Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/89/01. The contractual start date was in December 2010. The draft report began editorial review in February 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David G Jayne was a member of the Health Technology Assessment (HTA) Surgery Themed Call Board from 2012 to 2013 and a member of the Efficacy and Mechanism Evaluation Strategy Group from 2015 to 2018. John Scholefield was a member of the HTA Surgery Themed Call Board from 2012 to 2013. Claire T Hulme was a member of the HTA Commissioning Board from 2012 to 2017.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Jayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Fistula-in-ano is a common condition, affecting an estimated 1–2 people in 10,000 of the population. 1,2 It can arise spontaneously, when it is referred to as idiopathic or cryptoglandular, or as a result of an underlying pathology, such as inflammatory bowel disease, malignancy, trauma or irradiation. Idiopathic fistula-in-ano is the commonest aetiology. Anal fistula most commonly affects people in the third to fifth decades of life. It results in significant morbidity through chronic sepsis, causing pain and discharge, and financial implications through time off work and repeated hospital admissions. For those patients who suffer anal sphincter dysfunction as a result of fistula surgery, there is the added distress of faecal incontinence.
The majority of fistulas are low, incorporating only the lower portion of the anal sphincter complex and, as such, are amenable to simple fistulotomy (surgical laying open of the fistula tract), with a reasonable expectation of cure and little risk of incontinence. The remaining ‘high fistulas’, as determined by incorporation of more than one-third of the anal sphincter complex, present a management problem. To cure these fistulas, the tract connecting the internal and external fistula openings has to be eradicated with minimal sacrifice of the sphincter muscle in order to preserve continence.
A variety of surgical treatments have been described for high anal fistulas, but none offers the panacea of fistula eradication with guaranteed preservation of continence. Fistulotomy, cutting seton and advancement flap have all been advocated for high fistulas with varying degrees of success. Fistulotomy is associated with low recurrence rates, variously reported to be between 2% and 9%,3,4 but may be associated with a change in continence in up to 50% of patients. 5 The use of a cutting seton appears to reduce the rate of incontinence, but does not completely eliminate it, with recurrence rates reported to be between 0% and 8%. Minor incontinence is reported in 34–63% of patients treated with a cutting seton, with major incontinence rates between 2% and 26%. 6–12 In addition, the use of a cutting seton is often a protracted process, requiring repeated examination under anaesthesia (EUA) and frequently a completion fistulotomy. Rectal and anal advancement flaps have been advocated as a means of closing high fistulas with preservation of the external sphincter muscle. However, fistula recurrence rates of 25–54% have been reported, with a change of continence in 30–35% of patients. 13,14 Ligation of the intersphincteric fistula tract (LIFT) has recently been described for trans-sphincteric and complex fistulas. 15 Data are currently limited to single-centre studies and only one small randomised controlled trial (RCT). A systematic review and meta-analysis in 2014 reported successful fistula healing in 73% of patients with minimal morbidity and postoperative incontinence. 16
Anal fistula plugs offer an alternative approach to the treatment of anal fistulas. They are composed of bioprosthetic or synthetic materials and used to occlude the fistula tract and provide a physical scaffold for ingrowth of host regenerative cells to promote healing. Several fistula plugs have been developed, but the BioDesign Surgisis® anal fistula plug (Cook Medical, Bloomington, IN, USA), composed of acellular, lyophilised porcine intestinal submucosa, is the most established. Initial encouraging results from Johnson et al. ,17 reporting closure rates of up to 87%, were not reproduced in later studies. A systematic review and meta-analysis published in 2010, including 12 studies and 317 patients, reported a variable healing rate in complex fistula, ranging from 35% to 87%, but with minimal morbidity or incontinence. 18 The main factor determining failure appeared to be early plug extrusion, which was observed in 4–41% of cases. A subsequent meta-analysis in 2016, comparing the fistula plug with advancement flap, reported similar healing rates, but with less incontinence associated with the plug. 19 Although the use of the plug conferred a greater procedure cost, this was offset by a shorter recovery period.
In 2008, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme issued a call for research proposals to investigate the clinical effectiveness and cost-effectiveness of biosynthetic plugs in the treatment of high anal fistula. This was in response to the increasing adoption of the fistula plug into clinical practice against a background of uncertain clinical efficacy and potential increased costs associated with the new technology. An adequately powered RCT was needed to fill the evidence gap, with the primary outcome focusing on patient-reported quality of life (QoL) and secondary outcomes including fistula healing rates, complications, incontinence rates and cost-effectiveness.
The Fistula-In-Ano Trial (FIAT) was designed to address these criteria. The Surgisis anal fistula plug was chosen as the intervention, given its predominance in the market at that time. Owing to the number of alternative surgical techniques, a pragmatic comparator group was chosen, ‘surgeon’s preference’, to encompass the range of surgical practice. This initially included fistulotomy, cutting seton and advancement flap; subsequently, in 2011, the LIFT procedure was added as it gained clinical popularity.
This report presents the final results from the NIHR HTA FIAT. It is the largest known RCT assessing the fistula plug and the largest known trial evaluating different surgical techniques for fistula-in-ano.
Chapter 2 Methods
Trial-related information including the protocol, patient information sheets, consent forms and the case report forms (CRFs) are available at www.birmingham.ac.uk/fiat (accessed 1 February 2018). Please also see the protocol on the project page. 20 A list of protocol variations is given in Table 1.
| Revision number | From protocol version number; date | To protocol version number; date | Summary of changes |
|---|---|---|---|
| 1 | 1.0; 15 April 2010 | 1.1; 18 January 2011 |
|
| 2 | 1.1; 18 January 2011 | 2.0; 20 May 2011 |
|
| 3 | 2.0; 20 May 2011 | 3.0; 8 February 2012 |
|
| 4 | 3.0; 8 February 2012 | 3.1; 7 April 2014 |
|
| 5 | 3.1; 7 April 2014 | 4.0; 22 February 2016 |
|
Objectives
The aim of the FIAT was to compare the Surgisis anal fistula plug with standard surgical treatments for high trans-sphincteric anal fistulas. Standard surgical treatments were the surgeon’s preference of advancement flap, cutting seton, fistulotomy and LIFT procedure.
The specific trial objectives were:
-
to determine whether or not the use of the Surgisis anal fistula plug, compared with standard surgical techniques, results in an improvement in symptom-specific QoL
-
to determine whether or not the use of the Surgisis anal fistula plug, compared with standard surgical techniques, results in a difference in:
-
fistula healing rates
-
complication and reintervention rates
-
faecal incontinence rates
-
cost-effectiveness
-
health economic benefits.
-
Trial design
The FIAT is a pragmatic, Phase III, multicentre RCT with a health economic evaluation. Patients with a confirmed high trans-sphincteric fistula at risk of incontinence with fistulotomy (involving approximately one-third or more of the external sphincter complex) were randomised between the insertion of the Surgisis anal fistula plug and the surgeon’s preference of advancement flap, cutting seton, fistulotomy and LIFT procedure (Figure 1).
FIGURE 1.
Trial schema. MRI, magnetic resonance imaging.

Participants
Inclusion criteria
-
Clinical diagnosis of high trans-sphincteric cryptoglandular fistula-in-ano.
-
Patients must have undergone a prior EUA to characterise the nature of the fistula.
-
The fistula tract should be ≥ 2 cm in length.
-
Only a single internal fistula opening should be present at EUA, such that the fistula is suitable for treatment by insertion of a single fistula plug.
-
Patients must have been treated with a draining seton for a minimum period of 6 weeks prior to randomisation.
-
Patients must be aged ≥ 18 years and able to provide informed consent.
-
Fistulas must be of cryptoglandular aetiology.
Exclusion criteria
-
Unable/unwilling to provide informed consent.
-
Contraindication to general anaesthesia.
-
Low trans-sphincteric fistulas.
-
Non-cryptoglandular fistulas (e.g. Crohn’s disease, obstetric, irradiation, malignant, etc.).
-
Other perineal fistulas (e.g. rectovaginal fistulas, pouch-vaginal fistulas, etc.).
-
Complex disease in which more than one internal fistula opening is present and requiring concurrent insertion of more than one fistula plug.
-
Clinical evidence of active perianal sepsis. In the event that there is disagreement between clinical and radiological assessment of active sepsis/collection, the clinical opinion will prevail.
-
Cultural or religious objection to the use of pig tissue.
-
Absolute contraindication to magnetic resonance imaging (MRI) (e.g. cardiac pacemaker).
-
Patients with recurrent anal fistulas previously treated with a fistula plug.
As it is not known how the presence of an extension or secondary track (defined as an area of sepsis branching away from the primary fistula track, which may include a horseshoe extension or blind sinus track) affects the healing rates of the fistula plug, for the purposes of the FIAT these findings on EUA or MRI were not considered exclusion criteria. However, no evidence of undrained sepsis, either clinically or radiologically, prior to randomisation into the trial was permitted.
Rationale for choice of inclusion and exclusion criteria
The FIAT recruited patients with cryptogenic trans-sphincteric anal fistulas at risk of incontinence with fistulotomy (involving approximately one-third or more of the external sphincter complex).
Patients with recurrent fistulas previously treated by any means other than a fistula plug were eligible for participation in the trial. Patients were not eligible if insertion of a second fistula plug for the treatment of recurrent fistulation was planned.
For the purposes of this trial, a high fistula was defined as one that, on clinical grounds, runs a significant risk of incontinence if treated with fistulotomy (i.e. potentially involves a significant portion of the external sphincter complex). A low fistula is defined as one that can be treated with fistulotomy with minimal risk of long-term incontinence (i.e. has minimal involvement of the external sphincter complex).
Magnetic resonance imaging fistulography prior to randomisation
All patients must have had MRI within 6 months prior to randomisation.
The purpose of the initial MRI was:
-
to provide assessment for evidence of ongoing active perianal sepsis or undrained collection after seton insertion
-
to provide baseline imaging for comparison with the scan either at 12 months for the assessment of healing or sooner if there is treatment failure (recurrence)
-
to confirm the findings at EUA (i.e. consistent with a trans-sphincteric fistula of cryptoglandular origin involving approximately one-third or more of the external sphincter muscle).
All MRI was performed in a minimum of two planes, which included axial and coronal orientations with the imaging plane inclined to the anal canal, using either a STIR (short tau inversion recovery) or fat-saturated T2 sequence with a maximum slice thickness of 5 mm. Thinner slices or additional sequences and imaging planes were permitted according to local radiologist preference, type of magnetic resonance scanner and patient factors.
If undrained collections/extensions were identified on the initial MRI scan, MRI was repeated (using the same parameters described above) after surgical intervention to ensure resolution prior to randomisation.
Standardisation of MRI technique among recruiting sites was assured by holding dedicated training sessions led by the named FIAT radiologist (Tolan). Central review of all MRI studies (pre-randomisation and 12-month follow-up) was performed.
Recruitment and randomisation
Trial sites
Fifty-three UK hospital trusts opened to recruitment to the FIAT (see Appendix 1 for recruiting centres).
Standardisation of fistula plug insertion was ensured through mandatory attendance at a FIAT surgical workshop. All participating surgeons were also required to have performed a minimum of three fistula plug insertions.
All participating radiologists were required to attend a FIAT radiology workshop to quality assure interpretation of the MRI scans used to confirm trial eligibility.
Patient screening
As part of routine investigation, patients underwent EUA to characterise the fistula, according to Parks et al. ’s21 classification, to drain any accompanying sepsis and to insert a draining seton. The seton was left in situ for a minimum of 6 weeks, during which time MRI was performed to further characterise the fistula.
Informed consent
Potential participants were identified from three settings:
-
from the outpatient clinic, in the case of patients presenting with de novo or recurrent perianal sepsis/fistula in whom a high anal fistula was suspected or established
-
from the outpatient clinic, in the case of patients referred specifically for treatment of complex anal fistulas
-
following acute admission for treatment of perianal sepsis.
The informed consent process was supported by the use of patient information sheets. Potential participants received a full explanation of the aim, trial treatment, anticipated benefits and potential hazards of taking part in the trial. It was stressed that the patient was free to refuse to take part or withdraw from the trial at any time. Owing to the length of the screening process for inclusion in the FIAT, all patients had an appropriate length of time to consider inclusion, to read the patient information sheet and to discuss participation with others outside the site research team. Adequate opportunity was given to ask questions.
Written consent was obtained from the participants using the latest version of the informed consent form. Copies of the form were filed in the hospital notes and investigator site file and sent to the Birmingham Clinical Trials Unit (BCTU); the original was given to the patient.
Randomisation
Randomisation was performed once the EUA, seton insertion and MRI assessment had been completed and informed consent obtained. It was recommended that patients were randomised on admission for surgery or as close to the date of surgery as was possible.
Trial participants were randomised online via a secure 24-hour internet-based randomisation service or by a telephone call to the BCTU.
Participants were randomised in a 1 : 1 ratio to either the surgeon’s preference or the fistula plug.
The randomisation used a minimisation algorithm to avoid chance imbalances in important stratification variables. The stratification variables were age (< 30, 30–39, 40–49, 50–59, 60–69, ≥ 70 years), American Society of Anesthesiologists (ASA) grade (P1, P2, P3, P4), planned type of surgery (advancement flap, cutting seton, LIFT procedure, fistulotomy) and presence of extensions (yes, no).
Interventions
Participants were randomised to receive either the fistula plug or the surgeon’s preference (advancement flap, fistulotomy, cutting seton or LIFT procedure).
The technique for the placement of the fistula plug was standardised in accordance with the manufacturer’s recommendations for best practice, with all participating surgeons attending a mandatory training session followed by preceptorship with the first cases.
Anal fistula plug
It was recommended that patients receive a preoperative phosphate enema as bowel preparation and a single dose of intravenous prophylactic antibiotics at the induction of anaesthesia. The choice of antibiotic prophylaxis was at the surgeon’s discretion.
The draining seton was cut and a silk suture secured to one end and the seton removed, which pulled the silk suture into the fistula tract. The silk suture was tied to the end of a Cook fistula brush (Cook Medical, Bloomington, IN, USA), which was used to gently debride the fistula tract. The surgeon could choose to irrigate the fistula tract with saline solution or hydrogen peroxide.
Based on the appearance of the fistula tract, the surgeon decided whether a 7 mm or a 4 mm button fistula plug was required. The selected Surgisis anal fistula plug was rehydrated for 2 minutes in saline solution and secured to the silk suture.
The plug was pulled into the internal opening until resistance was met. The button head of the plug was secured to the internal opening and internal sphincter with a 2/0 Vicryl (Ethicon Inc., Somerville, NJ, USA) or equivalent absorbable suture. At the surgeon’s discretion, a mucosal flap was raised to cover the button head. The tip of the plug was cut flush with the external opening and if necessary the external opening was enlarged to facilitate drainage.
Postoperatively, patients were permitted to eat and drink as tolerated. No further antibiotics were administered and analgesics were administered as necessary. On discharge patients were advised to avoid all strenuous exertion for a period of 2 weeks.
Control arm: surgeon’s preference
The standard surgical techniques used to treat high trans-sphincteric fistula were grouped together as a single comparator and termed ‘surgeon’s preference’. All four techniques were standardised for the trial.
Advancement flap
Patients received a preoperative phosphate enema as bowel preparation and a single dose of intravenous prophylactic antibiotics at the induction of anaesthesia. The choice of antibiotic prophylaxis was at the surgeon’s discretion.
The location of the internal opening was identified and the draining seton removed. A vascularised flap of rectal tissue (rectal flap) or anoderm (anal flap) was mobilised off the underlying internal sphincter or subcutaneous fat and the site of the internal opening on the flap was excised. It was permissible to close the fistula tract with an absorbable suture as it passed through the internal sphincter. The mobilised flap was advanced over the site of the internal opening and sutured to the underlying internal sphincter with an absorbable suture. Postoperatively, patients were permitted to eat and drink as tolerated. No further antibiotics were administered. Stool softeners, bulking agents and analgesics were administered as necessary.
Fistulotomy
Patients received a preoperative phosphate enema as bowel preparation. No perioperative antibiotics were administered unless there was a specific indication (e.g. prosthetic heart valve). The location of the internal opening was identified and the draining seton removed. The course of the primary tract, and of any secondary tracts, was delineated with a fistula probe and the tract(s) laid open. The fistulotomy wound was permitted to be marsupialised as required. Postoperatively, patients were permitted to eat and drink as tolerated. No further antibiotics were be administered. Stool softeners, bulking agents and analgesics were administered as necessary.
Cutting seton
Patients received a preoperative phosphate enema as bowel preparation. No perioperative antibiotics were administered unless there was a specific indication (e.g. prosthetic heart valve). The location of the internal opening was identified and the draining seton removed. The course of the fistula tract was delineated with a fistula probe and a 1/0 Prolene (Ethicon Inc., Somerville, NJ, USA) or equivalent non-absorbable seton material passed through the external opening, primary tract and internal opening. If necessary, the skin bridge between the external opening and the external sphincter was divided. The seton was tied firmly around the fistula tract and the contained sphincter muscle. Postoperatively, patients were permitted to eat and drink as tolerated. Analgesics were administered as necessary. No further antibiotics were administered.
Ligation of intersphincteric fistula tract procedure
Patients received a preoperative phosphate enema as bowel preparation and a single dose of intravenous prophylactic antibiotics at the induction of anaesthesia. The choice of antibiotic prophylaxis was at the surgeon’s discretion. The draining seton was removed and, if helpful, the fistula tract marked by a probe. An intersphincteric dissection was performed to identify and isolate the fistula tract. The tract was ligated and divided. A suture may be placed to secure fistula closure at the surgeon’s discretion. The external fistula tract was curetted and left open to allow drainage. The intersphincteric wound was permitted to be left open or closed.
Blinding
Given the interventions and the outcomes, it was not possible to blind the surgeons, the participants or the outcome assessors.
Trial procedures and assessments
Following recruitment into the trial, participants underwent a clinical examination and measurement of St Mark’s incontinence scores. The baseline Faecal Incontinence Quality of Life (FIQoL) and the EQ-5D were completed.
Data on fistula classification according to Parks et al. 21 and on the use of antibiotics and bowel preparation at the induction of anaesthesia, along with technical details of the surgical procedures, were collected intraoperatively. The surgeon’s opinion of the usefulness of the baseline MRI scan as a guide to surgery was also recorded.
Information on the use of postoperative analgesia, immediate complications and reinterventions was collected at the time of discharge.
Participants were followed up at 6 weeks, 6 months and 12 months post randomisation. The trial ended once all participants had completed 12-month follow-up.
At each follow-up visit, a physical examination was performed to determine evidence of fistula healing. Data on bleeding, unexplained pain and septic events, as well as any other complication thought to be related to the intervention, were collected. Medicinal and surgical reintervention rates (i.e. intervention required for an ongoing complication) and St Mark’s incontinence scores were collected at each follow-up visit. Additionally, the FIQoL and EQ-5D questionnaires were completed at the same time points.
At 12 months, all patients underwent follow-up MRI.
Data on serious adverse events (SAEs) were collected at all time points.
Serious adverse events
Any adverse events meeting the definition of a SAE were recorded on a standardised SAE form and faxed to the BCTU within 24 hours of the local principal investigator (PI) or a member of their research team becoming aware of the event. The PI was responsible for assigning causality to the SAE before reporting.
For the purposes of the FIAT, SAEs included, but were not limited to:
-
unexpected events occurring during the surgical intervention (e.g. excessive bleeding)
-
significant postoperative bleeding above that normally expected following the surgical intervention, and any bleeding requiring transfusion or surgical intervention for haemostasis
-
urinary retention requiring catheterisation
-
postoperative pain above that normally expected following the surgical intervention
-
perianal or perineal sepsis requiring hospitalisation or surgical intervention
-
faecal incontinence or defecatory disturbance above that normally expected following the surgical intervention
-
complications related to the administration of the general anaesthetic or other medications (e.g. allergic response to antibiotics)
-
unexpected events related to MRI fistulography.
Outcome measures
The primary outcome measure for the trial was QoL measured using the validated, symptom-specific FIQoL. QoL was assessed at baseline, at 6 weeks and at 6 and 12 months post randomisation.
Symptom-specific QoL was chosen as the primary outcome, rather than fistula healing rates, as it reflects the primary aim of fistula surgery (to produce symptom relief while maintaining anal sphincter function and preserving symptom-specific QoL).
The secondary outcome measures were:
-
fistula healing rate at 12 months
-
faecal incontinence rates (as measured by St Mark’s incontinence score) at baseline and at 6 and 12 months
-
complication rates at 6 weeks and at 6 and 12 months
-
rates of reintervention at 6 and 12 months
-
generic QoL assessed EQ-5D and visual analogue scale scores at baseline, 6 weeks, 6 months and 12 months.
Fistula healing at 12 months was assessed by clinical examination. To be deemed to have healed, there had to be no visible external opening and no sign of ongoing sepsis or discharge.
Sample size
The primary outcome measure in the FIAT is symptom-specific QoL measured using the FIQoL questionnaire at baseline, 6 weeks, 6 months and 12 months.
It was estimated that a total of 400 patients would need to be recruited in a 1 : 1 ratio (200 patients to the fistula plug group and 200 patients to the surgeon’s preference group) to be able to detect a small to moderate treatment effect [effect size 0.3 standard deviation (SD)] (i.e. a difference in the primary end point between the two arms of the trial). To allow for a 20% non-compliance rate (non-acceptance, loss to follow-up, incomplete data), the aim was to recruit a total of 500 patients.
The choice of the 0.3 SD treatment effect size was pragmatic. An effect size of 0.2 SD is considered small, 0.5 moderate and 1.0 large. 23 Randomisation of 500 patients in total would provide good statistical power (80% at p < 0.05) to detect an effect size of 0.25 SD, high power (78% at p < 0.01) to detect an effect size of 0.3 SD and very high power (97% at p < 0.01) to detect an effect size of 0.4 SD.
Recruitment to the FIAT was slower than anticipated for a variety of reasons. Lack of surgical equipoise and availability of the plug outside the trial contributed to the low recruitment rate, but the main reason was a lower prevalence of eligible patients than expected. The most common reasons for ineligibility were complex fistula and reclassification owing to MRI results; all patients underwent MRI and a higher proportion than anticipated were reclassified. This led to a change in the diagnostic threshold. The assumptions in the original application were based on routine practice and traditional clinical classification at the time; most surgeons were not using MRI. Practice changed during, and potentially as a result of, the trial, and the unintended consequence was that MRI became more commonplace.
In January 2015, it was agreed with the HTA programme that the sample size for the FIAT would be reduced. If 300 patients were randomised (270 plus 10% dropout), then the FIAT would have 69% power to detect a small to moderate (0.3 SD) treatment effect, or 98% power to detect a moderate (0.5 SD) treatment effect (with α = 0.05).
Statistical methods
The primary outcome measure in the FIAT is symptom-specific QoL measured using the FIQoL questionnaire at baseline and at 6 weeks, 6 months and 12 months. The questionnaire comprises 29 multiple-choice questions grouped into four domains: lifestyle, coping/behaviour, depression/self-perception and embarrassment. Data obtained from the questionnaire were converted into scores using the validated method provided by the developers. Longitudinal plots of mean scores at baseline and over time by treatment group were produced for visual presentation of the data. The primary analysis is a comparison of the mean difference in FIQoL scores between the treatment groups from a repeated measures model (a statistically efficient approach that allows all of the follow-up data collated during the trial to be used, which further enhances statistical power). The model incorporates the 6-week, 6-month and 12-month time points. In addition, the baseline score is included as a covariate in the model. Separate models were constructed for each of the four domains of the FIQoL questionnaire. Further models that included a time-by-treatment interaction term were also fitted. Mean differences and 95% confidence intervals (CIs) were reported.
In addition to the primary adjusted intention-to-treat (ITT) analysis, a ‘per-protocol’ analysis was undertaken for the primary outcome as a sensitivity analysis to explore the potential effect of non-adherence to the randomised allocation. Participants were classified with respect to the first intervention they received, rather than the intervention to which they were randomised. Participants who did not have surgery were excluded from the analysis.
Four a priori subgroup analyses were planned for the primary outcome. These subgroups were for the minimisation variables: age at randomisation (< 30, 30–39, 40–49, 50–59, 60–69, ≥ 70 years), ASA grade (P1, P2, P3, P4), planned type of surgery (advancement flap, cutting seton, LIFT procedure, fistulotomy) and presence of extensions (yes, no). A treatment group-by-subgroup interaction parameter was included in the repeated measures model to assess whether or not there were any differences in the treatment effect across the different strata. Mean differences and 95% CIs were reported.
Data regarding fistula healing were recorded at the 6-week, 6-month and 12-month time points. The fistula healing rates in the two treatment groups were compared using a chi-squared test. Relative risks and 95% CIs were reported.
Faecal incontinence was measured using the St Mark’s incontinence score at baseline and at 6 weeks, 6 months and 12 months. St Mark’s incontinence scores were modelled at each time point, including treatment as a covariate. Mean differences and 95% CIs were reported.
Complication data relating to bleeding, unexplained pain and septic events were recorded at discharge (following operative procedure) and at 6 weeks, 6 months and 12 months. Complication data relating to urinary retention were recorded at discharge (following operative procedure) only. Data regarding participants’ need for reintervention were also recorded at the same time points. Reinterventions were classified as medicinal or surgical by the chief investigator of the trial. The overall complication rates and reintervention rates in the two treatment groups were compared at each time point separately using a chi-squared test. Relative risks and 95% CIs were reported. The proportions of participants experiencing each individual type of complication were also presented, as were the proportions of participants receiving medicinal or surgical reintervention. Time to first reintervention and time to first surgical reintervention were both analysed using a Cox regression model. Hazard ratios and 95% CIs were reported in both cases.
General QoL was assessed using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire at baseline and at 6 weeks, 6 months and 12 months. The questionnaire comprises five multiple-choice questions, each with three possible responses, and a visual analogue scale from 0 to 100. Data obtained from the questionnaire were converted into scores using the validated method provided by the developers. Longitudinal plots of mean scores at baseline and over time by treatment group were produced for visual presentation of the data. The scores in the two treatment groups were compared using repeated-measures models in the same manner as described for the primary outcome. Separate models were constructed for the health status score and visual analogue scale.
Subgroup analyses were carried out for fistula healing, faecal incontinence and EQ-5D-3L in the same manner as described for the analysis of subgroups for the primary outcome.
The SAE data were summarised descriptively. The SAE data were also analysed as a dichotomous variable, with each participant classed as either having or not having experienced a SAE. The two treatment groups were compared using a chi-squared test.
Estimates of treatment effects are presented with 95% CIs and p-values are two-tailed with a p-value < 0.05 considered to be statistically significant. No corrections for multiple tests were made. All analyses were carried out using SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA) or Stata® 14 (StataCorp LP, College Station, TX, USA).
Cost-effectiveness analysis
The aim of the economic evaluation was to assess the cost-effectiveness of the Surgisis anal fistula plug compared with surgeon’s preference for the treatment of trans-sphincteric fistula-in-ano.
The evaluation was performed using a UK NHS and Personal Social Services (PSS) perspective. The evaluation estimated the incremental cost-effectiveness ratios (ICERs) of the fistula plug compared with the surgeon’s preference at 12 months. It was planned that the results would be extrapolated using a decision-analytic model to estimate lifetime cost-effectiveness.
Resource use data
Resource use data collected from patients at 6 weeks, 6 months and 12 months were combined with data collected during the trial, including operation costs. Unit costs to estimate the total health resource use cost for each participant were informed by national sources, such as the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2017,24 NHS Reference Costs25 and the British National Formulary (BNF) 2018. 26
Utilities and quality-adjusted life-years
Health-related quality of life (HRQoL) of the trial participants was estimated using the EQ-5D-3L, which was administered alongside the participant resource use questionnaires. EQ-5D-3L scores were obtained at baseline and at 6 weeks, 6 months and 12 months, with differences between treatment arms assessed using two sample t-tests.
The primary health-related outcome measure used in the cost-effectiveness analysis was the quality-adjusted life-year (QALY), measured using the QoL scores obtained from the EQ-5D-3L questionnaires [in line with the National Institute for Health and Care Excellence’s (NICE) reference case27]. The responses to the EQ-5D-3L questionnaire were converted to utilities using standard UK tariff values. 28 QALYs were calculated by multiplying these values by the time spent in each state, with QoL linearly interpolated for the periods between the four observations provided in the trial data. Average QALYs between adjacent time points were calculated to generate smoothed estimates between the time points.
Missing data
First, patient-level analysis on complete cases was conducted; this required data on total QALYs and total costs. The total cost per participant was calculated from the UK NHS and PSS perspectives by adding the costs associated with the operations, including the costs of inpatient stay and postoperative costs, as well as the costs of further consultations, prescriptions, treatment and applicable intervention costs for all participants for whom response data were available. Multiple imputation by chained equations was then used to impute missing EQ-5D-3L data and individual components of total costs at all three time points. No imputation was undertaken for baseline values.
Economic model
It was anticipated that the cost-effectiveness of the Surgisis anal fistula plug beyond the trial period would be assessed through Markov modelling, allowing the outcomes to be extrapolated beyond the trial period. The Markov model would be formed using the 12-month data from the trial in addition to the published literature on longer-term outcomes and expert judgement, as necessary.
Sensitivity analysis
Probabilistic sensitivity analysis was used to assess uncertainty and the results presented using a cost-effectiveness plane and cost-effectiveness acceptability curve.
Patient and public involvement
The FIAT was developed by the Research and Audit Committee of the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and supported by the ACPGBI membership and Executive Council (which includes a patient liaison group). Two independent patient representatives were involved in the trial from conception as members of the Trial Management Group.
Ethics approval, regulations and trial registration
Ethics approval for the trial was granted by the National Research Ethics Service Committee East Midlands – Derby Research Ethics Committee (reference number 10/H0405/29).
The trial was conducted in accordance with the recommendations guiding physicians in biomedical research involving human subjects, adopted by the 18th World Medical Association General Assembly in Helsinki, Finland, June 1964,29 and its subsequent amendments, the Research Governance Framework for Health and Social Care,30 and the applicable UK statutory instruments including the Data Protection Act 199831 and the International Conference on Harmonisation Guidelines for Good Clinical Practice.
The trial was prospectively registered as ISRCTN78352529.
Chapter 3 Results
Screening
A total of 1355 patients were assessed for eligibility. Of these, 731 patients did not meet the eligibility criteria, 100 patients were eligible but were not randomised and 220 patients were still undergoing screening at the time of trial closure.
Reasons for ineligibility were available for 581 (79%) out of the 731 ineligible patients (Figure 2). Of these 581 patients, 437 (75%) presented with ineligible fistula morphology: complex fistula disease (n = 92), extrasphincteric fistulas (n = 3), fistula healed (n = 23), fistula tract too short (n = 18), high supralevator fistula (n = 1), intrasphincteric fistula (n = 43), low fistula (n = 122), non-cryptoglandular fistula (n = 71), other perineal fistula (n = 32), other unspecified fistula reason (n = 2), no evidence of fistula (n = 19), superficial fistula (n = 9) and suprasphincteric fistula (n = 2).
FIGURE 2.
Reasons for ineligibility.

A total of 94 (16%) patients did not meet the eligibility requirements because of the presence of coexistent anorectal pathology (e.g. anal fissure, haemorrhoids, pilonidal sinus).
A total of 33 (6%) patients were ineligible because of their treatment pathway: previously treated with the fistula plug (n = 18), draining seton not required (n = 5), draining seton not yet in place for 6 weeks (n = 1), cutting seton already in place (n = 1), draining seton to remain in situ (n = 1), contraindication to MRI (n = 5) and MRI outside acceptable time frame (n = 2).
Thirteen (2%) patients were not suitable for surgery; one patient was excluded because she was pregnant and three patients were unable to give informed consent.
Of the 100 patients who were eligible but not randomised, 79 did not want to participate in the trial and the remainder indicated a preference for one of the treatment allocations (12 wanted to receive a fistula plug and nine wanted to receive the surgeon’s preferred treatment).
Recruitment
The FIAT opened to recruitment in May 2011, and the first participant was recruited into the trial on 24 May 2011. A total of 304 participants were recruited and randomised, with the last patient entering the trial on 10 March 2016. Participants were split equally between the two randomised treatment allocations: 152 participants were randomised to receive the fistula plug and 152 were randomised to the surgeon’s preference. The 304 participants were recruited from 40 centres, 75% of those open to recruitment. The number of participants recruited at each site ranged from 1 to 32 (see Appendix 2). Recruitment figures by month are shown in Figure 3 and recruitment figures by centre are shown in Table 2. All participants had reached the 12-month follow-up time point by March 2017.
FIGURE 3.
Recruitment by month.

| Centre | Number of participants randomised |
|---|---|
| Southend University Hospital NHS Foundation Trust (Southend Hospital) | 32 |
| Leeds Teaching Hospitals NHS Trust (St James’s University Hospital/Leeds General Infirmary) | 28 |
| Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital) | 20 |
| Nottingham University Hospitals NHS Trust (Queen’s Medical Centre) | 20 |
| Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary) | 17 |
| Sandwell and West Birmingham Hospitals NHS Trust (Sandwell General Hospital) | 16 |
| Cardiff & Vale University Health Board (Llandough University Hospital/University Hospital of Wales) | 13 |
| Taunton & Somerset NHS Foundation Trust (Musgrove Park Hospital) | 11 |
| Burton Hospitals NHS Foundation Trust (Queen’s Hospital Burton) | 10 |
| University Hospitals Bristol NHS Foundation Trust (Bristol Royal Infirmary) | 9 |
| Dorset County Hospital NHS Foundation Trust (Dorset County Hospital) | 9 |
| Royal United Hospitals Bath NHS Foundation Trust (Royal United Hospitals Bath) | 8 |
| NHS Highland (Raigmore Hospital) | 8 |
| Chesterfield Royal Hospital NHS Foundation Trust (Chesterfield Royal Hospital) | 8 |
| Oxford University Hospitals NHS Foundation Trust (Churchill Hospital/John Radcliffe Hospital) | 8 |
| Wirral University Teaching Hospital NHS Foundation Trust (Arrowe Park Hospital) | 7 |
| University Hospitals Birmingham NHS Foundation Trust (Queen Elizabeth Hospital Birmingham) | 7 |
| Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich University Hospital) | 6 |
| Homerton University Hospital NHS Foundation Trust (Homerton University Hospital) | 6 |
| University Hospitals of Leicester NHS Trust (Leicester General Hospital) | 6 |
| Croydon Health Services NHS Trust (Croydon University Hospital) | 5 |
| Mid Essex Hospital Services NHS Trust (Broomfield Hospital) | 5 |
| The Mid Yorkshire Hospitals NHS Trust (Pinderfields General Hospital/Dewsbury and District Hospital) | 5 |
| Gloucestershire Hospitals NHS Foundation Trust (Cheltenham General Hospital) | 4 |
| Imperial College Healthcare NHS Trust (Charing Cross Hospital/St Mary’s Hospital) | 4 |
| Heart of England NHS Foundation Trust (Birmingham Heartlands Hospital/Good Hope Hospital) | 4 |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (St Peter’s Hospital) | 3 |
| Calderdale and Huddersfield NHS Foundation Trust (Huddersfield Royal Infirmary) | 3 |
| Southport and Ormskirk Hospital NHS Trust (Southport and Formby District General Hospital) | 3 |
| George Eliot Hospital NHS Trust (George Eliot Hospital) | 3 |
| Yeovil District Hospital NHS Trust (Yeovil District Hospital) | 2 |
| Hillingdon Hospitals NHS Foundation Trust (Hillingdon Hospital) | 2 |
| Ipswich Hospital NHS Trust (Ipswich Hospital) | 2 |
| Royal Liverpool and Broadgreen University Hospitals NHS Trust (The Royal Liverpool University Hospital) | 2 |
| South Tees Hospitals NHS Foundation Trust (The James Cook University Hospital) | 2 |
| Barking, Havering and Redbridge University Hospitals NHS Trust (Queen’s Hospital) | 2 |
| St Helens and Knowsley Teaching Hospitals NHS Trust (Whiston Hospital) | 1 |
| Poole Hospital NHS Foundation Trust (Poole Hospital) | 1 |
| The Royal Wolverhampton NHS Trust (New Cross Hospital) | 1 |
| Aneurin Bevan University Health Board (Nevill Hall Hospital) | 1 |
Participant flow
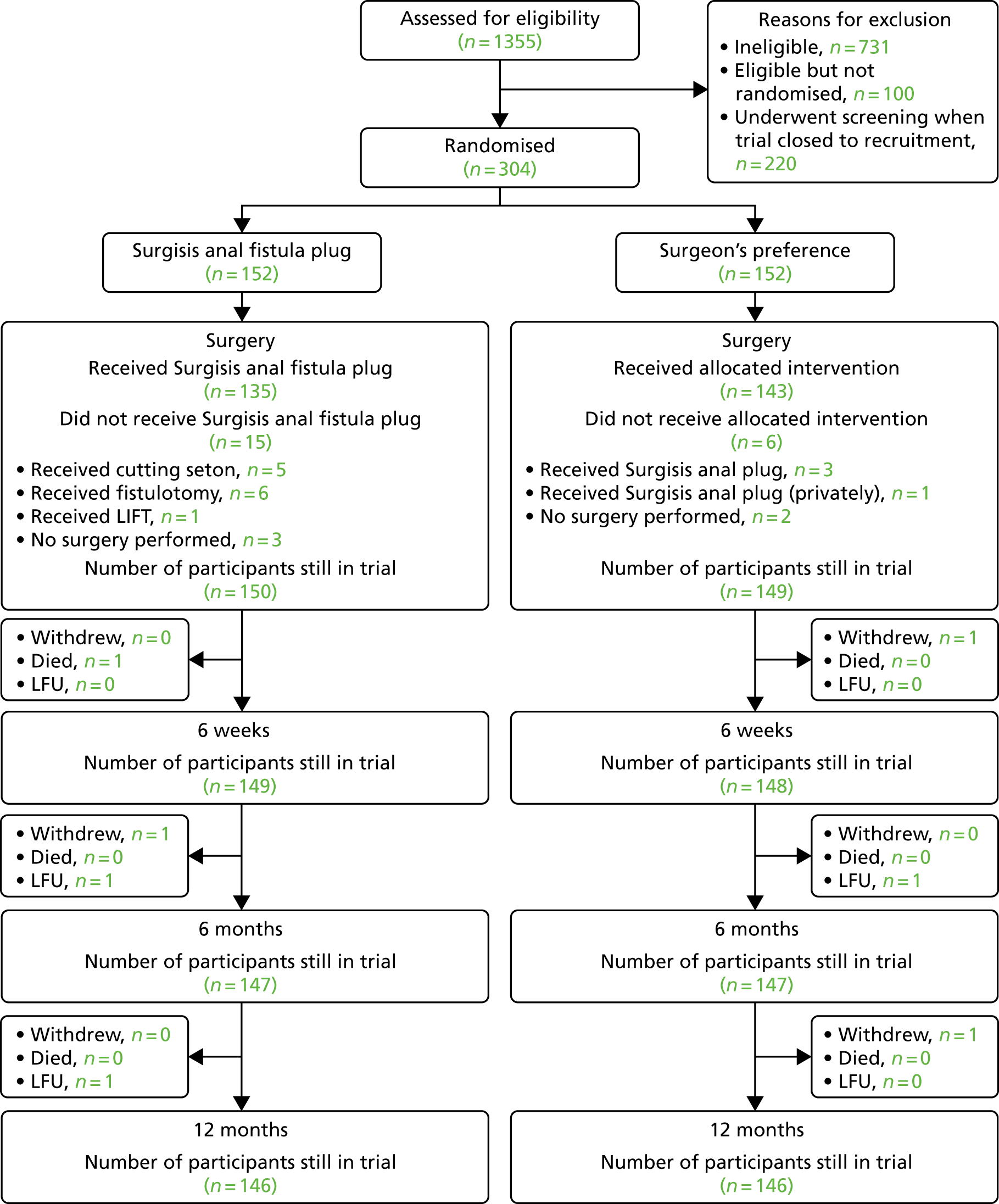
Of the 304 participants randomised into the FIAT, a total of eight (2.6%) withdrew their consent to remain in the trial. These withdrawals occurred at a range of time points through the trial: five participants withdrew consent prior to any trial treatment, one participant withdrew consent post surgery, one participant withdrew consent before the 6-month time point and one participant withdrew consent before the 12-month time point. Three patients were lost to follow-up: two participants prior to the 6-month time point and one participant before the 12-month time point. In the case of participants who withdrew consent or were lost to follow-up, the data collected up to the point of trial exit were used in the analyses. See Figure 4 for the Consolidated Standards of Reporting Trials (CONSORT) flow diagram with further details of participant flow.
FIGURE 4.
Consolidated Standards of Reporting Trials flow diagram. LFU, lost to follow-up.
Data completeness
Compliance with data collection was good (see Appendix 3). Baseline data were complete for 151 (99%) of the 152 participants in the fistula plug group and for 150 (99%) of the 152 participants in the surgeon’s preference group. Baseline radiology, which was mandatory for inclusion in the trial, was carried out in all participants. Follow-up MRI data were available for analysis for a total of 110 (75%) of the 146 of participants in the fistula group and 112 (77%) of the 146 participants in the surgeon’s preference group. Compliance with the collection of postoperative follow-up data was excellent, with complete data available for 99% of the trial population at the operative and postoperative time points. There was a gradual loss of surgical follow-up data with time, but levels remained acceptable (94% at 6 weeks, 88% at 6 months and 85% at 12 months). Similarly, the completeness of data collected from the patient-reported questionnaires (EQ-5D-3L and FIQoL) decreased with the increased length of follow-up (being 87% at 12 months).
Baseline data
The baseline characteristics of recruited participants, overall and by randomisation group, are shown in Table 3. The majority of participants in each group were classified as ASA I (normal, healthy patient) and were aged between 30 and 60 years (mean 45.1 years, range 18–83 years). There were more men (n = 167, 55%) than women (n = 137, 45%). The most frequently selected surgical procedures, prior to randomisation, were the LIFT procedure (n = 116, 38%) and cutting seton (n = 114, 38%), followed by advancement flap (n = 66, 22%) and fistulotomy (n = 8, 2%). There was no difference in comorbidity between the groups, with smokers making up 23% and 25% of the fistula plug and surgeon’s preference groups, respectively.
| Patient characteristic | Surgisis anal fistula plug (N = 152) | Surgeon’s preference (N = 152) | All participants (N = 304) |
|---|---|---|---|
| Minimisation variable | |||
| ASA grade, n (%) | |||
| P1 (normal, healthy patient) | 118 (78) | 117 (77) | 235 (77) |
| P2 (mild systemic disease) | 31 (20) | 30 (20) | 61 (20) |
| P3 (severe systemic disease) | 3 (2) | 5 (3) | 8 (3) |
| Age at randomisation (years), n (%) | |||
| < 30 | 23 (15) | 22 (15) | 45 (15) |
| 30–39 | 39 (26) | 36 (24) | 75 (25) |
| 40–49 | 35 (23) | 45 (30) | 80 (26) |
| 50–59 | 33 (22) | 29 (19) | 62 (21) |
| 60–69 | 12 (8) | 10 (6) | 22 (7) |
| ≥ 70 | 10 (6) | 10 (6) | 20 (6) |
| Type of surgery, n (%) | |||
| Advancement flap | 32 (21) | 34 (22) | 66 (22) |
| Fistulotomy | 6 (3) | 2 (1) | 8 (2) |
| Cutting seton | 57 (38) | 57 (38) | 114 (38) |
| LIFT procedure | 57 (38) | 59 (39) | 116 (38) |
| Secondary extensions at baseline EUA, n/N (%)a | 19/107 (18) | 17/105 (16) | 36/212 (17) |
| Patient characteristic | |||
| Age (years) | |||
| Age at randomisation, mean (SD, n) | 45.2 (14.1, 152) | 44.9 (13.7, 152) | 45.1 (13.9, 304) |
| Range | 20–83 | 18–80 | 18–83 |
| Sex, n (%) | |||
| Male | 86 (57) | 81 (53) | 167 (55) |
| Female | 66 (43) | 71 (47) | 137 (45) |
| Missing | 1 | 2 | 3 |
| Smoker, n (%) | 35 (23) | 38 (25) | 73 (24) |
| Missing | 1 | 2 | 3 |
| St Mark’s incontinence scoreb | |||
| Median (IQR, n) | 4 (1–6, 151) | 4 (2–8, 152) | 4 (2–7, 303) |
| Range | 0–21 | 0–18 | 0–21 |
| Incontinence for solid stools, n (%) | |||
| Never | 132 (89) | 134 (89) | 266 (89) |
| Rarely | 6 (4) | 6 (4) | 12 (4) |
| Sometimes | 8 (5) | 8 (5) | 16 (5) |
| Weekly | 0 | 1 (1) | 1 (1) |
| Daily | 3 (2) | 1 (1) | 4 (1) |
| Missing | 3 | 2 | 5 |
| Incontinence for liquid stools, n (%) | |||
| Never | 112 (75) | 103 (69) | 215 (72) |
| Rarely | 10 (7) | 18 (12) | 28 (9) |
| Sometimes | 21 (14) | 19 (13) | 40 (14) |
| Weekly | 4 (3) | 5 (3) | 9 (3) |
| Daily | 2 (1) | 5 (3) | 7 (2) |
| Missing | 3 | 2 | 5 |
| Incontinence for gas, n (%) | |||
| Never | 100 (67) | 91 (61) | 191 (64) |
| Rarely | 12 (8) | 10 (7) | 22 (7) |
| Sometimes | 24 (16) | 29 (19) | 53 (18) |
| Weekly | 3 (2) | 3 (2) | 6 (2) |
| Daily | 10 (7) | 17 (11) | 27 (9) |
| Missing | 3 | 2 | 5 |
| Alteration in lifestyle, n (%) | |||
| Never | 74 (50) | 72 (48) | 146 (49) |
| Rarely | 13 (9) | 10 (7) | 23 (8) |
| Sometimes | 20 (13) | 22 (15) | 42 (14) |
| Weekly | 7 (5) | 14 (9) | 21 (7) |
| Daily | 35 (23) | 32 (21) | 67 (22) |
| Missing | 3 | 2 | 5 |
| Wear a pad/plug, n (%) | 73 (49) | 63 (42) | 136 (46) |
| Missing | 4 | 2 | 6 |
| Taking constipation medicine, n (%) | 9 (6) | 19 (13) | 28 (9) |
| Missing | 4 | 2 | 6 |
| Lack of ability to defer defecation for 15 minutes, n (%) | 25 (17) | 34 (23) | 59 (20) |
| Missing | 4 | 2 | 6 |
| Fistula history | |||
| Acute sepsis/abscess, n (%) | 63 (42) | 71 (48) | 134 (45) |
| Missing | 2 | 4 | 6 |
| Chronic sepsis/fistula, n (%) | 98 (65) | 84 (56) | 182 (60) |
| Missing | 1 | 2 | 3 |
| First/recurrent fistula, n (%) | |||
| First | 101 (70) | 98 (70) | 199 (70) |
| Recurrent | 44 (30) | 42 (30) | 86 (30) |
| Missing/unknownc | 7 | 12 | 19 |
| Previous fistula surgery, n (%) | 64 (42) | 73 (48) | 137 (45) |
| Number of previous fistula surgeries | |||
| Median (IQR, n) | 2 (1–2, 63) | 1 (1–3, 73) | 2 (1–2, 136) |
| Range | 1–13 | 1–12 | 1–13 |
| Type of previous fistula surgery, n/N (%) | |||
| Fistulotomy | 10/63 (16) | 13/73 (18) | 23/136 (17) |
| Seton | 57/63 (90) | 64/73 (88) | 121/136 (89) |
| Advancement flap | 2/63 (3) | 2/73 (3) | 4/136 (3) |
| Fistula plug | 0/63 (0) | 0/73 (0) | 0/136 (0) |
| Other | 11/63 (17) | 23/73 (32) | 34/136 (25) |
| Missing | 1 | 0 | 1 |
| Previous anorectal surgery, n (%) | 29 (19) | 31 (21) | 60 (20) |
| Missing | 1 | 3 | 4 |
| EUA | |||
| Trans-sphincteric, n (%) | 150 (99) | 149 (99) | 299 (99) |
| Missing | 1 | 2 | 3 |
| Length of primary tract (cm) | |||
| Median (IQR, n) | 3.5 (3.0–4.0, 148) | 3.0 (2.5–4.0, 145) | 3.0 (3.0–4.0, 293) |
| Range | 1.5–12.0 | 1.5–8.0 | 1.5–12.0 |
| Level of internal opening in relation to dentate line, n (%) | |||
| Below | 12 (8) | 21 (14) | 33 (11) |
| At | 96 (64) | 99 (66) | 195 (65) |
| Above | 43 (28) | 30 (20) | 73 (24) |
| Missing | 1 | 2 | 3 |
| Extent of external sphincter involvement, n (%) | |||
| Less than one-third | 18 (12) | 20 (13) | 38 (12) |
| One-third | 5 (3) | 3 (2) | 8 (3) |
| More than one-third | 127 (85) | 127 (85) | 254 (85) |
| Missing | 2 | 2 | 4 |
| Secondary tracts, n (%) | 17 (11) | 19 (13) | 36 (12) |
| Missing | 1 | 2 | 3 |
| Number of secondary tracts | |||
| Median (IQR, n) | 1.0 (1.0–1.0, 17) | 1.0 (1.0–1.0, 19) | 1.0 (1.0–1.0, 36) |
| Range | 1.0–1.0 | 1.0–1.0 | 1.0–1.0 |
| Supralevator extension, n (%) | 4 (3) | 4 (3) | 8 (3) |
| Missing | 1 | 2 | 3 |
| Horseshoe extensions, n (%) | 10 (7) | 6 (4) | 16 (5) |
| Missing | 4 | 3 | 7 |
| Active sepsis/abscess, n (%) | 27 (18) | 26 (17) | 53 (18) |
| Missing | 1 | 2 | 3 |
| Seton inserted, n (%) | 149 (99) | 149 (99) | 298 (99) |
| Missing | 1 | 2 | 3 |
| Radiology MRI | |||
| Seton present in track, n (%) | |||
| No | 30 (20) | 28 (18) | 58 (19) |
| Yes | 90 (59) | 103 (68) | 193 (64) |
| Cannot identify | 32 (21) | 21 (14) | 53 (17) |
| Fistula type, n (%) | |||
| Superficial | 3 (2) | 1 (1) | 4 (1) |
| Intersphincteric | 14 (9) | 12 (8) | 26 (9) |
| Trans-sphincteric | 132 (87) | 138 (90) | 270 (89) |
| Supralevator | 0 (0) | 1 (1) | 1 (< 1) |
| Extrasphincteric | 1 (1) | 0 (0) | 1 (< 1) |
| Blind sinus | 1 (1) | 0 (0) | 1 (< 1) |
| Missing | 1 | 0 | 1 |
| Extensions present, n (%) | 41 (27) | 35 (23) | 76 (25) |
| Number of extensions | |||
| Median (IQR, n) | 1.0 (1.0–1.0, 41) | 1.0 (1.0–1.0, 35) | 1.0 (1.0–1.0, 76) |
| Range | 1.0–2.0 | 1.0–3.0 | 1.0–3.0 |
| Location of extensions, n/N (%) | |||
| Intersphincteric | 17/41 (41) | 19/35 (54) | 36/76 (47) |
| Ischioanal fossa | 24/41 (60) | 18/35 (51) | 42/76 (56) |
| Supralevator | 6/41 (15) | 2/35 (6) | 8/76 (11) |
| MRI concordant with EUA, n (%) | 50 (33) | 4 (3) | 102 (34) |
| Missing | 0 | 2 | 2 |
| MRI depicts additional findings vs. EUA, n (%) | 11 (7) | 10 (7) | 21 (7) |
| Missing | 0 | 2 | 2 |
The overall incidence of baseline incontinence symptoms, as judged by the St Mark’s incontinence score, was low and similar in the two groups [fistula plug, median incidence 4 (interquartile range 1–6); surgeon’s preference, median incidence 4 (interquartile range 2–8)]. A total of 136 (46%) of the 304 participants reported wearing a pad, which might reflect fistula discharge rather than true anal incontinence.
Chronic sepsis was reported in 98 (65%) patients in the fistula plug group and in 84 patients (56%) in the surgeon’s preference group, with the remainder reporting acute or acute on chronic sepsis. Recurrent fistulas were experienced by 30% of patients in each group, with 64 (42%) patients in the fistula plug group and 73 (48%) patients in the surgeon’s preference group undergoing previous fistula surgery. Overall, the number of prior fistula surgeries was similar between the groups (median 2, range 1–13), with the most frequent operations being seton drainage in 121 of 136 (89%) patients and fistulotomy in 23 of 136 (17%) patients.
All fistulas were deemed to be trans-sphincteric at EUA, although data were missing for one participant in the fistula plug group and two participants in the surgeon’s preference group. The morphology of the fistulas at baseline EUA was similar in both groups, with an overall median primary tract length of 3.0 cm (range 1.5–12.0 cm) and secondary tracts present in 36 (12%) patients. All patients underwent insertion of a draining seton, with the exception of one participant in the fistula plug group and two participants in the surgeon’s preference group for whom data were missing.
In around one-third of participants, gadolinium and oedema MRI sequences were obtained, with similar practices observed at baseline and on follow-up assessment. Baseline MRI characterised the fistula morphology as trans-sphincteric in 132 out of 152 (87%) participants in the fistula plug group and in 138 out of 152 (91%) participants in the surgeon’s preference group, with low numbers of intersphincteric (overall 26, 9%) and superficial (overall 4, 1%) fistulas reported. Secondary extensions on MRI were reported in 25% of cases and at EUA in 12% of cases, with similar numbers of secondary extensions reported in the two groups (overall median 1, range 1–3).
The majority of surgeons (overall 255/304, 84%) only reviewed the MRI report prior to surgery, with only around half actually reviewing the images. Approximately half of surgeons felt that MRI was a useful guide to surgery, with the other half reporting little or no benefit. Review of the MRI findings was reported to alter the surgical approach to a major degree in 12 (4%) of 304 cases and to a minor degree in 37 (12%) of 304 cases.
Compliance with randomisation allocation
Of the 152 participants randomised to the fistula plug group, 135 received their randomised allocation. In 15 participants (9.9%), treatment was non-compliant with the allocated treatment (five received a cutting seton, six received fistulotomy, one received LIFT and three did not receive any surgery). Two participants withdrew from the trial prior to surgery and their treatment is not known.
Of the 152 participants randomised to the surgeon’s preference group, 143 received some form of surgical intervention. Six participants (3.9%) were known to have been non-compliant with their randomised treatment allocation (four received a fistula plug and two did not receive any surgery). Three participants withdrew from the trial prior to surgery and their treatment is not known. At randomisation, all investigators had to indicate which of the four surgeon’s preference options (advancement flap, fistulotomy, LIFT or cutting seton) would be performed if the participant were to be randomised to the surgeon’s preference group. In total, 85% of participants received the type of surgery that was planned (Table 4). Only two fistulotomies were planned, but 13 were carried out.
| Planned surgery | Received surgery | ||||
|---|---|---|---|---|---|
| Advancement flap | Cutting seton | LIFT procedure | Fistulotomy | Total | |
| Advancement flap | 25 | 2 | 3 | 2 | 32 |
| Cutting seton | 0 | 43 | 3 | 7 | 53 |
| LIFT procedure | 0 | 2 | 52 | 2 | 56 |
| Fistulotomy | 0 | 0 | 0 | 2 | 2 |
| Total | 25 | 47 | 58 | 13 | 143 |
Overall compliance with randomised treatment was 91.4% (278/304).
Primary end point: quality of life
The primary objective of the FIAT was to compare the fistula plug with standard treatments for high trans-sphincteric anal fistulas in terms of QoL. Symptom-specific QoL was chosen rather than fistula healing rates because it reflects the primary aim of fistula surgery: to provide symptom relief while maintaining anal sphincter function and preserving symptom-specific QoL.
The validated questionnaire used in the FIAT to assess symptom-specific QoL was the FIQoL. Data from this questionnaire were collected at baseline and at 6 weeks, 6 months and 12 months. The questionnaire comprises 29 multiple-choice questions grouped into four domains: lifestyle, coping/behaviour, depression/self-perception and embarrassment. FIQoL domain scores range from 1 to 4, where higher scores indicate higher QoL.
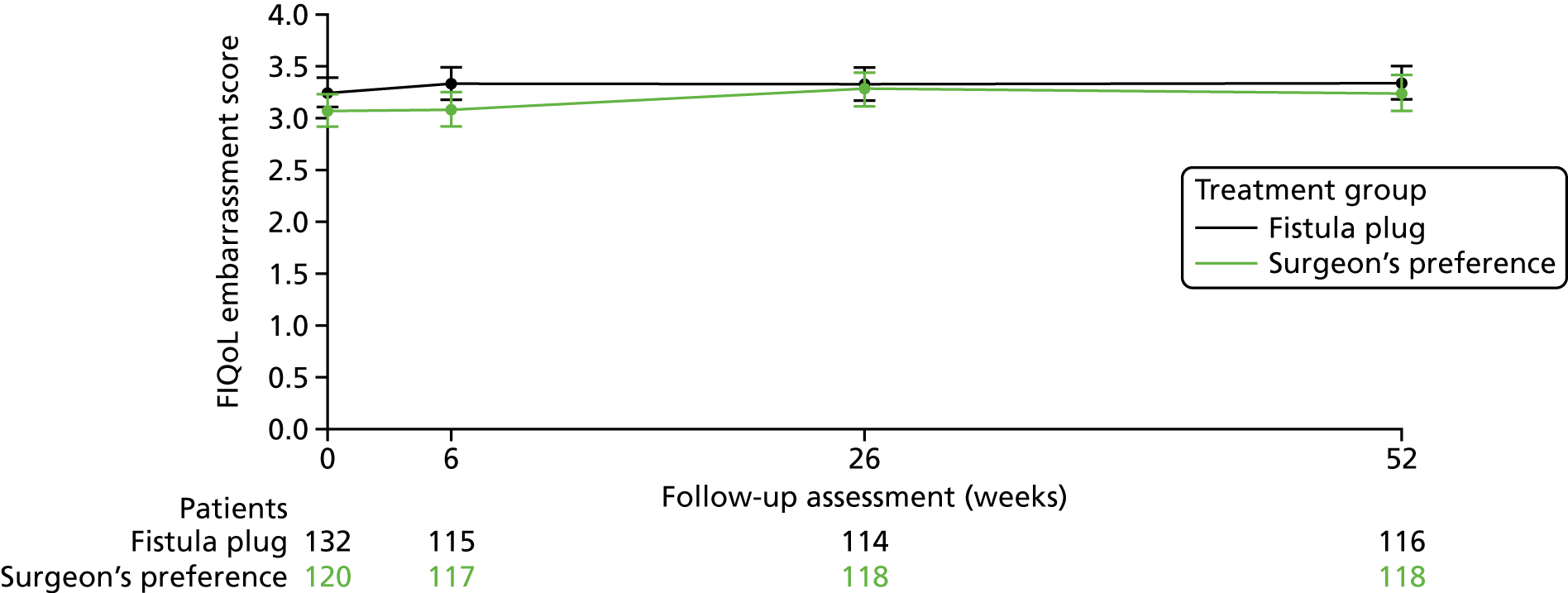
The primary analysis of the FIAT is a comparison of the mean difference in FIQoL scores between Surgisis anal fistula plug and surgeon’s preference from a repeated-measures model incorporating the 6-week, 6-month and 12-month time points, where the baseline score is included as a covariate in the model (Table 5). Separate models have been constructed for each of the four domains of the FIQoL questionnaire. Further models were also fitted, which included a time-by-treatment interaction term to identify any change in treatment over time.
| FIQoL domain | Surgisis anal fistula plug, mean (SD, n) | Surgeon’s preference, mean (SD, n) | Mean differencea (95% CI) | p-value | Treatment by time, p-value |
|---|---|---|---|---|---|
| FIQoL: lifestyle | |||||
| Baseline | 3.46 (0.75, 138) | 3.34 (0.83, 131) | 0.03 (–0.10 to 0.15) | 0.67 | 0.68 |
| 6 weeks | 3.49 (0.76, 127) | 3.42 (0.82, 126) | |||
| 6 months | 3.57 (0.73, 124) | 3.50 (0.77, 128) | |||
| 12 months | 3.60 (0.70, 125) | 3.54 (0.75, 128) | |||
| FIQoL: coping/behaviour | |||||
| Baseline | 3.30 (0.75, 138) | 3.14 (0.88, 131) | 0.11 (–0.03 to 0.24) | 0.11 | 0.31 |
| 6 weeks | 3.39 (0.76, 127) | 3.18 (0.89, 126) | |||
| 6 months | 3.44 (0.79, 124) | 3.31 (0.90, 128) | |||
| 12 months | 3.43 (0.83, 124) | 3.33 (0.85, 128) | |||
| FIQoL: depression/self-perception | |||||
| Baseline | 3.04 (0.77, 132) | 2.99 (0.81, 120) | 0.09 (–0.06 to 0.24) | 0.22 | 0.87 |
| 6 weeks | 3.13 (0.78, 115) | 3.03 (0.85, 118) | |||
| 6 months | 3.23 (0.76, 114) | 3.16 (0.91, 117) | |||
| 12 months | 3.29 (0.85, 115) | 3.20 (0.85, 118) | |||
| FIQoL: embarrassment | |||||
| Baseline | 3.26 (0.82, 132) | 3.08 (0.87, 120) | 0.12 (–0.05 to 0.29) | 0.18 | 0.06 |
| 6 weeks | 3.34 (0.84, 115) | 3.09 (0.92, 117) | |||
| 6 months | 3.34 (0.85, 114) | 3.29 (0.89, 118) | |||
| 12 months | 3.35 (0.89, 116) | 3.25 (0.95, 118) | |||
No significant differences were seen in any of the four domains between the fistula plug group and surgeon’s preference group. Models including the treatment-by-time interaction term were also non-significant and, thus, there is no evidence of a change in treatment effect over time.
Figures 5–8 provide longitudinal plots of mean FIQoL scores over time by treatment group for each domain of the FIQoL questionnaire.
FIGURE 5.
Mean FIQoL score: lifestyle domain.

FIGURE 6.
Mean FIQoL score: coping/behaviour domain.

FIGURE 7.
Mean FIQoL score: depression/self-perception domain.
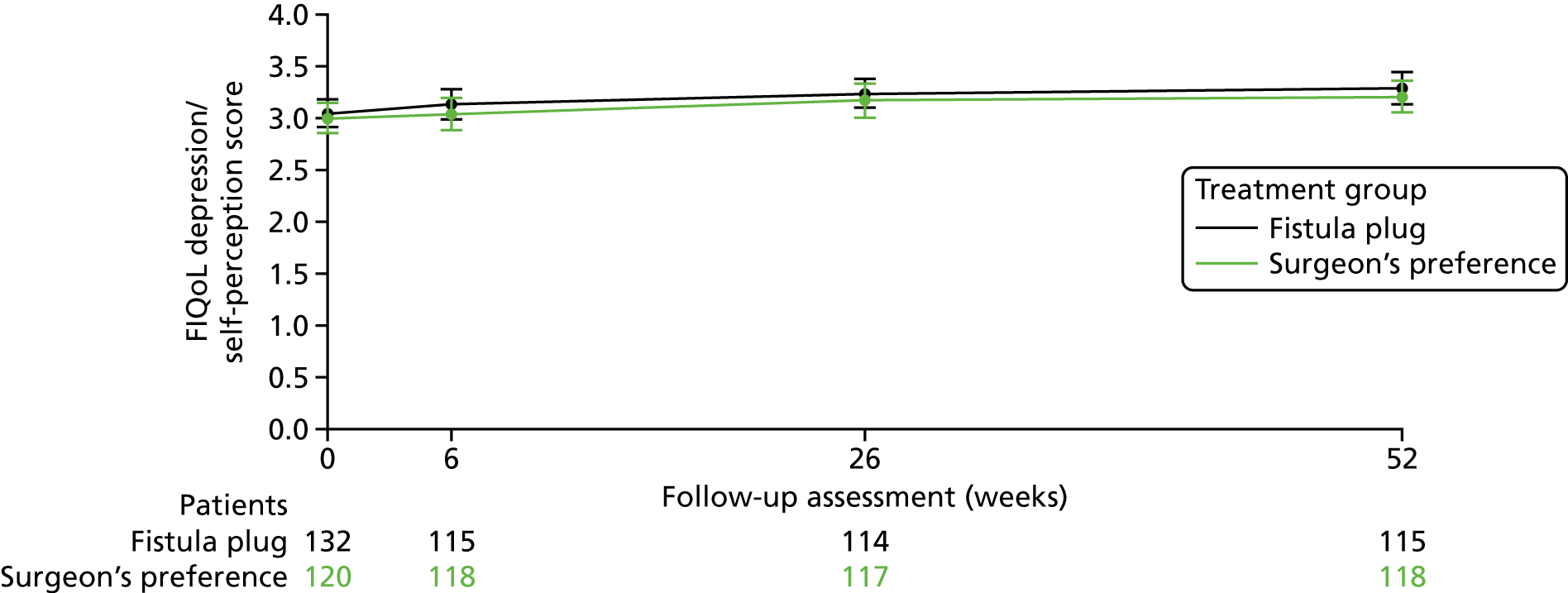
FIGURE 8.
Mean FIQoL score: embarrassment domain.
In addition to the primary-adjusted ITT analysis, a ‘per-protocol’ analysis was undertaken for the primary outcome as a sensitivity analysis to explore the potential effect of non-adherence to the randomised allocation (Table 6 and Appendix 4). Participants were classified with respect to the first intervention they received rather than the intervention to which they were randomised. In total, 155 participants received the surgeon’s preference (143 randomised to the surgeon’s preference; 12 randomised to the fistula plug) and 139 participants received a fistula plug (four randomised to the surgeon’s preference; 135 randomised to the fistula plug). Ten participants did not have surgery and have not been included in this analysis.
| FIQoL domain | Surgisis anal fistula plug, mean (SD, n) | Surgeon’s preference, mean (SD, n) | Mean differencea (95% CI) | p-value | Treatment by time, p-value |
|---|---|---|---|---|---|
| FIQoL: lifestyle | |||||
| Baseline | 3.46 (0.75, 130) | 3.35 (0.82, 136) | 0.09 (–0.04 to 0.21) | 0.17 | 0.54 |
| 6 weeks | 3.54 (0.71, 123) | 3.40 (0.83, 129) | |||
| 6 months | 3.59 (0.72, 118) | 3.48 (0.78, 132) | |||
| 12 months | 3.63 (0.67, 120) | 3.52 (0.78, 132) | |||
| FIQoL: coping/behaviour | |||||
| Baseline | 3.33 (0.73, 130) | 3.14 (0.87, 136) | 0.15 (0.01 to 0.28) | 0.03 | 0.12 |
| 6 weeks | 3.45 (0.72, 123) | 3.16 (0.89, 129) | |||
| 6 months | 3.46 (0.78, 118) | 3.29 (0.91, 132) | |||
| 12 months | 3.45 (0.82, 120) | 3.31 (0.86, 131) | |||
| FIQoL: depression/self-perception | |||||
| Baseline | 3.05 (0.78, 124) | 2.99 (0.79, 125) | 0.13 (–0.02 to 0.28) | 0.08 | 0.46 |
| 6 weeks | 3.18 (0.75, 111) | 3.01 (0.85, 121) | |||
| 6 months | 3.25 (0.76, 109) | 3.15 (0.91, 120) | |||
| 12 months | 3.31 (0.82, 110) | 3.19 (0.87, 122) | |||
| FIQoL: embarrassment | |||||
| Baseline | 3.26 (0.81, 124) | 3.09 (0.86, 125) | 0.17 (–0.001 to 0.34) | 0.051 | 0.02 |
| 6 weeks | 3.39 (0.81, 111) | 3.08 (0.92, 120) | |||
| 6 months | 3.37 (0.85, 109) | 3.27 (0.89, 121) | |||
| 12 months | 3.40 (0.87, 111) | 3.22 (0.95, 122) | |||
Similar to the ITT analysis, the per-protocol analysis failed to show significant differences in any of the domains of FIQoL across the four time points measured (see Appendix 4). A marginal improvement in FIQoL was observed in all domains at 6 weeks following surgery and was maintained until the 12-month follow-up assessment.
Prespecified subgroup analyses of the primary outcome were planned for the four variables for which the randomisation was minimised:
-
age at randomisation (< 30, 30–39, 40–49, 50–59, 60–69, ≥ 70 years)
-
ASA grade (P1, P2, P3, P4)
-
planned type of surgery (advancement flap, cutting seton, LIFT procedure, fistulotomy)
-
presence of extensions (yes, no).
There was no clear evidence to suggest that the treatment effect differed between the different patient subgroups for any of the four FIQoL domains (Tables 7–10).
| Variable | Interaction p-value | Mean differencea (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.32 | –0.22 (–0.53 to 0.10) | 0.17 |
| 30–39 | –0.02 (–0.28 to 0.24) | 0.88 | |
| 40–49 | 0.03 (–0.21 to 0.27) | 0.81 | |
| 50–59 | 0.19 (–0.08 to 0.46) | 0.17 | |
| 60–69 | 0.34 (–0.10 to 0.77) | 0.13 | |
| ≥ 70 | –0.06 (–0.55 to 0.44) | 0.82 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.58 | 0.01 (–0.14 to 0.15) | 0.94 |
| P2 (mild systemic disease) | 0.06 (–0.21 to 0.33) | 0.66 | |
| P3 (severe systemic disease) | 0.45 (–0.41 to 1.32) | 0.31 | |
| Type of surgery | |||
| Advancement flap | 0.60 | 0.07 (–0.21 to 0.36) | 0.61 |
| Cutting seton | 0.06 (–0.14 to 0.26) | 0.57 | |
| LIFT procedure | 0.01 (–0.19 to 0.21) | 0.90 | |
| Fistulotomy | –0.53 (–1.38 to 0.33) | 0.22 | |
| Presence of extensions | |||
| Yes | 0.16 | –0.23 (–0.61 to 0.15) | 0.24 |
| No | 0.07 (–0.11 to 0.25) | 0.42 | |
| Variable | Interaction p-value | Mean differencea (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.49 | –0.09 (–0.42 to 0.24) | 0.59 |
| 30–39 | –0.02 (–0.30 to 0.25) | 0.86 | |
| 40–49 | 0.12 (–0.13 to 0.38) | 0.34 | |
| 50–59 | 0.26 (–0.02 to 0.55) | 0.07 | |
| 60–69 | 0.17 (–0.29 to 0.63) | 0.46 | |
| ≥ 70 | 0.36 (–0.16 to 0.88) | 0.17 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.11 | 0.04 (–0.11 to 0.19) | 0.64 |
| P2 (mild systemic disease) | 0.29 (0.01 to 0.57) | 0.04 | |
| P3 (severe systemic disease) | 0.75 (–0.15 to 1.65) | 0.10 | |
| Type of surgery | |||
| Advancement flap | 0.44 | 0.11 (–0.19 to 0.41) | 0.48 |
| Cutting seton | 0.19 (–0.02 to 0.41) | 0.07 | |
| LIFT procedure | 0.05 (–0.16 to 0.26) | 0.63 | |
| Fistulotomy | –0.50 (–1.40 to 0.40) | 0.28 | |
| Presence of extensions | |||
| Yes | 0.53 | –0.04 (–0.43 to 0.35) | 0.84 |
| No | 0.10 (–0.09 to 0.28) | 0.29 | |
| Variable | Interaction p-value | Mean differencea (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.08 | –0.28 (–0.64 to 0.08) | 0.12 |
| 30–39 | –0.08 (–0.40 to 0.23) | 0.59 | |
| 40–49 | 0.15 (–0.14 to 0.43) | 0.32 | |
| 50–59 | 0.38 (0.08 to 0.68) | 0.01 | |
| 60–69 | 0.32 (–0.19 to 0.82) | 0.22 | |
| ≥ 70 | 0.21 (–0.36 to 0.77) | 0.48 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.19 | 0.02 (–0.15 to 0.19) | 0.82 |
| P2 (mild systemic disease) | 0.28 (–0.03 to 0.59) | 0.08 | |
| P3 (severe systemic disease) | 0.63 (–0.33 to 1.59) | 0.20 | |
| Type of surgery | |||
| Advancement flap | 0.12 | 0.21 (–0.12 to 0.54) | 0.21 |
| Cutting seton | 0.14 (–0.09 to 0.38) | 0.23 | |
| LIFT procedure | 0.05 (–0.18 to 0.28) | 0.68 | |
| Fistulotomy | –1.45 (–2.81 to –0.09) | 0.04 | |
| Presence of extensions | |||
| Yes | 0.92 | 0.05 (–0.37 to 0.47) | 0.81 |
| No | 0.07 (–0.13 to 0.28) | 0.47 | |
| Variable | Interaction p-value | Mean differencea (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.21 | –0.23 (–0.66 to 0.19) | 0.28 |
| 30–39 | –0.02 (–0.38 to 0.35) | 0.94 | |
| 40–49 | 0.22 (–0.12 to 0.56) | 0.21 | |
| 50–59 | 0.42 (0.06 to 0.78) | 0.02 | |
| 60–69 | 0.35 (–0.26 to 0.95) | 0.26 | |
| ≥ 70 | –0.11 (–0.78 to 0.56) | 0.75 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.48 | 0.09 (–0.11 to 0.29) | 0.36 |
| P2 (mild systemic disease) | 0.15 (–0.21 to 0.52) | 0.41 | |
| P3 (severe systemic disease) | 0.80 (–0.34 to 1.93) | 0.17 | |
| Type of surgery | |||
| Advancement flap | 0.12 | 0.19 (–0.20 to 0.58) | 0.34 |
| Cutting seton | 0.24 (–0.04 to 0.51) | 0.09 | |
| LIFT procedure | 0.05 (–0.22 to 0.32) | 0.70 | |
| Fistulotomy | –1.71 (–3.34 to –0.07) | 0.04 | |
| Presence of extensions | |||
| Yes | 0.77 | 0.13 (–0.37 to 0.63) | 0.61 |
| No | 0.05 (–0.19 to 0.29) | 0.70 | |
Secondary outcomes
Fistula healing (clinical assessment)
Fistula healing was recorded at 6 weeks and at 6 and 12 months (Table 11). At the 6-week time point approximately one-third of participants had clinical evidence of a healed fistula [42/141 (30%) in the fistula plug group vs. 45/137 (33%) in the surgeon’s preference group]. The proportion of fistulas that were reported as healed at 6 months was higher in the surgeon’s preference group, but this difference was not statistically significant. By 12 months, this trend was not apparent, with just over half of fistulas in both the fistula plug group (n/N = 66/122) and the surgeon’s preference group (n/N = 66/119) reported as healed. No significant differences between treatment groups in the proportion of patients whose fistula had healed were seen at any of the time points.
| Time | Surgisis anal fistula plug (N = 152) | Surgeon’s preference (N = 152) | Risk ratio (95% CI) | p-value |
|---|---|---|---|---|
| 6 weeks | ||||
| Fistula healing data available, n | 141 | 137 | 0.91 (0.64 to 1.29) | 0.58 |
| Fistula healing, n (%) | 42 (30) | 45 (33) | ||
| 6 months | ||||
| Fistula healing data available, n | 127 | 128 | 0.81 (0.61 to 1.08) | 0.14 |
| Fistula healing, n (%) | 50 (39) | 62 (48) | ||
| 12 months | ||||
| Fistula healing data available, n | 122 | 119 | 0.98 (0.78 to 1.23) | 0.83 |
| Fistula healing, n (%) | 66 (54) | 66 (55) | ||
Subgroup analyses of the effect of the four randomisation minimisation factors on 12-month fistula healing rates showed no clear evidence that the treatment effect differed between the different patient subgroups (Table 12).
| Subgroup | Interaction p-value | Risk ratio (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.91 | 0.74 (0.42 to 1.29) | 0.29 |
| 30–39 | 0.94 (0.60 to 1.48) | 0.80 | |
| 40–49 | 0.94 (0.62 to 1.41) | 0.76 | |
| 50–59 | 1.17 (0.67 to 2.04) | 0.57 | |
| 60–69 | 1.14 (0.42 to 3.08) | 0.80 | |
| ≥ 70 | 1.13 (0.41 to 3.08) | 0.82 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.42 | 0.89 (0.68 to 1.16) | 0.38 |
| P2 (mild systemic disease) | 1.28 (0.78 to 2.10) | 0.33 | |
| P3 (severe systemic disease) | 1.25 (0.22 to 7.22) | 0.80 | |
| Type of surgery | |||
| Advancement flap | Model did not converge | ||
| Cutting seton | |||
| LIFT procedure | |||
| Fistulotomy | |||
| Presence of extensions | |||
| Yes | 0.68 | 1.17 (0.59 to 2.31) | 0.66 |
| No | 0.99 (0.74 to 1.34) | 0.97 | |
Fistula healing rates by procedure
The fistula healing rates per received procedure and at the various follow-up time points are shown in Table 13. Forty-one (30%) of 136 participants in the fistula plug group were assessed as clinically healed by 6 weeks, with a gradual increase to 51 (41%) of 123 participants at 6 months and to 63 (55%) of 115 participants at 12 months. The best-performing procedure, accepting that the numbers treated were small, appeared to be fistulotomy, with 11 (65%) of 17 participants healed at 6 weeks and 12 (75%) of 16 participants healed at 12 months. Few participants receiving the cutting seton were healed at 6 weeks (7/48, 15%), but had a gradual increase in healing by 12 months (27/42, 64%). The LIFT procedure produced clinical healing in 16 (29%) of 55 participants at 6 weeks and in 17 (31%) of 55 participants at 6 months, increasing to 21 (42%) of 50 participants by 12 months.
| Time | Treatment received, n/N (%) | ||||
|---|---|---|---|---|---|
| Surgisis anal fistula plug | Cutting seton | Fistulotomy | Advancement flap | LIFT procedure | |
| 6 weeks | 41/136 (30) | 7/48 (15) | 11/17 (65) | 11/21 (52) | 16/55 (29) |
| 6 months | 51/123 (41) | 20/40 (50) | 14/17 (82) | 10/19 (53) | 17/55 (31) |
| 12 months | 63/115 (55) | 27/42 (64) | 12/16 (75) | 9/17 (53) | 21/50 (42) |
Fistula healing (radiological assessment)
Magnetic resonance imaging data were available for 110 (72%) of 152 participants in the fistula group and for 112 (74%) of 152 participants in the surgeon’s preference group. Overall, 192 (86%) of 220 participants underwent routine 12-month follow-up MRI, with 31 (14%) undergoing MRI for clinical relapse prior to the 12-month time point.
Follow-up MRI performed either for clinical relapse or at a routine 12-month follow-up revealed fistula healing in 54 (49%) of 110 participants in the fistula plug group, compared with 63 (56%) of 112 participants in the surgeon’s preference group (Table 14).
| Assessment | Treatment received, n/N (%) | ||||
|---|---|---|---|---|---|
| Surgisis anal fistula plug | Cutting seton | Fistulotomy | Advancement flap | LIFT procedure | |
| 12 months: clinical | 63/115 (55) | 27/42 (64) | 12/16 (75) | 9/17 (53) | 21/50 (42) |
| 12 months: MRI | 54/110 (49) | 63/112 (57) | |||
Faecal incontinence
Faecal incontinence was recorded at 6 weeks and at 6 and 12 months using the St Mark’s incontinence score. The score ranges from 0 to 24, where higher scores indicate a higher level of incontinence. The mean (SD) scores for each treatment group at each time point are given in Table 15. The baseline incontinence scores tended to be higher in the surgeon’s preference group than in the fistula plug group, but with similar SDs. No significant differences in mean incontinence score between treatment groups were seen at any of the follow-up time points. The numerically higher mean values in the surgeon’s preference group at all time points are of marginal significance and do not translate into a clinically meaningful difference.
| Time | Surgisis anal fistula plug, mean (SD, n) | Surgeon’s preference, mean (SD, n) | Mean differencea (95% CI) | p-value |
|---|---|---|---|---|
| Baseline | 4.54 (4.15, 151) | 5.24 (4.78, 152) | ||
| 6 weeks | 3.72 (4.22, 134) | 3.87 (4.97, 132) | –0.15 (–1.26 to 0.96) | 0.79 |
| 6 months | 3.06 (4.44, 120) | 3.61 (4.55, 117) | –0.55 (–1.70 to 0.60) | 0.35 |
| 12 months | 3.22 (4.54, 120) | 3.65 (4.91, 112) | –0.44 (–1.66 to 0.79) | 0.48 |
Subgroup analyses of the St Mark’s incontinence score data showed no clear evidence that the treatment effect differed between the different patient subgroups (Table 16).
| Subgroup | Interaction p-value | Mean differencea (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.51 | 1.54 (–1.90 to 4.98) | 0.38 |
| 30–39 | 0.82 (–1.67 to 3.30) | 0.52 | |
| 40–49 | –1.94 (–4.29 to 0.42) | 0.11 | |
| 50–59 | –0.30 (–3.06 to 2.45) | 0.83 | |
| 60–69 | –1.50 (–5.68 to 2.68) | 0.48 | |
| ≥ 70 | –1.50 (–6.17 to 3.17) | 0.53 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.75 | –0.49 (–1.87 to 0.90) | 0.49 |
| P2 (mild systemic disease) | –0.08 (–2.68 to 2.52) | 0.95 | |
| P3 (severe systemic disease) | 2.50 (–5.46 to 10.46) | 0.54 | |
| Type of surgery | |||
| Advancement flap | 0.40 | –1.48 (–4.26 to 1.30) | 0.30 |
| Cutting seton | –0.20 (–2.17 to 1.77) | 0.84 | |
| LIFT procedure | –0.57 (–2.54 to 1.40) | 0.57 | |
| Fistulotomy | 5.60 (–2.20 to 13.40) | 0.16 | |
| Presence of extensions | |||
| Yes | 0.59 | –0.09 (–3.81 to 3.63) | 0.96 |
| No | –1.20 (–2.90 to 0.49) | 0.16 | |
Complications
Data on bleeding, unexplained pain and septic events were recorded at discharge (following the operative procedure) and at 6 weeks, 6 months and 12 months (Table 17). Data on urinary retention were recorded at discharge only.
| Complication | Surgisis anal fistula plug (n = 152), n/N (%) | Surgeon’s preference (n = 152), n/N (%) | Risk ratioa (95% CI) | p-value |
|---|---|---|---|---|
| Postoperative | ||||
| Complications data available | 147 | 144 | ||
| Complications | 4 (3) | 2 (1) | 1.96 (0.36 to 10.53) | 0.42 |
| Bleeding | 2/4 (50) | 0/2 (0) | ||
| Urinary retention | 0/4 (0) | 1/2 (50) | ||
| Unexplained pain | 2/4 (50) | 1/2 (50) | ||
| Septic event | 0/4 (0) | 0/2 (0) | ||
| 6 weeks | ||||
| Complications data available | 142 | 137 | ||
| Complications | 49 (35) | 25 (18) | 1.89 (1.24 to 2.88) | 0.002 |
| Bleeding | 9/49 (18) | 5/25 (20) | ||
| Unexplained pain | 32/49 (65) | 9/25 (36) | ||
| Septic event | 15/49 (31) | 11/25 (44) | ||
| 6 months | ||||
| Complications data available | 129 | 129 | ||
| Complications | 27 (21) | 27 (21) | 1.00 (0.62 to 1.61) | 1.00 |
| Bleeding | 5/27 (19) | 4/27 (15) | ||
| Unexplained pain | 14/27 (52) | 7/27 (26) | ||
| Septic event | 5/27 (19) | 11/27 (41) | ||
| 12 months | ||||
| Complications data available | 124 | 121 | ||
| Complications | 28 (23) | 24 (20) | 1.14 (0.70 to 1.85) | 0.60 |
| Bleeding | 6/28 (21) | 4/24 (17) | ||
| Unexplained pain | 10/28 (36) | 8/24 (33) | ||
| Septic event | 14/28 (50) | 9/24 (38) | ||
Postoperative complications were few and rates were similar in both groups [fistula plug group, n/N = 4/147 (3%); surgeon’s preference group, n/N = 2/144 (1%)], indicating that fistula surgery carries a low level of morbidity.
The only significant difference observed between the two groups was at 6 weeks, when more participants in the fistula plug group than in the surgeon’s preference group had experienced complications (fistula plug group n/N = 49/142, 35%, vs. surgeon’s preference group n/N = 25/137, 18%; p = 0.002). This difference appeared to be due to a greater proportion of participants experiencing protracted pain in the fistula plug group.
By 6 and 12 months, the overall complication rates were similar in both groups, with unexpected pain continuing to be reported at 6 months, particularly in the fistula plug group. Even by 12 months, unexpected pain was still reported by around one-third of participants in both groups and septic complications were reported in 50% of the fistula plug group and 38% of the surgeon’s preference group.
Treatment-specific complications
The treatment-specific complications are reported in Table 18. Plug extrusion was an early complication reported in 20 (16%) of 126 participants in the fistula plug group, with persistent discharge from the fistula tract in 47 (45%) of 104 participants at 6 months and 40 (40%) of 101 participants at 12 months. Wound-related problems were reported in 2 (15%) of 13 participants in the fistulotomy group at 6 weeks, decreasing to 1 (7%) of 14 participants by 6 months and increasing again to 2 (14%) of 14 participants at 12 months. Similar rates of wound-related problems were reported following the LIFT procedure. Complications related to the advancement flap occurred in 4 (18%) of 22 participants at 6 weeks and persisted in 3 (16%) of 19 participants at 6 months’ follow-up and in 2 (13%) of 16 participants at 12-month follow-up.
| Procedure | 6 weeks, n/N (%) | 6 months, n/N (%) | 12 months, n/N (%) |
|---|---|---|---|
| Fistula plug extrusion | 20/126 (16) | ||
| Cutting seton extrusion | 9/49 (18) | ||
| Fistulotomy wound complications | 2/13 (15) | 1/14 (7) | 2/14 (14) |
| LIFT procedure wound problems | 8/53 (15) | 6/45 (13) | 8/44 (18) |
| Advancement flap complications | 4/22 (18) | 3/19 (16) | 2/16 (13) |
Reinterventions
Reintervention data were recorded at discharge (following operative procedure) and at 6 weeks, 6 months and 12 months, and each reintervention was classified as either medical (non-operative intervention) or surgical (operative intervention) by the clinical members of the Trial Management Team. The overall analyses of reintervention rates simply consider whether or not the participant received any reintervention at each time point (it is possible that participants could have received multiple reinterventions). In the breakdown of reinterventions, each type is counted separately. However, multiple reinterventions of the same type at the same time point (i.e. medical at 6 weeks) are counted only once. Reintervention data are shown in Table 19.
| Reintervention | Surgisis anal fistula plug (N = 152) | Surgeon’s preference (N = 152) | Risk ratioa (95% CI) | p-value |
|---|---|---|---|---|
| Postoperative | ||||
| Complications data available, n | 147 | 144 | ||
| Reintervention, n (%) | 2 (1) | 1 (1) | 1.96 (0.18 to 21.37) | 0.57 |
| Medical, n/N (%) | 1/2 (50) | 1/1 (100) | ||
| Surgical, n/N (%) | 1/2 (50) | 0/1 (0) | ||
| 6 weeks | ||||
| Complications data available, n | 142 | 137 | ||
| Reintervention, n (%)2 | 30 (21) | 16 (12) | 1.81 (1.03 to 3.17) | 0.03 |
| Medical, n/N (%) | 13/30 (43) | 8/16 (50) | ||
| Surgical, n/N (%) | 18/30 (60) | 8/16 (50) | ||
| 6 months | ||||
| Complication data available, n | 129 | 129 | ||
| Reintervention, n (%)2 | 25 (19) | 30 (23) | 0.83 (0.52 to 1.34) | 0.45 |
| Medical, n/N (%) | 3/25 (12) | 7/30 (23) | ||
| Surgical, n/N (%) | 24/25 (96) | 24/30 (80) | ||
| 12 months | ||||
| Complications data available, n | 124 | 121 | ||
| Reintervention, n (%)2 | 28 (23) | 27 (22) | 1.01 (0.64 to 1.61) | 0.96 |
| Medical, n/N (%) | 5/28 (18) | 2/27 (7) | ||
| Surgical, n/N (%) | 24/28 (86) | 26/27 (96) | ||
Postoperative reinterventions were rare (1% in both groups), highlighting the low immediate morbidity associated with fistula surgery. A significant difference in reinterventions was observed at the 6-week follow-up, being higher in the fistula plug group (n/N = 30/142, 21%) than in the surgeon’s preference group (n/N = 16/137, 12%). This significant difference was lost at 6- and 12-month follow-up, but reinterventions at 12 months were still common in both groups [fistula plug group, n/N = 28/124 (23%) vs. surgeon’s preference group, n/N = 27/121 (22%)]. Medical reinterventions were frequent at 6 weeks, accounting for almost 50% of reinterventions in both arms, but decreased as a proportion of the overall number of reinterventions with progressive follow-up.
Time to first reintervention and time to first surgical reintervention are presented in Figures 9 and 10, respectively. Hazard ratios are computed from a Cox regression model, where values < 1 favour the fistula plug. No significant differences were seen between the treatment groups.
FIGURE 9.
Time to first reintervention. Hazard ratio: fistula plug vs. surgeon’s preference 1.05 (95% CI 0.72 to 1.53).
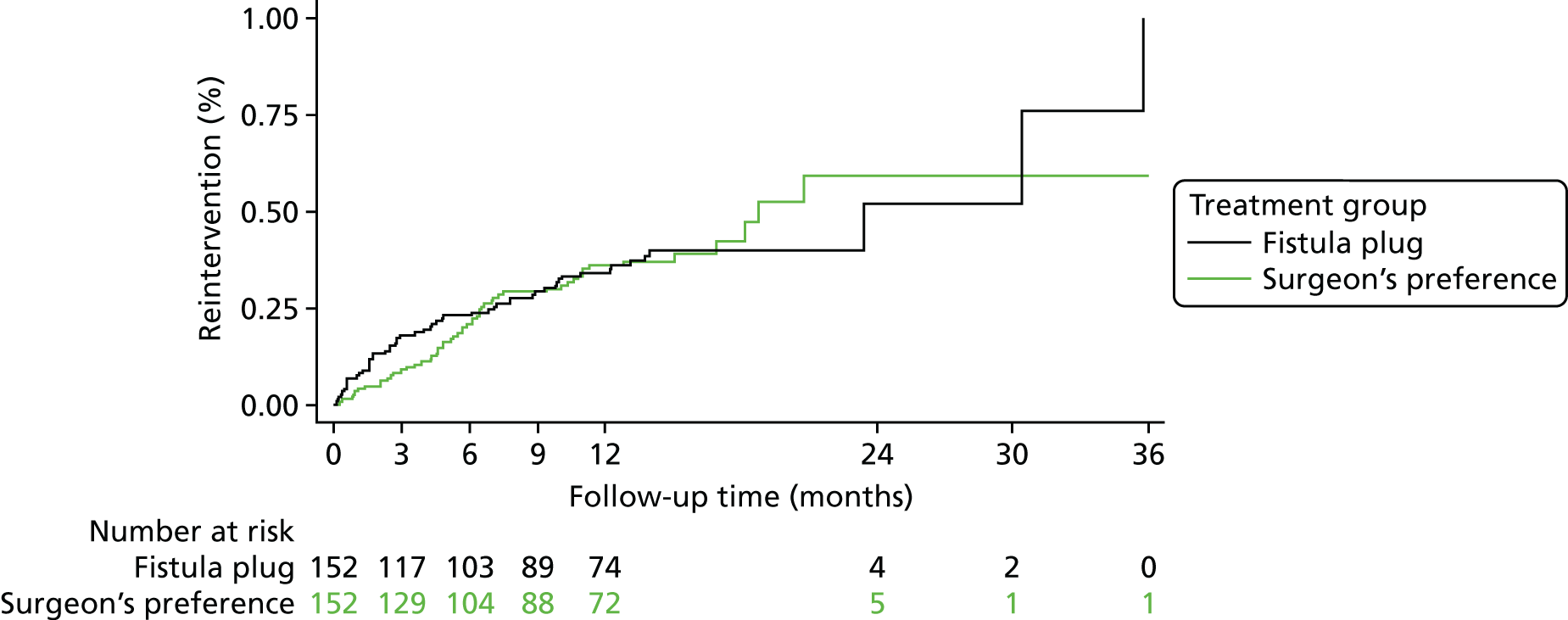
FIGURE 10.
Time to first surgical reintervention. Hazard ratio: fistula plug vs. surgeon’s preference 1.11 (95% CI 0.75 to 1.66).
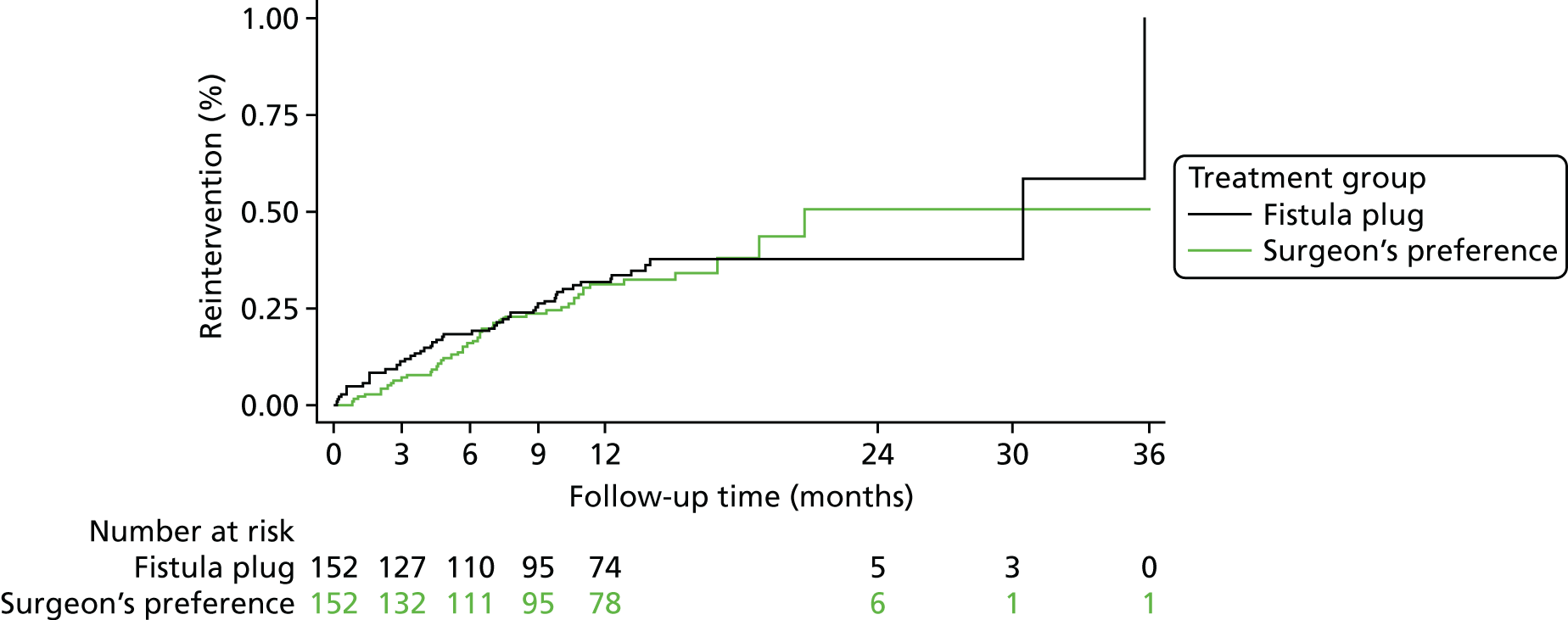
EuroQol-5 Dimensions, three-level version
The validated questionnaire used in the FIAT to assess general QoL was the EuroQol EQ-5D-3L. Data from this questionnaire were collected at baseline, and at 6 weeks, 6 months and 12 months. The questionnaire comprises five domains, each with three possible responses, and a visual analogue scale from 0 to 100. Responses to the domains are combined to give a HRQoL score, which can range from –0.594 to 1, where higher scores indicate a higher QoL. Higher values on the visual analogue scale also indicate a higher QoL.
Analyses of the EQ-5D-3L data compare the mean difference in EQ-5D-3L scores between the fistula plug group and the surgeon’s preference group from a repeated-measures model incorporating the 6-week, 6-month and 12-month time points, where the baseline score will be included as a covariate in the model (Table 20). Separate models have been constructed for both the visual analogue score and the health status score. Further models that included a time-by-treatment interaction term were also fitted to identify any change in treatment over time.
| Time | Surgisis anal fistula plug, mean (SD, n) | Surgeon’s preference, mean (SD, n) | Mean differencea (95% CI) | p-value | Treatment by time, p-value |
|---|---|---|---|---|---|
| Visual analogue scale | |||||
| Baseline | 73.30 (18.67, 139) | 74.61 (17.75, 131) | 1.66 (–1.45 to 4.77) | 0.29 | 0.41 |
| 6 weeks | 75.88 (18.44, 128) | 75.99 (18.22, 125) | |||
| 6 months | 80.14 (15.63, 124) | 77.64 (20.67, 129) | |||
| 12 months | 79.62 (19.04, 125) | 79.47 (15.62, 125) | |||
| EQ-5D-3L HRQoL | |||||
| Baseline | 0.77 (0.27, 136) | 0.76 (0.25, 130) | 0.01 (–0.04 to 0.05) | 0.76 | 0.54 |
| 6 weeks | 0.78 (0.24, 121) | 0.77 (0.25, 125) | |||
| 6 months | 0.83 (0.21, 121) | 0.79 (0.27, 129) | |||
| 12 months | 0.85 (0.21, 121) | 0.82 (0.24, 126) | |||
There was a marginal improvement in both the HRQoL and the visual analogue score between baseline and 12-month follow-up in both groups. No significant differences were seen for either the health status score or the visual analogue score between the fistula plug group and the surgeon’s preference group. Models including the treatment-by-time interaction term were also non-significant and, thus, there is no evidence of a change in treatment effect over time.
Subgroup analyses of the health status score and visual analogue score data showed no clear evidence that the treatment effect differed between the different patient subgroups (Tables 21 and 22).
| Subgroup | Interaction p-value | Mean difference (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.62 | –0.05 (–0.16 to 0.06) | 0.35 |
| 30–39 | 0.02 (–0.07 to 0.10) | 0.72 | |
| 40–49 | –0.002 (–0.08 to 0.08) | 0.97 | |
| 50–59 | 0.05 (–0.04 to 0.15) | 0.26 | |
| 60–69 | –0.06 (–0.20 to 0.09) | 0.45 | |
| ≥ 70 | 0.07 (–0.10 to 0.25) | 0.39 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.21 | 0.01 (–0.04 to 0.05) | 0.82 |
| P2 (mild systemic disease) | –0.02 (–0.11 to 0.07) | 0.66 | |
| P3 (severe systemic disease) | 0.25 (–0.03 to 0.54) | 0.09 | |
| Type of surgery | |||
| Advancement flap | 0.46 | 0.03 (–0.06 to 0.13) | 0.51 |
| Cutting seton | –0.0001 (–0.07 to 0.07) | 1.00 | |
| LIFT procedure | 0.01 (–0.06 to 0.08) | 0.72 | |
| Fistulotomy | –0.21 (–0.49 to 0.07) | 0.14 | |
| Presence of extensions | |||
| Yes | 0.28 | –0.06 (–0.18 to 0.06) | 0.34 |
| No | 0.02 (–0.04 to 0.07) | 0.61 | |
| Subgroup | Interaction p-value | Mean difference (95% CI) | p-value |
|---|---|---|---|
| Age at randomisation (years) | |||
| < 30 | 0.83 | 2.01 (–6.10 to 10.13) | 0.63 |
| 30–39 | –0.60 (–7.17 to 5.97) | 0.86 | |
| 40–49 | 2.35 (–3.67 to 8.37) | 0.44 | |
| 50–59 | 4.19 (–2.73 to 11.11) | 0.23 | |
| 60–69 | –3.49 (–14.70 to 7.72) | 0.54 | |
| ≥ 70 | 5.13 (–7.36 to 17.63) | 0.42 | |
| ASA grade | |||
| P1 (normal, healthy patient) | 0.85 | 1.40 (–2.13 to 4.93) | 0.43 |
| P2 (mild systemic disease) | 2.09 (–4.52 to 8.70) | 0.53 | |
| P3 (severe systemic disease) | –4.23 (–25.48 to 17.01) | 0.70 | |
| Type of surgery | |||
| Advancement flap | 0.29 | 3.44 (–3.52 to 10.39) | 0.33 |
| Cutting seton | 3.15 (–1.90 to 8.20) | 0.22 | |
| LIFT procedure | 0.34 (–4.63 to 5.30) | 0.89 | |
| Fistulotomy | –16.72 (–37.84 to 4.41) | 0.12 | |
| Presence of extensions | |||
| Yes | 0.84 | 2.29 (–6.84 to 11.43) | 0.62 |
| No | 1.23 (–3.00 to 5.46) | ||
Serious adverse events
Twenty-four SAEs were reported in 22 participants (7.2%): 14 SAEs in 14 participants (9.2%) in the fistula plug group and 10 SAEs in eight participants (5.3%) in the surgeon’s preference group (p = 0.19) (Table 23). All SAEs bar one (death in the fistula plug group) were related to trial treatment.
| SAE detail | Surgisis anal fistula plug (n = 152) | Surgeon’s preference (n = 152) |
|---|---|---|
| Septic complication | 9 | 8 |
| Unexpected pain | 4 | 0 |
| Urinary retention | 0 | 1 |
| Allergy to seton | 0 | 1 |
| Death | 1 | 0 |
| Total | 14 | 10 |
The most common reason for SAE reporting was a septic event related to the development of a perineal abscess or fistula recurrence, which accounted for 9 (64%) of 14 cases in the fistula plug group and 8 (80%) of 10 cases in the surgeon’s preference group. There was one accidental death in the fistula plug group, which was unrelated to the trial.
Economic evaluation
The economic evaluation was a within-trial analysis and examined the cost-effectiveness of the fistula plug compared with standard surgical techniques based on surgeon’s preference from a UK NHS and PSS perspective.
Unit cost data
Resource use data collected from patients at 6 weeks and at 6 and 12 months were combined with unit costs to estimate the total health resource use cost for each participant (Table 24).
| Resource item | Cost (£) | Source |
|---|---|---|
| GP surgery visit | 37.00 | PSSRU (2017):24 including direct care staff costs with qualification, per participant contact lasting 9.22 minutes |
| Nurse at GP surgery | 6.45 | PSSRU (2017):24 £42 per hour (cost including qualifications), per patient contact lasting 9.22 minutes |
| District nurse house visit | 77.35 |
PSSRU (2015):32 nurse specialist (community), £75 per hour with qualification of patient-related work (not updated in latest publication) Inflated to 2017 cost year |
| Walk-in centre | 32.94 | £28.2333 inflated to 2017 cost year |
| Hospital A&E department | 63.00 | Department of Health and Social Care34 |
Unit costs of patient-reported prescriptions
Prescriptions were reported by patients at 6 weeks and at 6 and 12 months, using a free-text field. This led to many varied entries by participants. Almost all of the prescriptions were for antibiotics, painkillers or dressings. Given that the price of dressings is negligible, it was assumed that, among participants who reported receiving one or more prescriptions, 50% received painkillers and 50% received antibiotics, as reported in Table 25.
Operation-associated costs
The resources used associated with the operations were collected by sites at the time of the operation and at 6 weeks’ follow-up. The costs are shown in Table 26. Given that no information was collected on the staff members present during the operations, it was assumed that the following health-care staff were present at the procedure: anaesthetist, anaesthetic assistant, surgeon, assistant surgeon, scrub nurse and a circulating nurse.
| Item | Cost (£) | Source |
|---|---|---|
| Surgisis anal fistula plug | 780 | |
| Theatre time (per hour) | 1144 | Information Services Division Scotland35 |
| Elective inpatient excess day | 392 | NHS Reference Costs, 2017:34 FZ22E Intermediate Anal Procedures, ≥ 19 years, with CC score 0 |
| Surgeon | 107 | PSSRU (2017)24 P213: hospital-based doctors – consultant surgeon |
| Assistant surgeon | 107 | PSSRU (2017)24 P213: hospital-based doctors – consultant surgeon |
| Anaesthetist | 107 | PSSRU (2017)24 P213: hospital-based doctors – consultant surgeon |
| Assistant anaesthetist | 54 | PSSRU (2017)24 P209: hospital-based nurses – grade 7 |
| Scrub nurse | 54 | PSSRU (2017)24 P209: hospital-based nurses – grade 7 |
| Circulating nurse | 54 | PSSRU (2017)24 P209: hospital-based nurses – grade 7 |
| Perioperative antibiotics: metronidazole 500 mg/100 ml infusion (100-ml bags), one dose = 500 mg | 62 (20 bags) | BNF (2018)26 |
| Postoperative antibiotics: oral co-amoxiclav (Augmentin, GlaxoSmithKline, London, UK) 250 mg/125 mg every 8 hours three times per day for 7 days | 1.59 (21 tablets) | BNF (2018)26 |
| Bowel preparation | ||
| Phosphates enema (Formula B) 128 ml, long tube | 27.93 | BNF (2018)26 |
| Oral preparation: MoviPrep® (Salix Pharmaceuticals, Bridgewater, NJ, USA) oral powder, one pair of sachets | 10.36 (4 sachets) | BNF (2018)26 |
| Analgesics | ||
| Morphine sulfate: 10 mg/10 ml solution for injection ampoules | 15 (10 ampoules) | BNF (2018)26 |
| Co-codamol: 15 mg/500 mg, two tablets | 4.93 (100 tablets) | BNF (2018)26 |
| Lactulose [Lacsa (Pty) Ltd, Durban, South Africa]: 10 g/15 ml oral solution (15-ml sachet, sugar free) | 2.52 (10 sachets) | BNF (2018)26 |
| Bulking Fybogel Mebeverine: effervescent granules in sachets [Reckitt Benckiser Healthcare (UK) Ltd] | 2.72 (30 sachets) | BNF (2018)26 |
| Tinzaparin (LEO Pharma, Ballerup, Denmark) sodium: 3500 units/0.35 ml solution for injection, pre-filled syringes | 27.71 (10 pre-filled disposable injections) | BNF (2018)26 |
Within-trial cost-effectiveness analysis
Cost-effectiveness results: base case
Complete resource use and QALY data were available for 177 participants, with 87 participants in the fistula plug group and 90 participants in the surgeon’s preference group.
Health-care resource use
The total costs associated with resource use are shown in Table 27. The mean total UK NHS and PSS resource use costs throughout the whole period of follow-up were £2738 for the fistula plug group and £2308 for the surgeon’s preference group, with the total mean costs for the fistula plug group being significantly higher (£430 difference; p = 0.0174). The mean costs attributable to readmissions were higher for the fistula plug group, but this difference was not significant. Likewise, although the mean costs attributable to health and social services use outside hospital were higher for the surgeon’s preference group, this difference was not significant.
| Cost type | Surgisis anal fistula plug (n = 87), mean (SD) (£) | Surgeon’s preference (n = 90), mean (SD) (£) | Difference p-value of t-test |
|---|---|---|---|
| Surgery-related costs | 2306 (610) | 1728 (502) | 0.0000 |
| Hospital-based costs due to readmissions | 159 (412) | 89 (363) | 0.2330 |
| Health and social services use | 267 (777) | 484 (1014) | 0.1092 |
| Total costs | 2738 (1151) | 2308 (1228) | 0.0174 |
Health outcomes
Table 28 shows the (unimputed) EQ-5D-3L scores at baseline, 6 weeks, 6 months and 12 months post operation. Both treatment groups showed increasing EQ-5D-3L scores from baseline up to 12 months post operation, with some variation at 6 weeks and 6 months post randomisation in both arms before increasing again.
| Time point | Surgisis anal fistula plug (n = 87) | Surgeon’s preference (n = 90) | Difference p-value of t-test |
|---|---|---|---|
| Baseline | |||
| Mean (SD) | 0.810 (0.238) | 0.750 (0.220) | 0.0818 |
| Median | 0.796 | 0.796 | |
| Minimum–maximum | –0.594 to 1.000 | –0.016 to 1.000 | |
| 6 weeks | |||
| Mean (SD) | 0.791 (0.222) | 0.780 (0.229) | 0.7330 |
| Median | 0.796 | 0.796 | |
| Minimum–maximum | 0.082 to 1.000 | –0.181 to 1.000 | |
| 6 months | |||
| Mean (SD) | 0.837 (0.210) | 0.775 (0.287) | 0.0998 |
| Median | 0.883 | 0.796 | |
| Minimum–maximum | 0.082 to 1.000 | –0.239 to 1.000 | |
| 12 months | |||
| Mean (SD) | 0.856 (0.195) | 0.836 (0.236) | 0.5372 |
| Median | 1.000 | 0.924 | |
| Minimum–maximum | –0.181 to 1.000 | –0.331 to 1.000 | |
| Total QALYs | |||
| Mean (SD) | 0.829 (0.174) | 0.790 (0.212) | 0.1820 |
| Median | 0.852 | 0.832 | |
| Minimum–maximum | 0.043 to 1.000 | –0.102 to 1.000 | |
On average, the difference between arms was marginal. Independent-sample t-tests indicated that the changes in the EQ-5D-3L score over time were not statistically significant. The average total QALY gain over the 12 months was marginally higher in the fistula plug group (0.829) than in the surgeon’s preference group (0.790), but this difference was not significant (p = 0.182).
Cost-effectiveness results (non-imputed)
Table 29 shows the total costs and EQ-5D-3L-generated QALYs for each of the treatment arms for the complete-case analysis. Differences in QALYs between groups were not significant, with marginal health decrements in the surgeon’s preference group compared with the fistula plug group. The mean total cost was significantly higher for the fistula plug group. The high SD for the deterministic cost estimates reflects the presence of a few outlying individuals who incurred significant health service costs.
| QALYs | Surgisis anal fistula plug (n = 87) | Surgeon’s preference (n = 90) |
|---|---|---|
| Total QALYs (SD) | 0.829 (0.174) | 0.790 (0.212) |
| Total cost, £ (SD) | 2738 (1151) | 2308 (1228) |
Table 30 provides the probabilistic cost-effectiveness results for the non-imputed data, showing the incremental costs and benefits as well as the ICER. The results suggest that the fistula plug is associated with an additional incremental cost of £430 and an incremental QALY gain of 0.039. The ICER shows that the cost per additional QALY gained is £10,993. Although this would be acceptable based on a willingness to pay (WTP) of £20,000 per QALY, this value is subject to much uncertainty. The overall net benefit associated with the fistula plug is positive (£352), suggesting that the fistula plug would be cost-effective over a 12-month time horizon.
| Strategy | Total cost, mean (SD) (£) | Incremental cost, mean (SD) (£) | QALY, mean (SD) | Incremental QALY, mean (SD) | ICER, mean (SD) (£/QALY) | Incremental net benefit, mean (SD) (£) |
|---|---|---|---|---|---|---|
| Surgeon’s preference | 2308 (129) | 0.790 (0.022) | ||||
| Surgisis anal fistula plug | 2738 (123) | 430 (178) | 0.829 (0.183) | 0.039 (0.029) | 10,993 (478,666) | 352 (622) |
Cost-effectiveness results (imputed)
Considering the imputed data values, the probabilistic cost-effectiveness results for the imputed data are shown in Table 27. There are only minor differences in the mean costs and mean QALYs for the surgeon’s preference group and the fistula plug group compared with the non-imputed data. The ICER is £17,279 per QALY, which is still below the NICE acceptance threshold of £20,000 per QALY, and the incremental net benefit is £71.
However, given the differences in the mean utility values at baseline, as informed by the EQ-5D-3L (see Table 24), the results were adjusted by baseline EQ-5D-3L values. Table 31 shows that when adjusting for baseline EQ-5D-3L values the ICER is £32,400, which is above the NICE acceptance threshold of £20,000 per QALY.
| Strategy | Total cost, mean (SD) (£) | Incremental cost, mean (SD) (£) | QALY, mean (SD) | Incremental QALY, mean (SD) | ICER, mean (SD) (£/QALY) | Incremental net benefit, mean (SD) |
|---|---|---|---|---|---|---|
| Surgeon’s preference | 2297 (118) | 0.800 (0.021) | ||||
| Surgisis anal fistula plug | 2750 (112) | 453 (163) | 0.826 (0.018) | 0.026 (0.027) | 17,279 (1,168,154) | 71 (578) |
| Adjusted baseline EQ-5D-3L | 32,400 | –168 |
The uncertainty of the cost-effectiveness estimate for the imputed data with baseline adjustment in the cost-effectiveness plane is shown graphically in Figure 11, using bootstrapping with 1000 iterations. For each bootstrap iteration, a new imputed data set was created, leading to 1000 incremental cost and incremental QALY estimates being produced.
FIGURE 11.
Cost-effectiveness plane showing the incremental cost and QALYs for the fistula plug group compared with the surgeon’s preference group from the bootstrap analysis of the imputed data adjusted for baseline EQ-5D-3L (mean values also shown).
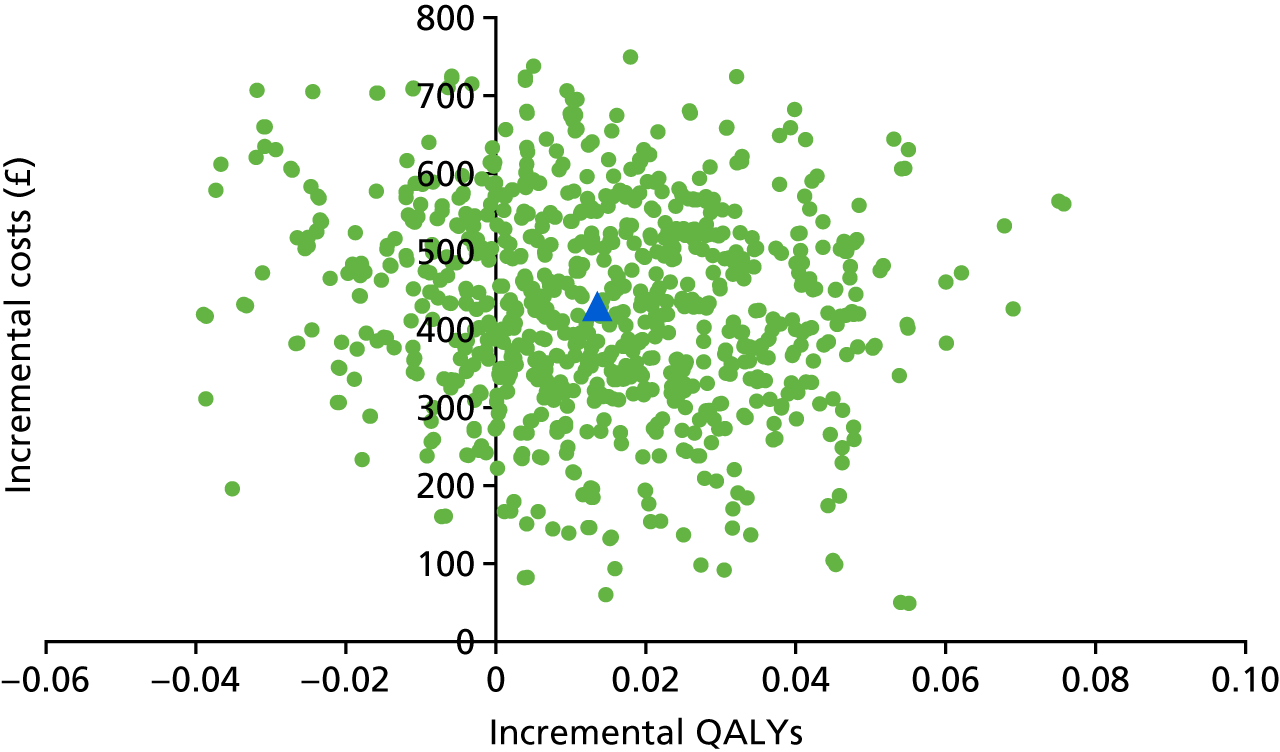
The majority of iterations have a positive incremental cost, which indicates that the fistula plug is very likely to be more costly than the surgeon’s preference. Moreover, the majority of iterations also see a positive incremental QALY estimate, which suggests that the fistula plug is likely to be more effective than surgeon’s preference in terms of QALYs gained. Finally, it is notable that for all 1000 iterations the fistula plug was always more expensive than surgeon’s preference.
The cost-effectiveness acceptability curve showing the probability that the fistula plug is cost-effective is presented in Figure 12 across a range of threshold values of WTP for a single QALY. The probability that the fistula plug is cost-effective is approximately 35–45% across the broader NICE acceptance threshold of £20,000–30,000 per QALY. At WTP thresholds for a QALY above £30,000, the probability that the fistula plug will be cost-effective gradually increases, plateauing at approximately 65%.
FIGURE 12.
Cost-effectiveness acceptability curve showing the probability that the fistula plug will be cost-effective compared with surgeon’s preference across different WTP values for a single QALY, using the bootstrap analysis of the imputed data adjusted for the baseline EQ-5D-3L.
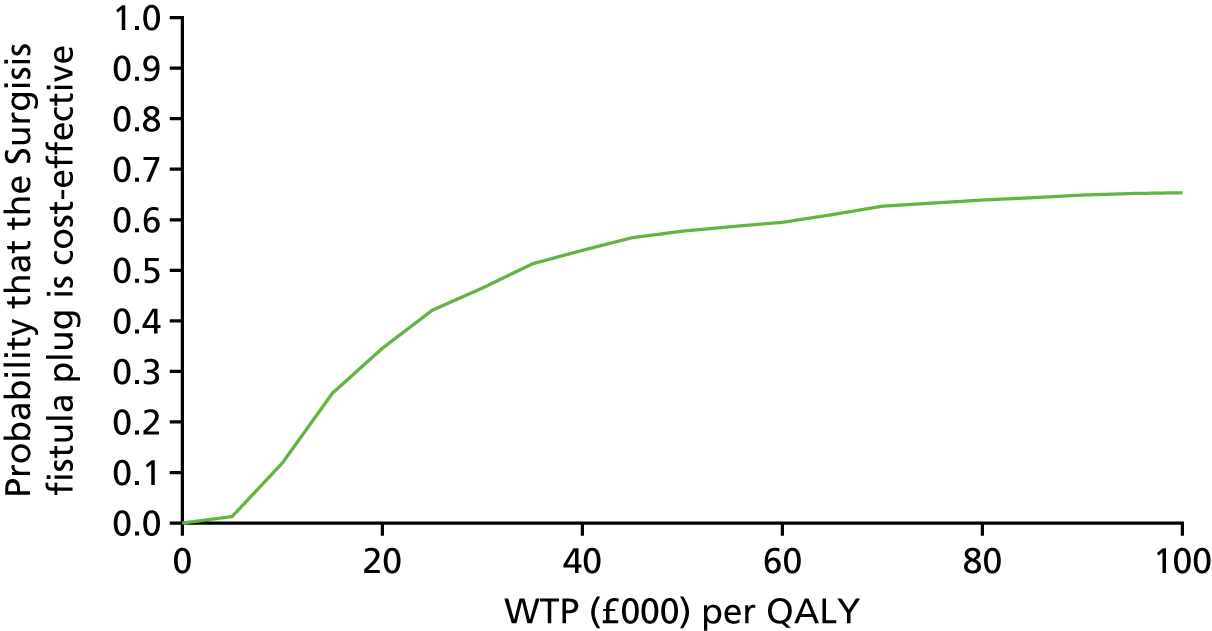
Economic model
Given the lack of secondary sources to inform patient outcomes beyond 12 months, no economic modelling was undertaken.
Chapter 4 Discussion
The FIAT is the largest known RCT trial investigating modern techniques for the treatment of trans-sphincteric fistula-in-ano. It provides important data on a recently introduced technology, the Surgisis anal fistula plug, which combines ease of application with the promise of minimal compromise to anal sphincter function and, therefore, maximal preservation of continence. However, the fistula plug comes with uncertainty regarding its efficacy and whether or not the potential benefits justify the additional cost of the device. In addition to answering the above questions, the FIAT provides valuable information about current treatment preferences for fistula treatment in the UK NHS (cutting seton, fistulotomy, advancement flap and LIFT procedure), along with an assessment of their efficacy and cost-effectiveness. The primary outcome measure, HRQoL, was chosen to reflect the impact of the various surgical techniques from a patient perspective; however, important secondary outcomes include not just the clinical rates of fistula healing but also radiological evidence of fistula healing, a feature unique to the FIAT that provides additional credence to the results.
The FIAT opened to recruitment in May 2011 with an anticipated accrual rate of 15 patients per month over 3 years, based on published incidence rates for trans-sphincteric fistulas of 1–2 people per 10,000 of the population. 1,2 The trial closed to recruitment in March 2016 having reached a revised recruitment target of 304 patients. The primary reason for the slower than expected recruitment is reflected in the CONSORT diagram (see Figure 3), with 1355 participants being screened for eligibility (a recruitment rate of approximately 1 in every 20 patients screened). By far the most common reason for ineligibility (76%) was the fistula morphology as assessed by a combination of clinical examination (EUA) and radiological assessment (baseline MRI). Clinical examination has previously been relied on to characterise fistula morphology, but is notoriously inaccurate and highly influenced by the experience of the individual surgeon. 36 MRI has been shown to improve the diagnostic accuracy for fistula-in-ano,22 although its routine use in the treatment algorithm for all anal fistulas is debated. The fistulas investigated in FIAT were all complex, by nature of their trans-sphincteric anatomy, and baseline MRI was mandatory prior to randomisation into the trial. Although most surgeons reviewed the MRI report, only around a half viewed the MRI scans, despite the fact that the MRI findings were felt to influence the surgical approach in around 16% of cases. The compulsory use of baseline MRI adds rigour to our analyses, but highlights the fact that fistulas considered to be trans-sphincteric can be more or less complex than apparent on clinical examination alone. Our data comparing EUA with MRI assessment revealed a concordance rate of only 34%, with MRI picking up more complex fistula disease or additional findings that justifies its use as a first-line investigation in all but the simplest of fistulas.
The baseline data show that the randomisation process in the FIAT produced a balanced proportion of participants in terms of pre-existing incontinence symptoms and risk factors for fistula healing. In particular, smokers, previous history of fistula surgery and fistula characteristics, factors that are considered to predispose to worse outcomes following fistula surgery,37–39 were equally represented in the fistula plug and surgeon’s preference groups. The baseline data also emphasise the difficulties with fistula surgery, in that it is infrequently amenable to a single intervention, but often multiple attempts are required to affect a cure. In the FIAT, 45% of participants had undergone a previous attempt at fistula eradication, with 60% suffering from chronic sepsis or recurrent fistulous disease.
The FIAT cohort is typical of the patient population suffering from fistula-in-ano, the majority of whom are healthy (ASA I) patients aged between 30 and 60 years, among whom there is a slight predominance of men. The young age of this affected population highlights the importance of finding effective treatments in this active, working population. Incontinence rates in this patient population are low and any disturbance of anal continence as a result of fistula treatment is likely to have a lifetime effect on physical and mental well-being and productivity, hence the importance attached to the preservation of continence when considering the efficacy of any fistula treatment. Although the St Mark’s incontinence scores in the FIAT cohort were higher than expected in comparison with an age-matched group without fistulous disease, they were generally low, indicating mild incontinence symptoms associated with the presence of a fistula. If anything, the rates of baseline incontinence symptoms reported in the FIAT are likely to be an overestimate, with symptoms, such as incontinence to gas, alteration in lifestyle and the wearing of pads, often attributable to the active fistula tract rather than true compromise to the anal sphincter mechanism.
In terms of the primary outcome measure, FIQoL at the 12-month follow-up, the FIAT found a marginal improvement in QoL in both the fistula plug group and the surgeon’s preference group, but no statistically significant difference between the groups (a finding that was reflected in all four domains of the FIQoL evaluation). Adamina et al. ,40 in a prospective cohort trial of 46 patients undergoing fistula plug treatment, reported a more marked improvement in QoL, as measured by the Short Form questionnaire-36 items version 2. Bondi et al. ,41 in a randomised trial comparing the fistula plug with advancement flap, found an improvement in QoL at 3 months, but no difference between the two techniques. The per-protocol analysis of the primary end point did not alter the findings, indicating that non-adherence to the randomisation allocation failed to have a material influence on the results. The prespecified subgroup analyses of the four randomisation minimisation factors (i.e. age, ASA grade, type of surgery, fistula extensions) also failed to demonstrate an influence on the primary outcome measure, which might be an unexpected finding given the perception that increasing age and ASA grade tend to be associated with poorer wound healing, and that certain techniques for fistula eradication (cutting seton and fistulotomy) are associated with high rates of incontinence compared with so-called sphincter-sparing techniques (LIFT procedure).
Fistula healing rates in both the fistula plug group and the surgeon’s preference group were at the lower end of the spectrum reported in the literature. Overall, the fistula healing rates reported in the FIAT might be viewed as disappointing, particularly when viewed against the literature, which is dominated by single-institution studies. However, in comparison with the other randomised trials evaluating the fistula plug, the results of the FIAT are consistent. In a randomised comparison of 94 patients treated with the fistula plug or advancement flap, the recurrence rate at 12 months was 66% with the fistula plug and 38% with the advancement flap,41 whereas in a similar randomised comparison of the fistula plug against advancement flap, involving 60 patients, the recurrence rates were 71% and 52% with the fistula plug and advancement flap, respectively. 42 Similarly, low rates of fistula healing using the fistula plug were reported in a non-randomised, multicentre prospective trial of 90 patients, with fistula healing at 12 months reported in 49% of patients. 43 Several systematic reviews and meta-analyses have now been published, documenting healing rates with the fistula plug varying between 35% and 87%, with higher rates in patients with single fistula tracts than in those with multiple fistula tracts. 18,19,44 In the FIAT there was no statistically significant difference between the fistula plug and surgeon’s preference groups at any of the time points, with only around one-third of fistulas healed by 6 weeks and just over half healed by 12 months. When the FIAT cohort was analysed for the influence of randomisation minimisation factors (i.e. age, ASA grade, type of surgery, fistula extensions) on healing rates, no significant differences were found. Again, this might seem somewhat surprising, given that age and ASA grade are believed to influence wound healing and the presence of more extensive disease (fistula extensions) is thought to predispose to fistula recurrence/persistence.
Although care has to be taken when drawing conclusions from our subgroup analysis of fistula healing by procedure undertaken, owing to the low numbers in each treatment group, some interesting observations emerge. The most effective surgical operation appeared to be fistulotomy [12/16 (75%) fistulas healed at 12 months], whereas the LIFT procedure, perhaps surprisingly in consideration of the literature, performed worst, with only 16 (29%) of 55 fistulas and 21 (45%) of 50 fistulas healed at 6 weeks and 12 months, respectively. By comparison, success rates reported in the literature, in terms of fistula healing, range between 70% and 80%,16,45,46 although most studies report only short-term follow-up. The cutting seton healed few fistulas (7/47 fistulas, 15%) at 6 weeks, but 27 (66%) of 41 fistulas by 12 months, in keeping with its mode of action, which is to produce a slow division of the included sphincter muscle. Although many UK surgeons are critical of the cutting seton, because of the fear of incontinence, encouraging results have been reported with little consequence on continence function. 47 The advancement flap healed 11 (52%) of 21 fistulas by 6 weeks, with a similar percentage (9/17 fistulas, 53%) remaining healed at 12 months. This is in keeping with reported rates in the literature, with one retrospective review reporting fistula healing rates of 63% with advancement flap, compared with 32% with the fistula plug, at a mean follow-up of 56 weeks,48 and an other trial reporting healing rates at 12 weeks of 59.3%, 60.4% and 32.6% with the use of the fistula plug, advancement flap and cutting seton, respectively. 49
The results from the FIAT were obtained by rigorous data collection as part of a RCT undertaken across 40 NHS hospitals and provide a fascinating insight into the efficacy of fistula surgery. The poor results of fistula surgery, regardless of surgical technique, are supported by the high percentage of FIAT participants who at baseline reported having previously undergone a fistula operation (overall 45% of participants) and the high rates of surgical reintervention reported at the 12-month follow-up (fistula plug group, n/N = 28/124, 23%; surgeon’s preference group, n/N = 27/121, 22%). Many of the surgical reinterventions were for septic complications secondary to fistula recurrence. It is known that many factors affect the outcomes of fistula surgery, including the characteristics of the fistula,50 the surgical technique used and the centre/surgeon undertaking the operation. 39,51 With specific reference to the technique of fistula plug insertion, there is evidence that the use of a prior draining seton does not affect healing rates, although the length of the fistula tract may be a determining feature. 50 Further in-depth analysis of the FIAT data set will undoubtedly give more insights into current practice and outcome variability within the UK NHS.
Although the long-term results of fistula surgery in terms of healing rates are poor, the surgical procedures themselves impart a low risk of morbidity in the early postoperative period, and repeated interventions in an attempt to eradicate the disease would appear to be justified. The main complication related to any type of fistula surgery appears to be protracted pain, which was the most reported complication up to 6 months’ follow-up (fistula plug group, n/N = 14/27, 52%; surgeon’s preference group, n/N = 7/27, 26%). By the 12-month follow-up, septic complications appeared to be more problematic, although unexpected pain continued to be represented, accepting that there is overlap in the reporting of these two symptoms given that sepsis is frequently accompanied by pain. The only significant difference in the complications rates between the two groups in the FIAT was observed at 6 weeks and was probably influenced by the higher rate of unexpected pain in the fistula plug group than in the surgeon’s preference group (fistula plug group, n/N = 32/49, 65%; surgeon’s preference group, n/N = 9/25, 36%; p = 0.002). This did not appear to be due to a higher rate of sepsis in the fistula group. Rather, it might be related to the method of securing the fistula plug, by stitching it to the internal anal sphincter, causing sphincter spasm, or a lower threshold for reporting pain in participants who might have perceived the fistula plug to be a minimally invasive, low-pain procedure. A complication specific to the fistula plug is plug extrusion early in the postoperative period, which is reported to occur in 10–15% of cases. 18,44,52,53 Despite our best efforts to standardise the method of fistula plug insertion in line with best practice techniques, including hands-on proctoring of investigators for the first three cases and the use of instruction videos, the plug extrusion rate in the FIAT remained at 16%. Further research into fistula plug fixation within the high-pressure anal canal is required to reduce the rate of plug extrusion. If this can be achieved, it might have a substantial impact on the overall healing rates achievable with the plug.
Preservation of continence is of paramount importance when considering surgery for fistula disease. On average, the St Mark’s incontinence scores in both the fistula plug group and the surgeon’s preference group were low at baseline. There appeared to be a numerical improvement in incontinence scores over time in both groups, which might reflect a positive change in certain symptoms (e.g. alteration in lifestyle, wearing a pad) associated with anal fistula, rather than a true improvement in anal continence. It is perhaps surprising that the surgeon’s preference group did not perform worse than the fistula plug group, given that it allowed the use of techniques (i.e. fistulotomy, advancement flap, cutting seton) known to injure the anal sphincter mechanism.
Given the overall lack of significant differences in efficacy and safety of the fistula plug, compared with other techniques for fistula treatment, the cost-effectiveness analysis is particularly important when considering widespread uptake of the plug within the UK NHS. Using EQ-5D to generate QALYs, a number of alternative results were obtained based on different approaches to the analysis of the data. The complete-case analysis showed that the fistula plug group experienced a slightly higher QALY gain than the surgeon’s preference group (0.829 vs. 0.790). The mean cost was higher in the fistula plug group than in the surgeon’s preference group (£2738 vs. £2308), driven by the additional cost of the Surgisis anal fistula plug. Applying probabilistic analysis to the complete data, the ICER was found to be £10,933 per QALY, indicating that the fistula plug may be considered to be more cost-effective than surgeon’s preference, although the SD (478,666) indicates the large uncertainty in this estimate. Using multiple imputation to increase the data set to 267 patients and probabilistic sensitivity analysis, the fistula plug was again found to be more costly (£2750 vs. £2297) and more effective (0.826 vs. 0.800 QALYs gained) compared with surgeon’s preference. In this case, the ICER was £17,279, again suggesting that the fistula plug may be considered to be more cost-effective than surgeon’s preference. However, when adjustment was made to account for differences in EQ-5D at baseline, the ICER increased to £32,400, suggesting that the fistula plug may not be considered to be cost-effective. Uncertainty in the results for the multiple imputed data adjusted for baseline EQ-5D suggests that the fistula plug is 35–45% more likely than surgeon’s preference to be cost-effective at thresholds of WTP for a QALY of £20,000–30,000. Thus, it can be concluded that the fistula plug may not be a cost-effective approach to the treatment of patients with high trans-sphincteric fistulas.
In terms of taking these results forward, a key approach would be to implement an economic model to examine how patient outcomes beyond the 1-year time horizon would impact on the conclusions drawn from the cost-effectiveness analysis. However, in order to do this, information would be required to inform the HRQoL of the patients beyond 1 year. At present, no such data are available and, consequently, in the FIAT a sensible economic model was not possible. Hypothetically, if it were assumed that the EQ-5D utility values collected at 12 months remained unchanged beyond that time, then it might be concluded that the fistula plug would become increasingly cost-effective as time passes. However, there is little evidence beyond the 12-month follow-up to justify this assumption. For example, a review in 2009 included 12 studies, all of which were small scale and had a median follow-up period of ≤ 12 months. 18 More recent studies have provided no indication of the longer-term costs and effects; a systematic review from 2015 identified no studies in which the follow-up period was longer than 14 months. 54 A subsequent review of another fistula plug (GORE® BIO-A®, Gore Medical, Newark, DE, USA) included one trial which had follow-up ranging from 3 to 19 months; however, the median was only 5 months and the trial included only 11 patients. 53 Nevertheless, as data become available, modelling approaches could be implemented to provide further insights to inform the cost basis for wider adoption of fistula plug technology.
Limitations
The limitations of the FIAT include the need to reduce the recruitment target because of the lower than anticipated accrual rate. As alluded to above, this was partly due to a high exclusion rate, probably associated with the mandatory use of baseline MRI and perhaps an overestimation in the literature about the true rate of trans-sphincteric fistulas compiled in an era when MRI was not available. Despite a reduction in target recruitment from 500 to 300 patients, the FIAT was still powered to detect a small to moderate difference in the primary outcome (FIQoL). In addition, the original sample size calculation factored in a 20% dropout rate, which did not materialise. Only 7 (2%) out of 304 participants were lost to follow-up and compliance with follow-up data was extremely good. Given the lack of any convincing difference in FIQoL between the fistula plug group and the surgeon’s preference group, it is unlikely that achieving the original sample size of 400 patients would have altered the results. Similarly, the crossover of patients between the treatment arms does not appear to have had a material influence on the results, as demonstrated by the per-protocol analysis.
The FIAT was an unblinded, parallel-group trial. It would obviously not have been possible to blind the surgeons to the treatment allocation. Similarly, it would have been difficult to blind the participants, given that some would have had an anodermal wound (LIFT procedure), whereas others did not (fistula plug and advancement flap) and others would have had a seton protruding from the anal canal (cutting seton). Likewise, blinding of the data collectors would have introduced problems in the collection of treatment-specific information, such as the rate of fistula plug extrusion. Although non-blinding might have introduced an element of bias, perhaps in patient reporting of postoperative pain, it is unlikely to have affected the primary outcome, for which there was no observable difference between the treatment arms.
Strenuous attempts were made to standardise the technique for the placement of the fistula plugs, including surgeon attendance at a mandatory training session, preceptorship with the first three procedures undertaken and video instruction. This was undertaken in an attempt to eliminate any learning curve effect associated with the fistula plug technique, which was probably small given that plug placement is a simple procedure that is well within the skill set of the coloproctologists participating in the FIAT. Standardisation was also considered to be important in reducing the occurrence of early fistula plug extrusion. Despite this, plug extrusion was still reported in 16% of cases of the fistula plug, perhaps indicating an intrinsic problem with the placement of prostheses within the high-pressure anal canal.
A criticism that could be levelled at the FIAT is the small number of patients recruited by several participating sites. This probably reflects the relatively low incidence of trans-sphincteric, cryptoglandular fistula-in-ano, but is mitigated to some degree by the simplicity of the fistula plug technique and the associated short learning curve.
One of the strengths of the FIAT was in the mandatory use of MRI at baseline and the 12-month follow-up, to overcome the recognised inaccuracies in relying solely on clinical examination to characterise fistulas and assess healing. Although we achieved 100% success in obtaining baseline MRI, despite our best efforts we were only able to obtain follow-up imaging for 73% of patients. This is probably still sufficient to obtain reasonable data accuracy and certainly far exceeds, to our knowledge, any previous attempts to use radiological imaging to obtain unequivocal evidence of fistula healing.
Conclusion
The FIAT failed to show a difference in the primary end point, FIQoL, between the fistula plug group and the surgeon’s preference group at the 12-month follow-up. Reassuringly, there was a low rate of early postoperative complications in both groups, indicating that fistula surgery generally carries a low level of morbidity and, therefore, it is justified to perform repeat procedures in an attempt to eradicate the disease. Similarly, it is reassuring to note that incontinence following fistula surgery is generally low, regardless of the surgical technique, and perhaps this should not be used exclusively as a rationale to support the use of the fistula plug. One of the stark highlights of the FIAT is the poor fistula healing rate obtained with all types of fistula surgery. The procedures included in the surgeon’s preference group are those most widely practised by colorectal surgeons in the UK. The results emphasise the inadequacies of current fistula surgery and the need for further research into the underlying pathophysiology of the disease in order to design new, more effective, therapies. Although alternative approaches to fistula eradication continue to be reported in the literature, there is no convincing evidence that they offer any advantage over those evaluated in the FIAT. Importantly, surgeons should learn to manage the expectations of patients suffering with fistula-in-ano, stressing the high failure rates in terms of fistula healing and the need for multiple reinterventions if the aim is to eradicate the disease. The FIAT has provided a health economics evaluation of the fistula plug in comparison with other commonly used surgical techniques. On the basis of the results presented, the fistula plug is more expensive than surgeon’s preference and more effective in terms of QALYs gained. Differences in QALY gains are very small and uncertain in our analysis. Taking account of parameter uncertainty and using some imputation to increase effective sample size, this study suggests that the fistula plug technology is unlikely to be considered a cost-effective use of UK NHS resources.
Recommendations for research
Further research might look at the long-term follow-up of the FIAT cohort to assess whether or not the outcomes change with time. In particular, the lower rates of fistula healing as assessed by MRI, compared with clinical evaluation, might manifest in further fistula recurrences in the future. As previously mentioned, it would also be informative to develop an economic model to examine how patient outcomes beyond the 1-year time horizon impact on the conclusions drawn from the cost-effectiveness analysis. Since the completion of the FIAT, a number of new technologies have been proposed for the treatment of trans-sphincteric fistula and it would be interesting to see if they offer any additional benefit.
Acknowledgements
The FIAT authors thank the NIHR HTA programme for funding the FIAT and the staff (Programme Manager Nick Eaton) for their support and advice during the course of the trial.
We are grateful to all members of the Trial Steering Committee [Simon Ambrose (Chairperson), John Spencer, James Hampton and Ly-Mee Yu] and the Data Monitoring and Ethics Committee [Steven Brown (Chairperson), Clive Kay and Louise Hiller] for their support and guidance.
A sincere thank-you to our patient representatives, Lucy Prodgers and Claire Roberts.
The authors would like to thank the staff of the trial office past and present, Peter Antonio (Data Manager, 2013–16) and Adrian Wilcockson (Programmer, 2009–17), for their information technology, database support and data management during the course of the trial. The BCTU Randomisation Team provided the randomisation service for the recruitment phase of the trial.
The authors would also like to thank the research nurses who played a vital role in the delivery of the trial by visiting the participating sites: Catherine Moriarty (2011–16), Julie Barnsley (2012–13), Margret Adrian (2012), Louise Warburton (2013–14) and Mandy Eyre (2014–16).
A sincere thank-you to Holly Speight, who helped upload all the MRI scans for Dr Damian Tolan.
The FIAT has been a collaborative effort and its success is a result of the enormous enthusiasm and support from all of the committee members and participating centres. We would therefore like to acknowledge the members of staff at recruitment sites who facilitated recruitment, treatment and follow-up of trial participants.
Sincere thanks are also due to the staff of the East Midlands – Derby Research Ethics Committee for considering our application and amendments during the course of the trial.
Finally, we would like to thank all the patients who agreed to participate in the trial.
Trial Management Group
Professor David G Jayne: chief investigator, applicant, Consultant Surgeon – The Leeds Teaching Hospitals NHS Trust, St James’s University Hospital/Leeds General Infirmary.
Professor John Scholefield: co-applicant, Consultant Surgeon – Nottingham University Hospitals NHS Trust, Queen’s Medical Centre.
Miss Asha Senapati: Consultant Surgeon – Portsmouth Hospitals NHS Trust, Queen Alexandra Hospital.
Dr Damian Tolan: Consultant Radiologist – The Leeds Teaching Hospitals NHS Trust, St James’s University Hospital/Leeds General Infirmary.
Professor Richard Gray: co-applicant, Clinical Trials and Medical Statistics – Nuffield Department of Population Health Medical Sciences Division, University of Oxford.
Dr Claire T Hulme: Health Economics – Academic Unit of Health Economics, Leeds Institute of Health Sciences, University of Leeds.
Dr Laura Magill: Surgical Portfolio Trials Team Leader – BCTU, University of Birmingham.
Dr Kelly Handley: Senior Statistician – BCTU, University of Birmingham.
Mrs Manjinder Kaur: Senior Trial Manager – BCTU, University of Birmingham.
Ms Lucy Prodgers: patient representative.
Ms Claire Roberts: patient representative.
Trial Steering Committee
Mr Simon Ambrose (Chairperson): Consultant Colorectal Surgeon (retired) – The Leeds Teaching Hospitals NHS Trust, St James’s University Hospital/Leeds General Infirmary.
Dr John Spencer (until December 2013): Consultant Radiologist – The Leeds Teaching Hospitals NHS Trust, St James’s University Hospital/Leeds General Infirmary.
Dr James Hampton (from December 2013): Consultant Gastrointestinal Radiologist – Sheffield Teaching Hospitals NHS Foundation Trust, Northern General Hospital.
Dr Ly-Mee Yu: Associate Professor and Deputy Director Academic (Primary Care Trials Unit) – Nuffield Department of Population Health Medical Sciences Division, University of Oxford.
Data Monitoring Committee
Mr Steven Brown (Chairperson): Consultant Surgeon – Sheffield Teaching Hospitals NHS Foundation Trust, Northern General Hospital.
Professor Clive Kay: Chief Executive of Bradford Teaching Hospitals NHS Foundation Trust and Consultant Radiologist – Bradford Teaching Hospitals NHS Foundation Trust, Bradford Royal Infirmary.
Dr Louise Hiller: Principal Research Fellow and Statistician – Warwick Clinical Trials Unit, University of Warwick.
Contributions of authors
Professor David G Jayne (Chief Investigator and Consultant Surgeon) contributed to the conception and design of all aspects of the project, the interpretation of the results and the writing and editing of the report. He also contributed to recruitment, data acquisition and the conduct of the trial.
Professor John Scholefield (Consultant Surgeon) contributed to the conception and design of all aspects of the project. He also contributed to recruitment, data acquisition and the conduct of the trial.
Dr Damian Tolan (Consultant Radiologist) contributed to the conception and design of all aspects of the project, the conduct of the trial, the interpretation of the results and the writing and editing of the report.
Professor Richard Gray (Professor of Statistics) contributed to the conception and design of all aspects of the project, the conduct of the trial, the interpretation of the results and the writing and editing of the report.
Dr Richard Edlin (Health Economist) contributed to the conception and design of all aspects of the project.
Professor Claire T Hulme (Health Economist) conducted and prepared the results of the trial-based cost-effectiveness analysis and contributed to the writing of the report.
Dr Andrew J Sutton (Health Economist) conducted and prepared the results of the trial-based cost-effectiveness analysis and contributed to the writing of the report.
Dr Kelly Handley (Senior Statistician) contributed to the design of the trial, supervised the statistical analyses and the interpretation of the results, and contributed to the writing of the report.
Ms Catherine A Hewitt (Trial Statistician) conducted the statistical analyses, prepared the results and contributed to the writing of the report.
Mrs Manjinder Kaur (Senior Trial Manager) contributed to the conduct and co-ordination of the trial, collation of the data and the interpretation of the results, and the writing and editing of the report.
Dr Laura Magill (Coloproctology/Surgical Portfolio Team Leader) contributed to the conception and design of all aspects of the project, the conduct of the trial, the interpretation of the results and the writing and editing of the report.
Publication
Simpson JA, Banerjea A, Scholefield JH. Management of anal fistula. BMJ 2012;345:e6705.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ewerth S, Ahlberg J, Collste G, Holmström B. Fistulain-ano. A six year follow up study of 143 operated patients. Acta Chir Scand Suppl 1978;482:53-5.
- Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol 1984;73:219-24.
- Showler PJGR, Keighley MRB, Alexander-Williams J. Fistula-in-ano is usually easy to treat surgically. Int J Colorect Dis 1986;1.
- Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum 1996;39:723-9. https://doi.org/10.1007/BF02054434.
- Lunniss PJ, Kamm MA, Phillips RK. Factors affecting continence after surgery for anal fistula. Br J Surg 1994;81:1382-5. https://doi.org/10.1002/bjs.1800810947.
- Garcia-Aguilar J, Belmonte C, Wong DW, Goldberg SM, Madoff RD. Cutting seton versus two-stage seton fistulotomy in the surgical management of high anal fistula. Br J Surg 1998;85:243-5. https://doi.org/10.1046/j.1365-2168.1998.02877.x.
- Williams JG, MacLeod CA, Rothenberger DA, Goldberg SM. Seton treatment of high anal fistulae. Br J Surg 1991;78:1159-61. https://doi.org/10.1002/bjs.1800781004.
- Pearl RK, Andrews JR, Orsay CP, Weisman RI, Prasad ML, Nelson RL, et al. Role of the seton in the management of anorectal fistulas. Dis Colon Rectum 1993;36:573-7. https://doi.org/10.1007/BF02049864.
- Dziki A, Bartos M. Seton treatment of anal fistula: experience with a new modification. Eur J Surg 1998;164:543-8. https://doi.org/10.1080/110241598750005930.
- Isbister WH, Al Sanea N. The cutting seton: an experience at King Faisal Specialist Hospital. Dis Colon Rectum 2001;44:722-7. https://doi.org/10.1007/BF02234574.
- Van Tets WF, Kuijpers JH. Seton treatment of perineal fistula with high anal or rectal opening. Br J Surg 1995;82:895-7. https://doi.org/10.1002/bjs.1800820711.
- Hamalainen KP, Sainio AP. Cutting seton for anal fistulas: high risk of minor control defects. Dis Colon Rectum 1997;40:1443-6. https://doi.org/10.1007/BF02070710.
- Schouten WR, Zimmerman DD, Briel JW. Transanal advancement flap repair of transsphincteric fistulas. Dis Colon Rectum 1999;42:1419-22. https://doi.org/10.1007/BF02235039.
- Zimmerman DD, Briel JW, Gosselink MP, Schouten WR. Anocutaneous advancement flap repair of transsphincteric fistulas. Dis Colon Rectum 2001;44:1474-80. https://doi.org/10.1007/BF02234601.
- Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol 2009;13:237-40. https://doi.org/10.1007/s10151-009-0522-2.
- Hong KD, Kang S, Kalaskar S, Wexner SD. Ligation of intersphincteric fistula tract (LIFT) to treat anal fistula: systematic review and meta-analysis. Tech Coloproctol 2014;18:685-91. https://doi.org/10.1007/s10151-014-1183-3.
- Johnson EK, Gaw JU, Armstrong DN. Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum 2006;49:371-6. https://doi.org/10.1007/s10350-005-0288-1.
- Garg P, Song J, Bhatia A, Kalia H, Menon GR. The efficacy of anal fistula plug in fistula-in-ano: a systematic review. Colorectal Dis 2010;12:965-70. https://doi.org/10.1111/j.1463-1318.2009.01933.x.
- Xu Y, Tang W. Comparison of an anal fistula plug and mucosa advancement flap for complex anal fistulas: a meta-analysis. ANZ J Surg 2016;86:978-82. https://doi.org/10.1111/ans.13751.
- FIAT (Fistula-In-Ano Trial) Comparing Surgisis® Anal Fistula Plug Versus Surgeon’s Preference (Advancement Flap, Fistulotomy, Cutting Seton) for Transsphincteric Fistula-In-Ano. Southampton: NIHR; n.d.
- Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg 1976;63:1-12. http://dx.doi.org/10.1002/bjs.1800630102.
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum 2000;43:9-16. https://doi.org/10.1007/BF02237236.
- Cohen J. Statistical Power Analyses for the Behavioural Sciences. London: Routledge; 1977.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- National Schedule of Reference Costs: 2015–16 – All NHS Trusts and NHS Foundation Trusts – HRG2017. London: Department of Health and Social Care; n.d.
- National Institute for Health and Care Excellence . British National Formulary 2018 n.d. https://bnf.nice.org.uk (accessed 6 April 2019).
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Dolan P. Modeling valuation for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Research Governance Framework for Health and Social Care. London: Department of Health and Social Care; 2015.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Lambert R, Fordham R, Large S, Gaffney B. A cost-minimisation study of 1,001 NHS Direct users. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-300.
- NHS Reference Costs. 2017–18 Tariff Information Spreadsheet – Index. London: Department of Health and Social Care; 2017.
- Information Services Division . Theatres n.d. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 16 January 2018).
- Ding JH, Bi LX, Zhao K, Feng YY, Zhu J, Zhang B, et al. Impact of three-dimensional endoanal ultrasound on the outcome of anal fistula surgery: a prospective cohort study. Colorectal Dis 2015;17:1104-12. https://doi.org/10.1111/codi.13108.
- Schwandner T, Roblick MH, Kierer W, Brom A, Padberg W, Hirschburger M. Surgical treatment of complex anal fistulas with the anal fistula plug: a prospective, multicenter study. Dis Colon Rectum 2009;52:1578-83. https://doi.org/10.1007/DCR.0b013e3181a8fbb7.
- Ellis CN, Rostas JW, Greiner FG. Long-term outcomes with the use of bioprosthetic plugs for the management of complex anal fistulas. Dis Colon Rectum 2010;53:798-802. https://doi.org/10.1007/DCR.0b013e3181d43b7d.
- Ommer A, Herold A, Joos A, Schmidt C, Weyand G, Bussen D. Gore BioA fistula plug in the treatment of high anal fistulas – initial results from a German multicenter-study. Ger Med Sci 2012;10. https://doi.org/10.3205/000164.
- Adamina M, Ross T, Guenin MO, Warschkow R, Rodger C, Cohen Z, et al. Anal fistula plug: a prospective evaluation of success, continence and quality of life in the treatment of complex fistulae. Colorectal Dis 2014;16:547-54. https://doi.org/10.1111/codi.12594.
- Bondi J, Avdagic J, Karlbom U, Hallböök O, Kalman D, Šaltytė Benth J, et al. Randomized clinical trial comparing collagen plug and advancement flap for trans-sphincteric anal fistula. Br J Surg 2017;104:1160-6. https://doi.org/10.1002/bjs.10549.
- van Koperen PJ, Bemelman WA, Gerhards MF, Janssen LW, van Tets WF, van Dalsen AD, et al. The anal fistula plug treatment compared with the mucosal advancement flap for cryptoglandular high transsphincteric perianal fistula: a double-blinded multicenter randomized trial. Dis Colon Rectum 2011;54:387-93. https://doi.org/10.1007/DCR.0b013e318206043e.
- Stamos MJ, Snyder M, Robb BW, Ky A, Singer M, Stewart DB, et al. Prospective multicenter study of a synthetic bioabsorbable anal fistula plug to treat cryptoglandular transsphincteric anal fistulas. Dis Colon Rectum 2015;58:344-51. https://doi.org/10.1097/DCR.0000000000000288.
- O’Riordan JM, Datta I, Johnston C, Baxter NN. A systematic review of the anal fistula plug for patients with Crohn’s and non-Crohn’s related fistula-in-ano. Dis Colon Rectum 2012;55:351-8. https://doi.org/10.1097/DCR.0b013e318239d1e4.
- Chen HJ, Sun GD, Zhu P, Zhou ZL, Chen YG, Yang BL. Effective and long-term outcome following ligation of the intersphincteric fistula tract (LIFT) for transsphincteric fistula. Int J Colorectal Dis 2017;32:583-5. https://doi.org/10.1007/s00384-016-2723-2.
- Han JG, Wang ZJ, Zheng Y, Chen CW, Wang XQ, Che XM, et al. Ligation of intersphincteric fistula tract vs ligation of the intersphincteric fistula tract plus a bioprosthetic anal fistula plug procedure in patients with transsphincteric anal fistula: early results of a multicenter prospective randomized trial. Ann Surg 2016;264:917-22. https://doi.org/10.1097/SLA.0000000000001562.
- Rosen DR, Kaiser AM. Definitive seton management for transsphincteric fistula-in-ano: harm or charm?. Colorectal Dis 2016;18:488-95. https://doi.org/10.1111/codi.13120.
- Christoforidis D, Pieh MC, Madoff RD, Mellgren AF. Treatment of transsphincteric anal fistulas by endorectal advancement flap or collagen fistula plug: a comparative study. Dis Colon Rectum 2009;52:18-22. https://doi.org/10.1007/DCR.0b013e31819756ac.
- Chung W, Kazemi P, Ko D, Sun C, Brown CJ, Raval M, et al. Anal fistula plug and fibrin glue versus conventional treatment in repair of complex anal fistulas. Am J Surg 2009;197:604-8. https://doi.org/10.1016/j.amjsurg.2008.12.013.
- McGee MF, Champagne BJ, Stulberg JJ, Reynolds H, Marderstein E, Delaney CP. Tract length predicts successful closure with anal fistula plug in cryptoglandular fistulas. Dis Colon Rectum 2010;53:1116-20. https://doi.org/10.1007/DCR.0b013e3181d972a9.
- Blom J, Husberg-Sellberg B, Lindelius A, Gustafsson UM, Carlens S, Oppelstrup H, et al. Results of collagen plug occlusion of anal fistula: a multicentre study of 126 patients. Colorectal Dis 2014;16:626-30. https://doi.org/10.1111/codi.12585.
- Herold A, Ommer A, Fürst A, Pakravan F, Hahnloser D, Strittmatter B, et al. Results of the Gore Bio-A fistula plug implantation in the treatment of anal fistula: a multicentre study. Tech Coloproctol 2016;20:585-90. https://doi.org/10.1007/s10151-016-1505-8.
- Narang SK, Jones C, Alam NN, Daniels IR, Smart NJ. Delayed absorbable synthetic plug (GORE® BIO-A®) for the treatment of fistula-in-ano: a systematic review. Colorectal Dis 2016;18:37-44. https://doi.org/10.1111/codi.13208.
- Köckerling F, Alam NN, Narang SK, Daniels IR, Smart NJ. Treatment of fistula-in-ano with fistula plug – a review under special consideration of the technique. Front Surg 2015;2. https://doi.org/10.3389/fsurg.2015.00055.
Appendix 1 Recruiting centres
Aneurin Bevan University Health Board (Nevill Hall Hospital)
-
Mr Chokkalingam Arun, PI and Lead Surgeon.
-
Dr Nick Cross, Lead Radiologist.
-
Mr Graham Sturgeon, Lead Research Nurse.
Ashford and St Peter’s Hospitals NHS Foundation Trust (St Peter’s Hospital)
-
Mr Philip Bearn, PI and Lead Surgeon.
-
Mr Nisar Pasha, Consultant Colorectal Surgeon.
-
Dr Allan Irvine, Lead Radiologist.
-
Mrs Vitoria Frost, Lead Research Nurse.
Barking, Havering and Redbridge University Hospitals NHS Trust (Queen’s Hospital)
-
Mr Joseph Huang, PI and Lead Surgeon.
-
Dr Jacques Gutmann, Lead Radiologist.
-
Ms Theresa McCluskey, Lead Research Nurse.
Burton Hospitals NHS Foundation Trust (Queen’s Hospital Burton)
-
Miss Anna Sverrisdottir, PI and Lead Surgeon (until September 2015).
-
Mr Pradeep Thomas, PI and Lead Surgeon (from September 2015).
-
Dr Shahzad Khan, Lead Radiologist.
-
Ms Gillian Bell, Research Nurse.
-
Ms Julie Birch, Research Nurse.
-
Ms Clare Mewies, Clinical Trials Co-ordinator.
Calderdale and Huddersfield NHS Foundation Trust (Huddersfield Royal Infirmary)
-
Mr Peter Holdsworth, PI and Lead Surgeon.
-
Dr Sarah Gurney, Lead Radiologist.
Cardiff & Vale University Health Board (Llandough University Hospital/University Hospital of Wales)
-
Mr Michael Davies, PI and Lead Surgeon.
-
Dr Robert Bleehen, Lead Radiologist.
-
Ms Linda Hazel, Research Nurse.
-
Mr Matthew Williams, Research Nurse.
-
Ms Micki Palmer, Research Nurse.
Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary)
-
Mr Finlay Curran, PI and Lead Surgeon.
-
Professor Jim Hill, Consultant Surgeon.
-
Dr Sarah O Shea, Lead Radiologist.
-
Ms Glaxy Gray, Lead Research Nurse.
Chesterfield Royal Hospital NHS Foundation Trust (Chesterfield Royal Hospital)
-
Mr Robin Gupta, PI and Lead Surgeon.
-
Mr Talalakukoppa Amarnath, Consultant Surgeon.
-
Mr Harjeet Narula, Consultant Surgeon.
-
Dr Heather Harris, Lead Radiologist.
-
Ms Julie Toms, Clinical Research Practitioner.
Croydon Health Services NHS Trust (Croydon University Hospital)
-
Mr Muti Abulafi, PI and Lead Surgeon.
-
Dr Helena Blake, Lead Radiologist.
Dorset County Hospital NHS Foundation Trust (Dorset County Hospital)
-
Mr Michael Lamparelli, PI and Lead Surgeon.
-
Dr Peter Taylor, Lead Radiologist.
-
Ms Arabis Oglesby, Research Nurse.
-
Ms Karen Hogben, Research Nurse.
George Eliot Hospital NHS Trust (George Eliot Hospital)
-
Mr Kalimuthu Marimuthu, PI and Lead Surgeon.
-
Dr Amitabh Palit, Lead Radiologist.
-
Ms Rosemary Musanhu, Lead Research Nurse.
-
Ms Emma Brannan, Clinical Trials Co-ordinator.
Gloucestershire Hospitals NHS Foundation Trust (Cheltenham General Hospital)
-
Mr James Wheeler, PI and Lead Surgeon.
-
Dr Richard Hopkins, Lead Radiologist.
Heart of England NHS Foundation Trust (Birmingham Heartlands Hospital/Good Hope Hospital)
-
Mr Haney Youssef, PI and Lead Surgeon.
-
Mr David McArthur, Consultant Surgeon.
-
Dr Mark Goldstein, Lead Radiologist.
-
Ms Linda Webber, Lead Research Nurse.
Homerton University Hospital NHS Foundation Trust (Homerton University Hospital)
-
Ms Tamzin Cuming, PI and Lead Surgeon.
-
Dr Peter Boavida, Lead Radiologist.
-
Ms Sophia Hans, Research Assistant.
Imperial College Healthcare NHS Trust (Charing Cross Hospital/St Mary’s Hospital)
-
Mr Gordon Buchanan, PI and Lead Surgeon (until October 2015).
-
Mr George Reese, PI and Lead Surgeon (from October 2015).
-
Mr Peter Dawson, Lead Radiologist.
-
Ms Melloney Allnutt, Research Nurse.
-
Ms Gillian Hornzee, Research Nurse.
-
Ms Byiravey Pathmanathan, Research Nurse.
Mid Essex Hospital Services NHS Trust (Broomfield Hospital)
-
Mr Toby Hammond, PI and Lead Surgeon.
-
Dr Peng Lee, Lead Radiologist.
-
Ms Joanne Topliffe, Lead Research Nurse.
The Royal Wolverhampton NHS Trust (New Cross Hospital)
-
Mr Graham Williams, PI and Lead Surgeon.
-
Dr Peter Li, Lead Radiologist.
-
Ms Stella Metherell, Lead Research Nurse.
NHS Highland (Raigmore Hospital)
-
Professor Angus Watson, PI and Lead Surgeon.
-
Mr Michael Lim, Consultant Surgeon.
-
Mr Kenneth Walker, Consultant Surgeon.
-
Mr James Docherty, Consultant Surgeon.
-
Dr Jason Walker, Lead Radiologist.
-
Ms Kathleen Macleod, Lead Research Nurse.
Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich University Hospital)
-
Mr Christopher Speakman, PI and Lead Surgeon.
-
Dr Stuart William, Lead Radiologist.
-
Ms Georgina Glister, Lead Research Nurse.
Nottingham University Hospitals NHS Trust (Queen’s Medical Centre)
-
Professor John Scholefield, PI and Lead Surgeon.
-
Mr Mike Robinson, Consultant Surgeon.
-
Mr Ayan Banerjea, Consultant Surgeon.
-
Dr William Dunn, Lead Radiologist.
-
Ms Mandy Eyre, Lead Research Nurse.
Oxford University Hospitals NHS Foundation Trust (Churchill Hospital/John Radcliffe Hospital)
-
Mr Christopher Cunningham, PI and Lead Surgeon.
-
Dr Andrew Slater, Lead Radiologist.
Poole Hospital NHS Foundation Trust (Poole Hospital)
-
Mr Guy Nash, PI and Lead Surgeon.
-
Dr David Tarver, Lead Radiologist.
Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital)
-
Miss Asha Senapti, PI and Lead Surgeon.
-
Dr Anthony Higginson, Lead Radiologist.
-
Ms Elizabeth Hawes, Research Nurse.
-
Ms Sheeba Babu, Research Nurse.
-
Ms Karen Flashman, Data Manager.
Royal United Hospitals Bath NHS Foundation Trust (Royal United Hospitals Bath)
-
Mr Michael Williamson, PI and Lead Surgeon.
-
Dr Andrea Phillips, Lead Radiologist.
-
Ms Joyce Katebe, Research Nurse.
-
Ms Dawne Chandler, Research Nurse.
Sandwell and West Birmingham Hospitals NHS Trust (Sandwell General Hospital)
-
Miss Kathryn Gill, PI and Lead Surgeon.
-
Dr Rosamund Donovan, Lead Radiologist.
-
Ms Julie Colley, Lead Research Nurse.
-
Ms Elzbieta Zulueta, Surgical Care Practitioner Colorectal/General Surgery.
South Tees Hospitals NHS Foundation Trust (The James Cook University Hospital)
-
Mr Douglas Atkin, PI and Lead Surgeon.
-
Dr Sumeet Miranda, Lead Radiologist.
Southend University Hospital NHS Foundation Trust (Southend Hospital)
-
Mr Bandipalyam Praveen, PI and Lead Surgeon.
-
Mr Manoj Jacob, Speciality Doctor in Colorectal Surgery.
-
Dr Saman Perera, Lead Radiologist.
-
Ms Sharon Tysoe, Lead Research Nurse.
Southport and Ormskirk Hospital NHS Trust (Southport and Formby District General Hospital)
-
Dr Dimitri Artioukh, PI and Lead Surgeon.
-
Dr Apam Chiphang, Lead Radiologist.
-
Ms Anna Morris, Research Nurse.
-
Ms Dawn Baker, Research Nurse.
St Helens and Knowsley Teaching Hospitals NHS Trust (Whiston Hospital)
-
Mr Raj Rajaganeshan, PI and Lead Surgeon.
-
Dr Kirsty Slaven, Lead Radiologist.
Taunton & Somerset NHS Foundation Trust (Musgrove Park Hospital)
-
Ms Louise Hunt, PI and Lead Surgeon.
-
Mr Paul Mackey, Consultant Surgeon.
-
Dr Paul Burn, Lead Radiologist.
-
Ms Jayne Foot, Lead Research Nurse.
Hillingdon Hospitals NHS Foundation Trust (Hillingdon Hospital)
-
Mr Yasser Mohsen, PI and Lead Surgeon.
-
Mr Alistair Myers, Consultant Surgeon.
-
Dr Ziad Meer, Lead Radiologist.
-
Miss Alex Diaz, Research Nurse.
-
Ms Mariam Nasseri, Research Nurse.
-
Ms Melinda Holden, Research Nurse.
Ipswich Hospital NHS Trust (Ipswich Hospital)
-
Mr James Pitt, PI and Lead Surgeon.
-
Dr Simon Smith, Lead Radiologist.
-
Ms Claire Swann, Lead Research Nurse.
Leeds Teaching Hospitals NHS Trust (St James’s University Hospital/Leeds General Hospital)
-
Professor David Jayne, PI and Lead Surgeon.
-
Dr Damian Tolan, Lead Radiologist.
-
Ms Catherine Moriarty, Lead Research Nurse.
The Mid Yorkshire Hospitals NHS Trust (Pinderfields General Hospital/Dewsbury and District Hospital)
-
Mr Chris Macklin, PI and Lead Surgeon.
-
Dr Katherine Naik, Lead Radiologist.
-
Ms Stephanie Lupton, Lead Research Nurse.
Royal Liverpool and Broadgreen University Hospitals NHS Trust (The Royal Liverpool University Hospital)
-
Mr Paul Rooney, PI and Lead Surgeon.
-
Dr Mark Hughes, Lead Radiologist.
-
Dr Priya Healey, Lead Radiologist.
University Hospitals Birmingham NHS Foundation Trust (Queen Elizabeth Hospital Birmingham)
-
Mr Nigel Suggett, PI and Lead Surgeon.
-
Professor Dion Morton, Consultant Surgeon.
-
Dr Deborah Tattersall, Lead Radiologist.
-
Dr Manijeh Ghodds, Data Contact.
University Hospitals Bristol NHS Foundation Trust (Bristol Royal Infirmary)
-
Mr Michael Thomas, PI and Lead Surgeon.
-
Dr Huw Roach, Lead Radiologist.
-
Dr Mark Callaway, Lead Radiologist.
-
Ms Rebecca Houlihan, Research Nurse.
-
Ms Karen Bobruk, Research Nurse.
-
Ms Catherine Phillpott, Research Nurse.
University Hospitals of Leicester NHS Trust (Leicester General Hospital)
-
Mr Baljit Singh, PI and Lead Surgeon.
-
Dr Ratan Verma, Lead Radiologist.
Wirral University Teaching Hospital NHS Foundation Trust (Arrowe Park Hospital)
-
Mr Liviu Titu, PI and Lead Surgeon.
-
Dr Nicholas Day, Lead Radiologist.
-
Ms Helyn Evans, Lead Research Nurse.
Yeovil District Hospital NHS Trust (Yeovil District Hospital)
-
Mr Nader Francis, PI and Lead Surgeon.
-
Dr Charles Hopkins, Lead Radiologist.
-
Mrs Joanna Alison, Research Nurse.
-
Ms Alison Lewis, Research Nurse.
Appendix 2 Site recruitment
| Site name | Date site opened | Date first participant randomised | Date last participant randomised | Number of participants randomised |
|---|---|---|---|---|
| Leeds Teaching Hospitals NHS Trust (St James’s University Hospital/Leeds General Infirmary) | 1 November 2010 | 4 July 2011 | 26 February 2016 | 28 |
| Dorset County Hospital NHS Foundation Trust (Dorset County Hospital) | 17 May 2011 | 24 May 2011 | 5 February 2015 | 9 |
| Royal Devon and Exeter NHS Foundation Trust (Royal Devon and Exeter Hospital) | 17 May 2011 | – | – | 0 |
| Sandwell and West Birmingham Hospitals NHS Trust (Sandwell General Hospital) | 31 May 2011 | 7 December 2011 | 11 July 2014 | 16 |
| Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital) | 17 June 2011 | 7 February 2012 | 18 February 2016 | 20 |
| Southport and Ormskirk Hospital NHS Trust (Southport and Formby District General Hospital) | 28 June 2011 | 11 January 2013 | 24 January 2014 | 3 |
| Ipswich Hospital NHS Trust (Ipswich Hospital) | 1 July 2011 | 21 October 2013 | 10 October 2014 | 2 |
| Nottingham University Hospitals NHS Trust (Queen’s Medical Centre) | 13 July 2011 | 27 September 2011 | 2 March 2016 | 20 |
| South Tees Hospitals NHS Foundation Trust (The James Cook University Hospital) | 21 July 2011 | 5 November 2012 | 23 December 2014 | 2 |
| North Devon Healthcare NHS Trust (North Devon District Hospital) | 25 July 2011 | – | – | 0 |
| Norfolk and Norwich University Hospitals NHS Foundation Trust (Norfolk and Norwich University Hospital) | 28 July 2011 | 16 May 2012 | 22 October 2015 | 6 |
| Croydon Health Services NHS Trust (Croydon University Hospital) | 23 August 2011 | 9 May 2012 | 21 March 2014 | 5 |
| University Hospitals of Leicester NHS Trust (Leicester General Hospital) | 24 August 2011 | 21 January 2013 | 22 August 2014 | 6 |
| University Hospitals Bristol NHS Foundation Trust (Bristol Royal Infirmary) | 6 October 2011 | 26 January 2012 | 29 January 2015 | 9 |
| Wirral University Teaching Hospital NHS Foundation Trust (Arrowe Park Hospital) | 14 October 2011 | 6 January 2012 | 19 June 2015 | 7 |
| Poole Hospital NHS Foundation Trust (Poole Hospital) | 24 October 2011 | 29 November 2011 | 29 November 2011 | 1 |
| Cardiff & Vale University Health Board (Llandough University Hospital/University Hospital of Wales) | 26 October 2011 | 10 April 2012 | 6 October 2015 | 13 |
| University Hospitals Birmingham NHS Foundation Trust (Queen Elizabeth Hospital Birmingham) | 1 November 2011 | 22 May 2012 | 14 January 2016 | 7 |
| County Durham and Darlington NHS Foundation Trust (University Hospital of North Durham) | 17 November 2011 | – | – | 0 |
| Gloucestershire Hospitals NHS Foundation Trust (Cheltenham General Hospital) | 2 March 2012 | 28 January 2013 | 8 April 2013 | 4 |
| Chesterfield Royal Hospital NHS Foundation Trust (Chesterfield Royal Hospital) | 2 March 2012 | 30 July 2012 | 1 December 2015 | 8 |
| Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary) | 2 March 2012 | 11 March 2013 | 29 January 2016 | 17 |
| Royal Liverpool and Broadgreen University Hospitals NHS Trust (The Royal Liverpool University Hospital) | 2 March 2012 | 18 July 2013 | 2 September 2015 | 2 |
| University Hospitals of Coventry and Warwickshire NHS Trust (University Hospital Coventry and Warwickshire) | 2 March 2012 | – | – | 0 |
| South Warwickshire NHS Foundation Trust (Warwick Hospital) | 2 March 2012 | – | – | 0 |
| NHS Grampian (Aberdeen Royal Infirmary) | 21 March 2012 | – | – | 0 |
| NHS Highland (Raigmore Hospital) | 22 March 2012 | 30 August 2012 | 24 February 2016 | 8 |
| The Mid Yorkshire Hospitals NHS Trust (Pinderfields General Hospital/Dewsbury and District Hospital) | 22 March 2012 | 4 December 2012 | 23 September 2015 | 5 |
| Calderdale and Huddersfield NHS Foundation Trust (Huddersfield Royal Infirmary) | 27 March 2012 | 4 May 2012 | 1 March 2013 | 3 |
| Aneurin Bevan University Health Board (Royal Gwent Hospital) | 17 April 2012 | – | – | 0 |
| Aneurin Bevan University Health Board (Nevill Hall Hospital) | 17 April 2012 | 3 February 2014 | 3 February 2014 | 1 |
| Southend University Hospital NHS Foundation Trust (Southend Hospital) | 18 May 2012 | 1 August 2012 | 18 February 2016 | 32 |
| Frimley Health NHS Foundation Trust (Wexham Park Hospital) | 15 August 2012 | – | – | 0 |
| Aintree University Hospital NHS Foundation Trust (Aintree University Hospital) | 1 October 2012 | – | – | 0 |
| Barking, Havering and Redbridge University Hospitals NHS Trust (Queen’s Hospital) | 3 October 2012 | 18 March 2013 | 10 March 2015 | 2 |
| Royal United Hospitals Bath NHS Foundation Trust (Royal United Hospitals Bath) | 22 January 2013 | 26 February 2014 | 29 February 2016 | 8 |
| Salford Royal NHS Foundation Trust (Salford Royal Hospital) | 13 March 2013 | – | – | 0 |
| Yeovil District Hospital NHS Trust (Yeovil District Hospital) | 28 March 2013 | 4 October 2013 | 11 February 2015 | 2 |
| Burton Hospitals NHS Foundation Trust (Queen’s Hospital Burton) | 3 June 2013 | 3 June 2013 | 3 August 2015 | 10 |
| Hillingdon Hospitals NHS Foundation Trust (Hillingdon Hospital) | 5 June 2013 | 8 August 2014 | 24 November 2015 | 2 |
| Heart of England NHS Foundation Trust (Birmingham Heartlands Hospital/Good Hope Hospital) | 30 August 2013 | 21 October 2013 | 30 June 2015 | 4 |
| Taunton & Somerset NHS Foundation Trust (Musgrove Park Hospital) | 22 October 2013 | 3 February 2014 | 11 December 2015 | 11 |
| Imperial College Healthcare NHS Trust (Charing Cross Hospital/St Mary’s Hospital) | 15 November 2013 | 12 February 2014 | 4 February 2015 | 4 |
| Oxford University Hospitals NHS Foundation Trust (Churchill Hospital/John Radcliffe Hospital) | 27 January 2014 | 12 June 2014 | 29 February 2016 | 8 |
| The Royal Wolverhampton NHS Trust (New Cross Hospital) | 10 February 2014 | 18 January 2016 | 18 January 2016 | 1 |
| St Helens and Knowsley Teaching Hospitals NHS Trust (Whiston Hospital) | 1 April 2014 | 19 May 2014 | 19 May 2014 | 1 |
| Homerton University Hospital NHS Foundation Trust (Homerton University Hospital) | 21 May 2014 | 15 September 2014 | 10 March 2016 | 6 |
| Mid Essex Hospital Services NHS Trust (Broomfield Hospital) | 12 June 2014 | 19 June 2014 | 7 August 2015 | 5 |
| Ashford and St Peter’s Hospitals NHS Foundation Trust (St Peter’s Hospital) | 12 June 2014 | 9 January 2015 | 5 October 2015 | 3 |
| George Eliot Hospital NHS Trust (George Eliot Hospital) | 1 December 2014 | 23 December 2014 | 13 April 2015 | 3 |
| North Bristol NHS Trust (Frenchay Hospital/Southmead Hospital) | 10 August 2015 | – | – | 0 |
| North Cumbria University Hospitals NHS Trust (Cumberland Infirmary) | 18 September 2015 | – | – | 0 |
| East Lancashire Hospitals NHS Trust (Royal Blackburn Hospital) | 18 September 2015 | – | – | 0 |
| Total | 53 (sites) | 40 (sites) | – | 304 |
Appendix 3 Data completeness
| Data form | Surgisis anal fistula plug (N = 152) | Surgeon’s preference (N = 152) | ||
|---|---|---|---|---|
| Expected, n | Received, n (%) | Expected, n | Received, n (%) | |
| Baseline data form | 152 | 151 (99) | 152 | 150 (99) |
| Radiology MRI form | ||||
| Baseline | 152 | 152 (100) | 152 | 152 (100) |
| Follow-up | 146 | 110 (75) | 146 | 112 (77) |
| Intraoperative form | 150 | 148 (99) | 149 | 147 (99) |
| Postoperative form | 148 | 147 (99) | 147 | 144 (98) |
| Follow-up | ||||
| 6 weeks | 149 | 142 (95) | 148 | 137 (93) |
| 6 months | 147 | 129 (88) | 147 | 129 (88) |
| 12 months | 146 | 124 (85) | 146 | 122 (84) |
| EQ-5D-3L | ||||
| Baseline | 152 | 139 (91) | 152 | 134 (88) |
| 6 weeks | 149 | 131 (88) | 148 | 127 (86) |
| 6 months | 147 | 126 (86) | 147 | 130 (88) |
| 12 months | 146 | 127 (87) | 146 | 127 (87) |
| FIQoL | ||||
| Baseline | 152 | 139 (91) | 152 | 134 (88) |
| 6 weeks | 149 | 128 (86) | 148 | 127 (86) |
| 6 months | 147 | 125 (85) | 147 | 129 (88) |
| 12 months | 146 | 126 (86) | 146 | 128 (88) |
Appendix 4 Per-protocol analysis of the Faecal Incontinence Quality of Life questionnaire by treatment group
The per-protocol analysis failed to show any statistical difference in FIQoL by treatment group at any of the follow-up time points and, therefore, did not change the interpretation of the main ITT analysis.
| FIQoL domain | Surgisis anal fistula plug, mean (SD, n) | Surgeon’s preference, mean (SD, n) | Mean difference (95% CI) | p-value | Treatment by time, p-value |
|---|---|---|---|---|---|
| FIQoL: lifestyle | |||||
| Baseline | 3.46 (0.75, 130) | 3.35 (0.82, 136) | 0.09 (–0.04 to 0.21) | 0.17 | 0.54 |
| 6 weeks | 3.54 (0.71, 123) | 3.40 (0.83, 129) | |||
| 6 months | 3.59 (0.72, 118) | 3.48 (0.78, 132) | |||
| 12 months | 3.63 (0.67, 120) | 3.52 (0.78, 132) | |||
| FIQoL: coping/behaviour | |||||
| Baseline | 3.33 (0.73, 130) | 3.14 (0.87, 136) | 0.15 (0.01 to 0.28) | 0.03 | 0.12 |
| 6 weeks | 3.45 (0.72, 123) | 3.16 (0.89, 129) | |||
| 6 months | 3.46 (0.78, 118) | 3.29 (0.91, 132) | |||
| 12 months | 3.45 (0.82, 120) | 3.31 (0.86, 131) | |||
| FIQoL: depression/self-perception | |||||
| Baseline | 3.05 (0.78, 124) | 2.99 (0.79, 125) | 0.13 (–0.02 to 0.28) | 0.08 | 0.46 |
| 6 weeks | 3.18 (0.75, 111) | 3.01 (0.85, 121) | |||
| 6 months | 3.25 (0.76, 109) | 3.15 (0.91, 120) | |||
| 12 months | 3.31 (0.82, 110) | 3.19 (0.87, 122) | |||
| FIQoL: embarrassment | |||||
| Baseline | 3.26 (0.81, 124) | 3.09 (0.86, 125) | 0.17 (–0.001 to 0.34) | 0.051 | 0.02 |
| 6 weeks | 3.39 (0.81, 111) | 3.08 (0.92, 120) | |||
| 6 months | 3.37 (0.85, 109) | 3.27 (0.89, 121) | |||
| 12 months | 3.40 (0.87, 111) | 3.22 (0.95, 122) | |||
List of abbreviations
- ACPGBI
- Association of Coloproctology of Great Britain and Ireland
- ASA
- American Society of Anesthesiologists
- BCTU
- Birmingham Clinical Trials Unit
- BNF
- British National Formulary
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EUA
- examination under anaesthesia
- FIAT
- Fistula-In-Ano Trial
- FIQoL
- Faecal Incontinence Quality of Life
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LIFT
- ligation of intersphincteric fistula tract
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PI
- principal investigator
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- WTP
- willingness to pay
