Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/88/13. The contractual start date was in January 2015. The draft report began editorial review in January 2018 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alastair D Hay and William Hollingworth are members of the Health Technology Assessment Clinical Trials Board. Chris Metcalfe is a member of a clinical trials unit in receipt of National Institute for Health Research (NIHR) support funding. Desmond Nunez is an author of a related Cochrane review protocol. Paul Little is the Director of the NIHR Programme Grants for Applied Research programme and a member of the NIHR Journals Library Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Hay et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Structure of this report
In this chapter, the rationale for reducing antibiotic prescriptions by treating ear pain in acute otitis media (AOM) with anaesthetic–analgesic is presented. Chapter 2 describes the study population and gives details of the interventions being compared and the study design. Chapter 3 presents the results of the study, followed by discussion and conclusions in Chapter 4.
Background
There is broad agreement that a high proportion of prescriptions for antibiotics are unjustified1,2 and likely to be harmful given the clear relationship between primary care prescribed antibiotics and bacterial resistance. 3 Furthermore, resistant bacteria are transmitted to social contacts, which is a problem with young children, who are unaware of hygiene conventions and who have high contact rates with other children, parents and grandparents. 4,5
In September 2013, the Department of Health and Social Care published the UK Five Year Antimicrobial Resistance Strategy 2013 to 2018,6 which calls for change in the understanding and response to antimicrobial resistance by the public, the NHS and the government in the UK. Its overarching goal is to slow the spread of antimicrobial resistance through three strategies: (1) improving the knowledge and understanding of antimicrobial resistance, (2) conserving and stewarding the effects of existing antibiotics and (3) stimulating the development of new antibiotics. Conserving and stewarding the effects of antibiotics can be achieved in five ways: (1) reducing the overall quantity of antibiotics prescribed and consumed; (2) when antibiotics are needed, promoting the use of narrow-spectrum agents; (3) when antibiotics are in demand but are ineffective, providing alternatives; (4) reducing the transmission of antibiotic-resistant bacteria; and (5) vaccinating against antibiotic-resistant bacteria.
Acute otitis media is a common, painful condition of childhood that has an impact on the family because of disrupted sleep and time off work and school. Primary care consultation and prescription of an antibiotic have been the mainstay of management; one UK study found that between 80% and 84% of children presenting to primary care with AOM were prescribed an antibiotic during the years 1995–2011. 1,2 This is despite the available evidence of benefit being restricted to children < 2 years old with bilateral AOM and those with otorrhoea,7 leading the National Institute for Health and Care Excellence (NICE) to conclude that these are the only children warranting a same-day full course of antibiotic treatment. 8 For other children, the use of a ‘wait-and-see’ strategy, with or without a delayed prescription for an antibiotic, has been shown to be safe in terms of treatment failure and in the frequency of complications of AOM including mastoiditis. 9,10
Evidence of the effectiveness for alternatives to antibiotics is urgently needed to reduce the largely inappropriate reliance on antibiotics and to relieve the most common and distressing symptoms of AOM. The results of the Children’s Ear Pain Study (CEDAR) are presented in this report, which investigated if anaesthetic ear drops are effective in reducing the consumption of antibiotics by children with AOM.
Literature review
Three previous trials have assessed the effectiveness of topical analgesia against placebo in relieving pain due to AOM. 11–13 All were included in a Cochrane review (updated in 2011) that concluded that ‘the evidence from [these] RCTs is insufficient to know whether ear drops are effective’. 14 In any case, those studies did not assess the impact of topical analgesia on antibiotic prescribing when used as an approach to the management of an episode of AOM, and so it is unknown whether or not parents will see topical analgesia as an effective and preferable alternative to antibiotics and, therefore, reduce unnecessary antibiotic use.
Benzocaine–phenazone otic solution (Auralgan®, currently manufactured by Pfizer Consumer Healthcare) drops have been used in Australia for > 40 years with little or no evidence of harm. Prior to commencing this study, as part of the trial risk assessment and because of the theoretical potential for induced methaemoglobinaemia, we undertook a search of the Australian Therapeutic Goods Administration Database of Adverse Event Notifications (DAEN) (www.tga.gov.au/database-adverse-event-notifications-daen). On 2 December 2015, the DAEN was searched for adverse event reports associated with Auralgan between 1 January 1971 and 19 August 2015. A total of 11 cases were reported, all of which appear to be minor adverse effects (some patients had more than one adverse effect): ear pain (two cases), discomfort (two cases), pruritus (one case), hyperacusis (one case), hearing impaired (one case), eye pain (one case), tinnitus (one case), burning sensation (two cases), ear canal erythema (one case), accidental exposure and overdose by child (one case), deafness (two cases), depressed mood (one case). This suggests a low risk of adverse reactions to Auralgan ear drops. There is a further potential for adverse effects arising from reduced antibiotic prescribing to children with AOM. However, previous observational data suggest that the frequency of such adverse effects is likely to be extremely low. 1 Moreover, in a survey of Australian pharmacies undertaken as part of preparation for the trial, it was established that these drops are widely available over the counter and sometimes used to avoid the need for a GP consultation (Harriet Downing, University of Bristol, 2015; unpublished survey data).
Study aim
The aim of the CEDAR trial is to investigate the clinical effectiveness and cost-effectiveness of benzocaine plus phenazone (active) ear drops compared with usual care (no drops) for reducing antibiotic consumption in children aged between 12 months and 10 years presenting to primary care with AOM.
Specific research questions
Following a presentation to primary care with AOM and compared with usual care:
-
Do active drops lead to a lower proportion of children consuming antibiotics in the first 8 days?
-
Do active drops lead to reduced oral analgesic consumption in the first 8 days?
-
Do active drops provide superior pain relief in the first 24–36 hours?
-
Do active drops provide superior pain relief in the first 24 hours?
-
Do active drops alter the number of days before starting antibiotics in the first 7 days?
-
Do active drops reduce the overall symptom burden, including episodes of crying or distress, disturbed sleep, interference with normal activity, appetite and fever, in the first 7 days?
-
Do active drops alter overall illness duration?
-
What are the net incremental costs to the NHS and society in the short (7 days) and medium (3 months) term?
-
Are the net incremental costs of active ear drops justified by improved pain relief, symptom burden, antibiotic use or quality of life?
Further objectives were to use qualitative methods to investigate parents’ and clinicians’ views and experiences of AOM in children in the CEDAR trial and to investigate the representativeness of the CEDAR trial sample by describing the presentation, management and outcome of children with AOM in primary care.
Chapter 2 Methods
Study design
The CEDAR trial was a multicentre randomised controlled trial (RCT) and the participating children were randomly allocated between three study groups: active treatment (benzocaine-based ear drops), placebo treatment (drops without the ‘active’ ingredient) and no drops (current standard care). Parents who did not want their children to take part in the trial but were willing to complete the study diary were offered enrolment in an observational cohort study (Figure 1).
FIGURE 1.
The CEDAR trial three-group randomised study with observational cohort study. OCS, observational cohort study.

Recruitment sites and site training
General practitioners at primary care research network practices in Bristol, Cardiff and Southampton were invited to take part in the study.
Training was delivered to each clinician who was involved in the trial. The training provided an outline of all trial recruitment and baseline data collection procedures, including how to train parents in administering the ear drops (for parents of children allocated to one of the treatment groups) and completing the Symptom and Recovery Questionnaire. A clinician training log was maintained at sites and within the Trial Master File reflecting the staff who had been trained. Clinicians at all participating primary care sites were also offered ongoing support, recruitment advice and refresher training on request by the study team.
Participants and recruitment
Eligibility
Children were eligible if they met all of the following criteria:
-
Aged ≥ 12 months and < 10 years.
-
Presenting within 1 week of suspected AOM onset (other preceding respiratory tract infection symptoms may be longer).
-
Parent/legal guardian available to give consent.
-
Parent-reported ear pain in 24 hours pre enrolment (or parent-suspected ear pain if child is too young to report pain).
-
Clinician diagnosis of AOM.
-
Child is immunocompetent.
-
Clinician willing to use a NICE-recommended ‘no’ oral antibiotic prescribing strategy or a ‘delayed’ oral antibiotic prescribing strategy (as per NICE guidelines15) for the AOM and other elements of the underlying acute respiratory tract infection. NICE recommends a ‘no’ or ‘delayed’ antibiotic prescribing strategy for most immune-competent children with AOM.
-
Parent able to give ear drops.
-
Parent willing in principle to use ear drops before oral antibiotics and to wait before giving delayed antibiotics as per NICE guidelines.
-
Parent able to report the child’s ear pain.
-
Parent able and willing to complete daily Symptom and Recovery Questionnaire and receive regular follow-up telephone calls in the English language, on the first day of the trial and every 2 or 3 days for up to 7 more days (or until child has been free of ear pain without medicines for 2 days running).
Children were not recruited if they matched any of the following exclusion criteria:
-
Child requires immediate hospitalisation.
-
Child requires same-day oral antibiotic treatment for AOM or other elements of the underlying acute respiratory tract infection (these children assessed for observational study eligibility). NICE recommends same-day antibiotic treatment for:
-
child < 2 years with bilateral AOM
-
otorrhoea (discharge from the ear)
-
child who is systemically very unwell or showing signs of respiratory distress (e.g. tachypnoea, hypoxia or recession)
-
child who has symptoms and signs suggestive of serious illness and/or complications (particularly mastoiditis)
-
child who is at a high risk of serious complications because of pre-existing comorbidity. NICE guidelines recommend that the following children receive same-day antibiotics:
-
– child who has significant heart, lung, renal, liver or neuromuscular disease (defined for the purposes of this study as requiring ongoing inpatient or outpatient care from specialist teams)
-
– child who has immunosuppression (defined for the purposes of this study as a formal diagnosis of immunosuppression)
-
– child who has cystic fibrosis
-
– child who was born prematurely (defined for the purposes of this study as born < 34 weeks and presenting within the first year of life)
-
– note: children with other conditions who are at higher risk of AOM (e.g. Down’s syndrome, cleft palate) may take part if the responsible clinician feels that they meet the inclusion criteria above.
-
-
Child who requires same-day oral antibiotics for another (non-AOM) infection or topical antibiotic ear drops.
-
Child who is currently receiving (or has received in the past 7 days) oral or ear drop (to the AOM ear) antibiotic treatment.
-
Suspected or confirmed perforation (owing to theoretical and unconfirmed risk of ototoxicity from active drops) or grommets still in situ.
-
Known sensitivity to trial medicine (Auralgan) or to its ingredients (benzocaine, phenazone, glycerine, hydroxyquinoline sulphate) or similar substances (e.g. other ester-type anaesthetics, such as procaine, tetracaine).
-
Known porphyria or haemoglobinopathy or glucose-6-phosphate dehydrogenase (G6PD) deficiency or methaemoglobinaemia.
-
Known family history of G6PD deficiency (noting that G6PD deficiency is more common in African, Asian and Mediterranean populations).
-
Current use of sulphonamides, antimalarials, hyaluronidase or St John’s wort.
-
Child who needs to continue taking other medicinal products containing benzocaine.
-
Child who has proven alternative source(s) of pain other than, and more severe than, the ear symptoms with which they are presenting.
-
Otoscopic appearances (as ascertained by clinician, when possible) consistent with observed fever (i.e. likely non-specific viral illness only, for example with just a slightly perfused or pink drum only).
-
Child who has normal ear on examination.
-
Child who has otitis externa, or other disorder of the outer ear or tympanic membrane for which the CEDAR trial ear drops should not be prescribed, in the AOM ear.
-
Child who has a hearing aid and parent feels hearing aid should remain in place in the AOM ear.
-
Symptoms (i.e. hearing loss and longer duration of illness) more suggestive of a diagnosis of otitis media with effusion (glue ear).
-
Child who has previously taken part in the CEDAR trial.
-
Child who has taken part in any research involving medicines within the last 90 days, or any other AOM-related research within the last 30 days.
-
Changes to the eligibility criteria
The team investigated methaemoglobinaemia and its association with benzocaine consumption in children. Although the potential risk was deemed very small, a decision was made to exclude children under the age of 12 months, rather than 6 months as previously planned.
Interventions
The investigational medicinal product (IMP) for this trial was a benzocaine and phenazone otic solution. This is an oil-based, combined local anaesthetic (benzocaine) and analgesic (phenazone) ear drop. One millilitre contains 14 mg (1.4%) of benzocaine and 54 mg (5.4%) of phenazone suspended in a glycerine-based liquid along with a preservative (hydroxyquinolone sulphate). Despite an absence of published evidence of effectiveness, it is available as a pharmacy medicine in Australia and New Zealand, and has been marketed since 1947 under Auralgan and other brand names. For this trial we intended to test Auralgan sold in 15-ml bottles.
The placebo was glycerine, with packaging as close in appearance as possible to that used for the active drops.
Outcomes
Primary outcome
Proportion of children who consumed antibiotics by day 8: parents of the children recruited into the CEDAR trial were asked to complete an 8-day Symptom and Recovery Questionnaire which asked, on each day, whether or not they had given the child antibiotics. Completion of all eight questions was required to create an overall consumed/did not consume binary variable.
Secondary outcomes
Ear pain score on day 2
The 8-day Symptom and Recovery Questionnaire also asked the parent to rate their child’s ear pain on a scale of 0–10 (Figure 2). Although active drops should have an almost immediate effect, the evening of day 2 was chosen as the time at which the drops had enough time to ease ear pain if they were going to. Active and placebo drops were compared to see whether or not the active ingredient provided relief over and above the soothing effects of the oily liquid in which they are contained. Placebo drops were also compared with usual care to test whether or not the oily liquid itself was more effective than nothing at all. The key secondary outcome tested the difference in ear pain scores between active and placebo drops, whereas an additional secondary outcome tested difference in ear pain between placebo and usual-care groups.
FIGURE 2.
Parent-completed assessment of pain.

Ear pain score on day 1
Another objective was to see whether or not the drops had an immediate effect on pain; therefore, parents were asked to score their child’s ear pain 1 hour after giving the first dose of ear drops. Active and placebo drops were compared to see whether or not the active ingredient provided relief over and above the soothing effects of the oily liquid in which they are contained. Pain scores were on a scale of 0–10. This was then compared between active and placebo groups.
Analgesic consumption
On each day of the symptom diary, parents were asked whether or not they had given their child any analgesics (e.g. ibuprofen or paracetamol). The number of doses was also recorded. A binary variable was created to identify children who had received analgesics on any of the first 8 days versus children who had not received any oral analgesics during any of the first 8 days; this measure was not derived for children with any missing oral analgesic data during the first 8 days. This measure was then compared between active and placebo groups.
Number of days before taking antibiotics
The number of days before taking antibiotics was obtained from the child’s symptom questionnaire. This was then compared between active and placebo groups, and between active and usual-care groups.
Mean overall symptom severity on days 1–4
Parents were asked to complete questions concerning other symptoms, such as episodes of crying, disturbed sleep and fever in their symptom diaries. Symptoms were rated on a scale of 0 (no problem) to 6 (extremely bad). All symptom scores were combined for each patient per day before calculating the area under the curve for days 1 to 8. This was then compared between active and placebo groups, and between active and usual-care groups.
Overall illness duration
The number of days until the parent rated score was 0 for 2 consecutive days was calculated for all children. This was then compared between active and placebo groups, and between active and usual-care groups.
Adverse events
Details of serious adverse events were collected, and the trial team notified immediately, using adverse event forms. Parents were also asked on the last day of their questionnaire whether their child had experienced any new or worsening symptoms during the trial.
Patient satisfaction
Parents were asked about their satisfaction with, and opinion of, treatment allocation and future intention to use drops (with/without prior GP consultation if drops were to become available over the counter) 8 days post randomisation.
NHS and societal costs
The net incremental costs to the NHS and society of using active ear drops compared with no drops (usual care) in the short (8 days) and medium term (3 months). NHS resource use (e.g. antibiotic or analgesic use, GP visits) and societal costs (school/nursery absences, parent lost productivity and other family expenses) were reported by parents in the 8-day questionnaire. Medium-term NHS resource use was collated from a review of the child’s GP records.
Child’s quality of life
Preference-based quality of child life was measured (baseline, 24–36 hours, 8 days and 3 months post randomisation) using the CHU-9D. 16 The CHU-9D is not designed for, or validated for, preschool children; therefore, this measure was used only in children aged ≥ 5 years. The OMQ-1417 was given at baseline and 3 months post randomisation for children aged ≥ 2 years (clinical report form and postal/online questionnaire at 3 months).
Sample size
Previous studies have suggested that 80–90% of children presenting to UK GPs with AOM receive a prescription for an antibiotic. 18,19 It has also been argued that a 20% change in consumption could have important effects on antimicrobial resistance. 3 The number of children needed in each group (active ear drops and usual care) to demonstrate a true 20% fall in antibiotic consumption, from 80–90% in the control group to 60–70% in the active ear drop group, with 90% power and two-sided significance level of 0.05, ranged from 92 to 119. Using the more conservative estimate of 119 children, and making provision for 20% attrition, this gave a target sample size of 149 children per group.
Our key secondary outcome was pain on day 2, measured using a validated 0–10 numerical rating scale. Based on the findings of a previous study using this measure,20 and the opinions of our patient and public involvement group, it was felt that a reduction in pain of 1 point was a clinically important difference. With 90% power and α = 0.05, we can detect a true mean difference of 1 point [standard deviation (SD) 2.5 points] in pain score between the active and placebo ear drop groups, detected with 90% of power at the two-sided significance level of 0.05, with 133 children per group. Accommodating 20% attrition, and assuming equal numbers in the three groups, we needed 167 children per group (more than for the primary outcome) and 501 children in total.
Random allocation
Each pack contained two bottles of active medicine, two bottles of placebo medicine or no bottles but a non-medicinal item of similar weight. Randomisation was stratified by centre in blocks of 30 packs, each block having the packs arranged in a random and consecutively numbered sequence. The Bristol Randomised Trials Collaboration provided the pharmaceutical supplier with a set of patient IDs, which were allocated to a pack according to the sequence for the current block for a centre. The supplier provided the pharmacy with medicine packs that had each been prelabelled with the patient ID and medicine pack ID numbers.
Patients were enrolled by their GP or research nurse who, at the stage of enrolment, was unaware of the contents of the next treatment pack in the sequence (allocation concealment). When the informed consent process was completed and signed, the trial pack was opened and allocation to drops (active or placebo) or not was confirmed.
Blinding
Although parents were aware if their child had been allocated to the usual-care group, if they received drops they were not aware if they had received active or placebo drops. An unblinded comparison of parents provided with ear drops and those allocated to usual care was the appropriate approach to establish if the provision of ear drops would reduce antibiotic consumption in children with ear pain due to AOM. All parents, researchers and clinicians were blinded to the treatment allocation between active and inactive drops as far as possible. The statistician had unblinded access to the data in February 2017, to report to the Data Monitoring Committee, when approximately 50 patients had been recruited.
Statistical methods
The main statistical analyses were prespecified in a statistical analysis plan. The final version of the statistical analysis plan was signed off on 19 September 2017. A confidential interim analysis was completed in February 2017 for the Data Monitoring Committee. Final data analysis started in October 2017 and finished in January 2018. Stata® 14.2 (StataCorp LP, College Station, TX, USA) was used for all statistical analyses.
Statistical primary analysis
All parents of recruited children were asked to complete an 8-day symptom diary that asked, on each day, ‘Did your child take any antibiotics by mouth today?’. The primary analysis was conducted using the modified intention-to-treat (ITT) approach, including only children with full 8-day data for this question. We used logistic regression to compare children who had taken antibiotics on any of the 8 days with those who had taken no antibiotics during the first 8 days.
H0: the proportion of children consuming antibiotics is the same in both groups.
H1: the proportion of children consuming antibiotics is different between the groups.
The primary hypothesis was whether or not active drops reduced the proportion of children consuming antibiotics compared with usual care (no drops).
Statistical secondary analyses
As for the primary analysis, secondary outcome measures were compared between the study groups using an appropriate regression model. When data were skewed, the square root transformation was used to obtain a difference in means. Descriptive statistics were presented for adverse events and patient satisfaction.
Sensitivity analyses
We prespecified a per-protocol analysis, as a sensitivity analysis, including participants in the full analysis set who were deemed to have no major protocol violations that could interfere with the objectives of the study. Compliance per day was measured only for days when the pain score was ≥ 1. Any volume of liquid used on that day was considered compliant. Therefore, if there was patient-reported pain for 5 out of 8 days, and drops were used for all five of the days with pain, then we classed this as 100% compliance. We carried out a per-protocol analysis for which we removed any patients from the active group who were considered non-compliant. This was prespecified as those taking their trial medication on < 50% of the days that they reported pain.
In a further sensitivity analysis, those who were lost to follow-up in the active drops group were assumed to have taken antibiotics and those in the usual-care group were assumed not to have done so.
As a post hoc sensitivity analysis we removed patients who were found to be ineligible after having been recruited to the study and allocated to a group.
Subgroup analyses
The following subgroups of children were prespecified for investigation of any treatment effect differences on the primary outcome measure. A treatment subgroup interaction term was added to the appropriate regression model:
-
prior duration of illness (≤ 72 hours vs. > 72 hours)
-
visibility of the ear drum (yes or no)
-
extent of AOM (unilateral or bilateral)
-
whether or not they had been given a delayed antibiotic script.
Recurrence of AOM was listed in the analysis plan in error (as it is a measure of outcome); therefore, this subgroup analysis did not go ahead.
Exploratory analyses
Exploratory analyses were used to help us contextualise our main findings, by investigating the effects of antibiotics and analgesics on pain.
Health economic methods
The prespecified economic analysis consisted of a cost-effectiveness analysis and cost–consequence study. As antimicrobial resistance is such an important concern,21 our primary economic analysis was a cost-effectiveness analysis with antibiotic use as a proxy for this outcome. The economic case for or against the active drops is most likely to hinge on externalities, that is antibiotic use and resistance, in the population, rather than purely on within trial estimates of costs and quality of life. The cost–consequence study (including quality of life) was a secondary economic analysis.
The primary comparison was the NHS and societal cost per antibiotic consumption avoided during the acute episode between those children receiving active ear drops and those receiving usual care. The active treatment (Auralgan) is currently not in the British National Formulary (BNF)22 or available in UK pharmacies and, therefore, there is no national unit cost available. Auralgan is available to purchase over the counter in Australian pharmacies. The median retail cost for a 15-ml bottle in December 2017 at 10 online Australian pharmacies was AU$10.99, equivalent to approximately £5.20 when converted using 2016 Organisation for Economic Co-operation and Development purchasing power parity statistics23 or £10.40 retail cost for the two 15-ml bottles given to each participant in the active drops group of the trial. We assumed that, if used in routine UK clinical practice, Auralgan would be prescribed by a GP and, therefore, the cost would be borne by the NHS rather than by the family. For many medicines, the NHS drug tariff paid to pharmacies is much lower than the retail cost. For example, for 120 mg/5 ml of oral suspension paracetamol the drug tariff cost is approximately one-third of the retail cost. Therefore, in our primary analysis we assumed that the cost to the NHS of two 15-ml bottles of Auralgan would be £3.54 and we conducted a sensitivity analysis assuming that the NHS would pay the retail cost of £10.40.
The incremental NHS treatment costs during the first 8 days after randomisation were ascertained from the symptom and recovery questionnaire and valued using national unit costs22,24 using 2016/17 values, when available. Parents recorded whether or not their child received an antibiotic on each day that symptoms persisted. We assumed that the antibiotic prescribed was 250 mg of amoxicillin, three times a day (age 1–4 years) or 500 mg three times a day (age ≥ 5 years) for 7 days. Using the NHS drug tariff price,25 this equates to a cost of £1.17 (age 1–4 years) or £2.34 (≥ age 5 years) per antibiotic prescription.
Parents also recorded daily use of ibuprofen or paracetamol during the first 8 days. When use was reported, we assumed this was prescribed (i.e. it was a cost borne by the NHS) and that age-appropriate doses were given. For example, based on national drug tariff prices,25 the cost of a 2-year-old child taking 100 mg/5 ml of ibuprofen three times a day for a total of 20 doses is £1.28.
For Auralgan and antibiotics, we assumed that the whole cost would be incurred no matter how frequently parents administered the medicine (i.e. the remaining medication would be disposed of after the acute episode rather than reused during later episodes). For paracetamol and ibuprofen, we assumed that the cost of the medication would be proportional to the number of times it was administered (i.e. the remaining medication would be stored and reused during later episodes).
On the eighth day after randomisation, parents were asked to report any further consultations with their GP, the NHS 111 telephone service or hospital ambulatory care relating to their child’s ear symptoms. Parent productivity costs were measured on the 8-day questionnaire and valued using average earnings. Bootstrapping was used to calculate confidence intervals (CIs) around the point estimate of the incremental cost-effectiveness ratio and cost-effectiveness acceptability curves.
In the secondary economic analysis, we prespecified a cost–consequence study tabulating 3-month treatment costs, including data from the 3-month GP note review, alongside other important outcomes including 24- to 36-hour ear pain relief, overall symptom burden, acute illness duration and CHU-9D utility scores. At the request of the funder, the GP note review was not conducted; therefore, we did not estimate 3-month treatment costs and instead present only CHU-9D utility scores at 3 months.
Qualitative methods
To examine the views and experiences of AOM and its treatment, qualitative interviews were conducted with parents and clinicians involved in the care of trial participants. The interview topic guide included the perceived effectiveness and acceptability of treatment and exploration of any barriers to treatment uptake outside the trial.
Sampling
All parents agreeing to participate in the trial were asked, at the time of consent, if they were willing to be contacted about taking part in a telephone interview. From willing participants, a purposive sample was to be drawn in relation to (1) trial site (Bristol, Southampton, Cardiff), (2) trial group (active/placebo or usual care) and (3) sociodemographic factors including child’s age, ethnicity and socioeconomic status (with participants being selected from areas of high and low social economic deprivation, based on an Index of Multiple Deprivation score). 26 Health-care professionals (GP’s and nurse practitioners) involved in the trial were to be purposively sampled in relation to (1) trial site and (2) length of time since qualification. Sample sizes would be determined by the need to achieve data saturation, such that no new themes emerged by the end of data collection. 27 Interviews were to be analysed in batches and sampling to continue until no new themes emerged.
Interview conduct
In-depth telephone interviews were conducted with parents 14 days afer randomisation. Lines of questioning within interviews focused on views and experiences of (1) the disease, (2) its diagnosis, (3) treatment and recovery, (4) information and support needs and (5) views and experiences of participation within the trial. Interviews also explored the potential implications of making the CEDAR drops available over the counter, following which the costs will shift from the NHS to the individual. Health-care professionals were to be interviewed to explore their views and experiences of the trial, information and support needs and their attitudes to the future implementation of treatments.
All interviews were conducted by telephone. At interview, participants were asked to give verbal consent, following which a flexible interview topic guide was used to ensure that primary issues were covered during all interviews, but without dictating data collection. This allowed participants to introduce unanticipated issues. The interviewer (CC) used open-ended questioning techniques to elicit participants’ experiences and views of key events and participants were asked to provide examples. Interviews were recorded using a digital voice recorder, transcribed using a professional transcription service and anonymised to protect confidentiality. Parents who took part in interviews received a £10 high street voucher.
Qualitative data analysis
Interview transcripts were checked for accuracy and then imported into NVivo 11 (QSR International, Warrington, UK) qualitative analysis software to aid management and indexing of data. Analysis began after the first interview and was ongoing and iterative. Earlier data were to inform further data collection; for instance, analytical insights from data gathered in earlier interviews would help identify any changes that needed to be made to the topic guide during later interviews. Thematic analysis28 utilising a data-driven inductive approach29 was used to identify and analyse patterns and themes of salience to participants and across the data set using constant comparison techniques. 30,31 First transcripts were read several times, to gain familiarisation with the data and initial ideas. Transcripts were then examined line by line, with inductive codes being assigned to data segments that provided insight to participants’ views and understandings. An initial coding frame was developed and newer data were compared with previous data and then with the properties of emerging categories that contain the main themes. The process of constant comparison allowed the generation of new themes, reclassification of themes and incorporation of themes within other themes, with the coding frame being modified as analysis developed. A subset of transcripts was to be independently double-coded by other members of the research team and compared, and discrepancies discussed and resolved, to achieve a coding consensus and to maximise rigour.
Chapter 3 Results
Study progress
Study recruitment had been planned to take place between June 2015 and June 2017. Owing to a delay in the supply of a suitable placebo, recruitment began in October 2016 across 27 GP practices with a two-group internal pilot phase. Children were randomly allocated to the active treatment group or the usual-care group. The protocol change necessary for the two-group trial (protocol version 1.4) received approval from the ethics committee in August 2016 (NHS HRA reference 15/SC/0376). When the placebo became available in March 2017, the three-group study began recruiting across 35 GP practices. Although the two- and three-group designs ran concurrently for 3 months, the two studies did not run at the same time within any given practice. Owing to the late start of the three-group trial, the observational cohort for parents who did not want their children to take part in that trial but were willing to complete the study diary (see Figure 1) was not initiated. Recruitment to the trial closed as planned in June 2017, with a recruitment period of 9 months having been achieved rather than the planned 24 months.
In April 2017, a Data Monitoring Committee meeting was held that highlighted that antibiotic prescribing rates were much lower than assumed in all study groups combined and, consequently, that the sample size target would not provide 90% statistical power. This may in part have been due to children requiring immediate antibiotics, and so ineligible for the trial, accounting for a bigger than assumed proportion of those prescribed antibiotics in the studies used to inform the sample size calculation. Following this finding, the Trial Management Group, along with the Trial Steering Committee, jointly agreed that the data collected should serve as internal pilot data (i.e. the data would be included in the primary analysis) and an application made to extend the study to allow further recruitment with a revised exclusion criterion relating to antibiotic prescribing.
In Autumn 2017, this application to extend the study was declined by the funders, and the study was required to close by the 31 December 2017, several months earlier than originally planned. Owing to this, no 3-month reviews of primary care records were conducted and so planned analyses relying on those reviews were not possible. The following presentation of results has adhered as closely as possible to the prespecified statistical analysis plan, including an amendment written as part of the study close-down plan, which adapts the original plan to data available from the internal pilot and full three-group stages.
Recruitment and completion of follow-up assessments
Seventy-four children were recruited during the two-group internal pilot, which ran from October 2016 to the end of April 2017, in 27 GP practices situated in all three centres. Thirty-two children were recruited to the three-group study, which ran from March 2017 to June 2017, in 35 GP practices in Bristol and Southampton. All 106 patients were recruited by 17 GPs, 18 nurses and 3 other health-care professionals, with each recruiting between 1 and 19 children. Our interim recruitment target at the end of June was 150 participants. After initially recruiting to target, recruitment fell short of the June target in part because of the time taken to switch practices from the two-group to the three-group trial and in part because of the limited supply of treatment packs (see Appendix 1 for the chart used to monitor recruitment).
Figure 3 shows the stages of the trial and the different levels of dropout that led to the final analysis sample. Overall, five patients randomised into the trial were found to be ineligible post randomisation. For three of these patients (two active two-group patients and one usual-care two-group patient), this was discovered shortly after randomisation and the patients were advised to return the drops and not complete the diary. For the other two of these patients, this was noticed only after follow-up had been completed. Given that our analysis was on an ITT basis, these patients were included in all analyses but excluded in a sensitivity analysis. 32 Further detail of the availability of data on the two key outcomes of antibiotic use over eight days and pain reported is given in Table 1.
FIGURE 3.
The CEDAR trial CONSORT (Consolidated Standards of Reporting Trials) flow chart. a, Been using dexamethasone/neomycin sulphate/acetic acid spray (Otomize®; Teva UK Ltd.), temporary resident so clinician decided not to recruit, previously taken part in the CEDAR trial, grommets, history of bloody discharge, dental issue causing ear pain, patient seen at branch surgery and unable to come to main surgery for appointment; b, child not happy about taking part, parent did not want to risk getting placebo, parent did not want child to participate; c, child too unwell (clinician’s view), risk of complications due to pre-existing comorbidities, right ear otitis externa, child too old, no reason stated; d, child too unwell (clinician’s view), child not happy about taking part, parent did not have time for recruitment, no reason listed; e, recruitment to the two-group pilot ran from October 2016 to the end of April 2017 (in 27 sites) while recruitment to the three-group pilot ran from March 2017 to June 2017 (in 35 sites); 12 sites recruiting to the three-group pilot were previously involved in the two-group pilot. Although the two-group and three-group trials ran concurrently between March 2017 and June 2017, the two trials did not run at the same time within a given site; f, one patient who completed follow-up was later found to be ineligible (bilateral AOM and < 2 years of age); andg, one patient who completed follow-up was later found to be ineligible (otorrhoea in right ear).

| Outcome measure | Treatment arm, n (%) | p-value | ||
|---|---|---|---|---|
| Active drops | Placebo drops | Usual care | ||
| Two-group study | ||||
| Number randomised | 38 | 36 | – | |
| Found to be ineligible | 1 (3) | 1 (3) | 0.74 | |
| Withdrew from study | 2 (5) | 2 (6) | 0.67 | |
| Lost to follow-up | 6 (16) | 5 (14) | 0.54 | |
| 8 days of antibiotic data | 29 (76) | 30 (83) | 0.32 | |
| Pain data on days 1 and 2 | 32 (84) | 30 (83) | 0.58 | |
| Three-group study | ||||
| Number randomised | 12 | 10 | 10 | – |
| Found to be ineligible | 1 (8) | 0 | 0 | – |
| Withdrew from study | 0 | 0 | 0 | – |
| Lost to follow-up | 1 (8) | 3 (30) | 1 (10) | 0.48 |
| 8 days of antibiotic data | 10 (83) | 7 (70) | 8 (80) | 0.87 |
| Pain data on days 1 and 2 | 10 (83) | 7 (70) | 9 (90) | 0.63 |
Baseline data
A total of 106 patients were randomised to the CEDAR two- and three-group studies and baseline characteristics are shown in Tables 2–5, respectively. The team prespecified in the analysis plan that any baseline characteristics that differed by at least 10%/0.5 SDs would be adjusted for in a sensitivity analysis. However, as the study recruited only one-fifth of the target sample size, the criterion was increased to 20%/0.75 SDs to counteract the increased chance of finding an imbalance.
| Characteristic | Treatment arm | |||
|---|---|---|---|---|
| Active drops (N = 38) | Usual care (N = 36) | |||
| N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | |
| Centre | ||||
| Bristol | 38 | 23 (61%) | 36 | 23 (64%) |
| Cardiff | 38 | 5 (13%) | 36 | 4 (11%) |
| Southampton | 38 | 10 (26%) | 36 | 9 (25%) |
| Child’s demographics | ||||
| Sex: male | 38 | 18 (47%) | 36 | 16 (44%) |
| Age (years) | 38 | 4.6 (2.5) | 36 | 4.6 (2.5) |
| Aged ≥ 5 years | 38 | 17 (45%) | 36 | 14 (39%) |
| White ethnicity | 34 | 33 (97%) | 31 | 31 (100%) |
| Living in an area of deprivationa | 37 | 3 (8%) | 35 | 3 (9%) |
| Exposure to potential risk factors | ||||
| Smokers in household | 34 | 2 (6%) | 31 | 1 (3%) |
| Additional children in household | 34 | 22 (65%) | 31 | 21 (68%) |
| Breastfed at 3 months | 34 | 14 (41%) | 31 | 9 (29%) |
| Does the child wear a hearing aid | 34 | 0 (0%) | 31 | 0 (0%) |
| Flu vaccination in last 12 months | 34 | 15 (44%) | 31 | 16 (52%) |
| Accompanying adult demographics | ||||
| Mother attended with child | 29 | 26 (90%) | 28 | 25 (89%) |
| Accompanying adult’s age (years) | 29 | 35.0 (6.0) | 28 | 36.4 (6.7) |
| Accompanying adult employed/full time education/retired | 34 | 25 (74%) | 31 | 26 (84%) |
| Accompanying adult a university graduate | 29 | 11 (34%) | 28 | 11 (39%) |
| Characteristic | Treatment arm | |||
|---|---|---|---|---|
| Active drops (N = 38) | Usual care (N = 36) | |||
| N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | |
| Ear pain/medication | ||||
| Child ear pain score (1–10)a | 14 | 6.0 (2.6) | 15 | 6.1 (3.1) |
| Parent ear pain score (1–10) | 38 | 6.9 (1.5) | 36 | 6.3 (1.7) |
| Number of days in pain | 38 | 2.7 (2.0) | 36 | 2.5 (1.4) |
| Received painkillers todayb | 37 | 32 (86%) | 34 | 25 (74%) |
| Symptoms (scale 0–6) | ||||
| Episodes of distress/crying | 37 | 3.8 (1.5) | 34 | 3.3 (1.3) |
| Disturbed sleep | 37 | 4.1 (1.6) | 34 | 3.7 (1.6) |
| Interference with normal activities | 37 | 3.1 (1.5) | 34 | 2.7 (1.5) |
| Eating/drinking less than normal | 37 | 2.7 (1.7) | 34 | 2.2 (1.6) |
| Fever | 37 | 1.9 (1.7) | 34 | 2.7 (1.7) |
| Hearing problems | 37 | 1.2 (1.4) | 34 | 1.4 (1.8) |
| Cough | 37 | 2.5 (1.8) | 34 | 1.8 (1.7) |
| Blocked/runny nose | 37 | 2.3 (1.8) | 34 | 2.1 (1.8) |
| Vomiting | 37 | 0.5 (1.5) | 34 | 0.4 (1.0) |
| Clinical examination | ||||
| AOM in both ears/bilateral | 38 | 8 (22%) | 36 | 5 (14%) |
| General health of child (0–10)c | 38 | 3.6 (1.9) | 36 | 3.3 (1.8) |
| Temperature | 38 | 37.0 (0.6) | 36 | 36.9 (0.7) |
| Given a delayed antibiotic | 38 | 4 (11%) | 36 | 11 (31%) |
| Characteristic | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active drops | Placebo drops | Usual care | ||||
| N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | |
| Centre | ||||||
| Bristol | 8 (67%) | 8 (80%) | 6 (60%) | |||
| Southampton | 4 (33%) | 2 (20%) | 4 (40%) | |||
| Child’s demographics | ||||||
| Sex: male | 12 | 11 (92%) | 10 | 3 (30%) | 10 | 5 (50%) |
| Age (years) | 12 | 4.9 (2.5) | 10 | 5.0 (1.9) | 10 | 4.6 (2.9) |
| Aged ≥ 5 years | 12 | 7 (58%) | 10 | 5 (50%) | 10 | 7 (70%) |
| White ethnicity | 10 | 9 (90%) | 7 | 6 (86%) | 9 | 9 (100%) |
| Living in an area of deprivationa | 11 | 2 (18%) | 10 | 0 (0%) | 10 | 4 (40%) |
| Exposure to potential risk factors | ||||||
| Smokers in household | 10 | 1 (10%) | 7 | 1 (14%) | 9 | 2 (22%) |
| Additional children in household | 10 | 8 (80%) | 7 | 4 (57%) | 9 | 7 (78%) |
| Breastfed at 3 months | 10 | 7 (70%) | 7 | 4 (57%) | 9 | 2 (22%) |
| Does the child wear a hearing aid? | 10 | 0 (0%) | 7 | 0 (0%) | 0 | 0 (0%) |
| Flu vaccination in last 12 months | 10 | 4 (40%) | 7 | 4 (57%) | 9 | 4 (44%) |
| Accompanying adult demographics | ||||||
| Mother attended with child | 9 | 9 (89%) | 7 | 5 (71%) | 8 | 8 (100%) |
| Accompanying adult’s age (years) | 9 | 38.7 (6.8) | 7 | 37.6 (8.2) | 8 | 31.5 (3.1) |
| Accompanying adult employed | 10 | 7 (70%) | 7 | 6 (86%) | 9 | 3 (33%) |
| Accompanying adult a university graduate | 9 | 3 (33%) | 7 | 3 (43%) | 8 | 3 (38%) |
| Characteristic | Treatment arm | |||||
|---|---|---|---|---|---|---|
| Active drops | Placebo drops | Usual care | ||||
| N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | N | Mean (SD) or n (%) | |
| Ear pain/medication | ||||||
| Child ear pain score (1–10)a | 7 | 6.4 (2.3) | 4 | 5.5 (3.0) | 4 | 7.5 (2.5) |
| Parent ear pain score (1–10) | 11 | 6.3 (1.8) | 10 | 5.3 (1.3) | 10 | 6.2 (2.2) |
| Number of days in pain | 11 | 1.5 (0.9) | 10 | 2.7 (1.5) | 10 | 2.3 (1.8) |
| Received painkillers todayb | 11 | 7 (64%) | 9 | 4 (44%) | 10 | 7 (70%) |
| Symptoms (scale 0–6) | ||||||
| Episodes of distress/crying | 11 | 3.0 (1.7) | 9 | 2.4 (1.3) | 10 | 4.2 (1.7) |
| Disturbed sleep | 11 | 2.9 (1.5) | 9 | 2.7 (1.3) | 10 | 4.2 (1.3) |
| Interference with normal activities | 11 | 2.5 (1.8) | 9 | 2.9 (1.5) | 10 | 3.3 (1.3) |
| Eating/drinking less than normal | 11 | 1.7 (1.6) | 9 | 2.4 (1.8) | 10 | 2.0 (1.7) |
| Fever | 11 | 1.2 (1.6) | 9 | 2.1 (1.5) | 10 | 2.1 (1.9) |
| Hearing problems | 11 | 1.4 (1.6) | 9 | 1.0 (1.6) | 10 | 0.7 (1.1) |
| Cough | 11 | 1.0 (1.0) | 9 | 2.2 (1.7) | 10 | 1.5 (1.6) |
| Blocked/runny nose | 11 | 1.8 (1.9) | 9 | 1.7 (1.1) | 10 | 2.9 (1.8) |
| Vomiting | 11 | 0.2 (0.6) | 9 | 0.4 (1.3) | 10 | 0.5 (1.1) |
| Clinical examination | ||||||
| AOM in both ears/bilateral | 11 | 2 (18%) | 10 | 4 (40%) | 10 | 2 (20%) |
| General health of child (0–10)c | 11 | 3.9 (1.7) | 10 | 3.3 (1.5) | 9 | 3.1 (1.2) |
| Temperature | 11 | 37.1 (0.8) | 10 | 37.5 (1.3) | 9 | 37.1 (0.6) |
| Given a delayed antibiotic | 11 | 3 (27%) | 10 | 1 (10%) | 10 | 3 (30%) |
In the two-group study, the only measure to have a difference exceeding the prespecified criterion of 20% or 0.75 SDs was receipt of a delayed prescription for an antibiotic (see Table 3). Those prescriptions should have been issued, and recorded, prior to randomisation and so it should not be the case that allocation to study group influenced antibiotic prescription, although we are unable to confirm this for sure.
Prior to analysis, the criterion for imbalance for the three-group study was increased to 30%/1 SD to counteract the increased chance of finding an imbalance given the small sample size. Variables that exceeded this criterion were sex, accompanying adult’s age and employment status, living in an area of deprivation, breastfeeding status at 3 months (see Table 4), episodes of distress/crying and disturbed sleep (see Table 5).
Outcomes and estimation
Primary outcome: antibiotic consumption
The primary research hypothesis was to find whether or not active ear drops would reduce antibiotic consumption compared with usual care. In the two-group study, 30% of patients in the usual-care group consumed antibiotics by day 8, compared with only 3% in the active drops group (Table 6). The results from the three-group study, although based on a much smaller sample size, were very similar: 25% in the usual-care group versus 0% in the active drops group. These two results were combined in a meta-analysis (with a continuity correction of 0.4444 to allow calculation of an odds ratio in the three-group study). The combined odds ratio supports the conclusion that, with 95% confidence, the crude odds of taking antibiotics is between 45% and 98% lower in the active drops group than in the usual care group (see Table 6). After controlling for the effect of administering a delayed antibiotic script at randomisation, the CI widens, weakening the evidence against the null hypothesis (see Table 6).
| Group | Treatment arm, n/N (%) | OR (95% CI) | p-value | OR (95% CI)a | p-valuea | |
|---|---|---|---|---|---|---|
| Active drops | Usual care | |||||
| Two-group | 1/29 (3) | 9/30 (30) | 0.08 (0.01 to 0.71) | 0.023 | 0.12 (0.01 to 1.18) | 0.069 |
| Three-group | 0/10 (0) | 2/8 (25) | 0.11 (0.00 to 3.17)b | 0.201 | 0.20 (0.01 to 3.49)b | 0.270 |
| Combined | 1/39 (3) | 11/38 (29) | 0.09 (0.02 to 0.55)c | 0.009c | 0.15 (0.03 to 0.87)d | 0.035d |
Receipt of a delayed antibiotic script was prespecified as a potential confounding variable in the statistical analysis plan; Table 7 shows a stratified analysis investigating the impact that the delayed antibiotic script had on the proportion of children consuming antibiotics. Overall, 47% (9/19) of those children given a delayed antibiotic script went on to consume an antibiotic, compared with 9% (6/65) of those not given a delayed antibiotic script. In the two-group study, 70% (7/10) of children given a delayed antibiotic went on to consume antibiotics if they were in the usual-care group, compared with 0% (0/3) of the children in the active drops group.
| Antibiotic consumption | Group, n/N (%) | ||||
|---|---|---|---|---|---|
| Two-group study | Three-group study | ||||
| Active drops | Usual care | Active drops | Placebo drops | Usual care | |
| Prescribed delayed antibiotic | 0/3 (0) | 7/10 (70) | 0/3 (0) | 0/0 (0) | 2/3 (67) |
| No prescribed delayed antibiotic | 1/26 (4) | 2/20 (10) | 0/7 (0) | 3/7 (43) | 0/5 (0) |
Data from the three-group study were used to investigate whether or not active ear drops would reduce antibiotic consumption compared with placebo drops. A total of 43% (3/7) of patients in the placebo drops group consumed antibiotics by day 8, compared with 0% (0/10) in the active drops group. Using a Fisher’s exact test, there is moderate evidence to suggest that active drops reduce antibiotic consumption compared with placebo drops (p = 0.051).
Key secondary outcome: ear pain on day 2
Parent-reported pain scores at day 2, in the three-group study, are presented in Table 8. There was no evidence to suggest that pain was reduced in active compared with placebo drops groups. Unadjusted and adjusted results were in favour of the placebo drops, with pain scores observed to be, on average, 0.96 points higher in the active group. There was evidence to suggest that pain was reduced in the placebo drops group compared with the usual-care group. Unadjusted and adjusted results were in favour of the placebo drops with pain scores on average 2.86 points higher in the usual-care group (see Table 8), and the 95% CI excluded the prespecified 1-point minimum clinically important difference.
| Treatment arm | Ear pain, mean (SD); n | Mean difference (95% CI) | p-value | Mean difference (95% CI)a | p-valuea |
|---|---|---|---|---|---|
| Placebo drops | 2.14 (1.07); 7 | Comparison | |||
| Active drops | 3.10 (2.23); 10 | 0.96 (–0.99 to 2.91) | 0.312 | 0.67 (–1.44 to 2.79) | 0.506 |
| Usual care | 5.00 (1.73); 9 | 2.86 (1.25 to 4.46) | 0.002 | 2.86 (1.13 to 4.60) | 0.003 |
We carried out a post hoc secondary analysis comparing ear pain between the active and usual-care groups. There was evidence (Table 9) to suggest that active ear drops reduced ear pain, which was strengthened after adjusting for baseline ear pain (at consultation). After adjustment, the combined mean difference was almost 2 points on the 0- to 6-point scale, with the 95% CI almost excluding the minimum clinically important difference and close to the effect of the placebo drops reported in Table 8.
| Group | Treatment arm, mean (SD); n | Mean difference (95% CI) | p-value | Mean difference (95% CI)a,b | p-valuea,b | |
|---|---|---|---|---|---|---|
| Active drops | Usual care | |||||
| Two-group | 2.81 (2.32); 32 | 4.43 (2.54); 30 | –1.62 (–2.86 to –0.39) | 0.011 | –2.01 (–3.23 to –0.78) | 0.002 |
| Three-group | 3.10 (2.23); 10 | 5.00 (1.73); 9 | –1.90 (–3.85 to 0.05) | 0.056 | –1.93 (–3.92 to 0.05) | 0.055 |
| Pooled | 2.88 (2.28); 42 | 4.56 (2.37); 39 | –1.70 (–2.74 to –0.66) | 0.001 | –1.99 (–3.01 to –0.95)b | < 0.001b |
Secondary outcome: ear pain on day 1
There was no evidence to suggest that pain was reduced in the active drops group compared with the placebo drops group on day 1 (Table 10).
| Group | Treatment arm, mean (SD); n | Mean difference (95% CI) | p-value | Mean difference (95% CI)a | p-valuea | |
|---|---|---|---|---|---|---|
| Active drops | Usual care | |||||
| Ear pain | 2.70 (1.16); 10 | 3.42 (1.62); 7 | –0.73 (–2.16 to 0.70) | 0.295 | –0.74 (–2.32 to 0.85) | 0.338 |
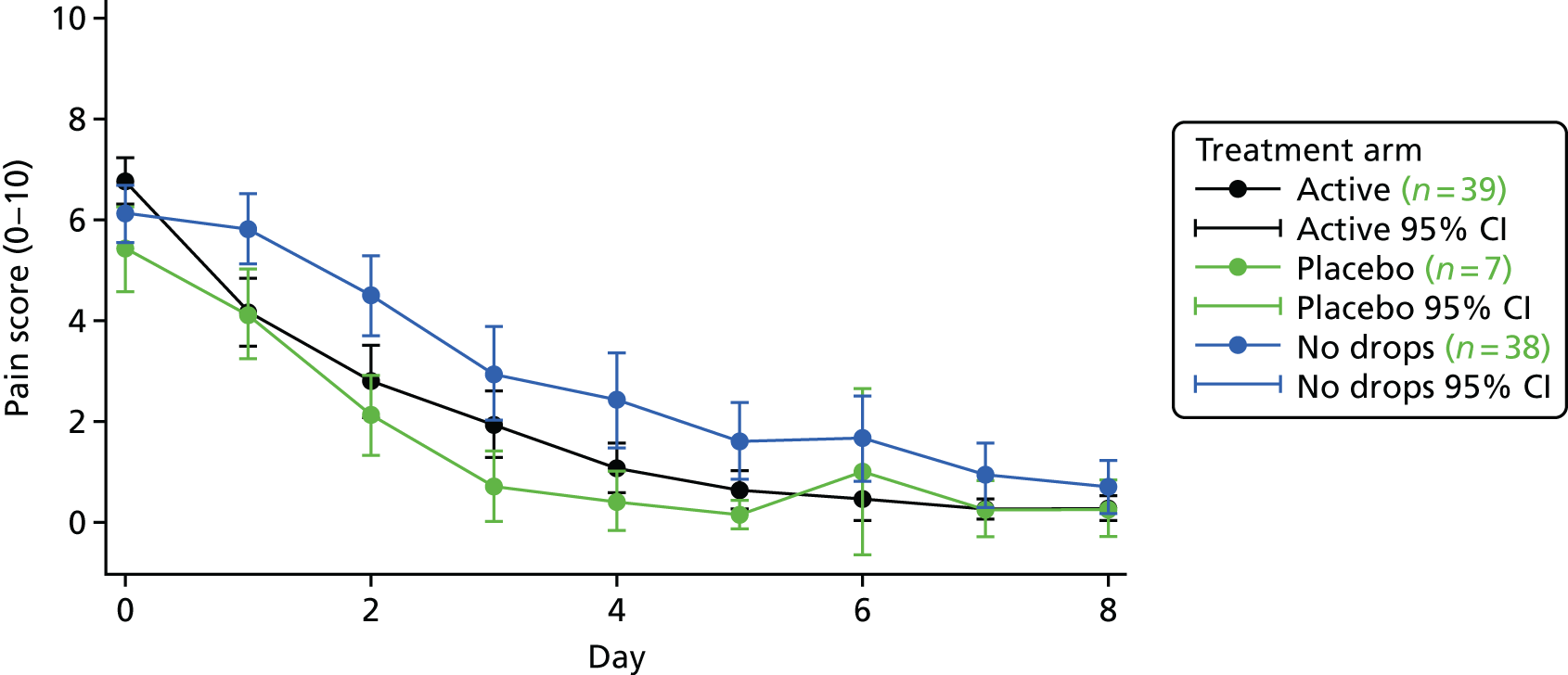
Combining the two- and three-group study data, Figure 4 shows the average daily pain scores for each of the three groups. Over the 8 days, lower levels of pain were reported by the active drop and placebo drop groups than by the no drops group.
FIGURE 4.
Mean pain scores by day and group, combining the two- and three-group children and including only those with full 8 days of data on ear pain.
Secondary outcome: oral analgesic consumption
Oral analgesic consumption was high, with 88% of children taking paracetamol or ibuprofen during the first 8 days after consultation. There was no evidence to suggest a difference in oral analgesic consumption between those taking active and placebo ear drops (Table 11).
| Group | Treatment arm, n/N (%) | OR (95% CI) | p-value | OR (95% CI)a | p-valuea | |
|---|---|---|---|---|---|---|
| Active drops | Placebo drops | |||||
| Analgesic consumption | 8/9 (89) | 6/7 (86) | 1.33 (0.07 to 25.91) | 0.849 | 1.21 (0.04 to 34.00) | 0.911 |
| Ibuprofen | 4/9 (44) | 3/7 (43) | ||||
| Paracetamol | 6/9 (67) | 6/7 (86) | ||||
During the recruitment process, parents were asked if their child had received any painkilling medicine prior to the recruitment consultation and 56 out of 79 (71%) participants said that they had. All 56 of these children subsequently received analgesics during the study. Out of the 23 who did not give their child painkilling medicine before taking part in the study, five continued to not use analgesics during the trial (active drops, n = 2; placebo, n = 1; and usual care, n = 2).
Considering the number of doses of oral analgesic that the children were receiving over the 8 days, there were no clear differences in the two-group study (Figure 5). In the three-group study, children allocated to the active drops appeared to take fewer doses than their placebo and usual-care counterparts (see Figure 5). However, these observed differences were consistent with chance (Table 12).
FIGURE 5.
Analgesic consumption up to day 8 for the two- and three-group studies. The box and whisker plots show the distributions of analgesic consumption. The box represents the IQR, the line within the box is the median, the upper and lower ‘whiskers’ are defined as the 75th percentile + 1.5 × IQR and the 25th percentile – 1.5 × IQR, respectively. The dots outside these ‘whiskers’ are the outliers observed in the data. IQR, interquartile range.

| Group | Treatment arm, median (IQR); n | Difference in meansa (95% CI) | p-valuea | |
|---|---|---|---|---|
| Active | Placebo | |||
| Number of doses | 2.0 (1.0–5.0); 9 | 6.5 (2.0–9.0); 6 | –0.54 (–1.86 to 0.787) | 0.397 |
Parents were also asked if they had given their children any other painkilling remedies. Only one additional treatment was reported for one child: xylometazoline (Otrivine, GlaxoSmithKline) nasal drops over 2 days.
Secondary outcome: overall symptom burden
For a first exploration of symptom burden, each child’s scores (high scores indicate greater severity, maximum score of 36 on each day) on each of the symptom measures were added together to give a total for each of the 8 days following recruitment. An ‘area under the curve’ approach (broadly equivalent to taking the average score for each child over the 8 days) was taken, with the resulting scores being positively skewed (Figure 6) and so were transformed (by taking the square root) to allow analysis with linear regression.
FIGURE 6.
Mean symptom burden in the two-group [(a) and (b)] and three-group studies [(c), (d) and (e)].


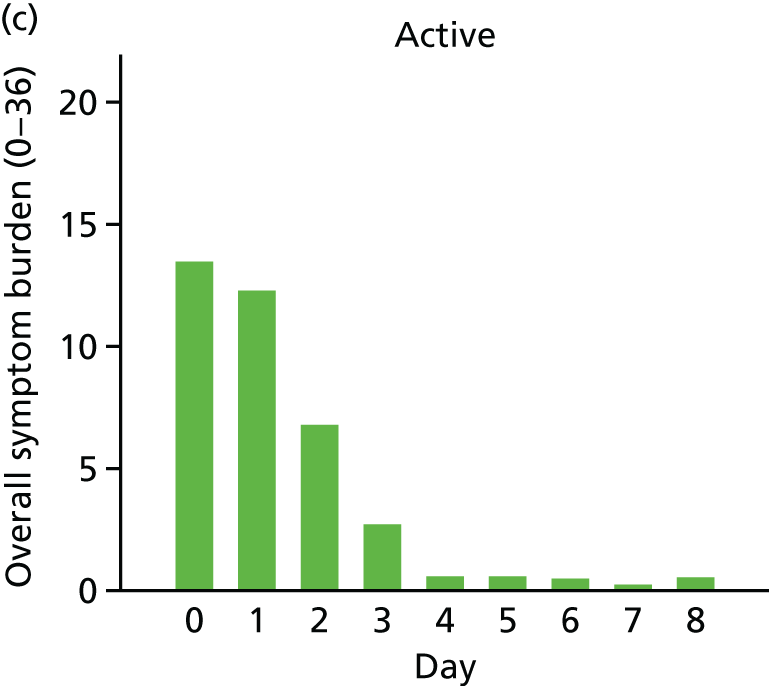
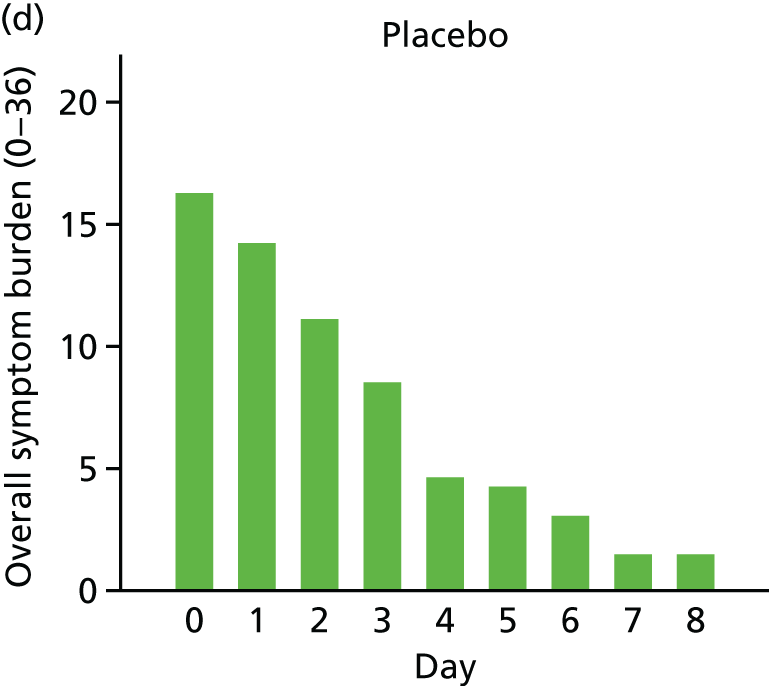

For both the two- and the three-group studies, the active drops appeared to lead to a faster resolution of symptoms, and so a lower symptom burden over the 8 days (see Figure 6), with modest evidence in support of these differences (Table 13).
| Treatment arm (study) | Symptom burden, median (IQR); n | Difference in meansa (95% CI) | p-valuea |
|---|---|---|---|
| Active drops (three-group) | 15.8 (8.5–21.5); 10 | Comparison | |
| Placebo drops (three-group) | 24.5 (10.5–50.5); 7 | 1.81 (–0.28 to 3.90) | 0.085 |
| Usual care (three-group) | 28.5 (14.0–42.0); 9 | 1.35 (–0.13 to 2.84) | 0.072 |
| Active drops (two-group) | 11.5 (5.8–33.5); 32 | Comparison | |
| Usual care (two-group) | 30.3 (6.3–45.0); 28 | 1.14 (–0.20 to 2.49) | 0.094 |
Secondary outcome: overall illness duration
Children in the active group appeared to be recovering at a faster rate than those in the usual-care group (Figure 7), but this difference was consistent with chance (Table 14). There was no evidence against the proportional hazards assumption.
FIGURE 7.
Kaplan–Meier curve for the time to recovery from pain in the two-group trial. Time to recovery (score = 0).

| Treatment arm (study) | Pain duration, median (IQR); n | Hazard ratio (95% CI) | p-value |
|---|---|---|---|
| Active drops (three-group) | 3 (3–5); 10 | Comparison | |
| Placebo drops (three-group) | 2 (2–4); 7 | 1.70 (0.61 to 4.75) | 0.31 |
| Usual care (three-group) | 3 (2–6); 9 | 0.94 (0.38 to 2.61) | 0.90 |
| Active drops (two-group) | 3 (2–5); 34 | Comparison | |
| Usual care (two-group) | 4 (3–Xa); 31 | 0.62 (0.34 to 1.11) | 0.11 |
Secondary outcome: time to antibiotic consumption
This measure was only reported for 15 children across the two studies, with no reports for some groups; consequently, the data are uninformative and not presented here.
Secondary outcome: adverse events
There was one serious adverse event experienced during the trial as a result of which a child was admitted to hospital due to breathing issues. The child was discharged from hospital the next day and the parent continued to complete the questionnaire. This child was allocated to the usual-care group, and so the event can be considered as unrelated to the ear drops.
At the end of the week, parents were asked to report any new or worsening symptoms, and six reports were made. For the active drops group, these included a head cold (moderate) and chickenpox (mild). Neither of these symptoms was thought to be related to the drops. For the usual-care group, there were reports of ringing in the ears (mild); a sore, snotty and bleeding nose (moderate); problem with balance (moderate); and itching around the neck (moderate). The last two symptoms were experienced by the same patient. No new or worsening symptoms were reported for the placebo drops group.
Secondary outcome: parent satisfaction
When parents were asked if they were satisfied with the trial ear drops, 93% (27/29) of parents receiving active drops in the two-group trial said that they were satisfied and 7% (2/29) of parents reported that they were neither satisfied nor dissatisfied. Parents of children allocated to active drops in the three-group trial reported 90% (9/10) satisfaction, with 10% (1/10) reporting that they were neither satisfied nor dissatisfied. Only 57% (4/7) of parents with children allocated to placebo drops reported satisfaction, with 29% (2/7) reporting that they were neither satisfied nor dissatisfied and 14% (1/7) reporting that they were not satisfied.
In the three-group trial, parents of children recruited to the active or placebo drops were asked on day 8 if they thought that their child was taking the painkilling ear drops. In the active group, 60% (6/10) thought that their child was taking the painkilling ear drops, and in the placebo group 29% (2/7) thought that they were.
When asked if they would use the drops if they were to be available over the counter, all parents in the two-group trial said ‘Yes’, with 69% in the active group saying that they would take them without GP advice compared with 50% in the usual-care group. Of the 26 children recruited in the three-group trial, two parents said that they would not use the drops if they became available; both were in the blinded placebo group. When asked what they would use in the future, 79% (23/29) of those in the active group of the two-group trial said just the trial drops, compared with 40% (4/10) of those active group patients in the three-group trial; these results are given in Figure 8. When comparing the answers to this question between those who gave their children antibiotics during the study and those who did not, it was found that those who gave antibiotics were more likely to say that they would use antibiotics again (20%, 3/15) than those who had not (3%, 2/69).
FIGURE 8.
By trial group: what would you want to use for your child for a similar illness in the future?

Sensitivity analyses
Per-protocol analysis
The trial team, together with the Data Monitoring Committee, defined compliance as taking study medication on ≥ 50% of the days that they reported pain. The per-protocol analysis excluded patients not achieving this level of compliance.
Overall, 78% of parents reported using the drops on all of the days when their children reported pain. There was only one child who did not comply at least 50% of the time, and this was the one child in the active drops group (two-group study) who consumed antibiotics. When we repeat the analysis without this child, the evidence of reduced antibiotic use in the active drops group remains (Table 15).
Sensitivity analysis: missing data imputed under best- and worst-case scenarios
Twenty-two children withdrew or were lost to follow-up before completing 8 days of reporting, and their antibiotic consumption status was therefore unknown. For a best-case scenario, the 11 children in the active group were assumed to have completed the trial without taking antibiotics, and the eight children in the usual-care group were assumed to have taken antibiotics at some point during the 8-day follow-up. For the worst-case scenario, the 11 children in the active group were assumed to have taken antibiotics and the eight children in the active drops group were assumed to have completed follow-up without consuming antibiotics. These assumptions are at the two extremes and the results, as expected, show that the best-case scenario strengthens the evidence for reduced antibiotic use in the active drops group, whereas the worst-case scenario weakens the evidence such that a reduction in antibiotic use is no longer clearly apparent (Table 16).
| Variable | Treatment arm, n/N (%) | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Active drops | Usual care | |||
| Best-case scenario | ||||
| Two-group trial | 1/38 (3) | 15/36 (42) | 0.04 (0.00 to 0.31) | 0.002 |
| Three-group trial | 0/12 (0) | 4/10 (40) | 0.05 (0.00 to 1.29)a | 0.071a |
| Worst-case scenario | ||||
| Two-group trial | 10/38 (26) | 9/36 (25) | 1.07 (0.38 to 3.04) | 0.897 |
| Three-group trial | 2/12 (17) | 2/10 (20) | 0.80 (0.09 to 7.00) | 0.840 |
Sensitivity analysis: exclusion of children found to be ineligible following allocation
The primary analysis was repeated without two children who were found to be ineligible only after data collection was complete: one child in the usual-care group of the two-group trial (who had bilateral AOM and was < 2 years old) and one child in the usual-care group of the three-group trial (who had otorrhoea in one ear). Table 17 shows the results when these two children were removed from the analysis. After removing the two ineligible children, the evidence for a reduction in antibiotic use in the active drops group was strengthened slightly.
Exploratory analysis: reported pain by antibiotic use
For those with pain scores for all 8 days, children who took antibiotics (n = 15) appeared to have higher pain scores than those who did not take antibiotics (n = 69) during the study (Figure 9). Although the direction of cause and effect could not be established from these data, it is possible that the use of antibiotics resulted from the higher pain.
FIGURE 9.
Mean pain scores by day and antibiotic consumption, combining the two- and three-group children and including only those with full 8-day data on ear pain.

Subgroup analyses
The evidence for subgroup effects is difficult to interpret in the context of low statistical power, which was the case for this study because of the modest sample size realised and the dimensions investigated having one subgroup much larger than the other. Consequently, the summary statistics are presented in Table 18.
| Subgroup | Treatment arm | |||
|---|---|---|---|---|
| Active drops | Usual care | |||
| N | Consumed antibiotics, n (%) | N | Consumed antibiotics, n (%) | |
| Prior duration of illness | ||||
| ≤ 72 hours | 20 | 1 (5) | 23 | 7 (30) |
| > 72 hours | 9 | 0 (0) | 7 | 2 (29) |
| Ear drum visible | ||||
| Yes | 28 | 1 (4) | 30 | 9 (30) |
| No | 1 | 0 (0) | 0 | 0 (0) |
| AOM ear(s) | ||||
| Unilateral | 20 | 1 (5) | 27 | 8 (30) |
| Bilateral | 8 | 0 (0) | 3 | 1 (33) |
| Antibiotic prescribing | ||||
| Delayed | 3 | 0 (0) | 10 | 7 (70) |
| None | 26 | 1 (4) | 20 | 2 (10) |
Health economic results
Cost-effectiveness findings
The unit costs of resources are presented in Table 19. There were no substantial differences in the total costs of health care or parental time off work during days 1–8 (Table 20). The reduction in antibiotic use among participants allocated to receive active drops leads to only a very small reduction (£0.38) in antibiotic prescribing costs in the short term. A small number of children had repeat consultations with their GP, the NHS 111 telephone consultation service or hospital ambulatory care services, but there was no difference in the cost of this care between groups. The mean number of parent/carer days off work was similar in the active drops (0.58 days) and usual-care (0.61 days) groups, but varied widely among respondents (range 0–6 days). Owing to the large variations in repeat consultations and time off work, the CI around the difference in total costs is wide and includes £0.
| Resource | Unit cost | Perspective | Source of cost |
|---|---|---|---|
| Active drops | £3.54 per 15-ml bottle | NHS | Online pharmacies |
| Antibiotic | £1.17 to £2.34 per prescriptiona | NHS | BNF22 |
| Paracetamol | £0.24 to £0.87 per daya | NHS | BNF22 |
| Ibuprofen | £0.19 to £0.58 per daya | NHS | BNF22 |
| GP visit | £36.00 | NHS | PSSRU24 |
| NHS 111 | £12.26 | NHS | Evaluation of NHS 111 study report33 |
| Hospital-related services | £199.00 | NHS | PSSRU24 |
| Time off work | £539.00 per weekb | Societal | Office for National Statistics34 |
| Resource | Treatment arm, mean (SD) | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Active drops (n = 38)a | Usual care (n = 37)a | |||
| Active drops | £3.54 (0) | £0 (0) | ||
| Antibioticsb | £0.06 (0.38) | £0.44 (0.80) | –£0.38 (–0.67 to –0.10) | 0.01 |
| Ibuprofenb | £0.44 (0.57) | £0.49 (0.62) | –£0.05 (–0.32 to 0.23) | 0.73 |
| Paracetamolb | £0.84 (0.90) | £1.17 (1.09) | –£0.32 (–0.78 to 0.13) | 0.16 |
| GP/NHS 111/ambulatory | £7.78 (40.66) | £9.27 (44.99) | –£1.49 (–21.21 to 18.23) | 0.88 |
| Time off work | £62.41 (75.74) | £65.55 (141.55) | –£3.14 (–55.20 to 48.91) | 0.91 |
| Total | £75.07 (99.47) | £76.92 (154.94) | –£1.85 (–61.61 to 57.91) | 0.95 |
Among the 75 children (active drops, n = 38; and usual care, n = 37) with complete data, our findings suggest that, from a NHS and societal perspective, active drops have the potential to both save money (£75.07 active drops; £76.92 usual care) and reduce antibiotic prescriptions (2.6%, active drops; 27.0%, usual care). Restricting the analysis to NHS costs (£12.66, active drops; £11.36, usual care) leads to an estimated cost of £5.19 per antibiotic prescription avoided, although this is associated with a high degree of uncertainty [95% CI lower limit (cost saving) undefined to upper limit £110]. The estimate is also sensitive to the unit cost of Auralgan; in a sensitivity analysis, assuming that the NHS pays the approximate retail cost of £10.40, the cost per antibiotic avoided increased to £33.43.
Other outcomes
Children in both the active drops and the usual-care groups of the trial had improvements in health-related quality of life, measured by the CHU-9D, at 2 days after randomisation and further improvements were observed by day 8 (Table 21). As this questionnaire was completed only for children aged ≥ 5 years, the sample size is small. However, there is weak evidence that CHU-9D scores were higher (better) among children in the active drops group at 8 days. There was no difference in the mean number of days off school or preschool childcare (Table 22).
| Group | Treatment arm, mean (SD); n | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Active drops | Usual care | |||
| Baseline | 0.696 (0.121); 21 | 0.657 (0.148); 19 | 0.039 (–0.047 to 0.125) | 0.367 |
| Day 2 | 0.829 (0.146); 17 | 0.807 (0.154); 15 | 0.023 (–0.086 to 0.131) | 0.672 |
| Day 8 | 0.983 (0.030); 21 | 0.923 (0.109); 15 | 0.060 (0.010 to 0.110) | 0.021 |
| Group | Treatment arm, mean (SD); n | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|
| Active drops | Usual care | |||
| Number of days | 0.95 (0.92); 39 | 0.91 (1.29); 38 | 0.04 (–0.47 to 0.55) | 0.873 |
Qualitative data results
Number and characteristics of parents
Three parents were interviewed, all of whom had taken part in the three-group trial. Table 23 displays characteristics of the parents and their children, including the trial participant child.
| Parent | Interviewee | ||
|---|---|---|---|
| PA01 | PA02 | PA03 | |
| Number of children | 3 | 1 | 3 |
| Age range of children (years) | 2–11 | 7 | 3–6 |
| Age of participating child (years) | 2 | 7 | 3 |
| Previous history of AOM | Siblings and study child | Study child | Siblings |
| Active or placebo drops | Active | Placebo | Placebo |
| Index of multiple deprivation decilea | 4 | 7 | 8 |
Owing to the primary aim of the qualitative evaluation (examining parents’ views and experiences of active, placebo and usual-care groups of the trial), interviews did not proceed until the three-group trial started. Six trial parents were contacted to take part in the qualitative interview; one did not respond, two agreed but could not conduct the interview within the study time period, and three agreed and took part in an interview. Owing to limited number of interviews, data saturation was not reached.
Themes developed from the analysis are described below with the use of verbatim quotes.
Views and experiences of trial
Parents spoke positively about the trial, saying that they thought it was a good idea. All the parents were pleased to be part of the trial and welcomed being invited to take part:
It sounded great and actually . . . it’s such a great idea.
PA01
I was quite open to it.
PA02
I think that the trial was good.
PA03
Parents demonstrated a good understanding of the aims of the trial:
Well I guess it was to test this local, local anaesthetic stuff on kids, um to see if it helped um kids that regularly suffer from ear pain, um whether it just you know to sort of um, kind of um avoid using antibiotics in the future.
PA03
When deciding to participate in the trial, perceived safety of the medicine was as important to the parents as the strength of the parents’ faith and trust in doctors and ‘scientists’ (PA02). Although safety was a concern, information provided within the information sheet and by practice staff during discussions at the point of recruitment, about established safety of use of the treatment, calmed any fears. Parents also felt comforted by having choice and control over whether or not the drops were administered and when:
I think it you know they [drops] were proven to help I was told in Australia and I thought well if they’re available elsewhere . . . and they’re safe, I’m quite happy. My boy was quite happy . . . to do it, so yeah I was quite happy.
PA02
I thought it [the CEDAR trial] was good . . . and I always think it’s as long as it’s not, you know like it’s a safe trial then I’m perfectly happy with that yeah.
PA03
I mean like I think that the trial was good, in that you know it did mention that if you don’t want to take the ear drops, then you can just put zero on that day . . . that was kind of a very positive kind of thing for me because you know I wouldn’t, I didn’t feel the pressure to have to like to do it if it was really just she was really hard to hold down or something, I’d just like right that’s that done and not to have to have committed to doing it for five or six or seven, do you know what I mean like kind of kept, that was kind of a choice which I kind of yeah appreciated.
PA03
Parents also felt that, as the drops were being externally applied as opposed to an oral medication, any risk was lowered and it was safer for the child to use. This added to their comfort for their child being involved in the trial:
I guess because it wasn’t like they were taking medicine orally, they were kind of, it was sort of an external thing that made me think that it was probably safer . . . but it [sic.] they were giving me something, a trial that was a medicine you consumed, then maybe I’d be a bit more concerned about that.
PA03
Parents felt that they had sufficient information to enable them and their child to take part in the trial. Information provided through the information sheet and by the study team was clear and well received. Parents appreciated that they could contact the study team with any queries. One parent used this resource to check whether or not they needed to continue with the drops when they felt that they did not need to any more:
It [study information] was fairly straightforward really, it just – it’s quite – it was pretty self-explanatory . . . The only thing was I checked um about like how long to use them once he’s got better, if you think – once you felt they’d got better . . . she [research nurse] was really good and she just messaged me back and just said that – carry on using it probably for like a couple of days after, like you would do with anything really, you know.
PA01
Parents interviewed did not use the online video that was available either because they felt that they did not need it as they already had sufficient information or did not have the time. The length of time and detail provided by the GP was important to parents and welcomed:
He [GP] was really good and really thorough about everything really . . . He went through it all of it actually . . . I think we were in there for ages, bless him, it made him very late . . . but he was really good and went through everything and checked, like went through it all with me.
PA01
Reasons for and experiences of consulting
Parents all consulted the GP because of their child being upset and in pain. One parent commented that because the child had a history of previous serious ear infection resulting in hospitalisation, she wanted ‘piece [sic.] of mind’ (PA01). Parents had no expectations of the consultation other than that the doctor would check the ear(s) and there would be discussion about any treatment. The main concern was to ease the child’s pain, whether by use of antibiotics, as in the past, or by other means:
Really like that if it was an ear infection and it, um – that he would get antibiotics possibly, if that was right – it’s really hard ‘cause there’s no – I wouldn’t say I’d go for antibiotics. But it’s a discussion you would have with the doctor.
PA01
Thing was he was in pain, I just wanted him not to be in pain you know.
PA02
There’s nothing worse than earache is there in kids?
PA02
Parents did not express an expectation of antibiotics from the consultation even if their child had used them in the past and were aware of the issue of antibiotic resistance:
You don’t just want to give them antibiotics because they get resist – you know, then the resistance come up and stuff.
PA01
I didn’t really [expect antibiotics], I don’t really, I’m not overly keen on the idea of constantly giving kids antibiotics so you know as long as I don’t think that there’s any danger of the infection getting worse, then I’d prefer to let yeah, I’d prefer to them to sort of recover by themselves yeah.
PA03
Parents were aware of attempts to decrease antibiotic use and embraced advice provided by the GP concerning their use. GPs had explained, within the initial trial consultation, that the type of infection being experienced would clear up on its own and that antibiotics may be of little use. They felt this was a discussion that needed to happen between themselves and the doctor and that they would be happy with what the doctor recommended:
I wouldn’t always say I’d go for antibiotics. But it’s a discussion that you would have, I guess, with the doctor and see actually knowing his history that possibly antibiotics might be the right course. But if it’s not that bad.
PA01
They [drops] help with pain with earache because if it’s a viral earache then antibiotics don’t necessarily work and the earache will go away on its own.
PA02
Well the doctor then, he told my husband that they were trying to not use antibiotics anymore and to leave it to clear up itself . . . I was happy with that to be honest . . . I mean I’d usually just take the doctor’s advice and go with what they think is best, so on that account I was like right if they think it’s going to clear up without antibiotics, that’s fine.
PA03
However, being able to have a delayed antibiotics prescription meant that they felt reassured when given the drops in case the child’s condition worsened. Parents did not use this prescription as they felt administering the trial drops was sufficient:
He [the GP] put my mind a bit more at ease because he gave me the delayed prescription . . . Because that was my only concern, that actually sometimes with [child’s name] with his ear infections, they go from nothing to sort of like really bad, that we could get him a prescription without having to go back to the doctor if needed.
PA01
Views and experience of treatments
Pain relief for the child was important to the parents and was discussed most when exploring experience of using the drops. All parents believed that the drops that they had received had relieved pain levels within the first day and parents (including those who had received the placebo drops) reported reduced use of other forms of pain relief, for example paracetamol (Calpol®, Johnson & Johnson Limited). When asked whether they thought they had the active or the placebo drops, all parents felt that, because of the quick reduction in pain and not using additional pain relief, they had the active drops:
Obviously it was uncomfortable ‘cause he didn’t really like the drops being put in. But there wasn’t a lot of pain, he didn’t seem in pain with it and we didn’t need to use much Calpol . . . it seemed to clear up quite quickly.
PA01
But he did say, he said his ear felt I don’t know after about 20 minutes, it wasn’t as painful as what it was before he had the drops . . . whereas he’d had an ear infection, earache before, it was a constant pain. And I hadn’t been giving him Calpol, so I couldn’t say that was taking the pain away.
PA02
It became apparent she was fine, like it got a bit, it became apparent that her ear wasn’t hurting as much, she was like after you know a couple of days you could touch her ear without her complaining whereas before you couldn’t even touch her without her crying.
PA03
Some parents discussed difficulties administering the drops as children were young and did not like the drops, or that parents had problems with the size of the bottles or drops:
The actual droplet thing was quite big for his ear . . . I think if it was a different bottle with a different, much smaller nozzle . . . it would have been a bit easier, because they were quite gloopy the drops were. So, I think if you could have got then [sic.] in, the droplet was just a bit too fat, do you know what I mean?
PA02
Well it was fine, if I could get it into her ear, to begin with it was quite hard because she’s only 3, she didn’t, she doesn’t like taking any type of medication or put cream on even you know, she’s awkward with everything, so I had to you know we had to kind of hold her in position to get the ear drops in her ear . . . so that was quite awkward to be honest, I know they said you take up to 12 doses a day or something, but we could barely get in that many to begin with because of just the fight and stuff.
PA03
Parents compared the use of the drops with previous experiences of antibiotics and felt the drops were beneficial and would be happy to use them for pain relief:
With the drops he appeared that the pain subsided whereas with antibiotics it takes a couple of days for it to start kicking in, so your topping them up with you know Calpol.
PA02
Yeah I probably would [use drops] because you know if the doctor’s saying to me you know if he has antibiotics, it’s only going to take a day off the pain, you know if he is going to be pain free for just one less day . . . instead of pumping, instead of putting antibiotics in, if I could put just a painkiller and it would take the pain away . . . if the infection if going to clear up on its own anyway, I think it would be good to have a painkiller for him . . . to take the pain away.
PA02
Parents with the confidence that the benzocaine/phenazone drops had reduced pain in their children would be happy to buy the drops from a pharmacy (over the counter) rather than consult the GP. This was on the premise that a pharmacist would be available for advice and information:
You know you’re giving it to a child, I think a little bit of advice from the pharmacist, how many times you can give it in a day and you know if the pain doesn’t go away within so many days you know contact your doctor sort of thing.
PA02
I’d probably like to ask the pharmacist that you know what they thought, and you know if they knew about the product and everything, it would just be sort of how to use it, you know not to overdose on it, just kind of basic information you get on most medicines.
PA03
Chapter 4 Discussion
Summary of main findings
Despite the CEDAR trial falling short of its sample size target, and demonstrating a lower than expected antibiotic consumption rate, the results provide evidence of a reduction in antibiotic consumption with the use of anaesthetic–analgesic ear drops compared with children who were not provided with ear drops.
The primary analysis (active drops vs. usual care) found modest evidence of a difference between groups when combining the results of the two- and three-group studies, with 11 out of 38 (29%) children in the usual-care group consuming antibiotics, compared with 1 out of 39 (3%) children in the anaesthetic–analgesic drops group. This represents an 87% reduction in the odds of consuming antibiotics (odds ratio 0.13, 95% CI 0.01 to 1.13). If this reflects a true effect of the ear drops, the most likely explanation for reduced antibiotic consumption is reduced pain. However, secondary analyses showed both placebo (glycerol) and active drops to be superior to no drops for pain relief at day 2. The former could be explained by the placebo effect or a soothing anti-inflammatory effect of glycerol. Secondary outcome measures provided modest evidence of reductions in oral analgesic consumption, symptom burden and illness duration in the active drops group compared with the usual care group.
Our economic analyses demonstrated that the use of active drops is unlikely to reduce medication costs in the short term, primarily because antibiotics are cheap. The more substantial economic benefits are likely to come in the longer term, via reduced antimicrobial resistance. Nevertheless, the ‘cost per antibiotic avoided’ of active drops is low and, if parental productivity costs are included in the analysis, it may even save money. Weak evidence was found that active drops improved health-related quality-of-life scores, measured by the CHU-9D at 8 days.
Interviews conducted with three participating parents revealed their views and experiences of AOM, its treatment and the CEDAR trial. The study aim was viewed positively by parents, who felt the treatment of pain to reduce the use of antibiotics was a good idea and who were happy for their child to participate. Parents’ main reason for taking their child to the doctor was that their child was upset and in pain and they wanted pain relief for their child. Parents did not express any preconceptions of whether or not the child should receive antibiotics and were happy to discuss treatment options with the doctor. Parents spoke positively about the trial drops, and all believed that they reduced pain in the child, although this was also the case for parents whose children received placebo drops. Parents stated that they would be happy to purchase the drops over the counter from a pharmacy, provided that pharmacist advice was available.
Strengths and weaknesses
The CEDAR trial was a pragmatic RCT conducted in UK primary care practices; allocation was concealed during recruitment and around 80% of parents provided full data on their child’s antibiotic consumption during the key 8-day follow-up period. However, the study was subject to a number of limitations.
The clear weaknesses of the CEDAR trial were the small sample size achieved (particularly with the planned three-group study), not being able to collect data at the planned 3-month follow-up point and not being able to commence recruitment in emergency departments as planned. Although comparisons of the raw data suggest a substantial treatment effect on antibiotic consumption, the results need to be interpreted with caution as estimates are imprecise and we are not able to confirm the probable mechanism through reduced pain. This weakness is likely to be purely one of low statistical power; although the study did close early, this is unlikely to have caused bias as early closure did not curtail the planned recruitment period and was a decision of the funder, who was unaware of the findings of the confidential interim analysis (although it had been made aware of the lower than expected antibiotic prescription rate across the three study groups combined). The lack of data at the 3-month follow-up means that the safety of the intervention in the period immediately after the infection, and the incidence of recurrent infection, remain unknown.
We observed a greater proportion of children in the usual-care group than in the drops groups who received a delayed prescription for an antibiotic. We had intended for children to receive these prescriptions prior to being allocated to a study group, although we cannot guarantee that this always occurred and, in hindsight, whether or not the child had received a delayed prescription should have been included in the information provided when logging the child into the study, in preparation for allocation to a study group. Those children provided with a delayed prescription were more likely to consume antibiotics during the 8-day follow-up; our prespecified primary analysis attempted to control for any confounding through this route. Of the small number of children in one of the drops groups who received a delayed prescription, none subsequently consumed antibiotics; this suggests that if receipt of a delayed prescription been equally low in the usual-care group, then this may have reduced antibiotic consumption in that group and hence reduced the difference in antibiotic consumption between study groups. However, taking a different view, although the intention had been to standardise antibiotic prescribing across the study groups, what actually happened in the trial would be closer to routine clinical practice, in which GPs might prescribe fewer antibiotics if they can provide anaesthetic–analgesic ear drops instead, with the lower prescription rate resulting in lower antibiotic consumption.
In fact, antibiotic consumption was already lower than anticipated in the usual-care group, potentially owing to the overcautious exclusion of children considered to need immediate antibiotics, to reduced use of delayed prescriptions by participating GPs who are perhaps already keen to reduce antibiotic consumption, or to informed consent procedures including the study rationale, which would have given parents the chance to opt out if they were keen to use antibiotics (the planned but unrealised observational cohort would have addressed this) or may have convinced some parents of the unsuitability of antibiotics for the treatment of AOM ear pain.
The larger number of participating children were recruited to the two-group study, which did not include the placebo drops group and so could not provide reliable evidence that the anaesthetic–analgesic drops were reducing antibiotic consumption through a painkilling mechanism. However, despite the modest sample size realised, the study does provide weak evidence that parents of children presenting to primary care with ear pain due to AOM find ear drops to be an acceptable treatment and are consequently less likely to use antibiotics. It seems unlikely that a different pattern of results would have been observed had children in the drops groups been more often provided with a delayed prescription for antibiotics, and indeed the study shows that parents of children in the drops group found it acceptable not to receive such a delayed prescription.
From the perspective of the economic analysis, as Auralgan is not in the BNF,22 it is currently unclear how much the NHS would pay for it if GPs were to prescribe it. Plausible assumptions have been made based on drug prices in other high-income countries and potential NHS discounts. However, if the actual procurement price were higher, the intervention would be less cost-effective than we have estimated. Owing to falling short of the recruitment target, the evidence of a reduction in health-care use up to 8 days was very weak, and with the review of medical records not going ahead we were unable to evaluate whether or not the possible reduction in health-care use might have been amplified over a 3-month period.
The strengths of the qualitative study are that it included parents who took part in the trial and it was therefore able to provide views and experiences from those directly involved. Reported themes were present across all interviews, strengthening their standing. All parents had previous experience of children with AOM and treatment and were therefore well placed to comment on whether or not the treatment was acceptable and whether or not they believed it worked. The main limitation is the small number of interviews conducted. Although more were planned, only three interviews with trial parents could be conducted within the timeframe of the three-group trial. This does provide useful insight into how parents experienced the trial and using the trial drops. However, caution needs to be exercised when taking findings forward. Views were captured only from those who agreed and continued to participate in the trial. Further interviews need to be conducted with a larger number of parents to determine whether or not findings continue to be prevalent and important. Further issues including divergent views and experiences may also be uncovered. As the sample was small, it was not possible to utilise a maximum variation approach and parents with different characteristics, for example socioeconomic backgrounds, child age, trial centre and trial group, need also to be interviewed. Owing to the delay with the trial it was also not possible to capture views and experiences of health-care professionals. Therefore, important insights into how the people directly involved in the care of the children and who were key to successful trial implementation were not gleaned. Future research needs to capture the views and experiences of health-care professionals from a range of trial sites.
Comparison with other literature
No new randomised studies of anaesthetic–analgesic ear drops have been published since the last Cochrane review in 2011. The three previous studies have assessed the effectiveness of topical analgesia against placebo in relieving pain due to AOM;11–13 all were included in the Cochrane review (updated 2011), which concluded that ‘the evidence from [these] RCTs is insufficient to know whether ear drops are effective’. 14 These studies address the efficacy of drops for pain and not the impact on case management with antibiotics; none of the trials included an economic evaluation. That said, pain relief is important to parents, and this emerged in the qualitative interviews completed for this study. In a post hoc and unblinded comparison of ear pain between this study’s active and usual-care groups, there was moderate evidence to suggest that active ear drops reduced ear pain, and this was strengthened after adjusting for baseline ear pain (at consultation). After adjustment, the combined mean difference was almost 2 points on the 0- to 6-point scale. In the analysis of pain relief following first instillation of drops in the small sample of 17 patients, there was no evidence to suggest that pain was reduced in active compared with placebo drops groups. However, both unadjusted and adjusted results were in favour of the active drops; those in the active groups had pain scores that were, on average, 0.73 points lower than in the placebo group. Thus, despite the small sample size, the present study may make a further contribution to the Cochrane review.
There is a well-recognised variation in prescribing rates for otitis media: the median rate being 60%, while in the highest decile of prescribing practices the figure is 90%. 1 A previous study in AOM investigated consumption,20 finding that 24% of children consumed antibiotics following a delayed prescription, compared with 33% in this usual-care group.
Our analyses suggest that anaesthetic–analgesic drops might increase medication costs during the acute episode. However, the wider societal costs of antimicrobial resistance were not quantified here. A literature review concluded that current estimates of the costs of resistance are likely to be underestimates and that an accurate forecast of costs may not be possible. 21 A recent trial evaluating the use of antibiotics in lower respiratory tract infections demonstrated that strategies to avoid antibiotic use become dramatically more cost-effective once the costs of resistance are included in the estimate. 35
Implications for clinical practice and research
Tackling antibiotic resistance is an international priority and reducing unnecessary antibiotic use for self-limiting illness is one potential mechanism to address this. This study suggests that substantial reduction in antibiotic use might be achieved in AOM in children by combining a no or delayed prescribing strategy with anaesthetic–analgesic ear drops.
A benefit of the analgesic effect of the active drops remains unproven when compared with placebo, although both active and glycerol drops may have important pain-relieving properties. Further research is needed to establish if effects are mediated by the active ingredients or if they could be achieved using a non-pharmacologically active oil.
Conclusion
This study has provided evidence that anaesthetic–analgesic ear drops result in a substantial reduction in antibiotic consumption in children presenting to primary care with AOM. The small number of children allocated to the placebo drops group was insufficient to establish whether or not the anaesthetic–analgesic ear drops reduced pain. Replication of these findings in a larger study would give greater confidence for informing clinical guidelines, would establish the safety of the intervention beyond the first 8 days and, if the mechanism of action can be established, may allow refinements to the intervention.
Acknowledgements
The CEDAR trial was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration, a UK Clinical Research Collaboration Registered Clinical Trials Unit in receipt of National Institute for Health Research (NIHR) Clinical Trials Unit support funding.
The study sponsor was the University of Bristol, and we would particularly like to acknowledge the support of Birgit Whitman. We would like to thank the independent members of our oversight committees:
Trial Steering Committee: Jonathan Mant, Cambridge University, UK (chairperson); Kay Wang, Oxford University, UK; Alan Smyth, Nottingham University, UK; and Victoria Wilson, patient and public involvement member.
Data Monitoring Committee: Toby Prevost, Imperial College London, UK (chairperson); Christian Mallen, Keele University, UK; and Alecia Nickless, Oxford University, UK.
We would like to acknowledge the essential contributions made to the CEDAR trial by the administrators at Bristol (Annie Sadoo, Kate Rowley, Penny Seume) Cardiff (Catherine Aymar) and Southampton (Oyinkan Alli-Balogen).
Study data were collected and managed using REDCap (Research Electronic Data Capture) tools (University of Colorado, Denver, CO, USA) hosted at the University of Bristol. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for importing data from external sources.
Funding history
The CEDAR trial was funded by the NIHR Health Technology Assessment programme. The project start date was 1 January 2015 and the planned study end date was 31 March 2018.
The project closed early, on 31 December 2017, as required by the funders. Issues were encountered with a lengthy delay to the IMP supply. Although the IMP supplier was identified through a competitive procurement process, the supplier failed to deliver the active drops and placebo in line with expected and revised timeframes. There were particular problems with the production of the matched placebo and the ability of the supplier to source materials to replicate the active drops. This contributed to the early closure of this important trial.
Contributions of authors
Alastair D Hay (University of Bristol) was the chief investigator chaired study management group, and contributed to the primary care expertise, study design, protocol development, trial monitoring, writing and approval of final report.
Harriet Downing (University of Bristol) was the trial manager and study management group member, and contributed to the protocol development and trial monitoring.
Nick A Francis (Cardiff University) was the principal investigator for Cardiff Centre and study management group member, and contributed to the primary care expertise, protocol development, trial monitoring, writing and approval of final report.
Grace J Young (University of Bristol) was the study statistician, and contributed to the statistical analysis, reporting to the data monitoring committee, writing and approval of final report.
Clare Clement (University of Bristol) was the qualitative researcher, and contributed to the qualitative interviews and analysis, writing and approval of final report.
Sue D Harris (University of Bristol) was the study nurse and study management group member, and contributed to the primary care expertise and approval of final report.
Aideen Ahern (University of Bristol) was the health economics researcher, and contributed to the health economics analysis and writing and approval of final report.
Behnaz Schofield (Cardiff University) was a study management group member, and contributed to the primary care expertise, protocol development, trial monitoring and approval of final report.
Tammy E Thomas (Southampton University) was a study management group member, and contributed to the primary care expertise, protocol development, trial monitoring and approval of final report.
Jeremy Horwood (University of Bristol) was the senior qualitative researcher, and contributed to the protocol development, study management group member, qualitative analysis, writing and approval of final report.
Peter S Blair (University of Bristol) was the senior statistician, and contributed to the protocol development, study management group, statistical analysis, writing and approval of final report.
William Hollingworth (University of Bristol) was the senior health economist, and contributed to the protocol development, study management group, health economic analysis, writing and approval of final report.
Victoria Wilson (University of Bristol) was the trial manager and study management group member, and contributed to the trial monitoring, writing and approval of final report.
Chris Metcalfe (University of Bristol) was the senior trials methodologist, and contributed to the study management group, writing and approval of final report.
Peter Stoddart (Bristol Royal Hospital for Children) was a study management group member, and contributed to the secondary care expertise, protocol development and approval of final report.
Desmond Nunez (University of British Columbia) was a study management group member, and contributed to the primary care expertise, protocol development, writing and approval of final report.
Mark D Lyttle (Bristol Royal Hospital for Children) was a study management group member, and contributed to the secondary care expertise, protocol development, approval of final report.
Paul Little (Southampton University) was a study management group member, and contributed to the primary care expertise, protocol development and approval of final report.
Michael V Moore (Southampton University) was the principal investigator for the Southampton Centre and was a study management group member, and contributed to the primary care expertise, protocol development, trial monitoring, writing and approval of final report.
Publication
Venekamp RP, Prasad V, Hay AD. Are topical antibiotics an alternative to oral antibiotics for children with acute otitis media and ear discharge? BMJ 2016;352:i308.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Gulliford MC, Dregan A, Moore MV, Ashworth M, Staa Tv, McCann G, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006245.
- Hawker JI, Smith S, Smith GE, Morbey R, Johnson AP, Fleming DM, et al. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995–2011: analysis of a large database of primary care consultations. J Antimicrob Chemother 2014;69:3423-30. https://doi.org/10.1093/jac/dku291.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340. https://doi.org/10.1136/bmj.c2096.
- Lietzau S, Raum E, von Baum H, Marre R, Brenner H. Household contacts were key factor for children’s colonization with resistant Escherichia coli in community setting. J Clin Epidemiol 2007;60:1149-55. https://doi.org/10.1016/j.jclinepi.2007.01.016.
- Fornasini M, Reves RR, Murray BE, Morrow AL, Pickering LK. Trimethoprim-resistant Escherichia coli in households of children attending day care centers. J Infect Dis 1992;166:326-30. https://doi.org/10.1093/infdis/166.2.326.
- UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. London: Department of Health and Social Care; 2013.
- Rovers MM, Glasziou P, Appelman CL, Burke P, McCormick DP, Damoiseaux RA, et al. Antibiotics for acute otitis media: a meta-analysis with individual patient data. Lancet 2006;368:1429-35. https://doi.org/10.1016/S0140-6736(06)69606-2.
- Clinical Knowledge Summary: Acute Otitis Media. London: NICE; 2013.
- Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD000219.pub4.
- Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the United Kingdom general practice research database. Pediatrics 2009;123:424-30. https://doi.org/10.1542/peds.2007-3349.
- Hoberman A, Paradise JL, Reynolds EA, Urkin J. Efficacy of Auralgan for treating ear pain in children with acute otitis media. Arch Pediatr Adolesc Med 1997;151:675-8. https://doi.org/10.1001/archpedi.1997.02170440037006.
- Bolt P, Barnett P, Babl FE, Sharwood LN. Topical lignocaine for pain relief in acute otitis media: results of a double-blind placebo-controlled randomised trial. Arch Dis Child 2008;93:40-4. https://doi.org/10.1136/adc.2006.110429.
- Sarrell EM, Cohen HA, Kahan E. Naturopathic treatment for ear pain in children. Pediatrics 2003;111:e574-9. https://doi.org/10.1542/peds.111.5.e574.
- Foxlee R, Johansson A, Wejfalk J, Dooley L, Mar Del C. Topical analgesia for acute otitis media (Review). Cochrane Database Syst Rev 2011;19.
- Respiratory Tract Infections (Self-Limiting): Prescribing Antibiotics. London: NICE; 2008.
- Ratcliffe J, Flynn T, Terlich F, Stevens K, Brazier J, Sawyer M. Developing adolescent-specific health state values for economic evaluation: an application of profile case best-worst scaling to the Child Health Utility 9D. PharmacoEconomics 2012;30:713-27. https://doi.org/10.2165/11597900-000000000-00000.
- Dakin H, Petrou S, Haggard M, Benge S, Williamson I. Mapping analyses to estimate health utilities based on responses to the OM8–30 Otitis Media Questionnaire. Qual Life Res 2010;19:65-80. https://doi.org/10.1007/s11136-009-9558-z.
- Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 2005;55:603-8.
- Williamson I, Benge S, Mullee M, Little P. Consultations for middle ear disease, antibiotic prescribing and risk factors for reattendance: a case-linked cohort study. Br J Gen Pract 2006;56:170-5.
- Little P, Gould C, Williamson I, Moore M, Warner G, Dunleavey J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001;322:336-42. https://doi.org/10.1136/bmj.322.7282.336.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ 2013;346. https://doi.org/10.1136/bmj.f1493.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Purchasing Power Parities. Paris: Organisation for Economic Co-operation and Development; 2016.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- BNF. London: NICE; 2017.
- Noble M, McLennan D, Wilkinson K, Whitworth A, Barnes H, Dibben C. The English Indices of Deprivation 2007. London: Communities and Local Government Publications; 2008.
- Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179-83. https://doi.org/10.1002/nur.4770180211.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Boyatzis R. Transforming Qualitative Information: Thematic Analysis and Code Development. Sage, CA: Thousand Oaks; 1998.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine; 1967.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage; 2006.
- Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652-4. https://doi.org/10.1136/bmj.325.7365.652.
- Turner J, O’Cathain A, Knowles E, Nicholl J, Tosh J, Sampson F, et al. Evaluation of NHS 111 Pilot Sites: Final Report 2012. www.sheffield.ac.uk/polopoly_fs/1.227404!/file/NHS_111_final_report_August_2012.pdf.
- Sickness Absence in the Labour Market, 2011. Newport: ONS; 2012.
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost-effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016;66:e633-9. https://doi.org/10.3399/bjgp16X686533.
Appendix 1 Recruitment to the CEDAR trial
FIGURE 10.
Projected and actual recruitment to the CEDAR trial: October 2016 to April 2017. Between October and December 2016, prior to the supply of IMP running low, randomisations to the two-group study reached target.

List of abbreviations
- AOM
- acute otitis media
- BNF
- British National Formulary
- CEDAR
- Children’s Ear Pain Study
- CI
- confidence interval
- DAEN
- Database of Adverse Event Notifications
- G6PD
- glucose-6-phosphate dehydrogenase
- GP
- general practitioner
- ID
- identifier
- IMP
- investigational medicinal product
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- RCT
- randomised controlled trial
- REDCap
- Research Electronic Data Capture
- SD
- standard deviation
