoulder muscle strength and control.
Incidence rates were higher in the UK than in Canada for all combinations of age groups and sex, except for men aged 16–20 years (UK men, 80.5 per 100,000 person-years; vs. Canadian men, 98.3 per 100,000 person-years). These higher incidence rates may be explained by UK data being based on primary care records in contrast to Canadian data, which are based on accident and emergency hospital records. 16
A study conducted in Denmark identified the same bimodal age distribution of incidence, and also specifically noted that older people most frequently dislocated their shoulders at home by falling on their arm, whereas young people most frequently suffered a shoulder dislocation while playing sports. 21 However, the increasing incidence of shoulder dislocations seen in UK women aged > 50 years is a new finding that is of both interest and concern because the reasons for it are not known. Such injuries in the elderly are usually associated with rotator cuff tears and fractures with subsequent loss of function, as well as instability. However, further work will be required to examine the reasons that may explain this increased risk of shoulder dislocations in ageing women. Possible reasons include biological differences between ageing men and women, including differences in joint proprioception, soft tissue tendon quality and protective muscle bulk. Other possibilities might be a difference in the incidence of falls between men and women. This is of particular importance, given that the population of the UK continues to change to include more elderly people. The increasing population priority needs to be given to increasing the safety of the elderly to reduce falls, dislocations and fractures, as advocated by the National Institute for Health and Care Excellence (NICE),22 which suggests that this is a finding that needs further investigation and research.
The main strength of this study is its large population-based cohort that uses real-world data from primary care. The CPRD is representative of the UK general population by age and sex and the age- and sex-specific incidence of primary shoulder dislocations observed are similar to those observed in Canada and the USA. Although some differences were observed, the incidence of traumatic shoulder dislocations in these other countries has only been calculated using regional data or hospital data. This is, therefore, the first time, to our knowledge, that the incidence of shoulder dislocations has been studied using population-based primary care data and the first time, to our knowledge, that results for the UK have been produced. The findings in this chapter and Chapter 2 support the use of CPRD for the subsequent study chapters and work package 2 studies.
In summary, in the UK most primary shoulder dislocations during the selected time period occurred in young men. An unexpected finding was that incidence increased in women aged > 50 years but not in men of the same age. The reasons for this are unknown. Priority and attention should be directed towards increasing preventative measures for young people playing contact sports, and to the research of the possible causes of the increase in primary shoulder dislocation incidence for women aged > 50 years.
Chapter 4 The impact of surgical treatment within 6 months or no surgical treatment on the rates of shoulder re-dislocations in young people aged 16–35 years with first-time traumatic anterior shoulder dislocation in England
This chapter begins the main analysis of the commissioned research (work package 2). The previous chapters described the internal and external validation of the data to be used in this and the following chapters.
Objectives of Chapter 4
To study the effect of surgical treatment on the 2-year recurrent shoulder dislocation rates in young adults in England when surgical treatment took place within 6 months of the first episode of TASD.
Methods
Study design
A population-based propensity-score-matched cohort study to control for confounding at baseline has been conducted. Young adults (aged 16–35 years) who presented with a first-time TASD were selected from two computerised NHS databases (CPRD and HES). Figure 8 shows a detailed illustration of the study plan, which is described in more detail in the following paragraphs.
FIGURE 8.
The UK TASH-D phase 2 plan design for patients who received surgery or no surgical treatment within 6 months of a diagnosis of a first-time TASD during 1 April 1997–26 April 2014, in England.
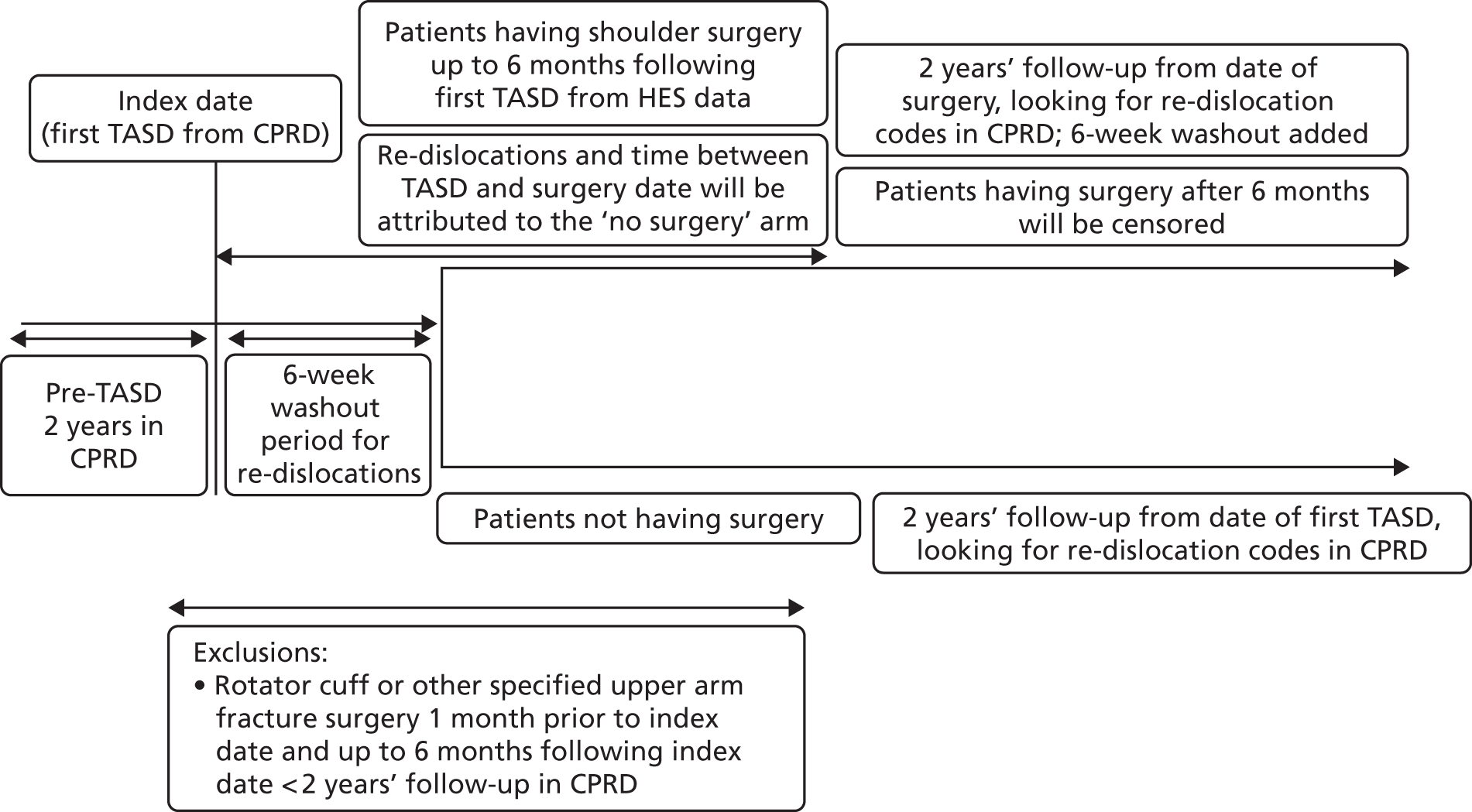
Using methodology identical to the internal validation study described in Chapter 2, to ensure that only primary dislocations had been captured, all participants had to have 2 years of clinical data within CPRD before their first TASD and at least 2 years’ follow-up from the event (re-dislocation). This 2-year washout period is required to ensure that a first dislocation code actually represents a first-time dislocation, as a recurrent dislocation usually occurs within 2 years of the primary event. This period therefore minimises the risk of a code being a second dislocation code. The period was defined using the date that the general practice was classified as ‘up to standard’ and the date that the patient was first registered at the practice. Patients had to be registered at ‘active’ practices, as defined in Chapter 2, Data source. Taking into account this washout period, the first Read code entry in CPRD for shoulder dislocation was then defined as the first dislocation. All events were collected using a pre-agreed validated list of CPRD Read codes (see Appendix 1). Patients experienced their first-time TASD between 1 April 1997 and 26 April 2014, allowing at least 2 years of follow-up for each patient to the end of the study on 26 April 2016.
A 6-week washout period for re-dislocation codes within CPRD was applied to all patients following their TASD to avoid duplicate records. Patients were highly unlikely to re-dislocate their shoulder during this period as their arms would be in slings and they would be following rehabilitation protocols, but would probably return to visit their GP for prescriptions for painkillers or referrals to physiotherapy and secondary care.
Surgical group
The surgical group comprised patients in CPRD with a first-time TASD who underwent shoulder stabilisation surgery after their first dislocation. Early surgical repair in this NHS context means ‘a decision to treat surgically after the first TASD’ (as per the approved study protocol) and receiving surgery within 6 months of injury before any subsequent dislocations. This meant linking HES data to CPRD data in such a way that a HES surgical OPCS 4.7 code was seen to occur after a single first dislocation code in CPRD before that surgical date. The timelines between first dislocation codes and OPCS 4.7 codes were recorded. If a re-dislocation occurred prior to their surgery date, the patient was allocated to the non-surgical arm.
A further 6-week washout period for re-dislocation codes within CPRD was applied to all patients in the surgical group following their surgery date to avoid duplicate records because they would have been asked to immobilise their arm for this period and, thus, a re-dislocation would be highly unlikely to occur. During these 6 weeks, patients would most probably return to visit their GPs for painkillers or referral to physiotherapy. Surgery patients were followed up for at least 2 years from the date of their surgery. Patients who had surgery more than 6 months following their TASD were censored on their date of surgery. The surgery dates for these patients ranged from 25 December 1997 to 18 August 2014.
Non-surgical group
Although the most desirable control group would have been physiotherapy, the internal validation study identified that the referral codes for physiotherapy are lacking and unreliable in CPRD. Thus, conservative care has been defined as ‘non-surgical intervention’, with no linked OPCS 4.7 surgical shoulder codes, producing a control cohort of patients whose first-time shoulder dislocation has been treated non-operatively. Non-surgical patients were followed up for at least 2 years following the date of their first-time TASD, as illustrated in Figure 8.
Outcome
The outcome was time to a shoulder re-dislocation, as defined by the CPRD Read codes given in Appendix 1. For the surgical group, the shoulder re-dislocation can occur between 6 weeks and 2 years following the date of surgery. For the non-surgical group, the shoulder re-dislocation could occur between 6 weeks and 2 years following the date of first-time TASD.
Any patients who died during the study were censored on their date of death.
Data sources
The analysis utilised two computerised NHS databases, one from primary care (the CPRD) and the other from secondary care (the HES). The characteristics of the CPRD have been described in Chapter 2, Data source. For HES data, each time a patient sees a health professional in a hospital, a record or ‘episode’ is created and added to the HES database. The HES record contains patient details, some diagnosis codes, treatments and lengths of hospital stay.
Clinical Practice Research Datalink has been linked with HES data to provide a HES-linked patient identifier. About 60% of CPRD-contributing practices have been linked to HES data. HES data provide a general patient identifier to facilitate linkage of hospital records belonging to the same individual. Management of the CPRD and HES databases was carried out by a senior data manager with expertise in the use of these data sets. The senior data manager developed an ad hoc code using Python (version 3.6, developed by Python Software Foundation, Wilmington, DE, USA) and Structured Query Language (SQL) (version 5.6.12, developed by the International Electrotechnical Commission and the International Organization for Standardisation; Geneva, Switzerland) to produce a final working data set that was analysed using standard statistical software packages Stata® version 14.1 and R (version 3; The R Foundation for Statistical Computing, Vienna, Austria).
Sample size
The original sample size considerations for this study were based on data from a Cochrane systematic review comparing surgical and non-surgical treatment for an acute TASD. 5 From the pooled results, 3 out of 58 patients in the surgical arm had subsequent further surgery (5.17%) compared with 17 out of 61 patients in the non-surgical arm (27.9%), at a minimum follow-up of 2 years (risk ratio 0.22, 95% CI 0.08 to 0.64). The large effect size is based on the pooled results of three randomised controlled trials with uncertainty around the true size of the effect outside a clinical trial setting in routine general practice. Therefore, values were set to detect a smaller difference in subsequent surgery within 2 years, with a 25% re-dislocation rate in the non-surgical group, compared with a 20% re-dislocation rate in the surgical group (an absolute difference of 5%). A two-sided, log-rank test for equality of survival curves was used, with 90% power at a 5% significance level (alpha) and for which the outcome is time to re-dislocation with an anticipated 25% re-dislocation rate in the non-surgical control group compared with a 20% re-dislocation rate in the surgical group [equivalent to a hazard ratio (HR) of 0.78]. Allowing for a 10% loss to follow-up and assuming equal group sizes meant that the study required a total sample size of 3065 participants, with 656 expected re-dislocations. 5 It was assumed that 35% of the patients would receive surgery within 6 months after one dislocation (n = 1073). 23
Participants
Inclusion criteria
The CPRD records of 6046 patients, aged 16–35 years with 2 years of data in the CPRD before a first-time TASD, identified in the internal validation study (see Table 1) were linked to HES records.
Exclusion criteria
The following patients were excluded:
-
those aged 16–35 years with a first-time TASD who cannot be linked by CPRD-HES
-
those with < 2 years of follow-up in the CPRD
-
those with prior shoulder surgery for a shoulder dislocation
-
those whose instability was treated with rotator cuff repair surgery or fracture surgery prior to or following a TASD.
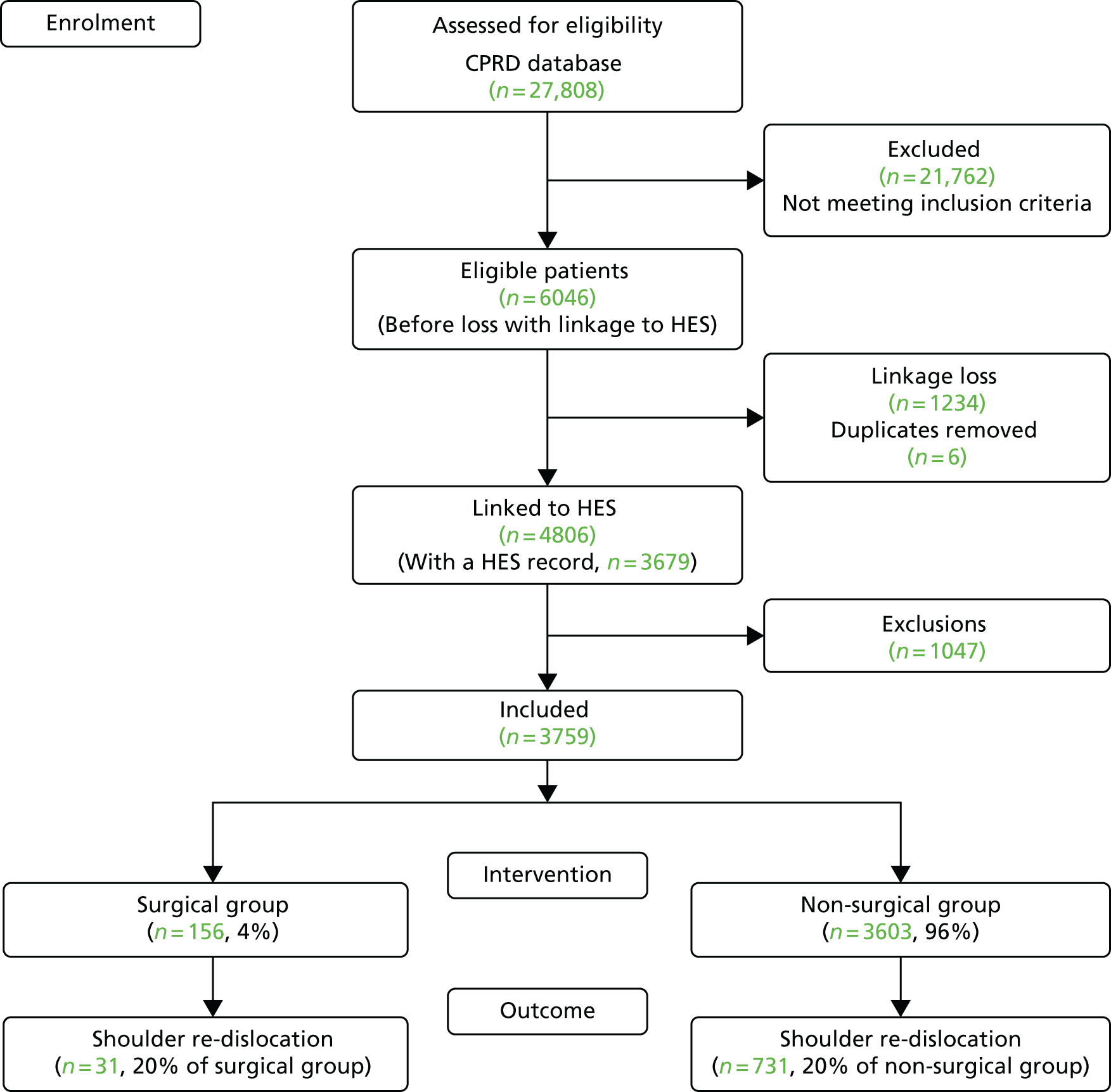
Exclusions were made in accordance with the criteria above and are described in Table 14. Following linkage to HES, there was a linkage loss of 1234 patients and six duplicates were identified and removed. A substantial number of patients had < 2 years of follow-up within the CPRD (n = 854), which may relate to these young people moving away from home to attend university or to start new jobs in new locations, which, in turn, requires a change of GP. A very small proportion of patients (3%) were excluded for having shoulder surgery for rotator cuff tears, fractures or prior dislocations. In total, 3759 patients remained available for analysis, which was greater than the minimum number of patients (n = 3065) required for a sufficient sample size and power (Figure 9).
| Exclusions | Excluded (n) | Total (N) | % |
|---|---|---|---|
| Eligible patients in the CPRD cohort for the internal validation study, following exclusions made in Table 1 | 6046 | 100 | |
| HES linkage loss | 1234 | 20 | |
| Duplicate removal | 6 | < 1 | |
| < 2 years of follow-up within the CPRD | 854 | 14 | |
| Prior shoulder surgery for a shoulder dislocation | 155 | 3 | |
| Surgery for rotator cuff tear prior to TASD | 1 | < 1 | |
| Surgery for rotator cuff tear in the 6 months following TASD | 4 | < 1 | |
| Surgery for a shoulder fracture prior to TASD | 14 | < 1 | |
| Surgery for a shoulder fracture in the 6 months following TASD | 19 | < 1 | |
| Total exclusions | 2287 | ||
| Patients included in subsequent analyses | 3759 | 62 |
FIGURE 9.
Shoulder dislocation exclusions flow chart for patients aged 16–35 years during 1 April 1997–26 April 2014, within CPRD in England with linkage to HES records.
Statistical analysis
The aim of this study was to investigate the effect of surgical intervention within 6 months compared with non-surgical intervention on the rates of re-dislocation in young patients with a first-time TASD over a 2-year period. In addressing this research question, the exposure is whether or not a patient received surgery, and the primary outcome of interest is the time from date of surgery for surgical patients or date of the first-time TASD for non-surgical patients to having a subsequent re-dislocation within 2 years.
Covariates
Demographic data were available from the CPRD on age, sex, BMI, IMD 2004,19 smoking status (i.e. current smoker, ex-smoker, non-smoker), drinking status (i.e. current drinker, non-drinker), geographic region, epilepsy and prescriptions for painkillers in the 3 months preceding the first-time TASD and 1 month following the first-time TASD. The CCI score was calculated using a list of predefined CPRD Read codes.
A consensus survey was conducted of specialist shoulder surgeons and shoulder physiotherapists who were all members of the British Elbow and Shoulder Society. The saturation point and a list of predictors was reached rapidly and this list is tabulated in Appendix 4. The list highlights the risk factors (and covariates) deemed most important. However, data were only reliably available on the following factors from the list: age, sex, geographic region, deprivation scores, time between first dislocation and surgery.
Missing data
Multiple imputation using chained equations was used to address potential bias and increase precision as a result of missing data on BMI, smoking, drinking and IMD. 24 The imputation equations included all potential factors, including the outcome and length of follow-up time. Fifty imputed data sets were generated and the resulting estimates were combined using Rubin’s rules.
Confounding by indication
In randomised controlled trials, each person has an equal probability of being in the treatment or the control group. Observational study designs, such as the one used for this study, are limited by an inherent imbalance of both known and unknown confounders, making some patients more likely to receive surgery than others. A surgeon typically uses information and risk factors on the patient at baseline to make a decision about whether or not to operate. Whether or not a patient receives surgery is therefore not random in this population-based setting.
As the type of surgery received is not randomly allocated in this study, propensity score matching methods were used to minimise confounding by indication. These propensity score methods were used to achieve comparability of groups with and without the intervention with respect to their observed baseline covariates, thus, controlling for confounding in estimating treatment effects. The use of these methods for the assessment of causality in epidemiological studies has been previously described. 25
The propensity score represents the probability that a patient received the intervention (surgery) conditionally, based on their covariate values. One feature of the propensity score is that it provides balance, so that at each value of the propensity score, the distribution of the covariates (used to define the score) is expected to be similar in the intervention group and non-surgical-intervention group. Comparing patients with the intervention and those without the intervention with the same propensity score gives an unbiased estimate of the effect of treatment.
A logistic equation was fitted for which the outcome was actually the main study exposure (surgical compared with non-surgical intervention) and an agreed list of covariates were introduced as potential confounders of the study outcome. 26,27 All of the covariates described in Covariates were included in the model.
Propensity scores were then used to match each patient receiving surgery to comparable non-surgical controls using a 0.2 standard deviations calliper, as demonstrated in previous simulation studies. 28 Matching was performed without replacement using the MatchIt package in R. 29 Each patient receiving surgery for TASD was matched to three comparable non-surgical controls.
The balance of covariates before and after matching was assessed by calculating the absolute standardised mean difference (SMD) for each covariate. The commonly used boundary for the absolute SMD to indicate acceptable balance is 10%, meaning that a standardised difference of < 10% is considered a good balance. The distribution of propensity scores before and after matching were also judged subjectively for sufficient overlap following matching using density plots.
If the analysis includes participants outside the boundaries of the overlap, this can lead to biased estimates. Participants outside these boundaries will be patients with very high or very low propensity scores. Thus, the best approach is to trim the patients included in the analysis, removing those with extreme propensity scores. This was carried out by calculating 1% of the extremes of the propensity score tails and removing patients outside the limits.
This is a standard method for minimising confounding by indication that not only provides participants with balanced baseline characteristics in both surgical and non-surgical groups, but also eliminates surgical patients with no comparable controls. 30
This methodology is now widely used in pharmacoepidemiology and drug safety, and has both strengths and limitations. The main advantages of propensity score matching are:
-
Exclusion of non-comparable subjects (e.g. non-surgical participants with a very low propensity score who probably have some contraindication or are not fit for surgery and, therefore, should not be compared with those who actually underwent surgical repair).
-
This method produces clearly comparable cohorts in terms of observed confounders and is highly visual and intuitive.
The main disadvantage (when compared with randomised trials) is the lack of adjustment/matching for unobserved confounders. In an observational setting, there is the potential risk that the choice of patient treatment by skilled clinicians is driven by unmeasured patient characteristics and risk factors that are not recorded in the observational data sets. This can affect the precision of the estimate of treatment efficacy and external validity. 31 The performance of a propensity score can be examined for homogeneity at different points on the propensity score scale. If the analysis has worked as anticipated, a similar treatment effect should be observed across the range of propensity score values. Another potential limitation is the potential loss of power if patients cannot be matched as part of the propensity score analysis.
The association between surgery and time to re-dislocation within a 2-year time frame was described using a Cox regression survival model, including the surgical patients and their three matched non-surgical controls. The model was stratified on matched sets, to allow for the correlation between matched pairs of surgical patients and controls. An assumption in the use of the Cox regression model is that of proportional hazards, which was assessed using Schoenfeld Residuals Test. Probability of survival up to 2 years was estimated in the surgical and non-surgical groups using Kaplan–Meier plots.
Immortal time bias
A common issue in epidemiological studies is that of immortal time bias, which describes a form of bias introduced from a period of time when the outcome or event of interest cannot occur by design. It usually occurs in a cohort study with two index dates, when the passing of time from the first date (i.e. inclusion date) to the date when a patient receives the intervention (i.e. exposure/treatment date) is by definition immortal in the exposed group. In the present study, immortal time bias would be introduced in the surgical group, arising in the time following a first dislocation to when they receive surgery, because during this time they cannot have the outcome of interest (otherwise they would have been classified as ‘non-surgical’). Although the patient is not truly ‘immortal’, they had to remain free from re-dislocations prior to receiving surgery, which introduces a bias of offering guaranteed survival time to the surgical group. The surgical patients will be artificially ‘safeguarded’ from having a re-dislocation until their date of surgery. By not being correctly classified, this immortal time would produce an artificial increase in re-dislocations in the non-surgical group, suggesting that surgery has a better outcome. The effects of immortal time bias have been confirmed and quantified by Suissa. 32
To address the problem of immortal time bias, time-varying exposures have been used. In the survival analysis, the time prior to surgery for the surgical group has been reclassified as ‘non-surgical’, and new (‘twin’) patients have been created and added to the ‘non-surgical’ group. This is deemed the best available method for the minimisation of immortal time bias. 33
Results
Descriptive characteristics
A cohort of 3759 patients diagnosed with a first-time TASD during 1 April 1997–26 April 2014 was identified in the CPRD. The CPRD Read codes used to identify these patients can be found in Appendix 5.
An unexpected finding at this stage was that only 4% (n = 156) of the remaining patients had received surgery for their dislocation within 6 months. This was only 15% of the expected number of surgical patients. In addition, an identical proportion of patients (20%) had suffered a re-dislocation in the surgical and non-surgical groups instead of the anticipated 5% difference. Although this finding is useful for this commissioned study, indicating that surgery after one traumatic dislocation is not common in the general NHS population, it also means that, overall, this study was underpowered for the primary question at 6 months. The analysis was conducted as per the approved protocol, but a protocol amendment was added to carry out an additional sensitivity analysis at 12 months.
For the 156 patients who underwent shoulder surgery within 6 months, the OPCS 4.7 surgical codes identified within HES are given in Appendix 6. The descriptive characteristics of patients categorised by receiving surgery within 6 months or no surgery are described in Table 15. In the first 4 years, fewer than five patients underwent surgery per year. This increased from 2001 to a maximum of 18 (out of a total of 156 surgical patients) in 2008. More men (83%) were diagnosed with a primary shoulder dislocation than women, and more men underwent surgery within 6 months (40 men vs. 16 women). The majority of patients were aged 17–21 years at the time of their first-time TASD, but similar numbers were operated on among those aged 18–25 years.
| Characteristic | Patient group, n (%) | ||
|---|---|---|---|
| Whole data set | No surgery | Surgery within 6 months of TASD | |
| Total | 3759 (100) | 3603 (96) | 156 (4) |
| Year of shoulder dislocation | |||
| 1997 | 62 (2) | 61 (2) | 1 (1) |
| 1998 | 106 (3) | 102 (3) | 4 (3) |
| 1999 | 120 (3) | 118 (3) | 2 (1) |
| 2000 | 143 (4) | 139 (4) | 4 (3) |
| 2001 | 192 (5) | 185 (5) | 7 (4) |
| 2002 | 226 (6) | 221 (6) | 5 (3) |
| 2003 | 270 (7) | 262 (7) | 8 (5) |
| 2004 | 276 (7) | 269 (7) | 7 (4) |
| 2005 | 258 (7) | 252 (7) | 6 (4) |
| 2006 | 280 (7) | 270 (7) | 10 (6) |
| 2007 | 303 (8) | 290 (8) | 13 (8) |
| 2008 | 297 (8) | 279 (8) | 18 (12) |
| 2009 | 275 (7) | 261 (7) | 14 (9) |
| 2010 | 294 (8) | 279 (8) | 5 (10) |
| 2011 | 265 (7) | 249 (7) | 16 (10) |
| 2012 | 212 (6) | 197 (5) | 15 (10) |
| 2013 | 159 (4) | 149 (4) | 10 (6) |
| 2014 | 21 (1) | 20 (1) | 1 (1) |
| Sex | |||
| Male | 3115 (83) | 2975 (83) | 140 (90) |
| Female | 644 (17) | 628 (17) | 16 (10) |
| Age at shoulder dislocation (years) | |||
| 16 | 204 (5) | 195 (5) | 9 (6) |
| 17 | 257 (7) | 251 (7) | 6 (4) |
| 18 | 241 (6) | 230 (6) | 11 (7) |
| 19 | 266 (7) | 255 (7) | 11 (7) |
| 20 | 254 (7) | 241 (7) | 13 (8) |
| 21 | 276 (7) | 266 (7) | 10 (6) |
| 22 | 242 (6) | 225 (6) | 17 (11) |
| 23 | 212 (6) | 203 (6) | 9 (6) |
| 24 | 222 (6) | 214 (6) | 8 (5) |
| 25 | 198 (5) | 187 (5) | 11 (7) |
| 26 | 180 (5) | 173 (5) | 7 (4) |
| 27 | 156 (4) | 148 (4) | 8 (5) |
| 28 | 128 (3) | 125 (3) | 3 (2) |
| 29 | 145 (4) | 138 (4) | 7 (4) |
| 30 | 147 (4) | 141 (4) | 6 (4) |
| 31 | 109 (3) | 105 (3) | 4 (3) |
| 32 | 137 (4) | 133 (4) | 4 (3) |
| 33 | 117 (3) | 113 (3) | 4 (3) |
| 34 | 131 (3) | 127 (4) | 4 (3) |
| 35 | 137 (4) | 133 (4) | 4 (3) |
| BMI (kg/m2) | |||
| < 25 | 1285 (34) | 1233 (34) | 52 (33) |
| 25.0–29.9 | 646 (17) | 617 (17) | 29 (19) |
| ≥ 30 | 309 (8) | 296 (8) | 13 (8) |
| Missing | 1519 (40) | 1457 (40) | 62 (40) |
| IMD 2004 (quintile of deprivation) | |||
| 1 (affluent) | 987 (26) | 959 (27) | 28 (18) |
| 2 | 840 (22) | 798 (22) | 42 (27) |
| 3 | 746 (20) | 723 (20) | 23 (15) |
| 4 | 691 (18) | 656 (18) | 35 (22) |
| 5 (deprived) | 492 (13) | 464 (13) | 28 (18) |
| Missing | 3 (< 1) | 3 (0) | 0 (0) |
| Smoking status | |||
| No | 2029 (54) | 1951 (54) | 78 (50) |
| Yes | 1137 (30) | 1086 (30) | 51 (33) |
| Ex-smoker | 314 (8) | 301 (8) | 13 (8) |
| Missing | 279 (7) | 265 (7) | 14 (9) |
| Drinking status | |||
| Yes | 1847 (49) | 1782 (49) | 65 (42) |
| No | 356 (9) | 340 (9) | 16 (10) |
| Missing | 1556 (41) | 1481 (41) | 75 (48) |
| CCI score | |||
| 0 | 3484 (93) | 3338 (93) | 146 (94) |
| 1 | 172 (5) | 166 (5) | 6 (4) |
| 2 | 66 (2) | 64 (2) | 2 (1) |
| ≥ 3 | 37 (1) | 35 (1) | 2 (1) |
| Region | |||
| East Midlands | 114 (3) | 108 (3) | 6 (4) |
| East of England | 444 (12) | 430 (12) | 14 (9) |
| London | 464 (12) | 445 (12) | 19 (12) |
| North East | 96 (3) | 89 (2) | 7 (4) |
| North West | 636 (17) | 603 (17) | 33 (21) |
| South Central | 543 (14) | 521 (14) | 22 (14) |
| South East Coast | 430 (11) | 420 (12) | 10 (6) |
| South West | 454 (12) | 430 (12) | 24 (15) |
| West Midlands | 441 (12) | 424 (12) | 17 (11) |
| Yorkshire and the Humber | 137 (4) | 133 (4) | 4 (3) |
| Epilepsy | 130 (3) | 118 (3) | 12 (8) |
| Painkiller prescriptions | |||
| Prescribed 3 months prior to TASD | 210 (6) | 198 (5) | 12 (8) |
| Prescribed 1 month following TASD | 446 (12) | 420 (12) | 26 (17) |
| Mortality | 22 (1) | 21 (< 1) | 1 (1) |
Only 8% of patients were overweight or obese, with missing data on BMI for 40% of all patients. There was a pattern of decreasing numbers of patients by deprivation quintile, but this pattern was not evident for surgical patients. Only three patients did not have a recorded IMD score. Most patients were non-smokers (54%) and there were missing data on smoking for 7% of all patients. Most patients drank alcohol (49%) and there were missing data on alcohol consumption for 41% of all patients. Most patients had no comorbid conditions (93%). Many patients were from the North West (n = 636) and South Central regions (n = 543), and fewer were from the North East region (n = 96). A small proportion of patients had been diagnosed with epilepsy (3%), but a higher proportion of all surgical patients had been diagnosed with epilepsy (8%). A small proportion (6%) of patients were prescribed painkillers in the 3 months prior to their first-time TASD, which increased to 12% in the 1 month following TASD. Of the patients prescribed painkillers, both 3 months prior to and 1 month after their TASD, only 1% of patients were prescribed a different painkiller. Only 22 patients died during the study period.
Multiply imputed data analyses
Multiple imputation by chained equations was conducted for patients with missing data on BMI, smoking, drinking and IMD. Appendix 7 presents the HRs, 95% CIs and p-values for each of these variables using all available data, a complete-case analysis and the multiply imputed data. The HRs are similar for each group, indicating that the multiple imputation process was successful.
Cox survival estimates for complete cases and the multiply imputed data, with the factors that had an impact on re-dislocations, are presented in Appendix 8. In total, only 1790 cases had complete data on all factors. For these patients, a protective but non-significant adjusted effect of surgery was observed (HR 0.55, 95% CI 0.29 to 1.04; p = 0.068). Year of first-time TASD, younger age at first-time TASD and an epilepsy diagnosis were significant risk factors for a re-dislocation.
Using the multiply imputed data resulted in similar HRs, 95% CIs and the same risk factors. The protective effect of surgery was less marked and remained non-significant (HR 0.76, 95% CI 0.52 to 1.11; p = 0.151).
Propensity score analysis
Logistic regression was used to calculate the propensity scores. The distribution of propensity scores in patients receiving surgery within 6 months and in those who had non-operative treatment are presented in Figure 10. Prior to matching, the propensity scores tended to be higher in the surgery group, but there was a substantial amount of overlap, indicating that it is possible to proceed with propensity score matching to estimate the treatment effect. Following matching, the propensity score density plots are very similar between the surgery and non-surgery patient groups. The propensity score matching does not include the non-surgical patients in the left-hand peak of the distribution.
FIGURE 10.
Propensity scores for first-time TASD patients diagnosed during 1 April 1997–26 April 2014, who had surgery within 6 months or no surgery within CPRD-HES, in England. (a) Prior to PS matching; and (b) following PS matching. PS, propensity score; SD, standard deviation.
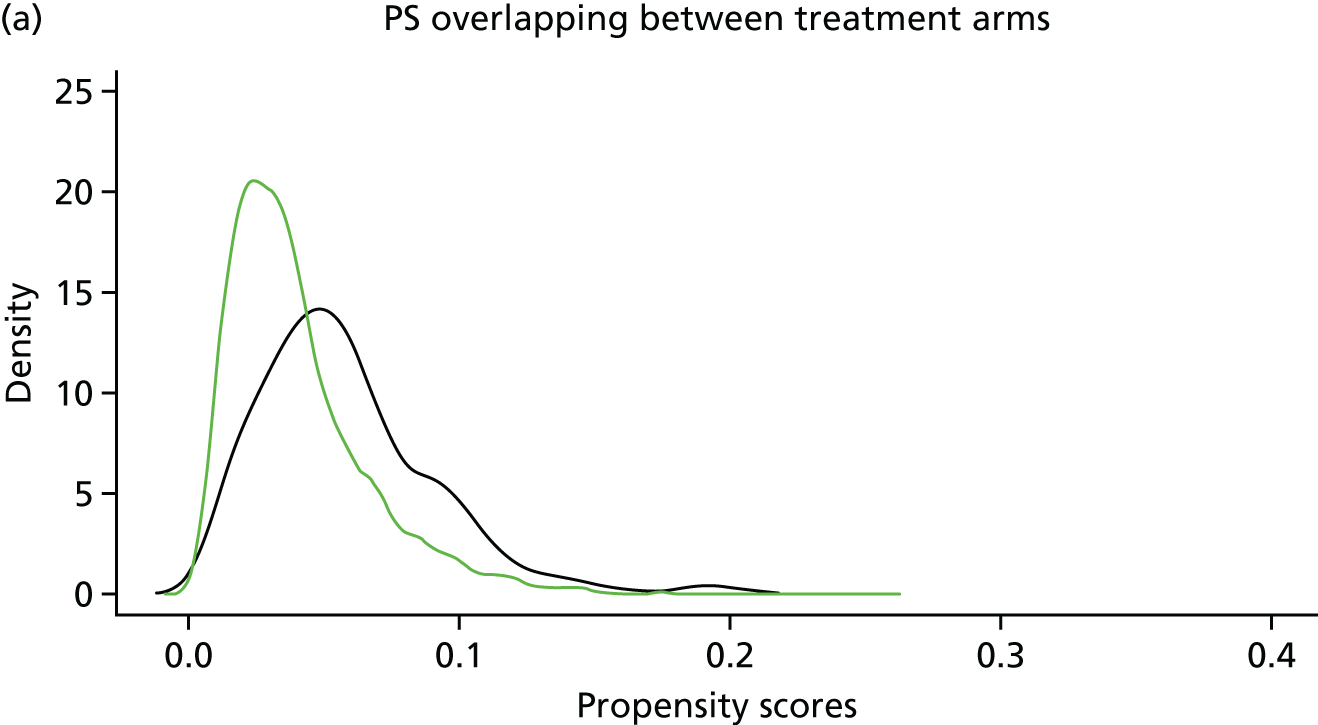
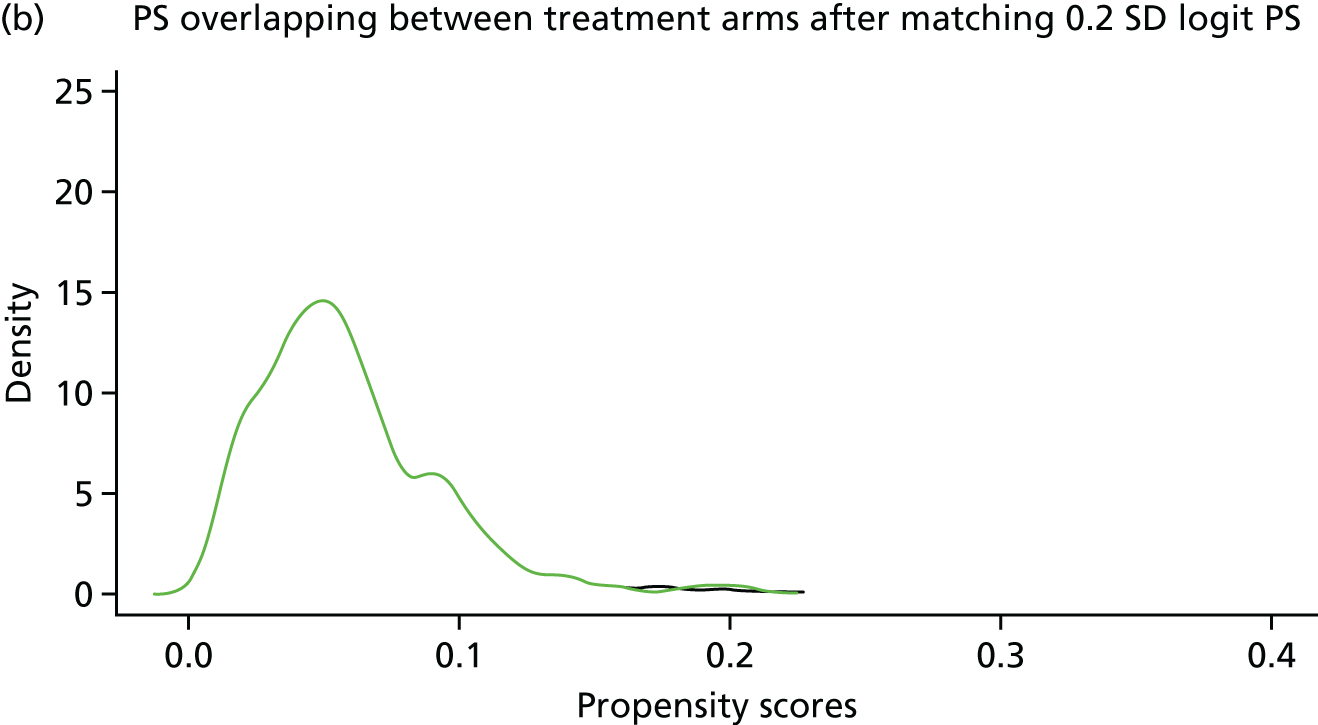
Table 16 presents the baseline characteristics of first-time TASD patients who had surgery or non-operative treatment within 6 months. The SMDs, prior to propensity score matching, show that the two groups of patients do differ for most characteristics (SMD > 0.1). During propensity score matching, each surgical patient (n = 156) was matched to three non-surgical patients (n = 468). In addition to this, the time between the date of the first-time TASD and the date of surgery was allocated to the non-surgical patients for 102 surgical patients, making a total of 570 non-surgical patients. The remaining 54 surgical patients had no time to be re-allocated because the date of their first-time TASD was the same as their date of surgery. Following propensity score matching, the SMDs were much smaller than before (all < 0.1), suggesting that the balancing was successful.
| Characteristic | All eligible primary shoulder dislocation patients (N = 3784)a | Matched analysis (3 : 1): patients matched on PS (N = 726)a,b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (n = 156) | Non-surgery (n = 3603) | SMD | Surgery (n = 156) | Non-surgery (n = 570) | SMD | |||||
| n (%) | Re-dislocation, n (%) | n (%) | Re-dislocation, n (%) | n (%) | Re-dislocation, n (%) | n (%) | Re-dislocation, n (%) | |||
| Re-dislocation | 156 (100) | 31 (20) | 3603 (100) | 731 (20) | 156 (100) | 31 (20) | 570 (100) | 102 (18) | ||
| Calendar year of shoulder dislocation | 156 (100) | 31 (20) | 3603 (100) | 731 (20) | 0.344 | 156 (100) | 31 (20) | 570 (100) | 102 (18) | –0.046 |
| Sex | ||||||||||
| Male | 140 (90) | 26 (19) | 2975 (83) | 618 (21) | –0.209 | 140 (90) | 26 (19) | 500 (88) | 89 (18) | –0.064 |
| Female | 16 (10) | 5 (31) | 628 (17) | 113 (18) | 16 (10) | 5 (31) | 70 (12) | 13 (19) | ||
| Age at shoulder dislocation (16- to 35-year-olds) | 156 (100) | 31 (20) | 3603 (100) | 731 (20) | –0.067 | 156 (100) | 31 (20) | 570 (100) | 102 (18) | 0.016 |
| BMI (kg/m2) | ||||||||||
| < 25 | 86 (55) | 20 (23) | 2159 (60) | 461 (21) | 0.114 | 86 (55) | 20 (23) | 320 (56) | 58 (18) | 0.030 |
| 25.0–29.9 | 44 (28) | 6 (14) | 972 (27) | 170 (17) | 44 (28) | 6 (14) | 162 (28) | 23 (14) | ||
| ≥ 30 | 26 (17) | 5 (19) | 472 (13) | 100 (21) | 26 (17) | 5 (19) | 88 (15) | 21 (24) | ||
| IMD 2004 (quintile of deprivation) | ||||||||||
| 1 (affluent) | 28 (18) | 2 (7) | 961 (27) | 189 (20) | 0.196 | 28 (18) | 2 (7) | 91 (16) | 18 (20) | –0.011 |
| 2 | 42 (27) | 7 (17) | 799 (22) | 168 (21) | 42 (27) | 7 (17) | 169 (30) | 33 (20) | ||
| 3 | 23 (15) | 8 (35) | 723 (20) | 139 (19) | 23 (15) | 8 (35) | 78 (14) | 8 (10) | ||
| 4 | 35 (22) | 8 (23) | 656 (18) | 133 (20) | 35 (22) | 8 (23) | 130 (23) | 21 (16) | ||
| 5 (deprived) | 28 (18) | 6 (21) | 464 (13) | 102 (22) | 28 (18) | 6 (21) | 102 (18) | 22 (22) | ||
| Smoking status | ||||||||||
| No | 85 (54) | 19 (22) | 2090 (58) | 446 (21) | 0.051 | 85 (54) | 19 (22) | 324 (57) | 59 (18) | 0.056 |
| Yes | 57 (37) | 10 (18) | 1184 (33) | 222 (19) | 57 (37) | 10 (18) | 202 (35) | 36 (18) | ||
| Ex-smoker | 14 (9) | 2 (14) | 329 (9) | 63 (19) | 14 (9) | 2 (14) | 44 (8) | 7 (16) | ||
| Drinking status | ||||||||||
| Yes | 128 (82) | 23 (18) | 2985 (83) | 597 (20) | 0.021 | 128 (82) | 23 (18) | 479 (84) | 80 (17) | 0.053 |
| No | 28 (18) | 8 (29) | 618 (17) | 134 (22) | 28 (18) | 8 (29) | 91 (16) | 22 (24) | ||
| CCI score | ||||||||||
| 0 | 146 (94) | 30 (21) | 3338 (93) | 682 (20) | –0.019 | 146 (94) | 30 (21) | 535 (94) | 96 (18) | 0.010 |
| 1 | 6 (4) | 0 (0) | 166 (5) | 31 (19) | 6 (4) | 0 (0) | 23 (4) | 5 (22) | ||
| 2 | 2 (1) | 0 (0) | 64 (2) | 11 (17) | 2 (1) | 0 (0) | 3 (1) | 0 (0) | ||
| ≥ 3 | 2 (1) | 1 (50) | 35 (1) | 7 (20) | 2 (1) | 1 (50) | 9 (2) | 1 (11) | ||
| Region | ||||||||||
| East Midlands | 6 (4) | 2 (33) | 108 (3) | 27 (25) | –0.024 | 6 (4) | 2 (33) | 24 (4) | 6 (25) | –0.003 |
| East of England | 14 (9) | 2 (14) | 430 (12) | 84 (20) | 14 (9) | 2 (14) | 44 (8) | 11 (25) | ||
| London | 19 (12) | 3 (16) | 445 (12) | 85 (19) | 19 (12) | 3 (16) | 76 (13) | 10 (13) | ||
| North East | 7 (4) | 0 (0) | 89 (2) | 24 (27) | 7 (4) | 0 (0) | 24 (4) | 5 (21) | ||
| North West | 33 (21) | 7 (21) | 603 (17) | 115 (19) | 33 (21) | 7 (21) | 126 (22) | 21 (17) | ||
| South Central | 22 (14) | 2 (9) | 521 (14) | 104 (20) | 22 (14) | 2 (9) | 74 (13) | 14 (19) | ||
| South East Coast | 10 (6) | 5 (50) | 420 (12) | 73 (17) | 10 (6) | 5 (50) | 42 (7) | 4 (10) | ||
| South West | 24 (15) | 7 (29) | 430 (12) | 93 (22) | 24 (15) | 7 (29) | 74 (13) | 10 (14) | ||
| West Midlands | 17 (11) | 3 (18) | 424 (12) | 100 (24) | 17 (11) | 3 (18) | 70 (12) | 18 (26) | ||
| Yorkshire and the Humber | 4 (3) | 0 (0) | 133 (4) | 26 (20) | 4 (3) | 0 (0) | 16 (3) | 3 (19) | ||
| Epilepsy | 12 (8) | 4 (33) | 118 (3) | 38 (32) | 0.195 | 12 (8) | 4 (33) | 42 (7) | 9 (21) | 0.012 |
| Painkiller prescriptions | ||||||||||
| 3 months prior to TASD | 12 (8) | 3 (25) | 198 (5) | 45 (23) | 0.088 | 12 (8) | 3 (25) | 48 (8) | 10 (21) | –0.027 |
| 1 month following TASD | 26 (17) | 7 (27) | 420 (12) | 75 (18) | 0.144 | 26 (17) | 7 (27) | 92 (16) | 18 (20) | 0.014 |
Figure 11 presents Kaplan–Meier estimates of probability of survival in the surgical and non-surgical groups; the hazards were proportional (Schoenfeld Residuals Test; p = 0.39). There appears to be a small survival advantage for surgical patients that is not statistically significant.
FIGURE 11.
Kaplan–Meier survival estimates for first-time TASD patients diagnosed during 1 April 1997–26 April 2014 who had surgery within 6 months or no surgery within CPRD-HES, in England.
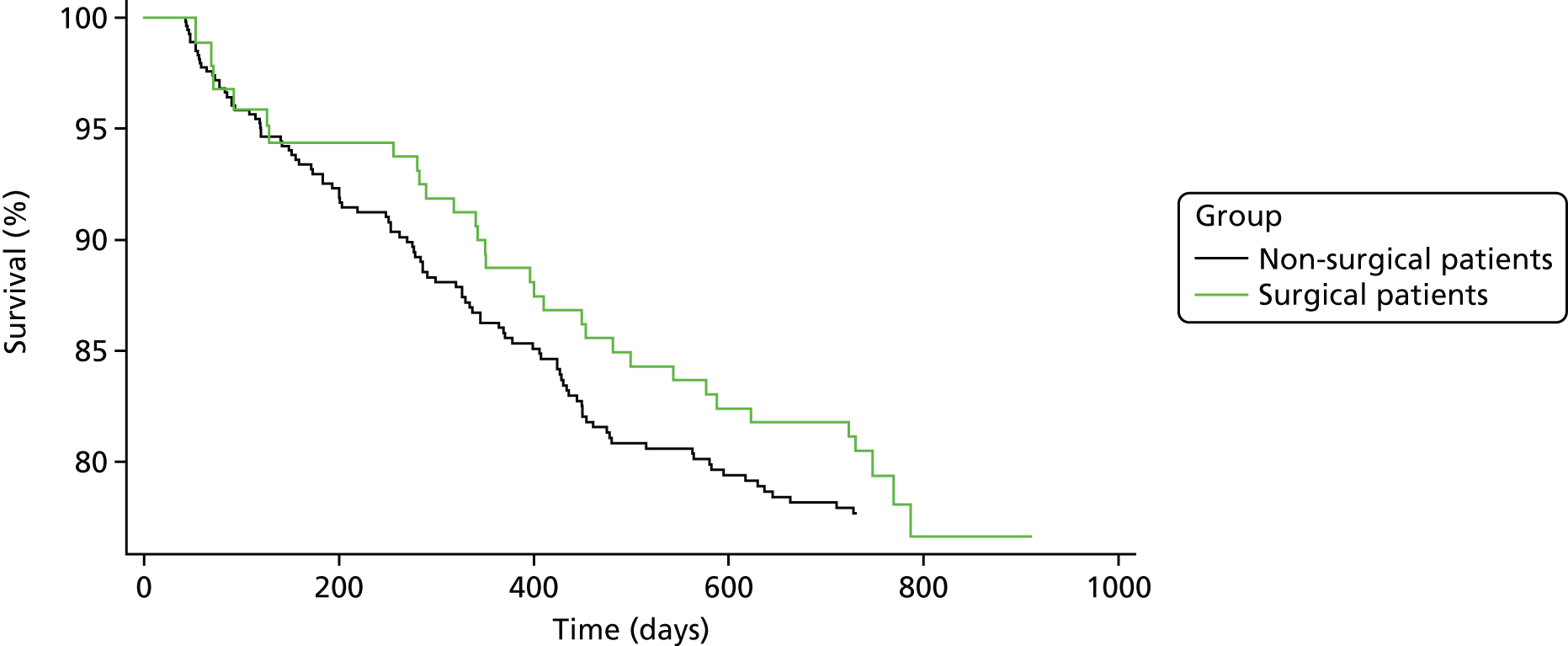
Table 17 presents the final results of the effect of surgery within 6 months compared with no surgery among first-time TASD patients. The median follow-up in both groups was similar and the rate of shoulder re-dislocations was slightly higher in the non-surgical group [0.36 per 1000 person-years (95% CI 0.29 to 0.43 per 1000 person-years) in the non-surgical group compared with 0.30 per 1000 person-years (95% CI 0.21 to 0.43 per 1000 person-years) in the surgical group], although this was not statistically significant as demonstrated by the wide CIs. Overall, the effect of surgery within 6 months appeared slightly protective, at HR 0.88 (95% CI 0.58 to 1.35; p = 0.565), but this was not statistically significant.
| N | Patient group | HR (95% CI); p-value | HRa (95% CI); p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Surgery | Non-surgery | |||||||
| Events (n) | Median follow-up (days) (IQR) | Rate (per 1000 person-years) (IQR) | Events | Median follow-up (days) (IQR) | Rate (per 1000 person-years) (IQR) | |||
| Shoulder re-dislocations | 31 | 731 (731–815) | 0.30 (0.21–0.43) | 102 | 731 (495–731) | 0.36 (0.29–0.43) | 0.84 (0.55 to 1.29); p = 0.429 | 0.88 (0.58 to 1.35); p = 0.565 |
Discussion
A population-based cohort of 3759 patients diagnosed with a first-time TASD during 1 April 1997–26 April 2014, with CPRD-HES linked records and 2 years of follow-up in England were identified. In total, only 156 patients in this data set received surgical treatment within 6 months of first-time TASD. Thus, despite the commissioned question, our findings conclude that early surgery after only one shoulder dislocation is uncommon in the NHS.
The overall finding from the propensity-score-matched analysis was that although surgery within 6 months appeared to be slightly protective, it was not a statistically significant deterrent for re-dislocations (HR 0.88, 95% CI 0.58 to 1.35; p = 0.565). The wide CI indicates that this study was underpowered. Owing to the unexpected small numbers receiving surgery after only one dislocation and the subsequent loss of study power, we are not able to confirm whether or not surgery within 6 months of a first-time anterior shoulder dislocation has any additional benefit on whether or not a patient suffers a re-dislocation.
This is the first time a large, primary care, national, observational data set has been used to examine the role of surgery on treating shoulder dislocations in England. The main strength of this study is that it uses a population-based cohort using real-world data from linked primary and secondary care databases and these databases are representative of the English population with respect to age and sex. The study also uses the latest statistical methods to account for missing data, confounding by indication and immortal time bias. There was a considerable number of missing data for BMI (40%) and for alcohol consumption (41%); fewer data were missing for smoking (7%) and IMD (n = 3). Data were successfully imputed for these covariates.
Although the number of patients (n = 3759) included in the analysis was greater than the minimum number required for statistical power (n = 3065), disappointingly, the main weakness of the study was the unexpected low number of NHS patients having surgery after one dislocation. In total, only 156 patients had undergone surgical treatment within 6 months of the date of their first-time TASD. The lack of surgical patients in the cohort resulted in an overall lack of statistical power. It was also observed that a substantial number of patients in this cohort of 16- to 35-year-olds had < 2 years of follow-up within the CPRD (n = 854), which may be related to moving location and changing GPs because of going to university or finding jobs.
The main disadvantage of using propensity score matching methods is that confounders for which no data are available result in a lack of adjustment. Other than age and sex, the risk factors recorded and available in the CPRD were considered less important risk factors. Many factors considered important by surgeons, including original cause/mechanism of shoulder dislocation, imaging findings of structural problems, anterior apprehension, occupation, sports played and level of sports, were not recorded in the observational data. This links with the findings later in Chapter 6, Prediction models. Finally, in this cohort, 20% of patients in the surgery and non-surgery group had suffered a shoulder re-dislocation. Responses to the GP questionnaire validation study (reported in Chapter 2) indicate that about one-third of patients suffered a re-dislocation within that CPRD cohort. It is possible that either shoulder re-dislocations have not been reported to GPs or the re-dislocations were not coded in the general practice’s computer system.
Conclusions
Overall, relatively few patients have surgery within 6 months of a first-time TASD in the NHS. This is probably a reflection of many GPs not referring patients with only one dislocation to secondary care and also because of NHS operative waiting times.
This study was underpowered, lacked sufficient follow-up data on many patients and did not include data on many of the important risk factors used by surgeons to make clinical decisions on the best care.
Based on these findings, and in an attempt to maximise the use of this data set to further examine the commissioned question of surgery after first-time shoulder dislocation, a further sensitivity analysis was planned and approved by the HTA programme and Independent Scientific Advisory Committee (ISAC) and is discussed in the following chapter.
Chapter 5 Sensitivity analysis: the impact of surgical treatment within 12 months or no surgery on shoulder re-dislocations in young people aged 16–35 years with first-time traumatic anterior shoulder dislocation in England
In view of the findings in the previous chapter, and to try to maximise the potential conclusions using the data set, a further sensitivity analysis was planned and approved. This analysis included a new sample size power calculation.
Objective of Chapter 5
To study the effect of surgical treatment within 12 months of diagnosis of a first-time episode of TASD on recurrence rates in the 3 years that follow the diagnosis, among young adults in England.
Methods
Study design
A population-based, propensity-score-matched cohort study to control for confounding at baseline has been conducted. Young adults (aged 16–35 years) who presented with a first-time occurrence of TASD were selected from two NHS computerised databases: the CPRD and HES. Figure 12 shows a detailed illustration of the study plan that is described in more detail in the following sections.
FIGURE 12.
The UK TASH-D phase 2 plan design for patients who received surgery or no surgical treatment within 12 months of diagnosis of a first-time TASD during 1 April 1997–31 March 2015, in England.
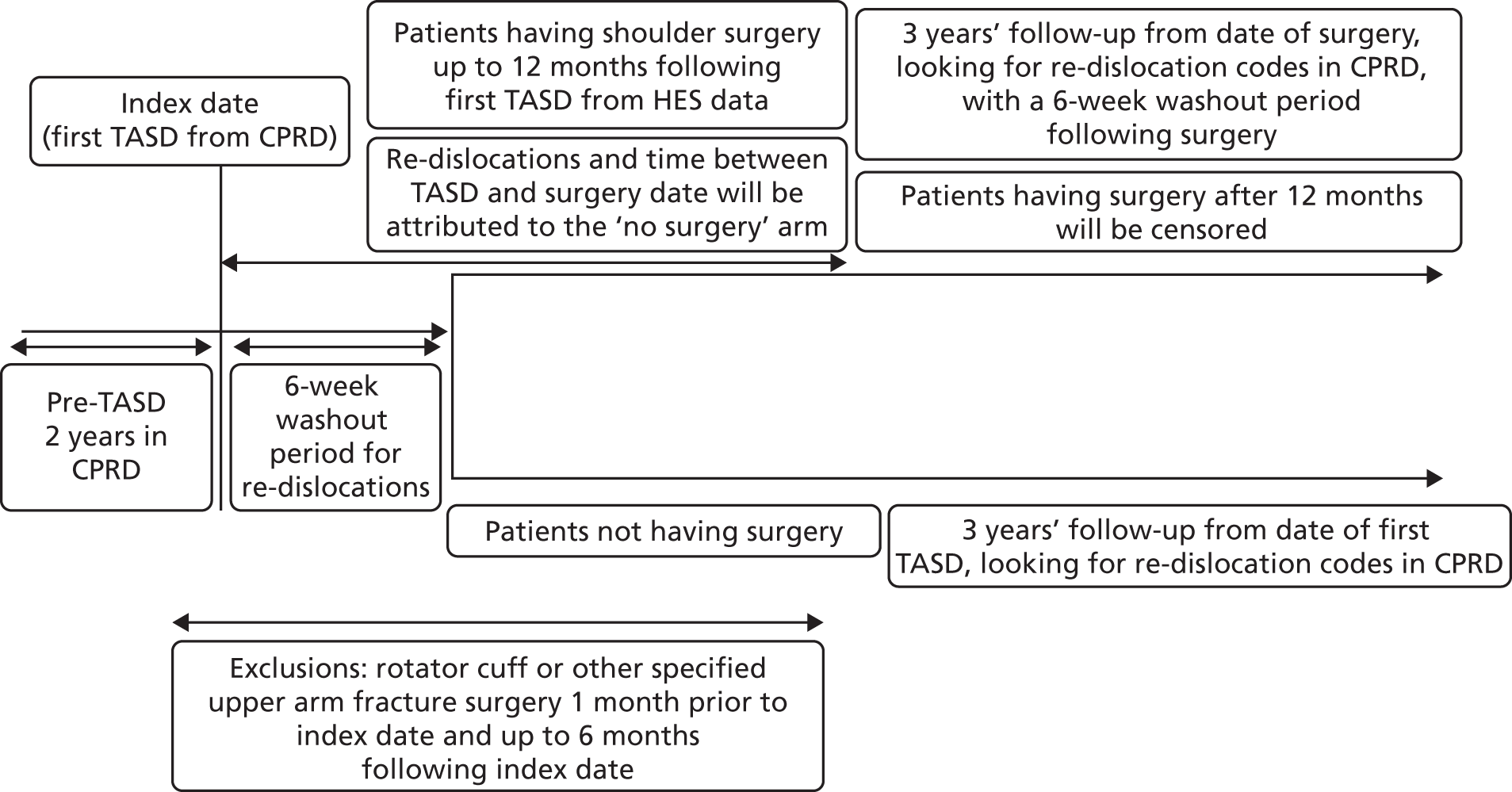
This study used methodology identical to that described in the internal validation study in Chapter 2 and the 6-month analysis in Chapter 4; all participants had to have 2 years of clinical data within the CPRD before their first-time TASD to ensure that only ‘primary’ dislocations had been captured. The same rules were followed and the first Read code entry in the CPRD for shoulder dislocation was defined as the ‘first’ dislocation. All events were collected using the pre-agreed, validated list of CPRD Read codes (see Appendix 1). Patients experienced their first-time TASD between 1 April 1997 and 31 March 2015, allowing up to 3 years of follow-up for each patient to the end of the study on 26 April 2016. As in the previous analysis, a 6-week washout period for re-dislocation codes within the CPRD was applied to all patients following their TASD to avoid duplicate records.
The key changes from the previous analysis described in Chapter 4 were to include:
-
patients having surgery up to 12 months from the date of their first-time TASD
-
patients with any length of follow-up, rather than at least 2 full years
-
follow-up up to 3 years, rather than 2 years.
Surgical group
The surgical group comprised patients in the CPRD with a first-time TASD who underwent shoulder stabilisation surgery after their first dislocation. This meant linking HES data to CPRD data in such a way that a HES surgical OPCS 4.7 code was seen to occur after a single first dislocation code in the CPRD before that surgical date. The timelines between first dislocation codes and OPCS 4.7 codes were recorded. If a re-dislocation occurred prior to their surgery date, the patient was allocated to the non-surgical arm.
A 6-week washout period for re-dislocation codes within the CPRD was applied to all patients in the surgical group following their surgery date to avoid duplicate records. Surgery patients were followed up for up to 3 years from the date of their surgery. Patients having surgery > 6 months following their TASD were censored on their date of surgery. The surgery dates for these patients ranged from 25 December 1997 to 30 September 2015.
Non-surgical group
As in the 6-month analysis, conservative care has been defined as ‘non-surgical intervention’ with no linked OPCS 4.7 surgical shoulder codes, producing a control cohort of patients whose first-time shoulder dislocation has been treated non-operatively. Non-surgical patients were followed up for up to 3 years following the date of their first-time TASD, as illustrated in Figure 12.
Outcome
The outcome was time to a shoulder re-dislocation as defined by the CPRD Read codes given in Appendix 1. For the surgical group, the shoulder re-dislocation can occur between 6 weeks and 2 years following the date of surgery. For the non-surgical group, the shoulder re-dislocation could occur between 6 weeks and 2 years from the date of the first-time TASD.
Any patients who died during the study were censored on their date of death.
Data sources
The analysis utilised two computerised NHS databases: the CPRD and HES, as described in Chapter 4. The linked databases were managed by a senior data manager using Python and SQL, and statistical analyses were conducted using Stata and R.
Sample size
The sample size calculation in the study plan, described fully in the previous chapter, required a total sample size of 3065 patients with 656 re-dislocations, with 90% power at the 5% significance level and allowing for a 10% loss to follow-up, to detect an absolute difference of 5% between the surgical and non-surgical groups. It was assumed that 1073 (35% of the total) patients would receive surgery. The 6-month analysis was underpowered on this basis.
A senior statistician reran the sample size calculation, reducing the power to 80%, having unequal groups (1 : 10 ratio), extending follow-up to 3 years from 2 years and looking at surgical intervention up to 12 months (rather than 6 months). To detect a HR of 0.73 (26% surgical re-dislocations vs. 19.5% non-surgical re-dislocations) would require a total of 3456 patients, of whom 314 were surgical patients and 3142 were non-surgical patients, and at least 695 re-dislocations.
Participants
Inclusion criteria
The CPRD records of 6046 patients aged 16–35 years with 2 years of data in the CPRD before a first-time TASD were identified in the internal validation study (see Table 1) and were linked to HES records.
Exclusion criteria
The following patients were excluded.
-
those aged 16–35 years with a first-time TASD who could not be linked by CPRD-HES
-
those with prior shoulder surgery for a shoulder dislocation
-
those whose instability was treated with rotator cuff repair surgery or fracture surgery prior to or following a TASD.
Exclusions were made in accordance with the criteria above and these are described in Table 18. Following linkage to HES data, there was a linkage loss of 1234 patients and six duplicates were identified and removed. A very small proportion of patients (n = 193) was excluded for having shoulder surgery for prior dislocations, rotator cuff tears or fractures.
| Exclusions | Excluded (n) | Total (n) | % |
|---|---|---|---|
| Eligible patients in the CPRD cohort for the internal validation study following exclusions made in Table 1 | 6046 | 100 | |
| HES data linkage loss | 1234 | 20 | |
| Duplicate removal | 6 | < 1 | |
| Prior shoulder surgery for a shoulder dislocation | 155 | 3 | |
| Surgery for rotator cuff tear prior to TASD | 1 | < 1 | |
| Surgery for rotator cuff tear 6 months following TASD | 4 | < 1 | |
| Surgery for a shoulder fracture prior to TASD | 14 | < 1 | |
| Surgery for shoulder fracture in the 6 months following TASD | 19 | < 1 | |
| Total exclusions | 1433 | 24 | |
| Patients included in subsequent analyses | 4613 | 76 |
In total, 4613 patients remained available for analysis, which was greater than the minimum number of patients (n = 3456) required for a sufficient sample size at 80% power (Figure 13). Of these, 342 were surgical patients (slightly more than the minimum required, n = 314) and 4271 were non-surgical patients (much greater than the minimum required, n = 3142). Re-dislocations were observed in 912 patients (much greater than the minimum required, n = 695). Among the surgical group, 18% of patients suffered a re-dislocation, and 20% of patients suffered a re-dislocation in the non-surgical group. The difference in re-dislocation proportions between the surgical and non-surgical groups was only 2%, rather than the 5% difference used in the power calculation, which means that the power is reduced. However, overall, this study of surgical intervention up to 12 months on shoulder re-dislocations should have had sufficient power.
FIGURE 13.
Shoulder dislocation exclusions flow chart for patients aged 16–35 years during 1 April 1997–31 March 2016 within CPRD in England, with linkage to HES records.
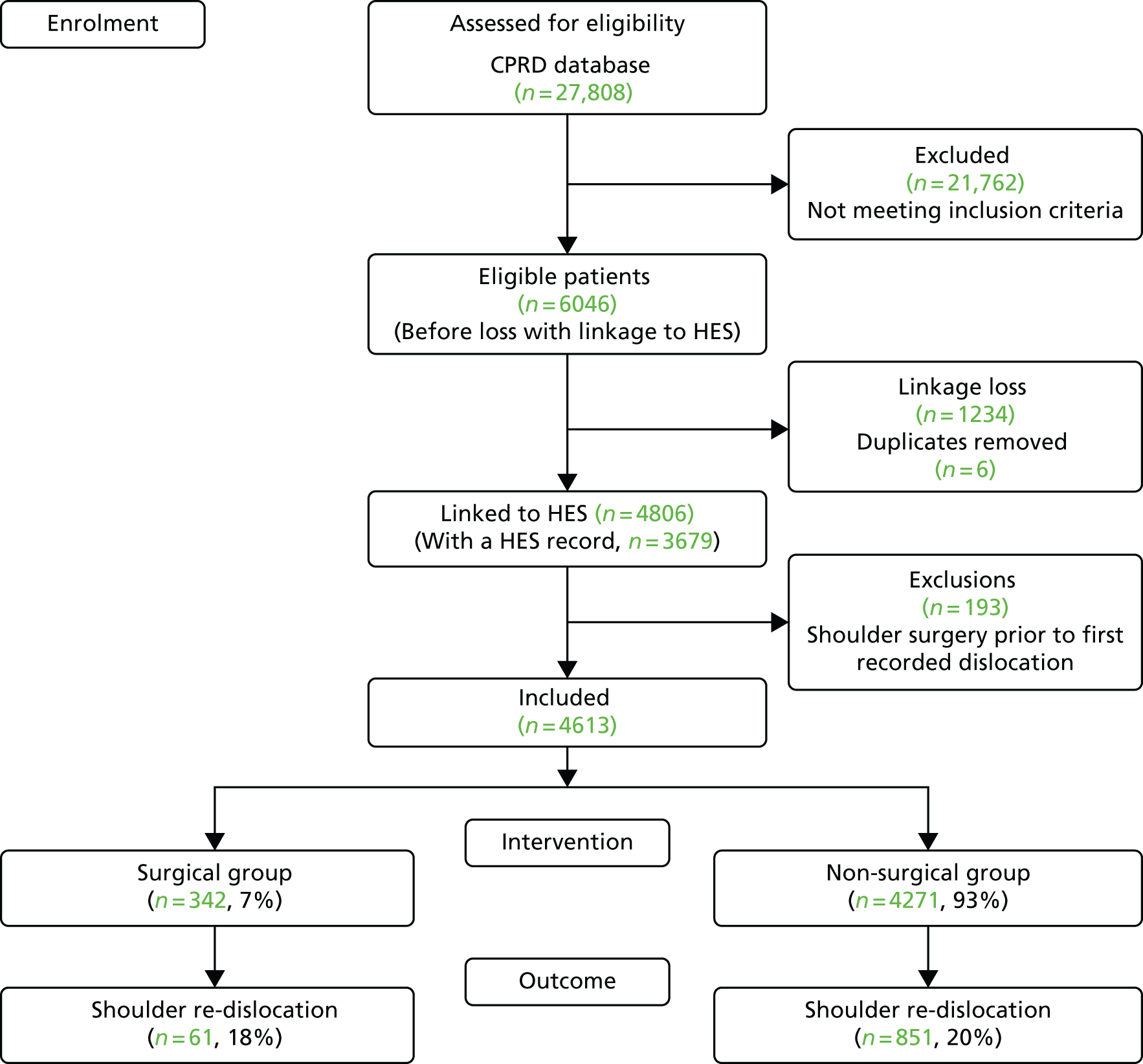
Statistical analysis
The statistical analysis used for this sensitivity analysis was identical to that described in Chapter 4, with respect to covariates, missing data, confounding by indication and immortal time bias.
Results
Descriptive characteristics
A cohort of 4613 patients diagnosed with a first-time TASD during 1 April 1997–31 March 2015 was identified in the CPRD. The CPRD Read codes used to identify these patients are listed in Appendix 9. Of these patients, 342 underwent shoulder surgery within 6 months. The OPCS 4.7 surgical codes identified within HES are given in Appendix 10.
The descriptive characteristics of patients categorised by receiving surgery within 12 months or no surgery are described in Table 19. In the first 4 years, < 10 patients underwent surgery per year, but this increased from 2001 to 2010 to a maximum of 34 patients out of a total of 342 surgical patients. More men (82%) were diagnosed with a primary shoulder dislocation than women, and more men underwent surgery within 12 months (n = 302 men vs. n = 40 women). The majority of patients were aged 17–21 years at the time of their first-time TASD, but similar numbers were operated on among those aged 18–25 years.
| Characteristic | Patient group, n (%) | ||
|---|---|---|---|
| Whole data set | No surgery | Surgery within 12 months of TASD | |
| Total | 4613 (100) | 4271 (93) | 342 (7) |
| Year of shoulder dislocation | |||
| 1997 | 65 (1) | 64 (1) | 1 (< 1) |
| 1998 | 115 (2) | 109 (3) | 6 (2) |
| 1999 | 128 (3) | 125 (3) | 3 (1) |
| 2000 | 161 (3) | 155 (4) | 6 (2) |
| 2001 | 209 (5) | 199 (5) | 10 (3) |
| 2002 | 250 (5) | 239 (6) | 11 (3) |
| 2003 | 309 (7) | 288 (7) | 21 (6) |
| 2004 | 301 (7) | 290 (7) | 11 (3) |
| 2005 | 288 (6) | 271 (6) | 17 (5) |
| 2006 | 315 (7) | 292 (7) | 23 (7) |
| 2007 | 334 (7) | 308 (7) | 26 (8) |
| 2008 | 339 (7) | 306 (7) | 33 (10) |
| 2009 | 331 (7) | 303 (7) | 28 (8) |
| 2010 | 348 (8) | 314 (7) | 34 (10) |
| 2011 | 329 (7) | 301 (7) | 28 (8) |
| 2012 | 293 (6) | 261 (6) | 32 (9) |
| 2013 | 237 (5) | 209 (5) | 28 (8) |
| 2014 | 217 (5) | 195 (5) | 22 (6) |
| 2015 | 44 (1) | 42 (1) | 2 (1) |
| Sex | |||
| Male | 3794 (82) | 3492 (82) | 302 (88) |
| Female | 819 (18) | 779 (18) | 40 (12) |
| Age at shoulder dislocation (years) | |||
| 16 | 245 (5) | 225 (5) | 20 (6) |
| 17 | 309 (7) | 294 (7) | 15 (4) |
| 18 | 308 (7) | 281 (7) | 27 (8) |
| 19 | 324 (7) | 293 (7) | 31 (9) |
| 20 | 295 (6) | 271 (6) | 24 (7) |
| 21 | 315 (7) | 298 (7) | 17 (5) |
| 22 | 290 (6) | 260 (6) | 30 (9) |
| 23 | 258 (6) | 242 (6) | 16 (5) |
| 24 | 277 (6) | 262 (6) | 15 (4) |
| 25 | 246 (5) | 216 (5) | 30 (9) |
| 26 | 230 (5) | 212 (5) | 18 (5) |
| 27 | 207 (4) | 190 (4) | 17 (5) |
| 28 | 165 (4) | 156 (4) | 9 (3) |
| 29 | 177 (4) | 159 (4) | 18 (5) |
| 30 | 178 (4) | 169 (4) | 9 (3) |
| 31 | 148 (3) | 137 (3) | 11 (3) |
| 32 | 164 (4) | 152 (4) | 12 (4) |
| 33 | 149 (3) | 143 (3) | 6 (2) |
| 34 | 161 (3) | 152 (4) | 9 (3) |
| 35 | 167 (4) | 159 (4) | 8 (2) |
| BMI (kg/m2) | |||
| < 25 | 1542 (33) | 1439 (34) | 103 (30) |
| 25.0–29.9 | 763 (17) | 708 (17) | 55 (16) |
| ≥ 30 | 364 (8) | 338 (8) | 26 (8) |
| Missing | 1944 (42) | 1786 (42) | 158 (46) |
| IMD 2004 (quintile of deprivation) | |||
| 1 (affluent) | 1241 (27) | 1173 (27) | 68 (20) |
| 2 | 1028 (22) | 949 (22) | 79 (23) |
| 3 | 915 (20) | 846 (20) | 69 (20) |
| 4 | 834 (18) | 752 (18) | 82 (24) |
| 5 (deprived) | 592 (13) | 548 (13) | 44 (13) |
| Missing | 3 (< 1) | 3 (< 1) | 0 (0) |
| Smoking status | |||
| No | 2461 (53) | 2287 (54) | 174 (51) |
| Yes | 1335 (29) | 1233 (29) | 102 (30) |
| Ex-smoker | 392 (8) | 362 (8) | 30 (9) |
| Missing | 425 (9) | 389 (9) | 36 (11) |
| Drinking status | |||
| Yes | 2172 (47) | 2033 (48) | 139 (41) |
| No | 432 (9) | 403 (9) | 29 (8) |
| Missing | 2009 (44) | 1835 (43) | 174 (51) |
| CCI score | |||
| 0 | 4275 (93) | 3945 (92) | 330 (96) |
| 1 | 205 (4) | 199 (5) | 6 (2) |
| 2 | 84 (2) | 80 (2) | 4 (1) |
| ≥ 3 | 49 (1) | 47 (1) | 2 (1) |
| Region | |||
| East Midlands | 152 (3) | 142 (3) | 10 (3) |
| East of England | 549 (12) | 522 (12) | 27 (8) |
| London | 560 (12) | 520 (12) | 40 (12) |
| North East | 113 (2) | 104 (2) | 9 (3) |
| North West | 743 (16) | 669 (16) | 74 (22) |
| South Central | 669 (15) | 628 (15) | 41 (12) |
| South East Coast | 537 (12) | 502 (12) | 35 (10) |
| South West | 580 (13) | 524 (12) | 56 (16) |
| West Midlands | 525 (11) | 488 (11) | 37 (11) |
| Yorkshire and the Humber | 185 (4) | 172 (4) | 13 (4) |
| Epilepsy | 156 (3) | 134 (3) | 22 (6) |
| Painkiller prescriptions | |||
| Prescribed 3 months prior to TASD | 257 (6) | 235 (6) | 22 (6) |
| Prescribed 1 month following TASD | 542 (12) | 499 (12) | 43 (13) |
| Mortality | 22 (< 1) | 20 (< 1) | 2 (< 1) |
Only 8% of patients were overweight or obese, with missing data on BMI for 42% of all patients. There was a pattern of decreasing numbers of patients by deprivation quintile, but this pattern was not evident for surgical patients. Only three patients did not have a recorded IMD score. Most patients were non-smokers (53%) and there were missing data on smoking for 9% of all patients. Most patients drank alcohol (47%) and there were missing data on alcohol consumption for 44% of all patients. Most patients had no comorbid conditions (93%). Many patients were from the North West (n = 743) and South Central regions (n = 669), and fewer were from the North East region (n = 113). A small proportion of patients had been diagnosed with epilepsy (3%), but a higher proportion of all surgical patients had been diagnosed with epilepsy (6%). A small proportion (6%) of patients were prescribed painkillers in the 3 months prior to their first-time TASD, which increased to 12% in the 1 month following a TASD. Of the patients prescribed painkillers, both 3 months prior to and 1 month after their TASD, only 1% were prescribed a different painkiller. Only 22 patients died during the study period.
Multiply imputed data analyses
Multiple imputation by chained equations was conducted for patients with missing data on BMI, smoking, drinking and IMD. Appendix 11 presents the HRs, 95% CIs and p-values for each of these variables using all available data, a complete-case analysis and the multiply imputed data. The HRs are similar for each group, indicating that the multiple imputation process was successful.
Cox survival estimates for complete cases and the multiply imputed data with the factors that had an impact on re-dislocations are presented in Appendix 12. Only 2100 cases had complete data on all factors. For these patients, a protective but non-significant adjusted effect of surgery was observed (HR 0.63, 95% CI 0.40 to 1.00; p = 0.052). A younger age at first-time TASD and an epilepsy diagnosis were significant risk factors for a re-dislocation.
Using the multiply imputed data resulted in similar HRs, 95% CIs and the same risk factors. The protective effect of surgery was less marked but was significant (HR 0.73, 95% CI 0.55 to 0.95; p = 0.022).
Propensity score analysis
Logistic regression was used to calculate the propensity scores. The distributions of propensity scores in the patients receiving surgery within 12 months and in those patients who had non-operative treatment are presented in Figure 14. Prior to matching, the propensity scores tended to be higher in the surgery group, but there is a substantial amount of overlap, indicating that it is possible to proceed with propensity score matching to estimate the treatment effect. Following matching, the propensity score density plots are very similar between the surgery and non-surgery patient groups. The propensity score matching will not include the non-surgical patients in the left-hand peak of the distribution.
FIGURE 14.
Propensity scores for first-time TASD patients diagnosed during 1 April 1997–31 March 2015 who had surgery within 12 months (black) or no surgery (green) within CPRD-HES, in England. (a) Prior to PS matching; and (b) following PS matching. PS, propensity score; SD, standard deviation.

Table 20 presents the baseline characteristics of first-time TASD patients who had surgery within 12 months or non-operative treatment. The SMDs, prior to propensity score matching, show that the two groups of patients do differ for most characteristics (SMD > 0.1). During propensity score matching, 295 surgical patients were each matched to 10 non-surgical patients (n = 2950). In addition to this, the time between the date of the first-time TASD and the date of surgery was allocated to the non-surgical patients for 233 surgical patients, making a total of 3183 non-surgical patients. The remaining 62 surgical patients had no time to be re-allocated because the date of their first-time TASD was the same date as their surgery. Following propensity score matching, the SMDs were much smaller than before (all < 0.1), which suggested that the balancing was successful.
| Characteristic | All eligible primary shoulder dislocation patients (N = 4613)a | Matched analysis (10 : 1): patients matched on propensity score (N = 3478)a,b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (n = 342) | Non-surgery (n = 4271) | SMD | Surgery (n = 295) | Non-surgery (n = 3183) | SMD | |||||
| n (%) | Re-dislocation (%) | n (%) | Re-dislocation (%) | n (%) | Re-dislocation (%) | n (%) | Re-dislocation (%) | |||
| Re-dislocation | 342 (100) | 61 (18) | 4271 (100) | 851 (20) | 295 (100) | 58 (20) | 3183 (100) | 590 (19) | ||
| Calendar year of shoulder dislocation | 342 (100) | 61 (18) | 4271 (100) | 851 (20) | 0.347 | 295 (100) | 58 (20) | 3183 (100) | 590 (19) | 0.011 |
| Sex | ||||||||||
| Male | 302 (88) | 52 (17) | 3492 (82) | 720 (21) | –0.184 | 258 (87) | 50 (19) | 2770 (87) | 528 (19) | –0.013 |
| Female | 40 (12) | 9 (23) | 779 (18) | 131 (17) | 37 (13) | 8 (22) | 413 (13) | 62 (15) | ||
| Age at shoulder dislocation (16- to 35-year-olds) | 342 (100) | 61 (18) | 4271 (100) | 851 (20) | –0.083 | 295 (100) | 58 (20) | 3183 (100) | 590 (19) | –0.019 |
| BMI (kg/m2) | ||||||||||
| < 25 | 184 (54) | 35 (19) | 2506 (59) | 523 (21) | 0.035 | 166 (56) | 34 (20) | 1835 (58) | 367 (20) | 0.013 |
| 25.0–29.9 | 106 (31) | 21 (20) | 1191 (28) | 231 (19) | 92 (31) | 19 (21) | 934 (29) | 163 (17) | ||
| ≥ 30 | 52 (15) | 5 (10) | 574 (13) | 97 (17) | 37 (13) | 5 (14) | 414 (13) | 60 (14) | ||
| IMD (quintile of deprivation) | ||||||||||
| 1 (affluent) | 68 (20) | 6 (9) | 1173 (27) | 225 (19) | 0.153 | 61 (21) | 6 (10) | 814 (26) | 144 (18) | 0.058 |
| 2 | 79 (23) | 14 (18) | 950 (22) | 201 (21) | 72 (24) | 14 (20) | 690 (22) | 146 (21) | ||
| 3 | 69 (20) | 17 (25) | 846 (20) | 158 (19) | 55 (19) | 15 (27) | 626 (20) | 104 (17) | ||
| 4 | 82 (24) | 13 (16) | 754 (18) | 150 (20) | 72 (24) | 13 (18) | 599 (19) | 102 (17) | ||
| 5 (deprived) | 44 (13) | 11 (25) | 548 (13) | 117 (21) | 35 (12) | 10 (29) | 454 (14) | 94 (26) | ||
| Smoking status | ||||||||||
| No | 197 (58) | 39 (20) | 2521 (59) | 528 (21) | –0.010 | 177 (60) | 37 (21) | 1888 (59) | 365 (19) | –0.002 |
| Yes | 112 (33) | 17 (15) | 1356 (32) | 252 (19) | 90 (31) | 16 (18) | 1011 (32) | 176 (17) | ||
| Ex-smoker | 33 (10) | 5 (15) | 394 (9) | 71 (18) | 28 (10) | 5 (18) | 284 (9) | 49 (17) | ||
| Drinking status | ||||||||||
| Yes | 280 (82) | 44 (16) | 3506 (82) | 677 (19) | 0.091 | 237 (80) | 42 (18) | 2541 (80) | 473 (19) | –0.013 |
| No | 62 (18) | 17 (27) | 765 (18) | 174 (23) | 58 (20) | 16 (28) | 642 (20) | 117 (18) | ||
| CCI score | ||||||||||
| 0 | 330 (96) | 60 (18) | 3945 (92) | 792 (20) | –0.146 | 283 (96) | 57 (20) | 3066 (96) | 567 (18) | 0.014 |
| 1 | 6 (2) | 0 (0) | 199 (5) | 37 (19) | 6 (2) | 0 (0) | 55 (2) | 15 (27) | ||
| 2 | 4 (1) | 0 (0) | 80 (2) | 15 (19) | 4 (1) | 0 (0) | 41 (1) | 5 (12) | ||
| ≥ 3 | 2 (1) | 1 (50) | 47 (1) | 7 (15) | 2 (< 1) | 1 (50) | 21 (< 1) | 3 (14) | ||
| Region | ||||||||||
| East Midlands | 10 (3) | 2 (20) | 142 (3) | 30 (21) | 0.075 | 10 (3) | 2 (20) | 103 (3) | 20 (19) | –0.012 |
| East of England | 27 (8) | 5 (19) | 522 (12) | 98 (19) | 26 (9) | 5 (19) | 268 (8) | 46 (17) | ||
| London | 40 (12) | 6 (15) | 520 (12) | 102 (20) | 37 (13) | 6 (16) | 396 (12) | 70 (18) | ||
| North East | 9 (3) | 3 (33) | 104 (2) | 26 (25) | 9 (3) | 3 (33) | 81 (3) | 18 (22) | ||
| North West | 74 (22) | 14 (19) | 669 (16) | 129 (19) | 53 (18) | 12 (23) | 592 (19) | 108 (18) | ||
| South Central | 41 (12) | 2 (5) | 628 (15) | 132 (21) | 36 (12) | 2 (6) | 433 (14) | 82 (19) | ||
| South East Coast | 35 (10) | 9 (26) | 502 (12) | 84 (17) | 34 (12) | 9 (26) | 359 (11) | 63 (18) | ||
| South West | 56 (16) | 10 (18) | 524 (12) | 110 (21) | 47 (16) | 9 (19) | 459 (14) | 88 (19) | ||
| West Midlands | 37 (11) | 7 (19) | 488 (11) | 110 (23) | 33 (11) | 7 (21) | 367 (12) | 76 (21) | ||
| Yorkshire and the Humber | 13 (4) | 3 (23) | 172 (4) | 30 (17) | 10 (3) | 3 (30) | 125 (4) | 19 (15) | ||
| Epilepsy | 22 (6) | 8 (36) | 134 (3) | 42 (31) | 0.155 | 10 (3) | 5 (50) | 128 (4) | 38 (30) | –0.033 |
| Painkiller prescriptions | ||||||||||
| 3 months prior to TASD | 22 (6) | 5 (23) | 235 (6) | 56 (24) | 0.039 | 15 (5) | 5 (33) | 181 (6) | 42 (23) | –0.027 |
| 1 month following TASD | 43 (13) | 10 (23) | 499 (12) | 89 (18) | 0.027 | 31 (11) | 9 (29) | 353 (11) | 57 (16) | –0.019 |
Figure 15 presents Kaplan–Meier estimates of probability of survival in the surgical and non-surgical groups. The hazards were not proportional as shown by the two lines crossing at approximately 750 days and by the Schoenfeld’s Residuals Test (p = 0.003). We estimated time-varying hazards, which showed that there was no effect in the first year of follow-up (p = 0.458). This means that, initially, surgical patients had a similar rate of re-dislocations to non-surgical patients. Between 1 and 3 years of follow-up, the non-surgical group was more likely to re-dislocate (p = 0.022). Overall, there appears to be a small survival advantage for surgical patients, but this is not statistically significant.
FIGURE 15.
Kaplan–Meier survival estimates for first-time TASD patients diagnosed during 1 April 1997–31 March 2015 who had surgery within 12 months (green) or no surgery (black) within CPRD-HES, in England.
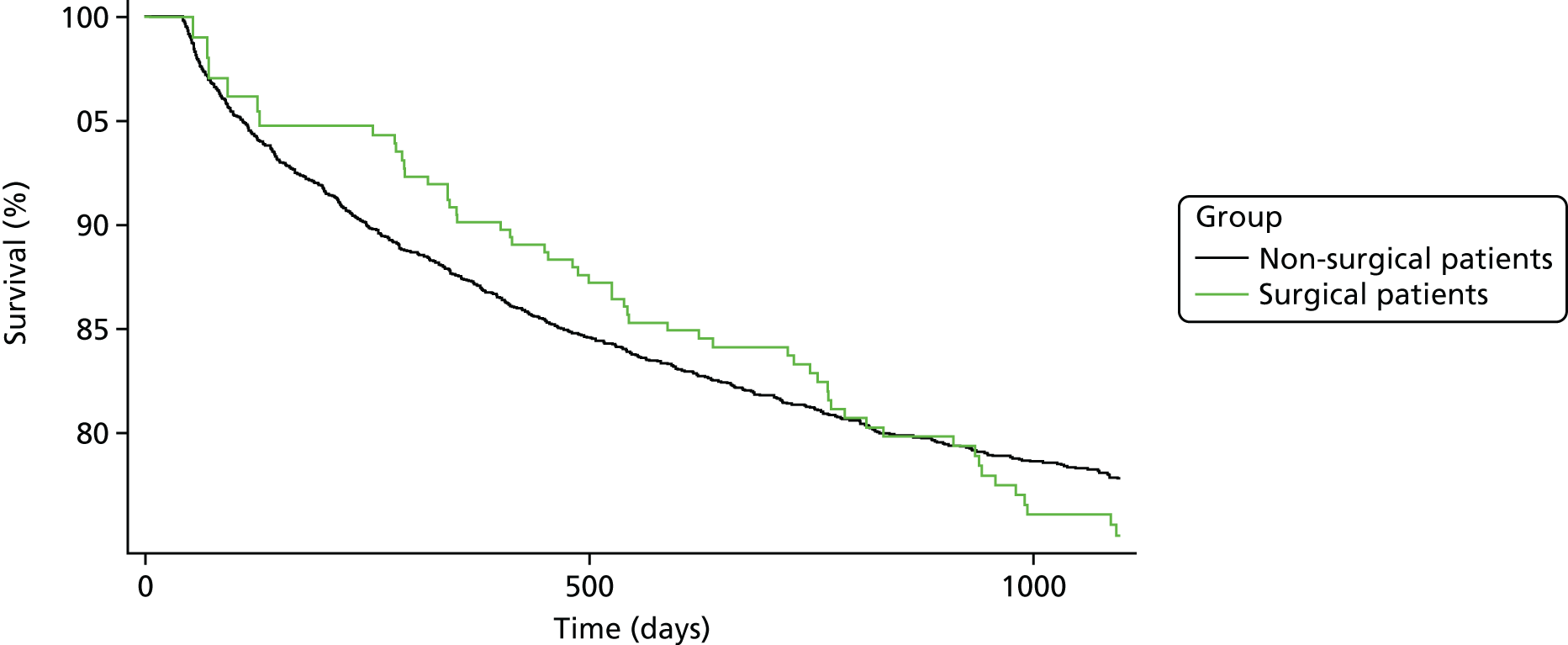
Table 21 presents the final results of the effect of surgery within 12 months compared with no surgery among first-time TASD patients. The median follow-up was less in the non-surgical group. The rate of shoulder re-dislocations was similar between the surgical and non-surgical groups (0.26 per 1000 person-years, 95% CI 0.20 to 0.33 per 100,000 person-years, compared with 0.26 per 1000 person-years, 95% CI 0.24 to 0.28 per 100,000 person-years, respectively). Overall, there was no difference between the effect of surgery and non-surgery within 12 months on shoulder re-dislocations (HR 1.17, 95% CI 0.88 to 1.55; p = 0.274).
| N | Patient group | HR (IQR) | HRa (IQR) | |||||
|---|---|---|---|---|---|---|---|---|
| Surgery | Non-surgery | |||||||
| Events (n) | Median follow-up (days) (IQR) | Rate (per 1000 person-years) (IQR) | Events (n) | Median follow-up (days) (IQR) | Rate (per 1000 person-years) (IQR) | |||
| Shoulder re-dislocations | 58 | 1096 (558–1214) | 0.26 (0.20–0.33) | 590 | 1028 (430–1096) | 0.26 (0.24–0.28) | 1.18 (0.89–1.56) | 1.17 (0.88–1.55) |
To test for residual confounding, the patients were split into quintiles, based on the value of the propensity score, and HRs were produced for each quintile (see Appendix 13). The numbers of surgeries were equal in each quintile. HRs were found to differ between quintiles, suggesting that there was unmeasured confounding in the study consistent with the a priori risk factors not available in the CPRD or HES. Quintile 5 was found to have a twofold increase in the risk of dislocation after surgery (HR 2.07, 95% CI 1.23 to 3.46). Some key differences between the characteristics of patients within each stratum are shown in Appendix 14. Quintile 5, for instance, included more men, people who had a more recent first-time TASD, fewer alcohol drinkers and more people with epilepsy than the other quintiles.
Discussion
A population-based cohort of 4613 patients diagnosed with a first-time TASD during 1 April 1997–31 March 2015 with CPRD-HES linked records and up to 3 years of follow-up in England were identified. Only 342 patients in this data set received surgical treatment within 12 months of a first-time TASD. This again confirms that, in the NHS, even when extending surgery to 12 months, it is still an uncommon treatment after only one shoulder dislocation. However, extending to 12 months for this sensitivity analysis did provide some more power to the analysis, even though 47 surgical patients were not matched to controls in the propensity score analysis. On this occasion, non-proportionality of the HRs was observed, which suggests that further, more complex, statistical techniques could be considered; however, the issues of small patient numbers in the surgical group, similar proportions of re-dislocations and residual confounding mean that further analysis is unlikely to change any conclusions.
The main finding from this further propensity-score-matched analysis was that surgery within 12 months of a first-time TASD had no obvious beneficial effect compared with non-surgical interventions (HR 1.17, 95% CI 0.88 to 1.55; p = 0.274). The lack of association meant that adjusting for clustering at the general practice level and conducting a Rosenbaum bounds sensitivity analysis was inappropriate. However, when the propensity scores were split into quintiles, one quintile in particular (5) was found to be at a significantly increased risk of shoulder re-dislocation. The fact that one quintile had a different risk of the outcome is highly suggestive of the existence of residual confounding. This quintile included more men, people who had their first-time TASD more recently and fewer alcohol drinkers and had most of the people with epilepsy. So although the study now has more power, residual confounding continues. The confounders are likely to be contained within the a priori list of important risk factors, used by surgeons to make clinical decisions on the best care, that could not be identified from the data, and so these confounders could not be taken into account during the propensity scoring.
Chapter 6 Prediction modelling
Introduction
This chapter describes the development and internal validation of a model to predict the risk of re-dislocation. Prediction models using routinely collected data from primary care were developed in the surgical and non-surgical cohorts separately, using the CPRD-HES linked data set.
Methods
Sample
The collated sample comprised patients aged 16–35 years with a first-time TASD who had at least 2 years of data (washout period) before a first-time entry Read code for shoulder dislocation and with up to 3 years of follow-up coding, who were registered at ‘active’ general practices in England (see Figure 11).
Definition of the primary outcome
The primary outcome was re-dislocation following a first-time TASD. We determined an entry date for each participant, which was the date of surgery for the surgical cohort and the date of first shoulder dislocation for the non-surgical cohort. Observation time was calculated from the entry date to an exit date, which was defined as the earliest date of recorded re-dislocation or 3 years after the index date.
Candidate predictors
Potential predictors of re-dislocation were defined a priori by expert consensus and informed by the validation study (see Appendix 4). Only eight of these predictors were available in the CPRD and were used as candidate predictors in the multivariable prediction model (Table 22). Owing to the sparseness of the CCI score in the surgical cohort, it was not considered in the model building in this cohort.
| Candidate predictors (surgical patients) | Candidate predictors (non-surgical patients) |
|---|---|
| Age (years) | Age (years) |
| Sex | Sex |
| BMI (kg/m2) | BMI (kg/m2) |
| Smoking status (non-smoker, current smoker, ex-smoker) | Smoking status (non-smoker, current smoker, ex-smoker) |
| Alcohol consumption (reference: non-drinker) | Alcohol consumption (reference: non-drinker) |
| Epilepsy | Epilepsy |
| Painkillers within 1 month of index date | Painkillers within 3 months of index date |
| IMD | IMD |
| CCI score |
Continuous predictors
Fractional polynomials were used to explore the presence of non-linear relationships of continuous predictors (e.g. age, BMI); however, a linear relationship was found to be a good approximation. 34
Missing data
We assumed that missing data occurred at random and we carried out multiple imputation. 24 Missing values were predicted on the basis of all other predictors as well as the outcome. One hundred imputed data sets were generated with imputed values, reflecting the uncertainty associated with the imputations. Models were fitted on each imputed data set and coefficients combined using Rubin’s rules.
Model development
All candidate predictors in Table 22 were included in the multivariable Cox regression models for predicting re-dislocation. Because predictors were chosen a priori based on clinical consensus, and only a small number of candidate predictors were available in the CPRD, no reduction of predictors was considered.
Assessment of model performance and internal validation
The predictive ability of the model was assessed in terms of discrimination. 35 Discrimination is the ability of the model to differentiate between individuals who have a re-dislocation and those who do not. Discrimination was assessed by calculating the concordance (c)-index; a value of 0.5 indicates no discrimination (equivalent to tossing a coin) and a value of 1 indicates perfect discrimination.
Optimism in the performance was assessed by bootstrap resampling. 35 We drew 200 samples with replacement from the original data, with the same size as the original derivation data. In each bootstrap sample the entire modelling process was repeated. This process was repeated over each of the 100 imputed data sets, and an averaged, optimism-corrected c-index was taken.
The R software environment (version 3.5.0) was used for all analyses. We followed the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement for reporting our analyses. 36,37
Results
The characteristics of the surgical and non-surgical groups are described in Table 23. There were 342 eligible individuals in the surgical cohort, of which 61 (18%) went on to have a re-dislocation within 3 years of the index date. There were 4271 individuals in the non-surgical cohort, of which 851 (20%) went on to have a re-dislocation within the study period. There were large numbers of missing data, most notably for BMI (46% missing in the surgical cohort and 42% missing in the non-surgical cohort) and alcohol consumption (51% missing in the surgical cohort and 43% missing in the non-surgical cohort). Forty-one per cent (n = 140) of individuals have no missing information on all eight predictors for the model developed in the surgical cohort, and 46% (n = 1960) have no missing information on all nine predictors for the model developed in the non-surgical cohort.
| Variable | Patient group, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| No surgery | Surgery | ||||
| No re-dislocation | Re-dislocation | No re-dislocation | Re-dislocation | ||
| Total number of participants | 3420 (80.1) | 851 (19.9) | 281 (82.2) | 61 (17.8) | 4613 |
| Year of shoulder dislocation | |||||
| 1997 | 54 (1.6) | 10 (1.2) | 1 (0.4) | 0 (0.0) | 65 (1.4) |
| 1998 | 82 (2.4) | 27 (3.2) | 5 (1.8) | 1 (1.6) | 115 (2.5) |
| 1999 | 101 (3.0) | 24 (2.8) | 2 (0.7) | 1 (1.6) | 128 (2.8) |
| 2000 | 127 (3.7) | 28 (3.3) | 5 (1.8) | 1 (1.6) | 161 (3.5) |
| 2001 | 153 (4.5) | 46 (5.4) | 7 (2.5) | 3 (4.9) | 209 (4.5) |
| 2002 | 189 (5.5) | 50 (5.9) | 10 (3.6) | 1 (1.6) | 250 (5.4) |
| 2003 | 234 (6.8) | 54 (6.3) | 17 (6.0) | 4 (6.6) | 309 (6.7) |
| 2004 | 232 (6.8) | 58 (6.8) | 10 (3.6) | 1 (1.6) | 301 (6.5) |
| 2005 | 213 (6.2) | 58 (6.8) | 13 (4.6) | 4 (6.6) | 288 (6.2) |
| 2006 | 230 (6.7) | 62 (7.3) | 21 (7.5) | 2 (3.3) | 315 (6.8) |
| 2007 | 240 (7.0) | 68 (8.0) | 20 (7.1) | 6 (9.8) | 334 (7.2) |
| 2008 | 239 (7.0) | 67 (7.9) | 28 (10.0) | 5 (8.2) | 339 (7.3) |
| 2009 | 238 (7.0) | 65 (7.6) | 20 (7.1) | 8 (13.1) | 331 (7.2) |
| 2010 | 244 (7.1) | 70 (8.2) | 29 (10.3) | 5 (8.2) | 348 (7.5) |
| 2011 | 251 (7.3) | 50 (5.9) | 24 (8.5) | 4 (6.6) | 329 (7.1) |
| 2012 | 207 (6.1) | 54 (6.3) | 26 (9.3) | 6 (9.8) | 293 (6.4) |
| 2013 | 170 (5.0) | 39 (4.6) | 22 (7.8) | 6 (9.8) | 237 (5.1) |
| 2014 | 174 (5.1) | 21 (2.5) | 19 (6.8) | 3 (4.9) | 217 (4.7) |
| 2015 | 42 (1.2) | 0 (0.0) | 2 (0.7) | 0 (0.0) | 44 (1.0) |
| Age (years), median (IQR) | 24 (20–29) | 22 (19–26) | 24 (20–28) | 21 (19–23) | 23 (19–28) |
| Sex | |||||
| Male | 2772 (81.1) | 720 (84.6) | 250 (89.0) | 52 (85.2) | 3794 (82.2) |
| Female | 648 (18.9) | 131 (18.9) | 31 (11.0) | 9 (14.8) | 819 (17.8) |
| BMI (kg/m2) | |||||
| < 25 | 1132 (33.1) | 307 (36.1) | 87 (31.0) | 16 (26.2) | 1542 (33.4) |
| 25.0–29.9 | 584 (17.1) | 124 (14.6) | 46 (16.4) | 9 (14.8) | 753 (16.5) |
| ≥ 30.0 | 277 (8.1) | 61 (7.2) | 24 (8.5) | 2 (3.3) | 364 (7.9) |
| Missing | 1427 (41.7) | 359 (42.2) | 124 (44.1) | 34 (55.7) | 1944 (42.1) |
| IMD 2004 (quintile of deprivation) | |||||
| 1 (affluent) | 948 (27.7) | 225 (26.4) | 62 (22.1) | 6 (9.8) | 1241 (26.9) |
| 2 | 748 (21.9) | 201 (23.6) | 65 (23.1) | 14 (23.0) | 1028 (22.3) |
| 3 | 688 (20.1) | 158 (18.6) | 52 (18.5) | 17 (27.9) | 915 (19.8) |
| 4 | 602 (17.6) | 150 (17.6) | 69 (24.6) | 13 (21.3) | 834 (18.1) |
| 5 (deprived) | 431 (12.6) | 117 (13.7) | 33 (11.7) | 11 (18.0) | 592 (12.8) |
| Missing | 3 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.1) |
| Smoking status | |||||
| Non-smoker | 1795 (52.5) | 492 (57.8) | 137 (48.8) | 37 (60.7) | 2461 (53.3) |
| Current smoker | 996 (29.1) | 237 (27.8) | 86 (30.6) | 16 (26.2) | 1335 (28.9) |
| Ex-smoker | 295 (8.6) | 67 (7.9) | 25 (8.9) | 5 (8.2) | 392 (8.5) |
| Missing | 334 (9.8) | 55 (6.5) | 33 (11.7) | 3 (4.9) | 426 (9.2) |
| Alcohol consumption? | |||||
| No | 312 (9.1) | 91 (10.7) | 21 (7.5) | 8 (13.1) | 432 (9.4) |
| Yes | 1628 (47.6) | 405 (47.6) | 122 (43.4) | 17 (27.9) | 2172 (47.1) |
| Missing | 1480 (43.3) | 355 (41.7) | 138 (49.1) | 36 (59.0) | 2009 (43.6) |
| CCI score | |||||
| 0 | 3153 (92.2) | 792 (93.1) | 270 (96.1) | 60 (98.4) | 4275 (92.7) |
| 1 | 162 (4.7) | 37 (4.3) | 6 (2.1) | 0 (0.0) | 205 (4.4) |
| 2 | 65 (1.9) | 15 (1.8) | 4 (1.4) | 0 (0.0) | 84 (1.8) |
| ≥ 3 | 40 (1.2) | 7 (0.8) | 1 (0.4) | 1 (1.6) | 49 (1.1) |
| Region | |||||
| East Midlands | 112 (3.3) | 30 (3.5) | 8 (2.8) | 2 (3.3) | 152 (3.3) |
| East of England | 424 (12.4) | 98 (11.5) | 22 (7.8) | 5 (8.2) | 549 (11.9) |
| London | 418 (12.2) | 102 (12.0) | 34 (12.1) | 6 (9.8) | 560 (12.1) |
| North East | 78 (2.3) | 26 (3.1) | 6 (2.1) | 3 (4.9) | 113 (2.4) |
| North West | 540 (15.8) | 129 (15.2) | 60 (21.4) | 14 (23.0) | 743 (16.1) |
| South Central | 496 (14.5) | 132 (15.5) | 39 (13.9) | 2 (3.3) | 669 (14.5) |
| South East Coast | 418 (12.2) | 84 (9.9) | 26 (9.3) | 9 (14.8) | 537 (11.6) |
| South West | 414 (12.1) | 110 (12.9) | 46 (16.4) | 10 (16.4) | 580 (12.6) |
| West Midlands | 378 (11.1) | 110 (12.9) | 30 (10.7) | 7 (11.5) | 525 (11.4) |
| Yorkshire and the Humber | 142 (4.2) | 30 (3.5) | 10 (3.6) | 3 (4.9) | 185 (4.0) |
| Epilepsy? | |||||
| No | 3328 (97.3) | 809 (95.1) | 267 (95.0) | 53 (86.9) | 4457 (96.6) |
| Yes | 92 (2.7) | 42 (4.9) | 14 (5.0) | 8 (13.1) | 156 (3.4) |
| Prescribed painkillers 3 months after the index date? | |||||
| No | 3241 (94.8) | 795 (93.4) | 264 (94.0) | 56 (91.8) | 4356 (94.4) |
| Yes | 179 (5.2) | 56 (6.6) | 17 (6.0) | 5 (8.2) | 257 (5.6) |
| Prescribed painkillers 1 month after the index date? | |||||
| No | – | – | 248 (88.3) | 51 (83.6) | – |
| Yes | – | – | 33 (11.7) | 10 (16.4) | – |
| First-time TASD | |||||
| 14G5: H/O dislocated shouldera | 393 (11.5) | 84 (9.9) | 33 (11.7) | 5 (8.2) | 515 |
| 7K6G300: closed reduction of dislocation of shoulder | 58 (1.7) | 24 (2.8) | 3 (1.1) | 2 (3.3) | 87 |
| N083100: recurrent joint dislocation, of shoulder region | 29 (0.8) | 8 (0.9) | 5 (1.8) | 1 (1.6) | 43 |
| N083A00: recurrent dislocation of shoulder – anterior | 113 (3.3) | 25 (2.9) | 25 (8.9) | 4 (6.6) | 167 |
| N083C00: recurrent subluxation of shoulder – anterior | 52 (1.5) | 14 (1.6) | 7 (2.5) | 1 (1.6) | 74 |
| S41..00: dislocation or subluxation of shoulder | 2081 (60.8) | 496 (58.3) | 147 (52.3) | 36 (53.5) | 2760 |
| S410.00: closed traumatic dislocation of shoulder | 75 (2.2) | 31 (3.6) | 4 (1.4) | 2 (3.3) | 112 |
| S410000: closed traumatic dislocation of shoulder joint, unspecified | 21 (0.6) | 7 (0.8) | 1 (0.4) | 0 (0.0) | 29 |
| S410100: closed traumatic dislocation of shoulder joint, anterior (subcoracoid) | 13 (0.4) | 2 (0.2) | 2 (0.7) | 0 (0.0) | 17 |
| S410111: anterior dislocation of shoulder | 87 (2.5) | 37 (4.3) | 11 (3.9) | 2 (3.3) | 137 |
| S412.00: closed traumatic subluxation, shoulder | 18 (0.5) | 2 (0.2) | 1 (0.4) | 0 (0.0) | 21 |
| S41z.00: dislocation of shoulder NOSa | 480 (14.0) | 121 (14.2) | 42 (14.9) | 8 (13.1) | 651 |
Prediction model: surgical cohort
Eight predictors were included in the model to predict re-dislocation in the surgery cohort (Table 24). With an effective sample size of 61 re-dislocation events, this yields an events-per-variable (EPV) number of 5.1 (61 events/12 regression coefficients), which is much smaller than the widely recommended EPV number of 10, indicating the likelihood of overfitting because of a small sample size.
| Predictor | Coefficient (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| Age (years) | –0.1029 (0.0366) | 0.90 (0.84 to 0.97) | 0.0050 |
| Sex | 0.10275 (0.3825) | 1.11 (0.52 to 2.34) | 0.7887 |
| BMI (kg/m2) | –0.0777 (0.0606) | 0.93 (0.82 to 1.04) | 0.2015 |
| Smoking status (reference: non-smoker) | |||
| Smoker | –0.4653 (0.3444) | 0.63 (0.32 to 1.23) | 0.1767 |
| Ex-smoker | –0.0989 (0.5322) | 0.91 (0.32 to 2.57) | 0.8526 |
| Alcohol consumption (reference: non-drinker) | –0.3965 (0.4968) | 0.67 (0.25 to 1.79) | 0.4255 |
| Epilepsy | 0.9216 (0.4434) | 2.51 (1.05 to 5.99) | 0.0377 |
| Painkillers within 1 month of index date | 0.5171 (0.3848) | 1.68 (0.79 to 3.57) | 0.1791 |
| IMD 2004 [reference: 1 (affluent)] | |||
| 2 | 0.7099 (0.5146) | 2.03 (0.74 to 5.58) | 0.1677 |
| 3 | 1.0641 (0.5029) | 2.90 (1.08 to 7.77) | 0.0344 |
| 4 | 0.6600 (0.5127) | 1.93 (0.71 to 5.29) | 0.1980 |
| 5 (deprived) | 1.0757 (0.5667) | 2.93 (0.97 to 8.90) | 0.0577 |
Age and epilepsy were the only statistically significant predictors (at the p < 0.05 level). The apparent predictive performance of the model, as measured by the c-index, was moderate, with a c-index of 0.72 (95% CI 0.65 to 0.80), which dropped slightly to 0.67 after correcting for optimism (because of overfitting).
Prediction model: non-surgical cohort
Nine predictors were included in the model to predict re-dislocation in the non-surgery cohort (Table 25). With an effective sample size of 851 re-dislocation events, this yields an EPV number of 56.7 (851 events/15 regression coefficients), which is higher than the widely recommended EPV number of 10, indicating a sufficient sample size for model development and the minimal likelihood of overfitting.
| Predictor | Coefficient (SE) | HR (95% CI) | p-value |
|---|---|---|---|
| Age (years) | –0.0379 (0.0069) | 0.96 (0.95 to 0.98) | < 0.0001 |
| Sex | –0.2179 (0.0959) | 0.80 (0.67 to 0.97) | 0.0230 |
| BMI (kg/m2) | –0.0122 (0.0094) | 0.99 (0.97 to 1.01) | 0.1964 |
| Smoking status (reference: non-smoker) | |||
| Smoker | –0.0775 (0.0828) | 0.93 (0.79 to 1.09) | 0.3496 |
| Ex-smoker | –0.0039 (0.1340) | 1.00 (0.77 to 1.21) | 0.9770 |
| Alcohol consumption (reference: non-drinker) | –0.0336 (0.1142) | 0.97 (0.77 to 1.21) | 0.7687 |
| Epilepsy | 0.6377 (0.1598) | 1.89 (1.38 to 2.59) | 0.0001 |
| Painkillers within 1 month of index date | –0.0726 (0.1139) | 0.93 (0.74 to 1.16) | 0.5242 |
| CCI score (reference: 0) | |||
| 1 | –0.1202 (0.1687) | 0.89 (0.64 to 1.23) | 0.4763 |
| 2 | –0.0464 (0.2616) | 0.95 (0.57 to 1.59) | 0.8593 |
| 3 | –0.2470 (0.3804) | 0.78 (0.37 to 1.65) | 0.5161 |
| IMD 2004 [reference: 1 (affluent)] | |||
| 2 | 0.1178 (0.0975) | 1.12 (0.93 to 1.36) | 0.2271 |
| 3 | 0.0031 (0.1046) | 1.00 (0.82 to 1.23) | 0.9766 |
| 4 | 0.0770 (0.1065) | 1.08 (0.88 to 1.33) | 0.4697 |
| 5 (deprived) | 0.1619 (0.1169) | 1.18 (0.93 to 1.48) | 0.1662 |
Age, sex and epilepsy were statistically significant predictors (at the p < 0.05 level). The apparent predictive performance of the model, as measured by the c-index, was low (c-index 0.58, 95% CI 0.56 to 0.60), which dropped to 0.56 after correcting for optimism (because of overfitting).
Discussion
Main findings
The use of primary care data to predict the risk of re-dislocation following a first-time TASD is limited. However, the performances of the two models were markedly different, with better performance in the surgery cohort. Both models identified age and epilepsy as statistically significant predictors of re-dislocation, but in the non-surgery cohort sex was also identified as a statistically significant predictor.
The model developed in the surgery cohort showed moderate performance, with a c-index of 0.67, suggesting that the information collected has some predictive capacity but that additional information (risk factors) is needed to allow better predictions of re-dislocation. For the model developed in the non-surgery cohort, the predictive ability of the model was poor, with a c-index of only 0.57, and, therefore, has no use for the risk factors available in predicting re-dislocation in this cohort of patients.
Strengths and limitations
This study has several strengths. The models were developed using data routinely collected in electronic health-care records in primary care. Widely recommended statistical methodology was followed to develop and evaluate the models, including the exploration of complex relationships (e.g. non-linearity) in continuous measurements (e.g. age and BMI). Bootstrapping techniques were used to internally validate the models.
There are also some limitations. We were only able to include a small number of predictors, fewer than half of those identified in the clinical consensus. This had a considerable impact on our ability to develop models to accurately predict the risk of re-dislocation. There was also a considerable number of missing data (e.g. BMI, alcohol consumption). However, we followed recommended guidance and increased the number of imputations to 100 to account for the large number of missing data and any uncertainty in the imputation. 24 Despite using a large electronic health records database (CPRD linked to HES), the number of eligible individuals, notably in the surgical cohort, was surprisingly small, with only 61 re-dislocation events during the study period. A small sample size can lead to overfitting. However, to counter the risk of overfitting, internal validation was carried out using bootstrapping to obtain unbiased estimates of model performance. Another limitation was the lack of a separate data set to carry out external validation, particularly to evaluate the model in the surgery cohort, which showed some predictive accuracy in the internal validation.
Conclusion
An insufficient number of data are routinely collected in CPRD and HES to allow for any reliable prediction modelling for shoulder re-dislocations after a first-time TASD in 16- to 35-year-olds.
Chapter 7 Discussion and conclusions
With regard to first-time TASDs, previous research has been limited to small cohort studies, case series and systematic reviews. The relevant background information supporting the need for research into the efficacy of management options for patients with a first-time TASD has been described. It highlighted that the use of traditional conservative management approaches after the initial reduction and joint immobilisation after a TASD seem to result in high rates of recurrent dislocation in some population groups. 3,38–40 In younger patients, rates of recurrence as high as 92–96% have been reported. 6 An incidence study of shoulder instability among athletes at a US military academy showed that 85% experienced a recurrent event within a 9-month period. 7 A systematic review showed that there were some limited data to support primary surgery following a first-time TASD in young adults engaged in demanding physical activities (i.e. military personnel and athletes). 5 A later systematic review also showed that in younger patients a significantly lower rate of recurrent instability was identified in the 2-year period following a first-time TASD for those having surgery than for those having no surgery (7% vs. 46%). 8
Consequently, there appears to be some limited evidence for surgical intervention following a first-time TASD in younger and/or highly active patients. This research was, therefore, commissioned by the NIHR HTA programme as an ongoing research uncertainty, and probably with concern that this type of surgery is becoming more frequent after a first-time TASD, based on the positive suggestions of the lower-quality evidence described above.
Internal and external validation study
We conducted both an internal and an external validation assessment of the CPRD and found it an acceptable data set to identify and study shoulder dislocation patients. It provided a large, population-based, primary care cohort that is representative of the UK general population. We found the GP coding internally valid for shoulder dislocations and the incidence rates externally valid against data from Canada and the USA.
Our large UK population-based cohort of 16,763 patients aged 16–70 years during 1995–2015 allowed us to identify, for the first time (to our knowledge), the overall incidence rates in the UK. Most shoulder dislocations occurred in men (72%). The overall incidence rate in men was 40.4 per 100,000 person-years, and in women this was 15.5 per 100,000 person-years. The highest incidence was observed in 16- to 20-year-old men (80.5 per 100,000 person-years). Although this was similar to other world data (Canadian, US and Norwegian cohorts15,16,20), an unexpected finding was that the incidence in women aged > 50 years increased to 28.1 per 100,000 person-years among those aged 61–70 years.
Our new finding of the increasing incidence of shoulder dislocations among women aged > 50 years is of both interest and concern because the reasons for it are not known. Such injuries in more elderly people are usually associated with rotator cuff tears and fractures, with the subsequent loss of function as well as instability. However, this finding suggests that further work is now required to examine the reasons that may underpin this increased risk of shoulder dislocations in ageing women. Biological differences between ageing men and women in relation to joint proprioception, soft tissue tendon quality and protective muscle bulk and differences in the incidence of falls between men and women are all factors that could be examined. With an increasing ageing population, priority needs to be given to increasing the safety of the elderly to reduce falls, dislocations and fractures, as advocated by NICE;22 this is a new finding that warrants exploration.
Stage 2: propensity score analysis
One weakness identified during stage 1 of our study was that not all a priori risk factors were identifiable in the CPRD and the impact this would have on the stage 2 propensity score analysis was unclear because linkage to HES did not take place until stage 2 of this study. However, besides this weakness, once CPRD and HES linkage had taken place, a population-based cohort of 3759 patients diagnosed with a first-time TASD during 1 April 1997–26 April 2014 with 2 years of follow-up in England was produced.
Therefore, it was surprising that for a commissioned research question we could find only 156 patients in this large data set who had received surgical treatment within 6 months of a first-time TASD. On the one hand, this information is useful and informative and allows us to conclude that within the NHS early surgery after only one shoulder dislocation is uncommon. It also means that the study would be underpowered to demonstrate any real differences in re-dislocation rates between surgical and non-surgical treatments. Therefore, although the number of patients (n = 3759) included in the analysis was greater than the minimum number required for statistical power (n = 3065), the unexpected low number of NHS patients having surgery after one dislocation was a disappointing and surprising finding. It was also observed that a substantial number of patients in the young cohort of 16- to 35-year-olds had < 2 years of follow-up within the CPRD (n = 854), which may be related to them going away to university or finding jobs in different locations, resulting in the need to change their GP and this, in turn, resulted in a further loss of numbers. Overall, relatively few patients have surgery within 6 months of a first-time TASD in the NHS. This is probably a reflection of many GPs not referring patients with only one dislocation to secondary care and also due to NHS operative waiting times.
Combining this finding with the confounding risk factors not available in either the CPRD or HES had a large impact on the study. Although the overall finding from the propensity-score-matched analysis was that surgery within 6 months was slightly protective, it did not reach statistical significance (HR 0.88, 95% CI 0.58 to 1.35; p = 0.565) and the wide CI further indicates that this study was underpowered. With regard to the missing risk factors, the main disadvantage of using propensity-score-matching methods is that confounders for which no data are available result in a lack of adjustment. Other than age and sex, the risk factors recorded and available in the CPRD are considered less important risk factors for this particular condition. Other important factors identified in our expert survey, such as cause of shoulder dislocation, imaging findings of structural problems, anterior apprehension, occupation, sports played and level of sports, were not recorded in the observational data. Outcome data on ongoing instability symptoms without dislocation were also not available and, thus, only the hard outcome of re-dislocation could be used. This is another layer of potential confounding that could not be accounted for.
Sensitivity analysis
Based on the primary analysis findings, and in an attempt to maximise the use of this data set to further examine the commissioned question of surgery after a first-time TASD, a further sensitivity analysis was planned and approved by the HTA programme and ISAC. We looked at surgery within 12 months of a first-time TASD and increased the follow-up to up to 3 years. This produced a population-based cohort of 4613 patients diagnosed with a first-time TASD during 1 April 1997–31 March 2015 in England within CPRD-HES linked records. The overall finding from this propensity-score-matched analysis was that surgery within 12 months had a similar effect to non-surgical interventions (HR 1.17, 95% CI 0.88 to 1.55; p = 0.274) and did not seem to offer any additional benefit on whether or not a patient suffers a re-dislocation. However, the number of patients receiving surgery only increased to 342 (from 156 patients) and residual confounding was present.
There are two further observations to note from this analysis. First, re-dislocations in the surgical group seemed to occur later. This is unlikely to be due to any benefits of surgery wearing off but more likely to be related to the return to contact sports, which is a true test of stability. This is often delayed for > 6 months after surgery as part of the rehabilitation process. Second, the same process tends not to be in place for any non-operative patients; such patients may not even return to contact sports having decided to change their lifestyle instead of considering surgery. Recording such outcome metrics would be important for any future trials on the treatment of this condition. Although risk factors and residual confounding existed and it was not possible to reliably compare surgery with no surgery, it is still worth noting the 20% re-dislocation rate in the surgical cohort. The re-dislocation recurrence rate after surgery is probably higher than many surgeons and patients would expect and will help inform shared decision-making processes with patients.
Prediction models
Although some risk factor data were not available, it was still possible to construct prediction models, but these were limited. A non-surgical and a surgical model were developed and although the performances of the two models were markedly different, with a better performance in the surgery cohort, both models identified age and epilepsy as statistically significant predictors of re-dislocation. In the non-surgery cohort, sex (male) was also identified as a statistically significant predictor of re-dislocation. The modelling study has several strengths, as the models were developed using data routinely collected in electronic health-care records in primary care. Widely recommended statistical methodology was used to develop and evaluate the models, including the exploration of complex relationships in continuous measurements and bootstrapping techniques to internally validate them. The results indicate that using prediction models for this condition holds promise, but more risk factors are needed for more accurate prediction of outcome information for patients and surgeons.
Conclusions
-
This study provides the first-time age- and sex-specific UK incidence rates for TASD, with most TASDs occurring in men, but with women aged > 50 years unexpectedly showing an increased incidence.
-
Far fewer patients received surgery after a first-time TASD than expected, leading to an underpowered study.
-
Surgery after a first-time TASD is not common in the NHS. Re-dislocation rates for surgical patients after a first-time TASD are higher than previously expected (at around 20%).
-
A sensitivity analysis at 12 months suggests that there is little difference in re-dislocation rates between surgical patients and non-surgical patients, but important residual confounding risk factors were present and not recorded in NHS primary and secondary care databases.
-
Missing risk factor data limited the value of the prediction modelling; however, age and epilepsy were identified as statistically significant predictors of re-dislocation.
A randomised controlled trial and/or a carefully constructed national shoulder dislocation registry documenting all appropriate risk factors and outcome metrics is needed to answer this commissioned research question reliably.
Acknowledgements
The authors would like to express their gratitude for the work of Margaret Smith and Clare Bankhead at the Primary Care Unit of the University of Oxford for facilitating access to CPRD data on shoulder dislocations. The authors would also like to express their gratitude for the work of Robin May and Emma Boyle at the CPRD for facilitating the internal validation study using GP questionnaires.
Patient and public involvement statement
A patient representative was involved in managing the study and sat on the Project Management Board. Although other patient and public involvement activity was planned in the protocol of this study, the findings have been such that widespread dissemination of the results and the development of specific patient decision-making information for patients has not been possible or worthwhile. The research question remains unanswered and is one of the top 20 James Lind Alliance Surgery for Common Shoulder Conditions41 research priorities.
Contributions of authors
Jonathan L Rees contributed to the conception and design of the study, oversaw the acquisition of data, analysis and interpretation of the findings.
Anjali Shah analysed the study data and contributed to the interpretation of the findings.
Katherine Edwards assisted with the literature review, data access and the internal validation of the study.
Maria T Sanchez-Santos assisted with analysing the data and interpreting the findings.
Danielle E Robinson assisted with analysing the data and interpreting the findings.
Antonella Delmestri contributed to the acquisition, cleaning and management of the data.
Andrew Carr contributed to the interpretation of the study findings.
Nigel Arden contributed to the interpretation of the study findings.
Sarah E Lamb contributed to the interpretation of the study findings.
Amar Rangan contributed to the interpretation of the study findings.
Andrew Judge contributed to the interpretation of the study findings.
Rafael Pinedo-Villanueva contributed to the interpretation of the study findings.
Tim Holt contributed to the interpretation of the study findings.
Sally Hopewell contributed to the interpretation of the study findings.
Daniel Prieto-Alhambra contributed to the interpretation of the study findings.
Gary Collins analysed the study data and contributed to the interpretation of the study findings.
All authors contributed to the drafting of this report or revising its content and have approved the final version.
Publication
Shah A, Judge A, Delmestri A, Edwards K, Arden NK, Prieto-Alhambra D, et al. Incidence of shoulder dislocations in the UK, 1995–2015: a population-based cohort study. BMJ Open 2017;7:e016112.
Data-sharing statement
All of the available data are included in the report. All queries should be submitted to the corresponding author for consideration.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Pope EJ, Ward JP, Rokito AS. Anterior shoulder instability – a history of arthroscopic treatment. Bull NYU Hosp Jt Dis 2011;69:44-9.
- Handoll HH, Hanchard NC, Goodchild L, Feary J. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD004962.pub2.
- Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am 1956;38-A:957-77. https://doi.org/10.2106/00004623-195638050-00001.
- Boffano M, Mortera S, Piana R. Management of the first episode of traumatic shoulder dislocation. EFORT Open Rev 2017;2:35-40. https://doi.org/10.1302/2058-5241.2.160018.
- Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev 2004;1. https://doi.org/10.1002/14651858.CD004325.pub2.
- Avila Lafuente JL, Moros Marco S, García Pequerul JM. Controversies in the management of the first time shoulder dislocation. Open Orthop J 2017;11:1001-10. https://doi.org/10.2174/1874325001711011001.
- Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med 2007;35:1168-73. https://doi.org/10.1177/0363546506295179.
- Brophy RH, Marx RG. The treatment of traumatic anterior instability of the shoulder: non-operative and surgical treatment. Arthroscopy 2009;25:298-304. https://doi.org/10.1016/j.arthro.2008.12.007.
- Itoi E, Hatakeyama Y, Sato T, Kido T, Minagawa H, Yamamoto N, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence. A randomized controlled trial. J Bone Joint Surg Am 2007;89:2124-31. https://doi.org/10.2106/JBJS.F.00654.
- Bankart ASB. Recurrent or habitual dislocation of the shoulder-joint. Br Med J 1923;2:1132-3. https://doi.org/10.1136/bmj.2.3285.1132.
- Mahiroğulları M, Ozkan H, Akyüz M, Uğraş AA, Güney A, Kuşkucu M. Comparison between the results of open and arthroscopic repair of isolated traumatic anterior instability of the shoulder. Acta Orthop Traumatol Turc 2010;44:180-5. https://doi.org/10.3944/AOTT.2010.2289.
- Pulavarti RS, Symes TH, Rangan A. Surgical interventions for anterior shoulder instability in adults. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD005077.pub2.
- Hovelius L. Incidence of shoulder dislocation in Sweden. Clin Orthop Relat Res 1982;166:127-31. https://doi.org/10.1097/00003086-198206000-00021.
- Hovelius L, Augustini BG, Fredin H, Johansson O, Norlin R, Thorling J. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am 1996;78:1677-84. https://doi.org/10.2106/00004623-199611000-00006.
- Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am 2010;92:542-9. https://doi.org/10.2106/JBJS.I.00450.
- Leroux T, Wasserstein D, Veillette C, Khoshbin A, Henry P, Chahal J, et al. Epidemiology of primary anterior shoulder dislocation requiring closed reduction in Ontario, Canada. Am J Sports Med 2014;42:442-50. https://doi.org/10.1177/0363546513510391.
- Shah A, Judge A, Delmestri A, Edwards K, Arden NK, Prieto-Alhambra D, et al. Incidence of shoulder dislocations in the UK, 1995–2015: a population-based cohort study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016112.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- The English Indices of Deprivation 2004 (Revised). London: Neighbourhood Renewal Unit, Office for the Deputy Prime Minister; 2004.
- Liavaag S, Svenningsen S, Reikerås O, Enger M, Fjalestad T, Pripp AH, et al. The epidemiology of shoulder dislocations in Oslo. Scand J Med Sci Sports 2011;21:e334-40. https://doi.org/10.1111/j.1600-0838.2011.01300.x.
- Krøner K, Lind T, Jensen J. The epidemiology of shoulder dislocations. Arch Orthop Trauma Surg 1989;108:288-90. https://doi.org/10.1007/BF00932317.
- Falls in Older People: Assessing Risk and Prevention (CG161). London: NICE; 2013.
- Malhotra A, Freudmann MS, Hay SM. Management of traumatic anterior shoulder dislocation in the 17- to 25-year age group: a dramatic evolution of practice. J Shoulder Elbow Surg 2012;21:545-53. https://doi.org/10.1016/j.jse.2011.01.006.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Rosenbaum PR, Rubin DB. Assessing sensitivity to an unobserved binary covariate in an observational study with binary outcome. J Royal Stat Soc 1983;45:212-18.
- Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 2006;163:262-70. https://doi.org/10.1093/aje/kwj047.
- Lunt M, Solomon D, Rothman K, Glynn R, Hyrich K, Symmons DP, et al. Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol 2009;169:909-17. https://doi.org/10.1093/aje/kwn391.
- Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009;51:171-84. https://doi.org/10.1002/bimj.200810488.
- Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1-28. https://doi.org/10.18637/jss.v042.i08.
- Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253-9. https://doi.org/10.1111/j.1742-7843.2006.pto_293.x.
- Freemantle N, Marston L, Walters K, Wood J, Reynolds MR, Petersen I. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ 2013;347. https://doi.org/10.1136/bmj.f6409.
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167:492-9. https://doi.org/10.1093/aje/kwm324.
- Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005;162:1016-23. https://doi.org/10.1093/aje/kwi307.
- Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. J Royal Stat Soc Series C (Applied Statistics) 1994;43:429-67. https://doi.org/10.2307/2986270.
- Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128-38. https://doi.org/10.1097/EDE.0b013e3181c30fb2.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55-63. https://doi.org/10.7326/M14-0697.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. https://doi.org/10.7326/M14-0698.
- Henry JH, Genung JA. Natural history of glenohumeral dislocation – revisited. Am J Sports Med 1982;10:135-7. https://doi.org/10.1177/036354658201000301.
- Robinson CM, Howes J, Murdoch H, Will E, Graham C. Functional outcome and risk of recurrent instability after primary traumatic anterior shoulder dislocation in young patients. J Bone Joint Surg Am 2006;88:2326-36. https://doi.org/10.2106/JBJS.E.01327.
- Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am 2009;91:791-6. https://doi.org/10.2106/JBJS.H.00514.
- Surgery for Common Shoulder Problems Top 10. Southampton: James Lind Alliance; n.d.
Appendix 1 Clinical Practice Research Datalink and Hospital Episode Statistics codes
Clinical Practice Research Datalink dislocation Read codes
| Description | Read code |
|---|---|
| Dislocation or subluxation of shoulder | S41..00 |
| Dislocation of shoulder NOSa | S41z.00 |
| H/O: dislocated shouldera | 14G5.00 |
| Closed reduction of dislocation of shoulder | 7K6G300 |
| Closed traumatic dislocation of shoulder | S410.00 |
| Recurrent dislocation of shoulder, anterior | N083A00 |
| Anterior dislocation of shoulder | S410111 |
| Recurrent joint dislocation of shoulder region | N083100 |
| Recurrent subluxation of shoulder, anterior | N083C00 |
| Closed traumatic dislocation of shoulder joint, anterior (subcoracoid) | S410100 |
| Closed traumatic dislocation shoulder joint, unspecified | S410000 |
| Closed traumatic subluxation, shoulder | S412.00 |
Hospital Episode Statistics Office of Population Censuses and Surveys 4.7 codes
Shown below is a list of the HES OPCS 4.7 codes used to identify shoulder dislocation events and outcomes. These codes have been provided by the expert advisor in orthopaedics to the NHS Digital Clinical Classifications Service and Collaborating Centres for the World Health Organization Family of International Classifications. The codes listed below are based on the following procedures: labral repair, stabilisation, capsular shift, Latarjet procedure, bone transfer, SLAP (Superior Labrum Anterior to Posterior) repair and Bankart repair. The following site codes should be present in cases when the codes do not define the anatomical site: Z81.3 (glenohumeral joint) or Z81.4 (shoulder joint).
| Operative description | OPCS 4.7 code |
|---|---|
| Other bones and joints: primary closed reduction of traumatic dislocation of joint, primary closed reduction of traumatic dislocation of joint and skeletal traction | W66.2 |
| Other bones and joints: primary closed reduction of traumatic dislocation of joint, other specified | W66.8 |
| Other bones and joints: primary closed reduction of traumatic dislocation of joint, unspecified | W66.9 |
| Other bones and joints: secondary reduction of traumatic dislocation of joint, re-manipulation of traumatic dislocation of joint | W67.6 |
| Stabilising operations on joint | W77 |
| Repair of capsule of joint for stabilisation of joint NEC | W77.1 |
| Transposition of muscle for stabilisation of joint | W77.2 |
| Blocking operations on joint using prosthesis for stabilisation of joint | W77.3 |
| Blocking operations on joint using bone for stabilisation of joint | W77.4 |
| Periarticular osteotomy for stabilisation of joint | W77.5 |
| Transposition of ligament for stabilisation of joint | W77.7 |
| Other specified stabilising operations on joint | W77.8 |
| Unspecified stabilising operations on joint | W77.9 |
| Prosthetic replacement of ligament | W72 |
| Primary prosthetic replacement of multiple ligaments | W72.1 |
| Prosthetic replacement of multiple ligaments NEC | W72.2 |
| Primary prosthetic replacement of intra-articular ligament | W72.3 |
| Prosthetic replacement of intra-articular ligament NEC | W72.4 |
| Primary prosthetic replacement of extra-articular ligament | W72.5 |
| Prosthetic replacement of extra-articular ligament NEC | W72.6 |
| Other specified prosthetic replacement of ligament | W72.8 |
| Unspecified prosthetic replacement of ligament | W72.9 |
| Other stabilising operations on joint | O27 |
| Extra-articular ligament reconstruction for stabilisation of joint | O27.1 |
| Repair of capsule and anterior and posterior labrum for stabilisation of glenohumeral joint | O27.2 |
| Repair of capsule and anterior labrum for stabilisation of glenohumeral joint | O27.3 |
| Repair of capsule and posterior labrum for stabilisation of glenohumeral joint | O27.4 |
| Other reconstruction of ligament | W74 |
| Reconstruction of multiple ligaments NEC | W74.1 |
| Reconstruction of intra-articular ligament NEC | W74.2 |
| Other specified other reconstruction of ligament | W74.8 |
| Unspecified other reconstruction of ligament | W74.9 |
| Other open repair of ligament | W75 |
| Open repair of multiple ligaments NEC | W75.1 |
| Open repair of intra-articular ligament NEC | W75.2 |
| Open repair of extra-articular ligament NEC | W75.3 |
| Other specified other open repair of ligament | W75.8 |
| Unspecified other open repair of ligament | W75.9 |
| Therapeutic endoscopic operations on other joint structure | W84 |
| Endoscopic repair of intra-articular ligament | W84.1 |
| Endoscopic re-attachment of intra-articular ligament | W84.2 |
| Endoscopic repair of superior labrum anterior to posterior tear | W84.7 |
| Other specified therapeutic endoscopic operations on other joint structure | W84.8 |
| Unspecified therapeutic endoscopic operations on other joint structure | W84.9 |
| Capsulorrhaphy of joint | W81.6 |
| Other bones and joints: therapeutic endoscopic operations on cavity of other joint, other specified | W86.8 |
| Other bones and joints: other manipulation of joint, unspecified | W91.9 |
Appendix 2 Validation algorithm
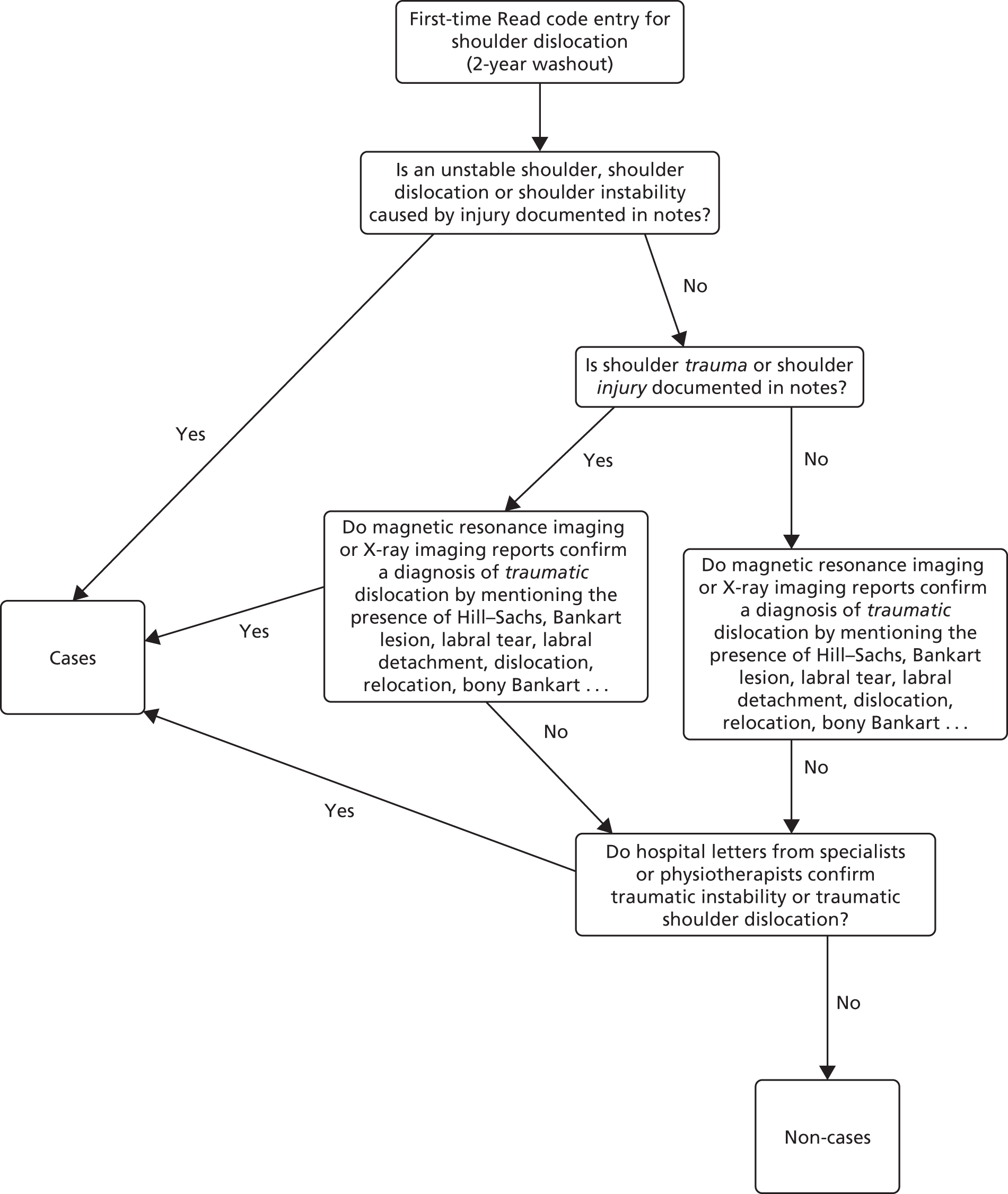
Appendix 3 General practitioner validation questionnaire of the UK TASH-D study distributed by the Clinical Practice Research Datalink questionnaire service
Appendix 4 List of risk factors identified by expert consensus
| Risk factors for re-dislocation after first dislocation | Risk factors for re-dislocation after surgery |
|---|---|
| Age (years) | Age (years) |
| Sex | Sex |
| UK region | UK region |
| Deprivation scores | Deprivation scores |
| Glenoid and/or humeral bone loss | Glenoid and/or humeral bone loss |
| Mechanism of injury | Number of dislocations pre surgical repair |
| Rotator cuff tears | Time between first dislocation and surgery |
| Imaging findings | Anterior apprehension |
| Anterior apprehension | Occupation |
| Occupation | Sport type and level |
| Sport type and level | Operation type |
| Neurological injury | Laxity/Beighton score |
| Laxity/Beighton score | Insufficient physiotherapy/rehabilitation after surgery |
| Insufficient physiotherapy/rehabilitation after first dislocation | Time at return to sports |
| Young rugby player (aged < 20 years) | Number of anchors used at surgery |
| Time at return to sports | Incorrect positioning of anchors |
| Post-dislocation immobilisation | Not addressing capsular laxity at surgery |
| Previous lower limb or back injury |
Appendix 5 Clinical Practice Research Datalink Read codes of primary shoulder dislocation patients who had surgery within 6 months or no surgery within Clinical Practice Research Datalink-Hospital Episode Statistics during 1 April 1997–26 April 2014, in England
| Diagnosis codes and descriptions for first-time TASD within the CPRD | Patient group, n (%) | ||
|---|---|---|---|
| Whole data set | No surgery | Surgery within 6 months of TASD | |
| Total | 3759 (100) | 3603 (96) | 156 (4) |
| S41..00: dislocation or subluxation of shoulder | 2269 (60) | 2187 (61) | 82 (53) |
| S41z.00: dislocation of shoulder NOSa | 510 (14) | 482 (13) | 28 (18) |
| 14G5.00: H/O – dislocated shouldera | 413 (11) | 398 (11) | 15 (10) |
| N083A00: recurrent dislocation of shoulder – anterior | 139 (4) | 126 (3) | 13 (8) |
| S410111: anterior dislocation of shoulder | 111 (3) | 104 (3) | 7 (4) |
| S410.00: closed traumatic dislocation of shoulder | 94 (3) | 90 (2) | 4 (3) |
| 7K6G300: closed reduction of dislocation of shoulder | 69 (2) | 67 (2) | 2 (1) |
| N083C00: recurrent subluxation of shoulder – anterior | 64 (2) | 60 (2) | 4 (3) |
| N083100: recurrent joint dislocation, of shoulder region | 36 (1) | 35 (1) | 1 (1) |
| S410000: closed traumatic dislocation shoulder joint, unspecified | 22 (1) | 22 (1) | 0 (0) |
| S410100: closed traumatic dislocation shoulder joint, anterior (subcoracoid) | 16 (< 1) | 16 (< 1) | 0 (0) |
| S412.00: closed traumatic subluxation, shoulder | 16 (< 1) | 16 (< 1) | 0 (0) |
Appendix 6 Hospital Episode Statistics Office of Population Censuses and Surveys 4.7 codes for primary shoulder dislocation patients diagnosed during 1 April 1997–26 April 2014 who had surgery within 6 months within Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Surgical codes and descriptions for first-time TASD patients within HES | n (%) |
|---|---|
| W77.1: repair of capsule of joint for stabilisation of joint NEC | 49 (31) |
| W66.9: other bones and joints – primary closed reduction of traumatic dislocation of joint – unspecified | 49 (31) |
| O27.3: repair of capsule and anterior labrum for stabilisation of glenohumeral joint | 15 (10) |
| W77.9: unspecified stabilising operations on joint | 8 (5) |
| W77.8: other specified stabilising operations on joint | 7 (4) |
| W91.9: other bones and joints – other manipulation of joint – unspecified | 7 (4) |
| W66.8: other bones and joints – primary closed reduction of traumatic dislocation of joint – other specified | 5 (3) |
| W84.7: endoscopic repair of superior labrum anterior to posterior tear | 3 (2) |
| W84.8: other specified therapeutic endoscopic operations on other joint structure | 3 (2) |
| W66.2: other bones and joints – primary closed reduction of traumatic dislocation of joint – primary closed reduction of traumatic dislocation of joint and skeletal traction | 2 (1) |
| W67.6: other bones and joints – secondary reduction of traumatic dislocation of joint – remanipulation of traumatic dislocation of joint | 2 (1) |
| W86.8: other bones and joints – therapeutic endoscopic operations on cavity of other joint – other specified | 2 (1) |
| O27.2: repair of capsule and anterior and posterior labrum for stabilisation of glenohumeral joint | 1 (1) |
| O27.4: repair of capsule and posterior labrum for stabilisation of glenohumeral joint | 1 (1) |
| W75.9: unspecified other open repair of ligament | 1 (1) |
| W77.4: blocking operations on joint using bone for stabilisation of joint | 1 (1) |
| Total | 156 (100) |
Appendix 7 Estimated univariate hazard ratios, 95% confidence intervals and p-values of patients suffering a shoulder re-dislocation following a primary traumatic anterior shoulder dislocation diagnosed during 1 April 2014–26 April 2014 for variables with missing data, in England
| Variables | All available data | Complete-case data (n = 1790) | Multiply imputed data (n = 3759) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| BMI (kg/m2) | |||||||||
| < 25 | 1.00 | 1.00 | 1.00 | ||||||
| 25.0–29.9 | 0.77 | 0.62 to 0.96 | 0.021 | 0.83 | 0.65 to 1.06 | 0.128 | 0.80 | 0.64 to 0.99 | 0.039 |
| ≥ 30 | 0.84 | 0.63 to 1.13 | 0.247 | 0.91 | 0.66 to 1.26 | 0.579 | 0.87 | 0.64 to 1.17 | 0.353 |
| IMD 2004 (quintile of deprivation) | |||||||||
| 1 (affluent) | 1.00 | 1.00 | 1.00 | ||||||
| 2 | 1.07 | 0.87 to 1.32 | 0.501 | 1.08 | 0.79 to 1.46 | 0.633 | 1.07 | 0.87 to 1.32 | 0.503 |
| 3 | 1.03 | 0.83 to 1.27 | 0.804 | 1.06 | 0.77 to 1.47 | 0.712 | 1.03 | 0.83 to 1.27 | 0.804 |
| 4 | 1.07 | 0.86 to 1.33 | 0.535 | 1.07 | 0.77 to 1.48 | 0.690 | 1.07 | 0.86 to 1.33 | 0.538 |
| 5 (deprived) | 1.16 | 0.92 to 1.47 | 0.206 | 1.09 | 0.78 to 1.54 | 0.609 | 1.16 | 0.92 to 1.47 | 0.208 |
| Smoking status | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.86 | 0.73 to 1.02 | 0.079 | 0.92 | 0.73 to 1.16 | 0.478 | 0.87 | 0.74 to 1.03 | 0.101 |
| Ex-smoker | 0.91 | 0.70 to 1.19 | 0.509 | 0.94 | 0.66 to 1.35 | 0.734 | 0.91 | 0.70 to 1.19 | 0.506 |
| Drinking status | |||||||||
| Yes | 1.00 | 1.00 | 1.00 | ||||||
| No | 1.20 | 0.95 to 1.53 | 0.129 | 1.01 | 0.75 to 1.35 | 0.971 | 1.20 | 0.97 to 1.48 | 0.087 |
Appendix 8 Cox survival estimates (hazard ratios), 95% confidence intervals and p-values for complete cases and multiply imputed data on primary traumatic anterior shoulder dislocation patients diagnosed during 1 April 1997–26 April 2014 who may have suffered a re-dislocation in Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Variables | Complete-case analysis (N = 1790) | Multiply imputed data analysis (N = 3759) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Re-dislocations, n (%) | Unadjusted | Adjusted model | Unadjusted | Adjusted model | |||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| No surgery | 1714 (96) | 340 (20) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Surgery | 76 (4) | 11 (14) | 0.63 | 0.34 to 1.18 | 0.148 | 0.55 | 0.29 to 1.04 | 0.068 | 0.83 | 0.57 to 1.21 | 0.326 | 0.76 | 0.52 to 1.11 | 0.151 |
| Calendar year of shoulder dislocation | 1.03 | 1.00 to 1.05 | 0.047 | 1.03 | 1.00 to 1.05 | 0.034 | 1.03 | 1.01 to 1.04 | 0.003 | 1.02 | 1.01 to 1.04 | 0.008 | ||
| Sex | ||||||||||||||
| Male | 1359 (76) | 265 (19) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Female | 431 (24) | 86 (20) | 1.01 | 0.79 to 1.29 | 0.917 | 1.00 | 0.78 to 1.27 | 0.970 | 0.87 | 0.71 to 1.05 | 0.151 | 0.87 | 0.71 to 1.05 | 0.153 |
| Age at shoulder dislocation (16- to 35-year-olds) | 0.96 | 0.95 to 0.98 | < 0.001 | 0.96 | 0.94 to 0.98 | < 0.001 | 0.97 | 0.95 to 0.98 | < 0.001 | 0.97 | 0.95 to 0.98 | < 0.001 | ||
| Epilepsy diagnosis? | ||||||||||||||
| No | 1708 (95) | 326 (19) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 82 (5) | 25 (30) | 1.77 | 1.76 to 2.65 | 0.006 | 1.93 | 1.28 to 2.91 | 0.002 | 1.82 | 1.34 to 2.49 | < 0.001 | 1.98 | 1.45 to 2.71 | < 0.001 |
Appendix 9 Clinical Practice Research Datalink Read codes of primary shoulder dislocation patients who had surgery within 12 months or no surgery within Clinical Practice Research Datalink-Hospital Episode Statistics during 1 April 1997–31 March 2015, in England
| Diagnosis codes and descriptions for first-time TASD within the CPRD | Patient group, n (%) | ||
|---|---|---|---|
| Whole data set | No surgery | Surgery within 12 months of TASD | |
| Total | 4613 (100) | 4271 (93) | 342 (7) |
| S41..00: dislocation or subluxation of shoulder | 2760 (60) | 2577 (60) | 183 (54) |
| S41z.00: dislocation of shoulder NOSa | 651 (14) | 601 (14) | 50 (15) |
| 14G5.00: H/O – dislocated shouldera | 515 (11) | 477 (11) | 38 (11) |
| N083A00: recurrent dislocation of shoulder – anterior | 167 (4) | 138 (3) | 29 (8) |
| S410111: anterior dislocation of shoulder | 137 (3) | 124 (3) | 13 (4) |
| S410.00: closed traumatic dislocation of shoulder | 112 (2) | 106 (2) | 6 (2) |
| 7K6G300: closed reduction of dislocation of shoulder | 87 (2) | 82 (2) | 5 (1) |
| N083C00: recurrent subluxation of shoulder – anterior | 74 (2) | 66 (2) | 8 (2) |
| N083100: recurrent joint dislocation, of shoulder region | 43 (1) | 37 (1) | 6 (2) |
| S410000: closed traumatic dislocation shoulder joint, unspecified | 29 (1) | 28 (1) | 1 (< 1) |
| S410100: closed traumatic dislocation shoulder joint, anterior (subcoracoid) | 17 (< 1) | 15 (< 1) | 2 (< 1) |
| S412.00: closed traumatic subluxation, shoulder | 21 (< 1) | 20 (< 1) | 1 (< 1) |
Appendix 10 Hospital Episode Statistics Office of Population Censuses and Surveys 4.7 codes for primary shoulder dislocation patients diagnosed during 1 April 1997–31 March 2015 who had surgery within 12 months within Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Surgical codes and descriptions for first-time TASD patients within HES | n (%) |
|---|---|
| W77.1: repair of capsule of joint for stabilisation of joint NEC | 127 (37) |
| W66.9: other bones and joints – primary closed reduction of traumatic dislocation of joint – unspecified | 69 (20) |
| O27.3: repair of capsule and anterior labrum for stabilisation of glenohumeral joint | 43 (13) |
| W77.9: unspecified stabilising operations on joint | 19 (6) |
| W77.8: other specified stabilising operations on joint | 16 (5) |
| W84.8: other specified therapeutic endoscopic operations on other joint structure | 15 (4) |
| O27.2: repair of capsule and anterior and posterior labrum for stabilisation of glenohumeral joint | 11 (3) |
| W91.9: other bones and joints – other manipulation of joint – unspecified | 8 (2) |
| W84.7: endoscopic repair of superior labrum anterior to posterior tear | 8 (2) |
| O27.4: repair of capsule and posterior labrum for stabilisation of glenohumeral joint | 6 (2) |
| W66.8: other bones and joints – primary closed reduction of traumatic dislocation of joint – other specified | 5 (1) |
| W86.8: other bones and joints – therapeutic endoscopic operations on cavity of other joint – other specified | 4 (1) |
| W77.4: blocking operations on joint using bone for stabilisation of joint | 3 (1) |
| W67.6: other bones and joints – secondary reduction of traumatic dislocation of joint – remanipulation of traumatic dislocation of joint | 3 (1) |
| W66.2: other bones and joints – primary closed reduction of traumatic dislocation of joint – primary closed reduction of traumatic dislocation of joint and skeletal traction | 2 (1) |
| W75.9: unspecified other open repair of ligament | 1 (< 1) |
| O27.1: extra-articular ligament reconstruction for stabilisation of joint | 1 (< 1) |
| W77.3: blocking operations on joint using prosthesis for stabilisation of joint | 1 (< 1) |
| Total | 100 (100) |
Appendix 11 Estimated univariate hazard ratios, 95% confidence intervals and p-values of patients suffering a shoulder re-dislocation following a primary traumatic anterior shoulder dislocation diagnosed during 1 April 2014–31 March 2015 for variables with missing data, in England
| Variables | All available data | Complete-case data (n = 2100) | Multiply imputed data (n = 4613) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| BMI (kg/m2) | |||||||||
| < 25 | 1.00 | 1.00 | 1.00 | ||||||
| 25.0–29.9 | 0.80 | 0.66 to 0.98 | 0.032 | 0.88 | 0.70 to 1.10 | 0.247 | 0.83 | 0.67 to 1.02 | 0.077 |
| ≥ 30 | 0.82 | 0.63 to 1.07 | 0.147 | 0.93 | 0.69 to 1.26 | 0.645 | 0.81 | 0.62 to 1.06 | 0.119 |
| IMD 2004 (quintile of deprivation) | |||||||||
| 1 (affluent) | 1.00 | 1.00 | 1.00 | ||||||
| 2 | 1.11 | 0.93 to 1.34 | 0.252 | 1.10 | 0.83 to 1.46 | 0.499 | 1.12 | 0.93 to 1.34 | 0.250 |
| 3 | 1.02 | 0.83 to 1.24 | 0.872 | 1.05 | 0.78 to 1.41 | 0.732 | 1.02 | 0.83 to 1.24 | 0.872 |
| 4 | 1.04 | 0.85 to 1.27 | 0.712 | 0.99 | 0.73 to 1.35 | 0.969 | 1.04 | 0.85 to 1.27 | 0.712 |
| 5 (deprived) | 1.18 | 0.95 to 1.46 | 0.140 | 1.06 | 0.78 to 1.46 | 0.700 | 1.18 | 0.95 to 1.46 | 0.140 |
| Smoking status | |||||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.86 | 0.74 to 0.99 | 0.042 | 0.88 | 0.71 to 1.09 | 0.248 | 0.86 | 0.74 to 1.00 | 0.048 |
| Ex-smoker | 0.86 | 0.67 to 1.10 | 0.221 | 0.88 | 0.63 to 1.22 | 0.438 | 0.87 | 0.68 to 1.11 | 0.255 |
| Drinking status | |||||||||
| Yes | 1.00 | 1.00 | 1.00 | ||||||
| No | 1.25 | 1.00 to 1.55 | 0.049 | 1.14 | 0.88 to 1.48 | 0.318 | 1.22 | 1.00 to 1.48 | 0.054 |
Appendix 12 Cox survival estimates (hazard ratios), 95% confidence intervals and p-values for complete cases and multiply imputed data on primary traumatic anterior shoulder dislocation patients diagnosed during 1 April 1997–31 March 2015 who may have suffered a re-dislocation in Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Variables | Complete-case analysis (N = 2100) | Multiply imputed data analysis (N = 4613) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Re-dislocations, n (%) | Unadjusted | Adjusted model | Unadjusted | Adjusted model | |||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| No surgery | 1960 (93) | 393 (20) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Surgery | 140 (7) | 19 (14) | 0.67 | 0.42 to 1.05 | 0.083 | 0.63 | 0.40 to 1.00 | 0.052 | 0.77 | 0.59 to 1.01 | 0.059 | 0.73 | 0.55 to 0.95 | 0.022 |
| Calendar year of shoulder dislocation | 1.00 | 0.98 to 1.02 | 0.833 | 1.00 | 0.98 to 1.03 | 0.816 | 1.00 | 0.99 to 1.02 | 0.845 | 1.00 | 0.98 to 1.01 | 0.952 | ||
| Sex | ||||||||||||||
| Male | 1582 (75) | 309 (20) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Female | 518 (25) | 103 (20) | 1.02 | 0.82 to 1.27 | 0.869 | 1.02 | 0.81 to 1.27 | 0.891 | 0.83 | 0.69 to 0.99 | 0.039 | 0.83 | 0.69 to 0.99 | 0.039 |
| Age at shoulder dislocation (16- to 35-year-olds) | 0.95 | 0.93 to 0.97 | < 0.001 | 0.95 | 0.93 to 0.97 | < 0.001 | 0.96 | 0.95 to 0.97 | < 0.001 | 0.96 | 0.95 to 0.97 | < 0.001 | ||
| Epilepsy diagnosis? | ||||||||||||||
| No | 2005 (95) | 383 (19) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 95 (5) | 29 (31) | 1.74 | 1.19 to 2.53 | 0.004 | 1.93 | 1.32 to 2.82 | 0.001 | 1.81 | 1.36 to 2.40 | < 0.001 | 2.00 | 1.51 to 2.68 | < 0.001 |
Appendix 13 The effect of surgery within 12 months by stratified quintiles of the propensity score compared with non-surgical treatment on re-dislocations among primary traumatic anterior shoulder dislocation patients who were diagnosed during 1 April 1997–31 March 2015 in Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Propensity score quintiles | HR | 95% CI | p-value |
|---|---|---|---|
| Overall | 1.17 | 0.88 to 1.55 | 0.260 |
| 1 | 0.58 | 0.24 to 1.42 | 0.233 |
| 2 | 1.11 | 0.61 to 2.01 | 0.735 |
| 3 | 0.73 | 0.32 to 1.67 | 0.458 |
| 4 | 1.39 | 0.80 to 2.42 | 0.247 |
| 5 | 2.07 | 1.23 to 3.46 | 0.006 |
Appendix 14 Characteristics of patients stratified by quintiles of the propensity score among those having surgery within 12 months compared with non-surgical treatment on re-dislocations among primary traumatic anterior shoulder dislocation patients who were diagnosed during 1 April 1997–31 March 2015 in Clinical Practice Research Datalink-Hospital Episode Statistics, in England
| Characteristic | Quintiles of the propensity score | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Year of shoulder dislocation, median (IQR) | 2003 (2001–6) | 2006 (2004–8) | 2008 (2006–10) | 2010 (2007–12) | 2012 (2010–13) |
| Sex, n (%) | |||||
| Male | 471 (68) | 589 (85) | 641 (92) | 651 (94) | 676 (97) |
| Female | 225 (32) | 107 (15) | 55 (8) | 44 (6) | 19 (3) |
| Age at shoulder dislocation (years), median (IQR) | 25 (20–30) | 23 (20–29) | 23 (19–27) | 22 (19–26) | 22 (19–26) |
| Drinking status, n (%) | |||||
| Yes | 618 (89) | 569 (82) | 572 (82) | 529 (76) | 490 (71) |
| No | 78 (11) | 127 (18) | 124 (18) | 166 (24) | 205 (30) |
| Epilepsy, n (%) | 9 (1) | 6 (1) | 10 (1) | 19 (3) | 94 (14) |
Glossary
- Clinical Practice Research Datalink
- A database that routinely collects observational data in UK primary care.
- Clinical Practice Research Datalink Read codes
- The dictionary of codes used in Clinical Practice Research Datalink.
- Hospital Episode Statistics
- A secondary care database covering the main types of patient-level NHS hospital activity.
- Hospital Episode Statistics Office of Population Censuses and Surveys 4.7 codes
- The dictionary of secondary care operative codes used in Hospital Episode Statistics.
List of abbreviations
- BMI
- body mass index
- CCI
- Charlson Comorbidity Index
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- EPV
- events per variable
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- ISAC
- Independent Scientific Advisory Committee
- NEISS
- National Electronic Injury Surveillance System
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OPCS
- Office of Population Censuses and Surveys
- SMD
- standardised mean difference
- SQL
- Structured Query Language
- TASD
- traumatic anterior shoulder dislocation
