Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/129/197. The contractual start date was in November 2016. The draft report began editorial review in May 2018 and was accepted for publication in September 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Manjit S Gohel has received personal fees from Medtronic pLc (Minneapolis, MN, USA) and Cook Medical LLC (Bloomington, IN, USA), plus a grant from Laboratoires Urgo S.A. (Chenôve, France). Andrew Bradbury had committee membership for the National Institute for Health Research Health Technology Assessment (HTA) Prioritisation Group and HTA Surgery Themed Call Board 2012–13, HTA Efficient Study Designs Board 2014–16, HTA Interventional Procedures Methods Group 2015–19 and HTA IP Panel 2015–19. In addition, Andrew Bradbury has received funding from STD Pharmaceutical Products Ltd (Hereford, UK) to travel to a foam sclerotherapy workshop in Tehran, Iran, in October 2016 and a grant to cover costs of undertaking a post-authorisation safety study in the UK and Europe. He also sat on the National Institute for Health and Care Excellence (NICE) committee for a clinical guideline (CG168) for the diagnosis and management of varicose veins. Nicky Cullum had committee membership on the HTA Commissioning Board from 2003 to 2008. David M Epstein has received grant funding from Vascular Insights LLC (Quincy, MA, USA) which was administered by the University of Granada. Alun H Davies has received grant funding from Medtronic, Vascular Insights, Laboratoires Urgo, Vascutek (Inchinnan, UK) and Actegy Health Ltd (Bracknell, UK), which are administered by Imperial College London. In addition, Alun H Davies has chaired the NICE clinical guideline (CG168) for the diagnosis and management of varicose veins.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gohel et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background of venous leg ulcers
Leg ulcers are open ‘sores’ on the lower limbs situated between the ankles and knees, and were defined in this trial as those that fail to heal within 6 weeks. These ulcers represent a source of great discomfort and social isolation to patients, who often complain of associated pain, odour and wound discharge. Ulcers often take many months to heal, meaning that the condition is also frustrating for health-care professionals involved in their management in hospital and community settings. In 70% of cases, the underlying cause of leg ulceration is lower limb venous disease, sometimes evident as varicose veins but often undetectable by visual examination alone. 1 The prevalence of venous leg ulcers in the adult population overall has been estimated at 0.03–1%, rising dramatically in those aged > 80 years. 2–4 As patients with venous ulceration often suffer episodes of recurrence, the number of patients at high risk of ulceration may actually be four- to fivefold higher. 5 It should also be noted that, with an ageing and increasingly obese population,6 the incidence and prevalence of venous ulceration are both likely to increase. Treatment of the condition in the UK incurs a substantial cost burden, estimated at £400M–600M per annum,7 although the figure could be higher. 8
Venous ulcers are characterised by protracted healing times. Despite recent advances in the management of patients with venous ulcers, 24-week healing rates in published randomised trials are around 60–65%,9,10 and the true population healing rates are likely to be significantly lower. Some ulcers may never heal, and patients whose ulcers do heal are at high risk of recurrent ulceration. These poor outcomes are likely to be a reflection of the severe underlying venous disease (reflux and, less commonly, obstruction) in this patient group, although inadequate assessment and suboptimal treatment of the venous disease are also likely to be important contributing factors.
Pathophysiology of venous leg ulcers
The venous circulation of the lower limb has two components: the deep and superficial systems. Blood normally flows from the superficial to the deep veins, stimulated by calf and foot muscle contractions. Blood is prevented from flowing back down the leg under the influence of gravity by ‘one-way’ bicuspid valves along the deep and superficial veins.
When these valves are damaged, they become incompetent, resulting in venous flow away from the heart. This results in the superficial veins usually becoming dilated and tortuous (varicose), and the resulting sustained high venous and capillary pressures lead to skin inflammation and breakdown of the skin, visible as ulceration. 11 The deep veins also have valves, which may also become incompetent and cause high venous pressure, but are not visible on the skin.
Duplex Doppler ultrasonography studies12–14 of patients attending leg ulcer clinics suggest that around 50% of patients with venous leg ulcers have disease only of the superficial veins, with a further 30–40% having a mixture of superficial and deep-venous disease. Surgical treatment of the superficial venous reflux can benefit both of these groups of patients, in terms of reducing ulcer recurrence. 15 Approximately 5–10% of patients with venous ulcers have diseased deep-venous systems only and are not amenable to surgical correction with current technology. These patients are usually treated with compression therapy alone.
Conservative management
Ulcer healing strategies are based on efforts to reduce the reflux of blood back down the leg and into the skin, as this is considered the most significant cause of high venous pressure and ulceration in most patients. Longstanding venous hypertension has been shown to cause a number of changes to the microcirculation in the lower leg, which can contribute to the chronic skin changes or eventual ulceration associated with chronic venous disease. 16
The mainstay of therapy for venous ulceration is compression therapy, which was first described around 2000 years ago. Compression bandaging is used to heal venous ulceration by counteracting the gravitational force on the blood, in effect temporarily replacing the incompetent valves. 17 Bandages are usually reapplied once to four times per week.
A Cochrane review18 of the effectiveness of compression reviewed 48 randomised controlled trials (RCTs) and found that the use of compression improved healing rates compared with no compression use and that multicomponent bandages are more effective than single-component systems, with two-component systems’ healing rates being equivalent to four-layer bandaging. An individual patient data meta-analysis18 found faster healing with four-layer bandaging use than with short-stretch bandaging use, and improved healing rates at 2–4 months using high-compression stockings compared with short-stretch bandaging. In addition, the meta-analysis showed the four-layer bandaging to be more cost-effective than short-stretch bandaging. 18
The haemodynamic benefit of compression is lost almost immediately after removal of compression, and so compression offers a treatment benefit only while in situ. 19 There are also side effects associated with compression, such as pressure damage, which can lead to reduced concordance rates, as highlighted by a recent Cochrane review. 20
Treatment options for superficial venous reflux
The treatment of superficial venous reflux offers a logical strategy for reducing chronic venous hypertension and so improving the healing of venous leg ulcers. Diseased superficial veins can be surgically removed (or ‘stripped’) by open varicose vein surgery or ablated using endovenous interventions without harming the overall venous function of the leg, theoretically removing a causative factor for recurrence of the ulcer after the compression bandaging has ceased.
Open surgery
For over a century, the treatment of superficial venous reflux has involved operative ligation and stripping of the vein and avulsion of bulging varicose veins. 21 Until recent years, open surgery has been considered the definitive treatment option for superficial venous reflux. However, the operation usually requires general anaesthesia, and patients often suffer discomfort, bruising and significant time off work in the postoperative period. Long-term studies have also identified significant complications of open surgery, including nerve damage and recurrence of varicose veins, seen in > 60% of patients at 11 years in one randomised study. 22
Endovenous interventions
In response to this high complication rate and a growing patient desire for less invasive treatments, a range of novel, minimally invasive, endovenous treatment options have been developed and have gained in popularity over the last 10–15 years. Interventions such as ultrasonography-guided foam sclerotherapy (UGFS),23 endovenous laser ablation (EVLA)24 or radiofrequency laser ablation (RFA)25 can be performed using local anaesthesia in an outpatient setting. Newer endovenous interventions include mechanochemical endovenous ablation (MOCA) and cyanoacrylate glue closure. These treatments involve cannulation of the vein to be treated, usually under ultrasonography guidance, obliteration and closure of the refluxing superficial veins by either chemical (e.g. foam sclerosant, glue) or thermal ablation (e.g. RFA, EVLA, steam). Numerous randomised studies have demonstrated that endovenous modalities result in comparable vein closure rates to open surgery, but are clearly superior in terms of complications and recovery. 26–28
Each of the different endovenous modalities has potential advantages and potential disadvantages, although all are less invasive than traditional open surgery. This is of particular relevance to patients with venous ulcers, who are often elderly and may have several comorbidities and for whom surgical procedures involving general anaesthesia may be inappropriate. Endovenous techniques can also be performed without discontinuing anticoagulation therapy, which is increasingly prescribed in this patient population.
Existing research
The ESCHAR randomised controlled trial
Aims and results
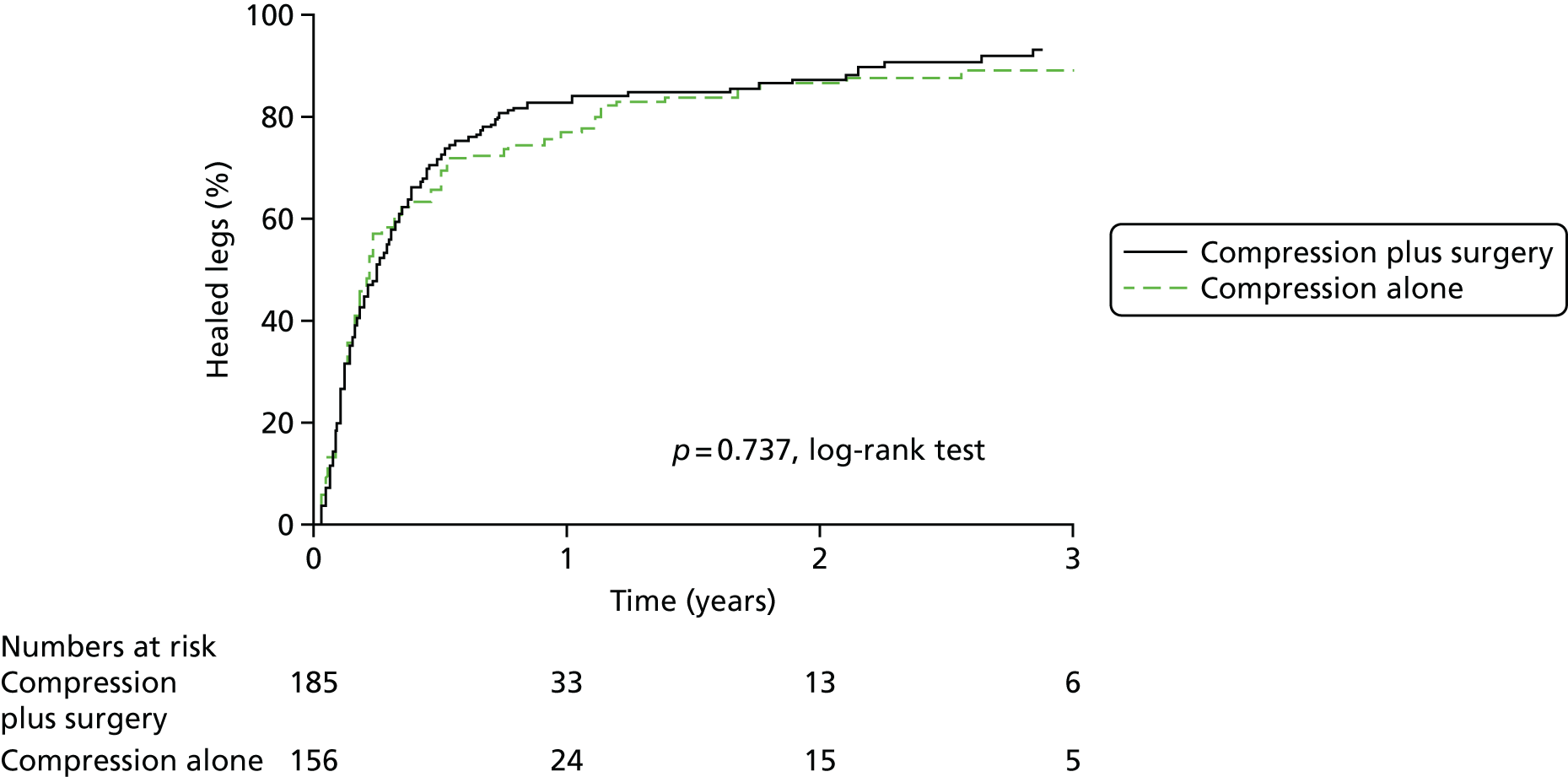
The most significant trial of superficial venous intervention in patients with venous ulceration is the Effect of Surgery and Compression on Healing And Recurrence (ESCHAR) trial (ISRCTN07549334). 9,15 The trial aimed to evaluate the role of traditional superficial venous surgery in reducing ulcer recurrence in patients with open or recently healed venous ulcers. Following prospective observational studies to inform power calculations, a total of 500 participants were randomised to compression therapy alone or to compression with open surgery for superficial venous reflux. The group randomised to surgical treatment had significantly lower venous ulcer recurrence rates at 4 years (Figure 1).
FIGURE 1.
The ESCHAR trial: ulcer recurrence. Reproduced from Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial, Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. ,15 vol. 335, p. 83, 2018, with permission from BMJ Publishing Group Ltd.

Analysis stratified by pattern of venous reflux demonstrated that this clinical benefit was present for patients with isolated superficial venous reflux and patients with superficial and segmental deep reflux. This clearly indicated that the majority of patients with venous ulceration could benefit from superficial venous intervention.
The ESCHAR trial was unable to detect an effect of surgery on ulcer healing (Figure 2). This finding has led many to conclude that treatment of venous reflux does not have a role in patients with open ulcers.
FIGURE 2.
The ESCHAR trial: ulcer healing. Reproduced from Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial, Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. ,15 vol. 335, p. 83, 2018, with permission from BMJ Publishing Group Ltd. 15
Weaknesses
There were, however, several limitations to the evidence from the ESCHAR trial. The trial was not powered to assess ulcer healing, as both patients with open ulcers and those with healed ulcers were included. The statistical power was further weakened by a high crossover rate, as around one-fifth of participants randomised to surgery later decided that they did not want to have the operation. Moreover, participants who consented to surgery waited a median of 7 weeks for intervention and so did not receive an immediate benefit. Consequently, some smaller ulcers might have already healed with compression bandaging. Finally, some of the surgical procedures used were suboptimal when judged by current standards and the use of local anaesthetic may have meant that some legs were left with residual venous incompetence. Thus, it is likely that the benefits of treating superficial venous reflux were underestimated in this trial, particularly for the assessment of ulcer healing. The poor patient acceptance of surgery emphasises the need for a minimally invasive superficial venous treatment modality in this patient group.
Other relevant research
In a smaller Dutch randomised trial, 170 patients (200 legs) were randomised to compression alone or compression with surgical treatment of superficial reflux (including subfascial endoscopic perforator surgery). 10 Although there was no statistically significant difference between healing rates with compression and surgery, the trial was underpowered and the results were compatible with improved ulcer healing rates and greater ulcer-free time in the group randomised to surgery.
The Ulcer Surgery as Adjuvant to compression Bandaging for Leg UlcErs (USABLE) trial29 randomised 76 patients with venous ulceration to four-layer compression bandaging or compression plus superficial venous surgery. Time to ulcer healing was similar between the groups. 29
Despite the widespread acceptance of endovenous modalities, few published prospective studies have reported outcomes in patients with leg ulcers. The Cochrane systematic review did not identify any eligible RCTs;30 another systematic review31 identified one RCT32 and, although this trial did not meet the quality criteria for inclusion in the Cochrane review, it found that endovenous thermal ablation significantly increased the probability of ulcer healing compared with compression alone [risk ratio 3.40, 95% confidence interval (CI) 1.65 to 6.98].
One retrospective cohort study of 170 patients with active or healed leg ulceration (195 legs) treated with EVLA achieved excellent healing rates and low recurrence rates of 16%, as did another study of 173 legs, which noted that ulcer healing and recurrence rates were similar to those seen with surgical stripping. 33,34
In a prospective study of 186 patients with leg ulceration treated with UGFS, the ulcer healing rate was > 70% and the patient acceptability of treatment was excellent. 35 In a further study of foam sclerotherapy in 130 patients, a healing rate of 82% was achieved. 36
Unsurprisingly, endovenous interventions are very acceptable to patients, and reported complication rates are low. 37 A recent meta-analysis demonstrated that clinical outcomes following endovenous interventions outcomes are comparable with those achieved with open surgery, but with lower complication rates of pain, infection and bruising, and faster/earlier return to work. 38
Although these studies lend support to the hypothesis that early endovenous ablation to correct superficial venous reflux may accelerate venous ulcer healing, a large randomised trial is required to provide reliable evidence and guide modern practice.
Current UK national guidelines
Scottish Intercollegiate Guidelines Network: 2010
The most current ulcer-specific guidance, issued by the Scottish Intercollegiate Guidelines Network in 2010,39 concluded that the optimal management of patients with venous ulceration includes the treatment of refluxing superficial veins to reduce the risk of ulcer recurrence based on the results of the ESCHAR trial.
National Institute for Health and Care Excellence guidelines: 2013
The National Institute for Health and Care Excellence (NICE) published guidance on the diagnosis and management of varicose veins in July 2013;40 it recommends the referral of patients with symptomatic varicose veins (including current or healed ulceration) to a vascular service within 2 weeks. Vascular service has been defined by NICE as:
. . . a team of healthcare professionals who have the skills to undertake a full clinical and duplex ultrasound assessment and provide a full range of treatment.
Despite a study noting an increase in referrals to secondary care in the period after implementation, results were unable to demonstrate an impact on early referral. 40,41 The NICE guidance also recommends the use of venous duplex ultrasonography to confirm the presence of venous insufficiency and endovenous intervention as first-line treatment. 42
National Institute for Health and Care Excellence quality standard: 2014
The NICE quality standard on the diagnosis and management of varicose veins of the legs43 was published in August 2014 and provides specific, concise and measurable statements to improve the process and care of patients with varicose veins. This quality standard echoed the 2013 NICE guidance in terms of referral, diagnosis and treatment choice.
Rationale for the Early Venous Reflux Ablation trial
Despite the evidence that the treatment of superficial venous reflux reduces ulcer recurrence in patients with venous leg ulcers, there is currently no level 1 evidence demonstrating reductions in time to healing. 21 With this void in evidence, superficial venous reflux is often treated after ulcers have healed following conservative treatment involving compression bandaging. The danger of taking this approach is that, once the ulcer is healed and the symptoms have resolved, patients may not be referred. The resulting untreated superficial venous reflux contributes to an increased risk of ulcer recurrence, which is both costly for the health service and distressing for the patient. The previous RCT literature may have underestimated the clinical benefit of intervention, with recent prospective cohort studies of endovenous intervention in active leg ulceration clearly suggesting an adjuvant benefit compared with compression alone in terms of healing rates. Time to healing has been highlighted as the end point that is most important to patients, as demonstrated in the patient and public involvement (PPI) work (see Appendix 1) of this trial and even a modest improvement in ulcer healing would significantly reduce the health-service costs associated with the condition.
As the incidence and prevalence of venous ulcers are likely to increase as a result of the ageing population, it is important to clarify the role and timing of superficial endovenous ablation in venous ulceration to guide treatment recommendations and referral pathways. 44,45
Summary of main points
Venous leg ulcers are open wounds that have a detrimental effect on the quality of life of patients. Treatment of the condition in the UK represents a substantial economic burden to the NHS and Personal Social Services, amounting to many hundreds of millions of pounds per year.
Until recently, superficial venous reflux could be treated only by open surgery. Newer, endovenous techniques have been shown to be just as effective as open surgery in terms of clinical improvement, but with reduced complications and pain. These techniques do not need to be performed under general anaesthetic and therefore may be more suitable for elderly patients with significant comorbidities. The most recent UK guidelines for varicose veins43 recommend early referral to a vascular service for diagnosis and first-line treatment by means of endovenous interventions.
The ESCHAR trial9,15 indicated that the majority of patients with venous ulceration could benefit from superficial venous intervention with respect to ulcer recurrence; however, the study was not powered to detect an effect on ulcer healing and therefore further research into ulcer healing was required.
Chapter 2 Methods
Research objectives
Primary objective
The primary objective was to determine the clinical effectiveness and cost-effectiveness of compression therapy with early endovenous ablation of superficial venous reflux compared with compression therapy with deferred endovenous ablation in patients with venous ulceration.
Secondary objective
The secondary objective was to investigate ulcer-free time, quality of life, and the clinical and technical success of endovenous ablation to 1 year.
Trial design
We conducted a pragmatic, multicentre, open RCT with participants randomised 1 : 1 to either (1) deferred (standard) therapy, consisting of multilayer elastic compression therapy, with deferred endovenous ablation of superficial reflux once the ulcer has healed, or (2) early endovenous ablation of superficial venous reflux (within 2 weeks) in addition to standard compression therapy.
Amendments to the protocol
Substantial amendments to the trial protocol were submitted after the initial approval, in order to increase recruitment and retention, correct the sample size calculation and clarify the health economic evaluation:
-
Version 2.0, dated 6 January 2014: amended to provide a clearer definition of ulcer healing, clarify the per-protocol analyses and safety sections, and to clarify that participants could be offered endovenous ablation of superficial venous reflux in the deferred group if their ulcer had not healed at 6 months.
-
Version 3.0, dated 10 March 2014: amended in order to allow the display of posters and dissemination of leaflets and participant information sheets in primary care sites.
-
Version 4.0, dated 16 March 2016: amended to correct the sample size from 500 participants to 450 participants (which was originally calculated erroneously), and to allow for a reduction in the number of photograph verification visits performed if the core laboratory confirms that the ulcer is healed in order to prevent unnecessary visits and enhance participant retention.
-
Version 5.0, dated 6 April 2017 [see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 23 April 2019)]: amended to (1) incorporate a Health Technology Assessment (HTA) funding extension to allow for the collection of longer-term follow-up during October 2018 and March 2019 and (2) make revisions to the health economics section to clarify and update the protocol to reflect new National Institute for Health Research (NIHR) guidelines. The follow-up period is now complete (31 March 2019) and, at the time of publication, we are cleaning and locking the database prior to data analysis.
Ethics and research and development approvals
A favourable ethics opinion was given by the National Research Ethics Service Committee South West – Central Bristol on 15 August 2013 (reference number 13/SW/0199). For a copy of the original approval see Report Supplementary Material 1. Annual reports were submitted to this committee, which confirmed that the ethics approval continued to apply.
The study-wide governance review was undertaken by the Clinical Research Network North West London in August 2013. Research and development NHS approvals were granted at participating sites between October 2013 and March 2015. The trial was granted the new Health Research Authority approval on 30 June 2016.
Sponsorship
The trial was sponsored by Imperial College London.
Trial management
The trial was supported by the Imperial Clinical Trials Unit (ICTU) and the day-to-day trial management was performed by the trial manager based in the academic vascular department of Charing Cross Hospital, London. The trial manager was responsible for co-ordinating the data collection; follow-up; data cleaning; monitoring visits; communication with the sites, participants and collaborators; and answering trial-specific queries. The trial manager and chief investigator met at least monthly during the course of the trial.
Trial Management Group
The trial was supervised by the Trial Management Group, which comprises the chief investigator, lead statistician, trial statistician, health economist and trial manager. The Trial Management Group met in person or by teleconference on a regular basis.
Trial Steering Committee
An independent Trial Steering Committee (TSC) was established as per the HTA TSC terms of reference to oversee trial conduct. The membership comprises five independent members (see Acknowledgements), the chief investigator, trial manager and lead statistician. The TSC met at least annually or more regularly if required, as decided by the committee. For the meeting dates see Appendix 2.
Data Monitoring Committee
An independent Data Monitoring Committee (DMC) was established as per the HTA DMC terms of reference, to monitor trial data and safety. The membership comprised four independent members (see Acknowledgements). The members met once prior to the start of the trial to agree the DMC charter and then on an annual basis to review recruitment, fidelity, retention and unblinded comparative data (for both safety and efficacy). No interim analyses were planned and the trial statistician was the only member of the trial team to have access to the unblinded data. Following each meeting, the DMC recommended continuation of the trial to the TSC. For the meeting dates see Appendix 2.
Participants
All patients aged ≥ 18 years presenting with a leg ulcer of venous origin who were able to tolerate compression therapy and were suitable for endovenous ablation of superficial venous reflux could be included.
Inclusion criteria
Patients with all of the criteria listed below were deemed eligible:
-
current leg ulceration duration of > 6 weeks’ but < 6 months
-
able to give informed consent to participate in the trial after reading the patient information documentation
-
patient aged ≥ 18 years
-
ankle–brachial pressure index (ABPI) of ≥ 0.8
-
primary or recurrent superficial truncal venous reflux on colour duplex assessment deemed by the treating clinician to be significant enough to warrant endovenous ablation.
Patients who could not speak/understand English were eligible for inclusion. Informed consent was obtained with assistance from translation services as per standard clinical practice; however, in view of the lack of cross-cultural validation for quality-of-life tools, only healing outcome data were collected.
Exclusion criteria
Patients meeting any of the criteria listed below were ineligible:
-
presence of deep-venous occlusive disease or other conditions precluding endovenous superficial venous ablation (at the discretion of the treating clinician)
-
patients unable to tolerate multilayer compression therapy (as concordance with compression therapy can be variable for patients at different times, patients who were generally concordant with compression, but unable to tolerate short periods, were still deemed eligible)
-
inability of the patient to receive prompt endovenous ablation by recruiting centre
-
pregnancy
-
leg ulcer of non-venous aetiology as assessed by the treating clinician
-
patients deemed to require skin grafting as assessed by the treating clinician.
Sample size
The sample size calculation for this trial was based on the primary outcome of time to ulcer healing. In the ESCHAR trial, the 24-week healing rate in participants randomised to compression alone was approximately 60%. 46 Two prospective studies evaluating the early endovenous ablation of superficial venous reflux suggested that the 24-week healing rate may be as high as 82%. 35,36
In order to calculate a sample size for this trial, the desirable absolute benefit associated with early endovenous ablation of superficial truncal reflux was estimated to be 15%. Assuming that the 24-week healing rate in the deferred (standard) group is 60%, to identify an absolute difference in 24-week healing rates between the two groups of 15% (60% vs. 75%), with 90% power and allowing for 10% dropout, the trial required 416 subjects (208 in each group, 254 healed leg ulcers in total). 47 To incorporate further allowances for protocol violations and unexpected dropouts, the target sample size was set at 450 participants.
Settings and locations
Participants were recruited from the vascular departments of 20 secondary care NHS trusts throughout England: Bradford Teaching Hospitals NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust, Frimley Health NHS Foundation Trust, Gloucestershire Hospitals NHS Foundation Trust, Heart of England NHS Trust (now University Hospitals Birmingham NHS Foundation Trust), Hull and East Yorkshire Hospitals NHS Trust, Imperial College Healthcare NHS Trust, Leeds Teaching Hospitals NHS Trust, North Cumbria University Hospitals NHS Trust, North West London Hospitals NHS Trust, University Hospitals Plymouth NHS Trust, Salisbury NHS Foundation Trust, Sheffield Teaching Hospitals NHS Foundation Trust, Taunton & Somerset NHS Foundation Trust, The Dudley Group NHS Foundation Trust, the Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust, Royal Wolverhampton NHS Trust, University Hospital Birmingham NHS Trust, Worcestershire Acute Hospitals NHS Trust, and York Teaching Hospital NHS Foundation Trust. For a list of participating hospitals see Acknowledgements, Local vascular research teams.
Recruitment procedure
Prior to commencing the trial, information was disseminated to general practices in each recruiting region. In addition, selected primary care trusts (PCTs) not currently involved in the trial were set up as patient identification centre sites displaying posters and leaflets and disseminating patient information sheets to patients once the protocol amendment had been approved. As per the July 2013 NICE guidelines on varicose veins,40 patients with venous ulcers were required to be referred from primary to secondary care as part of the standard care pathway.
Patients were screened from secondary care vascular, ulcer and tissue viability clinics. As part of standard care, patients are evaluated by clinical assessment and colour duplex examination. Depending on the results of these tests, the patients were given a short leaflet containing a summary of the trial and, if interested, then given the more detailed patient information sheet to read.
The details of patients who were eligible for the trial but did not agree to participate, and patients with ulcers who were not eligible for the trial, were recorded anonymously on screening logs along with a minimal data set (including age, ulcer duration and venous duplex/ABPI findings, if known, and reason for non-inclusion).
Informed consent
Patients were given a minimum of 24 hours to consider the trial in addition to the opportunity to discuss all aspects of the trial with their family and/or general practitioner (GP). Patients were then contacted by telephone by the research nurse so that any further questions could be answered. All willing patients were booked in to the leg ulcer clinic to undergo a baseline visit.
Written consent was obtained from each participant at the baseline visit. The patient information sheet and informed consent form (see Report Supplementary Material 2) both refer to the possibility of long-term follow-up if the trial is extended and seek permission to access to their NHS records for these purposes. With the participant’s consent, a letter was also sent to the participant’s GP (see Report Supplementary Material 3). A copy of the patient information sheet and informed consent form was filed in the participant’s hospital notes and the local research file and a copy was also given to the participant.
All trial documentation contained the contact details of the Early Venous Reflux Ablation (EVRA) trial chief investigator and trial manager to enable participants to obtain further information from the trial team if required.
Baseline assessment
Once written consent was given by the participant, eligibility was confirmed and baseline data were collected by the research nurse using the case report form (CRF) (see Report Supplementary Material 4).
Participant demographic and contact details
Data collected included participant contact details, GP details, age, sex, ethnicity and work status. Pregnancy tests were taken by women of child-bearing potential. Participants were provided with a reminder wallet card, which contained the contact details of the local research nurse with a reminder message to call the nurse when they thought that their ulcer had healed.
General medical and ulcer history
This included body mass index (BMI), ABPI, medical history and current medications. An ulcer history was taken, including any previous ulcers and interventions.
Current ulcer and venous assessment
Ulcer duration and size
For the leg to be randomised, the duration of the current ulcer (according to the participant and available medical records) and ulcer size were recorded.
To measure the total ulcer area, tracing grids of 1 cm2 squares were placed over all the ulcers on the randomised leg and the outside perimeter of the wounds was traced using an indelible pen. The ulcer area was determined by totalling the number of squares contained within the traced ulcer/s area. Where more than one ulcer was present, the total area was calculated by combining each individual area.
In addition, photographs of all the ulcers on the randomised leg were taken with a digital camera, alongside a measuring scale. Sony Cyber-shot DSC-WX60 16.2 Megapixel Digital Cameras (Sony Electronics Inc., San Diego, CA, USA) were provided to all sites and a simple photography protocol was detailed in the site handbook. The tracings and photographs were assigned pseudonyms (trial number) and transferred via a secure server to the trial manager.
The tracing and photograph protocol is detailed in Appendix 3.
Once follow-up was complete, an exact ulcer area was calculated from the wound grid and photograph by the use of a software program, ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA; open source). 48 By reviewing the photographs and tracings in combination, a judgement was made of the most accurate measurement to be taken as the total ulcer area.
Clinical ulcer assessment
Clinical, aetiological, anatomical and pathophysiological
Clinical, aetiological, anatomical and pathophysiological (CEAP) is a descriptive classification that was developed in 1994 by an ad hoc committee of the American Venous Forum in order to standardise the classification of chronic venous disease. 49 The classification was updated in 2004 to refine some of the definitions and introduce the simpler basis CEAP. 50 All participants were classified according to the basic CEAP. An active ulcer is described by C6, and a healed ulcer as C5.
Venous Clinical Severity Score
The Venous Clinical Severity Score (VCSS) is a component of the Venous Severity Scoring System designed in 2000 by an ad hoc American Venous Forum committee consensus, in order to compliment the CEAP classification and quantify the severity of disease and subsequent improvement or decline. 51 The VCSS has 10 components (pain, varicose veins, venous oedema, skin pigmentation, inflammation, induration, compression used and active ulcer, duration, number and size), each with four categories assigned values of 0–3. The overall scores can range from 0 (lowest severity) to 30 (highest severity). A score was recorded at baseline for each participant (Tables 1 and 2).
| Details of outcome measure | Type of assessment | Range of scores | Comments |
|---|---|---|---|
| VCSS52 | Physician-assessed clinical severity evaluation | 0–30 | Higher scores indicate more severe venous disease |
| AVVQ53 | Patient-reported disease-specific quality of life | 0–100a | Higher scores indicate worse health related to varicose veins |
| EQ-5D-5L55 | Patient-reported generic quality of life | 0–100 (health scale) | Consists of a health scale and health index (with higher scores indicating better health) |
| SF-3656 | Patient-reported generic quality of life | 0–100 (for each domain) | Eight scores covering different domains of health, with higher scores indicating better health |
| Score | None (0) | Mild (1) | Moderate (2) | Severe (3) |
|---|---|---|---|---|
| Pain or other discomfort (i.e. aching, heaviness, fatigue, soreness, burning). Presumes venous origin | None | Occasional pain or other discomfort (i.e. not restricting regular daily activity) | Daily pain or other discomfort (i.e. interfering with but not preventing regular daily activities) | Daily pain or discomfort (i.e. limits most regular daily activities) |
| Varicose veins: ‘varicose’ veins must be ≥ 3 mm in diameter to qualify in the standing position | None | Few: scattered (i.e. isolated branch varicosities or clusters). Also includes corona phlebectatica (ankle flare) | Confined to calf or thigh | Involves calf and thigh |
| Venous oedema: presumes venous origin | None | Limited to foot and ankle area | Extends above ankle but below knee | Extends to knee and above |
| Skin pigmentation: presumes venous origin. Does not include focal pigmentation over varicose veins or pigmentation due to other chronic diseases (i.e. vasculitis purpura) | None or focal | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf |
| Inflammation: more than just recent pigmentation (i.e. erythema, cellulitis, venous eczema, dermatitis) | None | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf |
| Induration: presumes venous origin of secondary skin and subcutaneous changes (i.e. chronic oedema with fibrosis, hypodermitis). Includes white atrophy and lipodermatosclerosis | None | Limited to perimalleolar area | Diffuse over lower third of calf | Wider distribution above lower third of calf |
| Active ulcer number | None | 1 | 2 | ≥ 3 |
| Active ulcer duration (longest active) | N/A | < 3 months | > 3 months but < 1 year | Not healed for > 1 year |
| Active ulcer size: diameter (largest active) | N/A | < 2 cm | 2–6 cm | > 6 cm |
| Use of compression therapy | Not used | Intermittent use of stockings | Wears stockings most days | Full compliance: with stockings |
Suitability for intervention
Details of venous disease were also collected, including previous deep-vein thrombosis (DVT) and pattern of venous reflux identified on the duplex ultrasound, to assess suitability for ablation. Duplex ultrasonography scanning was performed as per standard care at the randomising site.
Participant-completed questionnaires
To provide a comparator for participant-reported outcomes, enrolled participants completed three health questionnaires at baseline. The baseline health questionnaires were administered prior to the participants being told of their treatment allocation (see Report Supplementary Material 4).
EuroQol-5 Dimensions
The EuroQol-5 Dimensions (EQ-5D) is a widely recognised, generic tool to measure health outcomes and has been validated in a variety of patient groups, including those with venous leg ulcers. 58 The EQ-5D questionnaire comprises two sections; the first assesses the participant’s mobility, self-care, ability to perform usual activities, pain/discomfort and anxiety/depression levels, and the second records the participant’s self-rated health on a vertical score of 0 to 100 (see Table 1).
Short Form questionnaire-36 items
The Short Form questionnaire-36 items (SF-36) is a generic quality-of-life tool used to determine people’s physical and mental health. It has been validated in many patient groups, including those with varicose veins. 56 The physical domain measures physical functioning, physical role limitations, body pain and general health, whereas the mental dimension measures vitality, social functioning, mental health role limitations and general mental health. Two separate scores are produced (separate physical/mental component summary scores), in addition to the eight separate domain scores. Each score is measured on a scale of 0 to 100 (worst to best). Scores represent the percentage of total possible score achieved (see Table 1).
Aberdeen Varicose Vein Questionnaire
The Aberdeen Varicose Vein Questionnaire (AVVQ) is a validated patient-reported disease-specific health questionnaire to assess quality of life in patients with varicose veins. The AVVQ comprises a diagram on which patients draw on their varicose veins and a questionnaire with 12 questions, half of which require a response for each leg. The scores range from 0 to 100 (no effect to severe effect)53 (see Table 1).
Randomisation and treatment allocation
Separate randomisation lists for each centre were prepared by a statistician prior to recruitment using randomly permuted blocks in two block sizes (‘ralloc’ command; Stata® v14.2, StataCorp LP, College Station, TX, USA) and loaded onto the InForm™ version 4.6 (Oracle® Health Sciences, CA, USA) system. Access to the allocation sequence was strictly restricted to the statistician and appropriate members of the InForm technical support team to maintain allocation concealment.
Consenting participants were registered on the InForm integrated trial management system, a web-based data entry system maintained by the ICTU, and their eligibility for the trial verified. Once eligibility was confirmed, online randomisation was performed remotely by the research nurse.
Each participant was automatically assigned the next available treatment allocation in the appropriate randomisation list and allocated a unique trial number. The randomisation ratio was 1 : 1 with participants allocated to either:
-
early (within 2 weeks) endovenous ablation of superficial venous reflux in addition to compression therapy or
-
deferred (standard) therapy consisting of multilayer elastic compression therapy with deferred endovenous ablation of superficial reflux once the ulcer healed.
Blinding
It was not possible to blind either the treating team or the participant to the allocated treatment. The primary outcome, time to ulcer healing, was determined by two expert assessors who were blinded to participant details, including the treatment group.
Deferred ablation (standard care): control group
Participants in the deferred (standard) care group were randomised to receive multilayer compression therapy alone with endovenous ablation of superficial reflux once ulcer healing had been confirmed. Participants whose ulcer had not healed at 6 months post randomisation or who experienced clinical deterioration in the active leg ulcer during the control treatment, could be offered endovenous interventions if it was felt that the participant would benefit from expedited endovenous ablation (at the discretion of the local responsible clinician). The post-ablation duplex ultrasonography strategy for participants in the standard care group was left to local policy.
Early ablation: interventional group
Participants in the interventional group were randomised to receive endovenous ablation of superficial truncal reflux within 2 weeks of randomisation in addition to compression therapy. Post-ablation duplex ultrasonography was performed 6 weeks from randomisation.
Standardisation of compression therapy
As a wide range of compression types are currently used within the NHS, the specific therapy was left to the discretion of individual centres and primary care professionals. Multilayer elastic (two, three or four layer), short stretch and hosiery compression were all deemed acceptable for inclusion in the trial. All participants were advised to use compression hosiery post healing, in line with local policy.
Endovenous interventions
A wide range of endovenous ablation modalities are currently available and in widespread use. The following interventions were permitted in the trial: EVLA or RFA, UGFS, mechanochemical ablation and cyanoacrylate glue closure. These interventions could be performed alone or in combination, as directed by clinical need at the discretion of the responsible vascular specialist.
It was noted that the interventional strategies varied between institutions and between individual clinicians within the same department. Heterogeneity existed for site of vein cannulation (and, therefore, the length of vein ablated), the location of intervention (‘office’ or clinic based vs. operating theatre), interventional strategy for subulcer venous plexus (to ablate or not), the ablation of visible varicose veins (no treatment, UGFS or surgical avulsion) and the timing of any secondary interventions. As there was neither current research evidence nor consensus as to a single, optimal endovenous interventional strategy for superficial reflux in patients with leg ulceration, local and individual variation was allowed, subject to the following stipulations:
-
The endovenous strategy had to include ablation of the main truncal venous reflux.
-
Truncal venous reflux had to be treated to the lowest point of incompetence, where possible.
-
Significant (as deemed by the treating clinician) residual/recurrent superficial reflux on the 6-week duplex scan was to be ablated.
-
Participants had to continue with multilayer compression/stockings immediately after ablation.
Participant follow-up
All randomised participants were followed up until one of the following:
-
1 year after randomisation
-
the participant chose to withdraw from the trial
-
death.
The trial design is summarised in Appendix 4.
As per standard care, participants received routine leg ulcer care in the community and/or hospitals in accordance with local policies.
Monthly telephone calls/follow-up
Participants were followed up on a monthly basis by research nurses at each local site. The aim of the telephone follow-up was to assess whether or not the reference ulcer had healed (for ulcers that were unhealed at the last follow-up), and, in the case of ulcers that were known to have healed, to confirm that the ulcer remained healed. In cases of ulcer recurrence, the telephone follow-up was used to ascertain the date of recurrence and of subsequent healing. Information on utility and resource use, dressing changes, adverse events (AEs) and serious adverse events (SAEs) were also collected.
Six-week clinic visit
All participants underwent a clinical assessment of the reference leg at 6 weeks post randomisation to determine ulcer healing, in addition to VCSS evaluation and documentation of the current ulcer compression regimen. A wound tracing was drawn and photographs were taken to document the size of any unhealed ulcers. Disease-specific and generic quality of life were assessed by means of self-completed questionnaires (AVVQ, EQ-5D and SF-36).
Venous duplex ultrasonography was performed in participants in the early-ablation group to verify if any residual superficial venous reflux was present and guide whether or not further interventions were warranted.
Participant withdrawal
Participants could withdraw from the trial at any time without giving a reason; however, efforts were made to identify the reason for withdrawal whenever possible.
Participants who expressed a wish to withdraw from the trial visits were asked to confirm if they agreed to the trial team retaining their existing trial data and accessing trial-related NHS data; this was documented in the patient notes. If possible, participants were asked for permission to retain primary outcome data.
Participants who declined endovenous ablation remained in the trial for assessment of primary and secondary outcomes [and analysis on intention to treat (ITT)] unless they specifically withdrew their consent.
Measurement and verification of primary outcome measure
Time to healing of the reference ulcer (blinded)
The primary outcome measure of this trial was time from randomisation to complete healing of ulcers on the reference leg. Healing was defined in the protocol as complete re-epithelialisation of all ulceration on the randomised leg in the absence of a scab (as defined in the ESCHAR trial) with no dressing required.
If either the community nurse or the participant believed that ulcer healing had been achieved, they were asked to contact the local research centre immediately to trigger an urgent verification assessment by the research nurse within 1 week.
Ulcer healing was verified by clinical assessment and digital photography repeated weekly for 4 weeks, unless otherwise agreed by the trial manager. Digital photographs were assigned pseudonyms by trial number only and transferred via a secure server to the ICTU.
All digital images were assessed by two vascular surgeons blinded to treatment allocation. Each independently assessed the reference ulcer using a predefined set of decision rules based on those utilised in Venous leg Ulcer Study IV (VenUS IV)59 (see Appendix 5) to allocate each to one of three categories (healed, not healed or unsure). Disagreements were resolved through discussion with a third blinded expert reviewer.
When a reference ulcer was deemed to have healed, the date of the photograph in which healing was recorded was taken to be the date of healing. If healing was confirmed at the first verification visit, the date of healing notification (by participant or community nurse) was taken as the date of ulcer healing.
Measurement and verification of secondary outcome measures
Ulcer healing
The number of ulcers healed at 24 weeks was reported, in addition to time to ulcer healing, to allow comparison with other published studies.
Ulcer recurrence/ulcer-free time
Participant-reported ulcer recurrence on the reference leg was recorded by the research nurses for up to 12 months from randomisation or until trial exit, by means of monthly telephone calls to the participant. Recurrence was verified using patient notes from recent clinic visits whenever possible. When there had been a recurrence of venous leg ulceration on the reference leg, the dates of recurrence and subsequent healing, if applicable, were recorded and used to determine ulcer-free time.
Health-related quality of life
In addition to the baseline assessment, health-related quality of life (HRQoL) was measured at 6 weeks, 6 months and 12 months using questionnaires either administered in clinic by the research nurse or sent by mail to the participant along with a pre-addressed and prepaid envelope. Each questionnaire pack was identical in content to the baseline questionnaire pack containing the EQ-5D, SF-36 and AVVQ. When necessary, reminder letters were sent by post to participants if the questionnaires had not been returned.
Utility and resource use
Participant-reported utility and resource use was collected by the research nurses up to 12 months from randomisation or trial exit via monthly telephone calls to the participant, or at clinic visits if these occurred as part of clinical care. The participants were provided at baseline with diaries in which any visits to health-care providers could be recorded. All utility and resource use data were collected, whether or not deemed to be related to the reference leg.
Markers of clinical success
Venous Clinical Severity Score
In addition to the baseline visit, the VCSS was assessed by the research nurse or treating clinician at the 6-week clinic visit to allow comparison with the baseline score.
Ablation success
Local principal investigators assessed the presence of residual/recurrent truncal superficial venous reflux in the early-ablation group at 6 weeks by means of a venous duplex. Residual reflux and any recanalised segments were noted. When the truncal vein was not successfully closed, further endovenous ablation procedures were organised. For other patterns of residual or recurrent reflux (such as reflux in tributaries or perforating veins), the decision whether or not to perform additional endovenous interventions was left to the discretion of the treating clinician.
Safety monitoring of early ablation
Adverse events
The research nurses collected data regarding the occurrence of AEs during the monthly telephone calls and from clinic or surgery notes, and reported these to the ICTU via the web-based data capture system. Only AEs deemed by the local principal investigator to be related to the trial intervention or compression were recorded. The AEs thought to be related to the interventions are summarised in Table 3. AEs were reviewed and categorised by the trial manager and chief investigator as procedural complications.
| Systemic | Local |
|---|---|
| Allergic reaction required local/no treatment | Bleeding requiring intervention |
| Migraine | Blistering of skin |
| Visual disturbance | Pressure damage |
| Fainting | Nerve damage |
| Cough/chest tightness | DVT |
| Systemic infection | Haematoma |
| Pulmonary embolism | Participant-reported paraesthesia |
| Transient ischaemic attack | Pigmentation of skin |
| Stroke | Superficial thrombophlebitis |
| New ulcer | |
| Deterioration of ulcer | |
| Wound infection |
Serious adverse events
As per International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice (ICH–GCP) guidelines, SAEs were defined as those AEs that result in death; are life-threatening; require inpatient hospitalisation or prolongation of existing hospitalisation; result in persistent or significant disability or incapacity; result in congenital anomaly or birth defect; are cancer; or are other important medical events in the opinion of the responsible investigator (i.e. not life-threatening or resulting in hospitalisation, but may jeopardise the participant or require intervention to prevent one or more of the outcomes described previously). All SAEs were recorded, whether or not deemed by the local principal investigator to be related to the trial intervention or compression.
The research nurses collected data regarding the occurrence of all SAEs via the monthly telephone calls, clinic or surgery notes, and hospital admission records. These were reported to the ICTU via the web-based data capture system within 24 hours of the nurses becoming aware of the event and reviewed by the chief investigator.
All SAEs were also reported by the trial manager to the sponsor and chairperson of the DMC. SAEs were coded using Medical Dictionary for Regulatory Activities (MedDRA®) version 20.0 [URL: www.meddra.org (accessed 15 May 2019). MedDRA® terminology is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. MedDRA® trademark is registered by the International Federation of Pharmaceutical Manufacturers and Associations on behalf of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use].
Participant communications
Participants were kept updated on trial progress via the trial Facebook (Facebook, Inc., Menlo Park, CA, USA) and Twitter (Twitter, Inc., San Francisco, CA, USA) accounts. Two participant newsletters were circulated during the follow-up stage (for participants who had not withdrawn from the trial), to keep them updated with trial progress. A newsletter summarising the main results from the EVRA trial was also sent to non-withdrawn participants.
Statistical methods
The trial analysis was carried out on an ITT basis (all participants remained in the group allocated at randomisation). Histograms and box plots were used to check the distribution and possible outliers for continuous variables. Mathematical transformations were applied, when appropriate, in order to render the continuous variables distribution normally distributed. Continuous variables that follow an approximately normal distribution were summarised using means and standard deviations (SDs). Skewed continuous variables were summarised using medians and interquartile ranges (IQRs). Categorical variables were summarised using frequencies and percentages.
All hypothesis testing was planned to be two-tailed with a 5% significance level and no adjustment for multiple testing. Analyses were performed using Stata v14.2.
As the randomisation was stratified by centre, when possible, analyses are adjusted by trial centre. Potentially, this is done by including trial centre as either a fixed or a random effect in any regression models. As the centres that participated in the trial could be viewed as a random sample of all possible trial centres, random-effects models were preferred. However, in cases where random-effects models could not be fitted (e.g. owing to lack of convergence), trial centre was included in models as a fixed effect.
Baseline data
Baseline characteristics, including demographics, medical history, ulcer history and details of current ulcers, were summarised by treatment group using appropriate descriptive methods for all randomised participants. Ulcer duration was calculated as the difference between the date the current ulcer appeared (best estimate based on medical records, referral letters and participant recollection) and the date of randomisation. Deep vein reflux and/or obstruction was defined as iliac, femoral, popliteal or infrapopliteal deep vein reflux as shown on duplex scan [for details, see the statistical analysis plan on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)].
Trial completion
Reasons for trial exit were taken from the end-of-trial form and included completed trial (to 12 months), lost to follow-up, withdrawn and death.
Statistical analysis
Primary end point
The primary outcome was time to complete healing and we tested the hypothesis that there was no difference in time to ulcer healing between deferred- and early-ablation groups using a Cox proportional hazards model. As the randomisation was stratified by centre, centre was also included in the model as a random effect (shared frailty). The proportional hazards assumption was assessed graphically – by plotting –ln{–ln[Ŝ(t)]} versus ln(t) and checking that the curves for each level of the covariate are parallel – and also numerically using Grambsch and Therneau tests. Kaplan–Meier (KM) survival curves were also presented and, as a subsidiary analysis, we investigated the effect of participant age, ulcer size at baseline and duration of time to complete healing using Cox regression, with centre included in the model as a random effect to adjust for potential centre effect [for details, see the statistical analysis plan on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)].
Participants were censored at the time of last follow-up if they had died, withdrawn or were lost to follow-up before primary ulcer healing. The follow-up time was 1 year after randomisation, and thus observations of participants with an unhealed primary ulcer at 1 year after randomisation were also censored.
Secondary end points
Recurrence/ulcer-free time to 1 year and 24-week ulcer healing rate
The effect of the trial intervention on ulcer-free time was investigated after adjusting for potential confounders [for details, see the statistical analysis plan on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)], using multiple linear regression if the assumption of normality was met. If the assumption of normality was not met (there is no suitable transformation), ulcer-free time was categorised and analysed using appropriate regression methods to adjust for potential confounders. The number of ulcers healed at 24 weeks and associated 95% CIs were obtained from the KM analysis.
One-year ulcer-free time (in days) in those who had completed follow-up to 1 year was calculated as total follow-up time (i.e. 1 year) minus the total duration of ulcers, including the primary ulcer and any recurrences.
Quality of Life
The AVVQ was scored in accordance with the manual. 53
The SF-36 was scored using QualityMetric Health Outcomes™ scoring software 4.0 (QualityMetric, Lincoln, RI, USA) for the physical health and mental health dimensions and all eight scales: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain and general health.
The index-based values (‘utilities’) were calculated by the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), crosswalk index value calculator downloaded from the EQ-5D official website.
The HRQoL scores were presented using line plots for each trial group to illustrate trends in AVVQ score, SF-36 and EQ-5D-5L over time. We planned to report the means and 95% CI of means, or medians and interquartiles, at each time point (including baseline and 6 weeks and 6 and 12 months after randomisation), depending on the distribution of the data. Mixed models with time, age, ulcer size and duration as fixed effects, and trial centre and patient as nested random effects, were used to estimate differences in HRQoL scores between the trial groups at each time point and to calculate an overall p-value for the difference in HRQoL scores between the trial groups.
Markers for clinical success: Venous Clinical Severity Score
Clinical success was assessed using the VCSS, which was measured at baseline and 6 weeks post randomisation. Any change in VCSS was compared between the two groups using the t-test (assuming that change in VCSS is normally distributed) or appropriate non-parametric test (if change in VCSS is not normally distributed). The VCSS at 6 weeks post randomisation and baseline is summarised using box plots for both groups (see Figure 11).
Markers for clinical success: clinical, aetiological, anatomical and pathophysiological
The change in clinical classification in the CEAP score from baseline to 6 weeks post randomisation is reported (see Table 17) and the chi-squared test was used to compare the two groups.
Safety data
The safety data, including AEs and SAEs, were provided in a tabular format for the two groups [for details, see the statistical analysis plan on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)]. AEs were summarised by description and outcome and SAEs were summarised by SAE reason, frequency, severity, relationship to treatment, outcome and expectedness.
Sensitivity analysis
As a sensitivity analysis, a per-protocol analysis was performed by excluding participants with protocol deviations. This sensitivity analysis covered all primary and secondary outcomes.
Missing data
There was no imputation of missing data for the primary end point (time to healing) or the secondary end points of 24-week healing rate and ulcer-free time. However, multiple imputation of the quality-of-life measures and measures of clinical success was performed using chained equations as a sensitivity analysis. 60 The number of missing data were reported.
Health economic analysis
Overview of within-trial economic analysis
The within-trial health economic analysis compared early endovenous ablation with deferred endovenous ablation for superficial venous truncal reflux in patients with venous ulceration, within the 1-year time horizon of the clinical trial. A cost–utility analysis was performed. No subgroup analyses were undertaken. The analyses were performed from the perspective of the NHS and Personal Social Services in accordance with NICE methods guidance. 61
The total cost per patient aimed to include only items related to the endovenous ablation procedure or venous leg ulcer. The price year was 2015/16. No discounting was applied as the follow-up is 1 year. The trial was reported in accordance with guidelines for economic evaluation. 62 See Husereau et al. 62 for the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist and the project web page [www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)] for the health economic plan.
Data
Data were collected in the CRF by case note review and from questionnaires. The primary outcome measure was the quality-adjusted life-years (QALYs) gained at 1 year. Health state utilities were calculated from the EQ-5D-5L questionnaire administered to participants at baseline, and at 6 weeks and 6 and 12 months post randomisation. The base-case economic analysis uses the crosswalk tariff. 63 This is an algorithm that maps the EQ-5D-5L responses to the three-level responses and then values those health states using the original EQ-5D-3L tariff developed by Dolan. 64 This tariff was available from the EuroQol group and was recommended by NICE at the time of these analyses. 65 As a sensitivity analysis, an alternative five-level tariff recommended by Devlin et al. 55 was used. QALYs were estimated for each participant to 1 year as the ‘area under the curve’ of EQ-5D-5L index values.
Resource use items were recorded for each participant at monthly follow-up telephone calls. The health-care resource use collected in the trial and the assumptions made in the economic analysis are presented in Table 4.
| Resource use | Description |
|---|---|
| Trial vein ablation procedures | Time in operating theatre and the type of procedure (UGFS, RFA, EVLA or MOCA) were recorded. Participants could have more than one trial vein ablation procedure. Staff procedure costs were calculated from the time in operating theatre (recorded in the CRF) multiplied by standard unit costs (see Appendix 6) |
| Dressings and bandages for wound healing |
Dressings: classified in the CRF as NA dressing, Inadine™ [Systagenix (KCI company), San Antonio, TX, USA] (iodine impregnated), or other. For estimating costs, it was assumed that dressings were changed twice per week until wound healing Compression: the CRF recorded if the participant used compression bandages, stockings or no compression. If bandages, it was assumed that these were changed at each dressing change. Participants who used compression stockings were assumed to own two pairs (one to wash and one to wear), and that both were replaced every 3 months (Karen Dhillon, Imperial College London, 2017, personal communication). Bandages were assumed to have been used if the CRF did not state which mode of compression was applied (as these are the most common type of compression therapy in use) |
| Compression therapy to prevent recurrence after wound healing | The costs of compression therapy post healing were estimated in line with local policy. For estimating costs, it was assumed that stockings were changed every 3 months (Karen Dhillon, personal communication) |
| Visits to a district nurse or primary care nurse | All these visits, for any reason, were included in the total cost |
| Visits from a district nurse | All these visits, for any reason, were included in the total cost |
| Hospital admissions (inpatient and day case) | The trial collected data on the reason for the admission and any procedure undertaken as free text. Admissions were classified as ‘vein related’ if one of the text fields included one of these keyword fragments: ‘leg ulcer, vein, rf, abla, evlt, evla, sclero, screlo, vnus, foam, ugfs, angio, rehab, physio, conval, skin, antibio, sepsis, septic, infection, dvt’ (the list takes account of spelling errors in the text field). Vein ablation procedures were identified if one of the text fields included one of the following keywords: ‘vein, rf, abla, evlt, evla, sclero, screlo, vnus, foam, ugfs’. Admissions were cross-checked against protocol ablations so as not to double count the same event. The exact date of the admission was not recorded in the admissions CRF, only the month after randomisation. It was assumed that if two vein ablation procedures occurred in the same month, then they were duplicate records |
| Outpatient visits |
Outpatient visits were recorded, along with free text indicating the reason for the consultation and any procedure undertaken. Outpatient visits were classified as ‘not vein related’ if the reason for the consultation or the procedure contained one of these keywords: ‘tia, hernia, aaa, asth, aneurysm, ankle, opthal, arthritis, breast, bowel, bereavement, eye, breath, carpal, cpap, cancer, chest, colorectal, diab, diet, head, ent, endoscopy, endocrin, fall, fracture, gynae, gastro, heamat, hearing, heart, hyperdermic, immo, testic, kidney, knee, lung, lymph, facial, nasal, oncol, ortha, ortho, urology, pacemaker, parkinson, pessary, cateract, rheuma, renal, respiratory, reveal, recell, rhemat, spinal, sleep, wrist, thumb, shoulder, abdo, aorta, deaf, memory, migrane, ovary’ (note that ReCell and REVEAL are other concurrent clinical trials66,67) Vein procedures in outpatients were identified if one of the text fields included one of the following keywords: ‘sclero, foam, ugfs’ Outpatient visits were cross-checked against protocol ablations so as not to double count the same event. The exact date of the outpatient consultation was not recorded in the CRF, only the month after randomisation. It was assumed that if two vein ablation procedures occurred in the same month, then they were duplicate records |
| Visits to and from the GP | All these visits were included in the total cost, for any reason |
| Use of antiplatelet and anticoagulant medicines | The CRF recorded the drug used each month, but did not record the dose. It was assumed that doses (taking account age, sex and weight) were as recommended by the British National Formulary68 |
| Physiotherapy and occupational therapy | All these visits, for any reason, were included in the total cost |
| Home care visits (auxiliary nursing) | All these visits, for any reason, were included in the total cost |
| Home help visits for (personal care) | All these visits, for any reason, were included in the total cost |
| Out-of-pocket, informal care and personal expenses | Time lost from work and normal activities, informal care and whether or not out-of-pocket expenses were incurred were recorded in the CRF. These were tabulated but not included in the NHS and Personal Social Services total costs |
Ulcer-related health-care use
The participants in this trial tended to be elderly with comorbidities and, therefore, significant users of health-care resources. To obtain a precise estimate of the effect of the intervention on health-care use, and avoid statistical ‘noise’, the trial aimed to include only resource use related to the ulcer. The trial CRF collected the reason for the use of health-care resources and the procedure undertaken as free text. Keywords indicating ulcer-related activity included ulcer care, skin care, leg care, venous procedures, angiography, infection, rehabilitation, DVT and related keywords (see Table 4). Ulcer-related health care was included in the total cost per patient, whereas non-ulcer-related health care was tabulated but not included in total cost. Non-ulcer-related care was excluded from inpatient admissions, day case admissions and outpatient consultations. These health-care resources and costs, along with out-of-pocket expenses and time lost from usual activities, were tabulated but not included in the total mean cost per patient. It was assumed that all district nurse visits, primary care visits, physiotherapy and occupational therapy were definitely or probably ulcer related.
Unit costs
Costs were estimated by multiplying resource use by unit costs obtained from published literature, England and Wales Healthcare Resource Group costs and manufacturers’ list prices for catheters and other disposable kit (see Appendix 6).
Handling of missing data
A small number of trial data were missing because of withdrawal or for other reasons. The extent and pattern of missing data were assessed. Costs and EQ-5D-5L index were set to zero after the date of death. For the cost-effectiveness analysis, the base case uses ‘complete cases’ in an ITT analysis. A participant was considered to be a complete case if he or she completed all the EQ-5D questions at baseline, 6 weeks, 6 months and 1 year, and did not withdraw from the trial before 1 year.
As a sensitivity analysis, multiple imputation using chained equations was used to impute the remaining missing data by regression, under the assumption of ‘missingness at random’. 60 This means that missing costs are considered predictable from observed data, plus or minus a random error. For each participant lost to follow-up, costs were imputed at each month after the time of withdrawal and the EQ-5D-5L index was imputed at 6 weeks, 6 months and 1 year if these data were missing. Ten imputed data sets were created and analysed using Rubin’s rules (this was sufficient to give stable results allowing for Monte Carlo error). 60
Handling of protocol deviations
In the clinical trial, protocol deviations were seen in 117 patients (59 and 58 in early and deferred groups, respectively), the majority of which were late or missed follow-up appointments (n/N = 40/59 patients in the early-intervention group and n/N = 34/58 in the deferred-intervention group). A sensitivity analysis was carried out excluding these participants.
Cost-effectiveness analysis
The difference in mean total costs and mean total QALYs per participant between the treatment groups was estimated using bivariate normal regression (seemingly unrelated regression using the Stata command ‘surreg’), including baseline EQ-5D-5L in the QALY regression. 69
The incremental cost-effectiveness ratio (ICER) was calculated. The probability that early ablation was more cost-effective than deferred ablation was estimated at different cost-effectiveness thresholds using two methods. The first method assumed bivariate normality in the distribution of total costs and QALYs. The second method used the bootstrapping method, with 1000 Monte Carlo resamples. The bootstrap was used only for the analysis of complete cases. Bootstrap combined with multiple imputation can be very computationally demanding. If 1000 bootstrap resamples were used with 10 multiple imputations, 10,000 data sets would need to be generated and analysed. 70
Sensitivity analyses
Five models were estimated: (1) base case – complete cases with bootstrap standard errors (SEs) and crosswalk EQ-5D tariff; (2) complete case with bivariate normal SEs and crosswalk EQ-5D tariff; (3) multiple imputation with bivariate normal SEs and crosswalk EQ-5D tariff; (4) complete case with bootstrap SEs and EQ-5D-5L tariff estimated by Devlin et al. ;55 and (5) as model 1, excluding participants with protocol deviations.
Database and data processing
InForm database
Data were collected and managed using InForm, an electronic data capture system built around an Oracle database. The InForm system includes automated range checks and validation rules for data entry to help ensure data accuracy. A computer-generated audit trail is in place, which records the date, time, operator, operation and previous value of all manipulation of clinical data.
InForm storage and management was undertaken by the Imperial College London information and communication technologies team. InForm sits on a server behind a firewall connected to the college storage area network. The data are backed up regularly to removable media, allowing for disaster recovery. In addition to the college backup facility, every 20 minutes the activity logs for the trial are moved to another server in a different location to facilitate rapid recovery of data, should it become necessary (e.g. in a disaster recovery scenario).
Data were entered remotely into the database by research nurses at each site. Access to InForm is web based with role- and site-based security applied.
Data queries
During the recruitment and follow-up phases, inconsistent, implausible or missing data were investigated by the trial manager and further validation checks were carried out periodically by the trial statistician.
The trial manager performed quality control checks on the first two CRFs and participant questionnaires entered at each site to ensure the accuracy of data input and that data entry processes had been understood. Ongoing data checks using source data verification were performed at each monitoring visit as per the EVRA monitoring plan. Missing forms and data were flagged by the trial manager periodically and distributed to the appropriate sites on a regular basis.
Data cleaning
The data cleaning process included the following:
-
ensuring that missing/unknown values are labelled accurately
-
further ensuring that spurious values have not been included into data fields
-
check of inconsistencies in data not flagged by inbuilt edit checks
-
review of 100% of comments by the trial manager
-
review of 100% of anonymised duplex reports by the chief investigator assisted by the trial manager, to ensure that they were entered into InForm correctly
-
review of 100% of the data for the primary end point, final ulcer healing date, by an independent trial manager, to ensure that they were entered into InForm correctly.
Final data checks were performed by the statistician once the database had been soft locked before hard lock was complete. All outstanding queries were resolved prior to the database hard lock.
Chapter 3 Clinical results
Screening and recruitment
Recruitment commenced in October 2013 and was completed at the end of September 2016. In total, 6555 patients were screened for potential inclusion in the trial and, of these, 450 (6.9%) were randomised. The reasons for exclusion are presented in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 3).
FIGURE 3.
Consolidated Standards of Reporting Trials diagram of the trial population. The cumulative number of participants who had withdrawn, died, had failed to comply with the protocol or had been lost to follow-up by each time point are presented. Reproduced from The New England Journal of Medicine, Gohel et al. 57 A randomized trial of early endovenous ablation in venous ulceration, vol. 378, pp. 2105–14. Copyright © 2018 Massachusetts Medical Society. Reprinted with permission.

Trial site recruitment
Ten sites were initially activated for recruitment, with a further 11 sites activated as it became apparent that the original sites would be unable to reach their recruitment targets. Over the recruitment period, 21 sites participated in the trial, with one site failing to recruit any participants.
Appendix 7 shows the total number of participants recruited per site in order of the total number of weeks recruiting. The first six sites opened benefited from a dedicated part-time research nurse, whereas the remaining sites were supported by Clinical Research Network or local research nurses working across multiple studies.
Appendix 8 details the overall recruitment per month against the targets. At trial commencement, the monthly target recruitment was 24 participants per month. When it became apparent that this was not achievable (October 2015), the target was reduced to 13 participants per month, adding an additional 8 months to the recruitment period. The target of 450 participants was achieved on 30 September 2016.
Follow-up
Follow-up of the last recruited participant was competed on 28 September 2017. A total of 407 participants attended the 12-month follow-up and the median follow-up period for both the deferred- and early-ablation groups was 365 (IQR 364–370) days. Figure 3 details the trial exit time points. The cumulative numbers of participants who had withdrawn, died, failed to comply with the protocol or been lost to follow-up by each time point are presented.
Ineligible participants
Six ineligible participants were randomised to the trial: two participants in the early-ablation group (one with leg ulceration of > 6 months’ duration and one with no active ulceration) and four participants in the deferred-ablation group (two participants with leg ulceration of > 6 months’ duration, one participant with no active leg ulceration and one participant with deep-venous occlusive disease). These participants were included in the ITT analysis but excluded from the per-protocol analysis (see Figure 3).
Baseline characteristics of participants by trial group
The baseline characteristics, medical history, current medication, ulcer history and baseline compression therapy are summarised in Tables 5–7. The two trial groups were well matched in terms of baseline characteristics, including the following potential prognostic factors: ulcer duration, ulcer size, participant age and history of DVT.
| Characteristic | Early (N = 224) | Deferred (N = 226) | Total (N = 450) |
|---|---|---|---|
| Age (years), mean (SD) | 67.0 (15.5), n = 224 | 68.9 (14.0), n = 226 | 68.0 (14.8), n = 450 |
| Height (cm), mean (SD) | 171.9 (11.1), n = 220 | 170.5 (10.8), n = 220 | 171.2 (11.0), n = 440 |
| Weight (kg), mean (SD) | 89.5 (25.6), n = 218 | 88.8 (24.1), n = 219 | 89.1 (24.9), n = 437 |
| BMI (kg/m2), mean (SD) | 30.1 (7.8), n = 218 | 30.4 (7.4), n = 219 | 30.3 (7.6), n = 437 |
| Sex, n (%) | |||
| Female | 97 (43.3) | 106 (46.9) | 203 (45.1) |
| Male | 127 (56.7) | 120 (53.1) | 247 (54.9) |
| Smoking, n (%) | |||
| Current | 23 (10.3) | 19 (8.4) | 42 (9.3) |
| Former | 86 (38.4) | 101 (44.7) | 187 (41.6) |
| Never | 115 (51.3) | 106 (46.9) | 221 (49.1) |
| Ethnicity, n (%) | |||
| White | 206 (92.0) | 208 (92.0) | 414 (92.0) |
| Mixed | 1 (0.4) | 0 (0.0) | 1 (0.2) |
| Asian | 11 (4.9) | 12 (5.3) | 23 (5.1) |
| Black | 3 (1.3) | 5 (2.2) | 9 (1.8) |
| Other | 3 (1.3) | 1 (0.4) | 4 (0.9) |
| EQ-5D, mean (SD) | |||
| Health state score | 70.2 (17.7), n = 222 | 70.1 (17.1), n = 225 | 70.2 (17.4), n = 447 |
| Index value | 0.7 (0.2), n = 222 | 0.7 (0.2), n = 226 | 0.7 (0.2), n = 448 |
| SF-36, mean (SD) | |||
| Physical function | 37.3 (12.0), n = 223 | 37.5 (12.5), n = 225 | 37.4 (12.2), n = 448 |
| Role-physical | 39.0 (12.2), n = 223 | 39.7 (12.1), n = 224 | 39.4 (12.2), n = 447 |
| Body pain | 41.3 (11.1), n = 223 | 41.6 (11.9), n = 224 | 41.4 (11.5), n = 447 |
| General health | 45.8 (9.2), n = 223 | 46.0 (9.8), n = 225 | 45.8 (9.5), n = 448 |
| Vitality | 48.2 (10.2), n = 222 | 47.8 (10.6), n = 224 | 48.0 (10.4), n = 446 |
| Social functioning | 42.6 (12.4), n = 223 | 42.4 (13.5), n = 224 | 42.5 (13.0), n = 447 |
| Role-emotional | 42.7 (13.8), n = 222 | 43.7 (13.6), n = 224 | 43.2 (13.7), n = 446 |
| Mental health | 49.2 (10.3), n = 222 | 49.3 (10.7), n = 224 | 49.2 (10.5), n = 446 |
| Physical component summary | 38.5 (9.9), n = 222 | 38.8 (10.8), n = 223 | 38.6 (10.4), n = 445 |
| Mental component summary | 49.2 (10.9), n = 222 | 49.4 (11.6), n = 223 | 49.3 (11.2), n = 445 |
| Total AVVQ, mean (SD) | 44.1 (9.0), n = 200 | 44.3 (8.7), n = 192 | 44.2 (8.8), n = 392 |
| Variable | Early (N = 224) | Deferred (N = 226) | Total (N = 450) |
|---|---|---|---|
| Previous pregnancy, n (%)a | |||
| Yes | 85 (87.6) | 91 (85.9) | 172 (86.7) |
| History of DVT in pregnancy (yes) | 1 (1.2) | 2 (2.2) | 3 (1.7) |
| No | 12 (12.4) | 15 (14.) | 27 (13.3) |
| Hormone therapy, n (%)a | |||
| None | 66 (29.5) | 71 (31.4) | 137 (30.4) |
| Previous HRT | 16 (7.1) | 15 (6.6) | 31 (6.9) |
| Current HRT | 1 (0.4) | 3 (1.3) | 4 (0.9) |
| Previous OC | 21 (9.4) | 21 (9.3) | 42 (9.3) |
| Current OC | 2 (0.9) | 1 (0.4) | 3 (0.7) |
| Previous rheumatoid disease, n (%) | |||
| No | 204 (91.1) | 212 (93.8) | 416 (92.4) |
| Yes | 20 (8.9) | 14 (6.2) | 34 (7.6) |
| Previous DVT in either leg, n (%) | |||
| No | 206 (92.0) | 203 (89.8) | 409 (90.9) |
| Yes | 18 (8.0) | 23 (10.2) | 41 (9.1) |
| Previous DVT in trial leg, n (%) | |||
| No | 206 (93.3) | 203 (93.4) | 409 (93.2) |
| Yes | 15 (6.7) | 15 (6.6) | 30 (6.8) |
| Current antiplatelet therapy, n (%) | |||
| None | 172 (76.8) | 179 (79.2) | 351 (78.0) |
| Aspirin | 49 (21.9) | 44 (19.5) | 93 (20.7) |
| Clopidogrel | 5 (2.2) | 5 (2.2) | 10 (2.2) |
| Other | 1 (0.4) | 0 (0) | 1 (0.2) |
| Current anticoagulation therapy, n (%) | |||
| None | 196 (87.5) | 189 (83.6) | 385 (85.6) |
| Warfarin | 25 (11.2) | 32 (14.2) | 57 (12.7) |
| New oral anticoagulants | 2 (0.9) | 4 (1.8) | 6 (1.3) |
| Other | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Current steroids, n (%) | |||
| No | 211 (94.2) | 220 (97.4) | 431 (95.8) |
| Yes | 13 (5.8) | 6 (2.7) | 19 (4.2) |
| Current trental (pentoxifylline), n (%) | |||
| No | 224 (100) | 226 (100) | 450 (100) |
| Yes | 0 (0) | 0 (0) | 0 (0) |
| Diabetes, n (%) | |||
| No | 190 (84.8) | 198 (87.6) | 388 (86.2) |
| Yes | 34 (15.2) | 28 (12.4) | 62 (13.8) |
| Variable | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Previous ulcer (yes), n (%) | ||
| No | 106 (47.3) | 108 (48.0) |
| Yes | 118 (52.7) | 117 (52.0) |
| Ulcer dressing, n (%) | ||
| NA | 64 (28.6) | 55 (24.3) |
| Inadine | 28 (12.5) | 25 (11.1) |
| Other | 131 (58.5) | 146 (64.6) |
| Missing | 1 (0.4) | 0 (0) |
| Baseline compression, n (%) | ||
| Nonea | 3 (1.3) | 7 (3.1) |
| KTwo (Urgo Limited, Loughborough, UK) | 32 (14.3) | 29 (12.8) |
| Three-layer bandage | 42 (18.8) | 41 (18.1) |
| Four-layer bandage | 59 (26.3) | 59 (26.1) |
| European short stretch | 43 (19.2) | 36 (15.9) |
| Stocking, n (%) | 42 (18.8) | 53 (23.5) |
| Other | 2 (0.9) | 1 (0.4) |
| Missing | 1 (0.4) | 0 (0) |
| Time of wearing, n (%) | ||
| Day and night | 196 (87.5) | 185 (81.9) |
| Day only | 25 (11.2) | 39 (17.3) |
| Missing | 3 (1.3) | 2 (0.9) |
Slightly more men than women were randomised (55% vs. 45%). The mean participant BMI was 30.3 kg/m2 (clinically obese).
The baseline ulcer characteristics are summarised in Table 8. Ulcer duration was slightly greater in participants randomised to early ablation [median 3.2 months (IQR 2.3–4.2 months)] than in the deferred-ablation group [median 3.0 months (IQR 1.7–4.2 months)]. The median ulcer size in the early-ablation group was 2.4 cm2 (IQR 1.0–7.1 cm2), compared with 2.9 cm2 (IQR 1.1–8.2 cm2) in the deferred-ablation group.
| Characteristic | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Ulcer duration (months), median (IQR)a | 3.2 (2.3–4.2) | 3.0 (1.7–4.2) |
| Trial ulcer leg, n (%) | ||
| Right | 107 (47.8) | 115 (50.9) |
| Left | 117 (52.2) | 111 (49.1) |
| Ulcer location, n (%) | ||
| Lateral | 92 (41.1) | 93 (41.2) |
| Medial | 116 (51.8) | 118 (52.2) |
| Circumferential | 9 (4.0) | 7 (3.1) |
| Missing | 7 (3.1) | 8 (3.5) |
| Ulcer size (cm2), median (IQR)b | 2.4 (1.0–7.1) | 2.9 (1.1–8.2) |
| Duplex ultrasound scan: deep vein, n (%) | ||
| Normal | 150 (67.0) | 157 (69.5) |
| Abnormalc | 74 (33.0) | 69 (30.5) |
| Reflux | 74 (100) | 69 (100) |
| Outflow obstruction | 0 (0) | 0 (0) |
| CEAP score: clinical signs – grade, n (%) | ||
| C5 | 1 (0.4) | 1 (0.4) |
| C6 | 224 (99.6) | 225 (99.6) |
| CEAP score: clinical signs – presentation, n (%) | ||
| Asymptomatic | 0 (0) | 0 (0) |
| Symptomatic | 224 (100) | 226 (100) |
| Aetiological classification, n (%) | ||
| Primary | 217 (96.9) | 214 (94.7) |
| Secondary | 7 (3.1) | 12 (5.3) |
| Deep | 0 (0) | 0 (0) |
| No venous cause | 0 (0) | 0 (0) |
| Anatomical distribution, n (%) | ||
| Superficial | 220 (98.2) | 221 (97.8) |
| Perforator | 3 (1.3) | 3 (1.3) |
| Deep | 1 (0.4) | 2 (0.9) |
| Pathophysiological dysfunction, n (%) | ||
| Reflux | 224 (100) | 226 (100) |
| Obstruction | 0 (0) | 0 (0) |
| Both | 0 (0) | 1 (0.4) |
| No venous cause | 0 (0) | 0 (0) |
| VCSS, median (IQR) | 15 (14–18) | 16 (14–18) |
| Palpable pedal pulses, n (%) | ||
| No | 15 (6.7) | 14 (6.2) |
| Yes | 209 (93.3) | 212 (93.8) |
The six ineligible participants included two participants who had a healed ulcer at the time of randomisation (which was confirmed after randomisation). The ineligible participant with deep-venous occlusive disease was confirmed to have both deep vein reflux and outflow obstruction by baseline duplex ultrasonography scan. In general, ulcer characteristics were well matched between the two groups.
Table 9 details the patterns of superficial truncal venous reflux at baseline.
| Pattern of superficial reflux at baseline | Early (N = 224), n (%) | Deferred (N = 226), n (%) |
|---|---|---|
| GSV reflux alone | 123 (54.9) | 125 (55.4) |
| SSV reflux alone | 25 (11.2) | 30 (13.3) |
| GSV and SSV reflux | 65 (29.0) | 56 (24.8) |
| Other pattern of refluxa | 11 (4.9) | 15 (6.6) |
Interventions
Ablation method and timing of the first ablation are summarised in Table 10. There were 55 participants who did not undergo ablation in the deferred-intervention group and seven in the early-ablation group, including one in whom the procedure was abandoned before completion. The most common intervention was UGFS alone (47%), followed by endothermal ablation alone (29%).
| Variable | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Interventional ablation type, n (%) | ||
| No ablation | 6 (2.7) | 55 (24.3) |
| Endothermal onlya | 71 (31.7) | 54 (23.9) |
| UGFS onlyb | 111 (49.6) | 100 (44.3) |
| MOCA only | 5 (2.2) | 1 (0.4) |
| Endothermala and UGFSb | 27 (12.1) | 16 (7.1) |
| MOCA and UGFSb | 3 (1.3) | 0 (0) |
| Abandoned ablation | 1 (0.5) | 0 (0) |
| Timing of first ablation procedure,c n (%) | ||
| No ablation | 6 (2.7) | 55 (24.3)d |
| Within 2 weeks | 203 (90.6) | 1 (0.4) |
| Before ulcer healing | 200 | 1e |
| After ulcer healing | 3 | 0 |
| Between 2 and 4 weeks | 9 (4.0) | 1 (0.4) |
| Before ulcer healing | 9 | 1e |
| After ulcer healing | 0 | 0 |
| Between 4 weeks and 6 months | 6 (2.7) | 103 (45.6) |
| Before ulcer healing | 4 | 4e |
| After ulcer healing | 2 | 99 |
| After 6 months | 0 (0) | 66 (29.2) |
| Before ulcer healing | 0 | 19 |
| After ulcer healing | 0 | 47 |
Among the 55 participants in the deferred-ablation group who did not undergo endovenous ablation up to 1 year, 19 participants died, withdrew or were lost to follow-up from the trial and 36 participants completed the trial, including 27 participants with healed ulcer and nine participants with unhealed ulcer at 1 year (Table 11).
| Completion of the trial | Deferred (N = 55) | Early (N = 6) |
|---|---|---|
| Yes, n (%) | 36 (65.5) | 2 (33.3) |
| Ulcer healed by 12 months | 27 | 2 |
| Ulcer not healed by 12 months | 9 | 0 |
| No, n (%) | 19 (34.5) | 4 (66.7) |
| Withdrawal | 7 | 3 |
| Death | 7 | 1 |
| Other | 5 | 0 |
Regarding the timing of ablation, the majority of participants (90.6%) in the early-ablation group underwent ablation within 2 weeks of randomisation. In the deferred-ablation group, one participant was treated before 2 weeks and five participants were treated prior to ulcer healing between 2 weeks and 6 months. The reasons for the ablation before ulcer healing in the six participants in the deferred-ablation group were clinical deterioration of ulcer (n = 3), participant request for intervention (participant unwilling to continue with deferred ablation strategy) (n = 2) and participant treated early in error (n = 1).
Primary outcome: ulcer healing
Figure 4 shows the KM curve for time to ulcer healing. Among the 450 participants were two ineligible participants whose ulcer had healed by the time of randomisation and who did not contribute to the survival analysis. The median healing time was 56 (95% CI 49 to 66) days and 82 (95% CI 69 to 92) days in the early- and deferred-ablation groups, respectively.
FIGURE 4.
Kaplan–Meier curve showing ulcer healing time in the early- and deferred-ablation groups (p = 0.001, log-rank test). Ulcer healing rates were greater in participants randomised to early ablation. Reproduced from The New England Journal of Medicine, Gohel et al. 57 A randomized trial of early endovenous ablation in venous ulceration, vol. 378, pp. 2105–14. Copyright © 2018 Massachusetts Medical Society. Reprinted with permission.
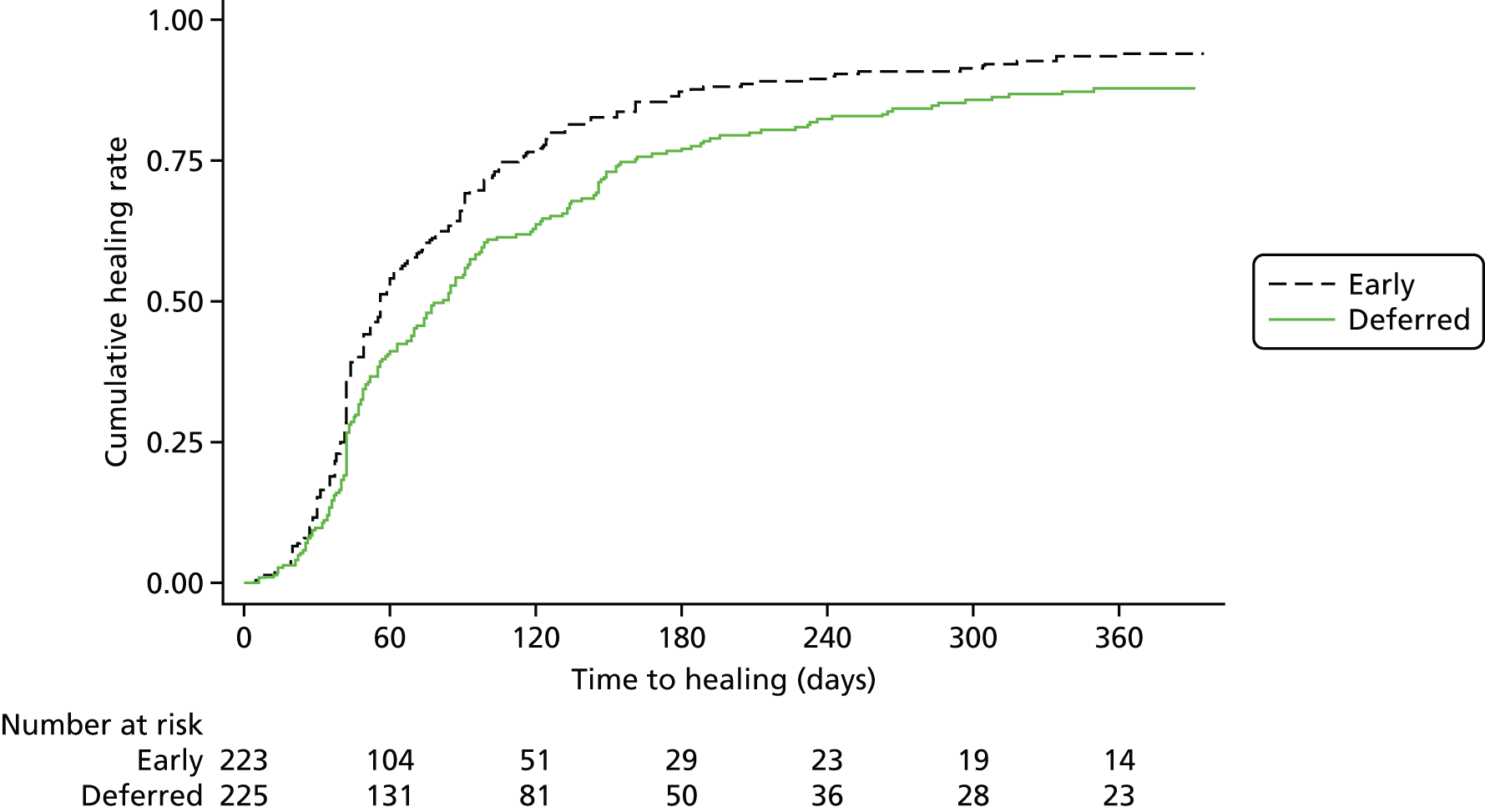
The proportional hazards assumption, assessed graphically, by plotting –ln{–ln[Ŝ(t)]} versus –ln(t), and numerically, using Grambsch and Therneau tests, was not violated.
Table 12 shows the Cox proportional hazards regression results. In the univariate model, with trial centre as a random effect, the hazard ratio (HR) for ulcer healing in the early-ablation group is 1.38 (95% CI 1.13 to 1.68) (p = 0.001) compared with participants randomised to deferred ablation. After further adjusting for age, ulcer duration and ulcer size at baseline, the HR is 1.42 (95% CI 1.16 to 1.73) (p = 0.001).
| Variable | N a | n a | Univariable modelb | Multivariable modelc | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Treatment | ||||||
| Deferred group | 226 | 194 | Reference | Reference | ||
| Early group | 224 | 210 | 1.38 (1.13 to 1.68) | 0.001 | 1.42 (1.16 to 1.73) | 0.001 |
| Age (years) | 448 | 402 | 1.00 (0.99 to 1.00) | 0.25 | 1.00 (0.99 to 1.01) | 0.69 |
| Ulcer duration (months) | ||||||
| First quartile (0.9–2.2) | 113 | 102 | Reference | Reference | ||
| Second quartile (2.3–3.1) | 114 | 101 | 1.01 (0.77 to 1.33) | 0.96 | 1.00 (0.76 to 1.33) | 0.97 |
| Third quartile (3.1–4.2) | 111 | 105 | 1.11 (0.85 to 1.47) | 0.44 | 1.14 (0.86 to 1.51) | 0.35 |
| Fourth quartile (4.2–8.4) | 112 | 96 | 0.75 (0.56 to 0.99) | 0.04 | 0.79 (0.59 to 1.05) | 0.10 |
| Ulcer size (cm2) | ||||||
| First quartile (0.4–1.5) | 113 | 108 | Reference | Reference | ||
| Second quartile (1.6–2.9) | 112 | 108 | 0.79 (0.61 to 1.04) | 0.09 | 0.72 (0.55 to 0.95) | 0.02 |
| Third quartile (3–7.5) | 113 | 101 | 0.52 (0.40 to 0.69) | < 0.001 | 0.51 (0.38 to 0.67) | < 0.001 |
| Fourth quartile (8–235) | 112 | 87 | 0.31 (0.23 to 0.41) | < 0.001 | 0.29 (0.22 to 0.39) | < 0.001 |
The HRs from the multivariable Cox regression model for specific (pre-planned) subgroups are presented in Figure 5. There is considerable consistency except for ulcer duration, where an interesting trend is observed. In the prespecified subgroup analysis to investigate any differential treatment effects of ulcer duration, the HR in the early ablation group increases across the quartiles of ulcer duration. In the first and second quartiles of ulcer duration, early ablation does not make a difference to ulcer healing relative to deferred ablation. However, this study is not powered to investigate any interactions and thus the above finding will need further studies to confirm.
FIGURE 5.
Forest plot of subgroup analysis for primary outcome. The healing advantage in prespecified subgroups was consistent with the overall healing benefit observed with early ablation. The broken line indicates overall HR for ulcer healing in entire study population. Reproduced from The New England Journal of Medicine, Gohel et al. 57 A randomized trial of early endovenous ablation in venous ulceration, vol. 378, pp. 2105–14. Copyright © 2018 Massachusetts Medical Society. Reprinted with permission.

Figure 6 shows the HRs for different treatments in the early ablation group compared with deferred ablation. As numbers in the MOCA only group, endothermal and UGFS group, and MOCA and UGFS group are small, the three ablation groups are merged into one group as ‘other ablation’. The HRs for the groups of endothermal only, UGFS only and other treatment are consistent.
FIGURE 6.
Forest plot of different endovenous ablation techniques for primary outcome. The healing advantage in prespecified subgroups treated with different ablation techniques was consistent with the overall healing benefit. The broken line indicates overall HR for ulcer healing in entire study population. Reproduced from The New England Journal of Medicine, Gohel et al. 57 A randomized trial of early endovenous ablation in venous ulceration, vol. 378, pp. 2105–14. Copyright © 2018 Massachusetts Medical Society. Reprinted with permission.
Secondary outcomes
Ulcer-free time to 1 year
Of the 450 participants, 407 attended the 12-month follow-up visit and were included in the analysis of ulcer-free time to 1 year. There were 203 and 204 participants in the deferred- and early-ablation groups, respectively. The median ulcer-free time to 1 year was 278 (IQR 175–324) days and 306 (IQR 240–328) days in the deferred- and early-ablation groups, respectively.
As the ulcer-free time to 1 year did not follow a normal distribution and mathematical transformation was not possible because of a few participants with zero days ulcer-free time, ulcer-free time to 1 year was categorised (into quartiles) and ordinal regression was used to assess the difference between the treatment groups. The proportionality assumption was not violated (assessed using the Brant test). The results are presented in Table 13. In the univariable analysis, with trial centre as a random effect, the odds ratio (OR) for being in a higher quartile was 1.60 (95% CI 1.13 to 2.27) for the early-ablation group. Further adjustment for age, ulcer duration and size at baseline did not affect the result. The OR in the multivariable model is 1.54 (95% CI 1.07 to 2.21; p = 0.02).
| Variable | Univariable modela | Multivariable modelb | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Treatment group | ||||
| Deferred | Reference | Reference | ||
| Early | 1.60 (1.13 to 2.27) | 0.009 | 1.54 (1.07 to 2.21) | 0.02 |
| Age (years) | 0.99 (0.98 to 1.00) | 0.14 | 1.00 (0.98 to 1.01) | 0.57 |
| Ulcer duration (months) | ||||
| First quartile (0.9–2.2) | Reference | Reference | ||
| Second quartile (2.3–3.1) | 0.87 (0.53 to 1.44) | 0.59 | 0.94 (0.56 to 1.56) | 0.80 |
| Third quartile (3.1–4.2) | 0.94 (0.57 to 1.55) | 0.82 | 0.96 (0.58 to 1.60) | 0.89 |
| Fourth quartile (4.2–8.4) | 0.55 (0.33 to 0.92) | 0.02 | 0.64 (0.38 to 1.08) | 0.10 |
| Ulcer size (cm2) | ||||
| First quartile (0.4–1.5) | Reference | Reference | ||
| Second quartile (1.6–2.9) | 0.50 (0.30 to 0.82) | 0.006 | 0.48 (0.29 to 0.79) | 0.004 |
| Third quartile (3–7.5) | 0.23 (0.14 to 0.39) | < 0.001 | 0.23 (0.14 to 0.39) | < 0.001 |
| Fourth quartile (8–235) | 0.09 (0.05 to 0.16) | < 0.001 | 0.10 (0.06 to 0.17) | < 0.001 |
Figure 7 shows the results of ordinal logistic regression in different subgroups. The results are consistent across different subgroups. The pattern observed is similar to that seen in the subgroup analysis by ulcer size and duration.
FIGURE 7.
Forest plot showing the treatment effect on ulcer-free time by predefined subgroups.

Figure 8 shows the ORs for the treatment effect on ulcer-free time by type of endovenous ablation. The ORs are consistent in the groups of endothermal only and UGFS only, whereas the OR in the other treatment group is 1.06 (95% CI 0.56 to 2.02). The lack of treatment effect here may be due to the small number in the other treatment group.
FIGURE 8.
Forest plot showing the treatment effect on ulcer-free time by different ablation techniques.
Ulcer healing at 12 and 24 weeks
The unadjusted KM time-to-event ulcer healing analysis can be seen in Table 14. The healing rates at 24 weeks were higher in the early-ablation group (85.6%, 95% CI 80.6% to 89.8%) than in the deferred-ablation group (76.3%, 95% CI 70.5% to 81.7%).
| Variable | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Ulcer healing ratea (95% CI) (%) | ||
| 12 weeks | 63.5 (57.2 to 69.8) | 51.6 (45.2 to 58.3) |
| 24 weeks | 85.6 (80.6 to 89.8) | 76.3 (70.5 to 81.7) |
| Number of participants with a healed ulcer at 12 months, n (%) | 210 (93.8) | 194 (85.8) |
| Number of participants with recurrent ulcer, n (%)b | 24 (11.4) | 32 (16.5) |
| Ulcer-free time (days), median (IQR) | 306 (240–328), n = 204 | 278 (175–324), n = 203 |
In a post hoc analysis to allow comparison with published studies, the 12-week ulcer healing rate was 63.5% (95% CI 57.2% to 69.8%) in the early-ablation group and 51.6% (95% CI 45.2% to 58.3%) in the deferred group. A total of 404 (89.8%) of 450 randomised participants had healed by 1 year post randomisation [210/224 (93.8%) in the early-ablation group and 194/226 (85.8%) in the deferred-ablation group]. The absolute difference in healing rates between the groups was 7.9% (95% CI 2.3% to 13.5%).
Quality of life
Table 15 and Figures 9 and 10 summarise the HRQoL data at baseline, 6 weeks and 6 and 12 months for the two trial groups. The AVVQ has scores ranging from 0 to 100, with 0 representing the best score and 100 the worst score, whereas, for EQ-5D and SF-36, the higher the score, the better the HRQoL. A decreasing trend of AVVQ score across time is observed for both the deferred- and early-ablation groups.
| Variable | Baseline | 6 weeks | 6 months | 12 months | p-valuea |
|---|---|---|---|---|---|
| Early, n | 226 | 21 | 204 | 199 | |
| Deferred, n | 224 | 219 | 208 | 203 | |
| AVVQ score, mean (SD) | |||||
| Deferred | 44.3 (8.7), n = 192 | 41.2 (9.3), n = 170 | 39.5 (10.3), n = 140 | 34.3 (10.4), n = 130 | |
| Early | 44.1 (9.0), n = 200 | 39.4 (10.2), n = 176 | 34.6 (9.4) n = 139 | 32.4 (8.3), n = 127 | |
| Difference (95% CI)b | –0.2 (–2.0 to, 1.6) | –2.1 (–4.0 to –0.2) | –4.8 (–6.9 to –2.7) | –1.8 (–4.0 to 0.3) | 0.0008 |
| EQ-5D health score, mean (SD) | |||||
| Deferred | 70.1 (17.1), n = 225 | 71.1 (18.7), n = 205 | 71.4 (19.6), n = 193 | 73.7 (17.4), n = 184 | |
| Early | 70.2 (17.7), n = 222 | 72.7 (18.6), n = 212 | 74.1 (15.8), n = 185 | 74.8 (16.9), n = 183 | |
| Difference (95% CI)b | 0.1 (–3.1 to 3.4) | 1.7 (–1.6 to 5.1) | 1.8 (–1.6 to 5.2) | 1.3 (–2.1 to 4.8) | 0.72 |
| EQ-5D index value, mean (SD)c | |||||
| Deferred | 0.73 (0.2), n = 226 | 0.75 (0.2), n = 208 | 0.76 (0.2), n = 192 | 0.80 (0.2), n = 182 | |
| Early | 0.73 (0.2), n = 222 | 0.79 (0.2), n = 211 | 0.81 (0.2), n = 186 | 0.83 (0.2), n = 184 | |
| Difference (95% CI)b | –0.01 (–0.04 to 0.03) | 0.04 (0.00 to 0.08) | 0.04 (0.00 to 0.08) | 0.03 (–0.01 to 0.07) | 0.03 |
| SF-36 physical function score, mean (SD) | |||||
| Deferred | 37.5 (12.5), n = 225 | 37.4 (13.0), n = 207 | 37.4 (13.7), n = 193 | 38.7 (13.4), n = 180 | |
| Early | 37.3 (12.0), n = 223 | 39.1 (12.7), n = 212 | 39.1 (12.8), n = 187 | 39.4 (12.9), n = 182 | |
| Difference (95% CI)b | –1.0 (–3.1 to 1.1) | 1.0 (–1.2 to 3.1) | 0.7 (–1.5 to 2.8) | 0.3 (–1.9 to 2.6) | 0.09 |
| SF-36 role-physical score, mean (SD) | |||||
| Deferred | 39.7 (12.1), n = 224 | 41.4 (12.7), n = 207 | 42.4 (12.7), n = 192 | 44.3 (12.9), n = 180 | |
| Early | 39.0 (12.2), n = 223 | 40.3 (12.5), n = 211 | 43.6 (12.6), n = 187 | 43.0 (12.7), n = 181 | |
| Difference (95% CI)b | –1.3 (–3.5 to 0.9) | –1.7 (–4.0 to 0.6) | 0.4 (–2.0 to 2.7) | –1.7 (–4.1 to 0.7) | 0.28 |
| SF-36 body pain score, mean (SD) | |||||
| Deferred | 41.6 (11.9), n = 224 | 44.3 (12.3), n = 207 | 45.9 (12.2), n = 193 | 47.8 (11.2), n = 180 | |
| Early | 41.3 (11.1), n = 223 | 46.6 (10.6), n = 212 | 48.2 (11.0), n = 187 | 49.3 (11.0), n = 182 | |
| Difference (95% CI)b | –0.5 (–2.6 to 1.6) | 2.2 (0.1 to 4.4) | 2.1 (–0.2 to 4.3) | 1.1 (–1.1 to 3.3) | 0.05 |
| SF-36 general health score, mean (SD) | |||||
| Deferred | 46.0 (9.8), n = 225 | 45.6 (9.2), n = 207 | 44.5 (10.1), n = 193 | 45.1 (10), n = 181 | |
| Early | 45.8 (9.2), n = 223 | 45.7 (9.1), n = 212 | 44.9 (9.8), n = 187 | 45.3 (10), n = 183 | |
| Difference (95% CI)b | –0.3 (–2.0 to 1.5) | 0.0 (–1.8 to 1.8) | 0.0 (–1.9 to 1.8) | 0.4 (–1.5 to 2.3) | 0.86 |
| SF-36 vitality score, mean (SD) | |||||
| Deferred | 47.8 (10.6), n = 224 | 47.5 (11.3), n = 207 | 48.8 (10.8), n = 193 | 49.6 (9.8), n = 179 | |
| Early | 48.2 (10.2), n = 222 | 49.1 (10.0), n = 212 | 49.4 (9.5), n = 187 | 50.5 (9.4), n = 182 | |
| Difference (95% CI)b | 0.1 (–1.7 to 2.0) | 1.4 (–0.5 to 3.3) | 0.0 (–1.9 to 2.0) | 0.9 (–1.0 to 2.9) | 0.31 |
| SF-36 social functioning score, mean (SD) | |||||
| Deferred | 42.4 (13.5), n = 224 | 44.0 (12.1), n = 207 | 44.7 (12.5), n = 193 | 47.3 (11.4), n = 181 | |
| Early | 42.6 (12.4), n = 223 | 44.9 (11.6), n = 212 | 47.0 (10.5), n = 186 | 47.4 (10.7), n = 182 | |
| Difference (95% CI)b | –0.1 (–2.3 to 2.0) | 0.6 (–1.6 to 2.8) | 1.5 (–0.8 to 3.7) | –0.4 (–2.7 to 2.0) | 0.40 |
| SF-36 role-emotional score, mean (SD) | |||||
| Deferred | 43.7 (13.6), n = 224 | 45.9 (13.3), n = 207 | 45.1 (13.2), n = 193 | 47.5 (12.2), n = 179 | |
| Early | 42.7 (13.8), n = 222 | 46.1 (12.8), n = 212 | 47.2 (12.2), n = 187 | 45.9 (13.0), n = 182 | |
| Difference (95% CI)b | –1.4 (–3.8 to 1.0) | 0.0 (–2.5 to 2.5) | 1.7 (–0.9 to 4.2) | –1.7 (–4.3 to 0.9) | 0.08 |
| SF-36 mental health score, mean (SD) | |||||
| Deferred | 49.3 (10.7), n = 224 | 49.2 (10.8), n = 207 | 49.5 (10.4), n = 193 | 50.7 (10.1), n = 179 | |
| Early | 49.2 (10.3), n = 222 | 50.6 (10.4), n = 212 | 51.7 (9.7), n = 187 | 51.0 (9.3), n = 182 | |
| Difference (95% CI)b | –0.2 (–2.1 to 1.7) | 1.3 (–0.7 to 3.2) | 1.7 (–0.3 to 3.7) | –0.2 (–2.2 to 1.8) | 0.07 |
| SF-36 physical component summary score, mean (SD) | |||||
| Deferred | 38.8 (10.8), n = 223 | 39.6 (11.6), n = 207 | 40.4 (12.1), n = 193 | 41.8 (12.0), n = 178 | |
| Early | 38.5 (9.9), n = 222 | 40.4 (10.2), n = 212 | 41.5 (11.5), n = 187 | 42.1 (11.6), n = 181 | |
| Difference (95% CI)b | –0.8 (–2.8 to 1.1) | 0.3 (–1.7 to 2.2) | 0.3 (–1.7 to 2.3) | 0.3 (–1.7 to 2.3) | 0.41 |
| SF-36 mental component summary score, mean (SD) | |||||
| Deferred | 49.4 (11.6), n = 223 | 50.2 (11.0), n = 207 | 50.2 (10.4), n = 193 | 52.0 (10.0), n = 178 | |
| Early | 49.2 (10.9), n = 222 | 51.1 (10.4), n = 212 | 52.2 (9.8), n = 187 | 51.6 (9.5), n = 181 | |
| Difference (95% CI)b | –0.3 (–2.2 to 1.7) | 0.9 (–1.1 to 2.9) | 1.5 (–0.5 to 3.6) | –0.7 (–2.7 to 1.4) | 0.09 |
FIGURE 9.
The changes in AVVQ score over time in the early- and deferred-ablation groups.
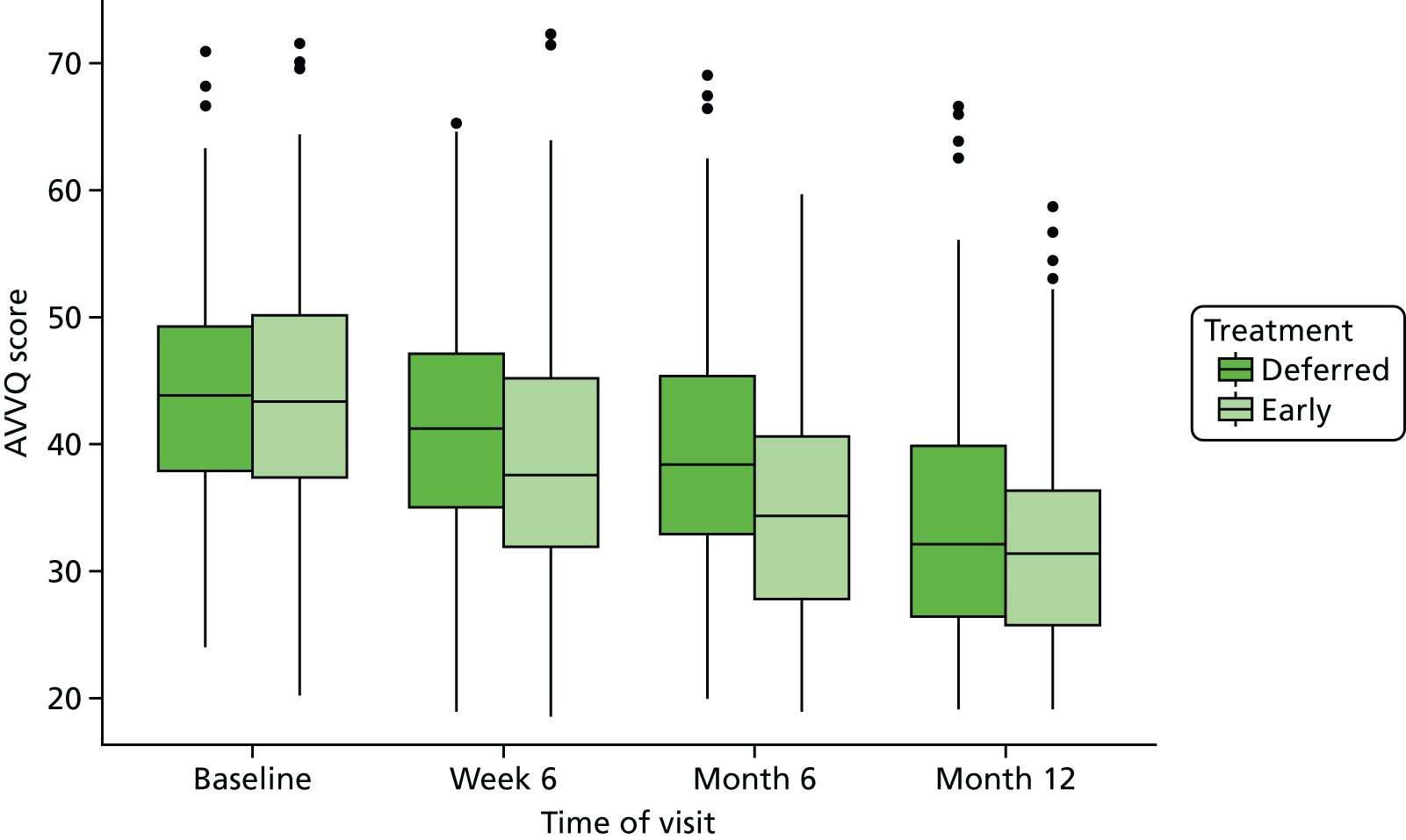
FIGURE 10.
The changes in EQ-5D: (a) health score and (b) index value over time in the early- and deferred-ablation groups.

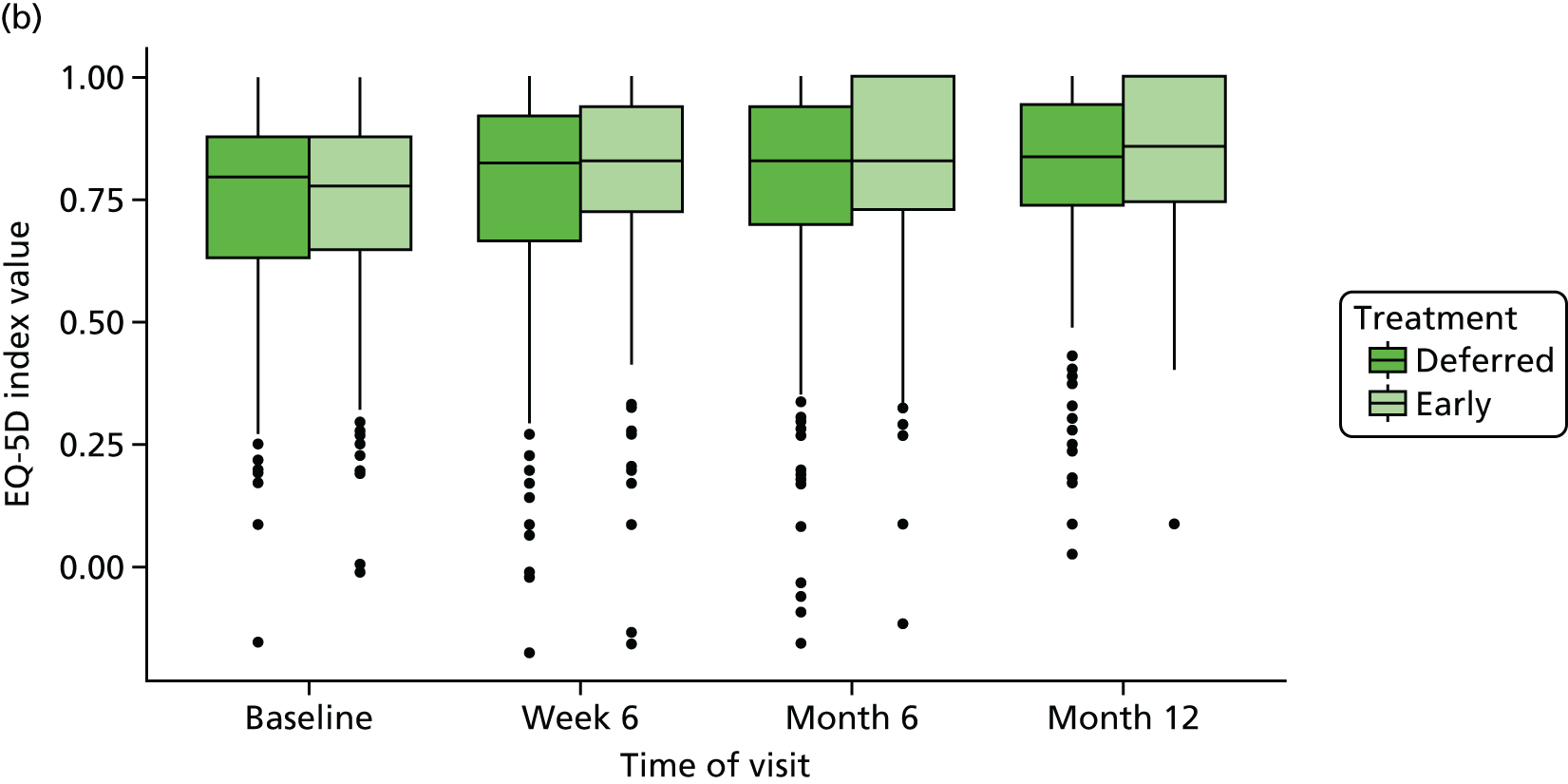
At baseline, AVVQ, EQ-5D-5L and SF-36 scores were similar in the early- and deferred-ablation groups. Overall, there was a significant difference in mean AVVQ scores between the treatment groups over time (p < 0.001), with lower mean scores, suggesting better disease-specific HRQoL, in the early-ablation group. There was a significant difference over time in mean EQ-5D index value between the treatment groups (p = 0.03), with more favourable scores in those randomised to early ablation, and in mean SF-36 body pain (p = 0.05). Observed differences between the groups for the other generic HRQoL measures were not statistically significant. However, as there was no control for multiple testing, these results should be interpreted with caution.
Table 16 summarises the HRQoL data with multiple imputation of missing values, which produces similar values.
| Variable | Baseline | 6 weeks | 6 months | 12 months |
|---|---|---|---|---|
| AVVQ score, mean (SD) | ||||
| Early ablation | 44.0 (9.0) | 39.1 (10.2) | 34.9 (10.1) | 33.0 (9.7) |
| Deferred ablation | 44.2 (8.9) | 41.2 (9.7) | 39.4 (10.3) | 34.8 (10.8) |
| Difference (95% CI)a | –0.2 (–2.1 to 1.7) | –2.2 (–4.7 to 0.3) | –4.5 (–6.5 to –2.5) | –1.8 (–4.1 to 0.5) |
| EQ-5D health score, mean (SD) | ||||
| Early ablation | 70.2 (17.7) | 72.6 (18.7) | 73.6 (16.3) | 74.8 (17.5) |
| Deferred ablation | 70.0 (17.1) | 70.7 (19.1) | 71.5 (19.4) | 73.0 (17.8) |
| Difference (95% CI)a | 0 (–3.3 to 3.3) | 1.8 (–1.8 to 5.4) | 1.8 (–2.0 to 5.7) | 1.8 (–1.6 to 5.1) |
| EQ-5D index value, mean (SD)b | ||||
| Early ablation | 0.73 (0.2) | 0.79 (0.2) | 0.81 (0.2) | 0.83 (0.2) |
| Deferred ablation | 0.73 (0.2) | 0.74 (0.2) | 0.77 (0.2) | 0.80 (0.2) |
| Difference (95% CI)a | –0.01 (–0.05 to 0.03) | 0.04 (0 to 0.09) | 0.04 (0 to 0.08) | 0.03 (–0.01 to 0.07) |
| SF-36 physical function score, mean (SD) | ||||
| Early ablation | 37.4 (12.0) | 39.1 (12.9) | 39.4 (12.9) | 39.7 (13.3) |
| Deferred ablation | 37.5 (12.5) | 37.4 (13.0) | 37.9 (13.6) | 38.3 (13.7) |
| Difference (95% CI)a | –1.0 (–3.2 to 1.1) | 0.8 (–1.4 to 3.1) | 0.6 (–1.7 to 3.0) | 0.7 (–1.6 to 3.0) |
| SF-36 role-physical score, mean (SD) | ||||
| Early ablation | 39.1 (12.2) | 40.3 (12.6) | 43.6 (12.6) | 43.3 (12.9) |
| Deferred ablation | 39.7 (12.1) | 41.5 (12.6) | 42.6 (12.8) | 43.8 (13.1) |
| Difference (95% CI)a | –1.2 (–3.5 to 1.0) | –1.9 (–4.1 to 0.4) | 0.4 (–2.6 to 3.3) | –0.9 (–3.4 to 1.5) |
| SF-36 body pain score, mean (SD) | ||||
| Early ablation | 41.3 (11.1) | 46.6 (10.6) | 48.3 (11) | 49.4 (11.1) |
| Deferred ablation | 41.6 (11.9) | 44.0 (12.2) | 46.1 (12) | 47.5 (11.5) |
| Difference (95% CI)a | –0.5 (–2.6 to 1.6) | 2.4 (0 to 4.7) | 2.1 (–0.2 to 4.4) | 1.9 (–0.3 to 4.0) |
| SF-36 general health score, mean (SD) | ||||
| Early ablation | 45.8 (9.2) | 45.5 (9.1) | 44.8 (9.8) | 45.1 (10.0) |
| Deferred ablation | 46.0 (9.8) | 45.5 (9.3) | 44.7 (10.2) | 44.6 (10.2) |
| Difference (95% CI)a | –0.3 (–2.1 to 1.5) | –0.1 (–1.9 to 1.8) | –0.1 (–2.1 to 2.0) | 0.4 (–1.4 to 2.2) |
| SF-36 vitality score, mean (SD) | ||||
| Early ablation | 48.2 (10.2) | 49.0 (10.2) | 49.1 (9.6) | 50.2 (9.7) |
| Deferred ablation | 47.9 (10.5) | 47.4 (11.2) | 48.7 (10.7) | 49.0 (10.0) |
| Difference (95% CI)a | 0.1 (–1.7 to 2.0) | 1.3 (–0.6 to 3.2) | 0.2 (–2.0 to 2.4) | 1.0 (–0.9 to 3.0) |
| SF-36 social functioning score, mean (SD) | ||||
| Early ablation | 42.6 (12.4) | 44.8 (11.6) | 46.9 (10.7) | 47.1 (11.0) |
| Deferred ablation | 42.4 (13.5) | 43.8 (12.1) | 44.9 (12.4) | 46.7 (11.7) |
| Difference (95% CI)a | –0.1 (–2.2 to 2.1) | 0.6 (–1.7 to 2.9) | 1.6 (–0.9 to 4.1) | 0.1 (–2.1 to 2.4) |
| SF-36 role-emotional score, mean (SD) | ||||
| Early ablation | 42.7 (13.7) | 46.1 (12.8) | 47.0 (12.5) | 45.6 (13.4) |
| Deferred ablation | 43.7 (13.6) | 45.8 (13.3) | 45.1 (13.1) | 47.1 (12.7) |
| Difference (95% CI)a | –1.4 (–3.8 to 1.0) | 0 (–2.7 to 2.7) | 1.4 (–1.3 to 4.1) | –1.9 (–4.5 to 0.8) |
| SF-36 mental health score, mean (SD) | ||||
| Early ablation | 49.2 (10.3) | 50.4 (10.5) | 51.2 (10.1) | 50.5 (10.2) |
| Deferred ablation | 49.3 (10.7) | 49.0 (10.8) | 49.4 (10.5) | 50.2 (10.6) |
| Difference (95% CI)a | –0.2 (–2.1 to 1.8) | 1.4 (–0.7 to 3.4) | 1.6 (–0.7 to 4.0) | 0.1 (–1.9 to 2.2) |
| SF-36 physical component summary score, mean (SD) | ||||
| Early ablation | 38.5 (10.0) | 40.4 (10.4) | 41.8 (11.4) | 42.6 (11.8) |
| Deferred ablation | 38.8 (10.7) | 39.6 (11.5) | 40.8 (12.1) | 41.2 (12.2) |
| Difference (95% CI)a | –0.8 (–2.7 to 1.2) | 0.2 (–1.8 to 2.2) | 0.4 (–1.9 to 2.7) | 1.0 (–1.1 to 3.1) |
| SF-36 mental component summary score, mean (SD) | ||||
| Early ablation | 49.2 (10.8) | 51 (10.4) | 51.7 (10.2) | 50.9 (10.2) |
| Deferred ablation | 49.4 (11.5) | 50 (11.1) | 50.1 (10.4) | 51.5 (10.4) |
| Difference (95% CI)a | –0.2 (–2.2 to 1.7) | 0.9 (–1.2 to 3.1) | 1.5 (–0.8 to 3.8) | –0.7 (–2.8 to 1.4) |
Clinical and technical success
Table 17 and Figure 11 show the clinical success at 6 weeks. The number of participants with improvement of clinical grade is 72 (31.9%) and 106 (47.3%) in deferred and early ablation groups, respectively.
| Variable | Treatment group | p-value | |
|---|---|---|---|
| Early (N = 224) | Deferred (N = 226) | ||
| VCSS total score, mean (SD) | |||
| Baseline | 15.8 (3.3), n = 223 | 15.7 (3.1), n = 226 | |
| Week 6 | 10.5 (4.7), n = 218 | 12.6 (4.4), n = 210 | < 0.001a |
| Clinical classification downgrade (C6 to C5), n (%) | |||
| Yes | 106 (47.3) | 72 (31.9) | 0.001b |
| No | 112 (50.0) | 139 (61.5) | |
| Missing | 6 (2.7) | 15 (6.6) | |
FIGURE 11.
Summary of VCSS score in early- and deferred-ablation groups: VCSS score at baseline and 6 weeks after randomisation.
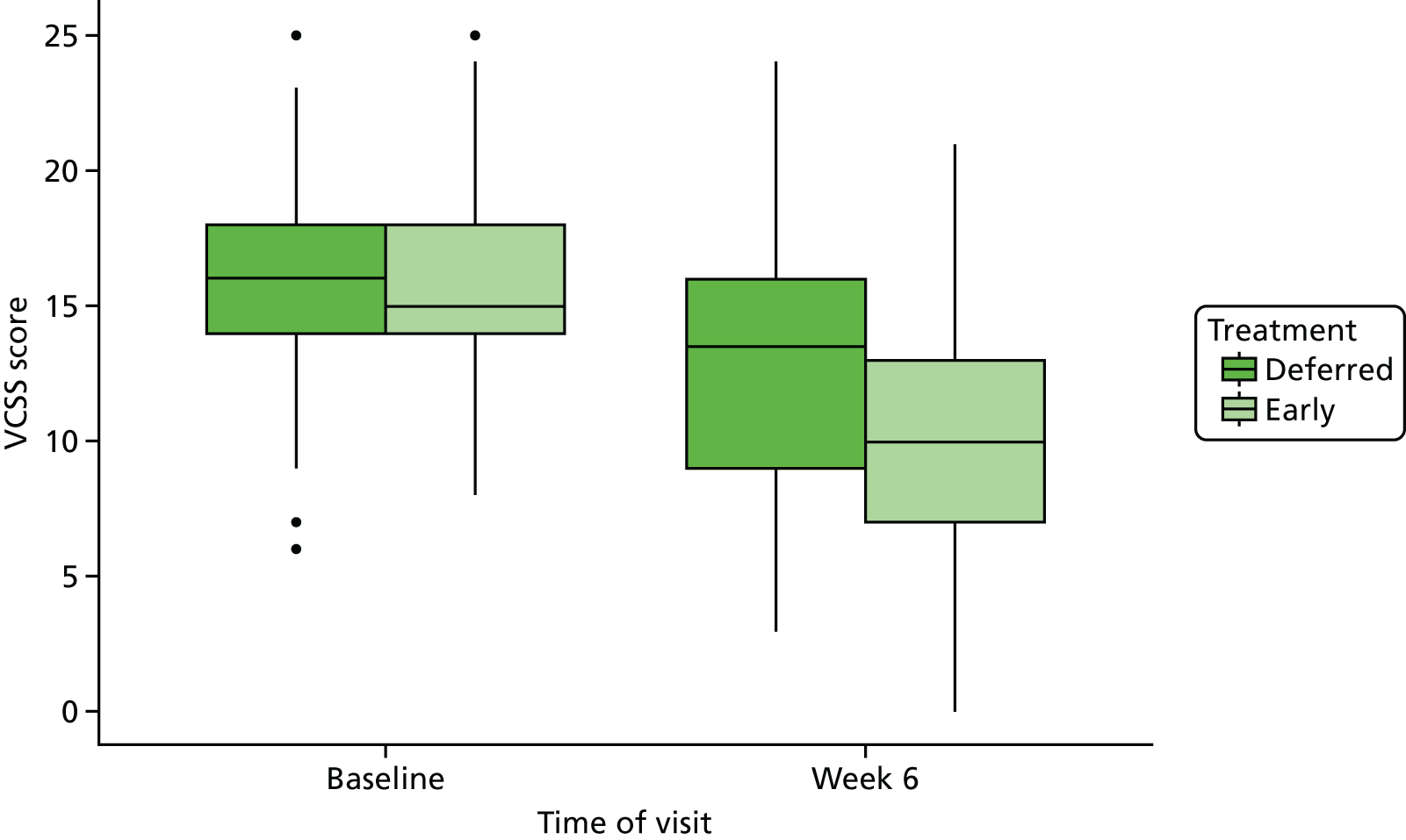
The VCSS evaluates changes in condition over time, with lower scores indicating better condition. Figure 11 clearly shows that early ablation was associated with a lower VCSS at week 6 than deferred ablation, whereas the VCSS at baseline was similar in both groups.
On assessment of post-ablation duplex ultrasound scans at 6 weeks, treated segments were completely ablated in 179 (83.3%) of 215 scanned participants and 74.8% of legs had no evidence of residual reflux.
Safety data
Table 18 summarises the ablation procedures received by trial participants in the early and deferred groups during 1 year of follow-up (AEs are shown). In the early-ablation group, 218 participants underwent at least one ablation treatment (97.3%), whereas in the deferred group 171 participants did so (75.7%).
| Variable | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Total number of procedures | 269 | 203 |
| Total number (%) of participants having a procedure | 218 (97.3) | 171 (75.7) |
| Number (%) of surgical procedures | ||
| One | 173 (79.4) | 147 (86.0) |
| Two | 39 (17.9) | 17 (9.9) |
| Three | 6 (2.8) | 6 (3.5) |
| Four | 0 (0) | 1 (0.6) |
| Total number of AEs | 117 | 130 |
| Total number (%) of participants with an AE | 67 (29.9) | 83 (36.7) |
| Description of AE, n (%) | ||
| Systemic | 7 (6.0) | 6 (4.6) |
| Local | 110 (94.2) | 124 (95.4) |
| Outcome, n (%) | ||
| Recovered | 111 (94.9) | 111 (85.4) |
| Not yet recovered | 6 (5.1) | 19 (14.6) |
| Death | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) |
| Missing | 0 (0) | 0 (0) |
Table 19 summarises the procedural complications after endovenous ablation. The most common complications were DVT and pain post ablation. The vast majority of DVTs were in crural veins and were asymptomatic.
| Complication | Early (N = 28) | Deferred (N = 24) |
|---|---|---|
| Allergic reaction requiring local or no treatment | 5 | 3 |
| Bleeding requiring intervention | 2 | 1 |
| Cough/chest tightness | 0 | 1 |
| DVT | 9a | 3b |
| Infectionc | 3 | 5 |
| Oedema | 1 | 0 |
| Pain | 6d | 6 |
| Participant-reported paraesthesia | 1 | 1 |
| Superficial thrombophlebitis | 1 | 4 |
Table 20 shows the summary of SAEs. The number of SAEs possibly, probably or definitely related to the ablation procedures was three in the deferred-ablation group and four in the early-ablation group, and all were expected. SAEs assessed as being related are categorised in Table 21.
| Variable | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| Number (%) of participants undergoing an ablation procedure | 218 (97.3) | 171 (75.7) |
| Total number of procedures | 269 | 203 |
| Total number of SAEs | 43 | 55 |
| Number (%) of participants with SAE | 30 (13.4) | 35 (15.5) |
| Serious reason, n (%) | ||
| Death | 3 (7.0) | 4 (7.3) |
| Life-threatening | 0 (0) | 0 (0) |
| Persistently disabling | 0 (0) | 0 (0) |
| Hospitalisation required | 38 (88.4) | 50 (90.9) |
| Congenital abnormality | 0 (0) | 0 (0) |
| Other | 2 (4.7) | 1 (1.8) |
| Frequency, n (%) | ||
| Single episode | 32 (74.4) | 49 (89.1) |
| Intermittent | 1 (2.3) | 1 (1.8) |
| Frequent | 1 (2.3) | 0 (0) |
| Continuous | 7 (16.3) | 5 (9.1) |
| Unknown | 2 (4.7) | 0 (0) |
| Severity, n (%) | ||
| Mild | 3 (7.0) | 4 (7.3) |
| Moderate | 17 (39.5) | 23 (41.8) |
| Severe | 18 (41.9) | 20 (36.4) |
| Life-threatening or disabling | 5 (11.6) | 8 (14.6) |
| Relation to procedure, n (%) | ||
| Not related | 38 (88.4) | 51 (92.7) |
| Unlikely | 1 (2.3) | 1 (1.8) |
| Possibly | 1 (2.3) | 3 (5.5) |
| Probably | 1 (2.3) | 0 (0) |
| Definite | 2 (4.7) | 0 (0) |
| Outcome, n (%) | ||
| Recovered | 36 (83.7) | 46 (83.6) |
| Not yet recovered | 1 (2.3) | 1 (1.8) |
| Death | 6 (14.0) | 8 (14.6) |
| Unknown | 0 (0) | 0 (0) |
| Expectedness, n (%)a | ||
| Expected | 4 (100) | 3 (100) |
| Treatment allocation | System organ classes term | Preferred term | Lowest-level term |
|---|---|---|---|
| Early | Musculoskeletal and connective tissue disorders | Pain in extremity | Leg pain |
| Early | Surgical and medical procedures | Vascular compression therapy | Compression dressing application |
| Early | General disorders and administration site conditions | Peripheral swelling | Swelling of legs |
| Early | Skin and subcutaneous tissue disorders | Skin ulcer | Leg ulcer |
| Deferred | Injury, poisoning and procedural complications | Laceration | Laceration of head |
| Deferred | Infections and infestations | Urinary tract infection | Urinary tract infection |
| Deferred | Infections and infestations | Infected skin ulcer | Infected skin ulcer |
Protocol deviations
There were 89 and 74 protocol deviations in early- and deferred-ablation groups, respectively. Table 22 shows the summary of the protocol deviations. The number of protocol deviations related to trial treatment was 38 (involving 32 participants) in the early-ablation group and 32 (involving 31 participants) in the deferred-ablation group. Participants with protocol deviations related to treatment were excluded from the per-protocol analysis.
| Variable | Early (N = 89a) | Deferred (N = 74a) |
|---|---|---|
| Number of participants with a protocol deviation | 59 | 58 |
| Deferred ablation in early group, n (%) | 17 (19.1) | 0 (0) |
| Non-concordance with bandaging, n (%) | 9 (10.1) | 12 (16.0) |
| Early ablation in deferred group, n (%) | 0 (0) | 16 (21.3) |
| Other, n (%) | 63 (70.8) | 46 (62.2) |
| Follow-up visit missing/late | 40 (63.5) | 34 (73.9) |
| Photograph/tracing not taken | 4 (6.4) | 4 (8.7) |
| Incorrect consent initially completed | 3 (4.8) | 4 (8.7) |
| Ineligible | 2 (3.2) | 4 (8.7) |
| Other | 14b (22.2) | 0 (0) |
Sensitivity analysis
The per-protocol analyses included 387 participants after excluding those with protocol deviation related to treatment. Figure 12 shows the KM curve based on the per-protocol analysis. The difference between the two groups is less pronounced than in the ITT analysis as the participants with protocol deviations in the deferred group experienced particularly poor healing (Figure 13). The smaller difference between the treatment groups in Table 23 also illustrates that the participants in the deferred group who did not have a protocol deviation had less severe ulcers. The 24-week ulcer healing rate in the deferred group was 76.3% in the ITT analysis and 82.6% in the per-protocol analysis. After adjusting for covariates in the Cox regression, in the per-protocol analysis the HR for time to healing associated with early compared with deferred ablation is 1.31 (95% CI 1.06 to 1.63; p = 0.01) (Table 24). In summary, although the deferred-intervention participants in the per-protocol analysis had less severe ulcers, it was still observed that early ablation led to more rapid ulcer healing in the per-protocol analysis.
FIGURE 12.
Per-protocol analysis (excluding participants with a protocol deviation) KM curve showing ulcer healing in the early and deferred (standard) ablation groups (p = 0.04).

FIGURE 13.
Kaplan–Meier curve showing ulcer healing time in the early- and deferred-ablation groups among participants with protocol deviations (p < 0.001).

| Variable | Early (N = 192) | Deferred (N = 195) |
|---|---|---|
| Ulcer healing rate (95% CI) (%)a | ||
| 12 weeks | 63.9% (57.1% to 70.6%) | 57.0% (50.2% to 64.1%) |
| 24 weeks | 86.4% (81.1% to 90.8%) | 82.6% (76.8% to 87.6%) |
| Number (%) of participants with healed ulcer at 1 year | 180 (93.8) | 170 (87.2) |
| Number (%) of participants with recurrent ulcerb | 23 (12.8) | 28 (16.5) |
| Ulcer-free time (days), median (IQR) | 309 (240–329), n = 177 | 286 (213–325), n = 176 |
| Variable | N a | n a | Univariable modelb | Multivariable modelc | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| Treatment group | ||||||
| Deferred | 195 | 170 | Reference | Reference | ||
| Early | 192 | 180 | 1.25 (1.01 to 1.55) | 0.04 | 1.31 (1.06 to 1.63) | 0.01 |
| Age (years) | 387 | 350 | 0.99 (0.98 to 1.00) | 0.02 | 1.00 (0.99 to 1.01) | 0.56 |
| Ulcer duration (months) | ||||||
| First quartile (0.9–2.2) | 101 | 91 | Reference | Reference | ||
| Second quartile (2.3–3.1) | 101 | 92 | 1.02 (0.76 to 1.37) | 0.88 | 1.03 (0.77 to 1.39) | 0.83 |
| Third quartile (3.1–4.2) | 96 | 91 | 1.09 (0.81 to 1.46) | 0.56 | 1.14 (0.85 to 1.53) | 0.38 |
| Fourth quartile (4.2–8.4) | 89 | 76 | 0.74 (0.54 to 1.00) | 0.05 | 0.84 (0.61 to 1.15) | 0.27 |
| Ulcer size (cm2) | ||||||
| First quartile (0.4–1.5) | 98 | 94 | Reference | Reference | ||
| Second quartile (1.6–2.9) | 96 | 93 | 0.80 (0.60 to 1.07) | 0.13 | 0.76 (0.57 to 1.03) | 0.07 |
| Third quartile (3–7.5) | 98 | 88 | 0.50 (0.37 to 0.67) | < 0.001 | 0.50 (0.37 to 0.67) | < 0.001 |
| Fourth quartile (8–235) | 95 | 75 | 0.30 (0.22 to 0.40) | < 0.001 | 0.29 (0.21 to 0.41) | < 0.001 |
Chapter 4 Economic evaluation results
Resource use and total cost analysis
Figure 14 and Appendix 9 show initial ablation procedures and overall subsequent resource use in the 450 randomised participants. Total mean cost per patient was calculated over 1 year. Participants who withdrew from the trial before 12 months were not included in the cost analysis. Participants who died during the year were included in the cost analysis, with costs set to £0 after the date of death. Hence, for the purposes of the total cost analysis, 211 participants in the deferred-ablation group (226 randomised minus 15 withdrawals, i.e. lost to follow-up or protocol deviations) and 208 participants in the early-ablation group (224 randomised minus 16 withdrawals, i.e. lost to follow-up or protocol deviations) completed 12 months of the trial or died (see Figure 3).
FIGURE 14.
NHS and Personal Social Services costs (£) of early vs. deferred strategies over 1 year, n = 208 (early-ablation group) and n = 211 (deferred-ablation group). Reproduced with permission from Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Epstein et al. 71 British Journal of Surgery, vol. 106, © 2019 BJS Society Ltd Published by John Wiley & Sons Ltd.
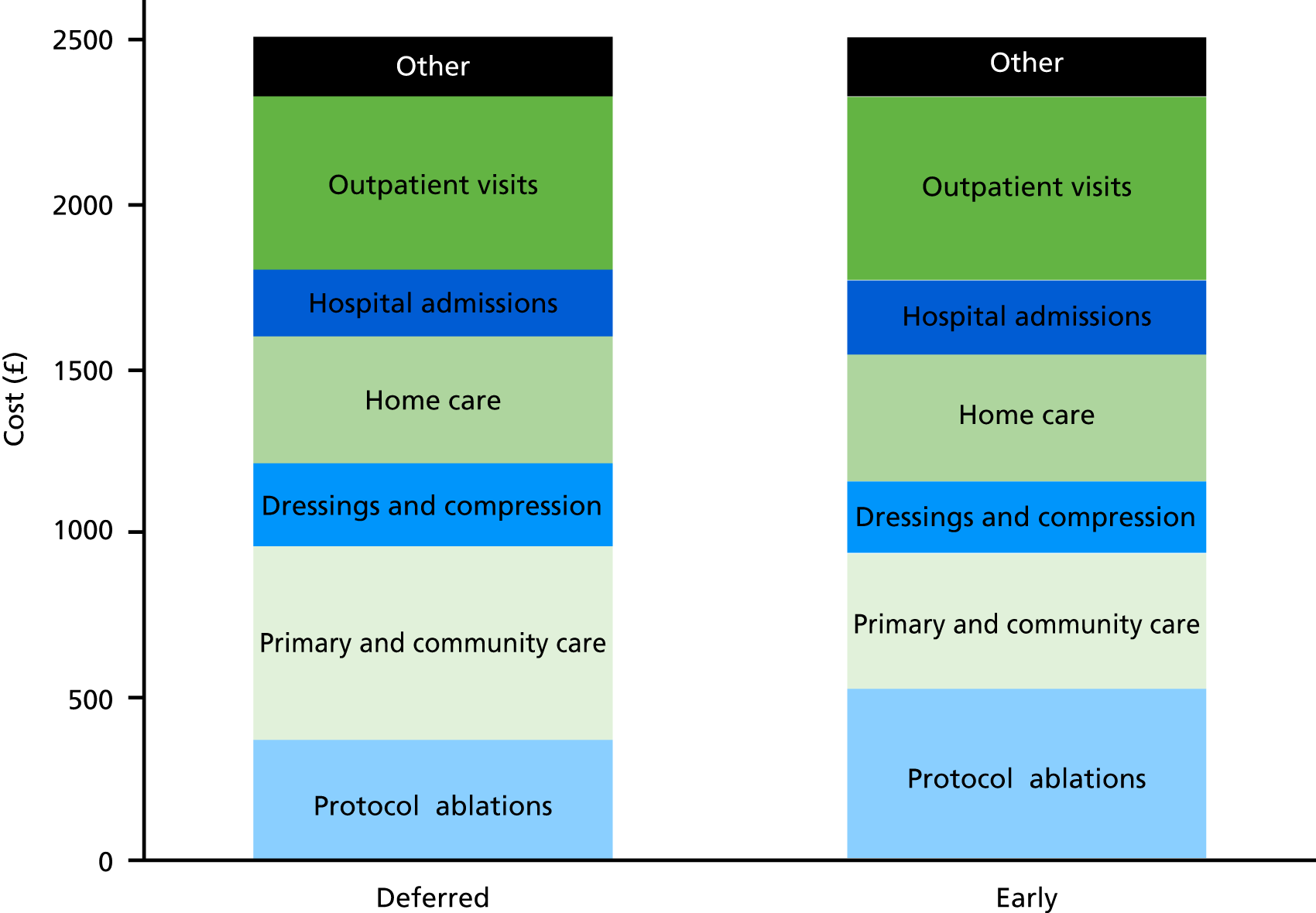
The total mean cost over 1 year was very similar in the two trial groups: £2514 (SD £2770) in participants randomised to early ablation and £2516 (SD £3242) in the deferred-ablation group.
The early-ablation group incurred a greater initial cost due to the allocated ablation procedure, even though the trial protocol suggested that participants in the deferred group should have an ablation procedure once the ulcer was healed. Reasons for non-ablation in participants randomised to deferred ablation are unclear, but both participant and clinician preferences are likely to have played a role. The greater initial costs in the early-ablation group were compensated for by the lower costs of district nurse visits and consumables to quicker wound healing. Other resource use was similar in the two groups.
Table 10 shows the number of index endovenous ablation procedures performed. The trial also recorded further interventions in the treatment visit CRF and in the monthly telephone follow-up. These may include, for example, reinterventions for return of symptoms. Some of these may be non-protocol ablations (e.g. ablation in the non-trial leg), but there is insufficient information to be certain.
Table 25 shows the total number of vein procedures recorded in the trial, including those reported in the monthly telephone follow-up. To avoid double-counting, a record was assumed to be duplicated if a participant reported a vein procedure in the same month both on the CRF and during the telephone follow-up.
| Number of ablation procedures per patient | Early (N = 224) | Deferred (N = 226) |
|---|---|---|
| No procedure | 6 | 55 |
| 1 | 150 | 120 |
| 2 | 30 | 30 |
| 3 | 32 | 14 |
| 4 | 6 | 6 |
| 5 | 0 | 1 |
Cost-effectiveness analysis
The cost-effectiveness analysis uses data on both total costs and QALYs over 1 year. A total of 344 (76%) of 450 participants were included in the complete-case cost-effectiveness analysis. Table 26 summarises the pattern of missing data. Thirty-one (7%) participants had missing data for costs (because either they withdrew from the trial or there was a protocol deviation). A greater proportion (16%) had some missing data at 12 months for EQ-5D-5L. This arose because of withdrawal and because not all participants fully completed all the questions in the HRQoL questionnaires at each follow-up. Overall, 24% had some missing EQ-5D or cost data over the year.
| Variable | Early | Deferred | Total |
|---|---|---|---|
| Randomised, n | 224 | 226 | 450 |
| Any missing cost data over the year, n (%) | 16 (7) | 15 (7) | 31 (7) |
| Missing EQ-5D-5L at baseline, n (%) | 2 (< 1) | 0 (0) | 2 (< 1) |
| Missing EQ-5D-5L at 6 weeks, n (%) | 13 (6) | 18 (8) | 31 (7) |
| Missing EQ-5D-5L at 6 months, n (%) | 36 (16) | 31 (14) | 67 (14) |
| Missing EQ-5D-5L at 12 months, n (%) | 36 (16) | 36 (16) | 72 (16) |
| Any missing data over the year, n (%) | 51 (23) | 55 (24) | 106 (24) |
| Complete cases, n | 173 | 171 | 344 |
Table 27 shows the results of the cost and QALY regressions for the cost-effectiveness analysis.
| Model 1 (base case) | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Description of model | Complete case (n = 344), with bootstrap SEs (1000 samples) and crosswalk EQ-5D tariff63 | Complete case (n = 344), with bivariate normal SEs and crosswalk EQ-5D tariff | 10 multiple imputations (n = 450), with bivariate normal SEs and crosswalk EQ-5D tariff | Complete case (n = 344) with bootstrap SEs and Devlin EQ-5D-5L tariff57 | Per-protocol compliers (n = 273) with bootstrap SEs |
| Difference in cost: mean (SE), p-value | 163 (318), 0.607 | 163 (322), 0.612 | –72 (290), 0.803 | 163 (322), 0.612 | 486 (326), 0.137 |
| Difference in QALY: mean (SE), p-value | 0.041 (0.017), 0.017 | 0.041 (0.018), 0.024 | 0.058 (0.018), 0.002 | 0.033 (0.016), 0.039 | 0.056 (0.019), 0.003 |
| ICER | £3976/QALY | £3976/QALY | n/ca | £4939/QALY | £8679/QALY |
In the complete-case analysis (model 1), the difference in cost was £163 (SE £318), the difference in QALYs gained at 1 year was 0.041 (SE 0.017) and the ICER was £3976 per QALY. There was an 89% probability that early venous surgery is cost-effective at the current willingness-to-pay (WTP) threshold of £20,000 per QALY (Figure 15). Assuming bivariate normality to estimate SEs gave very similar results (model 2). There was a significant negative correlation between costs and QALYs, indicating that participants with a worse quality of life were also those who tended to incur greater health-care costs (correlation –0.294; p < 0.001).
FIGURE 15.
Cost-effectiveness acceptability curves for each model. Model 1: complete case (dashed line); Model 2: complete case using bivariate normal model; Model 3: multiple imputation; Model 4: alternative EQ-5D-5L tariff; Model 5: Per-protocol. Reproduced with permission from Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Epstein et al. 71 British Journal of Surgery, vol. 106, © 2019 BJS Society Ltd Published by John Wiley & Sons Ltd.
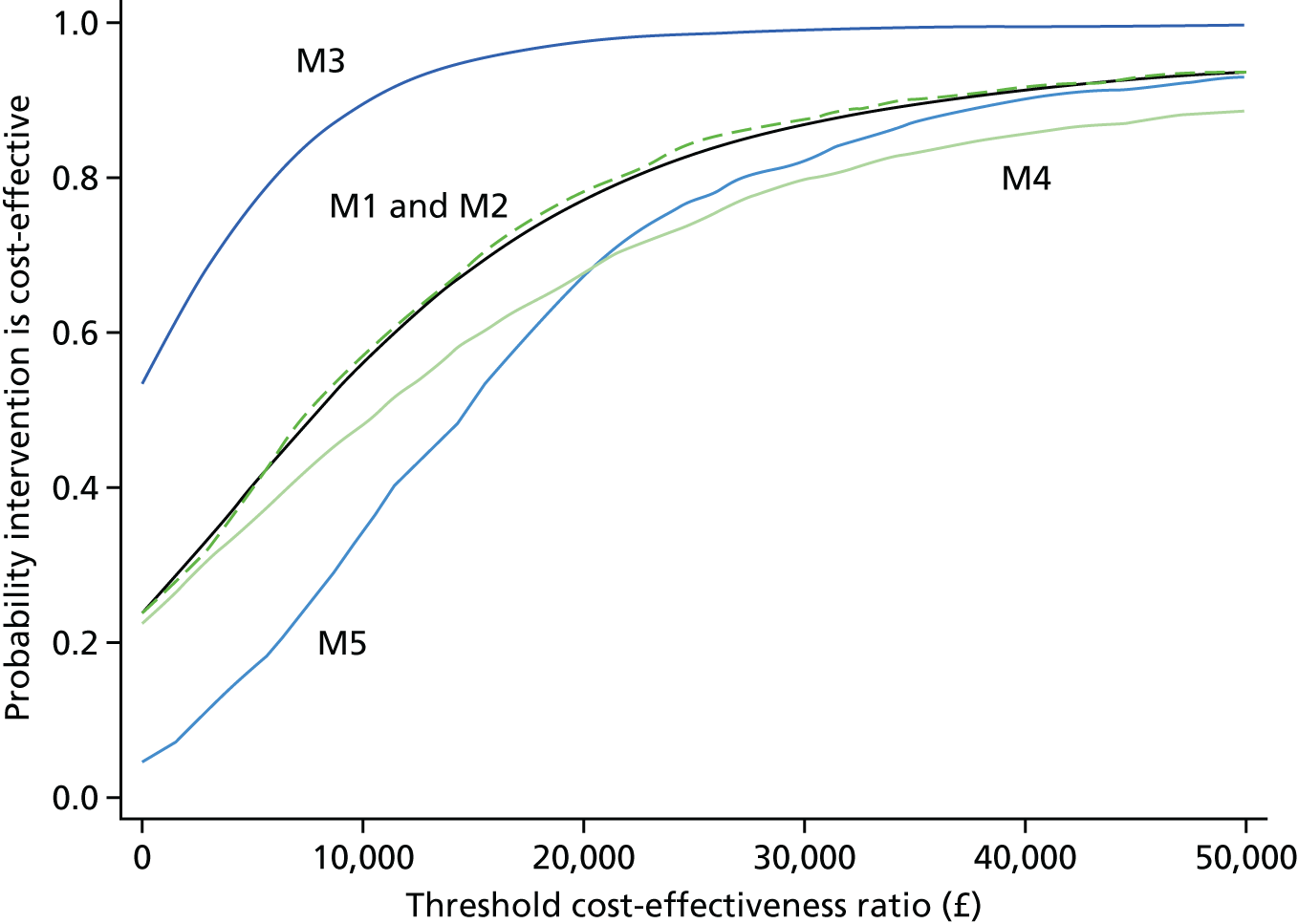
In model 3, missing data were imputed. The mean difference in total cost was –£72 (SE £290, i.e. early intervention was cheaper at 1 year), and the mean difference in QALYs gained over 1 year was 0.058 (SE 0.018). There was a 99% probability of early intervention being cost-effective at a threshold of £20,000 per QALY.
Using alternative tariff values for the EQ-5D-5L resulted in a slightly smaller difference in QALY between the treatment groups, but the ICER was similar to the base case (model 4).
The per-protocol analysis used the same approach as model 1, but excluded patients with protocol deviations. Protocol deviations were seen in 117 patients (59 and 58 in the early and deferred groups, respectively), of whom 71 had complete data. This left 273 patients for analysis (344 with complete data at 12 months minus 71 protocol deviations). The ICER in this model was £8679 per QALY (model 5).
Chapter 5 Discussion
Interpretation
The EVRA trial is the first multicentre RCT to assess the effect of early endovenous ablation for superficial venous reflux on ulcer healing in participants with venous ulceration. As standard care in the UK does not usually involve venous surgery (and, if surgery is performed, it is generally deferred until after ulcer healing with compression therapy), the results should be of interest to patients, clinicians and policy-makers.
The trial showed that early ablation of superficial reflux in addition to compression therapy significantly accelerates ulcer healing. Participants randomised to the early-ablation group also benefited from more ulcer-free time over the 12 months post randomisation.
Venous guidelines, worldwide,40,45 recommend the ablation of superficial venous reflux based on the results of the ESCHAR trial, which demonstrated that superficial venous surgery reduced ulcer recurrence compared with compression therapy alone. 9,15 ESCHAR, however, did not show a benefit in terms of ulcer healing, which may explain why leg ulcer care pathways usually do not include provisions for early assessment and treatment of superficial reflux. The exception is the US Society for Vascular Surgery and the American Venous Forum Guidelines, which make a weak recommendation (grade 2, level C) for endovenous ablation in active ulceration based on the results of some cohort studies. 45 In addition, the lack of standardised leg ulcer pathways and the involvement of a range of specialists may contribute to the inconsistent care delivered. 8,41,44
It is interesting to note that the healing rates at 12 and 24 weeks achieved in the deferred-ablation group (51.6% and 76.3%, respectively) are higher than those previously reported in the literature and seen in the general venous leg ulcer population. 72 This is likely to be as a result of good-quality compression applied by the specialised, highly trained research staff and not representative of usual care (which varies across regions and can suffer from lack of staffing and resource). 44,73
Despite the excellent healing rates in the deferred-ablation group in this trial, participants randomised to early ablation still demonstrated a shorter time to healing. A widespread strategy of early ablation is likely to show an even greater benefit, as endovenous interventions are usually delivered as a single treatment episode, in contrast with compression therapy, which requires ongoing compliance for optimal outcomes. Implementation of early endovenous ablation for patients with venous leg ulceration will require considerable changes to current care pathways and treatment paradigms. The EVRA trial results reinforce the NICE recommendation that patients with leg ulceration not healed within 2 weeks should be referred promptly to a vascular service for evaluation and treatment of venous disease.
Although the results of the subgroup analysis should be interpreted with caution, there is a clear trend for a greater benefit from early ablation as ulcer duration increases. The inclusion criteria for the trial stipulated an upper limit of 6 months in duration for ulcers. This was to minimise heterogeneity within the trial population, but also because investigators expressed concerns about withholding endovenous ablation from patients with ulcers that had failed to respond to 6 months of compression therapy. Whether or not an even greater benefit would exist in those with an ulcer duration of > 6 months remains unclear.
Adverse events
The most common complications of endovenous ablation were pain and DVT. The DVT rate seen in the early-ablation group was high compared with other literature. However, in six of the participants DVT was infrapopliteal, and in four of these the thrombosis was identified on routine post-UGFS duplex ultrasonography performed 7 days post ablation (as per the local scanning regimen in one of the recruiting centres). Therefore, it is likely that this represents a very high level of detection of subclinical DVT.
Although 98 SAEs were reported over the course of the trial, as may be expected given the age of the trial population, only seven were deemed to be possibly, probably or definitely related to the ablation procedures.
Health-related quality of life
Early ablation led to significant improvements in disease-specific (AVVQ) and general HRQoL (EQ-5D index value) and body pain (SF-36 body pain), over the follow-up period. Differences were most pronounced at 6 weeks and 6 months post randomisation, which is consistent with more rapid healing.
Costs and cost-effectiveness
The complete-case analysis shows little difference in total mean cost per patient over 1 year between early and deferred ablation [mean difference £163 (SE £318); p = 0.607]. The greater initial mean cost of the early-ablation strategy is mostly offset by the reduced cost of treating unhealed leg ulcers. There is, however, a substantial and statistically significant QALY gain over 1 year, with a mean difference of 0.041 (SE 0.017) QALYs; p = 0.017. The ICER of early ablation at 1 year is, therefore, £3976 per QALY, compared with deferred ablation, with a high probability (89%) that early ablation is more cost-effective at conventional UK WTP thresholds (£20,000 per QALY). Sensitivity analyses using alternative statistical models give qualitatively similar results.
The difference in HRQoL appears to narrow at 1 year. Further follow-up is required to understand whether or not the gains from early ablation are maintained in the longer term. If early ablation results in lower recurrence risk in addition to reducing the time to healing, then even greater cost-effectiveness may be present over the lifetime of the patient. 74
The economic analysis protocol envisaged a within-trial cost-effectiveness analysis at 1 year and a decision model [for details, see the health economic plan on the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/11129197/#/ (accessed 18 April 2019)]. The purpose of the decision model was to extrapolate recurrence rates in order to assess whether or not early ablation might be cost-effective over a patient’s lifetime. At 1 year, there were insufficient recurrence events to reliably compare early with delayed ablation. Hence, the decision model results based on EVRA trial data will be reported when the trial extension results become available in late 2019.
As it was not possible to construct an economic model incorporating recurrence rates based on EVRA trial data at 1 year, an interim analysis was undertaken based on recurrence and healing rates obtained from the literature. 31 These studies compared early ablation (with surgery or endothermal techniques) plus compression versus compression only. None of these studies compared early ablation with delayed ablation; hence, they are not directly comparable with the strategies under comparison in the EVRA trial and are therefore not described in detail in this report. Nevertheless, the analysis showed that even if early ablation reduced the rate of recurrence only, and did not have an impact on healing, this strategy would be very cost-effective over a patient’s lifetime. 74
Generalisability
The trial was designed to be as pragmatic as possible, with broad inclusion criteria and interventional strategies guided by the treating clinicians. Participants were recruited from 20 centres across England and, although the trial had only a 7% inclusion rate and screened > 6500 patients to randomise 450, the baseline characteristics of the trial participants appear representative of the target population, when compared with other leg ulcer studies. 46,75,76 Of those screened but not randomised, who were excluded for not meeting the eligibility criteria, the two largest groups were those who had their ulcer for > 6 months (1772/6105, 29%) and those whose ulcer had healed by the time of randomisation (610/6105, 10%), largely as a result of delays in referral from primary to secondary care. Those with ulcers already healed have been shown in the ESCHAR trial to benefit from superficial venous intervention. Findings from the pre-planned subgroup analyses suggest that those with an ulcer duration of > 6 months may also benefit from early endovenous ablation. The results from the EVRA trial may therefore be more generalisable than initially apparent. Furthermore, as > 90% of those in the early-ablation group were treated within 2 weeks and 79.4% of these participants required only one procedure, implementation in a NHS setting seems highly feasible. Patient concordance is also likely to be higher with early ablation than with compression alone, as treatment success is less dependent on ongoing patient compliance.
Strengths of the EVRA trial
Sample size and loss to follow-up
The EVRA trial is the largest and only RCT, to our knowledge, to evaluate the effect of early endovenous ablation of superficial venous reflux, and target sample size of 450 participants was achieved. An adequate number of healing events occurred. Loss to follow-ups, withdrawals and deaths did not exceed our estimated 10% of the total recruitment; therefore, the trial was powered effectively.
Missing data
Only 31 (7%) participants were lost to follow-up, withdrew or violated protocol during the 12 months of the trial and we were able to ascertain the primary and secondary clinical end points for the majority of participants. Missing data were mainly confined to patient-reported outcomes such as AVVQ and SF-36, for which there was marked attrition over time. This was addressed in the analysis by performing sensitivity analyses using imputed values. Findings based on multiple imputation were the same as for the complete-case analysis.
For the cost-effectiveness analysis, 24% of participants had some missing data (for EQ-5D or costs over 12 months). The base case used only participants with complete data and sensitivity analyses used multiple imputation of missing data. Both methods gave qualitatively similar results, showing that the difference in cost was not significantly different from zero, whereas early ablation was associated with a substantial and significantly greater QALY gain.
Verification visit and blinded outcome assessment
Although the treatment allocation could not be blinded, it is believed that the blinded outcome assessment is a key strength of the trial.
Limitations of the EVRA trial
Centre variation
Despite each centre having an established leg ulcer care pathway, variations of practice existed between centres, most importantly in the choice of endovenous modality. In order to minimise these variations, we stratified by centre and stipulated standardised ablation principles. Similarly, compression regimens varied across participants and for the same participant, who may have received multiple different compression therapies. In general, a pragmatic approach was adopted.
Superficial venous reflux patterns
The patterns of superficial venous reflux and presence and extent of deep venous incompetence varied. However, the results support previous studies that show clear benefits of treating superficial venous reflux, even in the presence of concomitant deep-venous incompetence. 46,77,78
Post-ablation duplex
The 6-week follow-up duplex ultrasonography was stipulated only in the early-ablation group, whereas the deferred group strategy was as per standard care. This may have led to more repeat procedures and a higher procedure success rate than in the deferred-ablation group; however, this is not relevant to the primary outcome of time to ulcer healing.
Ulcer recurrence
The trial follow-up period was only to 1 year and hence was too short to give meaningful recurrence data, as there is a potential bias against the early-ablation group. With ongoing follow-up and longer-term recurrence data, we anticipate that this bias will diminish. The follow-up period for the extension is now complete (as of 31 March 2019) and, at the time of publication, we are cleaning and locking the database prior to data analysis. No new data available to date.
Endovenous modality
The clinicians were permitted to use modalities of their choice subject to some core stipulations of ablation. Despite the trial showing an overall benefit for early ablation, there is no clear distinction of benefit between the various endovenous modalities. The common modality used in this trial was ultrasonography-guided sclerotherapy, most likely reflecting its low cost and versatility, although some large RCTs have shown that complete venous occlusion may be lower with UGFS than with endovenous ablation. 37,55 Longer-term follow-up is ongoing and should help determine whether or not this will affect longer-term clinical outcomes and recurrence rates.
Chapter 6 Conclusion
Overall conclusions
Early endovenous ablation of superficial truncal reflux in addition to compression therapy accelerates the healing of venous leg ulcers compared with deferred ablation.
Although there is little difference between early and deferred ablation for endovenous superficial venous ablation in terms of the total mean cost per patient over 1 year, early ablation results in a significant gain in QALYs compared with deferred ablation. Therefore, early ablation has a high probability of being cost-effective at NICE WTP thresholds.
Implications for health care
Findings from this trial suggest that, for people with venous leg ulcers, early assessment and ablation of superficial venous reflux, in addition to compression therapy, accelerates healing and produces health economic benefits. Implementation of early assessment and endovenous ablation of superficial venous reflux will require further development of care pathways between primary and secondary care.
Recommendations for research (numbered in order of priority)
-
Follow up patients for longer to determine if early endovenous ablation influences ulcer recurrence rates in the medium and long term.
-
Evaluate the benefit of early ablation for superficial venous reflux in patients with venous leg ulceration of > 6 months’ duration.
-
Determine the implications of deep-venous incompetence and occlusive, and the potential role of deep-venous stenting to improve venous outflow of the limb.
-
Evaluate the optimal technique and the extent of eradication of superficial venous incompetence in patients with venous ulceration.
Acknowledgements
Trial applicants
Alun H Davies, Manjit S Gohel, Jane Warwick, David M Epstein, Andrew Bradbury, Keith R Poskitt, Richard Bulbulia and Nicky Cullum.
Trial Management Group
Alun H Davies (chief investigator), Francine Heatley (trial manager), Xinxue Liu (statistician) and Jane Warwick (senior statistician).
Imperial Clinical Trials Unit
The following members were part of the wider ICTU trial team: Claire Smith, Hilda Tsang, Hema Collappen and Natalia Klimowska-Nassar (operation managers); Amanda Bravery, Sandra Griffiths, Joseph Fryer, Kayode Disu, Nayan Das, Ayse Depsen, Dinesh Sivakumar, Fiona Persaud (InForm database team); Ginny Picot (quality assurance).
Department of Surgery and Cancer, Imperial College London
The following members were part of the wider EVRA trial team: Rebecca Lawton (trial manager); Becky Ward (sponsor); Matt Ryan (research manager); and Kirti Patel and Zara Collard (contracts).
Trial Steering Committee
We would like to thank Professor Julie Brittenden (chairperson); Miss Rebecca Jane Winterborn (Consultant Vascular Surgeon); Professor Andrea Nelson (Head of School and Professor of Wound Healing); Dr Richard Haynes (Research Fellow and Honorary Consultant Nephrologist); and Mr Bruce Ley-Greaves (lay member), who all provided invaluable input and advice as an independent TSC member over the course of the trial.
Data Monitoring Committee
The team would also like to thank the independent DMC members: Professor Gerard Stansby (chairperson, Professor of Vascular Surgery), Professor Frank Smith (Professor of Vascular Surgery and Surgical Education), Professor Marcus Flather (Professor of Medicine, Clinical Trials) and Dr Ian Nunney (Medical Statistician).
Patient and public involvement
Bruce Ley-Greaves was involved in the original design during the grant application stages and was an active member of the TSC throughout the trial. Bruce’s involvement is detailed in Appendix 1.
Core laboratory
We are thankful to Chen Liu and Rahul Velineni for core laboratory review and data activity.
Health economics
David Epstein (Health Economist) conducted the analysis of economic models for the trial.
Data cleaning
We are thankful to Yujin Lee for assistance with data cleaning and validation.
Local vascular research teams
The EVRA team would like to thank the NHS trusts, the participating principal investigators and their colleagues for recruiting and monitoring trial participants. These include (in alphabetical order of participating hospitals followed by the local principal investigators and their colleagues): Mr Kevin Mercer, Fazah Gill, Alan Liu, Wendy Jepson, Amy Wormwell and Helen Rafferty (Bradford Royal Infirmary); Mr Manjit Gohel, Debbie Read, Simone Hargreaves, Karen Dhillon, Muzaffar Anwar, Ailsa Liddle and Helen Brown (Addenbrooke’s Hospital); Alun Davies, Karen Dhillon, Raman Kaur, Emma Solomon, Kaji Sritharan, Rahul Velineni, Chung Sim Lim, Andrew Busuttil and Roshan Bootun (Charing Cross Hospital); Keith Poskitt, Richard Bulbulia, Jo Waldron, Gina Wolfrey, Fiona Slim, Colin Davies, Lorraine Emerson, Marianne Grasty, Mark Whyman, Clare Wakley, Andrew Cooper, Jo Clapp, Nicola Hogg, Julia Howard, Jackie Dyer, Sheri Lyes, Danita Teemul, Kate Harvey, Mandy Pride, Andrew Kindon, Hannah Price, Laura Flemming, Gemma Birch, Helen Holmes and Jodie Weston (Cheltenham General Hospital); Thomas Joseph, Ron Eiffel, Theo Ojimba, Toni Wilson, Adrian Hodgson, Lesley Robinson, Jane Todhunter, Dean Heagarty, Anna Mckeane and Rachel McCarthy (Cumberland Infirmary); Jamie Barwell, Clare Northcott, Alan Elstone and Clare West (Derriford Hospital); Patrick Chong, David Gerrard, Andrea Croucher, Stephanie Levy, Claire Martin and Tracey Craig (Frimley Park Hospital); Daniel Carradice, Anna Firth, Emma Clarke, Angie Oswald, Judith Sinclair, Ian Chetter, Joseph El-Sheikha, Sandip Nandhra and Clement Leung (Hull Royal Infirmary); Julian Scott, Nikki Dewhirst, Janet Woods, David Russell, Rosie Darwood, Max Troxler, Julie Thackeray, Deborah Bell, David Watson and Louise Williamson (Leeds General Infirmary); James Coulston, Paul Eyers, Katy Darvall, Ian Hunter, Andrew Stewart, Alison Moss, Jane Rewbury, Claire Adams, Louise Vickery, Leanne Foote, Helen Durman, Frances Venn, Paula Hill, Kate James, Fliss Luxton, Denise Greenwell. Karen Roberts, Suzette Mitchell, Moira Tate and Helen Mills (Musgrove Park Hospital); Andrew Garnham, Simon Hobbs, Donna Mcintosh, Marie Green, Kate Collins, Jayne Rankin, Paula Poulton and Val Isgar (New Cross Hospital); Sophie Renton, Karen Dhillon, Manoj Trivedi, Marina Kafeza, Shadeh Parsapour, Hayley Moore, Mojahid Najem, Sean Connarty, Hazel Albon, Chris Lloyd and Jackie Trant (Northwick Park Hospital); Rajiv Vohra, Jo McCormack, Jeanette Marshall, Victoria Hardy, Radu Rogoveanu and Will Goff (Queen Elizabeth Hospital Birmingham); Andrew Garnham, Ranjit Gidda, Sue Merotra, Sandy Shiralkar, Agantha Jayatunga, Rajiv Pathak, Atiq Rehman, Kiran Randhawa, Joy Lewis, Sarah Fullwood, Stacey Jennings, Sharon Cole and Michael Wall (Russells Hall Hospital); Charles Ranaboldo, Sarah Hulin, Caroline Clarke, Ruth Fennelly, Robin Cooper, Ruth Boyes, Charlotte Draper, Linda Harris and Dee Mead (Salisbury District Hospital); Andrew Bradbury, Lisa Kelly, Gareth Bate, Huw Davies, Matt Popplewell, Martin Claridge, Mark Gannon, Harmeet Khaira, Mark Scriven, Teun Wilmink, Donald Adam and Hosaam Nasr (Solihull Hospital); Colin Bicknell, Mike Jenkins, Tristan Lane and Emily Serjeant (St Mary’s Hospital, London); Dominic Dodd, Shah Nawaz, John Humpreys, Mathew Barnes, Julie Sorrell, Diane Swift, Patrick Phillips, Hazel Trender and Nikki Fenwick (Northern General Hospital); Dynesh Rittoo, Sara Baker, Rebecca Mitchell, Sarah Andrews, Steve Williams and Jane Stephenson (Royal Bournemouth General Hospital); Isaac Nyamekye, Sarah Holloway, Wendy Hayes, Julie Day, Claire Clayton and Daniel Harding (Worcestershire Royal Hospital); Andrew Thompson, Andy Gibson, Zoe Murphy and Thomas Smith (York Hospital).
National Institute for Health Research Clinical Research Networks
We would like to thank the following Clinical Research Networks for their help and support throughout the study (in particular helping facilitate the set-up of patient identification centres): Clinical Research Network North West London (lead site); East Midlands; Eastern; Greater Manchester, Kent, Surrey and Sussex; North East and North Cumbria; North Thames; South London; South West Peninsular; Thames Valley and South Midlands; Wessex; West Midlands (in particular Shahnaz Khan); West of England; and Yorkshire and the Humber.
Patient identification centres
The EVRA team would like to thank the following NHS trusts for allowing some of their general practices to display posters and leaflets to help promote the trial and enhance recruitment: Lincolnshire Community Health Services NHS Trust, Lincolnshire Clinical Commissioning Groups (CCGs); Nottingham CityCare Partnership and County Health Partnerships within Nottinghamshire Healthcare NHS Foundation Trust, Nottingham City CCG and Nottinghamshire County CCGs; Derbyshire County and Derby City PCT; Derbyshire Community Health Services; Northamptonshire Healthcare NHS Foundation Trust; Leicestershire Partnership NHS Trust; Cambridgeshire Community Services NHS Trust; Cambridgeshire and Peterborough CCG; Hertfordshire Community NHS Trust; Hertfordshire Partnership NHS Foundation Trust; North Essex Partnership University NHS Foundation Trust; NHS South Norfolk CCG; Ashton Leigh and Wigan PCT; Bolton PCT; Bury PCT; Heywood, Middleton and Rochdale PCT; Manchester PCT; Oldham PCT; Salford PCT; Stockport PCT; Trafford PCT; Crawley CCG; Coastal West Sussex; Horsham & Mid Sussex; Eastbourne, Hailsham and Seaford CCG (formerly NHS East Sussex Downs and Weald); High Weald Lewes Havens CCG (formerly NHS East Sussex Downs and Weald); Hastings and Rother CCG (formerly NHS Hastings and Rother); East Surrey CCG (formerly NHS Surrey); Guildford and Waverley CCG (formerly NHS Surrey); North East Hampshire and Farnham CCG (formerly NHS Surrey); North West Surrey CCG (formerly NHS Surrey); Surrey Downs CCG (formerly NHS Surrey); Surrey Heath CCG (formerly NHS Surrey); Brighton and Hove CCG (formerly NHS Brighton and Hove); Ken and Medway CCGs; Medway Community Healthcare; North of England Commissioning CCGs; Central and North West London NHS Foundation Trust; Brent CCG; Central London Community Healthcare NHS Trust; Chelsea and Westminster Hospital NHS Foundation Trust; Hounslow and Richmond Community Healthcare NHS Trust; Bexley CCG; Lewisham CCG; Royal Devon and Exeter NHS Foundation Trust; Somerset CCG; Dorset CCG; Fareham and Gosport CCG; Isle of Wight CCG; North Hampshire CCG; North East Hampshire; Portsmouth CCG; Southampton CCG; South Eastern Hampshire CCG; Wilshire CCG; Birmingham Community Healthcare NHS Trust; Black Country Partnership NHS Foundation Trust; Worcestershire Health and Care NHS Trust; Worcestershire PCT; Walsall Healthcare NHS Trust; Royal Wolverhampton NHS Trust; Coventry and Warwickshire Partnership Trust; Bristol PCT; North Somerset and South Gloucestershire PCT; Banes CCG; NHS Swindon CCG; City Health Care Partnership; Humber NHS Foundation Trust; NHS Doncaster CCG; Airedale, Wharfedale and Craven CCG; Bradford City CCG; Bradford District CCG; Calderdale CCG; Leeds North CCG; Leeds South and East CCG; Leeds West CCG; Wakefield CCG; Vale of York CCG; Scarborough and Rydale CCG; Hambleton, Richmondshire and Whitby CCGs; Harrogate Rural and District CCG; Hull CCG; East Riding of Yorkshire CCG; and North Lincolnshire CCG.
Contributions of authors
Manjit S Gohel (Consultant Vascular Surgeon and co-applicant) was responsible for the design, conduct, supervision of the trial, acquisition of the data, interpretation of analysis and dissemination, drafting relevant chapters and final approval.
Francine Heatley (Trial Manager) managed and monitored the trial as trial manager, assisted with acquisition of the data, drafted relevant chapters and approved the final version of the report.
Xinxue Liu (Trial Statistician) was responsible for the conduct of the statistical analysis.
Andrew Bradbury (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the trial, acquisition of the data and review of the final draft.
Richard Bulbulia (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the trial and review of the final draft.
Nicky Cullum (Professor of Nursing, Head of the Division of Nursing, Midwifery & Social Work and co-applicant) was responsible for the design of the trial and review of the final draft.
David M Epstein (Lecturer, Applied Economics) was responsible for the design, conduct, analysis, dissemination and drafting of the cost-effectiveness chapter and review of the final draft.
Isaac Nyamekye (Consultant Vascular Surgeon) assisted with acquisition of the data and review of the final draft.
Keith R Poskitt (Consultant Vascular Surgeon and co-applicant) was responsible for the design of the trial, acquisition of the data and review of the final draft.
Sophie Renton (Consultant Vascular Surgeon) assisted with acquisition of the data and review of the final draft.
Jane Warwick (Senior Statistician and co-applicant) was involved in the design of both the trial and the statistical analysis plan, conduct of the statistical analysis and drafting of relevant chapters.
Alun H Davies (Professor of Vascular Surgery) was the chief investigator and was responsible for the design, conduct and supervision of the trial; interpretation of analysis and dissemination; drafting relevant chapters; and co-ordination of the report including final approval.
Francine Heatley, Alun H Davies, Manjit S Gohel, Jane Warwick and David M Epstein were responsible for drafting this report, although all authors provided comments on drafts and approved the final version.
Publications
Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018;378:2105–114.
Epstein DM, Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, et al. Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg 2019;106:555–62.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and appropriate agreements being in place.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Obermayer A, Garzon K. Identifying the source of superficial reflux in venous leg ulcers using duplex ultrasound. J Vasc Surg 2010;52:1255-61. https://doi.org/10.1016/j.jvs.2010.06.073.
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care 2009;18:154-61. https://doi.org/10.12968/jowc.2009.18.4.41607.
- Cullum N, Buckley H, Dumville J, Hall J, Lamb K, Madden M, et al. Wounds research for patient benefit: a 5-year programme of research. Programme Grants Appl Res 2016;4. https://doi.org/10.3310/pgfar04130.
- Graham ID, Harrison MB, Nelson EA, Lorimer K, Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care 2003;16:305-16. https://doi.org/10.1097/00129334-200311000-00013.
- Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg: clinical history. Br Med J 1987;294:1389-91. https://doi.org/10.1136/bmj.294.6584.1389.
- Statistics on Obesity, Physical Activity and Diet – England, 2012. Leeds: NHS Digital; 2012.
- Laing W. Chronic Venous Diseases of the Leg. London: Office of Health Economics; 1992.
- Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009283.
- Barwell JR, Davies CE, Deacon J, Harvey K, Minor J, Sassano A, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet 2004;363:1854-9. https://doi.org/10.1016/S0140-6736(04)16353-8.
- van Gent WB, Hop WC, van Praag MC, Mackaay AJ, de Boer EM, Wittens CH. Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg 2006;44:563-71. https://doi.org/10.1016/j.jvs.2006.04.053.
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014;130:333-46. https://doi.org/10.1161/CIRCULATIONAHA.113.006898.
- Grabs AJ, Wakely MC, Nyamekye I, Ghauri AS, Poskitt KR. Colour duplex ultrasonography in the rational management of chronic venous leg ulcers. Br J Surg 1996;83:1380-2. https://doi.org/10.1002/bjs.1800831016.
- Adam DJ, Naik J, Hartshorne T, Bello M, London NJ. The diagnosis and management of 689 chronic leg ulcers in a single-visit assessment clinic. Eur J Vasc Endovasc Surg 2003;25:462-8. https://doi.org/10.1053/ejvs.2002.1906.
- Tassiopoulos AK, Golts E, Oh DS, Labropoulos N. Current concepts in chronic venous ulceration. Eur J Vasc Endovasc Surg 2000;20:227-32. https://doi.org/10.1053/ejvs.2000.1157.
- Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007;335. https://doi.org/10.1136/bmj.39216.542442.BE.
- Browse NL, Burnand KG. The cause of venous ulceration. Lancet 1982;2:243-5. https://doi.org/10.1016/S0140-6736(82)90325-7.
- Ibegbuna V, Delis KT, Nicolaides AN, Aina O. Effect of elastic compression stockings on venous hemodynamics during walking. J Vas Surg 2003;37:420-5. https://doi.org/10.1067/mva.2003.104.
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11. https://doi.org/10.1002/14651858.CD000265.pub3.
- Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-23.
- Nelson EA, Bell-Syer SE. Compression for preventing recurrence of venous ulcers. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD002303.pub3.
- van den Bremer J, Moll FL. Historical overview of varicose vein surgery. Ann Vasc Surg 2010;24:426-32. https://doi.org/10.1016/j.avsg.2009.07.035.
- Winterborn RJ, Foy C, Earnshaw JJ. Causes of varicose vein recurrence: late results of a randomized controlled trial of stripping the long saphenous vein. J Vasc Surg 2004;40:634-9. https://doi.org/10.1016/j.jvs.2004.07.003.
- O’Hare JL, Earnshaw JJ. Randomised clinical trial of foam sclerotherapy for patients with a venous leg ulcer. Eur J Vasc Endovasc Surg 2010;39:495-9. https://doi.org/10.1016/j.ejvs.2009.11.025.
- Darwood RJ, Gough MJ. Endovenous laser treatment for uncomplicated varicose veins. Phlebology 2009;24:50-61. https://doi.org/10.1258/phleb.2009.09s006.
- Gohel MS, Davies AH. Radiofrequency ablation for uncomplicated varicose veins. Phlebology 2009;24:42-9. https://doi.org/10.1258/phleb.2009.09s005.
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:501-10. https://doi.org/10.1002/bjs.7394.
- Subramonia S, Lees T. Randomized clinical trial of radiofrequency ablation or conventional high ligation and stripping for great saphenous varicose veins. Br J Surg 2010;97:328-36. https://doi.org/10.1002/bjs.6867.
- van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg 2009;49:230-9. https://doi.org/10.1016/j.jvs.2008.06.030.
- Davies AH, Hawdon AJ, Greenhalgh RM, Thompson S. Failure of a trial evaluating the effect of venous surgery on healing and recurrence rates in venous ulcers? The USABLE trial: rationale, design and methodology, and reasons for failure. Phlebology 2004;19:137-42. https://doi.org/10.1258/0268355041753371.
- Samuel N, Carradice D, Wallace T, Smith GE, Chetter IC. Endovenous thermal ablation for healing venous ulcers and preventing recurrence. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD009494.pub2.
- Mauck KF, Asi N, Undavalli C, Elraiyah TA, Nabhan M, Altayar O, et al. Systematic review and meta-analysis of surgical interventions versus conservative therapy for venous ulcers. J Vasc Surg 2014;60:60-70.e1. https://doi.org/10.1016/j.jvs.2014.04.059.
- Viarengo LM, Potério-Filho J, Potério GM, Menezes FH, Meirelles GV. Endovenous laser treatment for varicose veins in patients with active ulcers: measurement of intravenous and perivenous temperatures during the procedure. Dermatol Surg 2007;33:1234-42. https://doi.org/10.1097/00042728-200710000-00014.
- Marston WA, Crowner J, Kouri A, Kalbaugh CA. Incidence of venous leg ulcer healing and recurrence after treatment with endovenous laser ablation. J Vasc Surg Venous Lymphat Disord 2017;5:525-32. https://doi.org/10.1016/j.jvsv.2017.02.007.
- Sinabulya H, Östmyren R, Blomgren L. Editor’s choice – mid-term outcomes of endovenous laser ablation in patients with active and healed venous ulcers: a follow-up study. Eur J Vasc Endovasc Surg 2017;53:710-16. https://doi.org/10.1016/j.ejvs.2017.02.028.
- Kulkarni SR, Slim FJ, Emerson LG, Davies C, Bulbulia RA, Whyman MR, et al. Effect of foam sclerotherapy on healing and long-term recurrence in chronic venous leg ulcers. Phlebology 2013;28:140-6. https://doi.org/10.1258/phleb.2011.011118.
- Pang KH, Bate GR, Darvall KA, Adam DJ, Bradbury AW. Healing and recurrence rates following ultrasound-guided foam sclerotherapy of superficial venous reflux in patients with chronic venous ulceration. Eur J Vasc Endovasc Surg 2010;40:790-5. https://doi.org/10.1016/j.ejvs.2010.08.011.
- Rasmussen L, Lawaetz M, Serup J, Bjoern L, Vennits B, Blemings A, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up. J Vasc Surg Venous Lymphat Disord 2013;1:349-56. https://doi.org/10.1016/j.jvsv.2013.04.008.
- Siribumrungwong B, Noorit P, Wilasrusmee C, Attia J, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials comparing endovenous ablation and surgical intervention in patients with varicose vein. Eur J Vasc Endovasc Surg 2012;44:214-23. https://doi.org/10.1016/j.ejvs.2012.05.017.
- Management of Chronic Venous Leg Ulcers. Edinburgh: SIGN; 2010.
- Varicose Veins: Diagnosis and Management. London: NICE; 2013.
- Davies HO, Popplewell M, Bate G, Kelly L, Darvall K, Bradbury AW. Impact of UK NICE clinical guidelines 168 on referrals to a specialist academic leg ulcer service. Phlebology 2018;33:84-8. https://doi.org/10.1177/0268355516688357.
- Onida S, Davies AH, Franklin I. Varicose veins – who should be referred?. Phlebology 2015;30:4-8. https://doi.org/10.1177/0268355515589249.
- Varicose Veins in the Legs. Quality Standard [QS67]. London: NICE; 2014.
- Bulbulia RA, Poskitt KR. The need for a National Service Framework for leg ulcers. Phlebology 2010;25:68-72. https://doi.org/10.1258/phleb.2010.010s10.
- O’Donnell TF, Passman MA. Clinical practice guidelines of the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF) – management of venous leg ulcers. Introduction. J Vasc Surg 2014;60:1-2. https://doi.org/10.1016/j.jvs.2014.04.058.
- Gohel MS, Barwell JR, Earnshaw JJ, Heather BP, Mitchell DC, Whyman MR, et al. Randomized clinical trial of compression plus surgery versus compression alone in chronic venous ulceration (ESCHAR study) – haemodynamic and anatomical changes. Br J Surg 2005;92:291-7. https://doi.org/10.1002/bjs.4837.
- Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med 1982;1:121-9. https://doi.org/10.1002/sim.4780010204.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671-5. https://doi.org/10.1038/nmeth.2089.
- Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg 1995;21:635-45. https://doi.org/10.1016/S0741-5214(95)70195-8.
- Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248-52. https://doi.org/10.1016/j.jvs.2004.09.027.
- Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg 2000;31:1307-12. https://doi.org/10.1067/mva.2000.107094.
- Marston WA, Vasquez MA, Lurie F, Wakefield TW, Rabe E, Shortell CK, et al. Multicenter assessment of the repeatability and reproducibility of the revised Venous Clinical Severity Score (rVCSS). J Vasc Surg Venous Lymphat Disord 2013;1:219-24. https://doi.org/10.1016/j.jvsv.2012.10.059.
- Garratt AM, Macdonald LM, Ruta DA, Russell IT, Buckingham JK, Krukowski ZH. Towards measurement of outcome for patients with varicose veins. Qual Health Care 1993;2:5-10. https://doi.org/10.1136/qshc.2.1.5.
- Brittenden J, Cotton SC, Elders A, Ramsay CR, Norrie J, Burr J, et al. A randomized trial comparing treatments for varicose veins. N Engl J Med 2014;371:1218-27. https://doi.org/10.1056/NEJMoa1400781.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Garratt AM, Ruta DA, Abdalla MI, Russell IT. Responsiveness of the SF-36 and a condition-specific measure of health for patients with varicose veins. Qual Life Res 1996;5:223-34. https://doi.org/10.1007/BF00434744.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med 2018;378:2105-14. https://doi.org/10.1056/NEJMoa1801214.
- Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res 2005;14:1705-18. https://doi.org/10.1007/s11136-005-2751-9.
- Ashby RL, Gabe R, Ali S, Saramago P, Chuang LH, Adderley U, et al. VenUS IV (Venous leg Ulcer Study IV) – compression hosiery compared with compression bandaging in the treatment of venous leg ulcers: a randomised controlled trial, mixed-treatment comparison and decision-analytic model. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18570.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013;29:117-22. https://doi.org/10.1017/S0266462313000160.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Position Statement on EQ-5D-5L 2017. London: NICE; 2017.
- ClinicalTrials.gov . Prospective Evaluation of the ReCell® Autologous Cell Harvesting Device For Specific Compassionate Use Cases n.d. https://clinicaltrials.gov/ct2/show/NCT02992249 (accessed 15 March 2019).
- ClinicalTrials.gov . REVEAL: Randomized EValuation of the Effects of Anacetrapib Through Lipid-Modification (REVEAL) n.d. https://clinicaltrials.gov/ct2/show/NCT01252953 (accessed 15 March 2019).
- British National Formulary 73. London: BMJ Group and Pharmaceutical Press; 2017.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018;37:2252-66. https://doi.org/10.1002/sim.7654.
- Epstein DM, Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, et al. Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg 2019;106:555-62.
- Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J 2018;15:29-37. https://doi.org/10.1111/iwj.12814.
- Chamanga E, Christie J, McKeown E. Community nurses’ experiences of treating patients with leg ulcers. J Community Nurs 2014;28:27-34.
- Epstein D, Gohel M, Heatley F, Davies AH. Cost-effectiveness of treatments for superficial venous reflux in patients with chronic venous ulceration. BJS Open 2018;2:203-12. https://doi.org/10.1002/bjs5.56.
- Ashby RL, Gabe R, Ali S, Adderley U, Bland JM, Cullum NA, et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014;383:871-9. https://doi.org/10.1016/S0140-6736(13)62368-5.
- Guest M, Smith JJ, Tripuraneni G, Howard A, Madden P, Greenhalgh RM, et al. Randomized clinical trial of varicose vein surgery with compression versus compression alone for the treatment of venous ulceration. Phlebology 2003;18:130-6. https://doi.org/10.1258/026835503322381333.
- Padberg FT, Pappas PJ, Araki CT, Back TL, Hobson RW. Hemodynamic and clinical improvement after superficial vein ablation in primary combined venous insufficiency with ulceration. J Vasc Surg 1996;24:711-18. https://doi.org/10.1016/S0741-5214(96)70002-2.
- Adam DJ, Bello M, Hartshorne T, London NJ. Role of superficial venous surgery in patients with combined superficial and segmental deep venous reflux. Eur J Vasc Endovasc Surg 2003;25:469-72. https://doi.org/10.1053/ejvs.2002.1894.
- de Wit M, Abma T, Koelewijn-van Loon M, Collins S, Kirwan J. Involving patient research partners has a significant impact on outcomes research: a responsive evaluation of the international OMERACT conferences. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002241.
- Bagley HJ, Short H, Harman NL, Hickey HR, Gamble CL, Woolfall K, et al. A patient and public involvement (PPI) toolkit for meaningful and flexible involvement in clinical trials – a work in progress. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0029-8.
- Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-89.
- Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect 2015;18:1151-66. https://doi.org/10.1111/hex.12090.
- National Institute for Health Research INVOLVE . INVOLVE Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research 2012. www.invo.org.uk/wp-content/uploads/2014/11/9938_INVOLVE_Briefing_Notes_WEB.pdf (accessed 5 October 2017).
- National Institute for Health Research INVOLVE . Jargon Buster n.d. www.invo.org.uk/resource-centre/jargon-buster/ (accessed 1 July 2018).
- Nuffield Department of Primary Care Health Sciences . Developing a Patient and Public Involvement Intervention to Enhance Recruitment and Retention in Surgical Trials (PIRRIST) n.d. www.phc.ox.ac.uk/research/health-experiences/research-projects/developing-a-patient-and-public-involvement-intervention-to-enhance-recruitment-and-retention-in-surgical-trials-pirrist (accessed 1 July 2018).
- Gamble C, Dudley L, Newman J. Evidence base for patient and public involvement in clinical trials (EPIC). Trials 2013;14. https://doi.org/10.1186/1745-6215-14-S1-O34.
- National Institute for Health Research INVOLVE . INVOLVE Policy on Payments and Expenses for Members of the Public 2016. www.invo.org.uk/posttypepublication/involve-policy-on-payments-and-expenses-for-members-of-the-public-including-involve-group-members-february-2016/ (accessed 5 October 2017).
- National Institute for Health Research . Going the Extra Mile: Improving the Nation’s Health and Wellbeing Through Public Involvement in Research 2015. www.nihr.ac.uk/patients-and-public/documents/Going-the-Extra-Mile.pdf.
- Brittenden J, Cotton SC, Elders A, Tassie E, Scotland G, Ramsay CR, et al. Clinical effectiveness and cost-effectiveness of foam sclerotherapy, endovenous laser ablation and surgery for varicose veins: results from the Comparison of LAser, Surgery and foam Sclerotherapy (CLASS) randomised controlled trial. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19270.
- NHS Supply Chain . Advanced Wound Care. Dressings Price List 2017. NHS Supply Chain n.d. www.supplychain.nhs.uk/product-news/contract-launch-briefs/price-ranking/contract-info/advanced-wound-care/ (accessed 11 April 2017).
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 1 September 2018).
- Curtis L. Unit Costs of Health and Social Care 2016. Canterbury: PSSRU, University of Kent; 2016.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Personal Social Services: Expenditure and Unit Costs 2014–15. Leeds: NHS Digital; 2016.
Appendix 1 Patient and public involvement
Introduction
In addition to the ethical obligation of researchers to include patients and the public in research, the NIHR grant application process requires an element of public consultation from the outset. The benefits of public involvement have been shown throughout all stages of research, including identifying outcome measures. 79,80 More recent systematic reviews of patient involvement in research highlighted that active participation in research can lead to more relevant research by identifying patient-important outcomes and more credible results. 81,82
In order to avoid a tokenistic approach to PPI, the INVOLVE Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research were consulted from the outset to plan PPI involvement in the trial. 83 The notes reinforce the importance of early engagement of lay members to enhance inclusivity, ownership of the study and a sense of purpose.
How patient and public involvement influenced the research design
Patient consultation
The 2004 ESCHAR trial suffered from a high crossover rate, as 19% of patients randomised to surgery refused an operation and this weakened the power of the trial. 15 It was assumed that the less invasive interventional modalities employed in the EVRA trial would not have the same rate of refusal. To corroborate this assumption, a small group of patients with active leg ulceration were consulted with the proposed trial design to see if they would be willing to undergo early intervention. Almost all the patients agreed that they would have been willing to participate in the trial, as they all wished to undergo intervention to heal their ulcer, and the trial offers the possibility of being treated sooner than standard care coupled with a less invasive strategy than open surgery.
Patient collaboration
A patient with healed leg ulceration (Bruce Ley-Greaves) who had previously shown an interest in research was approached to join as a lay member co-applicant to assist in the design of the research trial and ensure that the research question and outcomes were relevant to those affected by venous leg ulceration. Lay member involvement at the design stage helped the EVRA trial team gain insight into the following (quotations from Bruce Ley-Greaves):
-
patients’ fears and lack of knowledge about procedure and options
-
thoughts on early referral and intervention (‘my ulcer would have healed quicker if I had been referred and treated promptly as intervention had an immediate impact’)
-
deciding an appropriate primary outcome measure (‘time to healing is the most important outcome as the smell associated with the ulcer affected my social confidence’), as well as important secondary outcome measures, including patient quality of life and ulcer-free time
-
the frequency of follow-ups (‘most patients would benefit from a 6-week clinic visit and monthly telephone calls to give them reassurance that they were not lost in the system as most patients are discharged out into the community post procedure’).
Role description and expectations
In line with INVOLVE guidance, a role description was drafted to detail the expectations, commitment levels of the post, details of reimbursement for travel/time and some training and support resource links, including a link to the INVOLVE jargon buster (see Appendix 10). 84
The lay member co-applicant agreed to act as our trial-specific PPI representative for the duration of the trial and join the TSC that met on an annual basis at a minimum. The trial manager and PPI representative met several times on an informal basis throughout the trial. All out-of-pocket expenses were covered for travel to the meeting and refreshments were provided at each meeting.
Trial set-up phase
As part of the set-up phase, the PPI representative reviewed all the patient-facing documents, including, but not limited to, the patient information sheet, consent form and patient diaries prior to Research Ethics Committee submission, to ensure that the language was appropriate and easily understood and that jargon was eliminated. Feedback from sites during the recruiting phase indicated that this was a successful exercise as patients needed little clarification after reading the patient information sheet.
Recruitment phase
The PPI representative attended the first TSC meeting and contributed and co-approved the charter. During the recruitment phase, he was an active member of the committee and attended all the TSC meetings, either in person or via teleconference, depending on availability.
To aid recruitment, he suggested that recruitment posters and leaflets were placed in GP surgeries to help recruit patients from primary care and contributed to the design of these. The impact of the recruitment posters and leaflets was difficult to evaluate; however, several patients who saw the posters requested referral to the recruiting hospitals and subsequently participated in the trial.
The trial manager kept in regular contact with the PPI representative in between TSC meetings to keep him engaged and informed of trial progress, particularly recruitment numbers. A shopping voucher was offered as recognition for his time.
Follow-up phase
Trial participants were e-mailed a newsletter during the follow-up phase to keep them updated on trial progress and timelines, and when they could expect to find out the results of the trial. The PPI representative helped design this newsletter, which also included a one-page article on the PPI involvement within the trial.
Results
The PPI representative attended the TSC/DMC results meeting to help provide a public/patient perspective on the interpretation of trial findings.
Dissemination
The PPI representative contributed to the design of the dissemination plan to ensure that the research team will disseminate the results to trial participants, the general public and health professionals, and has contributed to and reviewed the plain English summary for this report.
Measuring the impact of the EVRA trial
As the HTA programme has granted an extension to the trial to collect recurrence data, there will be an opportunity for the PPI representative to be involved in the adoption of the trial results in clinical practice and measuring the impact of a trial’s findings and informing future trial design.
The EVRA trial team eagerly await the findings from the current University of Oxford study Patient and Public Involvement Intervention to Enhance Recruitment and Retention in Surgical Trials (PIRRIST),85 which aims to determine if PPI can improve recruitment and retention in clinical trials.
Evaluating patient and public involvement
A PPI involvement feedback meeting was convened in August 2017 to obtain the PPI representative’s views on his involvement to date. The results of this meeting are summarised in Table 28. Written consent was obtained to use direct quotations.
| What he enjoyed the most | What he would do differently |
|---|---|
| Education about leg ulcers:Learning more about leg ulcers and their treatment . . .And:. . . the chance to see better ways of doing something | More specific jargon buster, in addition to the INVOLVE dictionary:Some form of dictionary would help, as sometimes I sat there thinking ‘what does this mean?’ but didn’t necessarily want to jump in and say ‘sorry I don’t know what you’re talking about’. . . the jargon buster is more helpful than the PPI videos as I did lose interest easily as they seemed to go on a bit |
| Repaying health-care providers:Being able to [provide] feedback [on] treatment and say thank you for previous care | Clearer reimbursement schedule, payment for time:For the number of meetings payment was not necessary as I was learning and I was paying back and giving back to the system but if I was coming more often, though, there should be some sort of payment, for example £30 to £50 |
| Being able to offer insights into personal treatment experiences, referral problems, social concerns of having an leg ulcer:Sitting at GP getting frustrated and down about the whole thing, wondered what was going to happen. I’m rotting away here | Include another representative for support and understanding:Include a second person to gain a better understanding on the basis that the two reps got together outside the meeting to debrief with the Trial Manager. That would be the advantage of having a second person |
| Seeing that his input made a difference:I was pleased to contribute to the study design and that my ideas, such as posters in the GP surgeries, ideas [sic] were listened toParticipant documents were interesting to read and I was pleased to help make items more user friendly | Pre-TSC meeting to talk through the trial manager’s report and an after-meeting debrief:I had no problems with confidence speaking up but I usually only say something when I think I have some to contribute. I didn’t like to interrupt as I thought there was a job to be done and everyone is on short timescales and things did become clear late but a chance to speak before and after the meeting to go over detail I did not understand would be useful |
| Reimbursement for time and contribution:. . . the thank you voucher was a lovely touch and reinforced the collaboration | Ensure that all participants always attend in person as easier to engage:Better to have face-to-face meetings preference. Did not feel like a burden to attend meetings and combine with other things I wanted to do, like meet friends. . . much hard to understand items when discussed over a teleconference than in person |
Interestingly, these opinions are in line with results of the 2013 Evidence Base for Patient and Public Involvement in Clinical Trials (EPIC) study,86 which concluded that involvement from patients and the public is successful if the ‘goals are clear, if there are well developed plans for PPI in a trial, and if models of PPI are more responsive and managerial (e.g. membership of a Trial Management Group) rather than restricted to general oversight (e.g. membership of a TSC)’.
Summary
Based on the findings of the lay member involvement feedback, when designing future studies the research team would aim to:
-
involve more than one member from the outset (e.g. a patient representative, someone newly diagnosed with the condition and a member of the public)
-
include the members in the Trial Management Group discussions if appropriate
-
ensure that the trial manager spends time with the members before and after meetings to explain reports and debrief
-
ensure that TSC meetings are always held face to face
-
provide an additional study-specific ‘jargon buster’ dictionary
-
ensure that the reimbursement schedule is clear at the outset and calculated in line with the INVOLVE Policy on Payments and Expenses for Members of the Public87 advice, considering an hourly rate of payment for time.
By incorporating these findings in future trial design, the EVRA trial team hopes to aid the INVOLVE vision: by 2025 INVOLVE expect ‘all people using health and social care, and increasing numbers of the public, to be aware of and choosing to contribute to research by identifying future research priorities and research questions, informing the design and development of innovations, participating in research studies, advocating for the adoption and implementation of research in the NHS’. 88
Appendix 2 Trial committees’ meeting dates
Data Monitoring and Ethics Committee
-
21 October 2013.
-
30 June 2014.
-
22 April 2015.
-
15 January 2016.
-
26 July 2016.
-
17 January 2018.
Trial Steering Committee
-
12 December 2013.
-
24 April 2014.
-
5 November 2014.
-
19 October 2015.
-
23 June 2016.
-
4 May 2017.
-
17 January 2018.
Investigator meetings
-
25 April 2013.
-
20 June 2013.
-
27 November 2013.
-
24 April 2014.
-
28 November 2014.
-
25 May 2015.
-
11 November 2015.
-
8 July 2016.
-
11 July 2017.
-
28 February 2018.
Appendix 3 Digital photograph protocol
Ulcer tracing
The ulcer size was determined at the baseline and 6-week clinic visit via manual tracing:
-
Place planimetry (with 1 cm2 markers) grids over the wound.
-
Trace around end of ulcer with an indelible pen.
-
Count the square descriptive units (cm2) and enter into InForm.
-
Scan tracing and save as PtTrialnumber_Baseline_tracing_dd/mm/yy.
-
E-mail to EVRAtrial@imperial.ac.uk via the Imperial College FileExchange: https://icseclzt.cc.ic.ac.uk/.
Digital photograph of the ulcer
The ulcer size was determined at the baseline and 6-week clinic visit via digital photograph:
-
A digital camera (minimum 8 megapixel) should be used (recommended trial camera is the Sony Cyber-shot DSC-WX60 16.2 Megapixel Digital Camera).
-
Write the patient trial ID on the 3-cm calibration strip (red and white strips found in the site file) and place in the field of vision of camera on the leg but not obscuring wound edge.
-
Enable flash (all other camera macros should be disabled, i.e. general mode).
-
Position camera 15 cm from wound perpendicular to mid-point.
-
Capture two images to ensure one suitable image for analysis.
-
If wound cannot be captured in one single image, divide wound into two or more segments and summate images.
-
Save photo as PtTrialnumber_Baseline_Photo_dd/mm/yy.
-
E-mail to EVRAtrial@imperial.ac.uk via the Imperial College FileExchange: https://icseclzt.cc.ic.ac.uk/.
Appendix 4 The EVRA flow diagram
FIGURE 16.
Early Venous Reflux Ablation flow diagram. a, Assessments of ulcer healing will be ongoing throughout the trial follow-up period and will be performed by community nursing teams and research staff (at least every month); b, once the research team has been informed by the patient that the ulcer has healed (can occur any time during the 12 months).
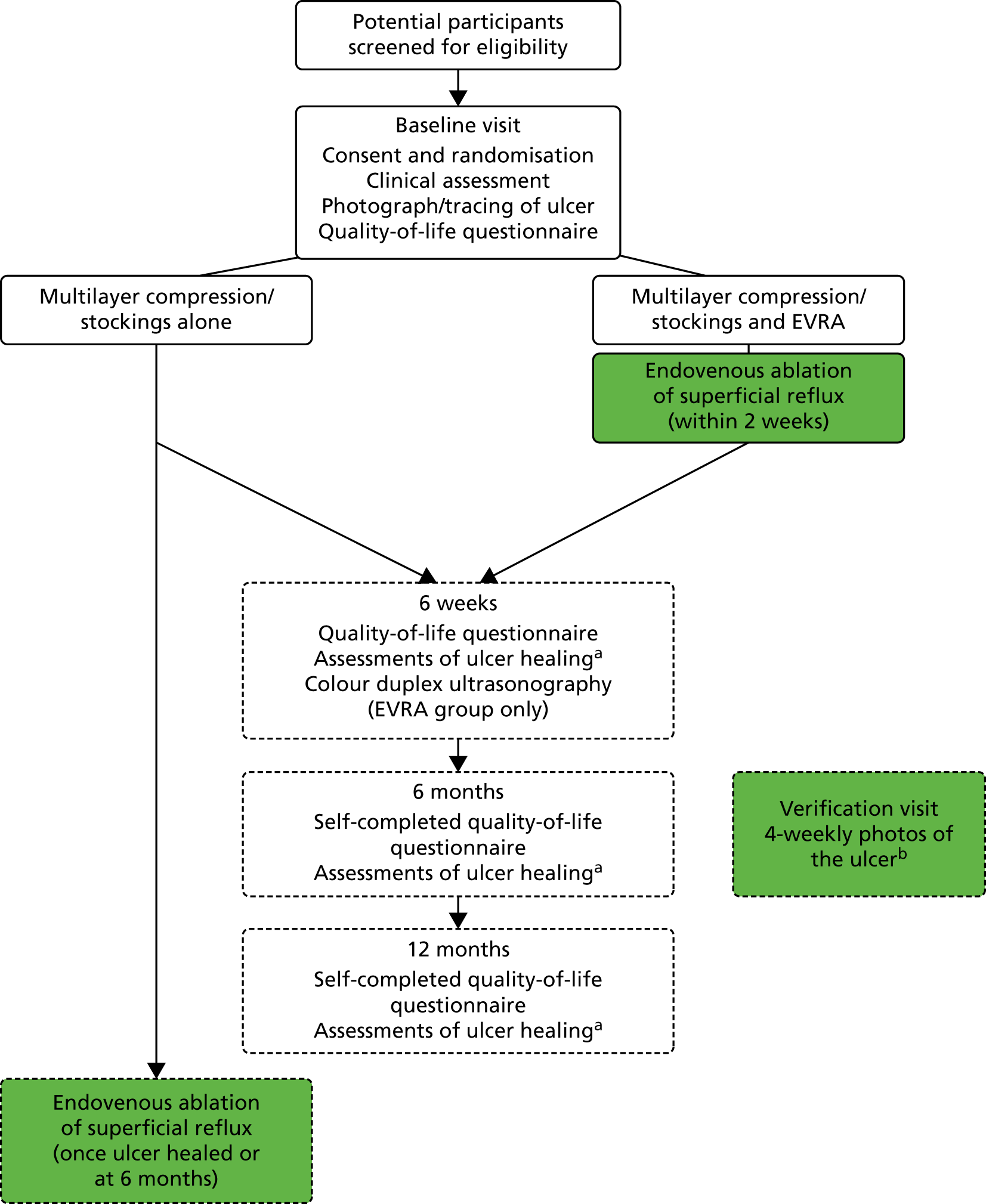
Appendix 5 Decision rules for verification of the primary outcome measure
Verification of ulcer healing will be by clinical assessment and digital photography, to be repeated weekly for 4 weeks. The digital images will be evaluated by two blinded expert assessors in order to ascertain the date of healing, which will be considered the primary healing end point. Disagreements will be resolved with involvement of a third blinded expert reviewer if necessary.
If the two blinded assessors agree that the reference ulcer has healed at the first photograph, the date of healing notification (by patient or community nurse) will be taken as the date of ulcer healing. If the two blinded assessors agree that the reference ulcer has healed at subsequent photographs, the date of those photographs will be used as the date of healing. If the two blinded assessors agree that the reference ulcer has healed at the first photograph, but the ulcer reoccurs at subsequent photographs, the date of healing from the first photograph will be used and the reoccurrence will be noted in the electronic CRF. Patients may undergo intervention for venous reflux after the first point the ulcer is confirmed healed (they do not have to wait until all four photographs are verified).
If the two assessors say ‘unsure’, then the ulcer has not healed at that point and the next photograph will be assessed.
If the two blinded assessors disagree on whether or not the reference ulcer has healed, there will be the following combinations with regard to healing:
-
Yes/unsure.
If the two assessors state ‘yes/unsure’ then the ulcer has healed, using the date provided by the assessor who said ‘yes’ or if the first photograph, the date of healing notification will be used.
-
No/unsure.
If the two assessors state ‘no/unsure’ then the ulcer has not healed.
-
Yes/no.
If the two assessors state ‘yes/no’ a third assessor will be consulted and will decide if the ulcer is healed or not. The third assessor’s decision will be final. If they are unsure whether or not the ulcer has healed, the ulcer will be considered unhealed.
If no photographs of the reference ulcer are available, the unblinded date and the treating nurse/GP recorded will be used if available.
If the (treating) nurses state that the wound is healed and stop taking photographs but blinded assessors say that the wound is not healed, then the wound is considered healed.
If photographs are taken of a participant for > 12 months and the date of healing occurs beyond 12 months post randomisation, then the participant will be regarded as unhealed at 12 months.
Photographs taken after a large interval of time has elapsed (i.e. ≥ 1 month) since the due date of the last healed photograph (post-healed photograph 4), will not be included in the blinded outcome assessment.
Appendix 6 Health economic unit costs
| Resource | Unit cost (£) | Assumption | Source |
|---|---|---|---|
| Index procedure | |||
| Staff procedure costs | |||
| EVLA | 5.49/minute | Assumed same cost/minute for RFA | Brittenden et al. 201589 |
| UGFS | 4.67/minute | Assumed same cost/minute for MOCA | Brittenden et al. 201589 |
| Disposable kit or catheter prices | |||
| EVLA | 238.60 | Angiodynamics (Caley Kitchen, 14 February 2018, personal communication). List price catheter £200. Generator £22,000 (assuming 2-year life, 600 procedures in total, cost of capital 3.5% per year). This gives an annuitised cost per procedure of £38.60 | |
| RFA | 543 | Harriet Ellis, Imperial College Healthcare NHS Trust, 16 November 2017, personal communication. Includes generator rental | |
| MOCA | 375 | Harriet Ellis, personal communication | |
| Other theatre consumables and anaesthetic | |||
| EVLA | 66 | Brittenden et al. 201589 | |
| RFA | 66 | Assumed same cost as EVLA | |
| UGFS | 50 | Brittenden et al. 201589 | |
| MOCA | 50 | Assumed same cost as UGFS | |
| Other costs of vein ablations (pre-procedure and recovery) | |||
| EVLA | 72 | Brittenden et al. 201589 | |
| RFA | 72 | Assumed same cost as EVLA | |
| UGFS | 42 | Brittenden et al. 201589 | |
| MOCA | 42 | Assumed same cost as UGFS | |
| Consumables ulcer healing | |||
| KTwo compression bandages | 7.84 | Assumed changed two times per week until healing | NHS supply chain90 |
| VenoTrain® ulcertec compression stockings (Bauerfeind, London UK) | 27.10 | Assumed two pairs changed every 3 months until healing | NHS Supply Chain90 |
| Ulcer dressing | Assumed changed two times per week until healing | ||
| NA dressing | 11.20 (for 40) | NHS Supply Chain90 | |
| Inadine 9.5 × 9.5 cm | 15 (for 25) | NHS Supply Chain90 | |
| Atrauman® dressing (Paul Hartmann Ltd., Heywood, UK) | 10.89 (for 30) | Assumed used if no other information provided | NHS Supply Chain90 |
| Consumables after healing to prevent recurrence | |||
| Class 2 compression stocking | 31.27 | Assumed changed every 3 months | NHS Supply Chain90 |
| Admissions to hospital (other than vein procedures) | |||
| Overnight stay without procedure | 265/night | NHR Reference Costs 2015 to 2016.91 Excess bed-day: peripheral vascular disorders with CC score 2–4 | |
| Spinal surgery | 4142 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Shoulder replacement | 5110 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient91 |
| Ankle surgery | 2667 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Hip replacement | 5877 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Knee replacement | 5745 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Cataract | 917 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Hernia repair | 1726 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Pacemaker | 2063 | Not ulcer related | NHR Reference Costs 2015 to 2016.91 Elective inpatient |
| Angiography and stent | 1449 | Ulcer related | NHR Reference Costs 2015 to 2016.91 Day case |
| Follow-up outpatient visit | |||
| Without procedure | 140/visit | NHR Reference Costs 2015 to 2016.91 Vascular surgery | |
| Office-based sclerotherapy | 245/visit | Brittenden et al. 201589 | |
| Primary care | |||
| Visit to district nurse/general practice nurse/vein clinic | 38/visit | Assume 15.5 minutes | NHR Reference Costs 2015 to 2016 91 |
| District nurse home visit | 72/visit | Includes travel time | NHR Reference Costs 2015 to 2016 91 |
| Visit to GP | 36/visit | PSSRU 201692 | |
| GP home visit | 88/visit | Includes travel time | PSSRU 2015.93 Expenditure and unit costs |
| Other health care use | |||
| Occupational therapist | 79/visit | NHR Reference Costs 2015 to 2016 91 | |
| Physiotherapist | 49/visit | NHR Reference Costs 2015 to 2016 91 | |
| Home carer visit | 38/visit | Nursing care | Assume same as district nurse |
| Home help visit | 29/visit | Personal care | NHS Digital.94 Expenditure and unit costs |
| Medicines68 | |||
| Apixiban 2.5 mg | 4.40/day | 5 mg BD every day | |
| Aspirin 75 mg | 0.03/day | 75 mg OD every day | |
| Clopidogrel 75 mg | 0.06/day | 75 mg OD every day | |
| Dalteparin 12,500 units/ml | 20.32/day | Males | For average weight 96 kg, 18,000 units/day |
| 14.12/day | Females | For average weight 80 kg, 12,500 units/day | |
| Warfarin | 0.04/day | ||
| Rivaroxaban 10 mg | 3.60/day | 20 mg OD | |
| Clexane (Enoxaparin) | 11.02/day | Male | 1.5 mg/kg OD |
| 7.84/day | Female | ||
| Dabigatran 150 mg | 1.70/day | 150 mg BD | |
Appendix 7 Recruitment per centre
| EVRA site | Participants recruited, n |
|---|---|
| Imperial College Healthcare NHS Trust | 45 |
| Cambridge University Hospitals NHS Foundation Trust | 27 |
| Worcestershire Acute Hospitals NHS Trust | 20 |
| North West London Hospitals NHS Trust | 29 |
| Gloucestershire Hospitals NHS Foundation Trust | 124 |
| Heart of England NHS Foundation Trust | 51 |
| University Hospitals Birmingham NHS Foundation Trust | 9 |
| North Cumbria University Hospitals NHS Trust | 32 |
| The Dudley Group NHS Foundation Trust | 8 |
| Royal Wolverhampton NHS Trust | 3 |
| York Teaching Hospital NHS Foundation Trust | 2 |
| Hull and East Yorkshire Hospitals NHS Trust | 7 |
| Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust | 22 |
| Frimley Health NHS Foundation Trust | 6 |
| University Hospitals Plymouth NHS Trust | 23 |
| Bradford Teaching Hospitals NHS Foundation Trust | 6 |
| Salisbury NHS Foundation Trust | 5 |
| Leeds Teaching Hospitals NHS Trust | 4 |
| Sheffield Teaching Hospitals NHS Foundation Trust | 6 |
| Taunton and Somerset NHS Foundation Trust | 21 |
Appendix 8 Recruitment graph
FIGURE 17.
The EVRA trial recruitment graph (target vs. actual recruitment). Initial target recruitment was 24 participants a month, which was revised to 13 participants a month in October 2015. The target of 450 participants was achieved in September 2016.
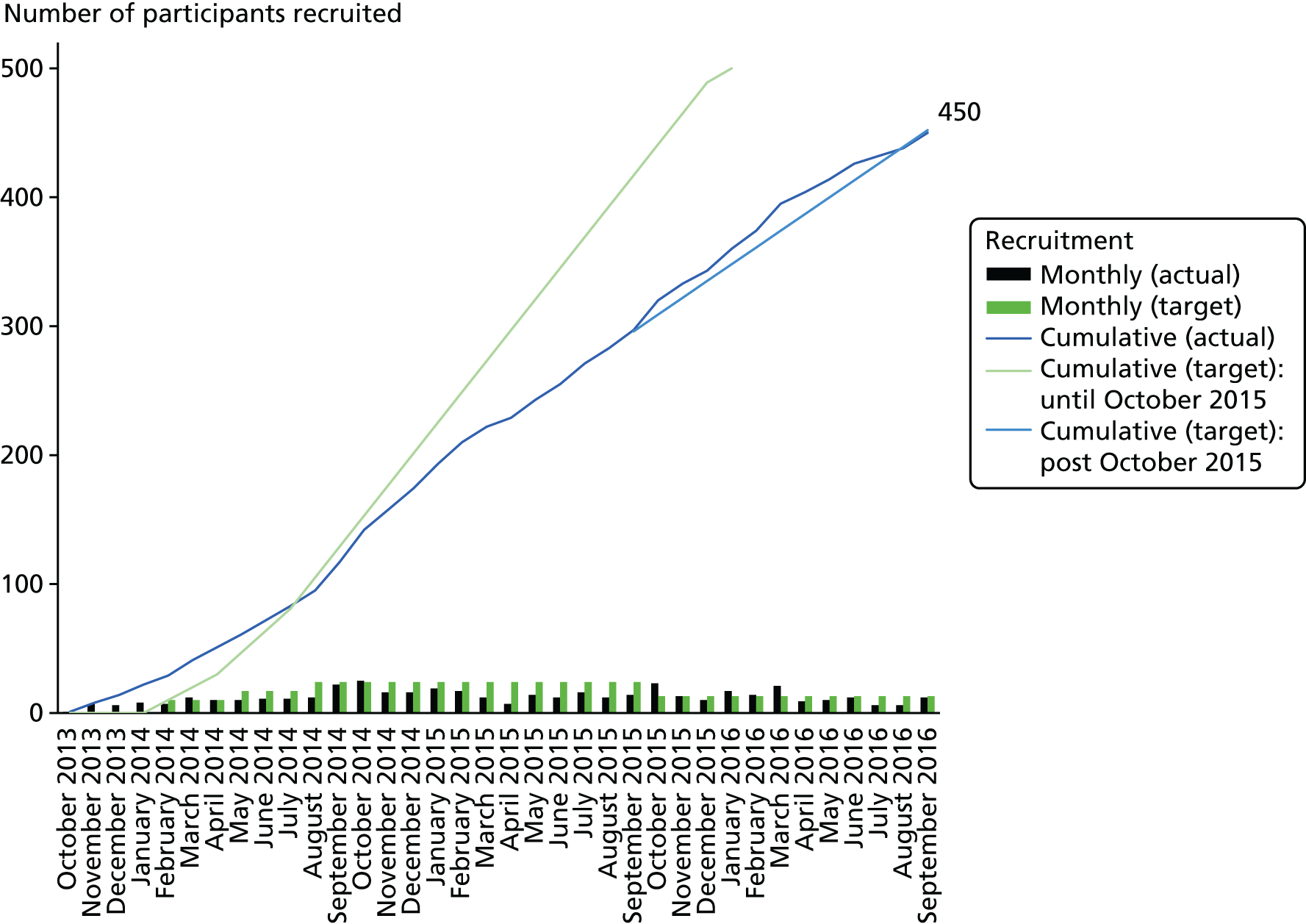
Appendix 9 Total resource use
The following table shows the total resource use reported during the trial (n = 450), and mean (SD) cost per participant with 1 year of follow-up (n = 419). Analyses without imputation.
| Resource type | Resource use (total) | Early (N = 208), mean cost (£) | SD (£) | Deferred (N = 211), mean cost (£) | SD (£) | |
|---|---|---|---|---|---|---|
| Early (N = 224) | Deferred (N = 226) | |||||
| Treatment visits in the trial leg | 523 | 368 | 370 | 369 | ||
| Number of procedures | 7 | 55 | ||||
| One or more procedure | 217 | 171 | ||||
| Two or more procedures | 45 | 24 | ||||
| Three or more procedures | 6 | 7 | ||||
| Four procedures | 0 | 1 | ||||
| Compression and dressings until healing (cost) | 229 | 230 | 255 | 242 | ||
| Compression stockings after healing (cost) | 87 | 33 | 77 | 39 | ||
| Hospital inpatient and day case admissions, not recorded as trial procedures | 27 | 16 | 227 | 693 | 207 | 1526 |
| Of which further ablation procedures, not recorded as trial procedures | 12 | 5 | ||||
| Visits to district nurse | 1947 | 2196 | 102 | 148 | 112 | 169 |
| Visits from district nurse | 624 | 1025 | 220 | 804 | 366 | 1263 |
| Visits to GP | 528 | 546 | 89 | 84 | 91 | 92 |
| Visits from GP | 23 | 49 | 9 | 28 | 20 | 56 |
| Outpatient consultations and procedures, not recorded as trial procedures | 807 | 731 | 588 | 851 | 527 | 952 |
| Of which further ablation procedures, not recorded as trial procedures | 73 | 69 | ||||
| Occupational therapy (visits) | 6 | 14 | 2 | 17 | 5 | 24 |
| Warfarin | 1 | 4 | 2 | 4 | ||
| Rivarox | 16 | 106 | 24 | 159 | ||
| Apixaban | 13 | 114 | 1 | 9 | ||
| Dalteparin | 2 | 29 | 10 | 65 | ||
| Dabigatran | 0 | 4 | 2 | 36 | ||
| Enoxaparin | 0 | 0 | 2 | 23 | ||
| Clopidogrel | 1 | 3 | 1 | 3 | ||
| Aspirin | 2 | 4 | 2 | 4 | ||
| Physiotherapy | 106 | 247 | 25 | 109 | 57 | 285 |
| Home care | 1413 | 1573 | 257 | 1593 | 262 | 1207 |
| Home help | 875 | 882 | 121 | 799 | 121 | 646 |
| Total cost | 2514 | 2770 | 2516 | 3242 | ||
| Hospital admissions unrelated to venous leg ulcer | 59 | 31 | 342 | 1435 | 192 | 1340 |
| Outpatient visits unrelated to venous leg ulcer | 151 | 156 | 98 | 207 | 103 | 414 |
| Out-of-pocket expenses | 87 | 122 | ||||
| Unpaid carer (days) | 4673 | 5132 | ||||
| Off-work days | 921 | 1458 | ||||
| Normal days lost | 4068 | 4947 | ||||
Appendix 10 Lay member role description
List of abbreviations
- ABPI
- ankle–brachial pressure index
- AE
- adverse event
- AVVQ
- Aberdeen Varicose Vein Questionnaire
- BMI
- body mass index
- CCG
- Clinical Commissioning Group
- CEAP
- clinical, aetiological, anatomical and pathophysiological
- CI
- confidence interval
- CRF
- case report form
- DMC
- Data Monitoring Committee
- DVT
- deep-vein thrombosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESCHAR
- Effect of Surgery and Compression on Healing And Recurrence
- EVLA
- endovenous laser ablation
- EVRA
- Early Venous Reflux Ablation
- GP
- general practitioner
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICTU
- Imperial Clinical Trials Unit
- IQR
- interquartile range
- ITT
- intention to treat
- KM
- Kaplan–Meier
- MOCA
- mechanochemical endovenous ablation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PCT
- primary care trust
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RFA
- radiofrequency laser ablation
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-36
- Short Form questionnaire-36 items
- TSC
- Trial Steering Committee
- UGFS
- ultrasonography-guided foam sclerotherapy
- VCSS
- Venous Clinical Severity Score
- WTP
- willingness to pay
