Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/106/02. The contractual start date was in March 2011. The draft report began editorial review in May 2016 and was accepted for publication in September 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ajit Lalvani is the named inventor for several patents underpinning T-cell-based diagnosis including interferon gamma, enzyme-linked immunospot assay, ESAT-6, CFP-10, Rv3615c, Rv3873 and Rv3879c. He has royalty entitlements from the University of Oxford spin-out company (Oxford Immunotec plc), in which he has held a minority share of equity and he is a member of the Efficacy and Mechanism Evaluation Board. Jonathan J Deeks is a member of the Health Technology Assessment (HTA) Commissioning Board and the HTA Efficient Study Designs Board. Onn Min Kon is chairperson of the UK Joint Tuberculosis Committee. Peter White has received research funding from Otsuka SA for a retrospective study of multidrug-resistant tuberculosis treatment in several eastern European countries outside the submitted work. He received grants from the Medical Research Council during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Takwoingi et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
In 2014, globally, an estimated 9.6 million cases of tuberculosis (TB) and 1.5 million deaths caused by the disease were reported to the World Health Organization. 1 Co-infection with TB and human immunodeficiency virus (HIV) accounts for a significant proportion of cases globally (12%) and together these infections are the biggest infectious causes of death. Health inequalities are exacerbated by the burden that TB places on the most vulnerable and poor around the world. Over the past 20 years, worldwide TB incidence and mortality have declined. However, despite this, the prevalence of TB still remains unacceptably high. Furthermore, there is not yet evidence of a reduction in the number of cases in England, particularly in major cities such as London and Birmingham.
In England, 6520 cases of TB were reported in 2014, with an incidence rate of 12.0 per 100,000. 2 Of these, 2572 cases were in London, where the incidence rate was 30.1 per 100,000. 2 Other urban high-incidence areas include Leicester, Birmingham, Luton, Manchester and Coventry. The majority of cases in England (72%) occurred in new entrants who were born outside the UK. 2
Tuberculosis is caused by active infection with Mycobacterium tuberculosis (Mtb). Upon initial infection with Mtb, a state of asymptomatic dormancy typically occurs. In most infected individuals this results in a prolonged and perhaps lifelong latent TB infection (LTBI). In others, however, a breakdown of immune control and activation of infection results in symptomatic disease, which can be fatal without treatment. Co-infection with HIV dramatically increases the likelihood of progression from latent to active disease.
Active TB can manifest with pulmonary or extrapulmonary phenotypes. In its active pulmonary form, TB is highly contagious. The diagnosis of active TB is central to preventing the spread of disease and thus controlling the TB epidemic. 3 However, the slow speed and poor sensitivity of existing diagnostic tools often lead to delays diagnosis and treatment of the disease. 4
Diagnosis of tuberculosis
Conventional methods of diagnosing active TB rely primarily on the identification of Mtb bacilli, as well as imaging affected areas. Smear microscopy is quick and inexpensive, but typically lacks sensitivity. Although cell culture is considered the ‘gold standard’ for diagnosis of active TB because of its higher sensitivity, it is often slow, taking up to 6 or 8 weeks to give a result. Polymerase chain reaction (PCR)-based tests for Mtb, such as the new GeneXpert Mtb/RIF® (Sunnyvale, Cepheid, CA, USA), can be quick and sensitive, but they are typically expensive and require a high standard of infrastructure. Imaging techniques such as chest radiography and computed tomography (CT) are quick and usually sensitive, but are not specific. Furthermore, they can be expensive, and, again, require a high standard of infrastructure (for example in the case of CT). All of these tests tend to be less accurate in cases of active TB with HIV co-infection, and also in cases of extrapulmonary TB.
Currently available tests for LTBI include the tuberculin skin test (TST) and interferon gamma release assays (IGRAs). The TST measures the in vivo delayed-type hypersensitivity response to intradermal inoculation of a crude mixture of mycobacterial antigens. Because this mixture contains antigens also present in bacillus Calmette–Guérin (BCG), the test can be confounded by prior BCG vaccination. IGRAs, on the other hand, detect ex vivo interferon gamma (IFN-γ) release from T cells (lymphocytes that play a key role in cell-mediated immunity) in response to Mtb-specific antigens, early secretory antigenic 6 kDa (ESAT-6) and culture filtrate protein 10 (CFP-10). These antigens are absent from the BCG vaccine and most environmental mycobacteria, and thus IGRAs tend to be more specific than the TST. 5 The two types of commercially available IGRAs are QuantiFERON GOLD In-Tube (QFT-GIT; Cellestis, Carnegie, VIC, Australia), a whole-blood enzyme-linked immunosorbent assay (ELISA), and T-SPOT. TB® (Oxford Immunotec, Abingdon, UK), an enzyme-linked immunospot assay (ELISpot); both IGRAs utilise peripheral blood mononuclear cells (PBMCs). These tests have been recommended by the UK, European and North American guidelines for diagnosis of LTBI. 6
Although typically used in the diagnosis of LTBI, TSTs and IGRAs actually detect Mtb infection in its entirety (i.e. active or latent). The role of the tests within published guidelines for diagnostic evaluation of suspected active TB to date has been limited because of their low specificity for the disease: they could never confirm a diagnosis of active TB because they cannot differentiate latent and active infection. However, because Mtb infection is a pre-requisite for TB disease, reliable determination of infection status could accelerate diagnostic assessment by enabling rapid exclusion of TB (within 24 hours) when the result is negative.
In order for the IGRA or TST to reliably rule out a diagnosis of Mtb infection and thus TB disease, the sensitivity of the test must be very high (> 95%). The sensitivity and specificity of IGRAs compared with the TST in active TB have been examined in a number of studies, varying in size and quality. 7 IGRAs are typically more specific than the TST for diagnosing Mtb infection and T-SPOT. TB is more sensitive than the TST for diagnosing TB. However, the diagnostic accuracy of T-SPOT. TB and QFT-GIT has not been compared directly head to head in suspected active TB in the UK, nor comprehensively assessed in immunosuppressed patients. Therefore, there is uncertainty in the role and clinical utility of IGRAs in the diagnostic workup of suspected TB, as well as their cost-effectiveness in UK NHS practice.
Aim and study objectives
Aim
To evaluate and compare the diagnostic accuracy and cost-effectiveness of IGRAs with conventional testing for diagnosis of active TB. Specifically, the study aimed to determine the sensitivity, specificity, positive predictive values (PPVs) and negative predictive values (NPVs), and likelihood ratios of T-SPOT. TB and QFT-GIT for diagnosis of active TB in routine NHS clinical practice. Second-generation IGRAs were also evaluated.
Study objectives
Primary objectives
-
To compare the diagnostic accuracy of T-SPOT. TB and QFT-GIT for the diagnosis of active pulmonary and extrapulmonary TB in routine clinical practice.
-
To develop an evidence-based optimal testing algorithm that defines the role of IGRAs in the diagnostic workup of suspected active TB.
-
To deliver the objectives above for a key subgroup: HIV co-infected patients (the highest-risk subgroup of TB).
-
To quantify and compare the cost-effectiveness of a range of possible testing strategies against the present testing regime.
Secondary objectives
-
To quantify the sensitivity, specificity, and positive and NPVs of T-SPOT. TB and QFT-GIT in a number of key patient subgroups, such as patients with pre-existing diabetes mellitus, end-stage renal failure and iatrogenic immunosuppression.
-
To quantify the use of second-generation IGRAs compared with existing commercially available assays.
Chapter 2 Methods
This chapter describes the study design and methods for the evaluation of the diagnostic accuracy of IGRAs in active TB. Our report adheres to the Standards for the Reporting of Diagnostic Accuracy Studies (STARD) guideline,8 as shown in Appendix 1. Methods for the health economic evaluation are described separately in Chapter 6.
Overview of the study design
This prospective multicentre study comparing the accuracy of IGRAs was conducted in routine clinical practice in the UK. Adults presenting with suspected active TB at NHS outpatient or inpatient services to participating hospitals in London, Slough, Oxford, Leicester and Birmingham were recruited. We used a within-patient design to compare test accuracy by performing all IGRAs on blood samples from each patient with the presence or absence of active TB verified using the reference standard. This design minimises between-patient variability while also allowing estimation of the accuracy of combinations of IGRAs. Blood samples for IGRA testing were collected from patients at baseline and follow-up (2 and 6 months). If necessary, and when available, TST results were used as part of the composite reference standard for verifying the final diagnosis of patients. The TST results were obtained from routine clinical care and so the availability of TST results reflects local practice in participating hospitals.
Participants
Inclusion criteria
Adults (aged ≥ 16 years) presenting with suspected (pulmonary or extrapulmonary) active TB to NHS outpatient or inpatient services were included. To replicate clinical practice, patients with a previous diagnosis of TB and/or history of TB treatment were recruited. However, they were excluded from the analyses on the basis that, when evaluating patients with suspected active TB, the clinician should not perform an IGRA because any immunological biomarker would remain positive and thus affect test accuracy. The study population was expected to be representative of the national TB burden in terms of ethnic mix and range of comorbidities. A key subgroup was HIV-positive patients.
Exclusion criteria
Participants aged < 16 years or those unable to give informed consent were excluded.
Setting
Patients were recruited at the point of diagnostic workup from 14 hospitals in 10 NHS trusts in the UK.
Recruitment process
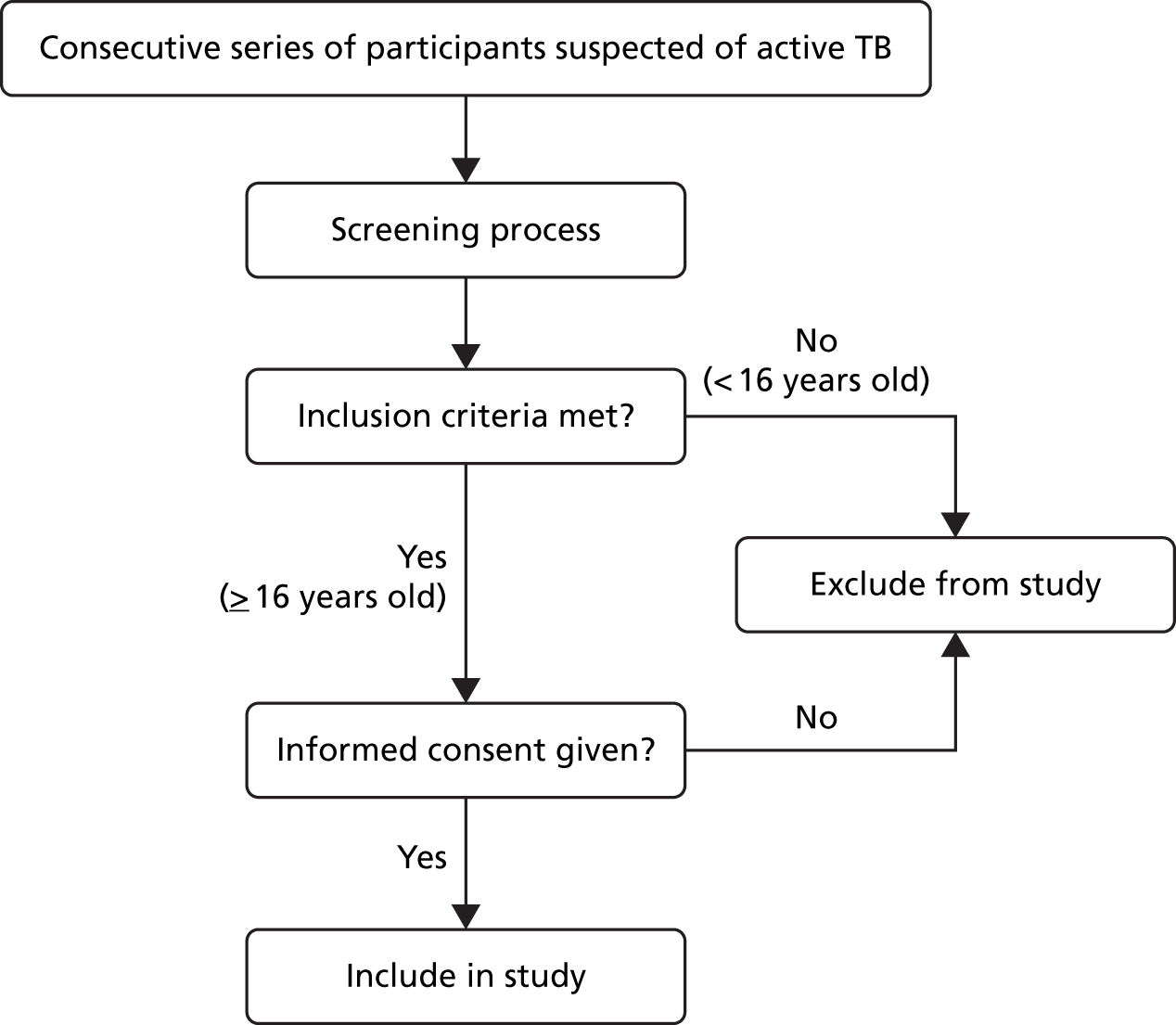
An overview of the recruitment process is shown in Figure 1. Potential participants presenting to participating NHS centres were referred to a TB research nurse by the attending clinician. The nurse then screened participants to ensure that they were eligible for the study according to the inclusion/exclusion criteria stated above. Each potential participant was provided with an information sheet and a verbal description of the study. Participants were included in the study if they were willing and informed consent was obtained.
FIGURE 1.
Overview of recruitment process.
Data collection and management
Follow-up
Participants were seen by research nurses for follow-up visits at 2 and 6 months after recruitment. Follow-up visits were scheduled to be carried out at the same time as the patient’s routine clinic appointments. At these visits blood was collected for IGRA testing and data on patient diagnosis were obtained. If patients were no longer being seen as part of their routine NHS care, they were not required to attend study follow-up visits. In such cases, information about a patient’s diagnosis was obtained from their medical notes. In addition to following up patients at 2 and 6 months, a review of patient records was performed up to 1 year post recruitment, if required, in order to obtain a final diagnosis.
Data management
A case report form (CRF) was used to collect patient data at each recruiting hospital after a patient consented to participate in the IGRAs for Diagnostic Evaluation of Active tuberculosis (IDEA) study. Following receipt of the CRFs, data were entered into an electronic password-protected database. The results of study IGRAs and all other study laboratory tests were entered into a separate secure database. This laboratory database was accessible only to specific laboratory staff. Thus, the Study Management Group (SMG) responsible for day-to-day management of the IDEA study was blinded to the IGRA results. Furthermore, NHS clinicians responsible for routine care and diagnosis of patients involved in the study were also blinded to study IGRA results. To enable preparation of study reports for meetings of the independent oversight committee [Study Steering Committee (SSC)], the study statistician was granted access to the clinical database and excerpts of the laboratory database at specific periods during the study.
Sample collection
Blood samples
Blood sampling was done at three time points: at baseline and at follow-up at 2 and 6 months. Only blood samples collected at baseline were used in the assessment of the index tests and for the reference standard. Samples taken at follow-up were used for further IGRA testing (but not analysed as part of the IDEA study) and also stored for future research as consented by patients. Blood-taking (venepuncture) procedures were carried out in accordance with local trust venepuncture guidelines. The baseline sample was obtained no later than 14 days after the start of treatment for TB or within 7 days of consent, whichever was earlier, depending on the patient’s diagnosis. For patients with a diagnosis of active TB, sarcoidosis or other non-TB diagnosis, follow-up study blood samples were taken 2 and 6 months after the start of treatment. For patients with a diagnosis of latent TB, when possible and if the patient returned to the same clinical team, blood samples were taken 3 and 6 months after the initiation of treatment (if treatment was indicated and given).
A 35-ml blood sample was taken at each time point. Blood was collected into heparinised collection tubes and QFT-GIT collection tubes for T-SPOT. TB and QFT-GIT assays, respectively. Furthermore, heparinised blood was collected for performance of second-generation IGRAs and to store plasma and PBMCs for future research. In addition, blood was collected into uncoated collection tubes in order to store serum for future research.
Blood samples were transported on the same day as sample collection either by a member of the research team or by courier in the appropriate United Nations-type approved packaging to the TB Research Centre for testing. All samples were processed within 6 hours of blood collection for London-based sites and within 8 hours for outer London sites. Excess PBMCs were stored in a liquid nitrogen tank, and serum and plasma were stored in a –80 °C freezer at the TB Research Centre (led by Professor Ajit Lalvani) at Imperial College London (St. Mary’s Hospital).
Diagnostic bronchoscopy samples
In patients with sputum smear-negative pulmonary TB, diagnostic bronchoscopies were performed and bronchoalveolar lavage (BAL) was obtained as part of routine clinical care. When surplus BAL samples were available and not required for diagnostic procedures, aliquots were cryopreserved and stored in the research biorepository for subsequent testing by IGRA. The bronchoscopic procedure, along with collection of surplus BAL samples, were applicable only for patients recruited at St. Mary’s Hospital, Imperial College Healthcare NHS Trust, in accordance with set clinical practice guidelines. The BAL sample consent was covered under the consent form as ‘tissue samples’. However, patients were informed if a surplus BAL sample was kept for the IDEA study.
Index tests
Interferon gamma release assays
Two types of commercially available IGRAs, QFT-GIT and T-SPOT. TB, were evaluated. In addition, a new ELISpot-based assay utilising novel antigens (Rv3615c, Rv2654, Rv3879c and Rv3873) was evaluated. The performance of each antigen was evaluated individually and in combinations that included either ESAT-6 and CFP-10, the two antigens that constitute T-SPOT. TB, or both. IGRA testing is not standard practice for HIV-negative patients suspected of having TB in the hospitals of our consortium and is not currently recommended for HIV-positive patients suspected of having TB. However, if IGRAs were used locally at participating hospitals as part of the routine diagnostic workup of patients, we recorded the tests done but we did not analyse the test results as study results for the IDEA study. Thus, only from IGRAs performed in our research laboratory specifically for this study were recorded and assessed. Laboratory staff performing the IGRAs and recording the test results were blinded to clinical information and reference standard results.
Analysis of T-SPOT.TB and novel antigens
Peripheral blood mononuclear cells were isolated from heparinised whole blood using the Ficoll-Paque™ density centrifugation method (GE Healthcare Bio-Science, Uppsala, Sweden), as described by Whitworth et al. 6 In brief, whole blood was diluted in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, Dorset, UK) and layered onto Ficoll-Paque™ Plus at a ratio of 2 : 1 in 50-ml Falcon® centrifuge tubes (Corning Science Mexico S.A. de C.V., Reynosa, USA). The tubes were centrifuged for 20 minutes at 18–25 °C and the cloudy PBMC layer was aspirated into fresh RPMI 1640 medium. Cells were washed with fresh RPMI 1640 medium and counted using trypan blue stain for use with the Countess® Automated Cell counter (Life Technologies, Eugene, OR, USA). T-SPOT. TB was applied to the freshly isolated PBMCs as per the manufacturer’s instructions9 and as described by Whitworth et al. 6
Cells were resuspended in AIM-V® Serum-Free Medium (Gibco by Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) at a concentration of 2.5 million cells/ml and 250,000 cells per well were incubated overnight (18 hours) at 37 °C with Mtb-specific antigens (ESAT-6, CFP-10, Rv3615c, Rv2654, Rv3879c and Rv3873) individually, and positive (phytohaemagglutinin) and negative (RPMI 1640 medium) controls in a 96-well plate, pre-coated with IFN-γ-specific monoclonal capture antibodies (included in T-SPOT. TB kit). Thus, a total of eight wells were used per patient and samples from 12 patients were included on a plate. Overnight incubation of the cells with antigens allows for IFN-γ secretion from activated Mtb-specific effector T cells present in the culture. Secreted IFN-γ binded to the pre-coated IFN-γ-specific monoclonal capture antibodies on the membrane of each well. After incubation, wells were washed with phosphate-buffered saline and an alkaline phosphatase-conjugated secondary IFN-γ-specific monoclonal antibody was added to bind to any captured IFN-γ. For a visible representation of the spots on the membrane, an alkaline phosphatase chromogen substrate was added.
Each spot formed on the membrane signifies IFN-γ release by a single activated Mtb antigen-specific T cell. Spot-forming cells (SFCs) are expected to be detected in positive control wells and absent in negative control wells. SFCs in TB antigen-stimulated wells indicate infection.
Spot-forming cells were counted using an automated ELISpot plate reader (AID ELISpot read system ELRIFL04; Advanced Imaging Devices GmbH, Strasbourg, Germany), with saturation level set at 60%. For individual antigens, test results were classified as negative, positive, borderline (equivocal) or indeterminate (invalid) by subtracting the spot count in the negative control well from the spot count in each panel, according to the algorithm illustrated in Figure 2 (based on the package insert for T-SPOT. TB). 9 Panels A, B, C, D, E and F correspond to ESAT-6, CFP-10, Rv3615c, Rv2654c, Rv3879c and Rv3873, respectively.
FIGURE 2.
Algorithm for defining test positivity of antigens. Panel X indicates one of the six panels, A to F, which correspond to the following antigens: A, ESAT-6; B, CFP-10; C, Rv3615c; D, Rv2654; E, Rv3879c; and F, Rv3873. TNTC, too numerous to count.

For T-SPOT. TB as well as other antigen combinations, if the positive control spot count was < 20, the result was deemed indeterminate, unless the response to one of the Mtb antigens was positive (or borderline), in which case the test result for the combination was deemed positive (or borderline). Thus, we applied an ‘OR’ rule (at least one antigen spot count deemed positive) for antigen combinations. For example, for T-SPOT. TB, a result was positive if the negative-control spot count was ≤ 10 and either ‘panel A minus negative control’ or ‘panel B minus negative control’ was ≥ 8 spots. This implies the T-SPOT. TB result was negative if both ‘panel A minus negative control’ and ‘panel B minus negative control’ were negative (≤ 4 spots). In the IDEA study, borderline test results (5–7 spots) were considered as positives.
Analysis of QuantiFERON GOLD In-Tube
The QFT-GIT assay was performed in two stages as per the manufacturer’s instructions10 and as described in Whitworth et al. 6 First, whole blood was collected from each participant into three QFT-GIT tubes containing a negative control, mitogen-positive control and Mtb antigens [ESAT-6, CFP-10 and TB7.7 (also known as Rv2654c, a possible PhiRv2 prophage protein) combined], as provided by the manufacturer. 10 The tubes were incubated at 37 °C for 16–24 hours to allow IFN-γ secretion from antigen-specific effector T cells into the extracellular fluid (plasma). After incubation, tubes were centrifuged and 150 µl of plasma was collected and stored in a 96-well plate for up to 4 weeks at 2–8 °C prior to performing the remainder of the assay.
To perform the ELISA step, 50 µl of plasma from each of the QFT-GIT tubes (i.e. containing a mitogen control, negative control and Mtb antigens) was transferred to wells of another 96-well plate pre-coated with IFN-γ-specific monoclonal capture antibodies and incubated with a conjugate [an IFN-γ-specific antibody conjugated to horseradish peroxidase (included in T-SPOT. TB kit)] for 2 hours at room temperature (22 °C). Plasma samples in each well were mixed thoroughly using a microplate shaker (PMS-1000i Microplate Shaker; Grant Instruments Ltd, Shepreth, UK) for 1 minute to ensure that any IFN-γ was evenly distributed throughout the sample. Secreted IFN-γ in the plasma will be sandwiched between the two antibodies.
After incubation and thorough washing with detergent, a photosensitive chromogen substrate solution (3,3’,5,5’-tetramethylbenzidine; included in QFT-GIT kit) was added, which converted the sample to a detectable form (blue colour signal). The reaction was stopped with a substrate stopping solution (sulphuric acid; included in QFT-GIT kit). The intensity of the colour is directly proportional to the levels of IFN-γ present in the plasma after activation of TB-specific T cells by Mtb antigens. Colour should develop in the positive control well and not in the negative control well. Colour in the TB antigen well indicates infection.
The optical density of each well was measured using a microplate reader (Elx800 Absorbance Reader; BioTek, Carnegie, VIC, Australia) with a 450-nm filter and a 620- to 650-nm reference filter. The concentration (IU/ml) of IFN-γ for the plasma sample from each of the three tubes (negative, mitogen and Mtb antigen) was determined against a series of standard concentrations (the standard curve). The test result (negative, positive or indeterminate) was calculated from the concentration values using a US Food and Drug Administration-approved algorithm (Figure 3) run on QuantiFERON-TB Gold In-Tube Analysis Software version 2.62 (Cellestis) in accordance with the manufacturer’s instructions. 10
FIGURE 3.
Determination of the test results for QFT-GIT. Reprinted from Methods, Vol. 61, Whitworth HS, Scott M, Connell DW, Dongés B, Lalvani A. IGRAs – the gateway to T cell based TB diagnosis, pp. 52–62. © 2013, with permission from Elsevier. 6

Tuberculin skin test
A TST was performed as part of routine clinical care. Each recruiting centre has its own policy for TST use based on National Institute for Health and Care Excellence (NICE) guidance. 11 Patients eligible for the TST, as defined by local or NICE guidance, received a single intradermal injection of two tuberculin units or 0.1 ml of unlicensed tuberculin Mantoux test [Tuberculin PPD RT23 SSI (Statens Serum Institut, Copenhagen, Denmark); this purified protein derivative (PPD) was used for the main site; however, other sites may have used other PPDs]. The test was administered by trained specialist TB nurses who were entitled to administer medicines within the trust under a Patient Group Directive. The degree of skin induration was measured 48–96 hours later. Test results were obtained from centres and we determined test positivity using three thresholds: ≥ 5 mm, ≥ 10 mm3,12 and a stratified threshold based on BCG vaccination status (≥ 6 mm for unvaccinated and ≥ 15 mm for vaccinated participants). 11 Patients were considered BCG vaccinated if they reported they had been vaccinated and/or had a BCG scar.
Reference standard
Participating hospitals followed the minimum set of tests defined within the NICE guideline11 for diagnosing active TB and in accordance with local routine practice. However, the final diagnosis of participants was verified using the composite reference standard defined in Appendix 2. The reference standard was applied by a panel of clinicians blinded to local (routine) and study IGRA results. The role of the clinical panel was to assess anonymised patient clinical data without knowledge of the IGRA results in order to confirm the diagnostic category of all study participants. The composition of the panel and the assessment process is described in Composition of the clinical panel and Assessment of final diagnosis.
Composition of the clinical panel
Clinical panel members were appointed from among the principal investigators (PIs) and co-investigators by the chief investigators. The panel included:
-
an independent chairperson
-
a chest physician
-
a HIV physician with knowledge of HIV/TB infection
-
an infectious disease physician.
All physicians had extensive TB expertise.
Structure of case panel meetings
Meetings were arranged and minutes were recorded by the study co-ordinator or a designated member of the study team. The panel meetings were held quarterly and the schedule was decided by the SMG depending on the number of data available for review. At each meeting, priority was given to assessment of indeterminate (category 3) cases. Additional meetings were arranged to ensure that all patients in categories 2 and 3, and some in category 4, were reviewed (see Assessment of final diagnosis). All panel members had to attend the meeting in person.
Assessment of final diagnosis
Diagnosis data (based on the Dosanjh categorisation3 outlined in Appendix 2) received from recruiting centres were reviewed as follows:
-
Category 1: all culture-confirmed cases were not reviewed, but were signed off at the end of the clinical panel meeting by the chief or co-investigators.
-
Category 2: all probable cases were reviewed.
-
Category 3: all indeterminate cases were reviewed.
-
Category 4: non-active TB cases with a confirmed alternative diagnosis were not reviewed by the panel. Complicated category 4 patients were reviewed by the panel.
Reviewing cases in this way ensured consistent final diagnosis categorisation.
Data available to the panel
For each patient who was reviewed, the following information was presented to the panel:
-
patient demographics
-
TB symptoms, previous TB information, TB exposure history, current medication, patient medical history, follow-up data, HIV infection status and relevant clinical information and travel data
-
relevant clinical correspondence and test results during diagnosis and follow-up (excluding results of routine and study IGRAs) such as culture, smear, PCR, TST, bronchoscopy, biopsy and/or radiological reports.
Documentation
Each panel member reviewed a patient’s documents and completed a form with the following information:
-
diagnosis category (based on the Dosanjh criteria)
-
body site of disease (only if final diagnosis was active TB)
-
method of diagnosis: included culture, PCR, imaging, smear microscopy, histology, clinical features, response to treatment, multiple and other. For multiple and other, details were to be specified.
Confirmation of diagnosis
Final diagnosis decisions were made by a majority vote, with the chairperson having a casting vote, if necessary. Final diagnoses were recorded by the study co-ordinator. If necessary, when a panel member had treated a patient being reviewed, the member was asked to provide information (without disclosing local or study IGRA results) and the member was excluded from final decision-making (their vote was replaced with a vote from the chairperson) for the patient.
Outcomes
Sensitivity, specificity, PPVs and NPVs and likelihood ratios for each test and combinations of tests were calculated to determine their diagnostic accuracy and clinical utility. The likely primary clinical utility of immune-based testing is to exclude TB. Thus, when interpreting the analyses and drawing conclusions, the focus was primarily on the sensitivities and NPVs. For test comparisons, relative test performance was assessed by comparing the sensitivity and specificity of one test with those of another test. These results were presented as relative sensitivities and relative specificities.
Statistical analyses
Sample size calculation
As stated in Outcomes, the primary clinical utility of IGRA results in the assessment of suspected active TB is likely to be in their NPV, which may enable clinicians to reliably rule out TB from the differential diagnoses. This, in turn, depends on the sensitivity of the test and the prevalence of active TB in the tested population. In a meta-analysis, the average sensitivity of T-SPOT. TB and QFT-GIT was 90% [95% confidence interval (CI) 86% to 93%] and 70% (95% CI 63% to 78%), respectively. 7 However, the estimates were mainly based on small studies and most studies included only patients without HIV infection. Furthermore, the estimates were not based on head-to-head comparative accuracy studies. Two large studies (n = 194 active TB cases diagnosed from n = 389 TB suspects;3 n = 216 active TB cases diagnosed from n = 413 TB suspects13) gave more robust estimates for T-SPOT. TB of 85.1% (95% CI 79.2% to 89.9%) and 85.2% (95% CI 76.1% to 91.9%), respectively. The latter study compared T-SPOT. TB and QFT-GIT, and gave an estimate of 78.1% (95% CI 70.7% to 84.3%) for QFT-GIT. 13
Given the available evidence, we powered the IDEA study to detect a conservatively estimated 10% difference in sensitivity between T-SPOT. TB and QFT-GIT, assuming a sensitivity of 85% for T-SPOT. TB and of 75% for QFT-GIT. To detect this difference at the 5% significance level (two-tailed) with 90% power, 855 patients were required (each receiving both tests), assuming a 40% prevalence of active TB in the study population. This calculation was done using a method that accounts for the paired nature of the data (based on McNemar’s test). 14 The method requires knowledge of the probability of positive T-SPOT. TB and positive QFT-GIT results among cases of active TB (concordance probability). A positive correlation, such as may be expected between both blood tests, would give a lower sample size than assuming independence or a negative correlation of test errors. However, as no pilot data were available to inform the choice of the concordance probability, we chose to be conservative and so assumed independence. To allow for missing data, indeterminate index test and reference standard results, withdrawal of consent and possible logistical errors, we aimed to recruit 1012 participants.
According to published evidence, the sensitivity of QFT-GIT decreases in HIV-positive subgroups, whereas that of T-SPOT. TB is unaffected in some studies and decreased in others. 15 Therefore, we computed sample size for the HIV-positive subgroup based on sensitivities of 85% and 65% for T-SPOT. TB and QFT-GIT, respectively. Assuming a 50% prevalence of active TB among these participants, we thus required 156 patients to detect a 20% difference between the IGRAs at the 5% significance level with 80% power. We aimed to recruit 200 patients for similar reasons to those outlined above.
Revision of sample size calculation and study extension for recruitment of HIV-positive participants
During the study recruitment period, the proportion of HIV-positive patients with a final diagnosis of active TB was found to be substantially lower (20% rather than 50%) than originally anticipated when the study was designed. This was attributed to a decrease in TB incidence in this population in recent years. 16 In order to answer a key objective regarding the utility of IGRAs in HIV-positive patients, the SSC and funder supported an extension of recruitment of HIV-positive participants to ensure that the study was adequately powered.
The sample size calculation was revised to take into account the reduced prevalence of active TB in this population. Given a prevalence of 20%, 390 HIV-positive participants will be required to detect a 20% difference between the sensitivity of T-SPOT. TB and QFT-GIT with 80% power at the 5% significance level. Thus, the sample size was increased from 156 to 390. Ethics approval was sought and an extension of 12 months was granted on 5 November 2014 to recruit and follow up only HIV-positive participants. Given the purposive recruitment of additional HIV-positive participants, the results are presented separately for the main cohort (including HIV-positive participants recruited during the first phase of the IDEA study prior to the extension period) in Chapters 3 and 4, and for the entire HIV-positive cohort in Chapter 5.
Data analysis
Sensitivity, specificity, PPV, NPV and likelihood ratios for each test and combination of tests were calculated to determine their diagnostic accuracy and clinical utility in the main cohort and within the key subgroups outlined in the study objectives. For all proportions, 95% CIs were calculated using the Wilson method. 17,18 CIs for positive and negative likelihood ratios were calculated using the method by Simel et al. 19 Separate analyses of the complete cohort of HIV-positive participants were also performed.
Patients classified as having culture-confirmed (category 1) or highly probable (category 2) active TB and those without active TB (category 4) were included in analyses of diagnostic accuracy (see definition of categories in Appendix 2). However, despite not being included in the analysis, the proportion of patients classified as clinically indeterminate (category 3) was reported. While borderline T-SPOT. TB results were included as test positives in primary analyses of the main study cohort, we examined the impact of this by excluding borderline test results in sensitivity analyses. For the primary analyses, patients with indeterminate IGRA results were excluded from the analyses. In clinical practice, if an IGRA was used as a rule-out test for active TB, then an indeterminate result would have the same implications as a positive result, that is, it could not rule out a diagnosis of the disease (and thus TB would remain a differential diagnosis). The impact of including indeterminate IGRA results as test positives (i.e. true positives and false positives depending on final diagnosis of active TB or no active TB) was assessed in sensitivity analyses. Sensitivity analyses were also conducted to investigate the impact of excluding category 2 patients on the sensitivity of IGRAs.
Comparisons between different IGRAs were performed using generalised estimating equation (GEE) models to exploit the paired nature of the data. Our analysis was based on all available data (i.e. it included patients who did not have a complete set of index test results). Separate GEE models were fitted for those with active TB (Dosanjh categories 1 and 2) and those with no active TB (category 4) to determine differences in sensitivity and specificity, respectively. The outcome variable in the GEE model was IGRA result (positive vs. negative) and the explanatory variable was type of IGRA, for example T-SPOT. TB versus QFT-GIT. The natural outputs from these models are odds ratios. For example, comparing the sensitivity of T-SPOT. TB to that of QFT-GIT, the odds ratio is the odds of a positive T-SPOT. TB result compared with the odds of a positive QFT-GIT result in patients with active TB. For the comparison of specificities, the odds ratio is the odds of a negative T-SPOT. TB result compared with the odds of a negative QFT-GIT result in non-TB patients. As odds ratios do not have an intuitive interpretation, we computed ratios of sensitivities (relative sensitivity) and ratios of specificities (relative specificity) using a function (nlcom) that computes point estimates and CIs for non-linear combinations of parameter estimates post estimation of the models. The CIs are computed using the delta method.
Variation in the relative performance of T-SPOT. TB and QFT-GIT with HIV infection status and other clinical characteristics was investigated by including one covariate at a time in the GEE models. 20 To assess the effect of a covariate on relative test performance, an interaction term for test type and the covariate was included in the model. In addition to these characteristics pre-specified in the protocol, we also investigated the effect of smoking, because this has been associated with a twofold increase in the risk of developing active TB. 21 We included both inpatients and outpatients in all our analyses of diagnostic accuracy to allow for generalisability of these tests as an initial test in any case of possible active TB. However, because disease severity and spectrum can influence the diagnostic performance of a test, we also investigated the effect of clinical setting (inpatients vs. outpatients) to determine if our approach was tenable. We were interested in exploring the effect of vitamin D, as it is an important cofactor for the intracellular killing of TB. It is associated with an increased resistance to TB infection, and with the phenotype of active TB. The value of vitamin D supplementation in active TB to improve disease outcome is unclear. However, we were unable to evaluate the effect of vitamin D status on IGRA performance because of variation between centres in the definition of vitamin D status.
In the subset of patients presenting with a TST result as part of their clinical diagnostic workup, the performance of the TST used in sequence with IGRAs was evaluated. The performance of this combination was assessed using logistic regression models constructed in the same form as Bayesian updating (post-test odds = pre-test odds × likelihood ratio) by including the log of the pre-test odds of prevalence (a constant term of known value) as an offset in the model. A linear predictor was then used to estimate log-likelihood ratios, rather than log-odds ratios, and bootstrap methods were used to obtain valid CIs. 22 Model parameterisations from Knottnerus23 were used to compute likelihood ratios for the additional diagnostic value of each test in a testing sequence. Non-parametric, bias-adjusted CIs for parameter estimates from 1000 bootstrap samples were computed.
We performed all analyses using Stata®, version 13.0 (StataCorp LP, College Station, TX, USA).
Patient and public involvement
Ms Nisha Karnani was our patient and public representative for the duration of the study. She was consulted at key points during the study and was invited to SSC meetings and the IDEA study presentation at the end of the study.
Study oversight and management arrangements
Study Management Group
The SMG included the chief investigators, study co-ordinator, lead research nurse and a post-doctoral research associate. The day-to-day management of the study was carried out by the study co-ordinator, with close support provided by the chief investigators and other members of the SMG. The SMG met monthly to discuss study progress and oversight.
Data Management Group
The Data Management Group (DMG) consisted of members of the statistical team and members of the SMG. The group met regularly to review data on recruitment and the prevalence of TB and HIV in the study cohort. The DMG reported to the SSC (see Study Steering Committee).
Study Steering Committee
Independent oversight was provided by the SSC. The committee included an independent chairperson (Professor Khalid Khan), three other independent members (Dr Stephen Gordon, Dr James Grey and Dr Johannes B Reitsma) and a patient and public involvement (PPI) representative (Ms Nisha Karnani).
Ethics arrangements and regulatory approvals
Ethics approval for this study
This study received ethics approval from the London – Camden Kings Cross Research Ethics Committee (REC) (reference number 11/H0722/8). The research study was submitted for site-specific assessment at each participating NHS trust. The chief investigators required a copy of the research and development (R&D) approval letter before accepting participants into the study. The study was conducted in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Declaration of Helsinki (1964) and later revisions. 24
Consent and study withdrawal
Consent to enter the study was sought from each participant only after a full explanation had been given, an information sheet offered and time allowed for consideration. Signed participant consent was then obtained. The right of the participant to refuse to participate without giving reasons was respected. After a participant was entered into the study the clinician remained free to give any treatment that he or she considered necessary, or to refer onto an appropriate health-care professional, at any stage if it was judged to be in the best interest of the participant. Reasons for such decisions were recorded. In such cases, participants remained in the study for the purposes of follow-up and data analysis. All participants were free to withdraw at any time from the study without giving reasons. Assurance was provided by the person taking consent that withdrawal will not affect the patient’s care. In accordance with good clinical practice guidance, participants who withdrew were not required to give a reason for withdrawal. Data were collected on participant’s final diagnosis unless consent was withdrawn for any data to be used.
Confidentiality
The chief investigator and all members of the research team abided by the Data Protection Act25 and preserved the confidentiality of participants involved in the study. Participants were allocated a unique identifying code (anonymised) on recruitment, with no personal identifiers recorded on any sample or data.
Indemnity
The Imperial College London, as sponsor of this study, holds negligent and non-negligent harm insurance policies that applied to this study. These were arranged through the Joint Research Office.
Protocol amendments
Between April 2011 and February 2015, the protocol underwent seven amendments as detailed in Appendix 3, Table 53. Six of the seven amendments were deemed substantial amendments that required ethics approval, whereas one was a minor amendment.
Chapter 3 Participant characteristics
The results presented in this chapter are based on all participants recruited prior to the study extension, including those with HIV infection. Thus, the chapter excludes HIV-positive patients recruited during the study extension period.
Recruitment of participants into main study cohort
A total of 1074 participants, including 177 (16.5%) who were HIV positive, were recruited from 10 NHS trusts into the main study between 25 November 2011 and 31 August 2013. The number of patients recruited at each centre is shown in Table 1. Over half of the study participants were recruited from two trusts: Imperial College Healthcare NHS Trust (26.4%) and London North West Healthcare NHS Trust (27.8%).
| Hospital trust | Patients recruited, n (%) |
|---|---|
| Imperial College Healthcare NHS Trust | 283 (26.4) |
| Heart of England NHS Foundation Trust | 106 (9.9) |
| Chelsea and Westminster Hospital NHS Foundation Trust | 52 (4.8) |
| Royal Free London NHS Foundation Trust | 59 (5.5) |
| St George’s Healthcare NHS Trust | 61 (5.7) |
| Frimley Health NHS Foundation Trust | 44 (4.1) |
| University Hospitals of Leicester NHS Trust | 116 (10.8) |
| London North West Healthcare NHS Trust | 299 (27.8) |
| Oxford University Hospitals NHS Trust | 4 (0.4) |
| Sandwell and West Birmingham Hospitals NHS Trust | 50 (4.7) |
| Total | 1074 (100) |
The flow of patients through the study is shown in Figure 4. Of the 1074 patients recruited, 845 were included in the analyses. Reasons for exclusion are shown in Figure 4. Forty patients from Frimley Health NHS Foundation Trust were excluded because patients all had diagnoses of confirmed or highly probable TB (categories 1 and 2) due to an error of implementation of recruitment criteria at this site (i.e. the natural spectrum of patients with suspected TB was not being recruited). The decision to exclude these patients was approved by the SSC following a SSC meeting during which the diagnosis of patients recruited at each centre was reviewed.
FIGURE 4.
Study flow diagram of patients with suspected active TB. The final four boxes show the number of patients with available IGRA results. a, Patients with previously diagnosed TB were excluded from analyses because IGRA results cannot be reliably interpreted in previously treated patients. The decision to exclude was taken by the expert diagnostic panel and study management group in consultation with the independent Study Steering Committee before unblinding of IGRA and next-generation IGRA results. b, On advice from the Study Steering Committee, and following consultation between the study management group and data management groups, 80 patients were excluded from analyses. Patients recruited from Frimley Health NHS Foundation Trust (n = 40) were excluded because they all had diagnoses of confirmed or highly probable TB (categories 1 and 2) due to an error of implementation of the recruitment criteria at this site, i.e. the natural spectrum of patients with suspected TB was not being recruited. A further subset of patients (n = 27) were, on review, considered by the expert diagnostic panel to be ineligible (before unblinding IGRA results) on the basis that they were being investigated for TB (due to an incidental abnormal chest X-ray, known contact with active TB, or screening for anti-TNF treatment), but did not present with symptoms or signs suggestive of TB. An additional 13 patients were excluded due to invalid consent forms. Republished with permission of Elsevier Science and Technology Journals, from Clinical utility of existing and second-generation interferon-γ release assays for diagnostic evaluation of tuberculosis: an observational cohort study, The Lancet Infectious Diseases, Whitworth HS, Badhan A, Boakye AA, Takwoingi Y, Rees-Roberts M, Partlett C, et al. , vol. 19, pp. 193–202, copyright 2019;27 permission conveyed through Copyright Clearance Center, Inc.
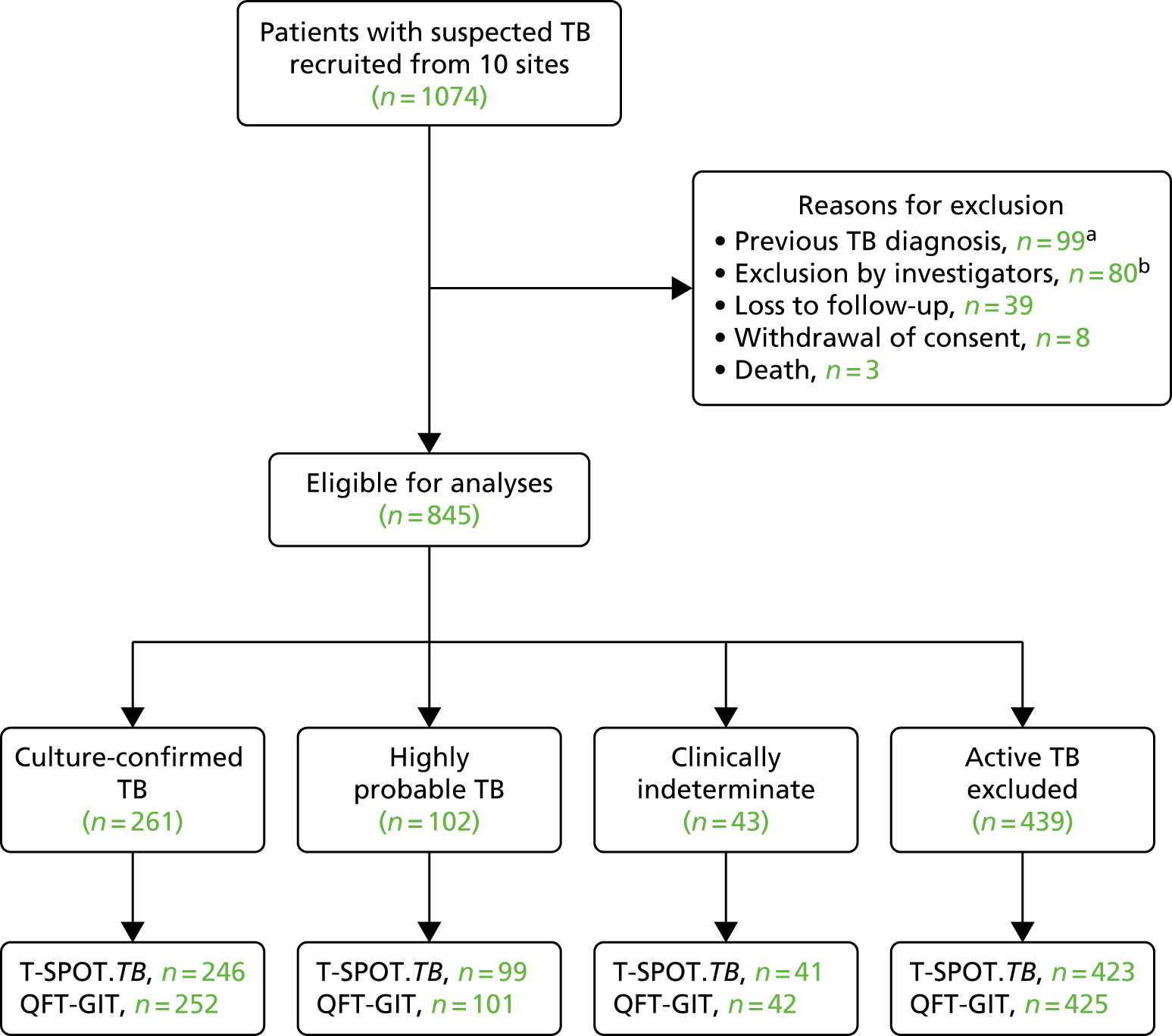
Table 2 shows the number of patients assigned to each diagnostic category. There were 43 (5.1%) patients with a clinically indeterminate (category 3) diagnosis. Of the remaining 802 patients, there were 363 (45.3%) cases of active TB [based on those with culture-confirmed (category 1) and highly probable (category 2) TB] and 439 (54.7%) in whom active TB was excluded (categories 4A to 4D). Of the 439 non-active TB cases, 117 (26.7%) were category 4D.
| Diagnostic category | Criteria | Number of patients |
|---|---|---|
| 1: Culture-confirmed TB | Microbiological culture of Mtb AND suggestive clinical and radiological findings | 261 |
| 2: Highly probable TB | Clinical and radiological features highly suggestive of TB and unlikely to be caused by other disease AND a decision to treat made by a clinician AND appropriate response to therapy AND histology supportive (if available) | 102 |
| 3: Clinically indeterminate | Final diagnosis of TB neither highly probable nor reliably excluded | 43 |
| 4: Active TB excluded | ||
| 4A: inactive TB | Stable CXR changes AND TST positivea (if done) AND bacteriologically negative (if done) AND no clinical evidence of active disease | 7 |
| 4B: one or more risk factors for TB exposure,b TST positivea | TST positivea AND bacteriologically negative (if done) AND no clinical evidence of active disease | 48 |
| 4C: one or more risk factors for TB exposure,b TST negative | History of TB exposure AND TST negative (if done) | 267 |
| 4D: no risk factors for TB exposure,b TST negative | No history of TB exposure AND TST negative (if done) | 117 |
| Total | 845 | |
Baseline characteristics of participants
The main demographic characteristics of the 845 patients are given in Table 3. Most patients (64.4%) were recruited from an outpatient setting, and the remaining were recruited from an inpatient setting. The median age of patients was 38 (range 16–86) years and most (59.3%) of the patients were male. Almost half (48.2%) of the study population were of Indian origin. Altogether, 75 countries of birth were represented in the study; 16 of the countries had at least 10 participants, with India (26.7%) and the UK (22.8%) accounting for almost half of the study population. The complete list of countries is given in Appendix 4, Table 54.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Clinical setting, n (%) | |||||
| Inpatient | 90 (34.5) | 30 (29.4) | 11 (25.6) | 170 (38.7) | 301 (35.6) |
| Outpatient | 171 (65.5) | 72 (70.6) | 32 (74.4) | 269 (61.3) | 544 (64.4) |
| Age (years), median (range) | 32 (16–81) | 36 (18–76) | 38 (16–79) | 44 (17–86) | 38 (16–86) |
| Male, n (%) | 177 (67.8) | 53 (52.0) | 21 (48.8) | 250 (56.9) | 501 (59.3) |
| Ethnic origin, n (%) | |||||
| Asian | 16 (6.1) | 6 (5.9) | 5 (11.6) | 14 (3.2) | 41 (4.9) |
| Black | 50 (19.2) | 22 (21.6) | 10 (23.3) | 102 (23.2) | 184 (21.8) |
| Hispanic | 1 (0.4) | 0 (0.0) | 0 (0.0) | 7 (1.6) | 8 (0.9) |
| Indian subcontinent | 167 (64.0) | 61 (59.8) | 16 (37.2) | 168 (38.3) | 412 (48.8) |
| Middle Eastern | 4 (1.5) | 0 (0.0) | 0 (0.0) | 12 (2.7) | 16 (1.9) |
| Mixed | 1 (0.4) | 4 (3.9) | 0 (0.0) | 8 (1.8) | 13 (1.5) |
| White | 22 (8.4) | 9 (8.8) | 12 (27.9) | 126 (28.7) | 169 (20.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 2 (0.2) |
| Years in the UK, median (range) | 4.9 (0.1–52.9) | 6.1 (0.3–59.7) | 10.5 (0.4–56.9) | 13.2 (0.0–60.3) | 8.3 (0.0–60.3) |
| Profession, n (%)a | |||||
| Paid employment | 130 (49.8) | 52 (51.0) | 21 (48.8) | 214 (48.7) | 417 (49.3) |
| Unpaid employment | 62 (23.8) | 24 (23.5) | 16 (37.2) | 164 (37.4) | 266 (31.5) |
| Student | 50 (19.2) | 13 (12.7) | 3 (7.0) | 26 (5.9) | 92 (10.9) |
| Health-care/laboratory worker | 16 (6.1) | 9 (8.8) | 2 (4.7) | 24 (5.5) | 51 (6.0) |
| Social/prison worker | 1 (0.4) | 1 (1.0) | 0 (0.0) | 2 (0.5) | 4 (0.5) |
| Sex worker | 0 (0.0) | 1 (1.0) | 0 (0.0) | 2 (0.5) | 3 (0.4) |
| Unknown | 2 (0.8) | 2 (2.0) | 1 (2.3) | 7 (1.6) | 12 (1.4) |
Table 4 shows the clinical characteristics of the patients. Of the 845 patients, 135 (16.0%) were HIV positive. Out of the 135 patients, there were two (1.5%) with a clinically indeterminate diagnosis. Of the remaining 133 patients, 25 (18.8%) were active TB cases and 108 (81.2%) were non-active TB cases.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Height (m), median (range) | 1.7 (1.4–2.0) | 1.7 (1.5–1.9) | 1.6 (1.5–1.8) | 1.7 (1.3–2.0) | 1.7 (1.3–2.0) |
| Weight (kg), median (range) | 63 (35–127) | 64 (40–116) | 71 (37–110) | 68 (38–157) | 65 (35–157) |
| BMI (kg/m2), median (range) | 22 (14–48) | 22 (16–42) | 24 (13–45) | 24 (15–47) | 23 (13–48) |
| BCG vaccinated, n (%) | 194 (74.3) | 79 (77.5) | 36 (83.7) | 340 (77.4) | 649 (76.8) |
| BCG scar visible, n (%) | |||||
| Yes | 172 (65.9) | 72 (70.6) | 29 (67.4) | 283 (64.5) | 556 (65.8) |
| No | 12 (4.6) | 3 (2.9) | 3 (7.0) | 19 (4.3) | 37 (4.4) |
| Unsure | 16 (6.1) | 8 (7.8) | 6 (14.0) | 44 (10.0) | 74 (8.8) |
| Missing | 61 (23.4) | 19 (18.6) | 5 (11.6) | 93 (21.2) | 178 (21.1) |
| Known TB contact, n (%) | 70 (26.8) | 25 (24.5) | 12 (27.9) | 83 (18.9) | 190 (22.5) |
| HIV positive, n (%) | 13 (5.0) | 12 (11.8) | 2 (4.7) | 108 (24.6) | 135 (16.0) |
| Other pre-existing conditions/comorbidities, n (%)a | |||||
| None | 169 (64.8) | 61 (59.8) | 19 (44.2) | 169 (38.5) | 418 (49.5) |
| Diabetes | 22 (8.4) | 5 (4.9) | 8 (18.6) | 53 (12.1) | 88 (10.4) |
| Hepatitis B | 5 (1.9) | 1 (1.0) | 0 (0.0) | 5 (1.1) | 11 (1.3) |
| Hepatitis C | 1 (0.4) | 1 (1.0) | 0 (0.0) | 10 (2.3) | 12 (1.4) |
| Chronic/end-stage renal failure | 5 (1.9) | 1 (1.0) | 2 (4.7) | 4 (0.9) | 12 (1.4) |
| Cancer | 1 (0.4) | 1 (1.0) | 0 (0.0) | 12 (2.7) | 14 (1.7) |
| Organ transplantation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 2 (0.2) |
| Asthma | 12 (4.6) | 5 (4.9) | 4 (9.3) | 50 (11.4) | 71 (8.4) |
| Sarcoidosis | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Other | 74 (28.4) | 37 (36.3) | 20 (46.5) | 228 (51.9) | 359 (42.5) |
| Vitamin D deficiency,b n (%) | |||||
| Deficient | 106 (40.6) | 35 (34.3) | 12 (27.9) | 59 (13.4) | 212 (25.1) |
| Insufficient | 49 (18.8) | 14 (13.7) | 7 (16.3) | 65 (14.8) | 135 (16.0) |
| Normal | 13 (5.0) | 8 (7.8) | 5 (11.6) | 34 (7.7) | 60 (7.1) |
| Not known | 93 (35.6) | 45 (44.1) | 19 (44.2) | 281 (64.0) | 438 (51.8) |
Of the 845 patients, over half had other comorbidities: 300 (35.5%) patients had a single comorbidity, 127 (15.0%) had multiple comorbidities and the remaining 418 (49.5%) had none. There were 88 (10.4%) patients with pre-existing diabetes mellitus, 12 (1.4%) patients with chronic/end-stage renal failure and 105 (12.4%) patients were on immunosuppressive therapy (Table 5). These were the three key subgroups that we had planned to investigate in the subgroup analyses. The thresholds used to categorise vitamin D status varied between hospital trusts, as detailed in Appendix 5, Table 55. Although vitamin D measurements were missing for a large number of patients (49.1%), when the results were available, many patients were categorised as either vitamin D deficient (26.5%) or insufficient (16.9%), with few (7.5%) having normal results (see Table 4).
| Medication | Dosanjh category, n (%)a | Total, n (%) | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| None | 63 (24.1) | 35 (34.3) | 13 (30.2) | 203 (46.2) | 314 (37.2) |
| Chemotherapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Corticosteroids ≥ 15 mg/day | 20 (7.7) | 5 (4.9) | 5 (11.6) | 20 (4.6) | 50 (5.9) |
| Corticosteroids < 15 mg/day | 13 (5.0) | 7 (6.9) | 1 (2.3) | 19 (4.3) | 40 (4.7) |
| Corticosteroids unknown | 1 (0.4) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) |
| Ciclosporin, tacrolimus or everolimus | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Other immune suppressants | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.1) | 5 (0.6) |
| Methotrexate | 1 (0.4) | 0 (0.0) | 0 (0.0) | 5 (1.1) | 6 (0.7) |
| Other | 191 (73.2) | 64 (62.7) | 30 (69.8) | 233 (53.1) | 518 (61.3) |
| Unknown | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 2 (0.2) |
The social history of participants is outlined in Table 6. Smoking history was missing for two patients; about two-thirds of the patients had never smoked, and the remaining patients were current or ex-smokers. Most (58.6%) patients also had no history of alcohol use. Almost all patients (97.6%) had no history of homelessness and a few patients (27/845, 3.2%) had a history of imprisonment.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Smoking history, n (%) | |||||
| Never smoked | 181 (69.3) | 81 (79.4) | 26 (60.5) | 248 (56.5) | 536 (63.4) |
| Ex-smoker | 31 (11.9) | 8 (7.8) | 6 (14.0) | 93 (21.2) | 138 (16.3) |
| Current smoker | 49 (18.8) | 13 (12.7) | 11 (25.6) | 96 (21.9) | 169 (20.0) |
| Unknown | 0 | 0 | 0 | 2 (0.5) | 2 (0.2) |
| Pack years if current smoker, n (%) | |||||
| ≤ 10 | 11 (22.4) | 6 (46.2) | 4 (36.4) | 27 (28.1) | 48 (28.4) |
| 11–20 | 1 (2.0) | 2 (15.4) | 1 (9.1) | 6 (6.3) | 10 (5.9) |
| 21–50 | 1 (2.0) | 1 (7.7) | 0 | 7 (7.3) | 9 (5.3) |
| > 51 | 1 (2.0) | 0 | 0 | 1 (1.0) | 2 (1.2) |
| Unknown | 35 (71.4) | 4 (30.8) | 6 (54.5) | 55 (57.3) | 100 (59.2) |
| History of alcohol use, n (%) | |||||
| Non-drinker | 163 (62.5) | 80 (78.4) | 27 (62.8) | 225 (51.3) | 495 (58.6) |
| Ex-drinker | 10 (3.8) | 2 (2.0) | 1 (2.3) | 35 (8.0) | 48 (5.7) |
| Current drinker | 88 (33.7) | 20 (19.6) | 15 (34.9) | 175 (39.9) | 298 (35.3) |
| Unknown | 0 | 0 | 0 | 4 (0.9) | 4 (0.5) |
| Units/week if current drinker, median (range) | 4 (0–250) | 5 (1–35) | 2 (0–140) | 5 (0–210) | 4 (0–250) |
| History of alcohol misuse, n (%) | 9 (3.4) | 0 | 1 (2.3) | 20 (4.6) | 30 (3.6) |
| History of recreational drug use, n (%) | |||||
| Non-user | 21 (8.0) | 10 (9.8) | 1 (2.3) | 18 (4.1) | 50 (5.9) |
| Ex-user | 2 (0.8) | 0 | 1 (2.3) | 5 (1.1) | 8 (0.9) |
| Current user | 5 (1.9) | 3 (2.9) | 1 (2.3) | 13 (3.0) | 22 (2.6) |
| Unknown | 233 (89.3) | 89 (87.3) | 40 (93.0) | 403 (91.8) | 765 (90.5) |
| History of homelessness, n (%) | |||||
| None | 256 (98.1) | 101 (99.0) | 43 (100.0) | 425 (96.8) | 825 (97.6) |
| Previously homeless | 2 (0.8) | 1 (1.0) | 0 | 11 (2.5) | 14 (1.7) |
| Currently homeless | 3 (1.1) | 0 | 0 | 3 (0.7) | 6 (0.7) |
| Years homeless if currently or previously homeless, median (range) | 4 (0–6) | 12 | – | 6 (0–24) | 6 (0–24) |
| History of imprisonment, n (%) | 4 (1.5) | 2 (2.0) | 0 | 21 (4.8) | 27 (3.2) |
Table 7 summarises the frequency of presenting symptoms for 827 patients. The main symptoms recorded were cough, fever, night sweats, weight loss, haemoptysis and lethargy. Patients generally presented with multiple symptoms, but a cough was often present (576/827, 69.6%). The median number of symptoms was four (range 1–10).
| Symptom | Diagnosis as per reference standard1 | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Cough, n (%) | 174 (68.0) | 53 (53.5) | 23 (53.5) | 326 (76.0) | 576 (69.6) |
| Fever, n (%) | 126 (49.2) | 49 (49.5) | 14 (32.6) | 195 (45.5) | 384 (46.4) |
| Night sweats, n (%) | 129 (50.4) | 53 (53.5) | 20 (46.5) | 215 (50.1) | 417 (50.4) |
| Weight loss, n (%) | 154 (60.2) | 54 (54.5) | 21 (48.8) | 211 (49.2) | 440 (53.2) |
| Haemoptysis, n (%) | 31 (12.1) | 8 (8.0) | 3 (7.0) | 65 (15.2) | 107 (12.9) |
| Lethargy, n (%) | 133 (52.0) | 56 (56.6) | 23 (53.5) | 222 (51.7) | 434 (52.5) |
| Other, n (%) | 163 (63.7) | 59 (59.46) | 25 (58.1) | 202 (47.1) | 449 (54.3) |
| Number of symptoms, median (range) | 4 (1–10) | 4 (1–8) | 3 (1–7) | 3 (1–10) | 4 (1–10) |
Final diagnosis
Table 8 shows the diagnostic tests performed during the diagnostic workup of patients. Chest radiography and culture were often performed (in 89.4% and 86.5% of patients, respectively), but cerebrospinal fluid testing and magnetic resonance imaging (MRI) were uncommon (in 3.6% and 12.0%, respectively). The number of T-SPOT. TB, QFT-GIT and TST tests performed as part of routine care at each centre is shown in Appendix 6, Table 56. However, for the purpose of the IDEA study, IGRAs were not used in determining the final diagnosis of patients. TSTs were performed in only 336 patients across the nine centres and results were available for 322 patients. Most of these 336 patients were recruited at London North West Healthcare NHS Trust (57%) and Imperial College Healthcare NHS Trust (26%).
| Test | Dosanjh category, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| BAL investigation | 51 (19.5) | 20 (19.6) | 7 (16.3) | 108 (24.6) | 186 (22.0) |
| CXR | 231 (88.5) | 95 (93.1) | 38 (88.4) | 391 (89.1) | 755 (89.3) |
| CSF investigation | 7 (2.7) | 6 (5.9) | 3 (7.0) | 14 (3.2) | 30 (3.6) |
| CT | 142 (54.4) | 69 (67.6) | 26 (60.5) | 273 (62.2) | 510 (60.4) |
| Culture | 261 (100) | 90 (88.2) | 29 (67.4) | 351 (80.0) | 731 (86.5) |
| Histology or biopsy | 72 (27.6) | 42 (41.2) | 15 (34.9) | 101 (23.0) | 230 (27.2) |
| MRI | 29 (11.1) | 17 (16.7) | 8 (18.6) | 47 (10.7) | 101 (12.0) |
| PCR | 85 (32.6) | 20 (19.6) | 6 (14.0) | 66 (15.0) | 177 (20.9) |
| Smear test | 232 (88.9) | 75 (73.5) | 24 (55.8) | 335 (76.3) | 666 (78.8) |
The final diagnosis of active TB patients is detailed in Table 9. Of the 363 patients with active TB, 237 (65.3%) had smear-negative TB. Forty-five (12.4%) active TB cases had both pulmonary and extrapulmonary TB. Approximately half (189/363, 52.1%) had only extrapulmonary TB. This occurred more often among those diagnosed as having highly probable TB (75/102, 73.5%) relative to culture-confirmed cases (114/261, 43.7%). In 129 (35.5%) active TB cases, patients had only pulmonary TB. In contrast to extrapulmonary TB, pulmonary TB was more common among culture-confirmed cases (110/261, 42.15%) than in highly probable TB cases (19/102, 18.6%). The most common sites of TB infection were the lungs (174/363, 47.9%) and lymph nodes (154/363, 42.4%). The drug sensitivity profile shows that of the 351 culture tests performed, 239 (65.8%) were fully sensitive and 22 (6.3%) were drug resistant.
| Characteristic | Category of TB, n (%) | Total, n (%) | |
|---|---|---|---|
| 1 | 2 | ||
| All TB | 261 (71.9) | 102 (28.1) | 363 (100) |
| Smear-positive TB | 67 (25.7) | 3 (2.9) | 70 (19.3) |
| Smear-negative TB | 165 (63.2) | 72 (70.6) | 237 (65.3) |
| Pulmonary TB | 110 (42.1) | 19 (18.6) | 129 (35.5) |
| Extrapulmonary TB | 114 (43.7) | 75 (73.5) | 189 (52.1) |
| Pulmonary and extrapulmonary TB | 37 (14.2) | 8 (7.8) | 45 (12.4) |
| Site of infectiona | |||
| Abdomen | 6 (2.3) | 3 (2.9) | 9 (2.5) |
| Bones | 5 (1.9) | 0 | 5 (1.4) |
| Brain | 2 (0.8) | 4 (3.9) | 6 (1.7) |
| Chest wall | 1 (0.4) | 1 (1.0) | 2 (0.6) |
| Lungs | 147 (56.3) | 27 (26.5) | 174 (47.9) |
| Lymph node | 105 (40.2) | 49 (48.0) | 154 (42.4) |
| Miliary TB (disseminated) | 11 (4.2) | 0 | 11 (3.0) |
| Pericardium | 4 (1.5) | 2 (2.0) | 6 (1.7) |
| Pleura | 15 (5.7) | 11 (10.8) | 26 (7.2) |
| Spine | 10 (3.8) | 6 (5.9) | 16 (4.4) |
| Other | 15 (5.7) | 16 (15.7) | 31 (8.5) |
| Drug sensitivity profileb | |||
| Fully sensitive | 239 (91.6) | 0 | 239 (68.1) |
| Drug resistant | 21 (8.1) | 0 | 21 (6.0) |
| MDR | 1 (0.4) | 0 | 1 (0.3) |
| Not tested | 0 | 90 (100) | 90 (25.6) |
Table 10 shows the final diagnosis of non-active TB patients. A patient may have multiple conditions. Of the seven conditions listed in the table, pneumonia was the most frequent diagnosis, with 104 of 439 (23.7%) patients having the condition. A higher proportion of inpatients were diagnosed with cancer (14.1%) or pneumonia (38.8%) than outpatients (4.5% and 14.1%, respectively). In contrast, a higher proportion of outpatients were diagnosed with chest infections, latent TB infection and sarcoidosis.
| Diagnosis | Non-active TB patients, n (%) | Total, n (%) (N = 439) | |
|---|---|---|---|
| Inpatients (n = 170) | Outpatients (n = 269) | ||
| Cancer | 24 (14.1) | 12 (4.5) | 36 (8.2) |
| Chest infection | 1 (0.6) | 15 (5.6) | 16 (3.6) |
| Lower respiratory tract infection | 10 (5.9) | 13 (4.8) | 23 (5.2) |
| Pneumonia | 66 (38.8) | 38 (14.1) | 104 (23.7) |
| Sarcoidosis | 5 (2.9) | 33 (12.3) | 38 (8.7) |
| Upper respiratory tract infection | 0 (0.0) | 13 (3.0) | 13 (3.0) |
| Other | 70 (41.2) | 153 (56.9) | 223 (50.8) |
Chapter 4 Diagnostic accuracy results
Overview
Estimates of the accuracy of IGRAs presented in this chapter are for the main cohort of patients (including HIV-positive patients recruited prior to the extension period). Estimates of the accuracy of T-SPOT. TB and QFT-GIT are presented individually, followed by comparisons of test accuracy (T-SPOT. TB vs. QFT-GIT). The results of subgroup analyses are then presented for T-SPOT. TB and QFT-GIT. Finally, test accuracy estimates are provided for ESAT-6, CFP-10 and second-generation IGRAs (Rv3615c, Rv2654, Rv3879c and Rv3873) individually and in combinations; the diagnostic accuracy of the test combinations were then compared with that of T-SPOT. TB. For completeness, we also briefly address the accuracy of the TST.
Completeness of interferon gamma release assay results
Of the 845 patients, T-SPOT. TB and QFT-GIT results were available for 809 (96%) and 820 (97%) patients, respectively. All 809 patients with T-SPOT. TB results also had results for the second-generation IGRAs (Rv3615c, Rv2654, Rv3879c and Rv3873). Table 11 shows the results for T-SPOT. TB and QFT-GIT by diagnostic category. For 805 patients, results were available for both IGRAs; reasons for missing T-SPOT. TB and QFT-GIT results are shown in Table 12. The cross-classified results of the two tests are given in Appendix 7 (see Table 57) for the 805 patients.
| Index test result | Dosanjh category | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| T-SPOT.TB | |||||||||
| Positive | 185 | 68 | 15 | 0 | 18 | 27 | 6 | 51 | 319 |
| Negative | 33 | 25 | 23 | 6 | 26 | 200 | 87 | 319 | 400 |
| Borderline | 16 | 1 | 0 | 0 | 1 | 14 | 1 | 16 | 33 |
| Indeterminate | 12 | 5 | 3 | 1 | 1 | 18 | 17 | 37 | 57 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| Median SFCs ESAT-6 (range) | 13 (0–387) | 13 (0–492) | 0 (0–325) | 0 (0–1) | 2 (0–274) | 0 (0–210) | 0 (0–147) | 0 (0–274) | 1 (0–492) |
| Median SFCs CFP-10 (range) | 17 (0–465) | 13 (0–437) | 1 (0–315) | 0 (0–3) | 1 (0–160) | 0 (0–166) | 0 (0–148) | 0 (0–166) | 1 (0–465) |
| QFT-GIT | |||||||||
| Positive | 163 | 57 | 14 | 0 | 19 | 49 | 6 | 74 | 308 |
| Negative | 68 | 39 | 22 | 7 | 25 | 187 | 85 | 304 | 433 |
| Indeterminate | 21 | 5 | 6 | 0 | 2 | 26 | 19 | 47 | 79 |
| Missing | 9 | 1 | 1 | 0 | 2 | 5 | 7 | 14 | 25 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| Median IFN-γ levels (range) | 0.69 (0–10) | 0.64 (0–10) | 0.15 (0–6.69) | 0.02 (0–0.34) | 0.15 (0–10) | 0.03 (0–10) | 0.01 (0–7.58) | 0.03 (0–10) | 0.12 (0–10) |
| Reason | Test, n | |
|---|---|---|
| QFT-GIT | T-SPOT.TB | |
| No sample could be taken | 1 | 0 |
| Sample destroyed for laboratory reasons | 2 | 11 |
| Sample unsuitable for testing | 6 | 8 |
| Unable to obtain sample from patient | 16 | 17 |
| Total | 25 | 36 |
Diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube
Table 13 shows the cross-tabulation of T-SPOT. TB and QFT-GIT results against active TB status (i.e. active TB or not). The proportion of indeterminate test results was 7.0% (57/809) for T-SPOT. TB and 9.6% (79/820) for QFT-GIT. The difference between the two proportions was 2.6% (95% CI –0.1% to 5.3%; p = 0.06). Based on all culture-confirmed and highly probable active TB cases and excluding indeterminate IGRA results, sensitivity was 82.3% (95% CI 77.8% to 86.1%) for T-SPOT. TB and 67.3% (95% CI 62.0% to 72.1%) for QFT-GIT. Among those in whom active TB was excluded, specificity was 82.6% (95% CI 78.6% to 86.1%) for T-SPOT. TB and 80.4% (95% CI 76.1% to 84.1%) for QFT-GIT (Table 14). The PPVs for T-SPOT. TB and QFT-GIT were 80.1% (95% CI 75.5% to 84.0%) and 74.8% (95% CI 69.6% to 79.5%), respectively, and the NPVs were 84.6% (95% CI 80.6% to 87.9%) and 74.0% (95% CI 69.5% to 78.0%), respectively. For T-SPOT. TB, 4.1% (33/809) of the test results were borderline (see Table 13). When these borderline results were excluded from the T-SPOT. TB analysis, sensitivity (95% CI) was 81.4% (76.6% to 85.3%), specificity (95% CI) was 86.2% (82.3% to 89.4%), PPV (95% CI) was 83.2% (78.6% to 87.0%) and NPV (95% CI) was 84.6% (80.6% to 87.9%). The full results of the analysis are shown in Appendix 7, Table 58.
| T-SPOT.TB, n | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active TB positive (categories 1 and 2) | Active TB negative (category 4) | ||||||||||||
| Positive | Negative | Borderline | Indeterminate | Missing | Total | Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT | Positive | 187 | 13 | 6 | 9 | 5 | 220 | 37 | 30 | 3 | 3 | 1 | 74 |
| Negative | 49 | 41 | 8 | 7 | 2 | 107 | 12 | 250 | 12 | 26 | 4 | 304 | |
| Indeterminate | 16 | 4 | 3 | 1 | 2 | 26 | 2 | 36 | 1 | 8 | 0 | 47 | |
| Missing | 1 | 0 | 0 | 0 | 9 | 10 | 0 | 3 | 0 | 0 | 11 | 14 | |
| Total | 253 | 58 | 17 | 17 | 18 | 363 | 51 | 319 | 16 | 37 | 16 | 439 | |
| Test performance | T-SPOT.TB | QFT-GIT | ||
|---|---|---|---|---|
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 270/328 | 82.3 (77.8 to 86.1) | 220/327 | 67.3 (62.0 to 72.1) |
| Culture-positive TB | 201/234 | 85.9 (80.9 to 89.8) | 163/231 | 70.6 (64.4 to 76.1) |
| Culture-negative TB | 59/83 | 71.1 (60.6 to 79.7) | 48/84 | 57.1 (46.5 to 67.2) |
| Smear-positive TB | 50/60 | 83.3 (72.0 to 90.7) | 42/56 | 75.0 (62.3 to 84.5) |
| Smear-negative TB | 179/216 | 82.9 (77.3 to 87.3) | 148/222 | 67.7 (60.2 to 72.5) |
| Pulmonary TB | 87/113 | 77.0 (68.4 to 83.8) | 78/114 | 68.4 (59.4 to 76.2) |
| Extrapulmonary TB | 147/175 | 84.0 (77.9 to 88.7) | 113/171 | 66.1 (58.7 to 72.8) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 319/386 | 82.6 (78.6 to 86.1) | 304/378 | 80.4 (76.1 to 84.1) |
| Active TB excluded, TST negative, no risk factors for LTBI | 87/94 | 92.3 (85.4 to 96.4) | 85/91 | 93.4 (86.4 to 96.9) |
| Predictive values | ||||
| PPV | 270/337 | 80.1 (75.5 to 84.0) | 220/294 | 74.8 (69.6 to 79.5) |
| NPV | 319/377 | 84.6 (80.6 to 87.9) | 304/411 | 74.0 (69.5 to 78.0) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 4.74 (3.79 to 5.93) | – | 3.44 (2.76 to 4.27) |
| Negative likelihood ratio | – | 0.21 (0.17 to 0.27) | – | 0.41 (0.35 to 0.48) |
Using only culture-confirmed active TB cases, the sensitivities of T-SPOT. TB and QFT-GIT increased slightly to 85.9% (95% CI 80.9% to 89.8%) and 70.6% (95% CI 64.4% to 76.1%), respectively. The sensitivity of T-SPOT. TB was 7.0% lower in patients diagnosed with pulmonary TB than in those with extrapulmonary TB [77.0% (95% CI 68.4% to 83.8%) vs. 84.0% (95% CI 77.9% to 88.7%)]. In contrast, although the difference was small (2.3%), the sensitivity of QFT-GIT was higher in patients who had pulmonary TB (68.4%, 95% CI 59.4% to 76.2%) than in those with extrapulmonary TB (66.1%, 95% CI 58.7% to 72.8%). Using only category 4D patients without active TB gave much higher specificities than using all patients without active TB (see Table 14).
In the sensitivity analyses with indeterminate test results included as test positives, the sensitivity of T-SPOT. TB was 83.2% (95% CI 78.9% to 86.8%) and 69.7% (95% CI 64.7% to 74.2%) for QFT-GIT. The specificity of T-SPOT. TB was 75.4% (95% CI 71.1% to 79.3%) and 71.5% (95% CI 67.1% to 75.6%) for QFT-GIT. Full results are provided in Appendix 7, Table 59.
Sensitivity, specificity and predictive values are presented as percentages. For TSPOT. TB, there were 69 test positives out of 94 highly probable TB cases, with sensitivity (95% CI) of 73.4% (63.7% to 81.3%). For QFT-GIT, there were 57 test positives out of 96 highly probable TB cases, with sensitivity (95% CI) of 59.4% (49.4% to 68.7%). Note that in the primary analyses of T-SPOT. TB, borderline test results were included as test positives. Sensitivity analyses were performed with borderline T-SPOT. TB results excluded and the results are shown in Appendix 7, Table 58. Indeterminate IGRA results were excluded from all analyses. See Appendix 7 for sensitivity analyses using all IGRA results with indeterminates included as test positives.
Comparison of diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube
Excluding indeterminate IGRA results, there were 714 T-SPOT. TB results and 705 QFT-GIT results. Based on analyses using GEE models, the sensitivity of T-SPOT. TB was superior to that of QFT-GIT (relative sensitivity 1.22, 95% CI 1.14 to 1.31; p < 0.001), but there was no statistical evidence of a difference in specificity (relative specificity 1.02, 95% CI 0.97 to 1.08; p = 0.3) (Table 15). Excluding the 33 borderline T-SPOT. TB results (see Appendix 7, Table 60), the results for relative sensitivity were similar to those from the main analysis but there was statistical evidence of a difference in specificity; the relative sensitivity (95% CI) was 1.20 (1.12 to 1.29; p < 0.001) and the relative specificity (95% CI) was 1.07 (1.02 to 1.12; p = 0.004). When all IGRA results were analysed and indeterminate IGRA results were included as test positives in a sensitivity analysis, the analysis included 768 T-SPOT. TB results and 778 QFT-GIT results. Similar to the primary analysis, there was statistical evidence of a difference in sensitivity (relative sensitivity 1.19, 95% CI 1.12 to 1.28; p < 0.001) and no evidence of a difference in specificity (relative specificity 1.05 95% CI 0.98 to 1.13; p = 0.1) (see Appendix 7, Table 61).
| Test | Number of test resultsa | Sensitivity (95% CI) | Number of test resultsb | Specificity (95% CI) |
|---|---|---|---|---|
| T-SPOT.TB | 328 | 82.2 (77.7 to 85.9) | 386 | 83.0 (78.9 to 86.4) |
| QFT-GIT | 327 | 67.3 (62.1 to 72.2) | 378 | 81.0 (76.8 to 84.6) |
| Ratio (95% CI);c p-value | – | 1.22 (1.14 to 1.31); < 0.001 | – | 1.02 (0.97 to 1.08); 0.3 |
Subgroup analyses for T-SPOT.TB and QuantiFERON GOLD In-Tube
Human immunodeficiency virus-positive and -negative patients
Human immunodeficiency virus co-infected patients are the highest-risk subgroup for TB. In this section we briefly present results for the 135 HIV-positive patients in the main cohort and address the primary objectives related to this subgroup in Chapter 5 using all HIV-positive patients recruited into the IDEA study. Appendix 8 (see Table 62) shows the results for T-SPOT. TB and QFT-GIT against active TB status. Appendix 8 (see Table 63) shows the cross-tabulation of T-SPOT. TB and QFT-GIT results in HIV-positive patients. For 134 patients, results were available for both T-SPOT. TB and QFT-GIT.
The number of test results for active TB and non-active TB patients who were available for the analyses of test performance in HIV-positive patients was small (see Appendix 8, Table 62). Using culture-confirmed and highly probable active TB cases and excluding indeterminate IGRA results, sensitivity was 63.2% (95% CI 41.0% to 80.9%) for T-SPOT. TB and 56.5% (95% CI 36.8% to 74.4%) for QFT-GIT. Among all non-active TB patients, specificity was 89.9% (95% CI 81.3% to 94.8%) for T-SPOT. TB and 92.0% (95% CI 84.3% to 96.1%) for QFT-GIT (Table 16). For T-SPOT. TB and QFT-GIT, the PPVs were 60.0% (95% CI 38.7% to 78.1%) and 65.0% (95% CI 43.3% to 81.9%), respectively, and the NPVs were 91.0% (95% CI 82.6% to 95.6%) and 88.9% (95% CI 80.7% to 93.9%), respectively.
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 12/19 | 63.2 (41.0 to 80.9) | 13/23 | 56.5 (36.8 to 74.4) |
| Culture-positive TB | 7/11 | 63.6 (35.4 to 84.8) | 8/13 | 61.5 (35.5 to 82.3) |
| Culture-negative TB | 5/8 | 62.5 (30.6 to 86.3) | 5/9 | 55.6 (26.7 to 81.1) |
| Smear-positive TB | 1/4 | 25.0 (4.6 to 69.9) | 3/5 | 60.0 (23.1 to 88.2) |
| Smear-negative TB | 7/11 | 63.6 (35.4 to 84.8) | 7/14 | 50.0 (26.8 to 73.2) |
| Pulmonary TB | 3/5 | 60.0 (23.1 to 88.2) | 5/8 | 62.5 (30.6 to 86.3) |
| Extrapulmonary TB | 7/10 | 70.0 (39.7 to 89.2) | 6/12 | 50.0 (25.4 to 74.6) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 71/79 | 89.9 (81.3 to 94.8) | 80/87 | 92.0 (84.3 to 96.1) |
| Active TB excluded, TST negative, no risk factors for LTBI | 28/29 | 96.6 (82.8 to 99.4) | 36/38 | 94.7 (82.7 to 98.5) |
| Predictive values | ||||
| PPV | 12/20 | 60.0 (38.7 to 78.1) | 13/20 | 65.0 (43.3 to 81.9) |
| NPV | 71/78 | 91.0 (82.6 to 95.6) | 80/90 | 88.9 (80.7 to 93.9) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 6.24 (2.97 to 13.1) | – | 7.03 (3.17 to 15.6) |
| Negative likelihood ratio | – | 0.41 (0.22 to 0.74) | – | 0.47 (0.30 to 0.76) |
For HIV-negative patients (Table 17), results were generally similar to those of the entire cohort that included both HIV-negative and -positive patients (see Table 14). For both subgroups, inclusion of indeterminate IGRA results in sensitivity analyses led to higher sensitivities, lower specificities and no change in NPVs, which is as expected because of the inclusion of indeterminates as test positives (see Appendix 8, Tables 64 and 65).
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 258/309 | 83.5 (79.0 to 87.2) | 207/304 | 68.1 (62.7 to 73.1) |
| Culture-positive TB | 194/223 | 87.0 (81.9 to 90.8) | 155/218 | 71.1 (64.8 to 76.7) |
| Culture-negative TB | 54/75 | 72.0 (61.0 to 80.9) | 43/75 | 57.3 (46.1 to 67.9) |
| Smear-positive TB | 49/56 | 87.5 (76.4 to 93.8) | 39/51 | 76.5 (63.2 to 86.0) |
| Smear-negative TB | 172/205 | 83.9 (78.3 to 88.3) | 141/208 | 67.8 (61.2 to 73.8) |
| Pulmonary TB | 84/108 | 77.8 (69.1 to 84.6) | 73/106 | 68.9 (59.5 to 76.9) |
| Extrapulmonary TB | 140/165 | 84.8 (78.6 to 89.5) | 107/159 | 67.3 (59.7 to 74.1) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 248/307 | 80.8 (76.0 to 84.8) | 224/291 | 77.0 (71.8 to 81.4) |
| Active TB excluded, TST negative, no risk factors for LTBI | 59/65 | 90.8 (81.3 to 95.7) | 49/53 | 92.5 (82.1 to 97.0) |
| Predictive values | ||||
| PPV | 258/317 | 81.4 (76.7 to 85.3) | 207/274 | 75.6 (70.1 to 80.3) |
| NPV | 248/299 | 82.9 (78.3 to 86.8) | 224/321 | 69.8 (64.6 to 74.6) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 4.35 (3.44 to 5.49) | – | 2.96 (2.37 to 3.70) |
| Negative likelihood ratio | – | 0.20 (0.16 to 0.26) | – | 0.42 (0.35 to 0.49) |
Other key patient subgroups
For our secondary objective of quantifying test accuracy in three key patient subgroups (patients with pre-existing diabetes mellitus, end-stage renal failure and iatrogenic immunosuppression), numbers of participants in the subgroups were small but, nonetheless, we evaluated the performance of IGRAs in patients with diabetes (see Table 4). The results for T-SPOT. TB and QFT-GIT against active TB status are shown in Appendix 9, Table 66. Owing to the limited number of data for the TST, only the performance of T-SPOT. TB and QFT-GIT is presented below (see Table 18). Table 67 in Appendix 9 shows the cross-tabulation of T-SPOT. TB and QFT-GIT results in the 88 patients with diabetes mellitus. For 86 patients, the results were available for both T-SPOT. TB and QFT-GIT.
Excluding indeterminate IGRA results, sensitivity was 68.0% (95% CI 48.4% to 82.8%) for T-SPOT. TB and 55.6% (95% CI 37.3% to 72.4%) for QFT-GIT among patients with culture-confirmed and highly probable active TB. Based on all non-active TB patients, specificity was 77.6% (95% CI 64.1% to 87.0%) for T-SPOT. TB and 78.7% (95% CI 65.1% to 88.0%) for QFT-GIT (Table 18). The PPVs were 60.7% (95% CI 42.4% to 76.4%) and 60.0% (95% CI 40.7% to 76.6%), and the NPVs were 82.6% (95% CI 69.3% to 90.9%) and 75.5% (95% CI 61.9% to 85.4%) for T-SPOT. TB and QFT-GIT, respectively. These analyses were based on limited data and so should be interpreted with caution. Appendix 9 (see Table 68) shows the performance of both tests when indeterminate IGRA results were included as test positives in sensitivity analyses.
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 17/25 | 68.0 (48.4 to 82.8) | 15/27 | 55.6 (37.3 to 72.4) |
| Culture-positive TB | 14/21 | 66.7 (45.4 to 82.8) | 12/22 | 54.5 (34.7 to 73.1) |
| Culture-negative TB | 3/4 | 75.0 (30.1 to 95.4) | 3/5 | 60.0 (23.1 to 88.2) |
| Smear-positive TB | 7/9 | 77.8 (45.3 to 93.7) | 6/9 | 66.7 (35.4 to 87.9) |
| Smear-negative TB | 9/14 | 64.3 (38.8 to 83.7) | 7/16 | 43.8 (23.1 to 66.8) |
| Pulmonary TB | 4/8 | 50.0 (21.5 to 78.5) | 5/9 | 55.6 (26.7 to 81.1) |
| Extrapulmonary TB | 10/13 | 76.9 (49.7 to 91.8) | 8/14 | 57.1 (32.6 to 78.6) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 38/49 | 77.6 (64.1 to 87.0) | 37/47 | 78.7 (65.1 to 88.0) |
| Active TB excluded, TST negative, no risk factors for LTBI | 6/7 | 85.7 (48.7 to 97.4) | 4/5 | 80.0 (37.6 to 96.4) |
| Predictive values | ||||
| PPV | 17/28 | 60.7 (42.4 to 76.4) | 15/25 | 60.0 (40.7 to 76.6) |
| NPV | 38/46 | 82.6 (69.3 to 90.9) | 37/49 | 75.5 (61.9 to 85.4) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 3.03 (1.69 to 5.44) | – | 2.61 (1.37 to 4.98) |
| Negative likelihood ratio | – | 0.41 (0.23 to 0.75) | – | 0.57 (0.36 to 0.88) |
Variation in relative performance of T-SPOT.TB and QuantiFERON-TB Gold Plus
We explored the effect of HIV co-infection, diabetes mellitus, smoking and clinical setting on the relative test performance of T-SPOT. TB and QFT-GIT. Each covariate was investigated in a separate regression model. The sensitivities of both tests were superior in HIV-negative patients than in HIV-positive patients. However, there was no statistical evidence of an effect of HIV infection status on relative sensitivity (p = 0.2). In contrast, specificities were superior in HIV-positive patients than in HIV-negative patients, but there was no statistical evidence of an effect of HIV infection status on relative specificity (p = 0.2).
Although the sensitivities and specificities of both tests were higher in those without diabetes mellitus than in those with diabetes mellitus, there was no statistical evidence of an effect on relative test performance (Table 19). Although the p-value from the Wald test of the interaction between test type and smoking gave a p-value of 0.01 for the difference in relative sensitivity between the group that had never smoked and the group of previous smokers, the global test of whether or not relative test performance varied across smoking subgroups was not statistically significant for sensitivity (p = 0.7) or for specificity (p = 0.4). The sensitivities of T-SPOT. TB and QFT-GIT were lower in inpatients than in outpatients; the converse was the case for their specificities.
| Covariate | N TD | N QD | Sensitivity (95% CI) | p-value | N TND | N QND | Specificity (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | T-SPOT.TB | QFT-GIT | |||||||
| None | 328 | 327 | 82.2 (77.7 to 85.9) | 67.3 (62.1 to 72.2) | N/A | 386 | 378 | 83.0 (78.9 to 86.4) | 81.0 (76.8 to 84.6) | N/A |
| HIV infection status | ||||||||||
| Negative | 309 | 304 | 83.5 (79.0 to 87.3) | 68.2 (62.8 to 73.2) | – | 307 | 291 | 80.8 (76.0 to 84.8) | 77.5 (72.4 to 81.9) | – |
| Positive | 19 | 23 | 60.1 (38.0 to 78.7) | 56.1 (36.1 to 74.35) | 0.2 | 79 | 87 | 90.0 (81.2 to 94.7) | 92.3 (84.7 to 96.3) | 0.2 |
| Diabetes | ||||||||||
| No | 303 | 300 | 83.3 (78.7 to 87.1) | 68.3 (62.9 to 73.3) | – | 337 | 331 | 83.8 (79.6 to 87.3) | 81.2 (76.7 to 85.0) | – |
| Yes | 25 | 27 | 67.8 (47.8 to 82.9) | 55.6 (36.9 to 72.8) | 0.5 | 49 | 47 | 76.9 (63.2 to 86.6) | 79.8 (66.0 to 88.8) | 0.4 |
| Smoking statusa | ||||||||||
| Never smoked | 238 | 239 | 83.8 (78.6 to 87.9) | 65.3 (59.1 to 71.1) | – | 220 | 216 | 83.0 (77.5 to 87.3) | 78.0 (72.1 to 83.0) | – |
| Previous smoke | 34 | 29 | 77.0 (60.2 to 88.1) | 82.2 (64.5 to 92.2) | 0.01 | 82 | 77 | 81.0 (71.1 to 88.0) | 80.4 (70.2 to 87.8) | 0.4 |
| Current smoke | 56 | 59 | 78.5 (66.0 to 87.3) | 68.3 (55.6 to 78.8) | 0.2 | 82 | 83 | 84.3 (75.0 to 90.6) | 88.8 (80.2 to 94.0) | 0.06 |
| Clinical setting | ||||||||||
| Inpatient | 104 | 96 | 77.7 (69.0 to 84.6) | 64.3 (54.5 to 73.1) | – | 140 | 132 | 86.8 (80.2 to 91.5) | 90.0 (83.6 to 94.1) | – |
| Outpatient | 224 | 231 | 84.2 (78.8 to 88.4) | 68.7 (62.4 to 74.3) | 0.4 | 246 | 246 | 80.6 (75.2 to 85.0) | 76.0 (70.3 to 80.9) | 0.09 |
Diagnostic accuracy of second-generation interferon gamma release assays
The diagnostic accuracy of ESAT-6, CFP-10 and four second-generation IGRAs (Rv3615c, Rv2654, Rv3879c and Rv3873) is presented in the following sections as individual tests and as test combinations. The accuracy of different test combinations was compared with that of T-SPOT. TB.
Individual antigens
The results of the six antigens cross-tabulated against active TB status are shown in Table 20 and their diagnostic accuracy in Table 21. Based on all culture-confirmed and highly probable active TB cases and excluding indeterminate IGRA results, the three antigens with the highest sensitivities were Rv3615c, ESAT-6 and CFP-10. The sensitivities were 78.0% (95% CI 73.1% to 82.1%), 69.4% (95% CI 64.1% to 74.1%) and 71.6% (95% CI 66.5% to 76.2%), and the specificities were 82.7% (95% CI 78.7% to 86.1%), 87.8% (95% CI 84.2% to 90.7%) and 86.3% (95% CI 82.5% to 89.4%) for Rv3615c, ESAT-6 and CFP-10, respectively. The performance of the remaining three antigens was very poor with sensitivities of 34.2% (95% CI 29.2% to 39.5%), 37.4% (95% CI 33.3% to 42.9%) and 38.2% (95% CI 33.1% to 43.7%) for Rv3873, Rv2654 and Rv3879c, respectively. However, their specificities were high: 95.1% (95% CI 92.4% to 96.8%), 91.7% (95% CI 88.5% to 94.0%) and 93.3% (95% CI 90.3% to 95.4%) for Rv3873, Rv2654 and Rv3879c, respectively. Table 21 also shows the performance of the tests in different clinical groups. For each antigen, sensitivity was higher in those with culture-confirmed active TB than in those with culture-negative active TB.
| IGRA result | Dosanjh category, n | Total, n | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| Rv3615c | |||||||||
| Positive | 171 | 53 | 13 | 0 | 13 | 35 | 6 | 54 | 291 |
| Negative | 38 | 33 | 24 | 6 | 28 | 200 | 86 | 320 | 415 |
| Borderline | 21 | 6 | 1 | 0 | 5 | 6 | 2 | 13 | 41 |
| Indeterminate | 16 | 7 | 3 | 1 | 0 | 18 | 17 | 36 | 62 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 25 (0–642) | 19 (0–450) | 1 (0–102) | 0 (0–4) | 1 (0–134) | 0 (0–493) | 0 (0–406) | 0 (0–493) | 2 (0–642) |
| Rv3873 | |||||||||
| Positive | 59 | 23 | 3 | 0 | 2 | 11 | 3 | 16 | 101 |
| Negative | 146 | 64 | 34 | 6 | 43 | 225 | 92 | 366 | 610 |
| Borderline | 22 | 5 | 0 | 0 | 0 | 3 | 0 | 3 | 30 |
| Indeterminate | 19 | 7 | 4 | 1 | 1 | 20 | 16 | 38 | 68 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 2 (0–178) | 1 (0–181) | 0 (0–145) | 0 (0–0) | 0 (0–30) | 0 (0–64) | 0 (0–71) | 0 (0–71) | 0 (0–181) |
| Rv2654 | |||||||||
| Positive | 74 | 27 | 6 | 0 | 5 | 13 | 5 | 23 | 130 |
| Negative | 138 | 61 | 29 | 6 | 36 | 222 | 88 | 352 | 580 |
| Borderline | 14 | 4 | 2 | 0 | 4 | 4 | 1 | 9 | 29 |
| Indeterminate | 20 | 7 | 4 | 1 | 1 | 20 | 17 | 39 | 70 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 2 (0–260) | 1 (0–395) | 1 (0–55) | 0 (0–1) | 1 (0–44) | 0 (0–370) | 0 (0–57) | 0 (0–370) | 0 (0–395) |
| Rv3879c | |||||||||
| Positive | 66 | 30 | 4 | 0 | 3 | 13 | 3 | 19 | 119 |
| Negative | 138 | 59 | 32 | 6 | 41 | 222 | 90 | 359 | 588 |
| Borderline | 23 | 3 | 1 | 0 | 1 | 4 | 2 | 7 | 34 |
| Indeterminate | 19 | 7 | 4 | 1 | 1 | 20 | 16 | 38 | 68 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 2 (0–241) | 1 (0–225) | 0 (0–154) | 0 (0–3) | 0 (0–42) | 0 (0–83) | 0 (0–58) | 0 (0–83) | 0 (0–241) |
| ESAT-6 | |||||||||
| Positive | 149 | 54 | 9 | 0 | 14 | 16 | 5 | 35 | 247 |
| Negative | 63 | 36 | 27 | 6 | 29 | 217 | 87 | 339 | 465 |
| Borderline | 18 | 3 | 1 | 0 | 2 | 8 | 2 | 12 | 34 |
| Indeterminate | 16 | 6 | 4 | 1 | 1 | 18 | 17 | 37 | 63 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 14 (0–642) | 13 (0–492) | 0 (0–325) | 0 (0–1) | 2 (0–274) | 0 (0–210) | 0 (0–147) | 0 (0–274) | 1 (0–642) |
| CFP-10 | |||||||||
| Positive | 157 | 57 | 11 | 0 | 14 | 24 | 4 | 42 | 267 |
| Negative | 57 | 35 | 26 | 6 | 29 | 208 | 90 | 333 | 451 |
| Borderline | 17 | 1 | 1 | 0 | 2 | 9 | 0 | 11 | 30 |
| Indeterminate | 15 | 6 | 3 | 1 | 1 | 18 | 17 | 37 | 61 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| SFCs, median (range) | 18 (0–642) | 13 (0–437) | 1 (0–315) | 0 (0–3) | 1 (0–160) | 0 (0–166) | 0 (0–148) | 0 (0–166) | 1 (0–642) |
| Test performance | Antigen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rv3615c | Rv3879c | Rv3873 | Rv2654 | ESAT-6 | CFP-10 | |||||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | |
| Sensitivity for a diagnosis of active TB | ||||||||||||
| All TB | 251/322 | 78.0 (73.1 to 82.1) | 122/319 | 38.2 (33.1 to 43.7) | 224/323 | 69.4 (64.1 to 74.1) | 224/323 | 69.4 (64.1 to 74.1) | 224/323 | 69.4 (64.1 to 74.1) | 232/324 | 71.6 (66.5 to 76.2) |
| Culture-positive TB | 192/230 | 83.5 (78.1 to 87.7) | 89/227 | 39.2 (33.1 to 45.7) | 167/230 | 72.6 (66.5 to 78.0) | 167/230 | 72.6 (66.5 to 78.0) | 167/230 | 72.6 (66.5 to 78.0) | 174/231 | 75.3 (69.4 to 80.4) |
| Culture-negative TB | 51/82 | 62.2 (51.4 to 71.9) | 28/82 | 34.1 (24.8 to 44.9) | 48/83 | 57.8 (47.1 to 67.9) | 48/83 | 57.8 (47.1 to 67.9) | 48/83 | 57.8 (47.1 to 67.9) | 50/82 | 61.0 (50.2 to 70.8) |
| Smear-positive TB | 48/58 | 82.8 (71.1 to 90.4) | 22/57 | 38.6 (27.1 to 51.6) | 39/58 | 67.2 (54.4 to 77.9) | 39/58 | 67.2 (54.4 to 77.9) | 39/58 | 67.2 (54.4 to 77.9) | 43/59 | 72.9 (60.4 to 82.6) |
| Smear-negative TB | 162/212 | 76.4 (70.3 to 81.6) | 86/212 | 40.6 (34.2 to 47.3) | 151/213 | 70.9 (64.5 to 76.6) | 151/213 | 70.9 (64.5 to 76.6) | 151/213 | 70.9 (64.5 to 76.6) | 155/215 | 72.1 (65.7 to 77.7) |
| Pulmonary TB | 85/111 | 76.6 (67.9 to 83.5) | 35/111 | 31.5 (23.6 to 40.7) | 71/111 | 64.0 (54.7 to 72.3) | 71/111 | 64.0 (54.7 to 72.3) | 71/111 | 64.0 (54.7 to 72.3) | 75/113 | 66.4 (57.3 to 74.4) |
| Extrapulmonary TB | 133/172 | 77.3 (70.5 to 83.0) | 71/170 | 41.8 (34.6 to 49.3) | 124/173 | 71.7 (64.5 to 77.9) | 124/173 | 71.7 (64.5 to 77.9) | 124/173 | 71.7 (64.5 to 77.9) | 127/172 | 73.8 (66.8 to 79.8) |
| Specificity for a diagnosis of active TB | ||||||||||||
| Active TB excluded | 320/387 | 82.7 (78.7 to 86.1) | 359/385 | 93.3 (90.3 to 95.4) | 339/386 | 87.8 (84.2 to 90.7) | 339/386 | 87.8 (84.2 to 90.7) | 339/386 | 87.8 (84.2 to 90.7) | 333/386 | 86.3 (82.5 to 89.4) |
| Active TB excluded, TST negative, no risk factors for LTBI | 86/94 | 91.5 (84.1 to 95.6) | 90/95 | 94.7 (88.3 to 97.7) | 87/94 | 92.6 (85.4 to 96.3) | 87/94 | 92.6 (85.4 to 96.3) | 87/94 | 92.6 (85.4 to 96.3) | 90/94 | 95.7 (89.6 to 98.3) |
| Predictive values | ||||||||||||
| PPV | 251/318 | 78.9 (74.1 to 83.1) | 122/148 | 82.4 (75.5 to 87.7) | 224/271 | 82.7 (77.7 to 86.7) | 224/271 | 82.7 (77.7 to 86.7) | 224/271 | 82.7 (77.7 to 86.7) | 232/285 | 82.4 (77.3 to 86.8) |
| NPV | 320/391 | 81.8 (77.7 to 85.4) | 359/556 | 64.6 (60.5 to 68.4) | 339/492 | 77.4 (73.3 to 81.1) | 339/492 | 77.4 (73.3 to 81.1) | 339/492 | 77.4 (73.3 to 81.1) | 333/425 | 78.4 (74.2 to 82.0) |
| Likelihood ratios | ||||||||||||
| Positive likelihood ratio | 4.50 (3.59 to 5.64) | 5.66 (3.81 to 8.42) | 5.70 (4.32 to 7.52) | 5.70 (4.32 to 7.52) | 5.70 (4.32 to 7.52) | 5.22 (4.02 to 6.76) | ||||||
| Negative likelihood ratio | 0.27 (0.22 to 0.33) | 0.66 (0.61 to 0.73) | 0.35 (0.30 to 0.41) | 0.35 (0.30 to 0.41) | 0.35 (0.30 to 0.41) | 0.33 (0.28 to 0.39) | ||||||
The results of the sensitivity analyses in which indeterminate test results were included as test positives are given in Appendix 10, Table 69. The results show slight increases in sensitivity with reductions in specificities.
Combinations of antigens
Various combinations of the antigens are shown cross-tabulated against diagnostic category in Table 22. Table 23 shows the diagnostic accuracy of the combinations. The sensitivity of the four-antigen (CFP-10, ESAT-6, Rv3615c and Rv3879c) and three-antigen (CFP-10, ESAT-6 and Rv3615c) combinations containing both CFP-10 and ESAT-6 were identical irrespective of whether all active TB cases or different subgroups were analysed, as shown in Table 23. For this three-antigen combination, the sensitivity was 89.9% (95% CI 86.2% to 92.7%) among all active TB cases and the specificity was 76.5% (95% CI 72.0% to 80.4%) in all non-active TB patients. Another three-antigen combination (CFP-10, Rv3615c and Rv3879c) gave a similar sensitivity and specificity of 89.0% (95% CI 85.1% to 91.9%) and 76.3% (95% CI 71.8% to 80.3%). Reducing this combination to a two-antigen combination of CFP-10 and Rv3615c gave a sensitivity of 88.4% (84.5% to 91.4%) and a specificity of 78.0% (95% CI 73.7% to 81.9%). See Appendix 10, Table 70, for sensitivity analyses with borderline test results excluded as test positives. For the results of the sensitivity analyses including indeterminate test results, see Appendix 10, Table 71.
| IGRA combination result | Dosanjh category, n | Total, n | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| CFP-10 + Rv3615c | |||||||||
| Positive | 197 | 66 | 15 | 0 | 19 | 44 | 7 | 70 | 348 |
| Negative | 15 | 23 | 23 | 6 | 22 | 188 | 86 | 302 | 363 |
| Borderline | 22 | 4 | 1 | 0 | 5 | 9 | 1 | 15 | 42 |
| Indeterminate | 12 | 6 | 2 | 1 | 0 | 18 | 17 | 36 | 56 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| CFP-10 + Rv3615c + Rv3879c | |||||||||
| Positive | 197 | 66 | 15 | 0 | 19 | 49 | 8 | 76 | 354 |
| Negative | 14 | 22 | 23 | 6 | 22 | 184 | 84 | 296 | 355 |
| Borderline | 23 | 5 | 1 | 0 | 5 | 8 | 3 | 16 | 45 |
| Indeterminate | 12 | 6 | 2 | 1 | 0 | 18 | 16 | 35 | 55 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| ESAT-6 + CFP-10 + Rv3615c | |||||||||
| Positive | 203 | 70 | 15 | 0 | 20 | 46 | 8 | 74 | 362 |
| Negative | 13 | 20 | 23 | 6 | 22 | 184 | 84 | 296 | 352 |
| Borderline | 18 | 4 | 1 | 0 | 4 | 11 | 2 | 17 | 40 |
| Indeterminate | 12 | 5 | 2 | 1 | 0 | 18 | 17 | 36 | 55 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | |||||||||
| Positive | 203 | 70 | 16 | 0 | 20 | 49 | 9 | 78 | 367 |
| Negative | 13 | 20 | 22 | 6 | 22 | 180 | 82 | 290 | 345 |
| Borderline | 18 | 4 | 1 | 0 | 4 | 12 | 4 | 20 | 43 |
| Indeterminate | 12 | 5 | 2 | 1 | 0 | 18 | 16 | 35 | 54 |
| Missing | 15 | 3 | 2 | 0 | 2 | 8 | 6 | 16 | 36 |
| Total | 261 | 102 | 43 | 7 | 48 | 267 | 117 | 439 | 845 |
| Test performance | Antigen combination | |||||||
|---|---|---|---|---|---|---|---|---|
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | ESAT-6 + CFP-10 + Rv3615 | CFP-10 + Rv3615c + Rv3879c | CFP-10 + Rv3615c | |||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||||
| All TB | 295/328 | 89.9 (86.2 to 92.7) | 295/328 | 89.9 (86.2 to 92.7) | 291/327 | 89.0 (85.1 to 91.9) | 289/327 | 88.4 (84.5 to 91.4) |
| Culture-positive TB | 221/234 | 94.4 (90.7 to 96.7) | 221/234 | 94.4 (90.7 to 96.7) | 220/234 | 94.0 (90.2 to 96.4) | 219/234 | 93.6 (89.7 to 96.1) |
| Culture-negative TB | 63/83 | 75.9 (65.7 to 83.8) | 63/83 | 75.9 (65.7 to 83.8) | 61/82 | 74.4 (64.0 to 82.6) | 60/82 | 73.2 (62.7 to 81.6) |
| Smear-positive TB | 57/60 | 95.0 (86.3 to 98.3) | 57/60 | 94.9 (86.1 to 98.3) | 57/60 | 95.0 (86.3 to 98.3) | 57/60 | 95.0 (86.3 to 98.3) |
| Smear-negative TB | 192/216 | 88.9 (84.0 to 92.4) | 192/216 | 88.9 (84.0 to 92.4) | 189/215 | 87.9 (82.9 to 91.6) | 187/215 | 87.0 (81.8 to 90.8) |
| Pulmonary TB | 101/113 | 89.4 (82.4 to 93.8) | 101/113 | 89.4 (82.4 to 93.8) | 101/113 | 89.4 (82.4 to 93.8) | 100/113 | 88.5 (81.3 to 93.2) |
| Extra pulmonary TB | 156/175 | 89.1 (83.7 to 92.9) | 156/175 | 89.1 (83.7 to 92.9) | 152/174 | 87.4 (81.6 to 91.5) | 151/174 | 86.8 (80.9 to 91) |
| Specificity for a diagnosis of active TB | ||||||||
| Active TB excluded | 290/388 | 74.7 (70.2 to 78.8) | 296/387 | 76.5 (72.0 to 80.4) | 296/388 | 76.3 (71.8 to 80.3) | 302/387 | 78.0 (73.7 to 81.9) |
| Active TB excluded, TST negative, no risk factors for LTBI | 82/95 | 86.3 (78.0 to 91.8) | 84/94 | 89.4 (81.5 to 94.1) | 84/95 | 88.4 (80.4 to 93.4) | 86/94 | 91.5 (84.1 to 95.6) |
| Predictive values | ||||||||
| PPV | 295/393 | 75.1 (70.6 to 79.1) | 295/386 | 76.4 (71.9 to 80.4) | 291/383 | 76.0 (71.5 to 80.0) | 289/374 | 77.3 (72.8 to 81.2) |
| NPV | 290/323 | 89.8 (86.0 to 92.6) | 296/329 | 90.0 (86.3 to 92.8) | 296/332 | 89.2 (85.4 to 92.1) | 302/340 | 88.8 (85.0 to 91.8) |
| Likelihood ratios | ||||||||
| Positive likelihood ratio | 3.56 (2.99 to 4.24) | 3.83 (3.18 to 4.59) | 3.75 (3.13 to 4.51) | 4.02 (3.32 to 4.88) | ||||
| Negative likelihood ratio | 0.14 (0.10 to 0.19) | 0.13 (0.10 to 0.18) | 0.14 (0.11 to 0.20) | 0.15 (0.11 to 0.20) | ||||
Sensitivity, specificity and predictive values are presented as percentages. For both the four-antigen (ESAT-6, CFP-10, Rv3615c and Rv3879c) and three-antigen (ESAT, CFP-10, Rv3615), there were 74 test positives out of 94 highly probable TB cases, with a sensitivity (95% CI) of 78.7% (69.4% to 85.8%). For the other three-antigen (CFP-10, Rv3615c, Rv3879c), there were 71 test positives out of 93 highly probable TB cases, with a sensitivity (95% CI) of 76.3% (66.8% to 83.8%). For the two-antigen combination of CFP-10 and Rv3615, there were 70 test positives out of 93 highly probable TB cases, with a sensitivity (95% CI) of 75.3% (65.6% to 82.9%). Note that in the primary analyses of combinations of next-generation IGRAs, borderline test results were included as test positives. Sensitivity analyses were performed with borderline IGRA results excluded and the results are shown in Appendix 10, Table 70. Indeterminate IGRA results were excluded from these analyses. See Appendix 10, Table 71, for the sensitivity analyses with indeterminates included as test positives.
Comparison of novel antigen combinations with T-SPOT.TB
The results of individual antigens classified against those of T-SPOT. TB show few discordant results between Rv3615c and T-SPOT. TB, unlike those of the other three novel antigens (Rv3873, Rv2654 and Rv3879c), which are not components of T-SPOT. TB (Table 24).
| T-SPOT.TB, n | ||||||
|---|---|---|---|---|---|---|
| IGRA result | Positive | Negative | Borderline | Indeterminate | Missing | Total |
| Rv3615c | ||||||
| Positive | 247 | 36 | 8 | 0 | 0 | 291 |
| Negative | 48 | 351 | 16 | 0 | 0 | 415 |
| Borderline | 18 | 13 | 8 | 2 | 0 | 41 |
| Indeterminate | 6 | 0 | 1 | 55 | 0 | 62 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
| Rv3873 | ||||||
| Positive | 95 | 4 | 1 | 1 | 0 | 101 |
| Negative | 187 | 393 | 30 | 0 | 0 | 610 |
| Borderline | 27 | 3 | 0 | 0 | 0 | 30 |
| Indeterminate | 10 | 0 | 2 | 56 | 0 | 68 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
| Rv2654 | ||||||
| Positive | 118 | 9 | 3 | 0 | 0 | 130 |
| Negative | 169 | 384 | 27 | 0 | 0 | 580 |
| Borderline | 21 | 7 | 1 | 0 | 0 | 29 |
| Indeterminate | 11 | 0 | 2 | 57 | 0 | 70 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
| Rv3879c | ||||||
| Positive | 109 | 6 | 3 | 1 | 0 | 119 |
| Negative | 170 | 390 | 28 | 0 | 0 | 588 |
| Borderline | 30 | 4 | 0 | 0 | 0 | 34 |
| Indeterminate | 10 | 0 | 2 | 56 | 0 | 68 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
| ESAT-6 | ||||||
| Positive | 247 | 0 | 0 | 0 | 0 | 247 |
| Negative | 46 | 400 | 19 | 0 | 0 | 465 |
| Borderline | 21 | 0 | 13 | 0 | 0 | 34 |
| Indeterminate | 5 | 0 | 1 | 57 | 0 | 63 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
| CFP-10 | ||||||
| Positive | 267 | 0 | 0 | 0 | 0 | 267 |
| Negative | 41 | 400 | 10 | 0 | 0 | 451 |
| Borderline | 8 | 0 | 22 | 0 | 0 | 30 |
| Indeterminate | 3 | 0 | 1 | 57 | 0 | 61 |
| Missing | 0 | 0 | 0 | 0 | 36 | 36 |
| Total | 319 | 400 | 33 | 57 | 36 | 845 |
Tables 25 and 26 show comparisons of the sensitivity and specificity of the different test combinations versus those of T-SPOT. TB. Indeterminate test results were excluded from these analyses (see Appendix 10, Tables 72 and 73 for the results of sensitivity analyses with indeterminates included). The sensitivity of T-SPOT. TB was lower than those of any of the four test combinations (see Table 25), but had higher specificity (see Table 26). The sensitivity of the two-antigen combination of CFP-10 and Rv3615c was 7% higher than that of T-SPOT. TB, with a relative sensitivity of 1.07 (95% CI 1.03 to 1.11). However, the specificity of the combined CFP-10 and Rv3615c was 5% lower than that of T-SPOT. TB, with a relative specificity of 0.95 (95% CI 0.92 to 0.98).
| Test | n a | Sensitivity, % (95% CI) | Relative sensitivityb (95% CI) | p-value |
|---|---|---|---|---|
| T-SPOT.TB (ESAT-6 + CFP-10) | 328 | 82.3 (77.7 to 86.1) | – | – |
| CFP-10 + Rv3615c | 327 | 88.4 (84.5 to 91.5) | 1.07 (1.03 to 1.12) | < 0.001 |
| CFP-10 + Rv3615c + Rv3879c | 327 | 89.0 (85.1 to 92.0) | 1.08 (1.04 to 1.12) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c | 328 | 89.9 (86.2 to 92.8) | 1.09 (1.06 to 1.13) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | 328 | 89.9 (86.2 to 92.8) | 1.09 (1.06 to 1.13) | < 0.001 |
| Test | n a | Specificity, % (95% CI) | Relative specificityb (95% CI) | p-value |
|---|---|---|---|---|
| T-SPOT.TB (ESAT-6 + CFP-10) | 386 | 82.5 (78.4 to 86.0) | – | – |
| CFP-10 + Rv3615c | 387 | 78.0 (73.6 to 81.9) | 0.95 (0.91 to 0.98) | 0.001 |
| CFP-10 + Rv3615c + Rv3879c | 388 | 76.3 (71.8 to 80.3) | 0.93 (0.89 to 0.96) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c | 387 | 76.5 (72.0 to 80.5) | 0.93 (0.90 to 0.96) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | 388 | 74.7 (70.2 to 78.8) | 0.91 (0.88 to 0.94) | < 0.001 |
Evaluation of the tuberculin skin test
The TST was not performed as part of the IDEA study. We did not aim to evaluate the accuracy of the TST and so we did not impose a standard protocol for the conduct of TSTs; each centre followed local trust policy. Five of the nine hospital trusts included in the analyses performed TSTs, but only on a subset of patients, ranging between 20% and 74% of those recruited. We do not know why certain patients were selected for the TST and others were not but the reasons may involve a mix of patient- and clinician-specific factors. Altogether, the TST results were available for only 38% of the study population. The TST was mainly performed at two centres (London North West Healthcare NHS Trust and Imperial College Healthcare NHS Trust), and these centres together accounted for 83% (266/322) of the available TST results. Furthermore, practice varied across the centres in the IDEA study.
Nonetheless, for completeness we estimated the diagnostic accuracy of the TST alone. In addition, because one of our primary objectives was to develop an evidence-based optimal testing algorithm that defines the role of IGRAs in the diagnostic workup of suspected active TB, we considered combinations with T-SPOT. TB or QFT-GIT. The results are presented in Appendix 13.
The sensitivity of the TST was evaluated at each of the three prespecified thresholds (≥ 5 mm, ≥ 10 mm and the stratified threshold). The findings presented in Appendix 13 should be interpreted cautiously given the limited number of data and variation in practice between centres. Moreover, the findings may be subject to bias. The final diagnosis of patients was verified using a composite reference standard applied by a panel of clinicians to anonymised patient clinical data. The panel were blinded to routine and study IGRA results. However, if TSTs were performed, then the results were available thus creating a potential for incorporation bias in the evaluation of the diagnostic accuracy of the TST.
Discussion
This large prospective study of the diagnostic accuracy of T-SPOT. TB, QFT-GIT, TSTs and second-generation IGRAs was conducted in secondary care in a population representative of clinical practice. The differences observed between inpatients and outpatients reflect the case mix of acute admissions, in which, for instance, pneumonia-like illnesses and advanced malignancy presentations are more common. However, the final TB diagnosis in these two groups was broadly similar and allows for generalisability of our findings. Figure 5 summarises the sensitivities of T-SPOT. TB, QFT-GIT, TSTs, and combinations of TSTs and IGRAs. Figure 5 also shows the sensitivities of combinations of novel antigens. T-SPOT. TB showed higher sensitivity than QFT-GIT, a relative increase of 22% (95% CI 14% to 31%), thus indicating greater utility as a rule-out test.
FIGURE 5.
Summary of sensitivities of IGRAs. The sensitivities of the six tests are sorted on the plot in increasing order.

Literature searches in PubMed were regularly conducted during the IDEA study to update the SSC on developments in the field. Appendix 11, Table 74, gives a summary of 31 IGRA studies and one systematic review published between 1 January 2013 and 16 March 2016. To the authors’ knowledge, the IDEA study is the largest prospective and most recent comparative evaluation of the accuracy of T-SPOT. TB and QFT-GIT for diagnosis of active TB in a high-income setting. None of the studies in Appendix 11 compared T-SPOT. TB and QFT-GIT either prospectively or retrospectively. QFT-GIT was compared with the TST in 14 (45.2%) studies and T-SPOT. TB was compared with the TST in four (12.9%) studies; the remaining 13 (41.9%) studies evaluated only T-SPOT. TB or QFT-GIT. According to the systematic review of pleural TB published in 2015,28 there was only one good-quality study out of the 21 studies and two small studies compared QFT-GIT and T-SPOT. TB. The authors of the review pooled results across all QuantiFERON and T-SPOT. TB assays and so results are not comparable with the findings of the IDEA study.
The rate of indeterminate IGRA results was higher for QFT-GIT (9.6%) than for T-SPOT. TB (7.0%). For both IGRAs, most of the indeterminate results were among patients without active TB, with 59% (47/79) and 65% (37/57) occurring for QFT-GIT and T-SPOT. TB. Appendix 12 (see Table 75) summarises some key characteristics of patients with indeterminate IGRA results. Indeterminates were excluded from the main analyses and the impact on findings was investigated in sensitivity analyses by including indeterminate IGRA results as test positives, that is, as true positives for those with culture-confirmed or highly probable active TB and as false positives for those without active TB. Based on this rule, an increase in sensitivities, a decrease in specificities and no change in NPVs was expected. Generally, small increases were seen in sensitivity accompanied by decreases in specificity.
Human immunodeficiency virus-positive patients were a key subgroup in which analyses of T-SPOT. TB and QFT-GIT were planned. However, the number of patients (n = 135) recruited in the main cohort was small. The IDEA study was extended to facilitate recruitment of additional patients. The results for the entire HIV cohort (i.e. including patients recruited during the extension period) are presented in Chapter 5. For the main study cohort, the sensitivities of T-SPOT. TB and QFT-GIT were lower in those with HIV co-infection than the HIV-negative subgroup. Similarly, the two IGRAs had lower sensitivities in those patients with diabetes mellitus than those without. Specificity was higher in HIV-positive patients than HIV-negative patients but was lower in those with diabetes mellitus than those without diabetes mellitus. Although there appeared to be differences in test performance between subgroups, there was no statistical evidence of an effect of HIV infection status or diabetes mellitus on relative test performance. The findings from these analyses should be taken with caution, as the number of test results in some subgroups was small. Subgroup analyses were not possible for the other two key subgroups: those with end-stage renal failure and those on immune suppressants.
The most promising novel antigen was RV3615c, with a sensitivity that was higher than that of the other five antigens, including CFP-10 and ESAT-6 (the two antigens that constitute T-SPOT. TB). Test combinations including Rv3615c showed higher sensitivity than T-SPOT. TB, with limited gains in sensitivity when more antigens were added to a combination. Thus, the two-antigen combination of CFP-10 and Rv3615c or the three-antigen combination of ESAT-6, CFP-10 and Rv3615c seem promising. The latter combination had a sensitivity and specificity of 90.5% (95% CI 86.9% to 93.2%) and 70.0% (95% CI 65.4% to 74.2%), compared with 83.2% (95% CI 78.9% to 86.8%) and 75.4% (95% CI 71.1% to 79.3%) for T-SPOT. TB. The added value of Rv3615c to T-SPOT. TB was a 9% (95% CI 5% to 12%) relative increase in sensitivity at the expense of specificity with a relative decrease of 7% (95% CI 4% to 10%). The incremental gain in sensitivity to 90% is likely to be clinically useful and warrants further investigation.
Chapter 5 Substudy of human immunodeficiency virus-positive participants
Recruitment of human immunodeficiency virus-positive patients
Following the closure of the main study to recruitment, two additional NHS trusts (King’s College Healthcare NHS Foundation Trust and Barts Health NHS Trust) were invited to participate in the study to facilitate the recruitment of HIV-positive patients. A total of 263 patients were recruited from 12 NHS trusts between 25 November 2011 and 19 December 2014. The number of patients recruited at each centre is shown in Table 27. Over half of the patients were recruited from two trusts: Royal Free London NHS Foundation Trust (29.3%) and Imperial College Healthcare NHS Trust (25.9%).
| Hospital trust | Patients recruited, n (%) |
|---|---|
| Imperial College Healthcare NHS Trust | 68 (25.9) |
| Heart of England NHS Foundation Trust | 7 (2.7) |
| Chelsea and Westminster Hospital NHS Foundation Trust | 49 (18.6) |
| Royal Free London NHS Foundation Trust | 77 (29.3) |
| St George’s University Hospitals NHS Foundation Trust | 6 (2.3) |
| Frimley Health NHS Foundation Trust | 2 (0.8) |
| University Hospitals of Leicester NHS Trust | 28 (10.7) |
| London North West Healthcare NHS Trust | 8 (3) |
| Oxford University Hospitals NHS Trust | 6 (2.3) |
| Sandwell and West Birmingham Hospitals NHS Trust | 1 (0.4) |
| King’s College Healthcare NHS Foundation Trust | 3 (1.1) |
| Barts Health NHS Trust | 8 (3) |
| Total | 263 (100) |
The flow of patients through the study is shown in Figure 6. Of the 263 HIV-positive patients recruited, 201 were included in the analyses. Reasons for exclusion are shown in Figure 6. Two patients from Frimley Health NHS Foundation Trust were excluded, as mentioned earlier in Chapter 3 (see Recruitment of participants into main study cohort).
FIGURE 6.
Study flow diagram of HIV-positive patients with suspected active TB. The final four boxes show the number of patients with available IGRA results. a, On advice from the Study Steering Committee, and following consultation between the study management group and data management groups, nine patients were excluded from analyses. Two patients recruited from Frimley Health NHS Foundation Trust were excluded because all patients recruited from that site had diagnoses of confirmed or highly probable TB (categories 1 and 2) due an error of implementation of recruitment criteria, i.e. the natural spectrum of patients with suspected TB was not being recruited. Two patients were, on review, considered by the expert diagnostic panel to be ineligible (before unblinding IGRA results) on the basis that they were being investigated for TB (due to an incidental abnormal chest X-ray, known contact with active TB, or screening for anti-TNF treatment), but did not present with symptoms or signs suggestive of TB. An additional five patients were excluded due to invalid consent forms.
The final diagnosis of four (2.0%) patients was clinically indeterminate (Figure 6 and Table 28). Among the remaining 197 patients, there were 32 (16.2%) cases of active TB and 165 (83.8%) cases of non-active TB. Of the 165 non-active TB cases, 68 (41.2%) were category 4D.
| Diagnostic category | Patients, n (%) |
|---|---|
| 1: Culture-confirmed TB | 18 (9.0) |
| 2: Highly probable TB | 14 (7.0) |
| 3: Clinically indeterminate | 4 (2.0) |
| 4: Active TB excluded | |
| 4A | 2 (1.0) |
| 4B | 2 (1.0) |
| 4C | 93 (46.3) |
| 4D | 68 (33.8) |
| Total | 201 (100) |
Baseline characteristics of the human immunodeficiency virus-positive cohort
The demographic characteristics of the 201 patients are given in Table 29. The median age of patients was 43 years (range 18–79 years) and the majority (67.7%) were male. A substantial number of HIV-positive patients were of black (45.3%) or white (37.8%) ethnicity. A total of 53 countries of birth were represented; countries with at least three participants are shown in Table 29. Many patients (97/201, 48.3%) were in paid employment.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Age (years), median (range) | 43 (26–62) | 40 (24–66) | 55 (30–62) | 44 (18–79) | 43 (18–79) |
| Male, n (%) | 13 (72.2) | 10 (71.4) | 2 (50.0) | 111 (67.3) | 136 (67.7) |
| Ethnic origin, n (%) | |||||
| Asian | 1 (5.6) | 2 (14.3) | 0 (0.0) | 3 (1.8) | 6 (3.0) |
| Black | 12 (66.7) | 7 (50.0) | 2 (50.0) | 70 (42.4) | 91 (45.3) |
| Hispanic | 0 (0.0) | 1 (7.1) | 0 (0.0) | 7 (4.2) | 8 (4.0) |
| Indian subcontinent | 3 (16.7) | 0 (0.0) | 1 (25.0) | 8 (4.8) | 12 (6.0) |
| Middle Eastern | 1 (5.6) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 3 (1.5) |
| Mixed | 0 (0.0) | 1 (7.1) | 0 (0.0) | 4 (2.4) | 5 (2.5) |
| White | 1 (5.6) | 3 (21.4) | 1 (25.0) | 71 (43.0) | 76 (37.8) |
| Country of birth, n (%) | |||||
| UK | 2 (11.1) | 1 (7.1) | 1 (25.0) | 57 (34.5) | 61 (30.3) |
| Zimbabwe | 1 (5.6) | 2 (14.3) | 0 (0.0) | 16 (9.7) | 19 (9.5) |
| Nigeria | 2 (11.1) | 0 (0.0) | 1 (25.0) | 6 (3.6) | 9 (4.5) |
| India | 2 (11.1) | 0 (0.0) | 1 (25.0) | 4 (2.4) | 7 (3.5) |
| Uganda | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (3.6) | 6 (3.0) |
| Ethiopia | 2 (11.1) | 2 (14.3) | 0 (0.0) | 1 (0.6) | 5 (2.5) |
| Ireland | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (3.0) | 5 (2.5) |
| Kenya | 0 (0.0) | 1 (7.1) | 0 (0.0) | 4 (2.4) | 5 (2.5) |
| Portugal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (3.0) | 5 (2.5) |
| South Africa | 0 (0.0) | 1 (7.1) | 0 (0.0) | 4 (2.4) | 5 (2.5) |
| Brazil | 0 (0.0) | 1 (7.1) | 0 (0.0) | 3 (1.8) | 4 (2.0) |
| Jamaica | 1 (5.6) | 1 (7.1) | 0 (0.0) | 2 (1.2) | 4 (2.0) |
| Poland | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (2.4) | 4 (2.0) |
| Angola | 1 (5.6) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 3 (1.5) |
| The Democratic Republic of the Congo | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.8) | 3 (1.5) |
| Malawi | 1 (5.6) | 1 (7.1) | 0 (0.0) | 1 (0.6) | 3 (1.5) |
| Sierra Leone | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.8) | 3 (1.5) |
| Sri Lanka | 1 (5.6) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 3 (1.5) |
| Thailand | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.8) | 3 (1.5) |
| Othera | 5 (27.8) | 4 (28.6) | 1 (25.0) | 32 (19.4) | 42 (20.9) |
| Years in the UK, median (range) | 9.6 (0.05–33.5) | 10.1 (1.0–37.1) | 8.8 (6.2–10.6) | 11.9 (0.2–60.3) | 11.6 (0.05–60.3) |
| Profession, n (%)b | |||||
| Paid employment | 9 (50.0) | 6 (42.9) | 1 (25.0) | 81 (49.1) | 97 (48.3) |
| Unpaid employment | 6 (33.3) | 3 (21.4) | 3 (75.0) | 61 (37.0) | 73 (36.3) |
| Student | 1 (5.6) | 0 | 0 | 8 (4.8) | 9 (4.5) |
| Health-care/laboratory worker | 1 (5.6) | 3 (21.4) | 0 | 9 (5.5) | 13 (6.5) |
| Social/prison worker | 1 (5.6) | 1 (7.1) | 0 | 3 (1.8) | 5 (2.5) |
| Sex worker | 0 | 1 (7.1) | 0 | 2 (1.2) | 3 (1.5) |
| Unknown | 0 | 0 | 0 | 1 (0.6) | 1 (0.5) |
The clinical characteristics of the patients are presented in Table 30. Of the 201 patients, 71 (35.3%) had no other comorbidities. There were 10 (5.0%) patients with pre-existing diabetes mellitus, four (2.0%) with chronic/end-stage renal failure and none was on immunosuppressive therapy. Vitamin D measurement was unknown for most (85.1%) patients (see Appendix 5, Table 55, for thresholds used to categorise vitamin D status). Table 30 shows CD4 (cluster of differentiation 4) count grouped into four categories. CD4 count was missing for eight patients. Most of the 193 patients had a CD4 count of ≥ 200 cells/µl (46.8%). The median CD4 count was 285 cells/µl (range 0–1228 cells/µl). The distribution of CD4 count in the cohort is shown in Figure 7.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Height (m), median (range) | 1.7 (1.4–1.8) | 1.8 (1.6–1.9) | 1.7 (1.6–1.8) | 1.7 (1.3–1.9) | 1.7 (1.3–1.9) |
| Weight (kg), median (range) | 69 (52–91) | 65 (42–116) | 62 (57–110) | 67 (43–112) | 67 (42–116) |
| BMI (kg/m2), median (range) | 24 (18–48) | 22 (16–40) | 21 (21–34) | 23 (12–39) | 23 (12–48) |
| BCG vaccinated, n (%) | 15 (83.3) | 12 (85.7) | 4 (100.0) | 136 (82.4) | 167 (83.1) |
| BCG scar visible, n (%) | |||||
| Yes | 13 (72.2) | 12 (85.7) | 2 (50.0) | 114 (69.1) | 141 (70.1) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (10.3) | 17 (8.5) |
| Unsure | 2 (11.1) | 0 (0.0) | 2 (50.0) | 17 (10.3) | 21 (10.4) |
| Missing | 3 (16.7) | 2 (14.3) | 0 (0.0) | 17 (10.3) | 22 (10.9) |
| Known TB contact, n (%) | 3 (16.7) | 2 (14.3) | 1 (25.0) | 19 (11.5) | 25 (12.4) |
| Other pre-existing conditions/comorbidities, n (%)a | |||||
| None | 9 (50.0) | 8 (57.1) | 2 (50.0) | 52 (31.5) | 71 (35.3) |
| Diabetes | 2 (11.1) | 0 (0.0) | 1 (25.0) | 7 (4.2) | 10 (5.0) |
| Hepatitis B | 4 (22.2) | 1 (7.1) | 0 (0.0) | 8 (4.8) | 13 (6.5) |
| Hepatitis C | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (6.1) | 10 (5.0) |
| Chronic/end-stage renal failure | 0 (0.0) | 0 (0.0) | 1 (25.0) | 3 (1.8) | 4 (2.0) |
| Cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (4.2) | 7 (3.5) |
| Organ transplantation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Asthma | 0 (0.0) | 2 (14.3) | 0 (0.0) | 12 (7.3) | 14 (7.0) |
| Other | 8 (44.4) | 4 (28.6) | 2 (50.0) | 101 (61.2) | 115 (57.2) |
| Medication, n (%)a | |||||
| None/missing | 4 (22.2) | 7 (50.0) | 0 (0.0) | 46 (27.9) | 57 (28.4) |
| Chemotherapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Corticosteroids ≥ 15 mg/day | 1 (5.6) | 1 (7.1) | 0 (0.0) | 14 (8.5) | 16 (8.0) |
| Corticosteroids < 15 mg/day | 0 (0.0) | 1 (7.1) | 0 (0.0) | 5 (3.0) | 6 (3.0) |
| Ciclosporin, tacrolimus or everolimus | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Methotrexate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Other | 18 (100.0) | 14 (100.0) | 4 (100.0) | 164 (99.4) | 200 (99.5) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Vitamin D deficiency, n (%) | |||||
| Deficient | 3 (16.7) | 1 (7.1) | 1 (25.0) | 9 (5.5) | 14 (7.0) |
| Insufficient | 1 (5.6) | 2 (14.3) | 0 (0.0) | 2 (1.2) | 5 (2.5) |
| Normal | 0 (0.0) | 1 (7.1) | 2 (50.0) | 7 (4.2) | 10 (5.0) |
| Not known | 14 (77.8) | 10 (71.4) | 1 (25.0) | 147 (89.1) | 172 (85.6) |
| CD4 count, n (%) | |||||
| < 50 cells/µl | 1 (5.6) | 4 (28.6) | 0 (0.0) | 48 (29.1) | 53 (26.4) |
| ≥ 50 cells/µl and < 100 cells/µl | 2 (5.6) | 0 (0.0) | 0 (0.0) | 15 (9.1) | 16 (8.0) |
| ≥ 100 cells/µl and < 200 cells/µl | 5 (27.8) | 3 (21.4) | 2 (50.0) | 20 (12.1) | 30 (14.9) |
| ≥ 200 cells/µl | 8 (44.4) | 6 (42.9) | 2 (50.0) | 78 (47.3) | 94 (46.8) |
| Missing | 3 (16.7) | 1 (7.14) | 0 (0.0) | 4 (2.4) | 8 (4.0) |
| CD4 count, cells/µl (range) | 293 (14–670) | 267 (0–669) | 370 (183–800) | 283 (0–1228) | 285 (0–1228) |
FIGURE 7.
Distribution of CD4 counts in HIV-positive substudy cohort.

Table 31 gives the social history of patients. Smoking history was missing for one patient; almost half (48.8%) of the patients had never smoked, whereas the remaining were current (31.3%) or ex-smokers (19.4%). Fourteen (7.0%) patients had a history of alcohol misuse and the history of recreational drug use was unknown for many (64.7%) patients. Few patients (6.0%) had a history of homelessness and 7.5% had a history of imprisonment.
| Characteristic | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Smoking history, n (%) | |||||
| Never smoked | 13 (72.2) | 7 (50.0) | 3 (75.0) | 75 (45.5) | 98 (48.8) |
| Ex-smoker | 3 (16.7) | 2 (14.3) | 0 (0.0) | 34 (20.6) | 39 (19.4) |
| Current smoker | 2 (11.1) | 5 (35.7) | 1 (25.0) | 55 (33.3) | 63 (31.3) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.5) |
| Pack years if current smoker, n (%) | |||||
| ≤ 10 years | 0 (0.0) | 1 (20.0) | 0 (0.0) | 9 (16.4) | 10 (15.9) |
| 11–20 years | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 2 (3.2) |
| 21–50 years | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.5) | 3 (4.8) |
| Unknown | 2 (100.0) | 4 (80.0) | 1 (100.0) | 41 (74.5) | 48 (76.2) |
| History of alcohol use, n (%) | |||||
| Non-drinker | 10 (55.6) | 6 (42.9) | 3 (75.0) | 67 (40.6) | 86 (42.8) |
| Ex-drinker | 1 (5.6) | 1 (7.1) | 0 (0.0) | 17 (10.3) | 19 (9.5) |
| Current drinker | 7 (38.9) | 7 (50.0) | 1 (25.0) | 79 (47.9) | 94 (46.8) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 2 (1.0) |
| Units/week if current drinker, median (range) | 5 (0–75) | 8 (0–19) | 10 (10–10) | 5 (0–126) | 5 (0–126) |
| History of alcohol misuse, n (%) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 13 (7.9) | 14 (7.0) |
| History of recreational drug use, n (%) | |||||
| Non-user | 6 (33.3) | 2 (14.3) | 2 (50.0) | 31 (18.8) | 41 (20.4) |
| Ex-user | 1 (5.6) | 0 (0.0) | 0 (0.0) | 7 (4.2) | 8 (4.0) |
| Current user | 0 (0.0) | 3 (21.4) | 0 (0.0) | 19 (11.5) | 22 (11.0) |
| Unknown | 11 (61.1) | 9 (64.3) | 2 (50.0) | 108 (65.5) | 130 (64.7) |
| History of homelessness, n (%) | 1 (5.6) | 1 (7.1) | 0 (0.0) | 10 (6.1) | 12 (6.0) |
| Years homeless if currently or previously homeless, median (range) | 0 (0.0) | 12 | – | 5 (0–12) | 5 (0–12) |
| History of imprisonment, n (%) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 14 (8.5) | 15 (7.5) |
The frequency of symptoms is shown in Table 32 based on data from 197 patients. The four other patients were recruited on the basis of abnormal clinical signs rather than symptoms. The main symptoms were cough, fever, night sweats, weight loss and lethargy. Patients generally presented with multiple symptoms; the median number of symptoms was three (range 1–10). Cough, as a symptom, was often present (68.5%) in patients.
| Symptoma | Dosanjh category | Total | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Cough, n (%) | 13 (72.2) | 4 (30.8) | 3 (75.0) | 115 (71.0) | 135 (68.5) |
| Fever, n (%) | 13 (72.2) | 8 (61.5) | 3 (75.0) | 85 (52.5) | 109 (55.3) |
| Night sweats, n (%) | 11 (61.1) | 9 (69.2) | 1 (25.0) | 86 (53.1) | 107 (54.3) |
| Weight loss, n (%) | 12 (66.7) | 11 (84.6) | 2 (50.0) | 89 (54.9) | 114 (57.9) |
| Haemoptysis, n (%) | 2 (11.1) | 1 (7.7) | 0 | 18 (11.1) | 21 (10.7) |
| Lethargy, n (%) | 7 (38.9) | 8 (61.5) | 1 (25.0) | 81 (50.0) | 97 (49.2) |
| Other, n (%) | 11 (61.1) | 5 (38.5) | 2 (50.0) | 79 (48.8) | 97 (49.2) |
| Number of symptoms, median (range) | 4 (1–8) | 4 (3–6) | 3.5 (1–5) | 3 (1–9) | 3 (1–10) |
Final diagnosis in human immunodeficiency virus-positive patients
The diagnostic tests performed during the diagnostic workup of patients are shown in Table 33. Chest radiography and culture were the most common tests performed (with each performed in 90.6% of patients). The number of T-SPOT. TB, QFT-GIT and TST tests performed as part of routine care, at each centre, is shown in Appendix 14 (see Table 78). These routine IGRA results were not used in the final diagnosis of patients in the IDEA study. TSTs were performed in only 12 patients at 3 of the 11 centres; TST results were available for all 12 patients.
| Test | Dosanjh category, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| BAL investigation | 5 (27.8) | 4 (28.6) | 3 (75.0) | 68 (41.2) | 80 (39.8) |
| CXR | 14 (77.8) | 14 (100.0) | 3 (75.0) | 151 (91.5) | 182 (90.5) |
| CSF investigation | 3 (16.7) | 6 (42.9) | 1 (25.0) | 17 (10.3) | 27 (13.4) |
| CT | 14 (77.8) | 12 (85.7) | 3 (75.0) | 122 (73.9) | 151 (75.1) |
| Culture | 18 (100.00) | 13 (92.9) | 3 (75.0) | 148 (89.7) | 182 (90.5) |
| Histology or biopsy | 3 (16.7) | 5 (35.7) | 0 (0.0) | 49 (29.7) | 57 (28.4) |
| MRI | 4 (22.2) | 9 (64.3) | 1 (25.0) | 28 (17.0) | 42 (20.9) |
| PCR | 10 (55.6) | 5 (35.7) | 1 (25.0) | 42 (25.5) | 58 (28.9) |
| Smear test | 14 (77.8) | 14 (100.0) | 2 (50.0) | 139 (84.2) | 169 (84.1) |
The final diagnoses of active TB patients are summarised in Table 34. Of the 32 patients with active TB, 19 (59.4%) had smear-negative TB. A total of 13 patients (40.6%) had pulmonary TB, 15 (46.9%) patients had extrapulmonary TB, and the remaining four (12.5%) patients had both forms of TB. The most common sites of infection were the lungs (53.1%) and lymph nodes (31.1%). Of the 31 culture tests performed, 20 (64.5%) had no drug resistance. Table 35 shows the final diagnosis of non-active TB patients. A patient may have multiple conditions, but pneumonia was the most frequent diagnosis (40.0%).
| Characteristic | Category of TB, n (%) | Total, n (%) | |
|---|---|---|---|
| 1 | 2 | ||
| All TB | 18 (56.3) | 14 (43.7) | 32 (100) |
| Smear-positive TB | 8 (44.4) | 1 (7.1) | 9 (28.1) |
| Smear-negative TB | 6 (33.3) | 13 (92.9) | 19 (59.4) |
| Pulmonary TB | 10 (55.6) | 3 (21.4) | 13 (40.6) |
| Extrapulmonary TB | 5 (27.8) | 10 (71.4) | 15 (46.9) |
| Pulmonary/extrapulmonary TB | 3 (16.7) | 1 (7.1) | 4 (12.5) |
| Site of infectiona | |||
| Brain | 0 (0.0) | 2 (14.3) | 2 (6.3) |
| Central nervous system | 0 (0.0) | 1 (7.1) | 1 (3.1) |
| Lung | 13 (72.2) | 4 (28.6) | 17 (53.1) |
| Lymph node | 4 (22.2) | 6 (42.9) | 10 (31.3) |
| Miliary TB (disseminated) | 3 (16.7) | 0 (0.0) | 3 (9.4) |
| Pericardium | 0 (0.0) | 1 (7.1) | 1 (3.1) |
| Pleura | 1 (5.6) | 0 (0.0) | 1 (3.1) |
| Spine | 1 (5.6) | 1 (7.1) | 2 (6.3) |
| Multidrug resistanceb | |||
| None | 18 (100) | 2 (15.4) | 20 (64.5) |
| Not tested | 0 (0.0) | 11 (84.6) | 11 (35.5) |
| Diagnosis | Number of patients (%) |
|---|---|
| Cancer | 17 (10.3) |
| Chest infection | 2 (1.2) |
| LRTI | 13 (7.9) |
| LTBI – treatment indicated | 1 (0.6) |
| Pneumonia | 66 (40.0) |
| Sarcoidosis | 3 (1.8) |
| Other | 75 (45.5) |
Test results for human immunodeficiency virus-positive patients
Of the 201 patients, 194 (96.5%) had results for both T-SPOT. TB and QFT-GIT; reasons for missing T-SPOT. TB and QFT-GIT results are shown in Table 36. The cross-classified results of the two tests are given in Appendix 14, Table 79. Table 37 shows the results for T-SPOT. TB, QFT-GIT and the TST according to the four diagnostic categories. Table 38 shows the cross-classification of the two tests for those with (categories 1 and 2) and without active TB (category 4).
| Reason | Test, n | |
|---|---|---|
| QFT-GIT | T-SPOT.TB | |
| No sample could be taken | 1 | – |
| Sample destroyed for laboratory reasons | – | 2 |
| Sample unsuitable for testing | – | 4 |
| Total | 1 | 6 |
| Index test | Dosanjh category | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| T-SPOT.TB | |||||||||
| Positive | 10 | 6 | 1 | 0 | 1 | 8 | 3 | 12 | 29 |
| Negative | 5 | 3 | 2 | 1 | 1 | 57 | 42 | 101 | 111 |
| Borderline | 0 | 1 | 1 | 0 | 0 | 6 | 2 | 8 | 10 |
| Indeterminate | 3 | 3 | 0 | 1 | 0 | 19 | 19 | 39 | 45 |
| Missing | 0 | 1 | 0 | 0 | 0 | 3 | 2 | 5 | 6 |
| Total | 18 | 14 | 4 | 2 | 2 | 93 | 68 | 165 | 201 |
| Median SFCs ESAT-6 (range) | 17 (0–462) | 8 (0–395) | 1 (0–3) | 0 | 0 | 0 (0–66) | 0 (0–74) | 0 (0–74) | 0 (0–462) |
| Median SFCs CFP-10 (range) | 6 (0–136) | 0 (0–315) | 3 (0–20) | 0 | 8 (0–16) | 0 (0–67) | 0 (0–56) | 0 (0–67) | 0 (0–315) |
| QFT-GIT | |||||||||
| Positive | 11 | 6 | 1 | 0 | 1 | 8 | 2 | 11 | 29 |
| Negative | 7 | 6 | 2 | 1 | 1 | 61 | 54 | 117 | 132 |
| Indeterminate | 0 | 2 | 1 | 1 | 0 | 24 | 11 | 36 | 39 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Total | 18 | 14 | 4 | 2 | 2 | 93 | 68 | 165 | 201 |
| Median IFN-γ levels (range) | 0.76 (0–3.78) | 0.03 (0–10) | 0.01 (0–0.54) | 0.04 (0–0.07) | 1.33 (0–2.65) | 0 (0–7.3) | 0 (0–1.56) | 0 (0–7.3) | 0.01 (0–10) |
| T-SPOT.TB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB positive (categories 1 and 2) | TB negative (category 4) | ||||||||||||
| Positive | Negative | Borderline | Indeterminate | Missing | Total | Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT | Positive | 14 | 1 | 0 | 2 | 0 | 17 | 4 | 3 | 1 | 3 | 0 | 11 |
| Negative | 1 | 6 | 1 | 4 | 1 | 13 | 5 | 75 | 7 | 26 | 4 | 117 | |
| Indeterminate | 1 | 1 | 0 | 0 | 0 | 2 | 3 | 22 | 0 | 10 | 1 | 36 | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Total | 16 | 8 | 1 | 6 | 1 | 32 | 12 | 101 | 8 | 39 | 5 | 165 | |
Diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
The proportion of indeterminate test results was 23.1% (45/195) for T-SPOT. TB and 19.5% (39/200) for QFT-GIT. The difference between the two proportions was 3.6% (95% CI –4.5% to 11.6%; p = 0.4). Sensitivity was 68.0% (95% CI 48.4% to 82.8%) for T-SPOT. TB and 56.7% (95% CI 39.2% to 72.6%) for QFT-GIT (Table 39). The specificities were 83.5% (95% CI 75.8% to 89.0%) and 91.4% (95% CI 85.3% to 95.1%). The PPVs for T-SPOT. TB and QFT-GIT were 46.0% (95% CI 31.0% to 61.6%) and 60.7% (95% CI 42.4% to 76.4%), respectively, and the NPVs were 92.7% (95% CI 86.2% to 96.2%) and 90.0% (95% CI 83.6% to 94.1%), respectively.
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 17/25 | 68.0 (48.4 to 82.8) | 17/30 | 56.7 (39.2 to 72.6) |
| Culture-positive TB | 10/15 | 66.7 (41.7 to 84.8) | 11/18 | 61.1 (38.6 to 79.7) |
| Culture-negative TB | 7/10 | 70.0 (39.7 to 89.2) | 6/11 | 54.5 (28.0 to 78.7) |
| Smear-positive TB | 4/8 | 50.0 (21.5 to 78.5) | 6/9 | 66.7 (35.4 to 87.9) |
| Smear-negative TB | 9/13 | 69.2 (42.4 to 87.3) | 8/17 | 47.1 (26.2 to 69.0) |
| Pulmonary TB | 7/10 | 70.0 (39.7 to 89.2) | 9/13 | 69.2 (42.4 to 87.3) |
| Extrapulmonary TB | 8/11 | 72.7 (43.4 to 90.3) | 6/14 | 42.9 (21.4 to 67.4) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 101/121 | 83.5 (75.8 to 89.0) | 117/128 | 91.4 (85.3 to 95.1) |
| Active TB excluded, TST negative, no risk factors for LTBI | 42/47 | 89.4 (77.4 to 95.4) | 54/56 | 96.4 (87.9 to 99.0) |
| Predictive value | ||||
| PPV | 17/37 | 46.0 (31.0 to 61.6) | 17/28 | 60.7 (42.4 to 76.4) |
| NPV | 101/109 | 92.7 (86.2 to 96.2) | 117/130 | 90.0 (83.6 to 94.1) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 4.11 (2.54 to 6.66) | – | 6.59 (3.46 to 12.6) |
| Negative likelihood ratio | – | 0.38 (0.22 to 0.68) | – | 0.47 (0.31 to 0.72) |
Using only culture-confirmed active TB cases, the sensitivity of T-SPOT. TB decreased slightly to 66.7% (95% CI 41.7% to 84.8%), whereas that of QFT-GIT increased to 61.1% (95% CI 38.6% to 79.7%). When the analyses were restricted to category 4D patients without active TB, specificities were higher than using all patients without active TB (see Table 39). In sensitivity analyses with indeterminate test results included as test positives, the sensitivity of T-SPOT. TB was 74.2% (95% CI 56.8% to 86.3%) and 59.4% (95% CI 42.3% to 74.5%) for QFT-GIT. The specificity of T-SPOT. TB was 63.1% (95% CI 55.4% to 70.2%) and 71.3% (95% CI 64.0% to 77.7%) for QFT-GIT. See Appendix 14, Table 80, for full results.
The diagnostic performance of the two IGRAs is shown stratified by CD4 count in Table 40. The estimates within each stratum give results comparable to the entire cohort in terms of the higher sensitivity of T-SPOT. TB and higher specificity of QFT-GIT. However, the estimates are based on very small numbers of active TB and non-active TB cases and are presented solely for illustrative purposes.
| CD4 count | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| < 50 cells/µl | 1/3 | 33.3 (6.15 to 79.2) | 1/4 | 25.0 (4.56 to 70.0) |
| ≥ 50 cells/µl and < 100 cells/µl | 0/1 | 0.00 (0.00 to 79.4) | 0/1 | 0.00 (0.00 to 79.4) |
| ≥ 100 cells/µl and < 200 cells/µl | 5/6 | 83.3 (43.7 to 97.0) | 4/7 | 57.1 (25.1 to 84.2) |
| ≥ 200 cells/µl | 10/12 | 83.3 (55.2 to 95.3) | 11/14 | 78.6 (52.4 to 92.4) |
| Specificity for a diagnosis of active TB | ||||
| < 50 cells/µl | 26/29 | 89.7 (73.6 to 96.4) | 26/29 | 89.7 (73.6 to 96.4) |
| ≥ 50 cells/µl and < 100 cells/µl | 11/13 | 84.6 (57.8 to 95.7) | 11/12 | 91.7 (64.6 to 98.5) |
| ≥ 100 cells/µl and < 200 cells/µl | 10/13 | 76.9 (49.7 to 91.8) | 14/15 | 93.3 (70.2 to 98.8) |
| ≥ 200 cells/µl | 53/64 | 83.1 (72.2 to 90.3) | 63/69 | 91.3 (82.3 to 96.0) |
Comparison of diagnostic accuracy of T-SPOT.TB and QuantiFERON GOLD In-Tube in human immunodeficiency virus-positive cohort
Excluding indeterminate IGRA results, there were 146 T-SPOT. TB results and 158 QFT-GIT results. The sensitivity of T-SPOT. TB was higher than that of QFT-GIT, with a relative sensitivity of 1.12 (95% CI 0.87 to 1.44). There was no statistical evidence of a difference (p = 0.4). In contrast, the specificity of T-SPOT. TB was significantly lower than that of QFT-GIT, with a relative specificity of 0.91 (95% CI 0.84 to 0.99) (Table 41). When indeterminate IGRA results were included as test positives in a sensitivity analysis, the analysis included 191 T-SPOT. TB results and 196 QFT-GIT results. Unlike the primary analysis, there was no statistical evidence of a difference in specificity (see Appendix 14, Table 81).
| Test | Number of test resultsa | Sensitivity (95% CI) | Number of test resultsb | Specificity (95% CI) |
|---|---|---|---|---|
| T-SPOT.TB | 25 | 62.8 (44.1 to 78.3) | 121 | 83.4 (75.7 to 88.9) |
| QFT-GIT | 30 | 56.1 (38.3 to 72.4) | 128 | 91.7 (85.4 to 95.4) |
| Ratio (95% CI);c p-value | – | 1.12 (0.87 to 1.44); 0.4 | – | 0.91 (0.84 to 0.99); 0.02 |
Discussion
Of the 263 patients recruited, 201 patients were included in the analyses. This is well below the target sample size of 390. Although T-SPOT. TB showed higher sensitivity than QFT-GIT, a relative increase of 12%, there was no statistical evidence of a difference in sensitivity. The sensitivity of QFT-GIT in the IDEA study was lower than that in other recent studies29–31 (see Appendix 11, Table 74).
The indeterminate rate was lower for QFT-GIT (19.5%) than for T-SPOT. TB (23.1%). The indeterminate results were mainly in those without active TB, with 92.3% (36/39) for QFT-GIT and 86.7% (39/45) for T-SPOT. TB. The impact of indeterminate results was explored in sensitivity analyses by including indeterminate results as test positives. There was a small increase in the sensitivity of QFT-GIT but a large increase in the sensitivity of T-SPOT. TB. This is because of the higher indeterminate rate for T-SPOT. TB (18.2%) among category 1 and 2 active TB cases than for QFT-GIT (5.9%).
Chapter 6 Economic evaluation methods
In this chapter we present methods for assessment of the cost-effectiveness of IGRAs as rule-out tests for active TB. That is, we consider using an IGRA as an initial test, with a negative result indicating that a patient does not have TB, thus accelerating diagnosis of the actual cause of disease in such patients. The use of QFT-GIT and T-SPOT. TB was compared against current practice, as determined by analysis of patient records. We considered which diagnostic tests were performed, their costs and the time taken between decision points involving each test. The time taken to diagnose or rule out TB is a key consideration. Our report adheres to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 32
Decision tree model
We developed a decision tree model to calculate the incremental costs and incremental health utilities (quality-adjusted life-year; QALYs) of changing from current practice to using an IGRA as an initial rule-out test. Current practice was determined by the analysis of patient records. The model structure representing current practice is shown in Figure 7. Adding a rule-out test to the diagnostic pathway introduces additional delay in the diagnosis of active TB in those patients who have the disease, as it introduces an additional step in the pathway. Patients who were not initially diagnosed with active TB have a follow-up consultation after approximately 2 months; those who had a false-negative rule-out test result, that is, they had TB incorrectly ruled out, can have TB identified at this point. The final diagnostic outcomes were the four categories described in Dosanjh et al. ,3 herein referred to as ‘Dosanjh categories’ (see Appendix 2, Table 52). The health economic analysis was undertaken from a NHS perspective. No discounting was required, as the diagnostic process occurs over a relatively short time period.
The model contained two levels of uncertainty.
-
Individual-level uncertainty: patient records revealed variation in the number and type of tests used for TB diagnosis and time to diagnosis.
-
Parameter uncertainty: uncertainty in the costs of tests and procedures, and the sensitivity and specificity of IGRAs.
A (balanced) bootstrap sample of TB and non-TB patients was created for each simulation of the decision tree, which retained the subsample sizes. Individual patient costs and time to diagnosis were jointly sampled to preserve the dependency between them. A Bayesian forward sampling approach generated values from all of these distributions to simulate a particular model outcome. This was then repeated in a Monte Carlo framework to obtain a sample of several thousand model runs that encapsulated the first-order (patient variability) and second-order (parameter) uncertainty in the model outputs. Table 42 summarises the model parameters. The model was implemented in the statistical programming language R (version R-3.3.0; R Foundation for Statistical Computing, Vienna, Austria).
| Parameter | Symbol | Main model values | Sensitivity range |
|---|---|---|---|
| Rule-out test unit cost (£) | C ruleout | T-SPOT.TB or QFT-GIT | 1–200 |
| Follow-up time for those not diagnosed with TB (days) | T FN | Direct estimate from the IDEA study clinical data set | 54–127a |
| Cohort active TB prevalence | n+/n | Direct estimate from the IDEA study clinical data set | 0.1–0.5 |
| Rule-out test time (days) | T ruleout | Uniform(2,14) | |
| Quality-of-life detriment for active TB symptoms | D QoL | Triangle(0.11,0.21,0.31)33,34 | |
| Current time to diagnosis by TB status | t¯i | Direct estimate from the IDEA study clinical data set | From the IDEA study data set |
| Current combined cost of diagnosis by TB status | c¯i | Direct estimate from the IDEA study clinical data set | From the IDEA study data set |
Distributional formulation of individual-level/sample uncertainty
The number of individuals who enter the diagnostic pathway was defined as n. Of these, we defined n+ as active TB cases and the remainder as non-active TB cases. We defined the probability of a given patient being an active TB case as n+/n and so the probability of being a non-active TB case is 1 – (n+/n). This can therefore be considered a draw from a Bernoulli distribution and so for the total sample the binomial distribution gives the number of TB cases in a sample population as:
that is,
By the same principle, patients are then randomly split between those who are ruled out and those who are not ruled out from the active TB and non-active TB subgroups:
This process results in a final random subdivision of the sample population into one of the end states.
Health economics outcomes
Each of the terminal nodes (outcomes) of the decision tree has an associated cost and health utility (measured in QALYs). Incremental QALY differences are due to differences in the time taken to start appropriate treatment, leading to differences in morbidity. For simplicity of notation, we shall denote 1 – DQoL by q. For the patient cohort these were defined as:
Estimation of costs used in the model
The costs and distributions used in the sensitivity analyses are summarised in Tables 43–45, using 2014/15 prices. When necessary, costs were inflated from previous years using the Hospital and Community Health Service pay and price index. 37
| Consultation type | Cost (£) | Distribution | Sources |
|---|---|---|---|
| First visit: respiratory medicine, multiprofessional | 241 | Gamma(53.3,4.52) | National Tariff Payment System 2014/15. Annex 5A35 |
| 167 (SE 33) | Hughes et al., 201236 | ||
| Follow-up visit: respiratory medicine, multiprofessional | 143 | Gamma(18.78,7.62) | National Tariff Payment System 2014/15. Annex 5A35 |
| 167 (SE 33) | Hughes et al., 201236 |
| Test | Unit cost (£) [min., max.] | Distribution | Sources |
|---|---|---|---|
| Culture | 22.29 (SE 2.23) | Gamma(100,0.22) | Drobniewski et al., 201538 |
| Sputum smear microscopy | 7 | Gamma(106,0.07) | NICE’s Tuberculosis: Prevention, Diagnosis, Management and Service Organisation. NICE Guideline 33, 201639 |
| 1.56 (SE 0.68) | Hughes et al., 201236 | ||
| TST | 17.48 | Auguste et al., 201640,41 | |
| 16 [8, 32] | Uniform(8,36) | NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| T-SPOT.TB | 59.57 | Sutcliffe, 201641 | |
| 55 [45, 99] | Uniform(50,106) | NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| QFT-GIT | 58 [29, 87] | Uniform(29,87) | Pareek et al., 201343 |
| CXR | 35 | NICE’s Tuberculosis: Prevention, Diagnosis, Management and Service Organisation. NICE Guideline 33, 201639 | |
| 28 [19, 34] | Uniform(23,43) | NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| Bronchoalveolar lavage | 23.24 | – | St Mary’s R&D office |
| [11.62, 46.48] | Uniform (11.62, 46.48) | Proportions from NICE’s Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33, 2006,44 Pareek et al., 201343 and NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| EBUS | 2634 | – | St Mary’s R&D office |
| Bronchoscopy procedure | 612 | – | St Mary’s R&D office |
| [306, 1224] | Uniform(306,1224) | Proportions from NICE’s Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33, 2006,44 Pareek et al., 201343 and NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| Histology from biopsy | 25 | – | St Mary’s R&D office |
| [12.5, 50] | Uniform(12.5,50) | Proportions from NICE’s Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33, 2006,44 Pareek et al., 201343 and NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| Needle aspirate | 90.21 | – | St Mary’s R&D office |
| [45.1, 180.42] | Uniform(45.1,180.42) | Proportions from NICE’s Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33, 2006,44 Pareek et al., 201343 and NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 | |
| PCR | 202.45 | – | St Mary’s R&D office |
| [101.2, 404.9] | Uniform(101.2,404.9) | Proportions from NICE’s Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33, 2006,44 Pareek et al., 201343 and NICE’s Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis, 201042 |
| Drug | Dosage (mg/day)a | Dosage by patient weight (mg/kg/day)a | Batch costb (£) | Quantity per batch (capsule or tablet)b | Dosage per capsule or tabletb | 60-day total cost (£) |
|---|---|---|---|---|---|---|
| Rifampicin | 600 | – | 48.00 | 100 | 300 | 57.60 |
| Isoniazid | 300 | – | 19.24 | 56 | 50 | 123.69 |
| Pyrazinamide | 2000 | – | 38.34 | 30 | 500 | 306.72 |
| Ethambutol hydrochloride | – | 15 | 42.74 | 56 | 400 | 116.74 |
When uncertainty bounds were not available in the recent sources used, uncertainty ranges were informed by previous studies. Uniform distributions were used when the upper and lower limits were available and gamma distributions were used when the standard error of the average cost was available.
When the lower, upper and mean values were available, the range between the lower and upper bound was defined by the proportional decrease and increase from the mean, respectively. These proportions were then used with an alternative, more appropriate, mean to calculate associated upper and lower limits. For example, a lower bound of half and an upper bound of twice the point estimate values were used. 42,43,46 Skewed distributions were represented using a gamma distribution.
As the end point of the analysis is diagnosis of TB or ruling out TB, treatment costs after final diagnosis are out of scope. However, when a patient was started on TB treatment and then a lack of response to that treatment informed a decision that the patient did not in fact have TB, the cost of this treatment was included, as it is part of the cost of ruling out TB for those patients.
Tests used in the diagnostic pathways are either specific to the diagnosis of active TB or, in the case of imaging tests [CT, MRI and positron emission tomography (PET)], can aid the diagnosis of multiple diseases. For patients who do not have TB, these imaging tests will be used to inform the ultimate diagnosis.
The treatment costs for those patients who have TB ruled out after starting treatment are given in Table 45. Following the NICE guidelines for active TB management,39 it was assumed that such patients are on treatment until their 2-month follow-up appointment, when they are reassessed for response to treatment. The regimen in this period is a daily treatment with rifampicin, isoniazid, pyrazinamide and ethambutol. The British National Formulary45 provides fixed dosages for adults, except for ethambutol hydrochloride, which is determined by patient weight. The mean weight at time of first presentation of 67.98 kg was used in the model. Table 46 summarises the sensitivity and specificity values previously given in Chapter 4, Comparison of diagnostic accuracy of T-SPOT. TB and QuantiFERON GOLD In-Tube and Tables 15 and 61 along with values for the beta PERT (project evaluation and review technique) distributions used for the probabilistic sensitivity analyses.
| Patient strata | Test | Indeterminate IGRA results | Sensitivity | 95% CI | Beta distribution, Beta (a, b) | Specificity | 95% CI | Beta distribution, Beta (a, b) |
|---|---|---|---|---|---|---|---|---|
| All | QFT-GIT | Excluded | 0.673 | 0.620 to 0.721 | (147,72) | 0.804 | 0.761 to 0.841 | (315,77) |
| All | T-SPOT.TB | Excluded | 0.823 | 0.778 to 0.861 | (299,64) | 0.826 | 0.786 to 0.861 | (293,60) |
| All | QFT-GIT | Included | 0.697 | 0.647 to 0.742 | (147,64) | 0.715 | 0.671 to 0.756 | (145,58) |
| All | T-SPOT.TB | Included | 0.832 | 0.789 to 0.868 | (290,59) | 0.754 | 0.711 to 0.793 | (348,112) |
| HIV positive | QFT-GIT | Excluded | 0.565 | 0.368 to 0.744 | (15,11) | 0.920 | 0.843 to 0.961 | (67,6) |
| HIV positive | T-SPOT.TB | Excluded | 0.632 | 0.410 to 0.809 | (14,8) | 0.899 | 0.813 to 0.948 | (74,7) |
| HIV positive | QFT-GIT | Included | 0.600 | 0.407 to 0.766 | (17,11) | 0.748 | 0.658 to 0.820 | (70,24) |
| HIV positive | T-SPOT.TB | Included | 0.708 | 0.508 to 0.851 | (18,7) | 0.664 | 0.570 to 0.746 | (73,36) |
| HIV negative | QFT-GIT | Excluded | 0.681 | 0.627 to 0.731 | (147,69) | 0.770 | 0.718 to 0.814 | (226,68) |
| HIV negative | T-SPOT.TB | Excluded | 0.835 | 0.790 to 0.872 | (287,57) | 0.808 | 0.760 to 0.848 | (250,59) |
| HIV negative | QFT-GIT | Included | 0.704 | 0.653 to 0.751 | (146,61) | 0.704 | 0.652 to 0.752 | (208,88) |
| HIV negative | T-SPOT.TB | Included | 0.841 | 0.797 to 0.877 | (280,53) | 0.785 | 0.736 to 0.827 | (264,72) |
Chapter 7 Economic evaluation results
Introduction
This chapter presents the results of the economic evaluation. First, a data analysis with a health economics focus is given. This demonstrates the patterns and variability in times and costs between patients and also indicates why a simple direct estimation of the relevant summary statistics is not appropriate. Second, the modelling results are given and, finally, the main base-case scenario results are presented.
Results
The idealised diagnostic pathway representing current practice is shown in Figure 8. Patient records were analysed to determine what proportion of patients followed the pathways in this diagram. For each Dosanjh category, the particular tests expected to be performed and their corresponding results were determined and then compared with patient records. Tables 47 and 48 present the frequencies of different tests performed, stratified by final diagnostic outcome. Importantly, the process of TB diagnosis rarely followed the idealised diagnostic pathways. Although culture, IGRAs (QFT-GIT and T-SPOT. TB), TSTs, sputum smear microscopy and chest radiography were frequently used, there was substantial variation between patients. These results are presented graphically in Figure 9, in which the number of patients who traverse each branch are indicated by the width of the branch. From the individual-level patient data, we used the empirical distributions of time to diagnosis and total test costs in the probabilistic sensitivity analysis in the following health economic evaluation.
FIGURE 8.
Idealised TB diagnostic pathway.
| Culture | Sputum smear | Chest radiography | Dosanjh category, n | Total, n | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Not performed | Not performed | Not performed | 0 | 0 | 3 | 13 | 16 |
| Not performed | Not performed | Indeterminate | 0 | 0 | 2 | 13 | 15 |
| Not performed | Not performed | Negative | 0 | 0 | 7 | 61 | 68 |
| Not performed | Not performed | Positive | 0 | 3 | 0 | 1 | 4 |
| Not performed | Negative | Not performed | 0 | 0 | 0 | 3 | 3 |
| Not performed | Negative | Indeterminate | 0 | 0 | 1 | 2 | 3 |
| Not performed | Negative | Negative | 0 | 0 | 1 | 6 | 7 |
| Not performed | Negative | Positive | 0 | 0 | 0 | 1 | 1 |
| Indeterminate | Not performed | Negative | 0 | 0 | 1 | 0 | 1 |
| Indeterminate | Negative | Negative | 0 | 0 | 0 | 1 | 1 |
| Indeterminate | Positive | Positive | 0 | 0 | 0 | 1 | 1 |
| Negative | Not performed | Not performed | 0 | 0 | 2 | 8 | 10 |
| Negative | Not performed | Indeterminate | 0 | 1 | 1 | 10 | 12 |
| Negative | Not performed | Negative | 0 | 1 | 3 | 18 | 22 |
| Negative | Not performed | Positive | 0 | 2 | 0 | 1 | 3 |
| Negative | Negative | Not performed | 0 | 2 | 2 | 34 | 38 |
| Negative | Negative | Indeterminate | 0 | 4 | 4 | 65 | 73 |
| Negative | Negative | Negative | 0 | 7 | 16 | 235 | 258 |
| Negative | Negative | Positive | 0 | 11 | 4 | 48 | 63 |
| Negative | Positive | Not performed | 0 | 0 | 0 | 2 | 2 |
| Negative | Positive | Indeterminate | 0 | 0 | 0 | 1 | 1 |
| Negative | Positive | Negative | 0 | 0 | 0 | 4 | 4 |
| Negative | Positive | Positive | 0 | 1 | 0 | 1 | 2 |
| Positive | Not performed | Not performed | 1 | 0 | 0 | 0 | 1 |
| Positive | Not performed | Indeterminate | 3 | 0 | 0 | 0 | 3 |
| Positive | Not performed | Positive | 4 | 0 | 0 | 0 | 4 |
| Positive | Negative | Not performed | 8 | 0 | 0 | 0 | 8 |
| Positive | Negative | Indeterminate | 17 | 0 | 0 | 0 | 17 |
| Positive | Negative | Negative | 14 | 0 | 0 | 0 | 14 |
| Positive | Negative | Positive | 57 | 0 | 0 | 0 | 57 |
| Positive | Positive | Not performed | 3 | 0 | 0 | 0 | 3 |
| Positive | Positive | Indeterminate | 5 | 0 | 0 | 0 | 5 |
| Positive | Positive | Negative | 2 | 0 | 0 | 0 | 2 |
| Positive | Positive | Positive | 47 | 0 | 0 | 0 | 47 |
| Total | 161 | 32 | 47 | 529 | 769 | ||
| Test/sampling method | Dosanjh category, n | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Culture | 161 (1.00) | 29 (0.91) | 33 (0.70) | 429 (0.81) |
| QFT-GIT | 15 (0.09) | 8 (0.25) | 9 (0.19) | 45 (0.09) |
| T-SPOT.TB | 29 (0.18) | 6 (0.19) | 19 (0.40) | 150 (0.28) |
| TST | 55 (0.34) | 10 (0.31) | 21 (0.45) | 164 (0.31) |
| Sputum smear microscopy | 153 (0.95) | 25 (0.78) | 28 (0.60) | 404 (0.76) |
| Bronchoalveolar lavage | 50 (0.31) | 14 (0.44) | 9 (0.19) | 135 (0.26) |
| Histology from biopsy | 23 (0.14) | 11 (0.34) | 14 (0.30) | 113 (0.21) |
| Needle aspirate | 28 (0.17) | 6 (0.19) | 10 (0.21) | 60 (0.11) |
| PCR | 70 (0.43) | 5 (0.16) | 7 (0.15) | 84 (0.16) |
| CXR | 148 (0.92) | 29 (0.91) | 40 (0.85) | 465 (0.88) |
| CT | 85 (0.53) | 22 (0.69) | 27 (0.57) | 323 (0.61) |
| MRI | 16 (0.10) | 3 (0.09) | 7 (0.15) | 57 (0.11) |
| PET | 1 (0.01) | 0 (0) | 5 (0.11) | 13 (0.02) |
FIGURE 9.
Representation of numbers of patients undergoing various TB tests, stratified by final diagnosis. (a) Culture-confirmed TB, Dosanjh category 1; (b) TB non-culture-confirmed, Dosanjh category 2; (c) Indeterminate, Dosanjh category 3; and (d) TB excluded, Dosnajh category 4. CXR, chest radiography. Patient numbers are shown on the branches. The frequencies of patients in each branch are proportional to the width of the branch. Upwards branches are for negative test results or if the decision was taken not to take a test (for chest radiography and culture) and the downwards branches are for positive results or a decision to test.



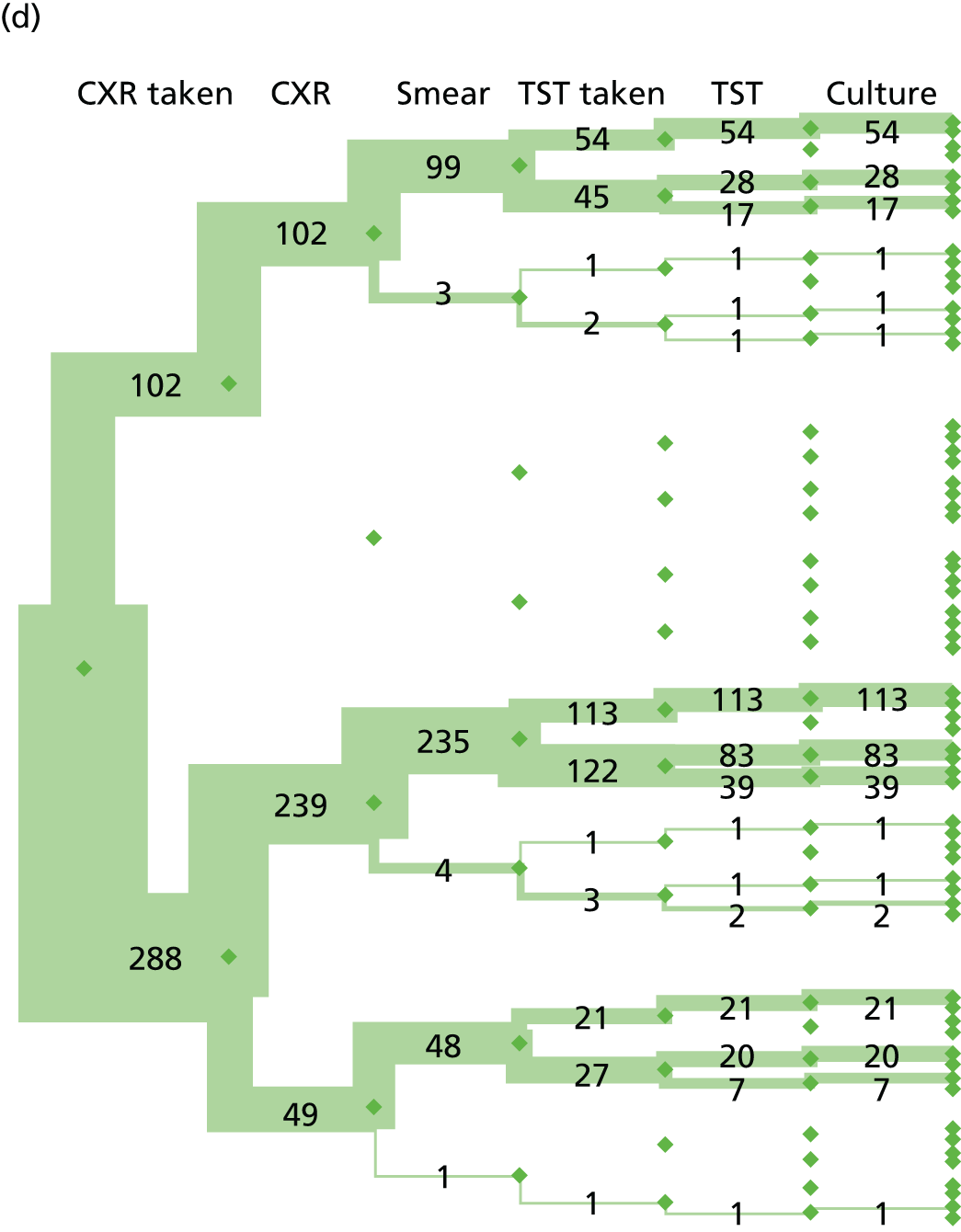
Table 49 gives distributional statistics of the cost of testing for each final diagnosis category. There are long right-hand tails, making single centrality summary statistics misleading.
| Dosanjh category | Diagnosis cost (£) | |||
|---|---|---|---|---|
| Lower quartile | Median | Mean | Upper quartile | |
| 1 | 292.31 (29.93) | 442.92 (17.64) | 469.77 (20.59) | 640.03 (30.78) |
| 2 | 383.73 (66.96) | 476.12 (29.6) | 474.73 (37.87) | 572.03 (53.04) |
| 3 | 254.08 (72.77) | 502.04 (49.58) | 535.48 (57.55) | 644.12 (83.71) |
| 4 | 202.00 (17.21) | 433.39 (7.69) | 445.61 (12.25) | 588.47 (17.75) |
The decision tree model structure is shown in Figure 10 and includes true-negative, false-negative, true-positive and false-positive rule-out test results. Patients receiving a positive rule-out test result (i.e. TB is not ruled out) then follow the standard diagnostic pathway, whereas patients receiving a negative rule-out test result are regarded as not having TB. If the rule-out test result is a false negative, then at the 2-month follow-up consultation the patient’s persistent symptoms leads to them entering the standard TB diagnostic pathway.
FIGURE 10.
Decision tree comparing current practice (‘no rule-out test’) with a diagnostic pathway incorporating an initial rule-out test (‘rule-out test’). PTB, pulmonary tuberculosis.
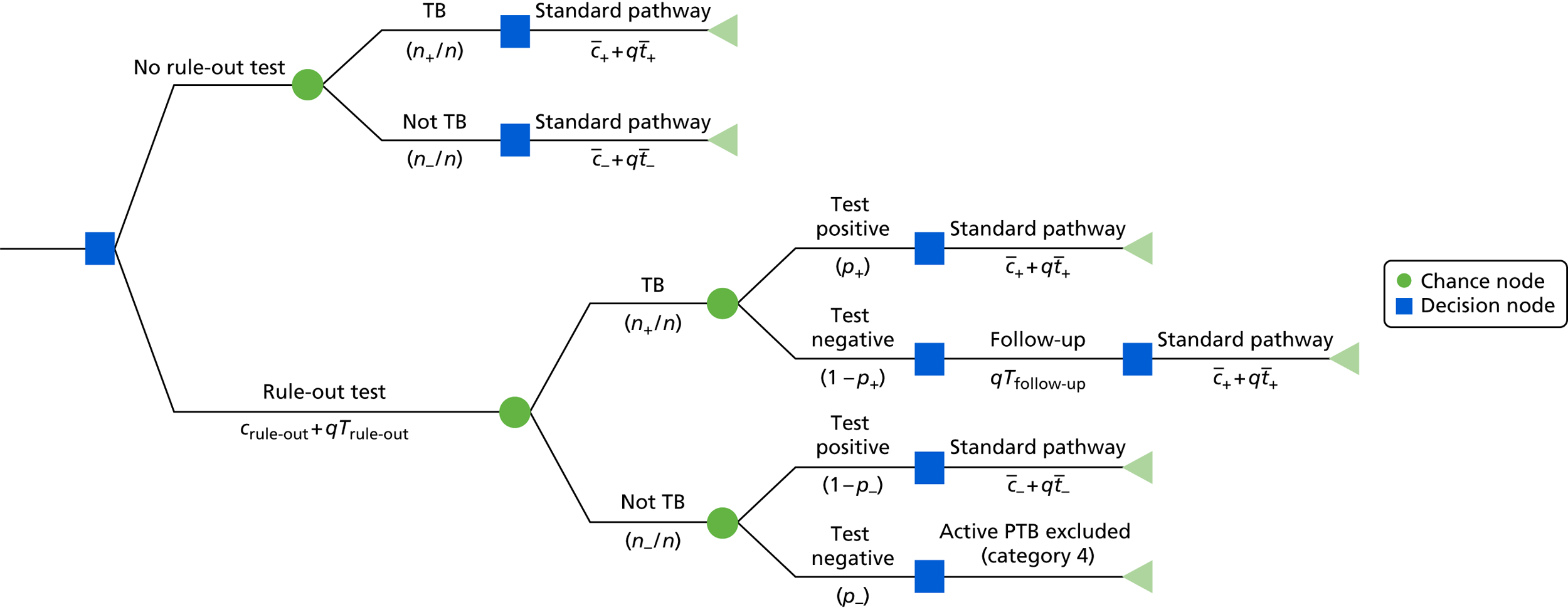
For patients who are TB negative and who receive a true negative in the rule-out test, the rule-out test results in a faster diagnosis of the true cause of their illness. However, for other patients, the rule-out test increases the delay in their ultimate diagnosis, both for those who have TB and those who do not.
The ‘standard pathway’ branch represents the range of variation observed in the patient cohort. Probabilities are shown below branches following a circular chance node and costs are below branches following a square decision node. The rule-out test is either T-SPOT. TB or QFT-GIT.
Results of the cost-effectiveness analysis are summarised in Table 50. Figure 11 shows cost-effectiveness planes for the use of T-SPOT. TB or QFT-GIT as rule-out tests, including three clouds of results for all patients, HIV-negative patients and HIV-positive patients. Figures 12 and 13 show the cost-effectiveness planes with uncertainty represented by ellipses showing the 95% and 50% ranges of uncertainty, respectively.
| Patient strata | Test | Indeterminate test results | Incremental QALYs | Incremental cost (£000) | ICER (× 103) | Probability cost-effective at £20,000 per QALY | Probability cost-effective at £30,000 per QALY |
|---|---|---|---|---|---|---|---|
| All | QFT-GIT | Excluded | –6.22 | –86.85 | 13.97 | 0.08 | 0.05 |
| All | T-SPOT.TB | Excluded | –3.58 | –78.81 | 22.01 | 0.26 | 0.22 |
| All | QFT-GIT | Included | –6.50 | –70.14 | 10.79 | 0.06 | 0.04 |
| All | T-SPOT.TB | Included | –4.14 | –65.12 | 15.73 | 0.21 | 0.17 |
| HIV positive | QFT-GIT | Excluded | –7.14 | –106.09 | 14.85 | 0.09 | 0.07 |
| HIV positive | T-SPOT.TB | Excluded | –5.91 | –86.87 | 14.70 | 0.12 | 0.08 |
| HIV positive | QFT-GIT | Included | –7.86 | –72.64 | 9.24 | 0.04 | 0.02 |
| HIV positive | T-SPOT.TB | Included | –6.34 | –42.11 | 6.64 | 0.04 | 0.03 |
| HIV negative | QFT-GIT | Excluded | –6.66 | –80.27 | 12.06 | 0.06 | 0.04 |
| HIV negative | T-SPOT.TB | Excluded | –3.52 | –75.31 | 21.40 | 0.26 | 0.21 |
| HIV negative | QFT-GIT | Included | –6.47 | –69.35 | 10.72 | 0.05 | 0.03 |
| HIV negative | T-SPOT.TB | Included | –3.57 | –71.45 | 20.04 | 0.24 | 0.19 |
FIGURE 11.
Cost-effectiveness planes comparing the use of T-SPOT. TB or QFT-GIT as rule-out tests with current practice. Results for (a) T-SPOT. TB with indeterminate diagnostic outcomes excluded; (b) T-SPOT. TB with indeterminate diagnostic outcomes included; (c) QFT-GIT with indeterminate diagnostic outcomes excluded; and (d) QFT-GIT with indeterminate diagnostic outcomes included. Statistics were calculated for the entire patient cohort. The upper panels show results for T-SPOT. TB, whereas the lower panels show results for QFT-GIT. Left- and right-hand panels present results with indeterminate diagnostic outcomes excluded and included, respectively. Note that an indeterminate IGRA result should not be confused with a diagnostic categorisation of a patient as being ‘clinically indeterminate’ with regard to having active TB (i.e. Dosanjh category 3). The analyses considered all patients (black circle), HIV-positive patients (green square) and HIV-negative patients (blue triangle), with each point showing the results of a single simulation result. The red points indicate the median values for each scenario. Diagonal lines indicate the cost-effectiveness thresholds of £20,000 per QALY (blue vertical line) and £30,000 per QALY (green vertical line). A total of 1000 simulations were run for each scenario.
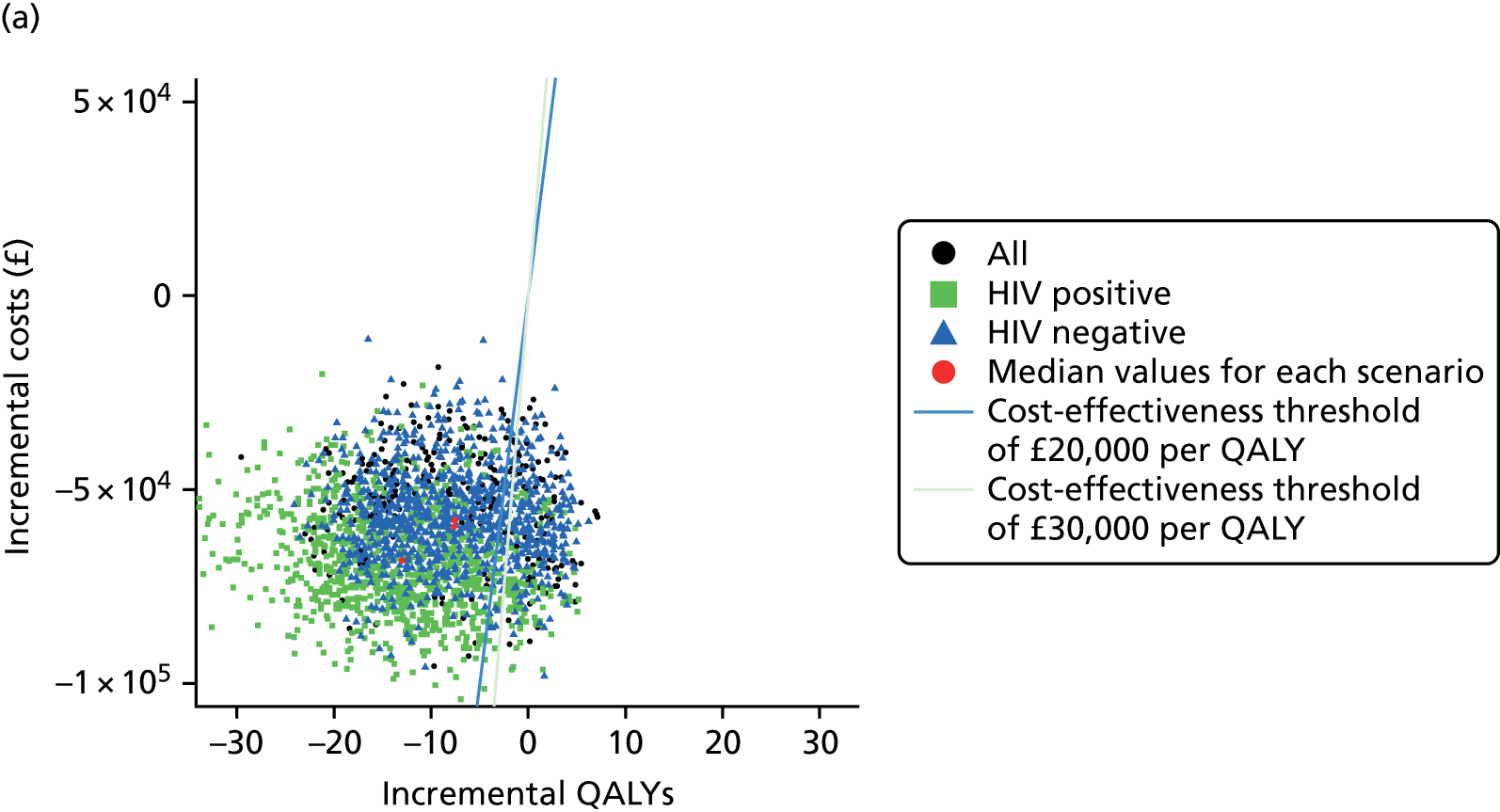
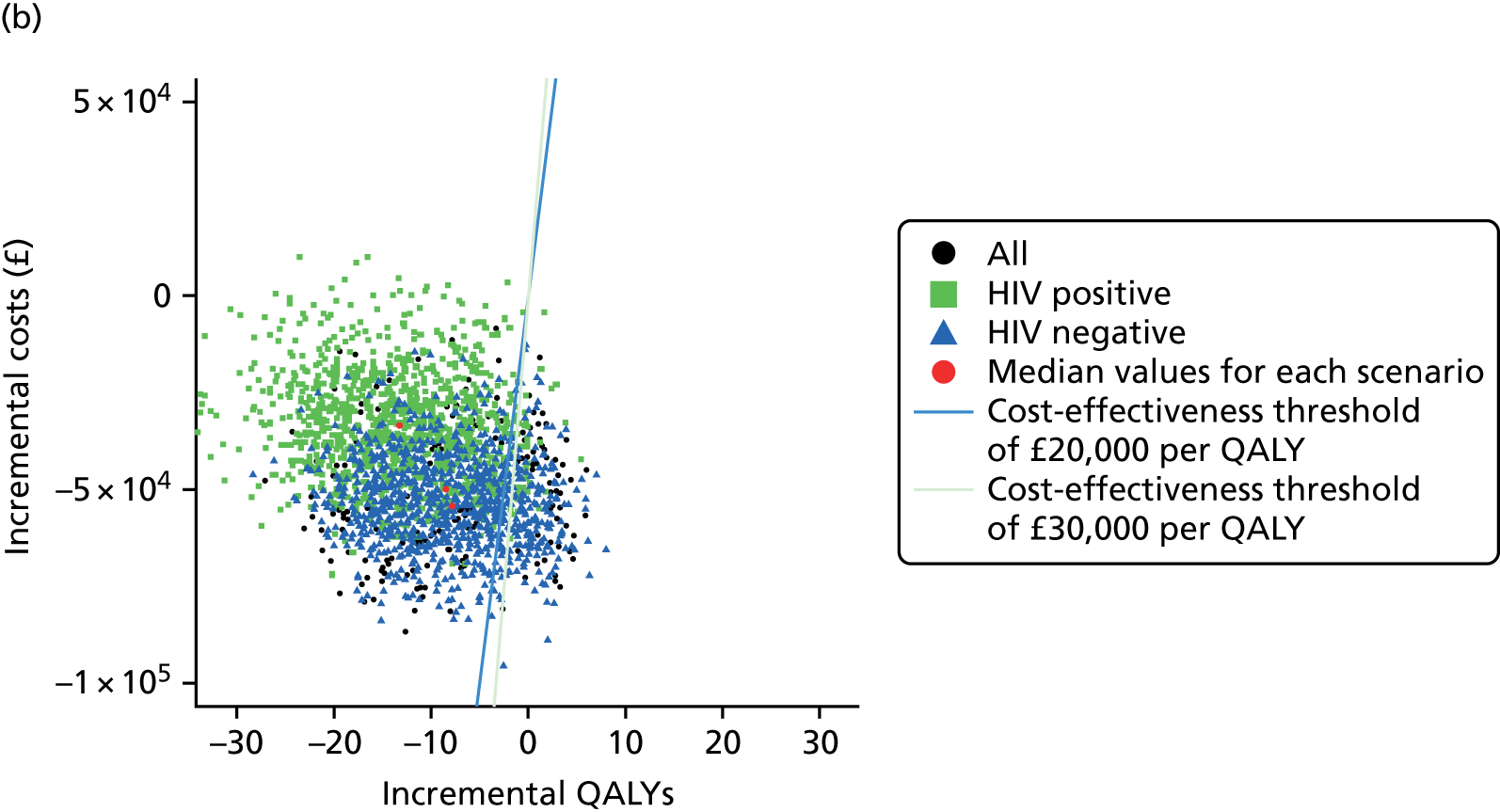
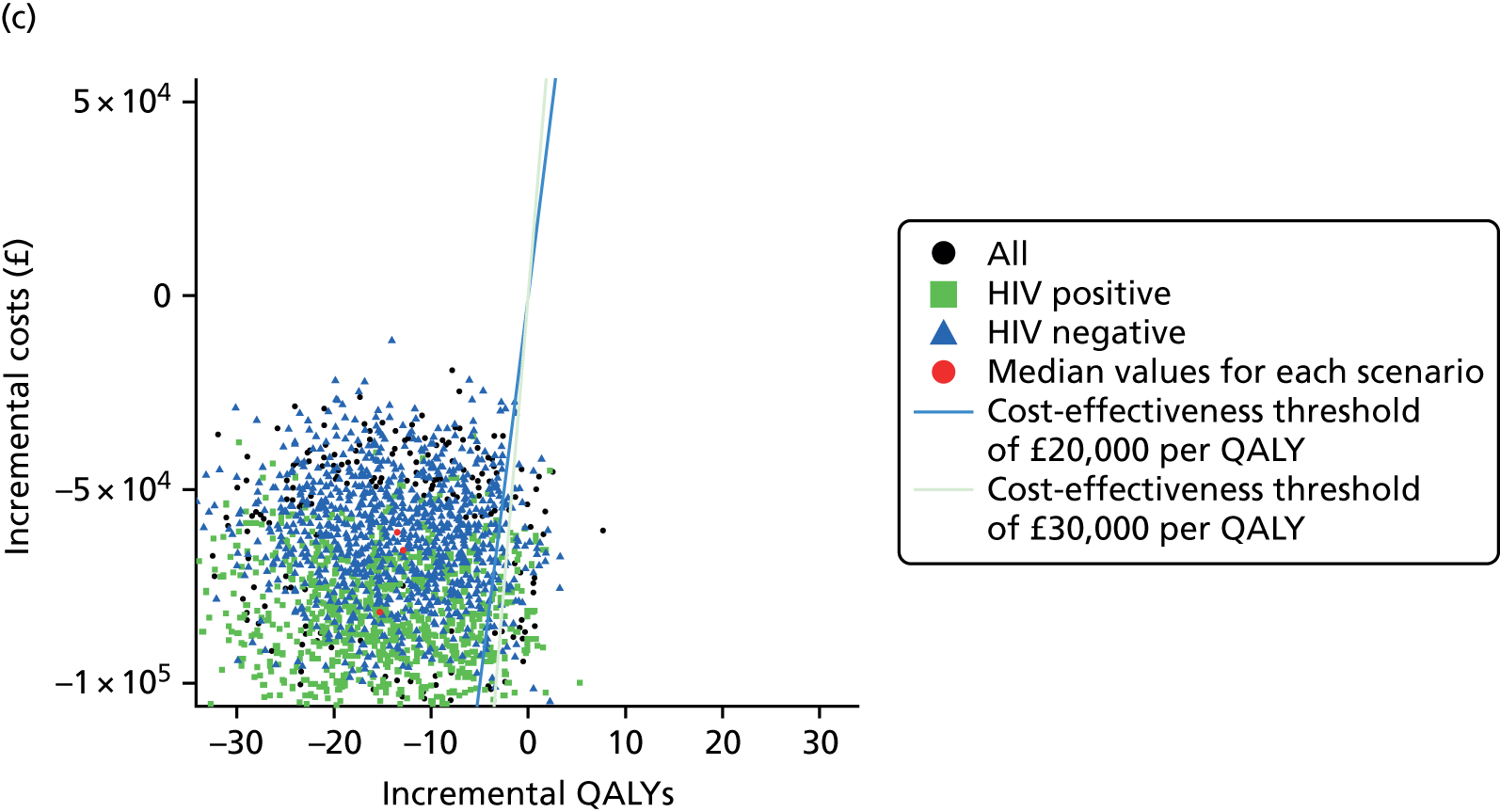
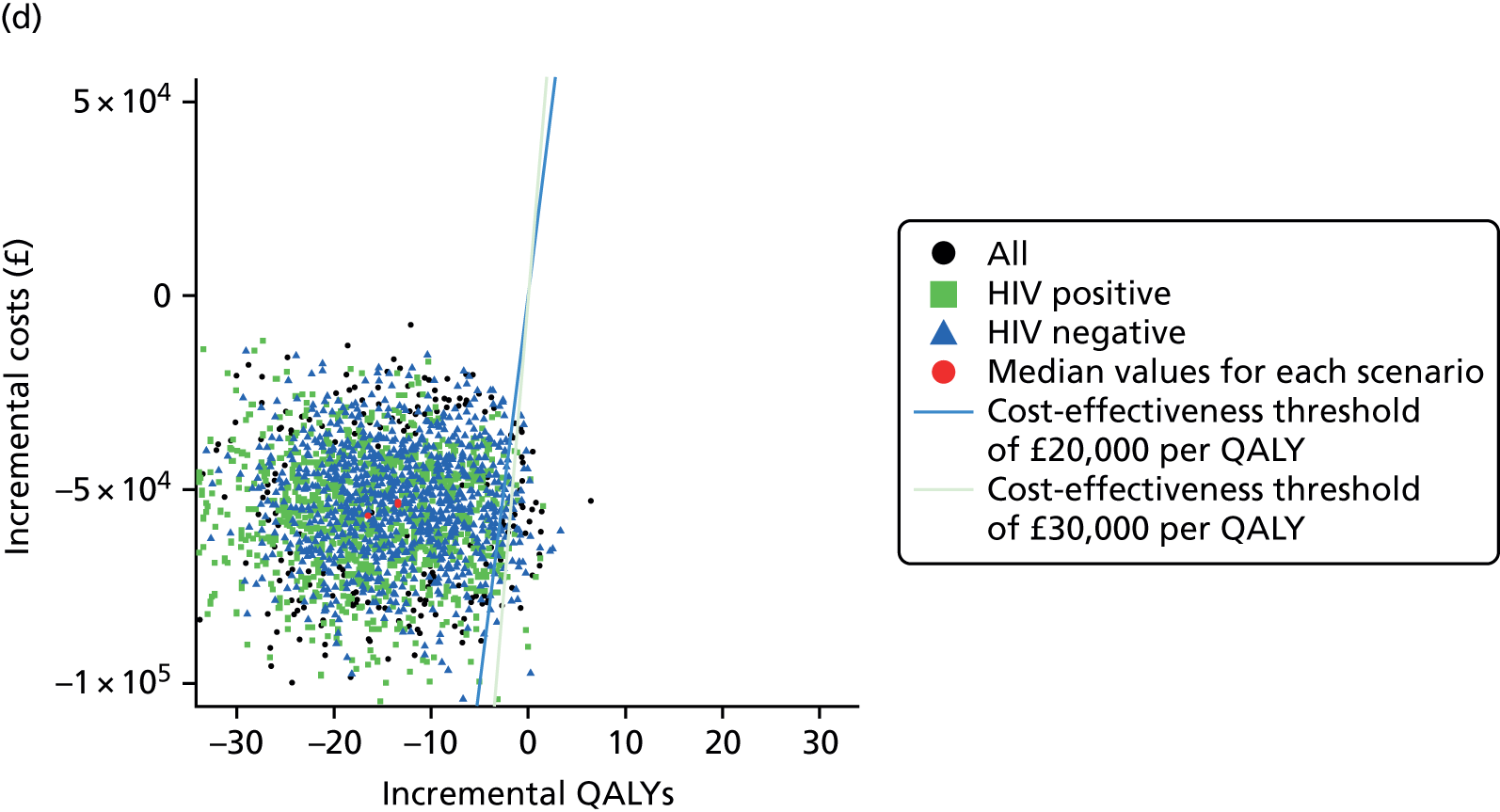
FIGURE 12.
Cost-effectiveness planes comparing the use of T-SPOT. TB or QFT-GIT as rule-out tests with current practice (95% density of simulation results). Results for (a) T-SPOT. TB with indeterminate diagnostic outcomes excluded; (b) T-SPOT. TB with indeterminate diagnostic outcomes included; (c) QFT-GIT with indeterminate diagnostic outcomes excluded; and (d) QFT-GIT with indeterminate diagnostic outcomes included. Statistics were calculated for the entire patient cohort. The upper panels show results for T-SPOT. TB, whereas the lower panels show results for QFT-GIT. Left- and right-hand panels present results with indeterminate diagnostic outcomes excluded and included, respectively. Ellipses show 95% density of simulation results. The analyses considered all patients (black lines), HIV-positive patients (green dashed lines) and HIV-negative patients (blue dotted lines). The green dots indicate the median values for each scenario. Diagonal lines indicate the cost-effectiveness thresholds of £20,000 per QALY (blue vertical line) and £30,000 per QALY (green vertical line). A total of 1000 simulations were run for each scenario.



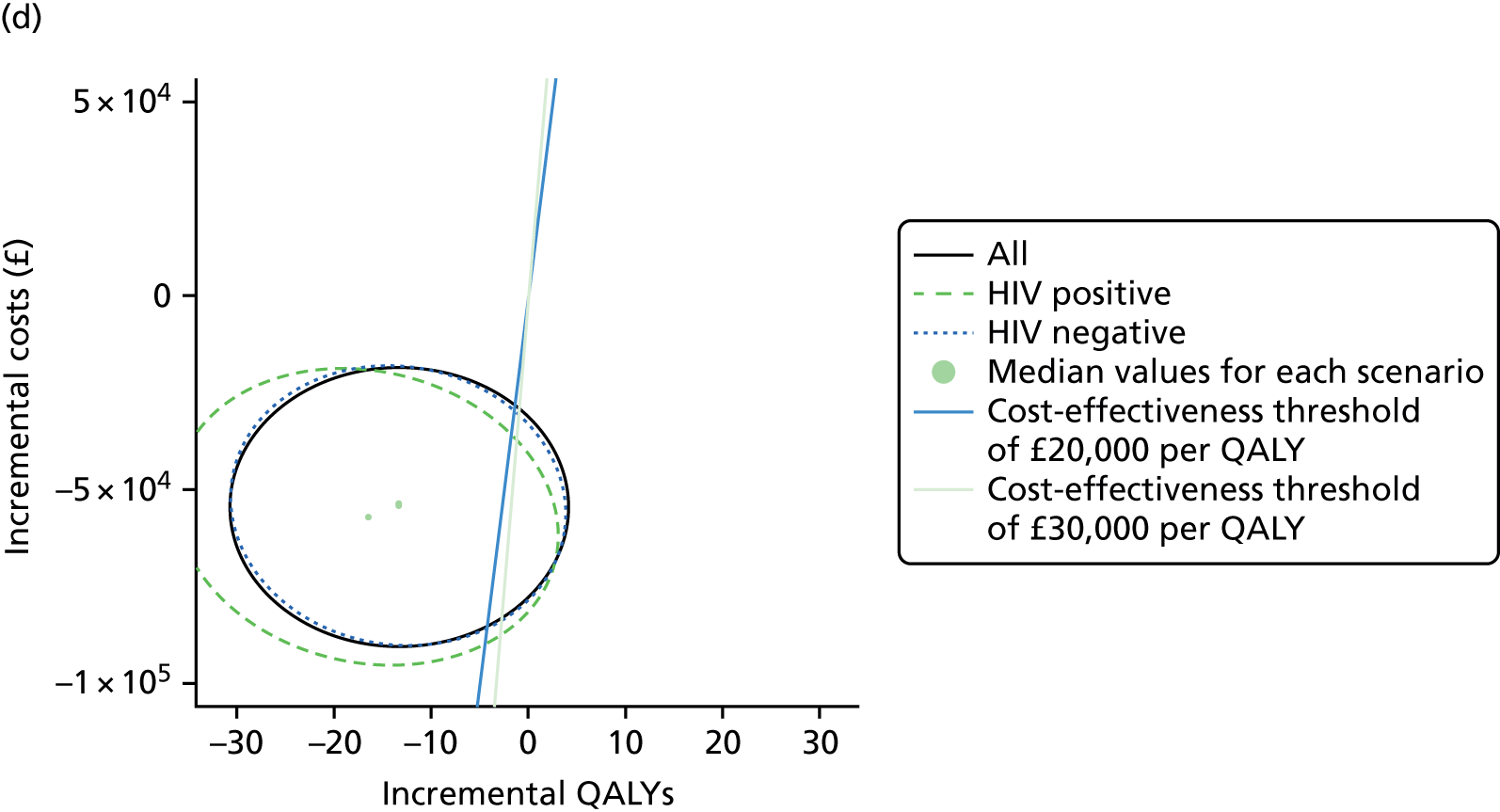
FIGURE 13.
Cost-effectiveness planes comparing the use of T-SPOT. TB or QFT-GIT as rule-out tests with current practice (50% density of simulation results). Results for (a) T-SPOT. TB with indeterminate diagnostic outcomes excluded; (b) T-SPOT. TB with indeterminate diagnostic outcomes included; (c) QFT-GIT with indeterminate diagnostic outcomes excluded; and (d) QFT-GIT with indeterminate diagnostic outcomes included. Upper panels show results for T-SPOT. TB, whereas lower panels show results for QFT-GIT. Left- and right-hand panels present results with indeterminate diagnostic outcomes excluded and included, respectively. Ellipses show 50% density of simulation results. The analyses considered all patients (black lines), HIV-positive patients (green dashed lines) and HIV-negative patients (blue dotted lines). The green dots indicate the median values for each scenario. Diagonal lines indicate the cost-effectiveness thresholds of £20,000 per QALY (blue vertical line) and £30,000 per QALY (green vertical line). A total of 1000 simulations were run for each scenario.


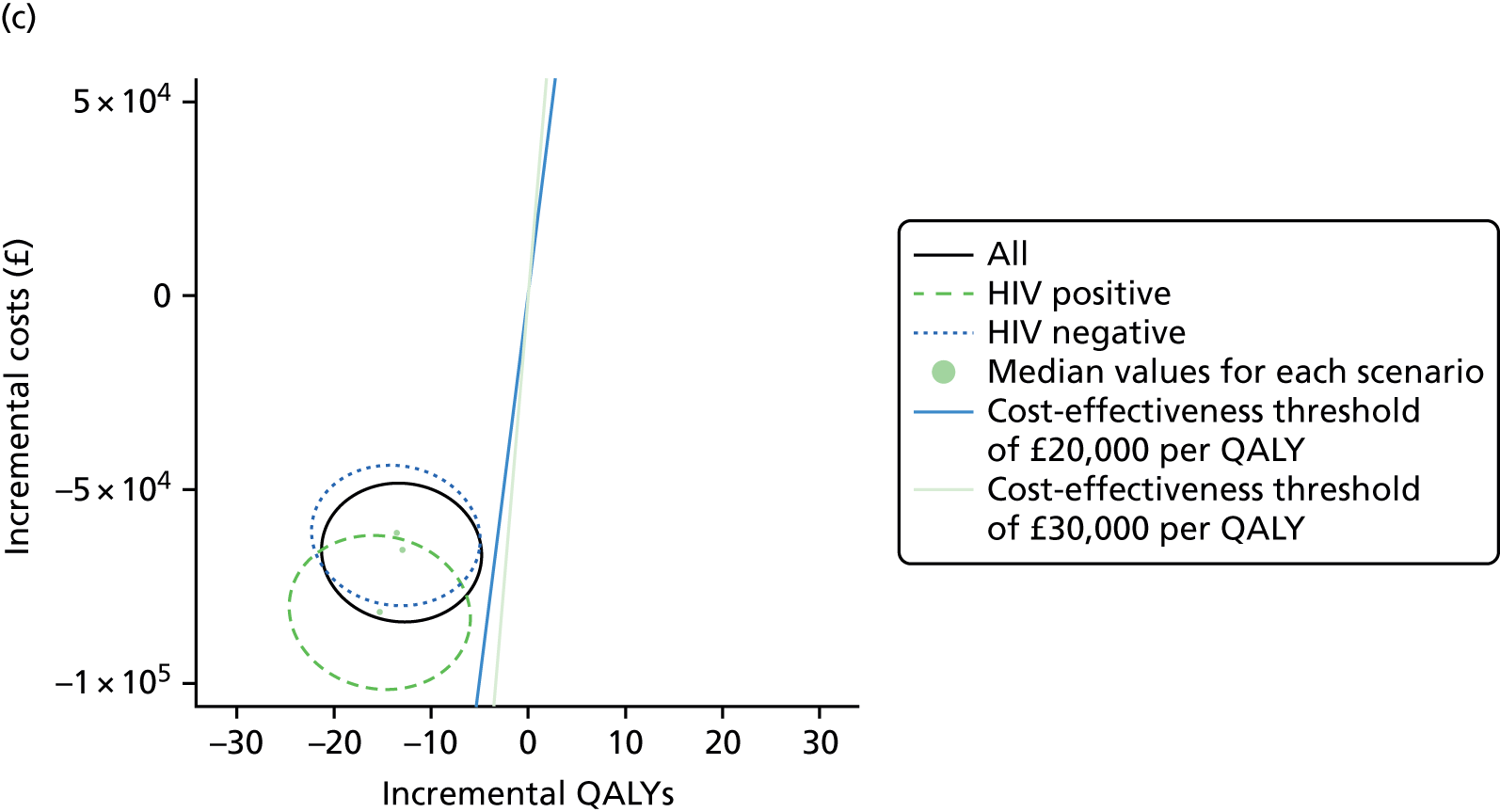
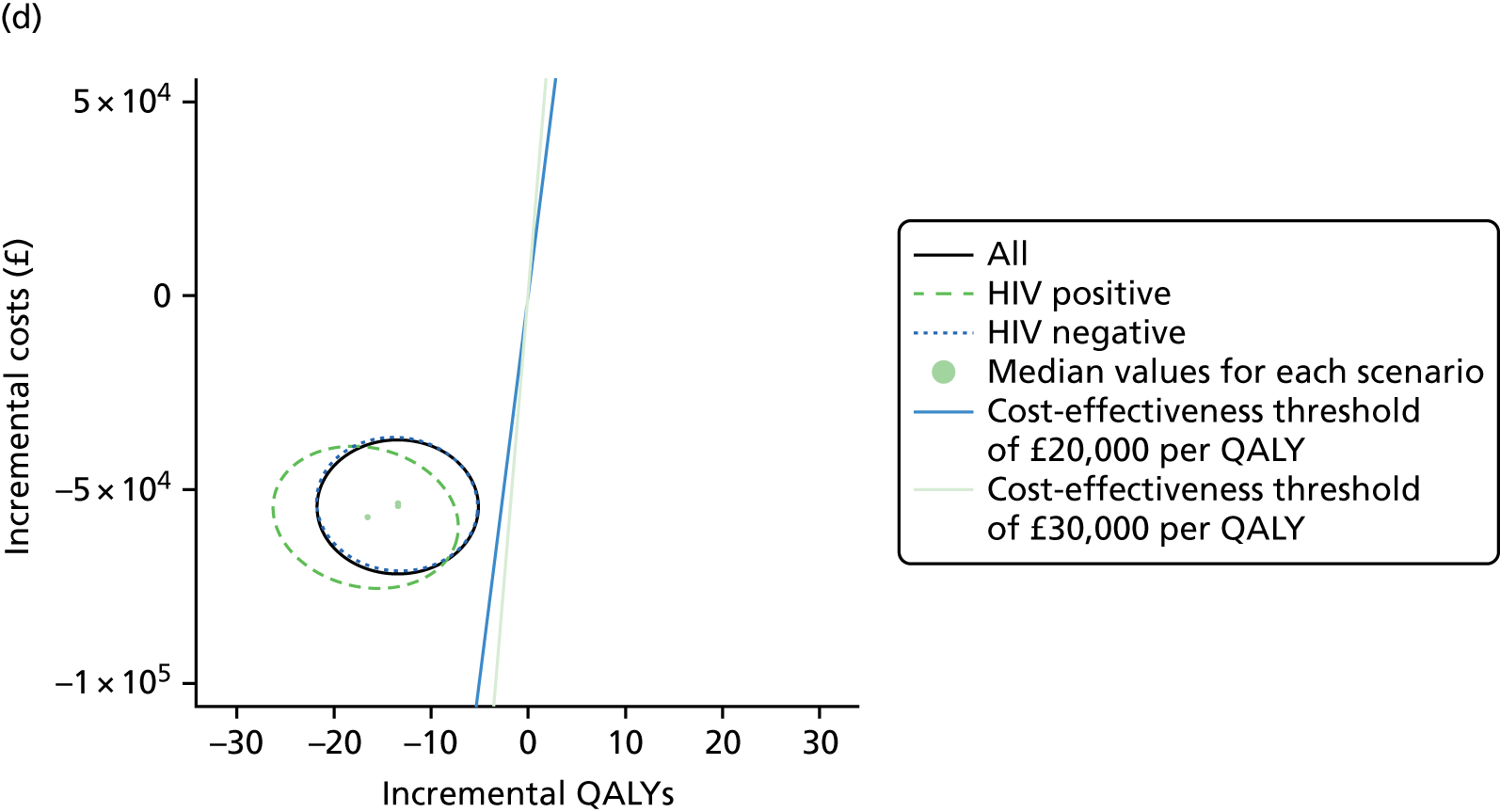
Using IGRAs as a rule-out test is likely to be cost saving (except perhaps for T-SPOT. TB when used with HIV-positive patients), but also harmful to health. The magnitude of the health detriment is such that it would not be cost-effective if a QALY is valued at either £20,000 or £30,000 (indicated by diagonal lines).
As the use of IGRAs as a rule-out test is detrimental to health (because of the increased average time to diagnosis) but cost saving, the cost-effectiveness acceptability curves (Figure 14) show a high probability of rule-out testing being cost-effective when the value of a QALY is low, with the probability declining steeply as the value of a QALY increases. This is because if a QALY has a low value then for a given cost saving a relatively large loss of QALYs would be considered acceptable, whereas if a QALY has a high value then such a QALY loss would not be acceptable. If a QALY is valued at £20,000, then all scenarios have a probability of < 30% of being cost-effective and many of them have a probability of < 10%. For £30,000 all scenarios have a probability of < 25% of being cost-effective, but many of them are in single figures. Figure 15 shows the corresponding upper and lower 95% binomial CIs for the cost-effectiveness acceptability curves in Figure 14, calculated using the normal approximation to the binomial distribution.
FIGURE 14.
Cost-effectiveness acceptability curves for T-SPOT. TB or QFT-GIT as rule-out tests compared with current practice. Results for (a) T-SPOT. TB with indeterminate IGRA result patients excluded; (b) T-SPOT. TB with indeterminate IGRA result patients included; (c) QFT-GIT with indeterminate IGRA result patients excluded; and (d) QFT-GIT with indeterminate IGRA result patients included. Upper panels show results for T-SPOT. TB, whereas lower panels show results for QFT-GIT. Left- and right-hand panels present results with indeterminate IGRA result patients excluded and included, respectively. The analyses considered all patients (black lines), HIV-positive patients (green dashed lines) and HIV-negative patients (blue dotted lines). Vertical lines indicate thresholds of £20,000 per QALY (green vertical line) and £30,000 per QALY (blue vertical line).

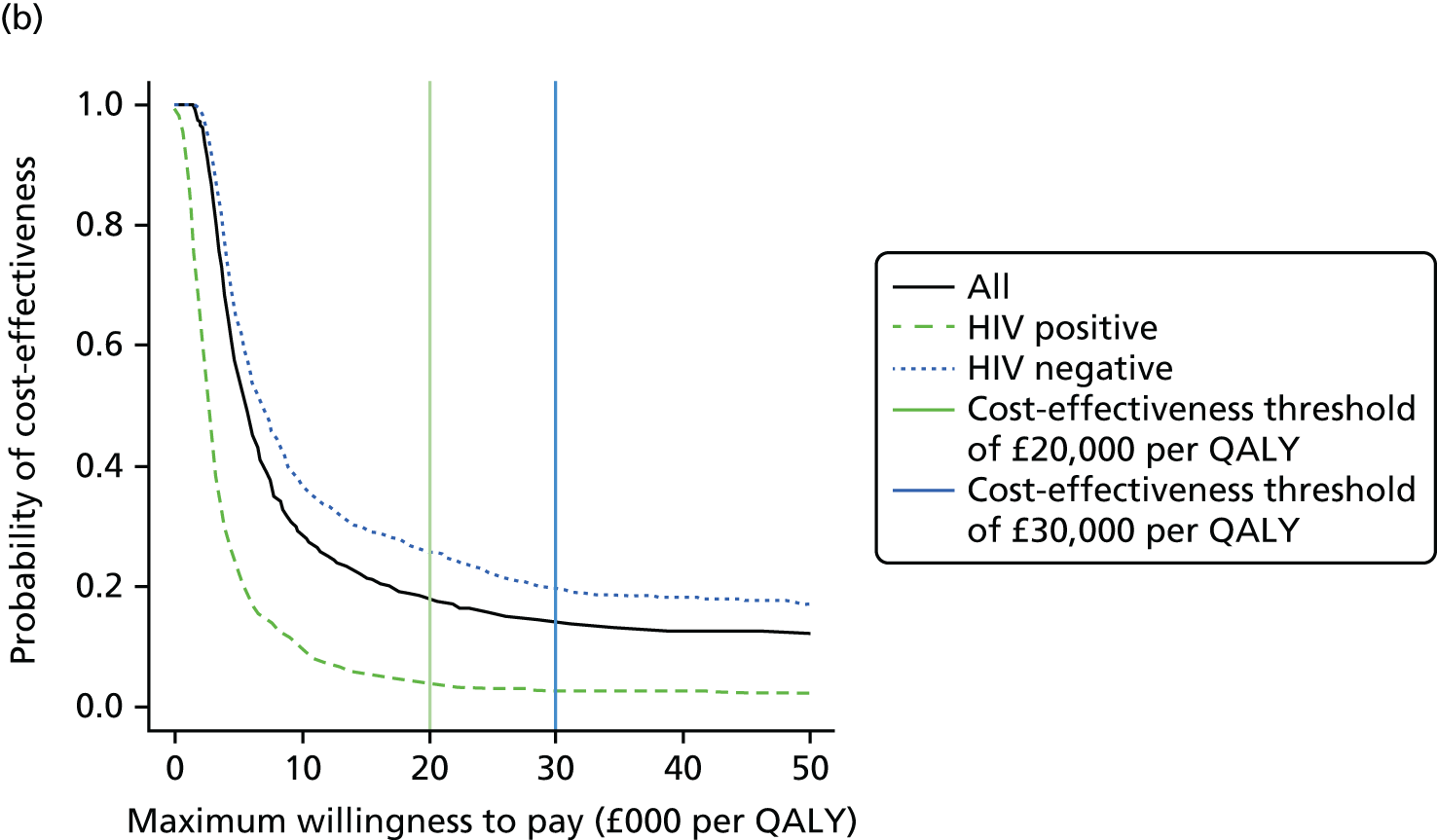
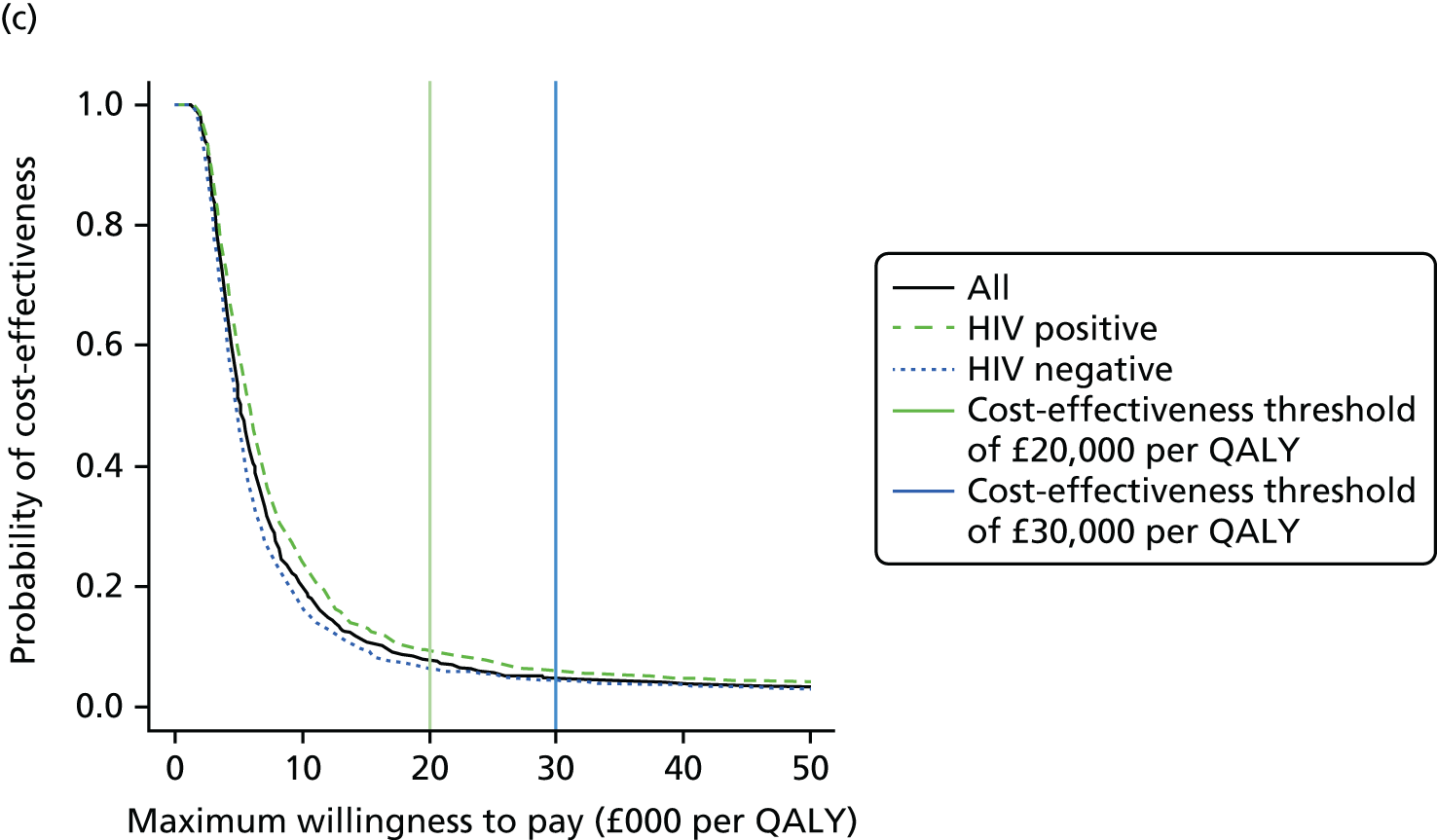
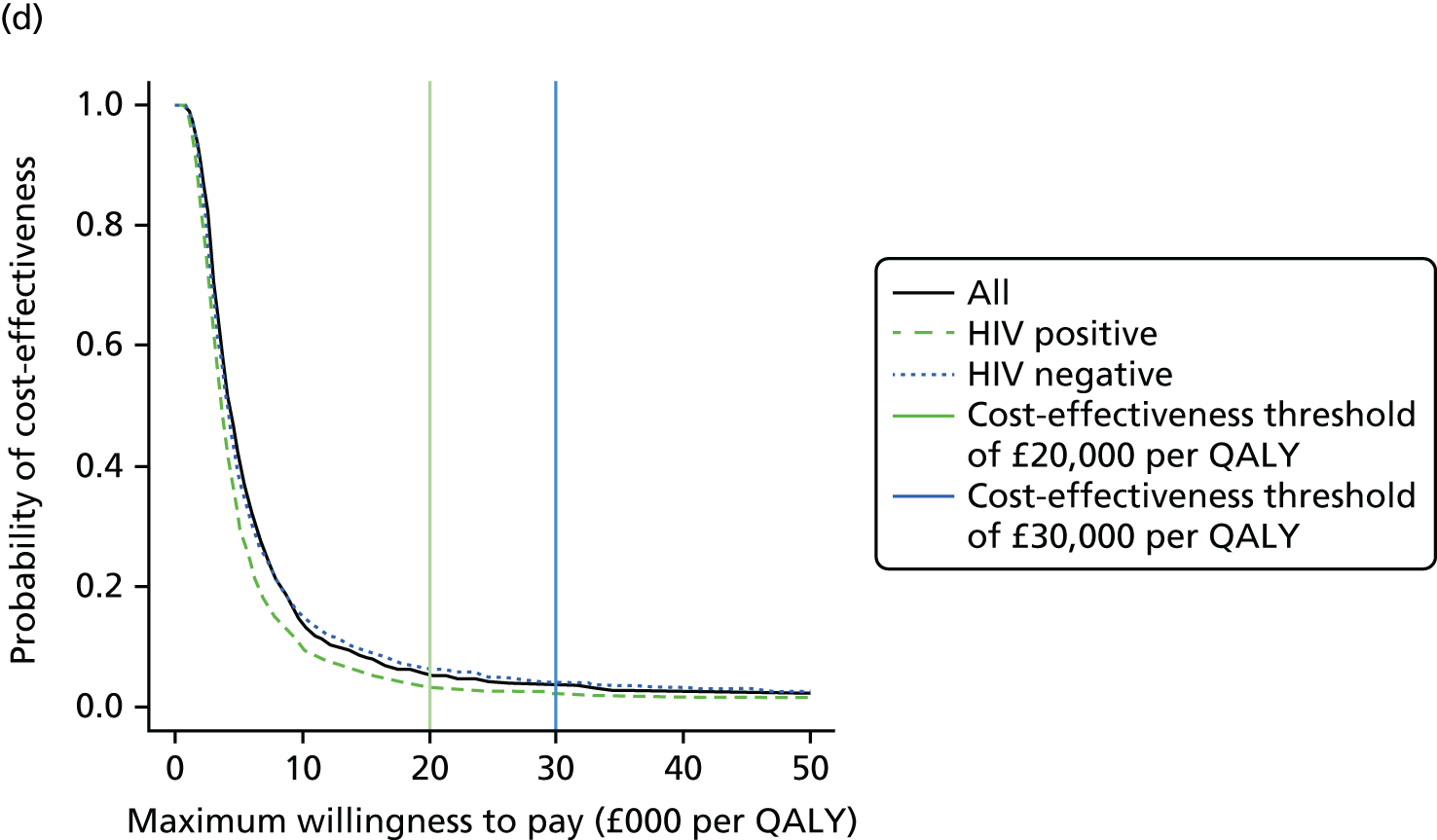
FIGURE 15.
Cost-effectiveness acceptability curves (with 95% CI values) for T-SPOT. TB or QFT-GIT as rule-out tests compared with current practice. Results for (a) T-SPOT. TB with indeterminate IGRA result patients excluded; (b) T-SPOT. TB with indeterminate IGRA result patients included; (c) QFT-GIT with indeterminate IGRA result patients excluded; and (d) QFT-GIT with indeterminate IGRA result patients included. Upper and lower 95% CI values shown assuming normal approximation to the binomial distribution. Upper panels show results for T-SPOT. TB, whereas lower panels show results for QFT-GIT. Left- and right-hand panels present results with indeterminate IGRA result patients excluded and included, respectively. The analyses considered all patients (black solid lines), HIV-positive patients (green lines) and HIV-negative patients (blue lines). Vertical lines indicate thresholds of £20,000 per QALY (green vertical line) and £30,000 per QALY (blue vertical line).

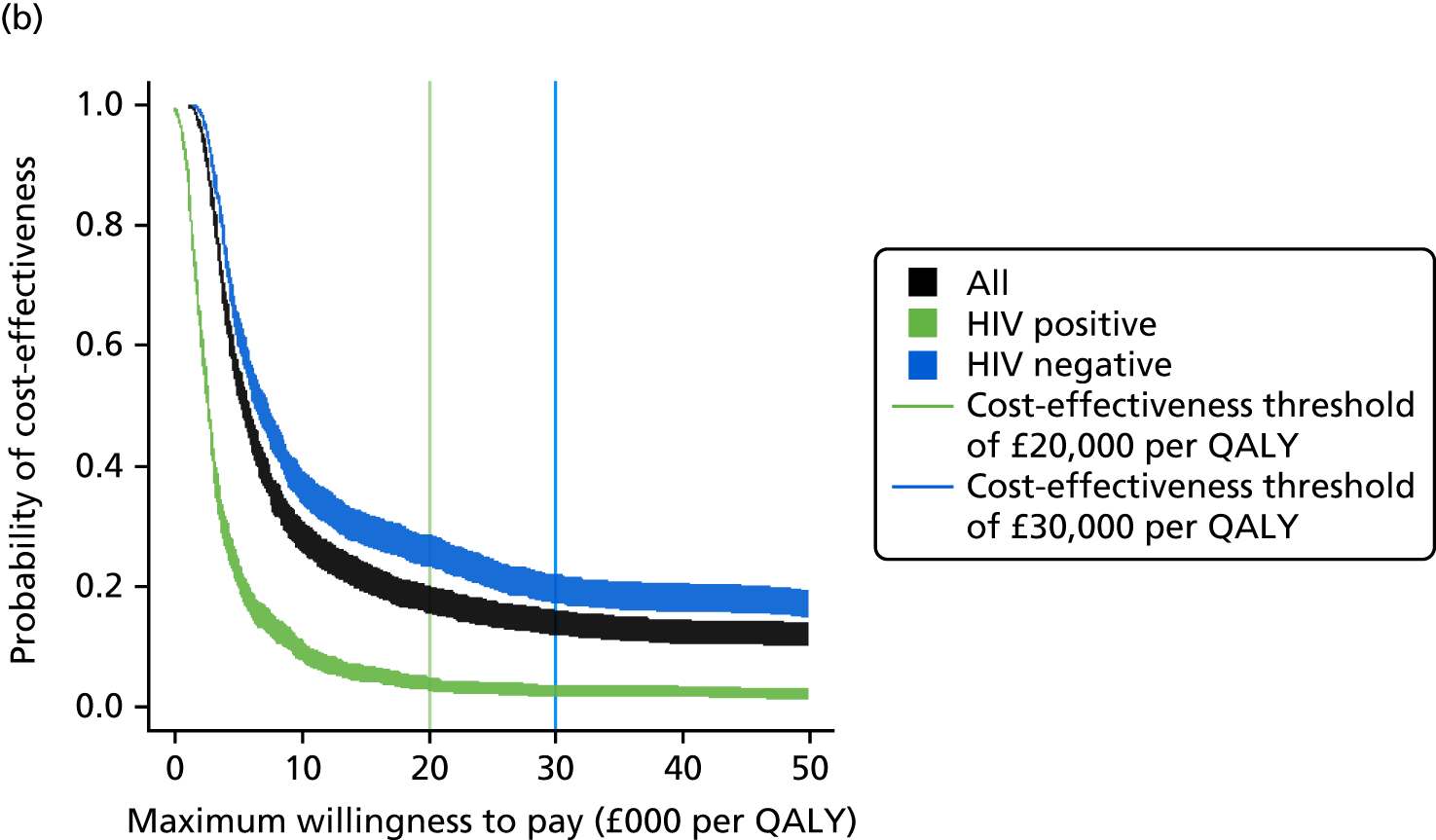
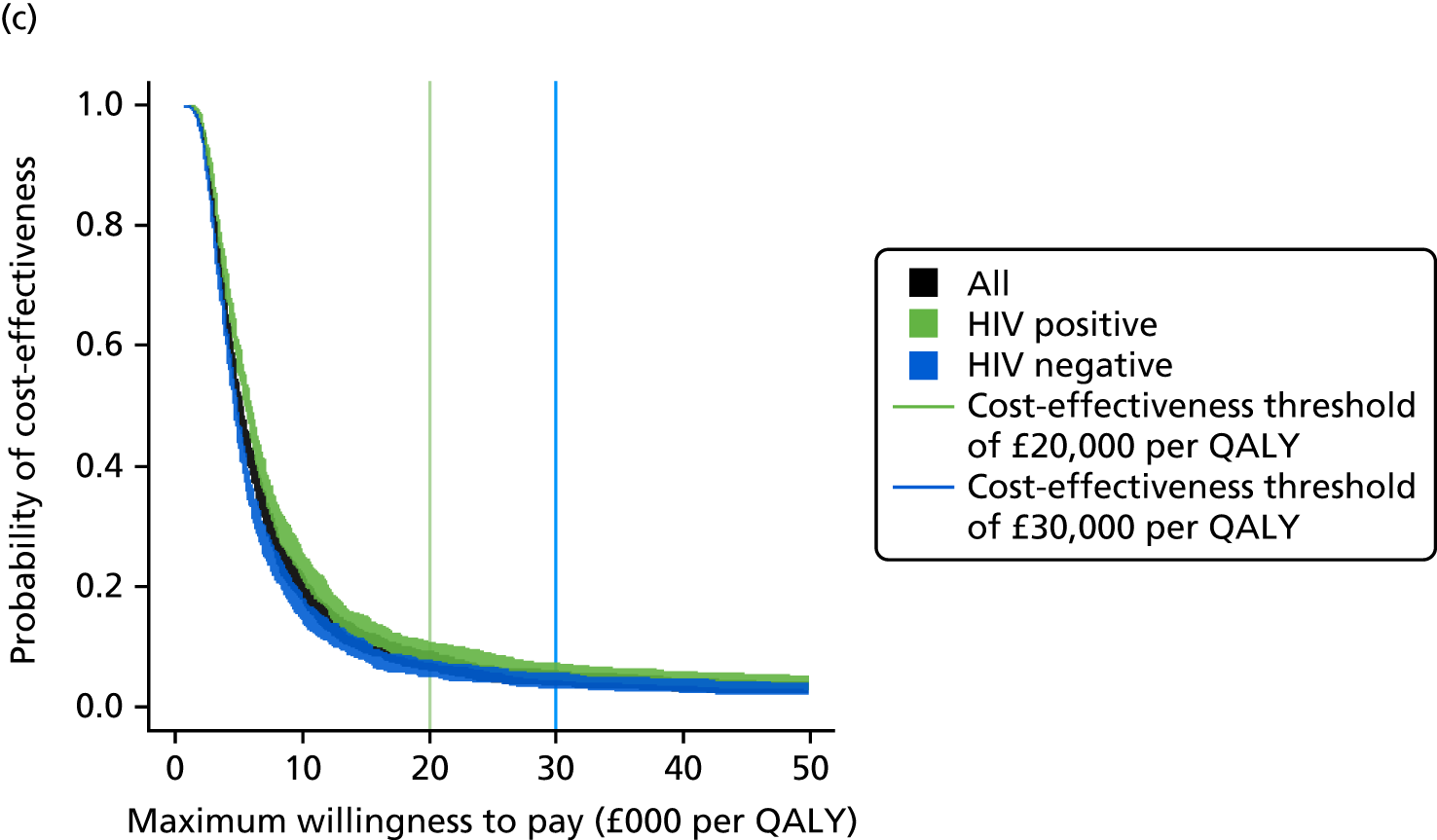

Discussion
An important finding of this study is that TB diagnosis rarely follows the idealised diagnostic pathways, meaning that costs and time delays until diagnosis may be very different from what economic analyses typically assume. In particular, costs of diagnosis may be typically underestimated, particularly when scanning is involved. Furthermore, there is considerable individual-level variation in the costs and time taken for TB diagnosis, which needs to be represented in the analysis. Therefore, we included the empirical distributions of time to diagnosis and total test costs in the probabilistic sensitivity analysis in the health economic evaluation.
The use of current IGRA tests for ruling out active TB would be unlikely to be considered cost-effective if a QALY were to be valued at £20,000 or £30,000; although it is cost saving, the health detriment is large. Health detriment occurs because of a delay in diagnosing active TB, prolonging illness, for two reasons. First, adding a rule-out test to the diagnostic pathway adds a step and increases the costs and time taken to ultimate diagnosis for all patients except those who do not have TB and receive a true-negative rule-out test result so that they do not undergo the remainder of TB-specific tests. Second, some patients with TB receive a false-negative result from the rule-out test, which delays their diagnosis of TB until after their follow-up appointment. However, for patients who do not have TB, and who have TB correctly ruled out by the initial test, the time to diagnosis of the cause of their illness is reduced, producing a health gain, and there is a cost saving owing to their not having further tests for TB. Whether there is a net health detriment or gain for the patient cohort as a whole depends on the prevalence of active TB in the patients, the performance characteristics of the rule-out test and the length of delay introduced by adding the initial rule-out test.
Limitations and generalisability
The multicentre design means that the study population is representative of the general population with clinically relevant risk of TB in the UK and is therefore representative of the greatest TB burden in the country. In areas where TB rates are low, the patient populations might be substantially different, and this has not been tested in the study. The model is flexible and is suitable for analysing rule-out tests with a range of performance characteristics, and could also be applied to patient populations in lower-burden settings if suitable data were available. A limitation is that, although we have quantified the health detriment of delayed treatment due to prolonged morbidity, we were unable to account for any additional detriment due to a potentially poorer prognosis, because of a lack of suitable data. In addition to a lack of data, the assumed time taken to apply the hypothetical rule-out test is an assumption based on expert opinion, and we have assumed that the time to follow-up of those patients who receive a negative hypothetical rule-out test result can be inferred from our existing data set. The timings of in-practice events were estimated from the study population. The variability in times between patients were explicitly included in the model.
Conclusions and recommendations
The use of current IGRA tests for ruling out active TB would be unlikely to be considered cost-effective if a QALY were to be valued at £20,000 or £30,000. A health detriment in patients with active TB whose diagnosis and treatment are delayed needs to be balanced against health gains in patients who do not have active TB whose diagnosis of the true cause of illness and commencement of appropriate treatment are accelerated, with consideration given to cost savings of faster ruling out of active TB. Although the performance of current IGRA tests means they are not cost-effective, improved test technology or alternative algorithms using current technology could potentially have a performance that is cost-effective.
Future research recommendations are that improved testing technology, including combinations of tests, be investigated with an equivalent analysis. The knowledge base in this field of research is improved data and understanding at the national and local level.
Chapter 8 Discussion
Diagnostic accuracy findings for T-SPOT. TB and QFT-GIT were described in detail in Chapters 4 and 5, and the results of the economic evaluation were discussed in Chapter 7. Therefore, this chapter focuses on the principal findings, strengths and limitations of the study, and implications for health care and research.
Principal findings
This large multicentre study of a consecutive series of patients being investigated for possible TB is representative of routine clinical practice in the UK. The study clearly showed that T-SPOT. TB is more sensitive than QFT-GIT (relative sensitivity 1.22, 95% CI 1.14 to 1.31; p < 0.001), but that specificities are similar (relative specificity 1.02, 95% CI 0.97 to 1.08; p = 0.3). For T-SPOT. TB and QFT-GIT, the sensitivities were 82.3% (95% CI 77.7% to 85.9%) and 67.3% (95% CI 62.1% to 72.2%), respectively, whereas the specificities were 82.6% (95% CI 78.6% to 86.1%) and 80.4% (95% CI 76.1% to 84.1%), respectively. In the substudy of HIV-positive patients, the highest-risk subgroup for TB, T-SPOT. TB also showed higher sensitivity than QFT-GIT (a relative increase of 12%), but there was no statistical evidence of a difference in sensitivity. The sensitivity of T-SPOT. TB in all patients (82.3%) or among HIV co-infected patients (68.0%) is not of sufficient clinical utility as a rule-out test for the diagnosis of active TB in routine clinical practice and cannot be used in isolation as a rule-out test. This is further supported by the economic evaluation. The use of current IGRA tests for ruling out active TB would be unlikely to be considered cost-effective if a QALY were to be valued at £20,000 or £30,000. There are cost savings, but the health detriment is large because of the delay in diagnosing active TB.
The specificities of the IGRAs were also not adequate for IGRAs to be recommended as rule-in tests. However, in category 4D patients (active TB excluded, TST negative and no risk factors for LTBI), both T-SPOT. TB and QFT-GIT showed high specificity: 92.3% (95% CI 85.4% to 96.4%) and 93.4% (95% CI 86.4% to 96.9%), respectively. This suggests that IGRAs may have potential value for ruling in TB in settings with a low probability of active TB. However, it should be noted that the number of category 4D patients was small.
For key subgroups in our main study cohort – patients with pre-existing diabetes mellitus, end-stage renal failure or iatrogenic immunosuppression – data were limited. Of the 845 patients included in our analyses, 88 (10.4%) had diabetes mellitus, 12 (1.4%) had chronic/end-stage renal failure and 105 (12.4%) patients were on immunosuppressive therapy. Analysis of patients with and without diabetes mellitus showed that the sensitivities and specificities of both tests were higher in those without diabetes mellitus than in those with diabetes mellitus. However, there was no statistical evidence of an effect on the relative test performance of T-SPOT. TB and QFT-GIT. Although this finding should be interpreted with caution because of the small number of active TB cases in the analyses, association between diabetes mellitus and IGRA performance has been reported. Faurholt-Jepsen et al. 47 reported an association between diabetes mellitus and lower levels of Mtb antigen-specific IFN-γ and the impact on QFT-GIT results. Our findings are similar, even though diabetes mellitus was self-reported in the IDEA study. We found no study that has evaluated the effect of diabetes mellitus on the diagnostic performance of T-SPOT. TB; the IDEA study appears to be the first study to suggest differences in the performance of T-SPOT. TB between diabetic and non-diabetic patients.
A new generation of QFT-GIT, QuantiFERON-TB Gold Plus (QFT®-Plus; Qiagen GmbH, Hilden, Germany), was recently launched. QFT-Plus includes a set of peptides designed to stimulate Mtb-specific cluster of differentiation 8-positive T cells. 48 There is very little published evidence about the performance of the test. According to Barcellini et al. ,48 who reported the first independent assessment of QFT-Plus, the test had sensitivity of 87.9% (95% CI 80.8% to 92.7%) in 116 TB patients and specificity of 97.2% (95% CI 92.0% to 99.0%) in 106 low-risk controls. In the IDEA study, second-generation IGRAs utilising novel antigens showed potential as rule-out tests. In particular, the use of a combination of existing antigens – ESAT-6 and CFP-10 – and the newer antigens – Rv3615c and Rv3879c – achieved a sensitivity of 89.9% (95% CI 86.2% to 92.7%) based on all active TB cases and 94.4% (95% CI 90.7% to 96.7%) among culture-confirmed cases. Similar results were obtained for the two-antigen combination of CFP-10 and Rv3615c and the three-antigen combination of ESAT-6, CFP-10 and Rv3615c. The added value of Rv3615c to T-SPOT. TB was a 9% (95% CI 5% to 12%) relative increase in sensitivity at the expense of specificity with a relative decrease of 7% (95% CI 4% to 10%). The incremental gain in sensitivity to 90% is likely to be clinically useful in ruling out active TB.
Strengths and limitations
To our knowledge, the IDEA study is the largest, prospective comparative accuracy study of the role of IGRAs for the diagnosis of active TB. Furthermore, we recruited a consecutive series of patients who were representative of UK clinical practice in a high-income setting. We ensured completeness and quality of the data such that missing data were minimal. Therefore, this well- designed and well-conducted study enabled robust and precise estimation of the relative performance of T-SPOT. TB and QFT-GIT in the main study cohort.
Our study has wide applicability as we did not exclude key subgroups, such as HIV-positive patients, but aimed to also compare the clinical performance of T-SPOT. TB and QFT-GIT in this population. In spite of not achieving the target sample size for the HIV-positive subgroup, compared with the five comparative studies identified in a systematic review published in 2012 and our literature review,15 the IDEA study remains the largest prospective head-to-head comparison of the two IGRAs in a HIV co-infected population.
The final diagnosis of TB is challenging in non-culture-confirmed cases and relies on a combination of epidemiological, radiological and diagnostic parameters. We used a composite reference standard applied by a panel of experienced clinicians. The panel followed a strict protocol. In the group of patients with non-microbiologically defined highly probable TB, there was a rigorous process to ensure that the clinical panel reviewed all cases using case histories, imaging, other test results and follow-up data to ensure that categorisation was as accurate as possible. The panel was blinded to the study and routine IGRA results to avoid potential for bias.
The IDEA study has limitations. First, owing to the low number of bronchoalveolar samples analysed, we were unable to fully characterise the value of IGRAs using BAL fluid. Therefore, we did not fulfil the secondary objective of determining the diagnostic accuracy of the two IGRAs applied to BAL samples in patients with suspected pulmonary TB who were sputum smear negative, as stated in the protocol.
Second, as already alluded to, we did not achieve our recruitment target for the HIV co-infected population. The IDEA study was extended to facilitate recruitment and more centres were included. We did achieve our original target of 200 patients, which was based on a prevalence of active TB of 50%. However, the final diagnosis of active TB in the 201 HIV-positive patients included in the analyses of the substudy was even lower (32/201, 15.9%) than the 20% used in our revised sample size calculation.
Finally, for the economic evaluation, we found that TB diagnosis rarely followed idealised diagnostic pathways, implying that costs and time delays until diagnosis may be very different from what economic analyses typically assume. In particular, costs of diagnosis may be typically underestimated, particularly when other modalities of imaging apart from plain chest radiology such as CT, ultrasound or magnetic resonance scanning are involved.
Implications for health care
Despite the significantly higher diagnostic sensitivity of T-SPOT. TB over QFT-GIT, neither of the two IGRAs can be used routinely as a reliable rule-out test for suspected active TB in this patient population in secondary care. Both IGRAs were also not cost-effective in this setting. However, for patients in which there is a suspicion of TB, but the pre-test probability is low, the NPV of a negative T-SPOT. TB result would be correspondingly higher.
The specificity of both IGRAs for a diagnosis of active TB was similar and too low to use as a rule-in test. However, in patients with suspected active TB, a positive IGRA result could help in certain circumstances to keep TB in the differential diagnosis and guide further diagnostic testing towards confirming or excluding a diagnosis of TB. A positive result in the setting of a HIV-infected patient with suspected TB provides clinically useful information, as specificity in this setting was higher than in HIV-negative patients, especially for QFT-GIT.
The incorporation of novel antigens into T-SPOT. TB, in particular Rv3615c, yielded a high sensitivity coupled with a modest reduction in specificity. The sensitivity and NPV of 90% in this high-prevalence population in secondary care are compatible with the use of this assay to exclude a diagnosis of TB in patients with lower pre-test probabilities. The 95% sensitivity in culture-confirmed TB is similar to that of the D-dimer assay, which is routinely used as a rapid rule-out test for suspected venous thromboembolism in patients with a low to moderate pre-test probability.
Notably, replacing ESAT-6 with Rv3615c also conferred higher sensitivity than T-SPOT. TB. Indeed, the diagnostic sensitivity and NPV of 89% were similar to that achieved by the incorporation of Rv3615c alongside both ESAT-6 and CFP-10. This observation is relevant for TB control internationally because one of the leading TB vaccine candidates currently in clinical trials, H56/IC31, incorporates ESAT-6. The vaccine is protective in the non-human primate model and if it proves to be protective in humans, it is likely to be licensed. If rolled out, vaccinated individuals will likely develop T-cell responses to ESAT-6, which would give false-positive IGRA results, akin to the current scenario with BCG vaccination inducing false-positive TST results. Replacing ESAT-6 with Rv3615c may be a potential solution because a CFP-10- and Rv3615c-based IGRA would have significantly higher sensitivity than existing IGRAs and specificity would not be compromised in H56/IC31-vaccinated individuals.
Recommendations for research
The second-generation IGRAs evaluated in this study do not need to be re-evaluated in a UK routine practice setting because this study enabled an equally rigorous evaluation of these novel assays as it did for conventional IGRAs. Precise estimates of diagnostic accuracy were obtained. However, it would be of interest to evaluate these new assays and their combinations in distinct clinical settings with much lower or much higher prevalence of active TB. It will also be important to assess how these novel IGRAs perform in immunosuppressed subgroups, including HIV-infected patients, diabetic patients with chronic renal impairment and those on immunosuppressive therapy. A comparative accuracy study of the novel assays and QFT-Plus may also be needed to determine how their sensitivity compares in routine practice.
Acknowledgements
We are grateful to clinical and research staff involved in facilitating recruitment and data collection at all participating centres. We would like to thank:
Independent members of the SSC for their advice and support throughout the study.
Lee Potiphar, Senior Research Nurse, for his contribution to the original study protocol and management of the IDEA study nursing team between September 2011 and June 2013.
Anil Sharma, Senior Research Nurse, for managing the IDEA study nursing team between June 2013 and September 2013.
The IDEA study Research Nurses: Samuel Bremang (December 2011 to February 2015), Anil Sharma (November 2011 to February 2014), Gary Hahn (November 2011 to November 2013), Lisa Grass (November 2011 to February 2014), Linda Haimbodi (September 2012 to January 2014) and Hiromi Uzu (May 2014 to February 2015).
Laboratory staff (Dennis Afram, Luigi Marongiu and Bianca Donges) and other staff (Ann-Kathrin Reuschl and Nazneen Siddiqui) involved in blood processing.
Dhansuklal Solanki and Denise Gardner for administrative assistance.
We thank the National Institute for Health Research (NIHR) Health Technology Assessment programme for funding, and the NIHR Health Protection Research Unit, Imperial College London for support. We especially thank all IDEA participants for their involvement in the study.
Staff who facilitated recruitment and data collection
| Centre | PI | Staff that recruited patients and performed data collection |
|---|---|---|
| Imperial College Healthcare NHS Trust | Professor Ajit Lalvani and Professor Onn Min Kon | Dr Matthew Berry, Dr Frances Sanderson, Dr Graham Cooke, Dr Tommy Pasvol, Mr Lee Potiphar, Mr Anil Sharma, Mrs Lisa Grass, Mr Samuel Bremang, Mrs Amarjit Badhan, Ms Hiromi Uzu, Ms Marie Francis, Miss Helen Piotrowski, Ms Marie O’Donoghue and Ms Irene Zondo |
| University Hospitals of Leicester NHS Trust | Dr Gerrit Woltmann and Dr Martin Wiselka | Mrs Kate Ellis, Mrs Hilary Pateman, Mrs Linda Mashonganyika and Mr Adam Lewszuk |
| Sandwell and West Birmingham Hospitals NHS Trust | Dr Naz Nathani | Mrs Harriet Goddard and Mrs Frances Lloyd |
| Heart of England NHS Foundation Trust | Dr Martin Dedicoat and Dr Heinke Kunst (co-investigator) | Ms Bridget Pointon and Ms Mary O’Sullivan |
| Oxford University Hospitals NHS Foundation Trust | Professor Christopher Conlon | Professor Christopher Conlon and Mr Lee Potiphar |
| Frimley Health NHS Foundation Trust | Dr Sarah Menzies | Dr John Wiggins, Mrs Amarjit Badhan, Mr Samuel Bremang and Mr Edmund Cox |
| King’s College Hospital NHS Foundation Trust | Dr Frank Post | Dr Frank Post, Ms Emily Wandolo and Ms Lucy Campbell |
| Barts Health NHS Trust | Dr Rebecca O’Connell | Mrs Nyasha Makoka, Mr Andrew Crawford-Jones and Ms Kaya Widuch |
| St George’s University Hospitals NHS Foundation Trust | Professor Derek Macallan and Dr Felix Chua (co-investigator) | Dr Carmen Cabeza, Miss Louise Wootton, Mr Gary Hahn, Mr Lee Potiphar and Mrs Amarjit Badhan |
| Chelsea and Westminster Hospital NHS Foundation Trust | Dr Anton Pozniak and Dr Mike Loebingher (co-investigator) | Mr Gary Hahn, Mr Lee Potiphar, Mr Anil Sharma, Mrs Amarjit Badhan, Ms Sanjeewa Basnayake, Mrs Lesley Ruta and Miss Kirsty Money |
| Royal Free London NHS Foundation Trust | Dr Marc Lipman | Dr Simon Bax, Mr Gary Hahn, Miss Janey Sewell, Mr Anil Sharma, Mr Lee Potiphar, Mrs Amarjit Badhan and Mrs Angelita Solamalai |
| London North West Healthcare NHS Trust | Dr Rob Davison and Dr David Abdoyeku (co-investigator) | Dr Laurence John, Dr Jim Buckley, Miss Linda Haimbodi, Mr Lee Potiphar, Mr Gary Hahn, Mr Anil Sharma, Mrs Amarjit Badhan, Mr Samuel Bremang and Ms Hiromi Uzu |
| Ealing Hospital for follow-up | Dr Howard Branley and Dr William Lynn | Mr Samuel Bremang |
Independent members of the Study Steering Committee
-
Professor Khalid Khan (Chairperson of the IDEA Steering Committee and Professor of Women’s Health and Clinical Epidemiology), Barts and the London School of Medicine and Dentistry, London, UK.
-
Professor Stephen Gordon (Professor of Respiratory Medicine and Director), Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Queen Elizabeth Central Hospital, Blantyre, Malawi and the Clinical Research Group, Liverpool School of Tropical Medicine, Liverpool, UK.
-
Dr James Gray (Consultant Microbiologist), Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust, Birmingham, UK.
-
Dr Johannes B Reitsma (Clinical Epidemiologist), Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands.
-
Ms Nisha Karnani (PPI member).
Members of the Data Management Group
Professor Ajit Lalvani, Professor Onn Min Kon, Professor Jon Deeks, Dr Yemisi Takwoingi, Dr Melanie Rees-Roberts, Mr Lee Potiphar, Mr Anil Sharma, Dr Hilary Whitworth, Mrs Amarjit Badhan, Miss Aime Boakye and Dr Christopher Partlett.
Members of the Study Management Group
Professor Ajit Lalvani, Professor Onn Min Kon, Dr Melanie Rees-Roberts, Mr Lee Potiphar, Dr Hilary Whitworth, Mr Anil Sharma and Mrs Amarjit Badhan.
The IDEA study collaborators
Dr Yemisi Takwoingi, Dr Hilary Whitworth, Dr Melanie Rees-Roberts, Mrs Amarjit Badhan, Dr Christopher Partlett, Dr Nathan Green, Miss Aime Boakye, Miss Heather Lambie, Mr Luigi Marongiu, Dr Mark Jit, Dr Peter White, Professor Jonathan Deeks, Professor Onn Min Kon, Dr Ajit Lalvani, Dr David Abdoyeku, Dr Howard Branley, Professor Felix Chua, Professor Christopher Conlon, Dr Graham Cooke, Professor Robert Davison, Dr Martin Dedicoat, Dr Heinke Kunst, Dr Marc Lipman, Dr Mike Loebingher, Dr William Lynn, Professor Derek Macallan, Dr Sarah Menzies, Dr Nazim Nathani, Dr Rebecca O’Connell, Dr Frank Post, Dr Anton Pozniak, Dr Martin Wiselka and Dr Gerrit Woltmann.
Contributions of authors
Dr Yemisi Takwoingi (Senior Study Statistician) led the statistical analysis, contributed to the interpretation of the diagnostic accuracy results, led the writing of Chapters 2–5 and produced the report.
Dr Hilary Whitworth (former Study Co-ordinator and Post-Doctoral Research Associate) was responsible for day-to-day management of the IDEA study and, management of the lead research nurse, writing of Chapter 1 and contributed to the writing of the report.
Dr Melanie Rees-Roberts (former IDEA Study Co-ordinator) contributed to the management of the IDEA study and built the study databases.
Mrs Amarjit Badhan (Senior Clinical Research Nurse) was responsible for the day-to-day management of the IDEA study with the study co-ordinator and for the management of the nursing team, led the writing of Chapters 1 and 8 and commented on the draft of the report.
Dr Christopher Partlett (Study Statistician) conducted the statistical analysis and contributed to the writing of the report.
Dr Nathan Green (Research Fellow) performed statistical analysis of diagnostic pathways and health economic modelling, and led the writing of Chapters 6 and 7.
Miss Aime Boakye (IDEA Administrator plus laboratory role) contributed to database management, data collection, data cleaning, quality assurance and the writing of Chapter 2.
Miss Heather Lambie (Laboratory Staff) performed blood and BAL processing, conducted index tests and entered laboratory data.
Mr Luigi Marongiu (Research Assistant) contributed to the management and analysis of blood samples and database management.
Dr Mark Jit (Senior Mathematical and Economic Modeller, Reader) contributed to the design and interpretation of analysis of diagnostic pathways and the health economic analysis.
Dr Peter White (Reader, Head of Public Health England Modelling and Economics Unit) contributed to the study design and led the statistical analysis of diagnostic pathways and the economic analysis.
Professor Jonathan J Deeks (Professor of Biostatistics and Co-applicant) contributed to the study design and analysis, the interpretation of results and the writing of the report.
Professor Onn Min Kon (Professor and Consultant Physician Imperial College Healthcare) was chief investigator, provided clinical expertise, and contributed to study design, execution and the writing of the report.
Professor Ajit Lalvani (Professor and Consultant Physician Imperial College Healthcare) was chief investigator, provided clinical expertise, and contributed to the study design, execution and the writing of the report.
Publications
Abubakar I, Stagg HR, Whitworth H, Lalvani A. How should I interpret an interferon gamma release assay result for tuberculosis infection? Thorax 2013;68:298–301.
Whitworth HS1, Scott M, Connell DW, Dongés B, Lalvani A. IGRAs – the gateway to T cell based TB diagnosis. Methods 2013;61:52–62.
Whitworth HS, Badhan A, Boakye AA, Takwoingi Y, Rees-Roberts M, Partlett C, et al. Clinical utility of existing and second-generation interferon-γ release-assays for diagnostic evaluation of tuberculosis: an observational cohort study. Lancet Infect Dis 2019;19:193–202.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Global Tuberculosis Report 2015. Geneva: World Health Organization; 2015.
- Tuberculosis in England 2015. Report Version 1.1. London: Public Health England; 2015.
- Dosanjh DP, Hinks TS, Innes JA, Deeks JJ, Pasvol G, Hackforth S, et al. Improved diagnostic evaluation of suspected tuberculosis. Ann Intern Med 2008;148:325-36. https://doi.org/10.7326/0003-4819-148-5-200803040-00003.
- Tuberculosis in the UK: Annual Report on Tuberculosis Surveillance in the UK 2008. London: Health Protection Agency; 2008.
- Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 1999;284:1520-3. https://doi.org/10.1126/science.284.5419.1520.
- Whitworth HS, Scott M, Connell DW, Dongés B, Lalvani A. IGRAs – the gateway to T cell based TB diagnosis. Methods 2013;61:52-6. https://doi.org/10.1016/j.ymeth.2012.12.012.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177-84. https://doi.org/10.7326/0003-4819-149-3-200808050-00241.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351. https://doi.org/10.1136/bmj.h5527.
- Oxford Immunotec . T-SPOT.TB Package Insert n.d. www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/PI-TB-IVD-UK-V2.pdf (accessed 4 April 2016).
- Qiagen . QuantiFERON TB Gold (QFT). ELISA Package Insert 2009. www.quantiferon.com/irm/content/PI/QFT/2PK/UK.pdf (accessed 4 April 2016).
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. London: NICE; 2011.
- Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 2002;57:804-9. https://doi.org/10.1136/thorax.57.9.804.
- Goletti D, Carrara S, Stefania C, Butera O, Amicosante M, Ernst M, et al. Accuracy of immunodiagnostic tests for active tuberculosis using single and combined results: a multicenter TBNET-Study. PLOS ONE 2008;3. https://doi.org/10.1371/journal.pone.0003417.
- Alonzo TA, Pepe MS, Moskowitz CS. Sample size calculations for comparative studies of medical tests for detecting presence of disease. Stat Med 2002;21:835-52. https://doi.org/10.1002/sim.1058.
- Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0032482.
- Rice B, Elford J, Yin Z, Kruijshaar M, Abubakar I, Lipman M, et al. Decreasing incidence of tuberculosis among heterosexuals living with diagnosed HIV in England and Wales. AIDS 2013;27:1151-7. https://doi.org/10.1097/QAD.0b013e32835e2cb1.
- Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209-12. https://doi.org/10.1080/01621459.1927.10502953.
- Brown LD, Cai TT, DasGupta A. Interval Estimation for a Binomial Proportion. Stat Sci 2001;16:101-17. https://doi.org/10.1214/ss/1009213286.
- Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 1991;44:763-70. https://doi.org/10.1016/0895-4356(91)90128-V.
- Leisenring W, Pepe MS, Longton G. A marginal regression modelling framework for evaluating medical diagnostic tests. Stat Med 1997;16:1263-81. https://doi.org/10.1002/(SICI)1097-0258(19970615)16:11<1263::AID-SIM550>3.0.CO;2-M.
- Yen YF, Yen MY, Lin YS, Lin YP, Shih HC, Li LH, et al. Smoking increases risk of recurrence after successful anti-tuberculosis treatment: a population-based study. Int J Tuberc Lung Dis 2014;18:492-8. https://doi.org/10.5588/ijtld.13.0694.
- Chan SF, Deeks JJ, Macaskill P, Irwig L. Three methods to construct predictive models using logistic regression and likelihood ratios to facilitate adjustment for pretest probability give similar results. J Clin Epidemiol 2008;61:52-63. https://doi.org/10.1016/j.jclinepi.2007.02.012.
- Knottnerus JA. Application of logistic regression to the analysis of diagnostic data: exact modeling of a probability tree of multiple binary variables. Med Decis Making 1992;12:93-108. https://doi.org/10.1177/0272989X9201200202.
- World Medical Association . Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Rose AM, Watson JM, Graham C, Nunn AJ, Drobniewski F, Ormerod LP, et al. Tuberculosis at the end of the 20th century in England and Wales: results of a national survey in 1998. Thorax 2001;56:173-9. https://doi.org/10.1136/thorax.56.3.173.
- Whitworth HS, Badhan A, Boakye AA, Takwoingi Y, Rees-Roberts M, Partlett C, et al. Clinical utility of existing and second-generation interferon-γ release-assays for diagnostic evaluation of tuberculosis: an observational cohort study. Lancet Infect Dis 2019;19:193-202. https://doi.org/10.1016/S1473-3099(18)30613-3.
- Aggarwal AN, Agarwal R, Gupta D, Dhooria S, Behera D. Interferon gamma release assays for diagnosis of pleural tuberculosis: a systematic review and meta-analysis. J Clin Microbiol 2015;53:2451-9. https://doi.org/10.1128/JCM.00823-15.
- Danel C, Kabran M, Inwoley A, Badje A, Herrmann JL, Moh R, et al. Quantiferon-TB Gold: performance for ruling out active tuberculosis in HIV-infected adults with high CD4 count in Côte d’Ivoire, West Africa. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0107245.
- Lagrange PH, Thangaraj SK, Dayal R, Deshpande A, Ganguly NK, Girardi E, et al. A toolbox for tuberculosis (TB) diagnosis: an Indian multicentric study (2006-2008). Evaluation of QuantiFERON-TB gold in tube for TB diagnosis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0073579.
- Sauzullo I, Mengoni F, Ermocida A, Massetti AP, D’Agostino C, Russo G, et al. Interferon-γ release assay in HIV-infected patients with active tuberculosis: impact of antituberculous drugs on host immune response. New Microbiol 2014;37:153-61.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 2013;16:e1-5. https://doi.org/10.1016/j.jval.2013.02.010.
- Kruijshaar ME, Lipman M, Essink-Bot ML, Lozewicz S, Creer D, Dart S, et al. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis 2010;14:296-302.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Discussion Paper 172. York: Centre for Health Economics, University of York; 1999.
- National Tariff Payment System 2014/15. London: Monitor; 2013.
- Hughes R, Wonderling D, Li B, Higgins B. The cost effectiveness of nucleic acid amplification techniques for the diagnosis of tuberculosis. Respir Med 2012;106:300-7. https://doi.org/10.1016/j.rmed.2011.10.005.
- Curtis L BA. Unit Costs of Health & Social Care. Canterbury: Personal Social Services Research Unit; 2015.
- Drobniewski F, Cooke M, Jordan J, Casali N, Mugwagwa T, Broda A, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19340.
- Tuberculosis: Prevention, Diagnosis, Management and Service Organisation. NICE Guideline 33. London: NICE; 2016.
- Auguste P, Tsertsvadze A, Pink J, Court R, Seedat F, Gurung T, et al. Accurate diagnosis of latent tuberculosis in children, people who are immunocompromised or at risk from immunosuppression and recent arrivals from countries with a high incidence of tuberculosis: systematic review and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20380.
- Auguste P, Tsertsvadze A, Pink J, Court R, Seedat F, Gurung T, et al. Accurate Diagnosis of Latent Tuberculosis in Children, in People Who are Immunocompromised or at Risk from Immunosuppression, and Recent Arrivals from Countries with a High Incidence of Tuberculosis: Systematic Review and Economic Evaluation. Appendix H: Warwick Evidence Diagnosis of LTBI. London: NICE; 2016.
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. NICE Guideline. Update of CG117 – Appendix 6. Cost Effectiveness Analysis of Interferon Gamma Release Assay (IGRA) Testing for Latent Tuberculosis. London: NICE; 2010.
- Pareek M, Bond M, Shorey J, Seneviratne S, Guy M, White P, et al. Community-based evaluation of immigrant tuberculosis screening using interferon γ release assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013;68:230-9. https://doi.org/10.1136/thoraxjnl-2011-201542.
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis and Measures for its Prevention and Control. NICE Guideline 33. London: NICE; 2006.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Pooran A, Booth H, Miller RF, Scott G, Badri M, Huggett JF, et al. Different screening strategies (single or dual) for the diagnosis of suspected latent tuberculosis: a cost effectiveness analysis. BMC Pulm Med 2010;10. https://doi.org/10.1186/1471-2466-10-7.
- Faurholt-Jepsen D, Aabye MG, Jensen AV, Range N, Praygod G, Jeremiah K, et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis 2014;46:384-91. https://doi.org/10.3109/00365548.2014.885657.
- Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, et al. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 2016;47:1587-90. https://doi.org/10.1183/13993003.02033-2015.
- Human Tissue Act 2004. London: The Stationery Office; 2004.
- Adewole OO, Erhabor GE, Sogaolu MO, Onipede AO, Owiafe PK, Awopeju FO, et al. Diagnostic utility of QuantiFERON-TB gold in-tube in active pulmonary tuberculosis in Nigeria. West Afr J Med 2013;32:180-5.
- Jeon YL, Nam YS, You E, Yang JJ, Kim MJ, Cho SY, et al. Factors influencing discordant results of the QuantiFERON-TB Gold In-tube test in patients with active TB. J Infect 2013;67:288-93. https://doi.org/10.1016/j.jinf.2013.06.005.
- Jia H, Pan L, Qin S, Liu F, Du F, Lan T, et al. Evaluation of interferon-γ release assay in the diagnosis of osteoarticular tuberculosis. Diagn Microbiol Infect Dis 2013;76:309-13. https://doi.org/10.1016/j.diagmicrobio.2013.03.030.
- Khalil KF, Ambreen A, Butt T. Comparison of sensitivity of QuantiFERON-TB gold test and tuberculin skin test in active pulmonary tuberculosis. J Coll Physicians Surg Pak 2013;23:633-6. https://doi.org/09.2013/JCPSP.633636.
- Kim JK, Bang WJ, Oh CY, Yoo C, Cho JS. Feasibility of the interferon-γ release assay for the diagnosis of genitourinary tuberculosis in an endemic area. Korean J Urol 2013;54:123-6. https://doi.org/10.4111/kju.2013.54.2.123.
- Lavender TW, Barrett A, Magee J, Ong EL. Interferon-γ release assays in the diagnosis of active tuberculosis disease in a low-incident setting: a 5-year review of data. Clin Microbiol Infect 2013;19:1078-81. https://doi.org/10.1111/1469-0691.12129.
- Lei Y, Yi FM, Zhao J, Luckheeram RV, Huang S, Chen M, et al. Utility of in vitro interferon-γ release assay in differential diagnosis between intestinal tuberculosis and Crohn’s disease. J Dig Dis 2013;14:68-75. https://doi.org/10.1111/1751-2980.12017.
- Liu F, Gao M, Zhang X, Du F, Jia H, Yang X, et al. Interferon-gamma release assay performance of pleural fluid and peripheral blood in pleural tuberculosis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0083857.
- Lodha R, Mukherjee A, Saini D, Saini S, Singh V, Singh S, et al. Role of the QuantiFERON®-TB Gold In-Tube test in the diagnosis of intrathoracic childhood tuberculosis. Int J Tuberc Lung Dis 2013;17:1383-8. https://doi.org/10.5588/ijtld.13.0348.
- Mahomed H, Ehrlich R, Hawkridge T, Hatherill M, Geiter L, Kafaar F, et al. Screening for TB in high school adolescents in a high burden setting in South Africa. Tuberculosis 2013;93:357-62. https://doi.org/10.1016/j.tube.2013.02.007.
- Fei B, Wu Z, Min K, Zhang J, Ding C, Wu H. Interferon-γ release assay in the diagnosis of laryngeal tuberculosis. Acta Otolaryngol 2014;134:314-7. https://doi.org/10.3109/00016489.2013.850174.
- Garazzino S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al. Performance of interferon-gamma release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J 2014;33:e226-31. https://doi.org/10.1097/INF.0000000000000353.
- Kim CH, Kim JY, Hwang YI, Lee CY, Choi JH, Park YB, et al. Interferon-γ enzyme-linked immunospot assay in patients with tuberculosis and healthy adults. Tuberc Respir Dis 2014;76:23-9. https://doi.org/10.4046/trd.2014.76.1.23.
- Kim CH, Lim JK, Yoo SS, Lee SY, Cha SI, Park JY, et al. Diagnostic performance of the QuantiFERON-TB Gold In-Tube assay and factors associated with nonpositive results in patients with miliary tuberculosis. Clin Infect Dis 2014;58:986-9. https://doi.org/10.1093/cid/ciu045.
- Park H, Shin JA, Kim HJ, Ahn CM, Chang YS. Whole blood interferon-γ release assay is insufficient for the diagnosis of sputum smear negative pulmonary tuberculosis. Yonsei Med J 2014;55:725-31. https://doi.org/10.3349/ymj.2014.55.3.725.
- Schopfer K, Rieder HL, Bodmer T, Steinlin-Schopfer JF, Chantana Y, Studer P, et al. The sensitivity of an interferon-γ release assay in microbiologically confirmed pediatric tuberculosis. Eur J Pediatr 2014;173:331-6. https://doi.org/10.1007/s00431-013-2161-x.
- Wang X, Wu Y, Wang M, Wang Y. The sensitivity of T-SPOT.TB assay in diagnosis of pediatric tuberculosis. Fetal Pediatr Pathol 2014;33:123-5. https://doi.org/10.3109/15513815.2013.878010.
- Wlodarczyk M, Rudnicka W, Janiszewska-Drobinska B, Kielnierowski G, Kowalewicz-Kulbat M, Fol M, et al. Interferon-gamma assay in combination with tuberculin skin test are insufficient for the diagnosis of culture-negative pulmonary tuberculosis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0107208.
- Anwar A, Hamdan AJ, Salim B, Yosra A, Hani M, Abdullah AH. Diagnostic utility of QuantiFERON-TB Gold (QFT-G) in active pulmonary tuberculosis. J Glob Infect Dis 2015;7:108-12. https://doi.org/10.4103/0974-777X.162231.
- Bao L, Li T, Diao N, Shen Y, Shao L, Zhang Y, et al. Fluctuating behavior and influential factors in the performance of the QuantiFERON-TB Gold In-Tube Assay in the diagnosis of tuberculosis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0103763.
- Sali M, Buonsenso D, Goletti D, D’Alfonso P, Zumbo A, Fadda G, et al. Accuracy of QuantiFERON-TB gold test for tuberculosis diagnosis in children. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0138952.
- Shin JA, Chang YS, Kim HJ, Ahn CM, Byun MK. Diagnostic utility of interferon-gamma release assay in extrapulmonary tuberculosis. Diagn Microbiol Infect Dis 2015;82:44-8. https://doi.org/10.1016/j.diagmicrobio.2015.02.002.
- Sun L, Tian JL, Yin QQ, Xiao J, Li JQ, Guo YJ, et al. Performance of the interferon gamma release assays in tuberculosis disease in children five years old or less. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0143820.
- Wong KS, Huang YC, Hu HC, Huang YC, Wen CH, Lin TY. Diagnostic utility of QuantiFERON-TB Gold In-Tube test in pediatric tuberculosis disease in Taiwanese children. J Microbiol Immunol Infect 2017;50:349-54. https://doi.org/10.1016/j.jmii.2015.07.012.
- Xia H, Wang X, Li F, Longuet C, Vernet G, Goletti D, et al. Diagnostic values of the QuantiFERON-TB Gold In-tube assay carried out in China for diagnosing pulmonary tuberculosis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0121021.
- Uzunhan O, Törün SH, Somer A, Salman N, Köksalan K. Comparison of tuberculin skin test and QuantiFERON®-TB Gold In-Tube for the diagnosis of childhood tuberculosis. Pediatr Int 2015;57:893-6. https://doi.org/10.1111/ped.12659.
- Azghay M, Bouchaud O, Mechaï F, Nicaise P, Fain O, Stirnemann J. Utility of QuantiFERON-TB Gold In-Tube assay in adult, pulmonary and extrapulmonary, active tuberculosis diagnosis. Int J Infect Dis 2016;44:25-30. https://doi.org/10.1016/j.ijid.2016.01.004.
- Jia H, Pan L, Du B, Sun Q, Wei R, Xing A, et al. Diagnostic performance of interferon-γ release assay for lymph node tuberculosis. Diagn Microbiol Infect Dis 2016;85:56-60. https://doi.org/10.1016/j.diagmicrobio.2016.02.001.
Appendix 1 Reporting checklist for diagnostic accuracy studies
| Section and topic | Number | Item | Reported on page number |
|---|---|---|---|
| Title or abstract | |||
| 1 | Identification as a study of diagnostic accuracy using at least one measure of accuracy (such as sensitivity, specificity, predictive values or AUC) | vii, viii | |
| Abstract | |||
| 2 | Structured summary of study design, methods, results and conclusions | vii, viii | |
| Introduction | |||
| 3 | Scientific and clinical background, including the intended use and clinical role of the index test | 1, 2 | |
| 4 | Study objectives and hypotheses | 2 | |
| Methods | |||
| Study design | 5 | Whether data collection was planned before the index test and reference standard were performed (prospective study) or after (retrospective study) | 3 |
| Participants | 6 | Eligibility criteria | 3 |
| 7 | On what basis potentially eligible participants were identified (such as symptoms, results from previous tests, inclusion in registry) | 3 | |
| 8 | Where and when potentially eligible participants were identified (setting, location and dates) | 3, 15 | |
| 9 | Whether participants formed a consecutive, random or convenience series | 3, Figure 1 | |
| Test methods | 10a | Index test, in sufficient detail to allow replication | 5–8 |
| 10b | Reference standard, in sufficient detail to allow replication | 8, 9 | |
| 11 | Rationale for choosing the reference standard (if alternatives exist) | Not applicable | |
| 12a | Definition of and rationale for test positivity cut-off points or result categories of the index test, distinguishing pre-specified from exploratory | 6–8 | |
| 12b | Definition of and rationale for test positivity cut-off points or result categories of the reference standard, distinguishing pre-specified from exploratory | Appendix 2 | |
| 13a | Whether clinical information and reference standard results were available to the performers/readers of the index test | 5 | |
| 13b | Whether clinical information and index test results were available to the assessors of the reference standard | 8, 9 | |
| Analysis | 14 | Methods for estimating or comparing measures of diagnostic accuracy | 10, 11 |
| 15 | How indeterminate index test or reference standard results were handled | 11 | |
| 16 | How missing data on the index test and reference standard were handled | 11 | |
| 17 | Any analyses of variability in diagnostic accuracy, distinguishing pre-specified from exploratory | 11 | |
| 18 | Intended sample size and how it was determined | 9, 10 | |
| Results | |||
| Participants | 19 | Flow of participants, using a diagram | 15, 16, Figure 4 |
| 20 | Baseline demographic and clinical characteristics of participants | 15–21, Tables 3–7, Appendix 4 | |
| 21a | Distribution of severity of disease in those with the target condition | 22, 23, Table 9 | |
| 21b | Distribution of alternative diagnoses in those without the target condition | 22, 24, Table 10 | |
| 22 | Time interval and any clinical interventions between index test and reference standard | 8 | |
| Test results | 23 | Cross-tabulation of the index test results (or their distribution) by the results of the reference standard | Tables 11, 13, 20, 22, 37, 38 and 62 |
| 24 | Estimates of diagnostic accuracy and their precision (such as 95% CIs) | 25–41, 54–57, Appendix 13 | |
| 25 | Any adverse events from performing the index test or the reference standard | Not applicable | |
| Discussion | |||
| 26 | Study limitations, including sources of potential bias, statistical uncertainty and generalisability | 86, 87 | |
| 27 | Implications for practice, including the intended use and clinical role of the index test | 87 | |
| Other information | |||
| 28 | Registration number and name of registry | Not registered | |
| 29 | Where the full study protocol can be accessed | www.nets.nihr.ac.uk/__data/assets/pdf_file/0011/51977/PRO-08-106-02.pdf | |
| 30 | Sources of funding and other support; role of funders | viii | |
Appendix 2 Composite reference standard for diagnosis of active tuberculosis
| Diagnostic category | Criteria |
|---|---|
| 1: Culture-confirmed TB | Microbiological culture of M. tuberculosis AND suggestive clinical and radiological findings |
| 2: Highly probable TB | Clinical and radiological features highly suggestive of TB and unlikely to be caused by other disease, AND a decision to treat made by a clinician, AND appropriate response to therapy AND histology supportive, if available |
| 3: Clinically indeterminate | Final diagnosis of TB neither highly probable nor reliably excluded |
| 4: Active TB excluded | |
| Subclassification | |
| 4A: inactive TB | Stable CXR changes, AND TST positivea (if done), AND bacteriologically negative (if done) AND no clinical evidence of active disease |
| 4B: one or more risk factors for TB exposure,a TST positiveb | TST positive,b AND bacteriologically negative (if done) AND no clinical evidence of active disease |
| 4C: one or more risk factors for TB exposure,a TST negative | History of TB exposure AND TST negative (if done) |
| 4D: no risk factors for TB exposure,a TST negative | No history of TB exposure AND TST negative (if done) |
Appendix 3 Protocol amendments
| Amendment number (date) | Details of changes |
|---|---|
| AM01 | |
| Substantial amendment (April 2011) |
|
| AM02 | |
| Substantial amendment (October 2011) |
|
| AM03 | |
| Substantial amendment (October 2012) |
|
| AM04 | |
| Non-substantial (February 2013) |
|
| AM05 | |
| Substantial amendment (October 2013) |
|
| AM06 | |
| Substantial amendment (April 2014) |
|
| AM07 | |
| Substantial amendment (February 2015) |
|
Appendix 4 Country of birth of patients
| Country of birth | Dosanjh category, n | Total, N | |||
|---|---|---|---|---|---|
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | ||
| Afghanistan | 3 | 0 | 0 | 3 | 6 |
| Algeria | 0 | 0 | 0 | 2 | 2 |
| Angola | 1 | 0 | 0 | 1 | 2 |
| Antigua and Barbuda | 0 | 0 | 0 | 1 | 1 |
| Argentina | 0 | 0 | 0 | 2 | 2 |
| Bangladesh | 4 | 1 | 1 | 13 | 19 |
| Belarus | 0 | 0 | 0 | 1 | 1 |
| Belgium | 1 | 0 | 0 | 0 | 1 |
| The Plurinational State of Bolivia | 1 | 0 | 0 | 1 | 2 |
| Brazil | 0 | 0 | 0 | 6 | 6 |
| Burundi | 2 | 0 | 0 | 0 | 2 |
| Cameroon | 0 | 0 | 0 | 1 | 1 |
| Chile | 0 | 0 | 0 | 1 | 1 |
| China | 1 | 0 | 0 | 0 | 1 |
| Colombia | 1 | 0 | 0 | 0 | 1 |
| Democratic Republic of the Congo | 1 | 0 | 0 | 2 | 3 |
| Cyprus | 0 | 0 | 0 | 3 | 3 |
| Denmark | 0 | 0 | 0 | 1 | 1 |
| Djibouti | 0 | 0 | 0 | 1 | 1 |
| Ecuador | 0 | 0 | 0 | 1 | 1 |
| Egypt | 0 | 0 | 0 | 1 | 1 |
| Eritrea | 5 | 2 | 3 | 3 | 13 |
| Estonia | 0 | 0 | 0 | 1 | 1 |
| Ethiopia | 2 | 2 | 0 | 4 | 8 |
| France | 0 | 0 | 0 | 2 | 2 |
| The Gambia | 0 | 0 | 0 | 1 | 1 |
| Germany | 0 | 0 | 0 | 2 | 2 |
| Ghana | 0 | 1 | 0 | 6 | 7 |
| Grenada | 1 | 0 | 0 | 0 | 1 |
| Guinea-Bissau | 0 | 0 | 0 | 1 | 1 |
| Hong Kong | 1 | 0 | 0 | 1 | 2 |
| India | 117 | 42 | 5 | 62 | 226 |
| Indonesia | 1 | 0 | 0 | 0 | 1 |
| Iran | 0 | 0 | 0 | 5 | 5 |
| Iraq | 1 | 0 | 0 | 4 | 5 |
| Ireland | 4 | 1 | 0 | 6 | 11 |
| Italy | 0 | 0 | 1 | 2 | 3 |
| Jamaica | 5 | 1 | 2 | 6 | 14 |
| Kazakhstan | 1 | 0 | 0 | 0 | 1 |
| Kenya | 5 | 2 | 0 | 19 | 26 |
| Kuwait | 2 | 0 | 1 | 0 | 3 |
| Libya | 1 | 0 | 0 | 0 | 1 |
| Lithuania | 0 | 0 | 0 | 1 | 1 |
| Malawi | 1 | 2 | 1 | 1 | 5 |
| Mauritius | 0 | 0 | 0 | 1 | 1 |
| Morocco | 1 | 0 | 0 | 4 | 5 |
| Mozambique | 0 | 0 | 0 | 1 | 1 |
| Nepal | 7 | 5 | 0 | 5 | 17 |
| Niger | 0 | 0 | 0 | 1 | 1 |
| Nigeria | 7 | 1 | 0 | 4 | 12 |
| Pakistan | 14 | 8 | 4 | 27 | 53 |
| Philippines | 9 | 5 | 1 | 6 | 21 |
| Poland | 1 | 1 | 1 | 10 | 13 |
| Portugal | 1 | 0 | 0 | 2 | 3 |
| Romania | 3 | 0 | 2 | 1 | 6 |
| Saudi Arabia | 0 | 0 | 0 | 1 | 1 |
| Sierra Leone | 0 | 0 | 0 | 3 | 3 |
| Somalia | 10 | 9 | 3 | 16 | 38 |
| South Africa | 1 | 2 | 1 | 5 | 9 |
| Spain | 0 | 0 | 1 | 0 | 1 |
| Sri Lanka | 7 | 0 | 1 | 13 | 21 |
| Sudan | 1 | 0 | 0 | 4 | 5 |
| Swaziland | 0 | 1 | 0 | 1 | 2 |
| Sweden | 0 | 0 | 0 | 1 | 1 |
| Switzerland | 0 | 0 | 0 | 1 | 1 |
| Syria | 0 | 0 | 0 | 1 | 1 |
| United Republic of Tanzania | 1 | 0 | 1 | 1 | 3 |
| Thailand | 0 | 1 | 0 | 2 | 3 |
| Uganda | 2 | 0 | 1 | 8 | 11 |
| UK | 32 | 11 | 12 | 138 | 193 |
| USA | 0 | 2 | 0 | 1 | 3 |
| Uruguay | 0 | 0 | 0 | 1 | 1 |
| Yemen | 0 | 0 | 0 | 1 | 1 |
| Zambia | 1 | 0 | 0 | 1 | 2 |
| Zimbabwe | 1 | 2 | 1 | 10 | 14 |
| Total | 261 | 102 | 43 | 439 | 845 |
Appendix 5 Thresholds used by centres for defining vitamin D status
| Hospital trust | Vitamin D status | ||
|---|---|---|---|
| Deficient | Insufficient | Normal | |
| Imperial College Healthcare NHS Trust | < 40 nmoI/l | 40–70 nmoI/l | 70–150 nmoI/l |
| Heart of England NHS Foundation Trust | < 30 nmoI/l | Not specified | > 49.9 nmoI/l |
| Chelsea and Westminster Hospital NHS Foundation Trust | < 40 nmoI/l | 40–70 nmoI/l | 70–150 nmoI/l |
| Royal Free London NHS Foundation Trust | < 25 nmoI/l | 25–75 nmoI/l | > 75 nmol/l |
| St George’s University Hospitals NHS Foundation Trust | < 50 nmoI/l | Not specified | 50–200 nmoI/l |
| Frimley Health NHS Foundation Trust | < 50 nmoI/l | Not specified | 50–150 nmoI/l |
| University Hospitals of Leicester NHS Trust | < 25 nmoI/l | 25–50 nmoI/l | > 50 nmoI/l |
| London North West Healthcare NHS Trust | < 12.5 nmoI/l | 12.5–50 nmoI/l | 50–140 nmoI/l |
| Oxford University Hospitals NHS Foundation Trust | < 50 nmoI/l | Not specified | > 50 nmoI/l |
| Sandwell and West Birmingham Hospitals NHS Trust | < 30 nmoI/l | 30–50 nmoI/l | > 50 nmoI/l |
| King’s College Hospital NHS Foundation Trust | < 20 µg/l | Not specified | 20–50 µg/l |
| Barts Health NHS Trust | < 30 nmoI/l | 30–50 nmoI/l | 80–150 nmoI/l |
| Ealing Hospital | < 25 nmoI/l | 25–50 nmoI/l | 51–163 nmoI/l |
Appendix 6 Interferon gamma release assays and tuberculin skin test performed in routine workup of active tuberculosis: main study cohort
| Hospital trust | Tests performed, n | Number of patientsa | ||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | TST | ||
| Imperial College Healthcare NHS Trust | 125 | 14 | 87 | 238 |
| Heart of England NHS Foundation Trust | 29 | 28 | 21 | 83 |
| Chelsea and Westminster Hospital NHS Foundation Trust | 26 | 0 | 0 | 40 |
| Royal Free London NHS Foundation Trust | 1 | 2 | 0 | 41 |
| St George’s University Hospitals NHS Foundation Trust | 29 | 1 | 29 | 43 |
| University Hospitals of Leicester NHS Trust | 4 | 22 | 0 | 100 |
| London North West Healthcare NHS Trust | 2 | 2 | 191 | 257 |
| Oxford University Hospitals NHS Foundation Trust | 0 | 0 | 0 | 2 |
| Sandwell and West Birmingham Hospitals NHS Trust | 6 | 2 | 8 | 41 |
| Total | 222 | 71 | 336b | 845 |
Appendix 7 Additional T-SPOT.TB and QFT-GIT results in all patients in the main study cohort
For T-SPOT. TB, there were 68 test positives out of 93 highly probable TB cases, with a sensitivity (95% CI) of 73.1% (63.3% to 81.1%). For QFT-GIT, there were 57 test positives out of 96 highly probable TB cases, with a sensitivity (95% CI) of 59.4% (49.4% to 68.7%). Indeterminate IGRA results were excluded from all analyses.
| T-SPOT.TB, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT, n (%) | Positive | 234 (73.4) | 45 (11.3) | 9 (27.3) | 13 (22.8) | 7 (19.4) | 308 (36.4) |
| Negative | 65 (20.4) | 307 (76.8) | 20 (60.6) | 35 (61.4) | 6 (16.7) | 433 (51.2) | |
| Indeterminate | 19 (6.0) | 45 (11.3) | 4 (12.1) | 9 (15.8) | 2 (5.6) | 79 (9.3) | |
| Missing | 1 (0.3) | 3 (0.8) | 0 (0.0) | 0 (0.0) | 21 (58.3) | 25 (3.0) | |
| Total | 319 (100) | 400 (100) | 33 (100) | 57 (100) | 36 (100) | 845 (100) | |
| Test performance | T-SPOT.TB | QFT-GIT | ||
|---|---|---|---|---|
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 253/311 | 81.4 (76.6 to 85.3) | 220/327 | 67.3 (62.0 to 72.1) |
| Culture-positive TB | 185/218 | 84.9 (79.5 to 89.0) | 163/231 | 70.6 (64.4 to 76.1) |
| Culture-negative TB | 58/83 | 69.9 (59.3 to 78.7) | 48/84 | 57.1 (46.5 to 67.2) |
| Smear-positive TB | 45/55 | 81.8 (69.7 to 89.8) | 42/56 | 75.0 (62.3 to 84.5) |
| Smear-negative TB | 169/206 | 82.0 (76.2 to 86.7) | 148/222 | 66.7 (60.2 to 72.5) |
| Pulmonary TB | 79/105 | 75.2 (66.2 to 82.5) | 79/115 | 68.7 (59.7 to 76.5) |
| Extrapulmonary TB | 141/169 | 83.4 (77.1 to 88.3) | 113/171 | 66.1 (58.7 to 72.8) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 319/370 | 86.2 (82.3 to 89.4) | 304/378 | 80.4 (76.1 to 84.1) |
| Active TB excluded, TST negative, no risk factors for LTBI | 87/93 | 93.5 (86.6 to 97.0) | 85/91 | 93.4 (86.4 to 96.9) |
| Predictive values | ||||
| PPV | 253/304 | 83.2 (78.6 to 87.0) | 220/294 | 74.8 (69.6 to 79.5) |
| NPV | 319/377 | 84.6 (80.6 to 87.9) | 304/411 | 74.0 (69.5 to 78.0) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 5.90 (4.55 to 7.66) | – | 3.44 (2.76 to 4.27) |
| Negative likelihood ratio | – | 0.22 (0.17 to 0.27) | – | 0.41 (0.35 to 0.48) |
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 287/345 | 83.2 (78.9 to 86.8) | 246/353 | 69.7 (64.7 to 74.2) |
| Culture-positive TB | 213/246 | 86.6 (81.8 to 90.3) | 184/252 | 73.0 (67.2 to 78.1) |
| Culture-negative TB | 64/88 | 72.7 (62.6 to 80.9) | 53/89 | 59.6 (49.2 to 69.1) |
| Smear-positive TB | 53/63 | 84.1 (73.2 to 91.1) | 53/67 | 79.1 (67.9 to 87.1) |
| Smear-negative TB | 192/229 | 83.8 (78.5 to 88.0) | 159/232 | 68.2 (62.0 to 73.9) |
| Pulmonary TB | 94/120 | 78.3 (70.2 to 84.8) | 89/125 | 71.2 (62.7 to 78.4) |
| Extrapulmonary TB | 155/183 | 84.7 (78.8 to 89.2) | 127/185 | 68.6 (61.6 to 74.9) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 319/423 | 75.4 (71.1 to 79.3) | 304/425 | 71.5 (67.1 to 75.6) |
| Active TB excluded, TST negative, no risk factors for LTBI | 87/111 | 78.4 (69.8 to 85.0) | 85/110 | 77.3 (68.6 to 84.1) |
| Predictive values | ||||
| PPV | 287/391 | 73.4 (68.8 to 77.5) | 246/367 | 67.0 (62.1 to 71.6) |
| NPV | 319/377 | 84.6 (80.6 to 87.9) | 304/411 | 74.0 (69.5 to 78.0) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 3.38 (2.85 to 4.03) | – | 2.45 (2.07 to 2.89) |
| Negative likelihood ratio | – | 0.22 (0.18 to 0.28) | – | 0.42 (0.36 to 0.50) |
| Test | Number of test resultsa | Sensitivity (95% CI) | Number of test resultsb | Specificity (95% CI) |
|---|---|---|---|---|
| T-SPOT.TB | 311 | 80.7 (76.1 to 84.7) | 370 | 86.5 (82.7 to 89.6) |
| QFT-GIT | 327 | 67.3 (62.0 to 72.1) | 378 | 81.1 (76.9 to 84.7) |
| Ratioc (95% CI); p-value | – | 1.20 (1.12 to 1.29); < 0.001 | – | 1.07 (1.02 to 1.12); 0.004 |
| Test | Number of test resultsa | Sensitivity (95% CI) | Number of test resultsb | Specificity (95% CI) |
|---|---|---|---|---|
| T-SPOT.TB | 345 | 83.3 (78.9 to 86.8) | 386 | 75.4 (71.1 to 79.3) |
| QFT-GIT | 353 | 69.7 (64.7 to 74.3) | 378 | 71.6 (67.1 to 75.7) |
| Ratioc (95% CI); p-value | – | 1.19 (1.12 to 1.28); < 0.001 | – | 1.05 (0.98 to 1.13); 0.1 |
Appendix 8 Additional T-SPOT.TB and QFT-GIT results in human immunodeficiency virus-positive and -negative patients in the main study cohort
| Index test result | Dosanjh category | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| T-SPOT.TB | |||||||||
| Positive | 7 | 5 | 0 | 0 | 0 | 4 | 1 | 5 | 17 |
| Negative | 4 | 3 | 2 | 0 | 1 | 42 | 28 | 71 | 80 |
| Borderline | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 |
| Indeterminate | 2 | 3 | 0 | 1 | 0 | 11 | 16 | 28 | 33 |
| Missing | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| Total | 13 | 12 | 2 | 1 | 1 | 61 | 45 | 108 | 135 |
| Median SFCs ESAT-6 (range) | 10 (0–94) | 8 (0–395) | 0 | 0 | 0 | 0 (0–42) | 0 (0–69) | 0 (0–69) | 0 (0–395) |
| Median SFCs CFP-10 (range) | 2 (0–136) | 0 (0–315) | 0 | 0 | 0 | 0 (0–37) | 0 (0–44) | 0 (0–44) | 0 (0–315) |
| QFT-GIT | |||||||||
| Positive | 8 | 5 | 0 | 0 | 0 | 5 | 2 | 7 | 20 |
| Negative | 5 | 5 | 1 | 1 | 1 | 42 | 36 | 80 | 91 |
| Indeterminate | 0 | 2 | 1 | 0 | 0 | 14 | 6 | 20 | 23 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Total | 13 | 12 | 2 | 1 | 1 | 61 | 45 | 108 | 135 |
| Median IFN-γ levels (range) | 0.95 (0–3.78) | 0.01 (0–10) | 0.01 (0–0.02) | 0.07 | 0 | 0.01 (0–3.82) | 0.01 (0–1.56) | 0.01 (0–3.82) | 0.02 (0–10) |
| T-SPOT.TB, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT, n (%) | Positive | 13 (76.4) | 3 (3.8) | 0 | 4 (12.1) | 0 | 20 (14.8) |
| Negative | 2 (11.8) | 60 (75.0) | 3 (100.0) | 24 (72.7) | 2 (100.0) | 91 (67.4) | |
| Indeterminate | 2 (11.8) | 16 (20.0) | 0 | 5 (15.2) | 0 | 23 (17.0) | |
| Missing | 0 | 1 (1.3) | 0 | 0 | 0 | 1 (0.7) | |
| Total | 17 (100.0) | 80 (100.0) | 3 (100.0) | 33 (100.0) | 2 (100.0) | 135 (100.0) | |
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 17/24 | 70.8 (50.8 to 85.1) | 15/25 | 60.0 (40.7 to 76.6) |
| Culture-positive TB | 9/13 | 69.2 (42.4 to 87.3) | 8/13 | 61.5 (35.5 to 82.3) |
| Culture-negative TB | 8/11 | 72.7 (43.4 to 90.3) | 7/11 | 63.6 (35.4 to 84.8) |
| Smear-positive TB | 2/5 | 40.0 (11.8 to 76.9) | 3/5 | 60.0 (23.1 to 88.2) |
| Smear-negative TB | 11/15 | 73.3 (48.0 to 89.1) | 9/16 | 56.2 (33.2 to 76.9) |
| Pulmonary TB | 6/8 | 75.0 (40.9 to 92.9) | 5/8 | 62.5 (30.6 to 86.3) |
| Extrapulmonary TB | 9/12 | 75.0 (46.8 to 91.1) | 7/13 | 53.8 (29.1 to 76.8) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 71/107 | 66.4 (57.0 to 74.6) | 80/107 | 74.8 (65.8 to 82.0) |
| Active TB excluded, TST negative, no risk factors for LTBI | 28/45 | 62.2 (47.6 to 74.9) | 36/44 | 81.8 (68.0 to 90.5) |
| Predictive values | ||||
| PPV | 17/53 | 32.1 (21.1 to 45.5) | 15/42 | 35.7 (23.0 to 50.8) |
| NPV | 71/78 | 91.0 (82.6 to 95.6) | 80/90 | 88.9 (80.7 to 93.9) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 2.11 (1.46 to 3.05) | – | 2.38 (1.51 to 3.76) |
| Negative likelihood ratio | – | 0.44 (0.23 to 0.83) | – | 0.54 (0.33 to 0.88) |
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 270/321 | 84.1 (79.7 to 87.7) | 231/328 | 70.4 (65.3 to 75.1) |
| Culture-positive TB | 204/233 | 87.6 (82.7 to 91.2) | 176/239 | 73.6 (67.7 to 78.8) |
| Culture-negative TB | 56/77 | 72.7 (61.9 to 81.4) | 46/78 | 59.0 (47.9 to 69.2) |
| Smear-positive TB | 51/58 | 87.9 (77.1 to 94.0) | 50/62 | 80.6 (69.1 to 88.6) |
| Smear-negative TB | 181/214 | 84.6 (79.1 to 88.8) | 150/217 | 69.1 (62.7 to 74.9) |
| Pulmonary TB | 88/112 | 78.6 (70.1 to 85.2) | 84/117 | 71.8 (63.0 to 79.2) |
| Extrapulmonary TB | 146/171 | 85.4 (79.3 to 89.9) | 120/172 | 69.8 (62.5 to 76.1) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 248/316 | 78.5 (73.6 to 82.7) | 224/318 | 70.4 (65.2 to 75.2) |
| Active TB excluded, TST negative, no risk factors for LTBI | 59/66 | 89.4 (79.7 to 94.8) | 49/66 | 74.2 (62.6 to 83.3) |
| Predictive values | ||||
| PPV | 270/338 | 79.9 (75.3 to 83.8) | 231/325 | 71.1 (65.9 to 75.7) |
| NPV | 248/299 | 82.9 (78.3 to 86.8) | 224/321 | 69.8 (64.6 to 74.6) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 3.91 (3.15 to 4.85) | – | 2.38 (1.98 to 2.86) |
| Negative likelihood ratio | – | 0.20 (0.16 to 0.26) | – | 0.42 (0.35 to 0.50) |
Appendix 9 Additional T-SPOT.TB and QFT-GIT results in patients with diabetes mellitus in the main study cohort
| Index test result | Dosanjh category | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4A | 4B | 4C | 4D | 4A–D | ||
| T-SPOT.TB | |||||||||
| Positive | 13 | 3 | 2 | 0 | 2 | 6 | 1 | 9 | 27 |
| Negative | 7 | 1 | 4 | 1 | 4 | 27 | 6 | 38 | 50 |
| Borderline | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 3 |
| Indeterminate | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 4 |
| Missing | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 4 |
| Total | 22 | 5 | 8 | 1 | 6 | 39 | 7 | 53 | 88 |
| Median SFCs ESAT-6 (range) | 8 (0–123) | 12 (1–26) | 0 (0–4) | 0 | 0 (0–9) | 0 (0–83) | 0 (0–22) | 0 (0–83) | 0 (0–123) |
| Median SFCs CFP-10 (range) | 5 (0–275) | 51 (2–103) | 1 (0–20) | 0 | 0 (0–20) | 0 (0–120) | 0 (0–4) | 0 (0–120) | 1 (0–275) |
| QFT-GIT | |||||||||
| Positive | 12 | 3 | 2 | 0 | 1 | 8 | 1 | 10 | 27 |
| Negative | 10 | 2 | 5 | 1 | 5 | 27 | 4 | 37 | 54 |
| Indeterminate | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 4 | 5 |
| Missing | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 |
| Total | 22 | 5 | 8 | 1 | 6 | 39 | 7 | 53 | 88 |
| Median IFN-γ levels (range) | 0.39 (0–10) | 1.1 (0.11–5.84) | 0.07 (0–3.58) | 0 | 0.01 (0–0.84) | 0.01 (0–10) | 0 (0–5.92) | 0 (0–10) | 0.07 (0–10) |
| T-SPOT.TB, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT, n (%) | Positive | 18 (66.7) | 5 (10.0) | 1 (33.3) | 2 (50.0) | 1 (25.0) | 27 (30.7) |
| Negative | 8 (29.6) | 41 (82.0) | 2 (66.7) | 2 (50.0) | 1 (25.0) | 54 (61.4) | |
| Indeterminate | 1 (3.7) | 4 (8.0) | 0 | 0 | 0 | 5 (5.7) | |
| Missing | 0 | 0 | 0 | 0 | 2 (50.0) | 2 (2.3) | |
| Total | 27 (100.0) | 50 (100.0) | 3 (100.0) | 4 (100.0) | 4 (100.0) | 88 (100.0) | |
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 19/27 | 70.4 (51.5 to 84.2) | 15/27 | 55.6 (37.3 to 72.4) |
| Culture-positive TB | 15/22 | 68.2 (47.3 to 83.6) | 12/22 | 54.5 (34.7 to 73.1) |
| Culture-negative TB | 4/5 | 80.0 (37.6 to 96.4) | 3/5 | 60.0 (23.1 to 88.2) |
| Smear-positive TB | 7/9 | 77.8 (45.3 to 93.7) | 6/9 | 66.7 (35.4 to 87.9) |
| Smear-negative TB | 11/16 | 68.8 (44.4 to 85.8) | 7/16 | 43.8 (23.1 to 66.8) |
| Pulmonary TB | 5/9 | 55.6 (26.7 to 81.1) | 5/9 | 55.6 (26.7 to 81.1) |
| Extrapulmonary TB | 11/14 | 78.6 (52.4 to 92.4) | 8/14 | 57.1 (32.6 to 78.6) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 38/49 | 77.6 (64.1 to 87.0) | 37/51 | 72.6 (59.1 to 82.9) |
| Active TB excluded, TST negative, no risk factors for LTBI | 6/7 | 85.7 (48.7 to 97.4) | 4/7 | 57.1 (25.0 to 84.2) |
| Predictive values | ||||
| PPV | 19/30 | 63.3 (45.5 to 78.1) | 15/29 | 51.7 (34.4 to 68.6) |
| NPV | 38/46 | 82.6 (69.3 to 90.9) | 37/49 | 75.5 (61.9 to 85.4) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 3.14 (1.76 to 5.57) | – | 2.02 (1.16 to 3.54) |
| Negative likelihood ratio | – | 70.4 (51.5 to 84.2) | – | 55.6 (37.3 to 72.4) |
Appendix 10 Additional results for evaluations of second-generation interferon gamma release assay in the main study cohort
For both the four-antigen (ESAT-6, CFP-10, Rv3615c and Rv3879c) and three-antigen (ESAT, CFP-10, Rv3615), there were 70 test positives out of 94 highly probable TB cases with sensitivity (95% CI) of 74.5% (64.8% to 82.2%). For the other three-antigen (CFP-10, Rv3615c, Rv3879c) and two-antigen combination of CFP-10 and Rv3615, there were 66 test positives out of 93 highly probable TB cases with sensitivity (95% CI) of 71.0% (61.1% to 79.2%). Indeterminate IGRA results were excluded from all analyses.
| Test performance | Antigens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rv3615c | Rv3879c | Rv3873 | Rv2654 | ESAT-6 | CFP-10 | |||||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||||||||
| All TB | 274/345 | 79.4 (74.8 to 83.4) | 148/345 | 42.9 (37.8 to 48.2) | 135/345 | 39.1 (34.1 to 44.4) | 146/345 | 42.3 (37.2 to 47.6) | 246/345 | 71.3 (66.3 to 75.8) | 253/345 | 73.3 (68.4 to 77.7) |
| Culture-positive TB | 208/246 | 84.6 (79.5 to 88.5) | 108/246 | 43.9 (37.8 to 50.2) | 100/246 | 40.7 (34.7 to 46.9) | 108/246 | 43.9 (37.8 to 50.2) | 183/246 | 74.4 (68.6 to 79.4) | 189/246 | 76.8 (71.2 to 81.7) |
| Culture-negative TB | 57/88 | 64.8 (54.4 to 73.9) | 34/88 | 38.6 (29.1 to 49.1) | 31/88 | 35.2 (26.1 to 45.6) | 3388 | 37.5 (28.1 to 47.9) | 53/88 | 60.2 (49.8 to 69.8) | 56/88 | 63.6 (53.2 to 72.9) |
| Smear-positive TB | 53/63 | 84.1 (73.2 to 91.1) | 28/63 | 44.4 (32.8 to 56.7) | 26/63 | 41.3 (30.0 to 53.6) | 21/63 | 33.3 (22.9 to 45.6) | 44/63 | 69.8 (57.6 to 79.8) | 47/63 | 74.6 (62.7 to 83.7) |
| Smear-negative TB | 179/229 | 78.2 (72.4 to 83.0) | 103/229 | 45.0 (38.7 to 51.5) | 88/229 | 38.4 (32.4 to 44.9) | 103/229 | 45.0 (38.7 to 51.5) | 167/229 | 72.9 (66.8 to 78.3) | 169/229 | 73.8 (67.7 to 79.1) |
| Pulmonary TB | 94/120 | 78.3 (70.1 to 84.8) | 44/120 | 36.7 (28.6 to 45.6) | 37/120 | 30.8 (23.3 to 39.6) | 50/120 | 41.7 (33.2 to 50.6) | 80/120 | 66.7 (57.8 to 74.5) | 82/120 | 68.3 (59.6 to 76.0) |
| Extra pulmonary TB | 144/183 | 78.7 (72.2 to 84.0) | 84/183 | 45.9 (38.8 to 53.1) | 77/183 | 42.1 (35.2 to 49.3) | 76/183 | 41.5 (34.6 to 48.8) | 134/183 | 73.2 (66.4 to 79.1) | 138/183 | 75.4 (68.7 to 81.1) |
| Specificity for a diagnosis of active TB | ||||||||||||
| Active TB excluded | 320/423 | 75.7 (71.3 to 79.5) | 359/423 | 84.9 (81.1 to 88.0) | 366/423 | 86.5 (82.9 to 89.5) | 352/423 | 83.2 (79.4 to 86.5) | 339/423 | 80.1 (76.1 to 83.7) | 333/423 | 78.7 (74.6 to 82.4) |
| Active TB excluded, TST negative, no risk factors for LTBI | 86/111 | 77.5 (68.9 to 84.3) | 90/111 | 81.1 (72.8 to 87.3) | 92/111 | 82.9 (74.8 to 88.8) | 88/111 | 79.3 (70.8 to 85.8) | 87/111 | 78.4 (69.8 to 85.0) | 90/111 | 81.1 (72.8 to 87.3) |
| Predictive values | ||||||||||||
| PPV | 274/377 | 72.7 (68 to 76.9) | 148/212 | 69.8 (63.3 to 75.6) | 135/192 | 70.3 (63.5 to 76.3) | 146/217 | 67.3 (60.8 to 73.2) | 246/330 | 74.5 (69.6 to 78.9) | 255/343 | 74.3 (69.5 to 78.7) |
| NPV | 320/391 | 81.8 (77.7 to 85.4) | 359/556 | 64.6 (60.5 to 68.4) | 366/576 | 63.5 (59.5 to 67.4) | 352/551 | 63.9 (59.8 to 67.8) | 339/438 | 77.4 (73.3 to 81.1) | 333/425 | 78.4 (74.2 to 82.0) |
| Likelihood ratios | ||||||||||||
| Positive likelihood ratio | 3.26 (2.73 to 3.89) | 2.83 (2.19 to 3.66) | 2.93 (2.23 to 3.86) | 2.52 (1.97 to 3.22) | 3.59 (2.93 to 4.40) | 3.45 (2.84 to 4.19) | ||||||
| Negative likelihood ratio | 0.27 (0.22 to 0.34) | 0.67 (0.61 to 0.74) | 0.70 (0.64 to 0.77) | 0.69 (0.63 to 0.77) | 0.36 (0.30 to 0.43) | 0.35 (0.28 to 0.41) | ||||||
| Test performance | Antigen combinations | |||||||
|---|---|---|---|---|---|---|---|---|
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | ESAT-6 + CFP-10 + Rv3615c | CFP-10 + Rv3615c + Rv3879c | CFP-10 + Rv3615c | |||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||||
| All TB | 273/306 | 89.2 (85.2 to 92.2) | 273/306 | 89.2 (85.2 to 92.2) | 263/299 | 88.0 (3.8 to 91.2) | 263/301 | 87.4 (83.1 to 90.7) |
| Culture-positive TB | 203/216 | 94.0 (90.0 to 96.4) | 203/216 | 94.0 (90.0 to 96.4) | 197/211 | 93.4 (89.2 to 96.0) | 197/212 | 92.9 (88.7 to 95.7) |
| Culture-negative TB | 60/80 | 75.0 (64.5 to 83.2) | 60/80 | 75.0 (64.5 to 83.2) | 57/78 | 73.1 (62.3 to 81.7) | 57/80 | 71.2 (60.5 to 80.0) |
| Smear-positive TB | 48/51 | 94.1 (84.1 to 98.0) | 48/51 | 94.1 (84.1 to 98.0) | 47/50 | 94.0 (83.8 to 97.9) | 47/50 | 94.0 (83.8 to 97.9) |
| Smear-negative TB | 183/207 | 88.4 (83.3 to 92.1) | 183/207 | 88.4 (83.3 to 92.1) | 176/202 | 87.11 (81.8 to 91.1) | 176/204 | 86.3 (80.9 to 90.3) |
| Pulmonary TB | 88/100 | 88.0 (80.2 to 93.0) | 88/100 | 88.0 (80.2 to 93.0) | 85/97 | 87.6 (79.6 to 92.8) | 85/98 | 86.7 (78.6 to 92.1) |
| Extra pulmonary TB | 148/167 | 88.6 (82.9 to 92.6) | 148/167 | 88.6 (82.9 to 92.6) | 142/164 | 86.6 (80.5 to 91.0) | 142/165 | 86.1 (80.0 to 90.5) |
| Specificity for a diagnosis of active TB | ||||||||
| Active TB excluded | 290/368 | 78.8 (74.3 to 82.7) | 296/370 | 80.0 (75.6 to 83.8) | 296/372 | 79.6 (75.2 to 83.4) | 302/372 | 81.2 (76.9 to 84.8) |
| Active TB excluded, TST negative, no risk factors for LTBI | 82/91 | 90.1 (82.3 to 94.7) | 84/92 | 91.3 (83.8 to 95.5) | 84/93 | 90.3 (82.6 to 94.8) | 86/93 | 92.5 (85.3 to 96.3) |
| Predictive values | ||||||||
| PPV | 273/351 | 77.8 (73.1 to 81.8) | 273/347 | 78.7 (74.1 to 82.7) | 263/339 | 77.6 (72.8 to 81.7) | 263/333 | 79.0 (74.3 to 83.0) |
| NPV | 290/323 | 89.8 (86.0 to 92.6) | 296/329 | 90.0 (86.2 to 92.8) | 296/332 | 89.2 (85.4 to 92.1) | 302/340 | 88.8 (85.0 to 91.7) |
| Likelihood ratios | ||||||||
| Positive likelihood ratio | 4.21 (3.44 to 5.15) | 4.46 (3.62 to 5.49) | 4.31 (3.51 to 5.28) | 4.64 (3.74 to 5.76) | ||||
| Negative likelihood ratio | 0.14 (0.10 to 0.19) | 0.13 (0.10 to 0.19) | 0.15 (0.11 to 0.21) | 0.16 (0.12 to 0.21) | ||||
| Test performance | Antigen combinations | |||||||
|---|---|---|---|---|---|---|---|---|
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | ESAT-6 + CFP-10 + Rv3615c | CFP-10 + Rv3615c + Rv3879c | CFP-10 + Rv3615c | |||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||||
| All TB | 312/345 | 90.4 (86.9 to 93.1) | 312/345 | 90.4 (86.9 to 93.1) | 309/345 | 89.6 (85.9 to 92.4) | 307/345 | 89.0 (85.2 to 91.9) |
| Culture-positive TB | 233/246 | 94.7 (91.2 to 96.9) | 233/246 | 94.7 (91.2 to 96.9) | 232/246 | 94.3 (90.7 to 96.6) | 231/246 | 93.9 (90.2 to 96.3) |
| Culture-negative TB | 68/88 | 77.3 (67.5 to 84.8) | 68/88 | 77.3 (67.5 to 84.8) | 67/88 | 76.1 (66.3 to 83.8) | 66/88 | 75.0 (65.0 to 82.9) |
| Smear-positive TB | 60/63 | 95.2 (86.9 to 98.4) | 60/63 | 95.2 (86.9 to 98.4) | 60/63 | 95.2 (86.9 to 98.4) | 60/63 | 95.2 (86.9 to 98.4) |
| Smear-negative TB | 205/229 | 89.5 (84.9 to 92.9) | 205/229 | 89.5 (84.9 to 92.9) | 203/229 | 88.6 (83.9 to 92.1) | 201/229 | 87.8 (82.9 to 91.4) |
| Pulmonary TB | 108/120 | 90.0 (83.3 to 94.2) | 108/120 | 90.0 (83.3 to 94.2) | 108/120 | 90.0 (83.3 to 94.2) | 107/120 | 89.2 (82.3 to 93.6) |
| Extra pulmonary TB | 164/183 | 89.6 (84.4 to 93.3) | 164/183 | 89.6 (84.4 to 93.3) | 161/183 | 88.0 (82.5 to 91.9) | 160/183 | 87.4 (81.8 to 91.5) |
| Specificity for a diagnosis of active TB | ||||||||
| Active TB excluded | 290/423 | 68.6 (64.0 to 72.8) | 296/423 | 70.0 (65.4 to 74.2) | 296/423 | 70.0 (65.4 to 74.2) | 302/423 | 71.4 (66.9 to 75.5) |
| Active TB excluded, TST negative, no risk factors for LTBI | 82/111 | 73.9 (65.0 to 81.2) | 84/111 | 75.7 (66.9 to 82.7) | 84/111 | 75.7 (66.9 to 82.7) | 83/111 | 74.8 (66.0 to 81.9) |
| Predictive values | ||||||||
| PPV | 312/445 | 70.1 (65.7 to 74.2) | 312/439 | 71.1 (66.7 to 75.1) | 309/436 | 70.9 (66.4 to 74.9) | 307/428 | 71.7 (67.3 to 75.8) |
| NPV | 290/323 | 89.8 (86.0 to 92.6) | 296/329 | 90.0 (86.3 to 92.8) | 296/332 | 89.2 (85.4 to 92.1) | 302/340 | 88.8 (85.0 to 91.8) |
| Likelihood ratios | ||||||||
| Positive likelihood ratio | 2.88 (2.49 to 3.33) | 3.01 (2.59 to 3.50) | 2.98 (2.57 to 3.47) | 3.11 (2.66 to 3.63) | ||||
| Negative likelihood ratio | 0.14 (0.10 to 0.19) | 0.14 (0.10 to 0.19) | 0.15 (0.11 to 0.20) | 0.15 (0.11 to 0.21) | ||||
| Test | Number of test results | Sensitivity (95% CI) | Relative sensitivitya (95% CI) | p-value |
|---|---|---|---|---|
| T-SPOT.TB (ESAT-6 + CFP-10) | 345 | 83.3 (79.0 to 86.9) | – | – |
| CFP-10 + Rv3615c | 345 | 89.0 (85.2 to 91.9) | 1.07 (1.03 to 1.11) | < 0.001 |
| CFP-10 + Rv3615c + Rv3879c | 345 | 89.6 (85.9 to 92.4) | 1.08 (1.04 to 1.11) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c | 345 | 90.4 (86.8 to 93.1) | 1.09 (1.05 to 1.12) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | 345 | 90.5 (86.9 to 93.2) | 1.09 (1.05 to 1.12) | < 0.001 |
| Test | Number of test results | Specificity (95% CI) | Relative specificitya (95% CI) | p-value |
|---|---|---|---|---|
| T-SPOT.TB (ESAT-6 + CFP-10) | 423 | 75.4 (71.1 to 79.3) | – | – |
| CFP-10 + Rv3615c | 423 | 71.4 (66.9 to 75.5) | 0.95 (0.92 to 0.98) | 0.002 |
| CFP-10 + Rv3615c + Rv3879c | 423 | 70.0 (65.4 to 74.2) | 0.93 (0.89 to 0.96) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c | 423 | 70.0 (65.4 to 74.2) | 0.93 (0.90 to 0.96) | < 0.001 |
| ESAT-6 + CFP-10 + Rv3615c + Rv3879c | 423 | 68.6 (64.0 to 72.8) | 0.91 (0.88 to 0.94) | < 0.001 |
Appendix 11 Studies of interferon gamma release assays for the diagnosis of active tuberculosis
| Reference (author and year of publication) | Study design | Population | Setting (recruitment period) | Test | ||
|---|---|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | TST | ||||
| Adewole et al., 201350 | Prospective study of QFT-GIT compared with the TST for diagnosis of pulmonary active TB | 61 smear-positive TB cases [mean age 35.1 years (SD 4.3 years)] and 41 healthy disease-free controls [mean age 27.8 years (SD 2.1 years)] were enrolled and analysed | Nigeria (full text was unavailable, so unable to extract more information) | NE |
Sensitivity: 76.0% (95% CI 61.8% to 85.2%) Specificity: 63.7% (95% CI 46.0% to 76.0%) Indeterminate rate: 3.5% |
Sensitivity: 96.6% (95% CI 88.5% to 98.3%) Specificity: 30.0% (95% CI 20.0% to 56.0%) |
| Jeon et al., 201351 | Retrospective analysis of laboratory and clinical records to evaluate factors associated with indeterminate and negative QFT-GIT results in active TB patients | 1301 patients including 168 confirmed active TB cases [mean age of active TB cases 54.8 years (SD 20.1 years)] | Kyung Hee University Hospital, Seoul, Korea (September 2009 and April 2012) | NE |
Sensitivity: 76.8% (95% CI 69.8% to 82.5%) Specificity: 58.3% (95% CI 55.3% to 61.4%) Indeterminate rate: 8% |
NE |
| Jia et al., 201352 | Prospective study of T-SPOT.TB for diagnosis of osteoarticular TB | 145 patients were enrolled and all were HIV negative. 18 possible cases and 17 indeterminates were excluded. 86 culture-confirmed or probable patients (age range 18–76 years) with osteoarticular TB and 24 without active TB (age range 16–80 years) were analysed | Beijing Chest Hospital, China (July 2011–June 2012) |
Sensitivity: 94.2% (95% CI 87.1% to 97.5%) Specificity: 70.8% (95% CI 50.8% to 85.1%) No indeterminates |
NE | NE |
| Khalil et al., 201353 | Comparison of QFT-GIT and the TST for the diagnosis of pulmonary active TB | 50 pulmonary active TB cases [mean age 41.8 years (SD 19.0 years)] | Fauji Foundation Hospital, Rawalpindi, Pakistan (July 2011–January 2012) | NE | Sensitivity: 80% | Sensitivity: 28% |
| Kim et al., 201354 | Retrospective study of QFT-GIT for diagnosis of GUTB | 57 patients [mean age 52 years (range 17–88 years)] with clinical or radiological features suspicious of GUTB | Urology clinic in Korea (March 2009–August 2011) | NE |
Sensitivity: 63.3% (95% CI 45.5% to 78.1%) Specificity: 59.3% (95% CI 40.7% to 75.5%) |
NE |
| Lagrange et al., 201330 | Prospective study of QFT-GIT compared with the TST for diagnosis of TB, stratified by HIV infection status | 2213 patients were enrolled. QFT-GIT was performed for 96 patients [median age 38.0 years (IQR 30.5–42.0 years)] with pulmonary active TB and 180 non-active TB cases. Of the 276 patients, a TST was performed in 53 active TB cases and 82 non-active TB cases | Nine centres in India (January 2006–July 2008) | NE |
HIV positive Sensitivity: 66.7% (95% CI 48.2% to 82.0%) Specificity: 64.8% (95% CI 50.6% to 77.3%) HIV negative Sensitivity: 95.0% (95% CI 75.1% to 99.9%) Specificity: 25.0% (95% CI 10.7% to 44.9%) Total Sensitivity: 77.4% (95% CI 63.8% to 87.7%) Specificity: 51.2% (95% CI 39.9% to 62.4%) Results above are from 135 patients in which data were available for both QFT-GIT and the TST Indeterminate rate among the 276 patients: 7% |
HIV positive Sensitivity: 51.5% (95% CI 33.5% to 69.2%) Specificity: 83.3% (95% CI 70.7% to 92.1%) HIV negative Sensitivity: 85.0% (62.1% to 96.8%) Specificity: 57.1% (95% CI 37.2% to 75.5%) Total Sensitivity: 64.2% (95% CI 49.8% to 76.9%) Specificity: 74.4% (95% CI 63.3% to 82.4%) Threshold: ≥ 10 mm |
| Lavender et al., 201355 | Retrospective review of clinical records of patients with QFT-GIT results for the diagnosis of active TB | 415 QFT-GIT requested, of which 120 were excluded. 295 patients [median age 40 years (range 16–90 years)] with and without HIV infection were analysed | Newcastle upon Tyne Hospitals, UK (clinical records of patients who had QFT-GIT requested between 29 June 2005 and 28 October 2010) | NE |
Sensitivity: 71.4% (95% CI 59.3% to 81.1%) Specificity: 81.0% (95% CI 75.5% to 85.6%) |
NE |
| Lei et al., 201356 | Case–control study of T-SPOT.TB for differential diagnosis of intestinal TB and Crohn’s disease | 88 patients with intestinal TB [mean age 36.2 years (SD 14.1 years)] and 103 with Crohn’s disease [mean age 37.0 years (SD 15.7 years)] | Inflammatory Bowel Disease Centre, Zhongnan Hospital, China (2003–11) |
Sensitivity: 86% (95% CI 75% to 96%) Specificity: 93% (95% CI 86% to 99%) |
NE |
Sensitivity: 60% (95% CI 49% to 71%) Specificity: 80% (95% CI 71% to 89%) Threshold: ≥ 10 mm |
| Liu et al., 201357 | Prospective study of T-SPOT.TB for diagnosis of pleural TB | 168 patients were enrolled, but 70 were excluded because they had no final diagnosis. 98 subjects with pleural effusion and no HIV co-infection were analysed. 55 patients [median age 39 years (range 25–59 years)] had pleural TB and 43 patients [median age 39 years (range 25–59 years)] without pleural TB | Beijing Chest Hospital, China (May 2012–June 2013) |
Sensitivity: 92.7% (95% CI 82.7% to 97.1%) Specificity: 62.8% (95% CI 47.9% to 76.0%) Indeterminate rate: 2% (These are results from the analysis of peripheral blood samples) |
NE | NE |
| Lodha et al., 201358 | Prospective study comparing QFT-GIT with the TST for diagnosis of intrathoracic childhood TB | 362 children [median age 115.5 months (IQR 73–144 months)] were enrolled in a RCT of micronutrient supplementation in children with intrathoracic TB | Two tertiary care hospitals in India (recruitment period not reported) | NE | Sensitivity: 82.0% (95% CI 77.8 to 85.6%) |
Sensitivity: 93.0% (95% CI 90.0% to 95.5%) Threshold: ≥ 10 mm |
| Mahomed et al., 201359 | Prospective study screening for active TB in adolescents | 6363 adolescents (age range 12–16 years) were screened. 21 had active TB. The TST and QFT-GIT results were available for 5071 and 5524 adolescents, respectively | 11 high schools in Worcester, South Africa (2005–7) | NE |
Sensitivity: 93.8% (95% CI 78.8% to 100.0%) Specificity: 46.8% (95% CI 36.3% to 57.2%) |
Sensitivity: 84.6% (95% CI 61.9% to 100.0%) Specificity: 58.1% (95% CI 49.5% to 66.7%) Threshold: ≥ 10 mm |
| Danel et al., 201429 | Nested cohort study of QFT-GIT for ruling out active TB in HIV-positive adults | 975 adults in Cote d’Ivoire (median age 35 years), 25 with active TB on day 0 | Nine clinical centres in Abidjan, Cote d’Ivoire (March 2008–August 2009) | NE |
Sensitivity: 88.0% (95% CI 75.3% to 100.0%) Specificity: 66.6% (95% CI 63.6% to 69.6%) Indeterminate rate: 3% |
NE |
| Fei et al., 201460 | Case–control study of T-SPOT.TB compared with the TST for diagnosis of laryngeal TB | 83 patients with laryngeal TB and 52 patients with vocal cord polyps as controls (age range 31–66 years), all without HIV infection | China (August 2007–December 2012) |
Sensitivity: 90.4% (95% CI 82.1% 95.0%) Specificity: 92.3% (81.8% to 97.0%) |
NE |
Sensitivity: 50.6% (95% CI 40.1% to 61.1%) Specificity: 61.5% (95% CI 48.0% to 73.5%) Threshold: ≥ 5 mm |
| Garazzino et al., 201461 | Retrospective multicentre study of children (aged 0–24 months) tested at least once with QFT-GIT and/or the TST for active TB | 823 children [median age 13.4 months (range 8.4–18.9 months)], 105 with confirmed TB. Results for both the TST and QFT-GIT were available for 616 children | 18 paediatric centres in Italy | NE |
Sensitivity: 91.1% Specificity: 98.1% (Results above are for the subset of patients with both the QFT-GIT and TST results) Indeterminate rate in entire cohort with QFT-GIT: 4.3% |
Sensitivity: 85.1% Specificity: 97.9% Threshold: ≥ 5 mm |
| Kim et al., 201462 | Prospective study of T-SPOT.TB for diagnosis of active TB | 134 patients [mean age 55.9 years (SD 20.2 years)] suspected of active TB and 62 healthy adults [mean age 30.6 years (SD 8.5 years)] were consecutively recruited. All had been BCG vaccinated at a very young age. All received T-SPOT.TB and 53 had TST results | Hallym University Han-gang Sacred Heart Hospital, Korea (June 2008–June 2010) |
Sensitivity: 87.8% (95% CI 74.5% to 94.7%) Specificity: 44.1% (95% CI 34.4% to 54.2%) Specificity in healthy adults: 75.8% (63.8% to 84.8%) |
NE |
Sensitivity: 70.0% (95% CI 48.1% to 85.5%) Specificity: 48.5% (95% CI 32.5% to 64.8%) Specificity in healthy adults: 40.3% (95% CI 29.0% to 52.7%) Threshold: ≥ 10 mm |
| Kim et al., 201463 | Prospective study of QFT-GIT for diagnosis of miliary TB | 44 patients [mean age 64 years (SD 19 years)] with miliary TB | Kyungpook National University Hospital, Daegu, South Korea (September 2009–July 2013) | NE |
Sensitivity: 68.2% (95% CI 53.4% to 80.0%) Indeterminate rate: 16% |
NE |
| Park et al., 201464 | Retrospective study of the QFT-GIT and TST for diagnosis of smear-negative active PTB | 224 sputum smear-negative PTB suspects, 94 confirmed as having active PTB [mean age 46.5 years (SD 20.4 years)] and 130 confirmed as non-PTB [mean age 56.6 years (SD 18.3 years)]. 106 patients received a TST | Gangnam Severance Hospital, Seoul, Korea (October 2007–April 2013) | NE |
Sensitivity: 81.9% (95% CI 74.1% to 89.7%) Specificity: 62.3% (95% CI 54.0% to 70.6%) |
Sensitivity: 58.1% (95% CI 45.8% to 70.4%) Specificity: 63.6% (95% CI 49.4% to 77.9%) Threshold: ≥ 10 mm |
| Sauzullo et al., 201431 | Prospective study of QFT-GIT in HIV patients with active TB | 44 patients infected with HIV [median age 42 years (range 31–62 years)] with active TB | Department of Public Health and Infectious Diseases, Sapienza University, Rome, Italy (September 2008–12) | NE |
Sensitivity: 65.9% (95% CI 51.1% to 78.1%) with indeterminates included as negatives Indeterminate rate: 22.7% |
Sensitivity: 54.5% (95% CI 40.1% to 68.3%) |
| Schopfer et al., 201465 | QFT-GIT evaluated in children with microbiologically confirmed TB | 52,400 children were admitted to hospital, of which 405 children had TB, including 91 children with microbiologically confirmed TB. 81 of these children were tested with QFT-GIT | Jayavarman VII Hospital, a large paediatric referral hospital in Cambodia (July 2005–March 2006) | NE | Sensitivity: 53.1% (95% CI 42.3% to 63.6%) | NE |
| Wang et al., 201466 | Retrospective study of T-SPOT.TB for diagnosis of paediatric TB | 102 patients with TB, aged ≤ 15 years | China (March 2012–September 2013) | Sensitivity: 58.8% (95% CI 49.1% to 67.9%) | NE | NE |
| Wlodarczyk et al., 201467 | Study of the QFT-GIT and TST for diagnosis of PTB | 126 adult patients admitted with a clinical diagnosis of pneumonia. Of these, 43 were culture positive [mean age 48.6 years (SD 18.2 years)], 37 culture negative [mean age 51.7 years (SD 15.5 years)], and 46 with non-mycobacterial, community-acquired lung diseases [mean age 52.7 years (SD 17.3 years)] | Regional Specialised Hospital of Tuberculosis and Lung Diseases in Tuszyn, Poland (January 2010–June 2011) | NE |
Sensitivity (culture positive): 65.1% Sensitivity (culture negative): 55.6% Specificity: 87% |
Sensitivity (culture positive): 55.8% Sensitivity (culture negative): 64.9% Specificity: 71.7% Threshold: ≥ 10 mm |
| Aggarwal et al., 201528 | Systematic review and meta-analysis of IGRAs for diagnosis of pleural TB | 20 evaluations of T-SPOT.TB including 1085 subjects; 14 evaluations of QFT assays including 727 subjects (only one high-quality study; considerable heterogeneity) | China, Taiwan, Egypt, Turkey, South Korea, Italy, South Africa, Norway, Italy, Germany and the Netherlands (included studies published between 2007 and 2014) | NE separately. Results pooled across QFT and T.SPOT.TB assays | NE separately | NE |
| Anwar et al., 201568 | Retrospective study of QFT-GIT for diagnosis of active PTB in hospital setting | 142 cases of confirmed TB and 226 pneumonia cases in Saudi Arabia (only patients with QFT-GIT result included) | King Abdulaziz Medical City in Riyadh, Saudi Arabia (January 2009–December 2013) | NE |
Sensitivity: 74.6% (95% CI 66.1% to 81.7%) Specificity: 76.5% (95% CI 69.9% to 82.2%) Indeterminate rate: 11.4% |
NE |
| Bao et al., 201569 | Prospective study of performance of QFT-GIT for diagnosis of TB in children and adults | 60 children and 212 adults with suspected active TB. 31 had confirmed TB. HIV-positive patients were excluded |
Children Shanghai Public Health Clinical Center and Children’s Hospital of Fudan University, China (December 2010–August 2011) Adults Huashan Hospital of Fudan University (December 2010– December 2011) |
NE |
Children Sensitivity: 83.9% (95% CI 66.3% to 94.6%) Specificity: 88.5% (95% CI 70.2% to 96.8%) Adults Sensitivity: 73.7% (95% CI 57.8% to 85.2%) Specificity: 70.4% (95% CI 62.9% to 77.0%) Indeterminate rate: 9.1% |
NE |
| Sali et al., 201570 | Retrospective study of QFT-GIT for diagnosis of TB infection or disease in children | 621 children with suspected active TB, screened for LTBI or clinically healthy, nationally or internationally adopted children evaluated by a national protocol for immigrants and nationally/internationally adopted children, with or without known history of contact with adult active TB cases; 140 active TB suspects; 19 confirmed TB cases | Paediatric Infectious Disease Unit and Catholic University of the Sacred Heart–A. Gemelli Hospital in Rome, Italy (January 2007–July 2010) | NE |
Sensitivity: 87.5% Specificity: 93.6% Indeterminate rate: 4.2% |
NE (a TST was performed in less than half of the patients. Hence, TST results were not included in the analysis) |
| Shin et al., 201571 | Retrospective review of clinical records to evaluate use of IGRAs for diagnosing EPTB in suspected cases | 418 patients with suspected EPTB in Korea; 324 with confirmed active EPTB. Only 56 had QFT-GIT results | Gangnam Severance Hospital in Seoul, South Korea. (July 2005–June 2012) | NE |
Sensitivity: 70.2% (95% CI 56.0% to 81.3%) Specificity: 66.7% (95% CI 35.4% to 87.9%) |
Sensitivity: 62.1% (95% CI 42.3% to 79.3%) Specificity: 87.5% (95% CI 52.9% to 97.8%) |
| Sun et al., 201572 | Prospective study to evaluate utility of T-SPOT.TB for diagnosis of paediatric TB in hospital setting | 117 children with active TB in China; 413 children with respiratory tract infection | Beijing Children’s Hospital (March 2011–June 2014) |
Sensitivity: 82.9% Specificity: 96.1% Indeterminate rate: 8.5% |
NE |
Sensitivity: 67.5% Specificity: 75.3 Threshold: ≥ 10 mm Results were also available for ≥ 5 mm and ≥ 15 mm and combinations of TST with T-SPOT. TB |
| Wong et al., 201573 | Retrospective chart analysis for paediatric patients who underwent the QFT-GIT and TST for confirmation of active TB | 70 paediatric patients in a population with high uptake of neonatal BCG vaccination; eight children had confirmed TB. 47 children had the QFT-GIT | Intermediate-burden region in Taiwan (January 2008–June 2014) | NE |
Sensitivity: 100% (95% CI 63.1% to 100%) Specificity: 97.1% (95% CI 85.1% to 99.9%) Indeterminate rate: 6.4% |
Sensitivity: 62.5% (95% CI 24.5% to 91.5%) Specificity: 95.2% (95% CI 76.2% to 99.9%) Threshold: ≥ 10 mm |
| Xia et al., 201574 | Prospective study of the QFT-GIT and TST for diagnosing PTB | 300 PTB, 41 disease controls and 59 health community controls were enrolled | Heilongjiang Province and south-east Zhejiang Province, China (May 2010–April 2011) | NE |
Sensitivity: 80.9% (95% CI 75.9% to 85.2%) Specificity: 36.6% (95% CI 22.1% to 53.1%) Indeterminate rate: 0.5% |
Sensitivity: 79.5% (95% CI 74.3% to 84.4%) Specificity: 36.6% (95% CI 22.1 to 53.1%) Threshold: ≥ 10 mm Results were also available for ≥ 5-mm and ≥ 15-mm thresholds, as well as combinations with QFT-GIT |
| Uzunhan et al., 201575 | Prospective comparison of the QFT-GIT and TST for diagnosis of childhood TB | 53 children with TB (16 culture positive); 92 healthy children with no risk factors for TB | Children referred to hospital paediatric clinics in Turkey (recruitment period not reported) | NE |
Sensitivity in all TB: 62.3% Specificity: 97.8% |
Sensitivity in all TB: 97.8% Specificity: 100% |
| Azghay et al., 201676 | Retrospective analysis of hospital records to analyse contribution of QFT-GIT to TB diagnosis | A total of 395 QFT-GIT assays were performed for suspected TB patients | Jean Verdier Hospital in Bondy, Paris, France (June 2008–June 2011) | NE |
Sensitivity: 85% (95% CI 73% to 92%) Specificity: 73.3% (95% CI 68% to 78%) Indeterminate rate: 11.6% |
Sensitivity: 78% (95% CI 57% to 91%) Combined test: 92.6% (95% CI 74% to 99%) |
| Jia et al., 201677 | Prospective study to evaluate T-SPOT.TB in lymph node TB | 405 patients with suspected lymph node TB; 83 with confirmed TB; 282 with TB excluded (21 clinically indeterminate and 19 clinical TB excluded from analyses) | Beijing Chest Hospital, China (July 2011–April 2015) |
Sensitivity: 90.4% Specificity: 70.5% Indeterminate rate: 3.8% |
NE | NE |
Appendix 12 Key characteristics of patients with indeterminate QFT-GIT and T-SPOT.TB results
| Characteristic | Test, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QFT-GIT | T-SPOT.TB | |||||||||
| Dosanjh category | Total | Dosanjh category | Total | |||||||
| Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | Culture-confirmed TB | Highly probable TB | Clinically indeterminate | Active TB excluded | |||
| All | 21 | 5 | 6 | 47 | 79 | 12 | 5 | 3 | 37 | 57 |
| HIV infection status | ||||||||||
| Positive | 0 (0) | 2 (40) | 1 (16.7) | 20 (42.6) | 23 (29.1) | 2 (16.7) | 3 (60) | 0 (0) | 28 (75.7) | 33 (57.9) |
| Negative | 21 (100) | 3 (60) | 5 (83.3) | 27 (57.4) | 56 (70.9) | 10 (83.3) | 2 (40) | 3 (100) | 9 (24.3) | 24 (42.1) |
| Diabetes | ||||||||||
| Yes | 0 (0) | 0 (0) | 1 (16.7) | 4 (8.5) | 5 (6.3) | 1 (8.3) | 1 (20) | 2 (66.7) | 0 (0) | 4 (7) |
| No | 21 (100) | 5 (100) | 5 (83.3) | 43 (91.5) | 74 (93.7) | 11 (91.7) | 4 (80) | 1 (33.3) | 37 (100) | 53 (93) |
| Immunosuppressive therapy | ||||||||||
| Yes | 0 (0) | 0 (0) | 0 (0) | 1 (2.1) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No | 21 (100) | 5 (100) | 6 (100) | 46 (97.9) | 78 (98.7) | 12 (100) | 5 (100) | 3 (100) | 37 (100) | 57 (100) |
| TST statusa | ||||||||||
| Positive | 6 (75) | 3 (75) | 0 (0) | 2 (18.2) | 11 (44) | 6 (100) | 1 (100) | 2 (100) | 1 (14.3) | 10 (62.5) |
| Negative | 2 (25) | 1 (25) | 2 (100) | 9 (81.8) | 14 (56) | 0 (0) | 0 (0) | 0 (0) | 6 (85.7) | 6 (37.5) |
| BCG scar | ||||||||||
| Yes | 14 (93.3) | 4 (100) | 4 (66.7) | 29 (80.6) | 51 (83.6) | 10 (100) | 3 (75) | 3 (100) | 21 (67.7) | 37 (77.1) |
| No | 1 (6.7) | 0 (0) | 1 (16.7) | 2 (5.6) | 4 (6.6) | 0 (0) | 0 (0) | 0 (0) | 3 (9.7) | 3 (6.3) |
| Unsure | 0 (0) | 0 (0) | 1 (16.7) | 5 (13.9) | 6 (9.8) | 0 (0) | 1 (25) | 0 (0) | 7 (22.6) | 8 (16.7) |
| BCG vaccinated | ||||||||||
| Yes | 15 (71.4) | 4 (80) | 6 (100) | 36 (76.6) | 61 (77.2) | 10 (83.3) | 4 (80) | 3 (100) | 31 (83.8) | 48 (84.2) |
| No | 6 (28.6) | 1 (20) | 0 (0) | 11 (23.4) | 18 (22.8) | 2 (16.7) | 1 (20) | 0 (0) | 6 (16.2) | 9 (15.8) |
Appendix 13 Evaluations of tuberculin skin test
Diagnostic accuracy of tuberculin skin test at different thresholds
At induration thresholds of ≥ 5 mm, ≥ 10 mm and the stratified threshold (≥ 6 mm for unvaccinated and ≥ 15 mm for BCG vaccinated patients), the TST had sensitivities of 95.4% (95% CI 90.9% to 97.8%), 94.8% (95% CI 90.0% to 97.3%) and 93.5% (95% CI 88.4% to 96.4%) in culture-positive and highly probable active TB patients (Table 76), respectively. In all non-active TB patients, the specificities for thresholds of ≥ 5 mm, ≥ 10 mm and the stratified threshold were 50.3% (95% CI 42.4% to 58.3%), 55.7% (95% CI 47.7% to 63.4%) and 66.4% (95% CI 58.5% to 73.5%). The TST gave high NPVs at the three thresholds; the NPV at the stratified threshold was 90.8% (95% CI 83.9% to 94.9%). Sensitivities were higher for extrapulmonary TB than pulmonary TB. There were large differences in specificity when analyses were restricted to category 4D non-active TB patients (Table 76), but there was very little change in the sensitivities when the analyses were limited to culture-positive TB patients. For further results of sensitivity analyses excluding highly probable (category 2) cases, see Table 77.
| Test performance | Threshold | |||||
|---|---|---|---|---|---|---|
| ≥ 5 mm | ≥ 10 mm threshold | Stratifieda | ||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||
| All TB | 146/153 | 95.4 (90.9 to 97.8) | 145/153 | 94.8 (90.0 to 97.3) | 143/153 | 93.5 (88.4 to 96.4) |
| Culture-positive TB | 98/104 | 94.2 (88.0 to 97.3) | 98/104 | 94.2 (88.0 to 97.3) | 96/104 | 92.3 (85.6 to 96.1) |
| Culture-negative TB | 44/45 | 97.8 (88.4 to 99.6) | 43/45 | 95.6 (85.2 to 98.8) | 43/45 | 95.6 (85.2 to 98.8) |
| Smear-positive TB | 23/26 | 88.5 (71.0 to 96.0) | 23/26 | 88.5 (71.0 to 96.0) | 23/26 | 88.5 (71.0 to 96.0) |
| Smear-negative TB | 106/110 | 96.4 (91.0 to 98.6) | 105/110 | 95.5 (89.9 to 98.0) | 104/110 | 94.6 (88.6 to 97.5) |
| Pulmonary TB | 42/47 | 89.4 (77.4 to 95.4) | 42/47 | 89.4 (77.4 to 95.4) | 40/47 | 85.1 (72.3 to 92.6) |
| Extrapulmonary TB | 89/90 | 98.9 (93.8 to 99.8) | 89/90 | 98.9 (94.0 to 99.8) | 88/90 | 97.8 (92.3 to 99.4) |
| Specificity for a diagnosis of active TB | ||||||
| Active TB excluded | 75/149 | 50.3 (42.4 to 58.3) | 83/149 | 55.7 (47.7 to 63.4) | 99/149 | 66.4 (58.5 to 73.5) |
| Active TB excluded, TST negative, no risk factors for LTBI | 16/21 | 76.2 (54.9 to 89.4) | 18/21 | 85.7 (65.4 to 95.0) | 21/21 | 100.0 (84.5 to 100.0) |
| Predictive values | ||||||
| PPV | 146/220 | 66.4 (59.9 to 72.3) | 145/211 | 68.7 (62.2 to 74.6) | 143/193 | 74.1 (67.5 to 79.8) |
| NPV | 75/82 | 91.5 (83.4 to 95.8) | 83/91 | 91.2 (83.6 to 95.5) | 99/109 | 90.8 (83.9 to 94.9) |
| Likelihood ratios | ||||||
| Positive likelihood ratio | – | 1.92 (1.63 to 2.27) | – | 2.14 (1.78 to 2.57) | – | 2.79 (2.21 to 3.51) |
| Negative likelihood ratio | – | 0.09 (0.04 to 0.19) | – | 0.09 (0.05 to 0.19) | – | 0.10 (0.05 to 0.18) |
| Test performance | Threshold | |||||
|---|---|---|---|---|---|---|
| ≥ 5 mm | ≥ 10 mm | Stratifieda | ||||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||||
| Culture-positive TB | 98/104 | 94.2 (88.0 to 97.3) | 98/104 | 94.2 (88.0 to 97.3) | 96/104 | 92.3 (85.6 to 96.1) |
| Smear-positive TB | 21/24 | 87.5 (69.0 to 95.7) | 21/24 | 87.5 (69.0 to 95.7) | 21/24 | 87.5 (69.0 to 95.7) |
| Smear-negative TB | 68/71 | 95.8 (88.3 to 98.6) | 68/71 | 95.8 (88.3 to 98.6) | 66/71 | 93.0 (84.6 to 97.0) |
| Pulmonary TB | 36/42 | 85.7 (72.2 to 93.3) | 36/40 | 90.0 (76.9 to 96.0) | 34/40 | 85.0 (70.9 to 92.9) |
| Extrapulmonary TB | 49/50 | 98.0 (89.5 to 99.6) | 49/50 | 98.0 (89.5 to 99.6) | 49/50 | 98.0 (89.5 to 99.6) |
| Specificity for a diagnosis of active TB | ||||||
| Active TB excluded | 75/149 | 50.3 (42.4 to 58.3) | 83/149 | 55.7 (47.7 to 63.4) | 99/149 | 66.4 (58.5 to 73.5) |
| Active TB excluded, TST negative, no risk factors for LTBI | 16/21 | 76.2 (54.9 to 89.4) | 18/21 | 85.7 (65.4 to 95.0) | 21/21 | 100.0 (84.5 to 100.0) |
| Predictive values | ||||||
| PPV | 98/172 | 57.0 (50.0 to 64.1) | 98/164 | 59.8 (52.1 to 67) | 96/146 | 65.8 (57.7 to 73.0) |
| NPV | 75/81 | 92.6 (84.8 to 96.6) | 83/89 | 93.3 (86.1 to 96.9) | 99/107 | 92.5 (85.9 to 96.2) |
| Likelihood ratios | ||||||
| Positive likelihood ratio | – | 1.90 (1.60 to 2.25) | – | 2.13 (1.77 to 2.56) | – | 2.75 (2.18 to 3.47) |
| Negative likelihood ratio | – | 0.12 (0.05 to 0.25) | – | 0.10 (0.05 to 0.23) | – | 0.12 (0.06 to 0.23) |
Combinations of tuberculin skin test and interferon gamma release assays
The TST and T-SPOT. TB had a combined sensitivity of 97.7% (95% CI 93.5% to 99.2%) and specificity of 62.0% (95% CI 53.4% to 69.9%). Results were similar for the combination of the TST and QFT-GIT, with a sensitivity of 96.9% (95% CI 92.4% to 98.8%) and specificity of 59.7% (95% CI 51.1% to 67.8%). Figure 16 shows sequential likelihood ratios for a testing strategy of a TST followed by either T-SPOT. TB or QFT-GIT. A positive result for both the TST and IGRAs gave positive likelihood ratios of 5.80 (95% CI 3.90 to 10.0) for TST followed by T-SPOT. TB and 4.77 (95% CI 3.25 to 8.16) for the TST followed by QFT-GIT. When both tests in a combination were negative, the negative likelihood ratios were 0.04 (95% CI 0.01 to 0.09) for the TST followed by T-SPOT. TB and 0.05 (95% CI 0.01 to 0 0.11) for the TST followed by QFT-GIT.
FIGURE 16.
Diagnostic performance of combinations of the TST with T-SPOT. TB or QFT-GIT. The analyses were based on 260 patients in whom results were available for both the TST and T-SPOT. TB, and for both the TST and QFT-GIT. Those with indeterminate IGRA results were excluded from the analyses. The likelihood ratios are presented with 95% CIs. The stratified threshold was used to determine the positivity of the TST. (a) A sequence of testing in which T-SPOT. TB follows the TST; and (b) a sequence of testing in which QFT-GIT follows the TST. The difference in the CI of the positive likelihood ratio for the TST is due to stochastic variation in the bootstrap method used for the computation of the likelihood ratios. ATB, active tuberculosis; LR, likelihood ratio.
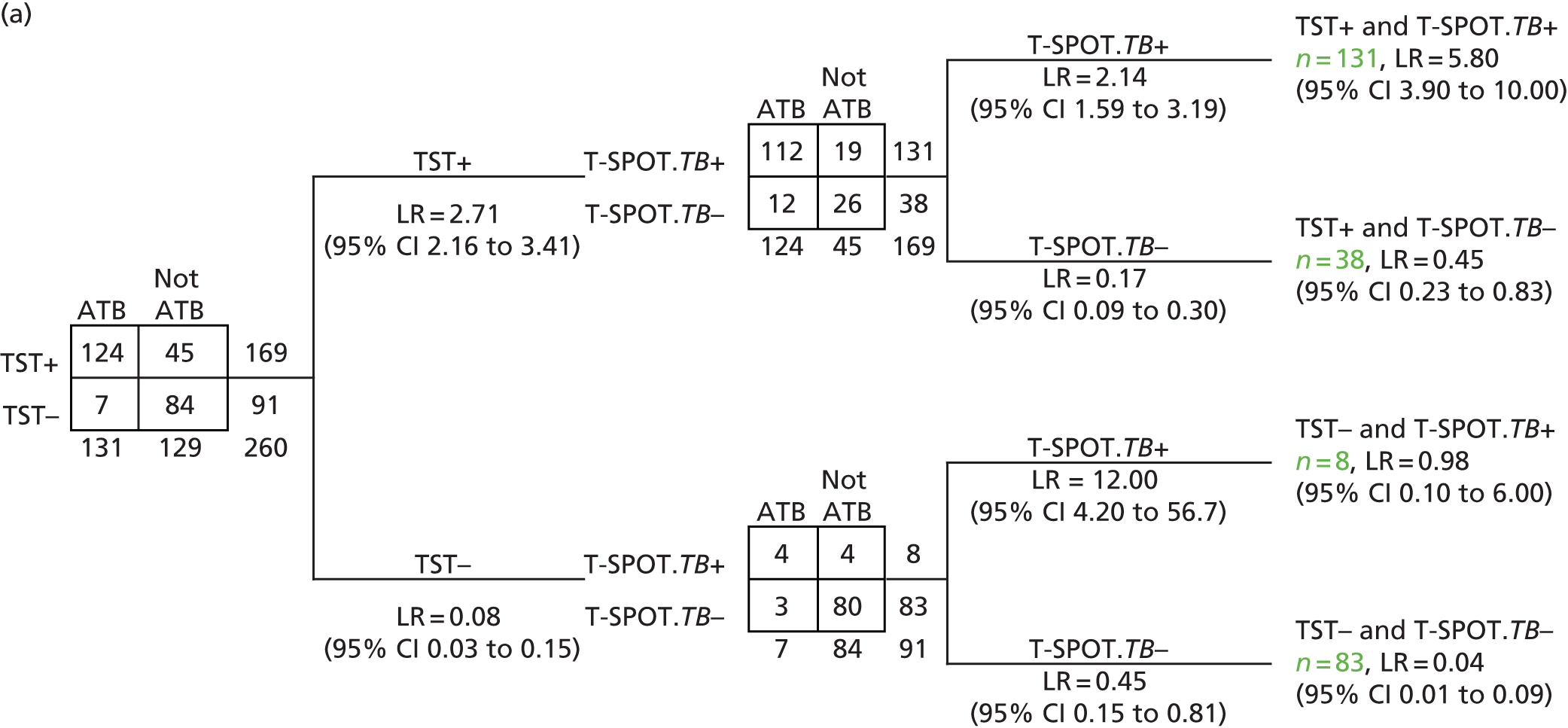

In sensitivity analyses excluding highly probable active TB cases (i.e. analyses of only culture-confirmed active TB cases and all non-active TB cases), the sensitivities and specificities of the combinations were largely unchanged. The TST and T-SPOT. TB had a combined sensitivity of 97.7% (95% CI 92.1% to 99.4%) and specificity of 62.0% (95% CI 53.4% to 69.9%). For the combination of a TST and QFT-GIT, sensitivity was 96.6% (95% CI 90.5% to 98.8%) and specificity was 59.7% (95% CI 51.1% to 67.8%). As can be seen in Figure 17, sequential likelihood ratios were generally similar to those from the analyses including both cultured-confirmed and highly probable active TB cases (see Figure 16).
FIGURE 17.
Diagnostic performance of combinations of the TST with T-SPOT. TB or QFT-GIT: sensitivity analyses excluding highly probable TB cases. The analyses were based on 217 patients in whom results were available for both the TST and T-SPOT. TB, and for both the TST and QFT-GIT. Those with highly probable active TB were excluded from the analyses. The likelihood ratios are presented with 95% CIs. The stratified threshold was used to determine the positivity of the TST. The difference in the CI of the positive likelihood ratio for the TST is due to stochastic variation in the bootstrap method used for the computation of the likelihood ratios. (a) A sequence of testing in which T-SPOT. TB follows the TST; and (b) a sequence of testing in which QFT-GIT follows the TST.


Appendix 14 Additional results in the human immunodeficiency virus-positive substudy cohort
| Hospital trust | Number of tests performed | Number of patients analyseda | ||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | TST | ||
| Imperial College Healthcare NHS Trust | 19 | 1 | 4 | 54 |
| The Heart of England NHS Foundation Trust | 3 | 0 | 0 | 6 |
| Chelsea and Westminster Hospital NHS Foundation Trust | 24 | 0 | 0 | 40 |
| Royal Free London NHS Foundation Trust | 6 | 3 | 5 | 53 |
| St George’s University Hospitals NHS Foundation Trust | 0 | 0 | 0 | 5 |
| King’s College Hospital NHS Foundation Trust | 0 | 0 | 0 | 2 |
| University Hospitals of Leicester NHS Trust | 0 | 2 | 0 | 23 |
| London North West Healthcare NHS Trust | 0 | 0 | 3 | 6 |
| Oxford University Hospitals NHS Foundation Trust | 0 | 0 | 0 | 4 |
| Sandwell and West Birmingham Hospitals NHS Trust | 0 | 0 | 0 | 1 |
| Barts Health NHS Trust | 3 | 0 | 0 | 7 |
| Total | 55 | 6 | 12 | 201 |
| T-SPOT.TB, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Borderline | Indeterminate | Missing | Total | ||
| QFT-GIT, n (%) | Positive | 19 (65.5) | 4 (3.6) | 1 (10.0) | 5 (11.1) | 0 | 29 (14.4) |
| Negative | 6 (20.7) | 82 (73.9) | 9 (90.0) | 30 (66.7) | 5 (83.3) | 132 (65.7) | |
| Indeterminate | 4 (13.8) | 24 (21.6) | 0 | 10 (22.2) | 1 (16.7) | 39 (19.4) | |
| Missing | 0 | 1 (0.9) | 0 | 0 | 0 | 1 (0.5) | |
| Total | 29 (100.0) | 111 (100.0) | 10 (100.0) | 45 (100.0) | 6 (100.0) | 201 (100.0) | |
| Test performance | Test | |||
|---|---|---|---|---|
| T-SPOT.TB | QFT-GIT | |||
| n/N | Estimate (95% CI) | n/N | Estimate (95% CI) | |
| Sensitivity for a diagnosis of active TB | ||||
| All TB | 23/31 | 74.2 (56.8 to 86.3) | 19/32 | 59.4 (42.3 to 74.5) |
| Culture-positive TB | 13/18 | 72.2 (49.1 to 87.5) | 11/18 | 61.1 (38.6 to 79.7) |
| Culture-negative TB | 10/13 | 76.9 (49.7 to 91.8) | 8/13 | 61.5 (35.5 to 82.3) |
| Smear-positive TB | 5/9 | 55.6 (26.7 to 81.1) | 6/9 | 66.7 (35.4 to 87.9) |
| Smear-negative TB | 14/18 | 77.8 (54.8 to 91.0) | 10/19 | 52.6 (31.7 to 72.7) |
| Pulmonary TB | 10/13 | 76.9 (49.7 to 91.8) | 9/13 | 69.2 (42.4 to 87.3) |
| Extrapulmonary TB | 11/14 | 78.6 (52.4 to 92.4) | 7/15 | 46.7 (24.8 to 69.9) |
| Specificity for a diagnosis of active TB | ||||
| Active TB excluded | 101/160 | 63.1 (55.4 to 70.2) | 117/164 | 71.3 (64.0 to 77.7) |
| Active TB excluded, TST negative, no risk factors for LTBI | 42/66 | 63.6 (51.6 to 74.2) | 54/67 | 80.6 (69.6 to 88.3) |
| Predictive value | ||||
| PPV | 23/82 | 28.0 (19.5 to 38.6) | 19/66 | 28.8 (19.3 to 40.6) |
| NPV | 101/109 | 92.3 (86.2 to 96.2) | 117/130 | 90.0 (83.6 to 94.1) |
| Likelihood ratios | ||||
| Positive likelihood ratio | – | 2.01 (1.51 to 2.69) | – | 2.07 (1.42 to 3.01) |
| Negative likelihood ratio | – | 0.41 (0.22 to 0.75) | – | 0.57 (0.37 to 0.88) |
| Test | Number of test resultsa | Sensitivity (95% CI) | Number of test resultsb | Specificity (95% CI) |
|---|---|---|---|---|
| T-SPOT.TB | 31 | 73.4 (55.4 to 86.0) | 160 | 63.2 (55.4 to 70.3) |
| QFT-GIT | 32 | 59.4 (41.6 to 75.0) | 164 | 71.4 (64.0 to 77.8) |
| Ratio (95% CI);c p-value | – | 1.23 (0.94 to 1.63); 0.1 | – | 0.88 (0.77 to 1.03); 0.1 |
List of abbreviations
- BAL
- bronchoalveolar lavage
- BCG
- bacillus Calmette–Guérin
- CD4
- cluster of differentiation 4
- CFP-10
- culture filtrate protein 10
- CI
- confidence interval
- CRF
- case report form
- CT
- computed tomography
- DMG
- Data Management Group
- ELISA
- enzyme-linked immunosorbent assay
- ELISpot
- enzyme-linked immunospot assay
- ESAT-6
- early secretory antigenic 6 kDa
- GEE
- generalised estimating equation
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- IDEA
- Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis study
- IFN-γ
- interferon gamma
- IGRA
- interferon gamma release assay
- LTBI
- latent tuberculosis infection
- MRI
- magnetic resonance imaging
- Mtb
- Mycobacterium tuberculosis
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- PBMC
- peripheral blood mononuclear cell
- PCR
- polymerase chain reaction
- PET
- positron emission tomography
- PI
- principal investigator
- PPD
- purified protein derivative
- PPI
- patient and public involvement
- PPV
- positive predictive value
- QALY
- quality-adjusted life-year
- QFT-GIT
- QuantiFERON GOLD In-Tube
- QFT-Plus
- QuantiFERON-TB Gold Plus
- R&D
- research and development
- RPMI
- Roswell Park Memorial Institute
- SFC
- spot-forming cell
- SMG
- Study Management Group
- SSC
- Study Steering Committee
- STARD
- Standards for the Reporting of Diagnostic Accuracy Studies
- TB
- tuberculosis
- TST
- tuberculin skin test