Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/53/31. The contractual start date was in May 2011. The draft report began editorial review in September 2017 and was accepted for publication in February 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Nicholas JA Webb has served on advisory boards within the past 5 years for AbbVie Inc. (North Chicago, IL, USA), Alexion Pharmaceuticals (New Haven, CT, USA), AMAG Pharmaceuticals Inc. (Waltham, MA, USA), Astellas Pharma Inc. (Tokyo, Japan), Raptor Pharmaceuticals (Novato, CA, USA), Takeda Pharmaceutical Company (Osaka, Japan) and UCB (Union Chimique Belge) (Brussels, Belgium). These have related to the design and conduct of early-phase trials in childhood kidney disease. None has been related to the treatment of corticosteroid-sensitive nephrotic syndrome. Since August 2018, Nicholas JA Webb has been Translational Medicine Discovery Director, Renal and Transplantation, at Novartis Institutes for BioMedical Research. Carole Cummins has received grants from Kidney Research UK and Kids Kidney Research.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Webb et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Idiopathic nephrotic syndrome (INS) is the most common glomerular disorder of childhood, with an incidence of 2 per 100,000 child population in the UK. The disease presents at a median age of 2 to 3 years and is twice as common in boys than in girls. 1,2 There is ethnic variability in the disease incidence, with a fourfold to sixfold higher incidence in the UK South Asian population. 1,3,4
The onset of INS is characterised by the acute onset of heavy proteinuria, resulting in the development of hypoalbuminaemia and generalised oedema. There is not infrequently a delay in diagnosis, with the child having being treated for allergy prior to eventual presentation to a paediatrician or paediatric nephrologist as an emergency. The disease pathogenesis is poorly understood; however, both in vitro and in vivo experiments have identified the immune system to be dysregulated at the time of disease onset. 5 The presence of nephrotic syndrome places the child at increased risk of a number of complications, including thromboembolic disease and infection, particularly with Streptococcus pneumoniae. Prior to the development of adequate antibiotic and remission-inducing therapy, the mortality rate from INS was of the order of 50%, the majority of deaths being related to infection. 6,7
In excess of 90% of children who present with INS will respond to a course of high-dose corticosteroid therapy. 8 For this reason, the majority are treated empirically with a course of corticosteroids without a renal biopsy being performed. Those who respond to the treatment are given the diagnostic label of having steroid-sensitive nephrotic syndrome (SSNS). Only those with atypical features at presentation (age < 12 months or over 12 years, persistent hypertension or impaired renal function, gross haematuria, low plasma C3, hepatitis B or C virus positivity) and those who do not respond to this initial course of corticosteroid therapy undergo renal biopsy. 9 This is in contrast to practice in adult patients with nephrotic syndrome, in whom the causes of nephrotic syndrome are diverse and biopsy at presentation is routinely performed to establish a histological diagnosis and to guide subsequent therapy. Little emphasis is placed upon histological diagnosis in children with SSNS, as it has been shown that corticosteroid sensitivity rather than histology is the key prognostic indicator. 10 Those children who respond to corticosteroids generally have a good long-term prognosis with a low risk of developing chronic kidney disease; in contrast, those who are corticosteroid unresponsive suffer significant morbidity, and around 50% will progress to end-stage kidney failure, necessitating dialysis and kidney transplantation over a 15-year period. 11 The majority of these children will have focal segmental glomerulosclerosis on renal biopsy. In an early seminal study, conducted by the International Study of Kidney Disease in Children (ISKDC),2 a large cohort of children underwent renal biopsy at presentation prior to the commencement of corticosteroid therapy. The majority of those who responded to corticosteroids were noted to have minimal change disease (MCD) histology, so called because the appearance of the kidney tissue at light microscopic level is essentially normal. Somewhat confusingly, in much of the published literature, the terms MCD and SSNS are used interchangeably, although this is not strictly correct, as a small number of children with MCD do not respond to corticosteroids and, similarly, a small number of corticosteroid-sensitive children have a histological diagnosis other than MCD.
Following initial successful treatment with corticosteroids, around 80% of children with SSNS develop disease relapses necessitating further courses of high-dose prednisolone, and around 50% develop frequently relapsing nephrotic syndrome (FRNS), which is defined as two or more relapses within the first 6 months following presentation or four relapses within any 12-month period, or steroid-dependent nephrotic syndrome (SDNS), which is defined as relapses occurring within 14 days of discontinuation of corticosteroid therapy. 12 Similar to the presenting episode, nephrotic syndrome relapses are associated with a risk of significant complications, including sepsis, thrombosis, dyslipidaemia and malnutrition. 7 The treatment of relapses with repeated courses of high-dose prednisolone is associated with major adverse effects, including hip avascular necrosis, growth failure, hypertension, obesity, diabetes and behavioural problems. 13,14 Furthermore, children frequently have to miss school during relapses, resulting in impaired academic performance and parental absence from work.
When complications of repeated courses of corticosteroids develop, or when they are expected, a range of immunosuppressive strategies are employed in an attempt to reduce the frequency of disease relapses. These include the use of long-term, low-dose, alternate-day prednisolone, as well as a range of non-corticosteroid immunosuppressive agents, including levamisole, cyclophosphamide, ciclosporin, tacrolimus, mycophenolate mofetil and rituximab.
Early follow-up studies8,15 suggested that the long-term prognosis for children with SSNS was excellent, with all retaining normal kidney function and over 90% achieving long-term remission, with complete cessation of relapses by the end of puberty. However, subsequent studies have reported higher rates of relapsing disease persisting beyond childhood, with 19% of UK patients suffering ongoing relapses into early adult life. 16 In the majority of patients who achieve permanent remission during childhood, relapse occurs at 13–16 years of age. 17 However, by this time, the majority of affected children will have received a significant cumulative corticosteroid dose, and many will have been exposed to other immunosuppressive agents. The role of the paediatric nephrologist is to maintain the child with SSNS as being well and free from relapses, while at the same time minimising the adverse effects of exposure to corticosteroids and other immunosuppressive therapies, thus ensuring that they emerge as healthy adults free from relapses and with no significant long-term treatment-related morbidity.
The ideal initial corticosteroid regimen for use at presentation of childhood INS should rapidly induce urinary remission (defined as 3 consecutive days of zero or trace proteinuria) with resolution of oedema. It must be sufficient to prevent frequent relapses necessitating the use of alternative immunosuppressive agents, although not so intensive that serious corticosteroid-related AEs develop. The first standardised corticosteroid treatment regimen was introduced by the ISKDC in the 1960s and consisted of a dose of 60 mg/m2 of prednisone (maximum 80 mg) given daily for 4 weeks followed by 40 mg/m2 (maximum 60 mg) on 3 consecutive days out of 7 for a total of 4 weeks. 18 Many centres made a minor modification whereby 40 mg/m2 was administered on alternate days rather than on 3 days out of 7 during the second 4-week period. Centres in the UK adopted the use of prednisolone rather than prednisone, as this was and remains the corticosteroid in routine use in UK paediatric practice, although children in the USA and other parts of Europe have continued to receive prednisone. These two agents are very closely related, with prednisone being metabolised to the active prednisolone following absorption.
Following the introduction of the ISKDC regimen, there has been significant debate regarding the optimal prednisolone regimen at the time of presentation of SSNS, and a number of randomised controlled trials (RCTs)19–28 have investigated whether giving a more or less intensive corticosteroid regimen at first presentation of INS affects the number of children suffering both disease relapses and adverse effects of corticosteroid therapy. One single RCT19 has shown that, compared with the ISKDC regimen, a less intensive regimen comprising a daily dose of 60 mg/m2 of prednisone only until the urine was negative for 3 consecutive days (urinary remission, median time 14 days) followed by 4 weeks of 40 mg/m2 of prednisone on alternate days resulted in a higher rate of disease relapse. No further studies have investigated this therapeutic strategy. By 2005, when the PREDnisolone in NephrOtic Syndrome (PREDNOS) study was being planned, a total of six RCTs20–25 had compared 2 months of prednisolone using the ISKDC regimen with a variety of different regimens of ≥ 3 months in duration. These regimens intensified the initial prednisolone regimen by increasing the duration of both the daily and alternate daily prednisolone phases. A Cochrane review29 of these six studies concluded that intensification of the initial corticosteroid therapy at disease presentation significantly reduced the rate of relapse at 12–24 months [risk ratio 0.7, 95% confidence interval (CI) 0.58 to 0.84]. There was an inverse linear relationship between treatment duration and risk of relapse [relative risk (RR) 1.26–0.112 duration; p = 0.03]. Furthermore, there was a significant reduction in the number of frequent relapsers and the mean relapse rate per participant per year. In addition to these six studies comparing the ISKDC regimen with longer duration corticosteroid regimens, a further four studies23,26–28 compared 3 months’ treatment with prednisolone with 6 months’ treatment; two were published only in abstract form. 27,28 Longer therapy duration resulted in a significantly decreased risk of relapse at 6 and 12 months (risk ratio 0.48, 95% CI 0.35 to 0.64, and risk ratio 0.57, 95% CI 0.45 to 0.71, respectively). Furthermore, the number of participants who developed FRNS was also lower in the 6-month group than in the 12-month group (risk ratio 0.55, 95% CI 0.38 to 0.80). Further analysis suggested that the benefits of more intensive corticosteroid therapy were more likely to be related to the increased duration of treatment than the higher cumulative dose; however, collinearity between treatment duration and dose prevented the Cochrane group29 from drawing definitive conclusions.
However, significant concerns were raised on a number of issues relating to the six studies contributing to the meta-analysis comparing 2 months of treatment with treatment for ≥ 3 months. The total number of participants was small, at only 520 participants across all six studies, and concerns were expressed about the quality of a number of these trials. One was (and remains) unpublished,22 with data available only in abstract form. None was placebo controlled or blinded in any way, and only two were at low risk of bias for allocation concealment. Trials with inadequate allocation concealment can exaggerate the efficacy of the experimental treatment by 30–40%, and meta-analysis of low-quality trials may overestimate the benefit of therapy. 30,31 Furthermore, only one of these trials was analysed on an intention-to-treat (ITT) basis; however, this same study indicated, in the discussion, that parents could exert some influence on which treatment group their child was allocated to, implying that the randomisation process was flawed. 23 It was also unclear whether or not there was a clinically useful reduction in the incidence of steroid-dependent disease and the use of second-line immunosuppressive agents. The studies also reported somewhat different corticosteroid-related adverse events, making interpretation of the impact that increased duration of corticosteroid therapy had on adverse effect profile difficult. Therefore, the authors of the Cochrane review29 (Dr Elisabeth Hodson and Professor Jonathan Craig) concluded that further well-designed and adequately powered RCTs were required to establish the optimum dose and duration of treatment, and were consulted from an early stage regarding the design of the PREDNOS study.
There has continued to be great debate regarding what the ideal corticosteroid regimen at disease presentation should be, and there is considerable variation in the treatment regimens used. Kidney Disease Improving Global Outcomes guidelines32 published in 2013 supported the conclusions of the Cochrane review,29 recommending that INS in children be treated initially with 60 mg/m2 or 2 mg/kg of prednisone or prednisolone for at least 12 weeks (4–6 weeks daily followed by 40 mg/m2 or 1.5 mg/kg every other day), followed by a slow tapering of dose over the next 2–5 months. Despite these recommendations, in the UK the majority of centres have continued to use the 8-week ISKDC regimen, as is the case in Canada, Nigeria and South Korea. In contrast, Germany, the Netherlands, Spain and other European nations have adopted a longer treatment regimen, as proposed by the Arbeitsgemainschaft für Pädiatrische Nephrologie (APN, the German Society for Paediatric Nephrology) and investigated in its RCT. 20 This consists of 6 weeks of daily prednisolone at a dose of 60 mg/m2 followed by 6 weeks of alternate daily prednisolone at 40 mg/m2. In France, a longer 18-week course of prednisone is in routine use. 33 A questionnaire survey reported significant heterogeneity in the regimens used in centres in the USA, with 13% using the ISKDC regimen, 7% using the APN regimen and many using either of these with a subsequent corticosteroid taper. 34 This genuine clinical equipoise confirmed the importance of conducting a high-quality RCT to determine whether or not extending the course of prednisolone beyond that recommended by the ISKDC was associated with improved clinical outcomes in UK children. We chose time to first relapse as our primary outcome measure and, following consultation with the British Association for Paediatric Nephrology and our patient group advisers from the Nephrotic Syndrome Trust (NeST) and the Renal Patient Support Group, we selected secondary outcome measures that were felt to be of clinical importance, including the incidence of FRNS and SDNS and the need for alternative, potentially more potent, immunosuppressive therapies. Given the paucity of high-quality information on the adverse effect profiles of standard course (SC) and extended course (EC) treatment courses of prednisolone, we also aimed to collect comprehensive adverse effect data, including abnormal behaviour, which was assessed through the use of the Achenbach Child Behaviour Checklist (ACBC). Abnormal behaviour is one of the most commonly reported adverse events (AEs) in routine clinical practice; however, it was rarely reported on in previously conducted clinical trials. Finally, we aimed to perform a detailed cost-effectiveness analysis to determine the relative cost and efficacy of the two regimens in quality-adjusted life-year (QALY) terms.
Since the commencement of the PREDNOS study, three further studies have reported their findings. 33,35,36 A well-conducted double-blind placebo-controlled RCT33 performed in the Netherlands aimed to ascertain whether the apparently better outcomes associated with prolonged prednisolone treatment occurred as a result of the increased duration of treatment or the higher cumulative dose of prednisolone administered. One hundred and fifty Dutch children were randomised to receive 3 months of prednisolone followed by 3 months of placebo or 6 months of prednisolone; both groups received equal cumulative doses of prednisolone (3360 mg/m2, the same dose as that administered in the APN regimen) and were followed up for a median of 47 months. One hundred and twenty-six children commenced trial medication. A primary end point of the development of FRNS was selected and no difference was detected (45% with 3 months of prednisolone vs. 50% with 6 months of prednisolone). There was no difference in the number of participants who developed relapses (77% vs. 80%), the number requiring alternative immunosuppressive agents or the number of AEs. The authors concluded that the reduced relapse rate associated with longer prednisolone regimens observed in previous studies most likely occurred as a result of the increased cumulative prednisolone dose administered rather than the lengthening of the duration of the treatment course.
More recently, two high-quality studies,35,36 published alongside one another in Kidney International with an accompanying editorial by Hoyer,37 reported outcomes that differed significantly from those reported in the Cochrane review. 13 Sinha et al. 35 from New Delhi and four other Indian centres enrolled 181 children aged 1–12 years presenting for the first time with INS. Participants were treated with a dose of prednisone 2 mg/kg daily for 6 weeks followed by 1.5 mg/kg on alternate days for a further 6 weeks, and were then randomised in a double-blind manner to receive either placebo or prednisone in decreasing doses for a further 3 months. The total dose of prednisone received was 3530 mg in the 6-month group and 2792 mg in the 3-month group. There was no difference between the two groups in the chosen primary end point [the number of relapses per 12 months of follow-up (1.26 vs. 1.54, respectively; p = 0.21)] or the percentage of participants with relapses or frequent relapses. There was no significant difference in the mean time to first relapse. The authors concluded that extending initial prednisolone treatment from 3 to 6 months did not influence the course of illness in children with SSNS. The second study36 randomised 255 Japanese children presenting with INS to either the ISKDC regimen (total dose of 2240 mg/m2) or a 6-month prednisolone regimen comprising 4 weeks of daily prednisolone followed by 20 weeks of tapering alternate-day prednisolone (total dose of 3885 mg/m2). Median follow-up was 36.7 months in the 2-month group and 38.2 months in the 6-month group. The chosen primary end point was the time to development of FRNS and was similar in both groups [hazard ratio (HR) 0.86, 90% CI 0.64 to 1.16]. The time to first relapse was also similar in both groups, as was the number of relapses per year. The frequency and severity of AEs were similar in both groups, despite the 6-month group receiving a significantly higher median cumulative dose of prednisolone over 2 years. Yoshikawa et al. 36 concluded that prolongation of the initial corticosteroid regimen from 2 to 6 months did not improve patient outcomes.
Following the publication of these studies, in 2015 the Cochrane group performed an update of their systematic review and meta-analysis. 13 They reported that the addition of these three well-designed studies had changed the conclusion of their review. They noted that studies of long versus shorter duration of corticosteroid treatment had heterogeneous treatment effects, with the older studies that were rated as having a higher risk of bias tending to overestimate the effect of longer-course therapy compared with more recently published studies rated as having a low risk of bias. Among the studies rated as having a low risk of bias, the group found that there was no significant difference in the risk of FRNS between those given prednisolone for 2 or 3 months and those receiving treatment for longer durations or a higher total dose, indicating that there is no benefit of increasing the duration of prednisolone beyond 2 or 3 months in the initial episode of SSNS. 13
Chapter 2 Methods
Trial-related information, including the protocol, study information sheets, consent and assent forms and the case report forms, is available at the PREDNOS website (www.birmingham.ac.uk/prednos; accessed 17 August 2017).
Objectives
The aim of the PREDNOS study was to compare treatment with an EC (16-week) prednisolone regimen with the SC (8-week) regimen, as proposed by the ISKDC for UK children presenting with their first episode of SSNS.
The specific study objectives were:
-
to determine whether or not an EC of prednisolone increases the time to first relapse in children presenting with SSNS
-
to determine whether or not an EC of prednisolone –
-
reduces the relapse rate
-
reduces the proportion of children who develop FRNS or SDNS
-
reduces the requirement for second- and third-line immunosuppressive agents, including levamisole, cyclophosphamide, ciclosporin, tacrolimus, mycophenolate mofetil and rituximab
-
is associated with an increased incidence of corticosteroid-related AEs, including behavioural problems
-
is more cost-effective than SC therapy.
-
Trial design
Randomised double-blind, parallel-group, placebo-controlled trial with health economic evaluation. The participant, clinician and study teams were masked to treatment allocation.
Participants
Inclusion criteria
Children presenting with their first episode of SSNS who met all of the following criteria were included in the study:
-
a urine albumin-to-creatinine ratio of > 200 mg/mmol or a protein-to-creatinine ratio of > 200 mg/mmol, determined quantitatively on an early-morning urine sample
-
a serum or plasma albumin level of < 25 g/l
-
aged between 1 and 15 years at the time of diagnosis
-
no prior therapy with corticosteroids or immunosuppressive or cytotoxic agents for any form of renal disease (other than the 28 days of prednisolone therapy given initially as routine clinical practice)
-
no evidence of underlying systemic disorder or exposure to agents known to be associated with newly presenting SSNS
-
informed consent by parent(s) (the term ’parent’ has been used throughout this report to reflect mother, father or legal guardian) and assent by participant when age appropriate.
Exclusion criteria
Study exclusion criteria were:
-
histological changes other than minimal lesion glomerulonephritis when renal biopsy has been undertaken
-
a prior history of poor adherence with medical therapy
-
known allergy to prednisolone.
Rationale for choice of inclusion and exclusion criteria
Children aged < 12 months were excluded, as nephrotic syndrome presenting in this age group is rarely corticosteroid sensitive and treatment with an empirical course of corticosteroids is not standard clinical practice. Infants in this age group are classified as having either congenital nephrotic syndrome (if < 3 months of age at presentation) or infantile nephrotic syndrome (if between 3 and 12 months of age at presentation). The causes of congenital and infantile nephrotic syndrome are frequently genetic, occurring as a consequence of mutations in genes expressing proteins in the podocyte, and are commonly unresponsive to any form of immunosuppressive therapy. Children ≥ 15 years of age were excluded because of the reduced likelihood of their nephrotic syndrome being corticosteroid sensitive. In this age group, the causes of INS are much more similar in relative frequency to the causes of INS in adults, with a higher incidence of membranous nephropathy and focal segmental glomerulosclerosis and a correspondingly lower incidence of MCD. Because of this, it is routine practice for renal biopsy to be performed to establish the histological diagnosis and to guide subsequent therapy, rather than the administration of an empirical course of prednisolone therapy.
The inclusion and exclusion criteria were otherwise selected to ensure that the study population was truly representative of the population of children presenting with SSNS in the UK for whom corticosteroid treatment would be appropriate. Given the significantly increased incidence of SSNS in the UK South Asian population, we made steps to ensure that we recruited a substantial number of participants from this group, specifically targeting study sites in areas with large South Asian communities.
Recruitment and randomisation
Study sites
One hundred and twenty-four district general hospitals and tertiary regional paediatric nephrology centres throughout the UK took part in the study. An additional centre participated in follow-up visits only. A flexible arrangement was set up whereby a child could be referred into the regional paediatric nephrology centre to allow them to participate in the study if this was not possible in the district general hospital.
Initial prednisolone treatment
Existing national and local protocols for the treatment for idiopathic childhood nephrotic syndrome all commence with the administration of 60 mg/m2 of prednisolone (maximum 80 mg) daily for a total of 4 weeks. Therefore, all children who presented to PREDNOS study sites with INS were treated with this regimen while consideration was given to whether or not they were a suitable candidate for the study. The most important determinant of this was whether or not their nephrotic syndrome proved to be corticosteroid sensitive, that is, the urine dipstick test became negative or trace for 3 consecutive days, indicating resolution of the proteinuria and establishment of a diagnosis of SSNS. As a result, children subsequently recruited into the study had all received a dose of 60 mg/m2 of prednisolone (maximum 80 mg) daily for 28 days in an open-label manner prior to the commencement of randomised study medications. Investigators were asked to attempt to standardise the prednisolone preparation that they used in all newly presenting children to ensure uniformity during this initial 4-week period. It was recommended that non-enteric-coated prednisolone tablets be used and that, if required for younger participants and others who were unable to swallow tablets whole, tablets be crushed with a proprietary tablet crusher. This ensured that potential participants would be able to take the randomised study drug, which was provided as a non-soluble non-dispersible tablet.
Recruitment and randomisation took place once it was thought that the child was corticosteroid sensitive; this generally occurred at between 14 and 21 days following commencement of open-label prednisolone therapy. This strategy ensured that participants recruited into the study had become, or were likely to become, corticosteroid sensitive and also allowed sufficient time for the principal investigator to obtain fully informed consent (see Informed consent). This timing of recruitment also meant that there was sufficient time for the study drug to be delivered to the family home (by Royal Mail Special Delivery) before day 29, the first day of scheduled treatment following the completion of 28 days of open-label prednisolone treatment. This approach to recruitment and randomisation was felt to be preferable to recruiting participants at the time of initial presentation, prior to the commencement of any prednisolone therapy, as the alternative would have resulted in more participants who were not steroid sensitive being included in the trial, which would have resulted in a higher drop-out rate.
Informed consent
The informed consent process was supported by the use of parent information sheets and patient information sheets for older participants who were felt by the study principal investigator to be able to understand these. Parents, and, when appropriate, participants, received a full explanation of the aim, trial treatment, expected benefits and potential hazards of taking part in the trial. It was stressed that the parent or participant was completely free to refuse to take part or withdraw from the trial at any time. Ample time (up to 1 week in some cases, but always more than 24 hours) was provided to read the parent/patient information sheet and to discuss participation with others outside the site research team. Adequate opportunity was given to ask questions.
Written consent was obtained from the parent and written assent was obtained from the participant, when age-appropriate, using the latest version of the informed consent/assent forms. Copies of these were given to the parents and filed in the hospital notes and the original was placed in the investigator site file.
Randomisation
Following confirmation of a diagnosis of nephrotic syndrome, and of ongoing treatment with corticosteroids without corticosteroid treatment failure at that point, and having obtained informed consent, study participants were randomised online via a secure 24-hour internet-based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit. Participants were randomised in a 1 : 1 ratio to either the 8-week SC of prednisolone or the 16-week EC of prednisolone. The randomisation used a minimisation algorithm to ensure that there was balance between the two treatment groups with regard to ethnicity (South Asian, white or other) and age (≤ 5 or ≥ 6 years). Both of these variables have previously been suggested to be linked to different outcomes following presentation. The incidence of SSNS is significantly higher among South Asian children than in white children or those of other ethnicities; however, there is some suggestion that their disease may follow a less complicated course, with a lower frequency of relapse and a lower incidence of frequently relapsing disease. 38 At least one study has suggested that children who are < 4 years of age benefit from prolonged initial prednisolone therapy26 and younger children have also previously been demonstrated to have a higher rate of disease relapse and development of FRNS. 39,40
Once the participant had been randomised, the local principal investigator sent a signed copy of the clinical trial prescription form and the consent/assent form(s) to the central pharmacy at the Birmingham Children’s Hospital to order the PREDNOS trial medication metered dose blister pack. Birmingham Children’s Hospital’s pharmacy was responsible for the dispensing of the randomised study drug for the entire UK; the study drug was sent by Royal Mail Special Delivery directly to the participant’s home. Only delegated staff within the pharmacy were able to view the treatment allocation in order to assemble the study drug treatment blister packs and dispatch these. This was performed via a secure login link to the randomisation programme once the participant had been randomised. This method of randomisation ensured that investigators and the co-ordinating centre were masked to the participant’s randomised treatment allocation.
Interventions
Treatment groups
Participants were randomised to receive either SC prednisolone therapy [the ISKDC regimen: a dose of 60 mg/m2/day of prednisolone (maximum dose 80 mg) for 4 weeks followed by 40 mg/m2 (maximum dose 60 mg) on alternate days for a further 4 weeks] or EC prednisolone therapy [a dose of 60 mg/m2/day of prednisolone (maximum 80 mg) for 4 weeks followed by 60 mg/m2 (maximum 60 mg) on alternate days for 2 weeks, with a subsequent gradual reduction in dose over a total of 12 weeks (tapering by 10 mg/m2 every 2 weeks), resulting in a total course of prednisolone of 16 weeks]. The trial schema is shown in Figure 1.
FIGURE 1.
Trial schema.

All participants received the initial 4 weeks of prednisolone as open-label treatment prior to recruitment and randomisation into the study and the dispensed study medication prescribed was of 12 weeks’ duration (4 weeks of prednisolone and 8 weeks of placebo in those randomised to the SC group and 12 weeks of prednisolone in those randomised to the EC group). The treatment schedule and the prednisolone dose administered at each time point is outlined in Table 1. Matching placebo tablets were used to maintain the double blind at each time point.
| Time (weeks) | Therapy prednisolone dose | |
|---|---|---|
| SC | EC | |
| Open-label routine clinical treatment | ||
| 0–4 | 60 mg/m2/day (maximum 80 mg) | 60 mg/m2/day (maximum 80 mg) |
| Randomised phase | ||
| 5–6 | 40 mg/m2/day (+placeboa) on alternate days | 60 mg/m2/day on alternate days |
| 7–8 | 40 mg/m2/day (+placeboa) on alternate days | 50 mg/m2/day on alternate days |
| 9–10 | Placebo on alternate days | 40 mg/m2/day on alternate days |
| 11–12 | Placebo on alternate days | 30 mg/m2/day on alternate days |
| 13–14 | Placebo on alternate days | 20 mg/m2/day on alternate days |
| 15–16 | Placebo on alternate days | 10 mg/m2/day on alternate days |
The entire course of study drug to be administered from weeks 5 to 16 was supplied in a blister pack by the central clinical trials pharmacy at the Birmingham Children’s Hospital (Figure 2). Prednisolone was supplied as 5-mg tablets alongside matching placebo, so that participants in both treatment groups received the same number of tablets at any time point in the study. The central pharmacy controlled allocation concealment and distributed trial medication once informed consent was obtained and randomisation had occurred. Both the study drug and the placebo were manufactured by Essential Nutrition Ltd (Brough, UK). Participants who were unable to swallow tablets whole were allowed to crush the study drug using a tablet crusher, which was supplied upon request. Parents were instructed to administer the study drug to participants first thing in the morning in keeping with routine clinical practice with prednisolone therapy.
FIGURE 2.
Blister pack of study drug as supplied to participants.

Delivered with the study drug, every participant also received a standard study pack containing a participant diary and a bottle of urinalysis sticks (Albustix®, Siemens Healthcare Limited, Frimley, UK; Bayer Diagnostics, Tarry Town, NY, USA) for daily morning testing for proteinuria.
Blinding
All those involved in treating the participant, the participant and their parents/guardians were masked as to the randomised treatment allocation.
Trial procedures and assessments
Following recruitment into the study, in keeping with routine clinical practice, families were asked to use a dipstick to test the study participant’s first morning urine sample for proteinuria on a daily basis and to record the results in the participant diary, alongside information about medications administered, any intercurrent illness and consultations with health-care professionals [general practitioner (GP), nurse, hospital emergency department, etc.] and details of all medicines prescribed or purchased over the counter. In keeping with routine clinical practice, families were instructed to contact their hospital clinical team if their child developed a relapse of nephrotic syndrome, which was defined using the internationally recognised definition of +++ proteinuria on Albustix for 3 consecutive days or the development of generalised oedema in association with +++ proteinuria on Albustix, so that relapse treatment could be prescribed. They were also instructed to call if there were other concerns, including the development of new AEs or other concerns about urine protein readings.
Participants were followed up with routine study visits at 4, 8, 12 and 16 weeks, and then at 5, 6, 8, 10, 12, 18, 24, 30, 36, 42 and 48 months after commencing prednisolone therapy. Participants were followed up for a minimum of 24 months and up to a maximum of 48 months; the study was completed once the last participant had completed 24 months of follow-up.
At each study visit, data were collected regarding recent relapses including prednisolone and other treatment for relapses, recent medication history and the development of AEs, including serious adverse events (SAEs). Specific information was documented regarding the development of significant bacterial, viral and fungal infections, as well as exposure to varicella infection requiring the administration of prophylactic therapy (intramuscular zoster immune globulin or oral aciclovir). A physical examination was performed, including measurement of height using a calibrated stadiometer with the child in bare or stocking feet, weight using calibrated scales and, from these, a calculation of body mass index (BMI) was made. Blood pressure was measured using whichever automated or manual device was in regular use within that outpatient clinic. There was a particular focus on the documentation of adverse effects of corticosteroid toxicity. This included assessment of increased appetite (parentally reported), Cushingoid features, striae and hypertrichosis, all assessed using a Likert scale (none, mild, moderate or severe). A parental subjective assessment of the presence of abdominal pain and behavioural problems (yes or no) was sought, and the parental subjective assessment was complemented by an objective questionnaire-based assessment. Dipstick analysis of the urine was performed to detect the presence of glycosuria; when this was significant and persistent, plasma glucose was measured, in keeping with routine clinical practice. A check of study medication adherence was made at 4, 8, 12 and 16 weeks; families were asked to bring the study medication blister pack with them to their child’s appointment. Ophthalmoscopy was performed by the principal investigator on an annual basis to look for evidence of corticosteroid-induced cataract and any other abnormality.
Quantitative data on behaviour were collected through the administration of the ACBC at weeks 4 and 16 and months 12, 24, 36 and 48. The ACBC is a standardised measure made up of 120 items measuring internalising (withdrawn, somatic complaints, anxiety/depression and thought problems) and externalising (social problems, attention problems and delinquent and aggressive conduct) behaviour problems. A total behavioural problem score is calculated from these problem scales and forms the basis of comparison with age- and gender-matched normative data. The ACBC has been used in over 8000 publications on over 500 topic areas in a diverse range of cultural groups and is supported by extensive research on service needs and outcomes, diagnosis, prevalence of problems, medical conditions, treatment efficacy, genetic and environmental effects and epidemiology.
For the purposes of the health economic analysis, data were collected regarding all contact with health professionals (in primary, secondary and tertiary care), prescriptions issued and over-the-counter medications purchased. The Pediatric Quality of Life Inventory (PedsQL) and Child Health Utility 9D (CHU-9D) quality-of-life (QoL) questionnaires were also completed by parents at weeks 4 and 16 and at months 12, 24, 36 and 48. The PedsQL Measurement Model is a modular approach to measuring health-related quality of life (HRQoL) in healthy children and adolescents and those with acute and chronic health conditions. The 23-item PedsQL Generic Core Scales were designed to measure the core dimensions of health as delineated by the World Health Organization, as well as role (school) functioning. The four multidimensional scales assess physical functioning, emotional functioning, social functioning and school functioning, generating three summary scores: total scale score, physical health summary score and psychosocial health summary score. The CHU-9D questionnaire is a generic preference-based HRQoL instrument for 7- to 17-year-old children. These questionnaires are discussed in further detail in Chapters 5 and 6.
Serious adverse events
Any AEs meeting the definition of a SAE were recorded on a standardised SAE form and faxed to the Birmingham Clinical Trials Unit within 24 hours of the local principal investigator or member of their research team becoming aware of the event. The principal investigator was responsible for assigning causality and expectedness to the SAE before reporting.
Blood samples
The study protocol included the collection of a single 10-ml ethylenediaminetetraacetic acid (EDTA) blood sample for a genetic substudy [not funded as part of the National Institute for Health Research (NIHR) award]. This was obtained at the time of routine venous sampling for clinical purposes whenever possible; however, the ethics approval did allow a standalone blood test to be collected solely for the purposes of the research project. Any potential discomfort associated with blood sampling was minimised by the use of clinical staff that were experienced in paediatric venepuncture and the use of both distraction therapy and topical anaesthetic agents as is routine clinical practice. The small volume of blood collected on one single occasion was not deemed sufficient to cause hypovolaemia or anaemia in participants of 1–14 years of age.
Study withdrawal
Participants were withdrawn from the study under the following circumstances:
-
Early withdrawal. Participants who initially appeared to be corticosteroid sensitive, that is, who developed at least 3 consecutive days of zero or trace proteinuria following the commencement of open-label prednisolone treatment and were, therefore, recruited and randomised, but who subsequently developed significant proteinuria again following randomisation. These participants were withdrawn from the study on the basis that they did not meet the standard definition of corticosteroid sensitivity and were likely to require additional therapy as part of their initial corticosteroid regimen, for example intravenous methylprednisolone, and also potentially require a renal biopsy and/or other investigations. As per the protocol, these participants were not included in any analyses.
-
Later withdrawal. Participants were withdrawn from the study at later time points if parental consent was withdrawn or at the request of the principal investigator. All of the data collected up until the time of withdrawal were included in the analysis.
Unblinding
Arrangements were made to facilitate the unblinding of participants should the need arise, for example a medical emergency when it was imperative that the treating clinician was aware of whether the participant was receiving active prednisolone or placebo. A code break was available through the Birmingham Children’s Hospital pharmacy.
Source data
The case report forms were not the source data for clinical information. However, in some instances, information on relapses and medication changes was entered directly onto case report forms from self-reported patient information from diaries, and so, in these instances, the case report forms were considered to be the source data. Source verification for diary data did not take place as the diaries were not retained. The questionnaires (ACBC, PedsQL and CHU-9D questionnaires) were considered source data; data were entered directly onto these case report forms. Source data were kept as part of the participants’ medical notes generated and maintained at each site.
Outcome measures
The primary outcome measure for the study was the time from commencement of open-label prednisolone therapy to first relapse of proteinuria. Relapse of proteinuria was defined as Albustix-positive proteinuria (+++ or greater) for 3 consecutive days or the presence of generalised oedema and +++ proteinuria.
Secondary outcome measures were:
-
relapse rate
-
incidence of FRNS (defined as two relapses or more in the first 6 months following presentation or four relapses within any 12-month period)
-
incidence of SDNS (defined as relapses on or within 14 days of completion of corticosteroid therapy)
-
incidence of use of second-line immunosuppressive agents, including levamisole, cyclophosphamide, ciclosporin, tacrolimus, mycophenolate mofetil and rituximab
-
rate of SAEs
-
rate of AEs
-
incidence of behavioural change (as assessed by the ACBC)
-
cost per relapse of proteinuria
-
cost per QALY gained.
Sample size
The primary analysis was based on a log-rank test of time to relapse. A relapse rate of 60% at 1 year was expected in the SC group. To detect an absolute difference of 20% (considered a clinically meaningful difference) in the relapse rate, from 60% in the SC group to 40% in the EC group, with 80% power and 2p = 0.05 required a total of 200 participants. Allowing for 10% drop-out, the total number of participants required was 224 (112 per group).
Monitoring of the drop-out rate during the trial showed that > 10% of participants were dropping out of the trial. Therefore, it was decided to increase the drop-out rate to 15%, which meant that the total number of participants required increased to 236. This change was implemented with the release of PREDNOS protocol version 2.1 (1 September 2013).
Statistical methods
The primary outcome measure was the time from commencement of open-label prednisolone therapy to first relapse. Kaplan–Meier survival curves were constructed for visual presentation of the time to first relapse. The primary analysis of time to first relapse was assessed across the two treatment groups and compared using a log-rank test. A Cox-proportional hazard model was fitted to obtain a HR and 95% CI. As a secondary analysis, a Cox-proportional hazard model, adjusting for the minimisation variables of ethnicity (South Asian, white or other) and age (≤ 5 or ≥ 6 years), was also fitted. If there were any other important prognostic factors that were unbalanced between the groups at baseline, then these would also be included in the model. It is unlikely that a participant will relapse while on corticosteroids; however, it is possible that participants in the SC group may experience an early relapse in weeks 9–16 when receiving placebo, which could potentially bias the results in favour of the EC group. Corticosteroid dependency was also a secondary outcome measure (defined as relapsing on or within 14 days of completion of corticosteroid therapy) and there may be a difference between the groups in corticosteroid dependency. Therefore, to avoid the potential for bias in these situations, if a participant relapsed before 18 weeks, their relapse time was set to 18 weeks. Participants in the EC group received corticosteroids up to week 16, so this also accounts for any possible difference between the groups in corticosteroid dependency. A secondary analysis was performed which analysed time to first relapse using the actual relapse date.
The relapse rate was reported as the mean number of relapses per participant. The relapse rate between the two groups was compared using a negative binomial model (as this was a better fit than the Poisson model, which was stated in the protocol) to obtain an incidence rate ratio (IRR); an offset was included in the model to allow for participants having different lengths of follow-up in the trial. Categorical data items (e.g. FRNS, SDNS) were compared between the groups using a chi-squared test and a RR was reported. If there were any imbalances between the groups in any prognostic factors, then a log-binomial model was fitted.
The SAE data were summarised descriptively. The SAE data were also analysed as a dichotomous variable, with each participant classed as either having experienced a SAE or not. The two groups were compared using a chi-squared test. The number of SAEs that a participant had experienced during the trial was compared between the groups using an appropriate count model. The AE data were reported using a Likert scale (none, mild, moderate or severe). For this report, these data were dichotomised into whether or not the participant had experienced an AE or not. The number of participants who had experienced an AE in the first 16 weeks (at the end of study medication), and then at 6, 12 and 24 months, was reported along with a RR and 95% CI.
The ACBC was analysed using repeated-measures methods, including the baseline score (4-week data) as a covariate in the model. Separate analyses, using t-tests, were carried out at each time point to allow for the possibility that adverse effects (as measured by the ACBC) had differing short- and long-term responses to the treatment. Mean differences and 95% CI were reported.
Two a priori subgroup analyses were planned for the primary outcome. These subgroups were for the minimisation variables of ethnicity (South Asian, white or other) and age (≤ 5 or ≥ 6 years). A treatment group by subgroup interaction parameter was included in the Cox proportional hazard model to assess whether or not there were any differences in the treatment effect across the different strata.
The other outcomes included height, weight, BMI and blood pressure. These were all expressed as standard deviation scores (SDSs) for the purposes of the analysis. For height, weight and BMI, SDSs were generated using UK World Health Organization data41 and as normal range data for blood pressure, according to age, sex and height, as produced by the US National High Blood Pressure Education Program Working Group on High Blood Pressure data. 42 These data are summarised descriptively and presented graphically using longitudinal plots.
Analyses were of all randomised participants using the ITT principle, except for those who, following randomisation, were subsequently found to be corticosteroid resistant. Exclusion of these participants results in no bias as (1) these drop-outs occurred prior to the commencement of randomised treatment and (2) clinicians were unaware of the treatment assigned to their participants. Estimates of treatment effects are presented with 95% CIs; p-values are two-tailed, with p < 0.05 considered statistically significant. No corrections for multiple tests were made. All analyses were carried out using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA) or Stata® version 14 (StataCorp LP, College Station, TX, USA). SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the US and other countries. ® indicates US registration.
Patient and public involvement
The trial protocol was discussed extensively with a number of representatives of the UK NeST and the UK Renal Patient Support Group, which provided valuable input regarding trial design, acceptability of study visit frequency and blood testing. It was the input of these groups that led us to perform such detailed investigation of the adverse behavioural effects of the two corticosteroid regimens.
Ethics approval, regulations and trial registration
Steroid-sensitive nephrotic syndrome is a disease that is of greatest prevalence during early childhood; therefore, it was considered ethically justified to use children rather than adults as study participants. Both the SC and EC prednisolone regimens under investigation in the PREDNOS study have been in use in multiple centres across the world for more than 10 years, and children participating in the study were considered to be at minimal risk.
The parents’ written informed consent for their child to participate in the trial and the participant’s assent, as appropriate to their assessed competence by the principal investigator, were both obtained prior to randomisation and after a full explanation had been given of the study, the treatment options and the manner of treatment allocation. This was supported by a parent information leaflet and information leaflets for both older and younger participants.
Ethics approval for the study was granted by the North West 7 Research Ethics Committee (reference number 10/H1008/122). The trial was carried out under a Clinical Trial Authorisation in accordance with the Medicines for Human Use (Clinical Trials) Regulations 2004 (21761/0255/001-0001). 43
The trial was conducted in accordance with the recommendations guiding physicians in biomedical research involving human subjects, adopted by the 18th World Medical Association General Assembly, Helsinki, Finland, June 1964, and its subsequent amendments, the Research Governance Framework for Health and Social Care; and the applicable UK Statutory Instruments including the Medicines for Human Use (Clinical Trials) Regulations 200443 and its subsequent amendments, the Data Protection Act 1998,44 the Human Tissue Act 200445 and the International Conference on Harmonisation’s Guidelines for Good Clinical Practice. 46
Chapter 3 Results
Recruitment
The PREDNOS study opened to recruitment in July 2011 and the first participant was recruited into the trial on 2 August 2011. Two hundred and thirty-seven participants were recruited and randomised, the last entering the study on 7 October 2014. The rate of recruitment was relatively constant with no evidence of seasonal variation (Figure 3). One hundred and eighteen participants were randomised to the SC group and 119 to EC group. The 237 participants were recruited from 86 (69%) of the 124 recruiting study sites; recruitment at the 86 sites varied between 1 and 19 participants. Individual site recruitment data are shown in Appendix 1. Participants had completed at least 2 years’ follow-up by October 2016.
FIGURE 3.
Recruitment of participants into the study.

Participant flow
Of the 237 participants randomised into the study, 44 (19%) were withdrawn from the trial (Figure 4). Following consent and randomisation, 14 participants (6%; nine in the SC group and five in the EC group) who had initially responded to open-label prednisolone, suggesting that they were corticosteroid sensitive, developed proteinuria (with time from commencing open-label prednisolone to withdrawal in these participants ranging from 26 to 35 days). These participants were deemed to be corticosteroid resistant and were withdrawn from the study as per the protocol. Their data were not included in any of the subsequent analysis, and so the ITT population was based on 223 participants.
FIGURE 4.
A Consolidated Standards of Reporting Trials diagram of participant flow through the trial. a, Two patients had discontinued their study medication. b, Two patients had discontinued their trial medication owing to relapse. Time points are at times post start of open-label treatment.
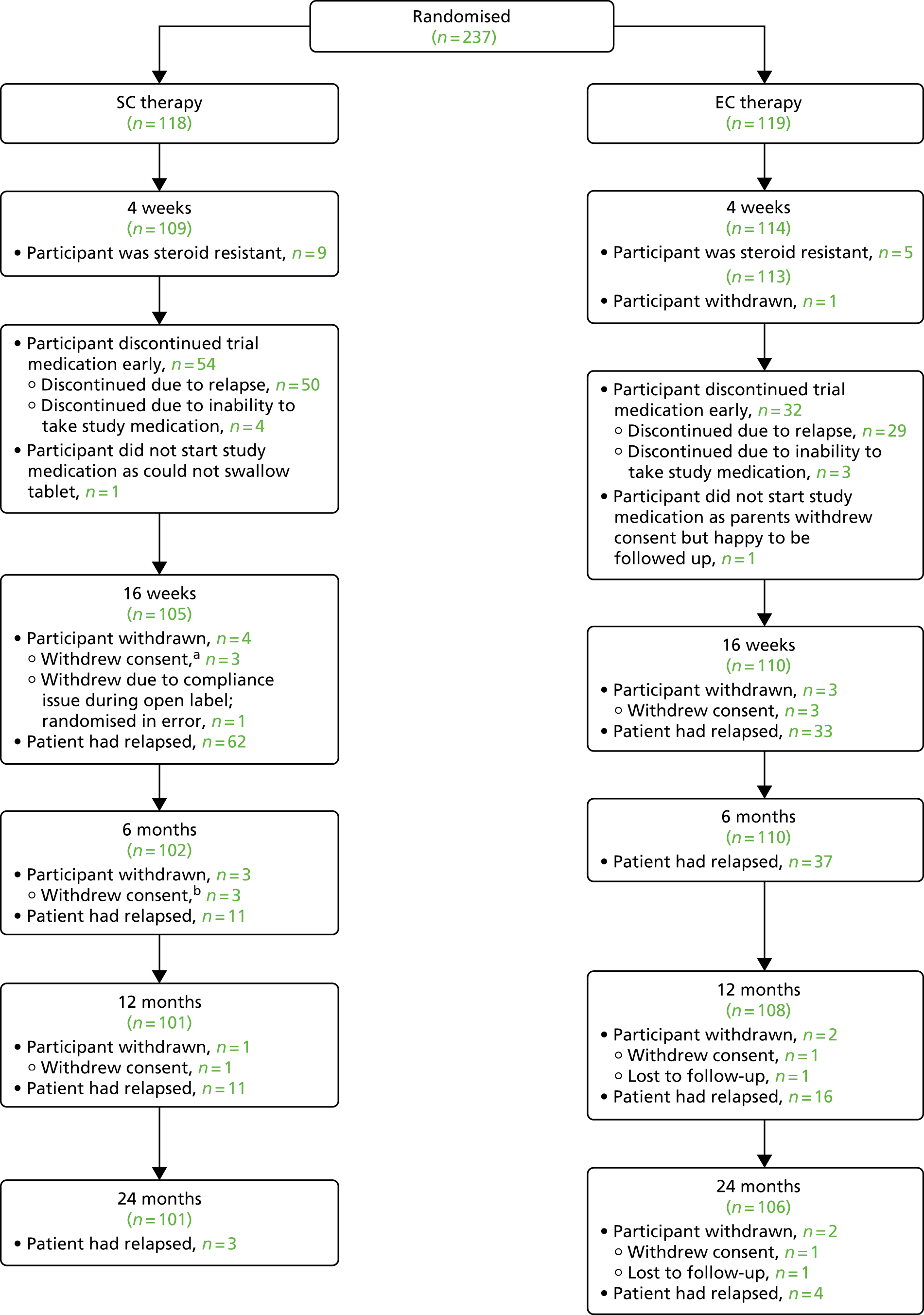
During the trial, 15 participants (6%) had their consent to participate in the study withdrawn (12 in year 1, one in year 2 and two in year 3), 11 participants (5%) became lost to follow-up (one in year 1, one in year 2, five in year 3 and four in year 4) and four participants (2%) withdrew for other reasons (two emigrated, one was withdrawn at the principal investigator’s discretion and one participant was randomised in error prior to entering remission with open-label treatment). For these 30 participants, the data collected up until the time of their withdrawal from the study were included in the analysis. Withdrawn participants were distributed evenly between the two groups (Table 2).
| Reason for withdrawal | Group (n) | Total (N = 237), n (%) | |
|---|---|---|---|
| SC | EC | ||
| Corticosteroid resistant | 9 | 5 | 14 (6) |
| Withdrew consent | 9 | 6 | 15 (6) |
| Lost to follow-up | 1 | 10 | 11 (5) |
| Other reason | 1 | 3 | 4 (2) |
| Exclusions (total) | 20 | 24 | 44 (19) |
Completeness of data
Attendance rates for follow-up study visits were high, as were submission rates of clinical data and participant questionnaires, with data completion rates being over 90% for all time points. There was no difference in data return rates between the two groups. The median length of follow-up was 37.5 months for the SC group and 36.7 months for the EC group.
Baseline data
The mean [standard deviation (SD)] age at randomisation of the ITT population was 4.9 (3.1) years, with 65% of participants being < 6 years of age. In keeping with the known pattern of presentation of SSNS, there was an excess of male participants (65%) and 20% were of South Asian origin and 14% were of other non-white origin. Fifty-four per cent of participants were overweight or obese; the median BMI percentile was 87.5. The mean dose of prednisolone administered in the open-label phase was 58.2 mg/m2/day (Table 3).
| Characteristic | Group | Total | |
|---|---|---|---|
| SC | EC | ||
| Total randomised | N = 118 | N = 119 | N = 237 |
| Corticosteroid sensitive participants (ITT cohort) | n = 109 | n = 114 | n = 223 |
| Age | |||
| Mean (years) (SD) | 4.7 (2.9) | 5.1 (3.2) | 4.9 (3.1) |
| 1–2 years, n (%) | 29 (27) | 28 (25) | 57 (26) |
| 3–5 years, n (%) | 43 (39) | 45 (39) | 88 (39) |
| 6–11 years, n (%) | 34 (31) | 35 (31) | 69 (31) |
| 12–17 years,a n (%) | 3 (3) | 6 (5) | 9 (4) |
| ≤ 5 years,b n (%) | 72 (66) | 73 (64) | 145 (65) |
| ≥ 6 years, n (%) | 37 (34) | 41 (36) | 78 (35) |
| Gender (male), n (%) | 78 (72) | 68 (60) | 146 (65) |
| Ethnicity,b n (%) | |||
| South Asian | 21 (19) | 23 (20) | 44 (20) |
| White | 73 (67) | 75 (66) | 148 (66) |
| Other/not stated | 15 (14) | 16 (14) | 31 (14) |
| BMI percentile, median (IQR) | 85.3 (66.3–97.3) | 90.0 (69.5–97.5) | 87.5 (66.6–97.3) |
| BMI percentile, n (%) | |||
| Underweight (< 5th) | 2 (2) | 0 (0) | 2 (1) |
| Healthy (5th–84th) | 52 (48) | 48 (42) | 100 (45) |
| Overweight (85th–95th) | 19 (17) | 24 (21) | 43 (19) |
| Obese (≥ 95th) | 36 (33) | 42 (37) | 78 (35) |
| Open-label prednisolone dose (mg/m2/day), mean (SD) | 58.5 (5.9) | 58.0 (6.8) | 58.2 (6.4) |
Discontinuation of study medication
Eighty-six (39%) out of the 223 participants did not complete their course of study medication. The number of participants discontinuing study medication was higher in the SC group than in the EC group (SC 50% vs. EC 28%; p = 0.001). The predominant reason for discontinuation was the development of relapse during the 12-week period when double-blind study medication was being administered (n = 79, 35%). When relapses developed during this period of study drug administration, the protocol stated that study medication was to be discontinued and relapse treatment was to be commenced. The incidence of study drug discontinuation was comparable in the two groups up until week 8 (week 4 of randomised study drug), when participants in both groups were receiving active prednisolone. However, thereafter, there was an increase in the number of discontinuations of study medication in the SC group (Figure 5). The number of participants who discontinued owing to relapse was higher in the SC group (n = 50) than in the EC group (n = 29). Participants in the SC group were scheduled to receive active prednisolone until week 8 (week 4 of randomised study drug) followed by placebo for weeks 8–16 (weeks 4–12 of randomised study drug), whereas those in the EC group were scheduled to receive active prednisolone right through until week 16 (week 12 of randomised study drug). The majority of participants in the SC group (38/50) who discontinued the study drug because of relapse over this 16-week period did so during weeks 9–16, once active prednisolone had been discontinued and they were receiving placebo. In contrast, in the EC group, discontinuations owing to relapse were spread over the 12-week study drug period.
FIGURE 5.
Time to discontinuation of study medication.
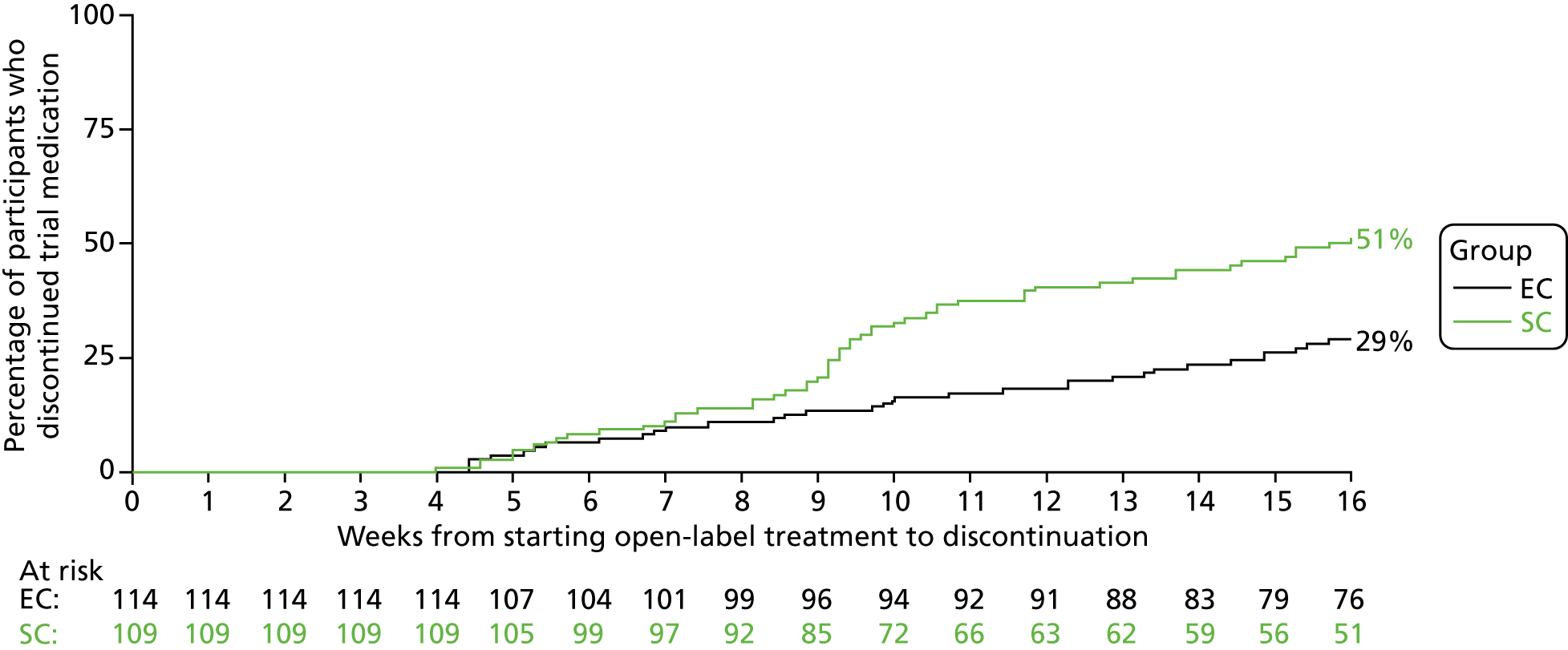
The other seven participants (3%; SC, n = 4, vs. EC, n = 3) who prematurely discontinued study medication did so in a deviation from the study protocol. In four participants, it proved impossible to administer the study medication, including in crushed form; one participant refused to take the study medication and two families withdrew consent during the 12-week period of study drug administration (one participant refused to take their medication and the other participant’s parent stopped the trial medication without consulting the site).
Adherence to study medication
Adherence to study medication was good, with only a small proportion of participants reporting missed doses (13%). In the majority of cases, only one or two doses were reported as missed; however, two participants in the EC group reported missing 12 and 14 doses. There was no difference between the two groups in the number of participants who reported missing doses (SC 10% vs. EC 16%; p = 0.2); however, the total number of doses missed was numerically greater in the EC group than in the SC group (30 vs. 57 missed doses; mainly owing to two participants in the EC group who missed 12 and 14 doses).
Primary outcome
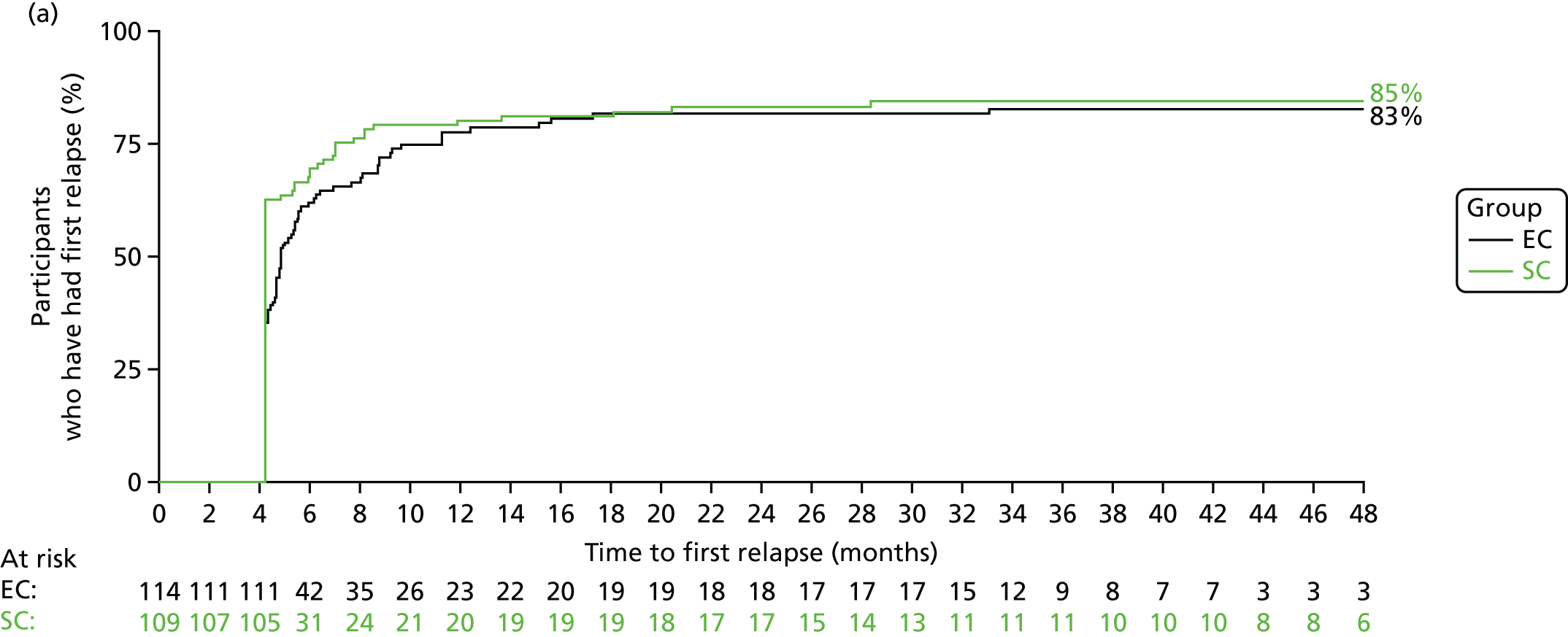
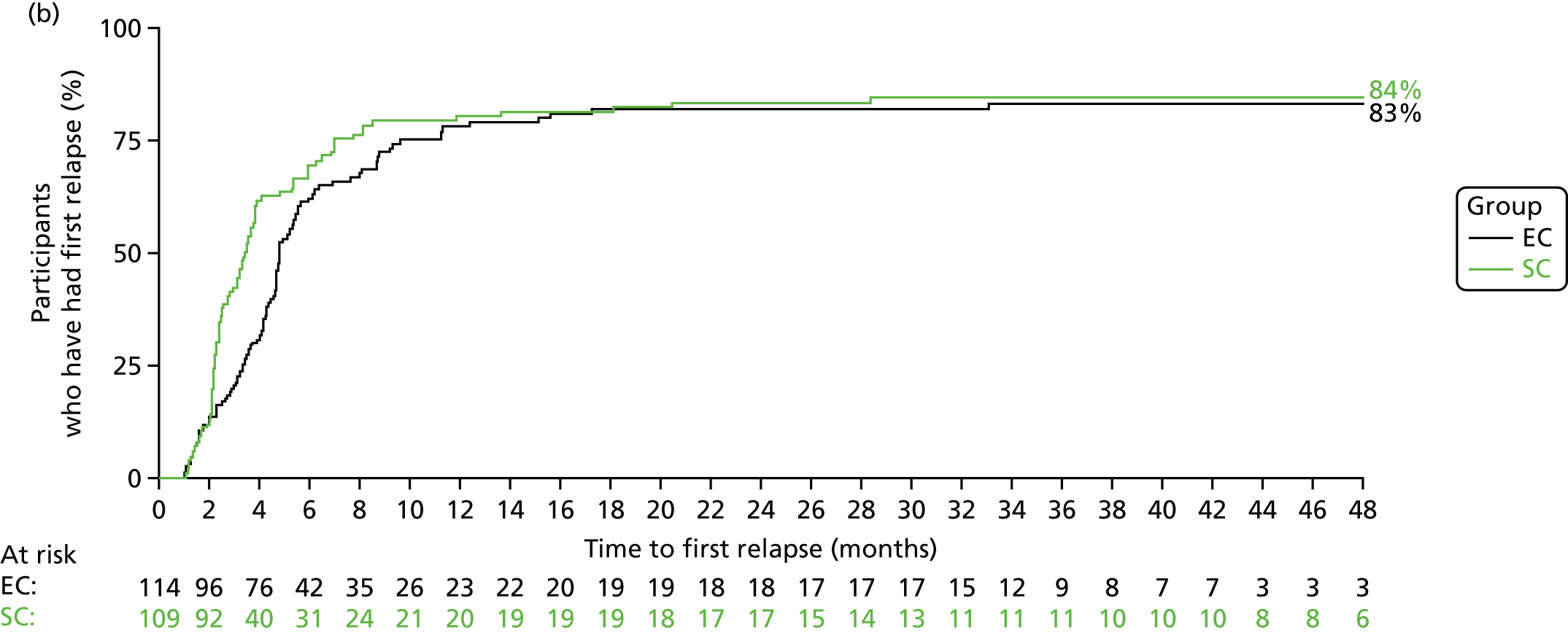
The number of participants who reported a relapse during the trial was 179: 88 out of 109 (81%) in the SC group and 91 out of 114 (80%) in the EC group. There was no significant difference in time to first relapse between the SC and EC groups (Figure 6a; unadjusted HR 0.87, 95% CI 0.65 to 1.17; log-rank p = 0.3). Analyses adjusting for the minimisation variables gave similar results (HR 0.86, 95% CI 0.64 to 1.16). The median [interquartile range (IQR)] time to first relapse was 87 (64.5–134) days for the SC group and 139 (90–179) days for the EC group. The time to first relapse using the actual relapse date is shown in Figure 6b.
FIGURE 6.
Time to first relapse. (a) When using time to relapse as 18 weeks if relapse occurred before 18 weeks; and (b) using actual date of relapse.
Secondary outcomes
The number of relapses experienced by participants in each treatment group is shown in Table 4. The number of relapses that participants experienced during the trial ranged from 0 to 15; there were eight participants in the SC group and nine in the EC group who experienced 10 or more relapses. The mean number of relapses did not differ (SC 3.61 vs. EC 3.98; IRR 1.09, 95% CI 0.86 to 1.39; p = 0.5) (see Table 4).
| Secondary outcome measures | Group | Estimate (95% CI) | p-value | |
|---|---|---|---|---|
| SC (N = 109) | EC (N = 114) | |||
| Relapses | ||||
| Number of relapses | 394 | 454 | ||
| Number of participants experiencing a relapse (%) | 88 (81) | 91 (80) | HR 0.87 (0.65 to 1.17) | 0.3 |
| Mean (SD) number of relapses per participant | 3.61 (3.25) | 3.98 (3.30) | IRR 1.09 (0.86 to 1.39) | 0.5 |
| Number of participants who developed FRNS (%) | 55 (50) | 60 (53) | RR 1.04 (0.81 to 1.35) | 0.7 |
| Number of participants who developed SDNS (%) | 48 (44) | 48 (42) | RR 0.96 (0.71 to 1.29) | 0.8 |
| Second-line immunosuppressive agents | ||||
| Number of participants who received second-line immunosuppressants (%) | 61 (56) | 62 (54) | RR 0.97 (0.77 to 1.23) | 0.8 |
| Type of immunosuppressant received | ||||
| Ciclosporin | 6 (6%) | 4 (4%) | ||
| Tacrolimus | 8 (7%) | 18 (16%) | ||
| Levamisole | 35 (32%) | 34 (30%) | ||
| Cyclophosphamide | 31 (28%) | 29 (25%) | ||
| Mycophenolate mofetil | 13 (12%) | 15 (13%) | ||
| Rituximab | 5 (5%) | 1 (1%) | ||
| Corticosteroid dose | n = 90 | n = 94 | ||
| Mean (SD) total prednisolone dose (mg)a | 5474.6 (3697.3) | 6674.1 (4998.2) | Mean difference 1199.5 (–83.8 to 2482.8) | 0.07 |
There were no significant differences between the two treatment groups in the proportion of participants developing FRNS (SC 50% vs. EC 53%, RR 1.04, 95% CI 0.81 to 1.35; p = 0.7) or SDNS (44% vs. 42%, RR 0.96, 95% CI 0.71 to 1.29; p = 0.8) (see Table 4). The median time to the development of FRNS was 129 days for the SC group and 173 days for the EC group (HR 0.93, 95% CI 0.64 to 1.34). There was no difference between groups in the proportion of participants requiring alternative immunosuppressive agents (56% vs. 54%; p = 0.8). The most common immunosuppressive agents used were levamisole and cyclophosphamide. The total dose of prednisolone received during the study (following completion of study medication) was larger in the EC group (6674.1 mg) than in the SC group (5474.6 mg) but this was not statistically significant (p = 0.07).
Serious adverse events
There were 67 SAEs reported in 46 participants (21%): 39 SAEs in 27 participants (25%) in the SC group and 28 SAEs in 19 participants (17%) in the EC group (p = 0.1). The most common reasons for SAE reporting were admission for disease relapse or bacterial infection. Five SAEs in the SC group and six SAEs in the EC group were felt by the principal investigator to be related to the study drug, although none resulted in study drug discontinuation. There was one accidental death that was unrelated to the trial.
Adverse events
The most common AEs reported were increased appetite, poor behaviour (parent reported), Cushingoid facies, hypertrichosis and abdominal pain (Table 5). In the first 16 weeks of the trial, increased appetite was reported in 87% of participants (SC 87% vs. EC 86%), poor behaviour in 83% (SC 90% vs. EC 75%), Cushingoid facies in 67% (SC 66%, vs. EC 68%), hypertrichosis in 26% (SC 23%, vs. EC 30%) and abdominal pain in 26% (SC 28%, vs. EC 25%). By 24 months, these had increased to 94% (SC 94%, vs. EC 93%) for increased appetite, 87% (SC 93%, vs. EC 82%) for poor behaviour, 72% (SC 72%, vs. EC 73%) for Cushingoid facies, 39% (SC 38%, vs. EC 39%) for hypertrichosis and 45% (SC 47%, vs. EC 43%) for abdominal pain. At 16 weeks and at 6, 12 and 24 months, there were no significant differences between the groups in the cumulative number of participants reporting any of the AEs, except for poor behaviour, which was lower in the EC group than in the SC group. In the first 16 weeks, 90% in the SC group reported poor behaviour, compared with 75% in the EC group (RR 0.85, 95% CI 0.76 to 0.96). Differences were also seen at 6 months (91% vs. 81%, RR 0.90, 95% CI 0.82 to 1.00), 12 months (92% vs. 82%, RR 0.90, 95% CI 0.82 to 0.98) and 24 months (93% vs. 82%, RR 0.90, 95% CI 0.82 to 0.98).
| AE | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 16 | 6 months | 12 months | 24 months | |||||||||
| Group, n reporting AEs (%) | RR (95% CI) | Group, n reporting AEs (%) | RR (95% CI) | Group, n reporting AEs (%) | RR (95% CI) | Group, n reporting AEs (%) | RR (95% CI) | |||||
| SC (N = 109) | EC (N = 114) | SC (N = 109) | EC (N = 114) | SC (N = 109) | EC (N = 114) | SC (N = 109) | EC (N = 114) | |||||
| Cushingoid facies | 72 (66) | 77 (68) | 1.04 (0.87 to 1.25) | 75 (69) | 79 (69) | 1.03 (0.87 to 1.22) | 76 (70) | 81 (71) | 1.05 (0.89 to 1.23) | 78 (72) | 83 (73) | 1.02 (0.88 to 1.19) |
| Striae | 3 (3) | 8 (7) | 2.69 (0.73 to 9.87) | 4 (4) | 11 (10) | 2.72 (0.90 to 8.27) | 6 (6) | 11 (10) | 1.80 (0.69 to 4.67) | 7 (6) | 14 (12) | 1.92 (0.81 to 4.54) |
| Hypertrichosis | 25 (23) | 34 (30) | 1.35 (0.87 to 2.09) | 30 (28) | 40 (35) | 1.28 (0.87 to 1.89) | 37 (34) | 42 (37) | 1.12 (0.80 to 1.59) | 41 (38) | 45 (39) | 1.05 (0.77 to 1.45) |
| Acne | 3 (3) | 6 (5) | 2.02 (0.52 to 7.86) | 6 (6) | 9 (8) | 1.49 (0.55 to 4.02) | 7 (6) | 11 (10) | 1.52 (0.62 to 3.77) | 7 (6) | 12 (11) | 1.64 (0.68 to 3.99) |
| Increased appetite | 95 (87) | 98 (86) | 0.99 (0.89 to 1.09) | 98 (90) | 100 (88) | 0.98 (0.90 to 1.07) | 102 (94) | 104 (91) | 0.99 (0.92 to 1.07) | 103 (94) | 106 (93) | 1.00 (0.94 to 1.07) |
| Poor behaviour | 98 (90) | 86 (75) | 0.85 (0.76 to 0.96) | 99 (91) | 92 (81) | 0.90 (0.82 to 1.00) | 100 (92) | 93 (82) | 0.90 (0.82 to 0.98) | 101 (93) | 94 (82) | 0.90 (0.82 to 0.98) |
| Glycosuria | 10 (9) | 9 (8) | 0.92 (0.39 to 2.16) | 11 (10) | 13 (11) | 1.24 (0.59 to 2.61) | 12 (11) | 17 (15) | 1.49 (0.76 to 2.91) | 14 (13) | 19 (17) | 1.34 (0.72 to 2.48) |
| Cataracta | – | – | – | – | – | – | 1 (1) | 0 (0) | – | 1 (1) | 1 (1) | 0.96 (0.06 to 15.00) |
| Abdominal pain | 31 (28) | 28 (25) | 0.92 (0.60 to 1.42) | 35 (32) | 38 (33) | 1.08 (0.74 to 1.56) | 46 (42) | 44 (39) | 0.95 (0.70 to 1.29) | 51 (47) | 49 (43) | 0.91 (0.69 to 1.20) |
Achenbach Child Behaviour Checklist
Quantitative data on behaviour were collected using the ACBC. There were no significant differences in the ACBC t-scores (p = 0.9) or total scores (p = 0.3) (Figure 7a and b; see also Table 21 in Appendix 2). There were also no differences in the proportion of participants reporting normal ACBC scores (see Table 22 in Appendix 2).
FIGURE 7.
ACBC scores. (a) t-score; and (b) total score. Higher scores indicate more abnormal behaviour.


Subgroup analyses
When prespecified subgroup analyses were performed for the primary outcome for the two minimisation variables of ethnicity (South Asian, white or other) and age (≤ 5 or ≥ 6 years), there was no clear evidence to suggest that the treatment effect differed between the different participant subgroups (Table 6). However, there may be some suggestion (p = 0.08) that in the EC group time to first relapse was extended in those participants aged ≤ 5 years (HR 0.72, 95% CI 0.50 to 1.05), with no difference between the two groups in participants aged ≥ 6 years (HR 1.26, 95% CI 0.77 to 2.07).
| Characteristic | Group, n/N (%) | Interaction p-value | HR (95% CI) | |
|---|---|---|---|---|
| SC (N = 109) | EC (N = 114) | |||
| Participants experiencing at least one relapse | ||||
| Age category (years) | ||||
| ≤ 5 | 60/72 (83) | 55/73 (75) | 0.08 | 0.72 (0.50 to 1.05) |
| ≥ 6 | 28/37 (76) | 36/41 (88) | 1.26 (0.77 to 2.07) | |
| Ethnicity | ||||
| South Asian | 16/21 (76) | 15/23 (65) | 0.6 | |
| White | 60/73 (82) | 63/75 (84) | ||
| Other | 12/15 (80) | 13/16 (81) | ||
| Gender | ||||
| Male | 64/78 (82) | 54/68 (79) | 0.5 | |
| Female | 24/31 (77) | 37/46 (80) | ||
A post hoc subgroup analysis was also performed for gender, as a number of non-randomised studies have reported that boys had a worse clinical outcome than girls. 16,47 We found no evidence of a difference in treatment effect according to gender (see Table 6).
Other outcomes
Growth data
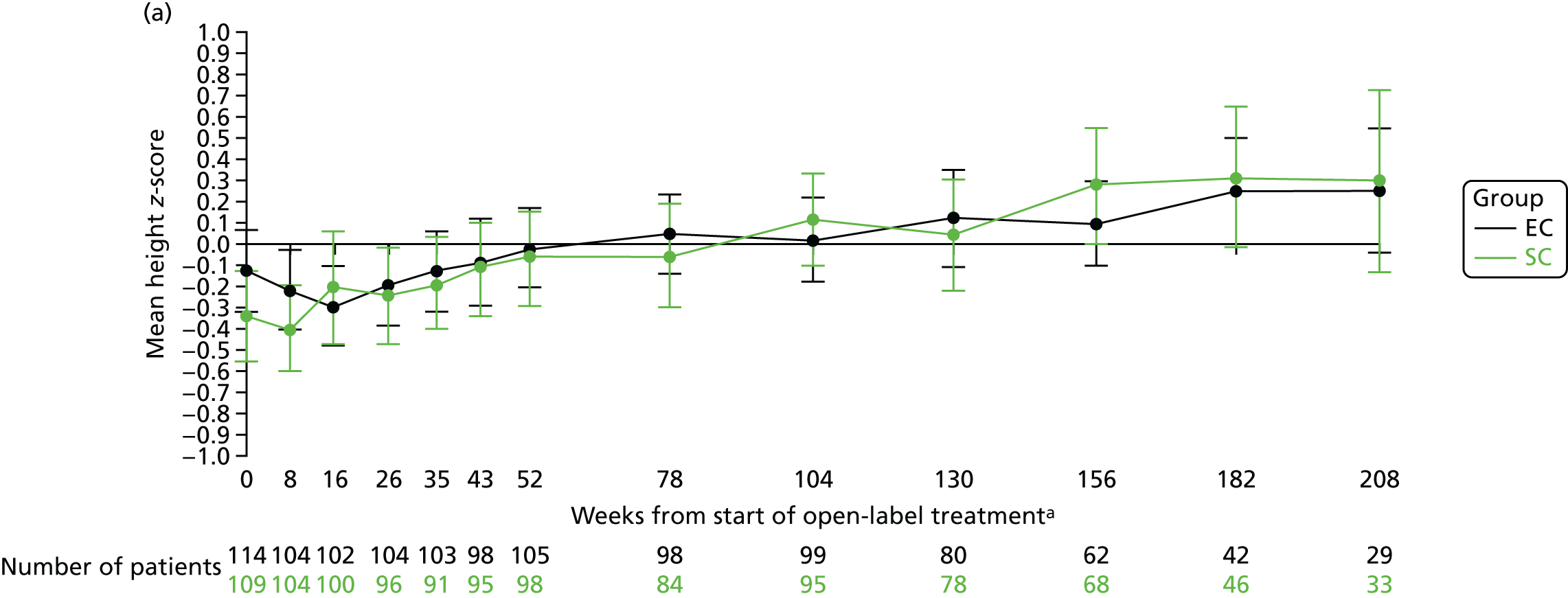
After the 16-week study medication period, the mean height z-score (Figure 8a) and percentile (see Figure 8b) scores gradually increased over time. At 12, 18 and 24 months, the mean height z-scores were –0.06 (SD 1.11), –0.06 (SD 1.13) and 0.12 (SD 1.09), respectively, in the SC group and –0.02 (SD 0.95), 0.05 (SD 0.93) and 0.02 (SD 0.99), respectively, in the EC group.
FIGURE 8.
Height. (a) z-score; and (b) percentile. a, Randomisation counted as week 0.
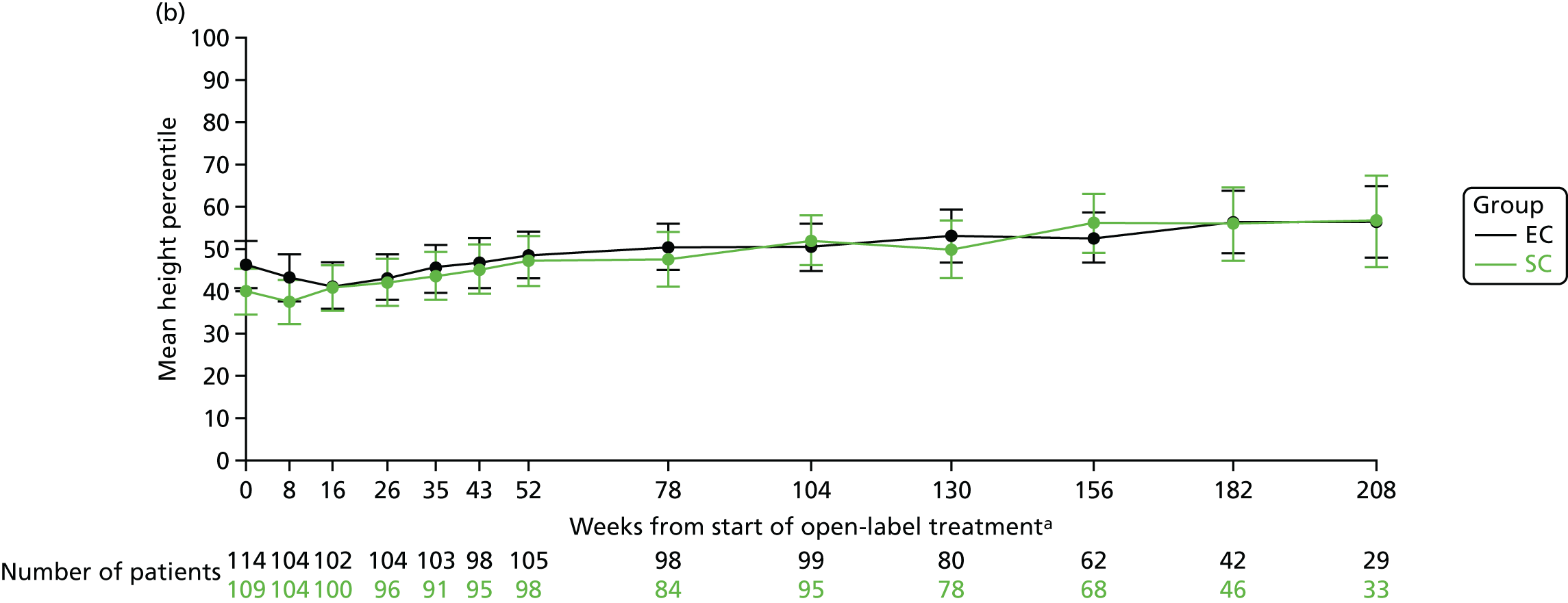
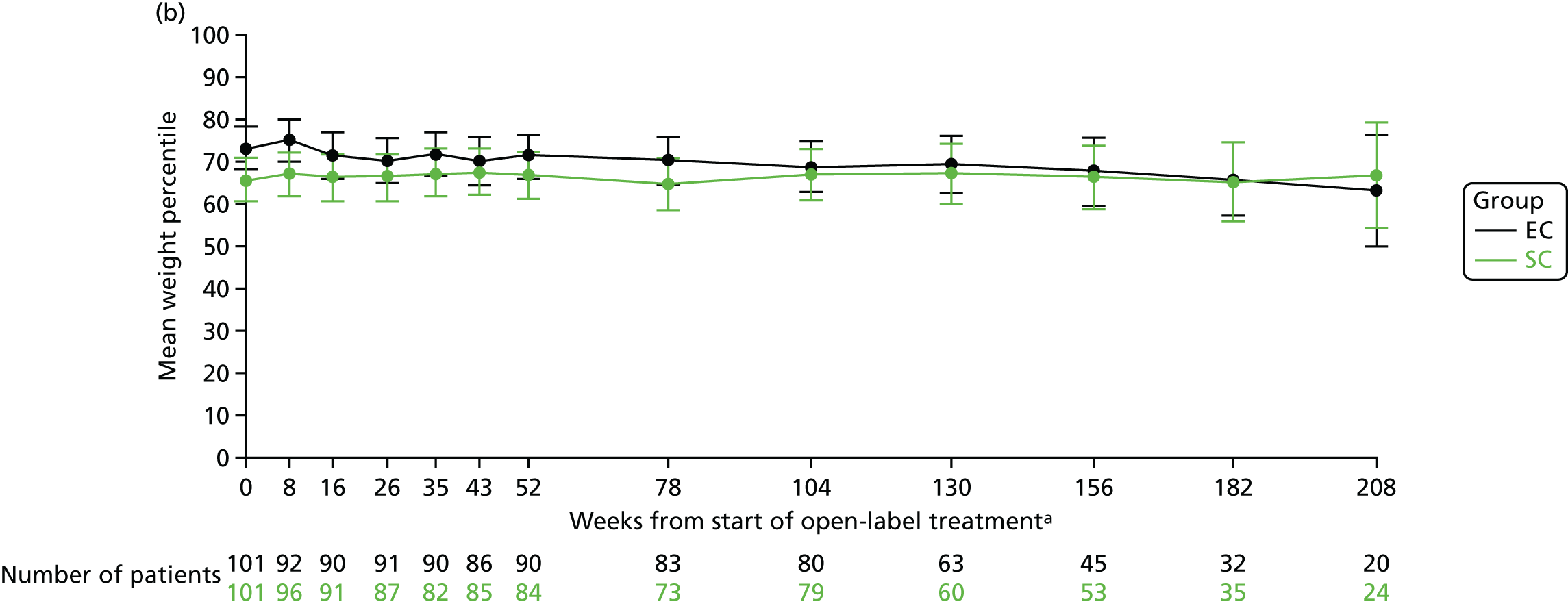
The mean weight z-scores at 8 weeks (when the corticosteroid dose between the two treatment arms are the most different, i.e. after 4 weeks of study medication) were 0.64 (SD 1.09) in the SC group and 0.98 (SD 1.03) in the EC group. At 12, 18 and 24 months, the mean weight z-scores were 0.64 (SD 1.04), 0.61 (SD 1.19) and 0.70 (SD 1.19), respectively, in the SC group and 0.85 (SD 1.09), 0.80 (SD 1.06) and 0.76 (SD 1.12), respectively, in the EC group (Figure 9a). The mean weight percentile data are shown in Figure 9b.
FIGURE 9.
Weight. (a) z-score; and (b) percentile. a, Randomisation counted as week 0.

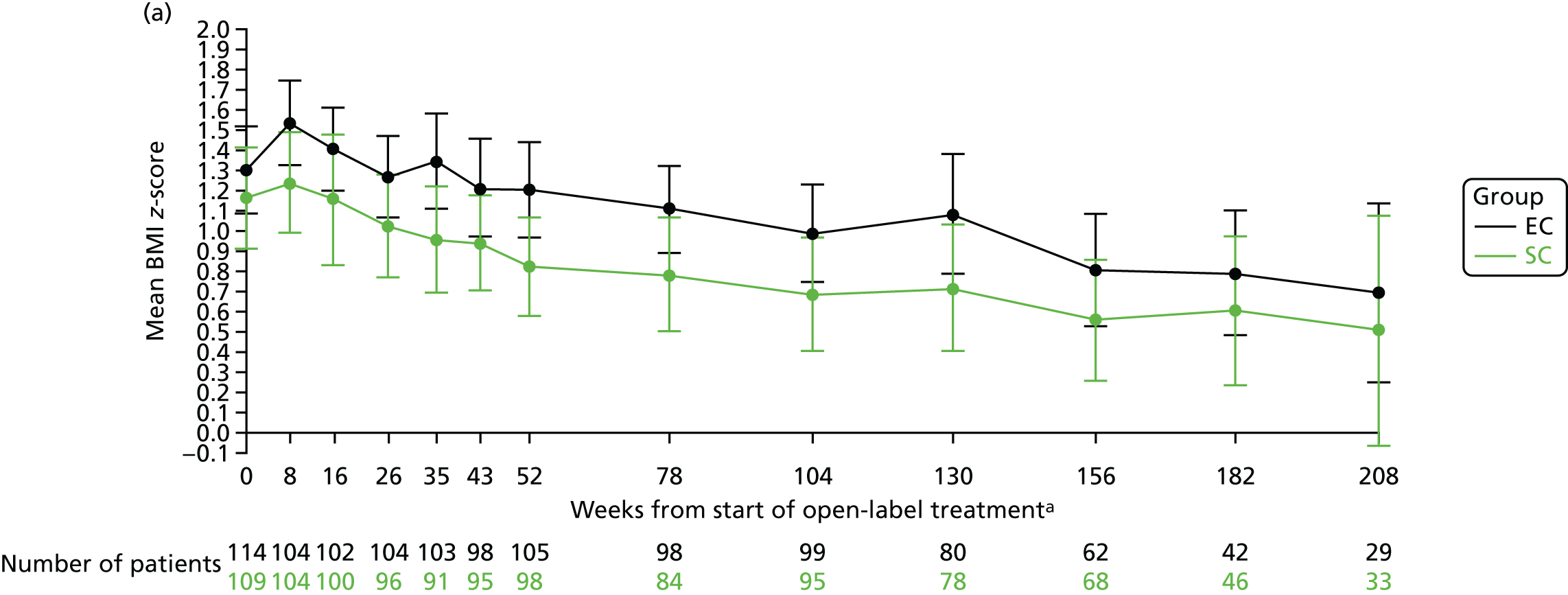
The mean BMI z-scores at 8 weeks (when the corticosteroid dose between the two treatment arms are the most different, i.e. after 4 weeks of study medication) were 1.24 (SD 1.27) in the SC group and 1.53 (SD 1.07) in the EC group. At 12, 18 and 24 months, the mean BMI z-scores were 0.82 (SD 1.21), 0.78 (SD 1.31) and 0.69 (SD 1.39), respectively, in the SC group and 1.20 (SD 1.22), 1.11 (SD 1.10) and 0.99 (SD 1.20), respectively, in the EC group (Figure 10a). The mean BMI percentile data are shown in Figure 10b.
FIGURE 10.
BMI. (a) z-score; and (b) percentile. a, Randomisation counted as week 0.

Blood pressure
The mean systolic blood pressure z-scores at 4 weeks (at the end of the open-label prednisolone period) were 1.25 (SD 0.86) in the SC group and 1.30 (SD 0.92) in the EC group. At 12, 18 and 24 months, the mean systolic blood pressure z-scores were 0.75 (SD 0.96), 0.67 (SD 1.03) and 0.70 (SD 0.97), respectively, in the SC group and 0.67 (SD 1.06), 0.53 (SD 0.96) and 0.53 (SD 0.89), respectively, in the EC group (Figure 11a). The mean systolic blood pressure percentile data are shown in Figure 11b.
FIGURE 11.
Systolic blood pressure. (a) z-score; and (b) percentile.

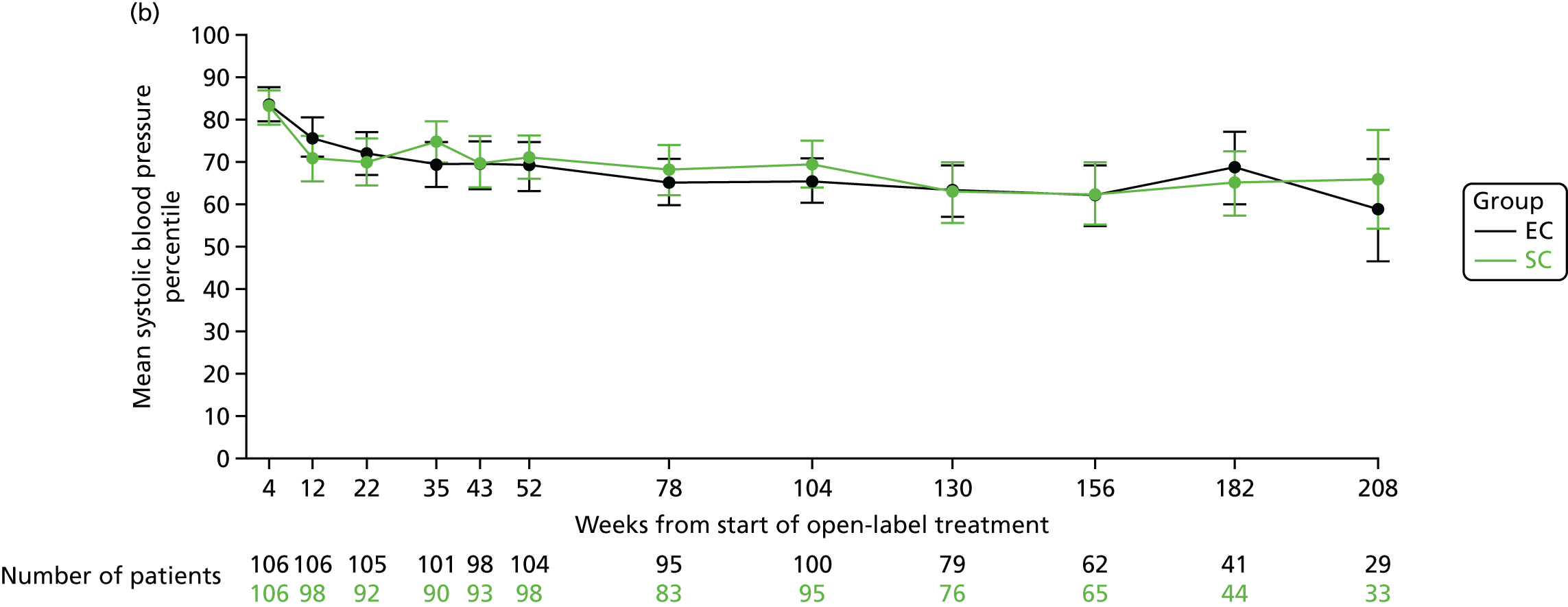
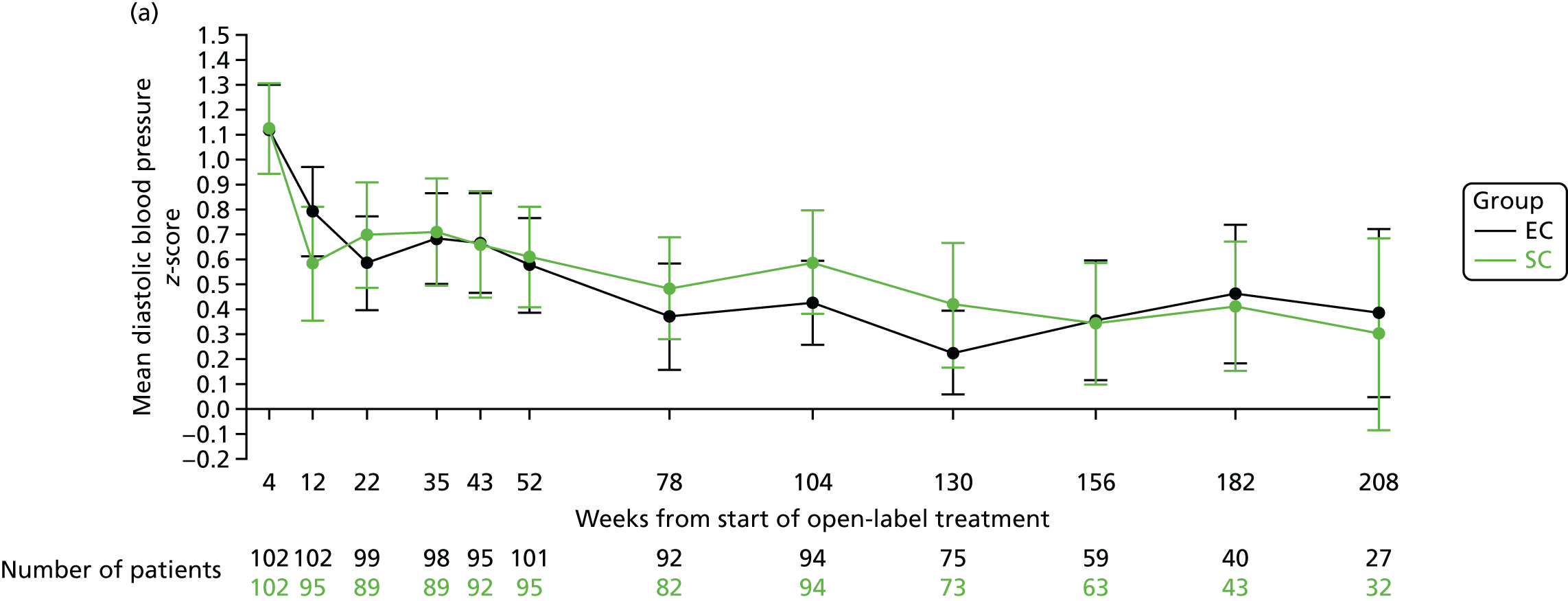
The mean diastolic blood pressure z-scores at 4 weeks (at the end of the open-label prednisolone period) were 1.12 (SD 0.93) in the SC group and 1.12 (SD 0.91) in the EC group. At 12, 18 and 24 months, the mean systolic blood pressure z-scores were 0.61 (SD 0.99), 0.48 (SD 0.93) and 0.58 (SD 1.02), respectively, in the SC group and 0.57 (SD 0.96), 0.37 (SD 1.03) and 0.42 (SD 0.83), respectively, in the EC group (Figure 12a). The mean diastolic blood pressure percentile data are shown in Figure 12b.
FIGURE 12.
Diastolic blood pressure. (a) z-score; and (b) percentile.

Chapter 4 Pilot study
Prior to submission of the application for NIHR funding for the PREDNOS study, a pilot study was conducted to test the proposed study methodology. At the time, the large majority of UK district general hospital paediatric departments had little or no experience in the conduct of RCTs involving an investigational medicinal product, and investigators were keen to ascertain that patients could be successfully recruited and followed in accordance with the study protocol. This was of great importance given that the majority of patients with nephrotic syndrome present to district general hospitals rather than to tertiary paediatric nephrology centres. This pilot study, which was funded jointly by Kidney Research UK and Kids Kidney Research, used an identical methodology to that of the proposed main study, randomising participants to either an 8-week SC or a 16-week EC of prednisolone therapy, and aimed to:
-
provide ‘proof of principle’ of successful recruitment of participants and collaboration in district general hospitals
-
develop a network of investigators, initially in the North West of the UK, although then extending beyond this region
-
provide information on recruitment rates
-
provide further evidence on the incidence of trial outcomes that could be used to inform the definitive trial design; these outcomes included sustained remission rate at 6 and 12 months, time to relapse, incidence of FRNS, incidence of SAEs and incidence of need for other immunosuppressive medications.
This pilot trial was carried out under a Clinical Trial Authorisation carried over from a Doctors and Dentist Exemption (reference number MF8000/13293), in accordance with the Medicines for Human Use Clinical Trials regulations. 43 Ethics approval for the study was granted by the Thames Valley Multi-centre Research Ethics Committee (reference number 04/12/025). The trial was sponsored by Great Ormond Street Hospital for Children NHS Foundation Trust (reference number 03/NU/13). Both study drug and placebo were manufactured by Essential Nutrition Ltd. The study was registered on ClinicalTrials.gov as reference number NCT00308321 and EudraCT as reference number 2004-001813-33.
The pilot study recruited its first participant in August 2006. Trial recruitment was assisted significantly by the development of the NIHR Medicines for Children Research Network (MCRN), which formally adopted the study into its portfolio in March 2007. A successful collaborative trial network was established, with principal investigators appointed at a total of 37 sites. By study completion, 26 sites were fully set up, and, by June 2008, 18 sites had recruited 55 participants. A further 13 sites had expressed active interest in participation in the study.
Of the 55 participants recruited, one was recruited and randomised in error before site set-up and never received the study drug or entered the trial and two proved resistant to corticosteroid therapy after providing informed consent, although prior to starting trial medication. These three participants were excluded from the trial and any analyses. Two experienced difficulties in taking solid tablets and withdrew from the study and two participants changed their area of residence and were lost to follow-up. Therefore, the ITT cohort consisted of 52 participants. The mean age was 6.1 (SD 3.0) years; 31 (60%) were male, 38 (73%) were white and 10 (19%) were South Asian. The median BMI percentile was 77.8 and the mean dose of prednisolone administered in the open-label phase was 60.3 mg/m2/day (Table 7).
| Baseline characteristic | Group | Total | |
|---|---|---|---|
| SC | EC | ||
| Total randomised | N = 27 | N = 27 | N = 54 |
| Steroid-sensitive participants (ITT cohort) | n = 27 | n = 25 | n = 52 |
| Age (years) | |||
| Mean (SD) | 5.7 (2.8) | 6.5 (3.1) | 6.1 (3.0) |
| ≤ 5 years, n (%) | 19 (70) | 10 (40) | 29 (56) |
| ≥ 6 years, n (%) | 8 (30) | 15 (60) | 23 (44) |
| Gender (male), n (%) | 16 (59) | 15 (60) | 31 (60) |
| Ethnicity, n (%) | |||
| South Asian | 5 (18) | 5 (20) | 10 (19) |
| White | 21 (78) | 17 (68) | 38 (73) |
| Other/not stated | 1 (4) | 3 (12) | 4 (8) |
| BMI percentile,a median (IQR) | 82.1 (62.6–90.7) | 77.0 (59.5–93.6) | 77.8 (59.5–93.6) |
| BMI percentile,a n (%) | |||
| Underweight (< 5th) | 0 (0) | 0 (0) | 0 (0) |
| Healthy (5th–84th) | 15 (60) | 16 (64) | 31 (62) |
| Overweight (85th–95th) | 5 (20) | 4 (16) | 9 (18) |
| Obese (≥ 95th) | 5 (20) | 5 (20) | 10 (20) |
| Open-label prednisolone dose (mg/m2/day), mean (SD) | 60.3 (7.2) | 60.4 (3.3) | 60.3 (5.5) |
There were 35 relapses in 52 participants. There were no suspected unexpected serious adverse reactions, although three participants experienced a SAE (trapped finger stitched in theatre, hospital admission for abdominal pain and hospital admission for viral wheeze).
A decision was made to not unblind the pilot data prior to the commencement of the main study, but to allow these data to be added to the results of the main study using meta-analysis methods. One minor modification was made to the protocol for the main study following the pilot study, in that the visit schedule between months 6 and 12 was reduced from monthly to 2-monthly; monthly visits were felt to be too onerous for participants and in excess of the visit schedule for routine clinical care for the large majority of patients with SSNS at this stage post presentation.
The data from the 223 participants in the PREDNOS study and the 52 participants in the PREDNOS pilot study were combined using random-effects meta-analysis methods. In the pilot study, date of relapse was not recorded, so the date that relapse treatment was commenced was used for the relapse date in the time to first relapse analysis here. This meta-analysis included 275 participants (136 in SC group and 139 in EC group) and showed no significant difference in time to first relapse between the two treatment groups (pooled HR 0.76, 95% CI 0.50 to 1.17; p = 0.21) (Figure 13).
FIGURE 13.
Meta-analysis of time to first relapse for the PREDNOS and PREDNOS pilot study. df, degrees of freedom; IV, inverse variance.
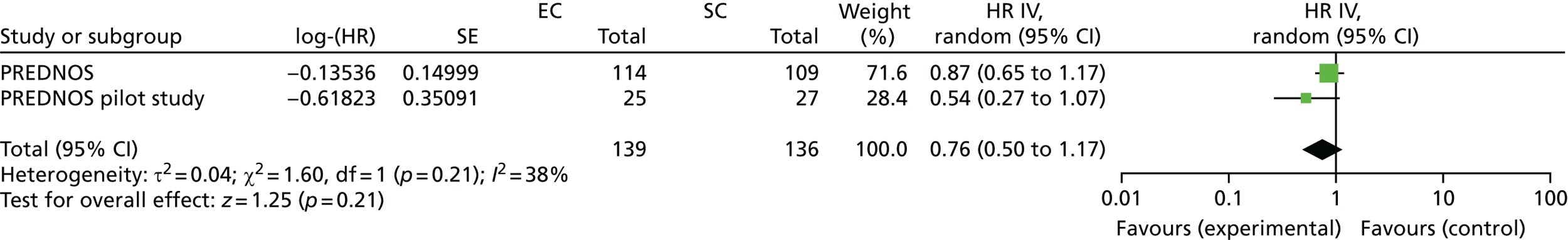
Chapter 5 Economic analysis: the mapping exercise
The economic analysis is organised into two chapters. Chapter 5 provides detail on the mapping exercise that was conducted to inform the outcomes for the main economic evaluation. Chapter 6 describes the economic evaluation to estimate the cost-effectiveness of extending prednisolone therapy over a 16-week period compared with the standard 8-week regimen for treating children with SSNS.
Background
The economic evaluation alongside the PREDNOS study consists of a cost–utility analysis with outcomes expressed as ‘cost per QALY’. To construct QALYs, utility values are derived from preference-based HRQoL instruments. The paediatric QoL PedsQL Generic Core Scale is a widely used instrument designed to measure HRQoL, and it is valid for children aged between 2 and 18 years. 48 However, as it is not a preference-based instrument, it is not suitable for estimating QALYs. The CHU-9D questionnaire is a preference-based instrument that has been developed primarily to support cost–utility analyses. However, it is valid only for 5- to 17-year-olds. As the population of the PREDNOS study included participants who were aged between 2 and 18 years, both instruments were employed. In order to derive utility scores for children aged < 5 years, a prediction algorithm, also known as a ‘mapping’ algorithm, was developed to estimate the CHU-9D score based on the responses to the PedsQL instrument. This chapter describes the method for constructing this prediction algorithm.
Condition-specific and generic instruments
Cost-effectiveness analysis is a comparative assessment of both costs and benefits linked to health-care interventions. Evidence of the benefits is often synthesised from clinical trials and may be captured as HRQoL using either ‘condition-specific’ or ‘generic’ survey instruments. Condition-specific instruments focus on health dimensions relevant to a particular disease, whereas generic instruments assess core dimensions of health that are relevant to all conditions. 49 Clinical trials often use condition-specific instruments as an outcome measure because these instruments are focused on the specific domains of QoL that are affected by a condition and are, therefore, sensitive to treatment effect in these domains. On the other hand, generic instruments measure a broader HRQoL construct;50 therefore, they allow comparisons of treatment benefit across a wide range of interventions across multiple conditions. Furthermore, generic instruments can be classed as either ‘preference-based’ or ‘non-preference-based’.
Preference-based versus non-preference-based instruments
Preference-based generic instruments attach weights to the domains of health to reflect a stronger preference for one domain of HRQoL over another, in order to generate a single weighted score, also known as a utility score. 51 In contrast, non-preference-based instruments simply sum the scores from all the health domains and, thus, assume an equal weighting. For cost–utility analysis, preference-based generic instruments are required to measure QoL, from which utility can be derived. The majority of generic instruments used in clinical trials are non-preference based,52 and are consequently of limited use for estimating the cost-effectiveness of diverse interventions on a common scale.
Validity of Child Health Utility 9D and Pediatric Quality of Life Inventory questionnaires across paediatric age groups
To capture both length and QoL associated with treatment, economists use QALYs,53,54 whereby cost-effectiveness of the treatment is expressed as cost per additional QALY gained. Within paediatric medicine, however, most HRQoL instruments developed for children and adolescents are non-preference based55 and, therefore, cannot be used for economic evaluation56 when QALYs are the desired outcome. However, a prediction algorithm/mapping function can be used to predict utility scores from responses to a non-preference-based instrument. This algorithm reflects the relationship between the preference and the non-preference-based instrument, using responses from a prior population.
Rationale for mapping within the PREDNOS study
Participants recruited into the study were aged between 2 and 15 years at baseline, with the oldest participant being 18 years old at the last follow-up. Therefore, in order to generate utility values for the2- to 18-year-olds within the trial, HRQoL information was collected either from both the PedsQL and the CHU-9D questionnaires for participants aged ≥ 5 years or from just the PedsQL instrument for participants aged 2–4 years. Therefore, utility values were directly elicited for all participants aged ≥ 5 years; for participants aged 2 to 4 years, the mapping algorithm was applied to predict the CHU-9D utility score based on the responses to the PedsQL instrument.
Methods
Outcome measures
The CHU-9D questionnaire was initially designed for children aged 7–11 years; however, further research has now extended its validity to children as young as 6 years57 and in adolescents up to age 17 years. 58 The self-reported and proxy-reported versions of the CHU-9D questionnaire each consist of nine dimensions: sad, worried, annoyed, tired, sleep, pain, school, routine and activity. Each dimension contains five severity levels, resulting in almost two million unique health states associated with the measure. Responses from the CHU-9D instrument are transformed into QoL (utility) weights derived from a UK general population sample using an algorithm developed by Stevens. 59 This gives a possible utility value set of between 0.33 (the worst health state) and 1 (the best health state).
The PedsQL Generic Core Scale is a well-validated non-preference-based measure. The self-reported version of the questionnaire has been validated in 5- to 18-year-olds, whereas the parent- or proxy-reported version is valid for use in 2- to 18-year-olds. Both versions of the instrument comprise 20 questions across four subscales or domains of health. There is a different PedsQL module for toddlers (aged 2–4 years), young children (aged 5–7 years), older children (aged 8–12 years) and adolescents (aged 13–18 years). The number of items within the health domain varies in some modules based on age of the respondent. The physical functioning domain has eight items, and both the emotional functioning and the social functioning domains have five items each. School functioning has five items for all age groups except toddlers, for whom there are only three items. Similar to the CHU-9D instrument, responses to each of the 23 items are on a five-point scale of increasing severity: never a problem, almost never a problem, sometimes a problem, often a problem and almost always a problem. Total scores are on a 0–100 scale, with 100 reflecting best-possible health state.
Data
In accordance with the study protocol, the proxy-reported version of the PedsQL and the CHU-9D questionnaires were used to collect HRQoL data at weeks 4 (baseline) and 16, and at months 12, 24, 36 and 48 for participants in both treatment groups. PedsQL was completed for participants across all the age groups in the trial (2–18 years), with the appropriate age-specific module for the instrument applied, whereas the CHU-9D questionnaire was completed only for participants who were aged ≥ 5 years. Data on participants who had completed both instruments across all the time points were considered relevant for the mapping exercise. In order to optimise the sample size, the data for this eligible cohort for the five time points in the RCT were combined and randomised into groups A and B. Observations with valid CHU-9D and PedsQL index scores, that is, after excluding missing items, in groups A and B will from here on be referred to as the estimation sample and the validation sample, respectively. Together, the two samples form the total mapping sample.
Model specification
First, to assess the conceptual overlap between the two instruments across the whole sample, the interdimensional correlations between the nine CHU-9D and the four PedsQL domains were explored using Spearman’s correlation. Next, the prediction mapping exercise involved regressing the CHU-9D utility scores (independent variable) against the PedsQL total, subscale or item scores (dependent variables) to generate an algorithm that could be subsequently used to predict the CHU-9D values. In order to select the model with the best goodness-of-fit statistic, three ‘functional forms’ were explored. The first was the ordinary least squares (OLS) regression with predicted utility scores censored at the value of 1. Although the OLS regression minimises the sum of squared errors, and represents the most common method within mapping studies,60 it has been shown that it does not cope well with multimodal distributions61 and does not always predict perfect health. The second was the generalised linear model (GLM),62 as it accommodates skewness in the estimation sample. The GLM requires specification of a distribution ‘family’ that captures the relationship between the mean and variance, and a link function between the linear part and the mean. The Modified Park test was applied to identify the preferred ‘family’ based on the lowest chi-squared value. The Hosmer–Lemeshow and Pearson correlation tests63,64 were used to select the link function, assumed as a good fit if both tests yielded non-significant p-values. The third form chosen for the prediction function was the tobit model, a censored regression that accommodates both the lower and upper limit utility scores. 65 Tobit models have been suggested for mapping despite concerns about inconsistencies in the presence of non-normality and heteroscedasticity. 66 In summary, six model specifications (covariates) were developed based on the OLS, tobit and the GLM ‘functional forms’, thus generating 18 models in total:
-
model 1 – PedsQL total scale score
-
model 2 – model 1, age, and sex
-
model 3 – PedsQL subscale scores
-
model 4 – model 3, age and sex
-
model 5 – PedsQL subscale score square terms and interaction terms
-
model 6 – model 5, age and sex.
The PREDNOS data are a longitudinal data set that can be viewed as having a two-level structure, for which the data collection time points (level 1 units) are nested within participants (level 2 unit). Random intercept mixed-effect models are often used to account for this hierarchical data structure, but this was not considered appropriate in this context because, for mapping purposes, the error variance was expected to be constant over time: the CHU-9D and the PedsQL data were collected from each individual at discrete time points and any variance in the estimation error over time was, therefore, assumed to be constant. In line with the assumption of constant variance over time, the PREDNOS data were considered to have only one hierarchical level, which is at the participant level. The within-participant correlation was taken into account by including the ‘clustering’ option for each of the 18 model specifications. For example, the model 1 specification was:
where [participant ID] was a unique participant identifier.
Assessing model performance
The following selection criteria were applied to assess the estimation performance of the models:67
-
Step 1. The models were assessed on the exactness of the predicted mean value in the estimation sample.
-
Step 2. One model from each functional form was selected based on its prediction accuracy in the estimation and validation sample. The indicators of prediction accuracy were the mean absolute error (MAE) and the mean squared error (MSE). The MAE is the mean absolute difference between the observed and the predicted values, while MSE is the mean squared difference between the observed and the predicted CHU-9D utility score. Larger MAE and MSE indicate poorer fit. MAE was prioritised over MSE, which has been shown to be less sensitive to outliers often found within the utility data. 68
-
Step 3. To assess and compare the shortlisted models estimated in step 2, a number of criteria were considered:
-
The distribution of the predicted and the observed CHU-9D scores were plotted to examine how well the predicted scores matched the observed scores.
-
The proportion of predictions deviating from observed values by < 0.03, < 0.05 and < 0.1 were calculated as a representation of how often the models produce reliable predictions.
-
The MAEs were presented for different CHU-9D value ranges to assess how well the models perform at the top and bottom of the index score range.
-
All of the analysis described follows the MApping onto Preference-based measures reporting Standards (MAPS). 69
Results
Sample characteristics
There were 643 observations across the five data collection time points from participants who were aged ≥ 5 years. These observations were randomised into groups A (n = 321) and B (n = 322). The longitudinal nature of the study meant that the number of missing data in the groups varied across the data collection points. After removing missing items, 279 observations with pairs of valid PedsQL and CHU-9D index scores in the first group formed the estimation sample, while the 284 observations in the second group formed the validation sample. The estimation and validation samples constitute the total mapping sample (n = 563). Figure 14 shows the distribution of the CHU-9D and PedsQL scores in the estimation and validation samples.
FIGURE 14.
Distribution of CHU-9D and PedsQL scores in the estimation and validation samples. (a) Estimation sample, CHU-9D; (b) validation sample, CHU-9D; (c) estimation sample, PedsQL; and (d) validation sample, PedsQL.



Table 8 reports the descriptive statistics for each sample at each time point. Overall, it seems that the randomisation ensured a balanced distribution of demographic characteristics between the estimation and the validation samples.
| Characteristic | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline (4 weeks) | 16 weeks | 12 months | 24 months | 36 months | 48 months | |
| Estimation sample | N = 55 | N = 47 | N = 58 | N = 54 | N = 39 | N = 26 |
| Age (year) | ||||||
| Mean (SD) | 7 (2.1) | 7.6 (2.1) | 7.4 (2.1) | 7.2 (1.9) | 7.3 (1.9) | 8.1 (2.0) |
| Median (IQR) | 6 (3) | 7 (4) | 7 (3) | 7 (2) | 7 (3) | 8 (3) |
| Range | 5–12 | 5–12 | 5–12 | 5–12 | 5–12 | 5–12 |
| Gender | ||||||
| Male, n (%) | 35 (63.6) | 33 (70.2) | 36 (62.1) | 32 (59.2) | 23 (58.9) | 18 (69.2) |
| CHU-9D score | ||||||
| Mean (SD) | 0.940 (0.063) | 0.929 (0.103) | 0.941 (0.080) | 0.950 (0.068) | 0.922 (0.081) | 0.937 (0.077) |
| Median (IQR) | 0.952 (0.106) | 0.952 (0.100) | 0.952 (0.081) | 0.968 (0.073) | 0.931 (0.108) | 0.967 (0.107) |
| Range | 0.786–1.000 | 0.534–1.000 | 0.509–1.000 | 0.68–1.000 | 0.702–1.000 | 0.697–1.000 |
| PedsQL score | ||||||
| Mean (SD) | 77.11 (16.16) | 82.4 (16.8) | 81.94 (15.91) | 84.24 (14.31) | 78.49 (20.58) | 80.85 (17.72) |
| Median (IQR) | 79.35 (28.26) | 89.13 (29.35) | 87.5 (20.65) | 88.04 (18.48) | 82.61 (30.43) | 82.61 (29.35) |
| Range | 40.22–100.00 | 45.65–100.00 | 41.3–100.00 | 43.48–100.00 | 31.52–100.00 | 39.13–100.00 |
| Validation sample | N = 36 | N = 46 | N = 50 | N = 70 | N = 56 | N = 26 |
| Age | ||||||
| Mean (SD) | 6.9 (1.8) | 7.1 (1.9) | 7.3 (2.0) | 7.6 (2.2) | 7.4 (2.2) | 8 (1.9) |
| Median (IQR) | 7 (3) | 7 (2) | 7 (3) | 7 (3) | 7 (3) | 8 (2) |
| Range | 5–11 | 5–12 | 5–12 | 5–12 | 5–13 | 5–13 |
| Gender | ||||||
| Male, n (%) | 25 (69.4) | 30 (65.2) | 32 (64.0) | 44 (62.9) | 29 (51.8) | 15 (57.7) |
| CHU-9D score | ||||||
| Mean (SD) | 0.924 (0.081) | 0.945 (0.067) | 0.941 (0.075) | 0.938 (0.076) | 0.951 (0.067) | 0.945 (0.06) |
| Median (IQR) | 0.952 (0.1) | 0.96 (0.079) | 0.96 (0.081) | 0.952 (0.102) | 0.967 (0.071) | 0.959 (0.097) |
| Range | 0.711–1 | 0.69–1 | 0.739–1 | 0.65–1 | 0.712–1 | 0.828–1 |
| PedsQL score | ||||||
| Mean (SD) | 75.88 (16.91) | 81.35 (14.53) | 78.28 (19.01) | 80.6 (17.25) | 83.13 (19.11) | 81.68 (20.3) |
| Median (IQR) | 77.72 (27.36) | 83.7 (18.48) | 83.7 (28.26) | 86.96 (27.17) | 91.85 (27.17) | 90.76 (20.65) |
| Range | 42.39–97.83 | 41.3–100 | 21.74–100 | 33.7–100 | 40.22–100 | 29.35–100 |
The mean CHU-9D utility score across all time points was 0.9374 (SD 0.0790) and 0.9409 (SD 0.0717) in the estimation and validation samples, respectively. The corresponding mean PedsQL score across all time points was 80.93 (SD 16.76) in the estimation sample and 80.31 (SD 17.79) in the validation sample. Within each sample, the mean PedsQL total score was lower than the mean CHU-9D utility score when both scores were standardised on a 100-point scale. Although both HRQoL measures were negatively skewed, the ceiling effect was more prominent with CHU-9D. Tables 23 and 24 in Appendix 3 summarise the CHU-9D responses for the estimation and validation samples across all data collection time points. Level 1 or ‘no problem’ always had the highest proportion of responses, hence the observed ceiling effect for the CHU-9D index score.
Performance and validation
Table 25 in Appendix 3 summarises the performance measures for all the model specifications, for both the estimation and the validation samples. Within the estimation sample, the models were able to reasonably predict the mean CHU-9D value (0.93742, SD 0.07898). Of the 18 models, 12 were able to predict the precise mean value by up to 1/10,000th of a QALY. The exceptions were the six tobit models. However, within the validation sample, the models were less able to predict the mean CHU-9D value (0.94094, SD 0.07174). The model GLM 2 had lowest mean predicted value (0.93409), whereas the model Tobit_3 had the highest mean predicted value (0.96575), giving a difference between the observed and predicted mean values of 0.0069 and 0.0245, respectively. These differences were below the threshold of 0.03, for which differences smaller than this level are considered to be a minimally important difference. 70,71 A further observation was that some OLS models and all the tobit models had maximum predicted values beyond the upper limit of the CHU-9D utility scale (0.33–1.00). However, none of the models predicted a utility value below the lower limit of the CHU-9D utility scale.
Models were initially assessed in terms of their ability to predict the mean value in the estimation sample. All GLM and OLS models were consequently shortlisted for further comparison and progress to ‘step 2’. The two models (GLM 6 and OLS 3) that had the ‘best’ performance in terms of MAE, in both the estimation and validation samples, were selected for a final comparison: ‘step 3’. Table 9 contains the model performance results for both of these models.
| Statistics | Sample | |||||
|---|---|---|---|---|---|---|
| Estimation | Validation | |||||
| Observed | GLM 6 | OLS 3 | Observed | GLM 6 | OLS 3 | |
| Mean | 0.937419 | 0.937419 | 0.937419 | 0.940941 | 0.937612 | 0.939018 |
| SD | 0.078978 | 0.051926 | 0.047318 | 0.071737 | 0.054762 | 0.046323 |
| CV | 0.084251 | 0.055393 | 0.050477 | 0.076240 | 0.058406 | 0.049331 |
| Minimum value | 0.509400 | 0.660930 | 0.812068 | 0.650000 | 0.705160 | 0.788717 |
| P 25 | 0.907600 | 0.910639 | 0.900076 | 0.912300 | 0.914908 | 0.905183 |
| P 50 | 0.952100 | 0.957229 | 0.946303 | 0.952100 | 0.958496 | 0.950063 |
| P 75 | 1.000000 | 0.978902 | 0.980433 | 1.000000 | 0.977276 | 0.977413 |
| Maximum value | 1.000000 | 0.989350 | 0.995221 | 1.000000 | 0.985504 | 0.993891 |
| MSE | – | 0.00353 | 0.00398 | – | 0.00345 | 0.00310 |
| MAE | – | 0.04078 | 0.04245 | – | 0.04182 | 0.03981 |
| < 0.03 AE (%) | – | 53.40 | 51.61 | – | 55.89 | 53.17 |
| < 0.05 AE (%) | – | 72.04 | 70.25 | – | 73.23 | 70.77 |
| < 0.10 AE (%) | – | 92.27 | 90.32 | – | 91.20 | 93.31 |
For the GLM, a logit transformation of the variable containing the CHU-9D utility scores was applied before the variable was used as the dependent in the prediction equation. As such, any predicted value from that equation will be a transformed value and, therefore, requires a back-transformation to estimate utility values. The information on the back-transformation step is as follows. Given that GLM 6 has a logit link, the CHU-9D utility values are calculated as shown below:
For the final models in step 3, in addition to assessing how accurately the models estimated the mean CHU-9D score in the validation sample, the distribution of the predicted score was also examined (see Figure 17, Appendix 3). GLM 6 had a wide range of predicted CHU-9D scores compared with OLS 3.
Approximately 56% of the predicted values from GLM model 6 in the validation sample had absolute errors less than the minimally important difference value of 0.03; the corresponding values for the OLS model 3 was 53%. GLM model 6 remained the preferred model specification when the error threshold was extended to 0.05.
Although the prediction accuracy of the mean scores was similar in both models, the accuracy level was not uniform across the CHU-9D utility range, as shown in Table 10. The number of observations with utility score of < 0.7 was small; therefore, the comparison between the best two models was restricted to observations with higher utility values. GLM 6 was superior to OLS 3 in the estimation sample; however, in the validation sample there were diverging results. OLS 3 had a better prediction accuracy when utility values were > 0.8, but less than full health, while the GLM 6 was superior at predicting full health and utility values between 0.7 and 0.8. So, although OLS 3 had a better prediction accuracy in the validation sample overall, it was found to be only marginally better than GLM 6.
| CHU-9D range | n | Model | |||
|---|---|---|---|---|---|
| GLM 6 | OLS 3 | ||||
| MSE | MAE | MSE | MAE | ||
| Estimation | |||||
| 0.5 ≤ value < 0.6 | 3 | 0.09443 | 0.30095 | 0.11168 | 0.33058 |
| 0.6 ≤ value < 0.7 | 6 | 0.01873 | 0.12096 | 0.02823 | 0.16497 |
| 0.7 ≤ value < 0.8 | 11 | 0.00726 | 0.07674 | 0.00988 | 0.09602 |
| 0.8 ≤ value < 0.9 | 49 | 0.00301 | 0.04441 | 0.00259 | 0.04034 |
| 0.9 ≤ value < 0.10 | 111 | 0.00154 | 0.02853 | 0.00155 | 0.03015 |
| Full health | 102 | 0.00242 | 0.03847 | 0.00279 | 0.03899 |
| Validation | |||||
| 0.6 ≤ value < 0.7 | 3 | 0.05502 | 0.23049 | 0.05277 | 0.22693 |
| 0.7 ≤ value < 0.8 | 12 | 0.01185 | 0.09583 | 0.01376 | 0.10958 |
| 0.8 ≤ value < 0.9 | 47 | 0.00468 | 0.05691 | 0.00329 | 0.04316 |
| 0.9 ≤ value < 0.10 | 115 | 0.00187 | 0.03057 | 0.00131 | 0.02942 |
| Full health | 107 | 0.00223 | 0.03593 | 0.00237 | 0.03643 |
In summary, relative to GLM 6, OLS 3 lacked the ability to predict the wider range of CHU-9D values (0.7–1), and a higher proportion of its predicted values had absolute errors above the minimally important difference. Furthermore, it was less able to predict full health, which is particularly important for utility data that tend to have ceiling effects. Taking all these factors into account, the GLM 6 model was selected as the preferred model for mapping from PedsQL to CHU-9D. Table 11 shows the coefficients for generating deterministic and probabilistic utility predictions using the GLM 6 model. Coefficients for OLS 3 have also been presented in situations in which this might be desired.
| Covariate | Model | |||
|---|---|---|---|---|
| GLM 6 | OLS 3 | |||
| Coefficient | SE | Coefficient | SE | |
| PedsQL PF squared | 0.0001615 | 0.000103 | – | – |
| PedsQL EF squared | 0.0004766*** | 0.000127 | – | – |
| PedsQL SF squared | –0.0000402 | 0.000145 | – | – |
| PedsQL FU squared | –0.0001646 | 0.000101 | – | – |
| PedsQL PF × EF | –0.0001103 | 0.000147 | – | – |
| PedsQL PF × SF | –0.000114 | 0.000173 | – | – |
| PedsQL PF × FU | 0.0000371 | 0.000143 | – | – |
| PedsQL EF × SF | –0.0002461 | 0.000209 | – | – |
| PedsQL EF × FU | –0.0001158 | 0.000167 | – | – |
| PedsQL SF × FU | 0.0004356*** | 0.00013 | – | – |
| PedsQL PF | – | – | 0.0007133* | 0.000297 |
| PedsQL EF | – | – | 0.0016477*** | 0.000228 |
| PedsQL SF | – | – | –0.00011 | 0.000383 |
| PedsQL FU | – | – | 0.000261 | 0.000276 |
| Age | 0.0279345 | 0.039717 | – | – |
| Sex | –0.0546336 | 0.146341 | – | – |
| Constant | 0.7135215 | 0.399623 | 0.7422337*** | 0.028841 |
Discussion
Although complying with current guidance for conducting and reporting mapping analyses,69 the results of this analysis show that CHU-9D utility scores can be estimated from PedsQL subscale scores with sufficient accuracy. Six models, each with three functional forms, were explored. All of the models produced reasonably similar predictions of the mean utility scores. The GLM 6 and OLS 3 models, with MAE of 0.04078 and 0.04245, respectively, were the two models that performed particularly well. Overall, GLM 6 was chosen as the preferred mapping model because of its better prediction accuracy over a wider range of CHU-9D utility scores.
In comparison with other similar published studies, the GLM 6 model (MAE 0.04078; MSE 0.00353) predicted the CHU-9D utility scores with more accuracy. For example, in one study that looked at the relationship between the CHU-9D and the Strengths and Difficulties Questionnaire, the MSE was 0.124;72,73 whereas another study that estimated CHU-9D utility scores from the KIDSCREEN questionnaire had a MAE of 0.095. 74 The GLM 6 model produced from this analysis also performed better than a previous model that had predicted EuroQoL-5 Dimension Youth version utility scores from PedsQL (MAE 0.115). 67
Despite these strengths, there are some limitations. The sample size was small compared with other mapping studies,52 thereby limiting the ability to robustly demonstrate the relationship between CHU-9D and PedsQL scores. A larger sample size may have reduced the prediction error of the model. Another caveat was the ceiling effect. A wider spectrum of health profiles was lacking because a considerable number of participants had perfect or near-perfect health, with none having utility scores of < 0.5 in the estimation sample. This was reflected in the distribution of scores across the five response levels for each of the CHU-9D domains and each PedsQL subscale score. This implies that caution should be exercised when using the algorithm in a less healthy population. Future research can focus on refining this mapping algorithm should data for children with more severe health states become available.
Mapping is not a substitute for direct utility estimation. Therefore, it is advised that, when possible, preference-based outcomes be collected for the measurement of cost-effectiveness. However, in the event that this is not feasible, the algorithm from the model presented here provides a valuable, justifiable and scientifically robust approach to predicting CHU-9D utility values. This mapping algorithm will be applied to generate utility scores for the PREDNOS trial population, who are aged between 2 and 4 years. The standard errors (SEs) for the coefficients have been reported, making it imperative for such evaluations to account for the uncertainty around the predicted values.
Chapter 6 Economic evaluation
Chapter 6 describes the economic evaluation alongside the PREDNOS trial. The overall aim was to estimate the cost-effectiveness of an extended prednisolone regimen over a 16-week period compared with a standard 8-week regimen for treating children with SSNS. The primary evaluation was a cost–utility analysis, with the outcome measured in terms of QALYs. All costs were expressed in 2015 prices.
Missing data were addressed using multiple imputation techniques. Uncertainty surrounding the cost-effectiveness estimates was examined using probabilistic sensitivity analysis75 and presented using cost-effectiveness acceptability curves (CEACs). The analysis of cost-effectiveness was conducted according to current best practice methods for conducting economic evaluations alongside clinical trials. 76
Aim
To estimate the cost-effectiveness of an extended prednisolone regimen over a 16-week period compared with the standard 8-week regimen for treating children with SSNS.
Methods
Data collection
To conduct the economic evaluation, data on both resource use and outcomes77 were collected within the study on case report forms. In line with standard practice, only resource use that was incurred in delivering the intervention was considered in the base-case scenario. To calculate the overall cost of the treatment, resource use data were multiplied by relevant unit costs. To calculate QALYs, health utility data were collected using the CHU-9D instrument59 at baseline (commencement of the study drug regimen at week 4), at week 16 and at months 12 and 24.
Health economic outcomes
Measuring quality-adjusted life-years
Health-related QoL information was collected using both the generic non-preference-based PedsQL Generic Core Scale and the preference-based CHU-9D questionnaire. The PedsQL and the CHU-9D (for children aged ≥ 5 years) questionnaires were proxy completed by parents or guardians at the relevant time points.
Two approaches were used to estimate utility values according to the age group of the trial population. For participants aged ≥ 5 years, utility values were estimated directly using the responses to the CHU-9D instrument and applying an existing UK value set59 (referred to as the direct estimation method). For participants aged between 2 and 4 years, a crosswalk/mapping technique was applied using the responses to the PedsQL questionnaire. For more detail on the mapping algorithm, see Chapter 5.
Assessing quality-adjusted life-year differences
Quality-adjusted life-years over 24 months were calculated for each participant using the area-under-the-curve method. 78 A univariate and multivariate regression model was then conducted to estimate QALY differences between the two treatment groups. In the univariate model, the utility scores over the trial period were regressed against intervention group status. In the multivariate regression model, the following variables were added as additional independent variables: baseline CHU-9D score, baseline age and gender.
To summarise, the two models reported are:
-
a univariate linear regression model
-
a multivariate linear regression model controlling for baseline utility, baseline age and gender.
Resource use data and cost analysis
A combination of micro (bottom-up) and macro (top-down) costing methodology was used to estimate the costs associated with each treatment group of the trial. Resource use items were grouped into three categories: primary care, secondary care and medication costs. Resource use data were collected at baseline, at weeks 4, 8, 12 and 16 and at months 5, 6, 8, 10, 12, 18 and 24. The cost analysis adopted a UK health sector perspective and costs reported are in 2015 prices.
Primary care resource cost
At each data collection time point, the primary care resource use was captured in terms of the recorded number of visits to the GP, practice nurse or other primary care staff for each participant between the relevant time point and the preceding data collection time point. Since the specific reason for the visit was not reported, the average unit cost was used and multiplied by the frequency of visits over each time period. The total primary care cost for each participant was then derived by simply summing the costs incurred by visits to the GP, nurse or other primary care staff over the 24-month period.
Secondary care cost
All resource use associated with secondary care was categorised into outpatient hospital care, emergency visits and hospital admissions. The cost of outpatient hospital care was then estimated by multiplying the number of visits by the unit cost of one visit. Different unit costs were used for consultant and non-consultant led visits. For emergency episodes and hospital admissions, these were further categorised into renal-related or non-renal-related admissions depending on whether or not the primary carer of the child reported that the admission was related to nephrotic syndrome. The cause of admission was assumed to be of renal aetiology if the carer did not report this information. Only nephrotic syndrome-related admissions were considered in the base-case analysis.
The cost of a hospital admission was estimated using the time interval between the admission and discharge date. An admission was considered as a zero bed-stay if the participant was admitted and discharged within 24 hours. A 5-day admission episode, which is the maximum expected length of stay for nephrotic syndrome in the UK,79 was assumed in situations in which the admission date was recorded but the discharge data were unavailable. The number of bed-days was considered as zero when both the admission and discharge dates were missing. Admission episodes with ≤ 5 bed-days were within what is known as a ‘trim point’ and were priced at a flat rate. Admission episodes with > 5 bed-days first incurred the trim-point flat rate, and then the excess bed-days were priced per additional bed-day at a ‘regular day rate’. Zero bed-stays incurred one regular day rate tariff. SAEs require either outpatient or inpatient hospital care. Therefore, to avoid double-counting, the cost of SAEs was not costed separately. Instead, it was assumed that this was captured by the recorded inpatient and outpatient hospital care data.
Medication cost
Prednisolone costs were estimated from the British National Formulary based on dose, quantity consumed and drug formulation. Information on other prescribed medications other than the study medication, such as second-line immunosuppressants, was captured as free text on the trial forms. The free-text sections were reviewed manually to establish the name, the dose and the formulation of the medication. This microcosting approach was considered important because the treatment of SSNS is associated with medications that have high daily costs. Therefore, it was considered essential to account for these high-cost drugs in the economic evaluation. After extracting prescription information from the free-text sections, the medications were classified into 31 groups. An index drug was identified in each group, to which a unit cost was attached. The unit cost of the index medication was then applied to all prescriptions within the group.
Data on non-prescribed medications were recorded as total out-of-pocket expenditure on medication. Costing of this item was not necessary, as the item was already captured in Great British pounds. This cost is included for descriptive purposes only and was not included in the economic evaluation, as this falls outside the health-care perspective for the analysis.
Unit costs
Primary care unit costs for pharmacist, nurse or GP consultations (telephone, home visit or practice based) were sourced from the Unit Costs of Health and Social Care 2015. 80 The National Schedule of Reference Costs79,81 was used to cost hospital-related activities. Within the reference cost schedule, resource use items within the NHS are coded into Healthcare Resource Group (HRG), onto which costs/tariffs are attached. The tariff for the nephrotic renal disease HRG code (PL69C) was chosen as the unit cost for all renal admissions. The British National Formulary82 provided unit costs for medicines by using doses reported in the case report form. Table 12 outlines the unit cost details that apply to all resource use items.
| Staff time | Details and assumptions | Source | |
|---|---|---|---|
| Mean duration of contact and mean cost of contact per hour | Cost per contact | ||
| GP | 17.2-minute contact with GP at £171/hour | £49.02 | PSSRU 201580 |
| Practice nurse | 15.5-minute contact with band 5 staff at £36/hour | £9.29 | PSSRU 201580 |
| Other staff | 15.5-minute contact with band 4 staff at £29/hour | £7.49 | PSSRU 201580 |
| Secondary care resource use | Details and assumptions | Cost per visit | |
| Renal emergency visit | HRG code PA69Z (non-elective spell) | £1321.00 | 2015 tariff81 |
| Renal elective admission | HRG code PA69Z (ordinary elective spell) | £648.00 | 2015 tariff81 |
| Renal elective admission after trim-point | HRG code PA69Z (per day long-stay tariff) | £285.00 | 2015 tariff81 |
| Paediatric outpatient | General paediatric outpatient | £220.00 | 2015 tariff81 |
| Medication | Drug (strength); dose/pack (cost in £); prescription | Cost per month | |
| Aciclovir | Acyclovir (800 mg); 35 doses (£4.38); 1 tablet QDS | £15.01 | BNF 201582 |
| Analgesics | Ibuprofen (200 mg); 84 doses (£3.50); 1 tablet TDS | £3.75 | BNF 201582 |
| Antacid and antiemetic | Ranitidine (150 mg); 60 doses (£34.09); 2 tablets BD | £34.09 | BNF 201582 |
| Antibiotics (other oral) | Cefixime (200 mg); 7 doses (£13.23); 1 tablet OD | £56.70 | BNF 201582 |
| Antibiotics (other topical) | Clindamycin (30 ml); 60 doses (£4.34); 1 application BD | £4.34 | BNF 201582 |
| Anticoagulants | Heparin (1000 IU); 1 dose (£1.49); 1 ampoule BD | £89.40 | BNF 201582 |
| Antihypertensive (angiotensin-converting enzyme inhibitor) | Lisinopril (2.5 mg); 28 doses (£0.89); 1 tablet OD | £0.95 | BNF 201582 |
| Antihypertensive (beta-blockers) | Atenolol (50 mg); 28 doses (£0.87); 1 tablet OD | £0.93 | BNF 201582 |
| Antihypertensive (calcium blockers) | Amlodipine (5 mg); 28 doses (£0.91); 1 tablet OD | £0.97 | BNF 201582 |
| Antihypertensive (others) | Metolazone (5 mg); 28 doses (£10.42); 1 tablet BD | £22.32 | BNF 201582 |
| Bath gel and Emollients | Calmurid® (Galderma, Watford) (100 g); 60 doses (£33.40); 1 application BD | £33.40 | BNF 201582 |
| Bronchodilators (inhaled) | Salbutamol (100 µg); 200 doses (£1.50); 2 puffs QDS | £1.80 | BNF 201582 |
| Ciclosporin | Ciclosporin (50 mg); 30 doses (£25.50); 1 tablet BD | £51.00 | BNF 201582 |
| Co-trimoxazole | Co-trimoxazole (480 mg); 28 doses (£3.14); 1 tablet BD | £6.73 | BNF 201582 |
| Cyclophosphamide | Cyclophosphamide (50 mg); 100 doses (£139.00); 1 tablet OD | £41.70 | BNF 201582 |
| Food supplements and probiotics | Abidec® (Omega Pharma Ltd, London) (25 ml); 20 doses (£3.33); 2 drops OD | £9.99 | BNF 201582 |
| Furosemide | Furosemide (40 mg); 28 doses (£0.83); 1 tablet TDS | £2.67 | BNF 201582 |
| Methylprednisolone (i.v.) | Methylprednisolone (500 mg); 1 dose (£9.60); 1 vial OD | £96.00 | BNF 201582 |
| Levamisole | Levamisole (50 mg); 1 dose (£1.18); 1 tablet SD | £11.16 | BNF 201582 |
| Mycophenolate mofetil | Mycophenolate (500 mg); 50 doses (£9.31); 1 tablet BD | £11.17 | BNF 201582 |
| Omeprazole | Omeprazole (10 mg); 28 doses (£1.18); 1 tablet OD | £1.26 | BNF 201582 |
| Penicillin | Penicillin V (250 mg); 28 doses (£1.18); 1 tablet QDS | £5.06 | BNF 201582 |
| Rituximab | Rituximab (375 mg); 1 dose (£873.15); 1 infusion weekly | £3492.60 | BNF 201582 |
| Spironolactone | Spironolactone (50 mg); 28 doses (£2.13); 1 tablet OD | £2.28 | BNF 201582 |
| Steroid (inhaled) | Seretide (250 µg); 60 doses (£35.00); 1 puff BD | £35.00 | BNF 201582 |
| Steroid (other oral) | Dexamethasone (500 µg); 28 doses (£56.54); 2 tablets BD | £121.16 | BNF 201582 |
| Steroid (topical) | Hydrocortisone (15 g); 30 doses (£5.39); 1 application BD | £10.78 | BNF 201582 |
| Tacrolimus | Capexion (1 mg); 50 doses (£68.20); 3 capsules BD | £245.52 | BNF 201582 |
| Trimethoprim | Trimethoprim (100 mg); 28 doses (£8.44); 1 tablet BD | £18.09 | BNF 201582 |
| Zoster immune globulin | Influenza vaccination (0.5 ml); single dose | £6.29 | BNF 201582 |
| Study drug | Drug (strength); dose/pack (cost in £); prescription | Cost per milligram | |
| Prednisolone | Prednisolone (5 mg); 28 doses (£1.61); as per study prescription | £0.01 | BNF 201582 |
Assessing cost differences
The total health-care cost at each time point was obtained by summing the medication cost and primary and secondary care costs. Similar to the estimation of between-group QALY differences, the mean cost difference between the two treatment groups was estimated using a regression-based technique while controlling for baseline age and gender.
Multiple imputation
The cost data were considered missing if participants reached a particular time point without having withdrawn from the study but did not return the study case report form. If information on resource use was missing from the returned questionnaires then the items in question were assumed not to have been used and consequently were allocated a cost of zero. In the case of the CHU-9D questionnaire, however, no response for one or more items resulted in a missing CHU-9D index score, as all nine items are necessary for computing the index score.
In line with the study protocol, missing data were imputed at each time point. 83 QALYs and resource use data were collected across 6 and 14 time points, respectively, over the study duration; therefore, these were imputed separately. While assuming that data were missing at random, multiple imputation was used to generate 40 imputed data sets for each cost item and QALY variable. The predictive mean matching method for multiple imputation was used to account for the non-normality of the distribution of costs and QALY scores. This method ensures that the imputations took only values from the data that were available in the original trial data. The imputation of costs was conducted at the level of resource group type (e.g. hospital admission, emergency visits, medication). Other variables included in the imputation model were age, sex and treatment group because these were thought a priori to be associated with missingness. Rubin’s rule84 was used to combine the 40 imputed data sets into one final imputed variable. A visual inspection of the histograms of the non-imputed and combined imputed variables was conducted to confirm that both distributions were similar.
Cost-effectiveness analysis
The mean differences in costs and QALYs between the extended and the standard prednisolone regimens over 24 months were estimated according to the ITT principle, meaning that data from all randomised participants were analysed irrespective of protocol deviations or participant withdrawal or death during the trial. The ratio of the mean difference in cost and the mean difference in QALYs between the two groups was calculated to estimate the cost per additional QALY gained. Resource use data (costs) and QALY data were skewed; therefore, the 95% CI of the arithmetic mean difference between the treatment groups for these parameters was obtained from non-parametric bootstraps, each with 5000 replications. The incremental cost-effectiveness ratio (ICER), which is the mean difference in cost and the mean difference in QALYs between the two groups, was then calculated to produce the cost per additional QALY gained.
A probabilistic sensitivity analysis was conducted by jointly bootstrapping the mean difference in cost and QALYs to produce 5000 paired estimates. This was to account for the uncertainty due to sampling variation in the participant-level data. The bootstrapped pairs of mean cost and QALYs were then graphically presented on a cost-effectiveness plane, and a CEAC was constructed from the plotted points. The incremental net benefit was used to construct the CEAC because of well-established limitations when ICERs from bootstrap replicates are spread over the four quadrants of the cost-effectiveness plane. 85,86 The CEAC shows the probability of the extended prednisolone regimen being cost-effective at different cost per QALY thresholds. In the UK, interventions are considered cost-effective if the cost per additional QALY gained is < £20,000. 76
A 3.5% discount rate was applied to all costs and outcomes, in accordance with National Institute for Health and Care Excellence’s guidelines for health technology appraisal. 76 Stata version 13 was used to perform the analysis.
Subgroup analysis
A series of sensitivity analyses (as per protocol) were conducted to assess the robustness of the base-case results to some assumptions made. To test the impact of imputing missing data, the analysis was conducted only on participants for whom we had complete data. Another subgroup analysis was based on the a priori assumption that prednisolone may have a different response in participants from different ethnic backgrounds. Ethnicity may also influence how households control exposure to infection in children who become immunosuppressed. The ethnic groups that were examined were South Asian, white and all other ethnic groups. The primary analysis results were also examined to see whether or not the results differed between participants who were 5 years or younger and those who were older. This is because the age at initial presentation has an important impact on the underlying cause of the disease, which may in turn have an impact on relapse rates, QoL and health-care consumption.
Results
Impact of extended prednisolone therapy on health-related quality of life
A total of 207 participants had been followed up to the 24-month primary end point, which was 92.8% of the ITT study population. The proportion of the study population that returned the study questionnaires at each time point gradually declined over the 24 months but did not fall below 90%, as shown in Table 13. Complete items on the entire eight CHU-9D domains were required for the calculation of the CHU-9D index score in older participants. The four PedsQL domain scores were also required for indirect estimation of CHU-9D score for younger participants. After excluding missing items, 90% of the participants within the study had a valid CHU-9D score at baseline. This proportion declined to 85% at month 24. One hundred and sixty-eight (75.3%) participants had a valid CHU-9D index score at baseline, at week 16 and at months 12 and 24.
| Time point | Followed up, n (%) | Followed up and returned questionnaire, n (%) | Valid CHU-9D index score calculated, n (%) | No valid CHU-9D index score (as % of participants in treatment group), n (%) | |
|---|---|---|---|---|---|
| SC (N = 109) | EC (N = 114) | ||||
| Week 4 | 223 (100.0) | 222 (99.6) | 201 (90.1) | 13 (11.9) | 9 (7.9) |
| Week 16 | 215 (96.4) | 211 (94.6) | 199 (89.2) | 12 (11.0) | 12 (10.5) |
| Month 12 | 209 (93.7) | 207 (92.8) | 202 (90.6) | 9 (8.3) | 12 (10.5) |
| Month 24 | 207 (92.8) | 203 (91.0) | 190 (85.2) | 13 (11.9) | 20 (17.5) |
| Complete case | – | – | 168 (75.3) | 30 (27.5) | 25 (21.9) |
Table 14 describes the mean utility value at each time point for each treatment group. It further classifies the data into utility scores for participants aged ≥ 5 years (collected using the CHU-9D questionnaire), and for all the participants in the study with a valid CHU-9D utility score – both mapped and direct CHU-9D utility scores. The mean utility at week 4 (baseline) for all participants across all age groups was 0.9242. At baseline, the mean utility for the SC group was 0.9231 compared with 0.9252 for the EC group. This baseline difference was adjusted for within the main cost–utility analyses. 78
| Time point | Group | Whole sample | ||||
|---|---|---|---|---|---|---|
| SC | EC | |||||
| n | Mean score (SD) | n | Mean score (SD) | n | Mean CHU-9D score (SD) | |
| Direct CHU-9D estimation | ||||||
| Baseline (week 4) | 42 | 0.9289 (0.0752) | 58 | 0.9301 (0.0686) | 101 | 0.9296 (0.0710) |
| Week 16 | 47 | 0.9394 (0.0665) | 54 | 0.9353 (0.0984) | 101 | 0.9372 (0.0846) |
| Month 12 | 56 | 0.9442 (0.0703) | 63 | 0.9396 (0.0826) | 119 | 0.9418 (0.0768) |
| Month 24 | 69 | 0.9341 (0.0784) | 70 | 0.9548 (0.0666) | 139 | 0.9445 (0.0732) |
| Direct and indirect CHU-9D estimation | ||||||
| Baseline | 96 | 0.9231 (0.0675) | 105 | 0.9252 (0.0656) | 201 | 0.9242 (0.0664) |
| Week 16 | 97 | 0.9333 (0.0567) | 102 | 0.9391 (0.0760) | 199 | 0.9363 (0.0672) |
| Month 12 | 100 | 0.9383 (0.0639) | 102 | 0.9425 (0.0688) | 202 | 0.9405 (0.0663) |
| Month 24 | 96 | 0.9316 (0.0708) | 94 | 0.9492 (0.0624) | 190 | 0.9403 (0.0672) |
Table 15 describes the unadjusted and the adjusted mean QALYs for each treatment group of the study. Participants in the study were required to have been followed up for at least 24 months. At month 24, the mean adjusted QALY difference between the groups was 0.0162 in favour of EC therapy; however, this difference was not significant at the 5% level. The lack of significance was attributable to the way the QALYs were calculated using the ‘area-under-the-curve’ method, which adjusts for imbalances in the CHU-9D score at baseline (week 4).
| Time point | Group | Bootstrapped difference (95% CI) | |
|---|---|---|---|
| SC | EC | ||
| Unimputed CHU-9D score, mean (SD) | |||
| Baseline | 0.9231 (0.0675) | 0.9252 (0.0656) | 0.0020 (–0.0162 to 0.0203) |
| Week 16 | 0.9333 (0.0567) | 0.9391 (0.0760) | 0.0058 (–0.0130 to 0.0246) |
| Month 12 | 0.9383 (0.0639) | 0.9425 (0.0688) | 0.0042 (–0.0141 to 0.0225) |
| Month 24 | 0.9316 (0.0708) | 0.9492 (0.0624) | 0.0176 (–0.0016 to 0.0368) |
| Unadjusted QALY over 24 months, mean (SE) | 1.7940 (0.0105) | 1.8046 (0.0105) | 0.0107 (–0.0183 to 0.0396) |
| Adjusted QALY over 24 months, mean (SE) | 1.7901 (0.0094) | 1.8027 (0.0095) | 0.0125 (–0.0137 to 0.0388) |
| Imputed CHU-9D score, mean (SD) | |||
| Baseline | 0.9228 (0.0640) | 0.9258 (0.0632) | 0.0031 (–0.0139 to 0.0200) |
| Week 16 | 0.9315 (0.0555) | 0.9404 (0.0725) | 0.0089 (–0.0083 to 0.0261) |
| Month 12 | 0.9393 (0.0614) | 0.9429 (0.0652) | 0.0036 (–0.0135 to 0.0208) |
| Month 24 | 0.9306 (0.0672) | 0.9488 (0.0594) | 0.0181 (0.0013 to 0.0349) |
| Unadjusted QALY over 24 months, mean (SE) | 1.7906 (0.0085) | 1.8072 (0.0083) | 0.0166 (–0.0067 to 0.0398) |
| Adjusted QALY over 24 months, mean (SE) | 1.7908 (0.0076) | 1.8070 (0.0074) | 0.0162 (–0.0047 to 0.0372) |
Resource use and cost
Participants in the EC group had fewer hospital admissions than participants in the SC group, and admissions were of shorter duration (Table 16). The average length of stay in the SC and EC groups over 24 months was 5.8 days and 2.5 days, respectively, which equates to a mean per-patient cost of £1539 and £691, respectively (Table 17). A breakdown of length of stay and cost from baseline to month 24 is shown in Tables 26 and 27 in Appendix 4. In addition to having fewer hospital admissions, participants in the EC group were also reported to have fewer hospital emergency visits than participants in the SC group (see Table 16). This led to a per-patient mean cost saving of £411 over the 24 months (see Table 17). In general, over the 24 months, participants in the EC group had fewer hospital outpatient visits than those in the SC group, and this led to a per-patient mean cost saving of £382 (see Table 17). Tables 16 and 17 also outline the disaggregated mean (SD) number of primary care visits and the corresponding mean per-patient cost by treatment group. Tables 28 and 29 in Appendix 4 show the hospital emergency visits and hospital outpatient visits at each follow-up time point until month 24. Overall, participants in the EC group had fewer primary care visits than participants in the SC group.
| Resource use | Group | Bootstrapped difference (95% CI) | |
|---|---|---|---|
| SC (n = 92) | EC (n = 94) | ||
| Primary care, n, mean (SD) | |||
| GP visits | 0.576 (1.030) | 0.383 (0.893) | –0.193 (–0.474 to 0.088) |
| Practice nurse visits | 1.554 (6.716) | 0.564 (1.535) | –0.991 (–2.369 to 0.388) |
| Other primary care staff visit | 0.065 (0.440) | 0.011 (0.103) | –0.055 (–0.148 to 0.039) |
| Secondary care, n, mean (SD) | |||
| Hospital admission bed-days | 5.826 (28.682) | 2.526 (5.307) | –3.299 (–8.742 to 2.144) |
| Hospital emergency visits | 0.630 (1.155) | 0.319 (0.832) | –0.311 (–0.594 to –0.029) |
| Hospital outpatient visits | 7.120 (11.607) | 5.383 (5.187) | –1.737 (–4.243 to 0.770) |
| Medication, mean (SD) | |||
| Ciclosporin (number of days) | 8.685 (51.922) | 0.638 (6.189) | –8.046 (–19.088 to 2.995) |
| Cyclophosphamide (number of days) | 14.380 (30.161) | 9.883 (23.684) | –4.497 (–12.972 to 3.977) |
| Levamisole (number of days) | 11.935 (44.831) | 13.234 (46.566) | 1.299 (–10.991 to 13.589) |
| Mycophenolate mofetil (number of days) | 6.696 (31.042) | 1.011 (9.799) | –5.685 (–12.541 to 1.171) |
| Rituximab (number of days) | 0.272 (2.204) | 0.000 (0.000) | –0.272 (–0.761 to 0.218) |
| Tacrolimus (number of days) | 0.500 (2.956) | 7.670 (46.942) | 7.170 (–2.617 to 16.957) |
| Prednisolone (mg consumed) | 3851.83 (2918.22) | 4274.81 (3311.25) | 422.988 (–499.172 to 1345.148) |
Reported figures are for participants who returned study questionnaire at all time points over the 24-month period.
| Cost component | Group, mean cost (£) (SD) | Bootstrapped difference (95% CI) | |
|---|---|---|---|
| SC (n = 92) | EC (n = 94) | ||
| Primary care visits | |||
| GP | 28.24 (50.47) | 18.77 (43.79) | –9.47 (–23.00 to 4.07) |
| Practice nurse | 14.46 (62.46) | 5.24 (14.28) | –9.21 (–21.99 to 3.57) |
| Other primary care staff | 0.49 (3.30) | 0.08 (0.77) | –0.41 (–1.11 to 0.30) |
| Secondary care | |||
| Hospital admission | 1539.36 (7603.47) | 691.13 (1308.60) | –848.23 (–2253.65 to 557.20) |
| Hospital emergency | 832.80 (1525.99) | 421.60 (1099.49) | –411.21 (–804.67 to –17.75) |
| Hospital outpatient | 1566.30 (2553.53) | 1184.26 (1141.15) | –382.05 (–946.98 to 182.88) |
| Medications | |||
| Ciclosporin | 14.76 (88.27) | 1.09 (10.52) | –13.68 (–32.21 to 4.85) |
| Cyclophosphamide | 19.99 (41.92) | 13.74 (32.92) | –6.25 (–17.43 to 4.93) |
| Levamisole | 4.44 (16.68) | 4.92 (17.32) | 0.48 (–4.01 to 5.06) |
| Mycophenolate mofetil | 2.49 (11.56) | 0.38 (3.64) | –2.11 (–4.42 to 0.191) |
| Rituximab | 31.64 (256.64) | 0.00 (0.00) | –31.64 (–82.07 to 18.80) |
| Tacrolimus | 4.09 (24.19) | 62.77 (384.17) | 58.68 (–19.51 to 136.88) |
| Prednisolone | 44.30 (33.56) | 49.16 (38.08) | 4.86 (–5.04 to 14.77) |
Extending the course of prednisolone over a longer duration meant that all participants in the EC group incurred a higher prednisolone drug cost over 24 months. The bootstrapped cost difference in Table 17 shows that mean cost difference (mean £4.86; 95% CI –£5.04 to £14.77) between the treatment groups was not significant. Ciclosporin, cyclophosphamide, levamisole, mycophenolate mofetil, rituximab and tacrolimus were the second-line immunosuppressants used in the treatment pathway. Table 16 shows that participants in the EC group were given levamisole and tacrolimus for longer than participants in the SC group and, overall, across the 24-month follow-up period, the per-patient mean cost difference attributed to just second-line immunosuppressant therapy was £11 lower in the SC group than in the EC group. The mean cost for other prescribed medications was £50 lower in the EC group.
The per-patient total mean cost was made up of costs associated with prednisolone prescriptions, hospital admissions, hospital outpatient visits, emergency visits, primary care visits, second-line immunosuppressants and other prescribed medications. To account for missing data, multiple imputation (5000 iterations) was applied at each follow-up time point. To estimate the difference in the per-patient total mean cost between the two treatment groups, adjustments were made for age, gender and the baseline (week 4) utility score. Over the 24-month study period, participants in the EC group incurred less cost than those in the SC group, by an average of £1673 per patient (Table 18).
| Imputed cost | Group, mean cost (£) (SD) | Bootstrapped cost difference (95% CI) | |
|---|---|---|---|
| SC (n = 109) | EC (n = 114) | ||
| Baseline | 45.54 (121.89) | 78.94 (202.20) | 35.24 (–7.57 to 78.05) |
| Week 8 | 132.10 (391.97) | 116.56 (458.72) | –21.53 (–137.43 to 94.38) |
| Week 12 | 401.43 (1285.11) | 56.54 (189.02) | –361.14 (–610.11 to –112.16) |
| Week 16 | 235.45 (410.97) | 73.74 (217.51) | –162.19 (–247.61 to –76.77) |
| Month 5 | 301.15 (700.77) | 265.89 (526.68) | –38 (–204.59 to 128.59) |
| Month 6 | 193.07 (435.69) | 255.04 (648.82) | 63.86 (–91.67 to 219.38) |
| Month 8 | 398.85 (624.72) | 255.98 (522.45) | –139.29 (–289.89 to 11.32) |
| Month 10 | 887.64 (5827.01) | 287.69 (519.73) | –653.39 (–1758.95 to 452.17) |
| Month 12 | 239.91 (434.46) | 258.11 (500.91) | 32.65 (–91.40 to 156.70) |
| Month 18 | 791.95 (1558.03) | 566.72 (880.52) | –225.15 (–569.61 to 119.32) |
| Month 24 | 666.18 (2103.73) | 466.64 (803.76) | –235.16 (–649.90 to 179.59) |
| Total imputed cost over 24 months | |||
| Unadjusted, mean (SE) | 4462.21 (748.13) | 2607.51 (731.46) | –1854.70 (–3959.44 to 250.03) |
| Adjusted, mean (SE) | 4369.20 (748.13) | 2696.43 (731.37) | –1672.77 (–3455.06 to 109.53) |
Cost–utility analysis
The incremental difference in costs was then offset against the difference in QALYs for the SC group versus the EC group. The EC group dominated the SC group, which means that, on average, participants incurred less cost and gained more QALYs. Cost–utility analysis combines the incremental costs with the incremental QALYs to produce an ICER, or a cost per additional QALY gained. In this situation, as costs were lower and QALYs gained higher, the mean ICER values were not calculated. The EC group was both more effective and cheaper (Table 19).
| Imputed values | Group | Bootstrapped difference (95% CI) | |
|---|---|---|---|
| SC (n = 109) | EC (n = 114) | ||
| Mean QALYs (SE) | 1.7908 (0.0076) | 1.8070 (0.0074) | 0.0162 (–0.0047 to 0.0372) |
| Mean cost, £ (SE) | 4369.20 (748.13) | 2696.43 (731.37) | –1672.77 (–3455.06 to 109.53) |
Values are mean (SE) unless stated otherwise. QALYs have been adjusted for age, gender and baseline utility value. Costs have been adjusted for age and gender. All costs are in Great British pounds (£).
Sensitivity analysis
To account for the uncertainty around the point estimates, an incremental cost-effectiveness plane and a CEAC were constructed using the net monetary benefit approach. The plane (Figure 15) shows 5000 jointly bootstrapped cost–QALY difference pairs scattered across all four quadrants.
FIGURE 15.
Cost–utility plane for the comparison of extended prednisolone therapy with standard prednisolone therapy, based on 5000 bootstrapped cost–effect pairs.

The majority of the points are in the south-east quadrant, indicating that the extended prednisolone course was less expensive and more effective than the standard prednisolone course. The dashed line represents the willingness-to-pay threshold of £20,000 per QALY and the solid line represents the willingness-to-pay threshold of £30,000 per QALY. Points to the right of both lines indicate situations when the extended prednisolone course is the preferred option, while points to the left indicate instances in which the SC is recommended. Figure 16 shows that the probability of extended prednisolone course being cost-effective is 98.8% and 99.9% at £20,000 and £30,000 per QALY, respectively.
FIGURE 16.
Cost-utility acceptability curve comparing extended and standard prednisolone therapy, based on 5000 bootstrapped cost–effect pairs.
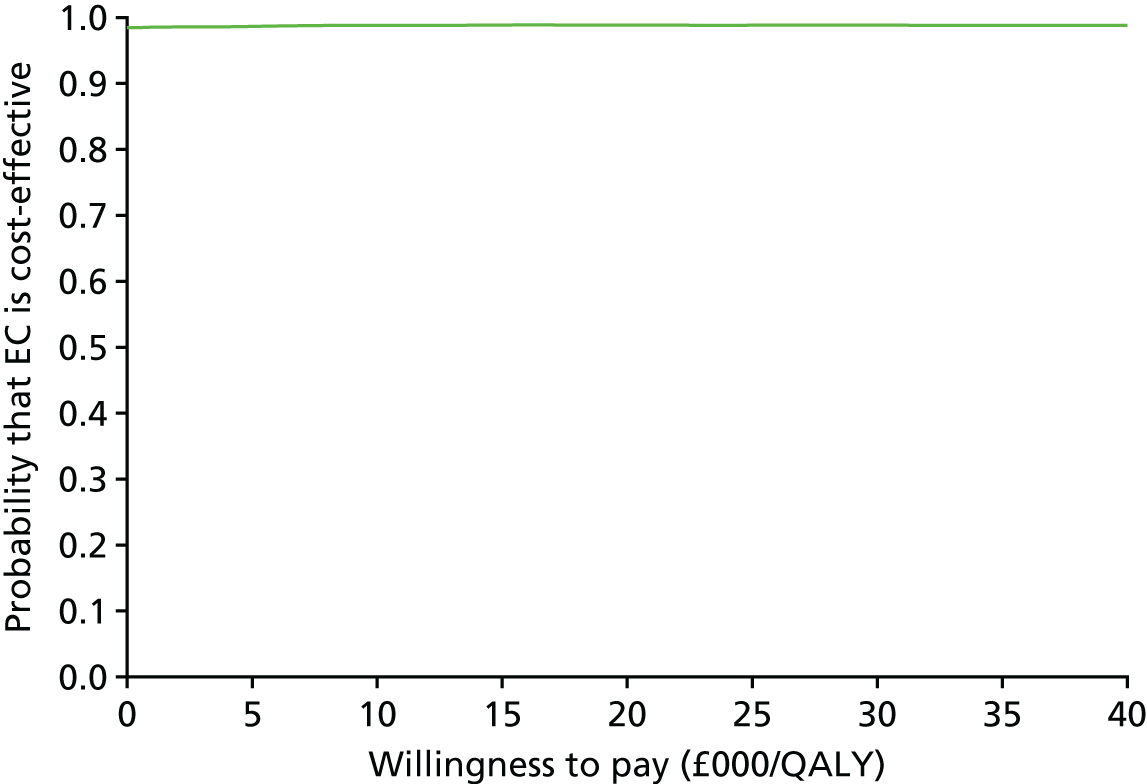
The EC group remained dominant at 24 months when the analysis was restricted to participants with complete cost and outcome data (Table 20). The main difference from the base-case analysis was the smaller incremental cost at 24 months (£582 vs £1673 cost saving). Overall, the probability of EC being cost-effective compared with SC, remained high, at 89%.
| Incremental costs and QALYs | Group, mean (SE) | Bootstrapped difference (95% CI) | |
|---|---|---|---|
| SC (n = 109) | EC (n = 114) | ||
| Base case | |||
| QALYs | 1.7908 (0.0076) | 1.8070 (0.0074) | 0.0162 (–0.0047 to 0.0372) |
| Cost (£) | 4369.20 (748.13) | 2696.43 (731.37) | –1672.77 (–3455.06 to 109.53) |
| Complete case | |||
| QALYs | 1.7931 (0.0098) | 1.8022 (0.0010) | 0.0091 (–0.0169 to 0.0350) |
| Cost (£) | 3078.10 (362.24) | 2495.61 (358.26) | –582.49 (–156.12 to 379.40) |
| Ethnicity | |||
| South Asian | |||
| QALYs | 1.7784 (0.0168) | 1.8290 (0.0154) | 0.0507 (–0.0073 to 0.0941) |
| Cost (£) | 2713.04 (527.78) | 1737.51 (484.10) | –975.52 (–2227.23 to 497.65) |
| White | |||
| QALYs | 1.7862 (0.0010) | 1.7968 (0.0095) | 0.0106 (–0.0147 to 0.0359) |
| Cost (£) | 3724.13 (376.31) | 3024.11 (362.57) | –700.02 (–1576.34 to 553.46) |
| Other | |||
| QALYs | 1.8088 (0.0160) | 1.8117 (0.0161) | 0.0029 (–0.0402 to 0.0460) |
| Cost (£) | 12,235.10 (4816.98) | 4494.64 (4847.47) | –7740.48 (–19,198.18 to 4017.14) |
| Age (years) | |||
| ≤ 5 | |||
| QALYs | 1.7982 (0.0128) | 1.8138 (0.0122) | 0.0156 (–0.0073 to 0.0386) |
| Cost (£) | 2381.26 (1621.93) | 377.27 (1546.25) | –2003.99 (–4235.41 to 427.12) |
| ≥ 6 | |||
| QALYs | 1.7627 (0.0204) | 1.7779 (0.0211) | 0.0152 (–0.0253 to 0.0557) |
| Cost (£) | 3534.97 (757.87) | 2690.46 (783.96) | –844.50 (–2400.28 to 629.32) |
Subgroup analysis
Participants from South Asian backgrounds had the highest QALY gains (see Table 20). Although the mean cost of the EC group was highest for the ‘other’ ethnicities category (non-white and non-South Asian), the intervention was associated with a considerable cost saving for this group. This group were also least likely to gain QALYs from extended prednisolone treatment.
The base-case result was robust to subgroup analysis according to age group, with the extended prednisolone therapy group retaining its dominance in participants who were ≤ 5 years at baseline and in older participants. It was cheaper and more effective to treat the younger age group than older participants, thereby making this population the more beneficial group to treat actively with extended prednisolone course.
Chapter 7 Discussion
The PREDNOS study has not shown any clinical benefit for a 16-week EC of prednisolone compared with the 8-week SC as described by the ISKDC in UK children presenting with SSNS. There was no significant difference between the two treatment groups in time to first relapse of nephrotic syndrome or in any other of the clinically important secondary end points, including the number of relapses experienced, the proportion of participants who went on to develop FRNS or SDNS or the requirement for alternative non-corticosteroid immunosuppressive therapies. However, despite showing no clinical benefit, the cost-effectiveness analysis suggested that EC therapy may be cheaper, with the possibility of a small QALY benefit.
These findings differ from the six studies published prior to the commencement of the PREDNOS study, which had compared the ISKDC regimen with prednisolone regimens of > 3 months’ duration. A Cochrane review29 of these studies performed in 2005 showed a benefit of longer-course prednisolone therapy, with a lower rate of relapse at 12–24 months, and a significant reduction in the number of children with FRNS. Based upon this, a recommendation was made that children presenting with SSNS should be treated with a minimum of 3 months of prednisolone therapy. This did not, however, lead to international consensus, and significant clinical equipoise and variation in practice persisted.
More recent studies have reported results that are consistent with PREDNOS. A Japanese study of 255 participants that compared the ISKDC regimen with a 6-month course of prednisolone found no benefit associated with longer-duration prednisolone therapy. 36 Yoshikawa et al. 36 chose a primary end point of time to the development of FRNS, a clinically important end point identifying those children who have developed a complicated disease course and who are likely to develop disease- and treatment-related morbidity and, therefore, require alternative, more potent, immunosuppressive therapies. There was no difference in FRNS, and the time to first relapse and the incidence of adverse effects were also similar in the two groups. An Indian study comparing 3 and 6 months of prednisolone did not show any benefit associated with increased duration of prednisolone,35 and neither did the Dutch study by Teeninga et al. ,33 which also compared 3 and 6 months of therapy. The inclusion of three well-designed studies in a 2015 update of the Cochrane review13 resulted in a change in the overall conclusions. It was noted that these studies of longer versus shorter duration of corticosteroids had heterogeneous treatment effects, with the older, higher risk of bias studies tending to overestimate the effect of longer-course therapy compared with the more recently published, lower risk of bias studies. Among studies at low risk of bias, there was no significant difference in the risk of FRNS between participants given prednisone for 2 or 3 months and those receiving therapy of longer duration or higher total dose, indicating that there is no benefit of increasing the duration of prednisone beyond 2 or 3 months in the initial episode of SSNS. However, when the meta-analysis was restricted to those addressing the same question as the PREDNOS study, comparing the 8-week ISKDC regimen with regimens of ≥ 3 months (i.e. adding the study of Yoshikawa et al. 36 to the six studies reported in the original Cochrane review), there remained a benefit for the longer (≥ 3 months) treatment regimen, although this only just reached statistical significance. The risk of FRNS was significantly lower (RR 0.68, 95% CI 0.47 to 1.00), as was the number of participants relapsing by 12–24 months (RR 0.8, 95% CI 0.64 to 1.00). It is likely that once the results of the PREDNOS study are added to the Cochrane review that the overall result will show no difference in outcome between the ISKDC and longer treatment regimens.
The data reported in our study are similar to those reported in previous studies. The proportion of participants experiencing a relapse was 80.3% (179/223) over a median follow-up of 37 months, which is comparable to the rate of 60–90% reported in the literature. 13,87 Teeninga et al. 33 reported a relapse rate of 78.6% (99/126) in a European population with a median follow-up of 47 months, with a median time to first relapse of 6 months for the 3-month prednisolone group and 8 months for the 6-month group. Sinha et al. 35 reported a lower relapse rate of 57.8% (104/180); however, participants were followed up for only 12 months. In the Japanese study, the overall relapse rate was not stated; however, the median time to relapse was 242 days and 243 days in the 2-month and 6-month groups, respectively, significantly longer than the 87 days for the SC group and 139 days for EC group observed in the PREDNOS study. It is noteworthy that Yoshikawa et al. used urine dipstick values of ++ or higher as their definition of relapse, although, if anything, this would have overdiagnosed relapse and reduced the time to first relapse. 36
We also found rates of FRNS similar (50% and 53%) to those previously reported. Using the same ISKDC definition as used in PREDNOS, Sinha et al. 35 reported a rate of FRNS at last follow-up of 50.4% in the 6-month group and 60.4% in the 3-month group. The time to FRNS was 23.0 months and 17.6 months, respectively. Teeninga et al. 33 reported FRNS in 45% of the 3-month prednisolone group and in 50% of the 6-month prednisolone group, commenting that this was higher than expected. In previous studies, FRNS has been reported in 32–78% of participants who received a 2-month course of prednisolone15,19–21,47,88 and in 18–44% of those who received prednisolone for 3 months. 20,26,47 It has been proposed that this variation may, in part, be explained by regional differences or variations in definitions of FRNS, length of observation and relapse treatments.
Interestingly, although we found no clinical benefit for the EC prednisolone treatment regimen, we did find that this regimen was cheaper and more effective in QALY terms. The cost analysis showed that, over a 24-month period, the EC treatment regimen cost less because of a lower rate of hospital admission, a shorter duration of hospital stay, fewer hospital emergency visits and fewer outpatient and primary care visits; therefore, on average, it was cheaper than the SC treatment regimen by £1673 per patient. Furthermore, the EC treatment regimen produced more QALYs than the SC treatment regimen. Using commonly applied threshold values for how much society is willing to pay for a QALY gain, the EC treatment regimen is cost-effective. At first glance, this result may seem surprising, as the clinical outcomes have shown little or no benefit of extending prednisolone treatment, yet the heath economics reveals possible evidence of cost-effectiveness. These differences, in part, relate to the differences in cost and QALYs, but also to the different methods of analysis adopted in the health economic and clinical evaluations.
Unlike the objectives of the clinical evaluation, which are about testing whether or not extending prednisolone therapy leads to an improved patient outcome relative to a control group, the objectives of the economic evaluation are to provide an estimation of the value of the extended therapy reflecting both efficiency and equity, and, thus, an estimate of whether or not the difference in cost between the treatment groups is worth the difference in effect, taking into account the opportunity cost of that investment and the fact that the resources could have been invested elsewhere across all parts of the NHS.
The key thing to note is that small insignificant clinical benefits can be cost-effective. The health economics focus is about comparing two things: costs and effects. For the health economic analysis, the effects are measured using QALYs, which reflect societal values and incorporate preferences for domains of QoL. These values are measured using preference-based QoL instruments, for example the CHU-9D questionnaire that was used in this study. Measuring cost differences between different treatment therapies has no meaning until these are offset against differences in effects: it is the simultaneous consideration of costs and effects and, therefore, the joint density of cost and effect differences76 that is the focus of a health economic evaluation.
Within the PREDNOS study, the clinical analysis quantified the difference in time to first relapse, at the individual participant level, using statistical inference. The economic analysis compared the per-participant cost of EC versus SC therapy, and found that the cost was, on average, £1673 lower in the EC group, and the QALYs gained were, on average, 0.0162 higher in the EC group. When the costs and QALY differences are assessed separately, these differences are not statistically significant; however, when assessed simultaneously, the ICER (the ratio of the mean cost and the mean QALY difference) produces a cost-effective result, as the EC regimen is cheaper and produces more QALYs, on average. Therefore, it is dominant, as it is not only more effective in QALY terms but also saves health-care resources, relative to the SC group.
Furthermore, there are different methods within the health economic analysis for representing the uncertainty in the cost and QALY differences. QALYs and cost data tend to have unusual distributional properties and are often skewed, exhibiting ceiling effects or having a bimodal distribution; for this reason, the stochastic bootstrapping method was applied. Bootstrapping generates multiple samples of joint cost and effect estimates from the same trial data, and these cost and effect pairs are then represented on a scatterplot on an incremental cost-effectiveness plane. Figure 15 in Chapter 6 presents the bootstrapped cost and QALY pairs from the PREDNOS study. It shows that most of the pairs lie in the south-east quadrant, indicating that there are cost-savings and QALY gains from the extended therapy versus the standard therapy; however, there are some points spread within the north-east quadrant (indicating that the extended therapy is more costly) and the south-west quadrant (indicating that the extended therapy leads to a QALY loss). This reflects some uncertainty regarding the cost savings and QALY gains to be achieved from extended therapy compared with standard therapy, which is consistent with the finding of a non-significant difference for both costs and effects, when considered independently, between the two treatment groups.
To account for the uncertainty in the cost and effect pairs, the proportions of points falling above and below a willingness-to-pay threshold line are simply counted and then the threshold line is varied to produce a CEAC. CEACs are regarded as an alternative method of calculating CIs and indicate the probability that the extended therapy is cost-effective, compared with standard therapy, for different threshold values of willingness to pay for a QALY gain. Using the PREDNOS trial data, the probability that the extended therapy is cost-effective, at the commonly applied threshold value of £20,000 per QALY, is 0.988. Therefore, despite there being no statistically significant differences in costs and effects for extended therapy compared with standard therapy, the CEAC shows that there is very little uncertainty, from a cost-effectiveness perspective, about the choice to treat patients with EC therapy compared with SC therapy. It is also worth noting that, regardless of benefit measured in QALYs, parents and children value avoidance of hospital admission. This is also valued by clinicians and reduces demand pressures on the NHS.
Previous studies have been somewhat inconsistent in their reporting of the adverse effects associated with using corticosteroids; however, the most recent (2015) Cochrane review13 found no significant differences in the risk of adverse events between extended duration and 2 or 3 months of prednisolone. We found no differences in the adverse effect profiles between the two treatment groups, with the exception of parentally reported poor behaviour, which was significantly more common in the SC group. At 24 months, the cumulative incidence of poor behaviour was 93% in the SC group, compared with 82% in the EC group (RR 0.90, 95% CI 0.82 to 0.98). There was no difference in the incidence of any other adverse effects including Cushingoid facies, striae, hypertrichosis, acne, increased appetite, glycosuria, cataract and abdominal pain. These findings are broadly comparable with those of multiple other larger-36 and smaller-scale20,21,23,25 trials addressing this same clinical question. The large majority of these have found no significant difference in the incidence of adverse effects; however, there was significant heterogeneity in the extent to which these were monitored. Most adverse events were transient and occurred relatively early on during the course of treatment, when the prednisolone dose was at its highest.
We were particularly interested in the impact that the two prednisolone regimens had on behaviour, as expert clinical opinion and advice from our patient and public involvement group indicated that this was the adverse effect of greatest prevalence and significance to families. When the PREDNOS study was designed, no previous study had objectively and systematically investigated this using a quantitative measure. In PREDNOS, we collected quantitative data on behaviour using the ACBC. Although parentally reported poor behaviour was significantly more common in the SC group, when behaviour was assessed objectively through the ACBC questionnaire completed by the parents, there was no significant difference in either the total behaviour score or t-score. The proportion of participants assessed as having abnormal behaviour by the ACBC was also not different between the two groups and varied between 21% and 31% at different time points throughout the study. The proportion of participants whose parents reported poor behaviour was higher than the proportion whose scores were outside the normal ACBC range. This provides some reassurance to parents that perceived poor behaviour is generally within normal bounds and is not greatly impacted by corticosteroid treatment, a finding of relevance in other paediatric conditions treated with corticosteroids. Teeninga et al. 33 assessed behaviour using visual analogue scales. Compared with baseline, participants scored significantly higher on eating, overactive behaviour and aggressive behaviour at 3 months’ follow-up (p < 0.01); however, these scores returned to baseline within 1 year in both groups. Scores for happiness temporarily dropped in the first 6 months, while scores for sleeping remained relatively stable over the entire observation period.
Subgroup analyses showed that there was no clear evidence to suggest that the treatment effect differed according to ethnicity, age or gender, although we were not powered to detect differences in subgroups. For age, there may be some suggestion that the time to first relapse was extended in those in the EC group in participants aged ≤ 5 years, with no difference between the two groups in participants aged ≥ 6 years. This remains a topic of some debate, as a number of studies have reported young age at diagnosis to be associated with an increased risk of FRNS and/or corticosteroid dependence,8,16,47,89 whereas others have not reported this association. 12,90,91 In a post hoc analysis of Sinha et al. ’s study,40 Cox regression suggested that participants aged ≤ 3 years benefited from prolonged therapy, with a reduction in the risk of a first relapse, but not of frequent relapses, and Poisson regression confirmed a higher relative relapse rate in younger participants. Other reports have strongly argued that age may be a predictor of disease severity, including FRNS, corticosteroid dependence and response to cyclophosphamide therapy. 92,93 A few non-randomised studies have investigated the role of gender in the disease course and have reported males to be at a disadvantage. 16,47 Cox regression analysis in the study of Teeninga et al. 33 did not identify boys as being at significantly greater risk of developing FRNS. We found also no evidence of a difference in treatment effect according to gender.
Systolic and diastolic blood pressure z-scores were similar in both treatment groups throughout the course of the study. z-scores were relatively high at the time of the week 4 visit, presumably as a result of the high dose of prednisolone being administered at this point. However, the z-scores decreased progressively during study follow-up. These observations are entirely consistent with the findings of other similar studies.
Interestingly, over the course of the study, following an initial slight fall during the first 16 weeks, the height z-scores increased in both treatment groups. This is an interesting observation, given the fact that these participants received multiple courses of prednisolone for treatment of relapses, a treatment that is known to have a negative impact on linear growth. Previous studies have also reported a fall in height velocity during the first few months of high-dose prednisolone treatment, with a subsequent return to baseline by 12 months. 33 Others have described a dose-dependent effect of corticosteroids on growth in children with SSNS. 94–96 A small number of studies have noted the baseline height SDS to be relatively low in children presenting with SSNS, although no satisfactory explanation has been found for this observation. 33,97 We did not observe this in PREDNOS study. Weight z-scores were relatively constant throughout the study, and BMI z-scores decreased over time.
The main strength of the PREDNOS study is its randomised, double-blind, placebo-controlled design. This ensured a low risk of selection, performance, detection and selective reporting bias. Inclusion criteria were defined to ensure that the study population was representative of the population of children presenting with SSNS in the UK. Outcomes were assessed using internationally accepted ISKDC definitions. Our primary outcome measure of time to first relapse was felt by UK clinicians to be of clinical importance, and previous studies88 have shown a link between timing of first relapse and subsequent clinical course. Baseline features were well balanced and there was a low rate of attrition. Regular safety assessment was ensured through regular clinical review. Prior to the commencement of PREDNOS, previous studies had been small (largest 184 participants) and no previous double-blind placebo controlled RCTs had ever been conducted in children with SSNS. PREDNOS successfully recruited 237 participants from 124 sites throughout all regions of the UK into a double-blind, placebo-controlled RCT. Since then, the studies of both Teeninga et al. 33 and Sinha et al. 35 were double-blinded, although one further study comparing the ISKDC regimen with longer duration therapy was not blinded. 36 The authors acknowledged that this may have introduced preconception bias; however, they proposed that because their design was a non-inferiority trial with regular visits and with relapses being measured objectively, they could not assume positive placebo effects. It must be remembered that this would not have been the case with the reporting of AEs, for which there is considerable scope for bias.
The sample size calculations for the study were based on detecting an absolute difference of 20% in relapse rate at 1 year from 60% in the SC group to 40% in the EC group using a log-rank test (80% power, α = 0.05). The 1-year relapse rate observed in the SC group was 77%. This means that the study has > 85% power to detect an absolute difference of 20% between groups (i.e. from 77% to 57%) using a log-rank test. This makes the likelihood of our results being the result of a type 2 statistical error small.
An additional strength of the PREDNOS study is the generalisability of its findings. We recruited participants with a first presentation of INS from across the UK with broad inclusion criteria. We selected an age range of 1–14 years as this is the range in which the large majority of patients present and are treated empirically with a course of corticosteroids without recourse to renal biopsy. One of the key purposes of the PREDNOS study was to ascertain the optimal prednisolone treatment regimen for UK children by comparing the 8-week SC ISKDC regimen with a longer 16-week EC regimen. We chose the ISKDC SC regimen for one group because this was the regimen in use in the very large majority of UK centres at the time of the planning of the PREDNOS study and chose a 16-week EC regimen as a comparator because longer duration treatment regimens have previously been shown to result in lower rates of relapse and FRNS. The ethnic mix of the study population broadly represented that of the wider population of children with nephrotic syndrome, including significant representation from the South Asian community. We recruited 44 participants (20% of the study population) from families of South Asian origin and 31 (14%) from families recorded as other non-white ethnic origin. This is a very similar figure to that reported in the study of Teeninga et al. ,33 which included 35% of participants of non-Western European descent. This is an important achievement, as the UK South Asian community in particular is significantly over-represented in the SSNS population, the incidence being around six times greater than in the UK white population. Furthermore, the UK South Asian population is generally under-represented in clinical trials and recruitment poses a number of particular challenges. 98 Finally, although formal screening logs were requested, in keeping with other studies these were not kept well; however, based on known epidemiological data, we estimate that we have managed to include 34% of newly presenting patients over a recruitment period of 3 years and 2 months. This indicates a high level of acceptability of the trial among both families and clinicians.
One of the greatest challenges in setting up the PREDNOS study was facilitating the recruitment of participants in district general hospitals. Children with INS have traditionally been, and continue to be, investigated and managed within district general hospitals rather than tertiary paediatric nephrology centres. Referral to tertiary centres generally takes place only if the presenting features are atypical or when investigation or management proves problematic. For this reason, any study that recruited solely from tertiary centres would not reach the large majority of potential participants and would risk sampling a preselected, somewhat atypical group of participants. In the early 2000s, when the PREDNOS study was being planned, there was little paediatric experience in the conduct of RCTs involving an investigational medicinal product and little funded infrastructure to support this work. Our Kidney Research UK and Kids Kidney Research jointly funded pilot study confirmed that there was great willingness among principal investigators to participate in the study and similar interest in the study from participants from both the white and South Asian communities. The pilot study allowed the trial design and infrastructure to be tested, including aspects such as the provision of study medication from a single national clinical trials pharmacy with delivery direct to the participant, attendance rates for study visits and the completion of questionnaires and the study case report forms. The success of the pilot study was significantly enhanced by the development of the NIHR Clinical Research Network: Children (formerly the NIHR MCRN), which commenced operating in 2005. 99 The PREDNOS pilot study was one of the first studies to be adopted onto the MCRN study portfolio and the infrastructure put in place facilitated study set-up and participant recruitment in many sites. The main PREDNOS study was also adopted onto the study portfolio and similarly benefited.
Possible weaknesses of the study include the possibility that the choice and preparation of study drug might have influenced the age profile of the population under study, with a potential trend towards the relative overinclusion of older participants. Because the PREDNOS study drug was supplied in crushable tablet form rather than in suspension or in a soluble or dispersible form, this may have resulted in younger children not participating in the study because of inability or perceived inability to swallow the crushed tablets. The initial ISKDC studies reported that the median age at presentation of MCD nephrotic syndrome was 3 years,2 and a UK series from the county of Yorkshire reported the incidence to be greatest in the 1–4 years age group. 1 The mean age of participants in our study was 4.9 years, with 65% of the study participants being < 6 years of age. This rather suggests that there was a trend towards the recruitment of slightly older participants, perhaps as a result of this study medication formulation issue. However, our study participant age profile is broadly comparable to those reported in the three most recent RCTs of corticosteroid therapy in SSNS by Teeninga et al. 33 (median age 4.2 years, IQR 3.2 to 6.2 years), Yoshikawa et al. 36 (mean age 6.7 years, SD 4.1 years, in the SC group and mean age 6.3 years, SD 4.1 years, in the EC group) and Sinha et al. 35 (median 42.4 months in 3-month treatment group and median 44.2 months in the 6-month treatment group).
The parents of two participants recruited at the chief investigator’s site commented that they felt that they knew which group their child had been randomised to because their child had noticed slight differences in taste between the active prednisolone and placebo tablets. Prednisolone and other oral corticosteroids tend to have a somewhat bitter taste and it may be that other study participants noticed this same phenomenon. This was not, however, reported by other principal investigators and, therefore, it seems unlikely that this would have had a significant impact on the study results.
One further potential minor limitation is the fact that study participants were, in the majority of cases, observed and treated at their local hospital, where the study visits took place. As such, observation and scoring of adverse effects in the study was performed by multiple observers. To avoid this issue would have meant that all study participants would have had to travel to a single or small number of centres, which would have proved a very significant barrier to participation. Randomisation was not minimised by centre, as individual centre contributions were difficult to predict.
In designing the study, we wanted to ensure that we objectively and comprehensively collected prednisolone-related AEs to adequately compare the two treatment regimens. Earlier studies lacked consistency in the level of information that was recorded and none reported quantitative data regarding behavioural change. Our adverse event reporting was, however, somewhat less comprehensive than that of some more recent studies and this warrants some further discussion. Yoshikawa et al. 36 performed formal ophthalmological assessment, including measurement of intraocular pressure, which was found to be elevated in 32 out of 246 participants (13%). 36 In the study of Teeninga et al. ,33 participants underwent formal ophthalmological assessment at diagnosis and after 6 months, specifically looking for evidence of cataract and glaucoma. 33 No cases of glaucoma were detected and only one single case of posterior subcapsular cataract was detected in the 3-month prednisolone group. In the PREDNOS study, we did not ask for participants to have a formal ophthalmological review, although principal investigators screened participants for cataract on an annual basis. Only one case of cataract was detected in each group, a similar frequency to the single case in the study of Sinha et al. 35 On the basis of their observations, Teeninga et al. 33 commented that cataract and glaucoma have been reported with much greater frequency in cohorts of Japanese children than those from other races and that their findings indicate that routine ophthalmological screening was not indicated at an early stage in Dutch children.
Yoshikawa et al. additionally performed regular blood tests and detected minor abnormalities of liver function tests and plasma amylase in up to 21% of participants. 36 We elected not to perform regular blood tests as part of the study protocol, principally because this is not routine clinical practice in the UK; children with SSNS generally have very few blood tests performed unless they are commenced on alternative immunosuppressive therapies mandating monitoring of drug levels or adverse effects. Furthermore, we were of the opinion that the introduction of regular blood tests into the study protocol would have a negative impact on study participation. We did include a single episode of blood sampling for the purpose of collecting deoxyribonucleic (DNA) samples for a separate study aiming to identify potential genetic changes associated with SSNS. A recommendation was made that this be performed at the time of venepuncture for other clinical reasons if this occurred, although our Research Ethics Committee approval permitted us to perform a standalone venepuncture solely for this sample. Sampling was successfully performed in 173 study participants.
In the study by Teeninga et al. 33 of Dutch children, lumbar spine bone mineral density was measured using dual-energy X-ray absorptiometry (DEXA) at baseline and after 6 months. We did not perform this investigation, principally because there is little in the published literature that indicates that significant abnormality of bone mineral density is likely to occur within the first 24 months of treatment, particularly in an unselected cohort of newly presenting SSNS patients. Although our own work has reported a minor reduction in trabecular bone mineral density in adult ‘survivors’ of childhood relapsing nephrotic syndrome, such changes required the use of peripheral quantitative computerised tomography, which is not widely available and would not have been detected using DEXA alone. 100 Many of the district general hospitals participating in PREDNOS would have also experienced difficulties in providing DEXA services for paediatric study participants. Furthermore, a high-quality prospective study examined 60 children with INS and 195 control children and found no deficits in spine or whole body bone mineral content. 101 In the study of Teeninga et al. , no difference was detected in bone mineral density from baseline to 6 months in either group, and they were not able to achieve bone assessment in all participants. 33
Conclusion
On the basis of the results of the PREDNOS study, it can be concluded that extending the duration of prednisolone beyond the 2-month ISKDC regimen that is currently being used in the large majority of UK centres does not result in a reduction in the time to first relapse, the number of participants developing FRNS or SDNS or the total dose of prednisolone administered. There were no differences between the two treatment regimens in the incidence of corticosteroid AEs. The cost-effectiveness analysis suggested that EC therapy may be cheaper, with the possibility of a small QALY benefit.
Future research recommendations
Our results, although not adequately powered to show a difference, suggest that children presenting with SSNS at < 6 years of age may benefit from receiving EC therapy, and this requires further investigation. This observation has previously been reported in other RCTs35 and is currently being investigated in an Indian trial that is ongoing (Professor Arvind Bagga, All India Institute of Medical Sciences, 2017, personal communication). This is of particular importance given that younger children appear to be at increased risk of FRNS and SDNS. 21,35,90,91
The lack of benefit of EC compared with SC therapy raises the issue of whether or not further studies should investigate if it is safe and efficacious to use even shorter corticosteroid regimens. This strategy has only once been previously addressed in a RCT, which showed the relapse rate and incidence of FRNS to be higher in those who received shorter course rather than SC therapy. However, like many of the earlier studies in this disease group, this was at significant risk of a number of areas of trial bias. 19 Finally, the disparate results between the health economic analysis and the clinical analysis requires further evaluation; the difficult question here is whether or not further RCTs comparing SC and EC are justified given the clear lack of clinical benefit.
Acknowledgements
The PREDNOS investigators would like to thank NIHR for funding this study and Kidney Research UK and Kids Kidney Research, who funded the pilot study. We thank all the parents and participants who agreed to enter the study, the investigators who contributed to the trial and the NIHR Clinical Research Network: Children for their support with study set-up and recruitment. We also thank the Service Users Group from the UK NeST and the Renal Patient Support Group, in particular Mrs Wendy Cook, Mrs Samantha Davies-Abbott and Dr Shahid Muhammad who provided valuable input regarding trial design and conduct and reviewed the plain English summary. The support of the British Association for Paediatric Nephrology and the Royal College of Paediatrics and Child Health is much appreciated.
PREDNOS Trial Management Group
Professor Nicholas Webb (Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust), Chief Investigator and Applicant; Dr Richard Trompeter (Paediatric Nephrology, Centre for Nephrology, University College London), Co-Chief Investigator and Co-Applicant; Professor Keith Wheatley (Clinical Trials and Statistics, Cancer Research UK Clinical Trials Unit, University of Birmingham), Co-Applicant; Dr Carole Cummins (Clinical Trials and Statistics, Institute of Applied Health Research, University of Birmingham), Co-Applicant; Miss Natalie Ives (Clinical Trials and Statistics, Birmingham Clinical Trials Unit, University of Birmingham), Co-Applicant; and Dr Emma Frew (Health Economics, Institute of Applied Health Research, University of Birmingham), Co-Applicant.
PREDNOS trial management and day-to-day co-ordination
Birmingham Clinical Trials Unit, University of Birmingham
Elizabeth Brettell, Team Leader; Emma Barsoum, Senior Trial Co-ordinator; Helen Bodenham-Chilton, Trial Co-ordinator; Noreen Akhtar, Trial Administrator; Charmaine Hunt (formerly Meek), Trial Administrator; Terry Hughes, Data Manager; Adam Khan, Data Manager; Nicholas Hilken, Programming Team Leader; Neil Winkles, Senior Analyst Programmer; Adrian Wilcockson, Senior Analyst Programmer; and Mark Hunter, Analyst Programmer.
PREDNOS trial statistics
Birmingham Clinical Trials Unit, University of Birmingham
Natalie Ives, Statistics Team Leader; Rebecca Woolley, Trial Statistician; Andrew Howman, Trial Statistician; and Natalie Marchevsky, Statistician.
PREDNOS trial health economics
Emma Frew, University of Birmingham (Institute of Applied Health Research), Health Economics Lead; Tosin Lambe, University of Birmingham (Institute of Applied Health Research), Health Economics; and Associated Professor Billingsley Kaambwa, Flinders University (School of Medicine), Health Economics (original Health Economics Lead and Co-Applicant).
Participating centres and PREDNOS collaborative group members
Abertawe Bro Morgannwg University Health Board
Morriston Hospital
Dr Michelle James-Ellison, Principal Investigator; and Hilary Marie Williams, Research Nurse.
Airedale NHS Foundation Trust
Airedale General Hospital
Dr Pronab Bala, Principal Investigator.
Alder Hey Children’s NHS Foundation Trust
Alder Hey Children’s Hospital
Dr Caroline Jones, Principal Investigator; Dr Richard Holt, Clinician; Dr Henry Morgan, Clinician; Elizabeth Bailey, Research Nurse; and Lauren Flanagan, Data Manager.
Ashford and St Peter’s Hospitals NHS Foundation Trust
St Peter’s Hospital
Dr Tariq Bhatti, Principal Investigator; Dr Shailini Bahl, Clinician; and Lorna Walding, Research Nurse.
Barnsley Hospital NHS Foundation Trust
Barnsley Hospital
Dr Ezzedin Gouta, Principal Investigator; Dr Rajeev Gupta, Clinician; and Dr Diarmuid Kerrin, Clinician.
Barts Health NHS Trust
Newham University Hospital, The Royal London Hospital, Whipps Cross University Hospital
Dr Ami Parikh, Principal Investigator; Dr Ann Duthie, previous Principal Investigator; Clinician (Whipps Cross University Hospital); Dr Nimze Gadong, previous Principal Investigator; Clinician (Newham University Hospital); Dr Bahadur Anjum, Clinician (Newham University Hospital); Bessie Crone, Research Nurse; Hilarious De Jesus, Research Nurse (The Royal London Hospital); Ivone Lancoma-Malcolm, Research Nurse (Newham University Hospital); and Nicolene Plaatjies, Research Nurse (Newham University Hospital, The Royal London Hospital).
Basildon and Thurrock University Hospitals NHS Foundation Trust
Basildon University Hospital
Dr Khorshid Khalifa, Principal Investigator.
Belfast Health and Social Care Trust
The Royal Belfast Hospital for Sick Children
Dr Karl McKeever, Principal Investigator; Dr Grace McCall, Clinician; Patricia McCreesh, Research Nurse; and Muriel Millar, Research Nurse.
Betsi Cadwaladr University Health Board
Glan Clwyd Hospital, Ysbyty Gwynedd
Dr Markus Hesseling, Principal Investigator (Glan Clwyd Hospital); Dr Madalitso Kubwalo, Principal Investigator (Ysbyty Gwynedd); Annette Bolger, Research Officer; and Lucie Hobson, Research Nurse.
Birmingham Women’s and Children’s NHS Foundation Trust
Birmingham Children’s Hospital
Dr David Milford, Principal Investigator; Dr Larissa Kerecuk, Clinician; Claire Norton, Central Pharmacist; Narinder Rahania, Central Pharmacist; Helen Williamson, Central Pharmacist; Fatima Bibi, Central Pharmacy Clinical Trials Assistant; Tracy Gazeley, Central Pharmacy Technician; and Julie Williams, Central Pharmacy Technician.
Bolton NHS Foundation Trust
Royal Bolton Hospital
Dr Fiona Watson, Principal Investigator; and Claire Abbot, Research Nurse.
Bradford Teaching Hospitals NHS Foundation Trust
Bradford Royal Infirmary
Dr Simon Frazer, Principal Investigator; and Louise Akeroyd, Research Nurse.
Burton Hospitals NHS Foundation Trust
Queen’s Hospital
Dr Mansoor Ahmed, Principal Investigator; Dr Dominic Muogbo, Clinician; Claire Backhouse, Research Nurse; and Stephanie Boswell, Research Nurse.
Calderdale and Huddersfield NHS Foundation Trust
Calderdale Royal Hospital, Huddersfield Royal Infirmary
Dr Eilean Crosbie, Principal Investigator.
Cambridge University Hospitals NHS Foundation Trust
Addenbrooke’s Hospital
Dr Peter Heinz, Principal Investigator; and Dr Birgit Ulbrich, Clinician.
Cardiff and Vale University Health Board
University Hospital of Wales
Dr Shivaram Hegde, Principal Investigator; and Pauline Jones, Research Nurse.
Central Manchester University Hospitals NHS Foundation Trust
Royal Manchester Children’s Hospital
Professor Nicholas JA Webb, Chief Investigator; Principal Investigator; Dr Rachel Lennon, Clinician; Laboratory; Dr Malcolm Lewis, Clinician; Dr Nicholas Plant, Clinician; Dr Mohan Shenoy, Clinician; Dr Shaila Sukthankar, Clinician; Charlotte Bryant, Research Co-ordinator; Sarah Douglas, Research Co-ordinator; Helen Sumner, Research Co-ordinator; and Jane Howell, Research Nurse.
Chelsea and Westminster Hospital NHS Foundation Trust
West Middlesex University Hospital
Dr Nour Elhadi, Principal Investigator; and Irene Bishton, Research Nurse.
Chesterfield Royal Hospital NHS Foundation Trust
Chesterfield Royal Hospital
Dr Oyekunle Ayonrinde, Principal Investigator.
Colchester Hospital University NHS Foundation Trust
Colchester General Hospital
Dr Andrea Turner, Principal Investigator; Dr Jonathan Campbell, Clinician; and Aine Turner, Research Nurse.
Countess of Chester Hospital NHS Foundation Trust
Countess of Chester Hospital
Dr John Gibbs, Principal Investigator; Dr Elizabeth Newby, Clinician; Dr Satyanarayana Saladi, Clinician; Dr Alison Timmis, Clinician; Caroline Burchett, Research Nurse; and Sarah De-Beger, Research Assistant.
County Durham and Darlington NHS Foundation Trust
Darlington Memorial Hospital, University Hospital of North Durham
Dr Asha Nair, Principal Investigator; and Dr Antima Banerjee, Clinician.
Croydon Health Services NHS Trust
Croydon University Hospital
Dr Theo Fenton, Principal Investigator.
Dartford and Gravesham NHS Trust
Darent Valley Hospital
Dr Selwyn D’Costa, Principal Investigator.
Derby Teaching Hospitals NHS Foundation Trust
Royal Derby Hospital
Dr Richard Bowker, Principal Investigator; Coral Smith, Research Nurse; and Vanessa Unsworth, Research Nurse.
Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust
Bassetlaw Hospital, Doncaster Royal Infirmary
Dr Anuja Natarajan, Principal Investigator; and Dr Lai Men Wong, Clinician.
Dorset County Hospital NHS Foundation Trust
Dorset County Hospital
Dr Phil Parslow, Principal Investigator.
East and North Hertfordshire NHS Trust
Lister Hospital
Dr Vijay Kandala, Principal Investigator; and Dr Salman Imran, previous Principal Investigator.
East Cheshire NHS Trust
Macclesfield District General Hospital
Dr Surendran Chandrasekaran, Principal Investigator; Dr Sheng-Ang Ho, previous Principal Investigator; Dr Ignatius Losa, Clinician; Dr Katrina Marinaki, Clinician; Natalie Keenan, Research Nurse; Jessica Nichols, Research Nurse; and Joanne Shippey, Research Nurse.
East Lancashire Hospitals NHS Trust
Royal Blackburn Hospital
Dr Javed Iqbal, Principal Investigator.
Epsom and St Helier University Hospitals NHS Trust
Epsom Hospital
Dr Kirsty Watts, Principal Investigator.
Frimley Health NHS Foundation Trust
Wexham Park Hospital
Dr Angela Yannoulias, Principal Investigator; and Dr Zilla Huma, previous Principal Investigator.
Gloucestershire Hospitals NHS Foundation Trust
Gloucestershire Royal Hospital
Dr Adamu Sambo, Principal Investigator; Dr Lyda Jadresic, previous Principal Investigator; and Susan Beames, Research Nurse.
Great Ormond Street Hospital for Children NHS Foundation Trust
Great Ormond Street Hospital
Dr Detlef Bockenhauer, Principal Investigator; Laboratory; and Dr Daljit Hothi, previous Principal Investigator.
Great Western Hospitals NHS Foundation Trust
Great Western Hospital
Dr Nick West, Principal Investigator; and Dr Helen Price, previous Principal Investigator.
Guy’s and St Thomas’ NHS Foundation Trust
Evelina London Children’s Hospital
Dr Manish Sinha, Principal Investigator; Emma Neal, Research Nurse; Elisabeth Reus, Research Nurse; and Paula Sofocleous, Research Nurse.
Harrogate and District NHS Foundation Trust
Harrogate District Hospital
Dr Ian Cannings, Principal Investigator.
Homerton University Hospital NHS Foundation Trust
Homerton University Hospital
Dr Rajiv Sood, Principal Investigator.
Hull and East Yorkshire Hospitals NHS Trust
Hull Royal Infirmary
Dr Verghese Mathew, Principal Investigator; and Dr Elsadeg Sharif, previous Principal Investigator.
Kettering General Hospital NHS Foundation Trust
Kettering General Hospital
Dr Harsha Bilolikar, Principal Investigator; and Sonia White, Research Nurse.
Kingston Hospital NHS Foundation Trust
Kingston Hospital
Dr Titi Ayeni, Principal Investigator.
Lancashire Teaching Hospitals NHS Foundation Trust
Royal Preston Hospital
Dr Ruth O’Connor, Principal Investigator.
Luton and Dunstable Hospital NHS Foundation Trust
Luton and Dunstable University Hospital
Dr Michael Eisenhut, Principal Investigator; Dr Tomasz Rajkowski, Clinician; Samantha Clough, Research Nurse; Rebecca Dixon, Research Nurse; and Karen Reep, Research Nurse.
Maidstone and Tunbridge Wells NHS Trust
Maidstone Hospital, Tunbridge Wells Hospital
Dr Krishnan Balasubramanian, Principal Investigator; and Dr Hamudi Kisat, Clinician.
Medway NHS Foundation Trust
Medway Maritime Hospital
Dr Janette Cansick, Principal Investigator.
Mid Cheshire Hospitals NHS Foundation Trust
Leighton Hospital
Dr Julie Ellison, Principal Investigator; Sally Smith, Research Nurse; and Samantha Tapscott, Research Nurse.
NHS Ayrshire and Arran
University Hospital Crosshouse
Dr Bridget Oates, Principal Investigator; and Claire Bell, Research Nurse.
NHS Dumfries & Galloway
Dumfries and Galloway Royal Infirmary
Dr Rajendran Shyam, Principal Investigator.
NHS Fife
Victoria Hospital
Dr Evelyn Menzies, Principal Investigator; and Pamela Cruickshanks, Clinical Nurse Specialist.
NHS Forth Valley
Forth Valley Royal Hospital
Dr John Schulga, Principal Investigator.
NHS Grampian
Royal Aberdeen Children’s Hospital
Dr Craig Oxley, Principal Investigator.
NHS Greater Glasgow and Clyde
Royal Alexandra Hospital, Royal Hospital for Children
Dr David Hughes, Principal Investigator (Royal Hospital for Children); Dr Amita Sharma, Principal Investigator (Royal Alexandra Hospital); Dr Leah Krischock, previous Principal Investigator (Royal Hospital for Children); Dr Jamie Houston, Clinician (Royal Alexandra Hospital); Diane Carroll, Research Nurse (Royal Hospital for Children); Sheryl Goddard, Research Nurse (Royal Hospital for Children); and Elizabeth Waxman, Research Nurse.
NHS Highland
Raigmore Hospital
Dr Alan Webb, Principal Investigator.
NHS Lanarkshire
Wishaw General Hospital
Dr Thin Thin Saing, Principal Investigator; Dr Colin Cunningham, Clinician; Angela Brown, Research Nurse; and Victoria Causer, Clinical Nurse Specialist.
NHS Lothian
Royal Hospital for Sick Children
Dr David Hughes, Principal Investigator; Dr Sepideh Taheri, previous Principal Investigator; Alyson Macdonald, Clinical Nurse Specialist; Tracey McGregor, Clinical Nurse Specialist; Emma Carson, Research Nurse; and Kay Riding, Research Nurse.
Norfolk and Norwich University Hospitals NHS Foundation Trust
Norfolk and Norwich University Hospital
Dr Nandu Thalange, Principal Investigator; Dr Chris Upton, previous Principal Investigator, Clinician; and Louisa Fear, Research Nurse.
North Cumbria University Hospitals NHS Trust
Cumberland Infirmary
Dr Glyn Jones, Principal Investigator.
North Tees and Hartlepool NHS Foundation Trust
University Hospital of North Tees
Dr Vijay Tandle, Principal Investigator; and Gabrielle Osborne, Research Nurse.
North West Anglia NHS Foundation Trust
Peterborough City Hospital
Dr Mona Aslam, Principal Investigator.
Northampton General Hospital NHS Trust
Northampton General Hospital
Dr Imogen Norton, Principal Investigator.
Northern Devon Healthcare NHS Trust
North Devon District Hospital
Dr Dermot Dalton, Principal Investigator; Dr Andrew Arend, previous Principal Investigator; and Dr Krishnakumar Thattakkat, previous Principal Investigator.
Northern Lincolnshire and Goole NHS Foundation Trust
Diana, Princess of Wales Hospital
Dr Bukar Wobi, Principal Investigator; Dr Bemigho Etuwewe, Clinician; Tara Walker, Data Manager; Julie Browning, Research Nurse; Marjorie Carling, Research Nurse; Emma Killingbeck, Research Nurse; Dorothy Ryan, Research Nurse; and Consolata Tsvangirai-Mahachi, Research Nurse.
Nottingham University Hospitals NHS Trust
Nottingham Children’s Hospital
Dr Andrew Lunn, Principal Investigator; and Dr Martin Christian, Clinician.
Oxford University Hospitals NHS Foundation Trust
John Radcliffe Hospital
Dr Janet Craze, Principal Investigator.
Plymouth Hospitals NHS Trust
Derriford Hospital
Dr Catherine Derry, Principal Investigator; Dr Robert Jones, previous Principal Investigator; Sarah-Jane Sharman, Research Nurse; and Sally Harwood, Trial Administrator.
Poole Hospital NHS Foundation Trust
Poole Hospital
Dr Steve Wadams, Principal Investigator; Heather Barham, Research Nurse; and Susan Power, Research Nurse.
Portsmouth Hospitals NHS Trust
Queen Alexandra Hospital
Dr Catherine Tuffrey, Principal Investigator; Dr Simon Birch, Clinician; Dr Judith Scanlan, Clinician; Andrew Gribbin, Research Nurse; and Sharon McCready, Research Nurse.
Royal Berkshire NHS Foundation Trust
Royal Berkshire Hospital
Dr Ann Gordon, Principal Investigator; Dr Gregory Boden, previous Principal Investigator; and Susan Hallett, Research Nurse.
Royal Cornwall Hospitals NHS Trust
Royal Cornwall Hospital
Dr Chris Williams, Principal Investigator; and Gillian Craig, Research Nurse.
Royal Devon and Exeter Hospital NHS Foundation Trust
Royal Devon and Exeter: Wonford Hospital
Dr Hannah Cottis, Principal Investigator; Dr Vaughan Lewis, previous Principal Investigator; Dr Christine McMillan, Clinician; Dr Nigel Osborne, Clinician; Caroline Harrill, Research Nurse; and Suzanne Wilkins, Research Nurse.
Royal Free London NHS Foundation Trust
Royal Free Hospital (non-recruiting centre)
Professor Robert Kleta, Laboratory.
Royal United Hospitals Bath NHS Foundation Trust
Royal United Hospital
Dr Lynn Diskin, Principal Investigator; Dr Amanda Billson, previous Principal Investigator; and Alison Barratt, Research Nurse.
Sheffield Children’s NHS Foundation Trust
Sheffield Children’s Hospital
Dr Gail Moss, Principal Investigator.
Sherwood Forest Hospitals NHS Foundation Trust
King’s Mill Hospital
Dr Simon Rhodes, Principal Investigator; Anna Coupe, Research Officer; and Caroline Moulds, Research Nurse.
Southern Health and Social Care Trust
Craigavon Area Hospital
Michael Smith, Principal Investigator; Sara Gilpin, Research Nurse; and Judith Ratcliffe, Research Nurse.
Southport and Ormskirk Hospital NHS Trust
Ormskirk and District General Hospital
Dr Sharryn Gardner, Principal Investigator.
St Helens and Knowsley Teaching Hospitals NHS Trust
Whiston Hospital
Dr Lakshmi Chilukuri, Principal Investigator.
Stockport NHS Foundation Trust
Stepping Hill Hospital
Dr Chris Cooper, Principal Investigator; and Sara Bennett, Research Nurse.
Tameside and Glossop Integrated Care NHS Foundation Trust
Tameside Hospital
Dr Anjali Petkar, Principal Investigator; Dr Ahsan Ul-Haq, Clinician; and Gill Waring, Research Nurse.
Taunton and Somerset NHS Foundation Trust
Musgrove Park Hospital
Dr Rebecca Mann, Principal Investigator; and Nicola Thorne, Research Nurse.
The Dudley Group NHS Foundation Trust
Russells Hall Hospital
Dr Zala Ibrahim, Principal Investigator.
The Ipswich Hospital NHS Trust
Ipswich Hospital
Dr Jackie Buck, Principal Investigator; and Deborah Beeby, Research Nurse.
The Leeds Teaching Hospitals NHS Trust
Leeds General Infirmary
Dr Eric Finlay, Principal Investigator; Majorie Allen, Research Nurse; and Rebecca Mottram, Research Nurse.
The Mid Yorkshire Hospitals NHS Trust
Dewsbury and District Hospital, Pinderfields Hospital
Dr Kathryn Deakin, Principal Investigator.
The Newcastle upon Tyne Hospitals NHS Foundation Trust
Great North Children’s Hospital
Dr Sally Johnson, Principal Investigator; Dr Yincent Tse, Clinician; Kathryn Bell, Research Nurse; and Denise Chisholm, Clinical Nurse Specialist.
The Pennine Acute Hospitals NHS Trust
North Manchester General Hospital, The Royal Oldham Hospital
Dr Beena Padmakumar, Principal Investigator; and Dr Talaivirichan Magadevan, Clinician (North Manchester General Hospital).
The Queen Elizabeth Hospital King’s Lynn NHS Foundation Trust
Queen Elizabeth Hospital
Dr Mandy Hughes, Principal Investigator; Dr Susan Rubin, previous Principal Investigator; and Amy Frary, Research Nurse.
The Rotherham NHS Foundation Trust
Rotherham Hospital
Dr Sanjay Suri, Principal Investigator; Dr Sherif El-Refee, previous Principal Investigator; and Dr Christine Harrison, Clinician.
The Royal Wolverhampton NHS Trust
Cannock Chase Hospital, New Cross Hospital
Dr Karen Davies, Principal Investigator; and Sharon Kempson, Research Nurse.
The Shrewsbury and Telford Hospital NHS Trust
Princess Royal Hospital, Royal Shrewsbury Hospital
Dr Naeem Ayub, Principal Investigator.
United Lincolnshire Hospitals NHS Trust
Lincoln County Hospital, Pilgrim Hospital
Dr Sourabh Mukhopadhyay, Principal Investigator (Lincoln County Hospital); Dr Margaret Crawford, Principal Investigator (Pilgrim Hospital); Dr Mujeeb Pervez, previous Principal Investigator (Pilgrim Hospital); and Dr Douglas Thomas, Clinician (Lincoln County Hospital).
University Hospital of South Manchester NHS Foundation Trust
Wythenshawe Hospital
Dr Gopikrishna Vemuri, Principal Investigator; Dr Radhika Puttha, previous Principal Investigator; Dr Alice Setti, previous Principal Investigator; Dr Faisal Al-Zidgali, Clinician; Claire Holliday, Research Nurse; and Louise Woodhead, Research Nurse.
University Hospital Southampton NHS Foundation Trust
Southampton Children’s Hospital
Rodney Gilbert, Principal Investigator.
University Hospitals Bristol NHS Foundation Trust
Bristol Royal Hospital for Children
Professor Moin Saleem, Principal Investigator; Dr Alison Kelly, Clinician; Michelle Ross, Research Nurse; and Heather Smee, Research Nurse.
University Hospitals Coventry and Warwickshire NHS Trust
University Hospital
Dr Nigel Coad, Principal Investigator.
University Hospitals of Leicester NHS Trust
Leicester Royal Infirmary
Dr Angela Hall, Principal Investigator; and Dr Peter Houtman, previous Principal Investigator.
University Hospitals of Morecambe Bay NHS Foundation Trust
Furness General Hospital, Royal Lancaster Infirmary
Dr Nayan Peepah Nardeosingh, Principal Investigator; Dr Mireille-Yvette Formosa, previous Principal Investigator; and Dr Patrick Ward, previous Principal Investigator.
University Hospitals of North Midlands NHS Trust
County Hospital, Royal Stoke University Hospital
Dr Yvonne Slater, Principal Investigator; Dr Saeeda Raja, previous Principal Investigator (County Hospital); Dr Kishor Tewary, previous Principal Investigator (County Hospital); and Dr Shiva Shankar, previous Principal Investigator (Royal Stoke University Hospital).
Warrington and Halton Hospitals NHS Foundation Trust
Warrington Hospital
Dr Rachel Delyth Webb, Principal Investigator; and Natalie Rogers, Research Nurse.
West Suffolk NHS Foundation Trust
West Suffolk Hospital
Dr Raman Lakshman, Principal Investigator; and Dr Karine Cesar, Clinician.
Western Health and Social Care Trust
Altnagelvin Area Hospital
Dr Damien Armstrong, Principal Investigator; and Julie Brown, Research Nurse.
Western Sussex Hospitals NHS Foundation Trust
St Richard’s Hospital, Worthing Hospital
Dr Nicholas Brennan, Principal Investigator; and Dr Stuart Nicholls, previous Principal Investigator (Worthing Hospital).
Wirral University Teaching Hospital NHS Foundation Trust
Arrowe Park Hospital
Dr Elizabeth Breen, Principal Investigator; Sharon Hughes, Research Nurse; and Lucy Lewis, Research Nurse.
Worcestershire Acute Hospitals NHS Trust
Alexandra Hospital, Worcestershire Royal Hospital
Dr John Scanlon, Principal Investigator; Dr Munir Ahmed, Clinician; and Dr Andrew Gallagher, Clinician.
Wrightington, Wigan And Leigh NHS Foundation Trust
Royal Albert Edward Infirmary
Dr Vineeta Joshi, Principal Investigator; Dr Madhumita Mukherjee, previous Principal Investigator; and Dr Singara Velmurugan, previous Principal Investigator.
Wye Valley NHS Trust
The County Hospital
Dr Simon Meyrick, Principal Investigator.
Yeovil District Hospital NHS Foundation Trust
Yeovil District Hospital
Dr Michael Fernando, Principal Investigator; Dr Christopher Zaborowski, Clinician; and Alex Stannett, Research Nurse.
York Teaching Hospital NHS Foundation Trust
Scarborough Hospital, The York Hospital
Dr Udupa Venkatesh, Principal Investigator (Scarborough Hospital); Dr Guy Millman, Principal Investigator (The York Hospital); Simon Dyer, Research Nurse (Scarborough Hospital); and Kerry Elliot, Research Nurse (Scarborough Hospital).
Other
University of Manchester (non-recruiting centre)
Professor David Ray, Laboratory.
Participant and public representatives
Wendy Cook, NeST Fundraising Co-ordinator; and Shahid Muhammad, The Renal Patient Support Group Co-founder and Chief in Research.
Trial Steering Committee
Professor Alan Watson, Nottingham University Hospitals NHS Trust (Nottingham Children’s Hospital), Chairperson, Emeritus Professor of Paediatric Nephrology; Professor Peter Clayton, University of Manchester (Faculty of Biology, Medicine and Health) and Central Manchester University Hospitals NHS Foundation Trust (Royal Manchester Children’s Hospital), Professor of Child Health & Paediatric Endocrinology; and Dr Kate Hillman, Central Manchester University Hospitals NHS Foundation Trust (Manchester Royal Infirmary), Consultant Renal Physician.
Data Monitoring Committee
Professor Philip Kalra, University of Manchester and Salford Royal NHS Foundation Trust (Salford Royal Hospital), Chairperson, Consultant Nephrologist/ Honorary Professor; Professor Timothy Barrett, University of Birmingham (Institute of Cancer and Genomic Sciences) and Birmingham Women’s and Children’s NHS Foundation Trust (Birmingham Children’s Hospital), Professor of Paediatric Endocrinology; and Dr Andrea Marshall University of Warwick (Warwick Clinical Trials Unit), Principal Research Fellow in Medical Statistics.
Pilot trial
Trial management
Dr Richard S Trompeter, Chief Investigator; Dr Nicholas Webb, Co-Investigator; and Dr Carole Cummins, Co-Investigator.
Principal investigators and collaborators
Dr C Mark Taylor, Birmingham Children’s Hospital; N Fineman, Bristol Royal Hospital for Children; Dr Javed Iqbal, Burnley General Hospital; Dr Nigel Coad, Coventry and Warwickshire Hospital; Dr Robert WA Jones, Derriford Hospital; Dr Richard S Trompeter, Great Ormond Street Hospital; Dr Jackie Buck, Ipswich Hospital; Dr Janet Craze, John Radcliffe Hospital; Dr Angela Hall, Leicester Royal Infirmary; Dr Peter Houtman; Dr Ian Spillman, Macclesfield District General; Dr Imogen Thornburgh, Northampton General Hospital; Dr Martin Christian, Nottingham City Hospital; Dr JH Evans; Dr Farida Hussain; Dr Alan Watson; Dr David Hughes, Royal Hospital for Sick Children Glasgow; Dr Richard Holt, Royal Liverpool Children’s Hospital; Dr Caroline Jones; Dr Ami Parikh, Royal London Hospital; Dr Mohan Shenoy, Royal Manchester Children’s Hospital; Dr Nicholas Webb; Dr Rodney D Gilbert, Southampton General Hospital; and Dr Eric Finlay, St James Hospital Leeds.
Data Monitoring Committee
Dr Martin Landray, University of Oxford; and Professor Adrian Woolf, University College London.
Trial co-ordinators
Dr Rachel Cook, University of Birmingham; and Dr Fazeelat Sharif, University of Birmingham.
Contributions of authors
Nicholas JA Webb (Professor of Paediatric Nephrology, Paediatric Nephrology; Chief Investigator) conceived the PREDNOS study and pilot study, led the protocol design, recruited participants and led the writing of the full report.
Rebecca L Woolley (Medical Statistician, Statistics) performed data analysis for the Data Monitoring Committee meetings throughout the duration of the trial and for the final report and helped to produce and commented on the final version of the full report.
Tosin Lambe (Research Fellow, Health Economics) conducted the mapping analysis, undertook the cost-effectiveness analyses and contributed to writing related sections of the report.
Emma Frew (Reader in Health Economics, Health Economics) developed the economic evaluation protocol, supervised the economic section of the report and contributed to the writing of the economic sections of the report.
Elizabeth A Brettell (Renal Team Leader, Clinical Trials Management) was responsible for overall co-ordination, management and oversight of the PREDNOS study at the Birmingham Clinical Trials Unit, provided significant input into the protocol and development of the case report forms, managed the delivery and execution of the study and helped to produce and commented on the final version of the full report.
Emma N Barsoum (Trial Co-ordinator, Clinical Trials Management) was responsible for overall co-ordination of the trial from set-up to the final report, provided significant input into the study set-up, monitoring and ensuring execution of the trial and was involved in producing the final version of the full report.
Richard S Trompeter (Emeritus Consultant Paediatric Nephrologist, Paediatric Nephrology) was initially the Chief Investigator and contributed to the trial design and management.
Carole Cummins (Senior Lecturer, Research Methods) was responsible for grant applications for pilot trial and for the design and execution of the pilot trial, provided major input to the grant application for the main trial and protocol and provided input into the final version of the full report.
Keith Wheatley (Professor of Clinical Trials, Clinical Trials and Medical Statistics) contributed to the design of the trial, interpretation of the results and review of the full report.
Natalie J Ives (Statistics Team Leader, Statistics) provided input into the protocol, was responsible for the statistical aspects of the study, provided oversight and supervision of the statistical analyses (Data Monitoring Committee and end-of-trial analyses), contributed to the interpretation of the results and provided major input into the writing of the full report.
Publications
Lambe T, Frew E, Ives NJ, Woolley RL, Cummins C, Brettell EA, et al. Mapping the Paediatric Quality of Life Generic Core Scales (PedsQL™) onto the Child Health Utility 9D (CHU-9D) index score for economic evaluation in children. PharmacoEconomics 2018;36:451–65.
Webb NJA, Woolley RL, Lambe T, Frew E, Brettell EA, Barsoum EN, et al. Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ 2019;365:l1800.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 2001;16:1040-4. https://doi.org/10.1007/s004670100021.
- International Study of Kidney Disease in Children . Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int 1978;13:159-65. https://doi.org/10.1038/ki.1978.23.
- Feehally J, Kendell NP, Swift PG, Walls J. High incidence of minimal change nephrotic syndrome in Asians. Arch Dis Child 1985;60:1018-20. https://doi.org/10.1136/adc.60.11.1018.
- Sharples PM, Poulton J, White RH. Steroid responsive nephrotic syndrome is more common in Asians. Arch Dis Child 1985;60:1014-17. https://doi.org/10.1136/adc.60.11.1014.
- Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol 2017;12:332-45. https://doi.org/10.2215/CJN.05000516.
- McCaffrey J, Lennon R, Webb NJ. The non-immunosuppressive management of childhood nephrotic syndrome. Pediatr Nephrol 2016;31:1383-402. https://doi.org/10.1007/s00467-015-3241-0.
- Webb NJA, Molony D, Craig J. Evidence-Based Nephrology. Oxford: Wiley-Blackwell; 2008.
- Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1985;1:368-70. https://doi.org/10.1016/S0140-6736(85)91387-X.
- Webb NJA, Rees L, Brogan PA, Bockenhauer D, Webb NJA. Pediatric Nephrology. Oxford: Oxford University Press; 2012.
- Webb NJ, Lewis MA, Iqbal J, Smart PJ, Lendon M, Postlethwaite RJ. Childhood steroid sensitive nephrotic syndrome: does the histology matter?. Am J Kid Dis 1996;27:484-8. https://doi.org/10.1016/S0272-6386(96)90157-2.
- Trautmann A, Schnaidt S, Lipska-Ziętkiewicz BS, Bodria M, Ozaltin F, Emma F, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol 2017;28:3055-65. https://doi.org/10.1681/ASN.2016101121.
- International Study of Kidney Disease in Children . Nephrotic syndrome in children: a randomised controlled trial comparing two prednisolone regimens in steroid responsive patients who relapse early. J Pediatr 1979;95:239-43.
- Hahn D, Hodson EM, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD001533.pub5.
- Hall AS, Thorley G, Houtman PN. The effects of corticosteroids on behavior in children with nephrotic syndrome. Pediatr Nephrol 2003;18:1220-3. https://doi.org/10.1007/s00467-003-1295-x.
- Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child 1982;57:544-8. https://doi.org/10.1136/adc.57.7.544.
- Lewis MA, Baildom EM, Davis N, Houston IB, Postlethwaite RJ. Nephrotic syndrome: from toddlers to twenties. Lancet 1989;1:255-9. https://doi.org/10.1016/S0140-6736(89)91266-X.
- Fakhouri F, Bocquet N, Taupin P, Presne C, Gagnadoux MF, Landais P, et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis 2003;41:550-7. https://doi.org/10.1053/ajkd.2003.50116.
- Abramowicz M, Barnett HL, Edelmann CM, Greifer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1:959-61.
- Arbeitsgemeinschaft für Padiatrische Nephrologie . Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1988;1:380-3.
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 1993;152:357-61.
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 1999;13:824-7.
- Jayantha UK. Comparison of ISKDC regime with a 7 months steroid regime in the first attack of nephrotic syndrome. Pediatr Nephrol 2004;19.
- Ksiazek J, Wyszyńska T. Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 1995;84:889-93.
- Norero C, Delucchi A, Lagos E, Rosati P. Initial therapy of primary nephrotic syndrome in children: evaluation in a period of 18 months of two prednisone treatment schedules. Chilean Co-operative Group of Study of Nephrotic Syndrome in Children. Rev Med Chil 1996;124:567-72.
- Ueda N, Chihara M, Kawaguchi S, Niinomi Y, Nonoda T, Matsumoto J, et al. Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr 1988;112:122-6.
- Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, et al. A randomized study of two long-course prednisolone regimens for nephrotic syndrome in children. Am J Kidney Dis 2003;41:1155-62.
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti AVS, Raddi G, Piscitelli A, et al. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial. Pediatr Nephrol 2004;19.
- Sharma RK, Ahmed M, Gupta A, Gulati S, Sharma AP. Comparison of abrupt withdrawal versus slow tapering regimens of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome. J Am Soc Nephrol 2000;11.
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2005;1. https://doi.org/1002/14651858.CD001533.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet 1998;352:609-13.
- Lombel RM, Gipson DS, Hodson EM. Kidney disease: improving global outcomes. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 2013;28:415-26. https://doi.org/10.1007/s00467-012-2310-x.
- Teeninga N, Kist-van Holthe JE, van Rijswijk N, de Mos NI, Hop WCJ, Wetzels JFM, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol 2012;24:149-59.
- Lande MB, Leonard MB. Variability among pediatric nephrologists in the initial therapy of nephrotic syndrome. Pediatr Nephrol 2000;14:766-9.
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, et al. Extending initial prednisolone treatment in a randomised controlled trial from 3 to 6 months did not significantly influence the course of illness. Kidney Int 2015;87:217-24.
- Yoshikawa N, Nakanishi K, Sako M, Oba MS, Mori R, Ota E, et al. A multicentre randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months in not inferior to six-month treatment. Kidney Int 2015;87:225-32.
- Hoyer PF. New lessons from randomized trials in steroid-sensitive nephrotic syndrome: clear evidence against long steroid therapy. Kidney Int 2015;87:17-9.
- Banh TH, Hussain-Shamsy N, Patel V, Vasilevska-Ristovska J, Borges K, Sibbald C, et al. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J Am Soc Nephrol 2016;11:1760-8. https://doi.org/10.2215/CJN.00380116.
- Takeda A, Matsutani H, Niimura F, Ohgushi H. Risk factors for relapse in childhood nephrotic syndrome. Pediatr Nephrol 1996;10:740-1.
- Sinha A, Hari P, Sharma PK, Gulati A, Kalaivani M, Mantan M, et al. Disease course in steroid sensitive nephrotic syndrome. Indian Pediatr 2012;49:881-7.
- Child Growth Standards. Geneva: World Health Organization; n.d.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555-76.
- Medicines for Human Use (Clinical Trials) Regulations 2004. London: The Stationery Office; 2004.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Human Tissue Act 2004. London: The Stationery Office; 2004.
- Guidelines for Good Clinical Practice. Geneva: International Conference on Harmonisation; 1996.
- Andersen RF, Thrane N, Noergaard K, Rytter L, Jespersen B, Rittig S. Early age at debut is a predictor of steroid-dependent and frequent relapsing nephrotic syndrome. Pediatr Nephrol 2010;25:1299-304. https://doi.org/10.1007/s00467-010-1537-7.
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329-41.
- Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care 1989;27:217-32.
- Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. PharmacoEconomics 2000;17:13-35.
- Mehrez A, Gafni A. Quality-adjusted life years, utility theory, and healthy-years equivalents. Med Decis Making 1989;9:142-9.
- Brazier JE, Yang Y, Tsuchiya A, Rowen DL. A review of studies mapping (or cross walking) non-preference based measures of health to generic preference-based measures. Eur J Health Econ 2010;11:215-25. https://doi.org/10.1007/s10198-009-0168-z.
- Gerard K, Mooney G. QALY league tables: handle with care. Health Econ 1993;2:59-64.
- Nord E. The QALY – a measure of social value rather than individual utility?. Health Econ 1994;3:89-93.
- Stevens KJ. Working with children to develop dimensions for a preference-based, generic, pediatric, health-related quality-of-life measure. Qual Health Res 2010;20:340-51. https://doi.org/10.1177/1049732309358328.
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6-7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83. https://doi.org/10.1007/s11136-012-0119-5.
- Stevens K, Ratcliffe J. Measuring and valuing health benefits for economic evaluation in adolescence: an assessment of the practicality and validity of the child health utility 9D in the Australian adolescent population. Value Health 2012;15:1092-9. https://doi.org/10.1016/j.jval.2012.07.011.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Dakin H. Review of studies mapping from quality of life or clinical measures to EQ-5D: an online database. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-151.
- Golub GH, Van Loan CF. An analysis of the total least squares problem. SIAM NUMER Anal 1980;17:883-93.
- Crawley MJ, Crawley MJ. The R Book. Chichester: John Wiley & Sons; n.d.
- Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The Hosmer–Lemeshow test revisited. Crit Care Med 2007;35:2052-6. https://doi.org/10.1097/01.CCM.0000275267.64078.B0.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New Jersey, NJ: John Wiley & Sons; 2004.
- Tobin J. Estimation of relationships for limited dependent variables. Econometrica 1958;26:24-36.
- Reynolds A, Shonkwiler J. Testing and correcting for distributional misspecifications in the tobit model: an application of the information matrix test. Empir Econ 1991;16:313-23.
- Khan KA, Petrou S, Rivero-Arias O, Walters SJ, Boyle SE. Mapping EQ-5D utility scores from the PedsQL™ generic core scales. PharmacoEconomics 2014;32:693-706. https://doi.org/10.1007/s40273-014-0153-y.
- Hyndman RJ, Koehler AB. Another look at measures of forecast accuracy. Int J Forecast 2006;22:679-88.
- Petrou S, Rivero-Arias O, Dakin H, Longworth L, Oppe M, Froud R, et al. The MAPS reporting statement for studies mapping onto generic preference-based outcome measures: explanation and elaboration. PharmacoEconomics 2015;33:993-1011. https://doi.org/10.1007/s40273-015-0312-9.
- Drummond M. Introducing economic and quality of life measurements into clinical studies. Ann Med 2001;33:344-9.
- Marra CA, Woolcott JC, Kopec JA, Shojania K, Offer R, Brazier JE, et al. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med 2005;60:1571-82.
- Boyer NR, Miller S, Connolly P, McIntosh E. Paving the way for the use of the SDQ in economic evaluations of school-based population health interventions: an empirical analysis of the external validity of SDQ mapping algorithms to the CHU9D in an educational setting. Qual Life Res 2016;25:913-23. https://doi.org/10.1007/s11136-015-1218-x.
- Furber G, Segal L, Leach M, Cocks J. Mapping scores from the Strengths and Difficulties Questionnaire (SDQ) to preference-based utility values. Qual Life Res 2014;23:403-11. https://doi.org/10.1007/s11136-013-0494-6.
- Chen G, Stevens K, Rowen D, Ratcliffe J. From KIDSCREEN-10 to CHU9D: creating a unique mapping algorithm for application in economic evaluation. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/s12955-014-0134-z.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. https://doi.org/10.1016/j.jval.2015.02.001.
- Guide to the Methods of Technology. London: National Institute for Health and Care Excellence; 2013.
- Torrance GW, Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- National Schedule of Reference Costs: 2015–16. London: Department of Health and Social Care; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- PbR Tariff Information Spreadsheet for 2013 to 2014. London: Department of Health and Social Care; 2014.
- Guidance on Actions and Uses of Drugs Prescribed in the UK. London: BMJ Group and Pharmaceutical Press; 2015.
- Burton A, Billingham LJ, Bryan S. Cost-effectiveness in clinical trials: using multiple imputation to deal with incomplete cost data. Clin Trials 2007;4:154-61.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Thompson AJ, Newman WG, Elliott RA, Roberts SA, Tricker K, Payne K. The cost-effectiveness of a pharmacogenetic test: a trial-based evaluation of TPMT genotyping for azathioprine. Value Health 2014;17:22-33. https://doi.org/10.1016/j.jval.2013.10.007.
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997;8:769-76.
- International Study of Kidney Disease in Children . Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 1982;101:514-18.
- Kabuki N, Okugawa T, Hayakawa H, Tomizawa S, Kasahara T, Uchiyama M. Influence of age at onset on the outcome of steroid-sensitive nephrotic syndrome. Pediatr Nephrol 1998;12:467-70.
- Yap HK, Han EJ, Heng CK, Gong WK. Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 2001;16:1049-52. https://doi.org/10.1007/s004670100024.
- Takeda A, Takimoto H, Mizusawa Y, Simoda M. Prediction of subsequent relapse in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 2001;16:888-93.
- Hoyer PF, Brodeh J. Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. J Am Soc Nephrol 2006;17:1151-7.
- Vester U, Kranz B, Zimmermann S, Hoyer PF. Cyclophosphamide in steroid-sensitive nephrotic syndrome: outcome and outlook. Pediatr Nephrol 2003;18:661-4. https://doi.org/10.1007/s00467-003-1170-9.
- Donatti TL, Koch VH. Final height of adults with childhood-onset steroid-responsive idiopathic nephrotic syndrome. Pediatr Nephrol 2009;24:2401-8. https://doi.org/10.1007/s00467-009-1301-z.
- Simmonds J, Grundy N, Trompeter R, Tullus K. Long-term steroid treatment and growth: a study in steroid-dependent nephrotic syndrome. Arch Dis Child 2010;95:146-9. https://doi.org/10.1136/adc.2007.129957.
- Hung YT, Yang LY. Follow-up of linear growth of body height in children with nephrotic syndrome. J Microbiol Immunol Infect 2006;39:422-5.
- Schärer K, Essigmann HC, Schaefer F. Body growth of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 1999;13:828-34.
- Macneill V, Nwokoro C, Griffiths C, Grigg J, Seale C. Recruiting ethnic minority participants to a clinical trial: a qualitative study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002750.
- Attar SG, Poustie V, Beresford MG. The medicines for children research network: building on current success as we move forward. Clin Invest 2014;4:399-405.
- Hegarty J, Mughal MZ, Adams J, Webb NJ. Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int 2005;68:2304-9.
- Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 2004;351:868-75. https://doi.org/10.1056/NEJMoa040367.
Appendix 1 Site recruitment
| Site name | Date | Number of participants randomised | ||
|---|---|---|---|---|
| Site opened | First participant randomised | Last participant randomised | ||
| Addenbrooke’s Hospital, Cambridge | 27 October 2011 | 14 October 2013 | 14 October 2013 | 1 |
| Airedale General Hospital, Keighley | 30 November 2011 | 16 January 2014 | 16 January 2014 | 1 |
| Alder Hey Children’s Hospital, Liverpool | 18 July 2011 | 22 August 2011 | 24 September 2014 | 10 |
| Alexandra Hospital, Redditch | 5 September 2013 | – | – | 0 |
| Altnagelvin Area Hospital, Derry | 3 September 2012 | – | – | 0 |
| Arrowe Park Hospital, Wirral | 19 July 2011 | 28 November 2011 | 28 November 2011 | 1 |
| Barnsley District General Hospital | 19 August 2011 | 25 August 2011 | 25 August 2011 | 1 |
| Basildon Hospital | 18 October 2011 | – | – | 0 |
| Bassetlaw Hospital | 9 August 2011 | – | – | 0 |
| Birmingham Children’s Hospital | 28 July 2011 | 2 December 2011 | 2 April 2014 | 5 |
| Bradford Royal Infirmary | 25 August 2011 | 7 September 2011 | 20 May 2013 | 5 |
| Bristol Royal Hospital for Children | 16 September 2011 | 10 October 2011 | 28 January 2014 | 8 |
| Calderdale Royal Hospital | 18 July 2012 | 12 February 2014 | 12 February 2014 | 1 |
| Chesterfield Royal Hospital | 26 July 2011 | – | – | 0 |
| City General Hospital, Stoke on Trent | 21 July 2011 | 2 August 2011 | 25 July 2013 | 2 |
| Colchester General Hospital | 10 February 2012 | 1 March 2012 | 3 April 2013 | 2 |
| Countess of Chester Hospital | 29 June 2011 | 27 October 2011 | 28 August 2014 | 3 |
| Craigavon Area Hospital | 18 April 2012 | 11 August 2014 | 11 August 2014 | 1 |
| Crosshouse Hospital, Kilmarnock | 23 January 2012 | – | – | 0 |
| Croydon University Hospital | 11 December 2013 | – | – | 0 |
| Cumberland Infirmary | 22 January 2013 | – | – | 0 |
| Darent Valley Hospital | 12 December 2013 | – | – | 0 |
| Darlington Memorial Hospital | 27 March 2013 | – | – | 0 |
| Derriford Hospital, Plymouth | 17 October 2011 | 20 October 2011 | 24 September 2014 | 2 |
| Dewsbury and District Hospital | 18 October 2011 | 29 May 2013 | 15 January 2014 | 2 |
| Diana, Princess of Wales Hospital, Grimsby | 14 November 2012 | 18 December 2013 | 8 July 2014 | 2 |
| Doncaster Royal Infirmary | 9 August 2011 | 11 June 2012 | 11 June 2012 | 1 |
| Dorset County Hospital | 30 June 2011 | 6 January 2014 | 6 January 2014 | 1 |
| Dumfries & Galloway Royal Infirmary | 30 November 2011 | – | – | 0 |
| Epsom Hospital | 24 May 2013 | – | – | 0 |
| Evelina Children’s Hospital, London | 2 November 2011 | 4 June 2013 | 13 June 2014 | 3 |
| Forth Park Hospital | 30 November 2011 | 18 June 2014 | 16 September 2014 | 2 |
| Forth Valley Royal Hospital | 10 August 2011 | – | – | 0 |
| Furness General Hospital | 27 October 2011 | – | – | 0 |
| Glan Clwyd Hospital, Rhyl | 6 July 2012 | 17 January 2013 | 21 May 2014 | 3 |
| Gloucestershire Royal Hospital | 29 July 2011 | 29 January 2013 | 10 June 2014 | 6 |
| Great Ormond Street Hospital, London | 6 December 2011 | 21 May 2012 | 21 May 2012 | 1 |
| Great Western Hospital, Swindon | 16 August 2012 | 21 October 2013 | 21 October 2013 | 1 |
| Harrogate District Hospital | 17 July 2013 | – | – | 0 |
| Hereford County Hospital | 17 October 2011 | 29 November 2011 | 29 November 2011 | 1 |
| Homerton University Hospital, London | 11 November 2011 | 8 October 2012 | 25 March 2014 | 2 |
| Huddersfield Royal Infirmary | 27 April 2012 | – | – | 0 |
| Hull Royal Infirmary | 9 December 2011 | 16 May 2012 | 16 May 2012 | 1 |
| John Radcliffe Hospital, Oxford | 6 February 2012 | 11 April 2012 | 30 October 2013 | 4 |
| Kettering General Hospital | 7 October 2011 | 14 August 2012 | 8 September 2014 | 4 |
| King’s Mill Hospital, Mansfield | 11 November 2011 | 31 May 2012 | 31 May 2012 | 1 |
| Kingston Hospital | 5 September 2014 | – | – | 0 |
| Leeds General Infirmary | 25 January 2012 | 7 November 2012 | 2 September 2013 | 3 |
| Leicester Royal Infirmary | 1 July 2011 | 23 August 2011 | 29 July 2014 | 16 |
| Leighton Hospital | 16 December 2011 | – | – | 0 |
| Lincoln County Hospital | 4 July 2012 | 20 November 2012 | 20 November 2012 | 1 |
| Lister Hospital, Stevenage | 2 June 2014 | – | – | 0 |
| Luton & Dunstable Hospital | 26 August 2011 | 14 December 2011 | 25 February 2013 | 2 |
| Macclesfield District General Hospital | 5 October 2011 | – | – | 0 |
| Maidstone Hospital | 10 August 2012 | 11 September 2012 | 15 May 2014 | 3 |
| Medway Maritime Hospital, Gillingham | 2 May 2014 | – | – | 0 |
| Morriston Hospital, Swansea | 14 May 2013 | 15 May 2013 | 15 May 2013 | 1 |
| Musgrove Park Hospital, Taunton | 27 July 2011 | 16 April 2012 | 16 April 2012 | 1 |
| New Cross Hospital, Wolverhampton | 27 April 2012 | 21 May 2012 | 28 June 2012 | 2 |
| Newham University Hospital, London | 28 August 2012 | 10 May 2013 | 12 July 2013 | 3 |
| Norfolk and Norwich University Hospital | 7 July 2011 | 17 December 2012 | 5 June 2013 | 3 |
| North Devon District Hospital | 7 August 2012 | 21 June 2013 | 21 June 2013 | 1 |
| North Manchester General Hospital | 5 March 2012 | 17 September 2012 | 17 September 2012 | 1 |
| Northampton General Hospital | 30 May 2012 | 10 September 2012 | 10 September 2012 | 1 |
| Nottingham Children’s Hospital | 2 August 2011 | 5 October 2011 | 25 June 2014 | 9 |
| Ormskirk & District General Hospital | 14 June 2012 | – | – | 0 |
| Peterborough District Hospital | 8 September 2011 | 26 March 2012 | 26 March 2012 | 1 |
| Pilgrim Hospital, Boston | 12 July 2012 | 3 September 2012 | 1 September 2014 | 3 |
| Pinderfields General Hospital, Wakefield | 18 October 2011 | 26 March 2013 | 26 March 2013 | 1 |
| Poole Hospital | 18 March 2013 | 4 October 2013 | 9 July 2014 | 2 |
| Princess Royal Hospital, Kent | 18 October 2011 | – | – | 0 |
| Queen Alexandra Hospital, Portsmouth | 24 April 2012 | 21 November 2013 | 20 May 2014 | 3 |
| Queen Elizabeth Hospital, King’s Lynn | 11 January 2012 | 27 May 2014 | 27 May 2014 | 1 |
| Queen’s Hospital, Burton | 26 July 2011 | 15 July 2013 | 15 July 2013 | 1 |
| Raigmore Hospital | 12 March 2012 | – | – | 0 |
| Rotherham General Hospital | 4 October 2011 | 9 February 2012 | 9 February 2012 | 1 |
| Royal Aberdeen Children’s Hospital | 8 February 2013 | – | – | 0 |
| Royal Albert Edward Infirmary, Wigan | 6 March 2012 | 25 May 2012 | 25 September 2014 | 2 |
| Royal Alexandra Hospital, Paisley | 29 May 2012 | 26 June 6012 | 26 June 6012 | 1 |
| Royal Belfast Hospital for Sick Children | 12 August 2013 | 16 September 2013 | 23 September 2014 | 4 |
| Royal Berkshire Hospital | 1 December 2011 | 8 August 2013 | 14 April 2013 | 3 |
| Royal Blackburn Hospital | 2 August 2011 | 16 March 2012 | 11 January 2013 | 5 |
| Royal Cornwall Hospital, Treliske | 2 February 2012 | 4 April 2013 | 4 April 2013 | 1 |
| Royal Derby Hospital | 7 November 2011 | 26 February 2014 | 15 July 2014 | 3 |
| Royal Devon & Exeter Hospital, Wonford | 26 July 2011 | 7 May 2014 | 7 May 2014 | 1 |
| Royal Hospital For Sick Children, Edinburgh | 23 May 2012 | 18 February 2013 | 27 March 2013 | 2 |
| Royal Hospital For Sick Children, Glasgow | 15 December 2011 | 28 June 2012 | 30 June 2014 | 7 |
| Royal Lancaster Infirmary | 1 March 2012 | 3 April 2012 | 28 August 2014 | 2 |
| Royal Manchester Children’s Hospital | 28 June 2011 | 23 February 2012 | 8 October 2014 | 19 |
| Royal Oldham Hospital | 8 March 2012 | 12 November 2012 | 25 September 2013 | 2 |
| Royal Preston Hospital | 28 March 2012 | – | – | 0 |
| Royal Shrewsbury Hospital | 18 October 2011 | – | – | 0 |
| Royal United Hospital, Bath | 7 July 2011 | 16 August 2012 | 16 August 2012 | 1 |
| Russells Hall Hospital, Dudley | 5 September 2011 | 3 December 2012 | 16 April 2013 | 2 |
| Scarborough Hospital | 7 February 2013 | 19 March 2013 | 19 March 2013 | 1 |
| Sheffield Children’s Hospital | 8 February 2013 | – | – | 0 |
| Southampton General Hospital | 24 February 2012 | 10 August 2012 | 10 August 2012 | 1 |
| St Peter’s Hospital, Chertsey | 2 August 2011 | 12 August 2011 | 28 March 2012 | 4 |
| St Richard’s Hospital, Chichester | 18 July 2012 | – | – | 0 |
| Stafford Hospital | 24 May 2012 | 24 May 2012 | 25 September 2012 | 2 |
| Stepping Hill Hospital, Stockport | 7 October 2011 | – | – | 0 |
| Tameside General Hospital | 19 August 2011 | 12 September 2011 | 7 June 2013 | 3 |
| The Great North Children’s Hospital, Newcastle | 13 October 2011 | 21 December 2011 | 20 February 2013 | 5 |
| The Ipswich Hospital | 13 July 2012 | 19 June 2013 | 19 June 2013 | 1 |
| The Royal Bolton Hospital | 24 August 2011 | 18 November 2011 | 21 January 2014 | 4 |
| The Royal London Hospital | 6 January 2012 | 2 February 2012 | 30 July 2012 | 2 |
| Tunbridge Wells Hospital | 26 September 2012 | 22 November 2012 | 23 September 2013 | 2 |
| University Hospital Coventry, Walsgrave | 26 October 2011 | 14 March 2012 | 6 June 2012 | 2 |
| University Hospital of North Durham | 27 March 2013 | – | – | 0 |
| University Hospital of North Tees | 6 September 2011 | 2 November 2011 | 23 September 2014 | 2 |
| University Hospital Of Wales, Cardiff | 7 October 2011 | 13 October 2011 | 17 September 2014 | 6 |
| Warrington Hospital | 8 September 2011 | 11 July 2012 | 11 July 2012 | 1 |
| West Middlesex University Hospital | 4 February 2014 | – | – | 0 |
| West Suffolk Hospital | 3 August 2011 | 13 March 2014 | 13 March 2014 | 1 |
| Wexham Park Hospital | 27 February 2013 | – | – | 0 |
| Whipps Cross Hospital, London | 30 April 2012 | – | – | 0 |
| Whiston Hospital, Merseyside | 29 May 2012 | – | – | 0 |
| Wishaw General Hospital | 29 September 2014 | – | – | 0 |
| Worcestershire Royal Hospital | 13 October 2011 | 10 February 2012 | 14 March 2014 | 2 |
| Worthing Hospital | 10 February 2012 | – | – | 0 |
| Wythenshawe Hospital | 8 July 2011 | 5 December 2011 | 5 December 2011 | 1 |
| Yeovil District Hospital | 30 September 2011 | 17 October 2011 | 30 April 2014 | 2 |
| York Hospital | 6 September 2011 | – | – | 0 |
| Ysbyty Gwynedd, Bangor | 4 May 2012 | 26 November 2012 | 7 December 2012 | 2 |
| Total | 124 sites | 86 sites | 237 patients | |
Appendix 2 Achenbach Child Behaviour Checklist
| Score | Group | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| SC | EC | |||
| t-score | ||||
| 4 weeks | ||||
| n | 104 | 109 | ||
| Mean (SD) | 53.28 (12.37) | 52.36 (12.36) | –0.92 (–4.26 to 2.42) | 0.6 |
| 16 weeks | ||||
| n | 97 | 104 | ||
| Mean (SD) | 51.47 (13.10) | 50.21 (13.91) | –1.26 (–5.03 to 2.50) | 0.5 |
| 12 months | ||||
| n | 98 | 102 | ||
| Mean (SD) | 50.84 (15.52) | 49.31 (13.31) | –1.52 (–5.55 to 2.50) | 0.5 |
| 24 months | ||||
| n | 92 | 94 | ||
| Mean (SD) | 48.95 (13.81) | 49.36 (13.54) | 0.42 (–3.54 to 4.37) | 0.8 |
| 36 months | ||||
| n | 61 | 61 | ||
| Mean (SD) | 49.07 (13.50) | 48.87 (13.74) | –0.20 (–5.08 to 4.69) | 0.9 |
| 48 months | ||||
| n | 32 | 28 | ||
| Mean (SD) | 50.13 (15.18) | 46.61 (13.06) | –3.52 (–10.89 to 3.86) | 0.3 |
| Total | ||||
| 4 weeks | ||||
| n | 105 | 111 | ||
| Mean (SD) | 37.28 (26.83) | 35.32 (25.86) | –1.96 (–9.03 to 5.11) | 0.6 |
| 16 weeks | ||||
| n | 97 | 105 | ||
| Mean (SD) | 34.30 (27.37) | 31.20 (27.10) | –3.10 (–10.66 to 4.46) | 0.4 |
| 12 months | ||||
| n | 98 | 102 | ||
| Mean (SD) | 34.34 (31.67) | 29.22 (24.88) | –5.12 (–13.09 to 2.85) | 0.2 |
| 24 months | ||||
| n | 93 | 95 | ||
| Mean (SD) | 28.92 (26.37) | 28.08 (25.15) | –0.84 (–8.25 to 6.57) | 0.8 |
| 36 months | ||||
| n | 61 | 61 | ||
| Mean (SD) | 27.11 (25.11) | 26.90 (26.15) | –0.21 (–9.40 to 8.98) | 1.0 |
| 48 months | ||||
| n | 32 | 28 | ||
| Mean (SD) | 29.56 (27.77) | 21.25 (21.06) | –8.31 (–21.20 to 4.57) | 0.2 |
| Score | Group | RRa (95% CI) | p-value | |
|---|---|---|---|---|
| SC | EC | |||
| Normal/abnormal scores | ||||
| 4 weeks | ||||
| n | 104 | 109 | ||
| Normal score (%) | 72 (69) | 79 (72) | ||
| 16 weeks | ||||
| n | 97 | 104 | ||
| Normal score (%) | 71 (73) | 77 (74) | 0.98 (0.86 to 1.10) | 0.9 |
| 12 months | ||||
| n | 98 | 102 | ||
| Normal score (%) | 69 (70) | 78 (76) | 0.97 (0.84 to 1.12) | 0.3 |
| 24 months | ||||
| n | 92 | 94 | ||
| Normal score (%) | 72 (78) | 70 (74) | 1.06 (0.92 to 1.23) | 0.8 |
| 36 months | ||||
| n | 61 | 61 | ||
| Normal score (%) | 47 (77) | 47 (77) | 0.98 (0.83 to 1.14) | 1.0 |
| 48 months | ||||
| n | 32 | 28 | ||
| Normal score (%) | 22 (69) | 22 (79) | 0.99 (0.73 to 1.35) | 0.4 |
Appendix 3 Economic analysis: the mapping exercise
| Characteristics | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline (N = 55) | 16 weeks (N = 47) | 12 months (N = 58) | 24 months (N = 54) | 36 months (N = 39) | 48 months (N = 26) | |
| Worried | ||||||
| Doesn’t feel worried today | 41 (74.55) | 42 (89.36) | 47 (81.03) | 48 (88.89) | 32 (82.05) | 21 (80.77) |
| Feels a little bit worried today | 10 (18.18) | 5 (10.64) | 7 (12.07) | 5 (9.26) | 5 (12.82) | 5 (19.23) |
| Feels a bit worried today | 3 (5.45) | 0 (0.00) | 3 (5.17) | 1 (1.85) | 1 (2.56) | 0 (0.00) |
| Feels quite worried today | 1 (1.82) | 0 (0.00) | 1 (1.72) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Feels very worried today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.56) | 0 (0.00) |
| Sad | ||||||
| Doesn’t feel sad today | 50 (90.91) | 42 (89.36) | 49 (84.48) | 49 (90.74) | 37 (94.87) | 25 (96.15) |
| Feels a little bit sad today | 3 (5.45) | 1 (2.13) | 6 (10.34) | 4 (7.41) | 1 (2.56) | 1 (3.85) |
| Feels a bit sad today | 1 (1.82) | 2 (4.26) | 3 (5.17) | 1 (1.85) | 0 (0.00) | 0 (0.00) |
| Feels quite sad today | 1 (1.82) | 2 (4.26) | 0 (0.00) | 0 (0.00) | 1 (2.56) | 0 (0.00) |
| Feels very sad today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Pain | ||||||
| Doesn’t have any pain today | 48 (87.27) | 41 (87.23) | 54 (93.1) | 48 (88.89) | 33 (84.62) | 20 (76.92) |
| Has a little bit of pain today | 6 (10.91) | 3 (6.38) | 3 (5.17) | 4 (7.41) | 6 (15.38) | 5 (19.23) |
| Has a bit of pain today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (3.7) | 0 (0.00) | 1 (3.85) |
| Has quite a lot of pain today | 1 (1.82) | 3 (6.38) | 1 (1.72) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Has a lot of pain today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | ||||||
| Doesn’t feel tired today | 33 (60.00) | 27 (57.45) | 33 (56.90) | 38 (70.37) | 22 (56.41) | 16 (61.54) |
| Feels a little bit tired today | 15 (27.27) | 13 (27.66) | 18 (31.03) | 11 (20.37) | 12 (30.77) | 7 (26.92) |
| Feels a bit tired today | 3 (5.45) | 5 (10.64) | 6 (10.34) | 3 (5.56) | 3 (7.69) | 3 (11.54) |
| Feels quite tired today | 2 (3.64) | 1 (2.13) | 1 (1.72) | 2 (3.7) | 2 (5.13) | 0 (0.00) |
| Feels very tired today | 2 (3.64) | 1 (2.13) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Annoyed | ||||||
| Doesn’t feel annoyed today | 44 (80) | 37 (78.72) | 52 (89.66) | 48 (88.89) | 36 (92.31) | 21 (80.77) |
| Feels a little bit annoyed today | 4 (7.27) | 6 (12.77) | 5 (8.62) | 5 (9.26) | 3 (7.69) | 3 (11.54) |
| Feels a bit annoyed today | 5 (9.09) | 1 (2.13) | 1 (1.72) | 1 (1.85) | 0 (0.00) | 2 (7.69) |
| Feels quite annoyed today | 0 (0.00) | 1 (2.13) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Feels very annoyed today | 2 (3.64) | 2 (4.26) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| School | ||||||
| Has no problems with schoolwork/homework today | 52 (94.55) | 41 (87.23) | 52 (89.66) | 45 (83.33) | 29 (74.36) | 23 (88.46) |
| Has a few problems with schoolwork/homework today | 1 (1.82) | 6 (12.77) | 3 (5.17) | 7 (12.96) | 6 (15.38) | 1 (3.85) |
| Has some problems with schoolwork/homework today | 1 (1.82) | 0 (0.00) | 2 (3.45) | 1 (1.85) | 3 (7.69) | 1 (3.85) |
| Has many problems with schoolwork/homework today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.85) | 1 (2.56) | 1 (3.85) |
| Cannot do schoolwork/homework today | 1 (1.82) | 0 (0.00) | 1 (1.72) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sleep | ||||||
| Had no problems sleeping last night | 43 (78.18) | 38 (80.85) | 40 (68.97) | 44 (81.48) | 25 (64.1) | 17 (65.38) |
| Had a few problems sleeping last night | 9 (16.36) | 5 (10.64) | 11 (18.97) | 5 (9.26) | 7 (17.95) | 4 (15.38) |
| Had some problems sleeping last night | 2 (3.64) | 2 (4.26) | 5 (8.62) | 3 (5.56) | 4 (10.26) | 0 (0.00) |
| Had many problems sleeping last night | 1 (1.82) | 2 (4.26) | 2 (3.45) | 2 (3.7) | 3 (7.69) | 5 (19.23) |
| Could not sleep at all last night | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Routine | ||||||
| Has no problems with daily routine today | 47 (85.45) | 38 (80.85) | 53 (91.38) | 49 (90.74) | 29 (74.36) | 23 (88.46) |
| Has a few problems with daily routine today | 8 (14.55) | 4 (8.51) | 4 (6.90) | 3 (5.56) | 6 (15.38) | 3 (11.54) |
| Has some problems with daily routine today | 0 (0.00) | 5 (10.64) | 1 (1.72) | 2 (3.7) | 2 (5.13) | 0 (0.00) |
| Has many problems with daily routine today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (5.13) | 0 (0.00) |
| Cannot do daily routine today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activity | ||||||
| Can join in with any activities today | 47 (85.45) | 41 (87.23) | 54 (93.1) | 48 (88.89) | 33 (84.62) | 25 (96.15) |
| Can join in with most activities today | 6 (10.91) | 2 (4.26) | 3 (5.17) | 4 (7.41) | 2 (5.13) | 1 (3.85) |
| Can join in with some activities today | 0 (0.00) | 2 (4.26) | 0 (0.00) | 1 (1.85) | 1 (2.56) | 0 (0.00) |
| Can join in with a few activities today | 2 (3.64) | 2 (4.26) | 0 (0.00) | 1 (1.85) | 2 (5.13) | 0 (0.00) |
| Can join in with no activities today | 0 (0.00) | 0 (0.00) | 1 (1.72) | 0 (0.00) | 1 (2.56) | 0 (0.00) |
| Characteristics | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline (N = 36) | 16 weeks (N = 46) | 12 months (N = 50) | 24 months (N = 70) | 36 months (N = 56) | 48 months (N = 26) | |
| Worried | ||||||
| Doesn’t feel worried today | 28 (77.78) | 34 (73.91) | 42 (84) | 59 (84.29) | 48 (85.71) | 24 (92.31) |
| Feels a little bit worried today | 7 (19.44) | 11 (23.91) | 5 (10) | 6 (8.57) | 5 (8.93) | 2 (7.69) |
| Feels a bit worried today | 0 (0.00) | 1 (2.17) | 2 (4) | 3 (4.29) | 1 (1.79) | 0 (0.00) |
| Feels quite worried today | 1 (2.78) | 0 (0.00) | 1 (2) | 2 (2.86) | 1 (1.79) | 0 (0.00) |
| Feels very worried today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.79) | 0 (0.00) |
| Sad | ||||||
| Doesn’t feel sad today | 32 (88.89) | 41 (89.13) | 48 (96.00) | 64 (91.43) | 52 (92.86) | 25 (96.15) |
| Feels a little bit sad today | 2 (5.56) | 4 (8.70) | 2 (4.00) | 3 (4.29) | 4 (7.14) | 1 (3.85) |
| Feels a bit sad today | 1 (2.78) | 1 (2.17) | 0 (0.00) | 3 (4.29) | 0 (0.00) | 0 (0.00) |
| Feels quite sad today | 1 (2.78) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Feels very sad today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Pain | ||||||
| Doesn’t have any pain today | 29 (80.56) | 39 (84.78) | 45 (90) | 61 (87.14) | 51 (91.07) | 21 (80.77) |
| Has a little bit of pain today | 5 (13.89) | 6 (13.04) | 4 (8) | 6 (8.57) | 5 (8.93) | 2 (7.69) |
| Has a bit of pain today | 1 (2.78) | 1 (2.17) | 1 (2) | 3 (4.29) | 0 (0.00) | 3 (11.54) |
| Has quite a lot of pain today | 1 (2.78) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Has a lot of pain today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tired | ||||||
| Doesn’t feel tired today | 17 (47.22) | 32 (69.57) | 29 (58) | 43 (61.43) | 37 (66.07) | 17 (65.38) |
| Feels a little bit tired today | 9 (25) | 10 (21.74) | 16 (32) | 21 (30) | 14 (25) | 7 (26.92) |
| Feels a bit tired today | 4 (11.11) | 2 (4.35) | 3 (6) | 3 (4.29) | 4 (7.14) | 1 (3.85) |
| Feels quite tired today | 6 (16.67) | 2 (4.35) | 2 (4) | 2 (2.86) | 1 (1.79) | 1 (3.85) |
| Feels very tired today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.43) | 0 (0.00) | 0 (0.00) |
| Annoyed | ||||||
| Doesn’t feel annoyed today | 29 (80.56) | 37 (80.43) | 45 (90) | 60 (85.71) | 54 (96.43) | 24 (92.31) |
| Feels a little bit annoyed today | 3 (8.33) | 6 (13.04) | 4 (8.00) | 7 (10.00) | 1 (1.79) | 2 (7.69) |
| Feels a bit annoyed today | 2 (5.56) | 1 (2.17) | 1 (2.00) | 2 (2.86) | 1 (1.79) | 0 (0.00) |
| Feels quite annoyed today | 0 (0.00) | 2 (4.35) | 0 (0.00) | 1 (1.43) | 0 (0.00) | 0 (0.00) |
| Feels very annoyed today | 2 (5.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| School | ||||||
| Has no problems with schoolwork/homework today | 34 (94.44) | 43 (93.48) | 41 (82.00) | 59 (84.29) | 48 (85.71) | 21 (80.77) |
| Has a few problems with schoolwork/homework today | 1 (2.78) | 1 (2.17) | 5 (10.00) | 8 (11.43) | 4 (7.14) | 4 (15.38) |
| Has some problems with schoolwork/homework today | 1 (2.78) | 2 (4.35) | 4 (8.00) | 1 (1.43) | 2 (3.57) | 0 (0.00) |
| Has many problems with schoolwork/homework today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (2.86) | 2 (3.57) | 1 (3.85) |
| Cannot do schoolwork/homework today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Sleep | ||||||
| Had no problems sleeping last night | 30 (83.33) | 37 (80.43) | 40 (80.00) | 54 (77.14) | 49 (87.5) | 21 (80.77) |
| Had a few problems sleeping last night | 4 (11.11) | 6 (13.04) | 6 (12.00) | 6 (8.57) | 4 (7.14) | 2 (7.69) |
| Had some problems sleeping last night | 0 (0.00) | 2 (4.35) | 2 (4.00) | 5 (7.14) | 1 (1.79) | 2 (7.69) |
| Had many problems sleeping last night | 2 (5.56) | 1 (2.17) | 1 (2.00) | 5 (7.14) | 2 (3.57) | 1 (3.85) |
| Could not sleep at all last night | 0 (0.00) | 0 (0.00) | 1 (2.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Routine | ||||||
| Has no problems with daily routine today | 31 (86.11) | 39 (84.78) | 44 (88.00) | 62 (88.57) | 48 (85.71) | 24 (92.31) |
| Has a few problems with daily routine today | 3 (8.33) | 4 (8.70) | 4 (8.00) | 7 (10.00) | 6 (10.71) | 0 (0.00) |
| Has some problems with daily routine today | 2 (5.56) | 2 (4.35) | 2 (4.00) | 1 (1.43) | 1 (1.79) | 1 (3.85) |
| Has many problems with daily routine today | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.79) | 1 (3.85) |
| Cannot do daily routine today | 0 (0.00) | 1 (2.17) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Activity | ||||||
| Can join in with any activities today | 27 (75.00) | 42 (91.3) | 43 (86) | 56 (80) | 49 (87.50) | 22 (84.62) |
| Can join in with most activities today | 5 (13.88) | 1 (2.17) | 3 (6) | 9 (12.86) | 4 (7.14) | 2 (7.69) |
| Can join in with some activities today | 2 (5.56) | 2 (4.35) | 2 (4) | 4 (5.71) | 3 (5.36) | 0 (0.00) |
| Can join in with a few activities today | 1 (2.78) | 1 (2.17) | 1 (2) | 0 (0.00) | 0 (0.00) | 2 (7.69) |
| Can join in with no activities today | 1 (2.78) | 0 (0.00) | 1 (2) | 1 (1.43) | 0 (0.00) | 0 (0.00) |
| Models | Sample | Average MAE across samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimation | Validation | ||||||||||||
| Mean (SD) | Minimum value | Maximum value | MSE | MAE | AIC | BIC | Mean (SD) | Minimum value | Maximum value | MSE | MAE | ||
| Observed | 0.93742 (0.07898) | 0.50940 | 1.00000 | – | – | – | – | 0.94094 (0.07174) | 0.65000 | 1.00000 | – | – | – |
| GLM_1 | 0.93742 (0.04433) | 0.74143 | 0.97345 | 0.00466 | 0.04789 | 98.12 | 105.38 | 0.93462 (0.05026) | 0.66582 | 0.97345 | 0.00366 | 0.04525 | 0.04657 |
| GLM_2 | 0.93742 (0.04535) | 0.72021 | 0.98126 | 0.00446 | 0.04704 | 101.78 | 116.30 | 0.93409 (0.05250) | 0.66769 | 0.98006 | 0.00372 | 0.04579 | 0.04642 |
| GLM_3 | 0.93742 (0.04978) | 0.72956 | 0.98071 | 0.00403 | 0.04313 | 101.91 | 120.06 | 0.93936 (0.05009) | 0.64983 | 0.97949 | 0.00326 | 0.04046 | 0.04180 |
| GLM_4 | 0.93742 (0.05019) | 0.73330 | 0.98309 | 0.00393 | 0.04254 | 105.77 | 131.19 | 0.93907 (0.05061) | 0.65902 | 0.98256 | 0.00324 | 0.04060 | 0.04157 |
| GLM_5 | 0.93742 (0.05176) | 0.65715 | 0.98975 | 0.00356 | 0.04109 | 112.75 | 152.70 | 0.93756 (0.05431) | 0.71233 | 0.98512 | 0.00344 | 0.04172 | 0.04141 |
| GLM_6 | 0.93742 (0.05193) | 0.66093 | 0.98935 | 0.00353 | 0.04078 | 116.70 | 163.90 | 0.93761 (0.05476) | 0.70516 | 0.98550 | 0.00345 | 0.04182 | 0.04130 |
| OLS_1 | 0.93742 (0.04266) | 0.81166 | 0.98597 | 0.00440 | 0.04595 | –718.03 | –710.77 | 0.93586 (0.04530) | 0.78676 | 0.98597 | 0.00348 | 0.04429 | 0.04512 |
| OLS_2 | 0.93742 (0.04338) | 0.80481 | 1.00366 | 0.00434 | 0.04575 | –717.97 | –703.44 | 0.93579 (0.04651) | 0.78818 | 1.00054 | 0.00348 | 0.04460 | 0.04518 |
| OLS_3 | 0.93742 (0.04732) | 0.81207 | 0.99522 | 0.00398 | 0.04245 | –739.82 | –721.67 | 0.93902 (0.04632) | 0.78872 | 0.99389 | 0.00310 | 0.03981 | 0.04113 |
| OLS_4 | 0.93742 (0.04762) | 0.81562 | 1.00483 | 0.00396 | 0.04236 | –737.82 | –712.40 | 0.93884 (0.04693) | 0.79050 | 1.00305 | 0.00310 | 0.03989 | 0.04113 |
| OLS_5 | 0.93742 (0.04924) | 0.76241 | 1.01474 | 0.00380 | 0.04218 | –741.10 | –701.16 | 0.93777 (0.05063) | 0.77988 | 1.01377 | 0.00327 | 0.04050 | 0.04134 |
| OLS_6 | 0.93742 (0.04935) | 0.76394 | 1.01301 | 0.00379 | 0.04219 | –737.88 | –690.67 | 0.93778 (0.05071) | 0.77576 | 1.01234 | 0.00326 | 0.04052 | 0.04136 |
| Tobit_1 | 0.96369 (0.05818) | 0.79220 | 1.02990 | 0.00533 | 0.05285 | –185.28 | –174.39 | 0.96156 (0.06177) | 0.75824 | 1.02990 | 0.00428 | 0.05003 | 0.05144 |
| Tobit_2 | 0.96348 (0.05855) | 0.78748 | 1.04827 | 0.00526 | 0.05242 | –183.12 | –164.97 | 0.96136 (0.06271) | 0.75878 | 1.04466 | 0.00431 | 0.05063 | 0.05153 |
| Tobit_3 | 0.96319 (0.06452) | 0.79299 | 1.04910 | 0.00496 | 0.05195 | –205.65 | –183.86 | 0.96575 (0.06269) | 0.76089 | 1.04170 | 0.00405 | 0.04816 | 0.05006 |
| Tobit_4 | 0.96307 (0.06456) | 0.79396 | 1.05047 | 0.00492 | 0.05159 | –202.25 | –173.20 | 0.96549 (0.06296) | 0.76257 | 1.04897 | 0.00403 | 0.04806 | 0.04983 |
| Tobit_5 | 0.96304 (0.06735) | 0.74322 | 1.07926 | 0.00482 | 0.05284 | –205.81 | –162.23 | 0.96387 (0.06842) | 0.76384 | 1.04872 | 0.00434 | 0.05107 | 0.05196 |
| Tobit_6 | 0.96300 (0.06734) | 0.74480 | 1.07843 | 0.00481 | 0.05270 | –201.93 | –151.09 | 0.96385 (0.06838) | 0.76169 | 1.05209 | 0.00433 | 0.05099 | 0.05185 |
FIGURE 17.
Distribution in the estimation and validation samples. Observed CHU-9D score, (a) estimation group and (b) validation group; GLM_6-predicted CHU-9D score, (c) estimation group and (d) validation group; and OLS_3-predicted CHU-9D score, (e) estimation group and (f) validation group.
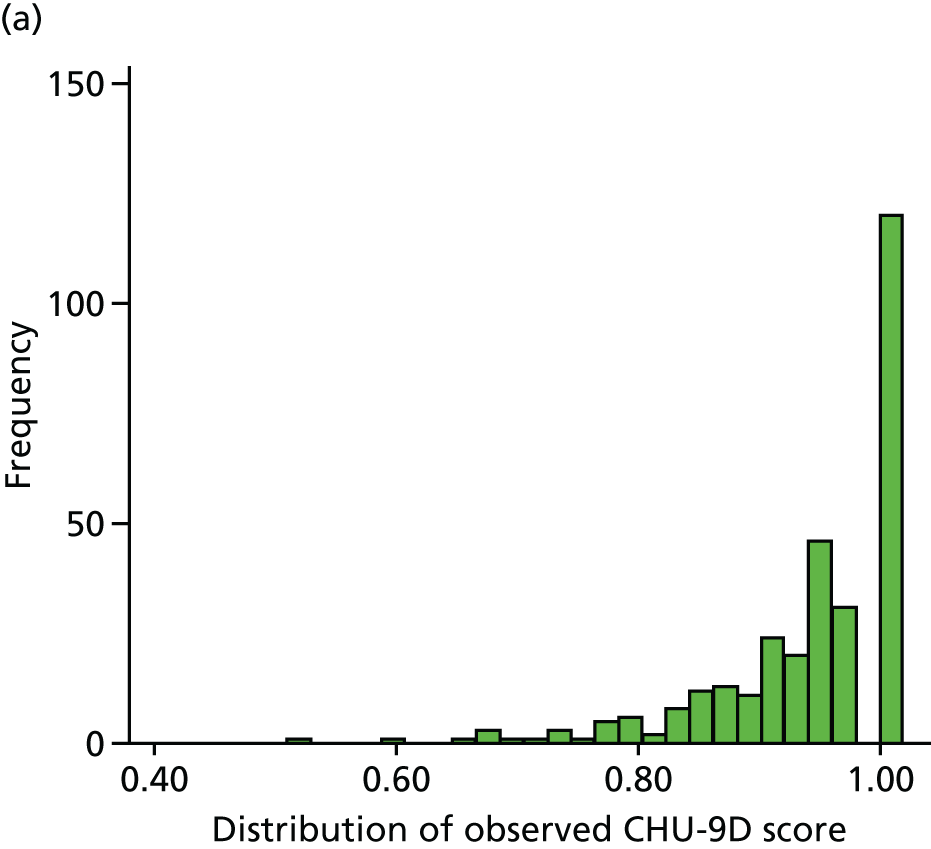
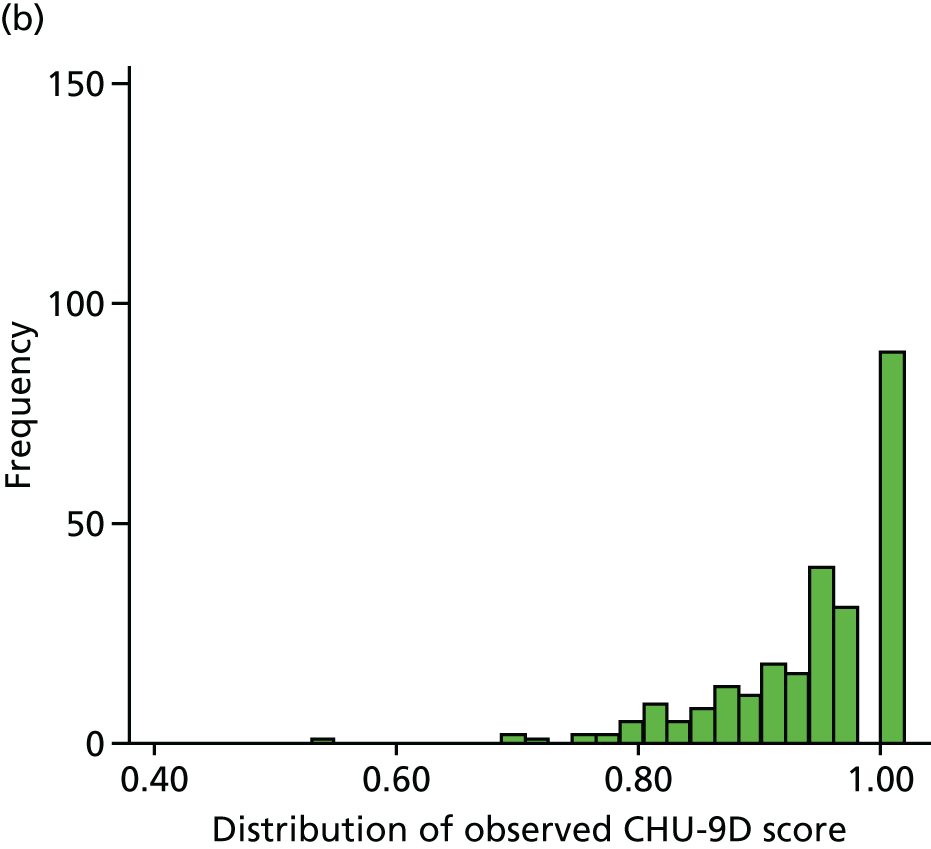

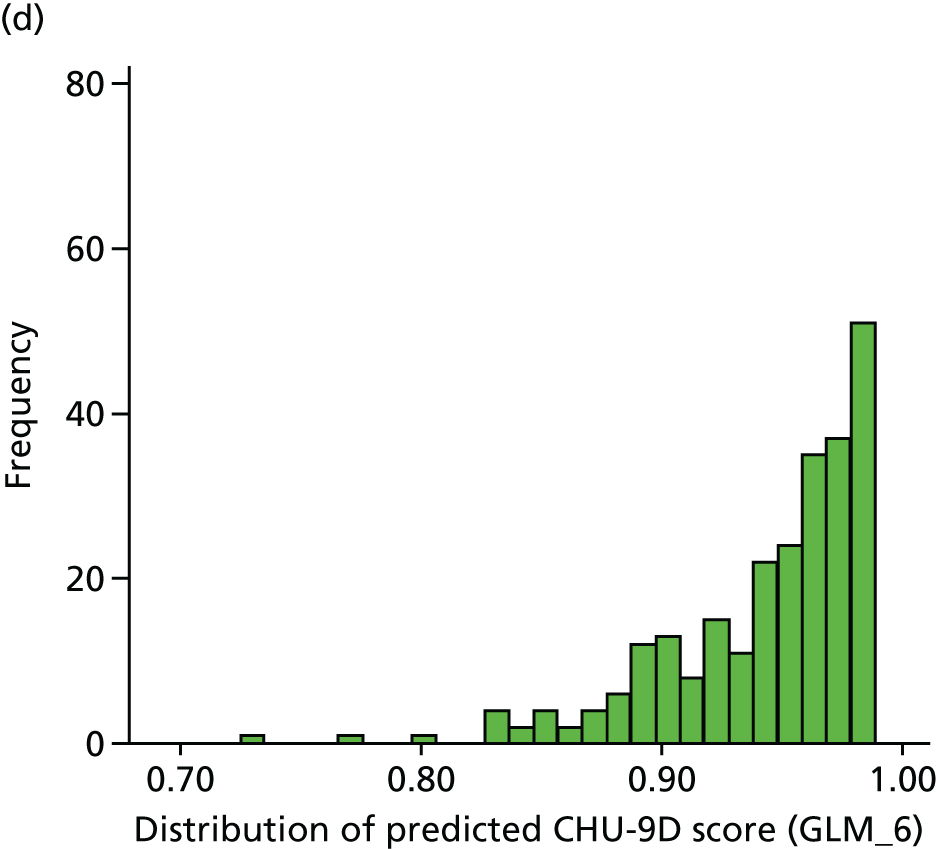

Appendix 4 Economic evaluation
| Time point | Group | Difference (bootstrapped 95% CI) | |||
|---|---|---|---|---|---|
| SC | EC | ||||
| n | Bed-days | n | Bed-days | ||
| Week 4 | 109 | 0.009 (0.096) | 113 | 0.027 (0.21) | 0.017 (–0.025 to 0.060) |
| Week 8 | 108 | 0.046 (0.317) | 106 | 0.34 (1.995) | 0.293 (–0.083 to 0.669) |
| Week 12 | 104 | 0.769 (4.282) | 109 | 0.018 (0.135) | –0.751 (–1.583 to 0.082) |
| Week 16 | 104 | 0.163 (0.698) | 107 | 0.112 (0.828) | –0.051 (–0.253 to 0.150) |
| Month 5 | 97 | 0.67 (2.688) | 109 | 0.312 (1.338) | –0.358 (–0.946 to 0.230) |
| Month 6 | 102 | 0.225 (1.052) | 109 | 0.56 (2.132) | 0.334 (–0.12 to 0.788) |
| Month 8 | 100 | 0.29 (1.233) | 109 | 0.165 (0.764) | –0.125 (–0.400 to 0.150) |
| Month 10 | 100 | 2.41 (21.408) | 107 | 0.271 (1.154) | –2.139 (–6.253 to 1.975) |
| Month 12 | 101 | 0.109 (0.527) | 106 | 0.33 (1.666) | 0.221 (–0.098 to 0.541) |
| Month 18 | 99 | 1.01 (3.663) | 105 | 0.352 (1.467) | –0.658 (–1.452 to 0.137) |
| Month 24 | 100 | 0.64 (3.463) | 103 | 0.233 (1.031) | –0.407 (–1.108 to 0.294) |
| Time point | Group | Difference (bootstrapped 95% CI) | |||
|---|---|---|---|---|---|
| SC | EC | ||||
| n | Episodes | n | Episodes | ||
| Week 4 | 109 | 0.009 (0.096) | 113 | 0.018 (0.132) | 0.009 (–0.021 to 0.038) |
| Week 8 | 108 | 0.046 (0.317) | 106 | 0.047 (0.213) | 0.001 (–0.074 to 0.075) |
| Week 12 | 104 | 0.096 (0.327) | 109 | 0.018 (0.135) | –0.078 (–0.144 to –0.012) |
| Week 16 | 104 | 0.067 (0.252) | 107 | 0.028 (0.166) | –0.039 (–0.096 to 0.017) |
| Month 5 | 97 | 0.093 (0.325) | 109 | 0.083 (0.277) | –0.01 (–0.091 to 0.071) |
| Month 6 | 102 | 0.078 (0.305) | 109 | 0.128 (0.363) | 0.05 (–0.042 to 0.142) |
| Month 8 | 100 | 0.08 (0.273) | 109 | 0.128 (0.579) | 0.048 (–0.077 to 0.174) |
| Month 10 | 100 | 0.11 (0.399) | 107 | 0.121 (0.428) | 0.011 (–0.103 to 0.126) |
| Month 12 | 101 | 0.069 (0.292) | 106 | 0.075 (0.299) | 0.006 (–0.07 to 0.083) |
| Month 18 | 99 | 0.202 (0.534) | 105 | 0.124 (0.409) | –0.078 (–0.212 to 0.056) |
| Month 24 | 100 | 0.2 (0.696) | 103 | 0.117 (0.449) | –0.083 (–0.243 to 0.076) |
| Time point | Group, mean (SD) | Bootstrapped cost difference (95% CI) | |||
|---|---|---|---|---|---|
| SC | EC | ||||
| Visits | Cost (£) | Visits | Cost (£) | ||
| Week 4 | 0 (0) | 0 (0) | 0.009 (0.094) | 11.69 (124.269) | 11.69 (–11.34 to 34.72) |
| Week 8 | 0.037 (0.234) | 48.926 (308.928) | 0.009 (0.097) | 12.462 (128.307) | –36.46 (–99.35 to 26.43) |
| Week 12 | 0.115 (0.425) | 152.423 (561.591) | 0.009 (0.096) | 12.119 (126.529) | –140.3 (–250.88 to –29.73) |
| Week 16 | 0.058 (0.234) | 76.212 (309.497) | 0.009 (0.097) | 12.346 (127.706) | –63.87 (–128.76 to 1.03) |
| Month 5 | 0.01 (0.102) | 13.619 (134.127) | 0.073 (0.262) | 96.954 (346.085) | 83.34 (11.05 to 155.62) |
| Month 6 | 0.029 (0.221) | 38.853 (291.314) | 0.037 (0.302) | 48.477 (399.006) | 9.62 (–85.76 to 105.01) |
| Month 8 | 0.08 (0.339) | 105.68 (447.481) | 0.028 (0.164) | 36.358 (217.116) | –69.32 (–162.81 to 24.17) |
| Month 10 | 0.08 (0.339) | 105.68 (447.481) | 0.028 (0.166) | 37.037 (219.096) | –68.64 (–168.73 to 31.44) |
| Month 12 | 0.04 (0.196) | 52.317 (258.915) | 0.019 (0.137) | 24.925 (180.587) | –27.39 (–90.65 to 35.87) |
| Month 18 | 0.091 (0.353) | 120.091 (465.742) | 0.086 (0.462) | 113.229 (610.72) | –6.86 (–152.1 to 138.37) |
| Month 24 | 0.11 (0.567) | 145.31 (748.567) | 0.078 (0.362) | 102.602 (478.419) | –42.71 (–213.66 to 128.24) |
| Time point | Group, mean (SD) | Bootstrapped cost difference (95% CI) | |||
|---|---|---|---|---|---|
| SC | EC | ||||
| Visits | Cost (£) | Visits | Cost (£) | ||
| Week 4 | 0.165 (0.481) | 36.33 (105.906) | 0.239 (0.555) | 52.566 (122.188) | 16.24 (–12.73 to 45.2) |
| Week 8 | 0.204 (0.448) | 44.815 (98.659) | 0.094 (0.325) | 20.755 (71.39) | –24.06 (–47.25 to –0.87) |
| Week 12 | 0.24 (0.566) | 52.885 (124.508) | 0.119 (0.424) | 26.239 (93.361) | –26.65 (–56.68 to 3.39) |
| Week 16 | 0.433 (1.086) | 95.192 (238.97) | 0.112 (0.372) | 24.673 (81.8) | –70.52 (–119.18 to –21.86) |
| Month 5 | 0.546 (1.267) | 120.206 (278.68) | 0.385 (0.815) | 84.771 (179.4) | –35.44 (–100.04 to 29.17) |
| Month 6 | 0.314 (0.731) | 69.02 (160.806) | 0.294 (0.724) | 64.587 (159.272) | –4.43 (–49.65 to 40.79) |
| Month 8 | 0.84 (1.461) | 184.8 (321.513) | 0.56 (0.966) | 123.119 (212.606) | –61.68 (–138.9 to 15.54) |
| Month 10 | 0.56 (1.175) | 123.2 (258.46) | 0.673 (1.472) | 148.037 (323.75) | 24.84 (–53.66 to 103.34) |
| Month 12 | 0.455 (0.985) | 100.198 (216.73) | 0.519 (1.08) | 114.151 (237.591) | 13.95 (–49 to 76.9) |
| Month 18 | 1.566 (2.639) | 344.444 (580.503) | 1.324 (1.842) | 291.238 (405.31) | –53.21 (–191.51 to 85.1) |
| Month 24 | 1.71 (7.55) | 376.2 (1660.903) | 1.155 (1.696) | 254.175 (373.196) | –122.03 (–448.77 to 204.72) |
List of abbreviations
- ACBC
- Achenbach Child Behaviour Checklist
- AE
- adverse event
- APN
- Arbeitsgemainschaft für Pädiatrische Nephrologie
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CHU-9D
- Child Health Utility 9D
- CI
- confidence interval
- DEXA
- dual-energy X-ray absorptiometry
- EC
- extended course
- FRNS
- frequently relapsing nephrotic syndrome
- GLM
- generalised linear model
- GP
- general practitioner
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- INS
- idiopathic nephrotic syndrome
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ISKDC
- International Study for Kidney Disease in Children
- ITT
- intention to treat
- MAE
- mean absolute error
- MCD
- minimal change disease
- MCRN
- Medicines for Children Research Network
- MSE
- mean squared error
- NeST
- Nephrotic Syndrome Trust
- NIHR
- National Institute for Health Research
- OLS
- ordinary least squares
- PedsQL
- Pediatric Quality of Life Inventory
- PREDNOS
- PREDnisolone in NephrOtic Syndrome
- QALY
- quality-adjusted life year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SC
- standard course
- SD
- standard deviation
- SDNS
- steroid-dependent nephrotic syndrome
- SDS
- standard deviation score
- SE
- standard error
- SSNS
- steroid-sensitive nephrotic syndrome
